User login
Why it’s important for dermatologists to learn about JAK inhibitors
PORTLAND, ORE. – according to Andrew Blauvelt, MD, MBA.
“In dermatology, you need to know about JAK inhibitors, and you need to know how to use them,” Dr. Blauvelt, president of Oregon Medical Research Center, Portland, said at the annual meeting of the Pacific Dermatologic Association. “Making the choice, ‘I’m not going to use those drugs because of safety concerns,’ may be okay in 2022, but we are going to be getting a lot more indications for these drugs. So instead of avoiding JAK inhibitors, I would say try to learn [about] them, understand them, and get your messaging out on safety.”
It’s difficult to imagine a clinician-researcher who has more experience with the use of biologics and JAK inhibitors in AD than Dr. Blauvelt, who has been the international investigator on several important trials of treatments that include dupilumab, tralokinumab, abrocitinib, and upadacitinib for AD such as CHRONOS, ECZTEND, JADE REGIMEN, and HEADS UP. At the meeting, he discussed his clinical approach to selecting systemic agents for AD and shared prescribing tips. He began by noting that the approval of dupilumab for moderate to severe AD in 2017 ushered in a new era of treating the disease systemically.
“When it was approved, experts went right to dupilumab if they could, and avoided the use of cyclosporine or methotrexate,” said Dr. Blauvelt, who is also an elected member of the American Society for Clinical Investigation and the International Eczema Council. “I still think that dupilumab is a great agent to start with. We’ve had a bit of difficulty improving upon it.”
Following dupilumab’s approval, three other systemic options became available for patients with moderate to severe AD: the human IgG4 monoclonal antibody tralokinumab that binds to interleukin-13, which is administered subcutaneously; and, more recently, the oral JAK inhibitors abrocitinib and upadacitinib, approved in January for moderate to severe AD.
“I’m a big fan of JAK inhibitors because I think they offer things that biologic and topical therapies can’t offer,” Dr. Blauvelt said. “Patients like the pills versus shots. They also like the speed; JAK inhibitors work faster than dupilumab and tralokinumab. So, if you have a patient with bad AD who wants to get better quickly, that would be a reason to choose a JAK inhibitor over a biologic if you can.”
When Dr. Blauvelt has asked AD clinical trial participants if they’d rather be treated with a biologic agent or with a JAK inhibitor, about half choose one over the other.
“Patients who shy away from the safety issues would choose the biologic trial while the ones who wanted the fast relief would choose the JAK trial,” he said. “But if you present both options and the patients prefer a pill, I think the JAK inhibitors do better with a rapid control of inflammation as well as pruritus – the latter within 2 days of taking the pills.”
When counseling patients initiating a JAK inhibitor, Dr. Blauvelt mentioned three advantages, compared with biologics: the pill formulation, the rapidity of response in pruritus control, and better efficacy. “The downside is the safety,” he said. “Safety is the elephant in the room for the JAK inhibitors.”
The risks listed in the boxed warning in the labeling for JAK inhibitors include: an increased risk of serious bacterial, fungal, and opportunistic infections such as TB; a higher rate of all-cause mortality, including cardiovascular death; a higher rate of MACE (major adverse cardiovascular events, defined as cardiovascular death, MI, and stroke); the potential for malignancy, including lymphoma; and the potential for thrombosis, including an increased incidence of pulmonary embolism (PE).
“Risk of thrombosis seems to be a class effect for all JAK inhibitors,” Dr. Blauvelt said. “As far as I know, it’s idiosyncratic. For nearly all the DVT [deep vein thrombosis] cases that have been reported, patients had baseline risk factors for DVT and PE, which are obesity, smoking, and use of oral contraceptives.”
Dr. Blauvelt pointed out that the boxed warning related to mortality, malignancies, and MACE stemmed from a long-term trial of the JAK inhibitor tofacitinib in RA patients. “Those patients had to be at least 50 years old, 75% of them were on concomitant methotrexate and/or prednisone, and they had to have at least one cardiac risk factor to get into the trial,” he said.
“I’m not saying those things can’t happen in dermatology patients, but if you look at the safety data of JAK inhibitors in the AD studies and in the alopecia areata studies, we are seeing a few cases of these things here and there, but not major signals,” he said. To date, “they look safer in dermatologic diseases compared to tofacitinib in RA data in older populations.”
He emphasized the importance of discussing each of the risks in the boxed warning with patients who are candidates for JAK inhibitor therapy.
Dr. Blauvelt likened the lab monitoring required for JAK inhibitors to that required for methotrexate. This means ordering at baseline, a CBC with differential, a chem-20, a lipid panel, and a QuantiFERON-TB Gold test. The JAK inhibitor labels do not include information on the frequency of monitoring, “but I have a distinct opinion on this because of my blood test monitoring experience in the trials for many years,” he said.
“I think it’s good to do follow-up testing at 1 month, then every 3 months in the first year. In my experience, the people who drop blood cell counts or increase their lipids tend to do it in the first year.”
After 1 year of treatment, he continued, follow-up testing once every 6 months is reasonable. “If CPK [creatine phosphokinase] goes up, I don’t worry about it; it’s not clinically relevant. There is no recommendation for CPK monitoring, so if you’re getting that on your chem-20, I’d say don’t worry about it.”
Dr. Blauvelt reported that he is an investigator and a scientific adviser for several pharmaceutical companies developing treatments for AD, including companies that are evaluating or marketing JAK inhibitors for AD, including AbbVie, Incyte, and Pfizer, as well as dupilumab’s joint developers Sanofi and Regeneron.
PORTLAND, ORE. – according to Andrew Blauvelt, MD, MBA.
“In dermatology, you need to know about JAK inhibitors, and you need to know how to use them,” Dr. Blauvelt, president of Oregon Medical Research Center, Portland, said at the annual meeting of the Pacific Dermatologic Association. “Making the choice, ‘I’m not going to use those drugs because of safety concerns,’ may be okay in 2022, but we are going to be getting a lot more indications for these drugs. So instead of avoiding JAK inhibitors, I would say try to learn [about] them, understand them, and get your messaging out on safety.”
It’s difficult to imagine a clinician-researcher who has more experience with the use of biologics and JAK inhibitors in AD than Dr. Blauvelt, who has been the international investigator on several important trials of treatments that include dupilumab, tralokinumab, abrocitinib, and upadacitinib for AD such as CHRONOS, ECZTEND, JADE REGIMEN, and HEADS UP. At the meeting, he discussed his clinical approach to selecting systemic agents for AD and shared prescribing tips. He began by noting that the approval of dupilumab for moderate to severe AD in 2017 ushered in a new era of treating the disease systemically.
“When it was approved, experts went right to dupilumab if they could, and avoided the use of cyclosporine or methotrexate,” said Dr. Blauvelt, who is also an elected member of the American Society for Clinical Investigation and the International Eczema Council. “I still think that dupilumab is a great agent to start with. We’ve had a bit of difficulty improving upon it.”
Following dupilumab’s approval, three other systemic options became available for patients with moderate to severe AD: the human IgG4 monoclonal antibody tralokinumab that binds to interleukin-13, which is administered subcutaneously; and, more recently, the oral JAK inhibitors abrocitinib and upadacitinib, approved in January for moderate to severe AD.
“I’m a big fan of JAK inhibitors because I think they offer things that biologic and topical therapies can’t offer,” Dr. Blauvelt said. “Patients like the pills versus shots. They also like the speed; JAK inhibitors work faster than dupilumab and tralokinumab. So, if you have a patient with bad AD who wants to get better quickly, that would be a reason to choose a JAK inhibitor over a biologic if you can.”
When Dr. Blauvelt has asked AD clinical trial participants if they’d rather be treated with a biologic agent or with a JAK inhibitor, about half choose one over the other.
“Patients who shy away from the safety issues would choose the biologic trial while the ones who wanted the fast relief would choose the JAK trial,” he said. “But if you present both options and the patients prefer a pill, I think the JAK inhibitors do better with a rapid control of inflammation as well as pruritus – the latter within 2 days of taking the pills.”
When counseling patients initiating a JAK inhibitor, Dr. Blauvelt mentioned three advantages, compared with biologics: the pill formulation, the rapidity of response in pruritus control, and better efficacy. “The downside is the safety,” he said. “Safety is the elephant in the room for the JAK inhibitors.”
The risks listed in the boxed warning in the labeling for JAK inhibitors include: an increased risk of serious bacterial, fungal, and opportunistic infections such as TB; a higher rate of all-cause mortality, including cardiovascular death; a higher rate of MACE (major adverse cardiovascular events, defined as cardiovascular death, MI, and stroke); the potential for malignancy, including lymphoma; and the potential for thrombosis, including an increased incidence of pulmonary embolism (PE).
“Risk of thrombosis seems to be a class effect for all JAK inhibitors,” Dr. Blauvelt said. “As far as I know, it’s idiosyncratic. For nearly all the DVT [deep vein thrombosis] cases that have been reported, patients had baseline risk factors for DVT and PE, which are obesity, smoking, and use of oral contraceptives.”
Dr. Blauvelt pointed out that the boxed warning related to mortality, malignancies, and MACE stemmed from a long-term trial of the JAK inhibitor tofacitinib in RA patients. “Those patients had to be at least 50 years old, 75% of them were on concomitant methotrexate and/or prednisone, and they had to have at least one cardiac risk factor to get into the trial,” he said.
“I’m not saying those things can’t happen in dermatology patients, but if you look at the safety data of JAK inhibitors in the AD studies and in the alopecia areata studies, we are seeing a few cases of these things here and there, but not major signals,” he said. To date, “they look safer in dermatologic diseases compared to tofacitinib in RA data in older populations.”
He emphasized the importance of discussing each of the risks in the boxed warning with patients who are candidates for JAK inhibitor therapy.
Dr. Blauvelt likened the lab monitoring required for JAK inhibitors to that required for methotrexate. This means ordering at baseline, a CBC with differential, a chem-20, a lipid panel, and a QuantiFERON-TB Gold test. The JAK inhibitor labels do not include information on the frequency of monitoring, “but I have a distinct opinion on this because of my blood test monitoring experience in the trials for many years,” he said.
“I think it’s good to do follow-up testing at 1 month, then every 3 months in the first year. In my experience, the people who drop blood cell counts or increase their lipids tend to do it in the first year.”
After 1 year of treatment, he continued, follow-up testing once every 6 months is reasonable. “If CPK [creatine phosphokinase] goes up, I don’t worry about it; it’s not clinically relevant. There is no recommendation for CPK monitoring, so if you’re getting that on your chem-20, I’d say don’t worry about it.”
Dr. Blauvelt reported that he is an investigator and a scientific adviser for several pharmaceutical companies developing treatments for AD, including companies that are evaluating or marketing JAK inhibitors for AD, including AbbVie, Incyte, and Pfizer, as well as dupilumab’s joint developers Sanofi and Regeneron.
PORTLAND, ORE. – according to Andrew Blauvelt, MD, MBA.
“In dermatology, you need to know about JAK inhibitors, and you need to know how to use them,” Dr. Blauvelt, president of Oregon Medical Research Center, Portland, said at the annual meeting of the Pacific Dermatologic Association. “Making the choice, ‘I’m not going to use those drugs because of safety concerns,’ may be okay in 2022, but we are going to be getting a lot more indications for these drugs. So instead of avoiding JAK inhibitors, I would say try to learn [about] them, understand them, and get your messaging out on safety.”
It’s difficult to imagine a clinician-researcher who has more experience with the use of biologics and JAK inhibitors in AD than Dr. Blauvelt, who has been the international investigator on several important trials of treatments that include dupilumab, tralokinumab, abrocitinib, and upadacitinib for AD such as CHRONOS, ECZTEND, JADE REGIMEN, and HEADS UP. At the meeting, he discussed his clinical approach to selecting systemic agents for AD and shared prescribing tips. He began by noting that the approval of dupilumab for moderate to severe AD in 2017 ushered in a new era of treating the disease systemically.
“When it was approved, experts went right to dupilumab if they could, and avoided the use of cyclosporine or methotrexate,” said Dr. Blauvelt, who is also an elected member of the American Society for Clinical Investigation and the International Eczema Council. “I still think that dupilumab is a great agent to start with. We’ve had a bit of difficulty improving upon it.”
Following dupilumab’s approval, three other systemic options became available for patients with moderate to severe AD: the human IgG4 monoclonal antibody tralokinumab that binds to interleukin-13, which is administered subcutaneously; and, more recently, the oral JAK inhibitors abrocitinib and upadacitinib, approved in January for moderate to severe AD.
“I’m a big fan of JAK inhibitors because I think they offer things that biologic and topical therapies can’t offer,” Dr. Blauvelt said. “Patients like the pills versus shots. They also like the speed; JAK inhibitors work faster than dupilumab and tralokinumab. So, if you have a patient with bad AD who wants to get better quickly, that would be a reason to choose a JAK inhibitor over a biologic if you can.”
When Dr. Blauvelt has asked AD clinical trial participants if they’d rather be treated with a biologic agent or with a JAK inhibitor, about half choose one over the other.
“Patients who shy away from the safety issues would choose the biologic trial while the ones who wanted the fast relief would choose the JAK trial,” he said. “But if you present both options and the patients prefer a pill, I think the JAK inhibitors do better with a rapid control of inflammation as well as pruritus – the latter within 2 days of taking the pills.”
When counseling patients initiating a JAK inhibitor, Dr. Blauvelt mentioned three advantages, compared with biologics: the pill formulation, the rapidity of response in pruritus control, and better efficacy. “The downside is the safety,” he said. “Safety is the elephant in the room for the JAK inhibitors.”
The risks listed in the boxed warning in the labeling for JAK inhibitors include: an increased risk of serious bacterial, fungal, and opportunistic infections such as TB; a higher rate of all-cause mortality, including cardiovascular death; a higher rate of MACE (major adverse cardiovascular events, defined as cardiovascular death, MI, and stroke); the potential for malignancy, including lymphoma; and the potential for thrombosis, including an increased incidence of pulmonary embolism (PE).
“Risk of thrombosis seems to be a class effect for all JAK inhibitors,” Dr. Blauvelt said. “As far as I know, it’s idiosyncratic. For nearly all the DVT [deep vein thrombosis] cases that have been reported, patients had baseline risk factors for DVT and PE, which are obesity, smoking, and use of oral contraceptives.”
Dr. Blauvelt pointed out that the boxed warning related to mortality, malignancies, and MACE stemmed from a long-term trial of the JAK inhibitor tofacitinib in RA patients. “Those patients had to be at least 50 years old, 75% of them were on concomitant methotrexate and/or prednisone, and they had to have at least one cardiac risk factor to get into the trial,” he said.
“I’m not saying those things can’t happen in dermatology patients, but if you look at the safety data of JAK inhibitors in the AD studies and in the alopecia areata studies, we are seeing a few cases of these things here and there, but not major signals,” he said. To date, “they look safer in dermatologic diseases compared to tofacitinib in RA data in older populations.”
He emphasized the importance of discussing each of the risks in the boxed warning with patients who are candidates for JAK inhibitor therapy.
Dr. Blauvelt likened the lab monitoring required for JAK inhibitors to that required for methotrexate. This means ordering at baseline, a CBC with differential, a chem-20, a lipid panel, and a QuantiFERON-TB Gold test. The JAK inhibitor labels do not include information on the frequency of monitoring, “but I have a distinct opinion on this because of my blood test monitoring experience in the trials for many years,” he said.
“I think it’s good to do follow-up testing at 1 month, then every 3 months in the first year. In my experience, the people who drop blood cell counts or increase their lipids tend to do it in the first year.”
After 1 year of treatment, he continued, follow-up testing once every 6 months is reasonable. “If CPK [creatine phosphokinase] goes up, I don’t worry about it; it’s not clinically relevant. There is no recommendation for CPK monitoring, so if you’re getting that on your chem-20, I’d say don’t worry about it.”
Dr. Blauvelt reported that he is an investigator and a scientific adviser for several pharmaceutical companies developing treatments for AD, including companies that are evaluating or marketing JAK inhibitors for AD, including AbbVie, Incyte, and Pfizer, as well as dupilumab’s joint developers Sanofi and Regeneron.
AT PDA 2022
FDA approves ‘rapid-acting’ oral drug for major depression
The U.S. Food and Drug Administration has approved the first oral N-methyl D-aspartate (NMDA) receptor antagonist for the treatment of major depressive disorder (MDD) in adults, its manufacturer has announced.
Auvelity (Axsome Therapeutics) is a proprietary extended-release oral tablet containing dextromethorphan (45 mg) and bupropion (105 mg).
,” the company said in a news release.
“The approval of Auvelity represents a milestone in depression treatment based on its novel oral NMDA antagonist mechanism, its rapid antidepressant efficacy demonstrated in controlled trials, and a relatively favorable safety profile,” Maurizio Fava, MD, psychiatrist-in-chief, Massachusetts General Hospital, Boston, added in the release.
‘Milestone’ in depression treatment?
Dr. Fava noted that nearly two-thirds of patients treated with currently available antidepressants fail to respond adequately, and those who do may not achieve clinically meaningful responses for up to 6-8 weeks.
“Given the debilitating nature of depression, the efficacy of Auvelity observed at 1 week and sustained thereafter may have a significant impact on the current treatment paradigm for this condition,” he said.
The company noted the drug was studied in a comprehensive clinical program that included more than 1,100 patients with MDD.
The efficacy of the drug was demonstrated in the GEMINI placebo-controlled study – with confirmatory evidence provided by the ASCEND study, which compared it with bupropion sustained-release tablets.
Axsome said it expects to launch the new oral medication in the fourth quarter of this year. It is not approved for use in children.
The full prescribing information and medication guide are available online.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved the first oral N-methyl D-aspartate (NMDA) receptor antagonist for the treatment of major depressive disorder (MDD) in adults, its manufacturer has announced.
Auvelity (Axsome Therapeutics) is a proprietary extended-release oral tablet containing dextromethorphan (45 mg) and bupropion (105 mg).
,” the company said in a news release.
“The approval of Auvelity represents a milestone in depression treatment based on its novel oral NMDA antagonist mechanism, its rapid antidepressant efficacy demonstrated in controlled trials, and a relatively favorable safety profile,” Maurizio Fava, MD, psychiatrist-in-chief, Massachusetts General Hospital, Boston, added in the release.
‘Milestone’ in depression treatment?
Dr. Fava noted that nearly two-thirds of patients treated with currently available antidepressants fail to respond adequately, and those who do may not achieve clinically meaningful responses for up to 6-8 weeks.
“Given the debilitating nature of depression, the efficacy of Auvelity observed at 1 week and sustained thereafter may have a significant impact on the current treatment paradigm for this condition,” he said.
The company noted the drug was studied in a comprehensive clinical program that included more than 1,100 patients with MDD.
The efficacy of the drug was demonstrated in the GEMINI placebo-controlled study – with confirmatory evidence provided by the ASCEND study, which compared it with bupropion sustained-release tablets.
Axsome said it expects to launch the new oral medication in the fourth quarter of this year. It is not approved for use in children.
The full prescribing information and medication guide are available online.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved the first oral N-methyl D-aspartate (NMDA) receptor antagonist for the treatment of major depressive disorder (MDD) in adults, its manufacturer has announced.
Auvelity (Axsome Therapeutics) is a proprietary extended-release oral tablet containing dextromethorphan (45 mg) and bupropion (105 mg).
,” the company said in a news release.
“The approval of Auvelity represents a milestone in depression treatment based on its novel oral NMDA antagonist mechanism, its rapid antidepressant efficacy demonstrated in controlled trials, and a relatively favorable safety profile,” Maurizio Fava, MD, psychiatrist-in-chief, Massachusetts General Hospital, Boston, added in the release.
‘Milestone’ in depression treatment?
Dr. Fava noted that nearly two-thirds of patients treated with currently available antidepressants fail to respond adequately, and those who do may not achieve clinically meaningful responses for up to 6-8 weeks.
“Given the debilitating nature of depression, the efficacy of Auvelity observed at 1 week and sustained thereafter may have a significant impact on the current treatment paradigm for this condition,” he said.
The company noted the drug was studied in a comprehensive clinical program that included more than 1,100 patients with MDD.
The efficacy of the drug was demonstrated in the GEMINI placebo-controlled study – with confirmatory evidence provided by the ASCEND study, which compared it with bupropion sustained-release tablets.
Axsome said it expects to launch the new oral medication in the fourth quarter of this year. It is not approved for use in children.
The full prescribing information and medication guide are available online.
A version of this article first appeared on Medscape.com.
Antibiotic before oral surgery spares endocarditis; study validates guidelines
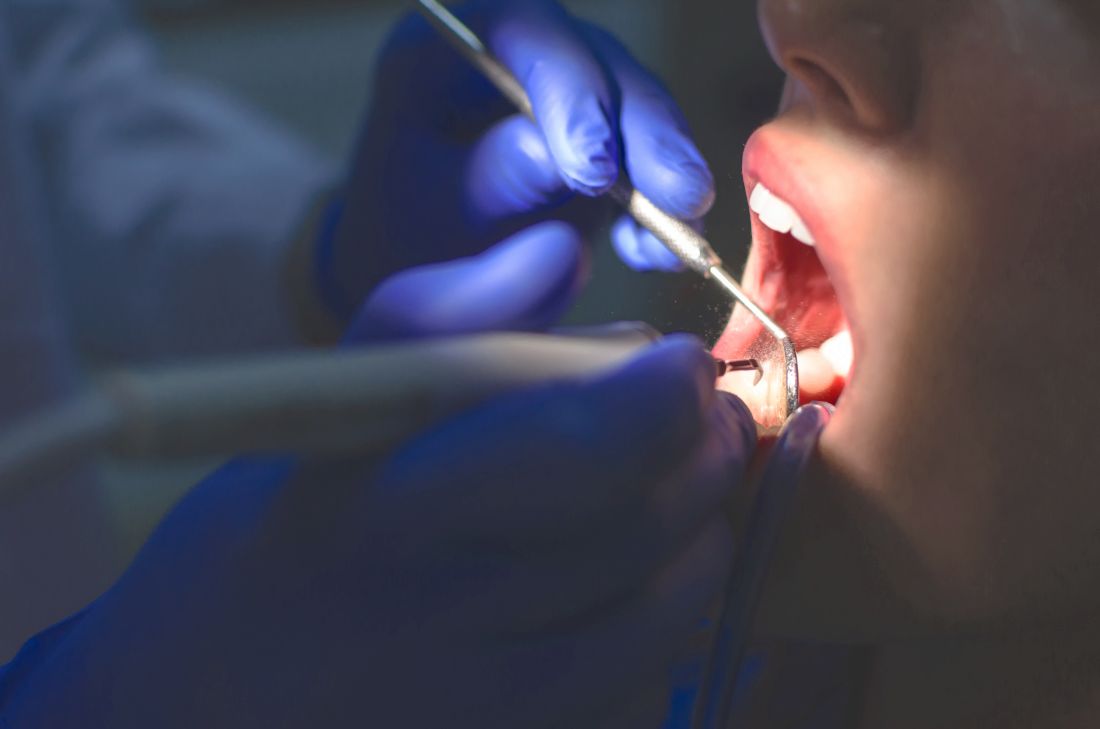
The strongest evidence yet to support clinical guidelines that recommend that people at high risk of endocarditis, such as those who’ve had previous episode the disease or who have a prosthetic cardiac valve, should take antibiotics before they have a tooth pulled or other types of oral surgery, comes from a new study that used two methodologies.
But it also pointed out that two-thirds of the time they aren’t getting that type of antibiotic coverage.
The researchers conducted a cohort study of almost 8 million retirees with employer-paid Medicare supplemental prescription benefits and dental benefits, then conducted a case-crossover study of 3,774 people from the cohort who’d been hospitalized with infectious endocarditis (IE) and who had invasive dental procedures. The bottom line is that the study supports the clinical guidelines from the American Heart Association and the European Society of Cardiology that recommend antibiotic prophylaxis (AP) before dental procedures for patients at high-risk of IE.
Likewise, lead author Martin Thornhill, MBBS, BDS, PhD, said in an interview, the findings also suggest that existing guidelines in the United Kingdom, which recommend against AP in these patients, “should be reconsidered.”
Those AHA and ESC guidelines, however, are “based on no good quality evidence,” said Dr. Thornhill, professor of translational research in dentistry at the University of Sheffield (England) School of Clinical Dentistry. “Other studies have looked at this, but we’ve done the largest study that has shown the clear association between invasive dental procedures and subsequent development of infective endocarditis.”
In the entire cohort of 7.95 million patients, 3,774 had cases of IE that required hospitalization. The study defined highest risk of IE as meeting one of these six criteria: a previous case of IE; a prosthetic cardiac valve or a valve repair that used prosthetic material; cyanotic congenital heart disease; palliative shunts or conduits to treat CHD; or a congenital heart defect that had been fully repaired, either by surgery or a transcatheter procedure, with prosthetic material or device – the latter within 6 months of the procedure.
Moderate IE risk included patients who had rheumatic heart disease, nonrheumatic valve disease or congenital valve anomalies—including mitral valve prolapse or aortic stenosis—or hypertrophic cardiomyopathy.
Risk classification and poor compliance
Highest-risk patients had significantly higher rates of IE a month after a dental procedure than lower-risk groups: 467.6 cases per 1 million procedures vs. 24.2 for moderate risk and 3.8 for low or unknown risk. A subanalysis found that the odds of IE were significantly increased for two specific dental procedures: extractions, with an odds ratio of 9.22 (95% confidence interval [CI], 5.54-15.88; P < .0001); and other oral surgical procedures, with an OR of 20.18 (95% CI, 11.22-37.74; P < .0001).
The study also found that 32.6% of the high-risk patients undergoing dental procedures got AP. “Clearly that shows a low level of compliance with the guidelines in the U.S.,” Dr. Thornhill said. “That’s something that needs to be addressed.”
The study was unique in that it used both a population cohort study and the case-crossover study. “It didn’t matter which of the two methods we used; we essentially came to the same result, which I think adds further weight to the findings,” Dr. Thornhill said.
This may be the best evidence to support the guidelines that clinicians may get. While the observational nature of this study has its limitations, conducting a randomized clinical trial to further validate the findings would be “logistically impossible,” he said, in that it would require an “absolutely enormous” cohort and coordination between medical and dental databases covering thousands of lives. An RCT would also require not using AP for some patients. “It’s not ethical to keep somebody off of antibiotic prophylaxis when there’s such a high risk of death and severe outcomes,” Dr. Thornhill said.
Ann Bolger, MD, emeritus professor of medicine at the University of California, San Francisco, and coauthor of an editorial comment on the study, said in an interview that this study is noteworthy not only for its dual methodology, but for the quality of the data that matched patients at high risk for IE with prescription and dental records. “The fact that they were able to have those details in enough granularity that they knew whether a dental procedure was likely to meet the criteria for these more invasive exposures really broke it open from my perspective,” she said.
She called the low compliance rate with AHA guidelines “one of the most sobering points of this,” and said it should put clinicians on notice that they must do more to educate and engage with high-risk patients. “The lines of communication here are somewhat fraught; it’s a little bit of a hot potato,” she said. “It’s a really great communications opportunity to get the provider’s attention back on this. You’re a cardiologist; you have to have this conversation when you see your patient with a prosthetic valve or who’s had endocarditis every time they come in. There’s a whole litany, and it takes 3 minutes, but you have to do it.”
The study received funding from Delta Dental of Michigan Research Committee and Renaissance Health Service Corp., and Dr. Thornhill received support from Delta Dental Research and Data Institute for the study. Dr. Bolger participated in the 2007 and 2021 AHA statements on AP to prevent IE.
The strongest evidence yet to support clinical guidelines that recommend that people at high risk of endocarditis, such as those who’ve had previous episode the disease or who have a prosthetic cardiac valve, should take antibiotics before they have a tooth pulled or other types of oral surgery, comes from a new study that used two methodologies.
But it also pointed out that two-thirds of the time they aren’t getting that type of antibiotic coverage.
The researchers conducted a cohort study of almost 8 million retirees with employer-paid Medicare supplemental prescription benefits and dental benefits, then conducted a case-crossover study of 3,774 people from the cohort who’d been hospitalized with infectious endocarditis (IE) and who had invasive dental procedures. The bottom line is that the study supports the clinical guidelines from the American Heart Association and the European Society of Cardiology that recommend antibiotic prophylaxis (AP) before dental procedures for patients at high-risk of IE.
Likewise, lead author Martin Thornhill, MBBS, BDS, PhD, said in an interview, the findings also suggest that existing guidelines in the United Kingdom, which recommend against AP in these patients, “should be reconsidered.”
Those AHA and ESC guidelines, however, are “based on no good quality evidence,” said Dr. Thornhill, professor of translational research in dentistry at the University of Sheffield (England) School of Clinical Dentistry. “Other studies have looked at this, but we’ve done the largest study that has shown the clear association between invasive dental procedures and subsequent development of infective endocarditis.”
In the entire cohort of 7.95 million patients, 3,774 had cases of IE that required hospitalization. The study defined highest risk of IE as meeting one of these six criteria: a previous case of IE; a prosthetic cardiac valve or a valve repair that used prosthetic material; cyanotic congenital heart disease; palliative shunts or conduits to treat CHD; or a congenital heart defect that had been fully repaired, either by surgery or a transcatheter procedure, with prosthetic material or device – the latter within 6 months of the procedure.
Moderate IE risk included patients who had rheumatic heart disease, nonrheumatic valve disease or congenital valve anomalies—including mitral valve prolapse or aortic stenosis—or hypertrophic cardiomyopathy.
Risk classification and poor compliance
Highest-risk patients had significantly higher rates of IE a month after a dental procedure than lower-risk groups: 467.6 cases per 1 million procedures vs. 24.2 for moderate risk and 3.8 for low or unknown risk. A subanalysis found that the odds of IE were significantly increased for two specific dental procedures: extractions, with an odds ratio of 9.22 (95% confidence interval [CI], 5.54-15.88; P < .0001); and other oral surgical procedures, with an OR of 20.18 (95% CI, 11.22-37.74; P < .0001).
The study also found that 32.6% of the high-risk patients undergoing dental procedures got AP. “Clearly that shows a low level of compliance with the guidelines in the U.S.,” Dr. Thornhill said. “That’s something that needs to be addressed.”
The study was unique in that it used both a population cohort study and the case-crossover study. “It didn’t matter which of the two methods we used; we essentially came to the same result, which I think adds further weight to the findings,” Dr. Thornhill said.
This may be the best evidence to support the guidelines that clinicians may get. While the observational nature of this study has its limitations, conducting a randomized clinical trial to further validate the findings would be “logistically impossible,” he said, in that it would require an “absolutely enormous” cohort and coordination between medical and dental databases covering thousands of lives. An RCT would also require not using AP for some patients. “It’s not ethical to keep somebody off of antibiotic prophylaxis when there’s such a high risk of death and severe outcomes,” Dr. Thornhill said.
Ann Bolger, MD, emeritus professor of medicine at the University of California, San Francisco, and coauthor of an editorial comment on the study, said in an interview that this study is noteworthy not only for its dual methodology, but for the quality of the data that matched patients at high risk for IE with prescription and dental records. “The fact that they were able to have those details in enough granularity that they knew whether a dental procedure was likely to meet the criteria for these more invasive exposures really broke it open from my perspective,” she said.
She called the low compliance rate with AHA guidelines “one of the most sobering points of this,” and said it should put clinicians on notice that they must do more to educate and engage with high-risk patients. “The lines of communication here are somewhat fraught; it’s a little bit of a hot potato,” she said. “It’s a really great communications opportunity to get the provider’s attention back on this. You’re a cardiologist; you have to have this conversation when you see your patient with a prosthetic valve or who’s had endocarditis every time they come in. There’s a whole litany, and it takes 3 minutes, but you have to do it.”
The study received funding from Delta Dental of Michigan Research Committee and Renaissance Health Service Corp., and Dr. Thornhill received support from Delta Dental Research and Data Institute for the study. Dr. Bolger participated in the 2007 and 2021 AHA statements on AP to prevent IE.
The strongest evidence yet to support clinical guidelines that recommend that people at high risk of endocarditis, such as those who’ve had previous episode the disease or who have a prosthetic cardiac valve, should take antibiotics before they have a tooth pulled or other types of oral surgery, comes from a new study that used two methodologies.
But it also pointed out that two-thirds of the time they aren’t getting that type of antibiotic coverage.
The researchers conducted a cohort study of almost 8 million retirees with employer-paid Medicare supplemental prescription benefits and dental benefits, then conducted a case-crossover study of 3,774 people from the cohort who’d been hospitalized with infectious endocarditis (IE) and who had invasive dental procedures. The bottom line is that the study supports the clinical guidelines from the American Heart Association and the European Society of Cardiology that recommend antibiotic prophylaxis (AP) before dental procedures for patients at high-risk of IE.
Likewise, lead author Martin Thornhill, MBBS, BDS, PhD, said in an interview, the findings also suggest that existing guidelines in the United Kingdom, which recommend against AP in these patients, “should be reconsidered.”
Those AHA and ESC guidelines, however, are “based on no good quality evidence,” said Dr. Thornhill, professor of translational research in dentistry at the University of Sheffield (England) School of Clinical Dentistry. “Other studies have looked at this, but we’ve done the largest study that has shown the clear association between invasive dental procedures and subsequent development of infective endocarditis.”
In the entire cohort of 7.95 million patients, 3,774 had cases of IE that required hospitalization. The study defined highest risk of IE as meeting one of these six criteria: a previous case of IE; a prosthetic cardiac valve or a valve repair that used prosthetic material; cyanotic congenital heart disease; palliative shunts or conduits to treat CHD; or a congenital heart defect that had been fully repaired, either by surgery or a transcatheter procedure, with prosthetic material or device – the latter within 6 months of the procedure.
Moderate IE risk included patients who had rheumatic heart disease, nonrheumatic valve disease or congenital valve anomalies—including mitral valve prolapse or aortic stenosis—or hypertrophic cardiomyopathy.
Risk classification and poor compliance
Highest-risk patients had significantly higher rates of IE a month after a dental procedure than lower-risk groups: 467.6 cases per 1 million procedures vs. 24.2 for moderate risk and 3.8 for low or unknown risk. A subanalysis found that the odds of IE were significantly increased for two specific dental procedures: extractions, with an odds ratio of 9.22 (95% confidence interval [CI], 5.54-15.88; P < .0001); and other oral surgical procedures, with an OR of 20.18 (95% CI, 11.22-37.74; P < .0001).
The study also found that 32.6% of the high-risk patients undergoing dental procedures got AP. “Clearly that shows a low level of compliance with the guidelines in the U.S.,” Dr. Thornhill said. “That’s something that needs to be addressed.”
The study was unique in that it used both a population cohort study and the case-crossover study. “It didn’t matter which of the two methods we used; we essentially came to the same result, which I think adds further weight to the findings,” Dr. Thornhill said.
This may be the best evidence to support the guidelines that clinicians may get. While the observational nature of this study has its limitations, conducting a randomized clinical trial to further validate the findings would be “logistically impossible,” he said, in that it would require an “absolutely enormous” cohort and coordination between medical and dental databases covering thousands of lives. An RCT would also require not using AP for some patients. “It’s not ethical to keep somebody off of antibiotic prophylaxis when there’s such a high risk of death and severe outcomes,” Dr. Thornhill said.
Ann Bolger, MD, emeritus professor of medicine at the University of California, San Francisco, and coauthor of an editorial comment on the study, said in an interview that this study is noteworthy not only for its dual methodology, but for the quality of the data that matched patients at high risk for IE with prescription and dental records. “The fact that they were able to have those details in enough granularity that they knew whether a dental procedure was likely to meet the criteria for these more invasive exposures really broke it open from my perspective,” she said.
She called the low compliance rate with AHA guidelines “one of the most sobering points of this,” and said it should put clinicians on notice that they must do more to educate and engage with high-risk patients. “The lines of communication here are somewhat fraught; it’s a little bit of a hot potato,” she said. “It’s a really great communications opportunity to get the provider’s attention back on this. You’re a cardiologist; you have to have this conversation when you see your patient with a prosthetic valve or who’s had endocarditis every time they come in. There’s a whole litany, and it takes 3 minutes, but you have to do it.”
The study received funding from Delta Dental of Michigan Research Committee and Renaissance Health Service Corp., and Dr. Thornhill received support from Delta Dental Research and Data Institute for the study. Dr. Bolger participated in the 2007 and 2021 AHA statements on AP to prevent IE.
FROM JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
What ketamine and psilocybin can and cannot do in depression
Recent studies with hallucinogens have raised hopes for an effective drug-based therapy to treat chronic depression. At the German Congress of Psychosomatic Medicine and Psychotherapy, Torsten Passie, MD, PhD, professor of psychiatry and psychotherapy at the Hannover (Germay) Medical School, gave a presentation on the current state of psilocybin and ketamine/esketamine research.
Dr. Passie, who also is head physician of the specialist unit for addiction and addiction prevention at the Diakonisches Werk in Hannover, has been investigating hallucinogenic substances and their application in psychotherapy for decades.
New therapies sought
In depression, gloom extends beyond the patient’s mood. For some time there has been little cause for joy with regard to chronic depression therapy. Established drug therapies hardly perform any better than placebo in meta-analyses, as a study recently confirmed. The pharmaceutical industry pulled out of psycho-pharmaceutical development more than 10 years ago. What’s more, the number of cases is rising, especially among young people, and there are long waiting times for psychotherapy appointments.
It is no wonder that some are welcoming new drug-based approaches with lysergic acid diethylamide (LSD)–like hallucinogens. In 2016, a study on psilocybin was published in The Lancet Psychiatry, although the study was unblinded and included only 24 patients.
Evoking emotions
A range of substances can be classed as hallucinogens, including psilocybin, mescaline, LSD, 3,4-methylenedioxy-methamphetamine (MDMA, also known as ecstasy), and ketamine.
Taking hallucinogens can cause a release of serotonin and dopamine, an increase in activity levels in the brain, a shift in stimulus filtering, an increase in the production of internal stimuli (inner experiences), and a change in sensory integration (for example, synesthesia).
Besides falling into a dreamlike state, patients can achieve an expansion or narrowing of consciousness if they focus on an inner experience. Internal perception increases. Perceptual routines are broken apart. Thought processes become more image-based and are more associative than normal.
Patients therefore are more capable of making new and unusual connections between different biographical or current situations. Previously unconscious ideas can become conscious. At higher doses, ego loss can occur, which can be associated with a mystical feeling of connectedness.
Hallucinogens mainly evoke and heighten emotions. Those effects may be experienced strongly as internal visions or in physical manifestations (for example, crying or laughing). In contrast, conventional antidepressants work by suppressing emotions (that is, emotional blunting).
These different mechanisms result in two contrasting management strategies. For example, SSRI antidepressants cause a patient to perceive workplace bullying as less severe and to do nothing to change the situation; the patient remains passive.
In contrast, a therapeutically guided, emotionally activating experience on hallucinogens can help the patient to try more actively to change the stressful situation.
Ketamine has a special place among hallucinogens. Unlike other hallucinogens, ketamine causes a strong clouding of consciousness, a reduction in physical sensory perception, and significant disruption in thinking and memory. It is therefore only suitable as a short-term intervention and is therapeutically impractical over the long term.
Ketamine’s effects
Ketamine, a racemic mixture of the enantiomers S-ketamine and R-ketamine, was originally used only as an analgesic and anesthetic. Owing to its rapid antidepressant effect, it has since also been used as an emergency medication for severe depression, sometimes in combination with SSRIs or serotonin noradrenaline reuptake inhibitors.
Approximately 60% of patients respond to the treatment. Whereas with conventional antidepressants, onset of action requires 10-14 days, ketamine is effective within a few hours. However, relapse always occurs, usually very quickly. After 2-3 days, the effect is usually approximately that of a placebo. An administration interval of about 2 days is optimal. However, “resistance” to the effect often develops after some time: the drug’s antidepressant effect diminishes.
Ketamine also has some unpleasant side effects, such as depersonalization, dissociation, impaired thinking, nystagmus, and psychotomimetic effects. Nausea and vomiting also occur. Interestingly, the latter does not bother the patient much, owing to the drug’s psychological effects, and it does not lead to treatment discontinuation, said Dr. Passie, who described his clinical experiences with ketamine.
Since ketamine causes a considerable clouding of consciousness, sensory disorders, and significant memory problems, it is not suitable for psychedelic-assisted psychotherapy, unlike LSD or psilocybin, he emphasized.
Ketamine 2.0?
Esketamine, the pure S-enantiomer of ketamine, has been on the market since 2019 in the form of a nasal spray (Spravato). Esketamine has been approved in combination with oral antidepressant therapy for adults with a moderate to severe episode of major depression for acute treatment of a psychiatric emergency.
A meta-analysis from 2022 concluded that the original racemic ketamine is better than the new esketamine in reducing symptoms of depression.
In his own comprehensive study, Dr. Passie concluded that the mental impairments that occur during therapy did not differ significantly between substances. The patients even felt that the side effects from esketamine therapy were much more mentally unpleasant, said Dr. Passie. He concluded that the R-enantiomer may have a kind of protective effect against some of the psychopathological effects of the S-enantiomer (esketamine).
In addition, preclinical studies have indicated that the antidepressant effects of R-enantiomer, which is not contained in esketamine, are longer lasting and stronger.
Another problem is absorption, which can be inconsistent with a nasal spray. It may differ, for example, depending on the ambient humidity or whether the patient has recently had a cold. In addition, the spray is far more expensive than the ketamine injection, said Dr. Passie. Patients must also use the nasal spray under supervision at a medical practice (as with the intravenous application) and must receive follow-up care there. It therefore offers no advantage over the ketamine injection.
According to the Institute for Quality and Efficiency in Healthcare, no additional benefit has been proven for esketamine over standard therapies for adults who have experienced a moderate to severe depressive episode when used as short-term treatment for the rapid reduction of depressive symptoms in a psychiatric emergency. The German Medical Association agreed with this evaluation in October 2021.
In the United Kingdom, the medication was never approved, owing to the fact that it was too expensive and that no studies comparing it with psychotherapy were available.
Add-on psilocybin?
What was experienced under the influence of psilocybin can also be subsequently processed and used in psychotherapy.
The acute effect of psilocybin begins after approximately 40 minutes and lasts for 4-6 hours. The antidepressant effect, if it occurs at all, is of immediate onset. Unlike ketamine/esketamine, psilocybin hardly has any physical side effects.
The neurologic mechanism of action has been investigated recently using fMRI and PET techniques. According to the investigations, the substance causes individual networks of activity in the patient’s brain to interconnect more strongly, said Dr. Passie. The thalamus, the filter station for sensory information, as well as the limbic and paralimbic structures, which generate emotions, and the cortex are all activated more strongly.
Two therapeutic settings
Psilocybin, at least in the context of studies, is used in two settings: psycholytic therapy and psychedelic therapy. Both settings originated in the 1950s and were also used with LSD as the active substance.
Psycholytic therapy with psilocybin entails multiple administrations at low doses (for example, 10-18 mg), incorporated into a longer, mostly psychodynamic therapy of around 50-100 hours (often on an inpatient basis at the beginning). It results in what is described as an extended encounter with oneself. The focus is on psychodynamic experiences, such as memories and internal conflicts. In addition, novel experiences with oneself and self-recognition are important.
Psychedelic therapy generally entails one or two sessions with a high dose (for example, 25-35 mg psilocybin). The preparation and follow-up are limited to a few sessions. These methods refer to so-called transpersonal psychology, which addresses extraordinary states of consciousness in line with religious experiences. It often leads to an intense self-confrontation as well as to new evaluations of self and world. The central element to this therapy is the experience of a mystical ego loss and the concomitant feeling of connectedness, which should help to expand one’s perspective.
Euphoria and disillusionment
The first promising studies with a few patients suffering from depression were followed by others in which the euphoria was allowed to fade away somewhat. In the first direct comparison in a methodically high-grade double-blind study, psilocybin was inferior to the SSRI antidepressant escitalopram.
“There is a great variation in response from person to person,” said Dr. Passie. “The better the study is methodically controlled, the worse the results,” he hypothesized.
“Since the method is up to 50 times more expensive in practice, compared to SSRI therapy over 6-12 weeks, the question clearly must be asked as to whether it really has any great future.”
Outlook for psilocybin
Nevertheless, Dr. Passie still sees potential in psilocybin. He considers an approach in which psilocybin therapy is more firmly incorporated into psychotherapy, with between four and 10 therapy sessions before and after administration of a lower therapeutic dose of the substance, to be more promising.
“With this kind of intensive preparation and follow-up, as well as the repeated psilocybin sessions, the patient can benefit much more than is possible with one or two high-dose sessions,” said Dr. Passie, who also is chair of the International Society for Substance-Assisted Psychotherapy. “The constant ‘in-depth work on the ego’ required for drastic therapeutic changes can be more effective and lead to permanent improvements. I have no doubt about this.”
In Dr. Passie’s opinion, the best approach would involve a dignified inpatient setting with a longer period of follow-up care and consistent posttreatment care, including group therapy. The shape of future psilocybin therapy depends on whether the rather abrupt change seen with high-dose psychedelic therapy is permanent. The answer to this question will be decisive for the method and manner of its future clinical use.
Because of the somewhat negative study results, however, the initial investors are pulling out. Dr. Passie is therefore skeptical about whether the necessary larger studies will take place and whether psilocybin will make it onto the market.
In Switzerland, which is not subject to EU restrictions, more than 30 physicians have been authorized to use psilocybin, LSD, and MDMA in psychotherapy sessions. Still, in some respects this is a special case that cannot be transferred easily to other countries, said Dr. Passie.
Possible psilocybin improvement?
Various chemical derivatives of psychoactive substances have been researched, including a psilocybin variant with the label CYB003. With CYB003, the length of the acute psychedelic experience is reduced from around 6 hours (such as with psilocybin) to 1 hour. The plasma concentration of the substance is less variable between different patients. It is assumed that its effects will also differ less from person to person.
In July, researchers began a study of the use of CYB003 in the treatment of major depression. In the randomized, double-blind, placebo-controlled study with 40 patients, multiple doses of the substance will be administered.
When asked, Dr. Passie was rather skeptical about the study. He considers the approaches with psilocybin derivatives to be the consequences of a “gold-rush atmosphere” and expects there will be no real additional benefit, especially not a reduction in the period of action.
A version of this article first appeared on Medscape.com.
Recent studies with hallucinogens have raised hopes for an effective drug-based therapy to treat chronic depression. At the German Congress of Psychosomatic Medicine and Psychotherapy, Torsten Passie, MD, PhD, professor of psychiatry and psychotherapy at the Hannover (Germay) Medical School, gave a presentation on the current state of psilocybin and ketamine/esketamine research.
Dr. Passie, who also is head physician of the specialist unit for addiction and addiction prevention at the Diakonisches Werk in Hannover, has been investigating hallucinogenic substances and their application in psychotherapy for decades.
New therapies sought
In depression, gloom extends beyond the patient’s mood. For some time there has been little cause for joy with regard to chronic depression therapy. Established drug therapies hardly perform any better than placebo in meta-analyses, as a study recently confirmed. The pharmaceutical industry pulled out of psycho-pharmaceutical development more than 10 years ago. What’s more, the number of cases is rising, especially among young people, and there are long waiting times for psychotherapy appointments.
It is no wonder that some are welcoming new drug-based approaches with lysergic acid diethylamide (LSD)–like hallucinogens. In 2016, a study on psilocybin was published in The Lancet Psychiatry, although the study was unblinded and included only 24 patients.
Evoking emotions
A range of substances can be classed as hallucinogens, including psilocybin, mescaline, LSD, 3,4-methylenedioxy-methamphetamine (MDMA, also known as ecstasy), and ketamine.
Taking hallucinogens can cause a release of serotonin and dopamine, an increase in activity levels in the brain, a shift in stimulus filtering, an increase in the production of internal stimuli (inner experiences), and a change in sensory integration (for example, synesthesia).
Besides falling into a dreamlike state, patients can achieve an expansion or narrowing of consciousness if they focus on an inner experience. Internal perception increases. Perceptual routines are broken apart. Thought processes become more image-based and are more associative than normal.
Patients therefore are more capable of making new and unusual connections between different biographical or current situations. Previously unconscious ideas can become conscious. At higher doses, ego loss can occur, which can be associated with a mystical feeling of connectedness.
Hallucinogens mainly evoke and heighten emotions. Those effects may be experienced strongly as internal visions or in physical manifestations (for example, crying or laughing). In contrast, conventional antidepressants work by suppressing emotions (that is, emotional blunting).
These different mechanisms result in two contrasting management strategies. For example, SSRI antidepressants cause a patient to perceive workplace bullying as less severe and to do nothing to change the situation; the patient remains passive.
In contrast, a therapeutically guided, emotionally activating experience on hallucinogens can help the patient to try more actively to change the stressful situation.
Ketamine has a special place among hallucinogens. Unlike other hallucinogens, ketamine causes a strong clouding of consciousness, a reduction in physical sensory perception, and significant disruption in thinking and memory. It is therefore only suitable as a short-term intervention and is therapeutically impractical over the long term.
Ketamine’s effects
Ketamine, a racemic mixture of the enantiomers S-ketamine and R-ketamine, was originally used only as an analgesic and anesthetic. Owing to its rapid antidepressant effect, it has since also been used as an emergency medication for severe depression, sometimes in combination with SSRIs or serotonin noradrenaline reuptake inhibitors.
Approximately 60% of patients respond to the treatment. Whereas with conventional antidepressants, onset of action requires 10-14 days, ketamine is effective within a few hours. However, relapse always occurs, usually very quickly. After 2-3 days, the effect is usually approximately that of a placebo. An administration interval of about 2 days is optimal. However, “resistance” to the effect often develops after some time: the drug’s antidepressant effect diminishes.
Ketamine also has some unpleasant side effects, such as depersonalization, dissociation, impaired thinking, nystagmus, and psychotomimetic effects. Nausea and vomiting also occur. Interestingly, the latter does not bother the patient much, owing to the drug’s psychological effects, and it does not lead to treatment discontinuation, said Dr. Passie, who described his clinical experiences with ketamine.
Since ketamine causes a considerable clouding of consciousness, sensory disorders, and significant memory problems, it is not suitable for psychedelic-assisted psychotherapy, unlike LSD or psilocybin, he emphasized.
Ketamine 2.0?
Esketamine, the pure S-enantiomer of ketamine, has been on the market since 2019 in the form of a nasal spray (Spravato). Esketamine has been approved in combination with oral antidepressant therapy for adults with a moderate to severe episode of major depression for acute treatment of a psychiatric emergency.
A meta-analysis from 2022 concluded that the original racemic ketamine is better than the new esketamine in reducing symptoms of depression.
In his own comprehensive study, Dr. Passie concluded that the mental impairments that occur during therapy did not differ significantly between substances. The patients even felt that the side effects from esketamine therapy were much more mentally unpleasant, said Dr. Passie. He concluded that the R-enantiomer may have a kind of protective effect against some of the psychopathological effects of the S-enantiomer (esketamine).
In addition, preclinical studies have indicated that the antidepressant effects of R-enantiomer, which is not contained in esketamine, are longer lasting and stronger.
Another problem is absorption, which can be inconsistent with a nasal spray. It may differ, for example, depending on the ambient humidity or whether the patient has recently had a cold. In addition, the spray is far more expensive than the ketamine injection, said Dr. Passie. Patients must also use the nasal spray under supervision at a medical practice (as with the intravenous application) and must receive follow-up care there. It therefore offers no advantage over the ketamine injection.
According to the Institute for Quality and Efficiency in Healthcare, no additional benefit has been proven for esketamine over standard therapies for adults who have experienced a moderate to severe depressive episode when used as short-term treatment for the rapid reduction of depressive symptoms in a psychiatric emergency. The German Medical Association agreed with this evaluation in October 2021.
In the United Kingdom, the medication was never approved, owing to the fact that it was too expensive and that no studies comparing it with psychotherapy were available.
Add-on psilocybin?
What was experienced under the influence of psilocybin can also be subsequently processed and used in psychotherapy.
The acute effect of psilocybin begins after approximately 40 minutes and lasts for 4-6 hours. The antidepressant effect, if it occurs at all, is of immediate onset. Unlike ketamine/esketamine, psilocybin hardly has any physical side effects.
The neurologic mechanism of action has been investigated recently using fMRI and PET techniques. According to the investigations, the substance causes individual networks of activity in the patient’s brain to interconnect more strongly, said Dr. Passie. The thalamus, the filter station for sensory information, as well as the limbic and paralimbic structures, which generate emotions, and the cortex are all activated more strongly.
Two therapeutic settings
Psilocybin, at least in the context of studies, is used in two settings: psycholytic therapy and psychedelic therapy. Both settings originated in the 1950s and were also used with LSD as the active substance.
Psycholytic therapy with psilocybin entails multiple administrations at low doses (for example, 10-18 mg), incorporated into a longer, mostly psychodynamic therapy of around 50-100 hours (often on an inpatient basis at the beginning). It results in what is described as an extended encounter with oneself. The focus is on psychodynamic experiences, such as memories and internal conflicts. In addition, novel experiences with oneself and self-recognition are important.
Psychedelic therapy generally entails one or two sessions with a high dose (for example, 25-35 mg psilocybin). The preparation and follow-up are limited to a few sessions. These methods refer to so-called transpersonal psychology, which addresses extraordinary states of consciousness in line with religious experiences. It often leads to an intense self-confrontation as well as to new evaluations of self and world. The central element to this therapy is the experience of a mystical ego loss and the concomitant feeling of connectedness, which should help to expand one’s perspective.
Euphoria and disillusionment
The first promising studies with a few patients suffering from depression were followed by others in which the euphoria was allowed to fade away somewhat. In the first direct comparison in a methodically high-grade double-blind study, psilocybin was inferior to the SSRI antidepressant escitalopram.
“There is a great variation in response from person to person,” said Dr. Passie. “The better the study is methodically controlled, the worse the results,” he hypothesized.
“Since the method is up to 50 times more expensive in practice, compared to SSRI therapy over 6-12 weeks, the question clearly must be asked as to whether it really has any great future.”
Outlook for psilocybin
Nevertheless, Dr. Passie still sees potential in psilocybin. He considers an approach in which psilocybin therapy is more firmly incorporated into psychotherapy, with between four and 10 therapy sessions before and after administration of a lower therapeutic dose of the substance, to be more promising.
“With this kind of intensive preparation and follow-up, as well as the repeated psilocybin sessions, the patient can benefit much more than is possible with one or two high-dose sessions,” said Dr. Passie, who also is chair of the International Society for Substance-Assisted Psychotherapy. “The constant ‘in-depth work on the ego’ required for drastic therapeutic changes can be more effective and lead to permanent improvements. I have no doubt about this.”
In Dr. Passie’s opinion, the best approach would involve a dignified inpatient setting with a longer period of follow-up care and consistent posttreatment care, including group therapy. The shape of future psilocybin therapy depends on whether the rather abrupt change seen with high-dose psychedelic therapy is permanent. The answer to this question will be decisive for the method and manner of its future clinical use.
Because of the somewhat negative study results, however, the initial investors are pulling out. Dr. Passie is therefore skeptical about whether the necessary larger studies will take place and whether psilocybin will make it onto the market.
In Switzerland, which is not subject to EU restrictions, more than 30 physicians have been authorized to use psilocybin, LSD, and MDMA in psychotherapy sessions. Still, in some respects this is a special case that cannot be transferred easily to other countries, said Dr. Passie.
Possible psilocybin improvement?
Various chemical derivatives of psychoactive substances have been researched, including a psilocybin variant with the label CYB003. With CYB003, the length of the acute psychedelic experience is reduced from around 6 hours (such as with psilocybin) to 1 hour. The plasma concentration of the substance is less variable between different patients. It is assumed that its effects will also differ less from person to person.
In July, researchers began a study of the use of CYB003 in the treatment of major depression. In the randomized, double-blind, placebo-controlled study with 40 patients, multiple doses of the substance will be administered.
When asked, Dr. Passie was rather skeptical about the study. He considers the approaches with psilocybin derivatives to be the consequences of a “gold-rush atmosphere” and expects there will be no real additional benefit, especially not a reduction in the period of action.
A version of this article first appeared on Medscape.com.
Recent studies with hallucinogens have raised hopes for an effective drug-based therapy to treat chronic depression. At the German Congress of Psychosomatic Medicine and Psychotherapy, Torsten Passie, MD, PhD, professor of psychiatry and psychotherapy at the Hannover (Germay) Medical School, gave a presentation on the current state of psilocybin and ketamine/esketamine research.
Dr. Passie, who also is head physician of the specialist unit for addiction and addiction prevention at the Diakonisches Werk in Hannover, has been investigating hallucinogenic substances and their application in psychotherapy for decades.
New therapies sought
In depression, gloom extends beyond the patient’s mood. For some time there has been little cause for joy with regard to chronic depression therapy. Established drug therapies hardly perform any better than placebo in meta-analyses, as a study recently confirmed. The pharmaceutical industry pulled out of psycho-pharmaceutical development more than 10 years ago. What’s more, the number of cases is rising, especially among young people, and there are long waiting times for psychotherapy appointments.
It is no wonder that some are welcoming new drug-based approaches with lysergic acid diethylamide (LSD)–like hallucinogens. In 2016, a study on psilocybin was published in The Lancet Psychiatry, although the study was unblinded and included only 24 patients.
Evoking emotions
A range of substances can be classed as hallucinogens, including psilocybin, mescaline, LSD, 3,4-methylenedioxy-methamphetamine (MDMA, also known as ecstasy), and ketamine.
Taking hallucinogens can cause a release of serotonin and dopamine, an increase in activity levels in the brain, a shift in stimulus filtering, an increase in the production of internal stimuli (inner experiences), and a change in sensory integration (for example, synesthesia).
Besides falling into a dreamlike state, patients can achieve an expansion or narrowing of consciousness if they focus on an inner experience. Internal perception increases. Perceptual routines are broken apart. Thought processes become more image-based and are more associative than normal.
Patients therefore are more capable of making new and unusual connections between different biographical or current situations. Previously unconscious ideas can become conscious. At higher doses, ego loss can occur, which can be associated with a mystical feeling of connectedness.
Hallucinogens mainly evoke and heighten emotions. Those effects may be experienced strongly as internal visions or in physical manifestations (for example, crying or laughing). In contrast, conventional antidepressants work by suppressing emotions (that is, emotional blunting).
These different mechanisms result in two contrasting management strategies. For example, SSRI antidepressants cause a patient to perceive workplace bullying as less severe and to do nothing to change the situation; the patient remains passive.
In contrast, a therapeutically guided, emotionally activating experience on hallucinogens can help the patient to try more actively to change the stressful situation.
Ketamine has a special place among hallucinogens. Unlike other hallucinogens, ketamine causes a strong clouding of consciousness, a reduction in physical sensory perception, and significant disruption in thinking and memory. It is therefore only suitable as a short-term intervention and is therapeutically impractical over the long term.
Ketamine’s effects
Ketamine, a racemic mixture of the enantiomers S-ketamine and R-ketamine, was originally used only as an analgesic and anesthetic. Owing to its rapid antidepressant effect, it has since also been used as an emergency medication for severe depression, sometimes in combination with SSRIs or serotonin noradrenaline reuptake inhibitors.
Approximately 60% of patients respond to the treatment. Whereas with conventional antidepressants, onset of action requires 10-14 days, ketamine is effective within a few hours. However, relapse always occurs, usually very quickly. After 2-3 days, the effect is usually approximately that of a placebo. An administration interval of about 2 days is optimal. However, “resistance” to the effect often develops after some time: the drug’s antidepressant effect diminishes.
Ketamine also has some unpleasant side effects, such as depersonalization, dissociation, impaired thinking, nystagmus, and psychotomimetic effects. Nausea and vomiting also occur. Interestingly, the latter does not bother the patient much, owing to the drug’s psychological effects, and it does not lead to treatment discontinuation, said Dr. Passie, who described his clinical experiences with ketamine.
Since ketamine causes a considerable clouding of consciousness, sensory disorders, and significant memory problems, it is not suitable for psychedelic-assisted psychotherapy, unlike LSD or psilocybin, he emphasized.
Ketamine 2.0?
Esketamine, the pure S-enantiomer of ketamine, has been on the market since 2019 in the form of a nasal spray (Spravato). Esketamine has been approved in combination with oral antidepressant therapy for adults with a moderate to severe episode of major depression for acute treatment of a psychiatric emergency.
A meta-analysis from 2022 concluded that the original racemic ketamine is better than the new esketamine in reducing symptoms of depression.
In his own comprehensive study, Dr. Passie concluded that the mental impairments that occur during therapy did not differ significantly between substances. The patients even felt that the side effects from esketamine therapy were much more mentally unpleasant, said Dr. Passie. He concluded that the R-enantiomer may have a kind of protective effect against some of the psychopathological effects of the S-enantiomer (esketamine).
In addition, preclinical studies have indicated that the antidepressant effects of R-enantiomer, which is not contained in esketamine, are longer lasting and stronger.
Another problem is absorption, which can be inconsistent with a nasal spray. It may differ, for example, depending on the ambient humidity or whether the patient has recently had a cold. In addition, the spray is far more expensive than the ketamine injection, said Dr. Passie. Patients must also use the nasal spray under supervision at a medical practice (as with the intravenous application) and must receive follow-up care there. It therefore offers no advantage over the ketamine injection.
According to the Institute for Quality and Efficiency in Healthcare, no additional benefit has been proven for esketamine over standard therapies for adults who have experienced a moderate to severe depressive episode when used as short-term treatment for the rapid reduction of depressive symptoms in a psychiatric emergency. The German Medical Association agreed with this evaluation in October 2021.
In the United Kingdom, the medication was never approved, owing to the fact that it was too expensive and that no studies comparing it with psychotherapy were available.
Add-on psilocybin?
What was experienced under the influence of psilocybin can also be subsequently processed and used in psychotherapy.
The acute effect of psilocybin begins after approximately 40 minutes and lasts for 4-6 hours. The antidepressant effect, if it occurs at all, is of immediate onset. Unlike ketamine/esketamine, psilocybin hardly has any physical side effects.
The neurologic mechanism of action has been investigated recently using fMRI and PET techniques. According to the investigations, the substance causes individual networks of activity in the patient’s brain to interconnect more strongly, said Dr. Passie. The thalamus, the filter station for sensory information, as well as the limbic and paralimbic structures, which generate emotions, and the cortex are all activated more strongly.
Two therapeutic settings
Psilocybin, at least in the context of studies, is used in two settings: psycholytic therapy and psychedelic therapy. Both settings originated in the 1950s and were also used with LSD as the active substance.
Psycholytic therapy with psilocybin entails multiple administrations at low doses (for example, 10-18 mg), incorporated into a longer, mostly psychodynamic therapy of around 50-100 hours (often on an inpatient basis at the beginning). It results in what is described as an extended encounter with oneself. The focus is on psychodynamic experiences, such as memories and internal conflicts. In addition, novel experiences with oneself and self-recognition are important.
Psychedelic therapy generally entails one or two sessions with a high dose (for example, 25-35 mg psilocybin). The preparation and follow-up are limited to a few sessions. These methods refer to so-called transpersonal psychology, which addresses extraordinary states of consciousness in line with religious experiences. It often leads to an intense self-confrontation as well as to new evaluations of self and world. The central element to this therapy is the experience of a mystical ego loss and the concomitant feeling of connectedness, which should help to expand one’s perspective.
Euphoria and disillusionment
The first promising studies with a few patients suffering from depression were followed by others in which the euphoria was allowed to fade away somewhat. In the first direct comparison in a methodically high-grade double-blind study, psilocybin was inferior to the SSRI antidepressant escitalopram.
“There is a great variation in response from person to person,” said Dr. Passie. “The better the study is methodically controlled, the worse the results,” he hypothesized.
“Since the method is up to 50 times more expensive in practice, compared to SSRI therapy over 6-12 weeks, the question clearly must be asked as to whether it really has any great future.”
Outlook for psilocybin
Nevertheless, Dr. Passie still sees potential in psilocybin. He considers an approach in which psilocybin therapy is more firmly incorporated into psychotherapy, with between four and 10 therapy sessions before and after administration of a lower therapeutic dose of the substance, to be more promising.
“With this kind of intensive preparation and follow-up, as well as the repeated psilocybin sessions, the patient can benefit much more than is possible with one or two high-dose sessions,” said Dr. Passie, who also is chair of the International Society for Substance-Assisted Psychotherapy. “The constant ‘in-depth work on the ego’ required for drastic therapeutic changes can be more effective and lead to permanent improvements. I have no doubt about this.”
In Dr. Passie’s opinion, the best approach would involve a dignified inpatient setting with a longer period of follow-up care and consistent posttreatment care, including group therapy. The shape of future psilocybin therapy depends on whether the rather abrupt change seen with high-dose psychedelic therapy is permanent. The answer to this question will be decisive for the method and manner of its future clinical use.
Because of the somewhat negative study results, however, the initial investors are pulling out. Dr. Passie is therefore skeptical about whether the necessary larger studies will take place and whether psilocybin will make it onto the market.
In Switzerland, which is not subject to EU restrictions, more than 30 physicians have been authorized to use psilocybin, LSD, and MDMA in psychotherapy sessions. Still, in some respects this is a special case that cannot be transferred easily to other countries, said Dr. Passie.
Possible psilocybin improvement?
Various chemical derivatives of psychoactive substances have been researched, including a psilocybin variant with the label CYB003. With CYB003, the length of the acute psychedelic experience is reduced from around 6 hours (such as with psilocybin) to 1 hour. The plasma concentration of the substance is less variable between different patients. It is assumed that its effects will also differ less from person to person.
In July, researchers began a study of the use of CYB003 in the treatment of major depression. In the randomized, double-blind, placebo-controlled study with 40 patients, multiple doses of the substance will be administered.
When asked, Dr. Passie was rather skeptical about the study. He considers the approaches with psilocybin derivatives to be the consequences of a “gold-rush atmosphere” and expects there will be no real additional benefit, especially not a reduction in the period of action.
A version of this article first appeared on Medscape.com.
Estrogen replacement therapy in endometrial cancer survivors
In the United States, uterine cancer is the fourth most common cancer among women, behind breast, lung/bronchus, and colorectal cancer. There are expected to be almost 66,000 new cases of uterine cancer in 2022.1 The majority of uterine cancers are endometrioid in histology and tend to be low grade, diagnosed at an early stage, and have a good prognosis. While our molecular understanding of endometrial cancers (EC) has changed significantly in recent years, low-grade endometrioid adenocarcinomas have historically been described as type 1 ECs. Type 1 ECs are typically caused by excess estrogen exposure (often unopposed or lacking progesterone protection) and are preceded by endometrial hyperplasia. Excess estrogen can come from exogenous sources (such as unopposed estrogen replacement therapy or tamoxifen, a commonly used treatment in estrogen receptor–positive breast cancer that acts as an estrogen agonist in the endometrium in postmenopausal patients) or endogenous ones (such as obesity).
Peripheral adipose tissue converts androgens into estrogens; paired with the decreased levels of sex hormone–binding globulin seen in obesity, there is more unbound or free serum estrogen (specifically estradiol) in obese women. Estrogen acts on the endometrium to cause proliferation and, if unopposed or imbalanced in relation to progesterone exposure, can ultimately lead to hyperplasia and malignancy.
If excess and unopposed estrogen exposure are major risk factors for the development of EC, is it safe to consider estrogen replacement therapy (ERT) in patients after EC treatment?
The short answer is the data are limited, but in a patient with a history of low-risk early-stage EC who undergoes appropriate counseling, it is likely safe to consider ERT.
Among EC survivors, there has been only one prospective randomized controlled trial that assessed the effect of recurrence rate and survival in women on ERT after EC treatment.2 Patients with stage I or occult stage II endometrial adenocarcinoma treated with at least a total hysterectomy and bilateral salpingo-oophorectomy were randomized to ERT versus placebo for 3 years of treatment, with therapy starting once recovered and within 20 weeks after surgery. Trial participation required an indication for ERT, such as vasomotor symptoms, vaginal atrophy, or increased risk of cardiovascular disease or osteoporosis.
The trial accrued 1,236 patients, falling short of its goal of 2,108 patients after enrollment decreased following the publication of the Women’s Health Initiative results in 2002. This publication prompted a review of the ERT study protocol that found that between decreased accrual and lower than expected recurrence rate, goal accrual would be impossible. Of those enrolled, participants were overwhelmingly white (84%-85%), 41-70 years old (80%-82%), and had stage IA or IB disease (88%). Median follow-up was almost 3 years.
Twenty-six (2.1%) patients experienced cancer recurrence, with similar rates in both groups. Three-year progression-free and overall survival were high overall among all study participants (94.8% and 96.5%). Unfortunately, because the study was closed early, definitive conclusions about the noninferiority of ERT versus placebo regarding oncologic outcomes in early-stage endometrial adenocarcinoma could not be made.
A subsequent meta-analysis looked at the effect of hormone therapy (HT) on recurrence rate in EC survivors.3 Five observational studies were included along with the previously discussed randomized controlled trial. Among 1,975 participants across six studies, there were cancer recurrences in 19 of 896 (2.1%) HT users and 64 of 1,079 (5.9%) controls. HT did not negatively affect cancer recurrence or overall survival. There was significant heterogeneity between studies as to dosing, duration, and type of HT given (some used estrogen-only replacement, others used estrogen and progesterone replacement, and some used both estrogen only and the combination of estrogen and progesterone replacement). Among the five nonrandomized studies included, a protective effect of combined HT on EC recurrence was noted. One study included patients with stage III disease, but only four patients received HT in this cohort.
Given the data we have, ERT does not appear to significantly affect oncologic outcomes in low-risk, early-stage EC survivors. We do not have data to support this same assertion in more advanced, high-risk disease. Before initiation of any ERT in an EC survivor, there should be a detailed discussion to weigh the risks and benefits of starting therapy. The goal of treatment should be to use the lowest dose of ERT possible to treat symptoms, with planned surveillance visits for symptom check-in and assessment of readiness to start tapering treatment.
Footnote: vaginal estrogen therapy
There are no randomized trials assessing the safety of vaginal estrogen preparations or their effect on oncologic outcomes in EC survivors. Observational data from the Women’s Health Initiative showed no increased risk of endometrial cancer in patients who used vaginal estrogen with an intact uterus.4 A recently published retrospective study among 244 gynecologic cancer survivors found low rates of disease recurrence and adverse outcomes among women who used vaginal estrogen for genitourinary symptoms.5 Among EC survivors, the incidence of recurrence was 2.4% for patients with stage I/II disease and 4.3% for stage III/IV disease, with a median follow-up of 80.2 months. While there appears to be some systemic absorption with vaginal estrogen use, this can be quite challenging to measure because of the current sensitivity of serum estradiol and estrone assays. Given the significantly lower serum levels with vaginal estrogen preparations compared with ERT, vaginal estrogen use appears to be safe in EC survivors.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Cancer Stat Facts: Uterine Cancer. National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Accessed 12 Aug. 2022. https://seer.cancer.gov/statfacts/html/corp.html.
2. Barakat RR et al. J Clin Oncol. 2006;24(4):587-92.
3. Shim SH et al. Eur J Cancer. 2014;50(9):1628-37.
4. Crandall CJ et al. Menopause. 2018 Jan;25(1):11-20.
5. Chambers LM et al. Int J Gynecol Cancer. 2020 Apr;30(4):515-24.
In the United States, uterine cancer is the fourth most common cancer among women, behind breast, lung/bronchus, and colorectal cancer. There are expected to be almost 66,000 new cases of uterine cancer in 2022.1 The majority of uterine cancers are endometrioid in histology and tend to be low grade, diagnosed at an early stage, and have a good prognosis. While our molecular understanding of endometrial cancers (EC) has changed significantly in recent years, low-grade endometrioid adenocarcinomas have historically been described as type 1 ECs. Type 1 ECs are typically caused by excess estrogen exposure (often unopposed or lacking progesterone protection) and are preceded by endometrial hyperplasia. Excess estrogen can come from exogenous sources (such as unopposed estrogen replacement therapy or tamoxifen, a commonly used treatment in estrogen receptor–positive breast cancer that acts as an estrogen agonist in the endometrium in postmenopausal patients) or endogenous ones (such as obesity).
Peripheral adipose tissue converts androgens into estrogens; paired with the decreased levels of sex hormone–binding globulin seen in obesity, there is more unbound or free serum estrogen (specifically estradiol) in obese women. Estrogen acts on the endometrium to cause proliferation and, if unopposed or imbalanced in relation to progesterone exposure, can ultimately lead to hyperplasia and malignancy.
If excess and unopposed estrogen exposure are major risk factors for the development of EC, is it safe to consider estrogen replacement therapy (ERT) in patients after EC treatment?
The short answer is the data are limited, but in a patient with a history of low-risk early-stage EC who undergoes appropriate counseling, it is likely safe to consider ERT.
Among EC survivors, there has been only one prospective randomized controlled trial that assessed the effect of recurrence rate and survival in women on ERT after EC treatment.2 Patients with stage I or occult stage II endometrial adenocarcinoma treated with at least a total hysterectomy and bilateral salpingo-oophorectomy were randomized to ERT versus placebo for 3 years of treatment, with therapy starting once recovered and within 20 weeks after surgery. Trial participation required an indication for ERT, such as vasomotor symptoms, vaginal atrophy, or increased risk of cardiovascular disease or osteoporosis.
The trial accrued 1,236 patients, falling short of its goal of 2,108 patients after enrollment decreased following the publication of the Women’s Health Initiative results in 2002. This publication prompted a review of the ERT study protocol that found that between decreased accrual and lower than expected recurrence rate, goal accrual would be impossible. Of those enrolled, participants were overwhelmingly white (84%-85%), 41-70 years old (80%-82%), and had stage IA or IB disease (88%). Median follow-up was almost 3 years.
Twenty-six (2.1%) patients experienced cancer recurrence, with similar rates in both groups. Three-year progression-free and overall survival were high overall among all study participants (94.8% and 96.5%). Unfortunately, because the study was closed early, definitive conclusions about the noninferiority of ERT versus placebo regarding oncologic outcomes in early-stage endometrial adenocarcinoma could not be made.
A subsequent meta-analysis looked at the effect of hormone therapy (HT) on recurrence rate in EC survivors.3 Five observational studies were included along with the previously discussed randomized controlled trial. Among 1,975 participants across six studies, there were cancer recurrences in 19 of 896 (2.1%) HT users and 64 of 1,079 (5.9%) controls. HT did not negatively affect cancer recurrence or overall survival. There was significant heterogeneity between studies as to dosing, duration, and type of HT given (some used estrogen-only replacement, others used estrogen and progesterone replacement, and some used both estrogen only and the combination of estrogen and progesterone replacement). Among the five nonrandomized studies included, a protective effect of combined HT on EC recurrence was noted. One study included patients with stage III disease, but only four patients received HT in this cohort.
Given the data we have, ERT does not appear to significantly affect oncologic outcomes in low-risk, early-stage EC survivors. We do not have data to support this same assertion in more advanced, high-risk disease. Before initiation of any ERT in an EC survivor, there should be a detailed discussion to weigh the risks and benefits of starting therapy. The goal of treatment should be to use the lowest dose of ERT possible to treat symptoms, with planned surveillance visits for symptom check-in and assessment of readiness to start tapering treatment.
Footnote: vaginal estrogen therapy
There are no randomized trials assessing the safety of vaginal estrogen preparations or their effect on oncologic outcomes in EC survivors. Observational data from the Women’s Health Initiative showed no increased risk of endometrial cancer in patients who used vaginal estrogen with an intact uterus.4 A recently published retrospective study among 244 gynecologic cancer survivors found low rates of disease recurrence and adverse outcomes among women who used vaginal estrogen for genitourinary symptoms.5 Among EC survivors, the incidence of recurrence was 2.4% for patients with stage I/II disease and 4.3% for stage III/IV disease, with a median follow-up of 80.2 months. While there appears to be some systemic absorption with vaginal estrogen use, this can be quite challenging to measure because of the current sensitivity of serum estradiol and estrone assays. Given the significantly lower serum levels with vaginal estrogen preparations compared with ERT, vaginal estrogen use appears to be safe in EC survivors.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Cancer Stat Facts: Uterine Cancer. National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Accessed 12 Aug. 2022. https://seer.cancer.gov/statfacts/html/corp.html.
2. Barakat RR et al. J Clin Oncol. 2006;24(4):587-92.
3. Shim SH et al. Eur J Cancer. 2014;50(9):1628-37.
4. Crandall CJ et al. Menopause. 2018 Jan;25(1):11-20.
5. Chambers LM et al. Int J Gynecol Cancer. 2020 Apr;30(4):515-24.
In the United States, uterine cancer is the fourth most common cancer among women, behind breast, lung/bronchus, and colorectal cancer. There are expected to be almost 66,000 new cases of uterine cancer in 2022.1 The majority of uterine cancers are endometrioid in histology and tend to be low grade, diagnosed at an early stage, and have a good prognosis. While our molecular understanding of endometrial cancers (EC) has changed significantly in recent years, low-grade endometrioid adenocarcinomas have historically been described as type 1 ECs. Type 1 ECs are typically caused by excess estrogen exposure (often unopposed or lacking progesterone protection) and are preceded by endometrial hyperplasia. Excess estrogen can come from exogenous sources (such as unopposed estrogen replacement therapy or tamoxifen, a commonly used treatment in estrogen receptor–positive breast cancer that acts as an estrogen agonist in the endometrium in postmenopausal patients) or endogenous ones (such as obesity).
Peripheral adipose tissue converts androgens into estrogens; paired with the decreased levels of sex hormone–binding globulin seen in obesity, there is more unbound or free serum estrogen (specifically estradiol) in obese women. Estrogen acts on the endometrium to cause proliferation and, if unopposed or imbalanced in relation to progesterone exposure, can ultimately lead to hyperplasia and malignancy.
If excess and unopposed estrogen exposure are major risk factors for the development of EC, is it safe to consider estrogen replacement therapy (ERT) in patients after EC treatment?
The short answer is the data are limited, but in a patient with a history of low-risk early-stage EC who undergoes appropriate counseling, it is likely safe to consider ERT.
Among EC survivors, there has been only one prospective randomized controlled trial that assessed the effect of recurrence rate and survival in women on ERT after EC treatment.2 Patients with stage I or occult stage II endometrial adenocarcinoma treated with at least a total hysterectomy and bilateral salpingo-oophorectomy were randomized to ERT versus placebo for 3 years of treatment, with therapy starting once recovered and within 20 weeks after surgery. Trial participation required an indication for ERT, such as vasomotor symptoms, vaginal atrophy, or increased risk of cardiovascular disease or osteoporosis.
The trial accrued 1,236 patients, falling short of its goal of 2,108 patients after enrollment decreased following the publication of the Women’s Health Initiative results in 2002. This publication prompted a review of the ERT study protocol that found that between decreased accrual and lower than expected recurrence rate, goal accrual would be impossible. Of those enrolled, participants were overwhelmingly white (84%-85%), 41-70 years old (80%-82%), and had stage IA or IB disease (88%). Median follow-up was almost 3 years.
Twenty-six (2.1%) patients experienced cancer recurrence, with similar rates in both groups. Three-year progression-free and overall survival were high overall among all study participants (94.8% and 96.5%). Unfortunately, because the study was closed early, definitive conclusions about the noninferiority of ERT versus placebo regarding oncologic outcomes in early-stage endometrial adenocarcinoma could not be made.
A subsequent meta-analysis looked at the effect of hormone therapy (HT) on recurrence rate in EC survivors.3 Five observational studies were included along with the previously discussed randomized controlled trial. Among 1,975 participants across six studies, there were cancer recurrences in 19 of 896 (2.1%) HT users and 64 of 1,079 (5.9%) controls. HT did not negatively affect cancer recurrence or overall survival. There was significant heterogeneity between studies as to dosing, duration, and type of HT given (some used estrogen-only replacement, others used estrogen and progesterone replacement, and some used both estrogen only and the combination of estrogen and progesterone replacement). Among the five nonrandomized studies included, a protective effect of combined HT on EC recurrence was noted. One study included patients with stage III disease, but only four patients received HT in this cohort.
Given the data we have, ERT does not appear to significantly affect oncologic outcomes in low-risk, early-stage EC survivors. We do not have data to support this same assertion in more advanced, high-risk disease. Before initiation of any ERT in an EC survivor, there should be a detailed discussion to weigh the risks and benefits of starting therapy. The goal of treatment should be to use the lowest dose of ERT possible to treat symptoms, with planned surveillance visits for symptom check-in and assessment of readiness to start tapering treatment.
Footnote: vaginal estrogen therapy
There are no randomized trials assessing the safety of vaginal estrogen preparations or their effect on oncologic outcomes in EC survivors. Observational data from the Women’s Health Initiative showed no increased risk of endometrial cancer in patients who used vaginal estrogen with an intact uterus.4 A recently published retrospective study among 244 gynecologic cancer survivors found low rates of disease recurrence and adverse outcomes among women who used vaginal estrogen for genitourinary symptoms.5 Among EC survivors, the incidence of recurrence was 2.4% for patients with stage I/II disease and 4.3% for stage III/IV disease, with a median follow-up of 80.2 months. While there appears to be some systemic absorption with vaginal estrogen use, this can be quite challenging to measure because of the current sensitivity of serum estradiol and estrone assays. Given the significantly lower serum levels with vaginal estrogen preparations compared with ERT, vaginal estrogen use appears to be safe in EC survivors.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Cancer Stat Facts: Uterine Cancer. National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Accessed 12 Aug. 2022. https://seer.cancer.gov/statfacts/html/corp.html.
2. Barakat RR et al. J Clin Oncol. 2006;24(4):587-92.
3. Shim SH et al. Eur J Cancer. 2014;50(9):1628-37.
4. Crandall CJ et al. Menopause. 2018 Jan;25(1):11-20.
5. Chambers LM et al. Int J Gynecol Cancer. 2020 Apr;30(4):515-24.
Obesity drug shortage triggers frustrations, workarounds
The glucagon-like peptide-1 (GLP-1) agonist semaglutide formulated for treating obesity (Wegovy) had a roaring takeoff a little more than a year ago, with surging patient demand after the U.S. Food and Drug Administration approved it in June 2021. But starting doses of the Wegovy form of semaglutide went missing in action starting late 2021 and continue to date, frustrating patients and their health care providers.
The arrival of Wegovy last year was hailed by obesity medicine specialists and others as a “game changer” for treating people with obesity because of semaglutide’s proven safety and efficacy at the subcutaneous dose of 2.4 mg delivered once a week to produce at least 15% weight loss in half the people who received it, as documented last year in results from one of the drug’s pivotal clinical trials.
But during the months following semaglutide’s approval for treating obesity (it also received an FDA marketing nod in late 2017 as Ozempic for treating type 2 diabetes), a worldwide shortage of Wegovy, including in the United States, emerged.
A manufacturing glitch shut down the primary location for production of U.S.-bound Wegovy injector pens for several months starting in late 2021, according to a December report from Novo Nordisk, the company that makes and markets the agent. (The Wegovy production issue appears to have had a very modest impact, especially in U.S. pharmacies, on the supply of semaglutide formulated as Ozempic, also marketed by Novo Nordisk, although Wegovy supply and demand have dramatically limited Ozempic availability in Australia.)
‘Unprecedented demand’ for Wegovy derailed when plant went offline
The supply side for Wegovy became so hopelessly broken that just months after U.S. sales began and immediately skyrocketed, Novo Nordisk made the remarkable decision to pull starting doses of Wegovy from the market to make it much harder to initiate patients (semaglutide and other GLP-1 agonists require gradual dose ramp-up to avoid gastrointestinal side effects), and the company publicly implored clinicians to not start new patients on the agent, which is where the status remains as of early August 2022.
Novo Nordisk’s financial report for the second quarter of 2022, released on Aug. 3, said the company “expects to make all Wegovy dose strengths available in the United States towards the end of 2022.”
A Dear Health Care Provider letter that Novo Nordisk posted on its U.S. Wegovy website last spring cited “unprecedented demand” that exceeded every prior product launch in the company’s history. It forced Novo Nordisk to pull the plug on all U.S. promotion of Wegovy and compelled the company to ask U.S. clinicians to halt new patient starts.
“I stopped offering Wegovy to new patients” since about the beginning of 2022, says Lauren D. Oshman, MD, a family and obesity medicine specialist at the University of Michigan, Ann Arbor. “It’s very frustrating to not have patients [with obesity] receive the optimal treatment available.” Although she adds that she tries to match obesity treatments to each patient’s clinical needs, and a GLP-1 agonist is not the first choice for every person with obesity.
“It was a disastrous rollout,” says Catherine W. Varney, DO, a family and obesity medicine specialist at the University of Virginia, Charlottesville. “It’s frustrating to know that the treatment is there but not being able to use it,” she said in an interview.
“I had about 800 patients on Wegovy” when the supply dropped earlier this year, and “I couldn’t handle the volume of messages that I got from patients,” recalls Angela Fitch, MD, associate director of the Massachusetts General Hospital Weight Center, Boston. “It was painful,” she said in an interview.
“Frustrating and chaotic,” is the description from Ivania M. Rizo, MD, director of obesity medicine at Boston Medical Center.
The liraglutide/Saxenda workaround
The upshot is that people with obesity and their health care providers have been busy devising workarounds to try to meet the intense demand for this drug-assisted approach to appetite control and weight loss. Their tactics run a wide gamut based on the crazy-quilt diversity of health insurance coverage across America.
Because the bottleneck for starting Wegovy resulted from unavailable starting doses (dosing starts at 0.25 mg delivered subcutaneously once a week, eventually ramping up to a maximum of 2.4 mg weekly), one option was to start patients on a different GLP-1 agonist, such as liraglutide (Saxenda, approved for obesity).
Starting a patient on liraglutide involves the same sort of up-titration and acclimation to a GLP-1 agonist that semaglutide requires, and transition between these agents seems feasible for at least some. It also means daily injections of liraglutide rather than the weekly schedule for semaglutide, although some patients prefer maintaining a daily dosing schedule. Another limitation of liraglutide is that evidence shows it is not nearly as effective for weight loss as semaglutide.
Results from the head-to-head STEP 8 trial, published in JAMA, showed an average weight loss from baseline of about 16% with semaglutide and about 6% with liraglutide (and about 2% with placebo).
A ‘reasonable’ evidence base, but more work
Changing from Saxenda to Wegovy, or from Wegovy to Saxenda, “would be reasonably evidence-based medicine,” said Dr. Oshman in an interview. She has managed a Wegovy-to-Saxenda switch for a “handful” of patients to deal with Wegovy shortages, but she has not yet moved anyone to Wegovy after a Saxenda initiation.
“No prospective study has looked at this transition,” but dose equivalence tables exist based on expert opinion, noted Dr. Oshman, as in this 2020 report.
Dr. Varney has several patients on the Saxenda-to-Wegovy track. She up-titrates patients on Saxenda to the maximum daily dose of 3.0 mg and then switches them to the 1.7 mg weekly dose of Wegovy, one of the “destination” Wegovy doses that has remained generally available during the shortage. But Dr. Varney’s experience is that only half of her patients made the changeover smoothly, with the others having “severe gastrointestinal distress,” including vomiting, she notes.
Dr. Fitch has also successfully used this Saxenda-to-Wegovy approach for some of her patients, but it hasn’t been easy.
“It’s more work and more prior authorizations. It’s harder and adds a layer of stress,” but, Dr. Fitch adds, “people are willing to work on it because the weight loss is worth it.”
The liraglutide to semaglutide shuffle is “doable,” says Dr. Rizo, “but I’m looking forward to not having to do it and being able to just start Wegovy.”
The tirzepatide coupon program works ‘off label’ for obesity
Another workaround depends on the FDA approval in May for tirzepatide (Mounjaro) for type 2 diabetes. Tirzepatide is a related GLP-1 agonist that also adds a second incretin-like agonist activity that mimics the glucose-dependent insulinotropic polypeptide.
Soon after approval, Lilly, the company that markets tirzepatide, started a U.S. coupon program geared exclusively to people with commercial insurance. Within certain refill and dollar limits, the program lets patients buy tirzepatide at pharmacies at an out-of-pocket cost of $25 for a 4-week supply (tirzepatide is also dosed by weekly subcutaneous injections). The program will extend into 2023.
Novo Nordisk offered U.S. patients with commercial insurance a similar discount when Wegovy first hit the U.S. market in 2021, but the program closed down once the supply shortage began.
Despite tirzepatide’s current approval only for type 2 diabetes, Dr. Varney has been successfully prescribing it to patients without diabetes off-label for weight loss.
“The coupons still work even when tirzepatide is used off-label,” she notes. And while the drug’s rollout is still only a couple of months old, so far, it’s gone “beautifully” with no hints of supply issues, she says.
But a major drawback to relying on an introductory coupon program that makes these agents affordable to patients is their ability to maintain treatment once the discounts inevitably end.
“We try to only prescribe agents that patients can continue to access,” says Dr. Fitch, who has had some patients with commercial insurance start on Wegovy with coupon discounts only to later lose access.
Many commercial U.S. insurers do not cover obesity treatments, a decision often driven by the employers who sponsor the coverage, she notes.
Study results have documented that when people with obesity stop taking a GLP-1 agonist their lost weight rebounds, as in a study that tracked people who stopped taking semaglutide.
Dr. Fitch has had success prescribing tirzepatide to patients with obesity but without diabetes who have certain types of Medicare drug coverage policies, which often do not deny off-label drug coverage. That approach works until patients reach the “donut hole” in their drug coverage and are faced with a certain level of out-of-pocket costs that can balloon to several thousand dollars.
Even more workarounds
Other approaches patients have used to acquire Wegovy include purchasing it in other countries, such as Canada or Brazil, says Dr. Fitch. But prices outside the United States, while substantially lower, can still be a barrier for many patients, notes Dr. Oshman.
Semaglutide in Canada goes for about $300 for a 4-week supply, roughly a quarter the U.S. price, she says, but is “still too high for many of my patients.”
Intense patient demand sometimes bordering on desperation has prompted some to seek semaglutide from private compounding pharmacies, a step clinicians regard as downright dangerous.
“Semaglutide from compounding pharmacies is not known to be safe. We feel strongly that it’s not something that people should do,” says Dr. Fitch.
“Compounding pharmacies have no FDA regulation. People don’t know what they’re getting. It’s dangerous,” agrees Dr. Varney. Physicians who refer people for privately compounded semaglutide “are taking advantage of desperate people,” she adds.
Although it seems likely that Novo Nordisk will soon sort out the supply problems and Wegovy will once again become more widely available, some of the issues patients have had with access to the weight loss medication stem from more systemic issues in the United States health insurance landscape: an unwillingness by payers to cover the costs of weight loss medications, a shortcoming that also exists for Medicare and Medicaid.
“We need to make obesity treatment a standard benefit, and not something that can be carved out,” says Dr. Fitch. People with obesity “deserve access to effective treatments for their disease,” she declares.
Dr. Oshman, Dr. Varney, and Dr. Rizo have reported no relevant financial relationships. Dr. Fitch has reported being an advisor to Jenny Craig.
A version of this article first appeared on Medscape.com.
The glucagon-like peptide-1 (GLP-1) agonist semaglutide formulated for treating obesity (Wegovy) had a roaring takeoff a little more than a year ago, with surging patient demand after the U.S. Food and Drug Administration approved it in June 2021. But starting doses of the Wegovy form of semaglutide went missing in action starting late 2021 and continue to date, frustrating patients and their health care providers.
The arrival of Wegovy last year was hailed by obesity medicine specialists and others as a “game changer” for treating people with obesity because of semaglutide’s proven safety and efficacy at the subcutaneous dose of 2.4 mg delivered once a week to produce at least 15% weight loss in half the people who received it, as documented last year in results from one of the drug’s pivotal clinical trials.
But during the months following semaglutide’s approval for treating obesity (it also received an FDA marketing nod in late 2017 as Ozempic for treating type 2 diabetes), a worldwide shortage of Wegovy, including in the United States, emerged.
A manufacturing glitch shut down the primary location for production of U.S.-bound Wegovy injector pens for several months starting in late 2021, according to a December report from Novo Nordisk, the company that makes and markets the agent. (The Wegovy production issue appears to have had a very modest impact, especially in U.S. pharmacies, on the supply of semaglutide formulated as Ozempic, also marketed by Novo Nordisk, although Wegovy supply and demand have dramatically limited Ozempic availability in Australia.)
‘Unprecedented demand’ for Wegovy derailed when plant went offline
The supply side for Wegovy became so hopelessly broken that just months after U.S. sales began and immediately skyrocketed, Novo Nordisk made the remarkable decision to pull starting doses of Wegovy from the market to make it much harder to initiate patients (semaglutide and other GLP-1 agonists require gradual dose ramp-up to avoid gastrointestinal side effects), and the company publicly implored clinicians to not start new patients on the agent, which is where the status remains as of early August 2022.
Novo Nordisk’s financial report for the second quarter of 2022, released on Aug. 3, said the company “expects to make all Wegovy dose strengths available in the United States towards the end of 2022.”
A Dear Health Care Provider letter that Novo Nordisk posted on its U.S. Wegovy website last spring cited “unprecedented demand” that exceeded every prior product launch in the company’s history. It forced Novo Nordisk to pull the plug on all U.S. promotion of Wegovy and compelled the company to ask U.S. clinicians to halt new patient starts.
“I stopped offering Wegovy to new patients” since about the beginning of 2022, says Lauren D. Oshman, MD, a family and obesity medicine specialist at the University of Michigan, Ann Arbor. “It’s very frustrating to not have patients [with obesity] receive the optimal treatment available.” Although she adds that she tries to match obesity treatments to each patient’s clinical needs, and a GLP-1 agonist is not the first choice for every person with obesity.
“It was a disastrous rollout,” says Catherine W. Varney, DO, a family and obesity medicine specialist at the University of Virginia, Charlottesville. “It’s frustrating to know that the treatment is there but not being able to use it,” she said in an interview.
“I had about 800 patients on Wegovy” when the supply dropped earlier this year, and “I couldn’t handle the volume of messages that I got from patients,” recalls Angela Fitch, MD, associate director of the Massachusetts General Hospital Weight Center, Boston. “It was painful,” she said in an interview.
“Frustrating and chaotic,” is the description from Ivania M. Rizo, MD, director of obesity medicine at Boston Medical Center.
The liraglutide/Saxenda workaround
The upshot is that people with obesity and their health care providers have been busy devising workarounds to try to meet the intense demand for this drug-assisted approach to appetite control and weight loss. Their tactics run a wide gamut based on the crazy-quilt diversity of health insurance coverage across America.
Because the bottleneck for starting Wegovy resulted from unavailable starting doses (dosing starts at 0.25 mg delivered subcutaneously once a week, eventually ramping up to a maximum of 2.4 mg weekly), one option was to start patients on a different GLP-1 agonist, such as liraglutide (Saxenda, approved for obesity).
Starting a patient on liraglutide involves the same sort of up-titration and acclimation to a GLP-1 agonist that semaglutide requires, and transition between these agents seems feasible for at least some. It also means daily injections of liraglutide rather than the weekly schedule for semaglutide, although some patients prefer maintaining a daily dosing schedule. Another limitation of liraglutide is that evidence shows it is not nearly as effective for weight loss as semaglutide.
Results from the head-to-head STEP 8 trial, published in JAMA, showed an average weight loss from baseline of about 16% with semaglutide and about 6% with liraglutide (and about 2% with placebo).
A ‘reasonable’ evidence base, but more work
Changing from Saxenda to Wegovy, or from Wegovy to Saxenda, “would be reasonably evidence-based medicine,” said Dr. Oshman in an interview. She has managed a Wegovy-to-Saxenda switch for a “handful” of patients to deal with Wegovy shortages, but she has not yet moved anyone to Wegovy after a Saxenda initiation.
“No prospective study has looked at this transition,” but dose equivalence tables exist based on expert opinion, noted Dr. Oshman, as in this 2020 report.
Dr. Varney has several patients on the Saxenda-to-Wegovy track. She up-titrates patients on Saxenda to the maximum daily dose of 3.0 mg and then switches them to the 1.7 mg weekly dose of Wegovy, one of the “destination” Wegovy doses that has remained generally available during the shortage. But Dr. Varney’s experience is that only half of her patients made the changeover smoothly, with the others having “severe gastrointestinal distress,” including vomiting, she notes.
Dr. Fitch has also successfully used this Saxenda-to-Wegovy approach for some of her patients, but it hasn’t been easy.
“It’s more work and more prior authorizations. It’s harder and adds a layer of stress,” but, Dr. Fitch adds, “people are willing to work on it because the weight loss is worth it.”
The liraglutide to semaglutide shuffle is “doable,” says Dr. Rizo, “but I’m looking forward to not having to do it and being able to just start Wegovy.”
The tirzepatide coupon program works ‘off label’ for obesity
Another workaround depends on the FDA approval in May for tirzepatide (Mounjaro) for type 2 diabetes. Tirzepatide is a related GLP-1 agonist that also adds a second incretin-like agonist activity that mimics the glucose-dependent insulinotropic polypeptide.
Soon after approval, Lilly, the company that markets tirzepatide, started a U.S. coupon program geared exclusively to people with commercial insurance. Within certain refill and dollar limits, the program lets patients buy tirzepatide at pharmacies at an out-of-pocket cost of $25 for a 4-week supply (tirzepatide is also dosed by weekly subcutaneous injections). The program will extend into 2023.
Novo Nordisk offered U.S. patients with commercial insurance a similar discount when Wegovy first hit the U.S. market in 2021, but the program closed down once the supply shortage began.
Despite tirzepatide’s current approval only for type 2 diabetes, Dr. Varney has been successfully prescribing it to patients without diabetes off-label for weight loss.
“The coupons still work even when tirzepatide is used off-label,” she notes. And while the drug’s rollout is still only a couple of months old, so far, it’s gone “beautifully” with no hints of supply issues, she says.
But a major drawback to relying on an introductory coupon program that makes these agents affordable to patients is their ability to maintain treatment once the discounts inevitably end.
“We try to only prescribe agents that patients can continue to access,” says Dr. Fitch, who has had some patients with commercial insurance start on Wegovy with coupon discounts only to later lose access.
Many commercial U.S. insurers do not cover obesity treatments, a decision often driven by the employers who sponsor the coverage, she notes.
Study results have documented that when people with obesity stop taking a GLP-1 agonist their lost weight rebounds, as in a study that tracked people who stopped taking semaglutide.
Dr. Fitch has had success prescribing tirzepatide to patients with obesity but without diabetes who have certain types of Medicare drug coverage policies, which often do not deny off-label drug coverage. That approach works until patients reach the “donut hole” in their drug coverage and are faced with a certain level of out-of-pocket costs that can balloon to several thousand dollars.
Even more workarounds
Other approaches patients have used to acquire Wegovy include purchasing it in other countries, such as Canada or Brazil, says Dr. Fitch. But prices outside the United States, while substantially lower, can still be a barrier for many patients, notes Dr. Oshman.
Semaglutide in Canada goes for about $300 for a 4-week supply, roughly a quarter the U.S. price, she says, but is “still too high for many of my patients.”
Intense patient demand sometimes bordering on desperation has prompted some to seek semaglutide from private compounding pharmacies, a step clinicians regard as downright dangerous.
“Semaglutide from compounding pharmacies is not known to be safe. We feel strongly that it’s not something that people should do,” says Dr. Fitch.
“Compounding pharmacies have no FDA regulation. People don’t know what they’re getting. It’s dangerous,” agrees Dr. Varney. Physicians who refer people for privately compounded semaglutide “are taking advantage of desperate people,” she adds.
Although it seems likely that Novo Nordisk will soon sort out the supply problems and Wegovy will once again become more widely available, some of the issues patients have had with access to the weight loss medication stem from more systemic issues in the United States health insurance landscape: an unwillingness by payers to cover the costs of weight loss medications, a shortcoming that also exists for Medicare and Medicaid.
“We need to make obesity treatment a standard benefit, and not something that can be carved out,” says Dr. Fitch. People with obesity “deserve access to effective treatments for their disease,” she declares.
Dr. Oshman, Dr. Varney, and Dr. Rizo have reported no relevant financial relationships. Dr. Fitch has reported being an advisor to Jenny Craig.
A version of this article first appeared on Medscape.com.
The glucagon-like peptide-1 (GLP-1) agonist semaglutide formulated for treating obesity (Wegovy) had a roaring takeoff a little more than a year ago, with surging patient demand after the U.S. Food and Drug Administration approved it in June 2021. But starting doses of the Wegovy form of semaglutide went missing in action starting late 2021 and continue to date, frustrating patients and their health care providers.
The arrival of Wegovy last year was hailed by obesity medicine specialists and others as a “game changer” for treating people with obesity because of semaglutide’s proven safety and efficacy at the subcutaneous dose of 2.4 mg delivered once a week to produce at least 15% weight loss in half the people who received it, as documented last year in results from one of the drug’s pivotal clinical trials.
But during the months following semaglutide’s approval for treating obesity (it also received an FDA marketing nod in late 2017 as Ozempic for treating type 2 diabetes), a worldwide shortage of Wegovy, including in the United States, emerged.
A manufacturing glitch shut down the primary location for production of U.S.-bound Wegovy injector pens for several months starting in late 2021, according to a December report from Novo Nordisk, the company that makes and markets the agent. (The Wegovy production issue appears to have had a very modest impact, especially in U.S. pharmacies, on the supply of semaglutide formulated as Ozempic, also marketed by Novo Nordisk, although Wegovy supply and demand have dramatically limited Ozempic availability in Australia.)
‘Unprecedented demand’ for Wegovy derailed when plant went offline
The supply side for Wegovy became so hopelessly broken that just months after U.S. sales began and immediately skyrocketed, Novo Nordisk made the remarkable decision to pull starting doses of Wegovy from the market to make it much harder to initiate patients (semaglutide and other GLP-1 agonists require gradual dose ramp-up to avoid gastrointestinal side effects), and the company publicly implored clinicians to not start new patients on the agent, which is where the status remains as of early August 2022.
Novo Nordisk’s financial report for the second quarter of 2022, released on Aug. 3, said the company “expects to make all Wegovy dose strengths available in the United States towards the end of 2022.”
A Dear Health Care Provider letter that Novo Nordisk posted on its U.S. Wegovy website last spring cited “unprecedented demand” that exceeded every prior product launch in the company’s history. It forced Novo Nordisk to pull the plug on all U.S. promotion of Wegovy and compelled the company to ask U.S. clinicians to halt new patient starts.
“I stopped offering Wegovy to new patients” since about the beginning of 2022, says Lauren D. Oshman, MD, a family and obesity medicine specialist at the University of Michigan, Ann Arbor. “It’s very frustrating to not have patients [with obesity] receive the optimal treatment available.” Although she adds that she tries to match obesity treatments to each patient’s clinical needs, and a GLP-1 agonist is not the first choice for every person with obesity.
“It was a disastrous rollout,” says Catherine W. Varney, DO, a family and obesity medicine specialist at the University of Virginia, Charlottesville. “It’s frustrating to know that the treatment is there but not being able to use it,” she said in an interview.
“I had about 800 patients on Wegovy” when the supply dropped earlier this year, and “I couldn’t handle the volume of messages that I got from patients,” recalls Angela Fitch, MD, associate director of the Massachusetts General Hospital Weight Center, Boston. “It was painful,” she said in an interview.
“Frustrating and chaotic,” is the description from Ivania M. Rizo, MD, director of obesity medicine at Boston Medical Center.
The liraglutide/Saxenda workaround
The upshot is that people with obesity and their health care providers have been busy devising workarounds to try to meet the intense demand for this drug-assisted approach to appetite control and weight loss. Their tactics run a wide gamut based on the crazy-quilt diversity of health insurance coverage across America.
Because the bottleneck for starting Wegovy resulted from unavailable starting doses (dosing starts at 0.25 mg delivered subcutaneously once a week, eventually ramping up to a maximum of 2.4 mg weekly), one option was to start patients on a different GLP-1 agonist, such as liraglutide (Saxenda, approved for obesity).
Starting a patient on liraglutide involves the same sort of up-titration and acclimation to a GLP-1 agonist that semaglutide requires, and transition between these agents seems feasible for at least some. It also means daily injections of liraglutide rather than the weekly schedule for semaglutide, although some patients prefer maintaining a daily dosing schedule. Another limitation of liraglutide is that evidence shows it is not nearly as effective for weight loss as semaglutide.
Results from the head-to-head STEP 8 trial, published in JAMA, showed an average weight loss from baseline of about 16% with semaglutide and about 6% with liraglutide (and about 2% with placebo).
A ‘reasonable’ evidence base, but more work
Changing from Saxenda to Wegovy, or from Wegovy to Saxenda, “would be reasonably evidence-based medicine,” said Dr. Oshman in an interview. She has managed a Wegovy-to-Saxenda switch for a “handful” of patients to deal with Wegovy shortages, but she has not yet moved anyone to Wegovy after a Saxenda initiation.
“No prospective study has looked at this transition,” but dose equivalence tables exist based on expert opinion, noted Dr. Oshman, as in this 2020 report.
Dr. Varney has several patients on the Saxenda-to-Wegovy track. She up-titrates patients on Saxenda to the maximum daily dose of 3.0 mg and then switches them to the 1.7 mg weekly dose of Wegovy, one of the “destination” Wegovy doses that has remained generally available during the shortage. But Dr. Varney’s experience is that only half of her patients made the changeover smoothly, with the others having “severe gastrointestinal distress,” including vomiting, she notes.
Dr. Fitch has also successfully used this Saxenda-to-Wegovy approach for some of her patients, but it hasn’t been easy.
“It’s more work and more prior authorizations. It’s harder and adds a layer of stress,” but, Dr. Fitch adds, “people are willing to work on it because the weight loss is worth it.”
The liraglutide to semaglutide shuffle is “doable,” says Dr. Rizo, “but I’m looking forward to not having to do it and being able to just start Wegovy.”
The tirzepatide coupon program works ‘off label’ for obesity
Another workaround depends on the FDA approval in May for tirzepatide (Mounjaro) for type 2 diabetes. Tirzepatide is a related GLP-1 agonist that also adds a second incretin-like agonist activity that mimics the glucose-dependent insulinotropic polypeptide.
Soon after approval, Lilly, the company that markets tirzepatide, started a U.S. coupon program geared exclusively to people with commercial insurance. Within certain refill and dollar limits, the program lets patients buy tirzepatide at pharmacies at an out-of-pocket cost of $25 for a 4-week supply (tirzepatide is also dosed by weekly subcutaneous injections). The program will extend into 2023.
Novo Nordisk offered U.S. patients with commercial insurance a similar discount when Wegovy first hit the U.S. market in 2021, but the program closed down once the supply shortage began.
Despite tirzepatide’s current approval only for type 2 diabetes, Dr. Varney has been successfully prescribing it to patients without diabetes off-label for weight loss.
“The coupons still work even when tirzepatide is used off-label,” she notes. And while the drug’s rollout is still only a couple of months old, so far, it’s gone “beautifully” with no hints of supply issues, she says.
But a major drawback to relying on an introductory coupon program that makes these agents affordable to patients is their ability to maintain treatment once the discounts inevitably end.
“We try to only prescribe agents that patients can continue to access,” says Dr. Fitch, who has had some patients with commercial insurance start on Wegovy with coupon discounts only to later lose access.
Many commercial U.S. insurers do not cover obesity treatments, a decision often driven by the employers who sponsor the coverage, she notes.
Study results have documented that when people with obesity stop taking a GLP-1 agonist their lost weight rebounds, as in a study that tracked people who stopped taking semaglutide.
Dr. Fitch has had success prescribing tirzepatide to patients with obesity but without diabetes who have certain types of Medicare drug coverage policies, which often do not deny off-label drug coverage. That approach works until patients reach the “donut hole” in their drug coverage and are faced with a certain level of out-of-pocket costs that can balloon to several thousand dollars.
Even more workarounds
Other approaches patients have used to acquire Wegovy include purchasing it in other countries, such as Canada or Brazil, says Dr. Fitch. But prices outside the United States, while substantially lower, can still be a barrier for many patients, notes Dr. Oshman.
Semaglutide in Canada goes for about $300 for a 4-week supply, roughly a quarter the U.S. price, she says, but is “still too high for many of my patients.”
Intense patient demand sometimes bordering on desperation has prompted some to seek semaglutide from private compounding pharmacies, a step clinicians regard as downright dangerous.
“Semaglutide from compounding pharmacies is not known to be safe. We feel strongly that it’s not something that people should do,” says Dr. Fitch.
“Compounding pharmacies have no FDA regulation. People don’t know what they’re getting. It’s dangerous,” agrees Dr. Varney. Physicians who refer people for privately compounded semaglutide “are taking advantage of desperate people,” she adds.
Although it seems likely that Novo Nordisk will soon sort out the supply problems and Wegovy will once again become more widely available, some of the issues patients have had with access to the weight loss medication stem from more systemic issues in the United States health insurance landscape: an unwillingness by payers to cover the costs of weight loss medications, a shortcoming that also exists for Medicare and Medicaid.
“We need to make obesity treatment a standard benefit, and not something that can be carved out,” says Dr. Fitch. People with obesity “deserve access to effective treatments for their disease,” she declares.
Dr. Oshman, Dr. Varney, and Dr. Rizo have reported no relevant financial relationships. Dr. Fitch has reported being an advisor to Jenny Craig.
A version of this article first appeared on Medscape.com.
FDA approves Enhertu (trastuzumab deruxtecan) for HER2 lung cancer
Patients with lung cancer now have another treatment option: If their tumors are found to carry HER2 mutations, they can now be treated with trastuzumab deruxtecan (Enhertu), a drug that specifically targets that defect.
This product is already approved for used in HER2-positive breast cancer and gastric cancer.
The FDA also approved companion diagnostic tests to detect HER2 mutations: Life Technologies Corporation’s Oncomine Dx Target Test for use in lung tissue and Guardant Health’s Guardant360 CDx for use on plasma samples. The agency notes that if no mutation is detected in a plasma specimen, the tumor tissue should be tested.
Specifically, the new indication is used in patients with unresectable or metastatic non–small cell lung cancer (NSCLC) whose tumors have activating HER2 (ERBB2) mutations, as detected by an FDA-approved test, and who have already received a prior systemic therapy.
About 3% of nonsquamous NSCLC tumors carry mutations in the HER2 gene, and they are associated with female sex, never-smokers, and a poor prognosis.
“HER2 mutant non–small cell lung cancer is an aggressive form of disease, which commonly affects young patients who have faced limited treatment options and a poor prognosis to date,” said Dave Fredrickson, executive vice-president of the oncology business unit at AstraZeneca.
The new approval “provides these patients with the opportunity to benefit from a targeted therapy and highlights the importance of testing for predictive markers, including HER2 in lung cancer, at the time of diagnosis to ensure patients receive the most appropriate treatment for their specific disease,” he commented in a company press release.
This is an accelerated approval, based on overall response rate data from the DESTINY-Lung02 phase 2 trial, the company noted. An interim efficacy analysis in a prespecified patient cohort showed that trastuzumab deruxtecan (at 5.4 mg/kg) demonstrated a confirmed overall response rate of 57.7% (n = 52; 95% confidence interval, 43.2%-71.3%) in patients with HER2-mutant unresectable or metastatic nonsquamous NSCLC who had received one prior systemic therapy as assessed by blinded independent central review. Complete responses were seen in 1.9% of patients (n = 1) and partial responses in 55.8% of patients (n = 29), with a median duration of response of 8.7 months (95% CI, 7.1-NE).
The FDA noted that for the 52 patients in the primary efficacy population of the DESTINY-Lung02 trial, the median age was 58 years (range, 30-78 years); 69% were female; and 79% were Asian, 12% were White, and 10% were of other races.
Clinical data welcomed by experts
Clinical data are already available from the DESTINY-Lung 01 trial, and the results were welcomed enthusiastically by experts when they were published in the New England Journal of Medicine earlier this year.
“These results establish the new standard of care for patients with NSCLC harboring HER2 mutations,” Antonio Passaro, MD, PhD, from the European Institute of Oncology IRCCS, Milan, and Solange Peters, MD, PhD, from Lausanne (Switzerland) University Hospital, wrote in an accompanying editorial.
This trial involved 91 patients, all treated with trastuzumab deruxtecan (at 6.4 mg/kg of body weight every 3 weeks). The median duration of treatment was 6.9 months, and the median follow-up was 13.1 months.
The results showed a 55% centrally confirmed objective response, and median duration of response was 9.3 months.
In addition, the investigators reported a median progression-free survival of 8.2 months and a median overall survival of almost 18 months, both of which they described as “encouraging” in this patient population.
However, the results also highlighted a problem with the drug in this patient population. Notably, 26% of patients experienced interstitial lung disease, which resulted in death in two patients. The drug was also withdrawn in 16 patients and interrupted in 8 patients because of this adverse event.
Editorialists Dr. Passaro and Dr. Peters described this finding as “a concern” and note that “the incidence of interstitial lung disease is significantly higher among patients with lung cancer than among those with breast or gastric cancers, which may indicate a role of smoking-related damage.”
They also highlighted the need for an “investigation of the clinical efficacy of a reduced dose of trastuzumab deruxtecan,” and so the dose was reduced for the DESTINY-Lung02 trial.
A version of this article first appeared on Medscape.com.
Patients with lung cancer now have another treatment option: If their tumors are found to carry HER2 mutations, they can now be treated with trastuzumab deruxtecan (Enhertu), a drug that specifically targets that defect.
This product is already approved for used in HER2-positive breast cancer and gastric cancer.
The FDA also approved companion diagnostic tests to detect HER2 mutations: Life Technologies Corporation’s Oncomine Dx Target Test for use in lung tissue and Guardant Health’s Guardant360 CDx for use on plasma samples. The agency notes that if no mutation is detected in a plasma specimen, the tumor tissue should be tested.
Specifically, the new indication is used in patients with unresectable or metastatic non–small cell lung cancer (NSCLC) whose tumors have activating HER2 (ERBB2) mutations, as detected by an FDA-approved test, and who have already received a prior systemic therapy.
About 3% of nonsquamous NSCLC tumors carry mutations in the HER2 gene, and they are associated with female sex, never-smokers, and a poor prognosis.
“HER2 mutant non–small cell lung cancer is an aggressive form of disease, which commonly affects young patients who have faced limited treatment options and a poor prognosis to date,” said Dave Fredrickson, executive vice-president of the oncology business unit at AstraZeneca.
The new approval “provides these patients with the opportunity to benefit from a targeted therapy and highlights the importance of testing for predictive markers, including HER2 in lung cancer, at the time of diagnosis to ensure patients receive the most appropriate treatment for their specific disease,” he commented in a company press release.
This is an accelerated approval, based on overall response rate data from the DESTINY-Lung02 phase 2 trial, the company noted. An interim efficacy analysis in a prespecified patient cohort showed that trastuzumab deruxtecan (at 5.4 mg/kg) demonstrated a confirmed overall response rate of 57.7% (n = 52; 95% confidence interval, 43.2%-71.3%) in patients with HER2-mutant unresectable or metastatic nonsquamous NSCLC who had received one prior systemic therapy as assessed by blinded independent central review. Complete responses were seen in 1.9% of patients (n = 1) and partial responses in 55.8% of patients (n = 29), with a median duration of response of 8.7 months (95% CI, 7.1-NE).
The FDA noted that for the 52 patients in the primary efficacy population of the DESTINY-Lung02 trial, the median age was 58 years (range, 30-78 years); 69% were female; and 79% were Asian, 12% were White, and 10% were of other races.
Clinical data welcomed by experts
Clinical data are already available from the DESTINY-Lung 01 trial, and the results were welcomed enthusiastically by experts when they were published in the New England Journal of Medicine earlier this year.
“These results establish the new standard of care for patients with NSCLC harboring HER2 mutations,” Antonio Passaro, MD, PhD, from the European Institute of Oncology IRCCS, Milan, and Solange Peters, MD, PhD, from Lausanne (Switzerland) University Hospital, wrote in an accompanying editorial.
This trial involved 91 patients, all treated with trastuzumab deruxtecan (at 6.4 mg/kg of body weight every 3 weeks). The median duration of treatment was 6.9 months, and the median follow-up was 13.1 months.
The results showed a 55% centrally confirmed objective response, and median duration of response was 9.3 months.
In addition, the investigators reported a median progression-free survival of 8.2 months and a median overall survival of almost 18 months, both of which they described as “encouraging” in this patient population.
However, the results also highlighted a problem with the drug in this patient population. Notably, 26% of patients experienced interstitial lung disease, which resulted in death in two patients. The drug was also withdrawn in 16 patients and interrupted in 8 patients because of this adverse event.
Editorialists Dr. Passaro and Dr. Peters described this finding as “a concern” and note that “the incidence of interstitial lung disease is significantly higher among patients with lung cancer than among those with breast or gastric cancers, which may indicate a role of smoking-related damage.”
They also highlighted the need for an “investigation of the clinical efficacy of a reduced dose of trastuzumab deruxtecan,” and so the dose was reduced for the DESTINY-Lung02 trial.
A version of this article first appeared on Medscape.com.
Patients with lung cancer now have another treatment option: If their tumors are found to carry HER2 mutations, they can now be treated with trastuzumab deruxtecan (Enhertu), a drug that specifically targets that defect.
This product is already approved for used in HER2-positive breast cancer and gastric cancer.
The FDA also approved companion diagnostic tests to detect HER2 mutations: Life Technologies Corporation’s Oncomine Dx Target Test for use in lung tissue and Guardant Health’s Guardant360 CDx for use on plasma samples. The agency notes that if no mutation is detected in a plasma specimen, the tumor tissue should be tested.
Specifically, the new indication is used in patients with unresectable or metastatic non–small cell lung cancer (NSCLC) whose tumors have activating HER2 (ERBB2) mutations, as detected by an FDA-approved test, and who have already received a prior systemic therapy.
About 3% of nonsquamous NSCLC tumors carry mutations in the HER2 gene, and they are associated with female sex, never-smokers, and a poor prognosis.
“HER2 mutant non–small cell lung cancer is an aggressive form of disease, which commonly affects young patients who have faced limited treatment options and a poor prognosis to date,” said Dave Fredrickson, executive vice-president of the oncology business unit at AstraZeneca.
The new approval “provides these patients with the opportunity to benefit from a targeted therapy and highlights the importance of testing for predictive markers, including HER2 in lung cancer, at the time of diagnosis to ensure patients receive the most appropriate treatment for their specific disease,” he commented in a company press release.
This is an accelerated approval, based on overall response rate data from the DESTINY-Lung02 phase 2 trial, the company noted. An interim efficacy analysis in a prespecified patient cohort showed that trastuzumab deruxtecan (at 5.4 mg/kg) demonstrated a confirmed overall response rate of 57.7% (n = 52; 95% confidence interval, 43.2%-71.3%) in patients with HER2-mutant unresectable or metastatic nonsquamous NSCLC who had received one prior systemic therapy as assessed by blinded independent central review. Complete responses were seen in 1.9% of patients (n = 1) and partial responses in 55.8% of patients (n = 29), with a median duration of response of 8.7 months (95% CI, 7.1-NE).
The FDA noted that for the 52 patients in the primary efficacy population of the DESTINY-Lung02 trial, the median age was 58 years (range, 30-78 years); 69% were female; and 79% were Asian, 12% were White, and 10% were of other races.
Clinical data welcomed by experts
Clinical data are already available from the DESTINY-Lung 01 trial, and the results were welcomed enthusiastically by experts when they were published in the New England Journal of Medicine earlier this year.
“These results establish the new standard of care for patients with NSCLC harboring HER2 mutations,” Antonio Passaro, MD, PhD, from the European Institute of Oncology IRCCS, Milan, and Solange Peters, MD, PhD, from Lausanne (Switzerland) University Hospital, wrote in an accompanying editorial.
This trial involved 91 patients, all treated with trastuzumab deruxtecan (at 6.4 mg/kg of body weight every 3 weeks). The median duration of treatment was 6.9 months, and the median follow-up was 13.1 months.
The results showed a 55% centrally confirmed objective response, and median duration of response was 9.3 months.
In addition, the investigators reported a median progression-free survival of 8.2 months and a median overall survival of almost 18 months, both of which they described as “encouraging” in this patient population.
However, the results also highlighted a problem with the drug in this patient population. Notably, 26% of patients experienced interstitial lung disease, which resulted in death in two patients. The drug was also withdrawn in 16 patients and interrupted in 8 patients because of this adverse event.
Editorialists Dr. Passaro and Dr. Peters described this finding as “a concern” and note that “the incidence of interstitial lung disease is significantly higher among patients with lung cancer than among those with breast or gastric cancers, which may indicate a role of smoking-related damage.”
They also highlighted the need for an “investigation of the clinical efficacy of a reduced dose of trastuzumab deruxtecan,” and so the dose was reduced for the DESTINY-Lung02 trial.
A version of this article first appeared on Medscape.com.
Value of a Pharmacy-Adjudicated Community Care Prior Authorization Drug Request Service
Veterans’ access to medical care was expanded outside of US Department of Veterans Affairs (VA) facilities with the inception of the 2014 Veterans Access, Choice, and Accountability Act (Choice Act).1 This legislation aimed to remove barriers some veterans were experiencing, specifically access to health care. In subsequent years, approximately 17% of veterans receiving care from the VA did so under the Choice Act.2 The Choice Act positively impacted medical care access for veterans but presented new challenges for VA pharmacies processing community care (CC) prescriptions, including limited access to outside health records, lack of interface between CC prescribers and the VA order entry system, and limited awareness of the VA national formulary.3,4 These factors made it difficult for VA pharmacies to assess prescriptions for clinical appropriateness, evaluate patient safety parameters, and manage expenditures.
In 2019, the Maintaining Internal Systems and Strengthening Integrated Outside Networks (MISSION) Act, which expanded CC support and better defined which veterans are able to receive care outside the VA, updated the Choice Act.4,5 However, VA pharmacies faced challenges in managing pharmacy drug costs and ensuring clinical appropriateness of prescription drug therapy. As a result, VA pharmacy departments have adjusted how they allocate workload, time, and funds.5
Pharmacists improve clinical outcomes and reduce health care costs by decreasing medication errors, unnecessary prescribing, and adverse drug events.6-12 Pharmacist-driven formulary management through evaluation of prior authorization drug requests (PADRs) has shown economic value.13,14 VA pharmacy review of community care PADRs is important because outside health care professionals (HCPs) might not be familiar with the VA formulary. This could lead to high volume of PADRs that do not meet criteria and could result in increased potential for medication misuse, adverse drug events, medication errors, and cost to the health system. It is imperative that CC orders are evaluated as critically as traditional orders.
The value of a centralized CC pharmacy team has not been assessed in the literature. The primary objective of this study was to assess the direct cost savings achieved through a centralized CC PADR process. Secondary objectives were to characterize the CC PADRs submitted to the site, including approval rate, reason for nonapproval, which medications were requested and by whom, and to compare CC prescriptions with other high-complexity (1a) VA facilities.
Community Care Pharmacy
VA health systems are stratified according to complexity, which reflects size, patient population, and services offered. This study was conducted at the Durham Veterans Affairs Health Care System (DVAHCS), North Carolina, a high-complexity, 251-bed, tertiary care referral, teaching, and research system. DVAHCS provides general and specialty medical, surgical, inpatient psychiatric, and ambulatory services, and serves as a major referral center.
DVAHCS created a centralized pharmacy team for processing CC prescriptions and managing customer service. This team’s goal is to increase CC prescription processing efficiency and transparency, ensure accountability of the health care team, and promote veteran-centric customer service. The pharmacy team includes a pharmacist program manager and a dedicated CC pharmacist with administrative support from a health benefits assistant and 4 pharmacy technicians. The CC pharmacy team assesses every new prescription to ensure the veteran is authorized to receive care in the community. Once eligibility is verified, a pharmacy technician or pharmacist evaluates the prescription to ensure it contains all required information, then contacts the prescriber for any missing data. If clinically appropriate, the pharmacist processes the prescription.
In 2020, the CC pharmacy team implemented a new process for reviewing and documenting CC prescriptions that require a PADR. The closed national VA formulary is set up so that all nonformulary medications and some formulary medications, including those that are restricted because of safety and/or cost, require a PADR.15 After a CC pharmacy technician confirms a veteran’s eligibility, the technician assesses whether the requested medication requires submitting a PADR to the VA internal electronic health record. The PADR is then adjudicated by a formulary management pharmacist, CC program manager, or CC pharmacist who reviews health records to determine whether the CC prescription meets VA medication use policy requirements.
If additional information is needed or an alternate medication is suggested, the pharmacist comments back on the PADR and a CC pharmacy technician contacts the prescriber. The PADR is canceled administratively then resubmitted once all information is obtained. While waiting for a response from the prescriber, the CC pharmacy technician contacts that veteran to give an update on the prescription status, as appropriate. Once there is sufficient information to adjudicate the PADR, the outcome is documented, and if approved, the order is processed.
Methods
The DVAHCS Institutional Review Board approved this retrospective review of CC PADRs submitted from June 1, 2020, through November 30, 2020. CC PADRs were excluded if they were duplicates or were reactivated administratively but had an initial submission date before the study period. Local data were collected for nonapproved CC PADRs including drug requested, dosage and directions, medication specialty, alternative drug recommended, drug acquisition cost, PADR submission date, PADR completion date, PADR nonapproval rationale, and documented time spent per PADR. Additional data was obtained for CC prescriptions at all 42 high-complexity VA facilities from the VA national CC prescription database for the study time interval and included total PADRs, PADR approval status, total CC prescription cost, and total CC fills.
Direct cost savings were calculated by assessing the cost of requested therapy that was not approved minus the cost of recommended therapy and cost to review all PADRs, as described by Britt and colleagues.13 The cost of the requested and recommended therapy was calculated based on VA drug acquisition cost at time of data collection and multiplied by the expected duration of therapy up to 1 year. For each CC prescription, duration of therapy was based on the duration limit in the prescription or annualized if no duration limit was documented. Cost of PADR review was calculated based on the total time pharmacists and pharmacy technicians documented for each step of the review process for a representative sample of 100 nonapproved PADRs and then multiplied by the salary plus benefits of an entry-level pharmacist and pharmacy technician.16 The eAppendix describes specific equations used for determining direct cost savings. Descriptive statistics were used to evaluate study results.
Results
During the 6-month study period, 611 CC PADRs were submitted to the pharmacy and 526 met inclusion criteria (Figure 1). Of those, 243 (46.2%) were approved and 283 (53.8%) were not approved. The cost of requested therapies for nonapproved CC PADRs totaled $584,565.48 and the cost of all recommended therapies was $57,473.59. The mean time per CC PADR was 24 minutes; 16 minutes for pharmacists and 8 minutes for pharmacy technicians. Given an hourly wage (plus benefits) of $67.25 for a pharmacist and $25.53 for a pharmacy technician, the total cost of review per CC PADR was $21.33. After subtracting the costs of all recommended therapies and review of all included CC PADRs, the process generated $515,872.31 in direct cost savings. After factoring in administrative lag time, such as HCP communication, an average of 8 calendar days was needed to complete a nonapproved PADR.
The most common rationale for PADR nonapproval was that the formulary alternative was not exhausted. Ondansetron orally disintegrating tablets was the most commonly nonapproved medication and azelastine was the most commonly approved medication. Dulaglutide was the most expensive nonapproved and tafamidis was the most expensive approved PADR (Table 1). Gastroenterology, endocrinology, and neurology were the top specialties for nonapproved PADRs while neurology, pulmonology, and endocrinology were the top specialties for approved PADRs (Table 2).
Several high-complexity VA facilities had no reported data; we used the median for the analysis to account for these outliers (Figure 2). The median (IQR) adjudicated CC PADRs for all facilities was 97 (20-175), median (IQR) CC PADR approval rate was 80.9% (63.7%-96.8%), median (IQR) total CC prescriptions was 8440 (2464-14,466), and median (IQR) cost per fill was $136.05 ($76.27-$221.28).
Discussion
This study demonstrated direct cost savings of $515,872.31 over 6 months with theadjudication of CC PADRs by a centralized CC pharmacy team. This could result in > $1,000,000 of cost savings per fiscal year.
The CC PADRs observed at DVAHCS had a 46.2% approval rate; almost one-half the approval rate of 84.1% of all PADRs submitted to the study site by VA HCPs captured by Britt and colleagues.13 Results from this study showed that coordination of care for nonapproved CC PADRs between the VA pharmacy and non-VA prescriber took an average of 8 calendar days. The noted CC PADR approval rate and administrative burden might be because of lack of familiarity of non-VA providers regarding the VA national formulary. The National VA Pharmacy Benefits Management determines the formulary using cost-effectiveness criteria that considers the medical literature and VA-specific contract pricing and prepares extensive guidance for restricted medications via relevant criteria for use.15 HCPs outside the VA might not know this information is available online. Because gastroenterology, endocrinology, and neurology specialty medications were among the most frequently nonapproved PADRs, VA formulary education could begin with CC HCPs in these practice areas.
This study showed that the CC PADR process was not solely driven by cost, but also included patient safety. Nonapproval rationale for some requests included submission without an indication, submission by a prescriber that did not have the authority to prescribe a type of medication, or contraindication based on patient-specific factors.
Compared with other VA high-complexity facilities, DVAHCS was among the top health care systems for total volume of CC prescriptions (n = 16,096) and among the lowest for cost/fill ($75.74). Similarly, DVAHCS was among the top sites for total adjudicated CC PADRs within the 6-month study period (n = 611) and the lowest approval rate (44.2%). This study shows that despite high volumes of overall CC prescriptions and CC PADRs, it is possible to maintain a low overall CC prescription cost/fill compared with other similarly complex sites across the country. Wide variance in reported results exists across high-complexity VA facilities because some sites had low to no CC fills and/or CC PADRs. This is likely a result of administrative differences when handling CC prescriptions and presents an opportunity to standardize this process nationally.
Limitations
CC PADRs were assessed during the COVID-19 pandemic, which might have resulted in lower-than-normal CC prescription and PADR volumes, therefore underestimating the potential for direct cost savings. Entry-level salary was used to demonstrate cost savings potential from the perspective of a newly hired CC team; however, the cost savings might have been less if the actual salaries of site personnel were higher. National contract pricing data were gathered at the time of data collection and might have been different than at the time of PADR submission. Chronic medication prescriptions were annualized, which could overestimate cost savings if the medication was discontinued or changed to an alternative therapy within that time period.
The study’s exclusion criteria could only be applied locally and did not include data received from the VA CC prescription database. This can be seen by the discrepancy in CC PADR approval rates from the local and national data (46.2% vs 44.2%, respectively) and CC PADR volume. High-complexity VA facility data were captured without assessing the CC prescription process at each site. As a result, definitive conclusions cannot be made regarding the impact of a centralized CC pharmacy team compared with other facilities.
Conclusions
Adjudication of CC PADRs by a centralized CC pharmacy team over a 6-month period provided > $500,000 in direct cost savings to a VA health care system. Considering the CC PADR approval rate seen in this study, the VA could allocate resources to educate CC providers about the VA formulary to increase the PADR approval rate and reduce administrative burden for VA pharmacies and prescribers. Future research should evaluate CC prescription handling practices at other VA facilities to compare the effectiveness among varying approaches and develop recommendations for a nationally standardized process.
Acknowledgments
Concept and design (AJJ, JNB, RBB, LAM, MD, MGH); acquisition of data (AJJ, MGH); analysis and interpretation of data (AJJ, JNB, RBB, LAM, MD, MGH); drafting of the manuscript (AJJ); critical revision of the manuscript for important intellectual content (AJJ, JNB, RBB, LAM, MD, MGH); statistical analysis (AJJ); administrative, technical, or logistic support (LAM, MGH); and supervision (MGH).
1. Gellad WF, Cunningham FE, Good CB, et al. Pharmacy use in the first year of the Veterans Choice Program: a mixed-methods evaluation. Med Care. 2017(7 suppl 1);55:S26. doi:10.1097/MLR.0000000000000661
2. Mattocks KM, Yehia B. Evaluating the veterans choice program: lessons for developing a high-performing integrated network. Med Care. 2017(7 suppl 1);55:S1-S3. doi:10.1097/MLR.0000000000000743.
3. Mattocks KM, Mengeling M, Sadler A, Baldor R, Bastian L. The Veterans Choice Act: a qualitative examination of rapid policy implementation in the Department of Veterans Affairs. Med Care. 2017;55(7 suppl 1):S71-S75. doi:10.1097/MLR.0000000000000667
4. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1108.08: VHA formulary management process. November 2, 2016. Accessed June 9, 2022. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3291
5. Massarweh NN, Itani KMF, Morris MS. The VA MISSION act and the future of veterans’ access to quality health care. JAMA. 2020;324:343-344. doi:10.1001/jama.2020.4505
6. Jourdan JP, Muzard A, Goyer I, et al. Impact of pharmacist interventions on clinical outcome and cost avoidance in a university teaching hospital. Int J Clin Pharm. 2018;40(6):1474-1481. doi:10.1007/s11096-018-0733-6
7. Lee AJ, Boro MS, Knapp KK, Meier JL, Korman NE. Clinical and economic outcomes of pharmacist recommendations in a Veterans Affairs medical center. Am J Health Syst Pharm. 2002;59(21):2070-2077. doi:10.1093/ajhp/59.21.2070
8. Dalton K, Byrne S. Role of the pharmacist in reducing healthcare costs: current insights. Integr Pharm Res Pract. 2017;6:37-46. doi:10.2147/IPRP.S108047
9. De Rijdt T, Willems L, Simoens S. Economic effects of clinical pharmacy interventions: a literature review. Am J Health Syst Pharm. 2008;65(12):1161-1172. doi:10.2146/ajhp070506
10. Perez A, Doloresco F, Hoffman J, et al. Economic evaluation of clinical pharmacy services: 2001-2005. Pharmacotherapy. 2009;29(1):128. doi:10.1592/phco.29.1.128
11. Nesbit TW, Shermock KM, Bobek MB, et al. Implementation and pharmacoeconomic analysis of a clinical staff pharmacist practice model. Am J Health Syst Pharm. 2001;58(9):784-790. doi:10.1093/ajhp/58.9.784
12. Yang S, Britt RB, Hashem MG, Brown JN. Outcomes of pharmacy-led hepatitis C direct-acting antiviral utilization management at a Veterans Affairs medical center. J Manag Care Pharm. 2017;23(3):364-369. doi:10.18553/jmcp.2017.23.3.364
13. Britt RB, Hashem MG, Bryan WE III, Kothapalli R, Brown JN. Economic outcomes associated with a pharmacist-adjudicated formulary consult service in a Veterans Affairs medical center. J Manag Care Pharm. 2016;22(9):1051-1061. doi:10.18553/jmcp.2016.22.9.1051
14. Jacob S, Britt RB, Bryan WE, Hashem MG, Hale JC, Brown JN. Economic outcomes associated with safety interventions by a pharmacist-adjudicated prior authorization consult service. J Manag Care Pharm. 2019;25(3):411-416. doi:10.18553/jmcp.2019.25.3.411
15. Aspinall SL, Sales MM, Good CB, et al. Pharmacy benefits management in the Veterans Health Administration revisited: a decade of advancements, 2004-2014. J Manag Care Spec Pharm. 2016;22(9):1058-1063. doi:10.18553/jmcp.2016.22.9.1058
16. US Department of Veterans Affairs, Office of the Chief Human Capital Officer. Title 38 Pay Schedules. Updated January 26, 2022. Accessed June 9, 2022. https://www.va.gov/ohrm/pay
Veterans’ access to medical care was expanded outside of US Department of Veterans Affairs (VA) facilities with the inception of the 2014 Veterans Access, Choice, and Accountability Act (Choice Act).1 This legislation aimed to remove barriers some veterans were experiencing, specifically access to health care. In subsequent years, approximately 17% of veterans receiving care from the VA did so under the Choice Act.2 The Choice Act positively impacted medical care access for veterans but presented new challenges for VA pharmacies processing community care (CC) prescriptions, including limited access to outside health records, lack of interface between CC prescribers and the VA order entry system, and limited awareness of the VA national formulary.3,4 These factors made it difficult for VA pharmacies to assess prescriptions for clinical appropriateness, evaluate patient safety parameters, and manage expenditures.
In 2019, the Maintaining Internal Systems and Strengthening Integrated Outside Networks (MISSION) Act, which expanded CC support and better defined which veterans are able to receive care outside the VA, updated the Choice Act.4,5 However, VA pharmacies faced challenges in managing pharmacy drug costs and ensuring clinical appropriateness of prescription drug therapy. As a result, VA pharmacy departments have adjusted how they allocate workload, time, and funds.5
Pharmacists improve clinical outcomes and reduce health care costs by decreasing medication errors, unnecessary prescribing, and adverse drug events.6-12 Pharmacist-driven formulary management through evaluation of prior authorization drug requests (PADRs) has shown economic value.13,14 VA pharmacy review of community care PADRs is important because outside health care professionals (HCPs) might not be familiar with the VA formulary. This could lead to high volume of PADRs that do not meet criteria and could result in increased potential for medication misuse, adverse drug events, medication errors, and cost to the health system. It is imperative that CC orders are evaluated as critically as traditional orders.
The value of a centralized CC pharmacy team has not been assessed in the literature. The primary objective of this study was to assess the direct cost savings achieved through a centralized CC PADR process. Secondary objectives were to characterize the CC PADRs submitted to the site, including approval rate, reason for nonapproval, which medications were requested and by whom, and to compare CC prescriptions with other high-complexity (1a) VA facilities.
Community Care Pharmacy
VA health systems are stratified according to complexity, which reflects size, patient population, and services offered. This study was conducted at the Durham Veterans Affairs Health Care System (DVAHCS), North Carolina, a high-complexity, 251-bed, tertiary care referral, teaching, and research system. DVAHCS provides general and specialty medical, surgical, inpatient psychiatric, and ambulatory services, and serves as a major referral center.
DVAHCS created a centralized pharmacy team for processing CC prescriptions and managing customer service. This team’s goal is to increase CC prescription processing efficiency and transparency, ensure accountability of the health care team, and promote veteran-centric customer service. The pharmacy team includes a pharmacist program manager and a dedicated CC pharmacist with administrative support from a health benefits assistant and 4 pharmacy technicians. The CC pharmacy team assesses every new prescription to ensure the veteran is authorized to receive care in the community. Once eligibility is verified, a pharmacy technician or pharmacist evaluates the prescription to ensure it contains all required information, then contacts the prescriber for any missing data. If clinically appropriate, the pharmacist processes the prescription.
In 2020, the CC pharmacy team implemented a new process for reviewing and documenting CC prescriptions that require a PADR. The closed national VA formulary is set up so that all nonformulary medications and some formulary medications, including those that are restricted because of safety and/or cost, require a PADR.15 After a CC pharmacy technician confirms a veteran’s eligibility, the technician assesses whether the requested medication requires submitting a PADR to the VA internal electronic health record. The PADR is then adjudicated by a formulary management pharmacist, CC program manager, or CC pharmacist who reviews health records to determine whether the CC prescription meets VA medication use policy requirements.
If additional information is needed or an alternate medication is suggested, the pharmacist comments back on the PADR and a CC pharmacy technician contacts the prescriber. The PADR is canceled administratively then resubmitted once all information is obtained. While waiting for a response from the prescriber, the CC pharmacy technician contacts that veteran to give an update on the prescription status, as appropriate. Once there is sufficient information to adjudicate the PADR, the outcome is documented, and if approved, the order is processed.
Methods
The DVAHCS Institutional Review Board approved this retrospective review of CC PADRs submitted from June 1, 2020, through November 30, 2020. CC PADRs were excluded if they were duplicates or were reactivated administratively but had an initial submission date before the study period. Local data were collected for nonapproved CC PADRs including drug requested, dosage and directions, medication specialty, alternative drug recommended, drug acquisition cost, PADR submission date, PADR completion date, PADR nonapproval rationale, and documented time spent per PADR. Additional data was obtained for CC prescriptions at all 42 high-complexity VA facilities from the VA national CC prescription database for the study time interval and included total PADRs, PADR approval status, total CC prescription cost, and total CC fills.
Direct cost savings were calculated by assessing the cost of requested therapy that was not approved minus the cost of recommended therapy and cost to review all PADRs, as described by Britt and colleagues.13 The cost of the requested and recommended therapy was calculated based on VA drug acquisition cost at time of data collection and multiplied by the expected duration of therapy up to 1 year. For each CC prescription, duration of therapy was based on the duration limit in the prescription or annualized if no duration limit was documented. Cost of PADR review was calculated based on the total time pharmacists and pharmacy technicians documented for each step of the review process for a representative sample of 100 nonapproved PADRs and then multiplied by the salary plus benefits of an entry-level pharmacist and pharmacy technician.16 The eAppendix describes specific equations used for determining direct cost savings. Descriptive statistics were used to evaluate study results.
Results
During the 6-month study period, 611 CC PADRs were submitted to the pharmacy and 526 met inclusion criteria (Figure 1). Of those, 243 (46.2%) were approved and 283 (53.8%) were not approved. The cost of requested therapies for nonapproved CC PADRs totaled $584,565.48 and the cost of all recommended therapies was $57,473.59. The mean time per CC PADR was 24 minutes; 16 minutes for pharmacists and 8 minutes for pharmacy technicians. Given an hourly wage (plus benefits) of $67.25 for a pharmacist and $25.53 for a pharmacy technician, the total cost of review per CC PADR was $21.33. After subtracting the costs of all recommended therapies and review of all included CC PADRs, the process generated $515,872.31 in direct cost savings. After factoring in administrative lag time, such as HCP communication, an average of 8 calendar days was needed to complete a nonapproved PADR.
The most common rationale for PADR nonapproval was that the formulary alternative was not exhausted. Ondansetron orally disintegrating tablets was the most commonly nonapproved medication and azelastine was the most commonly approved medication. Dulaglutide was the most expensive nonapproved and tafamidis was the most expensive approved PADR (Table 1). Gastroenterology, endocrinology, and neurology were the top specialties for nonapproved PADRs while neurology, pulmonology, and endocrinology were the top specialties for approved PADRs (Table 2).
Several high-complexity VA facilities had no reported data; we used the median for the analysis to account for these outliers (Figure 2). The median (IQR) adjudicated CC PADRs for all facilities was 97 (20-175), median (IQR) CC PADR approval rate was 80.9% (63.7%-96.8%), median (IQR) total CC prescriptions was 8440 (2464-14,466), and median (IQR) cost per fill was $136.05 ($76.27-$221.28).
Discussion
This study demonstrated direct cost savings of $515,872.31 over 6 months with theadjudication of CC PADRs by a centralized CC pharmacy team. This could result in > $1,000,000 of cost savings per fiscal year.
The CC PADRs observed at DVAHCS had a 46.2% approval rate; almost one-half the approval rate of 84.1% of all PADRs submitted to the study site by VA HCPs captured by Britt and colleagues.13 Results from this study showed that coordination of care for nonapproved CC PADRs between the VA pharmacy and non-VA prescriber took an average of 8 calendar days. The noted CC PADR approval rate and administrative burden might be because of lack of familiarity of non-VA providers regarding the VA national formulary. The National VA Pharmacy Benefits Management determines the formulary using cost-effectiveness criteria that considers the medical literature and VA-specific contract pricing and prepares extensive guidance for restricted medications via relevant criteria for use.15 HCPs outside the VA might not know this information is available online. Because gastroenterology, endocrinology, and neurology specialty medications were among the most frequently nonapproved PADRs, VA formulary education could begin with CC HCPs in these practice areas.
This study showed that the CC PADR process was not solely driven by cost, but also included patient safety. Nonapproval rationale for some requests included submission without an indication, submission by a prescriber that did not have the authority to prescribe a type of medication, or contraindication based on patient-specific factors.
Compared with other VA high-complexity facilities, DVAHCS was among the top health care systems for total volume of CC prescriptions (n = 16,096) and among the lowest for cost/fill ($75.74). Similarly, DVAHCS was among the top sites for total adjudicated CC PADRs within the 6-month study period (n = 611) and the lowest approval rate (44.2%). This study shows that despite high volumes of overall CC prescriptions and CC PADRs, it is possible to maintain a low overall CC prescription cost/fill compared with other similarly complex sites across the country. Wide variance in reported results exists across high-complexity VA facilities because some sites had low to no CC fills and/or CC PADRs. This is likely a result of administrative differences when handling CC prescriptions and presents an opportunity to standardize this process nationally.
Limitations
CC PADRs were assessed during the COVID-19 pandemic, which might have resulted in lower-than-normal CC prescription and PADR volumes, therefore underestimating the potential for direct cost savings. Entry-level salary was used to demonstrate cost savings potential from the perspective of a newly hired CC team; however, the cost savings might have been less if the actual salaries of site personnel were higher. National contract pricing data were gathered at the time of data collection and might have been different than at the time of PADR submission. Chronic medication prescriptions were annualized, which could overestimate cost savings if the medication was discontinued or changed to an alternative therapy within that time period.
The study’s exclusion criteria could only be applied locally and did not include data received from the VA CC prescription database. This can be seen by the discrepancy in CC PADR approval rates from the local and national data (46.2% vs 44.2%, respectively) and CC PADR volume. High-complexity VA facility data were captured without assessing the CC prescription process at each site. As a result, definitive conclusions cannot be made regarding the impact of a centralized CC pharmacy team compared with other facilities.
Conclusions
Adjudication of CC PADRs by a centralized CC pharmacy team over a 6-month period provided > $500,000 in direct cost savings to a VA health care system. Considering the CC PADR approval rate seen in this study, the VA could allocate resources to educate CC providers about the VA formulary to increase the PADR approval rate and reduce administrative burden for VA pharmacies and prescribers. Future research should evaluate CC prescription handling practices at other VA facilities to compare the effectiveness among varying approaches and develop recommendations for a nationally standardized process.
Acknowledgments
Concept and design (AJJ, JNB, RBB, LAM, MD, MGH); acquisition of data (AJJ, MGH); analysis and interpretation of data (AJJ, JNB, RBB, LAM, MD, MGH); drafting of the manuscript (AJJ); critical revision of the manuscript for important intellectual content (AJJ, JNB, RBB, LAM, MD, MGH); statistical analysis (AJJ); administrative, technical, or logistic support (LAM, MGH); and supervision (MGH).
Veterans’ access to medical care was expanded outside of US Department of Veterans Affairs (VA) facilities with the inception of the 2014 Veterans Access, Choice, and Accountability Act (Choice Act).1 This legislation aimed to remove barriers some veterans were experiencing, specifically access to health care. In subsequent years, approximately 17% of veterans receiving care from the VA did so under the Choice Act.2 The Choice Act positively impacted medical care access for veterans but presented new challenges for VA pharmacies processing community care (CC) prescriptions, including limited access to outside health records, lack of interface between CC prescribers and the VA order entry system, and limited awareness of the VA national formulary.3,4 These factors made it difficult for VA pharmacies to assess prescriptions for clinical appropriateness, evaluate patient safety parameters, and manage expenditures.
In 2019, the Maintaining Internal Systems and Strengthening Integrated Outside Networks (MISSION) Act, which expanded CC support and better defined which veterans are able to receive care outside the VA, updated the Choice Act.4,5 However, VA pharmacies faced challenges in managing pharmacy drug costs and ensuring clinical appropriateness of prescription drug therapy. As a result, VA pharmacy departments have adjusted how they allocate workload, time, and funds.5
Pharmacists improve clinical outcomes and reduce health care costs by decreasing medication errors, unnecessary prescribing, and adverse drug events.6-12 Pharmacist-driven formulary management through evaluation of prior authorization drug requests (PADRs) has shown economic value.13,14 VA pharmacy review of community care PADRs is important because outside health care professionals (HCPs) might not be familiar with the VA formulary. This could lead to high volume of PADRs that do not meet criteria and could result in increased potential for medication misuse, adverse drug events, medication errors, and cost to the health system. It is imperative that CC orders are evaluated as critically as traditional orders.
The value of a centralized CC pharmacy team has not been assessed in the literature. The primary objective of this study was to assess the direct cost savings achieved through a centralized CC PADR process. Secondary objectives were to characterize the CC PADRs submitted to the site, including approval rate, reason for nonapproval, which medications were requested and by whom, and to compare CC prescriptions with other high-complexity (1a) VA facilities.
Community Care Pharmacy
VA health systems are stratified according to complexity, which reflects size, patient population, and services offered. This study was conducted at the Durham Veterans Affairs Health Care System (DVAHCS), North Carolina, a high-complexity, 251-bed, tertiary care referral, teaching, and research system. DVAHCS provides general and specialty medical, surgical, inpatient psychiatric, and ambulatory services, and serves as a major referral center.
DVAHCS created a centralized pharmacy team for processing CC prescriptions and managing customer service. This team’s goal is to increase CC prescription processing efficiency and transparency, ensure accountability of the health care team, and promote veteran-centric customer service. The pharmacy team includes a pharmacist program manager and a dedicated CC pharmacist with administrative support from a health benefits assistant and 4 pharmacy technicians. The CC pharmacy team assesses every new prescription to ensure the veteran is authorized to receive care in the community. Once eligibility is verified, a pharmacy technician or pharmacist evaluates the prescription to ensure it contains all required information, then contacts the prescriber for any missing data. If clinically appropriate, the pharmacist processes the prescription.
In 2020, the CC pharmacy team implemented a new process for reviewing and documenting CC prescriptions that require a PADR. The closed national VA formulary is set up so that all nonformulary medications and some formulary medications, including those that are restricted because of safety and/or cost, require a PADR.15 After a CC pharmacy technician confirms a veteran’s eligibility, the technician assesses whether the requested medication requires submitting a PADR to the VA internal electronic health record. The PADR is then adjudicated by a formulary management pharmacist, CC program manager, or CC pharmacist who reviews health records to determine whether the CC prescription meets VA medication use policy requirements.
If additional information is needed or an alternate medication is suggested, the pharmacist comments back on the PADR and a CC pharmacy technician contacts the prescriber. The PADR is canceled administratively then resubmitted once all information is obtained. While waiting for a response from the prescriber, the CC pharmacy technician contacts that veteran to give an update on the prescription status, as appropriate. Once there is sufficient information to adjudicate the PADR, the outcome is documented, and if approved, the order is processed.
Methods
The DVAHCS Institutional Review Board approved this retrospective review of CC PADRs submitted from June 1, 2020, through November 30, 2020. CC PADRs were excluded if they were duplicates or were reactivated administratively but had an initial submission date before the study period. Local data were collected for nonapproved CC PADRs including drug requested, dosage and directions, medication specialty, alternative drug recommended, drug acquisition cost, PADR submission date, PADR completion date, PADR nonapproval rationale, and documented time spent per PADR. Additional data was obtained for CC prescriptions at all 42 high-complexity VA facilities from the VA national CC prescription database for the study time interval and included total PADRs, PADR approval status, total CC prescription cost, and total CC fills.
Direct cost savings were calculated by assessing the cost of requested therapy that was not approved minus the cost of recommended therapy and cost to review all PADRs, as described by Britt and colleagues.13 The cost of the requested and recommended therapy was calculated based on VA drug acquisition cost at time of data collection and multiplied by the expected duration of therapy up to 1 year. For each CC prescription, duration of therapy was based on the duration limit in the prescription or annualized if no duration limit was documented. Cost of PADR review was calculated based on the total time pharmacists and pharmacy technicians documented for each step of the review process for a representative sample of 100 nonapproved PADRs and then multiplied by the salary plus benefits of an entry-level pharmacist and pharmacy technician.16 The eAppendix describes specific equations used for determining direct cost savings. Descriptive statistics were used to evaluate study results.
Results
During the 6-month study period, 611 CC PADRs were submitted to the pharmacy and 526 met inclusion criteria (Figure 1). Of those, 243 (46.2%) were approved and 283 (53.8%) were not approved. The cost of requested therapies for nonapproved CC PADRs totaled $584,565.48 and the cost of all recommended therapies was $57,473.59. The mean time per CC PADR was 24 minutes; 16 minutes for pharmacists and 8 minutes for pharmacy technicians. Given an hourly wage (plus benefits) of $67.25 for a pharmacist and $25.53 for a pharmacy technician, the total cost of review per CC PADR was $21.33. After subtracting the costs of all recommended therapies and review of all included CC PADRs, the process generated $515,872.31 in direct cost savings. After factoring in administrative lag time, such as HCP communication, an average of 8 calendar days was needed to complete a nonapproved PADR.
The most common rationale for PADR nonapproval was that the formulary alternative was not exhausted. Ondansetron orally disintegrating tablets was the most commonly nonapproved medication and azelastine was the most commonly approved medication. Dulaglutide was the most expensive nonapproved and tafamidis was the most expensive approved PADR (Table 1). Gastroenterology, endocrinology, and neurology were the top specialties for nonapproved PADRs while neurology, pulmonology, and endocrinology were the top specialties for approved PADRs (Table 2).
Several high-complexity VA facilities had no reported data; we used the median for the analysis to account for these outliers (Figure 2). The median (IQR) adjudicated CC PADRs for all facilities was 97 (20-175), median (IQR) CC PADR approval rate was 80.9% (63.7%-96.8%), median (IQR) total CC prescriptions was 8440 (2464-14,466), and median (IQR) cost per fill was $136.05 ($76.27-$221.28).
Discussion
This study demonstrated direct cost savings of $515,872.31 over 6 months with theadjudication of CC PADRs by a centralized CC pharmacy team. This could result in > $1,000,000 of cost savings per fiscal year.
The CC PADRs observed at DVAHCS had a 46.2% approval rate; almost one-half the approval rate of 84.1% of all PADRs submitted to the study site by VA HCPs captured by Britt and colleagues.13 Results from this study showed that coordination of care for nonapproved CC PADRs between the VA pharmacy and non-VA prescriber took an average of 8 calendar days. The noted CC PADR approval rate and administrative burden might be because of lack of familiarity of non-VA providers regarding the VA national formulary. The National VA Pharmacy Benefits Management determines the formulary using cost-effectiveness criteria that considers the medical literature and VA-specific contract pricing and prepares extensive guidance for restricted medications via relevant criteria for use.15 HCPs outside the VA might not know this information is available online. Because gastroenterology, endocrinology, and neurology specialty medications were among the most frequently nonapproved PADRs, VA formulary education could begin with CC HCPs in these practice areas.
This study showed that the CC PADR process was not solely driven by cost, but also included patient safety. Nonapproval rationale for some requests included submission without an indication, submission by a prescriber that did not have the authority to prescribe a type of medication, or contraindication based on patient-specific factors.
Compared with other VA high-complexity facilities, DVAHCS was among the top health care systems for total volume of CC prescriptions (n = 16,096) and among the lowest for cost/fill ($75.74). Similarly, DVAHCS was among the top sites for total adjudicated CC PADRs within the 6-month study period (n = 611) and the lowest approval rate (44.2%). This study shows that despite high volumes of overall CC prescriptions and CC PADRs, it is possible to maintain a low overall CC prescription cost/fill compared with other similarly complex sites across the country. Wide variance in reported results exists across high-complexity VA facilities because some sites had low to no CC fills and/or CC PADRs. This is likely a result of administrative differences when handling CC prescriptions and presents an opportunity to standardize this process nationally.
Limitations
CC PADRs were assessed during the COVID-19 pandemic, which might have resulted in lower-than-normal CC prescription and PADR volumes, therefore underestimating the potential for direct cost savings. Entry-level salary was used to demonstrate cost savings potential from the perspective of a newly hired CC team; however, the cost savings might have been less if the actual salaries of site personnel were higher. National contract pricing data were gathered at the time of data collection and might have been different than at the time of PADR submission. Chronic medication prescriptions were annualized, which could overestimate cost savings if the medication was discontinued or changed to an alternative therapy within that time period.
The study’s exclusion criteria could only be applied locally and did not include data received from the VA CC prescription database. This can be seen by the discrepancy in CC PADR approval rates from the local and national data (46.2% vs 44.2%, respectively) and CC PADR volume. High-complexity VA facility data were captured without assessing the CC prescription process at each site. As a result, definitive conclusions cannot be made regarding the impact of a centralized CC pharmacy team compared with other facilities.
Conclusions
Adjudication of CC PADRs by a centralized CC pharmacy team over a 6-month period provided > $500,000 in direct cost savings to a VA health care system. Considering the CC PADR approval rate seen in this study, the VA could allocate resources to educate CC providers about the VA formulary to increase the PADR approval rate and reduce administrative burden for VA pharmacies and prescribers. Future research should evaluate CC prescription handling practices at other VA facilities to compare the effectiveness among varying approaches and develop recommendations for a nationally standardized process.
Acknowledgments
Concept and design (AJJ, JNB, RBB, LAM, MD, MGH); acquisition of data (AJJ, MGH); analysis and interpretation of data (AJJ, JNB, RBB, LAM, MD, MGH); drafting of the manuscript (AJJ); critical revision of the manuscript for important intellectual content (AJJ, JNB, RBB, LAM, MD, MGH); statistical analysis (AJJ); administrative, technical, or logistic support (LAM, MGH); and supervision (MGH).
1. Gellad WF, Cunningham FE, Good CB, et al. Pharmacy use in the first year of the Veterans Choice Program: a mixed-methods evaluation. Med Care. 2017(7 suppl 1);55:S26. doi:10.1097/MLR.0000000000000661
2. Mattocks KM, Yehia B. Evaluating the veterans choice program: lessons for developing a high-performing integrated network. Med Care. 2017(7 suppl 1);55:S1-S3. doi:10.1097/MLR.0000000000000743.
3. Mattocks KM, Mengeling M, Sadler A, Baldor R, Bastian L. The Veterans Choice Act: a qualitative examination of rapid policy implementation in the Department of Veterans Affairs. Med Care. 2017;55(7 suppl 1):S71-S75. doi:10.1097/MLR.0000000000000667
4. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1108.08: VHA formulary management process. November 2, 2016. Accessed June 9, 2022. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3291
5. Massarweh NN, Itani KMF, Morris MS. The VA MISSION act and the future of veterans’ access to quality health care. JAMA. 2020;324:343-344. doi:10.1001/jama.2020.4505
6. Jourdan JP, Muzard A, Goyer I, et al. Impact of pharmacist interventions on clinical outcome and cost avoidance in a university teaching hospital. Int J Clin Pharm. 2018;40(6):1474-1481. doi:10.1007/s11096-018-0733-6
7. Lee AJ, Boro MS, Knapp KK, Meier JL, Korman NE. Clinical and economic outcomes of pharmacist recommendations in a Veterans Affairs medical center. Am J Health Syst Pharm. 2002;59(21):2070-2077. doi:10.1093/ajhp/59.21.2070
8. Dalton K, Byrne S. Role of the pharmacist in reducing healthcare costs: current insights. Integr Pharm Res Pract. 2017;6:37-46. doi:10.2147/IPRP.S108047
9. De Rijdt T, Willems L, Simoens S. Economic effects of clinical pharmacy interventions: a literature review. Am J Health Syst Pharm. 2008;65(12):1161-1172. doi:10.2146/ajhp070506
10. Perez A, Doloresco F, Hoffman J, et al. Economic evaluation of clinical pharmacy services: 2001-2005. Pharmacotherapy. 2009;29(1):128. doi:10.1592/phco.29.1.128
11. Nesbit TW, Shermock KM, Bobek MB, et al. Implementation and pharmacoeconomic analysis of a clinical staff pharmacist practice model. Am J Health Syst Pharm. 2001;58(9):784-790. doi:10.1093/ajhp/58.9.784
12. Yang S, Britt RB, Hashem MG, Brown JN. Outcomes of pharmacy-led hepatitis C direct-acting antiviral utilization management at a Veterans Affairs medical center. J Manag Care Pharm. 2017;23(3):364-369. doi:10.18553/jmcp.2017.23.3.364
13. Britt RB, Hashem MG, Bryan WE III, Kothapalli R, Brown JN. Economic outcomes associated with a pharmacist-adjudicated formulary consult service in a Veterans Affairs medical center. J Manag Care Pharm. 2016;22(9):1051-1061. doi:10.18553/jmcp.2016.22.9.1051
14. Jacob S, Britt RB, Bryan WE, Hashem MG, Hale JC, Brown JN. Economic outcomes associated with safety interventions by a pharmacist-adjudicated prior authorization consult service. J Manag Care Pharm. 2019;25(3):411-416. doi:10.18553/jmcp.2019.25.3.411
15. Aspinall SL, Sales MM, Good CB, et al. Pharmacy benefits management in the Veterans Health Administration revisited: a decade of advancements, 2004-2014. J Manag Care Spec Pharm. 2016;22(9):1058-1063. doi:10.18553/jmcp.2016.22.9.1058
16. US Department of Veterans Affairs, Office of the Chief Human Capital Officer. Title 38 Pay Schedules. Updated January 26, 2022. Accessed June 9, 2022. https://www.va.gov/ohrm/pay
1. Gellad WF, Cunningham FE, Good CB, et al. Pharmacy use in the first year of the Veterans Choice Program: a mixed-methods evaluation. Med Care. 2017(7 suppl 1);55:S26. doi:10.1097/MLR.0000000000000661
2. Mattocks KM, Yehia B. Evaluating the veterans choice program: lessons for developing a high-performing integrated network. Med Care. 2017(7 suppl 1);55:S1-S3. doi:10.1097/MLR.0000000000000743.
3. Mattocks KM, Mengeling M, Sadler A, Baldor R, Bastian L. The Veterans Choice Act: a qualitative examination of rapid policy implementation in the Department of Veterans Affairs. Med Care. 2017;55(7 suppl 1):S71-S75. doi:10.1097/MLR.0000000000000667
4. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1108.08: VHA formulary management process. November 2, 2016. Accessed June 9, 2022. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3291
5. Massarweh NN, Itani KMF, Morris MS. The VA MISSION act and the future of veterans’ access to quality health care. JAMA. 2020;324:343-344. doi:10.1001/jama.2020.4505
6. Jourdan JP, Muzard A, Goyer I, et al. Impact of pharmacist interventions on clinical outcome and cost avoidance in a university teaching hospital. Int J Clin Pharm. 2018;40(6):1474-1481. doi:10.1007/s11096-018-0733-6
7. Lee AJ, Boro MS, Knapp KK, Meier JL, Korman NE. Clinical and economic outcomes of pharmacist recommendations in a Veterans Affairs medical center. Am J Health Syst Pharm. 2002;59(21):2070-2077. doi:10.1093/ajhp/59.21.2070
8. Dalton K, Byrne S. Role of the pharmacist in reducing healthcare costs: current insights. Integr Pharm Res Pract. 2017;6:37-46. doi:10.2147/IPRP.S108047
9. De Rijdt T, Willems L, Simoens S. Economic effects of clinical pharmacy interventions: a literature review. Am J Health Syst Pharm. 2008;65(12):1161-1172. doi:10.2146/ajhp070506
10. Perez A, Doloresco F, Hoffman J, et al. Economic evaluation of clinical pharmacy services: 2001-2005. Pharmacotherapy. 2009;29(1):128. doi:10.1592/phco.29.1.128
11. Nesbit TW, Shermock KM, Bobek MB, et al. Implementation and pharmacoeconomic analysis of a clinical staff pharmacist practice model. Am J Health Syst Pharm. 2001;58(9):784-790. doi:10.1093/ajhp/58.9.784
12. Yang S, Britt RB, Hashem MG, Brown JN. Outcomes of pharmacy-led hepatitis C direct-acting antiviral utilization management at a Veterans Affairs medical center. J Manag Care Pharm. 2017;23(3):364-369. doi:10.18553/jmcp.2017.23.3.364
13. Britt RB, Hashem MG, Bryan WE III, Kothapalli R, Brown JN. Economic outcomes associated with a pharmacist-adjudicated formulary consult service in a Veterans Affairs medical center. J Manag Care Pharm. 2016;22(9):1051-1061. doi:10.18553/jmcp.2016.22.9.1051
14. Jacob S, Britt RB, Bryan WE, Hashem MG, Hale JC, Brown JN. Economic outcomes associated with safety interventions by a pharmacist-adjudicated prior authorization consult service. J Manag Care Pharm. 2019;25(3):411-416. doi:10.18553/jmcp.2019.25.3.411
15. Aspinall SL, Sales MM, Good CB, et al. Pharmacy benefits management in the Veterans Health Administration revisited: a decade of advancements, 2004-2014. J Manag Care Spec Pharm. 2016;22(9):1058-1063. doi:10.18553/jmcp.2016.22.9.1058
16. US Department of Veterans Affairs, Office of the Chief Human Capital Officer. Title 38 Pay Schedules. Updated January 26, 2022. Accessed June 9, 2022. https://www.va.gov/ohrm/pay
Positive phase 3 results for novel schizophrenia drug
The investigational agent xanomeline-trospium (KarXT, Karuna Therapeutics), which combines a muscarinic receptor agonist with an anticholinergic agent, helps improve psychosis symptoms and is not associated with weight gain or sedation in adults with schizophrenia, new research shows.
Top-line results from the phase 3 EMERGENT-2 trial showed a significantly greater reduction from baseline on Positive and Negative Syndrome Scale (PANSS) total scores for those receiving the active drug than for those receiving placebo, meeting its primary endpoint.
and potentially usher in the first new class of medicine for these patients in more than 50 years,” Steve Paul, MD, chief executive officer, president, and chairman of Karuna Therapeutics, said in a press release.
Primary outcome met
About 20%-33% of patients with schizophrenia do not respond to conventional treatments, the company noted. Many have poor functional status and quality of life despite lifelong treatment with current antipsychotic agents.
Unlike current therapies, KarXT doesn’t rely on the dopaminergic or serotonergic pathways. It comprises the muscarinic agonist xanomeline and the muscarinic antagonist trospium and is designed to preferentially stimulate muscarinic receptors in the central nervous system.
Results from a phase 2 trial of almost 200 patients with schizophrenia were published last year in the New England Journal of Medicine. The findings showed that those who received xanomeline-trospium had a significantly greater reduction in psychosis symptoms than those who received placebo.
In the current phase 3 EMERGENT-2 trial, investigators included 252 adults aged 18-65 years who were diagnosed with schizophrenia and were experiencing symptoms of psychosis. Patients were randomly assigned to receive either a flexible dose of xanomeline-trospium or placebo twice daily.
The primary endpoint was change from baseline in the PANSS total score at week 5. Results showed a statistically significant and clinically meaningful 9.6-point reduction in the PANSS total score in participants taking the active drug, compared with those taking placebo (–21.2 vs. –11.6, respectively; P < .0001; Cohen’s d effect size, 0.61).
In addition, there was an early and sustained significant reduction of schizophrenia symptoms, as assessed by the PANSS total score, starting at week 2. This reduction was maintained through all trial timepoints.
Safety profile
The novel drug also met key secondary endpoints. In the active treatment group, there was a significant reduction on the PANSS subscales in both positive symptoms of schizophrenia, such as hallucinations or delusions, and negative symptoms, such as difficulty enjoying life or withdrawal from others.
Overall, the agent was generally well tolerated. The treatment-emergent adverse events (TEAEs) rate for xanomeline-trospium and placebo was 75% versus 58%, respectively.
The most common TEAEs for the active treatment were all mild-to-moderate in severity and included constipation, dyspepsia, nausea, vomiting, headache, increases in blood pressure, dizziness, gastroesophageal reflux disease, abdominal discomfort, and diarrhea.
As in prior trials, an increase in heart rate was also associated with the active treatment and decreased in magnitude by the end of the current study.
Discontinuation rates related to TEAEs were similar between xanomeline-trospium (7%) and placebo (6%), as were rates of serious TEAEs (2% in each group) – which included suicidal ideation, worsening of schizophrenia symptoms, and appendicitis.
Notably, the drug was not associated with common problematic adverse events of current therapies, such as weight gain, sedation, and movement disorders.
Karuna plans to submit a New Drug Application with the U.S. Food and Drug Administration for KarXT in mid-2023. In addition to schizophrenia, the drug is in development for the treatment of other psychiatric and neurological conditions, including Alzheimer’s disease.
A version of this article first appeared on Medscape.com.
The investigational agent xanomeline-trospium (KarXT, Karuna Therapeutics), which combines a muscarinic receptor agonist with an anticholinergic agent, helps improve psychosis symptoms and is not associated with weight gain or sedation in adults with schizophrenia, new research shows.
Top-line results from the phase 3 EMERGENT-2 trial showed a significantly greater reduction from baseline on Positive and Negative Syndrome Scale (PANSS) total scores for those receiving the active drug than for those receiving placebo, meeting its primary endpoint.
and potentially usher in the first new class of medicine for these patients in more than 50 years,” Steve Paul, MD, chief executive officer, president, and chairman of Karuna Therapeutics, said in a press release.
Primary outcome met
About 20%-33% of patients with schizophrenia do not respond to conventional treatments, the company noted. Many have poor functional status and quality of life despite lifelong treatment with current antipsychotic agents.
Unlike current therapies, KarXT doesn’t rely on the dopaminergic or serotonergic pathways. It comprises the muscarinic agonist xanomeline and the muscarinic antagonist trospium and is designed to preferentially stimulate muscarinic receptors in the central nervous system.
Results from a phase 2 trial of almost 200 patients with schizophrenia were published last year in the New England Journal of Medicine. The findings showed that those who received xanomeline-trospium had a significantly greater reduction in psychosis symptoms than those who received placebo.
In the current phase 3 EMERGENT-2 trial, investigators included 252 adults aged 18-65 years who were diagnosed with schizophrenia and were experiencing symptoms of psychosis. Patients were randomly assigned to receive either a flexible dose of xanomeline-trospium or placebo twice daily.
The primary endpoint was change from baseline in the PANSS total score at week 5. Results showed a statistically significant and clinically meaningful 9.6-point reduction in the PANSS total score in participants taking the active drug, compared with those taking placebo (–21.2 vs. –11.6, respectively; P < .0001; Cohen’s d effect size, 0.61).
In addition, there was an early and sustained significant reduction of schizophrenia symptoms, as assessed by the PANSS total score, starting at week 2. This reduction was maintained through all trial timepoints.
Safety profile
The novel drug also met key secondary endpoints. In the active treatment group, there was a significant reduction on the PANSS subscales in both positive symptoms of schizophrenia, such as hallucinations or delusions, and negative symptoms, such as difficulty enjoying life or withdrawal from others.
Overall, the agent was generally well tolerated. The treatment-emergent adverse events (TEAEs) rate for xanomeline-trospium and placebo was 75% versus 58%, respectively.
The most common TEAEs for the active treatment were all mild-to-moderate in severity and included constipation, dyspepsia, nausea, vomiting, headache, increases in blood pressure, dizziness, gastroesophageal reflux disease, abdominal discomfort, and diarrhea.
As in prior trials, an increase in heart rate was also associated with the active treatment and decreased in magnitude by the end of the current study.
Discontinuation rates related to TEAEs were similar between xanomeline-trospium (7%) and placebo (6%), as were rates of serious TEAEs (2% in each group) – which included suicidal ideation, worsening of schizophrenia symptoms, and appendicitis.
Notably, the drug was not associated with common problematic adverse events of current therapies, such as weight gain, sedation, and movement disorders.
Karuna plans to submit a New Drug Application with the U.S. Food and Drug Administration for KarXT in mid-2023. In addition to schizophrenia, the drug is in development for the treatment of other psychiatric and neurological conditions, including Alzheimer’s disease.
A version of this article first appeared on Medscape.com.
The investigational agent xanomeline-trospium (KarXT, Karuna Therapeutics), which combines a muscarinic receptor agonist with an anticholinergic agent, helps improve psychosis symptoms and is not associated with weight gain or sedation in adults with schizophrenia, new research shows.
Top-line results from the phase 3 EMERGENT-2 trial showed a significantly greater reduction from baseline on Positive and Negative Syndrome Scale (PANSS) total scores for those receiving the active drug than for those receiving placebo, meeting its primary endpoint.
and potentially usher in the first new class of medicine for these patients in more than 50 years,” Steve Paul, MD, chief executive officer, president, and chairman of Karuna Therapeutics, said in a press release.
Primary outcome met
About 20%-33% of patients with schizophrenia do not respond to conventional treatments, the company noted. Many have poor functional status and quality of life despite lifelong treatment with current antipsychotic agents.
Unlike current therapies, KarXT doesn’t rely on the dopaminergic or serotonergic pathways. It comprises the muscarinic agonist xanomeline and the muscarinic antagonist trospium and is designed to preferentially stimulate muscarinic receptors in the central nervous system.
Results from a phase 2 trial of almost 200 patients with schizophrenia were published last year in the New England Journal of Medicine. The findings showed that those who received xanomeline-trospium had a significantly greater reduction in psychosis symptoms than those who received placebo.
In the current phase 3 EMERGENT-2 trial, investigators included 252 adults aged 18-65 years who were diagnosed with schizophrenia and were experiencing symptoms of psychosis. Patients were randomly assigned to receive either a flexible dose of xanomeline-trospium or placebo twice daily.
The primary endpoint was change from baseline in the PANSS total score at week 5. Results showed a statistically significant and clinically meaningful 9.6-point reduction in the PANSS total score in participants taking the active drug, compared with those taking placebo (–21.2 vs. –11.6, respectively; P < .0001; Cohen’s d effect size, 0.61).
In addition, there was an early and sustained significant reduction of schizophrenia symptoms, as assessed by the PANSS total score, starting at week 2. This reduction was maintained through all trial timepoints.
Safety profile
The novel drug also met key secondary endpoints. In the active treatment group, there was a significant reduction on the PANSS subscales in both positive symptoms of schizophrenia, such as hallucinations or delusions, and negative symptoms, such as difficulty enjoying life or withdrawal from others.
Overall, the agent was generally well tolerated. The treatment-emergent adverse events (TEAEs) rate for xanomeline-trospium and placebo was 75% versus 58%, respectively.
The most common TEAEs for the active treatment were all mild-to-moderate in severity and included constipation, dyspepsia, nausea, vomiting, headache, increases in blood pressure, dizziness, gastroesophageal reflux disease, abdominal discomfort, and diarrhea.
As in prior trials, an increase in heart rate was also associated with the active treatment and decreased in magnitude by the end of the current study.
Discontinuation rates related to TEAEs were similar between xanomeline-trospium (7%) and placebo (6%), as were rates of serious TEAEs (2% in each group) – which included suicidal ideation, worsening of schizophrenia symptoms, and appendicitis.
Notably, the drug was not associated with common problematic adverse events of current therapies, such as weight gain, sedation, and movement disorders.
Karuna plans to submit a New Drug Application with the U.S. Food and Drug Administration for KarXT in mid-2023. In addition to schizophrenia, the drug is in development for the treatment of other psychiatric and neurological conditions, including Alzheimer’s disease.
A version of this article first appeared on Medscape.com.
FDA authorizes intradermal use of Jynneos vaccine for monkeypox
The Food and Drug Administration on Aug. 9 authorized intradermal administration of the Jynneos vaccine for the treatment of monkeypox. The process, approved specifically for high-risk patients, was passed under the administration’s Emergency Use Authorization. It follows the decision on Aug. 4 by the U.S. Department of Health and Human Services to declare monkeypox a public health emergency. Intradermal administration will allow providers to get five doses out of a one-dose vial.
This news organization will update this article as more information becomes available.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration on Aug. 9 authorized intradermal administration of the Jynneos vaccine for the treatment of monkeypox. The process, approved specifically for high-risk patients, was passed under the administration’s Emergency Use Authorization. It follows the decision on Aug. 4 by the U.S. Department of Health and Human Services to declare monkeypox a public health emergency. Intradermal administration will allow providers to get five doses out of a one-dose vial.
This news organization will update this article as more information becomes available.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration on Aug. 9 authorized intradermal administration of the Jynneos vaccine for the treatment of monkeypox. The process, approved specifically for high-risk patients, was passed under the administration’s Emergency Use Authorization. It follows the decision on Aug. 4 by the U.S. Department of Health and Human Services to declare monkeypox a public health emergency. Intradermal administration will allow providers to get five doses out of a one-dose vial.
This news organization will update this article as more information becomes available.
A version of this article first appeared on Medscape.com.