User login
Cancer risk assessment from family history: Gaps in primary care practice
OBJECTIVE: To determine whether an adequate amount of family history is being collected and recorded by family practitioners to appropriately identify patients at increased risk for cancer.
STUDY DESIGN: Retrospective chart audit.
POPULATION: Charts from 500 randomly chosen patients, 40 to 60 years of age, were audited. Of those charts, 400 were from a large academic family practice and 50 charts each were from 2 small community family practices in the greater Philadelphia area.
OUTCOMES MEASURED: General features of family history taking were recorded, including presence of a family history and date when recorded, evidence of updated family history data, and presence of a genogram. Cancer features recorded included mention of family history of cancer or colon polyps and, if positive, identification of which relative was affected, site of cancer, and age of diagnosis or death.
RESULTS: Most charts (89%) had some family history information recorded, and 55% listed a family history of cancer, either positive or negative. Of the 356 relatives affected with cancer, an age of diagnosis was documented in only 8%; of 183 first-degree relatives with cancer, only 7% had a documented age of diagnosis. Two percent of all charts had any mention of a family history of colon polyps. Sixty-five percent of family histories were recorded at the first visit, and only 35% had any updated family history information.
CONCLUSIONS: The number and type of family histories currently being recorded by family practitioners are not adequate to fully assess familial risk of cancer. New strategies will need to be developed to better prepare providers for risk-based clinical decision making.
Taking a family history is a significant component of providing comprehensive primary care because family history provides key psychosocial and medical risk information.1,2 As our understanding of the genetic basis for disease grows, obtaining an accurate and complete family history is likely to gain increasing relevance as a vital source of data to guide counseling and testing. Patients who have a first-degree relative with a colon neoplasm or prostate cancer are advised to screen differently from those who do not.3 Failure to gather accurate or complete family data prevents the clinician from providing advice that is consistent with screening guidelines. Understanding how primary care clinicians gather family history data is necessary to identify gaps in current performance and to develop strategies to bridge these gaps.
Several studies have used physician self-report to assess the current level of taking a family history in primary care. In 1 study, 90% of surveyed physicians stated that they obtain a family history of cancer from their patients, with 77% to 80% inquiring about a family history of colorectal cancer in their patients who are at least 40 years of age.3 In another study, 63% to 85% of responding physicians reported obtaining a family history of cancer from 76% to 100% of their patients.5 Respondents in 1 study reported obtaining family histories of colorectal cancer in only 30.7% of patients, breast cancer in 48.4% of patients, and coronary disease and hypertension in 94.3% of patients.6 However, data on actual performance of family history taking are sparse.7,8 The physicians in the Direct Observation of Primary Care study obtained a family history during 51% of new visits and 22% of established visits. A genogram was present in 11% of charts, and documentation of a family history of breast cancer or colorectal cancer was found in 40% of charts. Further analysis from this study showed that providers who more frequently obtained and recorded family history information performed more preventive care services for their patients.9
First-degree relatives of cancer patients appear to be interested in and receptive to information about their risk and in the possibility of genetic counseling.10-14 In fact, many patients overestimate their likelihood of getting cancer based on family history10; primary care providers thus have the opportunity to counsel and relieve anxiety in their patients. Family history is an important tool to define risk and guide referral, counseling, and testing.
This article presents the findings of a descriptive study of the review of 500 charts from 3 different family practice offices. We documented general family history components and completeness related to a family history of cancer, including whether enough family history information was collected to appropriately identify patients at increased risk for cancer.
Methods
Data were collected from 500 patient charts from 3 family practice offices in the greater Philadelphia area. Patients who were in the practice for at least 1 year, made a minimum of 2 visits between June 1, 1997 and June 1, 2000, and were between the ages of 40 and 60 years at 1 of those visits were included. Fifty charts were selected by using a random starting point at each of 2 small (1 to 3 providers) private practices, and 400 charts were randomly selected from all eligible charts in a large academic practice (more than 60 providers). The large practice had nearly 1500 patients who fit the selection criteria, and the total population did not differ from the random sample in mean age, sex, or race (P < .05). Total population characteristics were not available for the 2 smaller practices.
Family history data were collected from progress notes and designated family history spaces on flow sheets or chart covers. Family histories consisting only of “none” or “noncontributory” were counted only when they clearly referred to a specific condition. Family births or deaths recorded in a psychosocial context also were not counted as part of the family history unless death from a specific disease was mentioned. The first dated family history was considered to be first for the purpose of this study. Date of birth, race, sex, current primary care provider, if any, and the date first seen in the practice were recorded. The current primary care provider was determined by the physician seen for the majority of recent visits and/or notations in the chart at acute visits. Data collected for the primary care providers included practice site, sex, level of training, and years in practice. Variables collected from family history information included: date of first family history; date of most recent family history; presence of a genogram, presence of a patient-completed family history self-questionnaire; whether any mention was made, positive or negative, of cancer or colon polyps; and whether there was a positive family history of cancer or colon polyps. For individuals with a positive family history of cancer or colon polyps, all details recorded in the chart were abstracted; these included site of cancer or polyp, relationship to patient, age at diagnosis, and age at death. The data were entered into an Access 97 database and stored separately from chart number identifiers. All analyses and tests were done in SAS version 6.12.
Results
Demographic data from the 500 patients whose charts were audited are presented in Table 1. Ninety-seven percent of patients had a primary care provider, which included 60 physicians and 3 nurse practitioners. No significant associations were seen with practice site, sex, or level of training of the provider and the presence of family history information in the chart.
Most patients (89%) had some family history information in the chart and 63% had a genogram. This did not differ by sex or race of the patient. Fifty-seven percent of patients supplied family history information at the first visit to the office; 59% of these patients had no family history data recorded on subsequent visits. Only 31% of charts had updated family history information Table 2. For patients who had been in the same practice for at least 5 years and had some family history in the chart, 20% had some updated information within the past 3 years.
Of the 500 charts, 276 (55%) recorded a family history of cancer, positive or negative. Two hundred fifteen patients (43%) had a positive family history of cancer, with a total of 356 relatives affected. The site of cancer was listed for 88% of all family member cancers, with breast, colon, lung, and prostate being the most common cancer locations. The specific relative was identified in 92% of cases, with most being first (51%) or second (37%) degree. Although degree of relative and cancer location were usually recorded, age at diagnosis was listed for only 8% of affected relatives, and age at death was identified for 19% of relatives with cancer.
For patients with affected first-degree relatives, the group with the greatest clinical significance, primary care providers identified the location of the cancer in 93% of cases but listed the ages at diagnosis and death in only 7% and 31%, respectively Table 3. Only 7 medical records (1.4%) had any mention of a family history of polyps; of these, 5 (1%) were positive. None listed an age at diagnosis. Five patients in our study met the American Society of Clinical Oncology criteria to be evaluated for genetic breast and ovarian cancer syndrome, and no patients met the criteria for hereditary nonpolyposis colon cancer.
The 2 community practices intermittently used patient self-administered medical intake questionnaires. In our sample, 31 of 500 patients (6%) had a questionnaire in the chart. All patients who completed questionnaires had family history data in their charts. Use of a questionnaire was associated with a greater likelihood that the physician recorded the age of diagnosis for a relative with cancer, although this did not reach significance (20% vs 7%).
Discussion
Despite our finding that providers are documenting family histories in most charts, very few are recording the age of diagnosis in relatives diagnosed with cancer. Age of diagnosis plays a critical role in determining screening recommendations and identifying patients with possible genetic syndromes. For example, the Amsterdam criteria used to identify families with hereditary nonpolyposis colon cancer include knowing whether 1 of 3 relatives with colorectal cancer was diagnosed at younger than 50 years. Breast and ovarian cancer syndrome should be suspected when breast and/or ovarian cancer is diagnosed in 2 first-degree relatives younger than 50 years.15
Most physicians obtain family histories at initial visits. If the patient’s initial visit is for symptom- or disease-related care, an opportunity to gather family history data may be lost. New tools to consistently capture comprehensive family history data at this first visit may be beneficial. Patient self-administered intake questionnaires may prove valuable in this respect, but only 6% of charts in our study contained such a questionnaire, so we cannot draw conclusions about its impact. We did observe a trend toward gathering more complete family history data in patients who used a questionnaire.
There are no clear guidelines regarding when to update a family history. We arbitrarily chose 3 years as a reasonable period for primary care providers to explore changes in family history status. Updating at any subsequent visit was recorded for 35% of patient charts in which a family history was initially taken. It is not clear that primary care providers are documenting changes in family history in any systematic way. Opportunistic updating likely occurs when a new diagnosis of serious disease in a close family member produces anxiety, stress, or concern in the patient. The value of updating family history and the ideal interval to reexamine family history are unknown.
Several conditions would need to be met for family history updating to have value. (1) A close relative must have developed an important illness in the interval since the last family history was recorded or the update must discover family information that was previously missed. (2) The illness must have a familial component that affects the estimate of the risk of the identified patient. (3) The clinician would need to be aware of the updated information. (4) The clinician must change recommendations to the patient based on this new information. Discovering a new family history of colonic neoplasm satisfies these conditions. Process measures that have the potential to improve updating include adding an update of family history as an item on a preventive care flow sheet or using periodic self-administered patient questionnaires. Whether adequate improvements in health care would occur to justify these changes in process will need to be studied. If any updating has value, determining the appropriate intervals for systematic updates deserves attention.
Charts in this study included a genogram 63% of the time, a significant increase over the 11% noted in the Direct Observation of Primary Care study.9 This discrepancy may be explained by differences in practice types because 1 study suggested a higher genogram use in academic medical centers than in community practices.16 The genogram has been cited as an attractive and efficient way to document family history,17,18 but over one fourth of the charts that contained family history in our study used the more cumbersome narrative form. Many geneticists predict that our ability to apply genetic testing will grow dramatically over the next decade. Optimal application of this new knowledge will rely on the health care system’s capacity to accurately identify risk based on assessment of family history. The 3-generation pedigree is likely to be a key tool in finding individuals who may benefit from testing. However, there is currently no standardized education in family history taking in many undergraduate and graduate medical education programs.19
Although patients with a first-degree relative with a history of polyps diagnosed at younger than 60 years are considered to be at increased risk for colorectal cancer,3 providers infrequently asked about a family history of polyps. This may reflect a recent finding that only 36% of primary care providers recommend screening at the age of 40 years for their patients with a family history of polyps in relatives younger than 60 years.20 In fact, family history data do not consistently influence behavior: in the same study, gastroenterologists asked about a family history of polyps 93% of the time, but only 37% recommended earlier screening in those with such a history.
The study is limited in its use of only 3 primary care practices, 1 of which was a large academic family practice. However, because the charts of 63 different clinicians were represented, a range of educational backgrounds and personal philosophies toward family history taking was included. Most patients (97%) had a clearly identified primary care provider and patients had been members of the practice for an average of 7.6 years. The sample was specifically chosen to review the charts of individuals who had been enrolled in the same practice for at least 1 year. This may explain the higher rates of family history taking found in this study compared with previously published studies. Given that the vast majority of charts did contain some family history, it is even more compelling that age at diagnosis of cancer was inadequately recorded. This study reflected only what was documented in the patient chart and not direct observation of physician behavior regarding the family history. It is likely that physicians are not recording all responses to inquiries about family history, although the extent of this underreporting is unknown.
CONCLUSION
Findings in this chart review study are consistent with previous work showing that the quantity and type of family history currently being recorded in primary care charts are not adequate to fully assess familial risk. Bridging the gap between recommendations and actual practice will demand interventions to alter primary care practice or the introduction of new models to gather and analyze family data. Further research is also needed to evaluate the impact of improved family history taking on health care costs and outcomes.
Acknowledgments
The authors thank Howard Rabinowitz, MD, for providing helpful suggestions in the development and execution of this project and Aliza Mansolino for the preparation of the manuscript.
1. Brownson RC, Davis JR, Simms SG, Kern TG, Harmon RG. Cancer control knowledge and priorities among primary care physicians. J Cancer Educ 1993;8:35-41.
2. Emery J, Rose P. Expanding the role of the family history in primary care (editorial). Br J Gen Pract 1999;49(441):260-1.
3. Smith RA, von Eschenbach AC, Wender RC, et al. American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers. CA Cancer J Clin 2001;51:38-75.
4. Polednak AP. Screening for colorectal cancer by primary-care physicians in Long Island (New York) and Connecticut. Cancer Detect Prev 1989;13:301-9.
5. Acton RT, Burst NM, Casebeer L, et al. Knowledge, attitudes, and behaviors of Alabama’s primary care physicians regarding cancer genetics. Acad Med 2000;75:850-2.
6. Summerton N, Garrood PV. The family history in family practice: a questionnaire study. Fam Pract 1997;14:285-8.
7. Del Mar C, Lowe JB, Adkins P, Arnold E. What is the quality of general practitioner records in Australia? Aust Fam Phys 1996;suppl 1:S21-5.
8. Medalie JH, Zyzanski SJ, Langa D, Stange KC. The family in family practice: is it a reality?. J Fam Pract 1998;46:390-6.
9. Medalie JH, Zyzanski SJ, Goodwin MA, Stange KC. Two physician styles of focusing on the family. J Fam Pract 2000;49:209-15.
10. de Bock GH, Perk DC, Oosterwijk JC, Hageman GC, Kievit J, Springer MP. Women worried about their familial breast cancer risk-a study on genetic advice in general practice. Fam Pract 1997;14:40-3.
11. Graham ID, Logan DM, Hughes-Benzie R, et al. How interested is the public in genetic testing for colon cancer susceptibility? Report of a cross-sectional population survey. Cancer Prev Control 1998;2:167-72.
12. Bosompra K, Flynn BS, Ashikaga T, Rairikar CJ, Worden JK, Solomon LJ. Likelihood of undergoing genetic testing for cancer risk: a population-based study. Prev Med 2000;30:155-66.
13. Kinney AY, Choi YA, DeVellis B, Kobetz E, Millikan RC, Sandler RS. Interest in genetic testing among first-degree relatives of colorectal cancer patients. Am J Prev Med 2000;18:249-52.
14. Petersen GM, Larkin E, Codori AM, et al. Attitudes toward colon cancer gene testing: survey of relatives of colon cancer patients. Cancer Epidemiol Biomarkers Prev 1999;8(4 pt 2):337-44.
15. Statement of the American Society of Clinical Oncology: genetic testing for cancer susceptibility, adopted on February 20, 1996. J Clin Oncol 1996;14:1730-6.
16. Rogers J, Halloway R. Completion rate and reliability of the self-administered genogram. Fam Pract 1990;7:149-51.
17. Rogers J, Durkin M, Kelly K. The family genogram: an underutilized clinical tool. N J Med 1985;82:887-92.
18. Rogers J, Durkin M. The semi-structured genogram interview: I. Protocol, II. Evaluation. Fam Syst Med 1984;2:176-87.
19. Shore WB, Wilkie HA, Croughan-Minihane M. Family of origin genograms: evaluation of a teaching program for medical students. Fam Med 1994;26:238-43.
20. Physicians lax in screening patients with family history of adenomatous polyps. Reuters Health 2000;May 22.
Address reprint requests to Randa Sifri, MD, Department of Family Medicine, 1015 Walnut Street, Suite 401, Philadelphia, PA 19107. E-mail: [email protected].
To submit a letter to the editor on this topic, click here: [email protected].
OBJECTIVE: To determine whether an adequate amount of family history is being collected and recorded by family practitioners to appropriately identify patients at increased risk for cancer.
STUDY DESIGN: Retrospective chart audit.
POPULATION: Charts from 500 randomly chosen patients, 40 to 60 years of age, were audited. Of those charts, 400 were from a large academic family practice and 50 charts each were from 2 small community family practices in the greater Philadelphia area.
OUTCOMES MEASURED: General features of family history taking were recorded, including presence of a family history and date when recorded, evidence of updated family history data, and presence of a genogram. Cancer features recorded included mention of family history of cancer or colon polyps and, if positive, identification of which relative was affected, site of cancer, and age of diagnosis or death.
RESULTS: Most charts (89%) had some family history information recorded, and 55% listed a family history of cancer, either positive or negative. Of the 356 relatives affected with cancer, an age of diagnosis was documented in only 8%; of 183 first-degree relatives with cancer, only 7% had a documented age of diagnosis. Two percent of all charts had any mention of a family history of colon polyps. Sixty-five percent of family histories were recorded at the first visit, and only 35% had any updated family history information.
CONCLUSIONS: The number and type of family histories currently being recorded by family practitioners are not adequate to fully assess familial risk of cancer. New strategies will need to be developed to better prepare providers for risk-based clinical decision making.
Taking a family history is a significant component of providing comprehensive primary care because family history provides key psychosocial and medical risk information.1,2 As our understanding of the genetic basis for disease grows, obtaining an accurate and complete family history is likely to gain increasing relevance as a vital source of data to guide counseling and testing. Patients who have a first-degree relative with a colon neoplasm or prostate cancer are advised to screen differently from those who do not.3 Failure to gather accurate or complete family data prevents the clinician from providing advice that is consistent with screening guidelines. Understanding how primary care clinicians gather family history data is necessary to identify gaps in current performance and to develop strategies to bridge these gaps.
Several studies have used physician self-report to assess the current level of taking a family history in primary care. In 1 study, 90% of surveyed physicians stated that they obtain a family history of cancer from their patients, with 77% to 80% inquiring about a family history of colorectal cancer in their patients who are at least 40 years of age.3 In another study, 63% to 85% of responding physicians reported obtaining a family history of cancer from 76% to 100% of their patients.5 Respondents in 1 study reported obtaining family histories of colorectal cancer in only 30.7% of patients, breast cancer in 48.4% of patients, and coronary disease and hypertension in 94.3% of patients.6 However, data on actual performance of family history taking are sparse.7,8 The physicians in the Direct Observation of Primary Care study obtained a family history during 51% of new visits and 22% of established visits. A genogram was present in 11% of charts, and documentation of a family history of breast cancer or colorectal cancer was found in 40% of charts. Further analysis from this study showed that providers who more frequently obtained and recorded family history information performed more preventive care services for their patients.9
First-degree relatives of cancer patients appear to be interested in and receptive to information about their risk and in the possibility of genetic counseling.10-14 In fact, many patients overestimate their likelihood of getting cancer based on family history10; primary care providers thus have the opportunity to counsel and relieve anxiety in their patients. Family history is an important tool to define risk and guide referral, counseling, and testing.
This article presents the findings of a descriptive study of the review of 500 charts from 3 different family practice offices. We documented general family history components and completeness related to a family history of cancer, including whether enough family history information was collected to appropriately identify patients at increased risk for cancer.
Methods
Data were collected from 500 patient charts from 3 family practice offices in the greater Philadelphia area. Patients who were in the practice for at least 1 year, made a minimum of 2 visits between June 1, 1997 and June 1, 2000, and were between the ages of 40 and 60 years at 1 of those visits were included. Fifty charts were selected by using a random starting point at each of 2 small (1 to 3 providers) private practices, and 400 charts were randomly selected from all eligible charts in a large academic practice (more than 60 providers). The large practice had nearly 1500 patients who fit the selection criteria, and the total population did not differ from the random sample in mean age, sex, or race (P < .05). Total population characteristics were not available for the 2 smaller practices.
Family history data were collected from progress notes and designated family history spaces on flow sheets or chart covers. Family histories consisting only of “none” or “noncontributory” were counted only when they clearly referred to a specific condition. Family births or deaths recorded in a psychosocial context also were not counted as part of the family history unless death from a specific disease was mentioned. The first dated family history was considered to be first for the purpose of this study. Date of birth, race, sex, current primary care provider, if any, and the date first seen in the practice were recorded. The current primary care provider was determined by the physician seen for the majority of recent visits and/or notations in the chart at acute visits. Data collected for the primary care providers included practice site, sex, level of training, and years in practice. Variables collected from family history information included: date of first family history; date of most recent family history; presence of a genogram, presence of a patient-completed family history self-questionnaire; whether any mention was made, positive or negative, of cancer or colon polyps; and whether there was a positive family history of cancer or colon polyps. For individuals with a positive family history of cancer or colon polyps, all details recorded in the chart were abstracted; these included site of cancer or polyp, relationship to patient, age at diagnosis, and age at death. The data were entered into an Access 97 database and stored separately from chart number identifiers. All analyses and tests were done in SAS version 6.12.
Results
Demographic data from the 500 patients whose charts were audited are presented in Table 1. Ninety-seven percent of patients had a primary care provider, which included 60 physicians and 3 nurse practitioners. No significant associations were seen with practice site, sex, or level of training of the provider and the presence of family history information in the chart.
Most patients (89%) had some family history information in the chart and 63% had a genogram. This did not differ by sex or race of the patient. Fifty-seven percent of patients supplied family history information at the first visit to the office; 59% of these patients had no family history data recorded on subsequent visits. Only 31% of charts had updated family history information Table 2. For patients who had been in the same practice for at least 5 years and had some family history in the chart, 20% had some updated information within the past 3 years.
Of the 500 charts, 276 (55%) recorded a family history of cancer, positive or negative. Two hundred fifteen patients (43%) had a positive family history of cancer, with a total of 356 relatives affected. The site of cancer was listed for 88% of all family member cancers, with breast, colon, lung, and prostate being the most common cancer locations. The specific relative was identified in 92% of cases, with most being first (51%) or second (37%) degree. Although degree of relative and cancer location were usually recorded, age at diagnosis was listed for only 8% of affected relatives, and age at death was identified for 19% of relatives with cancer.
For patients with affected first-degree relatives, the group with the greatest clinical significance, primary care providers identified the location of the cancer in 93% of cases but listed the ages at diagnosis and death in only 7% and 31%, respectively Table 3. Only 7 medical records (1.4%) had any mention of a family history of polyps; of these, 5 (1%) were positive. None listed an age at diagnosis. Five patients in our study met the American Society of Clinical Oncology criteria to be evaluated for genetic breast and ovarian cancer syndrome, and no patients met the criteria for hereditary nonpolyposis colon cancer.
The 2 community practices intermittently used patient self-administered medical intake questionnaires. In our sample, 31 of 500 patients (6%) had a questionnaire in the chart. All patients who completed questionnaires had family history data in their charts. Use of a questionnaire was associated with a greater likelihood that the physician recorded the age of diagnosis for a relative with cancer, although this did not reach significance (20% vs 7%).
Discussion
Despite our finding that providers are documenting family histories in most charts, very few are recording the age of diagnosis in relatives diagnosed with cancer. Age of diagnosis plays a critical role in determining screening recommendations and identifying patients with possible genetic syndromes. For example, the Amsterdam criteria used to identify families with hereditary nonpolyposis colon cancer include knowing whether 1 of 3 relatives with colorectal cancer was diagnosed at younger than 50 years. Breast and ovarian cancer syndrome should be suspected when breast and/or ovarian cancer is diagnosed in 2 first-degree relatives younger than 50 years.15
Most physicians obtain family histories at initial visits. If the patient’s initial visit is for symptom- or disease-related care, an opportunity to gather family history data may be lost. New tools to consistently capture comprehensive family history data at this first visit may be beneficial. Patient self-administered intake questionnaires may prove valuable in this respect, but only 6% of charts in our study contained such a questionnaire, so we cannot draw conclusions about its impact. We did observe a trend toward gathering more complete family history data in patients who used a questionnaire.
There are no clear guidelines regarding when to update a family history. We arbitrarily chose 3 years as a reasonable period for primary care providers to explore changes in family history status. Updating at any subsequent visit was recorded for 35% of patient charts in which a family history was initially taken. It is not clear that primary care providers are documenting changes in family history in any systematic way. Opportunistic updating likely occurs when a new diagnosis of serious disease in a close family member produces anxiety, stress, or concern in the patient. The value of updating family history and the ideal interval to reexamine family history are unknown.
Several conditions would need to be met for family history updating to have value. (1) A close relative must have developed an important illness in the interval since the last family history was recorded or the update must discover family information that was previously missed. (2) The illness must have a familial component that affects the estimate of the risk of the identified patient. (3) The clinician would need to be aware of the updated information. (4) The clinician must change recommendations to the patient based on this new information. Discovering a new family history of colonic neoplasm satisfies these conditions. Process measures that have the potential to improve updating include adding an update of family history as an item on a preventive care flow sheet or using periodic self-administered patient questionnaires. Whether adequate improvements in health care would occur to justify these changes in process will need to be studied. If any updating has value, determining the appropriate intervals for systematic updates deserves attention.
Charts in this study included a genogram 63% of the time, a significant increase over the 11% noted in the Direct Observation of Primary Care study.9 This discrepancy may be explained by differences in practice types because 1 study suggested a higher genogram use in academic medical centers than in community practices.16 The genogram has been cited as an attractive and efficient way to document family history,17,18 but over one fourth of the charts that contained family history in our study used the more cumbersome narrative form. Many geneticists predict that our ability to apply genetic testing will grow dramatically over the next decade. Optimal application of this new knowledge will rely on the health care system’s capacity to accurately identify risk based on assessment of family history. The 3-generation pedigree is likely to be a key tool in finding individuals who may benefit from testing. However, there is currently no standardized education in family history taking in many undergraduate and graduate medical education programs.19
Although patients with a first-degree relative with a history of polyps diagnosed at younger than 60 years are considered to be at increased risk for colorectal cancer,3 providers infrequently asked about a family history of polyps. This may reflect a recent finding that only 36% of primary care providers recommend screening at the age of 40 years for their patients with a family history of polyps in relatives younger than 60 years.20 In fact, family history data do not consistently influence behavior: in the same study, gastroenterologists asked about a family history of polyps 93% of the time, but only 37% recommended earlier screening in those with such a history.
The study is limited in its use of only 3 primary care practices, 1 of which was a large academic family practice. However, because the charts of 63 different clinicians were represented, a range of educational backgrounds and personal philosophies toward family history taking was included. Most patients (97%) had a clearly identified primary care provider and patients had been members of the practice for an average of 7.6 years. The sample was specifically chosen to review the charts of individuals who had been enrolled in the same practice for at least 1 year. This may explain the higher rates of family history taking found in this study compared with previously published studies. Given that the vast majority of charts did contain some family history, it is even more compelling that age at diagnosis of cancer was inadequately recorded. This study reflected only what was documented in the patient chart and not direct observation of physician behavior regarding the family history. It is likely that physicians are not recording all responses to inquiries about family history, although the extent of this underreporting is unknown.
CONCLUSION
Findings in this chart review study are consistent with previous work showing that the quantity and type of family history currently being recorded in primary care charts are not adequate to fully assess familial risk. Bridging the gap between recommendations and actual practice will demand interventions to alter primary care practice or the introduction of new models to gather and analyze family data. Further research is also needed to evaluate the impact of improved family history taking on health care costs and outcomes.
Acknowledgments
The authors thank Howard Rabinowitz, MD, for providing helpful suggestions in the development and execution of this project and Aliza Mansolino for the preparation of the manuscript.
OBJECTIVE: To determine whether an adequate amount of family history is being collected and recorded by family practitioners to appropriately identify patients at increased risk for cancer.
STUDY DESIGN: Retrospective chart audit.
POPULATION: Charts from 500 randomly chosen patients, 40 to 60 years of age, were audited. Of those charts, 400 were from a large academic family practice and 50 charts each were from 2 small community family practices in the greater Philadelphia area.
OUTCOMES MEASURED: General features of family history taking were recorded, including presence of a family history and date when recorded, evidence of updated family history data, and presence of a genogram. Cancer features recorded included mention of family history of cancer or colon polyps and, if positive, identification of which relative was affected, site of cancer, and age of diagnosis or death.
RESULTS: Most charts (89%) had some family history information recorded, and 55% listed a family history of cancer, either positive or negative. Of the 356 relatives affected with cancer, an age of diagnosis was documented in only 8%; of 183 first-degree relatives with cancer, only 7% had a documented age of diagnosis. Two percent of all charts had any mention of a family history of colon polyps. Sixty-five percent of family histories were recorded at the first visit, and only 35% had any updated family history information.
CONCLUSIONS: The number and type of family histories currently being recorded by family practitioners are not adequate to fully assess familial risk of cancer. New strategies will need to be developed to better prepare providers for risk-based clinical decision making.
Taking a family history is a significant component of providing comprehensive primary care because family history provides key psychosocial and medical risk information.1,2 As our understanding of the genetic basis for disease grows, obtaining an accurate and complete family history is likely to gain increasing relevance as a vital source of data to guide counseling and testing. Patients who have a first-degree relative with a colon neoplasm or prostate cancer are advised to screen differently from those who do not.3 Failure to gather accurate or complete family data prevents the clinician from providing advice that is consistent with screening guidelines. Understanding how primary care clinicians gather family history data is necessary to identify gaps in current performance and to develop strategies to bridge these gaps.
Several studies have used physician self-report to assess the current level of taking a family history in primary care. In 1 study, 90% of surveyed physicians stated that they obtain a family history of cancer from their patients, with 77% to 80% inquiring about a family history of colorectal cancer in their patients who are at least 40 years of age.3 In another study, 63% to 85% of responding physicians reported obtaining a family history of cancer from 76% to 100% of their patients.5 Respondents in 1 study reported obtaining family histories of colorectal cancer in only 30.7% of patients, breast cancer in 48.4% of patients, and coronary disease and hypertension in 94.3% of patients.6 However, data on actual performance of family history taking are sparse.7,8 The physicians in the Direct Observation of Primary Care study obtained a family history during 51% of new visits and 22% of established visits. A genogram was present in 11% of charts, and documentation of a family history of breast cancer or colorectal cancer was found in 40% of charts. Further analysis from this study showed that providers who more frequently obtained and recorded family history information performed more preventive care services for their patients.9
First-degree relatives of cancer patients appear to be interested in and receptive to information about their risk and in the possibility of genetic counseling.10-14 In fact, many patients overestimate their likelihood of getting cancer based on family history10; primary care providers thus have the opportunity to counsel and relieve anxiety in their patients. Family history is an important tool to define risk and guide referral, counseling, and testing.
This article presents the findings of a descriptive study of the review of 500 charts from 3 different family practice offices. We documented general family history components and completeness related to a family history of cancer, including whether enough family history information was collected to appropriately identify patients at increased risk for cancer.
Methods
Data were collected from 500 patient charts from 3 family practice offices in the greater Philadelphia area. Patients who were in the practice for at least 1 year, made a minimum of 2 visits between June 1, 1997 and June 1, 2000, and were between the ages of 40 and 60 years at 1 of those visits were included. Fifty charts were selected by using a random starting point at each of 2 small (1 to 3 providers) private practices, and 400 charts were randomly selected from all eligible charts in a large academic practice (more than 60 providers). The large practice had nearly 1500 patients who fit the selection criteria, and the total population did not differ from the random sample in mean age, sex, or race (P < .05). Total population characteristics were not available for the 2 smaller practices.
Family history data were collected from progress notes and designated family history spaces on flow sheets or chart covers. Family histories consisting only of “none” or “noncontributory” were counted only when they clearly referred to a specific condition. Family births or deaths recorded in a psychosocial context also were not counted as part of the family history unless death from a specific disease was mentioned. The first dated family history was considered to be first for the purpose of this study. Date of birth, race, sex, current primary care provider, if any, and the date first seen in the practice were recorded. The current primary care provider was determined by the physician seen for the majority of recent visits and/or notations in the chart at acute visits. Data collected for the primary care providers included practice site, sex, level of training, and years in practice. Variables collected from family history information included: date of first family history; date of most recent family history; presence of a genogram, presence of a patient-completed family history self-questionnaire; whether any mention was made, positive or negative, of cancer or colon polyps; and whether there was a positive family history of cancer or colon polyps. For individuals with a positive family history of cancer or colon polyps, all details recorded in the chart were abstracted; these included site of cancer or polyp, relationship to patient, age at diagnosis, and age at death. The data were entered into an Access 97 database and stored separately from chart number identifiers. All analyses and tests were done in SAS version 6.12.
Results
Demographic data from the 500 patients whose charts were audited are presented in Table 1. Ninety-seven percent of patients had a primary care provider, which included 60 physicians and 3 nurse practitioners. No significant associations were seen with practice site, sex, or level of training of the provider and the presence of family history information in the chart.
Most patients (89%) had some family history information in the chart and 63% had a genogram. This did not differ by sex or race of the patient. Fifty-seven percent of patients supplied family history information at the first visit to the office; 59% of these patients had no family history data recorded on subsequent visits. Only 31% of charts had updated family history information Table 2. For patients who had been in the same practice for at least 5 years and had some family history in the chart, 20% had some updated information within the past 3 years.
Of the 500 charts, 276 (55%) recorded a family history of cancer, positive or negative. Two hundred fifteen patients (43%) had a positive family history of cancer, with a total of 356 relatives affected. The site of cancer was listed for 88% of all family member cancers, with breast, colon, lung, and prostate being the most common cancer locations. The specific relative was identified in 92% of cases, with most being first (51%) or second (37%) degree. Although degree of relative and cancer location were usually recorded, age at diagnosis was listed for only 8% of affected relatives, and age at death was identified for 19% of relatives with cancer.
For patients with affected first-degree relatives, the group with the greatest clinical significance, primary care providers identified the location of the cancer in 93% of cases but listed the ages at diagnosis and death in only 7% and 31%, respectively Table 3. Only 7 medical records (1.4%) had any mention of a family history of polyps; of these, 5 (1%) were positive. None listed an age at diagnosis. Five patients in our study met the American Society of Clinical Oncology criteria to be evaluated for genetic breast and ovarian cancer syndrome, and no patients met the criteria for hereditary nonpolyposis colon cancer.
The 2 community practices intermittently used patient self-administered medical intake questionnaires. In our sample, 31 of 500 patients (6%) had a questionnaire in the chart. All patients who completed questionnaires had family history data in their charts. Use of a questionnaire was associated with a greater likelihood that the physician recorded the age of diagnosis for a relative with cancer, although this did not reach significance (20% vs 7%).
Discussion
Despite our finding that providers are documenting family histories in most charts, very few are recording the age of diagnosis in relatives diagnosed with cancer. Age of diagnosis plays a critical role in determining screening recommendations and identifying patients with possible genetic syndromes. For example, the Amsterdam criteria used to identify families with hereditary nonpolyposis colon cancer include knowing whether 1 of 3 relatives with colorectal cancer was diagnosed at younger than 50 years. Breast and ovarian cancer syndrome should be suspected when breast and/or ovarian cancer is diagnosed in 2 first-degree relatives younger than 50 years.15
Most physicians obtain family histories at initial visits. If the patient’s initial visit is for symptom- or disease-related care, an opportunity to gather family history data may be lost. New tools to consistently capture comprehensive family history data at this first visit may be beneficial. Patient self-administered intake questionnaires may prove valuable in this respect, but only 6% of charts in our study contained such a questionnaire, so we cannot draw conclusions about its impact. We did observe a trend toward gathering more complete family history data in patients who used a questionnaire.
There are no clear guidelines regarding when to update a family history. We arbitrarily chose 3 years as a reasonable period for primary care providers to explore changes in family history status. Updating at any subsequent visit was recorded for 35% of patient charts in which a family history was initially taken. It is not clear that primary care providers are documenting changes in family history in any systematic way. Opportunistic updating likely occurs when a new diagnosis of serious disease in a close family member produces anxiety, stress, or concern in the patient. The value of updating family history and the ideal interval to reexamine family history are unknown.
Several conditions would need to be met for family history updating to have value. (1) A close relative must have developed an important illness in the interval since the last family history was recorded or the update must discover family information that was previously missed. (2) The illness must have a familial component that affects the estimate of the risk of the identified patient. (3) The clinician would need to be aware of the updated information. (4) The clinician must change recommendations to the patient based on this new information. Discovering a new family history of colonic neoplasm satisfies these conditions. Process measures that have the potential to improve updating include adding an update of family history as an item on a preventive care flow sheet or using periodic self-administered patient questionnaires. Whether adequate improvements in health care would occur to justify these changes in process will need to be studied. If any updating has value, determining the appropriate intervals for systematic updates deserves attention.
Charts in this study included a genogram 63% of the time, a significant increase over the 11% noted in the Direct Observation of Primary Care study.9 This discrepancy may be explained by differences in practice types because 1 study suggested a higher genogram use in academic medical centers than in community practices.16 The genogram has been cited as an attractive and efficient way to document family history,17,18 but over one fourth of the charts that contained family history in our study used the more cumbersome narrative form. Many geneticists predict that our ability to apply genetic testing will grow dramatically over the next decade. Optimal application of this new knowledge will rely on the health care system’s capacity to accurately identify risk based on assessment of family history. The 3-generation pedigree is likely to be a key tool in finding individuals who may benefit from testing. However, there is currently no standardized education in family history taking in many undergraduate and graduate medical education programs.19
Although patients with a first-degree relative with a history of polyps diagnosed at younger than 60 years are considered to be at increased risk for colorectal cancer,3 providers infrequently asked about a family history of polyps. This may reflect a recent finding that only 36% of primary care providers recommend screening at the age of 40 years for their patients with a family history of polyps in relatives younger than 60 years.20 In fact, family history data do not consistently influence behavior: in the same study, gastroenterologists asked about a family history of polyps 93% of the time, but only 37% recommended earlier screening in those with such a history.
The study is limited in its use of only 3 primary care practices, 1 of which was a large academic family practice. However, because the charts of 63 different clinicians were represented, a range of educational backgrounds and personal philosophies toward family history taking was included. Most patients (97%) had a clearly identified primary care provider and patients had been members of the practice for an average of 7.6 years. The sample was specifically chosen to review the charts of individuals who had been enrolled in the same practice for at least 1 year. This may explain the higher rates of family history taking found in this study compared with previously published studies. Given that the vast majority of charts did contain some family history, it is even more compelling that age at diagnosis of cancer was inadequately recorded. This study reflected only what was documented in the patient chart and not direct observation of physician behavior regarding the family history. It is likely that physicians are not recording all responses to inquiries about family history, although the extent of this underreporting is unknown.
CONCLUSION
Findings in this chart review study are consistent with previous work showing that the quantity and type of family history currently being recorded in primary care charts are not adequate to fully assess familial risk. Bridging the gap between recommendations and actual practice will demand interventions to alter primary care practice or the introduction of new models to gather and analyze family data. Further research is also needed to evaluate the impact of improved family history taking on health care costs and outcomes.
Acknowledgments
The authors thank Howard Rabinowitz, MD, for providing helpful suggestions in the development and execution of this project and Aliza Mansolino for the preparation of the manuscript.
1. Brownson RC, Davis JR, Simms SG, Kern TG, Harmon RG. Cancer control knowledge and priorities among primary care physicians. J Cancer Educ 1993;8:35-41.
2. Emery J, Rose P. Expanding the role of the family history in primary care (editorial). Br J Gen Pract 1999;49(441):260-1.
3. Smith RA, von Eschenbach AC, Wender RC, et al. American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers. CA Cancer J Clin 2001;51:38-75.
4. Polednak AP. Screening for colorectal cancer by primary-care physicians in Long Island (New York) and Connecticut. Cancer Detect Prev 1989;13:301-9.
5. Acton RT, Burst NM, Casebeer L, et al. Knowledge, attitudes, and behaviors of Alabama’s primary care physicians regarding cancer genetics. Acad Med 2000;75:850-2.
6. Summerton N, Garrood PV. The family history in family practice: a questionnaire study. Fam Pract 1997;14:285-8.
7. Del Mar C, Lowe JB, Adkins P, Arnold E. What is the quality of general practitioner records in Australia? Aust Fam Phys 1996;suppl 1:S21-5.
8. Medalie JH, Zyzanski SJ, Langa D, Stange KC. The family in family practice: is it a reality?. J Fam Pract 1998;46:390-6.
9. Medalie JH, Zyzanski SJ, Goodwin MA, Stange KC. Two physician styles of focusing on the family. J Fam Pract 2000;49:209-15.
10. de Bock GH, Perk DC, Oosterwijk JC, Hageman GC, Kievit J, Springer MP. Women worried about their familial breast cancer risk-a study on genetic advice in general practice. Fam Pract 1997;14:40-3.
11. Graham ID, Logan DM, Hughes-Benzie R, et al. How interested is the public in genetic testing for colon cancer susceptibility? Report of a cross-sectional population survey. Cancer Prev Control 1998;2:167-72.
12. Bosompra K, Flynn BS, Ashikaga T, Rairikar CJ, Worden JK, Solomon LJ. Likelihood of undergoing genetic testing for cancer risk: a population-based study. Prev Med 2000;30:155-66.
13. Kinney AY, Choi YA, DeVellis B, Kobetz E, Millikan RC, Sandler RS. Interest in genetic testing among first-degree relatives of colorectal cancer patients. Am J Prev Med 2000;18:249-52.
14. Petersen GM, Larkin E, Codori AM, et al. Attitudes toward colon cancer gene testing: survey of relatives of colon cancer patients. Cancer Epidemiol Biomarkers Prev 1999;8(4 pt 2):337-44.
15. Statement of the American Society of Clinical Oncology: genetic testing for cancer susceptibility, adopted on February 20, 1996. J Clin Oncol 1996;14:1730-6.
16. Rogers J, Halloway R. Completion rate and reliability of the self-administered genogram. Fam Pract 1990;7:149-51.
17. Rogers J, Durkin M, Kelly K. The family genogram: an underutilized clinical tool. N J Med 1985;82:887-92.
18. Rogers J, Durkin M. The semi-structured genogram interview: I. Protocol, II. Evaluation. Fam Syst Med 1984;2:176-87.
19. Shore WB, Wilkie HA, Croughan-Minihane M. Family of origin genograms: evaluation of a teaching program for medical students. Fam Med 1994;26:238-43.
20. Physicians lax in screening patients with family history of adenomatous polyps. Reuters Health 2000;May 22.
Address reprint requests to Randa Sifri, MD, Department of Family Medicine, 1015 Walnut Street, Suite 401, Philadelphia, PA 19107. E-mail: [email protected].
To submit a letter to the editor on this topic, click here: [email protected].
1. Brownson RC, Davis JR, Simms SG, Kern TG, Harmon RG. Cancer control knowledge and priorities among primary care physicians. J Cancer Educ 1993;8:35-41.
2. Emery J, Rose P. Expanding the role of the family history in primary care (editorial). Br J Gen Pract 1999;49(441):260-1.
3. Smith RA, von Eschenbach AC, Wender RC, et al. American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers. CA Cancer J Clin 2001;51:38-75.
4. Polednak AP. Screening for colorectal cancer by primary-care physicians in Long Island (New York) and Connecticut. Cancer Detect Prev 1989;13:301-9.
5. Acton RT, Burst NM, Casebeer L, et al. Knowledge, attitudes, and behaviors of Alabama’s primary care physicians regarding cancer genetics. Acad Med 2000;75:850-2.
6. Summerton N, Garrood PV. The family history in family practice: a questionnaire study. Fam Pract 1997;14:285-8.
7. Del Mar C, Lowe JB, Adkins P, Arnold E. What is the quality of general practitioner records in Australia? Aust Fam Phys 1996;suppl 1:S21-5.
8. Medalie JH, Zyzanski SJ, Langa D, Stange KC. The family in family practice: is it a reality?. J Fam Pract 1998;46:390-6.
9. Medalie JH, Zyzanski SJ, Goodwin MA, Stange KC. Two physician styles of focusing on the family. J Fam Pract 2000;49:209-15.
10. de Bock GH, Perk DC, Oosterwijk JC, Hageman GC, Kievit J, Springer MP. Women worried about their familial breast cancer risk-a study on genetic advice in general practice. Fam Pract 1997;14:40-3.
11. Graham ID, Logan DM, Hughes-Benzie R, et al. How interested is the public in genetic testing for colon cancer susceptibility? Report of a cross-sectional population survey. Cancer Prev Control 1998;2:167-72.
12. Bosompra K, Flynn BS, Ashikaga T, Rairikar CJ, Worden JK, Solomon LJ. Likelihood of undergoing genetic testing for cancer risk: a population-based study. Prev Med 2000;30:155-66.
13. Kinney AY, Choi YA, DeVellis B, Kobetz E, Millikan RC, Sandler RS. Interest in genetic testing among first-degree relatives of colorectal cancer patients. Am J Prev Med 2000;18:249-52.
14. Petersen GM, Larkin E, Codori AM, et al. Attitudes toward colon cancer gene testing: survey of relatives of colon cancer patients. Cancer Epidemiol Biomarkers Prev 1999;8(4 pt 2):337-44.
15. Statement of the American Society of Clinical Oncology: genetic testing for cancer susceptibility, adopted on February 20, 1996. J Clin Oncol 1996;14:1730-6.
16. Rogers J, Halloway R. Completion rate and reliability of the self-administered genogram. Fam Pract 1990;7:149-51.
17. Rogers J, Durkin M, Kelly K. The family genogram: an underutilized clinical tool. N J Med 1985;82:887-92.
18. Rogers J, Durkin M. The semi-structured genogram interview: I. Protocol, II. Evaluation. Fam Syst Med 1984;2:176-87.
19. Shore WB, Wilkie HA, Croughan-Minihane M. Family of origin genograms: evaluation of a teaching program for medical students. Fam Med 1994;26:238-43.
20. Physicians lax in screening patients with family history of adenomatous polyps. Reuters Health 2000;May 22.
Address reprint requests to Randa Sifri, MD, Department of Family Medicine, 1015 Walnut Street, Suite 401, Philadelphia, PA 19107. E-mail: [email protected].
To submit a letter to the editor on this topic, click here: [email protected].
Management of the low-grade abnormal Pap smear: What are women’s preferences?
- Any of several approaches may be used in managing women who have low-grade Pap smear abnormalities.
- Women’s preferences for a particular management approach to an abnormal Pap smear vary widely.
- Asking patients specific questions about their desire to avoid procedures and tolerance for uncertainty may help to clarify preferences.
The management of women who have low-grade cytologic abnormalities—including atypical squamous cells (ASC) and low-grade squamous intraepithelial lesions (LSIL)—is controversial.1-4 Without strong evidence favoring a single approach, some clinicians recommend immediate colposcopy to obtain a definitive diagnosis and to exclude the presence of a high-grade lesion, while others recommend observation with serial Pap smears, given the tendency for many low-grade lesions to regress spontaneously.5,6 Immediate colposcopy has the advantage of giving a patient a relatively rapid assessment of the nature and extent of her cervical dysplasia; however, the procedure is uncomfortable, and overall management may not be affected. Observation with serial Pap smears may avoid an invasive procedure, but it may also cause anxiety as time passes without a definitive diagnosis.
Eliciting and understanding patient preferences is an important part of clinical decision making. The clinician provides the best available information on the probability of clinical outcomes and the implications of each for the patient’s health. But only the patient knows what these outcomes mean to her well-being (also called “utility”).
Given the clinical disagreement over how to proceed with abnormal ASC and LSIL Pap smear results, the decision should be influenced by a patient’s preference, informed by knowledge of outcomes and costs of alternative approaches. It is unclear which approach women prefer, and whether women’s preferences for specific protocols are associated with sociodemographic characteristics. To understand better how women weigh these trade-offs, we evaluated the preferences of a diverse group of women for contrasting management approaches to the evaluation of a hypothetical low-grade abnormal Pap smear result.
Methods
Study population
Study participants were recruited from the waiting rooms of 5 family planning clinics in Northern California’s Central Valley. Women were eligible for the study if they were 18 years of age or older, or, if minors, they were emancipated and could thus provide informed consent. Potential subjects were excluded if they spoke neither English nor Spanish or if they had never had a Pap smear. The study protocol and informed consent procedures were reviewed and approved by the University of California, Davis, Human Subjects Committee.
Instruments and outcome measures
Interviews were conducted in English or Spanish. Information regarding demographic characteristics, level of education, past experiences with abnormal Pap smears and cervical cancer, and self-rated religiosity was collected with a self-administered questionnaire. The primary outcome measures were utilities (quantified preferences for specific health states) for 6 different scenarios. These were assessed by the standard gamble (SG) method, described in more detail below.7
Possible utility scores range from 0 to 1. A score of 0 represents immediate death; a score of 1 represents full (or ideal) health for the rest of one’s life. Because the scenarios under consideration in this study did not involve any meaningful level of risk of death, we expected utility scores for the scenarios to cluster toward the upper end of the scale. As a result, a measurement instrument based on an “immediate death” versus “full health” scale would be unable to discriminate between different scenarios. To avoid this problem, a scale was used in which the lower end point was a non-death state unambiguously less preferred than each of the scenarios under consideration.8 We used “invasive cervical cancer requiring hysterectomy” as the lower end point (utility of 0) contrasted with “full health with all normal Pap smears” (utility of 1) to generate the original score (SG Dys). In a separate standard gamble, subjects rated invasive cervical cancer versus immediate death (SG Ca), so that all utilities could be converted to the standard scale, using the formula: (1 – SG Ca) (SG Dys) + SG Ca.
The 6 scenarios rated in the study are shown in Table 1. The scenarios represent 3 sets of progressively more invasive interventions for a low-grade abnormal Pap smear: (1) resolution, representing spontaneous regression with treatment not required; (2) a low-grade abnormality requiring treatment with cryotherapy; (3) a more severe abnormality requiring a cervical cone biopsy. Following either spontaneous resolution or treatment, all scenarios assumed the abnormality was resolved. For each of the 3 results, a management strategy based on observation with serial Pap smears was applied in 1 instance, and a strategy of early colposcopy was applied in the other instance, resulting in 6 different pathways to the ultimate outcome; a normal Pap smear. The time frame for these scenarios was 18–36 months.
Trained interviewers used a standardized approach to elicit preferences from each subject. Subjects were read a description of all the procedures involved in the scenarios. Descriptions were accompanied by cards summarizing each procedure in pictures and words, and included information about the possibility of progression and spontaneous regression of the Pap smear abnormality. Subjects were encouraged to ask questions at any point during the interview. Procedure descriptions are available from the authors on request.
TABLE 1
Clinical scenarios classified by management approach and required treatment*
| Spontaneous resolution | Cryotherapy | Cone biopsy | |
|---|---|---|---|
| Observation | Pap smear: low-grade abnormal | Pap smear: low-grade abnormal | Pap smear: low-grade abnormal |
| ↓ | ↓ | ↓ | |
| Pap smear: normal | Pap smear: low-grade abnormal | Pap smear: normal | |
| ↓ | ↓ | ↓ | |
| 2 Pap smears every 6 months: normal | Pap smear: low-grade abnormal | Pap smear: normal | |
| ↓ | ↓ | ||
| Pap smear: low-grade abnormal | Pap smear: normal | ||
| ↓ | ↓ | ||
| Colposcopy and biopsy at 1 month | Colposcopy and biopsy at 1 month | ||
| ↓ | ↓ | ||
| Biopsy: low-grade abnormal | Biopsy: abnormal with ? ECC | ||
| ↓ | ↓ | ||
| Cryotherapy at 1 month | Cone biopsy at 1 month: moderately abnoramal cells | ||
| ↓ | ↓ | ||
| 3 Pap smears every 6 months: normal | Cure with cone biopsy | ||
| ↓ | |||
| 3 Pap smears every 6 months: normal | |||
| Early colposcopy | Pap smear: low-grade abnormal | Pap smear: low-grade abnormal | Pap smear: low-grade abnormal |
| ↓ | ↓ | ↓ | |
| Colposcopy and biopsy at 1 month | Colposcopy and biopsy at 1 month | Colposcopy and biopsy at 1 month | |
| ↓ | ↓ | ↓ | |
| Biopsy: normal | Biopsy: abnormal with ? ECC | Biopsy: abnormal with ? ECC | |
| ↓ | ↓ | ↓ | |
| Second colposcopy and biopsy | Cone biopsy at 1 month | Cone biopsy at 1 month | |
| ↓ | ↓ | ↓ | |
| Biopsy: normal | Biopsy: moderately abnormal | Biopsy: moderately abnormal | |
| ↓ | ↓ | ↓ | |
| 2 Pap smears every 6 months: normal | Cure with cone biopsy | Cure with cone biopsy | |
| ↓ | ↓ | ↓ | |
| Pap smear: low-grade abnormal | Colposcopy: normal | Colposcopy: normal | |
| ↓ | ↓ | ↓ | |
| Colposcopy and biopsy at 1 month | 2 Pap smears every 6 months: normal | 2 Pap smears every 6 months: normal | |
| ↓ | |||
| Biopsy: low-grade abnormal | |||
| ↓ | |||
| Colposcopy: normal | |||
| ↓ | |||
| 2 Pap smears every 6 months: normal | |||
| *Intervals are 6 months unless specified otherwise. ECC, endocervical curettage. | |||
Standard gamble
Subjects were asked their preference between the certainty of the scenario under consideration and an uncertain prospect of either having cervical cancer treated by hysterectomy or full health. A probability wheel was used as visual aid.9 The probability of cervical cancer was altered until the subject was indifferent between the certain scenario and the uncertain prospect. Once all 6 scenarios had been scored, each subject was asked about her preference between the certainty of cervical cancer treated by hysterectomy and the uncertain prospect of immediate death or full health, using the same method.
At the end of the interview, both the subject and the interviewer completed evaluation forms including ratings of how well the subject understood the standard gamble rating exercises. Subject confusion was also defined a priori as those placing a higher utility on scenario 3 (observation for a long period followed by cone biopsy), which represented the longest period of uncertainty followed by the most invasive procedure, than on scenario 1 (a single mildly abnormal Pap smear evaluated by observation which then resolved spontaneously), which represented the absence of any invasive procedure.
Statistical analysis
Descriptive statistics were generated for ratings of each scenario for the entire group and with the confused subjects removed. Confused subjects included those who reported they found the interview “very confusing,” those who were recorded by the interviewer as finding the interview “very confusing,” and those whose rankings met the criteria for subject confusion, as described above. Means, standard deviations, medians, and percentiles were calculated for each scenario. The mean differences in adjusted standard gamble ratings between paired scenarios was evaluated using a t distribution. Multiple regression analyses were used to explore how much between-subject variation in the standard gamble scores was explained by the variables listed above.
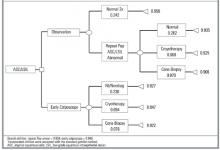
A simple decision tree (Figure 1) was constructed to contrast preferences for an observational approach vs early colposcopy. Outcome probabilities were derived from meta-analyses of the medical literature,5 from observational data obtained at the same Northern California family planning clinics,10 and, for cone biopsy outcomes, from expert opinion obtained using a modified Delphi process.11 Utilities were assigned to the decision tree based on the standard gamble results. Women having 2 consecutive low-grade abnormal Pap results followed by a normal Pap result were assigned the same utility value as that for women with a single abnormal result. Analysis of the tree, including 1-way and 2-way sensitivity analysis of key variables, was conducted with Data 3.5.
Results
One hundred seventy interviews were completed. Characteristics of the interview subjects are shown in Table 2. A total of 22 subjects were designated “confused.” Analyses including the confused subjects did not alter the pattern of results, but the range in responses was larger. All analyses are presented here with confused subjects removed (n = 148).
Median ratings with 25th–75th percentiles for the paired scenarios rated by the standard gamble are shown as box plots in Figure 2. Mean adjusted scores, standard deviations, and mean differences in scores between paired scenarios are shown in Table 3. Notable findings include the following. (1) For each scenario, the range of responses by either rating method was very large. (2) Mean differences in utilities for observation vs early colposcopy were small. (3) For the paired scenarios in which the outcome was spontaneous resolution, observation was preferred (P = .01); in the paired scenarios in which the outcome was cryotherapy, early colposcopy was preferred (P = .02). (4) In the multiple regression analyses for each scenario, age, education, ethnicity, religiosity, and having known someone with cervical cancer together explained only a small amount of the variability between subjects (range for R2, .09–.22).
The decision model with baseline probabilities is shown in Figure 1. The model was simplified to exclude the outcome of cervical cancer, which is a very rare outcome for women with ASC or LSIL cervical smears who have adequate follow-up.5 In the baseline analysis, the overall utility of early colposcopy was slightly favored over the overall utility of the observation approach (utility of observation = 0.932; utility of early colposcopy = 0.940).
Sensitivity analysis examines the effect of varying elements of the model on the outcome. In sensitivity analyses of probabilities, the early colposcopy branch was favored, but the differences were small. The maximum difference in utilities between branches was 0.012 in these sensitivity analyses. In 1-way sensitivity analysis of branch utilities, threshold utility values to favor the observation branch were 0.986 for spontaneous resolution after observation and 0.898 for early colposcopy. Threshold values for cryotherapy were 0.938 for observation and 0.938 for early colposcopy.
TABLE 2
Characteristics of study subjects (n = 170)
| Characteristics | n (%) |
|---|---|
| Mean age (range), y | 26 (14–53) |
| Education | |
| Less than high school | 58 (34%) |
| High school | 77 (45%) |
| Some college | 35 (20%) |
| Ethnicity | |
| African American | 21 (12%) |
| Caucasian | 84 (49%) |
| Latina | 46 (27%) |
| Other | 21 (12%) |
| Interview language, Spanish | 15 (9%) |
| Prior colposcopy | 23 (14%) |
| Moderately or very religious | 64 (38%) |
| Knows someone with cervical cancer | 43 (25%) |
TABLE 3
Adjusted standard gamble values and paired differences* (n=148)
| Management Strategy | ||||
|---|---|---|---|---|
| Short-term outcome | Observation Mean (SD) | Early colposcopy Mean (SD) | Difference | P value (2 sided) |
| Spontaneous resolution | .96 ±..13) | .93±.20) | .03 ±..15) | .01 |
| Cryotherapy | .93 ±..17) | .95 ±..14) | -.02 ±.11) | .02 |
| Cone biopsy | .91 ±..21) | .92 ±..16) | -.02 ±..17) | .23 |
| *Adjusted to scale so that immediate death had a utility of 0 and “full health with all normal Pap smears” had a utility of 1. | ||||
FIGURE 1Decision model comparing observation with early colposcopy *
FIGURE 2Distribution of individual utilities as assessed by the standard gamble*
Discussion
We found wide variation in women’s preferences for management approaches to a low-grade abnormal Pap smear result. The range of responses was very large and the variation between individuals rating the same scenario was substantially greater than the variation in mean ratings between different scenarios. Measured subject characteristics explained only a small proportion of the observed variation, indicating that other unmeasured factors contributed substantially to the variation. Although 25% of subjects stated they knew someone with cervical cancer, this high percentage seems improbable and more likely reflects knowledge of someone who had an abnormal Pap smear.
The decision model displayed a small preference for immediate colposcopy. This may be related to preference for quicker resolution of the concern about cancer, although it involves more procedures. Small changes in utilities for spontaneous resolution and cryotherapy influenced the model to prefer observation. For cryotherapy, these utility values were within 1 standard deviation of the mean.
Our finding of a wide variation in preferences is supported by other patient preference studies,12-14 including 2 on this subject. Ferris et al assessed triage preferences for the evaluation and management ASC and LSIL.13 They used a questionnaire with a sample of 968 women who presented for care at obstetrics and gynecology and family practice clinics. They found that more women preferred repeat Pap smear when the index smear was ASC, and more women preferred colposcopy when the index smear was LSIL. Among a group of 136 Canadian women with atypia or LSIL referred for colposcopy, Meana et al found that 64% preferred early colposcopy, while 17% preferred observation and 17% had no strong preference.14
The factors contributing to patient preferences are complex. Differences in preferences may be influenced by knowledge and understanding of the disease and possible interventions, risk aversion, access to services, socioeconomics, cultural background, and other factors. While 1 patient may be most interested in establishing a definitive diagnosis and undergoing treatment as soon as possible, another may place priority on avoiding invasive or uncomfortable procedures. How differences in patient preferences influence clinical choices is highlighted by the work of Kuppermann et al.15 These investigators found that utilities for outcomes of prenatal diagnostic testing predicted subsequent testing behavior.
Our findings are limited by our use of a convenience sample of women attending family planning clinics. They may not be representative of women’s preferences in general, or even those of women attending family planning clinics. Outcomes in our study were specified during the preference assessment process; in real decision making, the outcome is always unknown at the time the decision is made. We did not include HPV typing as an option in our clinical scenarios. While HPV typing may have a role for triage of ASC,6,16 it appears not to be useful in management of LSIL.17
Cost-effectiveness analysis would offer important information about which management approach might be favored in the context of resource allocation. For decision making by individual patients and doctors, however, decision analysis is often more relevant. In this case, the “preferred” decision is very sensitive to patient utilities, emphasizing the need for clear physician-patient communication.
Strengths of our study include the diversity of the subjects, the formal process for preference assessment, and the paired scenarios, which allow assessment of preferences for a single management decision, in which 2 separate paths lead to an equivalent ultimate outcome. Our findings are consistent with previous work showing that the sequence of events leading to an outcome will influence utilities for the outcome.18
Application to clinical practice
How might our findings be translated into clinical practice? In clinical situations where different approaches to management are unlikely to result in substantial outcome differences (a “toss-up”), patient preferences are a key aspect of the decision-making process.19 For women with lowgrade Pap abnormalities, several diagnostic options are available and no single option is strongly supported by evidence to offer better outcomes. Our study indicates that no single option is preferred by most women. Under these conditions, engaging the patient in the decision-making process may produce better health outcomes.20 Clinicians should anticipate highly varied preferences, and will need to adopt a flexible approach. Not all patients will want to be actively involved in the decision process, but the desire for information is nearly universal. Flexible use of the questions in Table 4 may help patients to define their preferences and will likely improve their satisfaction and adherence to the treatment plan.
TABLE 4
Questions for patients with an abnormal Pap smear
| What is your understanding of what it means for you to have an abnormal Pap smear showing _____________? |
| There are different options for the next step. Would you like to be involved in deciding which option is preferred for your case? |
| What questions do you have about these options? |
| How important is it to you to have a definite answer as soon as possible? |
| How do you feel about undergoing colposcopy? |
| Would you prefer to have a follow-up Pap smear in ____ months, which might avoid a colposcopy, or would you prefer to have a colposcopy sooner? |
· Acknowledgments ·
The authors thank the staff of Planned Parenthood Mar Monte East for their assistance with subject recruitment and interviews.
1. Woolf SH. Screening for cervical cancer. In: Goldbloom RB, Lawrence RS, eds. Preventing disease: beyond the rhetoric. New York: Spring-Verlag, 1990:319–23.
2. Kurman RJ, Henson DE, Herbst AL, Noller KL, Schiffman MH. Interim guidelines for management of abnormal cervical cytology. The 1992 National Cancer Institute Workshop. JAMA 1994;271:1866-69.
3. Miller AB, Anderson G, Brisson J, Laidlaw J, Le Pitre N, Malcolmson P, et al. Report of a national workshop on screening for cancer of the cervix. Can Med Assoc J 1991;145:1301-25.
4. American College of Obstetricians and Gynecologists. Cervical cytology: evaluation and management of abnormalities. ACOG technical bulletin no. 183. Washington, DC: American College of Obstetricians and Gynecologists, 1993.
5. Melnikow J, Nuovo J, Willan AR, Chan BK, Howell LP. Natural history of cervical squamous intraepithelial lesions: A meta-analysis. Obstet Gynecol 1998;92:727-34.
6. Wright TC, Cox TJ, Massad LS, Twiggs LB, Wilkonson EJ. Consensus guidelines for the management of women with cervical cytological abnormalities. JAMA 2002;287:2120-29.
7. Drummond MF, O’Brien BJ, Stoddart GL, Torrance GW, eds. Methods for the economic evaluation of health care programs. 2nd edition. New York: Oxford University Press, 1997.
8. Torrance G. Measurement of health state utilities for economic appraisal: A review. J Health Econ., 1986;5:1-30.
9. Furlong W, Feeny D, Torrance GW, Barr R, Horsman J. Guide to design and development of health state utility instrumentation. Centre for Health Economics and Policy Development. Working Paper Series # 90-9. Hamilton, McMaster University, 1990.
10. Melnikow J, Nuovo J, Paliescheskey M, Stewart GK, Howell L, Green B. Detection of high grade cervical dysplasia: Impact of age and Bethesda system-related follow-up criteria. Diagnostic Cytopathol 1997;17:321-25.
11. Fink A, Kosecoff J, Chassin M, Brook RH. Consensus methods: Characteristics and guidelines for use. Am J Pub Health 1984;74:979-83.
12. Nease RF, Kneeland T, O’Connor GT, Sumner W, Lumpkins C, Shaw L, et al. Variation in patient utilities for outcomes of the management of chronic stable angina. JAMA 1995;273:1185-90.
13. Ferris DG, Kriegel D, Cole L, Litaker M, Woodward L. Women’s triage and management p for cervical cytologic reports demonstrating atypical squamous cells of undetermined significance and low grade squamous intraepithelial lesions. Arch Fam Med 1997;6:348-53.
14. Meana M, Steward DE, Lickrish GM, Murphy J, Rosen B. Patient preference for the management of midly abnormal Papanicolaou smears. J Women’s Health and Gender Based Medicine 1999;8:941-7.
15. Kuppermann M, Nease RF, Learman LA, Gates E, Posner SF, Washington AE. How do women value Down syndrome-affected birth and miscarriage? The thirty-five-year-old question. Decis Making 1998;18:468.-
16. Solomon D, Schiffman M, Tarone R. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst 2001;93(4):252-3.
17. The atypical squamous cells of undetermined significance/low grade squamous intraepithelial lesions triage study (ALTS) group. Human papillomavirus testing for triage of women with cytologic evidence of low-grade squamous intra-epithelial lesions: baseline data from a randomized trial. J Natl Cancer Inst,. 2000;92:397-402.
18. Kuppermann M, Shiboski S, Feeny D, Elkin E, Washington AE. Can preference scores for discrete states be used to derive preference scores for an entire path of events? An application to prenatal diagnosis. Med Decis Making 1997;17:42-55.
19. Kassirer JP, Pauker SG. The toss up. N Engl J Med 1981;305:1457-9.
20. Kaplan SH, Greenfield S, Ware JE, Jr. Assessing the effects of physician patient interactions on the outcomes of care. Med Care 1989;27 (Suppl 3):S110-27.
- Any of several approaches may be used in managing women who have low-grade Pap smear abnormalities.
- Women’s preferences for a particular management approach to an abnormal Pap smear vary widely.
- Asking patients specific questions about their desire to avoid procedures and tolerance for uncertainty may help to clarify preferences.
The management of women who have low-grade cytologic abnormalities—including atypical squamous cells (ASC) and low-grade squamous intraepithelial lesions (LSIL)—is controversial.1-4 Without strong evidence favoring a single approach, some clinicians recommend immediate colposcopy to obtain a definitive diagnosis and to exclude the presence of a high-grade lesion, while others recommend observation with serial Pap smears, given the tendency for many low-grade lesions to regress spontaneously.5,6 Immediate colposcopy has the advantage of giving a patient a relatively rapid assessment of the nature and extent of her cervical dysplasia; however, the procedure is uncomfortable, and overall management may not be affected. Observation with serial Pap smears may avoid an invasive procedure, but it may also cause anxiety as time passes without a definitive diagnosis.
Eliciting and understanding patient preferences is an important part of clinical decision making. The clinician provides the best available information on the probability of clinical outcomes and the implications of each for the patient’s health. But only the patient knows what these outcomes mean to her well-being (also called “utility”).
Given the clinical disagreement over how to proceed with abnormal ASC and LSIL Pap smear results, the decision should be influenced by a patient’s preference, informed by knowledge of outcomes and costs of alternative approaches. It is unclear which approach women prefer, and whether women’s preferences for specific protocols are associated with sociodemographic characteristics. To understand better how women weigh these trade-offs, we evaluated the preferences of a diverse group of women for contrasting management approaches to the evaluation of a hypothetical low-grade abnormal Pap smear result.
Methods
Study population
Study participants were recruited from the waiting rooms of 5 family planning clinics in Northern California’s Central Valley. Women were eligible for the study if they were 18 years of age or older, or, if minors, they were emancipated and could thus provide informed consent. Potential subjects were excluded if they spoke neither English nor Spanish or if they had never had a Pap smear. The study protocol and informed consent procedures were reviewed and approved by the University of California, Davis, Human Subjects Committee.
Instruments and outcome measures
Interviews were conducted in English or Spanish. Information regarding demographic characteristics, level of education, past experiences with abnormal Pap smears and cervical cancer, and self-rated religiosity was collected with a self-administered questionnaire. The primary outcome measures were utilities (quantified preferences for specific health states) for 6 different scenarios. These were assessed by the standard gamble (SG) method, described in more detail below.7
Possible utility scores range from 0 to 1. A score of 0 represents immediate death; a score of 1 represents full (or ideal) health for the rest of one’s life. Because the scenarios under consideration in this study did not involve any meaningful level of risk of death, we expected utility scores for the scenarios to cluster toward the upper end of the scale. As a result, a measurement instrument based on an “immediate death” versus “full health” scale would be unable to discriminate between different scenarios. To avoid this problem, a scale was used in which the lower end point was a non-death state unambiguously less preferred than each of the scenarios under consideration.8 We used “invasive cervical cancer requiring hysterectomy” as the lower end point (utility of 0) contrasted with “full health with all normal Pap smears” (utility of 1) to generate the original score (SG Dys). In a separate standard gamble, subjects rated invasive cervical cancer versus immediate death (SG Ca), so that all utilities could be converted to the standard scale, using the formula: (1 – SG Ca) (SG Dys) + SG Ca.
The 6 scenarios rated in the study are shown in Table 1. The scenarios represent 3 sets of progressively more invasive interventions for a low-grade abnormal Pap smear: (1) resolution, representing spontaneous regression with treatment not required; (2) a low-grade abnormality requiring treatment with cryotherapy; (3) a more severe abnormality requiring a cervical cone biopsy. Following either spontaneous resolution or treatment, all scenarios assumed the abnormality was resolved. For each of the 3 results, a management strategy based on observation with serial Pap smears was applied in 1 instance, and a strategy of early colposcopy was applied in the other instance, resulting in 6 different pathways to the ultimate outcome; a normal Pap smear. The time frame for these scenarios was 18–36 months.
Trained interviewers used a standardized approach to elicit preferences from each subject. Subjects were read a description of all the procedures involved in the scenarios. Descriptions were accompanied by cards summarizing each procedure in pictures and words, and included information about the possibility of progression and spontaneous regression of the Pap smear abnormality. Subjects were encouraged to ask questions at any point during the interview. Procedure descriptions are available from the authors on request.
TABLE 1
Clinical scenarios classified by management approach and required treatment*
| Spontaneous resolution | Cryotherapy | Cone biopsy | |
|---|---|---|---|
| Observation | Pap smear: low-grade abnormal | Pap smear: low-grade abnormal | Pap smear: low-grade abnormal |
| ↓ | ↓ | ↓ | |
| Pap smear: normal | Pap smear: low-grade abnormal | Pap smear: normal | |
| ↓ | ↓ | ↓ | |
| 2 Pap smears every 6 months: normal | Pap smear: low-grade abnormal | Pap smear: normal | |
| ↓ | ↓ | ||
| Pap smear: low-grade abnormal | Pap smear: normal | ||
| ↓ | ↓ | ||
| Colposcopy and biopsy at 1 month | Colposcopy and biopsy at 1 month | ||
| ↓ | ↓ | ||
| Biopsy: low-grade abnormal | Biopsy: abnormal with ? ECC | ||
| ↓ | ↓ | ||
| Cryotherapy at 1 month | Cone biopsy at 1 month: moderately abnoramal cells | ||
| ↓ | ↓ | ||
| 3 Pap smears every 6 months: normal | Cure with cone biopsy | ||
| ↓ | |||
| 3 Pap smears every 6 months: normal | |||
| Early colposcopy | Pap smear: low-grade abnormal | Pap smear: low-grade abnormal | Pap smear: low-grade abnormal |
| ↓ | ↓ | ↓ | |
| Colposcopy and biopsy at 1 month | Colposcopy and biopsy at 1 month | Colposcopy and biopsy at 1 month | |
| ↓ | ↓ | ↓ | |
| Biopsy: normal | Biopsy: abnormal with ? ECC | Biopsy: abnormal with ? ECC | |
| ↓ | ↓ | ↓ | |
| Second colposcopy and biopsy | Cone biopsy at 1 month | Cone biopsy at 1 month | |
| ↓ | ↓ | ↓ | |
| Biopsy: normal | Biopsy: moderately abnormal | Biopsy: moderately abnormal | |
| ↓ | ↓ | ↓ | |
| 2 Pap smears every 6 months: normal | Cure with cone biopsy | Cure with cone biopsy | |
| ↓ | ↓ | ↓ | |
| Pap smear: low-grade abnormal | Colposcopy: normal | Colposcopy: normal | |
| ↓ | ↓ | ↓ | |
| Colposcopy and biopsy at 1 month | 2 Pap smears every 6 months: normal | 2 Pap smears every 6 months: normal | |
| ↓ | |||
| Biopsy: low-grade abnormal | |||
| ↓ | |||
| Colposcopy: normal | |||
| ↓ | |||
| 2 Pap smears every 6 months: normal | |||
| *Intervals are 6 months unless specified otherwise. ECC, endocervical curettage. | |||
Standard gamble
Subjects were asked their preference between the certainty of the scenario under consideration and an uncertain prospect of either having cervical cancer treated by hysterectomy or full health. A probability wheel was used as visual aid.9 The probability of cervical cancer was altered until the subject was indifferent between the certain scenario and the uncertain prospect. Once all 6 scenarios had been scored, each subject was asked about her preference between the certainty of cervical cancer treated by hysterectomy and the uncertain prospect of immediate death or full health, using the same method.
At the end of the interview, both the subject and the interviewer completed evaluation forms including ratings of how well the subject understood the standard gamble rating exercises. Subject confusion was also defined a priori as those placing a higher utility on scenario 3 (observation for a long period followed by cone biopsy), which represented the longest period of uncertainty followed by the most invasive procedure, than on scenario 1 (a single mildly abnormal Pap smear evaluated by observation which then resolved spontaneously), which represented the absence of any invasive procedure.
Statistical analysis
Descriptive statistics were generated for ratings of each scenario for the entire group and with the confused subjects removed. Confused subjects included those who reported they found the interview “very confusing,” those who were recorded by the interviewer as finding the interview “very confusing,” and those whose rankings met the criteria for subject confusion, as described above. Means, standard deviations, medians, and percentiles were calculated for each scenario. The mean differences in adjusted standard gamble ratings between paired scenarios was evaluated using a t distribution. Multiple regression analyses were used to explore how much between-subject variation in the standard gamble scores was explained by the variables listed above.
A simple decision tree (Figure 1) was constructed to contrast preferences for an observational approach vs early colposcopy. Outcome probabilities were derived from meta-analyses of the medical literature,5 from observational data obtained at the same Northern California family planning clinics,10 and, for cone biopsy outcomes, from expert opinion obtained using a modified Delphi process.11 Utilities were assigned to the decision tree based on the standard gamble results. Women having 2 consecutive low-grade abnormal Pap results followed by a normal Pap result were assigned the same utility value as that for women with a single abnormal result. Analysis of the tree, including 1-way and 2-way sensitivity analysis of key variables, was conducted with Data 3.5.
Results
One hundred seventy interviews were completed. Characteristics of the interview subjects are shown in Table 2. A total of 22 subjects were designated “confused.” Analyses including the confused subjects did not alter the pattern of results, but the range in responses was larger. All analyses are presented here with confused subjects removed (n = 148).
Median ratings with 25th–75th percentiles for the paired scenarios rated by the standard gamble are shown as box plots in Figure 2. Mean adjusted scores, standard deviations, and mean differences in scores between paired scenarios are shown in Table 3. Notable findings include the following. (1) For each scenario, the range of responses by either rating method was very large. (2) Mean differences in utilities for observation vs early colposcopy were small. (3) For the paired scenarios in which the outcome was spontaneous resolution, observation was preferred (P = .01); in the paired scenarios in which the outcome was cryotherapy, early colposcopy was preferred (P = .02). (4) In the multiple regression analyses for each scenario, age, education, ethnicity, religiosity, and having known someone with cervical cancer together explained only a small amount of the variability between subjects (range for R2, .09–.22).
The decision model with baseline probabilities is shown in Figure 1. The model was simplified to exclude the outcome of cervical cancer, which is a very rare outcome for women with ASC or LSIL cervical smears who have adequate follow-up.5 In the baseline analysis, the overall utility of early colposcopy was slightly favored over the overall utility of the observation approach (utility of observation = 0.932; utility of early colposcopy = 0.940).
Sensitivity analysis examines the effect of varying elements of the model on the outcome. In sensitivity analyses of probabilities, the early colposcopy branch was favored, but the differences were small. The maximum difference in utilities between branches was 0.012 in these sensitivity analyses. In 1-way sensitivity analysis of branch utilities, threshold utility values to favor the observation branch were 0.986 for spontaneous resolution after observation and 0.898 for early colposcopy. Threshold values for cryotherapy were 0.938 for observation and 0.938 for early colposcopy.
TABLE 2
Characteristics of study subjects (n = 170)
| Characteristics | n (%) |
|---|---|
| Mean age (range), y | 26 (14–53) |
| Education | |
| Less than high school | 58 (34%) |
| High school | 77 (45%) |
| Some college | 35 (20%) |
| Ethnicity | |
| African American | 21 (12%) |
| Caucasian | 84 (49%) |
| Latina | 46 (27%) |
| Other | 21 (12%) |
| Interview language, Spanish | 15 (9%) |
| Prior colposcopy | 23 (14%) |
| Moderately or very religious | 64 (38%) |
| Knows someone with cervical cancer | 43 (25%) |
TABLE 3
Adjusted standard gamble values and paired differences* (n=148)
| Management Strategy | ||||
|---|---|---|---|---|
| Short-term outcome | Observation Mean (SD) | Early colposcopy Mean (SD) | Difference | P value (2 sided) |
| Spontaneous resolution | .96 ±..13) | .93±.20) | .03 ±..15) | .01 |
| Cryotherapy | .93 ±..17) | .95 ±..14) | -.02 ±.11) | .02 |
| Cone biopsy | .91 ±..21) | .92 ±..16) | -.02 ±..17) | .23 |
| *Adjusted to scale so that immediate death had a utility of 0 and “full health with all normal Pap smears” had a utility of 1. | ||||
FIGURE 1Decision model comparing observation with early colposcopy *
FIGURE 2Distribution of individual utilities as assessed by the standard gamble*
Discussion
We found wide variation in women’s preferences for management approaches to a low-grade abnormal Pap smear result. The range of responses was very large and the variation between individuals rating the same scenario was substantially greater than the variation in mean ratings between different scenarios. Measured subject characteristics explained only a small proportion of the observed variation, indicating that other unmeasured factors contributed substantially to the variation. Although 25% of subjects stated they knew someone with cervical cancer, this high percentage seems improbable and more likely reflects knowledge of someone who had an abnormal Pap smear.
The decision model displayed a small preference for immediate colposcopy. This may be related to preference for quicker resolution of the concern about cancer, although it involves more procedures. Small changes in utilities for spontaneous resolution and cryotherapy influenced the model to prefer observation. For cryotherapy, these utility values were within 1 standard deviation of the mean.
Our finding of a wide variation in preferences is supported by other patient preference studies,12-14 including 2 on this subject. Ferris et al assessed triage preferences for the evaluation and management ASC and LSIL.13 They used a questionnaire with a sample of 968 women who presented for care at obstetrics and gynecology and family practice clinics. They found that more women preferred repeat Pap smear when the index smear was ASC, and more women preferred colposcopy when the index smear was LSIL. Among a group of 136 Canadian women with atypia or LSIL referred for colposcopy, Meana et al found that 64% preferred early colposcopy, while 17% preferred observation and 17% had no strong preference.14
The factors contributing to patient preferences are complex. Differences in preferences may be influenced by knowledge and understanding of the disease and possible interventions, risk aversion, access to services, socioeconomics, cultural background, and other factors. While 1 patient may be most interested in establishing a definitive diagnosis and undergoing treatment as soon as possible, another may place priority on avoiding invasive or uncomfortable procedures. How differences in patient preferences influence clinical choices is highlighted by the work of Kuppermann et al.15 These investigators found that utilities for outcomes of prenatal diagnostic testing predicted subsequent testing behavior.
Our findings are limited by our use of a convenience sample of women attending family planning clinics. They may not be representative of women’s preferences in general, or even those of women attending family planning clinics. Outcomes in our study were specified during the preference assessment process; in real decision making, the outcome is always unknown at the time the decision is made. We did not include HPV typing as an option in our clinical scenarios. While HPV typing may have a role for triage of ASC,6,16 it appears not to be useful in management of LSIL.17
Cost-effectiveness analysis would offer important information about which management approach might be favored in the context of resource allocation. For decision making by individual patients and doctors, however, decision analysis is often more relevant. In this case, the “preferred” decision is very sensitive to patient utilities, emphasizing the need for clear physician-patient communication.
Strengths of our study include the diversity of the subjects, the formal process for preference assessment, and the paired scenarios, which allow assessment of preferences for a single management decision, in which 2 separate paths lead to an equivalent ultimate outcome. Our findings are consistent with previous work showing that the sequence of events leading to an outcome will influence utilities for the outcome.18
Application to clinical practice
How might our findings be translated into clinical practice? In clinical situations where different approaches to management are unlikely to result in substantial outcome differences (a “toss-up”), patient preferences are a key aspect of the decision-making process.19 For women with lowgrade Pap abnormalities, several diagnostic options are available and no single option is strongly supported by evidence to offer better outcomes. Our study indicates that no single option is preferred by most women. Under these conditions, engaging the patient in the decision-making process may produce better health outcomes.20 Clinicians should anticipate highly varied preferences, and will need to adopt a flexible approach. Not all patients will want to be actively involved in the decision process, but the desire for information is nearly universal. Flexible use of the questions in Table 4 may help patients to define their preferences and will likely improve their satisfaction and adherence to the treatment plan.
TABLE 4
Questions for patients with an abnormal Pap smear
| What is your understanding of what it means for you to have an abnormal Pap smear showing _____________? |
| There are different options for the next step. Would you like to be involved in deciding which option is preferred for your case? |
| What questions do you have about these options? |
| How important is it to you to have a definite answer as soon as possible? |
| How do you feel about undergoing colposcopy? |
| Would you prefer to have a follow-up Pap smear in ____ months, which might avoid a colposcopy, or would you prefer to have a colposcopy sooner? |
· Acknowledgments ·
The authors thank the staff of Planned Parenthood Mar Monte East for their assistance with subject recruitment and interviews.
- Any of several approaches may be used in managing women who have low-grade Pap smear abnormalities.
- Women’s preferences for a particular management approach to an abnormal Pap smear vary widely.
- Asking patients specific questions about their desire to avoid procedures and tolerance for uncertainty may help to clarify preferences.
The management of women who have low-grade cytologic abnormalities—including atypical squamous cells (ASC) and low-grade squamous intraepithelial lesions (LSIL)—is controversial.1-4 Without strong evidence favoring a single approach, some clinicians recommend immediate colposcopy to obtain a definitive diagnosis and to exclude the presence of a high-grade lesion, while others recommend observation with serial Pap smears, given the tendency for many low-grade lesions to regress spontaneously.5,6 Immediate colposcopy has the advantage of giving a patient a relatively rapid assessment of the nature and extent of her cervical dysplasia; however, the procedure is uncomfortable, and overall management may not be affected. Observation with serial Pap smears may avoid an invasive procedure, but it may also cause anxiety as time passes without a definitive diagnosis.
Eliciting and understanding patient preferences is an important part of clinical decision making. The clinician provides the best available information on the probability of clinical outcomes and the implications of each for the patient’s health. But only the patient knows what these outcomes mean to her well-being (also called “utility”).
Given the clinical disagreement over how to proceed with abnormal ASC and LSIL Pap smear results, the decision should be influenced by a patient’s preference, informed by knowledge of outcomes and costs of alternative approaches. It is unclear which approach women prefer, and whether women’s preferences for specific protocols are associated with sociodemographic characteristics. To understand better how women weigh these trade-offs, we evaluated the preferences of a diverse group of women for contrasting management approaches to the evaluation of a hypothetical low-grade abnormal Pap smear result.
Methods
Study population
Study participants were recruited from the waiting rooms of 5 family planning clinics in Northern California’s Central Valley. Women were eligible for the study if they were 18 years of age or older, or, if minors, they were emancipated and could thus provide informed consent. Potential subjects were excluded if they spoke neither English nor Spanish or if they had never had a Pap smear. The study protocol and informed consent procedures were reviewed and approved by the University of California, Davis, Human Subjects Committee.
Instruments and outcome measures
Interviews were conducted in English or Spanish. Information regarding demographic characteristics, level of education, past experiences with abnormal Pap smears and cervical cancer, and self-rated religiosity was collected with a self-administered questionnaire. The primary outcome measures were utilities (quantified preferences for specific health states) for 6 different scenarios. These were assessed by the standard gamble (SG) method, described in more detail below.7
Possible utility scores range from 0 to 1. A score of 0 represents immediate death; a score of 1 represents full (or ideal) health for the rest of one’s life. Because the scenarios under consideration in this study did not involve any meaningful level of risk of death, we expected utility scores for the scenarios to cluster toward the upper end of the scale. As a result, a measurement instrument based on an “immediate death” versus “full health” scale would be unable to discriminate between different scenarios. To avoid this problem, a scale was used in which the lower end point was a non-death state unambiguously less preferred than each of the scenarios under consideration.8 We used “invasive cervical cancer requiring hysterectomy” as the lower end point (utility of 0) contrasted with “full health with all normal Pap smears” (utility of 1) to generate the original score (SG Dys). In a separate standard gamble, subjects rated invasive cervical cancer versus immediate death (SG Ca), so that all utilities could be converted to the standard scale, using the formula: (1 – SG Ca) (SG Dys) + SG Ca.
The 6 scenarios rated in the study are shown in Table 1. The scenarios represent 3 sets of progressively more invasive interventions for a low-grade abnormal Pap smear: (1) resolution, representing spontaneous regression with treatment not required; (2) a low-grade abnormality requiring treatment with cryotherapy; (3) a more severe abnormality requiring a cervical cone biopsy. Following either spontaneous resolution or treatment, all scenarios assumed the abnormality was resolved. For each of the 3 results, a management strategy based on observation with serial Pap smears was applied in 1 instance, and a strategy of early colposcopy was applied in the other instance, resulting in 6 different pathways to the ultimate outcome; a normal Pap smear. The time frame for these scenarios was 18–36 months.
Trained interviewers used a standardized approach to elicit preferences from each subject. Subjects were read a description of all the procedures involved in the scenarios. Descriptions were accompanied by cards summarizing each procedure in pictures and words, and included information about the possibility of progression and spontaneous regression of the Pap smear abnormality. Subjects were encouraged to ask questions at any point during the interview. Procedure descriptions are available from the authors on request.
TABLE 1
Clinical scenarios classified by management approach and required treatment*
| Spontaneous resolution | Cryotherapy | Cone biopsy | |
|---|---|---|---|
| Observation | Pap smear: low-grade abnormal | Pap smear: low-grade abnormal | Pap smear: low-grade abnormal |
| ↓ | ↓ | ↓ | |
| Pap smear: normal | Pap smear: low-grade abnormal | Pap smear: normal | |
| ↓ | ↓ | ↓ | |
| 2 Pap smears every 6 months: normal | Pap smear: low-grade abnormal | Pap smear: normal | |
| ↓ | ↓ | ||
| Pap smear: low-grade abnormal | Pap smear: normal | ||
| ↓ | ↓ | ||
| Colposcopy and biopsy at 1 month | Colposcopy and biopsy at 1 month | ||
| ↓ | ↓ | ||
| Biopsy: low-grade abnormal | Biopsy: abnormal with ? ECC | ||
| ↓ | ↓ | ||
| Cryotherapy at 1 month | Cone biopsy at 1 month: moderately abnoramal cells | ||
| ↓ | ↓ | ||
| 3 Pap smears every 6 months: normal | Cure with cone biopsy | ||
| ↓ | |||
| 3 Pap smears every 6 months: normal | |||
| Early colposcopy | Pap smear: low-grade abnormal | Pap smear: low-grade abnormal | Pap smear: low-grade abnormal |
| ↓ | ↓ | ↓ | |
| Colposcopy and biopsy at 1 month | Colposcopy and biopsy at 1 month | Colposcopy and biopsy at 1 month | |
| ↓ | ↓ | ↓ | |
| Biopsy: normal | Biopsy: abnormal with ? ECC | Biopsy: abnormal with ? ECC | |
| ↓ | ↓ | ↓ | |
| Second colposcopy and biopsy | Cone biopsy at 1 month | Cone biopsy at 1 month | |
| ↓ | ↓ | ↓ | |
| Biopsy: normal | Biopsy: moderately abnormal | Biopsy: moderately abnormal | |
| ↓ | ↓ | ↓ | |
| 2 Pap smears every 6 months: normal | Cure with cone biopsy | Cure with cone biopsy | |
| ↓ | ↓ | ↓ | |
| Pap smear: low-grade abnormal | Colposcopy: normal | Colposcopy: normal | |
| ↓ | ↓ | ↓ | |
| Colposcopy and biopsy at 1 month | 2 Pap smears every 6 months: normal | 2 Pap smears every 6 months: normal | |
| ↓ | |||
| Biopsy: low-grade abnormal | |||
| ↓ | |||
| Colposcopy: normal | |||
| ↓ | |||
| 2 Pap smears every 6 months: normal | |||
| *Intervals are 6 months unless specified otherwise. ECC, endocervical curettage. | |||
Standard gamble
Subjects were asked their preference between the certainty of the scenario under consideration and an uncertain prospect of either having cervical cancer treated by hysterectomy or full health. A probability wheel was used as visual aid.9 The probability of cervical cancer was altered until the subject was indifferent between the certain scenario and the uncertain prospect. Once all 6 scenarios had been scored, each subject was asked about her preference between the certainty of cervical cancer treated by hysterectomy and the uncertain prospect of immediate death or full health, using the same method.
At the end of the interview, both the subject and the interviewer completed evaluation forms including ratings of how well the subject understood the standard gamble rating exercises. Subject confusion was also defined a priori as those placing a higher utility on scenario 3 (observation for a long period followed by cone biopsy), which represented the longest period of uncertainty followed by the most invasive procedure, than on scenario 1 (a single mildly abnormal Pap smear evaluated by observation which then resolved spontaneously), which represented the absence of any invasive procedure.
Statistical analysis
Descriptive statistics were generated for ratings of each scenario for the entire group and with the confused subjects removed. Confused subjects included those who reported they found the interview “very confusing,” those who were recorded by the interviewer as finding the interview “very confusing,” and those whose rankings met the criteria for subject confusion, as described above. Means, standard deviations, medians, and percentiles were calculated for each scenario. The mean differences in adjusted standard gamble ratings between paired scenarios was evaluated using a t distribution. Multiple regression analyses were used to explore how much between-subject variation in the standard gamble scores was explained by the variables listed above.
A simple decision tree (Figure 1) was constructed to contrast preferences for an observational approach vs early colposcopy. Outcome probabilities were derived from meta-analyses of the medical literature,5 from observational data obtained at the same Northern California family planning clinics,10 and, for cone biopsy outcomes, from expert opinion obtained using a modified Delphi process.11 Utilities were assigned to the decision tree based on the standard gamble results. Women having 2 consecutive low-grade abnormal Pap results followed by a normal Pap result were assigned the same utility value as that for women with a single abnormal result. Analysis of the tree, including 1-way and 2-way sensitivity analysis of key variables, was conducted with Data 3.5.
Results
One hundred seventy interviews were completed. Characteristics of the interview subjects are shown in Table 2. A total of 22 subjects were designated “confused.” Analyses including the confused subjects did not alter the pattern of results, but the range in responses was larger. All analyses are presented here with confused subjects removed (n = 148).
Median ratings with 25th–75th percentiles for the paired scenarios rated by the standard gamble are shown as box plots in Figure 2. Mean adjusted scores, standard deviations, and mean differences in scores between paired scenarios are shown in Table 3. Notable findings include the following. (1) For each scenario, the range of responses by either rating method was very large. (2) Mean differences in utilities for observation vs early colposcopy were small. (3) For the paired scenarios in which the outcome was spontaneous resolution, observation was preferred (P = .01); in the paired scenarios in which the outcome was cryotherapy, early colposcopy was preferred (P = .02). (4) In the multiple regression analyses for each scenario, age, education, ethnicity, religiosity, and having known someone with cervical cancer together explained only a small amount of the variability between subjects (range for R2, .09–.22).
The decision model with baseline probabilities is shown in Figure 1. The model was simplified to exclude the outcome of cervical cancer, which is a very rare outcome for women with ASC or LSIL cervical smears who have adequate follow-up.5 In the baseline analysis, the overall utility of early colposcopy was slightly favored over the overall utility of the observation approach (utility of observation = 0.932; utility of early colposcopy = 0.940).
Sensitivity analysis examines the effect of varying elements of the model on the outcome. In sensitivity analyses of probabilities, the early colposcopy branch was favored, but the differences were small. The maximum difference in utilities between branches was 0.012 in these sensitivity analyses. In 1-way sensitivity analysis of branch utilities, threshold utility values to favor the observation branch were 0.986 for spontaneous resolution after observation and 0.898 for early colposcopy. Threshold values for cryotherapy were 0.938 for observation and 0.938 for early colposcopy.
TABLE 2
Characteristics of study subjects (n = 170)
| Characteristics | n (%) |
|---|---|
| Mean age (range), y | 26 (14–53) |
| Education | |
| Less than high school | 58 (34%) |
| High school | 77 (45%) |
| Some college | 35 (20%) |
| Ethnicity | |
| African American | 21 (12%) |
| Caucasian | 84 (49%) |
| Latina | 46 (27%) |
| Other | 21 (12%) |
| Interview language, Spanish | 15 (9%) |
| Prior colposcopy | 23 (14%) |
| Moderately or very religious | 64 (38%) |
| Knows someone with cervical cancer | 43 (25%) |
TABLE 3
Adjusted standard gamble values and paired differences* (n=148)
| Management Strategy | ||||
|---|---|---|---|---|
| Short-term outcome | Observation Mean (SD) | Early colposcopy Mean (SD) | Difference | P value (2 sided) |
| Spontaneous resolution | .96 ±..13) | .93±.20) | .03 ±..15) | .01 |
| Cryotherapy | .93 ±..17) | .95 ±..14) | -.02 ±.11) | .02 |
| Cone biopsy | .91 ±..21) | .92 ±..16) | -.02 ±..17) | .23 |
| *Adjusted to scale so that immediate death had a utility of 0 and “full health with all normal Pap smears” had a utility of 1. | ||||
FIGURE 1Decision model comparing observation with early colposcopy *
FIGURE 2Distribution of individual utilities as assessed by the standard gamble*
Discussion
We found wide variation in women’s preferences for management approaches to a low-grade abnormal Pap smear result. The range of responses was very large and the variation between individuals rating the same scenario was substantially greater than the variation in mean ratings between different scenarios. Measured subject characteristics explained only a small proportion of the observed variation, indicating that other unmeasured factors contributed substantially to the variation. Although 25% of subjects stated they knew someone with cervical cancer, this high percentage seems improbable and more likely reflects knowledge of someone who had an abnormal Pap smear.
The decision model displayed a small preference for immediate colposcopy. This may be related to preference for quicker resolution of the concern about cancer, although it involves more procedures. Small changes in utilities for spontaneous resolution and cryotherapy influenced the model to prefer observation. For cryotherapy, these utility values were within 1 standard deviation of the mean.
Our finding of a wide variation in preferences is supported by other patient preference studies,12-14 including 2 on this subject. Ferris et al assessed triage preferences for the evaluation and management ASC and LSIL.13 They used a questionnaire with a sample of 968 women who presented for care at obstetrics and gynecology and family practice clinics. They found that more women preferred repeat Pap smear when the index smear was ASC, and more women preferred colposcopy when the index smear was LSIL. Among a group of 136 Canadian women with atypia or LSIL referred for colposcopy, Meana et al found that 64% preferred early colposcopy, while 17% preferred observation and 17% had no strong preference.14
The factors contributing to patient preferences are complex. Differences in preferences may be influenced by knowledge and understanding of the disease and possible interventions, risk aversion, access to services, socioeconomics, cultural background, and other factors. While 1 patient may be most interested in establishing a definitive diagnosis and undergoing treatment as soon as possible, another may place priority on avoiding invasive or uncomfortable procedures. How differences in patient preferences influence clinical choices is highlighted by the work of Kuppermann et al.15 These investigators found that utilities for outcomes of prenatal diagnostic testing predicted subsequent testing behavior.
Our findings are limited by our use of a convenience sample of women attending family planning clinics. They may not be representative of women’s preferences in general, or even those of women attending family planning clinics. Outcomes in our study were specified during the preference assessment process; in real decision making, the outcome is always unknown at the time the decision is made. We did not include HPV typing as an option in our clinical scenarios. While HPV typing may have a role for triage of ASC,6,16 it appears not to be useful in management of LSIL.17
Cost-effectiveness analysis would offer important information about which management approach might be favored in the context of resource allocation. For decision making by individual patients and doctors, however, decision analysis is often more relevant. In this case, the “preferred” decision is very sensitive to patient utilities, emphasizing the need for clear physician-patient communication.
Strengths of our study include the diversity of the subjects, the formal process for preference assessment, and the paired scenarios, which allow assessment of preferences for a single management decision, in which 2 separate paths lead to an equivalent ultimate outcome. Our findings are consistent with previous work showing that the sequence of events leading to an outcome will influence utilities for the outcome.18
Application to clinical practice
How might our findings be translated into clinical practice? In clinical situations where different approaches to management are unlikely to result in substantial outcome differences (a “toss-up”), patient preferences are a key aspect of the decision-making process.19 For women with lowgrade Pap abnormalities, several diagnostic options are available and no single option is strongly supported by evidence to offer better outcomes. Our study indicates that no single option is preferred by most women. Under these conditions, engaging the patient in the decision-making process may produce better health outcomes.20 Clinicians should anticipate highly varied preferences, and will need to adopt a flexible approach. Not all patients will want to be actively involved in the decision process, but the desire for information is nearly universal. Flexible use of the questions in Table 4 may help patients to define their preferences and will likely improve their satisfaction and adherence to the treatment plan.
TABLE 4
Questions for patients with an abnormal Pap smear
| What is your understanding of what it means for you to have an abnormal Pap smear showing _____________? |
| There are different options for the next step. Would you like to be involved in deciding which option is preferred for your case? |
| What questions do you have about these options? |
| How important is it to you to have a definite answer as soon as possible? |
| How do you feel about undergoing colposcopy? |
| Would you prefer to have a follow-up Pap smear in ____ months, which might avoid a colposcopy, or would you prefer to have a colposcopy sooner? |
· Acknowledgments ·
The authors thank the staff of Planned Parenthood Mar Monte East for their assistance with subject recruitment and interviews.
1. Woolf SH. Screening for cervical cancer. In: Goldbloom RB, Lawrence RS, eds. Preventing disease: beyond the rhetoric. New York: Spring-Verlag, 1990:319–23.
2. Kurman RJ, Henson DE, Herbst AL, Noller KL, Schiffman MH. Interim guidelines for management of abnormal cervical cytology. The 1992 National Cancer Institute Workshop. JAMA 1994;271:1866-69.
3. Miller AB, Anderson G, Brisson J, Laidlaw J, Le Pitre N, Malcolmson P, et al. Report of a national workshop on screening for cancer of the cervix. Can Med Assoc J 1991;145:1301-25.
4. American College of Obstetricians and Gynecologists. Cervical cytology: evaluation and management of abnormalities. ACOG technical bulletin no. 183. Washington, DC: American College of Obstetricians and Gynecologists, 1993.
5. Melnikow J, Nuovo J, Willan AR, Chan BK, Howell LP. Natural history of cervical squamous intraepithelial lesions: A meta-analysis. Obstet Gynecol 1998;92:727-34.
6. Wright TC, Cox TJ, Massad LS, Twiggs LB, Wilkonson EJ. Consensus guidelines for the management of women with cervical cytological abnormalities. JAMA 2002;287:2120-29.
7. Drummond MF, O’Brien BJ, Stoddart GL, Torrance GW, eds. Methods for the economic evaluation of health care programs. 2nd edition. New York: Oxford University Press, 1997.
8. Torrance G. Measurement of health state utilities for economic appraisal: A review. J Health Econ., 1986;5:1-30.
9. Furlong W, Feeny D, Torrance GW, Barr R, Horsman J. Guide to design and development of health state utility instrumentation. Centre for Health Economics and Policy Development. Working Paper Series # 90-9. Hamilton, McMaster University, 1990.
10. Melnikow J, Nuovo J, Paliescheskey M, Stewart GK, Howell L, Green B. Detection of high grade cervical dysplasia: Impact of age and Bethesda system-related follow-up criteria. Diagnostic Cytopathol 1997;17:321-25.
11. Fink A, Kosecoff J, Chassin M, Brook RH. Consensus methods: Characteristics and guidelines for use. Am J Pub Health 1984;74:979-83.
12. Nease RF, Kneeland T, O’Connor GT, Sumner W, Lumpkins C, Shaw L, et al. Variation in patient utilities for outcomes of the management of chronic stable angina. JAMA 1995;273:1185-90.
13. Ferris DG, Kriegel D, Cole L, Litaker M, Woodward L. Women’s triage and management p for cervical cytologic reports demonstrating atypical squamous cells of undetermined significance and low grade squamous intraepithelial lesions. Arch Fam Med 1997;6:348-53.
14. Meana M, Steward DE, Lickrish GM, Murphy J, Rosen B. Patient preference for the management of midly abnormal Papanicolaou smears. J Women’s Health and Gender Based Medicine 1999;8:941-7.
15. Kuppermann M, Nease RF, Learman LA, Gates E, Posner SF, Washington AE. How do women value Down syndrome-affected birth and miscarriage? The thirty-five-year-old question. Decis Making 1998;18:468.-
16. Solomon D, Schiffman M, Tarone R. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst 2001;93(4):252-3.
17. The atypical squamous cells of undetermined significance/low grade squamous intraepithelial lesions triage study (ALTS) group. Human papillomavirus testing for triage of women with cytologic evidence of low-grade squamous intra-epithelial lesions: baseline data from a randomized trial. J Natl Cancer Inst,. 2000;92:397-402.
18. Kuppermann M, Shiboski S, Feeny D, Elkin E, Washington AE. Can preference scores for discrete states be used to derive preference scores for an entire path of events? An application to prenatal diagnosis. Med Decis Making 1997;17:42-55.
19. Kassirer JP, Pauker SG. The toss up. N Engl J Med 1981;305:1457-9.
20. Kaplan SH, Greenfield S, Ware JE, Jr. Assessing the effects of physician patient interactions on the outcomes of care. Med Care 1989;27 (Suppl 3):S110-27.
1. Woolf SH. Screening for cervical cancer. In: Goldbloom RB, Lawrence RS, eds. Preventing disease: beyond the rhetoric. New York: Spring-Verlag, 1990:319–23.
2. Kurman RJ, Henson DE, Herbst AL, Noller KL, Schiffman MH. Interim guidelines for management of abnormal cervical cytology. The 1992 National Cancer Institute Workshop. JAMA 1994;271:1866-69.
3. Miller AB, Anderson G, Brisson J, Laidlaw J, Le Pitre N, Malcolmson P, et al. Report of a national workshop on screening for cancer of the cervix. Can Med Assoc J 1991;145:1301-25.
4. American College of Obstetricians and Gynecologists. Cervical cytology: evaluation and management of abnormalities. ACOG technical bulletin no. 183. Washington, DC: American College of Obstetricians and Gynecologists, 1993.
5. Melnikow J, Nuovo J, Willan AR, Chan BK, Howell LP. Natural history of cervical squamous intraepithelial lesions: A meta-analysis. Obstet Gynecol 1998;92:727-34.
6. Wright TC, Cox TJ, Massad LS, Twiggs LB, Wilkonson EJ. Consensus guidelines for the management of women with cervical cytological abnormalities. JAMA 2002;287:2120-29.
7. Drummond MF, O’Brien BJ, Stoddart GL, Torrance GW, eds. Methods for the economic evaluation of health care programs. 2nd edition. New York: Oxford University Press, 1997.
8. Torrance G. Measurement of health state utilities for economic appraisal: A review. J Health Econ., 1986;5:1-30.
9. Furlong W, Feeny D, Torrance GW, Barr R, Horsman J. Guide to design and development of health state utility instrumentation. Centre for Health Economics and Policy Development. Working Paper Series # 90-9. Hamilton, McMaster University, 1990.
10. Melnikow J, Nuovo J, Paliescheskey M, Stewart GK, Howell L, Green B. Detection of high grade cervical dysplasia: Impact of age and Bethesda system-related follow-up criteria. Diagnostic Cytopathol 1997;17:321-25.
11. Fink A, Kosecoff J, Chassin M, Brook RH. Consensus methods: Characteristics and guidelines for use. Am J Pub Health 1984;74:979-83.
12. Nease RF, Kneeland T, O’Connor GT, Sumner W, Lumpkins C, Shaw L, et al. Variation in patient utilities for outcomes of the management of chronic stable angina. JAMA 1995;273:1185-90.
13. Ferris DG, Kriegel D, Cole L, Litaker M, Woodward L. Women’s triage and management p for cervical cytologic reports demonstrating atypical squamous cells of undetermined significance and low grade squamous intraepithelial lesions. Arch Fam Med 1997;6:348-53.
14. Meana M, Steward DE, Lickrish GM, Murphy J, Rosen B. Patient preference for the management of midly abnormal Papanicolaou smears. J Women’s Health and Gender Based Medicine 1999;8:941-7.
15. Kuppermann M, Nease RF, Learman LA, Gates E, Posner SF, Washington AE. How do women value Down syndrome-affected birth and miscarriage? The thirty-five-year-old question. Decis Making 1998;18:468.-
16. Solomon D, Schiffman M, Tarone R. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst 2001;93(4):252-3.
17. The atypical squamous cells of undetermined significance/low grade squamous intraepithelial lesions triage study (ALTS) group. Human papillomavirus testing for triage of women with cytologic evidence of low-grade squamous intra-epithelial lesions: baseline data from a randomized trial. J Natl Cancer Inst,. 2000;92:397-402.
18. Kuppermann M, Shiboski S, Feeny D, Elkin E, Washington AE. Can preference scores for discrete states be used to derive preference scores for an entire path of events? An application to prenatal diagnosis. Med Decis Making 1997;17:42-55.
19. Kassirer JP, Pauker SG. The toss up. N Engl J Med 1981;305:1457-9.
20. Kaplan SH, Greenfield S, Ware JE, Jr. Assessing the effects of physician patient interactions on the outcomes of care. Med Care 1989;27 (Suppl 3):S110-27.
Do written action plans improve patient outcomes in asthma? An evidence-based analysis
- Most studies of asthma self-management do not permit retrospective isolation of the independent effects of a written action plan or peak flow meter use.
- Studies designed to isolate the effect of these self-care activities are generally underpowered or prone to systematic bias.
- Available evidence suggests that peak flow meters and written action plans do not have a large impact on outcomes when applied to the general population of asthmatics.
- These interventions are most likely to have beneficial effect when applied to selected populations, particularly patients with high baseline utilization.
Self-management skills are widely promoted by health plans and specialty societies with the expectation that they will improve care. The 1997 National Heart, Lung, and Blood Institute guidelines on treating asthma emphasize self-management,1 although they do not recommend specific programs. To maximize therapeutic effectiveness, it would be useful to know which components of patient self-management improve outcomes. Written action plans and peak flow meters are commonly used in asthma self-management programs. While these are simple, low-cost interventions for an individual, the aggregate cost for the entire population of asthmatics may be high.2
Much literature has accumulated on the effectiveness of providing asthma education alone and on programs that actively engage patients in their own care.Several systematic reviews have found that providing educational information alone has had little effect on asthma outcomes.3-5 There is evidence, though, that self-management activities are more effective than educational information alone. A recent Cochrane review of 24 trials found that self-management with regular practitioner review reduces hospi-talizations and emergency room visits.6 This review did not identify specific components contributing to improved outcomes. In contrast to the aforementioned studies on patient education, a large case-control study of children in the Kaiser Permanente System,7 found that written action plans were associated with lower rates of hospitalization and emergency room use. However, such observational studies often include confounding factors and are not sufficient to establish a cause-effect relationship between written action plans and improved outcomes.
We report on a systematic review that attempts to isolate the independent effect of a written action plan on asthma outcomes. We address two key questions:
- Compared with medical management alone, does the addition of a written asthma action plan (with or without peak flow meter use) improve outcomes?
- Compared with a written action plan based on symptoms, does a written action plan based on peak flow monitoring improve outcomes?
Methods
This study is part of a broader evidence report on the management of chronic asthma prepared for the Agency of Health Care Research and Quality8. Complete details of the methodology are available in the full report8 (http://www.ahcpr.gov/clinic/epcix.htm).
Literature search and study selection
We performed a comprehensive literature search from 1980 to August 2000 using MEDLINE, Embase, the Cochrane Library, and a hand search of recent bibliographies. The search was limited to full-length, peer-reviewed articles with an English abstract. Two independent reviewers carried out each step of study selection and data abstraction. Disagreements were resolved by consensus of the two reviewers or, if necessary, by the decision of a third reviewer.
Initial study selection was limited to comparative full-length reports or abstracts in peer-reviewed medical journals, with at least 25 evaluable children or adults per arm, treated for at least 12 weeks. Relevant comparisons included a written action plan and no written action plan; a written action plan based on peak flow readings and a written action plan based on symptoms. Study designs varied: clinical trials, cohort comparisons, case-control analyses, cross-sectional evaluations, and before-after comparisons. Specific components of the management plan had to be described.
Relevant outcomes included measures of inpatient and outpatient utilization, lung function, symptoms, rescue medication or oral steroid use, and quality of life. Outcomes of greatest interest were utilization parameters, as the goals of self-management usually focus on improving these outcomes.
These initial selection criteria yielded many studies that were confounded by multiple asthma management interventions and thus did not isolate the comparisons of interest. Therefore, the research team collectively determined the study design features that would best isolate the effects of written action plans and used them as new criteria in a second round of study selection. The studies thus selected satisfied 4 criteria: 1) randomization of patients; (2) delivery of the same interventions to experimental and control groups, except that the experimental group also received a written action plan; (3) delivery of the same interventions to experimental and control groups, except that one group received a written action plan based on peak flow meter readings, and the comparison group received a written action plan based on symptom monitoring; and 4) inclusion of a written action plan that met our specified definition.
A written action plan, by our definition, had two components: an algorithm that identified specific clinical indicators signaling the need for adjustments in medication; and specific instructions on how to adjust medications in response to such indicators. Many publications lacked sufficient detail on the written plan, so a brief survey was sent to the primary author of each of the 36 studies. If no response was obtained (36%), the article was excluded only when it was clear from the publication that our definition was not met.
Assessment of study quality
High-quality studies were randomized controlled trials that met the 3 domains of study quality that have been demonstrated empirically to impact effect size: concealment of treatment allocation; double-blinding; and minimization of exclusion bias.9,10 However, we doubted the feasibility of double-blinding a written asthma plan intervention, and so relaxed this requirement. We considered exclusion bias to be minimized when a study either reported intent-to-treat analysis or excluded fewer than 10% of subjects from analysis, with the ratio of subjects excluded from each arm being less than 2:1.
To more fully evaluate study design issues that may be particularly important in asthma research,11,12 we constructed asthma-specific quality indicators in consultation with an expert panel. Controls for potential confounders of treatment effect included establishing reversibility of airway obstruction, controlling for other medication use, reporting compliance, and addressing seasonality. In addition, a priori reporting of power calculations and accounting for exclusions and withdrawals were judged to be study quality characteristics pertinent to this body of evidence.
Data analysis
We constructed evidence tables for the outcomes of interest, and performed a qualitative synthesis of the data. Meta-analysis was not appropriate due to wide discrepancies in the patient populations studied, the interventions employed, and measurement and reporting of outcomes.
Results
Our literature search yielded a total of 4578 citations. Of these, 36 studies met the initial selection criteria. Many of these qualifying studies, however, were confounded by multiple asthma management interventions applied inconsistently across treatment arms. For example, a common confounder was review of and change in long-term medication use in the treatment group, but not in the control group. This necessitated a refinement in our selection criteria to focus on studies that largely isolated the effect of written action plans.13-21 This step yielded a final evidence base of 9 randomized controlled trials with a total enrollment of 1501 patients.
Table 1 summarizes the characteristics, interventions, and outcomes of the 9 studies. Two studies were 3-arm trials,16,17 which raised the total number of comparisons among the 9 studies to 11. The largest study was the Grampian Asthma Study of Integrated Care (n=569),14 a community study conducted in the UK. Enrollment in the other 8 studies ranged from 43 to 64 patients per arm. Treatment duration ranged from 24 to 52 weeks.
None of the studies met our definition of high quality. In fact, no study met any of the generic quality criteria—none was blinded, none described concealment of allocation, and all excluded more than 10% of subjects. Furthermore, none reported an intention-to-treat analysis. Thus these trials were prone to withdrawal bias as well as overestimation of treatment effect due to lack of allocation concealment.
No study met the majority of asthma-specific indicators (Table 1). Of the 9 studies, only 5 met any asthma-specific indicator. Three reported prospective power calculations,13-15 but 2 of these substantially overestimated the expected effect.13,15 Two studies established reversibility;14,17 2 controlled for other medication use;13,15 and 2 reported compliance.17,21 Thus, the studies were also prone to a type II error (failing to detect a true effect) and to potential confounding of outcomes.
We performed sample power calculations for hospitalizations (Table 2), derived from baseline rates reported in 4 studies14,16-18 and standard deviations reported in 2.14,17 A study with 250 patients per arm could detect a reduction of 50% or more in hospitalization, given a control rate of at least 0.2 hospitalizations/patient/year. In actuality, GRASSIC,14 which is the largest available trial (N=569), had baseline hospitalization rates of 0.12 and 0.13. With this baseline rate, over 700 patients per arm are required, higher than the actual enrollment in GRASSIC. The other studies in this review would be adequately powered to detect a 50% difference only in the setting of even higher baseline utilization (eg, 0.30 hospitalizations/patient/year).
Table 3 displays utilization outcomes for the 11 comparisons in the 9 trials. In 5 studies (N=1019), medical management with a written action plan was compared with medical management without a written action plan.13-17 Two trials (N=185) compared a peak flow meter plus a written action plan with a peak flow meter and no written action plan18,19 In 4 studies (N=393), a written action plan based on peak flow monitoring was compared with a written action plan based on symptoms.
TABLE 1
Study characteristics
| Study | Patient popultation | Study Arms | Intervention components | Outcomes reported | Asthma quality indicators met |
|---|---|---|---|---|---|
| Optimal medical management vs. optimal medical management + PFM action plan | |||||
| Jones 199514 | Inclusions: patients using ICS | Usual care | SxD, FU | Ut, LF, Sx | Pow, Med |
| Exclusions: patients on oral steroids or using peak flow meters at home | PFM action plan | AP, PF, SxD, FU | |||
| Mean age: 29.5 years | |||||
| Severity level: Mild–moderate | |||||
| Drummond 1994 (GRASSIC)15 | Inclusion: FEV1 reversibility 20% or greater | Usual care | FU | Ut, LF, Med Ex | Pow, Rev |
| Exclusions: patients who already owned a PFM | PFM action plan | AP, PF, FU | |||
| Mean age: 50.8 years | |||||
| Severity level: Mild–severe | |||||
| Ayres 199516 | Inclusions: maximum PEF variability, 0.15%; minimum nights/week with symptoms, 3; minimum use of ICS or sodium cromoglycate, 3 months | Usual care | SxD, FU | LF, Sx, Ex | Pow, Med |
| Mean age: 45 years | PFM action plan | AP, PF, SxD, FU | |||
| Severity level: Moderate–severe | |||||
| Cowie 199713 | Inclusions: treatment for an exacerbation of asthma in an ER asthma clinic; history of receiving urgent treatment for asthma in the previous 12 months | Usual care | Ed, SxD, FU | Ut, PF, Med, Ex | None |
| Mean age: 37.8 years | PFM action plan | AP, PF, Ed, SxD, FU | |||
| Severity level: Mild–severe | |||||
| Cote 199717 | Inclusions: FEV1postbronchodilator 85-100 % of predicted; PEF, at minimum, 85 % of predicted; minimum PEF variability, 0%; Methacholine | Usual care | Ed | Ut, LF, Med | Exc, Rev, Com |
| Exclusions: patients having previously taken an asthma educational program | PFM action plan | Ed, Cn, AP, PF | |||
| Mean age: 36.5 years Severity level: Mild | |||||
| Usual care + PFM use alone vs. usual care + PFM action plan | |||||
| Ignacio-Garcia 199518 | Inclusions: patients from outpatient asthma clinic with asthma for 2 years | Usual care + PFM | PF, SxD, FU | Ut, LF, Med | None |
| Mean age: 41.9 years | Usual care + PFM action plan | PF, AP, Ed, SxD, FU | |||
| Severity level: Mild–severe | |||||
| Charlton 199419 | Inclusion: patients with inpatient or outpatient visit for asthma | Usual care + PFM | PF, Ed, SxD, FU | Ut, Sx, Med, Ex | None |
| Mean age: 6.5 years | Usual care + PFM action plan | PF, AP, Ed, SxD, FU | |||
| Severity level: Mild–moderate | |||||
| PFM action plan vs. Symptom action plan | |||||
| Turner 199820 | Inclusions: Maximum methacholine PC20, 7.9; using ICS | Symptom action plan | AP, Ed, SxD, Cn BM, EM | Ut, LF, Sx, Med | Exc, Com |
| Exclusions: previous PFM use; significant comorbid conditions | PFM action plan | PF, AP, Ed, SxD, Cn BM, EM | |||
| Mean age: 34.1 years | |||||
| Severity level: Mild–severe | |||||
| Charlton 199021 | Inclusions: patients on repeat prescribing register | Symptom action plan | AP, Ed, FU | Ut, Med | None |
| Mean age: NR | PFM action plan | PF, AP, Ed, FU, Cn | Ut, PF, Med, Ex | None | |
| Severity level: Mild–severe (?) | |||||
| Cowie 199716 | Inclusions: treatment for an exacerbation of asthma in an ER, or asthma clinic; history of receiving urgent treatment for asthma in the previous 12 months | Symptom action plan | AP, Ed, SxD, FU | ||
| PFM action plan | AP, PF, Ed, SxD, FU | ||||
| Cote 199717 | Inclusions: FEV1postbronchodilator, 85-100 % of predicted; PEF, at minimum, 85 % of predicted; minimum PEF variability, 0%; Methacholine | Symptom action plan | Ed, AP | Ut, LF, Med | Exc, Rev, Com |
| Exclusions: previous enrollment in an asthma educational program | PFM action plan | Ed, Cn, AP, PF | |||
| Eligibility criteria: ICS = inhaled corticosteroid; FEV1 = forced expiratory volume in 1 second; PEF = peak expiratory flow; PFM = peak flow meter; ER = emergency room; PC20 = 20% fall in FEV1 Intervention components: PF = Peak flow meter; AP = Written Action Plan; Ed = Education; SxD = Symptom diary; FU = Follow-up visits; Cn = Counseling; BM = Behavior modification; EM = Environmental modification | |||||
| Outcomes: Ut = Utilization measures; LF= Lung function measurements; Sx = Symptom=based measurements; Med = Medication use; Ex = Exacerbations of asth ma Asthma Quality Indicators: Exc = Accounted for excluded patients; Pow = Reported power calculations; Rev = Established reversibility of airway obstruction; Med = Controlled for other medication use; Com = Reported compliance; Sea = Addressed seasonality. | |||||
TABLE 2
Power calculations for hospitalizations per patient per year
| Assumed control mean | Possible treatment mean | % decrease | N needed per study arm |
|---|---|---|---|
| 0.10 | 0.075 | 25 | 3077 |
| 0.10 | 0.05 | 50 | 770 |
| 0.10 | 0.025 | 75 | 342 |
| 0.20 | 0.015 | 25 | 770 |
| 0.20 | 0.10 | 50 | 193 |
| 0.20 | 0.05 | 75 | 86 |
| 0.30 | 0.225 | 25 | 342 |
| 0.30 | 0.15 | 50 | 86 |
| 0.30 | 0.075 | 75 | 38 |
| Studies were identified that contained baseline rates on hospitalizations/patient/year, or information that allowed calculation of this parameter (Drummond, Abdalla, Beattie et al., 1994; Cote, Cartier, Robichaud et al. 1997; Cowie, Revitt, Underwood et al., 1997; Ignacio-Garcia and Gonzalez-Santos, 1995). Baseline rates of hospitalization varied in these studies from 0.04-0.29/patient/year. Standard deviations for this outcome were available only in two studies; Cote, Cartier, Robichaud et al. (1997) reported an SD of 0.30 for this variable, and an SD of 0.35 was calculated from the confidence intervals reported in GRASSIC (Drummond, Abdalla, Beattie et al., 1994). For the calculations, the more conservative 0.35 estimate for SD was used. | |||
| Number of patients per study arm were estimated for 80 percent power at the 5 percent significance level using control arm means of 0.10, 0.20, and 0.30 hospitalizations/patient/year. The expected reduction in this variable was tested along a spectrum from 25-75 percent. | |||
Written action plan versus no written action plan
All 5 studies used a peak flow meter based written action plan. All reported utilization outcomes, but the types and units of measurement were not consistent across studies (Table 2). Additionally, 4 studies reported on symptoms,13-16 and 3 reported lung function outcomes.13-15
With one notable exception, there were no statistically significant differences in outcomes among groups. Cowie et al16 reported an 11-fold decrease in total emergency room visits for the group using a peak-flow action plan (5 vs 55, P = .02), and also reported a reduction in hospitalizations of a similar magnitude (2 vs 12) that did not reach statistical significance. However, this study suffers from notable flaws that diminish confidence in the results. It is a post-intervention comparison among groups, which does not compare change from baseline, or incorporate baseline values as covariates in the analysis. Moreover baseline utilization data were provided by patient recall and not corroborated by medical records. There was a substantially larger variability in the baseline utilization rates for the peak flow group compared with the control group. This suggests that a subset of very high frequency users may have been over-represented in the peak flow group, and the reduction in emergency room visits may be concentrated in this subset.
Peak-flow meter-based written action plan versus peak flow meter with no written action plan
Two studies18,19 addressed the independent effect of a written action plan when added to peak flow self-monitoring (Table 3). Charlton19 reported no significant group differences for main outcomes, while Ignacio-Garcia18 reported large and statistically significant differences in most of the outcomes, favoring the group that used the written action plan.
The Ignacio-Garcia study, however, suffers from notable flaws suggesting the results may be attributable to bias. The sole participating physician, not blinded to treatment assignment, was highly involved in all phases of patient assessment, monitoring, and treatment. There was evidence of baseline differences between the two groups. A total of 25% of patients were withdrawn after randomization, and an unexplained decline in lung function occurred in the control group. Thus, the potential for selection bias, withdrawal bias, and ascertainment bias limits confidence in the results of this study
Symptom-based written action plan compared with peak flow-based written action plan
In 4 studies,16,17,20,21 reported outcomes were generally equivalent between groups and comparisons were not statistically significant, with one exception (Table 3). The 3-arm study by Cowie et al16 reported a striking reduction in the total number of emergency room visits with a peak flow meter-based written action plan compared with a symptom-based written action plan (5 versus 45, P
Discussion
The objective of this systematic review was to assess the independent effects of 2 specific components commonly included in asthma self-management plans—a written action plan and a peak flow meter. Few studies, however, are designed to permit reviewers to isolate the effects of these components. Moreover, the studies we reviewed did not clearly identify the population expected to benefit from interventions or specify the primary outcomes of interest; nor was the level of clinically meaningful improvement prospectively defined.
Most of the trials we reviewed, including the largest community study of 569 patients, did not demonstrate improved outcomes. The 2 trials that reported statistically significant results favoring a peak flow-based written action plan suffer from notable flaws suggesting the results may be attributable to bias. In the other 7 trials, there was little difference in outcomes between groups. However, these studies had insufficient power to detect group differences or confidently conclude equivalence between groups.
Thus, available evidence is insufficient to demonstrate that asthma outcomes are improved by use of a written asthma action plan, with or without peak flow monitoring. While this body of literature does not establish that these interventions are ineffective, it suggests they will not have a large effect on outcomes when applied to the general asthmatic population. The application of written action plans to all asthmatics indiscriminately may be a wasteful use of resources. This systematic review also questions the validity of written action plans as an indicator of asthma quality of care, or as a means to achieve quality improvement.
This analysis also highlights several obstacles to assessing the effects of disease management interventions. First, while the impact of whole intervention programs can be evaluated in controlled trials, it may be unfeasible to isolate each component of such programs and subject it to a rigorous analysis. Furthermore, as a behavioral intervention, the general principle of engaging patients in self-management may be more important that the specific components of these programs. Finally, regarding the optimization of medications (most obviously initiation of inhaled steroids) the impact of written action plans is likely to be relatively small, particularly on lung function or symptom control.
Future clinical trials should be done selectively, aimed at producing rigorous results that can improve the effectiveness of self-management interventions. Further study is warranted for specific subpopulations, such as those with higher baseline severity of illness or those with high baseline utilization rates. Available data suggest that, if there is benefit to be gained from self-management interventions, it will most likely be seen among these patients. Specific components of self-management that might be tested individually are those that are relatively high-cost, resource intensive, or risky for the patient.
Existing trials have tended to over-estimate the effects of action plan-based interventions, thus having invested resources for results inadequate for optimizing self-management strategies. Careful consideration needs to be taken in future trials to realistically estimate the expected impact of each intervention, and to specify the primary outcomes of interest and their baseline frequencies. Future trials should be large enough to detect a difference if one exists, or to confidently conclude that the intervention is ineffective.
Attention to these principles will help to advance our knowledge in this area most efficiently and to ultimately improve the quality of care for the entire population of patients with asthma.
· Acknowledgments ·
We acknowledge Kathleen Ziegler, Pharm.D, and Claudia Bonnell, RN, MSL, for their assistance in the research and preparation of this manuscript.
1. National Heart, Lung and Blood Institute. Expert panel report 2: guidelines for the diagnosis and management of asthma. Bethesda, MD: National Institutes of Health; 1997. NIH publication 97-4051.
2. Ruffin RE, Pierce RJ. Peak flow monitoring—which asthmatics, when, and how? Aust N Z J Med 1994;24:519-20.
3. Devine EC. Meta-analysis of the effects of psychoeducational care in adults with asthma. Res Nursing Health 1996;19:367-76.
4. Bernard-Bonnin AC, Stachenko S, Bonin D, et al. Self-management teaching programs and morbidity of pediatric asthma: a meta-analysis. J Allergy Clin Immunol 1995;95(1 Pt 1):34-41.
5. Gibson PG, Coughlan J, Wilson AJ, et al. Limited (information only) patient education programs for adults with asthma. Cochrane Database Syst Rev 2000a;(2):CD001005.-
6. Gibson PG, Coughlan J, Wilson AJ, et al. Self-management education and regular practitioner review for adults with asthma. Cochrane Database Syst Rev 2000b (2):CD001117.-
7. Lieu TA, Quesenberry CP, Jr, Capra AM, et al. Outpatient management practices associated with reduced risk of pediatric asthma hospitalization and emergency department visits. Pediatrics 1997;100(3 Pt 1):334-41.
8. Lefevre F, Piper M, Mark D, et al. Management of Chronic Asthma. AHRQ evidence report, contract number 290-97-001-5, 2001, http://www.ahcpr.gov/clinic/epcix.htm.
9. Mulrow CD, Oxman AD, editors. Cochrane Collaboration Handbook. Available in the Cochrane Library [database on disk and CD-ROM]. The Cochrane Collaboration; Issue 1. Oxford: Update Software; 1997.
10. Schulz KF, Chalmers I, Hayes RJ, et al. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408-12.
11. Berlin JA, Rennie D. Measuring the quality of trials: the quality of the quality scales. JAMA 1999;282(11):1083-5.
12. Juni P, Witschi A, Bloch R, et al. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA 1999;282(11):1054-60.
13. Jones KP, Mullee MA, Middleton M, et al. Peak flow based asthma self-management: a randomised controlled study in general practice. British Thoracic Society Research Committee. Thorax 1995;50(8):851-7.
14. Drummond N, Abdalla M, Beattie JAG, et al. Effectiveness of routine self monitoring of peak flow in patients with asthma. Grampian Asthma Study of Integrated Care GRASSIC). BMJ 1994 Feb. 26;308(6928):564-7.
15. Ayres JG, Campbell LM. A controlled assessment of an asthma self-management plan involving a budesonide dose regimen. OPTIONS Research Group. Eur Respir J 1996;886-92.
16. Cowie RL, Revitt SG, Underwood MF, et al. The effect of a peak flow-based action plan in the prevention of exacerbations of asthma. Chest 1997;112(6):1534-8.
17. Cote J, Cartier A, Robichaud P, et al. Influence on asthma morbidity of asthma education programs based on self-management plans following treatment optimization. Am J Respir Crit Care Med 1997;155(5):1509-14.
18. Ignacio-Garcia JM, Gonzalez-Santos P. Asthma self-management education program by home monitoring of peak expiratory flow. Am J Respir Crit Care Med 1995;151(2 Pt 1):353-9.
19. Charlton I, Antoniou AG, Atkinson J, et al. Asthma at the interface: bridging the gap between general practice and a district general hospital. Arch Dis Child 1994;70(4):313-8.
20. Turner MO, Taylor D, Bennett R, et al. A randomized trial comparing peak expiratory flow and symptom self-management plans for patients with asthma attending a primary care clinic. Am J Respir Crit Care Med 1998;157(2):540-6.
21. Charlton I, Charlton G, Broomfield J, et al. Evaluation of peak flow and symptoms only self-management plans for control of asthma in general practice. BMJ 1990;301(6765):1355-9.
- Most studies of asthma self-management do not permit retrospective isolation of the independent effects of a written action plan or peak flow meter use.
- Studies designed to isolate the effect of these self-care activities are generally underpowered or prone to systematic bias.
- Available evidence suggests that peak flow meters and written action plans do not have a large impact on outcomes when applied to the general population of asthmatics.
- These interventions are most likely to have beneficial effect when applied to selected populations, particularly patients with high baseline utilization.
Self-management skills are widely promoted by health plans and specialty societies with the expectation that they will improve care. The 1997 National Heart, Lung, and Blood Institute guidelines on treating asthma emphasize self-management,1 although they do not recommend specific programs. To maximize therapeutic effectiveness, it would be useful to know which components of patient self-management improve outcomes. Written action plans and peak flow meters are commonly used in asthma self-management programs. While these are simple, low-cost interventions for an individual, the aggregate cost for the entire population of asthmatics may be high.2
Much literature has accumulated on the effectiveness of providing asthma education alone and on programs that actively engage patients in their own care.Several systematic reviews have found that providing educational information alone has had little effect on asthma outcomes.3-5 There is evidence, though, that self-management activities are more effective than educational information alone. A recent Cochrane review of 24 trials found that self-management with regular practitioner review reduces hospi-talizations and emergency room visits.6 This review did not identify specific components contributing to improved outcomes. In contrast to the aforementioned studies on patient education, a large case-control study of children in the Kaiser Permanente System,7 found that written action plans were associated with lower rates of hospitalization and emergency room use. However, such observational studies often include confounding factors and are not sufficient to establish a cause-effect relationship between written action plans and improved outcomes.
We report on a systematic review that attempts to isolate the independent effect of a written action plan on asthma outcomes. We address two key questions:
- Compared with medical management alone, does the addition of a written asthma action plan (with or without peak flow meter use) improve outcomes?
- Compared with a written action plan based on symptoms, does a written action plan based on peak flow monitoring improve outcomes?
Methods
This study is part of a broader evidence report on the management of chronic asthma prepared for the Agency of Health Care Research and Quality8. Complete details of the methodology are available in the full report8 (http://www.ahcpr.gov/clinic/epcix.htm).
Literature search and study selection
We performed a comprehensive literature search from 1980 to August 2000 using MEDLINE, Embase, the Cochrane Library, and a hand search of recent bibliographies. The search was limited to full-length, peer-reviewed articles with an English abstract. Two independent reviewers carried out each step of study selection and data abstraction. Disagreements were resolved by consensus of the two reviewers or, if necessary, by the decision of a third reviewer.
Initial study selection was limited to comparative full-length reports or abstracts in peer-reviewed medical journals, with at least 25 evaluable children or adults per arm, treated for at least 12 weeks. Relevant comparisons included a written action plan and no written action plan; a written action plan based on peak flow readings and a written action plan based on symptoms. Study designs varied: clinical trials, cohort comparisons, case-control analyses, cross-sectional evaluations, and before-after comparisons. Specific components of the management plan had to be described.
Relevant outcomes included measures of inpatient and outpatient utilization, lung function, symptoms, rescue medication or oral steroid use, and quality of life. Outcomes of greatest interest were utilization parameters, as the goals of self-management usually focus on improving these outcomes.
These initial selection criteria yielded many studies that were confounded by multiple asthma management interventions and thus did not isolate the comparisons of interest. Therefore, the research team collectively determined the study design features that would best isolate the effects of written action plans and used them as new criteria in a second round of study selection. The studies thus selected satisfied 4 criteria: 1) randomization of patients; (2) delivery of the same interventions to experimental and control groups, except that the experimental group also received a written action plan; (3) delivery of the same interventions to experimental and control groups, except that one group received a written action plan based on peak flow meter readings, and the comparison group received a written action plan based on symptom monitoring; and 4) inclusion of a written action plan that met our specified definition.
A written action plan, by our definition, had two components: an algorithm that identified specific clinical indicators signaling the need for adjustments in medication; and specific instructions on how to adjust medications in response to such indicators. Many publications lacked sufficient detail on the written plan, so a brief survey was sent to the primary author of each of the 36 studies. If no response was obtained (36%), the article was excluded only when it was clear from the publication that our definition was not met.
Assessment of study quality
High-quality studies were randomized controlled trials that met the 3 domains of study quality that have been demonstrated empirically to impact effect size: concealment of treatment allocation; double-blinding; and minimization of exclusion bias.9,10 However, we doubted the feasibility of double-blinding a written asthma plan intervention, and so relaxed this requirement. We considered exclusion bias to be minimized when a study either reported intent-to-treat analysis or excluded fewer than 10% of subjects from analysis, with the ratio of subjects excluded from each arm being less than 2:1.
To more fully evaluate study design issues that may be particularly important in asthma research,11,12 we constructed asthma-specific quality indicators in consultation with an expert panel. Controls for potential confounders of treatment effect included establishing reversibility of airway obstruction, controlling for other medication use, reporting compliance, and addressing seasonality. In addition, a priori reporting of power calculations and accounting for exclusions and withdrawals were judged to be study quality characteristics pertinent to this body of evidence.
Data analysis
We constructed evidence tables for the outcomes of interest, and performed a qualitative synthesis of the data. Meta-analysis was not appropriate due to wide discrepancies in the patient populations studied, the interventions employed, and measurement and reporting of outcomes.
Results
Our literature search yielded a total of 4578 citations. Of these, 36 studies met the initial selection criteria. Many of these qualifying studies, however, were confounded by multiple asthma management interventions applied inconsistently across treatment arms. For example, a common confounder was review of and change in long-term medication use in the treatment group, but not in the control group. This necessitated a refinement in our selection criteria to focus on studies that largely isolated the effect of written action plans.13-21 This step yielded a final evidence base of 9 randomized controlled trials with a total enrollment of 1501 patients.
Table 1 summarizes the characteristics, interventions, and outcomes of the 9 studies. Two studies were 3-arm trials,16,17 which raised the total number of comparisons among the 9 studies to 11. The largest study was the Grampian Asthma Study of Integrated Care (n=569),14 a community study conducted in the UK. Enrollment in the other 8 studies ranged from 43 to 64 patients per arm. Treatment duration ranged from 24 to 52 weeks.
None of the studies met our definition of high quality. In fact, no study met any of the generic quality criteria—none was blinded, none described concealment of allocation, and all excluded more than 10% of subjects. Furthermore, none reported an intention-to-treat analysis. Thus these trials were prone to withdrawal bias as well as overestimation of treatment effect due to lack of allocation concealment.
No study met the majority of asthma-specific indicators (Table 1). Of the 9 studies, only 5 met any asthma-specific indicator. Three reported prospective power calculations,13-15 but 2 of these substantially overestimated the expected effect.13,15 Two studies established reversibility;14,17 2 controlled for other medication use;13,15 and 2 reported compliance.17,21 Thus, the studies were also prone to a type II error (failing to detect a true effect) and to potential confounding of outcomes.
We performed sample power calculations for hospitalizations (Table 2), derived from baseline rates reported in 4 studies14,16-18 and standard deviations reported in 2.14,17 A study with 250 patients per arm could detect a reduction of 50% or more in hospitalization, given a control rate of at least 0.2 hospitalizations/patient/year. In actuality, GRASSIC,14 which is the largest available trial (N=569), had baseline hospitalization rates of 0.12 and 0.13. With this baseline rate, over 700 patients per arm are required, higher than the actual enrollment in GRASSIC. The other studies in this review would be adequately powered to detect a 50% difference only in the setting of even higher baseline utilization (eg, 0.30 hospitalizations/patient/year).
Table 3 displays utilization outcomes for the 11 comparisons in the 9 trials. In 5 studies (N=1019), medical management with a written action plan was compared with medical management without a written action plan.13-17 Two trials (N=185) compared a peak flow meter plus a written action plan with a peak flow meter and no written action plan18,19 In 4 studies (N=393), a written action plan based on peak flow monitoring was compared with a written action plan based on symptoms.
TABLE 1
Study characteristics
| Study | Patient popultation | Study Arms | Intervention components | Outcomes reported | Asthma quality indicators met |
|---|---|---|---|---|---|
| Optimal medical management vs. optimal medical management + PFM action plan | |||||
| Jones 199514 | Inclusions: patients using ICS | Usual care | SxD, FU | Ut, LF, Sx | Pow, Med |
| Exclusions: patients on oral steroids or using peak flow meters at home | PFM action plan | AP, PF, SxD, FU | |||
| Mean age: 29.5 years | |||||
| Severity level: Mild–moderate | |||||
| Drummond 1994 (GRASSIC)15 | Inclusion: FEV1 reversibility 20% or greater | Usual care | FU | Ut, LF, Med Ex | Pow, Rev |
| Exclusions: patients who already owned a PFM | PFM action plan | AP, PF, FU | |||
| Mean age: 50.8 years | |||||
| Severity level: Mild–severe | |||||
| Ayres 199516 | Inclusions: maximum PEF variability, 0.15%; minimum nights/week with symptoms, 3; minimum use of ICS or sodium cromoglycate, 3 months | Usual care | SxD, FU | LF, Sx, Ex | Pow, Med |
| Mean age: 45 years | PFM action plan | AP, PF, SxD, FU | |||
| Severity level: Moderate–severe | |||||
| Cowie 199713 | Inclusions: treatment for an exacerbation of asthma in an ER asthma clinic; history of receiving urgent treatment for asthma in the previous 12 months | Usual care | Ed, SxD, FU | Ut, PF, Med, Ex | None |
| Mean age: 37.8 years | PFM action plan | AP, PF, Ed, SxD, FU | |||
| Severity level: Mild–severe | |||||
| Cote 199717 | Inclusions: FEV1postbronchodilator 85-100 % of predicted; PEF, at minimum, 85 % of predicted; minimum PEF variability, 0%; Methacholine | Usual care | Ed | Ut, LF, Med | Exc, Rev, Com |
| Exclusions: patients having previously taken an asthma educational program | PFM action plan | Ed, Cn, AP, PF | |||
| Mean age: 36.5 years Severity level: Mild | |||||
| Usual care + PFM use alone vs. usual care + PFM action plan | |||||
| Ignacio-Garcia 199518 | Inclusions: patients from outpatient asthma clinic with asthma for 2 years | Usual care + PFM | PF, SxD, FU | Ut, LF, Med | None |
| Mean age: 41.9 years | Usual care + PFM action plan | PF, AP, Ed, SxD, FU | |||
| Severity level: Mild–severe | |||||
| Charlton 199419 | Inclusion: patients with inpatient or outpatient visit for asthma | Usual care + PFM | PF, Ed, SxD, FU | Ut, Sx, Med, Ex | None |
| Mean age: 6.5 years | Usual care + PFM action plan | PF, AP, Ed, SxD, FU | |||
| Severity level: Mild–moderate | |||||
| PFM action plan vs. Symptom action plan | |||||
| Turner 199820 | Inclusions: Maximum methacholine PC20, 7.9; using ICS | Symptom action plan | AP, Ed, SxD, Cn BM, EM | Ut, LF, Sx, Med | Exc, Com |
| Exclusions: previous PFM use; significant comorbid conditions | PFM action plan | PF, AP, Ed, SxD, Cn BM, EM | |||
| Mean age: 34.1 years | |||||
| Severity level: Mild–severe | |||||
| Charlton 199021 | Inclusions: patients on repeat prescribing register | Symptom action plan | AP, Ed, FU | Ut, Med | None |
| Mean age: NR | PFM action plan | PF, AP, Ed, FU, Cn | Ut, PF, Med, Ex | None | |
| Severity level: Mild–severe (?) | |||||
| Cowie 199716 | Inclusions: treatment for an exacerbation of asthma in an ER, or asthma clinic; history of receiving urgent treatment for asthma in the previous 12 months | Symptom action plan | AP, Ed, SxD, FU | ||
| PFM action plan | AP, PF, Ed, SxD, FU | ||||
| Cote 199717 | Inclusions: FEV1postbronchodilator, 85-100 % of predicted; PEF, at minimum, 85 % of predicted; minimum PEF variability, 0%; Methacholine | Symptom action plan | Ed, AP | Ut, LF, Med | Exc, Rev, Com |
| Exclusions: previous enrollment in an asthma educational program | PFM action plan | Ed, Cn, AP, PF | |||
| Eligibility criteria: ICS = inhaled corticosteroid; FEV1 = forced expiratory volume in 1 second; PEF = peak expiratory flow; PFM = peak flow meter; ER = emergency room; PC20 = 20% fall in FEV1 Intervention components: PF = Peak flow meter; AP = Written Action Plan; Ed = Education; SxD = Symptom diary; FU = Follow-up visits; Cn = Counseling; BM = Behavior modification; EM = Environmental modification | |||||
| Outcomes: Ut = Utilization measures; LF= Lung function measurements; Sx = Symptom=based measurements; Med = Medication use; Ex = Exacerbations of asth ma Asthma Quality Indicators: Exc = Accounted for excluded patients; Pow = Reported power calculations; Rev = Established reversibility of airway obstruction; Med = Controlled for other medication use; Com = Reported compliance; Sea = Addressed seasonality. | |||||
TABLE 2
Power calculations for hospitalizations per patient per year
| Assumed control mean | Possible treatment mean | % decrease | N needed per study arm |
|---|---|---|---|
| 0.10 | 0.075 | 25 | 3077 |
| 0.10 | 0.05 | 50 | 770 |
| 0.10 | 0.025 | 75 | 342 |
| 0.20 | 0.015 | 25 | 770 |
| 0.20 | 0.10 | 50 | 193 |
| 0.20 | 0.05 | 75 | 86 |
| 0.30 | 0.225 | 25 | 342 |
| 0.30 | 0.15 | 50 | 86 |
| 0.30 | 0.075 | 75 | 38 |
| Studies were identified that contained baseline rates on hospitalizations/patient/year, or information that allowed calculation of this parameter (Drummond, Abdalla, Beattie et al., 1994; Cote, Cartier, Robichaud et al. 1997; Cowie, Revitt, Underwood et al., 1997; Ignacio-Garcia and Gonzalez-Santos, 1995). Baseline rates of hospitalization varied in these studies from 0.04-0.29/patient/year. Standard deviations for this outcome were available only in two studies; Cote, Cartier, Robichaud et al. (1997) reported an SD of 0.30 for this variable, and an SD of 0.35 was calculated from the confidence intervals reported in GRASSIC (Drummond, Abdalla, Beattie et al., 1994). For the calculations, the more conservative 0.35 estimate for SD was used. | |||
| Number of patients per study arm were estimated for 80 percent power at the 5 percent significance level using control arm means of 0.10, 0.20, and 0.30 hospitalizations/patient/year. The expected reduction in this variable was tested along a spectrum from 25-75 percent. | |||
Written action plan versus no written action plan
All 5 studies used a peak flow meter based written action plan. All reported utilization outcomes, but the types and units of measurement were not consistent across studies (Table 2). Additionally, 4 studies reported on symptoms,13-16 and 3 reported lung function outcomes.13-15
With one notable exception, there were no statistically significant differences in outcomes among groups. Cowie et al16 reported an 11-fold decrease in total emergency room visits for the group using a peak-flow action plan (5 vs 55, P = .02), and also reported a reduction in hospitalizations of a similar magnitude (2 vs 12) that did not reach statistical significance. However, this study suffers from notable flaws that diminish confidence in the results. It is a post-intervention comparison among groups, which does not compare change from baseline, or incorporate baseline values as covariates in the analysis. Moreover baseline utilization data were provided by patient recall and not corroborated by medical records. There was a substantially larger variability in the baseline utilization rates for the peak flow group compared with the control group. This suggests that a subset of very high frequency users may have been over-represented in the peak flow group, and the reduction in emergency room visits may be concentrated in this subset.
Peak-flow meter-based written action plan versus peak flow meter with no written action plan
Two studies18,19 addressed the independent effect of a written action plan when added to peak flow self-monitoring (Table 3). Charlton19 reported no significant group differences for main outcomes, while Ignacio-Garcia18 reported large and statistically significant differences in most of the outcomes, favoring the group that used the written action plan.
The Ignacio-Garcia study, however, suffers from notable flaws suggesting the results may be attributable to bias. The sole participating physician, not blinded to treatment assignment, was highly involved in all phases of patient assessment, monitoring, and treatment. There was evidence of baseline differences between the two groups. A total of 25% of patients were withdrawn after randomization, and an unexplained decline in lung function occurred in the control group. Thus, the potential for selection bias, withdrawal bias, and ascertainment bias limits confidence in the results of this study
Symptom-based written action plan compared with peak flow-based written action plan
In 4 studies,16,17,20,21 reported outcomes were generally equivalent between groups and comparisons were not statistically significant, with one exception (Table 3). The 3-arm study by Cowie et al16 reported a striking reduction in the total number of emergency room visits with a peak flow meter-based written action plan compared with a symptom-based written action plan (5 versus 45, P
Discussion
The objective of this systematic review was to assess the independent effects of 2 specific components commonly included in asthma self-management plans—a written action plan and a peak flow meter. Few studies, however, are designed to permit reviewers to isolate the effects of these components. Moreover, the studies we reviewed did not clearly identify the population expected to benefit from interventions or specify the primary outcomes of interest; nor was the level of clinically meaningful improvement prospectively defined.
Most of the trials we reviewed, including the largest community study of 569 patients, did not demonstrate improved outcomes. The 2 trials that reported statistically significant results favoring a peak flow-based written action plan suffer from notable flaws suggesting the results may be attributable to bias. In the other 7 trials, there was little difference in outcomes between groups. However, these studies had insufficient power to detect group differences or confidently conclude equivalence between groups.
Thus, available evidence is insufficient to demonstrate that asthma outcomes are improved by use of a written asthma action plan, with or without peak flow monitoring. While this body of literature does not establish that these interventions are ineffective, it suggests they will not have a large effect on outcomes when applied to the general asthmatic population. The application of written action plans to all asthmatics indiscriminately may be a wasteful use of resources. This systematic review also questions the validity of written action plans as an indicator of asthma quality of care, or as a means to achieve quality improvement.
This analysis also highlights several obstacles to assessing the effects of disease management interventions. First, while the impact of whole intervention programs can be evaluated in controlled trials, it may be unfeasible to isolate each component of such programs and subject it to a rigorous analysis. Furthermore, as a behavioral intervention, the general principle of engaging patients in self-management may be more important that the specific components of these programs. Finally, regarding the optimization of medications (most obviously initiation of inhaled steroids) the impact of written action plans is likely to be relatively small, particularly on lung function or symptom control.
Future clinical trials should be done selectively, aimed at producing rigorous results that can improve the effectiveness of self-management interventions. Further study is warranted for specific subpopulations, such as those with higher baseline severity of illness or those with high baseline utilization rates. Available data suggest that, if there is benefit to be gained from self-management interventions, it will most likely be seen among these patients. Specific components of self-management that might be tested individually are those that are relatively high-cost, resource intensive, or risky for the patient.
Existing trials have tended to over-estimate the effects of action plan-based interventions, thus having invested resources for results inadequate for optimizing self-management strategies. Careful consideration needs to be taken in future trials to realistically estimate the expected impact of each intervention, and to specify the primary outcomes of interest and their baseline frequencies. Future trials should be large enough to detect a difference if one exists, or to confidently conclude that the intervention is ineffective.
Attention to these principles will help to advance our knowledge in this area most efficiently and to ultimately improve the quality of care for the entire population of patients with asthma.
· Acknowledgments ·
We acknowledge Kathleen Ziegler, Pharm.D, and Claudia Bonnell, RN, MSL, for their assistance in the research and preparation of this manuscript.
- Most studies of asthma self-management do not permit retrospective isolation of the independent effects of a written action plan or peak flow meter use.
- Studies designed to isolate the effect of these self-care activities are generally underpowered or prone to systematic bias.
- Available evidence suggests that peak flow meters and written action plans do not have a large impact on outcomes when applied to the general population of asthmatics.
- These interventions are most likely to have beneficial effect when applied to selected populations, particularly patients with high baseline utilization.
Self-management skills are widely promoted by health plans and specialty societies with the expectation that they will improve care. The 1997 National Heart, Lung, and Blood Institute guidelines on treating asthma emphasize self-management,1 although they do not recommend specific programs. To maximize therapeutic effectiveness, it would be useful to know which components of patient self-management improve outcomes. Written action plans and peak flow meters are commonly used in asthma self-management programs. While these are simple, low-cost interventions for an individual, the aggregate cost for the entire population of asthmatics may be high.2
Much literature has accumulated on the effectiveness of providing asthma education alone and on programs that actively engage patients in their own care.Several systematic reviews have found that providing educational information alone has had little effect on asthma outcomes.3-5 There is evidence, though, that self-management activities are more effective than educational information alone. A recent Cochrane review of 24 trials found that self-management with regular practitioner review reduces hospi-talizations and emergency room visits.6 This review did not identify specific components contributing to improved outcomes. In contrast to the aforementioned studies on patient education, a large case-control study of children in the Kaiser Permanente System,7 found that written action plans were associated with lower rates of hospitalization and emergency room use. However, such observational studies often include confounding factors and are not sufficient to establish a cause-effect relationship between written action plans and improved outcomes.
We report on a systematic review that attempts to isolate the independent effect of a written action plan on asthma outcomes. We address two key questions:
- Compared with medical management alone, does the addition of a written asthma action plan (with or without peak flow meter use) improve outcomes?
- Compared with a written action plan based on symptoms, does a written action plan based on peak flow monitoring improve outcomes?
Methods
This study is part of a broader evidence report on the management of chronic asthma prepared for the Agency of Health Care Research and Quality8. Complete details of the methodology are available in the full report8 (http://www.ahcpr.gov/clinic/epcix.htm).
Literature search and study selection
We performed a comprehensive literature search from 1980 to August 2000 using MEDLINE, Embase, the Cochrane Library, and a hand search of recent bibliographies. The search was limited to full-length, peer-reviewed articles with an English abstract. Two independent reviewers carried out each step of study selection and data abstraction. Disagreements were resolved by consensus of the two reviewers or, if necessary, by the decision of a third reviewer.
Initial study selection was limited to comparative full-length reports or abstracts in peer-reviewed medical journals, with at least 25 evaluable children or adults per arm, treated for at least 12 weeks. Relevant comparisons included a written action plan and no written action plan; a written action plan based on peak flow readings and a written action plan based on symptoms. Study designs varied: clinical trials, cohort comparisons, case-control analyses, cross-sectional evaluations, and before-after comparisons. Specific components of the management plan had to be described.
Relevant outcomes included measures of inpatient and outpatient utilization, lung function, symptoms, rescue medication or oral steroid use, and quality of life. Outcomes of greatest interest were utilization parameters, as the goals of self-management usually focus on improving these outcomes.
These initial selection criteria yielded many studies that were confounded by multiple asthma management interventions and thus did not isolate the comparisons of interest. Therefore, the research team collectively determined the study design features that would best isolate the effects of written action plans and used them as new criteria in a second round of study selection. The studies thus selected satisfied 4 criteria: 1) randomization of patients; (2) delivery of the same interventions to experimental and control groups, except that the experimental group also received a written action plan; (3) delivery of the same interventions to experimental and control groups, except that one group received a written action plan based on peak flow meter readings, and the comparison group received a written action plan based on symptom monitoring; and 4) inclusion of a written action plan that met our specified definition.
A written action plan, by our definition, had two components: an algorithm that identified specific clinical indicators signaling the need for adjustments in medication; and specific instructions on how to adjust medications in response to such indicators. Many publications lacked sufficient detail on the written plan, so a brief survey was sent to the primary author of each of the 36 studies. If no response was obtained (36%), the article was excluded only when it was clear from the publication that our definition was not met.
Assessment of study quality
High-quality studies were randomized controlled trials that met the 3 domains of study quality that have been demonstrated empirically to impact effect size: concealment of treatment allocation; double-blinding; and minimization of exclusion bias.9,10 However, we doubted the feasibility of double-blinding a written asthma plan intervention, and so relaxed this requirement. We considered exclusion bias to be minimized when a study either reported intent-to-treat analysis or excluded fewer than 10% of subjects from analysis, with the ratio of subjects excluded from each arm being less than 2:1.
To more fully evaluate study design issues that may be particularly important in asthma research,11,12 we constructed asthma-specific quality indicators in consultation with an expert panel. Controls for potential confounders of treatment effect included establishing reversibility of airway obstruction, controlling for other medication use, reporting compliance, and addressing seasonality. In addition, a priori reporting of power calculations and accounting for exclusions and withdrawals were judged to be study quality characteristics pertinent to this body of evidence.
Data analysis
We constructed evidence tables for the outcomes of interest, and performed a qualitative synthesis of the data. Meta-analysis was not appropriate due to wide discrepancies in the patient populations studied, the interventions employed, and measurement and reporting of outcomes.
Results
Our literature search yielded a total of 4578 citations. Of these, 36 studies met the initial selection criteria. Many of these qualifying studies, however, were confounded by multiple asthma management interventions applied inconsistently across treatment arms. For example, a common confounder was review of and change in long-term medication use in the treatment group, but not in the control group. This necessitated a refinement in our selection criteria to focus on studies that largely isolated the effect of written action plans.13-21 This step yielded a final evidence base of 9 randomized controlled trials with a total enrollment of 1501 patients.
Table 1 summarizes the characteristics, interventions, and outcomes of the 9 studies. Two studies were 3-arm trials,16,17 which raised the total number of comparisons among the 9 studies to 11. The largest study was the Grampian Asthma Study of Integrated Care (n=569),14 a community study conducted in the UK. Enrollment in the other 8 studies ranged from 43 to 64 patients per arm. Treatment duration ranged from 24 to 52 weeks.
None of the studies met our definition of high quality. In fact, no study met any of the generic quality criteria—none was blinded, none described concealment of allocation, and all excluded more than 10% of subjects. Furthermore, none reported an intention-to-treat analysis. Thus these trials were prone to withdrawal bias as well as overestimation of treatment effect due to lack of allocation concealment.
No study met the majority of asthma-specific indicators (Table 1). Of the 9 studies, only 5 met any asthma-specific indicator. Three reported prospective power calculations,13-15 but 2 of these substantially overestimated the expected effect.13,15 Two studies established reversibility;14,17 2 controlled for other medication use;13,15 and 2 reported compliance.17,21 Thus, the studies were also prone to a type II error (failing to detect a true effect) and to potential confounding of outcomes.
We performed sample power calculations for hospitalizations (Table 2), derived from baseline rates reported in 4 studies14,16-18 and standard deviations reported in 2.14,17 A study with 250 patients per arm could detect a reduction of 50% or more in hospitalization, given a control rate of at least 0.2 hospitalizations/patient/year. In actuality, GRASSIC,14 which is the largest available trial (N=569), had baseline hospitalization rates of 0.12 and 0.13. With this baseline rate, over 700 patients per arm are required, higher than the actual enrollment in GRASSIC. The other studies in this review would be adequately powered to detect a 50% difference only in the setting of even higher baseline utilization (eg, 0.30 hospitalizations/patient/year).
Table 3 displays utilization outcomes for the 11 comparisons in the 9 trials. In 5 studies (N=1019), medical management with a written action plan was compared with medical management without a written action plan.13-17 Two trials (N=185) compared a peak flow meter plus a written action plan with a peak flow meter and no written action plan18,19 In 4 studies (N=393), a written action plan based on peak flow monitoring was compared with a written action plan based on symptoms.
TABLE 1
Study characteristics
| Study | Patient popultation | Study Arms | Intervention components | Outcomes reported | Asthma quality indicators met |
|---|---|---|---|---|---|
| Optimal medical management vs. optimal medical management + PFM action plan | |||||
| Jones 199514 | Inclusions: patients using ICS | Usual care | SxD, FU | Ut, LF, Sx | Pow, Med |
| Exclusions: patients on oral steroids or using peak flow meters at home | PFM action plan | AP, PF, SxD, FU | |||
| Mean age: 29.5 years | |||||
| Severity level: Mild–moderate | |||||
| Drummond 1994 (GRASSIC)15 | Inclusion: FEV1 reversibility 20% or greater | Usual care | FU | Ut, LF, Med Ex | Pow, Rev |
| Exclusions: patients who already owned a PFM | PFM action plan | AP, PF, FU | |||
| Mean age: 50.8 years | |||||
| Severity level: Mild–severe | |||||
| Ayres 199516 | Inclusions: maximum PEF variability, 0.15%; minimum nights/week with symptoms, 3; minimum use of ICS or sodium cromoglycate, 3 months | Usual care | SxD, FU | LF, Sx, Ex | Pow, Med |
| Mean age: 45 years | PFM action plan | AP, PF, SxD, FU | |||
| Severity level: Moderate–severe | |||||
| Cowie 199713 | Inclusions: treatment for an exacerbation of asthma in an ER asthma clinic; history of receiving urgent treatment for asthma in the previous 12 months | Usual care | Ed, SxD, FU | Ut, PF, Med, Ex | None |
| Mean age: 37.8 years | PFM action plan | AP, PF, Ed, SxD, FU | |||
| Severity level: Mild–severe | |||||
| Cote 199717 | Inclusions: FEV1postbronchodilator 85-100 % of predicted; PEF, at minimum, 85 % of predicted; minimum PEF variability, 0%; Methacholine | Usual care | Ed | Ut, LF, Med | Exc, Rev, Com |
| Exclusions: patients having previously taken an asthma educational program | PFM action plan | Ed, Cn, AP, PF | |||
| Mean age: 36.5 years Severity level: Mild | |||||
| Usual care + PFM use alone vs. usual care + PFM action plan | |||||
| Ignacio-Garcia 199518 | Inclusions: patients from outpatient asthma clinic with asthma for 2 years | Usual care + PFM | PF, SxD, FU | Ut, LF, Med | None |
| Mean age: 41.9 years | Usual care + PFM action plan | PF, AP, Ed, SxD, FU | |||
| Severity level: Mild–severe | |||||
| Charlton 199419 | Inclusion: patients with inpatient or outpatient visit for asthma | Usual care + PFM | PF, Ed, SxD, FU | Ut, Sx, Med, Ex | None |
| Mean age: 6.5 years | Usual care + PFM action plan | PF, AP, Ed, SxD, FU | |||
| Severity level: Mild–moderate | |||||
| PFM action plan vs. Symptom action plan | |||||
| Turner 199820 | Inclusions: Maximum methacholine PC20, 7.9; using ICS | Symptom action plan | AP, Ed, SxD, Cn BM, EM | Ut, LF, Sx, Med | Exc, Com |
| Exclusions: previous PFM use; significant comorbid conditions | PFM action plan | PF, AP, Ed, SxD, Cn BM, EM | |||
| Mean age: 34.1 years | |||||
| Severity level: Mild–severe | |||||
| Charlton 199021 | Inclusions: patients on repeat prescribing register | Symptom action plan | AP, Ed, FU | Ut, Med | None |
| Mean age: NR | PFM action plan | PF, AP, Ed, FU, Cn | Ut, PF, Med, Ex | None | |
| Severity level: Mild–severe (?) | |||||
| Cowie 199716 | Inclusions: treatment for an exacerbation of asthma in an ER, or asthma clinic; history of receiving urgent treatment for asthma in the previous 12 months | Symptom action plan | AP, Ed, SxD, FU | ||
| PFM action plan | AP, PF, Ed, SxD, FU | ||||
| Cote 199717 | Inclusions: FEV1postbronchodilator, 85-100 % of predicted; PEF, at minimum, 85 % of predicted; minimum PEF variability, 0%; Methacholine | Symptom action plan | Ed, AP | Ut, LF, Med | Exc, Rev, Com |
| Exclusions: previous enrollment in an asthma educational program | PFM action plan | Ed, Cn, AP, PF | |||
| Eligibility criteria: ICS = inhaled corticosteroid; FEV1 = forced expiratory volume in 1 second; PEF = peak expiratory flow; PFM = peak flow meter; ER = emergency room; PC20 = 20% fall in FEV1 Intervention components: PF = Peak flow meter; AP = Written Action Plan; Ed = Education; SxD = Symptom diary; FU = Follow-up visits; Cn = Counseling; BM = Behavior modification; EM = Environmental modification | |||||
| Outcomes: Ut = Utilization measures; LF= Lung function measurements; Sx = Symptom=based measurements; Med = Medication use; Ex = Exacerbations of asth ma Asthma Quality Indicators: Exc = Accounted for excluded patients; Pow = Reported power calculations; Rev = Established reversibility of airway obstruction; Med = Controlled for other medication use; Com = Reported compliance; Sea = Addressed seasonality. | |||||
TABLE 2
Power calculations for hospitalizations per patient per year
| Assumed control mean | Possible treatment mean | % decrease | N needed per study arm |
|---|---|---|---|
| 0.10 | 0.075 | 25 | 3077 |
| 0.10 | 0.05 | 50 | 770 |
| 0.10 | 0.025 | 75 | 342 |
| 0.20 | 0.015 | 25 | 770 |
| 0.20 | 0.10 | 50 | 193 |
| 0.20 | 0.05 | 75 | 86 |
| 0.30 | 0.225 | 25 | 342 |
| 0.30 | 0.15 | 50 | 86 |
| 0.30 | 0.075 | 75 | 38 |
| Studies were identified that contained baseline rates on hospitalizations/patient/year, or information that allowed calculation of this parameter (Drummond, Abdalla, Beattie et al., 1994; Cote, Cartier, Robichaud et al. 1997; Cowie, Revitt, Underwood et al., 1997; Ignacio-Garcia and Gonzalez-Santos, 1995). Baseline rates of hospitalization varied in these studies from 0.04-0.29/patient/year. Standard deviations for this outcome were available only in two studies; Cote, Cartier, Robichaud et al. (1997) reported an SD of 0.30 for this variable, and an SD of 0.35 was calculated from the confidence intervals reported in GRASSIC (Drummond, Abdalla, Beattie et al., 1994). For the calculations, the more conservative 0.35 estimate for SD was used. | |||
| Number of patients per study arm were estimated for 80 percent power at the 5 percent significance level using control arm means of 0.10, 0.20, and 0.30 hospitalizations/patient/year. The expected reduction in this variable was tested along a spectrum from 25-75 percent. | |||
Written action plan versus no written action plan
All 5 studies used a peak flow meter based written action plan. All reported utilization outcomes, but the types and units of measurement were not consistent across studies (Table 2). Additionally, 4 studies reported on symptoms,13-16 and 3 reported lung function outcomes.13-15
With one notable exception, there were no statistically significant differences in outcomes among groups. Cowie et al16 reported an 11-fold decrease in total emergency room visits for the group using a peak-flow action plan (5 vs 55, P = .02), and also reported a reduction in hospitalizations of a similar magnitude (2 vs 12) that did not reach statistical significance. However, this study suffers from notable flaws that diminish confidence in the results. It is a post-intervention comparison among groups, which does not compare change from baseline, or incorporate baseline values as covariates in the analysis. Moreover baseline utilization data were provided by patient recall and not corroborated by medical records. There was a substantially larger variability in the baseline utilization rates for the peak flow group compared with the control group. This suggests that a subset of very high frequency users may have been over-represented in the peak flow group, and the reduction in emergency room visits may be concentrated in this subset.
Peak-flow meter-based written action plan versus peak flow meter with no written action plan
Two studies18,19 addressed the independent effect of a written action plan when added to peak flow self-monitoring (Table 3). Charlton19 reported no significant group differences for main outcomes, while Ignacio-Garcia18 reported large and statistically significant differences in most of the outcomes, favoring the group that used the written action plan.
The Ignacio-Garcia study, however, suffers from notable flaws suggesting the results may be attributable to bias. The sole participating physician, not blinded to treatment assignment, was highly involved in all phases of patient assessment, monitoring, and treatment. There was evidence of baseline differences between the two groups. A total of 25% of patients were withdrawn after randomization, and an unexplained decline in lung function occurred in the control group. Thus, the potential for selection bias, withdrawal bias, and ascertainment bias limits confidence in the results of this study
Symptom-based written action plan compared with peak flow-based written action plan
In 4 studies,16,17,20,21 reported outcomes were generally equivalent between groups and comparisons were not statistically significant, with one exception (Table 3). The 3-arm study by Cowie et al16 reported a striking reduction in the total number of emergency room visits with a peak flow meter-based written action plan compared with a symptom-based written action plan (5 versus 45, P
Discussion
The objective of this systematic review was to assess the independent effects of 2 specific components commonly included in asthma self-management plans—a written action plan and a peak flow meter. Few studies, however, are designed to permit reviewers to isolate the effects of these components. Moreover, the studies we reviewed did not clearly identify the population expected to benefit from interventions or specify the primary outcomes of interest; nor was the level of clinically meaningful improvement prospectively defined.
Most of the trials we reviewed, including the largest community study of 569 patients, did not demonstrate improved outcomes. The 2 trials that reported statistically significant results favoring a peak flow-based written action plan suffer from notable flaws suggesting the results may be attributable to bias. In the other 7 trials, there was little difference in outcomes between groups. However, these studies had insufficient power to detect group differences or confidently conclude equivalence between groups.
Thus, available evidence is insufficient to demonstrate that asthma outcomes are improved by use of a written asthma action plan, with or without peak flow monitoring. While this body of literature does not establish that these interventions are ineffective, it suggests they will not have a large effect on outcomes when applied to the general asthmatic population. The application of written action plans to all asthmatics indiscriminately may be a wasteful use of resources. This systematic review also questions the validity of written action plans as an indicator of asthma quality of care, or as a means to achieve quality improvement.
This analysis also highlights several obstacles to assessing the effects of disease management interventions. First, while the impact of whole intervention programs can be evaluated in controlled trials, it may be unfeasible to isolate each component of such programs and subject it to a rigorous analysis. Furthermore, as a behavioral intervention, the general principle of engaging patients in self-management may be more important that the specific components of these programs. Finally, regarding the optimization of medications (most obviously initiation of inhaled steroids) the impact of written action plans is likely to be relatively small, particularly on lung function or symptom control.
Future clinical trials should be done selectively, aimed at producing rigorous results that can improve the effectiveness of self-management interventions. Further study is warranted for specific subpopulations, such as those with higher baseline severity of illness or those with high baseline utilization rates. Available data suggest that, if there is benefit to be gained from self-management interventions, it will most likely be seen among these patients. Specific components of self-management that might be tested individually are those that are relatively high-cost, resource intensive, or risky for the patient.
Existing trials have tended to over-estimate the effects of action plan-based interventions, thus having invested resources for results inadequate for optimizing self-management strategies. Careful consideration needs to be taken in future trials to realistically estimate the expected impact of each intervention, and to specify the primary outcomes of interest and their baseline frequencies. Future trials should be large enough to detect a difference if one exists, or to confidently conclude that the intervention is ineffective.
Attention to these principles will help to advance our knowledge in this area most efficiently and to ultimately improve the quality of care for the entire population of patients with asthma.
· Acknowledgments ·
We acknowledge Kathleen Ziegler, Pharm.D, and Claudia Bonnell, RN, MSL, for their assistance in the research and preparation of this manuscript.
1. National Heart, Lung and Blood Institute. Expert panel report 2: guidelines for the diagnosis and management of asthma. Bethesda, MD: National Institutes of Health; 1997. NIH publication 97-4051.
2. Ruffin RE, Pierce RJ. Peak flow monitoring—which asthmatics, when, and how? Aust N Z J Med 1994;24:519-20.
3. Devine EC. Meta-analysis of the effects of psychoeducational care in adults with asthma. Res Nursing Health 1996;19:367-76.
4. Bernard-Bonnin AC, Stachenko S, Bonin D, et al. Self-management teaching programs and morbidity of pediatric asthma: a meta-analysis. J Allergy Clin Immunol 1995;95(1 Pt 1):34-41.
5. Gibson PG, Coughlan J, Wilson AJ, et al. Limited (information only) patient education programs for adults with asthma. Cochrane Database Syst Rev 2000a;(2):CD001005.-
6. Gibson PG, Coughlan J, Wilson AJ, et al. Self-management education and regular practitioner review for adults with asthma. Cochrane Database Syst Rev 2000b (2):CD001117.-
7. Lieu TA, Quesenberry CP, Jr, Capra AM, et al. Outpatient management practices associated with reduced risk of pediatric asthma hospitalization and emergency department visits. Pediatrics 1997;100(3 Pt 1):334-41.
8. Lefevre F, Piper M, Mark D, et al. Management of Chronic Asthma. AHRQ evidence report, contract number 290-97-001-5, 2001, http://www.ahcpr.gov/clinic/epcix.htm.
9. Mulrow CD, Oxman AD, editors. Cochrane Collaboration Handbook. Available in the Cochrane Library [database on disk and CD-ROM]. The Cochrane Collaboration; Issue 1. Oxford: Update Software; 1997.
10. Schulz KF, Chalmers I, Hayes RJ, et al. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408-12.
11. Berlin JA, Rennie D. Measuring the quality of trials: the quality of the quality scales. JAMA 1999;282(11):1083-5.
12. Juni P, Witschi A, Bloch R, et al. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA 1999;282(11):1054-60.
13. Jones KP, Mullee MA, Middleton M, et al. Peak flow based asthma self-management: a randomised controlled study in general practice. British Thoracic Society Research Committee. Thorax 1995;50(8):851-7.
14. Drummond N, Abdalla M, Beattie JAG, et al. Effectiveness of routine self monitoring of peak flow in patients with asthma. Grampian Asthma Study of Integrated Care GRASSIC). BMJ 1994 Feb. 26;308(6928):564-7.
15. Ayres JG, Campbell LM. A controlled assessment of an asthma self-management plan involving a budesonide dose regimen. OPTIONS Research Group. Eur Respir J 1996;886-92.
16. Cowie RL, Revitt SG, Underwood MF, et al. The effect of a peak flow-based action plan in the prevention of exacerbations of asthma. Chest 1997;112(6):1534-8.
17. Cote J, Cartier A, Robichaud P, et al. Influence on asthma morbidity of asthma education programs based on self-management plans following treatment optimization. Am J Respir Crit Care Med 1997;155(5):1509-14.
18. Ignacio-Garcia JM, Gonzalez-Santos P. Asthma self-management education program by home monitoring of peak expiratory flow. Am J Respir Crit Care Med 1995;151(2 Pt 1):353-9.
19. Charlton I, Antoniou AG, Atkinson J, et al. Asthma at the interface: bridging the gap between general practice and a district general hospital. Arch Dis Child 1994;70(4):313-8.
20. Turner MO, Taylor D, Bennett R, et al. A randomized trial comparing peak expiratory flow and symptom self-management plans for patients with asthma attending a primary care clinic. Am J Respir Crit Care Med 1998;157(2):540-6.
21. Charlton I, Charlton G, Broomfield J, et al. Evaluation of peak flow and symptoms only self-management plans for control of asthma in general practice. BMJ 1990;301(6765):1355-9.
1. National Heart, Lung and Blood Institute. Expert panel report 2: guidelines for the diagnosis and management of asthma. Bethesda, MD: National Institutes of Health; 1997. NIH publication 97-4051.
2. Ruffin RE, Pierce RJ. Peak flow monitoring—which asthmatics, when, and how? Aust N Z J Med 1994;24:519-20.
3. Devine EC. Meta-analysis of the effects of psychoeducational care in adults with asthma. Res Nursing Health 1996;19:367-76.
4. Bernard-Bonnin AC, Stachenko S, Bonin D, et al. Self-management teaching programs and morbidity of pediatric asthma: a meta-analysis. J Allergy Clin Immunol 1995;95(1 Pt 1):34-41.
5. Gibson PG, Coughlan J, Wilson AJ, et al. Limited (information only) patient education programs for adults with asthma. Cochrane Database Syst Rev 2000a;(2):CD001005.-
6. Gibson PG, Coughlan J, Wilson AJ, et al. Self-management education and regular practitioner review for adults with asthma. Cochrane Database Syst Rev 2000b (2):CD001117.-
7. Lieu TA, Quesenberry CP, Jr, Capra AM, et al. Outpatient management practices associated with reduced risk of pediatric asthma hospitalization and emergency department visits. Pediatrics 1997;100(3 Pt 1):334-41.
8. Lefevre F, Piper M, Mark D, et al. Management of Chronic Asthma. AHRQ evidence report, contract number 290-97-001-5, 2001, http://www.ahcpr.gov/clinic/epcix.htm.
9. Mulrow CD, Oxman AD, editors. Cochrane Collaboration Handbook. Available in the Cochrane Library [database on disk and CD-ROM]. The Cochrane Collaboration; Issue 1. Oxford: Update Software; 1997.
10. Schulz KF, Chalmers I, Hayes RJ, et al. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408-12.
11. Berlin JA, Rennie D. Measuring the quality of trials: the quality of the quality scales. JAMA 1999;282(11):1083-5.
12. Juni P, Witschi A, Bloch R, et al. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA 1999;282(11):1054-60.
13. Jones KP, Mullee MA, Middleton M, et al. Peak flow based asthma self-management: a randomised controlled study in general practice. British Thoracic Society Research Committee. Thorax 1995;50(8):851-7.
14. Drummond N, Abdalla M, Beattie JAG, et al. Effectiveness of routine self monitoring of peak flow in patients with asthma. Grampian Asthma Study of Integrated Care GRASSIC). BMJ 1994 Feb. 26;308(6928):564-7.
15. Ayres JG, Campbell LM. A controlled assessment of an asthma self-management plan involving a budesonide dose regimen. OPTIONS Research Group. Eur Respir J 1996;886-92.
16. Cowie RL, Revitt SG, Underwood MF, et al. The effect of a peak flow-based action plan in the prevention of exacerbations of asthma. Chest 1997;112(6):1534-8.
17. Cote J, Cartier A, Robichaud P, et al. Influence on asthma morbidity of asthma education programs based on self-management plans following treatment optimization. Am J Respir Crit Care Med 1997;155(5):1509-14.
18. Ignacio-Garcia JM, Gonzalez-Santos P. Asthma self-management education program by home monitoring of peak expiratory flow. Am J Respir Crit Care Med 1995;151(2 Pt 1):353-9.
19. Charlton I, Antoniou AG, Atkinson J, et al. Asthma at the interface: bridging the gap between general practice and a district general hospital. Arch Dis Child 1994;70(4):313-8.
20. Turner MO, Taylor D, Bennett R, et al. A randomized trial comparing peak expiratory flow and symptom self-management plans for patients with asthma attending a primary care clinic. Am J Respir Crit Care Med 1998;157(2):540-6.
21. Charlton I, Charlton G, Broomfield J, et al. Evaluation of peak flow and symptoms only self-management plans for control of asthma in general practice. BMJ 1990;301(6765):1355-9.
Relationships between physician practice style, patient satisfaction, and attributes of primary care
- Different physician-patient interaction styles are actively used in community practice.
- A person-focused style is being used by almost half of the physicians observed, and this style is associated with greater patient-reported quality of primary care and greater patient satisfaction.
- This study provides further evidence to support the widespread implementation of this approach to the physician-patient interaction.
Over the past half century, changing medical technology, law, education, ethics, and research have influenced the current shape of physician-patient interactions.9 In 1956, the traditional model of Activity-Passivity (physician does something to the patient) was challenged with the revolutionary concept of active patient participation.10 The models of Guidance and Cooperation (physician tells patient what to do, patient cooperates) and Mutual Participation (physician enables patient to help him/herself, patient is a partner) were proposed10 and are reflected in modern theoretically-based interaction models. Numerous models have been proposed as variants of the Guidance/Cooperation model (eg, paternalistic model,11 priestly model,12 contractual model13) and the Mutual Participation model (eg, ethnographic model,14 consumerist model,11,15 family systems model16). Few of these models, though, have been empirically evaluated. The best-developed and most-studied mutual participation model is the patient-centered method.5,17-20
When data have been collected using quantitative or qualitative approaches, significant strides have been made in understanding physician-patient interaction3, 21-23 and the effect of such interactions on patient outcomes,5,24,25 primarily patient satisfaction.1,26-29 However, many studies have been limited by their focus on a narrow aspect of physician-patient communication, studying a small number of physicians or patients, and using medical students, residents, and hospital faculty as study subjects.
The purpose of this study was not to develop a new model of physician-patient interaction. Rather, variables characterizing physician style grounded by the direct observation of thousands of encounters for 138 community practicing family physicians were used to empirically cluster physicians into groups that represent distinct interaction styles. Because interaction style may be manifested in all phases of a patient encounter, we used as a guiding framework the 3 primary functions of an interview:30,31gathering information, enhancing a healing relationship, and making and implementing decisions. The importance of each of these functions varies depending on the nature of the encounter, but our overall approach provides a practical way of conceptualizing physician-patient interaction style. The association of the empirically derived and theoretically-based physician styles are tested with 3 outcomes: 1) patient report of delivery of attributes of primary care measured using the Components of Primary Care Instrument (CPCI), 2) patient satisfaction with the visit, and 3) the duration of the visit.
Methods
This study was part of the larger Direct Observation of Primary Care (DOPC) study, a cross sectional observational study that examined the content of 4454 outpatient visits to family physicians in northeast Ohio. Details of the methods of the DOPC study have been described extensively elsewhere.32,34 Briefly, 4 teams of 2 research nurses directly observed consecutive patient visits to 138 participating physicians in 84 practices between October 1994 and August 1995. The research nurses collected data on the content and context of consecutive office visits using the following methods: direct observation of the patient visit, patient exit questionnaire, medical record review, and collection of ethnographic field notes.33,34
Measures
Patients’ perception of the delivery 5 attributes of primary care was measured by the Components of Primary Care Instrument (CPCI). Interpersonal communication was an evaluation of the ease of exchange of information between patient and physician. The physician’s accumulated knowledge about the patient refers to the physician’s understanding of the patient’s medical history, health care needs, and values. Coordination of care refers to the information received from referrals to specialists and previous health care visits, and its incorporation into the current and future care of the patient. Preference to see usual physician refers to the degree to which patients believed and valued that they could go to their regular physician for almost all problems. Scale scores demonstrate good internal consistency reliability (Cronbach’s alpha: .68–.79).35 Continuity of care is measured by the Usual Provider Continuity index (UPC), which is the proportion of visits to the patient’s regular doctor in the past year out of the total number of physician visits in the past year.
Patient satisfaction was measured using the 4 physician-specific items from the MOS 9 Item Visit Rating Form36 (Cronbach’s alpha = .89).33 Also included on the patient survey was a single item assessing the degree to which patients’ expectations with the visit were met. Duration of the visit was the total face-to-face time the physician spent with the patient and was measured by direct observation.
Each physician’s interaction style was determined through a 2-step process. In the first step, ethnographic field notes were used to gather information that helps define core features of physician style. The field notes from 4 days of observation of 138 family physicians in 84 practices were transcribed and imported into FolioVIEWS37 for data management and coding. Analysis was conducted with an immersion-crystallization approach38 involving repetitive reading and summarization of the text data. Case summaries were constructed from a sample of practices selected to maximize variation among practice characteristics such as size, physician sex, and practice location. The case summaries were independently reviewed, and important features were identified. These features were cross-checked against the original data. This process, and the resulting 30 features, are described in detail elsewhere.32
Six of the features that emerged from the qualitative analyses pertain to physician style and are listed in Table 1. Each of the 3 primary interview functions30 is represented by at least 1 feature, ensuring good coverage of the core aspects of the interaction. Gathering information is shaped by physician orientation and the clinical information allowed or elicited in the visits. Enhancing healing relationships is realized in part through affective connection with patients. The final function, making and implementing decisions, is influenced by the level of control or shared power with patients, the physician’s openness to patients’ agendas, and the physician’s willingness to negotiate options with patients.
The second step involved a cluster analysis of the 6 variables. First a hierarchical approach was used to estimate the number of clusters. Then a non-hierarchical clustering approach was used to determine physician classification among the clusters and the features that distinguish the clusters.39 Analysis of variance was used to confirm that variables included in the cluster analysis significantly differed between at least 2 of the identified clusters, and thus were contributing to defining interaction style.
TABLE 1
Physician style variables
| Physician orientation: |
| Problem focused—physician focuses on the patient’s presenting complaint |
| Patient-focused—physician is open to a broader health care agenda with the patient and explores other possible issues |
| Scope of clinical information: |
| Biomedical—talk focuses on the biological information, diagnoses and treatments |
| Biopsychosocial—explores both the biological and social and psychological issues |
| Affective connection with patients: |
| Physician personable and friendly, connects with person on a personal level |
| Physician not personable and friendly, maintains professional distance |
| Openness to patient agenda: |
| Physician open to patient’s agenda |
| Physician sets and maintains the agenda |
| Sharing of control in interaction: |
| Physician shares control of the interaction |
| Physician controls the interaction |
| Negotiation of options with patient: |
| Physician negotiates options with patients |
| Physician does not negotiate options with patients |
Analyses
The association of physician and patient characteristics with interaction style was assessed by chi square for categorical variables and by analysis of variance for continuous variables. The association of physician style with each of the 5 attributes of primary care measured by the CPCI, the indicators of patient satisfaction, and duration of the visit were tested using multilevel modeling,40 to account for the hierarchical nature of data (ie, patients nested within physicians).
Results
Of the 4994 patients presenting for care by their family physicians, 4454 (89%) agreed to participate in the DOPC study. Physicians participating in the DOPC study were similar in age to national samples of family physicians, but over-represented female and residency-trained physicians.34 Patient age, sex, and race were similar to the population of patients seeing family physicians and general practitioners nationally as reported in the National Ambulatory Medical Care Survey.34 Patient questionnaires were returned by 3283 (74%) of the patients. Of those respondents, 2881 satisfactorily completed the CPCI, representing 88% of those returning a patient questionnaire and 65% of the total sample. The patients who completed the CPCI were more likely to be white, have private health care insurance, and be somewhat older than patients who did not complete the CPCI.35
The cluster analysis identified 4 distinct groups of physicians. Each of the 138 physicians was classified into 1 group. Each of the 6 variables in the analysis contributed to defining the 4 groups by significantly (P
Forty-nine percent of physicians were classified as person focused. These physicians were more focused on the person than the disease, were perceived as personable and friendly, were open to the patient’s agenda, and frequently negotiated options with the patient. Physicians classified as biopsychosocial (16%) were more focused on the patient’s disease, but elicited psychosocial clinical information. Physicians classified as biomedical (20%) were also more focused on the patient’s disease and were unlikely to elicit psychosocial information. These physicians also demonstrated a low level of friendliness and were unlikely to negotiate options with the patient. The high physician control group’s major characteristics were domination of the encounter and disregard of the patient’s agenda (14%).
Association of physician characteristics with the interaction styles is presented in Table 2. The percent of male and female physicians differed greatly among the 4 style groups. The proportion of female physicians in the person-focused group was almost 4 times that of the biopsychosocial group and the high physician control group (P
As reported in Table 3, physician style is significantly associated with 3 of the 5 patient reports of the attributes of primary care. Physicians classified as having a person-focused approach have the highest mean score of communication; the other 3 styles score lower, with the high-physician-control style scoring the lowest. Person-focused and biopsychosocial physicians scored highest on patient reports of accumulated knowledge; those in the biomedical group scored the lowest. Coordination of care was highest among the person-focused group and lowest among the high-control group Across the different types of physician style, there was no difference in patient report of preference for his or her regular physician or the measure of continuity of care.
The associations of physician style with 2 indicators of patient satisfaction are displayed in Table 4. The highest group mean of patient satisfaction is for the person-focused style, and the lowest is for the high-physician-control group. The indicator of the degree to which patient expectations were met also follows this pattern. Also displayed in Table 4, the person-focused style demonstrated the longest average duration of visit, at 11.5 minutes; the high-physician-control group visits were the shortest in duration, at about 9.5 minutes.
TABLE 2
Physician and patient characteristics associated with interaction style
| Characteristic | Total | Biopsychosocial | Biomedical | Person focused | High physician control | P |
|---|---|---|---|---|---|---|
| Physician | ||||||
| Number | 138 | 22 | 28 | 68 | 20 | |
| Age (mean years) | 43 | 45 | 43 | 42 | 46 | .06 |
| Female | 26% | 9% | 21% | 38% | 10% | |
| Residency trained | 90% | 86% | 86% | 94% | 85% | .44 |
| Patient | ||||||
| Number | 2881 | 504 | 578 | 1258 | 541 | |
| Age (mean years) | 42 | 44 | 41 | 42 | 43 | .11 |
| Female | 62% | 57% | 61% | 65% | 58% | |
Association of physician style with attributes of primary care1
| Attribute of primary care | Biopsychosocial | Biomedical | Person focused | High physician control | P |
|---|---|---|---|---|---|
| Communication | 4.27 | 4.26 | 4.43 | 4.21 | |
| Accumulated knowledge | 3.54 | 3.33 | 3.56 | 3.51 | |
| Coordination of care | 3.85 | 3.78 | 3.99 | 3.74 | |
| Preference for regular doctor | 4.46 | 4.45 | 4.46 | 4.39 | ns |
| Usual provider continuity2 | 0.67 | 0.66 | 0.64 | 0.65 | ns |
| 1Each row represents a separate multilevel regression model wherein each attribute of primary care is the outcome variable and the number in each column is the group mean of that attribute, adjusted for patient and physician age and sex, as well as the effect of the patients being nested within physicians. | |||||
| 2Usual provide continuity = total number of visits to regular physician in past year, divided by the total number of physician visits in the past year. | |||||
Association of physician interaction style with patient satisfaction and duration of visit1
| Outcome measures | Biopsychosocial | Biomedical | Person focused | High physician control | P |
|---|---|---|---|---|---|
| Patient satisfaction with physician | 4.38 | 4.39 | 4.49 | 4.30 | 002 |
| Patient expectations met | 4.36 | 4.33 | 4.45 | 4.31 | .02 |
| Length of visit (mean minutes) | 9.97 | 10.02 | 11.56 | 9.51 | .005 |
| 1Results from multilevel regression model, analyses include patient and physician age and gende as covariates, and controls for the nested nature of the data. | |||||
Discussion
These data indicate that a person-focused approach is actively used in community practice, and is the style most congruent with patient-reported quality of primary care and satisfaction with care. Our data, in concert with data reported by others,5,24 indicate strong support for the feasibility and value of the person-focused model. We found that, of the 4 distinct interaction styles, physicians with the person-focused style scored highest across all measures of the attributes of primary care and on the indicators of patient satisfaction, with the exception of continuity of care. In contrast, physicians with the high-control style were generally lowest on the primary care and satisfaction indicators.
It is important to emphasize that, even though the vast majority of patients in this sample are likely to have self-selected their primary care physician, patient rating of some attributes of primary care differed across the 4 physician styles. Patients of physicians with different styles equally valued seeing their regular physician, as reported by the preference-for-their-regular-doctor score; they exhibited similar proportions of continuity visits in the past year; and their satisfaction scores were all generally high. Patients appear to want to see their regular physician, regardless of interaction approach, even though some approaches—particularly the high-physician-control style—were rated poorer for communication, coordination of care, and accumulated knowledge.
There may be several explanations as to why a particular physician style is associated with specific patient reports of communication, accumulated knowledge, and coordination of care. Openness to the patient’s agenda and willingness to negotiate options—as was characteristic of the person-focused physicians—may facilitate good communication and convey an understanding of patient preferences and values regarding health. It is interesting to note that different groups scored lowest on some of the attributes of primary care. The high-physician-control group was the lowest on interpersonal communication and coordination of care. High-control physicians were more likely to dominate the agenda and the verbal exchanges. Patients may have felt they could not ask questions or that the physician did not listen to what they tried to say. The biomedical group of physicians were given the lowest scores by patients on accumulated knowledge, suggesting that patients thought these physicians were less likely to know their preferences and values regarding health care, know less about them as persons, and know less about their family and medical histories.
As others have proposed, we concur that interaction style is not a dichotomy or even a continuum of patient versus physician control, but is multidimensional, cutting across the main functions of the patient encounter (ie, information gathering, relationship building, and making and implementing decisions). These data provide some confirmation for the original scheme proposed by Szasz and Hollander,10 with the Mutual Participation model most represented by the person-focused approach and the Activity-Passivity model most represented by the high-physician-control group. The biopsychosocial and biomedical approaches represent different versions of the Guidance and Cooperative model.
The 4 types of physician style empirically derived from our data are similar to communication pattern types found by Roter et al,27 in a study with similar aims but different methods. Of the 5 types reported, narrowly biomedical and expanded biomedical accounted for 65% of visits, and biopsychosocial accounted for 20%. Psychosocial and consumerist (distinguished by a high degree of patient questions) accounted for only 8% each. It is interesting that in our data, we found the person-focused style was by far the most common approach (49%) among this group of family physicians. These differences in use of particular interaction styles may have several explanations. First, these data were collected more recently.27 Thus our data may reflect trends in a movement away from a paternalistic style and toward an increased patient participatory style. Second, our sample consisted entirely of family physicians practicing in the community, where the model of person-focused care may have a longer history of support and endorsement or be of greater importance to community family physicians, whose emphasis is on a breadth of care based on patient needs.6,7,18
Physicians with a person-focused style granted the longest visits, while high-control-physicians granted the shortest—a difference of more than 2 minutes per visit on average. The associations were not explained away by accounting for patient or physician characteristics, suggesting that a person-focused style may require more time. However, others have found that physicians engaging in a patient participatory style had office visits that were of similar duration as found with other approaches,23, 27 although the average duration of visit for both of these studies were considerably longer than the office visits among our sample.
This study has several strengths. The use of community practicing physicians in real world conditions for whom visits were similar in content to the visits reported by NAMCS34 adds to the generalizability of the findings. We have used an integration of qualitative and quantitative approaches to empirically derive categories of physician interaction style. Our data are based on nurse observation of an average of 32 encounters per physician and documented in rich and comprehensive qualitative fieldnotes. And finally, by using multilevel modeling, we have reported an honest estimate of the association of physician style and patient report of primary care by appropriately modeling the nested data structure.
The findings must be interpreted in light of potential study limitations. First, the patients who did not complete the patient questionnaire are somewhat different demographically than those patients who did complete it. However, non-completion of the questionnaire was not associated with physician style; therefore, it is unlikely that the associations would change, had these individuals been included. Second, because the study was cross-sectional we cannot control for patient self-selection of physicians. Nonetheless, since patients dissatisfied with the quality of care are likely to seek another physician, we would expect patient self-selection of physicians to bias the study toward the null, thus making our results even more remarkable.
These findings, in combination with the literature on the person-focused,24 patient-centered5,17,19,20,41 and relationship-centered approaches,42 provide strong evidence to support the widespread implementation of this physician-patient interaction approach. Further investigation in community practice may lead to identification of ways to support and encourage person-focused care and the time needed to provide such care.
· Acknowledgments ·
The authors are indebted to the physicians, office staff members, and patients without whose participation this study would not have been possible. This paper was improved by helpful suggestions on an earlier draft by Kurt C. Stange, MD, PhD. This study was supported by a grant from the National Cancer Institute (1R01 CA60862) and in part by the Center for Research in Family Practice and Primary Care and the American Academy of Family Practice.
1. Bertakis KD, Roter D, Putnam SM. The relationship of physician medical interview style to patient satisfaction. J Fam Pract. 1991;32:175-181.
2. Bertakis KD, Callahan EJ, Helms LJ, Azari R, Robbins JA, Miller J. Physician practice styles and patient outcomes. Med Care. 1998;36:879-891.
3. Stewart MA. What is a successful doctor-patient interview? A study of interactions and outcomes. Soc Sci Med. 1984;19:167-175.
4. Levinson W, Roter DL, Mullooly JP, Dull VT, Frankel RM. Physician-patient communication: the relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277:553-559.
5. Stewart M, Brown JB, Donner A, McWhinney IR, Oates J, Weston WW, Jordan J. The impact of patient-centered care on outcomes. J Fam Pract. 2000;49:796-804.
6. McWhinney IR. Through clinical method to a more humane medicine. In: White KL, ed. The task of medicine. Menlo Park, CA: The Henry J. Kaiser Family Foundation; 1988.
7. Stange KC, Jaén CR, Flocke SA, Miller WL, Crabtree BF, Zyzanski SJ. The value of a family physician. J Fam Pract. 1998;46:363-368.
8. Institute of Medicine. Primary Care: America’s Health in a New Era. Donaldson MS. YK, Lohr KN, Vanselow NA, ed Washington D.C.: National Academy Press; 1996.
9. Laine C, Davidoff F. Patient-centered medicine: A professional evolution. JAMA. 1996;275:152-156.
10. Szasz TS, Hollender MH. The basic models of the doctor-patient relationship. Arch Int Med. 1956;97:585-592.
11. Emanuel EJ, Emanuel LL. Four models of the physician-patient relationship. JAMA. 1992;267:2221-2226.
12. Veatch RM. Models for ethical medicine in a revolutionary age. What physician-patient roles foster the most ethical relationship? Hasting Center Reports. 1972;2:5-7.
13. Quill TE. Partnerships in patient care: a contractual approach. Ann Int Med. 1983;98:228-234.
14. Kleinman AM, Eisenberg L, Good B. Culture, illness, and care: Clinical lessons from anthropologic and cross-cultural research. Ann Int Med. 1978;88:251-258.
15. Lazare A, Eisenthal S, Wasserman L. The customer approach to patienthood: Attending to patient requests in a walk-in clinic. Archives of General Psychiatry. 1975;32:553-558.
16. McDaniel S, Campbell T, Seaburn D. Family-oriented primary care: a manual for medical providers. Berlin: Springer-Verlag; 1990.
17. Stewart M, Weston WW, Brown JB, McWhinney IR, McWilliam CL, Freeman TR. Patient-centered medicine: Transforming the clinical method. Thousand Oaks, CA: Sage Publications; 1995.
18. Levenstein JH, McCracken EC, McWhinney IR, Stewart MA, Brown JB. The patient-centred clinical method. 1. A model for the doctor-patient interaction in family medicine. Fam Pract. 1986;3:24-30.
19. Epstein RM. The science of patient-centered care. J Fam Pract. 2000;49:805-807.
20. Stewart M, Roter D. Communicating With Medical Patients. Knapp ML, ed second printing (1990) ed: Sage Publications; 1989.
21. Hall JA, Roter DL, Katz NR. Meta-analysis of correlates of provider behavior in medical encounters. Med Care. 1988;26:657-675.
22. Byrne PS, Long BEL. Doctors talking to patients. London: H.M.S.O.; 1976.
23. Marvel MK, Doherty WJ, Weiner E. Medical interviewing by exemplary family physicians. J Fam Pract. 1998;47:343-348.
24. Roter D. The enduring and evolving nature of the patient-physician relationship. Patient Educ and Counseling. 2000;39:5-15.
25. Kaplan SH, Greenfield S, Ware JE. Assessing the effects of physician-patient interactions on the outcomes of chronic disease. Med Care. 1989;27:S110-S127.
26. Buller MK, Buller DB. Physicians’ communication style and patient satisfaction. J Health Soc Behav. 1987;28:375-388.
27. Roter DL, Stewart M, Putnam SM, Lipkin M, Stiles W, Inui TS. Communication patterns of primary care physicians. JAMA. 1997;277:350-356.
28. Williams S, Weinman J, Dale J. Doctor-patient communication and patient satisfaction: A review. Fam Pract. 1998;15:480-492.
29. Greene MG, Adelman RD, Friedman E, Charon R. Older patient satisfaction with communication during an initial medical encounter. Soc Sci Med. 1994;38:1279-1288.
30. Cohen-Cole S. The medical interview: The three-function approach. St. Louis: Mosby Year Book; 1991.
31. Lazare A, Putnam SM, Lipkin M. Three functions of the medical interview. In: Lipkin M, Putnam S, Lazare A, eds. The medical interview: Clinical care, education and research. New York: Springer; 1995;3-19.
32. Crabtree BF, Miller WL, Aita V, Flocke SA, Stange KC. Primary care practice organization: A qualitative analysis. J Fam Pract. 1998;46:403-409.
33. Stange KC, Zyzanski SJ, Jaén CR, Callahan EJ, Kelly RB, Gillanders WR, Shank JC, Chao J, Medalie JH, Miller WL, Crabtree BF, Flocke SA, Gilchrist VJ, Langa DM, Goodwin MA. Illuminating the black box: a description of 4454 patient visits to 138 family physicians. J Fam Pract. 1998;46:377-389.
34. Stange KC, Zyzanski SJ, Smith TF, Kelly R, Langa DM, Flocke SA, Jaén CR. How valid are medical records and patient questionnaires for physician profiling and health services research? A comparison with direct observation of patient visits. Med Care. 1998;36:851-867.
35. Flocke SA. Measuring attributes of primary care: Development of a new instrument. J Fam Pract. 1997;45:64-74.
36. Rubin H, Gandek B, Roger WH, Kisinski M, McHorney C, Ware J. Patients’ ratings of outpatient visits in different practice settings. JAMA. 1993;270:835-840.
37. FolioVIEWS.. 3.1 ed. Provo, Utah: Folio Corporation; 1998.
38. Crabtree BF, Miller WL. Doing Qualitative Research. Newbury Park, California: Sage Publications; 1992.
39. Aldenderfer MS, Blashfield RK. Cluster Analysis. Lewis-Beck MS, ed Newbury Park: Sage; 1984.
40. Bryk AS, Raudenbush SW. Hierarchical linear models: applications and data analysis methods. Newbury Park: Sage Publications; 1992.
41. Stewart M, Brown JB, Boon H, Galajda J, Meredith L, Sangster M. Evidence on patient-doctor communication. Cancer Prevention and Control. 1999;3:25-30.
42. Carol P. Tresolini and the Pew-Fetzer Task Force on Advancing Psychosocial Health Education. Health profession education and relationship-centered care. San Francisco, CA: Pew Health Professions Commission; 1994.
- Different physician-patient interaction styles are actively used in community practice.
- A person-focused style is being used by almost half of the physicians observed, and this style is associated with greater patient-reported quality of primary care and greater patient satisfaction.
- This study provides further evidence to support the widespread implementation of this approach to the physician-patient interaction.
Over the past half century, changing medical technology, law, education, ethics, and research have influenced the current shape of physician-patient interactions.9 In 1956, the traditional model of Activity-Passivity (physician does something to the patient) was challenged with the revolutionary concept of active patient participation.10 The models of Guidance and Cooperation (physician tells patient what to do, patient cooperates) and Mutual Participation (physician enables patient to help him/herself, patient is a partner) were proposed10 and are reflected in modern theoretically-based interaction models. Numerous models have been proposed as variants of the Guidance/Cooperation model (eg, paternalistic model,11 priestly model,12 contractual model13) and the Mutual Participation model (eg, ethnographic model,14 consumerist model,11,15 family systems model16). Few of these models, though, have been empirically evaluated. The best-developed and most-studied mutual participation model is the patient-centered method.5,17-20
When data have been collected using quantitative or qualitative approaches, significant strides have been made in understanding physician-patient interaction3, 21-23 and the effect of such interactions on patient outcomes,5,24,25 primarily patient satisfaction.1,26-29 However, many studies have been limited by their focus on a narrow aspect of physician-patient communication, studying a small number of physicians or patients, and using medical students, residents, and hospital faculty as study subjects.
The purpose of this study was not to develop a new model of physician-patient interaction. Rather, variables characterizing physician style grounded by the direct observation of thousands of encounters for 138 community practicing family physicians were used to empirically cluster physicians into groups that represent distinct interaction styles. Because interaction style may be manifested in all phases of a patient encounter, we used as a guiding framework the 3 primary functions of an interview:30,31gathering information, enhancing a healing relationship, and making and implementing decisions. The importance of each of these functions varies depending on the nature of the encounter, but our overall approach provides a practical way of conceptualizing physician-patient interaction style. The association of the empirically derived and theoretically-based physician styles are tested with 3 outcomes: 1) patient report of delivery of attributes of primary care measured using the Components of Primary Care Instrument (CPCI), 2) patient satisfaction with the visit, and 3) the duration of the visit.
Methods
This study was part of the larger Direct Observation of Primary Care (DOPC) study, a cross sectional observational study that examined the content of 4454 outpatient visits to family physicians in northeast Ohio. Details of the methods of the DOPC study have been described extensively elsewhere.32,34 Briefly, 4 teams of 2 research nurses directly observed consecutive patient visits to 138 participating physicians in 84 practices between October 1994 and August 1995. The research nurses collected data on the content and context of consecutive office visits using the following methods: direct observation of the patient visit, patient exit questionnaire, medical record review, and collection of ethnographic field notes.33,34
Measures
Patients’ perception of the delivery 5 attributes of primary care was measured by the Components of Primary Care Instrument (CPCI). Interpersonal communication was an evaluation of the ease of exchange of information between patient and physician. The physician’s accumulated knowledge about the patient refers to the physician’s understanding of the patient’s medical history, health care needs, and values. Coordination of care refers to the information received from referrals to specialists and previous health care visits, and its incorporation into the current and future care of the patient. Preference to see usual physician refers to the degree to which patients believed and valued that they could go to their regular physician for almost all problems. Scale scores demonstrate good internal consistency reliability (Cronbach’s alpha: .68–.79).35 Continuity of care is measured by the Usual Provider Continuity index (UPC), which is the proportion of visits to the patient’s regular doctor in the past year out of the total number of physician visits in the past year.
Patient satisfaction was measured using the 4 physician-specific items from the MOS 9 Item Visit Rating Form36 (Cronbach’s alpha = .89).33 Also included on the patient survey was a single item assessing the degree to which patients’ expectations with the visit were met. Duration of the visit was the total face-to-face time the physician spent with the patient and was measured by direct observation.
Each physician’s interaction style was determined through a 2-step process. In the first step, ethnographic field notes were used to gather information that helps define core features of physician style. The field notes from 4 days of observation of 138 family physicians in 84 practices were transcribed and imported into FolioVIEWS37 for data management and coding. Analysis was conducted with an immersion-crystallization approach38 involving repetitive reading and summarization of the text data. Case summaries were constructed from a sample of practices selected to maximize variation among practice characteristics such as size, physician sex, and practice location. The case summaries were independently reviewed, and important features were identified. These features were cross-checked against the original data. This process, and the resulting 30 features, are described in detail elsewhere.32
Six of the features that emerged from the qualitative analyses pertain to physician style and are listed in Table 1. Each of the 3 primary interview functions30 is represented by at least 1 feature, ensuring good coverage of the core aspects of the interaction. Gathering information is shaped by physician orientation and the clinical information allowed or elicited in the visits. Enhancing healing relationships is realized in part through affective connection with patients. The final function, making and implementing decisions, is influenced by the level of control or shared power with patients, the physician’s openness to patients’ agendas, and the physician’s willingness to negotiate options with patients.
The second step involved a cluster analysis of the 6 variables. First a hierarchical approach was used to estimate the number of clusters. Then a non-hierarchical clustering approach was used to determine physician classification among the clusters and the features that distinguish the clusters.39 Analysis of variance was used to confirm that variables included in the cluster analysis significantly differed between at least 2 of the identified clusters, and thus were contributing to defining interaction style.
TABLE 1
Physician style variables
| Physician orientation: |
| Problem focused—physician focuses on the patient’s presenting complaint |
| Patient-focused—physician is open to a broader health care agenda with the patient and explores other possible issues |
| Scope of clinical information: |
| Biomedical—talk focuses on the biological information, diagnoses and treatments |
| Biopsychosocial—explores both the biological and social and psychological issues |
| Affective connection with patients: |
| Physician personable and friendly, connects with person on a personal level |
| Physician not personable and friendly, maintains professional distance |
| Openness to patient agenda: |
| Physician open to patient’s agenda |
| Physician sets and maintains the agenda |
| Sharing of control in interaction: |
| Physician shares control of the interaction |
| Physician controls the interaction |
| Negotiation of options with patient: |
| Physician negotiates options with patients |
| Physician does not negotiate options with patients |
Analyses
The association of physician and patient characteristics with interaction style was assessed by chi square for categorical variables and by analysis of variance for continuous variables. The association of physician style with each of the 5 attributes of primary care measured by the CPCI, the indicators of patient satisfaction, and duration of the visit were tested using multilevel modeling,40 to account for the hierarchical nature of data (ie, patients nested within physicians).
Results
Of the 4994 patients presenting for care by their family physicians, 4454 (89%) agreed to participate in the DOPC study. Physicians participating in the DOPC study were similar in age to national samples of family physicians, but over-represented female and residency-trained physicians.34 Patient age, sex, and race were similar to the population of patients seeing family physicians and general practitioners nationally as reported in the National Ambulatory Medical Care Survey.34 Patient questionnaires were returned by 3283 (74%) of the patients. Of those respondents, 2881 satisfactorily completed the CPCI, representing 88% of those returning a patient questionnaire and 65% of the total sample. The patients who completed the CPCI were more likely to be white, have private health care insurance, and be somewhat older than patients who did not complete the CPCI.35
The cluster analysis identified 4 distinct groups of physicians. Each of the 138 physicians was classified into 1 group. Each of the 6 variables in the analysis contributed to defining the 4 groups by significantly (P
Forty-nine percent of physicians were classified as person focused. These physicians were more focused on the person than the disease, were perceived as personable and friendly, were open to the patient’s agenda, and frequently negotiated options with the patient. Physicians classified as biopsychosocial (16%) were more focused on the patient’s disease, but elicited psychosocial clinical information. Physicians classified as biomedical (20%) were also more focused on the patient’s disease and were unlikely to elicit psychosocial information. These physicians also demonstrated a low level of friendliness and were unlikely to negotiate options with the patient. The high physician control group’s major characteristics were domination of the encounter and disregard of the patient’s agenda (14%).
Association of physician characteristics with the interaction styles is presented in Table 2. The percent of male and female physicians differed greatly among the 4 style groups. The proportion of female physicians in the person-focused group was almost 4 times that of the biopsychosocial group and the high physician control group (P
As reported in Table 3, physician style is significantly associated with 3 of the 5 patient reports of the attributes of primary care. Physicians classified as having a person-focused approach have the highest mean score of communication; the other 3 styles score lower, with the high-physician-control style scoring the lowest. Person-focused and biopsychosocial physicians scored highest on patient reports of accumulated knowledge; those in the biomedical group scored the lowest. Coordination of care was highest among the person-focused group and lowest among the high-control group Across the different types of physician style, there was no difference in patient report of preference for his or her regular physician or the measure of continuity of care.
The associations of physician style with 2 indicators of patient satisfaction are displayed in Table 4. The highest group mean of patient satisfaction is for the person-focused style, and the lowest is for the high-physician-control group. The indicator of the degree to which patient expectations were met also follows this pattern. Also displayed in Table 4, the person-focused style demonstrated the longest average duration of visit, at 11.5 minutes; the high-physician-control group visits were the shortest in duration, at about 9.5 minutes.
TABLE 2
Physician and patient characteristics associated with interaction style
| Characteristic | Total | Biopsychosocial | Biomedical | Person focused | High physician control | P |
|---|---|---|---|---|---|---|
| Physician | ||||||
| Number | 138 | 22 | 28 | 68 | 20 | |
| Age (mean years) | 43 | 45 | 43 | 42 | 46 | .06 |
| Female | 26% | 9% | 21% | 38% | 10% | |
| Residency trained | 90% | 86% | 86% | 94% | 85% | .44 |
| Patient | ||||||
| Number | 2881 | 504 | 578 | 1258 | 541 | |
| Age (mean years) | 42 | 44 | 41 | 42 | 43 | .11 |
| Female | 62% | 57% | 61% | 65% | 58% | |
Association of physician style with attributes of primary care1
| Attribute of primary care | Biopsychosocial | Biomedical | Person focused | High physician control | P |
|---|---|---|---|---|---|
| Communication | 4.27 | 4.26 | 4.43 | 4.21 | |
| Accumulated knowledge | 3.54 | 3.33 | 3.56 | 3.51 | |
| Coordination of care | 3.85 | 3.78 | 3.99 | 3.74 | |
| Preference for regular doctor | 4.46 | 4.45 | 4.46 | 4.39 | ns |
| Usual provider continuity2 | 0.67 | 0.66 | 0.64 | 0.65 | ns |
| 1Each row represents a separate multilevel regression model wherein each attribute of primary care is the outcome variable and the number in each column is the group mean of that attribute, adjusted for patient and physician age and sex, as well as the effect of the patients being nested within physicians. | |||||
| 2Usual provide continuity = total number of visits to regular physician in past year, divided by the total number of physician visits in the past year. | |||||
Association of physician interaction style with patient satisfaction and duration of visit1
| Outcome measures | Biopsychosocial | Biomedical | Person focused | High physician control | P |
|---|---|---|---|---|---|
| Patient satisfaction with physician | 4.38 | 4.39 | 4.49 | 4.30 | 002 |
| Patient expectations met | 4.36 | 4.33 | 4.45 | 4.31 | .02 |
| Length of visit (mean minutes) | 9.97 | 10.02 | 11.56 | 9.51 | .005 |
| 1Results from multilevel regression model, analyses include patient and physician age and gende as covariates, and controls for the nested nature of the data. | |||||
Discussion
These data indicate that a person-focused approach is actively used in community practice, and is the style most congruent with patient-reported quality of primary care and satisfaction with care. Our data, in concert with data reported by others,5,24 indicate strong support for the feasibility and value of the person-focused model. We found that, of the 4 distinct interaction styles, physicians with the person-focused style scored highest across all measures of the attributes of primary care and on the indicators of patient satisfaction, with the exception of continuity of care. In contrast, physicians with the high-control style were generally lowest on the primary care and satisfaction indicators.
It is important to emphasize that, even though the vast majority of patients in this sample are likely to have self-selected their primary care physician, patient rating of some attributes of primary care differed across the 4 physician styles. Patients of physicians with different styles equally valued seeing their regular physician, as reported by the preference-for-their-regular-doctor score; they exhibited similar proportions of continuity visits in the past year; and their satisfaction scores were all generally high. Patients appear to want to see their regular physician, regardless of interaction approach, even though some approaches—particularly the high-physician-control style—were rated poorer for communication, coordination of care, and accumulated knowledge.
There may be several explanations as to why a particular physician style is associated with specific patient reports of communication, accumulated knowledge, and coordination of care. Openness to the patient’s agenda and willingness to negotiate options—as was characteristic of the person-focused physicians—may facilitate good communication and convey an understanding of patient preferences and values regarding health. It is interesting to note that different groups scored lowest on some of the attributes of primary care. The high-physician-control group was the lowest on interpersonal communication and coordination of care. High-control physicians were more likely to dominate the agenda and the verbal exchanges. Patients may have felt they could not ask questions or that the physician did not listen to what they tried to say. The biomedical group of physicians were given the lowest scores by patients on accumulated knowledge, suggesting that patients thought these physicians were less likely to know their preferences and values regarding health care, know less about them as persons, and know less about their family and medical histories.
As others have proposed, we concur that interaction style is not a dichotomy or even a continuum of patient versus physician control, but is multidimensional, cutting across the main functions of the patient encounter (ie, information gathering, relationship building, and making and implementing decisions). These data provide some confirmation for the original scheme proposed by Szasz and Hollander,10 with the Mutual Participation model most represented by the person-focused approach and the Activity-Passivity model most represented by the high-physician-control group. The biopsychosocial and biomedical approaches represent different versions of the Guidance and Cooperative model.
The 4 types of physician style empirically derived from our data are similar to communication pattern types found by Roter et al,27 in a study with similar aims but different methods. Of the 5 types reported, narrowly biomedical and expanded biomedical accounted for 65% of visits, and biopsychosocial accounted for 20%. Psychosocial and consumerist (distinguished by a high degree of patient questions) accounted for only 8% each. It is interesting that in our data, we found the person-focused style was by far the most common approach (49%) among this group of family physicians. These differences in use of particular interaction styles may have several explanations. First, these data were collected more recently.27 Thus our data may reflect trends in a movement away from a paternalistic style and toward an increased patient participatory style. Second, our sample consisted entirely of family physicians practicing in the community, where the model of person-focused care may have a longer history of support and endorsement or be of greater importance to community family physicians, whose emphasis is on a breadth of care based on patient needs.6,7,18
Physicians with a person-focused style granted the longest visits, while high-control-physicians granted the shortest—a difference of more than 2 minutes per visit on average. The associations were not explained away by accounting for patient or physician characteristics, suggesting that a person-focused style may require more time. However, others have found that physicians engaging in a patient participatory style had office visits that were of similar duration as found with other approaches,23, 27 although the average duration of visit for both of these studies were considerably longer than the office visits among our sample.
This study has several strengths. The use of community practicing physicians in real world conditions for whom visits were similar in content to the visits reported by NAMCS34 adds to the generalizability of the findings. We have used an integration of qualitative and quantitative approaches to empirically derive categories of physician interaction style. Our data are based on nurse observation of an average of 32 encounters per physician and documented in rich and comprehensive qualitative fieldnotes. And finally, by using multilevel modeling, we have reported an honest estimate of the association of physician style and patient report of primary care by appropriately modeling the nested data structure.
The findings must be interpreted in light of potential study limitations. First, the patients who did not complete the patient questionnaire are somewhat different demographically than those patients who did complete it. However, non-completion of the questionnaire was not associated with physician style; therefore, it is unlikely that the associations would change, had these individuals been included. Second, because the study was cross-sectional we cannot control for patient self-selection of physicians. Nonetheless, since patients dissatisfied with the quality of care are likely to seek another physician, we would expect patient self-selection of physicians to bias the study toward the null, thus making our results even more remarkable.
These findings, in combination with the literature on the person-focused,24 patient-centered5,17,19,20,41 and relationship-centered approaches,42 provide strong evidence to support the widespread implementation of this physician-patient interaction approach. Further investigation in community practice may lead to identification of ways to support and encourage person-focused care and the time needed to provide such care.
· Acknowledgments ·
The authors are indebted to the physicians, office staff members, and patients without whose participation this study would not have been possible. This paper was improved by helpful suggestions on an earlier draft by Kurt C. Stange, MD, PhD. This study was supported by a grant from the National Cancer Institute (1R01 CA60862) and in part by the Center for Research in Family Practice and Primary Care and the American Academy of Family Practice.
- Different physician-patient interaction styles are actively used in community practice.
- A person-focused style is being used by almost half of the physicians observed, and this style is associated with greater patient-reported quality of primary care and greater patient satisfaction.
- This study provides further evidence to support the widespread implementation of this approach to the physician-patient interaction.
Over the past half century, changing medical technology, law, education, ethics, and research have influenced the current shape of physician-patient interactions.9 In 1956, the traditional model of Activity-Passivity (physician does something to the patient) was challenged with the revolutionary concept of active patient participation.10 The models of Guidance and Cooperation (physician tells patient what to do, patient cooperates) and Mutual Participation (physician enables patient to help him/herself, patient is a partner) were proposed10 and are reflected in modern theoretically-based interaction models. Numerous models have been proposed as variants of the Guidance/Cooperation model (eg, paternalistic model,11 priestly model,12 contractual model13) and the Mutual Participation model (eg, ethnographic model,14 consumerist model,11,15 family systems model16). Few of these models, though, have been empirically evaluated. The best-developed and most-studied mutual participation model is the patient-centered method.5,17-20
When data have been collected using quantitative or qualitative approaches, significant strides have been made in understanding physician-patient interaction3, 21-23 and the effect of such interactions on patient outcomes,5,24,25 primarily patient satisfaction.1,26-29 However, many studies have been limited by their focus on a narrow aspect of physician-patient communication, studying a small number of physicians or patients, and using medical students, residents, and hospital faculty as study subjects.
The purpose of this study was not to develop a new model of physician-patient interaction. Rather, variables characterizing physician style grounded by the direct observation of thousands of encounters for 138 community practicing family physicians were used to empirically cluster physicians into groups that represent distinct interaction styles. Because interaction style may be manifested in all phases of a patient encounter, we used as a guiding framework the 3 primary functions of an interview:30,31gathering information, enhancing a healing relationship, and making and implementing decisions. The importance of each of these functions varies depending on the nature of the encounter, but our overall approach provides a practical way of conceptualizing physician-patient interaction style. The association of the empirically derived and theoretically-based physician styles are tested with 3 outcomes: 1) patient report of delivery of attributes of primary care measured using the Components of Primary Care Instrument (CPCI), 2) patient satisfaction with the visit, and 3) the duration of the visit.
Methods
This study was part of the larger Direct Observation of Primary Care (DOPC) study, a cross sectional observational study that examined the content of 4454 outpatient visits to family physicians in northeast Ohio. Details of the methods of the DOPC study have been described extensively elsewhere.32,34 Briefly, 4 teams of 2 research nurses directly observed consecutive patient visits to 138 participating physicians in 84 practices between October 1994 and August 1995. The research nurses collected data on the content and context of consecutive office visits using the following methods: direct observation of the patient visit, patient exit questionnaire, medical record review, and collection of ethnographic field notes.33,34
Measures
Patients’ perception of the delivery 5 attributes of primary care was measured by the Components of Primary Care Instrument (CPCI). Interpersonal communication was an evaluation of the ease of exchange of information between patient and physician. The physician’s accumulated knowledge about the patient refers to the physician’s understanding of the patient’s medical history, health care needs, and values. Coordination of care refers to the information received from referrals to specialists and previous health care visits, and its incorporation into the current and future care of the patient. Preference to see usual physician refers to the degree to which patients believed and valued that they could go to their regular physician for almost all problems. Scale scores demonstrate good internal consistency reliability (Cronbach’s alpha: .68–.79).35 Continuity of care is measured by the Usual Provider Continuity index (UPC), which is the proportion of visits to the patient’s regular doctor in the past year out of the total number of physician visits in the past year.
Patient satisfaction was measured using the 4 physician-specific items from the MOS 9 Item Visit Rating Form36 (Cronbach’s alpha = .89).33 Also included on the patient survey was a single item assessing the degree to which patients’ expectations with the visit were met. Duration of the visit was the total face-to-face time the physician spent with the patient and was measured by direct observation.
Each physician’s interaction style was determined through a 2-step process. In the first step, ethnographic field notes were used to gather information that helps define core features of physician style. The field notes from 4 days of observation of 138 family physicians in 84 practices were transcribed and imported into FolioVIEWS37 for data management and coding. Analysis was conducted with an immersion-crystallization approach38 involving repetitive reading and summarization of the text data. Case summaries were constructed from a sample of practices selected to maximize variation among practice characteristics such as size, physician sex, and practice location. The case summaries were independently reviewed, and important features were identified. These features were cross-checked against the original data. This process, and the resulting 30 features, are described in detail elsewhere.32
Six of the features that emerged from the qualitative analyses pertain to physician style and are listed in Table 1. Each of the 3 primary interview functions30 is represented by at least 1 feature, ensuring good coverage of the core aspects of the interaction. Gathering information is shaped by physician orientation and the clinical information allowed or elicited in the visits. Enhancing healing relationships is realized in part through affective connection with patients. The final function, making and implementing decisions, is influenced by the level of control or shared power with patients, the physician’s openness to patients’ agendas, and the physician’s willingness to negotiate options with patients.
The second step involved a cluster analysis of the 6 variables. First a hierarchical approach was used to estimate the number of clusters. Then a non-hierarchical clustering approach was used to determine physician classification among the clusters and the features that distinguish the clusters.39 Analysis of variance was used to confirm that variables included in the cluster analysis significantly differed between at least 2 of the identified clusters, and thus were contributing to defining interaction style.
TABLE 1
Physician style variables
| Physician orientation: |
| Problem focused—physician focuses on the patient’s presenting complaint |
| Patient-focused—physician is open to a broader health care agenda with the patient and explores other possible issues |
| Scope of clinical information: |
| Biomedical—talk focuses on the biological information, diagnoses and treatments |
| Biopsychosocial—explores both the biological and social and psychological issues |
| Affective connection with patients: |
| Physician personable and friendly, connects with person on a personal level |
| Physician not personable and friendly, maintains professional distance |
| Openness to patient agenda: |
| Physician open to patient’s agenda |
| Physician sets and maintains the agenda |
| Sharing of control in interaction: |
| Physician shares control of the interaction |
| Physician controls the interaction |
| Negotiation of options with patient: |
| Physician negotiates options with patients |
| Physician does not negotiate options with patients |
Analyses
The association of physician and patient characteristics with interaction style was assessed by chi square for categorical variables and by analysis of variance for continuous variables. The association of physician style with each of the 5 attributes of primary care measured by the CPCI, the indicators of patient satisfaction, and duration of the visit were tested using multilevel modeling,40 to account for the hierarchical nature of data (ie, patients nested within physicians).
Results
Of the 4994 patients presenting for care by their family physicians, 4454 (89%) agreed to participate in the DOPC study. Physicians participating in the DOPC study were similar in age to national samples of family physicians, but over-represented female and residency-trained physicians.34 Patient age, sex, and race were similar to the population of patients seeing family physicians and general practitioners nationally as reported in the National Ambulatory Medical Care Survey.34 Patient questionnaires were returned by 3283 (74%) of the patients. Of those respondents, 2881 satisfactorily completed the CPCI, representing 88% of those returning a patient questionnaire and 65% of the total sample. The patients who completed the CPCI were more likely to be white, have private health care insurance, and be somewhat older than patients who did not complete the CPCI.35
The cluster analysis identified 4 distinct groups of physicians. Each of the 138 physicians was classified into 1 group. Each of the 6 variables in the analysis contributed to defining the 4 groups by significantly (P
Forty-nine percent of physicians were classified as person focused. These physicians were more focused on the person than the disease, were perceived as personable and friendly, were open to the patient’s agenda, and frequently negotiated options with the patient. Physicians classified as biopsychosocial (16%) were more focused on the patient’s disease, but elicited psychosocial clinical information. Physicians classified as biomedical (20%) were also more focused on the patient’s disease and were unlikely to elicit psychosocial information. These physicians also demonstrated a low level of friendliness and were unlikely to negotiate options with the patient. The high physician control group’s major characteristics were domination of the encounter and disregard of the patient’s agenda (14%).
Association of physician characteristics with the interaction styles is presented in Table 2. The percent of male and female physicians differed greatly among the 4 style groups. The proportion of female physicians in the person-focused group was almost 4 times that of the biopsychosocial group and the high physician control group (P
As reported in Table 3, physician style is significantly associated with 3 of the 5 patient reports of the attributes of primary care. Physicians classified as having a person-focused approach have the highest mean score of communication; the other 3 styles score lower, with the high-physician-control style scoring the lowest. Person-focused and biopsychosocial physicians scored highest on patient reports of accumulated knowledge; those in the biomedical group scored the lowest. Coordination of care was highest among the person-focused group and lowest among the high-control group Across the different types of physician style, there was no difference in patient report of preference for his or her regular physician or the measure of continuity of care.
The associations of physician style with 2 indicators of patient satisfaction are displayed in Table 4. The highest group mean of patient satisfaction is for the person-focused style, and the lowest is for the high-physician-control group. The indicator of the degree to which patient expectations were met also follows this pattern. Also displayed in Table 4, the person-focused style demonstrated the longest average duration of visit, at 11.5 minutes; the high-physician-control group visits were the shortest in duration, at about 9.5 minutes.
TABLE 2
Physician and patient characteristics associated with interaction style
| Characteristic | Total | Biopsychosocial | Biomedical | Person focused | High physician control | P |
|---|---|---|---|---|---|---|
| Physician | ||||||
| Number | 138 | 22 | 28 | 68 | 20 | |
| Age (mean years) | 43 | 45 | 43 | 42 | 46 | .06 |
| Female | 26% | 9% | 21% | 38% | 10% | |
| Residency trained | 90% | 86% | 86% | 94% | 85% | .44 |
| Patient | ||||||
| Number | 2881 | 504 | 578 | 1258 | 541 | |
| Age (mean years) | 42 | 44 | 41 | 42 | 43 | .11 |
| Female | 62% | 57% | 61% | 65% | 58% | |
Association of physician style with attributes of primary care1
| Attribute of primary care | Biopsychosocial | Biomedical | Person focused | High physician control | P |
|---|---|---|---|---|---|
| Communication | 4.27 | 4.26 | 4.43 | 4.21 | |
| Accumulated knowledge | 3.54 | 3.33 | 3.56 | 3.51 | |
| Coordination of care | 3.85 | 3.78 | 3.99 | 3.74 | |
| Preference for regular doctor | 4.46 | 4.45 | 4.46 | 4.39 | ns |
| Usual provider continuity2 | 0.67 | 0.66 | 0.64 | 0.65 | ns |
| 1Each row represents a separate multilevel regression model wherein each attribute of primary care is the outcome variable and the number in each column is the group mean of that attribute, adjusted for patient and physician age and sex, as well as the effect of the patients being nested within physicians. | |||||
| 2Usual provide continuity = total number of visits to regular physician in past year, divided by the total number of physician visits in the past year. | |||||
Association of physician interaction style with patient satisfaction and duration of visit1
| Outcome measures | Biopsychosocial | Biomedical | Person focused | High physician control | P |
|---|---|---|---|---|---|
| Patient satisfaction with physician | 4.38 | 4.39 | 4.49 | 4.30 | 002 |
| Patient expectations met | 4.36 | 4.33 | 4.45 | 4.31 | .02 |
| Length of visit (mean minutes) | 9.97 | 10.02 | 11.56 | 9.51 | .005 |
| 1Results from multilevel regression model, analyses include patient and physician age and gende as covariates, and controls for the nested nature of the data. | |||||
Discussion
These data indicate that a person-focused approach is actively used in community practice, and is the style most congruent with patient-reported quality of primary care and satisfaction with care. Our data, in concert with data reported by others,5,24 indicate strong support for the feasibility and value of the person-focused model. We found that, of the 4 distinct interaction styles, physicians with the person-focused style scored highest across all measures of the attributes of primary care and on the indicators of patient satisfaction, with the exception of continuity of care. In contrast, physicians with the high-control style were generally lowest on the primary care and satisfaction indicators.
It is important to emphasize that, even though the vast majority of patients in this sample are likely to have self-selected their primary care physician, patient rating of some attributes of primary care differed across the 4 physician styles. Patients of physicians with different styles equally valued seeing their regular physician, as reported by the preference-for-their-regular-doctor score; they exhibited similar proportions of continuity visits in the past year; and their satisfaction scores were all generally high. Patients appear to want to see their regular physician, regardless of interaction approach, even though some approaches—particularly the high-physician-control style—were rated poorer for communication, coordination of care, and accumulated knowledge.
There may be several explanations as to why a particular physician style is associated with specific patient reports of communication, accumulated knowledge, and coordination of care. Openness to the patient’s agenda and willingness to negotiate options—as was characteristic of the person-focused physicians—may facilitate good communication and convey an understanding of patient preferences and values regarding health. It is interesting to note that different groups scored lowest on some of the attributes of primary care. The high-physician-control group was the lowest on interpersonal communication and coordination of care. High-control physicians were more likely to dominate the agenda and the verbal exchanges. Patients may have felt they could not ask questions or that the physician did not listen to what they tried to say. The biomedical group of physicians were given the lowest scores by patients on accumulated knowledge, suggesting that patients thought these physicians were less likely to know their preferences and values regarding health care, know less about them as persons, and know less about their family and medical histories.
As others have proposed, we concur that interaction style is not a dichotomy or even a continuum of patient versus physician control, but is multidimensional, cutting across the main functions of the patient encounter (ie, information gathering, relationship building, and making and implementing decisions). These data provide some confirmation for the original scheme proposed by Szasz and Hollander,10 with the Mutual Participation model most represented by the person-focused approach and the Activity-Passivity model most represented by the high-physician-control group. The biopsychosocial and biomedical approaches represent different versions of the Guidance and Cooperative model.
The 4 types of physician style empirically derived from our data are similar to communication pattern types found by Roter et al,27 in a study with similar aims but different methods. Of the 5 types reported, narrowly biomedical and expanded biomedical accounted for 65% of visits, and biopsychosocial accounted for 20%. Psychosocial and consumerist (distinguished by a high degree of patient questions) accounted for only 8% each. It is interesting that in our data, we found the person-focused style was by far the most common approach (49%) among this group of family physicians. These differences in use of particular interaction styles may have several explanations. First, these data were collected more recently.27 Thus our data may reflect trends in a movement away from a paternalistic style and toward an increased patient participatory style. Second, our sample consisted entirely of family physicians practicing in the community, where the model of person-focused care may have a longer history of support and endorsement or be of greater importance to community family physicians, whose emphasis is on a breadth of care based on patient needs.6,7,18
Physicians with a person-focused style granted the longest visits, while high-control-physicians granted the shortest—a difference of more than 2 minutes per visit on average. The associations were not explained away by accounting for patient or physician characteristics, suggesting that a person-focused style may require more time. However, others have found that physicians engaging in a patient participatory style had office visits that were of similar duration as found with other approaches,23, 27 although the average duration of visit for both of these studies were considerably longer than the office visits among our sample.
This study has several strengths. The use of community practicing physicians in real world conditions for whom visits were similar in content to the visits reported by NAMCS34 adds to the generalizability of the findings. We have used an integration of qualitative and quantitative approaches to empirically derive categories of physician interaction style. Our data are based on nurse observation of an average of 32 encounters per physician and documented in rich and comprehensive qualitative fieldnotes. And finally, by using multilevel modeling, we have reported an honest estimate of the association of physician style and patient report of primary care by appropriately modeling the nested data structure.
The findings must be interpreted in light of potential study limitations. First, the patients who did not complete the patient questionnaire are somewhat different demographically than those patients who did complete it. However, non-completion of the questionnaire was not associated with physician style; therefore, it is unlikely that the associations would change, had these individuals been included. Second, because the study was cross-sectional we cannot control for patient self-selection of physicians. Nonetheless, since patients dissatisfied with the quality of care are likely to seek another physician, we would expect patient self-selection of physicians to bias the study toward the null, thus making our results even more remarkable.
These findings, in combination with the literature on the person-focused,24 patient-centered5,17,19,20,41 and relationship-centered approaches,42 provide strong evidence to support the widespread implementation of this physician-patient interaction approach. Further investigation in community practice may lead to identification of ways to support and encourage person-focused care and the time needed to provide such care.
· Acknowledgments ·
The authors are indebted to the physicians, office staff members, and patients without whose participation this study would not have been possible. This paper was improved by helpful suggestions on an earlier draft by Kurt C. Stange, MD, PhD. This study was supported by a grant from the National Cancer Institute (1R01 CA60862) and in part by the Center for Research in Family Practice and Primary Care and the American Academy of Family Practice.
1. Bertakis KD, Roter D, Putnam SM. The relationship of physician medical interview style to patient satisfaction. J Fam Pract. 1991;32:175-181.
2. Bertakis KD, Callahan EJ, Helms LJ, Azari R, Robbins JA, Miller J. Physician practice styles and patient outcomes. Med Care. 1998;36:879-891.
3. Stewart MA. What is a successful doctor-patient interview? A study of interactions and outcomes. Soc Sci Med. 1984;19:167-175.
4. Levinson W, Roter DL, Mullooly JP, Dull VT, Frankel RM. Physician-patient communication: the relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277:553-559.
5. Stewart M, Brown JB, Donner A, McWhinney IR, Oates J, Weston WW, Jordan J. The impact of patient-centered care on outcomes. J Fam Pract. 2000;49:796-804.
6. McWhinney IR. Through clinical method to a more humane medicine. In: White KL, ed. The task of medicine. Menlo Park, CA: The Henry J. Kaiser Family Foundation; 1988.
7. Stange KC, Jaén CR, Flocke SA, Miller WL, Crabtree BF, Zyzanski SJ. The value of a family physician. J Fam Pract. 1998;46:363-368.
8. Institute of Medicine. Primary Care: America’s Health in a New Era. Donaldson MS. YK, Lohr KN, Vanselow NA, ed Washington D.C.: National Academy Press; 1996.
9. Laine C, Davidoff F. Patient-centered medicine: A professional evolution. JAMA. 1996;275:152-156.
10. Szasz TS, Hollender MH. The basic models of the doctor-patient relationship. Arch Int Med. 1956;97:585-592.
11. Emanuel EJ, Emanuel LL. Four models of the physician-patient relationship. JAMA. 1992;267:2221-2226.
12. Veatch RM. Models for ethical medicine in a revolutionary age. What physician-patient roles foster the most ethical relationship? Hasting Center Reports. 1972;2:5-7.
13. Quill TE. Partnerships in patient care: a contractual approach. Ann Int Med. 1983;98:228-234.
14. Kleinman AM, Eisenberg L, Good B. Culture, illness, and care: Clinical lessons from anthropologic and cross-cultural research. Ann Int Med. 1978;88:251-258.
15. Lazare A, Eisenthal S, Wasserman L. The customer approach to patienthood: Attending to patient requests in a walk-in clinic. Archives of General Psychiatry. 1975;32:553-558.
16. McDaniel S, Campbell T, Seaburn D. Family-oriented primary care: a manual for medical providers. Berlin: Springer-Verlag; 1990.
17. Stewart M, Weston WW, Brown JB, McWhinney IR, McWilliam CL, Freeman TR. Patient-centered medicine: Transforming the clinical method. Thousand Oaks, CA: Sage Publications; 1995.
18. Levenstein JH, McCracken EC, McWhinney IR, Stewart MA, Brown JB. The patient-centred clinical method. 1. A model for the doctor-patient interaction in family medicine. Fam Pract. 1986;3:24-30.
19. Epstein RM. The science of patient-centered care. J Fam Pract. 2000;49:805-807.
20. Stewart M, Roter D. Communicating With Medical Patients. Knapp ML, ed second printing (1990) ed: Sage Publications; 1989.
21. Hall JA, Roter DL, Katz NR. Meta-analysis of correlates of provider behavior in medical encounters. Med Care. 1988;26:657-675.
22. Byrne PS, Long BEL. Doctors talking to patients. London: H.M.S.O.; 1976.
23. Marvel MK, Doherty WJ, Weiner E. Medical interviewing by exemplary family physicians. J Fam Pract. 1998;47:343-348.
24. Roter D. The enduring and evolving nature of the patient-physician relationship. Patient Educ and Counseling. 2000;39:5-15.
25. Kaplan SH, Greenfield S, Ware JE. Assessing the effects of physician-patient interactions on the outcomes of chronic disease. Med Care. 1989;27:S110-S127.
26. Buller MK, Buller DB. Physicians’ communication style and patient satisfaction. J Health Soc Behav. 1987;28:375-388.
27. Roter DL, Stewart M, Putnam SM, Lipkin M, Stiles W, Inui TS. Communication patterns of primary care physicians. JAMA. 1997;277:350-356.
28. Williams S, Weinman J, Dale J. Doctor-patient communication and patient satisfaction: A review. Fam Pract. 1998;15:480-492.
29. Greene MG, Adelman RD, Friedman E, Charon R. Older patient satisfaction with communication during an initial medical encounter. Soc Sci Med. 1994;38:1279-1288.
30. Cohen-Cole S. The medical interview: The three-function approach. St. Louis: Mosby Year Book; 1991.
31. Lazare A, Putnam SM, Lipkin M. Three functions of the medical interview. In: Lipkin M, Putnam S, Lazare A, eds. The medical interview: Clinical care, education and research. New York: Springer; 1995;3-19.
32. Crabtree BF, Miller WL, Aita V, Flocke SA, Stange KC. Primary care practice organization: A qualitative analysis. J Fam Pract. 1998;46:403-409.
33. Stange KC, Zyzanski SJ, Jaén CR, Callahan EJ, Kelly RB, Gillanders WR, Shank JC, Chao J, Medalie JH, Miller WL, Crabtree BF, Flocke SA, Gilchrist VJ, Langa DM, Goodwin MA. Illuminating the black box: a description of 4454 patient visits to 138 family physicians. J Fam Pract. 1998;46:377-389.
34. Stange KC, Zyzanski SJ, Smith TF, Kelly R, Langa DM, Flocke SA, Jaén CR. How valid are medical records and patient questionnaires for physician profiling and health services research? A comparison with direct observation of patient visits. Med Care. 1998;36:851-867.
35. Flocke SA. Measuring attributes of primary care: Development of a new instrument. J Fam Pract. 1997;45:64-74.
36. Rubin H, Gandek B, Roger WH, Kisinski M, McHorney C, Ware J. Patients’ ratings of outpatient visits in different practice settings. JAMA. 1993;270:835-840.
37. FolioVIEWS.. 3.1 ed. Provo, Utah: Folio Corporation; 1998.
38. Crabtree BF, Miller WL. Doing Qualitative Research. Newbury Park, California: Sage Publications; 1992.
39. Aldenderfer MS, Blashfield RK. Cluster Analysis. Lewis-Beck MS, ed Newbury Park: Sage; 1984.
40. Bryk AS, Raudenbush SW. Hierarchical linear models: applications and data analysis methods. Newbury Park: Sage Publications; 1992.
41. Stewart M, Brown JB, Boon H, Galajda J, Meredith L, Sangster M. Evidence on patient-doctor communication. Cancer Prevention and Control. 1999;3:25-30.
42. Carol P. Tresolini and the Pew-Fetzer Task Force on Advancing Psychosocial Health Education. Health profession education and relationship-centered care. San Francisco, CA: Pew Health Professions Commission; 1994.
1. Bertakis KD, Roter D, Putnam SM. The relationship of physician medical interview style to patient satisfaction. J Fam Pract. 1991;32:175-181.
2. Bertakis KD, Callahan EJ, Helms LJ, Azari R, Robbins JA, Miller J. Physician practice styles and patient outcomes. Med Care. 1998;36:879-891.
3. Stewart MA. What is a successful doctor-patient interview? A study of interactions and outcomes. Soc Sci Med. 1984;19:167-175.
4. Levinson W, Roter DL, Mullooly JP, Dull VT, Frankel RM. Physician-patient communication: the relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277:553-559.
5. Stewart M, Brown JB, Donner A, McWhinney IR, Oates J, Weston WW, Jordan J. The impact of patient-centered care on outcomes. J Fam Pract. 2000;49:796-804.
6. McWhinney IR. Through clinical method to a more humane medicine. In: White KL, ed. The task of medicine. Menlo Park, CA: The Henry J. Kaiser Family Foundation; 1988.
7. Stange KC, Jaén CR, Flocke SA, Miller WL, Crabtree BF, Zyzanski SJ. The value of a family physician. J Fam Pract. 1998;46:363-368.
8. Institute of Medicine. Primary Care: America’s Health in a New Era. Donaldson MS. YK, Lohr KN, Vanselow NA, ed Washington D.C.: National Academy Press; 1996.
9. Laine C, Davidoff F. Patient-centered medicine: A professional evolution. JAMA. 1996;275:152-156.
10. Szasz TS, Hollender MH. The basic models of the doctor-patient relationship. Arch Int Med. 1956;97:585-592.
11. Emanuel EJ, Emanuel LL. Four models of the physician-patient relationship. JAMA. 1992;267:2221-2226.
12. Veatch RM. Models for ethical medicine in a revolutionary age. What physician-patient roles foster the most ethical relationship? Hasting Center Reports. 1972;2:5-7.
13. Quill TE. Partnerships in patient care: a contractual approach. Ann Int Med. 1983;98:228-234.
14. Kleinman AM, Eisenberg L, Good B. Culture, illness, and care: Clinical lessons from anthropologic and cross-cultural research. Ann Int Med. 1978;88:251-258.
15. Lazare A, Eisenthal S, Wasserman L. The customer approach to patienthood: Attending to patient requests in a walk-in clinic. Archives of General Psychiatry. 1975;32:553-558.
16. McDaniel S, Campbell T, Seaburn D. Family-oriented primary care: a manual for medical providers. Berlin: Springer-Verlag; 1990.
17. Stewart M, Weston WW, Brown JB, McWhinney IR, McWilliam CL, Freeman TR. Patient-centered medicine: Transforming the clinical method. Thousand Oaks, CA: Sage Publications; 1995.
18. Levenstein JH, McCracken EC, McWhinney IR, Stewart MA, Brown JB. The patient-centred clinical method. 1. A model for the doctor-patient interaction in family medicine. Fam Pract. 1986;3:24-30.
19. Epstein RM. The science of patient-centered care. J Fam Pract. 2000;49:805-807.
20. Stewart M, Roter D. Communicating With Medical Patients. Knapp ML, ed second printing (1990) ed: Sage Publications; 1989.
21. Hall JA, Roter DL, Katz NR. Meta-analysis of correlates of provider behavior in medical encounters. Med Care. 1988;26:657-675.
22. Byrne PS, Long BEL. Doctors talking to patients. London: H.M.S.O.; 1976.
23. Marvel MK, Doherty WJ, Weiner E. Medical interviewing by exemplary family physicians. J Fam Pract. 1998;47:343-348.
24. Roter D. The enduring and evolving nature of the patient-physician relationship. Patient Educ and Counseling. 2000;39:5-15.
25. Kaplan SH, Greenfield S, Ware JE. Assessing the effects of physician-patient interactions on the outcomes of chronic disease. Med Care. 1989;27:S110-S127.
26. Buller MK, Buller DB. Physicians’ communication style and patient satisfaction. J Health Soc Behav. 1987;28:375-388.
27. Roter DL, Stewart M, Putnam SM, Lipkin M, Stiles W, Inui TS. Communication patterns of primary care physicians. JAMA. 1997;277:350-356.
28. Williams S, Weinman J, Dale J. Doctor-patient communication and patient satisfaction: A review. Fam Pract. 1998;15:480-492.
29. Greene MG, Adelman RD, Friedman E, Charon R. Older patient satisfaction with communication during an initial medical encounter. Soc Sci Med. 1994;38:1279-1288.
30. Cohen-Cole S. The medical interview: The three-function approach. St. Louis: Mosby Year Book; 1991.
31. Lazare A, Putnam SM, Lipkin M. Three functions of the medical interview. In: Lipkin M, Putnam S, Lazare A, eds. The medical interview: Clinical care, education and research. New York: Springer; 1995;3-19.
32. Crabtree BF, Miller WL, Aita V, Flocke SA, Stange KC. Primary care practice organization: A qualitative analysis. J Fam Pract. 1998;46:403-409.
33. Stange KC, Zyzanski SJ, Jaén CR, Callahan EJ, Kelly RB, Gillanders WR, Shank JC, Chao J, Medalie JH, Miller WL, Crabtree BF, Flocke SA, Gilchrist VJ, Langa DM, Goodwin MA. Illuminating the black box: a description of 4454 patient visits to 138 family physicians. J Fam Pract. 1998;46:377-389.
34. Stange KC, Zyzanski SJ, Smith TF, Kelly R, Langa DM, Flocke SA, Jaén CR. How valid are medical records and patient questionnaires for physician profiling and health services research? A comparison with direct observation of patient visits. Med Care. 1998;36:851-867.
35. Flocke SA. Measuring attributes of primary care: Development of a new instrument. J Fam Pract. 1997;45:64-74.
36. Rubin H, Gandek B, Roger WH, Kisinski M, McHorney C, Ware J. Patients’ ratings of outpatient visits in different practice settings. JAMA. 1993;270:835-840.
37. FolioVIEWS.. 3.1 ed. Provo, Utah: Folio Corporation; 1998.
38. Crabtree BF, Miller WL. Doing Qualitative Research. Newbury Park, California: Sage Publications; 1992.
39. Aldenderfer MS, Blashfield RK. Cluster Analysis. Lewis-Beck MS, ed Newbury Park: Sage; 1984.
40. Bryk AS, Raudenbush SW. Hierarchical linear models: applications and data analysis methods. Newbury Park: Sage Publications; 1992.
41. Stewart M, Brown JB, Boon H, Galajda J, Meredith L, Sangster M. Evidence on patient-doctor communication. Cancer Prevention and Control. 1999;3:25-30.
42. Carol P. Tresolini and the Pew-Fetzer Task Force on Advancing Psychosocial Health Education. Health profession education and relationship-centered care. San Francisco, CA: Pew Health Professions Commission; 1994.
Is a history of trauma associated with a reduced likelihood of cervical cancer screening?
- Women who had not had recommended cervical cancer screening were more likely to have been sexually abused in childhood.
- Women who were sexually abused in childhood may be at higher risk than other women for HPV and cervical cancer; therefore, screening is particularly important for these women.
- Not having cervical cancer screening may be a marker for childhood sexual abuse. Therefore, health care providers should consider investigating these issues with women who do not adhere to guidelines for routine Pap smears.
Unfortunately, 15% to 24% of US women do not receive recommended cervical cancer screening.1-3 Barriers to Pap screening include low income, low education, minority status;4 lack of cancer knowledge, attitudes, beliefs, low perceived cancer susceptibility, pain, embarrassment;5-7 language, and certain cultural beliefs.7-9 Sexual trauma has received little research attention as a factor contributing to lowered rates of Pap screening. Sexual trauma is reliably associated with subsequent poor health, which may be partially accounted for by poor preventive care.10-16 Childhood sexual abuse is strongly associated with negative health behaviors such as physical inactivity and smoking.13,17 Sexual violence is associated with lower rates of breast cancer screening18 and increased risk of posttraumatic stress disorder (PTSD).19-21 Avoidant coping styles (an aspect of PTSD) are associated with decreased health promotion behaviors such as screening.22-25
Gynecologic procedures may feel threatening to women with a history of sexual assault, and may be experienced as re-traumatizing.14,26-29 Women who had suffered childhood sexual abuse reported more anxiety, shame, and fear during a gynecologic examination than other women.28 Springs and Friedrich16 found a lower frequency of screening for cervical cancer among adult survivors of childhood sexual abuse, but did not assess the impact of other traumatic events in childhood or adulthood on Pap screening. Because previous research on correlates of sexual trauma has been criticized on the grounds that third variables could account for the observed associations,30 we evaluated associations of any traumatic event with low rates of Pap screening.
We hypothesized that having experienced traumatic events, in particular childhood sexual trauma, would function as barriers to Pap screening. We predicted that women who had not had medically appropriate Pap screening would report a greater number of traumatic events, especially sexual abuse trauma in this ethnically diverse random sample of women. We also expected that sexually traumatized women would express more negative attitudes toward Pap screening, and would be more likely to meet criteria for PTSD, both of which might contribute to lower levels of Pap screening.
Methods
Kaiser Permanente (KP), a pre-paid maintenance organization, offers cervical cancer screening at no cost to patients. KP’s clinical guidelines recommend Pap screening every 2 years for women over age 20 with average risk for cervical cancer. Self-report questionnaires were mailed to an age-stratified random sample of women 21–64 years old who were KP members at 3 locations. Women who had had a total hysterectomy were excluded. We compared women who had and who had not obtained Pap screening in the previous 2 years. In previous research18 we found that women who had not obtained mammography had a lower response rate to mailed questionnaires than women who had been screened. We therefore oversampled women who had not had Pap screening. We mailed questionnaires to 1314 women who had obtained Pap screening and 2897 who had not. The final sample included 364 women who had received screening in the past two years (28% response rate) and 372 who had not (13% response rate). Repeated sampling or telephoning of non-respondents was not allowed by KP policy.
Trauma history was measured in 2 ways. The Trauma History Questionnaire31,32 assesses a range of lifetime traumatic events. The Childhood Trauma Questionnaire33 assesses childhood physical abuse, physical neglect, sexual abuse, emotional abuse, and emotional neglect. PTSD was assessed with the Posttraumatic Stress Disorder Checklist.34 We inquired about attitudes toward Pap screening based on previous findings.
Data were analyzed using SAS.35 Contingency tables were analyzed to estimate the prevalence of traumatic events and their bivariate associations with Pap screening. Chi square analysis was used to evaluate the statistical significance of these associations. Hierarchical logistic regression was used to evaluate associations of traumatic events with screening, independent of clinic location, demographic characteristics, attitudes about screening, and PTSD.
Results
Sample demographics
Women who had been screened for cervical cancer and unscreened women were similar in age and education (Table 1). Unscreened women were more likely to be Asian American, to have incomes of $20,000 per year or less, and to have never been married.
TABLE 1
Demographic characteristics of women with and without Pap screening
| No Pap (%) n = 372a | Pap (%) n = 364a | P | |
|---|---|---|---|
| Ethnicity | .001 | ||
| African American | 10.1 | 11.6 | |
| Asian American | 20.1 | 8.0 | |
| European American | 60.6 | 71.8 | |
| Other | 9.2 | 8.6 | |
| Age | .076 | ||
| Mean (standard deviation) | 43.8 (12.8) | 45.5 (12.4) | |
| Education | .187 | ||
| Elementary school | 2.7 | 1.4 | |
| High school | 39.6 | 34.8 | |
| College | 41.5 | 43.2 | |
| Post-college | 16.9 | 20.6 | |
| Family income | .002 | ||
| $20,000/year or less | 12.4 | 5.8 | |
| $20,001–$50,000 | 41.3 | 38.2 | |
| More than $50,000 | 46.2 | 56.0 | |
| Marital status | .012 | ||
| Never married | 34.3 | 24.8 | |
| Married | 47.0 | 59.1 | |
| Separated | 1.6 | 0.6 | |
| Divorced | 13.8 | 13.1 | |
| Widowed | 3.2 | 2.5 | |
| aSample sizes vary slightly because of missing data on individual demographic items. | |||
Prevalence of trauma
Commonly reported events during childhood included natural disaster (reported by 13% of the women), sexual assault other than rape (11%), and news of a death or injury (10%). Childhood sexual abuse or sexual assault was reported by 18.4% of the respondents. The most common traumas in adulthood were receiving news of a death or serious injury (46%), natural disasters (33%), actual or attempted robbery (27%), and serious accidents (14%). Of the respondents, 8.3% reported sexual abuse or sexual assault in adulthood. Their overall rate of childhood and adult sexual assault was 26.7%.
Associations of trauma history with pap screening
We investigated the association of trauma with screening using chi square analyses. Women who had been raped before age 18 (36% vs. 50%, n = 713, P = .050) and women who had been subjected to other sexual assaults before age 18 (35% vs. 51%, n = 694, P = .009) were less likely to have been screened. Nonsexual childhood abuse and neglect were not related to screening. Women who experienced a natural disaster during childhood (36% vs. 52%, n = 571, P = .009) and those who experienced terrorist acts during adulthood (20% vs. 49%, n = 715, P = .024) were less likely to have been screened. (Although the association with a terrorist act was significant, exposures were reported by only 3% of unscreened women and 0.9% of screened women.) Women who reported a household break-in during adulthood were slightly more likely to have been screened (53% vs. 47%, n = 656, P = .032).
In a hierarchical logistic regression model (Table 2), childhood sexual abuse, but not other traumatic events, was associated with lower odds of screening when clinic location, demographic characteristics, attitudes, and PTSD were controlled. The logistic regression model was repeated using CTQ subscales to assess trauma, with similar results. Unmarried women were less likely than currently married women to have been screened, and Latina, Native American, Asian/Pacific, or multicultural women were less likely than European American women to have been screened. Women who endorsed the statement, “I have no symptoms so I do not need a Pap test” and those who anticipated embarrassment during screening were less likely than others to have been screened; women who believed that testing would ease their mind were more likely to have been screened.
TABLE 2
Hierarchical logistic regression model of sexual trauma and attitudes as predictors of pap screening
| Predictor | Adjusted odds ratio (95% CI) |
|---|---|
| Traumatic events | |
| Break-in (adult) | 1.14 (0.77, 1.70) |
| Natural disaster (child) | 0.78 (0.45, 1.38) |
| Terrorist act (adult) | 0.28 (0.07, 1.07) |
| Childhood sexual trauma | 0.56 (0.34, 0.91) * |
| Site | |
| Santa Rosa | 0.68 (0.44, 1.04) |
| San Francisco | 1.0 (referent) |
| Oakland | 1.27 (0.80, 2.02) |
| Education | |
| Less than college | 1.09 (0.71, 1.69) |
| College | 1.0 (referent) |
| More than college | 1.01 (0.65, 1.57) |
| Ethnicity | |
| European-American | 1.0 (referent) |
| African American | 0.59 (0.33, 1.06) |
| Other than African | |
| American or European | |
| American | 0.46 (0.29, 0.71) ** |
| Unmarried (compared with married) | 0.67 (0.48, 0.94) * |
| Attitudes toward Pap screening | |
| “I have no symptoms so I do not need a Pap test” | 0.66 (0.51, 0.85) ** |
| “I’ve had negative experiences with my health care provider” | 0.90 (0.73, 1.10) |
| “Getting a Pap test would ease my mind” | 1.54 (1.25, 1.89) *** |
| “There is danger of infection from a Pap test” | 1.09 (0.83, 1.43) |
| “I do not trust the health care system” | 1.06 (0.81, 1.39) |
| “I would be embarrassed to have a Pap test” | 0.67 (0.52, 0.84) *** |
| “Women who have many sexual | |
| partners are more likely to have cervical cancer” | 0.88 (0.73, 1.06) |
| “Pap would cause sexual assault flashbacks, or health care provider looks at me in a sexual way” | 1.05 (0.77, 1.45) |
| PTSD diagnosis | 1.62 (0.91, 2.90) |
| Missing data | 0.96 (0.80, 1.13) |
| *P | |
Discussion
Childhood sexual abuse is reliably associated with a decreased likelihood of cervical cancer screening. This association persisted despite controlling for demographic characteristics, attitudes about Pap screening, and PTSD symptoms. These findings are strengthened by the consistency with which childhood sexual abuse is associated with low rates of Pap screening using 2 measures of trauma in 3 clinics. Although cost has been a major barrier to access in previous studies of cervical cancer screening, it is not a barrier for women who are members of a pre-paid health plan. It was therefore possible for us to investigate known and suspected barriers to cervical cancer screening with fewer confounding co-variables.
This study clarifies the role of childhood sexual assault in Pap screening. Sexual assault, but not other traumatic events or other types of childhood abuse, is associated with lower rates of cervical cancer screening. Furthermore, sexual assault during childhood, but not during adulthood, is strongly associated with decreased Pap screening.
The relationship between childhood sexual abuse and Pap screening is particularly disturbing because women who were sexually assaulted as children are more likely to develop cervical dysplasia.36 Women who were sexually assaulted in childhood also tend to begin sexual activity at a young age and have more sexual partners.15,16,36 These are among the primary risk factors for human papillomavirus (HPV),37 an important cause of cervical cancer,38,39 and for cervical cancer.7 Women who were sexually abused in childhood are at increased risk of sexually transmitted disease,15,40 and HPV is the most common sexually transmitted viral disease.38 Therefore, women at higher risk for cervical cancer may be the same women who are least likely to be screened. Childhood sexual abuse may increase cervical cancer morbidity by reducing the probability of Pap screening, and by increasing the probability of disease. It may also decrease the likelihood that these women visit their physician for other routine health maintenance needs.
The low response rate in this study may have resulted from the questionnaire’s being sent to KP members once, without follow-up. Our response rate was comparable to a similar study of HMO members.16 Use of a mailed questionnaire probably resulted in underestimation of childhood sexual abuse prevalence.41 The relationship of sexual abuse to preventive health behaviors is comparable to that reported in studies with higher response rates.13,17
There is some evidence that the interpersonal climate between patient and clinician affects health outcomes,42 and we suspect it is a critical factor in increasing women’s comfort with Pap screening. One of our respondents commented: “I’ve always been treated professionally by my gynecologist and yet I still feel the need for the reassuring presence of a nurse during this procedure. I have asked the nurse to hold my hand during the test to calm me down. I find the hand holding or even her hand on my arm comforting.”
The most consistent predictor of cancer screening among women aged 40 and over was a health maintenance visit or regular source of care.43,44 Not having cervical cancer screening may be a marker for childhood sexual abuse. Therefore, health care providers should consider inquiring about a history of sexual abuse with women who do not follow guidelines for routine Pap screening. It is crucial to develop interventions that will lead to routine medical visits for women who have experienced sexual violence. As part of this process, we recommend education for physicians and other health care providers regarding sexual violence against women.
· Acknowledgments ·
Larry Walter, MA, and Sujaya Parthasarathy, PhD, of the Kaiser Permanente Division of Research in Oakland, California, contributed to our obtaining the random sample of women health plan members in this study. Howard Barkan, DrPH, helped design this project and participated in the data collection. We thank him for his insight and expertise.
1. American Cancer Society. Statistics: Table 3C.Pap Test, Women 18 and Older, by State, 1997 [website]. In; http://www3.cancer.org/cancerinfo/sitecenter.asp?ct=1&ctid=8&scp=8.3.8.42080&scs=4&scss=16&scdoc=42096&pnt=2&language=english [accessed 2001, 2/27], 2000.
2. American Cancer Society. Statistics: Cervical cancer [website]. In http://www3.cancer.org/cancerinfo/sitecenter.asp?ct=1&ctid=8&scp=8.3.4.4071&scs=4&scss=2&scdoc=42073&pnt=2&language=english [accessed 2001, 2/27]; 2000.
3. Hayward RA, Shapiro MF, Freeman HE, Corey CR. Who gets screened for cervical and breast cancer? Results from a new national survey. Arch Intern Med 1988;148:1177-81.
4. Breen N, B FJ. Stage of breast and cervical cancer diagnosis in disadvantaged neighborhoods: A prevention policy perspective. Am J Prev Med 1996;12(5):319-26.
5. Calle EE, Flanders WD, Thun MJ, Martin LM. Demographic predictors of mammography and Pap smear screening in US women. Am J Public Health 1993;83:53-60.
6. Peters RK, Bear MB, Thomas D. Barriers to screening for cancer of the cervix. Prev Med 1989;18:133-46.
7. Womeodu RJ, Bailey JE. Barriers to cancer screening. Med Clin North Am 1996;80(1):115-33.
8. Suarez L. Pap smear and mammogram screening in Mexican-American women: the effects of acculturation. Am J Public Health 1994;84:742-6.
9. Tang TW, Solomon LJ, Yeh CJ, Worden JK. The role of cultural variables in breast self-examination and cervical cancer screening behavior in young Asian women living in the United States. J Behav Med 1999;22(5):419-36.
10. Golding JM. Sexual assault history and physical health in randomly selected Los Angeles women. Health Psychol 1994;13:130-8.
11. Golding JM. Sexual assault history and women’s reproductive and sexual health. Psychol of Women Quarterly 1996;20:101-21.
12. Golding JM. Sexual assault history and long-term physical health: Evidence from clinical and population epidemiology. Curr Directions in Psychol Sci 1999;8:191-4.
13. Koss MP, Koss PG, Woodruff WJ. Deleterious effects of criminal victimization on women’s health and medical utilization. Arch Intern Med 1991;151:342-7.
14. Laws A. Sexual abuse history and women’s medical problems. J Gen Intern Med 1993;8:441-44.
15. Lechner ME, Vogel ME, Garcia-Shelton LM, Leichter JL, Steibel KR. Self-reported medical problems of adult female survivors of childhood sexual abuse. J Fam Pract 1993;36:633-8.
16. Springs FE, Friedrich WN. Health risk behaviors and medical sequelae of childhood sexual abuse. Mayo Clin Proc 1992;67:527-32.
17. Felitti V, Anda F, Nordenberg D, Williamson, Spitz A, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. Am J Prev Med 1998;14:245-58.
18. Farley M, Minkoff J, Barkan H. Breast cancer screening and trauma history. Women Health in press.
19. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 1995;52:1048-60.
20. Polusny MA, Follette VM. Long-term correlates of child sexual abuse: Theory and review of the empirical literature. Applied and Preventive Psychology 1995;4:143-66.
21. Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, Best CL. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. J Consulting Clin Psychol 1993;61:984-91.
22. Blake DD, Cook JD, Keane TM. Posttraumatic stress disorder and coping in veterans who are seeking medical treatment. J Clin Psychol 1992;48:695-704.
23. Fama LD, Blake DD, Gusman F. Coping and health behaviors in combat-related PTSD inpatients. In: Annual Meeting of the International Society for Traumatic Stress Studies; San Antonio; 1993.
24. Farley M, Barkan H. Somatization, dissociation, and tension-reducing behaviors in psychiatric outpatients. Psychother Psychosom 1997;66:133-40.
25. Wolfe J, Proctor SP, Brown P, Kimerling RD, J., Sullivan M, Chrestman K, et al. Relationship of physical health and posttraumatic stress disorder in young adult women. In: Annual Meeting of the International Society for Traumatic Stress Studies; 1994; Los Angeles; 1994.
26. Kitzinger J. Recalling the pain. Nursing Times 1990 January;17:38-40.
27. Menage J. Women’s perception of obstetric and gynaecological examinations. Br Med J 1993;306:1127-8.
28. Robohm JS, Buttenheim M. The gynecological care experiences of adult survivors of childhood sexual abuse: A preliminary investigation. Women Health 1996;24:59-75.
29. Wahlen SD. Adult survivors of childhood sexual abuse. In: Hendricks-Matthews M, editor. Violence education: Toward a solution. Kansas City, MO: Society of Teachers of Family Medicine; 1992. p. 89-102.
30. Briere J. Methodological issues in the study of sexual abuse effects. J Consulting Clin Psychol 1992;60:196-203.
31. Stamm BH, Varra ME. Instrumentation in the Field of Traumatic Stress. Oswego, NY: Research and Methodology Interest Group of the International Society for Traumatic Stress Studies; 1993.
32. Carlson EB, Briere J. Screening for traumatic experiences and trauma responses in mental health treatment settings. In: International Society for Traumatic Stress Studies; 1999 November 14; Miami, FL; 1999.
33. Bernstein DP, Fink L. Childhood Trauma Questionnaire: A Retrospective Self-Report (Manual). San Antonio, TX: Psychological Corporation; 1998.
34. Weathers FW, Litz BT, Herman DS, Huska JA, Keane TM. The PTSD Checklist (PCL): Reliability, Validity, and Diagnostic Utility. In: 9th Annual Meeting of the International Society for Traumatic Stress Studies; 1993; San Antonio, TX; 1993.
35. The SAS System for Windows. In. 8.02 ed. Cary, NC: SAS Institute; 2001.
36. Coker AL, Patel NJ, Krishnaswami W, Schmidt W, Richter DL. Childhood forced sex and cervical dysplasia among women prison inmates. Violence Against Women 1998;4(5):595-608.
37. Becker TM, Wheeler CM, McGough NS, Parmenter CA, Jordan SW, Stidley CA, et al. Sexually transmitted diseases and other risk factors for cervical dysplasia among southwestern Hispanic and non-Hispanic white women. JAMA 1994;271(15):1181-8.
38. Melnikow J, Nuovo J. Cancer prevention and screening in women. Women’s Health 1997;24(1):15-26.
39. Daling JR, Madeleine MM, McKnight B, Carter JJ, Wipf GC, Ashley R, et al. The relationship of human papillomavirus-related cervical tumors to cigarette smoking, oral contraceptive use, and prior herpes simplex virus type 2 infection. Cancer Epidemiol Biomarkers Prev 1996;5(7):541-8.
40. Plichta SB. Violence and abuse: Implications for women’s health. In: Falk, Collins, editors. Women’s health: The Commonwealth Fund survey. Baltimore, MD: Johns Hopkins University Press; 1996.
41. Peters SD, Wyatt GE, Finkelhor D. Prevalence. In: Finkelhor D, editor. A sourcebook on child sexual abuse. Beverly Hills, CA: Sage; 1986. p. 15-59.
42. DeBlasi Z, Harkness E, Ernst E, Georgiou A, Kleijnen J. Influence of context effects on health outcomes: A systematic review. Lancet 2001;357:757-62.
43. Mandelblatt JS, Gold K, O’Malley AS, Taylor K, Cagney K, Hopkins J, et al. Breast and cervical cancer screening among multiethnic women: Role of age, health and source of care. J Preventive Med 1999;28:418-25.
44. Ruffin MT, Gorenflo DW, Woodman B. Predictors of screening for breast, cervical, colorectal, and prostatic cancer among community-based primary care practices. J Am Board Fam Pract 2000;13:1-10.
- Women who had not had recommended cervical cancer screening were more likely to have been sexually abused in childhood.
- Women who were sexually abused in childhood may be at higher risk than other women for HPV and cervical cancer; therefore, screening is particularly important for these women.
- Not having cervical cancer screening may be a marker for childhood sexual abuse. Therefore, health care providers should consider investigating these issues with women who do not adhere to guidelines for routine Pap smears.
Unfortunately, 15% to 24% of US women do not receive recommended cervical cancer screening.1-3 Barriers to Pap screening include low income, low education, minority status;4 lack of cancer knowledge, attitudes, beliefs, low perceived cancer susceptibility, pain, embarrassment;5-7 language, and certain cultural beliefs.7-9 Sexual trauma has received little research attention as a factor contributing to lowered rates of Pap screening. Sexual trauma is reliably associated with subsequent poor health, which may be partially accounted for by poor preventive care.10-16 Childhood sexual abuse is strongly associated with negative health behaviors such as physical inactivity and smoking.13,17 Sexual violence is associated with lower rates of breast cancer screening18 and increased risk of posttraumatic stress disorder (PTSD).19-21 Avoidant coping styles (an aspect of PTSD) are associated with decreased health promotion behaviors such as screening.22-25
Gynecologic procedures may feel threatening to women with a history of sexual assault, and may be experienced as re-traumatizing.14,26-29 Women who had suffered childhood sexual abuse reported more anxiety, shame, and fear during a gynecologic examination than other women.28 Springs and Friedrich16 found a lower frequency of screening for cervical cancer among adult survivors of childhood sexual abuse, but did not assess the impact of other traumatic events in childhood or adulthood on Pap screening. Because previous research on correlates of sexual trauma has been criticized on the grounds that third variables could account for the observed associations,30 we evaluated associations of any traumatic event with low rates of Pap screening.
We hypothesized that having experienced traumatic events, in particular childhood sexual trauma, would function as barriers to Pap screening. We predicted that women who had not had medically appropriate Pap screening would report a greater number of traumatic events, especially sexual abuse trauma in this ethnically diverse random sample of women. We also expected that sexually traumatized women would express more negative attitudes toward Pap screening, and would be more likely to meet criteria for PTSD, both of which might contribute to lower levels of Pap screening.
Methods
Kaiser Permanente (KP), a pre-paid maintenance organization, offers cervical cancer screening at no cost to patients. KP’s clinical guidelines recommend Pap screening every 2 years for women over age 20 with average risk for cervical cancer. Self-report questionnaires were mailed to an age-stratified random sample of women 21–64 years old who were KP members at 3 locations. Women who had had a total hysterectomy were excluded. We compared women who had and who had not obtained Pap screening in the previous 2 years. In previous research18 we found that women who had not obtained mammography had a lower response rate to mailed questionnaires than women who had been screened. We therefore oversampled women who had not had Pap screening. We mailed questionnaires to 1314 women who had obtained Pap screening and 2897 who had not. The final sample included 364 women who had received screening in the past two years (28% response rate) and 372 who had not (13% response rate). Repeated sampling or telephoning of non-respondents was not allowed by KP policy.
Trauma history was measured in 2 ways. The Trauma History Questionnaire31,32 assesses a range of lifetime traumatic events. The Childhood Trauma Questionnaire33 assesses childhood physical abuse, physical neglect, sexual abuse, emotional abuse, and emotional neglect. PTSD was assessed with the Posttraumatic Stress Disorder Checklist.34 We inquired about attitudes toward Pap screening based on previous findings.
Data were analyzed using SAS.35 Contingency tables were analyzed to estimate the prevalence of traumatic events and their bivariate associations with Pap screening. Chi square analysis was used to evaluate the statistical significance of these associations. Hierarchical logistic regression was used to evaluate associations of traumatic events with screening, independent of clinic location, demographic characteristics, attitudes about screening, and PTSD.
Results
Sample demographics
Women who had been screened for cervical cancer and unscreened women were similar in age and education (Table 1). Unscreened women were more likely to be Asian American, to have incomes of $20,000 per year or less, and to have never been married.
TABLE 1
Demographic characteristics of women with and without Pap screening
| No Pap (%) n = 372a | Pap (%) n = 364a | P | |
|---|---|---|---|
| Ethnicity | .001 | ||
| African American | 10.1 | 11.6 | |
| Asian American | 20.1 | 8.0 | |
| European American | 60.6 | 71.8 | |
| Other | 9.2 | 8.6 | |
| Age | .076 | ||
| Mean (standard deviation) | 43.8 (12.8) | 45.5 (12.4) | |
| Education | .187 | ||
| Elementary school | 2.7 | 1.4 | |
| High school | 39.6 | 34.8 | |
| College | 41.5 | 43.2 | |
| Post-college | 16.9 | 20.6 | |
| Family income | .002 | ||
| $20,000/year or less | 12.4 | 5.8 | |
| $20,001–$50,000 | 41.3 | 38.2 | |
| More than $50,000 | 46.2 | 56.0 | |
| Marital status | .012 | ||
| Never married | 34.3 | 24.8 | |
| Married | 47.0 | 59.1 | |
| Separated | 1.6 | 0.6 | |
| Divorced | 13.8 | 13.1 | |
| Widowed | 3.2 | 2.5 | |
| aSample sizes vary slightly because of missing data on individual demographic items. | |||
Prevalence of trauma
Commonly reported events during childhood included natural disaster (reported by 13% of the women), sexual assault other than rape (11%), and news of a death or injury (10%). Childhood sexual abuse or sexual assault was reported by 18.4% of the respondents. The most common traumas in adulthood were receiving news of a death or serious injury (46%), natural disasters (33%), actual or attempted robbery (27%), and serious accidents (14%). Of the respondents, 8.3% reported sexual abuse or sexual assault in adulthood. Their overall rate of childhood and adult sexual assault was 26.7%.
Associations of trauma history with pap screening
We investigated the association of trauma with screening using chi square analyses. Women who had been raped before age 18 (36% vs. 50%, n = 713, P = .050) and women who had been subjected to other sexual assaults before age 18 (35% vs. 51%, n = 694, P = .009) were less likely to have been screened. Nonsexual childhood abuse and neglect were not related to screening. Women who experienced a natural disaster during childhood (36% vs. 52%, n = 571, P = .009) and those who experienced terrorist acts during adulthood (20% vs. 49%, n = 715, P = .024) were less likely to have been screened. (Although the association with a terrorist act was significant, exposures were reported by only 3% of unscreened women and 0.9% of screened women.) Women who reported a household break-in during adulthood were slightly more likely to have been screened (53% vs. 47%, n = 656, P = .032).
In a hierarchical logistic regression model (Table 2), childhood sexual abuse, but not other traumatic events, was associated with lower odds of screening when clinic location, demographic characteristics, attitudes, and PTSD were controlled. The logistic regression model was repeated using CTQ subscales to assess trauma, with similar results. Unmarried women were less likely than currently married women to have been screened, and Latina, Native American, Asian/Pacific, or multicultural women were less likely than European American women to have been screened. Women who endorsed the statement, “I have no symptoms so I do not need a Pap test” and those who anticipated embarrassment during screening were less likely than others to have been screened; women who believed that testing would ease their mind were more likely to have been screened.
TABLE 2
Hierarchical logistic regression model of sexual trauma and attitudes as predictors of pap screening
| Predictor | Adjusted odds ratio (95% CI) |
|---|---|
| Traumatic events | |
| Break-in (adult) | 1.14 (0.77, 1.70) |
| Natural disaster (child) | 0.78 (0.45, 1.38) |
| Terrorist act (adult) | 0.28 (0.07, 1.07) |
| Childhood sexual trauma | 0.56 (0.34, 0.91) * |
| Site | |
| Santa Rosa | 0.68 (0.44, 1.04) |
| San Francisco | 1.0 (referent) |
| Oakland | 1.27 (0.80, 2.02) |
| Education | |
| Less than college | 1.09 (0.71, 1.69) |
| College | 1.0 (referent) |
| More than college | 1.01 (0.65, 1.57) |
| Ethnicity | |
| European-American | 1.0 (referent) |
| African American | 0.59 (0.33, 1.06) |
| Other than African | |
| American or European | |
| American | 0.46 (0.29, 0.71) ** |
| Unmarried (compared with married) | 0.67 (0.48, 0.94) * |
| Attitudes toward Pap screening | |
| “I have no symptoms so I do not need a Pap test” | 0.66 (0.51, 0.85) ** |
| “I’ve had negative experiences with my health care provider” | 0.90 (0.73, 1.10) |
| “Getting a Pap test would ease my mind” | 1.54 (1.25, 1.89) *** |
| “There is danger of infection from a Pap test” | 1.09 (0.83, 1.43) |
| “I do not trust the health care system” | 1.06 (0.81, 1.39) |
| “I would be embarrassed to have a Pap test” | 0.67 (0.52, 0.84) *** |
| “Women who have many sexual | |
| partners are more likely to have cervical cancer” | 0.88 (0.73, 1.06) |
| “Pap would cause sexual assault flashbacks, or health care provider looks at me in a sexual way” | 1.05 (0.77, 1.45) |
| PTSD diagnosis | 1.62 (0.91, 2.90) |
| Missing data | 0.96 (0.80, 1.13) |
| *P | |
Discussion
Childhood sexual abuse is reliably associated with a decreased likelihood of cervical cancer screening. This association persisted despite controlling for demographic characteristics, attitudes about Pap screening, and PTSD symptoms. These findings are strengthened by the consistency with which childhood sexual abuse is associated with low rates of Pap screening using 2 measures of trauma in 3 clinics. Although cost has been a major barrier to access in previous studies of cervical cancer screening, it is not a barrier for women who are members of a pre-paid health plan. It was therefore possible for us to investigate known and suspected barriers to cervical cancer screening with fewer confounding co-variables.
This study clarifies the role of childhood sexual assault in Pap screening. Sexual assault, but not other traumatic events or other types of childhood abuse, is associated with lower rates of cervical cancer screening. Furthermore, sexual assault during childhood, but not during adulthood, is strongly associated with decreased Pap screening.
The relationship between childhood sexual abuse and Pap screening is particularly disturbing because women who were sexually assaulted as children are more likely to develop cervical dysplasia.36 Women who were sexually assaulted in childhood also tend to begin sexual activity at a young age and have more sexual partners.15,16,36 These are among the primary risk factors for human papillomavirus (HPV),37 an important cause of cervical cancer,38,39 and for cervical cancer.7 Women who were sexually abused in childhood are at increased risk of sexually transmitted disease,15,40 and HPV is the most common sexually transmitted viral disease.38 Therefore, women at higher risk for cervical cancer may be the same women who are least likely to be screened. Childhood sexual abuse may increase cervical cancer morbidity by reducing the probability of Pap screening, and by increasing the probability of disease. It may also decrease the likelihood that these women visit their physician for other routine health maintenance needs.
The low response rate in this study may have resulted from the questionnaire’s being sent to KP members once, without follow-up. Our response rate was comparable to a similar study of HMO members.16 Use of a mailed questionnaire probably resulted in underestimation of childhood sexual abuse prevalence.41 The relationship of sexual abuse to preventive health behaviors is comparable to that reported in studies with higher response rates.13,17
There is some evidence that the interpersonal climate between patient and clinician affects health outcomes,42 and we suspect it is a critical factor in increasing women’s comfort with Pap screening. One of our respondents commented: “I’ve always been treated professionally by my gynecologist and yet I still feel the need for the reassuring presence of a nurse during this procedure. I have asked the nurse to hold my hand during the test to calm me down. I find the hand holding or even her hand on my arm comforting.”
The most consistent predictor of cancer screening among women aged 40 and over was a health maintenance visit or regular source of care.43,44 Not having cervical cancer screening may be a marker for childhood sexual abuse. Therefore, health care providers should consider inquiring about a history of sexual abuse with women who do not follow guidelines for routine Pap screening. It is crucial to develop interventions that will lead to routine medical visits for women who have experienced sexual violence. As part of this process, we recommend education for physicians and other health care providers regarding sexual violence against women.
· Acknowledgments ·
Larry Walter, MA, and Sujaya Parthasarathy, PhD, of the Kaiser Permanente Division of Research in Oakland, California, contributed to our obtaining the random sample of women health plan members in this study. Howard Barkan, DrPH, helped design this project and participated in the data collection. We thank him for his insight and expertise.
- Women who had not had recommended cervical cancer screening were more likely to have been sexually abused in childhood.
- Women who were sexually abused in childhood may be at higher risk than other women for HPV and cervical cancer; therefore, screening is particularly important for these women.
- Not having cervical cancer screening may be a marker for childhood sexual abuse. Therefore, health care providers should consider investigating these issues with women who do not adhere to guidelines for routine Pap smears.
Unfortunately, 15% to 24% of US women do not receive recommended cervical cancer screening.1-3 Barriers to Pap screening include low income, low education, minority status;4 lack of cancer knowledge, attitudes, beliefs, low perceived cancer susceptibility, pain, embarrassment;5-7 language, and certain cultural beliefs.7-9 Sexual trauma has received little research attention as a factor contributing to lowered rates of Pap screening. Sexual trauma is reliably associated with subsequent poor health, which may be partially accounted for by poor preventive care.10-16 Childhood sexual abuse is strongly associated with negative health behaviors such as physical inactivity and smoking.13,17 Sexual violence is associated with lower rates of breast cancer screening18 and increased risk of posttraumatic stress disorder (PTSD).19-21 Avoidant coping styles (an aspect of PTSD) are associated with decreased health promotion behaviors such as screening.22-25
Gynecologic procedures may feel threatening to women with a history of sexual assault, and may be experienced as re-traumatizing.14,26-29 Women who had suffered childhood sexual abuse reported more anxiety, shame, and fear during a gynecologic examination than other women.28 Springs and Friedrich16 found a lower frequency of screening for cervical cancer among adult survivors of childhood sexual abuse, but did not assess the impact of other traumatic events in childhood or adulthood on Pap screening. Because previous research on correlates of sexual trauma has been criticized on the grounds that third variables could account for the observed associations,30 we evaluated associations of any traumatic event with low rates of Pap screening.
We hypothesized that having experienced traumatic events, in particular childhood sexual trauma, would function as barriers to Pap screening. We predicted that women who had not had medically appropriate Pap screening would report a greater number of traumatic events, especially sexual abuse trauma in this ethnically diverse random sample of women. We also expected that sexually traumatized women would express more negative attitudes toward Pap screening, and would be more likely to meet criteria for PTSD, both of which might contribute to lower levels of Pap screening.
Methods
Kaiser Permanente (KP), a pre-paid maintenance organization, offers cervical cancer screening at no cost to patients. KP’s clinical guidelines recommend Pap screening every 2 years for women over age 20 with average risk for cervical cancer. Self-report questionnaires were mailed to an age-stratified random sample of women 21–64 years old who were KP members at 3 locations. Women who had had a total hysterectomy were excluded. We compared women who had and who had not obtained Pap screening in the previous 2 years. In previous research18 we found that women who had not obtained mammography had a lower response rate to mailed questionnaires than women who had been screened. We therefore oversampled women who had not had Pap screening. We mailed questionnaires to 1314 women who had obtained Pap screening and 2897 who had not. The final sample included 364 women who had received screening in the past two years (28% response rate) and 372 who had not (13% response rate). Repeated sampling or telephoning of non-respondents was not allowed by KP policy.
Trauma history was measured in 2 ways. The Trauma History Questionnaire31,32 assesses a range of lifetime traumatic events. The Childhood Trauma Questionnaire33 assesses childhood physical abuse, physical neglect, sexual abuse, emotional abuse, and emotional neglect. PTSD was assessed with the Posttraumatic Stress Disorder Checklist.34 We inquired about attitudes toward Pap screening based on previous findings.
Data were analyzed using SAS.35 Contingency tables were analyzed to estimate the prevalence of traumatic events and their bivariate associations with Pap screening. Chi square analysis was used to evaluate the statistical significance of these associations. Hierarchical logistic regression was used to evaluate associations of traumatic events with screening, independent of clinic location, demographic characteristics, attitudes about screening, and PTSD.
Results
Sample demographics
Women who had been screened for cervical cancer and unscreened women were similar in age and education (Table 1). Unscreened women were more likely to be Asian American, to have incomes of $20,000 per year or less, and to have never been married.
TABLE 1
Demographic characteristics of women with and without Pap screening
| No Pap (%) n = 372a | Pap (%) n = 364a | P | |
|---|---|---|---|
| Ethnicity | .001 | ||
| African American | 10.1 | 11.6 | |
| Asian American | 20.1 | 8.0 | |
| European American | 60.6 | 71.8 | |
| Other | 9.2 | 8.6 | |
| Age | .076 | ||
| Mean (standard deviation) | 43.8 (12.8) | 45.5 (12.4) | |
| Education | .187 | ||
| Elementary school | 2.7 | 1.4 | |
| High school | 39.6 | 34.8 | |
| College | 41.5 | 43.2 | |
| Post-college | 16.9 | 20.6 | |
| Family income | .002 | ||
| $20,000/year or less | 12.4 | 5.8 | |
| $20,001–$50,000 | 41.3 | 38.2 | |
| More than $50,000 | 46.2 | 56.0 | |
| Marital status | .012 | ||
| Never married | 34.3 | 24.8 | |
| Married | 47.0 | 59.1 | |
| Separated | 1.6 | 0.6 | |
| Divorced | 13.8 | 13.1 | |
| Widowed | 3.2 | 2.5 | |
| aSample sizes vary slightly because of missing data on individual demographic items. | |||
Prevalence of trauma
Commonly reported events during childhood included natural disaster (reported by 13% of the women), sexual assault other than rape (11%), and news of a death or injury (10%). Childhood sexual abuse or sexual assault was reported by 18.4% of the respondents. The most common traumas in adulthood were receiving news of a death or serious injury (46%), natural disasters (33%), actual or attempted robbery (27%), and serious accidents (14%). Of the respondents, 8.3% reported sexual abuse or sexual assault in adulthood. Their overall rate of childhood and adult sexual assault was 26.7%.
Associations of trauma history with pap screening
We investigated the association of trauma with screening using chi square analyses. Women who had been raped before age 18 (36% vs. 50%, n = 713, P = .050) and women who had been subjected to other sexual assaults before age 18 (35% vs. 51%, n = 694, P = .009) were less likely to have been screened. Nonsexual childhood abuse and neglect were not related to screening. Women who experienced a natural disaster during childhood (36% vs. 52%, n = 571, P = .009) and those who experienced terrorist acts during adulthood (20% vs. 49%, n = 715, P = .024) were less likely to have been screened. (Although the association with a terrorist act was significant, exposures were reported by only 3% of unscreened women and 0.9% of screened women.) Women who reported a household break-in during adulthood were slightly more likely to have been screened (53% vs. 47%, n = 656, P = .032).
In a hierarchical logistic regression model (Table 2), childhood sexual abuse, but not other traumatic events, was associated with lower odds of screening when clinic location, demographic characteristics, attitudes, and PTSD were controlled. The logistic regression model was repeated using CTQ subscales to assess trauma, with similar results. Unmarried women were less likely than currently married women to have been screened, and Latina, Native American, Asian/Pacific, or multicultural women were less likely than European American women to have been screened. Women who endorsed the statement, “I have no symptoms so I do not need a Pap test” and those who anticipated embarrassment during screening were less likely than others to have been screened; women who believed that testing would ease their mind were more likely to have been screened.
TABLE 2
Hierarchical logistic regression model of sexual trauma and attitudes as predictors of pap screening
| Predictor | Adjusted odds ratio (95% CI) |
|---|---|
| Traumatic events | |
| Break-in (adult) | 1.14 (0.77, 1.70) |
| Natural disaster (child) | 0.78 (0.45, 1.38) |
| Terrorist act (adult) | 0.28 (0.07, 1.07) |
| Childhood sexual trauma | 0.56 (0.34, 0.91) * |
| Site | |
| Santa Rosa | 0.68 (0.44, 1.04) |
| San Francisco | 1.0 (referent) |
| Oakland | 1.27 (0.80, 2.02) |
| Education | |
| Less than college | 1.09 (0.71, 1.69) |
| College | 1.0 (referent) |
| More than college | 1.01 (0.65, 1.57) |
| Ethnicity | |
| European-American | 1.0 (referent) |
| African American | 0.59 (0.33, 1.06) |
| Other than African | |
| American or European | |
| American | 0.46 (0.29, 0.71) ** |
| Unmarried (compared with married) | 0.67 (0.48, 0.94) * |
| Attitudes toward Pap screening | |
| “I have no symptoms so I do not need a Pap test” | 0.66 (0.51, 0.85) ** |
| “I’ve had negative experiences with my health care provider” | 0.90 (0.73, 1.10) |
| “Getting a Pap test would ease my mind” | 1.54 (1.25, 1.89) *** |
| “There is danger of infection from a Pap test” | 1.09 (0.83, 1.43) |
| “I do not trust the health care system” | 1.06 (0.81, 1.39) |
| “I would be embarrassed to have a Pap test” | 0.67 (0.52, 0.84) *** |
| “Women who have many sexual | |
| partners are more likely to have cervical cancer” | 0.88 (0.73, 1.06) |
| “Pap would cause sexual assault flashbacks, or health care provider looks at me in a sexual way” | 1.05 (0.77, 1.45) |
| PTSD diagnosis | 1.62 (0.91, 2.90) |
| Missing data | 0.96 (0.80, 1.13) |
| *P | |
Discussion
Childhood sexual abuse is reliably associated with a decreased likelihood of cervical cancer screening. This association persisted despite controlling for demographic characteristics, attitudes about Pap screening, and PTSD symptoms. These findings are strengthened by the consistency with which childhood sexual abuse is associated with low rates of Pap screening using 2 measures of trauma in 3 clinics. Although cost has been a major barrier to access in previous studies of cervical cancer screening, it is not a barrier for women who are members of a pre-paid health plan. It was therefore possible for us to investigate known and suspected barriers to cervical cancer screening with fewer confounding co-variables.
This study clarifies the role of childhood sexual assault in Pap screening. Sexual assault, but not other traumatic events or other types of childhood abuse, is associated with lower rates of cervical cancer screening. Furthermore, sexual assault during childhood, but not during adulthood, is strongly associated with decreased Pap screening.
The relationship between childhood sexual abuse and Pap screening is particularly disturbing because women who were sexually assaulted as children are more likely to develop cervical dysplasia.36 Women who were sexually assaulted in childhood also tend to begin sexual activity at a young age and have more sexual partners.15,16,36 These are among the primary risk factors for human papillomavirus (HPV),37 an important cause of cervical cancer,38,39 and for cervical cancer.7 Women who were sexually abused in childhood are at increased risk of sexually transmitted disease,15,40 and HPV is the most common sexually transmitted viral disease.38 Therefore, women at higher risk for cervical cancer may be the same women who are least likely to be screened. Childhood sexual abuse may increase cervical cancer morbidity by reducing the probability of Pap screening, and by increasing the probability of disease. It may also decrease the likelihood that these women visit their physician for other routine health maintenance needs.
The low response rate in this study may have resulted from the questionnaire’s being sent to KP members once, without follow-up. Our response rate was comparable to a similar study of HMO members.16 Use of a mailed questionnaire probably resulted in underestimation of childhood sexual abuse prevalence.41 The relationship of sexual abuse to preventive health behaviors is comparable to that reported in studies with higher response rates.13,17
There is some evidence that the interpersonal climate between patient and clinician affects health outcomes,42 and we suspect it is a critical factor in increasing women’s comfort with Pap screening. One of our respondents commented: “I’ve always been treated professionally by my gynecologist and yet I still feel the need for the reassuring presence of a nurse during this procedure. I have asked the nurse to hold my hand during the test to calm me down. I find the hand holding or even her hand on my arm comforting.”
The most consistent predictor of cancer screening among women aged 40 and over was a health maintenance visit or regular source of care.43,44 Not having cervical cancer screening may be a marker for childhood sexual abuse. Therefore, health care providers should consider inquiring about a history of sexual abuse with women who do not follow guidelines for routine Pap screening. It is crucial to develop interventions that will lead to routine medical visits for women who have experienced sexual violence. As part of this process, we recommend education for physicians and other health care providers regarding sexual violence against women.
· Acknowledgments ·
Larry Walter, MA, and Sujaya Parthasarathy, PhD, of the Kaiser Permanente Division of Research in Oakland, California, contributed to our obtaining the random sample of women health plan members in this study. Howard Barkan, DrPH, helped design this project and participated in the data collection. We thank him for his insight and expertise.
1. American Cancer Society. Statistics: Table 3C.Pap Test, Women 18 and Older, by State, 1997 [website]. In; http://www3.cancer.org/cancerinfo/sitecenter.asp?ct=1&ctid=8&scp=8.3.8.42080&scs=4&scss=16&scdoc=42096&pnt=2&language=english [accessed 2001, 2/27], 2000.
2. American Cancer Society. Statistics: Cervical cancer [website]. In http://www3.cancer.org/cancerinfo/sitecenter.asp?ct=1&ctid=8&scp=8.3.4.4071&scs=4&scss=2&scdoc=42073&pnt=2&language=english [accessed 2001, 2/27]; 2000.
3. Hayward RA, Shapiro MF, Freeman HE, Corey CR. Who gets screened for cervical and breast cancer? Results from a new national survey. Arch Intern Med 1988;148:1177-81.
4. Breen N, B FJ. Stage of breast and cervical cancer diagnosis in disadvantaged neighborhoods: A prevention policy perspective. Am J Prev Med 1996;12(5):319-26.
5. Calle EE, Flanders WD, Thun MJ, Martin LM. Demographic predictors of mammography and Pap smear screening in US women. Am J Public Health 1993;83:53-60.
6. Peters RK, Bear MB, Thomas D. Barriers to screening for cancer of the cervix. Prev Med 1989;18:133-46.
7. Womeodu RJ, Bailey JE. Barriers to cancer screening. Med Clin North Am 1996;80(1):115-33.
8. Suarez L. Pap smear and mammogram screening in Mexican-American women: the effects of acculturation. Am J Public Health 1994;84:742-6.
9. Tang TW, Solomon LJ, Yeh CJ, Worden JK. The role of cultural variables in breast self-examination and cervical cancer screening behavior in young Asian women living in the United States. J Behav Med 1999;22(5):419-36.
10. Golding JM. Sexual assault history and physical health in randomly selected Los Angeles women. Health Psychol 1994;13:130-8.
11. Golding JM. Sexual assault history and women’s reproductive and sexual health. Psychol of Women Quarterly 1996;20:101-21.
12. Golding JM. Sexual assault history and long-term physical health: Evidence from clinical and population epidemiology. Curr Directions in Psychol Sci 1999;8:191-4.
13. Koss MP, Koss PG, Woodruff WJ. Deleterious effects of criminal victimization on women’s health and medical utilization. Arch Intern Med 1991;151:342-7.
14. Laws A. Sexual abuse history and women’s medical problems. J Gen Intern Med 1993;8:441-44.
15. Lechner ME, Vogel ME, Garcia-Shelton LM, Leichter JL, Steibel KR. Self-reported medical problems of adult female survivors of childhood sexual abuse. J Fam Pract 1993;36:633-8.
16. Springs FE, Friedrich WN. Health risk behaviors and medical sequelae of childhood sexual abuse. Mayo Clin Proc 1992;67:527-32.
17. Felitti V, Anda F, Nordenberg D, Williamson, Spitz A, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. Am J Prev Med 1998;14:245-58.
18. Farley M, Minkoff J, Barkan H. Breast cancer screening and trauma history. Women Health in press.
19. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 1995;52:1048-60.
20. Polusny MA, Follette VM. Long-term correlates of child sexual abuse: Theory and review of the empirical literature. Applied and Preventive Psychology 1995;4:143-66.
21. Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, Best CL. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. J Consulting Clin Psychol 1993;61:984-91.
22. Blake DD, Cook JD, Keane TM. Posttraumatic stress disorder and coping in veterans who are seeking medical treatment. J Clin Psychol 1992;48:695-704.
23. Fama LD, Blake DD, Gusman F. Coping and health behaviors in combat-related PTSD inpatients. In: Annual Meeting of the International Society for Traumatic Stress Studies; San Antonio; 1993.
24. Farley M, Barkan H. Somatization, dissociation, and tension-reducing behaviors in psychiatric outpatients. Psychother Psychosom 1997;66:133-40.
25. Wolfe J, Proctor SP, Brown P, Kimerling RD, J., Sullivan M, Chrestman K, et al. Relationship of physical health and posttraumatic stress disorder in young adult women. In: Annual Meeting of the International Society for Traumatic Stress Studies; 1994; Los Angeles; 1994.
26. Kitzinger J. Recalling the pain. Nursing Times 1990 January;17:38-40.
27. Menage J. Women’s perception of obstetric and gynaecological examinations. Br Med J 1993;306:1127-8.
28. Robohm JS, Buttenheim M. The gynecological care experiences of adult survivors of childhood sexual abuse: A preliminary investigation. Women Health 1996;24:59-75.
29. Wahlen SD. Adult survivors of childhood sexual abuse. In: Hendricks-Matthews M, editor. Violence education: Toward a solution. Kansas City, MO: Society of Teachers of Family Medicine; 1992. p. 89-102.
30. Briere J. Methodological issues in the study of sexual abuse effects. J Consulting Clin Psychol 1992;60:196-203.
31. Stamm BH, Varra ME. Instrumentation in the Field of Traumatic Stress. Oswego, NY: Research and Methodology Interest Group of the International Society for Traumatic Stress Studies; 1993.
32. Carlson EB, Briere J. Screening for traumatic experiences and trauma responses in mental health treatment settings. In: International Society for Traumatic Stress Studies; 1999 November 14; Miami, FL; 1999.
33. Bernstein DP, Fink L. Childhood Trauma Questionnaire: A Retrospective Self-Report (Manual). San Antonio, TX: Psychological Corporation; 1998.
34. Weathers FW, Litz BT, Herman DS, Huska JA, Keane TM. The PTSD Checklist (PCL): Reliability, Validity, and Diagnostic Utility. In: 9th Annual Meeting of the International Society for Traumatic Stress Studies; 1993; San Antonio, TX; 1993.
35. The SAS System for Windows. In. 8.02 ed. Cary, NC: SAS Institute; 2001.
36. Coker AL, Patel NJ, Krishnaswami W, Schmidt W, Richter DL. Childhood forced sex and cervical dysplasia among women prison inmates. Violence Against Women 1998;4(5):595-608.
37. Becker TM, Wheeler CM, McGough NS, Parmenter CA, Jordan SW, Stidley CA, et al. Sexually transmitted diseases and other risk factors for cervical dysplasia among southwestern Hispanic and non-Hispanic white women. JAMA 1994;271(15):1181-8.
38. Melnikow J, Nuovo J. Cancer prevention and screening in women. Women’s Health 1997;24(1):15-26.
39. Daling JR, Madeleine MM, McKnight B, Carter JJ, Wipf GC, Ashley R, et al. The relationship of human papillomavirus-related cervical tumors to cigarette smoking, oral contraceptive use, and prior herpes simplex virus type 2 infection. Cancer Epidemiol Biomarkers Prev 1996;5(7):541-8.
40. Plichta SB. Violence and abuse: Implications for women’s health. In: Falk, Collins, editors. Women’s health: The Commonwealth Fund survey. Baltimore, MD: Johns Hopkins University Press; 1996.
41. Peters SD, Wyatt GE, Finkelhor D. Prevalence. In: Finkelhor D, editor. A sourcebook on child sexual abuse. Beverly Hills, CA: Sage; 1986. p. 15-59.
42. DeBlasi Z, Harkness E, Ernst E, Georgiou A, Kleijnen J. Influence of context effects on health outcomes: A systematic review. Lancet 2001;357:757-62.
43. Mandelblatt JS, Gold K, O’Malley AS, Taylor K, Cagney K, Hopkins J, et al. Breast and cervical cancer screening among multiethnic women: Role of age, health and source of care. J Preventive Med 1999;28:418-25.
44. Ruffin MT, Gorenflo DW, Woodman B. Predictors of screening for breast, cervical, colorectal, and prostatic cancer among community-based primary care practices. J Am Board Fam Pract 2000;13:1-10.
1. American Cancer Society. Statistics: Table 3C.Pap Test, Women 18 and Older, by State, 1997 [website]. In; http://www3.cancer.org/cancerinfo/sitecenter.asp?ct=1&ctid=8&scp=8.3.8.42080&scs=4&scss=16&scdoc=42096&pnt=2&language=english [accessed 2001, 2/27], 2000.
2. American Cancer Society. Statistics: Cervical cancer [website]. In http://www3.cancer.org/cancerinfo/sitecenter.asp?ct=1&ctid=8&scp=8.3.4.4071&scs=4&scss=2&scdoc=42073&pnt=2&language=english [accessed 2001, 2/27]; 2000.
3. Hayward RA, Shapiro MF, Freeman HE, Corey CR. Who gets screened for cervical and breast cancer? Results from a new national survey. Arch Intern Med 1988;148:1177-81.
4. Breen N, B FJ. Stage of breast and cervical cancer diagnosis in disadvantaged neighborhoods: A prevention policy perspective. Am J Prev Med 1996;12(5):319-26.
5. Calle EE, Flanders WD, Thun MJ, Martin LM. Demographic predictors of mammography and Pap smear screening in US women. Am J Public Health 1993;83:53-60.
6. Peters RK, Bear MB, Thomas D. Barriers to screening for cancer of the cervix. Prev Med 1989;18:133-46.
7. Womeodu RJ, Bailey JE. Barriers to cancer screening. Med Clin North Am 1996;80(1):115-33.
8. Suarez L. Pap smear and mammogram screening in Mexican-American women: the effects of acculturation. Am J Public Health 1994;84:742-6.
9. Tang TW, Solomon LJ, Yeh CJ, Worden JK. The role of cultural variables in breast self-examination and cervical cancer screening behavior in young Asian women living in the United States. J Behav Med 1999;22(5):419-36.
10. Golding JM. Sexual assault history and physical health in randomly selected Los Angeles women. Health Psychol 1994;13:130-8.
11. Golding JM. Sexual assault history and women’s reproductive and sexual health. Psychol of Women Quarterly 1996;20:101-21.
12. Golding JM. Sexual assault history and long-term physical health: Evidence from clinical and population epidemiology. Curr Directions in Psychol Sci 1999;8:191-4.
13. Koss MP, Koss PG, Woodruff WJ. Deleterious effects of criminal victimization on women’s health and medical utilization. Arch Intern Med 1991;151:342-7.
14. Laws A. Sexual abuse history and women’s medical problems. J Gen Intern Med 1993;8:441-44.
15. Lechner ME, Vogel ME, Garcia-Shelton LM, Leichter JL, Steibel KR. Self-reported medical problems of adult female survivors of childhood sexual abuse. J Fam Pract 1993;36:633-8.
16. Springs FE, Friedrich WN. Health risk behaviors and medical sequelae of childhood sexual abuse. Mayo Clin Proc 1992;67:527-32.
17. Felitti V, Anda F, Nordenberg D, Williamson, Spitz A, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. Am J Prev Med 1998;14:245-58.
18. Farley M, Minkoff J, Barkan H. Breast cancer screening and trauma history. Women Health in press.
19. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 1995;52:1048-60.
20. Polusny MA, Follette VM. Long-term correlates of child sexual abuse: Theory and review of the empirical literature. Applied and Preventive Psychology 1995;4:143-66.
21. Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, Best CL. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. J Consulting Clin Psychol 1993;61:984-91.
22. Blake DD, Cook JD, Keane TM. Posttraumatic stress disorder and coping in veterans who are seeking medical treatment. J Clin Psychol 1992;48:695-704.
23. Fama LD, Blake DD, Gusman F. Coping and health behaviors in combat-related PTSD inpatients. In: Annual Meeting of the International Society for Traumatic Stress Studies; San Antonio; 1993.
24. Farley M, Barkan H. Somatization, dissociation, and tension-reducing behaviors in psychiatric outpatients. Psychother Psychosom 1997;66:133-40.
25. Wolfe J, Proctor SP, Brown P, Kimerling RD, J., Sullivan M, Chrestman K, et al. Relationship of physical health and posttraumatic stress disorder in young adult women. In: Annual Meeting of the International Society for Traumatic Stress Studies; 1994; Los Angeles; 1994.
26. Kitzinger J. Recalling the pain. Nursing Times 1990 January;17:38-40.
27. Menage J. Women’s perception of obstetric and gynaecological examinations. Br Med J 1993;306:1127-8.
28. Robohm JS, Buttenheim M. The gynecological care experiences of adult survivors of childhood sexual abuse: A preliminary investigation. Women Health 1996;24:59-75.
29. Wahlen SD. Adult survivors of childhood sexual abuse. In: Hendricks-Matthews M, editor. Violence education: Toward a solution. Kansas City, MO: Society of Teachers of Family Medicine; 1992. p. 89-102.
30. Briere J. Methodological issues in the study of sexual abuse effects. J Consulting Clin Psychol 1992;60:196-203.
31. Stamm BH, Varra ME. Instrumentation in the Field of Traumatic Stress. Oswego, NY: Research and Methodology Interest Group of the International Society for Traumatic Stress Studies; 1993.
32. Carlson EB, Briere J. Screening for traumatic experiences and trauma responses in mental health treatment settings. In: International Society for Traumatic Stress Studies; 1999 November 14; Miami, FL; 1999.
33. Bernstein DP, Fink L. Childhood Trauma Questionnaire: A Retrospective Self-Report (Manual). San Antonio, TX: Psychological Corporation; 1998.
34. Weathers FW, Litz BT, Herman DS, Huska JA, Keane TM. The PTSD Checklist (PCL): Reliability, Validity, and Diagnostic Utility. In: 9th Annual Meeting of the International Society for Traumatic Stress Studies; 1993; San Antonio, TX; 1993.
35. The SAS System for Windows. In. 8.02 ed. Cary, NC: SAS Institute; 2001.
36. Coker AL, Patel NJ, Krishnaswami W, Schmidt W, Richter DL. Childhood forced sex and cervical dysplasia among women prison inmates. Violence Against Women 1998;4(5):595-608.
37. Becker TM, Wheeler CM, McGough NS, Parmenter CA, Jordan SW, Stidley CA, et al. Sexually transmitted diseases and other risk factors for cervical dysplasia among southwestern Hispanic and non-Hispanic white women. JAMA 1994;271(15):1181-8.
38. Melnikow J, Nuovo J. Cancer prevention and screening in women. Women’s Health 1997;24(1):15-26.
39. Daling JR, Madeleine MM, McKnight B, Carter JJ, Wipf GC, Ashley R, et al. The relationship of human papillomavirus-related cervical tumors to cigarette smoking, oral contraceptive use, and prior herpes simplex virus type 2 infection. Cancer Epidemiol Biomarkers Prev 1996;5(7):541-8.
40. Plichta SB. Violence and abuse: Implications for women’s health. In: Falk, Collins, editors. Women’s health: The Commonwealth Fund survey. Baltimore, MD: Johns Hopkins University Press; 1996.
41. Peters SD, Wyatt GE, Finkelhor D. Prevalence. In: Finkelhor D, editor. A sourcebook on child sexual abuse. Beverly Hills, CA: Sage; 1986. p. 15-59.
42. DeBlasi Z, Harkness E, Ernst E, Georgiou A, Kleijnen J. Influence of context effects on health outcomes: A systematic review. Lancet 2001;357:757-62.
43. Mandelblatt JS, Gold K, O’Malley AS, Taylor K, Cagney K, Hopkins J, et al. Breast and cervical cancer screening among multiethnic women: Role of age, health and source of care. J Preventive Med 1999;28:418-25.
44. Ruffin MT, Gorenflo DW, Woodman B. Predictors of screening for breast, cervical, colorectal, and prostatic cancer among community-based primary care practices. J Am Board Fam Pract 2000;13:1-10.
Information about tests for breast cancer: What are we telling people?
We reviewed publications currently available about breast cancer screening to assess what information was provided about test accuracy and pretest and posttest disease probabilities, as this information is needed by consumers to make informed decisions about whether to undergo testing and to fully understand test results. A rating form was developed and used to assess 54 publications about their reports of breast cancer tests. A description of how the test is done was provided by almost all publications (93%). About half (48%) provided some information about possible adverse effects of the test. Eighteen percent of publications provided some (generally qualitative) information about test accuracy, and none provided quantitative information about the probability of disease given normal and abnormal test results.
It has been well established that patients want to participate in decisions about their treatment options1-3; therefore, they most likely also wish to participate in decisions about whether to undergo common diagnostic tests. A literature review (using the MESH headings Patient education, Consumer participation, and Sensitivity and Specificity) revealed only 1 study of patient knowledge and understanding of test accuracy for routine diagnostic tests.4 This study found that patients knew little about disease probabilities and diagnostic test characteristics, even if they had previous experience with the target disease. Some studies have addressed the information that people should be given about screening tests,5-8 and guidelines from the General Medical Council of the United Kingdom specify that information about the likelihood of positive or negative findings including false-negative and false-positive results must be provided.9 Logically, similar information should be available to individuals undergoing common diagnostic tests, but to our knowledge, no systematic assessment of the information available to consumers about common tests has been conducted. We therefore set out to assess the information provided about common tests in current consumer publications. Given the findings of the previous study,4 we were particularly interested to see whether information about test accuracy and about pretest and posttest probabilities was provided. We assessed breast cancer screening and diagnostic tests because much has been written for the public about breast cancer tests, and the information provided is usually relatively sophisticated.
Methods
The rating form
We developed a rating form to record the type of information in each publication. Following the recommendation that consumers’ questions should drive the content of information,3 we used “Questions to ask your physician about tests” in Smart Health Choices, a consumer-oriented book about making health decisions,10 and the General Medical Council guideline for providing information about screening tests9 to develop the rating form. In addition to assessing information about false-positive and false-negative results (test accuracy), pretest probabilities and posttest probabilities given a normal or abnormal test result, we assessed whether information was given about how the test is conducted, likely emotional responses to being tested, and shared clinical decision making. The rating form consisted of 16 items (see Table W1, available at http://www.jfponline.com). A 5-point Likert scale was used to rate the publications on each item from 1 (no information) to 5 (detailed information).
The publications
In December 1999 and January 2000 we telephoned the New South Wales (NSW) Cancer Council (the leading cancer advocacy center in NSW), the NSW BreastScreen Coordinating Unit (which coordinates all government-funded breast screening and assessment services in NSW, operating from 36 clinics) and 2 large private breast clinics. We also phoned the larger BreastScreen clinics directly. We asked for all pamphlets, booklets, or other written patient education materials about breast tests. Publications were received from 12 locations of 14 telephoned, (86%).
Rating agreement
We chose 10 publications at random and 2 of us (A.B. and P.B.) rated them independently. Overall, there was perfect agreement for 79% of the items, near agreement (1 point difference on the Likert scale) on 12% of items, and more than 1 point difference on the Likert scale on the remaining 9% of items. Based on these results we modified the scale slightly to reduce ambiguity and clarified how to rate information in a written guide. One of us (E.C.) rated all 54 publications using the guide and the rating form.
Results
We received 54 publications. Of these, 43% contained information on breast self-examination, 51% clinical examination, 69% screening mammography, 44% diagnostic mammography, 30% diagnostic ultrasound, 30% fine-needle aspiration biopsy, 28% core biopsy, 13% open surgical biopsy, and 7% genetic testing. The publications were written by cancer organisations, the BreastScreen Coordinating Unit, and by individual public and private clinics. Most were brief (1–4 A4 pages) although 1, on all aspects of breast cancer detection and treatment, was 44 pages long.
Almost all publications described how the test is done and half provided information about possible adverse effects of tests (Table). Only a minority of publications provided specific information about pretest probability (eg, how breast cancer risk changes with age), test accuracy, or posttest probability (Table). Where breast cancer risk was mentioned it was usually given in the form of a lifetime risk of a woman developing breast cancer. However, 13% of publications provided information about the risk of developing breast cancer in the next 5 to 10 years and one gave age-specific risks. Information about test accuracy was given as “not all cancers are detected” by the test (9%) or “9 out of 10 cancers are detected” by the test (7%). Quantitative estimates of specificity and positive predictive value were provided in 1 publication. Information about the probability of disease given a positive test result was given as “About 1 in 20 women are asked to come back for further tests. 9 out of 10 women who are recalled do not have breast cancer” in a few publications. Information about posttest probability given a normal test result was not given in any publication. Results of ratings on other items are available directly from the corresponding author.
TABLE
Percentage of 54 patient education publications rated as providing information on key items about breast cancer screening
| Information about | Publications providing any information (rating 2-5) | Publications providing substantial information (rating 4 or 5) |
|---|---|---|
| How the test is done (%) | 93 | 57 |
| How breast cancer risk varies by age (%) | 37 | 15 |
| Adverse effects of tests (%) | 48 | 9 |
| Test accuracy, ie, false-positive and false-negative results (%) | 18 | 2 |
| Posttest probability given a normal or abnormal test result (%) | 20 | 0 |
Discussion
We found that the quantitative information women need to make informed choices about whether to undergo a breast cancer screening and to fully understand the test results was lacking in most publications. The most commonly used format for expressing the prior probability of breast cancer was lifetime risk; age-specific information was rarely provided. As the risk of breast cancer varies greatly with age, age-specific prior probabilities, not lifetime risk, are needed for informed decision making. Few publications provided information about test accuracy. Failure to acknowledge that tests may give false-positive and false-negative results may mislead people in interpreting their results. For example, people may think that if the test is negative, disease is absent, whereas in reality a negative test reduces but does not eliminate the possibility of disease. Conversely, many tests give positive (or abnormal) results, which prompt anxiety even though disease is absent. In the absence of information about test accuracy it is likely that misconceptions about test results will persist.
Some limitations of the study should be noted. Although publications commonly used in New South Wales (the most populous state of Australia with approximately one third of the total national population) are unlikely to have been missed in this study, we cannot guarantee that all publications currently in use were obtained. Secondly, we acknowledge that the ratings may have been different if others rated these publications. However, it is unlikely that either of these considerations is substantial enough to affect the general direction of the findings.
In conclusion, we suggest that there is an urgent need to ascertain what information consumers need about screening and diagnostic tests so they can make rational, informed choices. Communicating information about pretest probability, test accuracy, and posttest probability to consumers will require careful development and evaluation work. However, this work is essential so that people can give truly informed consent to being tested. Further, good information is needed for those people who want to participate actively in decisions about whether to undergo a test, and to support more accurate patient understanding of test results.
1. Degner LF, Kristjanson LJ, Bowman D, et al. Information needs and decisional p in women with breast cancer. JAMA 1997;277:1485-92.
2. Coulter A. Evidence based patient information is important, so there needs to be a national strategy to ensure it. BMJ 1998;317:225-6.
3. Coulter A, Entwistle V, Gilbert D. Sharing decisions with patients: is the information good enough? BMJ 1999;318:318-22.
4. Hamm RM, Smith SL. The accuracy of patients’ judgements of disease probability and test sensitivity and specificity. J Fam Pract 1998;47:44-52.
5. Wolf AM, Becker DM. Cancer screening and informed patient discussions: truth and consequences. Arch Intern Med 1996;156:1069-72.
6. Raffle AE. Information about screening—is it to achieve high uptake or to ensure informed choice? Health Expect 2001;4:92-8.
7. Goyder E, Barratt A, Irwig LM. Telling people about screening programmes and screening test results: how can we do it better? J Med Screen 2000;7:123-6.
8. Marteau TM, Saidi G, Goodburn S, Lawton J, Michie S, Bobrow M. Numbers or words? A randomized controlled trial of presenting screen negative results to pregnant women. Prenat Diagn 2000;20:714-8.
9. General Medical Council. Seeking Patients’ Consent: The Ethical Considerations. London, UK: General Medical Council; 1999.
10. Irwig J, Irwig L, Sweet M. Smart Health Choices: How to Make Informed Health Decisions. Sydney, Australia: Allen & Unwin; 1999.
We reviewed publications currently available about breast cancer screening to assess what information was provided about test accuracy and pretest and posttest disease probabilities, as this information is needed by consumers to make informed decisions about whether to undergo testing and to fully understand test results. A rating form was developed and used to assess 54 publications about their reports of breast cancer tests. A description of how the test is done was provided by almost all publications (93%). About half (48%) provided some information about possible adverse effects of the test. Eighteen percent of publications provided some (generally qualitative) information about test accuracy, and none provided quantitative information about the probability of disease given normal and abnormal test results.
It has been well established that patients want to participate in decisions about their treatment options1-3; therefore, they most likely also wish to participate in decisions about whether to undergo common diagnostic tests. A literature review (using the MESH headings Patient education, Consumer participation, and Sensitivity and Specificity) revealed only 1 study of patient knowledge and understanding of test accuracy for routine diagnostic tests.4 This study found that patients knew little about disease probabilities and diagnostic test characteristics, even if they had previous experience with the target disease. Some studies have addressed the information that people should be given about screening tests,5-8 and guidelines from the General Medical Council of the United Kingdom specify that information about the likelihood of positive or negative findings including false-negative and false-positive results must be provided.9 Logically, similar information should be available to individuals undergoing common diagnostic tests, but to our knowledge, no systematic assessment of the information available to consumers about common tests has been conducted. We therefore set out to assess the information provided about common tests in current consumer publications. Given the findings of the previous study,4 we were particularly interested to see whether information about test accuracy and about pretest and posttest probabilities was provided. We assessed breast cancer screening and diagnostic tests because much has been written for the public about breast cancer tests, and the information provided is usually relatively sophisticated.
Methods
The rating form
We developed a rating form to record the type of information in each publication. Following the recommendation that consumers’ questions should drive the content of information,3 we used “Questions to ask your physician about tests” in Smart Health Choices, a consumer-oriented book about making health decisions,10 and the General Medical Council guideline for providing information about screening tests9 to develop the rating form. In addition to assessing information about false-positive and false-negative results (test accuracy), pretest probabilities and posttest probabilities given a normal or abnormal test result, we assessed whether information was given about how the test is conducted, likely emotional responses to being tested, and shared clinical decision making. The rating form consisted of 16 items (see Table W1, available at http://www.jfponline.com). A 5-point Likert scale was used to rate the publications on each item from 1 (no information) to 5 (detailed information).
The publications
In December 1999 and January 2000 we telephoned the New South Wales (NSW) Cancer Council (the leading cancer advocacy center in NSW), the NSW BreastScreen Coordinating Unit (which coordinates all government-funded breast screening and assessment services in NSW, operating from 36 clinics) and 2 large private breast clinics. We also phoned the larger BreastScreen clinics directly. We asked for all pamphlets, booklets, or other written patient education materials about breast tests. Publications were received from 12 locations of 14 telephoned, (86%).
Rating agreement
We chose 10 publications at random and 2 of us (A.B. and P.B.) rated them independently. Overall, there was perfect agreement for 79% of the items, near agreement (1 point difference on the Likert scale) on 12% of items, and more than 1 point difference on the Likert scale on the remaining 9% of items. Based on these results we modified the scale slightly to reduce ambiguity and clarified how to rate information in a written guide. One of us (E.C.) rated all 54 publications using the guide and the rating form.
Results
We received 54 publications. Of these, 43% contained information on breast self-examination, 51% clinical examination, 69% screening mammography, 44% diagnostic mammography, 30% diagnostic ultrasound, 30% fine-needle aspiration biopsy, 28% core biopsy, 13% open surgical biopsy, and 7% genetic testing. The publications were written by cancer organisations, the BreastScreen Coordinating Unit, and by individual public and private clinics. Most were brief (1–4 A4 pages) although 1, on all aspects of breast cancer detection and treatment, was 44 pages long.
Almost all publications described how the test is done and half provided information about possible adverse effects of tests (Table). Only a minority of publications provided specific information about pretest probability (eg, how breast cancer risk changes with age), test accuracy, or posttest probability (Table). Where breast cancer risk was mentioned it was usually given in the form of a lifetime risk of a woman developing breast cancer. However, 13% of publications provided information about the risk of developing breast cancer in the next 5 to 10 years and one gave age-specific risks. Information about test accuracy was given as “not all cancers are detected” by the test (9%) or “9 out of 10 cancers are detected” by the test (7%). Quantitative estimates of specificity and positive predictive value were provided in 1 publication. Information about the probability of disease given a positive test result was given as “About 1 in 20 women are asked to come back for further tests. 9 out of 10 women who are recalled do not have breast cancer” in a few publications. Information about posttest probability given a normal test result was not given in any publication. Results of ratings on other items are available directly from the corresponding author.
TABLE
Percentage of 54 patient education publications rated as providing information on key items about breast cancer screening
| Information about | Publications providing any information (rating 2-5) | Publications providing substantial information (rating 4 or 5) |
|---|---|---|
| How the test is done (%) | 93 | 57 |
| How breast cancer risk varies by age (%) | 37 | 15 |
| Adverse effects of tests (%) | 48 | 9 |
| Test accuracy, ie, false-positive and false-negative results (%) | 18 | 2 |
| Posttest probability given a normal or abnormal test result (%) | 20 | 0 |
Discussion
We found that the quantitative information women need to make informed choices about whether to undergo a breast cancer screening and to fully understand the test results was lacking in most publications. The most commonly used format for expressing the prior probability of breast cancer was lifetime risk; age-specific information was rarely provided. As the risk of breast cancer varies greatly with age, age-specific prior probabilities, not lifetime risk, are needed for informed decision making. Few publications provided information about test accuracy. Failure to acknowledge that tests may give false-positive and false-negative results may mislead people in interpreting their results. For example, people may think that if the test is negative, disease is absent, whereas in reality a negative test reduces but does not eliminate the possibility of disease. Conversely, many tests give positive (or abnormal) results, which prompt anxiety even though disease is absent. In the absence of information about test accuracy it is likely that misconceptions about test results will persist.
Some limitations of the study should be noted. Although publications commonly used in New South Wales (the most populous state of Australia with approximately one third of the total national population) are unlikely to have been missed in this study, we cannot guarantee that all publications currently in use were obtained. Secondly, we acknowledge that the ratings may have been different if others rated these publications. However, it is unlikely that either of these considerations is substantial enough to affect the general direction of the findings.
In conclusion, we suggest that there is an urgent need to ascertain what information consumers need about screening and diagnostic tests so they can make rational, informed choices. Communicating information about pretest probability, test accuracy, and posttest probability to consumers will require careful development and evaluation work. However, this work is essential so that people can give truly informed consent to being tested. Further, good information is needed for those people who want to participate actively in decisions about whether to undergo a test, and to support more accurate patient understanding of test results.
We reviewed publications currently available about breast cancer screening to assess what information was provided about test accuracy and pretest and posttest disease probabilities, as this information is needed by consumers to make informed decisions about whether to undergo testing and to fully understand test results. A rating form was developed and used to assess 54 publications about their reports of breast cancer tests. A description of how the test is done was provided by almost all publications (93%). About half (48%) provided some information about possible adverse effects of the test. Eighteen percent of publications provided some (generally qualitative) information about test accuracy, and none provided quantitative information about the probability of disease given normal and abnormal test results.
It has been well established that patients want to participate in decisions about their treatment options1-3; therefore, they most likely also wish to participate in decisions about whether to undergo common diagnostic tests. A literature review (using the MESH headings Patient education, Consumer participation, and Sensitivity and Specificity) revealed only 1 study of patient knowledge and understanding of test accuracy for routine diagnostic tests.4 This study found that patients knew little about disease probabilities and diagnostic test characteristics, even if they had previous experience with the target disease. Some studies have addressed the information that people should be given about screening tests,5-8 and guidelines from the General Medical Council of the United Kingdom specify that information about the likelihood of positive or negative findings including false-negative and false-positive results must be provided.9 Logically, similar information should be available to individuals undergoing common diagnostic tests, but to our knowledge, no systematic assessment of the information available to consumers about common tests has been conducted. We therefore set out to assess the information provided about common tests in current consumer publications. Given the findings of the previous study,4 we were particularly interested to see whether information about test accuracy and about pretest and posttest probabilities was provided. We assessed breast cancer screening and diagnostic tests because much has been written for the public about breast cancer tests, and the information provided is usually relatively sophisticated.
Methods
The rating form
We developed a rating form to record the type of information in each publication. Following the recommendation that consumers’ questions should drive the content of information,3 we used “Questions to ask your physician about tests” in Smart Health Choices, a consumer-oriented book about making health decisions,10 and the General Medical Council guideline for providing information about screening tests9 to develop the rating form. In addition to assessing information about false-positive and false-negative results (test accuracy), pretest probabilities and posttest probabilities given a normal or abnormal test result, we assessed whether information was given about how the test is conducted, likely emotional responses to being tested, and shared clinical decision making. The rating form consisted of 16 items (see Table W1, available at http://www.jfponline.com). A 5-point Likert scale was used to rate the publications on each item from 1 (no information) to 5 (detailed information).
The publications
In December 1999 and January 2000 we telephoned the New South Wales (NSW) Cancer Council (the leading cancer advocacy center in NSW), the NSW BreastScreen Coordinating Unit (which coordinates all government-funded breast screening and assessment services in NSW, operating from 36 clinics) and 2 large private breast clinics. We also phoned the larger BreastScreen clinics directly. We asked for all pamphlets, booklets, or other written patient education materials about breast tests. Publications were received from 12 locations of 14 telephoned, (86%).
Rating agreement
We chose 10 publications at random and 2 of us (A.B. and P.B.) rated them independently. Overall, there was perfect agreement for 79% of the items, near agreement (1 point difference on the Likert scale) on 12% of items, and more than 1 point difference on the Likert scale on the remaining 9% of items. Based on these results we modified the scale slightly to reduce ambiguity and clarified how to rate information in a written guide. One of us (E.C.) rated all 54 publications using the guide and the rating form.
Results
We received 54 publications. Of these, 43% contained information on breast self-examination, 51% clinical examination, 69% screening mammography, 44% diagnostic mammography, 30% diagnostic ultrasound, 30% fine-needle aspiration biopsy, 28% core biopsy, 13% open surgical biopsy, and 7% genetic testing. The publications were written by cancer organisations, the BreastScreen Coordinating Unit, and by individual public and private clinics. Most were brief (1–4 A4 pages) although 1, on all aspects of breast cancer detection and treatment, was 44 pages long.
Almost all publications described how the test is done and half provided information about possible adverse effects of tests (Table). Only a minority of publications provided specific information about pretest probability (eg, how breast cancer risk changes with age), test accuracy, or posttest probability (Table). Where breast cancer risk was mentioned it was usually given in the form of a lifetime risk of a woman developing breast cancer. However, 13% of publications provided information about the risk of developing breast cancer in the next 5 to 10 years and one gave age-specific risks. Information about test accuracy was given as “not all cancers are detected” by the test (9%) or “9 out of 10 cancers are detected” by the test (7%). Quantitative estimates of specificity and positive predictive value were provided in 1 publication. Information about the probability of disease given a positive test result was given as “About 1 in 20 women are asked to come back for further tests. 9 out of 10 women who are recalled do not have breast cancer” in a few publications. Information about posttest probability given a normal test result was not given in any publication. Results of ratings on other items are available directly from the corresponding author.
TABLE
Percentage of 54 patient education publications rated as providing information on key items about breast cancer screening
| Information about | Publications providing any information (rating 2-5) | Publications providing substantial information (rating 4 or 5) |
|---|---|---|
| How the test is done (%) | 93 | 57 |
| How breast cancer risk varies by age (%) | 37 | 15 |
| Adverse effects of tests (%) | 48 | 9 |
| Test accuracy, ie, false-positive and false-negative results (%) | 18 | 2 |
| Posttest probability given a normal or abnormal test result (%) | 20 | 0 |
Discussion
We found that the quantitative information women need to make informed choices about whether to undergo a breast cancer screening and to fully understand the test results was lacking in most publications. The most commonly used format for expressing the prior probability of breast cancer was lifetime risk; age-specific information was rarely provided. As the risk of breast cancer varies greatly with age, age-specific prior probabilities, not lifetime risk, are needed for informed decision making. Few publications provided information about test accuracy. Failure to acknowledge that tests may give false-positive and false-negative results may mislead people in interpreting their results. For example, people may think that if the test is negative, disease is absent, whereas in reality a negative test reduces but does not eliminate the possibility of disease. Conversely, many tests give positive (or abnormal) results, which prompt anxiety even though disease is absent. In the absence of information about test accuracy it is likely that misconceptions about test results will persist.
Some limitations of the study should be noted. Although publications commonly used in New South Wales (the most populous state of Australia with approximately one third of the total national population) are unlikely to have been missed in this study, we cannot guarantee that all publications currently in use were obtained. Secondly, we acknowledge that the ratings may have been different if others rated these publications. However, it is unlikely that either of these considerations is substantial enough to affect the general direction of the findings.
In conclusion, we suggest that there is an urgent need to ascertain what information consumers need about screening and diagnostic tests so they can make rational, informed choices. Communicating information about pretest probability, test accuracy, and posttest probability to consumers will require careful development and evaluation work. However, this work is essential so that people can give truly informed consent to being tested. Further, good information is needed for those people who want to participate actively in decisions about whether to undergo a test, and to support more accurate patient understanding of test results.
1. Degner LF, Kristjanson LJ, Bowman D, et al. Information needs and decisional p in women with breast cancer. JAMA 1997;277:1485-92.
2. Coulter A. Evidence based patient information is important, so there needs to be a national strategy to ensure it. BMJ 1998;317:225-6.
3. Coulter A, Entwistle V, Gilbert D. Sharing decisions with patients: is the information good enough? BMJ 1999;318:318-22.
4. Hamm RM, Smith SL. The accuracy of patients’ judgements of disease probability and test sensitivity and specificity. J Fam Pract 1998;47:44-52.
5. Wolf AM, Becker DM. Cancer screening and informed patient discussions: truth and consequences. Arch Intern Med 1996;156:1069-72.
6. Raffle AE. Information about screening—is it to achieve high uptake or to ensure informed choice? Health Expect 2001;4:92-8.
7. Goyder E, Barratt A, Irwig LM. Telling people about screening programmes and screening test results: how can we do it better? J Med Screen 2000;7:123-6.
8. Marteau TM, Saidi G, Goodburn S, Lawton J, Michie S, Bobrow M. Numbers or words? A randomized controlled trial of presenting screen negative results to pregnant women. Prenat Diagn 2000;20:714-8.
9. General Medical Council. Seeking Patients’ Consent: The Ethical Considerations. London, UK: General Medical Council; 1999.
10. Irwig J, Irwig L, Sweet M. Smart Health Choices: How to Make Informed Health Decisions. Sydney, Australia: Allen & Unwin; 1999.
1. Degner LF, Kristjanson LJ, Bowman D, et al. Information needs and decisional p in women with breast cancer. JAMA 1997;277:1485-92.
2. Coulter A. Evidence based patient information is important, so there needs to be a national strategy to ensure it. BMJ 1998;317:225-6.
3. Coulter A, Entwistle V, Gilbert D. Sharing decisions with patients: is the information good enough? BMJ 1999;318:318-22.
4. Hamm RM, Smith SL. The accuracy of patients’ judgements of disease probability and test sensitivity and specificity. J Fam Pract 1998;47:44-52.
5. Wolf AM, Becker DM. Cancer screening and informed patient discussions: truth and consequences. Arch Intern Med 1996;156:1069-72.
6. Raffle AE. Information about screening—is it to achieve high uptake or to ensure informed choice? Health Expect 2001;4:92-8.
7. Goyder E, Barratt A, Irwig LM. Telling people about screening programmes and screening test results: how can we do it better? J Med Screen 2000;7:123-6.
8. Marteau TM, Saidi G, Goodburn S, Lawton J, Michie S, Bobrow M. Numbers or words? A randomized controlled trial of presenting screen negative results to pregnant women. Prenat Diagn 2000;20:714-8.
9. General Medical Council. Seeking Patients’ Consent: The Ethical Considerations. London, UK: General Medical Council; 1999.
10. Irwig J, Irwig L, Sweet M. Smart Health Choices: How to Make Informed Health Decisions. Sydney, Australia: Allen & Unwin; 1999.
Improving influenza vaccination rates in the elderly
BACKGROUND: Vaccination coverage for influenza in the elderly remains low when the physician is the only person responsible for immunization. Integration of other health care workers may improve the coverage rate of at-risk groups.
OBJECTIVES: To estimate vaccination coverage rate by using a strategy based on the systematic intervention of a health care professional proposing vaccination before the doctor’s consultation, to evaluate the changes in coverage rates before and after introduction of this strategy, and to assess the feasibility of this intervention and the achieved coverage rate in family physician offices
STUDY DESIGN: Prospective study in a medical outpatient clinic and 5 family physician practices in Switzerland.
POPULATION: Participants consisted of all patients 65 years or older attending a medical outpatient clinic during the vaccination period in 1999 (n = 401), patients 65 years or older regularly followed at a medical outpatient clinic in 1998 and 1999 (n = 195), and patients 65 years or older presenting to 5 family physician offices in 1999 (n = 598).
OUTCOME MEASURED: Rates of vaccination coverage.
RESULTS: Among all participants, vaccination coverage rates in 1999 were 85% at the medical outpatient clinic and 83% in family physician offices. Among participants regularly followed at the medical outpatient clinic, vaccination coverage increased from 48% in 1998 to 76% in 1999. Rates of refusal were 9% at the medical outpatient clinic and 14% in the family physician offices.
CONCLUSIONS: The systematic intervention of a health care professional to suggest vaccination before the doctor’s visit is an effective measure to achieve high coverage rate. Such a strategy also improves outpatient clinic or private practice efficiency by reducing pressures on physicians.
Annual influenza vaccination is recommended for all persons 65 years and older.1-3 Unfortunately, coverage rate remains low. In Switzerland during the winter season of 1998-1999, estimated vaccination coverage was only 8% in the general population.4 In institutionalized elderly patients, coverage was 37% in the same study. In 1994, the rate estimated from a telephonic survey was only 36% in elderly patients in Geneva.5 Since then, an active promotional campaign among the public led to a coverage rate among persons older than 65 years of about 60% (L. Toscani, personal communication 2000), a rate that approaches that in the United States (67%).6
Apart from making the general population or the target groups aware of the importance of the vaccination to prevent influenza complications, a strategy commonly used to improve coverage consists of training the physicians. However, their knowledge about vaccination does not always explain their behavior; although doctors know that vaccines are efficacious and are convinced that they should offer vaccination to all at-risk patients, they do not propose it to all eligible patients.7 Reimbursement of vaccines, as done since 1996 in Switzerland, does not seem to increase vaccination rates.8
At the Medical Outpatient Clinic, University of Lausanne, we recorded the influenza vaccination coverage rate of patients 65 years and older who were followed regularly in 1997, after an intensive education of physicians including a state-of-the-art lecture and interactive seminars; the same was done in 1998, but letters also were sent to all patients who did not have an appointment during the vaccination period. The vaccination coverage increased from 39% in 1997 to 47% in 1998, presumably because of the reminder letter.9 This rate was still unsatisfactory. We postulated that the main reason for low rate of influenza vaccination coverage of elderly patients was the physicians’ omission to propose the vaccination rather than the patients’ refusal. To test this hypothesis and to improve the coverage at the same time, we introduced at the medical outpatient clinic a strategy coupling a systematic intervention of a medical person allowed to do injections (medical student) with the existing educational program and reminder letters. A similar method (except for the reminder letters) was applied in 5 general practices, with the receptionists providing the information and the paramedical staff performing the vaccination.
The specific objectives were (1) to estimate vaccination coverage rate by using a strategy based on the systematic intervention of a health care professional proposing vaccination before the doctor’s consultation, (2) to evaluate the changes in coverage rates before and after introduction of the strategy, and (3) to assess the feasibility of this intervention and the achieved coverage rate in family physicians’ offices.
Methods
The protocol was approved by the ethical committee of the Department of Internal Medicine, University Hospital, Lausanne. Table 1 summarizes the study profile, populations, strategy applied, and outcome measures that are described below. The study took place in the Medical Outpatient Clinic, University of Lausanne, which provides medical care to the general population (attendees are biased toward young people, refugees, foreigners, and elderly individuals with low incomes); and in 1 rural general practice office (Orbe) and 2 urban general practice offices (Neuchâtel), with 5 physicians.
Study populations
To estimate vaccination coverage rate after implementing the new strategy in the medical outpatient clinic (objective 1), we included all patients 65 years and older attending the 1999 vaccination period (401 patients). To evaluate the changes in coverage rates before and after the introduction of the strategy (objective 2), we included patients 65 years and older who were followed regularly at medical outpatient clinic in 1998 and 1999 (195 patients). To assess the feasibility of this intervention and the achieved coverage rate in family physicians’ offices, we included all patients 65 years and older attending family physicians’ offices in 1999 (598 patients).
Medical outpatient clinic procedures
Pre-intervention season (1998). During the vaccination period (mid-October to mid-December), all patients 65 years and older presented to the receptionist and then waited until they were seen by a first to fifth-year resident physician who proposed the vaccination during the consultation. The injection was then done by a nurse. Alternatively, anyone could go straight to the nurse (walk-in clinic) and ask for an influenza shot, which was done after checking that there was no contraindication (egg allergy, fever during the past 2 days, or use of anticoagulants, in which case subcutaneous injection was performed). To check that all patients regularly followed at the medical outpatient clinic had made contact during the vaccination period, an independent registrar in mid-November reviewed all files of patients 65 years and older. For those regularly followed, he checked whether an appointment had been made; if not, a reminder letter was sent to the patient.
Intervention season (1999). During the vaccination period, all patients 65 years and older were told by the receptionist that a medical student would propose an influenza vaccination before seeing the doctor. The medical student informed the patients about influenza complications and prevention by vaccination, and asked them whether they had been vaccinated in the previous year. The patient then had to decide whether to be vaccinated immediately, discuss the vaccination with the doctor first, or refuse the vaccination. In case of refusal, the reason was investigated. The patient who agreed to the vaccination received the injection from the medical student and a label was attached to the patient’s file to inform the doctor about the vaccination status. The same procedure performed in 1998 was applied for the reminder letter
General practice procedures
During the intervention season only (1999), the receptionist at the admission desk proposed influenza vaccination to all patients 65 years and older and the paramedical staff promptly performed the injection, if the patient agreed. The same information and questionnaires were applied in the family physician’s offices and the medical outpatient clinic.
Outcomes
Several outcomes were predefined: (1) vaccination coverage rate of all patients 65 years and older who attended the medical outpatient clinic spontaneously or on appointment during the 1999 vaccination period; (2) vaccination coverage rate of patients 65 years and older regularly followed (excluding those coming only once without a follow-up) at the medical outpatient clinic in 1998 and 1999 (all were contacted by a reminder letter, if not seen during vaccination period); (3) vaccination coverage rate of all patients 65 years and older who attended the family physicians’ offices during the 1999 vaccination period; and (4) reasons for nonvaccination among those interviewed.
Data management and analysis
The data were entered immediately into Epi Info 6.0 and analyzed with SPSS 7.5. Testing for differences between the 1998 and 1999 rates in the medical outpatient clinic was done with chi-square tests.
Results
Rate of vaccination coverage at the medical outpatient clinic during the intervention period
A total of 401 patients 65 years and older came to the outpatient clinic during the vaccination period in 1999. The median age was 74 years (range, 65-97 years) and most patients were male (56%). Table 2 shows that 85% (341/401) accepted the vaccination at the medical outpatient clinic. Of those, 52% were advised by the medical student before consultation, 26% came spontaneously to the nurse for influenza vaccination, and 19% came with advice from their physicians. The rate of refusal was 9% (see Table 2 for details).
Changes in rates of vaccination coverage of patients regularly followed at the medical outpatient clinic in 1998 and 1999
In 1998, 195 patients 65 years and older regularly followed at the medical outpatient clinic were monitored.9 A reminder letter was sent to patients who did not have an appointment during the vaccination period (73/195 patients). About one fifth (15/73) came for vaccination after the reminder letter. Overall, 48% (93/195) were vaccinated in 1998.
In 1999, 325 of 401 patients 65 years and older were regularly followed by a resident-physician. The larger number in 1999 (when compared 195 patients in 1998) was due to the fact that, in 1998, only those who followed in 1997 were monitored. Among the 325 patients, 268 came to the office during the vaccination period; 57 persons regularly followed did not have an appointment during the vaccination period and were sent a reminder letter. Overall, 76% of patients regularly followed (246/325) were vaccinated in 1999. Of those, 65% were advised by the medical student. The rate of refusal was 11%. Only 2 patients in the targeted population were missed during the target period, and 2 patients had medical contraindications for vaccination. Thus, the new strategy led to a relative increase of 58% for vaccination coverage (from 48% to 76%; P < .0001, Yates correction).
Rate of vaccination coverage in family physicians’ offices in 1999
A total of 598 patients 65 years and older attended their family physicians’ offices during the vaccination period. The median age was 74 years (range, 65-99 years) and most were female (62%). Eighty-three percent accepted the vaccination at the family physicians’ offices, 3% wished to be vaccinated somewhere else, and 14% refused.
Reasons for nonvaccination
The rates of refusal were 14% in the family physician’s offices and 9% in the medical outpatient clinic. Reasons for nonvaccination in the medical outpatient clinic were obtaining the influenza vaccination elsewhere (5%) and medical contraindications (1%; Table 2).
Discussion
Our study demonstrated that the systematic intervention of a paramedical person before the doctor’s consultation can lead to a considerable improvement in vaccination coverage in an ambulatory setting. A 58% relative increase (from 48% to 76%) over 1 year in the same institution has never been achieved. This suggested that failure of the physician to propose vaccination is an important reason for low vaccination coverage of high-risk patients in teaching institutions, where physician turnover is high. Thanks to our organizational strategy, we reached an 85% vaccination coverage among all outpatient attendees, which far surpassed that achieved by an ongoing educational program or alternative strategies such as reminder letters.
Until now, the highest coverage ever reported in Europe in persons 65 years and older was 82% in a study in Finland, where the investigators used an age-based strategy, with free vaccines and personnel-mailed reminders.10 Even when efficacious11 and cost effective,12 these strategies are insufficiently used.4 Other means to improve vaccination coverage such as centralized planning identification through computerized enrollment files at central registry and immunization clinics have improved vaccination coverage rates to almost 80% among chronically ill seniors in the United States.13
Two American studies showed that approaches incorporating administrative and organizational measures were more successful in improving vaccination rates than the education of providers. A pilot study14 and a 10-year follow-up15 done in the Minneapolis Department of Veterans Affairs Medical Center used a strategy including annual educational and publicity mailing to patients, walk-in clinics for vaccine administration, standing orders for nurses, and use of standardized patient information and medical record documentation forms. The follow-up study showed a remarkable improvement in the coverage rate, which reached 92% in 1996-1997 in specific groups. Another study done in the University Department of Emergency Medicine of Chicago that used standing orders for nurses at triage was only partly successful, with 47% coverage.16
Our study adds to the knowledge gathered in the United States, where administrative and organizational strategies (standing orders for nurses) improved vaccination coverage significantly.14-16 These studies were conducted in a different population sample, including hospitalized patients and other high-risk groups, which is quite different from the situation in general practice. Comparing vaccination coverage rates between different studies is always hazardous because of differences in inclusion criteria and reporting biases. The highest vaccination coverage ever achieved was 92%, but the method used for the rate estimation was a mailed survey where vaccinated persons may have been more likely to respond than nonvaccinated ones.15 The investigators exported their program from a teaching hospital to a community outpatient setting, with an increase in coverage from 56% to 72% in 1 clinic and no significant increase in the other clinic, which shows that several factors should be considered when a new strategy is implemented (staff, motivation, etc).17 The relatively low coverage (47%) reached in another study conducted in an emergency department was due in part to the fact that the nurse dedicated to providing vaccination was diverted from the preventive task of caring for patients when the workload increased. In addition, only one fourth of the emergency attendees was screened.16
To our knowledge, this is the first time that such an active strategy has been reported and applied in family physicians’ offices. The implementation in private practices documents its feasibility and effectiveness in those settings. The consistent coverage rates obtained in the medical outpatient clinic and the general practices (85% and 83%, respectively) confirmed the usefulness of the strategy, whatever the patient population and the medical staff involved. One may argue that patients had little choice between accepting or actively refusing the vaccination. We hope that the quality of information provided to the elderly, the possibility for them to ask specific questions that come to mind, and even to delay the decision until they can discuss the decision further with the doctor balances the potential enforcement.
The main disadvantage of our strategy is the need for an additional person in the outpatient clinic. The direct cost of an extra person in public service can be justified if it decreases the indirect costs for society, which is the case for influenza vaccination.12 More pragmatically, at the institutional level, the additional income generated by the increase of vaccine sales outweighed the salary of the medical student (about US$100/day for a total of 40 days, ie, 20 working days for 2 months). The implementation in the family physicians’ offices showed that such a procedure can work in a small setting without additional staff. Counseling about vaccination represents an additional task for the paramedical personnel, but it certainly provides more credit to the overall work and improves the therapeutic network. Also, because the vaccination has to be proposed, the time for discussion has to be taken by whoever is the most willing to do the job.
Some doctors may feel that this strategy excludes them from important duties. We must face the reality that physicians often do not accomplish their tasks properly. With our procedure, they remain informed about the vaccination status of their patients and maintain their important role in counseling patients who cannot decide about the vaccination or when problems arise.
One can argue that the increase in vaccination coverage rate was due, at least in part, to external factors such as media propaganda, and that the new strategy played only a marginal role. Of course, we cannot exclude such an influence, but it certainly unlikely was to be the cause of a 58% increase in coverage rate; indeed, no particular campaign was launched in 1999 and no change in coverage rate between 1998 and 1999 was observed in a similar institution in the same area. We chose to use historical controls because we were convinced that the new strategy would dramatically improve vaccination in the elderly and felt therefore that it was unethical not to offer this procedure to all our patients. Moreover, the main interest of the study was to investigate the maximum coverage rate that could be expected with a very active intervention and to estimate precisely the rate of true refusals, so that defined objectives in terms of coverage rates can be set in similar institutions.
The new strategy assessed in this study is in line with the recommendations of the Advisory Committee on Immunization Practices, published in March 2000, that promoted the use of standing orders to improve adult vaccination delivery.18 Evidence of effectiveness of such programs is increasing. They could be adapted to other preventive measures to improve delivery of those services, and they could be used to improve outpatient clinic efficiency by reducing pressures on physicians.
Acknowledgments
We thank M. Cheseaux and S. Martin, the medical students responsible for providing vaccine information and delivery and data entry, the resident-physicians at medical outpatient clinic, and the nurses and receptionists in the medical outpatient clinic and the family physicians’ practices.
1. WHO Influenza vaccination recommendations. Wkly Epidemiol Rec 1999;74:57-64.
2. Working Group Influenza. Recommendations for influenza vaccination during the 1999/2000 season. Bull OFSP 1999;39:737-9.
3. CDC Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 1998;47:1-26.
4. Ammon CE. Vaccination against influenza in Switzerland. A survey in health facilities. Bull OFSP 1999;4:62-4.
5. Gauthey L, Toscani L, Chamot E, Larequi T, Robert CF. Influenza vaccination coverage in the geriatric population of the State of Geneva, Switzerland. Eur J Pharmacol 1999;9:36-40.
6. Centers for Disease Control and Prevention. Influenza and pneumococcal vaccination levels among persons aged 65 years-United States, 1999. MMWR Morb Mortal Wkly Rep 2001;50:532-7.
7. McKinney WP, Barnas GP. Influenza immunization in the elderly: knowledge and attitudes do not explain physician behavior. Am J Pharmacol 1989;79:1422-4.
8. Etkind P, Simon M, Shannon S, et al. Impact of the Medicare Influenza Demonstration Project on influenza vaccination in a county in Massachusetts, 1988-1992. Community Health 1996;21:199-209.
9. Zorzoli M, Favrat B, D’Acremont V, Pécoud A, Genton B. Strategies to improve vaccination coverage among elderly patients. J Gen Intern Med 1999;14:83.-
10. Honkanen PO, Keistinen T, Kivela SL. The impact of vaccination strategy and methods of information on influenza and pneumococcal vaccination coverage in the elderly population. Vaccine 1997;15:317-20.
11. Armstrong K, Berlin M, Schwartz S, Propert K, Ubel P. Educational content and the effectiveness of influenza vaccination reminders. J Gen Intern Med 1999;14:695-7.
12. Baker AM, McCarthy B, Gurley VF, Yood MU. Influenza immunization in a managed care organization. J Gen Intern Med 1998;13:469-75.
13. Pearson DC, Jackson LA, Wagener B, Sarver L. A comprehensive influenza campaign in a managed care setting. Vaccine 1998;16:1718-21.
14. Margolis KL, Lofgren RP, Korn JE. Organizational strategies to improve influenza vaccine delivery. A standing order in a general medicine clinic. Arch Intern Med 1988;148:2205-7.
15. Nichol KL. Ten-year durability and success of an organized program to increase influenza and pneumococcal vaccination rates among high-risk adults. Am J Med 1998;105:385-92.
16. Slobodkin D, Kitlas J, Zielske P. Opportunities not missed systematic influenza and pneumococcal immunization in a public inner-city emergency department. Vaccine 1998;16:1795-802.
17. Margolis KL, Nichol KL, Wuorenma J. Exporting a successful influenza vaccination program from a teaching hospital to a community outpatient setting. J Am Geriatr Soc 1992;40:1021-3.
18. CDC Adult immunization programs in nontraditional settings: quality standard and guidance for program evaluation. A report of the national Vaccine Advisory Committee and Use of standing orders programs to increase adult vaccination rates. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2000;49:18-27.
Address reprint requests to Blaise Genton, MD, PhD, Policlinique Médicale Universitaire, César Roux 19, 1005 Lausanne, Switzerland. E-mail: [email protected].
To submit a letter to the editor on this topic, click here: [email protected].
BACKGROUND: Vaccination coverage for influenza in the elderly remains low when the physician is the only person responsible for immunization. Integration of other health care workers may improve the coverage rate of at-risk groups.
OBJECTIVES: To estimate vaccination coverage rate by using a strategy based on the systematic intervention of a health care professional proposing vaccination before the doctor’s consultation, to evaluate the changes in coverage rates before and after introduction of this strategy, and to assess the feasibility of this intervention and the achieved coverage rate in family physician offices
STUDY DESIGN: Prospective study in a medical outpatient clinic and 5 family physician practices in Switzerland.
POPULATION: Participants consisted of all patients 65 years or older attending a medical outpatient clinic during the vaccination period in 1999 (n = 401), patients 65 years or older regularly followed at a medical outpatient clinic in 1998 and 1999 (n = 195), and patients 65 years or older presenting to 5 family physician offices in 1999 (n = 598).
OUTCOME MEASURED: Rates of vaccination coverage.
RESULTS: Among all participants, vaccination coverage rates in 1999 were 85% at the medical outpatient clinic and 83% in family physician offices. Among participants regularly followed at the medical outpatient clinic, vaccination coverage increased from 48% in 1998 to 76% in 1999. Rates of refusal were 9% at the medical outpatient clinic and 14% in the family physician offices.
CONCLUSIONS: The systematic intervention of a health care professional to suggest vaccination before the doctor’s visit is an effective measure to achieve high coverage rate. Such a strategy also improves outpatient clinic or private practice efficiency by reducing pressures on physicians.
Annual influenza vaccination is recommended for all persons 65 years and older.1-3 Unfortunately, coverage rate remains low. In Switzerland during the winter season of 1998-1999, estimated vaccination coverage was only 8% in the general population.4 In institutionalized elderly patients, coverage was 37% in the same study. In 1994, the rate estimated from a telephonic survey was only 36% in elderly patients in Geneva.5 Since then, an active promotional campaign among the public led to a coverage rate among persons older than 65 years of about 60% (L. Toscani, personal communication 2000), a rate that approaches that in the United States (67%).6
Apart from making the general population or the target groups aware of the importance of the vaccination to prevent influenza complications, a strategy commonly used to improve coverage consists of training the physicians. However, their knowledge about vaccination does not always explain their behavior; although doctors know that vaccines are efficacious and are convinced that they should offer vaccination to all at-risk patients, they do not propose it to all eligible patients.7 Reimbursement of vaccines, as done since 1996 in Switzerland, does not seem to increase vaccination rates.8
At the Medical Outpatient Clinic, University of Lausanne, we recorded the influenza vaccination coverage rate of patients 65 years and older who were followed regularly in 1997, after an intensive education of physicians including a state-of-the-art lecture and interactive seminars; the same was done in 1998, but letters also were sent to all patients who did not have an appointment during the vaccination period. The vaccination coverage increased from 39% in 1997 to 47% in 1998, presumably because of the reminder letter.9 This rate was still unsatisfactory. We postulated that the main reason for low rate of influenza vaccination coverage of elderly patients was the physicians’ omission to propose the vaccination rather than the patients’ refusal. To test this hypothesis and to improve the coverage at the same time, we introduced at the medical outpatient clinic a strategy coupling a systematic intervention of a medical person allowed to do injections (medical student) with the existing educational program and reminder letters. A similar method (except for the reminder letters) was applied in 5 general practices, with the receptionists providing the information and the paramedical staff performing the vaccination.
The specific objectives were (1) to estimate vaccination coverage rate by using a strategy based on the systematic intervention of a health care professional proposing vaccination before the doctor’s consultation, (2) to evaluate the changes in coverage rates before and after introduction of the strategy, and (3) to assess the feasibility of this intervention and the achieved coverage rate in family physicians’ offices.
Methods
The protocol was approved by the ethical committee of the Department of Internal Medicine, University Hospital, Lausanne. Table 1 summarizes the study profile, populations, strategy applied, and outcome measures that are described below. The study took place in the Medical Outpatient Clinic, University of Lausanne, which provides medical care to the general population (attendees are biased toward young people, refugees, foreigners, and elderly individuals with low incomes); and in 1 rural general practice office (Orbe) and 2 urban general practice offices (Neuchâtel), with 5 physicians.
Study populations
To estimate vaccination coverage rate after implementing the new strategy in the medical outpatient clinic (objective 1), we included all patients 65 years and older attending the 1999 vaccination period (401 patients). To evaluate the changes in coverage rates before and after the introduction of the strategy (objective 2), we included patients 65 years and older who were followed regularly at medical outpatient clinic in 1998 and 1999 (195 patients). To assess the feasibility of this intervention and the achieved coverage rate in family physicians’ offices, we included all patients 65 years and older attending family physicians’ offices in 1999 (598 patients).
Medical outpatient clinic procedures
Pre-intervention season (1998). During the vaccination period (mid-October to mid-December), all patients 65 years and older presented to the receptionist and then waited until they were seen by a first to fifth-year resident physician who proposed the vaccination during the consultation. The injection was then done by a nurse. Alternatively, anyone could go straight to the nurse (walk-in clinic) and ask for an influenza shot, which was done after checking that there was no contraindication (egg allergy, fever during the past 2 days, or use of anticoagulants, in which case subcutaneous injection was performed). To check that all patients regularly followed at the medical outpatient clinic had made contact during the vaccination period, an independent registrar in mid-November reviewed all files of patients 65 years and older. For those regularly followed, he checked whether an appointment had been made; if not, a reminder letter was sent to the patient.
Intervention season (1999). During the vaccination period, all patients 65 years and older were told by the receptionist that a medical student would propose an influenza vaccination before seeing the doctor. The medical student informed the patients about influenza complications and prevention by vaccination, and asked them whether they had been vaccinated in the previous year. The patient then had to decide whether to be vaccinated immediately, discuss the vaccination with the doctor first, or refuse the vaccination. In case of refusal, the reason was investigated. The patient who agreed to the vaccination received the injection from the medical student and a label was attached to the patient’s file to inform the doctor about the vaccination status. The same procedure performed in 1998 was applied for the reminder letter
General practice procedures
During the intervention season only (1999), the receptionist at the admission desk proposed influenza vaccination to all patients 65 years and older and the paramedical staff promptly performed the injection, if the patient agreed. The same information and questionnaires were applied in the family physician’s offices and the medical outpatient clinic.
Outcomes
Several outcomes were predefined: (1) vaccination coverage rate of all patients 65 years and older who attended the medical outpatient clinic spontaneously or on appointment during the 1999 vaccination period; (2) vaccination coverage rate of patients 65 years and older regularly followed (excluding those coming only once without a follow-up) at the medical outpatient clinic in 1998 and 1999 (all were contacted by a reminder letter, if not seen during vaccination period); (3) vaccination coverage rate of all patients 65 years and older who attended the family physicians’ offices during the 1999 vaccination period; and (4) reasons for nonvaccination among those interviewed.
Data management and analysis
The data were entered immediately into Epi Info 6.0 and analyzed with SPSS 7.5. Testing for differences between the 1998 and 1999 rates in the medical outpatient clinic was done with chi-square tests.
Results
Rate of vaccination coverage at the medical outpatient clinic during the intervention period
A total of 401 patients 65 years and older came to the outpatient clinic during the vaccination period in 1999. The median age was 74 years (range, 65-97 years) and most patients were male (56%). Table 2 shows that 85% (341/401) accepted the vaccination at the medical outpatient clinic. Of those, 52% were advised by the medical student before consultation, 26% came spontaneously to the nurse for influenza vaccination, and 19% came with advice from their physicians. The rate of refusal was 9% (see Table 2 for details).
Changes in rates of vaccination coverage of patients regularly followed at the medical outpatient clinic in 1998 and 1999
In 1998, 195 patients 65 years and older regularly followed at the medical outpatient clinic were monitored.9 A reminder letter was sent to patients who did not have an appointment during the vaccination period (73/195 patients). About one fifth (15/73) came for vaccination after the reminder letter. Overall, 48% (93/195) were vaccinated in 1998.
In 1999, 325 of 401 patients 65 years and older were regularly followed by a resident-physician. The larger number in 1999 (when compared 195 patients in 1998) was due to the fact that, in 1998, only those who followed in 1997 were monitored. Among the 325 patients, 268 came to the office during the vaccination period; 57 persons regularly followed did not have an appointment during the vaccination period and were sent a reminder letter. Overall, 76% of patients regularly followed (246/325) were vaccinated in 1999. Of those, 65% were advised by the medical student. The rate of refusal was 11%. Only 2 patients in the targeted population were missed during the target period, and 2 patients had medical contraindications for vaccination. Thus, the new strategy led to a relative increase of 58% for vaccination coverage (from 48% to 76%; P < .0001, Yates correction).
Rate of vaccination coverage in family physicians’ offices in 1999
A total of 598 patients 65 years and older attended their family physicians’ offices during the vaccination period. The median age was 74 years (range, 65-99 years) and most were female (62%). Eighty-three percent accepted the vaccination at the family physicians’ offices, 3% wished to be vaccinated somewhere else, and 14% refused.
Reasons for nonvaccination
The rates of refusal were 14% in the family physician’s offices and 9% in the medical outpatient clinic. Reasons for nonvaccination in the medical outpatient clinic were obtaining the influenza vaccination elsewhere (5%) and medical contraindications (1%; Table 2).
Discussion
Our study demonstrated that the systematic intervention of a paramedical person before the doctor’s consultation can lead to a considerable improvement in vaccination coverage in an ambulatory setting. A 58% relative increase (from 48% to 76%) over 1 year in the same institution has never been achieved. This suggested that failure of the physician to propose vaccination is an important reason for low vaccination coverage of high-risk patients in teaching institutions, where physician turnover is high. Thanks to our organizational strategy, we reached an 85% vaccination coverage among all outpatient attendees, which far surpassed that achieved by an ongoing educational program or alternative strategies such as reminder letters.
Until now, the highest coverage ever reported in Europe in persons 65 years and older was 82% in a study in Finland, where the investigators used an age-based strategy, with free vaccines and personnel-mailed reminders.10 Even when efficacious11 and cost effective,12 these strategies are insufficiently used.4 Other means to improve vaccination coverage such as centralized planning identification through computerized enrollment files at central registry and immunization clinics have improved vaccination coverage rates to almost 80% among chronically ill seniors in the United States.13
Two American studies showed that approaches incorporating administrative and organizational measures were more successful in improving vaccination rates than the education of providers. A pilot study14 and a 10-year follow-up15 done in the Minneapolis Department of Veterans Affairs Medical Center used a strategy including annual educational and publicity mailing to patients, walk-in clinics for vaccine administration, standing orders for nurses, and use of standardized patient information and medical record documentation forms. The follow-up study showed a remarkable improvement in the coverage rate, which reached 92% in 1996-1997 in specific groups. Another study done in the University Department of Emergency Medicine of Chicago that used standing orders for nurses at triage was only partly successful, with 47% coverage.16
Our study adds to the knowledge gathered in the United States, where administrative and organizational strategies (standing orders for nurses) improved vaccination coverage significantly.14-16 These studies were conducted in a different population sample, including hospitalized patients and other high-risk groups, which is quite different from the situation in general practice. Comparing vaccination coverage rates between different studies is always hazardous because of differences in inclusion criteria and reporting biases. The highest vaccination coverage ever achieved was 92%, but the method used for the rate estimation was a mailed survey where vaccinated persons may have been more likely to respond than nonvaccinated ones.15 The investigators exported their program from a teaching hospital to a community outpatient setting, with an increase in coverage from 56% to 72% in 1 clinic and no significant increase in the other clinic, which shows that several factors should be considered when a new strategy is implemented (staff, motivation, etc).17 The relatively low coverage (47%) reached in another study conducted in an emergency department was due in part to the fact that the nurse dedicated to providing vaccination was diverted from the preventive task of caring for patients when the workload increased. In addition, only one fourth of the emergency attendees was screened.16
To our knowledge, this is the first time that such an active strategy has been reported and applied in family physicians’ offices. The implementation in private practices documents its feasibility and effectiveness in those settings. The consistent coverage rates obtained in the medical outpatient clinic and the general practices (85% and 83%, respectively) confirmed the usefulness of the strategy, whatever the patient population and the medical staff involved. One may argue that patients had little choice between accepting or actively refusing the vaccination. We hope that the quality of information provided to the elderly, the possibility for them to ask specific questions that come to mind, and even to delay the decision until they can discuss the decision further with the doctor balances the potential enforcement.
The main disadvantage of our strategy is the need for an additional person in the outpatient clinic. The direct cost of an extra person in public service can be justified if it decreases the indirect costs for society, which is the case for influenza vaccination.12 More pragmatically, at the institutional level, the additional income generated by the increase of vaccine sales outweighed the salary of the medical student (about US$100/day for a total of 40 days, ie, 20 working days for 2 months). The implementation in the family physicians’ offices showed that such a procedure can work in a small setting without additional staff. Counseling about vaccination represents an additional task for the paramedical personnel, but it certainly provides more credit to the overall work and improves the therapeutic network. Also, because the vaccination has to be proposed, the time for discussion has to be taken by whoever is the most willing to do the job.
Some doctors may feel that this strategy excludes them from important duties. We must face the reality that physicians often do not accomplish their tasks properly. With our procedure, they remain informed about the vaccination status of their patients and maintain their important role in counseling patients who cannot decide about the vaccination or when problems arise.
One can argue that the increase in vaccination coverage rate was due, at least in part, to external factors such as media propaganda, and that the new strategy played only a marginal role. Of course, we cannot exclude such an influence, but it certainly unlikely was to be the cause of a 58% increase in coverage rate; indeed, no particular campaign was launched in 1999 and no change in coverage rate between 1998 and 1999 was observed in a similar institution in the same area. We chose to use historical controls because we were convinced that the new strategy would dramatically improve vaccination in the elderly and felt therefore that it was unethical not to offer this procedure to all our patients. Moreover, the main interest of the study was to investigate the maximum coverage rate that could be expected with a very active intervention and to estimate precisely the rate of true refusals, so that defined objectives in terms of coverage rates can be set in similar institutions.
The new strategy assessed in this study is in line with the recommendations of the Advisory Committee on Immunization Practices, published in March 2000, that promoted the use of standing orders to improve adult vaccination delivery.18 Evidence of effectiveness of such programs is increasing. They could be adapted to other preventive measures to improve delivery of those services, and they could be used to improve outpatient clinic efficiency by reducing pressures on physicians.
Acknowledgments
We thank M. Cheseaux and S. Martin, the medical students responsible for providing vaccine information and delivery and data entry, the resident-physicians at medical outpatient clinic, and the nurses and receptionists in the medical outpatient clinic and the family physicians’ practices.
BACKGROUND: Vaccination coverage for influenza in the elderly remains low when the physician is the only person responsible for immunization. Integration of other health care workers may improve the coverage rate of at-risk groups.
OBJECTIVES: To estimate vaccination coverage rate by using a strategy based on the systematic intervention of a health care professional proposing vaccination before the doctor’s consultation, to evaluate the changes in coverage rates before and after introduction of this strategy, and to assess the feasibility of this intervention and the achieved coverage rate in family physician offices
STUDY DESIGN: Prospective study in a medical outpatient clinic and 5 family physician practices in Switzerland.
POPULATION: Participants consisted of all patients 65 years or older attending a medical outpatient clinic during the vaccination period in 1999 (n = 401), patients 65 years or older regularly followed at a medical outpatient clinic in 1998 and 1999 (n = 195), and patients 65 years or older presenting to 5 family physician offices in 1999 (n = 598).
OUTCOME MEASURED: Rates of vaccination coverage.
RESULTS: Among all participants, vaccination coverage rates in 1999 were 85% at the medical outpatient clinic and 83% in family physician offices. Among participants regularly followed at the medical outpatient clinic, vaccination coverage increased from 48% in 1998 to 76% in 1999. Rates of refusal were 9% at the medical outpatient clinic and 14% in the family physician offices.
CONCLUSIONS: The systematic intervention of a health care professional to suggest vaccination before the doctor’s visit is an effective measure to achieve high coverage rate. Such a strategy also improves outpatient clinic or private practice efficiency by reducing pressures on physicians.
Annual influenza vaccination is recommended for all persons 65 years and older.1-3 Unfortunately, coverage rate remains low. In Switzerland during the winter season of 1998-1999, estimated vaccination coverage was only 8% in the general population.4 In institutionalized elderly patients, coverage was 37% in the same study. In 1994, the rate estimated from a telephonic survey was only 36% in elderly patients in Geneva.5 Since then, an active promotional campaign among the public led to a coverage rate among persons older than 65 years of about 60% (L. Toscani, personal communication 2000), a rate that approaches that in the United States (67%).6
Apart from making the general population or the target groups aware of the importance of the vaccination to prevent influenza complications, a strategy commonly used to improve coverage consists of training the physicians. However, their knowledge about vaccination does not always explain their behavior; although doctors know that vaccines are efficacious and are convinced that they should offer vaccination to all at-risk patients, they do not propose it to all eligible patients.7 Reimbursement of vaccines, as done since 1996 in Switzerland, does not seem to increase vaccination rates.8
At the Medical Outpatient Clinic, University of Lausanne, we recorded the influenza vaccination coverage rate of patients 65 years and older who were followed regularly in 1997, after an intensive education of physicians including a state-of-the-art lecture and interactive seminars; the same was done in 1998, but letters also were sent to all patients who did not have an appointment during the vaccination period. The vaccination coverage increased from 39% in 1997 to 47% in 1998, presumably because of the reminder letter.9 This rate was still unsatisfactory. We postulated that the main reason for low rate of influenza vaccination coverage of elderly patients was the physicians’ omission to propose the vaccination rather than the patients’ refusal. To test this hypothesis and to improve the coverage at the same time, we introduced at the medical outpatient clinic a strategy coupling a systematic intervention of a medical person allowed to do injections (medical student) with the existing educational program and reminder letters. A similar method (except for the reminder letters) was applied in 5 general practices, with the receptionists providing the information and the paramedical staff performing the vaccination.
The specific objectives were (1) to estimate vaccination coverage rate by using a strategy based on the systematic intervention of a health care professional proposing vaccination before the doctor’s consultation, (2) to evaluate the changes in coverage rates before and after introduction of the strategy, and (3) to assess the feasibility of this intervention and the achieved coverage rate in family physicians’ offices.
Methods
The protocol was approved by the ethical committee of the Department of Internal Medicine, University Hospital, Lausanne. Table 1 summarizes the study profile, populations, strategy applied, and outcome measures that are described below. The study took place in the Medical Outpatient Clinic, University of Lausanne, which provides medical care to the general population (attendees are biased toward young people, refugees, foreigners, and elderly individuals with low incomes); and in 1 rural general practice office (Orbe) and 2 urban general practice offices (Neuchâtel), with 5 physicians.
Study populations
To estimate vaccination coverage rate after implementing the new strategy in the medical outpatient clinic (objective 1), we included all patients 65 years and older attending the 1999 vaccination period (401 patients). To evaluate the changes in coverage rates before and after the introduction of the strategy (objective 2), we included patients 65 years and older who were followed regularly at medical outpatient clinic in 1998 and 1999 (195 patients). To assess the feasibility of this intervention and the achieved coverage rate in family physicians’ offices, we included all patients 65 years and older attending family physicians’ offices in 1999 (598 patients).
Medical outpatient clinic procedures
Pre-intervention season (1998). During the vaccination period (mid-October to mid-December), all patients 65 years and older presented to the receptionist and then waited until they were seen by a first to fifth-year resident physician who proposed the vaccination during the consultation. The injection was then done by a nurse. Alternatively, anyone could go straight to the nurse (walk-in clinic) and ask for an influenza shot, which was done after checking that there was no contraindication (egg allergy, fever during the past 2 days, or use of anticoagulants, in which case subcutaneous injection was performed). To check that all patients regularly followed at the medical outpatient clinic had made contact during the vaccination period, an independent registrar in mid-November reviewed all files of patients 65 years and older. For those regularly followed, he checked whether an appointment had been made; if not, a reminder letter was sent to the patient.
Intervention season (1999). During the vaccination period, all patients 65 years and older were told by the receptionist that a medical student would propose an influenza vaccination before seeing the doctor. The medical student informed the patients about influenza complications and prevention by vaccination, and asked them whether they had been vaccinated in the previous year. The patient then had to decide whether to be vaccinated immediately, discuss the vaccination with the doctor first, or refuse the vaccination. In case of refusal, the reason was investigated. The patient who agreed to the vaccination received the injection from the medical student and a label was attached to the patient’s file to inform the doctor about the vaccination status. The same procedure performed in 1998 was applied for the reminder letter
General practice procedures
During the intervention season only (1999), the receptionist at the admission desk proposed influenza vaccination to all patients 65 years and older and the paramedical staff promptly performed the injection, if the patient agreed. The same information and questionnaires were applied in the family physician’s offices and the medical outpatient clinic.
Outcomes
Several outcomes were predefined: (1) vaccination coverage rate of all patients 65 years and older who attended the medical outpatient clinic spontaneously or on appointment during the 1999 vaccination period; (2) vaccination coverage rate of patients 65 years and older regularly followed (excluding those coming only once without a follow-up) at the medical outpatient clinic in 1998 and 1999 (all were contacted by a reminder letter, if not seen during vaccination period); (3) vaccination coverage rate of all patients 65 years and older who attended the family physicians’ offices during the 1999 vaccination period; and (4) reasons for nonvaccination among those interviewed.
Data management and analysis
The data were entered immediately into Epi Info 6.0 and analyzed with SPSS 7.5. Testing for differences between the 1998 and 1999 rates in the medical outpatient clinic was done with chi-square tests.
Results
Rate of vaccination coverage at the medical outpatient clinic during the intervention period
A total of 401 patients 65 years and older came to the outpatient clinic during the vaccination period in 1999. The median age was 74 years (range, 65-97 years) and most patients were male (56%). Table 2 shows that 85% (341/401) accepted the vaccination at the medical outpatient clinic. Of those, 52% were advised by the medical student before consultation, 26% came spontaneously to the nurse for influenza vaccination, and 19% came with advice from their physicians. The rate of refusal was 9% (see Table 2 for details).
Changes in rates of vaccination coverage of patients regularly followed at the medical outpatient clinic in 1998 and 1999
In 1998, 195 patients 65 years and older regularly followed at the medical outpatient clinic were monitored.9 A reminder letter was sent to patients who did not have an appointment during the vaccination period (73/195 patients). About one fifth (15/73) came for vaccination after the reminder letter. Overall, 48% (93/195) were vaccinated in 1998.
In 1999, 325 of 401 patients 65 years and older were regularly followed by a resident-physician. The larger number in 1999 (when compared 195 patients in 1998) was due to the fact that, in 1998, only those who followed in 1997 were monitored. Among the 325 patients, 268 came to the office during the vaccination period; 57 persons regularly followed did not have an appointment during the vaccination period and were sent a reminder letter. Overall, 76% of patients regularly followed (246/325) were vaccinated in 1999. Of those, 65% were advised by the medical student. The rate of refusal was 11%. Only 2 patients in the targeted population were missed during the target period, and 2 patients had medical contraindications for vaccination. Thus, the new strategy led to a relative increase of 58% for vaccination coverage (from 48% to 76%; P < .0001, Yates correction).
Rate of vaccination coverage in family physicians’ offices in 1999
A total of 598 patients 65 years and older attended their family physicians’ offices during the vaccination period. The median age was 74 years (range, 65-99 years) and most were female (62%). Eighty-three percent accepted the vaccination at the family physicians’ offices, 3% wished to be vaccinated somewhere else, and 14% refused.
Reasons for nonvaccination
The rates of refusal were 14% in the family physician’s offices and 9% in the medical outpatient clinic. Reasons for nonvaccination in the medical outpatient clinic were obtaining the influenza vaccination elsewhere (5%) and medical contraindications (1%; Table 2).
Discussion
Our study demonstrated that the systematic intervention of a paramedical person before the doctor’s consultation can lead to a considerable improvement in vaccination coverage in an ambulatory setting. A 58% relative increase (from 48% to 76%) over 1 year in the same institution has never been achieved. This suggested that failure of the physician to propose vaccination is an important reason for low vaccination coverage of high-risk patients in teaching institutions, where physician turnover is high. Thanks to our organizational strategy, we reached an 85% vaccination coverage among all outpatient attendees, which far surpassed that achieved by an ongoing educational program or alternative strategies such as reminder letters.
Until now, the highest coverage ever reported in Europe in persons 65 years and older was 82% in a study in Finland, where the investigators used an age-based strategy, with free vaccines and personnel-mailed reminders.10 Even when efficacious11 and cost effective,12 these strategies are insufficiently used.4 Other means to improve vaccination coverage such as centralized planning identification through computerized enrollment files at central registry and immunization clinics have improved vaccination coverage rates to almost 80% among chronically ill seniors in the United States.13
Two American studies showed that approaches incorporating administrative and organizational measures were more successful in improving vaccination rates than the education of providers. A pilot study14 and a 10-year follow-up15 done in the Minneapolis Department of Veterans Affairs Medical Center used a strategy including annual educational and publicity mailing to patients, walk-in clinics for vaccine administration, standing orders for nurses, and use of standardized patient information and medical record documentation forms. The follow-up study showed a remarkable improvement in the coverage rate, which reached 92% in 1996-1997 in specific groups. Another study done in the University Department of Emergency Medicine of Chicago that used standing orders for nurses at triage was only partly successful, with 47% coverage.16
Our study adds to the knowledge gathered in the United States, where administrative and organizational strategies (standing orders for nurses) improved vaccination coverage significantly.14-16 These studies were conducted in a different population sample, including hospitalized patients and other high-risk groups, which is quite different from the situation in general practice. Comparing vaccination coverage rates between different studies is always hazardous because of differences in inclusion criteria and reporting biases. The highest vaccination coverage ever achieved was 92%, but the method used for the rate estimation was a mailed survey where vaccinated persons may have been more likely to respond than nonvaccinated ones.15 The investigators exported their program from a teaching hospital to a community outpatient setting, with an increase in coverage from 56% to 72% in 1 clinic and no significant increase in the other clinic, which shows that several factors should be considered when a new strategy is implemented (staff, motivation, etc).17 The relatively low coverage (47%) reached in another study conducted in an emergency department was due in part to the fact that the nurse dedicated to providing vaccination was diverted from the preventive task of caring for patients when the workload increased. In addition, only one fourth of the emergency attendees was screened.16
To our knowledge, this is the first time that such an active strategy has been reported and applied in family physicians’ offices. The implementation in private practices documents its feasibility and effectiveness in those settings. The consistent coverage rates obtained in the medical outpatient clinic and the general practices (85% and 83%, respectively) confirmed the usefulness of the strategy, whatever the patient population and the medical staff involved. One may argue that patients had little choice between accepting or actively refusing the vaccination. We hope that the quality of information provided to the elderly, the possibility for them to ask specific questions that come to mind, and even to delay the decision until they can discuss the decision further with the doctor balances the potential enforcement.
The main disadvantage of our strategy is the need for an additional person in the outpatient clinic. The direct cost of an extra person in public service can be justified if it decreases the indirect costs for society, which is the case for influenza vaccination.12 More pragmatically, at the institutional level, the additional income generated by the increase of vaccine sales outweighed the salary of the medical student (about US$100/day for a total of 40 days, ie, 20 working days for 2 months). The implementation in the family physicians’ offices showed that such a procedure can work in a small setting without additional staff. Counseling about vaccination represents an additional task for the paramedical personnel, but it certainly provides more credit to the overall work and improves the therapeutic network. Also, because the vaccination has to be proposed, the time for discussion has to be taken by whoever is the most willing to do the job.
Some doctors may feel that this strategy excludes them from important duties. We must face the reality that physicians often do not accomplish their tasks properly. With our procedure, they remain informed about the vaccination status of their patients and maintain their important role in counseling patients who cannot decide about the vaccination or when problems arise.
One can argue that the increase in vaccination coverage rate was due, at least in part, to external factors such as media propaganda, and that the new strategy played only a marginal role. Of course, we cannot exclude such an influence, but it certainly unlikely was to be the cause of a 58% increase in coverage rate; indeed, no particular campaign was launched in 1999 and no change in coverage rate between 1998 and 1999 was observed in a similar institution in the same area. We chose to use historical controls because we were convinced that the new strategy would dramatically improve vaccination in the elderly and felt therefore that it was unethical not to offer this procedure to all our patients. Moreover, the main interest of the study was to investigate the maximum coverage rate that could be expected with a very active intervention and to estimate precisely the rate of true refusals, so that defined objectives in terms of coverage rates can be set in similar institutions.
The new strategy assessed in this study is in line with the recommendations of the Advisory Committee on Immunization Practices, published in March 2000, that promoted the use of standing orders to improve adult vaccination delivery.18 Evidence of effectiveness of such programs is increasing. They could be adapted to other preventive measures to improve delivery of those services, and they could be used to improve outpatient clinic efficiency by reducing pressures on physicians.
Acknowledgments
We thank M. Cheseaux and S. Martin, the medical students responsible for providing vaccine information and delivery and data entry, the resident-physicians at medical outpatient clinic, and the nurses and receptionists in the medical outpatient clinic and the family physicians’ practices.
1. WHO Influenza vaccination recommendations. Wkly Epidemiol Rec 1999;74:57-64.
2. Working Group Influenza. Recommendations for influenza vaccination during the 1999/2000 season. Bull OFSP 1999;39:737-9.
3. CDC Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 1998;47:1-26.
4. Ammon CE. Vaccination against influenza in Switzerland. A survey in health facilities. Bull OFSP 1999;4:62-4.
5. Gauthey L, Toscani L, Chamot E, Larequi T, Robert CF. Influenza vaccination coverage in the geriatric population of the State of Geneva, Switzerland. Eur J Pharmacol 1999;9:36-40.
6. Centers for Disease Control and Prevention. Influenza and pneumococcal vaccination levels among persons aged 65 years-United States, 1999. MMWR Morb Mortal Wkly Rep 2001;50:532-7.
7. McKinney WP, Barnas GP. Influenza immunization in the elderly: knowledge and attitudes do not explain physician behavior. Am J Pharmacol 1989;79:1422-4.
8. Etkind P, Simon M, Shannon S, et al. Impact of the Medicare Influenza Demonstration Project on influenza vaccination in a county in Massachusetts, 1988-1992. Community Health 1996;21:199-209.
9. Zorzoli M, Favrat B, D’Acremont V, Pécoud A, Genton B. Strategies to improve vaccination coverage among elderly patients. J Gen Intern Med 1999;14:83.-
10. Honkanen PO, Keistinen T, Kivela SL. The impact of vaccination strategy and methods of information on influenza and pneumococcal vaccination coverage in the elderly population. Vaccine 1997;15:317-20.
11. Armstrong K, Berlin M, Schwartz S, Propert K, Ubel P. Educational content and the effectiveness of influenza vaccination reminders. J Gen Intern Med 1999;14:695-7.
12. Baker AM, McCarthy B, Gurley VF, Yood MU. Influenza immunization in a managed care organization. J Gen Intern Med 1998;13:469-75.
13. Pearson DC, Jackson LA, Wagener B, Sarver L. A comprehensive influenza campaign in a managed care setting. Vaccine 1998;16:1718-21.
14. Margolis KL, Lofgren RP, Korn JE. Organizational strategies to improve influenza vaccine delivery. A standing order in a general medicine clinic. Arch Intern Med 1988;148:2205-7.
15. Nichol KL. Ten-year durability and success of an organized program to increase influenza and pneumococcal vaccination rates among high-risk adults. Am J Med 1998;105:385-92.
16. Slobodkin D, Kitlas J, Zielske P. Opportunities not missed systematic influenza and pneumococcal immunization in a public inner-city emergency department. Vaccine 1998;16:1795-802.
17. Margolis KL, Nichol KL, Wuorenma J. Exporting a successful influenza vaccination program from a teaching hospital to a community outpatient setting. J Am Geriatr Soc 1992;40:1021-3.
18. CDC Adult immunization programs in nontraditional settings: quality standard and guidance for program evaluation. A report of the national Vaccine Advisory Committee and Use of standing orders programs to increase adult vaccination rates. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2000;49:18-27.
Address reprint requests to Blaise Genton, MD, PhD, Policlinique Médicale Universitaire, César Roux 19, 1005 Lausanne, Switzerland. E-mail: [email protected].
To submit a letter to the editor on this topic, click here: [email protected].
1. WHO Influenza vaccination recommendations. Wkly Epidemiol Rec 1999;74:57-64.
2. Working Group Influenza. Recommendations for influenza vaccination during the 1999/2000 season. Bull OFSP 1999;39:737-9.
3. CDC Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 1998;47:1-26.
4. Ammon CE. Vaccination against influenza in Switzerland. A survey in health facilities. Bull OFSP 1999;4:62-4.
5. Gauthey L, Toscani L, Chamot E, Larequi T, Robert CF. Influenza vaccination coverage in the geriatric population of the State of Geneva, Switzerland. Eur J Pharmacol 1999;9:36-40.
6. Centers for Disease Control and Prevention. Influenza and pneumococcal vaccination levels among persons aged 65 years-United States, 1999. MMWR Morb Mortal Wkly Rep 2001;50:532-7.
7. McKinney WP, Barnas GP. Influenza immunization in the elderly: knowledge and attitudes do not explain physician behavior. Am J Pharmacol 1989;79:1422-4.
8. Etkind P, Simon M, Shannon S, et al. Impact of the Medicare Influenza Demonstration Project on influenza vaccination in a county in Massachusetts, 1988-1992. Community Health 1996;21:199-209.
9. Zorzoli M, Favrat B, D’Acremont V, Pécoud A, Genton B. Strategies to improve vaccination coverage among elderly patients. J Gen Intern Med 1999;14:83.-
10. Honkanen PO, Keistinen T, Kivela SL. The impact of vaccination strategy and methods of information on influenza and pneumococcal vaccination coverage in the elderly population. Vaccine 1997;15:317-20.
11. Armstrong K, Berlin M, Schwartz S, Propert K, Ubel P. Educational content and the effectiveness of influenza vaccination reminders. J Gen Intern Med 1999;14:695-7.
12. Baker AM, McCarthy B, Gurley VF, Yood MU. Influenza immunization in a managed care organization. J Gen Intern Med 1998;13:469-75.
13. Pearson DC, Jackson LA, Wagener B, Sarver L. A comprehensive influenza campaign in a managed care setting. Vaccine 1998;16:1718-21.
14. Margolis KL, Lofgren RP, Korn JE. Organizational strategies to improve influenza vaccine delivery. A standing order in a general medicine clinic. Arch Intern Med 1988;148:2205-7.
15. Nichol KL. Ten-year durability and success of an organized program to increase influenza and pneumococcal vaccination rates among high-risk adults. Am J Med 1998;105:385-92.
16. Slobodkin D, Kitlas J, Zielske P. Opportunities not missed systematic influenza and pneumococcal immunization in a public inner-city emergency department. Vaccine 1998;16:1795-802.
17. Margolis KL, Nichol KL, Wuorenma J. Exporting a successful influenza vaccination program from a teaching hospital to a community outpatient setting. J Am Geriatr Soc 1992;40:1021-3.
18. CDC Adult immunization programs in nontraditional settings: quality standard and guidance for program evaluation. A report of the national Vaccine Advisory Committee and Use of standing orders programs to increase adult vaccination rates. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2000;49:18-27.
Address reprint requests to Blaise Genton, MD, PhD, Policlinique Médicale Universitaire, César Roux 19, 1005 Lausanne, Switzerland. E-mail: [email protected].
To submit a letter to the editor on this topic, click here: [email protected].
Candida albicans Intralesional Injection Immunotherapy of Warts (See Erratum 2002;70:294.)
Stage-matched nutrition guidance for patients at elevated risk for cardiovascular disease: A randomized intervention study in family practice
OBJECTIVE: To examine stage-matched nutrition counseling by family physicians and its effect on dietary intake, anthropometry, and serum lipid levels in patients at elevated risk for cardiovascular disease.
METHODS: In this controlled trial, patients randomized to intervention practices received nutrition information following the Stages-of-Change Model, and patients randomized to control practices received usual care.
RESULTS: At both 6 and 12 months after baseline, total fat intake and saturated fat intake declined significantly more in the intervention group than in the control group: -5.7% and -2.6% of energy, respectively, at 6 months, and -3.6% and -1.7% of energy, respectively, at 12 months. For energy intake, body weight, and BMI, there were significant differences between groups only at 6 months: -0.8 megajoules (MJ), -0.7 kg, and -0.3 kg/m2, respectively. None of the serum lipid values changed significantly between groups at 12 months.
CONCLUSIONS: Nutritional counseling based on stages of change led to reductions in dietary fat intake and weight loss in the short term. However, we found no corresponding changes in serum lipid concentrations.
- Family physicians can select patients for nutrition counseling by a dietician by using a simple questionnaire based on the Stages-of-Change Model.
- With dietary intervention, a decline in fat intake was sustained at 1 year in a population with a high percentage of poorly educated subjects.
- We found no changes in serum lipids after 1 year of dietary intervention.
Cholesterol-lowering diet therapy is an important part of cardiovascular disease prevention.1 Family physicians (FPs) are uniquely positioned to provide nutrition information to persons at risk because of their expertise as perceived by consumers, and because they reach into nearly all segments of the population.2 It is important to build nutrition guidance into a model that enables change, and, considering the number of contacts between FP and patient,3,4 that is linked to the FP’s continuity of care over time.5 FPs can raise patient consciousness about dietary behavior, motivate patients to change their behavior, and, when appropriate, refer patients to a dietician or patient associations.6
The Transtheoretical Model of Behavior Change is increasingly being used to examine health behavior change.7 According to this model, people are assigned to 1 of the following 5 stages on the basis of their behavior and current intention for future action: (i) precontemplation, not even considering changing one’s behavior; (ii) contemplation, thinking about it; (iii) preparation, making definite plans to change; (iv) action, initiating behavior change; and (v) maintenance, maintaining desired behaviors.8
Evidence available on dietary applications studied in cross-sectional and not stage-matched intervention studies is sufficiently encouraging to warrant the inclusion of Transtheoretical Model constructs in prospective studies.7 As far as we know, only 2 stage-matched dietary intervention studies have been published. Both studies were carried out in primary care settings and both studies showed that tailored nutrition information is effective in dietary fat reduction.9,10 However, in these studies the intervention was not managed by the FP. We think the FP is the most appropriate person to manage such intervention in the family practice. Therefore, we conducted a controlled dietary intervention, based on the Stages-of-Change Model and managed by the FP with selective referral to a dietician. We examined the effects of dietary counseling on changes in dietary intake, anthropometry, and serum lipid levels in patients at elevated cardiovascular risk.
Methods
Participants and design
In this randomized controlled trial, men and women at elevated risk for cardiovascular disease were recruited from the 9 family practices joining the Nijmegen Monitoring Project, the research network of the Department of Family Medicine, University Medical Centre St. Radboud.11 Selection and flow of participants are described in Figure 1. Seventy-one patients were initially included in the intervention group and 72 patients in the control group (Table 1). Consequently, we had enough power (0.90) to detect a difference in change between groups for total fat intake of 3% of energy and 11.6 mg% of serum total cholesterol.12
After selection and recruitment of participants, the family practices were randomly divided into intervention (4) and control (5) practices. The practices, and not the patients, were the units of randomization, to avoid contamination of the information between intervention and control groups. Patients in the control practices received usual care.13-15 Each patient in the intervention arm received nutrition information according to his or her stage of change. All participants signed an informed consent form before entering the study. The Medical Ethical Committee of the Department of Human Nutrition and Epidemiology, Wageningen University, approved the study protocol. The study lasted from August 1998 until April 2000.
TABLE 1
Baseline characteristics of patients in the intervention and control groups
| Intervention n=71 | Control n=72 | P1 | |
|---|---|---|---|
| Sex (%) | |||
| Male | 24 | 29 | 0.48 |
| Female | 76 | 71 | |
| Age (years) | 58.5±7.1 | 58.2±6.9 | 0.83 |
| Disorder (%) | |||
| Hypertension | 94 | 89 | 0.34 |
| Diabetes mellitus II | 6 | 7 | |
| Hypertension & diabetes mellitus II | 0 | 4 | |
| Marital status (%) | |||
| Single | 6 | 3 | 0.83 |
| Married/cohabiting | 82 | 86 | |
| Divorced | 1 | 1 | |
| Widowed | 11 | 10 | |
| Education (%)2 | |||
| Low | 68 | 68 | 0.91 |
| Intermediate | 20 | 18 | |
| High | 13 | 15 | |
| Family history of heart disease (%)3 | 27 | 22 | 0.81 |
| Smoking (%)4 | |||
| Not smoking | 79 | 78 | 0.70 |
| Light smoker | 13 | 10 | |
| Heavy smoker | 9 | 13 | |
| Exercise, more than 20 minutes | |||
| No exercise | 20 | 13 | 0.42 |
| Less than 3 times a week | 37 | 30 | |
| 3 times a week | 18 | 26 | |
| More than 3 times a week | 25 | 31 | |
| 1Two-sided P values for differences in baseline characteristics between intervention and control groups. | |||
| 2Low: primary school, lower level of secondary school, lower vocational training. Intermediate: higher level of secondary school, intermediate vocational training. High: higher vocational training, university. | |||
| 3First-degree relatives younger than 60 years. | |||
| 4Light smoker: 0-10 cigarettes a day, or smoking pipe or cigars. Heavy smoker: > 10 cigarettes a day. | |||
FIGURE 1
Selection and flow of participants
Measurements
Specially trained practice assistants measured anthropometry data, and presented patients with a self-administered questionnaire on demographics, medical history, food frequency, and a stages-of-change algorithm at baseline, 6 months, and 12 months. Blood samples were taken at baseline and at 12 months.
Physical assessment. Anthropometry consisted of body weight to the nearest 0.5 kg, height, and waist and hip circumferences to the nearest 0.5 cm. Patients wore no shoes and only light clothing when weighed. Fasting blood samples were taken twice per measurement period at a 1-week interval, with the patient in the sitting position. The samples were stored at -80°C. Lipids were analyzed enzymatically for total cholesterol,16 HDL cholesterol,17 LDL cholesterol, and triglycerides18 with the Cobas Intergra 700 (Roche Diagnostics, Switzerland), at the laboratory of the Canisius Wilhelmina Hospital (Nijmegen, The Netherlands). The coefficient of variation within runs was 2.3% for total cholesterol, 1.6% for HDL cholesterol, and 1.8% for triglycerides. The LDL cholesterol level was calculated using the equation of Friedewald et al.19
Questionnaires. The questionnaire asked for demographic data, family history of heart disease, smoking status, physical activity, drug use, and diet history at baseline. At follow-up we checked for changes in smoking status, physical activity, and drug use. Patients in the control group were asked if they had visited a dietician during the study period. The intake of energy, total fat, fatty acids, and cholesterol during the preceding 4 weeks was assessed by asking patients to fill out a food frequency questionnaire that included 104 food items. The questionnaire was validated20 and recently revised according to the Dutch National Food Survey 1992.21 Dieticians carried out nutrient calculations with a computerized version of the Dutch food composition tables22 phoned patients in cases of inconsistency.
Stages of change for reduction of fat intake were assessed with a 4-item algorithm based on measures used in previous studies23,23 in combination with the results of the food frequency questionnaire. According to the algorithm, participants were judged to be in precontemplation if they did not consider their diet to be low in fat, they were not in the process of cutting down on fat, and they had no intention of reducing their fat consumption. Participants were considered to be in the contemplation phase if they intended to decrease their fat intake within 6 months but not within 30 days, and to be in preparation when they intended to decrease their fat intake within 30 days. Participants who reported they were currently trying to eat less fat were classified as in action, and participants who reported they had been eating less fat for at least 6 months were classified in maintenance. If participants in maintenance consumed 37% total fat or 12% saturated fat expressed as percent of energy intake, they were reclassified in precontemplation.
After completing the study, all patients filled in an evaluation questionnaire. Patients in the control group were also asked about which nutrition information they had received during the last year.
Intervention
The intervention consisted of nutrition counseling based on stages of change, directed by the FP with selective referral to a dietician. FPs were supported by a protocol that included Prochaska’s8 processes of change. Preparation and action stages were considered 1 stage (action stage) in this study, given the required nutrition education. (Figure 2) summarizes the intervention procedure: 1) counseling aimed at raising consciousness about dietary behavior in the precontemplation stage, 2) motivation to change dietary behavior in the contemplation stage, and 3) if a patient decided to change (action stage), information about practical aspects of dietary change and discussion of referral to a dietician. The intervention was conducted by the patient’s own FP. All patients were referred to the same dietician. Protocols for the FPs and the dietician had been developed and tested prior to the study and were discussed by FPs and the dietician in pre-study group sessions.
FIGURE 2
Intervention scheme with clarification
Data analyses
Differences between groups at baseline and follow-up were tested with unpaired t-tests for continuous variables and with chi-square tests for categorized variables. If the number of observations within 1 cell was less then 5, a Fisher’s exact test was used instead of a chi-square test. Differences within subjects were tested with a paired t-test. P values less than 0.05 were considered significant. Because of clustering of patients within practices, a multilevel analysis was also carried out (level 1 patient, level 2 practice). All analyses were performed on the basis of intention to treat. SAS version 6.12 was used for the statistical analyses (SAS Institute Inc., Cary, NC, USA).
Results
Study population
The study sample was predominantly female (73%), poorly educated (68%), with an average age of 58 years (see Table 1) for definitions of educational level). Of the cardiovascular risk factors, hypertension was present in 92% of participants, type 2 diabetes mellitus in 6%, both disorders in 2%, and a family history of heart disease in 25%. No significant differences were found between the intervention group (n=71) and the control group (n=72) (Table 1). Table 2 demonstrates that the 2 study groups also showed comparable baseline measures according to dietary intake, anthropometry, and serum lipid levels. The mean BMI of the total group of subjects was 28.7 kg/m 2 ; 83% had a BMI higher than 25 and the majority had high total cholesterol and dietary fat intake.
Intervention-related measures
At baseline, 51% of the patients in the intervention group were classified in the precontemplation stage, 24% in the contemplation stage, and 25% in the action stage. They had consulted their FP once (n=53) or twice (n=18) before they were referred to the study dietician (n=60). Eleven patients were not referred to the dietician because they did not reach the action stage. All of the referred patients but one received 3 consultations with the dietician. In the control group, 24% of the patients discussed nutrition issues with their FP, 57% read nutrition brochures related to cardiovascular topics, and 1% (7) were referred to a dietician.
Changes at follow-up measurements
After 6 months (Table 2) total energy intake was reduced by 1.4 and 0.6 MJ in the intervention and control groups, respectively; total fat intake by 7.9% and 2.2 % of total energy, and saturated fat intake by 3.4% and 0.8% of total energy. The reductions were significantly larger in the intervention group, except for unsaturated fat. This was also reflected in risk factors: body weight and BMI declined significantly more in the intervention group (1.5 kg body weight) than in the control group (0.6 kg body weight). We found no significant differences between groups for waist circumference and waist-hip ratio.
The reduced fat intake in the intervention group was maintained at 12 months, although the differences were smaller. Changes in energy intake and anthropometric values at this time no longer differed significantly with multilevel analysis. During the 12 months of the study, slight reductions were found for serum total cholesterol (intervention group: 2.3 mg/dL, controls: 6.2 mg/dL), LDL cholesterol (intervention group: 6.2 mg/dL, controls: 7.7 mg/dL), and triglycerides (intervention group: 0.8 mg/dL, controls: 3.1 mg/dL). HDL cholesterol increased slightly in both groups (3.9 mg/dL in the intervention group, 2.7 mg/dL in the control group). However, none of these differences were significant. There were no significant changes in smoking or physical activity (P values of chi-square tests per measurement moment were >0.85), and none of the patients was prescribed a cholesterol-lowering drug. The significance of the P values of the differences in variables between the first and last measurement moment did not change when multilevel analysis was performed, except for body weight (Table 2).
TABLE 2
Baseline measures and changes after 6 months and 12 months in dietary intake and anthropometry
| At Baseline | At 6 months | At 12 months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | P* | Intervention | Control | P** | Intervention | Control | P** | P*** | |
| n=71 Mean ± SD | n=72 Mean ± SD | n=70 Mean ± SD | n=67 Mean ± SD | n=67 Mean ± SD | n=63 Mean ± SD | |||||
| Dietary intake (per day) | ||||||||||
| Total energy (MJ/d)1 | 9.1 ± 2.7 | 9.6 ± 2.6 | 0.25 | -1.4 ± 1.9† | -0.6 ± 1.8† | 0.01 | -0.7 ± 3.0 | -0.9 ± 2.4† | 0.09 | 0.00 |
| Total fat (% of energy) | 42.1 ± 6.3 | 42.6 ± 5.2 | 0.64 | -7.9 ± 6.5† | -2.2 ± 4.9† | 0.00 | -5.6 ± 6.9† | -2.0 ± 6.7† | 0.00 | 0.00 |
| Saturated fat (% of energy) | 15.2 ± 2.6 | 15.5 ± 2.3 | 0.42 | -3.4 ± 2.7† | -0.8 ± 2.2† | 0.00 | -2.6 ± 2.7† | -0.9 ± 2.6† | 0.00 | 0.00 |
| Monounsaturated fat (% of energy) | 14.6 ± 3.3 | 14.9 ± 2.6 | 0.53 | -3.4 ± 3.3† | -0.7 ± 2.4† | 0.00 | -1.9 ± 4.1† | -0.3 ± 3.3 | 0.01 | 0.00 |
| Unsaturated fat (% of energy) | 9.4 ± 3.0 | 9.3 ± 3.0 | 0.79 | -1.0 ± 3.1† | 0.8 ± 3.0† | 0.37 | -1.0 ± 2.7† | -0.7 ± 3.7 | 0.73 | 0.73 |
| Cholesterol (mg) | 239.1 ± 91.5 | 254.8 ± 90.8 | 0.31 | -62.0 ± 68.9† | -22.8 ± 66.4† | 0.00 | -46.4 ± 77.1† | -33.4 ± 83.1† | 0.02 | 0.03 |
| Anthropometry | ||||||||||
| Body weight (kg) | 79.2 ± 14.9 | 80.3 ± 12.0 | 0.63 | -1.3 ± 1.8† | -0.6 ± 1.9† | 0.01 | 0.2 ± 3.0 | -0.6 ± 2.8† | 0.02 | 0.06 |
| Body Mass Index (kg/m2) | 28.1 ± 4.3 | 29.2 ± 4.8 | 0.15 | -0.5 ± 0.6† | -0.2 ± 0.7† | 0.01 | 0.0 ± 1.1 | -0.2 ± 1.0† | 0.03 | 0.08 |
| Waist circumference (cm) | 94.3 ± 12.1 | 97.7 ± 10.3 | 0.08 | -1.6 ± 4.9† | -1.7 ± 5.2† | 0.43 | -1.6 ± 6.6† | -1.8 ± 5.6† | 0.61 | 0.86 |
| Waist-hip circumference ratio | 0.89 ± 0.07 | 0.90 ± 0.09 | 0.36 | -0.0 ± 0.04 | -0.01 ± 0.05† | 0.13 | 0.01 ± 0.05† | -0.02 ± 0.05† | 0.15 | 0.15 |
| 1 Joule=0.24 cal | ||||||||||
| *Two-sided P values for differences in baseline measures between intervention and control group. | ||||||||||
| **One-sided P value for difference in change from baseline between intervention and control group. | ||||||||||
| ***P value with multilevel analysis | ||||||||||
| †Significant difference in changes after 6 and 12 months compared to baseline within group (one-sided P value<0.05). | ||||||||||
DISCUSSION
Nutrition counseling by an FP based on the Stages-of-Change Model, with referral to a dietician in the action stage, successfully changed dietary behavior after 6 months in patients at elevated risk for cardiovascular disease. This success was accompanied by reductions in body weight. Differences in fat intake were sustained at 12 months, but this was not reflected in lower serum lipid concentrations. Initial reductions in energy intake and anthropometric values did not persist after 1 year. Our findings are in line with other dietary intervention studies in family practice that report improved dietary habits but no significant effect on objective cardiovascular risk factors such as body weight and blood lipids.10,25-27 The uniqueness of our study is that it is the first randomized controlled trial in family practice based on the Stages-of-Change Model in which nutrition counseling is managed by the FP.
The reductions we found in total serum cholesterol concentrations (0.9% in the intervention group and 2.3% in the control group after 12 months) were smaller than the 3%-6% suggested by a systematic review of individualized nutrition counseling in free-living subjects.28 The observed reduction in our study is also less than predicted by the Keys equation.29 We do not have a clear explanation for this. It is possible that patients in the intervention group gave more socially desirable answers to the food frequency questionnaire than patients in the control group, due to the more extensive nutrition guidance in the intervention group.
The dropout rate in our study was low: 91% of the patients completed the trial, a notable strength of the study. This may be due to the fact that the participating patients were recruited and treated by their own FP, and may in part account for the small effect size as we avoided the selective participation of those patients who were most motivated for change. The education level of the study sample was low compared with the Dutch population at similar age,30 and this could also have resulted in smaller differences between intervention and control groups. In addition, it has been found that CHD patients who are obese and do not use lipid lowering drugs are less likely to follow recommended cholesterol-lowering diets.31 However, all of these factors make our study representative of the circumstances FPs can expect when managing nutritional intervention in routine care for patients at elevated risk for cardiovascular disease. Nutritional counseling on the basis of the Transtheoretical Model of stages of change7 is effective in the short term, but it is disappointing to have to conclude that this effect appears to be temporary, with eventual rebound to pre-intervention status. No sustained effects on the target outcomes such as body weight and serum lipids were found, possibly due to the relatively short (1-year) observation period. Improved effectiveness might be achieved with the development of patient protocols and education materials that are better aimed at poorly educated persons, and with more extensive use of modern forms of communications to implement lasting changes.
Conclusions
Nutritional counseling based on stages of change in patients at elevated risk for cardiovascular disease, provided by an FP with referral to a dietician in the action stage, led to reductions in dietary fat intake in the short and long term and to weight loss in the short term. In the absence of long-term effects on serum cholesterol levels, the emphasis remains on treating elevated lipids with drugs. However, research on effective and inexpensive dietary interventions remains important because of promising results for the short term and the important advantages of such intervention. The emphasis for future research should be testing new methods to maintain (dietary) behavioral changes and to investigate differences in susceptibility between individuals with unhealthy lifestyles. The model based on stages of change seems well suited for this sort of intervention, and the experience of this study is that it can easily be incorporated into the routines of family practice at low cost. As such, it is a simple instrument for selecting patients who are willing to change their food habits. Further, we reached a high percentage of poorly educated people, who are particularly vulnerable.32 We recommend examining whether education materials need to be better aimed at people with a low socio-economic status. Long-term nutrition counseling is needed for maintenance and further improvements.
Acknowledgments
This research was supported by the Netherlands Heart Foundation under grant no. 97.106 and by Bayer. We are grateful to the staff of the NMP family practices and their patients, without whom this study would not have been possible. We extend special thanks to the dieticians José Veen and Els Siebelink and to all the research assistants, especially to Marjolein Homs.
1. Pyorala K. CHD prevention in clinical practice Lancet 1996;348 Suppl 1:s26-s28.
2. Hiddink GJ, Hautvast JG, van-Woerkum CM, Fieren CJ, van-’t-Hof MA. Consumers’ expectations about nutrition guidance: the importance of primary care physicians Am J Clin Nutr 1997;65:1974S-1979S.
3. Gray DP. Dietary advice in British General Practice Eur J Clin Nutr 1999;53 Suppl 2:S3-S8.
4. De Bakker D., Abrahamse H., Van den Hoogen H., Braspenning J., Van Althuis T, Rutten R. Jaarrapport LINH 1998: contactfrequenties en verrichtingen in het landelijk informatie netwerk huisartsenzorg (LINH). Utrecht/Nijmegen, The Netherlands: NIVEL/WOK; 1999.
5. Van-Weel C. Nutritional guidance in general practice—a conceptual framework Eur J Clin Nutr 1999;53 Suppl 2:S108-S111.
6. Hiddink GJ, Hautvast JG, van-Woerkum CM, Fieren CJ, van-’t-Hof MA. Nutrition guidance by primary-care physicians: perceived barriers and low involvement. Eur J Clin Nutr 1995;49:842-851.
7. Horwath CC. Applying the transtheoretical model to eating behaviour change: challenges and opportunities Nutrition Research Reviews. 1999;12:281-317.
8. Prochaska JO, DiClemente CC. In search of how people change: applications to addictive behaviors American Psychologist 1992;47:1102-1114.
9. Cambell MK, DeVellis B, Strecher V, Ammerman A, DeVellis R, Sandler R. Improving dietary behaviour: the effectiveness of tailored messages in primary care settings Am J Public Health 1994;84:783-787.
10. Steptoe A, Doherty S, Rink E, Kerry S, Kendrick T, Hilton S. Behavioural counselling in general practice for the promotion of healthy behaviour among adults at increased risk of coronary heart disease: randomised trial. Br Med J 1999;319:943-947.
11. Van Weel C, Smith H, Beasley H. Family practice research networks. Experiences from 3 countries. J Fam Pract 2000;49:938-943.
12. Snedecor G, Cochran W. Statistical methods Iowa, USA: Iowa State University Press; 1991.
13. Rutten GEHM, Verhoeven S, Heine RJ, et al. NHG-Standaard Diabetes Mellitus Type 2 (eerste herziening) Huisarts Wet 1999;42:67-84.
14. Walma EP, Grundmeijer HGLM, Thomas S, Prins A, Van den Hoogen J, Van der laan JR. NHG-Standaard Hypertensie (eerste herziening) Huisarts Wet 1997;40:598-617.
15. Thomas S, Van der Weijden T, Van Drenth BB, Haverkort AFM, Hooi JD, Van der Laan JD. NHG-standaard Cholesterol Huisarts Wet 1999;42:406-417.
16. Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol Clin Chem 1974;20:470-475.
17. Burstein M, Scholnick HR, Morfin R. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyan-ions. J Lipid Res 1970;11:583-595.
18. Fossati P, Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide Clin Chem 1982;28:2077-2080.
19. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge Clin Chem 1972;18:499-502.
20. Feunekes GI, van-Staveren WA, De-Vries JH, Burema J, Hautvast JG. Relative and biomarker-based validity of a food-frequency questionnaire estimating intake of fats and cholesterol Am J Clin Nutr 1993;58:489-496.
21. Voorlichtingsbureau voor de Voeding. Zo eet Nederland 1992: resultaten van de voedselconsumptiepeiling 1992 (results of the 1992 Dutch National Food Consumption Survey). The Hague, The Netherlands: Voorlichtingsbureau voor de Voeding (The Netherlands Nutrition Centre);1993.
22. Voorlichtingsbureau voor de Voeding. Nederlandse Voedings-middelentabel 1997 (Dutch nutrient data base). The Hague, The Netherlands: Voorlichtingsbureau voor de Voeding (The Netherlands Nutrition Centre); 1997.
23. Curry S, Kristal A, Bowen D. An application of the stage model of behavior change to dietary fat reduction Health Education Research 1992;1:97-105.
24. Sporny LA, Contento I. Stages of change in dietary fat reduction: social psychological correlates J Nutr Educ 1995;27:191-199.
25. Cupples ME, McKnight A. Randomised controlled trial of health promotion in general practice for patients at high cardiovascular risk Br Med J 1994;309:993-996.
26. Neil HA, Roe L, Godlee RJ, et al. Randomised trial of lipid lowering dietary advice in general practice: the effects on serum lipids, lipoproteins and antioxidants. Br Med J 1995;310:569-573.
27. Hellénius M-L, Krakau I, De Faire U. Favourable long-term effects from advice on diet and exercise given to healthy men with raised cardiovascular risk factors. Nutr Metab Cardiovasc Dis 1997;7:293-300.
28. Tang JL, Armitage JM, Lancaster T, Silagy CA, Fowler GH, Neil HAW. Systematic review of dietary intervention trials to lower blood total cholesterol in free-living subjects. Br Med J 1998;316:1213-1220.
29. Keys A, Anderson J, Grande F. Serum cholesterol response to changes in the diet IV: particularly saturated fatty acids in diet 2S-P. Metabolism 1965;14:776-787.
30. Dickman, A., Eijkhout, M. P., Loeve J.A. Werken en leren 1999-2000: feiten en cijfers over de arbeidsmarkt en het onderwijs in Nederland. Alphen a.d. Rijn, The Netherlands, Centraal Bureau voor de Statistiek (Statistics The Netherlands);2000.
31. Erkkila AT, Sarkkinen ES, Koukkunen H, et al. Concordance of diet with the recommended cholesterol lowering diet in patients with coronary heart disease. Eur J Clin Nutr 1998;52:279-285.
32. Lynch JW, Kaplan GA, Cohen RD, Tuomilehto J, Salonen JT. Do cardiovascular risk factors explain the relation between socioeconomic status, risk of all-cause mortality, cardiovascular mortality and acute myocardial infarction? Am J Epidemiol 1996;144:934-942.
OBJECTIVE: To examine stage-matched nutrition counseling by family physicians and its effect on dietary intake, anthropometry, and serum lipid levels in patients at elevated risk for cardiovascular disease.
METHODS: In this controlled trial, patients randomized to intervention practices received nutrition information following the Stages-of-Change Model, and patients randomized to control practices received usual care.
RESULTS: At both 6 and 12 months after baseline, total fat intake and saturated fat intake declined significantly more in the intervention group than in the control group: -5.7% and -2.6% of energy, respectively, at 6 months, and -3.6% and -1.7% of energy, respectively, at 12 months. For energy intake, body weight, and BMI, there were significant differences between groups only at 6 months: -0.8 megajoules (MJ), -0.7 kg, and -0.3 kg/m2, respectively. None of the serum lipid values changed significantly between groups at 12 months.
CONCLUSIONS: Nutritional counseling based on stages of change led to reductions in dietary fat intake and weight loss in the short term. However, we found no corresponding changes in serum lipid concentrations.
- Family physicians can select patients for nutrition counseling by a dietician by using a simple questionnaire based on the Stages-of-Change Model.
- With dietary intervention, a decline in fat intake was sustained at 1 year in a population with a high percentage of poorly educated subjects.
- We found no changes in serum lipids after 1 year of dietary intervention.
Cholesterol-lowering diet therapy is an important part of cardiovascular disease prevention.1 Family physicians (FPs) are uniquely positioned to provide nutrition information to persons at risk because of their expertise as perceived by consumers, and because they reach into nearly all segments of the population.2 It is important to build nutrition guidance into a model that enables change, and, considering the number of contacts between FP and patient,3,4 that is linked to the FP’s continuity of care over time.5 FPs can raise patient consciousness about dietary behavior, motivate patients to change their behavior, and, when appropriate, refer patients to a dietician or patient associations.6
The Transtheoretical Model of Behavior Change is increasingly being used to examine health behavior change.7 According to this model, people are assigned to 1 of the following 5 stages on the basis of their behavior and current intention for future action: (i) precontemplation, not even considering changing one’s behavior; (ii) contemplation, thinking about it; (iii) preparation, making definite plans to change; (iv) action, initiating behavior change; and (v) maintenance, maintaining desired behaviors.8
Evidence available on dietary applications studied in cross-sectional and not stage-matched intervention studies is sufficiently encouraging to warrant the inclusion of Transtheoretical Model constructs in prospective studies.7 As far as we know, only 2 stage-matched dietary intervention studies have been published. Both studies were carried out in primary care settings and both studies showed that tailored nutrition information is effective in dietary fat reduction.9,10 However, in these studies the intervention was not managed by the FP. We think the FP is the most appropriate person to manage such intervention in the family practice. Therefore, we conducted a controlled dietary intervention, based on the Stages-of-Change Model and managed by the FP with selective referral to a dietician. We examined the effects of dietary counseling on changes in dietary intake, anthropometry, and serum lipid levels in patients at elevated cardiovascular risk.
Methods
Participants and design
In this randomized controlled trial, men and women at elevated risk for cardiovascular disease were recruited from the 9 family practices joining the Nijmegen Monitoring Project, the research network of the Department of Family Medicine, University Medical Centre St. Radboud.11 Selection and flow of participants are described in Figure 1. Seventy-one patients were initially included in the intervention group and 72 patients in the control group (Table 1). Consequently, we had enough power (0.90) to detect a difference in change between groups for total fat intake of 3% of energy and 11.6 mg% of serum total cholesterol.12
After selection and recruitment of participants, the family practices were randomly divided into intervention (4) and control (5) practices. The practices, and not the patients, were the units of randomization, to avoid contamination of the information between intervention and control groups. Patients in the control practices received usual care.13-15 Each patient in the intervention arm received nutrition information according to his or her stage of change. All participants signed an informed consent form before entering the study. The Medical Ethical Committee of the Department of Human Nutrition and Epidemiology, Wageningen University, approved the study protocol. The study lasted from August 1998 until April 2000.
TABLE 1
Baseline characteristics of patients in the intervention and control groups
| Intervention n=71 | Control n=72 | P1 | |
|---|---|---|---|
| Sex (%) | |||
| Male | 24 | 29 | 0.48 |
| Female | 76 | 71 | |
| Age (years) | 58.5±7.1 | 58.2±6.9 | 0.83 |
| Disorder (%) | |||
| Hypertension | 94 | 89 | 0.34 |
| Diabetes mellitus II | 6 | 7 | |
| Hypertension & diabetes mellitus II | 0 | 4 | |
| Marital status (%) | |||
| Single | 6 | 3 | 0.83 |
| Married/cohabiting | 82 | 86 | |
| Divorced | 1 | 1 | |
| Widowed | 11 | 10 | |
| Education (%)2 | |||
| Low | 68 | 68 | 0.91 |
| Intermediate | 20 | 18 | |
| High | 13 | 15 | |
| Family history of heart disease (%)3 | 27 | 22 | 0.81 |
| Smoking (%)4 | |||
| Not smoking | 79 | 78 | 0.70 |
| Light smoker | 13 | 10 | |
| Heavy smoker | 9 | 13 | |
| Exercise, more than 20 minutes | |||
| No exercise | 20 | 13 | 0.42 |
| Less than 3 times a week | 37 | 30 | |
| 3 times a week | 18 | 26 | |
| More than 3 times a week | 25 | 31 | |
| 1Two-sided P values for differences in baseline characteristics between intervention and control groups. | |||
| 2Low: primary school, lower level of secondary school, lower vocational training. Intermediate: higher level of secondary school, intermediate vocational training. High: higher vocational training, university. | |||
| 3First-degree relatives younger than 60 years. | |||
| 4Light smoker: 0-10 cigarettes a day, or smoking pipe or cigars. Heavy smoker: > 10 cigarettes a day. | |||
FIGURE 1
Selection and flow of participants
Measurements
Specially trained practice assistants measured anthropometry data, and presented patients with a self-administered questionnaire on demographics, medical history, food frequency, and a stages-of-change algorithm at baseline, 6 months, and 12 months. Blood samples were taken at baseline and at 12 months.
Physical assessment. Anthropometry consisted of body weight to the nearest 0.5 kg, height, and waist and hip circumferences to the nearest 0.5 cm. Patients wore no shoes and only light clothing when weighed. Fasting blood samples were taken twice per measurement period at a 1-week interval, with the patient in the sitting position. The samples were stored at -80°C. Lipids were analyzed enzymatically for total cholesterol,16 HDL cholesterol,17 LDL cholesterol, and triglycerides18 with the Cobas Intergra 700 (Roche Diagnostics, Switzerland), at the laboratory of the Canisius Wilhelmina Hospital (Nijmegen, The Netherlands). The coefficient of variation within runs was 2.3% for total cholesterol, 1.6% for HDL cholesterol, and 1.8% for triglycerides. The LDL cholesterol level was calculated using the equation of Friedewald et al.19
Questionnaires. The questionnaire asked for demographic data, family history of heart disease, smoking status, physical activity, drug use, and diet history at baseline. At follow-up we checked for changes in smoking status, physical activity, and drug use. Patients in the control group were asked if they had visited a dietician during the study period. The intake of energy, total fat, fatty acids, and cholesterol during the preceding 4 weeks was assessed by asking patients to fill out a food frequency questionnaire that included 104 food items. The questionnaire was validated20 and recently revised according to the Dutch National Food Survey 1992.21 Dieticians carried out nutrient calculations with a computerized version of the Dutch food composition tables22 phoned patients in cases of inconsistency.
Stages of change for reduction of fat intake were assessed with a 4-item algorithm based on measures used in previous studies23,23 in combination with the results of the food frequency questionnaire. According to the algorithm, participants were judged to be in precontemplation if they did not consider their diet to be low in fat, they were not in the process of cutting down on fat, and they had no intention of reducing their fat consumption. Participants were considered to be in the contemplation phase if they intended to decrease their fat intake within 6 months but not within 30 days, and to be in preparation when they intended to decrease their fat intake within 30 days. Participants who reported they were currently trying to eat less fat were classified as in action, and participants who reported they had been eating less fat for at least 6 months were classified in maintenance. If participants in maintenance consumed 37% total fat or 12% saturated fat expressed as percent of energy intake, they were reclassified in precontemplation.
After completing the study, all patients filled in an evaluation questionnaire. Patients in the control group were also asked about which nutrition information they had received during the last year.
Intervention
The intervention consisted of nutrition counseling based on stages of change, directed by the FP with selective referral to a dietician. FPs were supported by a protocol that included Prochaska’s8 processes of change. Preparation and action stages were considered 1 stage (action stage) in this study, given the required nutrition education. (Figure 2) summarizes the intervention procedure: 1) counseling aimed at raising consciousness about dietary behavior in the precontemplation stage, 2) motivation to change dietary behavior in the contemplation stage, and 3) if a patient decided to change (action stage), information about practical aspects of dietary change and discussion of referral to a dietician. The intervention was conducted by the patient’s own FP. All patients were referred to the same dietician. Protocols for the FPs and the dietician had been developed and tested prior to the study and were discussed by FPs and the dietician in pre-study group sessions.
FIGURE 2
Intervention scheme with clarification
Data analyses
Differences between groups at baseline and follow-up were tested with unpaired t-tests for continuous variables and with chi-square tests for categorized variables. If the number of observations within 1 cell was less then 5, a Fisher’s exact test was used instead of a chi-square test. Differences within subjects were tested with a paired t-test. P values less than 0.05 were considered significant. Because of clustering of patients within practices, a multilevel analysis was also carried out (level 1 patient, level 2 practice). All analyses were performed on the basis of intention to treat. SAS version 6.12 was used for the statistical analyses (SAS Institute Inc., Cary, NC, USA).
Results
Study population
The study sample was predominantly female (73%), poorly educated (68%), with an average age of 58 years (see Table 1) for definitions of educational level). Of the cardiovascular risk factors, hypertension was present in 92% of participants, type 2 diabetes mellitus in 6%, both disorders in 2%, and a family history of heart disease in 25%. No significant differences were found between the intervention group (n=71) and the control group (n=72) (Table 1). Table 2 demonstrates that the 2 study groups also showed comparable baseline measures according to dietary intake, anthropometry, and serum lipid levels. The mean BMI of the total group of subjects was 28.7 kg/m 2 ; 83% had a BMI higher than 25 and the majority had high total cholesterol and dietary fat intake.
Intervention-related measures
At baseline, 51% of the patients in the intervention group were classified in the precontemplation stage, 24% in the contemplation stage, and 25% in the action stage. They had consulted their FP once (n=53) or twice (n=18) before they were referred to the study dietician (n=60). Eleven patients were not referred to the dietician because they did not reach the action stage. All of the referred patients but one received 3 consultations with the dietician. In the control group, 24% of the patients discussed nutrition issues with their FP, 57% read nutrition brochures related to cardiovascular topics, and 1% (7) were referred to a dietician.
Changes at follow-up measurements
After 6 months (Table 2) total energy intake was reduced by 1.4 and 0.6 MJ in the intervention and control groups, respectively; total fat intake by 7.9% and 2.2 % of total energy, and saturated fat intake by 3.4% and 0.8% of total energy. The reductions were significantly larger in the intervention group, except for unsaturated fat. This was also reflected in risk factors: body weight and BMI declined significantly more in the intervention group (1.5 kg body weight) than in the control group (0.6 kg body weight). We found no significant differences between groups for waist circumference and waist-hip ratio.
The reduced fat intake in the intervention group was maintained at 12 months, although the differences were smaller. Changes in energy intake and anthropometric values at this time no longer differed significantly with multilevel analysis. During the 12 months of the study, slight reductions were found for serum total cholesterol (intervention group: 2.3 mg/dL, controls: 6.2 mg/dL), LDL cholesterol (intervention group: 6.2 mg/dL, controls: 7.7 mg/dL), and triglycerides (intervention group: 0.8 mg/dL, controls: 3.1 mg/dL). HDL cholesterol increased slightly in both groups (3.9 mg/dL in the intervention group, 2.7 mg/dL in the control group). However, none of these differences were significant. There were no significant changes in smoking or physical activity (P values of chi-square tests per measurement moment were >0.85), and none of the patients was prescribed a cholesterol-lowering drug. The significance of the P values of the differences in variables between the first and last measurement moment did not change when multilevel analysis was performed, except for body weight (Table 2).
TABLE 2
Baseline measures and changes after 6 months and 12 months in dietary intake and anthropometry
| At Baseline | At 6 months | At 12 months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | P* | Intervention | Control | P** | Intervention | Control | P** | P*** | |
| n=71 Mean ± SD | n=72 Mean ± SD | n=70 Mean ± SD | n=67 Mean ± SD | n=67 Mean ± SD | n=63 Mean ± SD | |||||
| Dietary intake (per day) | ||||||||||
| Total energy (MJ/d)1 | 9.1 ± 2.7 | 9.6 ± 2.6 | 0.25 | -1.4 ± 1.9† | -0.6 ± 1.8† | 0.01 | -0.7 ± 3.0 | -0.9 ± 2.4† | 0.09 | 0.00 |
| Total fat (% of energy) | 42.1 ± 6.3 | 42.6 ± 5.2 | 0.64 | -7.9 ± 6.5† | -2.2 ± 4.9† | 0.00 | -5.6 ± 6.9† | -2.0 ± 6.7† | 0.00 | 0.00 |
| Saturated fat (% of energy) | 15.2 ± 2.6 | 15.5 ± 2.3 | 0.42 | -3.4 ± 2.7† | -0.8 ± 2.2† | 0.00 | -2.6 ± 2.7† | -0.9 ± 2.6† | 0.00 | 0.00 |
| Monounsaturated fat (% of energy) | 14.6 ± 3.3 | 14.9 ± 2.6 | 0.53 | -3.4 ± 3.3† | -0.7 ± 2.4† | 0.00 | -1.9 ± 4.1† | -0.3 ± 3.3 | 0.01 | 0.00 |
| Unsaturated fat (% of energy) | 9.4 ± 3.0 | 9.3 ± 3.0 | 0.79 | -1.0 ± 3.1† | 0.8 ± 3.0† | 0.37 | -1.0 ± 2.7† | -0.7 ± 3.7 | 0.73 | 0.73 |
| Cholesterol (mg) | 239.1 ± 91.5 | 254.8 ± 90.8 | 0.31 | -62.0 ± 68.9† | -22.8 ± 66.4† | 0.00 | -46.4 ± 77.1† | -33.4 ± 83.1† | 0.02 | 0.03 |
| Anthropometry | ||||||||||
| Body weight (kg) | 79.2 ± 14.9 | 80.3 ± 12.0 | 0.63 | -1.3 ± 1.8† | -0.6 ± 1.9† | 0.01 | 0.2 ± 3.0 | -0.6 ± 2.8† | 0.02 | 0.06 |
| Body Mass Index (kg/m2) | 28.1 ± 4.3 | 29.2 ± 4.8 | 0.15 | -0.5 ± 0.6† | -0.2 ± 0.7† | 0.01 | 0.0 ± 1.1 | -0.2 ± 1.0† | 0.03 | 0.08 |
| Waist circumference (cm) | 94.3 ± 12.1 | 97.7 ± 10.3 | 0.08 | -1.6 ± 4.9† | -1.7 ± 5.2† | 0.43 | -1.6 ± 6.6† | -1.8 ± 5.6† | 0.61 | 0.86 |
| Waist-hip circumference ratio | 0.89 ± 0.07 | 0.90 ± 0.09 | 0.36 | -0.0 ± 0.04 | -0.01 ± 0.05† | 0.13 | 0.01 ± 0.05† | -0.02 ± 0.05† | 0.15 | 0.15 |
| 1 Joule=0.24 cal | ||||||||||
| *Two-sided P values for differences in baseline measures between intervention and control group. | ||||||||||
| **One-sided P value for difference in change from baseline between intervention and control group. | ||||||||||
| ***P value with multilevel analysis | ||||||||||
| †Significant difference in changes after 6 and 12 months compared to baseline within group (one-sided P value<0.05). | ||||||||||
DISCUSSION
Nutrition counseling by an FP based on the Stages-of-Change Model, with referral to a dietician in the action stage, successfully changed dietary behavior after 6 months in patients at elevated risk for cardiovascular disease. This success was accompanied by reductions in body weight. Differences in fat intake were sustained at 12 months, but this was not reflected in lower serum lipid concentrations. Initial reductions in energy intake and anthropometric values did not persist after 1 year. Our findings are in line with other dietary intervention studies in family practice that report improved dietary habits but no significant effect on objective cardiovascular risk factors such as body weight and blood lipids.10,25-27 The uniqueness of our study is that it is the first randomized controlled trial in family practice based on the Stages-of-Change Model in which nutrition counseling is managed by the FP.
The reductions we found in total serum cholesterol concentrations (0.9% in the intervention group and 2.3% in the control group after 12 months) were smaller than the 3%-6% suggested by a systematic review of individualized nutrition counseling in free-living subjects.28 The observed reduction in our study is also less than predicted by the Keys equation.29 We do not have a clear explanation for this. It is possible that patients in the intervention group gave more socially desirable answers to the food frequency questionnaire than patients in the control group, due to the more extensive nutrition guidance in the intervention group.
The dropout rate in our study was low: 91% of the patients completed the trial, a notable strength of the study. This may be due to the fact that the participating patients were recruited and treated by their own FP, and may in part account for the small effect size as we avoided the selective participation of those patients who were most motivated for change. The education level of the study sample was low compared with the Dutch population at similar age,30 and this could also have resulted in smaller differences between intervention and control groups. In addition, it has been found that CHD patients who are obese and do not use lipid lowering drugs are less likely to follow recommended cholesterol-lowering diets.31 However, all of these factors make our study representative of the circumstances FPs can expect when managing nutritional intervention in routine care for patients at elevated risk for cardiovascular disease. Nutritional counseling on the basis of the Transtheoretical Model of stages of change7 is effective in the short term, but it is disappointing to have to conclude that this effect appears to be temporary, with eventual rebound to pre-intervention status. No sustained effects on the target outcomes such as body weight and serum lipids were found, possibly due to the relatively short (1-year) observation period. Improved effectiveness might be achieved with the development of patient protocols and education materials that are better aimed at poorly educated persons, and with more extensive use of modern forms of communications to implement lasting changes.
Conclusions
Nutritional counseling based on stages of change in patients at elevated risk for cardiovascular disease, provided by an FP with referral to a dietician in the action stage, led to reductions in dietary fat intake in the short and long term and to weight loss in the short term. In the absence of long-term effects on serum cholesterol levels, the emphasis remains on treating elevated lipids with drugs. However, research on effective and inexpensive dietary interventions remains important because of promising results for the short term and the important advantages of such intervention. The emphasis for future research should be testing new methods to maintain (dietary) behavioral changes and to investigate differences in susceptibility between individuals with unhealthy lifestyles. The model based on stages of change seems well suited for this sort of intervention, and the experience of this study is that it can easily be incorporated into the routines of family practice at low cost. As such, it is a simple instrument for selecting patients who are willing to change their food habits. Further, we reached a high percentage of poorly educated people, who are particularly vulnerable.32 We recommend examining whether education materials need to be better aimed at people with a low socio-economic status. Long-term nutrition counseling is needed for maintenance and further improvements.
Acknowledgments
This research was supported by the Netherlands Heart Foundation under grant no. 97.106 and by Bayer. We are grateful to the staff of the NMP family practices and their patients, without whom this study would not have been possible. We extend special thanks to the dieticians José Veen and Els Siebelink and to all the research assistants, especially to Marjolein Homs.
OBJECTIVE: To examine stage-matched nutrition counseling by family physicians and its effect on dietary intake, anthropometry, and serum lipid levels in patients at elevated risk for cardiovascular disease.
METHODS: In this controlled trial, patients randomized to intervention practices received nutrition information following the Stages-of-Change Model, and patients randomized to control practices received usual care.
RESULTS: At both 6 and 12 months after baseline, total fat intake and saturated fat intake declined significantly more in the intervention group than in the control group: -5.7% and -2.6% of energy, respectively, at 6 months, and -3.6% and -1.7% of energy, respectively, at 12 months. For energy intake, body weight, and BMI, there were significant differences between groups only at 6 months: -0.8 megajoules (MJ), -0.7 kg, and -0.3 kg/m2, respectively. None of the serum lipid values changed significantly between groups at 12 months.
CONCLUSIONS: Nutritional counseling based on stages of change led to reductions in dietary fat intake and weight loss in the short term. However, we found no corresponding changes in serum lipid concentrations.
- Family physicians can select patients for nutrition counseling by a dietician by using a simple questionnaire based on the Stages-of-Change Model.
- With dietary intervention, a decline in fat intake was sustained at 1 year in a population with a high percentage of poorly educated subjects.
- We found no changes in serum lipids after 1 year of dietary intervention.
Cholesterol-lowering diet therapy is an important part of cardiovascular disease prevention.1 Family physicians (FPs) are uniquely positioned to provide nutrition information to persons at risk because of their expertise as perceived by consumers, and because they reach into nearly all segments of the population.2 It is important to build nutrition guidance into a model that enables change, and, considering the number of contacts between FP and patient,3,4 that is linked to the FP’s continuity of care over time.5 FPs can raise patient consciousness about dietary behavior, motivate patients to change their behavior, and, when appropriate, refer patients to a dietician or patient associations.6
The Transtheoretical Model of Behavior Change is increasingly being used to examine health behavior change.7 According to this model, people are assigned to 1 of the following 5 stages on the basis of their behavior and current intention for future action: (i) precontemplation, not even considering changing one’s behavior; (ii) contemplation, thinking about it; (iii) preparation, making definite plans to change; (iv) action, initiating behavior change; and (v) maintenance, maintaining desired behaviors.8
Evidence available on dietary applications studied in cross-sectional and not stage-matched intervention studies is sufficiently encouraging to warrant the inclusion of Transtheoretical Model constructs in prospective studies.7 As far as we know, only 2 stage-matched dietary intervention studies have been published. Both studies were carried out in primary care settings and both studies showed that tailored nutrition information is effective in dietary fat reduction.9,10 However, in these studies the intervention was not managed by the FP. We think the FP is the most appropriate person to manage such intervention in the family practice. Therefore, we conducted a controlled dietary intervention, based on the Stages-of-Change Model and managed by the FP with selective referral to a dietician. We examined the effects of dietary counseling on changes in dietary intake, anthropometry, and serum lipid levels in patients at elevated cardiovascular risk.
Methods
Participants and design
In this randomized controlled trial, men and women at elevated risk for cardiovascular disease were recruited from the 9 family practices joining the Nijmegen Monitoring Project, the research network of the Department of Family Medicine, University Medical Centre St. Radboud.11 Selection and flow of participants are described in Figure 1. Seventy-one patients were initially included in the intervention group and 72 patients in the control group (Table 1). Consequently, we had enough power (0.90) to detect a difference in change between groups for total fat intake of 3% of energy and 11.6 mg% of serum total cholesterol.12
After selection and recruitment of participants, the family practices were randomly divided into intervention (4) and control (5) practices. The practices, and not the patients, were the units of randomization, to avoid contamination of the information between intervention and control groups. Patients in the control practices received usual care.13-15 Each patient in the intervention arm received nutrition information according to his or her stage of change. All participants signed an informed consent form before entering the study. The Medical Ethical Committee of the Department of Human Nutrition and Epidemiology, Wageningen University, approved the study protocol. The study lasted from August 1998 until April 2000.
TABLE 1
Baseline characteristics of patients in the intervention and control groups
| Intervention n=71 | Control n=72 | P1 | |
|---|---|---|---|
| Sex (%) | |||
| Male | 24 | 29 | 0.48 |
| Female | 76 | 71 | |
| Age (years) | 58.5±7.1 | 58.2±6.9 | 0.83 |
| Disorder (%) | |||
| Hypertension | 94 | 89 | 0.34 |
| Diabetes mellitus II | 6 | 7 | |
| Hypertension & diabetes mellitus II | 0 | 4 | |
| Marital status (%) | |||
| Single | 6 | 3 | 0.83 |
| Married/cohabiting | 82 | 86 | |
| Divorced | 1 | 1 | |
| Widowed | 11 | 10 | |
| Education (%)2 | |||
| Low | 68 | 68 | 0.91 |
| Intermediate | 20 | 18 | |
| High | 13 | 15 | |
| Family history of heart disease (%)3 | 27 | 22 | 0.81 |
| Smoking (%)4 | |||
| Not smoking | 79 | 78 | 0.70 |
| Light smoker | 13 | 10 | |
| Heavy smoker | 9 | 13 | |
| Exercise, more than 20 minutes | |||
| No exercise | 20 | 13 | 0.42 |
| Less than 3 times a week | 37 | 30 | |
| 3 times a week | 18 | 26 | |
| More than 3 times a week | 25 | 31 | |
| 1Two-sided P values for differences in baseline characteristics between intervention and control groups. | |||
| 2Low: primary school, lower level of secondary school, lower vocational training. Intermediate: higher level of secondary school, intermediate vocational training. High: higher vocational training, university. | |||
| 3First-degree relatives younger than 60 years. | |||
| 4Light smoker: 0-10 cigarettes a day, or smoking pipe or cigars. Heavy smoker: > 10 cigarettes a day. | |||
FIGURE 1
Selection and flow of participants
Measurements
Specially trained practice assistants measured anthropometry data, and presented patients with a self-administered questionnaire on demographics, medical history, food frequency, and a stages-of-change algorithm at baseline, 6 months, and 12 months. Blood samples were taken at baseline and at 12 months.
Physical assessment. Anthropometry consisted of body weight to the nearest 0.5 kg, height, and waist and hip circumferences to the nearest 0.5 cm. Patients wore no shoes and only light clothing when weighed. Fasting blood samples were taken twice per measurement period at a 1-week interval, with the patient in the sitting position. The samples were stored at -80°C. Lipids were analyzed enzymatically for total cholesterol,16 HDL cholesterol,17 LDL cholesterol, and triglycerides18 with the Cobas Intergra 700 (Roche Diagnostics, Switzerland), at the laboratory of the Canisius Wilhelmina Hospital (Nijmegen, The Netherlands). The coefficient of variation within runs was 2.3% for total cholesterol, 1.6% for HDL cholesterol, and 1.8% for triglycerides. The LDL cholesterol level was calculated using the equation of Friedewald et al.19
Questionnaires. The questionnaire asked for demographic data, family history of heart disease, smoking status, physical activity, drug use, and diet history at baseline. At follow-up we checked for changes in smoking status, physical activity, and drug use. Patients in the control group were asked if they had visited a dietician during the study period. The intake of energy, total fat, fatty acids, and cholesterol during the preceding 4 weeks was assessed by asking patients to fill out a food frequency questionnaire that included 104 food items. The questionnaire was validated20 and recently revised according to the Dutch National Food Survey 1992.21 Dieticians carried out nutrient calculations with a computerized version of the Dutch food composition tables22 phoned patients in cases of inconsistency.
Stages of change for reduction of fat intake were assessed with a 4-item algorithm based on measures used in previous studies23,23 in combination with the results of the food frequency questionnaire. According to the algorithm, participants were judged to be in precontemplation if they did not consider their diet to be low in fat, they were not in the process of cutting down on fat, and they had no intention of reducing their fat consumption. Participants were considered to be in the contemplation phase if they intended to decrease their fat intake within 6 months but not within 30 days, and to be in preparation when they intended to decrease their fat intake within 30 days. Participants who reported they were currently trying to eat less fat were classified as in action, and participants who reported they had been eating less fat for at least 6 months were classified in maintenance. If participants in maintenance consumed 37% total fat or 12% saturated fat expressed as percent of energy intake, they were reclassified in precontemplation.
After completing the study, all patients filled in an evaluation questionnaire. Patients in the control group were also asked about which nutrition information they had received during the last year.
Intervention
The intervention consisted of nutrition counseling based on stages of change, directed by the FP with selective referral to a dietician. FPs were supported by a protocol that included Prochaska’s8 processes of change. Preparation and action stages were considered 1 stage (action stage) in this study, given the required nutrition education. (Figure 2) summarizes the intervention procedure: 1) counseling aimed at raising consciousness about dietary behavior in the precontemplation stage, 2) motivation to change dietary behavior in the contemplation stage, and 3) if a patient decided to change (action stage), information about practical aspects of dietary change and discussion of referral to a dietician. The intervention was conducted by the patient’s own FP. All patients were referred to the same dietician. Protocols for the FPs and the dietician had been developed and tested prior to the study and were discussed by FPs and the dietician in pre-study group sessions.
FIGURE 2
Intervention scheme with clarification
Data analyses
Differences between groups at baseline and follow-up were tested with unpaired t-tests for continuous variables and with chi-square tests for categorized variables. If the number of observations within 1 cell was less then 5, a Fisher’s exact test was used instead of a chi-square test. Differences within subjects were tested with a paired t-test. P values less than 0.05 were considered significant. Because of clustering of patients within practices, a multilevel analysis was also carried out (level 1 patient, level 2 practice). All analyses were performed on the basis of intention to treat. SAS version 6.12 was used for the statistical analyses (SAS Institute Inc., Cary, NC, USA).
Results
Study population
The study sample was predominantly female (73%), poorly educated (68%), with an average age of 58 years (see Table 1) for definitions of educational level). Of the cardiovascular risk factors, hypertension was present in 92% of participants, type 2 diabetes mellitus in 6%, both disorders in 2%, and a family history of heart disease in 25%. No significant differences were found between the intervention group (n=71) and the control group (n=72) (Table 1). Table 2 demonstrates that the 2 study groups also showed comparable baseline measures according to dietary intake, anthropometry, and serum lipid levels. The mean BMI of the total group of subjects was 28.7 kg/m 2 ; 83% had a BMI higher than 25 and the majority had high total cholesterol and dietary fat intake.
Intervention-related measures
At baseline, 51% of the patients in the intervention group were classified in the precontemplation stage, 24% in the contemplation stage, and 25% in the action stage. They had consulted their FP once (n=53) or twice (n=18) before they were referred to the study dietician (n=60). Eleven patients were not referred to the dietician because they did not reach the action stage. All of the referred patients but one received 3 consultations with the dietician. In the control group, 24% of the patients discussed nutrition issues with their FP, 57% read nutrition brochures related to cardiovascular topics, and 1% (7) were referred to a dietician.
Changes at follow-up measurements
After 6 months (Table 2) total energy intake was reduced by 1.4 and 0.6 MJ in the intervention and control groups, respectively; total fat intake by 7.9% and 2.2 % of total energy, and saturated fat intake by 3.4% and 0.8% of total energy. The reductions were significantly larger in the intervention group, except for unsaturated fat. This was also reflected in risk factors: body weight and BMI declined significantly more in the intervention group (1.5 kg body weight) than in the control group (0.6 kg body weight). We found no significant differences between groups for waist circumference and waist-hip ratio.
The reduced fat intake in the intervention group was maintained at 12 months, although the differences were smaller. Changes in energy intake and anthropometric values at this time no longer differed significantly with multilevel analysis. During the 12 months of the study, slight reductions were found for serum total cholesterol (intervention group: 2.3 mg/dL, controls: 6.2 mg/dL), LDL cholesterol (intervention group: 6.2 mg/dL, controls: 7.7 mg/dL), and triglycerides (intervention group: 0.8 mg/dL, controls: 3.1 mg/dL). HDL cholesterol increased slightly in both groups (3.9 mg/dL in the intervention group, 2.7 mg/dL in the control group). However, none of these differences were significant. There were no significant changes in smoking or physical activity (P values of chi-square tests per measurement moment were >0.85), and none of the patients was prescribed a cholesterol-lowering drug. The significance of the P values of the differences in variables between the first and last measurement moment did not change when multilevel analysis was performed, except for body weight (Table 2).
TABLE 2
Baseline measures and changes after 6 months and 12 months in dietary intake and anthropometry
| At Baseline | At 6 months | At 12 months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | P* | Intervention | Control | P** | Intervention | Control | P** | P*** | |
| n=71 Mean ± SD | n=72 Mean ± SD | n=70 Mean ± SD | n=67 Mean ± SD | n=67 Mean ± SD | n=63 Mean ± SD | |||||
| Dietary intake (per day) | ||||||||||
| Total energy (MJ/d)1 | 9.1 ± 2.7 | 9.6 ± 2.6 | 0.25 | -1.4 ± 1.9† | -0.6 ± 1.8† | 0.01 | -0.7 ± 3.0 | -0.9 ± 2.4† | 0.09 | 0.00 |
| Total fat (% of energy) | 42.1 ± 6.3 | 42.6 ± 5.2 | 0.64 | -7.9 ± 6.5† | -2.2 ± 4.9† | 0.00 | -5.6 ± 6.9† | -2.0 ± 6.7† | 0.00 | 0.00 |
| Saturated fat (% of energy) | 15.2 ± 2.6 | 15.5 ± 2.3 | 0.42 | -3.4 ± 2.7† | -0.8 ± 2.2† | 0.00 | -2.6 ± 2.7† | -0.9 ± 2.6† | 0.00 | 0.00 |
| Monounsaturated fat (% of energy) | 14.6 ± 3.3 | 14.9 ± 2.6 | 0.53 | -3.4 ± 3.3† | -0.7 ± 2.4† | 0.00 | -1.9 ± 4.1† | -0.3 ± 3.3 | 0.01 | 0.00 |
| Unsaturated fat (% of energy) | 9.4 ± 3.0 | 9.3 ± 3.0 | 0.79 | -1.0 ± 3.1† | 0.8 ± 3.0† | 0.37 | -1.0 ± 2.7† | -0.7 ± 3.7 | 0.73 | 0.73 |
| Cholesterol (mg) | 239.1 ± 91.5 | 254.8 ± 90.8 | 0.31 | -62.0 ± 68.9† | -22.8 ± 66.4† | 0.00 | -46.4 ± 77.1† | -33.4 ± 83.1† | 0.02 | 0.03 |
| Anthropometry | ||||||||||
| Body weight (kg) | 79.2 ± 14.9 | 80.3 ± 12.0 | 0.63 | -1.3 ± 1.8† | -0.6 ± 1.9† | 0.01 | 0.2 ± 3.0 | -0.6 ± 2.8† | 0.02 | 0.06 |
| Body Mass Index (kg/m2) | 28.1 ± 4.3 | 29.2 ± 4.8 | 0.15 | -0.5 ± 0.6† | -0.2 ± 0.7† | 0.01 | 0.0 ± 1.1 | -0.2 ± 1.0† | 0.03 | 0.08 |
| Waist circumference (cm) | 94.3 ± 12.1 | 97.7 ± 10.3 | 0.08 | -1.6 ± 4.9† | -1.7 ± 5.2† | 0.43 | -1.6 ± 6.6† | -1.8 ± 5.6† | 0.61 | 0.86 |
| Waist-hip circumference ratio | 0.89 ± 0.07 | 0.90 ± 0.09 | 0.36 | -0.0 ± 0.04 | -0.01 ± 0.05† | 0.13 | 0.01 ± 0.05† | -0.02 ± 0.05† | 0.15 | 0.15 |
| 1 Joule=0.24 cal | ||||||||||
| *Two-sided P values for differences in baseline measures between intervention and control group. | ||||||||||
| **One-sided P value for difference in change from baseline between intervention and control group. | ||||||||||
| ***P value with multilevel analysis | ||||||||||
| †Significant difference in changes after 6 and 12 months compared to baseline within group (one-sided P value<0.05). | ||||||||||
DISCUSSION
Nutrition counseling by an FP based on the Stages-of-Change Model, with referral to a dietician in the action stage, successfully changed dietary behavior after 6 months in patients at elevated risk for cardiovascular disease. This success was accompanied by reductions in body weight. Differences in fat intake were sustained at 12 months, but this was not reflected in lower serum lipid concentrations. Initial reductions in energy intake and anthropometric values did not persist after 1 year. Our findings are in line with other dietary intervention studies in family practice that report improved dietary habits but no significant effect on objective cardiovascular risk factors such as body weight and blood lipids.10,25-27 The uniqueness of our study is that it is the first randomized controlled trial in family practice based on the Stages-of-Change Model in which nutrition counseling is managed by the FP.
The reductions we found in total serum cholesterol concentrations (0.9% in the intervention group and 2.3% in the control group after 12 months) were smaller than the 3%-6% suggested by a systematic review of individualized nutrition counseling in free-living subjects.28 The observed reduction in our study is also less than predicted by the Keys equation.29 We do not have a clear explanation for this. It is possible that patients in the intervention group gave more socially desirable answers to the food frequency questionnaire than patients in the control group, due to the more extensive nutrition guidance in the intervention group.
The dropout rate in our study was low: 91% of the patients completed the trial, a notable strength of the study. This may be due to the fact that the participating patients were recruited and treated by their own FP, and may in part account for the small effect size as we avoided the selective participation of those patients who were most motivated for change. The education level of the study sample was low compared with the Dutch population at similar age,30 and this could also have resulted in smaller differences between intervention and control groups. In addition, it has been found that CHD patients who are obese and do not use lipid lowering drugs are less likely to follow recommended cholesterol-lowering diets.31 However, all of these factors make our study representative of the circumstances FPs can expect when managing nutritional intervention in routine care for patients at elevated risk for cardiovascular disease. Nutritional counseling on the basis of the Transtheoretical Model of stages of change7 is effective in the short term, but it is disappointing to have to conclude that this effect appears to be temporary, with eventual rebound to pre-intervention status. No sustained effects on the target outcomes such as body weight and serum lipids were found, possibly due to the relatively short (1-year) observation period. Improved effectiveness might be achieved with the development of patient protocols and education materials that are better aimed at poorly educated persons, and with more extensive use of modern forms of communications to implement lasting changes.
Conclusions
Nutritional counseling based on stages of change in patients at elevated risk for cardiovascular disease, provided by an FP with referral to a dietician in the action stage, led to reductions in dietary fat intake in the short and long term and to weight loss in the short term. In the absence of long-term effects on serum cholesterol levels, the emphasis remains on treating elevated lipids with drugs. However, research on effective and inexpensive dietary interventions remains important because of promising results for the short term and the important advantages of such intervention. The emphasis for future research should be testing new methods to maintain (dietary) behavioral changes and to investigate differences in susceptibility between individuals with unhealthy lifestyles. The model based on stages of change seems well suited for this sort of intervention, and the experience of this study is that it can easily be incorporated into the routines of family practice at low cost. As such, it is a simple instrument for selecting patients who are willing to change their food habits. Further, we reached a high percentage of poorly educated people, who are particularly vulnerable.32 We recommend examining whether education materials need to be better aimed at people with a low socio-economic status. Long-term nutrition counseling is needed for maintenance and further improvements.
Acknowledgments
This research was supported by the Netherlands Heart Foundation under grant no. 97.106 and by Bayer. We are grateful to the staff of the NMP family practices and their patients, without whom this study would not have been possible. We extend special thanks to the dieticians José Veen and Els Siebelink and to all the research assistants, especially to Marjolein Homs.
1. Pyorala K. CHD prevention in clinical practice Lancet 1996;348 Suppl 1:s26-s28.
2. Hiddink GJ, Hautvast JG, van-Woerkum CM, Fieren CJ, van-’t-Hof MA. Consumers’ expectations about nutrition guidance: the importance of primary care physicians Am J Clin Nutr 1997;65:1974S-1979S.
3. Gray DP. Dietary advice in British General Practice Eur J Clin Nutr 1999;53 Suppl 2:S3-S8.
4. De Bakker D., Abrahamse H., Van den Hoogen H., Braspenning J., Van Althuis T, Rutten R. Jaarrapport LINH 1998: contactfrequenties en verrichtingen in het landelijk informatie netwerk huisartsenzorg (LINH). Utrecht/Nijmegen, The Netherlands: NIVEL/WOK; 1999.
5. Van-Weel C. Nutritional guidance in general practice—a conceptual framework Eur J Clin Nutr 1999;53 Suppl 2:S108-S111.
6. Hiddink GJ, Hautvast JG, van-Woerkum CM, Fieren CJ, van-’t-Hof MA. Nutrition guidance by primary-care physicians: perceived barriers and low involvement. Eur J Clin Nutr 1995;49:842-851.
7. Horwath CC. Applying the transtheoretical model to eating behaviour change: challenges and opportunities Nutrition Research Reviews. 1999;12:281-317.
8. Prochaska JO, DiClemente CC. In search of how people change: applications to addictive behaviors American Psychologist 1992;47:1102-1114.
9. Cambell MK, DeVellis B, Strecher V, Ammerman A, DeVellis R, Sandler R. Improving dietary behaviour: the effectiveness of tailored messages in primary care settings Am J Public Health 1994;84:783-787.
10. Steptoe A, Doherty S, Rink E, Kerry S, Kendrick T, Hilton S. Behavioural counselling in general practice for the promotion of healthy behaviour among adults at increased risk of coronary heart disease: randomised trial. Br Med J 1999;319:943-947.
11. Van Weel C, Smith H, Beasley H. Family practice research networks. Experiences from 3 countries. J Fam Pract 2000;49:938-943.
12. Snedecor G, Cochran W. Statistical methods Iowa, USA: Iowa State University Press; 1991.
13. Rutten GEHM, Verhoeven S, Heine RJ, et al. NHG-Standaard Diabetes Mellitus Type 2 (eerste herziening) Huisarts Wet 1999;42:67-84.
14. Walma EP, Grundmeijer HGLM, Thomas S, Prins A, Van den Hoogen J, Van der laan JR. NHG-Standaard Hypertensie (eerste herziening) Huisarts Wet 1997;40:598-617.
15. Thomas S, Van der Weijden T, Van Drenth BB, Haverkort AFM, Hooi JD, Van der Laan JD. NHG-standaard Cholesterol Huisarts Wet 1999;42:406-417.
16. Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol Clin Chem 1974;20:470-475.
17. Burstein M, Scholnick HR, Morfin R. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyan-ions. J Lipid Res 1970;11:583-595.
18. Fossati P, Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide Clin Chem 1982;28:2077-2080.
19. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge Clin Chem 1972;18:499-502.
20. Feunekes GI, van-Staveren WA, De-Vries JH, Burema J, Hautvast JG. Relative and biomarker-based validity of a food-frequency questionnaire estimating intake of fats and cholesterol Am J Clin Nutr 1993;58:489-496.
21. Voorlichtingsbureau voor de Voeding. Zo eet Nederland 1992: resultaten van de voedselconsumptiepeiling 1992 (results of the 1992 Dutch National Food Consumption Survey). The Hague, The Netherlands: Voorlichtingsbureau voor de Voeding (The Netherlands Nutrition Centre);1993.
22. Voorlichtingsbureau voor de Voeding. Nederlandse Voedings-middelentabel 1997 (Dutch nutrient data base). The Hague, The Netherlands: Voorlichtingsbureau voor de Voeding (The Netherlands Nutrition Centre); 1997.
23. Curry S, Kristal A, Bowen D. An application of the stage model of behavior change to dietary fat reduction Health Education Research 1992;1:97-105.
24. Sporny LA, Contento I. Stages of change in dietary fat reduction: social psychological correlates J Nutr Educ 1995;27:191-199.
25. Cupples ME, McKnight A. Randomised controlled trial of health promotion in general practice for patients at high cardiovascular risk Br Med J 1994;309:993-996.
26. Neil HA, Roe L, Godlee RJ, et al. Randomised trial of lipid lowering dietary advice in general practice: the effects on serum lipids, lipoproteins and antioxidants. Br Med J 1995;310:569-573.
27. Hellénius M-L, Krakau I, De Faire U. Favourable long-term effects from advice on diet and exercise given to healthy men with raised cardiovascular risk factors. Nutr Metab Cardiovasc Dis 1997;7:293-300.
28. Tang JL, Armitage JM, Lancaster T, Silagy CA, Fowler GH, Neil HAW. Systematic review of dietary intervention trials to lower blood total cholesterol in free-living subjects. Br Med J 1998;316:1213-1220.
29. Keys A, Anderson J, Grande F. Serum cholesterol response to changes in the diet IV: particularly saturated fatty acids in diet 2S-P. Metabolism 1965;14:776-787.
30. Dickman, A., Eijkhout, M. P., Loeve J.A. Werken en leren 1999-2000: feiten en cijfers over de arbeidsmarkt en het onderwijs in Nederland. Alphen a.d. Rijn, The Netherlands, Centraal Bureau voor de Statistiek (Statistics The Netherlands);2000.
31. Erkkila AT, Sarkkinen ES, Koukkunen H, et al. Concordance of diet with the recommended cholesterol lowering diet in patients with coronary heart disease. Eur J Clin Nutr 1998;52:279-285.
32. Lynch JW, Kaplan GA, Cohen RD, Tuomilehto J, Salonen JT. Do cardiovascular risk factors explain the relation between socioeconomic status, risk of all-cause mortality, cardiovascular mortality and acute myocardial infarction? Am J Epidemiol 1996;144:934-942.
1. Pyorala K. CHD prevention in clinical practice Lancet 1996;348 Suppl 1:s26-s28.
2. Hiddink GJ, Hautvast JG, van-Woerkum CM, Fieren CJ, van-’t-Hof MA. Consumers’ expectations about nutrition guidance: the importance of primary care physicians Am J Clin Nutr 1997;65:1974S-1979S.
3. Gray DP. Dietary advice in British General Practice Eur J Clin Nutr 1999;53 Suppl 2:S3-S8.
4. De Bakker D., Abrahamse H., Van den Hoogen H., Braspenning J., Van Althuis T, Rutten R. Jaarrapport LINH 1998: contactfrequenties en verrichtingen in het landelijk informatie netwerk huisartsenzorg (LINH). Utrecht/Nijmegen, The Netherlands: NIVEL/WOK; 1999.
5. Van-Weel C. Nutritional guidance in general practice—a conceptual framework Eur J Clin Nutr 1999;53 Suppl 2:S108-S111.
6. Hiddink GJ, Hautvast JG, van-Woerkum CM, Fieren CJ, van-’t-Hof MA. Nutrition guidance by primary-care physicians: perceived barriers and low involvement. Eur J Clin Nutr 1995;49:842-851.
7. Horwath CC. Applying the transtheoretical model to eating behaviour change: challenges and opportunities Nutrition Research Reviews. 1999;12:281-317.
8. Prochaska JO, DiClemente CC. In search of how people change: applications to addictive behaviors American Psychologist 1992;47:1102-1114.
9. Cambell MK, DeVellis B, Strecher V, Ammerman A, DeVellis R, Sandler R. Improving dietary behaviour: the effectiveness of tailored messages in primary care settings Am J Public Health 1994;84:783-787.
10. Steptoe A, Doherty S, Rink E, Kerry S, Kendrick T, Hilton S. Behavioural counselling in general practice for the promotion of healthy behaviour among adults at increased risk of coronary heart disease: randomised trial. Br Med J 1999;319:943-947.
11. Van Weel C, Smith H, Beasley H. Family practice research networks. Experiences from 3 countries. J Fam Pract 2000;49:938-943.
12. Snedecor G, Cochran W. Statistical methods Iowa, USA: Iowa State University Press; 1991.
13. Rutten GEHM, Verhoeven S, Heine RJ, et al. NHG-Standaard Diabetes Mellitus Type 2 (eerste herziening) Huisarts Wet 1999;42:67-84.
14. Walma EP, Grundmeijer HGLM, Thomas S, Prins A, Van den Hoogen J, Van der laan JR. NHG-Standaard Hypertensie (eerste herziening) Huisarts Wet 1997;40:598-617.
15. Thomas S, Van der Weijden T, Van Drenth BB, Haverkort AFM, Hooi JD, Van der Laan JD. NHG-standaard Cholesterol Huisarts Wet 1999;42:406-417.
16. Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol Clin Chem 1974;20:470-475.
17. Burstein M, Scholnick HR, Morfin R. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyan-ions. J Lipid Res 1970;11:583-595.
18. Fossati P, Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide Clin Chem 1982;28:2077-2080.
19. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge Clin Chem 1972;18:499-502.
20. Feunekes GI, van-Staveren WA, De-Vries JH, Burema J, Hautvast JG. Relative and biomarker-based validity of a food-frequency questionnaire estimating intake of fats and cholesterol Am J Clin Nutr 1993;58:489-496.
21. Voorlichtingsbureau voor de Voeding. Zo eet Nederland 1992: resultaten van de voedselconsumptiepeiling 1992 (results of the 1992 Dutch National Food Consumption Survey). The Hague, The Netherlands: Voorlichtingsbureau voor de Voeding (The Netherlands Nutrition Centre);1993.
22. Voorlichtingsbureau voor de Voeding. Nederlandse Voedings-middelentabel 1997 (Dutch nutrient data base). The Hague, The Netherlands: Voorlichtingsbureau voor de Voeding (The Netherlands Nutrition Centre); 1997.
23. Curry S, Kristal A, Bowen D. An application of the stage model of behavior change to dietary fat reduction Health Education Research 1992;1:97-105.
24. Sporny LA, Contento I. Stages of change in dietary fat reduction: social psychological correlates J Nutr Educ 1995;27:191-199.
25. Cupples ME, McKnight A. Randomised controlled trial of health promotion in general practice for patients at high cardiovascular risk Br Med J 1994;309:993-996.
26. Neil HA, Roe L, Godlee RJ, et al. Randomised trial of lipid lowering dietary advice in general practice: the effects on serum lipids, lipoproteins and antioxidants. Br Med J 1995;310:569-573.
27. Hellénius M-L, Krakau I, De Faire U. Favourable long-term effects from advice on diet and exercise given to healthy men with raised cardiovascular risk factors. Nutr Metab Cardiovasc Dis 1997;7:293-300.
28. Tang JL, Armitage JM, Lancaster T, Silagy CA, Fowler GH, Neil HAW. Systematic review of dietary intervention trials to lower blood total cholesterol in free-living subjects. Br Med J 1998;316:1213-1220.
29. Keys A, Anderson J, Grande F. Serum cholesterol response to changes in the diet IV: particularly saturated fatty acids in diet 2S-P. Metabolism 1965;14:776-787.
30. Dickman, A., Eijkhout, M. P., Loeve J.A. Werken en leren 1999-2000: feiten en cijfers over de arbeidsmarkt en het onderwijs in Nederland. Alphen a.d. Rijn, The Netherlands, Centraal Bureau voor de Statistiek (Statistics The Netherlands);2000.
31. Erkkila AT, Sarkkinen ES, Koukkunen H, et al. Concordance of diet with the recommended cholesterol lowering diet in patients with coronary heart disease. Eur J Clin Nutr 1998;52:279-285.
32. Lynch JW, Kaplan GA, Cohen RD, Tuomilehto J, Salonen JT. Do cardiovascular risk factors explain the relation between socioeconomic status, risk of all-cause mortality, cardiovascular mortality and acute myocardial infarction? Am J Epidemiol 1996;144:934-942.
On the front lines: Family physicians’ preparedness for bioterrorism
OBJECTIVE: The events of September 11, 2001, and the nation’s recent experience with anthrax assaults made bioterrorism preparedness a national priority. Because primary care physicians are among the sentinel responders to bioterrorist attacks, we sought to determine family physicians’ beliefs about their preparedness for such an attack.
STUDY DESIGN: In October 2001 we conducted a national survey of 976 family physicians randomly selected from the American Academy of Family Physicians’ active membership directory.
POPULATION: 614 (63%) family physicians responded to the survey.
OUTCOMES MEASURED: Physicians’ self-reported ability to “know what to do as a doctor in the event of a suspected bioterrorist attack, recognize signs and symptoms of an illness due to bioterrorism, and know where to call to report a suspected bioterrorist attack.”
RESULTS: Ninety-five percent of physicians agreed that a bioterrorist attack is a real threat within the United States. However, only 27% of family physicians believed that the US health care system could respond effectively to a bioterrorist attack; fewer (17%) thought that their local medical communities could respond effectively. Twenty-six percent of physicians reported that they would know what to do as a doctor in the event of a bioterrorist attack. Only 18% had previous training in bioterrorism preparedness. In a multivariate analysis, physicians’ reported that preparedness for a bioterrorist attack was significantly associated with previous bioterrorism preparedness training (OR 3.9 [95% CI 2.4–6.3]) and knowing how to obtain information in the event of a bioterrorist attack (OR 6.4 [95% CI 3.9–10.6]).
CONCLUSIONS: Only one quarter of family physicians felt prepared to respond to a bioterrorist event. However, training in bioterrorism preparedness was significantly associated with physicians’ perceived ability to respond effectively to an attack. Primary care physicians need more training in bioterrorism preparedness and easy access to public health and medical information in the event of a bioterrorist attack.
- Only one quarter of family physicians believe they are prepared to respond to a bioterrorist event.
- Family physicians who have received training in bioterrorism preparedness are more confident than their untrained peers that they would respond effectively to a bioterrorist attack.
- Primary care physicians, who would be on the front line in a bioterrorism attack, should seek training in detection, surveillance, and response activities.
With the events of September 11, 2001, and the anthrax attacks that followed, the once seemingly remote threat of a bioterrorist attack in the United States is now a reality.13 As with infectious disease outbreaks and other public health emergencies, early detection and reporting are critical to a timely and effective response to a bioterrorist event.4-7 For most Americans, their first point of contact with the health care system is the primary care physician, who is therefore on the front line in this new era of bioterrorism.8,9 Because victims of a bioterrorist attack may not know they have been affected, and because the symptoms caused by many bioterrorism-related agents mimic those of common condi-tions, primary care physicians will likely be in the position of diagnosing and managing the initial cases of a bioterrorist-related illness.10 Physicians’ ability to identify cases and activate the public health system are crucial steps in effectively responding to a bioterrorist attack.6,11,12
Recent studies have concluded that the preparedness and infrastructure of the public health system are inadequate to deal with a bioterrorist attack and need improvement.7,13-16 One survey found that fewer than 20% of emergency departments in the Pacific Northwest had plans for responding to a bioterrorist event.17 While the emphasis on the public health system is appropriate, these studies failed to discuss the critical role of primary care providers in responding to bioterrorism.18-20
While physician experience with the public health system in managing natural disasters and infectious disease outbreaks may be helpful, the unique features of a bioterrorist attack require that primary care physicians be able to obtain and use information from public health and intelligence sources.4,21 To date, no studies have assessed primary care physi-cians’ ability to respond to a bioterrorist event. In this national survey we assessed family physicians’ personal sense of preparedness for responding to a bioterrorist attack.
Methods
In March 2001, the National Network for Family Practice and Primary Care Research of the American Academy of Family Physicians (AAFP) conducted 2 focus groups of family physicians to explore the issue of bioterrorism preparedness. Using the results of these focus groups, we designed a 37-item questionnaire to be completed by practicing family physicians. The survey was pilot-tested for clarity by 10 academic family physicians and revised accordingly. The questionnaire used 5-category Likert scales, ranging from “strongly agree” to “strongly disagree” or from “excellent” to “poor,” to measure physicians’ assessment of bioterrorist risk and preparedness, specific clinical competencies, and their prior level of interaction with the public health system. Physicians were also asked to list 4 biologic agents that might be used in a terrorist attack. Physicians’ demographic information, including age, gender, training level, and board certification, was obtained from the membership database of the AAFP. Physician age was divided into 3 categories because of its asymmetric distribution. Physicians were asked to describe their location as rural, urban, or suburban, and to describe the size of the population in their area. Using the physicians’ zip codes, we geocoded the respondents to 1 of 4 regions of the country. The study was approved by the Social Science Institutional Review Board at the University of Missouri – Kansas City.
The confidential survey was mailed to a national sample of 976 physicians randomly selected from the computerized database of approximately 53,900 active members of the AAFP. Approximately 85% of active members spend at least 70% of their professional time in direct patient care. Two subsequent mailings were sent to non-respondents. The initial survey was mailed in October 2001, before the first case of anthrax was reported to the Centers for Disease Control and Prevention.1
Three survey items were the main outcomes of the study because they represented the key features of family physician preparedness: (1) “knowing what to do as a doctor in the event of a suspected bioterrorist attack in my community,” (2) “recognizing signs and symptoms of an illness due to bioterrorism in my own patients,” and (3) “knowing where to call to report a suspected bioterrorist attack.” For analysis, Likert scale responses of “strongly agree” and “agree” were collapsed into a single category because of the small number of “strongly agree” responses. Similarly, “strongly disagree” and “disagree” responses were combined. Student’s t-test and Pearson’s chi-square test were used to assess statistical significance in bivariate analyses. Multivariate logistic regression was performed to assess the effects of age, sex, geographic location, risk assessment, ability to gather information, and previous training in bioterrorism preparedness on the main outcomes of interest. These variables were selected a priori from the conceptual model of the survey. Analyses were conducted using STATA, v. 7.0 (Stata Corp., College Station, TX).
Results
Of the 976 family physicians sent the bioterrorism survey, 614 (63%) responded. The average age of the respondents was 45 years (range 28–76 years) and 70% were male. Respondents were distributed among rural, suburban, and urban geographic locations (Table 1). Respondents did not differ significantly from non-respondents with respect to age, gender, medical training, or board certification (Table 1).
Although 95% of physicians agreed that a bioterrorist attack is a real threat within the United States, only 27% believed the United States health care system could respond effectively to such an attack (Table 2). Thirty-nine percent believed that an attack is a real threat in their local communities; however, only 19% thought their local medical community could respond effectively. Sixty percent thought it likely that current public health surveillance systems could quickly identify a bioterrorist attack. Physicians’ thoughts about the biochemical agents most likely to be used in an attack are listed in Table 3.
Almost three quarters of physicians did not feel prepared to respond to a bioterrorist attack. Only 24% of those surveyed believed they could recognize signs and symptoms of an illness in their patients due to bioterrorism, and 38% rated their current knowledge of the diagnosis and management of bioterrorism-related illness as poor. Moreover, only 18% of physicians had received previous training in bioterrorism preparedness (Table 2).
When asked about their ability to deal with natural disasters or infectious disease outbreaks, a significantly higher percentage of physicians reported they would know how to respond to these major public health events (Table 2). Twenty-six percent of physicians reported they would know what to do in the event of a bioterrorist attack, compared with 65% (P <0.001) of physicians who reported they would know what to do in the event of a natural disaster and 66% (P <0.001) who reported knowing what to do in an infectious disease outbreak. After combining responses for local hospitals and community preparedness, only 17% believed that both their hospitals and their medical communities could respond effectively to a bioterrorism attack, compared with 60% (P <0.001) for a natural disaster and 56% (P <0.001) for an infectious disease outbreak. Physicians who felt prepared for natural disasters were 4 times more likely than other doctors to know how to respond to a bioterrorist attack (36% vs. 9%, P <0.001). Physicians who felt prepared for infectious disease outbreaks were 6 times more likely than other doctors to know how to respond to a bioterrorist attack (37% vs. 6%, P <0.001).
Importantly, physicians felt better prepared for a bioterrorist attack if they had training in bioterrorism preparedness. Physicians who had received such training were 3 times more likely than other doctors to know how to respond to a bioterrorist attack (55% vs. 20%, P <0.001). Ninety-eight percent thought it was important for them to be trained to identify a bioterrorist attack, and 93% of physicians said they would like such training.
Familiarity with the public health system was not necessarily associated with physicians’ preparedness for bioterrorism. While 93% of physicians report notifiable infectious disease cases to the health department, only 57% (P <0.001) reported knowing whom to call to report a suspected bioterrorist attack. Fifty-six percent of physicians reported knowing how to get information if they suspected an attack in their community.
In the multivariate model, having received training in bioterrorism preparedness (OR 3.9 [95%CI 2.4–6.3]) and knowing how to obtain information in the event of a bioterrorist attack (OR 6.4 [95%CI 3.9–10.6]) were significantly associated with physicians’ knowing what to do in the event of an attack (Table 4). These factors were also significantly associated with physicians’ ability to recognize signs and symptoms of a bioterrorism-related illness and knowledge of how to report a bioterrorist attack. Believing that bioterrorism was a real threat to their communities was also significantly associated with a physician’s ability to recognize signs and symptoms of a bioterrorism-related illness (OR 1.9 [95%CI 1.2–2.9]). Physicians’ preparedness was not associated with age, gender, geographic location, or residence in a rural, urban, or suburban area.
TABLE 1
Comparison of survey respondents and non-respondents
| % Respondents (n=614) | % Non-respondents (n=362) | P value | ||
|---|---|---|---|---|
| Mean age (SD) | 45 (9.6) | 44 (9.6) | .70 | |
| Age categories | <40 | 32 | 33 | .57 |
| 40–50 | 43 | 45 | ||
| >50 | 26 | 23 | ||
| Gender | Male | 70 | 76 | .07 |
| Medical training | MD degree | 90 | 91 | .53 |
| International | ||||
| Medical Graduate | 17 | 14 | .30 | |
| Board status | Board certified | 86 | 82 | .09 |
| Mean years since certification (SD) | 12 (7.9) | 11 (7.6) | .56 | |
| Geographic setting | Northeast | 14 | ||
| Midwest | 27 | |||
| South | 38 | |||
| West | 21 | |||
| Rural | 35 | |||
| Suburban | 37 | |||
| Urban | 29 | |||
| Population | <25,000 | 36 | ||
| 25,000–350,000 | 41 | |||
| 350,000 | 24 |
TABLE 2
Physicians’ responses to selected survey items
| Strongly agree or agree (%) | Neutral (%) | Strongly disagree or disagree (%) | ||
|---|---|---|---|---|
| Risk assessment | ||||
| “A bioterrorist attack is a real threat...” | in the United States | 95 | 3 | 2 |
| in my local community | 39 | 34 | 27 | |
| Preparedness | ||||
| “Could respond effectively to a bioterrorist attack” | United States | |||
| health care system | 27 | 32 | 42 | |
| My local medical community | 19 | 34 | 47 | |
| My local hospital | 21 | 33 | 46 | |
| “Know what to do as a doctor in the event of a suspected bioterrorist attack.” | 26 | 25 | 49 | |
| “Could respond effectively to a natural disaster” | My local medical community | 62 | 21 | 17 |
| My local hospital | 66 | 19 | 14 | |
| Self | 65 | 20 | 15 | |
| “Could respond effectively to an infectious disease outbreak “ | My local medical community | 60 | 27 | 14 |
| My local hospital | 60 | 25 | 15 | |
| Self | 66 | 22 | 12 | |
| Capabilities in bioterrorism response | ||||
| “Know where to call to report suspected attack” | 57 | 13 | 30 | |
| “Would recognize signs and symptoms” | 24 | 36 | 40 | |
| “Know how to get information about attack” | 56 | 17 | 27 | |
| “Know how to get clinical information about bioterrorism” | 54 | 18 | 28 | |
| Received prior training in bioterrorism preparedness | “Yes” 18 | “No” 82 | ||
| Current knowledge of management of bioterroristrelated illness | “Excellent or Very good” 5 | “Poor” 38 | ||
TABLE 3
Biologic agents physicians consider most likely to be used in a bioterrorist attack
| Agent | Survey respondents (%) |
|---|---|
| Anthrax | 96 |
| Smallpox | 82 |
| Plague | 28 |
| Botulism | 22 |
| Ebola | 16 |
| Nerve gas | 14 |
| Tularemia | 11 |
| Escherichia coli | 7 |
| Salmonella | 5 |
| Influenza virus | 4 |
TABLE 4
Predictors of preparedness in 3 areas of responsibility
| Knowing what to do as a doctor | Recognizing signs and symptoms | Knowing whom to contact | ||||
|---|---|---|---|---|---|---|
| Factor | OR* | 95% CI | OR* | 95% CI | OR* | 95% CI |
| Age <40 | 1.0 | referent | 1.0 | referent | 1.0 | referent |
| Age 40–50 | 1.1 | 0.6–1.7 | 1.0 | 0.6–1.7 | .9 | 0.6–1.4 |
| Age >50 | 1.9 | 1.1–3.3 | 1.8 | 1.0–3.2 | 1.3 | 0.8–2.1 |
| Female | 1.0 | referent | 1.0 | referent | 1.0 | referent |
| Male | 1.9 | 1.0–2.6 | 1.6 | 0.9–2.6 | .8 | 0.5–1.2 |
| Believe bioterrorist attack is real threat | ||||||
| in community | 1.3 | 0.9–2.0 | 1.9 | 1.2–2.9 | 1.4 | 1.0–2.1 |
| Know how to get info in suspected bio attack | 6.4 | 3.9–10.6 | 6.2 | 3.7–10.5 | 6.3 | 4.3–9.1 |
| Had prior bioterrorism preparedness training | 3.9 | 2.4–6.3 | 2.9 | 1.8–4.7 | 3.3 | 1.9–5.9 |
| Live in urban area | 1.0 | referent | 1.0 | referent | 1.0 | referent |
| Live in rural area | 1.2 | 0.7–1.9 | 1.1 | 0.7–1.9 | 1.2 | 0.7–1.9 |
| Live in suburban area | 1.1 | 0.7–1.9 | 1.0 | 0.6–1.6 | 1.0 | 0.6–1.6 |
| * Adjusted for other factors in table. OR=odds ratio. CI=confidence interval. | ||||||
Discussion
Only one quarter of family physicians in this national survey felt prepared to respond to a bioterrorist event. The majority of respondents did not feel confident in diagnosing or managing a bioterrorism-related illness, and fewer than 60% reported knowing how to report a bioterrorist event or obtain information about such an event. In addition, only one quarter of physicians were confident that local or national health care systems could respond effectively to a bioterrorist attack.
Those physicians who had received bioterrorism preparedness training were more likely to report having the skills and knowledge to respond to a bioterrorist attack. Knowing how to get information in the event of a suspected attack was the greatest predictor of being able to diagnose and report cases. Although we did not assess the nature of the training or test physicians’ actual preparedness, these data suggest that training may improve physicians’ abilities to diagnose and treat victims of bioterrorism. Finally, there are no published validated measures of bioterrorism preparedness, and there are few data to demonstrate the effectiveness of particular training interventions.21
Physicians felt more comfortable responding to other types of public health emergencies, such as natural disasters or infectious disease outbreaks. This may be due in part to their personal experiences in dealing with these events, or may reflect the formalized training in public health response that is part of medical school curricula. The reporting and response skills physicians would use in dealing with the public health system during a bioterrorist event are similar to the ones they use during natural disasters and infectious disease outbreaks. However, further emphasis should be placed on the importance of information-gathering and pre-incident intelligence for physicians.4
Because the survey instrument did not define bioterrorism, we relied on the respondents’ personal definitions of bioterrorism. While the timing of the survey coincided with national media attention on the recent anthrax cases, we did not detect a high level of knowledge or confidence in dealing with bioterrorism. In fact, despite the timing, we believe the results are valid and may reflect all physicians’ heightened awareness of the threat of bioterrorism and especially their limitations in dealing with it. Physicians clearly acknowledge the need for more training in bioterrorism response.
Primary care physicians have an important role in the public health response to bioterrorism. The results of this study indicate physicians should be trained in how to identify and manage illnesses caused by biologic weapons, how to obtain information about bioterrorism quickly, and how to activate the public health system in the event of a suspected attack. As the public health infrastructure is improved through increased funding, it should integrate training for front-line primary care physicians in detection, surveillance, and response activities.22 The AAFP has already begun to promote web-based training resources for practicing physicians (www.aafp.org/btresponse). Further study is warranted to test educational interventions designed to improve physicians’ preparedness for bioterrorism and their interactions with the public health sector.
Acknowledgments
The views expressed are those of the authors. No official endorsement by the Agency for Healthcare Research and Quality or the American Academy of Family Physicians is intended or should be inferred. This study was funded by AHRQ grant P20 HS11182-S. The authors thank Tom Stewart for his assistance with data collection, Phyllis Naragon and Jon Temte for conducting the focus groups, and Clark Hanmer for his assistance with data analysis and manuscript revisions.
1. CDC Update: Investigation of bioterrorism-related anthrax and interim guidelines for clinical evaluation of persons with possible anthrax. Morb Mortal Wkly Rep MMWR 2001;50(43):941-8.
2. Borio L, Frank D, Mani V, et al. Death due to bioterrorism-related inhalational anthrax. JAMA 2001;286(20):2554-9.
3. Mayer TA, Bersoff-Matcha S, Murphy C, et al. Clinical presentation of inhalational anthrax following bioterrorism exposure. JAMA 2001;286(20):2549-53.
4. Committee on R&D needs for improving civilian medical response to chemical and biological terrorism incidents hpp Institute of Medicine, and Board on Environmental Studies and Toxicology, Commission on Life Sciences, National Research Council. Chemical and biological terrorism: research and development to improve civilian medical response.Washington DC: National Academy Press; 1999.
5. Khan AS, Morse S, Lillibridge S. Public-health preparedness for biological terrorism in the USA. Lancet 2000;356(9236):1179-82.
6. Franz DR, Jahrling PB, Friedlander AM, McClain DJ, Hoover DL, Bryne WR, et al. Clinical recognition and management of patients exposed to biological warfare agents. JAMA 1997;278(5):399-411.
7. CDC Biological and chemical terrorism: Strategic plan for preparedness and response. MMWR Morb Mortal Wkly Rep 2000;49(RR04):1-14.
8. Green LA, Fryer GE, , Jr. , Yawn BP, Lanier D, Dovey SM. The ecology of medical care revisited [see comments]. N Engl J Med 2001;344(26):2021-5.
9. Lane HC, Fauci AS. Bioterrorism on the home front. A new challenge for American medicine. JAMA 2001;286(20):2595-7.
10. Gourlay M, Siwek J. Resources in the war against bioterrorism. Am Fam Physician 2001;64(10):1676-8.
11. Gordon SM. The threat of bioterrorism: a reason to learn more about anthrax and smallpox. Cleve Clin J Med 1999;66(10):592-5.
12. Haines JD, Pitts K, Crutcher JM. Medical response to bioterrorism: are we prepared? J Okla State Med Assoc 2000;93(5):187-96.
13. Inglesby T, Grossman R, O’Toole T. A plague on your city: observations from topoff. Clin Infect Dis 2001;32(3):436-45.
14. Khan AS, Ashford DA. Ready or not—preparedness for bioterrorism. N Engl J Med 2001;345(4):287-9.
15. Garrett LC, Magruder C, Molgard CA. Taking the terror out of bioterrorism: planning for a bioterrorist event from a local perspective. I Public Health Manag Pract 2000;6(4):1-7.
16. Rosen P. Coping with bioterrorism is difficult, but may help us respond to new epidemics [see comments]. BMJ 2000;320(7227):71-2.
17. Wetter DC, Daniell WE, Treser CD. Hospital preparedness for victims of chemical or biological terrorism. Am J Public Health 2001;91(5):710-6.
18. Geiger HJ. Terrorism, biological weapons, and bonanzas: assessing the real threat to public health. [letter; comment]. Am J Public Health. 2001;91(5):708-9.
19. Henretig F. Biological and chemical terrorism defense: a view from the “front lines” of public health. [letter; comment]. Am J Public Health 2001;91(5):718-20.
20. Sidel VW, Cohen HW, Gould RM. Good intentions and the road to bioterrorism preparedness. Am J Public Health 2001;91(5):716-8.
21. Training of Clinicians for Public Health Events Relevant to Bioterrorism Preparedness. Rockville, MD: Agency for Healthcare Research and Quality; 2001. AHRQ Publication No. 02-E007.
22. Isaacs B. A boost for public health agencies. Philadelphia Inquirer 2001 November 26, 2001;Sect. A9.
OBJECTIVE: The events of September 11, 2001, and the nation’s recent experience with anthrax assaults made bioterrorism preparedness a national priority. Because primary care physicians are among the sentinel responders to bioterrorist attacks, we sought to determine family physicians’ beliefs about their preparedness for such an attack.
STUDY DESIGN: In October 2001 we conducted a national survey of 976 family physicians randomly selected from the American Academy of Family Physicians’ active membership directory.
POPULATION: 614 (63%) family physicians responded to the survey.
OUTCOMES MEASURED: Physicians’ self-reported ability to “know what to do as a doctor in the event of a suspected bioterrorist attack, recognize signs and symptoms of an illness due to bioterrorism, and know where to call to report a suspected bioterrorist attack.”
RESULTS: Ninety-five percent of physicians agreed that a bioterrorist attack is a real threat within the United States. However, only 27% of family physicians believed that the US health care system could respond effectively to a bioterrorist attack; fewer (17%) thought that their local medical communities could respond effectively. Twenty-six percent of physicians reported that they would know what to do as a doctor in the event of a bioterrorist attack. Only 18% had previous training in bioterrorism preparedness. In a multivariate analysis, physicians’ reported that preparedness for a bioterrorist attack was significantly associated with previous bioterrorism preparedness training (OR 3.9 [95% CI 2.4–6.3]) and knowing how to obtain information in the event of a bioterrorist attack (OR 6.4 [95% CI 3.9–10.6]).
CONCLUSIONS: Only one quarter of family physicians felt prepared to respond to a bioterrorist event. However, training in bioterrorism preparedness was significantly associated with physicians’ perceived ability to respond effectively to an attack. Primary care physicians need more training in bioterrorism preparedness and easy access to public health and medical information in the event of a bioterrorist attack.
- Only one quarter of family physicians believe they are prepared to respond to a bioterrorist event.
- Family physicians who have received training in bioterrorism preparedness are more confident than their untrained peers that they would respond effectively to a bioterrorist attack.
- Primary care physicians, who would be on the front line in a bioterrorism attack, should seek training in detection, surveillance, and response activities.
With the events of September 11, 2001, and the anthrax attacks that followed, the once seemingly remote threat of a bioterrorist attack in the United States is now a reality.13 As with infectious disease outbreaks and other public health emergencies, early detection and reporting are critical to a timely and effective response to a bioterrorist event.4-7 For most Americans, their first point of contact with the health care system is the primary care physician, who is therefore on the front line in this new era of bioterrorism.8,9 Because victims of a bioterrorist attack may not know they have been affected, and because the symptoms caused by many bioterrorism-related agents mimic those of common condi-tions, primary care physicians will likely be in the position of diagnosing and managing the initial cases of a bioterrorist-related illness.10 Physicians’ ability to identify cases and activate the public health system are crucial steps in effectively responding to a bioterrorist attack.6,11,12
Recent studies have concluded that the preparedness and infrastructure of the public health system are inadequate to deal with a bioterrorist attack and need improvement.7,13-16 One survey found that fewer than 20% of emergency departments in the Pacific Northwest had plans for responding to a bioterrorist event.17 While the emphasis on the public health system is appropriate, these studies failed to discuss the critical role of primary care providers in responding to bioterrorism.18-20
While physician experience with the public health system in managing natural disasters and infectious disease outbreaks may be helpful, the unique features of a bioterrorist attack require that primary care physicians be able to obtain and use information from public health and intelligence sources.4,21 To date, no studies have assessed primary care physi-cians’ ability to respond to a bioterrorist event. In this national survey we assessed family physicians’ personal sense of preparedness for responding to a bioterrorist attack.
Methods
In March 2001, the National Network for Family Practice and Primary Care Research of the American Academy of Family Physicians (AAFP) conducted 2 focus groups of family physicians to explore the issue of bioterrorism preparedness. Using the results of these focus groups, we designed a 37-item questionnaire to be completed by practicing family physicians. The survey was pilot-tested for clarity by 10 academic family physicians and revised accordingly. The questionnaire used 5-category Likert scales, ranging from “strongly agree” to “strongly disagree” or from “excellent” to “poor,” to measure physicians’ assessment of bioterrorist risk and preparedness, specific clinical competencies, and their prior level of interaction with the public health system. Physicians were also asked to list 4 biologic agents that might be used in a terrorist attack. Physicians’ demographic information, including age, gender, training level, and board certification, was obtained from the membership database of the AAFP. Physician age was divided into 3 categories because of its asymmetric distribution. Physicians were asked to describe their location as rural, urban, or suburban, and to describe the size of the population in their area. Using the physicians’ zip codes, we geocoded the respondents to 1 of 4 regions of the country. The study was approved by the Social Science Institutional Review Board at the University of Missouri – Kansas City.
The confidential survey was mailed to a national sample of 976 physicians randomly selected from the computerized database of approximately 53,900 active members of the AAFP. Approximately 85% of active members spend at least 70% of their professional time in direct patient care. Two subsequent mailings were sent to non-respondents. The initial survey was mailed in October 2001, before the first case of anthrax was reported to the Centers for Disease Control and Prevention.1
Three survey items were the main outcomes of the study because they represented the key features of family physician preparedness: (1) “knowing what to do as a doctor in the event of a suspected bioterrorist attack in my community,” (2) “recognizing signs and symptoms of an illness due to bioterrorism in my own patients,” and (3) “knowing where to call to report a suspected bioterrorist attack.” For analysis, Likert scale responses of “strongly agree” and “agree” were collapsed into a single category because of the small number of “strongly agree” responses. Similarly, “strongly disagree” and “disagree” responses were combined. Student’s t-test and Pearson’s chi-square test were used to assess statistical significance in bivariate analyses. Multivariate logistic regression was performed to assess the effects of age, sex, geographic location, risk assessment, ability to gather information, and previous training in bioterrorism preparedness on the main outcomes of interest. These variables were selected a priori from the conceptual model of the survey. Analyses were conducted using STATA, v. 7.0 (Stata Corp., College Station, TX).
Results
Of the 976 family physicians sent the bioterrorism survey, 614 (63%) responded. The average age of the respondents was 45 years (range 28–76 years) and 70% were male. Respondents were distributed among rural, suburban, and urban geographic locations (Table 1). Respondents did not differ significantly from non-respondents with respect to age, gender, medical training, or board certification (Table 1).
Although 95% of physicians agreed that a bioterrorist attack is a real threat within the United States, only 27% believed the United States health care system could respond effectively to such an attack (Table 2). Thirty-nine percent believed that an attack is a real threat in their local communities; however, only 19% thought their local medical community could respond effectively. Sixty percent thought it likely that current public health surveillance systems could quickly identify a bioterrorist attack. Physicians’ thoughts about the biochemical agents most likely to be used in an attack are listed in Table 3.
Almost three quarters of physicians did not feel prepared to respond to a bioterrorist attack. Only 24% of those surveyed believed they could recognize signs and symptoms of an illness in their patients due to bioterrorism, and 38% rated their current knowledge of the diagnosis and management of bioterrorism-related illness as poor. Moreover, only 18% of physicians had received previous training in bioterrorism preparedness (Table 2).
When asked about their ability to deal with natural disasters or infectious disease outbreaks, a significantly higher percentage of physicians reported they would know how to respond to these major public health events (Table 2). Twenty-six percent of physicians reported they would know what to do in the event of a bioterrorist attack, compared with 65% (P <0.001) of physicians who reported they would know what to do in the event of a natural disaster and 66% (P <0.001) who reported knowing what to do in an infectious disease outbreak. After combining responses for local hospitals and community preparedness, only 17% believed that both their hospitals and their medical communities could respond effectively to a bioterrorism attack, compared with 60% (P <0.001) for a natural disaster and 56% (P <0.001) for an infectious disease outbreak. Physicians who felt prepared for natural disasters were 4 times more likely than other doctors to know how to respond to a bioterrorist attack (36% vs. 9%, P <0.001). Physicians who felt prepared for infectious disease outbreaks were 6 times more likely than other doctors to know how to respond to a bioterrorist attack (37% vs. 6%, P <0.001).
Importantly, physicians felt better prepared for a bioterrorist attack if they had training in bioterrorism preparedness. Physicians who had received such training were 3 times more likely than other doctors to know how to respond to a bioterrorist attack (55% vs. 20%, P <0.001). Ninety-eight percent thought it was important for them to be trained to identify a bioterrorist attack, and 93% of physicians said they would like such training.
Familiarity with the public health system was not necessarily associated with physicians’ preparedness for bioterrorism. While 93% of physicians report notifiable infectious disease cases to the health department, only 57% (P <0.001) reported knowing whom to call to report a suspected bioterrorist attack. Fifty-six percent of physicians reported knowing how to get information if they suspected an attack in their community.
In the multivariate model, having received training in bioterrorism preparedness (OR 3.9 [95%CI 2.4–6.3]) and knowing how to obtain information in the event of a bioterrorist attack (OR 6.4 [95%CI 3.9–10.6]) were significantly associated with physicians’ knowing what to do in the event of an attack (Table 4). These factors were also significantly associated with physicians’ ability to recognize signs and symptoms of a bioterrorism-related illness and knowledge of how to report a bioterrorist attack. Believing that bioterrorism was a real threat to their communities was also significantly associated with a physician’s ability to recognize signs and symptoms of a bioterrorism-related illness (OR 1.9 [95%CI 1.2–2.9]). Physicians’ preparedness was not associated with age, gender, geographic location, or residence in a rural, urban, or suburban area.
TABLE 1
Comparison of survey respondents and non-respondents
| % Respondents (n=614) | % Non-respondents (n=362) | P value | ||
|---|---|---|---|---|
| Mean age (SD) | 45 (9.6) | 44 (9.6) | .70 | |
| Age categories | <40 | 32 | 33 | .57 |
| 40–50 | 43 | 45 | ||
| >50 | 26 | 23 | ||
| Gender | Male | 70 | 76 | .07 |
| Medical training | MD degree | 90 | 91 | .53 |
| International | ||||
| Medical Graduate | 17 | 14 | .30 | |
| Board status | Board certified | 86 | 82 | .09 |
| Mean years since certification (SD) | 12 (7.9) | 11 (7.6) | .56 | |
| Geographic setting | Northeast | 14 | ||
| Midwest | 27 | |||
| South | 38 | |||
| West | 21 | |||
| Rural | 35 | |||
| Suburban | 37 | |||
| Urban | 29 | |||
| Population | <25,000 | 36 | ||
| 25,000–350,000 | 41 | |||
| 350,000 | 24 |
TABLE 2
Physicians’ responses to selected survey items
| Strongly agree or agree (%) | Neutral (%) | Strongly disagree or disagree (%) | ||
|---|---|---|---|---|
| Risk assessment | ||||
| “A bioterrorist attack is a real threat...” | in the United States | 95 | 3 | 2 |
| in my local community | 39 | 34 | 27 | |
| Preparedness | ||||
| “Could respond effectively to a bioterrorist attack” | United States | |||
| health care system | 27 | 32 | 42 | |
| My local medical community | 19 | 34 | 47 | |
| My local hospital | 21 | 33 | 46 | |
| “Know what to do as a doctor in the event of a suspected bioterrorist attack.” | 26 | 25 | 49 | |
| “Could respond effectively to a natural disaster” | My local medical community | 62 | 21 | 17 |
| My local hospital | 66 | 19 | 14 | |
| Self | 65 | 20 | 15 | |
| “Could respond effectively to an infectious disease outbreak “ | My local medical community | 60 | 27 | 14 |
| My local hospital | 60 | 25 | 15 | |
| Self | 66 | 22 | 12 | |
| Capabilities in bioterrorism response | ||||
| “Know where to call to report suspected attack” | 57 | 13 | 30 | |
| “Would recognize signs and symptoms” | 24 | 36 | 40 | |
| “Know how to get information about attack” | 56 | 17 | 27 | |
| “Know how to get clinical information about bioterrorism” | 54 | 18 | 28 | |
| Received prior training in bioterrorism preparedness | “Yes” 18 | “No” 82 | ||
| Current knowledge of management of bioterroristrelated illness | “Excellent or Very good” 5 | “Poor” 38 | ||
TABLE 3
Biologic agents physicians consider most likely to be used in a bioterrorist attack
| Agent | Survey respondents (%) |
|---|---|
| Anthrax | 96 |
| Smallpox | 82 |
| Plague | 28 |
| Botulism | 22 |
| Ebola | 16 |
| Nerve gas | 14 |
| Tularemia | 11 |
| Escherichia coli | 7 |
| Salmonella | 5 |
| Influenza virus | 4 |
TABLE 4
Predictors of preparedness in 3 areas of responsibility
| Knowing what to do as a doctor | Recognizing signs and symptoms | Knowing whom to contact | ||||
|---|---|---|---|---|---|---|
| Factor | OR* | 95% CI | OR* | 95% CI | OR* | 95% CI |
| Age <40 | 1.0 | referent | 1.0 | referent | 1.0 | referent |
| Age 40–50 | 1.1 | 0.6–1.7 | 1.0 | 0.6–1.7 | .9 | 0.6–1.4 |
| Age >50 | 1.9 | 1.1–3.3 | 1.8 | 1.0–3.2 | 1.3 | 0.8–2.1 |
| Female | 1.0 | referent | 1.0 | referent | 1.0 | referent |
| Male | 1.9 | 1.0–2.6 | 1.6 | 0.9–2.6 | .8 | 0.5–1.2 |
| Believe bioterrorist attack is real threat | ||||||
| in community | 1.3 | 0.9–2.0 | 1.9 | 1.2–2.9 | 1.4 | 1.0–2.1 |
| Know how to get info in suspected bio attack | 6.4 | 3.9–10.6 | 6.2 | 3.7–10.5 | 6.3 | 4.3–9.1 |
| Had prior bioterrorism preparedness training | 3.9 | 2.4–6.3 | 2.9 | 1.8–4.7 | 3.3 | 1.9–5.9 |
| Live in urban area | 1.0 | referent | 1.0 | referent | 1.0 | referent |
| Live in rural area | 1.2 | 0.7–1.9 | 1.1 | 0.7–1.9 | 1.2 | 0.7–1.9 |
| Live in suburban area | 1.1 | 0.7–1.9 | 1.0 | 0.6–1.6 | 1.0 | 0.6–1.6 |
| * Adjusted for other factors in table. OR=odds ratio. CI=confidence interval. | ||||||
Discussion
Only one quarter of family physicians in this national survey felt prepared to respond to a bioterrorist event. The majority of respondents did not feel confident in diagnosing or managing a bioterrorism-related illness, and fewer than 60% reported knowing how to report a bioterrorist event or obtain information about such an event. In addition, only one quarter of physicians were confident that local or national health care systems could respond effectively to a bioterrorist attack.
Those physicians who had received bioterrorism preparedness training were more likely to report having the skills and knowledge to respond to a bioterrorist attack. Knowing how to get information in the event of a suspected attack was the greatest predictor of being able to diagnose and report cases. Although we did not assess the nature of the training or test physicians’ actual preparedness, these data suggest that training may improve physicians’ abilities to diagnose and treat victims of bioterrorism. Finally, there are no published validated measures of bioterrorism preparedness, and there are few data to demonstrate the effectiveness of particular training interventions.21
Physicians felt more comfortable responding to other types of public health emergencies, such as natural disasters or infectious disease outbreaks. This may be due in part to their personal experiences in dealing with these events, or may reflect the formalized training in public health response that is part of medical school curricula. The reporting and response skills physicians would use in dealing with the public health system during a bioterrorist event are similar to the ones they use during natural disasters and infectious disease outbreaks. However, further emphasis should be placed on the importance of information-gathering and pre-incident intelligence for physicians.4
Because the survey instrument did not define bioterrorism, we relied on the respondents’ personal definitions of bioterrorism. While the timing of the survey coincided with national media attention on the recent anthrax cases, we did not detect a high level of knowledge or confidence in dealing with bioterrorism. In fact, despite the timing, we believe the results are valid and may reflect all physicians’ heightened awareness of the threat of bioterrorism and especially their limitations in dealing with it. Physicians clearly acknowledge the need for more training in bioterrorism response.
Primary care physicians have an important role in the public health response to bioterrorism. The results of this study indicate physicians should be trained in how to identify and manage illnesses caused by biologic weapons, how to obtain information about bioterrorism quickly, and how to activate the public health system in the event of a suspected attack. As the public health infrastructure is improved through increased funding, it should integrate training for front-line primary care physicians in detection, surveillance, and response activities.22 The AAFP has already begun to promote web-based training resources for practicing physicians (www.aafp.org/btresponse). Further study is warranted to test educational interventions designed to improve physicians’ preparedness for bioterrorism and their interactions with the public health sector.
Acknowledgments
The views expressed are those of the authors. No official endorsement by the Agency for Healthcare Research and Quality or the American Academy of Family Physicians is intended or should be inferred. This study was funded by AHRQ grant P20 HS11182-S. The authors thank Tom Stewart for his assistance with data collection, Phyllis Naragon and Jon Temte for conducting the focus groups, and Clark Hanmer for his assistance with data analysis and manuscript revisions.
OBJECTIVE: The events of September 11, 2001, and the nation’s recent experience with anthrax assaults made bioterrorism preparedness a national priority. Because primary care physicians are among the sentinel responders to bioterrorist attacks, we sought to determine family physicians’ beliefs about their preparedness for such an attack.
STUDY DESIGN: In October 2001 we conducted a national survey of 976 family physicians randomly selected from the American Academy of Family Physicians’ active membership directory.
POPULATION: 614 (63%) family physicians responded to the survey.
OUTCOMES MEASURED: Physicians’ self-reported ability to “know what to do as a doctor in the event of a suspected bioterrorist attack, recognize signs and symptoms of an illness due to bioterrorism, and know where to call to report a suspected bioterrorist attack.”
RESULTS: Ninety-five percent of physicians agreed that a bioterrorist attack is a real threat within the United States. However, only 27% of family physicians believed that the US health care system could respond effectively to a bioterrorist attack; fewer (17%) thought that their local medical communities could respond effectively. Twenty-six percent of physicians reported that they would know what to do as a doctor in the event of a bioterrorist attack. Only 18% had previous training in bioterrorism preparedness. In a multivariate analysis, physicians’ reported that preparedness for a bioterrorist attack was significantly associated with previous bioterrorism preparedness training (OR 3.9 [95% CI 2.4–6.3]) and knowing how to obtain information in the event of a bioterrorist attack (OR 6.4 [95% CI 3.9–10.6]).
CONCLUSIONS: Only one quarter of family physicians felt prepared to respond to a bioterrorist event. However, training in bioterrorism preparedness was significantly associated with physicians’ perceived ability to respond effectively to an attack. Primary care physicians need more training in bioterrorism preparedness and easy access to public health and medical information in the event of a bioterrorist attack.
- Only one quarter of family physicians believe they are prepared to respond to a bioterrorist event.
- Family physicians who have received training in bioterrorism preparedness are more confident than their untrained peers that they would respond effectively to a bioterrorist attack.
- Primary care physicians, who would be on the front line in a bioterrorism attack, should seek training in detection, surveillance, and response activities.
With the events of September 11, 2001, and the anthrax attacks that followed, the once seemingly remote threat of a bioterrorist attack in the United States is now a reality.13 As with infectious disease outbreaks and other public health emergencies, early detection and reporting are critical to a timely and effective response to a bioterrorist event.4-7 For most Americans, their first point of contact with the health care system is the primary care physician, who is therefore on the front line in this new era of bioterrorism.8,9 Because victims of a bioterrorist attack may not know they have been affected, and because the symptoms caused by many bioterrorism-related agents mimic those of common condi-tions, primary care physicians will likely be in the position of diagnosing and managing the initial cases of a bioterrorist-related illness.10 Physicians’ ability to identify cases and activate the public health system are crucial steps in effectively responding to a bioterrorist attack.6,11,12
Recent studies have concluded that the preparedness and infrastructure of the public health system are inadequate to deal with a bioterrorist attack and need improvement.7,13-16 One survey found that fewer than 20% of emergency departments in the Pacific Northwest had plans for responding to a bioterrorist event.17 While the emphasis on the public health system is appropriate, these studies failed to discuss the critical role of primary care providers in responding to bioterrorism.18-20
While physician experience with the public health system in managing natural disasters and infectious disease outbreaks may be helpful, the unique features of a bioterrorist attack require that primary care physicians be able to obtain and use information from public health and intelligence sources.4,21 To date, no studies have assessed primary care physi-cians’ ability to respond to a bioterrorist event. In this national survey we assessed family physicians’ personal sense of preparedness for responding to a bioterrorist attack.
Methods
In March 2001, the National Network for Family Practice and Primary Care Research of the American Academy of Family Physicians (AAFP) conducted 2 focus groups of family physicians to explore the issue of bioterrorism preparedness. Using the results of these focus groups, we designed a 37-item questionnaire to be completed by practicing family physicians. The survey was pilot-tested for clarity by 10 academic family physicians and revised accordingly. The questionnaire used 5-category Likert scales, ranging from “strongly agree” to “strongly disagree” or from “excellent” to “poor,” to measure physicians’ assessment of bioterrorist risk and preparedness, specific clinical competencies, and their prior level of interaction with the public health system. Physicians were also asked to list 4 biologic agents that might be used in a terrorist attack. Physicians’ demographic information, including age, gender, training level, and board certification, was obtained from the membership database of the AAFP. Physician age was divided into 3 categories because of its asymmetric distribution. Physicians were asked to describe their location as rural, urban, or suburban, and to describe the size of the population in their area. Using the physicians’ zip codes, we geocoded the respondents to 1 of 4 regions of the country. The study was approved by the Social Science Institutional Review Board at the University of Missouri – Kansas City.
The confidential survey was mailed to a national sample of 976 physicians randomly selected from the computerized database of approximately 53,900 active members of the AAFP. Approximately 85% of active members spend at least 70% of their professional time in direct patient care. Two subsequent mailings were sent to non-respondents. The initial survey was mailed in October 2001, before the first case of anthrax was reported to the Centers for Disease Control and Prevention.1
Three survey items were the main outcomes of the study because they represented the key features of family physician preparedness: (1) “knowing what to do as a doctor in the event of a suspected bioterrorist attack in my community,” (2) “recognizing signs and symptoms of an illness due to bioterrorism in my own patients,” and (3) “knowing where to call to report a suspected bioterrorist attack.” For analysis, Likert scale responses of “strongly agree” and “agree” were collapsed into a single category because of the small number of “strongly agree” responses. Similarly, “strongly disagree” and “disagree” responses were combined. Student’s t-test and Pearson’s chi-square test were used to assess statistical significance in bivariate analyses. Multivariate logistic regression was performed to assess the effects of age, sex, geographic location, risk assessment, ability to gather information, and previous training in bioterrorism preparedness on the main outcomes of interest. These variables were selected a priori from the conceptual model of the survey. Analyses were conducted using STATA, v. 7.0 (Stata Corp., College Station, TX).
Results
Of the 976 family physicians sent the bioterrorism survey, 614 (63%) responded. The average age of the respondents was 45 years (range 28–76 years) and 70% were male. Respondents were distributed among rural, suburban, and urban geographic locations (Table 1). Respondents did not differ significantly from non-respondents with respect to age, gender, medical training, or board certification (Table 1).
Although 95% of physicians agreed that a bioterrorist attack is a real threat within the United States, only 27% believed the United States health care system could respond effectively to such an attack (Table 2). Thirty-nine percent believed that an attack is a real threat in their local communities; however, only 19% thought their local medical community could respond effectively. Sixty percent thought it likely that current public health surveillance systems could quickly identify a bioterrorist attack. Physicians’ thoughts about the biochemical agents most likely to be used in an attack are listed in Table 3.
Almost three quarters of physicians did not feel prepared to respond to a bioterrorist attack. Only 24% of those surveyed believed they could recognize signs and symptoms of an illness in their patients due to bioterrorism, and 38% rated their current knowledge of the diagnosis and management of bioterrorism-related illness as poor. Moreover, only 18% of physicians had received previous training in bioterrorism preparedness (Table 2).
When asked about their ability to deal with natural disasters or infectious disease outbreaks, a significantly higher percentage of physicians reported they would know how to respond to these major public health events (Table 2). Twenty-six percent of physicians reported they would know what to do in the event of a bioterrorist attack, compared with 65% (P <0.001) of physicians who reported they would know what to do in the event of a natural disaster and 66% (P <0.001) who reported knowing what to do in an infectious disease outbreak. After combining responses for local hospitals and community preparedness, only 17% believed that both their hospitals and their medical communities could respond effectively to a bioterrorism attack, compared with 60% (P <0.001) for a natural disaster and 56% (P <0.001) for an infectious disease outbreak. Physicians who felt prepared for natural disasters were 4 times more likely than other doctors to know how to respond to a bioterrorist attack (36% vs. 9%, P <0.001). Physicians who felt prepared for infectious disease outbreaks were 6 times more likely than other doctors to know how to respond to a bioterrorist attack (37% vs. 6%, P <0.001).
Importantly, physicians felt better prepared for a bioterrorist attack if they had training in bioterrorism preparedness. Physicians who had received such training were 3 times more likely than other doctors to know how to respond to a bioterrorist attack (55% vs. 20%, P <0.001). Ninety-eight percent thought it was important for them to be trained to identify a bioterrorist attack, and 93% of physicians said they would like such training.
Familiarity with the public health system was not necessarily associated with physicians’ preparedness for bioterrorism. While 93% of physicians report notifiable infectious disease cases to the health department, only 57% (P <0.001) reported knowing whom to call to report a suspected bioterrorist attack. Fifty-six percent of physicians reported knowing how to get information if they suspected an attack in their community.
In the multivariate model, having received training in bioterrorism preparedness (OR 3.9 [95%CI 2.4–6.3]) and knowing how to obtain information in the event of a bioterrorist attack (OR 6.4 [95%CI 3.9–10.6]) were significantly associated with physicians’ knowing what to do in the event of an attack (Table 4). These factors were also significantly associated with physicians’ ability to recognize signs and symptoms of a bioterrorism-related illness and knowledge of how to report a bioterrorist attack. Believing that bioterrorism was a real threat to their communities was also significantly associated with a physician’s ability to recognize signs and symptoms of a bioterrorism-related illness (OR 1.9 [95%CI 1.2–2.9]). Physicians’ preparedness was not associated with age, gender, geographic location, or residence in a rural, urban, or suburban area.
TABLE 1
Comparison of survey respondents and non-respondents
| % Respondents (n=614) | % Non-respondents (n=362) | P value | ||
|---|---|---|---|---|
| Mean age (SD) | 45 (9.6) | 44 (9.6) | .70 | |
| Age categories | <40 | 32 | 33 | .57 |
| 40–50 | 43 | 45 | ||
| >50 | 26 | 23 | ||
| Gender | Male | 70 | 76 | .07 |
| Medical training | MD degree | 90 | 91 | .53 |
| International | ||||
| Medical Graduate | 17 | 14 | .30 | |
| Board status | Board certified | 86 | 82 | .09 |
| Mean years since certification (SD) | 12 (7.9) | 11 (7.6) | .56 | |
| Geographic setting | Northeast | 14 | ||
| Midwest | 27 | |||
| South | 38 | |||
| West | 21 | |||
| Rural | 35 | |||
| Suburban | 37 | |||
| Urban | 29 | |||
| Population | <25,000 | 36 | ||
| 25,000–350,000 | 41 | |||
| 350,000 | 24 |
TABLE 2
Physicians’ responses to selected survey items
| Strongly agree or agree (%) | Neutral (%) | Strongly disagree or disagree (%) | ||
|---|---|---|---|---|
| Risk assessment | ||||
| “A bioterrorist attack is a real threat...” | in the United States | 95 | 3 | 2 |
| in my local community | 39 | 34 | 27 | |
| Preparedness | ||||
| “Could respond effectively to a bioterrorist attack” | United States | |||
| health care system | 27 | 32 | 42 | |
| My local medical community | 19 | 34 | 47 | |
| My local hospital | 21 | 33 | 46 | |
| “Know what to do as a doctor in the event of a suspected bioterrorist attack.” | 26 | 25 | 49 | |
| “Could respond effectively to a natural disaster” | My local medical community | 62 | 21 | 17 |
| My local hospital | 66 | 19 | 14 | |
| Self | 65 | 20 | 15 | |
| “Could respond effectively to an infectious disease outbreak “ | My local medical community | 60 | 27 | 14 |
| My local hospital | 60 | 25 | 15 | |
| Self | 66 | 22 | 12 | |
| Capabilities in bioterrorism response | ||||
| “Know where to call to report suspected attack” | 57 | 13 | 30 | |
| “Would recognize signs and symptoms” | 24 | 36 | 40 | |
| “Know how to get information about attack” | 56 | 17 | 27 | |
| “Know how to get clinical information about bioterrorism” | 54 | 18 | 28 | |
| Received prior training in bioterrorism preparedness | “Yes” 18 | “No” 82 | ||
| Current knowledge of management of bioterroristrelated illness | “Excellent or Very good” 5 | “Poor” 38 | ||
TABLE 3
Biologic agents physicians consider most likely to be used in a bioterrorist attack
| Agent | Survey respondents (%) |
|---|---|
| Anthrax | 96 |
| Smallpox | 82 |
| Plague | 28 |
| Botulism | 22 |
| Ebola | 16 |
| Nerve gas | 14 |
| Tularemia | 11 |
| Escherichia coli | 7 |
| Salmonella | 5 |
| Influenza virus | 4 |
TABLE 4
Predictors of preparedness in 3 areas of responsibility
| Knowing what to do as a doctor | Recognizing signs and symptoms | Knowing whom to contact | ||||
|---|---|---|---|---|---|---|
| Factor | OR* | 95% CI | OR* | 95% CI | OR* | 95% CI |
| Age <40 | 1.0 | referent | 1.0 | referent | 1.0 | referent |
| Age 40–50 | 1.1 | 0.6–1.7 | 1.0 | 0.6–1.7 | .9 | 0.6–1.4 |
| Age >50 | 1.9 | 1.1–3.3 | 1.8 | 1.0–3.2 | 1.3 | 0.8–2.1 |
| Female | 1.0 | referent | 1.0 | referent | 1.0 | referent |
| Male | 1.9 | 1.0–2.6 | 1.6 | 0.9–2.6 | .8 | 0.5–1.2 |
| Believe bioterrorist attack is real threat | ||||||
| in community | 1.3 | 0.9–2.0 | 1.9 | 1.2–2.9 | 1.4 | 1.0–2.1 |
| Know how to get info in suspected bio attack | 6.4 | 3.9–10.6 | 6.2 | 3.7–10.5 | 6.3 | 4.3–9.1 |
| Had prior bioterrorism preparedness training | 3.9 | 2.4–6.3 | 2.9 | 1.8–4.7 | 3.3 | 1.9–5.9 |
| Live in urban area | 1.0 | referent | 1.0 | referent | 1.0 | referent |
| Live in rural area | 1.2 | 0.7–1.9 | 1.1 | 0.7–1.9 | 1.2 | 0.7–1.9 |
| Live in suburban area | 1.1 | 0.7–1.9 | 1.0 | 0.6–1.6 | 1.0 | 0.6–1.6 |
| * Adjusted for other factors in table. OR=odds ratio. CI=confidence interval. | ||||||
Discussion
Only one quarter of family physicians in this national survey felt prepared to respond to a bioterrorist event. The majority of respondents did not feel confident in diagnosing or managing a bioterrorism-related illness, and fewer than 60% reported knowing how to report a bioterrorist event or obtain information about such an event. In addition, only one quarter of physicians were confident that local or national health care systems could respond effectively to a bioterrorist attack.
Those physicians who had received bioterrorism preparedness training were more likely to report having the skills and knowledge to respond to a bioterrorist attack. Knowing how to get information in the event of a suspected attack was the greatest predictor of being able to diagnose and report cases. Although we did not assess the nature of the training or test physicians’ actual preparedness, these data suggest that training may improve physicians’ abilities to diagnose and treat victims of bioterrorism. Finally, there are no published validated measures of bioterrorism preparedness, and there are few data to demonstrate the effectiveness of particular training interventions.21
Physicians felt more comfortable responding to other types of public health emergencies, such as natural disasters or infectious disease outbreaks. This may be due in part to their personal experiences in dealing with these events, or may reflect the formalized training in public health response that is part of medical school curricula. The reporting and response skills physicians would use in dealing with the public health system during a bioterrorist event are similar to the ones they use during natural disasters and infectious disease outbreaks. However, further emphasis should be placed on the importance of information-gathering and pre-incident intelligence for physicians.4
Because the survey instrument did not define bioterrorism, we relied on the respondents’ personal definitions of bioterrorism. While the timing of the survey coincided with national media attention on the recent anthrax cases, we did not detect a high level of knowledge or confidence in dealing with bioterrorism. In fact, despite the timing, we believe the results are valid and may reflect all physicians’ heightened awareness of the threat of bioterrorism and especially their limitations in dealing with it. Physicians clearly acknowledge the need for more training in bioterrorism response.
Primary care physicians have an important role in the public health response to bioterrorism. The results of this study indicate physicians should be trained in how to identify and manage illnesses caused by biologic weapons, how to obtain information about bioterrorism quickly, and how to activate the public health system in the event of a suspected attack. As the public health infrastructure is improved through increased funding, it should integrate training for front-line primary care physicians in detection, surveillance, and response activities.22 The AAFP has already begun to promote web-based training resources for practicing physicians (www.aafp.org/btresponse). Further study is warranted to test educational interventions designed to improve physicians’ preparedness for bioterrorism and their interactions with the public health sector.
Acknowledgments
The views expressed are those of the authors. No official endorsement by the Agency for Healthcare Research and Quality or the American Academy of Family Physicians is intended or should be inferred. This study was funded by AHRQ grant P20 HS11182-S. The authors thank Tom Stewart for his assistance with data collection, Phyllis Naragon and Jon Temte for conducting the focus groups, and Clark Hanmer for his assistance with data analysis and manuscript revisions.
1. CDC Update: Investigation of bioterrorism-related anthrax and interim guidelines for clinical evaluation of persons with possible anthrax. Morb Mortal Wkly Rep MMWR 2001;50(43):941-8.
2. Borio L, Frank D, Mani V, et al. Death due to bioterrorism-related inhalational anthrax. JAMA 2001;286(20):2554-9.
3. Mayer TA, Bersoff-Matcha S, Murphy C, et al. Clinical presentation of inhalational anthrax following bioterrorism exposure. JAMA 2001;286(20):2549-53.
4. Committee on R&D needs for improving civilian medical response to chemical and biological terrorism incidents hpp Institute of Medicine, and Board on Environmental Studies and Toxicology, Commission on Life Sciences, National Research Council. Chemical and biological terrorism: research and development to improve civilian medical response.Washington DC: National Academy Press; 1999.
5. Khan AS, Morse S, Lillibridge S. Public-health preparedness for biological terrorism in the USA. Lancet 2000;356(9236):1179-82.
6. Franz DR, Jahrling PB, Friedlander AM, McClain DJ, Hoover DL, Bryne WR, et al. Clinical recognition and management of patients exposed to biological warfare agents. JAMA 1997;278(5):399-411.
7. CDC Biological and chemical terrorism: Strategic plan for preparedness and response. MMWR Morb Mortal Wkly Rep 2000;49(RR04):1-14.
8. Green LA, Fryer GE, , Jr. , Yawn BP, Lanier D, Dovey SM. The ecology of medical care revisited [see comments]. N Engl J Med 2001;344(26):2021-5.
9. Lane HC, Fauci AS. Bioterrorism on the home front. A new challenge for American medicine. JAMA 2001;286(20):2595-7.
10. Gourlay M, Siwek J. Resources in the war against bioterrorism. Am Fam Physician 2001;64(10):1676-8.
11. Gordon SM. The threat of bioterrorism: a reason to learn more about anthrax and smallpox. Cleve Clin J Med 1999;66(10):592-5.
12. Haines JD, Pitts K, Crutcher JM. Medical response to bioterrorism: are we prepared? J Okla State Med Assoc 2000;93(5):187-96.
13. Inglesby T, Grossman R, O’Toole T. A plague on your city: observations from topoff. Clin Infect Dis 2001;32(3):436-45.
14. Khan AS, Ashford DA. Ready or not—preparedness for bioterrorism. N Engl J Med 2001;345(4):287-9.
15. Garrett LC, Magruder C, Molgard CA. Taking the terror out of bioterrorism: planning for a bioterrorist event from a local perspective. I Public Health Manag Pract 2000;6(4):1-7.
16. Rosen P. Coping with bioterrorism is difficult, but may help us respond to new epidemics [see comments]. BMJ 2000;320(7227):71-2.
17. Wetter DC, Daniell WE, Treser CD. Hospital preparedness for victims of chemical or biological terrorism. Am J Public Health 2001;91(5):710-6.
18. Geiger HJ. Terrorism, biological weapons, and bonanzas: assessing the real threat to public health. [letter; comment]. Am J Public Health. 2001;91(5):708-9.
19. Henretig F. Biological and chemical terrorism defense: a view from the “front lines” of public health. [letter; comment]. Am J Public Health 2001;91(5):718-20.
20. Sidel VW, Cohen HW, Gould RM. Good intentions and the road to bioterrorism preparedness. Am J Public Health 2001;91(5):716-8.
21. Training of Clinicians for Public Health Events Relevant to Bioterrorism Preparedness. Rockville, MD: Agency for Healthcare Research and Quality; 2001. AHRQ Publication No. 02-E007.
22. Isaacs B. A boost for public health agencies. Philadelphia Inquirer 2001 November 26, 2001;Sect. A9.
1. CDC Update: Investigation of bioterrorism-related anthrax and interim guidelines for clinical evaluation of persons with possible anthrax. Morb Mortal Wkly Rep MMWR 2001;50(43):941-8.
2. Borio L, Frank D, Mani V, et al. Death due to bioterrorism-related inhalational anthrax. JAMA 2001;286(20):2554-9.
3. Mayer TA, Bersoff-Matcha S, Murphy C, et al. Clinical presentation of inhalational anthrax following bioterrorism exposure. JAMA 2001;286(20):2549-53.
4. Committee on R&D needs for improving civilian medical response to chemical and biological terrorism incidents hpp Institute of Medicine, and Board on Environmental Studies and Toxicology, Commission on Life Sciences, National Research Council. Chemical and biological terrorism: research and development to improve civilian medical response.Washington DC: National Academy Press; 1999.
5. Khan AS, Morse S, Lillibridge S. Public-health preparedness for biological terrorism in the USA. Lancet 2000;356(9236):1179-82.
6. Franz DR, Jahrling PB, Friedlander AM, McClain DJ, Hoover DL, Bryne WR, et al. Clinical recognition and management of patients exposed to biological warfare agents. JAMA 1997;278(5):399-411.
7. CDC Biological and chemical terrorism: Strategic plan for preparedness and response. MMWR Morb Mortal Wkly Rep 2000;49(RR04):1-14.
8. Green LA, Fryer GE, , Jr. , Yawn BP, Lanier D, Dovey SM. The ecology of medical care revisited [see comments]. N Engl J Med 2001;344(26):2021-5.
9. Lane HC, Fauci AS. Bioterrorism on the home front. A new challenge for American medicine. JAMA 2001;286(20):2595-7.
10. Gourlay M, Siwek J. Resources in the war against bioterrorism. Am Fam Physician 2001;64(10):1676-8.
11. Gordon SM. The threat of bioterrorism: a reason to learn more about anthrax and smallpox. Cleve Clin J Med 1999;66(10):592-5.
12. Haines JD, Pitts K, Crutcher JM. Medical response to bioterrorism: are we prepared? J Okla State Med Assoc 2000;93(5):187-96.
13. Inglesby T, Grossman R, O’Toole T. A plague on your city: observations from topoff. Clin Infect Dis 2001;32(3):436-45.
14. Khan AS, Ashford DA. Ready or not—preparedness for bioterrorism. N Engl J Med 2001;345(4):287-9.
15. Garrett LC, Magruder C, Molgard CA. Taking the terror out of bioterrorism: planning for a bioterrorist event from a local perspective. I Public Health Manag Pract 2000;6(4):1-7.
16. Rosen P. Coping with bioterrorism is difficult, but may help us respond to new epidemics [see comments]. BMJ 2000;320(7227):71-2.
17. Wetter DC, Daniell WE, Treser CD. Hospital preparedness for victims of chemical or biological terrorism. Am J Public Health 2001;91(5):710-6.
18. Geiger HJ. Terrorism, biological weapons, and bonanzas: assessing the real threat to public health. [letter; comment]. Am J Public Health. 2001;91(5):708-9.
19. Henretig F. Biological and chemical terrorism defense: a view from the “front lines” of public health. [letter; comment]. Am J Public Health 2001;91(5):718-20.
20. Sidel VW, Cohen HW, Gould RM. Good intentions and the road to bioterrorism preparedness. Am J Public Health 2001;91(5):716-8.
21. Training of Clinicians for Public Health Events Relevant to Bioterrorism Preparedness. Rockville, MD: Agency for Healthcare Research and Quality; 2001. AHRQ Publication No. 02-E007.
22. Isaacs B. A boost for public health agencies. Philadelphia Inquirer 2001 November 26, 2001;Sect. A9.