User login
CDC issues new return-to-work guidelines
The Centers for Disease Control and Prevention is releasing new guidance on return-to-work rules for critical workers exposed to a COVID-19 case, or a suspected case, replacing previous guidance to stay home for 14 days.
“One of the most important things we can do is keep our critical workforce working,” CDC Director Robert Redfield said at a White House briefing on April 8. “In certain circumstances they can go back to work,” he said.
Neither Redfield nor the other governmental officials specified what counts as an essential worker, although it has generally referred to food-service and health care workers.
They must take their temperature before work, wear a facial mask at all times and practice social distancing when at work, the new guidance says. They cannot share headsets or other objects used near the face.
Employers must take the worker’s temperature and assess each one for symptoms before work starts, sending a worker home if he or she is sick. Employers must increase the cleaning of frequently used surfaces, increase air exchange in the building and test the use of face masks to be sure they do not interfere with workflow.
Pressed on whether he would reopen the country at the end of the 30-day Stop the Spread effort on April 30 — since one model has revised the U.S. death toll down from 100,000-240,000 to 61,000 — President Donald Trump said meetings will take place soon to discuss the decision and that he will ‘’rely very heavily” on health experts.
“We know now for sure that the mitigation we have been doing is having a positive effect,” said Anthony Fauci, MD, a coronavirus task force member and director of the National Institute of Allergy and Infectious Diseases.
This article first appeared on WebMD.
The Centers for Disease Control and Prevention is releasing new guidance on return-to-work rules for critical workers exposed to a COVID-19 case, or a suspected case, replacing previous guidance to stay home for 14 days.
“One of the most important things we can do is keep our critical workforce working,” CDC Director Robert Redfield said at a White House briefing on April 8. “In certain circumstances they can go back to work,” he said.
Neither Redfield nor the other governmental officials specified what counts as an essential worker, although it has generally referred to food-service and health care workers.
They must take their temperature before work, wear a facial mask at all times and practice social distancing when at work, the new guidance says. They cannot share headsets or other objects used near the face.
Employers must take the worker’s temperature and assess each one for symptoms before work starts, sending a worker home if he or she is sick. Employers must increase the cleaning of frequently used surfaces, increase air exchange in the building and test the use of face masks to be sure they do not interfere with workflow.
Pressed on whether he would reopen the country at the end of the 30-day Stop the Spread effort on April 30 — since one model has revised the U.S. death toll down from 100,000-240,000 to 61,000 — President Donald Trump said meetings will take place soon to discuss the decision and that he will ‘’rely very heavily” on health experts.
“We know now for sure that the mitigation we have been doing is having a positive effect,” said Anthony Fauci, MD, a coronavirus task force member and director of the National Institute of Allergy and Infectious Diseases.
This article first appeared on WebMD.
The Centers for Disease Control and Prevention is releasing new guidance on return-to-work rules for critical workers exposed to a COVID-19 case, or a suspected case, replacing previous guidance to stay home for 14 days.
“One of the most important things we can do is keep our critical workforce working,” CDC Director Robert Redfield said at a White House briefing on April 8. “In certain circumstances they can go back to work,” he said.
Neither Redfield nor the other governmental officials specified what counts as an essential worker, although it has generally referred to food-service and health care workers.
They must take their temperature before work, wear a facial mask at all times and practice social distancing when at work, the new guidance says. They cannot share headsets or other objects used near the face.
Employers must take the worker’s temperature and assess each one for symptoms before work starts, sending a worker home if he or she is sick. Employers must increase the cleaning of frequently used surfaces, increase air exchange in the building and test the use of face masks to be sure they do not interfere with workflow.
Pressed on whether he would reopen the country at the end of the 30-day Stop the Spread effort on April 30 — since one model has revised the U.S. death toll down from 100,000-240,000 to 61,000 — President Donald Trump said meetings will take place soon to discuss the decision and that he will ‘’rely very heavily” on health experts.
“We know now for sure that the mitigation we have been doing is having a positive effect,” said Anthony Fauci, MD, a coronavirus task force member and director of the National Institute of Allergy and Infectious Diseases.
This article first appeared on WebMD.
Almost 90% of COVID-19 admissions involve comorbidities
The hospitalization rate for COVID-19 is 4.6 per 100,000 population, and almost 90% of hospitalized patients have some type of underlying condition, according to the Centers for Disease Control and Prevention.
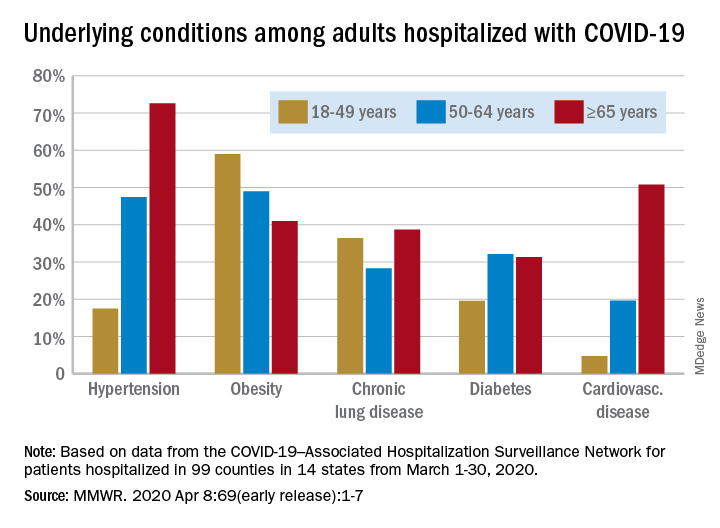
Data collected by the newly created COVID-19–Associated Hospitalization Surveillance Network (COVID-NET) put the exact prevalence of underlying conditions at 89.3% for patients hospitalized during March 1-30, 2020, Shikha Garg, MD, of the CDC’s COVID-NET team and associates wrote in the MMWR.
The hospitalization rate, based on COVID-NET data for March 1-28, increased with patient age. Those aged 65 years and older were admitted at a rate of 13.8 per 100,000, with 50- to 64-year-olds next at 7.4 per 100,000 and 18- to 49-year-olds at 2.5, they wrote.
The patients aged 65 years and older also were the most likely to have one or more underlying conditions, at 94.4%, compared with 86.4% of those aged 50-64 years and 85.4% of individuals who were aged 18-44 years, the investigators reported.
Hypertension was the most common comorbidity among the oldest patients, with a prevalence of 72.6%, followed by cardiovascular disease at 50.8% and obesity at 41%. In the two younger groups, obesity was the condition most often seen in COVID-19 patients, with prevalences of 49% in 50- to 64-year-olds and 59% in those aged 18-49, Dr. Garg and associates wrote.
“These findings underscore the importance of preventive measures (e.g., social distancing, respiratory hygiene, and wearing face coverings in public settings where social distancing measures are difficult to maintain) to protect older adults and persons with underlying medical conditions,” the investigators wrote.
COVID-NET surveillance includes laboratory-confirmed hospitalizations in 99 counties in 14 states: California, Colorado, Connecticut, Georgia, Iowa, Maryland, Michigan, Minnesota, New Mexico, New York, Ohio, Oregon, Tennessee, and Utah. Those counties represent about 10% of the U.S. population.
SOURCE: Garg S et al. MMWR. 2020 Apr 8;69(early release):1-7.
The hospitalization rate for COVID-19 is 4.6 per 100,000 population, and almost 90% of hospitalized patients have some type of underlying condition, according to the Centers for Disease Control and Prevention.

Data collected by the newly created COVID-19–Associated Hospitalization Surveillance Network (COVID-NET) put the exact prevalence of underlying conditions at 89.3% for patients hospitalized during March 1-30, 2020, Shikha Garg, MD, of the CDC’s COVID-NET team and associates wrote in the MMWR.
The hospitalization rate, based on COVID-NET data for March 1-28, increased with patient age. Those aged 65 years and older were admitted at a rate of 13.8 per 100,000, with 50- to 64-year-olds next at 7.4 per 100,000 and 18- to 49-year-olds at 2.5, they wrote.
The patients aged 65 years and older also were the most likely to have one or more underlying conditions, at 94.4%, compared with 86.4% of those aged 50-64 years and 85.4% of individuals who were aged 18-44 years, the investigators reported.
Hypertension was the most common comorbidity among the oldest patients, with a prevalence of 72.6%, followed by cardiovascular disease at 50.8% and obesity at 41%. In the two younger groups, obesity was the condition most often seen in COVID-19 patients, with prevalences of 49% in 50- to 64-year-olds and 59% in those aged 18-49, Dr. Garg and associates wrote.
“These findings underscore the importance of preventive measures (e.g., social distancing, respiratory hygiene, and wearing face coverings in public settings where social distancing measures are difficult to maintain) to protect older adults and persons with underlying medical conditions,” the investigators wrote.
COVID-NET surveillance includes laboratory-confirmed hospitalizations in 99 counties in 14 states: California, Colorado, Connecticut, Georgia, Iowa, Maryland, Michigan, Minnesota, New Mexico, New York, Ohio, Oregon, Tennessee, and Utah. Those counties represent about 10% of the U.S. population.
SOURCE: Garg S et al. MMWR. 2020 Apr 8;69(early release):1-7.
The hospitalization rate for COVID-19 is 4.6 per 100,000 population, and almost 90% of hospitalized patients have some type of underlying condition, according to the Centers for Disease Control and Prevention.

Data collected by the newly created COVID-19–Associated Hospitalization Surveillance Network (COVID-NET) put the exact prevalence of underlying conditions at 89.3% for patients hospitalized during March 1-30, 2020, Shikha Garg, MD, of the CDC’s COVID-NET team and associates wrote in the MMWR.
The hospitalization rate, based on COVID-NET data for March 1-28, increased with patient age. Those aged 65 years and older were admitted at a rate of 13.8 per 100,000, with 50- to 64-year-olds next at 7.4 per 100,000 and 18- to 49-year-olds at 2.5, they wrote.
The patients aged 65 years and older also were the most likely to have one or more underlying conditions, at 94.4%, compared with 86.4% of those aged 50-64 years and 85.4% of individuals who were aged 18-44 years, the investigators reported.
Hypertension was the most common comorbidity among the oldest patients, with a prevalence of 72.6%, followed by cardiovascular disease at 50.8% and obesity at 41%. In the two younger groups, obesity was the condition most often seen in COVID-19 patients, with prevalences of 49% in 50- to 64-year-olds and 59% in those aged 18-49, Dr. Garg and associates wrote.
“These findings underscore the importance of preventive measures (e.g., social distancing, respiratory hygiene, and wearing face coverings in public settings where social distancing measures are difficult to maintain) to protect older adults and persons with underlying medical conditions,” the investigators wrote.
COVID-NET surveillance includes laboratory-confirmed hospitalizations in 99 counties in 14 states: California, Colorado, Connecticut, Georgia, Iowa, Maryland, Michigan, Minnesota, New Mexico, New York, Ohio, Oregon, Tennessee, and Utah. Those counties represent about 10% of the U.S. population.
SOURCE: Garg S et al. MMWR. 2020 Apr 8;69(early release):1-7.
FROM THE MMWR
FDA approves first generic albuterol inhaler
The Food and Drug Administration has approved the first generic of Proventil HFA (albuterol sulfate) metered-dose inhaler, 90 mcg/inhalation, according to a release from the agency. This inhaler is indicated for prevention of bronchospasm in patients aged 4 years and older. Specifically, these are patients with reversible obstructive airway disease or exercise-induced bronchospasm.
“The FDA recognizes the increased demand for albuterol products during the novel coronavirus pandemic,” said FDA Commissioner Stephen M. Hahn, MD.
The most common side effects include upper respiratory tract infection, rhinitis, nausea, vomiting, rapid heart rate, tremor, and nervousness.
This approval comes as part of FDA’s efforts to guide industry through the development process of generic products, according to the release. Complex combination products – such as this inhaler, which comprises both medication and a delivery system – can be more challenging to develop than solid oral dosage forms, such as tablets.
The FDA released a draft guidance in March 2020 specific to proposed generic albuterol sulfate metered-dose inhalers, including drug products referencing Proventil HFA. As with other similar guidances, it details the steps companies need to take in developing generics in order to submit complete applications for those products. The full news release regarding this approval is available on the FDA website.
This article was updated 4/8/20.
The Food and Drug Administration has approved the first generic of Proventil HFA (albuterol sulfate) metered-dose inhaler, 90 mcg/inhalation, according to a release from the agency. This inhaler is indicated for prevention of bronchospasm in patients aged 4 years and older. Specifically, these are patients with reversible obstructive airway disease or exercise-induced bronchospasm.
“The FDA recognizes the increased demand for albuterol products during the novel coronavirus pandemic,” said FDA Commissioner Stephen M. Hahn, MD.
The most common side effects include upper respiratory tract infection, rhinitis, nausea, vomiting, rapid heart rate, tremor, and nervousness.
This approval comes as part of FDA’s efforts to guide industry through the development process of generic products, according to the release. Complex combination products – such as this inhaler, which comprises both medication and a delivery system – can be more challenging to develop than solid oral dosage forms, such as tablets.
The FDA released a draft guidance in March 2020 specific to proposed generic albuterol sulfate metered-dose inhalers, including drug products referencing Proventil HFA. As with other similar guidances, it details the steps companies need to take in developing generics in order to submit complete applications for those products. The full news release regarding this approval is available on the FDA website.
This article was updated 4/8/20.
The Food and Drug Administration has approved the first generic of Proventil HFA (albuterol sulfate) metered-dose inhaler, 90 mcg/inhalation, according to a release from the agency. This inhaler is indicated for prevention of bronchospasm in patients aged 4 years and older. Specifically, these are patients with reversible obstructive airway disease or exercise-induced bronchospasm.
“The FDA recognizes the increased demand for albuterol products during the novel coronavirus pandemic,” said FDA Commissioner Stephen M. Hahn, MD.
The most common side effects include upper respiratory tract infection, rhinitis, nausea, vomiting, rapid heart rate, tremor, and nervousness.
This approval comes as part of FDA’s efforts to guide industry through the development process of generic products, according to the release. Complex combination products – such as this inhaler, which comprises both medication and a delivery system – can be more challenging to develop than solid oral dosage forms, such as tablets.
The FDA released a draft guidance in March 2020 specific to proposed generic albuterol sulfate metered-dose inhalers, including drug products referencing Proventil HFA. As with other similar guidances, it details the steps companies need to take in developing generics in order to submit complete applications for those products. The full news release regarding this approval is available on the FDA website.
This article was updated 4/8/20.
Autism prevalence: ‘Diminishing disparity’ between black and white children
For the first time since detailed measurement began in 2000, there was no significant difference in autism prevalence between black and white 8-year-olds in 2016, according to data from the Centers for Disease Control and Prevention.
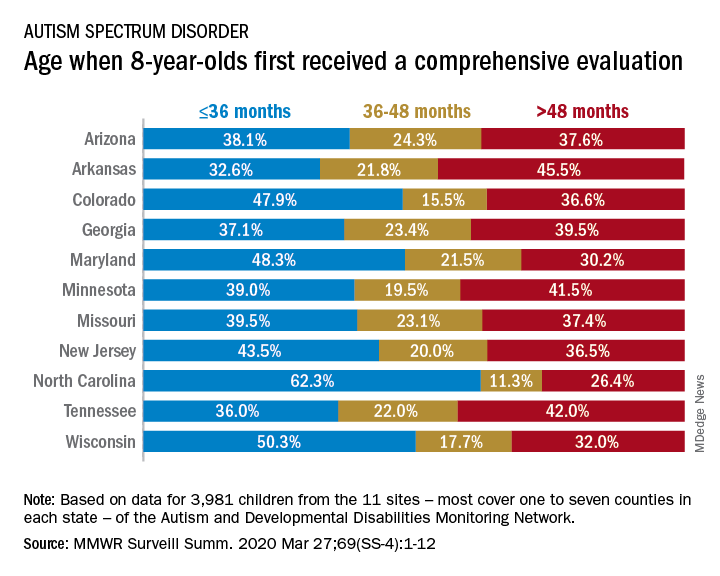
The latest analysis from the CDC’s Autism and Developmental Disabilities Monitoring (ADDM) Network puts the prevalence of autism spectrum disorder (ASD) at 18.3 per 1,000 children aged 8 years among black children and 18.5 per 1,000 in white children, Matthew J. Maenner, PhD, and associates said in MMWR Surveillance Summaries. Overall prevalence was 18.5 per 1,000 children, or 1 in 54 children, aged 8 years.
“This diminishing disparity in ASD prevalence might signify progress toward earlier and more equitable identification of ASD,” they wrote, while also noting that “black children with ASD were more likely than white children to have an intellectual disability” and were less likely to undergo evaluation by age 36 months.
and 42.9% of Hispanic children, said Dr. Maenner of the CDC’s National Center on Birth Defects and Developmental Disabilities.
The overall rate of early evaluation was 44% for the cohort of 3,981 children who were born in 2008 and included in the 2016 analysis of the 11 ADDM Network sites, they reported.
There was, however, considerable variation in the timing of that initial evaluation for ASD among the sites, which largely consisted of one to seven counties in most states, except for Arkansas (all 75 counties), Tennessee (11 counties), and Wisconsin (10 counties), Dr. Maenner and associates noted.
The two ADDM Network sites at the extremes of that variation were North Carolina and Arkansas. In North Carolina, almost twice as many children (62.3%) had an evaluation by 36 months than in Arkansas (32.6%), although Arkansas closed the gap a bit by evaluating 21.8% of children aged 37-48 months, compared with 11.3% in North Carolina, the investigators said.
“ASD continues to be a public health concern; the latest data from the ADDM Network underscore the ongoing need for timely and accessible developmental assessments, educational supports, and services for persons with ASD and their families,” they concluded.
SOURCE: Maenner MJ et al. MMWR Surveill Summ. 2020 Mar 27;69(SS-4):1-12.
For the first time since detailed measurement began in 2000, there was no significant difference in autism prevalence between black and white 8-year-olds in 2016, according to data from the Centers for Disease Control and Prevention.

The latest analysis from the CDC’s Autism and Developmental Disabilities Monitoring (ADDM) Network puts the prevalence of autism spectrum disorder (ASD) at 18.3 per 1,000 children aged 8 years among black children and 18.5 per 1,000 in white children, Matthew J. Maenner, PhD, and associates said in MMWR Surveillance Summaries. Overall prevalence was 18.5 per 1,000 children, or 1 in 54 children, aged 8 years.
“This diminishing disparity in ASD prevalence might signify progress toward earlier and more equitable identification of ASD,” they wrote, while also noting that “black children with ASD were more likely than white children to have an intellectual disability” and were less likely to undergo evaluation by age 36 months.
and 42.9% of Hispanic children, said Dr. Maenner of the CDC’s National Center on Birth Defects and Developmental Disabilities.
The overall rate of early evaluation was 44% for the cohort of 3,981 children who were born in 2008 and included in the 2016 analysis of the 11 ADDM Network sites, they reported.
There was, however, considerable variation in the timing of that initial evaluation for ASD among the sites, which largely consisted of one to seven counties in most states, except for Arkansas (all 75 counties), Tennessee (11 counties), and Wisconsin (10 counties), Dr. Maenner and associates noted.
The two ADDM Network sites at the extremes of that variation were North Carolina and Arkansas. In North Carolina, almost twice as many children (62.3%) had an evaluation by 36 months than in Arkansas (32.6%), although Arkansas closed the gap a bit by evaluating 21.8% of children aged 37-48 months, compared with 11.3% in North Carolina, the investigators said.
“ASD continues to be a public health concern; the latest data from the ADDM Network underscore the ongoing need for timely and accessible developmental assessments, educational supports, and services for persons with ASD and their families,” they concluded.
SOURCE: Maenner MJ et al. MMWR Surveill Summ. 2020 Mar 27;69(SS-4):1-12.
For the first time since detailed measurement began in 2000, there was no significant difference in autism prevalence between black and white 8-year-olds in 2016, according to data from the Centers for Disease Control and Prevention.

The latest analysis from the CDC’s Autism and Developmental Disabilities Monitoring (ADDM) Network puts the prevalence of autism spectrum disorder (ASD) at 18.3 per 1,000 children aged 8 years among black children and 18.5 per 1,000 in white children, Matthew J. Maenner, PhD, and associates said in MMWR Surveillance Summaries. Overall prevalence was 18.5 per 1,000 children, or 1 in 54 children, aged 8 years.
“This diminishing disparity in ASD prevalence might signify progress toward earlier and more equitable identification of ASD,” they wrote, while also noting that “black children with ASD were more likely than white children to have an intellectual disability” and were less likely to undergo evaluation by age 36 months.
and 42.9% of Hispanic children, said Dr. Maenner of the CDC’s National Center on Birth Defects and Developmental Disabilities.
The overall rate of early evaluation was 44% for the cohort of 3,981 children who were born in 2008 and included in the 2016 analysis of the 11 ADDM Network sites, they reported.
There was, however, considerable variation in the timing of that initial evaluation for ASD among the sites, which largely consisted of one to seven counties in most states, except for Arkansas (all 75 counties), Tennessee (11 counties), and Wisconsin (10 counties), Dr. Maenner and associates noted.
The two ADDM Network sites at the extremes of that variation were North Carolina and Arkansas. In North Carolina, almost twice as many children (62.3%) had an evaluation by 36 months than in Arkansas (32.6%), although Arkansas closed the gap a bit by evaluating 21.8% of children aged 37-48 months, compared with 11.3% in North Carolina, the investigators said.
“ASD continues to be a public health concern; the latest data from the ADDM Network underscore the ongoing need for timely and accessible developmental assessments, educational supports, and services for persons with ASD and their families,” they concluded.
SOURCE: Maenner MJ et al. MMWR Surveill Summ. 2020 Mar 27;69(SS-4):1-12.
FROM MMWR SURVEILLANCE SUMMARIES
Many children with COVID-19 don’t have cough or fever
according to the Centers for Disease and Prevention Control.
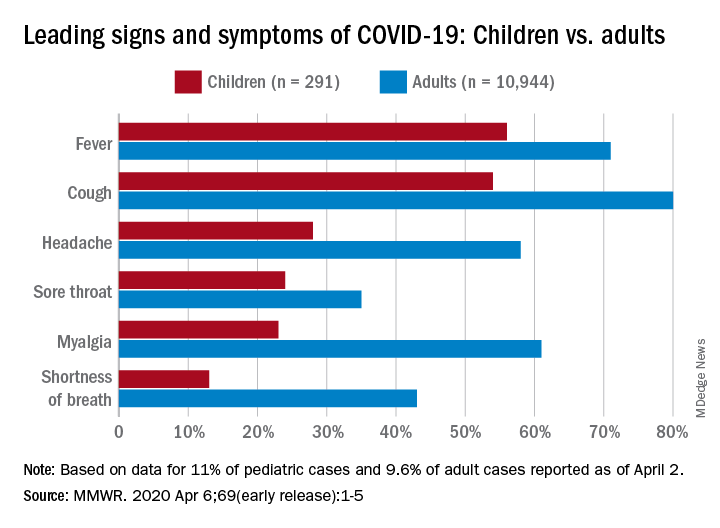
Among pediatric patients younger than 18 years in the United States, 73% had at least one of the trio of symptoms, compared with 93% of adults aged 18-64, noted Lucy A. McNamara, PhD, and the CDC’s COVID-19 response team, based on a preliminary analysis of the 149,082 cases reported as of April 2.
By a small margin, fever – present in 58% of pediatric patients – was the most common sign or symptom of COVID-19, compared with cough at 54% and shortness of breath in 13%. In adults, cough (81%) was seen most often, followed by fever (71%) and shortness of breath (43%), the investigators reported in the MMWR.
In both children and adults, headache and myalgia were more common than shortness of breath, as was sore throat in children, the team added.
“These findings are largely consistent with a report on pediatric COVID-19 patients aged <16 years in China, which found that only 41.5% of pediatric patients had fever [and] 48.5% had cough,” they wrote.
The CDC analysis of pediatric patients was limited by its small sample size, with data on signs and symptoms available for only 11% (291) of the 2,572 children known to have COVID-19 as of April 2. The adult population included 10,944 individuals, who represented 9.6% of the 113,985 U.S. patients aged 18-65, the response team said.
“As the number of COVID-19 cases continues to increase in many parts of the United States, it will be important to adapt COVID-19 surveillance strategies to maintain collection of critical case information without overburdening jurisdiction health departments,” they said.
SOURCE: McNamara LA et al. MMWR 2020 Apr 6;69(early release):1-5.
according to the Centers for Disease and Prevention Control.

Among pediatric patients younger than 18 years in the United States, 73% had at least one of the trio of symptoms, compared with 93% of adults aged 18-64, noted Lucy A. McNamara, PhD, and the CDC’s COVID-19 response team, based on a preliminary analysis of the 149,082 cases reported as of April 2.
By a small margin, fever – present in 58% of pediatric patients – was the most common sign or symptom of COVID-19, compared with cough at 54% and shortness of breath in 13%. In adults, cough (81%) was seen most often, followed by fever (71%) and shortness of breath (43%), the investigators reported in the MMWR.
In both children and adults, headache and myalgia were more common than shortness of breath, as was sore throat in children, the team added.
“These findings are largely consistent with a report on pediatric COVID-19 patients aged <16 years in China, which found that only 41.5% of pediatric patients had fever [and] 48.5% had cough,” they wrote.
The CDC analysis of pediatric patients was limited by its small sample size, with data on signs and symptoms available for only 11% (291) of the 2,572 children known to have COVID-19 as of April 2. The adult population included 10,944 individuals, who represented 9.6% of the 113,985 U.S. patients aged 18-65, the response team said.
“As the number of COVID-19 cases continues to increase in many parts of the United States, it will be important to adapt COVID-19 surveillance strategies to maintain collection of critical case information without overburdening jurisdiction health departments,” they said.
SOURCE: McNamara LA et al. MMWR 2020 Apr 6;69(early release):1-5.
according to the Centers for Disease and Prevention Control.

Among pediatric patients younger than 18 years in the United States, 73% had at least one of the trio of symptoms, compared with 93% of adults aged 18-64, noted Lucy A. McNamara, PhD, and the CDC’s COVID-19 response team, based on a preliminary analysis of the 149,082 cases reported as of April 2.
By a small margin, fever – present in 58% of pediatric patients – was the most common sign or symptom of COVID-19, compared with cough at 54% and shortness of breath in 13%. In adults, cough (81%) was seen most often, followed by fever (71%) and shortness of breath (43%), the investigators reported in the MMWR.
In both children and adults, headache and myalgia were more common than shortness of breath, as was sore throat in children, the team added.
“These findings are largely consistent with a report on pediatric COVID-19 patients aged <16 years in China, which found that only 41.5% of pediatric patients had fever [and] 48.5% had cough,” they wrote.
The CDC analysis of pediatric patients was limited by its small sample size, with data on signs and symptoms available for only 11% (291) of the 2,572 children known to have COVID-19 as of April 2. The adult population included 10,944 individuals, who represented 9.6% of the 113,985 U.S. patients aged 18-65, the response team said.
“As the number of COVID-19 cases continues to increase in many parts of the United States, it will be important to adapt COVID-19 surveillance strategies to maintain collection of critical case information without overburdening jurisdiction health departments,” they said.
SOURCE: McNamara LA et al. MMWR 2020 Apr 6;69(early release):1-5.
FROM MMWR
First advance in MDS for decade: Luspatercept for anemia
The US Food and Drug Administration has approved luspatercept (Reblozyl, Bristol-Myers Squibb/Acceleron) for the treatment of anemia in patients with myelodysplastic syndromes (MDS).
The green light represents the first treatment advancement in MDS in more than a decade, says an expert in the field.
Luspatercept is the first and so far only erythroid maturation agent (EMA), and was launched last year when it was approved for the treatment of anemia in adults with beta thalassemia, who require regular red blood cell transfusions.
The new approval is for the treatment of anemia in adult patients with very low- to intermediate-risk MDS with ring sideroblasts and patients with myelodysplastic/myeloproliferative neoplasm with ring sideroblasts and thrombocytosis, after they have progressed on treatment with an erythropoiesis-stimulating agent and who require two or more red blood cell (RBC) units over 8 weeks.
Luspatercept is not a substitute for RBC transfusions in patients who require immediate correction of anemia.
The FDA approval in MDS is based on results from the pivotal, placebo-controlled, phase 3 MEDALIST trial, conducted in 229 patients with very-low–, low- and intermediate-risk non-del(5q) MDS with ring sideroblasts. All patients were RBC transfusion-dependent and had disease that was refractory to, or unlikely to respond to, erythropoiesis-stimulating agents. Results were published in January in the New England Journal of Medicine. The study was funded by Acceleron Pharma and Celgene, which was later acquired by Bristol-Myers Squibb.
These results were first presented at the 2018 annual meeting of the American Society of Hematology (ASH), as reported by Medscape Medical News. At the time, ASH President Alexis Thompson, MD, said it appears that luspatercept can improve the production of endogenous RBCs by enhancing the maturation of these cells in the bone marrow. The drug significantly reduced the need for RBC transfusions, and “this is a very exciting advance for patients who would have few other treatment options,” she said.
“Anemia and the chronic need for transfusions is a very big issue for these patients,” commented lead study author Pierre Fenaux, MD, PhD, from Hôpital Saint-Louis in Paris, France. “With low hemoglobin levels, patients are tired all the time and have an increased risk of falls and cardiovascular events. When you can improve hemoglobin levels, you really see a difference in quality of life.”
The MEDALIST trial is an important milestone for patients with lower-risk, transfusion-dependent MDS, commented Elizabeth Griffiths, MD, associate professor of oncology and director of MDS, Roswell Park Comprehensive Cancer Center, Buffalo, New York.
“No new agents have been approved for MDS in the last 10 years, highlighting this development as a substantial step forward for the MDS community,” she told Medscape Medical News. “Current therapies are time-intensive and only modestly beneficial.”
“The availability of a new, effective drug — particularly relevant to those harboring SF3B1 mutations — is an exciting development and is likely to offer meaningful improvements in quality of life,” Griffiths said. “Since these patients tend to live longer than others with MDS, there are many patients in my clinical practice who would have fit the enrollment criteria for this study. Such patients are eagerly awaiting the opportunity for a decrease in transfusion burden.”
Study Details
In the trial, luspatercept reduced the severity of anemia — 38% of the 153 patients who received luspatercept achieved transfusion independence for 8 weeks or longer compared with 13% of the 76 patients receiving placebo (P < .001).
In the study, patients received luspatercept at a starting dose of 1.0 mg/kg with titration up to 1.75 mg/kg, if needed, or placebo, subcutaneously every 3 weeks for at least 24 weeks.
During the 16 weeks before the initiation of treatment, study patients had received a median of 5 RBC units transfusions during an 8-week period (43.2% of patients had ≥ 6 RBC units, 27.9% had ≥ 4 to < 6 RBC units, and 28.8% had < 4 RBC units). At baseline, 138 (60.3%), 58 (25.3%), and 32 (14%) patients had serum erythropoietin levels less than 200 IU/L, 200-500 IU/L, and greater than 500 IU/L, respectively.
The most common luspatercept-associated adverse events (any grade) in the trial were fatigue, diarrhea, asthenia, nausea, and dizziness. Grade 3 or 4 treatment-emergent adverse events were reported in 42.5% of patients who received luspatercept and 44.7% of patients who received placebo. The incidence of adverse events decreased over time, according to the study authors.
This article first appeared on Medscape.com.
The US Food and Drug Administration has approved luspatercept (Reblozyl, Bristol-Myers Squibb/Acceleron) for the treatment of anemia in patients with myelodysplastic syndromes (MDS).
The green light represents the first treatment advancement in MDS in more than a decade, says an expert in the field.
Luspatercept is the first and so far only erythroid maturation agent (EMA), and was launched last year when it was approved for the treatment of anemia in adults with beta thalassemia, who require regular red blood cell transfusions.
The new approval is for the treatment of anemia in adult patients with very low- to intermediate-risk MDS with ring sideroblasts and patients with myelodysplastic/myeloproliferative neoplasm with ring sideroblasts and thrombocytosis, after they have progressed on treatment with an erythropoiesis-stimulating agent and who require two or more red blood cell (RBC) units over 8 weeks.
Luspatercept is not a substitute for RBC transfusions in patients who require immediate correction of anemia.
The FDA approval in MDS is based on results from the pivotal, placebo-controlled, phase 3 MEDALIST trial, conducted in 229 patients with very-low–, low- and intermediate-risk non-del(5q) MDS with ring sideroblasts. All patients were RBC transfusion-dependent and had disease that was refractory to, or unlikely to respond to, erythropoiesis-stimulating agents. Results were published in January in the New England Journal of Medicine. The study was funded by Acceleron Pharma and Celgene, which was later acquired by Bristol-Myers Squibb.
These results were first presented at the 2018 annual meeting of the American Society of Hematology (ASH), as reported by Medscape Medical News. At the time, ASH President Alexis Thompson, MD, said it appears that luspatercept can improve the production of endogenous RBCs by enhancing the maturation of these cells in the bone marrow. The drug significantly reduced the need for RBC transfusions, and “this is a very exciting advance for patients who would have few other treatment options,” she said.
“Anemia and the chronic need for transfusions is a very big issue for these patients,” commented lead study author Pierre Fenaux, MD, PhD, from Hôpital Saint-Louis in Paris, France. “With low hemoglobin levels, patients are tired all the time and have an increased risk of falls and cardiovascular events. When you can improve hemoglobin levels, you really see a difference in quality of life.”
The MEDALIST trial is an important milestone for patients with lower-risk, transfusion-dependent MDS, commented Elizabeth Griffiths, MD, associate professor of oncology and director of MDS, Roswell Park Comprehensive Cancer Center, Buffalo, New York.
“No new agents have been approved for MDS in the last 10 years, highlighting this development as a substantial step forward for the MDS community,” she told Medscape Medical News. “Current therapies are time-intensive and only modestly beneficial.”
“The availability of a new, effective drug — particularly relevant to those harboring SF3B1 mutations — is an exciting development and is likely to offer meaningful improvements in quality of life,” Griffiths said. “Since these patients tend to live longer than others with MDS, there are many patients in my clinical practice who would have fit the enrollment criteria for this study. Such patients are eagerly awaiting the opportunity for a decrease in transfusion burden.”
Study Details
In the trial, luspatercept reduced the severity of anemia — 38% of the 153 patients who received luspatercept achieved transfusion independence for 8 weeks or longer compared with 13% of the 76 patients receiving placebo (P < .001).
In the study, patients received luspatercept at a starting dose of 1.0 mg/kg with titration up to 1.75 mg/kg, if needed, or placebo, subcutaneously every 3 weeks for at least 24 weeks.
During the 16 weeks before the initiation of treatment, study patients had received a median of 5 RBC units transfusions during an 8-week period (43.2% of patients had ≥ 6 RBC units, 27.9% had ≥ 4 to < 6 RBC units, and 28.8% had < 4 RBC units). At baseline, 138 (60.3%), 58 (25.3%), and 32 (14%) patients had serum erythropoietin levels less than 200 IU/L, 200-500 IU/L, and greater than 500 IU/L, respectively.
The most common luspatercept-associated adverse events (any grade) in the trial were fatigue, diarrhea, asthenia, nausea, and dizziness. Grade 3 or 4 treatment-emergent adverse events were reported in 42.5% of patients who received luspatercept and 44.7% of patients who received placebo. The incidence of adverse events decreased over time, according to the study authors.
This article first appeared on Medscape.com.
The US Food and Drug Administration has approved luspatercept (Reblozyl, Bristol-Myers Squibb/Acceleron) for the treatment of anemia in patients with myelodysplastic syndromes (MDS).
The green light represents the first treatment advancement in MDS in more than a decade, says an expert in the field.
Luspatercept is the first and so far only erythroid maturation agent (EMA), and was launched last year when it was approved for the treatment of anemia in adults with beta thalassemia, who require regular red blood cell transfusions.
The new approval is for the treatment of anemia in adult patients with very low- to intermediate-risk MDS with ring sideroblasts and patients with myelodysplastic/myeloproliferative neoplasm with ring sideroblasts and thrombocytosis, after they have progressed on treatment with an erythropoiesis-stimulating agent and who require two or more red blood cell (RBC) units over 8 weeks.
Luspatercept is not a substitute for RBC transfusions in patients who require immediate correction of anemia.
The FDA approval in MDS is based on results from the pivotal, placebo-controlled, phase 3 MEDALIST trial, conducted in 229 patients with very-low–, low- and intermediate-risk non-del(5q) MDS with ring sideroblasts. All patients were RBC transfusion-dependent and had disease that was refractory to, or unlikely to respond to, erythropoiesis-stimulating agents. Results were published in January in the New England Journal of Medicine. The study was funded by Acceleron Pharma and Celgene, which was later acquired by Bristol-Myers Squibb.
These results were first presented at the 2018 annual meeting of the American Society of Hematology (ASH), as reported by Medscape Medical News. At the time, ASH President Alexis Thompson, MD, said it appears that luspatercept can improve the production of endogenous RBCs by enhancing the maturation of these cells in the bone marrow. The drug significantly reduced the need for RBC transfusions, and “this is a very exciting advance for patients who would have few other treatment options,” she said.
“Anemia and the chronic need for transfusions is a very big issue for these patients,” commented lead study author Pierre Fenaux, MD, PhD, from Hôpital Saint-Louis in Paris, France. “With low hemoglobin levels, patients are tired all the time and have an increased risk of falls and cardiovascular events. When you can improve hemoglobin levels, you really see a difference in quality of life.”
The MEDALIST trial is an important milestone for patients with lower-risk, transfusion-dependent MDS, commented Elizabeth Griffiths, MD, associate professor of oncology and director of MDS, Roswell Park Comprehensive Cancer Center, Buffalo, New York.
“No new agents have been approved for MDS in the last 10 years, highlighting this development as a substantial step forward for the MDS community,” she told Medscape Medical News. “Current therapies are time-intensive and only modestly beneficial.”
“The availability of a new, effective drug — particularly relevant to those harboring SF3B1 mutations — is an exciting development and is likely to offer meaningful improvements in quality of life,” Griffiths said. “Since these patients tend to live longer than others with MDS, there are many patients in my clinical practice who would have fit the enrollment criteria for this study. Such patients are eagerly awaiting the opportunity for a decrease in transfusion burden.”
Study Details
In the trial, luspatercept reduced the severity of anemia — 38% of the 153 patients who received luspatercept achieved transfusion independence for 8 weeks or longer compared with 13% of the 76 patients receiving placebo (P < .001).
In the study, patients received luspatercept at a starting dose of 1.0 mg/kg with titration up to 1.75 mg/kg, if needed, or placebo, subcutaneously every 3 weeks for at least 24 weeks.
During the 16 weeks before the initiation of treatment, study patients had received a median of 5 RBC units transfusions during an 8-week period (43.2% of patients had ≥ 6 RBC units, 27.9% had ≥ 4 to < 6 RBC units, and 28.8% had < 4 RBC units). At baseline, 138 (60.3%), 58 (25.3%), and 32 (14%) patients had serum erythropoietin levels less than 200 IU/L, 200-500 IU/L, and greater than 500 IU/L, respectively.
The most common luspatercept-associated adverse events (any grade) in the trial were fatigue, diarrhea, asthenia, nausea, and dizziness. Grade 3 or 4 treatment-emergent adverse events were reported in 42.5% of patients who received luspatercept and 44.7% of patients who received placebo. The incidence of adverse events decreased over time, according to the study authors.
This article first appeared on Medscape.com.
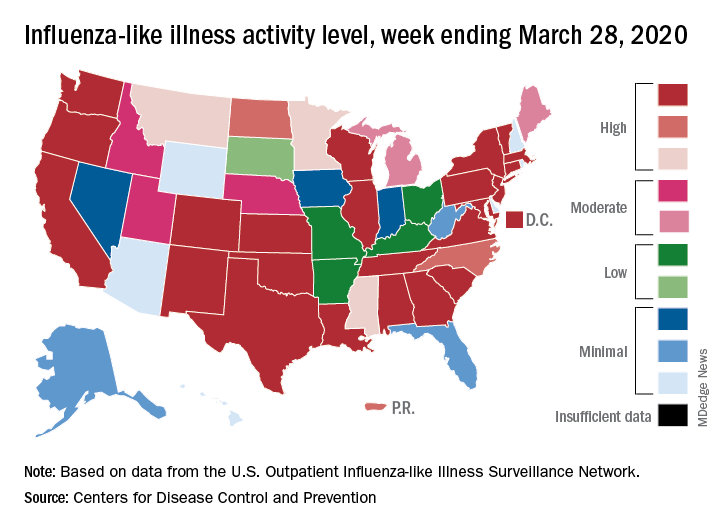
Flu activity down from its third peak of the season, COVID-19 still a factor
Influenza activity measures dropped during the week ending March 28, but the percentage of deaths attributed to pneumonia and influenza (P&I) has risen into epidemic territory, according to the Centers for Disease Control and Prevention.
This influenza news, however, needs to be viewed through a COVID-19 lens.
The P&I mortality data are reported together and are always a week behind the other measures, in this case covering the week ending March 21, but they show influenza deaths dropping to 0.8% as the overall P&I rate rose from 7.4% to 8.2%, a pneumonia-fueled increase that was “likely associated with COVID-19 rather than influenza,” the CDC’s influenza division noted.
The two main activity measures, at least, are on the same page for the first time since the end of February.
The rate of outpatient visits for influenza-like illness (ILI) had been dropping up to that point but then rose for an unprecedented third time this season, a change probably brought about by COVID-related health care–seeking behavior, the influenza division reported in its weekly FluView report.
This corresponding third drop in ILI activity brought the rate down to 5.4% this week from 6.2% the previous week, the CDC reported. The two previous high points occurred during the weeks ending Dec. 28 (7.0%) and Feb. 8 (6.7%)
The COVID-related changes, such as increased use of telemedicine and social distancing, “impact data from [the Outpatient Influenza-Like Illness Surveillance Network] in ways that are difficult to differentiate from changes in illness levels and should be interpreted with caution,” the CDC investigators noted.
The other activity measure, positive tests of respiratory specimens for influenza at clinical laboratories, continued the decline that started in mid-February by falling from 7.3% to 2.1%, its lowest rate since October, CDC data show.
Overall flu-related deaths may be down, but mortality in children continued at a near-record level. Seven such deaths were reported this past week, which brings the total for the 2019-2020 season to 162. “This number is higher than recorded at the same time in every season since reporting began in 2004-05, except for the 2009 pandemic,” the CDC noted.
Influenza activity measures dropped during the week ending March 28, but the percentage of deaths attributed to pneumonia and influenza (P&I) has risen into epidemic territory, according to the Centers for Disease Control and Prevention.
This influenza news, however, needs to be viewed through a COVID-19 lens.
The P&I mortality data are reported together and are always a week behind the other measures, in this case covering the week ending March 21, but they show influenza deaths dropping to 0.8% as the overall P&I rate rose from 7.4% to 8.2%, a pneumonia-fueled increase that was “likely associated with COVID-19 rather than influenza,” the CDC’s influenza division noted.
The two main activity measures, at least, are on the same page for the first time since the end of February.
The rate of outpatient visits for influenza-like illness (ILI) had been dropping up to that point but then rose for an unprecedented third time this season, a change probably brought about by COVID-related health care–seeking behavior, the influenza division reported in its weekly FluView report.
This corresponding third drop in ILI activity brought the rate down to 5.4% this week from 6.2% the previous week, the CDC reported. The two previous high points occurred during the weeks ending Dec. 28 (7.0%) and Feb. 8 (6.7%)
The COVID-related changes, such as increased use of telemedicine and social distancing, “impact data from [the Outpatient Influenza-Like Illness Surveillance Network] in ways that are difficult to differentiate from changes in illness levels and should be interpreted with caution,” the CDC investigators noted.
The other activity measure, positive tests of respiratory specimens for influenza at clinical laboratories, continued the decline that started in mid-February by falling from 7.3% to 2.1%, its lowest rate since October, CDC data show.
Overall flu-related deaths may be down, but mortality in children continued at a near-record level. Seven such deaths were reported this past week, which brings the total for the 2019-2020 season to 162. “This number is higher than recorded at the same time in every season since reporting began in 2004-05, except for the 2009 pandemic,” the CDC noted.
Influenza activity measures dropped during the week ending March 28, but the percentage of deaths attributed to pneumonia and influenza (P&I) has risen into epidemic territory, according to the Centers for Disease Control and Prevention.
This influenza news, however, needs to be viewed through a COVID-19 lens.
The P&I mortality data are reported together and are always a week behind the other measures, in this case covering the week ending March 21, but they show influenza deaths dropping to 0.8% as the overall P&I rate rose from 7.4% to 8.2%, a pneumonia-fueled increase that was “likely associated with COVID-19 rather than influenza,” the CDC’s influenza division noted.
The two main activity measures, at least, are on the same page for the first time since the end of February.
The rate of outpatient visits for influenza-like illness (ILI) had been dropping up to that point but then rose for an unprecedented third time this season, a change probably brought about by COVID-related health care–seeking behavior, the influenza division reported in its weekly FluView report.
This corresponding third drop in ILI activity brought the rate down to 5.4% this week from 6.2% the previous week, the CDC reported. The two previous high points occurred during the weeks ending Dec. 28 (7.0%) and Feb. 8 (6.7%)
The COVID-related changes, such as increased use of telemedicine and social distancing, “impact data from [the Outpatient Influenza-Like Illness Surveillance Network] in ways that are difficult to differentiate from changes in illness levels and should be interpreted with caution,” the CDC investigators noted.
The other activity measure, positive tests of respiratory specimens for influenza at clinical laboratories, continued the decline that started in mid-February by falling from 7.3% to 2.1%, its lowest rate since October, CDC data show.
Overall flu-related deaths may be down, but mortality in children continued at a near-record level. Seven such deaths were reported this past week, which brings the total for the 2019-2020 season to 162. “This number is higher than recorded at the same time in every season since reporting began in 2004-05, except for the 2009 pandemic,” the CDC noted.
COVID-19 transmission can occur before symptom onset
based on clinical and epidemiologic data for all cases reported in the country by March 16.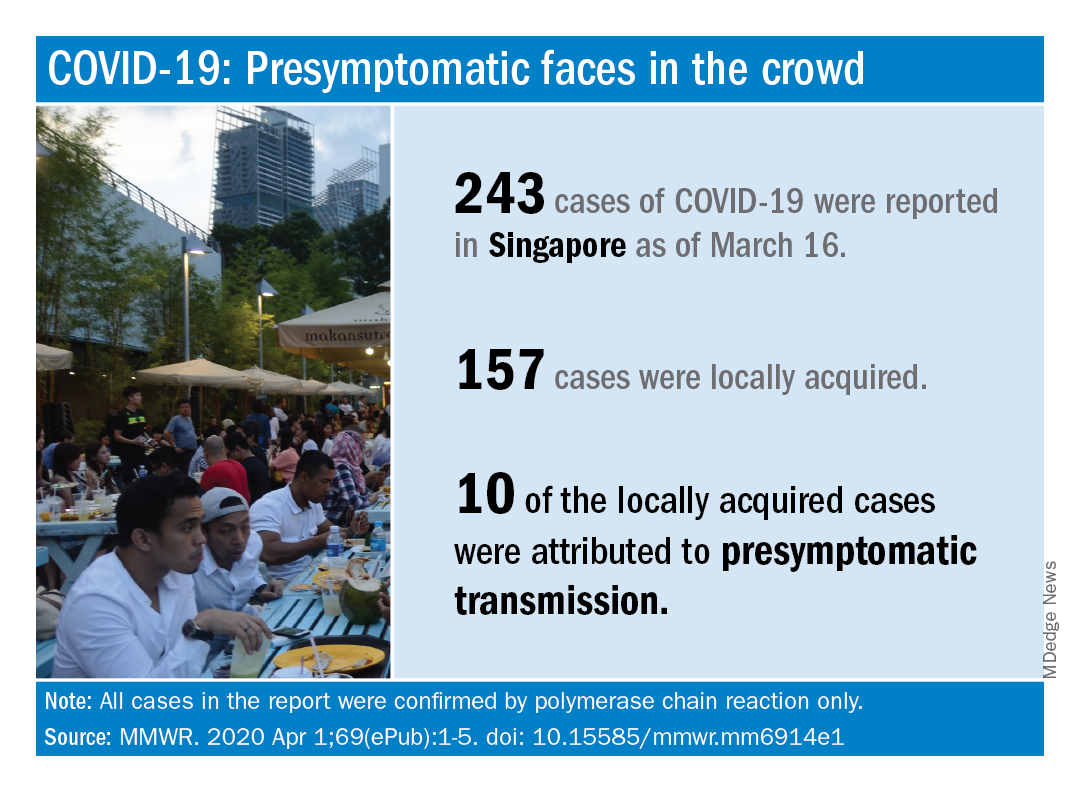
As of that date, there had been 243 cases of COVID-19, of which 157 were locally acquired. Among those 157 were 10 cases (6.4%) that involved probable presymptomatic transmission, Wycliffe E. Wei, MPH, and associates said April 1 in the Morbidity and Mortality Weekly Report.
They defined presymptomatic transmission “as the transmission of SARS-CoV-2 from an infected person (source patient) to a secondary patient before the source patient developed symptoms, as ascertained by exposure and symptom onset dates, with no evidence that the secondary patient had been exposed to anyone else with COVID-19.”
Investigation of all 243 cases in Singapore identified seven clusters, each involving two to five patients, as sources of presymptomatic transmission. In four of the clusters, the “exposure occurred 1-3 days before the source patient developed symptoms,” said Mr. Wei of the Singapore Ministry of Health and associates.
These findings, along with evidence from Chinese studies – one of which reported presymptomatic transmission in 12.6% of cases – support “the likelihood that viral shedding can occur in the absence of symptoms and before symptom onset,” they said.
SOURCE: Wei WE et al. MMWR. 2020 Apr 1;69(ePub):1-5. doi: 10.15585/mmwr.mm6914e1.
based on clinical and epidemiologic data for all cases reported in the country by March 16.
As of that date, there had been 243 cases of COVID-19, of which 157 were locally acquired. Among those 157 were 10 cases (6.4%) that involved probable presymptomatic transmission, Wycliffe E. Wei, MPH, and associates said April 1 in the Morbidity and Mortality Weekly Report.
They defined presymptomatic transmission “as the transmission of SARS-CoV-2 from an infected person (source patient) to a secondary patient before the source patient developed symptoms, as ascertained by exposure and symptom onset dates, with no evidence that the secondary patient had been exposed to anyone else with COVID-19.”
Investigation of all 243 cases in Singapore identified seven clusters, each involving two to five patients, as sources of presymptomatic transmission. In four of the clusters, the “exposure occurred 1-3 days before the source patient developed symptoms,” said Mr. Wei of the Singapore Ministry of Health and associates.
These findings, along with evidence from Chinese studies – one of which reported presymptomatic transmission in 12.6% of cases – support “the likelihood that viral shedding can occur in the absence of symptoms and before symptom onset,” they said.
SOURCE: Wei WE et al. MMWR. 2020 Apr 1;69(ePub):1-5. doi: 10.15585/mmwr.mm6914e1.
based on clinical and epidemiologic data for all cases reported in the country by March 16.
As of that date, there had been 243 cases of COVID-19, of which 157 were locally acquired. Among those 157 were 10 cases (6.4%) that involved probable presymptomatic transmission, Wycliffe E. Wei, MPH, and associates said April 1 in the Morbidity and Mortality Weekly Report.
They defined presymptomatic transmission “as the transmission of SARS-CoV-2 from an infected person (source patient) to a secondary patient before the source patient developed symptoms, as ascertained by exposure and symptom onset dates, with no evidence that the secondary patient had been exposed to anyone else with COVID-19.”
Investigation of all 243 cases in Singapore identified seven clusters, each involving two to five patients, as sources of presymptomatic transmission. In four of the clusters, the “exposure occurred 1-3 days before the source patient developed symptoms,” said Mr. Wei of the Singapore Ministry of Health and associates.
These findings, along with evidence from Chinese studies – one of which reported presymptomatic transmission in 12.6% of cases – support “the likelihood that viral shedding can occur in the absence of symptoms and before symptom onset,” they said.
SOURCE: Wei WE et al. MMWR. 2020 Apr 1;69(ePub):1-5. doi: 10.15585/mmwr.mm6914e1.
FROM MMWR
Comorbidities more common in hospitalized COVID-19 patients
Greater prevalence of underlying health conditions such as diabetes and chronic lung disease was seen among nearly 7,200 Americans hospitalized with coronavirus disease 2019 (COVID-19), according to the Centers for Disease Control and Prevention.
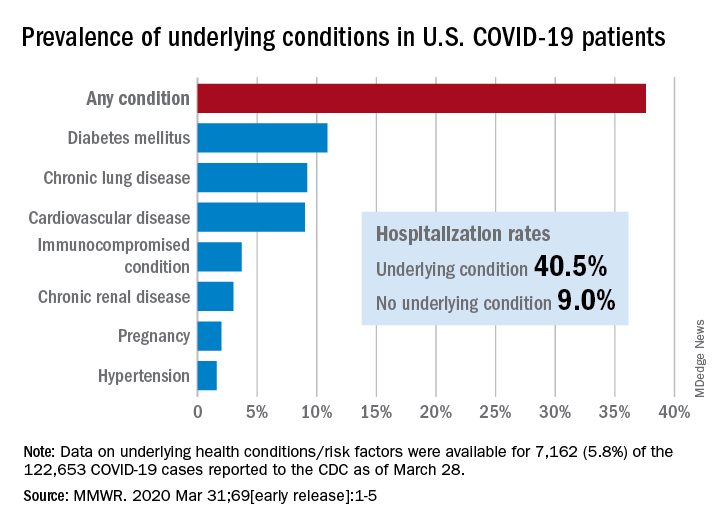
Of the 122,653 laboratory-confirmed COVID-19 cases reported to the CDC as of March 28, the COVID-19 Response Team had access to data on the presence or absence of underlying health conditions and other recognized risk factors for severe outcomes from respiratory infections for 7,162 (5.8%) patients.
“Among these patients, higher percentages of patients with underlying conditions were admitted to the hospital and to an ICU than patients without reported underlying conditions. These results are consistent with findings from China and Italy,” Katherine Fleming-Dutra, MD, and associates said in the MMWR.
Individuals with underlying health conditions/risk factors made up 37.6% of all COVID-19 patients in the study but represented a majority of ICU (78%) and non-ICU (71%) hospital admissions. In contrast, 73% of COVID-19 patients who were not hospitalized had no underlying conditions, Dr. Fleming-Dutra and the CDC COVID-19 Response Team reported.
With a prevalence of 10.9%, diabetes mellitus was the most common condition reported among all COVID-19 patients, followed by chronic lung disease (9.2%) and cardiovascular disease (9.0%), the investigators said.
Another look at the data shows that 40.5% of those with underlying conditions were hospitalized, compared with 9.0% of the 4,470 COVID-19 patients without any risk factors.
“Strategies to protect all persons and especially those with underlying health conditions, including social distancing and handwashing, should be implemented by all communities and all persons to help slow the spread of COVID-19,” the response team wrote.
SOURCE: Fleming-Dutra K et al. MMWR. 2020 Mar 31;69 (early release):1-5.
Greater prevalence of underlying health conditions such as diabetes and chronic lung disease was seen among nearly 7,200 Americans hospitalized with coronavirus disease 2019 (COVID-19), according to the Centers for Disease Control and Prevention.

Of the 122,653 laboratory-confirmed COVID-19 cases reported to the CDC as of March 28, the COVID-19 Response Team had access to data on the presence or absence of underlying health conditions and other recognized risk factors for severe outcomes from respiratory infections for 7,162 (5.8%) patients.
“Among these patients, higher percentages of patients with underlying conditions were admitted to the hospital and to an ICU than patients without reported underlying conditions. These results are consistent with findings from China and Italy,” Katherine Fleming-Dutra, MD, and associates said in the MMWR.
Individuals with underlying health conditions/risk factors made up 37.6% of all COVID-19 patients in the study but represented a majority of ICU (78%) and non-ICU (71%) hospital admissions. In contrast, 73% of COVID-19 patients who were not hospitalized had no underlying conditions, Dr. Fleming-Dutra and the CDC COVID-19 Response Team reported.
With a prevalence of 10.9%, diabetes mellitus was the most common condition reported among all COVID-19 patients, followed by chronic lung disease (9.2%) and cardiovascular disease (9.0%), the investigators said.
Another look at the data shows that 40.5% of those with underlying conditions were hospitalized, compared with 9.0% of the 4,470 COVID-19 patients without any risk factors.
“Strategies to protect all persons and especially those with underlying health conditions, including social distancing and handwashing, should be implemented by all communities and all persons to help slow the spread of COVID-19,” the response team wrote.
SOURCE: Fleming-Dutra K et al. MMWR. 2020 Mar 31;69 (early release):1-5.
Greater prevalence of underlying health conditions such as diabetes and chronic lung disease was seen among nearly 7,200 Americans hospitalized with coronavirus disease 2019 (COVID-19), according to the Centers for Disease Control and Prevention.

Of the 122,653 laboratory-confirmed COVID-19 cases reported to the CDC as of March 28, the COVID-19 Response Team had access to data on the presence or absence of underlying health conditions and other recognized risk factors for severe outcomes from respiratory infections for 7,162 (5.8%) patients.
“Among these patients, higher percentages of patients with underlying conditions were admitted to the hospital and to an ICU than patients without reported underlying conditions. These results are consistent with findings from China and Italy,” Katherine Fleming-Dutra, MD, and associates said in the MMWR.
Individuals with underlying health conditions/risk factors made up 37.6% of all COVID-19 patients in the study but represented a majority of ICU (78%) and non-ICU (71%) hospital admissions. In contrast, 73% of COVID-19 patients who were not hospitalized had no underlying conditions, Dr. Fleming-Dutra and the CDC COVID-19 Response Team reported.
With a prevalence of 10.9%, diabetes mellitus was the most common condition reported among all COVID-19 patients, followed by chronic lung disease (9.2%) and cardiovascular disease (9.0%), the investigators said.
Another look at the data shows that 40.5% of those with underlying conditions were hospitalized, compared with 9.0% of the 4,470 COVID-19 patients without any risk factors.
“Strategies to protect all persons and especially those with underlying health conditions, including social distancing and handwashing, should be implemented by all communities and all persons to help slow the spread of COVID-19,” the response team wrote.
SOURCE: Fleming-Dutra K et al. MMWR. 2020 Mar 31;69 (early release):1-5.
FROM MMWR
FDA calls for market removal of ranitidine
A problem with both branded and generic over-the-counter and prescription forms, from the market.
The NDMA contamination does not stem from a manufacturing concern, but rather the levels have been found to increase over time depending on how the ranitidine is stored.
In particular, the FDA found through product testing that the NDMA impurity developed over time when the ranitidine was stored above room temperature.
“The testing also showed that the older a ranitidine product is, or the longer the length of time since it was manufactured, the greater the level of NDMA,” FDA said in a statement announcing the call for product withdrawal.
The FDA has been investigating NDMA contamination since September 2019 when the agency first announced the contamination in ranitidine. Manufacturers have been withdrawing their products from the market since the first reports of contamination surfaced. Despite these recalls, there were still ranitidine products on the market, according to an FDA spokesperson, necessitating the further action taken by the agency.
In addition to products being removed from the market, FDA is asking consumers to discard any ranitidine products they may have.
“There are still questions about how the impurity is formed in ranitidine over time during storage,” Janet Woodcock, MD, director of the FDA Center for Drug Evaluation and Research, said during an April 1 conference call with reporters announcing the withdrawal request. “For example, what impact does the drug packaging have on the development or the specific formulation have on the development of NDMA.”
She said the issue may be fixable over time, and the agency is open to reformulations that demonstrate that ranitidine is stable over time and under various storage conditions.
Dr. Woodcock stressed that the products at the point of manufacture do not have unacceptable levels of NDMA.
“This is a market withdrawal, this is not a recall because technically the products are okay. They met all their specs,” she said. “It is only when they are subjected generally to heat stress do they manifest higher levels” of NDMA.
“Clearly, we can’t have products on the market that if they are stored under conditions consumers might store them under that they would become unacceptable.”
Dr. Woodcock said FDA is not withdrawing approvals for the products, but manufacturers would need to show the product remains stable under normal storage conditions.
This article was updated 4/7/20.
A problem with both branded and generic over-the-counter and prescription forms, from the market.
The NDMA contamination does not stem from a manufacturing concern, but rather the levels have been found to increase over time depending on how the ranitidine is stored.
In particular, the FDA found through product testing that the NDMA impurity developed over time when the ranitidine was stored above room temperature.
“The testing also showed that the older a ranitidine product is, or the longer the length of time since it was manufactured, the greater the level of NDMA,” FDA said in a statement announcing the call for product withdrawal.
The FDA has been investigating NDMA contamination since September 2019 when the agency first announced the contamination in ranitidine. Manufacturers have been withdrawing their products from the market since the first reports of contamination surfaced. Despite these recalls, there were still ranitidine products on the market, according to an FDA spokesperson, necessitating the further action taken by the agency.
In addition to products being removed from the market, FDA is asking consumers to discard any ranitidine products they may have.
“There are still questions about how the impurity is formed in ranitidine over time during storage,” Janet Woodcock, MD, director of the FDA Center for Drug Evaluation and Research, said during an April 1 conference call with reporters announcing the withdrawal request. “For example, what impact does the drug packaging have on the development or the specific formulation have on the development of NDMA.”
She said the issue may be fixable over time, and the agency is open to reformulations that demonstrate that ranitidine is stable over time and under various storage conditions.
Dr. Woodcock stressed that the products at the point of manufacture do not have unacceptable levels of NDMA.
“This is a market withdrawal, this is not a recall because technically the products are okay. They met all their specs,” she said. “It is only when they are subjected generally to heat stress do they manifest higher levels” of NDMA.
“Clearly, we can’t have products on the market that if they are stored under conditions consumers might store them under that they would become unacceptable.”
Dr. Woodcock said FDA is not withdrawing approvals for the products, but manufacturers would need to show the product remains stable under normal storage conditions.
This article was updated 4/7/20.
A problem with both branded and generic over-the-counter and prescription forms, from the market.
The NDMA contamination does not stem from a manufacturing concern, but rather the levels have been found to increase over time depending on how the ranitidine is stored.
In particular, the FDA found through product testing that the NDMA impurity developed over time when the ranitidine was stored above room temperature.
“The testing also showed that the older a ranitidine product is, or the longer the length of time since it was manufactured, the greater the level of NDMA,” FDA said in a statement announcing the call for product withdrawal.
The FDA has been investigating NDMA contamination since September 2019 when the agency first announced the contamination in ranitidine. Manufacturers have been withdrawing their products from the market since the first reports of contamination surfaced. Despite these recalls, there were still ranitidine products on the market, according to an FDA spokesperson, necessitating the further action taken by the agency.
In addition to products being removed from the market, FDA is asking consumers to discard any ranitidine products they may have.
“There are still questions about how the impurity is formed in ranitidine over time during storage,” Janet Woodcock, MD, director of the FDA Center for Drug Evaluation and Research, said during an April 1 conference call with reporters announcing the withdrawal request. “For example, what impact does the drug packaging have on the development or the specific formulation have on the development of NDMA.”
She said the issue may be fixable over time, and the agency is open to reformulations that demonstrate that ranitidine is stable over time and under various storage conditions.
Dr. Woodcock stressed that the products at the point of manufacture do not have unacceptable levels of NDMA.
“This is a market withdrawal, this is not a recall because technically the products are okay. They met all their specs,” she said. “It is only when they are subjected generally to heat stress do they manifest higher levels” of NDMA.
“Clearly, we can’t have products on the market that if they are stored under conditions consumers might store them under that they would become unacceptable.”
Dr. Woodcock said FDA is not withdrawing approvals for the products, but manufacturers would need to show the product remains stable under normal storage conditions.
This article was updated 4/7/20.