User login
Silent Epidemic: Loneliness a Serious Threat to Both Brain and Body
In a world that is more connected than ever, a silent epidemic is taking its toll. Overall, one in three US adults report chronic loneliness — a condition so detrimental that it rivals smoking and obesity with respect to its negative effect on health and well-being. From anxiety and depression to life-threatening conditions like cardiovascular disease, stroke, and Alzheimer’s and Parkinson’s diseases, loneliness is more than an emotion — it’s a serious threat to both the brain and body.
In 2023, a US Surgeon General advisory raised the alarm about the national problem of loneliness and isolation, describing it as an epidemic.
“Given the significant health consequences of loneliness and isolation, we must prioritize building social connection in the same way we have prioritized other critical public health issues such as tobacco, obesity, and substance use disorders. Together, we can build a country that’s healthier, more resilient, less lonely, and more connected,” the report concluded.
But how, exactly, does chronic loneliness affect the physiology and function of the brain? What does the latest research reveal about the link between loneliness and neurologic and psychiatric illness, and what can clinicians do to address the issue?
This news organization spoke to multiple experts in the field to explore these issues.
A Major Risk Factor
Anna Finley, PhD, assistant professor of psychology at North Dakota State University, Fargo, explained that loneliness and social isolation are different entities. Social isolation is an objective measure of the number of people someone interacts with on a regular basis, whereas loneliness is a subjective feeling that occurs when close connections are lacking.
“These two things are not actually as related as you think they would be. People can feel lonely in a crowd or feel well connected with only a few friendships. It’s more about the quality of the connection and the quality of your perception of it. So someone could be in some very supportive relationships but still feel that there’s something missing,” she said in an interview.
So what do we know about how loneliness affects health? Evidence supporting the hypothesis that loneliness is an emerging risk factor for many diseases is steadily building.
Recently, the American Heart Association published a statement summarizing the evidence for a direct association between social isolation and loneliness and coronary heart disease and stroke mortality.
In addition, many studies have shown that individuals experiencing social isolation or loneliness have an increased risk for anxiety and depression, dementia, infectious disease, hospitalization, and all-cause death, even after adjusting for age and many other traditional risk factors.
One study revealed that eliminating loneliness has the potential to prevent nearly 20% of cases of depression in adults aged 50 years or older.
Indu Subramanian, MD, professor of neurology at the University of California, Los Angeles, and colleagues conducted a study involving patients with Parkinson’s disease, which showed that the negative impact of loneliness on disease severity was as significant as the positive effects of 30 minutes of daily exercise.
“The importance of loneliness is under-recognized and undervalued, and it poses a major risk for health outcomes and quality of life,” said Subramanian.
Subramanian noted that loneliness is stigmatizing, causing people to feel unlikable and blame themselves, which prevents them from opening up to doctors or loved ones about their struggle. At the same time, healthcare providers may not think to ask about loneliness or know about potential interventions. She emphasized that much more work is needed to address this issue.
Early Mortality Risk
Julianne Holt-Lunstad, PhD, professor of psychology and neuroscience at Brigham Young University in Provo, Utah, is the author of two large meta-analyses that suggest loneliness, social isolation, or living alone are independent risk factors for early mortality, increasing this risk by about a third — the equivalent to the risk of smoking 15 cigarettes per day.
“We have quite robust evidence across a number of health outcomes implicating the harmful effects of loneliness and social isolation. While these are observational studies and show mainly associations, we do have evidence from longitudinal studies that show lacking social connection, whether that be loneliness or social isolation, predicts subsequent worse outcomes, and most of these studies have adjusted for alternative kinds of explanations, like age, initial health status, lifestyle factors,” Holt-Lunstad said.
There is some evidence to suggest that isolation is more predictive of physical health outcomes, whereas loneliness is more predictive of mental health outcomes. That said, both isolation and loneliness have significant effects on mental and physical health outcomes, she noted.
There is also the question of whether loneliness is causing poor health or whether people who are in poor health feel lonely because poor health can lead to social isolation.
Finley said there’s probably a bit of both going on, but longitudinal studies, where loneliness is measured at a fixed timepoint then health outcomes are reported a few years later, suggest that loneliness is contributing to these adverse outcomes.
She added that there is also some evidence in animal models to suggest that loneliness is a causal risk factor for adverse health outcomes. “But you can’t ask a mouse or rat how lonely they’re feeling. All you can do is house them individually — removing them from social connection. This isn’t necessarily the same thing as loneliness in humans.”
Finley is studying mechanisms in the brain that may be involved in mediating the adverse health consequences of loneliness.
“What I’ve been seeing in the data so far is that it tends to be the self-report of how lonely folks are feeling that has the associations with differences in the brain, as opposed to the number of social connections people have. It does seem to be the more subjective, emotional perception of loneliness that is important.”
In a review of potential mechanisms involved, she concluded that it is dysregulated emotions and altered perceptions of social interactions that has profound impacts on the brain, suggesting that people who are lonely may have a tendency to interpret social cues in a negative way, preventing them from forming productive positive relationships.
Lack of Trust
One researcher who has studied this phenomenon is Dirk Scheele, PhD, professor of social neuroscience at Ruhr University Bochum in Germany.
“We were interested to find out why people remained lonely,” he said in an interview. “Loneliness is an unpleasant experience, and there are so many opportunities for social contacts nowadays, it’s not really clear at first sight why people are chronically lonely.”
To examine this question, Scheele and his team conducted a study in which functional MRI was used to examine the brain in otherwise healthy individuals with high or low loneliness scores while they played a trust game.
They also simulated a positive social interaction between participants and researchers, in which they talked about plans for a fictitious lottery win, and about their hobbies and interests, during which mood was measured with questionnaires, and saliva samples were collected to measure hormone levels.
Results showed that the high-lonely individuals had reduced activation in the insula cortex during the trust decisions. “This area of the brain is involved in the processing of bodily signals, such as ‘gut feelings.’ So reduced activity here could be interpreted as fewer gut feelings on who can be trusted,” Scheele explained.
The high-lonely individuals also had reduced responsiveness to the positive social interaction with a lower release of oxytocin and a smaller elevation in mood compared with the control individuals.
Scheele pointed out that there is some evidence that oxytocin might increase trust, and there is reduced release of endogenous oxytocin in high loneliness.
“Our results are consistent with the idea that loneliness is associated with negative biases about other people. So if we expect negative things from other people — for instance, that they cannot be trusted — then that would hamper further social interactions and could lead to loneliness,” he added.
A Role for Oxytocin?
In another study, the same researchers tested short-term (five weekly sessions) group psychotherapy to reduce loneliness using established techniques to target these negative biases. They also investigated whether the effects of this group psychotherapy could be augmented by administering intranasal oxytocin (vs placebo) before the group psychotherapy sessions.
Results showed that the group psychotherapy intervention reduced trait loneliness (loneliness experienced over a prolonged period). The oxytocin did not show a significant effect on trait loneliness, but there was a suggestion that it may enhance the reduction in state loneliness (how someone is feeling at a specific time) brought about by the psychotherapy sessions.
“We found that bonding within the groups was experienced as more positive in the oxytocin treated groups. It is possible that a longer intervention would be helpful for longer-term results,” Scheele concluded. “It’s not going to be a quick fix for loneliness, but there may be a role for oxytocin as an adjunct to psychotherapy.”
A Basic Human Need
Another loneliness researcher, Livia Tomova, PhD, assistant professor of psychology at Cardiff University in Wales, has used social isolation to induce loneliness in young people and found that this intervention was linked to brain patterns similar to those associated with hunger.
“We know that the drive to eat food is a very basic human need. We know quite well how it is represented in the brain,” she explained.
The researchers tested how the brains of the participants responded to seeing pictures of social interactions after they underwent a prolonged period of social isolation. In a subsequent session, the same people were asked to undergo food fasting and then underwent brain scans when looking at pictures of food. Results showed that the neural patterns were similar in the two situations with increased activity in the substantia nigra area within the midbrain.
“This area of the brain processes rewards and motivation. It consists primarily of dopamine neurons and increased activity corresponds to a feeling of craving something. So this area of the brain that controls essential homeostatic needs is activated when people feel lonely, suggesting that our need for social contact with others is potentially a very basic need similar to eating,” Tomova said.
Lower Gray Matter Volumes in Key Brain Areas
And another group from Germany has found that higher loneliness scores are negatively associated with specific brain regions responsible for memory, emotion regulation, and social processing.
Sandra Düzel, PhD, and colleagues from the Max Planck Institute for Human Development and the Charité – Universitätsmedizin Berlin, both in Berlin, Germany, reported a study in which individuals who reported higher loneliness had smaller gray matter volumes in brain regions such as the left amygdala, anterior hippocampus, and cerebellum, regions which are crucial for both emotional regulation and higher-order cognitive processes, such as self-reflection and executive function.
Düzel believes that possible mechanisms behind the link between loneliness and brain volume differences could include stress-related damage, with prolonged loneliness associated with elevated levels of stress hormones, which can damage the hippocampus over time, and reduced cognitive and social stimulation, which may contribute to brain volume reductions in regions critical for memory and emotional processing.
“Loneliness is often characterized by reduced social and environmental diversity, leading to less engagement with novel experiences and potentially lower hippocampal-striatal connectivity.
Since novelty-seeking and environmental diversity are associated with positive emotional states, individuals experiencing loneliness might benefit from increased exposure to new environments which could stimulate the brain’s reward circuits, fostering positive affect and potentially mitigating the emotional burden of loneliness,” she said.
Is Social Prescribing the Answer?
So are there enough data now to act and attempt to develop interventions to reduce loneliness? Most of these researchers believe so.
“I think we have enough information to act on this now. There are a number of national academies consensus reports, which suggest that, while certainly there are still gaps in our evidence and more to be learned, there is sufficient evidence that a concerning portion of the population seems to lack connection, and that the consequences are serious enough that we need to do something about it,” said Holt-Lunstad.
Some countries have introduced social prescribing where doctors can prescribe a group activity or a regular visit or telephone conversation with a supportive person.
Subramanian pointed out that it’s easier to implement in countries with national health services and may be more difficult to embrace in the US healthcare system.
“We are not so encouraged from a financial perspective to think about preventive care in the US. We don’t have an easy way to recognize in any tangible way the downstream of such activities in terms of preventing future problems. That is something we need to work on,” she said.
Finley cautioned that to work well, social prescribing will require an understanding of each person’s individual situation.
“Some people may only receive benefit of interacting with others if they are also getting some sort of support to address the social and emotional concerns that are tagging along with loneliness. I’m not sure that just telling people to go join their local gardening club or whatever will be the correct answer for everyone.”
She pointed out that many people will have issues in their life that are making it hard for them to be social. These could be mobility or financial challenges, care responsibilities, or concerns about illnesses or life events. “We need to figure out what would have the most bang for the person’s buck, so to speak, as an intervention. That could mean connecting them to a group relevant to their individual situation.”
Opportunity to Connect Not Enough?
Tomova believes that training people in social skills may be a better option. “It appears that some people who are chronically lonely seem to struggle to make relationships with others. So just encouraging them to interact with others more will not necessarily help. We need to better understand the pathways involved and who are the people who become ill. We can then develop and target better interventions and teach people coping strategies for that situation.”
Scheele agreed. “While just giving people the opportunity to connect may work for some, others who are experiencing really chronic loneliness may not benefit very much from this unless their negative belief systems are addressed.” He suggested some sort of psychotherapy may be helpful in this situation.
But at least all seem to agree that healthcare providers need to be more aware of loneliness as a health risk factor, try to identify people at risk, and to think about how best to support them.
Holt-Lunstad noted that one of the recommendations in the US Surgeon General’s advisory was to increase the education, training, and resources on loneliness for healthcare providers.
“If we want this to be addressed, we need to give healthcare providers the time, resources, and training in order to do that, otherwise, we are adding one more thing to an already overburdened system. They need to understand how important it is, and how it might help them take care of the patient.”
“Our hope is that we can start to reverse some of the trends that we are seeing, both in terms of the prevalence rates of loneliness, but also that we could start seeing improvements in health and other kinds of outcomes,” she concluded.
Progress is being made in increasing awareness about the dangers of chronic loneliness. It’s now recognized as a serious health risk, but there are actionable steps that can help. Loneliness doesn’t have to be a permanent condition for anyone, said Scheele.
Holt-Lunstad served as an adviser for Foundation for Social Connection, Global Initiative on Loneliness and Connection, and Nextdoor Neighborhood Vitality Board and received research grants/income from Templeton Foundation, Eventbrite, Foundation for Social Connection, and Triple-S Foundation. Subramanian served as a speaker bureau for Acorda Pharma. The other researchers reported no disclosures.
A version of this article first appeared on Medscape.com.
In a world that is more connected than ever, a silent epidemic is taking its toll. Overall, one in three US adults report chronic loneliness — a condition so detrimental that it rivals smoking and obesity with respect to its negative effect on health and well-being. From anxiety and depression to life-threatening conditions like cardiovascular disease, stroke, and Alzheimer’s and Parkinson’s diseases, loneliness is more than an emotion — it’s a serious threat to both the brain and body.
In 2023, a US Surgeon General advisory raised the alarm about the national problem of loneliness and isolation, describing it as an epidemic.
“Given the significant health consequences of loneliness and isolation, we must prioritize building social connection in the same way we have prioritized other critical public health issues such as tobacco, obesity, and substance use disorders. Together, we can build a country that’s healthier, more resilient, less lonely, and more connected,” the report concluded.
But how, exactly, does chronic loneliness affect the physiology and function of the brain? What does the latest research reveal about the link between loneliness and neurologic and psychiatric illness, and what can clinicians do to address the issue?
This news organization spoke to multiple experts in the field to explore these issues.
A Major Risk Factor
Anna Finley, PhD, assistant professor of psychology at North Dakota State University, Fargo, explained that loneliness and social isolation are different entities. Social isolation is an objective measure of the number of people someone interacts with on a regular basis, whereas loneliness is a subjective feeling that occurs when close connections are lacking.
“These two things are not actually as related as you think they would be. People can feel lonely in a crowd or feel well connected with only a few friendships. It’s more about the quality of the connection and the quality of your perception of it. So someone could be in some very supportive relationships but still feel that there’s something missing,” she said in an interview.
So what do we know about how loneliness affects health? Evidence supporting the hypothesis that loneliness is an emerging risk factor for many diseases is steadily building.
Recently, the American Heart Association published a statement summarizing the evidence for a direct association between social isolation and loneliness and coronary heart disease and stroke mortality.
In addition, many studies have shown that individuals experiencing social isolation or loneliness have an increased risk for anxiety and depression, dementia, infectious disease, hospitalization, and all-cause death, even after adjusting for age and many other traditional risk factors.
One study revealed that eliminating loneliness has the potential to prevent nearly 20% of cases of depression in adults aged 50 years or older.
Indu Subramanian, MD, professor of neurology at the University of California, Los Angeles, and colleagues conducted a study involving patients with Parkinson’s disease, which showed that the negative impact of loneliness on disease severity was as significant as the positive effects of 30 minutes of daily exercise.
“The importance of loneliness is under-recognized and undervalued, and it poses a major risk for health outcomes and quality of life,” said Subramanian.
Subramanian noted that loneliness is stigmatizing, causing people to feel unlikable and blame themselves, which prevents them from opening up to doctors or loved ones about their struggle. At the same time, healthcare providers may not think to ask about loneliness or know about potential interventions. She emphasized that much more work is needed to address this issue.
Early Mortality Risk
Julianne Holt-Lunstad, PhD, professor of psychology and neuroscience at Brigham Young University in Provo, Utah, is the author of two large meta-analyses that suggest loneliness, social isolation, or living alone are independent risk factors for early mortality, increasing this risk by about a third — the equivalent to the risk of smoking 15 cigarettes per day.
“We have quite robust evidence across a number of health outcomes implicating the harmful effects of loneliness and social isolation. While these are observational studies and show mainly associations, we do have evidence from longitudinal studies that show lacking social connection, whether that be loneliness or social isolation, predicts subsequent worse outcomes, and most of these studies have adjusted for alternative kinds of explanations, like age, initial health status, lifestyle factors,” Holt-Lunstad said.
There is some evidence to suggest that isolation is more predictive of physical health outcomes, whereas loneliness is more predictive of mental health outcomes. That said, both isolation and loneliness have significant effects on mental and physical health outcomes, she noted.
There is also the question of whether loneliness is causing poor health or whether people who are in poor health feel lonely because poor health can lead to social isolation.
Finley said there’s probably a bit of both going on, but longitudinal studies, where loneliness is measured at a fixed timepoint then health outcomes are reported a few years later, suggest that loneliness is contributing to these adverse outcomes.
She added that there is also some evidence in animal models to suggest that loneliness is a causal risk factor for adverse health outcomes. “But you can’t ask a mouse or rat how lonely they’re feeling. All you can do is house them individually — removing them from social connection. This isn’t necessarily the same thing as loneliness in humans.”
Finley is studying mechanisms in the brain that may be involved in mediating the adverse health consequences of loneliness.
“What I’ve been seeing in the data so far is that it tends to be the self-report of how lonely folks are feeling that has the associations with differences in the brain, as opposed to the number of social connections people have. It does seem to be the more subjective, emotional perception of loneliness that is important.”
In a review of potential mechanisms involved, she concluded that it is dysregulated emotions and altered perceptions of social interactions that has profound impacts on the brain, suggesting that people who are lonely may have a tendency to interpret social cues in a negative way, preventing them from forming productive positive relationships.
Lack of Trust
One researcher who has studied this phenomenon is Dirk Scheele, PhD, professor of social neuroscience at Ruhr University Bochum in Germany.
“We were interested to find out why people remained lonely,” he said in an interview. “Loneliness is an unpleasant experience, and there are so many opportunities for social contacts nowadays, it’s not really clear at first sight why people are chronically lonely.”
To examine this question, Scheele and his team conducted a study in which functional MRI was used to examine the brain in otherwise healthy individuals with high or low loneliness scores while they played a trust game.
They also simulated a positive social interaction between participants and researchers, in which they talked about plans for a fictitious lottery win, and about their hobbies and interests, during which mood was measured with questionnaires, and saliva samples were collected to measure hormone levels.
Results showed that the high-lonely individuals had reduced activation in the insula cortex during the trust decisions. “This area of the brain is involved in the processing of bodily signals, such as ‘gut feelings.’ So reduced activity here could be interpreted as fewer gut feelings on who can be trusted,” Scheele explained.
The high-lonely individuals also had reduced responsiveness to the positive social interaction with a lower release of oxytocin and a smaller elevation in mood compared with the control individuals.
Scheele pointed out that there is some evidence that oxytocin might increase trust, and there is reduced release of endogenous oxytocin in high loneliness.
“Our results are consistent with the idea that loneliness is associated with negative biases about other people. So if we expect negative things from other people — for instance, that they cannot be trusted — then that would hamper further social interactions and could lead to loneliness,” he added.
A Role for Oxytocin?
In another study, the same researchers tested short-term (five weekly sessions) group psychotherapy to reduce loneliness using established techniques to target these negative biases. They also investigated whether the effects of this group psychotherapy could be augmented by administering intranasal oxytocin (vs placebo) before the group psychotherapy sessions.
Results showed that the group psychotherapy intervention reduced trait loneliness (loneliness experienced over a prolonged period). The oxytocin did not show a significant effect on trait loneliness, but there was a suggestion that it may enhance the reduction in state loneliness (how someone is feeling at a specific time) brought about by the psychotherapy sessions.
“We found that bonding within the groups was experienced as more positive in the oxytocin treated groups. It is possible that a longer intervention would be helpful for longer-term results,” Scheele concluded. “It’s not going to be a quick fix for loneliness, but there may be a role for oxytocin as an adjunct to psychotherapy.”
A Basic Human Need
Another loneliness researcher, Livia Tomova, PhD, assistant professor of psychology at Cardiff University in Wales, has used social isolation to induce loneliness in young people and found that this intervention was linked to brain patterns similar to those associated with hunger.
“We know that the drive to eat food is a very basic human need. We know quite well how it is represented in the brain,” she explained.
The researchers tested how the brains of the participants responded to seeing pictures of social interactions after they underwent a prolonged period of social isolation. In a subsequent session, the same people were asked to undergo food fasting and then underwent brain scans when looking at pictures of food. Results showed that the neural patterns were similar in the two situations with increased activity in the substantia nigra area within the midbrain.
“This area of the brain processes rewards and motivation. It consists primarily of dopamine neurons and increased activity corresponds to a feeling of craving something. So this area of the brain that controls essential homeostatic needs is activated when people feel lonely, suggesting that our need for social contact with others is potentially a very basic need similar to eating,” Tomova said.
Lower Gray Matter Volumes in Key Brain Areas
And another group from Germany has found that higher loneliness scores are negatively associated with specific brain regions responsible for memory, emotion regulation, and social processing.
Sandra Düzel, PhD, and colleagues from the Max Planck Institute for Human Development and the Charité – Universitätsmedizin Berlin, both in Berlin, Germany, reported a study in which individuals who reported higher loneliness had smaller gray matter volumes in brain regions such as the left amygdala, anterior hippocampus, and cerebellum, regions which are crucial for both emotional regulation and higher-order cognitive processes, such as self-reflection and executive function.
Düzel believes that possible mechanisms behind the link between loneliness and brain volume differences could include stress-related damage, with prolonged loneliness associated with elevated levels of stress hormones, which can damage the hippocampus over time, and reduced cognitive and social stimulation, which may contribute to brain volume reductions in regions critical for memory and emotional processing.
“Loneliness is often characterized by reduced social and environmental diversity, leading to less engagement with novel experiences and potentially lower hippocampal-striatal connectivity.
Since novelty-seeking and environmental diversity are associated with positive emotional states, individuals experiencing loneliness might benefit from increased exposure to new environments which could stimulate the brain’s reward circuits, fostering positive affect and potentially mitigating the emotional burden of loneliness,” she said.
Is Social Prescribing the Answer?
So are there enough data now to act and attempt to develop interventions to reduce loneliness? Most of these researchers believe so.
“I think we have enough information to act on this now. There are a number of national academies consensus reports, which suggest that, while certainly there are still gaps in our evidence and more to be learned, there is sufficient evidence that a concerning portion of the population seems to lack connection, and that the consequences are serious enough that we need to do something about it,” said Holt-Lunstad.
Some countries have introduced social prescribing where doctors can prescribe a group activity or a regular visit or telephone conversation with a supportive person.
Subramanian pointed out that it’s easier to implement in countries with national health services and may be more difficult to embrace in the US healthcare system.
“We are not so encouraged from a financial perspective to think about preventive care in the US. We don’t have an easy way to recognize in any tangible way the downstream of such activities in terms of preventing future problems. That is something we need to work on,” she said.
Finley cautioned that to work well, social prescribing will require an understanding of each person’s individual situation.
“Some people may only receive benefit of interacting with others if they are also getting some sort of support to address the social and emotional concerns that are tagging along with loneliness. I’m not sure that just telling people to go join their local gardening club or whatever will be the correct answer for everyone.”
She pointed out that many people will have issues in their life that are making it hard for them to be social. These could be mobility or financial challenges, care responsibilities, or concerns about illnesses or life events. “We need to figure out what would have the most bang for the person’s buck, so to speak, as an intervention. That could mean connecting them to a group relevant to their individual situation.”
Opportunity to Connect Not Enough?
Tomova believes that training people in social skills may be a better option. “It appears that some people who are chronically lonely seem to struggle to make relationships with others. So just encouraging them to interact with others more will not necessarily help. We need to better understand the pathways involved and who are the people who become ill. We can then develop and target better interventions and teach people coping strategies for that situation.”
Scheele agreed. “While just giving people the opportunity to connect may work for some, others who are experiencing really chronic loneliness may not benefit very much from this unless their negative belief systems are addressed.” He suggested some sort of psychotherapy may be helpful in this situation.
But at least all seem to agree that healthcare providers need to be more aware of loneliness as a health risk factor, try to identify people at risk, and to think about how best to support them.
Holt-Lunstad noted that one of the recommendations in the US Surgeon General’s advisory was to increase the education, training, and resources on loneliness for healthcare providers.
“If we want this to be addressed, we need to give healthcare providers the time, resources, and training in order to do that, otherwise, we are adding one more thing to an already overburdened system. They need to understand how important it is, and how it might help them take care of the patient.”
“Our hope is that we can start to reverse some of the trends that we are seeing, both in terms of the prevalence rates of loneliness, but also that we could start seeing improvements in health and other kinds of outcomes,” she concluded.
Progress is being made in increasing awareness about the dangers of chronic loneliness. It’s now recognized as a serious health risk, but there are actionable steps that can help. Loneliness doesn’t have to be a permanent condition for anyone, said Scheele.
Holt-Lunstad served as an adviser for Foundation for Social Connection, Global Initiative on Loneliness and Connection, and Nextdoor Neighborhood Vitality Board and received research grants/income from Templeton Foundation, Eventbrite, Foundation for Social Connection, and Triple-S Foundation. Subramanian served as a speaker bureau for Acorda Pharma. The other researchers reported no disclosures.
A version of this article first appeared on Medscape.com.
In a world that is more connected than ever, a silent epidemic is taking its toll. Overall, one in three US adults report chronic loneliness — a condition so detrimental that it rivals smoking and obesity with respect to its negative effect on health and well-being. From anxiety and depression to life-threatening conditions like cardiovascular disease, stroke, and Alzheimer’s and Parkinson’s diseases, loneliness is more than an emotion — it’s a serious threat to both the brain and body.
In 2023, a US Surgeon General advisory raised the alarm about the national problem of loneliness and isolation, describing it as an epidemic.
“Given the significant health consequences of loneliness and isolation, we must prioritize building social connection in the same way we have prioritized other critical public health issues such as tobacco, obesity, and substance use disorders. Together, we can build a country that’s healthier, more resilient, less lonely, and more connected,” the report concluded.
But how, exactly, does chronic loneliness affect the physiology and function of the brain? What does the latest research reveal about the link between loneliness and neurologic and psychiatric illness, and what can clinicians do to address the issue?
This news organization spoke to multiple experts in the field to explore these issues.
A Major Risk Factor
Anna Finley, PhD, assistant professor of psychology at North Dakota State University, Fargo, explained that loneliness and social isolation are different entities. Social isolation is an objective measure of the number of people someone interacts with on a regular basis, whereas loneliness is a subjective feeling that occurs when close connections are lacking.
“These two things are not actually as related as you think they would be. People can feel lonely in a crowd or feel well connected with only a few friendships. It’s more about the quality of the connection and the quality of your perception of it. So someone could be in some very supportive relationships but still feel that there’s something missing,” she said in an interview.
So what do we know about how loneliness affects health? Evidence supporting the hypothesis that loneliness is an emerging risk factor for many diseases is steadily building.
Recently, the American Heart Association published a statement summarizing the evidence for a direct association between social isolation and loneliness and coronary heart disease and stroke mortality.
In addition, many studies have shown that individuals experiencing social isolation or loneliness have an increased risk for anxiety and depression, dementia, infectious disease, hospitalization, and all-cause death, even after adjusting for age and many other traditional risk factors.
One study revealed that eliminating loneliness has the potential to prevent nearly 20% of cases of depression in adults aged 50 years or older.
Indu Subramanian, MD, professor of neurology at the University of California, Los Angeles, and colleagues conducted a study involving patients with Parkinson’s disease, which showed that the negative impact of loneliness on disease severity was as significant as the positive effects of 30 minutes of daily exercise.
“The importance of loneliness is under-recognized and undervalued, and it poses a major risk for health outcomes and quality of life,” said Subramanian.
Subramanian noted that loneliness is stigmatizing, causing people to feel unlikable and blame themselves, which prevents them from opening up to doctors or loved ones about their struggle. At the same time, healthcare providers may not think to ask about loneliness or know about potential interventions. She emphasized that much more work is needed to address this issue.
Early Mortality Risk
Julianne Holt-Lunstad, PhD, professor of psychology and neuroscience at Brigham Young University in Provo, Utah, is the author of two large meta-analyses that suggest loneliness, social isolation, or living alone are independent risk factors for early mortality, increasing this risk by about a third — the equivalent to the risk of smoking 15 cigarettes per day.
“We have quite robust evidence across a number of health outcomes implicating the harmful effects of loneliness and social isolation. While these are observational studies and show mainly associations, we do have evidence from longitudinal studies that show lacking social connection, whether that be loneliness or social isolation, predicts subsequent worse outcomes, and most of these studies have adjusted for alternative kinds of explanations, like age, initial health status, lifestyle factors,” Holt-Lunstad said.
There is some evidence to suggest that isolation is more predictive of physical health outcomes, whereas loneliness is more predictive of mental health outcomes. That said, both isolation and loneliness have significant effects on mental and physical health outcomes, she noted.
There is also the question of whether loneliness is causing poor health or whether people who are in poor health feel lonely because poor health can lead to social isolation.
Finley said there’s probably a bit of both going on, but longitudinal studies, where loneliness is measured at a fixed timepoint then health outcomes are reported a few years later, suggest that loneliness is contributing to these adverse outcomes.
She added that there is also some evidence in animal models to suggest that loneliness is a causal risk factor for adverse health outcomes. “But you can’t ask a mouse or rat how lonely they’re feeling. All you can do is house them individually — removing them from social connection. This isn’t necessarily the same thing as loneliness in humans.”
Finley is studying mechanisms in the brain that may be involved in mediating the adverse health consequences of loneliness.
“What I’ve been seeing in the data so far is that it tends to be the self-report of how lonely folks are feeling that has the associations with differences in the brain, as opposed to the number of social connections people have. It does seem to be the more subjective, emotional perception of loneliness that is important.”
In a review of potential mechanisms involved, she concluded that it is dysregulated emotions and altered perceptions of social interactions that has profound impacts on the brain, suggesting that people who are lonely may have a tendency to interpret social cues in a negative way, preventing them from forming productive positive relationships.
Lack of Trust
One researcher who has studied this phenomenon is Dirk Scheele, PhD, professor of social neuroscience at Ruhr University Bochum in Germany.
“We were interested to find out why people remained lonely,” he said in an interview. “Loneliness is an unpleasant experience, and there are so many opportunities for social contacts nowadays, it’s not really clear at first sight why people are chronically lonely.”
To examine this question, Scheele and his team conducted a study in which functional MRI was used to examine the brain in otherwise healthy individuals with high or low loneliness scores while they played a trust game.
They also simulated a positive social interaction between participants and researchers, in which they talked about plans for a fictitious lottery win, and about their hobbies and interests, during which mood was measured with questionnaires, and saliva samples were collected to measure hormone levels.
Results showed that the high-lonely individuals had reduced activation in the insula cortex during the trust decisions. “This area of the brain is involved in the processing of bodily signals, such as ‘gut feelings.’ So reduced activity here could be interpreted as fewer gut feelings on who can be trusted,” Scheele explained.
The high-lonely individuals also had reduced responsiveness to the positive social interaction with a lower release of oxytocin and a smaller elevation in mood compared with the control individuals.
Scheele pointed out that there is some evidence that oxytocin might increase trust, and there is reduced release of endogenous oxytocin in high loneliness.
“Our results are consistent with the idea that loneliness is associated with negative biases about other people. So if we expect negative things from other people — for instance, that they cannot be trusted — then that would hamper further social interactions and could lead to loneliness,” he added.
A Role for Oxytocin?
In another study, the same researchers tested short-term (five weekly sessions) group psychotherapy to reduce loneliness using established techniques to target these negative biases. They also investigated whether the effects of this group psychotherapy could be augmented by administering intranasal oxytocin (vs placebo) before the group psychotherapy sessions.
Results showed that the group psychotherapy intervention reduced trait loneliness (loneliness experienced over a prolonged period). The oxytocin did not show a significant effect on trait loneliness, but there was a suggestion that it may enhance the reduction in state loneliness (how someone is feeling at a specific time) brought about by the psychotherapy sessions.
“We found that bonding within the groups was experienced as more positive in the oxytocin treated groups. It is possible that a longer intervention would be helpful for longer-term results,” Scheele concluded. “It’s not going to be a quick fix for loneliness, but there may be a role for oxytocin as an adjunct to psychotherapy.”
A Basic Human Need
Another loneliness researcher, Livia Tomova, PhD, assistant professor of psychology at Cardiff University in Wales, has used social isolation to induce loneliness in young people and found that this intervention was linked to brain patterns similar to those associated with hunger.
“We know that the drive to eat food is a very basic human need. We know quite well how it is represented in the brain,” she explained.
The researchers tested how the brains of the participants responded to seeing pictures of social interactions after they underwent a prolonged period of social isolation. In a subsequent session, the same people were asked to undergo food fasting and then underwent brain scans when looking at pictures of food. Results showed that the neural patterns were similar in the two situations with increased activity in the substantia nigra area within the midbrain.
“This area of the brain processes rewards and motivation. It consists primarily of dopamine neurons and increased activity corresponds to a feeling of craving something. So this area of the brain that controls essential homeostatic needs is activated when people feel lonely, suggesting that our need for social contact with others is potentially a very basic need similar to eating,” Tomova said.
Lower Gray Matter Volumes in Key Brain Areas
And another group from Germany has found that higher loneliness scores are negatively associated with specific brain regions responsible for memory, emotion regulation, and social processing.
Sandra Düzel, PhD, and colleagues from the Max Planck Institute for Human Development and the Charité – Universitätsmedizin Berlin, both in Berlin, Germany, reported a study in which individuals who reported higher loneliness had smaller gray matter volumes in brain regions such as the left amygdala, anterior hippocampus, and cerebellum, regions which are crucial for both emotional regulation and higher-order cognitive processes, such as self-reflection and executive function.
Düzel believes that possible mechanisms behind the link between loneliness and brain volume differences could include stress-related damage, with prolonged loneliness associated with elevated levels of stress hormones, which can damage the hippocampus over time, and reduced cognitive and social stimulation, which may contribute to brain volume reductions in regions critical for memory and emotional processing.
“Loneliness is often characterized by reduced social and environmental diversity, leading to less engagement with novel experiences and potentially lower hippocampal-striatal connectivity.
Since novelty-seeking and environmental diversity are associated with positive emotional states, individuals experiencing loneliness might benefit from increased exposure to new environments which could stimulate the brain’s reward circuits, fostering positive affect and potentially mitigating the emotional burden of loneliness,” she said.
Is Social Prescribing the Answer?
So are there enough data now to act and attempt to develop interventions to reduce loneliness? Most of these researchers believe so.
“I think we have enough information to act on this now. There are a number of national academies consensus reports, which suggest that, while certainly there are still gaps in our evidence and more to be learned, there is sufficient evidence that a concerning portion of the population seems to lack connection, and that the consequences are serious enough that we need to do something about it,” said Holt-Lunstad.
Some countries have introduced social prescribing where doctors can prescribe a group activity or a regular visit or telephone conversation with a supportive person.
Subramanian pointed out that it’s easier to implement in countries with national health services and may be more difficult to embrace in the US healthcare system.
“We are not so encouraged from a financial perspective to think about preventive care in the US. We don’t have an easy way to recognize in any tangible way the downstream of such activities in terms of preventing future problems. That is something we need to work on,” she said.
Finley cautioned that to work well, social prescribing will require an understanding of each person’s individual situation.
“Some people may only receive benefit of interacting with others if they are also getting some sort of support to address the social and emotional concerns that are tagging along with loneliness. I’m not sure that just telling people to go join their local gardening club or whatever will be the correct answer for everyone.”
She pointed out that many people will have issues in their life that are making it hard for them to be social. These could be mobility or financial challenges, care responsibilities, or concerns about illnesses or life events. “We need to figure out what would have the most bang for the person’s buck, so to speak, as an intervention. That could mean connecting them to a group relevant to their individual situation.”
Opportunity to Connect Not Enough?
Tomova believes that training people in social skills may be a better option. “It appears that some people who are chronically lonely seem to struggle to make relationships with others. So just encouraging them to interact with others more will not necessarily help. We need to better understand the pathways involved and who are the people who become ill. We can then develop and target better interventions and teach people coping strategies for that situation.”
Scheele agreed. “While just giving people the opportunity to connect may work for some, others who are experiencing really chronic loneliness may not benefit very much from this unless their negative belief systems are addressed.” He suggested some sort of psychotherapy may be helpful in this situation.
But at least all seem to agree that healthcare providers need to be more aware of loneliness as a health risk factor, try to identify people at risk, and to think about how best to support them.
Holt-Lunstad noted that one of the recommendations in the US Surgeon General’s advisory was to increase the education, training, and resources on loneliness for healthcare providers.
“If we want this to be addressed, we need to give healthcare providers the time, resources, and training in order to do that, otherwise, we are adding one more thing to an already overburdened system. They need to understand how important it is, and how it might help them take care of the patient.”
“Our hope is that we can start to reverse some of the trends that we are seeing, both in terms of the prevalence rates of loneliness, but also that we could start seeing improvements in health and other kinds of outcomes,” she concluded.
Progress is being made in increasing awareness about the dangers of chronic loneliness. It’s now recognized as a serious health risk, but there are actionable steps that can help. Loneliness doesn’t have to be a permanent condition for anyone, said Scheele.
Holt-Lunstad served as an adviser for Foundation for Social Connection, Global Initiative on Loneliness and Connection, and Nextdoor Neighborhood Vitality Board and received research grants/income from Templeton Foundation, Eventbrite, Foundation for Social Connection, and Triple-S Foundation. Subramanian served as a speaker bureau for Acorda Pharma. The other researchers reported no disclosures.
A version of this article first appeared on Medscape.com.
IBS: Understanding a Common Yet Misunderstood Condition
Irritable bowel syndrome (IBS) is one of the most common conditions encountered by both primary care providers and gastroenterologists, with a pooled global prevalence of 11.2%. This functional bowel disorder is characterized by abdominal pain or discomfort, diarrhea and/or constipation, and bloating.
Unfortunately, , according to Alan Desmond, MB, consultant in gastroenterology and general internal medicine, Torbay Hospital, UK National Health Service.
Desmond regularly sees patients who either haven’t been accurately diagnosed or have been told, “Don’t worry, it’s ‘just’ irritable bowel syndrome,” he said at the recent International Conference on Nutrition in Medicine.
A 2017 study involving nearly 2000 patients with a history of gastrointestinal (GI) symptoms found that 43.1% of those who met the criteria for IBS were undiagnosed, and among those who were diagnosed, 26% were not receiving treatment.
“Many clinicians vastly underestimate the impact functional GI symptoms have on our patients in lack of productivity, becoming homebound or losing employment, the inability to enjoy a meal with friends or family, and always needing to know where the nearest bathroom is, for example,” Desmond said in an interview.
IBS can profoundly affect patients’ mental health. One study found that 38% of patients with IBS attending a tertiary care clinic contemplated suicide because they felt hopeless about ever achieving symptom relief.
Today, several dietary, pharmacologic, and psychological/behavioral approaches are available to treat patients with IBS, noted William D. Chey, MD, AGAF, chief of the Division of Gastroenterology and Hepatology, University of Michigan, Ann Arbor, Michigan.
“Each individual patient may need a different combination of these foundational treatments,” he said. “One size doesn’t fit all.”
Diagnostic Pathway
One reason IBS is so hard to diagnose is that it’s a “symptom-based disorder, with identification of the condition predicated upon certain key characteristics that are heterogeneous,” Chey said in an interview. “IBS in patient ‘A’ may not present the same way as IBS in patient ‘B,’ although there are certain foundational common characteristics.”
IBS involves “abnormalities in the motility and contractility of the GI tract,” he said. It can present with diarrhea (IBS-D), constipation (IBS-C), or a mixture or alternation of diarrhea and constipation (IBS-M).
Patients with IBS-D often have an exaggerated gastro-colonic response, while those with IBS-C often have a blunted response.
Beyond stool abnormalities and abdominal pain/discomfort, patients often report bloating/distension, low backache, lethargy, nausea, thigh pain, and urinary and gynecologic symptoms.
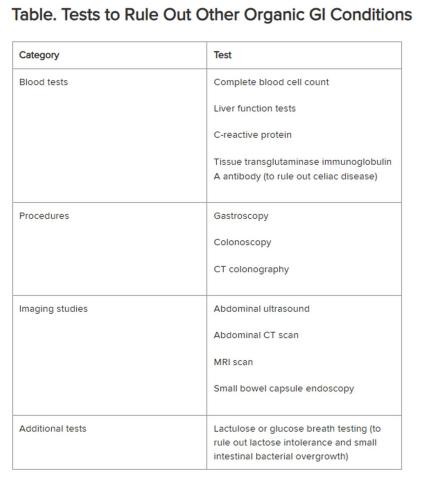
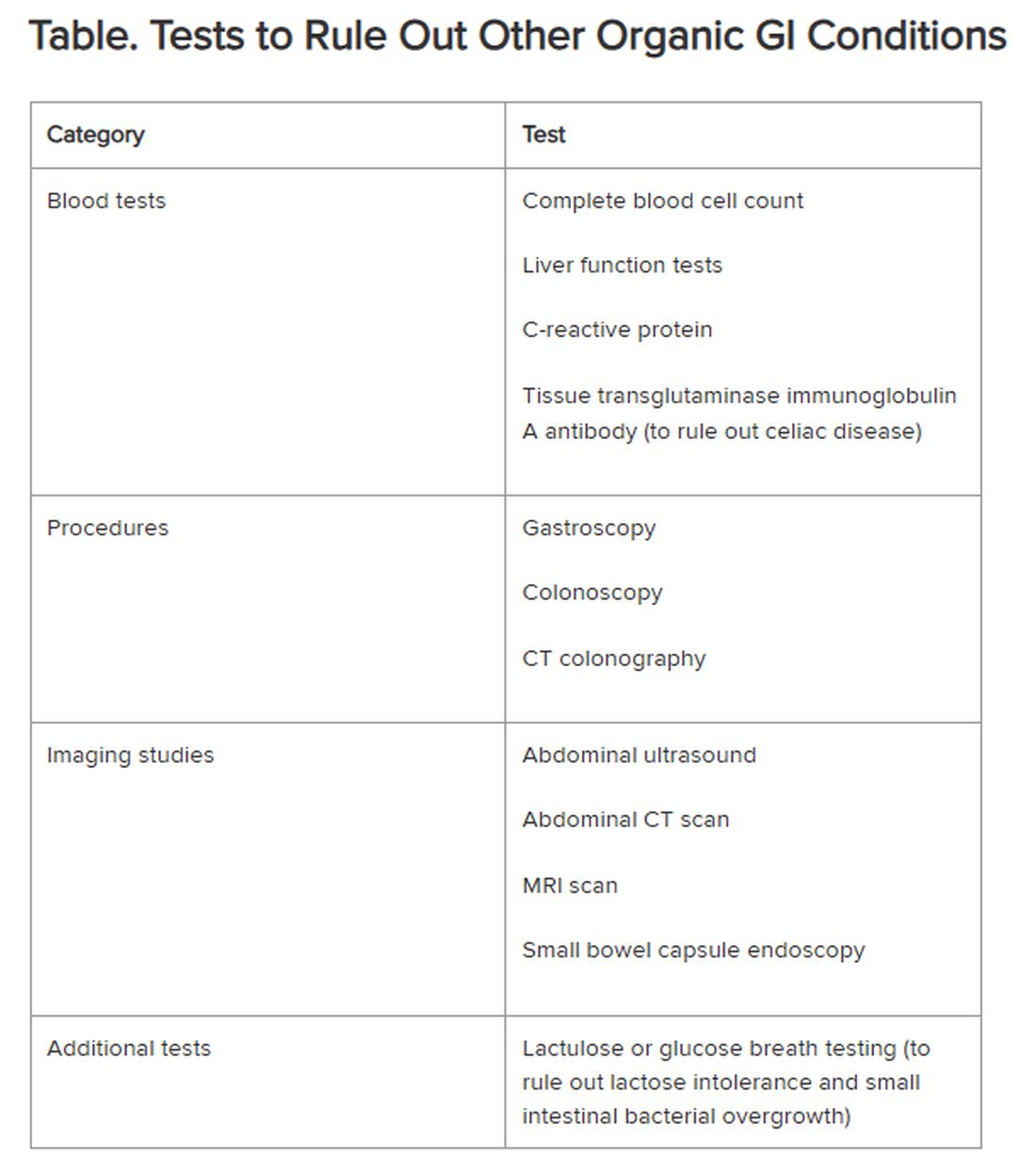
Historically, IBS has been regarded as a “diagnosis of exclusion” because classic diagnostic tests typically yield no concrete findings. Desmond noted that several blood tests, procedures, imaging studies, and other tests are available to rule out other organic GI conditions, as outlined in the Table.
If the patient comes from a geographical region where giardia is endemic, clinicians also should consider testing for the parasite, Chey said.
New Understanding of IBS Etiology
Now, advances in the understanding of IBS are changing the approach to the disease.
“The field is moving away from seeing IBS as a ‘wastebasket diagnosis,’ recognizing that there are other causes of a patient’s symptoms,” Mark Pimentel, MD, associate professor of medicine and gastroenterology, Cedars-Sinai, Los Angeles, said in an interview. “What’s made IBS so difficult to diagnose has been the absence of biological markers and hallmark findings on endoscopy.”
Recent research points to novel bacterial causes as culprits in the development of IBS. In particular, altered small bowel microbiota can be triggered by acute gastroenteritis.
Food poisoning can trigger the onset of IBS — a phenomenon called “postinfectious IBS (PI-IBS),” said Pimentel, who is also executive director of the Medically Associated Science and Technology Program at Cedars-Sinai. PI-IBS almost always takes the form of IBS-D, with up to 60% of patients with IBS-D suffering the long-term sequelae of food poisoning.
The types of bacteria most commonly associated with gastroenteritis are Shigella, Campylobacter, Salmonella, and Escherichia coli, Pimentel said. All of them release cytolethal distending toxin B (CdtB), causing the body to produce antibodies to the toxin.
CdtB resembles vinculin, a naturally occurring protein critical for healthy gut function. “Because of this molecular resemblance, the immune system often mistakes one for the other, producing anti-vinculin,” Pimentel explained.
This autoimmune response leads to disruptions in the gut microbiome, ultimately resulting in PI-IBS. The chain of events “doesn’t necessarily happen immediately,” Pimentel said. “You might have developed food poisoning at a party weeks or months ago.”
Acute gastroenteritis is common, affecting as many as 179 million people in the United States annually. A meta-analysis of 47 studies, incorporating 28,270 patients, found that those who had experienced acute gastroenteritis had a fourfold higher risk of developing IBS compared with nonexposed controls.
“The problem isn’t only the IBS itself, but the fact that people with PI-IBS are four times as likely to contract food poisoning again, which can further exacerbate IBS symptoms,” Pimentel said.
Diarrhea-predominant IBS can be detected through the presence of two blood biomarkers — anti-CdtB and anti-vinculin — in a blood test developed by Pimentel and his group.
“Elevation in either of these biomarkers establishes the diagnosis,” Pimentel said. “This is a breakthrough because it represents the first test that can make IBS a ‘diagnosis of inclusion.’”
The blood test also can identify IBS-M but not IBS-C.
Pimentel said that IBS-C is associated with increased levels of methanogenic archaea, which can be diagnosed by a positive methane breath test. “Methane gas slows intestinal contractility, which might result in constipation,” he said.
Diet as a Treatment Option
Diet is usually the starting point for IBS treatment, Chey said. “The standard dietary recommendations, as defined by the National Institute for Health and Care Excellence Guidance for managing IBS, are reasonable and common sense — eating three meals a day, avoiding carbonated beverages, excess alcohol, and excess caffeine, and avoiding hard-to-digest foods that can be gas producing.”
A diet low in fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs), which are carbohydrates that aren’t completely absorbed in the intestines, has been shown to be effective in alleviating GI distress in as many as 86% of patients with IBS, leading to improvements in overall GI symptoms as well as individual symptoms (eg, abdominal pain, bloating, constipation, diarrhea, and flatulence).
Desmond recommends the low FODMAP program delineated by Monash University in Australia. The diet should be undertaken only under the supervision of a dietitian, he warned. Moreover, following it on a long-term basis can have an adverse impact on dietary quality and the gut microbiome. Therefore, “it’s important to embark on stepwise reintroduction of FODMAPS under supervision to find acceptable thresholds that don’t cause a return of symptoms.”
A growing body of research suggests that following the Mediterranean diet can be helpful in reducing IBS symptoms. Chey said that some patients who tend to over-restrict their eating might benefit from a less restrictive diet than the typical low FODMAPs diet. For them, the Mediterranean diet may be a good option.
Pharmacotherapy for IBS
Nutritional approaches aren’t for everyone, Chey noted. “Some people don’t want to be on a highly restricted diet.” For them, medications addressing symptoms might be a better option.
Antispasmodics — either anticholinergics (hyoscine and dicyclomine) or smooth muscle relaxants (alverine, mebeverine, and peppermint oil) — can be helpful, although they can worsen constipation in a dose-dependent manner. It is advisable to use them on an as-needed rather than long-term basis.
Antidiarrheal agents include loperamide and diphenoxylate.
For constipation, laxatives (eg, senna, bisacodyl, polyethylene glycol, and sodium picosulfate) can be helpful.
Desmond noted that the American Gastroenterological Association does not recommend routine use of probiotics for most GI disorders, including IBS. Exceptions include prevention of Clostridioides difficile, ulcerative colitis, and pouchitis.
Targeting the Gut-Brain Relationship
Stress plays a role in exacerbating symptoms in patients with IBS and is an important target for intervention.
“If patients are living with a level of stress that’s impairing, we won’t be able to solve their gut issues until we resolve their stress issues,” Desmond said. “We need to calm the gut-microbiome-brain axis, which is multidimensional and bidirectional.”
Many people — even those without IBS — experience queasiness or diarrhea prior to a major event they’re nervous about, Chey noted. These events activate the brain, which activates the nervous system, which interacts with the GI tract. Indeed, IBS is now recognized as a disorder of gut-brain interaction, he said.
“We now know that the microbiome in the GI tract influences cognition and emotional function, depression, and anxiety. One might say that the gut is the ‘center of the universe’ to human beings,” Chey said.
Evidence-based psychological approaches for stress reduction in patients with IBS include cognitive behavioral therapy, specifically tailored to helping the patient identify associations between IBS symptoms and thoughts, emotions, and actions, as well as learning new behaviors and engaging in stress management. Psychodynamic (interpersonal) therapy enables patients to understand the connection between GI symptoms and interpersonal conflicts, emotional factors, or relationship difficulties.
Gut-directed hypnotherapy (GDH) is a “proven modality for IBS,” Desmond said. Unlike other forms of hypnotherapy, GDH focuses specifically on controlling and normalizing GI function. Studies have shown a reduction of ≥ 30% in abdominal pain in two thirds of participants, with overall response rates up to 85%. It can be delivered in an individual or group setting or via a smartphone.
Desmond recommends mindfulness-based therapy (MBT) for IBS. MBT focuses on the “cultivation of mindfulness, defined as intentional, nonjudgmental, present-focused awareness.” It has been found effective in reducing flares and the markers of gut inflammation in ulcerative colitis, as well as reducing symptoms of IBS.
Chey noted that an emerging body of literature supports the potential role of acupuncture in treating IBS, and his clinic employs it. “I would like to see further research into other areas of CAM [complementary and alternative medicine], including herbal approaches to IBS symptoms as well as stress.”
Finally, all the experts agree that more research is needed.
“The real tragedy is that the NIH invests next to nothing in IBS, in contrast to inflammatory bowel disease and many other conditions,” Pimentel said. “Yet IBS is 45 times more common than inflammatory bowel disease.”
Pimentel hopes that with enough advocacy and recognition that IBS isn’t “just stress-related,” more resources will be devoted to understanding this debilitating condition.
Desmond is the author of a book on the benefits of a plant-based diet. He has also received honoraria, speaking, and consultancy fees from the European Space Agency, Dyson Institute of Engineering and Technology, Riverford Organic Farmers, Ltd., Salesforce Inc., Sentara Healthcare, Saudi Sports for All Federation, the Physicians Committee for Responsible Medicine, The Plantrician Project, Doctors for Nutrition, and The Happy Pear.
Pimentel is a consultant for Bausch Health, Ferring Pharmaceuticals, and Ardelyx. He holds equity in and is also a consultant for Dieta Health, Salvo Health, Cylinder Health, and Gemelli Biotech. Cedars-Sinai has a licensing agreement with Gemelli Biotech and Hobbs Medical.
Chey is a consultant to AbbVie, Ardelyx, Atmo, Biomerica, Gemelli Biotech, Ironwood Pharmaceuticals, Nestlé, QOL Medical, Phathom Pharmaceuticals, Redhill, Salix/Valeant, Takeda, and Vibrant. He receives grant/research funding from Commonwealth Diagnostics International, Inc., US Food and Drug Administration, National Institutes of Health, QOL Medical, and Salix/Valeant. He holds stock options in Coprata, Dieta Health, Evinature, FoodMarble, Kiwi Biosciences, and ModifyHealth. He is a board or advisory panel member of the American College of Gastroenterology, GI Health Foundation, International Foundation for Gastrointestinal Disorders, Rome. He holds patents on My Nutrition Health, Digital Manometry, and Rectal Expulsion Device.
A version of this article appeared on Medscape.com.
Irritable bowel syndrome (IBS) is one of the most common conditions encountered by both primary care providers and gastroenterologists, with a pooled global prevalence of 11.2%. This functional bowel disorder is characterized by abdominal pain or discomfort, diarrhea and/or constipation, and bloating.
Unfortunately, , according to Alan Desmond, MB, consultant in gastroenterology and general internal medicine, Torbay Hospital, UK National Health Service.
Desmond regularly sees patients who either haven’t been accurately diagnosed or have been told, “Don’t worry, it’s ‘just’ irritable bowel syndrome,” he said at the recent International Conference on Nutrition in Medicine.
A 2017 study involving nearly 2000 patients with a history of gastrointestinal (GI) symptoms found that 43.1% of those who met the criteria for IBS were undiagnosed, and among those who were diagnosed, 26% were not receiving treatment.
“Many clinicians vastly underestimate the impact functional GI symptoms have on our patients in lack of productivity, becoming homebound or losing employment, the inability to enjoy a meal with friends or family, and always needing to know where the nearest bathroom is, for example,” Desmond said in an interview.
IBS can profoundly affect patients’ mental health. One study found that 38% of patients with IBS attending a tertiary care clinic contemplated suicide because they felt hopeless about ever achieving symptom relief.
Today, several dietary, pharmacologic, and psychological/behavioral approaches are available to treat patients with IBS, noted William D. Chey, MD, AGAF, chief of the Division of Gastroenterology and Hepatology, University of Michigan, Ann Arbor, Michigan.
“Each individual patient may need a different combination of these foundational treatments,” he said. “One size doesn’t fit all.”
Diagnostic Pathway
One reason IBS is so hard to diagnose is that it’s a “symptom-based disorder, with identification of the condition predicated upon certain key characteristics that are heterogeneous,” Chey said in an interview. “IBS in patient ‘A’ may not present the same way as IBS in patient ‘B,’ although there are certain foundational common characteristics.”
IBS involves “abnormalities in the motility and contractility of the GI tract,” he said. It can present with diarrhea (IBS-D), constipation (IBS-C), or a mixture or alternation of diarrhea and constipation (IBS-M).
Patients with IBS-D often have an exaggerated gastro-colonic response, while those with IBS-C often have a blunted response.
Beyond stool abnormalities and abdominal pain/discomfort, patients often report bloating/distension, low backache, lethargy, nausea, thigh pain, and urinary and gynecologic symptoms.
Historically, IBS has been regarded as a “diagnosis of exclusion” because classic diagnostic tests typically yield no concrete findings. Desmond noted that several blood tests, procedures, imaging studies, and other tests are available to rule out other organic GI conditions, as outlined in the Table.
If the patient comes from a geographical region where giardia is endemic, clinicians also should consider testing for the parasite, Chey said.
New Understanding of IBS Etiology
Now, advances in the understanding of IBS are changing the approach to the disease.
“The field is moving away from seeing IBS as a ‘wastebasket diagnosis,’ recognizing that there are other causes of a patient’s symptoms,” Mark Pimentel, MD, associate professor of medicine and gastroenterology, Cedars-Sinai, Los Angeles, said in an interview. “What’s made IBS so difficult to diagnose has been the absence of biological markers and hallmark findings on endoscopy.”
Recent research points to novel bacterial causes as culprits in the development of IBS. In particular, altered small bowel microbiota can be triggered by acute gastroenteritis.
Food poisoning can trigger the onset of IBS — a phenomenon called “postinfectious IBS (PI-IBS),” said Pimentel, who is also executive director of the Medically Associated Science and Technology Program at Cedars-Sinai. PI-IBS almost always takes the form of IBS-D, with up to 60% of patients with IBS-D suffering the long-term sequelae of food poisoning.
The types of bacteria most commonly associated with gastroenteritis are Shigella, Campylobacter, Salmonella, and Escherichia coli, Pimentel said. All of them release cytolethal distending toxin B (CdtB), causing the body to produce antibodies to the toxin.
CdtB resembles vinculin, a naturally occurring protein critical for healthy gut function. “Because of this molecular resemblance, the immune system often mistakes one for the other, producing anti-vinculin,” Pimentel explained.
This autoimmune response leads to disruptions in the gut microbiome, ultimately resulting in PI-IBS. The chain of events “doesn’t necessarily happen immediately,” Pimentel said. “You might have developed food poisoning at a party weeks or months ago.”
Acute gastroenteritis is common, affecting as many as 179 million people in the United States annually. A meta-analysis of 47 studies, incorporating 28,270 patients, found that those who had experienced acute gastroenteritis had a fourfold higher risk of developing IBS compared with nonexposed controls.
“The problem isn’t only the IBS itself, but the fact that people with PI-IBS are four times as likely to contract food poisoning again, which can further exacerbate IBS symptoms,” Pimentel said.
Diarrhea-predominant IBS can be detected through the presence of two blood biomarkers — anti-CdtB and anti-vinculin — in a blood test developed by Pimentel and his group.
“Elevation in either of these biomarkers establishes the diagnosis,” Pimentel said. “This is a breakthrough because it represents the first test that can make IBS a ‘diagnosis of inclusion.’”
The blood test also can identify IBS-M but not IBS-C.
Pimentel said that IBS-C is associated with increased levels of methanogenic archaea, which can be diagnosed by a positive methane breath test. “Methane gas slows intestinal contractility, which might result in constipation,” he said.
Diet as a Treatment Option
Diet is usually the starting point for IBS treatment, Chey said. “The standard dietary recommendations, as defined by the National Institute for Health and Care Excellence Guidance for managing IBS, are reasonable and common sense — eating three meals a day, avoiding carbonated beverages, excess alcohol, and excess caffeine, and avoiding hard-to-digest foods that can be gas producing.”
A diet low in fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs), which are carbohydrates that aren’t completely absorbed in the intestines, has been shown to be effective in alleviating GI distress in as many as 86% of patients with IBS, leading to improvements in overall GI symptoms as well as individual symptoms (eg, abdominal pain, bloating, constipation, diarrhea, and flatulence).
Desmond recommends the low FODMAP program delineated by Monash University in Australia. The diet should be undertaken only under the supervision of a dietitian, he warned. Moreover, following it on a long-term basis can have an adverse impact on dietary quality and the gut microbiome. Therefore, “it’s important to embark on stepwise reintroduction of FODMAPS under supervision to find acceptable thresholds that don’t cause a return of symptoms.”
A growing body of research suggests that following the Mediterranean diet can be helpful in reducing IBS symptoms. Chey said that some patients who tend to over-restrict their eating might benefit from a less restrictive diet than the typical low FODMAPs diet. For them, the Mediterranean diet may be a good option.
Pharmacotherapy for IBS
Nutritional approaches aren’t for everyone, Chey noted. “Some people don’t want to be on a highly restricted diet.” For them, medications addressing symptoms might be a better option.
Antispasmodics — either anticholinergics (hyoscine and dicyclomine) or smooth muscle relaxants (alverine, mebeverine, and peppermint oil) — can be helpful, although they can worsen constipation in a dose-dependent manner. It is advisable to use them on an as-needed rather than long-term basis.
Antidiarrheal agents include loperamide and diphenoxylate.
For constipation, laxatives (eg, senna, bisacodyl, polyethylene glycol, and sodium picosulfate) can be helpful.
Desmond noted that the American Gastroenterological Association does not recommend routine use of probiotics for most GI disorders, including IBS. Exceptions include prevention of Clostridioides difficile, ulcerative colitis, and pouchitis.
Targeting the Gut-Brain Relationship
Stress plays a role in exacerbating symptoms in patients with IBS and is an important target for intervention.
“If patients are living with a level of stress that’s impairing, we won’t be able to solve their gut issues until we resolve their stress issues,” Desmond said. “We need to calm the gut-microbiome-brain axis, which is multidimensional and bidirectional.”
Many people — even those without IBS — experience queasiness or diarrhea prior to a major event they’re nervous about, Chey noted. These events activate the brain, which activates the nervous system, which interacts with the GI tract. Indeed, IBS is now recognized as a disorder of gut-brain interaction, he said.
“We now know that the microbiome in the GI tract influences cognition and emotional function, depression, and anxiety. One might say that the gut is the ‘center of the universe’ to human beings,” Chey said.
Evidence-based psychological approaches for stress reduction in patients with IBS include cognitive behavioral therapy, specifically tailored to helping the patient identify associations between IBS symptoms and thoughts, emotions, and actions, as well as learning new behaviors and engaging in stress management. Psychodynamic (interpersonal) therapy enables patients to understand the connection between GI symptoms and interpersonal conflicts, emotional factors, or relationship difficulties.
Gut-directed hypnotherapy (GDH) is a “proven modality for IBS,” Desmond said. Unlike other forms of hypnotherapy, GDH focuses specifically on controlling and normalizing GI function. Studies have shown a reduction of ≥ 30% in abdominal pain in two thirds of participants, with overall response rates up to 85%. It can be delivered in an individual or group setting or via a smartphone.
Desmond recommends mindfulness-based therapy (MBT) for IBS. MBT focuses on the “cultivation of mindfulness, defined as intentional, nonjudgmental, present-focused awareness.” It has been found effective in reducing flares and the markers of gut inflammation in ulcerative colitis, as well as reducing symptoms of IBS.
Chey noted that an emerging body of literature supports the potential role of acupuncture in treating IBS, and his clinic employs it. “I would like to see further research into other areas of CAM [complementary and alternative medicine], including herbal approaches to IBS symptoms as well as stress.”
Finally, all the experts agree that more research is needed.
“The real tragedy is that the NIH invests next to nothing in IBS, in contrast to inflammatory bowel disease and many other conditions,” Pimentel said. “Yet IBS is 45 times more common than inflammatory bowel disease.”
Pimentel hopes that with enough advocacy and recognition that IBS isn’t “just stress-related,” more resources will be devoted to understanding this debilitating condition.
Desmond is the author of a book on the benefits of a plant-based diet. He has also received honoraria, speaking, and consultancy fees from the European Space Agency, Dyson Institute of Engineering and Technology, Riverford Organic Farmers, Ltd., Salesforce Inc., Sentara Healthcare, Saudi Sports for All Federation, the Physicians Committee for Responsible Medicine, The Plantrician Project, Doctors for Nutrition, and The Happy Pear.
Pimentel is a consultant for Bausch Health, Ferring Pharmaceuticals, and Ardelyx. He holds equity in and is also a consultant for Dieta Health, Salvo Health, Cylinder Health, and Gemelli Biotech. Cedars-Sinai has a licensing agreement with Gemelli Biotech and Hobbs Medical.
Chey is a consultant to AbbVie, Ardelyx, Atmo, Biomerica, Gemelli Biotech, Ironwood Pharmaceuticals, Nestlé, QOL Medical, Phathom Pharmaceuticals, Redhill, Salix/Valeant, Takeda, and Vibrant. He receives grant/research funding from Commonwealth Diagnostics International, Inc., US Food and Drug Administration, National Institutes of Health, QOL Medical, and Salix/Valeant. He holds stock options in Coprata, Dieta Health, Evinature, FoodMarble, Kiwi Biosciences, and ModifyHealth. He is a board or advisory panel member of the American College of Gastroenterology, GI Health Foundation, International Foundation for Gastrointestinal Disorders, Rome. He holds patents on My Nutrition Health, Digital Manometry, and Rectal Expulsion Device.
A version of this article appeared on Medscape.com.
Irritable bowel syndrome (IBS) is one of the most common conditions encountered by both primary care providers and gastroenterologists, with a pooled global prevalence of 11.2%. This functional bowel disorder is characterized by abdominal pain or discomfort, diarrhea and/or constipation, and bloating.
Unfortunately, , according to Alan Desmond, MB, consultant in gastroenterology and general internal medicine, Torbay Hospital, UK National Health Service.
Desmond regularly sees patients who either haven’t been accurately diagnosed or have been told, “Don’t worry, it’s ‘just’ irritable bowel syndrome,” he said at the recent International Conference on Nutrition in Medicine.
A 2017 study involving nearly 2000 patients with a history of gastrointestinal (GI) symptoms found that 43.1% of those who met the criteria for IBS were undiagnosed, and among those who were diagnosed, 26% were not receiving treatment.
“Many clinicians vastly underestimate the impact functional GI symptoms have on our patients in lack of productivity, becoming homebound or losing employment, the inability to enjoy a meal with friends or family, and always needing to know where the nearest bathroom is, for example,” Desmond said in an interview.
IBS can profoundly affect patients’ mental health. One study found that 38% of patients with IBS attending a tertiary care clinic contemplated suicide because they felt hopeless about ever achieving symptom relief.
Today, several dietary, pharmacologic, and psychological/behavioral approaches are available to treat patients with IBS, noted William D. Chey, MD, AGAF, chief of the Division of Gastroenterology and Hepatology, University of Michigan, Ann Arbor, Michigan.
“Each individual patient may need a different combination of these foundational treatments,” he said. “One size doesn’t fit all.”
Diagnostic Pathway
One reason IBS is so hard to diagnose is that it’s a “symptom-based disorder, with identification of the condition predicated upon certain key characteristics that are heterogeneous,” Chey said in an interview. “IBS in patient ‘A’ may not present the same way as IBS in patient ‘B,’ although there are certain foundational common characteristics.”
IBS involves “abnormalities in the motility and contractility of the GI tract,” he said. It can present with diarrhea (IBS-D), constipation (IBS-C), or a mixture or alternation of diarrhea and constipation (IBS-M).
Patients with IBS-D often have an exaggerated gastro-colonic response, while those with IBS-C often have a blunted response.
Beyond stool abnormalities and abdominal pain/discomfort, patients often report bloating/distension, low backache, lethargy, nausea, thigh pain, and urinary and gynecologic symptoms.
Historically, IBS has been regarded as a “diagnosis of exclusion” because classic diagnostic tests typically yield no concrete findings. Desmond noted that several blood tests, procedures, imaging studies, and other tests are available to rule out other organic GI conditions, as outlined in the Table.
If the patient comes from a geographical region where giardia is endemic, clinicians also should consider testing for the parasite, Chey said.
New Understanding of IBS Etiology
Now, advances in the understanding of IBS are changing the approach to the disease.
“The field is moving away from seeing IBS as a ‘wastebasket diagnosis,’ recognizing that there are other causes of a patient’s symptoms,” Mark Pimentel, MD, associate professor of medicine and gastroenterology, Cedars-Sinai, Los Angeles, said in an interview. “What’s made IBS so difficult to diagnose has been the absence of biological markers and hallmark findings on endoscopy.”
Recent research points to novel bacterial causes as culprits in the development of IBS. In particular, altered small bowel microbiota can be triggered by acute gastroenteritis.
Food poisoning can trigger the onset of IBS — a phenomenon called “postinfectious IBS (PI-IBS),” said Pimentel, who is also executive director of the Medically Associated Science and Technology Program at Cedars-Sinai. PI-IBS almost always takes the form of IBS-D, with up to 60% of patients with IBS-D suffering the long-term sequelae of food poisoning.
The types of bacteria most commonly associated with gastroenteritis are Shigella, Campylobacter, Salmonella, and Escherichia coli, Pimentel said. All of them release cytolethal distending toxin B (CdtB), causing the body to produce antibodies to the toxin.
CdtB resembles vinculin, a naturally occurring protein critical for healthy gut function. “Because of this molecular resemblance, the immune system often mistakes one for the other, producing anti-vinculin,” Pimentel explained.
This autoimmune response leads to disruptions in the gut microbiome, ultimately resulting in PI-IBS. The chain of events “doesn’t necessarily happen immediately,” Pimentel said. “You might have developed food poisoning at a party weeks or months ago.”
Acute gastroenteritis is common, affecting as many as 179 million people in the United States annually. A meta-analysis of 47 studies, incorporating 28,270 patients, found that those who had experienced acute gastroenteritis had a fourfold higher risk of developing IBS compared with nonexposed controls.
“The problem isn’t only the IBS itself, but the fact that people with PI-IBS are four times as likely to contract food poisoning again, which can further exacerbate IBS symptoms,” Pimentel said.
Diarrhea-predominant IBS can be detected through the presence of two blood biomarkers — anti-CdtB and anti-vinculin — in a blood test developed by Pimentel and his group.
“Elevation in either of these biomarkers establishes the diagnosis,” Pimentel said. “This is a breakthrough because it represents the first test that can make IBS a ‘diagnosis of inclusion.’”
The blood test also can identify IBS-M but not IBS-C.
Pimentel said that IBS-C is associated with increased levels of methanogenic archaea, which can be diagnosed by a positive methane breath test. “Methane gas slows intestinal contractility, which might result in constipation,” he said.
Diet as a Treatment Option
Diet is usually the starting point for IBS treatment, Chey said. “The standard dietary recommendations, as defined by the National Institute for Health and Care Excellence Guidance for managing IBS, are reasonable and common sense — eating three meals a day, avoiding carbonated beverages, excess alcohol, and excess caffeine, and avoiding hard-to-digest foods that can be gas producing.”
A diet low in fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs), which are carbohydrates that aren’t completely absorbed in the intestines, has been shown to be effective in alleviating GI distress in as many as 86% of patients with IBS, leading to improvements in overall GI symptoms as well as individual symptoms (eg, abdominal pain, bloating, constipation, diarrhea, and flatulence).
Desmond recommends the low FODMAP program delineated by Monash University in Australia. The diet should be undertaken only under the supervision of a dietitian, he warned. Moreover, following it on a long-term basis can have an adverse impact on dietary quality and the gut microbiome. Therefore, “it’s important to embark on stepwise reintroduction of FODMAPS under supervision to find acceptable thresholds that don’t cause a return of symptoms.”
A growing body of research suggests that following the Mediterranean diet can be helpful in reducing IBS symptoms. Chey said that some patients who tend to over-restrict their eating might benefit from a less restrictive diet than the typical low FODMAPs diet. For them, the Mediterranean diet may be a good option.
Pharmacotherapy for IBS
Nutritional approaches aren’t for everyone, Chey noted. “Some people don’t want to be on a highly restricted diet.” For them, medications addressing symptoms might be a better option.
Antispasmodics — either anticholinergics (hyoscine and dicyclomine) or smooth muscle relaxants (alverine, mebeverine, and peppermint oil) — can be helpful, although they can worsen constipation in a dose-dependent manner. It is advisable to use them on an as-needed rather than long-term basis.
Antidiarrheal agents include loperamide and diphenoxylate.
For constipation, laxatives (eg, senna, bisacodyl, polyethylene glycol, and sodium picosulfate) can be helpful.
Desmond noted that the American Gastroenterological Association does not recommend routine use of probiotics for most GI disorders, including IBS. Exceptions include prevention of Clostridioides difficile, ulcerative colitis, and pouchitis.
Targeting the Gut-Brain Relationship
Stress plays a role in exacerbating symptoms in patients with IBS and is an important target for intervention.
“If patients are living with a level of stress that’s impairing, we won’t be able to solve their gut issues until we resolve their stress issues,” Desmond said. “We need to calm the gut-microbiome-brain axis, which is multidimensional and bidirectional.”
Many people — even those without IBS — experience queasiness or diarrhea prior to a major event they’re nervous about, Chey noted. These events activate the brain, which activates the nervous system, which interacts with the GI tract. Indeed, IBS is now recognized as a disorder of gut-brain interaction, he said.
“We now know that the microbiome in the GI tract influences cognition and emotional function, depression, and anxiety. One might say that the gut is the ‘center of the universe’ to human beings,” Chey said.
Evidence-based psychological approaches for stress reduction in patients with IBS include cognitive behavioral therapy, specifically tailored to helping the patient identify associations between IBS symptoms and thoughts, emotions, and actions, as well as learning new behaviors and engaging in stress management. Psychodynamic (interpersonal) therapy enables patients to understand the connection between GI symptoms and interpersonal conflicts, emotional factors, or relationship difficulties.
Gut-directed hypnotherapy (GDH) is a “proven modality for IBS,” Desmond said. Unlike other forms of hypnotherapy, GDH focuses specifically on controlling and normalizing GI function. Studies have shown a reduction of ≥ 30% in abdominal pain in two thirds of participants, with overall response rates up to 85%. It can be delivered in an individual or group setting or via a smartphone.
Desmond recommends mindfulness-based therapy (MBT) for IBS. MBT focuses on the “cultivation of mindfulness, defined as intentional, nonjudgmental, present-focused awareness.” It has been found effective in reducing flares and the markers of gut inflammation in ulcerative colitis, as well as reducing symptoms of IBS.
Chey noted that an emerging body of literature supports the potential role of acupuncture in treating IBS, and his clinic employs it. “I would like to see further research into other areas of CAM [complementary and alternative medicine], including herbal approaches to IBS symptoms as well as stress.”
Finally, all the experts agree that more research is needed.
“The real tragedy is that the NIH invests next to nothing in IBS, in contrast to inflammatory bowel disease and many other conditions,” Pimentel said. “Yet IBS is 45 times more common than inflammatory bowel disease.”
Pimentel hopes that with enough advocacy and recognition that IBS isn’t “just stress-related,” more resources will be devoted to understanding this debilitating condition.
Desmond is the author of a book on the benefits of a plant-based diet. He has also received honoraria, speaking, and consultancy fees from the European Space Agency, Dyson Institute of Engineering and Technology, Riverford Organic Farmers, Ltd., Salesforce Inc., Sentara Healthcare, Saudi Sports for All Federation, the Physicians Committee for Responsible Medicine, The Plantrician Project, Doctors for Nutrition, and The Happy Pear.
Pimentel is a consultant for Bausch Health, Ferring Pharmaceuticals, and Ardelyx. He holds equity in and is also a consultant for Dieta Health, Salvo Health, Cylinder Health, and Gemelli Biotech. Cedars-Sinai has a licensing agreement with Gemelli Biotech and Hobbs Medical.
Chey is a consultant to AbbVie, Ardelyx, Atmo, Biomerica, Gemelli Biotech, Ironwood Pharmaceuticals, Nestlé, QOL Medical, Phathom Pharmaceuticals, Redhill, Salix/Valeant, Takeda, and Vibrant. He receives grant/research funding from Commonwealth Diagnostics International, Inc., US Food and Drug Administration, National Institutes of Health, QOL Medical, and Salix/Valeant. He holds stock options in Coprata, Dieta Health, Evinature, FoodMarble, Kiwi Biosciences, and ModifyHealth. He is a board or advisory panel member of the American College of Gastroenterology, GI Health Foundation, International Foundation for Gastrointestinal Disorders, Rome. He holds patents on My Nutrition Health, Digital Manometry, and Rectal Expulsion Device.
A version of this article appeared on Medscape.com.
Lawmakers Rush to Stave Off Doctor Pay Cuts as Medicare Finalizes 2025 Rates
Federal lawmakers are rushing to soften the blow of Medicare’s 2025 effective pay cut for doctors in 2025, introducing a bill that could limit the cut. But they have little time to act.
In 2025, the conversion factor used to calculate payment to doctors and hospitals caring for Medicare patients will drop to $32.35, a nearly 3% decrease from the current level.
Congress likely will act before the cuts take effect, said Rep. Larry Bucshon, MD (R-IN), who specialized in cardiothoracic surgery before joining Congress. Lawmakers in past years have typically tinkered with the Medicare physician fee schedule at the last minute, tucking in fixes to December legislative packages and spending bills.
“I’m pretty optimistic that a good portion of the fee cuts will be mitigated and they won’t go through,” Bucshon told this news organization in an interview.
Bruce A. Scott, MD, president of the American Medical Association (AMA) said in a statement that CMS’ release of the final fee schedule on November 1 should trigger serious work on a change to the 2025 Medicare physician fee schedule.
“The fee schedule rule released [on November 1] starts the clock — with January 1 looming,” Scott said. “A legislative remedy will require hard work and compromise. The 66 million patients who rely on Medicare are counting on that.”
Both Bucshon and Scott also joined many lawmakers and medical associations in calling on Congress for a larger overhaul of the Medicare physician fee schedule, well beyond whatever temporary adjustment may be made in the months ahead to avoid or soften the 2025 cuts.
The physician fee schedule sets formulas and rules regarding how the largest US buyer of health services pays the almost 1.3 million clinicians who bill Medicare. Of these, 51% are physicians. The physician fee schedule also covers payments for nurse practitioners, physician assistants, physical therapists, and other health professionals.
Last Major Overhaul Unpopular
There’s broad dissatisfaction with Congress’ last major overhaul of the Medicare physician fee schedule. The 2015 Medicare Access and CHIP Reauthorization Act (MACRA) aimed to shift clinicians toward programs tying pay increases to quality measures. But the implementation of that aim through the Merit-based Incentive Payment System is widely considered a disappointment.
MACRA was intended to end the need for annual “doc fixes,” as Congress’ last-minute Medicare adjustments are known. Seventeen such tweaks passed before MACRA took effect.
But MACRA did not include a broad-based inflation adjuster, and some clinicians’ incomes are lagging as inflation rates — and practice costs — have risen. Scott said the Medicare Economic Index, which is a measure used to gauge increases in practice costs for clinicians, is expected to rise by 3.5%.
“To put it bluntly, Medicare plans to pay us less while costs go up. You don’t have to be an economist to know that is an unsustainable trend, though one that has been going on for decades,” Scott said. “For physician practices operating on small margins already, this means it is harder to acquire new equipment, harder to retain staff, harder to take on new Medicare patients, and harder to keep the doors open, particularly in rural and underserved areas.”
In a statement, Jen Brull, MD, president of the American Academy of Family Physicians, noted that this likely will be the fifth year in a row that Congress will need to do a patch to prevent cuts in pay to clinicians.
Bucshon, who will retire from the House in January, said he expects Congress to pass legislation tying Medicare payment rates to inflation — eventually.
“People want to find a way to fix this problem, but also do it in a way that does not cut benefits to anyone, and that’s the key,” Bucshon said. “We’re going to have to find a way to make sure that providers are properly reimbursed.”
A version of this article first appeared on Medscape.com.
Federal lawmakers are rushing to soften the blow of Medicare’s 2025 effective pay cut for doctors in 2025, introducing a bill that could limit the cut. But they have little time to act.
In 2025, the conversion factor used to calculate payment to doctors and hospitals caring for Medicare patients will drop to $32.35, a nearly 3% decrease from the current level.
Congress likely will act before the cuts take effect, said Rep. Larry Bucshon, MD (R-IN), who specialized in cardiothoracic surgery before joining Congress. Lawmakers in past years have typically tinkered with the Medicare physician fee schedule at the last minute, tucking in fixes to December legislative packages and spending bills.
“I’m pretty optimistic that a good portion of the fee cuts will be mitigated and they won’t go through,” Bucshon told this news organization in an interview.
Bruce A. Scott, MD, president of the American Medical Association (AMA) said in a statement that CMS’ release of the final fee schedule on November 1 should trigger serious work on a change to the 2025 Medicare physician fee schedule.
“The fee schedule rule released [on November 1] starts the clock — with January 1 looming,” Scott said. “A legislative remedy will require hard work and compromise. The 66 million patients who rely on Medicare are counting on that.”
Both Bucshon and Scott also joined many lawmakers and medical associations in calling on Congress for a larger overhaul of the Medicare physician fee schedule, well beyond whatever temporary adjustment may be made in the months ahead to avoid or soften the 2025 cuts.
The physician fee schedule sets formulas and rules regarding how the largest US buyer of health services pays the almost 1.3 million clinicians who bill Medicare. Of these, 51% are physicians. The physician fee schedule also covers payments for nurse practitioners, physician assistants, physical therapists, and other health professionals.
Last Major Overhaul Unpopular
There’s broad dissatisfaction with Congress’ last major overhaul of the Medicare physician fee schedule. The 2015 Medicare Access and CHIP Reauthorization Act (MACRA) aimed to shift clinicians toward programs tying pay increases to quality measures. But the implementation of that aim through the Merit-based Incentive Payment System is widely considered a disappointment.
MACRA was intended to end the need for annual “doc fixes,” as Congress’ last-minute Medicare adjustments are known. Seventeen such tweaks passed before MACRA took effect.
But MACRA did not include a broad-based inflation adjuster, and some clinicians’ incomes are lagging as inflation rates — and practice costs — have risen. Scott said the Medicare Economic Index, which is a measure used to gauge increases in practice costs for clinicians, is expected to rise by 3.5%.
“To put it bluntly, Medicare plans to pay us less while costs go up. You don’t have to be an economist to know that is an unsustainable trend, though one that has been going on for decades,” Scott said. “For physician practices operating on small margins already, this means it is harder to acquire new equipment, harder to retain staff, harder to take on new Medicare patients, and harder to keep the doors open, particularly in rural and underserved areas.”
In a statement, Jen Brull, MD, president of the American Academy of Family Physicians, noted that this likely will be the fifth year in a row that Congress will need to do a patch to prevent cuts in pay to clinicians.
Bucshon, who will retire from the House in January, said he expects Congress to pass legislation tying Medicare payment rates to inflation — eventually.
“People want to find a way to fix this problem, but also do it in a way that does not cut benefits to anyone, and that’s the key,” Bucshon said. “We’re going to have to find a way to make sure that providers are properly reimbursed.”
A version of this article first appeared on Medscape.com.
Federal lawmakers are rushing to soften the blow of Medicare’s 2025 effective pay cut for doctors in 2025, introducing a bill that could limit the cut. But they have little time to act.
In 2025, the conversion factor used to calculate payment to doctors and hospitals caring for Medicare patients will drop to $32.35, a nearly 3% decrease from the current level.
Congress likely will act before the cuts take effect, said Rep. Larry Bucshon, MD (R-IN), who specialized in cardiothoracic surgery before joining Congress. Lawmakers in past years have typically tinkered with the Medicare physician fee schedule at the last minute, tucking in fixes to December legislative packages and spending bills.
“I’m pretty optimistic that a good portion of the fee cuts will be mitigated and they won’t go through,” Bucshon told this news organization in an interview.
Bruce A. Scott, MD, president of the American Medical Association (AMA) said in a statement that CMS’ release of the final fee schedule on November 1 should trigger serious work on a change to the 2025 Medicare physician fee schedule.
“The fee schedule rule released [on November 1] starts the clock — with January 1 looming,” Scott said. “A legislative remedy will require hard work and compromise. The 66 million patients who rely on Medicare are counting on that.”
Both Bucshon and Scott also joined many lawmakers and medical associations in calling on Congress for a larger overhaul of the Medicare physician fee schedule, well beyond whatever temporary adjustment may be made in the months ahead to avoid or soften the 2025 cuts.
The physician fee schedule sets formulas and rules regarding how the largest US buyer of health services pays the almost 1.3 million clinicians who bill Medicare. Of these, 51% are physicians. The physician fee schedule also covers payments for nurse practitioners, physician assistants, physical therapists, and other health professionals.
Last Major Overhaul Unpopular
There’s broad dissatisfaction with Congress’ last major overhaul of the Medicare physician fee schedule. The 2015 Medicare Access and CHIP Reauthorization Act (MACRA) aimed to shift clinicians toward programs tying pay increases to quality measures. But the implementation of that aim through the Merit-based Incentive Payment System is widely considered a disappointment.
MACRA was intended to end the need for annual “doc fixes,” as Congress’ last-minute Medicare adjustments are known. Seventeen such tweaks passed before MACRA took effect.
But MACRA did not include a broad-based inflation adjuster, and some clinicians’ incomes are lagging as inflation rates — and practice costs — have risen. Scott said the Medicare Economic Index, which is a measure used to gauge increases in practice costs for clinicians, is expected to rise by 3.5%.
“To put it bluntly, Medicare plans to pay us less while costs go up. You don’t have to be an economist to know that is an unsustainable trend, though one that has been going on for decades,” Scott said. “For physician practices operating on small margins already, this means it is harder to acquire new equipment, harder to retain staff, harder to take on new Medicare patients, and harder to keep the doors open, particularly in rural and underserved areas.”
In a statement, Jen Brull, MD, president of the American Academy of Family Physicians, noted that this likely will be the fifth year in a row that Congress will need to do a patch to prevent cuts in pay to clinicians.
Bucshon, who will retire from the House in January, said he expects Congress to pass legislation tying Medicare payment rates to inflation — eventually.
“People want to find a way to fix this problem, but also do it in a way that does not cut benefits to anyone, and that’s the key,” Bucshon said. “We’re going to have to find a way to make sure that providers are properly reimbursed.”
A version of this article first appeared on Medscape.com.
Can We Repurpose Obesity Drugs to Reverse Liver Disease?
Metabolic dysfunction–associated steatotic liver disease (MASLD) has become the most common liver disease worldwide, with a global prevalence of 32.4%. Its growth over the past three decades has occurred in tandem with increasing rates of obesity and type 2 diabetes — two cornerstones of MASLD.
Higher rates of MASLD and metabolic dysfunction–associated steatohepatitis (MASH) with fibrosis are present in adults with obesity and diabetes, noted Arun Sanyal, MD, professor and director of the Stravitz-Sanyal Institute for Liver Disease and Metabolic Health, Virginia Commonwealth University, Richmond, Virginia.
The success surrounding the medications for obesity and type 2 diabetes, including glucagon-like peptide 1 receptor agonists (GLP-1 RAs), has sparked studies investigating whether they could also be an effective treatment for liver disease.
In particular, GLP-1 RAs help patients lose weight and/or control diabetes by mimicking the function of the gut hormone GLP-1, released in response to nutrient intake, and are able to increase insulin secretion and reduce glucagon secretion, delay gastric emptying, and reduce appetite and caloric intake.
The studies for MASLD are testing whether these functions will also work against liver disease, either directly or indirectly, through obesity and diabetes control. The early results are promising.
More Than One Risk Factor in Play
MASLD is defined by the presence of hepatic steatosis and at least one of five cardiometabolic risk factors: Overweight/obesity, hypertension, hyperglycemia, dyslipidemia with either low-plasma high-density lipoprotein cholesterol or high triglycerides, or treatment for these conditions.
It is a grim trajectory if the disease progresses to MASH, as the patient may accumulate hepatic fibrosis and go on to develop cirrhosis and/or hepatocellular carcinoma.
Typically, more than one risk factor is at play in MASLD, noted Adnan Said, MD, chief of the Division of Gastroenterology and Hepatology at the William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin.
“It most commonly occurs in the setting of weight gain and obesity, which are epidemics in the United States and worldwide, as well as the associated condition — metabolic syndrome — which goes along with obesity and includes type 2 diabetes, hyperlipidemia, hypertension, and sleep apnea,” Said, a hepatology and gastroenterology professor at the University of Wisconsin–Madison, told this news organization.
The research surrounding MASLD is investigating GLP-1 RAs as single agents and in combination with other drugs.
Finding treatment is critical, as there is only one drug — resmetirom — approved for the treatment of MASH with moderate to advanced fibrosis. But because it’s not approved for earlier stages, a treatment gap exists. The drug also doesn’t produce weight loss, which is key to treating MASLD. And while GLP-1 RAs help patients with the weight loss that is critical to MASLD, they are only approved by the US Food and Drug Administration (FDA) for obesity and type 2 diabetes.
Single Agents
The GLP-1 RAs liraglutide and semaglutide, both approved for diabetes and weight loss, are being studied as single agents against liver disease, Said said.
“Their action in the setting of MASLD and MASH is primarily indirect, through systemic pathways, improving these conditions via weight loss, as well as by improving insulin sensitivity and reducing lipotoxicity,” he added.
One of the first trials of these agents for liver disease was in 2016. In that double-blind, randomized, 48-week clinical trial of liraglutide in patients with MASH and overweight, 39% of patients who received liraglutide had a resolution of MASH compared with only 9% of those who received placebo. Moreover, only 9% vs 36% of patients in the treatment vs placebo group had progression of fibrosis.
Since then, a 72-week phase 2 trial in patients with MASH, liver fibrosis (stages F1-F3), and overweight or obesity found that once-daily subcutaneous semaglutide (0.1, 0.2, or 0.4 mg) outperformed placebo on MASH resolution without worsening of fibrosis (36%-59% vs 17%) and on weight loss (5%-13% vs 1%), with the greatest benefits at the largest dose. However, neoplasms were reported in 15% of patients receiving semaglutide vs 8% of those receiving placebo.
A phase 1 trial involving patients with liver stiffness, steatosis, and overweight or obesity found significantly greater reductions in liver fat at 48 weeks with semaglutide vs placebo, as well as decreases in liver enzymes, body weight, and A1c. There was no significant difference in liver stiffness.
Furthermore, a meta-analysis of eight studies found that treatment with 24 weeks of semaglutide significantly improved liver enzymes, reduced liver stiffness, and improved metabolic parameters in patients with MASLD/MASH. The authors cautioned that gastrointestinal adverse effects “could be a major concern.”
Several studies have found other GLP-1 RAs, including exenatide and dulaglutide, have a beneficial impact on liver injury indices and liver steatosis.
A new retrospective observational study offers evidence that GLP-1 RAs may have a direct impact on MASLD, independent of weight loss. Among the 28% of patients with type 2 diabetes and MASLD who received a GLP-1 RA, there was a significant reduction not only in body mass index but also in A1c, liver enzymes, and controlled attenuation parameter scores. A beneficial impact on liver parameters was observed even in patients who didn’t lose weight. While there was no difference in liver stiffness measurement, the median 60-month follow-up time may not have been long enough to capture such changes.
Another study indicated that the apparent benefits of GLP-1 RAs, in this case semaglutide, may not extend to patients whose disease has progressed to cirrhosis.
Dual and Triple Mechanisms of Action
Newer agents with double or triple mechanisms of action appear to have a more direct effect on the liver.
“Dual agents may have an added effect by improving MASLD directly through adipose regulation and thermogenesis, thereby improving fibrosis,” Said said.
An example is tirzepatide, a GLP-1 RA and an agonist of glucose-dependent insulinotropic polypeptide (GIP). Like GLP-1, GIP is an incretin. When used together as co-agonists, GLP-1 and GIP have been shown to increase insulin and glucagonostatic response and may work synergistically.
A new phase 2 trial that randomly assigned patients with biopsy-confirmed MASH and moderate or severe fibrosis to receive either once-weekly subcutaneous tirzepatide at one of three doses (5, 10, or 15 mg) or placebo found that tirzepatide at each dosage outperformed placebo in resolution of MASH without worsening of fibrosis.
“These findings were encouraging,” Said said. “We’ll see if the results continue into phase 3 trials.”
The combination of GLP-1 RAs with glucagon (GCG) receptor agonists also has garnered interest.
In a phase 2 trial, adults with biopsy-confirmed MASH and fibrosis stages F1-F3 were randomly assigned to receive either one of three doses of the GLP-1/GCG RA survodutide (2.4, 4.8, or 6 mg) or placebo. Survodutide at each dose was found to be superior to placebo in improving MASH without the worsening of fibrosis, reducing liver fat content by at least 30%, and decreasing liver fibrosis by at least one stage, with the 4.8-mg dose showing the best performance for each measure. However, adverse events, including nausea, diarrhea, and vomiting, were more frequent with survodutide than with placebo.
Trials of triple-action agents (GLP-1/GIP/GCG RAs) are underway too.
The hope is the triple agonists could deliver greater reduction in hepatic fat in patients with MASLD, Sanyal said.
Sanyal further noted that a reduction in liver fat is important, citing a meta-analysis that showed ≥ 30% relative decline in liver fat is associated with higher odds of histologic response and MASH resolution.
Sanyal pointed to efocipegtrutide (HM15211), a GLP-1/GIP/GCG RA, which demonstrated significant liver fat reduction after 12 weeks in patients with MASLD in a phase 1b/2a randomized, placebo-controlled trial and is now in phase 2 development.
Another example is retatrutide (LY3437943), a once-weekly injectable, that was associated with up to a 24.2% reduction in body weight at 48 weeks, compared with 2.1% with placebo, in a phase 2 trial involving patients with obesity.
A sub-study assessed the mean relative change from baseline in liver fat at 24 weeks. These participants, who also had MASLD and ≥ 10% of liver fat content, were randomly assigned to receive either retatrutide in one of four doses (1, 4, 8, or 12 mg) or placebo for 48 weeks. All doses of retatrutide showed significantly greater reduction in liver fat content compared with placebo in weeks 24-48, with a mean relative liver fat reduction > 80% at the two higher doses. Moreover, ≥ 80% of participants on the higher retatrutide doses experienced ≥ 70% reduction in liver fat at 48 weeks, compared with 0% reduction in those on placebo, and hepatic steatosis resolved in > 85% of these participants.
This space “continues to evolve at a rapid rate,” Sanyal said. For example, oral dual-action agents are under development.
Obstacles and Warnings
Sanyal warned that GLP-1 RAs can cause nausea, so they have to be introduced at a low dose and slowly titrated upward. They should be used with caution in people with a history of multiple endocrine neoplasia. There is also a small but increased risk for gallstone formation and gallstone-induced pancreatitis with rapid weight loss.
GLP-1 RAs may increase the risk for suicidal ideation, with the authors of a recent study calling for “urgent clarification” regarding this possibility.
Following reports of suicidality submitted through its Adverse Events Reporting System, the FDA concluded that it could find no causal relationship between these agents and increased risk for suicidal ideation but also that it could not “definitively rule out that a small risk may exist” and would continue to investigate.
Access to GLP-1 RAs is an obstacle as well. Semaglutide continues to be on the FDA’s shortage list.
“This is improving, but there are still issues around getting approval from insurance companies,” Sanyal said.
Many patients discontinue use because of tolerability or access issues, which is problematic because most regain the weight they had lost while on the medication.
“Right now, we see GLP-1 RAs as a long-term therapeutic commitment, but there is a lot of research interest in figuring out if there’s a more modest benefit — almost an induction-remission maintenance approach to weight loss,” Sanyal said. These are “evolving trends,” and it’s unclear how they will unfold.
“As of now, you have to decide that if you’re putting your patient on these medications, they will have to take them on a long-term basis and include that consideration in your risk-benefit analysis, together with any concerns about adverse effects,” he said.
Sanyal reported consulting for Boehringer Ingelheim, Eli Lilly, and Novo Nordisk. Said received research support from Exact Sciences, Boehringer Ingelheim, and Mallinckrodt.
A version of this article first appeared on Medscape.com.
Metabolic dysfunction–associated steatotic liver disease (MASLD) has become the most common liver disease worldwide, with a global prevalence of 32.4%. Its growth over the past three decades has occurred in tandem with increasing rates of obesity and type 2 diabetes — two cornerstones of MASLD.
Higher rates of MASLD and metabolic dysfunction–associated steatohepatitis (MASH) with fibrosis are present in adults with obesity and diabetes, noted Arun Sanyal, MD, professor and director of the Stravitz-Sanyal Institute for Liver Disease and Metabolic Health, Virginia Commonwealth University, Richmond, Virginia.
The success surrounding the medications for obesity and type 2 diabetes, including glucagon-like peptide 1 receptor agonists (GLP-1 RAs), has sparked studies investigating whether they could also be an effective treatment for liver disease.
In particular, GLP-1 RAs help patients lose weight and/or control diabetes by mimicking the function of the gut hormone GLP-1, released in response to nutrient intake, and are able to increase insulin secretion and reduce glucagon secretion, delay gastric emptying, and reduce appetite and caloric intake.
The studies for MASLD are testing whether these functions will also work against liver disease, either directly or indirectly, through obesity and diabetes control. The early results are promising.
More Than One Risk Factor in Play
MASLD is defined by the presence of hepatic steatosis and at least one of five cardiometabolic risk factors: Overweight/obesity, hypertension, hyperglycemia, dyslipidemia with either low-plasma high-density lipoprotein cholesterol or high triglycerides, or treatment for these conditions.
It is a grim trajectory if the disease progresses to MASH, as the patient may accumulate hepatic fibrosis and go on to develop cirrhosis and/or hepatocellular carcinoma.
Typically, more than one risk factor is at play in MASLD, noted Adnan Said, MD, chief of the Division of Gastroenterology and Hepatology at the William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin.
“It most commonly occurs in the setting of weight gain and obesity, which are epidemics in the United States and worldwide, as well as the associated condition — metabolic syndrome — which goes along with obesity and includes type 2 diabetes, hyperlipidemia, hypertension, and sleep apnea,” Said, a hepatology and gastroenterology professor at the University of Wisconsin–Madison, told this news organization.
The research surrounding MASLD is investigating GLP-1 RAs as single agents and in combination with other drugs.
Finding treatment is critical, as there is only one drug — resmetirom — approved for the treatment of MASH with moderate to advanced fibrosis. But because it’s not approved for earlier stages, a treatment gap exists. The drug also doesn’t produce weight loss, which is key to treating MASLD. And while GLP-1 RAs help patients with the weight loss that is critical to MASLD, they are only approved by the US Food and Drug Administration (FDA) for obesity and type 2 diabetes.
Single Agents
The GLP-1 RAs liraglutide and semaglutide, both approved for diabetes and weight loss, are being studied as single agents against liver disease, Said said.
“Their action in the setting of MASLD and MASH is primarily indirect, through systemic pathways, improving these conditions via weight loss, as well as by improving insulin sensitivity and reducing lipotoxicity,” he added.
One of the first trials of these agents for liver disease was in 2016. In that double-blind, randomized, 48-week clinical trial of liraglutide in patients with MASH and overweight, 39% of patients who received liraglutide had a resolution of MASH compared with only 9% of those who received placebo. Moreover, only 9% vs 36% of patients in the treatment vs placebo group had progression of fibrosis.
Since then, a 72-week phase 2 trial in patients with MASH, liver fibrosis (stages F1-F3), and overweight or obesity found that once-daily subcutaneous semaglutide (0.1, 0.2, or 0.4 mg) outperformed placebo on MASH resolution without worsening of fibrosis (36%-59% vs 17%) and on weight loss (5%-13% vs 1%), with the greatest benefits at the largest dose. However, neoplasms were reported in 15% of patients receiving semaglutide vs 8% of those receiving placebo.
A phase 1 trial involving patients with liver stiffness, steatosis, and overweight or obesity found significantly greater reductions in liver fat at 48 weeks with semaglutide vs placebo, as well as decreases in liver enzymes, body weight, and A1c. There was no significant difference in liver stiffness.
Furthermore, a meta-analysis of eight studies found that treatment with 24 weeks of semaglutide significantly improved liver enzymes, reduced liver stiffness, and improved metabolic parameters in patients with MASLD/MASH. The authors cautioned that gastrointestinal adverse effects “could be a major concern.”
Several studies have found other GLP-1 RAs, including exenatide and dulaglutide, have a beneficial impact on liver injury indices and liver steatosis.
A new retrospective observational study offers evidence that GLP-1 RAs may have a direct impact on MASLD, independent of weight loss. Among the 28% of patients with type 2 diabetes and MASLD who received a GLP-1 RA, there was a significant reduction not only in body mass index but also in A1c, liver enzymes, and controlled attenuation parameter scores. A beneficial impact on liver parameters was observed even in patients who didn’t lose weight. While there was no difference in liver stiffness measurement, the median 60-month follow-up time may not have been long enough to capture such changes.
Another study indicated that the apparent benefits of GLP-1 RAs, in this case semaglutide, may not extend to patients whose disease has progressed to cirrhosis.
Dual and Triple Mechanisms of Action
Newer agents with double or triple mechanisms of action appear to have a more direct effect on the liver.
“Dual agents may have an added effect by improving MASLD directly through adipose regulation and thermogenesis, thereby improving fibrosis,” Said said.
An example is tirzepatide, a GLP-1 RA and an agonist of glucose-dependent insulinotropic polypeptide (GIP). Like GLP-1, GIP is an incretin. When used together as co-agonists, GLP-1 and GIP have been shown to increase insulin and glucagonostatic response and may work synergistically.
A new phase 2 trial that randomly assigned patients with biopsy-confirmed MASH and moderate or severe fibrosis to receive either once-weekly subcutaneous tirzepatide at one of three doses (5, 10, or 15 mg) or placebo found that tirzepatide at each dosage outperformed placebo in resolution of MASH without worsening of fibrosis.
“These findings were encouraging,” Said said. “We’ll see if the results continue into phase 3 trials.”
The combination of GLP-1 RAs with glucagon (GCG) receptor agonists also has garnered interest.
In a phase 2 trial, adults with biopsy-confirmed MASH and fibrosis stages F1-F3 were randomly assigned to receive either one of three doses of the GLP-1/GCG RA survodutide (2.4, 4.8, or 6 mg) or placebo. Survodutide at each dose was found to be superior to placebo in improving MASH without the worsening of fibrosis, reducing liver fat content by at least 30%, and decreasing liver fibrosis by at least one stage, with the 4.8-mg dose showing the best performance for each measure. However, adverse events, including nausea, diarrhea, and vomiting, were more frequent with survodutide than with placebo.
Trials of triple-action agents (GLP-1/GIP/GCG RAs) are underway too.
The hope is the triple agonists could deliver greater reduction in hepatic fat in patients with MASLD, Sanyal said.
Sanyal further noted that a reduction in liver fat is important, citing a meta-analysis that showed ≥ 30% relative decline in liver fat is associated with higher odds of histologic response and MASH resolution.
Sanyal pointed to efocipegtrutide (HM15211), a GLP-1/GIP/GCG RA, which demonstrated significant liver fat reduction after 12 weeks in patients with MASLD in a phase 1b/2a randomized, placebo-controlled trial and is now in phase 2 development.
Another example is retatrutide (LY3437943), a once-weekly injectable, that was associated with up to a 24.2% reduction in body weight at 48 weeks, compared with 2.1% with placebo, in a phase 2 trial involving patients with obesity.
A sub-study assessed the mean relative change from baseline in liver fat at 24 weeks. These participants, who also had MASLD and ≥ 10% of liver fat content, were randomly assigned to receive either retatrutide in one of four doses (1, 4, 8, or 12 mg) or placebo for 48 weeks. All doses of retatrutide showed significantly greater reduction in liver fat content compared with placebo in weeks 24-48, with a mean relative liver fat reduction > 80% at the two higher doses. Moreover, ≥ 80% of participants on the higher retatrutide doses experienced ≥ 70% reduction in liver fat at 48 weeks, compared with 0% reduction in those on placebo, and hepatic steatosis resolved in > 85% of these participants.
This space “continues to evolve at a rapid rate,” Sanyal said. For example, oral dual-action agents are under development.
Obstacles and Warnings
Sanyal warned that GLP-1 RAs can cause nausea, so they have to be introduced at a low dose and slowly titrated upward. They should be used with caution in people with a history of multiple endocrine neoplasia. There is also a small but increased risk for gallstone formation and gallstone-induced pancreatitis with rapid weight loss.
GLP-1 RAs may increase the risk for suicidal ideation, with the authors of a recent study calling for “urgent clarification” regarding this possibility.
Following reports of suicidality submitted through its Adverse Events Reporting System, the FDA concluded that it could find no causal relationship between these agents and increased risk for suicidal ideation but also that it could not “definitively rule out that a small risk may exist” and would continue to investigate.
Access to GLP-1 RAs is an obstacle as well. Semaglutide continues to be on the FDA’s shortage list.
“This is improving, but there are still issues around getting approval from insurance companies,” Sanyal said.
Many patients discontinue use because of tolerability or access issues, which is problematic because most regain the weight they had lost while on the medication.
“Right now, we see GLP-1 RAs as a long-term therapeutic commitment, but there is a lot of research interest in figuring out if there’s a more modest benefit — almost an induction-remission maintenance approach to weight loss,” Sanyal said. These are “evolving trends,” and it’s unclear how they will unfold.
“As of now, you have to decide that if you’re putting your patient on these medications, they will have to take them on a long-term basis and include that consideration in your risk-benefit analysis, together with any concerns about adverse effects,” he said.
Sanyal reported consulting for Boehringer Ingelheim, Eli Lilly, and Novo Nordisk. Said received research support from Exact Sciences, Boehringer Ingelheim, and Mallinckrodt.
A version of this article first appeared on Medscape.com.
Metabolic dysfunction–associated steatotic liver disease (MASLD) has become the most common liver disease worldwide, with a global prevalence of 32.4%. Its growth over the past three decades has occurred in tandem with increasing rates of obesity and type 2 diabetes — two cornerstones of MASLD.
Higher rates of MASLD and metabolic dysfunction–associated steatohepatitis (MASH) with fibrosis are present in adults with obesity and diabetes, noted Arun Sanyal, MD, professor and director of the Stravitz-Sanyal Institute for Liver Disease and Metabolic Health, Virginia Commonwealth University, Richmond, Virginia.
The success surrounding the medications for obesity and type 2 diabetes, including glucagon-like peptide 1 receptor agonists (GLP-1 RAs), has sparked studies investigating whether they could also be an effective treatment for liver disease.
In particular, GLP-1 RAs help patients lose weight and/or control diabetes by mimicking the function of the gut hormone GLP-1, released in response to nutrient intake, and are able to increase insulin secretion and reduce glucagon secretion, delay gastric emptying, and reduce appetite and caloric intake.
The studies for MASLD are testing whether these functions will also work against liver disease, either directly or indirectly, through obesity and diabetes control. The early results are promising.
More Than One Risk Factor in Play
MASLD is defined by the presence of hepatic steatosis and at least one of five cardiometabolic risk factors: Overweight/obesity, hypertension, hyperglycemia, dyslipidemia with either low-plasma high-density lipoprotein cholesterol or high triglycerides, or treatment for these conditions.
It is a grim trajectory if the disease progresses to MASH, as the patient may accumulate hepatic fibrosis and go on to develop cirrhosis and/or hepatocellular carcinoma.
Typically, more than one risk factor is at play in MASLD, noted Adnan Said, MD, chief of the Division of Gastroenterology and Hepatology at the William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin.
“It most commonly occurs in the setting of weight gain and obesity, which are epidemics in the United States and worldwide, as well as the associated condition — metabolic syndrome — which goes along with obesity and includes type 2 diabetes, hyperlipidemia, hypertension, and sleep apnea,” Said, a hepatology and gastroenterology professor at the University of Wisconsin–Madison, told this news organization.
The research surrounding MASLD is investigating GLP-1 RAs as single agents and in combination with other drugs.
Finding treatment is critical, as there is only one drug — resmetirom — approved for the treatment of MASH with moderate to advanced fibrosis. But because it’s not approved for earlier stages, a treatment gap exists. The drug also doesn’t produce weight loss, which is key to treating MASLD. And while GLP-1 RAs help patients with the weight loss that is critical to MASLD, they are only approved by the US Food and Drug Administration (FDA) for obesity and type 2 diabetes.
Single Agents
The GLP-1 RAs liraglutide and semaglutide, both approved for diabetes and weight loss, are being studied as single agents against liver disease, Said said.
“Their action in the setting of MASLD and MASH is primarily indirect, through systemic pathways, improving these conditions via weight loss, as well as by improving insulin sensitivity and reducing lipotoxicity,” he added.
One of the first trials of these agents for liver disease was in 2016. In that double-blind, randomized, 48-week clinical trial of liraglutide in patients with MASH and overweight, 39% of patients who received liraglutide had a resolution of MASH compared with only 9% of those who received placebo. Moreover, only 9% vs 36% of patients in the treatment vs placebo group had progression of fibrosis.
Since then, a 72-week phase 2 trial in patients with MASH, liver fibrosis (stages F1-F3), and overweight or obesity found that once-daily subcutaneous semaglutide (0.1, 0.2, or 0.4 mg) outperformed placebo on MASH resolution without worsening of fibrosis (36%-59% vs 17%) and on weight loss (5%-13% vs 1%), with the greatest benefits at the largest dose. However, neoplasms were reported in 15% of patients receiving semaglutide vs 8% of those receiving placebo.
A phase 1 trial involving patients with liver stiffness, steatosis, and overweight or obesity found significantly greater reductions in liver fat at 48 weeks with semaglutide vs placebo, as well as decreases in liver enzymes, body weight, and A1c. There was no significant difference in liver stiffness.
Furthermore, a meta-analysis of eight studies found that treatment with 24 weeks of semaglutide significantly improved liver enzymes, reduced liver stiffness, and improved metabolic parameters in patients with MASLD/MASH. The authors cautioned that gastrointestinal adverse effects “could be a major concern.”
Several studies have found other GLP-1 RAs, including exenatide and dulaglutide, have a beneficial impact on liver injury indices and liver steatosis.
A new retrospective observational study offers evidence that GLP-1 RAs may have a direct impact on MASLD, independent of weight loss. Among the 28% of patients with type 2 diabetes and MASLD who received a GLP-1 RA, there was a significant reduction not only in body mass index but also in A1c, liver enzymes, and controlled attenuation parameter scores. A beneficial impact on liver parameters was observed even in patients who didn’t lose weight. While there was no difference in liver stiffness measurement, the median 60-month follow-up time may not have been long enough to capture such changes.
Another study indicated that the apparent benefits of GLP-1 RAs, in this case semaglutide, may not extend to patients whose disease has progressed to cirrhosis.
Dual and Triple Mechanisms of Action
Newer agents with double or triple mechanisms of action appear to have a more direct effect on the liver.
“Dual agents may have an added effect by improving MASLD directly through adipose regulation and thermogenesis, thereby improving fibrosis,” Said said.
An example is tirzepatide, a GLP-1 RA and an agonist of glucose-dependent insulinotropic polypeptide (GIP). Like GLP-1, GIP is an incretin. When used together as co-agonists, GLP-1 and GIP have been shown to increase insulin and glucagonostatic response and may work synergistically.
A new phase 2 trial that randomly assigned patients with biopsy-confirmed MASH and moderate or severe fibrosis to receive either once-weekly subcutaneous tirzepatide at one of three doses (5, 10, or 15 mg) or placebo found that tirzepatide at each dosage outperformed placebo in resolution of MASH without worsening of fibrosis.
“These findings were encouraging,” Said said. “We’ll see if the results continue into phase 3 trials.”
The combination of GLP-1 RAs with glucagon (GCG) receptor agonists also has garnered interest.
In a phase 2 trial, adults with biopsy-confirmed MASH and fibrosis stages F1-F3 were randomly assigned to receive either one of three doses of the GLP-1/GCG RA survodutide (2.4, 4.8, or 6 mg) or placebo. Survodutide at each dose was found to be superior to placebo in improving MASH without the worsening of fibrosis, reducing liver fat content by at least 30%, and decreasing liver fibrosis by at least one stage, with the 4.8-mg dose showing the best performance for each measure. However, adverse events, including nausea, diarrhea, and vomiting, were more frequent with survodutide than with placebo.
Trials of triple-action agents (GLP-1/GIP/GCG RAs) are underway too.
The hope is the triple agonists could deliver greater reduction in hepatic fat in patients with MASLD, Sanyal said.
Sanyal further noted that a reduction in liver fat is important, citing a meta-analysis that showed ≥ 30% relative decline in liver fat is associated with higher odds of histologic response and MASH resolution.
Sanyal pointed to efocipegtrutide (HM15211), a GLP-1/GIP/GCG RA, which demonstrated significant liver fat reduction after 12 weeks in patients with MASLD in a phase 1b/2a randomized, placebo-controlled trial and is now in phase 2 development.
Another example is retatrutide (LY3437943), a once-weekly injectable, that was associated with up to a 24.2% reduction in body weight at 48 weeks, compared with 2.1% with placebo, in a phase 2 trial involving patients with obesity.
A sub-study assessed the mean relative change from baseline in liver fat at 24 weeks. These participants, who also had MASLD and ≥ 10% of liver fat content, were randomly assigned to receive either retatrutide in one of four doses (1, 4, 8, or 12 mg) or placebo for 48 weeks. All doses of retatrutide showed significantly greater reduction in liver fat content compared with placebo in weeks 24-48, with a mean relative liver fat reduction > 80% at the two higher doses. Moreover, ≥ 80% of participants on the higher retatrutide doses experienced ≥ 70% reduction in liver fat at 48 weeks, compared with 0% reduction in those on placebo, and hepatic steatosis resolved in > 85% of these participants.
This space “continues to evolve at a rapid rate,” Sanyal said. For example, oral dual-action agents are under development.
Obstacles and Warnings
Sanyal warned that GLP-1 RAs can cause nausea, so they have to be introduced at a low dose and slowly titrated upward. They should be used with caution in people with a history of multiple endocrine neoplasia. There is also a small but increased risk for gallstone formation and gallstone-induced pancreatitis with rapid weight loss.
GLP-1 RAs may increase the risk for suicidal ideation, with the authors of a recent study calling for “urgent clarification” regarding this possibility.
Following reports of suicidality submitted through its Adverse Events Reporting System, the FDA concluded that it could find no causal relationship between these agents and increased risk for suicidal ideation but also that it could not “definitively rule out that a small risk may exist” and would continue to investigate.
Access to GLP-1 RAs is an obstacle as well. Semaglutide continues to be on the FDA’s shortage list.
“This is improving, but there are still issues around getting approval from insurance companies,” Sanyal said.
Many patients discontinue use because of tolerability or access issues, which is problematic because most regain the weight they had lost while on the medication.
“Right now, we see GLP-1 RAs as a long-term therapeutic commitment, but there is a lot of research interest in figuring out if there’s a more modest benefit — almost an induction-remission maintenance approach to weight loss,” Sanyal said. These are “evolving trends,” and it’s unclear how they will unfold.
“As of now, you have to decide that if you’re putting your patient on these medications, they will have to take them on a long-term basis and include that consideration in your risk-benefit analysis, together with any concerns about adverse effects,” he said.
Sanyal reported consulting for Boehringer Ingelheim, Eli Lilly, and Novo Nordisk. Said received research support from Exact Sciences, Boehringer Ingelheim, and Mallinckrodt.
A version of this article first appeared on Medscape.com.
Gardasil 9 at 10 Years: Vaccine Protects Against Multiple Cancers
Vaccination against human papilloma virus (HPV), a group of more than 200 viruses infecting at least 50% of sexually active people over their lifetimes, has proved more than 90% effective for preventing several diseases caused by high-risk HPV types.
Gardasil 4: 2006
It started in 2006 with the approval of Human Papillomavirus Quadrivalent, types 6, 11, 16, and 18 (Gardasil 4). Merck’s vaccine began to lower rates of cervical cancer, a major global killer of women.
“It’s fair to say the vaccine has been an American and a global public health success story in reducing rates of cervical cancer,” Paula M. Cuccaro, PhD, assistant professor of health promotion and behavioral sciences at University of Texas School of Public Health, Houston, said in an interview.
How does a common virus trigger such a lethal gynecologic malignancy? “It knocks out two important cancer suppressor genes in cells,” explained Christina Annunziata,MD, PhD, a medical oncologist and senior vice president of extramural discovery science for the American Cancer Society. HPV oncoproteins are encoded by the E6 and E7 genes. As in other DNA tumor viruses, the E6 and E7 proteins functionally inactivate the tumor suppressor proteins p53 and pRB, respectively.
US Prevalence
Despite screening and vaccination, cervical cancer is still very much around. This year, 13,820 new cases of invasive cervical cancer will be diagnosed in the United States, and approximately 4360 women will die of it, according to the American Cancer Society. Even before the advent of Gardasil 4, incidence rates had already decreased by more than half from the mid-1970s to the mid-2000s, thanks largely to Pap smear screening programs for treatable premalignant lesions. “The US rate had dropped to about 20 per 100,000 women even before Gardasil 4,” said Annunziata. “After the introduction of the first vaccine, it decreased to 7 per 100,000, a decrease of about 30%, but it remains plateaued now at about the same level.”
Although the past decade has seen rates generally stabilize, there have been some changes in different age groups. In women ages 30-44, rates increased 1.7% each year from 2012 to 2019, while rates declined 11% each year for women ages 20-24— probably reflecting the impact of the first wave of prevention from Gardasil 4.
In one 2021 population-based study of US cancer registry data from 1999 to 2017, rates of both cervical squamous cell carcinoma and adenocarcinoma dropped. The largest declines occurred in females 15-20 years old, the age group most likely to be vaccinated against HPV but not typically screened, suggesting a vaccine-related effect.
Gardasil 9: 2014
With the 2014 approval of the vaccine’s second iteration, Gardasil 9, which replaced Gardasil 4 and targeted 9 HPV strains, immunization has taken broader aim. The strains covered by Gardasil 9 protect against oropharyngeal and other head and neck cancers — as well as penile, anal, vulvar, and vaginal malignancies and premalignancies, and genital warts in both sexes ages 9-45.
It may be years, however, before the impact of the newer polyvalent formulation is felt. “While the first vaccine has been successful against the prevalent strains of HPV linked to cervical cancer, it’s a little early to call it for the newer vaccine since oropharyngeal cancers tend to develop later in older men,” Cuccaro said. “But the types of HPV linked to mouth and throat cancers and covered by the newer vaccines are much less prevalent in those who are vaccinated. The strains not covered in the vaccine you see are equally present in the vaccinated and non-vaccinated.”
Angela L. Myers, MD, MPH, division director of infectious diseases and medical director of the Center for Wellbeing at Children’s Mercy in Kansas City, Missouri, added, “Unlike for cervical cancer, there are no screening programs for oropharyngeal lesions, so you have to wait to see rates until actual cancer develops.”
A 2023 review reported that HPV vaccination reduced levels of oropharyngeal HPV positivity in men, strengthening the case for pangender immunization.
And in a recent phase 3 doubled-blind trial, GARDASIL 9 reduced the incidence of anogenital persistent infection caused by nine types of HPV compared with a placebo.
Increasing Uptake
The current public health aim is to have 80% of young people in the targeted age group vaccinated with two doses. Today, uptake among those 9-26 years old stands at about 78% of girls and 75% of boys for the first dose, said Annunziata. “But it’s only about 61% for the two doses in the current series, and we want to improve that.”
Some parents may still harbor fears that immunizing teens and tweens — both the American Academy of Pediatrics and the American Cancer Society recommend immunization at age 9 — will open the door to precocious sexual activity.
“But overall, uptake in tweens and young teens has increased because the messaging has changed,” said Myers, with the rationale now focusing on cancer prevention not sexual-infection prophylaxis. “This is similar to the hepatitis B vaccine, which used to be given to young adults and is now given to newborns to prevent cancer.”
Cuccaro added that a proactive presentation by healthcare professionals has a significant effect on vaccine uptake and increases the odds of vaccination ninefold. “Providers should take a presumptive approach and avoid just offering the vaccine as an option. It should be included with regular childhood vaccinations,” she said. “And the advantage of starting early at age 9 is that you can spread the doses out across other regular childhood vaccinations, whereas if you start at age 11, you need to add the HPV vaccine to three other vaccines that are given at that time.”
After age 15, three doses are necessary. “Providers should stress to parents that it’s most effective when given before young people become sexually active and exposed to HPV,” Cuccaro said. And Myers stressed that despite the vaccine’s effectiveness, routine screening for cervical premalignancies is still important.
Despite increasing coverage, vaccination rates have some distance to go before the public health target of at least 80% uptake of the series in the targeted age group, Cuccaro cautioned.
On the global stage, barriers to immunization remain, but the World Health Organization has endorsed a campaign to eradicate cervical cancer through HPV vaccination. It has predicted that the 21st century may be the last to experience HPV-associated cancers, currently responsible for more than 300,000 annual deaths worldwide.
A Brief History of HPV Vaccines
- 1951. Cervical cancer patient Henrietta Lacks’ rapidly dividing cervical cells are collected by George Otto Gey at Johns Hopkins Hospital. They create the first immortal cell line (HeLa) used to study cancers and vaccines worldwide.
- 1976. Harald zur Hausen suggests that genital wart-associated HPV, not herpes simplex, is the probable cause of cervical cancer.
- 1983. HPV is confirmed as a cause of cancer.
- 1991. The first HPV vaccine is developed.
- 2002. Proof of principle and protective efficacy for the monovalent HPV 16 are shown.
- 2006. Merck’s Gardasil 4 (HPV 4) is FDA approved in girls ages 9-26 for protection against strains 6, 11, 16, and 18 — the cause of more than 70% of cervical cancer cases.
- 2009. Approval of Gardasil 4 is expanded to boys ages 9-26 for the prevention of genital warts.
- 2009. The FDA approves GlaxoSmithKline’s Cervarix (HPV 16 and 18) for girls and young women. The vaccine was withdrawn from the US market in 2016 following the success of Gardasil 9 but is used abroad for HPV cancer prevention.
- 2014. The 9-valent recombinant vaccine Gardasil 9 is FDA approved for protection against several low-risk, wart-causing HPV strains as well as the high-risk cancer strains targeted by HPV 4.
- 2018. The FDA expands approval to include females and males 27-45 years old.
- 2020. The FDA extends approval of Gardasil 9 to include prevention not only of cervical cancer but also, vaginal, vulvar, anal, oropharyngeal, and other head and neck cancers.
Annunziata, Cuccaro, and Myers had no competing interests to declare.
A version of this article appeared on Medscape.com.
Vaccination against human papilloma virus (HPV), a group of more than 200 viruses infecting at least 50% of sexually active people over their lifetimes, has proved more than 90% effective for preventing several diseases caused by high-risk HPV types.
Gardasil 4: 2006
It started in 2006 with the approval of Human Papillomavirus Quadrivalent, types 6, 11, 16, and 18 (Gardasil 4). Merck’s vaccine began to lower rates of cervical cancer, a major global killer of women.
“It’s fair to say the vaccine has been an American and a global public health success story in reducing rates of cervical cancer,” Paula M. Cuccaro, PhD, assistant professor of health promotion and behavioral sciences at University of Texas School of Public Health, Houston, said in an interview.
How does a common virus trigger such a lethal gynecologic malignancy? “It knocks out two important cancer suppressor genes in cells,” explained Christina Annunziata,MD, PhD, a medical oncologist and senior vice president of extramural discovery science for the American Cancer Society. HPV oncoproteins are encoded by the E6 and E7 genes. As in other DNA tumor viruses, the E6 and E7 proteins functionally inactivate the tumor suppressor proteins p53 and pRB, respectively.
US Prevalence
Despite screening and vaccination, cervical cancer is still very much around. This year, 13,820 new cases of invasive cervical cancer will be diagnosed in the United States, and approximately 4360 women will die of it, according to the American Cancer Society. Even before the advent of Gardasil 4, incidence rates had already decreased by more than half from the mid-1970s to the mid-2000s, thanks largely to Pap smear screening programs for treatable premalignant lesions. “The US rate had dropped to about 20 per 100,000 women even before Gardasil 4,” said Annunziata. “After the introduction of the first vaccine, it decreased to 7 per 100,000, a decrease of about 30%, but it remains plateaued now at about the same level.”
Although the past decade has seen rates generally stabilize, there have been some changes in different age groups. In women ages 30-44, rates increased 1.7% each year from 2012 to 2019, while rates declined 11% each year for women ages 20-24— probably reflecting the impact of the first wave of prevention from Gardasil 4.
In one 2021 population-based study of US cancer registry data from 1999 to 2017, rates of both cervical squamous cell carcinoma and adenocarcinoma dropped. The largest declines occurred in females 15-20 years old, the age group most likely to be vaccinated against HPV but not typically screened, suggesting a vaccine-related effect.
Gardasil 9: 2014
With the 2014 approval of the vaccine’s second iteration, Gardasil 9, which replaced Gardasil 4 and targeted 9 HPV strains, immunization has taken broader aim. The strains covered by Gardasil 9 protect against oropharyngeal and other head and neck cancers — as well as penile, anal, vulvar, and vaginal malignancies and premalignancies, and genital warts in both sexes ages 9-45.
It may be years, however, before the impact of the newer polyvalent formulation is felt. “While the first vaccine has been successful against the prevalent strains of HPV linked to cervical cancer, it’s a little early to call it for the newer vaccine since oropharyngeal cancers tend to develop later in older men,” Cuccaro said. “But the types of HPV linked to mouth and throat cancers and covered by the newer vaccines are much less prevalent in those who are vaccinated. The strains not covered in the vaccine you see are equally present in the vaccinated and non-vaccinated.”
Angela L. Myers, MD, MPH, division director of infectious diseases and medical director of the Center for Wellbeing at Children’s Mercy in Kansas City, Missouri, added, “Unlike for cervical cancer, there are no screening programs for oropharyngeal lesions, so you have to wait to see rates until actual cancer develops.”
A 2023 review reported that HPV vaccination reduced levels of oropharyngeal HPV positivity in men, strengthening the case for pangender immunization.
And in a recent phase 3 doubled-blind trial, GARDASIL 9 reduced the incidence of anogenital persistent infection caused by nine types of HPV compared with a placebo.
Increasing Uptake
The current public health aim is to have 80% of young people in the targeted age group vaccinated with two doses. Today, uptake among those 9-26 years old stands at about 78% of girls and 75% of boys for the first dose, said Annunziata. “But it’s only about 61% for the two doses in the current series, and we want to improve that.”
Some parents may still harbor fears that immunizing teens and tweens — both the American Academy of Pediatrics and the American Cancer Society recommend immunization at age 9 — will open the door to precocious sexual activity.
“But overall, uptake in tweens and young teens has increased because the messaging has changed,” said Myers, with the rationale now focusing on cancer prevention not sexual-infection prophylaxis. “This is similar to the hepatitis B vaccine, which used to be given to young adults and is now given to newborns to prevent cancer.”
Cuccaro added that a proactive presentation by healthcare professionals has a significant effect on vaccine uptake and increases the odds of vaccination ninefold. “Providers should take a presumptive approach and avoid just offering the vaccine as an option. It should be included with regular childhood vaccinations,” she said. “And the advantage of starting early at age 9 is that you can spread the doses out across other regular childhood vaccinations, whereas if you start at age 11, you need to add the HPV vaccine to three other vaccines that are given at that time.”
After age 15, three doses are necessary. “Providers should stress to parents that it’s most effective when given before young people become sexually active and exposed to HPV,” Cuccaro said. And Myers stressed that despite the vaccine’s effectiveness, routine screening for cervical premalignancies is still important.
Despite increasing coverage, vaccination rates have some distance to go before the public health target of at least 80% uptake of the series in the targeted age group, Cuccaro cautioned.
On the global stage, barriers to immunization remain, but the World Health Organization has endorsed a campaign to eradicate cervical cancer through HPV vaccination. It has predicted that the 21st century may be the last to experience HPV-associated cancers, currently responsible for more than 300,000 annual deaths worldwide.
A Brief History of HPV Vaccines
- 1951. Cervical cancer patient Henrietta Lacks’ rapidly dividing cervical cells are collected by George Otto Gey at Johns Hopkins Hospital. They create the first immortal cell line (HeLa) used to study cancers and vaccines worldwide.
- 1976. Harald zur Hausen suggests that genital wart-associated HPV, not herpes simplex, is the probable cause of cervical cancer.
- 1983. HPV is confirmed as a cause of cancer.
- 1991. The first HPV vaccine is developed.
- 2002. Proof of principle and protective efficacy for the monovalent HPV 16 are shown.
- 2006. Merck’s Gardasil 4 (HPV 4) is FDA approved in girls ages 9-26 for protection against strains 6, 11, 16, and 18 — the cause of more than 70% of cervical cancer cases.
- 2009. Approval of Gardasil 4 is expanded to boys ages 9-26 for the prevention of genital warts.
- 2009. The FDA approves GlaxoSmithKline’s Cervarix (HPV 16 and 18) for girls and young women. The vaccine was withdrawn from the US market in 2016 following the success of Gardasil 9 but is used abroad for HPV cancer prevention.
- 2014. The 9-valent recombinant vaccine Gardasil 9 is FDA approved for protection against several low-risk, wart-causing HPV strains as well as the high-risk cancer strains targeted by HPV 4.
- 2018. The FDA expands approval to include females and males 27-45 years old.
- 2020. The FDA extends approval of Gardasil 9 to include prevention not only of cervical cancer but also, vaginal, vulvar, anal, oropharyngeal, and other head and neck cancers.
Annunziata, Cuccaro, and Myers had no competing interests to declare.
A version of this article appeared on Medscape.com.
Vaccination against human papilloma virus (HPV), a group of more than 200 viruses infecting at least 50% of sexually active people over their lifetimes, has proved more than 90% effective for preventing several diseases caused by high-risk HPV types.
Gardasil 4: 2006
It started in 2006 with the approval of Human Papillomavirus Quadrivalent, types 6, 11, 16, and 18 (Gardasil 4). Merck’s vaccine began to lower rates of cervical cancer, a major global killer of women.
“It’s fair to say the vaccine has been an American and a global public health success story in reducing rates of cervical cancer,” Paula M. Cuccaro, PhD, assistant professor of health promotion and behavioral sciences at University of Texas School of Public Health, Houston, said in an interview.
How does a common virus trigger such a lethal gynecologic malignancy? “It knocks out two important cancer suppressor genes in cells,” explained Christina Annunziata,MD, PhD, a medical oncologist and senior vice president of extramural discovery science for the American Cancer Society. HPV oncoproteins are encoded by the E6 and E7 genes. As in other DNA tumor viruses, the E6 and E7 proteins functionally inactivate the tumor suppressor proteins p53 and pRB, respectively.
US Prevalence
Despite screening and vaccination, cervical cancer is still very much around. This year, 13,820 new cases of invasive cervical cancer will be diagnosed in the United States, and approximately 4360 women will die of it, according to the American Cancer Society. Even before the advent of Gardasil 4, incidence rates had already decreased by more than half from the mid-1970s to the mid-2000s, thanks largely to Pap smear screening programs for treatable premalignant lesions. “The US rate had dropped to about 20 per 100,000 women even before Gardasil 4,” said Annunziata. “After the introduction of the first vaccine, it decreased to 7 per 100,000, a decrease of about 30%, but it remains plateaued now at about the same level.”
Although the past decade has seen rates generally stabilize, there have been some changes in different age groups. In women ages 30-44, rates increased 1.7% each year from 2012 to 2019, while rates declined 11% each year for women ages 20-24— probably reflecting the impact of the first wave of prevention from Gardasil 4.
In one 2021 population-based study of US cancer registry data from 1999 to 2017, rates of both cervical squamous cell carcinoma and adenocarcinoma dropped. The largest declines occurred in females 15-20 years old, the age group most likely to be vaccinated against HPV but not typically screened, suggesting a vaccine-related effect.
Gardasil 9: 2014
With the 2014 approval of the vaccine’s second iteration, Gardasil 9, which replaced Gardasil 4 and targeted 9 HPV strains, immunization has taken broader aim. The strains covered by Gardasil 9 protect against oropharyngeal and other head and neck cancers — as well as penile, anal, vulvar, and vaginal malignancies and premalignancies, and genital warts in both sexes ages 9-45.
It may be years, however, before the impact of the newer polyvalent formulation is felt. “While the first vaccine has been successful against the prevalent strains of HPV linked to cervical cancer, it’s a little early to call it for the newer vaccine since oropharyngeal cancers tend to develop later in older men,” Cuccaro said. “But the types of HPV linked to mouth and throat cancers and covered by the newer vaccines are much less prevalent in those who are vaccinated. The strains not covered in the vaccine you see are equally present in the vaccinated and non-vaccinated.”
Angela L. Myers, MD, MPH, division director of infectious diseases and medical director of the Center for Wellbeing at Children’s Mercy in Kansas City, Missouri, added, “Unlike for cervical cancer, there are no screening programs for oropharyngeal lesions, so you have to wait to see rates until actual cancer develops.”
A 2023 review reported that HPV vaccination reduced levels of oropharyngeal HPV positivity in men, strengthening the case for pangender immunization.
And in a recent phase 3 doubled-blind trial, GARDASIL 9 reduced the incidence of anogenital persistent infection caused by nine types of HPV compared with a placebo.
Increasing Uptake
The current public health aim is to have 80% of young people in the targeted age group vaccinated with two doses. Today, uptake among those 9-26 years old stands at about 78% of girls and 75% of boys for the first dose, said Annunziata. “But it’s only about 61% for the two doses in the current series, and we want to improve that.”
Some parents may still harbor fears that immunizing teens and tweens — both the American Academy of Pediatrics and the American Cancer Society recommend immunization at age 9 — will open the door to precocious sexual activity.
“But overall, uptake in tweens and young teens has increased because the messaging has changed,” said Myers, with the rationale now focusing on cancer prevention not sexual-infection prophylaxis. “This is similar to the hepatitis B vaccine, which used to be given to young adults and is now given to newborns to prevent cancer.”
Cuccaro added that a proactive presentation by healthcare professionals has a significant effect on vaccine uptake and increases the odds of vaccination ninefold. “Providers should take a presumptive approach and avoid just offering the vaccine as an option. It should be included with regular childhood vaccinations,” she said. “And the advantage of starting early at age 9 is that you can spread the doses out across other regular childhood vaccinations, whereas if you start at age 11, you need to add the HPV vaccine to three other vaccines that are given at that time.”
After age 15, three doses are necessary. “Providers should stress to parents that it’s most effective when given before young people become sexually active and exposed to HPV,” Cuccaro said. And Myers stressed that despite the vaccine’s effectiveness, routine screening for cervical premalignancies is still important.
Despite increasing coverage, vaccination rates have some distance to go before the public health target of at least 80% uptake of the series in the targeted age group, Cuccaro cautioned.
On the global stage, barriers to immunization remain, but the World Health Organization has endorsed a campaign to eradicate cervical cancer through HPV vaccination. It has predicted that the 21st century may be the last to experience HPV-associated cancers, currently responsible for more than 300,000 annual deaths worldwide.
A Brief History of HPV Vaccines
- 1951. Cervical cancer patient Henrietta Lacks’ rapidly dividing cervical cells are collected by George Otto Gey at Johns Hopkins Hospital. They create the first immortal cell line (HeLa) used to study cancers and vaccines worldwide.
- 1976. Harald zur Hausen suggests that genital wart-associated HPV, not herpes simplex, is the probable cause of cervical cancer.
- 1983. HPV is confirmed as a cause of cancer.
- 1991. The first HPV vaccine is developed.
- 2002. Proof of principle and protective efficacy for the monovalent HPV 16 are shown.
- 2006. Merck’s Gardasil 4 (HPV 4) is FDA approved in girls ages 9-26 for protection against strains 6, 11, 16, and 18 — the cause of more than 70% of cervical cancer cases.
- 2009. Approval of Gardasil 4 is expanded to boys ages 9-26 for the prevention of genital warts.
- 2009. The FDA approves GlaxoSmithKline’s Cervarix (HPV 16 and 18) for girls and young women. The vaccine was withdrawn from the US market in 2016 following the success of Gardasil 9 but is used abroad for HPV cancer prevention.
- 2014. The 9-valent recombinant vaccine Gardasil 9 is FDA approved for protection against several low-risk, wart-causing HPV strains as well as the high-risk cancer strains targeted by HPV 4.
- 2018. The FDA expands approval to include females and males 27-45 years old.
- 2020. The FDA extends approval of Gardasil 9 to include prevention not only of cervical cancer but also, vaginal, vulvar, anal, oropharyngeal, and other head and neck cancers.
Annunziata, Cuccaro, and Myers had no competing interests to declare.
A version of this article appeared on Medscape.com.
Outpatient CAR T: Safe, Effective, Accessible
In one recent study, an industry-funded phase 2 trial, researchers found similar outcomes from outpatient and inpatient CAR T-cell therapy for relapsed/refractory large B-cell lymphoma with lisocabtagene maraleucel (Breyanzi).
Another recent study reported that outpatient treatment of B cell non-Hodgkin lymphoma with tisagenlecleucel (Kymriah) had similar efficacy to inpatient treatment. Meanwhile, a 2023 review of CAR T-cell therapy in various settings found similar outcomes in outpatient and inpatient treatment.
“The future of CAR T-cell therapy lies in balancing safety with accessibility,” said Rayne Rouce, MD, a pediatric oncologist at Texas Children’s Cancer Center in Houston, Texas, in an interview. “Expanding CAR T-cell therapy beyond large medical centers is a critical next step.”
Great Outcomes, Low Access
Since 2017, the FDA has approved six CAR T-cell therapies, which target cancer by harnessing the power of a patient’s own T cells. As an Oregon Health & Sciences University/Knight Cancer Center website explains, T cells are removed from the patient’s body, “genetically modified to make the chimeric antigen receptor, or CAR, [which] protein binds to specific proteins on the surface of cancer cells.”
Modified cells are grown and then infused back into the body, where they “multiply and may be able to destroy all the cancer cells.”
As Rouce puts it, “CAR T-cells have revolutionized the treatment of relapsed or refractory blood cancers.” One or more of the therapies have been approved to treat types of lymphoblastic leukemia, B-cell lymphoma, follicular lymphoma, mantle cell lymphoma, and multiple myeloma.
A 2023 review of clinical trial data reported complete response rates of 40%-54% in aggressive B-cell lymphoma, 67% in mantle cell lymphoma, and 69%-74% in indolent B cell lymphoma.
“Commercialization of CAR T-cell therapy brought hope that access would expand beyond the major academic medical centers with the highly specialized infrastructure and advanced laboratories required to manufacture and ultimately treat patients,” Rouce said. “However, it quickly became clear that patients who are underinsured or uninsured — or who live outside the network of the well-resourced institutions that house these therapies — are still unable to access these potentially life-saving therapies.”
A 2024 report estimated the cost of CAR T-cell therapy as $700,000-$1 million and said only a small percentage of those who could benefit from the treatment actually get it. For example, an estimated 10,000 patients with diffuse large B-cell lymphoma alone could benefit from CAR T therapy annually, but a survey of 200 US healthcare centers in 2021 found that 1900 procedures were performed overall for all indications.
Distance to Treatment Is a Major Obstacle
Even if patients have insurance plans willing to cover CAR T-cell therapy, they may not be able get care. While more than 150 US centers are certified to administer the therapy, “distance to major medical centers with CAR T capabilities is a major obstacle,” Yuliya Linhares, MD, chief of lymphoma at Miami Cancer Institute in Miami, Florida, said in an interview.
“I have had patients who chose to not proceed with CAR T therapy due to inability to travel the distance to the medical center for pre-CAR T appointments and assessments and a lack of caretakers who are available to stay nearby,” Linhares said.
Indeed, the challenges facing patients in rural and underserved urban areas can be overwhelming, Hoda Badr, PhD, professor of medicine at Baylor College of Medicine in Houston, Texas, said in an interview.
“They must take time off work, arrange accommodations near treatment sites, and manage travel costs, all of which strain limited financial resources. The inability to afford these additional expenses can lead to delays in receiving care or patients forgoing the treatment altogether,” Badr said. She added that “the psychological and social burden of being away from family and community support systems during treatment can intensify the stress of an already difficult situation.”
A statistic tells the story of the urban/community divide. CAR T-cell therapy administration at academic centers after leukapheresis — the separation and collection of white blood cells — is reported to be at around 90%, while it’s only 47% in community-based practices that have to refer patients elsewhere, Linhares noted.
Researchers Explore CAR T-Cell Therapy in the Community
Linhares is lead author of the phase 2 trial that explored administration of lisocabtagene maraleucel in 82 patients with relapsed/refractory large B-cell lymphoma. The findings were published Sept. 30 in Blood Advances.
The OUTREACH trial, funded by Juno/Bristol-Myers Squibb, treated patients in the third line and beyond at community medical centers (outpatient-monitored, 70%; inpatient-monitored, 30%). The trial didn’t require facilities to be certified by the Foundation for the Accreditation of Cellular Therapy (FACT); all had to be non-tertiary cancer centers that weren’t associated with a university. In order to administer therapy on the outpatient basis, the centers had to have phase 1 or hematopoietic stem cell transplant capabilities.
As Linhares explained, 72% of participating centers hadn’t provided CAR T-cell therapy before, and 44% did not have FACT accreditation. “About 32% of patients received CAR T at CAR T naive sites, while 70% of patients received CAR T as outpatients. Investigators had to decide whether patients qualified for the outpatient observation or had to be admitted for the inpatient observation,” she noted.
Community Outcomes Were Comparable to Major Trial
As for the results, grade 3 or higher adverse events occurred at a similar frequency among outpatients and inpatients at 74% and 76%, Linhares said. There were no grade 5 adverse events, and 25% of patients treated as outpatients were never hospitalized.
Response rates were similar to those in the major TRANSCEND trial with the objective response rates rate of 80% and complete response rates of 54%.
“Overall,” Linhares said, “our study demonstrated that with the availability of standard operating procedures, specially trained staff and a multidisciplinary team trained in CAR T toxicity management, inpatient and outpatient CAR T administration is feasible at specialized community medical centers.”
In 2023, another study examined patients with B-cell non-Hodgkin lymphoma who were treated on an outpatient basis with tisagenlecleucel. Researchers reported that outpatient therapy was “feasible and associated with similar efficacy outcomes as inpatient treatment.”
And a 2023 systematic literature review identified 11 studies that reported outpatient vs inpatient outcomes in CAR T-cell therapy and found “comparable response rates (80-82% in outpatient and 72-80% in inpatient).” Costs were cheaper in the outpatient setting.
Research findings like these are good news, Baylor College of Medicine’s Badr said. “Outpatient administration could help to scale the availability of this therapy to a broader range of healthcare settings, including those serving underserved populations. Findings indicate promising safety profiles, which is encouraging for expanding access.”
Not Every Patient Can Tolerate Outpatient Care
Linhares noted that the patients who received outpatient care in the lisocabtagene maraleucel study were in better shape than those in the inpatient group. Those selected for inpatient care had “higher disease risk characteristics, including high grade B cell lymphoma histology, higher disease burden, and having received bridging therapy. This points to the fact that the investigators properly selected patients who were at a higher risk of complications for inpatient observation. Additionally, some patients stayed as inpatient due to social factors, which increases length of stay independently of disease characteristics.”
Specifically, reasons for inpatient monitoring were disease characteristics (48%) including tumor burden and risk of adverse events; psychosocial factors (32%) including lack of caregiver support or transportation; COVID-19 precautions (8%); pre-infusion adverse events (8%) of fever and vasovagal reaction; and principal investigator decision (4%) due to limited hospital experience with CAR T-cell therapy.
Texas Children’s Cancer Center’s Rouce said “certain patients, particularly those with higher risk for complications or those who require intensive monitoring, may not be suited for outpatient CAR T-cell therapy. This may be due to other comorbidities or baseline factors known to predispose to CAR T-related toxicities. However, evidence-based risk mitigation algorithms may still allow closely monitored outpatient treatment, with recognition that hospital admission for incipient side effects may be necessary.”
What’s Next for Access to Therapy?
Rouce noted that her institution, like many others, is offering CAR T-cell therapy on an outpatient basis. “Additionally, continued scientific innovation, such as immediately available, off-the-shelf cell therapies and inducible safety switches, will ultimately improve access,” she said.
Linhares noted a recent advance and highlighted research that’s now in progress. “CAR Ts now have an indication as a second-line therapy in relapsed/refractory large B-cell lymphoma, and there are ongoing clinical trials that will potentially move CAR Ts into the first line,” she said. “Some trials are exploring allogeneic, readily available off-the-shelf CAR T for the treatment of minimal residual disease positive large B-cell lymphoma after completion of first-line therapy.”
These potential advances “are increasing the need for CAR T-capable medical centers,” Linhares noted. “More and more medical centers with expert hematology teams are becoming CAR T-certified, with more patients having access to CAR T.”
Still, she said, “I don’t think access is nearly as good as it should be. Many patients in rural areas are still unable to get this life-saving treatment. “However, “it is very possible that other novel targeted therapies, such as bispecific antibodies, will be used in place of CAR T in areas with poor CAR T access. Bispecific antibody efficacy in various B cell lymphoma histologies are being currently explored.”
Rouce discloses relationships with Novartis and Pfizer. Linhares reports ties with Kyowa Kirin, AbbVie, ADC, BeiGene, Genentech, Gilead, GlaxoSmithKline, Seagen, and TG. Badr has no disclosures.
A version of this article appeared on Medscape.com.
In one recent study, an industry-funded phase 2 trial, researchers found similar outcomes from outpatient and inpatient CAR T-cell therapy for relapsed/refractory large B-cell lymphoma with lisocabtagene maraleucel (Breyanzi).
Another recent study reported that outpatient treatment of B cell non-Hodgkin lymphoma with tisagenlecleucel (Kymriah) had similar efficacy to inpatient treatment. Meanwhile, a 2023 review of CAR T-cell therapy in various settings found similar outcomes in outpatient and inpatient treatment.
“The future of CAR T-cell therapy lies in balancing safety with accessibility,” said Rayne Rouce, MD, a pediatric oncologist at Texas Children’s Cancer Center in Houston, Texas, in an interview. “Expanding CAR T-cell therapy beyond large medical centers is a critical next step.”
Great Outcomes, Low Access
Since 2017, the FDA has approved six CAR T-cell therapies, which target cancer by harnessing the power of a patient’s own T cells. As an Oregon Health & Sciences University/Knight Cancer Center website explains, T cells are removed from the patient’s body, “genetically modified to make the chimeric antigen receptor, or CAR, [which] protein binds to specific proteins on the surface of cancer cells.”
Modified cells are grown and then infused back into the body, where they “multiply and may be able to destroy all the cancer cells.”
As Rouce puts it, “CAR T-cells have revolutionized the treatment of relapsed or refractory blood cancers.” One or more of the therapies have been approved to treat types of lymphoblastic leukemia, B-cell lymphoma, follicular lymphoma, mantle cell lymphoma, and multiple myeloma.
A 2023 review of clinical trial data reported complete response rates of 40%-54% in aggressive B-cell lymphoma, 67% in mantle cell lymphoma, and 69%-74% in indolent B cell lymphoma.
“Commercialization of CAR T-cell therapy brought hope that access would expand beyond the major academic medical centers with the highly specialized infrastructure and advanced laboratories required to manufacture and ultimately treat patients,” Rouce said. “However, it quickly became clear that patients who are underinsured or uninsured — or who live outside the network of the well-resourced institutions that house these therapies — are still unable to access these potentially life-saving therapies.”
A 2024 report estimated the cost of CAR T-cell therapy as $700,000-$1 million and said only a small percentage of those who could benefit from the treatment actually get it. For example, an estimated 10,000 patients with diffuse large B-cell lymphoma alone could benefit from CAR T therapy annually, but a survey of 200 US healthcare centers in 2021 found that 1900 procedures were performed overall for all indications.
Distance to Treatment Is a Major Obstacle
Even if patients have insurance plans willing to cover CAR T-cell therapy, they may not be able get care. While more than 150 US centers are certified to administer the therapy, “distance to major medical centers with CAR T capabilities is a major obstacle,” Yuliya Linhares, MD, chief of lymphoma at Miami Cancer Institute in Miami, Florida, said in an interview.
“I have had patients who chose to not proceed with CAR T therapy due to inability to travel the distance to the medical center for pre-CAR T appointments and assessments and a lack of caretakers who are available to stay nearby,” Linhares said.
Indeed, the challenges facing patients in rural and underserved urban areas can be overwhelming, Hoda Badr, PhD, professor of medicine at Baylor College of Medicine in Houston, Texas, said in an interview.
“They must take time off work, arrange accommodations near treatment sites, and manage travel costs, all of which strain limited financial resources. The inability to afford these additional expenses can lead to delays in receiving care or patients forgoing the treatment altogether,” Badr said. She added that “the psychological and social burden of being away from family and community support systems during treatment can intensify the stress of an already difficult situation.”
A statistic tells the story of the urban/community divide. CAR T-cell therapy administration at academic centers after leukapheresis — the separation and collection of white blood cells — is reported to be at around 90%, while it’s only 47% in community-based practices that have to refer patients elsewhere, Linhares noted.
Researchers Explore CAR T-Cell Therapy in the Community
Linhares is lead author of the phase 2 trial that explored administration of lisocabtagene maraleucel in 82 patients with relapsed/refractory large B-cell lymphoma. The findings were published Sept. 30 in Blood Advances.
The OUTREACH trial, funded by Juno/Bristol-Myers Squibb, treated patients in the third line and beyond at community medical centers (outpatient-monitored, 70%; inpatient-monitored, 30%). The trial didn’t require facilities to be certified by the Foundation for the Accreditation of Cellular Therapy (FACT); all had to be non-tertiary cancer centers that weren’t associated with a university. In order to administer therapy on the outpatient basis, the centers had to have phase 1 or hematopoietic stem cell transplant capabilities.
As Linhares explained, 72% of participating centers hadn’t provided CAR T-cell therapy before, and 44% did not have FACT accreditation. “About 32% of patients received CAR T at CAR T naive sites, while 70% of patients received CAR T as outpatients. Investigators had to decide whether patients qualified for the outpatient observation or had to be admitted for the inpatient observation,” she noted.
Community Outcomes Were Comparable to Major Trial
As for the results, grade 3 or higher adverse events occurred at a similar frequency among outpatients and inpatients at 74% and 76%, Linhares said. There were no grade 5 adverse events, and 25% of patients treated as outpatients were never hospitalized.
Response rates were similar to those in the major TRANSCEND trial with the objective response rates rate of 80% and complete response rates of 54%.
“Overall,” Linhares said, “our study demonstrated that with the availability of standard operating procedures, specially trained staff and a multidisciplinary team trained in CAR T toxicity management, inpatient and outpatient CAR T administration is feasible at specialized community medical centers.”
In 2023, another study examined patients with B-cell non-Hodgkin lymphoma who were treated on an outpatient basis with tisagenlecleucel. Researchers reported that outpatient therapy was “feasible and associated with similar efficacy outcomes as inpatient treatment.”
And a 2023 systematic literature review identified 11 studies that reported outpatient vs inpatient outcomes in CAR T-cell therapy and found “comparable response rates (80-82% in outpatient and 72-80% in inpatient).” Costs were cheaper in the outpatient setting.
Research findings like these are good news, Baylor College of Medicine’s Badr said. “Outpatient administration could help to scale the availability of this therapy to a broader range of healthcare settings, including those serving underserved populations. Findings indicate promising safety profiles, which is encouraging for expanding access.”
Not Every Patient Can Tolerate Outpatient Care
Linhares noted that the patients who received outpatient care in the lisocabtagene maraleucel study were in better shape than those in the inpatient group. Those selected for inpatient care had “higher disease risk characteristics, including high grade B cell lymphoma histology, higher disease burden, and having received bridging therapy. This points to the fact that the investigators properly selected patients who were at a higher risk of complications for inpatient observation. Additionally, some patients stayed as inpatient due to social factors, which increases length of stay independently of disease characteristics.”
Specifically, reasons for inpatient monitoring were disease characteristics (48%) including tumor burden and risk of adverse events; psychosocial factors (32%) including lack of caregiver support or transportation; COVID-19 precautions (8%); pre-infusion adverse events (8%) of fever and vasovagal reaction; and principal investigator decision (4%) due to limited hospital experience with CAR T-cell therapy.
Texas Children’s Cancer Center’s Rouce said “certain patients, particularly those with higher risk for complications or those who require intensive monitoring, may not be suited for outpatient CAR T-cell therapy. This may be due to other comorbidities or baseline factors known to predispose to CAR T-related toxicities. However, evidence-based risk mitigation algorithms may still allow closely monitored outpatient treatment, with recognition that hospital admission for incipient side effects may be necessary.”
What’s Next for Access to Therapy?
Rouce noted that her institution, like many others, is offering CAR T-cell therapy on an outpatient basis. “Additionally, continued scientific innovation, such as immediately available, off-the-shelf cell therapies and inducible safety switches, will ultimately improve access,” she said.
Linhares noted a recent advance and highlighted research that’s now in progress. “CAR Ts now have an indication as a second-line therapy in relapsed/refractory large B-cell lymphoma, and there are ongoing clinical trials that will potentially move CAR Ts into the first line,” she said. “Some trials are exploring allogeneic, readily available off-the-shelf CAR T for the treatment of minimal residual disease positive large B-cell lymphoma after completion of first-line therapy.”
These potential advances “are increasing the need for CAR T-capable medical centers,” Linhares noted. “More and more medical centers with expert hematology teams are becoming CAR T-certified, with more patients having access to CAR T.”
Still, she said, “I don’t think access is nearly as good as it should be. Many patients in rural areas are still unable to get this life-saving treatment. “However, “it is very possible that other novel targeted therapies, such as bispecific antibodies, will be used in place of CAR T in areas with poor CAR T access. Bispecific antibody efficacy in various B cell lymphoma histologies are being currently explored.”
Rouce discloses relationships with Novartis and Pfizer. Linhares reports ties with Kyowa Kirin, AbbVie, ADC, BeiGene, Genentech, Gilead, GlaxoSmithKline, Seagen, and TG. Badr has no disclosures.
A version of this article appeared on Medscape.com.
In one recent study, an industry-funded phase 2 trial, researchers found similar outcomes from outpatient and inpatient CAR T-cell therapy for relapsed/refractory large B-cell lymphoma with lisocabtagene maraleucel (Breyanzi).
Another recent study reported that outpatient treatment of B cell non-Hodgkin lymphoma with tisagenlecleucel (Kymriah) had similar efficacy to inpatient treatment. Meanwhile, a 2023 review of CAR T-cell therapy in various settings found similar outcomes in outpatient and inpatient treatment.
“The future of CAR T-cell therapy lies in balancing safety with accessibility,” said Rayne Rouce, MD, a pediatric oncologist at Texas Children’s Cancer Center in Houston, Texas, in an interview. “Expanding CAR T-cell therapy beyond large medical centers is a critical next step.”
Great Outcomes, Low Access
Since 2017, the FDA has approved six CAR T-cell therapies, which target cancer by harnessing the power of a patient’s own T cells. As an Oregon Health & Sciences University/Knight Cancer Center website explains, T cells are removed from the patient’s body, “genetically modified to make the chimeric antigen receptor, or CAR, [which] protein binds to specific proteins on the surface of cancer cells.”
Modified cells are grown and then infused back into the body, where they “multiply and may be able to destroy all the cancer cells.”
As Rouce puts it, “CAR T-cells have revolutionized the treatment of relapsed or refractory blood cancers.” One or more of the therapies have been approved to treat types of lymphoblastic leukemia, B-cell lymphoma, follicular lymphoma, mantle cell lymphoma, and multiple myeloma.
A 2023 review of clinical trial data reported complete response rates of 40%-54% in aggressive B-cell lymphoma, 67% in mantle cell lymphoma, and 69%-74% in indolent B cell lymphoma.
“Commercialization of CAR T-cell therapy brought hope that access would expand beyond the major academic medical centers with the highly specialized infrastructure and advanced laboratories required to manufacture and ultimately treat patients,” Rouce said. “However, it quickly became clear that patients who are underinsured or uninsured — or who live outside the network of the well-resourced institutions that house these therapies — are still unable to access these potentially life-saving therapies.”
A 2024 report estimated the cost of CAR T-cell therapy as $700,000-$1 million and said only a small percentage of those who could benefit from the treatment actually get it. For example, an estimated 10,000 patients with diffuse large B-cell lymphoma alone could benefit from CAR T therapy annually, but a survey of 200 US healthcare centers in 2021 found that 1900 procedures were performed overall for all indications.
Distance to Treatment Is a Major Obstacle
Even if patients have insurance plans willing to cover CAR T-cell therapy, they may not be able get care. While more than 150 US centers are certified to administer the therapy, “distance to major medical centers with CAR T capabilities is a major obstacle,” Yuliya Linhares, MD, chief of lymphoma at Miami Cancer Institute in Miami, Florida, said in an interview.
“I have had patients who chose to not proceed with CAR T therapy due to inability to travel the distance to the medical center for pre-CAR T appointments and assessments and a lack of caretakers who are available to stay nearby,” Linhares said.
Indeed, the challenges facing patients in rural and underserved urban areas can be overwhelming, Hoda Badr, PhD, professor of medicine at Baylor College of Medicine in Houston, Texas, said in an interview.
“They must take time off work, arrange accommodations near treatment sites, and manage travel costs, all of which strain limited financial resources. The inability to afford these additional expenses can lead to delays in receiving care or patients forgoing the treatment altogether,” Badr said. She added that “the psychological and social burden of being away from family and community support systems during treatment can intensify the stress of an already difficult situation.”
A statistic tells the story of the urban/community divide. CAR T-cell therapy administration at academic centers after leukapheresis — the separation and collection of white blood cells — is reported to be at around 90%, while it’s only 47% in community-based practices that have to refer patients elsewhere, Linhares noted.
Researchers Explore CAR T-Cell Therapy in the Community
Linhares is lead author of the phase 2 trial that explored administration of lisocabtagene maraleucel in 82 patients with relapsed/refractory large B-cell lymphoma. The findings were published Sept. 30 in Blood Advances.
The OUTREACH trial, funded by Juno/Bristol-Myers Squibb, treated patients in the third line and beyond at community medical centers (outpatient-monitored, 70%; inpatient-monitored, 30%). The trial didn’t require facilities to be certified by the Foundation for the Accreditation of Cellular Therapy (FACT); all had to be non-tertiary cancer centers that weren’t associated with a university. In order to administer therapy on the outpatient basis, the centers had to have phase 1 or hematopoietic stem cell transplant capabilities.
As Linhares explained, 72% of participating centers hadn’t provided CAR T-cell therapy before, and 44% did not have FACT accreditation. “About 32% of patients received CAR T at CAR T naive sites, while 70% of patients received CAR T as outpatients. Investigators had to decide whether patients qualified for the outpatient observation or had to be admitted for the inpatient observation,” she noted.
Community Outcomes Were Comparable to Major Trial
As for the results, grade 3 or higher adverse events occurred at a similar frequency among outpatients and inpatients at 74% and 76%, Linhares said. There were no grade 5 adverse events, and 25% of patients treated as outpatients were never hospitalized.
Response rates were similar to those in the major TRANSCEND trial with the objective response rates rate of 80% and complete response rates of 54%.
“Overall,” Linhares said, “our study demonstrated that with the availability of standard operating procedures, specially trained staff and a multidisciplinary team trained in CAR T toxicity management, inpatient and outpatient CAR T administration is feasible at specialized community medical centers.”
In 2023, another study examined patients with B-cell non-Hodgkin lymphoma who were treated on an outpatient basis with tisagenlecleucel. Researchers reported that outpatient therapy was “feasible and associated with similar efficacy outcomes as inpatient treatment.”
And a 2023 systematic literature review identified 11 studies that reported outpatient vs inpatient outcomes in CAR T-cell therapy and found “comparable response rates (80-82% in outpatient and 72-80% in inpatient).” Costs were cheaper in the outpatient setting.
Research findings like these are good news, Baylor College of Medicine’s Badr said. “Outpatient administration could help to scale the availability of this therapy to a broader range of healthcare settings, including those serving underserved populations. Findings indicate promising safety profiles, which is encouraging for expanding access.”
Not Every Patient Can Tolerate Outpatient Care
Linhares noted that the patients who received outpatient care in the lisocabtagene maraleucel study were in better shape than those in the inpatient group. Those selected for inpatient care had “higher disease risk characteristics, including high grade B cell lymphoma histology, higher disease burden, and having received bridging therapy. This points to the fact that the investigators properly selected patients who were at a higher risk of complications for inpatient observation. Additionally, some patients stayed as inpatient due to social factors, which increases length of stay independently of disease characteristics.”
Specifically, reasons for inpatient monitoring were disease characteristics (48%) including tumor burden and risk of adverse events; psychosocial factors (32%) including lack of caregiver support or transportation; COVID-19 precautions (8%); pre-infusion adverse events (8%) of fever and vasovagal reaction; and principal investigator decision (4%) due to limited hospital experience with CAR T-cell therapy.
Texas Children’s Cancer Center’s Rouce said “certain patients, particularly those with higher risk for complications or those who require intensive monitoring, may not be suited for outpatient CAR T-cell therapy. This may be due to other comorbidities or baseline factors known to predispose to CAR T-related toxicities. However, evidence-based risk mitigation algorithms may still allow closely monitored outpatient treatment, with recognition that hospital admission for incipient side effects may be necessary.”
What’s Next for Access to Therapy?
Rouce noted that her institution, like many others, is offering CAR T-cell therapy on an outpatient basis. “Additionally, continued scientific innovation, such as immediately available, off-the-shelf cell therapies and inducible safety switches, will ultimately improve access,” she said.
Linhares noted a recent advance and highlighted research that’s now in progress. “CAR Ts now have an indication as a second-line therapy in relapsed/refractory large B-cell lymphoma, and there are ongoing clinical trials that will potentially move CAR Ts into the first line,” she said. “Some trials are exploring allogeneic, readily available off-the-shelf CAR T for the treatment of minimal residual disease positive large B-cell lymphoma after completion of first-line therapy.”
These potential advances “are increasing the need for CAR T-capable medical centers,” Linhares noted. “More and more medical centers with expert hematology teams are becoming CAR T-certified, with more patients having access to CAR T.”
Still, she said, “I don’t think access is nearly as good as it should be. Many patients in rural areas are still unable to get this life-saving treatment. “However, “it is very possible that other novel targeted therapies, such as bispecific antibodies, will be used in place of CAR T in areas with poor CAR T access. Bispecific antibody efficacy in various B cell lymphoma histologies are being currently explored.”
Rouce discloses relationships with Novartis and Pfizer. Linhares reports ties with Kyowa Kirin, AbbVie, ADC, BeiGene, Genentech, Gilead, GlaxoSmithKline, Seagen, and TG. Badr has no disclosures.
A version of this article appeared on Medscape.com.
RA Prevention: A Decade of Trials Provides Insights on What’s to Come
With the discovery of autoantibodies and other risk factors for rheumatoid arthritis (RA), researchers developed clinical trials to see whether the disease can be prevented entirely. In the past 10 years, a number of these trials have concluded, with variable results.
While some trials demonstrated no effect at all, others showed that medical intervention can delay the onset of disease in certain populations and even reduce the rates of progression to RA. These completed trials also offer researchers the chance to identify opportunities to improve RA prevention trials moving forward.
“We’re looking at all that data and trying to figure out what the next step is going to be,” said Kevin Deane, MD, PhD, a professor of medicine and a rheumatologist at the University of Colorado School of Medicine, Aurora.
Key lessons include the need for improved risk stratification tools and better understanding of RA pathogenesis, he said.
The Research So Far
All RA prevention trials except for one have been completed and/or published within the past decade, bringing valuable insights to the field. (See chart below.)
Atorvastatin (STAPRA) and hydroxychloroquine (StopRA) proved ineffective in preventing the onset of RA, and both trials were stopped early. Rituximab and methotrexate (MTX) both delayed the onset of RA, but the effect disappeared by the end of the follow-up periods.
However, the 2-year results from the TREAT EARLIER trial showed that compared with patients given placebo, those given MTX showed improved MRI-detected joint inflammation, physical functioning, and reported symptoms.
The 4-year analysis of the trial further risk stratified participants and found that MTX showed a preventive effect in anti–citrullinated protein antibody (ACPA)–negative participants at an increased risk for RA.
Abatacept also showed promise in preventing RA in two separate trials. In the ARIAA trial, compared with placebo, 6 months of treatment with abatacept reduced MRI inflammation and symptoms and lowered the rates of progression to RA. This treatment effect lessened during the 1-year follow-up period, but the difference between the two groups was still significant at 18 months.
In the APIPPRA trial, 12 months of treatment with abatacept improved subclinical inflammation and quality-of-life measures in participants and reduced the rates of progression to RA through another 12 months of observation. However, during this post-treatment follow-up period, the treatment effect began to diminish.
While there have been some promising findings — not only in disease prevention but also in disease modification — these studies all looked at different patient groups, noted Kulveer Mankia, MA, DM, an associate professor and consulting rheumatologist at the Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds in England.
“You have disparate, different inclusion criteria in different studies, all of which take years to complete,” he said. For example, while the TREAT EARLIER trial recruited patients with joint pain and subclinical joint inflammation via MRI, regardless of autoantibody status, the APIPPRA trial enrolled patients that were both ACPA+ and rheumatoid factor (RF)+ with joint pain.
“You’re left extrapolating as to whether [these interventions] will work in different at-risk populations,” he said.
Even with specific inclusion criteria in each study, there can still be heterogeneity in risk within a study group, Deane said. In the TREAT EARLIER study, 18%-20% of participants ultimately developed RA over the study period, which is lower than expected.
“While it seemed like a pretty high-risk group, it wasn’t as high risk as we thought,” he said, “and that’s why we’ve gone back to the drawing board.”
Risk Stratification Efforts
There are now two ongoing joint efforts by the American College of Rheumatology (ACR) and the European Alliance of Associations for Rheumatology (EULAR) to define these populations and “bring some consensus to the field,” Mankia said.
The first aims to create a unanimous risk stratification tool for future RA prevention studies. The proposed system, devised for individuals with new joint symptoms who are at a risk for RA, was presented at the EULAR 2024 annual meeting and will be further discussed at the upcoming ACR 2024 annual meeting in Washington, DC.
The system uses a point system based on six criteria — three lab tests and three criteria commonly assessed in clinical practice:
- Morning stiffness
- Patient-reported joint swelling
- Difficulty making a fist
- Increased C-reactive protein
- RF positivity
- ACPA positivity
These criteria were picked so that the risk stratification tool can be used without imaging; however, the inclusion of MRI can further refine the score.
The ACR-EULAR task force that created the tool has emphasized that this criterion is specifically designed for research purposes and should not be used in clinical practice. Using this stratification tool should allow future clinical studies to group patients by similar risk, Deane said.
“Not that all studies have to look at exactly the same people, but each study should have similar risk stratification,” he said.
The second ACR-EULAR joint effort is taking a population-based approach to risk stratification, Deane said, to better predict RA risk in individuals without common symptoms like joint pain.
The aim is to create something analogous to the Framingham Risk Score in predicting cardiovascular disease, in which simple variables like total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, and smoking status can be used to calculate an individual’s 10-year risk for CVD, Deane explained.
The second approach could also identify patients earlier in the progression to RA, which may be easier to treat than later stages of disease.
Understanding RA Origins
However, treating an earlier stage of disease might require a different approach. Up to this point, medical interventions for RA prevention used drugs approved to treat RA, but inventions during the pre-RA stage — before any joint symptoms appear — might require targeting different immunologic pathways.
“The general concept is if there is a pre-RA stage when joints are not involved, that means all the immunologic abnormalities are probably happening somewhere else in the body,” he said. “The big question is: Where is that, and how exactly is that happening?”
One theory is that RA begins to develop in mucosal sites, such as the intestines or lungs, before it involves synovial joints.
“In the absence of resolution, these localized immune processes transition into a systemic process that targets the joints, either by direct effects of microbiota, molecular mimicry, and/or immune amplification,” wrote Deane and coauthors in a recent review article in Annals of the Rheumatic Diseases. “This, in turn, leads to inappropriate engagement of a range of effector mechanisms in both synovium and periarticular sites.”
Following this logic, the progression of the at-risk stage of RA could be considered a continuum along which there are multiple possible points for intervention. It’s also probable that the disease can develop through multiple pathways, Deane said.
“If you look at all the people who get rheumatoid arthritis, there’s probably no way those could have the same exact pathways,” he said. “There’s probably going to be different endotypes and understanding that is going to help us prevent disease in a better way.”
Looking Forward
Beyond improving risk stratification and understanding RA pathogenesis, researchers are also considering novel therapeutic approaches for future trials. Glucagon-like peptide 1 (GLP-1) receptor agonists could be worth exploring in RA prevention and treatment, said Jeffrey A. Sparks, MD, MMSc, a rheumatologist at Brigham and Women’s Hospital, Boston, Massachusetts.
These drugs — initially developed for diabetes — have already shown anti-inflammatory effects, and one study suggested that GLP-1s lowered the risk for major adverse cardiovascular events and all-cause mortality in individuals with immune-mediated inflammatory diseases. Obesity is a known risk factor for RA, so weight loss aided by GLP-1 drugs could also help reduce risk in certain patients. Clinical trials are needed to explore GLP-1s for both RA prevention and treatment, he said.
While prevention trials up to this point have used one-time, time-limited interventions, longer durations of medication or multiple rounds of therapy may be more efficacious. Even for trials that demonstrated the intervention arms had less progression to RA, this effect diminished once participants stopped the medication. In the ARIAA and APIPPRA trials using abatacept, “it wasn’t like we hit a reset button and [patients] just permanently now did not get rheumatoid arthritis,” Deane said, suggesting that alternative approaches should be explored.
“Future studies need to look at potentially longer doses of drug or lower doses of drug, or some combination that might be effective,” he said.
Deane received honoraria from Bristol-Myers Squibb, Thermo Fisher, and Werfen and grant funding from Janssen Research and Development and Gilead Sciences. Mankia received grant support from Gilead, Lilly, AstraZeneca, and Serac Life Sciences and honoraria or consultant fees from AbbVie, UCB, Lilly, Galapagos, DeepCure, Serac Life Sciences, AstraZeneca, and Zura Bio. Sparks received research support from Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, and Sonoma Biotherapeutics. He consulted for AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Gilead, Inova Diagnostics, Janssen, Merck, Mustang, Optum, Pfizer, ReCor Medical, Sana, Sobi, and UCB.
A version of this article first appeared on Medscape.com.
With the discovery of autoantibodies and other risk factors for rheumatoid arthritis (RA), researchers developed clinical trials to see whether the disease can be prevented entirely. In the past 10 years, a number of these trials have concluded, with variable results.
While some trials demonstrated no effect at all, others showed that medical intervention can delay the onset of disease in certain populations and even reduce the rates of progression to RA. These completed trials also offer researchers the chance to identify opportunities to improve RA prevention trials moving forward.
“We’re looking at all that data and trying to figure out what the next step is going to be,” said Kevin Deane, MD, PhD, a professor of medicine and a rheumatologist at the University of Colorado School of Medicine, Aurora.
Key lessons include the need for improved risk stratification tools and better understanding of RA pathogenesis, he said.
The Research So Far
All RA prevention trials except for one have been completed and/or published within the past decade, bringing valuable insights to the field. (See chart below.)

Atorvastatin (STAPRA) and hydroxychloroquine (StopRA) proved ineffective in preventing the onset of RA, and both trials were stopped early. Rituximab and methotrexate (MTX) both delayed the onset of RA, but the effect disappeared by the end of the follow-up periods.
However, the 2-year results from the TREAT EARLIER trial showed that compared with patients given placebo, those given MTX showed improved MRI-detected joint inflammation, physical functioning, and reported symptoms.
The 4-year analysis of the trial further risk stratified participants and found that MTX showed a preventive effect in anti–citrullinated protein antibody (ACPA)–negative participants at an increased risk for RA.
Abatacept also showed promise in preventing RA in two separate trials. In the ARIAA trial, compared with placebo, 6 months of treatment with abatacept reduced MRI inflammation and symptoms and lowered the rates of progression to RA. This treatment effect lessened during the 1-year follow-up period, but the difference between the two groups was still significant at 18 months.
In the APIPPRA trial, 12 months of treatment with abatacept improved subclinical inflammation and quality-of-life measures in participants and reduced the rates of progression to RA through another 12 months of observation. However, during this post-treatment follow-up period, the treatment effect began to diminish.
While there have been some promising findings — not only in disease prevention but also in disease modification — these studies all looked at different patient groups, noted Kulveer Mankia, MA, DM, an associate professor and consulting rheumatologist at the Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds in England.
“You have disparate, different inclusion criteria in different studies, all of which take years to complete,” he said. For example, while the TREAT EARLIER trial recruited patients with joint pain and subclinical joint inflammation via MRI, regardless of autoantibody status, the APIPPRA trial enrolled patients that were both ACPA+ and rheumatoid factor (RF)+ with joint pain.
“You’re left extrapolating as to whether [these interventions] will work in different at-risk populations,” he said.
Even with specific inclusion criteria in each study, there can still be heterogeneity in risk within a study group, Deane said. In the TREAT EARLIER study, 18%-20% of participants ultimately developed RA over the study period, which is lower than expected.
“While it seemed like a pretty high-risk group, it wasn’t as high risk as we thought,” he said, “and that’s why we’ve gone back to the drawing board.”
Risk Stratification Efforts
There are now two ongoing joint efforts by the American College of Rheumatology (ACR) and the European Alliance of Associations for Rheumatology (EULAR) to define these populations and “bring some consensus to the field,” Mankia said.
The first aims to create a unanimous risk stratification tool for future RA prevention studies. The proposed system, devised for individuals with new joint symptoms who are at a risk for RA, was presented at the EULAR 2024 annual meeting and will be further discussed at the upcoming ACR 2024 annual meeting in Washington, DC.
The system uses a point system based on six criteria — three lab tests and three criteria commonly assessed in clinical practice:
- Morning stiffness
- Patient-reported joint swelling
- Difficulty making a fist
- Increased C-reactive protein
- RF positivity
- ACPA positivity
These criteria were picked so that the risk stratification tool can be used without imaging; however, the inclusion of MRI can further refine the score.
The ACR-EULAR task force that created the tool has emphasized that this criterion is specifically designed for research purposes and should not be used in clinical practice. Using this stratification tool should allow future clinical studies to group patients by similar risk, Deane said.
“Not that all studies have to look at exactly the same people, but each study should have similar risk stratification,” he said.
The second ACR-EULAR joint effort is taking a population-based approach to risk stratification, Deane said, to better predict RA risk in individuals without common symptoms like joint pain.
The aim is to create something analogous to the Framingham Risk Score in predicting cardiovascular disease, in which simple variables like total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, and smoking status can be used to calculate an individual’s 10-year risk for CVD, Deane explained.
The second approach could also identify patients earlier in the progression to RA, which may be easier to treat than later stages of disease.
Understanding RA Origins
However, treating an earlier stage of disease might require a different approach. Up to this point, medical interventions for RA prevention used drugs approved to treat RA, but inventions during the pre-RA stage — before any joint symptoms appear — might require targeting different immunologic pathways.
“The general concept is if there is a pre-RA stage when joints are not involved, that means all the immunologic abnormalities are probably happening somewhere else in the body,” he said. “The big question is: Where is that, and how exactly is that happening?”
One theory is that RA begins to develop in mucosal sites, such as the intestines or lungs, before it involves synovial joints.
“In the absence of resolution, these localized immune processes transition into a systemic process that targets the joints, either by direct effects of microbiota, molecular mimicry, and/or immune amplification,” wrote Deane and coauthors in a recent review article in Annals of the Rheumatic Diseases. “This, in turn, leads to inappropriate engagement of a range of effector mechanisms in both synovium and periarticular sites.”
Following this logic, the progression of the at-risk stage of RA could be considered a continuum along which there are multiple possible points for intervention. It’s also probable that the disease can develop through multiple pathways, Deane said.
“If you look at all the people who get rheumatoid arthritis, there’s probably no way those could have the same exact pathways,” he said. “There’s probably going to be different endotypes and understanding that is going to help us prevent disease in a better way.”
Looking Forward
Beyond improving risk stratification and understanding RA pathogenesis, researchers are also considering novel therapeutic approaches for future trials. Glucagon-like peptide 1 (GLP-1) receptor agonists could be worth exploring in RA prevention and treatment, said Jeffrey A. Sparks, MD, MMSc, a rheumatologist at Brigham and Women’s Hospital, Boston, Massachusetts.
These drugs — initially developed for diabetes — have already shown anti-inflammatory effects, and one study suggested that GLP-1s lowered the risk for major adverse cardiovascular events and all-cause mortality in individuals with immune-mediated inflammatory diseases. Obesity is a known risk factor for RA, so weight loss aided by GLP-1 drugs could also help reduce risk in certain patients. Clinical trials are needed to explore GLP-1s for both RA prevention and treatment, he said.
While prevention trials up to this point have used one-time, time-limited interventions, longer durations of medication or multiple rounds of therapy may be more efficacious. Even for trials that demonstrated the intervention arms had less progression to RA, this effect diminished once participants stopped the medication. In the ARIAA and APIPPRA trials using abatacept, “it wasn’t like we hit a reset button and [patients] just permanently now did not get rheumatoid arthritis,” Deane said, suggesting that alternative approaches should be explored.
“Future studies need to look at potentially longer doses of drug or lower doses of drug, or some combination that might be effective,” he said.
Deane received honoraria from Bristol-Myers Squibb, Thermo Fisher, and Werfen and grant funding from Janssen Research and Development and Gilead Sciences. Mankia received grant support from Gilead, Lilly, AstraZeneca, and Serac Life Sciences and honoraria or consultant fees from AbbVie, UCB, Lilly, Galapagos, DeepCure, Serac Life Sciences, AstraZeneca, and Zura Bio. Sparks received research support from Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, and Sonoma Biotherapeutics. He consulted for AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Gilead, Inova Diagnostics, Janssen, Merck, Mustang, Optum, Pfizer, ReCor Medical, Sana, Sobi, and UCB.
A version of this article first appeared on Medscape.com.
With the discovery of autoantibodies and other risk factors for rheumatoid arthritis (RA), researchers developed clinical trials to see whether the disease can be prevented entirely. In the past 10 years, a number of these trials have concluded, with variable results.
While some trials demonstrated no effect at all, others showed that medical intervention can delay the onset of disease in certain populations and even reduce the rates of progression to RA. These completed trials also offer researchers the chance to identify opportunities to improve RA prevention trials moving forward.
“We’re looking at all that data and trying to figure out what the next step is going to be,” said Kevin Deane, MD, PhD, a professor of medicine and a rheumatologist at the University of Colorado School of Medicine, Aurora.
Key lessons include the need for improved risk stratification tools and better understanding of RA pathogenesis, he said.
The Research So Far
All RA prevention trials except for one have been completed and/or published within the past decade, bringing valuable insights to the field. (See chart below.)

Atorvastatin (STAPRA) and hydroxychloroquine (StopRA) proved ineffective in preventing the onset of RA, and both trials were stopped early. Rituximab and methotrexate (MTX) both delayed the onset of RA, but the effect disappeared by the end of the follow-up periods.
However, the 2-year results from the TREAT EARLIER trial showed that compared with patients given placebo, those given MTX showed improved MRI-detected joint inflammation, physical functioning, and reported symptoms.
The 4-year analysis of the trial further risk stratified participants and found that MTX showed a preventive effect in anti–citrullinated protein antibody (ACPA)–negative participants at an increased risk for RA.
Abatacept also showed promise in preventing RA in two separate trials. In the ARIAA trial, compared with placebo, 6 months of treatment with abatacept reduced MRI inflammation and symptoms and lowered the rates of progression to RA. This treatment effect lessened during the 1-year follow-up period, but the difference between the two groups was still significant at 18 months.
In the APIPPRA trial, 12 months of treatment with abatacept improved subclinical inflammation and quality-of-life measures in participants and reduced the rates of progression to RA through another 12 months of observation. However, during this post-treatment follow-up period, the treatment effect began to diminish.
While there have been some promising findings — not only in disease prevention but also in disease modification — these studies all looked at different patient groups, noted Kulveer Mankia, MA, DM, an associate professor and consulting rheumatologist at the Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds in England.
“You have disparate, different inclusion criteria in different studies, all of which take years to complete,” he said. For example, while the TREAT EARLIER trial recruited patients with joint pain and subclinical joint inflammation via MRI, regardless of autoantibody status, the APIPPRA trial enrolled patients that were both ACPA+ and rheumatoid factor (RF)+ with joint pain.
“You’re left extrapolating as to whether [these interventions] will work in different at-risk populations,” he said.
Even with specific inclusion criteria in each study, there can still be heterogeneity in risk within a study group, Deane said. In the TREAT EARLIER study, 18%-20% of participants ultimately developed RA over the study period, which is lower than expected.
“While it seemed like a pretty high-risk group, it wasn’t as high risk as we thought,” he said, “and that’s why we’ve gone back to the drawing board.”
Risk Stratification Efforts
There are now two ongoing joint efforts by the American College of Rheumatology (ACR) and the European Alliance of Associations for Rheumatology (EULAR) to define these populations and “bring some consensus to the field,” Mankia said.
The first aims to create a unanimous risk stratification tool for future RA prevention studies. The proposed system, devised for individuals with new joint symptoms who are at a risk for RA, was presented at the EULAR 2024 annual meeting and will be further discussed at the upcoming ACR 2024 annual meeting in Washington, DC.
The system uses a point system based on six criteria — three lab tests and three criteria commonly assessed in clinical practice:
- Morning stiffness
- Patient-reported joint swelling
- Difficulty making a fist
- Increased C-reactive protein
- RF positivity
- ACPA positivity
These criteria were picked so that the risk stratification tool can be used without imaging; however, the inclusion of MRI can further refine the score.
The ACR-EULAR task force that created the tool has emphasized that this criterion is specifically designed for research purposes and should not be used in clinical practice. Using this stratification tool should allow future clinical studies to group patients by similar risk, Deane said.
“Not that all studies have to look at exactly the same people, but each study should have similar risk stratification,” he said.
The second ACR-EULAR joint effort is taking a population-based approach to risk stratification, Deane said, to better predict RA risk in individuals without common symptoms like joint pain.
The aim is to create something analogous to the Framingham Risk Score in predicting cardiovascular disease, in which simple variables like total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, and smoking status can be used to calculate an individual’s 10-year risk for CVD, Deane explained.
The second approach could also identify patients earlier in the progression to RA, which may be easier to treat than later stages of disease.
Understanding RA Origins
However, treating an earlier stage of disease might require a different approach. Up to this point, medical interventions for RA prevention used drugs approved to treat RA, but inventions during the pre-RA stage — before any joint symptoms appear — might require targeting different immunologic pathways.
“The general concept is if there is a pre-RA stage when joints are not involved, that means all the immunologic abnormalities are probably happening somewhere else in the body,” he said. “The big question is: Where is that, and how exactly is that happening?”
One theory is that RA begins to develop in mucosal sites, such as the intestines or lungs, before it involves synovial joints.
“In the absence of resolution, these localized immune processes transition into a systemic process that targets the joints, either by direct effects of microbiota, molecular mimicry, and/or immune amplification,” wrote Deane and coauthors in a recent review article in Annals of the Rheumatic Diseases. “This, in turn, leads to inappropriate engagement of a range of effector mechanisms in both synovium and periarticular sites.”
Following this logic, the progression of the at-risk stage of RA could be considered a continuum along which there are multiple possible points for intervention. It’s also probable that the disease can develop through multiple pathways, Deane said.
“If you look at all the people who get rheumatoid arthritis, there’s probably no way those could have the same exact pathways,” he said. “There’s probably going to be different endotypes and understanding that is going to help us prevent disease in a better way.”
Looking Forward
Beyond improving risk stratification and understanding RA pathogenesis, researchers are also considering novel therapeutic approaches for future trials. Glucagon-like peptide 1 (GLP-1) receptor agonists could be worth exploring in RA prevention and treatment, said Jeffrey A. Sparks, MD, MMSc, a rheumatologist at Brigham and Women’s Hospital, Boston, Massachusetts.
These drugs — initially developed for diabetes — have already shown anti-inflammatory effects, and one study suggested that GLP-1s lowered the risk for major adverse cardiovascular events and all-cause mortality in individuals with immune-mediated inflammatory diseases. Obesity is a known risk factor for RA, so weight loss aided by GLP-1 drugs could also help reduce risk in certain patients. Clinical trials are needed to explore GLP-1s for both RA prevention and treatment, he said.
While prevention trials up to this point have used one-time, time-limited interventions, longer durations of medication or multiple rounds of therapy may be more efficacious. Even for trials that demonstrated the intervention arms had less progression to RA, this effect diminished once participants stopped the medication. In the ARIAA and APIPPRA trials using abatacept, “it wasn’t like we hit a reset button and [patients] just permanently now did not get rheumatoid arthritis,” Deane said, suggesting that alternative approaches should be explored.
“Future studies need to look at potentially longer doses of drug or lower doses of drug, or some combination that might be effective,” he said.
Deane received honoraria from Bristol-Myers Squibb, Thermo Fisher, and Werfen and grant funding from Janssen Research and Development and Gilead Sciences. Mankia received grant support from Gilead, Lilly, AstraZeneca, and Serac Life Sciences and honoraria or consultant fees from AbbVie, UCB, Lilly, Galapagos, DeepCure, Serac Life Sciences, AstraZeneca, and Zura Bio. Sparks received research support from Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, and Sonoma Biotherapeutics. He consulted for AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Gilead, Inova Diagnostics, Janssen, Merck, Mustang, Optum, Pfizer, ReCor Medical, Sana, Sobi, and UCB.
A version of this article first appeared on Medscape.com.
What’s the Evidence Behind Popular Supplements in Rheumatology? Experts Weigh in
Many people with rheumatologic diseases try supplements for symptom relief. Here’s what you need to know about some common picks.
Dietary supplements were a $159 billion business in the United States in 2023, and many people with rheumatologic diseases are buying in. Research suggests more than 6 in 10 people with fibromyalgia, nearly 8 in 10 people with Sjögren’s disease, and more than 8 in 10 people with rheumatoid arthritis (RA) take dietary supplements.
Whatever the symptom — pain, swelling, or fatigue — you can probably find a supplement purporting to relieve it. But do these supplements work, and are they safe? A study review in RMD Open comprising 24 systematic reviews and 150 original articles suggests more high-quality research is needed on the effects of dietary supplements on rheumatologic diseases. Most studies have focused on RA or osteoarthritis (OA), where the evidence level is moderate at best.
“The studies in this space are usually not very high quality because there’s no money to support them, among other things, plus the products are disparate,” said Janet Funk, MD, MS, professor in the School of Nutritional Sciences and Wellness at the University of Arizona, Tucson. She recommended brushing up on supplements and finding out what patients are taking so you can offer advice and watch for drug-supplement interactions.
When asked for a medication list, many patients forget to report supplements, Funk said. “You have to prompt them specifically. I think some physicians have very negative views about supplements because so little data is known, and patients might pick up on that and decide not to report their use.” She recommended saying something like: “To give you the best possible care, I want to know everything you’re taking, including supplements. The things I’m prescribing could maybe interact with the things you’re taking, so I want to make sure I know about all of it so that together we can figure out if the combination of things is safe.”
The quality of dietary supplements varies, and they aren’t regulated like drugs by the Food and Drug Administration. Funk recommended selecting products verified by NSF or ConsumerLab. They test supplements to ensure the label reflects what’s inside.
This news organization scoured the literature and asked experts to weigh in on the evidence behind popular supplements in rheumatology today.
The Essential Nutrients
Vitamin supplements are a staple in many homes — but are they helpful? “Individual vitamin supplements will not provide any benefit unless the person is deficient in a specific vitamin or mineral,” according to Elena Philippou, PhD, RD, associate professor of nutrition-dietetics at the University of Nicosia in Cyprus, and Elena Nikiphorou, MBBS, a rheumatologist at King’s College London in England. For some patients, deficiency is a reality. A retrospective cohort study in The Journal of Clinical Medicine found that people with RA were 17% more likely than age-matched control individuals to have nutrient deficiencies, perhaps because symptoms like fatigue, pain, and nausea affect their eating habits. Here’s what the science says about common vitamin supplements.
Vitamin D. This hormone-like vitamin, which attaches to receptors on immune cells to tamp down inflammation, was the most popular dietary supplement among rheumatology patients in a recent study from the United Kingdom. Vitamin D deficiency is common in people with RA, lupus, Sjögren’s disease, ankylosing spondylitis, systemic sclerosis, and fibromyalgia. In some cases, vitamin D levels track with disease activity, research suggests. Corticosteroids can also make vitamin D deficiency more likely. Can supplements help?
In RA, evidence points to small improvements. A systematic review of 11 studies including 3049 patients published in Nutrition Reviews showed that vitamin D supplements significantly reduced patients’ pain and Disease Activity Score in 28 joints (DAS28) using both C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR).
The research is mixed on the benefits of vitamin D supplementation for fibromyalgia symptoms, according to a study review in SN Comprehensive Clinical Medicine that included two studies and 80 patients on supplementation. However, researchers said it’s still worth discussing the potential benefits of taking vitamin D.
“Vitamin D supplementation is important in the context of various rheumatic diseases to prevent or treat bone disease,” said Philippou and Nikiphorou. “People with rheumatic disease should speak to their healthcare provider and ask to check their blood vitamin D concentration.” The results can help you recommend a dose.
Folate. Patients on methotrexate should take folic acid supplements under the guidance of a healthcare provider, said Philippou and Nikiphorou. The reason: Methotrexate can deplete folic acid levels, increasing the risk for side effects. An analysis of adverse event reports published showed that methotrexate users who took folic acid (or tumor necrosis factor–alpha inhibitors) had a reduced risk for hepatotoxicity or myelosuppression. A commonly recommended dose is 1 mg/d.
Vitamin B12. In a 2024 perspective paper in Rheumatology International, researchers said physicians should assess vitamin B12 levels early in the diagnostic process of rheumatologic diseases. One reason: Many symptoms of pernicious anemia, like fatigue, mimic symptoms of rheumatologic diseases. The gastrointestinal (GI) effects of systemic sclerosis could bring on vitamin B12 deficiency. In a small study in The Journal of Clinical Rheumatology, 44 of 62 patients with systemic sclerosis had low vitamin B12 levels.
Vitamin E. Vitamin E deficiency is rare in healthy adults. However, some medical conditions, like inflammatory bowel disease and malabsorption disorders, can make vitamin E deficiency more likely. In RA, a vitamin E supplement could help reduce joint swelling and sensitivity, according to a systematic review of nine studies including 39,845 patients in The European Journal of Clinical Nutrition. Researchers credit the nutrient’s role in aiding intestinal repair. Use with caution, as this supplement can increase bleeding risk in doses over 1000 mg/d.
Vitamin A. Like vitamin E, vitamin A deficiency is rare in the United States. The risk of oversupplementing is higher than undersupplementing. However, vitamin A deficiency can happen in people with chronic pancreatic, liver, or GI problems. In people with deficiency, a vitamin A supplement can help relieve dry eye symptoms common in Sjögren’s disease, suggests a narrative review published in Nutrients. Vitamin A might help reduce ocular surface changes by supporting the production of proteins that protect the outermost surfaces of the eyes. The recommended daily allowance for vitamin A is 900 μg. High-dose supplements can cause toxicity, resulting in GI symptoms and problems like lethargy, drowsiness, increased intracranial pressure, and skin changes.
The Replacements
These substances are similar to naturally occurring compounds in our bodies. The question is whether ingesting them yields benefits.
Glucosamine and chondroitin. Glucosamine and chondroitin occur naturally in our bodies and help us form and protect connective tissues. In pill form, this combo is the most popular dietary supplement for OA, according to research in the journal Maturitas. But studies of its effectiveness yield mixed results. A systematic review of 25 studies published in Inflammopharmacology showed that, in patients with knee OA, supplementation with about 1500 mg of glucosamine per day reduced tibiofemoral joint space narrowing, while supplementation with about 800 mg/d of chondroitin reduced pain intensity and improved physical function, compared with placebo. The duo of glucosamine and chondroitin did not bring significant benefits, perhaps because more studies are needed. Most side effects were mild, but some literature points to the potential for glucosamine to increase warfarin’s blood-thinning effects.
Omega-3 fatty acids. Fish oil is a top-selling supplement, and it might be helpful in inflammatory rheumatologic diseases. A systematic review of 30 studies including 710 patients published in Arthritis Research & Therapy showed that omega-3 fatty acid supplements can improve pain, swollen and tender joint count, DAS28 scores, and Health Assessment Questionnaire scores in patients with RA, psoriatic arthritis, or ankylosing spondylitis. In patients with lupus, a study review that included five studies and 284 patients in The International Journal of Environmental Research and Public Health suggested omega-3 fatty acid supplements could improve ESR, CRP, disease activity, inflammatory markers, oxidative stress, lipid levels, and endothelial function.
Omega-3 fatty acids have anti-inflammatory effects that might explain their benefits. In patients with RA, for example, fish oil supplementation was associated with elevated blood levels of resolvins and protectins, which help quell inflammation, according to a study in Prostaglandins, Leukotrienes and Essential Fatty Acids.
Philippou and Nikiphorou recommended combining food and supplements: Eat oily fish at least twice a week, regularly consume plant-based sources of omega-3s — like chia seeds, flaxseeds, or walnuts — and consider a daily supplement that contains 2 g of omega-3s from docosahexaenoic acid and eicosapentaenoic acid. Most fish oil side effects are mild, like heartburn and bad breath. Fish oil can have blood-thinning effects at high doses, so special attention is needed for patients on anticoagulants.
Probiotics. Building up the good bacteria in your gut might help you fight the effects of rheumatologic diseases. A systematic review of 80 randomized controlled trials in BMC Medicine suggested that therapies targeting the gut microbiota might improve the symptoms or inflammatory factors in celiac disease, lupus, juvenile idiopathic arthritis, psoriasis, Sjögren’s disease, multiple sclerosis, systemic sclerosis, Crohn’s disease, and ulcerative colitis. Probiotics were also shown to relieve pain in fibromyalgia, but they didn’t affect scores on the Fibromyalgia Impact Questionnaire. Probiotics were not helpful in spondyloarthritis or RA. There were no adverse events. By improving the balance of bacteria in the gut, probiotics might inhibit pro-inflammatory factors and signaling pathways and regulate CD4+ T-cell differentiation, the researchers wrote.
Not all probiotic supplements are created equal. Effects can vary by microorganism and dose. Until more high-quality studies are published, Philippou and Nikiphorou recommend daily consumption of probiotic food sources such as yogurt, kefir, sauerkraut, kimchi, tempeh, miso, and kombucha, along with prebiotic food sources such as bananas, onion, artichokes, asparagus, oats, leeks, and garlic.
Collagen. An increasingly popular supplement for hair, skin, and nails, some collagen peptide or hydrolyzed collagen supplements come with claims about joint health, too. Inside our bodies, collagen helps build joints. As a supplement, the jury is still out. A systematic review of 19 studies in The International Journal of Rheumatic Diseases suggested more research is needed to determine whether collagen supplements are harmful or helpful in OA or RA. Studies haven’t shown adverse events, and doses typically range from 2.5 to 15 g/d.
Coenzyme Q10 (CoQ10). This antioxidant occurs naturally in our cells and is produced through microbial fermentation for use in dietary supplements. A study review of 20 articles including 483 patients in Clinical Nutrition ESPEN concluded that CoQ10 supplementation up to 300 mg/d was beneficial in RA, fibromyalgia, or antiphospholipid syndrome (APS).
In RA, CoQ10 supplementation improved disease activity index, ESR, and cytokine levels and decreased malondialdehyde. CoQ10 might protect against the overproduction of reactive oxygen species that can promote inflammation and joint damage, the researchers said. In fibromyalgia, CoQ10 was linked with improvements in pain, fatigue, sleep, tender points count, mood disorders, and scores on the Fibromyalgia Impact Questionnaire in most of the included studies. CoQ10 might help in fibromyalgia by improving mitochondrial dysfunction. In APS, CoQ10 improved endothelial function and decreased prothrombotic and pro-inflammatory mediators. CoQ10 might change the expression of genes that promote atherosclerosis. A few patients had GI side effects like nausea and diarrhea, but the supplements were generally well tolerated.
Melatonin. Commonly touted as a sleep aid, this hormone has immune and anti-inflammatory activities that could benefit people with rheumatologic diseases. A study review of 13 articles including 533 patients in Clinical Nutrition ESPEN concluded that melatonin can help improve sleep, pain, and mood in fibromyalgia, OA, and osteoporosis but not in RA. Side effects were minimal, but a few people experienced nausea, drowsiness, nightmares, or headaches. Doses of 5-6 mg/d are likely safe for most adults.
The Plant-Derived Antioxidants
Many supplements used in rheumatology are antioxidants derived from herbs, spices, or other plants. When plants encounter stressors, like temperature changes or hungry insects, their secondary metabolism revs up and creates compounds with biological properties. Some of these substances influence inflammatory pathways in the human body, said Luís Silva, PhD, a medicinal chemistry researcher at the Polytechnic Institute of Guarda in Portugal. “If it is possible to reduce these kinds of anti-inflammatory processes, it is also possible that we could help people with inflammatory diseases to a good life, or a better life.”
Turmeric and curcumin. You might see this supplement labeled as turmeric, a golden spice in curry powder, or curcumin, an antioxidant compound known as a curcuminoid in turmeric. Curcuminoids might reduce inflammation by scavenging free radicals and inhibiting enzymes that make prostaglandins, Silva said.
Turmeric is the most popular herbal supplement for people with RA, according to Funk’s research. A study review of six publications including 539 patients in Frontiers in Immunology showed that curcumin supplements improved RA patients’ ESR, DAS, swollen joint count, and tender joint count. Turmeric could help patients with OA, too. Patients with OA who took 1000 mg/d of curcumin improved their pain and function, according to a systematic review including 12 studies and 1438 participants in the journal Nutrients. In lupus, small studies are promising but inconclusive, suggested a study review in Frontiers in Immunology.
Watch patients taking turmeric and methotrexate closely, Funk said. Both have been associated with liver problems. Some users also experience GI symptoms like diarrhea because turmeric doesn’t absorb well in the GI tract.
Milk thistle (silymarin). This flowering plant is often marketed as a liver-supporting supplement, but research also suggests promise in RA and OA. A systematic review of 12 studies in Current Rheumatology Reviews suggested that silymarin supplements might help relieve pain, reduce inflammation, and protect the cartilage matrix, synovial membrane, and cartilage cells in joints. This supplement might help via immunomodulatory, anti-inflammatory, antioxidant, and anti-apoptotic properties, the researchers said. Doses of 250-750 mg appear to be safe. Side effects such as gastroenteritis, diarrhea, bloating, and headache can occur.
Boswellia serrata. Sourced from the resin of a tree that grows in dry, mountainous regions of Asia and Africa, Boswellia serrata can help relieve joint pain and stiffness and improve joint function in OA, suggested a systematic review of seven trials involving 545 patients in BMC Complementary Medicine and Therapies. Users saw benefits when taking 100-250 mg/d for 4 weeks or more. Compounds in Boswellia serrata may inhibit 5-lipoxygenase, an enzyme involved in producing inflammatory leukotrienes. No adverse events were reported. In some studies, users have reported GI side effects.
Ginger. Ginger is a popular herbal supplement among people with RA, Funk’s research suggested. One small clinical trial involving 70 patients with RA in the journal Gene showed that taking 1500 mg/d of ginger for 12 weeks improved their DAS and boosted their expression of FoxP3 genes, which are linked with the function of regulatory T cells. A meta-analysis including three studies with 330 patients taking ginger published in the journal Nutrients suggested ginger can reduce pain and systemic inflammation in people with OA. Preclinical studies suggested phenolic compounds in this spicy root, such as gingerols, reduce inflammation through multiple mechanisms.
Funk’s research revealed wide variation in the quality of ginger supplements, reinforcing the importance of selecting an independently verified product. Research suggested a safe dose is up to 2-2.5 g/kg body weight.
Resveratrol. Found in red grapes and red wine, this compound is particularly good at blocking COX-2 enzymes, an important step in the inflammatory cascade, Silva said. “Because of their chemical structure, they have great affinity to these enzymes to lead to their inhibition,” he said. A study review of five articles including 481 patients in The European Journal of Rheumatology showed that people with OA, RA, or Takayasu arteritis who took 250-1000 mg/d of resveratrol saw improvements in pain, function, disease activity, joint swelling, and inflammation, with no side effects.
Cinnamon. This warming spice is gaining popularity as a supplement, reported the American Botanical Council. Cinnamon is often marketed as lowering blood sugar and supporting bone health. In a small study of 36 women with RA published in The Journal of the American College of Nutrition, participants who consumed 2 g/d of cinnamon powder had reduced DASs along with reduced pain and tender and swollen joint counts. Cinnamon may reduce pain by inhibiting prostaglandin and blunt inflammation by reducing the release of arachidonic acid from cell membranes, according to a study review in Frontiers in Pharmacology. GI problems and allergic reactions are among the most common side effects.
Funk, Nikiphorou, Philippou, and Silva all had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Many people with rheumatologic diseases try supplements for symptom relief. Here’s what you need to know about some common picks.
Dietary supplements were a $159 billion business in the United States in 2023, and many people with rheumatologic diseases are buying in. Research suggests more than 6 in 10 people with fibromyalgia, nearly 8 in 10 people with Sjögren’s disease, and more than 8 in 10 people with rheumatoid arthritis (RA) take dietary supplements.
Whatever the symptom — pain, swelling, or fatigue — you can probably find a supplement purporting to relieve it. But do these supplements work, and are they safe? A study review in RMD Open comprising 24 systematic reviews and 150 original articles suggests more high-quality research is needed on the effects of dietary supplements on rheumatologic diseases. Most studies have focused on RA or osteoarthritis (OA), where the evidence level is moderate at best.
“The studies in this space are usually not very high quality because there’s no money to support them, among other things, plus the products are disparate,” said Janet Funk, MD, MS, professor in the School of Nutritional Sciences and Wellness at the University of Arizona, Tucson. She recommended brushing up on supplements and finding out what patients are taking so you can offer advice and watch for drug-supplement interactions.
When asked for a medication list, many patients forget to report supplements, Funk said. “You have to prompt them specifically. I think some physicians have very negative views about supplements because so little data is known, and patients might pick up on that and decide not to report their use.” She recommended saying something like: “To give you the best possible care, I want to know everything you’re taking, including supplements. The things I’m prescribing could maybe interact with the things you’re taking, so I want to make sure I know about all of it so that together we can figure out if the combination of things is safe.”
The quality of dietary supplements varies, and they aren’t regulated like drugs by the Food and Drug Administration. Funk recommended selecting products verified by NSF or ConsumerLab. They test supplements to ensure the label reflects what’s inside.
This news organization scoured the literature and asked experts to weigh in on the evidence behind popular supplements in rheumatology today.
The Essential Nutrients
Vitamin supplements are a staple in many homes — but are they helpful? “Individual vitamin supplements will not provide any benefit unless the person is deficient in a specific vitamin or mineral,” according to Elena Philippou, PhD, RD, associate professor of nutrition-dietetics at the University of Nicosia in Cyprus, and Elena Nikiphorou, MBBS, a rheumatologist at King’s College London in England. For some patients, deficiency is a reality. A retrospective cohort study in The Journal of Clinical Medicine found that people with RA were 17% more likely than age-matched control individuals to have nutrient deficiencies, perhaps because symptoms like fatigue, pain, and nausea affect their eating habits. Here’s what the science says about common vitamin supplements.
Vitamin D. This hormone-like vitamin, which attaches to receptors on immune cells to tamp down inflammation, was the most popular dietary supplement among rheumatology patients in a recent study from the United Kingdom. Vitamin D deficiency is common in people with RA, lupus, Sjögren’s disease, ankylosing spondylitis, systemic sclerosis, and fibromyalgia. In some cases, vitamin D levels track with disease activity, research suggests. Corticosteroids can also make vitamin D deficiency more likely. Can supplements help?
In RA, evidence points to small improvements. A systematic review of 11 studies including 3049 patients published in Nutrition Reviews showed that vitamin D supplements significantly reduced patients’ pain and Disease Activity Score in 28 joints (DAS28) using both C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR).
The research is mixed on the benefits of vitamin D supplementation for fibromyalgia symptoms, according to a study review in SN Comprehensive Clinical Medicine that included two studies and 80 patients on supplementation. However, researchers said it’s still worth discussing the potential benefits of taking vitamin D.
“Vitamin D supplementation is important in the context of various rheumatic diseases to prevent or treat bone disease,” said Philippou and Nikiphorou. “People with rheumatic disease should speak to their healthcare provider and ask to check their blood vitamin D concentration.” The results can help you recommend a dose.
Folate. Patients on methotrexate should take folic acid supplements under the guidance of a healthcare provider, said Philippou and Nikiphorou. The reason: Methotrexate can deplete folic acid levels, increasing the risk for side effects. An analysis of adverse event reports published showed that methotrexate users who took folic acid (or tumor necrosis factor–alpha inhibitors) had a reduced risk for hepatotoxicity or myelosuppression. A commonly recommended dose is 1 mg/d.
Vitamin B12. In a 2024 perspective paper in Rheumatology International, researchers said physicians should assess vitamin B12 levels early in the diagnostic process of rheumatologic diseases. One reason: Many symptoms of pernicious anemia, like fatigue, mimic symptoms of rheumatologic diseases. The gastrointestinal (GI) effects of systemic sclerosis could bring on vitamin B12 deficiency. In a small study in The Journal of Clinical Rheumatology, 44 of 62 patients with systemic sclerosis had low vitamin B12 levels.
Vitamin E. Vitamin E deficiency is rare in healthy adults. However, some medical conditions, like inflammatory bowel disease and malabsorption disorders, can make vitamin E deficiency more likely. In RA, a vitamin E supplement could help reduce joint swelling and sensitivity, according to a systematic review of nine studies including 39,845 patients in The European Journal of Clinical Nutrition. Researchers credit the nutrient’s role in aiding intestinal repair. Use with caution, as this supplement can increase bleeding risk in doses over 1000 mg/d.
Vitamin A. Like vitamin E, vitamin A deficiency is rare in the United States. The risk of oversupplementing is higher than undersupplementing. However, vitamin A deficiency can happen in people with chronic pancreatic, liver, or GI problems. In people with deficiency, a vitamin A supplement can help relieve dry eye symptoms common in Sjögren’s disease, suggests a narrative review published in Nutrients. Vitamin A might help reduce ocular surface changes by supporting the production of proteins that protect the outermost surfaces of the eyes. The recommended daily allowance for vitamin A is 900 μg. High-dose supplements can cause toxicity, resulting in GI symptoms and problems like lethargy, drowsiness, increased intracranial pressure, and skin changes.
The Replacements
These substances are similar to naturally occurring compounds in our bodies. The question is whether ingesting them yields benefits.
Glucosamine and chondroitin. Glucosamine and chondroitin occur naturally in our bodies and help us form and protect connective tissues. In pill form, this combo is the most popular dietary supplement for OA, according to research in the journal Maturitas. But studies of its effectiveness yield mixed results. A systematic review of 25 studies published in Inflammopharmacology showed that, in patients with knee OA, supplementation with about 1500 mg of glucosamine per day reduced tibiofemoral joint space narrowing, while supplementation with about 800 mg/d of chondroitin reduced pain intensity and improved physical function, compared with placebo. The duo of glucosamine and chondroitin did not bring significant benefits, perhaps because more studies are needed. Most side effects were mild, but some literature points to the potential for glucosamine to increase warfarin’s blood-thinning effects.
Omega-3 fatty acids. Fish oil is a top-selling supplement, and it might be helpful in inflammatory rheumatologic diseases. A systematic review of 30 studies including 710 patients published in Arthritis Research & Therapy showed that omega-3 fatty acid supplements can improve pain, swollen and tender joint count, DAS28 scores, and Health Assessment Questionnaire scores in patients with RA, psoriatic arthritis, or ankylosing spondylitis. In patients with lupus, a study review that included five studies and 284 patients in The International Journal of Environmental Research and Public Health suggested omega-3 fatty acid supplements could improve ESR, CRP, disease activity, inflammatory markers, oxidative stress, lipid levels, and endothelial function.
Omega-3 fatty acids have anti-inflammatory effects that might explain their benefits. In patients with RA, for example, fish oil supplementation was associated with elevated blood levels of resolvins and protectins, which help quell inflammation, according to a study in Prostaglandins, Leukotrienes and Essential Fatty Acids.
Philippou and Nikiphorou recommended combining food and supplements: Eat oily fish at least twice a week, regularly consume plant-based sources of omega-3s — like chia seeds, flaxseeds, or walnuts — and consider a daily supplement that contains 2 g of omega-3s from docosahexaenoic acid and eicosapentaenoic acid. Most fish oil side effects are mild, like heartburn and bad breath. Fish oil can have blood-thinning effects at high doses, so special attention is needed for patients on anticoagulants.
Probiotics. Building up the good bacteria in your gut might help you fight the effects of rheumatologic diseases. A systematic review of 80 randomized controlled trials in BMC Medicine suggested that therapies targeting the gut microbiota might improve the symptoms or inflammatory factors in celiac disease, lupus, juvenile idiopathic arthritis, psoriasis, Sjögren’s disease, multiple sclerosis, systemic sclerosis, Crohn’s disease, and ulcerative colitis. Probiotics were also shown to relieve pain in fibromyalgia, but they didn’t affect scores on the Fibromyalgia Impact Questionnaire. Probiotics were not helpful in spondyloarthritis or RA. There were no adverse events. By improving the balance of bacteria in the gut, probiotics might inhibit pro-inflammatory factors and signaling pathways and regulate CD4+ T-cell differentiation, the researchers wrote.
Not all probiotic supplements are created equal. Effects can vary by microorganism and dose. Until more high-quality studies are published, Philippou and Nikiphorou recommend daily consumption of probiotic food sources such as yogurt, kefir, sauerkraut, kimchi, tempeh, miso, and kombucha, along with prebiotic food sources such as bananas, onion, artichokes, asparagus, oats, leeks, and garlic.
Collagen. An increasingly popular supplement for hair, skin, and nails, some collagen peptide or hydrolyzed collagen supplements come with claims about joint health, too. Inside our bodies, collagen helps build joints. As a supplement, the jury is still out. A systematic review of 19 studies in The International Journal of Rheumatic Diseases suggested more research is needed to determine whether collagen supplements are harmful or helpful in OA or RA. Studies haven’t shown adverse events, and doses typically range from 2.5 to 15 g/d.
Coenzyme Q10 (CoQ10). This antioxidant occurs naturally in our cells and is produced through microbial fermentation for use in dietary supplements. A study review of 20 articles including 483 patients in Clinical Nutrition ESPEN concluded that CoQ10 supplementation up to 300 mg/d was beneficial in RA, fibromyalgia, or antiphospholipid syndrome (APS).
In RA, CoQ10 supplementation improved disease activity index, ESR, and cytokine levels and decreased malondialdehyde. CoQ10 might protect against the overproduction of reactive oxygen species that can promote inflammation and joint damage, the researchers said. In fibromyalgia, CoQ10 was linked with improvements in pain, fatigue, sleep, tender points count, mood disorders, and scores on the Fibromyalgia Impact Questionnaire in most of the included studies. CoQ10 might help in fibromyalgia by improving mitochondrial dysfunction. In APS, CoQ10 improved endothelial function and decreased prothrombotic and pro-inflammatory mediators. CoQ10 might change the expression of genes that promote atherosclerosis. A few patients had GI side effects like nausea and diarrhea, but the supplements were generally well tolerated.
Melatonin. Commonly touted as a sleep aid, this hormone has immune and anti-inflammatory activities that could benefit people with rheumatologic diseases. A study review of 13 articles including 533 patients in Clinical Nutrition ESPEN concluded that melatonin can help improve sleep, pain, and mood in fibromyalgia, OA, and osteoporosis but not in RA. Side effects were minimal, but a few people experienced nausea, drowsiness, nightmares, or headaches. Doses of 5-6 mg/d are likely safe for most adults.
The Plant-Derived Antioxidants
Many supplements used in rheumatology are antioxidants derived from herbs, spices, or other plants. When plants encounter stressors, like temperature changes or hungry insects, their secondary metabolism revs up and creates compounds with biological properties. Some of these substances influence inflammatory pathways in the human body, said Luís Silva, PhD, a medicinal chemistry researcher at the Polytechnic Institute of Guarda in Portugal. “If it is possible to reduce these kinds of anti-inflammatory processes, it is also possible that we could help people with inflammatory diseases to a good life, or a better life.”
Turmeric and curcumin. You might see this supplement labeled as turmeric, a golden spice in curry powder, or curcumin, an antioxidant compound known as a curcuminoid in turmeric. Curcuminoids might reduce inflammation by scavenging free radicals and inhibiting enzymes that make prostaglandins, Silva said.
Turmeric is the most popular herbal supplement for people with RA, according to Funk’s research. A study review of six publications including 539 patients in Frontiers in Immunology showed that curcumin supplements improved RA patients’ ESR, DAS, swollen joint count, and tender joint count. Turmeric could help patients with OA, too. Patients with OA who took 1000 mg/d of curcumin improved their pain and function, according to a systematic review including 12 studies and 1438 participants in the journal Nutrients. In lupus, small studies are promising but inconclusive, suggested a study review in Frontiers in Immunology.
Watch patients taking turmeric and methotrexate closely, Funk said. Both have been associated with liver problems. Some users also experience GI symptoms like diarrhea because turmeric doesn’t absorb well in the GI tract.
Milk thistle (silymarin). This flowering plant is often marketed as a liver-supporting supplement, but research also suggests promise in RA and OA. A systematic review of 12 studies in Current Rheumatology Reviews suggested that silymarin supplements might help relieve pain, reduce inflammation, and protect the cartilage matrix, synovial membrane, and cartilage cells in joints. This supplement might help via immunomodulatory, anti-inflammatory, antioxidant, and anti-apoptotic properties, the researchers said. Doses of 250-750 mg appear to be safe. Side effects such as gastroenteritis, diarrhea, bloating, and headache can occur.
Boswellia serrata. Sourced from the resin of a tree that grows in dry, mountainous regions of Asia and Africa, Boswellia serrata can help relieve joint pain and stiffness and improve joint function in OA, suggested a systematic review of seven trials involving 545 patients in BMC Complementary Medicine and Therapies. Users saw benefits when taking 100-250 mg/d for 4 weeks or more. Compounds in Boswellia serrata may inhibit 5-lipoxygenase, an enzyme involved in producing inflammatory leukotrienes. No adverse events were reported. In some studies, users have reported GI side effects.
Ginger. Ginger is a popular herbal supplement among people with RA, Funk’s research suggested. One small clinical trial involving 70 patients with RA in the journal Gene showed that taking 1500 mg/d of ginger for 12 weeks improved their DAS and boosted their expression of FoxP3 genes, which are linked with the function of regulatory T cells. A meta-analysis including three studies with 330 patients taking ginger published in the journal Nutrients suggested ginger can reduce pain and systemic inflammation in people with OA. Preclinical studies suggested phenolic compounds in this spicy root, such as gingerols, reduce inflammation through multiple mechanisms.
Funk’s research revealed wide variation in the quality of ginger supplements, reinforcing the importance of selecting an independently verified product. Research suggested a safe dose is up to 2-2.5 g/kg body weight.
Resveratrol. Found in red grapes and red wine, this compound is particularly good at blocking COX-2 enzymes, an important step in the inflammatory cascade, Silva said. “Because of their chemical structure, they have great affinity to these enzymes to lead to their inhibition,” he said. A study review of five articles including 481 patients in The European Journal of Rheumatology showed that people with OA, RA, or Takayasu arteritis who took 250-1000 mg/d of resveratrol saw improvements in pain, function, disease activity, joint swelling, and inflammation, with no side effects.
Cinnamon. This warming spice is gaining popularity as a supplement, reported the American Botanical Council. Cinnamon is often marketed as lowering blood sugar and supporting bone health. In a small study of 36 women with RA published in The Journal of the American College of Nutrition, participants who consumed 2 g/d of cinnamon powder had reduced DASs along with reduced pain and tender and swollen joint counts. Cinnamon may reduce pain by inhibiting prostaglandin and blunt inflammation by reducing the release of arachidonic acid from cell membranes, according to a study review in Frontiers in Pharmacology. GI problems and allergic reactions are among the most common side effects.
Funk, Nikiphorou, Philippou, and Silva all had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Many people with rheumatologic diseases try supplements for symptom relief. Here’s what you need to know about some common picks.
Dietary supplements were a $159 billion business in the United States in 2023, and many people with rheumatologic diseases are buying in. Research suggests more than 6 in 10 people with fibromyalgia, nearly 8 in 10 people with Sjögren’s disease, and more than 8 in 10 people with rheumatoid arthritis (RA) take dietary supplements.
Whatever the symptom — pain, swelling, or fatigue — you can probably find a supplement purporting to relieve it. But do these supplements work, and are they safe? A study review in RMD Open comprising 24 systematic reviews and 150 original articles suggests more high-quality research is needed on the effects of dietary supplements on rheumatologic diseases. Most studies have focused on RA or osteoarthritis (OA), where the evidence level is moderate at best.
“The studies in this space are usually not very high quality because there’s no money to support them, among other things, plus the products are disparate,” said Janet Funk, MD, MS, professor in the School of Nutritional Sciences and Wellness at the University of Arizona, Tucson. She recommended brushing up on supplements and finding out what patients are taking so you can offer advice and watch for drug-supplement interactions.
When asked for a medication list, many patients forget to report supplements, Funk said. “You have to prompt them specifically. I think some physicians have very negative views about supplements because so little data is known, and patients might pick up on that and decide not to report their use.” She recommended saying something like: “To give you the best possible care, I want to know everything you’re taking, including supplements. The things I’m prescribing could maybe interact with the things you’re taking, so I want to make sure I know about all of it so that together we can figure out if the combination of things is safe.”
The quality of dietary supplements varies, and they aren’t regulated like drugs by the Food and Drug Administration. Funk recommended selecting products verified by NSF or ConsumerLab. They test supplements to ensure the label reflects what’s inside.
This news organization scoured the literature and asked experts to weigh in on the evidence behind popular supplements in rheumatology today.
The Essential Nutrients
Vitamin supplements are a staple in many homes — but are they helpful? “Individual vitamin supplements will not provide any benefit unless the person is deficient in a specific vitamin or mineral,” according to Elena Philippou, PhD, RD, associate professor of nutrition-dietetics at the University of Nicosia in Cyprus, and Elena Nikiphorou, MBBS, a rheumatologist at King’s College London in England. For some patients, deficiency is a reality. A retrospective cohort study in The Journal of Clinical Medicine found that people with RA were 17% more likely than age-matched control individuals to have nutrient deficiencies, perhaps because symptoms like fatigue, pain, and nausea affect their eating habits. Here’s what the science says about common vitamin supplements.
Vitamin D. This hormone-like vitamin, which attaches to receptors on immune cells to tamp down inflammation, was the most popular dietary supplement among rheumatology patients in a recent study from the United Kingdom. Vitamin D deficiency is common in people with RA, lupus, Sjögren’s disease, ankylosing spondylitis, systemic sclerosis, and fibromyalgia. In some cases, vitamin D levels track with disease activity, research suggests. Corticosteroids can also make vitamin D deficiency more likely. Can supplements help?
In RA, evidence points to small improvements. A systematic review of 11 studies including 3049 patients published in Nutrition Reviews showed that vitamin D supplements significantly reduced patients’ pain and Disease Activity Score in 28 joints (DAS28) using both C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR).
The research is mixed on the benefits of vitamin D supplementation for fibromyalgia symptoms, according to a study review in SN Comprehensive Clinical Medicine that included two studies and 80 patients on supplementation. However, researchers said it’s still worth discussing the potential benefits of taking vitamin D.
“Vitamin D supplementation is important in the context of various rheumatic diseases to prevent or treat bone disease,” said Philippou and Nikiphorou. “People with rheumatic disease should speak to their healthcare provider and ask to check their blood vitamin D concentration.” The results can help you recommend a dose.
Folate. Patients on methotrexate should take folic acid supplements under the guidance of a healthcare provider, said Philippou and Nikiphorou. The reason: Methotrexate can deplete folic acid levels, increasing the risk for side effects. An analysis of adverse event reports published showed that methotrexate users who took folic acid (or tumor necrosis factor–alpha inhibitors) had a reduced risk for hepatotoxicity or myelosuppression. A commonly recommended dose is 1 mg/d.
Vitamin B12. In a 2024 perspective paper in Rheumatology International, researchers said physicians should assess vitamin B12 levels early in the diagnostic process of rheumatologic diseases. One reason: Many symptoms of pernicious anemia, like fatigue, mimic symptoms of rheumatologic diseases. The gastrointestinal (GI) effects of systemic sclerosis could bring on vitamin B12 deficiency. In a small study in The Journal of Clinical Rheumatology, 44 of 62 patients with systemic sclerosis had low vitamin B12 levels.
Vitamin E. Vitamin E deficiency is rare in healthy adults. However, some medical conditions, like inflammatory bowel disease and malabsorption disorders, can make vitamin E deficiency more likely. In RA, a vitamin E supplement could help reduce joint swelling and sensitivity, according to a systematic review of nine studies including 39,845 patients in The European Journal of Clinical Nutrition. Researchers credit the nutrient’s role in aiding intestinal repair. Use with caution, as this supplement can increase bleeding risk in doses over 1000 mg/d.
Vitamin A. Like vitamin E, vitamin A deficiency is rare in the United States. The risk of oversupplementing is higher than undersupplementing. However, vitamin A deficiency can happen in people with chronic pancreatic, liver, or GI problems. In people with deficiency, a vitamin A supplement can help relieve dry eye symptoms common in Sjögren’s disease, suggests a narrative review published in Nutrients. Vitamin A might help reduce ocular surface changes by supporting the production of proteins that protect the outermost surfaces of the eyes. The recommended daily allowance for vitamin A is 900 μg. High-dose supplements can cause toxicity, resulting in GI symptoms and problems like lethargy, drowsiness, increased intracranial pressure, and skin changes.
The Replacements
These substances are similar to naturally occurring compounds in our bodies. The question is whether ingesting them yields benefits.
Glucosamine and chondroitin. Glucosamine and chondroitin occur naturally in our bodies and help us form and protect connective tissues. In pill form, this combo is the most popular dietary supplement for OA, according to research in the journal Maturitas. But studies of its effectiveness yield mixed results. A systematic review of 25 studies published in Inflammopharmacology showed that, in patients with knee OA, supplementation with about 1500 mg of glucosamine per day reduced tibiofemoral joint space narrowing, while supplementation with about 800 mg/d of chondroitin reduced pain intensity and improved physical function, compared with placebo. The duo of glucosamine and chondroitin did not bring significant benefits, perhaps because more studies are needed. Most side effects were mild, but some literature points to the potential for glucosamine to increase warfarin’s blood-thinning effects.
Omega-3 fatty acids. Fish oil is a top-selling supplement, and it might be helpful in inflammatory rheumatologic diseases. A systematic review of 30 studies including 710 patients published in Arthritis Research & Therapy showed that omega-3 fatty acid supplements can improve pain, swollen and tender joint count, DAS28 scores, and Health Assessment Questionnaire scores in patients with RA, psoriatic arthritis, or ankylosing spondylitis. In patients with lupus, a study review that included five studies and 284 patients in The International Journal of Environmental Research and Public Health suggested omega-3 fatty acid supplements could improve ESR, CRP, disease activity, inflammatory markers, oxidative stress, lipid levels, and endothelial function.
Omega-3 fatty acids have anti-inflammatory effects that might explain their benefits. In patients with RA, for example, fish oil supplementation was associated with elevated blood levels of resolvins and protectins, which help quell inflammation, according to a study in Prostaglandins, Leukotrienes and Essential Fatty Acids.
Philippou and Nikiphorou recommended combining food and supplements: Eat oily fish at least twice a week, regularly consume plant-based sources of omega-3s — like chia seeds, flaxseeds, or walnuts — and consider a daily supplement that contains 2 g of omega-3s from docosahexaenoic acid and eicosapentaenoic acid. Most fish oil side effects are mild, like heartburn and bad breath. Fish oil can have blood-thinning effects at high doses, so special attention is needed for patients on anticoagulants.
Probiotics. Building up the good bacteria in your gut might help you fight the effects of rheumatologic diseases. A systematic review of 80 randomized controlled trials in BMC Medicine suggested that therapies targeting the gut microbiota might improve the symptoms or inflammatory factors in celiac disease, lupus, juvenile idiopathic arthritis, psoriasis, Sjögren’s disease, multiple sclerosis, systemic sclerosis, Crohn’s disease, and ulcerative colitis. Probiotics were also shown to relieve pain in fibromyalgia, but they didn’t affect scores on the Fibromyalgia Impact Questionnaire. Probiotics were not helpful in spondyloarthritis or RA. There were no adverse events. By improving the balance of bacteria in the gut, probiotics might inhibit pro-inflammatory factors and signaling pathways and regulate CD4+ T-cell differentiation, the researchers wrote.
Not all probiotic supplements are created equal. Effects can vary by microorganism and dose. Until more high-quality studies are published, Philippou and Nikiphorou recommend daily consumption of probiotic food sources such as yogurt, kefir, sauerkraut, kimchi, tempeh, miso, and kombucha, along with prebiotic food sources such as bananas, onion, artichokes, asparagus, oats, leeks, and garlic.
Collagen. An increasingly popular supplement for hair, skin, and nails, some collagen peptide or hydrolyzed collagen supplements come with claims about joint health, too. Inside our bodies, collagen helps build joints. As a supplement, the jury is still out. A systematic review of 19 studies in The International Journal of Rheumatic Diseases suggested more research is needed to determine whether collagen supplements are harmful or helpful in OA or RA. Studies haven’t shown adverse events, and doses typically range from 2.5 to 15 g/d.
Coenzyme Q10 (CoQ10). This antioxidant occurs naturally in our cells and is produced through microbial fermentation for use in dietary supplements. A study review of 20 articles including 483 patients in Clinical Nutrition ESPEN concluded that CoQ10 supplementation up to 300 mg/d was beneficial in RA, fibromyalgia, or antiphospholipid syndrome (APS).
In RA, CoQ10 supplementation improved disease activity index, ESR, and cytokine levels and decreased malondialdehyde. CoQ10 might protect against the overproduction of reactive oxygen species that can promote inflammation and joint damage, the researchers said. In fibromyalgia, CoQ10 was linked with improvements in pain, fatigue, sleep, tender points count, mood disorders, and scores on the Fibromyalgia Impact Questionnaire in most of the included studies. CoQ10 might help in fibromyalgia by improving mitochondrial dysfunction. In APS, CoQ10 improved endothelial function and decreased prothrombotic and pro-inflammatory mediators. CoQ10 might change the expression of genes that promote atherosclerosis. A few patients had GI side effects like nausea and diarrhea, but the supplements were generally well tolerated.
Melatonin. Commonly touted as a sleep aid, this hormone has immune and anti-inflammatory activities that could benefit people with rheumatologic diseases. A study review of 13 articles including 533 patients in Clinical Nutrition ESPEN concluded that melatonin can help improve sleep, pain, and mood in fibromyalgia, OA, and osteoporosis but not in RA. Side effects were minimal, but a few people experienced nausea, drowsiness, nightmares, or headaches. Doses of 5-6 mg/d are likely safe for most adults.
The Plant-Derived Antioxidants
Many supplements used in rheumatology are antioxidants derived from herbs, spices, or other plants. When plants encounter stressors, like temperature changes or hungry insects, their secondary metabolism revs up and creates compounds with biological properties. Some of these substances influence inflammatory pathways in the human body, said Luís Silva, PhD, a medicinal chemistry researcher at the Polytechnic Institute of Guarda in Portugal. “If it is possible to reduce these kinds of anti-inflammatory processes, it is also possible that we could help people with inflammatory diseases to a good life, or a better life.”
Turmeric and curcumin. You might see this supplement labeled as turmeric, a golden spice in curry powder, or curcumin, an antioxidant compound known as a curcuminoid in turmeric. Curcuminoids might reduce inflammation by scavenging free radicals and inhibiting enzymes that make prostaglandins, Silva said.
Turmeric is the most popular herbal supplement for people with RA, according to Funk’s research. A study review of six publications including 539 patients in Frontiers in Immunology showed that curcumin supplements improved RA patients’ ESR, DAS, swollen joint count, and tender joint count. Turmeric could help patients with OA, too. Patients with OA who took 1000 mg/d of curcumin improved their pain and function, according to a systematic review including 12 studies and 1438 participants in the journal Nutrients. In lupus, small studies are promising but inconclusive, suggested a study review in Frontiers in Immunology.
Watch patients taking turmeric and methotrexate closely, Funk said. Both have been associated with liver problems. Some users also experience GI symptoms like diarrhea because turmeric doesn’t absorb well in the GI tract.
Milk thistle (silymarin). This flowering plant is often marketed as a liver-supporting supplement, but research also suggests promise in RA and OA. A systematic review of 12 studies in Current Rheumatology Reviews suggested that silymarin supplements might help relieve pain, reduce inflammation, and protect the cartilage matrix, synovial membrane, and cartilage cells in joints. This supplement might help via immunomodulatory, anti-inflammatory, antioxidant, and anti-apoptotic properties, the researchers said. Doses of 250-750 mg appear to be safe. Side effects such as gastroenteritis, diarrhea, bloating, and headache can occur.
Boswellia serrata. Sourced from the resin of a tree that grows in dry, mountainous regions of Asia and Africa, Boswellia serrata can help relieve joint pain and stiffness and improve joint function in OA, suggested a systematic review of seven trials involving 545 patients in BMC Complementary Medicine and Therapies. Users saw benefits when taking 100-250 mg/d for 4 weeks or more. Compounds in Boswellia serrata may inhibit 5-lipoxygenase, an enzyme involved in producing inflammatory leukotrienes. No adverse events were reported. In some studies, users have reported GI side effects.
Ginger. Ginger is a popular herbal supplement among people with RA, Funk’s research suggested. One small clinical trial involving 70 patients with RA in the journal Gene showed that taking 1500 mg/d of ginger for 12 weeks improved their DAS and boosted their expression of FoxP3 genes, which are linked with the function of regulatory T cells. A meta-analysis including three studies with 330 patients taking ginger published in the journal Nutrients suggested ginger can reduce pain and systemic inflammation in people with OA. Preclinical studies suggested phenolic compounds in this spicy root, such as gingerols, reduce inflammation through multiple mechanisms.
Funk’s research revealed wide variation in the quality of ginger supplements, reinforcing the importance of selecting an independently verified product. Research suggested a safe dose is up to 2-2.5 g/kg body weight.
Resveratrol. Found in red grapes and red wine, this compound is particularly good at blocking COX-2 enzymes, an important step in the inflammatory cascade, Silva said. “Because of their chemical structure, they have great affinity to these enzymes to lead to their inhibition,” he said. A study review of five articles including 481 patients in The European Journal of Rheumatology showed that people with OA, RA, or Takayasu arteritis who took 250-1000 mg/d of resveratrol saw improvements in pain, function, disease activity, joint swelling, and inflammation, with no side effects.
Cinnamon. This warming spice is gaining popularity as a supplement, reported the American Botanical Council. Cinnamon is often marketed as lowering blood sugar and supporting bone health. In a small study of 36 women with RA published in The Journal of the American College of Nutrition, participants who consumed 2 g/d of cinnamon powder had reduced DASs along with reduced pain and tender and swollen joint counts. Cinnamon may reduce pain by inhibiting prostaglandin and blunt inflammation by reducing the release of arachidonic acid from cell membranes, according to a study review in Frontiers in Pharmacology. GI problems and allergic reactions are among the most common side effects.
Funk, Nikiphorou, Philippou, and Silva all had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Minor Progress in Gender Pay Equity, But a Big Gap Persists
Despite some recent progress in compensation equity, women in medicine continue to be paid significantly lower salaries than men.
According to the Female Compensation Report 2024 by Medscape, male doctors of any kind earned an average salary of about $400,000, whereas female doctors earned approximately $309,000 — a 29% gap.
The report analyzed survey data from 7000 practicing physicians who were recruited over a 4-month period starting in October 2023. The respondents comprised roughly 60% women representing over 29 specialties.
In the 2022 report, the pay gap between the genders was 32%. But some women in the field argued substantial headway is still needed.
“You can try and pick apart the data, but I’d say we’re not really making progress,” said Susan T. Hingle, MD, an internist in Illinois and president of the American Medical Women’s Association. “A decline by a couple of percentage points is not significantly addressing this pay gap that over a lifetime is huge, can be millions of dollars.”
The gender gap was narrower among female primary care physicians (PCPs) vs medical specialists. Female PCPs earned around $253,000 per year, whereas male PCPs earned about $295,000 per year. Hingle suggested that female PCPs may enjoy more pay equity because health systems have a harder time filling these positions.
On the other hand, the gap for specialists rose from 27% in 2022 to 31% in 2023. Differences in how aggressively women and men negotiate compensation packages may play a role, said Hingle.
“Taking negotiation out of the equation would be progress to me,” said Hingle.
Pay disparity did not appear to be the result of time spent on the job — female doctors reported an average of 49 work hours per week, whereas their male counterparts reported 50 work hours per week.
Meanwhile, the pay gap progressively worsened over time. Among doctors aged 28-34 years, men earned an average of $53,000 more than women. By ages 46-49, men earned an average of $157,000 more than women.
“I had to take my employer to court to get equal compensation, sad as it is to say,” said a hospitalist in North Carolina.
Nearly 60% of women surveyed felt they were not being paid fairly for their efforts, up from less than half reported in Medscape’s 2021 report. Hingle said that this figure may not only reflect sentiments about the compensation gap, but also less support on the job, including fewer physician assistants (PAs), nurses, and administrative staff.
“At my job, I do the work of multiple people,” said a survey respondent. “Junior resident, senior resident, social worker, nurse practitioner, PA — as well as try to be a teacher, researcher, [and] an excellent doctor and have the time to make patients feel as if they are not in a rush.”
Roughly 30% of women physicians said they would not choose to go into medicine again if given the chance compared with 26% of male physicians.
“Gender inequities in our profession have a direct impact,” said Shikha Jain, MD, an oncologist in Chicago and founder of the Women in Medicine nonprofit. “I think women in general don’t feel valued in the care they’re providing.”
Jain cited bullying, harassment, and fewer opportunities for leadership and recognition as factors beyond pay that affect female physicians’ feelings of being valued.
A version of this article first appeared on Medscape.com.
Despite some recent progress in compensation equity, women in medicine continue to be paid significantly lower salaries than men.
According to the Female Compensation Report 2024 by Medscape, male doctors of any kind earned an average salary of about $400,000, whereas female doctors earned approximately $309,000 — a 29% gap.
The report analyzed survey data from 7000 practicing physicians who were recruited over a 4-month period starting in October 2023. The respondents comprised roughly 60% women representing over 29 specialties.
In the 2022 report, the pay gap between the genders was 32%. But some women in the field argued substantial headway is still needed.
“You can try and pick apart the data, but I’d say we’re not really making progress,” said Susan T. Hingle, MD, an internist in Illinois and president of the American Medical Women’s Association. “A decline by a couple of percentage points is not significantly addressing this pay gap that over a lifetime is huge, can be millions of dollars.”
The gender gap was narrower among female primary care physicians (PCPs) vs medical specialists. Female PCPs earned around $253,000 per year, whereas male PCPs earned about $295,000 per year. Hingle suggested that female PCPs may enjoy more pay equity because health systems have a harder time filling these positions.
On the other hand, the gap for specialists rose from 27% in 2022 to 31% in 2023. Differences in how aggressively women and men negotiate compensation packages may play a role, said Hingle.
“Taking negotiation out of the equation would be progress to me,” said Hingle.
Pay disparity did not appear to be the result of time spent on the job — female doctors reported an average of 49 work hours per week, whereas their male counterparts reported 50 work hours per week.
Meanwhile, the pay gap progressively worsened over time. Among doctors aged 28-34 years, men earned an average of $53,000 more than women. By ages 46-49, men earned an average of $157,000 more than women.
“I had to take my employer to court to get equal compensation, sad as it is to say,” said a hospitalist in North Carolina.
Nearly 60% of women surveyed felt they were not being paid fairly for their efforts, up from less than half reported in Medscape’s 2021 report. Hingle said that this figure may not only reflect sentiments about the compensation gap, but also less support on the job, including fewer physician assistants (PAs), nurses, and administrative staff.
“At my job, I do the work of multiple people,” said a survey respondent. “Junior resident, senior resident, social worker, nurse practitioner, PA — as well as try to be a teacher, researcher, [and] an excellent doctor and have the time to make patients feel as if they are not in a rush.”
Roughly 30% of women physicians said they would not choose to go into medicine again if given the chance compared with 26% of male physicians.
“Gender inequities in our profession have a direct impact,” said Shikha Jain, MD, an oncologist in Chicago and founder of the Women in Medicine nonprofit. “I think women in general don’t feel valued in the care they’re providing.”
Jain cited bullying, harassment, and fewer opportunities for leadership and recognition as factors beyond pay that affect female physicians’ feelings of being valued.
A version of this article first appeared on Medscape.com.
Despite some recent progress in compensation equity, women in medicine continue to be paid significantly lower salaries than men.
According to the Female Compensation Report 2024 by Medscape, male doctors of any kind earned an average salary of about $400,000, whereas female doctors earned approximately $309,000 — a 29% gap.
The report analyzed survey data from 7000 practicing physicians who were recruited over a 4-month period starting in October 2023. The respondents comprised roughly 60% women representing over 29 specialties.
In the 2022 report, the pay gap between the genders was 32%. But some women in the field argued substantial headway is still needed.
“You can try and pick apart the data, but I’d say we’re not really making progress,” said Susan T. Hingle, MD, an internist in Illinois and president of the American Medical Women’s Association. “A decline by a couple of percentage points is not significantly addressing this pay gap that over a lifetime is huge, can be millions of dollars.”
The gender gap was narrower among female primary care physicians (PCPs) vs medical specialists. Female PCPs earned around $253,000 per year, whereas male PCPs earned about $295,000 per year. Hingle suggested that female PCPs may enjoy more pay equity because health systems have a harder time filling these positions.
On the other hand, the gap for specialists rose from 27% in 2022 to 31% in 2023. Differences in how aggressively women and men negotiate compensation packages may play a role, said Hingle.
“Taking negotiation out of the equation would be progress to me,” said Hingle.
Pay disparity did not appear to be the result of time spent on the job — female doctors reported an average of 49 work hours per week, whereas their male counterparts reported 50 work hours per week.
Meanwhile, the pay gap progressively worsened over time. Among doctors aged 28-34 years, men earned an average of $53,000 more than women. By ages 46-49, men earned an average of $157,000 more than women.
“I had to take my employer to court to get equal compensation, sad as it is to say,” said a hospitalist in North Carolina.
Nearly 60% of women surveyed felt they were not being paid fairly for their efforts, up from less than half reported in Medscape’s 2021 report. Hingle said that this figure may not only reflect sentiments about the compensation gap, but also less support on the job, including fewer physician assistants (PAs), nurses, and administrative staff.
“At my job, I do the work of multiple people,” said a survey respondent. “Junior resident, senior resident, social worker, nurse practitioner, PA — as well as try to be a teacher, researcher, [and] an excellent doctor and have the time to make patients feel as if they are not in a rush.”
Roughly 30% of women physicians said they would not choose to go into medicine again if given the chance compared with 26% of male physicians.
“Gender inequities in our profession have a direct impact,” said Shikha Jain, MD, an oncologist in Chicago and founder of the Women in Medicine nonprofit. “I think women in general don’t feel valued in the care they’re providing.”
Jain cited bullying, harassment, and fewer opportunities for leadership and recognition as factors beyond pay that affect female physicians’ feelings of being valued.
A version of this article first appeared on Medscape.com.
Which Specialists Should Lead BP Control Efforts?
Current efforts to control high blood pressure (BP) are failing in the United States and globally.
The first World Health Organization (WHO) global report on hypertension found that only 54% of adults with hypertension are diagnosed, 42% get treatment, and just 21% have their hypertension controlled.
In the United States, almost half (48%) of adults have high BP, defined as a systolic BP > 130 mm Hg, or a diastolic BP > 80 mm Hg, or are taking medication for high BP, according to the Centers for Disease Control and Prevention. Only about one in four adults (22.5%) with high BP have their BP under control.
High BP is a major risk factor for coronary heart disease, heart failure, and stroke, and the problem of controlling it is only getting worse. In 2024, the American Heart Association estimates that, “among adults, prevalence of hypertension will increase from 51.2% in 2020 to 61.0% in 2050.”
Pharmacists Most Effective
Though many factors contribute to hypertension, researchers have found that the kind of specialist leading the hypertension team may play a role in success. Currently, most BP control teams are led by physicians in primary care.
In a recent meta-analysis involving 100 randomized controlled trials and more than 90,000 patients in Circulation, Katherine T. Mills, PhD, School of Public Health, Tulane University, New Orleans, Louisiana, and colleagues found that, while all the groups studied who led BP control efforts were successful in reducing BP, pharmacist- and community health worker–led teams saw the biggest reductions.
Those groups’ efforts resulted in the greatest systolic BP drops: −7.3 mm Hg (pharmacists) and −7.1 mm Hg (community health workers). Groups led by nurses and physicians saw systolic changes of −3 and −2.4 mm Hg, respectively.
Similarly, pharmacist- and community health worker–led efforts saw the greatest diastolic BP reductions (−3.8 and −3.1 mm Hg), compared with nurse-led (−1.6) and physician-led (−1.2) efforts.
Reductions Enough to Cut Cardiovascular Disease Risk
The reduction numbers for pharmacists are clinically meaningful, Mills said in an interview. “It’s greater than a lot of what we see from individual lifestyle changes,” such as reducing sodium intake or increasing physical activity.
“It’s a big enough blood pressure change to have meaningful reduction in risk of cardiovascular disease,” she said.
This evidence that the leader of the team matters is particularly important because the treatment of hypertension is not in doubt. Something else is not working the way it should.
“We have basically all the scientific evidence we need in terms of what interventions work. But there’s a big gap between that and what’s actually being done in the real world,” she said.
Mills said she was not surprised that pharmacists got the best results “because so much of it has to do with titrating medications and finding the right kind of medications for each patient.”
Additionally, BP management and control falls right into pharmacists’ wheelhouse, Mills noted, including evaluating medication side effects and talking to patients about medication adherence.
Why Pharmacists May Be More Successful
In an accompanying editorial, Ross T. Tsuyuki, PharmD, with the EPICORE Centre, Division of Cardiology, University of Alberta in Edmonton, Canada, and coauthors said the Mills study provides further data to support pharmacists leading BP control efforts, but it’s not the data that have been keeping the model from changing. The barriers include turf wars and lack of legislative change.
The editorialists also said having pharmacist-led BP teams is only the first step. “We need pharmacists to independently prescribe,” they wrote.
“Since individual states govern the scope of practice of pharmacists,” the editorialists wrote, “we have the enormous task of changing regulations to allow pharmacists to independently prescribe for hypertension. But it can be done. The Canadian province of Alberta allows pharmacists to prescribe. And more recently, Idaho. While most states allow some sort of collaborative (dependent) prescribing, that is only a first step.”
Allowing pharmacists to independently prescribe will help populations who do not have a physician or can’t get access to a physician, the editorialists wrote. But changing state legislation would be a lengthy and complex effort.
Physician-Led BP Control Model ‘Seems to Fail Miserably’
Coauthor of the editorial, Florian Rader, MD, MSc, medical director of the Hypertension Center of Excellence at Cedars-Sinai Medical Center in Los Angeles, California, said in an interview that, currently, physician-led teams are the norm, “and that model seems to fail miserably.”
He offered several key reasons for that. In primary care, patients with hypertension often have other problems — they may have high cholesterol or diabetes. “They may have acute illnesses that bother them as well as hypertension that doesn’t bother them,” he said.
Physicians tend to find excuses not to increase or add BP medications, Rader said. “We tend then to blame ‘white coat effect’ or say ‘you’re just nervous today.’ ”
Pharmacists, comparatively, are more protocol driven, he said. “They essentially look at blood pressure and they have an algorithm in their mind. If the blood pressure hits the guideline-stated bar, start this medication. If it hits another bar, increase or add another medication.”
Rader said turf wars are also keeping physician-led teams from changing, fueled by fears that patients will seek care from pharmacists instead of physicians.
“I don’t think the pharmacists will steal a single patient,” Rader said. “If a physician had a healthcare partner like a pharmacist to optimize blood pressure, then [patients] come back to the physician with normalized BP on the right medications. I think it’s a total win-win. I think we just have to get over that.”
Pharmacist-led warfarin clinics are very well established, Rader said, “but for whatever reason, when it comes to blood pressure, physicians are a little bit more hesitant.”
Collaboration Yes, Independent No
Hypertension expert Donald J. DiPette, MD, Health Sciences Distinguished Professor in the Department of Internal Medicine at University of South Carolina, Columbia, said he completely agrees with Mills and colleagues’ conclusion. “Pharmacists and community health workers are most effective at leading BP intervention implementation and should be prioritized in future hypertension control efforts.”
The conclusion “is in line with the thinking of major organizations,” said DiPette, who helped develop the WHO’s most recent pharmacological treatment of hypertension guidelines. “WHO suggests that pharmacological treatment of hypertension can be provided by nonphysician professionals such as pharmacists and nurses as long as the following conditions are met: Proper training, prescribing authority, specific management protocols, and physician oversight.”
DiPette strongly believes BP control efforts should be supervised by a physician, but that could come in different ways. He suggested a collaborative but physician-supervised development of a protocol. Everyone contributes, but the physician signs off on it.
As for the Idaho example of independent practice for pharmacists, DiPette said he doesn’t think that will make a big difference in control rates. “That’s still not team-based care.”
Community Health Workers Key
He said he was also glad to see community healthcare workers emerge as the next-most-effective group after pharmacists to lead BP control teams. This is particularly important as BP control efforts globally need to consider the cultural experience of individual communities. “The community worker is on the ground, and can help overcome some of the cultural barriers,” he said.
“The key is to focus on team-based care and moving away from silo practice,” DiPette said.
Physicians, he said, often fall into “clinical or therapeutic inertia,” where BP is concerned. “We fail to titrate or add additional hypertensive medications even when they’re clearly indicated by the blood pressure. This is a problem not with the individual patient or the healthcare system, this is on us as physicians.”
Nonphysicians are more aligned with following protocols and guidelines, irrespective of the dynamics of what’s going on, he said.
And following protocols rigidly is a good thing for hypertension. “We’re not overtreating hypertension,” he emphasized. “We’re undertreating it.”
Reversing the trend on hypertension will take a sea change in medicine — changing institutions, systems, and individuals who have been doing things the same way for decades, he said.
“Our hypertension control rates are dismal,” DiPette said. “What’s more alarming is they’re going down. That’s the urgency. That’s the burning platform. We must strongly consider doing something different.”
Tsuyuki has received investigator-initiated arm’s length research grants from Merck, Pfizer, AstraZeneca, and Sanofi. He has been a speaker/consultant for Merck, Emergent BioSolutions, and Shoppers Drug Mart/Loblaw Companies Limited. Rader has been a consultant for Bristol Meyers Squibb, Cytokinetics, Idorsia, Medtronic, and ReCor Medical. Mills and coauthors reported no relevant financial relationships. DiPette declared no relevant financial relationships. He was part of a leadership team that developed WHO guidelines on hypertension.
A version of this article first appeared on Medscape.com.
Current efforts to control high blood pressure (BP) are failing in the United States and globally.
The first World Health Organization (WHO) global report on hypertension found that only 54% of adults with hypertension are diagnosed, 42% get treatment, and just 21% have their hypertension controlled.
In the United States, almost half (48%) of adults have high BP, defined as a systolic BP > 130 mm Hg, or a diastolic BP > 80 mm Hg, or are taking medication for high BP, according to the Centers for Disease Control and Prevention. Only about one in four adults (22.5%) with high BP have their BP under control.
High BP is a major risk factor for coronary heart disease, heart failure, and stroke, and the problem of controlling it is only getting worse. In 2024, the American Heart Association estimates that, “among adults, prevalence of hypertension will increase from 51.2% in 2020 to 61.0% in 2050.”
Pharmacists Most Effective
Though many factors contribute to hypertension, researchers have found that the kind of specialist leading the hypertension team may play a role in success. Currently, most BP control teams are led by physicians in primary care.
In a recent meta-analysis involving 100 randomized controlled trials and more than 90,000 patients in Circulation, Katherine T. Mills, PhD, School of Public Health, Tulane University, New Orleans, Louisiana, and colleagues found that, while all the groups studied who led BP control efforts were successful in reducing BP, pharmacist- and community health worker–led teams saw the biggest reductions.
Those groups’ efforts resulted in the greatest systolic BP drops: −7.3 mm Hg (pharmacists) and −7.1 mm Hg (community health workers). Groups led by nurses and physicians saw systolic changes of −3 and −2.4 mm Hg, respectively.
Similarly, pharmacist- and community health worker–led efforts saw the greatest diastolic BP reductions (−3.8 and −3.1 mm Hg), compared with nurse-led (−1.6) and physician-led (−1.2) efforts.
Reductions Enough to Cut Cardiovascular Disease Risk
The reduction numbers for pharmacists are clinically meaningful, Mills said in an interview. “It’s greater than a lot of what we see from individual lifestyle changes,” such as reducing sodium intake or increasing physical activity.
“It’s a big enough blood pressure change to have meaningful reduction in risk of cardiovascular disease,” she said.
This evidence that the leader of the team matters is particularly important because the treatment of hypertension is not in doubt. Something else is not working the way it should.
“We have basically all the scientific evidence we need in terms of what interventions work. But there’s a big gap between that and what’s actually being done in the real world,” she said.
Mills said she was not surprised that pharmacists got the best results “because so much of it has to do with titrating medications and finding the right kind of medications for each patient.”
Additionally, BP management and control falls right into pharmacists’ wheelhouse, Mills noted, including evaluating medication side effects and talking to patients about medication adherence.
Why Pharmacists May Be More Successful
In an accompanying editorial, Ross T. Tsuyuki, PharmD, with the EPICORE Centre, Division of Cardiology, University of Alberta in Edmonton, Canada, and coauthors said the Mills study provides further data to support pharmacists leading BP control efforts, but it’s not the data that have been keeping the model from changing. The barriers include turf wars and lack of legislative change.
The editorialists also said having pharmacist-led BP teams is only the first step. “We need pharmacists to independently prescribe,” they wrote.
“Since individual states govern the scope of practice of pharmacists,” the editorialists wrote, “we have the enormous task of changing regulations to allow pharmacists to independently prescribe for hypertension. But it can be done. The Canadian province of Alberta allows pharmacists to prescribe. And more recently, Idaho. While most states allow some sort of collaborative (dependent) prescribing, that is only a first step.”
Allowing pharmacists to independently prescribe will help populations who do not have a physician or can’t get access to a physician, the editorialists wrote. But changing state legislation would be a lengthy and complex effort.
Physician-Led BP Control Model ‘Seems to Fail Miserably’
Coauthor of the editorial, Florian Rader, MD, MSc, medical director of the Hypertension Center of Excellence at Cedars-Sinai Medical Center in Los Angeles, California, said in an interview that, currently, physician-led teams are the norm, “and that model seems to fail miserably.”
He offered several key reasons for that. In primary care, patients with hypertension often have other problems — they may have high cholesterol or diabetes. “They may have acute illnesses that bother them as well as hypertension that doesn’t bother them,” he said.
Physicians tend to find excuses not to increase or add BP medications, Rader said. “We tend then to blame ‘white coat effect’ or say ‘you’re just nervous today.’ ”
Pharmacists, comparatively, are more protocol driven, he said. “They essentially look at blood pressure and they have an algorithm in their mind. If the blood pressure hits the guideline-stated bar, start this medication. If it hits another bar, increase or add another medication.”
Rader said turf wars are also keeping physician-led teams from changing, fueled by fears that patients will seek care from pharmacists instead of physicians.
“I don’t think the pharmacists will steal a single patient,” Rader said. “If a physician had a healthcare partner like a pharmacist to optimize blood pressure, then [patients] come back to the physician with normalized BP on the right medications. I think it’s a total win-win. I think we just have to get over that.”
Pharmacist-led warfarin clinics are very well established, Rader said, “but for whatever reason, when it comes to blood pressure, physicians are a little bit more hesitant.”
Collaboration Yes, Independent No
Hypertension expert Donald J. DiPette, MD, Health Sciences Distinguished Professor in the Department of Internal Medicine at University of South Carolina, Columbia, said he completely agrees with Mills and colleagues’ conclusion. “Pharmacists and community health workers are most effective at leading BP intervention implementation and should be prioritized in future hypertension control efforts.”
The conclusion “is in line with the thinking of major organizations,” said DiPette, who helped develop the WHO’s most recent pharmacological treatment of hypertension guidelines. “WHO suggests that pharmacological treatment of hypertension can be provided by nonphysician professionals such as pharmacists and nurses as long as the following conditions are met: Proper training, prescribing authority, specific management protocols, and physician oversight.”
DiPette strongly believes BP control efforts should be supervised by a physician, but that could come in different ways. He suggested a collaborative but physician-supervised development of a protocol. Everyone contributes, but the physician signs off on it.
As for the Idaho example of independent practice for pharmacists, DiPette said he doesn’t think that will make a big difference in control rates. “That’s still not team-based care.”
Community Health Workers Key
He said he was also glad to see community healthcare workers emerge as the next-most-effective group after pharmacists to lead BP control teams. This is particularly important as BP control efforts globally need to consider the cultural experience of individual communities. “The community worker is on the ground, and can help overcome some of the cultural barriers,” he said.
“The key is to focus on team-based care and moving away from silo practice,” DiPette said.
Physicians, he said, often fall into “clinical or therapeutic inertia,” where BP is concerned. “We fail to titrate or add additional hypertensive medications even when they’re clearly indicated by the blood pressure. This is a problem not with the individual patient or the healthcare system, this is on us as physicians.”
Nonphysicians are more aligned with following protocols and guidelines, irrespective of the dynamics of what’s going on, he said.
And following protocols rigidly is a good thing for hypertension. “We’re not overtreating hypertension,” he emphasized. “We’re undertreating it.”
Reversing the trend on hypertension will take a sea change in medicine — changing institutions, systems, and individuals who have been doing things the same way for decades, he said.
“Our hypertension control rates are dismal,” DiPette said. “What’s more alarming is they’re going down. That’s the urgency. That’s the burning platform. We must strongly consider doing something different.”
Tsuyuki has received investigator-initiated arm’s length research grants from Merck, Pfizer, AstraZeneca, and Sanofi. He has been a speaker/consultant for Merck, Emergent BioSolutions, and Shoppers Drug Mart/Loblaw Companies Limited. Rader has been a consultant for Bristol Meyers Squibb, Cytokinetics, Idorsia, Medtronic, and ReCor Medical. Mills and coauthors reported no relevant financial relationships. DiPette declared no relevant financial relationships. He was part of a leadership team that developed WHO guidelines on hypertension.
A version of this article first appeared on Medscape.com.
Current efforts to control high blood pressure (BP) are failing in the United States and globally.
The first World Health Organization (WHO) global report on hypertension found that only 54% of adults with hypertension are diagnosed, 42% get treatment, and just 21% have their hypertension controlled.
In the United States, almost half (48%) of adults have high BP, defined as a systolic BP > 130 mm Hg, or a diastolic BP > 80 mm Hg, or are taking medication for high BP, according to the Centers for Disease Control and Prevention. Only about one in four adults (22.5%) with high BP have their BP under control.
High BP is a major risk factor for coronary heart disease, heart failure, and stroke, and the problem of controlling it is only getting worse. In 2024, the American Heart Association estimates that, “among adults, prevalence of hypertension will increase from 51.2% in 2020 to 61.0% in 2050.”
Pharmacists Most Effective
Though many factors contribute to hypertension, researchers have found that the kind of specialist leading the hypertension team may play a role in success. Currently, most BP control teams are led by physicians in primary care.
In a recent meta-analysis involving 100 randomized controlled trials and more than 90,000 patients in Circulation, Katherine T. Mills, PhD, School of Public Health, Tulane University, New Orleans, Louisiana, and colleagues found that, while all the groups studied who led BP control efforts were successful in reducing BP, pharmacist- and community health worker–led teams saw the biggest reductions.
Those groups’ efforts resulted in the greatest systolic BP drops: −7.3 mm Hg (pharmacists) and −7.1 mm Hg (community health workers). Groups led by nurses and physicians saw systolic changes of −3 and −2.4 mm Hg, respectively.
Similarly, pharmacist- and community health worker–led efforts saw the greatest diastolic BP reductions (−3.8 and −3.1 mm Hg), compared with nurse-led (−1.6) and physician-led (−1.2) efforts.
Reductions Enough to Cut Cardiovascular Disease Risk
The reduction numbers for pharmacists are clinically meaningful, Mills said in an interview. “It’s greater than a lot of what we see from individual lifestyle changes,” such as reducing sodium intake or increasing physical activity.
“It’s a big enough blood pressure change to have meaningful reduction in risk of cardiovascular disease,” she said.
This evidence that the leader of the team matters is particularly important because the treatment of hypertension is not in doubt. Something else is not working the way it should.
“We have basically all the scientific evidence we need in terms of what interventions work. But there’s a big gap between that and what’s actually being done in the real world,” she said.
Mills said she was not surprised that pharmacists got the best results “because so much of it has to do with titrating medications and finding the right kind of medications for each patient.”
Additionally, BP management and control falls right into pharmacists’ wheelhouse, Mills noted, including evaluating medication side effects and talking to patients about medication adherence.
Why Pharmacists May Be More Successful
In an accompanying editorial, Ross T. Tsuyuki, PharmD, with the EPICORE Centre, Division of Cardiology, University of Alberta in Edmonton, Canada, and coauthors said the Mills study provides further data to support pharmacists leading BP control efforts, but it’s not the data that have been keeping the model from changing. The barriers include turf wars and lack of legislative change.
The editorialists also said having pharmacist-led BP teams is only the first step. “We need pharmacists to independently prescribe,” they wrote.
“Since individual states govern the scope of practice of pharmacists,” the editorialists wrote, “we have the enormous task of changing regulations to allow pharmacists to independently prescribe for hypertension. But it can be done. The Canadian province of Alberta allows pharmacists to prescribe. And more recently, Idaho. While most states allow some sort of collaborative (dependent) prescribing, that is only a first step.”
Allowing pharmacists to independently prescribe will help populations who do not have a physician or can’t get access to a physician, the editorialists wrote. But changing state legislation would be a lengthy and complex effort.
Physician-Led BP Control Model ‘Seems to Fail Miserably’
Coauthor of the editorial, Florian Rader, MD, MSc, medical director of the Hypertension Center of Excellence at Cedars-Sinai Medical Center in Los Angeles, California, said in an interview that, currently, physician-led teams are the norm, “and that model seems to fail miserably.”
He offered several key reasons for that. In primary care, patients with hypertension often have other problems — they may have high cholesterol or diabetes. “They may have acute illnesses that bother them as well as hypertension that doesn’t bother them,” he said.
Physicians tend to find excuses not to increase or add BP medications, Rader said. “We tend then to blame ‘white coat effect’ or say ‘you’re just nervous today.’ ”
Pharmacists, comparatively, are more protocol driven, he said. “They essentially look at blood pressure and they have an algorithm in their mind. If the blood pressure hits the guideline-stated bar, start this medication. If it hits another bar, increase or add another medication.”
Rader said turf wars are also keeping physician-led teams from changing, fueled by fears that patients will seek care from pharmacists instead of physicians.
“I don’t think the pharmacists will steal a single patient,” Rader said. “If a physician had a healthcare partner like a pharmacist to optimize blood pressure, then [patients] come back to the physician with normalized BP on the right medications. I think it’s a total win-win. I think we just have to get over that.”
Pharmacist-led warfarin clinics are very well established, Rader said, “but for whatever reason, when it comes to blood pressure, physicians are a little bit more hesitant.”
Collaboration Yes, Independent No
Hypertension expert Donald J. DiPette, MD, Health Sciences Distinguished Professor in the Department of Internal Medicine at University of South Carolina, Columbia, said he completely agrees with Mills and colleagues’ conclusion. “Pharmacists and community health workers are most effective at leading BP intervention implementation and should be prioritized in future hypertension control efforts.”
The conclusion “is in line with the thinking of major organizations,” said DiPette, who helped develop the WHO’s most recent pharmacological treatment of hypertension guidelines. “WHO suggests that pharmacological treatment of hypertension can be provided by nonphysician professionals such as pharmacists and nurses as long as the following conditions are met: Proper training, prescribing authority, specific management protocols, and physician oversight.”
DiPette strongly believes BP control efforts should be supervised by a physician, but that could come in different ways. He suggested a collaborative but physician-supervised development of a protocol. Everyone contributes, but the physician signs off on it.
As for the Idaho example of independent practice for pharmacists, DiPette said he doesn’t think that will make a big difference in control rates. “That’s still not team-based care.”
Community Health Workers Key
He said he was also glad to see community healthcare workers emerge as the next-most-effective group after pharmacists to lead BP control teams. This is particularly important as BP control efforts globally need to consider the cultural experience of individual communities. “The community worker is on the ground, and can help overcome some of the cultural barriers,” he said.
“The key is to focus on team-based care and moving away from silo practice,” DiPette said.
Physicians, he said, often fall into “clinical or therapeutic inertia,” where BP is concerned. “We fail to titrate or add additional hypertensive medications even when they’re clearly indicated by the blood pressure. This is a problem not with the individual patient or the healthcare system, this is on us as physicians.”
Nonphysicians are more aligned with following protocols and guidelines, irrespective of the dynamics of what’s going on, he said.
And following protocols rigidly is a good thing for hypertension. “We’re not overtreating hypertension,” he emphasized. “We’re undertreating it.”
Reversing the trend on hypertension will take a sea change in medicine — changing institutions, systems, and individuals who have been doing things the same way for decades, he said.
“Our hypertension control rates are dismal,” DiPette said. “What’s more alarming is they’re going down. That’s the urgency. That’s the burning platform. We must strongly consider doing something different.”
Tsuyuki has received investigator-initiated arm’s length research grants from Merck, Pfizer, AstraZeneca, and Sanofi. He has been a speaker/consultant for Merck, Emergent BioSolutions, and Shoppers Drug Mart/Loblaw Companies Limited. Rader has been a consultant for Bristol Meyers Squibb, Cytokinetics, Idorsia, Medtronic, and ReCor Medical. Mills and coauthors reported no relevant financial relationships. DiPette declared no relevant financial relationships. He was part of a leadership team that developed WHO guidelines on hypertension.
A version of this article first appeared on Medscape.com.