User login
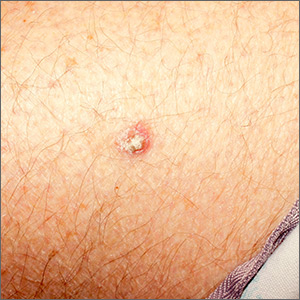
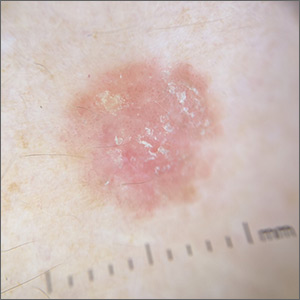
Rough lesion on the thigh
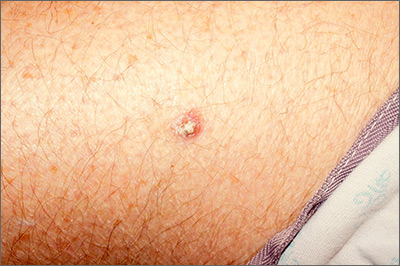
A shave biopsy was performed and revealed a well-differentiated, invasive squamous cell carcinoma (SCC). Complete excision was performed with a 4-mm margin.
In the United States, SCC is the most common skin cancer in Black patients, as well as the second most common skin cancer overall. A history of UV exposure from the sun or artificial tanning beds is the most significant risk factor.1 Radiation, carcinogenic chemical exposure, longstanding inflammation caused by burns, and immunosuppression are also risk factors for SCC.
When caring for a patient with SCC, the best initial work-up is a biopsy. A punch, shave, or excisional biopsy may all be appropriate if the dermis is adequately sampled. However, with a shave or shallow punch biopsy, thick keratin debris can unintentionally lead to a superficial sampling.
Cutaneous SCC should be treated by excision with 4- to 6-mm margins or Mohs microsurgery. Tumors that are smaller than 2 cm, lack aggressive histologic features, and are located in low-risk areas (eg, trunk or extremities) may be treated with standard excision. Larger tumors, recurrent tumors, higher risk histologic subtypes, and tumors on the head, neck, genitals, hands, or feet are candidates for Mohs surgery.
This patient was counseled to practice sun protection and to schedule regular follow-up visits every 6 months for the next 2 years.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68:957-966. doi: 10.1016/j.jaad.2012.11.037

A shave biopsy was performed and revealed a well-differentiated, invasive squamous cell carcinoma (SCC). Complete excision was performed with a 4-mm margin.
In the United States, SCC is the most common skin cancer in Black patients, as well as the second most common skin cancer overall. A history of UV exposure from the sun or artificial tanning beds is the most significant risk factor.1 Radiation, carcinogenic chemical exposure, longstanding inflammation caused by burns, and immunosuppression are also risk factors for SCC.
When caring for a patient with SCC, the best initial work-up is a biopsy. A punch, shave, or excisional biopsy may all be appropriate if the dermis is adequately sampled. However, with a shave or shallow punch biopsy, thick keratin debris can unintentionally lead to a superficial sampling.
Cutaneous SCC should be treated by excision with 4- to 6-mm margins or Mohs microsurgery. Tumors that are smaller than 2 cm, lack aggressive histologic features, and are located in low-risk areas (eg, trunk or extremities) may be treated with standard excision. Larger tumors, recurrent tumors, higher risk histologic subtypes, and tumors on the head, neck, genitals, hands, or feet are candidates for Mohs surgery.
This patient was counseled to practice sun protection and to schedule regular follow-up visits every 6 months for the next 2 years.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

A shave biopsy was performed and revealed a well-differentiated, invasive squamous cell carcinoma (SCC). Complete excision was performed with a 4-mm margin.
In the United States, SCC is the most common skin cancer in Black patients, as well as the second most common skin cancer overall. A history of UV exposure from the sun or artificial tanning beds is the most significant risk factor.1 Radiation, carcinogenic chemical exposure, longstanding inflammation caused by burns, and immunosuppression are also risk factors for SCC.
When caring for a patient with SCC, the best initial work-up is a biopsy. A punch, shave, or excisional biopsy may all be appropriate if the dermis is adequately sampled. However, with a shave or shallow punch biopsy, thick keratin debris can unintentionally lead to a superficial sampling.
Cutaneous SCC should be treated by excision with 4- to 6-mm margins or Mohs microsurgery. Tumors that are smaller than 2 cm, lack aggressive histologic features, and are located in low-risk areas (eg, trunk or extremities) may be treated with standard excision. Larger tumors, recurrent tumors, higher risk histologic subtypes, and tumors on the head, neck, genitals, hands, or feet are candidates for Mohs surgery.
This patient was counseled to practice sun protection and to schedule regular follow-up visits every 6 months for the next 2 years.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68:957-966. doi: 10.1016/j.jaad.2012.11.037
1. Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68:957-966. doi: 10.1016/j.jaad.2012.11.037
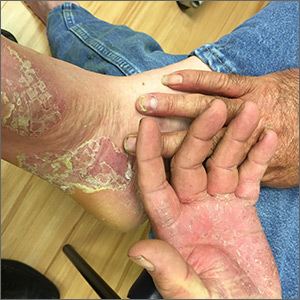
Painful hand and foot plaques
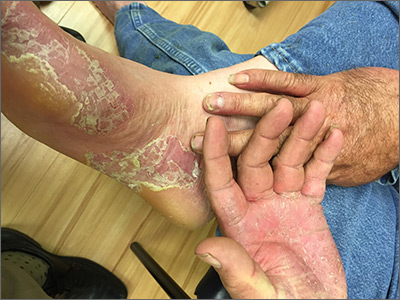
This patient had hand and foot psoriasis with the classic thick scale and erythema on his palms and soles. Additionally, in the area of the sole toward the heel, he had hyperpigmented macules called mahogany spots that are another hallmark of psoriasis. Pitting and distal onycholysis were also visible on his right ring finger.
This case illustrates how the painful plaques seen in hand and foot psoriasis—and other forms of psoriasis—can interfere with work and usual daily activities. UVA or narrowband UVB light therapy is a treatment option but requires 3 visits per week, which is not conducive to most people’s work schedules. Acitretin can be prescribed to decrease the abnormal proliferation of keratinocytes; however, adverse reactions can be expected, like this patient’s dry skin and itching. Furthermore, acitretin is a retinoid, like isotretinoin, which can cause severe birth defects, as well as hypertriglyceridemia and transaminitis. Pregnancy needs to be avoided for 3 years due to the teratogenicity and long washout period, so it should not be used in women with reproductive potential.1
This patient was initially treated with topical calcipotriene (a vitamin D derivative) and clobetasol (high-potency topical steroid) bid but did not have adequate improvement. Screening lab tests showed elevated liver enzymes, precluding treatment with methotrexate (and acitretin, which he’d received previously). He was started on apremilast, an oral phosphodiesterase inhibitor, because his insurance denied adalimumab. Apremilast can cause diarrhea, depression, nausea, and headache. Other than some loose stools, the patient tolerated apremilast well and showed significant improvement in his psoriasis at his 3-month follow-up visit.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Kaushik SB, Lebwohl MG. Review of safety and efficacy of approved systemic psoriasis therapies. Int J Dermatol. 2019;58:649-658. doi: 10.1111/ijd.14246.

This patient had hand and foot psoriasis with the classic thick scale and erythema on his palms and soles. Additionally, in the area of the sole toward the heel, he had hyperpigmented macules called mahogany spots that are another hallmark of psoriasis. Pitting and distal onycholysis were also visible on his right ring finger.
This case illustrates how the painful plaques seen in hand and foot psoriasis—and other forms of psoriasis—can interfere with work and usual daily activities. UVA or narrowband UVB light therapy is a treatment option but requires 3 visits per week, which is not conducive to most people’s work schedules. Acitretin can be prescribed to decrease the abnormal proliferation of keratinocytes; however, adverse reactions can be expected, like this patient’s dry skin and itching. Furthermore, acitretin is a retinoid, like isotretinoin, which can cause severe birth defects, as well as hypertriglyceridemia and transaminitis. Pregnancy needs to be avoided for 3 years due to the teratogenicity and long washout period, so it should not be used in women with reproductive potential.1
This patient was initially treated with topical calcipotriene (a vitamin D derivative) and clobetasol (high-potency topical steroid) bid but did not have adequate improvement. Screening lab tests showed elevated liver enzymes, precluding treatment with methotrexate (and acitretin, which he’d received previously). He was started on apremilast, an oral phosphodiesterase inhibitor, because his insurance denied adalimumab. Apremilast can cause diarrhea, depression, nausea, and headache. Other than some loose stools, the patient tolerated apremilast well and showed significant improvement in his psoriasis at his 3-month follow-up visit.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

This patient had hand and foot psoriasis with the classic thick scale and erythema on his palms and soles. Additionally, in the area of the sole toward the heel, he had hyperpigmented macules called mahogany spots that are another hallmark of psoriasis. Pitting and distal onycholysis were also visible on his right ring finger.
This case illustrates how the painful plaques seen in hand and foot psoriasis—and other forms of psoriasis—can interfere with work and usual daily activities. UVA or narrowband UVB light therapy is a treatment option but requires 3 visits per week, which is not conducive to most people’s work schedules. Acitretin can be prescribed to decrease the abnormal proliferation of keratinocytes; however, adverse reactions can be expected, like this patient’s dry skin and itching. Furthermore, acitretin is a retinoid, like isotretinoin, which can cause severe birth defects, as well as hypertriglyceridemia and transaminitis. Pregnancy needs to be avoided for 3 years due to the teratogenicity and long washout period, so it should not be used in women with reproductive potential.1
This patient was initially treated with topical calcipotriene (a vitamin D derivative) and clobetasol (high-potency topical steroid) bid but did not have adequate improvement. Screening lab tests showed elevated liver enzymes, precluding treatment with methotrexate (and acitretin, which he’d received previously). He was started on apremilast, an oral phosphodiesterase inhibitor, because his insurance denied adalimumab. Apremilast can cause diarrhea, depression, nausea, and headache. Other than some loose stools, the patient tolerated apremilast well and showed significant improvement in his psoriasis at his 3-month follow-up visit.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Kaushik SB, Lebwohl MG. Review of safety and efficacy of approved systemic psoriasis therapies. Int J Dermatol. 2019;58:649-658. doi: 10.1111/ijd.14246.
1. Kaushik SB, Lebwohl MG. Review of safety and efficacy of approved systemic psoriasis therapies. Int J Dermatol. 2019;58:649-658. doi: 10.1111/ijd.14246.
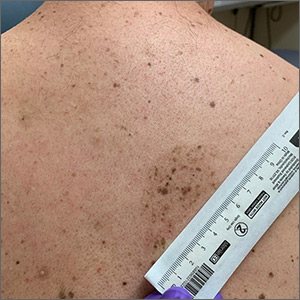
Cluster of hyperpigmented spots
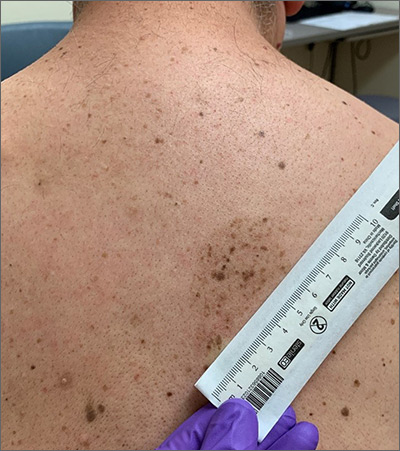
A large hyperpigmented patch with overlying darker macules and papules is characteristic of a speckled lentiginous nevus (SLN), also called a nevus spilus.
SLN is a cafe-au-lait˗like nevus that initially appears with a hyperpigmented background, usually at or around birth. Later, a speckled or polka-dot pattern of dark macules and papules appears over time. SLN is believed to be a form of congenital melanocytic nevus. There are believed to be 2 subtypes of SLN: nevus spilus maculosus and nevus spilus papulosus.
The maculosus subtype is characterized by flat and evenly distributed macules, that look like polka-dots. Histopathology reveals elongated interpapillary ridges containing increased numbers of melanocytes that form nests at the dermo-epidermal junction.
The papulosus subtype (which this patient had) is differentiated by superimposed speckles and papules whose size and distribution vary; this subype looks similar to a starry sky. Histopathology of the papulosus subtype shows melanocytic nevi of either the dermal or compound type—hence the papular appearance.
Given that SLN is a congenital melanocytic nevus, there is a small risk of transformation to malignant melanoma. The papulosus subtype is believed to have a more dynamic course with more lesions appearing over time. The maculosus subtype is considered to have a slightly higher risk of transformation into malignant melanoma compared to the papulosus subtype.
It is important to recognize that SLN is a distinct clinical entity, rather than a large irregular nevus. Mistaking it for a large suspicious nevus would require multiple biopsies of the most suspicious areas or excision of the entire lesion. Treatment for SLN includes serial surveillance with biopsy or excision of any suspicious areas that arise. In this case, the patient did not have any areas warranting biopsy, so the plan was to have him followed with annual clinical surveillance.
Photo and text courtesy of Erik Unruh, MD, MPH, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Happl, R. Speckled lentiginous naevus: which of the two disorders do you mean? Clin Exp Dermatol. 2009;34:133-135. doi: 10.1111/j.1365-2230.2008.02966.x.

A large hyperpigmented patch with overlying darker macules and papules is characteristic of a speckled lentiginous nevus (SLN), also called a nevus spilus.
SLN is a cafe-au-lait˗like nevus that initially appears with a hyperpigmented background, usually at or around birth. Later, a speckled or polka-dot pattern of dark macules and papules appears over time. SLN is believed to be a form of congenital melanocytic nevus. There are believed to be 2 subtypes of SLN: nevus spilus maculosus and nevus spilus papulosus.
The maculosus subtype is characterized by flat and evenly distributed macules, that look like polka-dots. Histopathology reveals elongated interpapillary ridges containing increased numbers of melanocytes that form nests at the dermo-epidermal junction.
The papulosus subtype (which this patient had) is differentiated by superimposed speckles and papules whose size and distribution vary; this subype looks similar to a starry sky. Histopathology of the papulosus subtype shows melanocytic nevi of either the dermal or compound type—hence the papular appearance.
Given that SLN is a congenital melanocytic nevus, there is a small risk of transformation to malignant melanoma. The papulosus subtype is believed to have a more dynamic course with more lesions appearing over time. The maculosus subtype is considered to have a slightly higher risk of transformation into malignant melanoma compared to the papulosus subtype.
It is important to recognize that SLN is a distinct clinical entity, rather than a large irregular nevus. Mistaking it for a large suspicious nevus would require multiple biopsies of the most suspicious areas or excision of the entire lesion. Treatment for SLN includes serial surveillance with biopsy or excision of any suspicious areas that arise. In this case, the patient did not have any areas warranting biopsy, so the plan was to have him followed with annual clinical surveillance.
Photo and text courtesy of Erik Unruh, MD, MPH, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

A large hyperpigmented patch with overlying darker macules and papules is characteristic of a speckled lentiginous nevus (SLN), also called a nevus spilus.
SLN is a cafe-au-lait˗like nevus that initially appears with a hyperpigmented background, usually at or around birth. Later, a speckled or polka-dot pattern of dark macules and papules appears over time. SLN is believed to be a form of congenital melanocytic nevus. There are believed to be 2 subtypes of SLN: nevus spilus maculosus and nevus spilus papulosus.
The maculosus subtype is characterized by flat and evenly distributed macules, that look like polka-dots. Histopathology reveals elongated interpapillary ridges containing increased numbers of melanocytes that form nests at the dermo-epidermal junction.
The papulosus subtype (which this patient had) is differentiated by superimposed speckles and papules whose size and distribution vary; this subype looks similar to a starry sky. Histopathology of the papulosus subtype shows melanocytic nevi of either the dermal or compound type—hence the papular appearance.
Given that SLN is a congenital melanocytic nevus, there is a small risk of transformation to malignant melanoma. The papulosus subtype is believed to have a more dynamic course with more lesions appearing over time. The maculosus subtype is considered to have a slightly higher risk of transformation into malignant melanoma compared to the papulosus subtype.
It is important to recognize that SLN is a distinct clinical entity, rather than a large irregular nevus. Mistaking it for a large suspicious nevus would require multiple biopsies of the most suspicious areas or excision of the entire lesion. Treatment for SLN includes serial surveillance with biopsy or excision of any suspicious areas that arise. In this case, the patient did not have any areas warranting biopsy, so the plan was to have him followed with annual clinical surveillance.
Photo and text courtesy of Erik Unruh, MD, MPH, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Happl, R. Speckled lentiginous naevus: which of the two disorders do you mean? Clin Exp Dermatol. 2009;34:133-135. doi: 10.1111/j.1365-2230.2008.02966.x.
Happl, R. Speckled lentiginous naevus: which of the two disorders do you mean? Clin Exp Dermatol. 2009;34:133-135. doi: 10.1111/j.1365-2230.2008.02966.x.
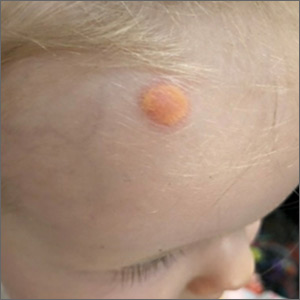
Child with yellow nodule
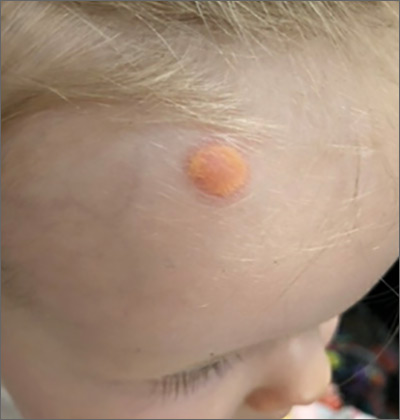
The characteristic orange-yellow color is the tip-off to the diagnosis of juvenile xanthogranuloma (JXG). It manifests as asymptomatic solitary or scattered papules or nodules, congenitally, or most commonly during the first year of life.
JXG is an unusual non-Langerhans cell histiocytosis that more commonly affects males. The etiology of JXG is unclear; it is presumed to be due to physical or infectious stimuli that produce a granulomatous histiocytic reaction. JXG typically manifests on the head, neck, upper extremities, and trunk. The appearance of JXG may be similar to that of Langerhans cell histiocytosis. If necessary, the diagnosis of JXG can be confirmed with a skin biopsy, which will reveal Touton-type giant cells and foamy histiocytes.
JXG is a benign and self-limiting disorder and spontaneously regresses within a few years. In rare cases, it can be systemic. If there are multiple lesions, relevant history, or physical exam features suggesting space-occupying lesions, imaging should be performed to rule out lesions in internal organs or structures. Treatment is indicated when there is systemic or symptomatic ocular involvement and may include surgical excision, radiotherapy, and/or systemic chemotherapy. In this case, the patient’s JXG management involved routine monitoring in anticipation of spontaneous resolution.
Image courtesy of John Durkin, MD, FAAD, Department of Dermatology, University of New Mexico School of Medicine, Albuquerque. Text courtesy of Kerry Song, BS, University of New Mexico School of Medicine, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Collie JS, Harper CD, Fillman EP. Juvenile Xanthogranuloma. In: StatPearls [Internet]. StatPearls Publishing; 2020 Jan. Accessed January 29, 2021. https://www.ncbi.nlm.nih.gov/books/NBK526103/#_NBK526103_pubdet

The characteristic orange-yellow color is the tip-off to the diagnosis of juvenile xanthogranuloma (JXG). It manifests as asymptomatic solitary or scattered papules or nodules, congenitally, or most commonly during the first year of life.
JXG is an unusual non-Langerhans cell histiocytosis that more commonly affects males. The etiology of JXG is unclear; it is presumed to be due to physical or infectious stimuli that produce a granulomatous histiocytic reaction. JXG typically manifests on the head, neck, upper extremities, and trunk. The appearance of JXG may be similar to that of Langerhans cell histiocytosis. If necessary, the diagnosis of JXG can be confirmed with a skin biopsy, which will reveal Touton-type giant cells and foamy histiocytes.
JXG is a benign and self-limiting disorder and spontaneously regresses within a few years. In rare cases, it can be systemic. If there are multiple lesions, relevant history, or physical exam features suggesting space-occupying lesions, imaging should be performed to rule out lesions in internal organs or structures. Treatment is indicated when there is systemic or symptomatic ocular involvement and may include surgical excision, radiotherapy, and/or systemic chemotherapy. In this case, the patient’s JXG management involved routine monitoring in anticipation of spontaneous resolution.
Image courtesy of John Durkin, MD, FAAD, Department of Dermatology, University of New Mexico School of Medicine, Albuquerque. Text courtesy of Kerry Song, BS, University of New Mexico School of Medicine, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

The characteristic orange-yellow color is the tip-off to the diagnosis of juvenile xanthogranuloma (JXG). It manifests as asymptomatic solitary or scattered papules or nodules, congenitally, or most commonly during the first year of life.
JXG is an unusual non-Langerhans cell histiocytosis that more commonly affects males. The etiology of JXG is unclear; it is presumed to be due to physical or infectious stimuli that produce a granulomatous histiocytic reaction. JXG typically manifests on the head, neck, upper extremities, and trunk. The appearance of JXG may be similar to that of Langerhans cell histiocytosis. If necessary, the diagnosis of JXG can be confirmed with a skin biopsy, which will reveal Touton-type giant cells and foamy histiocytes.
JXG is a benign and self-limiting disorder and spontaneously regresses within a few years. In rare cases, it can be systemic. If there are multiple lesions, relevant history, or physical exam features suggesting space-occupying lesions, imaging should be performed to rule out lesions in internal organs or structures. Treatment is indicated when there is systemic or symptomatic ocular involvement and may include surgical excision, radiotherapy, and/or systemic chemotherapy. In this case, the patient’s JXG management involved routine monitoring in anticipation of spontaneous resolution.
Image courtesy of John Durkin, MD, FAAD, Department of Dermatology, University of New Mexico School of Medicine, Albuquerque. Text courtesy of Kerry Song, BS, University of New Mexico School of Medicine, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Collie JS, Harper CD, Fillman EP. Juvenile Xanthogranuloma. In: StatPearls [Internet]. StatPearls Publishing; 2020 Jan. Accessed January 29, 2021. https://www.ncbi.nlm.nih.gov/books/NBK526103/#_NBK526103_pubdet
Collie JS, Harper CD, Fillman EP. Juvenile Xanthogranuloma. In: StatPearls [Internet]. StatPearls Publishing; 2020 Jan. Accessed January 29, 2021. https://www.ncbi.nlm.nih.gov/books/NBK526103/#_NBK526103_pubdet
Scaly lesion on forearm
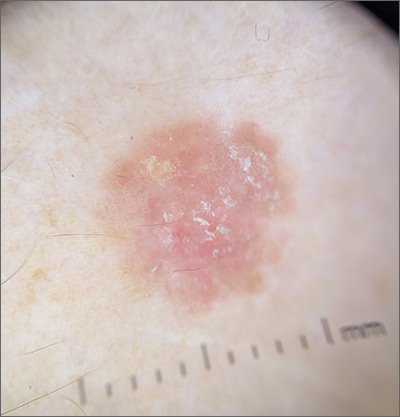
The suspicion raised by the dermoscopy results led to a shave biopsy, which confirmed the diagnosis of squamous cell carcinoma (SCC) in situ, also known as Bowen disease.
Bowen disease typically manifests as a scaly erythematous patch, often on sun-exposed skin. If untreated, these lesions have the potential to develop into invasive SCCs. Generally, the lesions are preceded by the formation of actinic keratosis (AK). In a 2009 trial performed by the Department of Veterans Affairs, up to 65% of SCCs were found to have previously been diagnosed as AK lesions.1
The selection of treatments includes excision, electrodessication and curettage, cryotherapy, and topical options such as fluorouracil bid for 4 weeks or imiquimod qd for 9 weeks.
After the physician outlined the treatment options, this patient opted for an elliptical excision. At the patient’s next follow-up appointment, she was found to have multiple AKs on her face; they were treated with cryotherapy. Patients with a diagnosis of precancerous or cancerous skin lesions are at high risk for additional AKs and skin cancer, so they should be counseled regarding the use of sun protective measures and the importance of annual screening for new lesions.
Image courtesy of Carlos Cano, MD, and text courtesy of Carlos Cano, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the veterans affairs topical tretinoin chemoprevention trial. Cancer. 2009;115:2523-2530. doi: 10.1002/cncr.24284.

The suspicion raised by the dermoscopy results led to a shave biopsy, which confirmed the diagnosis of squamous cell carcinoma (SCC) in situ, also known as Bowen disease.
Bowen disease typically manifests as a scaly erythematous patch, often on sun-exposed skin. If untreated, these lesions have the potential to develop into invasive SCCs. Generally, the lesions are preceded by the formation of actinic keratosis (AK). In a 2009 trial performed by the Department of Veterans Affairs, up to 65% of SCCs were found to have previously been diagnosed as AK lesions.1
The selection of treatments includes excision, electrodessication and curettage, cryotherapy, and topical options such as fluorouracil bid for 4 weeks or imiquimod qd for 9 weeks.
After the physician outlined the treatment options, this patient opted for an elliptical excision. At the patient’s next follow-up appointment, she was found to have multiple AKs on her face; they were treated with cryotherapy. Patients with a diagnosis of precancerous or cancerous skin lesions are at high risk for additional AKs and skin cancer, so they should be counseled regarding the use of sun protective measures and the importance of annual screening for new lesions.
Image courtesy of Carlos Cano, MD, and text courtesy of Carlos Cano, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

The suspicion raised by the dermoscopy results led to a shave biopsy, which confirmed the diagnosis of squamous cell carcinoma (SCC) in situ, also known as Bowen disease.
Bowen disease typically manifests as a scaly erythematous patch, often on sun-exposed skin. If untreated, these lesions have the potential to develop into invasive SCCs. Generally, the lesions are preceded by the formation of actinic keratosis (AK). In a 2009 trial performed by the Department of Veterans Affairs, up to 65% of SCCs were found to have previously been diagnosed as AK lesions.1
The selection of treatments includes excision, electrodessication and curettage, cryotherapy, and topical options such as fluorouracil bid for 4 weeks or imiquimod qd for 9 weeks.
After the physician outlined the treatment options, this patient opted for an elliptical excision. At the patient’s next follow-up appointment, she was found to have multiple AKs on her face; they were treated with cryotherapy. Patients with a diagnosis of precancerous or cancerous skin lesions are at high risk for additional AKs and skin cancer, so they should be counseled regarding the use of sun protective measures and the importance of annual screening for new lesions.
Image courtesy of Carlos Cano, MD, and text courtesy of Carlos Cano, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the veterans affairs topical tretinoin chemoprevention trial. Cancer. 2009;115:2523-2530. doi: 10.1002/cncr.24284.
1. Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the veterans affairs topical tretinoin chemoprevention trial. Cancer. 2009;115:2523-2530. doi: 10.1002/cncr.24284.
Pelvic pain
A 34-year-old woman with no significant past medical history presented as a new patient to our family medicine clinic with 2 weeks of intermittent lower abdominal and pelvic pain. She was sexually active with 1 partner and denied abnormal vaginal discharge or bleeding. She mentioned she’d had an intrauterine contraceptive device (IUD) placed a few weeks ago. The patient was afebrile, and her pelvic examination was unremarkable.
Physical examination showed mild tenderness to palpation over the lower abdomen without rebound tenderness or guarding. A complete metabolic panel revealed no significant abnormalities, and her human chorionic gonadotropin levels were normal.
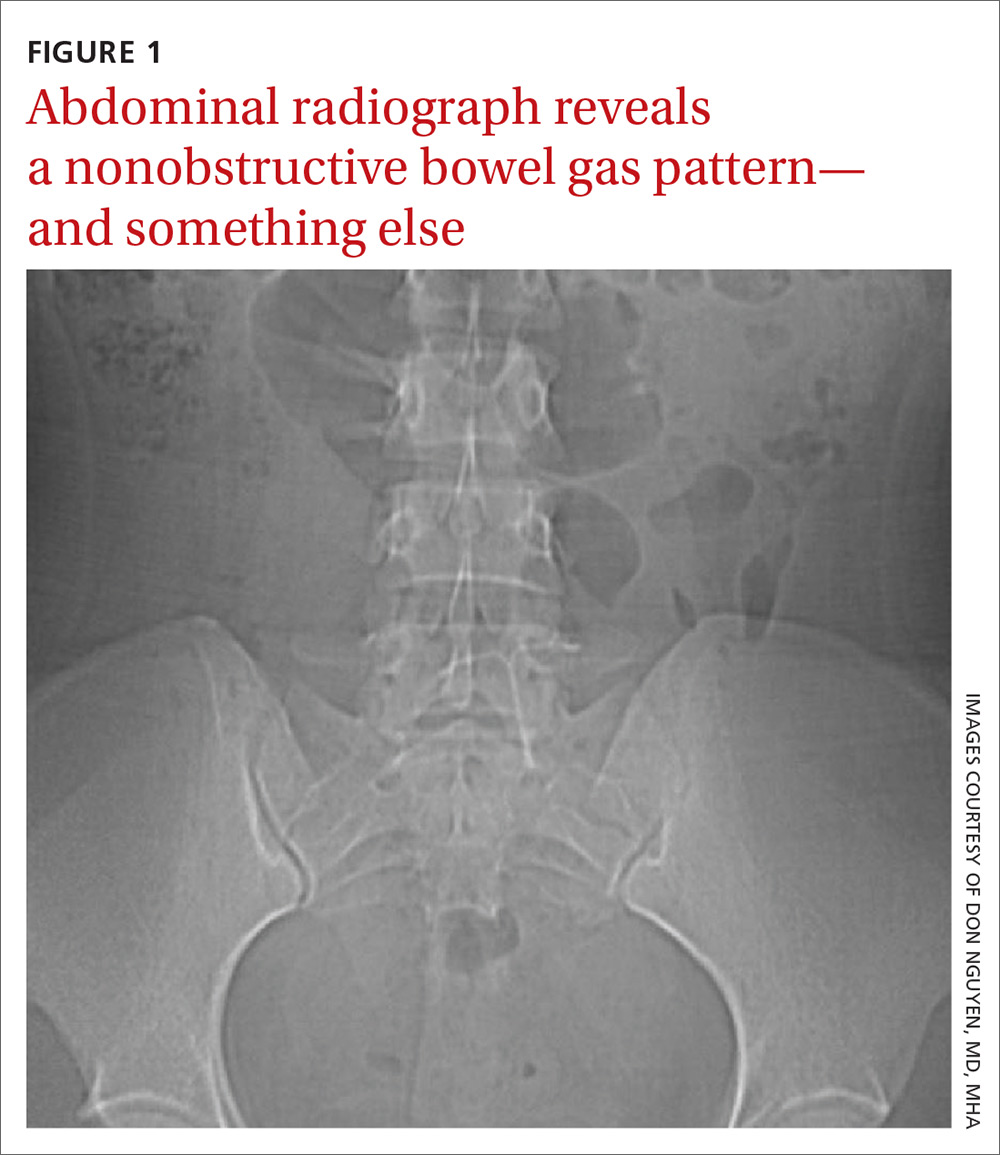
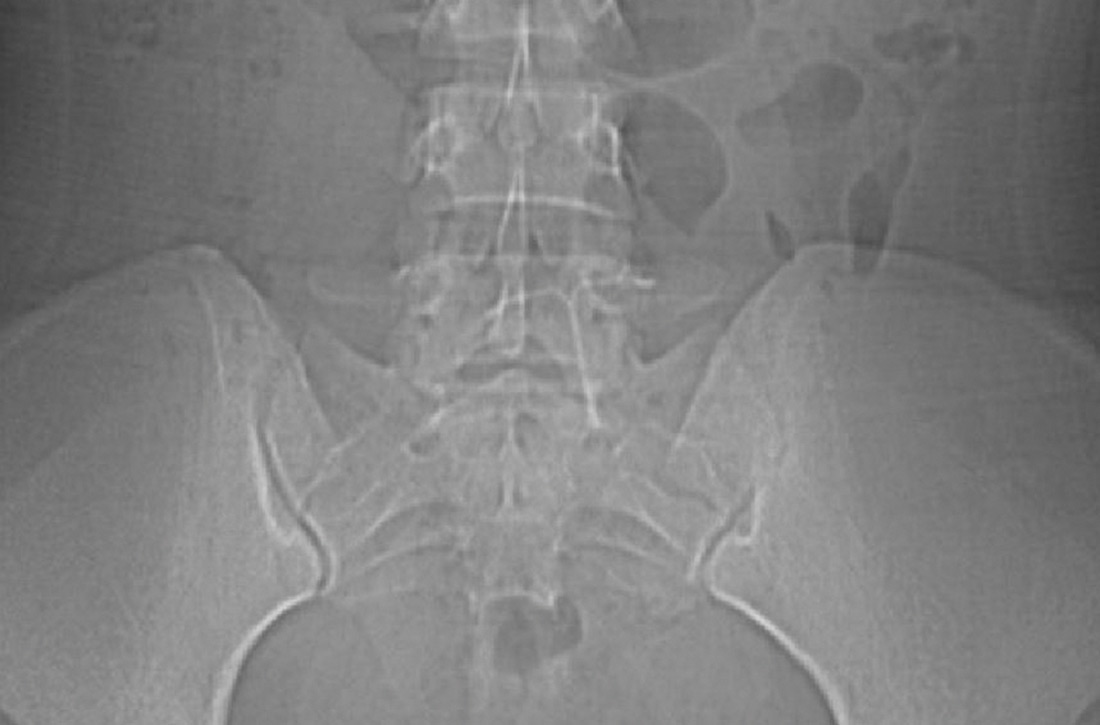
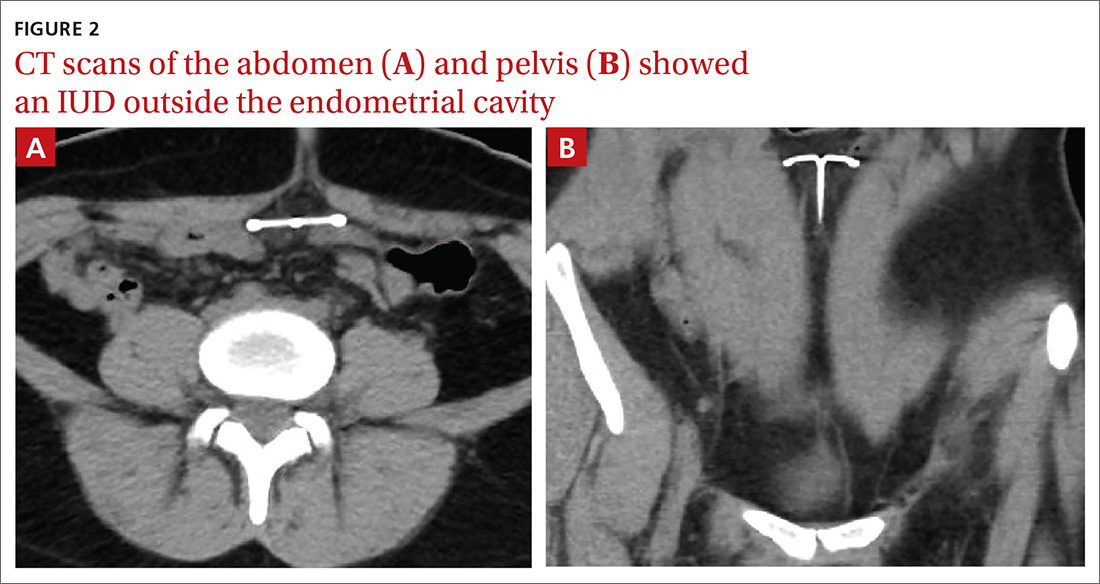
Findings from the physical exam and her clinical history prompted the need for imaging. An abdominal radiograph (FIGURE 1) and noncontrast computed tomography (FIGURES 2A and 2B) were subsequently ordered.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Intra-abdominal IUD migration
The abdominal radiograph revealed a nonobstructive bowel gas pattern with an IUD overlaying the central lower abdomen and pelvis at the L5-S1 level (FIGURE 1). Computed tomography (CT) of her abdomen and pelvis showed that the IUD was outside the endometrial cavity (FIGURES 2A and 2B). There was no evidence of pneumoperitoneum or bowel perforation. Based on the work-up and imaging, the patient’s pain was due to intra-abdominal IUD malpositioning.
Diagnostic criteria for IUD malpositioning include device migration into 1 of several locations, such as the lower uterine segment or cervix. IUD malpositioning can involve the rotation or protrusion of the device into or through the myometrium. On imaging, a well-positioned IUD should have a straight stem contained within the endometrial cavity, with the arms of the IUD extending laterally at the uterine fundus.
For our patient, an abdominal radiograph showed that her IUD was superiorly displaced outside the expected region of the endometrial cavity. CT helped to confirm this.
Complications with IUDs are few
Using an IUD is an increasingly popular method of contraception because it is effective and generally well tolerated, with minimal adverse effects or complications. In a multicenter retrospective chart review of 2138 patients who had IUDs, Aoun et al found that serious complications included pelvic inflammatory disease (2%), IUD expulsion (6%), and pregnancy (1%).1 In a retrospective cohort study examining complications among 90,489 women with IUDs, Berenson et al found ectopic pregnancy and uterine perforation affected < 1%.2
A less serious complication is IUD malpositioning. Although it does seem to occur more often than other, more serious complications, the exact incidence is unknown. In a retrospective case-control study, Braaten et al reported the rate for IUD malpositioning was 10.4% among 182 women.3 Malpositioned IUDs may be more likely to occur in those with suspected adenomyosis.3 In a study by de Kroon et al, the estimated prevalence rate for an abnormal IUD position ranged from 4% to 7.7% among 195 patients.4
Continue to: The clinical presentation of IUD migration
The clinical presentation of IUD migration
Identification of a malpositioned IUD is needed to avoid the possible increased risk for uterine perforation, IUD expulsion, or pregnancy.5
IUDs that have perforated the uterus float freely in the pelvis or abdomen and can result in injury to adjacent structures as well as peritonitis, fistulas, and hemorrhage.5-7 In addition, adhesion formation over the IUD can lead to intestinal obstruction, infertility, and chronic pain.6
Common symptoms of IUD malpositioning include abdominal or pelvic pain and abnormal bleeding, although many patients may be asymptomatic.8 In a retrospective study of 167 patients with IUDs who underwent pelvic ultrasound, 28 patients were found to have an IUD in an abnormal position.8 Rates of bleeding and pain were higher in patients with malpositioned IUDs (35.7% and 39.3%, respectively) than in those with a normally positioned IUD (15.1% and 19.4%, respectively).8
The differential Dx includes endometriosis and fibroids
IUD malpositioning can be distinguished from other diagnoses that cause pelvic pain and have similar presentations—including endometriosis, ectopic pregnancy, and fibroids—through imaging study findings, clinical history, and presentation.
Other conditions that may need to be ruled out include pelvic inflammatory disease, acute appendicitis, and ovarian cysts.9 A thorough history and physical examination can help rule out these conditions by organ system, and laboratory and imaging studies can help to confirm the diagnosis.
Continue to: Which imaging tool to use, and when
Which imaging tool to use, and when
Assessment of intrauterine contraception placement requires evaluation of the uterine cavity; gynecologic examination alone is not sufficient to fully evaluate for IUD position. Certain imaging studies are particularly helpful for revealing possible IUD migration.
Ultrasound—a widely available, radiation-free modality—is the first-line imaging tool for evaluation of an IUD’s position.10 In addition, ultrasound can provide effective evaluation of other pelvic structures, which is helpful in identifying or eliminating other causes of pain or abnormal bleeding.
Conventional radiography. If the IUD is not visualized on ultrasound, the American College of Obstetricians and Gynecologists (ACOG) recommends radiography to determine if the IUD has been expelled or has migrated to an extra-uterine position.6
CT may be best suited for the evaluation of more severe complications of IUD malpositioning, including visceral perforation, abscess formation, or bowel obstruction. CT should be considered if the patient’s clinical presentation is suspicious for a more serious intra-abdominal pathology.
Management depends on the IUD’s position
For patients whose IUD has an uncertain position or nonvisualized intravaginal strings, ACOG’s first-line recommendations include ruling out pregnancy, using an alternative method for contraception, and ordering pelvic ultrasonography.6 ACOG recommendations for the management of IUD malpositioning depend on the device’s location and the patient’s symptomatology.
Continue to: Management of low-lying IUDs
Management of low-lying IUDs is complex. An IUD that is malpositioned in the cervix is considered partially expelled and should be completely removed.6 For asymptomatic patients with an IUD located in the lower uterine segment and above the internal cervical os, there should be strong consideration given to leaving the IUD in place because removal is associated with higher rates of pregnancy given the low rates of initiation of effective contraception following removal.6
IUD malpositioning in the peritoneal cavity requires surgical intervention. Although ACOG’s first-line recommendation is laparoscopic intervention, laparotomy can be considered if laparoscopy does not result in the removal of the IUD or the patient has more severe complications (sepsis or bowel perforation).6 At the time of IUD removal, the clinician should also discuss and/or prescribe interim contraception.
Treatment for our patient included uncomplicated laparoscopic surgical removal of the intra-abdominal IUD. The patient’s symptoms went away following the procedure, and she was subsequently switched to an oral contraceptive.
1. Aoun J, Dines VA, Stovall DW, et al. Effects of age, parity, and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol. 2014;123:585-592.
2. Berenson AB, Tan A, Hirth JM, et al. Complications and continuation of intrauterine device use among commercially insured teenagers. Obstet Gynecol. 2013;121:951-958.
3. Braaten KP, Benson CB, Maurer R, et al. Malpositioned intrauterine contraceptive devices: risk factors, outcomes, and future pregnancies. Obstet Gynecol. 2011;118:1014-1020.
4. de Kroon CD, van Houwelingen JC, Trimbos JB, et al. The value of transvaginal ultrasound to monitor the position of an intrauterine device after insertion. A technology assessment study. Hum Reprod. 2003;18:2323-2327.
5. Thonneau P, Almont T, de La Rochebrochard E, et al. Risk factors for IUD failure: results of a large multicentre case-control study. Hum Reprod. 2006;21:2612-2616.
6. ACOG Committee on Gynecologic Practice. Committee Opinion No 672: clinical challenges of long-acting reversible contraceptive methods. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2016;128:e69-e77.
7. Heinemann K, Reed S, Moehner S, et al. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception. 2015;91:274-279.
8. Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices which are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110-115.
9. Bhavasr AK, Felner EJ, Shorma T. Common questions about the evaluation of acute pelvic pain. Am Fam Physician. 2016;93:41-48.
10. Peri N, Graham D, Levine D. Imaging of intrauterine contraceptive devices. J Ultrasound Med. 2007;26:1389-1401.
A 34-year-old woman with no significant past medical history presented as a new patient to our family medicine clinic with 2 weeks of intermittent lower abdominal and pelvic pain. She was sexually active with 1 partner and denied abnormal vaginal discharge or bleeding. She mentioned she’d had an intrauterine contraceptive device (IUD) placed a few weeks ago. The patient was afebrile, and her pelvic examination was unremarkable.
Physical examination showed mild tenderness to palpation over the lower abdomen without rebound tenderness or guarding. A complete metabolic panel revealed no significant abnormalities, and her human chorionic gonadotropin levels were normal.

Findings from the physical exam and her clinical history prompted the need for imaging. An abdominal radiograph (FIGURE 1) and noncontrast computed tomography (FIGURES 2A and 2B) were subsequently ordered.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Intra-abdominal IUD migration
The abdominal radiograph revealed a nonobstructive bowel gas pattern with an IUD overlaying the central lower abdomen and pelvis at the L5-S1 level (FIGURE 1). Computed tomography (CT) of her abdomen and pelvis showed that the IUD was outside the endometrial cavity (FIGURES 2A and 2B). There was no evidence of pneumoperitoneum or bowel perforation. Based on the work-up and imaging, the patient’s pain was due to intra-abdominal IUD malpositioning.
Diagnostic criteria for IUD malpositioning include device migration into 1 of several locations, such as the lower uterine segment or cervix. IUD malpositioning can involve the rotation or protrusion of the device into or through the myometrium. On imaging, a well-positioned IUD should have a straight stem contained within the endometrial cavity, with the arms of the IUD extending laterally at the uterine fundus.
For our patient, an abdominal radiograph showed that her IUD was superiorly displaced outside the expected region of the endometrial cavity. CT helped to confirm this.
Complications with IUDs are few
Using an IUD is an increasingly popular method of contraception because it is effective and generally well tolerated, with minimal adverse effects or complications. In a multicenter retrospective chart review of 2138 patients who had IUDs, Aoun et al found that serious complications included pelvic inflammatory disease (2%), IUD expulsion (6%), and pregnancy (1%).1 In a retrospective cohort study examining complications among 90,489 women with IUDs, Berenson et al found ectopic pregnancy and uterine perforation affected < 1%.2
A less serious complication is IUD malpositioning. Although it does seem to occur more often than other, more serious complications, the exact incidence is unknown. In a retrospective case-control study, Braaten et al reported the rate for IUD malpositioning was 10.4% among 182 women.3 Malpositioned IUDs may be more likely to occur in those with suspected adenomyosis.3 In a study by de Kroon et al, the estimated prevalence rate for an abnormal IUD position ranged from 4% to 7.7% among 195 patients.4
Continue to: The clinical presentation of IUD migration
The clinical presentation of IUD migration
Identification of a malpositioned IUD is needed to avoid the possible increased risk for uterine perforation, IUD expulsion, or pregnancy.5
IUDs that have perforated the uterus float freely in the pelvis or abdomen and can result in injury to adjacent structures as well as peritonitis, fistulas, and hemorrhage.5-7 In addition, adhesion formation over the IUD can lead to intestinal obstruction, infertility, and chronic pain.6
Common symptoms of IUD malpositioning include abdominal or pelvic pain and abnormal bleeding, although many patients may be asymptomatic.8 In a retrospective study of 167 patients with IUDs who underwent pelvic ultrasound, 28 patients were found to have an IUD in an abnormal position.8 Rates of bleeding and pain were higher in patients with malpositioned IUDs (35.7% and 39.3%, respectively) than in those with a normally positioned IUD (15.1% and 19.4%, respectively).8
The differential Dx includes endometriosis and fibroids
IUD malpositioning can be distinguished from other diagnoses that cause pelvic pain and have similar presentations—including endometriosis, ectopic pregnancy, and fibroids—through imaging study findings, clinical history, and presentation.
Other conditions that may need to be ruled out include pelvic inflammatory disease, acute appendicitis, and ovarian cysts.9 A thorough history and physical examination can help rule out these conditions by organ system, and laboratory and imaging studies can help to confirm the diagnosis.
Continue to: Which imaging tool to use, and when
Which imaging tool to use, and when
Assessment of intrauterine contraception placement requires evaluation of the uterine cavity; gynecologic examination alone is not sufficient to fully evaluate for IUD position. Certain imaging studies are particularly helpful for revealing possible IUD migration.
Ultrasound—a widely available, radiation-free modality—is the first-line imaging tool for evaluation of an IUD’s position.10 In addition, ultrasound can provide effective evaluation of other pelvic structures, which is helpful in identifying or eliminating other causes of pain or abnormal bleeding.
Conventional radiography. If the IUD is not visualized on ultrasound, the American College of Obstetricians and Gynecologists (ACOG) recommends radiography to determine if the IUD has been expelled or has migrated to an extra-uterine position.6
CT may be best suited for the evaluation of more severe complications of IUD malpositioning, including visceral perforation, abscess formation, or bowel obstruction. CT should be considered if the patient’s clinical presentation is suspicious for a more serious intra-abdominal pathology.
Management depends on the IUD’s position
For patients whose IUD has an uncertain position or nonvisualized intravaginal strings, ACOG’s first-line recommendations include ruling out pregnancy, using an alternative method for contraception, and ordering pelvic ultrasonography.6 ACOG recommendations for the management of IUD malpositioning depend on the device’s location and the patient’s symptomatology.
Continue to: Management of low-lying IUDs
Management of low-lying IUDs is complex. An IUD that is malpositioned in the cervix is considered partially expelled and should be completely removed.6 For asymptomatic patients with an IUD located in the lower uterine segment and above the internal cervical os, there should be strong consideration given to leaving the IUD in place because removal is associated with higher rates of pregnancy given the low rates of initiation of effective contraception following removal.6
IUD malpositioning in the peritoneal cavity requires surgical intervention. Although ACOG’s first-line recommendation is laparoscopic intervention, laparotomy can be considered if laparoscopy does not result in the removal of the IUD or the patient has more severe complications (sepsis or bowel perforation).6 At the time of IUD removal, the clinician should also discuss and/or prescribe interim contraception.
Treatment for our patient included uncomplicated laparoscopic surgical removal of the intra-abdominal IUD. The patient’s symptoms went away following the procedure, and she was subsequently switched to an oral contraceptive.
A 34-year-old woman with no significant past medical history presented as a new patient to our family medicine clinic with 2 weeks of intermittent lower abdominal and pelvic pain. She was sexually active with 1 partner and denied abnormal vaginal discharge or bleeding. She mentioned she’d had an intrauterine contraceptive device (IUD) placed a few weeks ago. The patient was afebrile, and her pelvic examination was unremarkable.
Physical examination showed mild tenderness to palpation over the lower abdomen without rebound tenderness or guarding. A complete metabolic panel revealed no significant abnormalities, and her human chorionic gonadotropin levels were normal.

Findings from the physical exam and her clinical history prompted the need for imaging. An abdominal radiograph (FIGURE 1) and noncontrast computed tomography (FIGURES 2A and 2B) were subsequently ordered.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Intra-abdominal IUD migration
The abdominal radiograph revealed a nonobstructive bowel gas pattern with an IUD overlaying the central lower abdomen and pelvis at the L5-S1 level (FIGURE 1). Computed tomography (CT) of her abdomen and pelvis showed that the IUD was outside the endometrial cavity (FIGURES 2A and 2B). There was no evidence of pneumoperitoneum or bowel perforation. Based on the work-up and imaging, the patient’s pain was due to intra-abdominal IUD malpositioning.
Diagnostic criteria for IUD malpositioning include device migration into 1 of several locations, such as the lower uterine segment or cervix. IUD malpositioning can involve the rotation or protrusion of the device into or through the myometrium. On imaging, a well-positioned IUD should have a straight stem contained within the endometrial cavity, with the arms of the IUD extending laterally at the uterine fundus.
For our patient, an abdominal radiograph showed that her IUD was superiorly displaced outside the expected region of the endometrial cavity. CT helped to confirm this.
Complications with IUDs are few
Using an IUD is an increasingly popular method of contraception because it is effective and generally well tolerated, with minimal adverse effects or complications. In a multicenter retrospective chart review of 2138 patients who had IUDs, Aoun et al found that serious complications included pelvic inflammatory disease (2%), IUD expulsion (6%), and pregnancy (1%).1 In a retrospective cohort study examining complications among 90,489 women with IUDs, Berenson et al found ectopic pregnancy and uterine perforation affected < 1%.2
A less serious complication is IUD malpositioning. Although it does seem to occur more often than other, more serious complications, the exact incidence is unknown. In a retrospective case-control study, Braaten et al reported the rate for IUD malpositioning was 10.4% among 182 women.3 Malpositioned IUDs may be more likely to occur in those with suspected adenomyosis.3 In a study by de Kroon et al, the estimated prevalence rate for an abnormal IUD position ranged from 4% to 7.7% among 195 patients.4
Continue to: The clinical presentation of IUD migration
The clinical presentation of IUD migration
Identification of a malpositioned IUD is needed to avoid the possible increased risk for uterine perforation, IUD expulsion, or pregnancy.5
IUDs that have perforated the uterus float freely in the pelvis or abdomen and can result in injury to adjacent structures as well as peritonitis, fistulas, and hemorrhage.5-7 In addition, adhesion formation over the IUD can lead to intestinal obstruction, infertility, and chronic pain.6
Common symptoms of IUD malpositioning include abdominal or pelvic pain and abnormal bleeding, although many patients may be asymptomatic.8 In a retrospective study of 167 patients with IUDs who underwent pelvic ultrasound, 28 patients were found to have an IUD in an abnormal position.8 Rates of bleeding and pain were higher in patients with malpositioned IUDs (35.7% and 39.3%, respectively) than in those with a normally positioned IUD (15.1% and 19.4%, respectively).8
The differential Dx includes endometriosis and fibroids
IUD malpositioning can be distinguished from other diagnoses that cause pelvic pain and have similar presentations—including endometriosis, ectopic pregnancy, and fibroids—through imaging study findings, clinical history, and presentation.
Other conditions that may need to be ruled out include pelvic inflammatory disease, acute appendicitis, and ovarian cysts.9 A thorough history and physical examination can help rule out these conditions by organ system, and laboratory and imaging studies can help to confirm the diagnosis.
Continue to: Which imaging tool to use, and when
Which imaging tool to use, and when
Assessment of intrauterine contraception placement requires evaluation of the uterine cavity; gynecologic examination alone is not sufficient to fully evaluate for IUD position. Certain imaging studies are particularly helpful for revealing possible IUD migration.
Ultrasound—a widely available, radiation-free modality—is the first-line imaging tool for evaluation of an IUD’s position.10 In addition, ultrasound can provide effective evaluation of other pelvic structures, which is helpful in identifying or eliminating other causes of pain or abnormal bleeding.
Conventional radiography. If the IUD is not visualized on ultrasound, the American College of Obstetricians and Gynecologists (ACOG) recommends radiography to determine if the IUD has been expelled or has migrated to an extra-uterine position.6
CT may be best suited for the evaluation of more severe complications of IUD malpositioning, including visceral perforation, abscess formation, or bowel obstruction. CT should be considered if the patient’s clinical presentation is suspicious for a more serious intra-abdominal pathology.
Management depends on the IUD’s position
For patients whose IUD has an uncertain position or nonvisualized intravaginal strings, ACOG’s first-line recommendations include ruling out pregnancy, using an alternative method for contraception, and ordering pelvic ultrasonography.6 ACOG recommendations for the management of IUD malpositioning depend on the device’s location and the patient’s symptomatology.
Continue to: Management of low-lying IUDs
Management of low-lying IUDs is complex. An IUD that is malpositioned in the cervix is considered partially expelled and should be completely removed.6 For asymptomatic patients with an IUD located in the lower uterine segment and above the internal cervical os, there should be strong consideration given to leaving the IUD in place because removal is associated with higher rates of pregnancy given the low rates of initiation of effective contraception following removal.6
IUD malpositioning in the peritoneal cavity requires surgical intervention. Although ACOG’s first-line recommendation is laparoscopic intervention, laparotomy can be considered if laparoscopy does not result in the removal of the IUD or the patient has more severe complications (sepsis or bowel perforation).6 At the time of IUD removal, the clinician should also discuss and/or prescribe interim contraception.
Treatment for our patient included uncomplicated laparoscopic surgical removal of the intra-abdominal IUD. The patient’s symptoms went away following the procedure, and she was subsequently switched to an oral contraceptive.
1. Aoun J, Dines VA, Stovall DW, et al. Effects of age, parity, and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol. 2014;123:585-592.
2. Berenson AB, Tan A, Hirth JM, et al. Complications and continuation of intrauterine device use among commercially insured teenagers. Obstet Gynecol. 2013;121:951-958.
3. Braaten KP, Benson CB, Maurer R, et al. Malpositioned intrauterine contraceptive devices: risk factors, outcomes, and future pregnancies. Obstet Gynecol. 2011;118:1014-1020.
4. de Kroon CD, van Houwelingen JC, Trimbos JB, et al. The value of transvaginal ultrasound to monitor the position of an intrauterine device after insertion. A technology assessment study. Hum Reprod. 2003;18:2323-2327.
5. Thonneau P, Almont T, de La Rochebrochard E, et al. Risk factors for IUD failure: results of a large multicentre case-control study. Hum Reprod. 2006;21:2612-2616.
6. ACOG Committee on Gynecologic Practice. Committee Opinion No 672: clinical challenges of long-acting reversible contraceptive methods. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2016;128:e69-e77.
7. Heinemann K, Reed S, Moehner S, et al. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception. 2015;91:274-279.
8. Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices which are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110-115.
9. Bhavasr AK, Felner EJ, Shorma T. Common questions about the evaluation of acute pelvic pain. Am Fam Physician. 2016;93:41-48.
10. Peri N, Graham D, Levine D. Imaging of intrauterine contraceptive devices. J Ultrasound Med. 2007;26:1389-1401.
1. Aoun J, Dines VA, Stovall DW, et al. Effects of age, parity, and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol. 2014;123:585-592.
2. Berenson AB, Tan A, Hirth JM, et al. Complications and continuation of intrauterine device use among commercially insured teenagers. Obstet Gynecol. 2013;121:951-958.
3. Braaten KP, Benson CB, Maurer R, et al. Malpositioned intrauterine contraceptive devices: risk factors, outcomes, and future pregnancies. Obstet Gynecol. 2011;118:1014-1020.
4. de Kroon CD, van Houwelingen JC, Trimbos JB, et al. The value of transvaginal ultrasound to monitor the position of an intrauterine device after insertion. A technology assessment study. Hum Reprod. 2003;18:2323-2327.
5. Thonneau P, Almont T, de La Rochebrochard E, et al. Risk factors for IUD failure: results of a large multicentre case-control study. Hum Reprod. 2006;21:2612-2616.
6. ACOG Committee on Gynecologic Practice. Committee Opinion No 672: clinical challenges of long-acting reversible contraceptive methods. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2016;128:e69-e77.
7. Heinemann K, Reed S, Moehner S, et al. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception. 2015;91:274-279.
8. Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices which are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110-115.
9. Bhavasr AK, Felner EJ, Shorma T. Common questions about the evaluation of acute pelvic pain. Am Fam Physician. 2016;93:41-48.
10. Peri N, Graham D, Levine D. Imaging of intrauterine contraceptive devices. J Ultrasound Med. 2007;26:1389-1401.
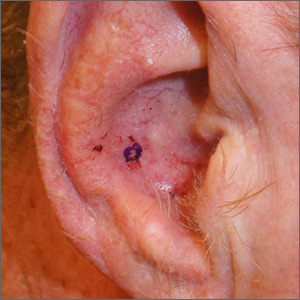
A hard-to-reach bleeding lesion
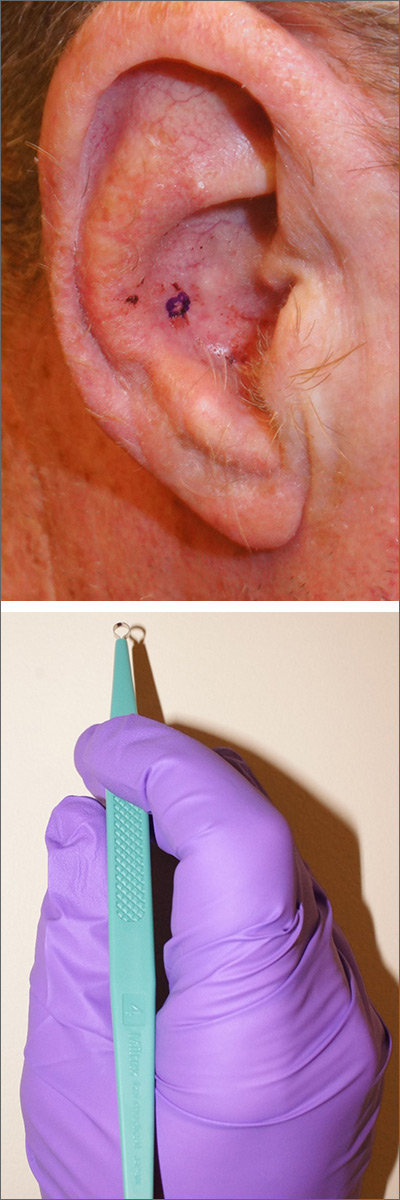
The history and exam were all suspicious for basal cell carcinoma (BCC), the most common of all cancers in the United States. (In African Americans, the most common skin cancer is squamous cell carcinoma). The suspicious lesion in this case was in a crevice, making it difficult to obtain a shave or punch biopsy.
As a result, a 4-mm disposable sterile curette was used (Figure). After informed consent was obtained, the lesion was cleansed with alcohol and marked with a surgical marker. Then, buffered lidocaine 1% with epinephrine was injected with a small syringe. Using a firm scraping motion with gentle rotation, a 4-mm sample of the lesion was quickly obtained and placed in standard formalin. Hemostasis was immediately obtained with firm application of 70% aluminum chloride in water using a cotton-tipped applicator. Heavy petrolatum was applied as a dressing.
The patient was confirmed to have a BCC and underwent Mohs surgery with clear margins after 1 stage. This case demonstrates the utility of a curette as a biopsy instrument for patients presenting with suspicious lesions in hard-to-reach places.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)
Yang YW, DiCaudo DJ. Effects of curettage after shave biopsy of unexpected melanoma: a retrospective review. J Am Acad Dermatol. 2018;78:1000-1002.

The history and exam were all suspicious for basal cell carcinoma (BCC), the most common of all cancers in the United States. (In African Americans, the most common skin cancer is squamous cell carcinoma). The suspicious lesion in this case was in a crevice, making it difficult to obtain a shave or punch biopsy.
As a result, a 4-mm disposable sterile curette was used (Figure). After informed consent was obtained, the lesion was cleansed with alcohol and marked with a surgical marker. Then, buffered lidocaine 1% with epinephrine was injected with a small syringe. Using a firm scraping motion with gentle rotation, a 4-mm sample of the lesion was quickly obtained and placed in standard formalin. Hemostasis was immediately obtained with firm application of 70% aluminum chloride in water using a cotton-tipped applicator. Heavy petrolatum was applied as a dressing.
The patient was confirmed to have a BCC and underwent Mohs surgery with clear margins after 1 stage. This case demonstrates the utility of a curette as a biopsy instrument for patients presenting with suspicious lesions in hard-to-reach places.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)

The history and exam were all suspicious for basal cell carcinoma (BCC), the most common of all cancers in the United States. (In African Americans, the most common skin cancer is squamous cell carcinoma). The suspicious lesion in this case was in a crevice, making it difficult to obtain a shave or punch biopsy.
As a result, a 4-mm disposable sterile curette was used (Figure). After informed consent was obtained, the lesion was cleansed with alcohol and marked with a surgical marker. Then, buffered lidocaine 1% with epinephrine was injected with a small syringe. Using a firm scraping motion with gentle rotation, a 4-mm sample of the lesion was quickly obtained and placed in standard formalin. Hemostasis was immediately obtained with firm application of 70% aluminum chloride in water using a cotton-tipped applicator. Heavy petrolatum was applied as a dressing.
The patient was confirmed to have a BCC and underwent Mohs surgery with clear margins after 1 stage. This case demonstrates the utility of a curette as a biopsy instrument for patients presenting with suspicious lesions in hard-to-reach places.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)
Yang YW, DiCaudo DJ. Effects of curettage after shave biopsy of unexpected melanoma: a retrospective review. J Am Acad Dermatol. 2018;78:1000-1002.
Yang YW, DiCaudo DJ. Effects of curettage after shave biopsy of unexpected melanoma: a retrospective review. J Am Acad Dermatol. 2018;78:1000-1002.
Generalized pruritic blisters and bullous lesions
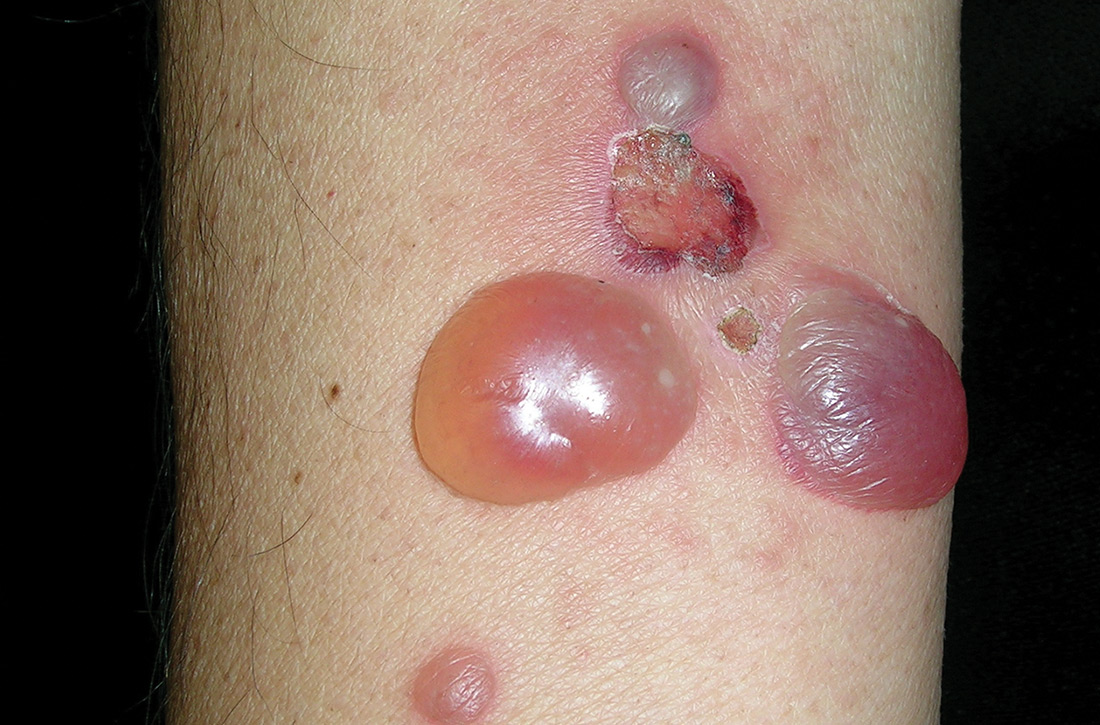
A 62-year-old man presented to our skin clinic with multiple pruritic, tense, bullous lesions that manifested on his arms, abdomen, back, and upper thighs over a 1-month period. There were no lesions in his oral cavity or around his eyes, nose, or penile region. He denied dysphagia.
The patient had multiple comorbidities, including diabetes, hypertension, recent stroke, and end-stage renal disease. He was being prepared for dialysis. His medications included torsemide, warfarin, amiodarone, metoprolol, pantoprozole, atorvastatin, and nifedipine. About 3 months prior to this presentation, he was started on oral linaglipton 5 mg/d, an oral antihyperglycemic medication. He had no history of skin disease or cancer, and his family history was not significant.
Physical examination showed multiple 5-mm to 2-cm blisters and bullae on the flexural surface of both of his arms (FIGURE), back, lower abdomen, and upper thighs. His palms and soles were not involved. The lesions were nontender, tense, and filled with clear fluid. Some were intact and others were rupturing. There was no mucocutaneous involvement. Nikolsky sign was negative. There were no signs of bleeding.
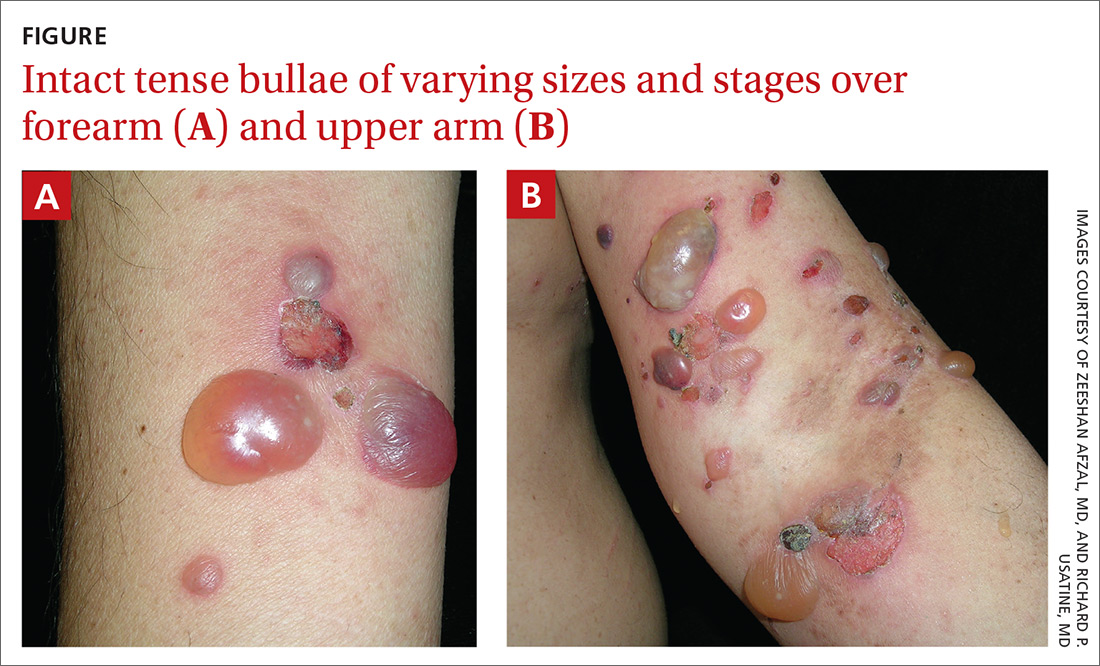
The family physician (FP) obtained a 4-mm punch biopsy at the edge of a 6-mm blister for light microscopy and a 3-mm perilesional punch biopsy for direct immunofluorescence (DIF) microscopy.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Bullous pemphigoid secondary to linagliptin use
DIF of the biopsy sample demonstrated linear deposition of complement 3 (C3) and immunoglobulin (Ig) G along the basement membrane zone. Indirect immunofluorescence on salt-split skin demonstrated linear deposition of IgG and C3 on both the roof and floor of the induced blisters. These findings and the patient’s clinical presentation met the criteria for bullous pemphigoid (BP), which is the most common autoimmune skin-blistering disease.1
BP is associated with subepidermal blistering, which can occur in reaction to a variety of triggers. Pathogenesis of this condition involves IgG anti-basement membrane autoantibody complex formation with the hemidesmosomal antigens BP230 and BP180—a process that activates C3 and the release of proteases that can be destructive to tissue along the dermo-epidermal junction.1
Growing incidence. BP usually occurs in patients > 60 years, with no racial or gender preference.1 The incidence rate of BP ranges from 2.4 to 21.7 new cases per 1 million individuals among various worldwide populations.2 The incidence appears to have increased 1.9- to 4.3-fold over the past 2 decades.2
What you’ll see, who’s at risk
Symptoms of BP include localized areas of erythema or pruritic urticarial plaques that gradually become more extensive. A patient may have pruritis alone for an extended period prior to developing blisters and bullae. The bullae are tense and normally 1 to 7 cm in size.1 Eruption is generalized, mostly affecting the lower abdomen, as well as the flexural parts of the extremities. The palms and soles also can be affected.
FPs should be aware of the atypical clinical variants of BP. In a review by Kridin and Ludwig, variants can be prurigo-like, eczema-like, urticaria-like, dyshidrosiform type, erosive type, and erythema annulare centrifugum–like type.2 At-risk populations, such as elderly patients (> 70 years), whose pruritis manifests with or without bullous formation, should be screened for BP.3,4
Continue to: Risk factors for BP
Risk factors for BP. Certain conditions linked to developing BP include neurologic disorders (dementia and Parkinson disease) and psychiatric disorders (unipolar and bipolar disorder).4 Further, it is important to note any medications that could be the cause of a patient’s BP, including dipeptidyl peptidase-4 (DPP-4) inhibitors, psychotropic medications, spironolactone, furosemide, beta-blockers, and antibiotics.3 This patient was taking a beta-blocker (metoprolol) and a DPP-4 inhibitor (linagliptin). Because he was most recently started on linagliptin, we suspected it may have had a causal role in the development of BP.
The association of DPP-4 inhibitors and BP
FPs are increasingly using DPP-4 inhibitors—including sitagliptin, vildagliptin, and linagliptin—as oral antihyperglycemic agents for type 2 diabetes mellitus. Therefore, it’s important to recognize this medication class’s association with BP.5 In a case-control study of 165 patients with BP, Benzaquen et al reported that 28 patients who were taking DPP-4 inhibitors had an associated increased risk for BP (adjusted odds ratio = 2.64; 95% confidence interval [CI], 1.19-5.85).3
The pathophysiology of BP associated with DPP-4 inhibitors remains unclear, but mechanisms have been proposed. The DPP-4 enzyme is expressed on many cells, including keratinocytes, T cells, and endothelial cells.3 It is possible that DPP-4 inhibition at these cells could stimulate activity of inflammatory cytokines, which can lead to enhanced local eosinophil activation and trigger bullous formation. DPP-4 enzymes are also involved in forming plasmin, which is a protease that cleaves BP180.3 Inhibition of this process can affect proper cleavage of BP180, impacting its function and antigenicity.3,6
Other conditions that also exhibit blisters
There are some skin conditions with similar presentations that need to be ruled out in the work-up.
Bullous diabeticorum is a rare, spontaneous, noninflammatory condition found in patients with diabetes.1 Blisters usually manifest as large, tense, asymmetrical, mildly tender lesions that commonly affect the feet and lower legs but can involve the trunk. These usually develop overnight without preceding trauma. Biopsy would show both intra-epidermal and subepidermal bulla with normal DIF findings.1 This condition usually has an excellent prognosis.
Continue to: Pemphigus vulgaris
Pemphigus vulgaris is characterized by nonpruritic, flaccid, painful blisters. This condition usually begins with manifestation of painful oral lesions that evolve into skin blisters. Some patients can develop mucocutaneous lesions.1 Nikolsky sign is positive in these cases. Light microscopy would show intra-epidermal bullae.
Dermatitis herpetiformis. This condition—usually affecting middle-age patients—is associated with severe pruritis and burning. It may start with a few pruritic papules or vesicles that later evolve into urticarial papules, vesicles, or bullae. Dermatitis herpetiformis can resemble herpes simplex virus. It can also be associated with gluten-sensitive enteropathy and small bowel lymphoma.1 DIF of a biopsy sample would show granular deposition of IgA within the tips of the dermal papillae and along the basement membrane of perilesional skin.1
Epidermolysis bullosa acquisita is a rare, severe, chronic condition with subepidermal mucocutaneous blistering.1 It is associated with skin fragility and spontaneous trauma-induced blisters that heal with scar formation and milia. IgG autoantibodies reacting to proteins in the basement membrane zone can cause the disease. It is also associated with Crohn disease.1 DIF findings are similar in BP, but they are differentiated by location of IgG deposits; they can be found on the dermal side of separation in epidermolysis bullosa acquisita, as compared with the epidermal side in BP.1
How to make the Dx in 3 steps
To effectively diagnose and classify BP, use the following 3-step method:
- Establish the presence of 3 of 4 clinical characteristics: patient’s age > 60 years, absence of atrophic scars, absence of mucosal involvement, and absence of bullous lesions on the head and neck.
- Order light microscopy. Findings should be consistent with eosinophils and neutrophils containing subepidermal bullae.
- Order a punch biopsy to obtain a perilesional specimen. DIF of the biopsy findings should feature linear deposits of IgG with or without C3 along the dermo-epidermal junction. This step is essential for an accurate diagnosis.
There also is benefit in ordering supplemental studies, such as an enzyme-linked immunosorbent assay for the detection of anti-BP180 or anti-BP230 IgG autoantibodies.7 However, for this patient, we did not order this study.
Continue to: Management focuses on steroids
Management focuses on steroids
The offending agent should be discontinued immediately. Depending on the severity of disease, treatment can include the use of potent topical corticosteroids alone or in combination with systemic corticosteroids and anti-inflammatory antibiotics (eg, doxycycline, minocycline, erythromycin).1,7 For patients with resistant or refractory disease, consider azathioprine, methotrexate, dapsone, and chlorambucil.1,7 Exceptional cases may benefit from the use of mycophenolate mofetil, intravenous immunoglobulin, or plasmapheresis.1,7
For this patient, initial treatment included discontinuation of linagliption and introduction of topical clobetasol 0.05% and oral prednisone 40 mg/d for 7 days, followed by prednisone 20 mg for 7 days. He was also started on oral doxycycline 100 mg bid and oral nicotinamide 500 mg bid.
1. Habif TP. Vesicular and bullous diseases. In: Habif TP, ed. Clinical Dermatology: a Color Guide to Diagnosis and Therapy. 6th ed. Elsevier; 2016:635-666.
2. Kridin K, Ludwig RJ. The growing incidence of bullous pemphigoid: overview and potential explanations. Front Med (Lausanne). 2018;5:220.
3. Benzaquen M, Borradori L, Berbis P, et al. Dipeptidyl peptidase IV inhibitors, a risk factor for bullous pemphigoid: retrospective multicenter case-control study from France and Switzerland. J Am Acad Dermatol. 2017;78:1090-1096.
4. Bastuji-Garin S, Joly P, Lemordant P, et al. Risk factors for bullous pemphigoid in the elderly: a prospective case-control study. J Invest Dermatol. 2011;131:637-643.
5. Kridin K, Bergman R. Association of bullous pemphigoid with dipeptidyl-peptidase 4 inhibitors in patients with diabetes: estimating the risk of the new agents and characterizing the patients. JAMA Dermatol. 2018;154:1152-1158.
6. Haber R, Fayad AM, Stephan F, et al. Bullous pemphigoid associated with linagliptin treatment. JAMA Dermatol. 2016;152:224-226.Management of bullous pemphigoid: the European Dermatology Forum consensus in collaboration with the European Academy of Dermatology and Venereology. Br J Dermatol. 2015;172:867-877.
A 62-year-old man presented to our skin clinic with multiple pruritic, tense, bullous lesions that manifested on his arms, abdomen, back, and upper thighs over a 1-month period. There were no lesions in his oral cavity or around his eyes, nose, or penile region. He denied dysphagia.
The patient had multiple comorbidities, including diabetes, hypertension, recent stroke, and end-stage renal disease. He was being prepared for dialysis. His medications included torsemide, warfarin, amiodarone, metoprolol, pantoprozole, atorvastatin, and nifedipine. About 3 months prior to this presentation, he was started on oral linaglipton 5 mg/d, an oral antihyperglycemic medication. He had no history of skin disease or cancer, and his family history was not significant.
Physical examination showed multiple 5-mm to 2-cm blisters and bullae on the flexural surface of both of his arms (FIGURE), back, lower abdomen, and upper thighs. His palms and soles were not involved. The lesions were nontender, tense, and filled with clear fluid. Some were intact and others were rupturing. There was no mucocutaneous involvement. Nikolsky sign was negative. There were no signs of bleeding.

The family physician (FP) obtained a 4-mm punch biopsy at the edge of a 6-mm blister for light microscopy and a 3-mm perilesional punch biopsy for direct immunofluorescence (DIF) microscopy.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Bullous pemphigoid secondary to linagliptin use
DIF of the biopsy sample demonstrated linear deposition of complement 3 (C3) and immunoglobulin (Ig) G along the basement membrane zone. Indirect immunofluorescence on salt-split skin demonstrated linear deposition of IgG and C3 on both the roof and floor of the induced blisters. These findings and the patient’s clinical presentation met the criteria for bullous pemphigoid (BP), which is the most common autoimmune skin-blistering disease.1
BP is associated with subepidermal blistering, which can occur in reaction to a variety of triggers. Pathogenesis of this condition involves IgG anti-basement membrane autoantibody complex formation with the hemidesmosomal antigens BP230 and BP180—a process that activates C3 and the release of proteases that can be destructive to tissue along the dermo-epidermal junction.1
Growing incidence. BP usually occurs in patients > 60 years, with no racial or gender preference.1 The incidence rate of BP ranges from 2.4 to 21.7 new cases per 1 million individuals among various worldwide populations.2 The incidence appears to have increased 1.9- to 4.3-fold over the past 2 decades.2
What you’ll see, who’s at risk
Symptoms of BP include localized areas of erythema or pruritic urticarial plaques that gradually become more extensive. A patient may have pruritis alone for an extended period prior to developing blisters and bullae. The bullae are tense and normally 1 to 7 cm in size.1 Eruption is generalized, mostly affecting the lower abdomen, as well as the flexural parts of the extremities. The palms and soles also can be affected.
FPs should be aware of the atypical clinical variants of BP. In a review by Kridin and Ludwig, variants can be prurigo-like, eczema-like, urticaria-like, dyshidrosiform type, erosive type, and erythema annulare centrifugum–like type.2 At-risk populations, such as elderly patients (> 70 years), whose pruritis manifests with or without bullous formation, should be screened for BP.3,4
Continue to: Risk factors for BP
Risk factors for BP. Certain conditions linked to developing BP include neurologic disorders (dementia and Parkinson disease) and psychiatric disorders (unipolar and bipolar disorder).4 Further, it is important to note any medications that could be the cause of a patient’s BP, including dipeptidyl peptidase-4 (DPP-4) inhibitors, psychotropic medications, spironolactone, furosemide, beta-blockers, and antibiotics.3 This patient was taking a beta-blocker (metoprolol) and a DPP-4 inhibitor (linagliptin). Because he was most recently started on linagliptin, we suspected it may have had a causal role in the development of BP.
The association of DPP-4 inhibitors and BP
FPs are increasingly using DPP-4 inhibitors—including sitagliptin, vildagliptin, and linagliptin—as oral antihyperglycemic agents for type 2 diabetes mellitus. Therefore, it’s important to recognize this medication class’s association with BP.5 In a case-control study of 165 patients with BP, Benzaquen et al reported that 28 patients who were taking DPP-4 inhibitors had an associated increased risk for BP (adjusted odds ratio = 2.64; 95% confidence interval [CI], 1.19-5.85).3
The pathophysiology of BP associated with DPP-4 inhibitors remains unclear, but mechanisms have been proposed. The DPP-4 enzyme is expressed on many cells, including keratinocytes, T cells, and endothelial cells.3 It is possible that DPP-4 inhibition at these cells could stimulate activity of inflammatory cytokines, which can lead to enhanced local eosinophil activation and trigger bullous formation. DPP-4 enzymes are also involved in forming plasmin, which is a protease that cleaves BP180.3 Inhibition of this process can affect proper cleavage of BP180, impacting its function and antigenicity.3,6
Other conditions that also exhibit blisters
There are some skin conditions with similar presentations that need to be ruled out in the work-up.
Bullous diabeticorum is a rare, spontaneous, noninflammatory condition found in patients with diabetes.1 Blisters usually manifest as large, tense, asymmetrical, mildly tender lesions that commonly affect the feet and lower legs but can involve the trunk. These usually develop overnight without preceding trauma. Biopsy would show both intra-epidermal and subepidermal bulla with normal DIF findings.1 This condition usually has an excellent prognosis.
Continue to: Pemphigus vulgaris
Pemphigus vulgaris is characterized by nonpruritic, flaccid, painful blisters. This condition usually begins with manifestation of painful oral lesions that evolve into skin blisters. Some patients can develop mucocutaneous lesions.1 Nikolsky sign is positive in these cases. Light microscopy would show intra-epidermal bullae.
Dermatitis herpetiformis. This condition—usually affecting middle-age patients—is associated with severe pruritis and burning. It may start with a few pruritic papules or vesicles that later evolve into urticarial papules, vesicles, or bullae. Dermatitis herpetiformis can resemble herpes simplex virus. It can also be associated with gluten-sensitive enteropathy and small bowel lymphoma.1 DIF of a biopsy sample would show granular deposition of IgA within the tips of the dermal papillae and along the basement membrane of perilesional skin.1
Epidermolysis bullosa acquisita is a rare, severe, chronic condition with subepidermal mucocutaneous blistering.1 It is associated with skin fragility and spontaneous trauma-induced blisters that heal with scar formation and milia. IgG autoantibodies reacting to proteins in the basement membrane zone can cause the disease. It is also associated with Crohn disease.1 DIF findings are similar in BP, but they are differentiated by location of IgG deposits; they can be found on the dermal side of separation in epidermolysis bullosa acquisita, as compared with the epidermal side in BP.1
How to make the Dx in 3 steps
To effectively diagnose and classify BP, use the following 3-step method:
- Establish the presence of 3 of 4 clinical characteristics: patient’s age > 60 years, absence of atrophic scars, absence of mucosal involvement, and absence of bullous lesions on the head and neck.
- Order light microscopy. Findings should be consistent with eosinophils and neutrophils containing subepidermal bullae.
- Order a punch biopsy to obtain a perilesional specimen. DIF of the biopsy findings should feature linear deposits of IgG with or without C3 along the dermo-epidermal junction. This step is essential for an accurate diagnosis.
There also is benefit in ordering supplemental studies, such as an enzyme-linked immunosorbent assay for the detection of anti-BP180 or anti-BP230 IgG autoantibodies.7 However, for this patient, we did not order this study.
Continue to: Management focuses on steroids
Management focuses on steroids
The offending agent should be discontinued immediately. Depending on the severity of disease, treatment can include the use of potent topical corticosteroids alone or in combination with systemic corticosteroids and anti-inflammatory antibiotics (eg, doxycycline, minocycline, erythromycin).1,7 For patients with resistant or refractory disease, consider azathioprine, methotrexate, dapsone, and chlorambucil.1,7 Exceptional cases may benefit from the use of mycophenolate mofetil, intravenous immunoglobulin, or plasmapheresis.1,7
For this patient, initial treatment included discontinuation of linagliption and introduction of topical clobetasol 0.05% and oral prednisone 40 mg/d for 7 days, followed by prednisone 20 mg for 7 days. He was also started on oral doxycycline 100 mg bid and oral nicotinamide 500 mg bid.
A 62-year-old man presented to our skin clinic with multiple pruritic, tense, bullous lesions that manifested on his arms, abdomen, back, and upper thighs over a 1-month period. There were no lesions in his oral cavity or around his eyes, nose, or penile region. He denied dysphagia.
The patient had multiple comorbidities, including diabetes, hypertension, recent stroke, and end-stage renal disease. He was being prepared for dialysis. His medications included torsemide, warfarin, amiodarone, metoprolol, pantoprozole, atorvastatin, and nifedipine. About 3 months prior to this presentation, he was started on oral linaglipton 5 mg/d, an oral antihyperglycemic medication. He had no history of skin disease or cancer, and his family history was not significant.
Physical examination showed multiple 5-mm to 2-cm blisters and bullae on the flexural surface of both of his arms (FIGURE), back, lower abdomen, and upper thighs. His palms and soles were not involved. The lesions were nontender, tense, and filled with clear fluid. Some were intact and others were rupturing. There was no mucocutaneous involvement. Nikolsky sign was negative. There were no signs of bleeding.

The family physician (FP) obtained a 4-mm punch biopsy at the edge of a 6-mm blister for light microscopy and a 3-mm perilesional punch biopsy for direct immunofluorescence (DIF) microscopy.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Bullous pemphigoid secondary to linagliptin use
DIF of the biopsy sample demonstrated linear deposition of complement 3 (C3) and immunoglobulin (Ig) G along the basement membrane zone. Indirect immunofluorescence on salt-split skin demonstrated linear deposition of IgG and C3 on both the roof and floor of the induced blisters. These findings and the patient’s clinical presentation met the criteria for bullous pemphigoid (BP), which is the most common autoimmune skin-blistering disease.1
BP is associated with subepidermal blistering, which can occur in reaction to a variety of triggers. Pathogenesis of this condition involves IgG anti-basement membrane autoantibody complex formation with the hemidesmosomal antigens BP230 and BP180—a process that activates C3 and the release of proteases that can be destructive to tissue along the dermo-epidermal junction.1
Growing incidence. BP usually occurs in patients > 60 years, with no racial or gender preference.1 The incidence rate of BP ranges from 2.4 to 21.7 new cases per 1 million individuals among various worldwide populations.2 The incidence appears to have increased 1.9- to 4.3-fold over the past 2 decades.2
What you’ll see, who’s at risk
Symptoms of BP include localized areas of erythema or pruritic urticarial plaques that gradually become more extensive. A patient may have pruritis alone for an extended period prior to developing blisters and bullae. The bullae are tense and normally 1 to 7 cm in size.1 Eruption is generalized, mostly affecting the lower abdomen, as well as the flexural parts of the extremities. The palms and soles also can be affected.
FPs should be aware of the atypical clinical variants of BP. In a review by Kridin and Ludwig, variants can be prurigo-like, eczema-like, urticaria-like, dyshidrosiform type, erosive type, and erythema annulare centrifugum–like type.2 At-risk populations, such as elderly patients (> 70 years), whose pruritis manifests with or without bullous formation, should be screened for BP.3,4
Continue to: Risk factors for BP
Risk factors for BP. Certain conditions linked to developing BP include neurologic disorders (dementia and Parkinson disease) and psychiatric disorders (unipolar and bipolar disorder).4 Further, it is important to note any medications that could be the cause of a patient’s BP, including dipeptidyl peptidase-4 (DPP-4) inhibitors, psychotropic medications, spironolactone, furosemide, beta-blockers, and antibiotics.3 This patient was taking a beta-blocker (metoprolol) and a DPP-4 inhibitor (linagliptin). Because he was most recently started on linagliptin, we suspected it may have had a causal role in the development of BP.
The association of DPP-4 inhibitors and BP
FPs are increasingly using DPP-4 inhibitors—including sitagliptin, vildagliptin, and linagliptin—as oral antihyperglycemic agents for type 2 diabetes mellitus. Therefore, it’s important to recognize this medication class’s association with BP.5 In a case-control study of 165 patients with BP, Benzaquen et al reported that 28 patients who were taking DPP-4 inhibitors had an associated increased risk for BP (adjusted odds ratio = 2.64; 95% confidence interval [CI], 1.19-5.85).3
The pathophysiology of BP associated with DPP-4 inhibitors remains unclear, but mechanisms have been proposed. The DPP-4 enzyme is expressed on many cells, including keratinocytes, T cells, and endothelial cells.3 It is possible that DPP-4 inhibition at these cells could stimulate activity of inflammatory cytokines, which can lead to enhanced local eosinophil activation and trigger bullous formation. DPP-4 enzymes are also involved in forming plasmin, which is a protease that cleaves BP180.3 Inhibition of this process can affect proper cleavage of BP180, impacting its function and antigenicity.3,6
Other conditions that also exhibit blisters
There are some skin conditions with similar presentations that need to be ruled out in the work-up.
Bullous diabeticorum is a rare, spontaneous, noninflammatory condition found in patients with diabetes.1 Blisters usually manifest as large, tense, asymmetrical, mildly tender lesions that commonly affect the feet and lower legs but can involve the trunk. These usually develop overnight without preceding trauma. Biopsy would show both intra-epidermal and subepidermal bulla with normal DIF findings.1 This condition usually has an excellent prognosis.
Continue to: Pemphigus vulgaris
Pemphigus vulgaris is characterized by nonpruritic, flaccid, painful blisters. This condition usually begins with manifestation of painful oral lesions that evolve into skin blisters. Some patients can develop mucocutaneous lesions.1 Nikolsky sign is positive in these cases. Light microscopy would show intra-epidermal bullae.
Dermatitis herpetiformis. This condition—usually affecting middle-age patients—is associated with severe pruritis and burning. It may start with a few pruritic papules or vesicles that later evolve into urticarial papules, vesicles, or bullae. Dermatitis herpetiformis can resemble herpes simplex virus. It can also be associated with gluten-sensitive enteropathy and small bowel lymphoma.1 DIF of a biopsy sample would show granular deposition of IgA within the tips of the dermal papillae and along the basement membrane of perilesional skin.1
Epidermolysis bullosa acquisita is a rare, severe, chronic condition with subepidermal mucocutaneous blistering.1 It is associated with skin fragility and spontaneous trauma-induced blisters that heal with scar formation and milia. IgG autoantibodies reacting to proteins in the basement membrane zone can cause the disease. It is also associated with Crohn disease.1 DIF findings are similar in BP, but they are differentiated by location of IgG deposits; they can be found on the dermal side of separation in epidermolysis bullosa acquisita, as compared with the epidermal side in BP.1
How to make the Dx in 3 steps
To effectively diagnose and classify BP, use the following 3-step method:
- Establish the presence of 3 of 4 clinical characteristics: patient’s age > 60 years, absence of atrophic scars, absence of mucosal involvement, and absence of bullous lesions on the head and neck.
- Order light microscopy. Findings should be consistent with eosinophils and neutrophils containing subepidermal bullae.
- Order a punch biopsy to obtain a perilesional specimen. DIF of the biopsy findings should feature linear deposits of IgG with or without C3 along the dermo-epidermal junction. This step is essential for an accurate diagnosis.
There also is benefit in ordering supplemental studies, such as an enzyme-linked immunosorbent assay for the detection of anti-BP180 or anti-BP230 IgG autoantibodies.7 However, for this patient, we did not order this study.
Continue to: Management focuses on steroids
Management focuses on steroids
The offending agent should be discontinued immediately. Depending on the severity of disease, treatment can include the use of potent topical corticosteroids alone or in combination with systemic corticosteroids and anti-inflammatory antibiotics (eg, doxycycline, minocycline, erythromycin).1,7 For patients with resistant or refractory disease, consider azathioprine, methotrexate, dapsone, and chlorambucil.1,7 Exceptional cases may benefit from the use of mycophenolate mofetil, intravenous immunoglobulin, or plasmapheresis.1,7
For this patient, initial treatment included discontinuation of linagliption and introduction of topical clobetasol 0.05% and oral prednisone 40 mg/d for 7 days, followed by prednisone 20 mg for 7 days. He was also started on oral doxycycline 100 mg bid and oral nicotinamide 500 mg bid.
1. Habif TP. Vesicular and bullous diseases. In: Habif TP, ed. Clinical Dermatology: a Color Guide to Diagnosis and Therapy. 6th ed. Elsevier; 2016:635-666.
2. Kridin K, Ludwig RJ. The growing incidence of bullous pemphigoid: overview and potential explanations. Front Med (Lausanne). 2018;5:220.
3. Benzaquen M, Borradori L, Berbis P, et al. Dipeptidyl peptidase IV inhibitors, a risk factor for bullous pemphigoid: retrospective multicenter case-control study from France and Switzerland. J Am Acad Dermatol. 2017;78:1090-1096.
4. Bastuji-Garin S, Joly P, Lemordant P, et al. Risk factors for bullous pemphigoid in the elderly: a prospective case-control study. J Invest Dermatol. 2011;131:637-643.
5. Kridin K, Bergman R. Association of bullous pemphigoid with dipeptidyl-peptidase 4 inhibitors in patients with diabetes: estimating the risk of the new agents and characterizing the patients. JAMA Dermatol. 2018;154:1152-1158.
6. Haber R, Fayad AM, Stephan F, et al. Bullous pemphigoid associated with linagliptin treatment. JAMA Dermatol. 2016;152:224-226.Management of bullous pemphigoid: the European Dermatology Forum consensus in collaboration with the European Academy of Dermatology and Venereology. Br J Dermatol. 2015;172:867-877.
1. Habif TP. Vesicular and bullous diseases. In: Habif TP, ed. Clinical Dermatology: a Color Guide to Diagnosis and Therapy. 6th ed. Elsevier; 2016:635-666.
2. Kridin K, Ludwig RJ. The growing incidence of bullous pemphigoid: overview and potential explanations. Front Med (Lausanne). 2018;5:220.
3. Benzaquen M, Borradori L, Berbis P, et al. Dipeptidyl peptidase IV inhibitors, a risk factor for bullous pemphigoid: retrospective multicenter case-control study from France and Switzerland. J Am Acad Dermatol. 2017;78:1090-1096.
4. Bastuji-Garin S, Joly P, Lemordant P, et al. Risk factors for bullous pemphigoid in the elderly: a prospective case-control study. J Invest Dermatol. 2011;131:637-643.
5. Kridin K, Bergman R. Association of bullous pemphigoid with dipeptidyl-peptidase 4 inhibitors in patients with diabetes: estimating the risk of the new agents and characterizing the patients. JAMA Dermatol. 2018;154:1152-1158.
6. Haber R, Fayad AM, Stephan F, et al. Bullous pemphigoid associated with linagliptin treatment. JAMA Dermatol. 2016;152:224-226.Management of bullous pemphigoid: the European Dermatology Forum consensus in collaboration with the European Academy of Dermatology and Venereology. Br J Dermatol. 2015;172:867-877.