User login
Painful Nodules With a Crawling Sensation
The Diagnosis: Cutaneous Furuncular Myiasis
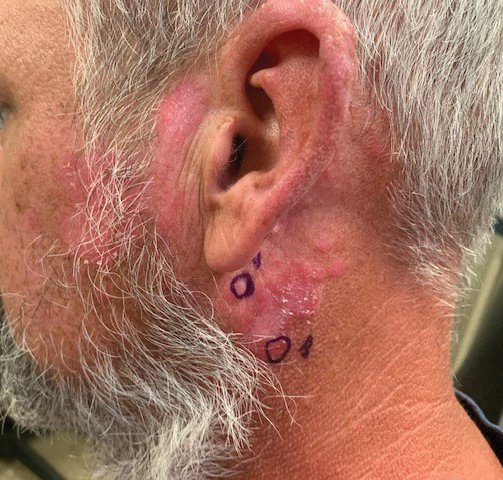
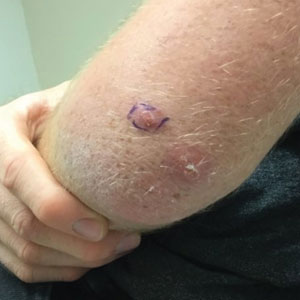
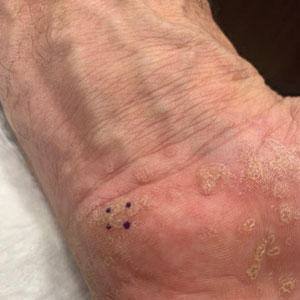
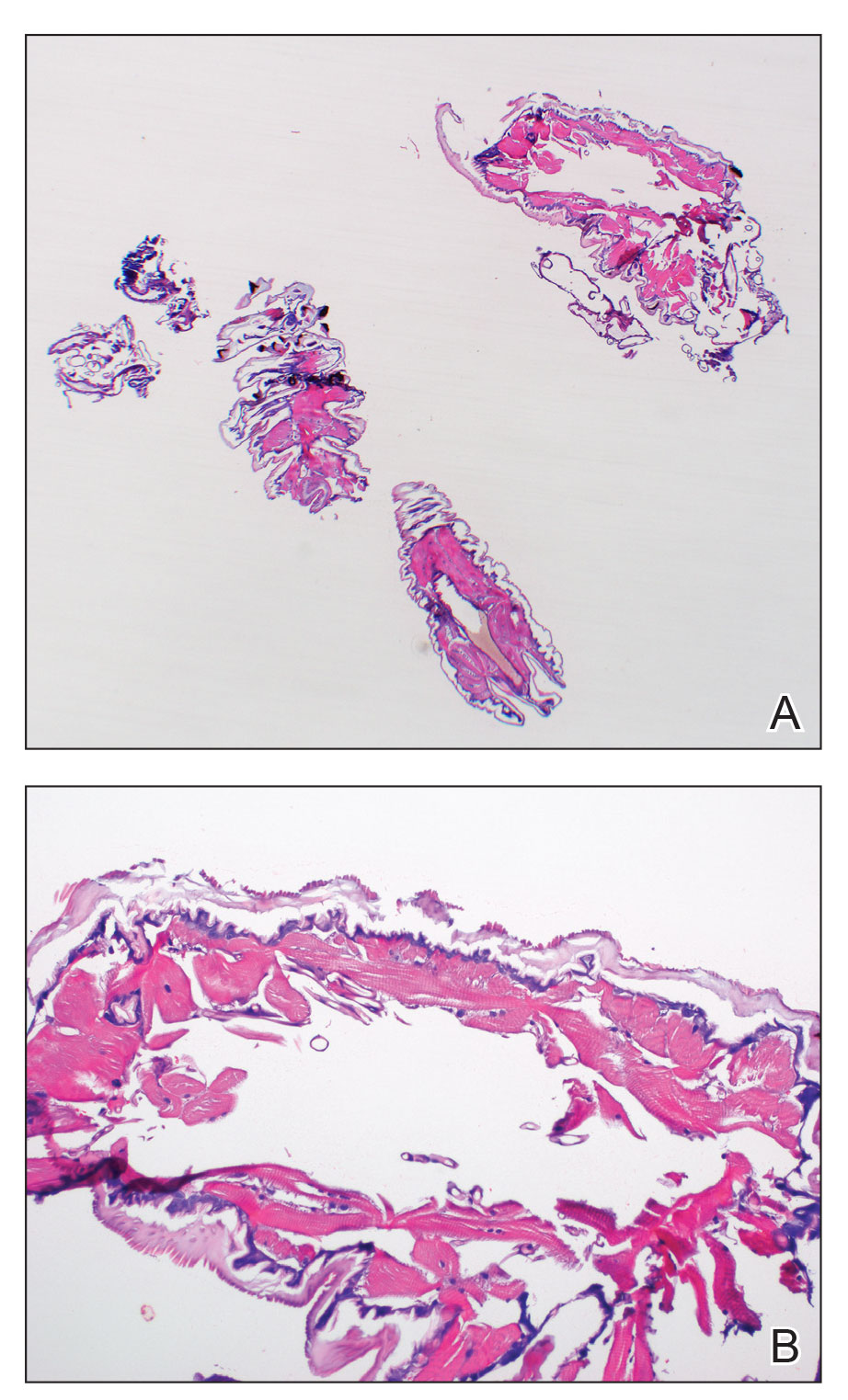
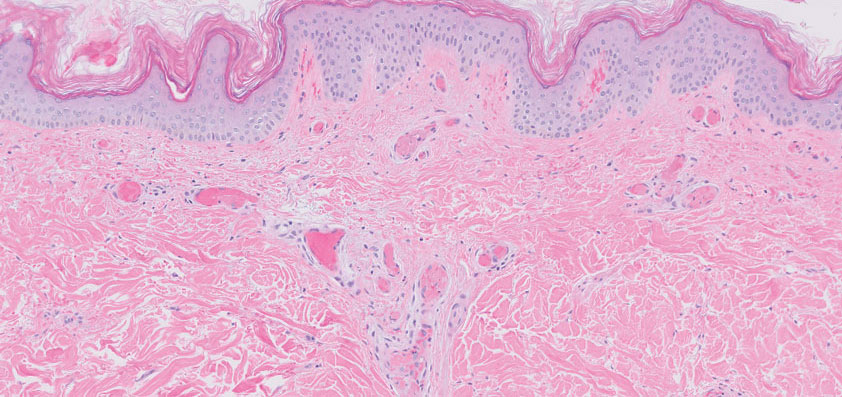
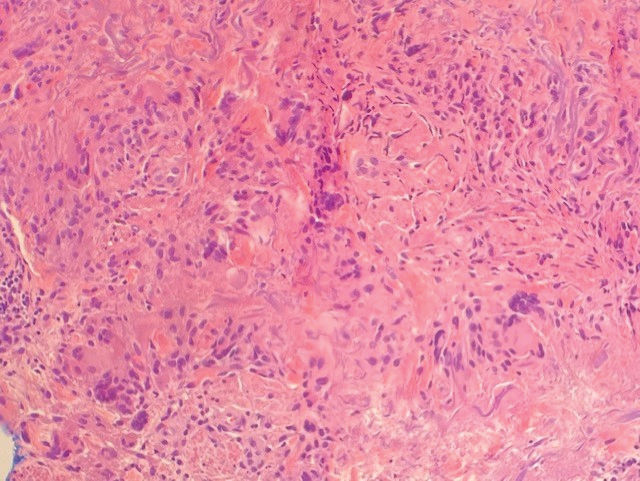
Histopathology of the punch biopsy showed an undulating chitinous exoskeleton and pigmented spines (setae) protruding from the exoskeleton with associated superficial perivascular lymphohistiocytic infiltrates on hematoxylin and eosin stain (Figure 1). Live insect larvae were observed and extracted, which immediately relieved the crawling sensation (Figure 2). Light microscopy of the larva showed a row of hooks surrounding a tapered body with a head attached anteriorly (Figure 3).
Myiasis is a parasitic infestation of the dipterous fly’s larvae in the host organ and tissue. There are 5 types of myiasis based on the location of the infestation: wound myiasis occurs with egg infestations on an open wound; furuncular myiasis results from egg placement by penetration of healthy skin by a mosquito vector; plaque myiasis comprises the placement of eggs on clothing through several maggots and flies; creeping myiasis involves the Gasterophilus fly delivering the larva intradermally; and body cavity myiasis may develop in the orbit, nasal cavity, urogenital system, and gastrointestinal tract.1-3

Furuncular myiasis infestation occurs via a complex life cycle in which mosquitoes act as a vector and transfer the eggs to the human or animal host.1-3 Botfly larvae then penetrate the skin and reside within the subdermis to mature. Adults then emerge after 1 month to repeat the cycle.1 Dermatobia hominis and Cordylobia anthropophaga are the most common causes of furuncular myiasis.2,3 Furuncular myiasis commonly presents in travelers that are returning from tropical countries. Initially, an itching erythematous papule develops. After the larvae mature, they can appear as boil-like lesions with a small central punctum.1-3 Dermoscopy can be utilized for visualization of different larvae anatomy such as a furuncularlike lesion, spines, and posterior breathing spiracle from the central punctum.4
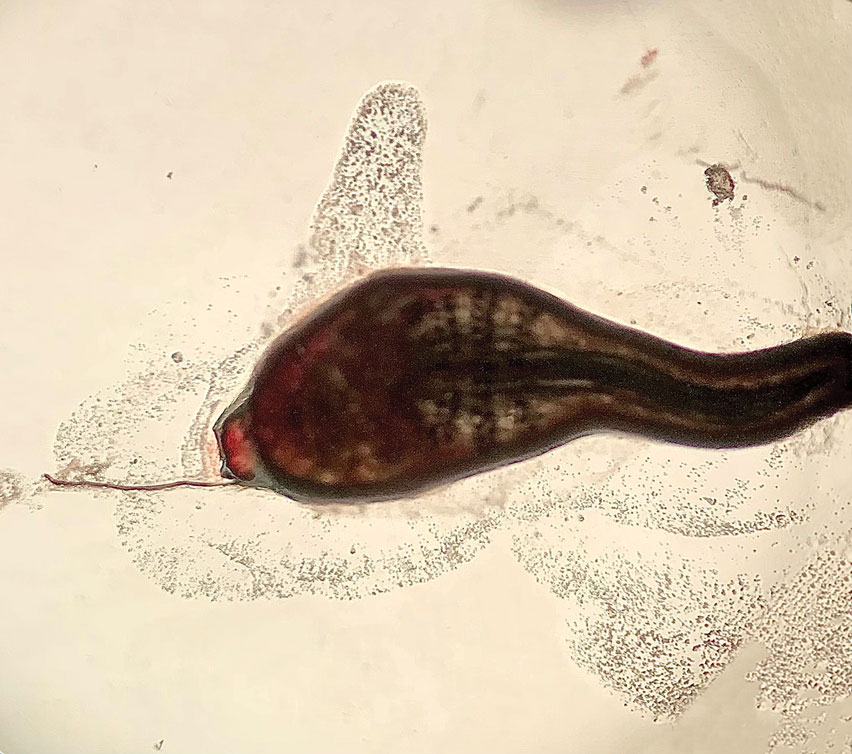
Our patient’s recent travel to the Amazon in Brazil, clinical history, and histopathologic findings ruled out other differential diagnoses such as cutaneous larva migrans, gnathostomiasis, loiasis, and tungiasis.
Treatment is curative with the extraction of the intact larva from the nodule. Localized skin anesthetic injection can be used to bulge the larva outward for easier extraction. A single dose of ivermectin 15 mg can treat the parasitic infestation of myiasis.1-3
- John DT, Petri WA, Markell EK, et al. Markell and Voge’s Medical Parasitology. 9th ed. Saunders Elsevier; 2006.
- Caissie R, Beaulieu F, Giroux M, et al. Cutaneous myiasis: diagnosis, treatment, and prevention. J Oral Maxillofac Surg. 2008;66:560-568.
- Lachish T, Marhoom E, Mumcuoglu KY, et al. Myiasis in travelers. J Travel Med. 2015;22:232-236.
- Mello C, Magalhães R. Triangular black dots in dermoscopy of furuncular myiasis. JAAD Case Rep. 2021;12:49-50.
The Diagnosis: Cutaneous Furuncular Myiasis
Histopathology of the punch biopsy showed an undulating chitinous exoskeleton and pigmented spines (setae) protruding from the exoskeleton with associated superficial perivascular lymphohistiocytic infiltrates on hematoxylin and eosin stain (Figure 1). Live insect larvae were observed and extracted, which immediately relieved the crawling sensation (Figure 2). Light microscopy of the larva showed a row of hooks surrounding a tapered body with a head attached anteriorly (Figure 3).

Myiasis is a parasitic infestation of the dipterous fly’s larvae in the host organ and tissue. There are 5 types of myiasis based on the location of the infestation: wound myiasis occurs with egg infestations on an open wound; furuncular myiasis results from egg placement by penetration of healthy skin by a mosquito vector; plaque myiasis comprises the placement of eggs on clothing through several maggots and flies; creeping myiasis involves the Gasterophilus fly delivering the larva intradermally; and body cavity myiasis may develop in the orbit, nasal cavity, urogenital system, and gastrointestinal tract.1-3

Furuncular myiasis infestation occurs via a complex life cycle in which mosquitoes act as a vector and transfer the eggs to the human or animal host.1-3 Botfly larvae then penetrate the skin and reside within the subdermis to mature. Adults then emerge after 1 month to repeat the cycle.1 Dermatobia hominis and Cordylobia anthropophaga are the most common causes of furuncular myiasis.2,3 Furuncular myiasis commonly presents in travelers that are returning from tropical countries. Initially, an itching erythematous papule develops. After the larvae mature, they can appear as boil-like lesions with a small central punctum.1-3 Dermoscopy can be utilized for visualization of different larvae anatomy such as a furuncularlike lesion, spines, and posterior breathing spiracle from the central punctum.4

Our patient’s recent travel to the Amazon in Brazil, clinical history, and histopathologic findings ruled out other differential diagnoses such as cutaneous larva migrans, gnathostomiasis, loiasis, and tungiasis.
Treatment is curative with the extraction of the intact larva from the nodule. Localized skin anesthetic injection can be used to bulge the larva outward for easier extraction. A single dose of ivermectin 15 mg can treat the parasitic infestation of myiasis.1-3
The Diagnosis: Cutaneous Furuncular Myiasis
Histopathology of the punch biopsy showed an undulating chitinous exoskeleton and pigmented spines (setae) protruding from the exoskeleton with associated superficial perivascular lymphohistiocytic infiltrates on hematoxylin and eosin stain (Figure 1). Live insect larvae were observed and extracted, which immediately relieved the crawling sensation (Figure 2). Light microscopy of the larva showed a row of hooks surrounding a tapered body with a head attached anteriorly (Figure 3).

Myiasis is a parasitic infestation of the dipterous fly’s larvae in the host organ and tissue. There are 5 types of myiasis based on the location of the infestation: wound myiasis occurs with egg infestations on an open wound; furuncular myiasis results from egg placement by penetration of healthy skin by a mosquito vector; plaque myiasis comprises the placement of eggs on clothing through several maggots and flies; creeping myiasis involves the Gasterophilus fly delivering the larva intradermally; and body cavity myiasis may develop in the orbit, nasal cavity, urogenital system, and gastrointestinal tract.1-3

Furuncular myiasis infestation occurs via a complex life cycle in which mosquitoes act as a vector and transfer the eggs to the human or animal host.1-3 Botfly larvae then penetrate the skin and reside within the subdermis to mature. Adults then emerge after 1 month to repeat the cycle.1 Dermatobia hominis and Cordylobia anthropophaga are the most common causes of furuncular myiasis.2,3 Furuncular myiasis commonly presents in travelers that are returning from tropical countries. Initially, an itching erythematous papule develops. After the larvae mature, they can appear as boil-like lesions with a small central punctum.1-3 Dermoscopy can be utilized for visualization of different larvae anatomy such as a furuncularlike lesion, spines, and posterior breathing spiracle from the central punctum.4

Our patient’s recent travel to the Amazon in Brazil, clinical history, and histopathologic findings ruled out other differential diagnoses such as cutaneous larva migrans, gnathostomiasis, loiasis, and tungiasis.
Treatment is curative with the extraction of the intact larva from the nodule. Localized skin anesthetic injection can be used to bulge the larva outward for easier extraction. A single dose of ivermectin 15 mg can treat the parasitic infestation of myiasis.1-3
- John DT, Petri WA, Markell EK, et al. Markell and Voge’s Medical Parasitology. 9th ed. Saunders Elsevier; 2006.
- Caissie R, Beaulieu F, Giroux M, et al. Cutaneous myiasis: diagnosis, treatment, and prevention. J Oral Maxillofac Surg. 2008;66:560-568.
- Lachish T, Marhoom E, Mumcuoglu KY, et al. Myiasis in travelers. J Travel Med. 2015;22:232-236.
- Mello C, Magalhães R. Triangular black dots in dermoscopy of furuncular myiasis. JAAD Case Rep. 2021;12:49-50.
- John DT, Petri WA, Markell EK, et al. Markell and Voge’s Medical Parasitology. 9th ed. Saunders Elsevier; 2006.
- Caissie R, Beaulieu F, Giroux M, et al. Cutaneous myiasis: diagnosis, treatment, and prevention. J Oral Maxillofac Surg. 2008;66:560-568.
- Lachish T, Marhoom E, Mumcuoglu KY, et al. Myiasis in travelers. J Travel Med. 2015;22:232-236.
- Mello C, Magalhães R. Triangular black dots in dermoscopy of furuncular myiasis. JAAD Case Rep. 2021;12:49-50.
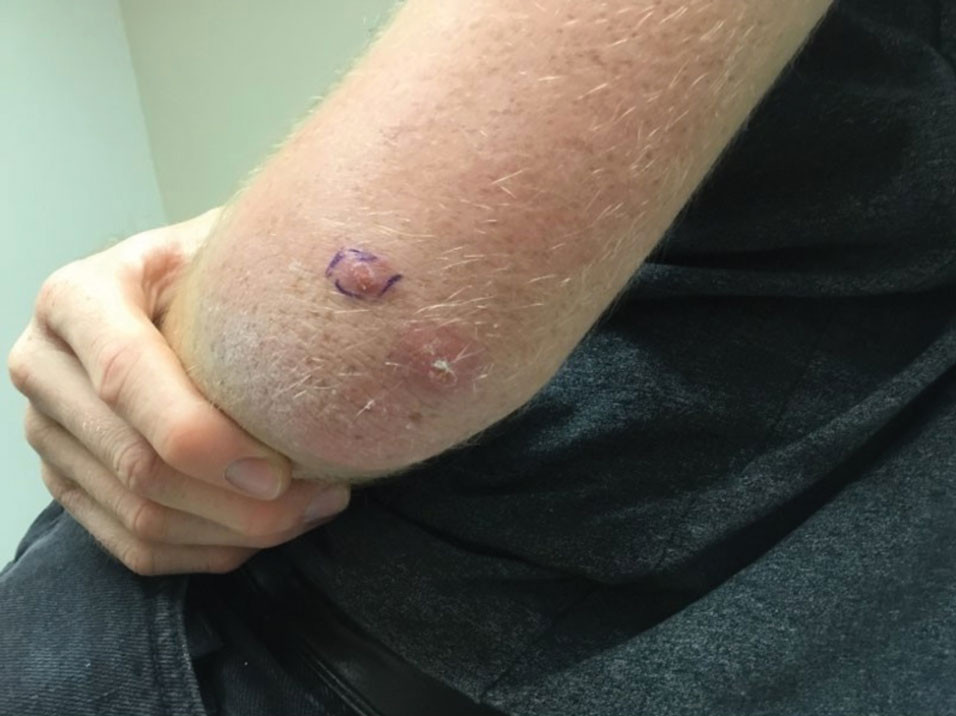
A 20-year-old man presented with progressively enlarging, painful lesions on the arm with a crawling sensation of 3 weeks’ duration. The lesions appeared after a recent trip to Brazil where he was hiking in the Amazon. He noted that the pain occurred suddenly and there was some serous drainage from the lesions. He denied any trauma to the area and reported no history of similar eruptions, treatments, or systemic symptoms. Physical examination revealed 2 tender erythematous nodules, each measuring 0.6 cm in diameter, with associated crust and a reported crawling sensation on the posterior aspect of the left arm. No drainage was seen. A punch biopsy was performed.
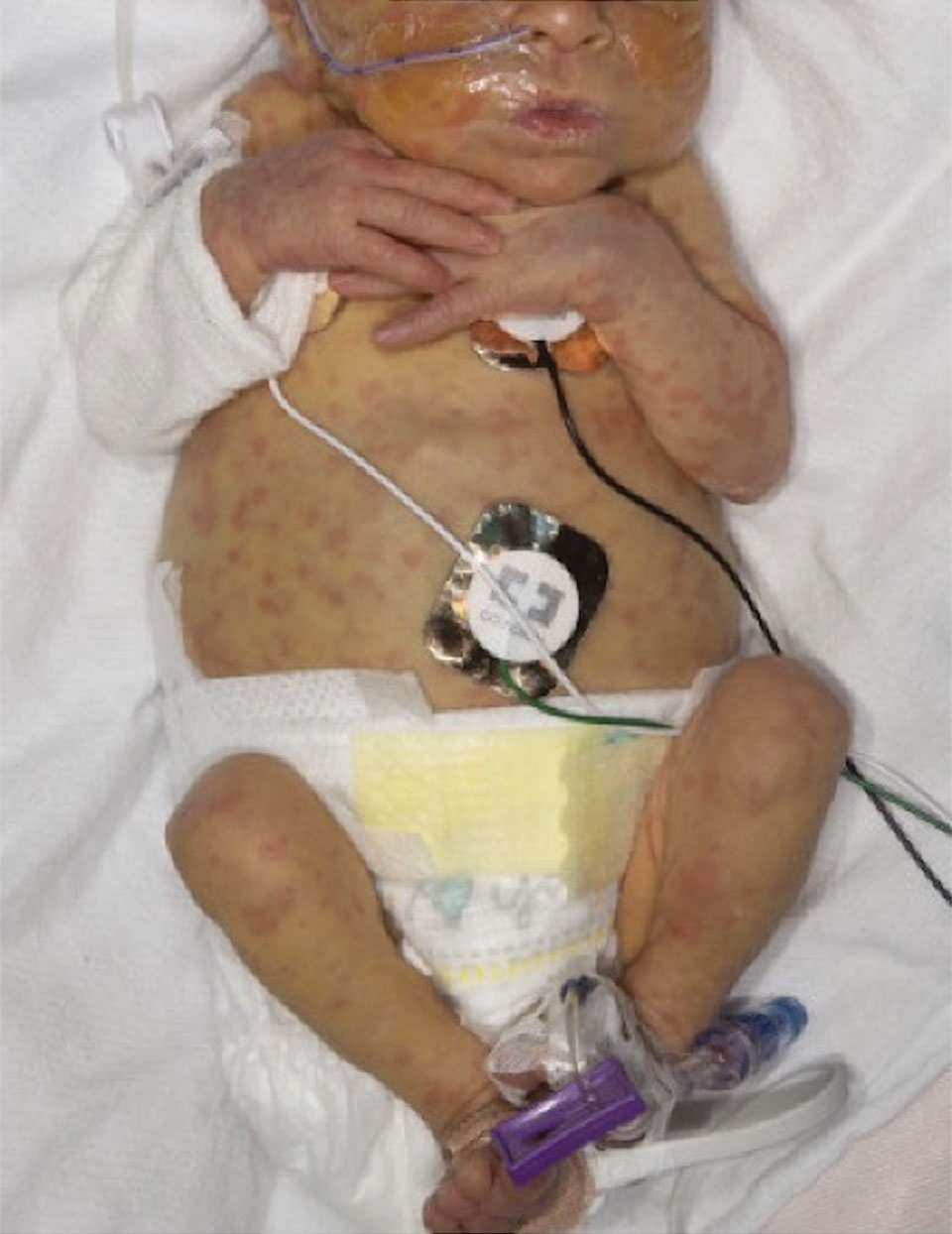
Scattered Red-Brown, Centrally Violaceous, Blanching Papules on an Infant
The Diagnosis: Neonatal-Onset Multisystem Inflammatory Disorder (NOMID)
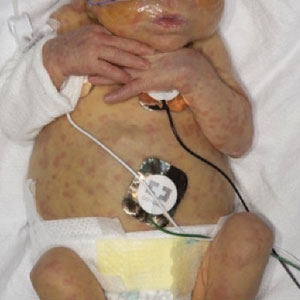
The punch biopsy demonstrated a predominantly deep but somewhat superficial, periadnexal, neutrophilic and eosinophilic infiltrate (Figure). The eruption resolved 3 days later with supportive treatment, including appropriate wound care. Genetic analysis revealed an autosomal-dominant NLR family pyrin domain containing 3 gene, NLRP3, de novo variant associated with neonatal-onset multisystem inflammatory disorder (NOMID). Additional workup to characterize our patient’s inflammatory profile revealed elevated IL-18, CD3, CD4, S100A12, and S100A8/A9 levels. On day 48 of life, she was started on anakinra, an IL-1 inhibitor, at a dose of 1 mg/kg subcutaneously, which eventually was titrated to 10 mg/kg at hospital discharge. Hearing screenings were within normal limits.

Cryopyrin-associated periodic syndromes (CAPS) consist of 3 rare, IL-1–associated, autoinflammatory disorders, including familial cold autoinflammatory syndrome (FCAS), Muckle-Wells syndrome (MWS), and NOMID (also known as chronic infantile neurologic cutaneous and articular syndrome). These conditions result from a sporadic or autosomal-dominant gain-of-function mutations in a single gene, NLRP3, on chromosome 1q44. NLRP3 encodes for cryopyrin, an important component of an IL-1 and IL-18 activating inflammasome.1 The most severe manifestation of CAPS is NOMID, which typically presents at birth as a migratory urticarial eruption, growth failure, myalgia, fever, and abnormal facial features, including frontal bossing, saddle-shaped nose, and protruding eyes.2 The illness also can manifest with hepatosplenomegaly, lymphadenopathy, uveitis, sensorineural hearing loss, cerebral atrophy, and other neurologic manifestations.3 A diagnosis of chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE) syndrome was less likely given that our patient remained afebrile and did not show signs of lipodystrophy and persistent violaceous eyelid swelling. Both FCAS and MWS are less severe forms of CAPS when compared to NOMID. Familial cold autoinflammatory syndrome was less likely given the absence of the typical periodic fever pattern associated with the condition and severity of our patient’s symptoms. Muckle-Wells syndrome typically presents in adolescence with symptoms of FCAS, painful urticarial plaques, and progressive sensorinueral hearing loss. Tumor necrosis factor receptor–associated periodic fever (TRAPS) usually is associated with episodic fevers, abdominal pain, periorbital edema, migratory erythema, and arthralgia.1,3,4
Diagnostic criteria for CAPS include elevated inflammatory markers and serum amyloid, plus at least 2 of the typical CAPS symptoms: urticarial rash, cold-triggered episodes, sensorineural hearing loss, musculoskeletal symptoms, chronic aseptic meningitis, and skeletal abnormalities.4 The sensitivity and specificity of these diagnostic criteria are 84% and 91%, respectively. Additional findings that can be seen but are not part of the diagnostic criteria include intermittent fever, transient joint swelling, bony overgrowths, uveitis, optic disc edema, impaired growth, and hepatosplenomegaly.5 Laboratory findings may reveal leukocytosis, eosinophilia, anemia, and/or thrombocytopenia.3,5
Genetic testing, skin biopsies, ophthalmic examinations, neuroimaging, joint radiography, cerebrospinal fluid tests, and hearing examinations can be performed for confirmation of diagnosis and evaluation of systemic complications.4 A skin biopsy may reveal a neutrophilic infiltrate. Ophthalmic examination can demonstrate uveitis and optic disk edema. Neuroimaging may reveal cerebral atrophy or ventricular dilation. Lastly, joint radiography can be used to evaluate for the presence of premature long bone ossification or osseous overgrowth.4
In summary, NOMID is a multisystemic disorder with cutaneous manifestations. Early recognition of this entity is important given the severe sequelae and available efficacious therapy. Dermatologists should be aware of these manifestations, as dermatologic consultation and a skin biopsy may aid in diagnosis.
- Lachmann HJ. Periodic fever syndromes. Best Pract Res Clin Rheumatol. 2017;31:596-609. doi:10.1016/j.berh.2017.12.001
- Hull KM, Shoham N, Jin Chae J, et al. The expanding spectrum of systemic autoinflammatory disorders and their rheumatic manifestations. Curr Opin Rheumatol. 2003;15:61-69. doi:10.1097/00002281-200301000-00011
- Ahmadi N, Brewer CC, Zalewski C, et al. Cryopyrin-associated periodic syndromes: otolaryngologic and audiologic manifestations. Otolaryngol Head Neck Surg. 2011;145:295-302. doi:10.1177/0194599811402296
- Kuemmerle-Deschner JB, Ozen S, Tyrrell PN, et al. Diagnostic criteria for cryopyrin-associated periodic syndrome (CAPS). Ann Rheum Dis. 2017;76:942-947. doi:10.1136/annrheumdis-2016-209686
- Aksentijevich I, Nowak M, Mallah M, et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrinassociated autoinflammatory diseases. Arthritis Rheum. 2002; 46:3340-3348. doi:10.1002/art.10688
The Diagnosis: Neonatal-Onset Multisystem Inflammatory Disorder (NOMID)
The punch biopsy demonstrated a predominantly deep but somewhat superficial, periadnexal, neutrophilic and eosinophilic infiltrate (Figure). The eruption resolved 3 days later with supportive treatment, including appropriate wound care. Genetic analysis revealed an autosomal-dominant NLR family pyrin domain containing 3 gene, NLRP3, de novo variant associated with neonatal-onset multisystem inflammatory disorder (NOMID). Additional workup to characterize our patient’s inflammatory profile revealed elevated IL-18, CD3, CD4, S100A12, and S100A8/A9 levels. On day 48 of life, she was started on anakinra, an IL-1 inhibitor, at a dose of 1 mg/kg subcutaneously, which eventually was titrated to 10 mg/kg at hospital discharge. Hearing screenings were within normal limits.

Cryopyrin-associated periodic syndromes (CAPS) consist of 3 rare, IL-1–associated, autoinflammatory disorders, including familial cold autoinflammatory syndrome (FCAS), Muckle-Wells syndrome (MWS), and NOMID (also known as chronic infantile neurologic cutaneous and articular syndrome). These conditions result from a sporadic or autosomal-dominant gain-of-function mutations in a single gene, NLRP3, on chromosome 1q44. NLRP3 encodes for cryopyrin, an important component of an IL-1 and IL-18 activating inflammasome.1 The most severe manifestation of CAPS is NOMID, which typically presents at birth as a migratory urticarial eruption, growth failure, myalgia, fever, and abnormal facial features, including frontal bossing, saddle-shaped nose, and protruding eyes.2 The illness also can manifest with hepatosplenomegaly, lymphadenopathy, uveitis, sensorineural hearing loss, cerebral atrophy, and other neurologic manifestations.3 A diagnosis of chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE) syndrome was less likely given that our patient remained afebrile and did not show signs of lipodystrophy and persistent violaceous eyelid swelling. Both FCAS and MWS are less severe forms of CAPS when compared to NOMID. Familial cold autoinflammatory syndrome was less likely given the absence of the typical periodic fever pattern associated with the condition and severity of our patient’s symptoms. Muckle-Wells syndrome typically presents in adolescence with symptoms of FCAS, painful urticarial plaques, and progressive sensorinueral hearing loss. Tumor necrosis factor receptor–associated periodic fever (TRAPS) usually is associated with episodic fevers, abdominal pain, periorbital edema, migratory erythema, and arthralgia.1,3,4
Diagnostic criteria for CAPS include elevated inflammatory markers and serum amyloid, plus at least 2 of the typical CAPS symptoms: urticarial rash, cold-triggered episodes, sensorineural hearing loss, musculoskeletal symptoms, chronic aseptic meningitis, and skeletal abnormalities.4 The sensitivity and specificity of these diagnostic criteria are 84% and 91%, respectively. Additional findings that can be seen but are not part of the diagnostic criteria include intermittent fever, transient joint swelling, bony overgrowths, uveitis, optic disc edema, impaired growth, and hepatosplenomegaly.5 Laboratory findings may reveal leukocytosis, eosinophilia, anemia, and/or thrombocytopenia.3,5
Genetic testing, skin biopsies, ophthalmic examinations, neuroimaging, joint radiography, cerebrospinal fluid tests, and hearing examinations can be performed for confirmation of diagnosis and evaluation of systemic complications.4 A skin biopsy may reveal a neutrophilic infiltrate. Ophthalmic examination can demonstrate uveitis and optic disk edema. Neuroimaging may reveal cerebral atrophy or ventricular dilation. Lastly, joint radiography can be used to evaluate for the presence of premature long bone ossification or osseous overgrowth.4
In summary, NOMID is a multisystemic disorder with cutaneous manifestations. Early recognition of this entity is important given the severe sequelae and available efficacious therapy. Dermatologists should be aware of these manifestations, as dermatologic consultation and a skin biopsy may aid in diagnosis.
The Diagnosis: Neonatal-Onset Multisystem Inflammatory Disorder (NOMID)
The punch biopsy demonstrated a predominantly deep but somewhat superficial, periadnexal, neutrophilic and eosinophilic infiltrate (Figure). The eruption resolved 3 days later with supportive treatment, including appropriate wound care. Genetic analysis revealed an autosomal-dominant NLR family pyrin domain containing 3 gene, NLRP3, de novo variant associated with neonatal-onset multisystem inflammatory disorder (NOMID). Additional workup to characterize our patient’s inflammatory profile revealed elevated IL-18, CD3, CD4, S100A12, and S100A8/A9 levels. On day 48 of life, she was started on anakinra, an IL-1 inhibitor, at a dose of 1 mg/kg subcutaneously, which eventually was titrated to 10 mg/kg at hospital discharge. Hearing screenings were within normal limits.

Cryopyrin-associated periodic syndromes (CAPS) consist of 3 rare, IL-1–associated, autoinflammatory disorders, including familial cold autoinflammatory syndrome (FCAS), Muckle-Wells syndrome (MWS), and NOMID (also known as chronic infantile neurologic cutaneous and articular syndrome). These conditions result from a sporadic or autosomal-dominant gain-of-function mutations in a single gene, NLRP3, on chromosome 1q44. NLRP3 encodes for cryopyrin, an important component of an IL-1 and IL-18 activating inflammasome.1 The most severe manifestation of CAPS is NOMID, which typically presents at birth as a migratory urticarial eruption, growth failure, myalgia, fever, and abnormal facial features, including frontal bossing, saddle-shaped nose, and protruding eyes.2 The illness also can manifest with hepatosplenomegaly, lymphadenopathy, uveitis, sensorineural hearing loss, cerebral atrophy, and other neurologic manifestations.3 A diagnosis of chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE) syndrome was less likely given that our patient remained afebrile and did not show signs of lipodystrophy and persistent violaceous eyelid swelling. Both FCAS and MWS are less severe forms of CAPS when compared to NOMID. Familial cold autoinflammatory syndrome was less likely given the absence of the typical periodic fever pattern associated with the condition and severity of our patient’s symptoms. Muckle-Wells syndrome typically presents in adolescence with symptoms of FCAS, painful urticarial plaques, and progressive sensorinueral hearing loss. Tumor necrosis factor receptor–associated periodic fever (TRAPS) usually is associated with episodic fevers, abdominal pain, periorbital edema, migratory erythema, and arthralgia.1,3,4
Diagnostic criteria for CAPS include elevated inflammatory markers and serum amyloid, plus at least 2 of the typical CAPS symptoms: urticarial rash, cold-triggered episodes, sensorineural hearing loss, musculoskeletal symptoms, chronic aseptic meningitis, and skeletal abnormalities.4 The sensitivity and specificity of these diagnostic criteria are 84% and 91%, respectively. Additional findings that can be seen but are not part of the diagnostic criteria include intermittent fever, transient joint swelling, bony overgrowths, uveitis, optic disc edema, impaired growth, and hepatosplenomegaly.5 Laboratory findings may reveal leukocytosis, eosinophilia, anemia, and/or thrombocytopenia.3,5
Genetic testing, skin biopsies, ophthalmic examinations, neuroimaging, joint radiography, cerebrospinal fluid tests, and hearing examinations can be performed for confirmation of diagnosis and evaluation of systemic complications.4 A skin biopsy may reveal a neutrophilic infiltrate. Ophthalmic examination can demonstrate uveitis and optic disk edema. Neuroimaging may reveal cerebral atrophy or ventricular dilation. Lastly, joint radiography can be used to evaluate for the presence of premature long bone ossification or osseous overgrowth.4
In summary, NOMID is a multisystemic disorder with cutaneous manifestations. Early recognition of this entity is important given the severe sequelae and available efficacious therapy. Dermatologists should be aware of these manifestations, as dermatologic consultation and a skin biopsy may aid in diagnosis.
- Lachmann HJ. Periodic fever syndromes. Best Pract Res Clin Rheumatol. 2017;31:596-609. doi:10.1016/j.berh.2017.12.001
- Hull KM, Shoham N, Jin Chae J, et al. The expanding spectrum of systemic autoinflammatory disorders and their rheumatic manifestations. Curr Opin Rheumatol. 2003;15:61-69. doi:10.1097/00002281-200301000-00011
- Ahmadi N, Brewer CC, Zalewski C, et al. Cryopyrin-associated periodic syndromes: otolaryngologic and audiologic manifestations. Otolaryngol Head Neck Surg. 2011;145:295-302. doi:10.1177/0194599811402296
- Kuemmerle-Deschner JB, Ozen S, Tyrrell PN, et al. Diagnostic criteria for cryopyrin-associated periodic syndrome (CAPS). Ann Rheum Dis. 2017;76:942-947. doi:10.1136/annrheumdis-2016-209686
- Aksentijevich I, Nowak M, Mallah M, et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrinassociated autoinflammatory diseases. Arthritis Rheum. 2002; 46:3340-3348. doi:10.1002/art.10688
- Lachmann HJ. Periodic fever syndromes. Best Pract Res Clin Rheumatol. 2017;31:596-609. doi:10.1016/j.berh.2017.12.001
- Hull KM, Shoham N, Jin Chae J, et al. The expanding spectrum of systemic autoinflammatory disorders and their rheumatic manifestations. Curr Opin Rheumatol. 2003;15:61-69. doi:10.1097/00002281-200301000-00011
- Ahmadi N, Brewer CC, Zalewski C, et al. Cryopyrin-associated periodic syndromes: otolaryngologic and audiologic manifestations. Otolaryngol Head Neck Surg. 2011;145:295-302. doi:10.1177/0194599811402296
- Kuemmerle-Deschner JB, Ozen S, Tyrrell PN, et al. Diagnostic criteria for cryopyrin-associated periodic syndrome (CAPS). Ann Rheum Dis. 2017;76:942-947. doi:10.1136/annrheumdis-2016-209686
- Aksentijevich I, Nowak M, Mallah M, et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrinassociated autoinflammatory diseases. Arthritis Rheum. 2002; 46:3340-3348. doi:10.1002/art.10688
A 2-week-old infant girl was transferred to a specialty pediatric hospital where dermatology was consulted for evaluation of a diffuse eruption triggered by cold that was similar to an eruption present at birth. She was born at 31 weeks and 2 days’ gestation at an outside hospital via caesarean delivery. Early delivery was prompted by superimposed pre-eclampsia with severe hypertension after administration of antenatal steroids. At birth, the infant was cyanotic and apneic and had a documented skin eruption, according to the medical record. She had thrombocytopenia, elevated C-reactive protein, and an elevated temperature without fever. Extensive septic workup, including blood, urine, and cerebrospinal fluid cultures; herpes simplex virus and cytomegalovirus screening; and Toxoplasma polymerase chain reaction were negative. Magnetic resonance imaging of the brain revealed no evidence of intracranial congenital infection. Ampicillinsulbactam was initiated for presumed culture-negative sepsis. On day 2 of hospitalization, she developed conjunctival icterus, hepatomegaly, and jaundice. Direct hyperbilirubinemia; anemia; and elevated triglycerides, ferritin, and ammonia all were present. Coagulation studies were normal. Subsequent workup, including abdominal ultrasonography and hepatobiliary iminodiacetic acid scan, was concerning for biliary atresia. Despite appropriate treatment, her condition did not improve and she was transferred. Repeat abdominal ultrasonography on day 24 of life confirmed hepatomegaly but did not demonstrate other findings of biliary atresia. At the current presentation, physical examination revealed many scattered, redbrown and centrally violaceous, blanching papules measuring a few millimeters involving the trunk, arms, buttocks, and legs. A punch biopsy was obtained.
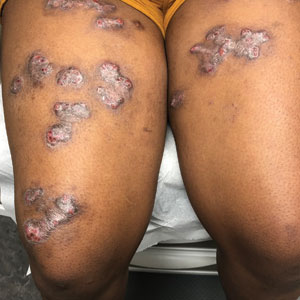
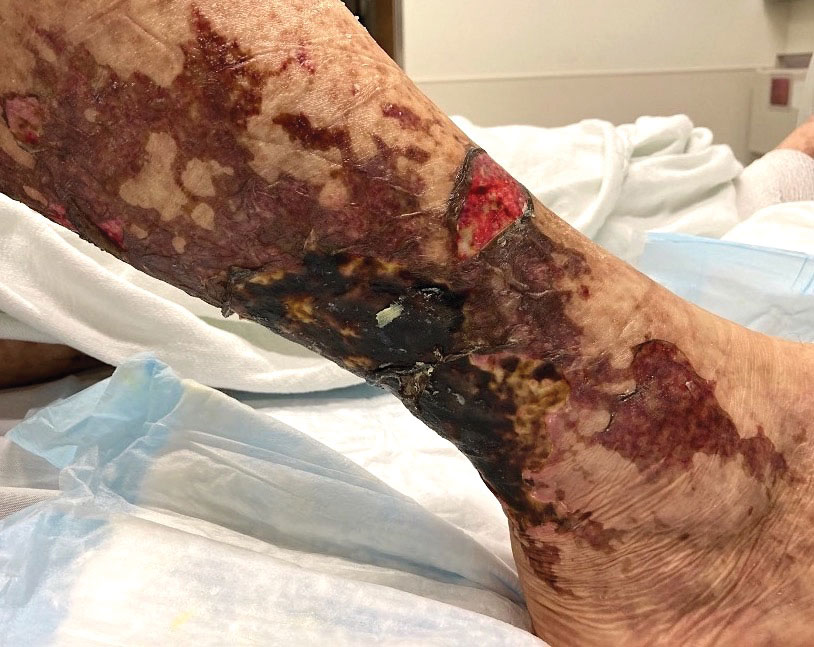
Retiform Purpura on the Lower Legs
The Diagnosis: Type I Cryoglobulinemia
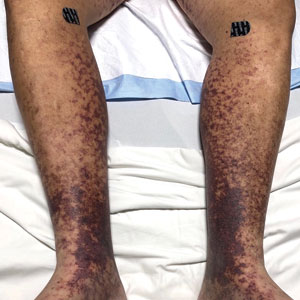
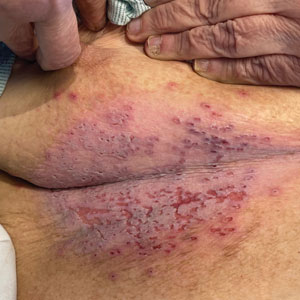
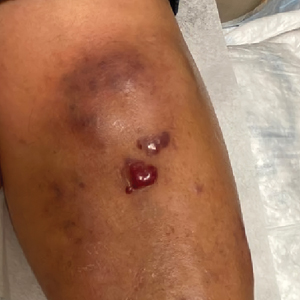
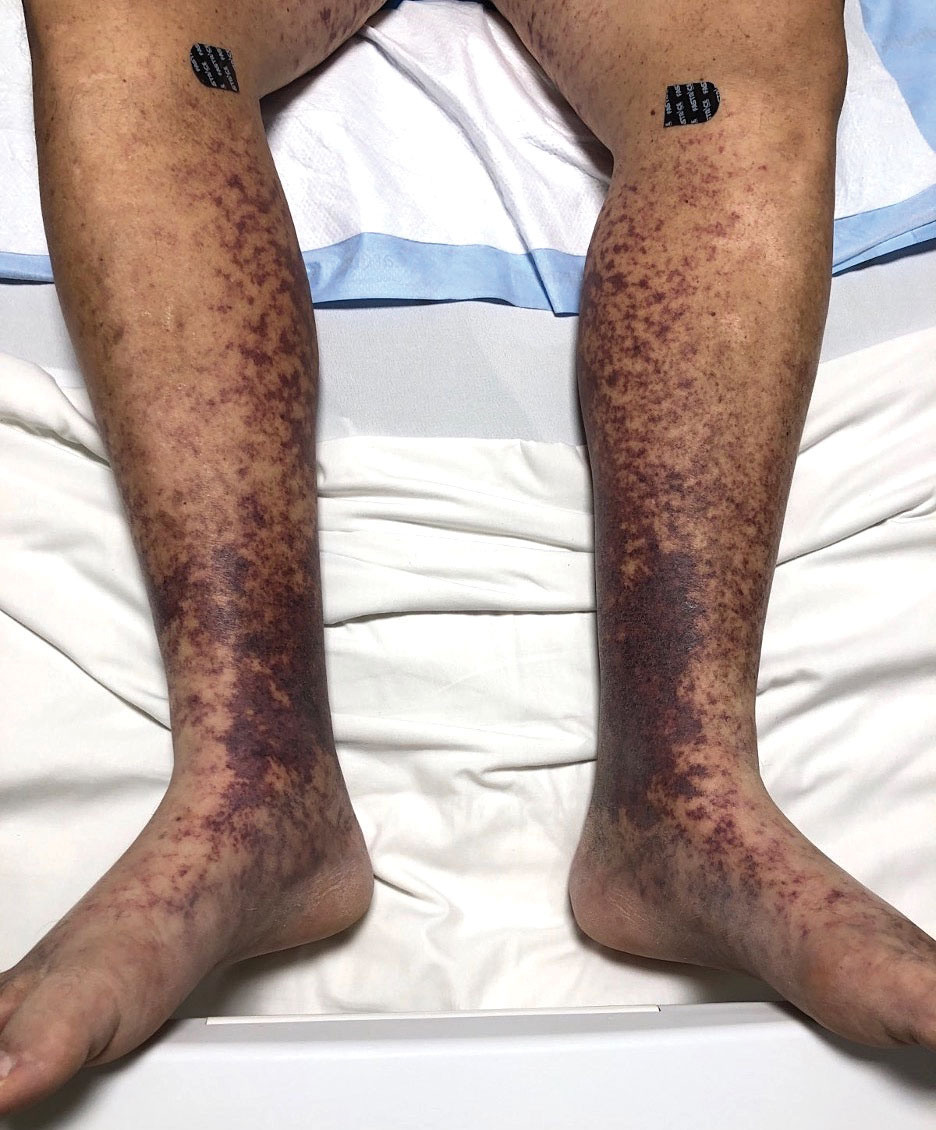
Retiform purpura with overlying necrosis subsequently developed over the course of a week following presentation (Figure 1). A skin biopsy showed fibrin thrombi and congestion of small- and medium-sized blood vessels, consistent with vasculopathy (Figure 2). Urinalysis revealed hematuria and proteinuria. A renal biopsy performed due to a continually elevated serum creatinine level revealed glomerulonephritis with numerous IgG1 lambda–restricted glomerular capillary hyaline thrombi, compatible with a lymphoproliferative disorder–associated type I cryoglobulinemia. A serum cryoglobulin immunofixation test confirmed type I cryoglobulinemia involving monoclonal IgG lambda. The combination of cutaneous, renal, and hematologic findings was consistent with type I cryoglobulinemia. A subsequent bone marrow biopsy demonstrated a CD20+ lambda–restricted plasma cell neoplasm. Initial treatment with high-dose corticosteroids followed by targeted treatment of the underlying hematologic condition with bortezomib, rituximab, and dexamethasone improved the skin disease.
Cryoglobulins are abnormal immunoglobulins that precipitate at temperatures below 37 °C. The persistent presence of cryoglobulins in the serum is termed cryoglobulinemia.1 Type I cryoglobulinemia is distinguished from mixed cryoglobulinemia—types II and III—by the presence of a single monoclonal immunoglobulin, typically IgM or IgG. It is associated with lymphoproliferative disorders, most commonly monoclonal gammopathy of undetermined significance and B-cell malignancies such as Waldenström macroglobulinemia, multiple myeloma, or chronic lymphocytic leukemia. Histopathology shows occlusion of small vessel lumina with homogenous eosinophilic material containing the monoclonal cryoprecipitate.2 Disease manifestations are caused by small vessel occlusion, which leads to ischemia and tissue damage.
Retiform purpura, livedo reticularis/racemosa, and necrosis leading to ulcers are the most common cutaneous clinical findings. Extracutaneous signs include peripheral neuropathy, arthralgia, Raynaud phenomenon, and acrocyanosis. Renal involvement, most commonly glomerulonephritis with associated proteinuria, is noted in 14% to 20% of cases.3,4 An elevated cryocrit can lead to symptoms of hyperviscosity syndrome.2
Treatment is difficult and primarily is focused on addressing the underlying hematologic condition, which is responsible for synthesis of the cryoglobulin. Decreasing cryoglobulin production leads to decreased occlusion of blood vessels, thus alleviating the ischemia and skin damage. Monoclonal gammopathy of undetermined significance–related type I cryoglobulinemia initially is treated with corticosteroids followed by rituximab if a CD20+ B-cell clone is identified.2 Bortezomib is recommended for cases associated with Waldenström macroglobulinemia and cases associated with multiple myeloma with concurrent renal failure. In patients with neuropathy, a lenalidomide-based treatment can be employed. Patients should be instructed to keep extremities warm.2 Diabetic foot care guidelines should be followed to prevent wound complications. The differential diagnosis for type I cryoglobulinemia includes other causes of retiform purpura–like angioinvasive fungal infection, antiphospholipid antibody syndrome, calciphylaxis, and livedoid vasculopathy.5 Angioinvasive fungal infections are caused by Candida, Aspergillus, and Mucorales species, as well as other hyaline molds. They typically occur in immunocompromised patients and invade the blood vessels via direct inoculation or dissemination.6 Patients present with retiform purpura but typically will be acutely ill with fevers and vital sign abnormalities. Histopathology with special stains often will identify the fungal organisms in the dermis or inside blood vessel walls with vessel wall destruction and hemorrhage.7 Accurate diagnosis is essential to selecting appropriate antifungal agents. If angioinvasive fungal infection is clinically suspected, treatment should begin before culture and histopathologic data are available.7
Antiphospholipid antibody syndrome is an autoimmune thrombophilia that can occur as primary disease or in association with other autoimmune conditions, most commonly systemic lupus erythematosus. Diagnosis requires the presence of antiphospholipid antibodies, such as lupus anticoagulant, anticardiolipin antibody, anti–β2-glycoprotein-1 antibody, with arterial or venous thrombosis and/or recurrent pregnancy loss. Paraproteinemia is not seen. The most common cutaneous finding is livedo reticularis, with livedo racemosa being a more distinctive finding.8 Small vessel thrombosis is seen histopathologically. Treatment includes antiplatelet and anticoagulant medications. Patients with refractory disease may benefit from additional therapy with hydroxychloroquine or intravenous immunoglobulins.8
Calciphylaxis is a rare depositional vasculopathy that often occurs in patients with end-stage renal disease on dialysis. Patients present with painful and poor-healing skin lesions including indurated nodules, violaceous plaques, and retiform purpura that typically affect areas of high adiposity such as the thighs, abdomen, and buttocks.9 Ulceration and superimposed infections are common complications. Histopathologically, small dermal and subcutaneous vessels demonstrate calcification, microthrombosis, and fibrointimal hyperplasia.9 Wound management is critically important in patients with calciphylaxis. Treatment with intravenous sodium thiosulfate is typical, but prognosis remains poor. Although livedoid vasculopathy may present with retiform purpura in the ankles, paraproteinemia is not seen and patients frequently present with punched-out ulcerations that tend to heal into atrophie blanche.10 Livedoid vasculopathy has been associated with underlying hypercoagulable states, connective tissue diseases, and chronic venous hypertension. Hypercoagulability and endothelial cell damage contribute to the formation of fibrin thrombi in the superficial dermal blood vessels. Histopathology demonstrates thickening of vessel walls and intraluminal hyaline thrombi. Successful treatment in most cases is achieved with anticoagulation therapy, typically rivaroxaban, especially in patients with underlying hypercoagulability. Antiplatelet therapy also may be considered, while anabolic agents have been shown to be helpful in patients with connective tissue disease.10
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j .jbspin.2019.01.016
- Muchtar E, Magen H, Gertz MA. How I treat cryoglobulinemia. Blood. 2017;129:289-298. doi:10.1182/blood-2016-09-719773
- Sidana S, Rajkumar SV, Dispenzieri A, et al. Clinical presentation and outcomes of patients with type 1 monoclonal cryoglobulinemia. Am J Hematol. 2017;92:668-673. doi:10.1002/ajh.24745
- Harel S, Mohr M, Jahn I, et al. Clinico-biological characteristics and treatment of type I monoclonal cryoglobulinaemia: a study of 64 cases. Br J Haematol. 2015;168:671-678. doi:10.1111/bjh.13196
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796. doi:10.1016/j.jaad.2019.07.112
- Shields BE, Rosenbach M, Brown-Joel Z, et al. Angioinvasive fungal infections impacting the skin: background, epidemiology, and clinical presentation. J Am Acad Dermatol. 2019;80:869-880.e5. doi:10.1016/j.jaad.2018.04.059
- Berger AP, Ford BA, Brown-Joel Z, et al. Angioinvasive fungal infections impacting the skin: diagnosis, management, and complications. J Am Acad Dermatol. 2019;80:883-898.e2. doi:10.1016/j.jaad.2018.04.058
- Negrini S, Pappalardo F, Murdaca G, et al. The antiphospholipid syndrome: from pathophysiology to treatment. Clin Exp Med. 2017;17:257-267. doi:10.1007/s10238-016-0430-5
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Georgesen C, Fox LP, Harp J. Retiform purpura: workup and therapeutic considerations in select conditions. J Am Acad Dermatol. 2020;82:799-816. doi:10.1016/j.jaad.2019.07.113
The Diagnosis: Type I Cryoglobulinemia
Retiform purpura with overlying necrosis subsequently developed over the course of a week following presentation (Figure 1). A skin biopsy showed fibrin thrombi and congestion of small- and medium-sized blood vessels, consistent with vasculopathy (Figure 2). Urinalysis revealed hematuria and proteinuria. A renal biopsy performed due to a continually elevated serum creatinine level revealed glomerulonephritis with numerous IgG1 lambda–restricted glomerular capillary hyaline thrombi, compatible with a lymphoproliferative disorder–associated type I cryoglobulinemia. A serum cryoglobulin immunofixation test confirmed type I cryoglobulinemia involving monoclonal IgG lambda. The combination of cutaneous, renal, and hematologic findings was consistent with type I cryoglobulinemia. A subsequent bone marrow biopsy demonstrated a CD20+ lambda–restricted plasma cell neoplasm. Initial treatment with high-dose corticosteroids followed by targeted treatment of the underlying hematologic condition with bortezomib, rituximab, and dexamethasone improved the skin disease.

Cryoglobulins are abnormal immunoglobulins that precipitate at temperatures below 37 °C. The persistent presence of cryoglobulins in the serum is termed cryoglobulinemia.1 Type I cryoglobulinemia is distinguished from mixed cryoglobulinemia—types II and III—by the presence of a single monoclonal immunoglobulin, typically IgM or IgG. It is associated with lymphoproliferative disorders, most commonly monoclonal gammopathy of undetermined significance and B-cell malignancies such as Waldenström macroglobulinemia, multiple myeloma, or chronic lymphocytic leukemia. Histopathology shows occlusion of small vessel lumina with homogenous eosinophilic material containing the monoclonal cryoprecipitate.2 Disease manifestations are caused by small vessel occlusion, which leads to ischemia and tissue damage.

Retiform purpura, livedo reticularis/racemosa, and necrosis leading to ulcers are the most common cutaneous clinical findings. Extracutaneous signs include peripheral neuropathy, arthralgia, Raynaud phenomenon, and acrocyanosis. Renal involvement, most commonly glomerulonephritis with associated proteinuria, is noted in 14% to 20% of cases.3,4 An elevated cryocrit can lead to symptoms of hyperviscosity syndrome.2
Treatment is difficult and primarily is focused on addressing the underlying hematologic condition, which is responsible for synthesis of the cryoglobulin. Decreasing cryoglobulin production leads to decreased occlusion of blood vessels, thus alleviating the ischemia and skin damage. Monoclonal gammopathy of undetermined significance–related type I cryoglobulinemia initially is treated with corticosteroids followed by rituximab if a CD20+ B-cell clone is identified.2 Bortezomib is recommended for cases associated with Waldenström macroglobulinemia and cases associated with multiple myeloma with concurrent renal failure. In patients with neuropathy, a lenalidomide-based treatment can be employed. Patients should be instructed to keep extremities warm.2 Diabetic foot care guidelines should be followed to prevent wound complications. The differential diagnosis for type I cryoglobulinemia includes other causes of retiform purpura–like angioinvasive fungal infection, antiphospholipid antibody syndrome, calciphylaxis, and livedoid vasculopathy.5 Angioinvasive fungal infections are caused by Candida, Aspergillus, and Mucorales species, as well as other hyaline molds. They typically occur in immunocompromised patients and invade the blood vessels via direct inoculation or dissemination.6 Patients present with retiform purpura but typically will be acutely ill with fevers and vital sign abnormalities. Histopathology with special stains often will identify the fungal organisms in the dermis or inside blood vessel walls with vessel wall destruction and hemorrhage.7 Accurate diagnosis is essential to selecting appropriate antifungal agents. If angioinvasive fungal infection is clinically suspected, treatment should begin before culture and histopathologic data are available.7
Antiphospholipid antibody syndrome is an autoimmune thrombophilia that can occur as primary disease or in association with other autoimmune conditions, most commonly systemic lupus erythematosus. Diagnosis requires the presence of antiphospholipid antibodies, such as lupus anticoagulant, anticardiolipin antibody, anti–β2-glycoprotein-1 antibody, with arterial or venous thrombosis and/or recurrent pregnancy loss. Paraproteinemia is not seen. The most common cutaneous finding is livedo reticularis, with livedo racemosa being a more distinctive finding.8 Small vessel thrombosis is seen histopathologically. Treatment includes antiplatelet and anticoagulant medications. Patients with refractory disease may benefit from additional therapy with hydroxychloroquine or intravenous immunoglobulins.8
Calciphylaxis is a rare depositional vasculopathy that often occurs in patients with end-stage renal disease on dialysis. Patients present with painful and poor-healing skin lesions including indurated nodules, violaceous plaques, and retiform purpura that typically affect areas of high adiposity such as the thighs, abdomen, and buttocks.9 Ulceration and superimposed infections are common complications. Histopathologically, small dermal and subcutaneous vessels demonstrate calcification, microthrombosis, and fibrointimal hyperplasia.9 Wound management is critically important in patients with calciphylaxis. Treatment with intravenous sodium thiosulfate is typical, but prognosis remains poor. Although livedoid vasculopathy may present with retiform purpura in the ankles, paraproteinemia is not seen and patients frequently present with punched-out ulcerations that tend to heal into atrophie blanche.10 Livedoid vasculopathy has been associated with underlying hypercoagulable states, connective tissue diseases, and chronic venous hypertension. Hypercoagulability and endothelial cell damage contribute to the formation of fibrin thrombi in the superficial dermal blood vessels. Histopathology demonstrates thickening of vessel walls and intraluminal hyaline thrombi. Successful treatment in most cases is achieved with anticoagulation therapy, typically rivaroxaban, especially in patients with underlying hypercoagulability. Antiplatelet therapy also may be considered, while anabolic agents have been shown to be helpful in patients with connective tissue disease.10
The Diagnosis: Type I Cryoglobulinemia
Retiform purpura with overlying necrosis subsequently developed over the course of a week following presentation (Figure 1). A skin biopsy showed fibrin thrombi and congestion of small- and medium-sized blood vessels, consistent with vasculopathy (Figure 2). Urinalysis revealed hematuria and proteinuria. A renal biopsy performed due to a continually elevated serum creatinine level revealed glomerulonephritis with numerous IgG1 lambda–restricted glomerular capillary hyaline thrombi, compatible with a lymphoproliferative disorder–associated type I cryoglobulinemia. A serum cryoglobulin immunofixation test confirmed type I cryoglobulinemia involving monoclonal IgG lambda. The combination of cutaneous, renal, and hematologic findings was consistent with type I cryoglobulinemia. A subsequent bone marrow biopsy demonstrated a CD20+ lambda–restricted plasma cell neoplasm. Initial treatment with high-dose corticosteroids followed by targeted treatment of the underlying hematologic condition with bortezomib, rituximab, and dexamethasone improved the skin disease.

Cryoglobulins are abnormal immunoglobulins that precipitate at temperatures below 37 °C. The persistent presence of cryoglobulins in the serum is termed cryoglobulinemia.1 Type I cryoglobulinemia is distinguished from mixed cryoglobulinemia—types II and III—by the presence of a single monoclonal immunoglobulin, typically IgM or IgG. It is associated with lymphoproliferative disorders, most commonly monoclonal gammopathy of undetermined significance and B-cell malignancies such as Waldenström macroglobulinemia, multiple myeloma, or chronic lymphocytic leukemia. Histopathology shows occlusion of small vessel lumina with homogenous eosinophilic material containing the monoclonal cryoprecipitate.2 Disease manifestations are caused by small vessel occlusion, which leads to ischemia and tissue damage.

Retiform purpura, livedo reticularis/racemosa, and necrosis leading to ulcers are the most common cutaneous clinical findings. Extracutaneous signs include peripheral neuropathy, arthralgia, Raynaud phenomenon, and acrocyanosis. Renal involvement, most commonly glomerulonephritis with associated proteinuria, is noted in 14% to 20% of cases.3,4 An elevated cryocrit can lead to symptoms of hyperviscosity syndrome.2
Treatment is difficult and primarily is focused on addressing the underlying hematologic condition, which is responsible for synthesis of the cryoglobulin. Decreasing cryoglobulin production leads to decreased occlusion of blood vessels, thus alleviating the ischemia and skin damage. Monoclonal gammopathy of undetermined significance–related type I cryoglobulinemia initially is treated with corticosteroids followed by rituximab if a CD20+ B-cell clone is identified.2 Bortezomib is recommended for cases associated with Waldenström macroglobulinemia and cases associated with multiple myeloma with concurrent renal failure. In patients with neuropathy, a lenalidomide-based treatment can be employed. Patients should be instructed to keep extremities warm.2 Diabetic foot care guidelines should be followed to prevent wound complications. The differential diagnosis for type I cryoglobulinemia includes other causes of retiform purpura–like angioinvasive fungal infection, antiphospholipid antibody syndrome, calciphylaxis, and livedoid vasculopathy.5 Angioinvasive fungal infections are caused by Candida, Aspergillus, and Mucorales species, as well as other hyaline molds. They typically occur in immunocompromised patients and invade the blood vessels via direct inoculation or dissemination.6 Patients present with retiform purpura but typically will be acutely ill with fevers and vital sign abnormalities. Histopathology with special stains often will identify the fungal organisms in the dermis or inside blood vessel walls with vessel wall destruction and hemorrhage.7 Accurate diagnosis is essential to selecting appropriate antifungal agents. If angioinvasive fungal infection is clinically suspected, treatment should begin before culture and histopathologic data are available.7
Antiphospholipid antibody syndrome is an autoimmune thrombophilia that can occur as primary disease or in association with other autoimmune conditions, most commonly systemic lupus erythematosus. Diagnosis requires the presence of antiphospholipid antibodies, such as lupus anticoagulant, anticardiolipin antibody, anti–β2-glycoprotein-1 antibody, with arterial or venous thrombosis and/or recurrent pregnancy loss. Paraproteinemia is not seen. The most common cutaneous finding is livedo reticularis, with livedo racemosa being a more distinctive finding.8 Small vessel thrombosis is seen histopathologically. Treatment includes antiplatelet and anticoagulant medications. Patients with refractory disease may benefit from additional therapy with hydroxychloroquine or intravenous immunoglobulins.8
Calciphylaxis is a rare depositional vasculopathy that often occurs in patients with end-stage renal disease on dialysis. Patients present with painful and poor-healing skin lesions including indurated nodules, violaceous plaques, and retiform purpura that typically affect areas of high adiposity such as the thighs, abdomen, and buttocks.9 Ulceration and superimposed infections are common complications. Histopathologically, small dermal and subcutaneous vessels demonstrate calcification, microthrombosis, and fibrointimal hyperplasia.9 Wound management is critically important in patients with calciphylaxis. Treatment with intravenous sodium thiosulfate is typical, but prognosis remains poor. Although livedoid vasculopathy may present with retiform purpura in the ankles, paraproteinemia is not seen and patients frequently present with punched-out ulcerations that tend to heal into atrophie blanche.10 Livedoid vasculopathy has been associated with underlying hypercoagulable states, connective tissue diseases, and chronic venous hypertension. Hypercoagulability and endothelial cell damage contribute to the formation of fibrin thrombi in the superficial dermal blood vessels. Histopathology demonstrates thickening of vessel walls and intraluminal hyaline thrombi. Successful treatment in most cases is achieved with anticoagulation therapy, typically rivaroxaban, especially in patients with underlying hypercoagulability. Antiplatelet therapy also may be considered, while anabolic agents have been shown to be helpful in patients with connective tissue disease.10
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j .jbspin.2019.01.016
- Muchtar E, Magen H, Gertz MA. How I treat cryoglobulinemia. Blood. 2017;129:289-298. doi:10.1182/blood-2016-09-719773
- Sidana S, Rajkumar SV, Dispenzieri A, et al. Clinical presentation and outcomes of patients with type 1 monoclonal cryoglobulinemia. Am J Hematol. 2017;92:668-673. doi:10.1002/ajh.24745
- Harel S, Mohr M, Jahn I, et al. Clinico-biological characteristics and treatment of type I monoclonal cryoglobulinaemia: a study of 64 cases. Br J Haematol. 2015;168:671-678. doi:10.1111/bjh.13196
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796. doi:10.1016/j.jaad.2019.07.112
- Shields BE, Rosenbach M, Brown-Joel Z, et al. Angioinvasive fungal infections impacting the skin: background, epidemiology, and clinical presentation. J Am Acad Dermatol. 2019;80:869-880.e5. doi:10.1016/j.jaad.2018.04.059
- Berger AP, Ford BA, Brown-Joel Z, et al. Angioinvasive fungal infections impacting the skin: diagnosis, management, and complications. J Am Acad Dermatol. 2019;80:883-898.e2. doi:10.1016/j.jaad.2018.04.058
- Negrini S, Pappalardo F, Murdaca G, et al. The antiphospholipid syndrome: from pathophysiology to treatment. Clin Exp Med. 2017;17:257-267. doi:10.1007/s10238-016-0430-5
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Georgesen C, Fox LP, Harp J. Retiform purpura: workup and therapeutic considerations in select conditions. J Am Acad Dermatol. 2020;82:799-816. doi:10.1016/j.jaad.2019.07.113
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j .jbspin.2019.01.016
- Muchtar E, Magen H, Gertz MA. How I treat cryoglobulinemia. Blood. 2017;129:289-298. doi:10.1182/blood-2016-09-719773
- Sidana S, Rajkumar SV, Dispenzieri A, et al. Clinical presentation and outcomes of patients with type 1 monoclonal cryoglobulinemia. Am J Hematol. 2017;92:668-673. doi:10.1002/ajh.24745
- Harel S, Mohr M, Jahn I, et al. Clinico-biological characteristics and treatment of type I monoclonal cryoglobulinaemia: a study of 64 cases. Br J Haematol. 2015;168:671-678. doi:10.1111/bjh.13196
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796. doi:10.1016/j.jaad.2019.07.112
- Shields BE, Rosenbach M, Brown-Joel Z, et al. Angioinvasive fungal infections impacting the skin: background, epidemiology, and clinical presentation. J Am Acad Dermatol. 2019;80:869-880.e5. doi:10.1016/j.jaad.2018.04.059
- Berger AP, Ford BA, Brown-Joel Z, et al. Angioinvasive fungal infections impacting the skin: diagnosis, management, and complications. J Am Acad Dermatol. 2019;80:883-898.e2. doi:10.1016/j.jaad.2018.04.058
- Negrini S, Pappalardo F, Murdaca G, et al. The antiphospholipid syndrome: from pathophysiology to treatment. Clin Exp Med. 2017;17:257-267. doi:10.1007/s10238-016-0430-5
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Georgesen C, Fox LP, Harp J. Retiform purpura: workup and therapeutic considerations in select conditions. J Am Acad Dermatol. 2020;82:799-816. doi:10.1016/j.jaad.2019.07.113
A 58-year-old man presented with a petechial and purpuric rash limited to the lower extremities. He reported that the rash had been present for months but worsened acutely over the last 3 days with new-onset dark urine, joint pain, and edema limiting his ability to walk. Physical examination showed areas of violaceous macules and papules on the legs and dorsal feet in a reticular distribution. Laboratory findings were remarkable for an elevated serum creatinine level of 2.75 mg/dL (reference range, 0.70–1.30 mg/dL), and serum immunofixation revealed the presence of markedly elevated IgG lambda monoclonal proteins. He was afebrile and his vital signs were stable. Dermatology, nephrology, and rheumatology services were consulted.
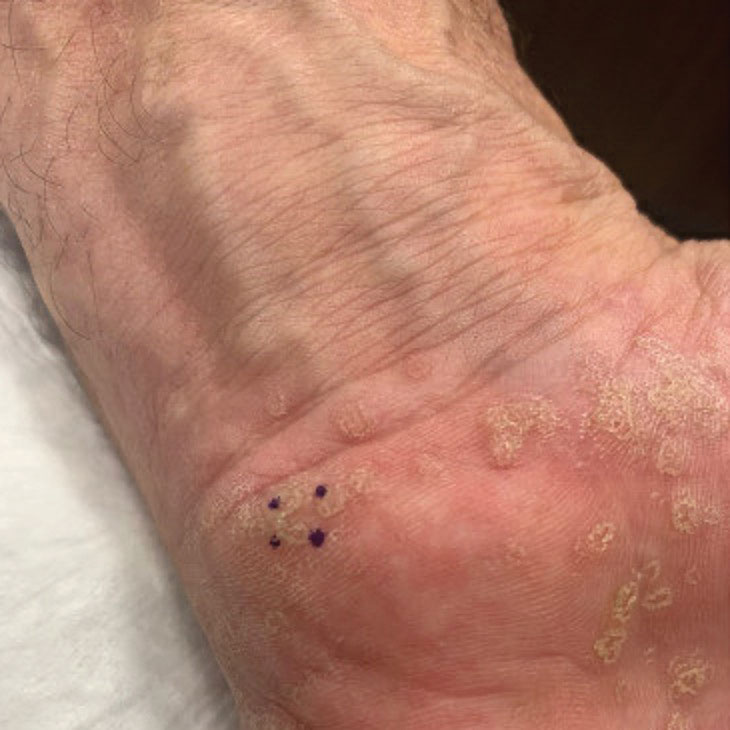
Symmetric Palmoplantar Papules With a Keratotic Border
The Diagnosis: Porokeratosis Plantaris Palmaris et Disseminata
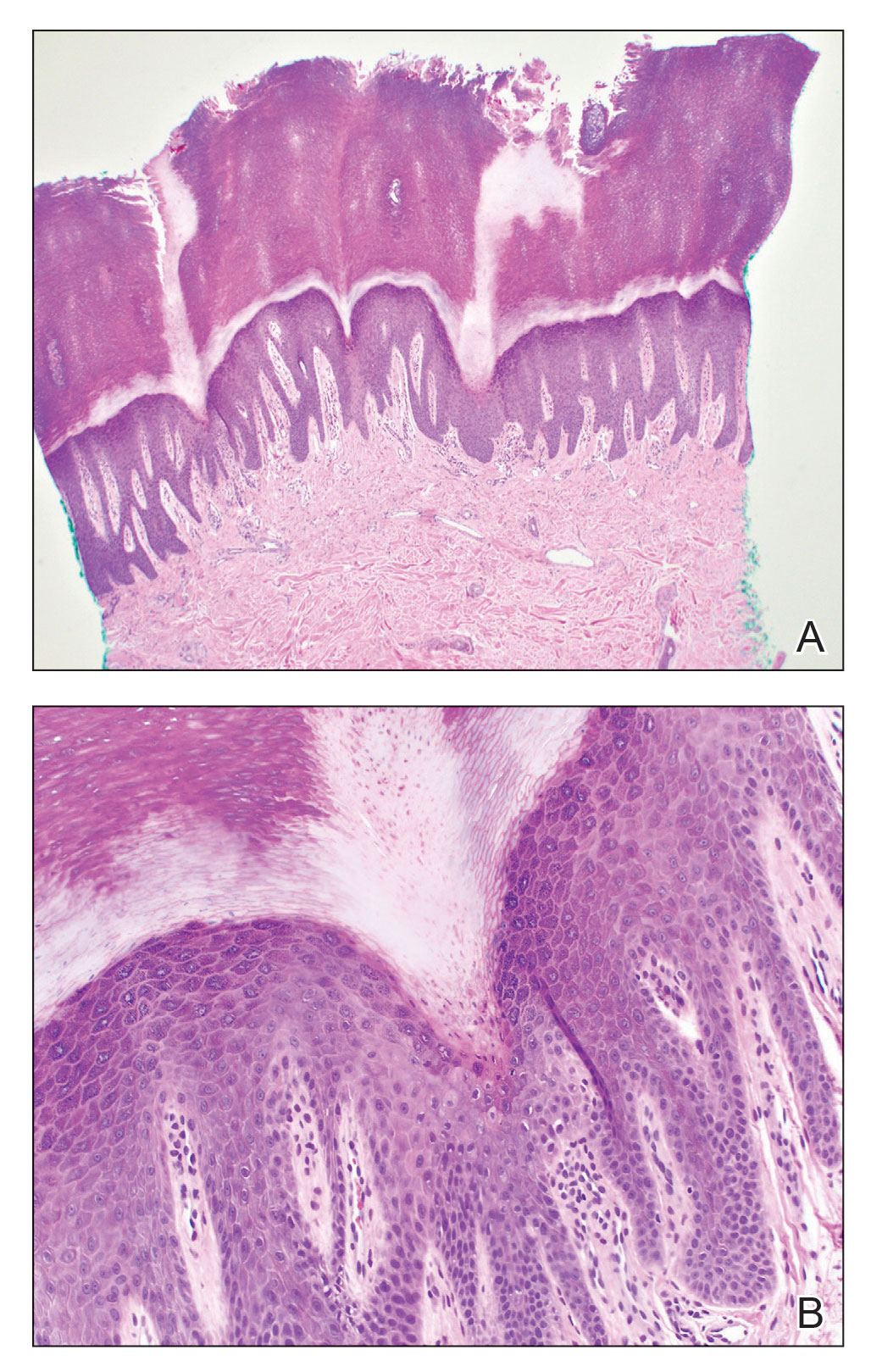
A 3-mm punch biopsy of the right upper arm showed incipient cornoid lamellae formation, pigment incontinence, and sparse dermal lymphocytic inflammation (Figure), suggestive of porokeratosis plantaris palmaris et disseminata (PPPD). The dermatopathologist recommended a second biopsy to confirm the diagnosis and to confirm that the lesions on the palms and soles also were suggestive of porokeratosis. A second 4-mm punch biopsy of the left palm was consistent with PPPD.
The risks of PPPD as a precancerous entity along with the benefits and side effects of the various management options were discussed with our patient. We recommended that he start low-dose isotretinoin (20 mg/d) due to the large body surface area affected, making focal and field treatments likely insufficient. However, our patient opted not to treat and did not return for follow-up.
Subtypes of porokeratosis, including disseminated superficial actinic porokeratosis (DSAP) and PPPD, are conditions that disrupt the normal maturation of keratin and present clinically with symmetric, crusted, annular papules.1 The signature but nonspecific histopathologic feature shared among the subtypes is the presence of a cornoid lamellae.2 Several triggers of porokeratosis have been proposed, including trauma and exposure to UV and ionizing radiation.2,3 The clinical variants of porokeratosis are important conditions to diagnose correctly because they portend a risk for Bowen disease and invasive squamous cell carcinoma and may indicate the presence of an underlying hematologic and/or solid organ malignancy.4 Management of porokeratosis is difficult, as treatments have shown limited efficacy and variable recurrence rates. Treatment options include focal, field, and systemic options, such as 5-fluorouracil, topical compound of cholesterol and lovastatin, isotretinoin, and acitretin.1,2
Porokeratoses may arise from gene mutations in the mevalonate pathway,5 which is essential for the production of cholesterol.6 Topical cholesterol alone has not been shown to improve porokeratosis, but the combination topical therapy of cholesterol and lovastatin is promising. It is theorized to deliver benefit by both providing the essential end product of the pathway and simultaneously reducing the number of potentially toxic intermediates.6
Porokeratosis plantaris palmaris et disseminata (also known as porokeratosis plantaris) is unique among the subtypes of porokeratosis in that its annular, red-pink, papular rash with scaling and a keratotic border tends to start distally, involving the palms and soles, and progresses proximally to the trunk with smaller lesions.1,7 This centripetal progression can take years, as was seen in our patient.1 The disease is uncommon, with a dearth of published reports on PPPD.2 However, case reports have shown that PPPD is strongly linked to family history and may have an autosomal-dominant inheritance pattern. Penetrance is greater in men than in women, as PPPD is twice as common in men.8 Most cases of PPPD have been diagnosed in patients in their 20s and 30s, but Hartman et al9 reported a case wherein a patient was diagnosed with PPPD after 65 years of age, similar to our patient.
Although the lesions in DSAP can appear similar to those in PPPD, DSAP is more common among the family of porokeratotic conditions, affecting women twice as often as men, with a sporadic pattern of inheritance.2 These same features are present in some other types of porokeratosis but not PPPD. Furthermore, DSAP progresses proximally to distally but often with truncal sparing.2
Akin to PPPD, pityriasis rubra pilaris (PRP) often presents with palmoplantar keratoderma.10 There are at least 6 types of PRP with varying degrees of similarity to PPPD. However, in many cases PRP is associated with a background of diffuse erythema on the body with islands of spared skin. In addition, cases of PRP have been linked to extracutaneous findings such as ectropion and joint pain.11
Darier disease, especially the acrokeratosis verruciformis of Hopf variant, is more common in men and involves younger populations, as in PPPD.11 However, the crusted lesions seen in Darier disease frequently involve the skin folds. These intertriginous lesions may coalesce, mimicking warts in appearance, and are at risk for secondary infection. Nail findings in Darier disease also are distinct and include longitudinal white or red stripes running along the nail bed, in addition to V-shaped nicks at the nail tips.
Psoriasis can occur anywhere on the body and is associated with silver scaling atop a salmon-colored dermatitis.12 It results from aberrant proliferation of keratinocytes. Some distinguishing features of psoriasis include a disease course that waxes and wanes as well as pitting of the nails.
Although PPPD typically affects young adults, we presented a case of PPPD in an older man. Porokeratosis plantaris palmaris et disseminata in older adults may represent a delayed diagnosis, imply a broader range for the age of onset, or suggest its manifestation secondary to radiation treatment or another phenomenon. For example, our patient received 35 radiotherapy cycles for tongue cancer more than 5 years prior to the onset of PPPD.
- Irisawa R, Yamazaki M, Yamamoto T, et al. A case of porokeratosis plantaris palmaris et disseminata and literature review. Dermatol Online J. 2012;18:5.
- Vargas-Mora P, Morgado-Carrasco D, Fusta-Novell X. Porokeratosis: a review of its pathophysiology, clinical manifestations, diagnosis, and treatment. Actas Dermosifiliogr. 2020;111:545-560.
- James AJ, Clarke LE, Elenitsas R, et al. Segmental porokeratosis after radiation therapy for follicular lymphoma. J Am Acad Dermatol. 2008;58(2 suppl):S49-S50.
- Schena D, Papagrigoraki A, Frigo A, et al. Eruptive disseminated porokeratosis associated with internal malignancies: a case report. Cutis. 2010;85:156-159.
- Zhang Z, Li C, Wu F, et al. Genomic variations of the mevalonate pathway in porokeratosis. Elife. 2015;4:E06322. doi:10.7554/eLife.06322
- Atzmony L, Lim YH, Hamilton C, et al. Topical cholesterol/lovastatin for the treatment of porokeratosis: a pathogenesis-directed therapy. J Am Acad Dermatol. 2020;82:123-131. doi:10.1016/j.jaad.2019.08.043
- Guss SB, Osbourn RA, Lutzner MA. Porokeratosis plantaris, palmaris, et disseminata. a third type of porokeratosis. Arch Dermatol. 1971;104:366-373.
- Kanitakis J. Porokeratoses: an update of clinical, aetiopathogenic and therapeutic features. Eur J Dermatol. 2014;24:533-544.
- Hartman R, Mandal R, Sanchez M, et al. Porokeratosis plantaris, palmaris, et disseminata. Dermatol Online J. 2010;16:22.
- Suryawanshi H, Dhobley A, Sharma A, et al. Darier disease: a rare genodermatosis. J Oral Maxillofac Pathol. 2017;21:321. doi:10.4103/jomfp.JOMFP_170_16
- Eastham AB. Pityriasis rubra pilaris. JAMA Dermatol. 2019;155:404. doi:10.1001/jamadermatol.2018.5030
- Nair PA, Badri T. Psoriasis. StatPearls Publishing; 2022. Updated April 6, 2022. Accessed March 13, 2023. https://www.ncbi.nlm.nih.gov/books/NBK448194/
The Diagnosis: Porokeratosis Plantaris Palmaris et Disseminata
A 3-mm punch biopsy of the right upper arm showed incipient cornoid lamellae formation, pigment incontinence, and sparse dermal lymphocytic inflammation (Figure), suggestive of porokeratosis plantaris palmaris et disseminata (PPPD). The dermatopathologist recommended a second biopsy to confirm the diagnosis and to confirm that the lesions on the palms and soles also were suggestive of porokeratosis. A second 4-mm punch biopsy of the left palm was consistent with PPPD.

The risks of PPPD as a precancerous entity along with the benefits and side effects of the various management options were discussed with our patient. We recommended that he start low-dose isotretinoin (20 mg/d) due to the large body surface area affected, making focal and field treatments likely insufficient. However, our patient opted not to treat and did not return for follow-up.
Subtypes of porokeratosis, including disseminated superficial actinic porokeratosis (DSAP) and PPPD, are conditions that disrupt the normal maturation of keratin and present clinically with symmetric, crusted, annular papules.1 The signature but nonspecific histopathologic feature shared among the subtypes is the presence of a cornoid lamellae.2 Several triggers of porokeratosis have been proposed, including trauma and exposure to UV and ionizing radiation.2,3 The clinical variants of porokeratosis are important conditions to diagnose correctly because they portend a risk for Bowen disease and invasive squamous cell carcinoma and may indicate the presence of an underlying hematologic and/or solid organ malignancy.4 Management of porokeratosis is difficult, as treatments have shown limited efficacy and variable recurrence rates. Treatment options include focal, field, and systemic options, such as 5-fluorouracil, topical compound of cholesterol and lovastatin, isotretinoin, and acitretin.1,2
Porokeratoses may arise from gene mutations in the mevalonate pathway,5 which is essential for the production of cholesterol.6 Topical cholesterol alone has not been shown to improve porokeratosis, but the combination topical therapy of cholesterol and lovastatin is promising. It is theorized to deliver benefit by both providing the essential end product of the pathway and simultaneously reducing the number of potentially toxic intermediates.6
Porokeratosis plantaris palmaris et disseminata (also known as porokeratosis plantaris) is unique among the subtypes of porokeratosis in that its annular, red-pink, papular rash with scaling and a keratotic border tends to start distally, involving the palms and soles, and progresses proximally to the trunk with smaller lesions.1,7 This centripetal progression can take years, as was seen in our patient.1 The disease is uncommon, with a dearth of published reports on PPPD.2 However, case reports have shown that PPPD is strongly linked to family history and may have an autosomal-dominant inheritance pattern. Penetrance is greater in men than in women, as PPPD is twice as common in men.8 Most cases of PPPD have been diagnosed in patients in their 20s and 30s, but Hartman et al9 reported a case wherein a patient was diagnosed with PPPD after 65 years of age, similar to our patient.
Although the lesions in DSAP can appear similar to those in PPPD, DSAP is more common among the family of porokeratotic conditions, affecting women twice as often as men, with a sporadic pattern of inheritance.2 These same features are present in some other types of porokeratosis but not PPPD. Furthermore, DSAP progresses proximally to distally but often with truncal sparing.2
Akin to PPPD, pityriasis rubra pilaris (PRP) often presents with palmoplantar keratoderma.10 There are at least 6 types of PRP with varying degrees of similarity to PPPD. However, in many cases PRP is associated with a background of diffuse erythema on the body with islands of spared skin. In addition, cases of PRP have been linked to extracutaneous findings such as ectropion and joint pain.11
Darier disease, especially the acrokeratosis verruciformis of Hopf variant, is more common in men and involves younger populations, as in PPPD.11 However, the crusted lesions seen in Darier disease frequently involve the skin folds. These intertriginous lesions may coalesce, mimicking warts in appearance, and are at risk for secondary infection. Nail findings in Darier disease also are distinct and include longitudinal white or red stripes running along the nail bed, in addition to V-shaped nicks at the nail tips.
Psoriasis can occur anywhere on the body and is associated with silver scaling atop a salmon-colored dermatitis.12 It results from aberrant proliferation of keratinocytes. Some distinguishing features of psoriasis include a disease course that waxes and wanes as well as pitting of the nails.
Although PPPD typically affects young adults, we presented a case of PPPD in an older man. Porokeratosis plantaris palmaris et disseminata in older adults may represent a delayed diagnosis, imply a broader range for the age of onset, or suggest its manifestation secondary to radiation treatment or another phenomenon. For example, our patient received 35 radiotherapy cycles for tongue cancer more than 5 years prior to the onset of PPPD.
The Diagnosis: Porokeratosis Plantaris Palmaris et Disseminata
A 3-mm punch biopsy of the right upper arm showed incipient cornoid lamellae formation, pigment incontinence, and sparse dermal lymphocytic inflammation (Figure), suggestive of porokeratosis plantaris palmaris et disseminata (PPPD). The dermatopathologist recommended a second biopsy to confirm the diagnosis and to confirm that the lesions on the palms and soles also were suggestive of porokeratosis. A second 4-mm punch biopsy of the left palm was consistent with PPPD.

The risks of PPPD as a precancerous entity along with the benefits and side effects of the various management options were discussed with our patient. We recommended that he start low-dose isotretinoin (20 mg/d) due to the large body surface area affected, making focal and field treatments likely insufficient. However, our patient opted not to treat and did not return for follow-up.
Subtypes of porokeratosis, including disseminated superficial actinic porokeratosis (DSAP) and PPPD, are conditions that disrupt the normal maturation of keratin and present clinically with symmetric, crusted, annular papules.1 The signature but nonspecific histopathologic feature shared among the subtypes is the presence of a cornoid lamellae.2 Several triggers of porokeratosis have been proposed, including trauma and exposure to UV and ionizing radiation.2,3 The clinical variants of porokeratosis are important conditions to diagnose correctly because they portend a risk for Bowen disease and invasive squamous cell carcinoma and may indicate the presence of an underlying hematologic and/or solid organ malignancy.4 Management of porokeratosis is difficult, as treatments have shown limited efficacy and variable recurrence rates. Treatment options include focal, field, and systemic options, such as 5-fluorouracil, topical compound of cholesterol and lovastatin, isotretinoin, and acitretin.1,2
Porokeratoses may arise from gene mutations in the mevalonate pathway,5 which is essential for the production of cholesterol.6 Topical cholesterol alone has not been shown to improve porokeratosis, but the combination topical therapy of cholesterol and lovastatin is promising. It is theorized to deliver benefit by both providing the essential end product of the pathway and simultaneously reducing the number of potentially toxic intermediates.6
Porokeratosis plantaris palmaris et disseminata (also known as porokeratosis plantaris) is unique among the subtypes of porokeratosis in that its annular, red-pink, papular rash with scaling and a keratotic border tends to start distally, involving the palms and soles, and progresses proximally to the trunk with smaller lesions.1,7 This centripetal progression can take years, as was seen in our patient.1 The disease is uncommon, with a dearth of published reports on PPPD.2 However, case reports have shown that PPPD is strongly linked to family history and may have an autosomal-dominant inheritance pattern. Penetrance is greater in men than in women, as PPPD is twice as common in men.8 Most cases of PPPD have been diagnosed in patients in their 20s and 30s, but Hartman et al9 reported a case wherein a patient was diagnosed with PPPD after 65 years of age, similar to our patient.
Although the lesions in DSAP can appear similar to those in PPPD, DSAP is more common among the family of porokeratotic conditions, affecting women twice as often as men, with a sporadic pattern of inheritance.2 These same features are present in some other types of porokeratosis but not PPPD. Furthermore, DSAP progresses proximally to distally but often with truncal sparing.2
Akin to PPPD, pityriasis rubra pilaris (PRP) often presents with palmoplantar keratoderma.10 There are at least 6 types of PRP with varying degrees of similarity to PPPD. However, in many cases PRP is associated with a background of diffuse erythema on the body with islands of spared skin. In addition, cases of PRP have been linked to extracutaneous findings such as ectropion and joint pain.11
Darier disease, especially the acrokeratosis verruciformis of Hopf variant, is more common in men and involves younger populations, as in PPPD.11 However, the crusted lesions seen in Darier disease frequently involve the skin folds. These intertriginous lesions may coalesce, mimicking warts in appearance, and are at risk for secondary infection. Nail findings in Darier disease also are distinct and include longitudinal white or red stripes running along the nail bed, in addition to V-shaped nicks at the nail tips.
Psoriasis can occur anywhere on the body and is associated with silver scaling atop a salmon-colored dermatitis.12 It results from aberrant proliferation of keratinocytes. Some distinguishing features of psoriasis include a disease course that waxes and wanes as well as pitting of the nails.
Although PPPD typically affects young adults, we presented a case of PPPD in an older man. Porokeratosis plantaris palmaris et disseminata in older adults may represent a delayed diagnosis, imply a broader range for the age of onset, or suggest its manifestation secondary to radiation treatment or another phenomenon. For example, our patient received 35 radiotherapy cycles for tongue cancer more than 5 years prior to the onset of PPPD.
- Irisawa R, Yamazaki M, Yamamoto T, et al. A case of porokeratosis plantaris palmaris et disseminata and literature review. Dermatol Online J. 2012;18:5.
- Vargas-Mora P, Morgado-Carrasco D, Fusta-Novell X. Porokeratosis: a review of its pathophysiology, clinical manifestations, diagnosis, and treatment. Actas Dermosifiliogr. 2020;111:545-560.
- James AJ, Clarke LE, Elenitsas R, et al. Segmental porokeratosis after radiation therapy for follicular lymphoma. J Am Acad Dermatol. 2008;58(2 suppl):S49-S50.
- Schena D, Papagrigoraki A, Frigo A, et al. Eruptive disseminated porokeratosis associated with internal malignancies: a case report. Cutis. 2010;85:156-159.
- Zhang Z, Li C, Wu F, et al. Genomic variations of the mevalonate pathway in porokeratosis. Elife. 2015;4:E06322. doi:10.7554/eLife.06322
- Atzmony L, Lim YH, Hamilton C, et al. Topical cholesterol/lovastatin for the treatment of porokeratosis: a pathogenesis-directed therapy. J Am Acad Dermatol. 2020;82:123-131. doi:10.1016/j.jaad.2019.08.043
- Guss SB, Osbourn RA, Lutzner MA. Porokeratosis plantaris, palmaris, et disseminata. a third type of porokeratosis. Arch Dermatol. 1971;104:366-373.
- Kanitakis J. Porokeratoses: an update of clinical, aetiopathogenic and therapeutic features. Eur J Dermatol. 2014;24:533-544.
- Hartman R, Mandal R, Sanchez M, et al. Porokeratosis plantaris, palmaris, et disseminata. Dermatol Online J. 2010;16:22.
- Suryawanshi H, Dhobley A, Sharma A, et al. Darier disease: a rare genodermatosis. J Oral Maxillofac Pathol. 2017;21:321. doi:10.4103/jomfp.JOMFP_170_16
- Eastham AB. Pityriasis rubra pilaris. JAMA Dermatol. 2019;155:404. doi:10.1001/jamadermatol.2018.5030
- Nair PA, Badri T. Psoriasis. StatPearls Publishing; 2022. Updated April 6, 2022. Accessed March 13, 2023. https://www.ncbi.nlm.nih.gov/books/NBK448194/
- Irisawa R, Yamazaki M, Yamamoto T, et al. A case of porokeratosis plantaris palmaris et disseminata and literature review. Dermatol Online J. 2012;18:5.
- Vargas-Mora P, Morgado-Carrasco D, Fusta-Novell X. Porokeratosis: a review of its pathophysiology, clinical manifestations, diagnosis, and treatment. Actas Dermosifiliogr. 2020;111:545-560.
- James AJ, Clarke LE, Elenitsas R, et al. Segmental porokeratosis after radiation therapy for follicular lymphoma. J Am Acad Dermatol. 2008;58(2 suppl):S49-S50.
- Schena D, Papagrigoraki A, Frigo A, et al. Eruptive disseminated porokeratosis associated with internal malignancies: a case report. Cutis. 2010;85:156-159.
- Zhang Z, Li C, Wu F, et al. Genomic variations of the mevalonate pathway in porokeratosis. Elife. 2015;4:E06322. doi:10.7554/eLife.06322
- Atzmony L, Lim YH, Hamilton C, et al. Topical cholesterol/lovastatin for the treatment of porokeratosis: a pathogenesis-directed therapy. J Am Acad Dermatol. 2020;82:123-131. doi:10.1016/j.jaad.2019.08.043
- Guss SB, Osbourn RA, Lutzner MA. Porokeratosis plantaris, palmaris, et disseminata. a third type of porokeratosis. Arch Dermatol. 1971;104:366-373.
- Kanitakis J. Porokeratoses: an update of clinical, aetiopathogenic and therapeutic features. Eur J Dermatol. 2014;24:533-544.
- Hartman R, Mandal R, Sanchez M, et al. Porokeratosis plantaris, palmaris, et disseminata. Dermatol Online J. 2010;16:22.
- Suryawanshi H, Dhobley A, Sharma A, et al. Darier disease: a rare genodermatosis. J Oral Maxillofac Pathol. 2017;21:321. doi:10.4103/jomfp.JOMFP_170_16
- Eastham AB. Pityriasis rubra pilaris. JAMA Dermatol. 2019;155:404. doi:10.1001/jamadermatol.2018.5030
- Nair PA, Badri T. Psoriasis. StatPearls Publishing; 2022. Updated April 6, 2022. Accessed March 13, 2023. https://www.ncbi.nlm.nih.gov/books/NBK448194/
A 67-year-old man presented to our office with a rash on the hands, feet, and periungual skin that began with wartlike growths many years prior and recently had started to involve the proximal arms and legs up to the thighs as well as the trunk. He had a medical history of essential hypertension and chronic obstructive pulmonary disease. He had an 18-year smoking history and had quit more than 25 years prior, with tongue cancer diagnosed more than 5 years prior that was treated with surgery, chemotherapy, and radiation. The lesions occasionally were itchy but not painful. He also reported that his nails frequently split down the middle. He denied any oral lesions and was not using any treatments for the rash. He had no history of skin cancer or other skin conditions. His family history was unclear. Physical examination revealed annular red-pink scaling with a keratotic border on the soles of the feet, palms, and periungual skin. There also were small hyperpigmented papules on the arms, legs, thighs, and trunk over a background of dry and discolored skin, as well as dystrophy of all nails.
Annular Erythematous Plaques With Central Hypopigmentation on Sun-Exposed Skin
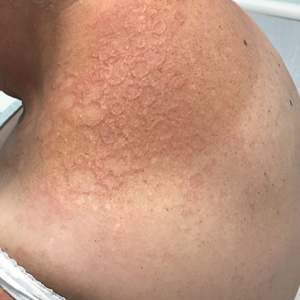
A biopsy showed a markedly elastotic dermis consisting of a palisading granulomatous inflammatory infiltrate and numerous multinucleated histiocytes (Figure). These histopathologic findings along with the clinical presentation confirmed a diagnosis of annular elastolytic granuloma (AEG). Treatment consisting of 3 months of oral minocycline, 2 months of oral doxycycline, and clobetasol ointment all failed. At that point, oral hydroxychloroquine was recommended. Our patient was lost to follow-up by dermatology, then subsequently was placed on hydroxychloroquine by rheumatology to treat both the osteoarthritis and AEG. A follow-up appointment with dermatology was planned for 3 months to monitor hydroxychloroquine treatment and monitor treatment progress; however, she did not follow-up or seek further treatment.
Annular elastolytic granuloma clinically is similar to granuloma annulare (GA), with both presenting as annular plaques surrounded by an elevated border.1 Although AEG clinically is distinct with hypopigmented atrophied plaque centers,2 a biopsy is required to confirm the lack of elastic tissue in zones of atrophy and the presence of multinucleated histiocytes.1,3 Lesions most commonly are seen clinically on sun-exposed areas in middle-aged White women; however, they rarely have been seen on frequently covered skin.4 Our case illustrates the striking photodistribution of AEG, especially on the posterior neck area. The clinical diagnoses of AEG, annular elastolytic giant cell granuloma, and GA in sun-exposed areas are synonymous and can be used interchangeably.5,6
Pathologies considered in the diagnosis of AEG include but are not limited to tinea corporis, annular lichen planus, erythema annulare centrifugum, and necrobiosis lipoidica. Scaling typically is absent in AEG, while tinea corporis presents with hyphae within the stratum corneum of the plaques.7 Papules along the periphery of annular lesions are more typical of annular lichen planus than AEG, and they tend to have a more purple hue.8 Erythema annulare centrifugum has annular erythematous plaques similar to those found in AEG but differs with scaling on the inner margins of these plaques. Histopathology presenting with a lymphocytic infiltrate surrounding vasculature and no indication of elastolytic degradation would further indicate a diagnosis of erythema annulare centrifugum.9 Histopathology showing necrobiosis, lipid depositions, and vascular wall thickenings is indicative of necrobiosis lipoidica.10
Similar to GA,11 the cause of AEG is idiopathic.2 Annular elastolytic granuloma and GA differ in the fact that elastin degradation is characteristic of AEG compared to collagen degradation in GA. It is suspected that elastin degradation in AEG patients is caused by an immune response triggering phagocytosis of elastin by multinucleated histiocytes.2 Actinic damage also is considered a possible cause of elastin fiber degradation in AEG.12 Granuloma annulare can be ruled out and the diagnosis of AEG confirmed with the absence of elastin fibers and mucin on pathology.13
Although there is no established first-line treatment of AEG, successful treatment has been achieved with antimalarial drugs paired with topical steroids.14 Treatment recommendations for AEG include minocycline, chloroquine, hydroxychloroquine, tranilast, and oral retinoids, as well as oral and topical steroids. In clinical cases where AEG occurs in the setting of a chronic disease such as diabetes mellitus, vascular occlusion, arthritis, or hypertension, treatment of underlying disease has been shown to resolve AEG symptoms.14
Although light therapy is not common for AEG, UV light radiation has demonstrated success in treating AEG.15,16 One study showed complete clearance of granulomatous papules after narrowband UVB treatment.15 Another study showed that 2 patients treated with psoralen plus UVA therapy reached complete clearance of AEG lasting at least 3 months after treatment.16
1. Lai JH, Murray SJ, Walsh NM. Evolution of granuloma annulare to mid-dermal elastolysis: report of a case and review of the literature. J Cutan Pathol. 2014;41:462-468. doi:10.1111/cup.12292 2. Klemke CD, Siebold D, Dippel E, et al. Generalised annular elastolytic giant cell granuloma. Dermatology. 2003;207:420-422. doi:10.1159/000074132 3. Limas C. The spectrum of primary cutaneous elastolytic granulomas and their distinction from granuloma annulare: a clinicopathological analysis. Histopathology. 2004;44:277-282. doi:10.1111/j.0309-0167.2004.01755.x 4. Revenga F, Rovira I, Pimentel J, et al. Annular elastolytic giant cell granuloma—actinic granuloma? Clin Exp Dermatol. 1996;21:51-53. 5. Hawryluk EB, Izikson L, English JC 3rd. Non-infectious granulomatous diseases of the skin and their associated systemic diseases: an evidence-based update to important clinical questions. Am J Clin Dermatol. 2010;11:171-181. doi:10.2165/11530080-000000000-00000 6. Berliner JG, Haemel A, LeBoit PE, et al. The sarcoidal variant of annular elastolytic granuloma. J Cutan Pathol. 2013;40:918-920. doi:10.1111/cup.12237 7. Pflederer RT, Ahmed S, Tonkovic-Capin V, et al. Annular polycyclic plaques on the chest and upper back [published online April 24, 2018]. JAAD Case Rep. 2018;4:405-407. doi:10.1016/j.jdcr.2017.07.022 8. Trayes KP, Savage K, Studdiford JS. Annular lesions: diagnosis and treatment. Am Fam Physician. 2018;98:283-291. 9. Weyers W, Diaz-Cascajo C, Weyers I. Erythema annulare centrifugum: results of a clinicopathologic study of 73 patients. Am J Dermatopathol. 2003;25:451-462. doi:10.1097/00000372-200312000-00001 10. Dowling GB, Jones EW. Atypical (annular) necrobiosis lipoidica of the face and scalp. a report of the clinical and histological features of 7 cases. Dermatologica. 1967;135:11-26. doi:10.1159/000254156 11. Piette EW, Rosenbach M. Granuloma annulare: pathogenesis, disease associations and triggers, and therapeutic options. J Am Acad Dermatol. 2016;75:467-479. doi:10.1016/j.jaad.2015 .03.055 12. O’Brien JP, Regan W. Actinically degenerate elastic tissue is the likely antigenic basis of actinic granuloma of the skin and of temporal arteritis [published correction appears in J Am Acad Dermatol. 2000; 42(1 pt 1):148]. J Am Acad Dermatol. 1999;40(2 pt 1):214-222. doi:10.1016/s0190-9622(99)70191-x 13. Rencic A, Nousari CH. Other rheumatologic diseases. In: Bolognia JL, Jorizzo JL, Rapini RP, et al, eds. Dermatology. 2nd ed. Elsevier Limited; 2008:600-601. 14. Burlando M, Herzum A, Cozzani E, et al. Can methotrexate be a successful treatment for unresponsive generalized annular elastolytic giant cell granuloma? case report and review of the literature. Dermatol Ther. 2021;34:E14705. doi:10.1111/dth.14705 15. Takata T, Ikeda M, Kodama H, et al. Regression of papular elastolytic giant cell granuloma using narrow-band UVB irradiation. Dermatology. 2006;212:77-79. doi:10.1159/000089028 16. Pérez-Pérez L, García-Gavín J, Allegue F, et al. Successful treatment of generalized elastolytic giant cell granuloma with psoralenultraviolet A. Photodermatol Photoimmunol Photomed. 2012;28:264-266. doi:10.1111/j.1600-0781.2012.00680.x
A biopsy showed a markedly elastotic dermis consisting of a palisading granulomatous inflammatory infiltrate and numerous multinucleated histiocytes (Figure). These histopathologic findings along with the clinical presentation confirmed a diagnosis of annular elastolytic granuloma (AEG). Treatment consisting of 3 months of oral minocycline, 2 months of oral doxycycline, and clobetasol ointment all failed. At that point, oral hydroxychloroquine was recommended. Our patient was lost to follow-up by dermatology, then subsequently was placed on hydroxychloroquine by rheumatology to treat both the osteoarthritis and AEG. A follow-up appointment with dermatology was planned for 3 months to monitor hydroxychloroquine treatment and monitor treatment progress; however, she did not follow-up or seek further treatment.

Annular elastolytic granuloma clinically is similar to granuloma annulare (GA), with both presenting as annular plaques surrounded by an elevated border.1 Although AEG clinically is distinct with hypopigmented atrophied plaque centers,2 a biopsy is required to confirm the lack of elastic tissue in zones of atrophy and the presence of multinucleated histiocytes.1,3 Lesions most commonly are seen clinically on sun-exposed areas in middle-aged White women; however, they rarely have been seen on frequently covered skin.4 Our case illustrates the striking photodistribution of AEG, especially on the posterior neck area. The clinical diagnoses of AEG, annular elastolytic giant cell granuloma, and GA in sun-exposed areas are synonymous and can be used interchangeably.5,6
Pathologies considered in the diagnosis of AEG include but are not limited to tinea corporis, annular lichen planus, erythema annulare centrifugum, and necrobiosis lipoidica. Scaling typically is absent in AEG, while tinea corporis presents with hyphae within the stratum corneum of the plaques.7 Papules along the periphery of annular lesions are more typical of annular lichen planus than AEG, and they tend to have a more purple hue.8 Erythema annulare centrifugum has annular erythematous plaques similar to those found in AEG but differs with scaling on the inner margins of these plaques. Histopathology presenting with a lymphocytic infiltrate surrounding vasculature and no indication of elastolytic degradation would further indicate a diagnosis of erythema annulare centrifugum.9 Histopathology showing necrobiosis, lipid depositions, and vascular wall thickenings is indicative of necrobiosis lipoidica.10
Similar to GA,11 the cause of AEG is idiopathic.2 Annular elastolytic granuloma and GA differ in the fact that elastin degradation is characteristic of AEG compared to collagen degradation in GA. It is suspected that elastin degradation in AEG patients is caused by an immune response triggering phagocytosis of elastin by multinucleated histiocytes.2 Actinic damage also is considered a possible cause of elastin fiber degradation in AEG.12 Granuloma annulare can be ruled out and the diagnosis of AEG confirmed with the absence of elastin fibers and mucin on pathology.13
Although there is no established first-line treatment of AEG, successful treatment has been achieved with antimalarial drugs paired with topical steroids.14 Treatment recommendations for AEG include minocycline, chloroquine, hydroxychloroquine, tranilast, and oral retinoids, as well as oral and topical steroids. In clinical cases where AEG occurs in the setting of a chronic disease such as diabetes mellitus, vascular occlusion, arthritis, or hypertension, treatment of underlying disease has been shown to resolve AEG symptoms.14
Although light therapy is not common for AEG, UV light radiation has demonstrated success in treating AEG.15,16 One study showed complete clearance of granulomatous papules after narrowband UVB treatment.15 Another study showed that 2 patients treated with psoralen plus UVA therapy reached complete clearance of AEG lasting at least 3 months after treatment.16
A biopsy showed a markedly elastotic dermis consisting of a palisading granulomatous inflammatory infiltrate and numerous multinucleated histiocytes (Figure). These histopathologic findings along with the clinical presentation confirmed a diagnosis of annular elastolytic granuloma (AEG). Treatment consisting of 3 months of oral minocycline, 2 months of oral doxycycline, and clobetasol ointment all failed. At that point, oral hydroxychloroquine was recommended. Our patient was lost to follow-up by dermatology, then subsequently was placed on hydroxychloroquine by rheumatology to treat both the osteoarthritis and AEG. A follow-up appointment with dermatology was planned for 3 months to monitor hydroxychloroquine treatment and monitor treatment progress; however, she did not follow-up or seek further treatment.

Annular elastolytic granuloma clinically is similar to granuloma annulare (GA), with both presenting as annular plaques surrounded by an elevated border.1 Although AEG clinically is distinct with hypopigmented atrophied plaque centers,2 a biopsy is required to confirm the lack of elastic tissue in zones of atrophy and the presence of multinucleated histiocytes.1,3 Lesions most commonly are seen clinically on sun-exposed areas in middle-aged White women; however, they rarely have been seen on frequently covered skin.4 Our case illustrates the striking photodistribution of AEG, especially on the posterior neck area. The clinical diagnoses of AEG, annular elastolytic giant cell granuloma, and GA in sun-exposed areas are synonymous and can be used interchangeably.5,6
Pathologies considered in the diagnosis of AEG include but are not limited to tinea corporis, annular lichen planus, erythema annulare centrifugum, and necrobiosis lipoidica. Scaling typically is absent in AEG, while tinea corporis presents with hyphae within the stratum corneum of the plaques.7 Papules along the periphery of annular lesions are more typical of annular lichen planus than AEG, and they tend to have a more purple hue.8 Erythema annulare centrifugum has annular erythematous plaques similar to those found in AEG but differs with scaling on the inner margins of these plaques. Histopathology presenting with a lymphocytic infiltrate surrounding vasculature and no indication of elastolytic degradation would further indicate a diagnosis of erythema annulare centrifugum.9 Histopathology showing necrobiosis, lipid depositions, and vascular wall thickenings is indicative of necrobiosis lipoidica.10
Similar to GA,11 the cause of AEG is idiopathic.2 Annular elastolytic granuloma and GA differ in the fact that elastin degradation is characteristic of AEG compared to collagen degradation in GA. It is suspected that elastin degradation in AEG patients is caused by an immune response triggering phagocytosis of elastin by multinucleated histiocytes.2 Actinic damage also is considered a possible cause of elastin fiber degradation in AEG.12 Granuloma annulare can be ruled out and the diagnosis of AEG confirmed with the absence of elastin fibers and mucin on pathology.13
Although there is no established first-line treatment of AEG, successful treatment has been achieved with antimalarial drugs paired with topical steroids.14 Treatment recommendations for AEG include minocycline, chloroquine, hydroxychloroquine, tranilast, and oral retinoids, as well as oral and topical steroids. In clinical cases where AEG occurs in the setting of a chronic disease such as diabetes mellitus, vascular occlusion, arthritis, or hypertension, treatment of underlying disease has been shown to resolve AEG symptoms.14
Although light therapy is not common for AEG, UV light radiation has demonstrated success in treating AEG.15,16 One study showed complete clearance of granulomatous papules after narrowband UVB treatment.15 Another study showed that 2 patients treated with psoralen plus UVA therapy reached complete clearance of AEG lasting at least 3 months after treatment.16
1. Lai JH, Murray SJ, Walsh NM. Evolution of granuloma annulare to mid-dermal elastolysis: report of a case and review of the literature. J Cutan Pathol. 2014;41:462-468. doi:10.1111/cup.12292 2. Klemke CD, Siebold D, Dippel E, et al. Generalised annular elastolytic giant cell granuloma. Dermatology. 2003;207:420-422. doi:10.1159/000074132 3. Limas C. The spectrum of primary cutaneous elastolytic granulomas and their distinction from granuloma annulare: a clinicopathological analysis. Histopathology. 2004;44:277-282. doi:10.1111/j.0309-0167.2004.01755.x 4. Revenga F, Rovira I, Pimentel J, et al. Annular elastolytic giant cell granuloma—actinic granuloma? Clin Exp Dermatol. 1996;21:51-53. 5. Hawryluk EB, Izikson L, English JC 3rd. Non-infectious granulomatous diseases of the skin and their associated systemic diseases: an evidence-based update to important clinical questions. Am J Clin Dermatol. 2010;11:171-181. doi:10.2165/11530080-000000000-00000 6. Berliner JG, Haemel A, LeBoit PE, et al. The sarcoidal variant of annular elastolytic granuloma. J Cutan Pathol. 2013;40:918-920. doi:10.1111/cup.12237 7. Pflederer RT, Ahmed S, Tonkovic-Capin V, et al. Annular polycyclic plaques on the chest and upper back [published online April 24, 2018]. JAAD Case Rep. 2018;4:405-407. doi:10.1016/j.jdcr.2017.07.022 8. Trayes KP, Savage K, Studdiford JS. Annular lesions: diagnosis and treatment. Am Fam Physician. 2018;98:283-291. 9. Weyers W, Diaz-Cascajo C, Weyers I. Erythema annulare centrifugum: results of a clinicopathologic study of 73 patients. Am J Dermatopathol. 2003;25:451-462. doi:10.1097/00000372-200312000-00001 10. Dowling GB, Jones EW. Atypical (annular) necrobiosis lipoidica of the face and scalp. a report of the clinical and histological features of 7 cases. Dermatologica. 1967;135:11-26. doi:10.1159/000254156 11. Piette EW, Rosenbach M. Granuloma annulare: pathogenesis, disease associations and triggers, and therapeutic options. J Am Acad Dermatol. 2016;75:467-479. doi:10.1016/j.jaad.2015 .03.055 12. O’Brien JP, Regan W. Actinically degenerate elastic tissue is the likely antigenic basis of actinic granuloma of the skin and of temporal arteritis [published correction appears in J Am Acad Dermatol. 2000; 42(1 pt 1):148]. J Am Acad Dermatol. 1999;40(2 pt 1):214-222. doi:10.1016/s0190-9622(99)70191-x 13. Rencic A, Nousari CH. Other rheumatologic diseases. In: Bolognia JL, Jorizzo JL, Rapini RP, et al, eds. Dermatology. 2nd ed. Elsevier Limited; 2008:600-601. 14. Burlando M, Herzum A, Cozzani E, et al. Can methotrexate be a successful treatment for unresponsive generalized annular elastolytic giant cell granuloma? case report and review of the literature. Dermatol Ther. 2021;34:E14705. doi:10.1111/dth.14705 15. Takata T, Ikeda M, Kodama H, et al. Regression of papular elastolytic giant cell granuloma using narrow-band UVB irradiation. Dermatology. 2006;212:77-79. doi:10.1159/000089028 16. Pérez-Pérez L, García-Gavín J, Allegue F, et al. Successful treatment of generalized elastolytic giant cell granuloma with psoralenultraviolet A. Photodermatol Photoimmunol Photomed. 2012;28:264-266. doi:10.1111/j.1600-0781.2012.00680.x
1. Lai JH, Murray SJ, Walsh NM. Evolution of granuloma annulare to mid-dermal elastolysis: report of a case and review of the literature. J Cutan Pathol. 2014;41:462-468. doi:10.1111/cup.12292 2. Klemke CD, Siebold D, Dippel E, et al. Generalised annular elastolytic giant cell granuloma. Dermatology. 2003;207:420-422. doi:10.1159/000074132 3. Limas C. The spectrum of primary cutaneous elastolytic granulomas and their distinction from granuloma annulare: a clinicopathological analysis. Histopathology. 2004;44:277-282. doi:10.1111/j.0309-0167.2004.01755.x 4. Revenga F, Rovira I, Pimentel J, et al. Annular elastolytic giant cell granuloma—actinic granuloma? Clin Exp Dermatol. 1996;21:51-53. 5. Hawryluk EB, Izikson L, English JC 3rd. Non-infectious granulomatous diseases of the skin and their associated systemic diseases: an evidence-based update to important clinical questions. Am J Clin Dermatol. 2010;11:171-181. doi:10.2165/11530080-000000000-00000 6. Berliner JG, Haemel A, LeBoit PE, et al. The sarcoidal variant of annular elastolytic granuloma. J Cutan Pathol. 2013;40:918-920. doi:10.1111/cup.12237 7. Pflederer RT, Ahmed S, Tonkovic-Capin V, et al. Annular polycyclic plaques on the chest and upper back [published online April 24, 2018]. JAAD Case Rep. 2018;4:405-407. doi:10.1016/j.jdcr.2017.07.022 8. Trayes KP, Savage K, Studdiford JS. Annular lesions: diagnosis and treatment. Am Fam Physician. 2018;98:283-291. 9. Weyers W, Diaz-Cascajo C, Weyers I. Erythema annulare centrifugum: results of a clinicopathologic study of 73 patients. Am J Dermatopathol. 2003;25:451-462. doi:10.1097/00000372-200312000-00001 10. Dowling GB, Jones EW. Atypical (annular) necrobiosis lipoidica of the face and scalp. a report of the clinical and histological features of 7 cases. Dermatologica. 1967;135:11-26. doi:10.1159/000254156 11. Piette EW, Rosenbach M. Granuloma annulare: pathogenesis, disease associations and triggers, and therapeutic options. J Am Acad Dermatol. 2016;75:467-479. doi:10.1016/j.jaad.2015 .03.055 12. O’Brien JP, Regan W. Actinically degenerate elastic tissue is the likely antigenic basis of actinic granuloma of the skin and of temporal arteritis [published correction appears in J Am Acad Dermatol. 2000; 42(1 pt 1):148]. J Am Acad Dermatol. 1999;40(2 pt 1):214-222. doi:10.1016/s0190-9622(99)70191-x 13. Rencic A, Nousari CH. Other rheumatologic diseases. In: Bolognia JL, Jorizzo JL, Rapini RP, et al, eds. Dermatology. 2nd ed. Elsevier Limited; 2008:600-601. 14. Burlando M, Herzum A, Cozzani E, et al. Can methotrexate be a successful treatment for unresponsive generalized annular elastolytic giant cell granuloma? case report and review of the literature. Dermatol Ther. 2021;34:E14705. doi:10.1111/dth.14705 15. Takata T, Ikeda M, Kodama H, et al. Regression of papular elastolytic giant cell granuloma using narrow-band UVB irradiation. Dermatology. 2006;212:77-79. doi:10.1159/000089028 16. Pérez-Pérez L, García-Gavín J, Allegue F, et al. Successful treatment of generalized elastolytic giant cell granuloma with psoralenultraviolet A. Photodermatol Photoimmunol Photomed. 2012;28:264-266. doi:10.1111/j.1600-0781.2012.00680.x
A 67-year-old White woman presented to our dermatology clinic with pruritic annular erythematous plaques with central hypopigmentation on the forearms, dorsal aspect of the hands, neck, and fingers of 3 to 4 months’ duration. The patient rated the severity of pruritus an 8 on a 10-point scale. A review of symptoms was positive for fatigue, joint pain, and headache. The patient had a history of type 2 diabetes mellitus, osteoarthritis, thyroid disease, and stage 3 renal failure. A punch biopsy from the left forearm was performed.
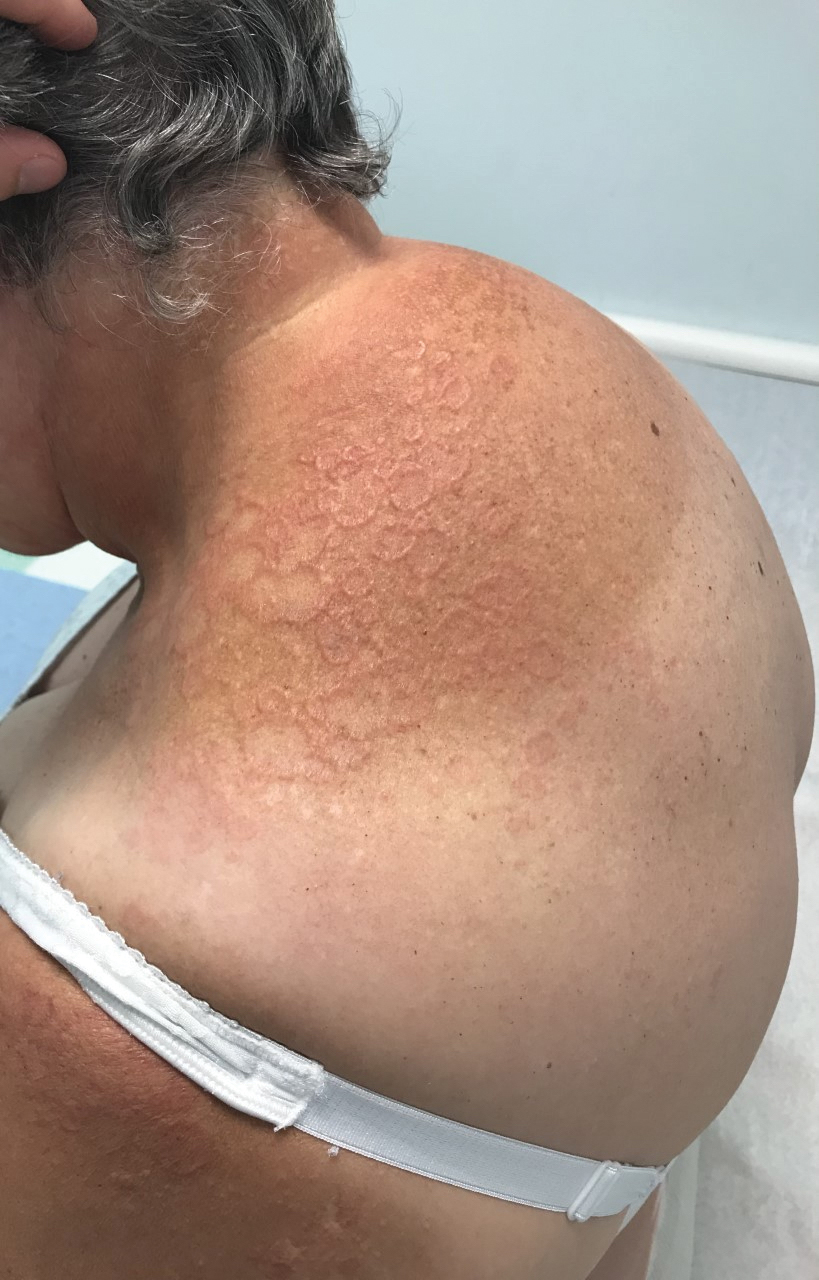
Papular Rash in a New Tattoo
The Diagnosis: Allergic Contact Dermatitis
This patient’s history and physical examination were most consistent with a diagnosis of allergic contact dermatitis, likely from an additive or diluent solution within the tattoo ink. Her history of a similar transient reaction following tattooing 2 weeks prior lent credence to an allergic etiology. She was treated with triamcinolone cream 0.1% as well as mupirocin ointment 2% for use as both an emollient and for precautionary antimicrobial coverage. The rash resolved within 2 days, and she reported no recurrence at a 6-month follow-up. The cosmesis of her tattoo was preserved.
Acute cellulitis may follow tattooing, but the absence of warmth, pain, or purulence on physical examination made this diagnosis less likely in this patient. Sarcoidosis or other granulomatous reactions may present as papules or nodules arising within a tattoo but would be unlikely to occur the next day. Nontuberculous mycobacterial infection likewise tends to present subacutely or chronically rather than immediately following tattoo application.
Tattooing has existed for millennia and is becoming increasingly popular.1,2 The tattooing process entails introduction of insoluble pigment compounds into the dermis to create a permanent design on the skin, which most often is accomplished via needling. As a result, tattooed skin is susceptible to both acute and chronic complications. Acute complications prominently include allergic hypersensitivity reactions and pyogenic bacterial infections. Chronic granulomatous, inflammatory, or infectious complications also can occur.
Allergic eczematous reactions to tattooing are well documented in the literature and are thought to originate from sensitization to pigment molecules themselves or alternatively to ink diluent compounds.3 Although reactions to ink diluent chemicals typically are self-resolving, allergic reactions to pigment can persist beyond the acute phase, as these insoluble compounds intentionally remain embedded in the dermis. The mechanism of action may involve haptenization of pigment molecules that then induces allergic hypersensitivity.3,4 Black pigment typically is derived from carbon black (ie, amorphous combustion byproducts such as soot). Colored inks historically consisted of inorganic heavy metal–containing salts prior to the modern introduction of synthetic azo and polycyclic dyes. These newer colored pigments appear to be less allergenic than their metallic predecessors; however, epidemiologic studies have suggested that allergic reactions still occur more commonly in colored tattoos than black tattoos.1 Overall, these reactions may occur in as many as one-third of individuals who receive tattoos.2,4
As with any process that disrupts skin integrity, tattooing carries a risk for transmitting various infectious pathogens. Microbes may originate from adjacent skin, contaminated needles, ink bottles, or nonsterile ink diluents. Although tattoo parlors and artists may undergo licensing to demonstrate adherence to hygienic standards, regulations vary between states and do not include testing of ink or ink additives to ensure sterility.4,5 Staphylococci and streptococci commonly are implicated in acute pyogenic skin infections following tattooing.5,6 Nontuberculous mycobacteria increasingly are being recognized as causative organisms for granulomatous lesions developing subacutely or even months after receiving a new tattoo.5,7 Local and systemic viral infections also may be transmitted during tattooing; cases of tattoo-transmitted viral warts, molluscum contagiosum, and hepatitis B and C viruses all have been observed.5,6,8 Herpes simplex virus transmission (colloquially termed herpes compunctorum) and HIV transmission through tattooing also are hypothesized to be possible, though there is a paucity of known cases for each.8,9
Chronic inflammatory, granulomatous, or neoplastic lesions may arise within tattooed skin months or years after tattooing. Foreign body granulomas, sarcoidosis, pseudolymphoma, pseudoepitheliomatous hyperplasia, and keratoacanthoma are some representative entities.3,5 Cases of cancerous lesions in tattooed skin have been documented, but their incidence appears similar to nontattooed skin.3 These broad categories of lesions are clinically diverse but may be difficult to definitively diagnose on examination alone; therefore, a biopsy should be strongly considered for any subacute to chronic skin lesions within a tattoo. Patients may be hesitant to disrupt the cosmesis of a tattoo but should be counseled on the attendant risks and benefits to make an informed decision regarding biopsy.
- Wenzel SM, Rittmann I, Landthaler M, et al. Adverse reactions after tattooing: review of the literature and comparison to results of a survey. Dermatology. 2013;226:138-147.
- Liszewski W, Kream E, Helland S, et al. The demographics and rates of tattoo complications, regret, and unsafe tattooing practices: a crosssectional study. Dermatol Surg. 2015;41:1283-1289.
- Islam PS, Chang C, Selmi C, et al. Medical complications of tattoos: a comprehensive review. Clin Rev Allergy Immunol. 2016;50:273-286.
- Serup J, Carlsen KH, Sepehri M. Tattoo complaints and complications: diagnosis and clinical spectrum. Curr Probl Dermatol. 2015;48:48-60.
- Simunovic C, Shinohara MM. Complications of decorative tattoos: recognition and management. Am J Clin Dermatol. 2014;15:525-536.
- Kazandjieva J, Tsankov N. Tattoos: dermatological complications. Clin Dermatol. 2007;25:375-382.
- Sergeant A, Conaglen P, Laurenson IF, et al. Mycobacterium chelonae infection: a complication of tattooing. Clin Exp Dermatol. 2013;38:140-142.
- Cohen PR. Tattoo-associated viral infections: a review. Clin Cosmet Investig Dermatol. 2021;14:1529-1540.
- Doll DC. Tattooing in prison and HIV infection. Lancet. 1988;1:66-67.
The Diagnosis: Allergic Contact Dermatitis
This patient’s history and physical examination were most consistent with a diagnosis of allergic contact dermatitis, likely from an additive or diluent solution within the tattoo ink. Her history of a similar transient reaction following tattooing 2 weeks prior lent credence to an allergic etiology. She was treated with triamcinolone cream 0.1% as well as mupirocin ointment 2% for use as both an emollient and for precautionary antimicrobial coverage. The rash resolved within 2 days, and she reported no recurrence at a 6-month follow-up. The cosmesis of her tattoo was preserved.
Acute cellulitis may follow tattooing, but the absence of warmth, pain, or purulence on physical examination made this diagnosis less likely in this patient. Sarcoidosis or other granulomatous reactions may present as papules or nodules arising within a tattoo but would be unlikely to occur the next day. Nontuberculous mycobacterial infection likewise tends to present subacutely or chronically rather than immediately following tattoo application.
Tattooing has existed for millennia and is becoming increasingly popular.1,2 The tattooing process entails introduction of insoluble pigment compounds into the dermis to create a permanent design on the skin, which most often is accomplished via needling. As a result, tattooed skin is susceptible to both acute and chronic complications. Acute complications prominently include allergic hypersensitivity reactions and pyogenic bacterial infections. Chronic granulomatous, inflammatory, or infectious complications also can occur.
Allergic eczematous reactions to tattooing are well documented in the literature and are thought to originate from sensitization to pigment molecules themselves or alternatively to ink diluent compounds.3 Although reactions to ink diluent chemicals typically are self-resolving, allergic reactions to pigment can persist beyond the acute phase, as these insoluble compounds intentionally remain embedded in the dermis. The mechanism of action may involve haptenization of pigment molecules that then induces allergic hypersensitivity.3,4 Black pigment typically is derived from carbon black (ie, amorphous combustion byproducts such as soot). Colored inks historically consisted of inorganic heavy metal–containing salts prior to the modern introduction of synthetic azo and polycyclic dyes. These newer colored pigments appear to be less allergenic than their metallic predecessors; however, epidemiologic studies have suggested that allergic reactions still occur more commonly in colored tattoos than black tattoos.1 Overall, these reactions may occur in as many as one-third of individuals who receive tattoos.2,4
As with any process that disrupts skin integrity, tattooing carries a risk for transmitting various infectious pathogens. Microbes may originate from adjacent skin, contaminated needles, ink bottles, or nonsterile ink diluents. Although tattoo parlors and artists may undergo licensing to demonstrate adherence to hygienic standards, regulations vary between states and do not include testing of ink or ink additives to ensure sterility.4,5 Staphylococci and streptococci commonly are implicated in acute pyogenic skin infections following tattooing.5,6 Nontuberculous mycobacteria increasingly are being recognized as causative organisms for granulomatous lesions developing subacutely or even months after receiving a new tattoo.5,7 Local and systemic viral infections also may be transmitted during tattooing; cases of tattoo-transmitted viral warts, molluscum contagiosum, and hepatitis B and C viruses all have been observed.5,6,8 Herpes simplex virus transmission (colloquially termed herpes compunctorum) and HIV transmission through tattooing also are hypothesized to be possible, though there is a paucity of known cases for each.8,9
Chronic inflammatory, granulomatous, or neoplastic lesions may arise within tattooed skin months or years after tattooing. Foreign body granulomas, sarcoidosis, pseudolymphoma, pseudoepitheliomatous hyperplasia, and keratoacanthoma are some representative entities.3,5 Cases of cancerous lesions in tattooed skin have been documented, but their incidence appears similar to nontattooed skin.3 These broad categories of lesions are clinically diverse but may be difficult to definitively diagnose on examination alone; therefore, a biopsy should be strongly considered for any subacute to chronic skin lesions within a tattoo. Patients may be hesitant to disrupt the cosmesis of a tattoo but should be counseled on the attendant risks and benefits to make an informed decision regarding biopsy.
The Diagnosis: Allergic Contact Dermatitis
This patient’s history and physical examination were most consistent with a diagnosis of allergic contact dermatitis, likely from an additive or diluent solution within the tattoo ink. Her history of a similar transient reaction following tattooing 2 weeks prior lent credence to an allergic etiology. She was treated with triamcinolone cream 0.1% as well as mupirocin ointment 2% for use as both an emollient and for precautionary antimicrobial coverage. The rash resolved within 2 days, and she reported no recurrence at a 6-month follow-up. The cosmesis of her tattoo was preserved.
Acute cellulitis may follow tattooing, but the absence of warmth, pain, or purulence on physical examination made this diagnosis less likely in this patient. Sarcoidosis or other granulomatous reactions may present as papules or nodules arising within a tattoo but would be unlikely to occur the next day. Nontuberculous mycobacterial infection likewise tends to present subacutely or chronically rather than immediately following tattoo application.
Tattooing has existed for millennia and is becoming increasingly popular.1,2 The tattooing process entails introduction of insoluble pigment compounds into the dermis to create a permanent design on the skin, which most often is accomplished via needling. As a result, tattooed skin is susceptible to both acute and chronic complications. Acute complications prominently include allergic hypersensitivity reactions and pyogenic bacterial infections. Chronic granulomatous, inflammatory, or infectious complications also can occur.
Allergic eczematous reactions to tattooing are well documented in the literature and are thought to originate from sensitization to pigment molecules themselves or alternatively to ink diluent compounds.3 Although reactions to ink diluent chemicals typically are self-resolving, allergic reactions to pigment can persist beyond the acute phase, as these insoluble compounds intentionally remain embedded in the dermis. The mechanism of action may involve haptenization of pigment molecules that then induces allergic hypersensitivity.3,4 Black pigment typically is derived from carbon black (ie, amorphous combustion byproducts such as soot). Colored inks historically consisted of inorganic heavy metal–containing salts prior to the modern introduction of synthetic azo and polycyclic dyes. These newer colored pigments appear to be less allergenic than their metallic predecessors; however, epidemiologic studies have suggested that allergic reactions still occur more commonly in colored tattoos than black tattoos.1 Overall, these reactions may occur in as many as one-third of individuals who receive tattoos.2,4
As with any process that disrupts skin integrity, tattooing carries a risk for transmitting various infectious pathogens. Microbes may originate from adjacent skin, contaminated needles, ink bottles, or nonsterile ink diluents. Although tattoo parlors and artists may undergo licensing to demonstrate adherence to hygienic standards, regulations vary between states and do not include testing of ink or ink additives to ensure sterility.4,5 Staphylococci and streptococci commonly are implicated in acute pyogenic skin infections following tattooing.5,6 Nontuberculous mycobacteria increasingly are being recognized as causative organisms for granulomatous lesions developing subacutely or even months after receiving a new tattoo.5,7 Local and systemic viral infections also may be transmitted during tattooing; cases of tattoo-transmitted viral warts, molluscum contagiosum, and hepatitis B and C viruses all have been observed.5,6,8 Herpes simplex virus transmission (colloquially termed herpes compunctorum) and HIV transmission through tattooing also are hypothesized to be possible, though there is a paucity of known cases for each.8,9
Chronic inflammatory, granulomatous, or neoplastic lesions may arise within tattooed skin months or years after tattooing. Foreign body granulomas, sarcoidosis, pseudolymphoma, pseudoepitheliomatous hyperplasia, and keratoacanthoma are some representative entities.3,5 Cases of cancerous lesions in tattooed skin have been documented, but their incidence appears similar to nontattooed skin.3 These broad categories of lesions are clinically diverse but may be difficult to definitively diagnose on examination alone; therefore, a biopsy should be strongly considered for any subacute to chronic skin lesions within a tattoo. Patients may be hesitant to disrupt the cosmesis of a tattoo but should be counseled on the attendant risks and benefits to make an informed decision regarding biopsy.
- Wenzel SM, Rittmann I, Landthaler M, et al. Adverse reactions after tattooing: review of the literature and comparison to results of a survey. Dermatology. 2013;226:138-147.
- Liszewski W, Kream E, Helland S, et al. The demographics and rates of tattoo complications, regret, and unsafe tattooing practices: a crosssectional study. Dermatol Surg. 2015;41:1283-1289.
- Islam PS, Chang C, Selmi C, et al. Medical complications of tattoos: a comprehensive review. Clin Rev Allergy Immunol. 2016;50:273-286.
- Serup J, Carlsen KH, Sepehri M. Tattoo complaints and complications: diagnosis and clinical spectrum. Curr Probl Dermatol. 2015;48:48-60.
- Simunovic C, Shinohara MM. Complications of decorative tattoos: recognition and management. Am J Clin Dermatol. 2014;15:525-536.
- Kazandjieva J, Tsankov N. Tattoos: dermatological complications. Clin Dermatol. 2007;25:375-382.
- Sergeant A, Conaglen P, Laurenson IF, et al. Mycobacterium chelonae infection: a complication of tattooing. Clin Exp Dermatol. 2013;38:140-142.
- Cohen PR. Tattoo-associated viral infections: a review. Clin Cosmet Investig Dermatol. 2021;14:1529-1540.
- Doll DC. Tattooing in prison and HIV infection. Lancet. 1988;1:66-67.
- Wenzel SM, Rittmann I, Landthaler M, et al. Adverse reactions after tattooing: review of the literature and comparison to results of a survey. Dermatology. 2013;226:138-147.
- Liszewski W, Kream E, Helland S, et al. The demographics and rates of tattoo complications, regret, and unsafe tattooing practices: a crosssectional study. Dermatol Surg. 2015;41:1283-1289.
- Islam PS, Chang C, Selmi C, et al. Medical complications of tattoos: a comprehensive review. Clin Rev Allergy Immunol. 2016;50:273-286.
- Serup J, Carlsen KH, Sepehri M. Tattoo complaints and complications: diagnosis and clinical spectrum. Curr Probl Dermatol. 2015;48:48-60.
- Simunovic C, Shinohara MM. Complications of decorative tattoos: recognition and management. Am J Clin Dermatol. 2014;15:525-536.
- Kazandjieva J, Tsankov N. Tattoos: dermatological complications. Clin Dermatol. 2007;25:375-382.
- Sergeant A, Conaglen P, Laurenson IF, et al. Mycobacterium chelonae infection: a complication of tattooing. Clin Exp Dermatol. 2013;38:140-142.
- Cohen PR. Tattoo-associated viral infections: a review. Clin Cosmet Investig Dermatol. 2021;14:1529-1540.
- Doll DC. Tattooing in prison and HIV infection. Lancet. 1988;1:66-67.
A healthy 21-year-old woman presented with a pruritic papulovesicular rash on the left arm of 2 days’ duration. The day before rash onset, she received a black ink tattoo on the left arm to complete the second half of a monochromatic sleevestyle design. She previously underwent initial tattooing of the left arm by the same artist 2 weeks prior and experienced a similar but less extensive rash that self-resolved after 1 week. She had 8 older tattoos on various other body parts and denied any reactions. Physical examination showed numerous scattered papules and papulovesicles confined to areas of newly tattooed skin throughout the left arm. In the larger swaths of the tattoo, the papules coalesced into well-defined plaques. There was a discrete rim of faint erythema bordering the newly tattooed skin. No erosions, ulcerations, or purulent areas were observed, and there was no tenderness or excess warmth of the affected skin. Adjacent previously tattooed areas of the left arm were unaffected.

Widespread Erosions in Intertriginous Areas
The Diagnosis: Darier Disease
A clinical diagnosis of Darier disease was made from the skin findings of pruritic, malodorous, keratotic papules in a seborrheic distribution and pathognomonic nail dystrophy, along with a family history that demonstrated autosomal-dominant inheritance. The ulcerations were suspected to be caused by a superimposed herpes simplex virus (HSV) infection in the form of eczema herpeticum. The clinical diagnosis was later confirmed via punch biopsy. Pathology results demonstrated focal acantholytic dyskeratosis, which was consistent with Darier disease given the focal nature and lack of acanthosis. The patient’s father and sister also were confirmed to have Darier disease by an outside dermatologist.
Darier disease is a rare keratinizing autosomaldominant genodermatosis that occurs due to a mutation in the ATP2A2 gene, which encodes a sarco/endoplasmic reticulum calcium ATPase pump that decreases cell adhesion between keratinocytes, leading to epidermal acantholysis and dyskeratosis and ultimately a disrupted skin barrier.1,2 Darier disease often presents in childhood and adolescence with papules in a seborrheic distribution on the central chest and back (Figure, A); the intertriginous folds also may be involved. Darier disease can manifest with palmoplantar pits (Figure, B), a cobblestonelike texture of the oral mucosa, acrokeratosis verruciformis of Hopf, and nail findings with alternating red and white longitudinal streaks in the nail bed resembling a candy cane along with characteristic V nicking deformities of the nails themselves (Figure, C). Chronic flares may occur throughout one’s lifetime, with patients experiencing more symptoms in the summer months due to heat, sweat, and UV light exposure, as well as infections that irritate the skin and worsen dyskeratosis. Studies have revealed an association between Darier disease and neuropsychiatric conditions, including major depressive disorder, schizophrenia, and bipolar disorder.3,4
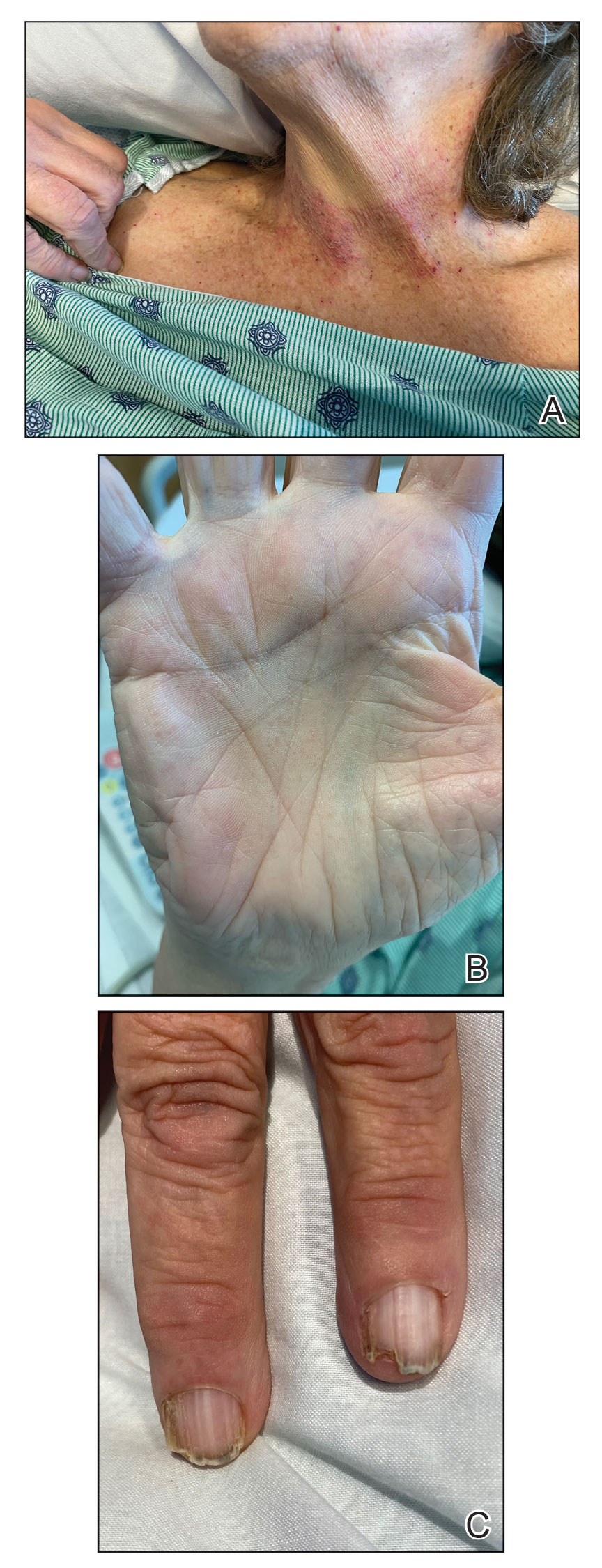
The skin barrier is compromised in patients with Darier disease, thereby making secondary infection more likely to occur. Polymerase chain reaction swabs of our patient’s purulent ulcerations were positive for HSV type 1, further strengthening a diagnosis of secondary eczema herpeticum, which occurs when patients have widespread HSV superinfecting pre-existing skin conditions such as atopic dermatitis, Darier disease, and Hailey-Hailey disease.5-7 The lesions are characterized by a monomorphic eruption of umbilicated vesicles on an erythematous base. Lesions can progress to punched-out ulcers and erosions with hemorrhagic crusts that coalesce, forming scalloped borders, similar to our patient’s presentation.8
Hailey-Hailey disease, a genodermatosis that alters calcium signaling with an autosomal-dominant inheritance pattern, was unlikely in our patient due to the presence of nail abnormalities and palmar pits that are characteristic of Darier disease. From a purely histopathologic standpoint, Grover disease was considered with skin biopsy demonstrating acantholytic dyskeratosis but was not compatible with the clinical context. Furthermore, trials of antibiotics with group A Streptococcus and Staphylococcus aureus coverage failed in our patient, and she lacked systemic symptoms that would be supportive of a cellulitis diagnosis. The punched-out lesions suggested that an isolated exacerbation of atopic dermatitis was not sufficient to explain all of the clinical findings.
Eczema herpeticum must be considered in the differential diagnosis for patients with underlying Darier disease and widespread ulcerations. Our patient had more recent punched-out ulcerations in the intertriginous regions, with other areas showing later stages of confluent ulcers with scalloped borders. Delayed diagnosis and treatment of eczema herpeticum combined with severe Darier disease can lead to increased risk for hospitalization and rarely fatality.8,9
Our patient was started on intravenous acyclovir until the lesions crusted and then was transitioned to a suppressive dose of oral valacyclovir given the widespread distribution. The Darier disease itself was managed with topical steroids and a zinc oxide barrier, serving as protectants to pathogens through microscopic breaks in the skin. Our patient also had a mild case of candidal intertrigo that was exacerbated by obesity and managed with topical ketoconazole. Gabapentin, hydromorphone, and acetaminophen were used for pain. She was discharged 10 days after admission with substantial improvement of both the HSV lesions and the irritation from her Darier disease. At follow-up visits 20 days later and again 6 months after discharge, she had been feeling well without any HSV flares.
The eczema herpeticum likely arose from our patient’s chronic skin barrier impairment attributed to Darier disease, leading to the cutaneous inoculation of HSV. Our patient and her family members had never been evaluated by a dermatologist until late in life during this hospitalization. Medication compliance with a suppressive dose of oral valacyclovir and topical steroids is vital to prevent flares of both eczema herpeticum and Darier disease, respectively. This case highlights the importance of dermatology consultation for complex cutaneous findings, as delayed diagnosis and treatment can lead to increased morbidity and mortality.
- Cooper SM, Burge SM. Darier’s disease: epidemiology, pathophysiology, and management. Am J Clin Dermatol. 2003;4:97-105. doi:10.2165/00128071-200304020-00003
- Dhitavat J, Cobbold C, Leslie N, et al. Impaired trafficking of the desmoplakins in cultured Darier’s disease keratinocytes. J Invest Dermatol. 2003;121:1349-1355. doi:10.1046/j.1523-1747.2003.12557.x
- Nakamura T, Kazuno AA, Nakajima K, et al. Loss of function mutations in ATP2A2 and psychoses: a case report and literature survey. Psychiatry Clin Neurosci. 2016;70:342-350. doi:10.1111/pcn.12395
- Gordon-Smith K, Jones LA, Burge SM, et al. The neuropsychiatric phenotype in Darier disease. Br J Dermatol. 2010;163:515-522. doi:10.1111/j.1365-2133.2010.09834.x
- Hemani SA, Edmond MB, Jaggi P, et al. Frequency and clinical features associated with eczema herpeticum in hospitalized children with presumed atopic dermatitis skin infection. Pediatr Infect Dis J. 2020;39:263-266. doi:10.1097/INF.0000000000002542
- Tayabali K, Pothiwalla H, Lowitt M. Eczema herpeticum in Darier’s disease: a topical storm. J Community Hosp Intern Med Perspect. 2019;9:347-350. doi:10.1080/20009666.2019.1650590
- Lee GH, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey disease. Ann Dermatol. 2009;21:311-314. doi:10.5021/ad.2009.21.3.311
- Nikkels AF, Beauthier F, Quatresooz P, et al. Fatal herpes simplex virus infection in Darier disease under corticotherapy. Eur J Dermatol. 2005;15:293-297.
- Vogt KA, Lohse CM, El-Azhary RA, et al. Kaposi varicelliform eruption in patients with Darier disease: a 20-year retrospective study. J Am Acad Dermatol. 2015;72:481-484. doi:10.1016/j.jaad.2014.12.001
The Diagnosis: Darier Disease
A clinical diagnosis of Darier disease was made from the skin findings of pruritic, malodorous, keratotic papules in a seborrheic distribution and pathognomonic nail dystrophy, along with a family history that demonstrated autosomal-dominant inheritance. The ulcerations were suspected to be caused by a superimposed herpes simplex virus (HSV) infection in the form of eczema herpeticum. The clinical diagnosis was later confirmed via punch biopsy. Pathology results demonstrated focal acantholytic dyskeratosis, which was consistent with Darier disease given the focal nature and lack of acanthosis. The patient’s father and sister also were confirmed to have Darier disease by an outside dermatologist.
Darier disease is a rare keratinizing autosomaldominant genodermatosis that occurs due to a mutation in the ATP2A2 gene, which encodes a sarco/endoplasmic reticulum calcium ATPase pump that decreases cell adhesion between keratinocytes, leading to epidermal acantholysis and dyskeratosis and ultimately a disrupted skin barrier.1,2 Darier disease often presents in childhood and adolescence with papules in a seborrheic distribution on the central chest and back (Figure, A); the intertriginous folds also may be involved. Darier disease can manifest with palmoplantar pits (Figure, B), a cobblestonelike texture of the oral mucosa, acrokeratosis verruciformis of Hopf, and nail findings with alternating red and white longitudinal streaks in the nail bed resembling a candy cane along with characteristic V nicking deformities of the nails themselves (Figure, C). Chronic flares may occur throughout one’s lifetime, with patients experiencing more symptoms in the summer months due to heat, sweat, and UV light exposure, as well as infections that irritate the skin and worsen dyskeratosis. Studies have revealed an association between Darier disease and neuropsychiatric conditions, including major depressive disorder, schizophrenia, and bipolar disorder.3,4

The skin barrier is compromised in patients with Darier disease, thereby making secondary infection more likely to occur. Polymerase chain reaction swabs of our patient’s purulent ulcerations were positive for HSV type 1, further strengthening a diagnosis of secondary eczema herpeticum, which occurs when patients have widespread HSV superinfecting pre-existing skin conditions such as atopic dermatitis, Darier disease, and Hailey-Hailey disease.5-7 The lesions are characterized by a monomorphic eruption of umbilicated vesicles on an erythematous base. Lesions can progress to punched-out ulcers and erosions with hemorrhagic crusts that coalesce, forming scalloped borders, similar to our patient’s presentation.8
Hailey-Hailey disease, a genodermatosis that alters calcium signaling with an autosomal-dominant inheritance pattern, was unlikely in our patient due to the presence of nail abnormalities and palmar pits that are characteristic of Darier disease. From a purely histopathologic standpoint, Grover disease was considered with skin biopsy demonstrating acantholytic dyskeratosis but was not compatible with the clinical context. Furthermore, trials of antibiotics with group A Streptococcus and Staphylococcus aureus coverage failed in our patient, and she lacked systemic symptoms that would be supportive of a cellulitis diagnosis. The punched-out lesions suggested that an isolated exacerbation of atopic dermatitis was not sufficient to explain all of the clinical findings.
Eczema herpeticum must be considered in the differential diagnosis for patients with underlying Darier disease and widespread ulcerations. Our patient had more recent punched-out ulcerations in the intertriginous regions, with other areas showing later stages of confluent ulcers with scalloped borders. Delayed diagnosis and treatment of eczema herpeticum combined with severe Darier disease can lead to increased risk for hospitalization and rarely fatality.8,9
Our patient was started on intravenous acyclovir until the lesions crusted and then was transitioned to a suppressive dose of oral valacyclovir given the widespread distribution. The Darier disease itself was managed with topical steroids and a zinc oxide barrier, serving as protectants to pathogens through microscopic breaks in the skin. Our patient also had a mild case of candidal intertrigo that was exacerbated by obesity and managed with topical ketoconazole. Gabapentin, hydromorphone, and acetaminophen were used for pain. She was discharged 10 days after admission with substantial improvement of both the HSV lesions and the irritation from her Darier disease. At follow-up visits 20 days later and again 6 months after discharge, she had been feeling well without any HSV flares.
The eczema herpeticum likely arose from our patient’s chronic skin barrier impairment attributed to Darier disease, leading to the cutaneous inoculation of HSV. Our patient and her family members had never been evaluated by a dermatologist until late in life during this hospitalization. Medication compliance with a suppressive dose of oral valacyclovir and topical steroids is vital to prevent flares of both eczema herpeticum and Darier disease, respectively. This case highlights the importance of dermatology consultation for complex cutaneous findings, as delayed diagnosis and treatment can lead to increased morbidity and mortality.
The Diagnosis: Darier Disease
A clinical diagnosis of Darier disease was made from the skin findings of pruritic, malodorous, keratotic papules in a seborrheic distribution and pathognomonic nail dystrophy, along with a family history that demonstrated autosomal-dominant inheritance. The ulcerations were suspected to be caused by a superimposed herpes simplex virus (HSV) infection in the form of eczema herpeticum. The clinical diagnosis was later confirmed via punch biopsy. Pathology results demonstrated focal acantholytic dyskeratosis, which was consistent with Darier disease given the focal nature and lack of acanthosis. The patient’s father and sister also were confirmed to have Darier disease by an outside dermatologist.
Darier disease is a rare keratinizing autosomaldominant genodermatosis that occurs due to a mutation in the ATP2A2 gene, which encodes a sarco/endoplasmic reticulum calcium ATPase pump that decreases cell adhesion between keratinocytes, leading to epidermal acantholysis and dyskeratosis and ultimately a disrupted skin barrier.1,2 Darier disease often presents in childhood and adolescence with papules in a seborrheic distribution on the central chest and back (Figure, A); the intertriginous folds also may be involved. Darier disease can manifest with palmoplantar pits (Figure, B), a cobblestonelike texture of the oral mucosa, acrokeratosis verruciformis of Hopf, and nail findings with alternating red and white longitudinal streaks in the nail bed resembling a candy cane along with characteristic V nicking deformities of the nails themselves (Figure, C). Chronic flares may occur throughout one’s lifetime, with patients experiencing more symptoms in the summer months due to heat, sweat, and UV light exposure, as well as infections that irritate the skin and worsen dyskeratosis. Studies have revealed an association between Darier disease and neuropsychiatric conditions, including major depressive disorder, schizophrenia, and bipolar disorder.3,4

The skin barrier is compromised in patients with Darier disease, thereby making secondary infection more likely to occur. Polymerase chain reaction swabs of our patient’s purulent ulcerations were positive for HSV type 1, further strengthening a diagnosis of secondary eczema herpeticum, which occurs when patients have widespread HSV superinfecting pre-existing skin conditions such as atopic dermatitis, Darier disease, and Hailey-Hailey disease.5-7 The lesions are characterized by a monomorphic eruption of umbilicated vesicles on an erythematous base. Lesions can progress to punched-out ulcers and erosions with hemorrhagic crusts that coalesce, forming scalloped borders, similar to our patient’s presentation.8
Hailey-Hailey disease, a genodermatosis that alters calcium signaling with an autosomal-dominant inheritance pattern, was unlikely in our patient due to the presence of nail abnormalities and palmar pits that are characteristic of Darier disease. From a purely histopathologic standpoint, Grover disease was considered with skin biopsy demonstrating acantholytic dyskeratosis but was not compatible with the clinical context. Furthermore, trials of antibiotics with group A Streptococcus and Staphylococcus aureus coverage failed in our patient, and she lacked systemic symptoms that would be supportive of a cellulitis diagnosis. The punched-out lesions suggested that an isolated exacerbation of atopic dermatitis was not sufficient to explain all of the clinical findings.
Eczema herpeticum must be considered in the differential diagnosis for patients with underlying Darier disease and widespread ulcerations. Our patient had more recent punched-out ulcerations in the intertriginous regions, with other areas showing later stages of confluent ulcers with scalloped borders. Delayed diagnosis and treatment of eczema herpeticum combined with severe Darier disease can lead to increased risk for hospitalization and rarely fatality.8,9
Our patient was started on intravenous acyclovir until the lesions crusted and then was transitioned to a suppressive dose of oral valacyclovir given the widespread distribution. The Darier disease itself was managed with topical steroids and a zinc oxide barrier, serving as protectants to pathogens through microscopic breaks in the skin. Our patient also had a mild case of candidal intertrigo that was exacerbated by obesity and managed with topical ketoconazole. Gabapentin, hydromorphone, and acetaminophen were used for pain. She was discharged 10 days after admission with substantial improvement of both the HSV lesions and the irritation from her Darier disease. At follow-up visits 20 days later and again 6 months after discharge, she had been feeling well without any HSV flares.
The eczema herpeticum likely arose from our patient’s chronic skin barrier impairment attributed to Darier disease, leading to the cutaneous inoculation of HSV. Our patient and her family members had never been evaluated by a dermatologist until late in life during this hospitalization. Medication compliance with a suppressive dose of oral valacyclovir and topical steroids is vital to prevent flares of both eczema herpeticum and Darier disease, respectively. This case highlights the importance of dermatology consultation for complex cutaneous findings, as delayed diagnosis and treatment can lead to increased morbidity and mortality.
- Cooper SM, Burge SM. Darier’s disease: epidemiology, pathophysiology, and management. Am J Clin Dermatol. 2003;4:97-105. doi:10.2165/00128071-200304020-00003
- Dhitavat J, Cobbold C, Leslie N, et al. Impaired trafficking of the desmoplakins in cultured Darier’s disease keratinocytes. J Invest Dermatol. 2003;121:1349-1355. doi:10.1046/j.1523-1747.2003.12557.x
- Nakamura T, Kazuno AA, Nakajima K, et al. Loss of function mutations in ATP2A2 and psychoses: a case report and literature survey. Psychiatry Clin Neurosci. 2016;70:342-350. doi:10.1111/pcn.12395
- Gordon-Smith K, Jones LA, Burge SM, et al. The neuropsychiatric phenotype in Darier disease. Br J Dermatol. 2010;163:515-522. doi:10.1111/j.1365-2133.2010.09834.x
- Hemani SA, Edmond MB, Jaggi P, et al. Frequency and clinical features associated with eczema herpeticum in hospitalized children with presumed atopic dermatitis skin infection. Pediatr Infect Dis J. 2020;39:263-266. doi:10.1097/INF.0000000000002542
- Tayabali K, Pothiwalla H, Lowitt M. Eczema herpeticum in Darier’s disease: a topical storm. J Community Hosp Intern Med Perspect. 2019;9:347-350. doi:10.1080/20009666.2019.1650590
- Lee GH, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey disease. Ann Dermatol. 2009;21:311-314. doi:10.5021/ad.2009.21.3.311
- Nikkels AF, Beauthier F, Quatresooz P, et al. Fatal herpes simplex virus infection in Darier disease under corticotherapy. Eur J Dermatol. 2005;15:293-297.
- Vogt KA, Lohse CM, El-Azhary RA, et al. Kaposi varicelliform eruption in patients with Darier disease: a 20-year retrospective study. J Am Acad Dermatol. 2015;72:481-484. doi:10.1016/j.jaad.2014.12.001
- Cooper SM, Burge SM. Darier’s disease: epidemiology, pathophysiology, and management. Am J Clin Dermatol. 2003;4:97-105. doi:10.2165/00128071-200304020-00003
- Dhitavat J, Cobbold C, Leslie N, et al. Impaired trafficking of the desmoplakins in cultured Darier’s disease keratinocytes. J Invest Dermatol. 2003;121:1349-1355. doi:10.1046/j.1523-1747.2003.12557.x
- Nakamura T, Kazuno AA, Nakajima K, et al. Loss of function mutations in ATP2A2 and psychoses: a case report and literature survey. Psychiatry Clin Neurosci. 2016;70:342-350. doi:10.1111/pcn.12395
- Gordon-Smith K, Jones LA, Burge SM, et al. The neuropsychiatric phenotype in Darier disease. Br J Dermatol. 2010;163:515-522. doi:10.1111/j.1365-2133.2010.09834.x
- Hemani SA, Edmond MB, Jaggi P, et al. Frequency and clinical features associated with eczema herpeticum in hospitalized children with presumed atopic dermatitis skin infection. Pediatr Infect Dis J. 2020;39:263-266. doi:10.1097/INF.0000000000002542
- Tayabali K, Pothiwalla H, Lowitt M. Eczema herpeticum in Darier’s disease: a topical storm. J Community Hosp Intern Med Perspect. 2019;9:347-350. doi:10.1080/20009666.2019.1650590
- Lee GH, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey disease. Ann Dermatol. 2009;21:311-314. doi:10.5021/ad.2009.21.3.311
- Nikkels AF, Beauthier F, Quatresooz P, et al. Fatal herpes simplex virus infection in Darier disease under corticotherapy. Eur J Dermatol. 2005;15:293-297.
- Vogt KA, Lohse CM, El-Azhary RA, et al. Kaposi varicelliform eruption in patients with Darier disease: a 20-year retrospective study. J Am Acad Dermatol. 2015;72:481-484. doi:10.1016/j.jaad.2014.12.001
A 72-year-old woman presented to the emergency department with painful, erythematic, pruritic, and purulent lesions in intertriginous regions including the inframammary, infra-abdominal, and inguinal folds with a burning sensation of 1 week’s duration. Her medical history was notable for obesity and major depressive disorder. She was empirically treated for cellulitis, but there was no improvement with cefazolin or clindamycin. Dermatology was consulted. Physical examination revealed gray-brown, slightly umbilicated papules in the inframammary region that were malodorous upon lifting the folds. Grouped, punched-out ulcerations with scalloped borders were superimposed onto these papules. Further examination revealed a macerated erythematous plaque in the infra-abdominal and inguinal regions with punched-out ulcers. Hemecrusted papules were observed in seborrheic areas including the anterior neck, hairline, and trunk. Few subtle keratotic pits were localized on the palms. She reported similar flares in the past but never saw a dermatologist and noted that her father and sister had similar papules in a seborrheic distribution. Nail abnormalities included red and white alternating subungual streaks with irregular texture including V nicking of the distal nails.
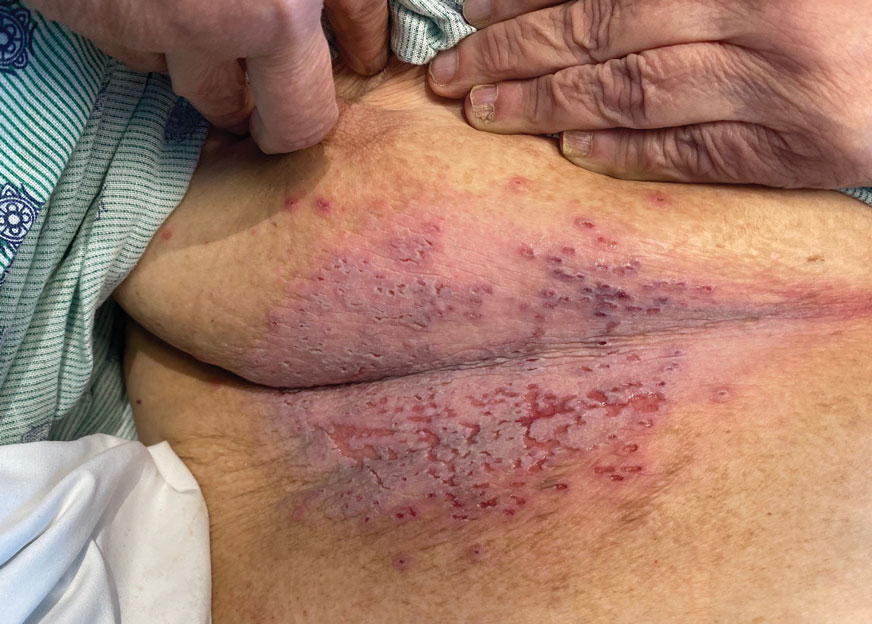
Spreading Painful Lesions on the Legs
The Diagnosis: Cutaneous Leishmaniasis
A punch biopsy of the skin showed pseudoepitheliomatous hyperplasia of the epidermis with dermal granulomatous and suppurative inflammation; tissue cultures remained sterile. Polymerase chain reaction testing of the skin revealed the presence of Leishmania guyanensis complex. Leishmaniasis is a widespread parasitic disease transmitted via sandflies that often is seen in children and young adults.1 Although leishmaniasis is endemic to several countries within Southeast Asia, East Africa, and Latin America, an increase in international travel has brought the disease to nonendemic regions. Therefore, it is crucial to obtain a detailed history of travel and exposure to sandflies in patients who have recently returned from endemic regions.
Leishmaniasis may present in 3 forms: cutaneous, mucocutaneous, or visceral. Cutaneous clinical findings vary depending on disease stage, causative species, and host immune activation. Presentation following a sandfly bite typically includes a papule that progresses to an erythematous nodule. Cutaneous leishmaniasis commonly occurs in areas of the body that are easily accessible to sandflies, such as the face, neck, and limbs. Mucocutaneous leishmaniasis presents with nasal or oral involvement several years after the onset of cutaneous leishmaniasis; however, it can coexist with cutaneous involvement. Without treatment, mucocutaneous leishmaniasis may lead to perforation of the nasal septum, destruction of the mouth, and life-threatening airway obstruction.1 Determining the specific species is important due to the variation in treatment options and prognosis. Because Leishmania organisms are fastidious, obtaining a positive culture often is challenging. Polymerase chain reaction can be utilized for identification, with detection rates of 97%.1 Systemic treatment is indicated for patients with multiple or large lesions; lesions on the hands, feet, face, or joints; or immunocompromised patients. Antimonial drugs are the first-line treatment for most forms of leishmaniasis, though increasing resistance has led to a decrease in efficacy.1 Our patient ultimately was treated with 4 weeks of miltefosine 50 mg 3 times daily. She obtained full resolution of the lesions with no further treatment indicated.
Pemphigus vegetans may present with various clinical manifestations that often can lead to a delay in diagnosis. The Hallopeau subtype typically presents as pustular lesions, while the Neumann subtype may present as large vesiculobullous erosive lesions that rupture and form verrucous, crusted, vegetative plaques. The groin, inguinal folds, axillae, thighs, and flexural areas commonly are affected, but reports of nasal, vaginal, and conjunctival involvement also exist.2
Granuloma inguinale is a sexually transmitted ulcerative disease that is caused by infection with Klebsiella granulomatis. It typically is found in tropical and subtropical climates, including Australia, Brazil, India, and South Africa. The initial presentation includes a single papule or multiple papules or nodules in the genital area that progress to a painless ulcer. It can be diagnosed via biopsies or tissue smears, which will demonstrate the presence of inclusion bodies known as Donovan bodies.3
Cutaneous tuberculosis (TB) can have variable clinical presentations and may be acquired exogenously or endogenously. Cutaneous TB can be divided into 2 categories: exogenous TB caused by inoculation and endogenous TB due to direct spread or autoinoculation. Exogenous TB subtypes include tuberculous chancre and TB verrucosa cutis, while endogenous TB includes scrofuloderma, orificial TB, and lupus vulgaris.4 Patches and plaques are found in patients with lupus vulgaris and TB verrucosa cutis. Scrofuloderma, tuberculous chancre, and orificial TB can present as ulcerative or erosive lesions. Cutaneous TB infection can be diagnosed through a smear, culture, or polymerase chain reaction.4
Deep cutaneous fungal infections most commonly present in immunocompromised individuals, particularly those who are severely neutropenic and are receiving broad-spectrum systemic antimicrobial agents. Deep cutaneous fungal infections initially present as a papule and evolve into a pustule followed by a necrotic ulcer. The lesions typically are accompanied by a fever and/or vital sign abnormalities.5
- Pace D. Leishmaniasis [published online September 17, 2014]. J Infect. 2014;69(suppl 1):S10-S18. doi:10.1016/j.jinf.2014.07.016
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls. StatPearls Publishing; 2022.
- Ornelas J, Kiuru M, Konia T, et al. Granuloma inguinale in a 51-year-old man. Dermatol Online J. 2016;22:13030/qt52k0c4hj.
- Chen Q, Chen W, Hao F. Cutaneous tuberculosis: a great imitator. Clin Dermatol. 2019;37:192-199.
- Marcoux D, Jafarian F, Joncas V, et al. Deep cutaneous fungal infections in immunocompromised children. J Am Acad Dermatol. 2009;61:857-864.
The Diagnosis: Cutaneous Leishmaniasis
A punch biopsy of the skin showed pseudoepitheliomatous hyperplasia of the epidermis with dermal granulomatous and suppurative inflammation; tissue cultures remained sterile. Polymerase chain reaction testing of the skin revealed the presence of Leishmania guyanensis complex. Leishmaniasis is a widespread parasitic disease transmitted via sandflies that often is seen in children and young adults.1 Although leishmaniasis is endemic to several countries within Southeast Asia, East Africa, and Latin America, an increase in international travel has brought the disease to nonendemic regions. Therefore, it is crucial to obtain a detailed history of travel and exposure to sandflies in patients who have recently returned from endemic regions.
Leishmaniasis may present in 3 forms: cutaneous, mucocutaneous, or visceral. Cutaneous clinical findings vary depending on disease stage, causative species, and host immune activation. Presentation following a sandfly bite typically includes a papule that progresses to an erythematous nodule. Cutaneous leishmaniasis commonly occurs in areas of the body that are easily accessible to sandflies, such as the face, neck, and limbs. Mucocutaneous leishmaniasis presents with nasal or oral involvement several years after the onset of cutaneous leishmaniasis; however, it can coexist with cutaneous involvement. Without treatment, mucocutaneous leishmaniasis may lead to perforation of the nasal septum, destruction of the mouth, and life-threatening airway obstruction.1 Determining the specific species is important due to the variation in treatment options and prognosis. Because Leishmania organisms are fastidious, obtaining a positive culture often is challenging. Polymerase chain reaction can be utilized for identification, with detection rates of 97%.1 Systemic treatment is indicated for patients with multiple or large lesions; lesions on the hands, feet, face, or joints; or immunocompromised patients. Antimonial drugs are the first-line treatment for most forms of leishmaniasis, though increasing resistance has led to a decrease in efficacy.1 Our patient ultimately was treated with 4 weeks of miltefosine 50 mg 3 times daily. She obtained full resolution of the lesions with no further treatment indicated.
Pemphigus vegetans may present with various clinical manifestations that often can lead to a delay in diagnosis. The Hallopeau subtype typically presents as pustular lesions, while the Neumann subtype may present as large vesiculobullous erosive lesions that rupture and form verrucous, crusted, vegetative plaques. The groin, inguinal folds, axillae, thighs, and flexural areas commonly are affected, but reports of nasal, vaginal, and conjunctival involvement also exist.2
Granuloma inguinale is a sexually transmitted ulcerative disease that is caused by infection with Klebsiella granulomatis. It typically is found in tropical and subtropical climates, including Australia, Brazil, India, and South Africa. The initial presentation includes a single papule or multiple papules or nodules in the genital area that progress to a painless ulcer. It can be diagnosed via biopsies or tissue smears, which will demonstrate the presence of inclusion bodies known as Donovan bodies.3
Cutaneous tuberculosis (TB) can have variable clinical presentations and may be acquired exogenously or endogenously. Cutaneous TB can be divided into 2 categories: exogenous TB caused by inoculation and endogenous TB due to direct spread or autoinoculation. Exogenous TB subtypes include tuberculous chancre and TB verrucosa cutis, while endogenous TB includes scrofuloderma, orificial TB, and lupus vulgaris.4 Patches and plaques are found in patients with lupus vulgaris and TB verrucosa cutis. Scrofuloderma, tuberculous chancre, and orificial TB can present as ulcerative or erosive lesions. Cutaneous TB infection can be diagnosed through a smear, culture, or polymerase chain reaction.4
Deep cutaneous fungal infections most commonly present in immunocompromised individuals, particularly those who are severely neutropenic and are receiving broad-spectrum systemic antimicrobial agents. Deep cutaneous fungal infections initially present as a papule and evolve into a pustule followed by a necrotic ulcer. The lesions typically are accompanied by a fever and/or vital sign abnormalities.5
The Diagnosis: Cutaneous Leishmaniasis
A punch biopsy of the skin showed pseudoepitheliomatous hyperplasia of the epidermis with dermal granulomatous and suppurative inflammation; tissue cultures remained sterile. Polymerase chain reaction testing of the skin revealed the presence of Leishmania guyanensis complex. Leishmaniasis is a widespread parasitic disease transmitted via sandflies that often is seen in children and young adults.1 Although leishmaniasis is endemic to several countries within Southeast Asia, East Africa, and Latin America, an increase in international travel has brought the disease to nonendemic regions. Therefore, it is crucial to obtain a detailed history of travel and exposure to sandflies in patients who have recently returned from endemic regions.
Leishmaniasis may present in 3 forms: cutaneous, mucocutaneous, or visceral. Cutaneous clinical findings vary depending on disease stage, causative species, and host immune activation. Presentation following a sandfly bite typically includes a papule that progresses to an erythematous nodule. Cutaneous leishmaniasis commonly occurs in areas of the body that are easily accessible to sandflies, such as the face, neck, and limbs. Mucocutaneous leishmaniasis presents with nasal or oral involvement several years after the onset of cutaneous leishmaniasis; however, it can coexist with cutaneous involvement. Without treatment, mucocutaneous leishmaniasis may lead to perforation of the nasal septum, destruction of the mouth, and life-threatening airway obstruction.1 Determining the specific species is important due to the variation in treatment options and prognosis. Because Leishmania organisms are fastidious, obtaining a positive culture often is challenging. Polymerase chain reaction can be utilized for identification, with detection rates of 97%.1 Systemic treatment is indicated for patients with multiple or large lesions; lesions on the hands, feet, face, or joints; or immunocompromised patients. Antimonial drugs are the first-line treatment for most forms of leishmaniasis, though increasing resistance has led to a decrease in efficacy.1 Our patient ultimately was treated with 4 weeks of miltefosine 50 mg 3 times daily. She obtained full resolution of the lesions with no further treatment indicated.
Pemphigus vegetans may present with various clinical manifestations that often can lead to a delay in diagnosis. The Hallopeau subtype typically presents as pustular lesions, while the Neumann subtype may present as large vesiculobullous erosive lesions that rupture and form verrucous, crusted, vegetative plaques. The groin, inguinal folds, axillae, thighs, and flexural areas commonly are affected, but reports of nasal, vaginal, and conjunctival involvement also exist.2
Granuloma inguinale is a sexually transmitted ulcerative disease that is caused by infection with Klebsiella granulomatis. It typically is found in tropical and subtropical climates, including Australia, Brazil, India, and South Africa. The initial presentation includes a single papule or multiple papules or nodules in the genital area that progress to a painless ulcer. It can be diagnosed via biopsies or tissue smears, which will demonstrate the presence of inclusion bodies known as Donovan bodies.3
Cutaneous tuberculosis (TB) can have variable clinical presentations and may be acquired exogenously or endogenously. Cutaneous TB can be divided into 2 categories: exogenous TB caused by inoculation and endogenous TB due to direct spread or autoinoculation. Exogenous TB subtypes include tuberculous chancre and TB verrucosa cutis, while endogenous TB includes scrofuloderma, orificial TB, and lupus vulgaris.4 Patches and plaques are found in patients with lupus vulgaris and TB verrucosa cutis. Scrofuloderma, tuberculous chancre, and orificial TB can present as ulcerative or erosive lesions. Cutaneous TB infection can be diagnosed through a smear, culture, or polymerase chain reaction.4
Deep cutaneous fungal infections most commonly present in immunocompromised individuals, particularly those who are severely neutropenic and are receiving broad-spectrum systemic antimicrobial agents. Deep cutaneous fungal infections initially present as a papule and evolve into a pustule followed by a necrotic ulcer. The lesions typically are accompanied by a fever and/or vital sign abnormalities.5
- Pace D. Leishmaniasis [published online September 17, 2014]. J Infect. 2014;69(suppl 1):S10-S18. doi:10.1016/j.jinf.2014.07.016
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls. StatPearls Publishing; 2022.
- Ornelas J, Kiuru M, Konia T, et al. Granuloma inguinale in a 51-year-old man. Dermatol Online J. 2016;22:13030/qt52k0c4hj.
- Chen Q, Chen W, Hao F. Cutaneous tuberculosis: a great imitator. Clin Dermatol. 2019;37:192-199.
- Marcoux D, Jafarian F, Joncas V, et al. Deep cutaneous fungal infections in immunocompromised children. J Am Acad Dermatol. 2009;61:857-864.
- Pace D. Leishmaniasis [published online September 17, 2014]. J Infect. 2014;69(suppl 1):S10-S18. doi:10.1016/j.jinf.2014.07.016
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls. StatPearls Publishing; 2022.
- Ornelas J, Kiuru M, Konia T, et al. Granuloma inguinale in a 51-year-old man. Dermatol Online J. 2016;22:13030/qt52k0c4hj.
- Chen Q, Chen W, Hao F. Cutaneous tuberculosis: a great imitator. Clin Dermatol. 2019;37:192-199.
- Marcoux D, Jafarian F, Joncas V, et al. Deep cutaneous fungal infections in immunocompromised children. J Am Acad Dermatol. 2009;61:857-864.
A 14-year-old adolescent girl presented with spreading painful lesions on the legs and left forearm of 2 years’ duration. Her travel history included several countries in South and Central America, traversing the Colombian jungle on foot. Near the end of the jungle trip, she noted a skin lesion on the left forearm around the site of an insect bite. Within 1 month, the lesions spread to the legs. She was treated with topical corticosteroids without improvement. Physical examination revealed verrucous, reddish-brown plaques on the legs and left forearm. Intranasal examination revealed a red rounded lesion inside the left nostril.
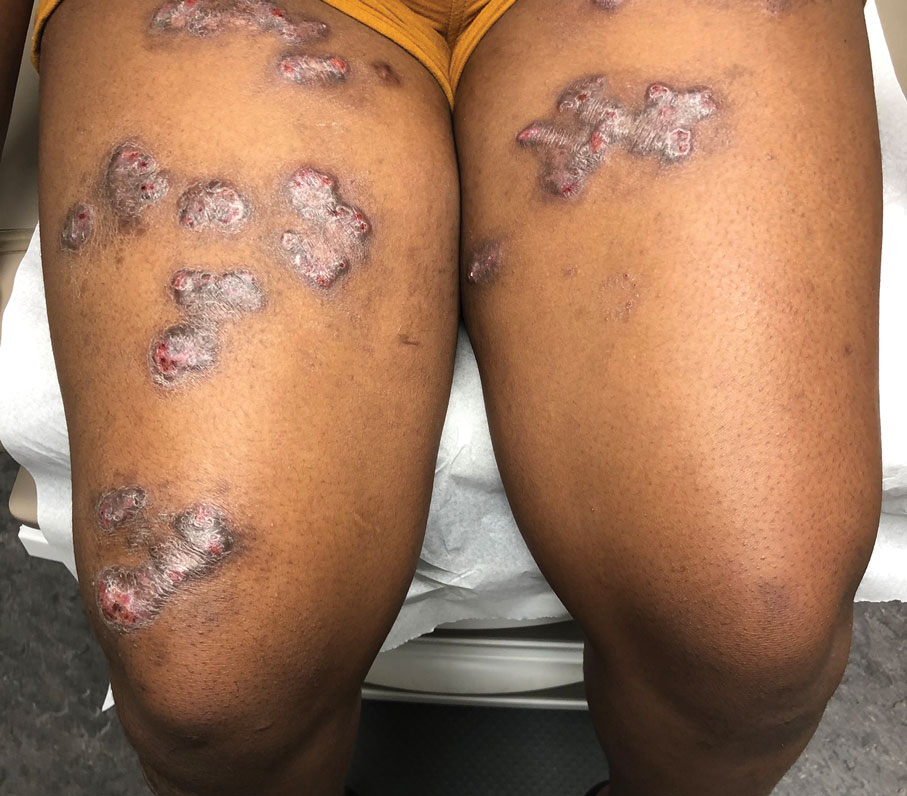
Violaceous Nodules on the Leg in a Patient with HIV
The Diagnosis: Plasmablastic Lymphoma
A punch biopsy of one of the leg nodules with hematoxylin and eosin staining revealed sheets of medium to large cells with plasmacytic differentiation (Figure, A and B). Immunohistochemistry showed CD79, epithelial membrane antigen, multiple myeloma 1, and CD138 positivity, as well as CD-19 negativity and positive staining on Epstein-Barr virus (EBV) in situ hybridization (Figure, C). Ki-67 stained greater than 90% of the neoplastic cells. Neoplastic cells were found to be λ restricted on κ and λ immunohistochemistry. Human herpesvirus 8 (HHV-8), CD3, and CD20 stains were negative. Subsequent fluorescent in situ hybridization was positive for MYC/immunoglobulin heavy chain (MYC/IGH) rearrangement t(8;14), confirming a diagnosis of plasmablastic lymphoma (PBL).
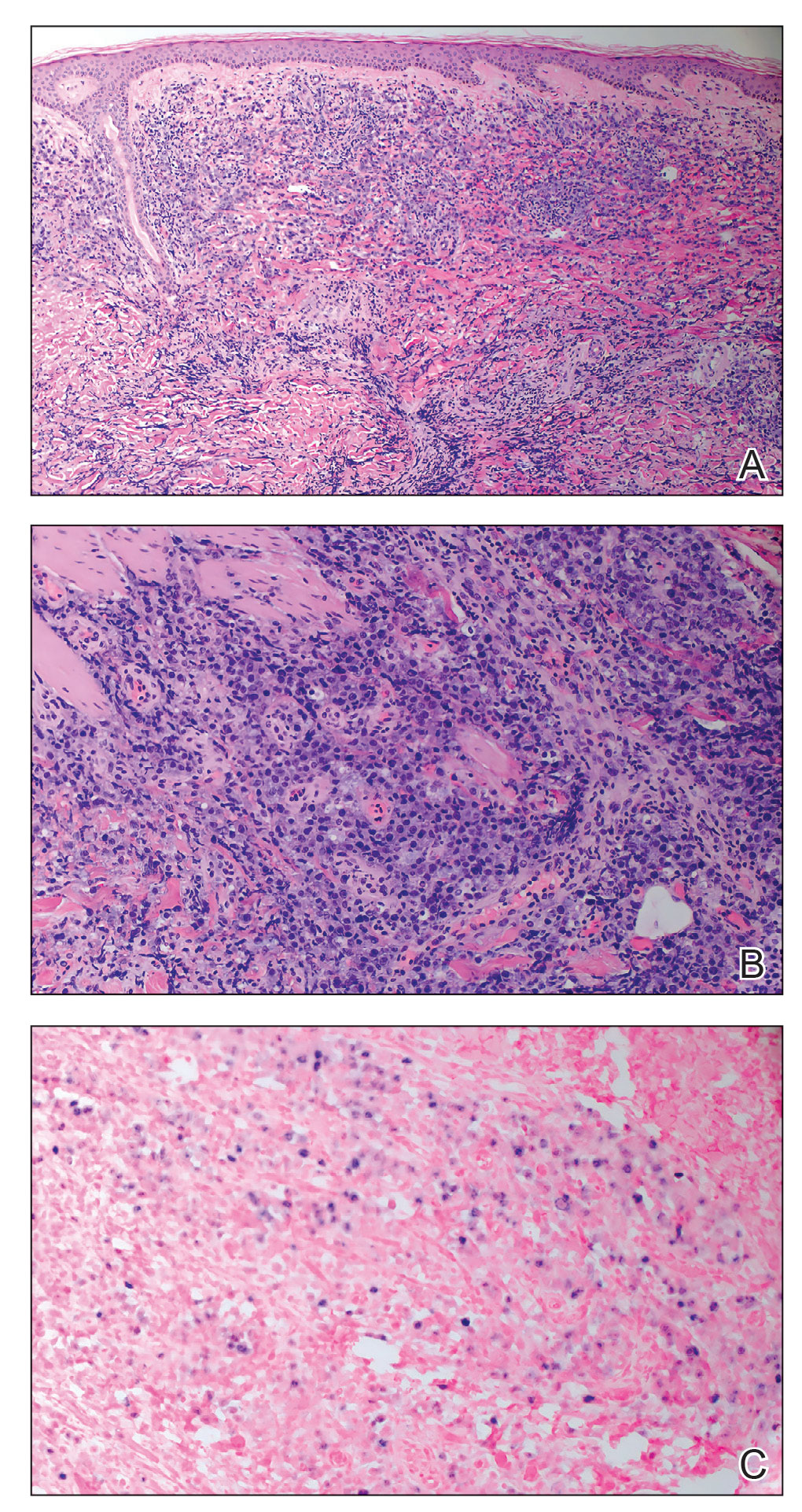
A bone marrow biopsy revealed normocellular bone marrow with trilineage hematopoiesis and no morphologic, immunophenotypic, or fluorescent in situ hybridization evidence of plasmablastic lymphoma or other pathology in the bone marrow. Our patient was started on hyper-CVAD (cyclophosphamide, vincristine, doxorubicin hydrochloride, dexamethasone) chemotherapy and was doing well with plans for a fourth course of chemotherapy. There is no standardized treatment course for cutaneous PBL, though excision with adjunctive chemotherapy treatment commonly has been reported in the literature.1
Plasmablastic lymphoma is a rare and aggressive diffuse large B-cell lymphoma associated with EBV infection that compromises approximately 2% to 3% of all HIV-related lymphomas.1,2 It frequently is associated with immunosuppression in patients with HIV or in transplant recipients on immunosuppression; however, it has been reported in immunocompetent individuals such as elderly patients.2 Plasmablastic lymphoma most commonly presents on the buccal mucosa but also can affect the gastrointestinal tract and occasionally has cutaneous manifestations.1,2 Cutaneous manifestations of PBL range from erythematous infiltrated plaques to ulcerated nodules presenting in an array of colors from flesh colored to violaceous.2 Primary cutaneous lesions can be seen on the legs, as in our patient.
Histopathologic examination reveals sheets of plasmablasts or large cells with eccentric nuclei and abundant basophilic cytoplasm.1 Plasmablastic lymphoma frequently is positive for mature B-cell markers such as CD38, CD138, multiple myeloma 1, and B lymphocyte–induced maturation protein 1.2,3 Uncommonly, PBL expresses paired box protein Pax-5 and CD20 markers.3 Although pathogenesis is poorly understood, it has been speculated that EBV infection is a common pathogenic factor. Epstein-Barr virus positivity has been noted in 60% of cases.2
Plasmablastic lymphoma and other malignant plasma cell processes such as plasmablastic myeloma (PBM) are morphologically similar. Proliferation of plasmablasts with rare plasmacytic cells is common in PBL, while plasmacytic cells are predominant in PBM. MYC rearrangement/ immunoglobulin heavy chain rearrangement t(8;14) was used to differentiate PBL from PBM in our patient; however, more cases of PBM with MYC/IGH rearrangement t(8;14) have been reported, making it an unreliable differentiating factor.4 A detailed clinical, pathologic, and genetic survey remains necessary for confirmatory diagnosis of PBL. Compared to other malignant plasma cell processes, PBL more commonly is seen in immunocompromised patients or those with HIV, such as our patient. Additionally, EBV testing is more likely to be positive in patients with PBL, further supporting this diagnosis in our patient.4
Presentations of bacillary angiomatosis, Kaposi sarcoma, and cutaneous lymphoma may be clinically similar; therefore, careful immunohistopathologic differentiation is necessary. Kaposi sarcoma is an angioproliferative disorder that develops from HHV-8 infection and commonly is associated with HIV. It presents as painless vascular lesions in a range of colors with typical progression from patch to plaque to nodules, frequently on the lower extremities. Histologically, admixtures of bland spindle cells, slitlike small vessel proliferation, and lymphocytic infiltration are typical. Neoplastic vessels lack basement membrane zones, resulting in microhemorrhages and hemosiderin deposition. Neoplastic vessels label with CD31 and CD34 endothelial markers in addition to HHV-8 antibodies, which is highly specific for Kaposi sarcoma and differentiates it from PBL.5
Bacillary angiomatosis is an infectious neovascular proliferation characterized by papular lesions that may resemble the lesions of PBL. Mixed cell infiltration in inflammatory cells with clumping of granular material is characteristic. Under Warthin-Starry staining, the granular material is abundant in gram-negative rods representing Bartonella species, which is the implicated infectious agent in bacillary angiomatosis.
Lymphomatoid papulosis (LyP) is the most common CD30+ lymphoproliferative disorder and also may present with exophytic nodules. The etiology of LyP remains unknown, but it is suspected that overexpression of CD30 plays a role. Lymphomatoid papulosis presents as red-violaceous papules and nodules in various stages of healing. Although variable histology among types of LyP exists, CD30+ T-cell lymphocytes remain the hallmark of LyP. Type A LyP, which accounts for 80% of LyP cases, reveals CD4+ and CD30+ cells scattered among neutrophils, eosinophils, and small lymphocytes.5 Lymphomatoid papulosis typically is self-healing, recurrent, and carries an excellent prognosis.
Plasmablastic lymphoma remains a rare and aggressive type of diffuse large B-cell lymphoma that can have primary cutaneous manifestations. It is prudent to consider PBL in the differential diagnosis of nodular lower extremity lesions, especially in immunosuppressed patients.
- Jambusaria A, Shafer D, Wu H, et al. Cutaneous plasmablastic lymphoma. J Am Acad Dermatol. 2008;58:676-678.
- Marques SA, Abbade LP, Guiotoku MM, et al. Primary cutaneous plasmablastic lymphoma revealing clinically unsuspected HIV infection. An Bras Dermatol. 2016;91:507-509.
- Bhatt R, Desai DS. Plasmablastic lymphoma. StatPearls. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK532975/
- Morris A, Monohan G. Plasmablastic myeloma versus plasmablastic lymphoma: different yet related diseases. Hematol Transfus Int J. 2018;6:25-28. doi:10.15406/htij.2018.06.00146
- Prieto-Torres L, Rodriguez-Pinilla SM, Onaindia A, et al. CD30-positive primary cutaneous lymphoproliferative disorders: molecular alterations and targeted therapies. Haematologica. 2019;104:226-235.
The Diagnosis: Plasmablastic Lymphoma
A punch biopsy of one of the leg nodules with hematoxylin and eosin staining revealed sheets of medium to large cells with plasmacytic differentiation (Figure, A and B). Immunohistochemistry showed CD79, epithelial membrane antigen, multiple myeloma 1, and CD138 positivity, as well as CD-19 negativity and positive staining on Epstein-Barr virus (EBV) in situ hybridization (Figure, C). Ki-67 stained greater than 90% of the neoplastic cells. Neoplastic cells were found to be λ restricted on κ and λ immunohistochemistry. Human herpesvirus 8 (HHV-8), CD3, and CD20 stains were negative. Subsequent fluorescent in situ hybridization was positive for MYC/immunoglobulin heavy chain (MYC/IGH) rearrangement t(8;14), confirming a diagnosis of plasmablastic lymphoma (PBL).

A bone marrow biopsy revealed normocellular bone marrow with trilineage hematopoiesis and no morphologic, immunophenotypic, or fluorescent in situ hybridization evidence of plasmablastic lymphoma or other pathology in the bone marrow. Our patient was started on hyper-CVAD (cyclophosphamide, vincristine, doxorubicin hydrochloride, dexamethasone) chemotherapy and was doing well with plans for a fourth course of chemotherapy. There is no standardized treatment course for cutaneous PBL, though excision with adjunctive chemotherapy treatment commonly has been reported in the literature.1
Plasmablastic lymphoma is a rare and aggressive diffuse large B-cell lymphoma associated with EBV infection that compromises approximately 2% to 3% of all HIV-related lymphomas.1,2 It frequently is associated with immunosuppression in patients with HIV or in transplant recipients on immunosuppression; however, it has been reported in immunocompetent individuals such as elderly patients.2 Plasmablastic lymphoma most commonly presents on the buccal mucosa but also can affect the gastrointestinal tract and occasionally has cutaneous manifestations.1,2 Cutaneous manifestations of PBL range from erythematous infiltrated plaques to ulcerated nodules presenting in an array of colors from flesh colored to violaceous.2 Primary cutaneous lesions can be seen on the legs, as in our patient.
Histopathologic examination reveals sheets of plasmablasts or large cells with eccentric nuclei and abundant basophilic cytoplasm.1 Plasmablastic lymphoma frequently is positive for mature B-cell markers such as CD38, CD138, multiple myeloma 1, and B lymphocyte–induced maturation protein 1.2,3 Uncommonly, PBL expresses paired box protein Pax-5 and CD20 markers.3 Although pathogenesis is poorly understood, it has been speculated that EBV infection is a common pathogenic factor. Epstein-Barr virus positivity has been noted in 60% of cases.2
Plasmablastic lymphoma and other malignant plasma cell processes such as plasmablastic myeloma (PBM) are morphologically similar. Proliferation of plasmablasts with rare plasmacytic cells is common in PBL, while plasmacytic cells are predominant in PBM. MYC rearrangement/ immunoglobulin heavy chain rearrangement t(8;14) was used to differentiate PBL from PBM in our patient; however, more cases of PBM with MYC/IGH rearrangement t(8;14) have been reported, making it an unreliable differentiating factor.4 A detailed clinical, pathologic, and genetic survey remains necessary for confirmatory diagnosis of PBL. Compared to other malignant plasma cell processes, PBL more commonly is seen in immunocompromised patients or those with HIV, such as our patient. Additionally, EBV testing is more likely to be positive in patients with PBL, further supporting this diagnosis in our patient.4
Presentations of bacillary angiomatosis, Kaposi sarcoma, and cutaneous lymphoma may be clinically similar; therefore, careful immunohistopathologic differentiation is necessary. Kaposi sarcoma is an angioproliferative disorder that develops from HHV-8 infection and commonly is associated with HIV. It presents as painless vascular lesions in a range of colors with typical progression from patch to plaque to nodules, frequently on the lower extremities. Histologically, admixtures of bland spindle cells, slitlike small vessel proliferation, and lymphocytic infiltration are typical. Neoplastic vessels lack basement membrane zones, resulting in microhemorrhages and hemosiderin deposition. Neoplastic vessels label with CD31 and CD34 endothelial markers in addition to HHV-8 antibodies, which is highly specific for Kaposi sarcoma and differentiates it from PBL.5
Bacillary angiomatosis is an infectious neovascular proliferation characterized by papular lesions that may resemble the lesions of PBL. Mixed cell infiltration in inflammatory cells with clumping of granular material is characteristic. Under Warthin-Starry staining, the granular material is abundant in gram-negative rods representing Bartonella species, which is the implicated infectious agent in bacillary angiomatosis.
Lymphomatoid papulosis (LyP) is the most common CD30+ lymphoproliferative disorder and also may present with exophytic nodules. The etiology of LyP remains unknown, but it is suspected that overexpression of CD30 plays a role. Lymphomatoid papulosis presents as red-violaceous papules and nodules in various stages of healing. Although variable histology among types of LyP exists, CD30+ T-cell lymphocytes remain the hallmark of LyP. Type A LyP, which accounts for 80% of LyP cases, reveals CD4+ and CD30+ cells scattered among neutrophils, eosinophils, and small lymphocytes.5 Lymphomatoid papulosis typically is self-healing, recurrent, and carries an excellent prognosis.
Plasmablastic lymphoma remains a rare and aggressive type of diffuse large B-cell lymphoma that can have primary cutaneous manifestations. It is prudent to consider PBL in the differential diagnosis of nodular lower extremity lesions, especially in immunosuppressed patients.
The Diagnosis: Plasmablastic Lymphoma
A punch biopsy of one of the leg nodules with hematoxylin and eosin staining revealed sheets of medium to large cells with plasmacytic differentiation (Figure, A and B). Immunohistochemistry showed CD79, epithelial membrane antigen, multiple myeloma 1, and CD138 positivity, as well as CD-19 negativity and positive staining on Epstein-Barr virus (EBV) in situ hybridization (Figure, C). Ki-67 stained greater than 90% of the neoplastic cells. Neoplastic cells were found to be λ restricted on κ and λ immunohistochemistry. Human herpesvirus 8 (HHV-8), CD3, and CD20 stains were negative. Subsequent fluorescent in situ hybridization was positive for MYC/immunoglobulin heavy chain (MYC/IGH) rearrangement t(8;14), confirming a diagnosis of plasmablastic lymphoma (PBL).

A bone marrow biopsy revealed normocellular bone marrow with trilineage hematopoiesis and no morphologic, immunophenotypic, or fluorescent in situ hybridization evidence of plasmablastic lymphoma or other pathology in the bone marrow. Our patient was started on hyper-CVAD (cyclophosphamide, vincristine, doxorubicin hydrochloride, dexamethasone) chemotherapy and was doing well with plans for a fourth course of chemotherapy. There is no standardized treatment course for cutaneous PBL, though excision with adjunctive chemotherapy treatment commonly has been reported in the literature.1
Plasmablastic lymphoma is a rare and aggressive diffuse large B-cell lymphoma associated with EBV infection that compromises approximately 2% to 3% of all HIV-related lymphomas.1,2 It frequently is associated with immunosuppression in patients with HIV or in transplant recipients on immunosuppression; however, it has been reported in immunocompetent individuals such as elderly patients.2 Plasmablastic lymphoma most commonly presents on the buccal mucosa but also can affect the gastrointestinal tract and occasionally has cutaneous manifestations.1,2 Cutaneous manifestations of PBL range from erythematous infiltrated plaques to ulcerated nodules presenting in an array of colors from flesh colored to violaceous.2 Primary cutaneous lesions can be seen on the legs, as in our patient.
Histopathologic examination reveals sheets of plasmablasts or large cells with eccentric nuclei and abundant basophilic cytoplasm.1 Plasmablastic lymphoma frequently is positive for mature B-cell markers such as CD38, CD138, multiple myeloma 1, and B lymphocyte–induced maturation protein 1.2,3 Uncommonly, PBL expresses paired box protein Pax-5 and CD20 markers.3 Although pathogenesis is poorly understood, it has been speculated that EBV infection is a common pathogenic factor. Epstein-Barr virus positivity has been noted in 60% of cases.2
Plasmablastic lymphoma and other malignant plasma cell processes such as plasmablastic myeloma (PBM) are morphologically similar. Proliferation of plasmablasts with rare plasmacytic cells is common in PBL, while plasmacytic cells are predominant in PBM. MYC rearrangement/ immunoglobulin heavy chain rearrangement t(8;14) was used to differentiate PBL from PBM in our patient; however, more cases of PBM with MYC/IGH rearrangement t(8;14) have been reported, making it an unreliable differentiating factor.4 A detailed clinical, pathologic, and genetic survey remains necessary for confirmatory diagnosis of PBL. Compared to other malignant plasma cell processes, PBL more commonly is seen in immunocompromised patients or those with HIV, such as our patient. Additionally, EBV testing is more likely to be positive in patients with PBL, further supporting this diagnosis in our patient.4
Presentations of bacillary angiomatosis, Kaposi sarcoma, and cutaneous lymphoma may be clinically similar; therefore, careful immunohistopathologic differentiation is necessary. Kaposi sarcoma is an angioproliferative disorder that develops from HHV-8 infection and commonly is associated with HIV. It presents as painless vascular lesions in a range of colors with typical progression from patch to plaque to nodules, frequently on the lower extremities. Histologically, admixtures of bland spindle cells, slitlike small vessel proliferation, and lymphocytic infiltration are typical. Neoplastic vessels lack basement membrane zones, resulting in microhemorrhages and hemosiderin deposition. Neoplastic vessels label with CD31 and CD34 endothelial markers in addition to HHV-8 antibodies, which is highly specific for Kaposi sarcoma and differentiates it from PBL.5
Bacillary angiomatosis is an infectious neovascular proliferation characterized by papular lesions that may resemble the lesions of PBL. Mixed cell infiltration in inflammatory cells with clumping of granular material is characteristic. Under Warthin-Starry staining, the granular material is abundant in gram-negative rods representing Bartonella species, which is the implicated infectious agent in bacillary angiomatosis.
Lymphomatoid papulosis (LyP) is the most common CD30+ lymphoproliferative disorder and also may present with exophytic nodules. The etiology of LyP remains unknown, but it is suspected that overexpression of CD30 plays a role. Lymphomatoid papulosis presents as red-violaceous papules and nodules in various stages of healing. Although variable histology among types of LyP exists, CD30+ T-cell lymphocytes remain the hallmark of LyP. Type A LyP, which accounts for 80% of LyP cases, reveals CD4+ and CD30+ cells scattered among neutrophils, eosinophils, and small lymphocytes.5 Lymphomatoid papulosis typically is self-healing, recurrent, and carries an excellent prognosis.
Plasmablastic lymphoma remains a rare and aggressive type of diffuse large B-cell lymphoma that can have primary cutaneous manifestations. It is prudent to consider PBL in the differential diagnosis of nodular lower extremity lesions, especially in immunosuppressed patients.
- Jambusaria A, Shafer D, Wu H, et al. Cutaneous plasmablastic lymphoma. J Am Acad Dermatol. 2008;58:676-678.
- Marques SA, Abbade LP, Guiotoku MM, et al. Primary cutaneous plasmablastic lymphoma revealing clinically unsuspected HIV infection. An Bras Dermatol. 2016;91:507-509.
- Bhatt R, Desai DS. Plasmablastic lymphoma. StatPearls. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK532975/
- Morris A, Monohan G. Plasmablastic myeloma versus plasmablastic lymphoma: different yet related diseases. Hematol Transfus Int J. 2018;6:25-28. doi:10.15406/htij.2018.06.00146
- Prieto-Torres L, Rodriguez-Pinilla SM, Onaindia A, et al. CD30-positive primary cutaneous lymphoproliferative disorders: molecular alterations and targeted therapies. Haematologica. 2019;104:226-235.
- Jambusaria A, Shafer D, Wu H, et al. Cutaneous plasmablastic lymphoma. J Am Acad Dermatol. 2008;58:676-678.
- Marques SA, Abbade LP, Guiotoku MM, et al. Primary cutaneous plasmablastic lymphoma revealing clinically unsuspected HIV infection. An Bras Dermatol. 2016;91:507-509.
- Bhatt R, Desai DS. Plasmablastic lymphoma. StatPearls. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK532975/
- Morris A, Monohan G. Plasmablastic myeloma versus plasmablastic lymphoma: different yet related diseases. Hematol Transfus Int J. 2018;6:25-28. doi:10.15406/htij.2018.06.00146
- Prieto-Torres L, Rodriguez-Pinilla SM, Onaindia A, et al. CD30-positive primary cutaneous lymphoproliferative disorders: molecular alterations and targeted therapies. Haematologica. 2019;104:226-235.
A 67-year-old man with long-standing hepatitis B virus and HIV managed with chronic antiretroviral therapy presented to an urgent care facility with worsening erythema and edema of the legs of 2 weeks’ duration. He was prescribed a 7-day course of cephalexin for presumed cellulitis. Two months later, he developed nodules on the lower extremities. He was seen by podiatry and prescribed a course of amoxicillin–clavulanic acid for presumed infection. Despite 2 courses of antibiotics, his symptoms progressed. The nodules expanded in number and some developed ulceration. Three months into his clinical course, he presented to our dermatology clinic. Physical examination revealed two 2- to 3-cm, violaceous, exophytic, tender nodules. He reported tactile allodynia of the lower extremities and denied fever, chills, night sweats, or weight loss. He also denied exposure to infectious or chemical agents and reported no recent travel. The patient was chronically taking lisinopril/hydrochlorothiazide, escitalopram, elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide, bupropion, and aspirin with no recent changes. A complete hematologic and biochemical survey largely was unremarkable. His HIV viral load was undetectable with a CD4 count greater than 400/mm3 (reference range, 490–1436/mm3). Lactate dehydrogenase was elevated at 568 IU/L (reference range, 135–225 IU/L). The lower leg lesions were biopsied for confirmatory diagnosis.
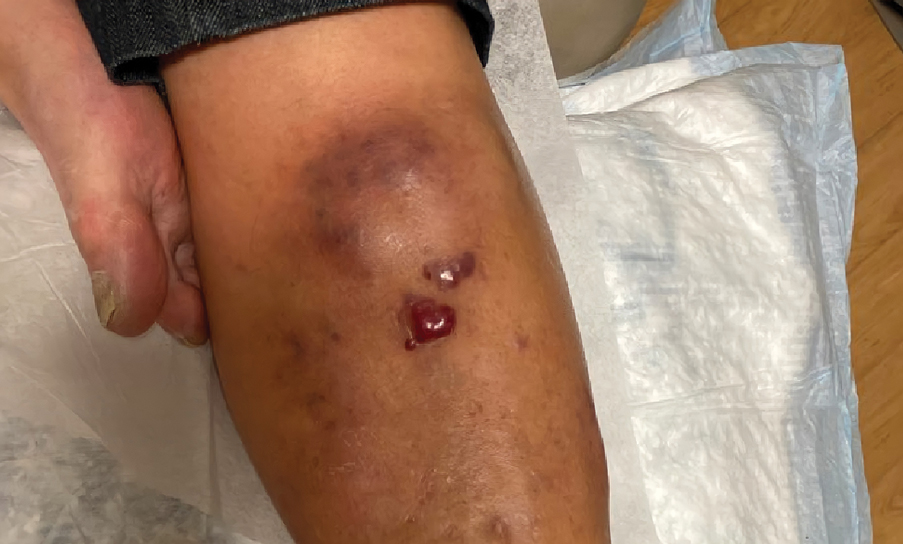
Chronic Erythematous Plaques Around the Ears
The Diagnosis: Discoid Lupus Erythematosus
The biopsies demonstrated vacuolar interface changes with superficial and deep perivascular and periadnexal inflammation as well as increased background mucin deposition. The clinical morphology and distributions of the plaques limited to the photoexposed areas of the head suggested a diagnosis of discoid lupus erythematosus (DLE). The interface changes on histopathology supported this clinical impression. Our patient was treated with limited application of triamcinolone ointment 0.1% twice daily around the ears and neck, tacrolimus ointment 0.1% twice daily on the face, and hydroxychloroquine, as well as sun protection instructions. Smoking cessation was strongly advised.
Discoid lupus erythematosus is a disorder with chronic, erythematous, scaly, coin-shaped (discoid) plaques and is the most common form of chronic cutaneous lupus erythematosus.1 Lesions usually present on sun-exposed areas of the face, scalp, neck, ears, lips, or upper torso. They expand slowly with an active peripheral margin and a central scar that can result in induration, pigmentation changes, telangiectases, pruritus, or tenderness. Hair-bearing areas may be involved, causing hair loss due to follicular plugging; irreversible scarring alopecia can result. Facial DLE often spares the nasolabial folds. Ear involvement characteristically includes the conchal bowl and the outer external auditory canal. Discoid lupus erythematosus is considered localized if most of the head and neck region is involved or generalized if lesions also are present below the neck. Risk factors for DLE include genetic and environmental factors such as UV exposure, hormones, or exposure to toxins such as cigarette smoke.1 The disorder most commonly affects females and has a higher prevalence in patients of African descent than in Asian and White patients. Disease can occur at any age but usually occurs between 20 and 40 years of age.2 Discoid lupus erythematosus and other forms of chronic cutaneous lupus can occur independently or in conjunction with systemic lupus erythematosus (SLE), and approximately 15% to 30% of SLE patients develop DLE.1
Discoid lupus erythematosus is clinically diagnosed by the presentation of plaques in the characteristic distribution with confirmation via skin biopsy.1 Elman et al3 created a system for DLE classification that was only clinical and did not involve histopathology. Histologically, DLE often includes basement membrane thickening, follicular keratin plugs, mucin deposition, and vacuolar change with an interface, and a perivascular and periadnexal lymphocytic infiltrate.3,4 Antibodies such as antinuclear antibodies, rheumatoid factor, anti–double-stranded DNA, anti-Smith, and Sjögren syndrome A and B antibodies may be present (albeit with low positive frequency) in cutaneous lupus erythematosus.4 Characteristics of SLE also may be present, helping to confirm the diagnosis. Because there is an association of DLE with SLE, various laboratory tests should be ordered, including complete blood cell count, renal function panel, inflammatory markers, antibodies, and urinalysis for proteinuria.2,4
Treatment of DLE consists of preventative measures, such as sun protection with vitamin D supplementation, avoidance of drug triggers, and smoking cessation, as well as pharmacotherapy. The importance of wearing sun protective hats and garments with sunscreen use cannot be understated.1 Smoking cessation should be advised because smoking reduces the efficacy of antimalarial treatment and potentially increases the likelihood of patients requiring a second antimalarial drug. Quinacrine often is noted in both the dermatology and rheumatology literature to be used for escalating cutaneous lupus erythematosus care when hydroxychloroquine is ineffective or not tolerated, but no US manufacturer produces this medication; thus, compounding is required, which may be financially prohibitive, making this recommendation difficult to translate into clinical practice.5 Firstline therapy for acute flares is high-potency topical corticosteroids. If lesions are primarily on areas other than the face, a medium-potency topical steroid may be used. Topical calcineurin inhibitors or intralesional corticosteroids may be used if minimal improvement is seen after initial topical corticosteroid therapy. Treatment for widespread disease or disease that is resistant to local treatment is systemic therapy with antimalarial agents, followed by antimetabolites, systemic retinoids, thalidomide, or dapsone.1,2 The Cutaneous Lupus Erythematosus Disease Area and Severity Index is a valid tool to gauge the degree of disease and to help with disease progression and treatment response by noting the features of the plaques.1
Patients also should be educated that this disease can last for years, and long-standing DLE plaques infrequently can give rise to squamous cell carcinoma. In addition, isolated DLE can progress to SLE in 5% to 28% of patients.2
The differential diagnosis in our patient included other diseases with violaceous annular lesions and central clearing. Majocchi granuloma usually presents in areas of prior trauma, possibly due to shaving the face in our patient, or in the setting of topical corticosteroid use or immunosuppression. Scaling often is present within lesions, and histology shows fungal elements.6 Cutaneous sarcoidosis usually presents on the face, with scarring alopecia when appearing on the scalp; histology shows noncaseating granulomas, and 70% of patients with cutaneous symptoms will have systemic sarcoidosis.7 Granuloma annulare most commonly presents on the extremities, and histology shows lymphohistiocytic granulomas in a palisaded or interstitial pattern with connective-tissue degeneration and mucinous deposits.8 Annular psoriasis often is scaly and symmetric with parakeratosis, epidermal hyperplasia, dilated dermal capillaries, loss of granular layer, perivascular mononuclear cell infiltrate, and elongation of rete ridges on histology.9 Drug-induced lupus erythematosus always should be considered in patients taking triggering drugs such as antihypertensives, lipid-lowering drugs, antifungals, anti–tumor necrosis factor drugs, and proton pump inhibitors—the latter being a drug our patient was taking.10
- Sontheimer CJ, Costner MI, Sontheimer RD. Lupus erythematosus. In: Kang S, Amagai M, Bruckner A, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw Hill; 2019:1037-1060.
- Lee KC. Discoid lupus. In: Ferri FF, ed. Ferri’s Clinical Advisor 2021. Elsevier; 2021:477.e15-477.e18.
- Elman SA, Joyce C, Braudis K, et al. Creation and validation of classification criteria for discoid lupus erythematosus. JAMA Dermatol. 2020;156:901-906. doi:10.1001/jamadermatol.2020.1698
- Patel P, Werth V. Cutaneous lupus erythematosus: a review. Dermatol Clin. 2002;20:373-385, v. doi:10.1016/s0733-8635(02)00016-5
- Mittal L, Werth VP. The quinacrine experience in a population of patients with cutaneous lupus erythematosus and dermatomyositis. J Am Acad Dermatol. 2017;77:374-377. doi:10.1016/j .jaad.2017.03.027
- Craddock LN, Schieke SM. Superficial fungal infection. In: Kang S, Amagai M, Bruckner A, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw Hill; 2019:2925-2951.
- Tan J, Vleugels R. Dermatologic findings in systemic disease. In: McKean S, Dressler D, Ross J, et al, eds. Principles and Practice of Hospital Medicine. 2nd ed. McGraw Hill; 2017:1145-1170.
- Prendiville JS. Granuloma annulare. In: Kang S, Amagai M, Bruckner A, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw Hill; 2019:564-571.
- Gudjonsson JE, Elder JT. Psoriasis. In: Kang S, Amagai M, Bruckner A, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw Hill; 2019:457-497.
- He Y, Sawalha AH. Drug-induced lupus erythematosus: an update on drugs and mechanisms. Curr Opin Rheumatol. 2018;30:490-497. doi:10.1097/BOR.0000000000000522
The Diagnosis: Discoid Lupus Erythematosus
The biopsies demonstrated vacuolar interface changes with superficial and deep perivascular and periadnexal inflammation as well as increased background mucin deposition. The clinical morphology and distributions of the plaques limited to the photoexposed areas of the head suggested a diagnosis of discoid lupus erythematosus (DLE). The interface changes on histopathology supported this clinical impression. Our patient was treated with limited application of triamcinolone ointment 0.1% twice daily around the ears and neck, tacrolimus ointment 0.1% twice daily on the face, and hydroxychloroquine, as well as sun protection instructions. Smoking cessation was strongly advised.
Discoid lupus erythematosus is a disorder with chronic, erythematous, scaly, coin-shaped (discoid) plaques and is the most common form of chronic cutaneous lupus erythematosus.1 Lesions usually present on sun-exposed areas of the face, scalp, neck, ears, lips, or upper torso. They expand slowly with an active peripheral margin and a central scar that can result in induration, pigmentation changes, telangiectases, pruritus, or tenderness. Hair-bearing areas may be involved, causing hair loss due to follicular plugging; irreversible scarring alopecia can result. Facial DLE often spares the nasolabial folds. Ear involvement characteristically includes the conchal bowl and the outer external auditory canal. Discoid lupus erythematosus is considered localized if most of the head and neck region is involved or generalized if lesions also are present below the neck. Risk factors for DLE include genetic and environmental factors such as UV exposure, hormones, or exposure to toxins such as cigarette smoke.1 The disorder most commonly affects females and has a higher prevalence in patients of African descent than in Asian and White patients. Disease can occur at any age but usually occurs between 20 and 40 years of age.2 Discoid lupus erythematosus and other forms of chronic cutaneous lupus can occur independently or in conjunction with systemic lupus erythematosus (SLE), and approximately 15% to 30% of SLE patients develop DLE.1
Discoid lupus erythematosus is clinically diagnosed by the presentation of plaques in the characteristic distribution with confirmation via skin biopsy.1 Elman et al3 created a system for DLE classification that was only clinical and did not involve histopathology. Histologically, DLE often includes basement membrane thickening, follicular keratin plugs, mucin deposition, and vacuolar change with an interface, and a perivascular and periadnexal lymphocytic infiltrate.3,4 Antibodies such as antinuclear antibodies, rheumatoid factor, anti–double-stranded DNA, anti-Smith, and Sjögren syndrome A and B antibodies may be present (albeit with low positive frequency) in cutaneous lupus erythematosus.4 Characteristics of SLE also may be present, helping to confirm the diagnosis. Because there is an association of DLE with SLE, various laboratory tests should be ordered, including complete blood cell count, renal function panel, inflammatory markers, antibodies, and urinalysis for proteinuria.2,4
Treatment of DLE consists of preventative measures, such as sun protection with vitamin D supplementation, avoidance of drug triggers, and smoking cessation, as well as pharmacotherapy. The importance of wearing sun protective hats and garments with sunscreen use cannot be understated.1 Smoking cessation should be advised because smoking reduces the efficacy of antimalarial treatment and potentially increases the likelihood of patients requiring a second antimalarial drug. Quinacrine often is noted in both the dermatology and rheumatology literature to be used for escalating cutaneous lupus erythematosus care when hydroxychloroquine is ineffective or not tolerated, but no US manufacturer produces this medication; thus, compounding is required, which may be financially prohibitive, making this recommendation difficult to translate into clinical practice.5 Firstline therapy for acute flares is high-potency topical corticosteroids. If lesions are primarily on areas other than the face, a medium-potency topical steroid may be used. Topical calcineurin inhibitors or intralesional corticosteroids may be used if minimal improvement is seen after initial topical corticosteroid therapy. Treatment for widespread disease or disease that is resistant to local treatment is systemic therapy with antimalarial agents, followed by antimetabolites, systemic retinoids, thalidomide, or dapsone.1,2 The Cutaneous Lupus Erythematosus Disease Area and Severity Index is a valid tool to gauge the degree of disease and to help with disease progression and treatment response by noting the features of the plaques.1
Patients also should be educated that this disease can last for years, and long-standing DLE plaques infrequently can give rise to squamous cell carcinoma. In addition, isolated DLE can progress to SLE in 5% to 28% of patients.2
The differential diagnosis in our patient included other diseases with violaceous annular lesions and central clearing. Majocchi granuloma usually presents in areas of prior trauma, possibly due to shaving the face in our patient, or in the setting of topical corticosteroid use or immunosuppression. Scaling often is present within lesions, and histology shows fungal elements.6 Cutaneous sarcoidosis usually presents on the face, with scarring alopecia when appearing on the scalp; histology shows noncaseating granulomas, and 70% of patients with cutaneous symptoms will have systemic sarcoidosis.7 Granuloma annulare most commonly presents on the extremities, and histology shows lymphohistiocytic granulomas in a palisaded or interstitial pattern with connective-tissue degeneration and mucinous deposits.8 Annular psoriasis often is scaly and symmetric with parakeratosis, epidermal hyperplasia, dilated dermal capillaries, loss of granular layer, perivascular mononuclear cell infiltrate, and elongation of rete ridges on histology.9 Drug-induced lupus erythematosus always should be considered in patients taking triggering drugs such as antihypertensives, lipid-lowering drugs, antifungals, anti–tumor necrosis factor drugs, and proton pump inhibitors—the latter being a drug our patient was taking.10
The Diagnosis: Discoid Lupus Erythematosus
The biopsies demonstrated vacuolar interface changes with superficial and deep perivascular and periadnexal inflammation as well as increased background mucin deposition. The clinical morphology and distributions of the plaques limited to the photoexposed areas of the head suggested a diagnosis of discoid lupus erythematosus (DLE). The interface changes on histopathology supported this clinical impression. Our patient was treated with limited application of triamcinolone ointment 0.1% twice daily around the ears and neck, tacrolimus ointment 0.1% twice daily on the face, and hydroxychloroquine, as well as sun protection instructions. Smoking cessation was strongly advised.
Discoid lupus erythematosus is a disorder with chronic, erythematous, scaly, coin-shaped (discoid) plaques and is the most common form of chronic cutaneous lupus erythematosus.1 Lesions usually present on sun-exposed areas of the face, scalp, neck, ears, lips, or upper torso. They expand slowly with an active peripheral margin and a central scar that can result in induration, pigmentation changes, telangiectases, pruritus, or tenderness. Hair-bearing areas may be involved, causing hair loss due to follicular plugging; irreversible scarring alopecia can result. Facial DLE often spares the nasolabial folds. Ear involvement characteristically includes the conchal bowl and the outer external auditory canal. Discoid lupus erythematosus is considered localized if most of the head and neck region is involved or generalized if lesions also are present below the neck. Risk factors for DLE include genetic and environmental factors such as UV exposure, hormones, or exposure to toxins such as cigarette smoke.1 The disorder most commonly affects females and has a higher prevalence in patients of African descent than in Asian and White patients. Disease can occur at any age but usually occurs between 20 and 40 years of age.2 Discoid lupus erythematosus and other forms of chronic cutaneous lupus can occur independently or in conjunction with systemic lupus erythematosus (SLE), and approximately 15% to 30% of SLE patients develop DLE.1
Discoid lupus erythematosus is clinically diagnosed by the presentation of plaques in the characteristic distribution with confirmation via skin biopsy.1 Elman et al3 created a system for DLE classification that was only clinical and did not involve histopathology. Histologically, DLE often includes basement membrane thickening, follicular keratin plugs, mucin deposition, and vacuolar change with an interface, and a perivascular and periadnexal lymphocytic infiltrate.3,4 Antibodies such as antinuclear antibodies, rheumatoid factor, anti–double-stranded DNA, anti-Smith, and Sjögren syndrome A and B antibodies may be present (albeit with low positive frequency) in cutaneous lupus erythematosus.4 Characteristics of SLE also may be present, helping to confirm the diagnosis. Because there is an association of DLE with SLE, various laboratory tests should be ordered, including complete blood cell count, renal function panel, inflammatory markers, antibodies, and urinalysis for proteinuria.2,4
Treatment of DLE consists of preventative measures, such as sun protection with vitamin D supplementation, avoidance of drug triggers, and smoking cessation, as well as pharmacotherapy. The importance of wearing sun protective hats and garments with sunscreen use cannot be understated.1 Smoking cessation should be advised because smoking reduces the efficacy of antimalarial treatment and potentially increases the likelihood of patients requiring a second antimalarial drug. Quinacrine often is noted in both the dermatology and rheumatology literature to be used for escalating cutaneous lupus erythematosus care when hydroxychloroquine is ineffective or not tolerated, but no US manufacturer produces this medication; thus, compounding is required, which may be financially prohibitive, making this recommendation difficult to translate into clinical practice.5 Firstline therapy for acute flares is high-potency topical corticosteroids. If lesions are primarily on areas other than the face, a medium-potency topical steroid may be used. Topical calcineurin inhibitors or intralesional corticosteroids may be used if minimal improvement is seen after initial topical corticosteroid therapy. Treatment for widespread disease or disease that is resistant to local treatment is systemic therapy with antimalarial agents, followed by antimetabolites, systemic retinoids, thalidomide, or dapsone.1,2 The Cutaneous Lupus Erythematosus Disease Area and Severity Index is a valid tool to gauge the degree of disease and to help with disease progression and treatment response by noting the features of the plaques.1
Patients also should be educated that this disease can last for years, and long-standing DLE plaques infrequently can give rise to squamous cell carcinoma. In addition, isolated DLE can progress to SLE in 5% to 28% of patients.2
The differential diagnosis in our patient included other diseases with violaceous annular lesions and central clearing. Majocchi granuloma usually presents in areas of prior trauma, possibly due to shaving the face in our patient, or in the setting of topical corticosteroid use or immunosuppression. Scaling often is present within lesions, and histology shows fungal elements.6 Cutaneous sarcoidosis usually presents on the face, with scarring alopecia when appearing on the scalp; histology shows noncaseating granulomas, and 70% of patients with cutaneous symptoms will have systemic sarcoidosis.7 Granuloma annulare most commonly presents on the extremities, and histology shows lymphohistiocytic granulomas in a palisaded or interstitial pattern with connective-tissue degeneration and mucinous deposits.8 Annular psoriasis often is scaly and symmetric with parakeratosis, epidermal hyperplasia, dilated dermal capillaries, loss of granular layer, perivascular mononuclear cell infiltrate, and elongation of rete ridges on histology.9 Drug-induced lupus erythematosus always should be considered in patients taking triggering drugs such as antihypertensives, lipid-lowering drugs, antifungals, anti–tumor necrosis factor drugs, and proton pump inhibitors—the latter being a drug our patient was taking.10
- Sontheimer CJ, Costner MI, Sontheimer RD. Lupus erythematosus. In: Kang S, Amagai M, Bruckner A, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw Hill; 2019:1037-1060.
- Lee KC. Discoid lupus. In: Ferri FF, ed. Ferri’s Clinical Advisor 2021. Elsevier; 2021:477.e15-477.e18.
- Elman SA, Joyce C, Braudis K, et al. Creation and validation of classification criteria for discoid lupus erythematosus. JAMA Dermatol. 2020;156:901-906. doi:10.1001/jamadermatol.2020.1698
- Patel P, Werth V. Cutaneous lupus erythematosus: a review. Dermatol Clin. 2002;20:373-385, v. doi:10.1016/s0733-8635(02)00016-5
- Mittal L, Werth VP. The quinacrine experience in a population of patients with cutaneous lupus erythematosus and dermatomyositis. J Am Acad Dermatol. 2017;77:374-377. doi:10.1016/j .jaad.2017.03.027
- Craddock LN, Schieke SM. Superficial fungal infection. In: Kang S, Amagai M, Bruckner A, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw Hill; 2019:2925-2951.
- Tan J, Vleugels R. Dermatologic findings in systemic disease. In: McKean S, Dressler D, Ross J, et al, eds. Principles and Practice of Hospital Medicine. 2nd ed. McGraw Hill; 2017:1145-1170.
- Prendiville JS. Granuloma annulare. In: Kang S, Amagai M, Bruckner A, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw Hill; 2019:564-571.
- Gudjonsson JE, Elder JT. Psoriasis. In: Kang S, Amagai M, Bruckner A, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw Hill; 2019:457-497.
- He Y, Sawalha AH. Drug-induced lupus erythematosus: an update on drugs and mechanisms. Curr Opin Rheumatol. 2018;30:490-497. doi:10.1097/BOR.0000000000000522
- Sontheimer CJ, Costner MI, Sontheimer RD. Lupus erythematosus. In: Kang S, Amagai M, Bruckner A, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw Hill; 2019:1037-1060.
- Lee KC. Discoid lupus. In: Ferri FF, ed. Ferri’s Clinical Advisor 2021. Elsevier; 2021:477.e15-477.e18.
- Elman SA, Joyce C, Braudis K, et al. Creation and validation of classification criteria for discoid lupus erythematosus. JAMA Dermatol. 2020;156:901-906. doi:10.1001/jamadermatol.2020.1698
- Patel P, Werth V. Cutaneous lupus erythematosus: a review. Dermatol Clin. 2002;20:373-385, v. doi:10.1016/s0733-8635(02)00016-5
- Mittal L, Werth VP. The quinacrine experience in a population of patients with cutaneous lupus erythematosus and dermatomyositis. J Am Acad Dermatol. 2017;77:374-377. doi:10.1016/j .jaad.2017.03.027
- Craddock LN, Schieke SM. Superficial fungal infection. In: Kang S, Amagai M, Bruckner A, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw Hill; 2019:2925-2951.
- Tan J, Vleugels R. Dermatologic findings in systemic disease. In: McKean S, Dressler D, Ross J, et al, eds. Principles and Practice of Hospital Medicine. 2nd ed. McGraw Hill; 2017:1145-1170.
- Prendiville JS. Granuloma annulare. In: Kang S, Amagai M, Bruckner A, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw Hill; 2019:564-571.
- Gudjonsson JE, Elder JT. Psoriasis. In: Kang S, Amagai M, Bruckner A, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw Hill; 2019:457-497.
- He Y, Sawalha AH. Drug-induced lupus erythematosus: an update on drugs and mechanisms. Curr Opin Rheumatol. 2018;30:490-497. doi:10.1097/BOR.0000000000000522
A 41-year-old man presented to the dermatology clinic with erythematous, pruritic, and painful plaques around the ears of 6 years’ duration. He reported that application of topical steroids, antifungals, and most recently a topical calcineurin inhibitor did not change the appearance or symptoms. His medical history was notable for tobacco smoking and gastroesophageal reflux disease, for which he was taking omeprazole for the last 3 years. He was unsure if the lesions changed with UV exposure. He was an active-duty US military service member, and his job required frequently working outdoors. A review of systems was otherwise unremarkable. Physical examination revealed annular, erythematous, indurated plaques on both the preauricular and postauricular skin on the left ear with associated central atrophy and hypopigmentation. No alopecia was appreciated. The remainder of the skin examination was unremarkable. Ancillary laboratory test results were notable for a negative antinuclear antibody screen but positive (low titer) for Sjögren syndrome A and B antibodies. Two punch biopsies were performed.