User login
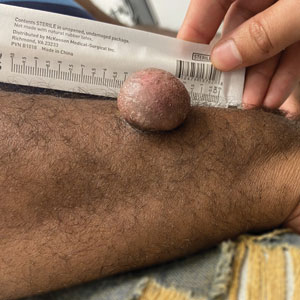
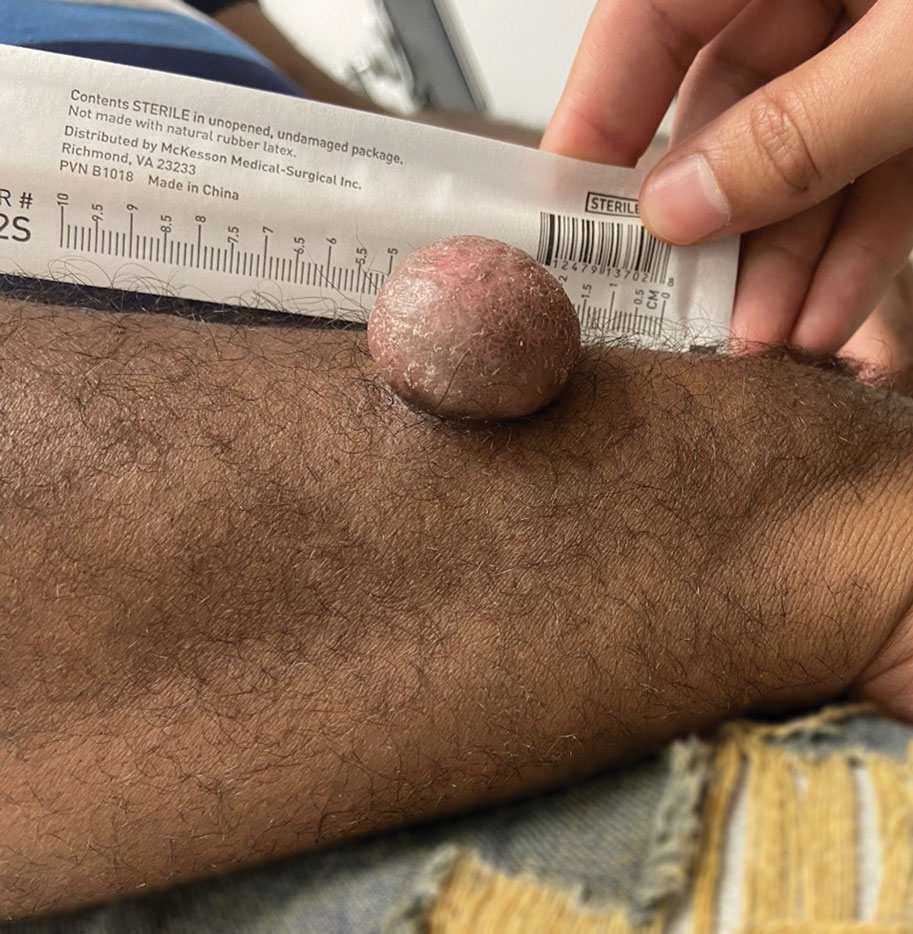
Asymptomatic Soft Tumor on the Forearm
The Diagnosis: Aneurysmal Dermatofibroma
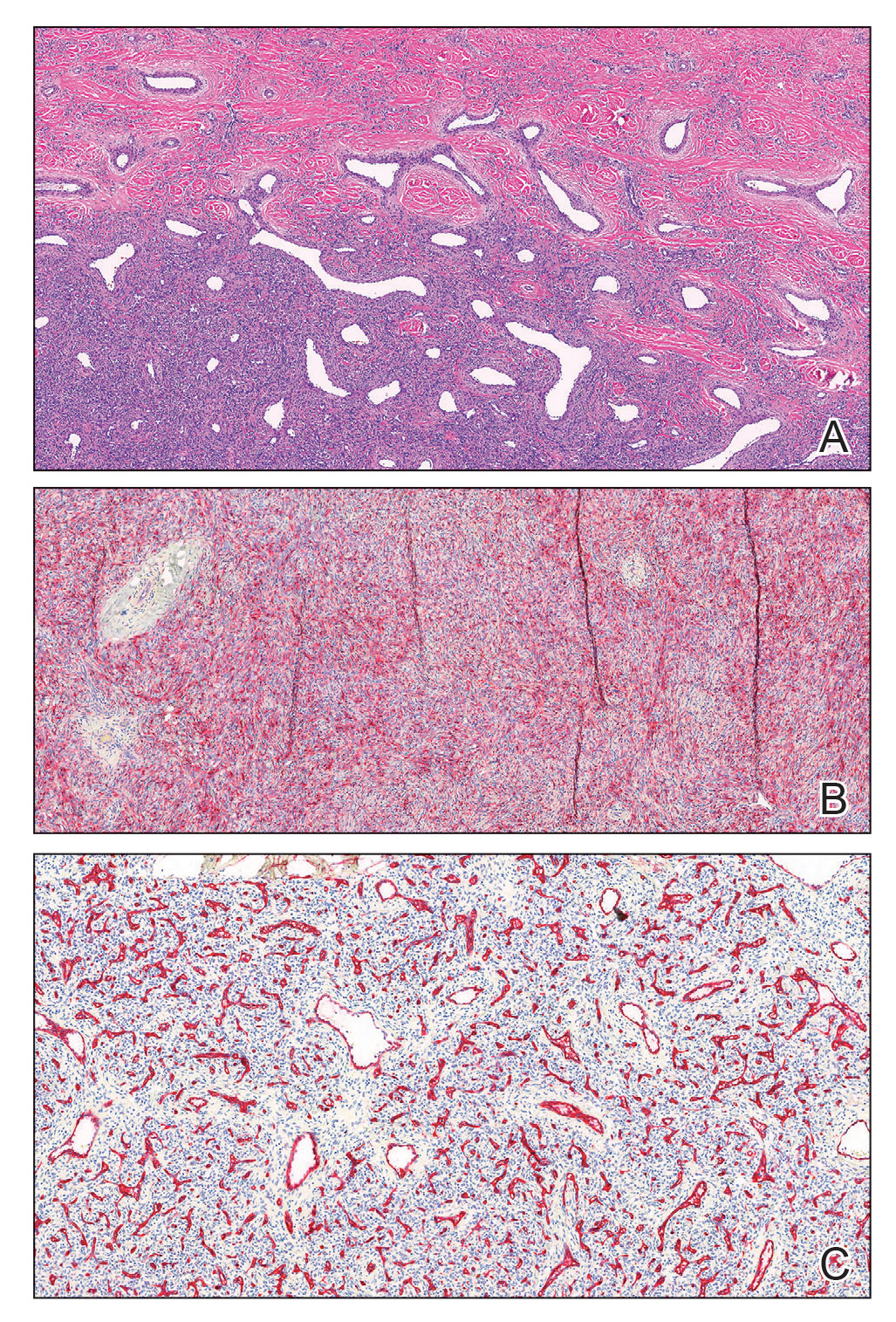
A shave biopsy of the entire tumor was performed at the initial visit. Histologic examination with hematoxylin and eosin staining revealed a fibrohistiocytic infiltrate containing cleftlike cavernous spaces lined by epithelial cells (Figure, A). Immunohistochemical staining revealed factor XIIIa expression on fibrohistiocytic cells (Figure, B). CD34 was expressed on vascular endothelial cells, but it failed to highlight the fibrohistiocytic space (Figure, C). Overall, these findings supported the diagnosis of aneurysmal dermatofibroma. The lesion healed without complications, and the patient was counseled on the risk for recurrence. He was offered localized excision but opted for conservative management without excision and close follow-up and monitoring.
Dermatofibromas are common benign cutaneous nodules that often are asymptomatic and occur on the extremities. Dermatofibromas also are known as cutaneous fibrous histiocytomas and have numerous histologic variants. Aneurysmal dermatofibroma (also called aneurysmal fibrous histiocytoma) is a rare histologic variant of dermatofibroma presenting as a slow-growing exophytic tumor that can be purple, red, brown, or blue. Although classic dermatofibromas typically constitute a straightforward diagnosis, aneurysmal dermatofibromas often are more challenging to clinically differentiate from other cutaneous neoplasms. Additionally, due to the exophytic nature and larger size (0.5–4.0 cm), aneurysmal dermatofibromas do not exhibit the characteristic dimple (Fitzpatrick) sign found in many dermatofibromas. Aneurysmal dermatofibromas are 10 times more likely to recur than classic dermatofibromas.1-4
Aneurysmal dermatofibromas can mimic other cutaneous neoplasms, some indolent and others more aggressive. Similar to aneurysmal dermatofibromas, solitary neurofibromas and nevi lipomatosus can appear as asymptomatic exophytic nodules with a similar spectrum of color and indolent clinical courses. In nevus lipomatosus, the dermis is almost entirely replaced by mature adipose tissue.5 Solitary neurofibromas represent a proliferation of neuromesenchymal cells with haphazardly arranged, wavy nuclei characteristic of nerve cells.6 Dermatofibrosarcoma protuberans can be distinguished from aneurysmal dermatofibroma by lack of factor XIIIa expression and diffuse positivity for CD34.7 Finally, aneurysmal dermatofibromas may resemble vascular tumors such as nodular Kaposi sarcoma. Kaposi sarcoma can be differentiated from an aneurysmal dermatofibroma by the presence of characteristic vascular wrapping, the absence of fibrohistiocytic cells, and expression of human herpesvirus 8 latent nuclear antigen-1.1,8 Although aneurysmal dermatofibromas are of low malignant potential, they are associated with a higher rate of recurrence compared to common dermatofibromas.9 Definitive treatment involves complete excision with follow-up to ensure no signs of recurrence.10 Incomplete excision can increase the likelihood of recurrence, especially for larger aneurysmal dermatofibromas. Aneurysmal dermatofibromas are one of the subtypes of dermatofibromas that may extend into the subcutaneous tissue. Han et al2 found that 77.8% of aneurysmal dermatofibromas extended into subcutaneous tissue. Recognizing the clinical and pathological features of this rare subtype of dermatofibroma can aid dermatologists in appropriate recognition and management.
- Burr DM, Peterson WA, Peterson MW. Aneurysmal fibrous histiocytoma: a case report and review of the literature. J Am Osteopath. June 2018;40. Accessed February 14, 2023. https://cdn.ymaws.com/www.aocd.org/resource/resmgr/jaocd/contents/volume40/40-04.pdf
- Han TY, Chang HS, Lee JHK, et al. A clinical and histopathological study of 122 cases of dermatofibroma (benign fibrous histiocytoma). Ann Dermatol. 2011;23:185-192.
- Morariu SH, Suciu M, Vartolomei MD, et al. Aneurysmal dermatofibroma mimicking both clinical and dermoscopic malignant melanoma and Kaposi’s sarcoma. Rom J Morphol Embryol. 2014;55:1221-1224.
- Calonje E, Fletcher CDM. Aneurysmal benign fibrous histiocytoma: clinicopathological analysis of 40 cases of a tumour frequently misdiagnosed as a vascular neoplasm. Histopathology. 1995;26:323-331.
- Pujani M, Choudhury M, Garg T, et al. Nevus lipomatosus superficialis: a rare cutaneous hamartoma. Indian Dermatol Online J. 2014;5:109-110.
- Strike SA, Puhaindran ME. Nerve tumors of the upper extremity. Clin Plast Surg. 2019;46:347-350.
- Cohen PR, Rapini RP, Farhood AI. Dermatofibroma and dermatofibrosarcoma protuberans: differential expression of CD34 and factor XIIIa. Am J Dermatopathol. 1994;16:573-574.
- Kandal S, Ozmen S, Demir HY, et al. Aneurysmal fibrous histiocytoma of the skin: a rare variant of dermatofibroma. Plast Reconstr Surg. 2005;116:2050-2051.
- Hornick JL. Cutaneous soft tissue tumors: how do we make sense of fibrous and “fibrohistiocytic” tumors with confusing names and similar appearances? Mod Pathol. 2020;33:56-65.
- Das A, Das A, Bandyopadhyay D, et al. Aneurysmal benign fibrous histiocytoma presenting as a giant acrochordon on thigh. Indian Dermatol Online J. 2015;6:436.
The Diagnosis: Aneurysmal Dermatofibroma
A shave biopsy of the entire tumor was performed at the initial visit. Histologic examination with hematoxylin and eosin staining revealed a fibrohistiocytic infiltrate containing cleftlike cavernous spaces lined by epithelial cells (Figure, A). Immunohistochemical staining revealed factor XIIIa expression on fibrohistiocytic cells (Figure, B). CD34 was expressed on vascular endothelial cells, but it failed to highlight the fibrohistiocytic space (Figure, C). Overall, these findings supported the diagnosis of aneurysmal dermatofibroma. The lesion healed without complications, and the patient was counseled on the risk for recurrence. He was offered localized excision but opted for conservative management without excision and close follow-up and monitoring.

Dermatofibromas are common benign cutaneous nodules that often are asymptomatic and occur on the extremities. Dermatofibromas also are known as cutaneous fibrous histiocytomas and have numerous histologic variants. Aneurysmal dermatofibroma (also called aneurysmal fibrous histiocytoma) is a rare histologic variant of dermatofibroma presenting as a slow-growing exophytic tumor that can be purple, red, brown, or blue. Although classic dermatofibromas typically constitute a straightforward diagnosis, aneurysmal dermatofibromas often are more challenging to clinically differentiate from other cutaneous neoplasms. Additionally, due to the exophytic nature and larger size (0.5–4.0 cm), aneurysmal dermatofibromas do not exhibit the characteristic dimple (Fitzpatrick) sign found in many dermatofibromas. Aneurysmal dermatofibromas are 10 times more likely to recur than classic dermatofibromas.1-4
Aneurysmal dermatofibromas can mimic other cutaneous neoplasms, some indolent and others more aggressive. Similar to aneurysmal dermatofibromas, solitary neurofibromas and nevi lipomatosus can appear as asymptomatic exophytic nodules with a similar spectrum of color and indolent clinical courses. In nevus lipomatosus, the dermis is almost entirely replaced by mature adipose tissue.5 Solitary neurofibromas represent a proliferation of neuromesenchymal cells with haphazardly arranged, wavy nuclei characteristic of nerve cells.6 Dermatofibrosarcoma protuberans can be distinguished from aneurysmal dermatofibroma by lack of factor XIIIa expression and diffuse positivity for CD34.7 Finally, aneurysmal dermatofibromas may resemble vascular tumors such as nodular Kaposi sarcoma. Kaposi sarcoma can be differentiated from an aneurysmal dermatofibroma by the presence of characteristic vascular wrapping, the absence of fibrohistiocytic cells, and expression of human herpesvirus 8 latent nuclear antigen-1.1,8 Although aneurysmal dermatofibromas are of low malignant potential, they are associated with a higher rate of recurrence compared to common dermatofibromas.9 Definitive treatment involves complete excision with follow-up to ensure no signs of recurrence.10 Incomplete excision can increase the likelihood of recurrence, especially for larger aneurysmal dermatofibromas. Aneurysmal dermatofibromas are one of the subtypes of dermatofibromas that may extend into the subcutaneous tissue. Han et al2 found that 77.8% of aneurysmal dermatofibromas extended into subcutaneous tissue. Recognizing the clinical and pathological features of this rare subtype of dermatofibroma can aid dermatologists in appropriate recognition and management.
The Diagnosis: Aneurysmal Dermatofibroma
A shave biopsy of the entire tumor was performed at the initial visit. Histologic examination with hematoxylin and eosin staining revealed a fibrohistiocytic infiltrate containing cleftlike cavernous spaces lined by epithelial cells (Figure, A). Immunohistochemical staining revealed factor XIIIa expression on fibrohistiocytic cells (Figure, B). CD34 was expressed on vascular endothelial cells, but it failed to highlight the fibrohistiocytic space (Figure, C). Overall, these findings supported the diagnosis of aneurysmal dermatofibroma. The lesion healed without complications, and the patient was counseled on the risk for recurrence. He was offered localized excision but opted for conservative management without excision and close follow-up and monitoring.

Dermatofibromas are common benign cutaneous nodules that often are asymptomatic and occur on the extremities. Dermatofibromas also are known as cutaneous fibrous histiocytomas and have numerous histologic variants. Aneurysmal dermatofibroma (also called aneurysmal fibrous histiocytoma) is a rare histologic variant of dermatofibroma presenting as a slow-growing exophytic tumor that can be purple, red, brown, or blue. Although classic dermatofibromas typically constitute a straightforward diagnosis, aneurysmal dermatofibromas often are more challenging to clinically differentiate from other cutaneous neoplasms. Additionally, due to the exophytic nature and larger size (0.5–4.0 cm), aneurysmal dermatofibromas do not exhibit the characteristic dimple (Fitzpatrick) sign found in many dermatofibromas. Aneurysmal dermatofibromas are 10 times more likely to recur than classic dermatofibromas.1-4
Aneurysmal dermatofibromas can mimic other cutaneous neoplasms, some indolent and others more aggressive. Similar to aneurysmal dermatofibromas, solitary neurofibromas and nevi lipomatosus can appear as asymptomatic exophytic nodules with a similar spectrum of color and indolent clinical courses. In nevus lipomatosus, the dermis is almost entirely replaced by mature adipose tissue.5 Solitary neurofibromas represent a proliferation of neuromesenchymal cells with haphazardly arranged, wavy nuclei characteristic of nerve cells.6 Dermatofibrosarcoma protuberans can be distinguished from aneurysmal dermatofibroma by lack of factor XIIIa expression and diffuse positivity for CD34.7 Finally, aneurysmal dermatofibromas may resemble vascular tumors such as nodular Kaposi sarcoma. Kaposi sarcoma can be differentiated from an aneurysmal dermatofibroma by the presence of characteristic vascular wrapping, the absence of fibrohistiocytic cells, and expression of human herpesvirus 8 latent nuclear antigen-1.1,8 Although aneurysmal dermatofibromas are of low malignant potential, they are associated with a higher rate of recurrence compared to common dermatofibromas.9 Definitive treatment involves complete excision with follow-up to ensure no signs of recurrence.10 Incomplete excision can increase the likelihood of recurrence, especially for larger aneurysmal dermatofibromas. Aneurysmal dermatofibromas are one of the subtypes of dermatofibromas that may extend into the subcutaneous tissue. Han et al2 found that 77.8% of aneurysmal dermatofibromas extended into subcutaneous tissue. Recognizing the clinical and pathological features of this rare subtype of dermatofibroma can aid dermatologists in appropriate recognition and management.
- Burr DM, Peterson WA, Peterson MW. Aneurysmal fibrous histiocytoma: a case report and review of the literature. J Am Osteopath. June 2018;40. Accessed February 14, 2023. https://cdn.ymaws.com/www.aocd.org/resource/resmgr/jaocd/contents/volume40/40-04.pdf
- Han TY, Chang HS, Lee JHK, et al. A clinical and histopathological study of 122 cases of dermatofibroma (benign fibrous histiocytoma). Ann Dermatol. 2011;23:185-192.
- Morariu SH, Suciu M, Vartolomei MD, et al. Aneurysmal dermatofibroma mimicking both clinical and dermoscopic malignant melanoma and Kaposi’s sarcoma. Rom J Morphol Embryol. 2014;55:1221-1224.
- Calonje E, Fletcher CDM. Aneurysmal benign fibrous histiocytoma: clinicopathological analysis of 40 cases of a tumour frequently misdiagnosed as a vascular neoplasm. Histopathology. 1995;26:323-331.
- Pujani M, Choudhury M, Garg T, et al. Nevus lipomatosus superficialis: a rare cutaneous hamartoma. Indian Dermatol Online J. 2014;5:109-110.
- Strike SA, Puhaindran ME. Nerve tumors of the upper extremity. Clin Plast Surg. 2019;46:347-350.
- Cohen PR, Rapini RP, Farhood AI. Dermatofibroma and dermatofibrosarcoma protuberans: differential expression of CD34 and factor XIIIa. Am J Dermatopathol. 1994;16:573-574.
- Kandal S, Ozmen S, Demir HY, et al. Aneurysmal fibrous histiocytoma of the skin: a rare variant of dermatofibroma. Plast Reconstr Surg. 2005;116:2050-2051.
- Hornick JL. Cutaneous soft tissue tumors: how do we make sense of fibrous and “fibrohistiocytic” tumors with confusing names and similar appearances? Mod Pathol. 2020;33:56-65.
- Das A, Das A, Bandyopadhyay D, et al. Aneurysmal benign fibrous histiocytoma presenting as a giant acrochordon on thigh. Indian Dermatol Online J. 2015;6:436.
- Burr DM, Peterson WA, Peterson MW. Aneurysmal fibrous histiocytoma: a case report and review of the literature. J Am Osteopath. June 2018;40. Accessed February 14, 2023. https://cdn.ymaws.com/www.aocd.org/resource/resmgr/jaocd/contents/volume40/40-04.pdf
- Han TY, Chang HS, Lee JHK, et al. A clinical and histopathological study of 122 cases of dermatofibroma (benign fibrous histiocytoma). Ann Dermatol. 2011;23:185-192.
- Morariu SH, Suciu M, Vartolomei MD, et al. Aneurysmal dermatofibroma mimicking both clinical and dermoscopic malignant melanoma and Kaposi’s sarcoma. Rom J Morphol Embryol. 2014;55:1221-1224.
- Calonje E, Fletcher CDM. Aneurysmal benign fibrous histiocytoma: clinicopathological analysis of 40 cases of a tumour frequently misdiagnosed as a vascular neoplasm. Histopathology. 1995;26:323-331.
- Pujani M, Choudhury M, Garg T, et al. Nevus lipomatosus superficialis: a rare cutaneous hamartoma. Indian Dermatol Online J. 2014;5:109-110.
- Strike SA, Puhaindran ME. Nerve tumors of the upper extremity. Clin Plast Surg. 2019;46:347-350.
- Cohen PR, Rapini RP, Farhood AI. Dermatofibroma and dermatofibrosarcoma protuberans: differential expression of CD34 and factor XIIIa. Am J Dermatopathol. 1994;16:573-574.
- Kandal S, Ozmen S, Demir HY, et al. Aneurysmal fibrous histiocytoma of the skin: a rare variant of dermatofibroma. Plast Reconstr Surg. 2005;116:2050-2051.
- Hornick JL. Cutaneous soft tissue tumors: how do we make sense of fibrous and “fibrohistiocytic” tumors with confusing names and similar appearances? Mod Pathol. 2020;33:56-65.
- Das A, Das A, Bandyopadhyay D, et al. Aneurysmal benign fibrous histiocytoma presenting as a giant acrochordon on thigh. Indian Dermatol Online J. 2015;6:436.
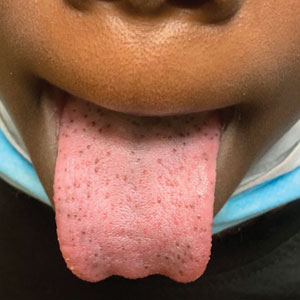
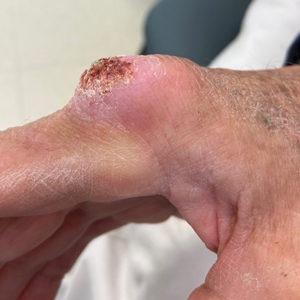
A 43-year-old Black man with no notable medical history presented to our clinic with a progressively enlarging tumor on the right forearm of 12 months’ duration. Despite its progressive growth, the tumor was asymptomatic. Physical examination of the right forearm revealed a 3.7×3.0-cm, well-circumscribed, exophytic tumor with a mildly erythematous hue, scaly surface, and rubbery consistency. There was no surrounding erythema, edema, localized lymphadenopathy, or concurrent lymphedema.
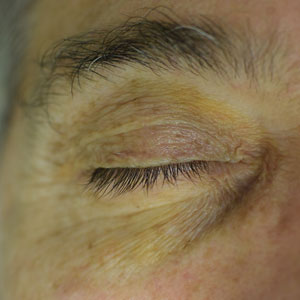
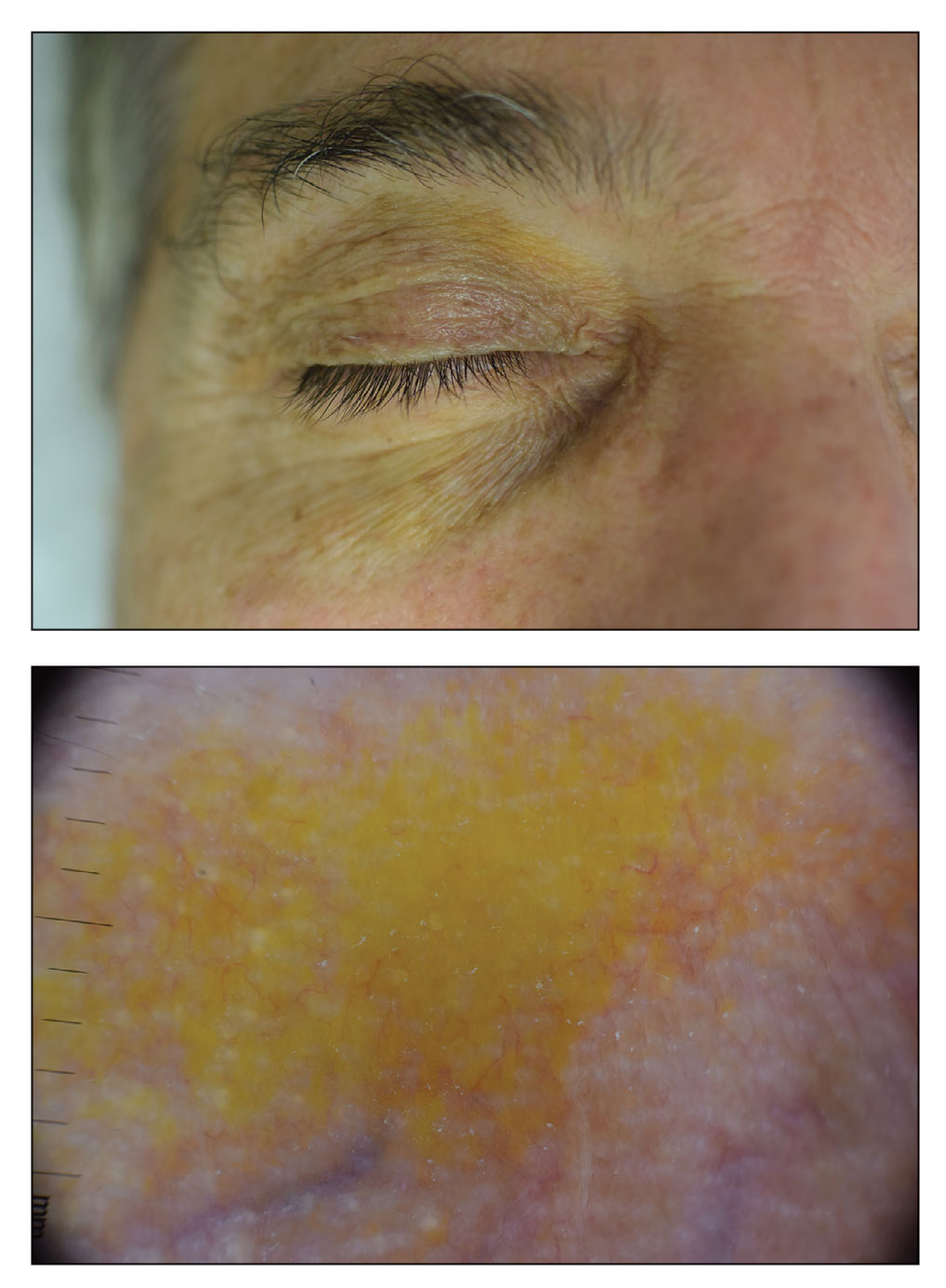
Periorbital Orange Spots
The Diagnosis: Orange Palpebral Spots
The clinical presentation of our patient was consistent with a diagnosis of orange palpebral spots (OPSs), an uncommon discoloration that most often appears in White patients in the fifth or sixth decades of life. Orange palpebral spots were first described in 2008 by Assouly et al1 in 27 patients (23 females and 4 males). In 2015, Belliveau et al2 expanded the designation to yellow-orange palpebral spots because they felt the term more fully expressed the color variations depicted in their patients; however, this term more frequently is used in ophthalmology.
Orange palpebral spots commonly appear as asymptomatic, yellow-orange, symmetric lesions with a predilection for the recessed areas of the superior eyelids but also can present on the canthi and inferior eyelids. The discolorations are more easily visible on fair skin and have been reported to measure from 10 to 15 mm in the long axis.3 Assouly et al1 described the orange spots as having indistinct margins, with borders similar to “sand on a sea shore.” Orange palpebral spots can be a persistent discoloration, and there are no reports of spontaneous regression. No known association with malignancy or systemic illness has been reported.
Case reports of OPSs describe histologic similarities between specimens, including increased adipose tissue and pigment-laden macrophages in the superficial dermis.2 The pigmented deposits sometimes may be found in the basal keratinocytes of the epidermis and turn black with Fontana-Masson stain.1 No inflammatory infiltrates, necrosis, or xanthomization are characteristically found. Stains for iron, mucin, and amyloid also have been negative.2
The cause of pigmentation in OPSs is unknown; however, lipofuscin deposits and high-situated adipocytes in the reticular dermis colored by carotenoids have been proposed as possible mechanisms.1 No unifying cause for pigmentation in the serum (eg, cholesterol, triglycerides, thyroid-stimulating hormone, free retinol, vitamin E, carotenoids) was found in 11 of 27 patients with OPSs assessed by Assouly et al.1 In one case, lipofuscin, a degradation product of lysosomes, was detected by microscopic autofluorescence in the superficial dermis. However, lipofuscin typically is a breakdown product associated with aging, and OPSs have been present in patients as young as 28 years.1 Local trauma related to eye rubbing is another theory that has been proposed due to the finding of melanin in the superficial dermis. However, the absence of hemosiderin deposits as well as the extensive duration of the discolorations makes local trauma a less likely explanation for the etiology of OPSs.2
The clinical differential diagnosis for OPSs includes xanthelasma, jaundice, and carotenoderma. Xanthelasma presents as elevated yellow plaques usually found over the medial aspect of the eyes. In contrast, OPSs are nonelevated with both orange and yellow hues typically present. Histologic samples of xanthelasma are characterized by lipid-laden macrophages (foam cells) in the dermis in contrast to the adipose tissue seen in OPSs that has not been phagocytized.1,2 The lack of scleral icterus made jaundice an unlikely diagnosis in our patient. Bilirubin elevations substantial enough to cause skin discoloration also would be expected to discolor the conjunctiva. In carotenoderma, carotenoids are deposited in the sweat and sebum of the stratum corneum with the orange pigmentation most prominent in regions of increased sweating such as the palms, soles, and nasolabial folds.4 Our patient’s lack of discoloration in places other than the periorbital region made carotenoderma less likely.
In the study by Assouly et al,1 10 of 11 patients who underwent laboratory analysis self-reported eating a diet rich in fruit and vegetables, though no standardized questionnaire was given. One patient was found to have an elevated vitamin E level, and in 5 cases there was an elevated level of β-cryptoxanthin. The significance of these elevations in such a small minority is unknown, and increased β-cryptoxanthin has been attributed to increased consumption of citrus fruits during the winter season. Our patient reported ingesting a daily oral supplement rich in carotenoids that constituted 60% of the daily value of vitamin E including mixed tocopherols as well as 90% of the daily value of vitamin A with many sources of carotenoids including beta-carotenes, lutein/zeaxanthin, lycopene, and astaxanthin. An invasive biopsy was not taken in this case, as OPSs largely are diagnosed clinically. Greater awareness and recognition of OPSs may help to identify common underlying causes for this unique diagnosis.
- Assouly P, Cavelier-Balloy B, Dupré T. Orange palpebral spots. Dermatology. 2008;216:166-170.
- Belliveau MJ, Odashiro AN, Harvey JT. Yellow-orange palpebral spots. Ophthalmology. 2015;122:2139-2140.
- Kluger N, Guillot B. Bilateral orange discoloration of the upper eyelids: a quiz. Acta Derm Venereol. 2011;91:211-212.
- Maharshak N, Shapiro J, Trau H. Carotenoderma—a review of the current literature. Int J Dermatol. 2003;42:178-181.
The Diagnosis: Orange Palpebral Spots
The clinical presentation of our patient was consistent with a diagnosis of orange palpebral spots (OPSs), an uncommon discoloration that most often appears in White patients in the fifth or sixth decades of life. Orange palpebral spots were first described in 2008 by Assouly et al1 in 27 patients (23 females and 4 males). In 2015, Belliveau et al2 expanded the designation to yellow-orange palpebral spots because they felt the term more fully expressed the color variations depicted in their patients; however, this term more frequently is used in ophthalmology.
Orange palpebral spots commonly appear as asymptomatic, yellow-orange, symmetric lesions with a predilection for the recessed areas of the superior eyelids but also can present on the canthi and inferior eyelids. The discolorations are more easily visible on fair skin and have been reported to measure from 10 to 15 mm in the long axis.3 Assouly et al1 described the orange spots as having indistinct margins, with borders similar to “sand on a sea shore.” Orange palpebral spots can be a persistent discoloration, and there are no reports of spontaneous regression. No known association with malignancy or systemic illness has been reported.
Case reports of OPSs describe histologic similarities between specimens, including increased adipose tissue and pigment-laden macrophages in the superficial dermis.2 The pigmented deposits sometimes may be found in the basal keratinocytes of the epidermis and turn black with Fontana-Masson stain.1 No inflammatory infiltrates, necrosis, or xanthomization are characteristically found. Stains for iron, mucin, and amyloid also have been negative.2
The cause of pigmentation in OPSs is unknown; however, lipofuscin deposits and high-situated adipocytes in the reticular dermis colored by carotenoids have been proposed as possible mechanisms.1 No unifying cause for pigmentation in the serum (eg, cholesterol, triglycerides, thyroid-stimulating hormone, free retinol, vitamin E, carotenoids) was found in 11 of 27 patients with OPSs assessed by Assouly et al.1 In one case, lipofuscin, a degradation product of lysosomes, was detected by microscopic autofluorescence in the superficial dermis. However, lipofuscin typically is a breakdown product associated with aging, and OPSs have been present in patients as young as 28 years.1 Local trauma related to eye rubbing is another theory that has been proposed due to the finding of melanin in the superficial dermis. However, the absence of hemosiderin deposits as well as the extensive duration of the discolorations makes local trauma a less likely explanation for the etiology of OPSs.2
The clinical differential diagnosis for OPSs includes xanthelasma, jaundice, and carotenoderma. Xanthelasma presents as elevated yellow plaques usually found over the medial aspect of the eyes. In contrast, OPSs are nonelevated with both orange and yellow hues typically present. Histologic samples of xanthelasma are characterized by lipid-laden macrophages (foam cells) in the dermis in contrast to the adipose tissue seen in OPSs that has not been phagocytized.1,2 The lack of scleral icterus made jaundice an unlikely diagnosis in our patient. Bilirubin elevations substantial enough to cause skin discoloration also would be expected to discolor the conjunctiva. In carotenoderma, carotenoids are deposited in the sweat and sebum of the stratum corneum with the orange pigmentation most prominent in regions of increased sweating such as the palms, soles, and nasolabial folds.4 Our patient’s lack of discoloration in places other than the periorbital region made carotenoderma less likely.
In the study by Assouly et al,1 10 of 11 patients who underwent laboratory analysis self-reported eating a diet rich in fruit and vegetables, though no standardized questionnaire was given. One patient was found to have an elevated vitamin E level, and in 5 cases there was an elevated level of β-cryptoxanthin. The significance of these elevations in such a small minority is unknown, and increased β-cryptoxanthin has been attributed to increased consumption of citrus fruits during the winter season. Our patient reported ingesting a daily oral supplement rich in carotenoids that constituted 60% of the daily value of vitamin E including mixed tocopherols as well as 90% of the daily value of vitamin A with many sources of carotenoids including beta-carotenes, lutein/zeaxanthin, lycopene, and astaxanthin. An invasive biopsy was not taken in this case, as OPSs largely are diagnosed clinically. Greater awareness and recognition of OPSs may help to identify common underlying causes for this unique diagnosis.
The Diagnosis: Orange Palpebral Spots
The clinical presentation of our patient was consistent with a diagnosis of orange palpebral spots (OPSs), an uncommon discoloration that most often appears in White patients in the fifth or sixth decades of life. Orange palpebral spots were first described in 2008 by Assouly et al1 in 27 patients (23 females and 4 males). In 2015, Belliveau et al2 expanded the designation to yellow-orange palpebral spots because they felt the term more fully expressed the color variations depicted in their patients; however, this term more frequently is used in ophthalmology.
Orange palpebral spots commonly appear as asymptomatic, yellow-orange, symmetric lesions with a predilection for the recessed areas of the superior eyelids but also can present on the canthi and inferior eyelids. The discolorations are more easily visible on fair skin and have been reported to measure from 10 to 15 mm in the long axis.3 Assouly et al1 described the orange spots as having indistinct margins, with borders similar to “sand on a sea shore.” Orange palpebral spots can be a persistent discoloration, and there are no reports of spontaneous regression. No known association with malignancy or systemic illness has been reported.
Case reports of OPSs describe histologic similarities between specimens, including increased adipose tissue and pigment-laden macrophages in the superficial dermis.2 The pigmented deposits sometimes may be found in the basal keratinocytes of the epidermis and turn black with Fontana-Masson stain.1 No inflammatory infiltrates, necrosis, or xanthomization are characteristically found. Stains for iron, mucin, and amyloid also have been negative.2
The cause of pigmentation in OPSs is unknown; however, lipofuscin deposits and high-situated adipocytes in the reticular dermis colored by carotenoids have been proposed as possible mechanisms.1 No unifying cause for pigmentation in the serum (eg, cholesterol, triglycerides, thyroid-stimulating hormone, free retinol, vitamin E, carotenoids) was found in 11 of 27 patients with OPSs assessed by Assouly et al.1 In one case, lipofuscin, a degradation product of lysosomes, was detected by microscopic autofluorescence in the superficial dermis. However, lipofuscin typically is a breakdown product associated with aging, and OPSs have been present in patients as young as 28 years.1 Local trauma related to eye rubbing is another theory that has been proposed due to the finding of melanin in the superficial dermis. However, the absence of hemosiderin deposits as well as the extensive duration of the discolorations makes local trauma a less likely explanation for the etiology of OPSs.2
The clinical differential diagnosis for OPSs includes xanthelasma, jaundice, and carotenoderma. Xanthelasma presents as elevated yellow plaques usually found over the medial aspect of the eyes. In contrast, OPSs are nonelevated with both orange and yellow hues typically present. Histologic samples of xanthelasma are characterized by lipid-laden macrophages (foam cells) in the dermis in contrast to the adipose tissue seen in OPSs that has not been phagocytized.1,2 The lack of scleral icterus made jaundice an unlikely diagnosis in our patient. Bilirubin elevations substantial enough to cause skin discoloration also would be expected to discolor the conjunctiva. In carotenoderma, carotenoids are deposited in the sweat and sebum of the stratum corneum with the orange pigmentation most prominent in regions of increased sweating such as the palms, soles, and nasolabial folds.4 Our patient’s lack of discoloration in places other than the periorbital region made carotenoderma less likely.
In the study by Assouly et al,1 10 of 11 patients who underwent laboratory analysis self-reported eating a diet rich in fruit and vegetables, though no standardized questionnaire was given. One patient was found to have an elevated vitamin E level, and in 5 cases there was an elevated level of β-cryptoxanthin. The significance of these elevations in such a small minority is unknown, and increased β-cryptoxanthin has been attributed to increased consumption of citrus fruits during the winter season. Our patient reported ingesting a daily oral supplement rich in carotenoids that constituted 60% of the daily value of vitamin E including mixed tocopherols as well as 90% of the daily value of vitamin A with many sources of carotenoids including beta-carotenes, lutein/zeaxanthin, lycopene, and astaxanthin. An invasive biopsy was not taken in this case, as OPSs largely are diagnosed clinically. Greater awareness and recognition of OPSs may help to identify common underlying causes for this unique diagnosis.
- Assouly P, Cavelier-Balloy B, Dupré T. Orange palpebral spots. Dermatology. 2008;216:166-170.
- Belliveau MJ, Odashiro AN, Harvey JT. Yellow-orange palpebral spots. Ophthalmology. 2015;122:2139-2140.
- Kluger N, Guillot B. Bilateral orange discoloration of the upper eyelids: a quiz. Acta Derm Venereol. 2011;91:211-212.
- Maharshak N, Shapiro J, Trau H. Carotenoderma—a review of the current literature. Int J Dermatol. 2003;42:178-181.
- Assouly P, Cavelier-Balloy B, Dupré T. Orange palpebral spots. Dermatology. 2008;216:166-170.
- Belliveau MJ, Odashiro AN, Harvey JT. Yellow-orange palpebral spots. Ophthalmology. 2015;122:2139-2140.
- Kluger N, Guillot B. Bilateral orange discoloration of the upper eyelids: a quiz. Acta Derm Venereol. 2011;91:211-212.
- Maharshak N, Shapiro J, Trau H. Carotenoderma—a review of the current literature. Int J Dermatol. 2003;42:178-181.
A 63-year-old White man with a history of melanoma presented to our dermatology clinic for evaluation of gradually worsening yellow discoloration around the eyes of 2 years’ duration. Physical examination revealed periorbital yellow-orange patches (top). The discolorations were nonelevated and nonpalpable. Dermoscopy revealed yellow blotches with sparing of the hair follicles (bottom). The remainder of the skin examination was unremarkable.
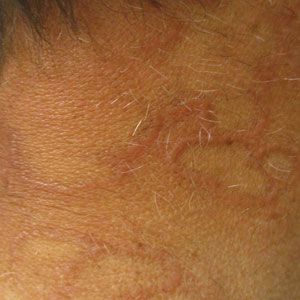
Annular Plaques Overlying Hyperpigmented Telangiectatic Patches on the Neck
The Diagnosis: Annular Elastolytic Giant Cell Granuloma
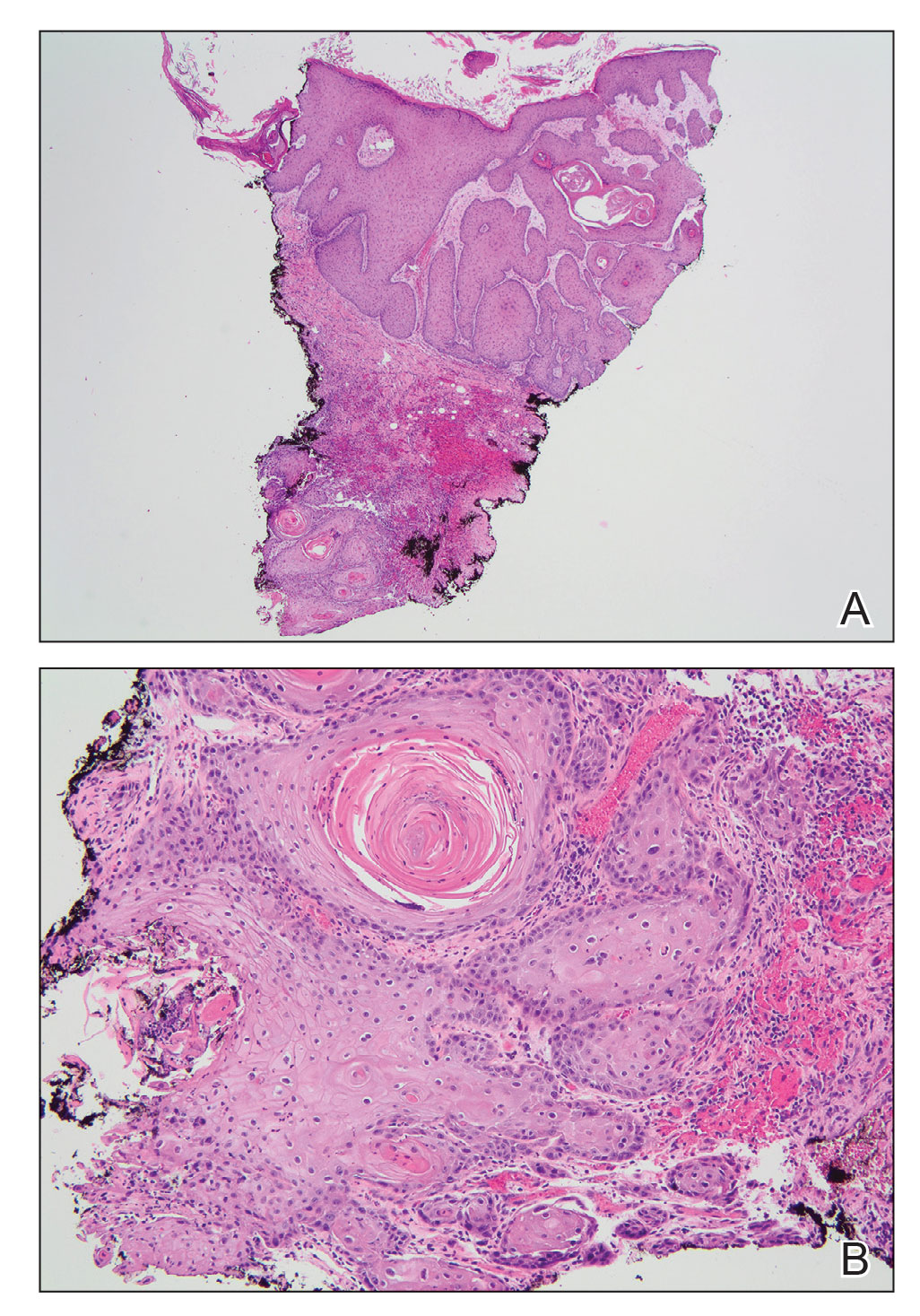
Histologic examination of the shave biopsies showed a granulomatous infiltrate of small lymphocytes, histiocytes, and multinucleated giant cells. The giant cells have abundant eosinophilic cytoplasm, with several also containing fragments of basophilic elastic fibers (elastophagocytosis)(Figure). Additionally, the granulomas revealed no signs of necrosis, making an infectious source unlikely, and examination under polarized light was negative for foreign material. These clinical and histologic findings were diagnostic for annular elastolytic giant cell granuloma (AEGCG).

Annular elastolytic giant cell granuloma is a rare chronic inflammatory disorder that classically presents on sun-exposed skin as annular plaques with elevated borders and atrophic centers.1-4 Histologically, AEGCG is characterized by diffuse granulomatous infiltrates composed of multinucleated giant cells, histiocytes, and lymphocytes in the dermis, along with phagocytosis of elastic fibers by multinucleated giant cells.5 The underlying etiology and pathogenesis of AEGCG remains unknown.6
Annular elastolytic giant cell granuloma commonly affects females aged 35 to 75 years; however, cases have been reported in the male and pediatric patient populations.1,2 Documented cases are known to last from 1 month to 10 years.7,8 Although the mechanisms underlying the development of AEGCG remain to be elucidated, studies have determined that the skin disorder is associated with sarcoidosis, molluscum contagiosum, amyloidosis, diabetes mellitus, and cutaneous T-cell lymphoma.9 Diabetes mellitus is the most common comorbidity associated with AEGCG, and it is theorized that diabetes contributes to the increased incidence of AEGCG in this population by inducing damage to elastic fibers in the skin.10 One study that examined 50 cases of AEGCG found that 38 patients had serum glucose levels evaluated, with 8 cases being subsequently diagnosed with diabetes mellitus and 6 cases with apparent impaired glucose tolerance, indicating that 37% of the sample population with AEGCG who were evaluated for metabolic disease were found to have definitive or latent type 2 diabetes mellitus.11 Although AEGCG is a rare disorder, a substantial number of patients diagnosed with AEGCG also have diabetes mellitus, making it important to consider screening all patients with AEGCG for diabetes given the ease and widely available resources to check glucose levels.
Actinic granuloma, granuloma annulare, atypical facial necrobiosis lipoidica, granuloma multiforme, secondary syphilis, tinea corporis, and erythema annulare centrifugum most commonly are included in the differential diagnosis with AEGCG; histopathology is the key determinant in discerning between these conditions.12 Our patient presented with typical annular plaques overlying hyperpigmented telangiectatic patches. With known type 2 diabetes mellitus and the clinical findings, granuloma annulare, erythema annulare centrifugum, and AEGCG remained high on the differential.
No standard of care exists for AEGCG due to its rare nature and tendency to spontaneously resolve. The most common first-line treatment includes topical and intralesional steroids, topical pimecrolimus, and the use of sunscreen and other sun-protective measures. UV radiation, specifically UVA, has been determined to be a causal factor for AEGCG by changing the antigenicity of elastic fibers and producing an immune response in individuals with fair skin.13 Further, resistant cases of AEGCG successfully have been treated with cyclosporine, systemic steroids, antimalarials, dapsone, and oral retinoids.14,15 Some studies reported partial regression or full resolution with topical tretinoin; adalimumab; clobetasol ointment; or a combination of corticosteroids, antihistamines, and hydroxychloroquine.2 Only 1 case series using sulfasalazine reported worsening symptoms after treatment initiation.16 Our patient deferred systemic medications and was treated with 4 weeks of topical triamcinolone followed by 4 weeks of topical tacrolimus with minimal improvement. At the time of diagnosis, our patient also was encouraged to use sun-protective measures. At 6-month follow-up, the lesions remained stable, and the decision was made to continue with photoprotection.
- Mistry AM, Patel R, Mistry M, et al. Annular elastolytic giant cell granuloma. Cureus. 2020;12:E11456. doi:10.7759/cureus.11456
- Chen WT, Hsiao PF, Wu YH. Spectrum and clinical variants of giant cell elastolytic granuloma. Int J Dermatol. 2017;56:738-745. doi:10.1111/ijd.13502
- Raposo I, Mota F, Lobo I, et al. Annular elastolytic giant cell granuloma: a “visible” diagnosis. Dermatol Online J. 2017;23:13030/qt9rq3j927
- Klemke CD, Siebold D, Dippel E, et al. Generalised annular elastolytic giant cell granuloma. Dermatology. 2003;207:420-422. doi:10.1159/000074132
- Hassan R, Arunprasath P, Padmavathy L, et al. Annular elastolytic giant cell granuloma in association with Hashimoto’s thyroiditis. Indian Dermatol Online J. 2016;7:107-110. doi:10.4103/2229-5178.178087
- Kaya Erdog˘ an H, Arık D, Acer E, et al. Clinicopathological features of annular elastolytic giant cell granuloma patients. Turkish J Dermatol. 2018;12:85-89.
- Can B, Kavala M, Türkog˘ lu Z, et al. Successful treatment of annular elastolytic giant cell granuloma with hydroxychloroquine. Int J Dermatol. 2013;52:509-511. doi:10.1111 /j.1365-4632.2011.04941.x
- Arora S, Malik A, Patil C, et al. Annular elastolytic giant cell granuloma: a report of 10 cases. Indian Dermatol Online J. 2015;6(suppl 1):S17-S20. doi:10.4103/2229-5178.171055
- Doulaveri G, Tsagroni E, Giannadaki M, et al. Annular elastolytic giant cell granuloma in a 70-year-old woman. Int J Dermatol. 2003;42:290-291. doi:10.1046/j.1365-4362.2003.01767.x
- Marmon S, O’Reilly KE, Fischer M, et al. Papular variant of annular elastolytic giant-cell granuloma. Dermatol Online J. 2012;18:23.
- Aso Y, Izaki S, Teraki Y. Annular elastolytic giant cell granuloma associated with diabetes mellitus: a case report and review of the Japanese literature. Clin Exp Dermatol. 2011;36:917-919. doi:10.1111 /j.1365-2230.2011.04094.x
- Liu X, Zhang W, Liu Y, et al. A case of annular elastolytic giant cell granuloma associated with syphilis. Case Rep Dermatol. 2018; 10:158-161. doi:10.1159/000489910
- Gutiérrez-González E, Pereiro M Jr, Toribio J. Elastolytic actinic giant cell granuloma. Dermatol Clin. 2015;33:331-341. doi:10.1016/j.det.2015.03.002
- de Oliveira FL, de Barros Silveira LK, Machado Ade M, et al. Hybrid clinical and histopathological pattern in annular lesions: an overlap between annular elastolytic giant cell granuloma and granuloma annulare? Case Rep Dermatol Med. 2012;2012:102915. doi:10.1155/2012/102915
- Wagenseller A, Larocca C, Vashi NA. Treatment of annular elastolytic giant cell granuloma with topical tretinoin. J Drugs Dermatol. 2017;16:699-700.
- Yang YW, Lehrer MD, Mangold AR, et al. Treatment of granuloma annulare and related granulomatous diseases with sulphasalazine: a series of 16 cases. J Eur Acad Dermatol Venereol. 2021;35:211-215. doi:10.1111/jdv.16356
The Diagnosis: Annular Elastolytic Giant Cell Granuloma
Histologic examination of the shave biopsies showed a granulomatous infiltrate of small lymphocytes, histiocytes, and multinucleated giant cells. The giant cells have abundant eosinophilic cytoplasm, with several also containing fragments of basophilic elastic fibers (elastophagocytosis)(Figure). Additionally, the granulomas revealed no signs of necrosis, making an infectious source unlikely, and examination under polarized light was negative for foreign material. These clinical and histologic findings were diagnostic for annular elastolytic giant cell granuloma (AEGCG).

Annular elastolytic giant cell granuloma is a rare chronic inflammatory disorder that classically presents on sun-exposed skin as annular plaques with elevated borders and atrophic centers.1-4 Histologically, AEGCG is characterized by diffuse granulomatous infiltrates composed of multinucleated giant cells, histiocytes, and lymphocytes in the dermis, along with phagocytosis of elastic fibers by multinucleated giant cells.5 The underlying etiology and pathogenesis of AEGCG remains unknown.6
Annular elastolytic giant cell granuloma commonly affects females aged 35 to 75 years; however, cases have been reported in the male and pediatric patient populations.1,2 Documented cases are known to last from 1 month to 10 years.7,8 Although the mechanisms underlying the development of AEGCG remain to be elucidated, studies have determined that the skin disorder is associated with sarcoidosis, molluscum contagiosum, amyloidosis, diabetes mellitus, and cutaneous T-cell lymphoma.9 Diabetes mellitus is the most common comorbidity associated with AEGCG, and it is theorized that diabetes contributes to the increased incidence of AEGCG in this population by inducing damage to elastic fibers in the skin.10 One study that examined 50 cases of AEGCG found that 38 patients had serum glucose levels evaluated, with 8 cases being subsequently diagnosed with diabetes mellitus and 6 cases with apparent impaired glucose tolerance, indicating that 37% of the sample population with AEGCG who were evaluated for metabolic disease were found to have definitive or latent type 2 diabetes mellitus.11 Although AEGCG is a rare disorder, a substantial number of patients diagnosed with AEGCG also have diabetes mellitus, making it important to consider screening all patients with AEGCG for diabetes given the ease and widely available resources to check glucose levels.
Actinic granuloma, granuloma annulare, atypical facial necrobiosis lipoidica, granuloma multiforme, secondary syphilis, tinea corporis, and erythema annulare centrifugum most commonly are included in the differential diagnosis with AEGCG; histopathology is the key determinant in discerning between these conditions.12 Our patient presented with typical annular plaques overlying hyperpigmented telangiectatic patches. With known type 2 diabetes mellitus and the clinical findings, granuloma annulare, erythema annulare centrifugum, and AEGCG remained high on the differential.
No standard of care exists for AEGCG due to its rare nature and tendency to spontaneously resolve. The most common first-line treatment includes topical and intralesional steroids, topical pimecrolimus, and the use of sunscreen and other sun-protective measures. UV radiation, specifically UVA, has been determined to be a causal factor for AEGCG by changing the antigenicity of elastic fibers and producing an immune response in individuals with fair skin.13 Further, resistant cases of AEGCG successfully have been treated with cyclosporine, systemic steroids, antimalarials, dapsone, and oral retinoids.14,15 Some studies reported partial regression or full resolution with topical tretinoin; adalimumab; clobetasol ointment; or a combination of corticosteroids, antihistamines, and hydroxychloroquine.2 Only 1 case series using sulfasalazine reported worsening symptoms after treatment initiation.16 Our patient deferred systemic medications and was treated with 4 weeks of topical triamcinolone followed by 4 weeks of topical tacrolimus with minimal improvement. At the time of diagnosis, our patient also was encouraged to use sun-protective measures. At 6-month follow-up, the lesions remained stable, and the decision was made to continue with photoprotection.
The Diagnosis: Annular Elastolytic Giant Cell Granuloma
Histologic examination of the shave biopsies showed a granulomatous infiltrate of small lymphocytes, histiocytes, and multinucleated giant cells. The giant cells have abundant eosinophilic cytoplasm, with several also containing fragments of basophilic elastic fibers (elastophagocytosis)(Figure). Additionally, the granulomas revealed no signs of necrosis, making an infectious source unlikely, and examination under polarized light was negative for foreign material. These clinical and histologic findings were diagnostic for annular elastolytic giant cell granuloma (AEGCG).

Annular elastolytic giant cell granuloma is a rare chronic inflammatory disorder that classically presents on sun-exposed skin as annular plaques with elevated borders and atrophic centers.1-4 Histologically, AEGCG is characterized by diffuse granulomatous infiltrates composed of multinucleated giant cells, histiocytes, and lymphocytes in the dermis, along with phagocytosis of elastic fibers by multinucleated giant cells.5 The underlying etiology and pathogenesis of AEGCG remains unknown.6
Annular elastolytic giant cell granuloma commonly affects females aged 35 to 75 years; however, cases have been reported in the male and pediatric patient populations.1,2 Documented cases are known to last from 1 month to 10 years.7,8 Although the mechanisms underlying the development of AEGCG remain to be elucidated, studies have determined that the skin disorder is associated with sarcoidosis, molluscum contagiosum, amyloidosis, diabetes mellitus, and cutaneous T-cell lymphoma.9 Diabetes mellitus is the most common comorbidity associated with AEGCG, and it is theorized that diabetes contributes to the increased incidence of AEGCG in this population by inducing damage to elastic fibers in the skin.10 One study that examined 50 cases of AEGCG found that 38 patients had serum glucose levels evaluated, with 8 cases being subsequently diagnosed with diabetes mellitus and 6 cases with apparent impaired glucose tolerance, indicating that 37% of the sample population with AEGCG who were evaluated for metabolic disease were found to have definitive or latent type 2 diabetes mellitus.11 Although AEGCG is a rare disorder, a substantial number of patients diagnosed with AEGCG also have diabetes mellitus, making it important to consider screening all patients with AEGCG for diabetes given the ease and widely available resources to check glucose levels.
Actinic granuloma, granuloma annulare, atypical facial necrobiosis lipoidica, granuloma multiforme, secondary syphilis, tinea corporis, and erythema annulare centrifugum most commonly are included in the differential diagnosis with AEGCG; histopathology is the key determinant in discerning between these conditions.12 Our patient presented with typical annular plaques overlying hyperpigmented telangiectatic patches. With known type 2 diabetes mellitus and the clinical findings, granuloma annulare, erythema annulare centrifugum, and AEGCG remained high on the differential.
No standard of care exists for AEGCG due to its rare nature and tendency to spontaneously resolve. The most common first-line treatment includes topical and intralesional steroids, topical pimecrolimus, and the use of sunscreen and other sun-protective measures. UV radiation, specifically UVA, has been determined to be a causal factor for AEGCG by changing the antigenicity of elastic fibers and producing an immune response in individuals with fair skin.13 Further, resistant cases of AEGCG successfully have been treated with cyclosporine, systemic steroids, antimalarials, dapsone, and oral retinoids.14,15 Some studies reported partial regression or full resolution with topical tretinoin; adalimumab; clobetasol ointment; or a combination of corticosteroids, antihistamines, and hydroxychloroquine.2 Only 1 case series using sulfasalazine reported worsening symptoms after treatment initiation.16 Our patient deferred systemic medications and was treated with 4 weeks of topical triamcinolone followed by 4 weeks of topical tacrolimus with minimal improvement. At the time of diagnosis, our patient also was encouraged to use sun-protective measures. At 6-month follow-up, the lesions remained stable, and the decision was made to continue with photoprotection.
- Mistry AM, Patel R, Mistry M, et al. Annular elastolytic giant cell granuloma. Cureus. 2020;12:E11456. doi:10.7759/cureus.11456
- Chen WT, Hsiao PF, Wu YH. Spectrum and clinical variants of giant cell elastolytic granuloma. Int J Dermatol. 2017;56:738-745. doi:10.1111/ijd.13502
- Raposo I, Mota F, Lobo I, et al. Annular elastolytic giant cell granuloma: a “visible” diagnosis. Dermatol Online J. 2017;23:13030/qt9rq3j927
- Klemke CD, Siebold D, Dippel E, et al. Generalised annular elastolytic giant cell granuloma. Dermatology. 2003;207:420-422. doi:10.1159/000074132
- Hassan R, Arunprasath P, Padmavathy L, et al. Annular elastolytic giant cell granuloma in association with Hashimoto’s thyroiditis. Indian Dermatol Online J. 2016;7:107-110. doi:10.4103/2229-5178.178087
- Kaya Erdog˘ an H, Arık D, Acer E, et al. Clinicopathological features of annular elastolytic giant cell granuloma patients. Turkish J Dermatol. 2018;12:85-89.
- Can B, Kavala M, Türkog˘ lu Z, et al. Successful treatment of annular elastolytic giant cell granuloma with hydroxychloroquine. Int J Dermatol. 2013;52:509-511. doi:10.1111 /j.1365-4632.2011.04941.x
- Arora S, Malik A, Patil C, et al. Annular elastolytic giant cell granuloma: a report of 10 cases. Indian Dermatol Online J. 2015;6(suppl 1):S17-S20. doi:10.4103/2229-5178.171055
- Doulaveri G, Tsagroni E, Giannadaki M, et al. Annular elastolytic giant cell granuloma in a 70-year-old woman. Int J Dermatol. 2003;42:290-291. doi:10.1046/j.1365-4362.2003.01767.x
- Marmon S, O’Reilly KE, Fischer M, et al. Papular variant of annular elastolytic giant-cell granuloma. Dermatol Online J. 2012;18:23.
- Aso Y, Izaki S, Teraki Y. Annular elastolytic giant cell granuloma associated with diabetes mellitus: a case report and review of the Japanese literature. Clin Exp Dermatol. 2011;36:917-919. doi:10.1111 /j.1365-2230.2011.04094.x
- Liu X, Zhang W, Liu Y, et al. A case of annular elastolytic giant cell granuloma associated with syphilis. Case Rep Dermatol. 2018; 10:158-161. doi:10.1159/000489910
- Gutiérrez-González E, Pereiro M Jr, Toribio J. Elastolytic actinic giant cell granuloma. Dermatol Clin. 2015;33:331-341. doi:10.1016/j.det.2015.03.002
- de Oliveira FL, de Barros Silveira LK, Machado Ade M, et al. Hybrid clinical and histopathological pattern in annular lesions: an overlap between annular elastolytic giant cell granuloma and granuloma annulare? Case Rep Dermatol Med. 2012;2012:102915. doi:10.1155/2012/102915
- Wagenseller A, Larocca C, Vashi NA. Treatment of annular elastolytic giant cell granuloma with topical tretinoin. J Drugs Dermatol. 2017;16:699-700.
- Yang YW, Lehrer MD, Mangold AR, et al. Treatment of granuloma annulare and related granulomatous diseases with sulphasalazine: a series of 16 cases. J Eur Acad Dermatol Venereol. 2021;35:211-215. doi:10.1111/jdv.16356
- Mistry AM, Patel R, Mistry M, et al. Annular elastolytic giant cell granuloma. Cureus. 2020;12:E11456. doi:10.7759/cureus.11456
- Chen WT, Hsiao PF, Wu YH. Spectrum and clinical variants of giant cell elastolytic granuloma. Int J Dermatol. 2017;56:738-745. doi:10.1111/ijd.13502
- Raposo I, Mota F, Lobo I, et al. Annular elastolytic giant cell granuloma: a “visible” diagnosis. Dermatol Online J. 2017;23:13030/qt9rq3j927
- Klemke CD, Siebold D, Dippel E, et al. Generalised annular elastolytic giant cell granuloma. Dermatology. 2003;207:420-422. doi:10.1159/000074132
- Hassan R, Arunprasath P, Padmavathy L, et al. Annular elastolytic giant cell granuloma in association with Hashimoto’s thyroiditis. Indian Dermatol Online J. 2016;7:107-110. doi:10.4103/2229-5178.178087
- Kaya Erdog˘ an H, Arık D, Acer E, et al. Clinicopathological features of annular elastolytic giant cell granuloma patients. Turkish J Dermatol. 2018;12:85-89.
- Can B, Kavala M, Türkog˘ lu Z, et al. Successful treatment of annular elastolytic giant cell granuloma with hydroxychloroquine. Int J Dermatol. 2013;52:509-511. doi:10.1111 /j.1365-4632.2011.04941.x
- Arora S, Malik A, Patil C, et al. Annular elastolytic giant cell granuloma: a report of 10 cases. Indian Dermatol Online J. 2015;6(suppl 1):S17-S20. doi:10.4103/2229-5178.171055
- Doulaveri G, Tsagroni E, Giannadaki M, et al. Annular elastolytic giant cell granuloma in a 70-year-old woman. Int J Dermatol. 2003;42:290-291. doi:10.1046/j.1365-4362.2003.01767.x
- Marmon S, O’Reilly KE, Fischer M, et al. Papular variant of annular elastolytic giant-cell granuloma. Dermatol Online J. 2012;18:23.
- Aso Y, Izaki S, Teraki Y. Annular elastolytic giant cell granuloma associated with diabetes mellitus: a case report and review of the Japanese literature. Clin Exp Dermatol. 2011;36:917-919. doi:10.1111 /j.1365-2230.2011.04094.x
- Liu X, Zhang W, Liu Y, et al. A case of annular elastolytic giant cell granuloma associated with syphilis. Case Rep Dermatol. 2018; 10:158-161. doi:10.1159/000489910
- Gutiérrez-González E, Pereiro M Jr, Toribio J. Elastolytic actinic giant cell granuloma. Dermatol Clin. 2015;33:331-341. doi:10.1016/j.det.2015.03.002
- de Oliveira FL, de Barros Silveira LK, Machado Ade M, et al. Hybrid clinical and histopathological pattern in annular lesions: an overlap between annular elastolytic giant cell granuloma and granuloma annulare? Case Rep Dermatol Med. 2012;2012:102915. doi:10.1155/2012/102915
- Wagenseller A, Larocca C, Vashi NA. Treatment of annular elastolytic giant cell granuloma with topical tretinoin. J Drugs Dermatol. 2017;16:699-700.
- Yang YW, Lehrer MD, Mangold AR, et al. Treatment of granuloma annulare and related granulomatous diseases with sulphasalazine: a series of 16 cases. J Eur Acad Dermatol Venereol. 2021;35:211-215. doi:10.1111/jdv.16356
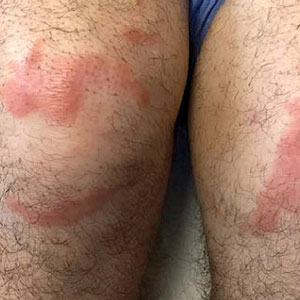
A 58-year-old man with a history of type 2 diabetes mellitus, nephrolithiasis, hypovitaminosis D, and hypercholesterolemia presented to our dermatology clinic for a follow-up total-body skin examination after a prior diagnosis of basal cell carcinoma on the vertex of the scalp. Physical examination revealed extensive photodamage and annular plaques overlying hyperpigmented telangiectatic patches on the dorsal portion of the neck. The eruption persisted for 1 year and failed to improve with clotrimazole cream. His medications included simvastatin, metformin, chlorthalidone, vitamin D, and tamsulosin. Two shave biopsies from the posterior neck were performed.

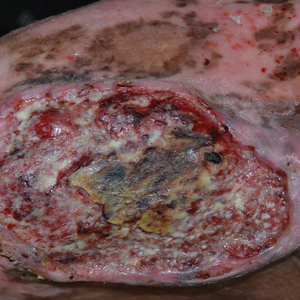
Chronic Ulcerative Lesion
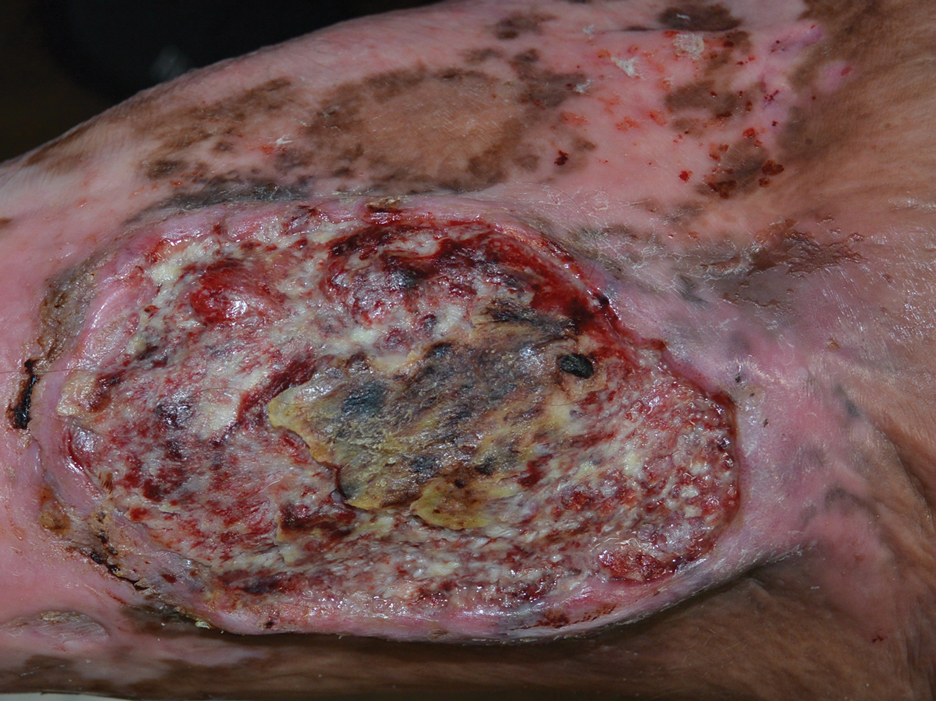
The Diagnosis: Marjolin Ulcer
A skin biopsy during his prior hospital admission demonstrated an ulcer with granulation tissue and mixed inflammation, and the patient was discharged with close outpatient follow-up. Two repeat skin biopsies from the peripheral margin at the time of the outpatient follow-up confirmed an invasive, well-differentiated squamous cell carcinoma (Figure), consistent with a Marjolin ulcer. Radiography demonstrated multiple left iliac chain and inguinal lymphadenopathies with extensive subcutaneous disease overlying the left medial tibia. After tumor board discussion, surgery was not recommended due to the size and likely penetration into the muscle. The patient began treatment with cemiplimab-rwlc, a PD-1 inhibitor. Within 4 cycles of treatment, he had improved pain and ambulation, and a 3-month follow-up positron emission tomography scan revealed decreased lymph node and cutaneous metabolic activity as well as clinical improvement.
Marjolin ulcers are rare and aggressive squamous cell carcinomas that arise from chronic wounds such as burn scars or pressure ulcers.1 Although an underlying well-differentiated squamous cell carcinoma is the most common etiology, patients also may present with underlying basal cell carcinomas, melanomas, or angiosarcomas.2 The exact pathogenesis underlying the malignant degeneration is unclear but appears to be driven by chronic inflammation. Patients classically present with a nonhealing ulcer associated with raised, friable, or crusty borders, as well as surrounding scar tissue. There is a median latency of 30 years after the trauma, though acute transformation within 12 months of an injury is possible.3 The diagnosis is confirmed with a peripheral wound biopsy. Surgical excision with wide margins remains the most common and effective intervention, especially for localized disease.1 The addition of lymph node dissection remains controversial, but treatment decisions can be guided by radiographic staging.4
The prognosis of Marjolin ulcers remains poor, with a predicted 5-year survival rate ranging from 43% to 58%.1 Dermatologists and trainees should be aware of Marjolin ulcers, especially as a mimicker of other chronic ulcerating conditions. Among the differential diagnosis, ulcerative lichen planus is a condition that commonly affects the oral and genital regions; however, patients with erosive lichen planus may develop an increased risk for the subsequent development of squamous cell carcinoma in the region.5 Furthermore, arterial ulcers typically develop on the distal lower extremities with other signs of chronic ischemia, including absent peripheral pulses, atrophic skin, hair loss, and ankle-brachial indices less than 0.5. Conversely, a venous ulcer classically affects the medial malleolus and will have evidence of venous insufficiency, including stasis dermatitis and peripheral edema.6
- Iqbal FM, Sinha Y, Jaffe W. Marjolin’s ulcer: a rare entity with a call for early diagnosis [published online July 15, 2015]. BMJ Case Rep. doi:10.1136/bcr-2014-208176
- Kanth AM, Heiman AJ, Nair L, et al. Current trends in management of Marjolin’s ulcer: a systematic review. J Burn Care Res. 2021;42:144-151. doi:10.1093/jbcr/iraa128
- Copcu E. Marjolin’s ulcer: a preventable complication of burns? Plast Reconstr Surg. 2009;124:E156-E164. doi:10.1097/PRS.0b013e3181a8082e
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Coll Certif Wound Spec. 2011; 3:60-64. doi:10.1016/j.jcws.2012.04.001
- Tziotzios C, Lee JYW, Brier T, et al. Lichen planus and lichenoid dermatoses: clinical overview and molecular basis. J Am Acad Dermatol. 2018;79:789-804.
- Spentzouris G, Labropoulos N. The evaluation of lower-extremity ulcers. Semin Intervent Radiol. 2009;26:286-295. doi:10.1055/s-0029-1242204
The Diagnosis: Marjolin Ulcer
A skin biopsy during his prior hospital admission demonstrated an ulcer with granulation tissue and mixed inflammation, and the patient was discharged with close outpatient follow-up. Two repeat skin biopsies from the peripheral margin at the time of the outpatient follow-up confirmed an invasive, well-differentiated squamous cell carcinoma (Figure), consistent with a Marjolin ulcer. Radiography demonstrated multiple left iliac chain and inguinal lymphadenopathies with extensive subcutaneous disease overlying the left medial tibia. After tumor board discussion, surgery was not recommended due to the size and likely penetration into the muscle. The patient began treatment with cemiplimab-rwlc, a PD-1 inhibitor. Within 4 cycles of treatment, he had improved pain and ambulation, and a 3-month follow-up positron emission tomography scan revealed decreased lymph node and cutaneous metabolic activity as well as clinical improvement.

Marjolin ulcers are rare and aggressive squamous cell carcinomas that arise from chronic wounds such as burn scars or pressure ulcers.1 Although an underlying well-differentiated squamous cell carcinoma is the most common etiology, patients also may present with underlying basal cell carcinomas, melanomas, or angiosarcomas.2 The exact pathogenesis underlying the malignant degeneration is unclear but appears to be driven by chronic inflammation. Patients classically present with a nonhealing ulcer associated with raised, friable, or crusty borders, as well as surrounding scar tissue. There is a median latency of 30 years after the trauma, though acute transformation within 12 months of an injury is possible.3 The diagnosis is confirmed with a peripheral wound biopsy. Surgical excision with wide margins remains the most common and effective intervention, especially for localized disease.1 The addition of lymph node dissection remains controversial, but treatment decisions can be guided by radiographic staging.4
The prognosis of Marjolin ulcers remains poor, with a predicted 5-year survival rate ranging from 43% to 58%.1 Dermatologists and trainees should be aware of Marjolin ulcers, especially as a mimicker of other chronic ulcerating conditions. Among the differential diagnosis, ulcerative lichen planus is a condition that commonly affects the oral and genital regions; however, patients with erosive lichen planus may develop an increased risk for the subsequent development of squamous cell carcinoma in the region.5 Furthermore, arterial ulcers typically develop on the distal lower extremities with other signs of chronic ischemia, including absent peripheral pulses, atrophic skin, hair loss, and ankle-brachial indices less than 0.5. Conversely, a venous ulcer classically affects the medial malleolus and will have evidence of venous insufficiency, including stasis dermatitis and peripheral edema.6
The Diagnosis: Marjolin Ulcer
A skin biopsy during his prior hospital admission demonstrated an ulcer with granulation tissue and mixed inflammation, and the patient was discharged with close outpatient follow-up. Two repeat skin biopsies from the peripheral margin at the time of the outpatient follow-up confirmed an invasive, well-differentiated squamous cell carcinoma (Figure), consistent with a Marjolin ulcer. Radiography demonstrated multiple left iliac chain and inguinal lymphadenopathies with extensive subcutaneous disease overlying the left medial tibia. After tumor board discussion, surgery was not recommended due to the size and likely penetration into the muscle. The patient began treatment with cemiplimab-rwlc, a PD-1 inhibitor. Within 4 cycles of treatment, he had improved pain and ambulation, and a 3-month follow-up positron emission tomography scan revealed decreased lymph node and cutaneous metabolic activity as well as clinical improvement.

Marjolin ulcers are rare and aggressive squamous cell carcinomas that arise from chronic wounds such as burn scars or pressure ulcers.1 Although an underlying well-differentiated squamous cell carcinoma is the most common etiology, patients also may present with underlying basal cell carcinomas, melanomas, or angiosarcomas.2 The exact pathogenesis underlying the malignant degeneration is unclear but appears to be driven by chronic inflammation. Patients classically present with a nonhealing ulcer associated with raised, friable, or crusty borders, as well as surrounding scar tissue. There is a median latency of 30 years after the trauma, though acute transformation within 12 months of an injury is possible.3 The diagnosis is confirmed with a peripheral wound biopsy. Surgical excision with wide margins remains the most common and effective intervention, especially for localized disease.1 The addition of lymph node dissection remains controversial, but treatment decisions can be guided by radiographic staging.4
The prognosis of Marjolin ulcers remains poor, with a predicted 5-year survival rate ranging from 43% to 58%.1 Dermatologists and trainees should be aware of Marjolin ulcers, especially as a mimicker of other chronic ulcerating conditions. Among the differential diagnosis, ulcerative lichen planus is a condition that commonly affects the oral and genital regions; however, patients with erosive lichen planus may develop an increased risk for the subsequent development of squamous cell carcinoma in the region.5 Furthermore, arterial ulcers typically develop on the distal lower extremities with other signs of chronic ischemia, including absent peripheral pulses, atrophic skin, hair loss, and ankle-brachial indices less than 0.5. Conversely, a venous ulcer classically affects the medial malleolus and will have evidence of venous insufficiency, including stasis dermatitis and peripheral edema.6
- Iqbal FM, Sinha Y, Jaffe W. Marjolin’s ulcer: a rare entity with a call for early diagnosis [published online July 15, 2015]. BMJ Case Rep. doi:10.1136/bcr-2014-208176
- Kanth AM, Heiman AJ, Nair L, et al. Current trends in management of Marjolin’s ulcer: a systematic review. J Burn Care Res. 2021;42:144-151. doi:10.1093/jbcr/iraa128
- Copcu E. Marjolin’s ulcer: a preventable complication of burns? Plast Reconstr Surg. 2009;124:E156-E164. doi:10.1097/PRS.0b013e3181a8082e
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Coll Certif Wound Spec. 2011; 3:60-64. doi:10.1016/j.jcws.2012.04.001
- Tziotzios C, Lee JYW, Brier T, et al. Lichen planus and lichenoid dermatoses: clinical overview and molecular basis. J Am Acad Dermatol. 2018;79:789-804.
- Spentzouris G, Labropoulos N. The evaluation of lower-extremity ulcers. Semin Intervent Radiol. 2009;26:286-295. doi:10.1055/s-0029-1242204
- Iqbal FM, Sinha Y, Jaffe W. Marjolin’s ulcer: a rare entity with a call for early diagnosis [published online July 15, 2015]. BMJ Case Rep. doi:10.1136/bcr-2014-208176
- Kanth AM, Heiman AJ, Nair L, et al. Current trends in management of Marjolin’s ulcer: a systematic review. J Burn Care Res. 2021;42:144-151. doi:10.1093/jbcr/iraa128
- Copcu E. Marjolin’s ulcer: a preventable complication of burns? Plast Reconstr Surg. 2009;124:E156-E164. doi:10.1097/PRS.0b013e3181a8082e
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Coll Certif Wound Spec. 2011; 3:60-64. doi:10.1016/j.jcws.2012.04.001
- Tziotzios C, Lee JYW, Brier T, et al. Lichen planus and lichenoid dermatoses: clinical overview and molecular basis. J Am Acad Dermatol. 2018;79:789-804.
- Spentzouris G, Labropoulos N. The evaluation of lower-extremity ulcers. Semin Intervent Radiol. 2009;26:286-295. doi:10.1055/s-0029-1242204
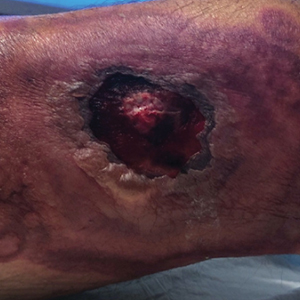
A 46-year-old man with a history of a left leg burn during childhood that was unsuccessfully treated with multiple skin grafts presented as a hospital follow-up for outpatient management of an ulcer. The patient had an ulcer that gradually increased in size over 7 years. Over the course of 2 weeks prior to the hospital presentation, he noted increased pain and severe difficulty with ambulation but remained afebrile without other systemic symptoms. Prior to the outpatient follow-up, he had been admitted to the hospital where he underwent imaging, laboratory studies, and skin biopsy, as well as treatment with empiric vancomycin. Physical examination revealed a large undermined ulcer with an elevated peripheral margin and crusting on the left lower leg with surrounding chronic scarring.
Exophytic Firm Papulonodule on the Labia in a Patient With Nonspecific Gastrointestinal Symptoms
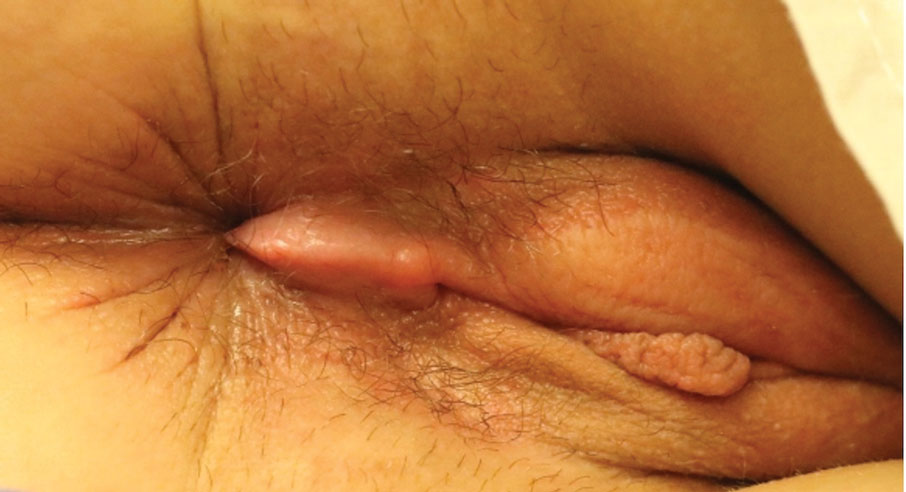
The Diagnosis: Cutaneous Crohn Disease
Kinyoun and Grocott-Gomori methenamine-silver staining of the labial biopsy were negative for mycobacteria and fungi, respectively. A complete blood cell count, erythrocyte sedimentation rate, C-reactive protein, celiac disease serologies, stool occult blood, and stool calprotectin laboratory test results were within reference range. Magnetic resonance imaging of the pelvis demonstrated an anal fissure extending from the anal verge at the 6 o’clock position, abnormal T2 bright signal in the skin of the buttocks and perineum extending to the labia, and mild mucosal enhancement of the rectal and anal mucosa. Esophagogastroduodenoscopy and magnetic resonance elastography were unremarkable. Colonoscopy demonstrated scattered superficial erythematous patches and erosions in the rectum. Histologically, there was mild to moderately active colitis in the rectum with no evidence of chronicity. Given our patient’s labial edema and exophytic papulonodule (Figure 1) in the setting of nonspecific gastrointestinal symptoms and granulomatous dermatitis seen on pathology (Figure 2), she was diagnosed with cutaneous Crohn disease (CD).
In our patient, labial biopsy was necessary to definitively diagnose CD. Prior to biopsy of the lesion, our patient was diagnosed with irritable bowel syndrome with constipation leading to an anal fissure and skin tag due to lack of laboratory, imaging, and colonoscopy findings commonly associated with CD. Her biopsy results and gastrointestinal symptoms made these diagnoses, as well as condyloma or a large sentinel skin tag, less likely.
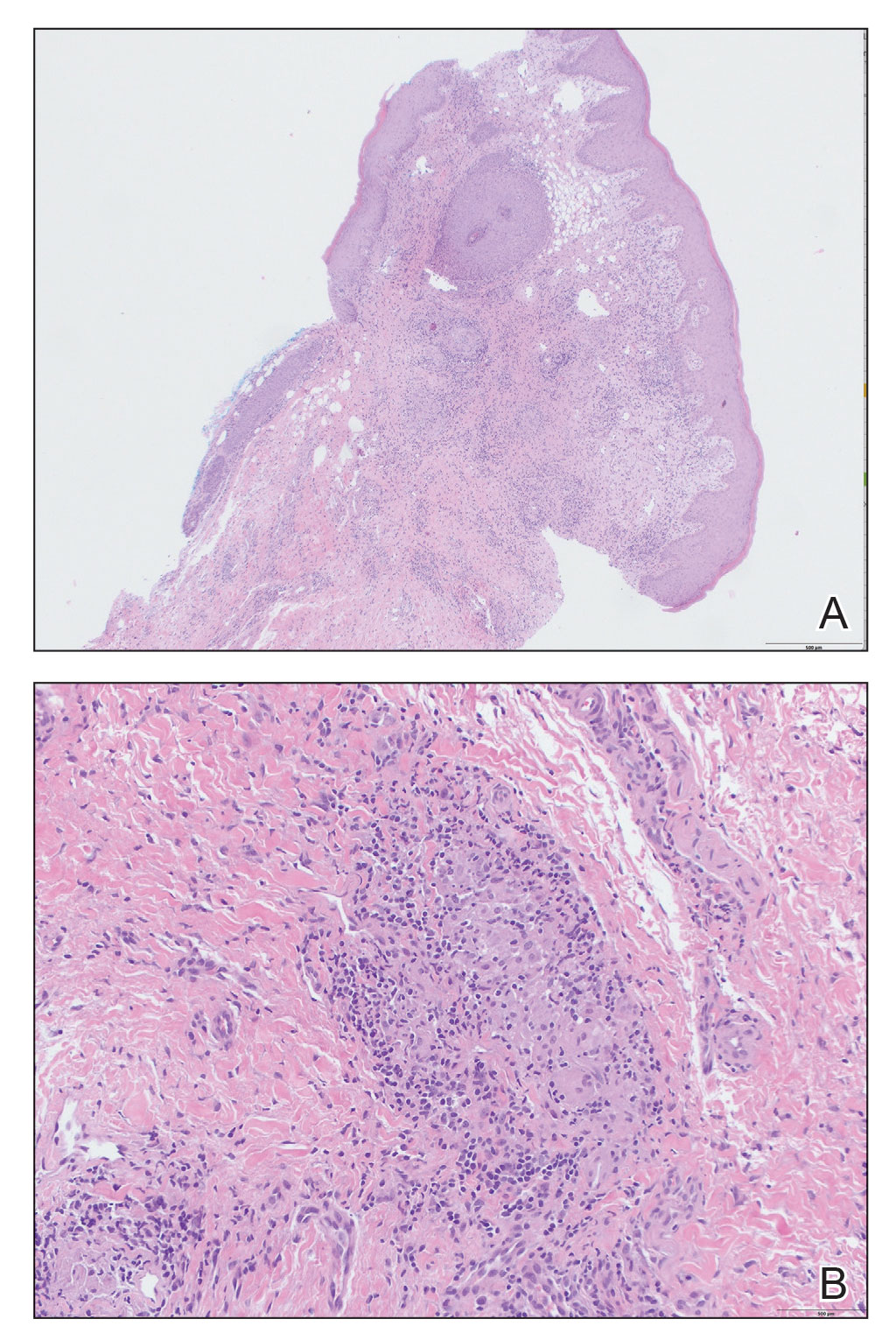
Extraintestinal findings of CD, especially cutaneous manifestations, are relatively frequent and may be present in as many as 44% of patients.1,2 Cutaneous CD often is characterized based on pathogenic mechanisms as either reactive, associated, or CD specific. Reactive cutaneous manifestations include erythema nodosum, pyoderma gangrenosum, and oral aphthae. Associated cutaneous manifestations include vitiligo, palmar erythema, and palmoplantar pustulosis.2 Crohn disease–specific manifestations, including genital or extragenital metastatic CD (MCD), fistulas, and oral involvement, are granulomatous in nature, similar to intestinal CD. Genital manifestations of MCD include edema, erythema, fissures, and/or ulceration of the vulva, penis, or scrotum. Labial swelling is the most common presenting symptom of MCD in females in both pediatric and adult age groups.2 Lymphedema, skin tags, and condylomalike growths also can be seen but are relatively less common.2
Given the labial edema, exophytic papulonodule, and granulomatous dermatitis seen on histopathology, our patient likely fit into the MCD category.2 In adults, most instances of MCD arise in the setting of well-established intestinal CD disease,3 whereas in children 86% of cases occur in patients without concurrent intestinal CD.2
Given the nonspecific and variable presentation of MCD, the differential diagnosis is broad. The differential diagnosis could include infectious etiologies such as condyloma acuminatum (human papillomavirus); syphilitic chancre; or mycobacterial, bacterial, fungal, or parasitic vulvovaginitis. Sexual abuse, sarcoidosis, Behçet disease, or hidradenitis suppurativa, among other diagnoses, also should be considered. Diagnostic workup should include biopsy of the lesion with special stains, polarizing microscopy, and tissue cultures.4 A thorough evaluation for gastrointestinal CD should be completed after diagnosis.3
The clinical course of vulvar CD can be unpredictable, with some cases healing spontaneously but most persisting despite treatment and sometimes prompting surgical removal.2,4 Early recognition is crucial, as long-standing MCD lesions can be therapy resistant.5 Due to the rarity of the condition and lack of data, there is a lack of treatment consensus for MCD. In 2014, the American Academy of Dermatology published treatment guidelines recommending superpotent topical steroids or topical tacrolimus as first-line therapy. Next-line therapy includes oral metronidazole, followed by prednisolone if still symptomatic.3 Treatment-resistant disease can warrant treatment with immunomodulators or tumor necrosis factor α inhibitors. Our patient was started on adalimumab; after just 2 months of therapy, the labial swelling decreased and the exophytic nodule was less firm and smaller.
Metastatic CD is a rare manifestation of cutaneous CD and can be present in the absence of gastrointestinal disease.3 This case demonstrates the importance of recognizing the cutaneous signs of CD and the necessity of lesional biopsy for the diagnosis of MCD, as our patient presented with nonspecific gastrointestinal symptoms and a diagnostic workup, including endoscopies, that proved inconclusive for the diagnosis of CD.
- Antonelli E, Bassotti G, Tramontana M, et al. Dermatological manifestations in inflammatory bowel diseases. J Clin Med. 2021;10:1-16. doi:10.3390/JCM10020364
- Schneider SL, Foster K, Patel D, et al. Cutaneous manifestations of metastatic Crohn’s disease. Pediatr Dermatol. 2018;35:566-574. doi:10.1111/PDE.13565
- Kurtzman DJB, Jones T, Lian F, et al. Metastatic Crohn’s disease: a review and approach to therapy. J Am Acad Dermatol. 2014;71:804-813. doi:10.1016/J.JAAD.2014.04.002
- Barret M, De Parades V, Battistella M, et al. Crohn’s disease of the vulva. J Crohns Colitis. 2014;8:563-570. doi:10.1016/J.CROHNS.2013.10.009
- Aberumand B, Howard J, Howard J. Metastatic Crohn’s disease: an approach to an uncommon but important cutaneous disorder [published online January 3, 2017]. Biomed Res Int. 2017;2017:8192150. doi:10.1155/2017/8192150
The Diagnosis: Cutaneous Crohn Disease
Kinyoun and Grocott-Gomori methenamine-silver staining of the labial biopsy were negative for mycobacteria and fungi, respectively. A complete blood cell count, erythrocyte sedimentation rate, C-reactive protein, celiac disease serologies, stool occult blood, and stool calprotectin laboratory test results were within reference range. Magnetic resonance imaging of the pelvis demonstrated an anal fissure extending from the anal verge at the 6 o’clock position, abnormal T2 bright signal in the skin of the buttocks and perineum extending to the labia, and mild mucosal enhancement of the rectal and anal mucosa. Esophagogastroduodenoscopy and magnetic resonance elastography were unremarkable. Colonoscopy demonstrated scattered superficial erythematous patches and erosions in the rectum. Histologically, there was mild to moderately active colitis in the rectum with no evidence of chronicity. Given our patient’s labial edema and exophytic papulonodule (Figure 1) in the setting of nonspecific gastrointestinal symptoms and granulomatous dermatitis seen on pathology (Figure 2), she was diagnosed with cutaneous Crohn disease (CD).

In our patient, labial biopsy was necessary to definitively diagnose CD. Prior to biopsy of the lesion, our patient was diagnosed with irritable bowel syndrome with constipation leading to an anal fissure and skin tag due to lack of laboratory, imaging, and colonoscopy findings commonly associated with CD. Her biopsy results and gastrointestinal symptoms made these diagnoses, as well as condyloma or a large sentinel skin tag, less likely.

Extraintestinal findings of CD, especially cutaneous manifestations, are relatively frequent and may be present in as many as 44% of patients.1,2 Cutaneous CD often is characterized based on pathogenic mechanisms as either reactive, associated, or CD specific. Reactive cutaneous manifestations include erythema nodosum, pyoderma gangrenosum, and oral aphthae. Associated cutaneous manifestations include vitiligo, palmar erythema, and palmoplantar pustulosis.2 Crohn disease–specific manifestations, including genital or extragenital metastatic CD (MCD), fistulas, and oral involvement, are granulomatous in nature, similar to intestinal CD. Genital manifestations of MCD include edema, erythema, fissures, and/or ulceration of the vulva, penis, or scrotum. Labial swelling is the most common presenting symptom of MCD in females in both pediatric and adult age groups.2 Lymphedema, skin tags, and condylomalike growths also can be seen but are relatively less common.2
Given the labial edema, exophytic papulonodule, and granulomatous dermatitis seen on histopathology, our patient likely fit into the MCD category.2 In adults, most instances of MCD arise in the setting of well-established intestinal CD disease,3 whereas in children 86% of cases occur in patients without concurrent intestinal CD.2
Given the nonspecific and variable presentation of MCD, the differential diagnosis is broad. The differential diagnosis could include infectious etiologies such as condyloma acuminatum (human papillomavirus); syphilitic chancre; or mycobacterial, bacterial, fungal, or parasitic vulvovaginitis. Sexual abuse, sarcoidosis, Behçet disease, or hidradenitis suppurativa, among other diagnoses, also should be considered. Diagnostic workup should include biopsy of the lesion with special stains, polarizing microscopy, and tissue cultures.4 A thorough evaluation for gastrointestinal CD should be completed after diagnosis.3
The clinical course of vulvar CD can be unpredictable, with some cases healing spontaneously but most persisting despite treatment and sometimes prompting surgical removal.2,4 Early recognition is crucial, as long-standing MCD lesions can be therapy resistant.5 Due to the rarity of the condition and lack of data, there is a lack of treatment consensus for MCD. In 2014, the American Academy of Dermatology published treatment guidelines recommending superpotent topical steroids or topical tacrolimus as first-line therapy. Next-line therapy includes oral metronidazole, followed by prednisolone if still symptomatic.3 Treatment-resistant disease can warrant treatment with immunomodulators or tumor necrosis factor α inhibitors. Our patient was started on adalimumab; after just 2 months of therapy, the labial swelling decreased and the exophytic nodule was less firm and smaller.
Metastatic CD is a rare manifestation of cutaneous CD and can be present in the absence of gastrointestinal disease.3 This case demonstrates the importance of recognizing the cutaneous signs of CD and the necessity of lesional biopsy for the diagnosis of MCD, as our patient presented with nonspecific gastrointestinal symptoms and a diagnostic workup, including endoscopies, that proved inconclusive for the diagnosis of CD.
The Diagnosis: Cutaneous Crohn Disease
Kinyoun and Grocott-Gomori methenamine-silver staining of the labial biopsy were negative for mycobacteria and fungi, respectively. A complete blood cell count, erythrocyte sedimentation rate, C-reactive protein, celiac disease serologies, stool occult blood, and stool calprotectin laboratory test results were within reference range. Magnetic resonance imaging of the pelvis demonstrated an anal fissure extending from the anal verge at the 6 o’clock position, abnormal T2 bright signal in the skin of the buttocks and perineum extending to the labia, and mild mucosal enhancement of the rectal and anal mucosa. Esophagogastroduodenoscopy and magnetic resonance elastography were unremarkable. Colonoscopy demonstrated scattered superficial erythematous patches and erosions in the rectum. Histologically, there was mild to moderately active colitis in the rectum with no evidence of chronicity. Given our patient’s labial edema and exophytic papulonodule (Figure 1) in the setting of nonspecific gastrointestinal symptoms and granulomatous dermatitis seen on pathology (Figure 2), she was diagnosed with cutaneous Crohn disease (CD).

In our patient, labial biopsy was necessary to definitively diagnose CD. Prior to biopsy of the lesion, our patient was diagnosed with irritable bowel syndrome with constipation leading to an anal fissure and skin tag due to lack of laboratory, imaging, and colonoscopy findings commonly associated with CD. Her biopsy results and gastrointestinal symptoms made these diagnoses, as well as condyloma or a large sentinel skin tag, less likely.

Extraintestinal findings of CD, especially cutaneous manifestations, are relatively frequent and may be present in as many as 44% of patients.1,2 Cutaneous CD often is characterized based on pathogenic mechanisms as either reactive, associated, or CD specific. Reactive cutaneous manifestations include erythema nodosum, pyoderma gangrenosum, and oral aphthae. Associated cutaneous manifestations include vitiligo, palmar erythema, and palmoplantar pustulosis.2 Crohn disease–specific manifestations, including genital or extragenital metastatic CD (MCD), fistulas, and oral involvement, are granulomatous in nature, similar to intestinal CD. Genital manifestations of MCD include edema, erythema, fissures, and/or ulceration of the vulva, penis, or scrotum. Labial swelling is the most common presenting symptom of MCD in females in both pediatric and adult age groups.2 Lymphedema, skin tags, and condylomalike growths also can be seen but are relatively less common.2
Given the labial edema, exophytic papulonodule, and granulomatous dermatitis seen on histopathology, our patient likely fit into the MCD category.2 In adults, most instances of MCD arise in the setting of well-established intestinal CD disease,3 whereas in children 86% of cases occur in patients without concurrent intestinal CD.2
Given the nonspecific and variable presentation of MCD, the differential diagnosis is broad. The differential diagnosis could include infectious etiologies such as condyloma acuminatum (human papillomavirus); syphilitic chancre; or mycobacterial, bacterial, fungal, or parasitic vulvovaginitis. Sexual abuse, sarcoidosis, Behçet disease, or hidradenitis suppurativa, among other diagnoses, also should be considered. Diagnostic workup should include biopsy of the lesion with special stains, polarizing microscopy, and tissue cultures.4 A thorough evaluation for gastrointestinal CD should be completed after diagnosis.3
The clinical course of vulvar CD can be unpredictable, with some cases healing spontaneously but most persisting despite treatment and sometimes prompting surgical removal.2,4 Early recognition is crucial, as long-standing MCD lesions can be therapy resistant.5 Due to the rarity of the condition and lack of data, there is a lack of treatment consensus for MCD. In 2014, the American Academy of Dermatology published treatment guidelines recommending superpotent topical steroids or topical tacrolimus as first-line therapy. Next-line therapy includes oral metronidazole, followed by prednisolone if still symptomatic.3 Treatment-resistant disease can warrant treatment with immunomodulators or tumor necrosis factor α inhibitors. Our patient was started on adalimumab; after just 2 months of therapy, the labial swelling decreased and the exophytic nodule was less firm and smaller.
Metastatic CD is a rare manifestation of cutaneous CD and can be present in the absence of gastrointestinal disease.3 This case demonstrates the importance of recognizing the cutaneous signs of CD and the necessity of lesional biopsy for the diagnosis of MCD, as our patient presented with nonspecific gastrointestinal symptoms and a diagnostic workup, including endoscopies, that proved inconclusive for the diagnosis of CD.
- Antonelli E, Bassotti G, Tramontana M, et al. Dermatological manifestations in inflammatory bowel diseases. J Clin Med. 2021;10:1-16. doi:10.3390/JCM10020364
- Schneider SL, Foster K, Patel D, et al. Cutaneous manifestations of metastatic Crohn’s disease. Pediatr Dermatol. 2018;35:566-574. doi:10.1111/PDE.13565
- Kurtzman DJB, Jones T, Lian F, et al. Metastatic Crohn’s disease: a review and approach to therapy. J Am Acad Dermatol. 2014;71:804-813. doi:10.1016/J.JAAD.2014.04.002
- Barret M, De Parades V, Battistella M, et al. Crohn’s disease of the vulva. J Crohns Colitis. 2014;8:563-570. doi:10.1016/J.CROHNS.2013.10.009
- Aberumand B, Howard J, Howard J. Metastatic Crohn’s disease: an approach to an uncommon but important cutaneous disorder [published online January 3, 2017]. Biomed Res Int. 2017;2017:8192150. doi:10.1155/2017/8192150
- Antonelli E, Bassotti G, Tramontana M, et al. Dermatological manifestations in inflammatory bowel diseases. J Clin Med. 2021;10:1-16. doi:10.3390/JCM10020364
- Schneider SL, Foster K, Patel D, et al. Cutaneous manifestations of metastatic Crohn’s disease. Pediatr Dermatol. 2018;35:566-574. doi:10.1111/PDE.13565
- Kurtzman DJB, Jones T, Lian F, et al. Metastatic Crohn’s disease: a review and approach to therapy. J Am Acad Dermatol. 2014;71:804-813. doi:10.1016/J.JAAD.2014.04.002
- Barret M, De Parades V, Battistella M, et al. Crohn’s disease of the vulva. J Crohns Colitis. 2014;8:563-570. doi:10.1016/J.CROHNS.2013.10.009
- Aberumand B, Howard J, Howard J. Metastatic Crohn’s disease: an approach to an uncommon but important cutaneous disorder [published online January 3, 2017]. Biomed Res Int. 2017;2017:8192150. doi:10.1155/2017/8192150
An 18-year-old woman with chronic constipation presented with an enlarging, painful, and edematous “lump” in the perineum of 1 year’s duration. The lesion became firmer and more painful with bowel movements. Physical examination revealed an enlarged right labia majora, as well as a pink to flesh-colored, exophytic, firm papulonodule in the perineum posterior to the right labia. The patient concomitantly was following with gastroenterology due to abdominal pain that worsened with eating, as well as constipation, nausea, weight loss, and rectal bleeding of 5 years’ duration. The patient denied rash, joint arthralgia, or oral ulcers. A biopsy from the labial lesion was performed.
Hyperpigmented Papules on the Tongue of a Child
The Diagnosis: Pigmented Fungiform Papillae of the Tongue
Our patient’s hyperpigmentation was confined to the fungiform papillae, leading to a diagnosis of pigmented fungiform papillae of the tongue (PFPT). A biopsy was not performed, and reassurance was provided regarding the benign nature of this finding, which did not require treatment.
Pigmented fungiform papillae of the tongue is a benign, nonprogressive, asymptomatic pigmentary condition that is most common among patients with skin of color and typically develops within the second or third decade of life.1,2 The pathogenesis is unclear, but activation of subepithelial melanophages without evidence of inflammation has been implicated.2 Although no standard treatment exists, cosmetic improvement with the use of the Q-switched ruby laser has been reported.3,4 Clinically, PFPT presents as asymptomatic hyperpigmentation confined to the fungiform papillae along the anterior and lateral portions of the tongue.1,2
Pigmented fungiform papillae of the tongue typically is an isolated finding but rarely can be associated with hyperpigmentation of the nails (as in our patient) or gingiva.2 Three different clinical patterns of presentation have been described: (1) a single well-circumscribed collection of pigmented fungiform papillae, (2) few scattered pigmented fungiform papillae admixed with many nonpigmented fungiform papillae, or (3) pigmentation of all fungiform papillae on the dorsal aspect of the tongue.2,5,6 Pigmented fungiform papillae of the tongue is a clinical diagnosis based on visual recognition. Dermoscopic examination revealing a cobblestonelike or rose petal–like pattern may be helpful in diagnosing PFPT.2,5-7 Although not typically recommended in the evaluation of PFPT, a biopsy will reveal papillary structures with hyperpigmentation of basilar keratinocytes as well as melanophages in the lamina propria.8 The latter finding suggests a transient inflammatory process despite the hallmark absence of inflammation.5 Melanocytic neoplasia and exogenous granules of pigment typically are not seen.8
Other conditions that may present with dark-colored macules or papules on the tongue should be considered in the evaluation of a patient with these clinical findings. Black hairy tongue (BHT), or lingua villosa nigra, is a benign finding due to filiform papillae hypertrophy on the dorsum of the tongue.9 Food particle debris caught in BHT can lead to porphyrin production by chromogenic bacteria and fungi. These porphyrins result in discoloration ranging from brown-black to yellow and green occurring anteriorly to the circumvallate papillae while usually sparing the tip and lateral sides of the tongue. Dermoscopy can show thin discolored fibers with a hairy appearance. Although normal filiform papillae are less than 1-mm long, 3-mm long papillae are considered diagnostic of BHT.9 Treatment includes effective oral hygiene and desquamation measures, which can lead to complete resolution.10
Peutz-Jeghers syndrome is a rare genodermatosis that is characterized by focal hyperpigmentation and multiple gastrointestinal mucosal hamartomatous polyps. Peutz-Jeghers syndrome should be suspected in a patient with discrete, 1- to 5-mm, brown to black macules on the perioral or periocular skin, tongue, genitals, palms, soles, and buccal mucosa with a history of abdominal symptoms.11,12
Addison disease, or primary adrenal insufficiency, may present with brown hyperpigmentation on chronically sun-exposed areas; regions of friction or pressure; surrounding scar tissue; and mucosal surfaces such as the tongue, inner surface of the lip, and buccal and gingival mucosa.13 Addison disease is differentiated from PFPT by a more generalized hyperpigmentation due to increased melanin production as well as the presence of systemic symptoms related to hypocortisolism. The pigmentation seen on the buccal mucosa in Addison disease is patchy and diffuse, and histology reveals basal melanin hyperpigmentation with superficial dermal melanophages.13
Hereditary hemorrhagic telangiectasia is an inherited disorder featuring telangiectasia and generally appears in the third decade of life.14 Telangiectases classically are 1 to 3 mm in diameter with or without slight elevation. Dermoscopic findings include small red clots, lacunae, and serpentine or linear vessels arranged in a radial conformation surrounding a homogenous pink center.15 These telangiectases typically occur on the skin or mucosa, particularly the face, lips, tongue, nail beds, and nasal mucosa; however, any organ can be affected with arteriovenous malformations. Recurrent epistaxis occurs in more than half of patients with hereditary hemorrhagic telangiectasia.14 Histopathology reveals dilated vessels and lacunae near the dermoepidermal junction displacing the epidermis and papillary dermis.15 It is distinguished from PFPT by the vascular nature of the lesions and by the presence of other characteristic symptoms such as recurrent epistaxis and visceral arteriovenous malformations.
- Romiti R, Molina De Medeiros L. Pigmented fungiform papillae of the tongue. Pediatr Dermatol. 2010;27:398-399. doi:10.1111/j .1525-1470.2010.01183.x
- Chessa MA, Patrizi A, Sechi A, et al. Pigmented fungiform lingual papillae: dermoscopic and clinical features. J Eur Acad Dermatol Venereol. 2018;32:935-939. doi:10.1111/jdv.14809
- Rice SM, Lal K. Successful treatment of pigmented fungiform papillae of the tongue with Q-switched ruby laser. Dermatol Surg. 2022;48:368-369. doi:10.1097/DSS.0000000000003371
- Mizawa M, Makino T, Furukawa F, et al. Efficacy of Q-switched ruby laser treatment for pigmented fungiform papillae of the tongue. J Dermatol. 2022;49:E133-E134. doi:10.1111/1346-8138.16270
- Holzwanger JM, Rudolph RI, Heaton CL. Pigmented fungiform papillae of the tongue: a common variant of oral pigmentation. Int J Dermatol. 1974;13:403-408. doi:10.1111/j.1365-4362.1974. tb05073.x
- Mukamal LV, Ormiga P, Ramos-E-Silva M. Dermoscopy of the pigmented fungiform papillae of the tongue. J Dermatol. 2012;39:397-399. doi:10.1111/j.1346-8138.2011.01328.x
- Surboyo MDC, Santosh ABR, Hariyani N, et al. Clinical utility of dermoscopy on diagnosing pigmented papillary fungiform papillae of the tongue: a systematic review. J Oral Biol Craniofac Res. 2021;11:618-623. doi:10.1016/j.jobcr.2021.09.008
- Chamseddin B, Vandergriff T. Pigmented fungiform papillae of the tongue: a clinical and histologic description [published online September 15, 2019]. Dermatol Online J. 2019;25:13030/qt8674c519.
- Jayasree P, Kaliyadan F, Ashique KT. Black hairy tongue. JAMA Dermatol. 2022;158:573. doi:10.1001/jamadermatol.2021.5314
- Schlager E, St Claire C, Ashack K, et al. Black hairy tongue: predisposing factors, diagnosis, and treatment. Am J Clin Dermatol. 2017;18:563-569. doi:10.1007/s40257-017-0268-y
- Sandru F, Petca A, Dumitrascu MC, et al. Peutz-Jeghers syndrome: skin manifestations and endocrine anomalies (review). Exp Ther Med. 2021;22:1387. doi:10.3892/etm.2021.10823
- Shah KR, Boland CR, Patel M, et al. Cutaneous manifestations of gastrointestinal disease: part I. J Am Acad Dermatol. 2013;68:189.e1-210. doi:10.1016/j.jaad.2012.10.037
- Lee K, Lian C, Vaidya A, et al. Oral mucosal hyperpigmentation. JAAD Case Rep. 2020;6:993-995. doi:10.1016/j.jdcr.2020.08.013
- Haitjema T, Westermann CJ, Overtoom TT, et al. Hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu disease): new insights in pathogenesis, complications, and treatment. Arch Intern Med. 1996;156:714-719.
- Tokoro S, Namiki T, Ugajin T, et al. Hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber’s disease): detailed assessment of skin lesions by dermoscopy and ultrasound. Int J Dermatol. 2019;58:E224-E226. doi:10.1111/ijd.14578
The Diagnosis: Pigmented Fungiform Papillae of the Tongue
Our patient’s hyperpigmentation was confined to the fungiform papillae, leading to a diagnosis of pigmented fungiform papillae of the tongue (PFPT). A biopsy was not performed, and reassurance was provided regarding the benign nature of this finding, which did not require treatment.
Pigmented fungiform papillae of the tongue is a benign, nonprogressive, asymptomatic pigmentary condition that is most common among patients with skin of color and typically develops within the second or third decade of life.1,2 The pathogenesis is unclear, but activation of subepithelial melanophages without evidence of inflammation has been implicated.2 Although no standard treatment exists, cosmetic improvement with the use of the Q-switched ruby laser has been reported.3,4 Clinically, PFPT presents as asymptomatic hyperpigmentation confined to the fungiform papillae along the anterior and lateral portions of the tongue.1,2
Pigmented fungiform papillae of the tongue typically is an isolated finding but rarely can be associated with hyperpigmentation of the nails (as in our patient) or gingiva.2 Three different clinical patterns of presentation have been described: (1) a single well-circumscribed collection of pigmented fungiform papillae, (2) few scattered pigmented fungiform papillae admixed with many nonpigmented fungiform papillae, or (3) pigmentation of all fungiform papillae on the dorsal aspect of the tongue.2,5,6 Pigmented fungiform papillae of the tongue is a clinical diagnosis based on visual recognition. Dermoscopic examination revealing a cobblestonelike or rose petal–like pattern may be helpful in diagnosing PFPT.2,5-7 Although not typically recommended in the evaluation of PFPT, a biopsy will reveal papillary structures with hyperpigmentation of basilar keratinocytes as well as melanophages in the lamina propria.8 The latter finding suggests a transient inflammatory process despite the hallmark absence of inflammation.5 Melanocytic neoplasia and exogenous granules of pigment typically are not seen.8
Other conditions that may present with dark-colored macules or papules on the tongue should be considered in the evaluation of a patient with these clinical findings. Black hairy tongue (BHT), or lingua villosa nigra, is a benign finding due to filiform papillae hypertrophy on the dorsum of the tongue.9 Food particle debris caught in BHT can lead to porphyrin production by chromogenic bacteria and fungi. These porphyrins result in discoloration ranging from brown-black to yellow and green occurring anteriorly to the circumvallate papillae while usually sparing the tip and lateral sides of the tongue. Dermoscopy can show thin discolored fibers with a hairy appearance. Although normal filiform papillae are less than 1-mm long, 3-mm long papillae are considered diagnostic of BHT.9 Treatment includes effective oral hygiene and desquamation measures, which can lead to complete resolution.10
Peutz-Jeghers syndrome is a rare genodermatosis that is characterized by focal hyperpigmentation and multiple gastrointestinal mucosal hamartomatous polyps. Peutz-Jeghers syndrome should be suspected in a patient with discrete, 1- to 5-mm, brown to black macules on the perioral or periocular skin, tongue, genitals, palms, soles, and buccal mucosa with a history of abdominal symptoms.11,12
Addison disease, or primary adrenal insufficiency, may present with brown hyperpigmentation on chronically sun-exposed areas; regions of friction or pressure; surrounding scar tissue; and mucosal surfaces such as the tongue, inner surface of the lip, and buccal and gingival mucosa.13 Addison disease is differentiated from PFPT by a more generalized hyperpigmentation due to increased melanin production as well as the presence of systemic symptoms related to hypocortisolism. The pigmentation seen on the buccal mucosa in Addison disease is patchy and diffuse, and histology reveals basal melanin hyperpigmentation with superficial dermal melanophages.13
Hereditary hemorrhagic telangiectasia is an inherited disorder featuring telangiectasia and generally appears in the third decade of life.14 Telangiectases classically are 1 to 3 mm in diameter with or without slight elevation. Dermoscopic findings include small red clots, lacunae, and serpentine or linear vessels arranged in a radial conformation surrounding a homogenous pink center.15 These telangiectases typically occur on the skin or mucosa, particularly the face, lips, tongue, nail beds, and nasal mucosa; however, any organ can be affected with arteriovenous malformations. Recurrent epistaxis occurs in more than half of patients with hereditary hemorrhagic telangiectasia.14 Histopathology reveals dilated vessels and lacunae near the dermoepidermal junction displacing the epidermis and papillary dermis.15 It is distinguished from PFPT by the vascular nature of the lesions and by the presence of other characteristic symptoms such as recurrent epistaxis and visceral arteriovenous malformations.
The Diagnosis: Pigmented Fungiform Papillae of the Tongue
Our patient’s hyperpigmentation was confined to the fungiform papillae, leading to a diagnosis of pigmented fungiform papillae of the tongue (PFPT). A biopsy was not performed, and reassurance was provided regarding the benign nature of this finding, which did not require treatment.
Pigmented fungiform papillae of the tongue is a benign, nonprogressive, asymptomatic pigmentary condition that is most common among patients with skin of color and typically develops within the second or third decade of life.1,2 The pathogenesis is unclear, but activation of subepithelial melanophages without evidence of inflammation has been implicated.2 Although no standard treatment exists, cosmetic improvement with the use of the Q-switched ruby laser has been reported.3,4 Clinically, PFPT presents as asymptomatic hyperpigmentation confined to the fungiform papillae along the anterior and lateral portions of the tongue.1,2
Pigmented fungiform papillae of the tongue typically is an isolated finding but rarely can be associated with hyperpigmentation of the nails (as in our patient) or gingiva.2 Three different clinical patterns of presentation have been described: (1) a single well-circumscribed collection of pigmented fungiform papillae, (2) few scattered pigmented fungiform papillae admixed with many nonpigmented fungiform papillae, or (3) pigmentation of all fungiform papillae on the dorsal aspect of the tongue.2,5,6 Pigmented fungiform papillae of the tongue is a clinical diagnosis based on visual recognition. Dermoscopic examination revealing a cobblestonelike or rose petal–like pattern may be helpful in diagnosing PFPT.2,5-7 Although not typically recommended in the evaluation of PFPT, a biopsy will reveal papillary structures with hyperpigmentation of basilar keratinocytes as well as melanophages in the lamina propria.8 The latter finding suggests a transient inflammatory process despite the hallmark absence of inflammation.5 Melanocytic neoplasia and exogenous granules of pigment typically are not seen.8
Other conditions that may present with dark-colored macules or papules on the tongue should be considered in the evaluation of a patient with these clinical findings. Black hairy tongue (BHT), or lingua villosa nigra, is a benign finding due to filiform papillae hypertrophy on the dorsum of the tongue.9 Food particle debris caught in BHT can lead to porphyrin production by chromogenic bacteria and fungi. These porphyrins result in discoloration ranging from brown-black to yellow and green occurring anteriorly to the circumvallate papillae while usually sparing the tip and lateral sides of the tongue. Dermoscopy can show thin discolored fibers with a hairy appearance. Although normal filiform papillae are less than 1-mm long, 3-mm long papillae are considered diagnostic of BHT.9 Treatment includes effective oral hygiene and desquamation measures, which can lead to complete resolution.10
Peutz-Jeghers syndrome is a rare genodermatosis that is characterized by focal hyperpigmentation and multiple gastrointestinal mucosal hamartomatous polyps. Peutz-Jeghers syndrome should be suspected in a patient with discrete, 1- to 5-mm, brown to black macules on the perioral or periocular skin, tongue, genitals, palms, soles, and buccal mucosa with a history of abdominal symptoms.11,12
Addison disease, or primary adrenal insufficiency, may present with brown hyperpigmentation on chronically sun-exposed areas; regions of friction or pressure; surrounding scar tissue; and mucosal surfaces such as the tongue, inner surface of the lip, and buccal and gingival mucosa.13 Addison disease is differentiated from PFPT by a more generalized hyperpigmentation due to increased melanin production as well as the presence of systemic symptoms related to hypocortisolism. The pigmentation seen on the buccal mucosa in Addison disease is patchy and diffuse, and histology reveals basal melanin hyperpigmentation with superficial dermal melanophages.13
Hereditary hemorrhagic telangiectasia is an inherited disorder featuring telangiectasia and generally appears in the third decade of life.14 Telangiectases classically are 1 to 3 mm in diameter with or without slight elevation. Dermoscopic findings include small red clots, lacunae, and serpentine or linear vessels arranged in a radial conformation surrounding a homogenous pink center.15 These telangiectases typically occur on the skin or mucosa, particularly the face, lips, tongue, nail beds, and nasal mucosa; however, any organ can be affected with arteriovenous malformations. Recurrent epistaxis occurs in more than half of patients with hereditary hemorrhagic telangiectasia.14 Histopathology reveals dilated vessels and lacunae near the dermoepidermal junction displacing the epidermis and papillary dermis.15 It is distinguished from PFPT by the vascular nature of the lesions and by the presence of other characteristic symptoms such as recurrent epistaxis and visceral arteriovenous malformations.
- Romiti R, Molina De Medeiros L. Pigmented fungiform papillae of the tongue. Pediatr Dermatol. 2010;27:398-399. doi:10.1111/j .1525-1470.2010.01183.x
- Chessa MA, Patrizi A, Sechi A, et al. Pigmented fungiform lingual papillae: dermoscopic and clinical features. J Eur Acad Dermatol Venereol. 2018;32:935-939. doi:10.1111/jdv.14809
- Rice SM, Lal K. Successful treatment of pigmented fungiform papillae of the tongue with Q-switched ruby laser. Dermatol Surg. 2022;48:368-369. doi:10.1097/DSS.0000000000003371
- Mizawa M, Makino T, Furukawa F, et al. Efficacy of Q-switched ruby laser treatment for pigmented fungiform papillae of the tongue. J Dermatol. 2022;49:E133-E134. doi:10.1111/1346-8138.16270
- Holzwanger JM, Rudolph RI, Heaton CL. Pigmented fungiform papillae of the tongue: a common variant of oral pigmentation. Int J Dermatol. 1974;13:403-408. doi:10.1111/j.1365-4362.1974. tb05073.x
- Mukamal LV, Ormiga P, Ramos-E-Silva M. Dermoscopy of the pigmented fungiform papillae of the tongue. J Dermatol. 2012;39:397-399. doi:10.1111/j.1346-8138.2011.01328.x
- Surboyo MDC, Santosh ABR, Hariyani N, et al. Clinical utility of dermoscopy on diagnosing pigmented papillary fungiform papillae of the tongue: a systematic review. J Oral Biol Craniofac Res. 2021;11:618-623. doi:10.1016/j.jobcr.2021.09.008
- Chamseddin B, Vandergriff T. Pigmented fungiform papillae of the tongue: a clinical and histologic description [published online September 15, 2019]. Dermatol Online J. 2019;25:13030/qt8674c519.
- Jayasree P, Kaliyadan F, Ashique KT. Black hairy tongue. JAMA Dermatol. 2022;158:573. doi:10.1001/jamadermatol.2021.5314
- Schlager E, St Claire C, Ashack K, et al. Black hairy tongue: predisposing factors, diagnosis, and treatment. Am J Clin Dermatol. 2017;18:563-569. doi:10.1007/s40257-017-0268-y
- Sandru F, Petca A, Dumitrascu MC, et al. Peutz-Jeghers syndrome: skin manifestations and endocrine anomalies (review). Exp Ther Med. 2021;22:1387. doi:10.3892/etm.2021.10823
- Shah KR, Boland CR, Patel M, et al. Cutaneous manifestations of gastrointestinal disease: part I. J Am Acad Dermatol. 2013;68:189.e1-210. doi:10.1016/j.jaad.2012.10.037
- Lee K, Lian C, Vaidya A, et al. Oral mucosal hyperpigmentation. JAAD Case Rep. 2020;6:993-995. doi:10.1016/j.jdcr.2020.08.013
- Haitjema T, Westermann CJ, Overtoom TT, et al. Hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu disease): new insights in pathogenesis, complications, and treatment. Arch Intern Med. 1996;156:714-719.
- Tokoro S, Namiki T, Ugajin T, et al. Hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber’s disease): detailed assessment of skin lesions by dermoscopy and ultrasound. Int J Dermatol. 2019;58:E224-E226. doi:10.1111/ijd.14578
- Romiti R, Molina De Medeiros L. Pigmented fungiform papillae of the tongue. Pediatr Dermatol. 2010;27:398-399. doi:10.1111/j .1525-1470.2010.01183.x
- Chessa MA, Patrizi A, Sechi A, et al. Pigmented fungiform lingual papillae: dermoscopic and clinical features. J Eur Acad Dermatol Venereol. 2018;32:935-939. doi:10.1111/jdv.14809
- Rice SM, Lal K. Successful treatment of pigmented fungiform papillae of the tongue with Q-switched ruby laser. Dermatol Surg. 2022;48:368-369. doi:10.1097/DSS.0000000000003371
- Mizawa M, Makino T, Furukawa F, et al. Efficacy of Q-switched ruby laser treatment for pigmented fungiform papillae of the tongue. J Dermatol. 2022;49:E133-E134. doi:10.1111/1346-8138.16270
- Holzwanger JM, Rudolph RI, Heaton CL. Pigmented fungiform papillae of the tongue: a common variant of oral pigmentation. Int J Dermatol. 1974;13:403-408. doi:10.1111/j.1365-4362.1974. tb05073.x
- Mukamal LV, Ormiga P, Ramos-E-Silva M. Dermoscopy of the pigmented fungiform papillae of the tongue. J Dermatol. 2012;39:397-399. doi:10.1111/j.1346-8138.2011.01328.x
- Surboyo MDC, Santosh ABR, Hariyani N, et al. Clinical utility of dermoscopy on diagnosing pigmented papillary fungiform papillae of the tongue: a systematic review. J Oral Biol Craniofac Res. 2021;11:618-623. doi:10.1016/j.jobcr.2021.09.008
- Chamseddin B, Vandergriff T. Pigmented fungiform papillae of the tongue: a clinical and histologic description [published online September 15, 2019]. Dermatol Online J. 2019;25:13030/qt8674c519.
- Jayasree P, Kaliyadan F, Ashique KT. Black hairy tongue. JAMA Dermatol. 2022;158:573. doi:10.1001/jamadermatol.2021.5314
- Schlager E, St Claire C, Ashack K, et al. Black hairy tongue: predisposing factors, diagnosis, and treatment. Am J Clin Dermatol. 2017;18:563-569. doi:10.1007/s40257-017-0268-y
- Sandru F, Petca A, Dumitrascu MC, et al. Peutz-Jeghers syndrome: skin manifestations and endocrine anomalies (review). Exp Ther Med. 2021;22:1387. doi:10.3892/etm.2021.10823
- Shah KR, Boland CR, Patel M, et al. Cutaneous manifestations of gastrointestinal disease: part I. J Am Acad Dermatol. 2013;68:189.e1-210. doi:10.1016/j.jaad.2012.10.037
- Lee K, Lian C, Vaidya A, et al. Oral mucosal hyperpigmentation. JAAD Case Rep. 2020;6:993-995. doi:10.1016/j.jdcr.2020.08.013
- Haitjema T, Westermann CJ, Overtoom TT, et al. Hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu disease): new insights in pathogenesis, complications, and treatment. Arch Intern Med. 1996;156:714-719.
- Tokoro S, Namiki T, Ugajin T, et al. Hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber’s disease): detailed assessment of skin lesions by dermoscopy and ultrasound. Int J Dermatol. 2019;58:E224-E226. doi:10.1111/ijd.14578
A 9-year-old Black boy presented to the dermatology clinic for evaluation of dark spots on the tongue. The family first noted these spots 5 months prior and reported that they remained stable during that time. The patient’s medical history was notable for autism spectrum disorder and multiple food allergies. His family history was negative for similar oral pigmentation or other pigmentary anomalies. A review of systems was positive only for selective eating and rare nosebleeds. Physical examination revealed numerous dark brown, pinpoint papules across the dorsal aspect of the tongue. No hyperpigmentation of the buccal mucosae, lips, palms, or soles was identified. Several light brown streaks were present on the fingernails and toenails, consistent with longitudinal melanonychia. A prior complete blood cell count was within reference range.
Atypical Keratotic Nodule on the Knuckle
The Diagnosis: Atypical Mycobacterial Infection
The history of rapid growth followed by shrinkage as well as the craterlike clinical appearance of our patient’s lesion were suspicious for the keratoacanthoma variant of squamous cell carcinoma (SCC). Periodic acid–Schiff green staining was negative for fungal or bacterial organisms, and the biopsy findings of keratinocyte atypia and irregular epidermal proliferation seemed to confirm our suspicion for well-differentiated SCC (Figure 1). Our patient subsequently was scheduled for Mohs micrographic surgery. Fortunately, a sample of tissue had been sent for panculture—bacterial, fungal, and mycobacterial—to rule out infectious etiologies, given the history of possible traumatic inoculation, and returned positive for Mycobacterium marinum infection prior to the surgery. Mohs surgery was canceled, and he was referred to an infectious disease specialist who started antibiotic treatment with azithromycin, ethambutol, and rifabutin. After 1 month of treatment the lesion substantially improved (Figure 2), further supporting the diagnosis of M marinum infection over SCC.
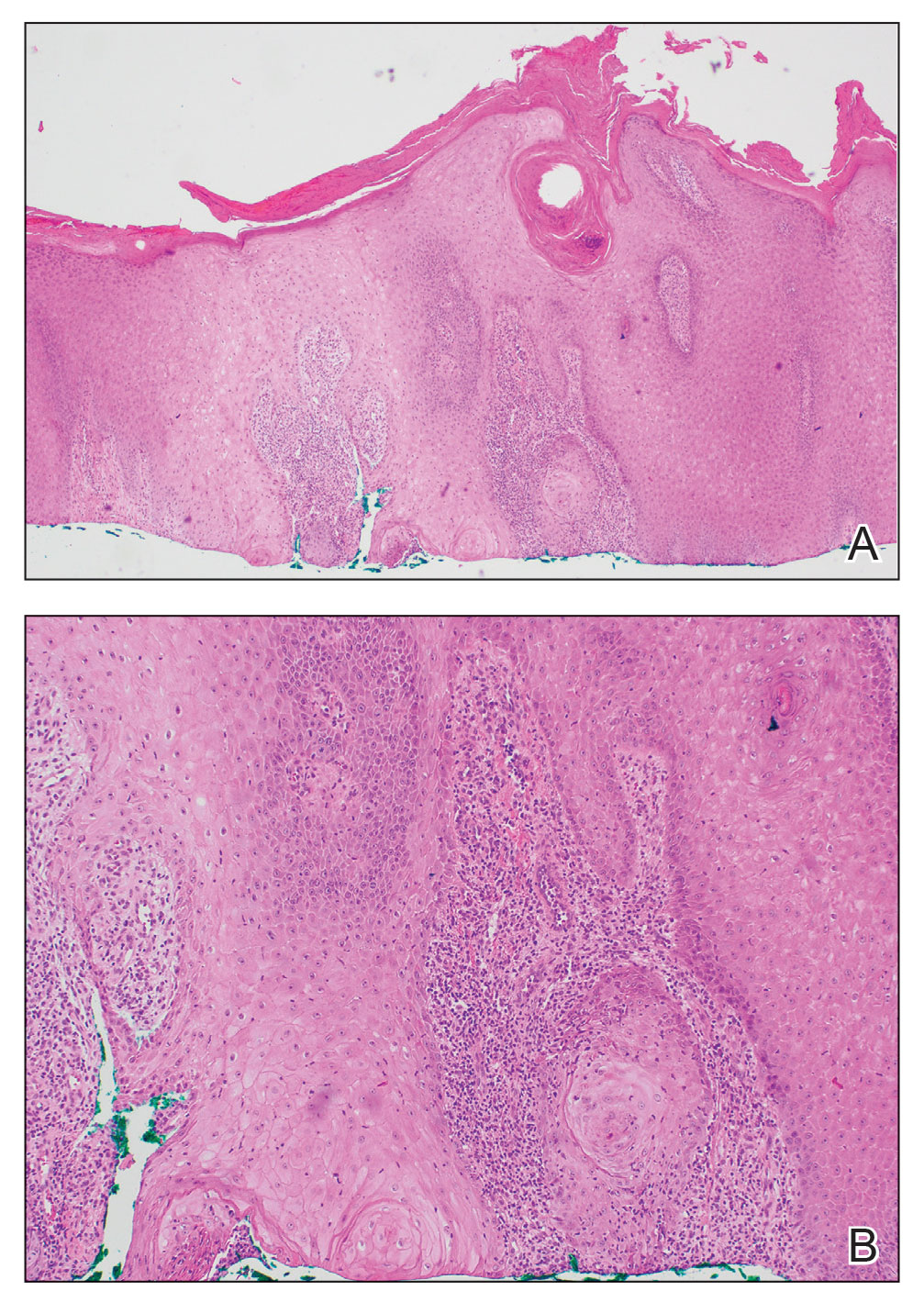
The differential diagnosis also included sporotrichosis, leishmaniasis, and chromoblastomycosis. Sporotrichosis lesions typically develop as multiple nodules and ulcers along a path of lymphatic drainage and can exhibit asteroid bodies and cigar-shaped yeast forms on histology. Chromoblastomycosis may display pseudoepitheliomatous hyperplasia and granulomatous inflammation; however, pathognomonic pigmented Medlar bodies also likely would be present.1 Leishmaniasis has a wide variety of presentations; however, it typically occurs in patients with exposure to endemic areas outside of the United States. Although leishmaniasis may demonstrate pseudoepitheliomatous hyperplasia, ulceration, and mixed inflammation on histology, it also likely would show amastigotes within dermal macrophages.2
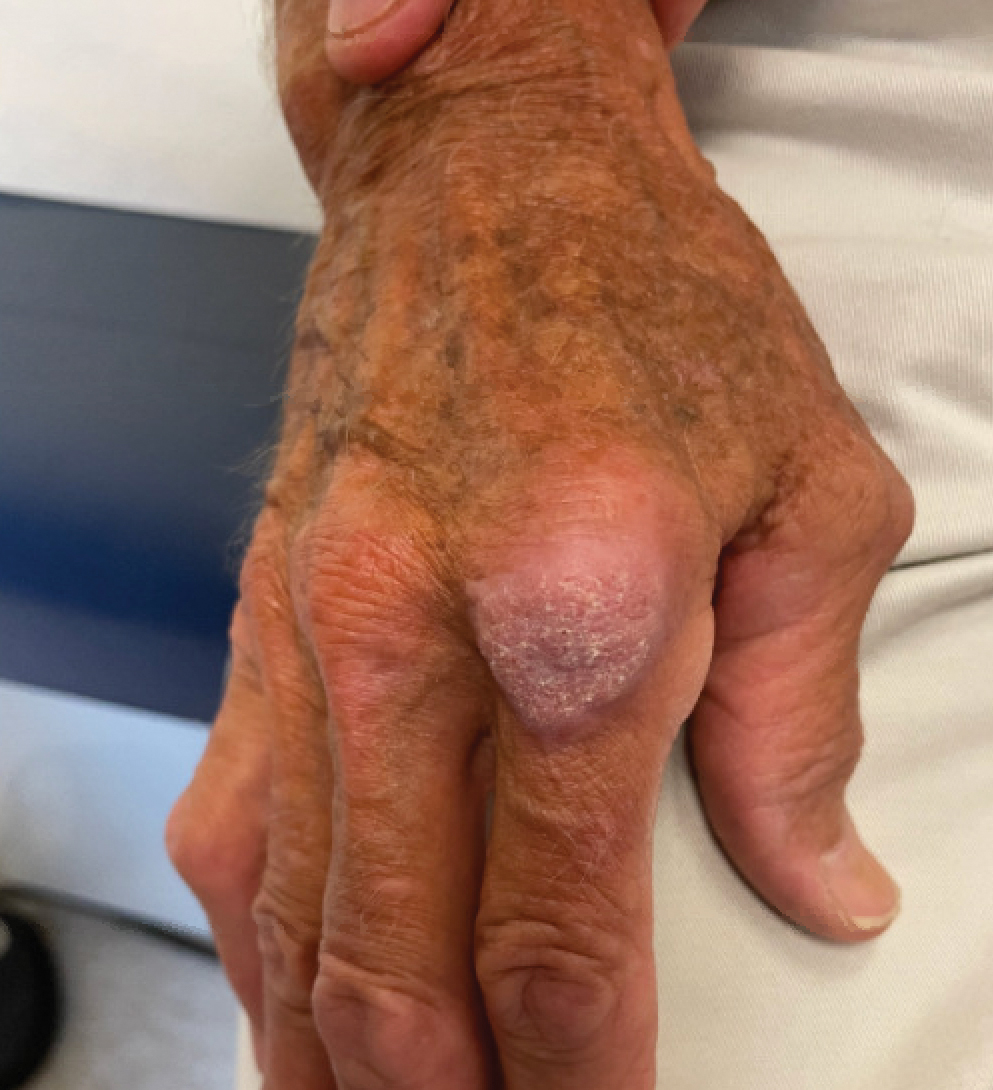
Atypical mycobacterial infections initially may be misdiagnosed as SCC due to their tendency to induce irregular acanthosis in the form of pseudoepitheliomatous hyperplasia as well as mild keratinocyte atypia secondary to inflammation.3,4 Our case is unique because it occurred with M marinum infection specifically. The histopathologic findings of M marinum infections are variable and may additionally include granulomas, most commonly suppurative; intraepithelial abscesses; small vessel proliferation; dermal fibrosis; multinucleated giant cells; and transepidermal elimination.4,5 Periodic acid–Schiff, Ziehl-Neelsen (acid-fast bacilli), and Fite staining may be used to distinguish M marinum infection from SCC but have low sensitivities (approximately 30%). Culture remains the most reliable test, with a sensitivity of nearly 80%.5-7 In our patient, a Periodic acid–Schiff stain was obtained prior to receiving culture results, and acid-fast bacilli and Fite staining were added after the culture returned positive; however, all 3 stains failed to highlight any mycobacteria.
The primary risk factor for infection with M marinum is contact with aquatic environments or marine animals, and most cases involve the fingers or the hand.6 After we reached the diagnosis and further discussed the patient’s history, he recalled fishing for and cleaning raw shrimp around the time that he had a splinter. The Infectious Diseases Society of America recommends a treatment course extending 1 to 2 months after clinical symptoms resolve with ethambutol in addition to clarithromycin or azithromycin.8 If the infection is near a joint, rifampin should be empirically added to account for a potentially deeper infection. Imaging should be obtained to evaluate for joint space involvement, with magnetic resonance imaging being the preferred modality. If joint space involvement is confirmed, surgical debridement is indicated. Surgical debridement also is indicated for infections that fail to respond to antibiotic therapy.8
This case highlights M marinum infection as a potential mimicker of SCC, particularly if the biopsy is relatively superficial, as often occurs when obtained via the common shave technique. The distinction is critical, as M marinum infection is highly treatable and inappropriate surgery on the typical hand and finger locations may subject patients to substantial morbidity, such as the need for a skin graft, reduced mobility from scarring, or risk for serious wound infection.9 For superficial biopsies of an atypical squamous process, pathologists also may consider routinely recommending tissue culture, especially for hand and finger locations or when a history of local trauma is reported, instead of recommending complete excision or repeat biopsy alone.
- Elewski BE, Hughey LC, Hunt KM, et al. Fungal diseases. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1329-1363.
- Bravo FG. Protozoa and worms. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1470-1502.
- Zayour M, Lazova R. Pseudoepitheliomatous hyperplasia: a review. Am J Dermatopathol. 2011;33:112-122; quiz 123-126. doi:10.1097 /DAD.0b013e3181fcfb47
- Li JJ, Beresford R, Fyfe J, et al. Clinical and histopathological features of cutaneous nontuberculous mycobacterial infection: a review of 13 cases. J Cutan Pathol. 2017;44:433-443. doi:10.1111/cup.12903
- Abbas O, Marrouch N, Kattar MM, et al. Cutaneous non-tuberculous mycobacterial infections: a clinical and histopathological study of 17 cases from Lebanon. J Eur Acad Dermatol Venereol. 2011;25:33-42. doi:10.1111/j.1468-3083.2010.03684.x
- Johnson MG, Stout JE. Twenty-eight cases of Mycobacterium marinum infection: retrospective case series and literature review. Infection. 2015;43:655-662. doi:10.1007/s15010-015-0776-8
- Aubry A, Mougari F, Reibel F, et al. Mycobacterium marinum. Microbiol Spectr. 2017;5. doi:10.1128/microbiolspec.TNMI7-0038-2016
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416. doi:10.1164/rccm.200604-571ST
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385. doi:10.1001/jamadermatol.2013.6255
The Diagnosis: Atypical Mycobacterial Infection
The history of rapid growth followed by shrinkage as well as the craterlike clinical appearance of our patient’s lesion were suspicious for the keratoacanthoma variant of squamous cell carcinoma (SCC). Periodic acid–Schiff green staining was negative for fungal or bacterial organisms, and the biopsy findings of keratinocyte atypia and irregular epidermal proliferation seemed to confirm our suspicion for well-differentiated SCC (Figure 1). Our patient subsequently was scheduled for Mohs micrographic surgery. Fortunately, a sample of tissue had been sent for panculture—bacterial, fungal, and mycobacterial—to rule out infectious etiologies, given the history of possible traumatic inoculation, and returned positive for Mycobacterium marinum infection prior to the surgery. Mohs surgery was canceled, and he was referred to an infectious disease specialist who started antibiotic treatment with azithromycin, ethambutol, and rifabutin. After 1 month of treatment the lesion substantially improved (Figure 2), further supporting the diagnosis of M marinum infection over SCC.

The differential diagnosis also included sporotrichosis, leishmaniasis, and chromoblastomycosis. Sporotrichosis lesions typically develop as multiple nodules and ulcers along a path of lymphatic drainage and can exhibit asteroid bodies and cigar-shaped yeast forms on histology. Chromoblastomycosis may display pseudoepitheliomatous hyperplasia and granulomatous inflammation; however, pathognomonic pigmented Medlar bodies also likely would be present.1 Leishmaniasis has a wide variety of presentations; however, it typically occurs in patients with exposure to endemic areas outside of the United States. Although leishmaniasis may demonstrate pseudoepitheliomatous hyperplasia, ulceration, and mixed inflammation on histology, it also likely would show amastigotes within dermal macrophages.2

Atypical mycobacterial infections initially may be misdiagnosed as SCC due to their tendency to induce irregular acanthosis in the form of pseudoepitheliomatous hyperplasia as well as mild keratinocyte atypia secondary to inflammation.3,4 Our case is unique because it occurred with M marinum infection specifically. The histopathologic findings of M marinum infections are variable and may additionally include granulomas, most commonly suppurative; intraepithelial abscesses; small vessel proliferation; dermal fibrosis; multinucleated giant cells; and transepidermal elimination.4,5 Periodic acid–Schiff, Ziehl-Neelsen (acid-fast bacilli), and Fite staining may be used to distinguish M marinum infection from SCC but have low sensitivities (approximately 30%). Culture remains the most reliable test, with a sensitivity of nearly 80%.5-7 In our patient, a Periodic acid–Schiff stain was obtained prior to receiving culture results, and acid-fast bacilli and Fite staining were added after the culture returned positive; however, all 3 stains failed to highlight any mycobacteria.
The primary risk factor for infection with M marinum is contact with aquatic environments or marine animals, and most cases involve the fingers or the hand.6 After we reached the diagnosis and further discussed the patient’s history, he recalled fishing for and cleaning raw shrimp around the time that he had a splinter. The Infectious Diseases Society of America recommends a treatment course extending 1 to 2 months after clinical symptoms resolve with ethambutol in addition to clarithromycin or azithromycin.8 If the infection is near a joint, rifampin should be empirically added to account for a potentially deeper infection. Imaging should be obtained to evaluate for joint space involvement, with magnetic resonance imaging being the preferred modality. If joint space involvement is confirmed, surgical debridement is indicated. Surgical debridement also is indicated for infections that fail to respond to antibiotic therapy.8
This case highlights M marinum infection as a potential mimicker of SCC, particularly if the biopsy is relatively superficial, as often occurs when obtained via the common shave technique. The distinction is critical, as M marinum infection is highly treatable and inappropriate surgery on the typical hand and finger locations may subject patients to substantial morbidity, such as the need for a skin graft, reduced mobility from scarring, or risk for serious wound infection.9 For superficial biopsies of an atypical squamous process, pathologists also may consider routinely recommending tissue culture, especially for hand and finger locations or when a history of local trauma is reported, instead of recommending complete excision or repeat biopsy alone.
The Diagnosis: Atypical Mycobacterial Infection
The history of rapid growth followed by shrinkage as well as the craterlike clinical appearance of our patient’s lesion were suspicious for the keratoacanthoma variant of squamous cell carcinoma (SCC). Periodic acid–Schiff green staining was negative for fungal or bacterial organisms, and the biopsy findings of keratinocyte atypia and irregular epidermal proliferation seemed to confirm our suspicion for well-differentiated SCC (Figure 1). Our patient subsequently was scheduled for Mohs micrographic surgery. Fortunately, a sample of tissue had been sent for panculture—bacterial, fungal, and mycobacterial—to rule out infectious etiologies, given the history of possible traumatic inoculation, and returned positive for Mycobacterium marinum infection prior to the surgery. Mohs surgery was canceled, and he was referred to an infectious disease specialist who started antibiotic treatment with azithromycin, ethambutol, and rifabutin. After 1 month of treatment the lesion substantially improved (Figure 2), further supporting the diagnosis of M marinum infection over SCC.

The differential diagnosis also included sporotrichosis, leishmaniasis, and chromoblastomycosis. Sporotrichosis lesions typically develop as multiple nodules and ulcers along a path of lymphatic drainage and can exhibit asteroid bodies and cigar-shaped yeast forms on histology. Chromoblastomycosis may display pseudoepitheliomatous hyperplasia and granulomatous inflammation; however, pathognomonic pigmented Medlar bodies also likely would be present.1 Leishmaniasis has a wide variety of presentations; however, it typically occurs in patients with exposure to endemic areas outside of the United States. Although leishmaniasis may demonstrate pseudoepitheliomatous hyperplasia, ulceration, and mixed inflammation on histology, it also likely would show amastigotes within dermal macrophages.2

Atypical mycobacterial infections initially may be misdiagnosed as SCC due to their tendency to induce irregular acanthosis in the form of pseudoepitheliomatous hyperplasia as well as mild keratinocyte atypia secondary to inflammation.3,4 Our case is unique because it occurred with M marinum infection specifically. The histopathologic findings of M marinum infections are variable and may additionally include granulomas, most commonly suppurative; intraepithelial abscesses; small vessel proliferation; dermal fibrosis; multinucleated giant cells; and transepidermal elimination.4,5 Periodic acid–Schiff, Ziehl-Neelsen (acid-fast bacilli), and Fite staining may be used to distinguish M marinum infection from SCC but have low sensitivities (approximately 30%). Culture remains the most reliable test, with a sensitivity of nearly 80%.5-7 In our patient, a Periodic acid–Schiff stain was obtained prior to receiving culture results, and acid-fast bacilli and Fite staining were added after the culture returned positive; however, all 3 stains failed to highlight any mycobacteria.
The primary risk factor for infection with M marinum is contact with aquatic environments or marine animals, and most cases involve the fingers or the hand.6 After we reached the diagnosis and further discussed the patient’s history, he recalled fishing for and cleaning raw shrimp around the time that he had a splinter. The Infectious Diseases Society of America recommends a treatment course extending 1 to 2 months after clinical symptoms resolve with ethambutol in addition to clarithromycin or azithromycin.8 If the infection is near a joint, rifampin should be empirically added to account for a potentially deeper infection. Imaging should be obtained to evaluate for joint space involvement, with magnetic resonance imaging being the preferred modality. If joint space involvement is confirmed, surgical debridement is indicated. Surgical debridement also is indicated for infections that fail to respond to antibiotic therapy.8
This case highlights M marinum infection as a potential mimicker of SCC, particularly if the biopsy is relatively superficial, as often occurs when obtained via the common shave technique. The distinction is critical, as M marinum infection is highly treatable and inappropriate surgery on the typical hand and finger locations may subject patients to substantial morbidity, such as the need for a skin graft, reduced mobility from scarring, or risk for serious wound infection.9 For superficial biopsies of an atypical squamous process, pathologists also may consider routinely recommending tissue culture, especially for hand and finger locations or when a history of local trauma is reported, instead of recommending complete excision or repeat biopsy alone.
- Elewski BE, Hughey LC, Hunt KM, et al. Fungal diseases. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1329-1363.
- Bravo FG. Protozoa and worms. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1470-1502.
- Zayour M, Lazova R. Pseudoepitheliomatous hyperplasia: a review. Am J Dermatopathol. 2011;33:112-122; quiz 123-126. doi:10.1097 /DAD.0b013e3181fcfb47
- Li JJ, Beresford R, Fyfe J, et al. Clinical and histopathological features of cutaneous nontuberculous mycobacterial infection: a review of 13 cases. J Cutan Pathol. 2017;44:433-443. doi:10.1111/cup.12903
- Abbas O, Marrouch N, Kattar MM, et al. Cutaneous non-tuberculous mycobacterial infections: a clinical and histopathological study of 17 cases from Lebanon. J Eur Acad Dermatol Venereol. 2011;25:33-42. doi:10.1111/j.1468-3083.2010.03684.x
- Johnson MG, Stout JE. Twenty-eight cases of Mycobacterium marinum infection: retrospective case series and literature review. Infection. 2015;43:655-662. doi:10.1007/s15010-015-0776-8
- Aubry A, Mougari F, Reibel F, et al. Mycobacterium marinum. Microbiol Spectr. 2017;5. doi:10.1128/microbiolspec.TNMI7-0038-2016
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416. doi:10.1164/rccm.200604-571ST
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385. doi:10.1001/jamadermatol.2013.6255
- Elewski BE, Hughey LC, Hunt KM, et al. Fungal diseases. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1329-1363.
- Bravo FG. Protozoa and worms. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1470-1502.
- Zayour M, Lazova R. Pseudoepitheliomatous hyperplasia: a review. Am J Dermatopathol. 2011;33:112-122; quiz 123-126. doi:10.1097 /DAD.0b013e3181fcfb47
- Li JJ, Beresford R, Fyfe J, et al. Clinical and histopathological features of cutaneous nontuberculous mycobacterial infection: a review of 13 cases. J Cutan Pathol. 2017;44:433-443. doi:10.1111/cup.12903
- Abbas O, Marrouch N, Kattar MM, et al. Cutaneous non-tuberculous mycobacterial infections: a clinical and histopathological study of 17 cases from Lebanon. J Eur Acad Dermatol Venereol. 2011;25:33-42. doi:10.1111/j.1468-3083.2010.03684.x
- Johnson MG, Stout JE. Twenty-eight cases of Mycobacterium marinum infection: retrospective case series and literature review. Infection. 2015;43:655-662. doi:10.1007/s15010-015-0776-8
- Aubry A, Mougari F, Reibel F, et al. Mycobacterium marinum. Microbiol Spectr. 2017;5. doi:10.1128/microbiolspec.TNMI7-0038-2016
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416. doi:10.1164/rccm.200604-571ST
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385. doi:10.1001/jamadermatol.2013.6255
A 75-year-old man presented with a lesion on the knuckle of 5 months’ duration. He reported that the lesion initially grew very quickly before shrinking down to its current size. He denied any bleeding or pain but thought he may have had a splinter in the area around the time the lesion appeared. He reported spending a lot of time outdoors and noted several recent insect and tick bites. He also owned a boat and frequently went fishing. He previously had been treated for actinic keratoses but had no history of skin cancer and no family history of melanoma. Physical examination revealed a 2-cm erythematous nodule with central hyperkeratosis overlying the metacarpophalangeal joint of the right index finger. A shave biopsy was performed.
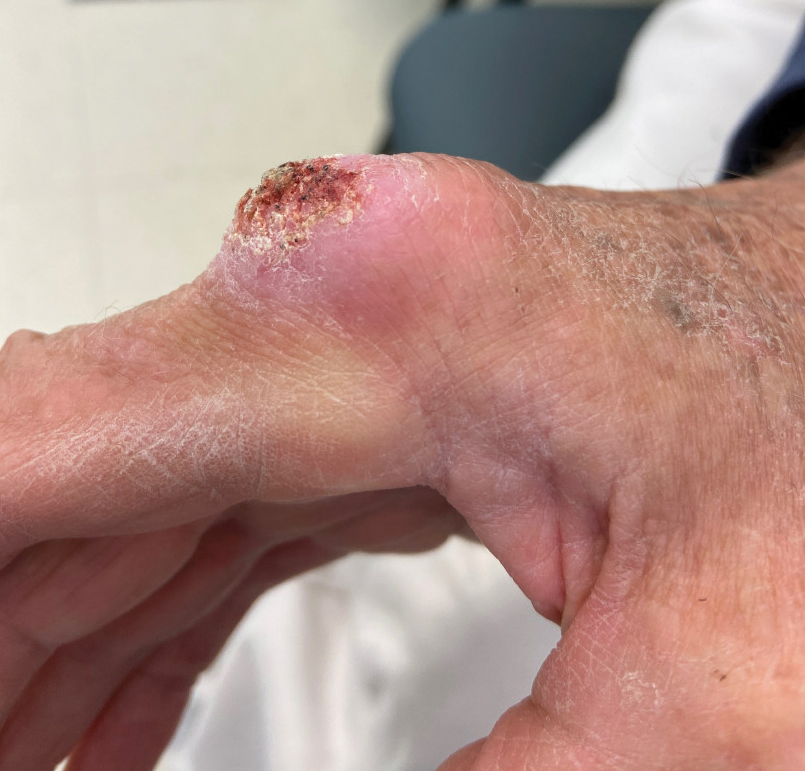
Violaceous-Purpuric Targetoid Macules and Patches With Bullae and Ulceration
The Diagnosis: Sweet Syndrome (Acute Febrile Neutrophilic Dermatosis)
A skin biopsy of the right lower extremity demonstrated diffuse interstitial, perivascular, and periadnexal neutrophilic dermal infiltrate in the reticular dermis (Figure 1), consistent with a diagnosis of Sweet syndrome without evidence of leukemia cutis or infection. The firm erythematous papulonodules with follicular accentuation on the face (Figure 2) also were confirmed as Sweet syndrome on histopathology. Concern for leukemic transformation was confirmed with bone biopsy revealing acute myeloid leukemia (AML). Our patient began a short course of prednisone, and the cutaneous lesions improved during hospitalization; however, he was lost to follow-up.
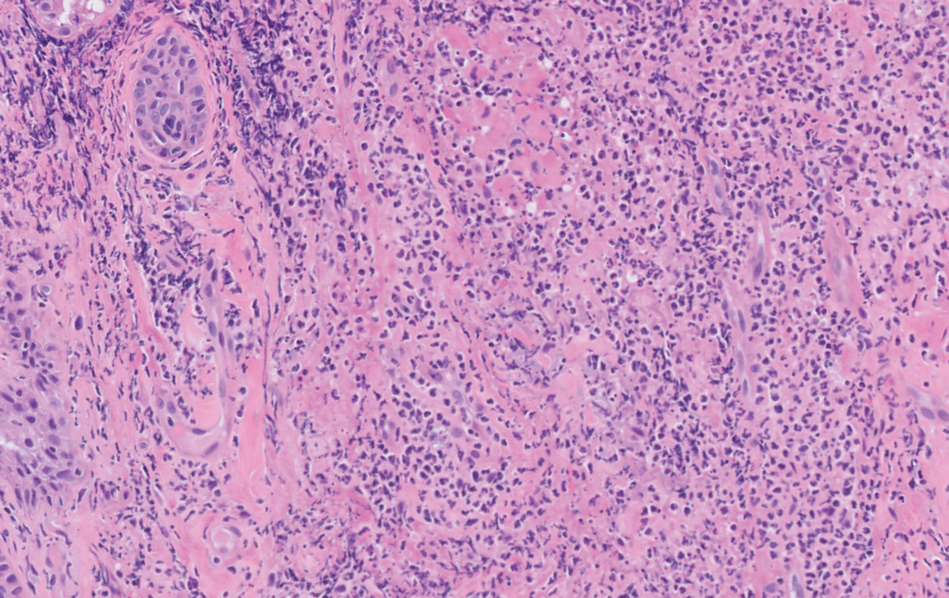
Sweet syndrome (also known as acute febrile neutrophilic dermatosis) is a rare inflammatory skin condition typically characterized by asymmetric, painful, erythematous to violaceous papules, plaques, or nodules involving the arms, face, and neck.1 It most commonly occurs in women and typically presents in patients aged 47 to 57 years. Although the pathogenesis of neutrophilic dermatoses is not completely understood, they are believed to be due to altered expression of inflammatory cytokines, irregular neutrophil function, and a genetic predisposition.2 There are 3 main categories of Sweet syndrome: classical (or idiopathic), drug induced, and malignancy associated.1 The lesions associated with Sweet syndrome vary from a few millimeters to several centimeters and may be annular or targetoid in the later stages. They also may form bullae and ulcerate. Fever, leukocytosis, and elevated acute-phase reactants also are common on presentation.1 Histopathologic analysis demonstrates an intense neutrophilic infiltrate within the reticular dermis with marked leukocytoclasia. Admixed within the neutrophil polymorphs are variable numbers of lymphocytes and histiocytes. Edema in the upper dermis also is characteristic.3 The exact pathogenesis of Sweet syndrome has yet to be elucidated but may involve a combination of cytokine dysregulation, hypersensitivity reactions, and genetics.4 Our case demonstrates 3 distinct morphologies of Sweet syndrome in a single patient, including classic edematous plaques, agminated targetoid plaques, and ulceration. Based on the clinical presentation, diagnostic workup for an undiagnosed malignancy was warranted, which confirmed AML. The malignancy-associated form of Sweet syndrome accounts for a substantial portion of cases, with approximately 21% of patients diagnosed with Sweet syndrome having an underlying malignancy, commonly a hematologic malignancy or myeloproliferative disorder with AML being the most common.1
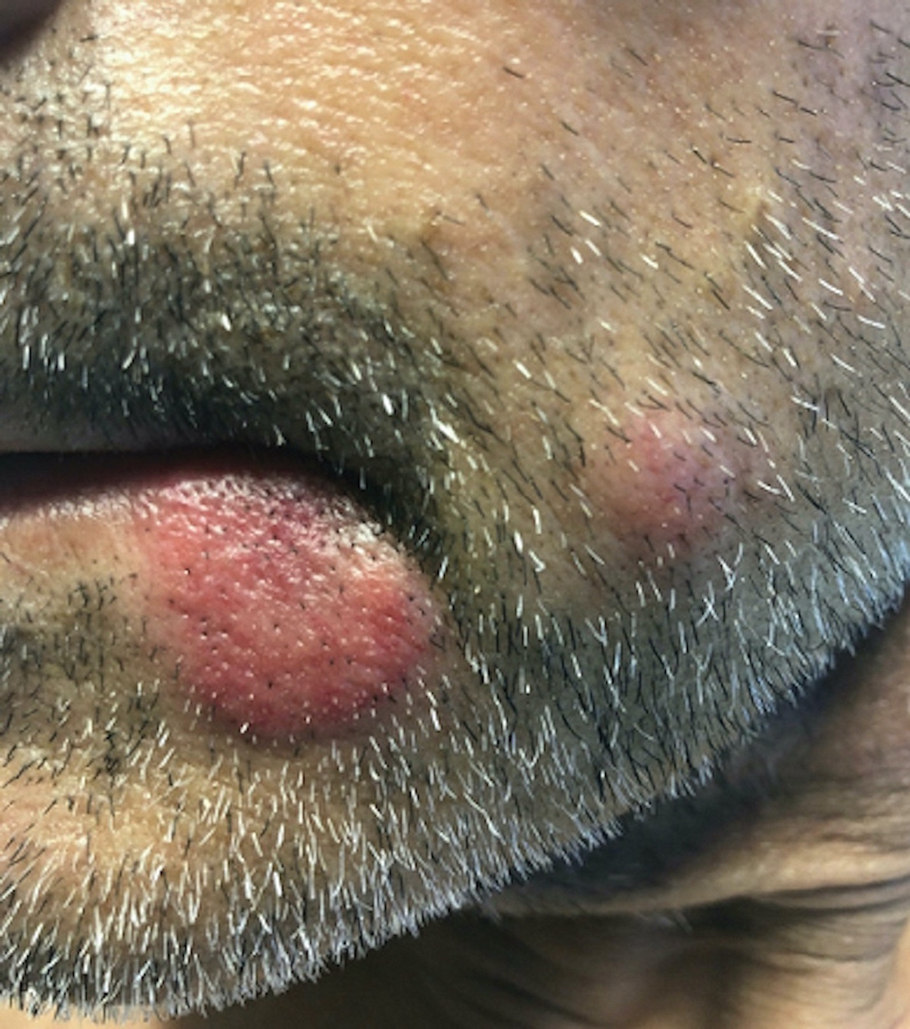
The differential diagnosis for Sweet syndrome includes cutaneous small vessel vasculitis, which commonly presents with symmetric palpable purpura of the legs. Lesions may be round, port wine–colored plaques and even may form ulcers, vesicles, and targetoid lesions. However, skin biopsy shows polymorphonuclear infiltrate affecting postcapillary venules, fibrinoid deposits, and extravasation of red blood cells.5 Leukemia cutis describes any type of leukemia that manifests in the skin. It typically presents as violaceous or red-brown papules, nodules, and plaques most commonly on the legs. Histopathology varies by immunophenotype but generally demonstrates perivascular or periadnexal involvement or a diffuse, interstitial, or nodular infiltrate of the dermis or subcutis.6 Neutrophilic eccrine hidradenitis describes an aseptic neutrophilic infiltration around eccrine coils and glands. It may present as papules or plaques that usually are erythematous but also may be pigmented. Lesions can be asymptomatic or painful as in Sweet syndrome and are distributed proximally or on the distal extremities. Histopathologic examination demonstrates the degeneration of the eccrine gland and neutrophilic inflammatory infiltrates.7 Lastly, necrotizing fasciitis is a life-threatening infection of the deep soft tissue and fascia, classically caused by group A Streptococcus. The infected site may have erythema, tenderness, fluctuance, necrosis, and bullae.8 Although our patient had a fever, he did not display the tachycardia, hypotension, tachypnea, and rapid deterioration that is common in necrotizing fasciitis.
Sweet syndrome may present with various morphologies within the same patient. Painful, erythematous to violaceous papules, plaques, nodules, bullae, and ulcers may be seen. A workup for an underlying malignancy may be warranted based on clinical presentation. Most patients have a rapid and dramatic response to systemic corticosteroids.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi:10.1186/1750-1172-2-34
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/J .JAAD.2017.11.064
- Pulido-Pérez A, Bergon-Sendin M, Sacks CA. Images in clinical medicine. N Engl J Med. 2020;16:382. doi:10.1056/NEJMicm1911025
- Marzano AV, Hilbrands L, Le ST, et al. Insights into the pathogenesis of Sweet’s syndrome. Front Immunol. 2019;10:414. doi:10.3389/fimmu.2019.00414
- Goeser MR, Laniosz V, Wetter DA. A practical approach to the diagnosis, evaluation, and management of cutaneous small-vessel vasculitis. Am J Clin Dermatol. 2014;15:299-306. doi:10.1007/s40257-014-0076-6
- Hee Cho-Vega J, Jeffrey Medeiros L, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142. doi:10.1309/WYAC YWF6NGM3WBRT
- Bachmeyer C, Aractingi S. Neutrophilic eccrine hidradenitis. Clin Dermatol. 2000;18:319-330. doi:10.1016/S0738-081X(99)00123-6
- Shimizu T, Tokuda Y. Necrotizing fasciitis. Intern Med. 2010; 49:1051-1057. doi:10.2169/internalmedicine.49.2964
The Diagnosis: Sweet Syndrome (Acute Febrile Neutrophilic Dermatosis)
A skin biopsy of the right lower extremity demonstrated diffuse interstitial, perivascular, and periadnexal neutrophilic dermal infiltrate in the reticular dermis (Figure 1), consistent with a diagnosis of Sweet syndrome without evidence of leukemia cutis or infection. The firm erythematous papulonodules with follicular accentuation on the face (Figure 2) also were confirmed as Sweet syndrome on histopathology. Concern for leukemic transformation was confirmed with bone biopsy revealing acute myeloid leukemia (AML). Our patient began a short course of prednisone, and the cutaneous lesions improved during hospitalization; however, he was lost to follow-up.

Sweet syndrome (also known as acute febrile neutrophilic dermatosis) is a rare inflammatory skin condition typically characterized by asymmetric, painful, erythematous to violaceous papules, plaques, or nodules involving the arms, face, and neck.1 It most commonly occurs in women and typically presents in patients aged 47 to 57 years. Although the pathogenesis of neutrophilic dermatoses is not completely understood, they are believed to be due to altered expression of inflammatory cytokines, irregular neutrophil function, and a genetic predisposition.2 There are 3 main categories of Sweet syndrome: classical (or idiopathic), drug induced, and malignancy associated.1 The lesions associated with Sweet syndrome vary from a few millimeters to several centimeters and may be annular or targetoid in the later stages. They also may form bullae and ulcerate. Fever, leukocytosis, and elevated acute-phase reactants also are common on presentation.1 Histopathologic analysis demonstrates an intense neutrophilic infiltrate within the reticular dermis with marked leukocytoclasia. Admixed within the neutrophil polymorphs are variable numbers of lymphocytes and histiocytes. Edema in the upper dermis also is characteristic.3 The exact pathogenesis of Sweet syndrome has yet to be elucidated but may involve a combination of cytokine dysregulation, hypersensitivity reactions, and genetics.4 Our case demonstrates 3 distinct morphologies of Sweet syndrome in a single patient, including classic edematous plaques, agminated targetoid plaques, and ulceration. Based on the clinical presentation, diagnostic workup for an undiagnosed malignancy was warranted, which confirmed AML. The malignancy-associated form of Sweet syndrome accounts for a substantial portion of cases, with approximately 21% of patients diagnosed with Sweet syndrome having an underlying malignancy, commonly a hematologic malignancy or myeloproliferative disorder with AML being the most common.1

The differential diagnosis for Sweet syndrome includes cutaneous small vessel vasculitis, which commonly presents with symmetric palpable purpura of the legs. Lesions may be round, port wine–colored plaques and even may form ulcers, vesicles, and targetoid lesions. However, skin biopsy shows polymorphonuclear infiltrate affecting postcapillary venules, fibrinoid deposits, and extravasation of red blood cells.5 Leukemia cutis describes any type of leukemia that manifests in the skin. It typically presents as violaceous or red-brown papules, nodules, and plaques most commonly on the legs. Histopathology varies by immunophenotype but generally demonstrates perivascular or periadnexal involvement or a diffuse, interstitial, or nodular infiltrate of the dermis or subcutis.6 Neutrophilic eccrine hidradenitis describes an aseptic neutrophilic infiltration around eccrine coils and glands. It may present as papules or plaques that usually are erythematous but also may be pigmented. Lesions can be asymptomatic or painful as in Sweet syndrome and are distributed proximally or on the distal extremities. Histopathologic examination demonstrates the degeneration of the eccrine gland and neutrophilic inflammatory infiltrates.7 Lastly, necrotizing fasciitis is a life-threatening infection of the deep soft tissue and fascia, classically caused by group A Streptococcus. The infected site may have erythema, tenderness, fluctuance, necrosis, and bullae.8 Although our patient had a fever, he did not display the tachycardia, hypotension, tachypnea, and rapid deterioration that is common in necrotizing fasciitis.
Sweet syndrome may present with various morphologies within the same patient. Painful, erythematous to violaceous papules, plaques, nodules, bullae, and ulcers may be seen. A workup for an underlying malignancy may be warranted based on clinical presentation. Most patients have a rapid and dramatic response to systemic corticosteroids.
The Diagnosis: Sweet Syndrome (Acute Febrile Neutrophilic Dermatosis)
A skin biopsy of the right lower extremity demonstrated diffuse interstitial, perivascular, and periadnexal neutrophilic dermal infiltrate in the reticular dermis (Figure 1), consistent with a diagnosis of Sweet syndrome without evidence of leukemia cutis or infection. The firm erythematous papulonodules with follicular accentuation on the face (Figure 2) also were confirmed as Sweet syndrome on histopathology. Concern for leukemic transformation was confirmed with bone biopsy revealing acute myeloid leukemia (AML). Our patient began a short course of prednisone, and the cutaneous lesions improved during hospitalization; however, he was lost to follow-up.

Sweet syndrome (also known as acute febrile neutrophilic dermatosis) is a rare inflammatory skin condition typically characterized by asymmetric, painful, erythematous to violaceous papules, plaques, or nodules involving the arms, face, and neck.1 It most commonly occurs in women and typically presents in patients aged 47 to 57 years. Although the pathogenesis of neutrophilic dermatoses is not completely understood, they are believed to be due to altered expression of inflammatory cytokines, irregular neutrophil function, and a genetic predisposition.2 There are 3 main categories of Sweet syndrome: classical (or idiopathic), drug induced, and malignancy associated.1 The lesions associated with Sweet syndrome vary from a few millimeters to several centimeters and may be annular or targetoid in the later stages. They also may form bullae and ulcerate. Fever, leukocytosis, and elevated acute-phase reactants also are common on presentation.1 Histopathologic analysis demonstrates an intense neutrophilic infiltrate within the reticular dermis with marked leukocytoclasia. Admixed within the neutrophil polymorphs are variable numbers of lymphocytes and histiocytes. Edema in the upper dermis also is characteristic.3 The exact pathogenesis of Sweet syndrome has yet to be elucidated but may involve a combination of cytokine dysregulation, hypersensitivity reactions, and genetics.4 Our case demonstrates 3 distinct morphologies of Sweet syndrome in a single patient, including classic edematous plaques, agminated targetoid plaques, and ulceration. Based on the clinical presentation, diagnostic workup for an undiagnosed malignancy was warranted, which confirmed AML. The malignancy-associated form of Sweet syndrome accounts for a substantial portion of cases, with approximately 21% of patients diagnosed with Sweet syndrome having an underlying malignancy, commonly a hematologic malignancy or myeloproliferative disorder with AML being the most common.1

The differential diagnosis for Sweet syndrome includes cutaneous small vessel vasculitis, which commonly presents with symmetric palpable purpura of the legs. Lesions may be round, port wine–colored plaques and even may form ulcers, vesicles, and targetoid lesions. However, skin biopsy shows polymorphonuclear infiltrate affecting postcapillary venules, fibrinoid deposits, and extravasation of red blood cells.5 Leukemia cutis describes any type of leukemia that manifests in the skin. It typically presents as violaceous or red-brown papules, nodules, and plaques most commonly on the legs. Histopathology varies by immunophenotype but generally demonstrates perivascular or periadnexal involvement or a diffuse, interstitial, or nodular infiltrate of the dermis or subcutis.6 Neutrophilic eccrine hidradenitis describes an aseptic neutrophilic infiltration around eccrine coils and glands. It may present as papules or plaques that usually are erythematous but also may be pigmented. Lesions can be asymptomatic or painful as in Sweet syndrome and are distributed proximally or on the distal extremities. Histopathologic examination demonstrates the degeneration of the eccrine gland and neutrophilic inflammatory infiltrates.7 Lastly, necrotizing fasciitis is a life-threatening infection of the deep soft tissue and fascia, classically caused by group A Streptococcus. The infected site may have erythema, tenderness, fluctuance, necrosis, and bullae.8 Although our patient had a fever, he did not display the tachycardia, hypotension, tachypnea, and rapid deterioration that is common in necrotizing fasciitis.
Sweet syndrome may present with various morphologies within the same patient. Painful, erythematous to violaceous papules, plaques, nodules, bullae, and ulcers may be seen. A workup for an underlying malignancy may be warranted based on clinical presentation. Most patients have a rapid and dramatic response to systemic corticosteroids.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi:10.1186/1750-1172-2-34
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/J .JAAD.2017.11.064
- Pulido-Pérez A, Bergon-Sendin M, Sacks CA. Images in clinical medicine. N Engl J Med. 2020;16:382. doi:10.1056/NEJMicm1911025
- Marzano AV, Hilbrands L, Le ST, et al. Insights into the pathogenesis of Sweet’s syndrome. Front Immunol. 2019;10:414. doi:10.3389/fimmu.2019.00414
- Goeser MR, Laniosz V, Wetter DA. A practical approach to the diagnosis, evaluation, and management of cutaneous small-vessel vasculitis. Am J Clin Dermatol. 2014;15:299-306. doi:10.1007/s40257-014-0076-6
- Hee Cho-Vega J, Jeffrey Medeiros L, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142. doi:10.1309/WYAC YWF6NGM3WBRT
- Bachmeyer C, Aractingi S. Neutrophilic eccrine hidradenitis. Clin Dermatol. 2000;18:319-330. doi:10.1016/S0738-081X(99)00123-6
- Shimizu T, Tokuda Y. Necrotizing fasciitis. Intern Med. 2010; 49:1051-1057. doi:10.2169/internalmedicine.49.2964
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi:10.1186/1750-1172-2-34
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/J .JAAD.2017.11.064
- Pulido-Pérez A, Bergon-Sendin M, Sacks CA. Images in clinical medicine. N Engl J Med. 2020;16:382. doi:10.1056/NEJMicm1911025
- Marzano AV, Hilbrands L, Le ST, et al. Insights into the pathogenesis of Sweet’s syndrome. Front Immunol. 2019;10:414. doi:10.3389/fimmu.2019.00414
- Goeser MR, Laniosz V, Wetter DA. A practical approach to the diagnosis, evaluation, and management of cutaneous small-vessel vasculitis. Am J Clin Dermatol. 2014;15:299-306. doi:10.1007/s40257-014-0076-6
- Hee Cho-Vega J, Jeffrey Medeiros L, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142. doi:10.1309/WYAC YWF6NGM3WBRT
- Bachmeyer C, Aractingi S. Neutrophilic eccrine hidradenitis. Clin Dermatol. 2000;18:319-330. doi:10.1016/S0738-081X(99)00123-6
- Shimizu T, Tokuda Y. Necrotizing fasciitis. Intern Med. 2010; 49:1051-1057. doi:10.2169/internalmedicine.49.2964
A 64-year-old man with long-standing myelofibrosis presented with neutropenic fevers as well as progressive painful lesions of 3 days’ duration on the legs. A bone marrow biopsy during this hospitalization demonstrated a recent progression of the patient’s myelofibrosis to acute myeloid leukemia. Physical examination revealed round to oval, violaceous, targetoid plaques. Within a week, new erythematous and nodular lesions appeared on the right arm and left vermilion border. The lesions on the legs enlarged, formed bullae, and ulcerated.
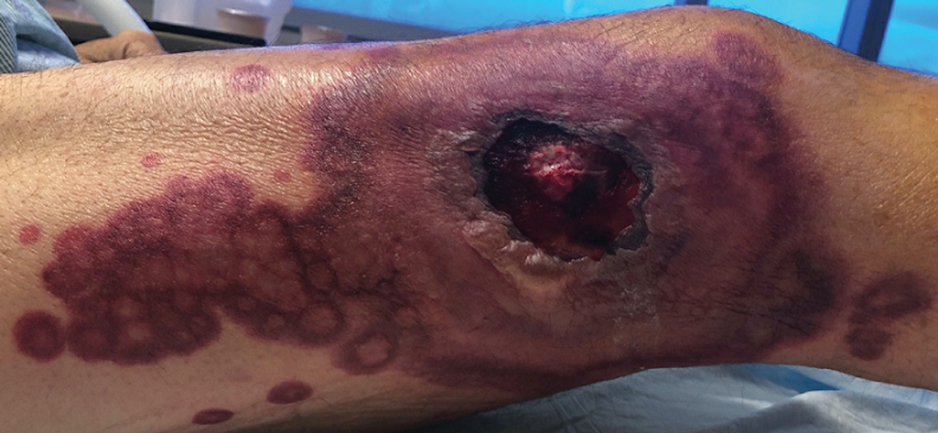
Multiple Annular Erythematous Plaques
The Diagnosis: Mid-Borderline Multibacillary Leprosy
The biopsies showed a granulomatous dermatitis involving the dermis and subcutaneous adipose tissue (Figure, A). Fite staining also revealed numerous acid-fast bacilli (AFB) throughout the dermis (Figure, B); however, polymerase chain reaction (PCR) for Mycobacterium tuberculosis was negative, and concomitant AFB tissue culture showed no growth after 8 weeks of incubation from the left wrist biopsy (Table). Interestingly, a left inguinal lymph node biopsy performed 6 months prior to presentation that helped to establish the diagnosis of follicular lymphoma also revealed nonnecrotizing granulomas and the presence of rare AFB; this formalin-fixed specimen subsequently tested negative for M tuberculosis and nontuberculous mycobacteria (NTM) by broad-range PCR. Due to a high index of suspicion, another unpreserved skin biopsy of the right knee was sent for NTM testing with PCR. Primers to 16S ribosomal RNA and the beta subunit of RNA polymerase, rpoB, gene detected Mycobacterium leprae DNA, leading to the diagnosis of mid-borderline (or borderline-borderline) multibacillary leprosy. Our patient subsequently reported subtle hypoesthesia of the plaques on the knees. He recalled eating undercooked armadillo meat in the southern United States more than 30 years prior to admission. In addition, he had a history of being incarcerated in the northeastern United States. This case was reported to the National Hansen’s Disease Program, and our patient was started on a 2-year course of daily clarithromycin, daily minocycline, and once-monthly moxifloxacin. His family also was evaluated and did not have any skin lesions concerning for leprosy.

Leprosy is a major global health concern, transmitted via breaks in the skin, respiratory secretions, and contact with armadillos. It continues to be endemic in India, Brazil, and Indonesia.1 In the United States where leprosy is nonendemic, 159 new cases were detected in 2020; the most notable risk factors in the United States are armadillo exposure and travel history.2,3 Mycobacterium leprae are intracellular bacilli that preferentially infect macrophages and Schwann cells, resulting in erythematous or hypopigmented skin lesions that often are anesthetic. Mycobacterium leprae has the longest doubling time of all bacteria with unknown in vitro growth requirements and a typical in vivo incubation period of 2 to 10 years.4 Therefore, in vitro cultures will yield no growth, as seen in our case. In our patient, Fite stain showed acid-fast organisms in multiple tissue specimens, but AFB cultures demonstrated no growth after 8 weeks of incubation. Although clinicopathologic correlation is most important, PCR analysis can help to assist in the diagnosis of leprosy. Unpreserved tissue should be used when possible, as the fixation process may adversely affect the analytic sensitivity of subsequent PCR-based assays.5 In our case, NTM were not detected by PCR in the inguinal lymph node specimen despite demonstrating rare AFB staining. This result likely was multifactorial, including the effect of formalin fixation and paraffin embedding as well as concomitant low biomass.

Leprosy is known as a great imitator, and clinical manifestations (both neurologic and cutaneous) depend on host immune response to the mycobacteria. Although tuberculoid leprosy (associated with T helper type 1 immune response) is distinguished by few asymmetric, well-demarcated, and often hypopigmented plaques, lepromatous leprosy (associated with T helper type 2 response) is characterized by numerous symmetric and poorly defined lesions. Borderline leprosy, as seen in our patient, is the most common type of leprosy and shows features of both tuberculoid and lepromatous leprosy.4 It also may be particularly difficult to diagnose.6,7 Borderline-borderline leprosy involves lesions that mostly are of the lepromatous type and symmetric but also may include raised plaques, as in tuberculoid leprosy.4 Plaques in an annular configuration with central clearing, as seen in our patient, are considered suggestive.8 Histopathology of borderline-borderline leprosy lesions shows subepidermal clear zones, and granulomas are more diffuse than in tuberculoid leprosy.4
Given the noncaseating granulomatous dermatitis seen on histopathology and the relatively higher incidence of sarcoidosis in our region of practice, our initial differential included sarcoidosis and other granulomatous disorders such as granuloma annulare. Interestingly, sarcoidosis has been misdiagnosed as leprosy on multiple occasions in countries where leprosy is endemic.9,10 Localized cutaneous leishmaniasis typically presents with infiltrated plaques and nodules that may ulcerate; diffuse and disseminated as well as mucocutaneous presentations may occur depending on the species and severity of infection. Parasitized macrophages containing amastigotes may be seen in the dermis highlighted by CD1a immunostaining. Mycosis fungoides presents as papulosquamous patches or plaques, often favoring sunprotected sites; the hypopigmented variant may mimic the central clearing seen in leprosy.
The diagnosis of leprosy can be challenging due to varying clinical presentation; indolent growth of the causative organism; and indeterminate nature of stains, including the Fite stain. Although leprosy is an uncommon diagnosis, this case underscores the need to keep it in the differential of granulomatous dermatoses in the appropriate clinical setting, particularly in patients with risk factors for exposure.8
- Blok DJ, De Vlas SJ, Richardus JH. Global elimination of leprosy by 2020: are we on track? Parasit Vectors. 2015;8:548. doi:10.1186/s13071-015-1143-4
- National Hansen’s disease (leprosy) program caring and curing since 1894. Health Resources and Services Administration website. Published April 13, 2017. Accessed November 17, 2022. https://www.hrsa.gov/hansens-disease/index.html
- Aslam S, Peraza J, Mekaiel A, et al. Major risk factors for leprosy in a non-endemic area of the United States: a case series. IDCases. 2019;17:E00557. doi:10.1016/j.idcr.2019.e00557
- Kundakci N, Erdem C. Leprosy: a great imitator. Clin Dermatol. 2019;37:200-212. doi:10.1016/j.clindermatol.2019.01.002
- Marchetti G, Gori A, Catozzi L, et al. Evaluation of PCR in detection of Mycobacterium tuberculosis from formalin-fixed, paraffin-embedded tissues: comparison of four amplification assays. J Clin Microbiol. 1998;36:1512-1517.
- Pawar M, Zawar V. Mid-borderline leprosy masquerading as an overlap syndrome. Rheumatology (Oxford). 2018;57:1686-1688. doi:10.1093 /rheumatology/key125
- Day W, Prodanovic E. Borderline lepromatous leprosy masking as tinea versicolor. Int J Dermatol. 2019;58:E125-E126. doi:10.1111/ijd.14439
- Lastória JC, de Abreu MAMM. Leprosy: review of the epidemiological, clinical, and etiopathogenic aspects: part 1. An Bras Dermatol. 2014;89:205-218. doi:10.1590/abd1806-4841.20142450
- Kaushik A, Vinay K, Narang T, et al. Ichthyosiform sarcoidosis: a mimic of leprosy? Clin Exp Dermatol. 2019;44:677-680. doi:10.1111/ced.13863
- Chowdhary KN, Rao R, Priya P, et al. Cutaneous sarcoidosis misdiagnosed as leprosy. report of two cases and review of literature. Indian J Lepr. 2016;88:177-183.
The Diagnosis: Mid-Borderline Multibacillary Leprosy
The biopsies showed a granulomatous dermatitis involving the dermis and subcutaneous adipose tissue (Figure, A). Fite staining also revealed numerous acid-fast bacilli (AFB) throughout the dermis (Figure, B); however, polymerase chain reaction (PCR) for Mycobacterium tuberculosis was negative, and concomitant AFB tissue culture showed no growth after 8 weeks of incubation from the left wrist biopsy (Table). Interestingly, a left inguinal lymph node biopsy performed 6 months prior to presentation that helped to establish the diagnosis of follicular lymphoma also revealed nonnecrotizing granulomas and the presence of rare AFB; this formalin-fixed specimen subsequently tested negative for M tuberculosis and nontuberculous mycobacteria (NTM) by broad-range PCR. Due to a high index of suspicion, another unpreserved skin biopsy of the right knee was sent for NTM testing with PCR. Primers to 16S ribosomal RNA and the beta subunit of RNA polymerase, rpoB, gene detected Mycobacterium leprae DNA, leading to the diagnosis of mid-borderline (or borderline-borderline) multibacillary leprosy. Our patient subsequently reported subtle hypoesthesia of the plaques on the knees. He recalled eating undercooked armadillo meat in the southern United States more than 30 years prior to admission. In addition, he had a history of being incarcerated in the northeastern United States. This case was reported to the National Hansen’s Disease Program, and our patient was started on a 2-year course of daily clarithromycin, daily minocycline, and once-monthly moxifloxacin. His family also was evaluated and did not have any skin lesions concerning for leprosy.

Leprosy is a major global health concern, transmitted via breaks in the skin, respiratory secretions, and contact with armadillos. It continues to be endemic in India, Brazil, and Indonesia.1 In the United States where leprosy is nonendemic, 159 new cases were detected in 2020; the most notable risk factors in the United States are armadillo exposure and travel history.2,3 Mycobacterium leprae are intracellular bacilli that preferentially infect macrophages and Schwann cells, resulting in erythematous or hypopigmented skin lesions that often are anesthetic. Mycobacterium leprae has the longest doubling time of all bacteria with unknown in vitro growth requirements and a typical in vivo incubation period of 2 to 10 years.4 Therefore, in vitro cultures will yield no growth, as seen in our case. In our patient, Fite stain showed acid-fast organisms in multiple tissue specimens, but AFB cultures demonstrated no growth after 8 weeks of incubation. Although clinicopathologic correlation is most important, PCR analysis can help to assist in the diagnosis of leprosy. Unpreserved tissue should be used when possible, as the fixation process may adversely affect the analytic sensitivity of subsequent PCR-based assays.5 In our case, NTM were not detected by PCR in the inguinal lymph node specimen despite demonstrating rare AFB staining. This result likely was multifactorial, including the effect of formalin fixation and paraffin embedding as well as concomitant low biomass.

Leprosy is known as a great imitator, and clinical manifestations (both neurologic and cutaneous) depend on host immune response to the mycobacteria. Although tuberculoid leprosy (associated with T helper type 1 immune response) is distinguished by few asymmetric, well-demarcated, and often hypopigmented plaques, lepromatous leprosy (associated with T helper type 2 response) is characterized by numerous symmetric and poorly defined lesions. Borderline leprosy, as seen in our patient, is the most common type of leprosy and shows features of both tuberculoid and lepromatous leprosy.4 It also may be particularly difficult to diagnose.6,7 Borderline-borderline leprosy involves lesions that mostly are of the lepromatous type and symmetric but also may include raised plaques, as in tuberculoid leprosy.4 Plaques in an annular configuration with central clearing, as seen in our patient, are considered suggestive.8 Histopathology of borderline-borderline leprosy lesions shows subepidermal clear zones, and granulomas are more diffuse than in tuberculoid leprosy.4
Given the noncaseating granulomatous dermatitis seen on histopathology and the relatively higher incidence of sarcoidosis in our region of practice, our initial differential included sarcoidosis and other granulomatous disorders such as granuloma annulare. Interestingly, sarcoidosis has been misdiagnosed as leprosy on multiple occasions in countries where leprosy is endemic.9,10 Localized cutaneous leishmaniasis typically presents with infiltrated plaques and nodules that may ulcerate; diffuse and disseminated as well as mucocutaneous presentations may occur depending on the species and severity of infection. Parasitized macrophages containing amastigotes may be seen in the dermis highlighted by CD1a immunostaining. Mycosis fungoides presents as papulosquamous patches or plaques, often favoring sunprotected sites; the hypopigmented variant may mimic the central clearing seen in leprosy.
The diagnosis of leprosy can be challenging due to varying clinical presentation; indolent growth of the causative organism; and indeterminate nature of stains, including the Fite stain. Although leprosy is an uncommon diagnosis, this case underscores the need to keep it in the differential of granulomatous dermatoses in the appropriate clinical setting, particularly in patients with risk factors for exposure.8
The Diagnosis: Mid-Borderline Multibacillary Leprosy
The biopsies showed a granulomatous dermatitis involving the dermis and subcutaneous adipose tissue (Figure, A). Fite staining also revealed numerous acid-fast bacilli (AFB) throughout the dermis (Figure, B); however, polymerase chain reaction (PCR) for Mycobacterium tuberculosis was negative, and concomitant AFB tissue culture showed no growth after 8 weeks of incubation from the left wrist biopsy (Table). Interestingly, a left inguinal lymph node biopsy performed 6 months prior to presentation that helped to establish the diagnosis of follicular lymphoma also revealed nonnecrotizing granulomas and the presence of rare AFB; this formalin-fixed specimen subsequently tested negative for M tuberculosis and nontuberculous mycobacteria (NTM) by broad-range PCR. Due to a high index of suspicion, another unpreserved skin biopsy of the right knee was sent for NTM testing with PCR. Primers to 16S ribosomal RNA and the beta subunit of RNA polymerase, rpoB, gene detected Mycobacterium leprae DNA, leading to the diagnosis of mid-borderline (or borderline-borderline) multibacillary leprosy. Our patient subsequently reported subtle hypoesthesia of the plaques on the knees. He recalled eating undercooked armadillo meat in the southern United States more than 30 years prior to admission. In addition, he had a history of being incarcerated in the northeastern United States. This case was reported to the National Hansen’s Disease Program, and our patient was started on a 2-year course of daily clarithromycin, daily minocycline, and once-monthly moxifloxacin. His family also was evaluated and did not have any skin lesions concerning for leprosy.

Leprosy is a major global health concern, transmitted via breaks in the skin, respiratory secretions, and contact with armadillos. It continues to be endemic in India, Brazil, and Indonesia.1 In the United States where leprosy is nonendemic, 159 new cases were detected in 2020; the most notable risk factors in the United States are armadillo exposure and travel history.2,3 Mycobacterium leprae are intracellular bacilli that preferentially infect macrophages and Schwann cells, resulting in erythematous or hypopigmented skin lesions that often are anesthetic. Mycobacterium leprae has the longest doubling time of all bacteria with unknown in vitro growth requirements and a typical in vivo incubation period of 2 to 10 years.4 Therefore, in vitro cultures will yield no growth, as seen in our case. In our patient, Fite stain showed acid-fast organisms in multiple tissue specimens, but AFB cultures demonstrated no growth after 8 weeks of incubation. Although clinicopathologic correlation is most important, PCR analysis can help to assist in the diagnosis of leprosy. Unpreserved tissue should be used when possible, as the fixation process may adversely affect the analytic sensitivity of subsequent PCR-based assays.5 In our case, NTM were not detected by PCR in the inguinal lymph node specimen despite demonstrating rare AFB staining. This result likely was multifactorial, including the effect of formalin fixation and paraffin embedding as well as concomitant low biomass.

Leprosy is known as a great imitator, and clinical manifestations (both neurologic and cutaneous) depend on host immune response to the mycobacteria. Although tuberculoid leprosy (associated with T helper type 1 immune response) is distinguished by few asymmetric, well-demarcated, and often hypopigmented plaques, lepromatous leprosy (associated with T helper type 2 response) is characterized by numerous symmetric and poorly defined lesions. Borderline leprosy, as seen in our patient, is the most common type of leprosy and shows features of both tuberculoid and lepromatous leprosy.4 It also may be particularly difficult to diagnose.6,7 Borderline-borderline leprosy involves lesions that mostly are of the lepromatous type and symmetric but also may include raised plaques, as in tuberculoid leprosy.4 Plaques in an annular configuration with central clearing, as seen in our patient, are considered suggestive.8 Histopathology of borderline-borderline leprosy lesions shows subepidermal clear zones, and granulomas are more diffuse than in tuberculoid leprosy.4
Given the noncaseating granulomatous dermatitis seen on histopathology and the relatively higher incidence of sarcoidosis in our region of practice, our initial differential included sarcoidosis and other granulomatous disorders such as granuloma annulare. Interestingly, sarcoidosis has been misdiagnosed as leprosy on multiple occasions in countries where leprosy is endemic.9,10 Localized cutaneous leishmaniasis typically presents with infiltrated plaques and nodules that may ulcerate; diffuse and disseminated as well as mucocutaneous presentations may occur depending on the species and severity of infection. Parasitized macrophages containing amastigotes may be seen in the dermis highlighted by CD1a immunostaining. Mycosis fungoides presents as papulosquamous patches or plaques, often favoring sunprotected sites; the hypopigmented variant may mimic the central clearing seen in leprosy.
The diagnosis of leprosy can be challenging due to varying clinical presentation; indolent growth of the causative organism; and indeterminate nature of stains, including the Fite stain. Although leprosy is an uncommon diagnosis, this case underscores the need to keep it in the differential of granulomatous dermatoses in the appropriate clinical setting, particularly in patients with risk factors for exposure.8
- Blok DJ, De Vlas SJ, Richardus JH. Global elimination of leprosy by 2020: are we on track? Parasit Vectors. 2015;8:548. doi:10.1186/s13071-015-1143-4
- National Hansen’s disease (leprosy) program caring and curing since 1894. Health Resources and Services Administration website. Published April 13, 2017. Accessed November 17, 2022. https://www.hrsa.gov/hansens-disease/index.html
- Aslam S, Peraza J, Mekaiel A, et al. Major risk factors for leprosy in a non-endemic area of the United States: a case series. IDCases. 2019;17:E00557. doi:10.1016/j.idcr.2019.e00557
- Kundakci N, Erdem C. Leprosy: a great imitator. Clin Dermatol. 2019;37:200-212. doi:10.1016/j.clindermatol.2019.01.002
- Marchetti G, Gori A, Catozzi L, et al. Evaluation of PCR in detection of Mycobacterium tuberculosis from formalin-fixed, paraffin-embedded tissues: comparison of four amplification assays. J Clin Microbiol. 1998;36:1512-1517.
- Pawar M, Zawar V. Mid-borderline leprosy masquerading as an overlap syndrome. Rheumatology (Oxford). 2018;57:1686-1688. doi:10.1093 /rheumatology/key125
- Day W, Prodanovic E. Borderline lepromatous leprosy masking as tinea versicolor. Int J Dermatol. 2019;58:E125-E126. doi:10.1111/ijd.14439
- Lastória JC, de Abreu MAMM. Leprosy: review of the epidemiological, clinical, and etiopathogenic aspects: part 1. An Bras Dermatol. 2014;89:205-218. doi:10.1590/abd1806-4841.20142450
- Kaushik A, Vinay K, Narang T, et al. Ichthyosiform sarcoidosis: a mimic of leprosy? Clin Exp Dermatol. 2019;44:677-680. doi:10.1111/ced.13863
- Chowdhary KN, Rao R, Priya P, et al. Cutaneous sarcoidosis misdiagnosed as leprosy. report of two cases and review of literature. Indian J Lepr. 2016;88:177-183.
- Blok DJ, De Vlas SJ, Richardus JH. Global elimination of leprosy by 2020: are we on track? Parasit Vectors. 2015;8:548. doi:10.1186/s13071-015-1143-4
- National Hansen’s disease (leprosy) program caring and curing since 1894. Health Resources and Services Administration website. Published April 13, 2017. Accessed November 17, 2022. https://www.hrsa.gov/hansens-disease/index.html
- Aslam S, Peraza J, Mekaiel A, et al. Major risk factors for leprosy in a non-endemic area of the United States: a case series. IDCases. 2019;17:E00557. doi:10.1016/j.idcr.2019.e00557
- Kundakci N, Erdem C. Leprosy: a great imitator. Clin Dermatol. 2019;37:200-212. doi:10.1016/j.clindermatol.2019.01.002
- Marchetti G, Gori A, Catozzi L, et al. Evaluation of PCR in detection of Mycobacterium tuberculosis from formalin-fixed, paraffin-embedded tissues: comparison of four amplification assays. J Clin Microbiol. 1998;36:1512-1517.
- Pawar M, Zawar V. Mid-borderline leprosy masquerading as an overlap syndrome. Rheumatology (Oxford). 2018;57:1686-1688. doi:10.1093 /rheumatology/key125
- Day W, Prodanovic E. Borderline lepromatous leprosy masking as tinea versicolor. Int J Dermatol. 2019;58:E125-E126. doi:10.1111/ijd.14439
- Lastória JC, de Abreu MAMM. Leprosy: review of the epidemiological, clinical, and etiopathogenic aspects: part 1. An Bras Dermatol. 2014;89:205-218. doi:10.1590/abd1806-4841.20142450
- Kaushik A, Vinay K, Narang T, et al. Ichthyosiform sarcoidosis: a mimic of leprosy? Clin Exp Dermatol. 2019;44:677-680. doi:10.1111/ced.13863
- Chowdhary KN, Rao R, Priya P, et al. Cutaneous sarcoidosis misdiagnosed as leprosy. report of two cases and review of literature. Indian J Lepr. 2016;88:177-183.
A 59-year-old man was admitted to the medical ward with multiple annular erythematous plaques and polyarthralgia of several months’ duration. His medical history included low-grade stage IIA follicular lymphoma diagnosed 6 months prior to presentation, substance abuse with opiates and cocaine, coronary artery disease, ascending aortic aneurysm, and chronic lower back pain. Physical examination revealed multiple red to red-brown papules and plaques, some in an annular configuration, that were distributed on the cheeks, left wrist, knees, dorsal feet, and soles. Bilateral inguinal lymphadenopathy also was noted. Serological testing for HIV, hepatitis B and C viruses, Treponema pallidum, Borrelia burgdorferi, and tuberculosis assay were negative. Arthrocentesis of the left wrist 1 week prior to admission noted 5333 nucleated cells/μL (reference range, <3000 cells/μL) and no crystals; culture of the fluid was sterile. Skin biopsies of plaques on the left wrist, left dorsal foot, and right knee were obtained for histopathologic analysis.
Yellow Nodule on the Scalp
The Diagnosis: Solitary Sclerotic Fibroma
Based on the clinical and histologic findings, the patient was diagnosed with solitary sclerotic fibroma (SF). Sclerotic fibroma is a rare benign tumor that first was described in 1972 by Weary et al1 in the oral mucosa of a patient with Cowden syndrome, a genodermatosis associated with multiple benign and malignant tumors. Rapini and Golitz2 reported solitary SF in 11 otherwise-healthy individuals with no signs of multiple hamartoma syndrome. Solitary SF is a sporadic benign condition, whereas multiple lesions are suggestive of Cowden syndrome. Solitary SF most commonly appears as an asymptomatic white-yellow papule or nodule on the head or neck, though larger tumors have been reported on the trunk and extremities.3 Histologic features of solitary SF include a well-circumscribed dermal nodule composed of eosinophilic dense collagen bundles arranged in a plywoodlike pattern (Figure). Immunohistochemistry is positive for CD34 and vimentin but negative for S-100, epithelial membrane antigen, and neuron-specific enolase.4
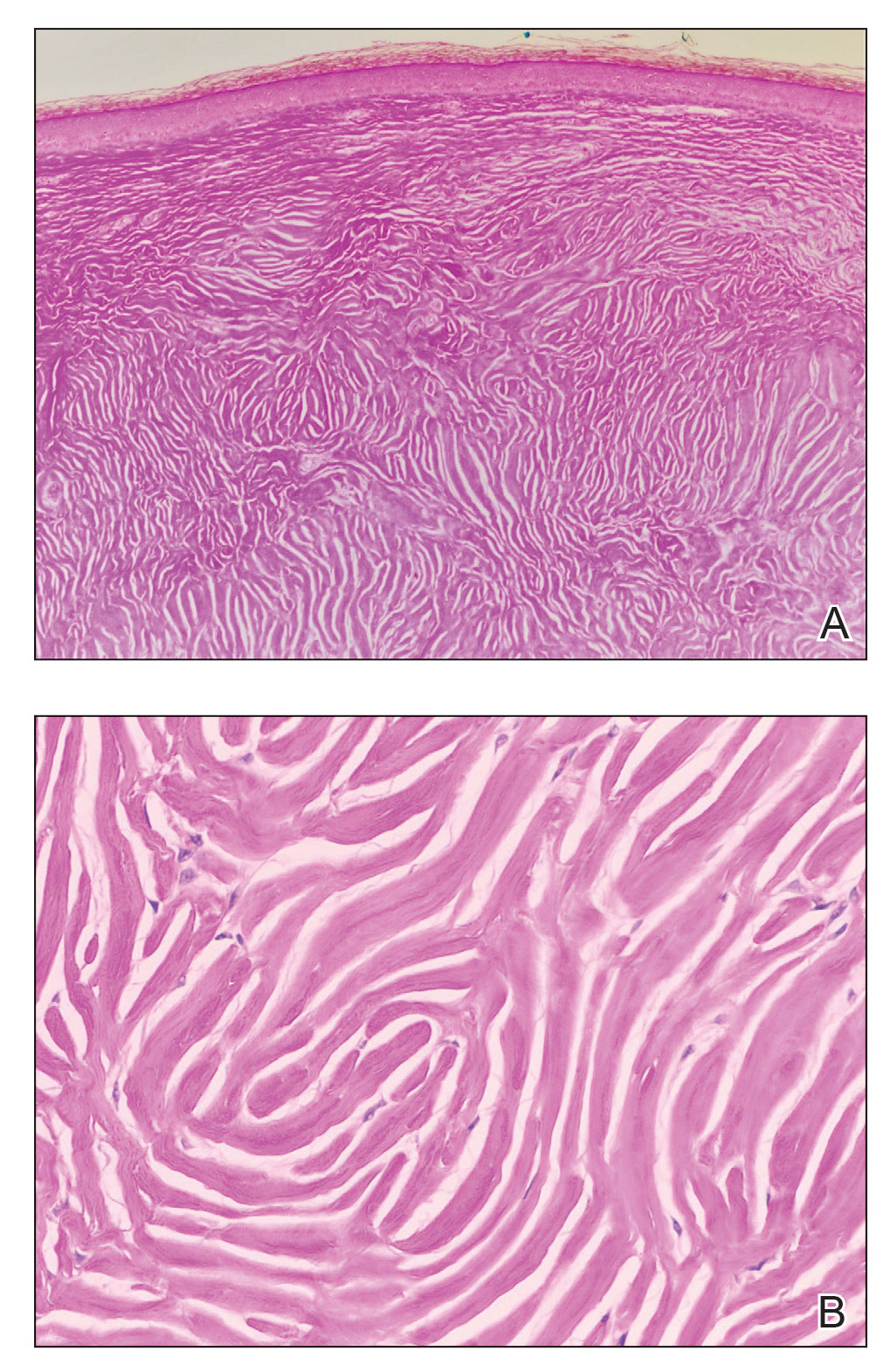
The differential diagnosis of solitary SF of the head and neck includes sebaceous adenoma, pilar cyst, nodular basal cell carcinoma, and giant molluscum contagiosum. Sebaceous adenomas usually are solitary yellow nodules less than 1 cm in diameter and located on the head and neck. They are the most common sebaceous neoplasm associated with Muir-Torre syndrome, an autosomal-dominant disorder characterized by sebaceous adenoma or carcinoma and colorectal cancer. Histopathology demonstrates well-circumscribed, round aggregations of mature lipid-filled sebocytes with a rim of basaloid germinative cells at the periphery. Pilar cysts typically are flesh-colored subcutaneous nodules on the scalp that are freely mobile over underlying tissue. Histopathology shows stratified squamous epithelium lining and trichilemmal keratinization. Nodular basal cell carcinoma has a pearly translucent appearance and arborizing telangiectases. Histopathology demonstrates nests of basaloid cells with palisading of the cells at the periphery. Giant solitary molluscum contagiosum is a dome-shaped, flesh-colored nodule with central umbilication. Histopathology reveals hyperplastic squamous epithelium with characteristic eosinophilic inclusion bodies above the basal layer.
Solitary SF can be difficult to diagnose based solely on the clinical presentation; thus biopsy with histologic evaluation is recommended. If SF is confirmed, the clinician should inquire about a family history of Cowden syndrome and then perform a total-body skin examination to check for multiple SF and other clinical hamartomas of Cowden syndrome such as trichilemmomas, acral keratosis, and oral papillomas.
- Weary PE, Gorlin RJ, Gentry Jr WC, et al. Multiple hamartoma syndrome (Cowden’s disease). Arch Dermatol. 1972;106:682-690.
- Rapini RP, Golitz LE. Sclerotic fibromas of the skin. J Am Acad Dermatol. 1989;20(2 pt 1):266-271.
- Tosa M, Ansai S, Kuwahara H, et al. Two cases of sclerotic fibroma of the skin that mimicked keloids clinically. J Nippon Med Sch. 2018;85:283-286.
- High WA, Stewart D, Essary LR, et al. Sclerotic fibroma-like changes in various neoplastic and inflammatory skin lesions: is sclerotic fibroma a distinct entity? J Cutan Pathol. 2004;31:373-378.
The Diagnosis: Solitary Sclerotic Fibroma
Based on the clinical and histologic findings, the patient was diagnosed with solitary sclerotic fibroma (SF). Sclerotic fibroma is a rare benign tumor that first was described in 1972 by Weary et al1 in the oral mucosa of a patient with Cowden syndrome, a genodermatosis associated with multiple benign and malignant tumors. Rapini and Golitz2 reported solitary SF in 11 otherwise-healthy individuals with no signs of multiple hamartoma syndrome. Solitary SF is a sporadic benign condition, whereas multiple lesions are suggestive of Cowden syndrome. Solitary SF most commonly appears as an asymptomatic white-yellow papule or nodule on the head or neck, though larger tumors have been reported on the trunk and extremities.3 Histologic features of solitary SF include a well-circumscribed dermal nodule composed of eosinophilic dense collagen bundles arranged in a plywoodlike pattern (Figure). Immunohistochemistry is positive for CD34 and vimentin but negative for S-100, epithelial membrane antigen, and neuron-specific enolase.4

The differential diagnosis of solitary SF of the head and neck includes sebaceous adenoma, pilar cyst, nodular basal cell carcinoma, and giant molluscum contagiosum. Sebaceous adenomas usually are solitary yellow nodules less than 1 cm in diameter and located on the head and neck. They are the most common sebaceous neoplasm associated with Muir-Torre syndrome, an autosomal-dominant disorder characterized by sebaceous adenoma or carcinoma and colorectal cancer. Histopathology demonstrates well-circumscribed, round aggregations of mature lipid-filled sebocytes with a rim of basaloid germinative cells at the periphery. Pilar cysts typically are flesh-colored subcutaneous nodules on the scalp that are freely mobile over underlying tissue. Histopathology shows stratified squamous epithelium lining and trichilemmal keratinization. Nodular basal cell carcinoma has a pearly translucent appearance and arborizing telangiectases. Histopathology demonstrates nests of basaloid cells with palisading of the cells at the periphery. Giant solitary molluscum contagiosum is a dome-shaped, flesh-colored nodule with central umbilication. Histopathology reveals hyperplastic squamous epithelium with characteristic eosinophilic inclusion bodies above the basal layer.
Solitary SF can be difficult to diagnose based solely on the clinical presentation; thus biopsy with histologic evaluation is recommended. If SF is confirmed, the clinician should inquire about a family history of Cowden syndrome and then perform a total-body skin examination to check for multiple SF and other clinical hamartomas of Cowden syndrome such as trichilemmomas, acral keratosis, and oral papillomas.
The Diagnosis: Solitary Sclerotic Fibroma
Based on the clinical and histologic findings, the patient was diagnosed with solitary sclerotic fibroma (SF). Sclerotic fibroma is a rare benign tumor that first was described in 1972 by Weary et al1 in the oral mucosa of a patient with Cowden syndrome, a genodermatosis associated with multiple benign and malignant tumors. Rapini and Golitz2 reported solitary SF in 11 otherwise-healthy individuals with no signs of multiple hamartoma syndrome. Solitary SF is a sporadic benign condition, whereas multiple lesions are suggestive of Cowden syndrome. Solitary SF most commonly appears as an asymptomatic white-yellow papule or nodule on the head or neck, though larger tumors have been reported on the trunk and extremities.3 Histologic features of solitary SF include a well-circumscribed dermal nodule composed of eosinophilic dense collagen bundles arranged in a plywoodlike pattern (Figure). Immunohistochemistry is positive for CD34 and vimentin but negative for S-100, epithelial membrane antigen, and neuron-specific enolase.4

The differential diagnosis of solitary SF of the head and neck includes sebaceous adenoma, pilar cyst, nodular basal cell carcinoma, and giant molluscum contagiosum. Sebaceous adenomas usually are solitary yellow nodules less than 1 cm in diameter and located on the head and neck. They are the most common sebaceous neoplasm associated with Muir-Torre syndrome, an autosomal-dominant disorder characterized by sebaceous adenoma or carcinoma and colorectal cancer. Histopathology demonstrates well-circumscribed, round aggregations of mature lipid-filled sebocytes with a rim of basaloid germinative cells at the periphery. Pilar cysts typically are flesh-colored subcutaneous nodules on the scalp that are freely mobile over underlying tissue. Histopathology shows stratified squamous epithelium lining and trichilemmal keratinization. Nodular basal cell carcinoma has a pearly translucent appearance and arborizing telangiectases. Histopathology demonstrates nests of basaloid cells with palisading of the cells at the periphery. Giant solitary molluscum contagiosum is a dome-shaped, flesh-colored nodule with central umbilication. Histopathology reveals hyperplastic squamous epithelium with characteristic eosinophilic inclusion bodies above the basal layer.
Solitary SF can be difficult to diagnose based solely on the clinical presentation; thus biopsy with histologic evaluation is recommended. If SF is confirmed, the clinician should inquire about a family history of Cowden syndrome and then perform a total-body skin examination to check for multiple SF and other clinical hamartomas of Cowden syndrome such as trichilemmomas, acral keratosis, and oral papillomas.
- Weary PE, Gorlin RJ, Gentry Jr WC, et al. Multiple hamartoma syndrome (Cowden’s disease). Arch Dermatol. 1972;106:682-690.
- Rapini RP, Golitz LE. Sclerotic fibromas of the skin. J Am Acad Dermatol. 1989;20(2 pt 1):266-271.
- Tosa M, Ansai S, Kuwahara H, et al. Two cases of sclerotic fibroma of the skin that mimicked keloids clinically. J Nippon Med Sch. 2018;85:283-286.
- High WA, Stewart D, Essary LR, et al. Sclerotic fibroma-like changes in various neoplastic and inflammatory skin lesions: is sclerotic fibroma a distinct entity? J Cutan Pathol. 2004;31:373-378.
- Weary PE, Gorlin RJ, Gentry Jr WC, et al. Multiple hamartoma syndrome (Cowden’s disease). Arch Dermatol. 1972;106:682-690.
- Rapini RP, Golitz LE. Sclerotic fibromas of the skin. J Am Acad Dermatol. 1989;20(2 pt 1):266-271.
- Tosa M, Ansai S, Kuwahara H, et al. Two cases of sclerotic fibroma of the skin that mimicked keloids clinically. J Nippon Med Sch. 2018;85:283-286.
- High WA, Stewart D, Essary LR, et al. Sclerotic fibroma-like changes in various neoplastic and inflammatory skin lesions: is sclerotic fibroma a distinct entity? J Cutan Pathol. 2004;31:373-378.
A 45-year-old woman was referred to dermatology by a primary care physician for evaluation of a raised skin lesion on the scalp. She was otherwise healthy. The lesion had been present for many years but recently grew in size. The patient reported that the lesion was subject to recurrent physical trauma and she wanted it removed. Physical examination revealed a 6×6-mm, domeshaped, yellow nodule on the left inferior parietal scalp. There were no similar lesions located elsewhere on the body. A shave removal was performed and sent for histopathologic evaluation.