User login
Granuloma Annulare: A Retrospective Series of 133 Patients
Granuloma annulare (GA) is a granulomatous skin disorder of uncertain etiology. A number of clinical variants exist, most commonly localized annular plaques on the hands or feet, generalized lesions, or subcutaneous nodules in children. Histologically, GA exhibits granulomatous inflammation with either interstitial or palisading lymphocytes and histiocytes with mucin deposition.
Few data exist regarding the epidemiology of GA. Although the pathogenesis of GA is unknown, associations between GA and underlying systemic processes, such as diabetes mellitus, hyperlipidemia, thyroid disease, and human immunodeficiency virus (HIV), have been suggested.
The purpose of this retrospective study was to determine the number of cases of GA seen annually at the Department of Dermatology at the University of Pennsylvania (Philadelphia, Pennsylvania) from 2008 to 2014. Additionally, we reviewed all cases of biopsy-proven GA from 2010 to 2014 and reported the demographics, underlying medical comorbidities, medications, treatments, and outcomes seen in this patient population.
Methods
We identified the number of outpatients presenting with GA annually using PennSeek, a tool developed by the Penn Medicine Data Analytics Center to search electronic medical records (EMRs). We queried the EMR database to determine the number of discrete patients seen at the Department of Dermatology at the University of Pennsylvania annually from 2008 (the year the EMR was established) to 2014. We then used PennSeek to determine the number of patients given a diagnosis of GA annually from 2008 to 2014 based on the International Classification of Diseases, Ninth Revision (ICD-9).
After using PennSeek to identify all patients given the ICD-9 diagnosis of GA from 2008 to 2014, we reviewed the EMRs of these patients to identify cases that were biopsy proven. For the biopsy-proven cases of GA seen at the University of Pennsylvania from 2010 to 2014, we reviewed the EMRs of these patients for clinical characteristics and treatment outcomes. For each case, we recorded the patient’s age, sex, medical comorbidities, GA subtype, and medications.
This study was approved by the University of Pennsylvania’s institutional review board.
Results
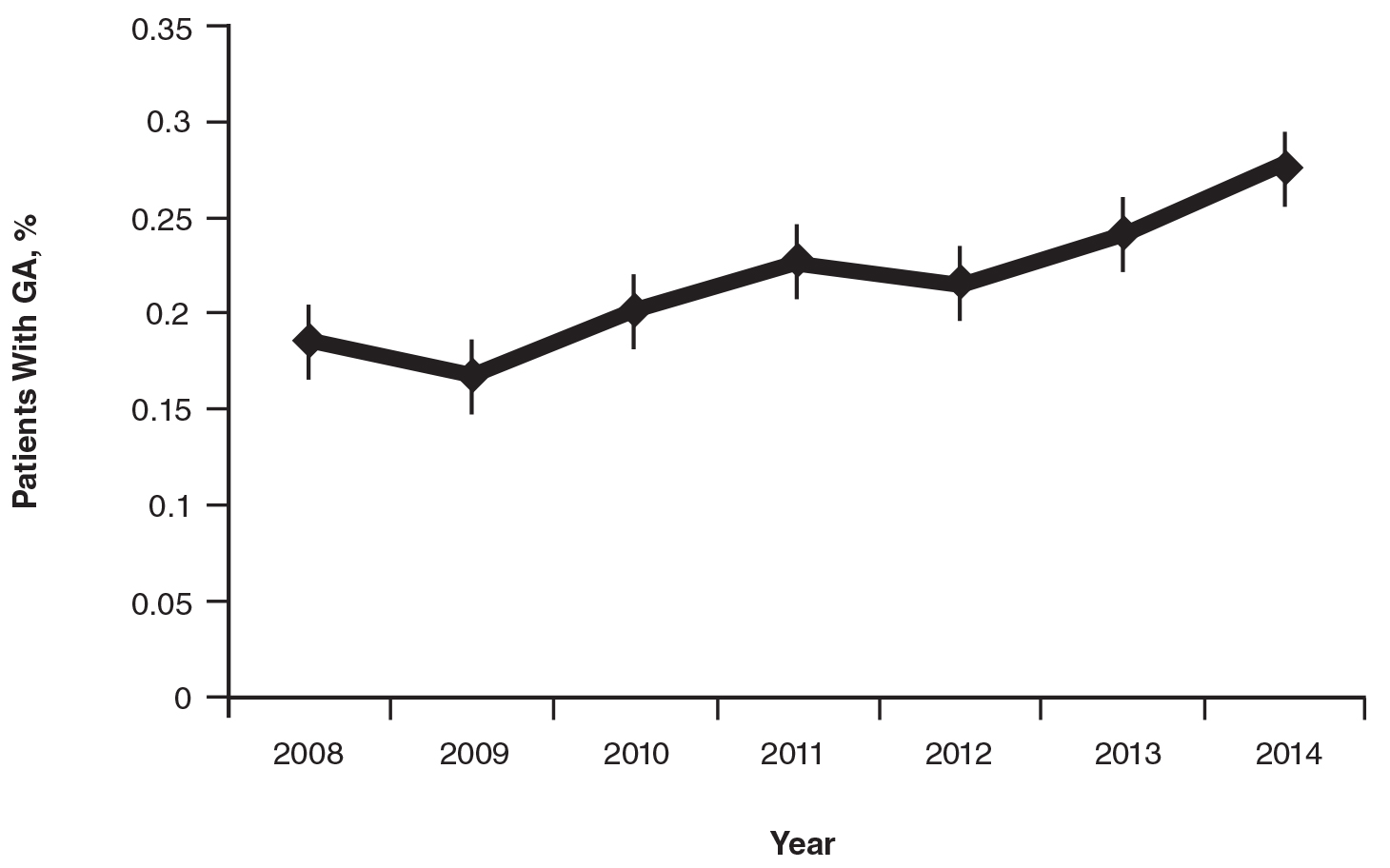
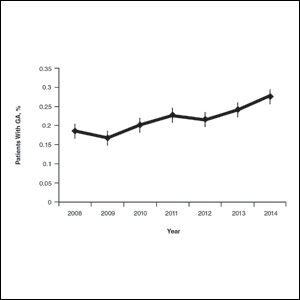
On average, the percentage of patients given a diagnosis of GA annually was 0.22% (95% CI, 0.19%-0.24%). A Pearson χ2 test was used to determine if any single annual percentage was significantly different from the others. We found a P value of .321, which suggests that the percentage of patients with GA seen annually has been stable from 2008 to 2014 (Figure).
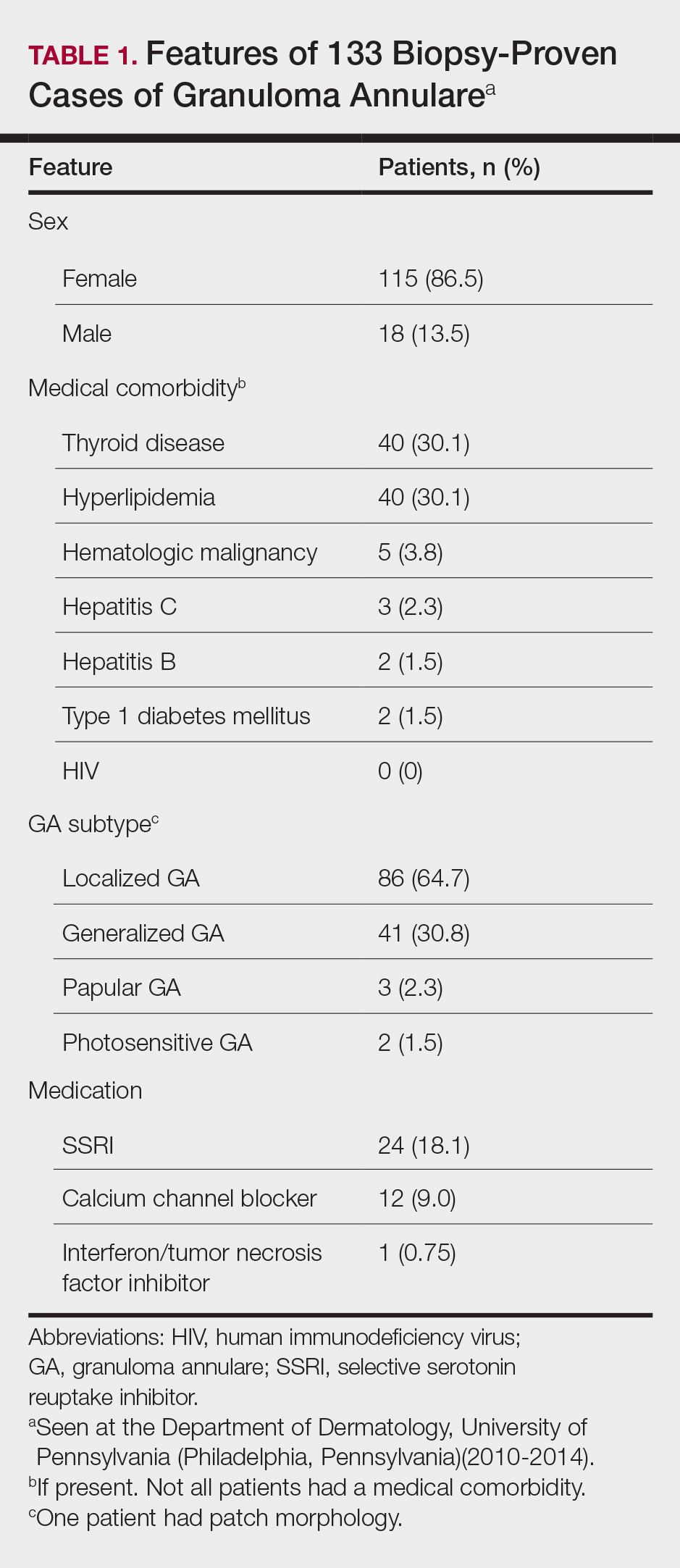
There were 133 cases of biopsy-proven GA that were reviewed for clinical characteristics; of them, 86.5% were female. Thyroid disease was noted in 30.1% of patients, hyperlipidemia in 30.1%, and hematologic malignancies in 3.8%. Type 1 diabetes mellitus was noted in 1.5% of patients. None of the patients were HIV-positive, 1.5% were hepatitis B–positive, and 2.3% were hepatitis C–positive. Of the 133 cases, 64.7% had localized GA and 30.8% had generalized GA. Photosensitive and papular GA were rarer (1.5% and 2.3% of cases, respectively). Use of a selective serotonin reuptake inhibitor (SSRI) was noted in 18.1% of patients; use of a calcium channel blocker was noted in 9.0% (Table 1).
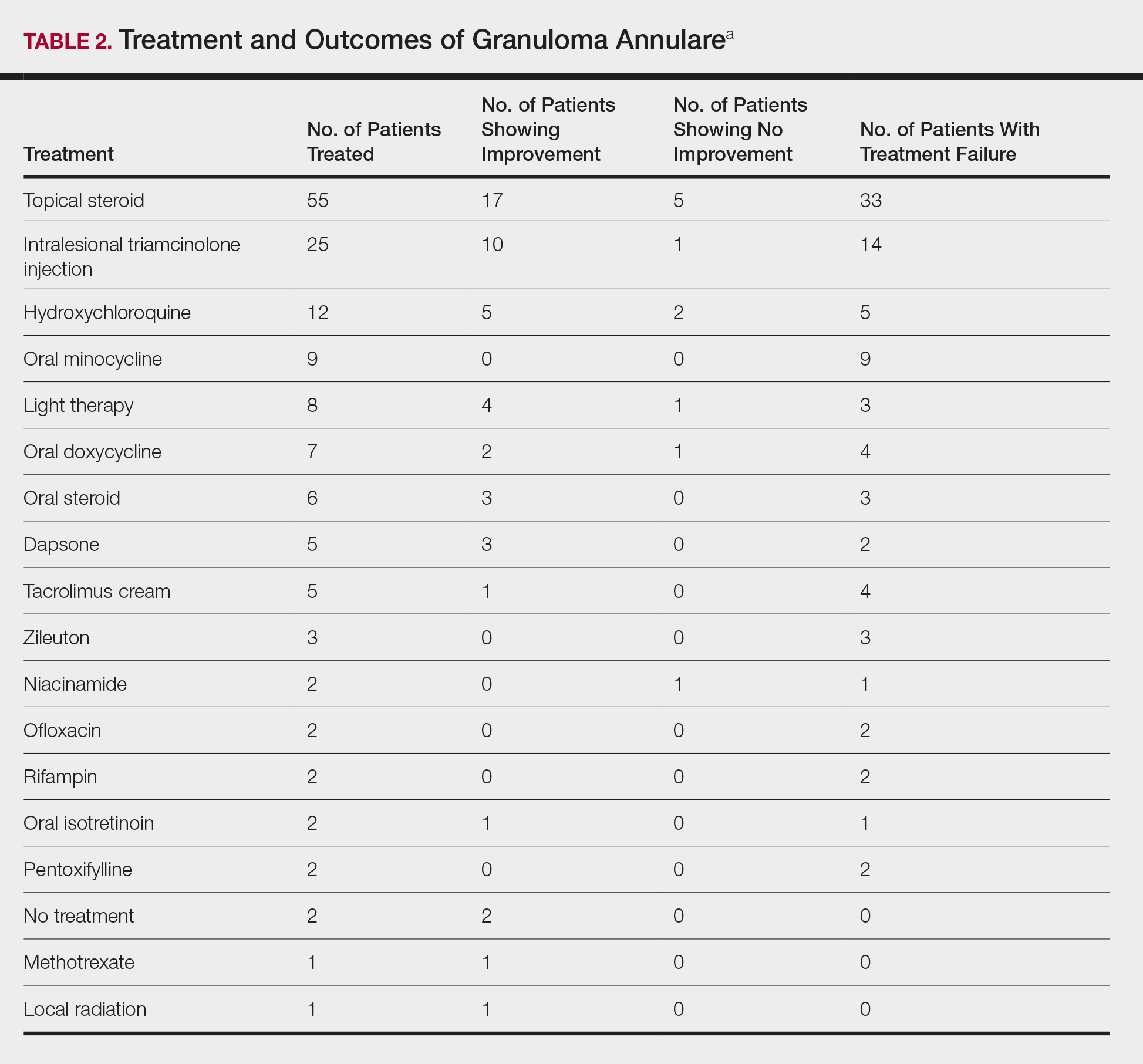
The most commonly prescribed treatment of GA was topical steroids; 30.9% of patients who were prescribed a topical steroid experienced improvement of their condition. Intralesional triamcinolone was the second most prescribed treatment of GA, with an improvement rate of 40.0% (Table 2).
Comment
We attempted to determine the period of prevalence of GA in a tertiary care, university-based referral practice and evaluate disease associations, treatments, and outcomes of patients with biopsy-proven GA. Our calculated period prevalence of GA of 0.22% to 0.27% is consistent with another review, which reported that 0.1% to 0.4% of new patients presenting to a dermatology practice were given a diagnosis of GA.1 More than 85% of the cases we reviewed were seen in females, a finding that is more heavily skewed compared to prior reports that have suggested a female to male ratio of approximately 1:1 to 2:1.1-7 Our findings suggest that GA is a female-predominant condition, or women may be more likely to seek evaluation for the condition.
More than 95% of the cases we reviewed were localized (64.7%) or generalized (30.8%) GA, making these variants the most common forms of GA, which is consistent with prior reports.1-3,8,9 Other varieties of GA—drug induced, patch, perforating, photosensitive, palmar, and papular—appear rare. Because this study was conducted at an adult hospital, subcutaneous GA, which often is seen in children, may be underrepresented. As a retrospective chart review, it is possible that documentation is insufficient to capture each rare variant.
Concomitant Disorders and Unrelated Medical Therapy
Hypothyroidism is statistically significantly overrepresented in our patient population (30.1%) compared with an average prevalence of 1% to 2% in iodine-replete populations (Fisher exact test, P<.001).10 This finding is consistent with prior small studies and cases series, which have suggested an association between autoimmune thyroiditis and GA.11-14
Despite prior reports of a possible association between HIV and GA,15-24 none of our patients had a diagnosis of HIV. However, many of our patients were not tested for HIV, which confounds our results and may represent a practice gap in the field.
At 1.5%, the prevalence of type 1 diabetes mellitus in our patients is slightly higher than the national average of 0.3%.25 However, based on a Fisher exact test of analysis of proportions, this difference is not statistically significant (P=.106).
At 1.5% and 2.3%, the prevalence of hepatitis B and hepatitis C, respectively, in our patients is slightly higher than the national average of 0.5% and 1%, respectively.26 However, based on a Fisher exact test of analysis of proportions, these differences are not statistically significant (P=.142 and P=.146, respectively).
Given the high prevalence of hyperlipidemia in the United States (31.7%), this disease is not overrepresented in our sample (30.1%), though others have suggested there may be a connection.27,28 Based on a Fisher exact test, this difference of proportions is not statistically significant (P=.780).
Selective serotonin reuptake inhibitor use is common in the United States; approximately 11% of Americans older than 12 years use an SSRI.29 At 18.1%, the use of SSRIs in our patient group was statistically significantly higher than the national average (Fisher exact test, P=.017), suggesting a possible association between SSRI use and development of GA, warranting further investigation.
The use of calcium channel blockers, interferon, and tumor necrosis factor inhibitors was not significantly associated with GA in our series.
GA Therapy
The most commonly used treatments for GA in our study were topical steroids and intralesional triamcinolone, followed by hydroxychloroquine; all treatments employed exhibited a widely variable response. Assessing treatment response via retrospective chart review is challenging and response rates may not be accurately captured.
Study Limitations
Our study had several limitations. In calculating the period prevalence of GA, our query was limited by the number of years that the EMR has been in place. The number of cases we reviewed for clinical characteristics was limited to 133, as many cases with the ICD-9 diagnosis of GA were not biopsy proven and therefore were not included in our review. Many of the cases we reviewed were lost to follow-up, which prevented us from determining treatment outcomes.
Another weakness of our study was that our query did not provide an estimate of incidence or prevalence of GA overall, as this analysis was not a population-based study. The power of our study was limited by the number of cases of GA seen annually and the number of patients lost to follow-up. Additionally, our study population may only be generalizable to other large academic centers.
Conclusion
This study further solidifies our understanding of the epidemiology of GA and diseases that can be associated with GA. We identified a higher female to male ratio than previous reports, and consistent with prior reports, we noted potential associations with conditions such as thyroid disease and hyperlipidemia. Our population demonstrated higher rates of SSRI use than expected, warranting further investigation. Dermatologists should be aware of potential disease associations with GA, but as a whole we need better data and larger studies to determine the appropriate evaluation and treatment for patients with GA.
- Muhlbauer JE. Granuloma annulare. J Am Acad Dermatol. 1980;3:217-230.
- Thornsberry LA, English JC 3rd. Etiology, diagnosis, and therapeutic management of granuloma annulare: an update. Am J Clin Dermatol. 2013;14:279-290.
- Wells RS, Smith MA. The natural history of granuloma annulare. Br J Dermatol. 1963;75:199-205.
- Wallet-Faber N, Farhi D, Gorin I, et al. Outcome of granuloma annulare: shorter duration is associated with younger age and recent onset. J Eur Acad Dermatol Venereol. 2010;24:103-104.
- Dahl MV. Granuloma annulare: long-term follow-up. Arch Dermatol. 2007;143:946-947.
- Yun JH, Lee JY, Kim MK, et al. Clinical and pathological features of generalized granuloma annulare with their correlation: a retrospective multicenter study in Korea. Ann Dermatol. 2009;21:113-119.
- Tan HH, Goh CL. Granuloma annulare: a review of 41 cases at the National Skin Centre. Ann Acad Med Singapore. 2000;29:714-718.
- Cyr PR. Diagnosis and management of granuloma annulare. Am Fam Physician. 2006;74:1729-1734.
- Smith MD, Downie JB, DiCostanzo D. Granuloma annulare. Int J Dermatol. 1997;36:326-333.
- Vanderpump MPJ. The epidemiology of thyroid diseases. In: Braverman LE, Utiger RD, eds. Werner and Ingbar’s The Thyroid: A Fundamental and Clinical Text. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005:398-496.
- Vázquez-López F, Pereiro M Jr, Manjón Haces JA, et al. Localized granuloma annulare and autoimmune thyroiditis in adult women: a case-control study. J Am Acad Dermatol. 2003;48:517-520.
- Vázquez-López F, González-López MA, Raya-Aguado C, et al. Localized granuloma annulare and autoimmune thyroiditis: a new case report. J Am Acad Dermatol. 2000;43(5, pt 2):943-945.
- Kappeler D, Troendle A, Mueller B. Localized granuloma annulare associated with autoimmune thyroid disease in a patient with a positive family history for autoimmune polyglandular syndrome type II. Eur J Endocrinol. 2001;145:101-102.
- Maschio M, Marigliano M, Sabbion A, et al. A rare case of granuloma annulare in a 5-year-old child with type 1 diabetes and autoimmune thyroiditis. Am J Dermatopathol. 2013;35:385-387.
- Smith NP. AIDS, Kaposi’s sarcoma and the dermatologist. J R Soc Med. 1985;78:97-99.
- Huerter CJ, Bass J, Bergfeld WF, et al. Perforating granuloma annulare in a patient with acquired immunodeficiency syndrome. Immunohistologic evaluation of the cellular infiltrate. Arch Dermatol. 1987;123:1217-1220.
- Jones SK, Harman RR. Atypical granuloma annulare in patients with the acquired immunodeficiency syndrome. J Am Acad Dermatol. 1989;20(2 pt 1):299-300.
- Devesa Parente JA, Dores JA, Aranha JM. Generalized perforating granuloma annulare: case report. Acta Dermatovenerol Croat. 2012;20:260-262.
- Ghadially R, Sibbald RG, Walter JB, et al. Granuloma annulare in patients with human immunodeficiency virus infections. J Am Acad Dermatol. 1989;20(2, pt 1):232-235.
- Toro JR, Chu P, Yen TS, et al. Granuloma annulare and human immunodeficiency virus infection. Arch Dermatol. 1999;135:1341-1346.
- Cohen PR. Granuloma annulare: a mucocutaneous condition in human immunodeficiency virus-infected patients. Arch Dermatol. 1999;135:1404-1407.
- O’Moore EJ, Nandawni R, Uthayakumar S, et al. HIV-associated granuloma annulare (HAGA): a report of six cases. Br J Dermatol. 2000;142:1054-1056.
- Kapembwa MS, Goolamali SK, Price A, et al. Granuloma annulare masquerading as molluscum contagiosum-like eruption in an HIV-positive African woman. J Am Acad Dermatol. 2003;49(suppl 2):S184-S186.
- Morris SD, Cerio R, Paige DG. An unusual presentation of diffuse granuloma annulare in an HIV-positive patient—immunohistochemical evidence of predominant CD8 lymphocytes. Clin Exp Dermatol. 2002;27:205-208.
- Maahs DM, West NA, Lawrence JM, et al. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39:481-497.
- Centers for Disease Control and Prevention. Viral hepatitis surveillance—United States, 2010. www.cdc.gov/hepatitis/statistics/2010surveillance/commentary.htm. Accessed November 10, 2018.
- Mozaffarian D, Benjamin EJ, Go AS, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:E29-E322.
- Wu W, Robinson-Bostom L, Kokkotou E, et al. Dyslipidemia in granuloma annulare: a case-control study. Arch Dermatol. 2012;148:1131-1136.
- Pratt LA, Brody DJ, Gu Q. Antidepressant Use in Persons Aged 12 and Over: United States, 2005-2008. NCHS Data Brief, No. 76. Hyattsville, MD: National Center for Health Statistics; 2011. http://www.cdc.gov/nchs/data/databriefs/db76.htm. Updated October 19, 2011. Accessed June 1, 2014.
Granuloma annulare (GA) is a granulomatous skin disorder of uncertain etiology. A number of clinical variants exist, most commonly localized annular plaques on the hands or feet, generalized lesions, or subcutaneous nodules in children. Histologically, GA exhibits granulomatous inflammation with either interstitial or palisading lymphocytes and histiocytes with mucin deposition.
Few data exist regarding the epidemiology of GA. Although the pathogenesis of GA is unknown, associations between GA and underlying systemic processes, such as diabetes mellitus, hyperlipidemia, thyroid disease, and human immunodeficiency virus (HIV), have been suggested.
The purpose of this retrospective study was to determine the number of cases of GA seen annually at the Department of Dermatology at the University of Pennsylvania (Philadelphia, Pennsylvania) from 2008 to 2014. Additionally, we reviewed all cases of biopsy-proven GA from 2010 to 2014 and reported the demographics, underlying medical comorbidities, medications, treatments, and outcomes seen in this patient population.
Methods
We identified the number of outpatients presenting with GA annually using PennSeek, a tool developed by the Penn Medicine Data Analytics Center to search electronic medical records (EMRs). We queried the EMR database to determine the number of discrete patients seen at the Department of Dermatology at the University of Pennsylvania annually from 2008 (the year the EMR was established) to 2014. We then used PennSeek to determine the number of patients given a diagnosis of GA annually from 2008 to 2014 based on the International Classification of Diseases, Ninth Revision (ICD-9).
After using PennSeek to identify all patients given the ICD-9 diagnosis of GA from 2008 to 2014, we reviewed the EMRs of these patients to identify cases that were biopsy proven. For the biopsy-proven cases of GA seen at the University of Pennsylvania from 2010 to 2014, we reviewed the EMRs of these patients for clinical characteristics and treatment outcomes. For each case, we recorded the patient’s age, sex, medical comorbidities, GA subtype, and medications.
This study was approved by the University of Pennsylvania’s institutional review board.
Results
On average, the percentage of patients given a diagnosis of GA annually was 0.22% (95% CI, 0.19%-0.24%). A Pearson χ2 test was used to determine if any single annual percentage was significantly different from the others. We found a P value of .321, which suggests that the percentage of patients with GA seen annually has been stable from 2008 to 2014 (Figure).
There were 133 cases of biopsy-proven GA that were reviewed for clinical characteristics; of them, 86.5% were female. Thyroid disease was noted in 30.1% of patients, hyperlipidemia in 30.1%, and hematologic malignancies in 3.8%. Type 1 diabetes mellitus was noted in 1.5% of patients. None of the patients were HIV-positive, 1.5% were hepatitis B–positive, and 2.3% were hepatitis C–positive. Of the 133 cases, 64.7% had localized GA and 30.8% had generalized GA. Photosensitive and papular GA were rarer (1.5% and 2.3% of cases, respectively). Use of a selective serotonin reuptake inhibitor (SSRI) was noted in 18.1% of patients; use of a calcium channel blocker was noted in 9.0% (Table 1).
The most commonly prescribed treatment of GA was topical steroids; 30.9% of patients who were prescribed a topical steroid experienced improvement of their condition. Intralesional triamcinolone was the second most prescribed treatment of GA, with an improvement rate of 40.0% (Table 2).
Comment
We attempted to determine the period of prevalence of GA in a tertiary care, university-based referral practice and evaluate disease associations, treatments, and outcomes of patients with biopsy-proven GA. Our calculated period prevalence of GA of 0.22% to 0.27% is consistent with another review, which reported that 0.1% to 0.4% of new patients presenting to a dermatology practice were given a diagnosis of GA.1 More than 85% of the cases we reviewed were seen in females, a finding that is more heavily skewed compared to prior reports that have suggested a female to male ratio of approximately 1:1 to 2:1.1-7 Our findings suggest that GA is a female-predominant condition, or women may be more likely to seek evaluation for the condition.
More than 95% of the cases we reviewed were localized (64.7%) or generalized (30.8%) GA, making these variants the most common forms of GA, which is consistent with prior reports.1-3,8,9 Other varieties of GA—drug induced, patch, perforating, photosensitive, palmar, and papular—appear rare. Because this study was conducted at an adult hospital, subcutaneous GA, which often is seen in children, may be underrepresented. As a retrospective chart review, it is possible that documentation is insufficient to capture each rare variant.
Concomitant Disorders and Unrelated Medical Therapy
Hypothyroidism is statistically significantly overrepresented in our patient population (30.1%) compared with an average prevalence of 1% to 2% in iodine-replete populations (Fisher exact test, P<.001).10 This finding is consistent with prior small studies and cases series, which have suggested an association between autoimmune thyroiditis and GA.11-14
Despite prior reports of a possible association between HIV and GA,15-24 none of our patients had a diagnosis of HIV. However, many of our patients were not tested for HIV, which confounds our results and may represent a practice gap in the field.
At 1.5%, the prevalence of type 1 diabetes mellitus in our patients is slightly higher than the national average of 0.3%.25 However, based on a Fisher exact test of analysis of proportions, this difference is not statistically significant (P=.106).
At 1.5% and 2.3%, the prevalence of hepatitis B and hepatitis C, respectively, in our patients is slightly higher than the national average of 0.5% and 1%, respectively.26 However, based on a Fisher exact test of analysis of proportions, these differences are not statistically significant (P=.142 and P=.146, respectively).
Given the high prevalence of hyperlipidemia in the United States (31.7%), this disease is not overrepresented in our sample (30.1%), though others have suggested there may be a connection.27,28 Based on a Fisher exact test, this difference of proportions is not statistically significant (P=.780).
Selective serotonin reuptake inhibitor use is common in the United States; approximately 11% of Americans older than 12 years use an SSRI.29 At 18.1%, the use of SSRIs in our patient group was statistically significantly higher than the national average (Fisher exact test, P=.017), suggesting a possible association between SSRI use and development of GA, warranting further investigation.
The use of calcium channel blockers, interferon, and tumor necrosis factor inhibitors was not significantly associated with GA in our series.
GA Therapy
The most commonly used treatments for GA in our study were topical steroids and intralesional triamcinolone, followed by hydroxychloroquine; all treatments employed exhibited a widely variable response. Assessing treatment response via retrospective chart review is challenging and response rates may not be accurately captured.
Study Limitations
Our study had several limitations. In calculating the period prevalence of GA, our query was limited by the number of years that the EMR has been in place. The number of cases we reviewed for clinical characteristics was limited to 133, as many cases with the ICD-9 diagnosis of GA were not biopsy proven and therefore were not included in our review. Many of the cases we reviewed were lost to follow-up, which prevented us from determining treatment outcomes.
Another weakness of our study was that our query did not provide an estimate of incidence or prevalence of GA overall, as this analysis was not a population-based study. The power of our study was limited by the number of cases of GA seen annually and the number of patients lost to follow-up. Additionally, our study population may only be generalizable to other large academic centers.
Conclusion
This study further solidifies our understanding of the epidemiology of GA and diseases that can be associated with GA. We identified a higher female to male ratio than previous reports, and consistent with prior reports, we noted potential associations with conditions such as thyroid disease and hyperlipidemia. Our population demonstrated higher rates of SSRI use than expected, warranting further investigation. Dermatologists should be aware of potential disease associations with GA, but as a whole we need better data and larger studies to determine the appropriate evaluation and treatment for patients with GA.
Granuloma annulare (GA) is a granulomatous skin disorder of uncertain etiology. A number of clinical variants exist, most commonly localized annular plaques on the hands or feet, generalized lesions, or subcutaneous nodules in children. Histologically, GA exhibits granulomatous inflammation with either interstitial or palisading lymphocytes and histiocytes with mucin deposition.
Few data exist regarding the epidemiology of GA. Although the pathogenesis of GA is unknown, associations between GA and underlying systemic processes, such as diabetes mellitus, hyperlipidemia, thyroid disease, and human immunodeficiency virus (HIV), have been suggested.
The purpose of this retrospective study was to determine the number of cases of GA seen annually at the Department of Dermatology at the University of Pennsylvania (Philadelphia, Pennsylvania) from 2008 to 2014. Additionally, we reviewed all cases of biopsy-proven GA from 2010 to 2014 and reported the demographics, underlying medical comorbidities, medications, treatments, and outcomes seen in this patient population.
Methods
We identified the number of outpatients presenting with GA annually using PennSeek, a tool developed by the Penn Medicine Data Analytics Center to search electronic medical records (EMRs). We queried the EMR database to determine the number of discrete patients seen at the Department of Dermatology at the University of Pennsylvania annually from 2008 (the year the EMR was established) to 2014. We then used PennSeek to determine the number of patients given a diagnosis of GA annually from 2008 to 2014 based on the International Classification of Diseases, Ninth Revision (ICD-9).
After using PennSeek to identify all patients given the ICD-9 diagnosis of GA from 2008 to 2014, we reviewed the EMRs of these patients to identify cases that were biopsy proven. For the biopsy-proven cases of GA seen at the University of Pennsylvania from 2010 to 2014, we reviewed the EMRs of these patients for clinical characteristics and treatment outcomes. For each case, we recorded the patient’s age, sex, medical comorbidities, GA subtype, and medications.
This study was approved by the University of Pennsylvania’s institutional review board.
Results
On average, the percentage of patients given a diagnosis of GA annually was 0.22% (95% CI, 0.19%-0.24%). A Pearson χ2 test was used to determine if any single annual percentage was significantly different from the others. We found a P value of .321, which suggests that the percentage of patients with GA seen annually has been stable from 2008 to 2014 (Figure).
There were 133 cases of biopsy-proven GA that were reviewed for clinical characteristics; of them, 86.5% were female. Thyroid disease was noted in 30.1% of patients, hyperlipidemia in 30.1%, and hematologic malignancies in 3.8%. Type 1 diabetes mellitus was noted in 1.5% of patients. None of the patients were HIV-positive, 1.5% were hepatitis B–positive, and 2.3% were hepatitis C–positive. Of the 133 cases, 64.7% had localized GA and 30.8% had generalized GA. Photosensitive and papular GA were rarer (1.5% and 2.3% of cases, respectively). Use of a selective serotonin reuptake inhibitor (SSRI) was noted in 18.1% of patients; use of a calcium channel blocker was noted in 9.0% (Table 1).
The most commonly prescribed treatment of GA was topical steroids; 30.9% of patients who were prescribed a topical steroid experienced improvement of their condition. Intralesional triamcinolone was the second most prescribed treatment of GA, with an improvement rate of 40.0% (Table 2).
Comment
We attempted to determine the period of prevalence of GA in a tertiary care, university-based referral practice and evaluate disease associations, treatments, and outcomes of patients with biopsy-proven GA. Our calculated period prevalence of GA of 0.22% to 0.27% is consistent with another review, which reported that 0.1% to 0.4% of new patients presenting to a dermatology practice were given a diagnosis of GA.1 More than 85% of the cases we reviewed were seen in females, a finding that is more heavily skewed compared to prior reports that have suggested a female to male ratio of approximately 1:1 to 2:1.1-7 Our findings suggest that GA is a female-predominant condition, or women may be more likely to seek evaluation for the condition.
More than 95% of the cases we reviewed were localized (64.7%) or generalized (30.8%) GA, making these variants the most common forms of GA, which is consistent with prior reports.1-3,8,9 Other varieties of GA—drug induced, patch, perforating, photosensitive, palmar, and papular—appear rare. Because this study was conducted at an adult hospital, subcutaneous GA, which often is seen in children, may be underrepresented. As a retrospective chart review, it is possible that documentation is insufficient to capture each rare variant.
Concomitant Disorders and Unrelated Medical Therapy
Hypothyroidism is statistically significantly overrepresented in our patient population (30.1%) compared with an average prevalence of 1% to 2% in iodine-replete populations (Fisher exact test, P<.001).10 This finding is consistent with prior small studies and cases series, which have suggested an association between autoimmune thyroiditis and GA.11-14
Despite prior reports of a possible association between HIV and GA,15-24 none of our patients had a diagnosis of HIV. However, many of our patients were not tested for HIV, which confounds our results and may represent a practice gap in the field.
At 1.5%, the prevalence of type 1 diabetes mellitus in our patients is slightly higher than the national average of 0.3%.25 However, based on a Fisher exact test of analysis of proportions, this difference is not statistically significant (P=.106).
At 1.5% and 2.3%, the prevalence of hepatitis B and hepatitis C, respectively, in our patients is slightly higher than the national average of 0.5% and 1%, respectively.26 However, based on a Fisher exact test of analysis of proportions, these differences are not statistically significant (P=.142 and P=.146, respectively).
Given the high prevalence of hyperlipidemia in the United States (31.7%), this disease is not overrepresented in our sample (30.1%), though others have suggested there may be a connection.27,28 Based on a Fisher exact test, this difference of proportions is not statistically significant (P=.780).
Selective serotonin reuptake inhibitor use is common in the United States; approximately 11% of Americans older than 12 years use an SSRI.29 At 18.1%, the use of SSRIs in our patient group was statistically significantly higher than the national average (Fisher exact test, P=.017), suggesting a possible association between SSRI use and development of GA, warranting further investigation.
The use of calcium channel blockers, interferon, and tumor necrosis factor inhibitors was not significantly associated with GA in our series.
GA Therapy
The most commonly used treatments for GA in our study were topical steroids and intralesional triamcinolone, followed by hydroxychloroquine; all treatments employed exhibited a widely variable response. Assessing treatment response via retrospective chart review is challenging and response rates may not be accurately captured.
Study Limitations
Our study had several limitations. In calculating the period prevalence of GA, our query was limited by the number of years that the EMR has been in place. The number of cases we reviewed for clinical characteristics was limited to 133, as many cases with the ICD-9 diagnosis of GA were not biopsy proven and therefore were not included in our review. Many of the cases we reviewed were lost to follow-up, which prevented us from determining treatment outcomes.
Another weakness of our study was that our query did not provide an estimate of incidence or prevalence of GA overall, as this analysis was not a population-based study. The power of our study was limited by the number of cases of GA seen annually and the number of patients lost to follow-up. Additionally, our study population may only be generalizable to other large academic centers.
Conclusion
This study further solidifies our understanding of the epidemiology of GA and diseases that can be associated with GA. We identified a higher female to male ratio than previous reports, and consistent with prior reports, we noted potential associations with conditions such as thyroid disease and hyperlipidemia. Our population demonstrated higher rates of SSRI use than expected, warranting further investigation. Dermatologists should be aware of potential disease associations with GA, but as a whole we need better data and larger studies to determine the appropriate evaluation and treatment for patients with GA.
- Muhlbauer JE. Granuloma annulare. J Am Acad Dermatol. 1980;3:217-230.
- Thornsberry LA, English JC 3rd. Etiology, diagnosis, and therapeutic management of granuloma annulare: an update. Am J Clin Dermatol. 2013;14:279-290.
- Wells RS, Smith MA. The natural history of granuloma annulare. Br J Dermatol. 1963;75:199-205.
- Wallet-Faber N, Farhi D, Gorin I, et al. Outcome of granuloma annulare: shorter duration is associated with younger age and recent onset. J Eur Acad Dermatol Venereol. 2010;24:103-104.
- Dahl MV. Granuloma annulare: long-term follow-up. Arch Dermatol. 2007;143:946-947.
- Yun JH, Lee JY, Kim MK, et al. Clinical and pathological features of generalized granuloma annulare with their correlation: a retrospective multicenter study in Korea. Ann Dermatol. 2009;21:113-119.
- Tan HH, Goh CL. Granuloma annulare: a review of 41 cases at the National Skin Centre. Ann Acad Med Singapore. 2000;29:714-718.
- Cyr PR. Diagnosis and management of granuloma annulare. Am Fam Physician. 2006;74:1729-1734.
- Smith MD, Downie JB, DiCostanzo D. Granuloma annulare. Int J Dermatol. 1997;36:326-333.
- Vanderpump MPJ. The epidemiology of thyroid diseases. In: Braverman LE, Utiger RD, eds. Werner and Ingbar’s The Thyroid: A Fundamental and Clinical Text. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005:398-496.
- Vázquez-López F, Pereiro M Jr, Manjón Haces JA, et al. Localized granuloma annulare and autoimmune thyroiditis in adult women: a case-control study. J Am Acad Dermatol. 2003;48:517-520.
- Vázquez-López F, González-López MA, Raya-Aguado C, et al. Localized granuloma annulare and autoimmune thyroiditis: a new case report. J Am Acad Dermatol. 2000;43(5, pt 2):943-945.
- Kappeler D, Troendle A, Mueller B. Localized granuloma annulare associated with autoimmune thyroid disease in a patient with a positive family history for autoimmune polyglandular syndrome type II. Eur J Endocrinol. 2001;145:101-102.
- Maschio M, Marigliano M, Sabbion A, et al. A rare case of granuloma annulare in a 5-year-old child with type 1 diabetes and autoimmune thyroiditis. Am J Dermatopathol. 2013;35:385-387.
- Smith NP. AIDS, Kaposi’s sarcoma and the dermatologist. J R Soc Med. 1985;78:97-99.
- Huerter CJ, Bass J, Bergfeld WF, et al. Perforating granuloma annulare in a patient with acquired immunodeficiency syndrome. Immunohistologic evaluation of the cellular infiltrate. Arch Dermatol. 1987;123:1217-1220.
- Jones SK, Harman RR. Atypical granuloma annulare in patients with the acquired immunodeficiency syndrome. J Am Acad Dermatol. 1989;20(2 pt 1):299-300.
- Devesa Parente JA, Dores JA, Aranha JM. Generalized perforating granuloma annulare: case report. Acta Dermatovenerol Croat. 2012;20:260-262.
- Ghadially R, Sibbald RG, Walter JB, et al. Granuloma annulare in patients with human immunodeficiency virus infections. J Am Acad Dermatol. 1989;20(2, pt 1):232-235.
- Toro JR, Chu P, Yen TS, et al. Granuloma annulare and human immunodeficiency virus infection. Arch Dermatol. 1999;135:1341-1346.
- Cohen PR. Granuloma annulare: a mucocutaneous condition in human immunodeficiency virus-infected patients. Arch Dermatol. 1999;135:1404-1407.
- O’Moore EJ, Nandawni R, Uthayakumar S, et al. HIV-associated granuloma annulare (HAGA): a report of six cases. Br J Dermatol. 2000;142:1054-1056.
- Kapembwa MS, Goolamali SK, Price A, et al. Granuloma annulare masquerading as molluscum contagiosum-like eruption in an HIV-positive African woman. J Am Acad Dermatol. 2003;49(suppl 2):S184-S186.
- Morris SD, Cerio R, Paige DG. An unusual presentation of diffuse granuloma annulare in an HIV-positive patient—immunohistochemical evidence of predominant CD8 lymphocytes. Clin Exp Dermatol. 2002;27:205-208.
- Maahs DM, West NA, Lawrence JM, et al. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39:481-497.
- Centers for Disease Control and Prevention. Viral hepatitis surveillance—United States, 2010. www.cdc.gov/hepatitis/statistics/2010surveillance/commentary.htm. Accessed November 10, 2018.
- Mozaffarian D, Benjamin EJ, Go AS, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:E29-E322.
- Wu W, Robinson-Bostom L, Kokkotou E, et al. Dyslipidemia in granuloma annulare: a case-control study. Arch Dermatol. 2012;148:1131-1136.
- Pratt LA, Brody DJ, Gu Q. Antidepressant Use in Persons Aged 12 and Over: United States, 2005-2008. NCHS Data Brief, No. 76. Hyattsville, MD: National Center for Health Statistics; 2011. http://www.cdc.gov/nchs/data/databriefs/db76.htm. Updated October 19, 2011. Accessed June 1, 2014.
- Muhlbauer JE. Granuloma annulare. J Am Acad Dermatol. 1980;3:217-230.
- Thornsberry LA, English JC 3rd. Etiology, diagnosis, and therapeutic management of granuloma annulare: an update. Am J Clin Dermatol. 2013;14:279-290.
- Wells RS, Smith MA. The natural history of granuloma annulare. Br J Dermatol. 1963;75:199-205.
- Wallet-Faber N, Farhi D, Gorin I, et al. Outcome of granuloma annulare: shorter duration is associated with younger age and recent onset. J Eur Acad Dermatol Venereol. 2010;24:103-104.
- Dahl MV. Granuloma annulare: long-term follow-up. Arch Dermatol. 2007;143:946-947.
- Yun JH, Lee JY, Kim MK, et al. Clinical and pathological features of generalized granuloma annulare with their correlation: a retrospective multicenter study in Korea. Ann Dermatol. 2009;21:113-119.
- Tan HH, Goh CL. Granuloma annulare: a review of 41 cases at the National Skin Centre. Ann Acad Med Singapore. 2000;29:714-718.
- Cyr PR. Diagnosis and management of granuloma annulare. Am Fam Physician. 2006;74:1729-1734.
- Smith MD, Downie JB, DiCostanzo D. Granuloma annulare. Int J Dermatol. 1997;36:326-333.
- Vanderpump MPJ. The epidemiology of thyroid diseases. In: Braverman LE, Utiger RD, eds. Werner and Ingbar’s The Thyroid: A Fundamental and Clinical Text. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005:398-496.
- Vázquez-López F, Pereiro M Jr, Manjón Haces JA, et al. Localized granuloma annulare and autoimmune thyroiditis in adult women: a case-control study. J Am Acad Dermatol. 2003;48:517-520.
- Vázquez-López F, González-López MA, Raya-Aguado C, et al. Localized granuloma annulare and autoimmune thyroiditis: a new case report. J Am Acad Dermatol. 2000;43(5, pt 2):943-945.
- Kappeler D, Troendle A, Mueller B. Localized granuloma annulare associated with autoimmune thyroid disease in a patient with a positive family history for autoimmune polyglandular syndrome type II. Eur J Endocrinol. 2001;145:101-102.
- Maschio M, Marigliano M, Sabbion A, et al. A rare case of granuloma annulare in a 5-year-old child with type 1 diabetes and autoimmune thyroiditis. Am J Dermatopathol. 2013;35:385-387.
- Smith NP. AIDS, Kaposi’s sarcoma and the dermatologist. J R Soc Med. 1985;78:97-99.
- Huerter CJ, Bass J, Bergfeld WF, et al. Perforating granuloma annulare in a patient with acquired immunodeficiency syndrome. Immunohistologic evaluation of the cellular infiltrate. Arch Dermatol. 1987;123:1217-1220.
- Jones SK, Harman RR. Atypical granuloma annulare in patients with the acquired immunodeficiency syndrome. J Am Acad Dermatol. 1989;20(2 pt 1):299-300.
- Devesa Parente JA, Dores JA, Aranha JM. Generalized perforating granuloma annulare: case report. Acta Dermatovenerol Croat. 2012;20:260-262.
- Ghadially R, Sibbald RG, Walter JB, et al. Granuloma annulare in patients with human immunodeficiency virus infections. J Am Acad Dermatol. 1989;20(2, pt 1):232-235.
- Toro JR, Chu P, Yen TS, et al. Granuloma annulare and human immunodeficiency virus infection. Arch Dermatol. 1999;135:1341-1346.
- Cohen PR. Granuloma annulare: a mucocutaneous condition in human immunodeficiency virus-infected patients. Arch Dermatol. 1999;135:1404-1407.
- O’Moore EJ, Nandawni R, Uthayakumar S, et al. HIV-associated granuloma annulare (HAGA): a report of six cases. Br J Dermatol. 2000;142:1054-1056.
- Kapembwa MS, Goolamali SK, Price A, et al. Granuloma annulare masquerading as molluscum contagiosum-like eruption in an HIV-positive African woman. J Am Acad Dermatol. 2003;49(suppl 2):S184-S186.
- Morris SD, Cerio R, Paige DG. An unusual presentation of diffuse granuloma annulare in an HIV-positive patient—immunohistochemical evidence of predominant CD8 lymphocytes. Clin Exp Dermatol. 2002;27:205-208.
- Maahs DM, West NA, Lawrence JM, et al. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39:481-497.
- Centers for Disease Control and Prevention. Viral hepatitis surveillance—United States, 2010. www.cdc.gov/hepatitis/statistics/2010surveillance/commentary.htm. Accessed November 10, 2018.
- Mozaffarian D, Benjamin EJ, Go AS, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:E29-E322.
- Wu W, Robinson-Bostom L, Kokkotou E, et al. Dyslipidemia in granuloma annulare: a case-control study. Arch Dermatol. 2012;148:1131-1136.
- Pratt LA, Brody DJ, Gu Q. Antidepressant Use in Persons Aged 12 and Over: United States, 2005-2008. NCHS Data Brief, No. 76. Hyattsville, MD: National Center for Health Statistics; 2011. http://www.cdc.gov/nchs/data/databriefs/db76.htm. Updated October 19, 2011. Accessed June 1, 2014.
Practice Points
- Although the pathogenesis of granuloma annulare (GA) is unknown, associations between the disorder and underlying systemic processes (eg, diabetes mellitus, hyperlipidemia, thyroid disease, human immunodeficiency virus) have been proposed.
- This study elicited a period prevalence of GA of 0.22% to 0.27%.
- The most commonly used treatments of GA were topical steroids and intralesional triamcinolone, followed by hydroxychloroquine.
Safety and Efficacy of Halobetasol Propionate Lotion 0.01% in the Treatment of Moderate to Severe Plaque Psoriasis: A Pooled Analysis of 2 Phase 3 Studies
Psoriasis is a chronic, immune-mediated, inflammatory disease affecting almost 2% of the population.1-3 It is characterized by patches of raised reddish skin covered by silvery-white scales. Most patients have limited disease (<5% body surface area [BSA] involvement) that can be managed with topical agents.4 Topical corticosteroids (TCSs) are considered first-line therapy for mild to moderate disease because of the inflammatory nature of the condition and often are used in conjunction with systemic agents in more severe psoriasis.4
As many as 20% to 30% of patients with moderate to severe plaque psoriasis have inadequate disease control.5 Several factors may affect patient outcomes; however, drug selection and patient adherence are important given the chronic nature of the disease. A survey of 1200 patients with psoriasis reported nonadherence rates of 73% with topical therapy.6 In addition, patients tend to apply less than the recommended dose or abandon treatment altogether if rapid improvement does not occur7,8; it is not uncommon for patients with psoriasis to mistakenly believe treatment will improve their condition within 1 to 2 weeks.9 Patient satisfaction with topical treatments is low, partly because of these false expectations and formulation issues. Treatments can be greasy and sticky, with unpleasant odors and the potential to stain clothes and linens.7,10 Safety concerns with TCSs also limit their consecutive use beyond 2 to 4 weeks, which is not ideal for a disease that requires a long-term management strategy.
A potent/superpotent TCS that is administered once daily and has a safety profile that affords longer-term, once-daily treatment in an aesthetically pleasing formulation would seem ideal. Herein, we investigate the safety and tolerability of a novel low-concentration (0.01%) lotion formulation of halobetasol propionate (HP), reporting on the pooled data from 2 phase 3 clinical studies in participants with moderate to severe psoriasis.
METHODS
Study Design
We conducted 2 multicenter, double-blind, randomized, parallel-group phase 3 studies to assess the safety, tolerability, and efficacy of HP lotion 0.01% in participants with a clinical diagnosis of moderate to severe psoriasis with an investigator global assessment (IGA) score of 3 or 4 and an affected BSA of 3% to 12%. Participants were randomized (2:1) to receive HP lotion or vehicle applied topically to the affected area once daily for 8 weeks.
Inclusion and Exclusion Criteria
The studies included individuals of either sex aged 18 years or older. A target lesion was defined primarily to assess signs of psoriasis, measuring 16 to 100 cm2, with a score of 3 (moderate) or higher for 2 of 3 different psoriasis signs—erythema, plaque elevation, and scaling—and summed score of 8 or higher, with no sign scoring less than 2. Participants who had pustular psoriasis or used phototherapy, photochemotherapy, or systemic psoriasis therapy within the prior 4 weeks or biologics within the prior 3 months, or those who were diagnosed with skin conditions that would interfere with the interpretation of results were excluded from the studies.
Study Oversight
Participants provided written informed consent before study-related procedures were performed, and the protocol and consent were approved by institutional review boards or ethics committees at all investigational sites. The study was conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki.
Efficacy Assessment
A 5-point scale ranging from 0 (clear) to 4 (severe) was used by the investigator at each study visit to assess the overall psoriasis severity of the treatable areas. Treatment success (the percentage of participants with at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]) was evaluated at weeks 2, 4, 6, and 8, w
Signs of psoriasis at the target lesion were assessed at each visit using individual 5-point scales ranging from 0 (clear) to 4 (severe). Treatment success was defined as at least a 2-grade improvement from baseline score for each of the key signs—erythema, plaque elevation, and scaling—and reported at weeks 2, 4, 6, and 8, with a posttreatment follow-up at week 12.
Affected BSA also was evaluated at each visit. In addition, an IGA×BSA composite score was calculated by multiplying the IGA by the BSA (range, 9–48 [eg, maximum IGA=4 and maximum BSA=12]) at each time point. The mean percentage change in IGA×BSA from baseline was calculated for each study visit. Additional end points included the achievement of a 50%, 75%, and 90% or greater reduction from baseline IGA×BSA score—IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90—at week 8.
Safety Assessment
Safety evaluations including adverse events (AEs), local skin reactions (LSRs), vital signs, laboratory evaluations, and physical examinations were performed. Information on reported and observed AEs was obtained at each visit. Routine safety laboratory tests were performed at screening, week 4, and week 8. An abbreviated physical examination was performed at baseline, week 8 (end of treatment), and week 12 (end of study). Treatment areas also were examined by the investigator at baseline and each subsequent visit for the presence or absence of marked known drug-related AEs including skin atrophy, striae, telangiectasia, and folliculitis.
LSR Assessment
Local skin reactions such as itching, dryness, and burning/stinging were evaluated at each study visit using 4-point scales ranging from 0 (clear) to 3 (severe). Given the nature of the disease, the presence of LSRs and symptoms at baseline is commonplace, and as such, these evaluations identified both improvement and any emergent issues.
Statistical Analysis
The primary study goal was to assess differences in treatment efficacy between HP lotion and vehicle with respect to IGA. All statistical processing was performed using SAS unless otherwise stated; statistical tests were 2-sided and performed at the 0.05 level of significance. Markov Chain Monte Carlo multiple imputation was the primary method used to handle missing efficacy data. No imputations were made for missing safety data. All participants were randomized, and the dispensed study drug was included in the intention-to-treat analysis set. This analysis was considered primary for the evaluation of efficacy. Data were analyzed using Cochran-Mantel-Haenszel tests, stratified by analysis center.
Body surface area data were analyzed in a post hoc analysis of covariance with factors of treatment and analysis center and baseline BSA as a covariate. P values for comparisons of percentage change in IGA×BSA were derived from a Wilcoxon rank sum test. For IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90, P values were derived from a Cochran-Mantel-Haenszel test. Last observation carried forward was used to impute data for IGA and BSA through week 8 prior to analysis.
The primary safety analysis was conducted at week 8 using the safety analysis set, which included all participants who were randomized, received at least 1 confirmed dose of the study drug, and had at least 1 postbaseline safety assessment. Adverse events were recorded and classified using the Medical Dictionary for Regulatory Activities (MedDRA, Version 18.0). A post hoc Wilcoxon rank sum test was conducted to compare itching, dryness, and burning/stinging scores at week 8 for HP lotion versus vehicle.
RESULTS
Participant Disposition
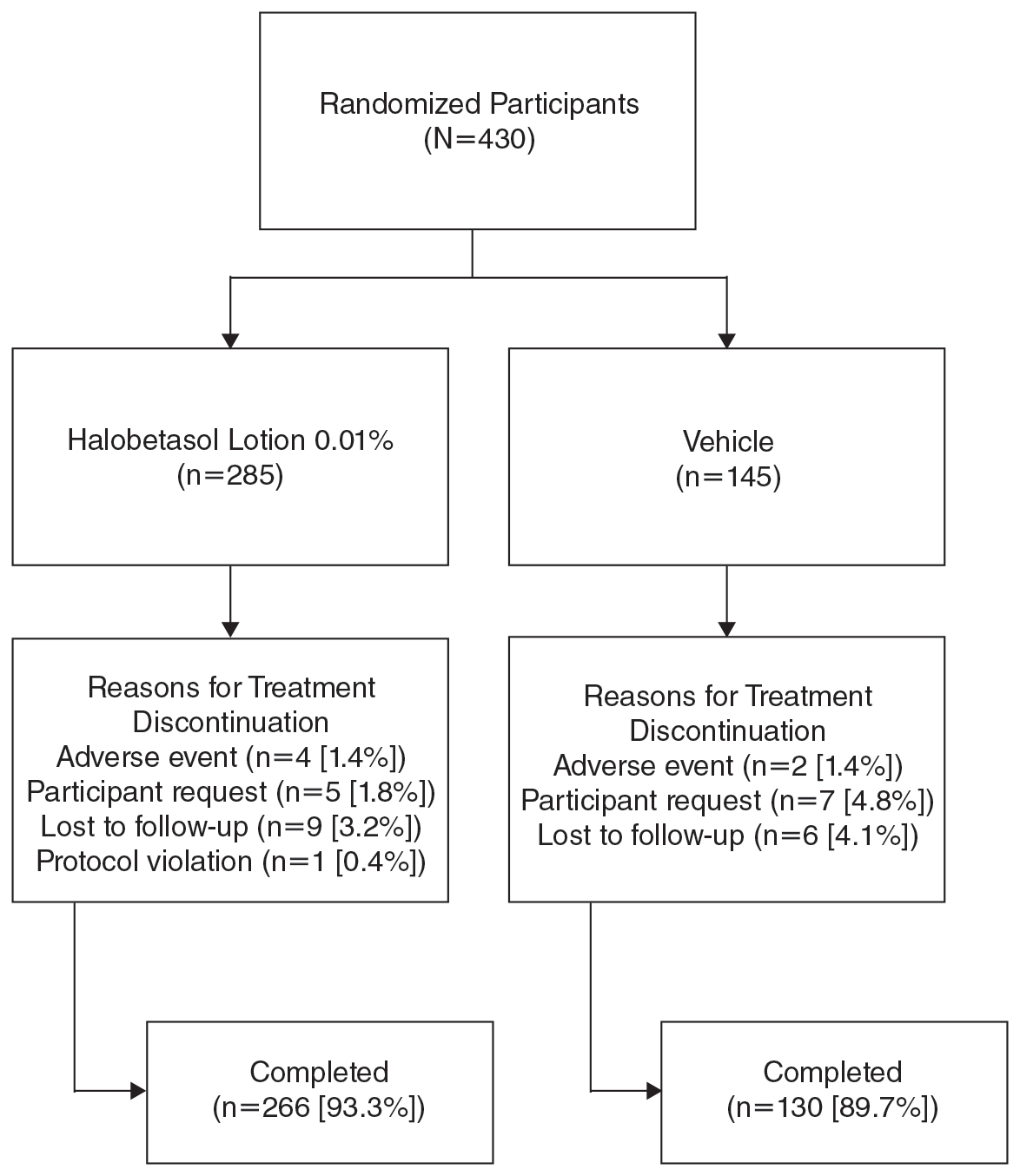
Overall, 430 participants were randomized (2:1) to HP lotion (n=285) or vehicle (n=145)(eFigure 1) and included in the intention-to-treat population. Across the 2 studies, 93.3% (n=266) of participants treated with HP lotion and 89.7% (n=130) of participants treated with vehicle completed treatment. The main reasons for study discontinuation with HP lotion were lost to follow-up (3.2%; n=9), participant request (1.8%; n=5), and AEs (1.4%; n=4). Participant request (4.8%; n=7), lost to follow-up (4.1%; n=6), and AEs (1.4%; n=2) also were the main reasons for treatment discontinuation in the vehicle arm.
A total of 426 participants were included in the safety population, with no postbaseline safety evaluation in 4 participants.
Baseline Participant Demographics
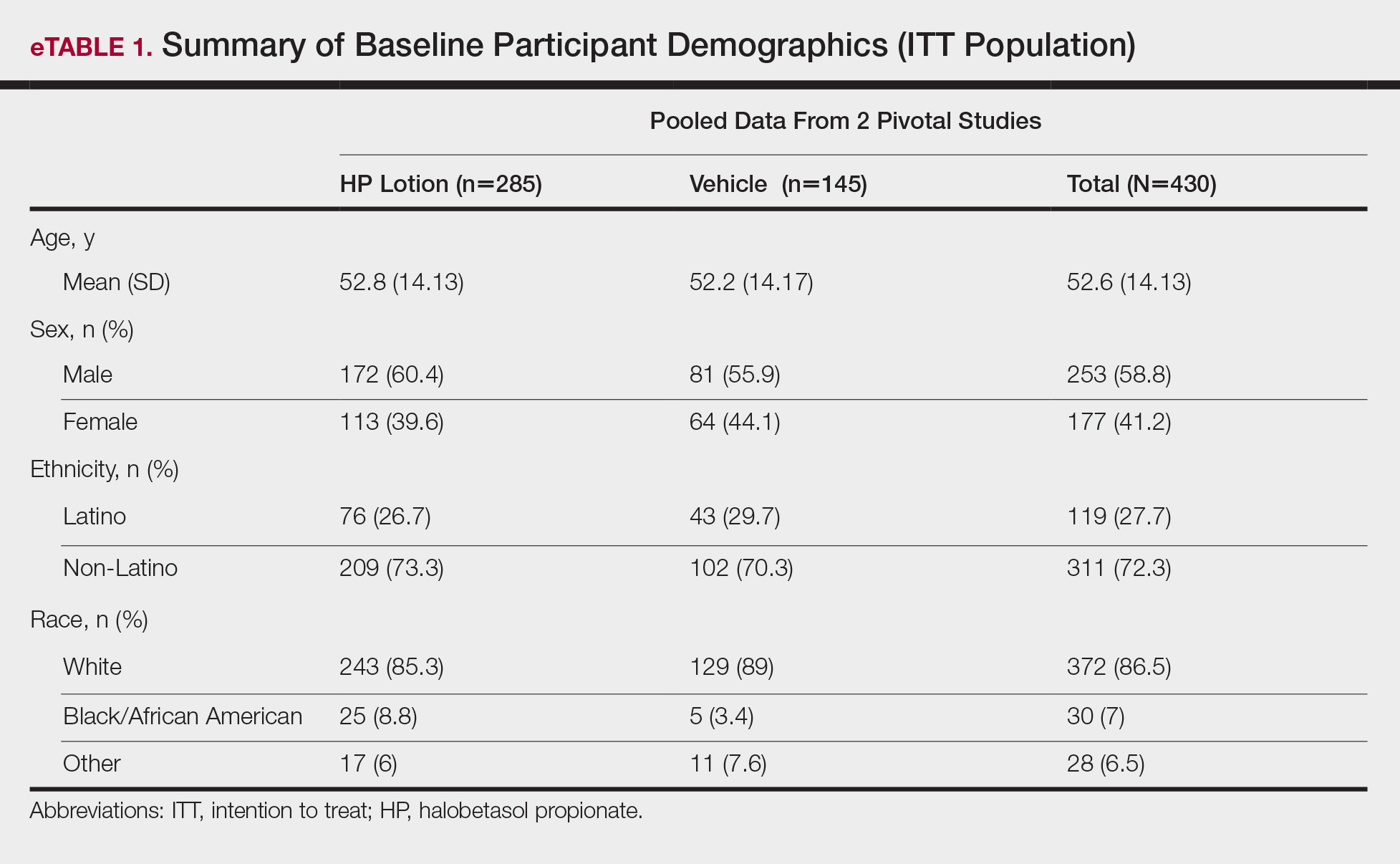
Demographic data were comparable across the 2 studies. The mean age (SD) was 52.6 (14.13) years. Overall, the majority of participants were male (58.8%; n=253) and white (86.5%; n=372)(eTable 1).
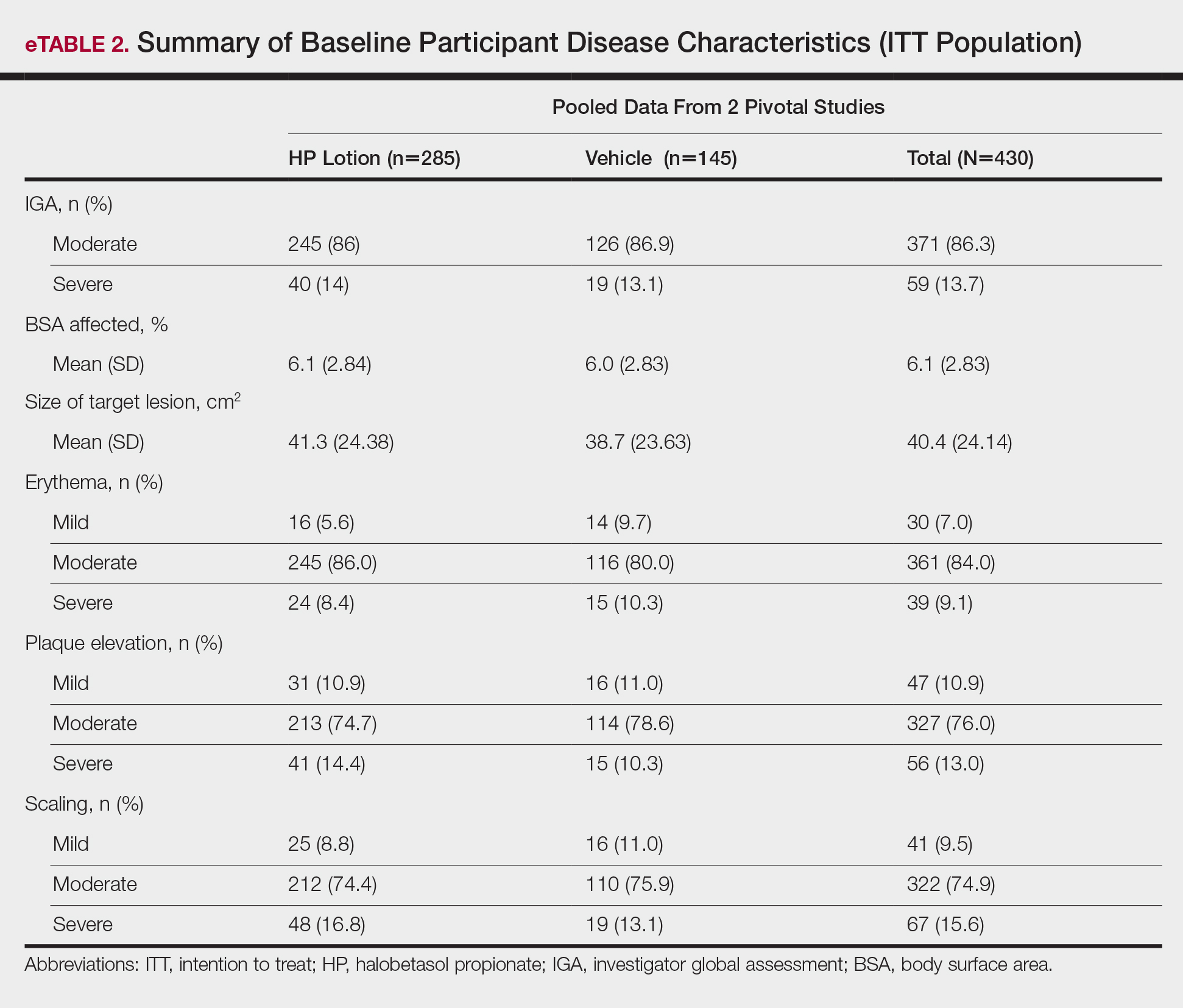
Baseline disease characteristics also were comparable across the treatment groups. Participants had moderate (86.3%; n=371) or severe (13.7%; n=59) disease, with a mean BSA (SD) of 6.1% (2.83) and mean size of target lesion (SD) of 40.4 cm2 (24.14). The majority of participants had moderate (erythema, 84.0%; plaque elevation, 76.0%; and scaling, 74.9%) or severe (erythema, 9.1%; plaque elevation, 13.0%; and scaling, 15.6%) signs of psoriasis at the target lesion site (eTable 2).
Efficacy Evaluation
IGA of Disease Severity
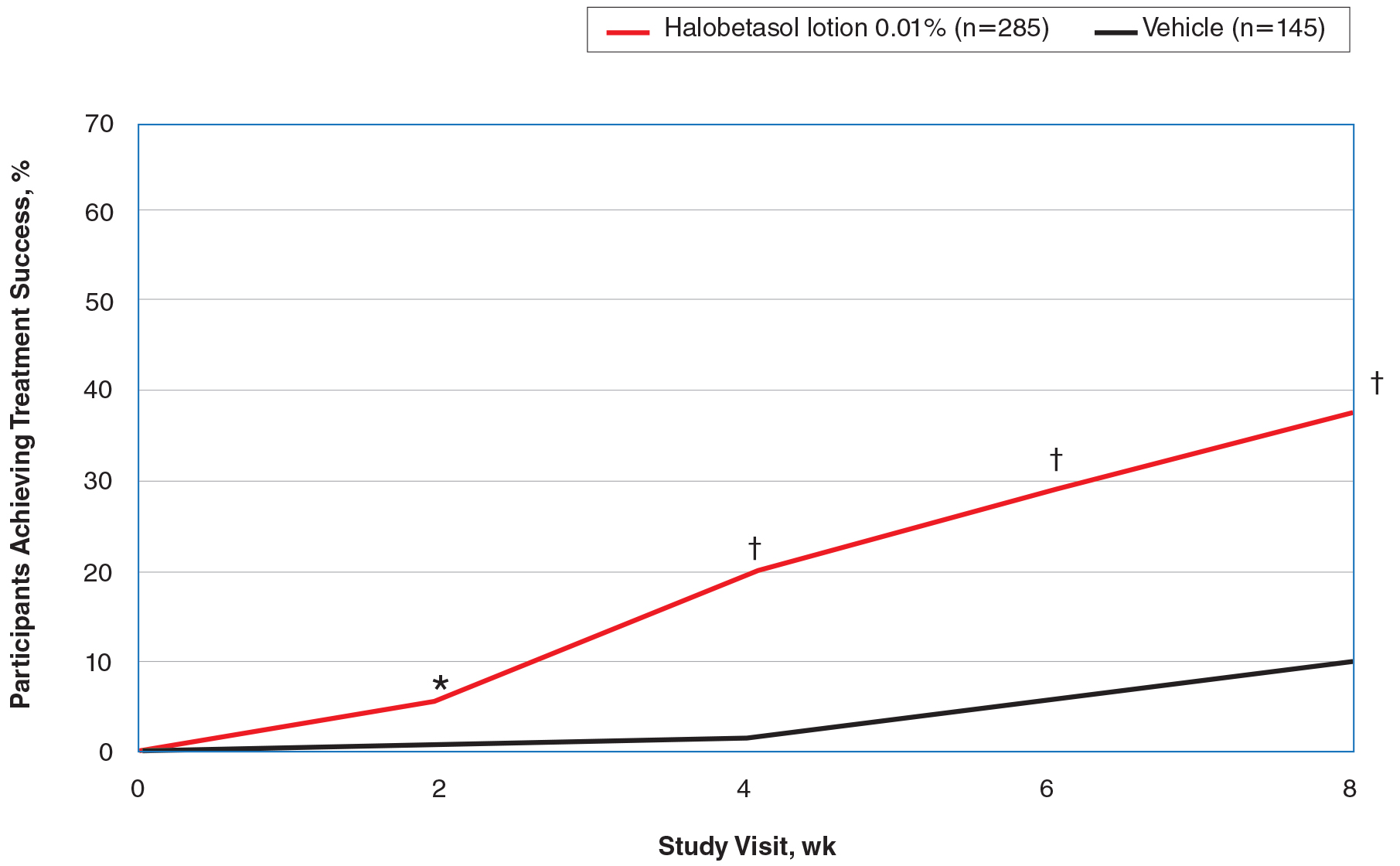
Halobetasol propionate lotion was consistently more effective than its vehicle in achieving treatment success (at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]). Halobetasol propionate lotion demonstrated statistically significant superiority over vehicle as early as week 2 (P=.003). By week 8, 37.43% of participants in the HP lotion group achieved treatment success compared with 10.03% in the vehicle group (P<.001)(Figure 1).
Overall, 39% of participants who had moderate disease (IGA score, 3) at baseline were treatment successes with HP lotion at week 8 compared with 11.53% of participants treated with vehicle; 27.97% of participants with severe disease (IGA score, 4) were treatment successes, with at least a 3-grade improvement in IGA. No participants with severe psoriasis who were treated with vehicle achieved treatment success at week 8. Efficacy was similar in female and male participants, allowing for vehicle effects.
Severity of Signs of Psoriasis (Erythema, Plaque Elevation, and Scaling) at Target Lesion Site
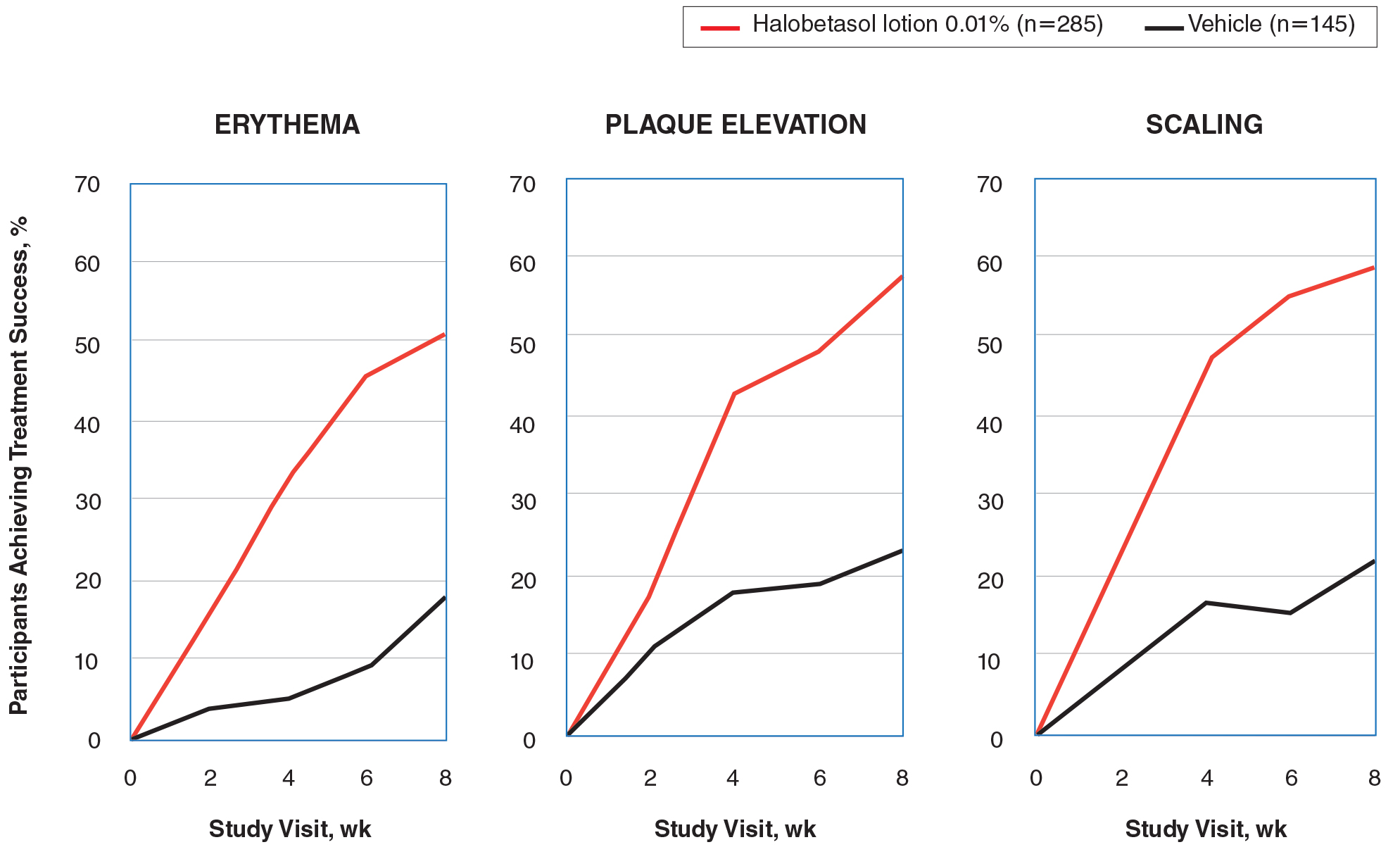
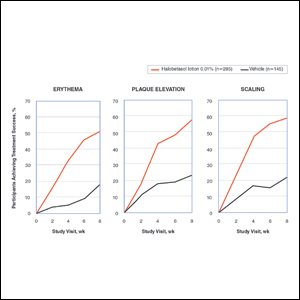
Halobetasol propionate lotion was statistically superior to vehicle in reducing the psoriasis signs of erythema, plaque elevation, and scaling at the target lesion from week 2. At week 8, treatment success (at least a 2-grade improvement from baseline) was achieved by 51.48% (erythema), 57.64% (plaque elevation), and 58.98% (scaling) of participants compared with 17.85%, 23.61%, and 22.82%, respectively, with vehicle (all P<.001)(Figure 2).
BSA Assessment
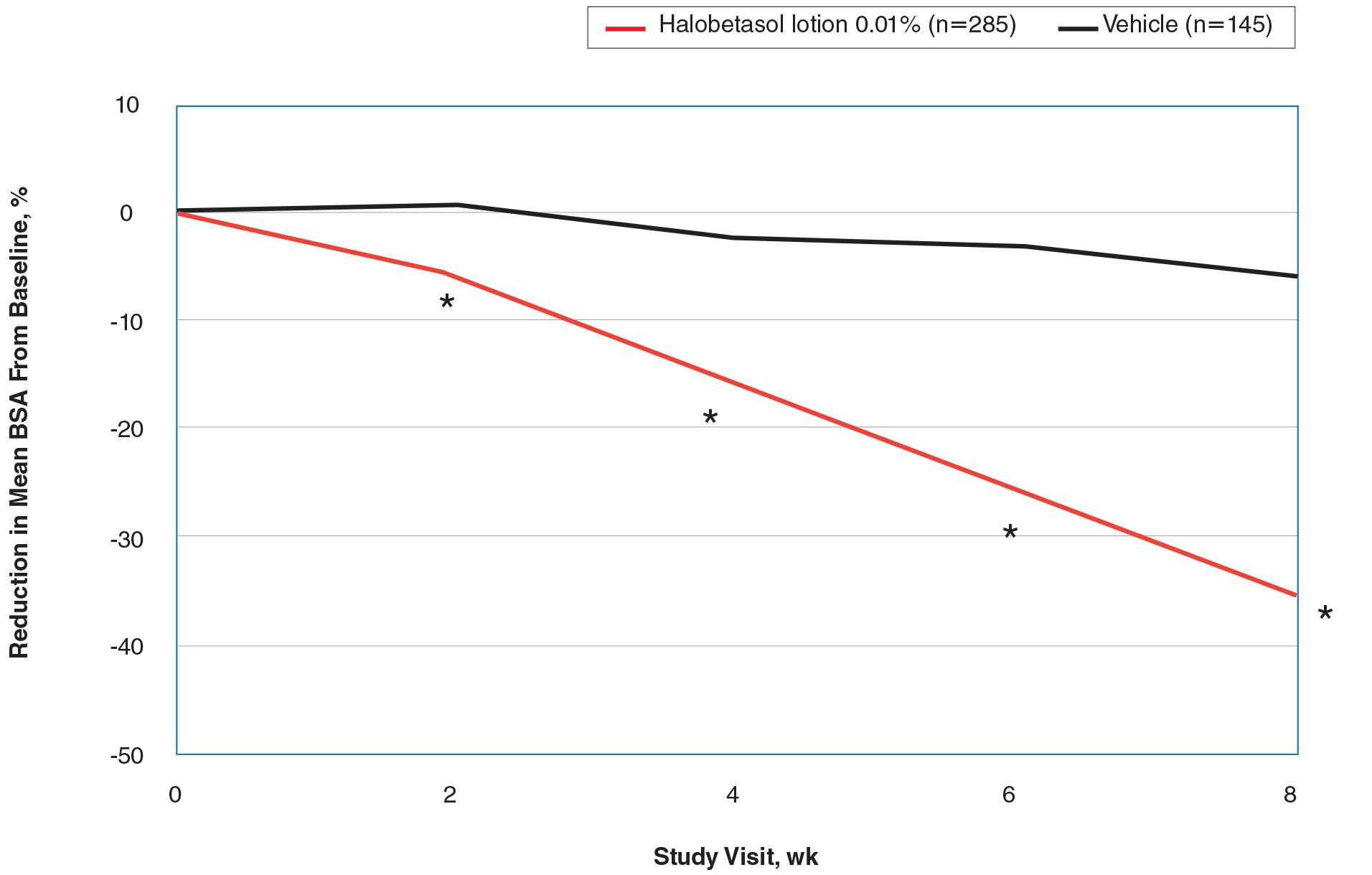
Halobetasol propionate lotion was statistically superior to vehicle in reducing BSA from week 2. At week 8 there was a 35.20% reduction in mean BSA for HP lotion compared to 5.85% for vehicle (P<.001)(eFigure 2).
IGA×BSA Composite Score
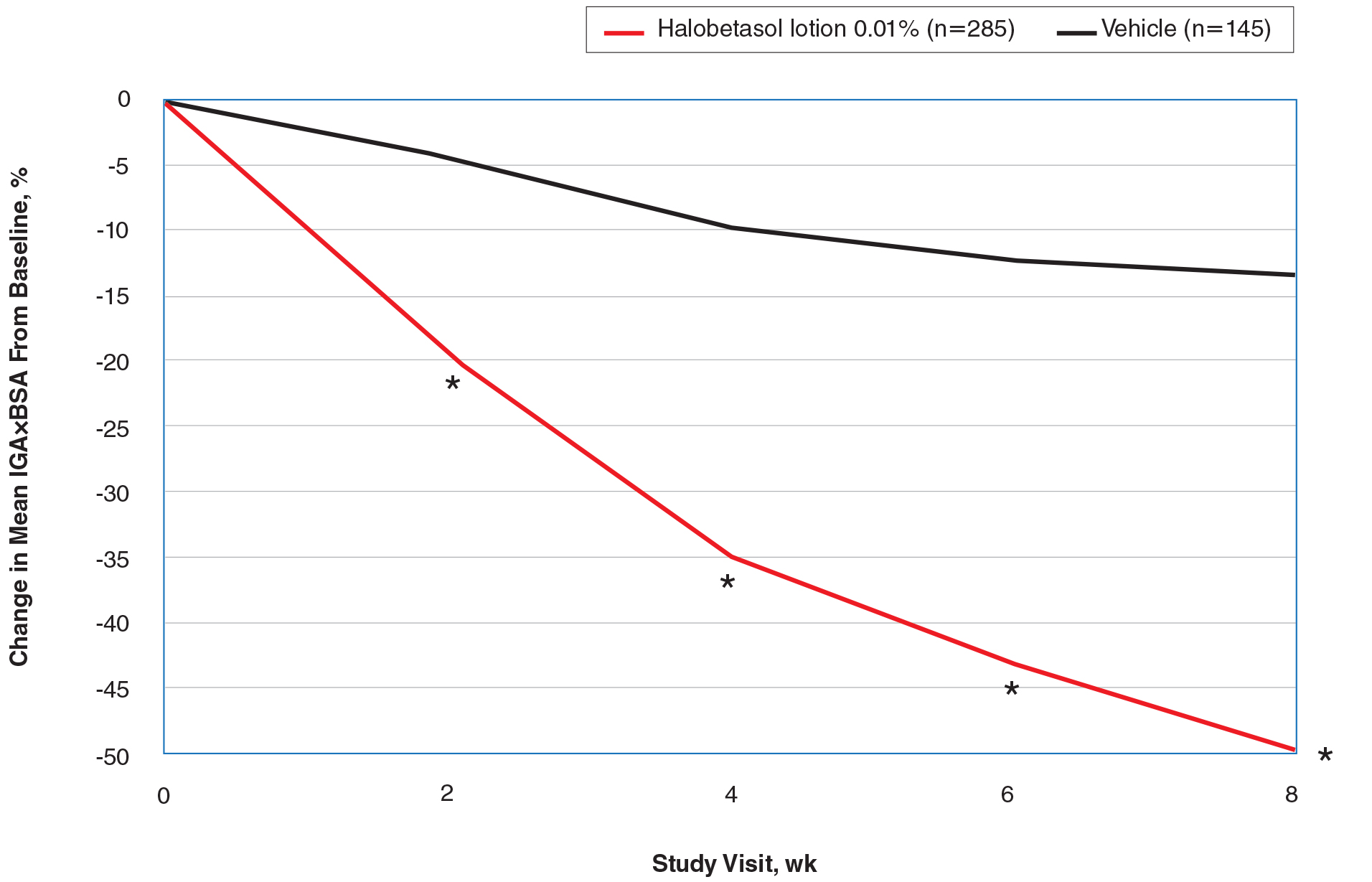
At baseline, the mean IGA×BSA scores for HP lotion and vehicle were similar: 19.3 and 18.8, respectively. By week 8, the percentage change in mean IGA×BSA score with HP lotion was 49.44% compared to 13.35% with vehicle (P<.001). Differences were significant from week 2 (P<.001)(Figure 3).
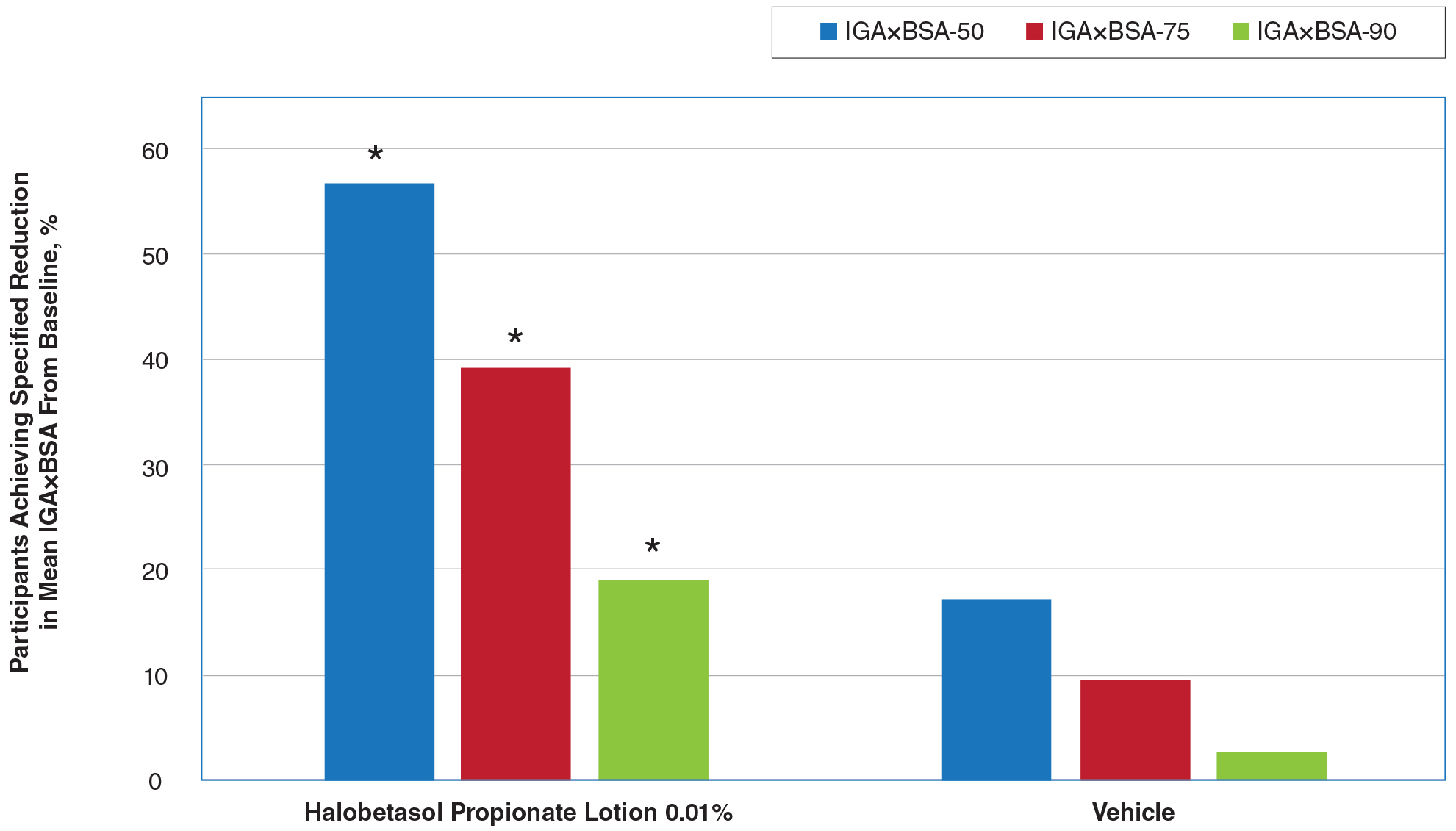
By week 8, 56.8% of participants (n=162) treated with HP lotion had achieved a 50% or greater reduction in baseline IGA×BSA compared to 17.2% of participants treated with vehicle (P<.001). Reductions of IGA×BSA-75 and IGA×BSA-90 were achieved in 39.3% and 19.3% of participants treated with HP lotion, respectively, compared with 9.7% and 2.8% of participants treated with vehicle (both P<.001)(eFigure 3).
Safety Evaluation
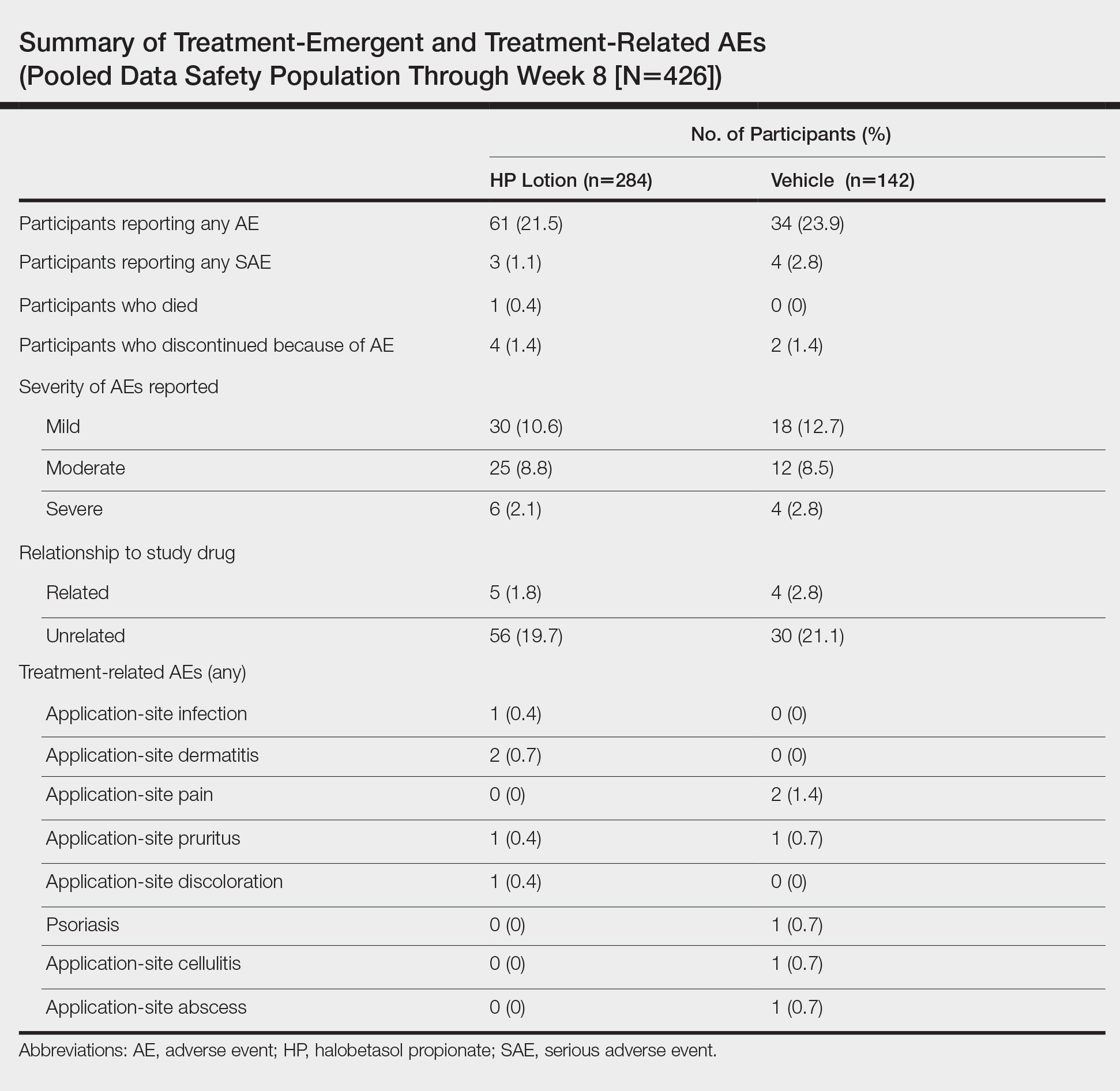
Adverse event reports were low and similar between the active and vehicle groups. Overall, 61 participants (21.5%) treated with HP lotion reported AEs compared with 34 participants (23.9%) treated with vehicle (Table). The majority of participants treated with HP lotion (90.2%) had AEs that were mild or moderate. There was 1 AE of telangiectasia, not considered treatment related. There were 5 treatment-related AEs for HP lotion, all at the application site: dermatitis (0.7%; n=2), infection (0.4%; n=1), pruritus (0.4%; n=1), and discoloration (0.4%; n=1). There were no AE reports of skin atrophy or folliculitis.
Local Skin Reactions
Most LSRs at baseline were mild to moderate in severity. Itching was the most common, present in 76.8% of participants. Participant-reported burning/stinging was less common, reported by 40.6% of participants. Investigator-reported dryness was noted in 65.7% of participants. There was a rapid improvement in participant-reported itching as early as week 2 that was sustained to the end of the studies, with more gradual improvements in skin dryness and burning/stinging.
COMMENT
Plaque psoriasis is a chronic condition. The rationale behind the development of HP lotion 0.01% was to provide optimal topical treatment of moderate to severe psoriasis, allowing for the potential of prolonged use beyond the 2-week consecutive use normally applied to HP cream 0.05% in a light, once-daily, aesthetically pleasing lotion formulation that patients would prefer.
Treatment success was rapid and achieved in more than 37% of participants by week 8, with significant improvements in psoriasis signs and symptoms (erythema, plaque elevation, and scaling) compared with vehicle. However, IGA does not consider BSA involvement, a key aspect of disease severity,11,12 and improvements in psoriasis signs of erythema, plaque elevation, and scaling were only assessed at the target lesion. Recently, the product of the IGA and BSA involvement (IGA×BSA) has been proposed as a simple alternative for assessing response to therapy that has been consistently shown to be highly correlated with the psoriasis area and severity index.13-19 Halobetasol propionate lotion 0.01% achieved a 50% reduction in IGA×BSA score by week 8. This efficacy compares well with results reported with apremilast in patients with moderate plaque psoriasis.20
Achieving clinically meaningful outcomes is an important aspect of disease management, especially in psoriasis with its disease burden and detriment to quality of life. It has been suggested that achieving a 75% or greater reduction from baseline IGA×BSA score (IGA×BSA-75) is an appropriate clinical goal.20 In our investigation, IGA×BSA-75 was achieved by 39% of participants treated with HP lotion by week 8, which again compares favorably with 35% of participants in the apremilast study who achieved IGA×BSA-75 at week 16.20
Physicians continue to have long-term safety concerns with TCSs,4,11,12 participants remain concerned about the risk for skin thinning,13 and product labelling restricts HP cream 0.05% consecutive use to 2 weeks. In clinical experience, HP cream 0.05% is well tolerated, with potential local AEs similar to those experienced with other superpotent TCSs. In short-term clinical trials, local AEs at the site of application were reported in up to 13% of patients21-26; itching, burning, or stinging were the most common local AEs (reported in 4.4% of patients).27
There were minimal safety concerns in our 2 studies using an 8-week, once-daily treatment regimen with HP lotion 0.01%. Local AEs at the application site were reported in less than 1% of participants. Baseline itching, dryness, and burning/stinging all improved with treatment.
CONCLUSION
Halobetasol propionate lotion 0.01% provides rapid improvement in disease severity. Halobetasol propionate lotion was consistently more effective than vehicle in achieving treatment success; reducing the BSA affected by the disease; reducing erythema, plaque elevation, and scaling at the target lesion; and improving IGA×BSA score over 8 weeks, which is a realistic time frame to see improvement in psoriasis with a topical steroid. There were minimal safety concerns with prolonged use. Halobetasol propionate lotion may provide an effective and reasonable treatment option in patients with moderate to severe plaque psoriasis.
Acknowledgment
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of this article. Ortho Dermatologics funded Mr. Bulley’s activities pertaining to this article.
- Gudjonsson JE, Elder JT. Psoriasis: epidemiology. Clin Dermatol. 2007;25:535-546.
- Liu Y, Krueger JG, Bowcock AM. Psoriasis: genetic associations and immune system changes. Genes Immun. 2007;8:1-12.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Alinia H, Moradi Tuchayi S, Smith JA, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176:759-764.
- Young M, Aldredge L, Parker P. Psoriasis for the primary care practitioner. J Am Assoc Nurse Pract. 2017;29:157-178.
- Devaux S, Castela A, Archier E, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):61-67.
- Ersser SJ, Cowdell FC, Latter SM, et al. Self-management experiences in adults with mild-moderate psoriasis: an exploratory study and implications for improved support. Br J Dermatol. 2010;163:1044-1049.
- Choi CW, Kim BR, Ohn J, et al. The advantage of cyclosporine A and methotrexate rotational therapy in long-term systemic treatment for chronic plaque psoriasis in a real world practice. Ann Dermatol. 2017;29:55-60.
- Callis Duffin K, Yeung H, Takeshita J, et al. Patient satisfaction with treatments for moderate-to-severe plaque psoriasis in clinical practice. Br J Dermatol. 2014;170:672-680.
- Spuls PI, Lecluse LL, Poulsen ML, et al. How good are clinical severity and outcome measures for psoriasis? quantitative evaluation in a systematic review. J Invest Dermatol. 2010;130:933-943.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Bozek A, Reich A. The reliability of three psoriasis assessment tools: psoriasis area severity index, body surface area and physician global assessment. Adv Clin Exp Med. 2017;26:851-856.
- Walsh JA, McFadden M, Woodcock J, et al. Product of the Physician Global Assessment and body surface area: a simple static measure of psoriasis severity in a longitudinal cohort. J Am Acad Dermatol. 2013;69:931-937.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis over 52 weeks: a phase III, randomized, controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Duffin KC, Papp KA, Bagel J, et al. Evaluation of the Physician Global Assessment and body surface area composite tool for assessing psoriasis response to apremilast therapy: results from ESTEEM 1 and ESTEEM 2. J Drugs Dermatol. 2017;16:147-153.
- Chiesa Fuxench ZC, Callis DK, Siegel M, et al. Validity of the Simple Measure for Assessing Psoriasis Activity (S-MAPA) for objectively evaluating disease severity in patients with plaque psoriasis. J Am Acad Dermatol. 2015;73:868-870.
- Walsh J. Comparative assessment of PASI and variations of PGA×BSA as measures of psoriasis severity in a clinical trial of moderate to severe psoriasis [poster 1830]. Presented at: Annual Meeting of the American Academy of Dermatology; March 20-24, 2015; San Francisco, CA.
- Gottlieb AB, Merola JF, Chen R, et al. Assessing clinical response and defining minimal disease activity in plaque psoriasis with the Physician Global Assessment and body surface area (PGA×BSA) composite tool: An analysis of apremilast phase 3 ESTEEM data. J Am Acad Dermatol. 2017;77:1178-1180.
- Strober B, Bagel J, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate plaque psoriasis with lower BSA: week 16 results from the UNVEIL study. J Drugs Dermatol. 2017;16:801-808.
- Bernhard J, Whitmore C, Guzzo C, et al. Evaluation of halobetasol propionate ointment in the treatment of plaque psoriasis: report on two double-blind, vehicle-controlled studies. J Am Acad Dermatol. 1991;25:1170-1174.
- Katz HI, Gross E, Buxman M, et al. A double-blind, vehicle-controlled paired comparison of halobetasol propionate cream on patients with plaque psoriasis. J Am Acad Dermatol. 1991;25:1175-1178.
- Blum G, Yawalkar S. A comparative, multicenter, double blind trial of 0.05% halobetasol propionate ointment and 0.1% betamethasone valerate ointment in the treatment of patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1153-1156.
- Goldberg B, Hartdegen R, Presbury D, et al. A double-blind, multicenter comparison of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1145-1148.
- Mensing H, Korsukewitz G, Yawalkar S. A double-blind, multicenter comparison between 0.05% halobetasol propionate ointment and 0.05% betamethasone dipropionate ointment in chronic plaque psoriasis. J Am Acad Dermatol. 1991;25:1149-1152.
- Herz G, Blum G, Yawalkar S. Halobetasol propionate cream by day and halobetasol propionate ointment at night for the treatment of pediatric patients with chronic, localized psoriasis and atopic dermatitis. J Am Acad Dermatol. 1991;25:1166-1169.
- Ultravate [package insert]. Jacksonville, FL: Ranbaxy; 2012.
Psoriasis is a chronic, immune-mediated, inflammatory disease affecting almost 2% of the population.1-3 It is characterized by patches of raised reddish skin covered by silvery-white scales. Most patients have limited disease (<5% body surface area [BSA] involvement) that can be managed with topical agents.4 Topical corticosteroids (TCSs) are considered first-line therapy for mild to moderate disease because of the inflammatory nature of the condition and often are used in conjunction with systemic agents in more severe psoriasis.4
As many as 20% to 30% of patients with moderate to severe plaque psoriasis have inadequate disease control.5 Several factors may affect patient outcomes; however, drug selection and patient adherence are important given the chronic nature of the disease. A survey of 1200 patients with psoriasis reported nonadherence rates of 73% with topical therapy.6 In addition, patients tend to apply less than the recommended dose or abandon treatment altogether if rapid improvement does not occur7,8; it is not uncommon for patients with psoriasis to mistakenly believe treatment will improve their condition within 1 to 2 weeks.9 Patient satisfaction with topical treatments is low, partly because of these false expectations and formulation issues. Treatments can be greasy and sticky, with unpleasant odors and the potential to stain clothes and linens.7,10 Safety concerns with TCSs also limit their consecutive use beyond 2 to 4 weeks, which is not ideal for a disease that requires a long-term management strategy.
A potent/superpotent TCS that is administered once daily and has a safety profile that affords longer-term, once-daily treatment in an aesthetically pleasing formulation would seem ideal. Herein, we investigate the safety and tolerability of a novel low-concentration (0.01%) lotion formulation of halobetasol propionate (HP), reporting on the pooled data from 2 phase 3 clinical studies in participants with moderate to severe psoriasis.
METHODS
Study Design
We conducted 2 multicenter, double-blind, randomized, parallel-group phase 3 studies to assess the safety, tolerability, and efficacy of HP lotion 0.01% in participants with a clinical diagnosis of moderate to severe psoriasis with an investigator global assessment (IGA) score of 3 or 4 and an affected BSA of 3% to 12%. Participants were randomized (2:1) to receive HP lotion or vehicle applied topically to the affected area once daily for 8 weeks.
Inclusion and Exclusion Criteria
The studies included individuals of either sex aged 18 years or older. A target lesion was defined primarily to assess signs of psoriasis, measuring 16 to 100 cm2, with a score of 3 (moderate) or higher for 2 of 3 different psoriasis signs—erythema, plaque elevation, and scaling—and summed score of 8 or higher, with no sign scoring less than 2. Participants who had pustular psoriasis or used phototherapy, photochemotherapy, or systemic psoriasis therapy within the prior 4 weeks or biologics within the prior 3 months, or those who were diagnosed with skin conditions that would interfere with the interpretation of results were excluded from the studies.
Study Oversight
Participants provided written informed consent before study-related procedures were performed, and the protocol and consent were approved by institutional review boards or ethics committees at all investigational sites. The study was conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki.
Efficacy Assessment
A 5-point scale ranging from 0 (clear) to 4 (severe) was used by the investigator at each study visit to assess the overall psoriasis severity of the treatable areas. Treatment success (the percentage of participants with at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]) was evaluated at weeks 2, 4, 6, and 8, w
Signs of psoriasis at the target lesion were assessed at each visit using individual 5-point scales ranging from 0 (clear) to 4 (severe). Treatment success was defined as at least a 2-grade improvement from baseline score for each of the key signs—erythema, plaque elevation, and scaling—and reported at weeks 2, 4, 6, and 8, with a posttreatment follow-up at week 12.
Affected BSA also was evaluated at each visit. In addition, an IGA×BSA composite score was calculated by multiplying the IGA by the BSA (range, 9–48 [eg, maximum IGA=4 and maximum BSA=12]) at each time point. The mean percentage change in IGA×BSA from baseline was calculated for each study visit. Additional end points included the achievement of a 50%, 75%, and 90% or greater reduction from baseline IGA×BSA score—IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90—at week 8.
Safety Assessment
Safety evaluations including adverse events (AEs), local skin reactions (LSRs), vital signs, laboratory evaluations, and physical examinations were performed. Information on reported and observed AEs was obtained at each visit. Routine safety laboratory tests were performed at screening, week 4, and week 8. An abbreviated physical examination was performed at baseline, week 8 (end of treatment), and week 12 (end of study). Treatment areas also were examined by the investigator at baseline and each subsequent visit for the presence or absence of marked known drug-related AEs including skin atrophy, striae, telangiectasia, and folliculitis.
LSR Assessment
Local skin reactions such as itching, dryness, and burning/stinging were evaluated at each study visit using 4-point scales ranging from 0 (clear) to 3 (severe). Given the nature of the disease, the presence of LSRs and symptoms at baseline is commonplace, and as such, these evaluations identified both improvement and any emergent issues.
Statistical Analysis
The primary study goal was to assess differences in treatment efficacy between HP lotion and vehicle with respect to IGA. All statistical processing was performed using SAS unless otherwise stated; statistical tests were 2-sided and performed at the 0.05 level of significance. Markov Chain Monte Carlo multiple imputation was the primary method used to handle missing efficacy data. No imputations were made for missing safety data. All participants were randomized, and the dispensed study drug was included in the intention-to-treat analysis set. This analysis was considered primary for the evaluation of efficacy. Data were analyzed using Cochran-Mantel-Haenszel tests, stratified by analysis center.
Body surface area data were analyzed in a post hoc analysis of covariance with factors of treatment and analysis center and baseline BSA as a covariate. P values for comparisons of percentage change in IGA×BSA were derived from a Wilcoxon rank sum test. For IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90, P values were derived from a Cochran-Mantel-Haenszel test. Last observation carried forward was used to impute data for IGA and BSA through week 8 prior to analysis.
The primary safety analysis was conducted at week 8 using the safety analysis set, which included all participants who were randomized, received at least 1 confirmed dose of the study drug, and had at least 1 postbaseline safety assessment. Adverse events were recorded and classified using the Medical Dictionary for Regulatory Activities (MedDRA, Version 18.0). A post hoc Wilcoxon rank sum test was conducted to compare itching, dryness, and burning/stinging scores at week 8 for HP lotion versus vehicle.
RESULTS
Participant Disposition
Overall, 430 participants were randomized (2:1) to HP lotion (n=285) or vehicle (n=145)(eFigure 1) and included in the intention-to-treat population. Across the 2 studies, 93.3% (n=266) of participants treated with HP lotion and 89.7% (n=130) of participants treated with vehicle completed treatment. The main reasons for study discontinuation with HP lotion were lost to follow-up (3.2%; n=9), participant request (1.8%; n=5), and AEs (1.4%; n=4). Participant request (4.8%; n=7), lost to follow-up (4.1%; n=6), and AEs (1.4%; n=2) also were the main reasons for treatment discontinuation in the vehicle arm.
A total of 426 participants were included in the safety population, with no postbaseline safety evaluation in 4 participants.
Baseline Participant Demographics
Demographic data were comparable across the 2 studies. The mean age (SD) was 52.6 (14.13) years. Overall, the majority of participants were male (58.8%; n=253) and white (86.5%; n=372)(eTable 1).
Baseline disease characteristics also were comparable across the treatment groups. Participants had moderate (86.3%; n=371) or severe (13.7%; n=59) disease, with a mean BSA (SD) of 6.1% (2.83) and mean size of target lesion (SD) of 40.4 cm2 (24.14). The majority of participants had moderate (erythema, 84.0%; plaque elevation, 76.0%; and scaling, 74.9%) or severe (erythema, 9.1%; plaque elevation, 13.0%; and scaling, 15.6%) signs of psoriasis at the target lesion site (eTable 2).
Efficacy Evaluation
IGA of Disease Severity
Halobetasol propionate lotion was consistently more effective than its vehicle in achieving treatment success (at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]). Halobetasol propionate lotion demonstrated statistically significant superiority over vehicle as early as week 2 (P=.003). By week 8, 37.43% of participants in the HP lotion group achieved treatment success compared with 10.03% in the vehicle group (P<.001)(Figure 1).
Overall, 39% of participants who had moderate disease (IGA score, 3) at baseline were treatment successes with HP lotion at week 8 compared with 11.53% of participants treated with vehicle; 27.97% of participants with severe disease (IGA score, 4) were treatment successes, with at least a 3-grade improvement in IGA. No participants with severe psoriasis who were treated with vehicle achieved treatment success at week 8. Efficacy was similar in female and male participants, allowing for vehicle effects.
Severity of Signs of Psoriasis (Erythema, Plaque Elevation, and Scaling) at Target Lesion Site
Halobetasol propionate lotion was statistically superior to vehicle in reducing the psoriasis signs of erythema, plaque elevation, and scaling at the target lesion from week 2. At week 8, treatment success (at least a 2-grade improvement from baseline) was achieved by 51.48% (erythema), 57.64% (plaque elevation), and 58.98% (scaling) of participants compared with 17.85%, 23.61%, and 22.82%, respectively, with vehicle (all P<.001)(Figure 2).
BSA Assessment
Halobetasol propionate lotion was statistically superior to vehicle in reducing BSA from week 2. At week 8 there was a 35.20% reduction in mean BSA for HP lotion compared to 5.85% for vehicle (P<.001)(eFigure 2).
IGA×BSA Composite Score
At baseline, the mean IGA×BSA scores for HP lotion and vehicle were similar: 19.3 and 18.8, respectively. By week 8, the percentage change in mean IGA×BSA score with HP lotion was 49.44% compared to 13.35% with vehicle (P<.001). Differences were significant from week 2 (P<.001)(Figure 3).
By week 8, 56.8% of participants (n=162) treated with HP lotion had achieved a 50% or greater reduction in baseline IGA×BSA compared to 17.2% of participants treated with vehicle (P<.001). Reductions of IGA×BSA-75 and IGA×BSA-90 were achieved in 39.3% and 19.3% of participants treated with HP lotion, respectively, compared with 9.7% and 2.8% of participants treated with vehicle (both P<.001)(eFigure 3).
Safety Evaluation
Adverse event reports were low and similar between the active and vehicle groups. Overall, 61 participants (21.5%) treated with HP lotion reported AEs compared with 34 participants (23.9%) treated with vehicle (Table). The majority of participants treated with HP lotion (90.2%) had AEs that were mild or moderate. There was 1 AE of telangiectasia, not considered treatment related. There were 5 treatment-related AEs for HP lotion, all at the application site: dermatitis (0.7%; n=2), infection (0.4%; n=1), pruritus (0.4%; n=1), and discoloration (0.4%; n=1). There were no AE reports of skin atrophy or folliculitis.
Local Skin Reactions
Most LSRs at baseline were mild to moderate in severity. Itching was the most common, present in 76.8% of participants. Participant-reported burning/stinging was less common, reported by 40.6% of participants. Investigator-reported dryness was noted in 65.7% of participants. There was a rapid improvement in participant-reported itching as early as week 2 that was sustained to the end of the studies, with more gradual improvements in skin dryness and burning/stinging.
COMMENT
Plaque psoriasis is a chronic condition. The rationale behind the development of HP lotion 0.01% was to provide optimal topical treatment of moderate to severe psoriasis, allowing for the potential of prolonged use beyond the 2-week consecutive use normally applied to HP cream 0.05% in a light, once-daily, aesthetically pleasing lotion formulation that patients would prefer.
Treatment success was rapid and achieved in more than 37% of participants by week 8, with significant improvements in psoriasis signs and symptoms (erythema, plaque elevation, and scaling) compared with vehicle. However, IGA does not consider BSA involvement, a key aspect of disease severity,11,12 and improvements in psoriasis signs of erythema, plaque elevation, and scaling were only assessed at the target lesion. Recently, the product of the IGA and BSA involvement (IGA×BSA) has been proposed as a simple alternative for assessing response to therapy that has been consistently shown to be highly correlated with the psoriasis area and severity index.13-19 Halobetasol propionate lotion 0.01% achieved a 50% reduction in IGA×BSA score by week 8. This efficacy compares well with results reported with apremilast in patients with moderate plaque psoriasis.20
Achieving clinically meaningful outcomes is an important aspect of disease management, especially in psoriasis with its disease burden and detriment to quality of life. It has been suggested that achieving a 75% or greater reduction from baseline IGA×BSA score (IGA×BSA-75) is an appropriate clinical goal.20 In our investigation, IGA×BSA-75 was achieved by 39% of participants treated with HP lotion by week 8, which again compares favorably with 35% of participants in the apremilast study who achieved IGA×BSA-75 at week 16.20
Physicians continue to have long-term safety concerns with TCSs,4,11,12 participants remain concerned about the risk for skin thinning,13 and product labelling restricts HP cream 0.05% consecutive use to 2 weeks. In clinical experience, HP cream 0.05% is well tolerated, with potential local AEs similar to those experienced with other superpotent TCSs. In short-term clinical trials, local AEs at the site of application were reported in up to 13% of patients21-26; itching, burning, or stinging were the most common local AEs (reported in 4.4% of patients).27
There were minimal safety concerns in our 2 studies using an 8-week, once-daily treatment regimen with HP lotion 0.01%. Local AEs at the application site were reported in less than 1% of participants. Baseline itching, dryness, and burning/stinging all improved with treatment.
CONCLUSION
Halobetasol propionate lotion 0.01% provides rapid improvement in disease severity. Halobetasol propionate lotion was consistently more effective than vehicle in achieving treatment success; reducing the BSA affected by the disease; reducing erythema, plaque elevation, and scaling at the target lesion; and improving IGA×BSA score over 8 weeks, which is a realistic time frame to see improvement in psoriasis with a topical steroid. There were minimal safety concerns with prolonged use. Halobetasol propionate lotion may provide an effective and reasonable treatment option in patients with moderate to severe plaque psoriasis.
Acknowledgment
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of this article. Ortho Dermatologics funded Mr. Bulley’s activities pertaining to this article.
Psoriasis is a chronic, immune-mediated, inflammatory disease affecting almost 2% of the population.1-3 It is characterized by patches of raised reddish skin covered by silvery-white scales. Most patients have limited disease (<5% body surface area [BSA] involvement) that can be managed with topical agents.4 Topical corticosteroids (TCSs) are considered first-line therapy for mild to moderate disease because of the inflammatory nature of the condition and often are used in conjunction with systemic agents in more severe psoriasis.4
As many as 20% to 30% of patients with moderate to severe plaque psoriasis have inadequate disease control.5 Several factors may affect patient outcomes; however, drug selection and patient adherence are important given the chronic nature of the disease. A survey of 1200 patients with psoriasis reported nonadherence rates of 73% with topical therapy.6 In addition, patients tend to apply less than the recommended dose or abandon treatment altogether if rapid improvement does not occur7,8; it is not uncommon for patients with psoriasis to mistakenly believe treatment will improve their condition within 1 to 2 weeks.9 Patient satisfaction with topical treatments is low, partly because of these false expectations and formulation issues. Treatments can be greasy and sticky, with unpleasant odors and the potential to stain clothes and linens.7,10 Safety concerns with TCSs also limit their consecutive use beyond 2 to 4 weeks, which is not ideal for a disease that requires a long-term management strategy.
A potent/superpotent TCS that is administered once daily and has a safety profile that affords longer-term, once-daily treatment in an aesthetically pleasing formulation would seem ideal. Herein, we investigate the safety and tolerability of a novel low-concentration (0.01%) lotion formulation of halobetasol propionate (HP), reporting on the pooled data from 2 phase 3 clinical studies in participants with moderate to severe psoriasis.
METHODS
Study Design
We conducted 2 multicenter, double-blind, randomized, parallel-group phase 3 studies to assess the safety, tolerability, and efficacy of HP lotion 0.01% in participants with a clinical diagnosis of moderate to severe psoriasis with an investigator global assessment (IGA) score of 3 or 4 and an affected BSA of 3% to 12%. Participants were randomized (2:1) to receive HP lotion or vehicle applied topically to the affected area once daily for 8 weeks.
Inclusion and Exclusion Criteria
The studies included individuals of either sex aged 18 years or older. A target lesion was defined primarily to assess signs of psoriasis, measuring 16 to 100 cm2, with a score of 3 (moderate) or higher for 2 of 3 different psoriasis signs—erythema, plaque elevation, and scaling—and summed score of 8 or higher, with no sign scoring less than 2. Participants who had pustular psoriasis or used phototherapy, photochemotherapy, or systemic psoriasis therapy within the prior 4 weeks or biologics within the prior 3 months, or those who were diagnosed with skin conditions that would interfere with the interpretation of results were excluded from the studies.
Study Oversight
Participants provided written informed consent before study-related procedures were performed, and the protocol and consent were approved by institutional review boards or ethics committees at all investigational sites. The study was conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki.
Efficacy Assessment
A 5-point scale ranging from 0 (clear) to 4 (severe) was used by the investigator at each study visit to assess the overall psoriasis severity of the treatable areas. Treatment success (the percentage of participants with at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]) was evaluated at weeks 2, 4, 6, and 8, w
Signs of psoriasis at the target lesion were assessed at each visit using individual 5-point scales ranging from 0 (clear) to 4 (severe). Treatment success was defined as at least a 2-grade improvement from baseline score for each of the key signs—erythema, plaque elevation, and scaling—and reported at weeks 2, 4, 6, and 8, with a posttreatment follow-up at week 12.
Affected BSA also was evaluated at each visit. In addition, an IGA×BSA composite score was calculated by multiplying the IGA by the BSA (range, 9–48 [eg, maximum IGA=4 and maximum BSA=12]) at each time point. The mean percentage change in IGA×BSA from baseline was calculated for each study visit. Additional end points included the achievement of a 50%, 75%, and 90% or greater reduction from baseline IGA×BSA score—IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90—at week 8.
Safety Assessment
Safety evaluations including adverse events (AEs), local skin reactions (LSRs), vital signs, laboratory evaluations, and physical examinations were performed. Information on reported and observed AEs was obtained at each visit. Routine safety laboratory tests were performed at screening, week 4, and week 8. An abbreviated physical examination was performed at baseline, week 8 (end of treatment), and week 12 (end of study). Treatment areas also were examined by the investigator at baseline and each subsequent visit for the presence or absence of marked known drug-related AEs including skin atrophy, striae, telangiectasia, and folliculitis.
LSR Assessment
Local skin reactions such as itching, dryness, and burning/stinging were evaluated at each study visit using 4-point scales ranging from 0 (clear) to 3 (severe). Given the nature of the disease, the presence of LSRs and symptoms at baseline is commonplace, and as such, these evaluations identified both improvement and any emergent issues.
Statistical Analysis
The primary study goal was to assess differences in treatment efficacy between HP lotion and vehicle with respect to IGA. All statistical processing was performed using SAS unless otherwise stated; statistical tests were 2-sided and performed at the 0.05 level of significance. Markov Chain Monte Carlo multiple imputation was the primary method used to handle missing efficacy data. No imputations were made for missing safety data. All participants were randomized, and the dispensed study drug was included in the intention-to-treat analysis set. This analysis was considered primary for the evaluation of efficacy. Data were analyzed using Cochran-Mantel-Haenszel tests, stratified by analysis center.
Body surface area data were analyzed in a post hoc analysis of covariance with factors of treatment and analysis center and baseline BSA as a covariate. P values for comparisons of percentage change in IGA×BSA were derived from a Wilcoxon rank sum test. For IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90, P values were derived from a Cochran-Mantel-Haenszel test. Last observation carried forward was used to impute data for IGA and BSA through week 8 prior to analysis.
The primary safety analysis was conducted at week 8 using the safety analysis set, which included all participants who were randomized, received at least 1 confirmed dose of the study drug, and had at least 1 postbaseline safety assessment. Adverse events were recorded and classified using the Medical Dictionary for Regulatory Activities (MedDRA, Version 18.0). A post hoc Wilcoxon rank sum test was conducted to compare itching, dryness, and burning/stinging scores at week 8 for HP lotion versus vehicle.
RESULTS
Participant Disposition
Overall, 430 participants were randomized (2:1) to HP lotion (n=285) or vehicle (n=145)(eFigure 1) and included in the intention-to-treat population. Across the 2 studies, 93.3% (n=266) of participants treated with HP lotion and 89.7% (n=130) of participants treated with vehicle completed treatment. The main reasons for study discontinuation with HP lotion were lost to follow-up (3.2%; n=9), participant request (1.8%; n=5), and AEs (1.4%; n=4). Participant request (4.8%; n=7), lost to follow-up (4.1%; n=6), and AEs (1.4%; n=2) also were the main reasons for treatment discontinuation in the vehicle arm.
A total of 426 participants were included in the safety population, with no postbaseline safety evaluation in 4 participants.
Baseline Participant Demographics
Demographic data were comparable across the 2 studies. The mean age (SD) was 52.6 (14.13) years. Overall, the majority of participants were male (58.8%; n=253) and white (86.5%; n=372)(eTable 1).
Baseline disease characteristics also were comparable across the treatment groups. Participants had moderate (86.3%; n=371) or severe (13.7%; n=59) disease, with a mean BSA (SD) of 6.1% (2.83) and mean size of target lesion (SD) of 40.4 cm2 (24.14). The majority of participants had moderate (erythema, 84.0%; plaque elevation, 76.0%; and scaling, 74.9%) or severe (erythema, 9.1%; plaque elevation, 13.0%; and scaling, 15.6%) signs of psoriasis at the target lesion site (eTable 2).
Efficacy Evaluation
IGA of Disease Severity
Halobetasol propionate lotion was consistently more effective than its vehicle in achieving treatment success (at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]). Halobetasol propionate lotion demonstrated statistically significant superiority over vehicle as early as week 2 (P=.003). By week 8, 37.43% of participants in the HP lotion group achieved treatment success compared with 10.03% in the vehicle group (P<.001)(Figure 1).
Overall, 39% of participants who had moderate disease (IGA score, 3) at baseline were treatment successes with HP lotion at week 8 compared with 11.53% of participants treated with vehicle; 27.97% of participants with severe disease (IGA score, 4) were treatment successes, with at least a 3-grade improvement in IGA. No participants with severe psoriasis who were treated with vehicle achieved treatment success at week 8. Efficacy was similar in female and male participants, allowing for vehicle effects.
Severity of Signs of Psoriasis (Erythema, Plaque Elevation, and Scaling) at Target Lesion Site
Halobetasol propionate lotion was statistically superior to vehicle in reducing the psoriasis signs of erythema, plaque elevation, and scaling at the target lesion from week 2. At week 8, treatment success (at least a 2-grade improvement from baseline) was achieved by 51.48% (erythema), 57.64% (plaque elevation), and 58.98% (scaling) of participants compared with 17.85%, 23.61%, and 22.82%, respectively, with vehicle (all P<.001)(Figure 2).
BSA Assessment
Halobetasol propionate lotion was statistically superior to vehicle in reducing BSA from week 2. At week 8 there was a 35.20% reduction in mean BSA for HP lotion compared to 5.85% for vehicle (P<.001)(eFigure 2).
IGA×BSA Composite Score
At baseline, the mean IGA×BSA scores for HP lotion and vehicle were similar: 19.3 and 18.8, respectively. By week 8, the percentage change in mean IGA×BSA score with HP lotion was 49.44% compared to 13.35% with vehicle (P<.001). Differences were significant from week 2 (P<.001)(Figure 3).
By week 8, 56.8% of participants (n=162) treated with HP lotion had achieved a 50% or greater reduction in baseline IGA×BSA compared to 17.2% of participants treated with vehicle (P<.001). Reductions of IGA×BSA-75 and IGA×BSA-90 were achieved in 39.3% and 19.3% of participants treated with HP lotion, respectively, compared with 9.7% and 2.8% of participants treated with vehicle (both P<.001)(eFigure 3).
Safety Evaluation
Adverse event reports were low and similar between the active and vehicle groups. Overall, 61 participants (21.5%) treated with HP lotion reported AEs compared with 34 participants (23.9%) treated with vehicle (Table). The majority of participants treated with HP lotion (90.2%) had AEs that were mild or moderate. There was 1 AE of telangiectasia, not considered treatment related. There were 5 treatment-related AEs for HP lotion, all at the application site: dermatitis (0.7%; n=2), infection (0.4%; n=1), pruritus (0.4%; n=1), and discoloration (0.4%; n=1). There were no AE reports of skin atrophy or folliculitis.
Local Skin Reactions
Most LSRs at baseline were mild to moderate in severity. Itching was the most common, present in 76.8% of participants. Participant-reported burning/stinging was less common, reported by 40.6% of participants. Investigator-reported dryness was noted in 65.7% of participants. There was a rapid improvement in participant-reported itching as early as week 2 that was sustained to the end of the studies, with more gradual improvements in skin dryness and burning/stinging.
COMMENT
Plaque psoriasis is a chronic condition. The rationale behind the development of HP lotion 0.01% was to provide optimal topical treatment of moderate to severe psoriasis, allowing for the potential of prolonged use beyond the 2-week consecutive use normally applied to HP cream 0.05% in a light, once-daily, aesthetically pleasing lotion formulation that patients would prefer.
Treatment success was rapid and achieved in more than 37% of participants by week 8, with significant improvements in psoriasis signs and symptoms (erythema, plaque elevation, and scaling) compared with vehicle. However, IGA does not consider BSA involvement, a key aspect of disease severity,11,12 and improvements in psoriasis signs of erythema, plaque elevation, and scaling were only assessed at the target lesion. Recently, the product of the IGA and BSA involvement (IGA×BSA) has been proposed as a simple alternative for assessing response to therapy that has been consistently shown to be highly correlated with the psoriasis area and severity index.13-19 Halobetasol propionate lotion 0.01% achieved a 50% reduction in IGA×BSA score by week 8. This efficacy compares well with results reported with apremilast in patients with moderate plaque psoriasis.20
Achieving clinically meaningful outcomes is an important aspect of disease management, especially in psoriasis with its disease burden and detriment to quality of life. It has been suggested that achieving a 75% or greater reduction from baseline IGA×BSA score (IGA×BSA-75) is an appropriate clinical goal.20 In our investigation, IGA×BSA-75 was achieved by 39% of participants treated with HP lotion by week 8, which again compares favorably with 35% of participants in the apremilast study who achieved IGA×BSA-75 at week 16.20
Physicians continue to have long-term safety concerns with TCSs,4,11,12 participants remain concerned about the risk for skin thinning,13 and product labelling restricts HP cream 0.05% consecutive use to 2 weeks. In clinical experience, HP cream 0.05% is well tolerated, with potential local AEs similar to those experienced with other superpotent TCSs. In short-term clinical trials, local AEs at the site of application were reported in up to 13% of patients21-26; itching, burning, or stinging were the most common local AEs (reported in 4.4% of patients).27
There were minimal safety concerns in our 2 studies using an 8-week, once-daily treatment regimen with HP lotion 0.01%. Local AEs at the application site were reported in less than 1% of participants. Baseline itching, dryness, and burning/stinging all improved with treatment.
CONCLUSION
Halobetasol propionate lotion 0.01% provides rapid improvement in disease severity. Halobetasol propionate lotion was consistently more effective than vehicle in achieving treatment success; reducing the BSA affected by the disease; reducing erythema, plaque elevation, and scaling at the target lesion; and improving IGA×BSA score over 8 weeks, which is a realistic time frame to see improvement in psoriasis with a topical steroid. There were minimal safety concerns with prolonged use. Halobetasol propionate lotion may provide an effective and reasonable treatment option in patients with moderate to severe plaque psoriasis.
Acknowledgment
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of this article. Ortho Dermatologics funded Mr. Bulley’s activities pertaining to this article.
- Gudjonsson JE, Elder JT. Psoriasis: epidemiology. Clin Dermatol. 2007;25:535-546.
- Liu Y, Krueger JG, Bowcock AM. Psoriasis: genetic associations and immune system changes. Genes Immun. 2007;8:1-12.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Alinia H, Moradi Tuchayi S, Smith JA, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176:759-764.
- Young M, Aldredge L, Parker P. Psoriasis for the primary care practitioner. J Am Assoc Nurse Pract. 2017;29:157-178.
- Devaux S, Castela A, Archier E, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):61-67.
- Ersser SJ, Cowdell FC, Latter SM, et al. Self-management experiences in adults with mild-moderate psoriasis: an exploratory study and implications for improved support. Br J Dermatol. 2010;163:1044-1049.
- Choi CW, Kim BR, Ohn J, et al. The advantage of cyclosporine A and methotrexate rotational therapy in long-term systemic treatment for chronic plaque psoriasis in a real world practice. Ann Dermatol. 2017;29:55-60.
- Callis Duffin K, Yeung H, Takeshita J, et al. Patient satisfaction with treatments for moderate-to-severe plaque psoriasis in clinical practice. Br J Dermatol. 2014;170:672-680.
- Spuls PI, Lecluse LL, Poulsen ML, et al. How good are clinical severity and outcome measures for psoriasis? quantitative evaluation in a systematic review. J Invest Dermatol. 2010;130:933-943.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Bozek A, Reich A. The reliability of three psoriasis assessment tools: psoriasis area severity index, body surface area and physician global assessment. Adv Clin Exp Med. 2017;26:851-856.
- Walsh JA, McFadden M, Woodcock J, et al. Product of the Physician Global Assessment and body surface area: a simple static measure of psoriasis severity in a longitudinal cohort. J Am Acad Dermatol. 2013;69:931-937.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis over 52 weeks: a phase III, randomized, controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Duffin KC, Papp KA, Bagel J, et al. Evaluation of the Physician Global Assessment and body surface area composite tool for assessing psoriasis response to apremilast therapy: results from ESTEEM 1 and ESTEEM 2. J Drugs Dermatol. 2017;16:147-153.
- Chiesa Fuxench ZC, Callis DK, Siegel M, et al. Validity of the Simple Measure for Assessing Psoriasis Activity (S-MAPA) for objectively evaluating disease severity in patients with plaque psoriasis. J Am Acad Dermatol. 2015;73:868-870.
- Walsh J. Comparative assessment of PASI and variations of PGA×BSA as measures of psoriasis severity in a clinical trial of moderate to severe psoriasis [poster 1830]. Presented at: Annual Meeting of the American Academy of Dermatology; March 20-24, 2015; San Francisco, CA.
- Gottlieb AB, Merola JF, Chen R, et al. Assessing clinical response and defining minimal disease activity in plaque psoriasis with the Physician Global Assessment and body surface area (PGA×BSA) composite tool: An analysis of apremilast phase 3 ESTEEM data. J Am Acad Dermatol. 2017;77:1178-1180.
- Strober B, Bagel J, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate plaque psoriasis with lower BSA: week 16 results from the UNVEIL study. J Drugs Dermatol. 2017;16:801-808.
- Bernhard J, Whitmore C, Guzzo C, et al. Evaluation of halobetasol propionate ointment in the treatment of plaque psoriasis: report on two double-blind, vehicle-controlled studies. J Am Acad Dermatol. 1991;25:1170-1174.
- Katz HI, Gross E, Buxman M, et al. A double-blind, vehicle-controlled paired comparison of halobetasol propionate cream on patients with plaque psoriasis. J Am Acad Dermatol. 1991;25:1175-1178.
- Blum G, Yawalkar S. A comparative, multicenter, double blind trial of 0.05% halobetasol propionate ointment and 0.1% betamethasone valerate ointment in the treatment of patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1153-1156.
- Goldberg B, Hartdegen R, Presbury D, et al. A double-blind, multicenter comparison of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1145-1148.
- Mensing H, Korsukewitz G, Yawalkar S. A double-blind, multicenter comparison between 0.05% halobetasol propionate ointment and 0.05% betamethasone dipropionate ointment in chronic plaque psoriasis. J Am Acad Dermatol. 1991;25:1149-1152.
- Herz G, Blum G, Yawalkar S. Halobetasol propionate cream by day and halobetasol propionate ointment at night for the treatment of pediatric patients with chronic, localized psoriasis and atopic dermatitis. J Am Acad Dermatol. 1991;25:1166-1169.
- Ultravate [package insert]. Jacksonville, FL: Ranbaxy; 2012.
- Gudjonsson JE, Elder JT. Psoriasis: epidemiology. Clin Dermatol. 2007;25:535-546.
- Liu Y, Krueger JG, Bowcock AM. Psoriasis: genetic associations and immune system changes. Genes Immun. 2007;8:1-12.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Alinia H, Moradi Tuchayi S, Smith JA, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176:759-764.
- Young M, Aldredge L, Parker P. Psoriasis for the primary care practitioner. J Am Assoc Nurse Pract. 2017;29:157-178.
- Devaux S, Castela A, Archier E, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):61-67.
- Ersser SJ, Cowdell FC, Latter SM, et al. Self-management experiences in adults with mild-moderate psoriasis: an exploratory study and implications for improved support. Br J Dermatol. 2010;163:1044-1049.
- Choi CW, Kim BR, Ohn J, et al. The advantage of cyclosporine A and methotrexate rotational therapy in long-term systemic treatment for chronic plaque psoriasis in a real world practice. Ann Dermatol. 2017;29:55-60.
- Callis Duffin K, Yeung H, Takeshita J, et al. Patient satisfaction with treatments for moderate-to-severe plaque psoriasis in clinical practice. Br J Dermatol. 2014;170:672-680.
- Spuls PI, Lecluse LL, Poulsen ML, et al. How good are clinical severity and outcome measures for psoriasis? quantitative evaluation in a systematic review. J Invest Dermatol. 2010;130:933-943.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Bozek A, Reich A. The reliability of three psoriasis assessment tools: psoriasis area severity index, body surface area and physician global assessment. Adv Clin Exp Med. 2017;26:851-856.
- Walsh JA, McFadden M, Woodcock J, et al. Product of the Physician Global Assessment and body surface area: a simple static measure of psoriasis severity in a longitudinal cohort. J Am Acad Dermatol. 2013;69:931-937.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis over 52 weeks: a phase III, randomized, controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Duffin KC, Papp KA, Bagel J, et al. Evaluation of the Physician Global Assessment and body surface area composite tool for assessing psoriasis response to apremilast therapy: results from ESTEEM 1 and ESTEEM 2. J Drugs Dermatol. 2017;16:147-153.
- Chiesa Fuxench ZC, Callis DK, Siegel M, et al. Validity of the Simple Measure for Assessing Psoriasis Activity (S-MAPA) for objectively evaluating disease severity in patients with plaque psoriasis. J Am Acad Dermatol. 2015;73:868-870.
- Walsh J. Comparative assessment of PASI and variations of PGA×BSA as measures of psoriasis severity in a clinical trial of moderate to severe psoriasis [poster 1830]. Presented at: Annual Meeting of the American Academy of Dermatology; March 20-24, 2015; San Francisco, CA.
- Gottlieb AB, Merola JF, Chen R, et al. Assessing clinical response and defining minimal disease activity in plaque psoriasis with the Physician Global Assessment and body surface area (PGA×BSA) composite tool: An analysis of apremilast phase 3 ESTEEM data. J Am Acad Dermatol. 2017;77:1178-1180.
- Strober B, Bagel J, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate plaque psoriasis with lower BSA: week 16 results from the UNVEIL study. J Drugs Dermatol. 2017;16:801-808.
- Bernhard J, Whitmore C, Guzzo C, et al. Evaluation of halobetasol propionate ointment in the treatment of plaque psoriasis: report on two double-blind, vehicle-controlled studies. J Am Acad Dermatol. 1991;25:1170-1174.
- Katz HI, Gross E, Buxman M, et al. A double-blind, vehicle-controlled paired comparison of halobetasol propionate cream on patients with plaque psoriasis. J Am Acad Dermatol. 1991;25:1175-1178.
- Blum G, Yawalkar S. A comparative, multicenter, double blind trial of 0.05% halobetasol propionate ointment and 0.1% betamethasone valerate ointment in the treatment of patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1153-1156.
- Goldberg B, Hartdegen R, Presbury D, et al. A double-blind, multicenter comparison of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1145-1148.
- Mensing H, Korsukewitz G, Yawalkar S. A double-blind, multicenter comparison between 0.05% halobetasol propionate ointment and 0.05% betamethasone dipropionate ointment in chronic plaque psoriasis. J Am Acad Dermatol. 1991;25:1149-1152.
- Herz G, Blum G, Yawalkar S. Halobetasol propionate cream by day and halobetasol propionate ointment at night for the treatment of pediatric patients with chronic, localized psoriasis and atopic dermatitis. J Am Acad Dermatol. 1991;25:1166-1169.
- Ultravate [package insert]. Jacksonville, FL: Ranbaxy; 2012.
Risk of Cancer-Associated Thrombosis and Bleeding in Veterans With Malignancy Who Are Receiving Direct Oral Anticoagulants (FULL)
Patients with cancer are at an increased risk of both venous thromboembolism (VTE) and bleeding complications. Risk factors for development of cancer-associated thrombosis (CAT) include indwelling lines, antineoplastic therapies, lack of mobility, and physical/chemical damage from the tumor.1 Venous thromboembolism may manifest as either deep vein thrombosis (DVT) or pulmonary embolism (PE). Cancer-associated thrombosis can lead to significant mortality in patients with cancer and may increase health care costs for additional medications and hospitalizations.
Zullig and colleagues estimated that 46,666 veterans received cancer care from the US Department of Veteran Affairs (VA) health care system in 2010. This number equates to about 3% of all patients with cancer in the US who receive at least some of their health care from the VA health care system.2 In addition to cancer care, these veterans receive treatment for various comorbid conditions. One such condition that is of concern in a prothrombotic state is atrial fibrillation (AF). For this condition, patients often require anticoagulation therapy with aspirin, warfarin, or one of the recently approved direct oral anticoagulant agents (DOACs), depending on risk factors.
Background
Due to their ease of administration, limited monitoring requirements, and proven safety and efficacy in patients with AF requiring anticoagulation, the American Heart Association (AHA) and American College of Cardiology recently switched their recommendations for rivaroxaban and dabigatran for oral stroke prevention to a class 1/level B recommendation.3
The American College of Chest Physicians (ACCP) recommends treatment with DOACs over warfarin therapy for acute VTE in patients without cancer; however, the ACCP prefers low molecular-weight heparin (LMWH) over the DOACs for treatment of CAT.4 Recently, the National Comprehensive Cancer Network (NCCN) updated its guidelines for the treatment of cancer-associated thromboembolic disease to recommend 2 of the DOACs (apixaban, rivaroxaban) for treatment of acute VTE over warfarin. These guidelines also recommend LMWH over DOACs for treatment of acute VTE in patients with cancer.5 These NCCN recommendations are largely based on prespecified subgroup meta-analyses of the DOACs compared with those of LMWH or warfarin in the cancer population.
In addition to stroke prevention in patients with AF, DOACs have additional FDA-approved indications, including treatment of acute VTE, prevention of recurrent VTE, and postoperative VTE treatment and prophylaxis. Due to a lack of head-to-head, randomized controlled trials comparing LMWH with DOACs in patients with cancer, these agents have not found their formal place in the treatment or prevention of CAT. Several meta-analyses have suggested similar efficacy and safety outcomes in patients with cancer compared with those of LMWH.6-8 These meta-analysis studies largely looked at subpopulations and compared the outcomes with those of the landmark CLOT (Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer Investigators) and CATCH (Comparison of Acute Treatments in Cancer Hemostasis) trials.9,10
As it is still unclear whether the DOACs are effective and safe for treatment/prevention of CAT, some confusion remains regarding the best management of these at-risk patients. In patients with cancer on DOAC therapy for an approved indication, it is assumed that the therapeutic benefit seen in approved indications would translate to treatment and prevention of CAT. This study aims to determine the incidence of VTE and rates of major and clinically relevant nonmajor bleeding (CRNMB) in veterans with cancer who received a DOAC.
Methods
This retrospective, single-center chart review was approved by the local institutional review board and research safety committee. A search within the VA Corporate Data Warehouse identified patients who had an active prescription for one of the DOACs (apixaban, dabigatran, edoxaban, and rivaroxaban) along with an ICD 9 or ICD 10 code corresponding to a malignancy.
Patients were included in the final analysis if they were aged 18 to 89 years at time of DOAC receipt, undergoing active treatment for malignancy, had evidence of a history of malignancy (either diagnostic or charted evidence of previous treatment), or received cancer-related surgery within 30 days of DOAC prescription with curative intent. Patients were excluded from the final analysis if they did not receive a DOAC prescription or have any clear evidence of malignancy documented in the medical chart.
Patients’ charts were evaluated for the following clinical endpoints: patient age, height (cm), weight (kg), type of malignancy, type of treatment for malignancy, serum creatinine (SCr), creatinine clearance (CrCl) calculated with the Cockcroft-Gault equation using actual body weight, serum hemoglobin, aspartate aminotransferase, alanine aminotransferase, total bilirubin, indication for DOAC, type of VTE, presence of a prior VTE, and diagnostic test performed for VTE. Major bleeding and CRNMB criteria were based on the definitions provided by the International Society on Thrombosis and Haemostasis (ISTH).11 All laboratory values and demographic information were gathered at the time of initial DOAC prescription.
The primary endpoint for this study was incidence of VTE. The secondary endpoints included major bleeding and CRNMB. All data collection and statistical analysis were done using Microsoft Excel 2016 (Redmond, WA). Comparisons of data between trials were done using the chi-squared calculation.
Results
From initial FDA approval of dabigatran (first DOAC on the market) on October 15, 2012, to January 1, 2017, there were 343 patients who met initial inclusion criteria. Of those, 115 did not have any clear evidence of malignancy, 22 did not have any records of DOAC receipt, 15 did not receive a DOAC within the date range, and 23 patients’ charts were unavailable.
The majority of the patients were males (96.6%), with an average age of 74.5 years. The average weight of all patients was 92.5 kg, with an average SCr of 1.1 mg/dL. This equated to an average CrCl of 85.5 mL/min based on the Cockcroft-Gault equation using actual bodyweight. Of the 177 patients evaluated, 30 (16.9%) were receiving active cancer treatment at time of DOAC initiation.
Two (1.1%) patients developed a VTE while receiving a DOAC.
Among the 177 evaluable patients in this study, there were 7 patients (4%) who developed a major bleed and 13 patients (7.3%) who developed a clinically relevant nonmajor bleed according to the definitions provided by ISTH.11
As previously mentioned, only 30 of the patients were actively receiving treatment during DOAC administration. Most of the documented cases of malignancy were either a history of nonmelanoma skin cancer (NMSC) or prostate cancer. The most common method of treatment was surgical resection for both malignancies. Of the 30 patients who received active malignancy treatment while on a DOAC, there were 4 patients with multiple myeloma, 6 patients with NMSC, 4 patients with colon cancer, 1 patient with chronic lymphocytic leukemia (CLL), 1 patient with chronic myelogenous leukemia (CML), 1 patient with small lymphocytic leukemia (SLL), 4 patients with non-small cell lung cancer (NSCLC), 1 patient with unspecified brain cancer, and 1 patient with breast cancer. The various characteristics of these patients are presented in Table 6.
Discussion
The CLOT and CATCH trials were chosen as historic comparators. Although the active treatment interventions and comparator arms were not similar between the patients included in this study and the CLOT and CATCH trials, the authors felt the comparison was appropriate as these trials were designed specifically for patients with malignancy. Additionally, these trials sought to assess rates of VTE formation and bleeding in the patient with malignancies—outcomes that aligned with this study. Alternative trials for comparison are the subgroup analyses of patients with malignancies in the AMPLIFY, RE-COVER, and EINSTEIN trials.12-14 Although these trials were designed to stratify patients based on presence of malignancy, they were not powered to account for increased risk of VTE in patients with malignancies.
There are multiple risk factors that increase the risk of CAT. Khoranna and colleagues identified primary stomach, pancreas, brain, lung, lymphoma, gynecologic, bladder, testicular, and renal carcinomas as a high risk of VTE formation.15 Additionally, Khoranna and colleagues noted that elderly patients and patients actively receiving treatment are at an increased risk of VTE formation.15 The low rate of VTE formation (1.1%) in the patients in this study may be due to the low risk for VTE formation. As previously mentioned, only 30 of the patients (16.9%) in this study were receiving active treatment.
Additionally, there were only 42 patients (23.7%) who had a high-risk malignancy. The increased age of the patient population (74.5 years old) in this study is one risk factor that could largely skew the risks of VTE formation in the patient population. In addition to age, the average body mass index (BMI) of this study’s patient population (30 kg/m2) may further increase risk of VTE. Although Khoranna and colleagues identified a BMI of 35 kg/m2 as the cutoff for increased risk of CAT, the increased risk based on a BMI of 30 kg/m2 cannot be ignored in the patients in this study.15
Another risk inherent in the treatment of patients with cancer is pancytopenia, which may lead to increased risks of bleeding and infection. When patients are exposed to an anticoagulant agent in the setting of decreased platelets and hemoglobin (from treatment or disease process), the risk for major bleeds and CRNMB are increased drastically. In this patient population, the combined rate of bleeding (11.3%) was relatively decreased compared with that of the CLOT (16.5% for all bleeding events) and CATCH (15.7% for all bleeding events) trials.9,10
Compared with the oncology subgroup analysis of the AMPLIFY, RE-COVER, and EINSTEIN trials, the differences are more noticeable. The AMPLIFY trial reported a 1.1% incidence of bleeding in patients with cancer on apixaban, whereas the RE-COVER trial did not report bleeding rates, and the EINSTEIN trial reported a 14% incidence of bleeding in all patients with cancer on rivaroxaban for VTE treatment.12-14 This study found a bleeding incidence of 12.2% with apixaban, 5.7% with dabigatran, and 14.7% with rivaroxaban. In this trial the incidence of bleeding with rivaroxaban were similar; however, the incidence of bleeding with apixaban was markedly higher. There is no obvious explanation for this, as the dosing of apixaban was appropriate in all patients in this trial except for one. There was no documented bleed in this patient’s medical chart.
A meta-analysis conducted by Vedovati and colleagues identified 6 studies in which patients with cancer received either a DOAC (with or without a heparin product) or vitamin K antagonist.16 That analysis found a nonsignificant reduction in VTE recurrence (odds ratio [OR], 0.63; 95% confidence interval [CI], 0.31-1.1), major bleeding (OR, 0.77; 95% CI, 0.41-1.44), and CRNMB (OR, 0.85; 95% CI, 0.62-1.18).16 The meta-analysis adds to the growing body of evidence in support of both safety and efficacy of DOACs in patients with cancer. Although the Vedovati and colleagues study does not directly compare rates between 2 treatment groups, the findings of similar rates of VTE recurrence, major bleed, and CRNMB are consistent with the current study. Despite differing patient characteristics, the meta-analysis by Vedovati and colleagues supports the ongoing use of DOACs in patients with malignancy, as does the current study.16
Limitations
Although it seems that apixaban, dabigatran, and rivaroxaban are effective in reducing the risk of VTE in veterans with malignancy, there are some inherent weaknesses in the current study. Most notably is the choice of comparator trials. The authors’ believe that the CLOT and CATCH trials were the most appropriate based on similarities in population and outcomes. Considering the CLOT and CATCH trials compared LMWH to coumarin products for treatment of VTE, future studies should compare use of these agents with DOACs in the cancer population. In addition, the study did not include outcomes that would adequately assess risks of VTE and bleeding formation. This information would have been beneficial to more effectively categorize this study’s patient population based on risks of each of its predetermined outcomes. Understanding safety and efficacy of DOACs in patients at various risks would help practitioners to choose more appropriate agents in practice. Last, this study did not assess the incidence of stroke in study patients. This is important because the DOACs were used mostly for stroke prevention in AF and atrial flutter. The increased risk of VTE in patients with cancer cannot directly correlate to risk of stroke with a comorbid cardiac condition, but the hypercoagulable state cannot be ignored in these patients.
Conclusion
This study provided some preliminary evidence for the safety and efficacy of DOACs in patients with cancer. The low incidence of VTE formation and similar rates of bleeding among other clinical trials indicate that DOACs are safe alternatives to currently recommended anticoagulation medication in patients with cancer.
1. Motykie GD, Zebala LP, Caprini JA, et al. A guide to venous thromboembolism risk factor assessment. J Thromb Thrombolysis. 2000;9(3):253-262.
2. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System: 2010 update. Mil Med. 2017;182(7):e1883-e1891.
3. January CT, Wann S, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. Circulation. 2014;130(23):2071-2104.
4. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352.
5. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Cancer-associated venous thromboembolic disease. Version 1.2018. https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/vte.pdf. Updated March 22, 2018. Accessed April 9, 2018.
6. Brunetti ND, Gesuete E, De Gennaro L, et al. Direct-acting oral anticoagulants compared to vitamin K inhibitors and low molecular weight heparin for the prevention of venous thromboembolism in patients with cancer: a meta-analysis study. Int J Cardiol. 2017;230:214-221.
7. Posch F, Konigsbrügge O, Zielinski C, Pabinger I, Ay C. Treatment of venous thromboembolism in patients with cancer: a network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015;136(3):582-589.
8. van Es N, Coppens M, Schulman S, Middledorp S, Büller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124(12):1968-1975.
9. Lee AY, Levine MN, Baker RI, et al; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low molecular weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
10. Lee AY, Kamphuisen PW, Meyer G, et al; CATCH Investigators. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314(7):677-686.
11. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S; Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(11):2119-2126.
12. Agnelli G, Büller HR, Cohen A, et al. Oral apixaban for the treatment of venous thromboembolism in cancer patients: results from the AMPLIFY trial. J Thromb Haemost. 2015;13(12):2187-2191.
13. Schulman S, Goldhaber SZ, Kearon C, et al. Treatment with dabigatran or warfarin in patients with venous thromboembolism and cancer. Thromb Haemost. 2015;114(1):150-157.
14. Prins MH, Lensing AW, Brighton TA, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PF): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematol. 2014;1(1):e37-e46.
15. Khoranna AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27(9):4839-4847.
16. Vedovati MC, Germini F, Agnelli G, Becattini C. Direct oral anticoagulants in patients with VTE and cancer: a systematic review and meta-analysis. Chest. 2015;147(2):475-483.
Patients with cancer are at an increased risk of both venous thromboembolism (VTE) and bleeding complications. Risk factors for development of cancer-associated thrombosis (CAT) include indwelling lines, antineoplastic therapies, lack of mobility, and physical/chemical damage from the tumor.1 Venous thromboembolism may manifest as either deep vein thrombosis (DVT) or pulmonary embolism (PE). Cancer-associated thrombosis can lead to significant mortality in patients with cancer and may increase health care costs for additional medications and hospitalizations.
Zullig and colleagues estimated that 46,666 veterans received cancer care from the US Department of Veteran Affairs (VA) health care system in 2010. This number equates to about 3% of all patients with cancer in the US who receive at least some of their health care from the VA health care system.2 In addition to cancer care, these veterans receive treatment for various comorbid conditions. One such condition that is of concern in a prothrombotic state is atrial fibrillation (AF). For this condition, patients often require anticoagulation therapy with aspirin, warfarin, or one of the recently approved direct oral anticoagulant agents (DOACs), depending on risk factors.
Background
Due to their ease of administration, limited monitoring requirements, and proven safety and efficacy in patients with AF requiring anticoagulation, the American Heart Association (AHA) and American College of Cardiology recently switched their recommendations for rivaroxaban and dabigatran for oral stroke prevention to a class 1/level B recommendation.3
The American College of Chest Physicians (ACCP) recommends treatment with DOACs over warfarin therapy for acute VTE in patients without cancer; however, the ACCP prefers low molecular-weight heparin (LMWH) over the DOACs for treatment of CAT.4 Recently, the National Comprehensive Cancer Network (NCCN) updated its guidelines for the treatment of cancer-associated thromboembolic disease to recommend 2 of the DOACs (apixaban, rivaroxaban) for treatment of acute VTE over warfarin. These guidelines also recommend LMWH over DOACs for treatment of acute VTE in patients with cancer.5 These NCCN recommendations are largely based on prespecified subgroup meta-analyses of the DOACs compared with those of LMWH or warfarin in the cancer population.
In addition to stroke prevention in patients with AF, DOACs have additional FDA-approved indications, including treatment of acute VTE, prevention of recurrent VTE, and postoperative VTE treatment and prophylaxis. Due to a lack of head-to-head, randomized controlled trials comparing LMWH with DOACs in patients with cancer, these agents have not found their formal place in the treatment or prevention of CAT. Several meta-analyses have suggested similar efficacy and safety outcomes in patients with cancer compared with those of LMWH.6-8 These meta-analysis studies largely looked at subpopulations and compared the outcomes with those of the landmark CLOT (Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer Investigators) and CATCH (Comparison of Acute Treatments in Cancer Hemostasis) trials.9,10
As it is still unclear whether the DOACs are effective and safe for treatment/prevention of CAT, some confusion remains regarding the best management of these at-risk patients. In patients with cancer on DOAC therapy for an approved indication, it is assumed that the therapeutic benefit seen in approved indications would translate to treatment and prevention of CAT. This study aims to determine the incidence of VTE and rates of major and clinically relevant nonmajor bleeding (CRNMB) in veterans with cancer who received a DOAC.
Methods
This retrospective, single-center chart review was approved by the local institutional review board and research safety committee. A search within the VA Corporate Data Warehouse identified patients who had an active prescription for one of the DOACs (apixaban, dabigatran, edoxaban, and rivaroxaban) along with an ICD 9 or ICD 10 code corresponding to a malignancy.
Patients were included in the final analysis if they were aged 18 to 89 years at time of DOAC receipt, undergoing active treatment for malignancy, had evidence of a history of malignancy (either diagnostic or charted evidence of previous treatment), or received cancer-related surgery within 30 days of DOAC prescription with curative intent. Patients were excluded from the final analysis if they did not receive a DOAC prescription or have any clear evidence of malignancy documented in the medical chart.
Patients’ charts were evaluated for the following clinical endpoints: patient age, height (cm), weight (kg), type of malignancy, type of treatment for malignancy, serum creatinine (SCr), creatinine clearance (CrCl) calculated with the Cockcroft-Gault equation using actual body weight, serum hemoglobin, aspartate aminotransferase, alanine aminotransferase, total bilirubin, indication for DOAC, type of VTE, presence of a prior VTE, and diagnostic test performed for VTE. Major bleeding and CRNMB criteria were based on the definitions provided by the International Society on Thrombosis and Haemostasis (ISTH).11 All laboratory values and demographic information were gathered at the time of initial DOAC prescription.
The primary endpoint for this study was incidence of VTE. The secondary endpoints included major bleeding and CRNMB. All data collection and statistical analysis were done using Microsoft Excel 2016 (Redmond, WA). Comparisons of data between trials were done using the chi-squared calculation.
Results
From initial FDA approval of dabigatran (first DOAC on the market) on October 15, 2012, to January 1, 2017, there were 343 patients who met initial inclusion criteria. Of those, 115 did not have any clear evidence of malignancy, 22 did not have any records of DOAC receipt, 15 did not receive a DOAC within the date range, and 23 patients’ charts were unavailable.
The majority of the patients were males (96.6%), with an average age of 74.5 years. The average weight of all patients was 92.5 kg, with an average SCr of 1.1 mg/dL. This equated to an average CrCl of 85.5 mL/min based on the Cockcroft-Gault equation using actual bodyweight. Of the 177 patients evaluated, 30 (16.9%) were receiving active cancer treatment at time of DOAC initiation.
Two (1.1%) patients developed a VTE while receiving a DOAC.
Among the 177 evaluable patients in this study, there were 7 patients (4%) who developed a major bleed and 13 patients (7.3%) who developed a clinically relevant nonmajor bleed according to the definitions provided by ISTH.11
As previously mentioned, only 30 of the patients were actively receiving treatment during DOAC administration. Most of the documented cases of malignancy were either a history of nonmelanoma skin cancer (NMSC) or prostate cancer. The most common method of treatment was surgical resection for both malignancies. Of the 30 patients who received active malignancy treatment while on a DOAC, there were 4 patients with multiple myeloma, 6 patients with NMSC, 4 patients with colon cancer, 1 patient with chronic lymphocytic leukemia (CLL), 1 patient with chronic myelogenous leukemia (CML), 1 patient with small lymphocytic leukemia (SLL), 4 patients with non-small cell lung cancer (NSCLC), 1 patient with unspecified brain cancer, and 1 patient with breast cancer. The various characteristics of these patients are presented in Table 6.
Discussion
The CLOT and CATCH trials were chosen as historic comparators. Although the active treatment interventions and comparator arms were not similar between the patients included in this study and the CLOT and CATCH trials, the authors felt the comparison was appropriate as these trials were designed specifically for patients with malignancy. Additionally, these trials sought to assess rates of VTE formation and bleeding in the patient with malignancies—outcomes that aligned with this study. Alternative trials for comparison are the subgroup analyses of patients with malignancies in the AMPLIFY, RE-COVER, and EINSTEIN trials.12-14 Although these trials were designed to stratify patients based on presence of malignancy, they were not powered to account for increased risk of VTE in patients with malignancies.
There are multiple risk factors that increase the risk of CAT. Khoranna and colleagues identified primary stomach, pancreas, brain, lung, lymphoma, gynecologic, bladder, testicular, and renal carcinomas as a high risk of VTE formation.15 Additionally, Khoranna and colleagues noted that elderly patients and patients actively receiving treatment are at an increased risk of VTE formation.15 The low rate of VTE formation (1.1%) in the patients in this study may be due to the low risk for VTE formation. As previously mentioned, only 30 of the patients (16.9%) in this study were receiving active treatment.
Additionally, there were only 42 patients (23.7%) who had a high-risk malignancy. The increased age of the patient population (74.5 years old) in this study is one risk factor that could largely skew the risks of VTE formation in the patient population. In addition to age, the average body mass index (BMI) of this study’s patient population (30 kg/m2) may further increase risk of VTE. Although Khoranna and colleagues identified a BMI of 35 kg/m2 as the cutoff for increased risk of CAT, the increased risk based on a BMI of 30 kg/m2 cannot be ignored in the patients in this study.15
Another risk inherent in the treatment of patients with cancer is pancytopenia, which may lead to increased risks of bleeding and infection. When patients are exposed to an anticoagulant agent in the setting of decreased platelets and hemoglobin (from treatment or disease process), the risk for major bleeds and CRNMB are increased drastically. In this patient population, the combined rate of bleeding (11.3%) was relatively decreased compared with that of the CLOT (16.5% for all bleeding events) and CATCH (15.7% for all bleeding events) trials.9,10
Compared with the oncology subgroup analysis of the AMPLIFY, RE-COVER, and EINSTEIN trials, the differences are more noticeable. The AMPLIFY trial reported a 1.1% incidence of bleeding in patients with cancer on apixaban, whereas the RE-COVER trial did not report bleeding rates, and the EINSTEIN trial reported a 14% incidence of bleeding in all patients with cancer on rivaroxaban for VTE treatment.12-14 This study found a bleeding incidence of 12.2% with apixaban, 5.7% with dabigatran, and 14.7% with rivaroxaban. In this trial the incidence of bleeding with rivaroxaban were similar; however, the incidence of bleeding with apixaban was markedly higher. There is no obvious explanation for this, as the dosing of apixaban was appropriate in all patients in this trial except for one. There was no documented bleed in this patient’s medical chart.
A meta-analysis conducted by Vedovati and colleagues identified 6 studies in which patients with cancer received either a DOAC (with or without a heparin product) or vitamin K antagonist.16 That analysis found a nonsignificant reduction in VTE recurrence (odds ratio [OR], 0.63; 95% confidence interval [CI], 0.31-1.1), major bleeding (OR, 0.77; 95% CI, 0.41-1.44), and CRNMB (OR, 0.85; 95% CI, 0.62-1.18).16 The meta-analysis adds to the growing body of evidence in support of both safety and efficacy of DOACs in patients with cancer. Although the Vedovati and colleagues study does not directly compare rates between 2 treatment groups, the findings of similar rates of VTE recurrence, major bleed, and CRNMB are consistent with the current study. Despite differing patient characteristics, the meta-analysis by Vedovati and colleagues supports the ongoing use of DOACs in patients with malignancy, as does the current study.16
Limitations
Although it seems that apixaban, dabigatran, and rivaroxaban are effective in reducing the risk of VTE in veterans with malignancy, there are some inherent weaknesses in the current study. Most notably is the choice of comparator trials. The authors’ believe that the CLOT and CATCH trials were the most appropriate based on similarities in population and outcomes. Considering the CLOT and CATCH trials compared LMWH to coumarin products for treatment of VTE, future studies should compare use of these agents with DOACs in the cancer population. In addition, the study did not include outcomes that would adequately assess risks of VTE and bleeding formation. This information would have been beneficial to more effectively categorize this study’s patient population based on risks of each of its predetermined outcomes. Understanding safety and efficacy of DOACs in patients at various risks would help practitioners to choose more appropriate agents in practice. Last, this study did not assess the incidence of stroke in study patients. This is important because the DOACs were used mostly for stroke prevention in AF and atrial flutter. The increased risk of VTE in patients with cancer cannot directly correlate to risk of stroke with a comorbid cardiac condition, but the hypercoagulable state cannot be ignored in these patients.
Conclusion
This study provided some preliminary evidence for the safety and efficacy of DOACs in patients with cancer. The low incidence of VTE formation and similar rates of bleeding among other clinical trials indicate that DOACs are safe alternatives to currently recommended anticoagulation medication in patients with cancer.
Patients with cancer are at an increased risk of both venous thromboembolism (VTE) and bleeding complications. Risk factors for development of cancer-associated thrombosis (CAT) include indwelling lines, antineoplastic therapies, lack of mobility, and physical/chemical damage from the tumor.1 Venous thromboembolism may manifest as either deep vein thrombosis (DVT) or pulmonary embolism (PE). Cancer-associated thrombosis can lead to significant mortality in patients with cancer and may increase health care costs for additional medications and hospitalizations.
Zullig and colleagues estimated that 46,666 veterans received cancer care from the US Department of Veteran Affairs (VA) health care system in 2010. This number equates to about 3% of all patients with cancer in the US who receive at least some of their health care from the VA health care system.2 In addition to cancer care, these veterans receive treatment for various comorbid conditions. One such condition that is of concern in a prothrombotic state is atrial fibrillation (AF). For this condition, patients often require anticoagulation therapy with aspirin, warfarin, or one of the recently approved direct oral anticoagulant agents (DOACs), depending on risk factors.
Background
Due to their ease of administration, limited monitoring requirements, and proven safety and efficacy in patients with AF requiring anticoagulation, the American Heart Association (AHA) and American College of Cardiology recently switched their recommendations for rivaroxaban and dabigatran for oral stroke prevention to a class 1/level B recommendation.3
The American College of Chest Physicians (ACCP) recommends treatment with DOACs over warfarin therapy for acute VTE in patients without cancer; however, the ACCP prefers low molecular-weight heparin (LMWH) over the DOACs for treatment of CAT.4 Recently, the National Comprehensive Cancer Network (NCCN) updated its guidelines for the treatment of cancer-associated thromboembolic disease to recommend 2 of the DOACs (apixaban, rivaroxaban) for treatment of acute VTE over warfarin. These guidelines also recommend LMWH over DOACs for treatment of acute VTE in patients with cancer.5 These NCCN recommendations are largely based on prespecified subgroup meta-analyses of the DOACs compared with those of LMWH or warfarin in the cancer population.
In addition to stroke prevention in patients with AF, DOACs have additional FDA-approved indications, including treatment of acute VTE, prevention of recurrent VTE, and postoperative VTE treatment and prophylaxis. Due to a lack of head-to-head, randomized controlled trials comparing LMWH with DOACs in patients with cancer, these agents have not found their formal place in the treatment or prevention of CAT. Several meta-analyses have suggested similar efficacy and safety outcomes in patients with cancer compared with those of LMWH.6-8 These meta-analysis studies largely looked at subpopulations and compared the outcomes with those of the landmark CLOT (Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer Investigators) and CATCH (Comparison of Acute Treatments in Cancer Hemostasis) trials.9,10
As it is still unclear whether the DOACs are effective and safe for treatment/prevention of CAT, some confusion remains regarding the best management of these at-risk patients. In patients with cancer on DOAC therapy for an approved indication, it is assumed that the therapeutic benefit seen in approved indications would translate to treatment and prevention of CAT. This study aims to determine the incidence of VTE and rates of major and clinically relevant nonmajor bleeding (CRNMB) in veterans with cancer who received a DOAC.
Methods
This retrospective, single-center chart review was approved by the local institutional review board and research safety committee. A search within the VA Corporate Data Warehouse identified patients who had an active prescription for one of the DOACs (apixaban, dabigatran, edoxaban, and rivaroxaban) along with an ICD 9 or ICD 10 code corresponding to a malignancy.
Patients were included in the final analysis if they were aged 18 to 89 years at time of DOAC receipt, undergoing active treatment for malignancy, had evidence of a history of malignancy (either diagnostic or charted evidence of previous treatment), or received cancer-related surgery within 30 days of DOAC prescription with curative intent. Patients were excluded from the final analysis if they did not receive a DOAC prescription or have any clear evidence of malignancy documented in the medical chart.
Patients’ charts were evaluated for the following clinical endpoints: patient age, height (cm), weight (kg), type of malignancy, type of treatment for malignancy, serum creatinine (SCr), creatinine clearance (CrCl) calculated with the Cockcroft-Gault equation using actual body weight, serum hemoglobin, aspartate aminotransferase, alanine aminotransferase, total bilirubin, indication for DOAC, type of VTE, presence of a prior VTE, and diagnostic test performed for VTE. Major bleeding and CRNMB criteria were based on the definitions provided by the International Society on Thrombosis and Haemostasis (ISTH).11 All laboratory values and demographic information were gathered at the time of initial DOAC prescription.
The primary endpoint for this study was incidence of VTE. The secondary endpoints included major bleeding and CRNMB. All data collection and statistical analysis were done using Microsoft Excel 2016 (Redmond, WA). Comparisons of data between trials were done using the chi-squared calculation.
Results
From initial FDA approval of dabigatran (first DOAC on the market) on October 15, 2012, to January 1, 2017, there were 343 patients who met initial inclusion criteria. Of those, 115 did not have any clear evidence of malignancy, 22 did not have any records of DOAC receipt, 15 did not receive a DOAC within the date range, and 23 patients’ charts were unavailable.
The majority of the patients were males (96.6%), with an average age of 74.5 years. The average weight of all patients was 92.5 kg, with an average SCr of 1.1 mg/dL. This equated to an average CrCl of 85.5 mL/min based on the Cockcroft-Gault equation using actual bodyweight. Of the 177 patients evaluated, 30 (16.9%) were receiving active cancer treatment at time of DOAC initiation.
Two (1.1%) patients developed a VTE while receiving a DOAC.
Among the 177 evaluable patients in this study, there were 7 patients (4%) who developed a major bleed and 13 patients (7.3%) who developed a clinically relevant nonmajor bleed according to the definitions provided by ISTH.11
As previously mentioned, only 30 of the patients were actively receiving treatment during DOAC administration. Most of the documented cases of malignancy were either a history of nonmelanoma skin cancer (NMSC) or prostate cancer. The most common method of treatment was surgical resection for both malignancies. Of the 30 patients who received active malignancy treatment while on a DOAC, there were 4 patients with multiple myeloma, 6 patients with NMSC, 4 patients with colon cancer, 1 patient with chronic lymphocytic leukemia (CLL), 1 patient with chronic myelogenous leukemia (CML), 1 patient with small lymphocytic leukemia (SLL), 4 patients with non-small cell lung cancer (NSCLC), 1 patient with unspecified brain cancer, and 1 patient with breast cancer. The various characteristics of these patients are presented in Table 6.
Discussion
The CLOT and CATCH trials were chosen as historic comparators. Although the active treatment interventions and comparator arms were not similar between the patients included in this study and the CLOT and CATCH trials, the authors felt the comparison was appropriate as these trials were designed specifically for patients with malignancy. Additionally, these trials sought to assess rates of VTE formation and bleeding in the patient with malignancies—outcomes that aligned with this study. Alternative trials for comparison are the subgroup analyses of patients with malignancies in the AMPLIFY, RE-COVER, and EINSTEIN trials.12-14 Although these trials were designed to stratify patients based on presence of malignancy, they were not powered to account for increased risk of VTE in patients with malignancies.
There are multiple risk factors that increase the risk of CAT. Khoranna and colleagues identified primary stomach, pancreas, brain, lung, lymphoma, gynecologic, bladder, testicular, and renal carcinomas as a high risk of VTE formation.15 Additionally, Khoranna and colleagues noted that elderly patients and patients actively receiving treatment are at an increased risk of VTE formation.15 The low rate of VTE formation (1.1%) in the patients in this study may be due to the low risk for VTE formation. As previously mentioned, only 30 of the patients (16.9%) in this study were receiving active treatment.
Additionally, there were only 42 patients (23.7%) who had a high-risk malignancy. The increased age of the patient population (74.5 years old) in this study is one risk factor that could largely skew the risks of VTE formation in the patient population. In addition to age, the average body mass index (BMI) of this study’s patient population (30 kg/m2) may further increase risk of VTE. Although Khoranna and colleagues identified a BMI of 35 kg/m2 as the cutoff for increased risk of CAT, the increased risk based on a BMI of 30 kg/m2 cannot be ignored in the patients in this study.15
Another risk inherent in the treatment of patients with cancer is pancytopenia, which may lead to increased risks of bleeding and infection. When patients are exposed to an anticoagulant agent in the setting of decreased platelets and hemoglobin (from treatment or disease process), the risk for major bleeds and CRNMB are increased drastically. In this patient population, the combined rate of bleeding (11.3%) was relatively decreased compared with that of the CLOT (16.5% for all bleeding events) and CATCH (15.7% for all bleeding events) trials.9,10
Compared with the oncology subgroup analysis of the AMPLIFY, RE-COVER, and EINSTEIN trials, the differences are more noticeable. The AMPLIFY trial reported a 1.1% incidence of bleeding in patients with cancer on apixaban, whereas the RE-COVER trial did not report bleeding rates, and the EINSTEIN trial reported a 14% incidence of bleeding in all patients with cancer on rivaroxaban for VTE treatment.12-14 This study found a bleeding incidence of 12.2% with apixaban, 5.7% with dabigatran, and 14.7% with rivaroxaban. In this trial the incidence of bleeding with rivaroxaban were similar; however, the incidence of bleeding with apixaban was markedly higher. There is no obvious explanation for this, as the dosing of apixaban was appropriate in all patients in this trial except for one. There was no documented bleed in this patient’s medical chart.
A meta-analysis conducted by Vedovati and colleagues identified 6 studies in which patients with cancer received either a DOAC (with or without a heparin product) or vitamin K antagonist.16 That analysis found a nonsignificant reduction in VTE recurrence (odds ratio [OR], 0.63; 95% confidence interval [CI], 0.31-1.1), major bleeding (OR, 0.77; 95% CI, 0.41-1.44), and CRNMB (OR, 0.85; 95% CI, 0.62-1.18).16 The meta-analysis adds to the growing body of evidence in support of both safety and efficacy of DOACs in patients with cancer. Although the Vedovati and colleagues study does not directly compare rates between 2 treatment groups, the findings of similar rates of VTE recurrence, major bleed, and CRNMB are consistent with the current study. Despite differing patient characteristics, the meta-analysis by Vedovati and colleagues supports the ongoing use of DOACs in patients with malignancy, as does the current study.16
Limitations
Although it seems that apixaban, dabigatran, and rivaroxaban are effective in reducing the risk of VTE in veterans with malignancy, there are some inherent weaknesses in the current study. Most notably is the choice of comparator trials. The authors’ believe that the CLOT and CATCH trials were the most appropriate based on similarities in population and outcomes. Considering the CLOT and CATCH trials compared LMWH to coumarin products for treatment of VTE, future studies should compare use of these agents with DOACs in the cancer population. In addition, the study did not include outcomes that would adequately assess risks of VTE and bleeding formation. This information would have been beneficial to more effectively categorize this study’s patient population based on risks of each of its predetermined outcomes. Understanding safety and efficacy of DOACs in patients at various risks would help practitioners to choose more appropriate agents in practice. Last, this study did not assess the incidence of stroke in study patients. This is important because the DOACs were used mostly for stroke prevention in AF and atrial flutter. The increased risk of VTE in patients with cancer cannot directly correlate to risk of stroke with a comorbid cardiac condition, but the hypercoagulable state cannot be ignored in these patients.
Conclusion
This study provided some preliminary evidence for the safety and efficacy of DOACs in patients with cancer. The low incidence of VTE formation and similar rates of bleeding among other clinical trials indicate that DOACs are safe alternatives to currently recommended anticoagulation medication in patients with cancer.
1. Motykie GD, Zebala LP, Caprini JA, et al. A guide to venous thromboembolism risk factor assessment. J Thromb Thrombolysis. 2000;9(3):253-262.
2. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System: 2010 update. Mil Med. 2017;182(7):e1883-e1891.
3. January CT, Wann S, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. Circulation. 2014;130(23):2071-2104.
4. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352.
5. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Cancer-associated venous thromboembolic disease. Version 1.2018. https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/vte.pdf. Updated March 22, 2018. Accessed April 9, 2018.
6. Brunetti ND, Gesuete E, De Gennaro L, et al. Direct-acting oral anticoagulants compared to vitamin K inhibitors and low molecular weight heparin for the prevention of venous thromboembolism in patients with cancer: a meta-analysis study. Int J Cardiol. 2017;230:214-221.
7. Posch F, Konigsbrügge O, Zielinski C, Pabinger I, Ay C. Treatment of venous thromboembolism in patients with cancer: a network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015;136(3):582-589.
8. van Es N, Coppens M, Schulman S, Middledorp S, Büller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124(12):1968-1975.
9. Lee AY, Levine MN, Baker RI, et al; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low molecular weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
10. Lee AY, Kamphuisen PW, Meyer G, et al; CATCH Investigators. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314(7):677-686.
11. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S; Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(11):2119-2126.
12. Agnelli G, Büller HR, Cohen A, et al. Oral apixaban for the treatment of venous thromboembolism in cancer patients: results from the AMPLIFY trial. J Thromb Haemost. 2015;13(12):2187-2191.
13. Schulman S, Goldhaber SZ, Kearon C, et al. Treatment with dabigatran or warfarin in patients with venous thromboembolism and cancer. Thromb Haemost. 2015;114(1):150-157.
14. Prins MH, Lensing AW, Brighton TA, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PF): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematol. 2014;1(1):e37-e46.
15. Khoranna AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27(9):4839-4847.
16. Vedovati MC, Germini F, Agnelli G, Becattini C. Direct oral anticoagulants in patients with VTE and cancer: a systematic review and meta-analysis. Chest. 2015;147(2):475-483.
1. Motykie GD, Zebala LP, Caprini JA, et al. A guide to venous thromboembolism risk factor assessment. J Thromb Thrombolysis. 2000;9(3):253-262.
2. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System: 2010 update. Mil Med. 2017;182(7):e1883-e1891.
3. January CT, Wann S, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. Circulation. 2014;130(23):2071-2104.
4. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352.
5. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Cancer-associated venous thromboembolic disease. Version 1.2018. https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/vte.pdf. Updated March 22, 2018. Accessed April 9, 2018.
6. Brunetti ND, Gesuete E, De Gennaro L, et al. Direct-acting oral anticoagulants compared to vitamin K inhibitors and low molecular weight heparin for the prevention of venous thromboembolism in patients with cancer: a meta-analysis study. Int J Cardiol. 2017;230:214-221.
7. Posch F, Konigsbrügge O, Zielinski C, Pabinger I, Ay C. Treatment of venous thromboembolism in patients with cancer: a network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015;136(3):582-589.
8. van Es N, Coppens M, Schulman S, Middledorp S, Büller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124(12):1968-1975.
9. Lee AY, Levine MN, Baker RI, et al; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low molecular weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
10. Lee AY, Kamphuisen PW, Meyer G, et al; CATCH Investigators. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314(7):677-686.
11. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S; Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(11):2119-2126.
12. Agnelli G, Büller HR, Cohen A, et al. Oral apixaban for the treatment of venous thromboembolism in cancer patients: results from the AMPLIFY trial. J Thromb Haemost. 2015;13(12):2187-2191.
13. Schulman S, Goldhaber SZ, Kearon C, et al. Treatment with dabigatran or warfarin in patients with venous thromboembolism and cancer. Thromb Haemost. 2015;114(1):150-157.
14. Prins MH, Lensing AW, Brighton TA, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PF): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematol. 2014;1(1):e37-e46.
15. Khoranna AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27(9):4839-4847.
16. Vedovati MC, Germini F, Agnelli G, Becattini C. Direct oral anticoagulants in patients with VTE and cancer: a systematic review and meta-analysis. Chest. 2015;147(2):475-483.
Prevalence of Suspicious Ultrasound Features in Hot Thyroid Nodules (FULL)
Although historically associated with a low risk of malignancy, hyperthyroidism is no longer thought to be protective against the occurrence of thyroid cancer. The incidence of malignancy has been reported in Graves disease at 2% and as high as 9% in toxic multinodular goiters.1,2
In evaluating patients with thyroid nodules and low thyroid stimulating hormone (TSH), which may indicate hyperthyroidism, the American Thyroid Association (ATA) recommends a radioiodine thyroid scan to determine whether a thyroid nodule is autonomous (hot) or nonfunctional (cold).3 Hot thyroid nodules are nodular areas of hyperfunctioning activity on radioiodine scan where tracer uptake is greater than the surrounding normal thyroid.
Historically, hot nodules have been associated with a low risk of malignancy and typically did not receive further ultrasound evaluation. However, recent studies have documented that the incidence of thyroid cancer in hot nodules may be underestimated. Mirfakhraee and colleagues performed a literature review in 2013 that revealed the prevalence of thyroid carcinoma in hot nodules managed by thyroidectomy ranged from 0% to 12.5% and averaged 3.1%.4 These findings may underestimate the prevalence of malignancy, because most hot nodules are not managed by thyroidectomy.
Given findings of hot nodules harboring malignancy, the authors investigated the role of thyroid ultrasound in patients with hyperthyroidism to identify suspicious features concerning for possible malignancies. The study objective was to estimate the prevalence of hot nodules with sonographic features concerning for malignancy in patients with hyperthyroidism in a Department of Veterans Affairs (VA) health care system.
Methods
This retrospective chart review consisted of 149,549 patients seen between January 2010 and December 2015 at the VA Northern California Health Care System (VANCHCS). The institutional review board approved the study and informed consent was waived.
Seven hundred sixty veterans were identified in the Computerized Patient Record System (CPRS) using the following ICD-9 codes: 242.9 (hyperthyroidism), 242.2 (toxic multinodular goiter), 242.3 (toxic nodular goiter), 242.1 (toxic uninodular goiter), and 241.9 (adenomatous goiter) (Figure 1).
Manual review of thyroid ultrasound scans for suspicious characteristics concerning for thyroid carcinoma were based on the 2015 ATA Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer.3 Per the ATA guidelines, sonographic patterns that are highly suspicious for malignancy were solid hypoechoic nodule or solid hypoechoic component of a partially cystic nodule with one or more of the following features: irregular margins (infiltrative, microlobulated), microcalcifications, taller than wide on transverse view, and rim calcifications with small extrusive soft-tissue component. Sonographic patterns with intermediate suspicion were hypoechoic solid nodule with smooth margins without microcalcifications, extrathyroidal extension, or taller than wide shape.3
Results
Of the 760 identified veterans, 230 had thyroid ultrasounds, and 113 had radioiodine thyroid scans. Of these, 70 patients had both ultrasound and radioiodine thyroid scans. This cohort consisted of 84.3% (59) males and 15.7% women (11). Ages ranged from 32 to 93 (mean age 62.9) years.
A total of 121 nodules were identified among the remaining 43 patients (11 individuals with cold thyroid scans and 16 individuals with no nodules were excluded). Of the 121 nodules, 44 were hot nodules, 29 were coexisting nodules found in patients with hot nodules, and 48 were other nodules found in patients without coexisting hot nodules (Figure 2).
Of the 44 hot nodules, the analysis identified 16 hot nodules with suspicious features on ultrasound and 28 nodules without suspicious findings. Breakdown of specific suspicious features included 11 that were solid hypoechoic, 3 nodules that had microcalcifications, and 2 nodules that had both characteristics (Table).
Twelve patients had hot nodules with suspicious ultrasound findings. Of this group, 6 patients had no further workup, 1 patient was lost to follow-up, and 1 patient was planned for fine needle aspiration (FNA) biopsy. Four patients underwent FNA, and all results were benign.
Discussion
Although most veterans identified with hyperthyroidism did not undergo imaging studies, of those who did, a remarkable number had unexpected ultrasonographically suspicious nodules. Of the 44 hot nodules identified on radioiodine studies, 16 had suspicious ultrasound findings that raised concern for malignancy based on the most recent ATA guidelines. In contrast to recent studies that have suggested an increased incidence of thyroid carcinoma in hot nodules, no cancers were detected in this cohort.4 However, only 4 patients in this study underwent FNA.
Worth noting is that the most common suspicious feature found in this study’s cohort was hypoechoic solid nodules, which is a feature that has a sensitivity of 81% however a low specificity of 53% in detecting thyroid malignancy.5 This appearance also is found in 55% of benign thyroid nodules.6 The overlap of hypoechoic nodules as a feature in both benign and malignant thyroid nodules can present as a diagnostic challenge in differentiating between the two.
The 2015 ATA guideline recommends that low TSH warrants a radioiodine scan, and FNA should be considered for isofunctioning or nonfunctioning nodules with suspicious sonographic features. Hot nodules found on scintigraphy need no further cytologic evaluation because they are mostly benign.3 There is no clear stance on the use of ultrasound in hot nodules.
The answer to whether patients with hot nodules should undergo ultrasound still remains unclear. This study showed a surprising number of hot nodules with worrisome architecture found on ultrasound. However, whether that correlates to actual malignant findings remains unknown as most individuals in the cohort did not undergo biopsy. Also, given the high prevalence of suspicious findings, it may be difficult to use ultrasound as a diagnostic tool in patients with hot nodules as false positives may lead to unnecessary interventions such as biopsy.
Limitations
The patient population consisted mostly of men (84.3%) and cannot be applied to the general population. Thyroid nodules are 4 times more common in women than they are in men.7 Another limitation was the lack of data on patients’ radiation exposure while in military service or as civilians. Finally, as a retrospective study, there was unavoidable selection bias.
Conclusion
The prevalence of suspicious findings concerning for malignancy in hot nodules was 36.3% (16/44) based on the 2015 ATA guidelines. This study’s preliminary observation suggests that although ultrasound is a noninvasive and relatively inexpensive diagnostic modality, it has a limited role in the evaluation of hot nodules given the high prevalence of suspicious findings. Clinicians may still consider its use in patients who also have high-risk historic features. This was a thought-generating, retrospective study, and further prospective studies in larger populations are needed to validate the study’s results.
1. Stocker DJ, Burch HB. Thyroid cancer yield in patients with Graves’ disease. Minerva Endocrinol. 2003;28(3):205-212.
2. Cerci C, Cerci SS, Eroglu E, et al. Thyroid cancer in toxic and non-toxic multinodular goiter. J Postgrad Med. 2007;53(3):157-160.
3. Haugen BRM, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133.
4. Mirfakhraee S, Mathews D, Peng L, Woodruff S, Zigman JM. A solitary hyperfunctioning thyroid nodule harboring thyroid carcinoma: review of the literature. Thyroid Res. 2013;6(1):7.
5. Papini E, Guglielmi R, Bianchini A, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002;87(5):1941-1946.
6. Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993;328(8):553-559.
7. Fish SA, Langer JE, Mandel SJ. Sonographic imaging of thyroid nodules and cervical lymph nodes. Endocrinol Metab Clin North Am. 2008;37(2):401-417.
Although historically associated with a low risk of malignancy, hyperthyroidism is no longer thought to be protective against the occurrence of thyroid cancer. The incidence of malignancy has been reported in Graves disease at 2% and as high as 9% in toxic multinodular goiters.1,2
In evaluating patients with thyroid nodules and low thyroid stimulating hormone (TSH), which may indicate hyperthyroidism, the American Thyroid Association (ATA) recommends a radioiodine thyroid scan to determine whether a thyroid nodule is autonomous (hot) or nonfunctional (cold).3 Hot thyroid nodules are nodular areas of hyperfunctioning activity on radioiodine scan where tracer uptake is greater than the surrounding normal thyroid.
Historically, hot nodules have been associated with a low risk of malignancy and typically did not receive further ultrasound evaluation. However, recent studies have documented that the incidence of thyroid cancer in hot nodules may be underestimated. Mirfakhraee and colleagues performed a literature review in 2013 that revealed the prevalence of thyroid carcinoma in hot nodules managed by thyroidectomy ranged from 0% to 12.5% and averaged 3.1%.4 These findings may underestimate the prevalence of malignancy, because most hot nodules are not managed by thyroidectomy.
Given findings of hot nodules harboring malignancy, the authors investigated the role of thyroid ultrasound in patients with hyperthyroidism to identify suspicious features concerning for possible malignancies. The study objective was to estimate the prevalence of hot nodules with sonographic features concerning for malignancy in patients with hyperthyroidism in a Department of Veterans Affairs (VA) health care system.
Methods
This retrospective chart review consisted of 149,549 patients seen between January 2010 and December 2015 at the VA Northern California Health Care System (VANCHCS). The institutional review board approved the study and informed consent was waived.
Seven hundred sixty veterans were identified in the Computerized Patient Record System (CPRS) using the following ICD-9 codes: 242.9 (hyperthyroidism), 242.2 (toxic multinodular goiter), 242.3 (toxic nodular goiter), 242.1 (toxic uninodular goiter), and 241.9 (adenomatous goiter) (Figure 1).
Manual review of thyroid ultrasound scans for suspicious characteristics concerning for thyroid carcinoma were based on the 2015 ATA Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer.3 Per the ATA guidelines, sonographic patterns that are highly suspicious for malignancy were solid hypoechoic nodule or solid hypoechoic component of a partially cystic nodule with one or more of the following features: irregular margins (infiltrative, microlobulated), microcalcifications, taller than wide on transverse view, and rim calcifications with small extrusive soft-tissue component. Sonographic patterns with intermediate suspicion were hypoechoic solid nodule with smooth margins without microcalcifications, extrathyroidal extension, or taller than wide shape.3
Results
Of the 760 identified veterans, 230 had thyroid ultrasounds, and 113 had radioiodine thyroid scans. Of these, 70 patients had both ultrasound and radioiodine thyroid scans. This cohort consisted of 84.3% (59) males and 15.7% women (11). Ages ranged from 32 to 93 (mean age 62.9) years.
A total of 121 nodules were identified among the remaining 43 patients (11 individuals with cold thyroid scans and 16 individuals with no nodules were excluded). Of the 121 nodules, 44 were hot nodules, 29 were coexisting nodules found in patients with hot nodules, and 48 were other nodules found in patients without coexisting hot nodules (Figure 2).
Of the 44 hot nodules, the analysis identified 16 hot nodules with suspicious features on ultrasound and 28 nodules without suspicious findings. Breakdown of specific suspicious features included 11 that were solid hypoechoic, 3 nodules that had microcalcifications, and 2 nodules that had both characteristics (Table).
Twelve patients had hot nodules with suspicious ultrasound findings. Of this group, 6 patients had no further workup, 1 patient was lost to follow-up, and 1 patient was planned for fine needle aspiration (FNA) biopsy. Four patients underwent FNA, and all results were benign.
Discussion
Although most veterans identified with hyperthyroidism did not undergo imaging studies, of those who did, a remarkable number had unexpected ultrasonographically suspicious nodules. Of the 44 hot nodules identified on radioiodine studies, 16 had suspicious ultrasound findings that raised concern for malignancy based on the most recent ATA guidelines. In contrast to recent studies that have suggested an increased incidence of thyroid carcinoma in hot nodules, no cancers were detected in this cohort.4 However, only 4 patients in this study underwent FNA.
Worth noting is that the most common suspicious feature found in this study’s cohort was hypoechoic solid nodules, which is a feature that has a sensitivity of 81% however a low specificity of 53% in detecting thyroid malignancy.5 This appearance also is found in 55% of benign thyroid nodules.6 The overlap of hypoechoic nodules as a feature in both benign and malignant thyroid nodules can present as a diagnostic challenge in differentiating between the two.
The 2015 ATA guideline recommends that low TSH warrants a radioiodine scan, and FNA should be considered for isofunctioning or nonfunctioning nodules with suspicious sonographic features. Hot nodules found on scintigraphy need no further cytologic evaluation because they are mostly benign.3 There is no clear stance on the use of ultrasound in hot nodules.
The answer to whether patients with hot nodules should undergo ultrasound still remains unclear. This study showed a surprising number of hot nodules with worrisome architecture found on ultrasound. However, whether that correlates to actual malignant findings remains unknown as most individuals in the cohort did not undergo biopsy. Also, given the high prevalence of suspicious findings, it may be difficult to use ultrasound as a diagnostic tool in patients with hot nodules as false positives may lead to unnecessary interventions such as biopsy.
Limitations
The patient population consisted mostly of men (84.3%) and cannot be applied to the general population. Thyroid nodules are 4 times more common in women than they are in men.7 Another limitation was the lack of data on patients’ radiation exposure while in military service or as civilians. Finally, as a retrospective study, there was unavoidable selection bias.
Conclusion
The prevalence of suspicious findings concerning for malignancy in hot nodules was 36.3% (16/44) based on the 2015 ATA guidelines. This study’s preliminary observation suggests that although ultrasound is a noninvasive and relatively inexpensive diagnostic modality, it has a limited role in the evaluation of hot nodules given the high prevalence of suspicious findings. Clinicians may still consider its use in patients who also have high-risk historic features. This was a thought-generating, retrospective study, and further prospective studies in larger populations are needed to validate the study’s results.
Although historically associated with a low risk of malignancy, hyperthyroidism is no longer thought to be protective against the occurrence of thyroid cancer. The incidence of malignancy has been reported in Graves disease at 2% and as high as 9% in toxic multinodular goiters.1,2
In evaluating patients with thyroid nodules and low thyroid stimulating hormone (TSH), which may indicate hyperthyroidism, the American Thyroid Association (ATA) recommends a radioiodine thyroid scan to determine whether a thyroid nodule is autonomous (hot) or nonfunctional (cold).3 Hot thyroid nodules are nodular areas of hyperfunctioning activity on radioiodine scan where tracer uptake is greater than the surrounding normal thyroid.
Historically, hot nodules have been associated with a low risk of malignancy and typically did not receive further ultrasound evaluation. However, recent studies have documented that the incidence of thyroid cancer in hot nodules may be underestimated. Mirfakhraee and colleagues performed a literature review in 2013 that revealed the prevalence of thyroid carcinoma in hot nodules managed by thyroidectomy ranged from 0% to 12.5% and averaged 3.1%.4 These findings may underestimate the prevalence of malignancy, because most hot nodules are not managed by thyroidectomy.
Given findings of hot nodules harboring malignancy, the authors investigated the role of thyroid ultrasound in patients with hyperthyroidism to identify suspicious features concerning for possible malignancies. The study objective was to estimate the prevalence of hot nodules with sonographic features concerning for malignancy in patients with hyperthyroidism in a Department of Veterans Affairs (VA) health care system.
Methods
This retrospective chart review consisted of 149,549 patients seen between January 2010 and December 2015 at the VA Northern California Health Care System (VANCHCS). The institutional review board approved the study and informed consent was waived.
Seven hundred sixty veterans were identified in the Computerized Patient Record System (CPRS) using the following ICD-9 codes: 242.9 (hyperthyroidism), 242.2 (toxic multinodular goiter), 242.3 (toxic nodular goiter), 242.1 (toxic uninodular goiter), and 241.9 (adenomatous goiter) (Figure 1).
Manual review of thyroid ultrasound scans for suspicious characteristics concerning for thyroid carcinoma were based on the 2015 ATA Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer.3 Per the ATA guidelines, sonographic patterns that are highly suspicious for malignancy were solid hypoechoic nodule or solid hypoechoic component of a partially cystic nodule with one or more of the following features: irregular margins (infiltrative, microlobulated), microcalcifications, taller than wide on transverse view, and rim calcifications with small extrusive soft-tissue component. Sonographic patterns with intermediate suspicion were hypoechoic solid nodule with smooth margins without microcalcifications, extrathyroidal extension, or taller than wide shape.3
Results
Of the 760 identified veterans, 230 had thyroid ultrasounds, and 113 had radioiodine thyroid scans. Of these, 70 patients had both ultrasound and radioiodine thyroid scans. This cohort consisted of 84.3% (59) males and 15.7% women (11). Ages ranged from 32 to 93 (mean age 62.9) years.
A total of 121 nodules were identified among the remaining 43 patients (11 individuals with cold thyroid scans and 16 individuals with no nodules were excluded). Of the 121 nodules, 44 were hot nodules, 29 were coexisting nodules found in patients with hot nodules, and 48 were other nodules found in patients without coexisting hot nodules (Figure 2).
Of the 44 hot nodules, the analysis identified 16 hot nodules with suspicious features on ultrasound and 28 nodules without suspicious findings. Breakdown of specific suspicious features included 11 that were solid hypoechoic, 3 nodules that had microcalcifications, and 2 nodules that had both characteristics (Table).
Twelve patients had hot nodules with suspicious ultrasound findings. Of this group, 6 patients had no further workup, 1 patient was lost to follow-up, and 1 patient was planned for fine needle aspiration (FNA) biopsy. Four patients underwent FNA, and all results were benign.
Discussion
Although most veterans identified with hyperthyroidism did not undergo imaging studies, of those who did, a remarkable number had unexpected ultrasonographically suspicious nodules. Of the 44 hot nodules identified on radioiodine studies, 16 had suspicious ultrasound findings that raised concern for malignancy based on the most recent ATA guidelines. In contrast to recent studies that have suggested an increased incidence of thyroid carcinoma in hot nodules, no cancers were detected in this cohort.4 However, only 4 patients in this study underwent FNA.
Worth noting is that the most common suspicious feature found in this study’s cohort was hypoechoic solid nodules, which is a feature that has a sensitivity of 81% however a low specificity of 53% in detecting thyroid malignancy.5 This appearance also is found in 55% of benign thyroid nodules.6 The overlap of hypoechoic nodules as a feature in both benign and malignant thyroid nodules can present as a diagnostic challenge in differentiating between the two.
The 2015 ATA guideline recommends that low TSH warrants a radioiodine scan, and FNA should be considered for isofunctioning or nonfunctioning nodules with suspicious sonographic features. Hot nodules found on scintigraphy need no further cytologic evaluation because they are mostly benign.3 There is no clear stance on the use of ultrasound in hot nodules.
The answer to whether patients with hot nodules should undergo ultrasound still remains unclear. This study showed a surprising number of hot nodules with worrisome architecture found on ultrasound. However, whether that correlates to actual malignant findings remains unknown as most individuals in the cohort did not undergo biopsy. Also, given the high prevalence of suspicious findings, it may be difficult to use ultrasound as a diagnostic tool in patients with hot nodules as false positives may lead to unnecessary interventions such as biopsy.
Limitations
The patient population consisted mostly of men (84.3%) and cannot be applied to the general population. Thyroid nodules are 4 times more common in women than they are in men.7 Another limitation was the lack of data on patients’ radiation exposure while in military service or as civilians. Finally, as a retrospective study, there was unavoidable selection bias.
Conclusion
The prevalence of suspicious findings concerning for malignancy in hot nodules was 36.3% (16/44) based on the 2015 ATA guidelines. This study’s preliminary observation suggests that although ultrasound is a noninvasive and relatively inexpensive diagnostic modality, it has a limited role in the evaluation of hot nodules given the high prevalence of suspicious findings. Clinicians may still consider its use in patients who also have high-risk historic features. This was a thought-generating, retrospective study, and further prospective studies in larger populations are needed to validate the study’s results.
1. Stocker DJ, Burch HB. Thyroid cancer yield in patients with Graves’ disease. Minerva Endocrinol. 2003;28(3):205-212.
2. Cerci C, Cerci SS, Eroglu E, et al. Thyroid cancer in toxic and non-toxic multinodular goiter. J Postgrad Med. 2007;53(3):157-160.
3. Haugen BRM, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133.
4. Mirfakhraee S, Mathews D, Peng L, Woodruff S, Zigman JM. A solitary hyperfunctioning thyroid nodule harboring thyroid carcinoma: review of the literature. Thyroid Res. 2013;6(1):7.
5. Papini E, Guglielmi R, Bianchini A, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002;87(5):1941-1946.
6. Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993;328(8):553-559.
7. Fish SA, Langer JE, Mandel SJ. Sonographic imaging of thyroid nodules and cervical lymph nodes. Endocrinol Metab Clin North Am. 2008;37(2):401-417.
1. Stocker DJ, Burch HB. Thyroid cancer yield in patients with Graves’ disease. Minerva Endocrinol. 2003;28(3):205-212.
2. Cerci C, Cerci SS, Eroglu E, et al. Thyroid cancer in toxic and non-toxic multinodular goiter. J Postgrad Med. 2007;53(3):157-160.
3. Haugen BRM, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133.
4. Mirfakhraee S, Mathews D, Peng L, Woodruff S, Zigman JM. A solitary hyperfunctioning thyroid nodule harboring thyroid carcinoma: review of the literature. Thyroid Res. 2013;6(1):7.
5. Papini E, Guglielmi R, Bianchini A, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002;87(5):1941-1946.
6. Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993;328(8):553-559.
7. Fish SA, Langer JE, Mandel SJ. Sonographic imaging of thyroid nodules and cervical lymph nodes. Endocrinol Metab Clin North Am. 2008;37(2):401-417.
Patient, Caregiver, and Clinician Perspectives on Expectations for Home Healthcare after Discharge: A Qualitative Case Study
Patients who are discharged from the hospital with home healthcare (HHC) are older, sicker, and more likely to be readmitted to the hospital than patients discharged home without HHC.1-3 Communication between clinicians in different settings is a key factor in successful transitions. In prior work, we focused on communication between primary care providers, hospitalists, and HHC nurses to inform efforts to improve care transitions.4,5 In one study, HHC nurses described that patients frequently have expectations beyond the scope of what skilled HHC provides,5 which prompted us to also question experiences of patients and caregivers after discharge with skilled HHC (eg, nursing and physical therapy).
In a prior qualitative study by Foust and colleagues, HHC patients and caregivers described disparate experiences around preparation for hospital discharge—patients expressed knowing about the timing and plans for discharge, and the caregivers frequently felt left out of this discussion.6 In other studies, caregivers of recently discharged patients have described feeling excluded from interactions with clinicians both before and after discharge.7,8 In another recent qualitative study, caregivers described uncertainty about their role compared with the HHC role in caring for the patient.9
As of 2016, a majority of states had passed the Caregiver Advise, Record, and Enable (CARE) Act, which requires hospitals to (1) record a family caregiver in the medical record, (2) inform this caregiver about discharge, and (3) deliver instructions with education about medical tasks that they will need to complete after discharge.10
METHODS
Study Design
In this qualitative descriptive case study, we interviewed HHC patients, an involved caregiver, and the HHC clinician completing the first HHC visit within 7-14 days following hospital discharge. We chose this timeframe to allow patients to receive one or more HHC visits following hospital discharge.
Population
- >65 years old,12 eligibility was initially limited to patients
- >65 years old. Due to recruitment challenges, the age range was broadened to
- >50 years old in October 2017.
Because our goal was to better understand the experience of general medicine patients with multiple comorbidities, we recruited patients from one general medicine unit at an academic hospital in Colorado. Patients on this unit were screened for eligibility Monday-Friday (excluding weekends and holidays) based on research assistant availability.
Criteria included are as follows: HHC referral, three or more comorbidities, resides in the community prior to admission (ie, not in a facility), cognitively intact, English speaking, and able to identify a caregiver participating in their care. Eligible patients were approached for written consent prior to discharge to allow us to contact them 7-14 days after discharge for an interview by phone or in their home, per their preference. At the time of consent, patients provided contact information for their informal caregiver. Caregiver eligibility criteria included the following: age ≥18 years and provides caregiving at least one hour a week before hospital discharge. HHC clinicians approached for interviews had completed the first HHC visit for the patient following discharge. Both caregivers and HHC clinicians provided verbal consent for interviews. All participants received a $25 gift card for participation in the study.
Framework and Data Collection
Our interview guides were organized by the Agency for Healthcare Research and Quality Care Coordination Framework, an approach we have taken in prior work.4,5,13 We added questions about patient preparation and self-management support to build on findings from a prior study with HHC nurses and on prior work by Coleman and colleagues.5,14 Sample questions from the interview guides for patients, caregivers, and HHC clinicians within key analysis domains are included in Appendix 1. The patient and caregiver interviews were completed by an individual with prior experience in social work and healthcare (SS). The HHC clinician interviews were completed by either this individual (SS) or a physician-researcher with experience in qualitative methods (CJ). Patients and caregivers could choose to be interviewed individually or together. All interviews were digitally recorded and transcribed verbatim.
Analysis
Patient interviews lasted an average of 43 minutes, caregiver interviews an average of 41 minutes, and HHC clinician interviews an average of 25 minutes
We observed the two main themes of clear and unclear expectations for HHC after discharge. Clear expectations occur when the patient and/or caregiver have expectations for HHC that align with the services they receive. Unclear expectations occur when the patient and/or caregiver expectations are either uncertain or misaligned with the services they receive. Although not all interviews yielded codes about clear or unclear expectations, patients described clear expectations in five cases and unclear expectations in another five cases.
In nine cases with more than one perspective available, expectations were compared within cases and found to be clear (three cases), unclear (three cases), or discordant (three cases) across perspectives. For the discordant cases, the description of clear and unclear expectations differed between patients and either their caregiver or their HHC clinician. Patients and caregivers with clear expectations for HHC frequently described prior experiences with skilled HHC or work experience within the healthcare field. In most cases with unclear expectations, the patient and caregiver did not have prior experience with HHC. In addition, the desire for assistance with personal care for patients such as showering and housekeeping was described by caregivers with unclear expectations. The results are organized into clear, unclear, and discordant expectations from the perspectives of patients, caregivers, and HHC clinicians within cases.
Clear Expectations within Cases
Clear expectations for HHC were identified across perspectives in three cases, with sample quotes provided in Table 2. In the case of patient 1, the patient and HHC nurse had known each other for over two years because the patient had a wound requiring long-term HHC services. A caregiver did not complete an interview in this case. With patient 2, the patient, caregiver, and HHC physical therapist (PT) all describe that the patient had clear expectations for HHC. In this case, the patient and caregiver describe feeling prepared because of previously receiving HHC, prior work experience in the healthcare field, and a caregiver with experience working in HHC. In the case of patient 3, the patient had previously received HHC from the same HHC nurse.
Unclear Expectations across Cases
For the three cases in which unclear expectations were described across perspectives, two of the patients described being new to HHC, with representative quotes in Table 2. Patient 4 and her caregiver are new to HHC and describe unclear expectations for both the HHC referral and the HHC role, which was also noted by the HHC clinician. Of note, the caregiver for patient 4 further described that she was unable to be present for the first HHC visit. In the case of patient 5, although the patient had previously received HHC, the patient describes not knowing why the HHC PT needs to see her after discharge, which is also noted by the HHC PT. Finally, both patient 6 and her HHC PT describe that the patient was not sure about their expectations for HHC and that HHC was a new experience for them.
Discordant Expectation Clarity across Cases
In three of the cases, the description of clear and unclear expectations was discrepant across roles. In case 7, the caregiver and patient are new to HHC and express different perspectives about expectations for HHC. The HHC clinician, in this case, did not complete an interview. The caregiver describes not being present for the first HHC visit and no awareness that the patient was being discharged with HHC:
Caregiver: Well, we didn’t even know she had home health until she got home.
The same caregiver also expresses unclear expectations for HHC:
Caregiver: It’s pretty cloudy. They (the HHC clinicians) don’t help her with her laundry, they don’t help with the housekeeping, they don’t help… with her showers so somebody is there when she showers. They don’t do anything. The only two things like I said is the…home healthcare comes in on Wednesdays to see what she needs and then the therapy comes in one day a week.
However, the patient expresses more clear expectations that are being met by HHC.
Patient: They (HHC) have met my expectations. They come in twice a week. They do vitals, take vitals and discuss with me, you know, what my feelings are, how I’m doing and I know they have met my expectations.
In case 8, although the patient describes knowing about the HHC PT involvement in her care, she expresses some unclear expectations about an HHC nurse after discharge.
Patient: As far as home health, I didn’t have a real …plan there at the hospital… They knew about (the HHC PT) coming once a week but as far as, you know, a nurse coming by to check on me, no.
However, the HHC PT describes feeling that the patient had clear expectations for HHC after discharge:
Interviewer: Can you reflect on whether she was prepared to receive home healthcare?
HHC PT: Yeah, she was ready.
Interviewer: …do you feel like she was prepared to know what to expect from you?
HHC PT: Yeah, but I think that comes from being a previous patient also.
Finally, in case 9, the patient describes clear expectations for HHC even though they were new to HHC:
Patient: …I knew what the PT was going to do and …I still need her because I’ve lost so much weight so she’s been really good, instrumental, at giving me exercises… Occupational therapist…she’s going to teach me how to shave, she’s going to teach me how to get ready for the day.
The HHC PT describes that although the patient knew the PT role, they reflect that the patient may have been somewhat unclear about expectations for the first HHC visit:
HHC PT: He knew all that it entailed with the exception of he didn’t really know what the first day was going to be like and the first day I don’t usually do treatment because it does take a long time to get all the paperwork signed, to do the evaluation and the fact that it takes two hours to do that note.
DISCUSSION
In this qualitative case study with HHC patients, caregivers, and clinicians, the participants described varying levels of expectation clarity for HHC after discharge. We triangulated across and within cases and found three cases with clear expectations and three cases with unclear expectations for HHC across perspectives. In three additional cases, we found discordant expectations across perspectives: patients and HHC clinician expectations differed in two of the cases and a patient and caregiver differed in one case. Of interest, in all three cases of clear expectations across perspectives, the patients and/or caregivers had prior HHC or healthcare work experience. In contrast,
Prior studies in this area have included a qualitative study HHC patients, caregivers, and clinicians by Foust and colleagues in which multiple caregivers described finding out about the discharge from the patient or other caregivers, rather than being actively engaged by clinicians.6 In another recent qualitative study by Arbaje and colleagues, a majority of caregivers described “mismatched expectations” about HHC services, in which caregivers
When caregivers have unclear expectations for HHC, they could be expressing the need for more support after hospital discharge, which suggests an active role for hospital teams to assess and address additional support needs with the patients and caregivers. For example, if the patient or caregiver request additional personal care services, a home health aide could help to reduce caregiver burden and improve the support network for the patient. In a prior study in which patients were asked what would help them to make informed decisions about postacute care options, the patients described wanting to receive practical information that could describe how it would apply to their specific situation and perceived needs.18 To provide this for patients and caregivers, it would follow that hospitals could provide information about skilled HHC nursing and therapies and information about services that could meet additional needs, such as home health aides.
Limitations of this study include that it was a small qualitative case study of patients, caregivers, and HHC clinicians from one medical unit at one academic medical center. Most patients in this study had Medicare insurance, were 65 years and older, white, and female.
In conclusion, to improve care transitions for HHC patients and their caregivers, emphasizing engagement of caregivers is key to ensure that they are educated about HHC, provided with additional support as needed, and included in initial HHC visits once the patients are at home. Even though patients and caregivers with prior HHC experience often had clear expectations for HHC, a strategy to uniformly engage caregivers across a range of experience can ensure caregivers have all the information and support needed to optimize care transitions to HHC.
Disclosures
The authors have nothing to disclose.
Funding
Dr. Christine Jones is supported by grant number K08HS024569 from the Agency for Healthcare Research and Quality. Jason Falvey was supported by grant F31AG056069 from the National Institute on Aging, National Institutes of Health and is currently supported by T32AG019134. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality or the National Institutes of Health.
1. Jones CD, Wald HL, Boxer RS, et al. Characteristics associated with home health care referrals at hospital discharge: results from the 2012 National Inpatient Sample. Health Serv Res. 2017;52(2):879-894. doi: 10.1111/1475-6773. PubMed
2. Avalere Health. Home Health Chartbook 2015: Prepared for the Alliance for Home Health Quality and Innovation. 2016.
3. Hospital Compare. https://www.medicare.gov/hospitalcompare/search.html. Accessed May 1, 2017.
4. Jones CD, Vu MB, O’Donnell CM, et al. A failure to communicate: a qualitative exploration of care coordination between hospitalists and primary care providers around patient hospitalizations. J Gen Intern Med. 2015;30(4):417-424. doi: 10.1007/s11606-014-3056-x. PubMed
5. Jones CD, Jones J, Richard A, et al. “Connecting the dots”: a qualitative study of home health nurse perspectives on coordinating care for recently discharged patients. J Gen Intern Med. 2017;32(10):1114-1121. doi: 10.1007/s11606-017-4104-0. PubMed
6. Foust JB, Vuckovic N, Henriquez E. Hospital to home health care transition: patient, caregiver, and clinician perspectives. West J Nurs Res. 2012;34(2):194-212. doi: 10.1177/0193945911400448. PubMed
7. Blair J, Volpe M, Aggarwal B. Challenges, needs, and experiences of recently hospitalized cardiac patients and their informal caregivers. J Cardiovasc Nurs. 2014;29(1):29-37. doi: 10.1097/JCN.0b013e3182784123. PubMed
8. Coleman EA, Roman SP. Family caregivers’ experiences during transitions out of hospital. J Healthc Qual. 2015;37(1):12-21. doi: 10.1097/01.JHQ.0000460117.83437.b3. PubMed
9. Arbaje AI, Hughes A, Werner N, et al. Information management goals and process failures during home visits for middle-aged and older adults receiving skilled home healthcare services after hospital discharge: a multisite, qualitative study. BMJ Qual Saf. 2018. doi: 10.1136/bmjqs-2018-008163. PubMed
10. Coleman EA. Family caregivers as partners in care transitions: the caregiver advise record and enable act. J Hosp Med. 2016;11(12):883-885. doi: 10.1002/jhm.2637. PubMed
11. Jones AL, Harris-Kojetin L, Valverde R. Characteristics and use of home health care by men and women aged 65 and over. Natl Health Stat Report. 2012(52):1-7. PubMed
12. Tian W. An all-payer view of hospital discharge to postacute care, 2013. HCUP Statistical Brief #205. Rockville, Maryland; 2016. PubMed
13. McDonald KM, Sundaram V, Bravata DM, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies (Vol. 7: Care Coordination). Rockville, Maryland; 2007. PubMed
14. Coleman EA, Smith JD, Frank JC, Eilertsen TB, Thiare JN, Kramer AM. Development and testing of a measure designed to assess the quality of care transitions. Int J Integr Care. 2002;2:e02. doi: 10.5334/ijic.60. PubMed
15. Jones J, Nowels CT, Sudore R, Ahluwalia S, Bekelman DB. The future as a series of transitions: qualitative study of heart failure patients and their informal caregivers. J Gen Intern Med. 2015;30(2):176-182. doi: 10.1007/s11606-014-3085-5. PubMed
16. Lum HD, Jones J, Lahoff D, et al. Unique challenges of hospice for patients with heart failure: a qualitative study of hospice clinicians. Am Heart J. 2015;170(3):524-530 e523. doi: 10.1016/j.ahj.2015.06.019. PubMed
17. Kerr C, Nixon A, Wild D. Assessing and demonstrating data saturation in qualitative inquiry supporting patient-reported outcomes research. Expert Rev Pharmacoecon Outcomes Res. 2010;10(3):269-281. doi: 10.1586/erp.10.30. PubMed
18. Sefcik JS, Nock RH, Flores EJ, et al. Patient preferences for information on post-acute care services. Res Gerontol Nurs. 2016;9(4):175-182. doi: 10.3928/19404921-20160120-01. PubMed
19. Coleman EA, Roman SP, Hall KA, Min SJ. Enhancing the care transitions intervention protocol to better address the needs of family caregivers. J Healthc Qual. 2015;37(1):2-11. doi: 10.1097/01.JHQ.0000460118.60567.fe. PubMed
Patients who are discharged from the hospital with home healthcare (HHC) are older, sicker, and more likely to be readmitted to the hospital than patients discharged home without HHC.1-3 Communication between clinicians in different settings is a key factor in successful transitions. In prior work, we focused on communication between primary care providers, hospitalists, and HHC nurses to inform efforts to improve care transitions.4,5 In one study, HHC nurses described that patients frequently have expectations beyond the scope of what skilled HHC provides,5 which prompted us to also question experiences of patients and caregivers after discharge with skilled HHC (eg, nursing and physical therapy).
In a prior qualitative study by Foust and colleagues, HHC patients and caregivers described disparate experiences around preparation for hospital discharge—patients expressed knowing about the timing and plans for discharge, and the caregivers frequently felt left out of this discussion.6 In other studies, caregivers of recently discharged patients have described feeling excluded from interactions with clinicians both before and after discharge.7,8 In another recent qualitative study, caregivers described uncertainty about their role compared with the HHC role in caring for the patient.9
As of 2016, a majority of states had passed the Caregiver Advise, Record, and Enable (CARE) Act, which requires hospitals to (1) record a family caregiver in the medical record, (2) inform this caregiver about discharge, and (3) deliver instructions with education about medical tasks that they will need to complete after discharge.10
METHODS
Study Design
In this qualitative descriptive case study, we interviewed HHC patients, an involved caregiver, and the HHC clinician completing the first HHC visit within 7-14 days following hospital discharge. We chose this timeframe to allow patients to receive one or more HHC visits following hospital discharge.
Population
- >65 years old,12 eligibility was initially limited to patients
- >65 years old. Due to recruitment challenges, the age range was broadened to
- >50 years old in October 2017.
Because our goal was to better understand the experience of general medicine patients with multiple comorbidities, we recruited patients from one general medicine unit at an academic hospital in Colorado. Patients on this unit were screened for eligibility Monday-Friday (excluding weekends and holidays) based on research assistant availability.
Criteria included are as follows: HHC referral, three or more comorbidities, resides in the community prior to admission (ie, not in a facility), cognitively intact, English speaking, and able to identify a caregiver participating in their care. Eligible patients were approached for written consent prior to discharge to allow us to contact them 7-14 days after discharge for an interview by phone or in their home, per their preference. At the time of consent, patients provided contact information for their informal caregiver. Caregiver eligibility criteria included the following: age ≥18 years and provides caregiving at least one hour a week before hospital discharge. HHC clinicians approached for interviews had completed the first HHC visit for the patient following discharge. Both caregivers and HHC clinicians provided verbal consent for interviews. All participants received a $25 gift card for participation in the study.
Framework and Data Collection
Our interview guides were organized by the Agency for Healthcare Research and Quality Care Coordination Framework, an approach we have taken in prior work.4,5,13 We added questions about patient preparation and self-management support to build on findings from a prior study with HHC nurses and on prior work by Coleman and colleagues.5,14 Sample questions from the interview guides for patients, caregivers, and HHC clinicians within key analysis domains are included in Appendix 1. The patient and caregiver interviews were completed by an individual with prior experience in social work and healthcare (SS). The HHC clinician interviews were completed by either this individual (SS) or a physician-researcher with experience in qualitative methods (CJ). Patients and caregivers could choose to be interviewed individually or together. All interviews were digitally recorded and transcribed verbatim.
Analysis
Patient interviews lasted an average of 43 minutes, caregiver interviews an average of 41 minutes, and HHC clinician interviews an average of 25 minutes
We observed the two main themes of clear and unclear expectations for HHC after discharge. Clear expectations occur when the patient and/or caregiver have expectations for HHC that align with the services they receive. Unclear expectations occur when the patient and/or caregiver expectations are either uncertain or misaligned with the services they receive. Although not all interviews yielded codes about clear or unclear expectations, patients described clear expectations in five cases and unclear expectations in another five cases.
In nine cases with more than one perspective available, expectations were compared within cases and found to be clear (three cases), unclear (three cases), or discordant (three cases) across perspectives. For the discordant cases, the description of clear and unclear expectations differed between patients and either their caregiver or their HHC clinician. Patients and caregivers with clear expectations for HHC frequently described prior experiences with skilled HHC or work experience within the healthcare field. In most cases with unclear expectations, the patient and caregiver did not have prior experience with HHC. In addition, the desire for assistance with personal care for patients such as showering and housekeeping was described by caregivers with unclear expectations. The results are organized into clear, unclear, and discordant expectations from the perspectives of patients, caregivers, and HHC clinicians within cases.
Clear Expectations within Cases
Clear expectations for HHC were identified across perspectives in three cases, with sample quotes provided in Table 2. In the case of patient 1, the patient and HHC nurse had known each other for over two years because the patient had a wound requiring long-term HHC services. A caregiver did not complete an interview in this case. With patient 2, the patient, caregiver, and HHC physical therapist (PT) all describe that the patient had clear expectations for HHC. In this case, the patient and caregiver describe feeling prepared because of previously receiving HHC, prior work experience in the healthcare field, and a caregiver with experience working in HHC. In the case of patient 3, the patient had previously received HHC from the same HHC nurse.
Unclear Expectations across Cases
For the three cases in which unclear expectations were described across perspectives, two of the patients described being new to HHC, with representative quotes in Table 2. Patient 4 and her caregiver are new to HHC and describe unclear expectations for both the HHC referral and the HHC role, which was also noted by the HHC clinician. Of note, the caregiver for patient 4 further described that she was unable to be present for the first HHC visit. In the case of patient 5, although the patient had previously received HHC, the patient describes not knowing why the HHC PT needs to see her after discharge, which is also noted by the HHC PT. Finally, both patient 6 and her HHC PT describe that the patient was not sure about their expectations for HHC and that HHC was a new experience for them.
Discordant Expectation Clarity across Cases
In three of the cases, the description of clear and unclear expectations was discrepant across roles. In case 7, the caregiver and patient are new to HHC and express different perspectives about expectations for HHC. The HHC clinician, in this case, did not complete an interview. The caregiver describes not being present for the first HHC visit and no awareness that the patient was being discharged with HHC:
Caregiver: Well, we didn’t even know she had home health until she got home.
The same caregiver also expresses unclear expectations for HHC:
Caregiver: It’s pretty cloudy. They (the HHC clinicians) don’t help her with her laundry, they don’t help with the housekeeping, they don’t help… with her showers so somebody is there when she showers. They don’t do anything. The only two things like I said is the…home healthcare comes in on Wednesdays to see what she needs and then the therapy comes in one day a week.
However, the patient expresses more clear expectations that are being met by HHC.
Patient: They (HHC) have met my expectations. They come in twice a week. They do vitals, take vitals and discuss with me, you know, what my feelings are, how I’m doing and I know they have met my expectations.
In case 8, although the patient describes knowing about the HHC PT involvement in her care, she expresses some unclear expectations about an HHC nurse after discharge.
Patient: As far as home health, I didn’t have a real …plan there at the hospital… They knew about (the HHC PT) coming once a week but as far as, you know, a nurse coming by to check on me, no.
However, the HHC PT describes feeling that the patient had clear expectations for HHC after discharge:
Interviewer: Can you reflect on whether she was prepared to receive home healthcare?
HHC PT: Yeah, she was ready.
Interviewer: …do you feel like she was prepared to know what to expect from you?
HHC PT: Yeah, but I think that comes from being a previous patient also.
Finally, in case 9, the patient describes clear expectations for HHC even though they were new to HHC:
Patient: …I knew what the PT was going to do and …I still need her because I’ve lost so much weight so she’s been really good, instrumental, at giving me exercises… Occupational therapist…she’s going to teach me how to shave, she’s going to teach me how to get ready for the day.
The HHC PT describes that although the patient knew the PT role, they reflect that the patient may have been somewhat unclear about expectations for the first HHC visit:
HHC PT: He knew all that it entailed with the exception of he didn’t really know what the first day was going to be like and the first day I don’t usually do treatment because it does take a long time to get all the paperwork signed, to do the evaluation and the fact that it takes two hours to do that note.
DISCUSSION
In this qualitative case study with HHC patients, caregivers, and clinicians, the participants described varying levels of expectation clarity for HHC after discharge. We triangulated across and within cases and found three cases with clear expectations and three cases with unclear expectations for HHC across perspectives. In three additional cases, we found discordant expectations across perspectives: patients and HHC clinician expectations differed in two of the cases and a patient and caregiver differed in one case. Of interest, in all three cases of clear expectations across perspectives, the patients and/or caregivers had prior HHC or healthcare work experience. In contrast,
Prior studies in this area have included a qualitative study HHC patients, caregivers, and clinicians by Foust and colleagues in which multiple caregivers described finding out about the discharge from the patient or other caregivers, rather than being actively engaged by clinicians.6 In another recent qualitative study by Arbaje and colleagues, a majority of caregivers described “mismatched expectations” about HHC services, in which caregivers
When caregivers have unclear expectations for HHC, they could be expressing the need for more support after hospital discharge, which suggests an active role for hospital teams to assess and address additional support needs with the patients and caregivers. For example, if the patient or caregiver request additional personal care services, a home health aide could help to reduce caregiver burden and improve the support network for the patient. In a prior study in which patients were asked what would help them to make informed decisions about postacute care options, the patients described wanting to receive practical information that could describe how it would apply to their specific situation and perceived needs.18 To provide this for patients and caregivers, it would follow that hospitals could provide information about skilled HHC nursing and therapies and information about services that could meet additional needs, such as home health aides.
Limitations of this study include that it was a small qualitative case study of patients, caregivers, and HHC clinicians from one medical unit at one academic medical center. Most patients in this study had Medicare insurance, were 65 years and older, white, and female.
In conclusion, to improve care transitions for HHC patients and their caregivers, emphasizing engagement of caregivers is key to ensure that they are educated about HHC, provided with additional support as needed, and included in initial HHC visits once the patients are at home. Even though patients and caregivers with prior HHC experience often had clear expectations for HHC, a strategy to uniformly engage caregivers across a range of experience can ensure caregivers have all the information and support needed to optimize care transitions to HHC.
Disclosures
The authors have nothing to disclose.
Funding
Dr. Christine Jones is supported by grant number K08HS024569 from the Agency for Healthcare Research and Quality. Jason Falvey was supported by grant F31AG056069 from the National Institute on Aging, National Institutes of Health and is currently supported by T32AG019134. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality or the National Institutes of Health.
Patients who are discharged from the hospital with home healthcare (HHC) are older, sicker, and more likely to be readmitted to the hospital than patients discharged home without HHC.1-3 Communication between clinicians in different settings is a key factor in successful transitions. In prior work, we focused on communication between primary care providers, hospitalists, and HHC nurses to inform efforts to improve care transitions.4,5 In one study, HHC nurses described that patients frequently have expectations beyond the scope of what skilled HHC provides,5 which prompted us to also question experiences of patients and caregivers after discharge with skilled HHC (eg, nursing and physical therapy).
In a prior qualitative study by Foust and colleagues, HHC patients and caregivers described disparate experiences around preparation for hospital discharge—patients expressed knowing about the timing and plans for discharge, and the caregivers frequently felt left out of this discussion.6 In other studies, caregivers of recently discharged patients have described feeling excluded from interactions with clinicians both before and after discharge.7,8 In another recent qualitative study, caregivers described uncertainty about their role compared with the HHC role in caring for the patient.9
As of 2016, a majority of states had passed the Caregiver Advise, Record, and Enable (CARE) Act, which requires hospitals to (1) record a family caregiver in the medical record, (2) inform this caregiver about discharge, and (3) deliver instructions with education about medical tasks that they will need to complete after discharge.10
METHODS
Study Design
In this qualitative descriptive case study, we interviewed HHC patients, an involved caregiver, and the HHC clinician completing the first HHC visit within 7-14 days following hospital discharge. We chose this timeframe to allow patients to receive one or more HHC visits following hospital discharge.
Population
- >65 years old,12 eligibility was initially limited to patients
- >65 years old. Due to recruitment challenges, the age range was broadened to
- >50 years old in October 2017.
Because our goal was to better understand the experience of general medicine patients with multiple comorbidities, we recruited patients from one general medicine unit at an academic hospital in Colorado. Patients on this unit were screened for eligibility Monday-Friday (excluding weekends and holidays) based on research assistant availability.
Criteria included are as follows: HHC referral, three or more comorbidities, resides in the community prior to admission (ie, not in a facility), cognitively intact, English speaking, and able to identify a caregiver participating in their care. Eligible patients were approached for written consent prior to discharge to allow us to contact them 7-14 days after discharge for an interview by phone or in their home, per their preference. At the time of consent, patients provided contact information for their informal caregiver. Caregiver eligibility criteria included the following: age ≥18 years and provides caregiving at least one hour a week before hospital discharge. HHC clinicians approached for interviews had completed the first HHC visit for the patient following discharge. Both caregivers and HHC clinicians provided verbal consent for interviews. All participants received a $25 gift card for participation in the study.
Framework and Data Collection
Our interview guides were organized by the Agency for Healthcare Research and Quality Care Coordination Framework, an approach we have taken in prior work.4,5,13 We added questions about patient preparation and self-management support to build on findings from a prior study with HHC nurses and on prior work by Coleman and colleagues.5,14 Sample questions from the interview guides for patients, caregivers, and HHC clinicians within key analysis domains are included in Appendix 1. The patient and caregiver interviews were completed by an individual with prior experience in social work and healthcare (SS). The HHC clinician interviews were completed by either this individual (SS) or a physician-researcher with experience in qualitative methods (CJ). Patients and caregivers could choose to be interviewed individually or together. All interviews were digitally recorded and transcribed verbatim.
Analysis
Patient interviews lasted an average of 43 minutes, caregiver interviews an average of 41 minutes, and HHC clinician interviews an average of 25 minutes
We observed the two main themes of clear and unclear expectations for HHC after discharge. Clear expectations occur when the patient and/or caregiver have expectations for HHC that align with the services they receive. Unclear expectations occur when the patient and/or caregiver expectations are either uncertain or misaligned with the services they receive. Although not all interviews yielded codes about clear or unclear expectations, patients described clear expectations in five cases and unclear expectations in another five cases.
In nine cases with more than one perspective available, expectations were compared within cases and found to be clear (three cases), unclear (three cases), or discordant (three cases) across perspectives. For the discordant cases, the description of clear and unclear expectations differed between patients and either their caregiver or their HHC clinician. Patients and caregivers with clear expectations for HHC frequently described prior experiences with skilled HHC or work experience within the healthcare field. In most cases with unclear expectations, the patient and caregiver did not have prior experience with HHC. In addition, the desire for assistance with personal care for patients such as showering and housekeeping was described by caregivers with unclear expectations. The results are organized into clear, unclear, and discordant expectations from the perspectives of patients, caregivers, and HHC clinicians within cases.
Clear Expectations within Cases
Clear expectations for HHC were identified across perspectives in three cases, with sample quotes provided in Table 2. In the case of patient 1, the patient and HHC nurse had known each other for over two years because the patient had a wound requiring long-term HHC services. A caregiver did not complete an interview in this case. With patient 2, the patient, caregiver, and HHC physical therapist (PT) all describe that the patient had clear expectations for HHC. In this case, the patient and caregiver describe feeling prepared because of previously receiving HHC, prior work experience in the healthcare field, and a caregiver with experience working in HHC. In the case of patient 3, the patient had previously received HHC from the same HHC nurse.
Unclear Expectations across Cases
For the three cases in which unclear expectations were described across perspectives, two of the patients described being new to HHC, with representative quotes in Table 2. Patient 4 and her caregiver are new to HHC and describe unclear expectations for both the HHC referral and the HHC role, which was also noted by the HHC clinician. Of note, the caregiver for patient 4 further described that she was unable to be present for the first HHC visit. In the case of patient 5, although the patient had previously received HHC, the patient describes not knowing why the HHC PT needs to see her after discharge, which is also noted by the HHC PT. Finally, both patient 6 and her HHC PT describe that the patient was not sure about their expectations for HHC and that HHC was a new experience for them.
Discordant Expectation Clarity across Cases
In three of the cases, the description of clear and unclear expectations was discrepant across roles. In case 7, the caregiver and patient are new to HHC and express different perspectives about expectations for HHC. The HHC clinician, in this case, did not complete an interview. The caregiver describes not being present for the first HHC visit and no awareness that the patient was being discharged with HHC:
Caregiver: Well, we didn’t even know she had home health until she got home.
The same caregiver also expresses unclear expectations for HHC:
Caregiver: It’s pretty cloudy. They (the HHC clinicians) don’t help her with her laundry, they don’t help with the housekeeping, they don’t help… with her showers so somebody is there when she showers. They don’t do anything. The only two things like I said is the…home healthcare comes in on Wednesdays to see what she needs and then the therapy comes in one day a week.
However, the patient expresses more clear expectations that are being met by HHC.
Patient: They (HHC) have met my expectations. They come in twice a week. They do vitals, take vitals and discuss with me, you know, what my feelings are, how I’m doing and I know they have met my expectations.
In case 8, although the patient describes knowing about the HHC PT involvement in her care, she expresses some unclear expectations about an HHC nurse after discharge.
Patient: As far as home health, I didn’t have a real …plan there at the hospital… They knew about (the HHC PT) coming once a week but as far as, you know, a nurse coming by to check on me, no.
However, the HHC PT describes feeling that the patient had clear expectations for HHC after discharge:
Interviewer: Can you reflect on whether she was prepared to receive home healthcare?
HHC PT: Yeah, she was ready.
Interviewer: …do you feel like she was prepared to know what to expect from you?
HHC PT: Yeah, but I think that comes from being a previous patient also.
Finally, in case 9, the patient describes clear expectations for HHC even though they were new to HHC:
Patient: …I knew what the PT was going to do and …I still need her because I’ve lost so much weight so she’s been really good, instrumental, at giving me exercises… Occupational therapist…she’s going to teach me how to shave, she’s going to teach me how to get ready for the day.
The HHC PT describes that although the patient knew the PT role, they reflect that the patient may have been somewhat unclear about expectations for the first HHC visit:
HHC PT: He knew all that it entailed with the exception of he didn’t really know what the first day was going to be like and the first day I don’t usually do treatment because it does take a long time to get all the paperwork signed, to do the evaluation and the fact that it takes two hours to do that note.
DISCUSSION
In this qualitative case study with HHC patients, caregivers, and clinicians, the participants described varying levels of expectation clarity for HHC after discharge. We triangulated across and within cases and found three cases with clear expectations and three cases with unclear expectations for HHC across perspectives. In three additional cases, we found discordant expectations across perspectives: patients and HHC clinician expectations differed in two of the cases and a patient and caregiver differed in one case. Of interest, in all three cases of clear expectations across perspectives, the patients and/or caregivers had prior HHC or healthcare work experience. In contrast,
Prior studies in this area have included a qualitative study HHC patients, caregivers, and clinicians by Foust and colleagues in which multiple caregivers described finding out about the discharge from the patient or other caregivers, rather than being actively engaged by clinicians.6 In another recent qualitative study by Arbaje and colleagues, a majority of caregivers described “mismatched expectations” about HHC services, in which caregivers
When caregivers have unclear expectations for HHC, they could be expressing the need for more support after hospital discharge, which suggests an active role for hospital teams to assess and address additional support needs with the patients and caregivers. For example, if the patient or caregiver request additional personal care services, a home health aide could help to reduce caregiver burden and improve the support network for the patient. In a prior study in which patients were asked what would help them to make informed decisions about postacute care options, the patients described wanting to receive practical information that could describe how it would apply to their specific situation and perceived needs.18 To provide this for patients and caregivers, it would follow that hospitals could provide information about skilled HHC nursing and therapies and information about services that could meet additional needs, such as home health aides.
Limitations of this study include that it was a small qualitative case study of patients, caregivers, and HHC clinicians from one medical unit at one academic medical center. Most patients in this study had Medicare insurance, were 65 years and older, white, and female.
In conclusion, to improve care transitions for HHC patients and their caregivers, emphasizing engagement of caregivers is key to ensure that they are educated about HHC, provided with additional support as needed, and included in initial HHC visits once the patients are at home. Even though patients and caregivers with prior HHC experience often had clear expectations for HHC, a strategy to uniformly engage caregivers across a range of experience can ensure caregivers have all the information and support needed to optimize care transitions to HHC.
Disclosures
The authors have nothing to disclose.
Funding
Dr. Christine Jones is supported by grant number K08HS024569 from the Agency for Healthcare Research and Quality. Jason Falvey was supported by grant F31AG056069 from the National Institute on Aging, National Institutes of Health and is currently supported by T32AG019134. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality or the National Institutes of Health.
1. Jones CD, Wald HL, Boxer RS, et al. Characteristics associated with home health care referrals at hospital discharge: results from the 2012 National Inpatient Sample. Health Serv Res. 2017;52(2):879-894. doi: 10.1111/1475-6773. PubMed
2. Avalere Health. Home Health Chartbook 2015: Prepared for the Alliance for Home Health Quality and Innovation. 2016.
3. Hospital Compare. https://www.medicare.gov/hospitalcompare/search.html. Accessed May 1, 2017.
4. Jones CD, Vu MB, O’Donnell CM, et al. A failure to communicate: a qualitative exploration of care coordination between hospitalists and primary care providers around patient hospitalizations. J Gen Intern Med. 2015;30(4):417-424. doi: 10.1007/s11606-014-3056-x. PubMed
5. Jones CD, Jones J, Richard A, et al. “Connecting the dots”: a qualitative study of home health nurse perspectives on coordinating care for recently discharged patients. J Gen Intern Med. 2017;32(10):1114-1121. doi: 10.1007/s11606-017-4104-0. PubMed
6. Foust JB, Vuckovic N, Henriquez E. Hospital to home health care transition: patient, caregiver, and clinician perspectives. West J Nurs Res. 2012;34(2):194-212. doi: 10.1177/0193945911400448. PubMed
7. Blair J, Volpe M, Aggarwal B. Challenges, needs, and experiences of recently hospitalized cardiac patients and their informal caregivers. J Cardiovasc Nurs. 2014;29(1):29-37. doi: 10.1097/JCN.0b013e3182784123. PubMed
8. Coleman EA, Roman SP. Family caregivers’ experiences during transitions out of hospital. J Healthc Qual. 2015;37(1):12-21. doi: 10.1097/01.JHQ.0000460117.83437.b3. PubMed
9. Arbaje AI, Hughes A, Werner N, et al. Information management goals and process failures during home visits for middle-aged and older adults receiving skilled home healthcare services after hospital discharge: a multisite, qualitative study. BMJ Qual Saf. 2018. doi: 10.1136/bmjqs-2018-008163. PubMed
10. Coleman EA. Family caregivers as partners in care transitions: the caregiver advise record and enable act. J Hosp Med. 2016;11(12):883-885. doi: 10.1002/jhm.2637. PubMed
11. Jones AL, Harris-Kojetin L, Valverde R. Characteristics and use of home health care by men and women aged 65 and over. Natl Health Stat Report. 2012(52):1-7. PubMed
12. Tian W. An all-payer view of hospital discharge to postacute care, 2013. HCUP Statistical Brief #205. Rockville, Maryland; 2016. PubMed
13. McDonald KM, Sundaram V, Bravata DM, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies (Vol. 7: Care Coordination). Rockville, Maryland; 2007. PubMed
14. Coleman EA, Smith JD, Frank JC, Eilertsen TB, Thiare JN, Kramer AM. Development and testing of a measure designed to assess the quality of care transitions. Int J Integr Care. 2002;2:e02. doi: 10.5334/ijic.60. PubMed
15. Jones J, Nowels CT, Sudore R, Ahluwalia S, Bekelman DB. The future as a series of transitions: qualitative study of heart failure patients and their informal caregivers. J Gen Intern Med. 2015;30(2):176-182. doi: 10.1007/s11606-014-3085-5. PubMed
16. Lum HD, Jones J, Lahoff D, et al. Unique challenges of hospice for patients with heart failure: a qualitative study of hospice clinicians. Am Heart J. 2015;170(3):524-530 e523. doi: 10.1016/j.ahj.2015.06.019. PubMed
17. Kerr C, Nixon A, Wild D. Assessing and demonstrating data saturation in qualitative inquiry supporting patient-reported outcomes research. Expert Rev Pharmacoecon Outcomes Res. 2010;10(3):269-281. doi: 10.1586/erp.10.30. PubMed
18. Sefcik JS, Nock RH, Flores EJ, et al. Patient preferences for information on post-acute care services. Res Gerontol Nurs. 2016;9(4):175-182. doi: 10.3928/19404921-20160120-01. PubMed
19. Coleman EA, Roman SP, Hall KA, Min SJ. Enhancing the care transitions intervention protocol to better address the needs of family caregivers. J Healthc Qual. 2015;37(1):2-11. doi: 10.1097/01.JHQ.0000460118.60567.fe. PubMed
1. Jones CD, Wald HL, Boxer RS, et al. Characteristics associated with home health care referrals at hospital discharge: results from the 2012 National Inpatient Sample. Health Serv Res. 2017;52(2):879-894. doi: 10.1111/1475-6773. PubMed
2. Avalere Health. Home Health Chartbook 2015: Prepared for the Alliance for Home Health Quality and Innovation. 2016.
3. Hospital Compare. https://www.medicare.gov/hospitalcompare/search.html. Accessed May 1, 2017.
4. Jones CD, Vu MB, O’Donnell CM, et al. A failure to communicate: a qualitative exploration of care coordination between hospitalists and primary care providers around patient hospitalizations. J Gen Intern Med. 2015;30(4):417-424. doi: 10.1007/s11606-014-3056-x. PubMed
5. Jones CD, Jones J, Richard A, et al. “Connecting the dots”: a qualitative study of home health nurse perspectives on coordinating care for recently discharged patients. J Gen Intern Med. 2017;32(10):1114-1121. doi: 10.1007/s11606-017-4104-0. PubMed
6. Foust JB, Vuckovic N, Henriquez E. Hospital to home health care transition: patient, caregiver, and clinician perspectives. West J Nurs Res. 2012;34(2):194-212. doi: 10.1177/0193945911400448. PubMed
7. Blair J, Volpe M, Aggarwal B. Challenges, needs, and experiences of recently hospitalized cardiac patients and their informal caregivers. J Cardiovasc Nurs. 2014;29(1):29-37. doi: 10.1097/JCN.0b013e3182784123. PubMed
8. Coleman EA, Roman SP. Family caregivers’ experiences during transitions out of hospital. J Healthc Qual. 2015;37(1):12-21. doi: 10.1097/01.JHQ.0000460117.83437.b3. PubMed
9. Arbaje AI, Hughes A, Werner N, et al. Information management goals and process failures during home visits for middle-aged and older adults receiving skilled home healthcare services after hospital discharge: a multisite, qualitative study. BMJ Qual Saf. 2018. doi: 10.1136/bmjqs-2018-008163. PubMed
10. Coleman EA. Family caregivers as partners in care transitions: the caregiver advise record and enable act. J Hosp Med. 2016;11(12):883-885. doi: 10.1002/jhm.2637. PubMed
11. Jones AL, Harris-Kojetin L, Valverde R. Characteristics and use of home health care by men and women aged 65 and over. Natl Health Stat Report. 2012(52):1-7. PubMed
12. Tian W. An all-payer view of hospital discharge to postacute care, 2013. HCUP Statistical Brief #205. Rockville, Maryland; 2016. PubMed
13. McDonald KM, Sundaram V, Bravata DM, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies (Vol. 7: Care Coordination). Rockville, Maryland; 2007. PubMed
14. Coleman EA, Smith JD, Frank JC, Eilertsen TB, Thiare JN, Kramer AM. Development and testing of a measure designed to assess the quality of care transitions. Int J Integr Care. 2002;2:e02. doi: 10.5334/ijic.60. PubMed
15. Jones J, Nowels CT, Sudore R, Ahluwalia S, Bekelman DB. The future as a series of transitions: qualitative study of heart failure patients and their informal caregivers. J Gen Intern Med. 2015;30(2):176-182. doi: 10.1007/s11606-014-3085-5. PubMed
16. Lum HD, Jones J, Lahoff D, et al. Unique challenges of hospice for patients with heart failure: a qualitative study of hospice clinicians. Am Heart J. 2015;170(3):524-530 e523. doi: 10.1016/j.ahj.2015.06.019. PubMed
17. Kerr C, Nixon A, Wild D. Assessing and demonstrating data saturation in qualitative inquiry supporting patient-reported outcomes research. Expert Rev Pharmacoecon Outcomes Res. 2010;10(3):269-281. doi: 10.1586/erp.10.30. PubMed
18. Sefcik JS, Nock RH, Flores EJ, et al. Patient preferences for information on post-acute care services. Res Gerontol Nurs. 2016;9(4):175-182. doi: 10.3928/19404921-20160120-01. PubMed
19. Coleman EA, Roman SP, Hall KA, Min SJ. Enhancing the care transitions intervention protocol to better address the needs of family caregivers. J Healthc Qual. 2015;37(1):2-11. doi: 10.1097/01.JHQ.0000460118.60567.fe. PubMed
© 2019 Society of Hospital Medicine
Deimplementation of Routine Chest X-rays in Adult Intensive Care Units
Despite increased awareness of Choosing Wisely (CW)® recommendations to reduce low-value care,1 there is limited published data about strategies to implement these guidelines or evidence that they have influenced ordering patterns or reduced healthcare spending
In ICUs, CXR ordering strategies may be routine (daily) or on-demand (with clinical indication). The former strategy’s principal advantage is the potential to detect life-threatening situations that may otherwise escape diagnosis.11 Disadvantages include cost, radiation exposure, patient inconvenience, false-positive workups, and low diagnostic and therapeutic value.12,13 On-demand strategies may safely reduce CXR ordering by 32% to 45%.11-17 Based on this evidence, the Critical Care Societies Collaborative and the American College of Radiology have recommended on-demand CXR ordering.18,19 Here, we describe the effectiveness of an intervention to reduce CXR ordering in two ICUs while evaluating the deimplementation strategies using a validated framework.
METHODS
Setting and Design
Vanderbilt University Medical Center (VUMC) is an academic referral center in Nashville, Tennessee. The cardiovascular ICU (CVICU) has 27 beds and the medical ICU (MICU) has 34 beds. Acute care nurse practitioners (ACNPs) and two critical care physicians staff the CVICU; cardiology fellows, anesthesia critical care fellows, and transplant and cardiac surgeons are also active in patient care. The MICU is staffed by two critical care physicians who supervise one team of ACNPs and two teams of medical residents who rotate through the unit every two weeks. Each MICU team is assigned a fellow in pulmonary and critical care.
We conducted a prospective, nonrandomized study in these units from October 2015 to June 2016. The VUMC Institutional Review Board approved the intervention as a quality improvement (QI) activity, waiving the requirement for informed consent.
Intervention
Following the top CW recommendation of the Critical Care Societies Collaborative—“Don’t order diagnostic tests at regular intervals (such as every day), but rather in response to specific clinical questions.”19—the VUMC resident-led CW Steering Committee designed a multifaceted approach to reduce ordering of routine CXRs in ICUs. The intervention included a didactic session on CW and proper CXR ordering practices, peer champions, data audits, and feedback to providers through weekly e-mails (see Supplemental Materials, 1 – Resident Presentation and 2 – CXR Flyer). 20
In September 2015, CVICU and MICU teams received a didactic session highlighting CW, current CXR ordering rates, and the plan for reducing CXR ordering. On October 5, 2015, teams began receiving weekly e-mails with ordering rates defined as CXRs ordered per patient per day and a brief rationale for reducing unnecessary CXRs. To encourage friendly competition, we provided weekly rates to the MICU teams, allowing for transparent benchmarking against one another. A similar competition strategy was not used in the CVICU due to the lack of multiple teams.
In the CVICU, two ACNPs volunteered as peer champions. These champions coordinated data feedback and advocated for the intervention among their colleagues. In the MICU, three internal medicine residents volunteered as peer champions and fulfilled similar roles.
To facilitate deimplementation, we conducted two Plan-Do-Study-Act (PDSA) cycles, the first from November to mid-December 2015 and the second from mid-December 2015 to mid-January 2016. During these cycles, we tailored our deimplementation strategy based on barriers identified by the peer champions and ICU leaders (described in the Qualitative Results section). Peer champions and the CW Steering Committee generated potential solutions by conversing with stakeholders and using the Expert Recommendations for Implementing Change (ERIC).20 Interventions included disseminating promotional flyers, holding meetings with stakeholders, and providing monthly CXR ordering rates. After the PDSA cycles, we continued reexamining the deimplementation efforts by reviewing ordering rates and soliciting feedback from ICU leaders and peer champions. However, no significant changes to the intervention were made during this time.
Quantitative Evaluation
We extracted data from VUMC’s Enterprise Data Warehouse during the intervention period (October 5, 2015 to May 24, 2016) and a historical control period (October 1, 2014 to October 4, 2015). Within each ICU, descriptive statistics were used to compare patient cohorts in the baseline and intervention periods by age, sex, and race.
The primary outcome was CXRs ordered per patient per day by hospital unit (CVICU or MICU). The baseline period included all data between October 1, 2014 and September 15, 2015. To account for priming of providers from didactic education, we allowed a washout period from September 16, 2015 to October 4, 2015. As a preliminary analysis, we compared CXR rates in the baseline and intervention periods using Wilcoxon rank-sum tests. We then conducted interrupted time-series analyses with segmented linear regression to assess differences in linear trends in CXR rates over the two periods. To account for different staffing models in the MICU, we stratified the impact of the intervention by team—medical resident (physician) or ACNP. R version 3.4.0 was used for statistical analysis.21
Qualitative Evaluation
Our qualitative evaluation consisted of embedded observation and semistructured interviews with stakeholders. The qualitative portion was guided by the Consolidated Framework for Implementation Research (CFIR), a widely used framework for design and evaluation of improvement initiatives that helped us to determine major facilitators and barriers to implementation.22,23
Embedded Observation
From November 2015 to January 2016, we observed morning rounds in the CVICU and MICU one to two times weekly to understand factors facilitating and inhibiting uptake of the intervention. Observations were recorded and organized using a CFIR-based template and directed toward understanding interactions among team members (eg, the decision-making process hierarchy), team workflows and decision-making processes, process of ordering CXRs, and providers’ knowledge and perceptions of the CXR intervention (see Supplemental Material, 3 – CFIR Table).22,23 After rounds, ICU team members were invited to share suggestions for improving the intervention. All observations occurred during and shortly following morning rounds when the vast majority of routine CXRs are ordered; we did not evaluate night or evening workflows. In the spirit of continuous improvement, we evaluated data in real-time.
Semistructured Interviews
Based on the direct observations, we developed semistructured interview questions to further evaluate provider perspectives (eg, “Do you believe ICU patients need a daily CXR?”) and constructs aligning with CFIR (eg, “intervention source—internally vs externally developed;” see Supplemental Material, 4 – Interview Questions).
Stakeholders from both ICUs were recruited through e-mail and in-person requests to participate in semistructured interviews. In the CVICU, we interviewed critical care physicians, anesthesia critical care fellows, and ACNPs. In the MICU, we interviewed medical students, interns, residents, critical care fellows, attending intensivist physicians, and ACNPs. We also interviewed X-ray technologists who routinely perform portable films in the units.
RESULTS
Quantitative Results
Cardiovascular Intensive Care Unit
The median baseline CXR ordering rate in the CVICU was 1.16 CXRs per patient per day, with interquartile range (IQR) 1.06-1.28. During the intervention period, the rate dropped to 1.07 (IQR 0.94-1.21; P < .001; Table 2). The time-series analysis suggested an essentially flat trend during the baseline period, followed by a small but significant drop in ordering rates during the intervention period (P < .001; Table 3 and Figure 1). Ordering rates appeared to increase slightly over the course of the intervention period, but this slight upward trend was not significantly different from the flat trend seen during the baseline period.
Medical Intensive Care Unit
For both physician and ACPN teams, the median baseline CXR ordering rates in the MICU were much lower than the baseline rate in the CVICU (Table 2). For the MICU physician care team, the baseline CXR ordering rate was 0.60 CXRs per patient per day (IQR 0.48-0.73). For the ACNP team, the median rate was 0.39 CXRs per patient per day (IQR 0.21-0.57). Both rates stayed approximately the same during the intervention period (Table 2). The time-series analysis suggested a statistically significant but very slight downward trend in CXR ordering rates during the baseline period, in the physician (P = .011) and ACNP (P = .022) teams (Table 3, Figure 2). Under this model, a small increase in CXR ordering initially occurred during the intervention period for both physician and ACNP teams (P = .010 and P = .055, respectively), after which the rates declined slightly. Trends in ordering rates during the intervention period were not significantly different from the slight downward trends seen during the baseline period.
Qualitative Results
We identified 25 of 39 CFIR constructs as relevant to the initiative (see Supplemental Materials, 3 – CFIR Table.) We determined the major facilitators of deimplementation to be peer champion discussions about CXR ordering on rounds and weekly data feedback, particularly if accompanied by in-person follow-up.
We also noted differences in CXR ordering rationales and decisions between the units. Generally, residents in the MICU and ACNPs in the CVICU made decisions to order CXRs. However, decisions were influenced by the expectations of attending physicians. While CVICU providers tended to order CXRs reflexively as part of morning labs, MICU providers—in particular, ACNPs who had been trained on indications for proper CXR ordering—generally ordered CXRs for specific indications (eg, worsening respiratory status). Of note, MICU ACNPs reported the use of bedside ultrasound as an alternate imaging modality and a reason for their higher threshold to order CXRs.
Deimplementation barriers in both units included the need to identify goal CXR ordering rates and the intervention’s limited visibility. To address these barriers, we conducted PDSA cycles and used the CFIR and ERIC to generate potential solutions.24 We established a goal of a 20% absolute reduction in the CVICU, added monthly CXR rates to weekly e-mail reports to better account for variations in patient populations and ordering practices, and circulated materials to promote on-demand CXR ordering. Promotional materials contained guidelines on CXR ordering and five “Frequently Held Misconceptions” about ordering practices with succinct, evidence-based explanations (see Supplemental Material, 2 – CXR Flyer).
Approximately four months after the start of intervention, some CVICU physicians became concerned that on-demand CXR ordering might be inappropriate for high-risk surgical patients, including those who are undergoing or have undergone heart transplants, lung transplants, or left-ventricular assist device placement. This concern arose following two adverse outcomes, which were not attributed to the CXR initiative, but which heightened concerns about patient safety. A rise in CXR ordering then occurred, and CVICU providers requested that we perform an analysis of these high-risk groups. While segmented linear regression in this subgroup suggested that average daily CXR ordering rates did decrease among the high-risk group at the start of the intervention period (P = .001), the difference between the rates in the two periods was not significant using the Wilcoxon rank-sum test. Exclusion of these patients from the main analysis did not alter the interpretation of the findings reported above for the CVICU.
DISCUSSION
A deimplementation intervention using provider education, peer champions, and data feedback was associated with fewer CXRs in the CVICU (P < .001) but not in the MICU. The CFIR-guided qualitative analysis was valuable for evaluating our deimplementation strategy and for identifying differences between the two ICUs.
Relatively few studies have demonstrated effective interventions that address CW recommendations.25-28 However, three population-level analyses of insurance claims show mixed results.3,4,29 Experts have thus proposed using implementation science to improve uptake of CW recommendations.2,3,7,8 Our study demonstrates the effectiveness of this approach. As expected, providers largely endorsed an on-demand CXR ordering strategy. Using the CFIR, however, we discovered barriers (eg, concern that data feedback did not reflect variations in patients’ needs). Using methods from implementation science allowed us to diagnose and tailor our approaches.
Our qualitative evaluation suggested that the intervention was ineffective mostly due to CFIR’s “inner setting” constructs, including resident fatigue, competing priorities, and decreased investment in QI projects because of the rotating nature of providers in training. Baseline CXR ordering rates in the MICU were also considerably lower than in the CVICU. We observed that CVICU providers ordered many CXRs following the placement of lines or tubes and that ACNPs in the MICU had received education on appropriate CXR ordering practices and had access to an alternate imaging modality in ultrasound. These factors may partially explain the difference in baseline rates.
As noted in a study of cardiac stress testing guidelines, the existence of high-value care recommendations does not mean overuse.30 Indeed, the lack of significant CXR over-ordering in the MICU highlights the importance of baseline measurement and partnering with information technology departments to create the best possible data feedback systems.30-32 Our experience shows that these systems should provide sufficient pre-implementation data (ideally >1 year), such that teams selecting QI projects can ensure that a project is a good use of institutional resources and change capital.
To inform future work, we informally assessed program costs and savings. We estimate the initiative cost $1,600, including $1,000 for curriculum development and teaching time, $300 for educational materials, and $300 for CXR tracking dashboard development. Hospital charges and reimbursements for CXR vary widely.33 We calculated savings using a range of rates, from a conservative $23 (the Medicare reimbursement rate for single-view CXR, CPT code 71010, global fee) to $50 (an approximate blended reimbursement rate across payers).34,35 In the CVICU, we estimate that 51 CXRs were avoided each month, saving $1,173-$2,550 per month or $9,384-$20,400 over eight months of follow-up. Annualizing these figures, we estimate net savings of $12,476-$29,000 in the first year in a 27-bed ICU. Costs to continue the program include education of new employees, booster training, and dashboard maintenance for an estimated annual cost of $1,000. It is difficult to estimate effectiveness over time, but if we conservatively assume that 30 CXRs were avoided each month, then the projected savings would be $8,280-$18,000 per year or an annual net savings of $7,280-$17,000 in the ICU. Although these amounts are modest, providing trainees with experiential learning opportunities in high-value care is valuable in its own right, meets curricular goals, may result in spill-over effects to other diagnostic and therapeutic decisions, and may influence long-term practice patterns. Institutional decisions to pursue projects such as this should take into account these potential benefits.
This evaluation is not without limitations. First, the study was conducted in a single tertiary-care hospital, potentially limiting its generalizability.36 Second, the study design lacked a concurrent control group, and observed outcomes may have been influenced by broader CXR utilization trends, increased awareness of low-value care generally or from previous CW projects at VUMC, seasonal effects, or the Hawthorne effect. Third, the study outcome was all CXRs ordered, rather than CXRs that were unnecessary or not clinically indicated. We chose all CXRs because it was more pragmatic, did not require clinical case review, and could be incorporated promptly into dashboards, enabling timely performance feedback. Other performance measures have taken a similar tack (eg, tracking all-cause readmissions rather than preventable readmissions). Given this approach, we did not track clinical indications for CXRs (eg, central line placement). Fourth, although we compared resident and APRN orders, we did not collect data on other provider characteristics such as years in/out of training or board certification status. These considerations should be addressed in future research.
Finally, the increase in CVICU CXR ordering at the end of the intervention period, which occurred following two adverse events, raises concerns about sustainability. While unrelated to CXR orders, the events resulted in increased ordering of diagnostic tests and showed the difficulty of deimplementation in ICUs. Indeed, some CVICU providers argued that on-demand CXR ordering represented minimal potential cost savings and had not been studied among heart and lung transplant patients. Subsequently, Tonna et al. have shown that on-demand CXR ordering can be safely implemented among such patients.37 Also similar to our study, Tonna et al. observed an initial decrease in CXR ordering, followed by a gradual increase toward baseline ordering rates. These findings highlight the need for sustained awareness and interventions and for the careful selection of high-value projects.
In conclusion, our study shows that a deimplementation intervention based on CW recommendations can reduce CXR ordering and that ongoing evaluation of contextual factors provides insights for both real-time modifications of current interventions and the design of future interventions. We found that messaging about reducing unnecessary tests works well when discussions are framed at the unit level but may be counterproductive if used to question individual ordering decisions.38 Additional lessons learned include the value of participation on rounds to build trust among stakeholders, the utility of monthly rather than weekly statistics for feedback, stakeholder input and peer champions, and differences in approach with physician and ACNP audiences.
Acknowledgments
The authors thank the VUMC Choosing Wisely committee; Mr. Bill Harrell in Advanced Data Analytics for developing the Tableau platform used in our data feedback strategy; Emily Feld, MD, Jerry Zifodya, MD, and Ryan Kindle, MD for assisting with data feedback to providers in the MICU; Todd Rice, MD, Director of the MICU, for his support of the initiative; and Beth Prusaczyk, PhD, MSW and David Stevenson, PhD for providing feedback on earlier drafts of this manuscript.
Disclosures
Dr. Kripalani reports personal fees from Verustat, personal fees from SAI Interactive, and equity from Bioscape Digital, outside the submitted work. All other authors have nothing to disclose.
Funding
This work was supported by an Innovation Grant from the Alliance for Academic Internal Medicine (AAIM, 2016) and by the Departments of Internal Medicine and Graduate Medical Education at Vanderbilt University Medical Center. The AAIM did not have a role in the study design, data collection, data analysis, data interpretation, or manuscript writing.
1. Cassel CK, Guest JA. Choosing Wisely: Helping physicians and patients make smart decisions about their care. JAMA. 2012;307(17):1801-1802.
2. Gonzales R, Cattamanchi A. Changing clinician behavior when less is more. JAMA Intern Med. 2015;175(12):1921-1922.
3. Rosenberg A, Agiro A, Gottlieb M, et al. Early trends among seven recommendations from the Choosing Wisely campaign. JAMA Intern Med. 2015;175(12):1913-1920.
4. Hong AS, Ross-Degnan D, Zhang F, Wharam JF. Small decline in low-value back imaging associated with the ‘Choosing Wisely’ campaign, 2012-14. Health Aff (Millwood). 2017;36(4):671-679. doi: 10.1377/hlthaff.2016.1263.
5. Parks AL, O’Malley PG. From choosing wisely to practicing value—more to the story. JAMA Intern Med. 2016;176(10):1571-1572.
6. Johnson PT, Pahwa AK, Feldman LS, Ziegelstein RC, Hellmann DB. Advancing high-value health care: a new AJM column dedicated to cost-conscious care quality improvement. Am J Med. 2017;130(6):619-621. doi: 10.1016/j.amjmed.2016.12.018.
7. Eccles MP, Mittman BS. Welcome to implementation science. Implement Sci. 2006;1:1. doi: 10.1186/1748-5908-1-1.
8. Bhatia RS, Levinson W, Shortt S, et al. Measuring the effect of Choosing Wisely: an integrated framework to assess campaign impact on low-value care. BMJ Qual Saf. 2015;24:523-531. doi: 10.1136/bmjqs-2015-004070.
9. Selby K, Barnes GD. Learning to de-adopt ineffective healthcare practices. Am J Med. 2018;131(7):721-722. doi: 10.1016/j.amjmed.2018.03.014.
10. Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs. Med Care. 2012;50(3):217-226. doi: 10.1097/MLR.0b013e3182408812.
11. Ganapathy A, Adhikari NK, Spiegelman J, Scales DC. Routine chest x-rays in intensive care units: a systematic review and meta-analysis. Crit Care. 2012;16(2):R68. doi: 10.1186/cc11321.
12. Graat ME, Kröner A, Spronk PE, et al. Elimination of daily routine chest radiographs in a mixed medical-surgical intensive care unit. Intensive Care Med. 2007;33(4):639-644. doi: 10.1007/s00134-007-0542-1.
13. Hendrikse KA, Gratama JWC, Ten Hove W, Rommes JH, Schultz MJ, Spronk PE. Low value of routine chest radiographs in a mixed medical-surgical ICU. Chest. 2007;132(3):823-828. doi: 10.1378/chest.07-1162.
14. Clec’h C, Simon P, Hamdi A, et al. Are daily routine chest radiographs useful in critically ill, mechanically ventilated patients? A randomized study. Intensive Care Med. 2008;34(2):264-270. doi: 10.1007/s00134-007-0919-1.
15. Oba Y, Zaza T. Abandoning daily routine chest radiography in the intensive care unit: meta-analysis. Radiology. 2010;255(2):386-395. doi: 10.1148/radiol.10090946.
16. Mets O, Spronk PE, Binnekade J, Stoker J, de Mol BAJM, Schultz MJ. Elimination of daily routine chest radiographs does not change on-demand radiography practice in post-cardiothoracic surgery patients. J Thorac Cardiovasc Surg. 2007;134(1):139-144. doi: 10.1016/j.jtcvs.2007.02.029.
17. Hejblum G, Chalumeau-Lemoine L, Ioos V, et al. Comparison of routine and on-demand prescription of chest radiographs in mechanically ventilated adults: a multicentre, cluster-randomised, two-period crossover study. Lancet. 2009;374(9702):1687-1693. doi: 10.1016/S0140-6736(09)61459-8.
18. McComb BL, Chung JH, Crabtree TD, et al. ACR appropriateness criteria® routine chest radiography. J Thorac Imaging. 2016;31(2):W13-W15. doi: 10.1097/RTI.0000000000000200.
19. Halpern SD, Becker D, Curtis JR, et al. An official American Thoracic Society/American Association of Critical-Care Nurses/American College of Chest Physicians/Society of Critical Care Medicine policy statement: the Choosing Wisely® top 5 list in critical care medicine. Am J Respir Crit Care Med. 2014;190(7):818-826. doi: 10.1164/rccm.201407-1317ST.
20. Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: Results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10:21. doi: 10.1186/s13012-015-0209-1.
21. R [computer program]. Version 3.4.0. Vienna, Austria: R Foundation for Statistical Computing; 2013.
22. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. doi: 10.1186/1748-5908-4-50.
23. Kirk MA, Kelley C, Yankey N, Birken SA, Abadie B, Damschroder L. A systematic review of the use of the Consolidated Framework for Implementation Research. Implement Sci. 2016;11:72. doi: 10.1186/s13012-016-0437-z.
24. Speroff T, James BC, Nelson EC, Headrick LA, Brommels M, Reed JE. Guidelines for appraisal and publication of PDSA quality improvement. Qual Manag Health Care. 2014;13(1):33-39. doi: 10.1097/00019514-200401000-00003.
25. Corson AH, Fan VS, White T, et al. A multifaceted hospitalist quality improvement intervention: Decreased frequency of common labs. J Hosp Med. 2015;10(6):390-395. doi: 10.1002/jhm.2354.
26. Ferrari R. Evaluation of the Canadian Rheumatology Association Choosing Wisely recommendation concerning anti-nuclear antibody (ANA) testing. Clin Rheumatol. 2015;34(9):1551-1556. doi: 10.1007/s10067-015-2985-z.
27. Ferrari R, Prosser C. Testing vitamin D levels and choosing wisely. JAMA Intern Med. 2016;176(7):1019-1020.
28. Iams W, Heck J, Kapp M, et al. A multidisciplinary housestaff-led initiative to safely reduce daily laboratory testing. Acad Med. 2016;91(6):813-820. doi: 10.1097/ACM.0000000000001149.
29. Kost A, Genao I, Lee JW, Smith SR. Clinical decisions made in primary care clinics before and after Choosing WiselyTM. J Am Board Fam Med. 2015;28(4):471-474. doi: 10.3122/jabfm.2015.05.140332.
30. Kerr EA, Chen J, Sussman JB, Klamerus ML, Nallamothu BK. Stress testing before low-risk surgery: so many recommendations, so little overuse. JAMA Intern Med. 2015;175(4):645-647. doi: 10.1001/jamainternmed.2014.7877.
31. Colla CH, Morden NE, Sequist TD, Schpero WL, Rosenthal MB. Choosing wisely: prevalence and correlates of low-value health care services in the United States. J Gen Intern Med. 2014;30(2):221-228. doi: 10.1007/s11606-014-3070-z.
32. Shetty KD, Meeker D, Schneider EC, Hussey PS, Damberg CL. Evaluating the feasibility and utility of translating Choosing Wisely recommendations into e-Measures. Healthcare. 2015;3(1):24-37. doi: 10.1016/j.hjdsi.2014.12.002.
33. Woodland DC, Cooper CR, Rashid MF, et al. Routine chest X-ray is unnecessary after ultrasound-guided central venous line placement in the operating room. J Crit Care. 2018;46:13-16. doi: 10.1016/j.jcrc.2018.03.027.
34. Krause TM, Ukhanova M, Revere FL. Private carriers’ physician payment rates compared with Medicare and Medicaid. Tex Med. 2016;112(6):e1.
35. American College of Radiology. Medicare physician fee schedule. Available at https://www.acr.org/Advocacy-and-Economics/Radiology-Economics/Medicare-Medicaid/MPFS. Accessed October 15, 2018.
36. Siegel MD, Rubinowitz AN. Routine daily vs on-demand chest radiographs in intensive care. Lancet. 2009;374(9702):1656-1658. doi: 10.1016/S0140-6736(09)61632-9.
37. Tonna JE, Kawamoto K, Presson AP, et al. Single intervention for a reduction in portable chest radiography (pCXR) in cardiovascular and surgical/trauma ICUs and associated outcomes. J Crit Care. 2018;44:18-23. doi: 10.1016/j.jcrc.2017.10.003.
38. Wolfson D, Santa J, Slass L. Engaging physicians and consumers in conversations about treatment overuse and waste: a short history of the choosing wisely campaign. Acad Med. 2014;89(7):990-995. doi: 10.1097/ACM.0000000000000270.
Despite increased awareness of Choosing Wisely (CW)® recommendations to reduce low-value care,1 there is limited published data about strategies to implement these guidelines or evidence that they have influenced ordering patterns or reduced healthcare spending
In ICUs, CXR ordering strategies may be routine (daily) or on-demand (with clinical indication). The former strategy’s principal advantage is the potential to detect life-threatening situations that may otherwise escape diagnosis.11 Disadvantages include cost, radiation exposure, patient inconvenience, false-positive workups, and low diagnostic and therapeutic value.12,13 On-demand strategies may safely reduce CXR ordering by 32% to 45%.11-17 Based on this evidence, the Critical Care Societies Collaborative and the American College of Radiology have recommended on-demand CXR ordering.18,19 Here, we describe the effectiveness of an intervention to reduce CXR ordering in two ICUs while evaluating the deimplementation strategies using a validated framework.
METHODS
Setting and Design
Vanderbilt University Medical Center (VUMC) is an academic referral center in Nashville, Tennessee. The cardiovascular ICU (CVICU) has 27 beds and the medical ICU (MICU) has 34 beds. Acute care nurse practitioners (ACNPs) and two critical care physicians staff the CVICU; cardiology fellows, anesthesia critical care fellows, and transplant and cardiac surgeons are also active in patient care. The MICU is staffed by two critical care physicians who supervise one team of ACNPs and two teams of medical residents who rotate through the unit every two weeks. Each MICU team is assigned a fellow in pulmonary and critical care.
We conducted a prospective, nonrandomized study in these units from October 2015 to June 2016. The VUMC Institutional Review Board approved the intervention as a quality improvement (QI) activity, waiving the requirement for informed consent.
Intervention
Following the top CW recommendation of the Critical Care Societies Collaborative—“Don’t order diagnostic tests at regular intervals (such as every day), but rather in response to specific clinical questions.”19—the VUMC resident-led CW Steering Committee designed a multifaceted approach to reduce ordering of routine CXRs in ICUs. The intervention included a didactic session on CW and proper CXR ordering practices, peer champions, data audits, and feedback to providers through weekly e-mails (see Supplemental Materials, 1 – Resident Presentation and 2 – CXR Flyer). 20
In September 2015, CVICU and MICU teams received a didactic session highlighting CW, current CXR ordering rates, and the plan for reducing CXR ordering. On October 5, 2015, teams began receiving weekly e-mails with ordering rates defined as CXRs ordered per patient per day and a brief rationale for reducing unnecessary CXRs. To encourage friendly competition, we provided weekly rates to the MICU teams, allowing for transparent benchmarking against one another. A similar competition strategy was not used in the CVICU due to the lack of multiple teams.
In the CVICU, two ACNPs volunteered as peer champions. These champions coordinated data feedback and advocated for the intervention among their colleagues. In the MICU, three internal medicine residents volunteered as peer champions and fulfilled similar roles.
To facilitate deimplementation, we conducted two Plan-Do-Study-Act (PDSA) cycles, the first from November to mid-December 2015 and the second from mid-December 2015 to mid-January 2016. During these cycles, we tailored our deimplementation strategy based on barriers identified by the peer champions and ICU leaders (described in the Qualitative Results section). Peer champions and the CW Steering Committee generated potential solutions by conversing with stakeholders and using the Expert Recommendations for Implementing Change (ERIC).20 Interventions included disseminating promotional flyers, holding meetings with stakeholders, and providing monthly CXR ordering rates. After the PDSA cycles, we continued reexamining the deimplementation efforts by reviewing ordering rates and soliciting feedback from ICU leaders and peer champions. However, no significant changes to the intervention were made during this time.
Quantitative Evaluation
We extracted data from VUMC’s Enterprise Data Warehouse during the intervention period (October 5, 2015 to May 24, 2016) and a historical control period (October 1, 2014 to October 4, 2015). Within each ICU, descriptive statistics were used to compare patient cohorts in the baseline and intervention periods by age, sex, and race.
The primary outcome was CXRs ordered per patient per day by hospital unit (CVICU or MICU). The baseline period included all data between October 1, 2014 and September 15, 2015. To account for priming of providers from didactic education, we allowed a washout period from September 16, 2015 to October 4, 2015. As a preliminary analysis, we compared CXR rates in the baseline and intervention periods using Wilcoxon rank-sum tests. We then conducted interrupted time-series analyses with segmented linear regression to assess differences in linear trends in CXR rates over the two periods. To account for different staffing models in the MICU, we stratified the impact of the intervention by team—medical resident (physician) or ACNP. R version 3.4.0 was used for statistical analysis.21
Qualitative Evaluation
Our qualitative evaluation consisted of embedded observation and semistructured interviews with stakeholders. The qualitative portion was guided by the Consolidated Framework for Implementation Research (CFIR), a widely used framework for design and evaluation of improvement initiatives that helped us to determine major facilitators and barriers to implementation.22,23
Embedded Observation
From November 2015 to January 2016, we observed morning rounds in the CVICU and MICU one to two times weekly to understand factors facilitating and inhibiting uptake of the intervention. Observations were recorded and organized using a CFIR-based template and directed toward understanding interactions among team members (eg, the decision-making process hierarchy), team workflows and decision-making processes, process of ordering CXRs, and providers’ knowledge and perceptions of the CXR intervention (see Supplemental Material, 3 – CFIR Table).22,23 After rounds, ICU team members were invited to share suggestions for improving the intervention. All observations occurred during and shortly following morning rounds when the vast majority of routine CXRs are ordered; we did not evaluate night or evening workflows. In the spirit of continuous improvement, we evaluated data in real-time.
Semistructured Interviews
Based on the direct observations, we developed semistructured interview questions to further evaluate provider perspectives (eg, “Do you believe ICU patients need a daily CXR?”) and constructs aligning with CFIR (eg, “intervention source—internally vs externally developed;” see Supplemental Material, 4 – Interview Questions).
Stakeholders from both ICUs were recruited through e-mail and in-person requests to participate in semistructured interviews. In the CVICU, we interviewed critical care physicians, anesthesia critical care fellows, and ACNPs. In the MICU, we interviewed medical students, interns, residents, critical care fellows, attending intensivist physicians, and ACNPs. We also interviewed X-ray technologists who routinely perform portable films in the units.
RESULTS
Quantitative Results
Cardiovascular Intensive Care Unit
The median baseline CXR ordering rate in the CVICU was 1.16 CXRs per patient per day, with interquartile range (IQR) 1.06-1.28. During the intervention period, the rate dropped to 1.07 (IQR 0.94-1.21; P < .001; Table 2). The time-series analysis suggested an essentially flat trend during the baseline period, followed by a small but significant drop in ordering rates during the intervention period (P < .001; Table 3 and Figure 1). Ordering rates appeared to increase slightly over the course of the intervention period, but this slight upward trend was not significantly different from the flat trend seen during the baseline period.
Medical Intensive Care Unit
For both physician and ACPN teams, the median baseline CXR ordering rates in the MICU were much lower than the baseline rate in the CVICU (Table 2). For the MICU physician care team, the baseline CXR ordering rate was 0.60 CXRs per patient per day (IQR 0.48-0.73). For the ACNP team, the median rate was 0.39 CXRs per patient per day (IQR 0.21-0.57). Both rates stayed approximately the same during the intervention period (Table 2). The time-series analysis suggested a statistically significant but very slight downward trend in CXR ordering rates during the baseline period, in the physician (P = .011) and ACNP (P = .022) teams (Table 3, Figure 2). Under this model, a small increase in CXR ordering initially occurred during the intervention period for both physician and ACNP teams (P = .010 and P = .055, respectively), after which the rates declined slightly. Trends in ordering rates during the intervention period were not significantly different from the slight downward trends seen during the baseline period.
Qualitative Results
We identified 25 of 39 CFIR constructs as relevant to the initiative (see Supplemental Materials, 3 – CFIR Table.) We determined the major facilitators of deimplementation to be peer champion discussions about CXR ordering on rounds and weekly data feedback, particularly if accompanied by in-person follow-up.
We also noted differences in CXR ordering rationales and decisions between the units. Generally, residents in the MICU and ACNPs in the CVICU made decisions to order CXRs. However, decisions were influenced by the expectations of attending physicians. While CVICU providers tended to order CXRs reflexively as part of morning labs, MICU providers—in particular, ACNPs who had been trained on indications for proper CXR ordering—generally ordered CXRs for specific indications (eg, worsening respiratory status). Of note, MICU ACNPs reported the use of bedside ultrasound as an alternate imaging modality and a reason for their higher threshold to order CXRs.
Deimplementation barriers in both units included the need to identify goal CXR ordering rates and the intervention’s limited visibility. To address these barriers, we conducted PDSA cycles and used the CFIR and ERIC to generate potential solutions.24 We established a goal of a 20% absolute reduction in the CVICU, added monthly CXR rates to weekly e-mail reports to better account for variations in patient populations and ordering practices, and circulated materials to promote on-demand CXR ordering. Promotional materials contained guidelines on CXR ordering and five “Frequently Held Misconceptions” about ordering practices with succinct, evidence-based explanations (see Supplemental Material, 2 – CXR Flyer).
Approximately four months after the start of intervention, some CVICU physicians became concerned that on-demand CXR ordering might be inappropriate for high-risk surgical patients, including those who are undergoing or have undergone heart transplants, lung transplants, or left-ventricular assist device placement. This concern arose following two adverse outcomes, which were not attributed to the CXR initiative, but which heightened concerns about patient safety. A rise in CXR ordering then occurred, and CVICU providers requested that we perform an analysis of these high-risk groups. While segmented linear regression in this subgroup suggested that average daily CXR ordering rates did decrease among the high-risk group at the start of the intervention period (P = .001), the difference between the rates in the two periods was not significant using the Wilcoxon rank-sum test. Exclusion of these patients from the main analysis did not alter the interpretation of the findings reported above for the CVICU.
DISCUSSION
A deimplementation intervention using provider education, peer champions, and data feedback was associated with fewer CXRs in the CVICU (P < .001) but not in the MICU. The CFIR-guided qualitative analysis was valuable for evaluating our deimplementation strategy and for identifying differences between the two ICUs.
Relatively few studies have demonstrated effective interventions that address CW recommendations.25-28 However, three population-level analyses of insurance claims show mixed results.3,4,29 Experts have thus proposed using implementation science to improve uptake of CW recommendations.2,3,7,8 Our study demonstrates the effectiveness of this approach. As expected, providers largely endorsed an on-demand CXR ordering strategy. Using the CFIR, however, we discovered barriers (eg, concern that data feedback did not reflect variations in patients’ needs). Using methods from implementation science allowed us to diagnose and tailor our approaches.
Our qualitative evaluation suggested that the intervention was ineffective mostly due to CFIR’s “inner setting” constructs, including resident fatigue, competing priorities, and decreased investment in QI projects because of the rotating nature of providers in training. Baseline CXR ordering rates in the MICU were also considerably lower than in the CVICU. We observed that CVICU providers ordered many CXRs following the placement of lines or tubes and that ACNPs in the MICU had received education on appropriate CXR ordering practices and had access to an alternate imaging modality in ultrasound. These factors may partially explain the difference in baseline rates.
As noted in a study of cardiac stress testing guidelines, the existence of high-value care recommendations does not mean overuse.30 Indeed, the lack of significant CXR over-ordering in the MICU highlights the importance of baseline measurement and partnering with information technology departments to create the best possible data feedback systems.30-32 Our experience shows that these systems should provide sufficient pre-implementation data (ideally >1 year), such that teams selecting QI projects can ensure that a project is a good use of institutional resources and change capital.
To inform future work, we informally assessed program costs and savings. We estimate the initiative cost $1,600, including $1,000 for curriculum development and teaching time, $300 for educational materials, and $300 for CXR tracking dashboard development. Hospital charges and reimbursements for CXR vary widely.33 We calculated savings using a range of rates, from a conservative $23 (the Medicare reimbursement rate for single-view CXR, CPT code 71010, global fee) to $50 (an approximate blended reimbursement rate across payers).34,35 In the CVICU, we estimate that 51 CXRs were avoided each month, saving $1,173-$2,550 per month or $9,384-$20,400 over eight months of follow-up. Annualizing these figures, we estimate net savings of $12,476-$29,000 in the first year in a 27-bed ICU. Costs to continue the program include education of new employees, booster training, and dashboard maintenance for an estimated annual cost of $1,000. It is difficult to estimate effectiveness over time, but if we conservatively assume that 30 CXRs were avoided each month, then the projected savings would be $8,280-$18,000 per year or an annual net savings of $7,280-$17,000 in the ICU. Although these amounts are modest, providing trainees with experiential learning opportunities in high-value care is valuable in its own right, meets curricular goals, may result in spill-over effects to other diagnostic and therapeutic decisions, and may influence long-term practice patterns. Institutional decisions to pursue projects such as this should take into account these potential benefits.
This evaluation is not without limitations. First, the study was conducted in a single tertiary-care hospital, potentially limiting its generalizability.36 Second, the study design lacked a concurrent control group, and observed outcomes may have been influenced by broader CXR utilization trends, increased awareness of low-value care generally or from previous CW projects at VUMC, seasonal effects, or the Hawthorne effect. Third, the study outcome was all CXRs ordered, rather than CXRs that were unnecessary or not clinically indicated. We chose all CXRs because it was more pragmatic, did not require clinical case review, and could be incorporated promptly into dashboards, enabling timely performance feedback. Other performance measures have taken a similar tack (eg, tracking all-cause readmissions rather than preventable readmissions). Given this approach, we did not track clinical indications for CXRs (eg, central line placement). Fourth, although we compared resident and APRN orders, we did not collect data on other provider characteristics such as years in/out of training or board certification status. These considerations should be addressed in future research.
Finally, the increase in CVICU CXR ordering at the end of the intervention period, which occurred following two adverse events, raises concerns about sustainability. While unrelated to CXR orders, the events resulted in increased ordering of diagnostic tests and showed the difficulty of deimplementation in ICUs. Indeed, some CVICU providers argued that on-demand CXR ordering represented minimal potential cost savings and had not been studied among heart and lung transplant patients. Subsequently, Tonna et al. have shown that on-demand CXR ordering can be safely implemented among such patients.37 Also similar to our study, Tonna et al. observed an initial decrease in CXR ordering, followed by a gradual increase toward baseline ordering rates. These findings highlight the need for sustained awareness and interventions and for the careful selection of high-value projects.
In conclusion, our study shows that a deimplementation intervention based on CW recommendations can reduce CXR ordering and that ongoing evaluation of contextual factors provides insights for both real-time modifications of current interventions and the design of future interventions. We found that messaging about reducing unnecessary tests works well when discussions are framed at the unit level but may be counterproductive if used to question individual ordering decisions.38 Additional lessons learned include the value of participation on rounds to build trust among stakeholders, the utility of monthly rather than weekly statistics for feedback, stakeholder input and peer champions, and differences in approach with physician and ACNP audiences.
Acknowledgments
The authors thank the VUMC Choosing Wisely committee; Mr. Bill Harrell in Advanced Data Analytics for developing the Tableau platform used in our data feedback strategy; Emily Feld, MD, Jerry Zifodya, MD, and Ryan Kindle, MD for assisting with data feedback to providers in the MICU; Todd Rice, MD, Director of the MICU, for his support of the initiative; and Beth Prusaczyk, PhD, MSW and David Stevenson, PhD for providing feedback on earlier drafts of this manuscript.
Disclosures
Dr. Kripalani reports personal fees from Verustat, personal fees from SAI Interactive, and equity from Bioscape Digital, outside the submitted work. All other authors have nothing to disclose.
Funding
This work was supported by an Innovation Grant from the Alliance for Academic Internal Medicine (AAIM, 2016) and by the Departments of Internal Medicine and Graduate Medical Education at Vanderbilt University Medical Center. The AAIM did not have a role in the study design, data collection, data analysis, data interpretation, or manuscript writing.
Despite increased awareness of Choosing Wisely (CW)® recommendations to reduce low-value care,1 there is limited published data about strategies to implement these guidelines or evidence that they have influenced ordering patterns or reduced healthcare spending
In ICUs, CXR ordering strategies may be routine (daily) or on-demand (with clinical indication). The former strategy’s principal advantage is the potential to detect life-threatening situations that may otherwise escape diagnosis.11 Disadvantages include cost, radiation exposure, patient inconvenience, false-positive workups, and low diagnostic and therapeutic value.12,13 On-demand strategies may safely reduce CXR ordering by 32% to 45%.11-17 Based on this evidence, the Critical Care Societies Collaborative and the American College of Radiology have recommended on-demand CXR ordering.18,19 Here, we describe the effectiveness of an intervention to reduce CXR ordering in two ICUs while evaluating the deimplementation strategies using a validated framework.
METHODS
Setting and Design
Vanderbilt University Medical Center (VUMC) is an academic referral center in Nashville, Tennessee. The cardiovascular ICU (CVICU) has 27 beds and the medical ICU (MICU) has 34 beds. Acute care nurse practitioners (ACNPs) and two critical care physicians staff the CVICU; cardiology fellows, anesthesia critical care fellows, and transplant and cardiac surgeons are also active in patient care. The MICU is staffed by two critical care physicians who supervise one team of ACNPs and two teams of medical residents who rotate through the unit every two weeks. Each MICU team is assigned a fellow in pulmonary and critical care.
We conducted a prospective, nonrandomized study in these units from October 2015 to June 2016. The VUMC Institutional Review Board approved the intervention as a quality improvement (QI) activity, waiving the requirement for informed consent.
Intervention
Following the top CW recommendation of the Critical Care Societies Collaborative—“Don’t order diagnostic tests at regular intervals (such as every day), but rather in response to specific clinical questions.”19—the VUMC resident-led CW Steering Committee designed a multifaceted approach to reduce ordering of routine CXRs in ICUs. The intervention included a didactic session on CW and proper CXR ordering practices, peer champions, data audits, and feedback to providers through weekly e-mails (see Supplemental Materials, 1 – Resident Presentation and 2 – CXR Flyer). 20
In September 2015, CVICU and MICU teams received a didactic session highlighting CW, current CXR ordering rates, and the plan for reducing CXR ordering. On October 5, 2015, teams began receiving weekly e-mails with ordering rates defined as CXRs ordered per patient per day and a brief rationale for reducing unnecessary CXRs. To encourage friendly competition, we provided weekly rates to the MICU teams, allowing for transparent benchmarking against one another. A similar competition strategy was not used in the CVICU due to the lack of multiple teams.
In the CVICU, two ACNPs volunteered as peer champions. These champions coordinated data feedback and advocated for the intervention among their colleagues. In the MICU, three internal medicine residents volunteered as peer champions and fulfilled similar roles.
To facilitate deimplementation, we conducted two Plan-Do-Study-Act (PDSA) cycles, the first from November to mid-December 2015 and the second from mid-December 2015 to mid-January 2016. During these cycles, we tailored our deimplementation strategy based on barriers identified by the peer champions and ICU leaders (described in the Qualitative Results section). Peer champions and the CW Steering Committee generated potential solutions by conversing with stakeholders and using the Expert Recommendations for Implementing Change (ERIC).20 Interventions included disseminating promotional flyers, holding meetings with stakeholders, and providing monthly CXR ordering rates. After the PDSA cycles, we continued reexamining the deimplementation efforts by reviewing ordering rates and soliciting feedback from ICU leaders and peer champions. However, no significant changes to the intervention were made during this time.
Quantitative Evaluation
We extracted data from VUMC’s Enterprise Data Warehouse during the intervention period (October 5, 2015 to May 24, 2016) and a historical control period (October 1, 2014 to October 4, 2015). Within each ICU, descriptive statistics were used to compare patient cohorts in the baseline and intervention periods by age, sex, and race.
The primary outcome was CXRs ordered per patient per day by hospital unit (CVICU or MICU). The baseline period included all data between October 1, 2014 and September 15, 2015. To account for priming of providers from didactic education, we allowed a washout period from September 16, 2015 to October 4, 2015. As a preliminary analysis, we compared CXR rates in the baseline and intervention periods using Wilcoxon rank-sum tests. We then conducted interrupted time-series analyses with segmented linear regression to assess differences in linear trends in CXR rates over the two periods. To account for different staffing models in the MICU, we stratified the impact of the intervention by team—medical resident (physician) or ACNP. R version 3.4.0 was used for statistical analysis.21
Qualitative Evaluation
Our qualitative evaluation consisted of embedded observation and semistructured interviews with stakeholders. The qualitative portion was guided by the Consolidated Framework for Implementation Research (CFIR), a widely used framework for design and evaluation of improvement initiatives that helped us to determine major facilitators and barriers to implementation.22,23
Embedded Observation
From November 2015 to January 2016, we observed morning rounds in the CVICU and MICU one to two times weekly to understand factors facilitating and inhibiting uptake of the intervention. Observations were recorded and organized using a CFIR-based template and directed toward understanding interactions among team members (eg, the decision-making process hierarchy), team workflows and decision-making processes, process of ordering CXRs, and providers’ knowledge and perceptions of the CXR intervention (see Supplemental Material, 3 – CFIR Table).22,23 After rounds, ICU team members were invited to share suggestions for improving the intervention. All observations occurred during and shortly following morning rounds when the vast majority of routine CXRs are ordered; we did not evaluate night or evening workflows. In the spirit of continuous improvement, we evaluated data in real-time.
Semistructured Interviews
Based on the direct observations, we developed semistructured interview questions to further evaluate provider perspectives (eg, “Do you believe ICU patients need a daily CXR?”) and constructs aligning with CFIR (eg, “intervention source—internally vs externally developed;” see Supplemental Material, 4 – Interview Questions).
Stakeholders from both ICUs were recruited through e-mail and in-person requests to participate in semistructured interviews. In the CVICU, we interviewed critical care physicians, anesthesia critical care fellows, and ACNPs. In the MICU, we interviewed medical students, interns, residents, critical care fellows, attending intensivist physicians, and ACNPs. We also interviewed X-ray technologists who routinely perform portable films in the units.
RESULTS
Quantitative Results
Cardiovascular Intensive Care Unit
The median baseline CXR ordering rate in the CVICU was 1.16 CXRs per patient per day, with interquartile range (IQR) 1.06-1.28. During the intervention period, the rate dropped to 1.07 (IQR 0.94-1.21; P < .001; Table 2). The time-series analysis suggested an essentially flat trend during the baseline period, followed by a small but significant drop in ordering rates during the intervention period (P < .001; Table 3 and Figure 1). Ordering rates appeared to increase slightly over the course of the intervention period, but this slight upward trend was not significantly different from the flat trend seen during the baseline period.
Medical Intensive Care Unit
For both physician and ACPN teams, the median baseline CXR ordering rates in the MICU were much lower than the baseline rate in the CVICU (Table 2). For the MICU physician care team, the baseline CXR ordering rate was 0.60 CXRs per patient per day (IQR 0.48-0.73). For the ACNP team, the median rate was 0.39 CXRs per patient per day (IQR 0.21-0.57). Both rates stayed approximately the same during the intervention period (Table 2). The time-series analysis suggested a statistically significant but very slight downward trend in CXR ordering rates during the baseline period, in the physician (P = .011) and ACNP (P = .022) teams (Table 3, Figure 2). Under this model, a small increase in CXR ordering initially occurred during the intervention period for both physician and ACNP teams (P = .010 and P = .055, respectively), after which the rates declined slightly. Trends in ordering rates during the intervention period were not significantly different from the slight downward trends seen during the baseline period.
Qualitative Results
We identified 25 of 39 CFIR constructs as relevant to the initiative (see Supplemental Materials, 3 – CFIR Table.) We determined the major facilitators of deimplementation to be peer champion discussions about CXR ordering on rounds and weekly data feedback, particularly if accompanied by in-person follow-up.
We also noted differences in CXR ordering rationales and decisions between the units. Generally, residents in the MICU and ACNPs in the CVICU made decisions to order CXRs. However, decisions were influenced by the expectations of attending physicians. While CVICU providers tended to order CXRs reflexively as part of morning labs, MICU providers—in particular, ACNPs who had been trained on indications for proper CXR ordering—generally ordered CXRs for specific indications (eg, worsening respiratory status). Of note, MICU ACNPs reported the use of bedside ultrasound as an alternate imaging modality and a reason for their higher threshold to order CXRs.
Deimplementation barriers in both units included the need to identify goal CXR ordering rates and the intervention’s limited visibility. To address these barriers, we conducted PDSA cycles and used the CFIR and ERIC to generate potential solutions.24 We established a goal of a 20% absolute reduction in the CVICU, added monthly CXR rates to weekly e-mail reports to better account for variations in patient populations and ordering practices, and circulated materials to promote on-demand CXR ordering. Promotional materials contained guidelines on CXR ordering and five “Frequently Held Misconceptions” about ordering practices with succinct, evidence-based explanations (see Supplemental Material, 2 – CXR Flyer).
Approximately four months after the start of intervention, some CVICU physicians became concerned that on-demand CXR ordering might be inappropriate for high-risk surgical patients, including those who are undergoing or have undergone heart transplants, lung transplants, or left-ventricular assist device placement. This concern arose following two adverse outcomes, which were not attributed to the CXR initiative, but which heightened concerns about patient safety. A rise in CXR ordering then occurred, and CVICU providers requested that we perform an analysis of these high-risk groups. While segmented linear regression in this subgroup suggested that average daily CXR ordering rates did decrease among the high-risk group at the start of the intervention period (P = .001), the difference between the rates in the two periods was not significant using the Wilcoxon rank-sum test. Exclusion of these patients from the main analysis did not alter the interpretation of the findings reported above for the CVICU.
DISCUSSION
A deimplementation intervention using provider education, peer champions, and data feedback was associated with fewer CXRs in the CVICU (P < .001) but not in the MICU. The CFIR-guided qualitative analysis was valuable for evaluating our deimplementation strategy and for identifying differences between the two ICUs.
Relatively few studies have demonstrated effective interventions that address CW recommendations.25-28 However, three population-level analyses of insurance claims show mixed results.3,4,29 Experts have thus proposed using implementation science to improve uptake of CW recommendations.2,3,7,8 Our study demonstrates the effectiveness of this approach. As expected, providers largely endorsed an on-demand CXR ordering strategy. Using the CFIR, however, we discovered barriers (eg, concern that data feedback did not reflect variations in patients’ needs). Using methods from implementation science allowed us to diagnose and tailor our approaches.
Our qualitative evaluation suggested that the intervention was ineffective mostly due to CFIR’s “inner setting” constructs, including resident fatigue, competing priorities, and decreased investment in QI projects because of the rotating nature of providers in training. Baseline CXR ordering rates in the MICU were also considerably lower than in the CVICU. We observed that CVICU providers ordered many CXRs following the placement of lines or tubes and that ACNPs in the MICU had received education on appropriate CXR ordering practices and had access to an alternate imaging modality in ultrasound. These factors may partially explain the difference in baseline rates.
As noted in a study of cardiac stress testing guidelines, the existence of high-value care recommendations does not mean overuse.30 Indeed, the lack of significant CXR over-ordering in the MICU highlights the importance of baseline measurement and partnering with information technology departments to create the best possible data feedback systems.30-32 Our experience shows that these systems should provide sufficient pre-implementation data (ideally >1 year), such that teams selecting QI projects can ensure that a project is a good use of institutional resources and change capital.
To inform future work, we informally assessed program costs and savings. We estimate the initiative cost $1,600, including $1,000 for curriculum development and teaching time, $300 for educational materials, and $300 for CXR tracking dashboard development. Hospital charges and reimbursements for CXR vary widely.33 We calculated savings using a range of rates, from a conservative $23 (the Medicare reimbursement rate for single-view CXR, CPT code 71010, global fee) to $50 (an approximate blended reimbursement rate across payers).34,35 In the CVICU, we estimate that 51 CXRs were avoided each month, saving $1,173-$2,550 per month or $9,384-$20,400 over eight months of follow-up. Annualizing these figures, we estimate net savings of $12,476-$29,000 in the first year in a 27-bed ICU. Costs to continue the program include education of new employees, booster training, and dashboard maintenance for an estimated annual cost of $1,000. It is difficult to estimate effectiveness over time, but if we conservatively assume that 30 CXRs were avoided each month, then the projected savings would be $8,280-$18,000 per year or an annual net savings of $7,280-$17,000 in the ICU. Although these amounts are modest, providing trainees with experiential learning opportunities in high-value care is valuable in its own right, meets curricular goals, may result in spill-over effects to other diagnostic and therapeutic decisions, and may influence long-term practice patterns. Institutional decisions to pursue projects such as this should take into account these potential benefits.
This evaluation is not without limitations. First, the study was conducted in a single tertiary-care hospital, potentially limiting its generalizability.36 Second, the study design lacked a concurrent control group, and observed outcomes may have been influenced by broader CXR utilization trends, increased awareness of low-value care generally or from previous CW projects at VUMC, seasonal effects, or the Hawthorne effect. Third, the study outcome was all CXRs ordered, rather than CXRs that were unnecessary or not clinically indicated. We chose all CXRs because it was more pragmatic, did not require clinical case review, and could be incorporated promptly into dashboards, enabling timely performance feedback. Other performance measures have taken a similar tack (eg, tracking all-cause readmissions rather than preventable readmissions). Given this approach, we did not track clinical indications for CXRs (eg, central line placement). Fourth, although we compared resident and APRN orders, we did not collect data on other provider characteristics such as years in/out of training or board certification status. These considerations should be addressed in future research.
Finally, the increase in CVICU CXR ordering at the end of the intervention period, which occurred following two adverse events, raises concerns about sustainability. While unrelated to CXR orders, the events resulted in increased ordering of diagnostic tests and showed the difficulty of deimplementation in ICUs. Indeed, some CVICU providers argued that on-demand CXR ordering represented minimal potential cost savings and had not been studied among heart and lung transplant patients. Subsequently, Tonna et al. have shown that on-demand CXR ordering can be safely implemented among such patients.37 Also similar to our study, Tonna et al. observed an initial decrease in CXR ordering, followed by a gradual increase toward baseline ordering rates. These findings highlight the need for sustained awareness and interventions and for the careful selection of high-value projects.
In conclusion, our study shows that a deimplementation intervention based on CW recommendations can reduce CXR ordering and that ongoing evaluation of contextual factors provides insights for both real-time modifications of current interventions and the design of future interventions. We found that messaging about reducing unnecessary tests works well when discussions are framed at the unit level but may be counterproductive if used to question individual ordering decisions.38 Additional lessons learned include the value of participation on rounds to build trust among stakeholders, the utility of monthly rather than weekly statistics for feedback, stakeholder input and peer champions, and differences in approach with physician and ACNP audiences.
Acknowledgments
The authors thank the VUMC Choosing Wisely committee; Mr. Bill Harrell in Advanced Data Analytics for developing the Tableau platform used in our data feedback strategy; Emily Feld, MD, Jerry Zifodya, MD, and Ryan Kindle, MD for assisting with data feedback to providers in the MICU; Todd Rice, MD, Director of the MICU, for his support of the initiative; and Beth Prusaczyk, PhD, MSW and David Stevenson, PhD for providing feedback on earlier drafts of this manuscript.
Disclosures
Dr. Kripalani reports personal fees from Verustat, personal fees from SAI Interactive, and equity from Bioscape Digital, outside the submitted work. All other authors have nothing to disclose.
Funding
This work was supported by an Innovation Grant from the Alliance for Academic Internal Medicine (AAIM, 2016) and by the Departments of Internal Medicine and Graduate Medical Education at Vanderbilt University Medical Center. The AAIM did not have a role in the study design, data collection, data analysis, data interpretation, or manuscript writing.
1. Cassel CK, Guest JA. Choosing Wisely: Helping physicians and patients make smart decisions about their care. JAMA. 2012;307(17):1801-1802.
2. Gonzales R, Cattamanchi A. Changing clinician behavior when less is more. JAMA Intern Med. 2015;175(12):1921-1922.
3. Rosenberg A, Agiro A, Gottlieb M, et al. Early trends among seven recommendations from the Choosing Wisely campaign. JAMA Intern Med. 2015;175(12):1913-1920.
4. Hong AS, Ross-Degnan D, Zhang F, Wharam JF. Small decline in low-value back imaging associated with the ‘Choosing Wisely’ campaign, 2012-14. Health Aff (Millwood). 2017;36(4):671-679. doi: 10.1377/hlthaff.2016.1263.
5. Parks AL, O’Malley PG. From choosing wisely to practicing value—more to the story. JAMA Intern Med. 2016;176(10):1571-1572.
6. Johnson PT, Pahwa AK, Feldman LS, Ziegelstein RC, Hellmann DB. Advancing high-value health care: a new AJM column dedicated to cost-conscious care quality improvement. Am J Med. 2017;130(6):619-621. doi: 10.1016/j.amjmed.2016.12.018.
7. Eccles MP, Mittman BS. Welcome to implementation science. Implement Sci. 2006;1:1. doi: 10.1186/1748-5908-1-1.
8. Bhatia RS, Levinson W, Shortt S, et al. Measuring the effect of Choosing Wisely: an integrated framework to assess campaign impact on low-value care. BMJ Qual Saf. 2015;24:523-531. doi: 10.1136/bmjqs-2015-004070.
9. Selby K, Barnes GD. Learning to de-adopt ineffective healthcare practices. Am J Med. 2018;131(7):721-722. doi: 10.1016/j.amjmed.2018.03.014.
10. Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs. Med Care. 2012;50(3):217-226. doi: 10.1097/MLR.0b013e3182408812.
11. Ganapathy A, Adhikari NK, Spiegelman J, Scales DC. Routine chest x-rays in intensive care units: a systematic review and meta-analysis. Crit Care. 2012;16(2):R68. doi: 10.1186/cc11321.
12. Graat ME, Kröner A, Spronk PE, et al. Elimination of daily routine chest radiographs in a mixed medical-surgical intensive care unit. Intensive Care Med. 2007;33(4):639-644. doi: 10.1007/s00134-007-0542-1.
13. Hendrikse KA, Gratama JWC, Ten Hove W, Rommes JH, Schultz MJ, Spronk PE. Low value of routine chest radiographs in a mixed medical-surgical ICU. Chest. 2007;132(3):823-828. doi: 10.1378/chest.07-1162.
14. Clec’h C, Simon P, Hamdi A, et al. Are daily routine chest radiographs useful in critically ill, mechanically ventilated patients? A randomized study. Intensive Care Med. 2008;34(2):264-270. doi: 10.1007/s00134-007-0919-1.
15. Oba Y, Zaza T. Abandoning daily routine chest radiography in the intensive care unit: meta-analysis. Radiology. 2010;255(2):386-395. doi: 10.1148/radiol.10090946.
16. Mets O, Spronk PE, Binnekade J, Stoker J, de Mol BAJM, Schultz MJ. Elimination of daily routine chest radiographs does not change on-demand radiography practice in post-cardiothoracic surgery patients. J Thorac Cardiovasc Surg. 2007;134(1):139-144. doi: 10.1016/j.jtcvs.2007.02.029.
17. Hejblum G, Chalumeau-Lemoine L, Ioos V, et al. Comparison of routine and on-demand prescription of chest radiographs in mechanically ventilated adults: a multicentre, cluster-randomised, two-period crossover study. Lancet. 2009;374(9702):1687-1693. doi: 10.1016/S0140-6736(09)61459-8.
18. McComb BL, Chung JH, Crabtree TD, et al. ACR appropriateness criteria® routine chest radiography. J Thorac Imaging. 2016;31(2):W13-W15. doi: 10.1097/RTI.0000000000000200.
19. Halpern SD, Becker D, Curtis JR, et al. An official American Thoracic Society/American Association of Critical-Care Nurses/American College of Chest Physicians/Society of Critical Care Medicine policy statement: the Choosing Wisely® top 5 list in critical care medicine. Am J Respir Crit Care Med. 2014;190(7):818-826. doi: 10.1164/rccm.201407-1317ST.
20. Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: Results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10:21. doi: 10.1186/s13012-015-0209-1.
21. R [computer program]. Version 3.4.0. Vienna, Austria: R Foundation for Statistical Computing; 2013.
22. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. doi: 10.1186/1748-5908-4-50.
23. Kirk MA, Kelley C, Yankey N, Birken SA, Abadie B, Damschroder L. A systematic review of the use of the Consolidated Framework for Implementation Research. Implement Sci. 2016;11:72. doi: 10.1186/s13012-016-0437-z.
24. Speroff T, James BC, Nelson EC, Headrick LA, Brommels M, Reed JE. Guidelines for appraisal and publication of PDSA quality improvement. Qual Manag Health Care. 2014;13(1):33-39. doi: 10.1097/00019514-200401000-00003.
25. Corson AH, Fan VS, White T, et al. A multifaceted hospitalist quality improvement intervention: Decreased frequency of common labs. J Hosp Med. 2015;10(6):390-395. doi: 10.1002/jhm.2354.
26. Ferrari R. Evaluation of the Canadian Rheumatology Association Choosing Wisely recommendation concerning anti-nuclear antibody (ANA) testing. Clin Rheumatol. 2015;34(9):1551-1556. doi: 10.1007/s10067-015-2985-z.
27. Ferrari R, Prosser C. Testing vitamin D levels and choosing wisely. JAMA Intern Med. 2016;176(7):1019-1020.
28. Iams W, Heck J, Kapp M, et al. A multidisciplinary housestaff-led initiative to safely reduce daily laboratory testing. Acad Med. 2016;91(6):813-820. doi: 10.1097/ACM.0000000000001149.
29. Kost A, Genao I, Lee JW, Smith SR. Clinical decisions made in primary care clinics before and after Choosing WiselyTM. J Am Board Fam Med. 2015;28(4):471-474. doi: 10.3122/jabfm.2015.05.140332.
30. Kerr EA, Chen J, Sussman JB, Klamerus ML, Nallamothu BK. Stress testing before low-risk surgery: so many recommendations, so little overuse. JAMA Intern Med. 2015;175(4):645-647. doi: 10.1001/jamainternmed.2014.7877.
31. Colla CH, Morden NE, Sequist TD, Schpero WL, Rosenthal MB. Choosing wisely: prevalence and correlates of low-value health care services in the United States. J Gen Intern Med. 2014;30(2):221-228. doi: 10.1007/s11606-014-3070-z.
32. Shetty KD, Meeker D, Schneider EC, Hussey PS, Damberg CL. Evaluating the feasibility and utility of translating Choosing Wisely recommendations into e-Measures. Healthcare. 2015;3(1):24-37. doi: 10.1016/j.hjdsi.2014.12.002.
33. Woodland DC, Cooper CR, Rashid MF, et al. Routine chest X-ray is unnecessary after ultrasound-guided central venous line placement in the operating room. J Crit Care. 2018;46:13-16. doi: 10.1016/j.jcrc.2018.03.027.
34. Krause TM, Ukhanova M, Revere FL. Private carriers’ physician payment rates compared with Medicare and Medicaid. Tex Med. 2016;112(6):e1.
35. American College of Radiology. Medicare physician fee schedule. Available at https://www.acr.org/Advocacy-and-Economics/Radiology-Economics/Medicare-Medicaid/MPFS. Accessed October 15, 2018.
36. Siegel MD, Rubinowitz AN. Routine daily vs on-demand chest radiographs in intensive care. Lancet. 2009;374(9702):1656-1658. doi: 10.1016/S0140-6736(09)61632-9.
37. Tonna JE, Kawamoto K, Presson AP, et al. Single intervention for a reduction in portable chest radiography (pCXR) in cardiovascular and surgical/trauma ICUs and associated outcomes. J Crit Care. 2018;44:18-23. doi: 10.1016/j.jcrc.2017.10.003.
38. Wolfson D, Santa J, Slass L. Engaging physicians and consumers in conversations about treatment overuse and waste: a short history of the choosing wisely campaign. Acad Med. 2014;89(7):990-995. doi: 10.1097/ACM.0000000000000270.
1. Cassel CK, Guest JA. Choosing Wisely: Helping physicians and patients make smart decisions about their care. JAMA. 2012;307(17):1801-1802.
2. Gonzales R, Cattamanchi A. Changing clinician behavior when less is more. JAMA Intern Med. 2015;175(12):1921-1922.
3. Rosenberg A, Agiro A, Gottlieb M, et al. Early trends among seven recommendations from the Choosing Wisely campaign. JAMA Intern Med. 2015;175(12):1913-1920.
4. Hong AS, Ross-Degnan D, Zhang F, Wharam JF. Small decline in low-value back imaging associated with the ‘Choosing Wisely’ campaign, 2012-14. Health Aff (Millwood). 2017;36(4):671-679. doi: 10.1377/hlthaff.2016.1263.
5. Parks AL, O’Malley PG. From choosing wisely to practicing value—more to the story. JAMA Intern Med. 2016;176(10):1571-1572.
6. Johnson PT, Pahwa AK, Feldman LS, Ziegelstein RC, Hellmann DB. Advancing high-value health care: a new AJM column dedicated to cost-conscious care quality improvement. Am J Med. 2017;130(6):619-621. doi: 10.1016/j.amjmed.2016.12.018.
7. Eccles MP, Mittman BS. Welcome to implementation science. Implement Sci. 2006;1:1. doi: 10.1186/1748-5908-1-1.
8. Bhatia RS, Levinson W, Shortt S, et al. Measuring the effect of Choosing Wisely: an integrated framework to assess campaign impact on low-value care. BMJ Qual Saf. 2015;24:523-531. doi: 10.1136/bmjqs-2015-004070.
9. Selby K, Barnes GD. Learning to de-adopt ineffective healthcare practices. Am J Med. 2018;131(7):721-722. doi: 10.1016/j.amjmed.2018.03.014.
10. Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs. Med Care. 2012;50(3):217-226. doi: 10.1097/MLR.0b013e3182408812.
11. Ganapathy A, Adhikari NK, Spiegelman J, Scales DC. Routine chest x-rays in intensive care units: a systematic review and meta-analysis. Crit Care. 2012;16(2):R68. doi: 10.1186/cc11321.
12. Graat ME, Kröner A, Spronk PE, et al. Elimination of daily routine chest radiographs in a mixed medical-surgical intensive care unit. Intensive Care Med. 2007;33(4):639-644. doi: 10.1007/s00134-007-0542-1.
13. Hendrikse KA, Gratama JWC, Ten Hove W, Rommes JH, Schultz MJ, Spronk PE. Low value of routine chest radiographs in a mixed medical-surgical ICU. Chest. 2007;132(3):823-828. doi: 10.1378/chest.07-1162.
14. Clec’h C, Simon P, Hamdi A, et al. Are daily routine chest radiographs useful in critically ill, mechanically ventilated patients? A randomized study. Intensive Care Med. 2008;34(2):264-270. doi: 10.1007/s00134-007-0919-1.
15. Oba Y, Zaza T. Abandoning daily routine chest radiography in the intensive care unit: meta-analysis. Radiology. 2010;255(2):386-395. doi: 10.1148/radiol.10090946.
16. Mets O, Spronk PE, Binnekade J, Stoker J, de Mol BAJM, Schultz MJ. Elimination of daily routine chest radiographs does not change on-demand radiography practice in post-cardiothoracic surgery patients. J Thorac Cardiovasc Surg. 2007;134(1):139-144. doi: 10.1016/j.jtcvs.2007.02.029.
17. Hejblum G, Chalumeau-Lemoine L, Ioos V, et al. Comparison of routine and on-demand prescription of chest radiographs in mechanically ventilated adults: a multicentre, cluster-randomised, two-period crossover study. Lancet. 2009;374(9702):1687-1693. doi: 10.1016/S0140-6736(09)61459-8.
18. McComb BL, Chung JH, Crabtree TD, et al. ACR appropriateness criteria® routine chest radiography. J Thorac Imaging. 2016;31(2):W13-W15. doi: 10.1097/RTI.0000000000000200.
19. Halpern SD, Becker D, Curtis JR, et al. An official American Thoracic Society/American Association of Critical-Care Nurses/American College of Chest Physicians/Society of Critical Care Medicine policy statement: the Choosing Wisely® top 5 list in critical care medicine. Am J Respir Crit Care Med. 2014;190(7):818-826. doi: 10.1164/rccm.201407-1317ST.
20. Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: Results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10:21. doi: 10.1186/s13012-015-0209-1.
21. R [computer program]. Version 3.4.0. Vienna, Austria: R Foundation for Statistical Computing; 2013.
22. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. doi: 10.1186/1748-5908-4-50.
23. Kirk MA, Kelley C, Yankey N, Birken SA, Abadie B, Damschroder L. A systematic review of the use of the Consolidated Framework for Implementation Research. Implement Sci. 2016;11:72. doi: 10.1186/s13012-016-0437-z.
24. Speroff T, James BC, Nelson EC, Headrick LA, Brommels M, Reed JE. Guidelines for appraisal and publication of PDSA quality improvement. Qual Manag Health Care. 2014;13(1):33-39. doi: 10.1097/00019514-200401000-00003.
25. Corson AH, Fan VS, White T, et al. A multifaceted hospitalist quality improvement intervention: Decreased frequency of common labs. J Hosp Med. 2015;10(6):390-395. doi: 10.1002/jhm.2354.
26. Ferrari R. Evaluation of the Canadian Rheumatology Association Choosing Wisely recommendation concerning anti-nuclear antibody (ANA) testing. Clin Rheumatol. 2015;34(9):1551-1556. doi: 10.1007/s10067-015-2985-z.
27. Ferrari R, Prosser C. Testing vitamin D levels and choosing wisely. JAMA Intern Med. 2016;176(7):1019-1020.
28. Iams W, Heck J, Kapp M, et al. A multidisciplinary housestaff-led initiative to safely reduce daily laboratory testing. Acad Med. 2016;91(6):813-820. doi: 10.1097/ACM.0000000000001149.
29. Kost A, Genao I, Lee JW, Smith SR. Clinical decisions made in primary care clinics before and after Choosing WiselyTM. J Am Board Fam Med. 2015;28(4):471-474. doi: 10.3122/jabfm.2015.05.140332.
30. Kerr EA, Chen J, Sussman JB, Klamerus ML, Nallamothu BK. Stress testing before low-risk surgery: so many recommendations, so little overuse. JAMA Intern Med. 2015;175(4):645-647. doi: 10.1001/jamainternmed.2014.7877.
31. Colla CH, Morden NE, Sequist TD, Schpero WL, Rosenthal MB. Choosing wisely: prevalence and correlates of low-value health care services in the United States. J Gen Intern Med. 2014;30(2):221-228. doi: 10.1007/s11606-014-3070-z.
32. Shetty KD, Meeker D, Schneider EC, Hussey PS, Damberg CL. Evaluating the feasibility and utility of translating Choosing Wisely recommendations into e-Measures. Healthcare. 2015;3(1):24-37. doi: 10.1016/j.hjdsi.2014.12.002.
33. Woodland DC, Cooper CR, Rashid MF, et al. Routine chest X-ray is unnecessary after ultrasound-guided central venous line placement in the operating room. J Crit Care. 2018;46:13-16. doi: 10.1016/j.jcrc.2018.03.027.
34. Krause TM, Ukhanova M, Revere FL. Private carriers’ physician payment rates compared with Medicare and Medicaid. Tex Med. 2016;112(6):e1.
35. American College of Radiology. Medicare physician fee schedule. Available at https://www.acr.org/Advocacy-and-Economics/Radiology-Economics/Medicare-Medicaid/MPFS. Accessed October 15, 2018.
36. Siegel MD, Rubinowitz AN. Routine daily vs on-demand chest radiographs in intensive care. Lancet. 2009;374(9702):1656-1658. doi: 10.1016/S0140-6736(09)61632-9.
37. Tonna JE, Kawamoto K, Presson AP, et al. Single intervention for a reduction in portable chest radiography (pCXR) in cardiovascular and surgical/trauma ICUs and associated outcomes. J Crit Care. 2018;44:18-23. doi: 10.1016/j.jcrc.2017.10.003.
38. Wolfson D, Santa J, Slass L. Engaging physicians and consumers in conversations about treatment overuse and waste: a short history of the choosing wisely campaign. Acad Med. 2014;89(7):990-995. doi: 10.1097/ACM.0000000000000270.
© 2019 Society of Hospital Medicine
Association of Weekend Admission and Weekend Discharge with Length of Stay and 30-Day Readmission in Children’s Hospitals
Increasingly, metrics such as length of stay (LOS) and readmissions are being utilized in the United States to assess quality of healthcare because these factors may represent opportunities to reduce cost and improve healthcare delivery.1-8 However, the relatively low rate of pediatric readmissions,9 coupled with limited data regarding recommended LOS or best practices to prevent readmissions in children, challenges the ability of hospitals to safely reduce LOS and readmission rates for children.10–12
In adults, weekend admission is associated with prolonged LOS, increased readmission rates, and increased risk of mortality.13-21 This association is referred to as the “weekend effect.” While the weekend effect has been examined in children, the results of these studies have been variable, with some studies supporting this association and others refuting it.22-31 In contrast to patient demographic and clinical characteristics that are known to affect LOS and readmissions,32 the weekend effect represents a potentially modifiable aspect of a hospitalization that could be targeted to improve healthcare delivery.
With increasing national attention toward improving quality of care and reducing LOS and healthcare costs, more definitive evidence of the weekend effect is necessary to prioritize resource use at both the local and national levels. Therefore, we sought to determine the association of weekend admission and weekend discharge on LOS and 30-day readmissions, respectively, among a national cohort of children. We hypothesized that children admitted on the weekend would have longer LOS, whereas those discharged on the weekend would have higher readmission rates.
METHODS
Study Design and Data Source
We conducted a multicenter, retrospective, cross-sectional study. Data were obtained from the Pediatric Health Information System (PHIS), an administrative and billing database of 46 free-standing tertiary care pediatric hospitals affiliated with the Children’s Hospital Association (Lenexa, Kansas). Patient data are de-identified within PHIS; however, encrypted patient identifiers allow individual patients to be followed across visits. This study was not considered human subjects research by the policies of the Cincinnati Children’s Hospital Institutional Review Board.
Participants
We included hospitalizations to a PHIS-participating hospital for children aged 0-17 years between October 1, 2014 and September 30, 2015. We excluded children who were transferred from/to another institution, left against medical advice, or died in the hospital because these indications may result in incomplete LOS information and would not consistently contribute to readmission rates. We also excluded birth hospitalizations and children admitted for planned procedures. Birth hospitalizations were defined as hospitalizations that began on the day of birth.
Main Exposures
No standard definition of weekend admission or discharge was identified in the literature.33 Thus, we defined a weekend admission as an admission between 3:00
Main Outcomes
Our outcomes included LOS for weekend admission and 30-day readmissions for weekend discharge. LOS, measured in hours, was defined using the reported admission and discharge times. Readmissions were defined as a return to the same hospital within the subsequent 30 days following discharge.
Patient Demographics and Other Study Variables
Patient demographics included age, gender, race/ethnicity, payer, and median household income quartile based on the patient’s home ZIP code. Other study variables included presence of a complex chronic condition (CCC),34 technology dependence,34 number of chronic conditions of any complexity, admission through the emergency department, intensive care unit (ICU) admission, and case mix index. ICU admission and case mix index were chosen as markers for severity of illness. ICU admission was defined as any child who incurred ICU charges at any time following admission. Case mix index in PHIS is a relative weight assigned to each discharge based on the All-Patient Refined Diagnostic Group (APR-DRG; 3M) assignment and APR-DRG severity of illness, which ranges from 1 (minor) to 4 (extreme). The weights are derived by the Children’s Hospital Association from the HCUP KID 2012 database as the ratio of the average cost for discharges within a specific APR-DRG severity of illness combination to the average cost for all discharges in the database.
Statistical Analysis
Continuous variables were summarized with medians and interquartile ranges, while categorical variables were summarized with frequencies and percentages. Differences in admission and discharge characteristics between weekend and weekday were assessed using Wilcoxon rank sum tests for continuous variables and chi-square tests of association for categorical variables. We used generalized linear mixed modeling (GLMM) techniques to assess the impact of weekend admission on LOS and weekend discharge on readmission, adjusting for important patient demographic and clinical characteristics. Furthermore, we used GLMM point estimates to describe the variation across hospitals of the impact of weekday versus weekend care on LOS and readmissions. We assumed an underlying log-normal distribution for LOS and an underlying binomial distribution for 30-day readmission. All GLMMs included a random intercept for each hospital to account for patient clustering within a hospital. All statistical analyses were performed using SAS v.9.4 (SAS Institute, Cary, North Carolina), and P values <.05 were considered statistically significant.
RESULTS
We identified 390,745 hospitalizations that met inclusion criteria (Supplementary Figure 1). The median LOS among our cohort was 41 hours (interquartile range [IQR] 24-71) and the median 30-day readmission rate was 8.2% (IQR 7.2-9.4).
Admission Demographics for Weekends and Weekdays
Among the included hospitalizations, 92,266 (23.6%) admissions occurred on a weekend (Supplementary Table 1). Overall, a higher percentage of children <5 years of age were admitted on a weekend compared with those admitted on a weekday (53.3% vs 49.1%, P < .001). We observed a small but statistically significant difference in the proportion of weekend versus weekday admissions according to gender, race/ethnicity, payer, and median household income quartile. Children with medical complexity and those with technology dependence were admitted less frequently on a weekend. A higher proportion of children were admitted through the emergency department on a weekend and a higher frequency of ICU utilization was observed for children admitted on a weekend compared with those admitted on a weekday.
Association Between Study Variables and Length of Stay
In comparing adjusted LOS for weekend versus weekday admissions across 43 hospitals, not only did LOS vary across hospitals (P < .001), but the association between LOS and weekend versus weekday care also varied across hospitals (P < .001) (Figure 1). Weekend admission was associated with a significantly longer LOS at eight (18.6%) hospitals and a significantly shorter LOS at four (9.3%) hospitals with nonstatistically significant differences at the remaining hospitals.
In adjusted analyses, we observed that infants ≤30 days of age, on average, had an adjusted LOS that was 24% longer than that of 15- to 17-year-olds, while children aged 1-14 years had an adjusted LOS that was 6%-18% shorter (Table 1). ICU utilization, admission through the emergency department, and number of chronic conditions had the greatest association with LOS. As the number of chronic conditions increased, the LOS increased. No association was found between weekend versus weekday admission and LOS (adjusted LOS [95% CI]: weekend 63.70 [61.01-66.52] hours versus weekday 63.40 [60.73-66.19] hours, P = .112).
Discharge Demographics for Weekends and Weekdays
Of the included hospitalizations, 127,421 (32.6%) discharges occurred on a weekend (Supplementary Table 2). Overall, a greater percentage of weekend discharges comprised children <5 years of age compared with the percentage of weekday discharges for children <5 years of age (51.5% vs 49.5%, P < .001). No statistically significant differences were found in gender, payer, or median household income quartile between those children discharged on a weekend versus those discharged on a weekday. We found small, statistically significant differences in the proportion of weekend versus weekday discharges according to race/ethnicity, with fewer non-Hispanic white children being discharged on the weekend versus weekday. Children with medical complexity, technology dependence, and patients with ICU utilization were less frequently discharged on a weekend compared with those discharged on a weekday.
Association Between Study Variables and Readmissions
In comparing the adjusted odds of readmissions for weekend versus weekday discharges across 43 PHIS hospitals, we observed significant variation (P < .001) in readmission rates from hospital to hospital (Figure 2). However, the direction of impact of weekend care on readmissions was similar (P = .314) across hospitals (ie, for 37 of 43 hospitals, the readmission rate was greater for weekend discharges compared with that for weekday discharges). For 17 (39.5%) of 43 hospitals, weekend discharge was associated with a significantly higher readmission rate, while the differences between weekday and weekend discharge were not statistically significant for the remaining hospitals.
In adjusted analyses, we observed that infants <1 year were more likely to be readmitted compared with 15- to 17-year-olds, while children 5-14 years of age were less likely to be readmitted (Table 2). Medical complexity and the number of chronic conditions had the greatest association with readmissions, with increased likelihood of readmission observed as the number of chronic conditions increased. Weekend discharge was associated with increased probability of readmission compared with weekday discharge (adjusted probability of readmission [95% CI]: weekend 0.13 [0.12-0.13] vs weekday 0.11 [0.11-0.12], P < .001).
DISCUSSION
While the reasons for the weekend effect are unclear, data supporting this difference have been observed across many diverse patient groups and health systems both nationally and internationally.13-27,31 Weekend care is thought to differ from weekday care because of differences in physician and nurse staffing, availability of ancillary services, access to diagnostic testing and therapeutic interventions, ability to arrange outpatient follow-up, and individual patient clinical factors, including acuity of illness. Few studies have assessed the effect of weekend discharges on patient or system outcomes. Among children within a single health system, readmission risk was associated with weekend admission but not with weekend discharge.22 This observation suggests that if differential care exists, then it occurs during initial clinical management rather than during discharge planning. Consequently, understanding the interaction of weekend admission and LOS is important. In addition, the relative paucity of pediatric data examining a weekend discharge effect limits the ability to generalize these findings across other hospitals or health systems.
In contrast to prior work, we observed a modest increased risk for readmission among those discharged on the weekend in a large sample of children. Auger and Davis reported a lack of association between weekend discharge and readmissions at one tertiary care children’s hospital, citing reduced discharge volumes on the weekend, especially among children with medical complexity, as a possible driver for their observation.22 The inclusion of a much larger population across 43 hospitals in our study may explain our different findings compared with previous research. In addition, the inclusion/exclusion criteria differed between the two studies; we excluded index admissions for planned procedures in this study (which are more likely to occur during the week), which may have contributed to the differing conclusions. Although Auger and Davis suggest that differences in initial clinical management may be responsible for the weekend effect,22 our observations suggest that discharge planning practices may also contribute to readmission risk. For example, a family’s inability to access compounded medications at a local pharmacy or to access primary care following discharge could reasonably contribute to treatment failure and increased readmission risk. Attention to improving and standardizing discharge practices may alleviate differences in readmission risk among children discharged on a weekend.
Individual patient characteristics greatly influence LOS and readmission risk. Congruent with prior studies, medical complexity and technology dependence were among the factors in our study that had the strongest association with LOS and readmission risk.32 As with prior studies22, we observed that children with medical complexity and technology dependence were less frequently admitted and discharged on a weekend than on a weekday, which suggests that physicians may avoid complicated discharges on the weekend. Children with medical complexity present a unique challenge to physicians when assessing discharge readiness, given that these children frequently require careful coordination of durable medical equipment, obtainment of special medication preparations, and possibly the resumption or establishment of home health services. Notably, we cannot discern from our data what proportion of discharges may be delayed over the weekend secondary to challenges involved in coordinating care for children with medical complexity. Future investigations aimed at assessing physician decision making and discharge readiness in relation to discharge timing among children with medical complexity may establish this relationship more clearly.
We observed substantial variation in LOS and readmission risk across 43 tertiary care children’s hospitals. Since the 1970s, numerous studies have reported worse outcomes among patients admitted on the weekend. While the majority of studies support the weekend effect, several recent studies suggest that patients admitted on the weekend are at no greater risk of adverse outcomes than those admitted during the week.35-37 Our work builds on the existing literature, demonstrating a complex and variable relationship between weekend admission/discharge, LOS, and readmission risk across hospitals. Notably, while many hospitals in our study experienced a significant weekend effect in LOS or readmission risk, only four hospitals experienced a statistically significant weekend effect for both LOS and readmission risk (three hospitals experienced increased risk for both, while one hospital experienced increased readmission risk but decreased LOS). Future investigations of the weekend effect should focus on exploring the differences in admission/discharge practices and staffing patterns of hospitals that did or did not experience a weekend effect.
This study has several limitations
CONCLUSION
In a study of 43 children’s hospitals, children discharged on the weekend had a slightly increased readmission risk compared with children discharged on a weekday. Wide variation in the weekend effect on LOS and readmission risk was evident across hospitals. Individual patient characteristics had a greater impact on LOS and readmission risk than the weekend effect. Future investigations aimed at understanding which factors contribute most strongly to a weekend effect within individual hospitals (eg, differences in institutional admission/discharge practices) may help alleviate the weekend effect and improve healthcare quality.
Acknowledgments
This manuscript resulted from “Paper in a Day,” a Pediatric Research in Inpatient Settings (PRIS) Network-sponsored workshop presented at the Pediatric Hospital Medicine 2017 annual meeting. Workshop participants learned how to ask and answer a health services research question and efficiently prepare a manuscript for publication. The following are the members of the PRIS Network who contributed to this work: Jessica L. Bettenhausen, MD; Rebecca M. Cantu, MD, MPH; Jillian M Cotter, MD; Megan Deisz, MD; Teresa Frazer, MD; Pratichi Goenka, MD; Ashley Jenkins, MD; Kathryn E. Kyler, MD; Janet T. Lau, MD; Brian E. Lee, MD; Christiane Lenzen, MD; Trisha Marshall, MD; John M. Morrison MD, PhD; Lauren Nassetta, MD; Raymond Parlar-Chun, MD; Sonya Tang Girdwood MD, PhD; Tony R Tarchichi, MD; Irina G. Trifonova, MD; Jacqueline M. Walker, MD, MHPE; and Susan C. Walley, MD. See appendix for contact information for members of the PRIS Network
Funding
The authors have no financial relationships relevant to this article to disclose.
Disclosures
The authors have no conflicts of interest to disclose.
1. Crossing the Quality Chasm: The IOM Health Care Quality Initiative : Health and Medicine Division. http://www.nationalacademies.org/hmd/Global/News%20Announcements/Crossing-the-Quality-Chasm-The-IOM-Health-Care-Quality-Initiative.aspx. Accessed November 20, 2017.
2. Institute for Healthcare Improvement: IHI Home Page. http://www.ihi.org:80/Pages/default.aspx. Accessed November 20, 2017.
3. Berry JG, Zaslavsky AM, Toomey SL, et al. Recognizing differences in hospital quality performance for pediatric inpatient care. Pediatrics. 2015;136(2):251-262. doi:10.1542/peds.2014-3131
4. NQF: All-Cause Admissions and Readmissions Measures - Final Report. http://www.qualityforum.org/Publications/2015/04/All-Cause_Admissions_and_Readmissions_Measures_-_Final_Report.aspx. Accessed March 24, 2018.
5. Hospital Inpatient Potentially Preventable Readmissions Information and Reports. https://www.illinois.gov/hfs/MedicalProviders/hospitals/PPRReports/Pages/default.aspx. Accessed November 6, 2016.
6. Potentially Preventable Readmissions in Texas Medicaid and CHIP Programs - Fiscal Year 2013 | Texas Health and Human Services. https://hhs.texas.gov/reports/2016/08/potentially-preventable-readmissions-texas-medicaid-and-chip-programs-fiscal-year. Accessed November 6, 2016.
7. Statewide Planning and Research Cooperative System. http://www.health.ny.gov/statistics/sparcs/sb/. Accessed November 6, 2016.
8. HCA Implements Potentially Preventable Readmission (PPR) Adjustments. Wash State Hosp Assoc. http://www.wsha.org/articles/hca-implements-potentially-preventable-readmission-ppr-adjustments/. Accessed November 8, 2016.
9. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. doi:10.1001/jama.2012.188351 PubMed
10. Bardach NS, Vittinghoff E, Asteria-Peñaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. doi:10.1542/peds.2012-3527 PubMed
11. Berry JG, Blaine K, Rogers J, et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice. JAMA Pediatr. 2014;168(10):955-962; quiz 965-966. doi:10.1001/jamapediatrics.2014.891 PubMed
12. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: seamless transitions and (Re)admissions network. Pediatrics. 2015;135(1):164. doi:10.1542/peds.2014-1887 PubMed
13. Freemantle N, Ray D, McNulty D, et al. Increased mortality associated with weekend hospital admission: a case for expanded seven day services? BMJ. 2015;351:h4596. doi:10.1136/bmj.h4596 PubMed
14. Schilling PL, Campbell DA, Englesbe MJ, Davis MM. A comparison of in-hospital mortality risk conferred by high hospital occupancy, differences in nurse staffing levels, weekend admission, and seasonal influenza. Med Care. 2010;48(3):224-232. doi:10.1097/MLR.0b013e3181c162c0 PubMed
15. Cram P, Hillis SL, Barnett M, Rosenthal GE. Effects of weekend admission and hospital teaching status on in-hospital mortality. Am J Med. 2004;117(3):151-157. doi:10.1016/j.amjmed.2004.02.035 PubMed
16. Zapf MAC, Kothari AN, Markossian T, et al. The “weekend effect” in urgent general operative procedures. Surgery. 2015;158(2):508-514. doi:10.1016/j.surg.2015.02.024 PubMed
17. Freemantle N, Richardson M, Wood J, et al. Weekend hospitalization and additional risk of death: an analysis of inpatient data. J R Soc Med. 2012;105(2):74-84. doi:10.1258/jrsm.2012.120009 PubMed
18. Bell CM, Redelmeier DA. Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345(9):663-668. doi:10.1056/NEJMsa003376 PubMed
19. Coiera E, Wang Y, Magrabi F, Concha OP, Gallego B, Runciman W. Predicting the cumulative risk of death during hospitalization by modeling weekend, weekday and diurnal mortality risks. BMC Health Serv Res. 2014;14:226. doi:10.1186/1472-6963-14-226 PubMed
20. Powell ES, Khare RK, Courtney DM, Feinglass J. The weekend effect for patients with sepsis presenting to the emergency department. J Emerg Med. 2013;45(5):641-648. doi:10.1016/j.jemermed.2013.04.042 PubMed
21. Ananthakrishnan AN, McGinley EL, Saeian K. Outcomes of weekend admissions for upper gastrointestinal hemorrhage: a nationwide analysis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2009;7(3):296-302e1. doi:10.1016/j.cgh.2008.08.013 PubMed
22. Auger KA, Davis MM. Pediatric weekend admission and increased unplanned readmission rates. J Hosp Med. 2015;10(11):743-745. doi:10.1002/jhm.2426 PubMed
23. Goldstein SD, Papandria DJ, Aboagye J, et al. The “weekend effect” in pediatric surgery - increased mortality for children undergoing urgent surgery during the weekend. J Pediatr Surg. 2014;49(7):1087-1091. doi:10.1016/j.jpedsurg.2014.01.001 PubMed
24. Adil MM, Vidal G, Beslow LA. Weekend effect in children with stroke in the nationwide inpatient sample. Stroke. 2016;47(6):1436-1443. doi:10.1161/STROKEAHA.116.013453 PubMed
25. McCrory MC, Spaeder MC, Gower EW, et al. Time of admission to the PICU and mortality. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2017;18(10):915-923. doi:10.1097/PCC.0000000000001268 PubMed
26. Mangold WD. Neonatal mortality by the day of the week in the 1974-75 Arkansas live birth cohort. Am J Public Health. 1981;71(6):601-605. PubMed
27. MacFarlane A. Variations in number of births and perinatal mortality by day of week in England and Wales. Br Med J. 1978;2(6153):1670-1673. PubMed
28. McShane P, Draper ES, McKinney PA, McFadzean J, Parslow RC, Paediatric intensive care audit network (PICANet). Effects of out-of-hours and winter admissions and number of patients per unit on mortality in pediatric intensive care. J Pediatr. 2013;163(4):1039-1044.e5. doi:10.1016/j.jpeds.2013.03.061 PubMed
29. Hixson ED, Davis S, Morris S, Harrison AM. Do weekends or evenings matter in a pediatric intensive care unit? Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2005;6(5):523-530. PubMed
30. Gonzalez KW, Dalton BGA, Weaver KL, Sherman AK, St Peter SD, Snyder CL. Effect of timing of cannulation on outcome for pediatric extracorporeal life support. Pediatr Surg Int. 2016;32(7):665-669. doi:10.1007/s00383-016-3901-6 PubMed
31. Desai V, Gonda D, Ryan SL, et al. The effect of weekend and after-hours surgery on morbidity and mortality rates in pediatric neurosurgery patients. J Neurosurg Pediatr. 2015;16(6):726-731. doi:10.3171/2015.6.PEDS15184 PubMed
32. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. doi:10.1001/jama.2011.122 PubMed
33. Hoshijima H, Takeuchi R, Mihara T, et al. Weekend versus weekday admission and short-term mortality: A meta-analysis of 88 cohort studies including 56,934,649 participants. Medicine (Baltimore). 2017;96(17):e6685. doi:10.1097/MD.0000000000006685 PubMed
34. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. doi:10.1186/1471-2431-14-199 PubMed
35. Li L, Rothwell PM, Oxford Vascular Study. Biases in detection of apparent “weekend effect” on outcome with administrative coding data: population based study of stroke. BMJ. 2016;353:i2648. doi: https://doi.org/10.1136/bmj.i2648 PubMed
36. Bray BD, Cloud GC, James MA, et al. Weekly variation in health-care quality by day and time of admission: a nationwide, registry-based, prospective cohort study of acute stroke care. The Lancet. 2016;388(10040):170-177. doi:10.1016/S0140-6736(16)30443-3 PubMed
37. Ko SQ, Strom JB, Shen C, Yeh RW. Mortality, Length of Stay, and Cost of Weekend Admissions. J Hosp Med. 2018. doi:10.12788/jhm.2906 PubMed
38. Tubbs-Cooley HL, Cimiotti JP, Silber JH, Sloane DM, Aiken LH. An observational study of nurse staffing ratios and hospital readmission among children admitted for common conditions. BMJ Qual Saf. 2013;22(9):735-742. doi:10.1136/bmjqs-2012-001610 PubMed
39. Ong M, Bostrom A, Vidyarthi A, McCulloch C, Auerbach A. House staff team workload and organization effects on patient outcomes in an academic general internal medicine inpatient service. Arch Intern Med. 2007;167(1):47-52. doi:10.1001/archinte.167.1.47 PubMed
Increasingly, metrics such as length of stay (LOS) and readmissions are being utilized in the United States to assess quality of healthcare because these factors may represent opportunities to reduce cost and improve healthcare delivery.1-8 However, the relatively low rate of pediatric readmissions,9 coupled with limited data regarding recommended LOS or best practices to prevent readmissions in children, challenges the ability of hospitals to safely reduce LOS and readmission rates for children.10–12
In adults, weekend admission is associated with prolonged LOS, increased readmission rates, and increased risk of mortality.13-21 This association is referred to as the “weekend effect.” While the weekend effect has been examined in children, the results of these studies have been variable, with some studies supporting this association and others refuting it.22-31 In contrast to patient demographic and clinical characteristics that are known to affect LOS and readmissions,32 the weekend effect represents a potentially modifiable aspect of a hospitalization that could be targeted to improve healthcare delivery.
With increasing national attention toward improving quality of care and reducing LOS and healthcare costs, more definitive evidence of the weekend effect is necessary to prioritize resource use at both the local and national levels. Therefore, we sought to determine the association of weekend admission and weekend discharge on LOS and 30-day readmissions, respectively, among a national cohort of children. We hypothesized that children admitted on the weekend would have longer LOS, whereas those discharged on the weekend would have higher readmission rates.
METHODS
Study Design and Data Source
We conducted a multicenter, retrospective, cross-sectional study. Data were obtained from the Pediatric Health Information System (PHIS), an administrative and billing database of 46 free-standing tertiary care pediatric hospitals affiliated with the Children’s Hospital Association (Lenexa, Kansas). Patient data are de-identified within PHIS; however, encrypted patient identifiers allow individual patients to be followed across visits. This study was not considered human subjects research by the policies of the Cincinnati Children’s Hospital Institutional Review Board.
Participants
We included hospitalizations to a PHIS-participating hospital for children aged 0-17 years between October 1, 2014 and September 30, 2015. We excluded children who were transferred from/to another institution, left against medical advice, or died in the hospital because these indications may result in incomplete LOS information and would not consistently contribute to readmission rates. We also excluded birth hospitalizations and children admitted for planned procedures. Birth hospitalizations were defined as hospitalizations that began on the day of birth.
Main Exposures
No standard definition of weekend admission or discharge was identified in the literature.33 Thus, we defined a weekend admission as an admission between 3:00
Main Outcomes
Our outcomes included LOS for weekend admission and 30-day readmissions for weekend discharge. LOS, measured in hours, was defined using the reported admission and discharge times. Readmissions were defined as a return to the same hospital within the subsequent 30 days following discharge.
Patient Demographics and Other Study Variables
Patient demographics included age, gender, race/ethnicity, payer, and median household income quartile based on the patient’s home ZIP code. Other study variables included presence of a complex chronic condition (CCC),34 technology dependence,34 number of chronic conditions of any complexity, admission through the emergency department, intensive care unit (ICU) admission, and case mix index. ICU admission and case mix index were chosen as markers for severity of illness. ICU admission was defined as any child who incurred ICU charges at any time following admission. Case mix index in PHIS is a relative weight assigned to each discharge based on the All-Patient Refined Diagnostic Group (APR-DRG; 3M) assignment and APR-DRG severity of illness, which ranges from 1 (minor) to 4 (extreme). The weights are derived by the Children’s Hospital Association from the HCUP KID 2012 database as the ratio of the average cost for discharges within a specific APR-DRG severity of illness combination to the average cost for all discharges in the database.
Statistical Analysis
Continuous variables were summarized with medians and interquartile ranges, while categorical variables were summarized with frequencies and percentages. Differences in admission and discharge characteristics between weekend and weekday were assessed using Wilcoxon rank sum tests for continuous variables and chi-square tests of association for categorical variables. We used generalized linear mixed modeling (GLMM) techniques to assess the impact of weekend admission on LOS and weekend discharge on readmission, adjusting for important patient demographic and clinical characteristics. Furthermore, we used GLMM point estimates to describe the variation across hospitals of the impact of weekday versus weekend care on LOS and readmissions. We assumed an underlying log-normal distribution for LOS and an underlying binomial distribution for 30-day readmission. All GLMMs included a random intercept for each hospital to account for patient clustering within a hospital. All statistical analyses were performed using SAS v.9.4 (SAS Institute, Cary, North Carolina), and P values <.05 were considered statistically significant.
RESULTS
We identified 390,745 hospitalizations that met inclusion criteria (Supplementary Figure 1). The median LOS among our cohort was 41 hours (interquartile range [IQR] 24-71) and the median 30-day readmission rate was 8.2% (IQR 7.2-9.4).
Admission Demographics for Weekends and Weekdays
Among the included hospitalizations, 92,266 (23.6%) admissions occurred on a weekend (Supplementary Table 1). Overall, a higher percentage of children <5 years of age were admitted on a weekend compared with those admitted on a weekday (53.3% vs 49.1%, P < .001). We observed a small but statistically significant difference in the proportion of weekend versus weekday admissions according to gender, race/ethnicity, payer, and median household income quartile. Children with medical complexity and those with technology dependence were admitted less frequently on a weekend. A higher proportion of children were admitted through the emergency department on a weekend and a higher frequency of ICU utilization was observed for children admitted on a weekend compared with those admitted on a weekday.
Association Between Study Variables and Length of Stay
In comparing adjusted LOS for weekend versus weekday admissions across 43 hospitals, not only did LOS vary across hospitals (P < .001), but the association between LOS and weekend versus weekday care also varied across hospitals (P < .001) (Figure 1). Weekend admission was associated with a significantly longer LOS at eight (18.6%) hospitals and a significantly shorter LOS at four (9.3%) hospitals with nonstatistically significant differences at the remaining hospitals.
In adjusted analyses, we observed that infants ≤30 days of age, on average, had an adjusted LOS that was 24% longer than that of 15- to 17-year-olds, while children aged 1-14 years had an adjusted LOS that was 6%-18% shorter (Table 1). ICU utilization, admission through the emergency department, and number of chronic conditions had the greatest association with LOS. As the number of chronic conditions increased, the LOS increased. No association was found between weekend versus weekday admission and LOS (adjusted LOS [95% CI]: weekend 63.70 [61.01-66.52] hours versus weekday 63.40 [60.73-66.19] hours, P = .112).
Discharge Demographics for Weekends and Weekdays
Of the included hospitalizations, 127,421 (32.6%) discharges occurred on a weekend (Supplementary Table 2). Overall, a greater percentage of weekend discharges comprised children <5 years of age compared with the percentage of weekday discharges for children <5 years of age (51.5% vs 49.5%, P < .001). No statistically significant differences were found in gender, payer, or median household income quartile between those children discharged on a weekend versus those discharged on a weekday. We found small, statistically significant differences in the proportion of weekend versus weekday discharges according to race/ethnicity, with fewer non-Hispanic white children being discharged on the weekend versus weekday. Children with medical complexity, technology dependence, and patients with ICU utilization were less frequently discharged on a weekend compared with those discharged on a weekday.
Association Between Study Variables and Readmissions
In comparing the adjusted odds of readmissions for weekend versus weekday discharges across 43 PHIS hospitals, we observed significant variation (P < .001) in readmission rates from hospital to hospital (Figure 2). However, the direction of impact of weekend care on readmissions was similar (P = .314) across hospitals (ie, for 37 of 43 hospitals, the readmission rate was greater for weekend discharges compared with that for weekday discharges). For 17 (39.5%) of 43 hospitals, weekend discharge was associated with a significantly higher readmission rate, while the differences between weekday and weekend discharge were not statistically significant for the remaining hospitals.
In adjusted analyses, we observed that infants <1 year were more likely to be readmitted compared with 15- to 17-year-olds, while children 5-14 years of age were less likely to be readmitted (Table 2). Medical complexity and the number of chronic conditions had the greatest association with readmissions, with increased likelihood of readmission observed as the number of chronic conditions increased. Weekend discharge was associated with increased probability of readmission compared with weekday discharge (adjusted probability of readmission [95% CI]: weekend 0.13 [0.12-0.13] vs weekday 0.11 [0.11-0.12], P < .001).
DISCUSSION
While the reasons for the weekend effect are unclear, data supporting this difference have been observed across many diverse patient groups and health systems both nationally and internationally.13-27,31 Weekend care is thought to differ from weekday care because of differences in physician and nurse staffing, availability of ancillary services, access to diagnostic testing and therapeutic interventions, ability to arrange outpatient follow-up, and individual patient clinical factors, including acuity of illness. Few studies have assessed the effect of weekend discharges on patient or system outcomes. Among children within a single health system, readmission risk was associated with weekend admission but not with weekend discharge.22 This observation suggests that if differential care exists, then it occurs during initial clinical management rather than during discharge planning. Consequently, understanding the interaction of weekend admission and LOS is important. In addition, the relative paucity of pediatric data examining a weekend discharge effect limits the ability to generalize these findings across other hospitals or health systems.
In contrast to prior work, we observed a modest increased risk for readmission among those discharged on the weekend in a large sample of children. Auger and Davis reported a lack of association between weekend discharge and readmissions at one tertiary care children’s hospital, citing reduced discharge volumes on the weekend, especially among children with medical complexity, as a possible driver for their observation.22 The inclusion of a much larger population across 43 hospitals in our study may explain our different findings compared with previous research. In addition, the inclusion/exclusion criteria differed between the two studies; we excluded index admissions for planned procedures in this study (which are more likely to occur during the week), which may have contributed to the differing conclusions. Although Auger and Davis suggest that differences in initial clinical management may be responsible for the weekend effect,22 our observations suggest that discharge planning practices may also contribute to readmission risk. For example, a family’s inability to access compounded medications at a local pharmacy or to access primary care following discharge could reasonably contribute to treatment failure and increased readmission risk. Attention to improving and standardizing discharge practices may alleviate differences in readmission risk among children discharged on a weekend.
Individual patient characteristics greatly influence LOS and readmission risk. Congruent with prior studies, medical complexity and technology dependence were among the factors in our study that had the strongest association with LOS and readmission risk.32 As with prior studies22, we observed that children with medical complexity and technology dependence were less frequently admitted and discharged on a weekend than on a weekday, which suggests that physicians may avoid complicated discharges on the weekend. Children with medical complexity present a unique challenge to physicians when assessing discharge readiness, given that these children frequently require careful coordination of durable medical equipment, obtainment of special medication preparations, and possibly the resumption or establishment of home health services. Notably, we cannot discern from our data what proportion of discharges may be delayed over the weekend secondary to challenges involved in coordinating care for children with medical complexity. Future investigations aimed at assessing physician decision making and discharge readiness in relation to discharge timing among children with medical complexity may establish this relationship more clearly.
We observed substantial variation in LOS and readmission risk across 43 tertiary care children’s hospitals. Since the 1970s, numerous studies have reported worse outcomes among patients admitted on the weekend. While the majority of studies support the weekend effect, several recent studies suggest that patients admitted on the weekend are at no greater risk of adverse outcomes than those admitted during the week.35-37 Our work builds on the existing literature, demonstrating a complex and variable relationship between weekend admission/discharge, LOS, and readmission risk across hospitals. Notably, while many hospitals in our study experienced a significant weekend effect in LOS or readmission risk, only four hospitals experienced a statistically significant weekend effect for both LOS and readmission risk (three hospitals experienced increased risk for both, while one hospital experienced increased readmission risk but decreased LOS). Future investigations of the weekend effect should focus on exploring the differences in admission/discharge practices and staffing patterns of hospitals that did or did not experience a weekend effect.
This study has several limitations
CONCLUSION
In a study of 43 children’s hospitals, children discharged on the weekend had a slightly increased readmission risk compared with children discharged on a weekday. Wide variation in the weekend effect on LOS and readmission risk was evident across hospitals. Individual patient characteristics had a greater impact on LOS and readmission risk than the weekend effect. Future investigations aimed at understanding which factors contribute most strongly to a weekend effect within individual hospitals (eg, differences in institutional admission/discharge practices) may help alleviate the weekend effect and improve healthcare quality.
Acknowledgments
This manuscript resulted from “Paper in a Day,” a Pediatric Research in Inpatient Settings (PRIS) Network-sponsored workshop presented at the Pediatric Hospital Medicine 2017 annual meeting. Workshop participants learned how to ask and answer a health services research question and efficiently prepare a manuscript for publication. The following are the members of the PRIS Network who contributed to this work: Jessica L. Bettenhausen, MD; Rebecca M. Cantu, MD, MPH; Jillian M Cotter, MD; Megan Deisz, MD; Teresa Frazer, MD; Pratichi Goenka, MD; Ashley Jenkins, MD; Kathryn E. Kyler, MD; Janet T. Lau, MD; Brian E. Lee, MD; Christiane Lenzen, MD; Trisha Marshall, MD; John M. Morrison MD, PhD; Lauren Nassetta, MD; Raymond Parlar-Chun, MD; Sonya Tang Girdwood MD, PhD; Tony R Tarchichi, MD; Irina G. Trifonova, MD; Jacqueline M. Walker, MD, MHPE; and Susan C. Walley, MD. See appendix for contact information for members of the PRIS Network
Funding
The authors have no financial relationships relevant to this article to disclose.
Disclosures
The authors have no conflicts of interest to disclose.
Increasingly, metrics such as length of stay (LOS) and readmissions are being utilized in the United States to assess quality of healthcare because these factors may represent opportunities to reduce cost and improve healthcare delivery.1-8 However, the relatively low rate of pediatric readmissions,9 coupled with limited data regarding recommended LOS or best practices to prevent readmissions in children, challenges the ability of hospitals to safely reduce LOS and readmission rates for children.10–12
In adults, weekend admission is associated with prolonged LOS, increased readmission rates, and increased risk of mortality.13-21 This association is referred to as the “weekend effect.” While the weekend effect has been examined in children, the results of these studies have been variable, with some studies supporting this association and others refuting it.22-31 In contrast to patient demographic and clinical characteristics that are known to affect LOS and readmissions,32 the weekend effect represents a potentially modifiable aspect of a hospitalization that could be targeted to improve healthcare delivery.
With increasing national attention toward improving quality of care and reducing LOS and healthcare costs, more definitive evidence of the weekend effect is necessary to prioritize resource use at both the local and national levels. Therefore, we sought to determine the association of weekend admission and weekend discharge on LOS and 30-day readmissions, respectively, among a national cohort of children. We hypothesized that children admitted on the weekend would have longer LOS, whereas those discharged on the weekend would have higher readmission rates.
METHODS
Study Design and Data Source
We conducted a multicenter, retrospective, cross-sectional study. Data were obtained from the Pediatric Health Information System (PHIS), an administrative and billing database of 46 free-standing tertiary care pediatric hospitals affiliated with the Children’s Hospital Association (Lenexa, Kansas). Patient data are de-identified within PHIS; however, encrypted patient identifiers allow individual patients to be followed across visits. This study was not considered human subjects research by the policies of the Cincinnati Children’s Hospital Institutional Review Board.
Participants
We included hospitalizations to a PHIS-participating hospital for children aged 0-17 years between October 1, 2014 and September 30, 2015. We excluded children who were transferred from/to another institution, left against medical advice, or died in the hospital because these indications may result in incomplete LOS information and would not consistently contribute to readmission rates. We also excluded birth hospitalizations and children admitted for planned procedures. Birth hospitalizations were defined as hospitalizations that began on the day of birth.
Main Exposures
No standard definition of weekend admission or discharge was identified in the literature.33 Thus, we defined a weekend admission as an admission between 3:00
Main Outcomes
Our outcomes included LOS for weekend admission and 30-day readmissions for weekend discharge. LOS, measured in hours, was defined using the reported admission and discharge times. Readmissions were defined as a return to the same hospital within the subsequent 30 days following discharge.
Patient Demographics and Other Study Variables
Patient demographics included age, gender, race/ethnicity, payer, and median household income quartile based on the patient’s home ZIP code. Other study variables included presence of a complex chronic condition (CCC),34 technology dependence,34 number of chronic conditions of any complexity, admission through the emergency department, intensive care unit (ICU) admission, and case mix index. ICU admission and case mix index were chosen as markers for severity of illness. ICU admission was defined as any child who incurred ICU charges at any time following admission. Case mix index in PHIS is a relative weight assigned to each discharge based on the All-Patient Refined Diagnostic Group (APR-DRG; 3M) assignment and APR-DRG severity of illness, which ranges from 1 (minor) to 4 (extreme). The weights are derived by the Children’s Hospital Association from the HCUP KID 2012 database as the ratio of the average cost for discharges within a specific APR-DRG severity of illness combination to the average cost for all discharges in the database.
Statistical Analysis
Continuous variables were summarized with medians and interquartile ranges, while categorical variables were summarized with frequencies and percentages. Differences in admission and discharge characteristics between weekend and weekday were assessed using Wilcoxon rank sum tests for continuous variables and chi-square tests of association for categorical variables. We used generalized linear mixed modeling (GLMM) techniques to assess the impact of weekend admission on LOS and weekend discharge on readmission, adjusting for important patient demographic and clinical characteristics. Furthermore, we used GLMM point estimates to describe the variation across hospitals of the impact of weekday versus weekend care on LOS and readmissions. We assumed an underlying log-normal distribution for LOS and an underlying binomial distribution for 30-day readmission. All GLMMs included a random intercept for each hospital to account for patient clustering within a hospital. All statistical analyses were performed using SAS v.9.4 (SAS Institute, Cary, North Carolina), and P values <.05 were considered statistically significant.
RESULTS
We identified 390,745 hospitalizations that met inclusion criteria (Supplementary Figure 1). The median LOS among our cohort was 41 hours (interquartile range [IQR] 24-71) and the median 30-day readmission rate was 8.2% (IQR 7.2-9.4).
Admission Demographics for Weekends and Weekdays
Among the included hospitalizations, 92,266 (23.6%) admissions occurred on a weekend (Supplementary Table 1). Overall, a higher percentage of children <5 years of age were admitted on a weekend compared with those admitted on a weekday (53.3% vs 49.1%, P < .001). We observed a small but statistically significant difference in the proportion of weekend versus weekday admissions according to gender, race/ethnicity, payer, and median household income quartile. Children with medical complexity and those with technology dependence were admitted less frequently on a weekend. A higher proportion of children were admitted through the emergency department on a weekend and a higher frequency of ICU utilization was observed for children admitted on a weekend compared with those admitted on a weekday.
Association Between Study Variables and Length of Stay
In comparing adjusted LOS for weekend versus weekday admissions across 43 hospitals, not only did LOS vary across hospitals (P < .001), but the association between LOS and weekend versus weekday care also varied across hospitals (P < .001) (Figure 1). Weekend admission was associated with a significantly longer LOS at eight (18.6%) hospitals and a significantly shorter LOS at four (9.3%) hospitals with nonstatistically significant differences at the remaining hospitals.
In adjusted analyses, we observed that infants ≤30 days of age, on average, had an adjusted LOS that was 24% longer than that of 15- to 17-year-olds, while children aged 1-14 years had an adjusted LOS that was 6%-18% shorter (Table 1). ICU utilization, admission through the emergency department, and number of chronic conditions had the greatest association with LOS. As the number of chronic conditions increased, the LOS increased. No association was found between weekend versus weekday admission and LOS (adjusted LOS [95% CI]: weekend 63.70 [61.01-66.52] hours versus weekday 63.40 [60.73-66.19] hours, P = .112).
Discharge Demographics for Weekends and Weekdays
Of the included hospitalizations, 127,421 (32.6%) discharges occurred on a weekend (Supplementary Table 2). Overall, a greater percentage of weekend discharges comprised children <5 years of age compared with the percentage of weekday discharges for children <5 years of age (51.5% vs 49.5%, P < .001). No statistically significant differences were found in gender, payer, or median household income quartile between those children discharged on a weekend versus those discharged on a weekday. We found small, statistically significant differences in the proportion of weekend versus weekday discharges according to race/ethnicity, with fewer non-Hispanic white children being discharged on the weekend versus weekday. Children with medical complexity, technology dependence, and patients with ICU utilization were less frequently discharged on a weekend compared with those discharged on a weekday.
Association Between Study Variables and Readmissions
In comparing the adjusted odds of readmissions for weekend versus weekday discharges across 43 PHIS hospitals, we observed significant variation (P < .001) in readmission rates from hospital to hospital (Figure 2). However, the direction of impact of weekend care on readmissions was similar (P = .314) across hospitals (ie, for 37 of 43 hospitals, the readmission rate was greater for weekend discharges compared with that for weekday discharges). For 17 (39.5%) of 43 hospitals, weekend discharge was associated with a significantly higher readmission rate, while the differences between weekday and weekend discharge were not statistically significant for the remaining hospitals.
In adjusted analyses, we observed that infants <1 year were more likely to be readmitted compared with 15- to 17-year-olds, while children 5-14 years of age were less likely to be readmitted (Table 2). Medical complexity and the number of chronic conditions had the greatest association with readmissions, with increased likelihood of readmission observed as the number of chronic conditions increased. Weekend discharge was associated with increased probability of readmission compared with weekday discharge (adjusted probability of readmission [95% CI]: weekend 0.13 [0.12-0.13] vs weekday 0.11 [0.11-0.12], P < .001).
DISCUSSION
While the reasons for the weekend effect are unclear, data supporting this difference have been observed across many diverse patient groups and health systems both nationally and internationally.13-27,31 Weekend care is thought to differ from weekday care because of differences in physician and nurse staffing, availability of ancillary services, access to diagnostic testing and therapeutic interventions, ability to arrange outpatient follow-up, and individual patient clinical factors, including acuity of illness. Few studies have assessed the effect of weekend discharges on patient or system outcomes. Among children within a single health system, readmission risk was associated with weekend admission but not with weekend discharge.22 This observation suggests that if differential care exists, then it occurs during initial clinical management rather than during discharge planning. Consequently, understanding the interaction of weekend admission and LOS is important. In addition, the relative paucity of pediatric data examining a weekend discharge effect limits the ability to generalize these findings across other hospitals or health systems.
In contrast to prior work, we observed a modest increased risk for readmission among those discharged on the weekend in a large sample of children. Auger and Davis reported a lack of association between weekend discharge and readmissions at one tertiary care children’s hospital, citing reduced discharge volumes on the weekend, especially among children with medical complexity, as a possible driver for their observation.22 The inclusion of a much larger population across 43 hospitals in our study may explain our different findings compared with previous research. In addition, the inclusion/exclusion criteria differed between the two studies; we excluded index admissions for planned procedures in this study (which are more likely to occur during the week), which may have contributed to the differing conclusions. Although Auger and Davis suggest that differences in initial clinical management may be responsible for the weekend effect,22 our observations suggest that discharge planning practices may also contribute to readmission risk. For example, a family’s inability to access compounded medications at a local pharmacy or to access primary care following discharge could reasonably contribute to treatment failure and increased readmission risk. Attention to improving and standardizing discharge practices may alleviate differences in readmission risk among children discharged on a weekend.
Individual patient characteristics greatly influence LOS and readmission risk. Congruent with prior studies, medical complexity and technology dependence were among the factors in our study that had the strongest association with LOS and readmission risk.32 As with prior studies22, we observed that children with medical complexity and technology dependence were less frequently admitted and discharged on a weekend than on a weekday, which suggests that physicians may avoid complicated discharges on the weekend. Children with medical complexity present a unique challenge to physicians when assessing discharge readiness, given that these children frequently require careful coordination of durable medical equipment, obtainment of special medication preparations, and possibly the resumption or establishment of home health services. Notably, we cannot discern from our data what proportion of discharges may be delayed over the weekend secondary to challenges involved in coordinating care for children with medical complexity. Future investigations aimed at assessing physician decision making and discharge readiness in relation to discharge timing among children with medical complexity may establish this relationship more clearly.
We observed substantial variation in LOS and readmission risk across 43 tertiary care children’s hospitals. Since the 1970s, numerous studies have reported worse outcomes among patients admitted on the weekend. While the majority of studies support the weekend effect, several recent studies suggest that patients admitted on the weekend are at no greater risk of adverse outcomes than those admitted during the week.35-37 Our work builds on the existing literature, demonstrating a complex and variable relationship between weekend admission/discharge, LOS, and readmission risk across hospitals. Notably, while many hospitals in our study experienced a significant weekend effect in LOS or readmission risk, only four hospitals experienced a statistically significant weekend effect for both LOS and readmission risk (three hospitals experienced increased risk for both, while one hospital experienced increased readmission risk but decreased LOS). Future investigations of the weekend effect should focus on exploring the differences in admission/discharge practices and staffing patterns of hospitals that did or did not experience a weekend effect.
This study has several limitations
CONCLUSION
In a study of 43 children’s hospitals, children discharged on the weekend had a slightly increased readmission risk compared with children discharged on a weekday. Wide variation in the weekend effect on LOS and readmission risk was evident across hospitals. Individual patient characteristics had a greater impact on LOS and readmission risk than the weekend effect. Future investigations aimed at understanding which factors contribute most strongly to a weekend effect within individual hospitals (eg, differences in institutional admission/discharge practices) may help alleviate the weekend effect and improve healthcare quality.
Acknowledgments
This manuscript resulted from “Paper in a Day,” a Pediatric Research in Inpatient Settings (PRIS) Network-sponsored workshop presented at the Pediatric Hospital Medicine 2017 annual meeting. Workshop participants learned how to ask and answer a health services research question and efficiently prepare a manuscript for publication. The following are the members of the PRIS Network who contributed to this work: Jessica L. Bettenhausen, MD; Rebecca M. Cantu, MD, MPH; Jillian M Cotter, MD; Megan Deisz, MD; Teresa Frazer, MD; Pratichi Goenka, MD; Ashley Jenkins, MD; Kathryn E. Kyler, MD; Janet T. Lau, MD; Brian E. Lee, MD; Christiane Lenzen, MD; Trisha Marshall, MD; John M. Morrison MD, PhD; Lauren Nassetta, MD; Raymond Parlar-Chun, MD; Sonya Tang Girdwood MD, PhD; Tony R Tarchichi, MD; Irina G. Trifonova, MD; Jacqueline M. Walker, MD, MHPE; and Susan C. Walley, MD. See appendix for contact information for members of the PRIS Network
Funding
The authors have no financial relationships relevant to this article to disclose.
Disclosures
The authors have no conflicts of interest to disclose.
1. Crossing the Quality Chasm: The IOM Health Care Quality Initiative : Health and Medicine Division. http://www.nationalacademies.org/hmd/Global/News%20Announcements/Crossing-the-Quality-Chasm-The-IOM-Health-Care-Quality-Initiative.aspx. Accessed November 20, 2017.
2. Institute for Healthcare Improvement: IHI Home Page. http://www.ihi.org:80/Pages/default.aspx. Accessed November 20, 2017.
3. Berry JG, Zaslavsky AM, Toomey SL, et al. Recognizing differences in hospital quality performance for pediatric inpatient care. Pediatrics. 2015;136(2):251-262. doi:10.1542/peds.2014-3131
4. NQF: All-Cause Admissions and Readmissions Measures - Final Report. http://www.qualityforum.org/Publications/2015/04/All-Cause_Admissions_and_Readmissions_Measures_-_Final_Report.aspx. Accessed March 24, 2018.
5. Hospital Inpatient Potentially Preventable Readmissions Information and Reports. https://www.illinois.gov/hfs/MedicalProviders/hospitals/PPRReports/Pages/default.aspx. Accessed November 6, 2016.
6. Potentially Preventable Readmissions in Texas Medicaid and CHIP Programs - Fiscal Year 2013 | Texas Health and Human Services. https://hhs.texas.gov/reports/2016/08/potentially-preventable-readmissions-texas-medicaid-and-chip-programs-fiscal-year. Accessed November 6, 2016.
7. Statewide Planning and Research Cooperative System. http://www.health.ny.gov/statistics/sparcs/sb/. Accessed November 6, 2016.
8. HCA Implements Potentially Preventable Readmission (PPR) Adjustments. Wash State Hosp Assoc. http://www.wsha.org/articles/hca-implements-potentially-preventable-readmission-ppr-adjustments/. Accessed November 8, 2016.
9. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. doi:10.1001/jama.2012.188351 PubMed
10. Bardach NS, Vittinghoff E, Asteria-Peñaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. doi:10.1542/peds.2012-3527 PubMed
11. Berry JG, Blaine K, Rogers J, et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice. JAMA Pediatr. 2014;168(10):955-962; quiz 965-966. doi:10.1001/jamapediatrics.2014.891 PubMed
12. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: seamless transitions and (Re)admissions network. Pediatrics. 2015;135(1):164. doi:10.1542/peds.2014-1887 PubMed
13. Freemantle N, Ray D, McNulty D, et al. Increased mortality associated with weekend hospital admission: a case for expanded seven day services? BMJ. 2015;351:h4596. doi:10.1136/bmj.h4596 PubMed
14. Schilling PL, Campbell DA, Englesbe MJ, Davis MM. A comparison of in-hospital mortality risk conferred by high hospital occupancy, differences in nurse staffing levels, weekend admission, and seasonal influenza. Med Care. 2010;48(3):224-232. doi:10.1097/MLR.0b013e3181c162c0 PubMed
15. Cram P, Hillis SL, Barnett M, Rosenthal GE. Effects of weekend admission and hospital teaching status on in-hospital mortality. Am J Med. 2004;117(3):151-157. doi:10.1016/j.amjmed.2004.02.035 PubMed
16. Zapf MAC, Kothari AN, Markossian T, et al. The “weekend effect” in urgent general operative procedures. Surgery. 2015;158(2):508-514. doi:10.1016/j.surg.2015.02.024 PubMed
17. Freemantle N, Richardson M, Wood J, et al. Weekend hospitalization and additional risk of death: an analysis of inpatient data. J R Soc Med. 2012;105(2):74-84. doi:10.1258/jrsm.2012.120009 PubMed
18. Bell CM, Redelmeier DA. Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345(9):663-668. doi:10.1056/NEJMsa003376 PubMed
19. Coiera E, Wang Y, Magrabi F, Concha OP, Gallego B, Runciman W. Predicting the cumulative risk of death during hospitalization by modeling weekend, weekday and diurnal mortality risks. BMC Health Serv Res. 2014;14:226. doi:10.1186/1472-6963-14-226 PubMed
20. Powell ES, Khare RK, Courtney DM, Feinglass J. The weekend effect for patients with sepsis presenting to the emergency department. J Emerg Med. 2013;45(5):641-648. doi:10.1016/j.jemermed.2013.04.042 PubMed
21. Ananthakrishnan AN, McGinley EL, Saeian K. Outcomes of weekend admissions for upper gastrointestinal hemorrhage: a nationwide analysis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2009;7(3):296-302e1. doi:10.1016/j.cgh.2008.08.013 PubMed
22. Auger KA, Davis MM. Pediatric weekend admission and increased unplanned readmission rates. J Hosp Med. 2015;10(11):743-745. doi:10.1002/jhm.2426 PubMed
23. Goldstein SD, Papandria DJ, Aboagye J, et al. The “weekend effect” in pediatric surgery - increased mortality for children undergoing urgent surgery during the weekend. J Pediatr Surg. 2014;49(7):1087-1091. doi:10.1016/j.jpedsurg.2014.01.001 PubMed
24. Adil MM, Vidal G, Beslow LA. Weekend effect in children with stroke in the nationwide inpatient sample. Stroke. 2016;47(6):1436-1443. doi:10.1161/STROKEAHA.116.013453 PubMed
25. McCrory MC, Spaeder MC, Gower EW, et al. Time of admission to the PICU and mortality. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2017;18(10):915-923. doi:10.1097/PCC.0000000000001268 PubMed
26. Mangold WD. Neonatal mortality by the day of the week in the 1974-75 Arkansas live birth cohort. Am J Public Health. 1981;71(6):601-605. PubMed
27. MacFarlane A. Variations in number of births and perinatal mortality by day of week in England and Wales. Br Med J. 1978;2(6153):1670-1673. PubMed
28. McShane P, Draper ES, McKinney PA, McFadzean J, Parslow RC, Paediatric intensive care audit network (PICANet). Effects of out-of-hours and winter admissions and number of patients per unit on mortality in pediatric intensive care. J Pediatr. 2013;163(4):1039-1044.e5. doi:10.1016/j.jpeds.2013.03.061 PubMed
29. Hixson ED, Davis S, Morris S, Harrison AM. Do weekends or evenings matter in a pediatric intensive care unit? Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2005;6(5):523-530. PubMed
30. Gonzalez KW, Dalton BGA, Weaver KL, Sherman AK, St Peter SD, Snyder CL. Effect of timing of cannulation on outcome for pediatric extracorporeal life support. Pediatr Surg Int. 2016;32(7):665-669. doi:10.1007/s00383-016-3901-6 PubMed
31. Desai V, Gonda D, Ryan SL, et al. The effect of weekend and after-hours surgery on morbidity and mortality rates in pediatric neurosurgery patients. J Neurosurg Pediatr. 2015;16(6):726-731. doi:10.3171/2015.6.PEDS15184 PubMed
32. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. doi:10.1001/jama.2011.122 PubMed
33. Hoshijima H, Takeuchi R, Mihara T, et al. Weekend versus weekday admission and short-term mortality: A meta-analysis of 88 cohort studies including 56,934,649 participants. Medicine (Baltimore). 2017;96(17):e6685. doi:10.1097/MD.0000000000006685 PubMed
34. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. doi:10.1186/1471-2431-14-199 PubMed
35. Li L, Rothwell PM, Oxford Vascular Study. Biases in detection of apparent “weekend effect” on outcome with administrative coding data: population based study of stroke. BMJ. 2016;353:i2648. doi: https://doi.org/10.1136/bmj.i2648 PubMed
36. Bray BD, Cloud GC, James MA, et al. Weekly variation in health-care quality by day and time of admission: a nationwide, registry-based, prospective cohort study of acute stroke care. The Lancet. 2016;388(10040):170-177. doi:10.1016/S0140-6736(16)30443-3 PubMed
37. Ko SQ, Strom JB, Shen C, Yeh RW. Mortality, Length of Stay, and Cost of Weekend Admissions. J Hosp Med. 2018. doi:10.12788/jhm.2906 PubMed
38. Tubbs-Cooley HL, Cimiotti JP, Silber JH, Sloane DM, Aiken LH. An observational study of nurse staffing ratios and hospital readmission among children admitted for common conditions. BMJ Qual Saf. 2013;22(9):735-742. doi:10.1136/bmjqs-2012-001610 PubMed
39. Ong M, Bostrom A, Vidyarthi A, McCulloch C, Auerbach A. House staff team workload and organization effects on patient outcomes in an academic general internal medicine inpatient service. Arch Intern Med. 2007;167(1):47-52. doi:10.1001/archinte.167.1.47 PubMed
1. Crossing the Quality Chasm: The IOM Health Care Quality Initiative : Health and Medicine Division. http://www.nationalacademies.org/hmd/Global/News%20Announcements/Crossing-the-Quality-Chasm-The-IOM-Health-Care-Quality-Initiative.aspx. Accessed November 20, 2017.
2. Institute for Healthcare Improvement: IHI Home Page. http://www.ihi.org:80/Pages/default.aspx. Accessed November 20, 2017.
3. Berry JG, Zaslavsky AM, Toomey SL, et al. Recognizing differences in hospital quality performance for pediatric inpatient care. Pediatrics. 2015;136(2):251-262. doi:10.1542/peds.2014-3131
4. NQF: All-Cause Admissions and Readmissions Measures - Final Report. http://www.qualityforum.org/Publications/2015/04/All-Cause_Admissions_and_Readmissions_Measures_-_Final_Report.aspx. Accessed March 24, 2018.
5. Hospital Inpatient Potentially Preventable Readmissions Information and Reports. https://www.illinois.gov/hfs/MedicalProviders/hospitals/PPRReports/Pages/default.aspx. Accessed November 6, 2016.
6. Potentially Preventable Readmissions in Texas Medicaid and CHIP Programs - Fiscal Year 2013 | Texas Health and Human Services. https://hhs.texas.gov/reports/2016/08/potentially-preventable-readmissions-texas-medicaid-and-chip-programs-fiscal-year. Accessed November 6, 2016.
7. Statewide Planning and Research Cooperative System. http://www.health.ny.gov/statistics/sparcs/sb/. Accessed November 6, 2016.
8. HCA Implements Potentially Preventable Readmission (PPR) Adjustments. Wash State Hosp Assoc. http://www.wsha.org/articles/hca-implements-potentially-preventable-readmission-ppr-adjustments/. Accessed November 8, 2016.
9. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. doi:10.1001/jama.2012.188351 PubMed
10. Bardach NS, Vittinghoff E, Asteria-Peñaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. doi:10.1542/peds.2012-3527 PubMed
11. Berry JG, Blaine K, Rogers J, et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice. JAMA Pediatr. 2014;168(10):955-962; quiz 965-966. doi:10.1001/jamapediatrics.2014.891 PubMed
12. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: seamless transitions and (Re)admissions network. Pediatrics. 2015;135(1):164. doi:10.1542/peds.2014-1887 PubMed
13. Freemantle N, Ray D, McNulty D, et al. Increased mortality associated with weekend hospital admission: a case for expanded seven day services? BMJ. 2015;351:h4596. doi:10.1136/bmj.h4596 PubMed
14. Schilling PL, Campbell DA, Englesbe MJ, Davis MM. A comparison of in-hospital mortality risk conferred by high hospital occupancy, differences in nurse staffing levels, weekend admission, and seasonal influenza. Med Care. 2010;48(3):224-232. doi:10.1097/MLR.0b013e3181c162c0 PubMed
15. Cram P, Hillis SL, Barnett M, Rosenthal GE. Effects of weekend admission and hospital teaching status on in-hospital mortality. Am J Med. 2004;117(3):151-157. doi:10.1016/j.amjmed.2004.02.035 PubMed
16. Zapf MAC, Kothari AN, Markossian T, et al. The “weekend effect” in urgent general operative procedures. Surgery. 2015;158(2):508-514. doi:10.1016/j.surg.2015.02.024 PubMed
17. Freemantle N, Richardson M, Wood J, et al. Weekend hospitalization and additional risk of death: an analysis of inpatient data. J R Soc Med. 2012;105(2):74-84. doi:10.1258/jrsm.2012.120009 PubMed
18. Bell CM, Redelmeier DA. Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345(9):663-668. doi:10.1056/NEJMsa003376 PubMed
19. Coiera E, Wang Y, Magrabi F, Concha OP, Gallego B, Runciman W. Predicting the cumulative risk of death during hospitalization by modeling weekend, weekday and diurnal mortality risks. BMC Health Serv Res. 2014;14:226. doi:10.1186/1472-6963-14-226 PubMed
20. Powell ES, Khare RK, Courtney DM, Feinglass J. The weekend effect for patients with sepsis presenting to the emergency department. J Emerg Med. 2013;45(5):641-648. doi:10.1016/j.jemermed.2013.04.042 PubMed
21. Ananthakrishnan AN, McGinley EL, Saeian K. Outcomes of weekend admissions for upper gastrointestinal hemorrhage: a nationwide analysis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2009;7(3):296-302e1. doi:10.1016/j.cgh.2008.08.013 PubMed
22. Auger KA, Davis MM. Pediatric weekend admission and increased unplanned readmission rates. J Hosp Med. 2015;10(11):743-745. doi:10.1002/jhm.2426 PubMed
23. Goldstein SD, Papandria DJ, Aboagye J, et al. The “weekend effect” in pediatric surgery - increased mortality for children undergoing urgent surgery during the weekend. J Pediatr Surg. 2014;49(7):1087-1091. doi:10.1016/j.jpedsurg.2014.01.001 PubMed
24. Adil MM, Vidal G, Beslow LA. Weekend effect in children with stroke in the nationwide inpatient sample. Stroke. 2016;47(6):1436-1443. doi:10.1161/STROKEAHA.116.013453 PubMed
25. McCrory MC, Spaeder MC, Gower EW, et al. Time of admission to the PICU and mortality. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2017;18(10):915-923. doi:10.1097/PCC.0000000000001268 PubMed
26. Mangold WD. Neonatal mortality by the day of the week in the 1974-75 Arkansas live birth cohort. Am J Public Health. 1981;71(6):601-605. PubMed
27. MacFarlane A. Variations in number of births and perinatal mortality by day of week in England and Wales. Br Med J. 1978;2(6153):1670-1673. PubMed
28. McShane P, Draper ES, McKinney PA, McFadzean J, Parslow RC, Paediatric intensive care audit network (PICANet). Effects of out-of-hours and winter admissions and number of patients per unit on mortality in pediatric intensive care. J Pediatr. 2013;163(4):1039-1044.e5. doi:10.1016/j.jpeds.2013.03.061 PubMed
29. Hixson ED, Davis S, Morris S, Harrison AM. Do weekends or evenings matter in a pediatric intensive care unit? Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2005;6(5):523-530. PubMed
30. Gonzalez KW, Dalton BGA, Weaver KL, Sherman AK, St Peter SD, Snyder CL. Effect of timing of cannulation on outcome for pediatric extracorporeal life support. Pediatr Surg Int. 2016;32(7):665-669. doi:10.1007/s00383-016-3901-6 PubMed
31. Desai V, Gonda D, Ryan SL, et al. The effect of weekend and after-hours surgery on morbidity and mortality rates in pediatric neurosurgery patients. J Neurosurg Pediatr. 2015;16(6):726-731. doi:10.3171/2015.6.PEDS15184 PubMed
32. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. doi:10.1001/jama.2011.122 PubMed
33. Hoshijima H, Takeuchi R, Mihara T, et al. Weekend versus weekday admission and short-term mortality: A meta-analysis of 88 cohort studies including 56,934,649 participants. Medicine (Baltimore). 2017;96(17):e6685. doi:10.1097/MD.0000000000006685 PubMed
34. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. doi:10.1186/1471-2431-14-199 PubMed
35. Li L, Rothwell PM, Oxford Vascular Study. Biases in detection of apparent “weekend effect” on outcome with administrative coding data: population based study of stroke. BMJ. 2016;353:i2648. doi: https://doi.org/10.1136/bmj.i2648 PubMed
36. Bray BD, Cloud GC, James MA, et al. Weekly variation in health-care quality by day and time of admission: a nationwide, registry-based, prospective cohort study of acute stroke care. The Lancet. 2016;388(10040):170-177. doi:10.1016/S0140-6736(16)30443-3 PubMed
37. Ko SQ, Strom JB, Shen C, Yeh RW. Mortality, Length of Stay, and Cost of Weekend Admissions. J Hosp Med. 2018. doi:10.12788/jhm.2906 PubMed
38. Tubbs-Cooley HL, Cimiotti JP, Silber JH, Sloane DM, Aiken LH. An observational study of nurse staffing ratios and hospital readmission among children admitted for common conditions. BMJ Qual Saf. 2013;22(9):735-742. doi:10.1136/bmjqs-2012-001610 PubMed
39. Ong M, Bostrom A, Vidyarthi A, McCulloch C, Auerbach A. House staff team workload and organization effects on patient outcomes in an academic general internal medicine inpatient service. Arch Intern Med. 2007;167(1):47-52. doi:10.1001/archinte.167.1.47 PubMed
© 2018 Society of Hospital Medicine
Health and Economic Burden of Nonalcoholic Fatty Liver Disease in the United States and Its Impact on Veterans
Nonalcoholic fatty liver disease (NAFLD) is the hepatic manifestation of the metabolic syndrome. NAFLD also is an independent risk factor for cardiovascular disease, type 2 diabetes mellitus (T2DM), chronic kidney disease, cirrhosis, liver cancer, and all-cause mortality.1-3 As the leading cause of liver disease in the US and globally, NAFLD is strongly associated with obesity and metabolic syndrome, with the rising prevalence of NAFLD closely mirroring the epidemic of obesity and T2DM.4,5 The unrelenting increase of NAFLD prevalence has led to a significant rise in associated health care and economic burdens, compounded by the boom in childhood obesity and an aging population. In this review, we will discuss the epidemiology and economic burden of NAFLD in the US and how it affects veteran health.
NAFLD Definition
NAFLD is defined as the presence of > 5% of hepatic steatosis in the absence of excessive alcohol use, steatosis-inducing medication, or other concurrent chronic liver diseases.
Compared with patients with NAFL, patients with NASH are at a much higher risk of developing fibrosis (scarring of the liver) and cirrhosis (significant scarring with distorted liver architecture). Patients with either NAFL or NASH, with or without advanced fibrosis, also can develop hepatocellular carcinoma (HCC). Severity of liver fibrosis (ie, fibrosis stage) is the most important predictor of liver-associated mortality and all-cause mortality; those with significant fibrosis (≥ F2 stage of fibrosis) are more likely to die of liver disease or to undergo a liver transplant compared with those with earlier stages of disease (ie, stages 0 to F1). Those with advanced scarring or cirrhosis (≥ F3 stage of fibrosis) exhibit an even higher risk of death or liver transplantation.6
NAFLD is a slow and often progressive disease. Time to progression between each stage of fibrosis is about 7 years; however, there has been a documented subset of patients with rapid progression to advanced fibrosis.7 The risk factors associated with this increased risk of fibrosis progression remain poorly understood.
Prevalence
The prevalence of NAFLD in the US is about 24% to 26%—about 85 million Americans. Up to 20% to 30% of these cases (about 17-25 million Americans) are thought to have NASH (Figure 2).
Although liver biopsy remains the current gold standard for diagnosis and histopathologic staging of NAFLD, alternatives to liver biopsy include elastography techniques (ie, transient elastography using Fibroscan[Paris, France], shear wave elastography using Supersonic Image Aixplorer [Weston, FL], and magnetic resonance elastography), magnetic resonance spectroscopy, liver enzymes, and noninvasive simple and complex (serologic) scoring systems such as the Fatty Liver Index. Among these, liver enzymes and serologic scores are most likely to underestimate NAFLD disease burden. Transient elastographyis widely used because the test is easy to perform, noninvasive, and reliably estimates the degree of liver fibrosis in patients with NAFLD by measuring the speed of a mechanically induced shear wave using pulse-echo ultrasonic acquisitions in a much larger portion of the tissue (about 100 times more than a liver biopsy core). Transient elastography also can objectively quantify the amount of liver fat by measuring a 3.5 MHz ultrasound coefficient of attenuation or controlled attenuation parameter (CAP). This correlates with the degree of liver fat, and a higher CAP level reflects a greater degree of steatosis.
The largest study of US veterans utilized abnormal (ie, elevated) liver enzymes as the diagnostic criteria and reviewed nearly 10 million veterans who were followed between 2003 and 2011. Investigators reported a NAFLD prevalence of 13.6% in this population and noted an overall increase in NAFLD prevalence from 6.3% in 2003 to 17.6% in 2011, which highlights the continued growth in NAFLD clinical burden.10 This study, however, is likely to have underestimated the prevalence of NAFLD among veterans because liver enzymes are often normal among those with NAFLD (ie, low sensitivity), and the prevalence of obesity and T2DM are significantly higher in the veteran population vs the general population.
Incidence
There are limited studies on NAFLD incidence. The largest study of US veterans to date used liver enzymes as its diagnostic criteria and reported an annual NAFLD incidence of 2 to 3 per 100 persons (over 9 years from 2003 to 2011).10 There are a few studies from Asia and Europe, and a recent pooled meta-analysis of these studies reported the incidence of NAFLD in Asia to be 52.3 per 1,000 person-years; the incidence in Western countries was 28 per 1,000 person-years.5 These variances may be explained, in part, to disparities in race/background. For example, Hispanics and South Asians (ie, people from Bangladesh, India, or Pakistan) are at higher risk for NAFLD/NASH.11 These findings reinforce the need for further studies to better estimate the true incidence of NAFLD among veterans.
Chronic Liver Disease, Cirrhosis, and Hepatocellular Carcinoma
The prevalence of NASH cirrhosis also has been evaluated using serologic scores, such as aspartate aminotransferase to platelet ratio index (APRI). The National Health and Nutrition Examination Survey (NHANES) database was reviewed, and data for adults in 2 separate periods were analyzed (1999-2002 and 2009-2012) and the prevalence of NASH cirrhosis was noted to have increased 2.5-fold over the period (0.072% vs 0.178%, P < .05).11 Based on data from the HealthCore Integrated Research Database from 2006 to 2014, about 15% of cirrhosis cases were attributed to NAFLD, and about 24% each were attributed to hepatitis C virus (HCV) and alcoholic liver disease.12 A review of about 60,000 veterans with cirrhosis between 2001 and 2013 revealed a prevalence of NAFLD-related cirrhosis of about 15%, while 48% were attributed to HCV.13 In contrast to the continued increase in NAFLD prevalence, the number of patients with HCV-associated liver disease has been in gradual decline since the advent of direct acting antiviral medications in 2011.12
Based on data from the United Network for Organ Sharing (UNOS), the number of patients awaiting liver transplant due to NAFLD nearly tripled from 2004 to 2013, and in 2013 NAFLD became the second leading disease among waitlisted patients for liver transplantation.14 Dynamic Markov modeling predicts that cases of decompensated NASH cirrhosis (ie, liver failure) will rise by 161%, from about 144,000 to 376,000 cases over the next 15 years.8 These projections predict that NAFLD will overtake HCV as the leading cause of chronic liver disease among patients awaiting a liver transplant and will pose a significant clinical and economic burden in the coming years.
Aside from cirrhosis due to NAFLD, NAFLD-related HCC has been on the rise and has overtaken HCV-related HCC. UNOS data from 2003 to 2015 have shown a 2-fold decline in liver transplantation for HCV-associated HCC; however, the same period saw a 10-fold increase in liver transplantation for NAFLD-associated HCC.15,16 This trend in NAFLD-related HCC is expected to grow from 5,000 to 6,000 cases in 2005 to 45,000 cases by 2025.9 More surprisingly, studies from the US veteran population have reported that patients with NAFLD-related HCC are less likely to have cirrhosis compared with patients with HCV- or alcohol-related HCC.17 Among all US veterans who developed HCC in the absence of cirrhosis between 2005 and 2010, NAFLD and metabolic syndrome seemed to be the leading risk factors for development of HCC.18 These trends raise concern for the rise in noncirrhotic HCC in the NAFLD population and highlight the need to improve current screening guidelines for this subset of patients.
Economic Burden
With such a heavy clinical burden and a projected increase in volume over the next decade, NAFLD is expected to have a similarly exponential impact on the economic burden. A review of 976 Medicare beneficiaries with NAFLD who were hospitalized from January 1, 2010 to December 31, 2010, noted a median annual total payment of about $11,000, with significantly lower payment for patients without cirrhosis compared with those with cirrhosis ($10,146 vs $18,804, P < .01).19 Another review of 29,528 Medicare beneficiaries with NAFLD who sought outpatient care between 2005 and 2010 saw a rise in mean yearly charges in 2005 of $2,624 ± 3,308 to $3,608 ± 5,132 in 2010 (P < .05).20
To place these costs in perspective, Allen and colleagues reviewed a large national claims database of individuals enrolled with private and Medicare advantage health plans.21 Comparing patients with NAFLD with a control matched group with similar metabolic comorbidities the study revealed annual median outpatient care costs of $5,363 for the patients with NAFLD with Medicare advantage plans, which was significantly higher than $4,111 for the control group. Projection models based on similar Medicare beneficiaries estimate a rise in annual US economic burden to $103 billion from direct medical care costs alone and another $188 billion in societal costs related to NAFLD.22 New NASH/antifibrotic therapies are being evaluated in clinical trials and are expected to lead to even higher costs. Given the similarities in the trends of NAFLD prevalence between veterans and the general population, it is anticipated that a similar growth and burden in health care utilization cost will affect the VHA.
Association With Other Chronic Medical Conditions
NAFLD is closely associated with metabolic syndrome (Figure 3). Concurrent diagnosis of NAFLD in patients with existing T2DM is associated with poor glycemic control, progressive diabetic retinopathy, diabetic nephropathy, increased risk of cardiovascular complications, and a 2-fold increase in all-cause mortality.1-3
Similar associations have been described between NAFLD and other metabolic conditions such as obesity, hypertension, hypothyroidism, polycystic ovarian syndrome, and chronic kidney disease.31 Identifying patients with NAFLD may help with screening for the above metabolic diseases because patients with NAFLD (by comparison with patients with non-NAFLD) are at higher risk for diabetic, cardiovascular, and pulmonary complications and may warrant a more intensive treatment approach.
Conclusion
A leading cause of chronic liver disease and cirrhosis in the US, NAFLD is independently associated with metabolic syndrome and all-cause mortality. The number of veterans with NAFLD is expected to grow significantly over the coming years given the ongoing epidemic of adult and childhood obesity and T2DM. It is associated with many other medical conditions, including cardiovascular disease and complications, incident metabolic diseases, and may progress to liver cirrhosis and cirrhosis associated complications like HCC and liver failure. The current lack of any approved drug treatment for NASH/fibrosis and the shortage of organs for liver transplant emphasize the need for comprehensive primary prevention measures to reduce the future health and economic costs associated with NAFLD.
There is a growing need to address the epidemic of metabolic syndrome, as heralded by the World Health Organization in its 2013 global action plan. To address this multifaceted disease, initial approach should be to improve NAFLD education among veterans, beginning with the primary care teams and extending to specialty care, including hepatologists. Future steps also should include the development of a comprehensive metabolic/NAFLD clinic in all US Department of Veterans Affairs medical centers that integrates multidisciplinary care, point-of-care evaluation (eg, elastography staging of disease), and access to clinical trials, and have NAFLD care/outcome as a key performance target for all providers.
1. Targher G, Bertolini L, Padovani R, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30(5):1212-1218.
2. Targher G, Bertolini L, Rodella S, et al. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia. 2008;51(3):444-450.
3. Adams LA, Harmsen S, St Sauver JL, et al. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community-based cohort study. Am J Gastroenterol. 2010. 105(7):1567-1573.
4. Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10(11):686-690.
5. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73-84.
6. Angulo P, Machado MV, Diehl AM. Fibrosis in nonalcoholic fatty liver disease: mechanisms and clinical implications. Semin Liver Dis. 2015;35(2):132-145.
7. Satapathy SK, Sanyal AJ. Epidemiology and natural history of nonalcoholic fatty liver disease. Semin Liver Dis. 2015;35(3):221-235.
8. Estes C, Anstee QM, Arias-Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69(4):896-904.
9. Ahmed O, Liu L, Gayed A, et al. The changing face of hepatocellular carcinoma: forecasting prevalence of nonalcoholic steatohepatitis and hepatitis C cirrhosis. J Clin Exp Hepatol. 2018. In press.
10. Kanwal F, Kramer JR, Duan Z, Yu X, White D, El-Serag HB. Trends in the burden of nonalcoholic fatty liver disease in a United States cohort of veterans. Clin Gastroenterol Hepatol. 2016;14(2):301-308.
11. Kabbany MN, Conjeevaram Selvakumar PK, Watt K, et al. Prevalence of nonalcoholic steatohepatitis-associated cirrhosis in the United States: an analysis of national health and nutrition examination survey data. Am J Gastroenterol. 2017;112(4):581-587.
12. Goldberg D, Ditah IC, Saeian K, et al. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology. 2017;152(5):1090-1099.
13. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149(6):1471-1482.
14. Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547-555.
15. Belli LS, Perricone G, Adam R, et al; all the contributing centers (www.eltr.org) and the European Liver and Intestine Transplant Association (ELITA). Impact of DAAs on liver transplantation: major effects on the evolution of indications and results. An ELITA study based on the ELTR registry. J Hepatol. 2018;69(4):810-817.
16. Flemming JA, Kim WR, Brosgart CL, Terrault NA. Reduction in liver transplant wait-listing in the era of direct-acting antiviral therapy. Hepatology. 2017;65(3):804-812.
17. Mittal S, Sada YH, El-Serag HB, et al. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the Veteran Affairs population. Clin Gastroenterol Hepatol. 2015;13(3):594-601.
18. Mittal S, El-Serag HB, Sada YH, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14(1):124-131.e1.
19. Sayiner M, Otgonsuren M, Cable R. Variables associated with inpatient and outpatient resource utilization among medicare beneficiaries with nonalcoholic fatty liver disease with or without cirrhosis. J Clin Gastroenterol. 2017;51(3):254-260.
20. Younossi ZM, Zheng L, Stepanova M, Henry L, Venkatesan C, Mishra A. Trends in outpatient resource utilizations and outcomes for Medicare beneficiaries with nonalcoholic fatty liver disease. J Clin Gastroenterol. 2015;49(3):222-227.
21. Allen AM, Van Houten HK, Sangaralingham LR, Talwalkar JA, McCoy RG. Healthcare cost and utilization in nonalcoholic fatty liver disease: real-world data from a large US claims database. Hepatology. 2018;68(6):2230-2238.
22. Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64(5):1577-1586.
23. Armstrong MJ, Hazlehurst JM, Parker R, et al. Severe asymptomatic non-alcoholic fatty liver disease in routine diabetes care; a multi-disciplinary team approach to diagnosis and management. QJM. 2014;107(1):33-41.
24. Ekstedt M, Franzén LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865-873.
25. Kim D, Choi SY, Park EH, et al. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology. 2012;56(2):605-613.
26. Stepanova M, Younossi ZM. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin Gastroenterol Hepatol. 2012;10(6):646-650.
27. Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363(14):1341-1350.
28. Mir HM, Stepanova M, Afendy H, Cable R, Younossi ZM. Association of sleep disorders with nonalcoholic fatty liver disease (NAFLD): a population-based study. J Clin Exp Hepatol. 2013;3(3):181-185.
29. Agrawal S, Duseja A, Aggarwal A, et al. Obstructive sleep apnea is an important predictor of hepatic fibrosis in patients with nonalcoholic fatty liver disease in a tertiary care center. Hepatol Int. 2015;9(2):283-291.
30. Sookoian S, Pirola CJ. Obstructive sleep apnea is associated with fatty liver and abnormal liver enzymes: a meta-analysis. Obes Surg. 2013;23(11):1815-1825.
31. Armstrong MJ, Adams LA, Canbay A, Syn WK. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology. 2014;59(3):1174-1197.
Nonalcoholic fatty liver disease (NAFLD) is the hepatic manifestation of the metabolic syndrome. NAFLD also is an independent risk factor for cardiovascular disease, type 2 diabetes mellitus (T2DM), chronic kidney disease, cirrhosis, liver cancer, and all-cause mortality.1-3 As the leading cause of liver disease in the US and globally, NAFLD is strongly associated with obesity and metabolic syndrome, with the rising prevalence of NAFLD closely mirroring the epidemic of obesity and T2DM.4,5 The unrelenting increase of NAFLD prevalence has led to a significant rise in associated health care and economic burdens, compounded by the boom in childhood obesity and an aging population. In this review, we will discuss the epidemiology and economic burden of NAFLD in the US and how it affects veteran health.
NAFLD Definition
NAFLD is defined as the presence of > 5% of hepatic steatosis in the absence of excessive alcohol use, steatosis-inducing medication, or other concurrent chronic liver diseases.
Compared with patients with NAFL, patients with NASH are at a much higher risk of developing fibrosis (scarring of the liver) and cirrhosis (significant scarring with distorted liver architecture). Patients with either NAFL or NASH, with or without advanced fibrosis, also can develop hepatocellular carcinoma (HCC). Severity of liver fibrosis (ie, fibrosis stage) is the most important predictor of liver-associated mortality and all-cause mortality; those with significant fibrosis (≥ F2 stage of fibrosis) are more likely to die of liver disease or to undergo a liver transplant compared with those with earlier stages of disease (ie, stages 0 to F1). Those with advanced scarring or cirrhosis (≥ F3 stage of fibrosis) exhibit an even higher risk of death or liver transplantation.6
NAFLD is a slow and often progressive disease. Time to progression between each stage of fibrosis is about 7 years; however, there has been a documented subset of patients with rapid progression to advanced fibrosis.7 The risk factors associated with this increased risk of fibrosis progression remain poorly understood.
Prevalence
The prevalence of NAFLD in the US is about 24% to 26%—about 85 million Americans. Up to 20% to 30% of these cases (about 17-25 million Americans) are thought to have NASH (Figure 2).
Although liver biopsy remains the current gold standard for diagnosis and histopathologic staging of NAFLD, alternatives to liver biopsy include elastography techniques (ie, transient elastography using Fibroscan[Paris, France], shear wave elastography using Supersonic Image Aixplorer [Weston, FL], and magnetic resonance elastography), magnetic resonance spectroscopy, liver enzymes, and noninvasive simple and complex (serologic) scoring systems such as the Fatty Liver Index. Among these, liver enzymes and serologic scores are most likely to underestimate NAFLD disease burden. Transient elastographyis widely used because the test is easy to perform, noninvasive, and reliably estimates the degree of liver fibrosis in patients with NAFLD by measuring the speed of a mechanically induced shear wave using pulse-echo ultrasonic acquisitions in a much larger portion of the tissue (about 100 times more than a liver biopsy core). Transient elastography also can objectively quantify the amount of liver fat by measuring a 3.5 MHz ultrasound coefficient of attenuation or controlled attenuation parameter (CAP). This correlates with the degree of liver fat, and a higher CAP level reflects a greater degree of steatosis.
The largest study of US veterans utilized abnormal (ie, elevated) liver enzymes as the diagnostic criteria and reviewed nearly 10 million veterans who were followed between 2003 and 2011. Investigators reported a NAFLD prevalence of 13.6% in this population and noted an overall increase in NAFLD prevalence from 6.3% in 2003 to 17.6% in 2011, which highlights the continued growth in NAFLD clinical burden.10 This study, however, is likely to have underestimated the prevalence of NAFLD among veterans because liver enzymes are often normal among those with NAFLD (ie, low sensitivity), and the prevalence of obesity and T2DM are significantly higher in the veteran population vs the general population.
Incidence
There are limited studies on NAFLD incidence. The largest study of US veterans to date used liver enzymes as its diagnostic criteria and reported an annual NAFLD incidence of 2 to 3 per 100 persons (over 9 years from 2003 to 2011).10 There are a few studies from Asia and Europe, and a recent pooled meta-analysis of these studies reported the incidence of NAFLD in Asia to be 52.3 per 1,000 person-years; the incidence in Western countries was 28 per 1,000 person-years.5 These variances may be explained, in part, to disparities in race/background. For example, Hispanics and South Asians (ie, people from Bangladesh, India, or Pakistan) are at higher risk for NAFLD/NASH.11 These findings reinforce the need for further studies to better estimate the true incidence of NAFLD among veterans.
Chronic Liver Disease, Cirrhosis, and Hepatocellular Carcinoma
The prevalence of NASH cirrhosis also has been evaluated using serologic scores, such as aspartate aminotransferase to platelet ratio index (APRI). The National Health and Nutrition Examination Survey (NHANES) database was reviewed, and data for adults in 2 separate periods were analyzed (1999-2002 and 2009-2012) and the prevalence of NASH cirrhosis was noted to have increased 2.5-fold over the period (0.072% vs 0.178%, P < .05).11 Based on data from the HealthCore Integrated Research Database from 2006 to 2014, about 15% of cirrhosis cases were attributed to NAFLD, and about 24% each were attributed to hepatitis C virus (HCV) and alcoholic liver disease.12 A review of about 60,000 veterans with cirrhosis between 2001 and 2013 revealed a prevalence of NAFLD-related cirrhosis of about 15%, while 48% were attributed to HCV.13 In contrast to the continued increase in NAFLD prevalence, the number of patients with HCV-associated liver disease has been in gradual decline since the advent of direct acting antiviral medications in 2011.12
Based on data from the United Network for Organ Sharing (UNOS), the number of patients awaiting liver transplant due to NAFLD nearly tripled from 2004 to 2013, and in 2013 NAFLD became the second leading disease among waitlisted patients for liver transplantation.14 Dynamic Markov modeling predicts that cases of decompensated NASH cirrhosis (ie, liver failure) will rise by 161%, from about 144,000 to 376,000 cases over the next 15 years.8 These projections predict that NAFLD will overtake HCV as the leading cause of chronic liver disease among patients awaiting a liver transplant and will pose a significant clinical and economic burden in the coming years.
Aside from cirrhosis due to NAFLD, NAFLD-related HCC has been on the rise and has overtaken HCV-related HCC. UNOS data from 2003 to 2015 have shown a 2-fold decline in liver transplantation for HCV-associated HCC; however, the same period saw a 10-fold increase in liver transplantation for NAFLD-associated HCC.15,16 This trend in NAFLD-related HCC is expected to grow from 5,000 to 6,000 cases in 2005 to 45,000 cases by 2025.9 More surprisingly, studies from the US veteran population have reported that patients with NAFLD-related HCC are less likely to have cirrhosis compared with patients with HCV- or alcohol-related HCC.17 Among all US veterans who developed HCC in the absence of cirrhosis between 2005 and 2010, NAFLD and metabolic syndrome seemed to be the leading risk factors for development of HCC.18 These trends raise concern for the rise in noncirrhotic HCC in the NAFLD population and highlight the need to improve current screening guidelines for this subset of patients.
Economic Burden
With such a heavy clinical burden and a projected increase in volume over the next decade, NAFLD is expected to have a similarly exponential impact on the economic burden. A review of 976 Medicare beneficiaries with NAFLD who were hospitalized from January 1, 2010 to December 31, 2010, noted a median annual total payment of about $11,000, with significantly lower payment for patients without cirrhosis compared with those with cirrhosis ($10,146 vs $18,804, P < .01).19 Another review of 29,528 Medicare beneficiaries with NAFLD who sought outpatient care between 2005 and 2010 saw a rise in mean yearly charges in 2005 of $2,624 ± 3,308 to $3,608 ± 5,132 in 2010 (P < .05).20
To place these costs in perspective, Allen and colleagues reviewed a large national claims database of individuals enrolled with private and Medicare advantage health plans.21 Comparing patients with NAFLD with a control matched group with similar metabolic comorbidities the study revealed annual median outpatient care costs of $5,363 for the patients with NAFLD with Medicare advantage plans, which was significantly higher than $4,111 for the control group. Projection models based on similar Medicare beneficiaries estimate a rise in annual US economic burden to $103 billion from direct medical care costs alone and another $188 billion in societal costs related to NAFLD.22 New NASH/antifibrotic therapies are being evaluated in clinical trials and are expected to lead to even higher costs. Given the similarities in the trends of NAFLD prevalence between veterans and the general population, it is anticipated that a similar growth and burden in health care utilization cost will affect the VHA.
Association With Other Chronic Medical Conditions
NAFLD is closely associated with metabolic syndrome (Figure 3). Concurrent diagnosis of NAFLD in patients with existing T2DM is associated with poor glycemic control, progressive diabetic retinopathy, diabetic nephropathy, increased risk of cardiovascular complications, and a 2-fold increase in all-cause mortality.1-3
Similar associations have been described between NAFLD and other metabolic conditions such as obesity, hypertension, hypothyroidism, polycystic ovarian syndrome, and chronic kidney disease.31 Identifying patients with NAFLD may help with screening for the above metabolic diseases because patients with NAFLD (by comparison with patients with non-NAFLD) are at higher risk for diabetic, cardiovascular, and pulmonary complications and may warrant a more intensive treatment approach.
Conclusion
A leading cause of chronic liver disease and cirrhosis in the US, NAFLD is independently associated with metabolic syndrome and all-cause mortality. The number of veterans with NAFLD is expected to grow significantly over the coming years given the ongoing epidemic of adult and childhood obesity and T2DM. It is associated with many other medical conditions, including cardiovascular disease and complications, incident metabolic diseases, and may progress to liver cirrhosis and cirrhosis associated complications like HCC and liver failure. The current lack of any approved drug treatment for NASH/fibrosis and the shortage of organs for liver transplant emphasize the need for comprehensive primary prevention measures to reduce the future health and economic costs associated with NAFLD.
There is a growing need to address the epidemic of metabolic syndrome, as heralded by the World Health Organization in its 2013 global action plan. To address this multifaceted disease, initial approach should be to improve NAFLD education among veterans, beginning with the primary care teams and extending to specialty care, including hepatologists. Future steps also should include the development of a comprehensive metabolic/NAFLD clinic in all US Department of Veterans Affairs medical centers that integrates multidisciplinary care, point-of-care evaluation (eg, elastography staging of disease), and access to clinical trials, and have NAFLD care/outcome as a key performance target for all providers.
Nonalcoholic fatty liver disease (NAFLD) is the hepatic manifestation of the metabolic syndrome. NAFLD also is an independent risk factor for cardiovascular disease, type 2 diabetes mellitus (T2DM), chronic kidney disease, cirrhosis, liver cancer, and all-cause mortality.1-3 As the leading cause of liver disease in the US and globally, NAFLD is strongly associated with obesity and metabolic syndrome, with the rising prevalence of NAFLD closely mirroring the epidemic of obesity and T2DM.4,5 The unrelenting increase of NAFLD prevalence has led to a significant rise in associated health care and economic burdens, compounded by the boom in childhood obesity and an aging population. In this review, we will discuss the epidemiology and economic burden of NAFLD in the US and how it affects veteran health.
NAFLD Definition
NAFLD is defined as the presence of > 5% of hepatic steatosis in the absence of excessive alcohol use, steatosis-inducing medication, or other concurrent chronic liver diseases.
Compared with patients with NAFL, patients with NASH are at a much higher risk of developing fibrosis (scarring of the liver) and cirrhosis (significant scarring with distorted liver architecture). Patients with either NAFL or NASH, with or without advanced fibrosis, also can develop hepatocellular carcinoma (HCC). Severity of liver fibrosis (ie, fibrosis stage) is the most important predictor of liver-associated mortality and all-cause mortality; those with significant fibrosis (≥ F2 stage of fibrosis) are more likely to die of liver disease or to undergo a liver transplant compared with those with earlier stages of disease (ie, stages 0 to F1). Those with advanced scarring or cirrhosis (≥ F3 stage of fibrosis) exhibit an even higher risk of death or liver transplantation.6
NAFLD is a slow and often progressive disease. Time to progression between each stage of fibrosis is about 7 years; however, there has been a documented subset of patients with rapid progression to advanced fibrosis.7 The risk factors associated with this increased risk of fibrosis progression remain poorly understood.
Prevalence
The prevalence of NAFLD in the US is about 24% to 26%—about 85 million Americans. Up to 20% to 30% of these cases (about 17-25 million Americans) are thought to have NASH (Figure 2).
Although liver biopsy remains the current gold standard for diagnosis and histopathologic staging of NAFLD, alternatives to liver biopsy include elastography techniques (ie, transient elastography using Fibroscan[Paris, France], shear wave elastography using Supersonic Image Aixplorer [Weston, FL], and magnetic resonance elastography), magnetic resonance spectroscopy, liver enzymes, and noninvasive simple and complex (serologic) scoring systems such as the Fatty Liver Index. Among these, liver enzymes and serologic scores are most likely to underestimate NAFLD disease burden. Transient elastographyis widely used because the test is easy to perform, noninvasive, and reliably estimates the degree of liver fibrosis in patients with NAFLD by measuring the speed of a mechanically induced shear wave using pulse-echo ultrasonic acquisitions in a much larger portion of the tissue (about 100 times more than a liver biopsy core). Transient elastography also can objectively quantify the amount of liver fat by measuring a 3.5 MHz ultrasound coefficient of attenuation or controlled attenuation parameter (CAP). This correlates with the degree of liver fat, and a higher CAP level reflects a greater degree of steatosis.
The largest study of US veterans utilized abnormal (ie, elevated) liver enzymes as the diagnostic criteria and reviewed nearly 10 million veterans who were followed between 2003 and 2011. Investigators reported a NAFLD prevalence of 13.6% in this population and noted an overall increase in NAFLD prevalence from 6.3% in 2003 to 17.6% in 2011, which highlights the continued growth in NAFLD clinical burden.10 This study, however, is likely to have underestimated the prevalence of NAFLD among veterans because liver enzymes are often normal among those with NAFLD (ie, low sensitivity), and the prevalence of obesity and T2DM are significantly higher in the veteran population vs the general population.
Incidence
There are limited studies on NAFLD incidence. The largest study of US veterans to date used liver enzymes as its diagnostic criteria and reported an annual NAFLD incidence of 2 to 3 per 100 persons (over 9 years from 2003 to 2011).10 There are a few studies from Asia and Europe, and a recent pooled meta-analysis of these studies reported the incidence of NAFLD in Asia to be 52.3 per 1,000 person-years; the incidence in Western countries was 28 per 1,000 person-years.5 These variances may be explained, in part, to disparities in race/background. For example, Hispanics and South Asians (ie, people from Bangladesh, India, or Pakistan) are at higher risk for NAFLD/NASH.11 These findings reinforce the need for further studies to better estimate the true incidence of NAFLD among veterans.
Chronic Liver Disease, Cirrhosis, and Hepatocellular Carcinoma
The prevalence of NASH cirrhosis also has been evaluated using serologic scores, such as aspartate aminotransferase to platelet ratio index (APRI). The National Health and Nutrition Examination Survey (NHANES) database was reviewed, and data for adults in 2 separate periods were analyzed (1999-2002 and 2009-2012) and the prevalence of NASH cirrhosis was noted to have increased 2.5-fold over the period (0.072% vs 0.178%, P < .05).11 Based on data from the HealthCore Integrated Research Database from 2006 to 2014, about 15% of cirrhosis cases were attributed to NAFLD, and about 24% each were attributed to hepatitis C virus (HCV) and alcoholic liver disease.12 A review of about 60,000 veterans with cirrhosis between 2001 and 2013 revealed a prevalence of NAFLD-related cirrhosis of about 15%, while 48% were attributed to HCV.13 In contrast to the continued increase in NAFLD prevalence, the number of patients with HCV-associated liver disease has been in gradual decline since the advent of direct acting antiviral medications in 2011.12
Based on data from the United Network for Organ Sharing (UNOS), the number of patients awaiting liver transplant due to NAFLD nearly tripled from 2004 to 2013, and in 2013 NAFLD became the second leading disease among waitlisted patients for liver transplantation.14 Dynamic Markov modeling predicts that cases of decompensated NASH cirrhosis (ie, liver failure) will rise by 161%, from about 144,000 to 376,000 cases over the next 15 years.8 These projections predict that NAFLD will overtake HCV as the leading cause of chronic liver disease among patients awaiting a liver transplant and will pose a significant clinical and economic burden in the coming years.
Aside from cirrhosis due to NAFLD, NAFLD-related HCC has been on the rise and has overtaken HCV-related HCC. UNOS data from 2003 to 2015 have shown a 2-fold decline in liver transplantation for HCV-associated HCC; however, the same period saw a 10-fold increase in liver transplantation for NAFLD-associated HCC.15,16 This trend in NAFLD-related HCC is expected to grow from 5,000 to 6,000 cases in 2005 to 45,000 cases by 2025.9 More surprisingly, studies from the US veteran population have reported that patients with NAFLD-related HCC are less likely to have cirrhosis compared with patients with HCV- or alcohol-related HCC.17 Among all US veterans who developed HCC in the absence of cirrhosis between 2005 and 2010, NAFLD and metabolic syndrome seemed to be the leading risk factors for development of HCC.18 These trends raise concern for the rise in noncirrhotic HCC in the NAFLD population and highlight the need to improve current screening guidelines for this subset of patients.
Economic Burden
With such a heavy clinical burden and a projected increase in volume over the next decade, NAFLD is expected to have a similarly exponential impact on the economic burden. A review of 976 Medicare beneficiaries with NAFLD who were hospitalized from January 1, 2010 to December 31, 2010, noted a median annual total payment of about $11,000, with significantly lower payment for patients without cirrhosis compared with those with cirrhosis ($10,146 vs $18,804, P < .01).19 Another review of 29,528 Medicare beneficiaries with NAFLD who sought outpatient care between 2005 and 2010 saw a rise in mean yearly charges in 2005 of $2,624 ± 3,308 to $3,608 ± 5,132 in 2010 (P < .05).20
To place these costs in perspective, Allen and colleagues reviewed a large national claims database of individuals enrolled with private and Medicare advantage health plans.21 Comparing patients with NAFLD with a control matched group with similar metabolic comorbidities the study revealed annual median outpatient care costs of $5,363 for the patients with NAFLD with Medicare advantage plans, which was significantly higher than $4,111 for the control group. Projection models based on similar Medicare beneficiaries estimate a rise in annual US economic burden to $103 billion from direct medical care costs alone and another $188 billion in societal costs related to NAFLD.22 New NASH/antifibrotic therapies are being evaluated in clinical trials and are expected to lead to even higher costs. Given the similarities in the trends of NAFLD prevalence between veterans and the general population, it is anticipated that a similar growth and burden in health care utilization cost will affect the VHA.
Association With Other Chronic Medical Conditions
NAFLD is closely associated with metabolic syndrome (Figure 3). Concurrent diagnosis of NAFLD in patients with existing T2DM is associated with poor glycemic control, progressive diabetic retinopathy, diabetic nephropathy, increased risk of cardiovascular complications, and a 2-fold increase in all-cause mortality.1-3
Similar associations have been described between NAFLD and other metabolic conditions such as obesity, hypertension, hypothyroidism, polycystic ovarian syndrome, and chronic kidney disease.31 Identifying patients with NAFLD may help with screening for the above metabolic diseases because patients with NAFLD (by comparison with patients with non-NAFLD) are at higher risk for diabetic, cardiovascular, and pulmonary complications and may warrant a more intensive treatment approach.
Conclusion
A leading cause of chronic liver disease and cirrhosis in the US, NAFLD is independently associated with metabolic syndrome and all-cause mortality. The number of veterans with NAFLD is expected to grow significantly over the coming years given the ongoing epidemic of adult and childhood obesity and T2DM. It is associated with many other medical conditions, including cardiovascular disease and complications, incident metabolic diseases, and may progress to liver cirrhosis and cirrhosis associated complications like HCC and liver failure. The current lack of any approved drug treatment for NASH/fibrosis and the shortage of organs for liver transplant emphasize the need for comprehensive primary prevention measures to reduce the future health and economic costs associated with NAFLD.
There is a growing need to address the epidemic of metabolic syndrome, as heralded by the World Health Organization in its 2013 global action plan. To address this multifaceted disease, initial approach should be to improve NAFLD education among veterans, beginning with the primary care teams and extending to specialty care, including hepatologists. Future steps also should include the development of a comprehensive metabolic/NAFLD clinic in all US Department of Veterans Affairs medical centers that integrates multidisciplinary care, point-of-care evaluation (eg, elastography staging of disease), and access to clinical trials, and have NAFLD care/outcome as a key performance target for all providers.
1. Targher G, Bertolini L, Padovani R, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30(5):1212-1218.
2. Targher G, Bertolini L, Rodella S, et al. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia. 2008;51(3):444-450.
3. Adams LA, Harmsen S, St Sauver JL, et al. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community-based cohort study. Am J Gastroenterol. 2010. 105(7):1567-1573.
4. Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10(11):686-690.
5. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73-84.
6. Angulo P, Machado MV, Diehl AM. Fibrosis in nonalcoholic fatty liver disease: mechanisms and clinical implications. Semin Liver Dis. 2015;35(2):132-145.
7. Satapathy SK, Sanyal AJ. Epidemiology and natural history of nonalcoholic fatty liver disease. Semin Liver Dis. 2015;35(3):221-235.
8. Estes C, Anstee QM, Arias-Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69(4):896-904.
9. Ahmed O, Liu L, Gayed A, et al. The changing face of hepatocellular carcinoma: forecasting prevalence of nonalcoholic steatohepatitis and hepatitis C cirrhosis. J Clin Exp Hepatol. 2018. In press.
10. Kanwal F, Kramer JR, Duan Z, Yu X, White D, El-Serag HB. Trends in the burden of nonalcoholic fatty liver disease in a United States cohort of veterans. Clin Gastroenterol Hepatol. 2016;14(2):301-308.
11. Kabbany MN, Conjeevaram Selvakumar PK, Watt K, et al. Prevalence of nonalcoholic steatohepatitis-associated cirrhosis in the United States: an analysis of national health and nutrition examination survey data. Am J Gastroenterol. 2017;112(4):581-587.
12. Goldberg D, Ditah IC, Saeian K, et al. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology. 2017;152(5):1090-1099.
13. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149(6):1471-1482.
14. Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547-555.
15. Belli LS, Perricone G, Adam R, et al; all the contributing centers (www.eltr.org) and the European Liver and Intestine Transplant Association (ELITA). Impact of DAAs on liver transplantation: major effects on the evolution of indications and results. An ELITA study based on the ELTR registry. J Hepatol. 2018;69(4):810-817.
16. Flemming JA, Kim WR, Brosgart CL, Terrault NA. Reduction in liver transplant wait-listing in the era of direct-acting antiviral therapy. Hepatology. 2017;65(3):804-812.
17. Mittal S, Sada YH, El-Serag HB, et al. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the Veteran Affairs population. Clin Gastroenterol Hepatol. 2015;13(3):594-601.
18. Mittal S, El-Serag HB, Sada YH, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14(1):124-131.e1.
19. Sayiner M, Otgonsuren M, Cable R. Variables associated with inpatient and outpatient resource utilization among medicare beneficiaries with nonalcoholic fatty liver disease with or without cirrhosis. J Clin Gastroenterol. 2017;51(3):254-260.
20. Younossi ZM, Zheng L, Stepanova M, Henry L, Venkatesan C, Mishra A. Trends in outpatient resource utilizations and outcomes for Medicare beneficiaries with nonalcoholic fatty liver disease. J Clin Gastroenterol. 2015;49(3):222-227.
21. Allen AM, Van Houten HK, Sangaralingham LR, Talwalkar JA, McCoy RG. Healthcare cost and utilization in nonalcoholic fatty liver disease: real-world data from a large US claims database. Hepatology. 2018;68(6):2230-2238.
22. Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64(5):1577-1586.
23. Armstrong MJ, Hazlehurst JM, Parker R, et al. Severe asymptomatic non-alcoholic fatty liver disease in routine diabetes care; a multi-disciplinary team approach to diagnosis and management. QJM. 2014;107(1):33-41.
24. Ekstedt M, Franzén LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865-873.
25. Kim D, Choi SY, Park EH, et al. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology. 2012;56(2):605-613.
26. Stepanova M, Younossi ZM. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin Gastroenterol Hepatol. 2012;10(6):646-650.
27. Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363(14):1341-1350.
28. Mir HM, Stepanova M, Afendy H, Cable R, Younossi ZM. Association of sleep disorders with nonalcoholic fatty liver disease (NAFLD): a population-based study. J Clin Exp Hepatol. 2013;3(3):181-185.
29. Agrawal S, Duseja A, Aggarwal A, et al. Obstructive sleep apnea is an important predictor of hepatic fibrosis in patients with nonalcoholic fatty liver disease in a tertiary care center. Hepatol Int. 2015;9(2):283-291.
30. Sookoian S, Pirola CJ. Obstructive sleep apnea is associated with fatty liver and abnormal liver enzymes: a meta-analysis. Obes Surg. 2013;23(11):1815-1825.
31. Armstrong MJ, Adams LA, Canbay A, Syn WK. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology. 2014;59(3):1174-1197.
1. Targher G, Bertolini L, Padovani R, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30(5):1212-1218.
2. Targher G, Bertolini L, Rodella S, et al. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia. 2008;51(3):444-450.
3. Adams LA, Harmsen S, St Sauver JL, et al. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community-based cohort study. Am J Gastroenterol. 2010. 105(7):1567-1573.
4. Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10(11):686-690.
5. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73-84.
6. Angulo P, Machado MV, Diehl AM. Fibrosis in nonalcoholic fatty liver disease: mechanisms and clinical implications. Semin Liver Dis. 2015;35(2):132-145.
7. Satapathy SK, Sanyal AJ. Epidemiology and natural history of nonalcoholic fatty liver disease. Semin Liver Dis. 2015;35(3):221-235.
8. Estes C, Anstee QM, Arias-Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69(4):896-904.
9. Ahmed O, Liu L, Gayed A, et al. The changing face of hepatocellular carcinoma: forecasting prevalence of nonalcoholic steatohepatitis and hepatitis C cirrhosis. J Clin Exp Hepatol. 2018. In press.
10. Kanwal F, Kramer JR, Duan Z, Yu X, White D, El-Serag HB. Trends in the burden of nonalcoholic fatty liver disease in a United States cohort of veterans. Clin Gastroenterol Hepatol. 2016;14(2):301-308.
11. Kabbany MN, Conjeevaram Selvakumar PK, Watt K, et al. Prevalence of nonalcoholic steatohepatitis-associated cirrhosis in the United States: an analysis of national health and nutrition examination survey data. Am J Gastroenterol. 2017;112(4):581-587.
12. Goldberg D, Ditah IC, Saeian K, et al. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology. 2017;152(5):1090-1099.
13. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149(6):1471-1482.
14. Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547-555.
15. Belli LS, Perricone G, Adam R, et al; all the contributing centers (www.eltr.org) and the European Liver and Intestine Transplant Association (ELITA). Impact of DAAs on liver transplantation: major effects on the evolution of indications and results. An ELITA study based on the ELTR registry. J Hepatol. 2018;69(4):810-817.
16. Flemming JA, Kim WR, Brosgart CL, Terrault NA. Reduction in liver transplant wait-listing in the era of direct-acting antiviral therapy. Hepatology. 2017;65(3):804-812.
17. Mittal S, Sada YH, El-Serag HB, et al. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the Veteran Affairs population. Clin Gastroenterol Hepatol. 2015;13(3):594-601.
18. Mittal S, El-Serag HB, Sada YH, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14(1):124-131.e1.
19. Sayiner M, Otgonsuren M, Cable R. Variables associated with inpatient and outpatient resource utilization among medicare beneficiaries with nonalcoholic fatty liver disease with or without cirrhosis. J Clin Gastroenterol. 2017;51(3):254-260.
20. Younossi ZM, Zheng L, Stepanova M, Henry L, Venkatesan C, Mishra A. Trends in outpatient resource utilizations and outcomes for Medicare beneficiaries with nonalcoholic fatty liver disease. J Clin Gastroenterol. 2015;49(3):222-227.
21. Allen AM, Van Houten HK, Sangaralingham LR, Talwalkar JA, McCoy RG. Healthcare cost and utilization in nonalcoholic fatty liver disease: real-world data from a large US claims database. Hepatology. 2018;68(6):2230-2238.
22. Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64(5):1577-1586.
23. Armstrong MJ, Hazlehurst JM, Parker R, et al. Severe asymptomatic non-alcoholic fatty liver disease in routine diabetes care; a multi-disciplinary team approach to diagnosis and management. QJM. 2014;107(1):33-41.
24. Ekstedt M, Franzén LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865-873.
25. Kim D, Choi SY, Park EH, et al. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology. 2012;56(2):605-613.
26. Stepanova M, Younossi ZM. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin Gastroenterol Hepatol. 2012;10(6):646-650.
27. Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363(14):1341-1350.
28. Mir HM, Stepanova M, Afendy H, Cable R, Younossi ZM. Association of sleep disorders with nonalcoholic fatty liver disease (NAFLD): a population-based study. J Clin Exp Hepatol. 2013;3(3):181-185.
29. Agrawal S, Duseja A, Aggarwal A, et al. Obstructive sleep apnea is an important predictor of hepatic fibrosis in patients with nonalcoholic fatty liver disease in a tertiary care center. Hepatol Int. 2015;9(2):283-291.
30. Sookoian S, Pirola CJ. Obstructive sleep apnea is associated with fatty liver and abnormal liver enzymes: a meta-analysis. Obes Surg. 2013;23(11):1815-1825.
31. Armstrong MJ, Adams LA, Canbay A, Syn WK. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology. 2014;59(3):1174-1197.
Managing the Silent Epidemic of Nonalcoholic Fatty Liver Disease
For many years, viral hepatitis and particularly hepatitis C have been the bread and butter for clinicians dealing with chronic liver diseases. Over the past few years the Veterans Health Administration (VHA) has been incredibly successful in identifying, treating, and curing a significant proportion of veterans of this viral disease. However, nonalcoholic fatty liver disease (NAFLD) has become the most common cause of chronic liver disease worldwide and will soon overtake hepatitis C virus as the leading cause of liver transplantation. NAFLD covers a disease spectrum ranging from nonalcoholic fatty liver (NAFL) progressing to nonalcoholic steatohepatitis (NASH) to liver cirrhosis and liver cancer or liver failure. In the absence of effective treatment approaches, it is not surprising that NAFLD will create financial challenges for the VHA and US health care budgets. It is thus appropriate that Federal Practitioner has decided to publish a series of articles highlighting NAFLD and how it affects millions of Americans on its way to reaching quietly epidemic proportions within the VHA and across the globe.
Although NAFLD seems to have quietly and quickly reached epidemic proportions, its obscurity should not be surprising. NAFLD does not cause obvious symptoms in most patients, there is no simple test available for diagnosis of NASH, and disease-specific medications have not yet been approved for treatment. Primary care providers (PCPs) are the first point of medical contact for a majority of patients with or at risk for NAFLD; shockingly, NAFLD is greatly underrecognized, resulting in delayed diagnoses, which impact both health-related and quality-of-life outcomes in these patients. As emphasized in “Identifying and Treating Nonalcoholic Fatty Liver Disease” by Hunt and colleagues (page 20), PCPs should focus on 4 main aspects related to NAFLD: (1) Does my patient have NAFL? (2) Is my patient at risk for NASH and its ensuing manifestations? (3) Do simple noninvasive serum liver fibrosis markers suggest presence of clinically relevant liver fibrosis? and (4) Does my patient benefit from being referred to a specialist. The PCP is integral in optimally managing medical comorbidities and metabolic abnormalities as well as coordinating intense lifestyle and exercise interventions.
“Health and Economic Burden of Nonalcoholic Fatty Liver Disease in the United States and Its Impact on Veterans” by Shetty and Syn (page 14) discusses the epidemiology and economic burden of NAFLD in the US and how it will affect the health of veterans. Chronic liver disease is a major cause of mortality, morbidity, and health care resource utilization worldwide. Over the past 3 decades, NAFLD has gone from obscure liver diseases to the most common cause of chronic liver disease affecting 25% of the world’s population. Patients with NAFL who have advanced to NASH have an increased risk of liver-specific death. NASH is among the top etiologies for hepatocellular cancer and the fastest growing indication for liver transplantation, projected to overtake hepatitis C virus as the leading cause of liver transplantation. Most disturbing though is the fact that patients with NASH are the least likely to be surveyed for hepatocellular cancer development and the most likely to die while awaiting liver transplantation. Recent modeling estimates a 178% increase in liver deaths related to NASH by 2030.
The clinical burden of all stages of NAFLD is related to its prevalence, incidence, and progressiveness and has to be coupled with its tremendous economic burden based on inpatient, outpatient, professional services, emergency department, and pharmacy costs. It is thus not surprising that we are heading toward a serious health care crisis in the next few decades with the cost of managing NAFLD complications alone approaching an estimated 10-year economic burden of nearly $1 trillion.
The third article by Glass and colleagues (In press) puts the spectrum of NAFLD in the context of a disrupted systemic metabolic environment related to overnutrition alongside reduced physical activity. It is not surprising that type 2 diabetes mellitus (T2DM), obesity, and cardiovascular disease are frequent comorbidities present in a high proportion of patients with NAFLD. The prevalence of NAFLD among people with T2DM exceeds 60%. Importantly, convincing evidence has accumulated supporting the concept that interactions between these metabolic syndrome components and NAFLD are complex and bidirectional. Evidence from cross-sectional and longitudinal studies favors the presence of NAFLD and its severity preceding and/or promoting the development of metabolic comorbidities such as T2DM. Concomitantly, the presence of T2DM seems to accelerate the clinical course of NAFLD and is a predictor of advanced liver fibrosis and mortality. Compared with diseases that have a single etiology, such as viral hepatitis, NAFLD is a very complex disease with multiple interacting metabolic pathways that operate in an individual, leading to the clinical manifestation. Clearly, our present understanding of NAFLD/NASH as a single conglomerate disease is overly simplistic, and further study is warranted.
NAFLD and its variations comprise an increasing number and proportion of referral to hepatologists or providers with experience treating patients with chronic liver disease for the management of advanced disease stages; similarly, PCPs face the challenge to manage early stages of NAFLD. Given the magnitude of the problem of NAFLD, it is imperative that dedicated control efforts at the population level must intensify. As is emphasized in the fourth article of this series (In press), Puri and Fuchs call for a replacement of the traditional health care model of office visits with individual specialist working in silos. To overcome the narrow focus of a subspecialty outpatient clinic, time constraints, and gaps in NAFLD awareness, a patient-centered multidisciplinary approach to the treatment and coordination of care for the medically complex NAFLD patients is needed. The VHA is the largest integrated health care system in the US and is well positioned to implement an organizational strategy to facilitate standardized NAFLD care. The proposed model is centered on a broad assessment of the patient, involving the input from several disciplines; on completion of the assessment, a multidisciplinary team will formulate a personalized intervention plan.
The composition of this multidisciplinary team will vary based on expertise and resources available in each clinical setting. Once an intervention has been started, tracking and monitoring of intermediate and long-term functional outcomes will be helpful to modify the intervention in case outcomes are not achieved. Patient education, from the initial assessment until the intervention phase, plays a critical element to ensure that the patient has sufficient knowledge and skills to achieve the treatment goals set with their health care team.
Ultimately, integration of health care services will lead to better quality of care, increased patient satisfaction, and importantly to improved health care service utilization that will reduce health care resources and costs. Although such a proposal may seem ambitious, it is now the time for innovative thinking that will create sustainable solutions for the silent epidemic of NAFLD. Without advancing a proactive vision, the VA and the world will soon become saddled with an unmanageable economic and health care burden.
For many years, viral hepatitis and particularly hepatitis C have been the bread and butter for clinicians dealing with chronic liver diseases. Over the past few years the Veterans Health Administration (VHA) has been incredibly successful in identifying, treating, and curing a significant proportion of veterans of this viral disease. However, nonalcoholic fatty liver disease (NAFLD) has become the most common cause of chronic liver disease worldwide and will soon overtake hepatitis C virus as the leading cause of liver transplantation. NAFLD covers a disease spectrum ranging from nonalcoholic fatty liver (NAFL) progressing to nonalcoholic steatohepatitis (NASH) to liver cirrhosis and liver cancer or liver failure. In the absence of effective treatment approaches, it is not surprising that NAFLD will create financial challenges for the VHA and US health care budgets. It is thus appropriate that Federal Practitioner has decided to publish a series of articles highlighting NAFLD and how it affects millions of Americans on its way to reaching quietly epidemic proportions within the VHA and across the globe.
Although NAFLD seems to have quietly and quickly reached epidemic proportions, its obscurity should not be surprising. NAFLD does not cause obvious symptoms in most patients, there is no simple test available for diagnosis of NASH, and disease-specific medications have not yet been approved for treatment. Primary care providers (PCPs) are the first point of medical contact for a majority of patients with or at risk for NAFLD; shockingly, NAFLD is greatly underrecognized, resulting in delayed diagnoses, which impact both health-related and quality-of-life outcomes in these patients. As emphasized in “Identifying and Treating Nonalcoholic Fatty Liver Disease” by Hunt and colleagues (page 20), PCPs should focus on 4 main aspects related to NAFLD: (1) Does my patient have NAFL? (2) Is my patient at risk for NASH and its ensuing manifestations? (3) Do simple noninvasive serum liver fibrosis markers suggest presence of clinically relevant liver fibrosis? and (4) Does my patient benefit from being referred to a specialist. The PCP is integral in optimally managing medical comorbidities and metabolic abnormalities as well as coordinating intense lifestyle and exercise interventions.
“Health and Economic Burden of Nonalcoholic Fatty Liver Disease in the United States and Its Impact on Veterans” by Shetty and Syn (page 14) discusses the epidemiology and economic burden of NAFLD in the US and how it will affect the health of veterans. Chronic liver disease is a major cause of mortality, morbidity, and health care resource utilization worldwide. Over the past 3 decades, NAFLD has gone from obscure liver diseases to the most common cause of chronic liver disease affecting 25% of the world’s population. Patients with NAFL who have advanced to NASH have an increased risk of liver-specific death. NASH is among the top etiologies for hepatocellular cancer and the fastest growing indication for liver transplantation, projected to overtake hepatitis C virus as the leading cause of liver transplantation. Most disturbing though is the fact that patients with NASH are the least likely to be surveyed for hepatocellular cancer development and the most likely to die while awaiting liver transplantation. Recent modeling estimates a 178% increase in liver deaths related to NASH by 2030.
The clinical burden of all stages of NAFLD is related to its prevalence, incidence, and progressiveness and has to be coupled with its tremendous economic burden based on inpatient, outpatient, professional services, emergency department, and pharmacy costs. It is thus not surprising that we are heading toward a serious health care crisis in the next few decades with the cost of managing NAFLD complications alone approaching an estimated 10-year economic burden of nearly $1 trillion.
The third article by Glass and colleagues (In press) puts the spectrum of NAFLD in the context of a disrupted systemic metabolic environment related to overnutrition alongside reduced physical activity. It is not surprising that type 2 diabetes mellitus (T2DM), obesity, and cardiovascular disease are frequent comorbidities present in a high proportion of patients with NAFLD. The prevalence of NAFLD among people with T2DM exceeds 60%. Importantly, convincing evidence has accumulated supporting the concept that interactions between these metabolic syndrome components and NAFLD are complex and bidirectional. Evidence from cross-sectional and longitudinal studies favors the presence of NAFLD and its severity preceding and/or promoting the development of metabolic comorbidities such as T2DM. Concomitantly, the presence of T2DM seems to accelerate the clinical course of NAFLD and is a predictor of advanced liver fibrosis and mortality. Compared with diseases that have a single etiology, such as viral hepatitis, NAFLD is a very complex disease with multiple interacting metabolic pathways that operate in an individual, leading to the clinical manifestation. Clearly, our present understanding of NAFLD/NASH as a single conglomerate disease is overly simplistic, and further study is warranted.
NAFLD and its variations comprise an increasing number and proportion of referral to hepatologists or providers with experience treating patients with chronic liver disease for the management of advanced disease stages; similarly, PCPs face the challenge to manage early stages of NAFLD. Given the magnitude of the problem of NAFLD, it is imperative that dedicated control efforts at the population level must intensify. As is emphasized in the fourth article of this series (In press), Puri and Fuchs call for a replacement of the traditional health care model of office visits with individual specialist working in silos. To overcome the narrow focus of a subspecialty outpatient clinic, time constraints, and gaps in NAFLD awareness, a patient-centered multidisciplinary approach to the treatment and coordination of care for the medically complex NAFLD patients is needed. The VHA is the largest integrated health care system in the US and is well positioned to implement an organizational strategy to facilitate standardized NAFLD care. The proposed model is centered on a broad assessment of the patient, involving the input from several disciplines; on completion of the assessment, a multidisciplinary team will formulate a personalized intervention plan.
The composition of this multidisciplinary team will vary based on expertise and resources available in each clinical setting. Once an intervention has been started, tracking and monitoring of intermediate and long-term functional outcomes will be helpful to modify the intervention in case outcomes are not achieved. Patient education, from the initial assessment until the intervention phase, plays a critical element to ensure that the patient has sufficient knowledge and skills to achieve the treatment goals set with their health care team.
Ultimately, integration of health care services will lead to better quality of care, increased patient satisfaction, and importantly to improved health care service utilization that will reduce health care resources and costs. Although such a proposal may seem ambitious, it is now the time for innovative thinking that will create sustainable solutions for the silent epidemic of NAFLD. Without advancing a proactive vision, the VA and the world will soon become saddled with an unmanageable economic and health care burden.
For many years, viral hepatitis and particularly hepatitis C have been the bread and butter for clinicians dealing with chronic liver diseases. Over the past few years the Veterans Health Administration (VHA) has been incredibly successful in identifying, treating, and curing a significant proportion of veterans of this viral disease. However, nonalcoholic fatty liver disease (NAFLD) has become the most common cause of chronic liver disease worldwide and will soon overtake hepatitis C virus as the leading cause of liver transplantation. NAFLD covers a disease spectrum ranging from nonalcoholic fatty liver (NAFL) progressing to nonalcoholic steatohepatitis (NASH) to liver cirrhosis and liver cancer or liver failure. In the absence of effective treatment approaches, it is not surprising that NAFLD will create financial challenges for the VHA and US health care budgets. It is thus appropriate that Federal Practitioner has decided to publish a series of articles highlighting NAFLD and how it affects millions of Americans on its way to reaching quietly epidemic proportions within the VHA and across the globe.
Although NAFLD seems to have quietly and quickly reached epidemic proportions, its obscurity should not be surprising. NAFLD does not cause obvious symptoms in most patients, there is no simple test available for diagnosis of NASH, and disease-specific medications have not yet been approved for treatment. Primary care providers (PCPs) are the first point of medical contact for a majority of patients with or at risk for NAFLD; shockingly, NAFLD is greatly underrecognized, resulting in delayed diagnoses, which impact both health-related and quality-of-life outcomes in these patients. As emphasized in “Identifying and Treating Nonalcoholic Fatty Liver Disease” by Hunt and colleagues (page 20), PCPs should focus on 4 main aspects related to NAFLD: (1) Does my patient have NAFL? (2) Is my patient at risk for NASH and its ensuing manifestations? (3) Do simple noninvasive serum liver fibrosis markers suggest presence of clinically relevant liver fibrosis? and (4) Does my patient benefit from being referred to a specialist. The PCP is integral in optimally managing medical comorbidities and metabolic abnormalities as well as coordinating intense lifestyle and exercise interventions.
“Health and Economic Burden of Nonalcoholic Fatty Liver Disease in the United States and Its Impact on Veterans” by Shetty and Syn (page 14) discusses the epidemiology and economic burden of NAFLD in the US and how it will affect the health of veterans. Chronic liver disease is a major cause of mortality, morbidity, and health care resource utilization worldwide. Over the past 3 decades, NAFLD has gone from obscure liver diseases to the most common cause of chronic liver disease affecting 25% of the world’s population. Patients with NAFL who have advanced to NASH have an increased risk of liver-specific death. NASH is among the top etiologies for hepatocellular cancer and the fastest growing indication for liver transplantation, projected to overtake hepatitis C virus as the leading cause of liver transplantation. Most disturbing though is the fact that patients with NASH are the least likely to be surveyed for hepatocellular cancer development and the most likely to die while awaiting liver transplantation. Recent modeling estimates a 178% increase in liver deaths related to NASH by 2030.
The clinical burden of all stages of NAFLD is related to its prevalence, incidence, and progressiveness and has to be coupled with its tremendous economic burden based on inpatient, outpatient, professional services, emergency department, and pharmacy costs. It is thus not surprising that we are heading toward a serious health care crisis in the next few decades with the cost of managing NAFLD complications alone approaching an estimated 10-year economic burden of nearly $1 trillion.
The third article by Glass and colleagues (In press) puts the spectrum of NAFLD in the context of a disrupted systemic metabolic environment related to overnutrition alongside reduced physical activity. It is not surprising that type 2 diabetes mellitus (T2DM), obesity, and cardiovascular disease are frequent comorbidities present in a high proportion of patients with NAFLD. The prevalence of NAFLD among people with T2DM exceeds 60%. Importantly, convincing evidence has accumulated supporting the concept that interactions between these metabolic syndrome components and NAFLD are complex and bidirectional. Evidence from cross-sectional and longitudinal studies favors the presence of NAFLD and its severity preceding and/or promoting the development of metabolic comorbidities such as T2DM. Concomitantly, the presence of T2DM seems to accelerate the clinical course of NAFLD and is a predictor of advanced liver fibrosis and mortality. Compared with diseases that have a single etiology, such as viral hepatitis, NAFLD is a very complex disease with multiple interacting metabolic pathways that operate in an individual, leading to the clinical manifestation. Clearly, our present understanding of NAFLD/NASH as a single conglomerate disease is overly simplistic, and further study is warranted.
NAFLD and its variations comprise an increasing number and proportion of referral to hepatologists or providers with experience treating patients with chronic liver disease for the management of advanced disease stages; similarly, PCPs face the challenge to manage early stages of NAFLD. Given the magnitude of the problem of NAFLD, it is imperative that dedicated control efforts at the population level must intensify. As is emphasized in the fourth article of this series (In press), Puri and Fuchs call for a replacement of the traditional health care model of office visits with individual specialist working in silos. To overcome the narrow focus of a subspecialty outpatient clinic, time constraints, and gaps in NAFLD awareness, a patient-centered multidisciplinary approach to the treatment and coordination of care for the medically complex NAFLD patients is needed. The VHA is the largest integrated health care system in the US and is well positioned to implement an organizational strategy to facilitate standardized NAFLD care. The proposed model is centered on a broad assessment of the patient, involving the input from several disciplines; on completion of the assessment, a multidisciplinary team will formulate a personalized intervention plan.
The composition of this multidisciplinary team will vary based on expertise and resources available in each clinical setting. Once an intervention has been started, tracking and monitoring of intermediate and long-term functional outcomes will be helpful to modify the intervention in case outcomes are not achieved. Patient education, from the initial assessment until the intervention phase, plays a critical element to ensure that the patient has sufficient knowledge and skills to achieve the treatment goals set with their health care team.
Ultimately, integration of health care services will lead to better quality of care, increased patient satisfaction, and importantly to improved health care service utilization that will reduce health care resources and costs. Although such a proposal may seem ambitious, it is now the time for innovative thinking that will create sustainable solutions for the silent epidemic of NAFLD. Without advancing a proactive vision, the VA and the world will soon become saddled with an unmanageable economic and health care burden.