User login
Milestone Match Day sees record highs; soar in DO applicants
Unifying allopathic (MD) and osteopathic (DO) applicants for the first time in a single matching program, 2020’s Match Day results underscored the continuing growth of DOs in the field, boosting numbers in primary care medicine and the Match as a whole.
The 2020 Main Residency Match bested 2019’s record as the largest in the history of the National Resident Matching Program (NRMP), with 40,084 applicants submitting program choices for 37,256 positions. This compares with 38,376 applicants vying for 35,185 positions last year.
It’s the seventh consecutive year in which overall match numbers are up, according to the NRMP. Although the number of applicants increased, so did the number of positions, resulting in a slight drop in the percent of positions filled during 2019-2020.
Available first-year (PGY-1) positions rose to 34,266, an increase of 2,072 (6.4%) over 2019. “This was, in part, due to the last migration of osteopathic program positions into the Main Residency Match,” Donna L. Lamb, DHSc, NRMP president and CEO, said in an interview. An agreement the Accreditation Council for Graduate Medical Education, American Osteopathic Association and American Association of Colleges of Osteopathic Medicine reached in 2014 recognized ACGME as the primary accrediting body for graduate medical education programs by 2020.
This led to the first single match for U.S. MD and DO senior students and graduates and the inclusion of DO senior students as sponsored applicants in 2020, Dr. Lamb noted.
Gains, trends in 2020 match
Growth in U.S. DO senior participation also pushed this year’s Match to record highs. There were 6,581 U.S. DO medical school seniors who submitted rank order lists, 1,103 more than in 2019. Among those seniors, 90.7% matched to PGY-1 positions, driving the match rate for U.S. DO seniors up 2.6 percentage points from 2019.
Since 2016, the number of U.S. DO seniors seeking positions has risen by 3,599 or 120%. “Of course, the number of U.S. MD seniors who submitted program choices was also record-high: 19,326, an increase of 401 over 2019. The 93.7% match rate to first-year positions for this group has remained very consistent for many years,” Dr. Lamb said.
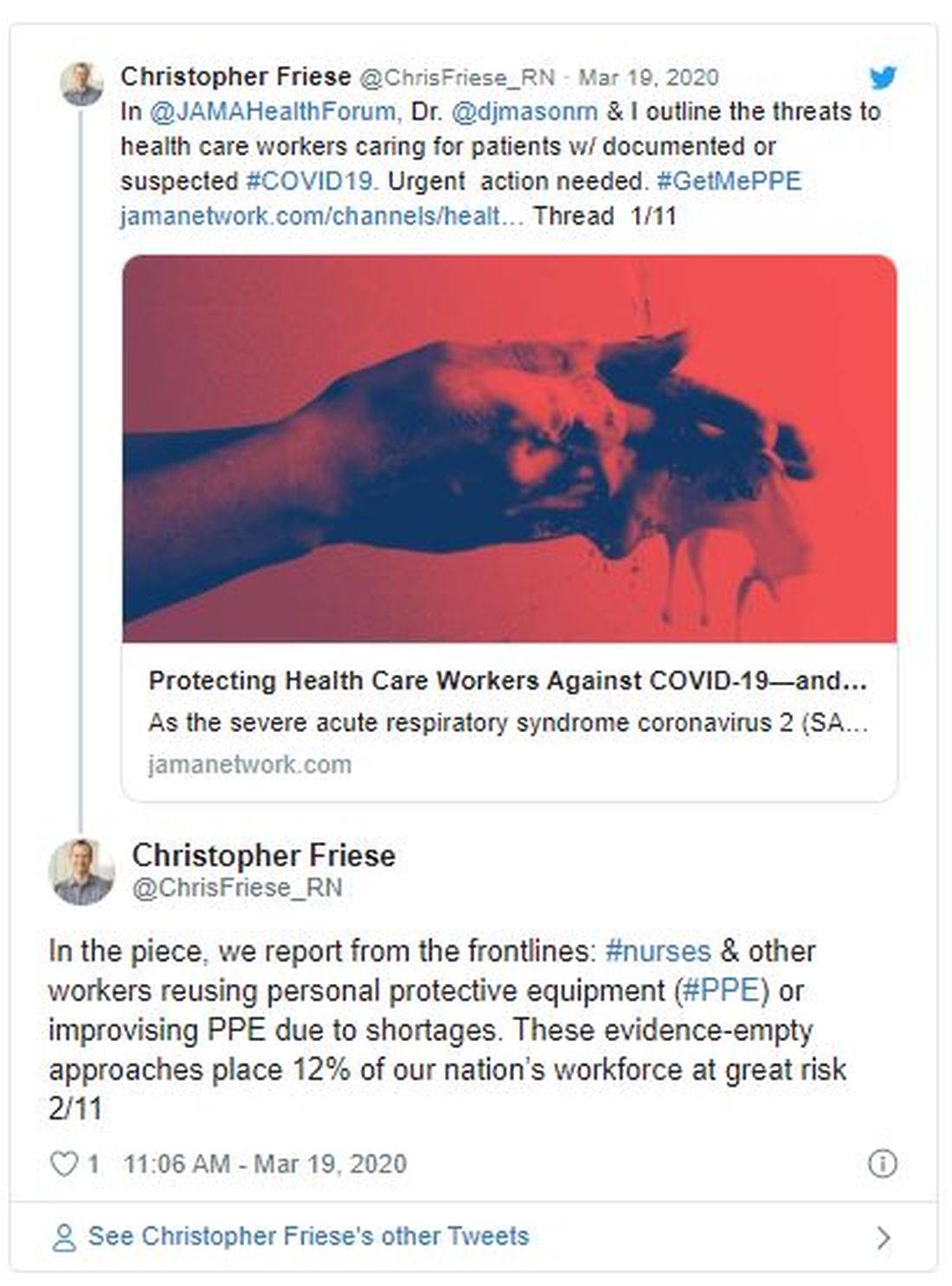
Among individual specialties, the NRMP reported extremely high fill rates for dermatology, medicine-emergency medicine, neurological surgery, physical medicine and rehabilitation (categorical), integrated plastic surgery, and thoracic surgery. Other competitive specialties included medicine-pediatrics, orthopedic surgery, otolaryngology, and vascular surgery.
Participation of international medical school students and graduates (IMGs) went up in 2020, breaking a 3-year cycle of decline. More than 61% matched to first-year positions, 2.5 percentage points higher than 2019 – and the highest match rate since 1990. “IMGs generally are having the most success matching to primary care specialties, including internal medicine, family medicine, and pediatrics,” Dr. Lamb said.
Primary care benefits from DO growth
DO candidates also helped drive up the numbers in primary care.
Internal medicine offered 8,697 categorical positions, 581 more than in 2019, reflecting a fill rate of 95.7%. More than 40% of these slots were filled by U.S. MD seniors, a category that’s seen decreases over the last 5 years, due in part to administrative and financial burdens associated with primary care internal medicine.
“In addition, the steady growth of internal medicine has increased the overall number of training positions available, and with the growth of other specialties in parallel, it has also likely had some effect on decreasing the percentage of U.S. graduates entering the field,” Phil Masters, MD, vice president of membership and global engagement at the American College of Physicians, said in an interview.
However, fill rates for U.S. DO seniors reached 16% in 2020, a notable rise from 6.9% in 2016. “As the number of osteopathic trainees increases, we are happy that more are choosing internal medicine as a career path,” Dr. Masters said, adding that the slightly different training and practice orientation of osteopathic physicians “complements that of their allopathic colleagues, and add richness to the many different practice settings that internal medicine encompasses.”
A record number of DO seniors also matched in family medicine (1,392), accounting for nearly 30% of all applicants. The single match led to an important net increase in filled family medicine residency positions, Clif Knight, MD, senior vice president for education at the American Academy of Family Physicians, said in an interview.
Overall, family medicine filled 92.5% of its 4,662 positions, 555 more than in 2019. The results show that family medicine and primary care are on solid footing, Dr. Knight said. “We are excited that the number of filled family medicine residency positions increased from last year. This is important as we work to meet the significant primary care workforce shortage,” he added.
In other specialties:
- Pediatrics filled more than 98% of its 2,864 categorical positions, 17 more than in 2019. U.S. MD seniors filled 1,731 (60.4%) of those slots. “We’re very excited about our newly matched pediatricians,” Sara “Sally” H. Goza, MD, president of the American Academy of Pediatrics, said in an interview. “The coronavirus outbreak has shown us how valuable the pediatric workforce is and how much we’re needed.’’
- Dermatology offered 478 positions, achieving a fill rate of 98.1%. “Looking at our own program’s Match results, I feel very satisfied that we are accomplishing our specific aim to serve rural populations and to create a diverse workforce in dermatology,” Erik Stratman, MD, an expert on dermatologic education in U.S. medical schools/residency programs, and a member of the American Academy of Dermatology, said in an interview. “It’s nice to see the fruits of the specialty’s expanding efforts to get the right people in the specialty who reflect those populations we serve.”
- Obstetrics-gynecology offered 1,433 first-year positions – 48 more than in 2019 – achieving a fill rate of 99.8%, with U.S. MD seniors filling more than 75% of those slots.
- Neurology filled more than 97.5% of 682 offered positions in 2020. However, U.S. MD seniors represented just under half of those filled positions (46.5%).
- Psychiatry offered 1,858 positions in 2020, achieving an overall fill rate of 98.9%, 61.2% for U.S. MD seniors.
- Emergency Medicine filled 99.5% of the 2,665 positions offered this year. In this profession, the U.S. MD fill rate was 64.3%. These new interns are sorely needed at a time when EM physicians are on the front lines of a pandemic, Hannah R. Hughes, MD, president of the Emergency Medicine Residents’ Association, said in an interview.
Unifying allopathic (MD) and osteopathic (DO) applicants for the first time in a single matching program, 2020’s Match Day results underscored the continuing growth of DOs in the field, boosting numbers in primary care medicine and the Match as a whole.

The 2020 Main Residency Match bested 2019’s record as the largest in the history of the National Resident Matching Program (NRMP), with 40,084 applicants submitting program choices for 37,256 positions. This compares with 38,376 applicants vying for 35,185 positions last year.
It’s the seventh consecutive year in which overall match numbers are up, according to the NRMP. Although the number of applicants increased, so did the number of positions, resulting in a slight drop in the percent of positions filled during 2019-2020.
Available first-year (PGY-1) positions rose to 34,266, an increase of 2,072 (6.4%) over 2019. “This was, in part, due to the last migration of osteopathic program positions into the Main Residency Match,” Donna L. Lamb, DHSc, NRMP president and CEO, said in an interview. An agreement the Accreditation Council for Graduate Medical Education, American Osteopathic Association and American Association of Colleges of Osteopathic Medicine reached in 2014 recognized ACGME as the primary accrediting body for graduate medical education programs by 2020.
This led to the first single match for U.S. MD and DO senior students and graduates and the inclusion of DO senior students as sponsored applicants in 2020, Dr. Lamb noted.
Gains, trends in 2020 match
Growth in U.S. DO senior participation also pushed this year’s Match to record highs. There were 6,581 U.S. DO medical school seniors who submitted rank order lists, 1,103 more than in 2019. Among those seniors, 90.7% matched to PGY-1 positions, driving the match rate for U.S. DO seniors up 2.6 percentage points from 2019.
Since 2016, the number of U.S. DO seniors seeking positions has risen by 3,599 or 120%. “Of course, the number of U.S. MD seniors who submitted program choices was also record-high: 19,326, an increase of 401 over 2019. The 93.7% match rate to first-year positions for this group has remained very consistent for many years,” Dr. Lamb said.
Among individual specialties, the NRMP reported extremely high fill rates for dermatology, medicine-emergency medicine, neurological surgery, physical medicine and rehabilitation (categorical), integrated plastic surgery, and thoracic surgery. Other competitive specialties included medicine-pediatrics, orthopedic surgery, otolaryngology, and vascular surgery.
Participation of international medical school students and graduates (IMGs) went up in 2020, breaking a 3-year cycle of decline. More than 61% matched to first-year positions, 2.5 percentage points higher than 2019 – and the highest match rate since 1990. “IMGs generally are having the most success matching to primary care specialties, including internal medicine, family medicine, and pediatrics,” Dr. Lamb said.
Primary care benefits from DO growth
DO candidates also helped drive up the numbers in primary care.
Internal medicine offered 8,697 categorical positions, 581 more than in 2019, reflecting a fill rate of 95.7%. More than 40% of these slots were filled by U.S. MD seniors, a category that’s seen decreases over the last 5 years, due in part to administrative and financial burdens associated with primary care internal medicine.
“In addition, the steady growth of internal medicine has increased the overall number of training positions available, and with the growth of other specialties in parallel, it has also likely had some effect on decreasing the percentage of U.S. graduates entering the field,” Phil Masters, MD, vice president of membership and global engagement at the American College of Physicians, said in an interview.
However, fill rates for U.S. DO seniors reached 16% in 2020, a notable rise from 6.9% in 2016. “As the number of osteopathic trainees increases, we are happy that more are choosing internal medicine as a career path,” Dr. Masters said, adding that the slightly different training and practice orientation of osteopathic physicians “complements that of their allopathic colleagues, and add richness to the many different practice settings that internal medicine encompasses.”
A record number of DO seniors also matched in family medicine (1,392), accounting for nearly 30% of all applicants. The single match led to an important net increase in filled family medicine residency positions, Clif Knight, MD, senior vice president for education at the American Academy of Family Physicians, said in an interview.
Overall, family medicine filled 92.5% of its 4,662 positions, 555 more than in 2019. The results show that family medicine and primary care are on solid footing, Dr. Knight said. “We are excited that the number of filled family medicine residency positions increased from last year. This is important as we work to meet the significant primary care workforce shortage,” he added.
In other specialties:
- Pediatrics filled more than 98% of its 2,864 categorical positions, 17 more than in 2019. U.S. MD seniors filled 1,731 (60.4%) of those slots. “We’re very excited about our newly matched pediatricians,” Sara “Sally” H. Goza, MD, president of the American Academy of Pediatrics, said in an interview. “The coronavirus outbreak has shown us how valuable the pediatric workforce is and how much we’re needed.’’
- Dermatology offered 478 positions, achieving a fill rate of 98.1%. “Looking at our own program’s Match results, I feel very satisfied that we are accomplishing our specific aim to serve rural populations and to create a diverse workforce in dermatology,” Erik Stratman, MD, an expert on dermatologic education in U.S. medical schools/residency programs, and a member of the American Academy of Dermatology, said in an interview. “It’s nice to see the fruits of the specialty’s expanding efforts to get the right people in the specialty who reflect those populations we serve.”
- Obstetrics-gynecology offered 1,433 first-year positions – 48 more than in 2019 – achieving a fill rate of 99.8%, with U.S. MD seniors filling more than 75% of those slots.
- Neurology filled more than 97.5% of 682 offered positions in 2020. However, U.S. MD seniors represented just under half of those filled positions (46.5%).
- Psychiatry offered 1,858 positions in 2020, achieving an overall fill rate of 98.9%, 61.2% for U.S. MD seniors.
- Emergency Medicine filled 99.5% of the 2,665 positions offered this year. In this profession, the U.S. MD fill rate was 64.3%. These new interns are sorely needed at a time when EM physicians are on the front lines of a pandemic, Hannah R. Hughes, MD, president of the Emergency Medicine Residents’ Association, said in an interview.
Unifying allopathic (MD) and osteopathic (DO) applicants for the first time in a single matching program, 2020’s Match Day results underscored the continuing growth of DOs in the field, boosting numbers in primary care medicine and the Match as a whole.

The 2020 Main Residency Match bested 2019’s record as the largest in the history of the National Resident Matching Program (NRMP), with 40,084 applicants submitting program choices for 37,256 positions. This compares with 38,376 applicants vying for 35,185 positions last year.
It’s the seventh consecutive year in which overall match numbers are up, according to the NRMP. Although the number of applicants increased, so did the number of positions, resulting in a slight drop in the percent of positions filled during 2019-2020.
Available first-year (PGY-1) positions rose to 34,266, an increase of 2,072 (6.4%) over 2019. “This was, in part, due to the last migration of osteopathic program positions into the Main Residency Match,” Donna L. Lamb, DHSc, NRMP president and CEO, said in an interview. An agreement the Accreditation Council for Graduate Medical Education, American Osteopathic Association and American Association of Colleges of Osteopathic Medicine reached in 2014 recognized ACGME as the primary accrediting body for graduate medical education programs by 2020.
This led to the first single match for U.S. MD and DO senior students and graduates and the inclusion of DO senior students as sponsored applicants in 2020, Dr. Lamb noted.
Gains, trends in 2020 match
Growth in U.S. DO senior participation also pushed this year’s Match to record highs. There were 6,581 U.S. DO medical school seniors who submitted rank order lists, 1,103 more than in 2019. Among those seniors, 90.7% matched to PGY-1 positions, driving the match rate for U.S. DO seniors up 2.6 percentage points from 2019.
Since 2016, the number of U.S. DO seniors seeking positions has risen by 3,599 or 120%. “Of course, the number of U.S. MD seniors who submitted program choices was also record-high: 19,326, an increase of 401 over 2019. The 93.7% match rate to first-year positions for this group has remained very consistent for many years,” Dr. Lamb said.
Among individual specialties, the NRMP reported extremely high fill rates for dermatology, medicine-emergency medicine, neurological surgery, physical medicine and rehabilitation (categorical), integrated plastic surgery, and thoracic surgery. Other competitive specialties included medicine-pediatrics, orthopedic surgery, otolaryngology, and vascular surgery.
Participation of international medical school students and graduates (IMGs) went up in 2020, breaking a 3-year cycle of decline. More than 61% matched to first-year positions, 2.5 percentage points higher than 2019 – and the highest match rate since 1990. “IMGs generally are having the most success matching to primary care specialties, including internal medicine, family medicine, and pediatrics,” Dr. Lamb said.
Primary care benefits from DO growth
DO candidates also helped drive up the numbers in primary care.
Internal medicine offered 8,697 categorical positions, 581 more than in 2019, reflecting a fill rate of 95.7%. More than 40% of these slots were filled by U.S. MD seniors, a category that’s seen decreases over the last 5 years, due in part to administrative and financial burdens associated with primary care internal medicine.
“In addition, the steady growth of internal medicine has increased the overall number of training positions available, and with the growth of other specialties in parallel, it has also likely had some effect on decreasing the percentage of U.S. graduates entering the field,” Phil Masters, MD, vice president of membership and global engagement at the American College of Physicians, said in an interview.
However, fill rates for U.S. DO seniors reached 16% in 2020, a notable rise from 6.9% in 2016. “As the number of osteopathic trainees increases, we are happy that more are choosing internal medicine as a career path,” Dr. Masters said, adding that the slightly different training and practice orientation of osteopathic physicians “complements that of their allopathic colleagues, and add richness to the many different practice settings that internal medicine encompasses.”
A record number of DO seniors also matched in family medicine (1,392), accounting for nearly 30% of all applicants. The single match led to an important net increase in filled family medicine residency positions, Clif Knight, MD, senior vice president for education at the American Academy of Family Physicians, said in an interview.
Overall, family medicine filled 92.5% of its 4,662 positions, 555 more than in 2019. The results show that family medicine and primary care are on solid footing, Dr. Knight said. “We are excited that the number of filled family medicine residency positions increased from last year. This is important as we work to meet the significant primary care workforce shortage,” he added.
In other specialties:
- Pediatrics filled more than 98% of its 2,864 categorical positions, 17 more than in 2019. U.S. MD seniors filled 1,731 (60.4%) of those slots. “We’re very excited about our newly matched pediatricians,” Sara “Sally” H. Goza, MD, president of the American Academy of Pediatrics, said in an interview. “The coronavirus outbreak has shown us how valuable the pediatric workforce is and how much we’re needed.’’
- Dermatology offered 478 positions, achieving a fill rate of 98.1%. “Looking at our own program’s Match results, I feel very satisfied that we are accomplishing our specific aim to serve rural populations and to create a diverse workforce in dermatology,” Erik Stratman, MD, an expert on dermatologic education in U.S. medical schools/residency programs, and a member of the American Academy of Dermatology, said in an interview. “It’s nice to see the fruits of the specialty’s expanding efforts to get the right people in the specialty who reflect those populations we serve.”
- Obstetrics-gynecology offered 1,433 first-year positions – 48 more than in 2019 – achieving a fill rate of 99.8%, with U.S. MD seniors filling more than 75% of those slots.
- Neurology filled more than 97.5% of 682 offered positions in 2020. However, U.S. MD seniors represented just under half of those filled positions (46.5%).
- Psychiatry offered 1,858 positions in 2020, achieving an overall fill rate of 98.9%, 61.2% for U.S. MD seniors.
- Emergency Medicine filled 99.5% of the 2,665 positions offered this year. In this profession, the U.S. MD fill rate was 64.3%. These new interns are sorely needed at a time when EM physicians are on the front lines of a pandemic, Hannah R. Hughes, MD, president of the Emergency Medicine Residents’ Association, said in an interview.
DIY masks: Worth the risk? Researchers are conflicted
In the midst of the rapidly spreading COVID-19 pandemic, hospitals and clinics are running out of masks. Health care workers are going online to beg for more, the hashtags #GetMePPE and #WeNeedPPE are trending on Twitter, and some hospitals have even put out public calls for mask donations. Health providers are working scared: They know that the moment the masks run out, they’re at increased risk for disease. So instead of waiting for mask shipments that may be weeks off, some people are making their own.
Using a simple template, they cut green surgical sheeting into half-moons, which they pin and sew before attaching elastic straps. Deaconess Health System in Evansville, Indiana, has posted instructions for fabric masks on their website and asked the public to step up and sew.
Elsewhere, health care workers have turned to diapers, maxi pads and other products to create masks. Social media channels are full of tips and sewing patterns. It’s an innovative strategy that is also contentious. Limited evidence suggests that homemade masks can offer some protection. But the DIY approach has also drawn criticism for providing a false sense of security, potentially putting wearers at risk.
The conflict points to an immediate need for more protective equipment, says Christopher Friese, PhD, RN, professor of nursing and public health at the University of Michigan, Ann Arbor. Also needed, he says, are new ideas for reducing strain on limited supplies, like adopting gear from other industries and finding innovative ways to provide care so that less protective gear is needed.
“We don’t want clinicians inventing and ‘MacGyvering’ their own device because we don’t want to put them at risk if we can avoid it,” says Friese, referring to the TV character who could build and assemble a vast array of tools/devices. “We have options that have been tested, and we have experience, maybe not in health care, but in other settings. We want to try that first before that frontline doctor, nurse, respiratory therapist decides to take matters into their own hands.
Increasingly, though, health care workers are finding they have no other choice — something even the CDC has acknowledged. In new guidelines, the agency recommends a bandanna, scarf, or other type of covering in cases where face masks are not available.
N95 respirators or surgical masks?
There are two main types of masks generally used in health care. N95 respirators filter out 95% of airborne particles, including bacteria and viruses. The lighter surgical or medical face masks are made to prevent spit and mucous from getting on patients or equipment.
Both types reduce rates of infection among health care workers, though comparisons (at least for influenza) have yet to show that one is superior to the other. One 2020 review by Chinese researchers, for example, analyzed six randomly controlled trials that included more than 9000 participants and found no added benefits of N95 masks over ordinary surgical masks for health care providers treating patients with the flu.
But COVID-19 is not influenza, and evidence suggests it may require more intensive protection, says Friese, who coauthored a blog post for JAMA about the country’s unpreparedness for protecting health care workers during a pandemic. The virus can linger in the air for hours, suggesting that N95 respirators are health care providers’ best option when treating infected patients.
The problem is there’s not enough to go around — of either mask type. In a March 5 survey, National Nurses United reported that just 30% of more than 6500 US respondents said their organizations had enough PPE to respond to a surge in patients. Another 38% did not know if their organizations were prepared. In a tweet, Friese estimated that 12% of nurses and other providers are at risk from reusing equipment or using equipment that is not backed by evidence.
Physicians and providers around the world have been sharing strategies online for how to make their own masks. Techniques vary, as do materials and plans for how to use the homemade equipment. At Phoebe Putney Health, DIY masks are intended to be worn over N95 respirators and then disposed of so that the respirators can be reused more safely, says Amanda Clements, the hospital’s public relations coordinator. Providers might also wear them to greet people at the front door.
Some evidence suggests that homemade masks can help in a pinch, at least for some illnesses. For a 2013 study by researchers in the UK, volunteers made surgical masks from cotton T-shirts, then put them on and coughed into a chamber that measured how much bacterial content got through. The team also assessed the aerosol-filtering ability of a variety of household materials, including scarfs, antimicrobial pillowcases, vacuum-cleaner bags, and tea towels. They tested each material with an aerosol containing two types of bacteria similar in size to influenza.
Commercial surgical masks performed three times better than homemade ones in the filtration test. Surgical masks worked twice as well at blocking droplets on the cough test. But all the makeshift materials — which also included silk, linen, and regular pillowcases — blocked some microbes. Vacuum-cleaner bags blocked the most bacteria, but their stiffness and thickness made them unsuitable for use as masks, the researchers reported. Tea towels showed a similar pattern. But pillowcases and cotton T-shirts were stretchy enough to fit well, thereby reducing the particles that could get through or around them.
Homemade masks should be used only as a last resort if commercial masks become unavailable, the researchers concluded. “Probably something is better than nothing for trained health care workers — for droplet contact avoidance, if nothing else,” says Anna Davies, BSc, a research facilitator at the University of Cambridge, UK, who is a former public health microbiologist and one of the study’s authors.
She recommends that members of the general public donate any stockpiles they have to health care workers, and make their own if they want masks for personal use. She is working with collaborators in the US to develop guidance for how best to do it.
“If people are quarantined and looking for something worthwhile to do, it probably wouldn’t be the worst thing to apply themselves to,” she wrote by email. “My suggestion would be for something soft and cotton, ideally with a bit of stretch (although it’s a pain to sew), and in two layers, marked ‘inside’ and ‘outside.’ ”
The idea that something is better than nothing was also the conclusion of a 2008 study by researchers in the Netherlands and the US. The study enlisted 28 healthy individuals who performed a variety of tasks while wearing N95 masks, surgical masks, or homemade masks sewn from teacloths. Effectiveness varied among individuals, but over a 90-second period, N95 masks worked best, with 25 times more protection than surgical masks and about 50 times more protection than homemade ones. Surgical masks were twice as effective as homemade masks. But the homemade masks offered at least some protection against large droplets.
Researchers emphasize that it’s not yet clear whether those findings are applicable to aerosolized COVID-19. In an influenza pandemic, at least, the authors posit that homemade masks could reduce transmission for the general public enough for some immunity to build. “It is important not to focus on a single intervention in case of a pandemic,” the researchers write, “but to integrate all effective interventions for optimal protection.”
For health care workers on the frontlines of COVID-19, Friese says, homemade masks might do more than nothing but they also might not work. Instead, he would rather see providers using construction or nuclear-engineering masks. And his best suggestion is something many providers are already doing: reducing physical contact with patients through telemedicine and other creative solutions, which is cutting down the overwhelming need for PPE.
Homemade mask production emphasizes the urgent need for more supplies, Friese adds.
“The government needs to step up and do a variety of things to increase production, and that needs to happen now, immediately,” he says. “We don’t we don’t want our clinicians to have to come up with these decisions.”
This article first appeared on Medscape.com.
In the midst of the rapidly spreading COVID-19 pandemic, hospitals and clinics are running out of masks. Health care workers are going online to beg for more, the hashtags #GetMePPE and #WeNeedPPE are trending on Twitter, and some hospitals have even put out public calls for mask donations. Health providers are working scared: They know that the moment the masks run out, they’re at increased risk for disease. So instead of waiting for mask shipments that may be weeks off, some people are making their own.
Using a simple template, they cut green surgical sheeting into half-moons, which they pin and sew before attaching elastic straps. Deaconess Health System in Evansville, Indiana, has posted instructions for fabric masks on their website and asked the public to step up and sew.
Elsewhere, health care workers have turned to diapers, maxi pads and other products to create masks. Social media channels are full of tips and sewing patterns. It’s an innovative strategy that is also contentious. Limited evidence suggests that homemade masks can offer some protection. But the DIY approach has also drawn criticism for providing a false sense of security, potentially putting wearers at risk.
The conflict points to an immediate need for more protective equipment, says Christopher Friese, PhD, RN, professor of nursing and public health at the University of Michigan, Ann Arbor. Also needed, he says, are new ideas for reducing strain on limited supplies, like adopting gear from other industries and finding innovative ways to provide care so that less protective gear is needed.
“We don’t want clinicians inventing and ‘MacGyvering’ their own device because we don’t want to put them at risk if we can avoid it,” says Friese, referring to the TV character who could build and assemble a vast array of tools/devices. “We have options that have been tested, and we have experience, maybe not in health care, but in other settings. We want to try that first before that frontline doctor, nurse, respiratory therapist decides to take matters into their own hands.
Increasingly, though, health care workers are finding they have no other choice — something even the CDC has acknowledged. In new guidelines, the agency recommends a bandanna, scarf, or other type of covering in cases where face masks are not available.
N95 respirators or surgical masks?
There are two main types of masks generally used in health care. N95 respirators filter out 95% of airborne particles, including bacteria and viruses. The lighter surgical or medical face masks are made to prevent spit and mucous from getting on patients or equipment.
Both types reduce rates of infection among health care workers, though comparisons (at least for influenza) have yet to show that one is superior to the other. One 2020 review by Chinese researchers, for example, analyzed six randomly controlled trials that included more than 9000 participants and found no added benefits of N95 masks over ordinary surgical masks for health care providers treating patients with the flu.
But COVID-19 is not influenza, and evidence suggests it may require more intensive protection, says Friese, who coauthored a blog post for JAMA about the country’s unpreparedness for protecting health care workers during a pandemic. The virus can linger in the air for hours, suggesting that N95 respirators are health care providers’ best option when treating infected patients.
The problem is there’s not enough to go around — of either mask type. In a March 5 survey, National Nurses United reported that just 30% of more than 6500 US respondents said their organizations had enough PPE to respond to a surge in patients. Another 38% did not know if their organizations were prepared. In a tweet, Friese estimated that 12% of nurses and other providers are at risk from reusing equipment or using equipment that is not backed by evidence.
Physicians and providers around the world have been sharing strategies online for how to make their own masks. Techniques vary, as do materials and plans for how to use the homemade equipment. At Phoebe Putney Health, DIY masks are intended to be worn over N95 respirators and then disposed of so that the respirators can be reused more safely, says Amanda Clements, the hospital’s public relations coordinator. Providers might also wear them to greet people at the front door.
Some evidence suggests that homemade masks can help in a pinch, at least for some illnesses. For a 2013 study by researchers in the UK, volunteers made surgical masks from cotton T-shirts, then put them on and coughed into a chamber that measured how much bacterial content got through. The team also assessed the aerosol-filtering ability of a variety of household materials, including scarfs, antimicrobial pillowcases, vacuum-cleaner bags, and tea towels. They tested each material with an aerosol containing two types of bacteria similar in size to influenza.
Commercial surgical masks performed three times better than homemade ones in the filtration test. Surgical masks worked twice as well at blocking droplets on the cough test. But all the makeshift materials — which also included silk, linen, and regular pillowcases — blocked some microbes. Vacuum-cleaner bags blocked the most bacteria, but their stiffness and thickness made them unsuitable for use as masks, the researchers reported. Tea towels showed a similar pattern. But pillowcases and cotton T-shirts were stretchy enough to fit well, thereby reducing the particles that could get through or around them.
Homemade masks should be used only as a last resort if commercial masks become unavailable, the researchers concluded. “Probably something is better than nothing for trained health care workers — for droplet contact avoidance, if nothing else,” says Anna Davies, BSc, a research facilitator at the University of Cambridge, UK, who is a former public health microbiologist and one of the study’s authors.
She recommends that members of the general public donate any stockpiles they have to health care workers, and make their own if they want masks for personal use. She is working with collaborators in the US to develop guidance for how best to do it.
“If people are quarantined and looking for something worthwhile to do, it probably wouldn’t be the worst thing to apply themselves to,” she wrote by email. “My suggestion would be for something soft and cotton, ideally with a bit of stretch (although it’s a pain to sew), and in two layers, marked ‘inside’ and ‘outside.’ ”
The idea that something is better than nothing was also the conclusion of a 2008 study by researchers in the Netherlands and the US. The study enlisted 28 healthy individuals who performed a variety of tasks while wearing N95 masks, surgical masks, or homemade masks sewn from teacloths. Effectiveness varied among individuals, but over a 90-second period, N95 masks worked best, with 25 times more protection than surgical masks and about 50 times more protection than homemade ones. Surgical masks were twice as effective as homemade masks. But the homemade masks offered at least some protection against large droplets.
Researchers emphasize that it’s not yet clear whether those findings are applicable to aerosolized COVID-19. In an influenza pandemic, at least, the authors posit that homemade masks could reduce transmission for the general public enough for some immunity to build. “It is important not to focus on a single intervention in case of a pandemic,” the researchers write, “but to integrate all effective interventions for optimal protection.”
For health care workers on the frontlines of COVID-19, Friese says, homemade masks might do more than nothing but they also might not work. Instead, he would rather see providers using construction or nuclear-engineering masks. And his best suggestion is something many providers are already doing: reducing physical contact with patients through telemedicine and other creative solutions, which is cutting down the overwhelming need for PPE.
Homemade mask production emphasizes the urgent need for more supplies, Friese adds.
“The government needs to step up and do a variety of things to increase production, and that needs to happen now, immediately,” he says. “We don’t we don’t want our clinicians to have to come up with these decisions.”
This article first appeared on Medscape.com.
In the midst of the rapidly spreading COVID-19 pandemic, hospitals and clinics are running out of masks. Health care workers are going online to beg for more, the hashtags #GetMePPE and #WeNeedPPE are trending on Twitter, and some hospitals have even put out public calls for mask donations. Health providers are working scared: They know that the moment the masks run out, they’re at increased risk for disease. So instead of waiting for mask shipments that may be weeks off, some people are making their own.
Using a simple template, they cut green surgical sheeting into half-moons, which they pin and sew before attaching elastic straps. Deaconess Health System in Evansville, Indiana, has posted instructions for fabric masks on their website and asked the public to step up and sew.
Elsewhere, health care workers have turned to diapers, maxi pads and other products to create masks. Social media channels are full of tips and sewing patterns. It’s an innovative strategy that is also contentious. Limited evidence suggests that homemade masks can offer some protection. But the DIY approach has also drawn criticism for providing a false sense of security, potentially putting wearers at risk.
The conflict points to an immediate need for more protective equipment, says Christopher Friese, PhD, RN, professor of nursing and public health at the University of Michigan, Ann Arbor. Also needed, he says, are new ideas for reducing strain on limited supplies, like adopting gear from other industries and finding innovative ways to provide care so that less protective gear is needed.
“We don’t want clinicians inventing and ‘MacGyvering’ their own device because we don’t want to put them at risk if we can avoid it,” says Friese, referring to the TV character who could build and assemble a vast array of tools/devices. “We have options that have been tested, and we have experience, maybe not in health care, but in other settings. We want to try that first before that frontline doctor, nurse, respiratory therapist decides to take matters into their own hands.
Increasingly, though, health care workers are finding they have no other choice — something even the CDC has acknowledged. In new guidelines, the agency recommends a bandanna, scarf, or other type of covering in cases where face masks are not available.
N95 respirators or surgical masks?
There are two main types of masks generally used in health care. N95 respirators filter out 95% of airborne particles, including bacteria and viruses. The lighter surgical or medical face masks are made to prevent spit and mucous from getting on patients or equipment.
Both types reduce rates of infection among health care workers, though comparisons (at least for influenza) have yet to show that one is superior to the other. One 2020 review by Chinese researchers, for example, analyzed six randomly controlled trials that included more than 9000 participants and found no added benefits of N95 masks over ordinary surgical masks for health care providers treating patients with the flu.
But COVID-19 is not influenza, and evidence suggests it may require more intensive protection, says Friese, who coauthored a blog post for JAMA about the country’s unpreparedness for protecting health care workers during a pandemic. The virus can linger in the air for hours, suggesting that N95 respirators are health care providers’ best option when treating infected patients.
The problem is there’s not enough to go around — of either mask type. In a March 5 survey, National Nurses United reported that just 30% of more than 6500 US respondents said their organizations had enough PPE to respond to a surge in patients. Another 38% did not know if their organizations were prepared. In a tweet, Friese estimated that 12% of nurses and other providers are at risk from reusing equipment or using equipment that is not backed by evidence.
Physicians and providers around the world have been sharing strategies online for how to make their own masks. Techniques vary, as do materials and plans for how to use the homemade equipment. At Phoebe Putney Health, DIY masks are intended to be worn over N95 respirators and then disposed of so that the respirators can be reused more safely, says Amanda Clements, the hospital’s public relations coordinator. Providers might also wear them to greet people at the front door.
Some evidence suggests that homemade masks can help in a pinch, at least for some illnesses. For a 2013 study by researchers in the UK, volunteers made surgical masks from cotton T-shirts, then put them on and coughed into a chamber that measured how much bacterial content got through. The team also assessed the aerosol-filtering ability of a variety of household materials, including scarfs, antimicrobial pillowcases, vacuum-cleaner bags, and tea towels. They tested each material with an aerosol containing two types of bacteria similar in size to influenza.
Commercial surgical masks performed three times better than homemade ones in the filtration test. Surgical masks worked twice as well at blocking droplets on the cough test. But all the makeshift materials — which also included silk, linen, and regular pillowcases — blocked some microbes. Vacuum-cleaner bags blocked the most bacteria, but their stiffness and thickness made them unsuitable for use as masks, the researchers reported. Tea towels showed a similar pattern. But pillowcases and cotton T-shirts were stretchy enough to fit well, thereby reducing the particles that could get through or around them.
Homemade masks should be used only as a last resort if commercial masks become unavailable, the researchers concluded. “Probably something is better than nothing for trained health care workers — for droplet contact avoidance, if nothing else,” says Anna Davies, BSc, a research facilitator at the University of Cambridge, UK, who is a former public health microbiologist and one of the study’s authors.
She recommends that members of the general public donate any stockpiles they have to health care workers, and make their own if they want masks for personal use. She is working with collaborators in the US to develop guidance for how best to do it.
“If people are quarantined and looking for something worthwhile to do, it probably wouldn’t be the worst thing to apply themselves to,” she wrote by email. “My suggestion would be for something soft and cotton, ideally with a bit of stretch (although it’s a pain to sew), and in two layers, marked ‘inside’ and ‘outside.’ ”
The idea that something is better than nothing was also the conclusion of a 2008 study by researchers in the Netherlands and the US. The study enlisted 28 healthy individuals who performed a variety of tasks while wearing N95 masks, surgical masks, or homemade masks sewn from teacloths. Effectiveness varied among individuals, but over a 90-second period, N95 masks worked best, with 25 times more protection than surgical masks and about 50 times more protection than homemade ones. Surgical masks were twice as effective as homemade masks. But the homemade masks offered at least some protection against large droplets.
Researchers emphasize that it’s not yet clear whether those findings are applicable to aerosolized COVID-19. In an influenza pandemic, at least, the authors posit that homemade masks could reduce transmission for the general public enough for some immunity to build. “It is important not to focus on a single intervention in case of a pandemic,” the researchers write, “but to integrate all effective interventions for optimal protection.”
For health care workers on the frontlines of COVID-19, Friese says, homemade masks might do more than nothing but they also might not work. Instead, he would rather see providers using construction or nuclear-engineering masks. And his best suggestion is something many providers are already doing: reducing physical contact with patients through telemedicine and other creative solutions, which is cutting down the overwhelming need for PPE.
Homemade mask production emphasizes the urgent need for more supplies, Friese adds.
“The government needs to step up and do a variety of things to increase production, and that needs to happen now, immediately,” he says. “We don’t we don’t want our clinicians to have to come up with these decisions.”
This article first appeared on Medscape.com.
Match Day 2020: Online announcements replace celebrations, champagne
The third Friday in March usually marks a time when medical students across the United States participate in envelope-opening ceremonies with peers and family members. This year, the ruthless onslaught of coronavirus has forced residency programs to rethink their celebrations, leveraging social media platforms and other technologies to toast Match Day in cyberspace.
In the absence of ceremonies taking place due to restrictions on mass gatherings, “we anticipate that students may be more emotional than they expect,” Hannah R. Hughes, MD, president of the Emergency Medicine Residents’ Association (EMRA) said in an interview. To support these students on their journey to residency, EMRA has launched a social media campaign, asking medical students “to share with us their envelope-opening moments – either a selfie, photo, or video – that we can share with our online networks,” Dr. Hughes said.
EMRA is also asking program coordinators to forward photos and congratulatory messages to their new residents “so that we can share them with our networks at large,” she added.
Going virtual, it seems, has become the new norm.
At the University of California, San Francisco, the medical school decided to cancel its Match Day celebration for new interns, echoing many other programs across the United States. “We always send out a welcome email and make phone calls to all of our new interns,” said Rebecca Berman, MD, director of UCSF’s internal medicine residency program, which houses 63 medicine interns and 181 residents. Traditionally, the program has hosted the celebration for current residents. That, of course, had to change this year.
Current interns like to join in the fun, “since it means their internship is rapidly coming to a close,” said Dr. Berman, who at press time was considering a virtual toast via Zoom as a possible alternative. “These are difficult times for everyone, and we are doing our best to make our residents feel united and connected while they take care of patients in the era of social distancing.”
Melissa Held, MD, associate dean of medical student affairs at the University of Connecticut’s School of Medicine, Farmington, had been planning a celebration in the school’s academic rotunda with food and champagne. “Students typically come with their family members or significant others. The dean and I usually say a few words and then at noon, students get envelopes and can open them to find out where they matched for residency,” Dr. Held said. This year, the school will be uploading Match letters to its online system. Students can remotely find out where they matched at noon. “I plan to put together a slide show of pictures and congratulatory remarks from faculty and staff that will be sent to them around 11:30 a.m.,” Dr. Held said.
Mark Miceli, EdD, who oversees Match Day for the 130-plus medical students at the University of Massachusetts Medical School, Worcester, is inviting faculty and staff to submit short videos of congratulations, which it will post on its student affairs Match Day Instagram account. Like other schools, it will share results with students in an email, said Dr. Miceli, assistant vice provost of student life. “This message will be more personalized to our school than the NRMP [National Resident Matching Program] message, and will also include links to our match stats, a map of our matched student locations, and a list of where folks matched,” he said.
Students can opt out of the list if they want to. The communications department has also provided templates for signs students can print out. “They can write in where they matched, and take pictures for social media. We are encouraging the use of various hashtags to help build a virtual community,” Dr. Miceli said.
In a state hit particularly hard by coronavirus, the University of Washington School of Medicine is spreading Match Day cheer through online meeting platforms and celebratory graphics. This five-state school, representing students from Washington, Wyoming, Alaska, Montana, and Idaho, usually hosts several events across the different states and students have their pick of which to attend, according to Sarah Wood, associate director of student affairs.
In lieu of in-person events, some states are hosting a Zoom online celebration, others are using social media networking systems. “We’re inviting everyone to take part in an online event ... where we’ll do a slide show of photos that one of our students put together,” Ms. Wood said.
Students are disappointed in this change of plans, she said. To make things more festive, Ms. Wood is adding graphics such as fireworks and photos to the emails containing the Match results. “I want this to be more exciting for them than just a basic letter,” she said.
For now, Ms. Wood is trying to focus on the Match Day celebration, but admits that “my bigger fear is if we have to cancel graduation – and what that might look like.”
The third Friday in March usually marks a time when medical students across the United States participate in envelope-opening ceremonies with peers and family members. This year, the ruthless onslaught of coronavirus has forced residency programs to rethink their celebrations, leveraging social media platforms and other technologies to toast Match Day in cyberspace.
In the absence of ceremonies taking place due to restrictions on mass gatherings, “we anticipate that students may be more emotional than they expect,” Hannah R. Hughes, MD, president of the Emergency Medicine Residents’ Association (EMRA) said in an interview. To support these students on their journey to residency, EMRA has launched a social media campaign, asking medical students “to share with us their envelope-opening moments – either a selfie, photo, or video – that we can share with our online networks,” Dr. Hughes said.
EMRA is also asking program coordinators to forward photos and congratulatory messages to their new residents “so that we can share them with our networks at large,” she added.
Going virtual, it seems, has become the new norm.
At the University of California, San Francisco, the medical school decided to cancel its Match Day celebration for new interns, echoing many other programs across the United States. “We always send out a welcome email and make phone calls to all of our new interns,” said Rebecca Berman, MD, director of UCSF’s internal medicine residency program, which houses 63 medicine interns and 181 residents. Traditionally, the program has hosted the celebration for current residents. That, of course, had to change this year.
Current interns like to join in the fun, “since it means their internship is rapidly coming to a close,” said Dr. Berman, who at press time was considering a virtual toast via Zoom as a possible alternative. “These are difficult times for everyone, and we are doing our best to make our residents feel united and connected while they take care of patients in the era of social distancing.”
Melissa Held, MD, associate dean of medical student affairs at the University of Connecticut’s School of Medicine, Farmington, had been planning a celebration in the school’s academic rotunda with food and champagne. “Students typically come with their family members or significant others. The dean and I usually say a few words and then at noon, students get envelopes and can open them to find out where they matched for residency,” Dr. Held said. This year, the school will be uploading Match letters to its online system. Students can remotely find out where they matched at noon. “I plan to put together a slide show of pictures and congratulatory remarks from faculty and staff that will be sent to them around 11:30 a.m.,” Dr. Held said.
Mark Miceli, EdD, who oversees Match Day for the 130-plus medical students at the University of Massachusetts Medical School, Worcester, is inviting faculty and staff to submit short videos of congratulations, which it will post on its student affairs Match Day Instagram account. Like other schools, it will share results with students in an email, said Dr. Miceli, assistant vice provost of student life. “This message will be more personalized to our school than the NRMP [National Resident Matching Program] message, and will also include links to our match stats, a map of our matched student locations, and a list of where folks matched,” he said.
Students can opt out of the list if they want to. The communications department has also provided templates for signs students can print out. “They can write in where they matched, and take pictures for social media. We are encouraging the use of various hashtags to help build a virtual community,” Dr. Miceli said.
In a state hit particularly hard by coronavirus, the University of Washington School of Medicine is spreading Match Day cheer through online meeting platforms and celebratory graphics. This five-state school, representing students from Washington, Wyoming, Alaska, Montana, and Idaho, usually hosts several events across the different states and students have their pick of which to attend, according to Sarah Wood, associate director of student affairs.
In lieu of in-person events, some states are hosting a Zoom online celebration, others are using social media networking systems. “We’re inviting everyone to take part in an online event ... where we’ll do a slide show of photos that one of our students put together,” Ms. Wood said.
Students are disappointed in this change of plans, she said. To make things more festive, Ms. Wood is adding graphics such as fireworks and photos to the emails containing the Match results. “I want this to be more exciting for them than just a basic letter,” she said.
For now, Ms. Wood is trying to focus on the Match Day celebration, but admits that “my bigger fear is if we have to cancel graduation – and what that might look like.”
The third Friday in March usually marks a time when medical students across the United States participate in envelope-opening ceremonies with peers and family members. This year, the ruthless onslaught of coronavirus has forced residency programs to rethink their celebrations, leveraging social media platforms and other technologies to toast Match Day in cyberspace.
In the absence of ceremonies taking place due to restrictions on mass gatherings, “we anticipate that students may be more emotional than they expect,” Hannah R. Hughes, MD, president of the Emergency Medicine Residents’ Association (EMRA) said in an interview. To support these students on their journey to residency, EMRA has launched a social media campaign, asking medical students “to share with us their envelope-opening moments – either a selfie, photo, or video – that we can share with our online networks,” Dr. Hughes said.
EMRA is also asking program coordinators to forward photos and congratulatory messages to their new residents “so that we can share them with our networks at large,” she added.
Going virtual, it seems, has become the new norm.
At the University of California, San Francisco, the medical school decided to cancel its Match Day celebration for new interns, echoing many other programs across the United States. “We always send out a welcome email and make phone calls to all of our new interns,” said Rebecca Berman, MD, director of UCSF’s internal medicine residency program, which houses 63 medicine interns and 181 residents. Traditionally, the program has hosted the celebration for current residents. That, of course, had to change this year.
Current interns like to join in the fun, “since it means their internship is rapidly coming to a close,” said Dr. Berman, who at press time was considering a virtual toast via Zoom as a possible alternative. “These are difficult times for everyone, and we are doing our best to make our residents feel united and connected while they take care of patients in the era of social distancing.”
Melissa Held, MD, associate dean of medical student affairs at the University of Connecticut’s School of Medicine, Farmington, had been planning a celebration in the school’s academic rotunda with food and champagne. “Students typically come with their family members or significant others. The dean and I usually say a few words and then at noon, students get envelopes and can open them to find out where they matched for residency,” Dr. Held said. This year, the school will be uploading Match letters to its online system. Students can remotely find out where they matched at noon. “I plan to put together a slide show of pictures and congratulatory remarks from faculty and staff that will be sent to them around 11:30 a.m.,” Dr. Held said.
Mark Miceli, EdD, who oversees Match Day for the 130-plus medical students at the University of Massachusetts Medical School, Worcester, is inviting faculty and staff to submit short videos of congratulations, which it will post on its student affairs Match Day Instagram account. Like other schools, it will share results with students in an email, said Dr. Miceli, assistant vice provost of student life. “This message will be more personalized to our school than the NRMP [National Resident Matching Program] message, and will also include links to our match stats, a map of our matched student locations, and a list of where folks matched,” he said.
Students can opt out of the list if they want to. The communications department has also provided templates for signs students can print out. “They can write in where they matched, and take pictures for social media. We are encouraging the use of various hashtags to help build a virtual community,” Dr. Miceli said.
In a state hit particularly hard by coronavirus, the University of Washington School of Medicine is spreading Match Day cheer through online meeting platforms and celebratory graphics. This five-state school, representing students from Washington, Wyoming, Alaska, Montana, and Idaho, usually hosts several events across the different states and students have their pick of which to attend, according to Sarah Wood, associate director of student affairs.
In lieu of in-person events, some states are hosting a Zoom online celebration, others are using social media networking systems. “We’re inviting everyone to take part in an online event ... where we’ll do a slide show of photos that one of our students put together,” Ms. Wood said.
Students are disappointed in this change of plans, she said. To make things more festive, Ms. Wood is adding graphics such as fireworks and photos to the emails containing the Match results. “I want this to be more exciting for them than just a basic letter,” she said.
For now, Ms. Wood is trying to focus on the Match Day celebration, but admits that “my bigger fear is if we have to cancel graduation – and what that might look like.”
Clinicians petition government for national quarantine
Clinicians across the United States are petitioning the federal government to follow the lead of South Korea, China, and other nations by imposing an immediate nationwide quarantine to slow the inevitable spread of COVID-19. Without federal action, the creators say, their lives and the lives of their colleagues, patients, and families are being put at increased risk.
In addition to the quarantine, the petition, posted on the website Change.org, calls on U.S. leaders to institute emergency production and distribution of personal protective equipment for healthcare workers and to rapidly increase access to testing.
The petition – which garnered more than 40,000 signatures in just 12 hours and as of this writing was approaching 94,000 – was started by an apolitical Facebook group to focus attention on what members see as the most critical issues for clinicians: slowing the spread of the virus through a coast-to-coast quarantine, protection of medical personnel with adequate supplies of essential equipment, and widespread testing.
“We started this group last Friday out of the realization that clinicians needed information about the outbreak and weren’t getting it,” said coadministrator Jessica McIntyre, MD, a pediatric hospitalist at Elliot Hospital in Manchester, N.H.
“We wanted to get ahead of it and connect with people before we were in the trenches experiencing it and to see what other programs were doing. From a local perspective, it has been really hard to see what people are doing in other states, especially when the protocols in our own states are changing every single day as we collect more information,” she said in an interview.
The Horse Has Bolted
A family medicine physician in Illinois helped launch the Facebook group. She asked that her name not be used but said in an interview that earlier actions may have prevented or at least delayed the need for the more draconian measures that her group is recommending.
“Clearly South Korea is one of the superstars as far as response has gone, but the concern we have in the United States is that we’re well beyond that point – we needed to be testing people over a month ago, in the hope of preventing a quarantine,” she said in an interview.
According to National Public Radio, as of March 13, South Korea had conducted 3,600 tests per million population, compared with five per million in the United States.
“I think the most concerning part is to see where Italy is now and where we are in comparison. Our ICUs have not yet overflowed, but I think we’re definitely looking at that in the next few weeks – hopefully longer, but I suspect that it will happen shortly,” she continued.
She cited work by Harvard University biostatistician Xihong Lin, PhD, that shows that when health authorities in Wuhan, China – widely cited as the epicenter of the global pandemic – cordoned off the city, the infection rate dropped from one person infecting 3.8 others to one infecting 1.25, thereby significantly slowing the rate of transmission.
“This is absolutely what we need to be doing,” she said.
Real News
Within 3 days of its creation, the online group had accrued more than 80,000 members with advanced medical training, including MDs, DOs, physician assistants, nurse practitioners, and certified registered nurse anesthetists.
“A lot of us were already very busy with our day-to-day work outside of COVID-19, and I think a lot of us felt unsure about where to get the best information,” said coadministrator David Janssen, MD, a family medicine physician in group practice in Sioux Center, Iowa,
“If you turn on the TV, there’s a lot of politicizing of the issue, and there’s a lot of good information, but also a lot of bad information. When health care providers talk to other health care providers, that’s often how we get our information and how we learn,” he said in an interview.
The COVID-19 U.S. Physicians/APP Facebook group includes 20 volunteer moderators who handle hundreds of posts per hour from persons seeking information on the novel coronavirus, what to tell patients, and how to protect themselves.
“It’s been wonderful to see how providers have been helping other providers sort through issues. Teaching hospitals have their hands on the latest research, but a lot of people like myself are at small community hospitals, critical-access hospitals, where we may have a lot of questions but don’t necessarily have the answers readily available to us,” Dr. Janssen said.
Dr. Janssen said that his community of about 8,000 residents initially had only four COVID-19 testing kits, or one for every 2,000 people. The situation has since improved, and more tests are now available, he added.
Dr. McIntyre, Dr. Janssen, and the Illinois family physician have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Clinicians across the United States are petitioning the federal government to follow the lead of South Korea, China, and other nations by imposing an immediate nationwide quarantine to slow the inevitable spread of COVID-19. Without federal action, the creators say, their lives and the lives of their colleagues, patients, and families are being put at increased risk.
In addition to the quarantine, the petition, posted on the website Change.org, calls on U.S. leaders to institute emergency production and distribution of personal protective equipment for healthcare workers and to rapidly increase access to testing.
The petition – which garnered more than 40,000 signatures in just 12 hours and as of this writing was approaching 94,000 – was started by an apolitical Facebook group to focus attention on what members see as the most critical issues for clinicians: slowing the spread of the virus through a coast-to-coast quarantine, protection of medical personnel with adequate supplies of essential equipment, and widespread testing.
“We started this group last Friday out of the realization that clinicians needed information about the outbreak and weren’t getting it,” said coadministrator Jessica McIntyre, MD, a pediatric hospitalist at Elliot Hospital in Manchester, N.H.
“We wanted to get ahead of it and connect with people before we were in the trenches experiencing it and to see what other programs were doing. From a local perspective, it has been really hard to see what people are doing in other states, especially when the protocols in our own states are changing every single day as we collect more information,” she said in an interview.
The Horse Has Bolted
A family medicine physician in Illinois helped launch the Facebook group. She asked that her name not be used but said in an interview that earlier actions may have prevented or at least delayed the need for the more draconian measures that her group is recommending.
“Clearly South Korea is one of the superstars as far as response has gone, but the concern we have in the United States is that we’re well beyond that point – we needed to be testing people over a month ago, in the hope of preventing a quarantine,” she said in an interview.
According to National Public Radio, as of March 13, South Korea had conducted 3,600 tests per million population, compared with five per million in the United States.
“I think the most concerning part is to see where Italy is now and where we are in comparison. Our ICUs have not yet overflowed, but I think we’re definitely looking at that in the next few weeks – hopefully longer, but I suspect that it will happen shortly,” she continued.
She cited work by Harvard University biostatistician Xihong Lin, PhD, that shows that when health authorities in Wuhan, China – widely cited as the epicenter of the global pandemic – cordoned off the city, the infection rate dropped from one person infecting 3.8 others to one infecting 1.25, thereby significantly slowing the rate of transmission.
“This is absolutely what we need to be doing,” she said.
Real News
Within 3 days of its creation, the online group had accrued more than 80,000 members with advanced medical training, including MDs, DOs, physician assistants, nurse practitioners, and certified registered nurse anesthetists.
“A lot of us were already very busy with our day-to-day work outside of COVID-19, and I think a lot of us felt unsure about where to get the best information,” said coadministrator David Janssen, MD, a family medicine physician in group practice in Sioux Center, Iowa,
“If you turn on the TV, there’s a lot of politicizing of the issue, and there’s a lot of good information, but also a lot of bad information. When health care providers talk to other health care providers, that’s often how we get our information and how we learn,” he said in an interview.
The COVID-19 U.S. Physicians/APP Facebook group includes 20 volunteer moderators who handle hundreds of posts per hour from persons seeking information on the novel coronavirus, what to tell patients, and how to protect themselves.
“It’s been wonderful to see how providers have been helping other providers sort through issues. Teaching hospitals have their hands on the latest research, but a lot of people like myself are at small community hospitals, critical-access hospitals, where we may have a lot of questions but don’t necessarily have the answers readily available to us,” Dr. Janssen said.
Dr. Janssen said that his community of about 8,000 residents initially had only four COVID-19 testing kits, or one for every 2,000 people. The situation has since improved, and more tests are now available, he added.
Dr. McIntyre, Dr. Janssen, and the Illinois family physician have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Clinicians across the United States are petitioning the federal government to follow the lead of South Korea, China, and other nations by imposing an immediate nationwide quarantine to slow the inevitable spread of COVID-19. Without federal action, the creators say, their lives and the lives of their colleagues, patients, and families are being put at increased risk.
In addition to the quarantine, the petition, posted on the website Change.org, calls on U.S. leaders to institute emergency production and distribution of personal protective equipment for healthcare workers and to rapidly increase access to testing.
The petition – which garnered more than 40,000 signatures in just 12 hours and as of this writing was approaching 94,000 – was started by an apolitical Facebook group to focus attention on what members see as the most critical issues for clinicians: slowing the spread of the virus through a coast-to-coast quarantine, protection of medical personnel with adequate supplies of essential equipment, and widespread testing.
“We started this group last Friday out of the realization that clinicians needed information about the outbreak and weren’t getting it,” said coadministrator Jessica McIntyre, MD, a pediatric hospitalist at Elliot Hospital in Manchester, N.H.
“We wanted to get ahead of it and connect with people before we were in the trenches experiencing it and to see what other programs were doing. From a local perspective, it has been really hard to see what people are doing in other states, especially when the protocols in our own states are changing every single day as we collect more information,” she said in an interview.
The Horse Has Bolted
A family medicine physician in Illinois helped launch the Facebook group. She asked that her name not be used but said in an interview that earlier actions may have prevented or at least delayed the need for the more draconian measures that her group is recommending.
“Clearly South Korea is one of the superstars as far as response has gone, but the concern we have in the United States is that we’re well beyond that point – we needed to be testing people over a month ago, in the hope of preventing a quarantine,” she said in an interview.
According to National Public Radio, as of March 13, South Korea had conducted 3,600 tests per million population, compared with five per million in the United States.
“I think the most concerning part is to see where Italy is now and where we are in comparison. Our ICUs have not yet overflowed, but I think we’re definitely looking at that in the next few weeks – hopefully longer, but I suspect that it will happen shortly,” she continued.
She cited work by Harvard University biostatistician Xihong Lin, PhD, that shows that when health authorities in Wuhan, China – widely cited as the epicenter of the global pandemic – cordoned off the city, the infection rate dropped from one person infecting 3.8 others to one infecting 1.25, thereby significantly slowing the rate of transmission.
“This is absolutely what we need to be doing,” she said.
Real News
Within 3 days of its creation, the online group had accrued more than 80,000 members with advanced medical training, including MDs, DOs, physician assistants, nurse practitioners, and certified registered nurse anesthetists.
“A lot of us were already very busy with our day-to-day work outside of COVID-19, and I think a lot of us felt unsure about where to get the best information,” said coadministrator David Janssen, MD, a family medicine physician in group practice in Sioux Center, Iowa,
“If you turn on the TV, there’s a lot of politicizing of the issue, and there’s a lot of good information, but also a lot of bad information. When health care providers talk to other health care providers, that’s often how we get our information and how we learn,” he said in an interview.
The COVID-19 U.S. Physicians/APP Facebook group includes 20 volunteer moderators who handle hundreds of posts per hour from persons seeking information on the novel coronavirus, what to tell patients, and how to protect themselves.
“It’s been wonderful to see how providers have been helping other providers sort through issues. Teaching hospitals have their hands on the latest research, but a lot of people like myself are at small community hospitals, critical-access hospitals, where we may have a lot of questions but don’t necessarily have the answers readily available to us,” Dr. Janssen said.
Dr. Janssen said that his community of about 8,000 residents initially had only four COVID-19 testing kits, or one for every 2,000 people. The situation has since improved, and more tests are now available, he added.
Dr. McIntyre, Dr. Janssen, and the Illinois family physician have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Physicians and health systems can reduce fear around COVID-19
A message from a Chief Wellness Officer
We are at a time, unfortunately, of significant public uncertainty and fear of “the coronavirus.” Mixed and inaccurate messages from national leaders in the setting of delayed testing availability have heightened fears and impeded a uniformity in responses, medical and preventive.
Despite this, physicians, nurses, and other health professionals across the country, and in many other countries, have been addressing the medical realities of this pandemic in a way that should make every one of us health professionals proud – from the Chinese doctors and nurses to the Italian intensivists and primary care physicians throughout many countries who have treated patients suffering from, or fearful of, a novel disease with uncertain transmission characteristics and unpredictable clinical outcomes.
It is now time for physicians and other health providers in the United States to step up to the plate and model appropriate transmission-reducing behavior for the general public. This will help reduce the overall morbidity and mortality associated with this pandemic and let us return to a more normal lifestyle as soon as possible. Physicians need to be reassuring but realistic, and there are concrete steps that we can take to demonstrate to the general public that there is a way forward.
First the basic facts. The United States does not have enough intensive care beds or ventilators to handle a major pandemic. We will also have insufficient physicians and nurses if many are quarantined. The tragic experience in Italy, where patients are dying from lack of ventilators, intensive care facilities, and staff, must not be repeated here.
Many health systems are canceling or reducing outpatient appointments and increasingly using video and other telehealth technologies, especially for assessing and triaging people who believe that they may have become infected and are relatively asymptomatic. While all of the disruptions may seem unsettling, they are actually good news for those of us in healthcare. Efforts to “flatten the curve” will slow the infection spread and help us better manage patients who become critical.
So, what can physicians do?
- Make sure you are getting good information about the situation. Access reliable information and data that are widely available through the Centers for Disease Control and Prevention, the National Institutes of Health, and the World Health Organization. Listen to professional news organizations, local and national. Pass this information to your patients and community.
- Obviously, when practicing clinically, follow all infection control protocols, which will inevitably change over time. Make it clear to your patients why you are following these protocols and procedures.
- Support and actively promote the public health responses to this pandemic. Systematic reviews of the evidence base have found that isolating ill persons, testing and tracing contacts, quarantining exposed persons, closing schools and workplaces, and avoiding crowding are more effective if implemented immediately, simultaneously (ie, school closures combined with teleworking for parents), and with high community compliance.
- Practice social distancing so that you remain as much in control as you can. This will make you feel psychologically better and safer, as well as reduce the risk for transmission. Take the essential precautionary measures that we are all being asked to take. Wash your hands. Do not shake hands. Clean shared items. Do not go to large public gatherings. Minimize large group travel as much as you can. Use video to see your patients or your own doctor.
- Connect and reconnect with people you trust and love. See your family, your partner, your children, your friends. Speak to them on the phone and nourish those relationships. See how they feel and care for each other. They will be worried about you. Reassure them. Be in the moment with them and use the importance of these relationships to give yourself a chance not to overthink any fears you might have.
- Look after yourself physically. Physical fitness is good for your mental health. While White House guidelines suggest avoiding gyms, you can still enjoy long walks and outdoor activities. Take the weekend off and don’t work excessively. Sleep well – at least 7-8 hours. which has a series of really excellent meditation and relaxation tools.
- Do not panic. Uncertainty surrounding the pandemic makes all of us anxious and afraid. It is normal to become hypervigilant, especially with our nonstop media. It is normal to be concerned when we feel out of control and when we are hearing about a possible future catastrophe, especially when fed with differing sets of information from multiple sources and countries.
- Be careful with any large decisions you are making that may affect the lives of yourself and your loved ones. Think about your decisions and try to take the long view; and run them by your spouse, partner, or friends. This is not a time to be making sudden big decisions that may be driven unconsciously, in part at least, by fear and anxiety.
- Realize that all of these societal disruptions are actually good for us in health care, and they help your family and friends understand the importance of slowing the disease’s spread. That’s good for health care and good for everyone.
Finally, remember that “this is what we do,” to quote Doug Kirk, MD, chief medical officer of UC Davis Health. We must look after our patients. But we also have to look after ourselves so that we can look after our patients. We should all be proud of our work and our caring. And we should model our personal behavior to our patients and to our families and friends so that they will model it to their community networks. That way, more people will keep well, and we will have more chance of “flattening the curve” and reducing the morbidity and mortality associated with COVID-19.
Peter M. Yellowlees, MBBS, MD, is a professor in the Department of Psychiatry at the University of California, Davis. He is a longtime Medscape contributor.
This article first appeared on Medscape.com.
A message from a Chief Wellness Officer
We are at a time, unfortunately, of significant public uncertainty and fear of “the coronavirus.” Mixed and inaccurate messages from national leaders in the setting of delayed testing availability have heightened fears and impeded a uniformity in responses, medical and preventive.
Despite this, physicians, nurses, and other health professionals across the country, and in many other countries, have been addressing the medical realities of this pandemic in a way that should make every one of us health professionals proud – from the Chinese doctors and nurses to the Italian intensivists and primary care physicians throughout many countries who have treated patients suffering from, or fearful of, a novel disease with uncertain transmission characteristics and unpredictable clinical outcomes.
It is now time for physicians and other health providers in the United States to step up to the plate and model appropriate transmission-reducing behavior for the general public. This will help reduce the overall morbidity and mortality associated with this pandemic and let us return to a more normal lifestyle as soon as possible. Physicians need to be reassuring but realistic, and there are concrete steps that we can take to demonstrate to the general public that there is a way forward.
First the basic facts. The United States does not have enough intensive care beds or ventilators to handle a major pandemic. We will also have insufficient physicians and nurses if many are quarantined. The tragic experience in Italy, where patients are dying from lack of ventilators, intensive care facilities, and staff, must not be repeated here.
Many health systems are canceling or reducing outpatient appointments and increasingly using video and other telehealth technologies, especially for assessing and triaging people who believe that they may have become infected and are relatively asymptomatic. While all of the disruptions may seem unsettling, they are actually good news for those of us in healthcare. Efforts to “flatten the curve” will slow the infection spread and help us better manage patients who become critical.
So, what can physicians do?
- Make sure you are getting good information about the situation. Access reliable information and data that are widely available through the Centers for Disease Control and Prevention, the National Institutes of Health, and the World Health Organization. Listen to professional news organizations, local and national. Pass this information to your patients and community.
- Obviously, when practicing clinically, follow all infection control protocols, which will inevitably change over time. Make it clear to your patients why you are following these protocols and procedures.
- Support and actively promote the public health responses to this pandemic. Systematic reviews of the evidence base have found that isolating ill persons, testing and tracing contacts, quarantining exposed persons, closing schools and workplaces, and avoiding crowding are more effective if implemented immediately, simultaneously (ie, school closures combined with teleworking for parents), and with high community compliance.
- Practice social distancing so that you remain as much in control as you can. This will make you feel psychologically better and safer, as well as reduce the risk for transmission. Take the essential precautionary measures that we are all being asked to take. Wash your hands. Do not shake hands. Clean shared items. Do not go to large public gatherings. Minimize large group travel as much as you can. Use video to see your patients or your own doctor.
- Connect and reconnect with people you trust and love. See your family, your partner, your children, your friends. Speak to them on the phone and nourish those relationships. See how they feel and care for each other. They will be worried about you. Reassure them. Be in the moment with them and use the importance of these relationships to give yourself a chance not to overthink any fears you might have.
- Look after yourself physically. Physical fitness is good for your mental health. While White House guidelines suggest avoiding gyms, you can still enjoy long walks and outdoor activities. Take the weekend off and don’t work excessively. Sleep well – at least 7-8 hours. which has a series of really excellent meditation and relaxation tools.
- Do not panic. Uncertainty surrounding the pandemic makes all of us anxious and afraid. It is normal to become hypervigilant, especially with our nonstop media. It is normal to be concerned when we feel out of control and when we are hearing about a possible future catastrophe, especially when fed with differing sets of information from multiple sources and countries.
- Be careful with any large decisions you are making that may affect the lives of yourself and your loved ones. Think about your decisions and try to take the long view; and run them by your spouse, partner, or friends. This is not a time to be making sudden big decisions that may be driven unconsciously, in part at least, by fear and anxiety.
- Realize that all of these societal disruptions are actually good for us in health care, and they help your family and friends understand the importance of slowing the disease’s spread. That’s good for health care and good for everyone.
Finally, remember that “this is what we do,” to quote Doug Kirk, MD, chief medical officer of UC Davis Health. We must look after our patients. But we also have to look after ourselves so that we can look after our patients. We should all be proud of our work and our caring. And we should model our personal behavior to our patients and to our families and friends so that they will model it to their community networks. That way, more people will keep well, and we will have more chance of “flattening the curve” and reducing the morbidity and mortality associated with COVID-19.
Peter M. Yellowlees, MBBS, MD, is a professor in the Department of Psychiatry at the University of California, Davis. He is a longtime Medscape contributor.
This article first appeared on Medscape.com.
A message from a Chief Wellness Officer
We are at a time, unfortunately, of significant public uncertainty and fear of “the coronavirus.” Mixed and inaccurate messages from national leaders in the setting of delayed testing availability have heightened fears and impeded a uniformity in responses, medical and preventive.
Despite this, physicians, nurses, and other health professionals across the country, and in many other countries, have been addressing the medical realities of this pandemic in a way that should make every one of us health professionals proud – from the Chinese doctors and nurses to the Italian intensivists and primary care physicians throughout many countries who have treated patients suffering from, or fearful of, a novel disease with uncertain transmission characteristics and unpredictable clinical outcomes.
It is now time for physicians and other health providers in the United States to step up to the plate and model appropriate transmission-reducing behavior for the general public. This will help reduce the overall morbidity and mortality associated with this pandemic and let us return to a more normal lifestyle as soon as possible. Physicians need to be reassuring but realistic, and there are concrete steps that we can take to demonstrate to the general public that there is a way forward.
First the basic facts. The United States does not have enough intensive care beds or ventilators to handle a major pandemic. We will also have insufficient physicians and nurses if many are quarantined. The tragic experience in Italy, where patients are dying from lack of ventilators, intensive care facilities, and staff, must not be repeated here.
Many health systems are canceling or reducing outpatient appointments and increasingly using video and other telehealth technologies, especially for assessing and triaging people who believe that they may have become infected and are relatively asymptomatic. While all of the disruptions may seem unsettling, they are actually good news for those of us in healthcare. Efforts to “flatten the curve” will slow the infection spread and help us better manage patients who become critical.
So, what can physicians do?
- Make sure you are getting good information about the situation. Access reliable information and data that are widely available through the Centers for Disease Control and Prevention, the National Institutes of Health, and the World Health Organization. Listen to professional news organizations, local and national. Pass this information to your patients and community.
- Obviously, when practicing clinically, follow all infection control protocols, which will inevitably change over time. Make it clear to your patients why you are following these protocols and procedures.
- Support and actively promote the public health responses to this pandemic. Systematic reviews of the evidence base have found that isolating ill persons, testing and tracing contacts, quarantining exposed persons, closing schools and workplaces, and avoiding crowding are more effective if implemented immediately, simultaneously (ie, school closures combined with teleworking for parents), and with high community compliance.
- Practice social distancing so that you remain as much in control as you can. This will make you feel psychologically better and safer, as well as reduce the risk for transmission. Take the essential precautionary measures that we are all being asked to take. Wash your hands. Do not shake hands. Clean shared items. Do not go to large public gatherings. Minimize large group travel as much as you can. Use video to see your patients or your own doctor.
- Connect and reconnect with people you trust and love. See your family, your partner, your children, your friends. Speak to them on the phone and nourish those relationships. See how they feel and care for each other. They will be worried about you. Reassure them. Be in the moment with them and use the importance of these relationships to give yourself a chance not to overthink any fears you might have.
- Look after yourself physically. Physical fitness is good for your mental health. While White House guidelines suggest avoiding gyms, you can still enjoy long walks and outdoor activities. Take the weekend off and don’t work excessively. Sleep well – at least 7-8 hours. which has a series of really excellent meditation and relaxation tools.
- Do not panic. Uncertainty surrounding the pandemic makes all of us anxious and afraid. It is normal to become hypervigilant, especially with our nonstop media. It is normal to be concerned when we feel out of control and when we are hearing about a possible future catastrophe, especially when fed with differing sets of information from multiple sources and countries.
- Be careful with any large decisions you are making that may affect the lives of yourself and your loved ones. Think about your decisions and try to take the long view; and run them by your spouse, partner, or friends. This is not a time to be making sudden big decisions that may be driven unconsciously, in part at least, by fear and anxiety.
- Realize that all of these societal disruptions are actually good for us in health care, and they help your family and friends understand the importance of slowing the disease’s spread. That’s good for health care and good for everyone.
Finally, remember that “this is what we do,” to quote Doug Kirk, MD, chief medical officer of UC Davis Health. We must look after our patients. But we also have to look after ourselves so that we can look after our patients. We should all be proud of our work and our caring. And we should model our personal behavior to our patients and to our families and friends so that they will model it to their community networks. That way, more people will keep well, and we will have more chance of “flattening the curve” and reducing the morbidity and mortality associated with COVID-19.
Peter M. Yellowlees, MBBS, MD, is a professor in the Department of Psychiatry at the University of California, Davis. He is a longtime Medscape contributor.
This article first appeared on Medscape.com.
Handoffs in Dermatology Residency
As a dermatologist, there are innumerable items to track after each patient encounter, such as results from biopsies, laboratory tests, cultures, and imaging, as well as ensuring follow-up with providers in other specialties. In residency, there is the complicating factor of switching rotations and therefore transitioning care to different providers (Figure). Ensuring organized handoff practices is especially important in residency. In a study of malpractice claims involving residents, handoff problems were a notable contributing factor in 19% of malpractice cases involving residents vs 13% of cases involving attending physicians.1 There still is a high percentage of malpractice cases involving handoff problems among attending physicians, highlighting the fact that these issues persist beyond residency.

This article will review a variety of handoff and organizational practices that dermatology residents currently use, discuss the evidence behind best practices, and highlight additional considerations relevant when selecting organizational tools.
Varied Practices
Based on personal discussions with residents from 7 dermatology residency programs across the country, there is marked variability in both the frequency of handoffs and organizational methods utilized. Two major factors that dictate these practices are the structure of the residency program and electronic health record (EHR) capacities.
Program structure and allocation of resident responsibilities affect the frequency of handoffs in the outpatient dermatology residency setting. In some programs, residents are responsible for all pending studies for patients they have seen, even after switching clinical sites. In other programs, residents sign out patients, including pending test results, when transitioning from one clinical rotation to another. The frequency of these handoffs varies, ranging from every few weeks to every 4 months.
Many dermatology residents report utilizing features in the EHR to organize outstanding tasks and results, obviating the need for additional documentation. Some EHRs have the capacity to assign proxies, which allows for a seamless transition to another provider. When the EHR lacks these capabilities, organization of outstanding tasks relies more heavily on supplemental documentation. Residents noted using spreadsheets, typed documents, electronic applications designed to organize handoffs outside of the EHR, and handwritten notes.
There is room for formal education on the best handoff and organizational practices in dermatology residency. A study of anesthesiology residents at a major academic institution suggested that education regarding sign-out practices is most effective when it is multimodal, using both formal and informal methods.2 Based on my discussions with other dermatology residents, these practices generally are informally learned; often, dermatology residents did not realize that organization practices varied so widely at other institutions.
Evidence Behind Handoff Practices
There are data in the dermatology literature to support utilizing electronic means for handoff practices. At a tertiary dermatology department in Melbourne, Australia, providers created a novel electronic handover system using Microsoft programs to be used alongside the main hospital EHR to help practitioners keep track of outpatient studies.3 An audit of this system demonstrated that its use provided a reliable system for follow-up on all outpatient results, with benefits in clinical, organizational, and health research domains.4 The investigators noted that residents, registrars, nurses, and consultants utilized the electronic handover system, with residents completing 90% of all tasks.3 Similarly, several residents I spoke with personally cited using Listrunner (www.listrunnerapp.com), a Health Insurance Portability and Accountability Act–compliant electronic tool outside of the EHR designed for collaborative management of patient lists.
Outside of the dermatology literature, resident handoff in the outpatient setting mainly has been studied in the primary care year-end transition of care, with findings that are certainly relevant to dermatology residency. Pincavage et al5 performed a targeted literature search on year-end handoff practices, and Donnelly et al6 studied internal medicine residents in an outpatient ambulatory clinic; both supported implementing a standardized process for sign-out. Pincavage et al5 also recommended focusing on high-risk patients, educating residents on handoff practices, preparing patients for the transition, and performing safety audits. Donnelly et al6 found that providing time dedicated to patient handoff and clear expectations improved handoff practices.
There is extensive literature on handoff practices in the inpatient setting sparked by an increasing number of handoffs after the implementation of Accreditation Council for Graduate Medical Education duty hour restrictions in 1989. Some of the guiding principles may be applied to the outpatient dermatology setting. Many residents may be familiar with mnemonics that have been developed to organize content during sign-out, which have been s
Other Considerations
An important consideration during patient handoffs is security, especially when implementing documentation and tools outside of the EHR. It is important for providers to be compliant with institutional policies as well as the Health Insurance Portability and Accountability Act and ensure protection against cyberattacks, which have been on the rise; 83% of 1300 physicians surveyed have been the victim of a cyberattack.9 Providers also should be mindful of redundancies in organizational and handoff practices. Multiple methods for keeping track of information helps ensure that important results do not fall through the cracks. However, too many redundancies may be wasteful of a practice’s resources and providers’ time.
Final Thoughts
There are varied practices regarding organization of handoff and follow-up. Residency should serve as an opportunity for physicians to become familiar with different practices. Becoming familiar with the varied options may be helpful to take forward in one’s career, especially given that dermatologists may enter a work setting postresidency with practices that are different from where they trained. Additionally, given rapid shifts in technologies, providers must change how they stay organized. This evolving landscape provides an opportunity for the next generation of dermatologists to take leadership to shape the future of organizational practices.
- Singh H, Thomas EJ, Petersen LA, et al. Medical errors involving trainees: a study of closed malpractice claims from 5 insurers. Arch Intern Med. 2007;167:2030-2036.
- Muralidharan M, Clapp JT, Pulos BP, et al. How does training in anesthesia residency shape residents’ approaches to patient care handoffs? a single-center qualitative interview study. BMC Med Educ. 2018;18:271.
- Poon F, Martyres R, Denahy A, et al. Improving patient safety: the impact of an outpatients’ electronic handover system in a tertiary dermatology department. Australas J Dermatol. 2018;59:E183-E188.
- Listrunner website. https://www.listrunnerapp.com. Accessed January 30, 2020.
- Pincavage AT, Donnelly MJ, Young JQ, et al. Year-end resident clinic handoffs: narrative review and recommendations for improvement. Jt Comm J Qual Patient Saf. 2017;43:71-79.
- Donnelly MJ, Clauser JM, Weissman NJ. An intervention to improve ambulatory care handoffs at the end of residency. J Grad Med Educ. 2012;4:381-384.
- Vidyarthi AR, Arora V, Schnipper JL, et al. Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign-out. J Hosp Med. 2006;1:257-266.
- Breaux J, Mclendon R, Stedman RB, et al. Developing a standardized and sustainable resident sign-out process: an AIAMC National Initiative IV Project. Ochsner J. 2014;14:563-568.
- American Medical Association and Accenture. Taking the physician’s pulse: tackling cyber threats in healthcare. https://www.accenture.com/_acnmedia/accenture/conversion-assets/dotcom/documents/local/en/accenture-health-taking-the-physicians-pulse.pdf. Accessed January 30, 2020.
As a dermatologist, there are innumerable items to track after each patient encounter, such as results from biopsies, laboratory tests, cultures, and imaging, as well as ensuring follow-up with providers in other specialties. In residency, there is the complicating factor of switching rotations and therefore transitioning care to different providers (Figure). Ensuring organized handoff practices is especially important in residency. In a study of malpractice claims involving residents, handoff problems were a notable contributing factor in 19% of malpractice cases involving residents vs 13% of cases involving attending physicians.1 There still is a high percentage of malpractice cases involving handoff problems among attending physicians, highlighting the fact that these issues persist beyond residency.

This article will review a variety of handoff and organizational practices that dermatology residents currently use, discuss the evidence behind best practices, and highlight additional considerations relevant when selecting organizational tools.
Varied Practices
Based on personal discussions with residents from 7 dermatology residency programs across the country, there is marked variability in both the frequency of handoffs and organizational methods utilized. Two major factors that dictate these practices are the structure of the residency program and electronic health record (EHR) capacities.
Program structure and allocation of resident responsibilities affect the frequency of handoffs in the outpatient dermatology residency setting. In some programs, residents are responsible for all pending studies for patients they have seen, even after switching clinical sites. In other programs, residents sign out patients, including pending test results, when transitioning from one clinical rotation to another. The frequency of these handoffs varies, ranging from every few weeks to every 4 months.
Many dermatology residents report utilizing features in the EHR to organize outstanding tasks and results, obviating the need for additional documentation. Some EHRs have the capacity to assign proxies, which allows for a seamless transition to another provider. When the EHR lacks these capabilities, organization of outstanding tasks relies more heavily on supplemental documentation. Residents noted using spreadsheets, typed documents, electronic applications designed to organize handoffs outside of the EHR, and handwritten notes.
There is room for formal education on the best handoff and organizational practices in dermatology residency. A study of anesthesiology residents at a major academic institution suggested that education regarding sign-out practices is most effective when it is multimodal, using both formal and informal methods.2 Based on my discussions with other dermatology residents, these practices generally are informally learned; often, dermatology residents did not realize that organization practices varied so widely at other institutions.
Evidence Behind Handoff Practices
There are data in the dermatology literature to support utilizing electronic means for handoff practices. At a tertiary dermatology department in Melbourne, Australia, providers created a novel electronic handover system using Microsoft programs to be used alongside the main hospital EHR to help practitioners keep track of outpatient studies.3 An audit of this system demonstrated that its use provided a reliable system for follow-up on all outpatient results, with benefits in clinical, organizational, and health research domains.4 The investigators noted that residents, registrars, nurses, and consultants utilized the electronic handover system, with residents completing 90% of all tasks.3 Similarly, several residents I spoke with personally cited using Listrunner (www.listrunnerapp.com), a Health Insurance Portability and Accountability Act–compliant electronic tool outside of the EHR designed for collaborative management of patient lists.
Outside of the dermatology literature, resident handoff in the outpatient setting mainly has been studied in the primary care year-end transition of care, with findings that are certainly relevant to dermatology residency. Pincavage et al5 performed a targeted literature search on year-end handoff practices, and Donnelly et al6 studied internal medicine residents in an outpatient ambulatory clinic; both supported implementing a standardized process for sign-out. Pincavage et al5 also recommended focusing on high-risk patients, educating residents on handoff practices, preparing patients for the transition, and performing safety audits. Donnelly et al6 found that providing time dedicated to patient handoff and clear expectations improved handoff practices.
There is extensive literature on handoff practices in the inpatient setting sparked by an increasing number of handoffs after the implementation of Accreditation Council for Graduate Medical Education duty hour restrictions in 1989. Some of the guiding principles may be applied to the outpatient dermatology setting. Many residents may be familiar with mnemonics that have been developed to organize content during sign-out, which have been s

Other Considerations
An important consideration during patient handoffs is security, especially when implementing documentation and tools outside of the EHR. It is important for providers to be compliant with institutional policies as well as the Health Insurance Portability and Accountability Act and ensure protection against cyberattacks, which have been on the rise; 83% of 1300 physicians surveyed have been the victim of a cyberattack.9 Providers also should be mindful of redundancies in organizational and handoff practices. Multiple methods for keeping track of information helps ensure that important results do not fall through the cracks. However, too many redundancies may be wasteful of a practice’s resources and providers’ time.
Final Thoughts
There are varied practices regarding organization of handoff and follow-up. Residency should serve as an opportunity for physicians to become familiar with different practices. Becoming familiar with the varied options may be helpful to take forward in one’s career, especially given that dermatologists may enter a work setting postresidency with practices that are different from where they trained. Additionally, given rapid shifts in technologies, providers must change how they stay organized. This evolving landscape provides an opportunity for the next generation of dermatologists to take leadership to shape the future of organizational practices.
As a dermatologist, there are innumerable items to track after each patient encounter, such as results from biopsies, laboratory tests, cultures, and imaging, as well as ensuring follow-up with providers in other specialties. In residency, there is the complicating factor of switching rotations and therefore transitioning care to different providers (Figure). Ensuring organized handoff practices is especially important in residency. In a study of malpractice claims involving residents, handoff problems were a notable contributing factor in 19% of malpractice cases involving residents vs 13% of cases involving attending physicians.1 There still is a high percentage of malpractice cases involving handoff problems among attending physicians, highlighting the fact that these issues persist beyond residency.

This article will review a variety of handoff and organizational practices that dermatology residents currently use, discuss the evidence behind best practices, and highlight additional considerations relevant when selecting organizational tools.
Varied Practices
Based on personal discussions with residents from 7 dermatology residency programs across the country, there is marked variability in both the frequency of handoffs and organizational methods utilized. Two major factors that dictate these practices are the structure of the residency program and electronic health record (EHR) capacities.
Program structure and allocation of resident responsibilities affect the frequency of handoffs in the outpatient dermatology residency setting. In some programs, residents are responsible for all pending studies for patients they have seen, even after switching clinical sites. In other programs, residents sign out patients, including pending test results, when transitioning from one clinical rotation to another. The frequency of these handoffs varies, ranging from every few weeks to every 4 months.
Many dermatology residents report utilizing features in the EHR to organize outstanding tasks and results, obviating the need for additional documentation. Some EHRs have the capacity to assign proxies, which allows for a seamless transition to another provider. When the EHR lacks these capabilities, organization of outstanding tasks relies more heavily on supplemental documentation. Residents noted using spreadsheets, typed documents, electronic applications designed to organize handoffs outside of the EHR, and handwritten notes.
There is room for formal education on the best handoff and organizational practices in dermatology residency. A study of anesthesiology residents at a major academic institution suggested that education regarding sign-out practices is most effective when it is multimodal, using both formal and informal methods.2 Based on my discussions with other dermatology residents, these practices generally are informally learned; often, dermatology residents did not realize that organization practices varied so widely at other institutions.
Evidence Behind Handoff Practices
There are data in the dermatology literature to support utilizing electronic means for handoff practices. At a tertiary dermatology department in Melbourne, Australia, providers created a novel electronic handover system using Microsoft programs to be used alongside the main hospital EHR to help practitioners keep track of outpatient studies.3 An audit of this system demonstrated that its use provided a reliable system for follow-up on all outpatient results, with benefits in clinical, organizational, and health research domains.4 The investigators noted that residents, registrars, nurses, and consultants utilized the electronic handover system, with residents completing 90% of all tasks.3 Similarly, several residents I spoke with personally cited using Listrunner (www.listrunnerapp.com), a Health Insurance Portability and Accountability Act–compliant electronic tool outside of the EHR designed for collaborative management of patient lists.
Outside of the dermatology literature, resident handoff in the outpatient setting mainly has been studied in the primary care year-end transition of care, with findings that are certainly relevant to dermatology residency. Pincavage et al5 performed a targeted literature search on year-end handoff practices, and Donnelly et al6 studied internal medicine residents in an outpatient ambulatory clinic; both supported implementing a standardized process for sign-out. Pincavage et al5 also recommended focusing on high-risk patients, educating residents on handoff practices, preparing patients for the transition, and performing safety audits. Donnelly et al6 found that providing time dedicated to patient handoff and clear expectations improved handoff practices.
There is extensive literature on handoff practices in the inpatient setting sparked by an increasing number of handoffs after the implementation of Accreditation Council for Graduate Medical Education duty hour restrictions in 1989. Some of the guiding principles may be applied to the outpatient dermatology setting. Many residents may be familiar with mnemonics that have been developed to organize content during sign-out, which have been s

Other Considerations
An important consideration during patient handoffs is security, especially when implementing documentation and tools outside of the EHR. It is important for providers to be compliant with institutional policies as well as the Health Insurance Portability and Accountability Act and ensure protection against cyberattacks, which have been on the rise; 83% of 1300 physicians surveyed have been the victim of a cyberattack.9 Providers also should be mindful of redundancies in organizational and handoff practices. Multiple methods for keeping track of information helps ensure that important results do not fall through the cracks. However, too many redundancies may be wasteful of a practice’s resources and providers’ time.
Final Thoughts
There are varied practices regarding organization of handoff and follow-up. Residency should serve as an opportunity for physicians to become familiar with different practices. Becoming familiar with the varied options may be helpful to take forward in one’s career, especially given that dermatologists may enter a work setting postresidency with practices that are different from where they trained. Additionally, given rapid shifts in technologies, providers must change how they stay organized. This evolving landscape provides an opportunity for the next generation of dermatologists to take leadership to shape the future of organizational practices.
- Singh H, Thomas EJ, Petersen LA, et al. Medical errors involving trainees: a study of closed malpractice claims from 5 insurers. Arch Intern Med. 2007;167:2030-2036.
- Muralidharan M, Clapp JT, Pulos BP, et al. How does training in anesthesia residency shape residents’ approaches to patient care handoffs? a single-center qualitative interview study. BMC Med Educ. 2018;18:271.
- Poon F, Martyres R, Denahy A, et al. Improving patient safety: the impact of an outpatients’ electronic handover system in a tertiary dermatology department. Australas J Dermatol. 2018;59:E183-E188.
- Listrunner website. https://www.listrunnerapp.com. Accessed January 30, 2020.
- Pincavage AT, Donnelly MJ, Young JQ, et al. Year-end resident clinic handoffs: narrative review and recommendations for improvement. Jt Comm J Qual Patient Saf. 2017;43:71-79.
- Donnelly MJ, Clauser JM, Weissman NJ. An intervention to improve ambulatory care handoffs at the end of residency. J Grad Med Educ. 2012;4:381-384.
- Vidyarthi AR, Arora V, Schnipper JL, et al. Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign-out. J Hosp Med. 2006;1:257-266.
- Breaux J, Mclendon R, Stedman RB, et al. Developing a standardized and sustainable resident sign-out process: an AIAMC National Initiative IV Project. Ochsner J. 2014;14:563-568.
- American Medical Association and Accenture. Taking the physician’s pulse: tackling cyber threats in healthcare. https://www.accenture.com/_acnmedia/accenture/conversion-assets/dotcom/documents/local/en/accenture-health-taking-the-physicians-pulse.pdf. Accessed January 30, 2020.
- Singh H, Thomas EJ, Petersen LA, et al. Medical errors involving trainees: a study of closed malpractice claims from 5 insurers. Arch Intern Med. 2007;167:2030-2036.
- Muralidharan M, Clapp JT, Pulos BP, et al. How does training in anesthesia residency shape residents’ approaches to patient care handoffs? a single-center qualitative interview study. BMC Med Educ. 2018;18:271.
- Poon F, Martyres R, Denahy A, et al. Improving patient safety: the impact of an outpatients’ electronic handover system in a tertiary dermatology department. Australas J Dermatol. 2018;59:E183-E188.
- Listrunner website. https://www.listrunnerapp.com. Accessed January 30, 2020.
- Pincavage AT, Donnelly MJ, Young JQ, et al. Year-end resident clinic handoffs: narrative review and recommendations for improvement. Jt Comm J Qual Patient Saf. 2017;43:71-79.
- Donnelly MJ, Clauser JM, Weissman NJ. An intervention to improve ambulatory care handoffs at the end of residency. J Grad Med Educ. 2012;4:381-384.
- Vidyarthi AR, Arora V, Schnipper JL, et al. Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign-out. J Hosp Med. 2006;1:257-266.
- Breaux J, Mclendon R, Stedman RB, et al. Developing a standardized and sustainable resident sign-out process: an AIAMC National Initiative IV Project. Ochsner J. 2014;14:563-568.
- American Medical Association and Accenture. Taking the physician’s pulse: tackling cyber threats in healthcare. https://www.accenture.com/_acnmedia/accenture/conversion-assets/dotcom/documents/local/en/accenture-health-taking-the-physicians-pulse.pdf. Accessed January 30, 2020.
Resident Pearl
- For dermatology residents, ensuring organized handoff and follow-up practices is essential. Residency provides an opportunity to become familiar with different practices to take forward in one’s career.
Ketamine and serotonin syndrome: A case report
Long utilized as a rapid anesthetic, ketamine has been increasingly used in sub-anesthetic doses for several psychiatric indications, including depression, suicidality, and chronic pain. Recently, an intranasal form of esketamine—the S-enantiomer of ketamine—was FDA-approved for treatment-resistant depression. Previously, researchers believed ketamine mediated its analgesic and psychotropic effects solely via N-methyl-
CASE REPORT
Ms. O, age 41, has a history of endometriosis, anticardiolipin antibody syndrome, major depressive disorder, and generalized anxiety disorder. She initially presented to an outside hospital and was admitted for chronic endometriosis pain. During that admission, her pain was treated with IV ketamine, 40 mg/hour, on hospital Days 1 through 4. While hospitalized, she continued to receive her home medications: fluoxetine, 40 mg/d, coumadin, 5 mg/d, and diphenhydramine, 25 mg/d. On Day 5, Ms. O experienced visual hallucinations and was diagnosed with ketamine-induced delirium. She was treated with haloperidol (dose unknown) with reportedly good effect. On Day 7, she was discharged home.
Upon returning home, she experienced persistent altered mental status. Her significant other brought her to our hospital for further workup. Ms. O’s body temperature was 37.6°C, and she was diaphoretic. Her blood pressure was 154/100 mm Hg, and her heart rate was 125 bpm. On physical examination, she had 4+ patellar and Achilles reflexes with left ankle clonus and crossed adductors. Her mental status exam showed increased latency of thought and speech, with bizarre affect as evidenced by illogical mannerisms and appearance. She said she was “not feeling myself” and would stare at walls for prolonged periods of time, appearing internally preoccupied and confused.
Ms. O was treated with IV lorazepam, 2 mg. Fourteen hours later, her temperature returned to normal, but she remained tachycardic, hypertensive, and altered. She received 2 additional doses of 2 mg and 1 mg. Seventeen hours after the initial dose of IV lorazepam was administered (and 3 hours after the second dose), Ms. O’s heart rate returned to normal. She was ultimately converted to oral lorazepam, 1 mg every 12 hours. Two hours later, Ms. O’s blood pressure returned to normal, and her physical exam showed normal reflexes.
Ms. O was given a presumptive diagnosis of ketamine-induced serotonin syndrome. She made a good recovery and was discharged home.
A suspected association
Serotonin syndrome is caused by increased levels of the neurotransmitter serotonin in the CNS. Clinical features of serotonin syndrome include agitation, restlessness, mydriasis, altered mental status or confusion, tachycardia, hypertension, muscle rigidity, diaphoresis, diarrhea, piloerection, headache, fasciculations, clonus, and shivering. Severe cases can be life-threatening and may present with high fever, seizures, arrhythmias, and loss of consciousness. Serotonin syndrome is a clinical diagnosis; the Hunter Serotonin Toxicity Criteria are often used to make the diagnosis. To meet these criteria, a patient must have received a serotonergic agent, and at least one of the following must be present4:
- spontaneous clonus
- inducible clonus and agitation or diaphoresis
- ocular clonus and agitation or diaphoresis
- tremor and hyperreflexia
- hypertonia, temperature >38°C, and ocular clonus or inducible clonus.
For Ms. O, we suspected that administration of ketamine in conjunction with fluoxetine, 40 mg/d, led to serotonin syndrome. Ms. O exhibited ocular clonus and diaphoresis, thus satisfying the Hunter Serotonin Toxicity Criteria, and she also had inducible clonus, altered mental status, hypertension, and tachycardia, which makes serotonin syndrome the most likely diagnosis. She improved after receiving lorazepam, which is often used to treat hypertonicity, decrease autonomic instability, and prevent seizures seen in serotonin syndrome.5
Continue to: There is sparse literature...
There is sparse literature describing serotonin syndrome related to ketamine use. Ketamine has been shown to increase levels of glutamate in the medial prefrontal cortex. Higher levels of glutamine in turn stimulate excitatory glutamatergic neurons that project to the dorsal raphe nucleus. When stimulated, the dorsal raphe nucleus releases serotonin.6 There is also evidence that ketamine inhibits uptake of serotonin in synapses.7 These mechanisms combine to create a net increase in CNS-wide serotonin.
Ketamine is being increasingly used to treat depression and other conditions. This case report underscores the importance of considering serotonin syndrome when treating patients receiving ketamine, especially when it is used in conjunction with selective serotonin reuptake inhibitors.
1. du Jardin KG, Müller HK, Elfving B, et al. Potential involvement of serotonergic signaling in ketamine’s antidepressant actions: A critical review. Prog Neuropsychopharmacol Biol Psychiatry. 2016;71:27-38.
2. Gigliucci V, O’Dowd G, Casey S, et al. Ketamine elicits sustained antidepressant-like activity via a serotonin-dependent mechanism. Psychopharmacology (Berl). 2013;228(1):157-166.
3. Warner ME, Naranjo J, Pollard EM, et al. Serotonergic medications, herbal supplements, and perioperative serotonin syndrome. Can J Anaesth. 2017;64(9):940-946.
4. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635-642.
5. Frank C. Recognition and treatment of serotonin syndrome. Can Fam Physician. 2008;54(7):988-992.
6. López-Gil X, Jiménez-Sánchez L, Campa L, et al. Role of serotonin and noradrenaline in the rapid antidepressant action of ketamine. ACS Chem Neurosci. 2019;10(7):3318-3326.
7. Martin LL, Bouchal RL, Smith DJ. Ketamine inhibits serotonin uptake in vivo. Neuropharmacology. 1982;21(2):113-118.
Long utilized as a rapid anesthetic, ketamine has been increasingly used in sub-anesthetic doses for several psychiatric indications, including depression, suicidality, and chronic pain. Recently, an intranasal form of esketamine—the S-enantiomer of ketamine—was FDA-approved for treatment-resistant depression. Previously, researchers believed ketamine mediated its analgesic and psychotropic effects solely via N-methyl-
CASE REPORT
Ms. O, age 41, has a history of endometriosis, anticardiolipin antibody syndrome, major depressive disorder, and generalized anxiety disorder. She initially presented to an outside hospital and was admitted for chronic endometriosis pain. During that admission, her pain was treated with IV ketamine, 40 mg/hour, on hospital Days 1 through 4. While hospitalized, she continued to receive her home medications: fluoxetine, 40 mg/d, coumadin, 5 mg/d, and diphenhydramine, 25 mg/d. On Day 5, Ms. O experienced visual hallucinations and was diagnosed with ketamine-induced delirium. She was treated with haloperidol (dose unknown) with reportedly good effect. On Day 7, she was discharged home.
Upon returning home, she experienced persistent altered mental status. Her significant other brought her to our hospital for further workup. Ms. O’s body temperature was 37.6°C, and she was diaphoretic. Her blood pressure was 154/100 mm Hg, and her heart rate was 125 bpm. On physical examination, she had 4+ patellar and Achilles reflexes with left ankle clonus and crossed adductors. Her mental status exam showed increased latency of thought and speech, with bizarre affect as evidenced by illogical mannerisms and appearance. She said she was “not feeling myself” and would stare at walls for prolonged periods of time, appearing internally preoccupied and confused.
Ms. O was treated with IV lorazepam, 2 mg. Fourteen hours later, her temperature returned to normal, but she remained tachycardic, hypertensive, and altered. She received 2 additional doses of 2 mg and 1 mg. Seventeen hours after the initial dose of IV lorazepam was administered (and 3 hours after the second dose), Ms. O’s heart rate returned to normal. She was ultimately converted to oral lorazepam, 1 mg every 12 hours. Two hours later, Ms. O’s blood pressure returned to normal, and her physical exam showed normal reflexes.
Ms. O was given a presumptive diagnosis of ketamine-induced serotonin syndrome. She made a good recovery and was discharged home.
A suspected association
Serotonin syndrome is caused by increased levels of the neurotransmitter serotonin in the CNS. Clinical features of serotonin syndrome include agitation, restlessness, mydriasis, altered mental status or confusion, tachycardia, hypertension, muscle rigidity, diaphoresis, diarrhea, piloerection, headache, fasciculations, clonus, and shivering. Severe cases can be life-threatening and may present with high fever, seizures, arrhythmias, and loss of consciousness. Serotonin syndrome is a clinical diagnosis; the Hunter Serotonin Toxicity Criteria are often used to make the diagnosis. To meet these criteria, a patient must have received a serotonergic agent, and at least one of the following must be present4:
- spontaneous clonus
- inducible clonus and agitation or diaphoresis
- ocular clonus and agitation or diaphoresis
- tremor and hyperreflexia
- hypertonia, temperature >38°C, and ocular clonus or inducible clonus.
For Ms. O, we suspected that administration of ketamine in conjunction with fluoxetine, 40 mg/d, led to serotonin syndrome. Ms. O exhibited ocular clonus and diaphoresis, thus satisfying the Hunter Serotonin Toxicity Criteria, and she also had inducible clonus, altered mental status, hypertension, and tachycardia, which makes serotonin syndrome the most likely diagnosis. She improved after receiving lorazepam, which is often used to treat hypertonicity, decrease autonomic instability, and prevent seizures seen in serotonin syndrome.5
Continue to: There is sparse literature...
There is sparse literature describing serotonin syndrome related to ketamine use. Ketamine has been shown to increase levels of glutamate in the medial prefrontal cortex. Higher levels of glutamine in turn stimulate excitatory glutamatergic neurons that project to the dorsal raphe nucleus. When stimulated, the dorsal raphe nucleus releases serotonin.6 There is also evidence that ketamine inhibits uptake of serotonin in synapses.7 These mechanisms combine to create a net increase in CNS-wide serotonin.
Ketamine is being increasingly used to treat depression and other conditions. This case report underscores the importance of considering serotonin syndrome when treating patients receiving ketamine, especially when it is used in conjunction with selective serotonin reuptake inhibitors.
Long utilized as a rapid anesthetic, ketamine has been increasingly used in sub-anesthetic doses for several psychiatric indications, including depression, suicidality, and chronic pain. Recently, an intranasal form of esketamine—the S-enantiomer of ketamine—was FDA-approved for treatment-resistant depression. Previously, researchers believed ketamine mediated its analgesic and psychotropic effects solely via N-methyl-
CASE REPORT
Ms. O, age 41, has a history of endometriosis, anticardiolipin antibody syndrome, major depressive disorder, and generalized anxiety disorder. She initially presented to an outside hospital and was admitted for chronic endometriosis pain. During that admission, her pain was treated with IV ketamine, 40 mg/hour, on hospital Days 1 through 4. While hospitalized, she continued to receive her home medications: fluoxetine, 40 mg/d, coumadin, 5 mg/d, and diphenhydramine, 25 mg/d. On Day 5, Ms. O experienced visual hallucinations and was diagnosed with ketamine-induced delirium. She was treated with haloperidol (dose unknown) with reportedly good effect. On Day 7, she was discharged home.
Upon returning home, she experienced persistent altered mental status. Her significant other brought her to our hospital for further workup. Ms. O’s body temperature was 37.6°C, and she was diaphoretic. Her blood pressure was 154/100 mm Hg, and her heart rate was 125 bpm. On physical examination, she had 4+ patellar and Achilles reflexes with left ankle clonus and crossed adductors. Her mental status exam showed increased latency of thought and speech, with bizarre affect as evidenced by illogical mannerisms and appearance. She said she was “not feeling myself” and would stare at walls for prolonged periods of time, appearing internally preoccupied and confused.
Ms. O was treated with IV lorazepam, 2 mg. Fourteen hours later, her temperature returned to normal, but she remained tachycardic, hypertensive, and altered. She received 2 additional doses of 2 mg and 1 mg. Seventeen hours after the initial dose of IV lorazepam was administered (and 3 hours after the second dose), Ms. O’s heart rate returned to normal. She was ultimately converted to oral lorazepam, 1 mg every 12 hours. Two hours later, Ms. O’s blood pressure returned to normal, and her physical exam showed normal reflexes.
Ms. O was given a presumptive diagnosis of ketamine-induced serotonin syndrome. She made a good recovery and was discharged home.
A suspected association
Serotonin syndrome is caused by increased levels of the neurotransmitter serotonin in the CNS. Clinical features of serotonin syndrome include agitation, restlessness, mydriasis, altered mental status or confusion, tachycardia, hypertension, muscle rigidity, diaphoresis, diarrhea, piloerection, headache, fasciculations, clonus, and shivering. Severe cases can be life-threatening and may present with high fever, seizures, arrhythmias, and loss of consciousness. Serotonin syndrome is a clinical diagnosis; the Hunter Serotonin Toxicity Criteria are often used to make the diagnosis. To meet these criteria, a patient must have received a serotonergic agent, and at least one of the following must be present4:
- spontaneous clonus
- inducible clonus and agitation or diaphoresis
- ocular clonus and agitation or diaphoresis
- tremor and hyperreflexia
- hypertonia, temperature >38°C, and ocular clonus or inducible clonus.
For Ms. O, we suspected that administration of ketamine in conjunction with fluoxetine, 40 mg/d, led to serotonin syndrome. Ms. O exhibited ocular clonus and diaphoresis, thus satisfying the Hunter Serotonin Toxicity Criteria, and she also had inducible clonus, altered mental status, hypertension, and tachycardia, which makes serotonin syndrome the most likely diagnosis. She improved after receiving lorazepam, which is often used to treat hypertonicity, decrease autonomic instability, and prevent seizures seen in serotonin syndrome.5
Continue to: There is sparse literature...
There is sparse literature describing serotonin syndrome related to ketamine use. Ketamine has been shown to increase levels of glutamate in the medial prefrontal cortex. Higher levels of glutamine in turn stimulate excitatory glutamatergic neurons that project to the dorsal raphe nucleus. When stimulated, the dorsal raphe nucleus releases serotonin.6 There is also evidence that ketamine inhibits uptake of serotonin in synapses.7 These mechanisms combine to create a net increase in CNS-wide serotonin.
Ketamine is being increasingly used to treat depression and other conditions. This case report underscores the importance of considering serotonin syndrome when treating patients receiving ketamine, especially when it is used in conjunction with selective serotonin reuptake inhibitors.
1. du Jardin KG, Müller HK, Elfving B, et al. Potential involvement of serotonergic signaling in ketamine’s antidepressant actions: A critical review. Prog Neuropsychopharmacol Biol Psychiatry. 2016;71:27-38.
2. Gigliucci V, O’Dowd G, Casey S, et al. Ketamine elicits sustained antidepressant-like activity via a serotonin-dependent mechanism. Psychopharmacology (Berl). 2013;228(1):157-166.
3. Warner ME, Naranjo J, Pollard EM, et al. Serotonergic medications, herbal supplements, and perioperative serotonin syndrome. Can J Anaesth. 2017;64(9):940-946.
4. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635-642.
5. Frank C. Recognition and treatment of serotonin syndrome. Can Fam Physician. 2008;54(7):988-992.
6. López-Gil X, Jiménez-Sánchez L, Campa L, et al. Role of serotonin and noradrenaline in the rapid antidepressant action of ketamine. ACS Chem Neurosci. 2019;10(7):3318-3326.
7. Martin LL, Bouchal RL, Smith DJ. Ketamine inhibits serotonin uptake in vivo. Neuropharmacology. 1982;21(2):113-118.
1. du Jardin KG, Müller HK, Elfving B, et al. Potential involvement of serotonergic signaling in ketamine’s antidepressant actions: A critical review. Prog Neuropsychopharmacol Biol Psychiatry. 2016;71:27-38.
2. Gigliucci V, O’Dowd G, Casey S, et al. Ketamine elicits sustained antidepressant-like activity via a serotonin-dependent mechanism. Psychopharmacology (Berl). 2013;228(1):157-166.
3. Warner ME, Naranjo J, Pollard EM, et al. Serotonergic medications, herbal supplements, and perioperative serotonin syndrome. Can J Anaesth. 2017;64(9):940-946.
4. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635-642.
5. Frank C. Recognition and treatment of serotonin syndrome. Can Fam Physician. 2008;54(7):988-992.
6. López-Gil X, Jiménez-Sánchez L, Campa L, et al. Role of serotonin and noradrenaline in the rapid antidepressant action of ketamine. ACS Chem Neurosci. 2019;10(7):3318-3326.
7. Martin LL, Bouchal RL, Smith DJ. Ketamine inhibits serotonin uptake in vivo. Neuropharmacology. 1982;21(2):113-118.
June Medical Services v. Russo: Understanding this high-stakes abortion case
On March 4, 2020, the Supreme Court of the United States (SCOTUS) will hear opening arguments for June Medical Services v. Russo. (Please note that this case was originally referred to as June Medical Services v. Gee. However, Secretary Rebekah Gee resigned from her position on January 31, 2020, and was replaced by Interim Secretary Stephen Russo.) The case will examine a Louisiana law (Louisiana Act 620, or LA 620), originally passed in 2014, that requires physicians to have hospital admitting privileges within 30 miles of where they provide abortion services.1 When LA 620 was signed into law in 2014, 5 of Louisiana’s 6 abortion clinics would not have met the standards created by this legislation and would have been forced to close, potentially leaving the vast majority of women in Louisiana without access to an abortion provider, and disproportionately impacting poor and rural women. Prior to enactment of this law, physicians at these 5 clinics attempted to obtain admitting privileges, and all were denied. The denials occurred due to two main reasons—because the providers admitted too few patients each year to qualify for hospital privileges or simply because they provided abortion care.2 Shortly after this legislation was signed into law, the Center for Reproductive Rights (CRR) challenged the law, citing the undue burden it created for patients attempting to access abortion care.
Prior case also considered question of hospital privileges for abortion providers
Interestingly, SCOTUS already has ruled on this very question. In 1992, the Court ruled in Planned Parenthood of Southeastern Pennsylvania v. Casey that it is unconstitutional for a state to create an “undue burden” on a woman’s right to abortion prior to fetal viability.3 And in 2016, when considering whether or not requiring abortion providers to obtain hospital privileges creates an undue burden in Whole Women’s Health (WWH) v. Hellerstedt, the Supreme Court’s answer was yes, it does. WWH, with legal aid from CRR, challenged Texas House Bill 2 (H.B. 2), which similar to LA 620, required abortion providers to have local admitting privileges. Based largely on the precedent set in Casey, SCOTUS ruled 5-3 in favor of WWH.
The Louisiana law currently in question was written and challenged in district court simultaneous to the Supreme Court’s review of WWH. The district court declared LA 620 invalid and permanently enjoined its enforcement, finding the law would “drastically burden women’s right to choose abortions.”4 However, the US Court of Appeals for the Fifth Circuit reviewed the case and overturned the district court decision, finding the lower court’s analysis erroneous and stating, “no clinics will likely be forced to close on account of [LA 620].” The Fifth Circuit panel ruled that, despite the precedent of WWH, LA 620 did not create an undue burden because of state-level differences in admitting privileges, demographics, and geography. They also found that only 30% of the 2 million women living in Louisiana would be impacted by the law, predominantly via longer wait times, and argued that this does not represent significant burden. The plaintiffs filed for an emergency stay with SCOTUS, who granted the stay pending a full hearing. On March 4, the Supreme Court will hear arguments to determine if the Fifth Circuit was correct in drawing a distinction between LA 620 and the SCOTUS verdict in WWH.
Targeted restrictions on abortion providers
LA 620 joins a long series of laws meant to enact targeted restrictions on abortion providers, or “TRAP” laws. TRAP laws are written to limit access to abortion under the guise of improving patient safety, despite ample evidence to the contrary, and include such various regulations as admitting privileges, facilities requirements, waiting periods, and parental or partner notification. Many such laws have been enacted in the last decade, and many struck down based on judicial precedent.
How the Supreme Court has ruled in the past
When a case is appealed to the Supreme Court, the court can either decline to hear the case, thereby leaving the lower courts’ ruling in place, or choose to hear the case in full and either affirm or overturn the lower court’s decision. After issuing a ruling in WWH, the 2016-2017 Roberts Court declined to hear challenges from other states with similarly overturned laws, leaving the laws struck down. In electing to hear June Medical Services v. Russo, the court has the opportunity to uphold or overturn the Fifth Circuit Court’s decision. However, today’s Supreme Court differs greatly from the Supreme Court in 2016.
In 2016, the court ruled 5-3 to overturn H.B. 2 in WWH shortly after the death of Justice Antonin Scalia. Scalia was replaced by Justice Neil Gorsuch, a Constitutional originalist who has never directly ruled on an abortion case.5 In 2018, Justice Anthony Kennedy, who authored the court’s majority opinion on Casey and was among the majority on WWH, retired, and was replaced by Justice Brett Kavanaugh. Kavanaugh has ruled once on the right to abortion in Garza v. Hargan in 2017, where he argued that precedent states that the government has “permissible interests in favoring fetal life…and refraining from facilitating abortion,” and that significant delay in care did not constitute undue burden.6 In regard to the 5-4 stay issued by the court in June Medical Services, Kavanaugh joined Gorsuch in voting to deny the application for stay, and was the only justice to issue an opinion alongside the ruling, arguing that because the doctors in question had not applied for and been denied admitting privileges since the WWH ruling, the case hinges on theoretical rather than demonstrable undue burden.7 Appointed by President Donald Trump, both Gorsuch and Kavanaugh are widely considered to be conservative judges, and while neither has a strong judicial record on abortion rights, both are anticipated to side with the conservative majority on the court.
The Supreme Court rarely overturns its own precedent, but concerns are high
The question of precedent will be central in SCOTUS hearing June Medical Services v. Russo so quickly after the WWH decision. Additionally, in hearing this case, the court will have the opportunity to reexamine all relevant precedent, including the Planned Parenthood of Southeastern Pennsylvania v. Casey decision and even Roe v. Wade. With a conservative court and an increasingly charged political environment, reproductive rights advocates fear that the June Medical Services v. Russo ruling may be the first step toward dismantling judicial protection of abortion rights in the United States.
If SCOTUS rules against June Medical Services, stating that admitting privileges do not cause an undue burden for women seeking to access abortion care, other states likely will introduce and enact similar legislation. These TRAP laws have the potential to limit or eliminate access to abortion for 25 million people of reproductive age. Numerous studies have demonstrated that limiting access to abortion care does not decrease the number of abortions but can result in patients using unsafe means to obtain an abortion.8
The medical community recognizes the danger of enacting restrictive legislation. The American College of Obstetricians and Gynecologists (ACOG), along with the American Medical Association, the Society of Maternal-Fetal Medicine, the Association for Sexual and Reproductive Medicine, the American Association of Family Practitioners, and many others, filed an amicus curiae in support of the June Medical Services plaintiffs.9 These brief filings are critical to ensuring the courts hear physician voices in this important legal decision, and ACOG’s briefs have been quoted in several previous Supreme Court opinions, concurrences, and dissents.
Action items
- Although June Medical Services v. Russo’s decision will not be made until early summer 2020, we can continue to use our voices and expertise to speak out against laws designed to limit access to abortion—at the state and federal levels. As women’s health clinicians, we see the impact abortion restrictions have on our patients, especially our low income and rural patients. Sharing these stories with our legislators, testifying for or against legislation, and speaking out in our communities can have a powerful impact. Check with your local ACOG chapter or with ACOG’s state and government affairs office for more information.
- Follow along with this case at SCOTUS Blog.
- Lastly, make sure you are registered to vote. We are in an election year, and using our voices in and out of the ballot box is critical. You can register here.
- HB338. Louisiana State Legislature. 2014. http://www.legis.la.gov/legis/BillInfo.aspx?s=14RS&b=ACT620&sbi=y. Accessed February 19, 2020.
- Nash E, Donovan MK. Admitting priveleges are back at the U.S. Supreme Court with serious implications for abortion access. Guttmacher Institute. Updated December 3, 2019.
- Planned Parenthood of Southeastern Pennsylvania v. Casey. Cornell Law School Legal Information Institute. https://www.law.cornell.edu/supremecourt/text/505/833. Accessed February 20, 2020.
- June Medical Services LLC v Gee. Oyez. www.oyez.org/cases/2019/18-1323. Accessed February 20, 2020.
- Neil Gorsuch. Oyez. https://www.oyez.org/justices/neil_gorsuch. Accessed February 20, 2020.
- Judge Kavanaugh’s Judicial Record on the Right to Abortion. Center for Reproductive Rights. https://www.reproductiverights.org/sites/crr.civicactions.net/files/documents/factsheets/Judge-Kavanaugh-Judicial-Record-on-the-Right-to-Abortion2.pdf. Accessed February 20, 2020.
- Kavanaugh B. (2019, February 7). June Medical Services, L.L.C, v. Gee, 586 U.S. ____ (2019). Supreme Court of the United States. https://www.supremecourt.gov/opinions/18pdf/18a774_3ebh.pdf. Accessed February 20, 2020.
- Cohen SA. Facts and consequences: Legality, incidence and safety of abortion worldwide. November 20, 2009.
- June Medical Services, LLC v. Russo. SCOTUSblog. February 6, 2020. https://www.scotusblog.com/case-files/cases/june-medical-services-llc-v-russo/. Accessed February 20, 2020.
On March 4, 2020, the Supreme Court of the United States (SCOTUS) will hear opening arguments for June Medical Services v. Russo. (Please note that this case was originally referred to as June Medical Services v. Gee. However, Secretary Rebekah Gee resigned from her position on January 31, 2020, and was replaced by Interim Secretary Stephen Russo.) The case will examine a Louisiana law (Louisiana Act 620, or LA 620), originally passed in 2014, that requires physicians to have hospital admitting privileges within 30 miles of where they provide abortion services.1 When LA 620 was signed into law in 2014, 5 of Louisiana’s 6 abortion clinics would not have met the standards created by this legislation and would have been forced to close, potentially leaving the vast majority of women in Louisiana without access to an abortion provider, and disproportionately impacting poor and rural women. Prior to enactment of this law, physicians at these 5 clinics attempted to obtain admitting privileges, and all were denied. The denials occurred due to two main reasons—because the providers admitted too few patients each year to qualify for hospital privileges or simply because they provided abortion care.2 Shortly after this legislation was signed into law, the Center for Reproductive Rights (CRR) challenged the law, citing the undue burden it created for patients attempting to access abortion care.
Prior case also considered question of hospital privileges for abortion providers
Interestingly, SCOTUS already has ruled on this very question. In 1992, the Court ruled in Planned Parenthood of Southeastern Pennsylvania v. Casey that it is unconstitutional for a state to create an “undue burden” on a woman’s right to abortion prior to fetal viability.3 And in 2016, when considering whether or not requiring abortion providers to obtain hospital privileges creates an undue burden in Whole Women’s Health (WWH) v. Hellerstedt, the Supreme Court’s answer was yes, it does. WWH, with legal aid from CRR, challenged Texas House Bill 2 (H.B. 2), which similar to LA 620, required abortion providers to have local admitting privileges. Based largely on the precedent set in Casey, SCOTUS ruled 5-3 in favor of WWH.
The Louisiana law currently in question was written and challenged in district court simultaneous to the Supreme Court’s review of WWH. The district court declared LA 620 invalid and permanently enjoined its enforcement, finding the law would “drastically burden women’s right to choose abortions.”4 However, the US Court of Appeals for the Fifth Circuit reviewed the case and overturned the district court decision, finding the lower court’s analysis erroneous and stating, “no clinics will likely be forced to close on account of [LA 620].” The Fifth Circuit panel ruled that, despite the precedent of WWH, LA 620 did not create an undue burden because of state-level differences in admitting privileges, demographics, and geography. They also found that only 30% of the 2 million women living in Louisiana would be impacted by the law, predominantly via longer wait times, and argued that this does not represent significant burden. The plaintiffs filed for an emergency stay with SCOTUS, who granted the stay pending a full hearing. On March 4, the Supreme Court will hear arguments to determine if the Fifth Circuit was correct in drawing a distinction between LA 620 and the SCOTUS verdict in WWH.
Targeted restrictions on abortion providers
LA 620 joins a long series of laws meant to enact targeted restrictions on abortion providers, or “TRAP” laws. TRAP laws are written to limit access to abortion under the guise of improving patient safety, despite ample evidence to the contrary, and include such various regulations as admitting privileges, facilities requirements, waiting periods, and parental or partner notification. Many such laws have been enacted in the last decade, and many struck down based on judicial precedent.
How the Supreme Court has ruled in the past
When a case is appealed to the Supreme Court, the court can either decline to hear the case, thereby leaving the lower courts’ ruling in place, or choose to hear the case in full and either affirm or overturn the lower court’s decision. After issuing a ruling in WWH, the 2016-2017 Roberts Court declined to hear challenges from other states with similarly overturned laws, leaving the laws struck down. In electing to hear June Medical Services v. Russo, the court has the opportunity to uphold or overturn the Fifth Circuit Court’s decision. However, today’s Supreme Court differs greatly from the Supreme Court in 2016.
In 2016, the court ruled 5-3 to overturn H.B. 2 in WWH shortly after the death of Justice Antonin Scalia. Scalia was replaced by Justice Neil Gorsuch, a Constitutional originalist who has never directly ruled on an abortion case.5 In 2018, Justice Anthony Kennedy, who authored the court’s majority opinion on Casey and was among the majority on WWH, retired, and was replaced by Justice Brett Kavanaugh. Kavanaugh has ruled once on the right to abortion in Garza v. Hargan in 2017, where he argued that precedent states that the government has “permissible interests in favoring fetal life…and refraining from facilitating abortion,” and that significant delay in care did not constitute undue burden.6 In regard to the 5-4 stay issued by the court in June Medical Services, Kavanaugh joined Gorsuch in voting to deny the application for stay, and was the only justice to issue an opinion alongside the ruling, arguing that because the doctors in question had not applied for and been denied admitting privileges since the WWH ruling, the case hinges on theoretical rather than demonstrable undue burden.7 Appointed by President Donald Trump, both Gorsuch and Kavanaugh are widely considered to be conservative judges, and while neither has a strong judicial record on abortion rights, both are anticipated to side with the conservative majority on the court.
The Supreme Court rarely overturns its own precedent, but concerns are high
The question of precedent will be central in SCOTUS hearing June Medical Services v. Russo so quickly after the WWH decision. Additionally, in hearing this case, the court will have the opportunity to reexamine all relevant precedent, including the Planned Parenthood of Southeastern Pennsylvania v. Casey decision and even Roe v. Wade. With a conservative court and an increasingly charged political environment, reproductive rights advocates fear that the June Medical Services v. Russo ruling may be the first step toward dismantling judicial protection of abortion rights in the United States.
If SCOTUS rules against June Medical Services, stating that admitting privileges do not cause an undue burden for women seeking to access abortion care, other states likely will introduce and enact similar legislation. These TRAP laws have the potential to limit or eliminate access to abortion for 25 million people of reproductive age. Numerous studies have demonstrated that limiting access to abortion care does not decrease the number of abortions but can result in patients using unsafe means to obtain an abortion.8
The medical community recognizes the danger of enacting restrictive legislation. The American College of Obstetricians and Gynecologists (ACOG), along with the American Medical Association, the Society of Maternal-Fetal Medicine, the Association for Sexual and Reproductive Medicine, the American Association of Family Practitioners, and many others, filed an amicus curiae in support of the June Medical Services plaintiffs.9 These brief filings are critical to ensuring the courts hear physician voices in this important legal decision, and ACOG’s briefs have been quoted in several previous Supreme Court opinions, concurrences, and dissents.
Action items
- Although June Medical Services v. Russo’s decision will not be made until early summer 2020, we can continue to use our voices and expertise to speak out against laws designed to limit access to abortion—at the state and federal levels. As women’s health clinicians, we see the impact abortion restrictions have on our patients, especially our low income and rural patients. Sharing these stories with our legislators, testifying for or against legislation, and speaking out in our communities can have a powerful impact. Check with your local ACOG chapter or with ACOG’s state and government affairs office for more information.
- Follow along with this case at SCOTUS Blog.
- Lastly, make sure you are registered to vote. We are in an election year, and using our voices in and out of the ballot box is critical. You can register here.
On March 4, 2020, the Supreme Court of the United States (SCOTUS) will hear opening arguments for June Medical Services v. Russo. (Please note that this case was originally referred to as June Medical Services v. Gee. However, Secretary Rebekah Gee resigned from her position on January 31, 2020, and was replaced by Interim Secretary Stephen Russo.) The case will examine a Louisiana law (Louisiana Act 620, or LA 620), originally passed in 2014, that requires physicians to have hospital admitting privileges within 30 miles of where they provide abortion services.1 When LA 620 was signed into law in 2014, 5 of Louisiana’s 6 abortion clinics would not have met the standards created by this legislation and would have been forced to close, potentially leaving the vast majority of women in Louisiana without access to an abortion provider, and disproportionately impacting poor and rural women. Prior to enactment of this law, physicians at these 5 clinics attempted to obtain admitting privileges, and all were denied. The denials occurred due to two main reasons—because the providers admitted too few patients each year to qualify for hospital privileges or simply because they provided abortion care.2 Shortly after this legislation was signed into law, the Center for Reproductive Rights (CRR) challenged the law, citing the undue burden it created for patients attempting to access abortion care.
Prior case also considered question of hospital privileges for abortion providers
Interestingly, SCOTUS already has ruled on this very question. In 1992, the Court ruled in Planned Parenthood of Southeastern Pennsylvania v. Casey that it is unconstitutional for a state to create an “undue burden” on a woman’s right to abortion prior to fetal viability.3 And in 2016, when considering whether or not requiring abortion providers to obtain hospital privileges creates an undue burden in Whole Women’s Health (WWH) v. Hellerstedt, the Supreme Court’s answer was yes, it does. WWH, with legal aid from CRR, challenged Texas House Bill 2 (H.B. 2), which similar to LA 620, required abortion providers to have local admitting privileges. Based largely on the precedent set in Casey, SCOTUS ruled 5-3 in favor of WWH.
The Louisiana law currently in question was written and challenged in district court simultaneous to the Supreme Court’s review of WWH. The district court declared LA 620 invalid and permanently enjoined its enforcement, finding the law would “drastically burden women’s right to choose abortions.”4 However, the US Court of Appeals for the Fifth Circuit reviewed the case and overturned the district court decision, finding the lower court’s analysis erroneous and stating, “no clinics will likely be forced to close on account of [LA 620].” The Fifth Circuit panel ruled that, despite the precedent of WWH, LA 620 did not create an undue burden because of state-level differences in admitting privileges, demographics, and geography. They also found that only 30% of the 2 million women living in Louisiana would be impacted by the law, predominantly via longer wait times, and argued that this does not represent significant burden. The plaintiffs filed for an emergency stay with SCOTUS, who granted the stay pending a full hearing. On March 4, the Supreme Court will hear arguments to determine if the Fifth Circuit was correct in drawing a distinction between LA 620 and the SCOTUS verdict in WWH.
Targeted restrictions on abortion providers
LA 620 joins a long series of laws meant to enact targeted restrictions on abortion providers, or “TRAP” laws. TRAP laws are written to limit access to abortion under the guise of improving patient safety, despite ample evidence to the contrary, and include such various regulations as admitting privileges, facilities requirements, waiting periods, and parental or partner notification. Many such laws have been enacted in the last decade, and many struck down based on judicial precedent.
How the Supreme Court has ruled in the past
When a case is appealed to the Supreme Court, the court can either decline to hear the case, thereby leaving the lower courts’ ruling in place, or choose to hear the case in full and either affirm or overturn the lower court’s decision. After issuing a ruling in WWH, the 2016-2017 Roberts Court declined to hear challenges from other states with similarly overturned laws, leaving the laws struck down. In electing to hear June Medical Services v. Russo, the court has the opportunity to uphold or overturn the Fifth Circuit Court’s decision. However, today’s Supreme Court differs greatly from the Supreme Court in 2016.
In 2016, the court ruled 5-3 to overturn H.B. 2 in WWH shortly after the death of Justice Antonin Scalia. Scalia was replaced by Justice Neil Gorsuch, a Constitutional originalist who has never directly ruled on an abortion case.5 In 2018, Justice Anthony Kennedy, who authored the court’s majority opinion on Casey and was among the majority on WWH, retired, and was replaced by Justice Brett Kavanaugh. Kavanaugh has ruled once on the right to abortion in Garza v. Hargan in 2017, where he argued that precedent states that the government has “permissible interests in favoring fetal life…and refraining from facilitating abortion,” and that significant delay in care did not constitute undue burden.6 In regard to the 5-4 stay issued by the court in June Medical Services, Kavanaugh joined Gorsuch in voting to deny the application for stay, and was the only justice to issue an opinion alongside the ruling, arguing that because the doctors in question had not applied for and been denied admitting privileges since the WWH ruling, the case hinges on theoretical rather than demonstrable undue burden.7 Appointed by President Donald Trump, both Gorsuch and Kavanaugh are widely considered to be conservative judges, and while neither has a strong judicial record on abortion rights, both are anticipated to side with the conservative majority on the court.
The Supreme Court rarely overturns its own precedent, but concerns are high
The question of precedent will be central in SCOTUS hearing June Medical Services v. Russo so quickly after the WWH decision. Additionally, in hearing this case, the court will have the opportunity to reexamine all relevant precedent, including the Planned Parenthood of Southeastern Pennsylvania v. Casey decision and even Roe v. Wade. With a conservative court and an increasingly charged political environment, reproductive rights advocates fear that the June Medical Services v. Russo ruling may be the first step toward dismantling judicial protection of abortion rights in the United States.
If SCOTUS rules against June Medical Services, stating that admitting privileges do not cause an undue burden for women seeking to access abortion care, other states likely will introduce and enact similar legislation. These TRAP laws have the potential to limit or eliminate access to abortion for 25 million people of reproductive age. Numerous studies have demonstrated that limiting access to abortion care does not decrease the number of abortions but can result in patients using unsafe means to obtain an abortion.8
The medical community recognizes the danger of enacting restrictive legislation. The American College of Obstetricians and Gynecologists (ACOG), along with the American Medical Association, the Society of Maternal-Fetal Medicine, the Association for Sexual and Reproductive Medicine, the American Association of Family Practitioners, and many others, filed an amicus curiae in support of the June Medical Services plaintiffs.9 These brief filings are critical to ensuring the courts hear physician voices in this important legal decision, and ACOG’s briefs have been quoted in several previous Supreme Court opinions, concurrences, and dissents.
Action items
- Although June Medical Services v. Russo’s decision will not be made until early summer 2020, we can continue to use our voices and expertise to speak out against laws designed to limit access to abortion—at the state and federal levels. As women’s health clinicians, we see the impact abortion restrictions have on our patients, especially our low income and rural patients. Sharing these stories with our legislators, testifying for or against legislation, and speaking out in our communities can have a powerful impact. Check with your local ACOG chapter or with ACOG’s state and government affairs office for more information.
- Follow along with this case at SCOTUS Blog.
- Lastly, make sure you are registered to vote. We are in an election year, and using our voices in and out of the ballot box is critical. You can register here.
- HB338. Louisiana State Legislature. 2014. http://www.legis.la.gov/legis/BillInfo.aspx?s=14RS&b=ACT620&sbi=y. Accessed February 19, 2020.
- Nash E, Donovan MK. Admitting priveleges are back at the U.S. Supreme Court with serious implications for abortion access. Guttmacher Institute. Updated December 3, 2019.
- Planned Parenthood of Southeastern Pennsylvania v. Casey. Cornell Law School Legal Information Institute. https://www.law.cornell.edu/supremecourt/text/505/833. Accessed February 20, 2020.
- June Medical Services LLC v Gee. Oyez. www.oyez.org/cases/2019/18-1323. Accessed February 20, 2020.
- Neil Gorsuch. Oyez. https://www.oyez.org/justices/neil_gorsuch. Accessed February 20, 2020.
- Judge Kavanaugh’s Judicial Record on the Right to Abortion. Center for Reproductive Rights. https://www.reproductiverights.org/sites/crr.civicactions.net/files/documents/factsheets/Judge-Kavanaugh-Judicial-Record-on-the-Right-to-Abortion2.pdf. Accessed February 20, 2020.
- Kavanaugh B. (2019, February 7). June Medical Services, L.L.C, v. Gee, 586 U.S. ____ (2019). Supreme Court of the United States. https://www.supremecourt.gov/opinions/18pdf/18a774_3ebh.pdf. Accessed February 20, 2020.
- Cohen SA. Facts and consequences: Legality, incidence and safety of abortion worldwide. November 20, 2009.
- June Medical Services, LLC v. Russo. SCOTUSblog. February 6, 2020. https://www.scotusblog.com/case-files/cases/june-medical-services-llc-v-russo/. Accessed February 20, 2020.
- HB338. Louisiana State Legislature. 2014. http://www.legis.la.gov/legis/BillInfo.aspx?s=14RS&b=ACT620&sbi=y. Accessed February 19, 2020.
- Nash E, Donovan MK. Admitting priveleges are back at the U.S. Supreme Court with serious implications for abortion access. Guttmacher Institute. Updated December 3, 2019.
- Planned Parenthood of Southeastern Pennsylvania v. Casey. Cornell Law School Legal Information Institute. https://www.law.cornell.edu/supremecourt/text/505/833. Accessed February 20, 2020.
- June Medical Services LLC v Gee. Oyez. www.oyez.org/cases/2019/18-1323. Accessed February 20, 2020.
- Neil Gorsuch. Oyez. https://www.oyez.org/justices/neil_gorsuch. Accessed February 20, 2020.
- Judge Kavanaugh’s Judicial Record on the Right to Abortion. Center for Reproductive Rights. https://www.reproductiverights.org/sites/crr.civicactions.net/files/documents/factsheets/Judge-Kavanaugh-Judicial-Record-on-the-Right-to-Abortion2.pdf. Accessed February 20, 2020.
- Kavanaugh B. (2019, February 7). June Medical Services, L.L.C, v. Gee, 586 U.S. ____ (2019). Supreme Court of the United States. https://www.supremecourt.gov/opinions/18pdf/18a774_3ebh.pdf. Accessed February 20, 2020.
- Cohen SA. Facts and consequences: Legality, incidence and safety of abortion worldwide. November 20, 2009.
- June Medical Services, LLC v. Russo. SCOTUSblog. February 6, 2020. https://www.scotusblog.com/case-files/cases/june-medical-services-llc-v-russo/. Accessed February 20, 2020.
Top Residents Selected for 2020 dermMentors™ Resident of Distinction Award™
The dermMentors™ Resident of Distinction Award™ was presented to 5 dermatology residents at the 19th Annual Caribbean Dermatology Symposium, January 21–25, 2020, in Paradise Island, Bahamas. Recipients of the award include Rachel Giesey, DO, Case Western Reserve University, Cleveland, Ohio, and University Hospitals Cleveland Medical Center, Cleveland, Ohio; Janice Tiao, MD, Boston University Medical Center, Boston, Massachusetts; Jordan V. Wang, MD, MBE, MBA, Thomas Jefferson University, Philadelphia, Pennsylvania; Jacqueline D. Watchmaker, MD, Boston University School of Medicine, Boston, Massachusetts; and Jennifer E. Yeh, MD, PhD, Brigham and Women’s Hospital, Boston, Massachusetts. The residents presented their research during the general sessions on January 25, 2020.
The overall grand prize was awarded to Dr. Yeh for her research entitled, “Topical Imiquimod in Combination With Brachytherapy for Unresectable Cutaneous Melanoma Metastases.” Dr. Yeh presented the utility of combining topical imiquimod with brachytherapy for locoregional control of cutaneous metastases through the presentation of 3 patients with scalp melanoma initially treated with wide local excision who developed numerous cutaneous metastases. “While surgical excision is the first-line treatment of single, discrete cutaneous metastases, it may not be practical in patients with multiple foci of disease distributed over large areas, as seen in the 3 patients presented here who achieved complete resolution of their cutaneous metastatic burden with concurrent topical imiquimod and brachytherapy,” Dr. Yeh reported.
Presentations by the other residents included a study of the burden of common skin diseases in the Caribbean and the potential correlation with a country’s socioeconomic status (Dr. Giesey), a study of the use of doxycycline in patients with lichen planopilaris and frontal fibrosing alopecia (Dr. Tiao), a discussion of counterfeit medical devices and injectables as well as medical spas in dermatology (Dr. Wang), and a study of the most common reasons patients are dissatisfied with minimally and noninvasive cosmetic procedures (Dr. Watchmaker). Access all of the abstracts presented by the top residents here.
The dermMentors™ Resident of Distinction Award™ recognizes top residents in dermatology. DermMentors.org and the dermMentors™ Resident of Distinction Award™ are sponsored by Beiersdorf Inc and administered by DermEd, Inc. The 2020 dermMentors™ Residents of Distinction™ presented new scientific research during the general sessions of the 19th Annual Caribbean Dermatology Symposium on January 25, 2020.
The dermMentors™ Resident of Distinction Award™ was presented to 5 dermatology residents at the 19th Annual Caribbean Dermatology Symposium, January 21–25, 2020, in Paradise Island, Bahamas. Recipients of the award include Rachel Giesey, DO, Case Western Reserve University, Cleveland, Ohio, and University Hospitals Cleveland Medical Center, Cleveland, Ohio; Janice Tiao, MD, Boston University Medical Center, Boston, Massachusetts; Jordan V. Wang, MD, MBE, MBA, Thomas Jefferson University, Philadelphia, Pennsylvania; Jacqueline D. Watchmaker, MD, Boston University School of Medicine, Boston, Massachusetts; and Jennifer E. Yeh, MD, PhD, Brigham and Women’s Hospital, Boston, Massachusetts. The residents presented their research during the general sessions on January 25, 2020.
The overall grand prize was awarded to Dr. Yeh for her research entitled, “Topical Imiquimod in Combination With Brachytherapy for Unresectable Cutaneous Melanoma Metastases.” Dr. Yeh presented the utility of combining topical imiquimod with brachytherapy for locoregional control of cutaneous metastases through the presentation of 3 patients with scalp melanoma initially treated with wide local excision who developed numerous cutaneous metastases. “While surgical excision is the first-line treatment of single, discrete cutaneous metastases, it may not be practical in patients with multiple foci of disease distributed over large areas, as seen in the 3 patients presented here who achieved complete resolution of their cutaneous metastatic burden with concurrent topical imiquimod and brachytherapy,” Dr. Yeh reported.
Presentations by the other residents included a study of the burden of common skin diseases in the Caribbean and the potential correlation with a country’s socioeconomic status (Dr. Giesey), a study of the use of doxycycline in patients with lichen planopilaris and frontal fibrosing alopecia (Dr. Tiao), a discussion of counterfeit medical devices and injectables as well as medical spas in dermatology (Dr. Wang), and a study of the most common reasons patients are dissatisfied with minimally and noninvasive cosmetic procedures (Dr. Watchmaker). Access all of the abstracts presented by the top residents here.
The dermMentors™ Resident of Distinction Award™ recognizes top residents in dermatology. DermMentors.org and the dermMentors™ Resident of Distinction Award™ are sponsored by Beiersdorf Inc and administered by DermEd, Inc. The 2020 dermMentors™ Residents of Distinction™ presented new scientific research during the general sessions of the 19th Annual Caribbean Dermatology Symposium on January 25, 2020.
The dermMentors™ Resident of Distinction Award™ was presented to 5 dermatology residents at the 19th Annual Caribbean Dermatology Symposium, January 21–25, 2020, in Paradise Island, Bahamas. Recipients of the award include Rachel Giesey, DO, Case Western Reserve University, Cleveland, Ohio, and University Hospitals Cleveland Medical Center, Cleveland, Ohio; Janice Tiao, MD, Boston University Medical Center, Boston, Massachusetts; Jordan V. Wang, MD, MBE, MBA, Thomas Jefferson University, Philadelphia, Pennsylvania; Jacqueline D. Watchmaker, MD, Boston University School of Medicine, Boston, Massachusetts; and Jennifer E. Yeh, MD, PhD, Brigham and Women’s Hospital, Boston, Massachusetts. The residents presented their research during the general sessions on January 25, 2020.
The overall grand prize was awarded to Dr. Yeh for her research entitled, “Topical Imiquimod in Combination With Brachytherapy for Unresectable Cutaneous Melanoma Metastases.” Dr. Yeh presented the utility of combining topical imiquimod with brachytherapy for locoregional control of cutaneous metastases through the presentation of 3 patients with scalp melanoma initially treated with wide local excision who developed numerous cutaneous metastases. “While surgical excision is the first-line treatment of single, discrete cutaneous metastases, it may not be practical in patients with multiple foci of disease distributed over large areas, as seen in the 3 patients presented here who achieved complete resolution of their cutaneous metastatic burden with concurrent topical imiquimod and brachytherapy,” Dr. Yeh reported.
Presentations by the other residents included a study of the burden of common skin diseases in the Caribbean and the potential correlation with a country’s socioeconomic status (Dr. Giesey), a study of the use of doxycycline in patients with lichen planopilaris and frontal fibrosing alopecia (Dr. Tiao), a discussion of counterfeit medical devices and injectables as well as medical spas in dermatology (Dr. Wang), and a study of the most common reasons patients are dissatisfied with minimally and noninvasive cosmetic procedures (Dr. Watchmaker). Access all of the abstracts presented by the top residents here.
The dermMentors™ Resident of Distinction Award™ recognizes top residents in dermatology. DermMentors.org and the dermMentors™ Resident of Distinction Award™ are sponsored by Beiersdorf Inc and administered by DermEd, Inc. The 2020 dermMentors™ Residents of Distinction™ presented new scientific research during the general sessions of the 19th Annual Caribbean Dermatology Symposium on January 25, 2020.
‘Momentous’ USMLE change: New pass/fail format stuns medicine
News that the United States Medical Licensing Examination (USMLE) program will change its Step 1 scoring from a 3-digit number to pass/fail starting Jan. 1, 2022, has set off a flurry of shocked responses from students and physicians.
J. Bryan Carmody, MD, MPH, an assistant professor at Eastern Virginia Medical School in Norfolk, said in an interview that he was “stunned” when he heard the news on Wednesday and said the switch presents “the single biggest opportunity for medical school education reform since the Flexner Report,” which in 1910 established standards for modern medical education.
Numbers will continue for some tests
The USMLE cosponsors – the Federation of State Medical Boards (FSMB) and the National Board of Medical Examiners (NBME) – said that the Step 2 Clinical Knowledge (CK) exam and Step 3 will continue to be scored numerically. Step 2 Clinical Skills (CS) will continue its pass/fail system.
The change was made after Step 1 had been roundly criticized as playing too big a role in the process of becoming a physician and for causing students to study for the test instead of engaging fully in their medical education.
Ramie Fathy, a third-year medical student at the University of Pennsylvania, Philadelphia, currently studying for Step 1, said in an interview that it would have been nice personally to have the pass/fail choice, but he predicts both good and unintended consequences in the change.
The positive news, Mr. Fathy said, is that less emphasis will be put on the Step 1 test, which includes memorizing basic science details that may or not be relevant depending on later specialty choice.
“It’s not necessarily measuring what the test makers intended, which was whether or not a student can understand and apply basic science concepts to the practice of medicine,” he said.
“The current system encourages students to get as high a score as possible, which – after a certain point – translates to memorizing many little details that become increasingly less practically relevant,” Mr. Fathy said.
Pressure may move elsewhere?
However, Mr. Fathy worries that, without a scoring system to help decide who stands out in Step 1, residency program directors will depend more on the reputation of candidates’ medical school and the clout of the person writing a letter of recommendation – factors that are often influenced by family resources and social standing. That could wedge a further economic divide into the path to becoming a physician.
Mr. Fathy said he and fellow students are watching for information on what the passing bar will be and what happens with Step 2 Clinical Knowledge exam. USMLE has promised more information as soon as it is available.
“The question is whether that test will replace Step 1 as the standardized metric of student competency,” Mr. Fathy said, which would put more pressure on students further down the medical path.
Will Step 2 anxiety increase?
Dr. Carmody agreed that there is the danger that students now will spend their time studying for Step 2 CK at the expense of other parts of their education.
Meaningful reform will depend on the pass/fail move being coupled with other reforms, most importantly application caps, said Dr. Carmody, who teaches preclinical medical students and works with the residency program.
He has been blogging about Step 1 pass/fail for the past year.
Currently students can apply for as many residencies as they can pay for and Carmody said the number of applications per student has been rising over the past decade.
“That puts program directors under an impossible burden,” he said. “With our Step 1-based system, there’s significant inequality in the number of interviews people get. Programs end up overinviting the same group of people who look good on paper.”
People outside that group respond by sending more applications than they need to just to get a few interviews, Dr. Carmody added.
With caps, students would have an incentive to apply to only those programs in which they had a sincere interest, he said. Program directors also would then be better able to evaluate each application.
Switching Step 1 to pass/fail may have some effect on medical school burnout, Dr. Carmody said.
“It’s one thing to work hard when you’re on call and your patients depend on it,” he said. “But I would have a hard time staying up late every night studying something that I know in my heart is not going to help my patients, but I have to do it because I have to do better than the person who’s studying in the apartment next to me.”
Test has strayed from original purpose
Joseph Safdieh, MD, an assistant dean for clinical curriculum and director of the medical student neurology clerkship for the Weill Cornell Medicine, New York, sees the move as positive overall.
“We should not be using any single metric to define or describe our students’ overall profile,” he said in an interview.
“This has been a very significant anxiety point for our medical students for quite a number of years,” Dr. Safdieh said. “They were frustrated that their entire 4 years of medical school seemingly came down to one number.”
The test was created originally as one of three parts of licensure, he pointed out.
“Over the past 10 or 15 years, the exam has morphed to become a litmus test for very specific residency programs,” he said.
However, Dr. Safdieh has concerns that Step 2 will cultivate the same anxiety and may get too big a spotlight without the Step 1 metric, “although one could argue that test does more accurately reflect clinical material,” he said.
He also worries that students who have selected a specialty by the time they take Step 2 may find late in the game that they are less competitive in their field than they thought they were and may have to make a last-minute switch.
Dr. Safdieh said he thinks Step 2 will be next to go the pass/fail route. In reading between the lines of the announcement, he believes the test cosponsors didn’t make both pass/fail at once because it would have been “a nuclear bomb to the system.”
He credited the cosponsors with making what he called a “bold and momentous decision to initiate radical change in the overall transition between undergraduate and graduate medical education.”
Dr. Safdieh added that few in medicine were expecting Wednesday’s announcement.
“I think many of us were expecting them to go to quartile grading, not to go this far,” he said.
Dr. Safdieh suggested that, among those who may see downstream effects from the pass/fail move are offshore schools, such as those in the Caribbean. “Those schools rely on Step 1 to demonstrate that their students are meeting the rigor,” he said. But he hopes that this will lead to more holistic review.
“We’re hoping that this will force change in the system so that residency directors will look at more than just test-taking ability. They’ll look at publications and scholarship, community service and advocacy and performance in medical school,” Dr. Safdieh said.
Alison J. Whelan, MD, chief medical education officer of the Association of American Medical Colleges said in a statement, “The transition from medical school to residency training is a matter of great concern throughout academic medicine.
“The decision by the NBME and FSMB to change USMLE Step 1 score reporting to pass/fail was very carefully considered to balance student learning and student well-being,” she said. “The medical education community must now work together to identify and implement additional changes to improve the overall UME-GME [undergraduate and graduate medical education] transition system for all stakeholders and the AAMC is committed to helping lead this work.”
Dr. Fathy, Dr. Carmody, and Dr. Safdieh have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
News that the United States Medical Licensing Examination (USMLE) program will change its Step 1 scoring from a 3-digit number to pass/fail starting Jan. 1, 2022, has set off a flurry of shocked responses from students and physicians.
J. Bryan Carmody, MD, MPH, an assistant professor at Eastern Virginia Medical School in Norfolk, said in an interview that he was “stunned” when he heard the news on Wednesday and said the switch presents “the single biggest opportunity for medical school education reform since the Flexner Report,” which in 1910 established standards for modern medical education.
Numbers will continue for some tests
The USMLE cosponsors – the Federation of State Medical Boards (FSMB) and the National Board of Medical Examiners (NBME) – said that the Step 2 Clinical Knowledge (CK) exam and Step 3 will continue to be scored numerically. Step 2 Clinical Skills (CS) will continue its pass/fail system.
The change was made after Step 1 had been roundly criticized as playing too big a role in the process of becoming a physician and for causing students to study for the test instead of engaging fully in their medical education.
Ramie Fathy, a third-year medical student at the University of Pennsylvania, Philadelphia, currently studying for Step 1, said in an interview that it would have been nice personally to have the pass/fail choice, but he predicts both good and unintended consequences in the change.
The positive news, Mr. Fathy said, is that less emphasis will be put on the Step 1 test, which includes memorizing basic science details that may or not be relevant depending on later specialty choice.
“It’s not necessarily measuring what the test makers intended, which was whether or not a student can understand and apply basic science concepts to the practice of medicine,” he said.
“The current system encourages students to get as high a score as possible, which – after a certain point – translates to memorizing many little details that become increasingly less practically relevant,” Mr. Fathy said.
Pressure may move elsewhere?
However, Mr. Fathy worries that, without a scoring system to help decide who stands out in Step 1, residency program directors will depend more on the reputation of candidates’ medical school and the clout of the person writing a letter of recommendation – factors that are often influenced by family resources and social standing. That could wedge a further economic divide into the path to becoming a physician.
Mr. Fathy said he and fellow students are watching for information on what the passing bar will be and what happens with Step 2 Clinical Knowledge exam. USMLE has promised more information as soon as it is available.
“The question is whether that test will replace Step 1 as the standardized metric of student competency,” Mr. Fathy said, which would put more pressure on students further down the medical path.
Will Step 2 anxiety increase?
Dr. Carmody agreed that there is the danger that students now will spend their time studying for Step 2 CK at the expense of other parts of their education.
Meaningful reform will depend on the pass/fail move being coupled with other reforms, most importantly application caps, said Dr. Carmody, who teaches preclinical medical students and works with the residency program.
He has been blogging about Step 1 pass/fail for the past year.
Currently students can apply for as many residencies as they can pay for and Carmody said the number of applications per student has been rising over the past decade.
“That puts program directors under an impossible burden,” he said. “With our Step 1-based system, there’s significant inequality in the number of interviews people get. Programs end up overinviting the same group of people who look good on paper.”
People outside that group respond by sending more applications than they need to just to get a few interviews, Dr. Carmody added.
With caps, students would have an incentive to apply to only those programs in which they had a sincere interest, he said. Program directors also would then be better able to evaluate each application.
Switching Step 1 to pass/fail may have some effect on medical school burnout, Dr. Carmody said.
“It’s one thing to work hard when you’re on call and your patients depend on it,” he said. “But I would have a hard time staying up late every night studying something that I know in my heart is not going to help my patients, but I have to do it because I have to do better than the person who’s studying in the apartment next to me.”
Test has strayed from original purpose
Joseph Safdieh, MD, an assistant dean for clinical curriculum and director of the medical student neurology clerkship for the Weill Cornell Medicine, New York, sees the move as positive overall.
“We should not be using any single metric to define or describe our students’ overall profile,” he said in an interview.
“This has been a very significant anxiety point for our medical students for quite a number of years,” Dr. Safdieh said. “They were frustrated that their entire 4 years of medical school seemingly came down to one number.”
The test was created originally as one of three parts of licensure, he pointed out.
“Over the past 10 or 15 years, the exam has morphed to become a litmus test for very specific residency programs,” he said.
However, Dr. Safdieh has concerns that Step 2 will cultivate the same anxiety and may get too big a spotlight without the Step 1 metric, “although one could argue that test does more accurately reflect clinical material,” he said.
He also worries that students who have selected a specialty by the time they take Step 2 may find late in the game that they are less competitive in their field than they thought they were and may have to make a last-minute switch.
Dr. Safdieh said he thinks Step 2 will be next to go the pass/fail route. In reading between the lines of the announcement, he believes the test cosponsors didn’t make both pass/fail at once because it would have been “a nuclear bomb to the system.”
He credited the cosponsors with making what he called a “bold and momentous decision to initiate radical change in the overall transition between undergraduate and graduate medical education.”
Dr. Safdieh added that few in medicine were expecting Wednesday’s announcement.
“I think many of us were expecting them to go to quartile grading, not to go this far,” he said.
Dr. Safdieh suggested that, among those who may see downstream effects from the pass/fail move are offshore schools, such as those in the Caribbean. “Those schools rely on Step 1 to demonstrate that their students are meeting the rigor,” he said. But he hopes that this will lead to more holistic review.
“We’re hoping that this will force change in the system so that residency directors will look at more than just test-taking ability. They’ll look at publications and scholarship, community service and advocacy and performance in medical school,” Dr. Safdieh said.
Alison J. Whelan, MD, chief medical education officer of the Association of American Medical Colleges said in a statement, “The transition from medical school to residency training is a matter of great concern throughout academic medicine.
“The decision by the NBME and FSMB to change USMLE Step 1 score reporting to pass/fail was very carefully considered to balance student learning and student well-being,” she said. “The medical education community must now work together to identify and implement additional changes to improve the overall UME-GME [undergraduate and graduate medical education] transition system for all stakeholders and the AAMC is committed to helping lead this work.”
Dr. Fathy, Dr. Carmody, and Dr. Safdieh have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
News that the United States Medical Licensing Examination (USMLE) program will change its Step 1 scoring from a 3-digit number to pass/fail starting Jan. 1, 2022, has set off a flurry of shocked responses from students and physicians.
J. Bryan Carmody, MD, MPH, an assistant professor at Eastern Virginia Medical School in Norfolk, said in an interview that he was “stunned” when he heard the news on Wednesday and said the switch presents “the single biggest opportunity for medical school education reform since the Flexner Report,” which in 1910 established standards for modern medical education.
Numbers will continue for some tests
The USMLE cosponsors – the Federation of State Medical Boards (FSMB) and the National Board of Medical Examiners (NBME) – said that the Step 2 Clinical Knowledge (CK) exam and Step 3 will continue to be scored numerically. Step 2 Clinical Skills (CS) will continue its pass/fail system.
The change was made after Step 1 had been roundly criticized as playing too big a role in the process of becoming a physician and for causing students to study for the test instead of engaging fully in their medical education.
Ramie Fathy, a third-year medical student at the University of Pennsylvania, Philadelphia, currently studying for Step 1, said in an interview that it would have been nice personally to have the pass/fail choice, but he predicts both good and unintended consequences in the change.
The positive news, Mr. Fathy said, is that less emphasis will be put on the Step 1 test, which includes memorizing basic science details that may or not be relevant depending on later specialty choice.
“It’s not necessarily measuring what the test makers intended, which was whether or not a student can understand and apply basic science concepts to the practice of medicine,” he said.
“The current system encourages students to get as high a score as possible, which – after a certain point – translates to memorizing many little details that become increasingly less practically relevant,” Mr. Fathy said.
Pressure may move elsewhere?
However, Mr. Fathy worries that, without a scoring system to help decide who stands out in Step 1, residency program directors will depend more on the reputation of candidates’ medical school and the clout of the person writing a letter of recommendation – factors that are often influenced by family resources and social standing. That could wedge a further economic divide into the path to becoming a physician.
Mr. Fathy said he and fellow students are watching for information on what the passing bar will be and what happens with Step 2 Clinical Knowledge exam. USMLE has promised more information as soon as it is available.
“The question is whether that test will replace Step 1 as the standardized metric of student competency,” Mr. Fathy said, which would put more pressure on students further down the medical path.
Will Step 2 anxiety increase?
Dr. Carmody agreed that there is the danger that students now will spend their time studying for Step 2 CK at the expense of other parts of their education.
Meaningful reform will depend on the pass/fail move being coupled with other reforms, most importantly application caps, said Dr. Carmody, who teaches preclinical medical students and works with the residency program.
He has been blogging about Step 1 pass/fail for the past year.
Currently students can apply for as many residencies as they can pay for and Carmody said the number of applications per student has been rising over the past decade.
“That puts program directors under an impossible burden,” he said. “With our Step 1-based system, there’s significant inequality in the number of interviews people get. Programs end up overinviting the same group of people who look good on paper.”
People outside that group respond by sending more applications than they need to just to get a few interviews, Dr. Carmody added.
With caps, students would have an incentive to apply to only those programs in which they had a sincere interest, he said. Program directors also would then be better able to evaluate each application.
Switching Step 1 to pass/fail may have some effect on medical school burnout, Dr. Carmody said.
“It’s one thing to work hard when you’re on call and your patients depend on it,” he said. “But I would have a hard time staying up late every night studying something that I know in my heart is not going to help my patients, but I have to do it because I have to do better than the person who’s studying in the apartment next to me.”
Test has strayed from original purpose
Joseph Safdieh, MD, an assistant dean for clinical curriculum and director of the medical student neurology clerkship for the Weill Cornell Medicine, New York, sees the move as positive overall.
“We should not be using any single metric to define or describe our students’ overall profile,” he said in an interview.
“This has been a very significant anxiety point for our medical students for quite a number of years,” Dr. Safdieh said. “They were frustrated that their entire 4 years of medical school seemingly came down to one number.”
The test was created originally as one of three parts of licensure, he pointed out.
“Over the past 10 or 15 years, the exam has morphed to become a litmus test for very specific residency programs,” he said.
However, Dr. Safdieh has concerns that Step 2 will cultivate the same anxiety and may get too big a spotlight without the Step 1 metric, “although one could argue that test does more accurately reflect clinical material,” he said.
He also worries that students who have selected a specialty by the time they take Step 2 may find late in the game that they are less competitive in their field than they thought they were and may have to make a last-minute switch.
Dr. Safdieh said he thinks Step 2 will be next to go the pass/fail route. In reading between the lines of the announcement, he believes the test cosponsors didn’t make both pass/fail at once because it would have been “a nuclear bomb to the system.”
He credited the cosponsors with making what he called a “bold and momentous decision to initiate radical change in the overall transition between undergraduate and graduate medical education.”
Dr. Safdieh added that few in medicine were expecting Wednesday’s announcement.
“I think many of us were expecting them to go to quartile grading, not to go this far,” he said.
Dr. Safdieh suggested that, among those who may see downstream effects from the pass/fail move are offshore schools, such as those in the Caribbean. “Those schools rely on Step 1 to demonstrate that their students are meeting the rigor,” he said. But he hopes that this will lead to more holistic review.
“We’re hoping that this will force change in the system so that residency directors will look at more than just test-taking ability. They’ll look at publications and scholarship, community service and advocacy and performance in medical school,” Dr. Safdieh said.
Alison J. Whelan, MD, chief medical education officer of the Association of American Medical Colleges said in a statement, “The transition from medical school to residency training is a matter of great concern throughout academic medicine.
“The decision by the NBME and FSMB to change USMLE Step 1 score reporting to pass/fail was very carefully considered to balance student learning and student well-being,” she said. “The medical education community must now work together to identify and implement additional changes to improve the overall UME-GME [undergraduate and graduate medical education] transition system for all stakeholders and the AAMC is committed to helping lead this work.”
Dr. Fathy, Dr. Carmody, and Dr. Safdieh have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.