User login
IRONMAN galvanizes case for IV iron repletion in heart failure
CHICAGO – Another major study appears to back the use of intravenous iron repletion in patients with heart failure (HF) and iron deficiency, strengthening largely consistent evidence, researchers say, that the treatment may improve symptoms and prevent some HF-related hospital admissions.
To be sure, the IRONMAN trial, which compared intravenous iron versus usual care in such patients – most with reduced ejection fraction and not hospitalized – failed to show a benefit for its primary endpoint. The 18% reduction in risk for HF hospitalization or cardiovascular (CV) death seen in the trial, however encouraging, can only be called a trend (P = .07).
But the intervention showed signs of benefit for some secondary endpoints, including quality of life scores, and hinted at such an effect on HF hospitalization. Risk for the latter endpoint dropped 20% (P = .085) over a median follow-up of 2.7 years.
The findings “build upon the other data we have that correcting iron deficiency can help improve well-being, and particularly reduce the risk of hospitalization, in a broad range of [HF] patients,” said Paul Kalra, MD, of the University of Glasgow and Portsmouth (England) Hospitals University NHS Trust.
The tested regimen “was well tolerated with no safety concerns” and offers “reassurance about the long-term safety” of the intravenous iron it used, ferric derisomaltose (MonoFerric), in patients with HF, Dr. Kalra said at a media briefing on the trial.
The remarks preceded his formal presentation of IRONMAN at the American Heart Association scientific sessions. Dr. Kalra is also lead author on the trial’s publication in The Lancet.
IRONMAN strengthens the base of evidence supporting intravenous iron in HF with iron deficiency, especially chronic HF in outpatients, Dr. Kalra and others said. It also supports efficacy for a form of intravenous iron not previously tested in a major HF trial.
Still, “the totality of data are now supporting intravenous iron per se,” regardless of the iron agent used, said Dr. Kalra. But ferric derisomaltose may have dosing advantages, he observed, “and we’ve now got these long-term safety data.”
The strongest prior support for intravenous iron in HF came from hospitalized patients who received it as ferric carboxymaltose (Ferinject) and were followed only 12 months. That was in the AFFIRM-AHF trial, published 2 years ago, which also missed its primary endpoint – the same one used in IRONMAN. Some outcomes in the two trials were similar.
The risk for HF hospitalization or CV death for intravenous iron therapy, compared with usual care, in AFFIRM-AHF fell 21% (P = .059), missing significance but apparently driven by a 26% drop in risk for HF readmissions (P = .013). But neither that trial nor IRONMAN suggested a benefit for CV mortality on its own.
The COVID effect
In IRONMAN, Dr. Kalra said, usual care could include oral iron supplementation, which 17% of patients in the control group received. That could potentially have kept the intravenous iron group from making a better showing for the primary endpoint, he proposed.
And some iron doses and other treatments were missed by a substantial number of patients in both groups who entered the trial after the United Kingdom’s national lockdown in response to the COVID-19 pandemic, he observed. “Patients were not able to come into hospitals for research visits, or in fact when they were able, may not have wanted to.”
So, the group conducted a “prespecified” sensitivity analysis that excluded the 9% of patients enrolled by the end of March 2020, about the time of the first lockdown, and followed the remainder for another 6 months.
In that analysis, risk for HF hospitalization or CV death declined 24% in the intravenous iron group, a marginal but significant result (P = .047) that was dominated by an improvement in HF hospitalizations.
Effects on guidelines
The intravenous iron recommendations in the European HF guidelines refer only to ferric carboxymaltose without mentioning other forms, such as ferric derisomaltose, “but this is now a class effect given the similarities between AFFIRM-AHF and IRONMAN,” said Gregory D. Lewis, MD, Mass General Brigham, Boston, invited discussant for Dr. Kalra’s presentation at the AHA session.
“In the United States, we relegate IV iron to improvement in functional capacity as a comorbidity of heart failure. Perhaps this role will expand,” added Dr. Lewis, who is medical director of his center’s heart transplant program.
He also wondered aloud whether the purported clinical benefits of intravenous iron in HF patients with iron deficiency, not as yet supported by a significant primary-endpoint showing in one of the major trials, currently justify expansion of its use in practice.
“With the benefits of IV iron on exercise capacity and quality of life, and the safety of administering high doses of IV iron,” potentially reducing HF polypharmacy, he noted, “should we be considering IV iron more commonly for utilization in our patients even if we find that heart failure hospitalizations and mortality are only modestly improved?”
IRONMAN “asked whether there’s benefit to IV iron in the longer term,” Kiran Musunuru, MD, PhD, MPH, University of Pennsylvania,Philadelphia, observed at the media briefing. As the trial was reported, “that does in fact, seem to be the case,” said Dr. Musunuru, who was not involved in IRONMAN.
Therefore, he said, “this study reinforces the message that we should be routinely monitoring our heart failure patients for iron deficiency and supplementing them as needed.”
A commentary linked to the IRONMAN publication agreed. The trial “increases the evidence base for the treatment of iron deficiency with intravenous iron supplementation,” wrote the editorialists, led by Theresa A. McDonagh, MD, King’s College Hospital and School of Cardiovascular Sciences, London.
Patients with acute or chronic HF, iron deficiency, and reduced or mildly reduced ejection fractions “should be offered treatment with intravenous iron to reduce their risk of hospital admission for heart failure,” they concluded.
Mostly reduced-EF outpatients
The open-label, blinded-endpoint IRONMAN trial, conducted at 70 centers in the United Kingdom, entered adults with HF, ejection fractions 45% or lower within the previous 2 years, and iron deficiency defined as transferrin saturation less than 20% or serum ferritin levels below 100 mcg/L, the report states. They were either hospitalized for HF, had such a hospitalization within the past 6 months, or were outpatients with elevated natriuretic peptide levels; the third category accounted for two thirds of the trial population.
Of the 1,137 randomized patients, 569 were assigned to receive intravenous ferric derisomaltose at weight- and hemoglobin-adjusted dosages; 568 went to the usual-care group.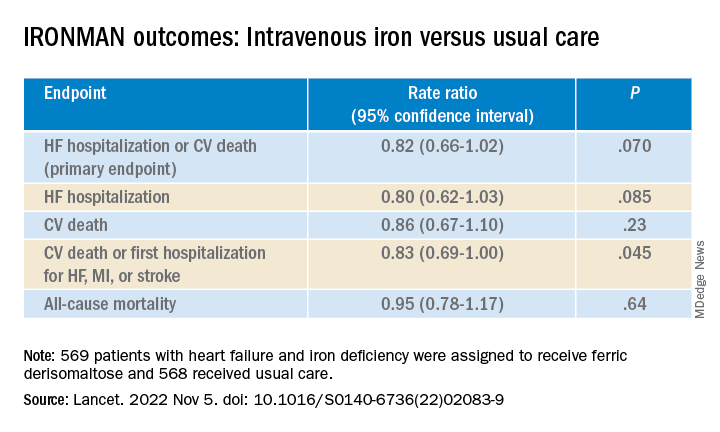
Those receiving intravenous iron visited the trial clinic 4 weeks later and then every 4 months. At those visits, they received a round of ferric derisomaltose if their ferritin levels were below 100 mcg/L, or 400 mcg/L or lower if transferrin saturation was below 25%, the published report states.
Mean scores on the Minnesota Living with Heart Failure Questionnaire improved by a marginally significant 3.33 points (P = .050) at 4 months in the intravenous iron group. The gain receded to a nonsignificant 2.57 points by 20 months (P = .23).
In COVID-related sensitivity analysis, the intravenous iron group showed a significant benefit for the primary endpoint and a trend for improved HF hospitalizations.
- HF hospitalization or CV death: RR, 0.76 (95% confidence interval, 0.58-1.00; P = .047)
- HF hospitalization: RR 0.76 (95% CI, 0.56-1.03; P = .077)
Fewer patients in the intravenous iron group experienced serious cardiac adverse events, 36% compared with 43% in for those on usual care, P = .016.
The recently updated European Society of Cardiology guidelines for HF made it a class 1 recommendation to assess iron status in every patient, Kalra observed. “It doesn›t specify how frequently, but I think we should be thinking about every 4-6 months.”
Dr. Kalra disclosed receiving research grants from Pharmacosmos; and consulting or lecturing for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, Pfizer, Pharmacosmos, Servier, and Vifor Pharma. Dr. Musunuru disclosed significant ownership interest in Verve Therapeutics and Variant Bio. Dr. Lewis disclosed relationships with NXT, American Regent, and RIVUS; and receiving research grants from Cytokinetics and Amgen.
A version of this article first appeared on Medscape.com.
CHICAGO – Another major study appears to back the use of intravenous iron repletion in patients with heart failure (HF) and iron deficiency, strengthening largely consistent evidence, researchers say, that the treatment may improve symptoms and prevent some HF-related hospital admissions.
To be sure, the IRONMAN trial, which compared intravenous iron versus usual care in such patients – most with reduced ejection fraction and not hospitalized – failed to show a benefit for its primary endpoint. The 18% reduction in risk for HF hospitalization or cardiovascular (CV) death seen in the trial, however encouraging, can only be called a trend (P = .07).
But the intervention showed signs of benefit for some secondary endpoints, including quality of life scores, and hinted at such an effect on HF hospitalization. Risk for the latter endpoint dropped 20% (P = .085) over a median follow-up of 2.7 years.
The findings “build upon the other data we have that correcting iron deficiency can help improve well-being, and particularly reduce the risk of hospitalization, in a broad range of [HF] patients,” said Paul Kalra, MD, of the University of Glasgow and Portsmouth (England) Hospitals University NHS Trust.
The tested regimen “was well tolerated with no safety concerns” and offers “reassurance about the long-term safety” of the intravenous iron it used, ferric derisomaltose (MonoFerric), in patients with HF, Dr. Kalra said at a media briefing on the trial.
The remarks preceded his formal presentation of IRONMAN at the American Heart Association scientific sessions. Dr. Kalra is also lead author on the trial’s publication in The Lancet.
IRONMAN strengthens the base of evidence supporting intravenous iron in HF with iron deficiency, especially chronic HF in outpatients, Dr. Kalra and others said. It also supports efficacy for a form of intravenous iron not previously tested in a major HF trial.
Still, “the totality of data are now supporting intravenous iron per se,” regardless of the iron agent used, said Dr. Kalra. But ferric derisomaltose may have dosing advantages, he observed, “and we’ve now got these long-term safety data.”
The strongest prior support for intravenous iron in HF came from hospitalized patients who received it as ferric carboxymaltose (Ferinject) and were followed only 12 months. That was in the AFFIRM-AHF trial, published 2 years ago, which also missed its primary endpoint – the same one used in IRONMAN. Some outcomes in the two trials were similar.
The risk for HF hospitalization or CV death for intravenous iron therapy, compared with usual care, in AFFIRM-AHF fell 21% (P = .059), missing significance but apparently driven by a 26% drop in risk for HF readmissions (P = .013). But neither that trial nor IRONMAN suggested a benefit for CV mortality on its own.
The COVID effect
In IRONMAN, Dr. Kalra said, usual care could include oral iron supplementation, which 17% of patients in the control group received. That could potentially have kept the intravenous iron group from making a better showing for the primary endpoint, he proposed.
And some iron doses and other treatments were missed by a substantial number of patients in both groups who entered the trial after the United Kingdom’s national lockdown in response to the COVID-19 pandemic, he observed. “Patients were not able to come into hospitals for research visits, or in fact when they were able, may not have wanted to.”
So, the group conducted a “prespecified” sensitivity analysis that excluded the 9% of patients enrolled by the end of March 2020, about the time of the first lockdown, and followed the remainder for another 6 months.
In that analysis, risk for HF hospitalization or CV death declined 24% in the intravenous iron group, a marginal but significant result (P = .047) that was dominated by an improvement in HF hospitalizations.
Effects on guidelines
The intravenous iron recommendations in the European HF guidelines refer only to ferric carboxymaltose without mentioning other forms, such as ferric derisomaltose, “but this is now a class effect given the similarities between AFFIRM-AHF and IRONMAN,” said Gregory D. Lewis, MD, Mass General Brigham, Boston, invited discussant for Dr. Kalra’s presentation at the AHA session.
“In the United States, we relegate IV iron to improvement in functional capacity as a comorbidity of heart failure. Perhaps this role will expand,” added Dr. Lewis, who is medical director of his center’s heart transplant program.
He also wondered aloud whether the purported clinical benefits of intravenous iron in HF patients with iron deficiency, not as yet supported by a significant primary-endpoint showing in one of the major trials, currently justify expansion of its use in practice.
“With the benefits of IV iron on exercise capacity and quality of life, and the safety of administering high doses of IV iron,” potentially reducing HF polypharmacy, he noted, “should we be considering IV iron more commonly for utilization in our patients even if we find that heart failure hospitalizations and mortality are only modestly improved?”
IRONMAN “asked whether there’s benefit to IV iron in the longer term,” Kiran Musunuru, MD, PhD, MPH, University of Pennsylvania,Philadelphia, observed at the media briefing. As the trial was reported, “that does in fact, seem to be the case,” said Dr. Musunuru, who was not involved in IRONMAN.
Therefore, he said, “this study reinforces the message that we should be routinely monitoring our heart failure patients for iron deficiency and supplementing them as needed.”
A commentary linked to the IRONMAN publication agreed. The trial “increases the evidence base for the treatment of iron deficiency with intravenous iron supplementation,” wrote the editorialists, led by Theresa A. McDonagh, MD, King’s College Hospital and School of Cardiovascular Sciences, London.
Patients with acute or chronic HF, iron deficiency, and reduced or mildly reduced ejection fractions “should be offered treatment with intravenous iron to reduce their risk of hospital admission for heart failure,” they concluded.
Mostly reduced-EF outpatients
The open-label, blinded-endpoint IRONMAN trial, conducted at 70 centers in the United Kingdom, entered adults with HF, ejection fractions 45% or lower within the previous 2 years, and iron deficiency defined as transferrin saturation less than 20% or serum ferritin levels below 100 mcg/L, the report states. They were either hospitalized for HF, had such a hospitalization within the past 6 months, or were outpatients with elevated natriuretic peptide levels; the third category accounted for two thirds of the trial population.
Of the 1,137 randomized patients, 569 were assigned to receive intravenous ferric derisomaltose at weight- and hemoglobin-adjusted dosages; 568 went to the usual-care group.
Those receiving intravenous iron visited the trial clinic 4 weeks later and then every 4 months. At those visits, they received a round of ferric derisomaltose if their ferritin levels were below 100 mcg/L, or 400 mcg/L or lower if transferrin saturation was below 25%, the published report states.
Mean scores on the Minnesota Living with Heart Failure Questionnaire improved by a marginally significant 3.33 points (P = .050) at 4 months in the intravenous iron group. The gain receded to a nonsignificant 2.57 points by 20 months (P = .23).
In COVID-related sensitivity analysis, the intravenous iron group showed a significant benefit for the primary endpoint and a trend for improved HF hospitalizations.
- HF hospitalization or CV death: RR, 0.76 (95% confidence interval, 0.58-1.00; P = .047)
- HF hospitalization: RR 0.76 (95% CI, 0.56-1.03; P = .077)
Fewer patients in the intravenous iron group experienced serious cardiac adverse events, 36% compared with 43% in for those on usual care, P = .016.
The recently updated European Society of Cardiology guidelines for HF made it a class 1 recommendation to assess iron status in every patient, Kalra observed. “It doesn›t specify how frequently, but I think we should be thinking about every 4-6 months.”
Dr. Kalra disclosed receiving research grants from Pharmacosmos; and consulting or lecturing for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, Pfizer, Pharmacosmos, Servier, and Vifor Pharma. Dr. Musunuru disclosed significant ownership interest in Verve Therapeutics and Variant Bio. Dr. Lewis disclosed relationships with NXT, American Regent, and RIVUS; and receiving research grants from Cytokinetics and Amgen.
A version of this article first appeared on Medscape.com.
CHICAGO – Another major study appears to back the use of intravenous iron repletion in patients with heart failure (HF) and iron deficiency, strengthening largely consistent evidence, researchers say, that the treatment may improve symptoms and prevent some HF-related hospital admissions.
To be sure, the IRONMAN trial, which compared intravenous iron versus usual care in such patients – most with reduced ejection fraction and not hospitalized – failed to show a benefit for its primary endpoint. The 18% reduction in risk for HF hospitalization or cardiovascular (CV) death seen in the trial, however encouraging, can only be called a trend (P = .07).
But the intervention showed signs of benefit for some secondary endpoints, including quality of life scores, and hinted at such an effect on HF hospitalization. Risk for the latter endpoint dropped 20% (P = .085) over a median follow-up of 2.7 years.
The findings “build upon the other data we have that correcting iron deficiency can help improve well-being, and particularly reduce the risk of hospitalization, in a broad range of [HF] patients,” said Paul Kalra, MD, of the University of Glasgow and Portsmouth (England) Hospitals University NHS Trust.
The tested regimen “was well tolerated with no safety concerns” and offers “reassurance about the long-term safety” of the intravenous iron it used, ferric derisomaltose (MonoFerric), in patients with HF, Dr. Kalra said at a media briefing on the trial.
The remarks preceded his formal presentation of IRONMAN at the American Heart Association scientific sessions. Dr. Kalra is also lead author on the trial’s publication in The Lancet.
IRONMAN strengthens the base of evidence supporting intravenous iron in HF with iron deficiency, especially chronic HF in outpatients, Dr. Kalra and others said. It also supports efficacy for a form of intravenous iron not previously tested in a major HF trial.
Still, “the totality of data are now supporting intravenous iron per se,” regardless of the iron agent used, said Dr. Kalra. But ferric derisomaltose may have dosing advantages, he observed, “and we’ve now got these long-term safety data.”
The strongest prior support for intravenous iron in HF came from hospitalized patients who received it as ferric carboxymaltose (Ferinject) and were followed only 12 months. That was in the AFFIRM-AHF trial, published 2 years ago, which also missed its primary endpoint – the same one used in IRONMAN. Some outcomes in the two trials were similar.
The risk for HF hospitalization or CV death for intravenous iron therapy, compared with usual care, in AFFIRM-AHF fell 21% (P = .059), missing significance but apparently driven by a 26% drop in risk for HF readmissions (P = .013). But neither that trial nor IRONMAN suggested a benefit for CV mortality on its own.
The COVID effect
In IRONMAN, Dr. Kalra said, usual care could include oral iron supplementation, which 17% of patients in the control group received. That could potentially have kept the intravenous iron group from making a better showing for the primary endpoint, he proposed.
And some iron doses and other treatments were missed by a substantial number of patients in both groups who entered the trial after the United Kingdom’s national lockdown in response to the COVID-19 pandemic, he observed. “Patients were not able to come into hospitals for research visits, or in fact when they were able, may not have wanted to.”
So, the group conducted a “prespecified” sensitivity analysis that excluded the 9% of patients enrolled by the end of March 2020, about the time of the first lockdown, and followed the remainder for another 6 months.
In that analysis, risk for HF hospitalization or CV death declined 24% in the intravenous iron group, a marginal but significant result (P = .047) that was dominated by an improvement in HF hospitalizations.
Effects on guidelines
The intravenous iron recommendations in the European HF guidelines refer only to ferric carboxymaltose without mentioning other forms, such as ferric derisomaltose, “but this is now a class effect given the similarities between AFFIRM-AHF and IRONMAN,” said Gregory D. Lewis, MD, Mass General Brigham, Boston, invited discussant for Dr. Kalra’s presentation at the AHA session.
“In the United States, we relegate IV iron to improvement in functional capacity as a comorbidity of heart failure. Perhaps this role will expand,” added Dr. Lewis, who is medical director of his center’s heart transplant program.
He also wondered aloud whether the purported clinical benefits of intravenous iron in HF patients with iron deficiency, not as yet supported by a significant primary-endpoint showing in one of the major trials, currently justify expansion of its use in practice.
“With the benefits of IV iron on exercise capacity and quality of life, and the safety of administering high doses of IV iron,” potentially reducing HF polypharmacy, he noted, “should we be considering IV iron more commonly for utilization in our patients even if we find that heart failure hospitalizations and mortality are only modestly improved?”
IRONMAN “asked whether there’s benefit to IV iron in the longer term,” Kiran Musunuru, MD, PhD, MPH, University of Pennsylvania,Philadelphia, observed at the media briefing. As the trial was reported, “that does in fact, seem to be the case,” said Dr. Musunuru, who was not involved in IRONMAN.
Therefore, he said, “this study reinforces the message that we should be routinely monitoring our heart failure patients for iron deficiency and supplementing them as needed.”
A commentary linked to the IRONMAN publication agreed. The trial “increases the evidence base for the treatment of iron deficiency with intravenous iron supplementation,” wrote the editorialists, led by Theresa A. McDonagh, MD, King’s College Hospital and School of Cardiovascular Sciences, London.
Patients with acute or chronic HF, iron deficiency, and reduced or mildly reduced ejection fractions “should be offered treatment with intravenous iron to reduce their risk of hospital admission for heart failure,” they concluded.
Mostly reduced-EF outpatients
The open-label, blinded-endpoint IRONMAN trial, conducted at 70 centers in the United Kingdom, entered adults with HF, ejection fractions 45% or lower within the previous 2 years, and iron deficiency defined as transferrin saturation less than 20% or serum ferritin levels below 100 mcg/L, the report states. They were either hospitalized for HF, had such a hospitalization within the past 6 months, or were outpatients with elevated natriuretic peptide levels; the third category accounted for two thirds of the trial population.
Of the 1,137 randomized patients, 569 were assigned to receive intravenous ferric derisomaltose at weight- and hemoglobin-adjusted dosages; 568 went to the usual-care group.
Those receiving intravenous iron visited the trial clinic 4 weeks later and then every 4 months. At those visits, they received a round of ferric derisomaltose if their ferritin levels were below 100 mcg/L, or 400 mcg/L or lower if transferrin saturation was below 25%, the published report states.
Mean scores on the Minnesota Living with Heart Failure Questionnaire improved by a marginally significant 3.33 points (P = .050) at 4 months in the intravenous iron group. The gain receded to a nonsignificant 2.57 points by 20 months (P = .23).
In COVID-related sensitivity analysis, the intravenous iron group showed a significant benefit for the primary endpoint and a trend for improved HF hospitalizations.
- HF hospitalization or CV death: RR, 0.76 (95% confidence interval, 0.58-1.00; P = .047)
- HF hospitalization: RR 0.76 (95% CI, 0.56-1.03; P = .077)
Fewer patients in the intravenous iron group experienced serious cardiac adverse events, 36% compared with 43% in for those on usual care, P = .016.
The recently updated European Society of Cardiology guidelines for HF made it a class 1 recommendation to assess iron status in every patient, Kalra observed. “It doesn›t specify how frequently, but I think we should be thinking about every 4-6 months.”
Dr. Kalra disclosed receiving research grants from Pharmacosmos; and consulting or lecturing for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, Pfizer, Pharmacosmos, Servier, and Vifor Pharma. Dr. Musunuru disclosed significant ownership interest in Verve Therapeutics and Variant Bio. Dr. Lewis disclosed relationships with NXT, American Regent, and RIVUS; and receiving research grants from Cytokinetics and Amgen.
A version of this article first appeared on Medscape.com.
AT AHA 2022
Optimize HF meds rapidly and fully after hospital discharge: STRONG-HF
CHICAGO – Clinicians who prescribe heart failure meds are holding the best hand they’ve ever had, but with so much underuse and suboptimal dosing in actual practice, it seems many may not appreciate the value of their cards. But a major randomized trial that has captured the field’s attention may embolden them to go all in.
Results showed that a strategy of early, rapid up-titration of multiple guideline-directed meds in patients hospitalized with heart failure, compared with a usual-care approach, cut their 6-month risk for death or HF readmission by a steep 34% (P = .002).
The drugs had been started and partly up-titrated in the hospital with the goal of full up-titration within 2 weeks after discharge.
Patients well tolerated the high-intensity approach, researchers said. Their quality-of-life scores improved (P < .0001) compared with the usual-care group, and adverse events were considered few and manageable in the international trial with more than 1,000 patients.
Safety on the high-intensity strategy depended on close patient monitoring at frequently planned clinic visits along with guidance for the up-titrations from clinical signs and natriuretic peptide levels, observed Alexandre Mebazaa, MD, PhD, University of Paris and Public Hospitals of Paris.
Dr. Mebazaa is principal investigator on the trial, called STRONG-HF, which he presented at the American Heart Association scientific sessions, held in Chicago and virtually. He is also lead author on the study’s same-day publication in the Lancet.
The high-intensity strategy’s superiority emerged early in the trial, which was halted early on the data safety monitoring board’s recommendation, with about 90% of follow-ups completed. The board “felt it was unethical to keep patients in usual care,” Dr. Mebazaa said at a press conference.
A dramatic change
The next step, he said, will be to educate the heart failure community on the high-intensity care technique so it can swiftly enter clinical practice. Currently in acute heart failure, “very few patients are monitored after discharge and treated with full doses of heart failure therapies.”
Adoption of the strategy “would be a dramatic change from what’s currently being done,” said Martin B. Leon, MD, NewYork-Presbyterian/Columbia University Irving Medical Center, New York, who moderated the press conference.
Only an estimated 5% of patients with HF in the United States receive full guideline-directed medical therapy, Dr. Leon said, “so the generalizability of this strategy, with careful follow-up that has safety involved in it, is absolutely crucial.”
But the potential impact of this high-intensity approach on resource use is unknown, raising questions about how widely and consistently it could be implemented, said Dr. Leon, who is not connected with STRONG-HF.
The trial called for in-hospital initiation of the three distinct drug classes that, at the time, were the core of guideline-directed HF therapy, with up-titration to 50% of recommended dosage by hospital discharge, and then to 100% within 2 weeks later.
The meds included a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and a renin-angiotensin system inhibitor (RASI). The latter could be an ACE inhibitor, angiotensin-receptor blocker (ARB), or angiotensin receptor-neprilysin inhibitor (ARNI).
How about a fourth drug?
Conspicuously absent from the list, for contemporary practice, was an SGLT2 inhibitor, a class that entered the HF guidelines well after STRONG-HF was designed. They would undoubtedly join the other three agents were the high-intensity strategy to enter practice, potentially changing its complexity and safety profile.
But Dr. Mebazaa and other experts don’t see that as a big challenge and would expect a smooth transition to a high-intensity approach that also includes the SGLT2 inhibitors.
STRONG-HF was necessary in part because many clinicians have been “reluctant” to take full advantage of three agents that had been the basis of guideline-directed therapy, he told this news organization.
That reluctance stemmed from concerns that beta-blockers might worsen the heart failure, ACE inhibitors could hurt the kidneys, or MRAs might cause hyperkalemia, Dr. Mebazaa said. The STRONG-HF high-intensity regimen, therefore, demanded multiple clinic visits for close follow-up.
But the SGLT2 inhibitors “are known to be rather safe drugs, at least much safer than the three others,” he said. So, it seems unlikely that their addition to a beta-blocker, RASI, and MRA in patients with HF would worsen the risk of adverse events.
John G.F. Cleland, MD, PhD, agrees. With addition of the fourth agent, “You may need to be a little bit more careful with renal function, just in that first couple of weeks,” he told this news organization. “But I think it would be easy to add an SGLT2 inhibitor into this regimen. And in general, there’s no titration with an SGLT2 inhibitor, so they’ll all be on full dose predischarge.”
Given the drugs’ diuretic-like action, moreover, some patients might be able to pull back on their loop diuretics, speculated Dr. Cleland, from the University of Glasgow’s School of Health and Wellbeing.
The prospect of a high-intensity strategy’s wide implementation in practice presents both “challenges and opportunities,” Amanda R. Vest, MBBS, MPH, Tufts University, Boston, told this news organization.
“There may be additional challenges in terms of ensuring we avoid hypotension or acute kidney injury in the up-titration phase,” said Dr. Vest, who is medical director of her center’s cardiac transplantation program but not connected with STRONG-HF.
“But it also gives us opportunities,” she added, “because there are some patients, especially in that vulnerable postdischarge phase, who are actually much more able to tolerate introduction of an SGLT2 inhibitor than, for example, an ACE inhibitor, ARB, or ARNI – or maybe a beta-blocker if they’ve been in a low cardiac-output state.” Effective dosing would depend on “the personalization and skill of the clinician in optimizing the medications in their correct sequence,” Dr. Vest said.
“It’s challenging to think that we would ever get to 100% up-titration,” she added, “and even in this excellent study, they didn’t get to 100%.” But as clinicians gain experience with the high-intensity strategy, especially as the SGLT2 inhibitors are included, “I think we can reasonably expect more progress to be made in these up-titration skills.”
No restrictions on LVEF
The researchers entered 1,078 patients hospitalized with acute HF in 14 countries across Africa, Europe, the Middle East, and South America, and randomly assigned them to the high-intensity management strategy or usual care.
About 60% of the patients were male and 77% were White. There were no entry restrictions based on left ventricular ejection fraction (LVEF), which exceeded 40% in almost a third of cases.
In the high-intensity care group’s 542 patients, the three agents were up-titrated to 50% of the maximum guideline-recommended dosage prior to hospital discharge, and to 100% within 2 weeks after discharge. Symptoms and laboratory biomarkers, including natriuretic peptides, were monitored closely at four planned clinical visits over the following 6 weeks.
The 536 patients assigned to usual care were discharged and managed according to local standards, with their meds handled by their own primary care doctors or cardiologists, the published report notes. They were reevaluated by STRONG-HF clinicians 90 days after discharge.
The number of clinic visits in the first 90 postdischarge days averaged 4.8 in the high-intensity care group and 1.0 for those receiving usual care. Full up-titration was far more likely in the high-intensity care group: 55% vs. 2% for RASI agents, 49% vs. 4% for beta-blockers, and 84% vs. 46% for MRAs.
They also fared significantly better on all measured parameters associated with decongestion, including weight, prevalence of peripheral edema, jugular venous pressure, NYHA functional class, and natriuretic peptide levels, the researchers said.
The primary endpoint of 180-day death from any cause or HF readmission was met by 15.2% of the high-intensity care group and 23.3% of usual-care patients, for an adjusted risk ratio (RR) of 0.66 (95% CI, 0.50-0.86; P = .0021).
Subgroup analyses saw no significant interactions by age, sex, race, geography, or baseline blood pressure, renal function, or LVEF. Patients with higher vs. lower baseline natriuretic peptide levels trend toward better responses to high-intensity care (P = .08)
The COVID effect
The group performed a sensitivity analysis that excluded deaths attributed to COVID-19 in STRONG-HF, which launched prior to the pandemic. The high-intensity strategy’s benefit for the primary endpoint grew, with an adjusted RR of 0.61 (95% CI, 0.46-0.82; P = .0005). There was no corresponding effect on death from any cause (P = .15).
Treatment-related adverse effects in the overall trial were seen in 41.1% of the high-intensity care group and in 29.5% of those assigned to usual care.
The higher rate in the high-intensity care arm “may be related to their higher number of [clinic] visits compared to usual care,” Dr. Mebazaa said. “However, serious adverse events and fatal adverse events were similar in both arms.”
Cardiac failure was the most common adverse event, developing in about 15% in both groups. It was followed by hypotension, hyperkalemia, and renal impairment, according to the published report.
Dr. Cleland cautioned that the risk of adverse events would potentially be higher should the high-intensity strategy become common clinical practice. The median age in STRONG-HF was 63, which is “10-15 years younger, on average, than the population with recently admitted heart failure that we see. There’s no doubt that older people have more multimorbidity.”
STRONG-HF was funded by Roche Diagnostics. Dr. Mebazaa discloses receiving grants from Roche Diagnostics, Abbott Laboratories, 4TEEN4, and Windtree Therapeutics; honoraria for lectures from Roche Diagnostics, Bayer, and Merck, Sharp & Dohme; and consulting for Corteria Pharmaceuticals, S-form Pharma, FIRE-1, Implicity, 4TEEN4, and Adrenomed; and to being a co-inventor on a patent involving combination therapy for patients having acute or persistent dyspnea.
Dr. Vest reports modest relationships with Boehringer Ingelheim, Corvia, and CareDx; and receiving research grants from the American Heart Association and the National Institutes of Health. Dr. Cleland discloses receiving honoraria from Idorsia; and research grants from Vifor Pharma, Medtronic, Bayer, and Bristol-Myers Squibb. Dr. Leon had no disclosures.
A version of this article first appeared on Medscape.com.
CHICAGO – Clinicians who prescribe heart failure meds are holding the best hand they’ve ever had, but with so much underuse and suboptimal dosing in actual practice, it seems many may not appreciate the value of their cards. But a major randomized trial that has captured the field’s attention may embolden them to go all in.
Results showed that a strategy of early, rapid up-titration of multiple guideline-directed meds in patients hospitalized with heart failure, compared with a usual-care approach, cut their 6-month risk for death or HF readmission by a steep 34% (P = .002).
The drugs had been started and partly up-titrated in the hospital with the goal of full up-titration within 2 weeks after discharge.
Patients well tolerated the high-intensity approach, researchers said. Their quality-of-life scores improved (P < .0001) compared with the usual-care group, and adverse events were considered few and manageable in the international trial with more than 1,000 patients.
Safety on the high-intensity strategy depended on close patient monitoring at frequently planned clinic visits along with guidance for the up-titrations from clinical signs and natriuretic peptide levels, observed Alexandre Mebazaa, MD, PhD, University of Paris and Public Hospitals of Paris.
Dr. Mebazaa is principal investigator on the trial, called STRONG-HF, which he presented at the American Heart Association scientific sessions, held in Chicago and virtually. He is also lead author on the study’s same-day publication in the Lancet.
The high-intensity strategy’s superiority emerged early in the trial, which was halted early on the data safety monitoring board’s recommendation, with about 90% of follow-ups completed. The board “felt it was unethical to keep patients in usual care,” Dr. Mebazaa said at a press conference.
A dramatic change
The next step, he said, will be to educate the heart failure community on the high-intensity care technique so it can swiftly enter clinical practice. Currently in acute heart failure, “very few patients are monitored after discharge and treated with full doses of heart failure therapies.”
Adoption of the strategy “would be a dramatic change from what’s currently being done,” said Martin B. Leon, MD, NewYork-Presbyterian/Columbia University Irving Medical Center, New York, who moderated the press conference.
Only an estimated 5% of patients with HF in the United States receive full guideline-directed medical therapy, Dr. Leon said, “so the generalizability of this strategy, with careful follow-up that has safety involved in it, is absolutely crucial.”
But the potential impact of this high-intensity approach on resource use is unknown, raising questions about how widely and consistently it could be implemented, said Dr. Leon, who is not connected with STRONG-HF.
The trial called for in-hospital initiation of the three distinct drug classes that, at the time, were the core of guideline-directed HF therapy, with up-titration to 50% of recommended dosage by hospital discharge, and then to 100% within 2 weeks later.
The meds included a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and a renin-angiotensin system inhibitor (RASI). The latter could be an ACE inhibitor, angiotensin-receptor blocker (ARB), or angiotensin receptor-neprilysin inhibitor (ARNI).
How about a fourth drug?
Conspicuously absent from the list, for contemporary practice, was an SGLT2 inhibitor, a class that entered the HF guidelines well after STRONG-HF was designed. They would undoubtedly join the other three agents were the high-intensity strategy to enter practice, potentially changing its complexity and safety profile.
But Dr. Mebazaa and other experts don’t see that as a big challenge and would expect a smooth transition to a high-intensity approach that also includes the SGLT2 inhibitors.
STRONG-HF was necessary in part because many clinicians have been “reluctant” to take full advantage of three agents that had been the basis of guideline-directed therapy, he told this news organization.
That reluctance stemmed from concerns that beta-blockers might worsen the heart failure, ACE inhibitors could hurt the kidneys, or MRAs might cause hyperkalemia, Dr. Mebazaa said. The STRONG-HF high-intensity regimen, therefore, demanded multiple clinic visits for close follow-up.
But the SGLT2 inhibitors “are known to be rather safe drugs, at least much safer than the three others,” he said. So, it seems unlikely that their addition to a beta-blocker, RASI, and MRA in patients with HF would worsen the risk of adverse events.
John G.F. Cleland, MD, PhD, agrees. With addition of the fourth agent, “You may need to be a little bit more careful with renal function, just in that first couple of weeks,” he told this news organization. “But I think it would be easy to add an SGLT2 inhibitor into this regimen. And in general, there’s no titration with an SGLT2 inhibitor, so they’ll all be on full dose predischarge.”
Given the drugs’ diuretic-like action, moreover, some patients might be able to pull back on their loop diuretics, speculated Dr. Cleland, from the University of Glasgow’s School of Health and Wellbeing.
The prospect of a high-intensity strategy’s wide implementation in practice presents both “challenges and opportunities,” Amanda R. Vest, MBBS, MPH, Tufts University, Boston, told this news organization.
“There may be additional challenges in terms of ensuring we avoid hypotension or acute kidney injury in the up-titration phase,” said Dr. Vest, who is medical director of her center’s cardiac transplantation program but not connected with STRONG-HF.
“But it also gives us opportunities,” she added, “because there are some patients, especially in that vulnerable postdischarge phase, who are actually much more able to tolerate introduction of an SGLT2 inhibitor than, for example, an ACE inhibitor, ARB, or ARNI – or maybe a beta-blocker if they’ve been in a low cardiac-output state.” Effective dosing would depend on “the personalization and skill of the clinician in optimizing the medications in their correct sequence,” Dr. Vest said.
“It’s challenging to think that we would ever get to 100% up-titration,” she added, “and even in this excellent study, they didn’t get to 100%.” But as clinicians gain experience with the high-intensity strategy, especially as the SGLT2 inhibitors are included, “I think we can reasonably expect more progress to be made in these up-titration skills.”
No restrictions on LVEF
The researchers entered 1,078 patients hospitalized with acute HF in 14 countries across Africa, Europe, the Middle East, and South America, and randomly assigned them to the high-intensity management strategy or usual care.
About 60% of the patients were male and 77% were White. There were no entry restrictions based on left ventricular ejection fraction (LVEF), which exceeded 40% in almost a third of cases.
In the high-intensity care group’s 542 patients, the three agents were up-titrated to 50% of the maximum guideline-recommended dosage prior to hospital discharge, and to 100% within 2 weeks after discharge. Symptoms and laboratory biomarkers, including natriuretic peptides, were monitored closely at four planned clinical visits over the following 6 weeks.
The 536 patients assigned to usual care were discharged and managed according to local standards, with their meds handled by their own primary care doctors or cardiologists, the published report notes. They were reevaluated by STRONG-HF clinicians 90 days after discharge.
The number of clinic visits in the first 90 postdischarge days averaged 4.8 in the high-intensity care group and 1.0 for those receiving usual care. Full up-titration was far more likely in the high-intensity care group: 55% vs. 2% for RASI agents, 49% vs. 4% for beta-blockers, and 84% vs. 46% for MRAs.
They also fared significantly better on all measured parameters associated with decongestion, including weight, prevalence of peripheral edema, jugular venous pressure, NYHA functional class, and natriuretic peptide levels, the researchers said.
The primary endpoint of 180-day death from any cause or HF readmission was met by 15.2% of the high-intensity care group and 23.3% of usual-care patients, for an adjusted risk ratio (RR) of 0.66 (95% CI, 0.50-0.86; P = .0021).
Subgroup analyses saw no significant interactions by age, sex, race, geography, or baseline blood pressure, renal function, or LVEF. Patients with higher vs. lower baseline natriuretic peptide levels trend toward better responses to high-intensity care (P = .08)
The COVID effect
The group performed a sensitivity analysis that excluded deaths attributed to COVID-19 in STRONG-HF, which launched prior to the pandemic. The high-intensity strategy’s benefit for the primary endpoint grew, with an adjusted RR of 0.61 (95% CI, 0.46-0.82; P = .0005). There was no corresponding effect on death from any cause (P = .15).
Treatment-related adverse effects in the overall trial were seen in 41.1% of the high-intensity care group and in 29.5% of those assigned to usual care.
The higher rate in the high-intensity care arm “may be related to their higher number of [clinic] visits compared to usual care,” Dr. Mebazaa said. “However, serious adverse events and fatal adverse events were similar in both arms.”
Cardiac failure was the most common adverse event, developing in about 15% in both groups. It was followed by hypotension, hyperkalemia, and renal impairment, according to the published report.
Dr. Cleland cautioned that the risk of adverse events would potentially be higher should the high-intensity strategy become common clinical practice. The median age in STRONG-HF was 63, which is “10-15 years younger, on average, than the population with recently admitted heart failure that we see. There’s no doubt that older people have more multimorbidity.”
STRONG-HF was funded by Roche Diagnostics. Dr. Mebazaa discloses receiving grants from Roche Diagnostics, Abbott Laboratories, 4TEEN4, and Windtree Therapeutics; honoraria for lectures from Roche Diagnostics, Bayer, and Merck, Sharp & Dohme; and consulting for Corteria Pharmaceuticals, S-form Pharma, FIRE-1, Implicity, 4TEEN4, and Adrenomed; and to being a co-inventor on a patent involving combination therapy for patients having acute or persistent dyspnea.
Dr. Vest reports modest relationships with Boehringer Ingelheim, Corvia, and CareDx; and receiving research grants from the American Heart Association and the National Institutes of Health. Dr. Cleland discloses receiving honoraria from Idorsia; and research grants from Vifor Pharma, Medtronic, Bayer, and Bristol-Myers Squibb. Dr. Leon had no disclosures.
A version of this article first appeared on Medscape.com.
CHICAGO – Clinicians who prescribe heart failure meds are holding the best hand they’ve ever had, but with so much underuse and suboptimal dosing in actual practice, it seems many may not appreciate the value of their cards. But a major randomized trial that has captured the field’s attention may embolden them to go all in.
Results showed that a strategy of early, rapid up-titration of multiple guideline-directed meds in patients hospitalized with heart failure, compared with a usual-care approach, cut their 6-month risk for death or HF readmission by a steep 34% (P = .002).
The drugs had been started and partly up-titrated in the hospital with the goal of full up-titration within 2 weeks after discharge.
Patients well tolerated the high-intensity approach, researchers said. Their quality-of-life scores improved (P < .0001) compared with the usual-care group, and adverse events were considered few and manageable in the international trial with more than 1,000 patients.
Safety on the high-intensity strategy depended on close patient monitoring at frequently planned clinic visits along with guidance for the up-titrations from clinical signs and natriuretic peptide levels, observed Alexandre Mebazaa, MD, PhD, University of Paris and Public Hospitals of Paris.
Dr. Mebazaa is principal investigator on the trial, called STRONG-HF, which he presented at the American Heart Association scientific sessions, held in Chicago and virtually. He is also lead author on the study’s same-day publication in the Lancet.
The high-intensity strategy’s superiority emerged early in the trial, which was halted early on the data safety monitoring board’s recommendation, with about 90% of follow-ups completed. The board “felt it was unethical to keep patients in usual care,” Dr. Mebazaa said at a press conference.
A dramatic change
The next step, he said, will be to educate the heart failure community on the high-intensity care technique so it can swiftly enter clinical practice. Currently in acute heart failure, “very few patients are monitored after discharge and treated with full doses of heart failure therapies.”
Adoption of the strategy “would be a dramatic change from what’s currently being done,” said Martin B. Leon, MD, NewYork-Presbyterian/Columbia University Irving Medical Center, New York, who moderated the press conference.
Only an estimated 5% of patients with HF in the United States receive full guideline-directed medical therapy, Dr. Leon said, “so the generalizability of this strategy, with careful follow-up that has safety involved in it, is absolutely crucial.”
But the potential impact of this high-intensity approach on resource use is unknown, raising questions about how widely and consistently it could be implemented, said Dr. Leon, who is not connected with STRONG-HF.
The trial called for in-hospital initiation of the three distinct drug classes that, at the time, were the core of guideline-directed HF therapy, with up-titration to 50% of recommended dosage by hospital discharge, and then to 100% within 2 weeks later.
The meds included a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and a renin-angiotensin system inhibitor (RASI). The latter could be an ACE inhibitor, angiotensin-receptor blocker (ARB), or angiotensin receptor-neprilysin inhibitor (ARNI).
How about a fourth drug?
Conspicuously absent from the list, for contemporary practice, was an SGLT2 inhibitor, a class that entered the HF guidelines well after STRONG-HF was designed. They would undoubtedly join the other three agents were the high-intensity strategy to enter practice, potentially changing its complexity and safety profile.
But Dr. Mebazaa and other experts don’t see that as a big challenge and would expect a smooth transition to a high-intensity approach that also includes the SGLT2 inhibitors.
STRONG-HF was necessary in part because many clinicians have been “reluctant” to take full advantage of three agents that had been the basis of guideline-directed therapy, he told this news organization.
That reluctance stemmed from concerns that beta-blockers might worsen the heart failure, ACE inhibitors could hurt the kidneys, or MRAs might cause hyperkalemia, Dr. Mebazaa said. The STRONG-HF high-intensity regimen, therefore, demanded multiple clinic visits for close follow-up.
But the SGLT2 inhibitors “are known to be rather safe drugs, at least much safer than the three others,” he said. So, it seems unlikely that their addition to a beta-blocker, RASI, and MRA in patients with HF would worsen the risk of adverse events.
John G.F. Cleland, MD, PhD, agrees. With addition of the fourth agent, “You may need to be a little bit more careful with renal function, just in that first couple of weeks,” he told this news organization. “But I think it would be easy to add an SGLT2 inhibitor into this regimen. And in general, there’s no titration with an SGLT2 inhibitor, so they’ll all be on full dose predischarge.”
Given the drugs’ diuretic-like action, moreover, some patients might be able to pull back on their loop diuretics, speculated Dr. Cleland, from the University of Glasgow’s School of Health and Wellbeing.
The prospect of a high-intensity strategy’s wide implementation in practice presents both “challenges and opportunities,” Amanda R. Vest, MBBS, MPH, Tufts University, Boston, told this news organization.
“There may be additional challenges in terms of ensuring we avoid hypotension or acute kidney injury in the up-titration phase,” said Dr. Vest, who is medical director of her center’s cardiac transplantation program but not connected with STRONG-HF.
“But it also gives us opportunities,” she added, “because there are some patients, especially in that vulnerable postdischarge phase, who are actually much more able to tolerate introduction of an SGLT2 inhibitor than, for example, an ACE inhibitor, ARB, or ARNI – or maybe a beta-blocker if they’ve been in a low cardiac-output state.” Effective dosing would depend on “the personalization and skill of the clinician in optimizing the medications in their correct sequence,” Dr. Vest said.
“It’s challenging to think that we would ever get to 100% up-titration,” she added, “and even in this excellent study, they didn’t get to 100%.” But as clinicians gain experience with the high-intensity strategy, especially as the SGLT2 inhibitors are included, “I think we can reasonably expect more progress to be made in these up-titration skills.”
No restrictions on LVEF
The researchers entered 1,078 patients hospitalized with acute HF in 14 countries across Africa, Europe, the Middle East, and South America, and randomly assigned them to the high-intensity management strategy or usual care.
About 60% of the patients were male and 77% were White. There were no entry restrictions based on left ventricular ejection fraction (LVEF), which exceeded 40% in almost a third of cases.
In the high-intensity care group’s 542 patients, the three agents were up-titrated to 50% of the maximum guideline-recommended dosage prior to hospital discharge, and to 100% within 2 weeks after discharge. Symptoms and laboratory biomarkers, including natriuretic peptides, were monitored closely at four planned clinical visits over the following 6 weeks.
The 536 patients assigned to usual care were discharged and managed according to local standards, with their meds handled by their own primary care doctors or cardiologists, the published report notes. They were reevaluated by STRONG-HF clinicians 90 days after discharge.
The number of clinic visits in the first 90 postdischarge days averaged 4.8 in the high-intensity care group and 1.0 for those receiving usual care. Full up-titration was far more likely in the high-intensity care group: 55% vs. 2% for RASI agents, 49% vs. 4% for beta-blockers, and 84% vs. 46% for MRAs.
They also fared significantly better on all measured parameters associated with decongestion, including weight, prevalence of peripheral edema, jugular venous pressure, NYHA functional class, and natriuretic peptide levels, the researchers said.
The primary endpoint of 180-day death from any cause or HF readmission was met by 15.2% of the high-intensity care group and 23.3% of usual-care patients, for an adjusted risk ratio (RR) of 0.66 (95% CI, 0.50-0.86; P = .0021).
Subgroup analyses saw no significant interactions by age, sex, race, geography, or baseline blood pressure, renal function, or LVEF. Patients with higher vs. lower baseline natriuretic peptide levels trend toward better responses to high-intensity care (P = .08)
The COVID effect
The group performed a sensitivity analysis that excluded deaths attributed to COVID-19 in STRONG-HF, which launched prior to the pandemic. The high-intensity strategy’s benefit for the primary endpoint grew, with an adjusted RR of 0.61 (95% CI, 0.46-0.82; P = .0005). There was no corresponding effect on death from any cause (P = .15).
Treatment-related adverse effects in the overall trial were seen in 41.1% of the high-intensity care group and in 29.5% of those assigned to usual care.
The higher rate in the high-intensity care arm “may be related to their higher number of [clinic] visits compared to usual care,” Dr. Mebazaa said. “However, serious adverse events and fatal adverse events were similar in both arms.”
Cardiac failure was the most common adverse event, developing in about 15% in both groups. It was followed by hypotension, hyperkalemia, and renal impairment, according to the published report.
Dr. Cleland cautioned that the risk of adverse events would potentially be higher should the high-intensity strategy become common clinical practice. The median age in STRONG-HF was 63, which is “10-15 years younger, on average, than the population with recently admitted heart failure that we see. There’s no doubt that older people have more multimorbidity.”
STRONG-HF was funded by Roche Diagnostics. Dr. Mebazaa discloses receiving grants from Roche Diagnostics, Abbott Laboratories, 4TEEN4, and Windtree Therapeutics; honoraria for lectures from Roche Diagnostics, Bayer, and Merck, Sharp & Dohme; and consulting for Corteria Pharmaceuticals, S-form Pharma, FIRE-1, Implicity, 4TEEN4, and Adrenomed; and to being a co-inventor on a patent involving combination therapy for patients having acute or persistent dyspnea.
Dr. Vest reports modest relationships with Boehringer Ingelheim, Corvia, and CareDx; and receiving research grants from the American Heart Association and the National Institutes of Health. Dr. Cleland discloses receiving honoraria from Idorsia; and research grants from Vifor Pharma, Medtronic, Bayer, and Bristol-Myers Squibb. Dr. Leon had no disclosures.
A version of this article first appeared on Medscape.com.
AT AHA 2022
Flu vaccination associated with reduced stroke risk
The risk of stroke was about 23% lower in the 6 months following a flu shot, regardless of the patient’s age, sex, or underlying health conditions.
“There is an established link between upper respiratory infection and both heart attack and stroke. This has been very salient in the past few years throughout the COVID-19 pandemic,” study author Jessalyn Holodinsky, PhD, a stroke epidemiologist and postdoctoral fellow in clinical neurosciences at the University of Calgary (Alta.) told this news organization.
“It is also known that the flu shot can reduce risk of heart attack and hospitalization for those with heart disease,” she said. “Given both of these [observations], we thought it prudent to study whether there is a link between vaccination for influenza and stroke.”
The study was published in the Lancet Public Health.
Large effect size
The investigators analyzed administrative data from 2009 through 2018 from the Alberta Health Care Insurance Plan, which covers all residents of Alberta. The province provides free seasonal influenza vaccines to residents under the insurance plan.
The research team looked for stroke events such as acute ischemic stroke, intracerebral hemorrhage, subarachnoid hemorrhage, and transient ischemic attack. They then analyzed the risk of stroke events among those with or without a flu shot in the previous 6 months. They accounted for multiple factors, including age, sex, income, location, and factors related to stroke risk, such as anticoagulant use, atrial fibrillation, chronic obstructive pulmonary disease, diabetes, and hypertension.
Among the 4.1 million adults included in the researchers’ analysis, about 1.8 million (43%) received at least one vaccination during the study period. Nearly 97,000 people received a flu vaccine in each year they were in the study, including 29,288 who received a shot in all 10 flu seasons included in the study.
About 38,000 stroke events were recorded, including about 34,000 (90%) first stroke events. Among the 10% of strokes that were recurrent events, the maximum number of stroke events in one person was nine.
Overall, patients who received at least one influenza vaccine were more likely to be older, be women, and have higher rates of comorbidities. The vaccinated group had a slightly higher proportion of people who lived in urban areas, but the income levels were similar between the vaccinated and unvaccinated groups.
The crude incidence of stroke was higher among people who had ever received an influenza vaccination, at 1.25%, compared with 0.52% among those who hadn’t been vaccinated. However, after adjusting for age, sex, underlying conditions, and socioeconomic status, recent flu vaccination (that is, in the previous 6 months) was associated with a 23% reduced risk of stroke.
The significant reduction in risk applied to all stroke types, particularly acute ischemic stroke and intracerebral hemorrhage. In addition, influenza vaccination was associated with a reduced risk across all ages and risk profiles, except patients without hypertension.
“What we were most surprised by was the sheer magnitude of the effect and that it existed across different adult age groups, for both sexes, and for those with and without risk factors for stroke,” said Dr. Holodinsky.
Vaccination was associated with a larger reduction in stroke risk in men than in women, perhaps because unvaccinated men had a significantly higher baseline risk for stroke than unvaccinated women, the study authors write.
Promoting cardiovascular health
In addition, vaccination was associated with a greater relative reduction in stroke risk in younger age groups, lower income groups, and those with diabetes, chronic obstructive pulmonary disease, and anticoagulant use.
Among 2.4 million people observed for the entire study period, vaccination protection increased with the number of vaccines received. People who were vaccinated serially each year had a significantly lower risk of stroke than those who received one shot.
Dr. Holodinsky and colleagues are conducting additional research into influenza vaccination, including stroke risk in children. They’re also investigating whether the reduced risk applies to other vaccinations for respiratory illnesses, such as COVID-19 and pneumonia.
“We hope that this added effect of vaccination encourages more adults to receive the flu shot,” she said. “One day, vaccinations might be considered a key pillar of cardiovascular health, along with diet, exercise, control of hypertension and high cholesterol, and smoking cessation.”
Future research should also investigate the reasons why adults – particularly people at high risk with underlying conditions – don’t receive recommended influenza vaccines, the study authors wrote.
‘Call to action’
Bahar Behrouzi, an MD-PhD candidate focused on clinical epidemiology at the Institute of Health Policy, Management, and Evaluation, University of Toronto, said: “There are a variety of observational studies around the world that show that flu vaccine uptake is low among the general population and high-risk persons. In studying these questions, our hope is that we can continue to build confidence in viral respiratory vaccines like the influenza vaccine by continuing to generate rigorous evidence with the latest data.”
Ms. Behrouzi, who wasn’t involved with this study, has researched influenza vaccination and cardiovascular risk. She and her colleagues have found that flu vaccines were associated with a 34% lower risk of major adverse cardiovascular events, including a 45% reduced risk among patients with recent acute coronary syndrome.
“The broader public health message is for people to advocate for themselves and get the seasonal flu vaccine, especially if they are part of an at-risk group,” she said. “In our studies, we have positioned this message as a call to action not only for the public, but also for health care professionals – particularly specialists such as cardiologists or neurologists – to encourage or remind them to engage in conversation about the broad benefits of vaccination beyond just preventing or reducing the severity of flu infection.”
The study was conducted without outside funding. Dr. Holodinsky and Ms. Behrouzi have reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
The risk of stroke was about 23% lower in the 6 months following a flu shot, regardless of the patient’s age, sex, or underlying health conditions.
“There is an established link between upper respiratory infection and both heart attack and stroke. This has been very salient in the past few years throughout the COVID-19 pandemic,” study author Jessalyn Holodinsky, PhD, a stroke epidemiologist and postdoctoral fellow in clinical neurosciences at the University of Calgary (Alta.) told this news organization.
“It is also known that the flu shot can reduce risk of heart attack and hospitalization for those with heart disease,” she said. “Given both of these [observations], we thought it prudent to study whether there is a link between vaccination for influenza and stroke.”
The study was published in the Lancet Public Health.
Large effect size
The investigators analyzed administrative data from 2009 through 2018 from the Alberta Health Care Insurance Plan, which covers all residents of Alberta. The province provides free seasonal influenza vaccines to residents under the insurance plan.
The research team looked for stroke events such as acute ischemic stroke, intracerebral hemorrhage, subarachnoid hemorrhage, and transient ischemic attack. They then analyzed the risk of stroke events among those with or without a flu shot in the previous 6 months. They accounted for multiple factors, including age, sex, income, location, and factors related to stroke risk, such as anticoagulant use, atrial fibrillation, chronic obstructive pulmonary disease, diabetes, and hypertension.
Among the 4.1 million adults included in the researchers’ analysis, about 1.8 million (43%) received at least one vaccination during the study period. Nearly 97,000 people received a flu vaccine in each year they were in the study, including 29,288 who received a shot in all 10 flu seasons included in the study.
About 38,000 stroke events were recorded, including about 34,000 (90%) first stroke events. Among the 10% of strokes that were recurrent events, the maximum number of stroke events in one person was nine.
Overall, patients who received at least one influenza vaccine were more likely to be older, be women, and have higher rates of comorbidities. The vaccinated group had a slightly higher proportion of people who lived in urban areas, but the income levels were similar between the vaccinated and unvaccinated groups.
The crude incidence of stroke was higher among people who had ever received an influenza vaccination, at 1.25%, compared with 0.52% among those who hadn’t been vaccinated. However, after adjusting for age, sex, underlying conditions, and socioeconomic status, recent flu vaccination (that is, in the previous 6 months) was associated with a 23% reduced risk of stroke.
The significant reduction in risk applied to all stroke types, particularly acute ischemic stroke and intracerebral hemorrhage. In addition, influenza vaccination was associated with a reduced risk across all ages and risk profiles, except patients without hypertension.
“What we were most surprised by was the sheer magnitude of the effect and that it existed across different adult age groups, for both sexes, and for those with and without risk factors for stroke,” said Dr. Holodinsky.
Vaccination was associated with a larger reduction in stroke risk in men than in women, perhaps because unvaccinated men had a significantly higher baseline risk for stroke than unvaccinated women, the study authors write.
Promoting cardiovascular health
In addition, vaccination was associated with a greater relative reduction in stroke risk in younger age groups, lower income groups, and those with diabetes, chronic obstructive pulmonary disease, and anticoagulant use.
Among 2.4 million people observed for the entire study period, vaccination protection increased with the number of vaccines received. People who were vaccinated serially each year had a significantly lower risk of stroke than those who received one shot.
Dr. Holodinsky and colleagues are conducting additional research into influenza vaccination, including stroke risk in children. They’re also investigating whether the reduced risk applies to other vaccinations for respiratory illnesses, such as COVID-19 and pneumonia.
“We hope that this added effect of vaccination encourages more adults to receive the flu shot,” she said. “One day, vaccinations might be considered a key pillar of cardiovascular health, along with diet, exercise, control of hypertension and high cholesterol, and smoking cessation.”
Future research should also investigate the reasons why adults – particularly people at high risk with underlying conditions – don’t receive recommended influenza vaccines, the study authors wrote.
‘Call to action’
Bahar Behrouzi, an MD-PhD candidate focused on clinical epidemiology at the Institute of Health Policy, Management, and Evaluation, University of Toronto, said: “There are a variety of observational studies around the world that show that flu vaccine uptake is low among the general population and high-risk persons. In studying these questions, our hope is that we can continue to build confidence in viral respiratory vaccines like the influenza vaccine by continuing to generate rigorous evidence with the latest data.”
Ms. Behrouzi, who wasn’t involved with this study, has researched influenza vaccination and cardiovascular risk. She and her colleagues have found that flu vaccines were associated with a 34% lower risk of major adverse cardiovascular events, including a 45% reduced risk among patients with recent acute coronary syndrome.
“The broader public health message is for people to advocate for themselves and get the seasonal flu vaccine, especially if they are part of an at-risk group,” she said. “In our studies, we have positioned this message as a call to action not only for the public, but also for health care professionals – particularly specialists such as cardiologists or neurologists – to encourage or remind them to engage in conversation about the broad benefits of vaccination beyond just preventing or reducing the severity of flu infection.”
The study was conducted without outside funding. Dr. Holodinsky and Ms. Behrouzi have reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
The risk of stroke was about 23% lower in the 6 months following a flu shot, regardless of the patient’s age, sex, or underlying health conditions.
“There is an established link between upper respiratory infection and both heart attack and stroke. This has been very salient in the past few years throughout the COVID-19 pandemic,” study author Jessalyn Holodinsky, PhD, a stroke epidemiologist and postdoctoral fellow in clinical neurosciences at the University of Calgary (Alta.) told this news organization.
“It is also known that the flu shot can reduce risk of heart attack and hospitalization for those with heart disease,” she said. “Given both of these [observations], we thought it prudent to study whether there is a link between vaccination for influenza and stroke.”
The study was published in the Lancet Public Health.
Large effect size
The investigators analyzed administrative data from 2009 through 2018 from the Alberta Health Care Insurance Plan, which covers all residents of Alberta. The province provides free seasonal influenza vaccines to residents under the insurance plan.
The research team looked for stroke events such as acute ischemic stroke, intracerebral hemorrhage, subarachnoid hemorrhage, and transient ischemic attack. They then analyzed the risk of stroke events among those with or without a flu shot in the previous 6 months. They accounted for multiple factors, including age, sex, income, location, and factors related to stroke risk, such as anticoagulant use, atrial fibrillation, chronic obstructive pulmonary disease, diabetes, and hypertension.
Among the 4.1 million adults included in the researchers’ analysis, about 1.8 million (43%) received at least one vaccination during the study period. Nearly 97,000 people received a flu vaccine in each year they were in the study, including 29,288 who received a shot in all 10 flu seasons included in the study.
About 38,000 stroke events were recorded, including about 34,000 (90%) first stroke events. Among the 10% of strokes that were recurrent events, the maximum number of stroke events in one person was nine.
Overall, patients who received at least one influenza vaccine were more likely to be older, be women, and have higher rates of comorbidities. The vaccinated group had a slightly higher proportion of people who lived in urban areas, but the income levels were similar between the vaccinated and unvaccinated groups.
The crude incidence of stroke was higher among people who had ever received an influenza vaccination, at 1.25%, compared with 0.52% among those who hadn’t been vaccinated. However, after adjusting for age, sex, underlying conditions, and socioeconomic status, recent flu vaccination (that is, in the previous 6 months) was associated with a 23% reduced risk of stroke.
The significant reduction in risk applied to all stroke types, particularly acute ischemic stroke and intracerebral hemorrhage. In addition, influenza vaccination was associated with a reduced risk across all ages and risk profiles, except patients without hypertension.
“What we were most surprised by was the sheer magnitude of the effect and that it existed across different adult age groups, for both sexes, and for those with and without risk factors for stroke,” said Dr. Holodinsky.
Vaccination was associated with a larger reduction in stroke risk in men than in women, perhaps because unvaccinated men had a significantly higher baseline risk for stroke than unvaccinated women, the study authors write.
Promoting cardiovascular health
In addition, vaccination was associated with a greater relative reduction in stroke risk in younger age groups, lower income groups, and those with diabetes, chronic obstructive pulmonary disease, and anticoagulant use.
Among 2.4 million people observed for the entire study period, vaccination protection increased with the number of vaccines received. People who were vaccinated serially each year had a significantly lower risk of stroke than those who received one shot.
Dr. Holodinsky and colleagues are conducting additional research into influenza vaccination, including stroke risk in children. They’re also investigating whether the reduced risk applies to other vaccinations for respiratory illnesses, such as COVID-19 and pneumonia.
“We hope that this added effect of vaccination encourages more adults to receive the flu shot,” she said. “One day, vaccinations might be considered a key pillar of cardiovascular health, along with diet, exercise, control of hypertension and high cholesterol, and smoking cessation.”
Future research should also investigate the reasons why adults – particularly people at high risk with underlying conditions – don’t receive recommended influenza vaccines, the study authors wrote.
‘Call to action’
Bahar Behrouzi, an MD-PhD candidate focused on clinical epidemiology at the Institute of Health Policy, Management, and Evaluation, University of Toronto, said: “There are a variety of observational studies around the world that show that flu vaccine uptake is low among the general population and high-risk persons. In studying these questions, our hope is that we can continue to build confidence in viral respiratory vaccines like the influenza vaccine by continuing to generate rigorous evidence with the latest data.”
Ms. Behrouzi, who wasn’t involved with this study, has researched influenza vaccination and cardiovascular risk. She and her colleagues have found that flu vaccines were associated with a 34% lower risk of major adverse cardiovascular events, including a 45% reduced risk among patients with recent acute coronary syndrome.
“The broader public health message is for people to advocate for themselves and get the seasonal flu vaccine, especially if they are part of an at-risk group,” she said. “In our studies, we have positioned this message as a call to action not only for the public, but also for health care professionals – particularly specialists such as cardiologists or neurologists – to encourage or remind them to engage in conversation about the broad benefits of vaccination beyond just preventing or reducing the severity of flu infection.”
The study was conducted without outside funding. Dr. Holodinsky and Ms. Behrouzi have reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM LANCET PUBLIC HEALTH
Tirzepatide cuts BP during obesity treatment
CHICAGO – compared with placebo, while causing modest increases in heart rate, in a prespecified substudy of the SURMOUNT-1 trial.
“The large effects on ambulatory 24-hour blood pressure raise the possibility that there may be important long-term benefits of [tirzepatide] on the complications of obesity,” said James A. de Lemos, MD, during a presentation at the American Heart Association scientific sessions.
“The findings are concordant with the [previously reported] office-based measurements, and the blood pressure reductions provide further evidence for the potential benefits of tirzepatide on cardiovascular health and outcomes,” said Dr. de Lemos, a cardiologist and professor at the University of Texas Southwestern Medical Center, Dallas.
The substudy included 600 of the 2,539 people enrolled in SURMOUNT-1, the first of two pivotal trials for tirzepatide (Mounjaro) in people without diabetes but with obesity or overweight (body mass index of 27-29 kg/m2) plus at least one weight-related complication. The primary endpoints of SURMOUNT-1 were the percent change in weight from baseline to 72 weeks on treatment with either of three different weekly injected doses of tirzepatide, compared with control subjects who received placebo, and the percentage of enrolled subjects achieving at least 5% loss in baseline weight, compared with the controls.
Tirzepatide treatment led to significant increases in both results, compared with controls, with the highest dose tested, 15 mg/week, resulting in an average 20.9% drop in weight from baseline after 72 weeks of treatment, and 91% of enrolled subjects on that dose achieving the 5% weight-loss threshold during the same time frame, in results published in 2022 in the New England Journal of Medicine.
24-hour ambulatory pressures from 494 people
The substudy enrolled 600 of the SURMOUNT-1 participants and involved 24-hour ambulatory BP and heart rate measurements at entry and after 36 weeks on treatment. Full results were available for 494 of these people. The substudy included only study participants who entered with a BP of less than 140/90 mm Hg. Enrollment in SURMOUNT-1 overall excluded people with a BP of 160/100 mm Hg or higher. The average BP among all enrolled participants was about 123/80 mm Hg, while heart rates averaged about 73 beats per minute.
Systolic BP measured with the ambulatory monitor fell from baseline by an average of 5.6, 8.8, and 6.2 mm Hg in the people who received tirzepatide in weekly doses of 5, 10, or 15 mg, respectively, and rose by an average 1.8 mm Hg among the controls, Dr. de Lemos reported. Diastolic BP dropped among the tirzepatide recipients by an average of 1.5, 2.4, and 0.0 mm Hg in the three ascending tirzepatide treatment arms, and rose by an average 0.5 mm Hg among the controls. All of the differences between the intervention groups and the controls were significant except for the change in diastolic BP among participants who received 15 mg of tirzepatide weekly.
The results showed that 36 weeks on tirzepatide treatment was associated with “arguably clinically meaningful” reductions in systolic and diastolic BPs, Dr. de Lemos said. “There is a lot of optimism that this will translate into clinical benefits.” He also noted that, “within the limits of cross-study comparisons, the blood pressure changes look favorable, compared with the single-incretin mechanism GLP-1 [glucagonlike peptide–1] receptor agonists.”
Heart rate fell by an average 1.8 bpm in the controls, and rose by an average 0.3, 0.5, and 3.6 bpm among the three groups receiving ascending weekly tirzepatide doses, effects that were “consistent with what’s been seen with the GLP-1 receptor agonists,” noted Dr. de Lemos.
Tirzepatide is known as a “twincretin” because it shares this GLP-1 receptor agonism and also has a second incretin agonist activity, to the receptor for the glucose-dependent insulinotropic polypeptide.
Lowering of blood pressure plateaus
Changes in BP over time during the 72 weeks on treatment, data first presented in the original report, showed that average systolic pressure in the people who received tirzepatide fell sharply during the first 24 weeks on treatment, and then leveled out with little further change over time. Furthermore, all three tirzepatide doses produced roughly similar systolic BP reductions. Changes in diastolic pressure over time showed a mostly similar pattern of reduction, although a modest ongoing decrease in average diastolic pressure continued beyond 24 weeks.
This pattern of a plateau in BP reduction has been seen before in studies using other treatments to produce weight loss, including bariatric surgery, said Naveed Sattar, MBChB, PhD, professor of metabolic medicine at the University of Glasgow, who was not involved in SURMOUNT-1. He attributed the plateau in BP reduction among tirzepatide-treated people to them hitting a wall in their BP nadir based on homeostatic limits. Dr. Sattar noted that most enrolled participants had normal BPs at entry based on the reported study averages.
“It’s hard to go lower, but the blood pressure reduction may be larger in people who start at higher pressure levels,” Dr. Sattar said in an interview.
Another inferred cap on BP reductions in the trial hypothesizes that the individual clinicians who managed the enrolled patients may have cut back on other BP-lowering agents as the pressures of the tirzepatide recipients fell to relatively low levels, suggested Darren McGuire, MD, a cardiologist and professor at UT Southwestern Medical Center, who also was not involved in the SURMOUNT-1 study.
Incretin agonists as antihypertensive drugs
The substantial BP-lowering seen with tirzepatide, as well as with other incretin agonist agents, suggests a new way to think about BP control in people with overweight or obesity, Dr. Sattar said.
“Until now, we haven’t had tools where people lose so much weight. Now that we have these tools [incretin agonists as well as bariatric surgery], we see substantial blood pressure reductions. It makes you think we should use weight-loss agents to lower blood pressure rather than a beta-blocker or angiotensin-converting enzyme inhibitor; then we’d also produce all the other benefits from weight loss,” Dr. Sattar suggested.
Dr. de Lemos said he sees signals that the BP reductions caused by tirzepatide and the GLP-1 receptor agonists may go beyond just weight-loss effects.
“There appears to be a larger blood pressure reduction than anticipated based on the change in weight,” he said during his presentation. “GLP-1 is active in most vascular tissues, so these [receptor agonist] agents likely have vascular or cardiac effects, or even effects on other tissues that may affect blood pressure.”
Heart rate increases were usually modest
The experiences with GLP-1 receptor agonists also suggest that the heart rate increases seen with tirzepatide treatment in SURMOUNT-1 will not have long-term effects. “The [Food and Drug Administration] mandated this heart rate substudy to make sure that the increase in heart rate was not larger than what would be anticipated” with a GLP-1 receptor agonist, Dr. de Lemos explained.
SURMOUNT-1 had a treatment-stopping rule to prevent a person’s heart rate from rising beyond 10 bpm from baseline. “Trivial numbers” of patients experienced a heart rate increase of this magnitude, he said. If used in routine practice, Dr. de Lemos said that he would closely investigate a patient with a heart rate increase greater than 10 mm Hg. The average increase seen with the highest dose, about 4 bpm above baseline, would generally not be concerning.
Tirzepatide received U.S. marketing approval from the FDA in May 2022 for treating people with type 2 diabetes. In October 2022, the FDA gave tirzepatide “Fast Track” designation for the pending application for approval of an indication to treat people with overweight or obesity who match the entry criteria for SURMOUNT-1 and for the second pivotal trial for this indication, SURMOUNT-2. According to a statement from Eli Lilly, the company that is developing and markets tirzepatide (Mounjaro), the FDA’s decision on the obesity indication will remain pending until the SURMOUNT-2 results are available, which the company expects will occur in 2023.
SURMOUNT-1 and SURMOUNT-2 were sponsored by Lilly, the company that markets tirzepatide. Dr. de Lemos has been a consultant to Lilly as well as to Amgen, AstraZeneca, Janssen, Novo Nordisk, Ortho, Quidel Cardiovascular, and Regeneron. Dr. Sattar has financial ties to Lilly, Afimmune, Amgen, AstraZeneca, Boehringer Ingelheim, Hammi, Merck Sharpe & Dohme, Novartis, Novo Nordisk, Pfizer, Roche, and Sanofi-Aventis. Dr. McGuire has ties to Lilly as well as to Altimmune, Applied Therapeutics, Bayer, Boehringer Ingelheim, CSL Behring, Lexicon, Merck, Metavant, Novo Nordisk, and Sanofi.
CHICAGO – compared with placebo, while causing modest increases in heart rate, in a prespecified substudy of the SURMOUNT-1 trial.
“The large effects on ambulatory 24-hour blood pressure raise the possibility that there may be important long-term benefits of [tirzepatide] on the complications of obesity,” said James A. de Lemos, MD, during a presentation at the American Heart Association scientific sessions.
“The findings are concordant with the [previously reported] office-based measurements, and the blood pressure reductions provide further evidence for the potential benefits of tirzepatide on cardiovascular health and outcomes,” said Dr. de Lemos, a cardiologist and professor at the University of Texas Southwestern Medical Center, Dallas.
The substudy included 600 of the 2,539 people enrolled in SURMOUNT-1, the first of two pivotal trials for tirzepatide (Mounjaro) in people without diabetes but with obesity or overweight (body mass index of 27-29 kg/m2) plus at least one weight-related complication. The primary endpoints of SURMOUNT-1 were the percent change in weight from baseline to 72 weeks on treatment with either of three different weekly injected doses of tirzepatide, compared with control subjects who received placebo, and the percentage of enrolled subjects achieving at least 5% loss in baseline weight, compared with the controls.
Tirzepatide treatment led to significant increases in both results, compared with controls, with the highest dose tested, 15 mg/week, resulting in an average 20.9% drop in weight from baseline after 72 weeks of treatment, and 91% of enrolled subjects on that dose achieving the 5% weight-loss threshold during the same time frame, in results published in 2022 in the New England Journal of Medicine.
24-hour ambulatory pressures from 494 people
The substudy enrolled 600 of the SURMOUNT-1 participants and involved 24-hour ambulatory BP and heart rate measurements at entry and after 36 weeks on treatment. Full results were available for 494 of these people. The substudy included only study participants who entered with a BP of less than 140/90 mm Hg. Enrollment in SURMOUNT-1 overall excluded people with a BP of 160/100 mm Hg or higher. The average BP among all enrolled participants was about 123/80 mm Hg, while heart rates averaged about 73 beats per minute.
Systolic BP measured with the ambulatory monitor fell from baseline by an average of 5.6, 8.8, and 6.2 mm Hg in the people who received tirzepatide in weekly doses of 5, 10, or 15 mg, respectively, and rose by an average 1.8 mm Hg among the controls, Dr. de Lemos reported. Diastolic BP dropped among the tirzepatide recipients by an average of 1.5, 2.4, and 0.0 mm Hg in the three ascending tirzepatide treatment arms, and rose by an average 0.5 mm Hg among the controls. All of the differences between the intervention groups and the controls were significant except for the change in diastolic BP among participants who received 15 mg of tirzepatide weekly.
The results showed that 36 weeks on tirzepatide treatment was associated with “arguably clinically meaningful” reductions in systolic and diastolic BPs, Dr. de Lemos said. “There is a lot of optimism that this will translate into clinical benefits.” He also noted that, “within the limits of cross-study comparisons, the blood pressure changes look favorable, compared with the single-incretin mechanism GLP-1 [glucagonlike peptide–1] receptor agonists.”
Heart rate fell by an average 1.8 bpm in the controls, and rose by an average 0.3, 0.5, and 3.6 bpm among the three groups receiving ascending weekly tirzepatide doses, effects that were “consistent with what’s been seen with the GLP-1 receptor agonists,” noted Dr. de Lemos.
Tirzepatide is known as a “twincretin” because it shares this GLP-1 receptor agonism and also has a second incretin agonist activity, to the receptor for the glucose-dependent insulinotropic polypeptide.
Lowering of blood pressure plateaus
Changes in BP over time during the 72 weeks on treatment, data first presented in the original report, showed that average systolic pressure in the people who received tirzepatide fell sharply during the first 24 weeks on treatment, and then leveled out with little further change over time. Furthermore, all three tirzepatide doses produced roughly similar systolic BP reductions. Changes in diastolic pressure over time showed a mostly similar pattern of reduction, although a modest ongoing decrease in average diastolic pressure continued beyond 24 weeks.
This pattern of a plateau in BP reduction has been seen before in studies using other treatments to produce weight loss, including bariatric surgery, said Naveed Sattar, MBChB, PhD, professor of metabolic medicine at the University of Glasgow, who was not involved in SURMOUNT-1. He attributed the plateau in BP reduction among tirzepatide-treated people to them hitting a wall in their BP nadir based on homeostatic limits. Dr. Sattar noted that most enrolled participants had normal BPs at entry based on the reported study averages.
“It’s hard to go lower, but the blood pressure reduction may be larger in people who start at higher pressure levels,” Dr. Sattar said in an interview.
Another inferred cap on BP reductions in the trial hypothesizes that the individual clinicians who managed the enrolled patients may have cut back on other BP-lowering agents as the pressures of the tirzepatide recipients fell to relatively low levels, suggested Darren McGuire, MD, a cardiologist and professor at UT Southwestern Medical Center, who also was not involved in the SURMOUNT-1 study.
Incretin agonists as antihypertensive drugs
The substantial BP-lowering seen with tirzepatide, as well as with other incretin agonist agents, suggests a new way to think about BP control in people with overweight or obesity, Dr. Sattar said.
“Until now, we haven’t had tools where people lose so much weight. Now that we have these tools [incretin agonists as well as bariatric surgery], we see substantial blood pressure reductions. It makes you think we should use weight-loss agents to lower blood pressure rather than a beta-blocker or angiotensin-converting enzyme inhibitor; then we’d also produce all the other benefits from weight loss,” Dr. Sattar suggested.
Dr. de Lemos said he sees signals that the BP reductions caused by tirzepatide and the GLP-1 receptor agonists may go beyond just weight-loss effects.
“There appears to be a larger blood pressure reduction than anticipated based on the change in weight,” he said during his presentation. “GLP-1 is active in most vascular tissues, so these [receptor agonist] agents likely have vascular or cardiac effects, or even effects on other tissues that may affect blood pressure.”
Heart rate increases were usually modest
The experiences with GLP-1 receptor agonists also suggest that the heart rate increases seen with tirzepatide treatment in SURMOUNT-1 will not have long-term effects. “The [Food and Drug Administration] mandated this heart rate substudy to make sure that the increase in heart rate was not larger than what would be anticipated” with a GLP-1 receptor agonist, Dr. de Lemos explained.
SURMOUNT-1 had a treatment-stopping rule to prevent a person’s heart rate from rising beyond 10 bpm from baseline. “Trivial numbers” of patients experienced a heart rate increase of this magnitude, he said. If used in routine practice, Dr. de Lemos said that he would closely investigate a patient with a heart rate increase greater than 10 mm Hg. The average increase seen with the highest dose, about 4 bpm above baseline, would generally not be concerning.
Tirzepatide received U.S. marketing approval from the FDA in May 2022 for treating people with type 2 diabetes. In October 2022, the FDA gave tirzepatide “Fast Track” designation for the pending application for approval of an indication to treat people with overweight or obesity who match the entry criteria for SURMOUNT-1 and for the second pivotal trial for this indication, SURMOUNT-2. According to a statement from Eli Lilly, the company that is developing and markets tirzepatide (Mounjaro), the FDA’s decision on the obesity indication will remain pending until the SURMOUNT-2 results are available, which the company expects will occur in 2023.
SURMOUNT-1 and SURMOUNT-2 were sponsored by Lilly, the company that markets tirzepatide. Dr. de Lemos has been a consultant to Lilly as well as to Amgen, AstraZeneca, Janssen, Novo Nordisk, Ortho, Quidel Cardiovascular, and Regeneron. Dr. Sattar has financial ties to Lilly, Afimmune, Amgen, AstraZeneca, Boehringer Ingelheim, Hammi, Merck Sharpe & Dohme, Novartis, Novo Nordisk, Pfizer, Roche, and Sanofi-Aventis. Dr. McGuire has ties to Lilly as well as to Altimmune, Applied Therapeutics, Bayer, Boehringer Ingelheim, CSL Behring, Lexicon, Merck, Metavant, Novo Nordisk, and Sanofi.
CHICAGO – compared with placebo, while causing modest increases in heart rate, in a prespecified substudy of the SURMOUNT-1 trial.
“The large effects on ambulatory 24-hour blood pressure raise the possibility that there may be important long-term benefits of [tirzepatide] on the complications of obesity,” said James A. de Lemos, MD, during a presentation at the American Heart Association scientific sessions.
“The findings are concordant with the [previously reported] office-based measurements, and the blood pressure reductions provide further evidence for the potential benefits of tirzepatide on cardiovascular health and outcomes,” said Dr. de Lemos, a cardiologist and professor at the University of Texas Southwestern Medical Center, Dallas.
The substudy included 600 of the 2,539 people enrolled in SURMOUNT-1, the first of two pivotal trials for tirzepatide (Mounjaro) in people without diabetes but with obesity or overweight (body mass index of 27-29 kg/m2) plus at least one weight-related complication. The primary endpoints of SURMOUNT-1 were the percent change in weight from baseline to 72 weeks on treatment with either of three different weekly injected doses of tirzepatide, compared with control subjects who received placebo, and the percentage of enrolled subjects achieving at least 5% loss in baseline weight, compared with the controls.
Tirzepatide treatment led to significant increases in both results, compared with controls, with the highest dose tested, 15 mg/week, resulting in an average 20.9% drop in weight from baseline after 72 weeks of treatment, and 91% of enrolled subjects on that dose achieving the 5% weight-loss threshold during the same time frame, in results published in 2022 in the New England Journal of Medicine.
24-hour ambulatory pressures from 494 people
The substudy enrolled 600 of the SURMOUNT-1 participants and involved 24-hour ambulatory BP and heart rate measurements at entry and after 36 weeks on treatment. Full results were available for 494 of these people. The substudy included only study participants who entered with a BP of less than 140/90 mm Hg. Enrollment in SURMOUNT-1 overall excluded people with a BP of 160/100 mm Hg or higher. The average BP among all enrolled participants was about 123/80 mm Hg, while heart rates averaged about 73 beats per minute.
Systolic BP measured with the ambulatory monitor fell from baseline by an average of 5.6, 8.8, and 6.2 mm Hg in the people who received tirzepatide in weekly doses of 5, 10, or 15 mg, respectively, and rose by an average 1.8 mm Hg among the controls, Dr. de Lemos reported. Diastolic BP dropped among the tirzepatide recipients by an average of 1.5, 2.4, and 0.0 mm Hg in the three ascending tirzepatide treatment arms, and rose by an average 0.5 mm Hg among the controls. All of the differences between the intervention groups and the controls were significant except for the change in diastolic BP among participants who received 15 mg of tirzepatide weekly.
The results showed that 36 weeks on tirzepatide treatment was associated with “arguably clinically meaningful” reductions in systolic and diastolic BPs, Dr. de Lemos said. “There is a lot of optimism that this will translate into clinical benefits.” He also noted that, “within the limits of cross-study comparisons, the blood pressure changes look favorable, compared with the single-incretin mechanism GLP-1 [glucagonlike peptide–1] receptor agonists.”
Heart rate fell by an average 1.8 bpm in the controls, and rose by an average 0.3, 0.5, and 3.6 bpm among the three groups receiving ascending weekly tirzepatide doses, effects that were “consistent with what’s been seen with the GLP-1 receptor agonists,” noted Dr. de Lemos.
Tirzepatide is known as a “twincretin” because it shares this GLP-1 receptor agonism and also has a second incretin agonist activity, to the receptor for the glucose-dependent insulinotropic polypeptide.
Lowering of blood pressure plateaus
Changes in BP over time during the 72 weeks on treatment, data first presented in the original report, showed that average systolic pressure in the people who received tirzepatide fell sharply during the first 24 weeks on treatment, and then leveled out with little further change over time. Furthermore, all three tirzepatide doses produced roughly similar systolic BP reductions. Changes in diastolic pressure over time showed a mostly similar pattern of reduction, although a modest ongoing decrease in average diastolic pressure continued beyond 24 weeks.
This pattern of a plateau in BP reduction has been seen before in studies using other treatments to produce weight loss, including bariatric surgery, said Naveed Sattar, MBChB, PhD, professor of metabolic medicine at the University of Glasgow, who was not involved in SURMOUNT-1. He attributed the plateau in BP reduction among tirzepatide-treated people to them hitting a wall in their BP nadir based on homeostatic limits. Dr. Sattar noted that most enrolled participants had normal BPs at entry based on the reported study averages.
“It’s hard to go lower, but the blood pressure reduction may be larger in people who start at higher pressure levels,” Dr. Sattar said in an interview.
Another inferred cap on BP reductions in the trial hypothesizes that the individual clinicians who managed the enrolled patients may have cut back on other BP-lowering agents as the pressures of the tirzepatide recipients fell to relatively low levels, suggested Darren McGuire, MD, a cardiologist and professor at UT Southwestern Medical Center, who also was not involved in the SURMOUNT-1 study.
Incretin agonists as antihypertensive drugs
The substantial BP-lowering seen with tirzepatide, as well as with other incretin agonist agents, suggests a new way to think about BP control in people with overweight or obesity, Dr. Sattar said.
“Until now, we haven’t had tools where people lose so much weight. Now that we have these tools [incretin agonists as well as bariatric surgery], we see substantial blood pressure reductions. It makes you think we should use weight-loss agents to lower blood pressure rather than a beta-blocker or angiotensin-converting enzyme inhibitor; then we’d also produce all the other benefits from weight loss,” Dr. Sattar suggested.
Dr. de Lemos said he sees signals that the BP reductions caused by tirzepatide and the GLP-1 receptor agonists may go beyond just weight-loss effects.
“There appears to be a larger blood pressure reduction than anticipated based on the change in weight,” he said during his presentation. “GLP-1 is active in most vascular tissues, so these [receptor agonist] agents likely have vascular or cardiac effects, or even effects on other tissues that may affect blood pressure.”
Heart rate increases were usually modest
The experiences with GLP-1 receptor agonists also suggest that the heart rate increases seen with tirzepatide treatment in SURMOUNT-1 will not have long-term effects. “The [Food and Drug Administration] mandated this heart rate substudy to make sure that the increase in heart rate was not larger than what would be anticipated” with a GLP-1 receptor agonist, Dr. de Lemos explained.
SURMOUNT-1 had a treatment-stopping rule to prevent a person’s heart rate from rising beyond 10 bpm from baseline. “Trivial numbers” of patients experienced a heart rate increase of this magnitude, he said. If used in routine practice, Dr. de Lemos said that he would closely investigate a patient with a heart rate increase greater than 10 mm Hg. The average increase seen with the highest dose, about 4 bpm above baseline, would generally not be concerning.
Tirzepatide received U.S. marketing approval from the FDA in May 2022 for treating people with type 2 diabetes. In October 2022, the FDA gave tirzepatide “Fast Track” designation for the pending application for approval of an indication to treat people with overweight or obesity who match the entry criteria for SURMOUNT-1 and for the second pivotal trial for this indication, SURMOUNT-2. According to a statement from Eli Lilly, the company that is developing and markets tirzepatide (Mounjaro), the FDA’s decision on the obesity indication will remain pending until the SURMOUNT-2 results are available, which the company expects will occur in 2023.
SURMOUNT-1 and SURMOUNT-2 were sponsored by Lilly, the company that markets tirzepatide. Dr. de Lemos has been a consultant to Lilly as well as to Amgen, AstraZeneca, Janssen, Novo Nordisk, Ortho, Quidel Cardiovascular, and Regeneron. Dr. Sattar has financial ties to Lilly, Afimmune, Amgen, AstraZeneca, Boehringer Ingelheim, Hammi, Merck Sharpe & Dohme, Novartis, Novo Nordisk, Pfizer, Roche, and Sanofi-Aventis. Dr. McGuire has ties to Lilly as well as to Altimmune, Applied Therapeutics, Bayer, Boehringer Ingelheim, CSL Behring, Lexicon, Merck, Metavant, Novo Nordisk, and Sanofi.
AT AHA 2022
Statins boost glycemia slightly, but CVD benefits prevail
CHICAGO – A new, expanded meta-analysis confirmed the long-known effect that statin treatment has on raising blood glucose levels and causing incident diabetes, but it also documented that these effects are small and any risk they pose to statin users is dwarfed by the cholesterol-lowering effect of statins and their ability to reduce risk for atherosclerotic cardiovascular disease (ASCVD).
This meta-analysis of 23 trials with a total of more than 150,000 participants showed that statin therapy significantly increased the risk for new-onset diabetes and worsening glycemia, driven by a “very small but generalized increase in glucose,” with a greater effect from high-intensity statin regimens and a similar but somewhat more muted effect from low- and moderate-intensity statin treatment, David Preiss, MBChB, PhD, reported at the American Heart Association scientific sessions.
Dr. Preiss also stressed that despite this, “the cardiovascular benefits of statin therapy remain substantial and profound” in people regardless of whether they have diabetes, prediabetes, or normoglycemia when they start statin treatment, noting that the impact of even high-intensity statin treatment is “absolutely tiny” increases in hemoglobin A1c and blood glucose.
“This does not detract from the substantial benefit of statin treatment,” declared Dr. Preiss, a metabolic medicine specialist and endocrinologist at Oxford (England) University.
Small glycemia increases ‘nudge’ some into diabetes
The data Dr. Preiss reported showed that high-intensity statin treatment (atorvastatin at a daily dose of at least 40 mg, or rosuvastatin at a daily dose of at least 20 mg) led to an average increase in A1c levels of 0.08 percentage points among people without diabetes when their treatment began and 0.24 percentage points among people already diagnosed with diabetes. Blood glucose levels rose by an average of 0.04 mmol/L (less than 1 mg/d) in those without diabetes, and by an average 0.22 mmol/L (about 4 mg/dL) in those with diabetes. People who received low- or moderate-intensity statin regimens had significant but smaller increases.
“We’re not talking about people going from no diabetes to frank diabetes. We’re talking about [statins] nudging a very small number of people across a diabetes threshold,” an A1c of 6.5% that is set somewhat arbitrarily based on an increased risk for developing retinopathy, Dr. Preiss said. ”A person just needs to lose a [daily] can of Coke’s worth of weight to eliminate any apparent diabetes risk,” he noted.
Benefit outweighs risks by three- to sevenfold
Dr. Preiss presented two other examples of what his findings showed to illustrate the relatively small risk posed by statin therapy compared with its potential benefits. Treating 10,000 people for 5 years with a high-intensity statin regimen in those with established ASCVD (secondary prevention) would result in an increment of 150 extra people developing diabetes because of the hyperglycemic effect of statins, compared with an expected prevention of 1,000 ASCVD events. Among 10,000 people at high ASCVD risk and taking a high-intensity statin regimen for primary prevention 5 years of treatment would result in roughly 130 extra cases of incident diabetes while preventing about 500 ASCVD events.
In addition, applying the new risk estimates to the people included in the UK Biobank database, whose median A1c is 5.5%, showed that a high-intensity statin regimen could be expected to raise the prevalence of those with an A1c of 6.5% or greater from 4.5% to 5.7%.
Several preventive cardiologists who heard the report and were not involved with the analysis agreed with Dr. Preiss that the benefits of statin treatment substantially offset this confirmed hyperglycemic effect.
Risk ‘more than counterbalanced by benefit’
“He clearly showed that the small hyperglycemia risk posed by statin use is more than counterbalanced by its benefit for reducing ASCVD events,” commented Neil J. Stone, MD, a cardiologist and professor of medicine at Northwestern University, Chicago. “I agree that, for those with prediabetes who are on the road to diabetes with or without a statin, the small increase in glucose with a statin should not dissuade statin usage because the benefit is so large. Rather, it should focus efforts to improve diet, increase physical activity, and keep weight controlled.”
Dr. Stone also noted in an interview that in the JUPITER trial, which examined the effects of a daily 20-mg dose of rosuvastatin (Crestor), a high-intensity regimen, study participants with diabetes risk factors who were assigned to rosuvastatin had an onset of diabetes that was earlier than people assigned to placebo by only about 5.4 weeks, yet this group had evidence of significant benefit.
“I agree with Dr. Preiss that the benefits of statins in reducing heart attack, stroke, and cardiovascular death far outweigh their modest effects on glycemia,” commented Brendan M. Everett, MD, a cardiologist and preventive medicine specialist at Brigham and Women’s Hospital in Boston. “This is particularly true for those with preexisting prediabetes or diabetes, who have an elevated risk of atherosclerotic events and thus stand to derive more significant benefit from statins. The benefits of lowering LDL cholesterol with a statin for preventing seriously morbid, and potentially fatal, cardiovascular events far outweigh the extremely modest, or even negligible, increases in the risk of diabetes that could be seen with the extremely small increases in A1c,” Dr. Everett said in an interview.
The new findings “reaffirm that there is a increased risk [from statins] but the most important point is that it is a very, very tiny difference in A1c,” commented Marc S. Sabatine, MD, a cardiologist and professor at Harvard Medical School, Boston. “These data have been known for quite some time, but this analysis was done in a more rigorous way.” The finding of “a small increase in risk for diabetes is really because diabetes has a biochemical threshold and statin treatment nudges some people a little past a line that is semi-arbitrary. It’s important to be cognizant of this, but it in no way dissuades me from treating patients aggressively with statins to reduce their ASCVD risk. I would monitor their A1c levels, and if they go higher and can’t be controlled with lifestyle we have plenty of medications that can control it,” he said in an interview.
No difference by statin type
The meta-analysis used data from 13 placebo-controlled statin trials that together involved 123,940 participants and had an average 4.3 years of follow-up, and four trials that compared one statin with another and collectively involved 30,734 participants with an average 4.9 years of follow-up.
The analyses showed that high-intensity statin treatment increased the rate of incident diabetes by a significant 36% relative to controls and increased the rate of worsening glycemia by a significant 24% compared with controls. Low- or moderate-intensity statin regimens increased incident diabetes by a significant 10% and raised the incidence of worsening glycemia by a significant 10% compared with controls, Dr. Preiss reported.
These effects did not significantly differ by type of statin (the study included people treated with atorvastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin, and simvastatin), nor across a variety of subgroups based on age, sex, race, body mass index, diabetes risk, renal function, cholesterol levels, or cardiovascular disease. The effect was also consistent regardless of the duration of treatment.
Dr. Preiss also downplayed the magnitude of the apparent difference in risk posed by high-intensity and less intense statin regimens. “I suspect the apparent heterogeneity is true, but not quite as big as what we see,” he said.
The mechanisms by which statins have this effect remain unclear, but evidence suggests that it may be a direct effect of the main action of statins, inhibition of the HMG-CoA reductase enzyme.
The study received no commercial funding. Dr. Preiss and Dr. Stone had no disclosures. Dr. Everett has been a consultant to Eli Lilly, Gilead, Ipsen, Janssen, and Provention. Dr. Sabatine has been a consultant to Althera, Amgen, Anthos Therapeutics, AstraZeneca, Beren Therapeutics, Bristol-Myers Squibb, DalCor, Dr Reddy’s Laboratories, Fibrogen, Intarcia, Merck, Moderna, Novo Nordisk, and Silence Therapeutics.
CHICAGO – A new, expanded meta-analysis confirmed the long-known effect that statin treatment has on raising blood glucose levels and causing incident diabetes, but it also documented that these effects are small and any risk they pose to statin users is dwarfed by the cholesterol-lowering effect of statins and their ability to reduce risk for atherosclerotic cardiovascular disease (ASCVD).
This meta-analysis of 23 trials with a total of more than 150,000 participants showed that statin therapy significantly increased the risk for new-onset diabetes and worsening glycemia, driven by a “very small but generalized increase in glucose,” with a greater effect from high-intensity statin regimens and a similar but somewhat more muted effect from low- and moderate-intensity statin treatment, David Preiss, MBChB, PhD, reported at the American Heart Association scientific sessions.
Dr. Preiss also stressed that despite this, “the cardiovascular benefits of statin therapy remain substantial and profound” in people regardless of whether they have diabetes, prediabetes, or normoglycemia when they start statin treatment, noting that the impact of even high-intensity statin treatment is “absolutely tiny” increases in hemoglobin A1c and blood glucose.
“This does not detract from the substantial benefit of statin treatment,” declared Dr. Preiss, a metabolic medicine specialist and endocrinologist at Oxford (England) University.
Small glycemia increases ‘nudge’ some into diabetes
The data Dr. Preiss reported showed that high-intensity statin treatment (atorvastatin at a daily dose of at least 40 mg, or rosuvastatin at a daily dose of at least 20 mg) led to an average increase in A1c levels of 0.08 percentage points among people without diabetes when their treatment began and 0.24 percentage points among people already diagnosed with diabetes. Blood glucose levels rose by an average of 0.04 mmol/L (less than 1 mg/d) in those without diabetes, and by an average 0.22 mmol/L (about 4 mg/dL) in those with diabetes. People who received low- or moderate-intensity statin regimens had significant but smaller increases.
“We’re not talking about people going from no diabetes to frank diabetes. We’re talking about [statins] nudging a very small number of people across a diabetes threshold,” an A1c of 6.5% that is set somewhat arbitrarily based on an increased risk for developing retinopathy, Dr. Preiss said. ”A person just needs to lose a [daily] can of Coke’s worth of weight to eliminate any apparent diabetes risk,” he noted.
Benefit outweighs risks by three- to sevenfold
Dr. Preiss presented two other examples of what his findings showed to illustrate the relatively small risk posed by statin therapy compared with its potential benefits. Treating 10,000 people for 5 years with a high-intensity statin regimen in those with established ASCVD (secondary prevention) would result in an increment of 150 extra people developing diabetes because of the hyperglycemic effect of statins, compared with an expected prevention of 1,000 ASCVD events. Among 10,000 people at high ASCVD risk and taking a high-intensity statin regimen for primary prevention 5 years of treatment would result in roughly 130 extra cases of incident diabetes while preventing about 500 ASCVD events.
In addition, applying the new risk estimates to the people included in the UK Biobank database, whose median A1c is 5.5%, showed that a high-intensity statin regimen could be expected to raise the prevalence of those with an A1c of 6.5% or greater from 4.5% to 5.7%.
Several preventive cardiologists who heard the report and were not involved with the analysis agreed with Dr. Preiss that the benefits of statin treatment substantially offset this confirmed hyperglycemic effect.
Risk ‘more than counterbalanced by benefit’
“He clearly showed that the small hyperglycemia risk posed by statin use is more than counterbalanced by its benefit for reducing ASCVD events,” commented Neil J. Stone, MD, a cardiologist and professor of medicine at Northwestern University, Chicago. “I agree that, for those with prediabetes who are on the road to diabetes with or without a statin, the small increase in glucose with a statin should not dissuade statin usage because the benefit is so large. Rather, it should focus efforts to improve diet, increase physical activity, and keep weight controlled.”
Dr. Stone also noted in an interview that in the JUPITER trial, which examined the effects of a daily 20-mg dose of rosuvastatin (Crestor), a high-intensity regimen, study participants with diabetes risk factors who were assigned to rosuvastatin had an onset of diabetes that was earlier than people assigned to placebo by only about 5.4 weeks, yet this group had evidence of significant benefit.
“I agree with Dr. Preiss that the benefits of statins in reducing heart attack, stroke, and cardiovascular death far outweigh their modest effects on glycemia,” commented Brendan M. Everett, MD, a cardiologist and preventive medicine specialist at Brigham and Women’s Hospital in Boston. “This is particularly true for those with preexisting prediabetes or diabetes, who have an elevated risk of atherosclerotic events and thus stand to derive more significant benefit from statins. The benefits of lowering LDL cholesterol with a statin for preventing seriously morbid, and potentially fatal, cardiovascular events far outweigh the extremely modest, or even negligible, increases in the risk of diabetes that could be seen with the extremely small increases in A1c,” Dr. Everett said in an interview.
The new findings “reaffirm that there is a increased risk [from statins] but the most important point is that it is a very, very tiny difference in A1c,” commented Marc S. Sabatine, MD, a cardiologist and professor at Harvard Medical School, Boston. “These data have been known for quite some time, but this analysis was done in a more rigorous way.” The finding of “a small increase in risk for diabetes is really because diabetes has a biochemical threshold and statin treatment nudges some people a little past a line that is semi-arbitrary. It’s important to be cognizant of this, but it in no way dissuades me from treating patients aggressively with statins to reduce their ASCVD risk. I would monitor their A1c levels, and if they go higher and can’t be controlled with lifestyle we have plenty of medications that can control it,” he said in an interview.
No difference by statin type
The meta-analysis used data from 13 placebo-controlled statin trials that together involved 123,940 participants and had an average 4.3 years of follow-up, and four trials that compared one statin with another and collectively involved 30,734 participants with an average 4.9 years of follow-up.
The analyses showed that high-intensity statin treatment increased the rate of incident diabetes by a significant 36% relative to controls and increased the rate of worsening glycemia by a significant 24% compared with controls. Low- or moderate-intensity statin regimens increased incident diabetes by a significant 10% and raised the incidence of worsening glycemia by a significant 10% compared with controls, Dr. Preiss reported.
These effects did not significantly differ by type of statin (the study included people treated with atorvastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin, and simvastatin), nor across a variety of subgroups based on age, sex, race, body mass index, diabetes risk, renal function, cholesterol levels, or cardiovascular disease. The effect was also consistent regardless of the duration of treatment.
Dr. Preiss also downplayed the magnitude of the apparent difference in risk posed by high-intensity and less intense statin regimens. “I suspect the apparent heterogeneity is true, but not quite as big as what we see,” he said.
The mechanisms by which statins have this effect remain unclear, but evidence suggests that it may be a direct effect of the main action of statins, inhibition of the HMG-CoA reductase enzyme.
The study received no commercial funding. Dr. Preiss and Dr. Stone had no disclosures. Dr. Everett has been a consultant to Eli Lilly, Gilead, Ipsen, Janssen, and Provention. Dr. Sabatine has been a consultant to Althera, Amgen, Anthos Therapeutics, AstraZeneca, Beren Therapeutics, Bristol-Myers Squibb, DalCor, Dr Reddy’s Laboratories, Fibrogen, Intarcia, Merck, Moderna, Novo Nordisk, and Silence Therapeutics.
CHICAGO – A new, expanded meta-analysis confirmed the long-known effect that statin treatment has on raising blood glucose levels and causing incident diabetes, but it also documented that these effects are small and any risk they pose to statin users is dwarfed by the cholesterol-lowering effect of statins and their ability to reduce risk for atherosclerotic cardiovascular disease (ASCVD).
This meta-analysis of 23 trials with a total of more than 150,000 participants showed that statin therapy significantly increased the risk for new-onset diabetes and worsening glycemia, driven by a “very small but generalized increase in glucose,” with a greater effect from high-intensity statin regimens and a similar but somewhat more muted effect from low- and moderate-intensity statin treatment, David Preiss, MBChB, PhD, reported at the American Heart Association scientific sessions.
Dr. Preiss also stressed that despite this, “the cardiovascular benefits of statin therapy remain substantial and profound” in people regardless of whether they have diabetes, prediabetes, or normoglycemia when they start statin treatment, noting that the impact of even high-intensity statin treatment is “absolutely tiny” increases in hemoglobin A1c and blood glucose.
“This does not detract from the substantial benefit of statin treatment,” declared Dr. Preiss, a metabolic medicine specialist and endocrinologist at Oxford (England) University.
Small glycemia increases ‘nudge’ some into diabetes
The data Dr. Preiss reported showed that high-intensity statin treatment (atorvastatin at a daily dose of at least 40 mg, or rosuvastatin at a daily dose of at least 20 mg) led to an average increase in A1c levels of 0.08 percentage points among people without diabetes when their treatment began and 0.24 percentage points among people already diagnosed with diabetes. Blood glucose levels rose by an average of 0.04 mmol/L (less than 1 mg/d) in those without diabetes, and by an average 0.22 mmol/L (about 4 mg/dL) in those with diabetes. People who received low- or moderate-intensity statin regimens had significant but smaller increases.
“We’re not talking about people going from no diabetes to frank diabetes. We’re talking about [statins] nudging a very small number of people across a diabetes threshold,” an A1c of 6.5% that is set somewhat arbitrarily based on an increased risk for developing retinopathy, Dr. Preiss said. ”A person just needs to lose a [daily] can of Coke’s worth of weight to eliminate any apparent diabetes risk,” he noted.
Benefit outweighs risks by three- to sevenfold
Dr. Preiss presented two other examples of what his findings showed to illustrate the relatively small risk posed by statin therapy compared with its potential benefits. Treating 10,000 people for 5 years with a high-intensity statin regimen in those with established ASCVD (secondary prevention) would result in an increment of 150 extra people developing diabetes because of the hyperglycemic effect of statins, compared with an expected prevention of 1,000 ASCVD events. Among 10,000 people at high ASCVD risk and taking a high-intensity statin regimen for primary prevention 5 years of treatment would result in roughly 130 extra cases of incident diabetes while preventing about 500 ASCVD events.
In addition, applying the new risk estimates to the people included in the UK Biobank database, whose median A1c is 5.5%, showed that a high-intensity statin regimen could be expected to raise the prevalence of those with an A1c of 6.5% or greater from 4.5% to 5.7%.
Several preventive cardiologists who heard the report and were not involved with the analysis agreed with Dr. Preiss that the benefits of statin treatment substantially offset this confirmed hyperglycemic effect.
Risk ‘more than counterbalanced by benefit’
“He clearly showed that the small hyperglycemia risk posed by statin use is more than counterbalanced by its benefit for reducing ASCVD events,” commented Neil J. Stone, MD, a cardiologist and professor of medicine at Northwestern University, Chicago. “I agree that, for those with prediabetes who are on the road to diabetes with or without a statin, the small increase in glucose with a statin should not dissuade statin usage because the benefit is so large. Rather, it should focus efforts to improve diet, increase physical activity, and keep weight controlled.”
Dr. Stone also noted in an interview that in the JUPITER trial, which examined the effects of a daily 20-mg dose of rosuvastatin (Crestor), a high-intensity regimen, study participants with diabetes risk factors who were assigned to rosuvastatin had an onset of diabetes that was earlier than people assigned to placebo by only about 5.4 weeks, yet this group had evidence of significant benefit.
“I agree with Dr. Preiss that the benefits of statins in reducing heart attack, stroke, and cardiovascular death far outweigh their modest effects on glycemia,” commented Brendan M. Everett, MD, a cardiologist and preventive medicine specialist at Brigham and Women’s Hospital in Boston. “This is particularly true for those with preexisting prediabetes or diabetes, who have an elevated risk of atherosclerotic events and thus stand to derive more significant benefit from statins. The benefits of lowering LDL cholesterol with a statin for preventing seriously morbid, and potentially fatal, cardiovascular events far outweigh the extremely modest, or even negligible, increases in the risk of diabetes that could be seen with the extremely small increases in A1c,” Dr. Everett said in an interview.
The new findings “reaffirm that there is a increased risk [from statins] but the most important point is that it is a very, very tiny difference in A1c,” commented Marc S. Sabatine, MD, a cardiologist and professor at Harvard Medical School, Boston. “These data have been known for quite some time, but this analysis was done in a more rigorous way.” The finding of “a small increase in risk for diabetes is really because diabetes has a biochemical threshold and statin treatment nudges some people a little past a line that is semi-arbitrary. It’s important to be cognizant of this, but it in no way dissuades me from treating patients aggressively with statins to reduce their ASCVD risk. I would monitor their A1c levels, and if they go higher and can’t be controlled with lifestyle we have plenty of medications that can control it,” he said in an interview.
No difference by statin type
The meta-analysis used data from 13 placebo-controlled statin trials that together involved 123,940 participants and had an average 4.3 years of follow-up, and four trials that compared one statin with another and collectively involved 30,734 participants with an average 4.9 years of follow-up.
The analyses showed that high-intensity statin treatment increased the rate of incident diabetes by a significant 36% relative to controls and increased the rate of worsening glycemia by a significant 24% compared with controls. Low- or moderate-intensity statin regimens increased incident diabetes by a significant 10% and raised the incidence of worsening glycemia by a significant 10% compared with controls, Dr. Preiss reported.
These effects did not significantly differ by type of statin (the study included people treated with atorvastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin, and simvastatin), nor across a variety of subgroups based on age, sex, race, body mass index, diabetes risk, renal function, cholesterol levels, or cardiovascular disease. The effect was also consistent regardless of the duration of treatment.
Dr. Preiss also downplayed the magnitude of the apparent difference in risk posed by high-intensity and less intense statin regimens. “I suspect the apparent heterogeneity is true, but not quite as big as what we see,” he said.
The mechanisms by which statins have this effect remain unclear, but evidence suggests that it may be a direct effect of the main action of statins, inhibition of the HMG-CoA reductase enzyme.
The study received no commercial funding. Dr. Preiss and Dr. Stone had no disclosures. Dr. Everett has been a consultant to Eli Lilly, Gilead, Ipsen, Janssen, and Provention. Dr. Sabatine has been a consultant to Althera, Amgen, Anthos Therapeutics, AstraZeneca, Beren Therapeutics, Bristol-Myers Squibb, DalCor, Dr Reddy’s Laboratories, Fibrogen, Intarcia, Merck, Moderna, Novo Nordisk, and Silence Therapeutics.
AT AHA 2022
The body of evidence for Paxlovid therapy
Dear Colleagues,
We have a mismatch. The evidence supporting treatment for Paxlovid is compelling for people aged 60 or over, but the older patients in the United States are much less likely to be treated. Not only was there a randomized, placebo-controlled trial of high-risk patients which showed 89% reduction of hospitalizations and deaths (median age, 45), but there have been multiple real-world effectiveness studies subsequently published that have partitioned the benefit for age 65 or older, such as the ones from Israel and Hong Kong (age 60+). Overall, the real-world effectiveness in the first month after treatment is at least as good, if not better, than in the high-risk randomized trial.
We’re doing the current survey to find out, but the most likely reasons include (1) lack of confidence of benefit; (2) medication interactions; and (3) concerns over rebound.
Let me address each of these briefly. The lack of confidence in benefit stems from the fact that the initial high-risk trial was in unvaccinated individuals. That concern can now be put aside because all of the several real-world studies confirming the protective benefit against hospitalizations and deaths are in people who have been vaccinated, and a significant proportion received booster shots.
The potential medication interactions due to the ritonavir component of the Paxlovid drug combination, attributable to its cytochrome P450 3A4 inhibition, have been unduly emphasized. There are many drug-interaction checkers for Paxlovid, but this one from the University of Liverpool is user friendly, color- and icon-coded, and shows that the vast majority of interactions can be sidestepped by discontinuing the medication of concern for the length of the Paxlovid treatment, 5 days. The simple chart is provided in my recent substack newsletter.
As far as rebound, this problem has unfortunately been exaggerated because of lack of prospective systematic studies and appreciation that a positive test of clinical symptom rebound can occur without Paxlovid. There are soon to be multiple reports that the incidence of Paxlovid rebound is fairly low, in the range of 10%. That concern should not be a reason to withhold treatment.
Now the plot thickens. A new preprint report from the Veterans Health Administration, the largest health care system in the United States, looks at 90-day outcomes of about 9,000 Paxlovid-treated patients and approximately 47,000 controls. Not only was there a 26% reduction in long COVID, but of the breakdown of 12 organs/systems and symptoms, 10 of 12 were significantly reduced with Paxlovid, including pulmonary embolism, deep vein thrombosis, and neurocognitive impairment. There was also a 48% reduction in death and a 30% reduction in hospitalizations after the first 30 days. I have reviewed all of these data and put them in context in a recent newsletter. A key point is that the magnitude of benefit was unaffected by vaccination or booster status, or prior COVID infections, or unvaccinated status. Also, it was the same for men and women, as well as for age > 70 and age < 60. These findings all emphasize a new reason to be using Paxlovid therapy, and if replicated, Paxlovid may even be indicated for younger patients (who are at low risk for hospitalizations and deaths but at increased risk for long COVID).
In summary, for older patients, we should be thinking of why we should be using Paxlovid rather than the reason not to treat. We’ll be interested in the survey results to understand the mismatch better, and we look forward to your ideas and feedback to make better use of this treatment for the people who need it the most.
Sincerely yours, Eric J. Topol, MD
Dr. Topol reports no conflicts of interest with Pfizer; he receives no honoraria or speaker fees, does not serve in an advisory role, and has no financial association with the company.
A version of this article first appeared on Medscape.com.
Dear Colleagues,
We have a mismatch. The evidence supporting treatment for Paxlovid is compelling for people aged 60 or over, but the older patients in the United States are much less likely to be treated. Not only was there a randomized, placebo-controlled trial of high-risk patients which showed 89% reduction of hospitalizations and deaths (median age, 45), but there have been multiple real-world effectiveness studies subsequently published that have partitioned the benefit for age 65 or older, such as the ones from Israel and Hong Kong (age 60+). Overall, the real-world effectiveness in the first month after treatment is at least as good, if not better, than in the high-risk randomized trial.
We’re doing the current survey to find out, but the most likely reasons include (1) lack of confidence of benefit; (2) medication interactions; and (3) concerns over rebound.
Let me address each of these briefly. The lack of confidence in benefit stems from the fact that the initial high-risk trial was in unvaccinated individuals. That concern can now be put aside because all of the several real-world studies confirming the protective benefit against hospitalizations and deaths are in people who have been vaccinated, and a significant proportion received booster shots.
The potential medication interactions due to the ritonavir component of the Paxlovid drug combination, attributable to its cytochrome P450 3A4 inhibition, have been unduly emphasized. There are many drug-interaction checkers for Paxlovid, but this one from the University of Liverpool is user friendly, color- and icon-coded, and shows that the vast majority of interactions can be sidestepped by discontinuing the medication of concern for the length of the Paxlovid treatment, 5 days. The simple chart is provided in my recent substack newsletter.
As far as rebound, this problem has unfortunately been exaggerated because of lack of prospective systematic studies and appreciation that a positive test of clinical symptom rebound can occur without Paxlovid. There are soon to be multiple reports that the incidence of Paxlovid rebound is fairly low, in the range of 10%. That concern should not be a reason to withhold treatment.
Now the plot thickens. A new preprint report from the Veterans Health Administration, the largest health care system in the United States, looks at 90-day outcomes of about 9,000 Paxlovid-treated patients and approximately 47,000 controls. Not only was there a 26% reduction in long COVID, but of the breakdown of 12 organs/systems and symptoms, 10 of 12 were significantly reduced with Paxlovid, including pulmonary embolism, deep vein thrombosis, and neurocognitive impairment. There was also a 48% reduction in death and a 30% reduction in hospitalizations after the first 30 days. I have reviewed all of these data and put them in context in a recent newsletter. A key point is that the magnitude of benefit was unaffected by vaccination or booster status, or prior COVID infections, or unvaccinated status. Also, it was the same for men and women, as well as for age > 70 and age < 60. These findings all emphasize a new reason to be using Paxlovid therapy, and if replicated, Paxlovid may even be indicated for younger patients (who are at low risk for hospitalizations and deaths but at increased risk for long COVID).
In summary, for older patients, we should be thinking of why we should be using Paxlovid rather than the reason not to treat. We’ll be interested in the survey results to understand the mismatch better, and we look forward to your ideas and feedback to make better use of this treatment for the people who need it the most.
Sincerely yours, Eric J. Topol, MD
Dr. Topol reports no conflicts of interest with Pfizer; he receives no honoraria or speaker fees, does not serve in an advisory role, and has no financial association with the company.
A version of this article first appeared on Medscape.com.
Dear Colleagues,
We have a mismatch. The evidence supporting treatment for Paxlovid is compelling for people aged 60 or over, but the older patients in the United States are much less likely to be treated. Not only was there a randomized, placebo-controlled trial of high-risk patients which showed 89% reduction of hospitalizations and deaths (median age, 45), but there have been multiple real-world effectiveness studies subsequently published that have partitioned the benefit for age 65 or older, such as the ones from Israel and Hong Kong (age 60+). Overall, the real-world effectiveness in the first month after treatment is at least as good, if not better, than in the high-risk randomized trial.
We’re doing the current survey to find out, but the most likely reasons include (1) lack of confidence of benefit; (2) medication interactions; and (3) concerns over rebound.
Let me address each of these briefly. The lack of confidence in benefit stems from the fact that the initial high-risk trial was in unvaccinated individuals. That concern can now be put aside because all of the several real-world studies confirming the protective benefit against hospitalizations and deaths are in people who have been vaccinated, and a significant proportion received booster shots.
The potential medication interactions due to the ritonavir component of the Paxlovid drug combination, attributable to its cytochrome P450 3A4 inhibition, have been unduly emphasized. There are many drug-interaction checkers for Paxlovid, but this one from the University of Liverpool is user friendly, color- and icon-coded, and shows that the vast majority of interactions can be sidestepped by discontinuing the medication of concern for the length of the Paxlovid treatment, 5 days. The simple chart is provided in my recent substack newsletter.
As far as rebound, this problem has unfortunately been exaggerated because of lack of prospective systematic studies and appreciation that a positive test of clinical symptom rebound can occur without Paxlovid. There are soon to be multiple reports that the incidence of Paxlovid rebound is fairly low, in the range of 10%. That concern should not be a reason to withhold treatment.
Now the plot thickens. A new preprint report from the Veterans Health Administration, the largest health care system in the United States, looks at 90-day outcomes of about 9,000 Paxlovid-treated patients and approximately 47,000 controls. Not only was there a 26% reduction in long COVID, but of the breakdown of 12 organs/systems and symptoms, 10 of 12 were significantly reduced with Paxlovid, including pulmonary embolism, deep vein thrombosis, and neurocognitive impairment. There was also a 48% reduction in death and a 30% reduction in hospitalizations after the first 30 days. I have reviewed all of these data and put them in context in a recent newsletter. A key point is that the magnitude of benefit was unaffected by vaccination or booster status, or prior COVID infections, or unvaccinated status. Also, it was the same for men and women, as well as for age > 70 and age < 60. These findings all emphasize a new reason to be using Paxlovid therapy, and if replicated, Paxlovid may even be indicated for younger patients (who are at low risk for hospitalizations and deaths but at increased risk for long COVID).
In summary, for older patients, we should be thinking of why we should be using Paxlovid rather than the reason not to treat. We’ll be interested in the survey results to understand the mismatch better, and we look forward to your ideas and feedback to make better use of this treatment for the people who need it the most.
Sincerely yours, Eric J. Topol, MD
Dr. Topol reports no conflicts of interest with Pfizer; he receives no honoraria or speaker fees, does not serve in an advisory role, and has no financial association with the company.
A version of this article first appeared on Medscape.com.
Tirzepatide lowers weight across all groups with obesity
SAN DIEGO – Weight loss with tirzepatide was fairly uniform across different body mass index ranges, ages, and number of obesity-related comorbidities in patients with overweight/obesity without type 2 diabetes.
These were the main findings in a session about tirzepatide – the dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) agonist – for obesity, presented at the annual meeting of the Obesity Society.
In May, tirzepatide (Mounjaro), a once-weekly subcutaneous injection, was approved by the Food and Drug Administration for glycemic control in patients with type 2 diabetes based on the SURPASS clinical trials.
Then in June, at the American Diabetes Association 2022 annual meeting, researchers reported “unprecedented” weight loss with tirzepatide in patients without type 2 diabetes, in the phase 3 SURMOUNT-1 clinical trial.
In early October, the FDA granted fast track status (expedited review) to tirzepatide for use as an antiobesity drug.
Now these new analyses from SURMOUNT-1 show that “regardless of BMI, regardless of age, regardless of number of obesity-related complications, there was a clear dose-related weight loss that was pretty consistent across groups,” Session Chair Patrick M. O’Neil, PhD, who was not involved with this research, summarized.
“The absolute levels of these weight losses are higher than we’ve seen thus far with [antiobesity] medications,” added Dr. O’Neil, professor of psychiatry and behavioral sciences and director of the Weight Management Center at the Medical University of South Carolina, Charleston.
“Semaglutide took things up one big notch, and this is up a little notch above that,” he said in an interview.
“I’m a psychologist. It should be remembered that in all cases, the FDA approvals are predicated to using [drugs] as an adjunct to diet and exercise change as well,” he stressed.
“I don’t think people should expect that any medication that is currently available will have a lasting effect when it’s no longer taken,” he continued.
“We don’t expect any of these [antiobesity] medications to be making any permanent physiological changes,” Dr. O’Neil added, but patients could “use this medication to help themselves make some long-lasting behavioral changes, so that when they come off the medication, hopefully they’ll be able to continue these new patterns.
“Clearly the medications are having a significant impact,” he emphasized.
BMI, age, comorbidity subgroups, and overall QoL in SURMOUNT-1
SURMOUNT-1 compared the efficacy and safety of tirzepatide 5, 10, and 15 mg subcutaneous once-weekly to placebo, as an adjunct to a reduced-calorie diet and increased physical activity. The study included 2,539 adults without type 2 diabetes who had obesity (BMI ≥ 30 kg/m2) or overweight (BMI ≥ 27 kg/m2) with at least one obesity-related complication (hypertension, dyslipidemia, obstructive sleep apnea, or cardiovascular disease).
Age subgroups
Robert F. Kushner, MD, of Northwestern University, Chicago, noted that “Excessive lean mass loss is a clinical concern in elderly individuals being treated for obesity,” so it’s important to know if weight loss with tirzepatide differs by age.
The researchers performed a post hoc analysis in patients who had dual-energy x-ray absorptiometry (DXA) readings at baseline and week 72 (oral abstract 109).
The three age groups in the current analysis were < 50 years old (99 patients), ≥ 50 to < 65 years old (41 patients), and ≥ 65 years old (20 patients). Overall, 63% of patients were age < 50 years, 31% were age 50 to < 65 years, and 6% were ≥ 65 years.
At 72 weeks, patients taking 5, 10, and 15 mg/week tirzepatide lost 21.5%, 20.8%, and 22% of their initial body weight, respectively.
“Tirzepatide significantly lowered total body mass versus placebo regardless of age subgroups,” and it “consistently lowered fat mass, lean mass, fat-mass-to-lean-mass ratio, and visceral fat mass across age subgroups,” Dr. Kushner reported.
BMI subgroups
Louis J. Aronne, MD, Weill Cornell Medicine, New York, presented findings from a prespecified analysis of BMI subgroups (oral abstract 110).
The four BMI subgroups were:
- ≥ 27 to < 30 kg/m2 (overweight), mean initial weight 178 pounds, mean weight reduction 29-30 pounds
- ≥ 30 to < 35 kg/m2 (class 1 obesity), mean initial weight 198 pounds, mean weight reduction 33-43 pounds
- 35 to < 40 kg/m2 (class 2 obesity), mean initial weight 228 pounds, mean reduction 34-56 pounds
- 40 kg/m2 (class 3 obesity), mean initial weight 280 pounds, mean weight reduction 44-64 pounds
Patients with an initial BMI of ≥ 35 to < 40 kg/m2 who received the 15-mg/week dose of tirzepatide had the greatest weight loss, at 24.5%, which is approximately what is seen with bariatric surgeries such as sleeve gastrectomy (25%).
The proportion of patients reaching ≥ 5% weight reduction was approximately 90% in all weight categories. “These numbers are unprecedented,” said Dr. Aronne.
In addition, overall, 73%-90% of patients receiving the 5- to 15-mg doses of tirzepatide achieved ≥ 10% body weight reduction, and “something we never thought we would see” is that 50%-78% of the patients receiving the drug lost 15% or more of their body weight.
In reply to an audience question, Dr. Aronne said it would take further study to determine who would respond well to tirzepatide.
And in reply to another question about whether it would make sense to treat to a target of a normal BMI, he said: “I think we are getting there.”
Patients in the 27- to 30-kg/m2 BMI category lost about the same amount of weight at a 5-mg dose as at a higher dose, suggesting they should stick to the lower dose, which would likely also have fewer side effects, he noted.
Number of comorbidities
Comorbidities in SURMOUNT-1 included hypertension, dyslipidemia, obstructive sleep apnea, atherosclerotic cardiovascular disease, osteoarthritis, anxiety/depression, polycystic ovary syndrome, nonalcoholic fatty liver disease, and asthma/chronic obstructive pulmonary disease. Of the patients with no comorbidities, 32.6% had prediabetes (oral abstract 111).
Sriram Machineni, MD, University of North Carolina at Chapel Hill, noted that obesity is associated with a significantly increased risk of clustering of at least two obesity-related complications, but little is known about how this affects outcomes.
The patients in SURMOUNT-1 were classified into three groups based on number of comorbidities:
- Zero comorbidities, 37% of patients: baseline mean age of 39, mean duration of obesity of 12 years, 29% men
- One comorbidity, 27% of patients: baseline mean age of 44, mean duration of obesity of 14 years, 31% men
- Two or more comorbidities, 36% of patients: baseline mean age of 52, duration of obesity 17 years, 37% men
Regardless of the number of comorbidities, all doses of tirzepatide resulted in a greater reduction in body weight compared with placebo.
Quality of life
Jiat Ling Poon, MD, an employee of Eli Lilly, presented findings from patient-reported replies to questionnaires including Impact of Weight on Quality of Life–Lite (IWQOL-Lite), which assesses physical and psychosocial health, and the Short Form–36 Health Survey, which assesses physical functioning, bodily pain, vitality, role-emotional, role-physical, general health, social functioning, and mental health (oral abstract 112).
Tirzepatide at all doses resulted in significantly greater improvements in patient-reported outcomes compared with placebo.
Meanwhile, the phase 3 SURMOUNT-2 clinical trial of tirzepatide for weight loss in patients with type 2 diabetes is projected to be completed in April 2023.
The studies were funded by Eli Lilly.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Weight loss with tirzepatide was fairly uniform across different body mass index ranges, ages, and number of obesity-related comorbidities in patients with overweight/obesity without type 2 diabetes.
These were the main findings in a session about tirzepatide – the dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) agonist – for obesity, presented at the annual meeting of the Obesity Society.
In May, tirzepatide (Mounjaro), a once-weekly subcutaneous injection, was approved by the Food and Drug Administration for glycemic control in patients with type 2 diabetes based on the SURPASS clinical trials.
Then in June, at the American Diabetes Association 2022 annual meeting, researchers reported “unprecedented” weight loss with tirzepatide in patients without type 2 diabetes, in the phase 3 SURMOUNT-1 clinical trial.
In early October, the FDA granted fast track status (expedited review) to tirzepatide for use as an antiobesity drug.
Now these new analyses from SURMOUNT-1 show that “regardless of BMI, regardless of age, regardless of number of obesity-related complications, there was a clear dose-related weight loss that was pretty consistent across groups,” Session Chair Patrick M. O’Neil, PhD, who was not involved with this research, summarized.
“The absolute levels of these weight losses are higher than we’ve seen thus far with [antiobesity] medications,” added Dr. O’Neil, professor of psychiatry and behavioral sciences and director of the Weight Management Center at the Medical University of South Carolina, Charleston.
“Semaglutide took things up one big notch, and this is up a little notch above that,” he said in an interview.
“I’m a psychologist. It should be remembered that in all cases, the FDA approvals are predicated to using [drugs] as an adjunct to diet and exercise change as well,” he stressed.
“I don’t think people should expect that any medication that is currently available will have a lasting effect when it’s no longer taken,” he continued.
“We don’t expect any of these [antiobesity] medications to be making any permanent physiological changes,” Dr. O’Neil added, but patients could “use this medication to help themselves make some long-lasting behavioral changes, so that when they come off the medication, hopefully they’ll be able to continue these new patterns.
“Clearly the medications are having a significant impact,” he emphasized.
BMI, age, comorbidity subgroups, and overall QoL in SURMOUNT-1
SURMOUNT-1 compared the efficacy and safety of tirzepatide 5, 10, and 15 mg subcutaneous once-weekly to placebo, as an adjunct to a reduced-calorie diet and increased physical activity. The study included 2,539 adults without type 2 diabetes who had obesity (BMI ≥ 30 kg/m2) or overweight (BMI ≥ 27 kg/m2) with at least one obesity-related complication (hypertension, dyslipidemia, obstructive sleep apnea, or cardiovascular disease).
Age subgroups
Robert F. Kushner, MD, of Northwestern University, Chicago, noted that “Excessive lean mass loss is a clinical concern in elderly individuals being treated for obesity,” so it’s important to know if weight loss with tirzepatide differs by age.
The researchers performed a post hoc analysis in patients who had dual-energy x-ray absorptiometry (DXA) readings at baseline and week 72 (oral abstract 109).
The three age groups in the current analysis were < 50 years old (99 patients), ≥ 50 to < 65 years old (41 patients), and ≥ 65 years old (20 patients). Overall, 63% of patients were age < 50 years, 31% were age 50 to < 65 years, and 6% were ≥ 65 years.
At 72 weeks, patients taking 5, 10, and 15 mg/week tirzepatide lost 21.5%, 20.8%, and 22% of their initial body weight, respectively.
“Tirzepatide significantly lowered total body mass versus placebo regardless of age subgroups,” and it “consistently lowered fat mass, lean mass, fat-mass-to-lean-mass ratio, and visceral fat mass across age subgroups,” Dr. Kushner reported.
BMI subgroups
Louis J. Aronne, MD, Weill Cornell Medicine, New York, presented findings from a prespecified analysis of BMI subgroups (oral abstract 110).
The four BMI subgroups were:
- ≥ 27 to < 30 kg/m2 (overweight), mean initial weight 178 pounds, mean weight reduction 29-30 pounds
- ≥ 30 to < 35 kg/m2 (class 1 obesity), mean initial weight 198 pounds, mean weight reduction 33-43 pounds
- 35 to < 40 kg/m2 (class 2 obesity), mean initial weight 228 pounds, mean reduction 34-56 pounds
- 40 kg/m2 (class 3 obesity), mean initial weight 280 pounds, mean weight reduction 44-64 pounds
Patients with an initial BMI of ≥ 35 to < 40 kg/m2 who received the 15-mg/week dose of tirzepatide had the greatest weight loss, at 24.5%, which is approximately what is seen with bariatric surgeries such as sleeve gastrectomy (25%).
The proportion of patients reaching ≥ 5% weight reduction was approximately 90% in all weight categories. “These numbers are unprecedented,” said Dr. Aronne.
In addition, overall, 73%-90% of patients receiving the 5- to 15-mg doses of tirzepatide achieved ≥ 10% body weight reduction, and “something we never thought we would see” is that 50%-78% of the patients receiving the drug lost 15% or more of their body weight.
In reply to an audience question, Dr. Aronne said it would take further study to determine who would respond well to tirzepatide.
And in reply to another question about whether it would make sense to treat to a target of a normal BMI, he said: “I think we are getting there.”
Patients in the 27- to 30-kg/m2 BMI category lost about the same amount of weight at a 5-mg dose as at a higher dose, suggesting they should stick to the lower dose, which would likely also have fewer side effects, he noted.
Number of comorbidities
Comorbidities in SURMOUNT-1 included hypertension, dyslipidemia, obstructive sleep apnea, atherosclerotic cardiovascular disease, osteoarthritis, anxiety/depression, polycystic ovary syndrome, nonalcoholic fatty liver disease, and asthma/chronic obstructive pulmonary disease. Of the patients with no comorbidities, 32.6% had prediabetes (oral abstract 111).
Sriram Machineni, MD, University of North Carolina at Chapel Hill, noted that obesity is associated with a significantly increased risk of clustering of at least two obesity-related complications, but little is known about how this affects outcomes.
The patients in SURMOUNT-1 were classified into three groups based on number of comorbidities:
- Zero comorbidities, 37% of patients: baseline mean age of 39, mean duration of obesity of 12 years, 29% men
- One comorbidity, 27% of patients: baseline mean age of 44, mean duration of obesity of 14 years, 31% men
- Two or more comorbidities, 36% of patients: baseline mean age of 52, duration of obesity 17 years, 37% men
Regardless of the number of comorbidities, all doses of tirzepatide resulted in a greater reduction in body weight compared with placebo.
Quality of life
Jiat Ling Poon, MD, an employee of Eli Lilly, presented findings from patient-reported replies to questionnaires including Impact of Weight on Quality of Life–Lite (IWQOL-Lite), which assesses physical and psychosocial health, and the Short Form–36 Health Survey, which assesses physical functioning, bodily pain, vitality, role-emotional, role-physical, general health, social functioning, and mental health (oral abstract 112).
Tirzepatide at all doses resulted in significantly greater improvements in patient-reported outcomes compared with placebo.
Meanwhile, the phase 3 SURMOUNT-2 clinical trial of tirzepatide for weight loss in patients with type 2 diabetes is projected to be completed in April 2023.
The studies were funded by Eli Lilly.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Weight loss with tirzepatide was fairly uniform across different body mass index ranges, ages, and number of obesity-related comorbidities in patients with overweight/obesity without type 2 diabetes.
These were the main findings in a session about tirzepatide – the dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) agonist – for obesity, presented at the annual meeting of the Obesity Society.
In May, tirzepatide (Mounjaro), a once-weekly subcutaneous injection, was approved by the Food and Drug Administration for glycemic control in patients with type 2 diabetes based on the SURPASS clinical trials.
Then in June, at the American Diabetes Association 2022 annual meeting, researchers reported “unprecedented” weight loss with tirzepatide in patients without type 2 diabetes, in the phase 3 SURMOUNT-1 clinical trial.
In early October, the FDA granted fast track status (expedited review) to tirzepatide for use as an antiobesity drug.
Now these new analyses from SURMOUNT-1 show that “regardless of BMI, regardless of age, regardless of number of obesity-related complications, there was a clear dose-related weight loss that was pretty consistent across groups,” Session Chair Patrick M. O’Neil, PhD, who was not involved with this research, summarized.
“The absolute levels of these weight losses are higher than we’ve seen thus far with [antiobesity] medications,” added Dr. O’Neil, professor of psychiatry and behavioral sciences and director of the Weight Management Center at the Medical University of South Carolina, Charleston.
“Semaglutide took things up one big notch, and this is up a little notch above that,” he said in an interview.
“I’m a psychologist. It should be remembered that in all cases, the FDA approvals are predicated to using [drugs] as an adjunct to diet and exercise change as well,” he stressed.
“I don’t think people should expect that any medication that is currently available will have a lasting effect when it’s no longer taken,” he continued.
“We don’t expect any of these [antiobesity] medications to be making any permanent physiological changes,” Dr. O’Neil added, but patients could “use this medication to help themselves make some long-lasting behavioral changes, so that when they come off the medication, hopefully they’ll be able to continue these new patterns.
“Clearly the medications are having a significant impact,” he emphasized.
BMI, age, comorbidity subgroups, and overall QoL in SURMOUNT-1
SURMOUNT-1 compared the efficacy and safety of tirzepatide 5, 10, and 15 mg subcutaneous once-weekly to placebo, as an adjunct to a reduced-calorie diet and increased physical activity. The study included 2,539 adults without type 2 diabetes who had obesity (BMI ≥ 30 kg/m2) or overweight (BMI ≥ 27 kg/m2) with at least one obesity-related complication (hypertension, dyslipidemia, obstructive sleep apnea, or cardiovascular disease).
Age subgroups
Robert F. Kushner, MD, of Northwestern University, Chicago, noted that “Excessive lean mass loss is a clinical concern in elderly individuals being treated for obesity,” so it’s important to know if weight loss with tirzepatide differs by age.
The researchers performed a post hoc analysis in patients who had dual-energy x-ray absorptiometry (DXA) readings at baseline and week 72 (oral abstract 109).
The three age groups in the current analysis were < 50 years old (99 patients), ≥ 50 to < 65 years old (41 patients), and ≥ 65 years old (20 patients). Overall, 63% of patients were age < 50 years, 31% were age 50 to < 65 years, and 6% were ≥ 65 years.
At 72 weeks, patients taking 5, 10, and 15 mg/week tirzepatide lost 21.5%, 20.8%, and 22% of their initial body weight, respectively.
“Tirzepatide significantly lowered total body mass versus placebo regardless of age subgroups,” and it “consistently lowered fat mass, lean mass, fat-mass-to-lean-mass ratio, and visceral fat mass across age subgroups,” Dr. Kushner reported.
BMI subgroups
Louis J. Aronne, MD, Weill Cornell Medicine, New York, presented findings from a prespecified analysis of BMI subgroups (oral abstract 110).
The four BMI subgroups were:
- ≥ 27 to < 30 kg/m2 (overweight), mean initial weight 178 pounds, mean weight reduction 29-30 pounds
- ≥ 30 to < 35 kg/m2 (class 1 obesity), mean initial weight 198 pounds, mean weight reduction 33-43 pounds
- 35 to < 40 kg/m2 (class 2 obesity), mean initial weight 228 pounds, mean reduction 34-56 pounds
- 40 kg/m2 (class 3 obesity), mean initial weight 280 pounds, mean weight reduction 44-64 pounds
Patients with an initial BMI of ≥ 35 to < 40 kg/m2 who received the 15-mg/week dose of tirzepatide had the greatest weight loss, at 24.5%, which is approximately what is seen with bariatric surgeries such as sleeve gastrectomy (25%).
The proportion of patients reaching ≥ 5% weight reduction was approximately 90% in all weight categories. “These numbers are unprecedented,” said Dr. Aronne.
In addition, overall, 73%-90% of patients receiving the 5- to 15-mg doses of tirzepatide achieved ≥ 10% body weight reduction, and “something we never thought we would see” is that 50%-78% of the patients receiving the drug lost 15% or more of their body weight.
In reply to an audience question, Dr. Aronne said it would take further study to determine who would respond well to tirzepatide.
And in reply to another question about whether it would make sense to treat to a target of a normal BMI, he said: “I think we are getting there.”
Patients in the 27- to 30-kg/m2 BMI category lost about the same amount of weight at a 5-mg dose as at a higher dose, suggesting they should stick to the lower dose, which would likely also have fewer side effects, he noted.
Number of comorbidities
Comorbidities in SURMOUNT-1 included hypertension, dyslipidemia, obstructive sleep apnea, atherosclerotic cardiovascular disease, osteoarthritis, anxiety/depression, polycystic ovary syndrome, nonalcoholic fatty liver disease, and asthma/chronic obstructive pulmonary disease. Of the patients with no comorbidities, 32.6% had prediabetes (oral abstract 111).
Sriram Machineni, MD, University of North Carolina at Chapel Hill, noted that obesity is associated with a significantly increased risk of clustering of at least two obesity-related complications, but little is known about how this affects outcomes.
The patients in SURMOUNT-1 were classified into three groups based on number of comorbidities:
- Zero comorbidities, 37% of patients: baseline mean age of 39, mean duration of obesity of 12 years, 29% men
- One comorbidity, 27% of patients: baseline mean age of 44, mean duration of obesity of 14 years, 31% men
- Two or more comorbidities, 36% of patients: baseline mean age of 52, duration of obesity 17 years, 37% men
Regardless of the number of comorbidities, all doses of tirzepatide resulted in a greater reduction in body weight compared with placebo.
Quality of life
Jiat Ling Poon, MD, an employee of Eli Lilly, presented findings from patient-reported replies to questionnaires including Impact of Weight on Quality of Life–Lite (IWQOL-Lite), which assesses physical and psychosocial health, and the Short Form–36 Health Survey, which assesses physical functioning, bodily pain, vitality, role-emotional, role-physical, general health, social functioning, and mental health (oral abstract 112).
Tirzepatide at all doses resulted in significantly greater improvements in patient-reported outcomes compared with placebo.
Meanwhile, the phase 3 SURMOUNT-2 clinical trial of tirzepatide for weight loss in patients with type 2 diabetes is projected to be completed in April 2023.
The studies were funded by Eli Lilly.
A version of this article first appeared on Medscape.com.
AT OBESITYWEEK® 2022
Clozapine underutilized in treatment-resistant schizophrenia
, and when it is used, the drug is often delayed by several crucial years, reducing chances of efficacy.
“Despite being the only pharmacological therapy approved for treatment-resistant schizophrenia, clozapine is underutilized globally, even in developed countries, where only about 30% of patients who would benefit from the drug receive it,” said John M. Kane, MD, of the department of psychiatry, Zucker Hillside Hospital, Northwell Health, Glen Oaks, N.Y., in a presentation on the subject at the 21st Annual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists in Cincinnati, Ohio.
Clozapine, a tricyclic dibenzodiazepine available in branded and various generic versions, is approved by the U.S. Food and Drug Administration as a third-line therapy for severe, treatment-resistant schizophrenia, with studies showing benefits exceeding those of any other antipsychotics for the indication.
But while recommendations suggest use after a trial of two or more antipsychotics, with at least one being an atypical antipsychotic, one recent review finds delays in clozapine commencement ranging from 19.3 weeks to 5.5 years, and the duration of illness prior to clozapine use ranging from 1.1 to 9.7 years.
Blood monitoring, side effects
The key deterrents preventing many clinicians and patients from trying clozapine sooner are the drug’s safety and tolerability profiles, and notably the requirement of regular blood testing due to an increased risk of agranulocytosis.
Specifically, the blood testing is required every week for 6 months, then every other week for the next 6 months, and then once a month after that; however, “many of us think that that’s excessive at this point in time,” Dr. Kane noted.
Various other potential side effects are also of concern, including myocarditis, seizures, constipation, arrhythmia, hypersalivation, pneumonia, and metabolic symptoms including diabetes.
In terms of the common strategies that clinicians turn to when patients fail to respond to their current antipsychotic, including increasing doses, combining agents, or treatment switching, “none of the strategies likely rival clozapine in terms of efficacy,” Dr. Kane said.
Regarding higher dosing: “There is very little data suggesting that higher doses of antipsychotic drugs will work when the moderate or recommended dose has not worked,” he said.
Combination therapy strategies may provide benefits, but “they’re not a substitute for clozapine,” Dr. Kane added, noting that the combinations that do appear to be the most effective involve clozapine.
And regarding drug switching, studies suggest the likelihood of response in switching from one drug to another is “actually very low,” Dr. Kane added.
Clozapine also doesn’t work for all – the response rate runs between about 30% and 60%, Dr. Kane said, but when it is effective, the benefits can be profound.
“There are some patients who have a very pronounced response to clozapine – some patients describe it as life-changing,” he said.
Treatment delays reduce efficacy
Importantly, the delays before receiving clozapine are not inconsequential – data show that each outpatient antipsychotic trial prior to clozapine reduces the likelihood of response by 8%-11%, and each hospital admission further reduces the likelihood of response by 4%-8%, underscoring the need to identify treatment resistance as early as possible, Dr. Kane said.
“It’s critically important to try to identify treatment resistance earlier than we usually do because if we can get it under control sooner, we have a better chance of improving the patient’s outcome, and this has been shown in a number of studies,” he said.
“The longer you wait, the less likely you are to see a good response even to clozapine.”
Despite the concerns about clozapine, Dr. Kane notes that even the blood monitoring does not appear to be a big complaint for patients, especially they are improving.
“In our experience, the patients who benefit from clozapine don’t really have a problem with the monitoring,” he said.
“In fact, patients who benefit from clozapine are much more adherent to the medication than other patients that we see, which is understandable, because if you feel you’re really getting a benefit from medicine, you’re going to be much more motivated to take it even if it has side effects.”
A recent systematic review of 13 studies and 1,487 patients backs that up, concluding that “patients generally have a favorable experience when being treated with clozapine,” with the caveat that “conclusions are limited by the risk of bias, particularly survivorship bias.”
Preference for clozapine over other antipsychotic medications was reported by 54%-86% of patients in the review, with specific improvements in mood (11%-78%) and cognition (5%-68%).
Clinicians the biggest ‘obstacle’
Dr. Kane notes that an important factor in underutilization could indeed be the manner in which clinicians discuss clozapine with their patients – often opening the discussion by focusing on the negative aspects that, without the context of the potential benefits, can be deal-breakers for patient from the start.
“The clinicians in my opinion are really the obstacle,” Dr. Kane said. “What we always hear from clinicians is ‘I can’t do it because the patient refuses, or the patient doesn’t like the side effects’.”
Dr. Kane notes that most side effects can indeed be managed – regarding the risk for metabolic syndrome, for instance, he recommends that patients should be given metformin from the beginning when they’re started on clozapine.
He adds that in most cases, a 3-month trial is enough to answer the question of whether clozapine is working or not.
“Three months is a good trial, but it may not even tell you the total response to clozapine because that may actually accrue over time,” he said. “We’ve seen patients who actually get better and better beyond 3 months.”
Not offering the drug to patients, however, is doing them a serious disservice, Dr. Kane added.
“What I tell patients and families is that it would be a shame to miss this opportunity for a potential treatment that could be life-changing,” he said. “Does it have potential side effects? Yes. Do you have to get blood tests? Yes. And I can’t tell by evaluating a patient’s history or examining that patient whether or not they’re going to be a good responder. But would you really want to miss an opportunity to find that out?”
“To me the argument is – let’s try this drug for 3 months and see what effect it has, and at that point you’ll be in a much better position to make a decision about the benefits versus risk,” Dr. Kane said.
The only FDA-approved drug for treatment-resistant schizophrenia
Remarkably, clozapine isn’t just the only drug to currently have approval from the FDA for treatment-resistant schizophrenia – it has been for the last 3 decades.
“There have been attempts to develop medications with similar efficacy, but they have not succeeded,” Dr. Kane said in an interview. “We are still uncertain as to what accounts for clozapine’s unique qualities.”
Yet, with treatment-resistant schizophrenia patients representing some of the most dire mental illness cases clinicians may face, the need for better treatment decisions – and additional options – is pressing, Dr. Kane said.
“[The lack of any other drugs] is a big embarrassment to our field, in my opinion,” he said. “I’m a big proponent of clozapine, but we should have found another substance by now that could substitute for clozapine, which obviously has a lot of side effects and is not the easiest drug to use.”
Dr. Kane reported relationships either as a speaker or consultant/advisory board member and/or receives research grant support from Alkermes, Allergan, Click Therapeutics, Dainippon Sumitomo, H. Lundbeck, HLS Therapeutics, Indivior, Intra-Cellular Therapies, Janssen Pharmaceutical, Johnson & Johnson, LB Pharmaceuticals, Merck, Minerva, Neurocrine, Neumora Therapeutics, Novartis Pharmaceuticals, Otsuka, Reviva, Roche, Saladax, Sunovion, Takeda, and Teva. Dr. Kane receives non-mutual funds stock ownership/stock options from LB Pharmaceuticals, Vanguard Research Group, and North Shore Therapeutics, and receives patent holder/royalties paid by UpToDate.
The Psychopharmacology Update was sponsored by Medscape Live. Medscape Live and this news organization are owned by the same parent company.
, and when it is used, the drug is often delayed by several crucial years, reducing chances of efficacy.
“Despite being the only pharmacological therapy approved for treatment-resistant schizophrenia, clozapine is underutilized globally, even in developed countries, where only about 30% of patients who would benefit from the drug receive it,” said John M. Kane, MD, of the department of psychiatry, Zucker Hillside Hospital, Northwell Health, Glen Oaks, N.Y., in a presentation on the subject at the 21st Annual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists in Cincinnati, Ohio.
Clozapine, a tricyclic dibenzodiazepine available in branded and various generic versions, is approved by the U.S. Food and Drug Administration as a third-line therapy for severe, treatment-resistant schizophrenia, with studies showing benefits exceeding those of any other antipsychotics for the indication.
But while recommendations suggest use after a trial of two or more antipsychotics, with at least one being an atypical antipsychotic, one recent review finds delays in clozapine commencement ranging from 19.3 weeks to 5.5 years, and the duration of illness prior to clozapine use ranging from 1.1 to 9.7 years.
Blood monitoring, side effects
The key deterrents preventing many clinicians and patients from trying clozapine sooner are the drug’s safety and tolerability profiles, and notably the requirement of regular blood testing due to an increased risk of agranulocytosis.
Specifically, the blood testing is required every week for 6 months, then every other week for the next 6 months, and then once a month after that; however, “many of us think that that’s excessive at this point in time,” Dr. Kane noted.
Various other potential side effects are also of concern, including myocarditis, seizures, constipation, arrhythmia, hypersalivation, pneumonia, and metabolic symptoms including diabetes.
In terms of the common strategies that clinicians turn to when patients fail to respond to their current antipsychotic, including increasing doses, combining agents, or treatment switching, “none of the strategies likely rival clozapine in terms of efficacy,” Dr. Kane said.
Regarding higher dosing: “There is very little data suggesting that higher doses of antipsychotic drugs will work when the moderate or recommended dose has not worked,” he said.
Combination therapy strategies may provide benefits, but “they’re not a substitute for clozapine,” Dr. Kane added, noting that the combinations that do appear to be the most effective involve clozapine.
And regarding drug switching, studies suggest the likelihood of response in switching from one drug to another is “actually very low,” Dr. Kane added.
Clozapine also doesn’t work for all – the response rate runs between about 30% and 60%, Dr. Kane said, but when it is effective, the benefits can be profound.
“There are some patients who have a very pronounced response to clozapine – some patients describe it as life-changing,” he said.
Treatment delays reduce efficacy
Importantly, the delays before receiving clozapine are not inconsequential – data show that each outpatient antipsychotic trial prior to clozapine reduces the likelihood of response by 8%-11%, and each hospital admission further reduces the likelihood of response by 4%-8%, underscoring the need to identify treatment resistance as early as possible, Dr. Kane said.
“It’s critically important to try to identify treatment resistance earlier than we usually do because if we can get it under control sooner, we have a better chance of improving the patient’s outcome, and this has been shown in a number of studies,” he said.
“The longer you wait, the less likely you are to see a good response even to clozapine.”
Despite the concerns about clozapine, Dr. Kane notes that even the blood monitoring does not appear to be a big complaint for patients, especially they are improving.
“In our experience, the patients who benefit from clozapine don’t really have a problem with the monitoring,” he said.
“In fact, patients who benefit from clozapine are much more adherent to the medication than other patients that we see, which is understandable, because if you feel you’re really getting a benefit from medicine, you’re going to be much more motivated to take it even if it has side effects.”
A recent systematic review of 13 studies and 1,487 patients backs that up, concluding that “patients generally have a favorable experience when being treated with clozapine,” with the caveat that “conclusions are limited by the risk of bias, particularly survivorship bias.”
Preference for clozapine over other antipsychotic medications was reported by 54%-86% of patients in the review, with specific improvements in mood (11%-78%) and cognition (5%-68%).
Clinicians the biggest ‘obstacle’
Dr. Kane notes that an important factor in underutilization could indeed be the manner in which clinicians discuss clozapine with their patients – often opening the discussion by focusing on the negative aspects that, without the context of the potential benefits, can be deal-breakers for patient from the start.
“The clinicians in my opinion are really the obstacle,” Dr. Kane said. “What we always hear from clinicians is ‘I can’t do it because the patient refuses, or the patient doesn’t like the side effects’.”
Dr. Kane notes that most side effects can indeed be managed – regarding the risk for metabolic syndrome, for instance, he recommends that patients should be given metformin from the beginning when they’re started on clozapine.
He adds that in most cases, a 3-month trial is enough to answer the question of whether clozapine is working or not.
“Three months is a good trial, but it may not even tell you the total response to clozapine because that may actually accrue over time,” he said. “We’ve seen patients who actually get better and better beyond 3 months.”
Not offering the drug to patients, however, is doing them a serious disservice, Dr. Kane added.
“What I tell patients and families is that it would be a shame to miss this opportunity for a potential treatment that could be life-changing,” he said. “Does it have potential side effects? Yes. Do you have to get blood tests? Yes. And I can’t tell by evaluating a patient’s history or examining that patient whether or not they’re going to be a good responder. But would you really want to miss an opportunity to find that out?”
“To me the argument is – let’s try this drug for 3 months and see what effect it has, and at that point you’ll be in a much better position to make a decision about the benefits versus risk,” Dr. Kane said.
The only FDA-approved drug for treatment-resistant schizophrenia
Remarkably, clozapine isn’t just the only drug to currently have approval from the FDA for treatment-resistant schizophrenia – it has been for the last 3 decades.
“There have been attempts to develop medications with similar efficacy, but they have not succeeded,” Dr. Kane said in an interview. “We are still uncertain as to what accounts for clozapine’s unique qualities.”
Yet, with treatment-resistant schizophrenia patients representing some of the most dire mental illness cases clinicians may face, the need for better treatment decisions – and additional options – is pressing, Dr. Kane said.
“[The lack of any other drugs] is a big embarrassment to our field, in my opinion,” he said. “I’m a big proponent of clozapine, but we should have found another substance by now that could substitute for clozapine, which obviously has a lot of side effects and is not the easiest drug to use.”
Dr. Kane reported relationships either as a speaker or consultant/advisory board member and/or receives research grant support from Alkermes, Allergan, Click Therapeutics, Dainippon Sumitomo, H. Lundbeck, HLS Therapeutics, Indivior, Intra-Cellular Therapies, Janssen Pharmaceutical, Johnson & Johnson, LB Pharmaceuticals, Merck, Minerva, Neurocrine, Neumora Therapeutics, Novartis Pharmaceuticals, Otsuka, Reviva, Roche, Saladax, Sunovion, Takeda, and Teva. Dr. Kane receives non-mutual funds stock ownership/stock options from LB Pharmaceuticals, Vanguard Research Group, and North Shore Therapeutics, and receives patent holder/royalties paid by UpToDate.
The Psychopharmacology Update was sponsored by Medscape Live. Medscape Live and this news organization are owned by the same parent company.
, and when it is used, the drug is often delayed by several crucial years, reducing chances of efficacy.
“Despite being the only pharmacological therapy approved for treatment-resistant schizophrenia, clozapine is underutilized globally, even in developed countries, where only about 30% of patients who would benefit from the drug receive it,” said John M. Kane, MD, of the department of psychiatry, Zucker Hillside Hospital, Northwell Health, Glen Oaks, N.Y., in a presentation on the subject at the 21st Annual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists in Cincinnati, Ohio.
Clozapine, a tricyclic dibenzodiazepine available in branded and various generic versions, is approved by the U.S. Food and Drug Administration as a third-line therapy for severe, treatment-resistant schizophrenia, with studies showing benefits exceeding those of any other antipsychotics for the indication.
But while recommendations suggest use after a trial of two or more antipsychotics, with at least one being an atypical antipsychotic, one recent review finds delays in clozapine commencement ranging from 19.3 weeks to 5.5 years, and the duration of illness prior to clozapine use ranging from 1.1 to 9.7 years.
Blood monitoring, side effects
The key deterrents preventing many clinicians and patients from trying clozapine sooner are the drug’s safety and tolerability profiles, and notably the requirement of regular blood testing due to an increased risk of agranulocytosis.
Specifically, the blood testing is required every week for 6 months, then every other week for the next 6 months, and then once a month after that; however, “many of us think that that’s excessive at this point in time,” Dr. Kane noted.
Various other potential side effects are also of concern, including myocarditis, seizures, constipation, arrhythmia, hypersalivation, pneumonia, and metabolic symptoms including diabetes.
In terms of the common strategies that clinicians turn to when patients fail to respond to their current antipsychotic, including increasing doses, combining agents, or treatment switching, “none of the strategies likely rival clozapine in terms of efficacy,” Dr. Kane said.
Regarding higher dosing: “There is very little data suggesting that higher doses of antipsychotic drugs will work when the moderate or recommended dose has not worked,” he said.
Combination therapy strategies may provide benefits, but “they’re not a substitute for clozapine,” Dr. Kane added, noting that the combinations that do appear to be the most effective involve clozapine.
And regarding drug switching, studies suggest the likelihood of response in switching from one drug to another is “actually very low,” Dr. Kane added.
Clozapine also doesn’t work for all – the response rate runs between about 30% and 60%, Dr. Kane said, but when it is effective, the benefits can be profound.
“There are some patients who have a very pronounced response to clozapine – some patients describe it as life-changing,” he said.
Treatment delays reduce efficacy
Importantly, the delays before receiving clozapine are not inconsequential – data show that each outpatient antipsychotic trial prior to clozapine reduces the likelihood of response by 8%-11%, and each hospital admission further reduces the likelihood of response by 4%-8%, underscoring the need to identify treatment resistance as early as possible, Dr. Kane said.
“It’s critically important to try to identify treatment resistance earlier than we usually do because if we can get it under control sooner, we have a better chance of improving the patient’s outcome, and this has been shown in a number of studies,” he said.
“The longer you wait, the less likely you are to see a good response even to clozapine.”
Despite the concerns about clozapine, Dr. Kane notes that even the blood monitoring does not appear to be a big complaint for patients, especially they are improving.
“In our experience, the patients who benefit from clozapine don’t really have a problem with the monitoring,” he said.
“In fact, patients who benefit from clozapine are much more adherent to the medication than other patients that we see, which is understandable, because if you feel you’re really getting a benefit from medicine, you’re going to be much more motivated to take it even if it has side effects.”
A recent systematic review of 13 studies and 1,487 patients backs that up, concluding that “patients generally have a favorable experience when being treated with clozapine,” with the caveat that “conclusions are limited by the risk of bias, particularly survivorship bias.”
Preference for clozapine over other antipsychotic medications was reported by 54%-86% of patients in the review, with specific improvements in mood (11%-78%) and cognition (5%-68%).
Clinicians the biggest ‘obstacle’
Dr. Kane notes that an important factor in underutilization could indeed be the manner in which clinicians discuss clozapine with their patients – often opening the discussion by focusing on the negative aspects that, without the context of the potential benefits, can be deal-breakers for patient from the start.
“The clinicians in my opinion are really the obstacle,” Dr. Kane said. “What we always hear from clinicians is ‘I can’t do it because the patient refuses, or the patient doesn’t like the side effects’.”
Dr. Kane notes that most side effects can indeed be managed – regarding the risk for metabolic syndrome, for instance, he recommends that patients should be given metformin from the beginning when they’re started on clozapine.
He adds that in most cases, a 3-month trial is enough to answer the question of whether clozapine is working or not.
“Three months is a good trial, but it may not even tell you the total response to clozapine because that may actually accrue over time,” he said. “We’ve seen patients who actually get better and better beyond 3 months.”
Not offering the drug to patients, however, is doing them a serious disservice, Dr. Kane added.
“What I tell patients and families is that it would be a shame to miss this opportunity for a potential treatment that could be life-changing,” he said. “Does it have potential side effects? Yes. Do you have to get blood tests? Yes. And I can’t tell by evaluating a patient’s history or examining that patient whether or not they’re going to be a good responder. But would you really want to miss an opportunity to find that out?”
“To me the argument is – let’s try this drug for 3 months and see what effect it has, and at that point you’ll be in a much better position to make a decision about the benefits versus risk,” Dr. Kane said.
The only FDA-approved drug for treatment-resistant schizophrenia
Remarkably, clozapine isn’t just the only drug to currently have approval from the FDA for treatment-resistant schizophrenia – it has been for the last 3 decades.
“There have been attempts to develop medications with similar efficacy, but they have not succeeded,” Dr. Kane said in an interview. “We are still uncertain as to what accounts for clozapine’s unique qualities.”
Yet, with treatment-resistant schizophrenia patients representing some of the most dire mental illness cases clinicians may face, the need for better treatment decisions – and additional options – is pressing, Dr. Kane said.
“[The lack of any other drugs] is a big embarrassment to our field, in my opinion,” he said. “I’m a big proponent of clozapine, but we should have found another substance by now that could substitute for clozapine, which obviously has a lot of side effects and is not the easiest drug to use.”
Dr. Kane reported relationships either as a speaker or consultant/advisory board member and/or receives research grant support from Alkermes, Allergan, Click Therapeutics, Dainippon Sumitomo, H. Lundbeck, HLS Therapeutics, Indivior, Intra-Cellular Therapies, Janssen Pharmaceutical, Johnson & Johnson, LB Pharmaceuticals, Merck, Minerva, Neurocrine, Neumora Therapeutics, Novartis Pharmaceuticals, Otsuka, Reviva, Roche, Saladax, Sunovion, Takeda, and Teva. Dr. Kane receives non-mutual funds stock ownership/stock options from LB Pharmaceuticals, Vanguard Research Group, and North Shore Therapeutics, and receives patent holder/royalties paid by UpToDate.
The Psychopharmacology Update was sponsored by Medscape Live. Medscape Live and this news organization are owned by the same parent company.
FROM PSYCHOPHARMACOLOGY UPDATE
Promising new antibiotic emerges for treating UTIs
A new antibiotic for urinary tract infections is heading toward government approval.
It would be the first new treatment in 20 years for UTIs, which affect more than half of women at least sometime in their lives, according to data compiled by the Department of Health and Human Services.
Called Gepotidacin, the antibiotic’s trial has halted enrollment early due to excellent effectiveness and safety results thus far, drugmaker GSK announced in a press release Nov. 3. GSK will seek approval and peer-reviewed publication early next year.
There is a need for new antibiotics such as this because of increasing antibiotic resistance. Antibiotic resistance to bacteria has become so prevalent that the World Health Organization recently began publishing a list of bacteria that pose the greatest public health threats.
“It’s definitely a big deal,” Cindy Liu, MD, MPH, PhD, of the Antibiotic Resistance Action Center at George Washington University, told CNN.
However, antibiotics are not a particularly profitable type of drug, The Wall Street Journal reported. The newspaper noted that they need to be used sparingly to limit resistance, and the cheapest option is usually prescribed. Some small companies that make antibiotics have even gone bankrupt recently, the Journal noted.
The U.S. government has invested in GSK’s development of Gepotidacin. The company predicts the drug could be a “blockbuster” and earn more than $1 billion due to UTI resistance to other drugs, the Journal reported.
“I think it will be really interesting and important to the field to see both how the drug companies sort of market this product and sort of how it does,” Dr. Liu said.
A version of this article first appeared on Medscape.com.
A new antibiotic for urinary tract infections is heading toward government approval.
It would be the first new treatment in 20 years for UTIs, which affect more than half of women at least sometime in their lives, according to data compiled by the Department of Health and Human Services.
Called Gepotidacin, the antibiotic’s trial has halted enrollment early due to excellent effectiveness and safety results thus far, drugmaker GSK announced in a press release Nov. 3. GSK will seek approval and peer-reviewed publication early next year.
There is a need for new antibiotics such as this because of increasing antibiotic resistance. Antibiotic resistance to bacteria has become so prevalent that the World Health Organization recently began publishing a list of bacteria that pose the greatest public health threats.
“It’s definitely a big deal,” Cindy Liu, MD, MPH, PhD, of the Antibiotic Resistance Action Center at George Washington University, told CNN.
However, antibiotics are not a particularly profitable type of drug, The Wall Street Journal reported. The newspaper noted that they need to be used sparingly to limit resistance, and the cheapest option is usually prescribed. Some small companies that make antibiotics have even gone bankrupt recently, the Journal noted.
The U.S. government has invested in GSK’s development of Gepotidacin. The company predicts the drug could be a “blockbuster” and earn more than $1 billion due to UTI resistance to other drugs, the Journal reported.
“I think it will be really interesting and important to the field to see both how the drug companies sort of market this product and sort of how it does,” Dr. Liu said.
A version of this article first appeared on Medscape.com.
A new antibiotic for urinary tract infections is heading toward government approval.
It would be the first new treatment in 20 years for UTIs, which affect more than half of women at least sometime in their lives, according to data compiled by the Department of Health and Human Services.
Called Gepotidacin, the antibiotic’s trial has halted enrollment early due to excellent effectiveness and safety results thus far, drugmaker GSK announced in a press release Nov. 3. GSK will seek approval and peer-reviewed publication early next year.
There is a need for new antibiotics such as this because of increasing antibiotic resistance. Antibiotic resistance to bacteria has become so prevalent that the World Health Organization recently began publishing a list of bacteria that pose the greatest public health threats.
“It’s definitely a big deal,” Cindy Liu, MD, MPH, PhD, of the Antibiotic Resistance Action Center at George Washington University, told CNN.
However, antibiotics are not a particularly profitable type of drug, The Wall Street Journal reported. The newspaper noted that they need to be used sparingly to limit resistance, and the cheapest option is usually prescribed. Some small companies that make antibiotics have even gone bankrupt recently, the Journal noted.
The U.S. government has invested in GSK’s development of Gepotidacin. The company predicts the drug could be a “blockbuster” and earn more than $1 billion due to UTI resistance to other drugs, the Journal reported.
“I think it will be really interesting and important to the field to see both how the drug companies sort of market this product and sort of how it does,” Dr. Liu said.
A version of this article first appeared on Medscape.com.
HPV vaccine effectiveness dependent on age at receipt
The effectiveness of the human papillomavirus (HPV) vaccine against HPV types 16 and 18 is highly dependent on the age at which it is given. Prevalence rates have been shown to be significantly lower among girls who are vaccinated at the recommended ages of 9-12 years, compared with those who are vaccinated after their sexual debut, data from the National Health and Nutrition Examination Survey (NHANES) indicate.
“HPV vaccination does not have any therapeutic effect on HPV infections already acquired, which is more likely to explain the difference in prevalence between predebut versus postdebut recipients than a lower immune response [among older recipients],” lead study author Didem Egemen, PhD, National Cancer Institute, Rockville, Md., told this news organization in an email.
“Still, among older females, the immune response of the vaccine is likely to still be quite strong, and we would encourage vaccination [of female patients] if unvaccinated, as our paper showed that vaccination post debut will still reduce HPV 16/18 prevalence by half,” she added.
The research letter was published online in JAMA Network Open.
National sample evaluated
Using data from NHANES, a biennial, cross-sectional sample (cycles 2011 through 2018), the researchers identified female persons who were aged 26 years or younger in 2006, when HPV vaccination was introduced, and who were eligible for routine vaccination or “catch-up” vaccination (given between the ages of 13 and 26 years), as per recommendations from the Advisory Committee on Immunization Practices. The investigators then compared the prevalence of HPV types 16 and 18 among unvaccinated female patients, female patients who had been vaccinated prior to their sexual debut (predebut group), and those who had been vaccinated after their sexual debut (postdebut group).
They also estimated vaccine uptake among those who were eligible for routine vaccination, as well as the proportion of vaccinated female patients with respect to racial and ethnic subgroups.
In the overall cohort, the prevalence of HPV types 16 and 18 decreased by 6% (95% confidence interval, 4%-7%) in the unvaccinated group to 3% (95% CI, 1%-6%) in the postdebut group and to less than 1% (95% CI, <1%-1%) in the predebut group, Dr. Egemen and colleagues report.
In real percentages, the prevalence of HPV 16 and 18 was 89% lower in the predebut group (P < .001) but only 41% lower in the postdebut group (P = .29) compared with unvaccinated female patients. And compared with female patients who were vaccinated after their sexual debut, the prevalence of HPV 16 and 18 was reduced by 82% among those who had received the vaccine at the recommended ages of 9-12 years (P = .08).
In the current study, Dr. Egeman acknowledged that only 38% of ever-eligible female patients received the vaccine, although the prevalence increased to 56% when only female patients who were eligible for routine vaccination were taken into account. On the other hand, only 21% (95% CI, 14%-28%) of female patients eligible for routine vaccination received their first dose by age 12 years.
Indeed, the mean age on receipt of the first vaccination dose was 14.5 years (95% CI, 14.1-14.8 years), the authors note, and only 59% of girls received their first dose prior to their sexual debut. Additionally, among routine vaccination–eligible girls aged 12 years or younger in 2006, 33% were vaccinated before and 23% after their sexual debut, and the rest were not vaccinated.
Interestingly, differences in the age at which the HPV vaccine was received by race and ethnicity were negligible, the investigators point out.
Vaccination rates increasing
Asked to comment on the findings, Rebecca Perkins, MD, professor of obstetrics and gynecology at Boston University, Boston Medical Center, pointed out that the investigators evaluated data from 2011 to 2018. “We know that HPV vaccination rates have increased over that period and continue to increase,” she emphasized in an email to this news organization.
Physicians also know that more persons are being vaccinated between the ages of 9 and 12 than was the case at the beginning of this study. “This is good news,” she said, “as it means that more adolescents now in 2022 are benefiting fully from vaccination than they were in 2011,” she added.
At the same time, Dr. Perkins acknowledged that many persons are still missing out on the chance to receive the vaccine on time – which means they are missing out on the chance to prevent cancer.
“Making sure that all adolescents receive vaccination between the ages of 9 to 12 has the potential to prevent up to 40,000 cancers every year in the U.S., [including] the most common HPV-related cancers, such as cervical cancer in women and tongue and tonsillar cancer in men,” Dr. Perkins noted.
“Thus, it’s critical that doctors and parents get the message that you can’t vaccinate too early, only too late,” she emphasized.
Dr. Edgman and Dr. Perkins report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The effectiveness of the human papillomavirus (HPV) vaccine against HPV types 16 and 18 is highly dependent on the age at which it is given. Prevalence rates have been shown to be significantly lower among girls who are vaccinated at the recommended ages of 9-12 years, compared with those who are vaccinated after their sexual debut, data from the National Health and Nutrition Examination Survey (NHANES) indicate.
“HPV vaccination does not have any therapeutic effect on HPV infections already acquired, which is more likely to explain the difference in prevalence between predebut versus postdebut recipients than a lower immune response [among older recipients],” lead study author Didem Egemen, PhD, National Cancer Institute, Rockville, Md., told this news organization in an email.
“Still, among older females, the immune response of the vaccine is likely to still be quite strong, and we would encourage vaccination [of female patients] if unvaccinated, as our paper showed that vaccination post debut will still reduce HPV 16/18 prevalence by half,” she added.
The research letter was published online in JAMA Network Open.
National sample evaluated
Using data from NHANES, a biennial, cross-sectional sample (cycles 2011 through 2018), the researchers identified female persons who were aged 26 years or younger in 2006, when HPV vaccination was introduced, and who were eligible for routine vaccination or “catch-up” vaccination (given between the ages of 13 and 26 years), as per recommendations from the Advisory Committee on Immunization Practices. The investigators then compared the prevalence of HPV types 16 and 18 among unvaccinated female patients, female patients who had been vaccinated prior to their sexual debut (predebut group), and those who had been vaccinated after their sexual debut (postdebut group).
They also estimated vaccine uptake among those who were eligible for routine vaccination, as well as the proportion of vaccinated female patients with respect to racial and ethnic subgroups.
In the overall cohort, the prevalence of HPV types 16 and 18 decreased by 6% (95% confidence interval, 4%-7%) in the unvaccinated group to 3% (95% CI, 1%-6%) in the postdebut group and to less than 1% (95% CI, <1%-1%) in the predebut group, Dr. Egemen and colleagues report.
In real percentages, the prevalence of HPV 16 and 18 was 89% lower in the predebut group (P < .001) but only 41% lower in the postdebut group (P = .29) compared with unvaccinated female patients. And compared with female patients who were vaccinated after their sexual debut, the prevalence of HPV 16 and 18 was reduced by 82% among those who had received the vaccine at the recommended ages of 9-12 years (P = .08).
In the current study, Dr. Egeman acknowledged that only 38% of ever-eligible female patients received the vaccine, although the prevalence increased to 56% when only female patients who were eligible for routine vaccination were taken into account. On the other hand, only 21% (95% CI, 14%-28%) of female patients eligible for routine vaccination received their first dose by age 12 years.
Indeed, the mean age on receipt of the first vaccination dose was 14.5 years (95% CI, 14.1-14.8 years), the authors note, and only 59% of girls received their first dose prior to their sexual debut. Additionally, among routine vaccination–eligible girls aged 12 years or younger in 2006, 33% were vaccinated before and 23% after their sexual debut, and the rest were not vaccinated.
Interestingly, differences in the age at which the HPV vaccine was received by race and ethnicity were negligible, the investigators point out.
Vaccination rates increasing
Asked to comment on the findings, Rebecca Perkins, MD, professor of obstetrics and gynecology at Boston University, Boston Medical Center, pointed out that the investigators evaluated data from 2011 to 2018. “We know that HPV vaccination rates have increased over that period and continue to increase,” she emphasized in an email to this news organization.
Physicians also know that more persons are being vaccinated between the ages of 9 and 12 than was the case at the beginning of this study. “This is good news,” she said, “as it means that more adolescents now in 2022 are benefiting fully from vaccination than they were in 2011,” she added.
At the same time, Dr. Perkins acknowledged that many persons are still missing out on the chance to receive the vaccine on time – which means they are missing out on the chance to prevent cancer.
“Making sure that all adolescents receive vaccination between the ages of 9 to 12 has the potential to prevent up to 40,000 cancers every year in the U.S., [including] the most common HPV-related cancers, such as cervical cancer in women and tongue and tonsillar cancer in men,” Dr. Perkins noted.
“Thus, it’s critical that doctors and parents get the message that you can’t vaccinate too early, only too late,” she emphasized.
Dr. Edgman and Dr. Perkins report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The effectiveness of the human papillomavirus (HPV) vaccine against HPV types 16 and 18 is highly dependent on the age at which it is given. Prevalence rates have been shown to be significantly lower among girls who are vaccinated at the recommended ages of 9-12 years, compared with those who are vaccinated after their sexual debut, data from the National Health and Nutrition Examination Survey (NHANES) indicate.
“HPV vaccination does not have any therapeutic effect on HPV infections already acquired, which is more likely to explain the difference in prevalence between predebut versus postdebut recipients than a lower immune response [among older recipients],” lead study author Didem Egemen, PhD, National Cancer Institute, Rockville, Md., told this news organization in an email.
“Still, among older females, the immune response of the vaccine is likely to still be quite strong, and we would encourage vaccination [of female patients] if unvaccinated, as our paper showed that vaccination post debut will still reduce HPV 16/18 prevalence by half,” she added.
The research letter was published online in JAMA Network Open.
National sample evaluated
Using data from NHANES, a biennial, cross-sectional sample (cycles 2011 through 2018), the researchers identified female persons who were aged 26 years or younger in 2006, when HPV vaccination was introduced, and who were eligible for routine vaccination or “catch-up” vaccination (given between the ages of 13 and 26 years), as per recommendations from the Advisory Committee on Immunization Practices. The investigators then compared the prevalence of HPV types 16 and 18 among unvaccinated female patients, female patients who had been vaccinated prior to their sexual debut (predebut group), and those who had been vaccinated after their sexual debut (postdebut group).
They also estimated vaccine uptake among those who were eligible for routine vaccination, as well as the proportion of vaccinated female patients with respect to racial and ethnic subgroups.
In the overall cohort, the prevalence of HPV types 16 and 18 decreased by 6% (95% confidence interval, 4%-7%) in the unvaccinated group to 3% (95% CI, 1%-6%) in the postdebut group and to less than 1% (95% CI, <1%-1%) in the predebut group, Dr. Egemen and colleagues report.
In real percentages, the prevalence of HPV 16 and 18 was 89% lower in the predebut group (P < .001) but only 41% lower in the postdebut group (P = .29) compared with unvaccinated female patients. And compared with female patients who were vaccinated after their sexual debut, the prevalence of HPV 16 and 18 was reduced by 82% among those who had received the vaccine at the recommended ages of 9-12 years (P = .08).
In the current study, Dr. Egeman acknowledged that only 38% of ever-eligible female patients received the vaccine, although the prevalence increased to 56% when only female patients who were eligible for routine vaccination were taken into account. On the other hand, only 21% (95% CI, 14%-28%) of female patients eligible for routine vaccination received their first dose by age 12 years.
Indeed, the mean age on receipt of the first vaccination dose was 14.5 years (95% CI, 14.1-14.8 years), the authors note, and only 59% of girls received their first dose prior to their sexual debut. Additionally, among routine vaccination–eligible girls aged 12 years or younger in 2006, 33% were vaccinated before and 23% after their sexual debut, and the rest were not vaccinated.
Interestingly, differences in the age at which the HPV vaccine was received by race and ethnicity were negligible, the investigators point out.
Vaccination rates increasing
Asked to comment on the findings, Rebecca Perkins, MD, professor of obstetrics and gynecology at Boston University, Boston Medical Center, pointed out that the investigators evaluated data from 2011 to 2018. “We know that HPV vaccination rates have increased over that period and continue to increase,” she emphasized in an email to this news organization.
Physicians also know that more persons are being vaccinated between the ages of 9 and 12 than was the case at the beginning of this study. “This is good news,” she said, “as it means that more adolescents now in 2022 are benefiting fully from vaccination than they were in 2011,” she added.
At the same time, Dr. Perkins acknowledged that many persons are still missing out on the chance to receive the vaccine on time – which means they are missing out on the chance to prevent cancer.
“Making sure that all adolescents receive vaccination between the ages of 9 to 12 has the potential to prevent up to 40,000 cancers every year in the U.S., [including] the most common HPV-related cancers, such as cervical cancer in women and tongue and tonsillar cancer in men,” Dr. Perkins noted.
“Thus, it’s critical that doctors and parents get the message that you can’t vaccinate too early, only too late,” she emphasized.
Dr. Edgman and Dr. Perkins report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN









