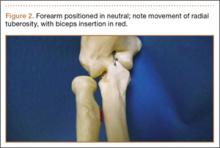
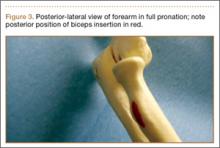
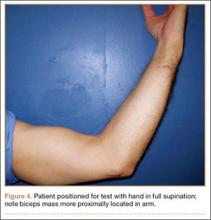
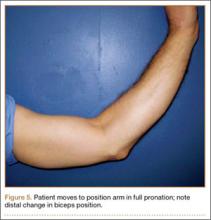
User login
Patient-Directed Valgus Stress Radiograph of the Knee: A New and Novel Technique
Medial-compartment partial knee arthroplasty (unicompartmental replacement) is an accepted surgical intervention for anteromedial osteoarthritis of the knee.1 The radiographic investigations required in the workup of these patients should include weight-bearing standing anteroposterior (AP), lateral, and sunrise (Merchant) views, as well as a valgus stress AP radiograph to assess the functionality of the lateral compartment. The method of properly obtaining the valgus stress film has been well described by the Oxford Group.2 Its recommended radiographic technique requires that a surgeon or a radiologic technologist perform the valgus stress maneuver, manually, while another technologist shoots the film. The 2 consequences of this technique are that it requires 2 individuals to obtain the film, and it subjects the individual who is applying the stress to some level of radiation exposure, which is undesirable. Because of this and the time inconvenience, many surgeons omit the valgus stress radiograph, which can lead to the adverse outcome of missing a lateral compartment that is functionally incompetent, resulting in the potential for early lateral compartment progression of osteoarthritis and the need for revision surgery, usually to a total knee arthroplasty.
In an attempt to mitigate these barriers to obtaining the necessary valgus stress radiograph, Dr. Mauerhan’s team developed a technique that could be done with the assistance of the patient and would require only 1 technologist to perform. Additionally, this project was a quality improvement initiative, because it lowered radiation exposure to all personnel involved in obtaining the correct films.
Materials and Methods
We initiated the project using weight-bearing strategies to impart the valgus stress view of the knee. After trying several different wedges and blocks, and varying patient instructions, we realized a different approach to this problem would be required to find an acceptable solution. We redirected our efforts to effectively performing the stress view with the patient in a supine position on the radiograph table. Ultimately, we decided that a much stiffer wedge and a denser object to squeeze would facilitate obtaining a proper film. Considering all available options, a youth size 4 soccer ball (diameter, 11 in) was introduced along with a slightly larger positioning wedge. The soccer ball was wrapped with 4-in Coban wrap (3M) to create a nonslip surface. This change in patient positioning, along with a standardized 7º to 10º cephalic radiographic tube angulation, helped to correct issues with tibial plateau visualization. Once these changes were enacted, we obtained fairly consistent positive results, and we instituted this patient-directed valgus stress view of the knee, along with a manual valgus stress view for comparison.
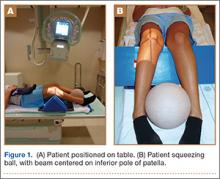
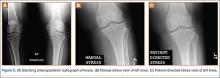
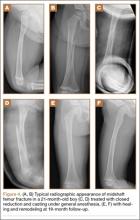
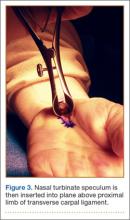
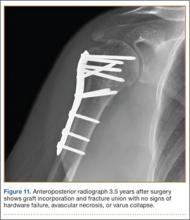
The protocol for obtaining the patient-directed valgus stress view of the knee is as follows: The patient lays supine with a dense 45º spine-positioning wedge (Burlington Medical Supplies) placed under both knees and the patient’s heels on the examining table. The radiographic tube is angled cephalad 7º to 10º centered on the inferior pole of the patella, using a 40-in source to image-receptor distance, collimated to part; the image receptor is placed under the affected knee, below the positioning wedge. The affected knee is rotated to the “true” AP position (the patella will be centered between the femoral condyles on the AP exposure), and the ball is placed between the patient’s legs just above the ankle joint. The technologist demonstrates to the patient how to squeeze the ball while maintaining contact of heels with the table. The technologist can exit the room and obtain the exposure, which is taken while the patient is squeezing the ball, as shown in Figures 1A and 1B. Examples of the standing AP, manual stress, and patient-directed valgus radiographs are shown in Figures 2A-2C. The entire technique is demonstrated in the Video.

Results
During the 9 months of this quality improvement project, 78 examinations were performed. Five studies did not show complete correction of the varus deformity. Of these, 3 showed complete correction on a manual valgus stress radiograph, and 2 did not, contraindicating the use of partial knee replacement. Three patients displayed collapse of the lateral compartment, indicating a nonfunctional lateral compartment, and, therefore, were also a contraindication to partial knee arthroplasty. The remaining 70 patients had identical radiographic results with both the manual and patient-directed valgus stress tests. There was no instance of examination failure or need to repeat as a result of difficulty of the examination for the patient. Repeat films because of positioning errors were very rare, usually early in the learning curve, and no more prevalent than when using the manual stress method. The technique was reproducible and easy to teach and adopt.
Discussion
In total, 73 patients (93.5%) with the patient-directed stress film showed the desired result, either correction of the medial compartment narrowing in conjunction with an intact lateral compartment or narrowing of the lateral compartment. Of the 5 patients (6.5%) whose patient-directed stress films did not show correction of the varus deformity, 3 patients displayed correction with a manually applied stress radiograph and 2 did not. Based on this observation, our recommendation would be for those patients who do not show adequate correction on the patient-directed stress radiograph to have a manual examination to establish the presence or absence of the desired correction.
Performing a valgus stress radiograph is an integral part of the investigation to determine if the patient is an appropriate candidate for partial knee arthroplasty.3 The historical, manually performed valgus stress radiograph requires 2 individuals, 1 to apply the stress with the patient on the table and 1 to shoot the exposure. For the individual or individuals applying this stress, there is an increased radiation exposure that would be undesirable over a long career. The authors developed a new technique using a commercially available spinal positioning wedge and 11-in youth soccer ball wrapped with Coban wrap, as described, which is economical and easy to obtain and use in the clinical setting. We believe this cost-effective method will offer surgeons who perform partial knee arthroplasty a novel method to obtain the important information gleaned from the valgus stress radiograph and to improve surgical outcomes through the preoperative assessment of the lateral compartment. Additionally, as a quality and safety improvement initiative, we believe this technique will reduce radiographic exposure for those performing these studies, and, because the examination can be carried out by a single technologist, it will significantly improve efficiency in the radiology suite.
Conclusion
We have developed a new method of obtaining the important valgus stress radiograph as part of the workup of patients with medial-compartment osteoarthritis of the knee. The technique can be performed with easily obtainable, commercially available products and is reliable 93.5% of the time. It also adds to the efficiency of the radiology suite and reduces radiographic exposure for technologists.
1. White SH, Ludkowski PF, Goodfellow JW. Anteromedial osteoarthritis of the knee. J Bone Joint Surg Br. 1991;73(4):582-586.
2. Goodfellow JW, O’Conner JJ, Dodd CA, Murray DW. Unicompartmental Arthroplasty with the Oxford Knee. Woodeaton, Oxford, England: Goodfellow Publishers Limited; 2006:38-39.
3. Gibson PH, Goodfellow JW. Stress radiography in degenerative arthritis of the knee. J Bone Joint Surg Br. 1986;68(4):608-609.
Medial-compartment partial knee arthroplasty (unicompartmental replacement) is an accepted surgical intervention for anteromedial osteoarthritis of the knee.1 The radiographic investigations required in the workup of these patients should include weight-bearing standing anteroposterior (AP), lateral, and sunrise (Merchant) views, as well as a valgus stress AP radiograph to assess the functionality of the lateral compartment. The method of properly obtaining the valgus stress film has been well described by the Oxford Group.2 Its recommended radiographic technique requires that a surgeon or a radiologic technologist perform the valgus stress maneuver, manually, while another technologist shoots the film. The 2 consequences of this technique are that it requires 2 individuals to obtain the film, and it subjects the individual who is applying the stress to some level of radiation exposure, which is undesirable. Because of this and the time inconvenience, many surgeons omit the valgus stress radiograph, which can lead to the adverse outcome of missing a lateral compartment that is functionally incompetent, resulting in the potential for early lateral compartment progression of osteoarthritis and the need for revision surgery, usually to a total knee arthroplasty.
In an attempt to mitigate these barriers to obtaining the necessary valgus stress radiograph, Dr. Mauerhan’s team developed a technique that could be done with the assistance of the patient and would require only 1 technologist to perform. Additionally, this project was a quality improvement initiative, because it lowered radiation exposure to all personnel involved in obtaining the correct films.
Materials and Methods
We initiated the project using weight-bearing strategies to impart the valgus stress view of the knee. After trying several different wedges and blocks, and varying patient instructions, we realized a different approach to this problem would be required to find an acceptable solution. We redirected our efforts to effectively performing the stress view with the patient in a supine position on the radiograph table. Ultimately, we decided that a much stiffer wedge and a denser object to squeeze would facilitate obtaining a proper film. Considering all available options, a youth size 4 soccer ball (diameter, 11 in) was introduced along with a slightly larger positioning wedge. The soccer ball was wrapped with 4-in Coban wrap (3M) to create a nonslip surface. This change in patient positioning, along with a standardized 7º to 10º cephalic radiographic tube angulation, helped to correct issues with tibial plateau visualization. Once these changes were enacted, we obtained fairly consistent positive results, and we instituted this patient-directed valgus stress view of the knee, along with a manual valgus stress view for comparison.
The protocol for obtaining the patient-directed valgus stress view of the knee is as follows: The patient lays supine with a dense 45º spine-positioning wedge (Burlington Medical Supplies) placed under both knees and the patient’s heels on the examining table. The radiographic tube is angled cephalad 7º to 10º centered on the inferior pole of the patella, using a 40-in source to image-receptor distance, collimated to part; the image receptor is placed under the affected knee, below the positioning wedge. The affected knee is rotated to the “true” AP position (the patella will be centered between the femoral condyles on the AP exposure), and the ball is placed between the patient’s legs just above the ankle joint. The technologist demonstrates to the patient how to squeeze the ball while maintaining contact of heels with the table. The technologist can exit the room and obtain the exposure, which is taken while the patient is squeezing the ball, as shown in Figures 1A and 1B. Examples of the standing AP, manual stress, and patient-directed valgus radiographs are shown in Figures 2A-2C. The entire technique is demonstrated in the Video.

Results
During the 9 months of this quality improvement project, 78 examinations were performed. Five studies did not show complete correction of the varus deformity. Of these, 3 showed complete correction on a manual valgus stress radiograph, and 2 did not, contraindicating the use of partial knee replacement. Three patients displayed collapse of the lateral compartment, indicating a nonfunctional lateral compartment, and, therefore, were also a contraindication to partial knee arthroplasty. The remaining 70 patients had identical radiographic results with both the manual and patient-directed valgus stress tests. There was no instance of examination failure or need to repeat as a result of difficulty of the examination for the patient. Repeat films because of positioning errors were very rare, usually early in the learning curve, and no more prevalent than when using the manual stress method. The technique was reproducible and easy to teach and adopt.
Discussion
In total, 73 patients (93.5%) with the patient-directed stress film showed the desired result, either correction of the medial compartment narrowing in conjunction with an intact lateral compartment or narrowing of the lateral compartment. Of the 5 patients (6.5%) whose patient-directed stress films did not show correction of the varus deformity, 3 patients displayed correction with a manually applied stress radiograph and 2 did not. Based on this observation, our recommendation would be for those patients who do not show adequate correction on the patient-directed stress radiograph to have a manual examination to establish the presence or absence of the desired correction.
Performing a valgus stress radiograph is an integral part of the investigation to determine if the patient is an appropriate candidate for partial knee arthroplasty.3 The historical, manually performed valgus stress radiograph requires 2 individuals, 1 to apply the stress with the patient on the table and 1 to shoot the exposure. For the individual or individuals applying this stress, there is an increased radiation exposure that would be undesirable over a long career. The authors developed a new technique using a commercially available spinal positioning wedge and 11-in youth soccer ball wrapped with Coban wrap, as described, which is economical and easy to obtain and use in the clinical setting. We believe this cost-effective method will offer surgeons who perform partial knee arthroplasty a novel method to obtain the important information gleaned from the valgus stress radiograph and to improve surgical outcomes through the preoperative assessment of the lateral compartment. Additionally, as a quality and safety improvement initiative, we believe this technique will reduce radiographic exposure for those performing these studies, and, because the examination can be carried out by a single technologist, it will significantly improve efficiency in the radiology suite.
Conclusion
We have developed a new method of obtaining the important valgus stress radiograph as part of the workup of patients with medial-compartment osteoarthritis of the knee. The technique can be performed with easily obtainable, commercially available products and is reliable 93.5% of the time. It also adds to the efficiency of the radiology suite and reduces radiographic exposure for technologists.
Medial-compartment partial knee arthroplasty (unicompartmental replacement) is an accepted surgical intervention for anteromedial osteoarthritis of the knee.1 The radiographic investigations required in the workup of these patients should include weight-bearing standing anteroposterior (AP), lateral, and sunrise (Merchant) views, as well as a valgus stress AP radiograph to assess the functionality of the lateral compartment. The method of properly obtaining the valgus stress film has been well described by the Oxford Group.2 Its recommended radiographic technique requires that a surgeon or a radiologic technologist perform the valgus stress maneuver, manually, while another technologist shoots the film. The 2 consequences of this technique are that it requires 2 individuals to obtain the film, and it subjects the individual who is applying the stress to some level of radiation exposure, which is undesirable. Because of this and the time inconvenience, many surgeons omit the valgus stress radiograph, which can lead to the adverse outcome of missing a lateral compartment that is functionally incompetent, resulting in the potential for early lateral compartment progression of osteoarthritis and the need for revision surgery, usually to a total knee arthroplasty.
In an attempt to mitigate these barriers to obtaining the necessary valgus stress radiograph, Dr. Mauerhan’s team developed a technique that could be done with the assistance of the patient and would require only 1 technologist to perform. Additionally, this project was a quality improvement initiative, because it lowered radiation exposure to all personnel involved in obtaining the correct films.
Materials and Methods
We initiated the project using weight-bearing strategies to impart the valgus stress view of the knee. After trying several different wedges and blocks, and varying patient instructions, we realized a different approach to this problem would be required to find an acceptable solution. We redirected our efforts to effectively performing the stress view with the patient in a supine position on the radiograph table. Ultimately, we decided that a much stiffer wedge and a denser object to squeeze would facilitate obtaining a proper film. Considering all available options, a youth size 4 soccer ball (diameter, 11 in) was introduced along with a slightly larger positioning wedge. The soccer ball was wrapped with 4-in Coban wrap (3M) to create a nonslip surface. This change in patient positioning, along with a standardized 7º to 10º cephalic radiographic tube angulation, helped to correct issues with tibial plateau visualization. Once these changes were enacted, we obtained fairly consistent positive results, and we instituted this patient-directed valgus stress view of the knee, along with a manual valgus stress view for comparison.
The protocol for obtaining the patient-directed valgus stress view of the knee is as follows: The patient lays supine with a dense 45º spine-positioning wedge (Burlington Medical Supplies) placed under both knees and the patient’s heels on the examining table. The radiographic tube is angled cephalad 7º to 10º centered on the inferior pole of the patella, using a 40-in source to image-receptor distance, collimated to part; the image receptor is placed under the affected knee, below the positioning wedge. The affected knee is rotated to the “true” AP position (the patella will be centered between the femoral condyles on the AP exposure), and the ball is placed between the patient’s legs just above the ankle joint. The technologist demonstrates to the patient how to squeeze the ball while maintaining contact of heels with the table. The technologist can exit the room and obtain the exposure, which is taken while the patient is squeezing the ball, as shown in Figures 1A and 1B. Examples of the standing AP, manual stress, and patient-directed valgus radiographs are shown in Figures 2A-2C. The entire technique is demonstrated in the Video.

Results
During the 9 months of this quality improvement project, 78 examinations were performed. Five studies did not show complete correction of the varus deformity. Of these, 3 showed complete correction on a manual valgus stress radiograph, and 2 did not, contraindicating the use of partial knee replacement. Three patients displayed collapse of the lateral compartment, indicating a nonfunctional lateral compartment, and, therefore, were also a contraindication to partial knee arthroplasty. The remaining 70 patients had identical radiographic results with both the manual and patient-directed valgus stress tests. There was no instance of examination failure or need to repeat as a result of difficulty of the examination for the patient. Repeat films because of positioning errors were very rare, usually early in the learning curve, and no more prevalent than when using the manual stress method. The technique was reproducible and easy to teach and adopt.
Discussion
In total, 73 patients (93.5%) with the patient-directed stress film showed the desired result, either correction of the medial compartment narrowing in conjunction with an intact lateral compartment or narrowing of the lateral compartment. Of the 5 patients (6.5%) whose patient-directed stress films did not show correction of the varus deformity, 3 patients displayed correction with a manually applied stress radiograph and 2 did not. Based on this observation, our recommendation would be for those patients who do not show adequate correction on the patient-directed stress radiograph to have a manual examination to establish the presence or absence of the desired correction.
Performing a valgus stress radiograph is an integral part of the investigation to determine if the patient is an appropriate candidate for partial knee arthroplasty.3 The historical, manually performed valgus stress radiograph requires 2 individuals, 1 to apply the stress with the patient on the table and 1 to shoot the exposure. For the individual or individuals applying this stress, there is an increased radiation exposure that would be undesirable over a long career. The authors developed a new technique using a commercially available spinal positioning wedge and 11-in youth soccer ball wrapped with Coban wrap, as described, which is economical and easy to obtain and use in the clinical setting. We believe this cost-effective method will offer surgeons who perform partial knee arthroplasty a novel method to obtain the important information gleaned from the valgus stress radiograph and to improve surgical outcomes through the preoperative assessment of the lateral compartment. Additionally, as a quality and safety improvement initiative, we believe this technique will reduce radiographic exposure for those performing these studies, and, because the examination can be carried out by a single technologist, it will significantly improve efficiency in the radiology suite.
Conclusion
We have developed a new method of obtaining the important valgus stress radiograph as part of the workup of patients with medial-compartment osteoarthritis of the knee. The technique can be performed with easily obtainable, commercially available products and is reliable 93.5% of the time. It also adds to the efficiency of the radiology suite and reduces radiographic exposure for technologists.
1. White SH, Ludkowski PF, Goodfellow JW. Anteromedial osteoarthritis of the knee. J Bone Joint Surg Br. 1991;73(4):582-586.
2. Goodfellow JW, O’Conner JJ, Dodd CA, Murray DW. Unicompartmental Arthroplasty with the Oxford Knee. Woodeaton, Oxford, England: Goodfellow Publishers Limited; 2006:38-39.
3. Gibson PH, Goodfellow JW. Stress radiography in degenerative arthritis of the knee. J Bone Joint Surg Br. 1986;68(4):608-609.
1. White SH, Ludkowski PF, Goodfellow JW. Anteromedial osteoarthritis of the knee. J Bone Joint Surg Br. 1991;73(4):582-586.
2. Goodfellow JW, O’Conner JJ, Dodd CA, Murray DW. Unicompartmental Arthroplasty with the Oxford Knee. Woodeaton, Oxford, England: Goodfellow Publishers Limited; 2006:38-39.
3. Gibson PH, Goodfellow JW. Stress radiography in degenerative arthritis of the knee. J Bone Joint Surg Br. 1986;68(4):608-609.
Complete Closing Wedge Osteotomy for Correction of Blount Disease (Tibia Vara): A Technique
Blount disease (tibia vara) is an angular tibia deformity that includes varus, increased posterior slope, and internal rotation. This deformity was first described in 1922 by Erlacher1 in Germany. In 1937, Walter Blount2 reported on it in the United States. It is the most common cause of pathologic genu varum in adolescence and childhood.
An oblique incomplete closing wedge osteotomy of the proximal tibial metaphysis was described by Wagner3 for the treatment of unicompartmental osteoarthrosis of the knee in adults. Laurencin and colleagues4 applied this technique to the treatment of pediatric tibia vara with favorable results. They spared the medial cortex of the tibia in their incomplete closing wedge osteotomy technique. In each of the 9 cases we treated and describe here, we accidentally completed the tibial osteotomy when attempting the Laurencin technique. Given that the osteotomy was completed, we modified the Laurencin technique by using a 6-hole, 4.5-mm compression plate rather than a 5-hole semitubular plate, and added a large oblique screw from the medial side to compress the osteotomy site and to protect the plate from fracture. In addition, in 2 patients who weighed more than 250 pounds, we used an external fixator for additional stability. In this article, we report the outcomes of correcting adolescent tibia vara with a complete closing wedge tibial osteotomy and an oblique fibular osteotomy.
Materials and Methods
This study was approved by the Institutional Review Board at Pennsylvania State University. Between 2009 and 2012, we performed 9 complete oblique proximal tibial lateral closing wedge osteotomies on 8 patients (2 girls, 6 boys). In each case, the primary diagnosis was Blount disease. One patient also had renal dysplasia and was receiving dialysis. Mean age at time of operation was 15 years (range, 13-17 years). Mean preoperative weight was 215 pounds (range, 119-317 lb). Mean weight gain at follow-up was 4.39 pounds (range, –10 to 19 lb). Mean body mass index (BMI) was 38 (range, 25-48) (Table). All patients had varus angulation of the proximal tibia before surgery. Mean preoperative varus on standing films was 22° (range, 10°-36°). Because of the patients’ size, we used standing long-leg radiographs, on individual cassettes, for each leg.
Surgical Technique
Before surgery, we use paper cutouts to template the osteotomy wedge. We also use perioperative antibiotics and a standard time-out. For visualization of the entire leg for accurate correction, we prepare and drape the entire leg. A sterile tourniquet is used. At the midshaft of the fibula, a 4-cm incision is made, and dissection is carefully carried down to the fibula. Subperiosteal dissection is performed about the fibula, allowing adequate clearance for an oblique osteotomy. The osteotomy removes about 1 cm of fibula, which is to be used as bone graft for the tibial osteotomy. In addition, a lateral compartment fasciotomy is performed to prevent swelling-related complications. The wound is irrigated and injected with bupivacaine and closed in routine fashion.
We then make an inverted hockey-stick incision over the proximal tibia, centered down to the tibial tubercle. After dissecting down to the anterior compartment, we perform a fasciotomy of about 8 cm to accommodate swelling. Subperiosteal dissection is then performed around the proximal tibia. The medial soft tissues are left attached to increase blood supply and healing. During subperiosteal dissection, soft elevators are used to gently retract the lateral soft tissues along with the inferior and posterior structures. We use fluoroscopic imaging to guide the osteotomy as well as screw and plate placement. We use a 6-hole, 4.5-mm compression plate and screws for fixation. The 2 proximal screws of the plate are predrilled in place to allow for application of the plate after completion of the osteotomy. The plate is then rotated out of position on 1 screw, and the osteotomy is identified under fluoroscopy with the appropriate position distal to the second hole of the 6-hole plate.
An oscillating saw and osteotomes are used to perform the oblique osteotomy. The pre-estimated bone wedge is removed. Wedge size is adjusted, if needed. The bone wedge is morselized for bone graft. The osteotomy is then closed, correcting both varus and internal tibial torsion. Our goal is 5° valgus. After correction is obtained, the plate is placed, and the proximal screw is snugly seated. Three cortical screws are placed distally to hold the plate in place under compression mode, and a cancellous screw is placed superiorly at the proximal portion of the plate for additional fixation. The screw placed proximal to the osteotomy site is a fully threaded cortical screw with excellent compression. Correction and proper placement of hardware are verified with fluoroscopy.
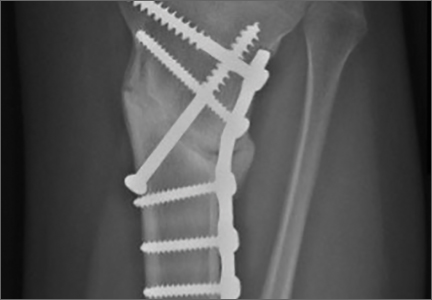
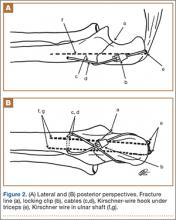
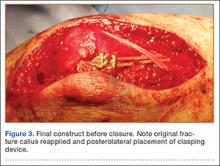
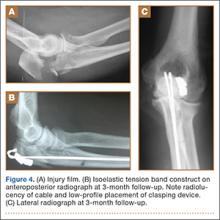
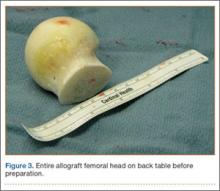
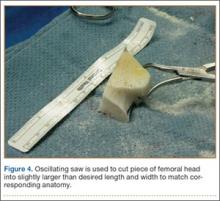
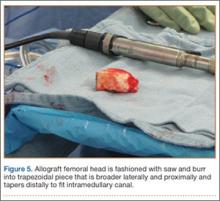
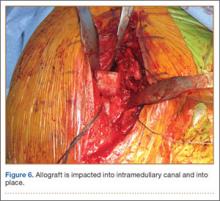
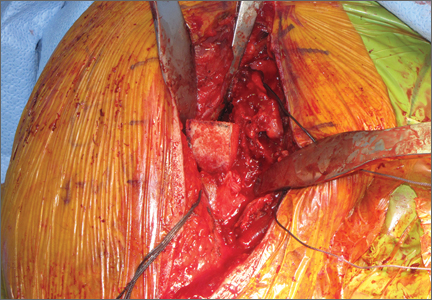
The wound is irrigated and injected with bupivacaine. Bone graft is then placed at the osteotomy site. Additional bone graft is placed posteriorly between the osteotomy site and the muscle mass to stimulate additional healing. Another screw is placed obliquely from the medial side across the osteotomy site to provide additional fixation (Figure 1).
A deep drain is placed and connected to bulb suction for 24 hours after surgery. The wound is then closed in routine fashion. In 2 patients who weighed more than 250 pounds, we used an external fixator for additional stability (Figure 2).
Postoperative Care
The incisions are dressed with antibiotic ointment and 4×4-in bandages and then wrapped with sterile cotton under-cast padding. The leg is placed into a well-padded cylinder cast with the knee flexed 10°. The leg is aligned to about 5° valgus. The cast is then split on the side and spread to allow for swelling and to prevent compartment syndrome.5 We also use a drain hooked to bulb suction, which is removed 24 hours after surgery. Toe-touch weight-bearing with crutches is allowed immediately after surgery. The cast is removed at 6 weeks, and a hinged range-of-motion knee brace is worn for another 6 weeks. All patients are allowed to resume normal activity after 4 months. In our 2 external-fixator cases, a cast was not used, and toe-touch weight-bearing and knee motion were allowed immediately. The external fixators were removed at about 10 weeks.
Results
Mean postoperative mechanical femoral-tibial angle was 3°, and mean correction was 26° (range, 16°-43°) (Table). Lateral distal femoral angle did not show significant femoral deformity in our sample. Mean medial proximal tibial angle was 74° (range, 63°-79°). In each case, the varus deformity was primarily in the tibia. Mean tourniquet time was 88 minutes (range, 50-119 min). Our complication rate was 11% (1 knee). In our first case, in which we did not use an extra medial screw, the 4.5-mm plate fractured at the osteotomy site 2.5 months after surgery. The 250-pound patient subsequently lost 17° of correction, and valgus alignment was not achieved. Preoperative varus was 25°, and postoperative alignment was 8° varus. This plate fracture led us to use an extra medial screw for additional stability in all subsequent cases and to consider using an external fixator for patients weighing more than 250 pounds. After the first case, there were no other plate fractures. A potential problem with closing wedge osteotomy is shortening, but varus correction restores some length. Mean postoperative leg-length difference was 10 mm (range, 0-16 mm). No patient complained of leg-length difference during the postoperative follow-up.
Eight and a half months after surgery, 1 patient had hardware removed, at the family’s request. No patient experienced perioperative infection or neurovascular damage. Our overall patient population was obese—mean BMI was 38 (range, 25-48), and mean postoperative weight was 219 pounds. Three of our 8 patients were overweight (BMI, 25-30), and 5 were obese (BMI, >30). For prevention of plate failure, we recommend using an extra oblique screw in all patients and considering an external fixator for patients who weigh more than 250 pounds.
Discussion
Correction of adolescent tibia vara can be challenging because of patient obesity. The technique described here—a modification of the technique of Laurencin and colleagues4—is practical and reproducible in this population. The goals in performing osteotomy are to correct the deformity, restore joint alignment, preserve leg length, and prevent recurrent deformity and other complications, such as neurovascular injury, nonunion, and infection.3,6-8 Our technique minimizes the risk for these complications. For example, the fasciotomy provides excellent decompression of the anterior and lateral compartments, minimizing neurovascular ischemia and the risk for compartment syndrome. During cast placement, splitting and spreading reduce the risk for compartment syndrome as well.5
Wagner3,9 demonstrated the utility of a closing wedge proximal tibial osteotomy in adults. Laurencin and colleagues4 showed this technique is effective in correcting tibia vara in a pediatric population. However, they did not specify patient weight and used a small semitubular plate for fixation, and some of their patients had infantile Blount disease. We modified the technique in 3 ways. First, we performed a complete osteotomy. Second, because our patients were adolescents and very large, we used a 6-hole, 4.5-mm compression plate and screws. Third, we used an external fixator for increased stability in patients who weighed more than 250 pounds.
The reported technique, using an oblique metaphyseal closing wedge osteotomy with internal fixation in obese patients, is practical, safe, and reliable. This technique is a useful alternative to an external fixator. We used it on 9 knees with tibia vara, and it was completely successful in 8 cases and partially successful in 1 (hardware breakage occurred). An external fixator was used to prevent hardware breakage in 2 patients who weighed more than 250 pounds. This technique is a valuable treatment option for surgical correction, especially in obese patients.
1. Erlacher P. Deformierende Prozesse der Epiphysengegend bei Kindem. Archiv Orthop Unfall-Chir. 1922;20:81-96.
2. Blount WP. Tibia vara. J Bone Joint Surg. 1937;29:1-28.
3. Wagner H. Principles of corrective osteotomies in osteoarthrosis of the knee. In: Weal UH, ed. Joint Preserving Procedures of the Lower Extremity. New York, NY: Springer; 1980:77-102.
4. Laurencin CT, Ferriter PJ, Millis MB. Oblique proximal tibial osteotomy for the correction of tibia vara in the young. Clin Orthop Relat Res. 1996;(327):218-224.
5. Garfin SR, Mubarak SJ, Evans KL, Hargens AR, Akeson WH. Quantification of intracompartmental pressure and volume under plaster casts. J Bone Joint Surg Am. 1981;63(3):449-453.
6. Mycoskie PJ. Complications of osteotomies about the knee in children. Orthopedics. 1981;4(9):1005-1015.
7. Matsen FA, Staheli LT. Neurovascular complications following tibial osteotomy in children. A case report. Clin Orthop Relat Res. 1975;(110):210-214.
8. Steel HH, Sandrew RE, Sullivan PD. Complications of tibial osteotomy in children for genu varum or valgum. Evidence that neurological changes are due to ischemia. J Bone Joint Surg Am. 1971;53(8):1629-1635.
9. Wagner H. The displacement osteotomy as a correction principle. In: Heirholzer G, Muller KH, eds. Corrective Osteotomies of the Lower Extremity After Trauma. Berlin, Germany: Springer; 1985:141-150.
Blount disease (tibia vara) is an angular tibia deformity that includes varus, increased posterior slope, and internal rotation. This deformity was first described in 1922 by Erlacher1 in Germany. In 1937, Walter Blount2 reported on it in the United States. It is the most common cause of pathologic genu varum in adolescence and childhood.
An oblique incomplete closing wedge osteotomy of the proximal tibial metaphysis was described by Wagner3 for the treatment of unicompartmental osteoarthrosis of the knee in adults. Laurencin and colleagues4 applied this technique to the treatment of pediatric tibia vara with favorable results. They spared the medial cortex of the tibia in their incomplete closing wedge osteotomy technique. In each of the 9 cases we treated and describe here, we accidentally completed the tibial osteotomy when attempting the Laurencin technique. Given that the osteotomy was completed, we modified the Laurencin technique by using a 6-hole, 4.5-mm compression plate rather than a 5-hole semitubular plate, and added a large oblique screw from the medial side to compress the osteotomy site and to protect the plate from fracture. In addition, in 2 patients who weighed more than 250 pounds, we used an external fixator for additional stability. In this article, we report the outcomes of correcting adolescent tibia vara with a complete closing wedge tibial osteotomy and an oblique fibular osteotomy.
Materials and Methods
This study was approved by the Institutional Review Board at Pennsylvania State University. Between 2009 and 2012, we performed 9 complete oblique proximal tibial lateral closing wedge osteotomies on 8 patients (2 girls, 6 boys). In each case, the primary diagnosis was Blount disease. One patient also had renal dysplasia and was receiving dialysis. Mean age at time of operation was 15 years (range, 13-17 years). Mean preoperative weight was 215 pounds (range, 119-317 lb). Mean weight gain at follow-up was 4.39 pounds (range, –10 to 19 lb). Mean body mass index (BMI) was 38 (range, 25-48) (Table). All patients had varus angulation of the proximal tibia before surgery. Mean preoperative varus on standing films was 22° (range, 10°-36°). Because of the patients’ size, we used standing long-leg radiographs, on individual cassettes, for each leg.
Surgical Technique
Before surgery, we use paper cutouts to template the osteotomy wedge. We also use perioperative antibiotics and a standard time-out. For visualization of the entire leg for accurate correction, we prepare and drape the entire leg. A sterile tourniquet is used. At the midshaft of the fibula, a 4-cm incision is made, and dissection is carefully carried down to the fibula. Subperiosteal dissection is performed about the fibula, allowing adequate clearance for an oblique osteotomy. The osteotomy removes about 1 cm of fibula, which is to be used as bone graft for the tibial osteotomy. In addition, a lateral compartment fasciotomy is performed to prevent swelling-related complications. The wound is irrigated and injected with bupivacaine and closed in routine fashion.
We then make an inverted hockey-stick incision over the proximal tibia, centered down to the tibial tubercle. After dissecting down to the anterior compartment, we perform a fasciotomy of about 8 cm to accommodate swelling. Subperiosteal dissection is then performed around the proximal tibia. The medial soft tissues are left attached to increase blood supply and healing. During subperiosteal dissection, soft elevators are used to gently retract the lateral soft tissues along with the inferior and posterior structures. We use fluoroscopic imaging to guide the osteotomy as well as screw and plate placement. We use a 6-hole, 4.5-mm compression plate and screws for fixation. The 2 proximal screws of the plate are predrilled in place to allow for application of the plate after completion of the osteotomy. The plate is then rotated out of position on 1 screw, and the osteotomy is identified under fluoroscopy with the appropriate position distal to the second hole of the 6-hole plate.
An oscillating saw and osteotomes are used to perform the oblique osteotomy. The pre-estimated bone wedge is removed. Wedge size is adjusted, if needed. The bone wedge is morselized for bone graft. The osteotomy is then closed, correcting both varus and internal tibial torsion. Our goal is 5° valgus. After correction is obtained, the plate is placed, and the proximal screw is snugly seated. Three cortical screws are placed distally to hold the plate in place under compression mode, and a cancellous screw is placed superiorly at the proximal portion of the plate for additional fixation. The screw placed proximal to the osteotomy site is a fully threaded cortical screw with excellent compression. Correction and proper placement of hardware are verified with fluoroscopy.
The wound is irrigated and injected with bupivacaine. Bone graft is then placed at the osteotomy site. Additional bone graft is placed posteriorly between the osteotomy site and the muscle mass to stimulate additional healing. Another screw is placed obliquely from the medial side across the osteotomy site to provide additional fixation (Figure 1).
A deep drain is placed and connected to bulb suction for 24 hours after surgery. The wound is then closed in routine fashion. In 2 patients who weighed more than 250 pounds, we used an external fixator for additional stability (Figure 2).
Postoperative Care
The incisions are dressed with antibiotic ointment and 4×4-in bandages and then wrapped with sterile cotton under-cast padding. The leg is placed into a well-padded cylinder cast with the knee flexed 10°. The leg is aligned to about 5° valgus. The cast is then split on the side and spread to allow for swelling and to prevent compartment syndrome.5 We also use a drain hooked to bulb suction, which is removed 24 hours after surgery. Toe-touch weight-bearing with crutches is allowed immediately after surgery. The cast is removed at 6 weeks, and a hinged range-of-motion knee brace is worn for another 6 weeks. All patients are allowed to resume normal activity after 4 months. In our 2 external-fixator cases, a cast was not used, and toe-touch weight-bearing and knee motion were allowed immediately. The external fixators were removed at about 10 weeks.
Results
Mean postoperative mechanical femoral-tibial angle was 3°, and mean correction was 26° (range, 16°-43°) (Table). Lateral distal femoral angle did not show significant femoral deformity in our sample. Mean medial proximal tibial angle was 74° (range, 63°-79°). In each case, the varus deformity was primarily in the tibia. Mean tourniquet time was 88 minutes (range, 50-119 min). Our complication rate was 11% (1 knee). In our first case, in which we did not use an extra medial screw, the 4.5-mm plate fractured at the osteotomy site 2.5 months after surgery. The 250-pound patient subsequently lost 17° of correction, and valgus alignment was not achieved. Preoperative varus was 25°, and postoperative alignment was 8° varus. This plate fracture led us to use an extra medial screw for additional stability in all subsequent cases and to consider using an external fixator for patients weighing more than 250 pounds. After the first case, there were no other plate fractures. A potential problem with closing wedge osteotomy is shortening, but varus correction restores some length. Mean postoperative leg-length difference was 10 mm (range, 0-16 mm). No patient complained of leg-length difference during the postoperative follow-up.
Eight and a half months after surgery, 1 patient had hardware removed, at the family’s request. No patient experienced perioperative infection or neurovascular damage. Our overall patient population was obese—mean BMI was 38 (range, 25-48), and mean postoperative weight was 219 pounds. Three of our 8 patients were overweight (BMI, 25-30), and 5 were obese (BMI, >30). For prevention of plate failure, we recommend using an extra oblique screw in all patients and considering an external fixator for patients who weigh more than 250 pounds.
Discussion
Correction of adolescent tibia vara can be challenging because of patient obesity. The technique described here—a modification of the technique of Laurencin and colleagues4—is practical and reproducible in this population. The goals in performing osteotomy are to correct the deformity, restore joint alignment, preserve leg length, and prevent recurrent deformity and other complications, such as neurovascular injury, nonunion, and infection.3,6-8 Our technique minimizes the risk for these complications. For example, the fasciotomy provides excellent decompression of the anterior and lateral compartments, minimizing neurovascular ischemia and the risk for compartment syndrome. During cast placement, splitting and spreading reduce the risk for compartment syndrome as well.5
Wagner3,9 demonstrated the utility of a closing wedge proximal tibial osteotomy in adults. Laurencin and colleagues4 showed this technique is effective in correcting tibia vara in a pediatric population. However, they did not specify patient weight and used a small semitubular plate for fixation, and some of their patients had infantile Blount disease. We modified the technique in 3 ways. First, we performed a complete osteotomy. Second, because our patients were adolescents and very large, we used a 6-hole, 4.5-mm compression plate and screws. Third, we used an external fixator for increased stability in patients who weighed more than 250 pounds.
The reported technique, using an oblique metaphyseal closing wedge osteotomy with internal fixation in obese patients, is practical, safe, and reliable. This technique is a useful alternative to an external fixator. We used it on 9 knees with tibia vara, and it was completely successful in 8 cases and partially successful in 1 (hardware breakage occurred). An external fixator was used to prevent hardware breakage in 2 patients who weighed more than 250 pounds. This technique is a valuable treatment option for surgical correction, especially in obese patients.
Blount disease (tibia vara) is an angular tibia deformity that includes varus, increased posterior slope, and internal rotation. This deformity was first described in 1922 by Erlacher1 in Germany. In 1937, Walter Blount2 reported on it in the United States. It is the most common cause of pathologic genu varum in adolescence and childhood.
An oblique incomplete closing wedge osteotomy of the proximal tibial metaphysis was described by Wagner3 for the treatment of unicompartmental osteoarthrosis of the knee in adults. Laurencin and colleagues4 applied this technique to the treatment of pediatric tibia vara with favorable results. They spared the medial cortex of the tibia in their incomplete closing wedge osteotomy technique. In each of the 9 cases we treated and describe here, we accidentally completed the tibial osteotomy when attempting the Laurencin technique. Given that the osteotomy was completed, we modified the Laurencin technique by using a 6-hole, 4.5-mm compression plate rather than a 5-hole semitubular plate, and added a large oblique screw from the medial side to compress the osteotomy site and to protect the plate from fracture. In addition, in 2 patients who weighed more than 250 pounds, we used an external fixator for additional stability. In this article, we report the outcomes of correcting adolescent tibia vara with a complete closing wedge tibial osteotomy and an oblique fibular osteotomy.
Materials and Methods
This study was approved by the Institutional Review Board at Pennsylvania State University. Between 2009 and 2012, we performed 9 complete oblique proximal tibial lateral closing wedge osteotomies on 8 patients (2 girls, 6 boys). In each case, the primary diagnosis was Blount disease. One patient also had renal dysplasia and was receiving dialysis. Mean age at time of operation was 15 years (range, 13-17 years). Mean preoperative weight was 215 pounds (range, 119-317 lb). Mean weight gain at follow-up was 4.39 pounds (range, –10 to 19 lb). Mean body mass index (BMI) was 38 (range, 25-48) (Table). All patients had varus angulation of the proximal tibia before surgery. Mean preoperative varus on standing films was 22° (range, 10°-36°). Because of the patients’ size, we used standing long-leg radiographs, on individual cassettes, for each leg.
Surgical Technique
Before surgery, we use paper cutouts to template the osteotomy wedge. We also use perioperative antibiotics and a standard time-out. For visualization of the entire leg for accurate correction, we prepare and drape the entire leg. A sterile tourniquet is used. At the midshaft of the fibula, a 4-cm incision is made, and dissection is carefully carried down to the fibula. Subperiosteal dissection is performed about the fibula, allowing adequate clearance for an oblique osteotomy. The osteotomy removes about 1 cm of fibula, which is to be used as bone graft for the tibial osteotomy. In addition, a lateral compartment fasciotomy is performed to prevent swelling-related complications. The wound is irrigated and injected with bupivacaine and closed in routine fashion.
We then make an inverted hockey-stick incision over the proximal tibia, centered down to the tibial tubercle. After dissecting down to the anterior compartment, we perform a fasciotomy of about 8 cm to accommodate swelling. Subperiosteal dissection is then performed around the proximal tibia. The medial soft tissues are left attached to increase blood supply and healing. During subperiosteal dissection, soft elevators are used to gently retract the lateral soft tissues along with the inferior and posterior structures. We use fluoroscopic imaging to guide the osteotomy as well as screw and plate placement. We use a 6-hole, 4.5-mm compression plate and screws for fixation. The 2 proximal screws of the plate are predrilled in place to allow for application of the plate after completion of the osteotomy. The plate is then rotated out of position on 1 screw, and the osteotomy is identified under fluoroscopy with the appropriate position distal to the second hole of the 6-hole plate.
An oscillating saw and osteotomes are used to perform the oblique osteotomy. The pre-estimated bone wedge is removed. Wedge size is adjusted, if needed. The bone wedge is morselized for bone graft. The osteotomy is then closed, correcting both varus and internal tibial torsion. Our goal is 5° valgus. After correction is obtained, the plate is placed, and the proximal screw is snugly seated. Three cortical screws are placed distally to hold the plate in place under compression mode, and a cancellous screw is placed superiorly at the proximal portion of the plate for additional fixation. The screw placed proximal to the osteotomy site is a fully threaded cortical screw with excellent compression. Correction and proper placement of hardware are verified with fluoroscopy.
The wound is irrigated and injected with bupivacaine. Bone graft is then placed at the osteotomy site. Additional bone graft is placed posteriorly between the osteotomy site and the muscle mass to stimulate additional healing. Another screw is placed obliquely from the medial side across the osteotomy site to provide additional fixation (Figure 1).
A deep drain is placed and connected to bulb suction for 24 hours after surgery. The wound is then closed in routine fashion. In 2 patients who weighed more than 250 pounds, we used an external fixator for additional stability (Figure 2).
Postoperative Care
The incisions are dressed with antibiotic ointment and 4×4-in bandages and then wrapped with sterile cotton under-cast padding. The leg is placed into a well-padded cylinder cast with the knee flexed 10°. The leg is aligned to about 5° valgus. The cast is then split on the side and spread to allow for swelling and to prevent compartment syndrome.5 We also use a drain hooked to bulb suction, which is removed 24 hours after surgery. Toe-touch weight-bearing with crutches is allowed immediately after surgery. The cast is removed at 6 weeks, and a hinged range-of-motion knee brace is worn for another 6 weeks. All patients are allowed to resume normal activity after 4 months. In our 2 external-fixator cases, a cast was not used, and toe-touch weight-bearing and knee motion were allowed immediately. The external fixators were removed at about 10 weeks.
Results
Mean postoperative mechanical femoral-tibial angle was 3°, and mean correction was 26° (range, 16°-43°) (Table). Lateral distal femoral angle did not show significant femoral deformity in our sample. Mean medial proximal tibial angle was 74° (range, 63°-79°). In each case, the varus deformity was primarily in the tibia. Mean tourniquet time was 88 minutes (range, 50-119 min). Our complication rate was 11% (1 knee). In our first case, in which we did not use an extra medial screw, the 4.5-mm plate fractured at the osteotomy site 2.5 months after surgery. The 250-pound patient subsequently lost 17° of correction, and valgus alignment was not achieved. Preoperative varus was 25°, and postoperative alignment was 8° varus. This plate fracture led us to use an extra medial screw for additional stability in all subsequent cases and to consider using an external fixator for patients weighing more than 250 pounds. After the first case, there were no other plate fractures. A potential problem with closing wedge osteotomy is shortening, but varus correction restores some length. Mean postoperative leg-length difference was 10 mm (range, 0-16 mm). No patient complained of leg-length difference during the postoperative follow-up.
Eight and a half months after surgery, 1 patient had hardware removed, at the family’s request. No patient experienced perioperative infection or neurovascular damage. Our overall patient population was obese—mean BMI was 38 (range, 25-48), and mean postoperative weight was 219 pounds. Three of our 8 patients were overweight (BMI, 25-30), and 5 were obese (BMI, >30). For prevention of plate failure, we recommend using an extra oblique screw in all patients and considering an external fixator for patients who weigh more than 250 pounds.
Discussion
Correction of adolescent tibia vara can be challenging because of patient obesity. The technique described here—a modification of the technique of Laurencin and colleagues4—is practical and reproducible in this population. The goals in performing osteotomy are to correct the deformity, restore joint alignment, preserve leg length, and prevent recurrent deformity and other complications, such as neurovascular injury, nonunion, and infection.3,6-8 Our technique minimizes the risk for these complications. For example, the fasciotomy provides excellent decompression of the anterior and lateral compartments, minimizing neurovascular ischemia and the risk for compartment syndrome. During cast placement, splitting and spreading reduce the risk for compartment syndrome as well.5
Wagner3,9 demonstrated the utility of a closing wedge proximal tibial osteotomy in adults. Laurencin and colleagues4 showed this technique is effective in correcting tibia vara in a pediatric population. However, they did not specify patient weight and used a small semitubular plate for fixation, and some of their patients had infantile Blount disease. We modified the technique in 3 ways. First, we performed a complete osteotomy. Second, because our patients were adolescents and very large, we used a 6-hole, 4.5-mm compression plate and screws. Third, we used an external fixator for increased stability in patients who weighed more than 250 pounds.
The reported technique, using an oblique metaphyseal closing wedge osteotomy with internal fixation in obese patients, is practical, safe, and reliable. This technique is a useful alternative to an external fixator. We used it on 9 knees with tibia vara, and it was completely successful in 8 cases and partially successful in 1 (hardware breakage occurred). An external fixator was used to prevent hardware breakage in 2 patients who weighed more than 250 pounds. This technique is a valuable treatment option for surgical correction, especially in obese patients.
1. Erlacher P. Deformierende Prozesse der Epiphysengegend bei Kindem. Archiv Orthop Unfall-Chir. 1922;20:81-96.
2. Blount WP. Tibia vara. J Bone Joint Surg. 1937;29:1-28.
3. Wagner H. Principles of corrective osteotomies in osteoarthrosis of the knee. In: Weal UH, ed. Joint Preserving Procedures of the Lower Extremity. New York, NY: Springer; 1980:77-102.
4. Laurencin CT, Ferriter PJ, Millis MB. Oblique proximal tibial osteotomy for the correction of tibia vara in the young. Clin Orthop Relat Res. 1996;(327):218-224.
5. Garfin SR, Mubarak SJ, Evans KL, Hargens AR, Akeson WH. Quantification of intracompartmental pressure and volume under plaster casts. J Bone Joint Surg Am. 1981;63(3):449-453.
6. Mycoskie PJ. Complications of osteotomies about the knee in children. Orthopedics. 1981;4(9):1005-1015.
7. Matsen FA, Staheli LT. Neurovascular complications following tibial osteotomy in children. A case report. Clin Orthop Relat Res. 1975;(110):210-214.
8. Steel HH, Sandrew RE, Sullivan PD. Complications of tibial osteotomy in children for genu varum or valgum. Evidence that neurological changes are due to ischemia. J Bone Joint Surg Am. 1971;53(8):1629-1635.
9. Wagner H. The displacement osteotomy as a correction principle. In: Heirholzer G, Muller KH, eds. Corrective Osteotomies of the Lower Extremity After Trauma. Berlin, Germany: Springer; 1985:141-150.
1. Erlacher P. Deformierende Prozesse der Epiphysengegend bei Kindem. Archiv Orthop Unfall-Chir. 1922;20:81-96.
2. Blount WP. Tibia vara. J Bone Joint Surg. 1937;29:1-28.
3. Wagner H. Principles of corrective osteotomies in osteoarthrosis of the knee. In: Weal UH, ed. Joint Preserving Procedures of the Lower Extremity. New York, NY: Springer; 1980:77-102.
4. Laurencin CT, Ferriter PJ, Millis MB. Oblique proximal tibial osteotomy for the correction of tibia vara in the young. Clin Orthop Relat Res. 1996;(327):218-224.
5. Garfin SR, Mubarak SJ, Evans KL, Hargens AR, Akeson WH. Quantification of intracompartmental pressure and volume under plaster casts. J Bone Joint Surg Am. 1981;63(3):449-453.
6. Mycoskie PJ. Complications of osteotomies about the knee in children. Orthopedics. 1981;4(9):1005-1015.
7. Matsen FA, Staheli LT. Neurovascular complications following tibial osteotomy in children. A case report. Clin Orthop Relat Res. 1975;(110):210-214.
8. Steel HH, Sandrew RE, Sullivan PD. Complications of tibial osteotomy in children for genu varum or valgum. Evidence that neurological changes are due to ischemia. J Bone Joint Surg Am. 1971;53(8):1629-1635.
9. Wagner H. The displacement osteotomy as a correction principle. In: Heirholzer G, Muller KH, eds. Corrective Osteotomies of the Lower Extremity After Trauma. Berlin, Germany: Springer; 1985:141-150.
US National Practice Patterns in Ambulatory Operative Management of Lateral Epicondylitis
First described by Runge1 in 1873 and later termed lawn-tennis arm by Major2 in 1883, lateral epicondylitis is a common cause of elbow pain, affecting 1% to 3% of the general population each year.3,4 Given that prevalence estimates are up to 15% among workers in repetitive hand task industries,5-7 symptoms of lateral epicondylitis are thought to be related to recurring wrist extension and alternating forearm pronation and supination.8 Between 80% and 90% of patients with lateral epicondylitis experience symptomatic improvement with conservative therapy,9-11 including rest and use of nonsteroidal anti-inflammatory medications,12 physical therapy,13,14 corticosteroid injections,10,15,16 orthoses,17,18 and shock wave therapy.19 However, between 4% and 11% of patients with newly diagnosed lateral epicondylitis do not respond to prolonged (6- to 12-month) conservative treatment and then require operative intervention,11,20,21 with some referral practices reporting rates as high as 25%.22
Traditionally, operative management of lateral epicondylitis involved open débridement of the extensor carpi radialis brevis (ECRB).11,20 More recently, the spectrum of operations for lateral epicondylitis has expanded to include procedures that repair the extensor origin after débridement of the torn tendon and angiofibroblastic dysplasia; procedures that use fasciotomy or direct release of the extensor origin from the epicondyle to relieve tension on the common extensor; procedures directed at the radial or posterior interosseous nerve; and procedures that use arthroscopic techniques to divide the orbicular ligament, reshape the radial head, or release the extensor origin.23 There has been debate about the value of repairing the ECRB, lengthening the ECRB, simultaneously decompressing the radial nerve or resecting epicondylar bone, and performing the procedures percutaneously, endoscopically, or arthroscopically.24-28 Despite multiple studies of the outcomes of these procedures,11,29-31 little is known regarding US national trends for operative treatment of lateral epicondylitis. Understanding national practice patterns and disease burden is essential to allocation of limited health care resources.
We conducted a study to determine US national trends in use of ambulatory surgery for lateral epicondylitis. We focused on age, sex, surgical setting, anesthetic type, and payment method.
Methods
As the National Survey of Ambulatory Surgery32 (NSAS) is an administrative dataset in which all data are deidentified and available for public use, this study was exempt from requiring institutional review board approval.
NSAS data were used to analyze trends in treatment of lateral epicondylitis between 1994 and 2006. NSAS was undertaken by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC) to obtain information about the use of ambulatory surgery in the United States. Since the early 1980s, ambulatory surgery has increased in the United States because of advances in medical technology and cost-containment initiatives.33 The number of procedures being performed in ambulatory surgery centers increased from 31.5 million in 1996 to 53.3 million in 2006.34 Funded by the CDC, NSAS is a national study that involves both hospital-based and freestanding ambulatory surgery centers and provides the most recent and comprehensive overview of ambulatory surgery in the United States.35 Because of budgetary limitations, 2006 was the last year in which data for NSAS were collected. Data for NSAS come from Medicare-participating, noninstitutional hospitals (excluding military hospitals, federal facilities, and Veteran Affairs hospitals) in all 50 states and the District of Columbia with a minimum of 6 beds staffed for patient use. NSAS used only short-stay hospitals (hospitals with an average length of stay for all patients of less than 30 days) or hospitals that had a specialty of general (medical or surgical) or children’s general. NSAS was conducted in 1994, 1996, and 2006 with medical information recorded on patient abstracts coded by contract staff. NSAS selected a sample of ambulatory surgery visits using a systematic random sampling procedure, and selection of visits within each facility was done separately for each location where ambulatory surgery was performed. In 1994, 751 facilities were sampled, and 88% of hospitals responded. In 1996, 750 facilities were sampled, and 91% of hospitals responded. In 2006, 696 facilities were sampled, and 75% responded. The surveys used International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes36 to classify medical diagnoses and procedures. To produce an unbiased national estimate, NCHS used multistage estimate procedures, including inflation by reciprocals of the probabilities of sample selection, population-weighting ratio adjustments, and adjustment for no response.37
Demographic and medical information was obtained for people with an ICD-9-CM diagnosis code of lateral epicondylitis (726.32), using previously described techniques.38 Data were then recorded for age, sex, facility type, insurance type, anesthesia type, diagnoses, and procedures.
Descriptive statistics consisted of means and standard deviations for continuous variables and frequency and percentages for discrete variables. Because NSAS data were collected on the basis of a probabilistic sample scheme, they were analyzed using a sampling weighting method. Sampling weights (inverse of selection probability) provided by the CDC were used to account for unequal sampling probabilities and to produce estimates for all visits in the United States. A Taylor linearization model provided by the CDC estimates was used to calculate standard error and confidence intervals (CIs) of the data. Standard error is a measure of sampling variability that occurs by chance because only a sample rather than the entire universe is surveyed. To define population parameters, NCHS chose 95% CIs along with a point estimate. Direct statistical comparison between years cannot be performed because of sampling differences in the database compared between years. The CIs, however, can suggest statistical differences if the data are nonoverlapping. US census data were used to obtain national population estimates for each year of the study (1994, 1996, 2006).39 Rates were presented as number of procedures per 100,000 standard population. For age, a direct adjustment procedure was used, and the US population in 2000 was selected as the standard population. Applying sex-specific rates to the standard population and dividing by the total in the standard population, we calculated sex-adjusted rates for each year. All data were analyzed using SPSS Version 20 software.
Results
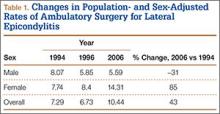
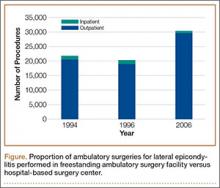
A total of 30,311 ambulatory surgical procedures (95% CI, 27,292-33,330) or 10.44 per 100,000 capita were recorded by NSAS for the treatment of lateral epicondylitis in 2006 (Table 1). This represents a large increase in the total number of ambulatory procedures, from 21,852 in 1994 (95% CI, 19,981-23,722; 7.29/100,000) and 20,372 in 1996 (95% CI, 18,660-22,083; 6.73/100,000).
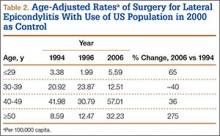
Between 1994 and 2006, the sex-adjusted rate of ambulatory surgery for lateral epicondylitis increased by 85% among females (7.74/100,000 to 14.31/100,000), whereas the rate decreased by 31% among males (8.07/100,000 to 5.59/100,000) (Table 1). The age-adjusted rate of ambulatory surgery for lateral epicondylitis increased among all age groups except the 30–39 years group (Table 2). The largest increase in age-adjusted rates was found for patients older than 50 years (275%) between 1994 and 2006.
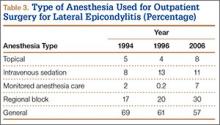
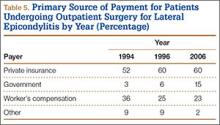
During the study period, use of regional anesthesia nearly doubled, from 17% to 30%, whereas use of general anesthesia decreased, from 69% to 57% (Table 3). At all time points, the most common procedure performed for lateral epicondylitis in ambulatory surgery centers was division/release of the joint capsule of the elbow (Table 4). Private insurance remained the most common source of payment for all study years, ranging from 52% to 60% (Table 5). The Figure shows that, between 1994 and 2006, the proportion of surgeries performed in a freestanding ambulatory center increased.
Discussion
In this descriptive epidemiologic study, we used NSAS data to investigate trends in ambulatory surgery for lateral epicondylitis between 1994 and 2006.32 Our results showed that total number of procedures and the population-adjusted rate of procedures for lateral epicondylitis increased during the study period. The largest increase in age-adjusted rates of surgery for lateral epicondylitis was found among patients older than 50 years, whereas the highest age-adjusted rate of ambulatory surgery for lateral epicondylitis was found among patients between ages 40 and 49 years. These findings are similar to those of previous studies, which have shown that most patients with lateral epicondylitis present in the fourth and fifth decades of life.22 Prior reports have suggested that the incidence of lateral epicondylitis in men and women is equal.22 The present study found a change in sex-adjusted rates of ambulatory surgery for lateral epicondylitis between 1994 and 2006. Specifically, in 1994, surgery rates for men and women were similar (8.07/100,000 and 7.74/100,000), but in 2006 the sex-adjusted rate of surgery for lateral epicondylitis was almost 3 times higher for women than for men (14.31/100,000 vs 5.59/100,000).
We also found that the population-adjusted rate of lateral epicondylectomy increased drastically, from 0.4 per 100,000 in 1994 to 3.53 per 100,000 in 2006. Lateral epicondylectomy involves excision of the tip of the lateral epicondyle (typically, 0.5 cm) to produce a cancellous bone surface to which the edges of the débrided extensor tendon can be approximated without tension.23 It is possible that the increased rate of lateral epicondylectomy reflects evidence-based practice changes during the study period,27 though denervation was found more favorable than epicondylectomy in a recent study by Berry and colleagues.40 Future studies should investigate whether rates of epicondylectomy have changed since 2006. In addition, the present study showed a correlation between the introduction of arthroscopic techniques for the treatment of lateral epicondylitis and the period when much research was being conducted on the topic.24,25,28 As arthroscopic techniques improve, their rates are likely to continue to increase.
Our results also showed an increase in procedures performed in freestanding facilities. The rise in ambulatory surgical volume, speculated to result from more procedures being performed in freestanding facilities,34 has been reported with knee and shoulder arthroscopy.41 In addition, though general anesthesia remained the most used technique, our results showed a shift toward peripheral nerve blocks. The increase in regional anesthesia, which has also been noted in joint arthroscopy, is thought to stem from the advent of nerve-localizing technology, such as nerve stimulation and ultrasound guidance.41 Peripheral nerve blocks are favorable on both economic and quality measures, are associated with fewer opioid-related side effects, and overall provide better analgesia in comparison with opioids, highlighting their importance in the ambulatory setting.42
Although large, national databases are well suited to epidemiologic research,43 our study had limitations. As with all databases, NSAS is subject to data entry errors and coding errors.44,45 However, the database administrators corrected for this by using a multistage estimate procedure with weighting adjustments for no response and population-weighting ratio adjustments.35 Another limitation of this study is its lack of clinical detail, as procedure codes are general and do not allow differentiation between specific patients. Because of the retrospective nature of the analysis and the heterogeneity of the data, assessment of specific surgeries for lateral epicondylitis was limited. Although a strength of using NSAS to perform epidemiologic analyses is its large sample size, this also sacrifices specificity in terms of clinical insight. The results of this study may influence investigations to distinguish differences between procedures used in the treatment of lateral epicondylitis. Furthermore, the results of this study are limited to ambulatory surgery practice patterns in the United States between 1996 and 2006. Last, our ability to perform economic analyses was limited, as data on total hospital cost were not recorded by the surveys.
Conclusion
The increase in ambulatory surgery for lateral epicondylitis, demonstrated in this study, emphasizes the importance of national funding for surveys such as NSAS beyond 2006, as utilization trends may have considerable effects on health care policies that influence the quality of patient care.
1. Runge F. Zur genese und behandlung des schreibekramfes. Berl Klin Wochenschr. 1873;10:245.
2. Major HP. Lawn-tennis elbow. Br Med J. 1883;2:557.
3. Allander E. Prevalence, incidence, and remission rates of some common rheumatic diseases or syndromes. Scand J Rheumatol. 1974;3(3):145-153.
4. Verhaar JA. Tennis elbow. Anatomical, epidemiological and therapeutic aspects. Int Orthop. 1994;18(5):263-267.
5. Kurppa K, Viikari-Juntura E, Kuosma E, Huuskonen M, Kivi P. Incidence of tenosynovitis or peritendinitis and epicondylitis in a meat-processing factory. Scand J Work Environ Health. 1991;17(1):32-37.
6. Ranney D, Wells R, Moore A. Upper limb musculoskeletal disorders in highly repetitive industries: precise anatomical physical findings. Ergonomics. 1995;38(7):1408-1423.
7. Haahr JP, Andersen JH. Physical and psychosocial risk factors for lateral epicondylitis: a population based case-referent study. Occup Environ Med. 2003;60(5):322-329.
8. Goldie I. Epicondylitis lateralis humeri (epicondylalgia or tennis elbow). A pathogenetical study. Acta Chir Scand Suppl. 1964;57(suppl 399):1+.
9. Binder AI, Hazleman BL. Lateral humeral epicondylitis—a study of natural history and the effect of conservative therapy. Br J Rheumatol. 1983;22(2):73-76.
10. Smidt N, van der Windt DA, Assendelft WJ, Devillé WL, Korthals-de Bos IB, Bouter LM. Corticosteroid injections, physiotherapy, or a wait-and-see policy for lateral epicondylitis: a randomised controlled trial. Lancet. 2002;359(9307):657-662.
11. Nirschl RP, Pettrone FA. Tennis elbow. The surgical treatment of lateral epicondylitis. J Bone Joint Surg Am. 1979;61(6):832-839.
12. Burnham R, Gregg R, Healy P, Steadward R. The effectiveness of topical diclofenac for lateral epicondylitis. Clin J Sport Med. 1998;8(2):78-81.
13. Martinez-Silvestrini JA, Newcomer KL, Gay RE, Schaefer MP, Kortebein P, Arendt KW. Chronic lateral epicondylitis: comparative effectiveness of a home exercise program including stretching alone versus stretching supplemented with eccentric or concentric strengthening. J Hand Ther. 2005;18(4):411-419.
14. Svernlöv B, Adolfsson L. Non-operative treatment regime including eccentric training for lateral humeral epicondylalgia. Scand J Med Sci Sports. 2001;11(6):328-334.
15. Hay EM, Paterson SM, Lewis M, Hosie G, Croft P. Pragmatic randomised controlled trial of local corticosteroid injection and naproxen for treatment of lateral epicondylitis of elbow in primary care. BMJ. 1999;319(7215):964-968.
16. Lewis M, Hay EM, Paterson SM, Croft P. Local steroid injections for tennis elbow: does the pain get worse before it gets better? Results from a randomized controlled trial. Clin J Pain. 2005;21(4):330-334.
17. Van De Streek MD, Van Der Schans CP, De Greef MH, Postema K. The effect of a forearm/hand splint compared with an elbow band as a treatment for lateral epicondylitis. Prosthet Orthot Int. 2004;28(2):183-189.
18. Struijs PA, Smidt N, Arola H, Dijk vC, Buchbinder R, Assendelft WJ. Orthotic devices for the treatment of tennis elbow. Cochrane Database Syst Rev. 2002;(1):CD001821.
19. Buchbinder R, Green SE, Youd JM, Assendelft WJ, Barnsley L, Smidt N. Shock wave therapy for lateral elbow pain. Cochrane Database Syst Rev. 2005;(4):CD003524.
20. Boyd HB, McLeod AC Jr. Tennis elbow. J Bone Joint Surg Am. 1973;55(6):1183-1187.
21. Coonrad RW, Hooper WR. Tennis elbow: its course, natural history, conservative and surgical management. J Bone Joint Surg Am. 1973;55(6):1177-1182.
22. Calfee RP, Patel A, DaSilva MF, Akelman E. Management of lateral epicondylitis: current concepts. J Am Acad Orthop Surg. 2008;16(1):19-29.
23. Plancher KD, Bishai SK. Open lateral epicondylectomy: a simple technique update for the 21st century. Tech Orthop. 2006;21(4):276-282.
24. Peart RE, Strickler SS, Schweitzer KM Jr. Lateral epicondylitis: a comparative study of open and arthroscopic lateral release. Am J Orthop. 2004;33(11):565-567.
25. Dunkow PD, Jatti M, Muddu BN. A comparison of open and percutaneous techniques in the surgical treatment of tennis elbow. J Bone Joint Surg Br. 2004;86(5):701-704.
26. Rosenberg N, Henderson I. Surgical treatment of resistant lateral epicondylitis. Follow-up study of 19 patients after excision, release and repair of proximal common extensor tendon origin. Arch Orthop Trauma Surg. 2002;122(9-10):514-517.
27. Almquist EE, Necking L, Bach AW. Epicondylar resection with anconeus muscle transfer for chronic lateral epicondylitis. J Hand Surg Am. 1998;23(4):723-731.
28. Smith AM, Castle JA, Ruch DS. Arthroscopic resection of the common extensor origin: anatomic considerations. J Shoulder Elbow Surg. 2003;12(4):375-379.
29. Baker CL Jr, Murphy KP, Gottlob CA, Curd DT. Arthroscopic classification and treatment of lateral epicondylitis: two-year clinical results. J Shoulder Elbow Surg. 2000;9(6):475-482.
30. Owens BD, Murphy KP, Kuklo TR. Arthroscopic release for lateral epicondylitis. Arthroscopy. 2001;17(6):582-587.
31. Mullett H, Sprague M, Brown G, Hausman M. Arthroscopic treatment of lateral epicondylitis: clinical and cadaveric studies. Clin Orthop Relat Res. 2005;(439):123-128.
32. National Survey of Ambulatory Surgery. Centers for Disease Control and Prevention website. http://www.cdc.gov/nchs/nsas/nsas_questionnaires.htm. Published May 4, 2010. Accessed November 10, 2015.
33. Leader S, Moon M. Medicare trends in ambulatory surgery. Health Aff. 1989;8(1):158-170.
34. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;(11):1-25.
35. Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg Am. 2011;93(11):994-1000.
36. Centers for Disease Control and Prevention, National Center for Health Statistics. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). http://www.cdc.gov/nchs/icd/icd9cm.htm. Updated June 18, 2013. Accessed October 28, 2015.
37. Dennison C, Pokras R. Design and operation of the National Hospital Discharge Survey: 1988 redesign. Vital Health Stat 1. 2000;(39):1-42.
38. Stundner O, Kirksey M, Chiu YL, et al. Demographics and perioperative outcome in patients with depression and anxiety undergoing total joint arthroplasty: a population-based study. Psychosomatics. 2013;54(2):149-157.
39. Population estimates. US Department of Commerce, United States Census Bureau website. http://www.census.gov/popest/index.html. Accessed November 16, 2015.
40. Berry N, Neumeister MW, Russell RC, Dellon AL. Epicondylectomy versus denervation for lateral humeral epicondylitis. Hand. 2011;6(2):174-178.
41. Memtsoudis SG, Kuo C, Ma Y, Edwards A, Mazumdar M, Liguori G. Changes in anesthesia-related factors in ambulatory knee and shoulder surgery: United States 1996–2006. Reg Anesth Pain Med. 2011;36(4):327-331.
42. Richman JM, Liu SS, Courpas G, et al. Does continuous peripheral nerve block provide superior pain control to opioids? A meta-analysis. Anesth Analg. 2006;102(1):248-257.
43. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680.
44. Gray DT, Hodge DO, Ilstrup DM, Butterfield LC, Baratz KH, Concordance of Medicare data and population-based clinical data on cataract surgery utilization in Olmsted County, Minnesota. Am J Epidemiol. 1997;145(12):1123-1126.
45. Memtsoudis SG. Limitations associated with the analysis of data from administrative databases. Anesthesiology. 2009;111(2):449.
First described by Runge1 in 1873 and later termed lawn-tennis arm by Major2 in 1883, lateral epicondylitis is a common cause of elbow pain, affecting 1% to 3% of the general population each year.3,4 Given that prevalence estimates are up to 15% among workers in repetitive hand task industries,5-7 symptoms of lateral epicondylitis are thought to be related to recurring wrist extension and alternating forearm pronation and supination.8 Between 80% and 90% of patients with lateral epicondylitis experience symptomatic improvement with conservative therapy,9-11 including rest and use of nonsteroidal anti-inflammatory medications,12 physical therapy,13,14 corticosteroid injections,10,15,16 orthoses,17,18 and shock wave therapy.19 However, between 4% and 11% of patients with newly diagnosed lateral epicondylitis do not respond to prolonged (6- to 12-month) conservative treatment and then require operative intervention,11,20,21 with some referral practices reporting rates as high as 25%.22
Traditionally, operative management of lateral epicondylitis involved open débridement of the extensor carpi radialis brevis (ECRB).11,20 More recently, the spectrum of operations for lateral epicondylitis has expanded to include procedures that repair the extensor origin after débridement of the torn tendon and angiofibroblastic dysplasia; procedures that use fasciotomy or direct release of the extensor origin from the epicondyle to relieve tension on the common extensor; procedures directed at the radial or posterior interosseous nerve; and procedures that use arthroscopic techniques to divide the orbicular ligament, reshape the radial head, or release the extensor origin.23 There has been debate about the value of repairing the ECRB, lengthening the ECRB, simultaneously decompressing the radial nerve or resecting epicondylar bone, and performing the procedures percutaneously, endoscopically, or arthroscopically.24-28 Despite multiple studies of the outcomes of these procedures,11,29-31 little is known regarding US national trends for operative treatment of lateral epicondylitis. Understanding national practice patterns and disease burden is essential to allocation of limited health care resources.
We conducted a study to determine US national trends in use of ambulatory surgery for lateral epicondylitis. We focused on age, sex, surgical setting, anesthetic type, and payment method.
Methods
As the National Survey of Ambulatory Surgery32 (NSAS) is an administrative dataset in which all data are deidentified and available for public use, this study was exempt from requiring institutional review board approval.
NSAS data were used to analyze trends in treatment of lateral epicondylitis between 1994 and 2006. NSAS was undertaken by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC) to obtain information about the use of ambulatory surgery in the United States. Since the early 1980s, ambulatory surgery has increased in the United States because of advances in medical technology and cost-containment initiatives.33 The number of procedures being performed in ambulatory surgery centers increased from 31.5 million in 1996 to 53.3 million in 2006.34 Funded by the CDC, NSAS is a national study that involves both hospital-based and freestanding ambulatory surgery centers and provides the most recent and comprehensive overview of ambulatory surgery in the United States.35 Because of budgetary limitations, 2006 was the last year in which data for NSAS were collected. Data for NSAS come from Medicare-participating, noninstitutional hospitals (excluding military hospitals, federal facilities, and Veteran Affairs hospitals) in all 50 states and the District of Columbia with a minimum of 6 beds staffed for patient use. NSAS used only short-stay hospitals (hospitals with an average length of stay for all patients of less than 30 days) or hospitals that had a specialty of general (medical or surgical) or children’s general. NSAS was conducted in 1994, 1996, and 2006 with medical information recorded on patient abstracts coded by contract staff. NSAS selected a sample of ambulatory surgery visits using a systematic random sampling procedure, and selection of visits within each facility was done separately for each location where ambulatory surgery was performed. In 1994, 751 facilities were sampled, and 88% of hospitals responded. In 1996, 750 facilities were sampled, and 91% of hospitals responded. In 2006, 696 facilities were sampled, and 75% responded. The surveys used International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes36 to classify medical diagnoses and procedures. To produce an unbiased national estimate, NCHS used multistage estimate procedures, including inflation by reciprocals of the probabilities of sample selection, population-weighting ratio adjustments, and adjustment for no response.37
Demographic and medical information was obtained for people with an ICD-9-CM diagnosis code of lateral epicondylitis (726.32), using previously described techniques.38 Data were then recorded for age, sex, facility type, insurance type, anesthesia type, diagnoses, and procedures.
Descriptive statistics consisted of means and standard deviations for continuous variables and frequency and percentages for discrete variables. Because NSAS data were collected on the basis of a probabilistic sample scheme, they were analyzed using a sampling weighting method. Sampling weights (inverse of selection probability) provided by the CDC were used to account for unequal sampling probabilities and to produce estimates for all visits in the United States. A Taylor linearization model provided by the CDC estimates was used to calculate standard error and confidence intervals (CIs) of the data. Standard error is a measure of sampling variability that occurs by chance because only a sample rather than the entire universe is surveyed. To define population parameters, NCHS chose 95% CIs along with a point estimate. Direct statistical comparison between years cannot be performed because of sampling differences in the database compared between years. The CIs, however, can suggest statistical differences if the data are nonoverlapping. US census data were used to obtain national population estimates for each year of the study (1994, 1996, 2006).39 Rates were presented as number of procedures per 100,000 standard population. For age, a direct adjustment procedure was used, and the US population in 2000 was selected as the standard population. Applying sex-specific rates to the standard population and dividing by the total in the standard population, we calculated sex-adjusted rates for each year. All data were analyzed using SPSS Version 20 software.
Results
A total of 30,311 ambulatory surgical procedures (95% CI, 27,292-33,330) or 10.44 per 100,000 capita were recorded by NSAS for the treatment of lateral epicondylitis in 2006 (Table 1). This represents a large increase in the total number of ambulatory procedures, from 21,852 in 1994 (95% CI, 19,981-23,722; 7.29/100,000) and 20,372 in 1996 (95% CI, 18,660-22,083; 6.73/100,000).
Between 1994 and 2006, the sex-adjusted rate of ambulatory surgery for lateral epicondylitis increased by 85% among females (7.74/100,000 to 14.31/100,000), whereas the rate decreased by 31% among males (8.07/100,000 to 5.59/100,000) (Table 1). The age-adjusted rate of ambulatory surgery for lateral epicondylitis increased among all age groups except the 30–39 years group (Table 2). The largest increase in age-adjusted rates was found for patients older than 50 years (275%) between 1994 and 2006.
During the study period, use of regional anesthesia nearly doubled, from 17% to 30%, whereas use of general anesthesia decreased, from 69% to 57% (Table 3). At all time points, the most common procedure performed for lateral epicondylitis in ambulatory surgery centers was division/release of the joint capsule of the elbow (Table 4). Private insurance remained the most common source of payment for all study years, ranging from 52% to 60% (Table 5). The Figure shows that, between 1994 and 2006, the proportion of surgeries performed in a freestanding ambulatory center increased.
Discussion
In this descriptive epidemiologic study, we used NSAS data to investigate trends in ambulatory surgery for lateral epicondylitis between 1994 and 2006.32 Our results showed that total number of procedures and the population-adjusted rate of procedures for lateral epicondylitis increased during the study period. The largest increase in age-adjusted rates of surgery for lateral epicondylitis was found among patients older than 50 years, whereas the highest age-adjusted rate of ambulatory surgery for lateral epicondylitis was found among patients between ages 40 and 49 years. These findings are similar to those of previous studies, which have shown that most patients with lateral epicondylitis present in the fourth and fifth decades of life.22 Prior reports have suggested that the incidence of lateral epicondylitis in men and women is equal.22 The present study found a change in sex-adjusted rates of ambulatory surgery for lateral epicondylitis between 1994 and 2006. Specifically, in 1994, surgery rates for men and women were similar (8.07/100,000 and 7.74/100,000), but in 2006 the sex-adjusted rate of surgery for lateral epicondylitis was almost 3 times higher for women than for men (14.31/100,000 vs 5.59/100,000).
We also found that the population-adjusted rate of lateral epicondylectomy increased drastically, from 0.4 per 100,000 in 1994 to 3.53 per 100,000 in 2006. Lateral epicondylectomy involves excision of the tip of the lateral epicondyle (typically, 0.5 cm) to produce a cancellous bone surface to which the edges of the débrided extensor tendon can be approximated without tension.23 It is possible that the increased rate of lateral epicondylectomy reflects evidence-based practice changes during the study period,27 though denervation was found more favorable than epicondylectomy in a recent study by Berry and colleagues.40 Future studies should investigate whether rates of epicondylectomy have changed since 2006. In addition, the present study showed a correlation between the introduction of arthroscopic techniques for the treatment of lateral epicondylitis and the period when much research was being conducted on the topic.24,25,28 As arthroscopic techniques improve, their rates are likely to continue to increase.
Our results also showed an increase in procedures performed in freestanding facilities. The rise in ambulatory surgical volume, speculated to result from more procedures being performed in freestanding facilities,34 has been reported with knee and shoulder arthroscopy.41 In addition, though general anesthesia remained the most used technique, our results showed a shift toward peripheral nerve blocks. The increase in regional anesthesia, which has also been noted in joint arthroscopy, is thought to stem from the advent of nerve-localizing technology, such as nerve stimulation and ultrasound guidance.41 Peripheral nerve blocks are favorable on both economic and quality measures, are associated with fewer opioid-related side effects, and overall provide better analgesia in comparison with opioids, highlighting their importance in the ambulatory setting.42
Although large, national databases are well suited to epidemiologic research,43 our study had limitations. As with all databases, NSAS is subject to data entry errors and coding errors.44,45 However, the database administrators corrected for this by using a multistage estimate procedure with weighting adjustments for no response and population-weighting ratio adjustments.35 Another limitation of this study is its lack of clinical detail, as procedure codes are general and do not allow differentiation between specific patients. Because of the retrospective nature of the analysis and the heterogeneity of the data, assessment of specific surgeries for lateral epicondylitis was limited. Although a strength of using NSAS to perform epidemiologic analyses is its large sample size, this also sacrifices specificity in terms of clinical insight. The results of this study may influence investigations to distinguish differences between procedures used in the treatment of lateral epicondylitis. Furthermore, the results of this study are limited to ambulatory surgery practice patterns in the United States between 1996 and 2006. Last, our ability to perform economic analyses was limited, as data on total hospital cost were not recorded by the surveys.
Conclusion
The increase in ambulatory surgery for lateral epicondylitis, demonstrated in this study, emphasizes the importance of national funding for surveys such as NSAS beyond 2006, as utilization trends may have considerable effects on health care policies that influence the quality of patient care.
First described by Runge1 in 1873 and later termed lawn-tennis arm by Major2 in 1883, lateral epicondylitis is a common cause of elbow pain, affecting 1% to 3% of the general population each year.3,4 Given that prevalence estimates are up to 15% among workers in repetitive hand task industries,5-7 symptoms of lateral epicondylitis are thought to be related to recurring wrist extension and alternating forearm pronation and supination.8 Between 80% and 90% of patients with lateral epicondylitis experience symptomatic improvement with conservative therapy,9-11 including rest and use of nonsteroidal anti-inflammatory medications,12 physical therapy,13,14 corticosteroid injections,10,15,16 orthoses,17,18 and shock wave therapy.19 However, between 4% and 11% of patients with newly diagnosed lateral epicondylitis do not respond to prolonged (6- to 12-month) conservative treatment and then require operative intervention,11,20,21 with some referral practices reporting rates as high as 25%.22
Traditionally, operative management of lateral epicondylitis involved open débridement of the extensor carpi radialis brevis (ECRB).11,20 More recently, the spectrum of operations for lateral epicondylitis has expanded to include procedures that repair the extensor origin after débridement of the torn tendon and angiofibroblastic dysplasia; procedures that use fasciotomy or direct release of the extensor origin from the epicondyle to relieve tension on the common extensor; procedures directed at the radial or posterior interosseous nerve; and procedures that use arthroscopic techniques to divide the orbicular ligament, reshape the radial head, or release the extensor origin.23 There has been debate about the value of repairing the ECRB, lengthening the ECRB, simultaneously decompressing the radial nerve or resecting epicondylar bone, and performing the procedures percutaneously, endoscopically, or arthroscopically.24-28 Despite multiple studies of the outcomes of these procedures,11,29-31 little is known regarding US national trends for operative treatment of lateral epicondylitis. Understanding national practice patterns and disease burden is essential to allocation of limited health care resources.
We conducted a study to determine US national trends in use of ambulatory surgery for lateral epicondylitis. We focused on age, sex, surgical setting, anesthetic type, and payment method.
Methods
As the National Survey of Ambulatory Surgery32 (NSAS) is an administrative dataset in which all data are deidentified and available for public use, this study was exempt from requiring institutional review board approval.
NSAS data were used to analyze trends in treatment of lateral epicondylitis between 1994 and 2006. NSAS was undertaken by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC) to obtain information about the use of ambulatory surgery in the United States. Since the early 1980s, ambulatory surgery has increased in the United States because of advances in medical technology and cost-containment initiatives.33 The number of procedures being performed in ambulatory surgery centers increased from 31.5 million in 1996 to 53.3 million in 2006.34 Funded by the CDC, NSAS is a national study that involves both hospital-based and freestanding ambulatory surgery centers and provides the most recent and comprehensive overview of ambulatory surgery in the United States.35 Because of budgetary limitations, 2006 was the last year in which data for NSAS were collected. Data for NSAS come from Medicare-participating, noninstitutional hospitals (excluding military hospitals, federal facilities, and Veteran Affairs hospitals) in all 50 states and the District of Columbia with a minimum of 6 beds staffed for patient use. NSAS used only short-stay hospitals (hospitals with an average length of stay for all patients of less than 30 days) or hospitals that had a specialty of general (medical or surgical) or children’s general. NSAS was conducted in 1994, 1996, and 2006 with medical information recorded on patient abstracts coded by contract staff. NSAS selected a sample of ambulatory surgery visits using a systematic random sampling procedure, and selection of visits within each facility was done separately for each location where ambulatory surgery was performed. In 1994, 751 facilities were sampled, and 88% of hospitals responded. In 1996, 750 facilities were sampled, and 91% of hospitals responded. In 2006, 696 facilities were sampled, and 75% responded. The surveys used International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes36 to classify medical diagnoses and procedures. To produce an unbiased national estimate, NCHS used multistage estimate procedures, including inflation by reciprocals of the probabilities of sample selection, population-weighting ratio adjustments, and adjustment for no response.37
Demographic and medical information was obtained for people with an ICD-9-CM diagnosis code of lateral epicondylitis (726.32), using previously described techniques.38 Data were then recorded for age, sex, facility type, insurance type, anesthesia type, diagnoses, and procedures.
Descriptive statistics consisted of means and standard deviations for continuous variables and frequency and percentages for discrete variables. Because NSAS data were collected on the basis of a probabilistic sample scheme, they were analyzed using a sampling weighting method. Sampling weights (inverse of selection probability) provided by the CDC were used to account for unequal sampling probabilities and to produce estimates for all visits in the United States. A Taylor linearization model provided by the CDC estimates was used to calculate standard error and confidence intervals (CIs) of the data. Standard error is a measure of sampling variability that occurs by chance because only a sample rather than the entire universe is surveyed. To define population parameters, NCHS chose 95% CIs along with a point estimate. Direct statistical comparison between years cannot be performed because of sampling differences in the database compared between years. The CIs, however, can suggest statistical differences if the data are nonoverlapping. US census data were used to obtain national population estimates for each year of the study (1994, 1996, 2006).39 Rates were presented as number of procedures per 100,000 standard population. For age, a direct adjustment procedure was used, and the US population in 2000 was selected as the standard population. Applying sex-specific rates to the standard population and dividing by the total in the standard population, we calculated sex-adjusted rates for each year. All data were analyzed using SPSS Version 20 software.
Results
A total of 30,311 ambulatory surgical procedures (95% CI, 27,292-33,330) or 10.44 per 100,000 capita were recorded by NSAS for the treatment of lateral epicondylitis in 2006 (Table 1). This represents a large increase in the total number of ambulatory procedures, from 21,852 in 1994 (95% CI, 19,981-23,722; 7.29/100,000) and 20,372 in 1996 (95% CI, 18,660-22,083; 6.73/100,000).
Between 1994 and 2006, the sex-adjusted rate of ambulatory surgery for lateral epicondylitis increased by 85% among females (7.74/100,000 to 14.31/100,000), whereas the rate decreased by 31% among males (8.07/100,000 to 5.59/100,000) (Table 1). The age-adjusted rate of ambulatory surgery for lateral epicondylitis increased among all age groups except the 30–39 years group (Table 2). The largest increase in age-adjusted rates was found for patients older than 50 years (275%) between 1994 and 2006.
During the study period, use of regional anesthesia nearly doubled, from 17% to 30%, whereas use of general anesthesia decreased, from 69% to 57% (Table 3). At all time points, the most common procedure performed for lateral epicondylitis in ambulatory surgery centers was division/release of the joint capsule of the elbow (Table 4). Private insurance remained the most common source of payment for all study years, ranging from 52% to 60% (Table 5). The Figure shows that, between 1994 and 2006, the proportion of surgeries performed in a freestanding ambulatory center increased.
Discussion
In this descriptive epidemiologic study, we used NSAS data to investigate trends in ambulatory surgery for lateral epicondylitis between 1994 and 2006.32 Our results showed that total number of procedures and the population-adjusted rate of procedures for lateral epicondylitis increased during the study period. The largest increase in age-adjusted rates of surgery for lateral epicondylitis was found among patients older than 50 years, whereas the highest age-adjusted rate of ambulatory surgery for lateral epicondylitis was found among patients between ages 40 and 49 years. These findings are similar to those of previous studies, which have shown that most patients with lateral epicondylitis present in the fourth and fifth decades of life.22 Prior reports have suggested that the incidence of lateral epicondylitis in men and women is equal.22 The present study found a change in sex-adjusted rates of ambulatory surgery for lateral epicondylitis between 1994 and 2006. Specifically, in 1994, surgery rates for men and women were similar (8.07/100,000 and 7.74/100,000), but in 2006 the sex-adjusted rate of surgery for lateral epicondylitis was almost 3 times higher for women than for men (14.31/100,000 vs 5.59/100,000).
We also found that the population-adjusted rate of lateral epicondylectomy increased drastically, from 0.4 per 100,000 in 1994 to 3.53 per 100,000 in 2006. Lateral epicondylectomy involves excision of the tip of the lateral epicondyle (typically, 0.5 cm) to produce a cancellous bone surface to which the edges of the débrided extensor tendon can be approximated without tension.23 It is possible that the increased rate of lateral epicondylectomy reflects evidence-based practice changes during the study period,27 though denervation was found more favorable than epicondylectomy in a recent study by Berry and colleagues.40 Future studies should investigate whether rates of epicondylectomy have changed since 2006. In addition, the present study showed a correlation between the introduction of arthroscopic techniques for the treatment of lateral epicondylitis and the period when much research was being conducted on the topic.24,25,28 As arthroscopic techniques improve, their rates are likely to continue to increase.
Our results also showed an increase in procedures performed in freestanding facilities. The rise in ambulatory surgical volume, speculated to result from more procedures being performed in freestanding facilities,34 has been reported with knee and shoulder arthroscopy.41 In addition, though general anesthesia remained the most used technique, our results showed a shift toward peripheral nerve blocks. The increase in regional anesthesia, which has also been noted in joint arthroscopy, is thought to stem from the advent of nerve-localizing technology, such as nerve stimulation and ultrasound guidance.41 Peripheral nerve blocks are favorable on both economic and quality measures, are associated with fewer opioid-related side effects, and overall provide better analgesia in comparison with opioids, highlighting their importance in the ambulatory setting.42
Although large, national databases are well suited to epidemiologic research,43 our study had limitations. As with all databases, NSAS is subject to data entry errors and coding errors.44,45 However, the database administrators corrected for this by using a multistage estimate procedure with weighting adjustments for no response and population-weighting ratio adjustments.35 Another limitation of this study is its lack of clinical detail, as procedure codes are general and do not allow differentiation between specific patients. Because of the retrospective nature of the analysis and the heterogeneity of the data, assessment of specific surgeries for lateral epicondylitis was limited. Although a strength of using NSAS to perform epidemiologic analyses is its large sample size, this also sacrifices specificity in terms of clinical insight. The results of this study may influence investigations to distinguish differences between procedures used in the treatment of lateral epicondylitis. Furthermore, the results of this study are limited to ambulatory surgery practice patterns in the United States between 1996 and 2006. Last, our ability to perform economic analyses was limited, as data on total hospital cost were not recorded by the surveys.
Conclusion
The increase in ambulatory surgery for lateral epicondylitis, demonstrated in this study, emphasizes the importance of national funding for surveys such as NSAS beyond 2006, as utilization trends may have considerable effects on health care policies that influence the quality of patient care.
1. Runge F. Zur genese und behandlung des schreibekramfes. Berl Klin Wochenschr. 1873;10:245.
2. Major HP. Lawn-tennis elbow. Br Med J. 1883;2:557.
3. Allander E. Prevalence, incidence, and remission rates of some common rheumatic diseases or syndromes. Scand J Rheumatol. 1974;3(3):145-153.
4. Verhaar JA. Tennis elbow. Anatomical, epidemiological and therapeutic aspects. Int Orthop. 1994;18(5):263-267.
5. Kurppa K, Viikari-Juntura E, Kuosma E, Huuskonen M, Kivi P. Incidence of tenosynovitis or peritendinitis and epicondylitis in a meat-processing factory. Scand J Work Environ Health. 1991;17(1):32-37.
6. Ranney D, Wells R, Moore A. Upper limb musculoskeletal disorders in highly repetitive industries: precise anatomical physical findings. Ergonomics. 1995;38(7):1408-1423.
7. Haahr JP, Andersen JH. Physical and psychosocial risk factors for lateral epicondylitis: a population based case-referent study. Occup Environ Med. 2003;60(5):322-329.
8. Goldie I. Epicondylitis lateralis humeri (epicondylalgia or tennis elbow). A pathogenetical study. Acta Chir Scand Suppl. 1964;57(suppl 399):1+.
9. Binder AI, Hazleman BL. Lateral humeral epicondylitis—a study of natural history and the effect of conservative therapy. Br J Rheumatol. 1983;22(2):73-76.
10. Smidt N, van der Windt DA, Assendelft WJ, Devillé WL, Korthals-de Bos IB, Bouter LM. Corticosteroid injections, physiotherapy, or a wait-and-see policy for lateral epicondylitis: a randomised controlled trial. Lancet. 2002;359(9307):657-662.
11. Nirschl RP, Pettrone FA. Tennis elbow. The surgical treatment of lateral epicondylitis. J Bone Joint Surg Am. 1979;61(6):832-839.
12. Burnham R, Gregg R, Healy P, Steadward R. The effectiveness of topical diclofenac for lateral epicondylitis. Clin J Sport Med. 1998;8(2):78-81.
13. Martinez-Silvestrini JA, Newcomer KL, Gay RE, Schaefer MP, Kortebein P, Arendt KW. Chronic lateral epicondylitis: comparative effectiveness of a home exercise program including stretching alone versus stretching supplemented with eccentric or concentric strengthening. J Hand Ther. 2005;18(4):411-419.
14. Svernlöv B, Adolfsson L. Non-operative treatment regime including eccentric training for lateral humeral epicondylalgia. Scand J Med Sci Sports. 2001;11(6):328-334.
15. Hay EM, Paterson SM, Lewis M, Hosie G, Croft P. Pragmatic randomised controlled trial of local corticosteroid injection and naproxen for treatment of lateral epicondylitis of elbow in primary care. BMJ. 1999;319(7215):964-968.
16. Lewis M, Hay EM, Paterson SM, Croft P. Local steroid injections for tennis elbow: does the pain get worse before it gets better? Results from a randomized controlled trial. Clin J Pain. 2005;21(4):330-334.
17. Van De Streek MD, Van Der Schans CP, De Greef MH, Postema K. The effect of a forearm/hand splint compared with an elbow band as a treatment for lateral epicondylitis. Prosthet Orthot Int. 2004;28(2):183-189.
18. Struijs PA, Smidt N, Arola H, Dijk vC, Buchbinder R, Assendelft WJ. Orthotic devices for the treatment of tennis elbow. Cochrane Database Syst Rev. 2002;(1):CD001821.
19. Buchbinder R, Green SE, Youd JM, Assendelft WJ, Barnsley L, Smidt N. Shock wave therapy for lateral elbow pain. Cochrane Database Syst Rev. 2005;(4):CD003524.
20. Boyd HB, McLeod AC Jr. Tennis elbow. J Bone Joint Surg Am. 1973;55(6):1183-1187.
21. Coonrad RW, Hooper WR. Tennis elbow: its course, natural history, conservative and surgical management. J Bone Joint Surg Am. 1973;55(6):1177-1182.
22. Calfee RP, Patel A, DaSilva MF, Akelman E. Management of lateral epicondylitis: current concepts. J Am Acad Orthop Surg. 2008;16(1):19-29.
23. Plancher KD, Bishai SK. Open lateral epicondylectomy: a simple technique update for the 21st century. Tech Orthop. 2006;21(4):276-282.
24. Peart RE, Strickler SS, Schweitzer KM Jr. Lateral epicondylitis: a comparative study of open and arthroscopic lateral release. Am J Orthop. 2004;33(11):565-567.
25. Dunkow PD, Jatti M, Muddu BN. A comparison of open and percutaneous techniques in the surgical treatment of tennis elbow. J Bone Joint Surg Br. 2004;86(5):701-704.
26. Rosenberg N, Henderson I. Surgical treatment of resistant lateral epicondylitis. Follow-up study of 19 patients after excision, release and repair of proximal common extensor tendon origin. Arch Orthop Trauma Surg. 2002;122(9-10):514-517.
27. Almquist EE, Necking L, Bach AW. Epicondylar resection with anconeus muscle transfer for chronic lateral epicondylitis. J Hand Surg Am. 1998;23(4):723-731.
28. Smith AM, Castle JA, Ruch DS. Arthroscopic resection of the common extensor origin: anatomic considerations. J Shoulder Elbow Surg. 2003;12(4):375-379.
29. Baker CL Jr, Murphy KP, Gottlob CA, Curd DT. Arthroscopic classification and treatment of lateral epicondylitis: two-year clinical results. J Shoulder Elbow Surg. 2000;9(6):475-482.
30. Owens BD, Murphy KP, Kuklo TR. Arthroscopic release for lateral epicondylitis. Arthroscopy. 2001;17(6):582-587.
31. Mullett H, Sprague M, Brown G, Hausman M. Arthroscopic treatment of lateral epicondylitis: clinical and cadaveric studies. Clin Orthop Relat Res. 2005;(439):123-128.
32. National Survey of Ambulatory Surgery. Centers for Disease Control and Prevention website. http://www.cdc.gov/nchs/nsas/nsas_questionnaires.htm. Published May 4, 2010. Accessed November 10, 2015.
33. Leader S, Moon M. Medicare trends in ambulatory surgery. Health Aff. 1989;8(1):158-170.
34. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;(11):1-25.
35. Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg Am. 2011;93(11):994-1000.
36. Centers for Disease Control and Prevention, National Center for Health Statistics. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). http://www.cdc.gov/nchs/icd/icd9cm.htm. Updated June 18, 2013. Accessed October 28, 2015.
37. Dennison C, Pokras R. Design and operation of the National Hospital Discharge Survey: 1988 redesign. Vital Health Stat 1. 2000;(39):1-42.
38. Stundner O, Kirksey M, Chiu YL, et al. Demographics and perioperative outcome in patients with depression and anxiety undergoing total joint arthroplasty: a population-based study. Psychosomatics. 2013;54(2):149-157.
39. Population estimates. US Department of Commerce, United States Census Bureau website. http://www.census.gov/popest/index.html. Accessed November 16, 2015.
40. Berry N, Neumeister MW, Russell RC, Dellon AL. Epicondylectomy versus denervation for lateral humeral epicondylitis. Hand. 2011;6(2):174-178.
41. Memtsoudis SG, Kuo C, Ma Y, Edwards A, Mazumdar M, Liguori G. Changes in anesthesia-related factors in ambulatory knee and shoulder surgery: United States 1996–2006. Reg Anesth Pain Med. 2011;36(4):327-331.
42. Richman JM, Liu SS, Courpas G, et al. Does continuous peripheral nerve block provide superior pain control to opioids? A meta-analysis. Anesth Analg. 2006;102(1):248-257.
43. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680.
44. Gray DT, Hodge DO, Ilstrup DM, Butterfield LC, Baratz KH, Concordance of Medicare data and population-based clinical data on cataract surgery utilization in Olmsted County, Minnesota. Am J Epidemiol. 1997;145(12):1123-1126.
45. Memtsoudis SG. Limitations associated with the analysis of data from administrative databases. Anesthesiology. 2009;111(2):449.
1. Runge F. Zur genese und behandlung des schreibekramfes. Berl Klin Wochenschr. 1873;10:245.
2. Major HP. Lawn-tennis elbow. Br Med J. 1883;2:557.
3. Allander E. Prevalence, incidence, and remission rates of some common rheumatic diseases or syndromes. Scand J Rheumatol. 1974;3(3):145-153.
4. Verhaar JA. Tennis elbow. Anatomical, epidemiological and therapeutic aspects. Int Orthop. 1994;18(5):263-267.
5. Kurppa K, Viikari-Juntura E, Kuosma E, Huuskonen M, Kivi P. Incidence of tenosynovitis or peritendinitis and epicondylitis in a meat-processing factory. Scand J Work Environ Health. 1991;17(1):32-37.
6. Ranney D, Wells R, Moore A. Upper limb musculoskeletal disorders in highly repetitive industries: precise anatomical physical findings. Ergonomics. 1995;38(7):1408-1423.
7. Haahr JP, Andersen JH. Physical and psychosocial risk factors for lateral epicondylitis: a population based case-referent study. Occup Environ Med. 2003;60(5):322-329.
8. Goldie I. Epicondylitis lateralis humeri (epicondylalgia or tennis elbow). A pathogenetical study. Acta Chir Scand Suppl. 1964;57(suppl 399):1+.
9. Binder AI, Hazleman BL. Lateral humeral epicondylitis—a study of natural history and the effect of conservative therapy. Br J Rheumatol. 1983;22(2):73-76.
10. Smidt N, van der Windt DA, Assendelft WJ, Devillé WL, Korthals-de Bos IB, Bouter LM. Corticosteroid injections, physiotherapy, or a wait-and-see policy for lateral epicondylitis: a randomised controlled trial. Lancet. 2002;359(9307):657-662.
11. Nirschl RP, Pettrone FA. Tennis elbow. The surgical treatment of lateral epicondylitis. J Bone Joint Surg Am. 1979;61(6):832-839.
12. Burnham R, Gregg R, Healy P, Steadward R. The effectiveness of topical diclofenac for lateral epicondylitis. Clin J Sport Med. 1998;8(2):78-81.
13. Martinez-Silvestrini JA, Newcomer KL, Gay RE, Schaefer MP, Kortebein P, Arendt KW. Chronic lateral epicondylitis: comparative effectiveness of a home exercise program including stretching alone versus stretching supplemented with eccentric or concentric strengthening. J Hand Ther. 2005;18(4):411-419.
14. Svernlöv B, Adolfsson L. Non-operative treatment regime including eccentric training for lateral humeral epicondylalgia. Scand J Med Sci Sports. 2001;11(6):328-334.
15. Hay EM, Paterson SM, Lewis M, Hosie G, Croft P. Pragmatic randomised controlled trial of local corticosteroid injection and naproxen for treatment of lateral epicondylitis of elbow in primary care. BMJ. 1999;319(7215):964-968.
16. Lewis M, Hay EM, Paterson SM, Croft P. Local steroid injections for tennis elbow: does the pain get worse before it gets better? Results from a randomized controlled trial. Clin J Pain. 2005;21(4):330-334.
17. Van De Streek MD, Van Der Schans CP, De Greef MH, Postema K. The effect of a forearm/hand splint compared with an elbow band as a treatment for lateral epicondylitis. Prosthet Orthot Int. 2004;28(2):183-189.
18. Struijs PA, Smidt N, Arola H, Dijk vC, Buchbinder R, Assendelft WJ. Orthotic devices for the treatment of tennis elbow. Cochrane Database Syst Rev. 2002;(1):CD001821.
19. Buchbinder R, Green SE, Youd JM, Assendelft WJ, Barnsley L, Smidt N. Shock wave therapy for lateral elbow pain. Cochrane Database Syst Rev. 2005;(4):CD003524.
20. Boyd HB, McLeod AC Jr. Tennis elbow. J Bone Joint Surg Am. 1973;55(6):1183-1187.
21. Coonrad RW, Hooper WR. Tennis elbow: its course, natural history, conservative and surgical management. J Bone Joint Surg Am. 1973;55(6):1177-1182.
22. Calfee RP, Patel A, DaSilva MF, Akelman E. Management of lateral epicondylitis: current concepts. J Am Acad Orthop Surg. 2008;16(1):19-29.
23. Plancher KD, Bishai SK. Open lateral epicondylectomy: a simple technique update for the 21st century. Tech Orthop. 2006;21(4):276-282.
24. Peart RE, Strickler SS, Schweitzer KM Jr. Lateral epicondylitis: a comparative study of open and arthroscopic lateral release. Am J Orthop. 2004;33(11):565-567.
25. Dunkow PD, Jatti M, Muddu BN. A comparison of open and percutaneous techniques in the surgical treatment of tennis elbow. J Bone Joint Surg Br. 2004;86(5):701-704.
26. Rosenberg N, Henderson I. Surgical treatment of resistant lateral epicondylitis. Follow-up study of 19 patients after excision, release and repair of proximal common extensor tendon origin. Arch Orthop Trauma Surg. 2002;122(9-10):514-517.
27. Almquist EE, Necking L, Bach AW. Epicondylar resection with anconeus muscle transfer for chronic lateral epicondylitis. J Hand Surg Am. 1998;23(4):723-731.
28. Smith AM, Castle JA, Ruch DS. Arthroscopic resection of the common extensor origin: anatomic considerations. J Shoulder Elbow Surg. 2003;12(4):375-379.
29. Baker CL Jr, Murphy KP, Gottlob CA, Curd DT. Arthroscopic classification and treatment of lateral epicondylitis: two-year clinical results. J Shoulder Elbow Surg. 2000;9(6):475-482.
30. Owens BD, Murphy KP, Kuklo TR. Arthroscopic release for lateral epicondylitis. Arthroscopy. 2001;17(6):582-587.
31. Mullett H, Sprague M, Brown G, Hausman M. Arthroscopic treatment of lateral epicondylitis: clinical and cadaveric studies. Clin Orthop Relat Res. 2005;(439):123-128.
32. National Survey of Ambulatory Surgery. Centers for Disease Control and Prevention website. http://www.cdc.gov/nchs/nsas/nsas_questionnaires.htm. Published May 4, 2010. Accessed November 10, 2015.
33. Leader S, Moon M. Medicare trends in ambulatory surgery. Health Aff. 1989;8(1):158-170.
34. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;(11):1-25.
35. Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg Am. 2011;93(11):994-1000.
36. Centers for Disease Control and Prevention, National Center for Health Statistics. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). http://www.cdc.gov/nchs/icd/icd9cm.htm. Updated June 18, 2013. Accessed October 28, 2015.
37. Dennison C, Pokras R. Design and operation of the National Hospital Discharge Survey: 1988 redesign. Vital Health Stat 1. 2000;(39):1-42.
38. Stundner O, Kirksey M, Chiu YL, et al. Demographics and perioperative outcome in patients with depression and anxiety undergoing total joint arthroplasty: a population-based study. Psychosomatics. 2013;54(2):149-157.
39. Population estimates. US Department of Commerce, United States Census Bureau website. http://www.census.gov/popest/index.html. Accessed November 16, 2015.
40. Berry N, Neumeister MW, Russell RC, Dellon AL. Epicondylectomy versus denervation for lateral humeral epicondylitis. Hand. 2011;6(2):174-178.
41. Memtsoudis SG, Kuo C, Ma Y, Edwards A, Mazumdar M, Liguori G. Changes in anesthesia-related factors in ambulatory knee and shoulder surgery: United States 1996–2006. Reg Anesth Pain Med. 2011;36(4):327-331.
42. Richman JM, Liu SS, Courpas G, et al. Does continuous peripheral nerve block provide superior pain control to opioids? A meta-analysis. Anesth Analg. 2006;102(1):248-257.
43. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680.
44. Gray DT, Hodge DO, Ilstrup DM, Butterfield LC, Baratz KH, Concordance of Medicare data and population-based clinical data on cataract surgery utilization in Olmsted County, Minnesota. Am J Epidemiol. 1997;145(12):1123-1126.
45. Memtsoudis SG. Limitations associated with the analysis of data from administrative databases. Anesthesiology. 2009;111(2):449.
Intra-articular Olecranon Fracture Fixed with an Iso-Elastic Tension Band
Surgical technique using isoelastic tension band for treatment of olecranon fractures.
To read the authors' full article click here.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Surgical technique using isoelastic tension band for treatment of olecranon fractures.
To read the authors' full article click here.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Surgical technique using isoelastic tension band for treatment of olecranon fractures.
To read the authors' full article click here.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Technique Using Isoelastic Tension Band for Treatment of Olecranon Fractures
Olecranon fractures are relatively common in adults and constitute 10% of all upper extremity injuries.1,2 An olecranon fracture may be sustained either directly (from blunt trauma or a fall onto the tip of the elbow) or indirectly (as a result of forceful hyperextension of the triceps during a fall onto an outstretched arm). Displaced olecranon fractures with extensor discontinuity require reduction and stabilization. One treatment option is tension band wiring (TBW), which is used to manage noncomminuted fractures.3 TBW, first described by Weber and Vasey4 in 1963, involves transforming the distractive forces of the triceps into dynamic compression forces across the olecranon articular surface using 2 intramedullary Kirschner wires (K-wires) and stainless steel wires looped in figure-of-8 fashion.
Various modifications of the TBW technique of Weber and Vasey4 have been proposed to reduce the frequency of complications. These modifications include substituting screws for K-wires, aiming the angle of the K-wires into the anterior coronoid cortex or loop configuration of the stainless steel wire, using double knots and twisting procedures to finalize fixation, and using alternative materials for the loop construct.5-8 In the literature and in our experience, patients often complain after surgery about prominent K-wires and the twisted knots used to tension the construct.9-12 Surgeons also must address the technical difficulties of positioning the brittle wire without kinking, and avoiding slack while tensioning.
In this article, we report on the clinical outcomes of a series of 7 patients with olecranon fracture treated with a US Food and Drug Administration–approved novel isoelastic ultrahigh-molecular-weight polyethylene (UHMWPE) cerclage cable (Iso-Elastic Cerclage System, Kinamed).
Materials and Methods
Surgical Technique
The patient is arranged in a sloppy lateral position to allow access to the posterior elbow. A nonsterile tourniquet is placed on the upper arm, and the limb is sterilely prepared and draped in standard fashion. A posterolateral incision is made around the olecranon and extended proximally 6 cm and distally 6 cm along the subcutaneous border of the ulna. The fracture is visualized and comminution identified.
To provide anchorage for a pointed reduction clamp, the surgeon drills a 2.5-mm hole in the subcutaneous border of the ulnar shaft. The fracture is reduced in extension and the clamp affixed. The elbow is then flexed and the reduction confirmed visually and by imaging. After realignment of the articular surfaces, 2 longitudinal, parallel K-wires (diameter, 1.6-2.0 mm) are passed in antegrade direction through the proximal olecranon within the medullary canal of the shaft. The proximal ends must not cross the cortex so they may fully capture the figure-of-8 wire during subsequent, final advancement, and the distal ends must not pierce the anterior cortex. A 2.5-mm transverse hole is created distal to the fracture in the dorsal aspect of the ulnar shaft from medial to lateral at 2 times the distance from the tip of the olecranon to the fracture site. This hole is expanded with a 3.5-mm drill bit, allowing both strands of the cable to be passed simultaneously medial to lateral, making the figure-of-8. The 3.5-mm hole represents about 20% of the overall width of the bone, which we have not found to create a significant stress riser in either laboratory or clinical tests of this construct. Proximally, the cables are placed on the periosteum of the olecranon but deep to the triceps tendon and adjacent to the K-wires. The locking clip is placed on the posterolateral aspect of the elbow joint in a location where it can be covered with local tissue for adequate padding. The cable is then threaded through the clamping bracket and tightened slowly and gradually with a tensioning device to low torque level (Figure 1). At this stage, tension may be released to make any necessary adjustments. Last, the locking clip is deployed, securing the tension band in the clip, and the excess cable is trimmed with a scalpel. Softening and pliability of the cable during its insertion and tensioning should be noted.
The ends of the K-wires are now curved in a hook configuration. The tines of the hooks should be parallel to accommodate the cable, and then the triceps is sharply incised to bone. If the bone is hard, an awl is used to create a pilot hole so the hook may be impaled into bone while capturing the cable. Next, the triceps is closed over the pins, minimizing the potential for pin migration and backout. The 2 K-wires are left in place to keep the fragments in proper anatomical alignment during healing and to prevent displacement with elbow motion. Figure 2 is a schematic of the final construct, and Figure 3 shows the construct in a patient.
Reduction of the olecranon fracture is assessed by imaging in full extension to check for possible implant impingement. Last, we apply the previously harvested fracture callus to the fracture site. Layered closure is performed, and bulky soft dressings are applied. Postoperative immobilization with a splint is used. Gentle range-of-motion exercises begin in about 2 weeks and progress as pain allows.
A case example with preoperative and postoperative images taken at 3-month follow-up is provided in Figure 4. The entire surgical technique can be viewed in the Video.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Clinical Cases
Between July 2007 and February 2011, 7 patients with displaced olecranon fractures underwent osteosynthesis using the isoelastic tension band (Table 1). According to the Mayo classification system, 5 of these patients had type 2A fractures, 1 had a type 2B fracture with an ipsilateral nondisplaced radial neck fracture, and 1 had a type 3B fracture. There were 4 female and 3 male patients. The injury was on the dominant side in 3 patients. All patients gave informed consent to evaluation at subsequent office visits and completed outcomes questionnaires by mail several years after surgery. Mean follow-up at which outcome measures questionnaires were obtained was 3.3 years (range, 2.1-6.8 years). Exclusion criteria were age under 18 years and inability to provide informed consent, fracture patterns with extensive articular comminution, and open fractures. Permission to conduct this research was granted by institutional review board.
At each visit, patients completed the Disabilities of the Arm, Shoulder, and Hand (DASH) functional outcome survey and were evaluated according to Broberg and Morrey’s elbow scoring system.13,14 Chart review consisted of evaluation of medical records, including radiographs and orthopedic physician notes in which preoperative examination was documented, mechanism of injury was noted, radiologic fracture pattern was evaluated, and time to bony union was recorded. Elbow motion was documented. Grip strength was measured with a calibrated Jamar dynamometer (Sammons Preston Rolyan) set at level 2, as delineated in Broberg and Morrey’s functional elbow scoring system.
Results
The 7 patients were assessed at a mean final follow-up of 19 months after surgery and received a mean Broberg and Morrey score of good (92.2/100) (Table 2). Restoration of motion and strength was excellent; compared with contralateral extremity, mean flexion arc was 96%, and mean forearm rotation was 96%. Grip was 99% of the noninjured side, perhaps the result of increased conditioning from physical therapy. Patients completed outcomes questionnaires at a mean of 3.3 years after surgery. Mean (SD) DASH score at this longest follow-up was 12.6 (17.2) (Table 2). Patients were satisfied (mean, 9.8/10; range, 9.5-10) and had little pain (mean, 0.8/10; range, 0-3). All fractures united, and there were no infections. One patient had a satisfactory union with complete restoration of motion and continued to play sports vocationally but developed pain over the locking clip 5 years after the index procedure and decided to have the implant removed. He had no radiographic evidence of K-wire or implant migration. Another patient had a minor degree of implant irritation at longest follow-up but did not request hardware removal.
Discussion
Stainless steel wire is often used in TBW because of its widespread availability, low cost, lack of immunogenicity, and relative strength.7 However, stainless steel wire has several disadvantages. It is susceptible to low-cycle fatigue failure, and fatigue strength may be seriously reduced secondary to incidental trauma to the wire on implantation.15,16 Other complications are kinking, skin irritation, implant prominence, fixation loss caused by wire loosening, and inadequate initial reduction potentially requiring revision.10,12,17-21
Isoelastic cable is a new type of cerclage cable that consists of UHMWPE strands braided over a nylon core. The particular property profile of the isoelastic tension band gives the cable intrinsic elastic and pliable qualities. In addition, unlike stainless steel, the band maintains a uniform, continuous compression force across a fracture site.22 Multifilament braided cables fatigue and fray, but the isoelastic cerclage cable showed no evidence of fraying or breakage after 1 million loading cycles.22,23 Compared with metal wire or braided metal cable, the band also has higher fatigue strength and higher ultimate tensile strength.7 Furthermore, the cable is less abrasive than stainless steel, so theoretically it is less irritating to surrounding subcutaneous tissue. Last, the pliability of the band allows the surgeon to create multiple loops of cable without the wire-failure side effects related to kinking, which is common with the metal construct.
In 2010, Ting and colleagues24 retrospectively studied implant failure complications associated with use of isoelastic cerclage cables in the treatment of periprosthetic fractures in total hip arthroplasty. They reported a breakage rate of 0% and noted that previously published breakage data for metallic cerclage devices ranged from 0% to 44%. They concluded that isoelastic cables were not associated with material failure, and there were no direct complications related to the cables. Similarly, Edwards and colleagues25 evaluated the same type of cable used in revision shoulder arthroplasty and reported excellent success and no failures. Although these data stem from use in the femur and humerus, we think the noted benefits apply to fractures of the elbow as well, as we observed a similar breakage rate (0%).
Various studies have addressed the clinical complaints and reoperation rates associated with retained metal implants after olecranon fixation. Traditional AO (Arbeitsgemeinschaft für Osteosynthesefragen) technique involves subcutaneous placement of stainless steel wires, which often results in tissue irritation. Reoperation rates as high as 80% have been reported, and a proportion of implant removals may in fact be caused by factors related to the subcutaneous placement of the metallic implants rather than K-wire migration alone.5,12,18 A nonmetallic isoelastic tension band can provide a more comfortable and less irritating implant, which could reduce the need for secondary intervention related to painful subcutaneous implant. One of our 7 patients had a symptomatic implant removed 5 years after surgery. This patient complained of pain over the area of the tension band device clip, so after fracture healing the entire fixation device was removed in the operating room. If reoperation is necessary, removal of intramedullary K-wires is relatively simple using a minimal incision; removal of stainless steel TBW may require a larger approach if the twisted knots cannot be easily retrieved.
A study of compression forces created by stainless steel wire demonstrated that a “finely tuned mechanical sense” was needed to produce optimal fixation compression when using stainless steel wire.26 It was observed that a submaximal twist created insufficient compressive force, while an ostensibly minimal increase in twisting force above optimum abruptly caused wire failure through breakage. Cerclage cables using clasping devices, such as the current isoelastic cerclage cable, were superior in ease of application. Furthermore, a clasping device allows for cable tension readjustment that is not possible with stainless steel wire. The clasping mechanism precludes the surgeon from having to bury the stainless steel knot and allows for the objective cable-tensioning not possible with stainless steel wire. Last, the tensioning device is titratable, which allows the surgeon to set the construct at a predetermined quantitative tension, which is of benefit in patients with osteopenia.
One limitation of this study is that it did not resolve the potential for K-wire migration, and we agree with previous recommendations that careful attention to surgical technique may avoid such a complication.10 In addition, the sample was small, and the study lacked a control group; a larger sample and a control group would have boosted study power. Nevertheless, the physical and functional outcomes associated with use of this technique were excellent. These results demonstrate an efficacious attempt to decrease secondary surgery rates and are therefore proof of concept that the isoelastic tension band may be used as an alternative to stainless steel in the TBW of displaced olecranon fractures with minimal or no comminution.
Conclusion
This easily reproducible technique for use of an isoelastic tension band in olecranon fracture fixation was associated with excellent physical and functional outcomes in a series of 7 patients. The rate of secondary intervention was slightly better for these patients than for patients treated with wire tension band fixation. Although more rigorous study of this device is needed, we think it is a promising alternative to wire tension band techniques.
1. Rommens PM, Küchle R, Schneider RU, Reuter M. Olecranon fractures in adults: factors influencing outcome. Injury. 2004;35(11):1149-1157.
2. Veillette CJ, Steinmann SP. Olecranon fractures. Orthop Clin North Am. 2008;39(2):229-236.
3. Newman SD, Mauffrey C, Krikler S. Olecranon fractures. Injury. 2009;40(6):575-581.
4. Weber BG, Vasey H. Osteosynthesis in olecranon fractures [in German]. Z Unfallmed Berufskr. 1963;56:90-96.
5. Netz P, Strömberg L. Non-sliding pins in traction absorbing wiring of fractures: a modified technique. Acta Orthop Scand. 1982;53(3):355-360.
6. Prayson MJ, Williams JL, Marshall MP, Scilaris TA, Lingenfelter EJ. Biomechanical comparison of fixation methods in transverse olecranon fractures: a cadaveric study. J Orthop Trauma. 1997;11(8):565-572.
7. Rothaug PG, Boston RC, Richardson DW, Nunamaker DM. A comparison of ultra-high-molecular weight polyethylene cable and stainless steel wire using two fixation techniques for repair of equine midbody sesamoid fractures: an in vitro biomechanical study. Vet Surg. 2002;31(5):445-454.
8. Harrell RM, Tong J, Weinhold PS, Dahners LE. Comparison of the mechanical properties of different tension band materials and suture techniques. J Orthop Trauma. 2003;17(2):119-122.
9. Nimura A, Nakagawa T, Wakabayashi Y, Sekiya I, Okawa A, Muneta T. Repair of olecranon fractures using FiberWire without metallic implants: report of two cases. J Orthop Surg Res. 2010;5:73.
10. Macko D, Szabo RM. Complications of tension-band wiring of olecranon fractures. J Bone Joint Surg Am. 1985;67(9):1396-1401.
11. Helm RH, Hornby R, Miller SW. The complications of surgical treatment of displaced fractures of the olecranon. Injury. 1987;18(1):48-50.
12. Romero JM, Miran A, Jensen CH. Complications and re-operation rate after tension-band wiring of olecranon fractures. J Orthop Sci. 2000;5(4):318-320.
13. Beaton DE, Katz JN, Fossel AH, Wright JG, Tarasuk V, Bombardier C. Measuring the whole or the parts? Validity, reliability, and responsiveness of the Disabilities of the Arm, Shoulder and Hand outcome measure in different regions of the upper extremity. J Hand Ther. 2001;14(2):128-146.
14. Broberg MA, Morrey BF. Results of delayed excision of the radial head after fracture. J Bone Joint Surg Am. 1986;68(5):669-674.
15. Bostrom MP, Asnis SE, Ernberg JJ, et al. Fatigue testing of cerclage stainless steel wire fixation. J Orthop Trauma. 1994;8(5):422-428.
16. Oh I, Sander TW, Treharne RW. The fatigue resistance of orthopaedic wire. Clin Orthop Relat Res. 1985;(192):228-236.
17. Amstutz HC, Maki S. Complications of trochanteric osteotomy in total hip replacement. J Bone Joint Surg Am. 1978;60(2):214-216.
18. Jensen CM, Olsen BB. Drawbacks of traction-absorbing wiring (TAW) in displaced fractures of the olecranon. Injury. 1986;17(3):174-175.
19. Kumar G, Mereddy PK, Hakkalamani S, Donnachie NJ. Implant removal following surgical stabilization of patella fracture. Orthopedics. 2010;33(5).
20. Hume MC, Wiss DA. Olecranon fractures. A clinical and radiographic comparison of tension band wiring and plate fixation. Clin Orthop Relat Res. 1992;(285):229-235.
21. Wolfgang G, Burke F, Bush D, et al. Surgical treatment of displaced olecranon fractures by tension band wiring technique. Clin Orthop Relat Res. 1987;(224):192-204.
22. Sarin VK, Mattchen TM, Hack B. A novel iso-elastic cerclage cable for treatment of fractures. Paper presented at: Annual Meeting of the Orthopaedic Research Society; February 20-23, 2005; Washington, DC. Paper 739.
23. Silverton CD, Jacobs JJ, Rosenberg AG, Kull L, Conley A, Galante JO. Complications of a cable grip system. J Arthroplasty. 1996;11(4):400-404.
24. Ting NT, Wera GD, Levine BR, Della Valle CJ. Early experience with a novel nonmetallic cable in reconstructive hip surgery. Clin Orthop Relat Res. 2010;468(9):2382-2386.
25. Edwards TB, Stuart KD, Trappey GJ, O’Connor DP, Sarin VK. Utility of polymer cerclage cables in revision shoulder arthroplasty. Orthopedics. 2011;34(4).
26. Shaw JA, Daubert HB. Compression capability of cerclage fixation systems. A biomechanical study. Orthopedics. 1988;11(8):1169-1174.
Olecranon fractures are relatively common in adults and constitute 10% of all upper extremity injuries.1,2 An olecranon fracture may be sustained either directly (from blunt trauma or a fall onto the tip of the elbow) or indirectly (as a result of forceful hyperextension of the triceps during a fall onto an outstretched arm). Displaced olecranon fractures with extensor discontinuity require reduction and stabilization. One treatment option is tension band wiring (TBW), which is used to manage noncomminuted fractures.3 TBW, first described by Weber and Vasey4 in 1963, involves transforming the distractive forces of the triceps into dynamic compression forces across the olecranon articular surface using 2 intramedullary Kirschner wires (K-wires) and stainless steel wires looped in figure-of-8 fashion.
Various modifications of the TBW technique of Weber and Vasey4 have been proposed to reduce the frequency of complications. These modifications include substituting screws for K-wires, aiming the angle of the K-wires into the anterior coronoid cortex or loop configuration of the stainless steel wire, using double knots and twisting procedures to finalize fixation, and using alternative materials for the loop construct.5-8 In the literature and in our experience, patients often complain after surgery about prominent K-wires and the twisted knots used to tension the construct.9-12 Surgeons also must address the technical difficulties of positioning the brittle wire without kinking, and avoiding slack while tensioning.
In this article, we report on the clinical outcomes of a series of 7 patients with olecranon fracture treated with a US Food and Drug Administration–approved novel isoelastic ultrahigh-molecular-weight polyethylene (UHMWPE) cerclage cable (Iso-Elastic Cerclage System, Kinamed).
Materials and Methods
Surgical Technique
The patient is arranged in a sloppy lateral position to allow access to the posterior elbow. A nonsterile tourniquet is placed on the upper arm, and the limb is sterilely prepared and draped in standard fashion. A posterolateral incision is made around the olecranon and extended proximally 6 cm and distally 6 cm along the subcutaneous border of the ulna. The fracture is visualized and comminution identified.
To provide anchorage for a pointed reduction clamp, the surgeon drills a 2.5-mm hole in the subcutaneous border of the ulnar shaft. The fracture is reduced in extension and the clamp affixed. The elbow is then flexed and the reduction confirmed visually and by imaging. After realignment of the articular surfaces, 2 longitudinal, parallel K-wires (diameter, 1.6-2.0 mm) are passed in antegrade direction through the proximal olecranon within the medullary canal of the shaft. The proximal ends must not cross the cortex so they may fully capture the figure-of-8 wire during subsequent, final advancement, and the distal ends must not pierce the anterior cortex. A 2.5-mm transverse hole is created distal to the fracture in the dorsal aspect of the ulnar shaft from medial to lateral at 2 times the distance from the tip of the olecranon to the fracture site. This hole is expanded with a 3.5-mm drill bit, allowing both strands of the cable to be passed simultaneously medial to lateral, making the figure-of-8. The 3.5-mm hole represents about 20% of the overall width of the bone, which we have not found to create a significant stress riser in either laboratory or clinical tests of this construct. Proximally, the cables are placed on the periosteum of the olecranon but deep to the triceps tendon and adjacent to the K-wires. The locking clip is placed on the posterolateral aspect of the elbow joint in a location where it can be covered with local tissue for adequate padding. The cable is then threaded through the clamping bracket and tightened slowly and gradually with a tensioning device to low torque level (Figure 1). At this stage, tension may be released to make any necessary adjustments. Last, the locking clip is deployed, securing the tension band in the clip, and the excess cable is trimmed with a scalpel. Softening and pliability of the cable during its insertion and tensioning should be noted.
The ends of the K-wires are now curved in a hook configuration. The tines of the hooks should be parallel to accommodate the cable, and then the triceps is sharply incised to bone. If the bone is hard, an awl is used to create a pilot hole so the hook may be impaled into bone while capturing the cable. Next, the triceps is closed over the pins, minimizing the potential for pin migration and backout. The 2 K-wires are left in place to keep the fragments in proper anatomical alignment during healing and to prevent displacement with elbow motion. Figure 2 is a schematic of the final construct, and Figure 3 shows the construct in a patient.
Reduction of the olecranon fracture is assessed by imaging in full extension to check for possible implant impingement. Last, we apply the previously harvested fracture callus to the fracture site. Layered closure is performed, and bulky soft dressings are applied. Postoperative immobilization with a splint is used. Gentle range-of-motion exercises begin in about 2 weeks and progress as pain allows.
A case example with preoperative and postoperative images taken at 3-month follow-up is provided in Figure 4. The entire surgical technique can be viewed in the Video.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Clinical Cases
Between July 2007 and February 2011, 7 patients with displaced olecranon fractures underwent osteosynthesis using the isoelastic tension band (Table 1). According to the Mayo classification system, 5 of these patients had type 2A fractures, 1 had a type 2B fracture with an ipsilateral nondisplaced radial neck fracture, and 1 had a type 3B fracture. There were 4 female and 3 male patients. The injury was on the dominant side in 3 patients. All patients gave informed consent to evaluation at subsequent office visits and completed outcomes questionnaires by mail several years after surgery. Mean follow-up at which outcome measures questionnaires were obtained was 3.3 years (range, 2.1-6.8 years). Exclusion criteria were age under 18 years and inability to provide informed consent, fracture patterns with extensive articular comminution, and open fractures. Permission to conduct this research was granted by institutional review board.
At each visit, patients completed the Disabilities of the Arm, Shoulder, and Hand (DASH) functional outcome survey and were evaluated according to Broberg and Morrey’s elbow scoring system.13,14 Chart review consisted of evaluation of medical records, including radiographs and orthopedic physician notes in which preoperative examination was documented, mechanism of injury was noted, radiologic fracture pattern was evaluated, and time to bony union was recorded. Elbow motion was documented. Grip strength was measured with a calibrated Jamar dynamometer (Sammons Preston Rolyan) set at level 2, as delineated in Broberg and Morrey’s functional elbow scoring system.
Results
The 7 patients were assessed at a mean final follow-up of 19 months after surgery and received a mean Broberg and Morrey score of good (92.2/100) (Table 2). Restoration of motion and strength was excellent; compared with contralateral extremity, mean flexion arc was 96%, and mean forearm rotation was 96%. Grip was 99% of the noninjured side, perhaps the result of increased conditioning from physical therapy. Patients completed outcomes questionnaires at a mean of 3.3 years after surgery. Mean (SD) DASH score at this longest follow-up was 12.6 (17.2) (Table 2). Patients were satisfied (mean, 9.8/10; range, 9.5-10) and had little pain (mean, 0.8/10; range, 0-3). All fractures united, and there were no infections. One patient had a satisfactory union with complete restoration of motion and continued to play sports vocationally but developed pain over the locking clip 5 years after the index procedure and decided to have the implant removed. He had no radiographic evidence of K-wire or implant migration. Another patient had a minor degree of implant irritation at longest follow-up but did not request hardware removal.
Discussion
Stainless steel wire is often used in TBW because of its widespread availability, low cost, lack of immunogenicity, and relative strength.7 However, stainless steel wire has several disadvantages. It is susceptible to low-cycle fatigue failure, and fatigue strength may be seriously reduced secondary to incidental trauma to the wire on implantation.15,16 Other complications are kinking, skin irritation, implant prominence, fixation loss caused by wire loosening, and inadequate initial reduction potentially requiring revision.10,12,17-21
Isoelastic cable is a new type of cerclage cable that consists of UHMWPE strands braided over a nylon core. The particular property profile of the isoelastic tension band gives the cable intrinsic elastic and pliable qualities. In addition, unlike stainless steel, the band maintains a uniform, continuous compression force across a fracture site.22 Multifilament braided cables fatigue and fray, but the isoelastic cerclage cable showed no evidence of fraying or breakage after 1 million loading cycles.22,23 Compared with metal wire or braided metal cable, the band also has higher fatigue strength and higher ultimate tensile strength.7 Furthermore, the cable is less abrasive than stainless steel, so theoretically it is less irritating to surrounding subcutaneous tissue. Last, the pliability of the band allows the surgeon to create multiple loops of cable without the wire-failure side effects related to kinking, which is common with the metal construct.
In 2010, Ting and colleagues24 retrospectively studied implant failure complications associated with use of isoelastic cerclage cables in the treatment of periprosthetic fractures in total hip arthroplasty. They reported a breakage rate of 0% and noted that previously published breakage data for metallic cerclage devices ranged from 0% to 44%. They concluded that isoelastic cables were not associated with material failure, and there were no direct complications related to the cables. Similarly, Edwards and colleagues25 evaluated the same type of cable used in revision shoulder arthroplasty and reported excellent success and no failures. Although these data stem from use in the femur and humerus, we think the noted benefits apply to fractures of the elbow as well, as we observed a similar breakage rate (0%).
Various studies have addressed the clinical complaints and reoperation rates associated with retained metal implants after olecranon fixation. Traditional AO (Arbeitsgemeinschaft für Osteosynthesefragen) technique involves subcutaneous placement of stainless steel wires, which often results in tissue irritation. Reoperation rates as high as 80% have been reported, and a proportion of implant removals may in fact be caused by factors related to the subcutaneous placement of the metallic implants rather than K-wire migration alone.5,12,18 A nonmetallic isoelastic tension band can provide a more comfortable and less irritating implant, which could reduce the need for secondary intervention related to painful subcutaneous implant. One of our 7 patients had a symptomatic implant removed 5 years after surgery. This patient complained of pain over the area of the tension band device clip, so after fracture healing the entire fixation device was removed in the operating room. If reoperation is necessary, removal of intramedullary K-wires is relatively simple using a minimal incision; removal of stainless steel TBW may require a larger approach if the twisted knots cannot be easily retrieved.
A study of compression forces created by stainless steel wire demonstrated that a “finely tuned mechanical sense” was needed to produce optimal fixation compression when using stainless steel wire.26 It was observed that a submaximal twist created insufficient compressive force, while an ostensibly minimal increase in twisting force above optimum abruptly caused wire failure through breakage. Cerclage cables using clasping devices, such as the current isoelastic cerclage cable, were superior in ease of application. Furthermore, a clasping device allows for cable tension readjustment that is not possible with stainless steel wire. The clasping mechanism precludes the surgeon from having to bury the stainless steel knot and allows for the objective cable-tensioning not possible with stainless steel wire. Last, the tensioning device is titratable, which allows the surgeon to set the construct at a predetermined quantitative tension, which is of benefit in patients with osteopenia.
One limitation of this study is that it did not resolve the potential for K-wire migration, and we agree with previous recommendations that careful attention to surgical technique may avoid such a complication.10 In addition, the sample was small, and the study lacked a control group; a larger sample and a control group would have boosted study power. Nevertheless, the physical and functional outcomes associated with use of this technique were excellent. These results demonstrate an efficacious attempt to decrease secondary surgery rates and are therefore proof of concept that the isoelastic tension band may be used as an alternative to stainless steel in the TBW of displaced olecranon fractures with minimal or no comminution.
Conclusion
This easily reproducible technique for use of an isoelastic tension band in olecranon fracture fixation was associated with excellent physical and functional outcomes in a series of 7 patients. The rate of secondary intervention was slightly better for these patients than for patients treated with wire tension band fixation. Although more rigorous study of this device is needed, we think it is a promising alternative to wire tension band techniques.
Olecranon fractures are relatively common in adults and constitute 10% of all upper extremity injuries.1,2 An olecranon fracture may be sustained either directly (from blunt trauma or a fall onto the tip of the elbow) or indirectly (as a result of forceful hyperextension of the triceps during a fall onto an outstretched arm). Displaced olecranon fractures with extensor discontinuity require reduction and stabilization. One treatment option is tension band wiring (TBW), which is used to manage noncomminuted fractures.3 TBW, first described by Weber and Vasey4 in 1963, involves transforming the distractive forces of the triceps into dynamic compression forces across the olecranon articular surface using 2 intramedullary Kirschner wires (K-wires) and stainless steel wires looped in figure-of-8 fashion.
Various modifications of the TBW technique of Weber and Vasey4 have been proposed to reduce the frequency of complications. These modifications include substituting screws for K-wires, aiming the angle of the K-wires into the anterior coronoid cortex or loop configuration of the stainless steel wire, using double knots and twisting procedures to finalize fixation, and using alternative materials for the loop construct.5-8 In the literature and in our experience, patients often complain after surgery about prominent K-wires and the twisted knots used to tension the construct.9-12 Surgeons also must address the technical difficulties of positioning the brittle wire without kinking, and avoiding slack while tensioning.
In this article, we report on the clinical outcomes of a series of 7 patients with olecranon fracture treated with a US Food and Drug Administration–approved novel isoelastic ultrahigh-molecular-weight polyethylene (UHMWPE) cerclage cable (Iso-Elastic Cerclage System, Kinamed).
Materials and Methods
Surgical Technique
The patient is arranged in a sloppy lateral position to allow access to the posterior elbow. A nonsterile tourniquet is placed on the upper arm, and the limb is sterilely prepared and draped in standard fashion. A posterolateral incision is made around the olecranon and extended proximally 6 cm and distally 6 cm along the subcutaneous border of the ulna. The fracture is visualized and comminution identified.
To provide anchorage for a pointed reduction clamp, the surgeon drills a 2.5-mm hole in the subcutaneous border of the ulnar shaft. The fracture is reduced in extension and the clamp affixed. The elbow is then flexed and the reduction confirmed visually and by imaging. After realignment of the articular surfaces, 2 longitudinal, parallel K-wires (diameter, 1.6-2.0 mm) are passed in antegrade direction through the proximal olecranon within the medullary canal of the shaft. The proximal ends must not cross the cortex so they may fully capture the figure-of-8 wire during subsequent, final advancement, and the distal ends must not pierce the anterior cortex. A 2.5-mm transverse hole is created distal to the fracture in the dorsal aspect of the ulnar shaft from medial to lateral at 2 times the distance from the tip of the olecranon to the fracture site. This hole is expanded with a 3.5-mm drill bit, allowing both strands of the cable to be passed simultaneously medial to lateral, making the figure-of-8. The 3.5-mm hole represents about 20% of the overall width of the bone, which we have not found to create a significant stress riser in either laboratory or clinical tests of this construct. Proximally, the cables are placed on the periosteum of the olecranon but deep to the triceps tendon and adjacent to the K-wires. The locking clip is placed on the posterolateral aspect of the elbow joint in a location where it can be covered with local tissue for adequate padding. The cable is then threaded through the clamping bracket and tightened slowly and gradually with a tensioning device to low torque level (Figure 1). At this stage, tension may be released to make any necessary adjustments. Last, the locking clip is deployed, securing the tension band in the clip, and the excess cable is trimmed with a scalpel. Softening and pliability of the cable during its insertion and tensioning should be noted.
The ends of the K-wires are now curved in a hook configuration. The tines of the hooks should be parallel to accommodate the cable, and then the triceps is sharply incised to bone. If the bone is hard, an awl is used to create a pilot hole so the hook may be impaled into bone while capturing the cable. Next, the triceps is closed over the pins, minimizing the potential for pin migration and backout. The 2 K-wires are left in place to keep the fragments in proper anatomical alignment during healing and to prevent displacement with elbow motion. Figure 2 is a schematic of the final construct, and Figure 3 shows the construct in a patient.
Reduction of the olecranon fracture is assessed by imaging in full extension to check for possible implant impingement. Last, we apply the previously harvested fracture callus to the fracture site. Layered closure is performed, and bulky soft dressings are applied. Postoperative immobilization with a splint is used. Gentle range-of-motion exercises begin in about 2 weeks and progress as pain allows.
A case example with preoperative and postoperative images taken at 3-month follow-up is provided in Figure 4. The entire surgical technique can be viewed in the Video.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Clinical Cases
Between July 2007 and February 2011, 7 patients with displaced olecranon fractures underwent osteosynthesis using the isoelastic tension band (Table 1). According to the Mayo classification system, 5 of these patients had type 2A fractures, 1 had a type 2B fracture with an ipsilateral nondisplaced radial neck fracture, and 1 had a type 3B fracture. There were 4 female and 3 male patients. The injury was on the dominant side in 3 patients. All patients gave informed consent to evaluation at subsequent office visits and completed outcomes questionnaires by mail several years after surgery. Mean follow-up at which outcome measures questionnaires were obtained was 3.3 years (range, 2.1-6.8 years). Exclusion criteria were age under 18 years and inability to provide informed consent, fracture patterns with extensive articular comminution, and open fractures. Permission to conduct this research was granted by institutional review board.
At each visit, patients completed the Disabilities of the Arm, Shoulder, and Hand (DASH) functional outcome survey and were evaluated according to Broberg and Morrey’s elbow scoring system.13,14 Chart review consisted of evaluation of medical records, including radiographs and orthopedic physician notes in which preoperative examination was documented, mechanism of injury was noted, radiologic fracture pattern was evaluated, and time to bony union was recorded. Elbow motion was documented. Grip strength was measured with a calibrated Jamar dynamometer (Sammons Preston Rolyan) set at level 2, as delineated in Broberg and Morrey’s functional elbow scoring system.
Results
The 7 patients were assessed at a mean final follow-up of 19 months after surgery and received a mean Broberg and Morrey score of good (92.2/100) (Table 2). Restoration of motion and strength was excellent; compared with contralateral extremity, mean flexion arc was 96%, and mean forearm rotation was 96%. Grip was 99% of the noninjured side, perhaps the result of increased conditioning from physical therapy. Patients completed outcomes questionnaires at a mean of 3.3 years after surgery. Mean (SD) DASH score at this longest follow-up was 12.6 (17.2) (Table 2). Patients were satisfied (mean, 9.8/10; range, 9.5-10) and had little pain (mean, 0.8/10; range, 0-3). All fractures united, and there were no infections. One patient had a satisfactory union with complete restoration of motion and continued to play sports vocationally but developed pain over the locking clip 5 years after the index procedure and decided to have the implant removed. He had no radiographic evidence of K-wire or implant migration. Another patient had a minor degree of implant irritation at longest follow-up but did not request hardware removal.
Discussion
Stainless steel wire is often used in TBW because of its widespread availability, low cost, lack of immunogenicity, and relative strength.7 However, stainless steel wire has several disadvantages. It is susceptible to low-cycle fatigue failure, and fatigue strength may be seriously reduced secondary to incidental trauma to the wire on implantation.15,16 Other complications are kinking, skin irritation, implant prominence, fixation loss caused by wire loosening, and inadequate initial reduction potentially requiring revision.10,12,17-21
Isoelastic cable is a new type of cerclage cable that consists of UHMWPE strands braided over a nylon core. The particular property profile of the isoelastic tension band gives the cable intrinsic elastic and pliable qualities. In addition, unlike stainless steel, the band maintains a uniform, continuous compression force across a fracture site.22 Multifilament braided cables fatigue and fray, but the isoelastic cerclage cable showed no evidence of fraying or breakage after 1 million loading cycles.22,23 Compared with metal wire or braided metal cable, the band also has higher fatigue strength and higher ultimate tensile strength.7 Furthermore, the cable is less abrasive than stainless steel, so theoretically it is less irritating to surrounding subcutaneous tissue. Last, the pliability of the band allows the surgeon to create multiple loops of cable without the wire-failure side effects related to kinking, which is common with the metal construct.
In 2010, Ting and colleagues24 retrospectively studied implant failure complications associated with use of isoelastic cerclage cables in the treatment of periprosthetic fractures in total hip arthroplasty. They reported a breakage rate of 0% and noted that previously published breakage data for metallic cerclage devices ranged from 0% to 44%. They concluded that isoelastic cables were not associated with material failure, and there were no direct complications related to the cables. Similarly, Edwards and colleagues25 evaluated the same type of cable used in revision shoulder arthroplasty and reported excellent success and no failures. Although these data stem from use in the femur and humerus, we think the noted benefits apply to fractures of the elbow as well, as we observed a similar breakage rate (0%).
Various studies have addressed the clinical complaints and reoperation rates associated with retained metal implants after olecranon fixation. Traditional AO (Arbeitsgemeinschaft für Osteosynthesefragen) technique involves subcutaneous placement of stainless steel wires, which often results in tissue irritation. Reoperation rates as high as 80% have been reported, and a proportion of implant removals may in fact be caused by factors related to the subcutaneous placement of the metallic implants rather than K-wire migration alone.5,12,18 A nonmetallic isoelastic tension band can provide a more comfortable and less irritating implant, which could reduce the need for secondary intervention related to painful subcutaneous implant. One of our 7 patients had a symptomatic implant removed 5 years after surgery. This patient complained of pain over the area of the tension band device clip, so after fracture healing the entire fixation device was removed in the operating room. If reoperation is necessary, removal of intramedullary K-wires is relatively simple using a minimal incision; removal of stainless steel TBW may require a larger approach if the twisted knots cannot be easily retrieved.
A study of compression forces created by stainless steel wire demonstrated that a “finely tuned mechanical sense” was needed to produce optimal fixation compression when using stainless steel wire.26 It was observed that a submaximal twist created insufficient compressive force, while an ostensibly minimal increase in twisting force above optimum abruptly caused wire failure through breakage. Cerclage cables using clasping devices, such as the current isoelastic cerclage cable, were superior in ease of application. Furthermore, a clasping device allows for cable tension readjustment that is not possible with stainless steel wire. The clasping mechanism precludes the surgeon from having to bury the stainless steel knot and allows for the objective cable-tensioning not possible with stainless steel wire. Last, the tensioning device is titratable, which allows the surgeon to set the construct at a predetermined quantitative tension, which is of benefit in patients with osteopenia.
One limitation of this study is that it did not resolve the potential for K-wire migration, and we agree with previous recommendations that careful attention to surgical technique may avoid such a complication.10 In addition, the sample was small, and the study lacked a control group; a larger sample and a control group would have boosted study power. Nevertheless, the physical and functional outcomes associated with use of this technique were excellent. These results demonstrate an efficacious attempt to decrease secondary surgery rates and are therefore proof of concept that the isoelastic tension band may be used as an alternative to stainless steel in the TBW of displaced olecranon fractures with minimal or no comminution.
Conclusion
This easily reproducible technique for use of an isoelastic tension band in olecranon fracture fixation was associated with excellent physical and functional outcomes in a series of 7 patients. The rate of secondary intervention was slightly better for these patients than for patients treated with wire tension band fixation. Although more rigorous study of this device is needed, we think it is a promising alternative to wire tension band techniques.
1. Rommens PM, Küchle R, Schneider RU, Reuter M. Olecranon fractures in adults: factors influencing outcome. Injury. 2004;35(11):1149-1157.
2. Veillette CJ, Steinmann SP. Olecranon fractures. Orthop Clin North Am. 2008;39(2):229-236.
3. Newman SD, Mauffrey C, Krikler S. Olecranon fractures. Injury. 2009;40(6):575-581.
4. Weber BG, Vasey H. Osteosynthesis in olecranon fractures [in German]. Z Unfallmed Berufskr. 1963;56:90-96.
5. Netz P, Strömberg L. Non-sliding pins in traction absorbing wiring of fractures: a modified technique. Acta Orthop Scand. 1982;53(3):355-360.
6. Prayson MJ, Williams JL, Marshall MP, Scilaris TA, Lingenfelter EJ. Biomechanical comparison of fixation methods in transverse olecranon fractures: a cadaveric study. J Orthop Trauma. 1997;11(8):565-572.
7. Rothaug PG, Boston RC, Richardson DW, Nunamaker DM. A comparison of ultra-high-molecular weight polyethylene cable and stainless steel wire using two fixation techniques for repair of equine midbody sesamoid fractures: an in vitro biomechanical study. Vet Surg. 2002;31(5):445-454.
8. Harrell RM, Tong J, Weinhold PS, Dahners LE. Comparison of the mechanical properties of different tension band materials and suture techniques. J Orthop Trauma. 2003;17(2):119-122.
9. Nimura A, Nakagawa T, Wakabayashi Y, Sekiya I, Okawa A, Muneta T. Repair of olecranon fractures using FiberWire without metallic implants: report of two cases. J Orthop Surg Res. 2010;5:73.
10. Macko D, Szabo RM. Complications of tension-band wiring of olecranon fractures. J Bone Joint Surg Am. 1985;67(9):1396-1401.
11. Helm RH, Hornby R, Miller SW. The complications of surgical treatment of displaced fractures of the olecranon. Injury. 1987;18(1):48-50.
12. Romero JM, Miran A, Jensen CH. Complications and re-operation rate after tension-band wiring of olecranon fractures. J Orthop Sci. 2000;5(4):318-320.
13. Beaton DE, Katz JN, Fossel AH, Wright JG, Tarasuk V, Bombardier C. Measuring the whole or the parts? Validity, reliability, and responsiveness of the Disabilities of the Arm, Shoulder and Hand outcome measure in different regions of the upper extremity. J Hand Ther. 2001;14(2):128-146.
14. Broberg MA, Morrey BF. Results of delayed excision of the radial head after fracture. J Bone Joint Surg Am. 1986;68(5):669-674.
15. Bostrom MP, Asnis SE, Ernberg JJ, et al. Fatigue testing of cerclage stainless steel wire fixation. J Orthop Trauma. 1994;8(5):422-428.
16. Oh I, Sander TW, Treharne RW. The fatigue resistance of orthopaedic wire. Clin Orthop Relat Res. 1985;(192):228-236.
17. Amstutz HC, Maki S. Complications of trochanteric osteotomy in total hip replacement. J Bone Joint Surg Am. 1978;60(2):214-216.
18. Jensen CM, Olsen BB. Drawbacks of traction-absorbing wiring (TAW) in displaced fractures of the olecranon. Injury. 1986;17(3):174-175.
19. Kumar G, Mereddy PK, Hakkalamani S, Donnachie NJ. Implant removal following surgical stabilization of patella fracture. Orthopedics. 2010;33(5).
20. Hume MC, Wiss DA. Olecranon fractures. A clinical and radiographic comparison of tension band wiring and plate fixation. Clin Orthop Relat Res. 1992;(285):229-235.
21. Wolfgang G, Burke F, Bush D, et al. Surgical treatment of displaced olecranon fractures by tension band wiring technique. Clin Orthop Relat Res. 1987;(224):192-204.
22. Sarin VK, Mattchen TM, Hack B. A novel iso-elastic cerclage cable for treatment of fractures. Paper presented at: Annual Meeting of the Orthopaedic Research Society; February 20-23, 2005; Washington, DC. Paper 739.
23. Silverton CD, Jacobs JJ, Rosenberg AG, Kull L, Conley A, Galante JO. Complications of a cable grip system. J Arthroplasty. 1996;11(4):400-404.
24. Ting NT, Wera GD, Levine BR, Della Valle CJ. Early experience with a novel nonmetallic cable in reconstructive hip surgery. Clin Orthop Relat Res. 2010;468(9):2382-2386.
25. Edwards TB, Stuart KD, Trappey GJ, O’Connor DP, Sarin VK. Utility of polymer cerclage cables in revision shoulder arthroplasty. Orthopedics. 2011;34(4).
26. Shaw JA, Daubert HB. Compression capability of cerclage fixation systems. A biomechanical study. Orthopedics. 1988;11(8):1169-1174.
1. Rommens PM, Küchle R, Schneider RU, Reuter M. Olecranon fractures in adults: factors influencing outcome. Injury. 2004;35(11):1149-1157.
2. Veillette CJ, Steinmann SP. Olecranon fractures. Orthop Clin North Am. 2008;39(2):229-236.
3. Newman SD, Mauffrey C, Krikler S. Olecranon fractures. Injury. 2009;40(6):575-581.
4. Weber BG, Vasey H. Osteosynthesis in olecranon fractures [in German]. Z Unfallmed Berufskr. 1963;56:90-96.
5. Netz P, Strömberg L. Non-sliding pins in traction absorbing wiring of fractures: a modified technique. Acta Orthop Scand. 1982;53(3):355-360.
6. Prayson MJ, Williams JL, Marshall MP, Scilaris TA, Lingenfelter EJ. Biomechanical comparison of fixation methods in transverse olecranon fractures: a cadaveric study. J Orthop Trauma. 1997;11(8):565-572.
7. Rothaug PG, Boston RC, Richardson DW, Nunamaker DM. A comparison of ultra-high-molecular weight polyethylene cable and stainless steel wire using two fixation techniques for repair of equine midbody sesamoid fractures: an in vitro biomechanical study. Vet Surg. 2002;31(5):445-454.
8. Harrell RM, Tong J, Weinhold PS, Dahners LE. Comparison of the mechanical properties of different tension band materials and suture techniques. J Orthop Trauma. 2003;17(2):119-122.
9. Nimura A, Nakagawa T, Wakabayashi Y, Sekiya I, Okawa A, Muneta T. Repair of olecranon fractures using FiberWire without metallic implants: report of two cases. J Orthop Surg Res. 2010;5:73.
10. Macko D, Szabo RM. Complications of tension-band wiring of olecranon fractures. J Bone Joint Surg Am. 1985;67(9):1396-1401.
11. Helm RH, Hornby R, Miller SW. The complications of surgical treatment of displaced fractures of the olecranon. Injury. 1987;18(1):48-50.
12. Romero JM, Miran A, Jensen CH. Complications and re-operation rate after tension-band wiring of olecranon fractures. J Orthop Sci. 2000;5(4):318-320.
13. Beaton DE, Katz JN, Fossel AH, Wright JG, Tarasuk V, Bombardier C. Measuring the whole or the parts? Validity, reliability, and responsiveness of the Disabilities of the Arm, Shoulder and Hand outcome measure in different regions of the upper extremity. J Hand Ther. 2001;14(2):128-146.
14. Broberg MA, Morrey BF. Results of delayed excision of the radial head after fracture. J Bone Joint Surg Am. 1986;68(5):669-674.
15. Bostrom MP, Asnis SE, Ernberg JJ, et al. Fatigue testing of cerclage stainless steel wire fixation. J Orthop Trauma. 1994;8(5):422-428.
16. Oh I, Sander TW, Treharne RW. The fatigue resistance of orthopaedic wire. Clin Orthop Relat Res. 1985;(192):228-236.
17. Amstutz HC, Maki S. Complications of trochanteric osteotomy in total hip replacement. J Bone Joint Surg Am. 1978;60(2):214-216.
18. Jensen CM, Olsen BB. Drawbacks of traction-absorbing wiring (TAW) in displaced fractures of the olecranon. Injury. 1986;17(3):174-175.
19. Kumar G, Mereddy PK, Hakkalamani S, Donnachie NJ. Implant removal following surgical stabilization of patella fracture. Orthopedics. 2010;33(5).
20. Hume MC, Wiss DA. Olecranon fractures. A clinical and radiographic comparison of tension band wiring and plate fixation. Clin Orthop Relat Res. 1992;(285):229-235.
21. Wolfgang G, Burke F, Bush D, et al. Surgical treatment of displaced olecranon fractures by tension band wiring technique. Clin Orthop Relat Res. 1987;(224):192-204.
22. Sarin VK, Mattchen TM, Hack B. A novel iso-elastic cerclage cable for treatment of fractures. Paper presented at: Annual Meeting of the Orthopaedic Research Society; February 20-23, 2005; Washington, DC. Paper 739.
23. Silverton CD, Jacobs JJ, Rosenberg AG, Kull L, Conley A, Galante JO. Complications of a cable grip system. J Arthroplasty. 1996;11(4):400-404.
24. Ting NT, Wera GD, Levine BR, Della Valle CJ. Early experience with a novel nonmetallic cable in reconstructive hip surgery. Clin Orthop Relat Res. 2010;468(9):2382-2386.
25. Edwards TB, Stuart KD, Trappey GJ, O’Connor DP, Sarin VK. Utility of polymer cerclage cables in revision shoulder arthroplasty. Orthopedics. 2011;34(4).
26. Shaw JA, Daubert HB. Compression capability of cerclage fixation systems. A biomechanical study. Orthopedics. 1988;11(8):1169-1174.
Reinforcing a Spica Cast With a Fiberglass Bar
Femur fractures (Orthopaedic Trauma Association classes 31, 32, 33)1 are common childhood injuries, occurring at a rate of 19 per 100,000 children in the United States.2 Peak occurrence is bimodal at ages 2 and 17 years. The most common mechanism of injury in children under 6 years is a fall, and hip spica casting is the preferred treatment modality in this group.3-5
A bar connecting the legs of the spica cast has been shown to facilitate patient transport5 and significantly decrease mechanical failure of the spica cast.6 This bar often consists of a broom handle or pipe that must be cut to size during the case and subsequently incorporated into the cast—tasks that are often inconvenient and time-consuming for on-call or emergency department staff unfamiliar with orthopedic tools.
In this article, we review a spica cast application that incorporates a low-cost, lightweight technique for fabricating a connecting bar from existing fiberglass casting material. The Institutional Review Board at Connecticut Children’s Medical Center approved this work.
Technique of Double-Leg Spica Casting With Fiberglass Bar
A spica casting table (Orthopedic Systems) with a well-padded post is placed on the operating room table and adjusted to the length of the patient from perineum to just below the shoulders. With the patient under general anesthesia, folded towels are used to provide 2 to 4 cm of padding on the anterior torso, atop which a waterproof pantaloon is applied. The patient is transferred to the spica table, and the patient’s arms are gently secured to the casting table with cast padding or tape in an abducted position at the shoulders. A surgeon controls the legs by holding the feet with the long fingers just above the heels, the index fingers on the anterior ankle, and the thumbs on the soles of the feet. Cast padding is wrapped from the nipple line to the supramalleolar region on each leg. The bony prominences of the malleoli, patella, fibular head, femoral condyles, iliac crests, and coccyx are well padded.
Fiberglass is then rolled without compression onto the patient, beginning with the torso and perineal areas. The injured leg is wrapped to its final length above the malleoli while the uninjured leg is kept free. Maintaining the position of the injured leg with simultaneous molding at the fracture site, typically to promote valgus, allows fracture reduction. The fracture position is then checked under image intensification. For femur fractures, hip abduction and flexion are set to 45° and 90°, respectively, while knee flexion is between 50° and 90°. The uninjured leg is then wrapped with fiberglass. Additional strips of fiberglass can be used to reinforce weak junctional regions between the torso and the legs, posteriorly over the “intern’s triangle” and anteriorly along the hip crease.
A connecting fiberglass bar is then created using a fiberglass roll once the cast is hardened. A 2-inch fiberglass roll is wrapped around one leg to secure its position (Figure 1A) and then rolled around the second limb (Figure 1B). Fiberglass is then pulled taut and rolled around the bridge that has been created in order to thicken the bar (Figure 2). The roll is again brought around the closest limb, wrapped back across the bridge to the other limb, and rolled out to its full length. Last, the legs are abducted 1 to 2 cm to tension the bar (Figure 3). Although this does not produce enough movement to cause a crease and a resultant ulcer, careful inspection of common pressure points (eg, popliteal fossa) should be performed after the cast is complete.
The chest towels are removed, and the final cast is inspected clinically and fluoroscopically at the fracture site before extubation. The cast is trimmed as needed to ensure room for perineal care, as well as full ankle flexion and extension without impingement. Cast edges are further petaled with plastic tape (Hy-Tape International) to provide padding and prevent the waterproof lining from tearing.
Postoperative care involves overnight observation and caregiver practice in perineal care. Frequent rotation from supine to prone is encouraged. Nurses confirm car-seat fit before discharge. If needed, radiographs are obtained 7 to 10 days later to help with wedging adjustment. The cast is removed in the clinic when adequate callus is appreciated on subsequent radiographs.
Case Series
Our experience with this technique in 16 unilateral femur fractures has been favorable (Table). Patient age ranged from 5 months to 3 years. Mean pretreatment angulation was 13° varus and 11° procurvatum. The majority of fractures were femoral shaft fractures; 1 was proximal, 2 distal.
All fractures united without cast revision. Mean cast time was 4.5 weeks (range, 16 days–6 weeks). Immediate postoperative alignment was 2.5° varus (range, 11° valgus to 16° varus) and 7° procurvatum (range, 1° recurvatum to 22° procurvatum). Mean shortening was 1.5 cm (range, 0-2.7 cm). Final alignment was 1° valgus (range, 9° valgus to 12° varus) and 5° procurvatum (range, 0° to 22°). Mean follow-up was 8 months. There were no cases of skin maceration or cast failure. No casts precluded use of a spica car-seat. Figure 4 shows a typical case with a midshaft fracture treated with closed reduction and casting for 4 weeks with good remodeling at final follow-up, 19 months after injury.
Discussion
Although single-leg walking spica casts have been shown to safely treat low-energy femur fractures in children 1 to 6 years old,7 length-unstable femur fractures, bilateral femur fractures, and patients with hip dysplasia continue to be managed with a double-leg hip spica construct. Cast integrity remains fundamental to the control of most fractures and prevention of cast-related complications, such as skin maceration and ulceration. Surgeons typically use spica cast reinforcement schemes—such as cast augments of the torso–limb junction, with multiple layers of casting material or incorporation of a connecting bar between the legs, typically constructed by overwrapping a wooden dowel in casting material—to improve the mechanical stability of casts.6 The present technique of creating a connecting bar from fiberglass casting material significantly simplifies the standard wooden dowel approach and provided excellent results in our treatment group in terms of cast integrity and fracture alignment. In addition, at our institution, a roll of fiberglass costs $2.10, whereas a wooden dowel costs $3 to $10 and can be difficult to locate if not frequently used. Other tube-shaped materials, such as the disposable material used to package implants and tubes, carry an even lower cost. However, we have found that a single fiberglass roll is most readily available and easiest to apply.
Although proper spica cast application remains important in managing pediatric trauma, it lacks a good technical description in the literature. In this technical report, we have presented our standard spica cast application method, which minimizes the range of cast complications that have been reported, from minor skin irritation to superior mesenteric artery syndrome. Two salient technical highlights are use of waterproof pantaloon liners and cast petaling, which we have found almost eliminate the morbidity of potential skin complications, reported to occur at a rate of 28%.8 In addition, we forgo applying the cast on the injured leg in segments. Application of a short-leg cast on the injured leg to allow traction on the leg during cast application is of dubious utility and may be potentially harmful, with described complications of peroneal nerve palsy and compartment syndrome.9-11 Further, it is important to use an abdominal spacer (eg, a stack of towels) under the cast padding to create room for abdominal expansion and minimize pressure thought to induce superior mesenteric artery syndrome. Plastic or rubber abdominal spacers have also been described.12,13 Last, leg position is important for reduction and maintenance of the fracture, as well as patient care. Literature advocates minimizing hip abduction to just that needed for perineal care and maximizing hip flexion and knee extension to optimize car-seat fit and safety.14
Conclusion
Construction of a spica cast lower limb connecting bar from readily available fiberglass casting material allows a facile and rapid addition to the mechanical stability of a spica cast in the treatment of pediatric femur fractures. The technique is low-cost and obviates the need for additional extraneous materials.
1. Slongo TF, Audigé L; AO Pediatric Classification Group. Fracture and dislocation classification compendium for children: the AO Pediatric Comprehensive Classification of Long Bone Fractures (PCCF). J Orthop Trauma. 2007;21(10):S135-S160.
2. Hinton RY, Lincoln A, Crockett MM, Sponseller P, Smith G. Fractures of the femoral shaft in children. Incidence, mechanisms, and sociodemographic risk factors. J Bone Joint Surg Am. 1999;81(4):500-509.
3. Campbell WC, Canale ST, Beaty JH, eds. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, PA: Mosby Elsevier; 2008.
4. Lovell WW, Winter RB, Morrissy RT, Weinstein SL. Lovell and Winter’s Pediatric Orthopaedics. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
5. Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children. 4th ed. Philadelphia, PA: Elsevier Health Sciences; 2009.
6. Hosalkar HS, Jones S, Chowdhury M, Chatoo M, Hill RA. Connecting bar for hip spica reinforcement: does it help? J Pediatr Orthop B. 2003;12(2):100-102.
7. Flynn JM, Garner MR, Jones KJ, et al. The treatment of low-energy femoral shaft fractures: a prospective study comparing the “walking spica” with the traditional spica cast. J Bone Joint Surg Am. 2011;93(23):2196-2202.
8. DiFazio R, Vessey J, Zurakowski D, Hresko MT, Matheney T. Incidence of skin complications and associated charges in children treated with hip spica casts for femur fractures. J Pediatr Orthop. 2011;31(1):17-22.
9. Weiss AP, Schenck RC Jr, Sponseller PD, Thompson JD. Peroneal nerve palsy after early cast application for femoral fractures in children. J Pediatr Orthop. 1992;12(1):25-28.
10. Mubarak SJ, Frick S, Sink E, Rathjen K, Noonan KJ. Volkmann contracture and compartment syndromes after femur fractures in children treated with 90/90 spica casts. J Pediatr Orthop. 2006;26(5):567-572.
11. Large TM, Frick SL. Compartment syndrome of the leg after treatment of a femoral fracture with an early sitting spica cast. A report of two cases. J Bone Joint Surg Am. 2003;85(11):2207-2210.
12. Sharma S, Azzopardi T. Reduction of abdominal pressure for prophylaxis of the mesenteric artery syndrome (cast syndrome) in a hip spica—a simple technique. Ann R Coll Surg Engl. 2006;88(3):317.
13. Kiter E, Demirkan F, Kiliç BA, Erkula G. A new technique for creating an abdominal window in a hip spica cast. J Orthop Trauma. 2003;17(6):442-443.
14. Zielinski J, Oliver G, Sybesma J, Walter N, Atkinson P. Casting technique and restraint choice influence child safety during transport of body casted children subjected to a simulated frontal MVA. J Trauma. 2009;66(6):1653-1665.
Femur fractures (Orthopaedic Trauma Association classes 31, 32, 33)1 are common childhood injuries, occurring at a rate of 19 per 100,000 children in the United States.2 Peak occurrence is bimodal at ages 2 and 17 years. The most common mechanism of injury in children under 6 years is a fall, and hip spica casting is the preferred treatment modality in this group.3-5
A bar connecting the legs of the spica cast has been shown to facilitate patient transport5 and significantly decrease mechanical failure of the spica cast.6 This bar often consists of a broom handle or pipe that must be cut to size during the case and subsequently incorporated into the cast—tasks that are often inconvenient and time-consuming for on-call or emergency department staff unfamiliar with orthopedic tools.
In this article, we review a spica cast application that incorporates a low-cost, lightweight technique for fabricating a connecting bar from existing fiberglass casting material. The Institutional Review Board at Connecticut Children’s Medical Center approved this work.
Technique of Double-Leg Spica Casting With Fiberglass Bar
A spica casting table (Orthopedic Systems) with a well-padded post is placed on the operating room table and adjusted to the length of the patient from perineum to just below the shoulders. With the patient under general anesthesia, folded towels are used to provide 2 to 4 cm of padding on the anterior torso, atop which a waterproof pantaloon is applied. The patient is transferred to the spica table, and the patient’s arms are gently secured to the casting table with cast padding or tape in an abducted position at the shoulders. A surgeon controls the legs by holding the feet with the long fingers just above the heels, the index fingers on the anterior ankle, and the thumbs on the soles of the feet. Cast padding is wrapped from the nipple line to the supramalleolar region on each leg. The bony prominences of the malleoli, patella, fibular head, femoral condyles, iliac crests, and coccyx are well padded.
Fiberglass is then rolled without compression onto the patient, beginning with the torso and perineal areas. The injured leg is wrapped to its final length above the malleoli while the uninjured leg is kept free. Maintaining the position of the injured leg with simultaneous molding at the fracture site, typically to promote valgus, allows fracture reduction. The fracture position is then checked under image intensification. For femur fractures, hip abduction and flexion are set to 45° and 90°, respectively, while knee flexion is between 50° and 90°. The uninjured leg is then wrapped with fiberglass. Additional strips of fiberglass can be used to reinforce weak junctional regions between the torso and the legs, posteriorly over the “intern’s triangle” and anteriorly along the hip crease.
A connecting fiberglass bar is then created using a fiberglass roll once the cast is hardened. A 2-inch fiberglass roll is wrapped around one leg to secure its position (Figure 1A) and then rolled around the second limb (Figure 1B). Fiberglass is then pulled taut and rolled around the bridge that has been created in order to thicken the bar (Figure 2). The roll is again brought around the closest limb, wrapped back across the bridge to the other limb, and rolled out to its full length. Last, the legs are abducted 1 to 2 cm to tension the bar (Figure 3). Although this does not produce enough movement to cause a crease and a resultant ulcer, careful inspection of common pressure points (eg, popliteal fossa) should be performed after the cast is complete.
The chest towels are removed, and the final cast is inspected clinically and fluoroscopically at the fracture site before extubation. The cast is trimmed as needed to ensure room for perineal care, as well as full ankle flexion and extension without impingement. Cast edges are further petaled with plastic tape (Hy-Tape International) to provide padding and prevent the waterproof lining from tearing.
Postoperative care involves overnight observation and caregiver practice in perineal care. Frequent rotation from supine to prone is encouraged. Nurses confirm car-seat fit before discharge. If needed, radiographs are obtained 7 to 10 days later to help with wedging adjustment. The cast is removed in the clinic when adequate callus is appreciated on subsequent radiographs.
Case Series
Our experience with this technique in 16 unilateral femur fractures has been favorable (Table). Patient age ranged from 5 months to 3 years. Mean pretreatment angulation was 13° varus and 11° procurvatum. The majority of fractures were femoral shaft fractures; 1 was proximal, 2 distal.
All fractures united without cast revision. Mean cast time was 4.5 weeks (range, 16 days–6 weeks). Immediate postoperative alignment was 2.5° varus (range, 11° valgus to 16° varus) and 7° procurvatum (range, 1° recurvatum to 22° procurvatum). Mean shortening was 1.5 cm (range, 0-2.7 cm). Final alignment was 1° valgus (range, 9° valgus to 12° varus) and 5° procurvatum (range, 0° to 22°). Mean follow-up was 8 months. There were no cases of skin maceration or cast failure. No casts precluded use of a spica car-seat. Figure 4 shows a typical case with a midshaft fracture treated with closed reduction and casting for 4 weeks with good remodeling at final follow-up, 19 months after injury.
Discussion
Although single-leg walking spica casts have been shown to safely treat low-energy femur fractures in children 1 to 6 years old,7 length-unstable femur fractures, bilateral femur fractures, and patients with hip dysplasia continue to be managed with a double-leg hip spica construct. Cast integrity remains fundamental to the control of most fractures and prevention of cast-related complications, such as skin maceration and ulceration. Surgeons typically use spica cast reinforcement schemes—such as cast augments of the torso–limb junction, with multiple layers of casting material or incorporation of a connecting bar between the legs, typically constructed by overwrapping a wooden dowel in casting material—to improve the mechanical stability of casts.6 The present technique of creating a connecting bar from fiberglass casting material significantly simplifies the standard wooden dowel approach and provided excellent results in our treatment group in terms of cast integrity and fracture alignment. In addition, at our institution, a roll of fiberglass costs $2.10, whereas a wooden dowel costs $3 to $10 and can be difficult to locate if not frequently used. Other tube-shaped materials, such as the disposable material used to package implants and tubes, carry an even lower cost. However, we have found that a single fiberglass roll is most readily available and easiest to apply.
Although proper spica cast application remains important in managing pediatric trauma, it lacks a good technical description in the literature. In this technical report, we have presented our standard spica cast application method, which minimizes the range of cast complications that have been reported, from minor skin irritation to superior mesenteric artery syndrome. Two salient technical highlights are use of waterproof pantaloon liners and cast petaling, which we have found almost eliminate the morbidity of potential skin complications, reported to occur at a rate of 28%.8 In addition, we forgo applying the cast on the injured leg in segments. Application of a short-leg cast on the injured leg to allow traction on the leg during cast application is of dubious utility and may be potentially harmful, with described complications of peroneal nerve palsy and compartment syndrome.9-11 Further, it is important to use an abdominal spacer (eg, a stack of towels) under the cast padding to create room for abdominal expansion and minimize pressure thought to induce superior mesenteric artery syndrome. Plastic or rubber abdominal spacers have also been described.12,13 Last, leg position is important for reduction and maintenance of the fracture, as well as patient care. Literature advocates minimizing hip abduction to just that needed for perineal care and maximizing hip flexion and knee extension to optimize car-seat fit and safety.14
Conclusion
Construction of a spica cast lower limb connecting bar from readily available fiberglass casting material allows a facile and rapid addition to the mechanical stability of a spica cast in the treatment of pediatric femur fractures. The technique is low-cost and obviates the need for additional extraneous materials.
Femur fractures (Orthopaedic Trauma Association classes 31, 32, 33)1 are common childhood injuries, occurring at a rate of 19 per 100,000 children in the United States.2 Peak occurrence is bimodal at ages 2 and 17 years. The most common mechanism of injury in children under 6 years is a fall, and hip spica casting is the preferred treatment modality in this group.3-5
A bar connecting the legs of the spica cast has been shown to facilitate patient transport5 and significantly decrease mechanical failure of the spica cast.6 This bar often consists of a broom handle or pipe that must be cut to size during the case and subsequently incorporated into the cast—tasks that are often inconvenient and time-consuming for on-call or emergency department staff unfamiliar with orthopedic tools.
In this article, we review a spica cast application that incorporates a low-cost, lightweight technique for fabricating a connecting bar from existing fiberglass casting material. The Institutional Review Board at Connecticut Children’s Medical Center approved this work.
Technique of Double-Leg Spica Casting With Fiberglass Bar
A spica casting table (Orthopedic Systems) with a well-padded post is placed on the operating room table and adjusted to the length of the patient from perineum to just below the shoulders. With the patient under general anesthesia, folded towels are used to provide 2 to 4 cm of padding on the anterior torso, atop which a waterproof pantaloon is applied. The patient is transferred to the spica table, and the patient’s arms are gently secured to the casting table with cast padding or tape in an abducted position at the shoulders. A surgeon controls the legs by holding the feet with the long fingers just above the heels, the index fingers on the anterior ankle, and the thumbs on the soles of the feet. Cast padding is wrapped from the nipple line to the supramalleolar region on each leg. The bony prominences of the malleoli, patella, fibular head, femoral condyles, iliac crests, and coccyx are well padded.
Fiberglass is then rolled without compression onto the patient, beginning with the torso and perineal areas. The injured leg is wrapped to its final length above the malleoli while the uninjured leg is kept free. Maintaining the position of the injured leg with simultaneous molding at the fracture site, typically to promote valgus, allows fracture reduction. The fracture position is then checked under image intensification. For femur fractures, hip abduction and flexion are set to 45° and 90°, respectively, while knee flexion is between 50° and 90°. The uninjured leg is then wrapped with fiberglass. Additional strips of fiberglass can be used to reinforce weak junctional regions between the torso and the legs, posteriorly over the “intern’s triangle” and anteriorly along the hip crease.
A connecting fiberglass bar is then created using a fiberglass roll once the cast is hardened. A 2-inch fiberglass roll is wrapped around one leg to secure its position (Figure 1A) and then rolled around the second limb (Figure 1B). Fiberglass is then pulled taut and rolled around the bridge that has been created in order to thicken the bar (Figure 2). The roll is again brought around the closest limb, wrapped back across the bridge to the other limb, and rolled out to its full length. Last, the legs are abducted 1 to 2 cm to tension the bar (Figure 3). Although this does not produce enough movement to cause a crease and a resultant ulcer, careful inspection of common pressure points (eg, popliteal fossa) should be performed after the cast is complete.
The chest towels are removed, and the final cast is inspected clinically and fluoroscopically at the fracture site before extubation. The cast is trimmed as needed to ensure room for perineal care, as well as full ankle flexion and extension without impingement. Cast edges are further petaled with plastic tape (Hy-Tape International) to provide padding and prevent the waterproof lining from tearing.
Postoperative care involves overnight observation and caregiver practice in perineal care. Frequent rotation from supine to prone is encouraged. Nurses confirm car-seat fit before discharge. If needed, radiographs are obtained 7 to 10 days later to help with wedging adjustment. The cast is removed in the clinic when adequate callus is appreciated on subsequent radiographs.
Case Series
Our experience with this technique in 16 unilateral femur fractures has been favorable (Table). Patient age ranged from 5 months to 3 years. Mean pretreatment angulation was 13° varus and 11° procurvatum. The majority of fractures were femoral shaft fractures; 1 was proximal, 2 distal.
All fractures united without cast revision. Mean cast time was 4.5 weeks (range, 16 days–6 weeks). Immediate postoperative alignment was 2.5° varus (range, 11° valgus to 16° varus) and 7° procurvatum (range, 1° recurvatum to 22° procurvatum). Mean shortening was 1.5 cm (range, 0-2.7 cm). Final alignment was 1° valgus (range, 9° valgus to 12° varus) and 5° procurvatum (range, 0° to 22°). Mean follow-up was 8 months. There were no cases of skin maceration or cast failure. No casts precluded use of a spica car-seat. Figure 4 shows a typical case with a midshaft fracture treated with closed reduction and casting for 4 weeks with good remodeling at final follow-up, 19 months after injury.
Discussion
Although single-leg walking spica casts have been shown to safely treat low-energy femur fractures in children 1 to 6 years old,7 length-unstable femur fractures, bilateral femur fractures, and patients with hip dysplasia continue to be managed with a double-leg hip spica construct. Cast integrity remains fundamental to the control of most fractures and prevention of cast-related complications, such as skin maceration and ulceration. Surgeons typically use spica cast reinforcement schemes—such as cast augments of the torso–limb junction, with multiple layers of casting material or incorporation of a connecting bar between the legs, typically constructed by overwrapping a wooden dowel in casting material—to improve the mechanical stability of casts.6 The present technique of creating a connecting bar from fiberglass casting material significantly simplifies the standard wooden dowel approach and provided excellent results in our treatment group in terms of cast integrity and fracture alignment. In addition, at our institution, a roll of fiberglass costs $2.10, whereas a wooden dowel costs $3 to $10 and can be difficult to locate if not frequently used. Other tube-shaped materials, such as the disposable material used to package implants and tubes, carry an even lower cost. However, we have found that a single fiberglass roll is most readily available and easiest to apply.
Although proper spica cast application remains important in managing pediatric trauma, it lacks a good technical description in the literature. In this technical report, we have presented our standard spica cast application method, which minimizes the range of cast complications that have been reported, from minor skin irritation to superior mesenteric artery syndrome. Two salient technical highlights are use of waterproof pantaloon liners and cast petaling, which we have found almost eliminate the morbidity of potential skin complications, reported to occur at a rate of 28%.8 In addition, we forgo applying the cast on the injured leg in segments. Application of a short-leg cast on the injured leg to allow traction on the leg during cast application is of dubious utility and may be potentially harmful, with described complications of peroneal nerve palsy and compartment syndrome.9-11 Further, it is important to use an abdominal spacer (eg, a stack of towels) under the cast padding to create room for abdominal expansion and minimize pressure thought to induce superior mesenteric artery syndrome. Plastic or rubber abdominal spacers have also been described.12,13 Last, leg position is important for reduction and maintenance of the fracture, as well as patient care. Literature advocates minimizing hip abduction to just that needed for perineal care and maximizing hip flexion and knee extension to optimize car-seat fit and safety.14
Conclusion
Construction of a spica cast lower limb connecting bar from readily available fiberglass casting material allows a facile and rapid addition to the mechanical stability of a spica cast in the treatment of pediatric femur fractures. The technique is low-cost and obviates the need for additional extraneous materials.
1. Slongo TF, Audigé L; AO Pediatric Classification Group. Fracture and dislocation classification compendium for children: the AO Pediatric Comprehensive Classification of Long Bone Fractures (PCCF). J Orthop Trauma. 2007;21(10):S135-S160.
2. Hinton RY, Lincoln A, Crockett MM, Sponseller P, Smith G. Fractures of the femoral shaft in children. Incidence, mechanisms, and sociodemographic risk factors. J Bone Joint Surg Am. 1999;81(4):500-509.
3. Campbell WC, Canale ST, Beaty JH, eds. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, PA: Mosby Elsevier; 2008.
4. Lovell WW, Winter RB, Morrissy RT, Weinstein SL. Lovell and Winter’s Pediatric Orthopaedics. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
5. Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children. 4th ed. Philadelphia, PA: Elsevier Health Sciences; 2009.
6. Hosalkar HS, Jones S, Chowdhury M, Chatoo M, Hill RA. Connecting bar for hip spica reinforcement: does it help? J Pediatr Orthop B. 2003;12(2):100-102.
7. Flynn JM, Garner MR, Jones KJ, et al. The treatment of low-energy femoral shaft fractures: a prospective study comparing the “walking spica” with the traditional spica cast. J Bone Joint Surg Am. 2011;93(23):2196-2202.
8. DiFazio R, Vessey J, Zurakowski D, Hresko MT, Matheney T. Incidence of skin complications and associated charges in children treated with hip spica casts for femur fractures. J Pediatr Orthop. 2011;31(1):17-22.
9. Weiss AP, Schenck RC Jr, Sponseller PD, Thompson JD. Peroneal nerve palsy after early cast application for femoral fractures in children. J Pediatr Orthop. 1992;12(1):25-28.
10. Mubarak SJ, Frick S, Sink E, Rathjen K, Noonan KJ. Volkmann contracture and compartment syndromes after femur fractures in children treated with 90/90 spica casts. J Pediatr Orthop. 2006;26(5):567-572.
11. Large TM, Frick SL. Compartment syndrome of the leg after treatment of a femoral fracture with an early sitting spica cast. A report of two cases. J Bone Joint Surg Am. 2003;85(11):2207-2210.
12. Sharma S, Azzopardi T. Reduction of abdominal pressure for prophylaxis of the mesenteric artery syndrome (cast syndrome) in a hip spica—a simple technique. Ann R Coll Surg Engl. 2006;88(3):317.
13. Kiter E, Demirkan F, Kiliç BA, Erkula G. A new technique for creating an abdominal window in a hip spica cast. J Orthop Trauma. 2003;17(6):442-443.
14. Zielinski J, Oliver G, Sybesma J, Walter N, Atkinson P. Casting technique and restraint choice influence child safety during transport of body casted children subjected to a simulated frontal MVA. J Trauma. 2009;66(6):1653-1665.
1. Slongo TF, Audigé L; AO Pediatric Classification Group. Fracture and dislocation classification compendium for children: the AO Pediatric Comprehensive Classification of Long Bone Fractures (PCCF). J Orthop Trauma. 2007;21(10):S135-S160.
2. Hinton RY, Lincoln A, Crockett MM, Sponseller P, Smith G. Fractures of the femoral shaft in children. Incidence, mechanisms, and sociodemographic risk factors. J Bone Joint Surg Am. 1999;81(4):500-509.
3. Campbell WC, Canale ST, Beaty JH, eds. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, PA: Mosby Elsevier; 2008.
4. Lovell WW, Winter RB, Morrissy RT, Weinstein SL. Lovell and Winter’s Pediatric Orthopaedics. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
5. Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children. 4th ed. Philadelphia, PA: Elsevier Health Sciences; 2009.
6. Hosalkar HS, Jones S, Chowdhury M, Chatoo M, Hill RA. Connecting bar for hip spica reinforcement: does it help? J Pediatr Orthop B. 2003;12(2):100-102.
7. Flynn JM, Garner MR, Jones KJ, et al. The treatment of low-energy femoral shaft fractures: a prospective study comparing the “walking spica” with the traditional spica cast. J Bone Joint Surg Am. 2011;93(23):2196-2202.
8. DiFazio R, Vessey J, Zurakowski D, Hresko MT, Matheney T. Incidence of skin complications and associated charges in children treated with hip spica casts for femur fractures. J Pediatr Orthop. 2011;31(1):17-22.
9. Weiss AP, Schenck RC Jr, Sponseller PD, Thompson JD. Peroneal nerve palsy after early cast application for femoral fractures in children. J Pediatr Orthop. 1992;12(1):25-28.
10. Mubarak SJ, Frick S, Sink E, Rathjen K, Noonan KJ. Volkmann contracture and compartment syndromes after femur fractures in children treated with 90/90 spica casts. J Pediatr Orthop. 2006;26(5):567-572.
11. Large TM, Frick SL. Compartment syndrome of the leg after treatment of a femoral fracture with an early sitting spica cast. A report of two cases. J Bone Joint Surg Am. 2003;85(11):2207-2210.
12. Sharma S, Azzopardi T. Reduction of abdominal pressure for prophylaxis of the mesenteric artery syndrome (cast syndrome) in a hip spica—a simple technique. Ann R Coll Surg Engl. 2006;88(3):317.
13. Kiter E, Demirkan F, Kiliç BA, Erkula G. A new technique for creating an abdominal window in a hip spica cast. J Orthop Trauma. 2003;17(6):442-443.
14. Zielinski J, Oliver G, Sybesma J, Walter N, Atkinson P. Casting technique and restraint choice influence child safety during transport of body casted children subjected to a simulated frontal MVA. J Trauma. 2009;66(6):1653-1665.
Open Carpal Tunnel Release With Use of a Nasal Turbinate Speculum
Carpal tunnel syndrome (CTS) is a disorder characterized by entrapment of the median nerve at the wrist, which may lead to symptoms of pain, paresthesia, and, ultimately, thenar muscle atrophy. Surgical intervention is indicated with persistent or progressive symptoms despite nonoperative management. Timely surgical decompression aims to halt progression of this disorder and prevent permanent peripheral nerve injury.
Carpal tunnel release (CTR) is the most common hand and wrist surgery in the United States, with about 400,000 operations performed annually.1,2 Several methods of decompressing the carpal tunnel have been described.3 These include standard open CTR (OCTR), mini-open approaches, and various endoscopic techniques. OCTR was initially described by Sir James Learmonth in 1933,4 and it remains the gold-standard surgical treatment for patients with symptomatic CTS. Uniform excellent results with high patient satisfaction and low complication rates have been reported in several series.5-9 Common to all techniques is complete proximal-to-distal division of the transverse carpal ligament (TCL). Magnetic resonance imaging studies have shown that TCL transection and the resulting diastasis between the radial and ulnar leaflets cause a significant increase in the volume of the carpal tunnel, leading to decreased pressure.10,11
Endoscopic CTR (ECTR) techniques were developed in an effort to reduce complications, scar sensitivity, and pillar pain and facilitate more rapid return to work.12-17 Outcome studies have demonstrated that both open and endoscopic releases yield patient-reported subjective improvements over preoperative symptoms.18-22 A randomized, controlled trial by Trumble and colleagues23 in 2002 found that ECTR led to improved patient outcomes in the early postoperative period (first 3 months), though differences in outcomes were reduced at final follow-up. More recently (2007), a Cochrane review of 33 trials concluded there was no strong evidence favoring use of alternative techniques over OCTR.3 Further, OCTR has been found to be technically less demanding and associated with decreased complications and costs.24
Indications
The benefit of median nerve decompression at the wrist for CTS is clear.6,7 Indications for surgery in patients with CTS include persistent symptoms despite nonoperative treatment, objective sensory disturbance or motor weakness, and thenar atrophy. Symptomatic response to corticosteroid injection is predictive of success after carpal tunnel surgery.25 More than 87% of patients who gain symptomatic relief from corticosteroid injection have an excellent surgical outcome.
Technique
OCTR allows direct visualization of the TCL and the distal volar forearm fascia (DVFF) and evaluation for the presence of anomalous branching patterns of the median nerve. OCTR traditionally was performed through a 4- to 5-cm longitudinal incision extending from the wrist crease proximally to the Kaplan cardinal line distally. The mini-open technique is identical with the exception of incision length. We routinely use a 2.5- to 3-cm incision. Regardless of incision length, each OCTR should proceed through the same reproducible steps.
We perform OCTR under tourniquet control. Choice of anesthesia is surgeon and patient preference. We prefer local anesthesia with conscious sedation. After conscious sedation is administered, we infiltrate the carpal tunnel and surrounding subcutaneous tissue with 10 mL of a 50:50 mixture of 0.5% bupivacaine and 1% lidocaine without epinephrine.
A 2.5- to 3-cm longitudinal incision is made along the axis of the radial border of the ring finger from the Kaplan cardinal line26 and extending about 3 cm proximally toward the wrist flexion crease ulnar to the palmaris longus if present (Figure 1).
After the skin is incised longitudinally, the subcutaneous fat is mobilized and cutaneous sensory branches identified and protected. The underlying superficial palmar fascia is incised in line with the skin incision. The underlying midportion of the TCL is now visualized.
Transverse Carpal Ligament Release
Occasionally, the investing fascia along the ulnar edge of the thenar musculature is mobilized radialward (if the thenar musculature is well developed) to visualize the proximal limb of the TCL. Injury to any anomalous motor branch of the median nerve is avoided by directly visualizing and then incising the TCL (Figure 2). The TCL is incised along its ulnar border just radial to the hook of hamate from distal to proximal in line with the radial border of the ring finger. Staying near the ulnar attachment of the TCL keeps the plane of ligament division farther away from the median nerve and its recurrent motor branches. Although the ulnar neurovascular bundle typically resides ulnar to the hook of hamate in the canal of Guyon, the surgeon must be aware that it can be located radial to the hook in some instances.27,28 In the elderly, the ulnar artery may be tortuous and enter the field and require retraction. The TCL is incised distally until the sentinel fat pad, which marks the superficial palmar arterial arch, is visualized. This bed of adipose tissue marks the distal edge of the TCL.29
Proximally, subcutaneous tissues above the proximal limb of the TCL and DVFF are mobilized to about 2 cm proximal to the wrist flexion crease to create a plane for the fine long nasal turbinate speculum. The nasal turbinate speculum is then inserted into this plane above the proximal limb of the TCL and DVFF (Figure 3). Once inserted to the level of the confluence of the TCL and the DVFF, the speculum is opened.
Topside visualization is now encountered with the ulnar neurovascular bundle protected by the ulnar blade of the speculum. A long-handle scalpel is used to incise the TCL and the DVFF under direct visualization from proximal to distal in line with the previously completed distal release (Figure 4). As the nasal turbinate speculum is stretching the TCL and putting it under tension, the TCL can be heard splitting as it is being incised. Once the TCL and the DVFF are divided, the speculum is slowly closed and removed. Wide diastasis of the radial and ulnar leaflets of the TCL and the DVFF is directly visualized. Complete decompression of the median nerve from the distal forearm fascia to the superficial palmar arch is confirmed.
Adhesions between the undersurface of the radial leaflet and the flexor tendons and median nerve are mobilized. The median nerve is assessed for “hourglass” morphology or atrophy. The flexor tendons can be swept radialward with a free elevator to inspect the floor of the carpal tunnel. Flexor tenosynovectomy is not routinely performed. The incision is closed with interrupted simple sutures using 4-0 nylon.
Study Results
This study was conducted at Hand Surgery PC, Newton-Wellesley Hospital, Tufts University School of Medicine. Over a 10-month interval, 101 consecutive mini-OCTRs (63 right hands, 38 left hands) were performed with this proximal release modification in 88 patients (51 females, 37 males) by Dr. Ruchelsman and Dr. Belsky (Table). CTRs performed in the setting of wrist and/or carpal trauma were excluded. Mean age was 62.8 years. Mean follow-up was 11.3 weeks (~3 months). For isolated cases of CTR, mean tourniquet time was 16 minutes. CTS symptoms were relieved in all patients with a high degree of satisfaction as measured with history and examination findings at follow-up visits. There were no major complications (eg, infection, neural or vascular damage, severe residual pain). Four patients reported minor residual numbness in the fingers at latest follow-up but nevertheless had major improvement over preoperative baseline. These 4 patients had preoperative electromyograms or nerve conduction studies documenting the extent of their disease. There was 1 case of minor wound complication. Three weeks after surgery, the patient had a 1-cm wound opening, which closed with local wound care. The patient did not develop any drainage, infection, bleeding, or neurologic symptoms.
Discussion
Open release of the TCL—the gold standard of surgical treatment for CTS—produces reliable symptom relief in the vast majority of patients.25,30 Given that the most common complication of carpal tunnel surgery is incomplete release of the TCL,31,32 this technique, which uses a nasal turbinate speculum to better visualize the median nerve, could potentially reduce the reoperation rate. The nasal turbinate speculum allows the surgeon to see the confluence of the TCL and the DVFF. In addition, as the complete release can be visualized, there is minimal chance of injury.
The 2007 Cochrane review3 found no strong evidence supporting replacing OCTR with endoscopic techniques. Previous investigators have questioned the utility of ECTR given that it is higher in cost and more resource-intensive than OCTR1,33,34 and is associated with higher rates of certain complications.5,22,35-37 A 2004 meta-analysis of 13 randomized, controlled trials found a higher rate of reversible nerve damage with an odds ratio of 3.1 for ECTR versus OCTR.35 A more recent (2006) review of more than 80 studies found transient neurapraxias in 1.45% of ECTR cases and 0.25% of OCTR cases.5 The same study reported overall complication rates (reversible and major neurovascular structural injuries) of 0.74% for OCTR and 1.63% for ECTR (P < .005). Another limitation of ECTR is that endoscopic techniques require a higher degree of surgical skill, which makes teaching residents and fellows more challenging.
The novel nasal turbinate speculum technique presented here is easily reproducible and allows first-time surgeons to visualize all important structures. Given that this technique does not require an endoscope or an endoscope-viewing tower, it is likely more cost-effective and requires less time for turnover between cases. Patients obtain good relief of their CTS symptoms with this technique, and most return to their daily activities within weeks after operation.
1. Ono S, Clapham PJ, Chung KC. Optimal management of carpal tunnel syndrome. Int J Gen Med. 2010;3(4):255-261.
2. Concannon MJ, Brownfield ML, Puckett CL. The incidence of recurrence after endoscopic carpal tunnel release. Plast Reconstr Surg. 2000;105(5):1662-1665.
3. Scholten RJ, Mink van der Molen A, Uitdehaag BM, Bouter LM, de Vet HC. Surgical treatment options for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;(4):CD003905.
4. In memoriam Sir James Learmonth, K.C.V.O., C.B.E., hon. F.R.C.S. (1895-1967). Ann R Coll Surg Engl. 1967;41(5):438-439.
5. Benson LS, Bare AA, Nagle DJ, Harder VS, Williams CS, Visotsky JL. Complications of endoscopic and open carpal tunnel release. Arthroscopy. 2006;22(9):919-924, 924.e1-e2.
6. Jarvik JG, Comstock BA, Kliot M, et al. Surgery versus non-surgical therapy for carpal tunnel syndrome: a randomised parallel-group trial. Lancet. 2009;374(9695):1074-1081.
7. Verdugo RJ, Salinas RA, Castillo JL, et al. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane Database Syst Rev. 2008;(4):CD001552.
8. Garland H, Langworth EP, Taverner D, et al. Surgical treatment for the carpal tunnel syndrome. Lancet. 1964;1(7343):1129-1130.
9. Gerritsen AA, de Vet HC, Scholten RJ, et al. Splinting vs surgery in the treatment of carpal tunnel syndrome: a randomized controlled trial. JAMA. 2002;288(10):1245-1251.
10. Gelberman RH, Hergenroeder PT, Hargens AR, et al. The carpal tunnel syndrome. A study of carpal canal pressures. J Bone Joint Surg Am. 1981;63(3):380-383.
11. Sucher BM. Myofascial manipulative release of carpal tunnel syndrome: documentation with magnetic resonance imaging. J Am Osteopath Assoc. 1993;93(12):1273-1278.
12. Pereira EE, Miranda DA, Sere I, et al. Endoscopic release of the carpal tunnel: a 2-portal-modified technique. Tech Hand Up Extrem Surg. 2010;14(4):263-265.
13. Louis DS, Greene TL, Noellert RC. Complications of carpal tunnel surgery. J Neurosurg. 1985;62(3):352-356.
14. Mirza MA, King ET Jr, Tanveer S. Palmar uniportal extrabursal endoscopic carpal tunnel release. Arthroscopy. 1995;11(1):82-90.
15. Brown MG, Keyser B, Rothenberg ES. Endoscopic carpal tunnel release. J Hand Surg Am. 1992;17(6):1009-1011.
16. Agee JM, McCarroll HR Jr, Tortosa RD, et al. Endoscopic release of the carpal tunnel: a randomized prospective multicenter study. J Hand Surg Am. 1992;17(6):987-995.
17. Okutsu I, Ninomiya S, Takatori Y, et al. Endoscopic management of carpal tunnel syndrome. Arthroscopy. 1989;5(1):11-18.
18. Ghaly RF, Saban KL, Haley DA, et al. Endoscopic carpal tunnel release surgery: report of patient satisfaction. Neurol Res. 2000;22(6):551-555.
19. Lee WP, Plancher KD, Strickland JW. Carpal tunnel release with a small palmar incision. Hand Clin. 1996;12(2):271-284.
20. Biyani A, Downes EM. An open twin incision technique of carpal tunnel decompression with reduced incidence of scar tenderness. J Hand Surg Br. 1993;18(3):331-334.
21. Brown RA, Gelberman RH, Seiler JG 3rd, et al. Carpal tunnel release. A prospective, randomized assessment of open and endoscopic methods. J Bone Joint Surg Am. 1993;75(9):1265-1275.
22. Chow JC. Endoscopic release of the carpal ligament for carpal tunnel syndrome: 22-month clinical result. Arthroscopy. 1990;6(4):288-296.
23. Trumble TE, Diao E, Abrams RA, et al. Single-portal endoscopic carpal tunnel release compared with open release: a prospective, randomized trial. J Bone Joint Surg Am. 2002;84(7):1107-1115.
24. Gerritsen AA, Uitdehaag BM, van Geldere D, et al. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg. 2001;88(10):1285-1295.
25. Edgell SE, McCabe SJ, Breidenbach WC, et al. Predicting the outcome of carpal tunnel release. J Hand Surg Am. 2003;28(2):255-261.
26. Vella JC, Hartigan BJ, Stern PJ. Kaplan’s cardinal line. J Hand Surg Am. 2006;31(6):912-918.
27. Kwon JY, Kim JY, Hong JT, et al. Position change of the neurovascular structures around the carpal tunnel with dynamic wrist motion. J Korean Neurosurg Soc. 2011;50(4):377-380.
28. Netscher D, Polsen C, Thornby J, et al. Anatomic delineation of the ulnar nerve and ulnar artery in relation to the carpal tunnel by axial magnetic resonance imaging scanning. J Hand Surg Am. 1996;21(2):273-276.
29. Madhav TJ, To P, Stern PJ. The palmar fat pad is a reliable intraoperative landmark during carpal tunnel release. J Hand Surg Am. 2009;34(7):1204-1209.
30. Kulick MI, Gordillo G, Javidi T, et al. Long-term analysis of patients having surgical treatment for carpal tunnel syndrome. J Hand Surg Am. 1986;11(1):59-66.
31. Bland JD. Treatment of carpal tunnel syndrome. Muscle Nerve. 2007;36(2):167-171.
32. MacDonald RI, Lichtman DM, Hanlon JJ, et al. Complications of surgical release for carpal tunnel syndrome. J Hand Surg Am. 1978;3(1):70-76.
33. Atroshi I, Larsson GU, Ornstein E, Hofer M, Johnsson R, Ranstam J. Outcomes of endoscopic surgery compared with open surgery for carpal tunnel syndrome among employed patients: randomised controlled trial. BMJ. 2006;332(7556):1473.
34. Ferdinand RD, MacLean JG. Endoscopic versus open carpal tunnel release in bilateral carpal tunnel syndrome. A prospective, randomised, blinded assessment. J Bone Joint Surg Br. 2002;84(3):375-379.
35. Thoma A, Veltri K, Haines T, et al. A meta-analysis of randomized controlled trials comparing endoscopic and open carpal tunnel decompression. Plast Reconstr Surg. 2004;114(5):1137-1146.
36. Murphy RX Jr, Jennings JF, Wukich DK. Major neurovascular complications of endoscopic carpal tunnel release. J Hand Surg Am. 1994;19(1):114-118.
37. Palmer DH, Paulson JC, Lane-Larsen CL, et al. Endoscopic carpal tunnel release: a comparison of two techniques with open release. Arthroscopy. 1993;9(5):498-508.
Carpal tunnel syndrome (CTS) is a disorder characterized by entrapment of the median nerve at the wrist, which may lead to symptoms of pain, paresthesia, and, ultimately, thenar muscle atrophy. Surgical intervention is indicated with persistent or progressive symptoms despite nonoperative management. Timely surgical decompression aims to halt progression of this disorder and prevent permanent peripheral nerve injury.
Carpal tunnel release (CTR) is the most common hand and wrist surgery in the United States, with about 400,000 operations performed annually.1,2 Several methods of decompressing the carpal tunnel have been described.3 These include standard open CTR (OCTR), mini-open approaches, and various endoscopic techniques. OCTR was initially described by Sir James Learmonth in 1933,4 and it remains the gold-standard surgical treatment for patients with symptomatic CTS. Uniform excellent results with high patient satisfaction and low complication rates have been reported in several series.5-9 Common to all techniques is complete proximal-to-distal division of the transverse carpal ligament (TCL). Magnetic resonance imaging studies have shown that TCL transection and the resulting diastasis between the radial and ulnar leaflets cause a significant increase in the volume of the carpal tunnel, leading to decreased pressure.10,11
Endoscopic CTR (ECTR) techniques were developed in an effort to reduce complications, scar sensitivity, and pillar pain and facilitate more rapid return to work.12-17 Outcome studies have demonstrated that both open and endoscopic releases yield patient-reported subjective improvements over preoperative symptoms.18-22 A randomized, controlled trial by Trumble and colleagues23 in 2002 found that ECTR led to improved patient outcomes in the early postoperative period (first 3 months), though differences in outcomes were reduced at final follow-up. More recently (2007), a Cochrane review of 33 trials concluded there was no strong evidence favoring use of alternative techniques over OCTR.3 Further, OCTR has been found to be technically less demanding and associated with decreased complications and costs.24
Indications
The benefit of median nerve decompression at the wrist for CTS is clear.6,7 Indications for surgery in patients with CTS include persistent symptoms despite nonoperative treatment, objective sensory disturbance or motor weakness, and thenar atrophy. Symptomatic response to corticosteroid injection is predictive of success after carpal tunnel surgery.25 More than 87% of patients who gain symptomatic relief from corticosteroid injection have an excellent surgical outcome.
Technique
OCTR allows direct visualization of the TCL and the distal volar forearm fascia (DVFF) and evaluation for the presence of anomalous branching patterns of the median nerve. OCTR traditionally was performed through a 4- to 5-cm longitudinal incision extending from the wrist crease proximally to the Kaplan cardinal line distally. The mini-open technique is identical with the exception of incision length. We routinely use a 2.5- to 3-cm incision. Regardless of incision length, each OCTR should proceed through the same reproducible steps.
We perform OCTR under tourniquet control. Choice of anesthesia is surgeon and patient preference. We prefer local anesthesia with conscious sedation. After conscious sedation is administered, we infiltrate the carpal tunnel and surrounding subcutaneous tissue with 10 mL of a 50:50 mixture of 0.5% bupivacaine and 1% lidocaine without epinephrine.
A 2.5- to 3-cm longitudinal incision is made along the axis of the radial border of the ring finger from the Kaplan cardinal line26 and extending about 3 cm proximally toward the wrist flexion crease ulnar to the palmaris longus if present (Figure 1).
After the skin is incised longitudinally, the subcutaneous fat is mobilized and cutaneous sensory branches identified and protected. The underlying superficial palmar fascia is incised in line with the skin incision. The underlying midportion of the TCL is now visualized.
Transverse Carpal Ligament Release
Occasionally, the investing fascia along the ulnar edge of the thenar musculature is mobilized radialward (if the thenar musculature is well developed) to visualize the proximal limb of the TCL. Injury to any anomalous motor branch of the median nerve is avoided by directly visualizing and then incising the TCL (Figure 2). The TCL is incised along its ulnar border just radial to the hook of hamate from distal to proximal in line with the radial border of the ring finger. Staying near the ulnar attachment of the TCL keeps the plane of ligament division farther away from the median nerve and its recurrent motor branches. Although the ulnar neurovascular bundle typically resides ulnar to the hook of hamate in the canal of Guyon, the surgeon must be aware that it can be located radial to the hook in some instances.27,28 In the elderly, the ulnar artery may be tortuous and enter the field and require retraction. The TCL is incised distally until the sentinel fat pad, which marks the superficial palmar arterial arch, is visualized. This bed of adipose tissue marks the distal edge of the TCL.29
Proximally, subcutaneous tissues above the proximal limb of the TCL and DVFF are mobilized to about 2 cm proximal to the wrist flexion crease to create a plane for the fine long nasal turbinate speculum. The nasal turbinate speculum is then inserted into this plane above the proximal limb of the TCL and DVFF (Figure 3). Once inserted to the level of the confluence of the TCL and the DVFF, the speculum is opened.
Topside visualization is now encountered with the ulnar neurovascular bundle protected by the ulnar blade of the speculum. A long-handle scalpel is used to incise the TCL and the DVFF under direct visualization from proximal to distal in line with the previously completed distal release (Figure 4). As the nasal turbinate speculum is stretching the TCL and putting it under tension, the TCL can be heard splitting as it is being incised. Once the TCL and the DVFF are divided, the speculum is slowly closed and removed. Wide diastasis of the radial and ulnar leaflets of the TCL and the DVFF is directly visualized. Complete decompression of the median nerve from the distal forearm fascia to the superficial palmar arch is confirmed.
Adhesions between the undersurface of the radial leaflet and the flexor tendons and median nerve are mobilized. The median nerve is assessed for “hourglass” morphology or atrophy. The flexor tendons can be swept radialward with a free elevator to inspect the floor of the carpal tunnel. Flexor tenosynovectomy is not routinely performed. The incision is closed with interrupted simple sutures using 4-0 nylon.
Study Results
This study was conducted at Hand Surgery PC, Newton-Wellesley Hospital, Tufts University School of Medicine. Over a 10-month interval, 101 consecutive mini-OCTRs (63 right hands, 38 left hands) were performed with this proximal release modification in 88 patients (51 females, 37 males) by Dr. Ruchelsman and Dr. Belsky (Table). CTRs performed in the setting of wrist and/or carpal trauma were excluded. Mean age was 62.8 years. Mean follow-up was 11.3 weeks (~3 months). For isolated cases of CTR, mean tourniquet time was 16 minutes. CTS symptoms were relieved in all patients with a high degree of satisfaction as measured with history and examination findings at follow-up visits. There were no major complications (eg, infection, neural or vascular damage, severe residual pain). Four patients reported minor residual numbness in the fingers at latest follow-up but nevertheless had major improvement over preoperative baseline. These 4 patients had preoperative electromyograms or nerve conduction studies documenting the extent of their disease. There was 1 case of minor wound complication. Three weeks after surgery, the patient had a 1-cm wound opening, which closed with local wound care. The patient did not develop any drainage, infection, bleeding, or neurologic symptoms.
Discussion
Open release of the TCL—the gold standard of surgical treatment for CTS—produces reliable symptom relief in the vast majority of patients.25,30 Given that the most common complication of carpal tunnel surgery is incomplete release of the TCL,31,32 this technique, which uses a nasal turbinate speculum to better visualize the median nerve, could potentially reduce the reoperation rate. The nasal turbinate speculum allows the surgeon to see the confluence of the TCL and the DVFF. In addition, as the complete release can be visualized, there is minimal chance of injury.
The 2007 Cochrane review3 found no strong evidence supporting replacing OCTR with endoscopic techniques. Previous investigators have questioned the utility of ECTR given that it is higher in cost and more resource-intensive than OCTR1,33,34 and is associated with higher rates of certain complications.5,22,35-37 A 2004 meta-analysis of 13 randomized, controlled trials found a higher rate of reversible nerve damage with an odds ratio of 3.1 for ECTR versus OCTR.35 A more recent (2006) review of more than 80 studies found transient neurapraxias in 1.45% of ECTR cases and 0.25% of OCTR cases.5 The same study reported overall complication rates (reversible and major neurovascular structural injuries) of 0.74% for OCTR and 1.63% for ECTR (P < .005). Another limitation of ECTR is that endoscopic techniques require a higher degree of surgical skill, which makes teaching residents and fellows more challenging.
The novel nasal turbinate speculum technique presented here is easily reproducible and allows first-time surgeons to visualize all important structures. Given that this technique does not require an endoscope or an endoscope-viewing tower, it is likely more cost-effective and requires less time for turnover between cases. Patients obtain good relief of their CTS symptoms with this technique, and most return to their daily activities within weeks after operation.
Carpal tunnel syndrome (CTS) is a disorder characterized by entrapment of the median nerve at the wrist, which may lead to symptoms of pain, paresthesia, and, ultimately, thenar muscle atrophy. Surgical intervention is indicated with persistent or progressive symptoms despite nonoperative management. Timely surgical decompression aims to halt progression of this disorder and prevent permanent peripheral nerve injury.
Carpal tunnel release (CTR) is the most common hand and wrist surgery in the United States, with about 400,000 operations performed annually.1,2 Several methods of decompressing the carpal tunnel have been described.3 These include standard open CTR (OCTR), mini-open approaches, and various endoscopic techniques. OCTR was initially described by Sir James Learmonth in 1933,4 and it remains the gold-standard surgical treatment for patients with symptomatic CTS. Uniform excellent results with high patient satisfaction and low complication rates have been reported in several series.5-9 Common to all techniques is complete proximal-to-distal division of the transverse carpal ligament (TCL). Magnetic resonance imaging studies have shown that TCL transection and the resulting diastasis between the radial and ulnar leaflets cause a significant increase in the volume of the carpal tunnel, leading to decreased pressure.10,11
Endoscopic CTR (ECTR) techniques were developed in an effort to reduce complications, scar sensitivity, and pillar pain and facilitate more rapid return to work.12-17 Outcome studies have demonstrated that both open and endoscopic releases yield patient-reported subjective improvements over preoperative symptoms.18-22 A randomized, controlled trial by Trumble and colleagues23 in 2002 found that ECTR led to improved patient outcomes in the early postoperative period (first 3 months), though differences in outcomes were reduced at final follow-up. More recently (2007), a Cochrane review of 33 trials concluded there was no strong evidence favoring use of alternative techniques over OCTR.3 Further, OCTR has been found to be technically less demanding and associated with decreased complications and costs.24
Indications
The benefit of median nerve decompression at the wrist for CTS is clear.6,7 Indications for surgery in patients with CTS include persistent symptoms despite nonoperative treatment, objective sensory disturbance or motor weakness, and thenar atrophy. Symptomatic response to corticosteroid injection is predictive of success after carpal tunnel surgery.25 More than 87% of patients who gain symptomatic relief from corticosteroid injection have an excellent surgical outcome.
Technique
OCTR allows direct visualization of the TCL and the distal volar forearm fascia (DVFF) and evaluation for the presence of anomalous branching patterns of the median nerve. OCTR traditionally was performed through a 4- to 5-cm longitudinal incision extending from the wrist crease proximally to the Kaplan cardinal line distally. The mini-open technique is identical with the exception of incision length. We routinely use a 2.5- to 3-cm incision. Regardless of incision length, each OCTR should proceed through the same reproducible steps.
We perform OCTR under tourniquet control. Choice of anesthesia is surgeon and patient preference. We prefer local anesthesia with conscious sedation. After conscious sedation is administered, we infiltrate the carpal tunnel and surrounding subcutaneous tissue with 10 mL of a 50:50 mixture of 0.5% bupivacaine and 1% lidocaine without epinephrine.
A 2.5- to 3-cm longitudinal incision is made along the axis of the radial border of the ring finger from the Kaplan cardinal line26 and extending about 3 cm proximally toward the wrist flexion crease ulnar to the palmaris longus if present (Figure 1).
After the skin is incised longitudinally, the subcutaneous fat is mobilized and cutaneous sensory branches identified and protected. The underlying superficial palmar fascia is incised in line with the skin incision. The underlying midportion of the TCL is now visualized.
Transverse Carpal Ligament Release
Occasionally, the investing fascia along the ulnar edge of the thenar musculature is mobilized radialward (if the thenar musculature is well developed) to visualize the proximal limb of the TCL. Injury to any anomalous motor branch of the median nerve is avoided by directly visualizing and then incising the TCL (Figure 2). The TCL is incised along its ulnar border just radial to the hook of hamate from distal to proximal in line with the radial border of the ring finger. Staying near the ulnar attachment of the TCL keeps the plane of ligament division farther away from the median nerve and its recurrent motor branches. Although the ulnar neurovascular bundle typically resides ulnar to the hook of hamate in the canal of Guyon, the surgeon must be aware that it can be located radial to the hook in some instances.27,28 In the elderly, the ulnar artery may be tortuous and enter the field and require retraction. The TCL is incised distally until the sentinel fat pad, which marks the superficial palmar arterial arch, is visualized. This bed of adipose tissue marks the distal edge of the TCL.29
Proximally, subcutaneous tissues above the proximal limb of the TCL and DVFF are mobilized to about 2 cm proximal to the wrist flexion crease to create a plane for the fine long nasal turbinate speculum. The nasal turbinate speculum is then inserted into this plane above the proximal limb of the TCL and DVFF (Figure 3). Once inserted to the level of the confluence of the TCL and the DVFF, the speculum is opened.
Topside visualization is now encountered with the ulnar neurovascular bundle protected by the ulnar blade of the speculum. A long-handle scalpel is used to incise the TCL and the DVFF under direct visualization from proximal to distal in line with the previously completed distal release (Figure 4). As the nasal turbinate speculum is stretching the TCL and putting it under tension, the TCL can be heard splitting as it is being incised. Once the TCL and the DVFF are divided, the speculum is slowly closed and removed. Wide diastasis of the radial and ulnar leaflets of the TCL and the DVFF is directly visualized. Complete decompression of the median nerve from the distal forearm fascia to the superficial palmar arch is confirmed.
Adhesions between the undersurface of the radial leaflet and the flexor tendons and median nerve are mobilized. The median nerve is assessed for “hourglass” morphology or atrophy. The flexor tendons can be swept radialward with a free elevator to inspect the floor of the carpal tunnel. Flexor tenosynovectomy is not routinely performed. The incision is closed with interrupted simple sutures using 4-0 nylon.
Study Results
This study was conducted at Hand Surgery PC, Newton-Wellesley Hospital, Tufts University School of Medicine. Over a 10-month interval, 101 consecutive mini-OCTRs (63 right hands, 38 left hands) were performed with this proximal release modification in 88 patients (51 females, 37 males) by Dr. Ruchelsman and Dr. Belsky (Table). CTRs performed in the setting of wrist and/or carpal trauma were excluded. Mean age was 62.8 years. Mean follow-up was 11.3 weeks (~3 months). For isolated cases of CTR, mean tourniquet time was 16 minutes. CTS symptoms were relieved in all patients with a high degree of satisfaction as measured with history and examination findings at follow-up visits. There were no major complications (eg, infection, neural or vascular damage, severe residual pain). Four patients reported minor residual numbness in the fingers at latest follow-up but nevertheless had major improvement over preoperative baseline. These 4 patients had preoperative electromyograms or nerve conduction studies documenting the extent of their disease. There was 1 case of minor wound complication. Three weeks after surgery, the patient had a 1-cm wound opening, which closed with local wound care. The patient did not develop any drainage, infection, bleeding, or neurologic symptoms.
Discussion
Open release of the TCL—the gold standard of surgical treatment for CTS—produces reliable symptom relief in the vast majority of patients.25,30 Given that the most common complication of carpal tunnel surgery is incomplete release of the TCL,31,32 this technique, which uses a nasal turbinate speculum to better visualize the median nerve, could potentially reduce the reoperation rate. The nasal turbinate speculum allows the surgeon to see the confluence of the TCL and the DVFF. In addition, as the complete release can be visualized, there is minimal chance of injury.
The 2007 Cochrane review3 found no strong evidence supporting replacing OCTR with endoscopic techniques. Previous investigators have questioned the utility of ECTR given that it is higher in cost and more resource-intensive than OCTR1,33,34 and is associated with higher rates of certain complications.5,22,35-37 A 2004 meta-analysis of 13 randomized, controlled trials found a higher rate of reversible nerve damage with an odds ratio of 3.1 for ECTR versus OCTR.35 A more recent (2006) review of more than 80 studies found transient neurapraxias in 1.45% of ECTR cases and 0.25% of OCTR cases.5 The same study reported overall complication rates (reversible and major neurovascular structural injuries) of 0.74% for OCTR and 1.63% for ECTR (P < .005). Another limitation of ECTR is that endoscopic techniques require a higher degree of surgical skill, which makes teaching residents and fellows more challenging.
The novel nasal turbinate speculum technique presented here is easily reproducible and allows first-time surgeons to visualize all important structures. Given that this technique does not require an endoscope or an endoscope-viewing tower, it is likely more cost-effective and requires less time for turnover between cases. Patients obtain good relief of their CTS symptoms with this technique, and most return to their daily activities within weeks after operation.
1. Ono S, Clapham PJ, Chung KC. Optimal management of carpal tunnel syndrome. Int J Gen Med. 2010;3(4):255-261.
2. Concannon MJ, Brownfield ML, Puckett CL. The incidence of recurrence after endoscopic carpal tunnel release. Plast Reconstr Surg. 2000;105(5):1662-1665.
3. Scholten RJ, Mink van der Molen A, Uitdehaag BM, Bouter LM, de Vet HC. Surgical treatment options for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;(4):CD003905.
4. In memoriam Sir James Learmonth, K.C.V.O., C.B.E., hon. F.R.C.S. (1895-1967). Ann R Coll Surg Engl. 1967;41(5):438-439.
5. Benson LS, Bare AA, Nagle DJ, Harder VS, Williams CS, Visotsky JL. Complications of endoscopic and open carpal tunnel release. Arthroscopy. 2006;22(9):919-924, 924.e1-e2.
6. Jarvik JG, Comstock BA, Kliot M, et al. Surgery versus non-surgical therapy for carpal tunnel syndrome: a randomised parallel-group trial. Lancet. 2009;374(9695):1074-1081.
7. Verdugo RJ, Salinas RA, Castillo JL, et al. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane Database Syst Rev. 2008;(4):CD001552.
8. Garland H, Langworth EP, Taverner D, et al. Surgical treatment for the carpal tunnel syndrome. Lancet. 1964;1(7343):1129-1130.
9. Gerritsen AA, de Vet HC, Scholten RJ, et al. Splinting vs surgery in the treatment of carpal tunnel syndrome: a randomized controlled trial. JAMA. 2002;288(10):1245-1251.
10. Gelberman RH, Hergenroeder PT, Hargens AR, et al. The carpal tunnel syndrome. A study of carpal canal pressures. J Bone Joint Surg Am. 1981;63(3):380-383.
11. Sucher BM. Myofascial manipulative release of carpal tunnel syndrome: documentation with magnetic resonance imaging. J Am Osteopath Assoc. 1993;93(12):1273-1278.
12. Pereira EE, Miranda DA, Sere I, et al. Endoscopic release of the carpal tunnel: a 2-portal-modified technique. Tech Hand Up Extrem Surg. 2010;14(4):263-265.
13. Louis DS, Greene TL, Noellert RC. Complications of carpal tunnel surgery. J Neurosurg. 1985;62(3):352-356.
14. Mirza MA, King ET Jr, Tanveer S. Palmar uniportal extrabursal endoscopic carpal tunnel release. Arthroscopy. 1995;11(1):82-90.
15. Brown MG, Keyser B, Rothenberg ES. Endoscopic carpal tunnel release. J Hand Surg Am. 1992;17(6):1009-1011.
16. Agee JM, McCarroll HR Jr, Tortosa RD, et al. Endoscopic release of the carpal tunnel: a randomized prospective multicenter study. J Hand Surg Am. 1992;17(6):987-995.
17. Okutsu I, Ninomiya S, Takatori Y, et al. Endoscopic management of carpal tunnel syndrome. Arthroscopy. 1989;5(1):11-18.
18. Ghaly RF, Saban KL, Haley DA, et al. Endoscopic carpal tunnel release surgery: report of patient satisfaction. Neurol Res. 2000;22(6):551-555.
19. Lee WP, Plancher KD, Strickland JW. Carpal tunnel release with a small palmar incision. Hand Clin. 1996;12(2):271-284.
20. Biyani A, Downes EM. An open twin incision technique of carpal tunnel decompression with reduced incidence of scar tenderness. J Hand Surg Br. 1993;18(3):331-334.
21. Brown RA, Gelberman RH, Seiler JG 3rd, et al. Carpal tunnel release. A prospective, randomized assessment of open and endoscopic methods. J Bone Joint Surg Am. 1993;75(9):1265-1275.
22. Chow JC. Endoscopic release of the carpal ligament for carpal tunnel syndrome: 22-month clinical result. Arthroscopy. 1990;6(4):288-296.
23. Trumble TE, Diao E, Abrams RA, et al. Single-portal endoscopic carpal tunnel release compared with open release: a prospective, randomized trial. J Bone Joint Surg Am. 2002;84(7):1107-1115.
24. Gerritsen AA, Uitdehaag BM, van Geldere D, et al. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg. 2001;88(10):1285-1295.
25. Edgell SE, McCabe SJ, Breidenbach WC, et al. Predicting the outcome of carpal tunnel release. J Hand Surg Am. 2003;28(2):255-261.
26. Vella JC, Hartigan BJ, Stern PJ. Kaplan’s cardinal line. J Hand Surg Am. 2006;31(6):912-918.
27. Kwon JY, Kim JY, Hong JT, et al. Position change of the neurovascular structures around the carpal tunnel with dynamic wrist motion. J Korean Neurosurg Soc. 2011;50(4):377-380.
28. Netscher D, Polsen C, Thornby J, et al. Anatomic delineation of the ulnar nerve and ulnar artery in relation to the carpal tunnel by axial magnetic resonance imaging scanning. J Hand Surg Am. 1996;21(2):273-276.
29. Madhav TJ, To P, Stern PJ. The palmar fat pad is a reliable intraoperative landmark during carpal tunnel release. J Hand Surg Am. 2009;34(7):1204-1209.
30. Kulick MI, Gordillo G, Javidi T, et al. Long-term analysis of patients having surgical treatment for carpal tunnel syndrome. J Hand Surg Am. 1986;11(1):59-66.
31. Bland JD. Treatment of carpal tunnel syndrome. Muscle Nerve. 2007;36(2):167-171.
32. MacDonald RI, Lichtman DM, Hanlon JJ, et al. Complications of surgical release for carpal tunnel syndrome. J Hand Surg Am. 1978;3(1):70-76.
33. Atroshi I, Larsson GU, Ornstein E, Hofer M, Johnsson R, Ranstam J. Outcomes of endoscopic surgery compared with open surgery for carpal tunnel syndrome among employed patients: randomised controlled trial. BMJ. 2006;332(7556):1473.
34. Ferdinand RD, MacLean JG. Endoscopic versus open carpal tunnel release in bilateral carpal tunnel syndrome. A prospective, randomised, blinded assessment. J Bone Joint Surg Br. 2002;84(3):375-379.
35. Thoma A, Veltri K, Haines T, et al. A meta-analysis of randomized controlled trials comparing endoscopic and open carpal tunnel decompression. Plast Reconstr Surg. 2004;114(5):1137-1146.
36. Murphy RX Jr, Jennings JF, Wukich DK. Major neurovascular complications of endoscopic carpal tunnel release. J Hand Surg Am. 1994;19(1):114-118.
37. Palmer DH, Paulson JC, Lane-Larsen CL, et al. Endoscopic carpal tunnel release: a comparison of two techniques with open release. Arthroscopy. 1993;9(5):498-508.
1. Ono S, Clapham PJ, Chung KC. Optimal management of carpal tunnel syndrome. Int J Gen Med. 2010;3(4):255-261.
2. Concannon MJ, Brownfield ML, Puckett CL. The incidence of recurrence after endoscopic carpal tunnel release. Plast Reconstr Surg. 2000;105(5):1662-1665.
3. Scholten RJ, Mink van der Molen A, Uitdehaag BM, Bouter LM, de Vet HC. Surgical treatment options for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;(4):CD003905.
4. In memoriam Sir James Learmonth, K.C.V.O., C.B.E., hon. F.R.C.S. (1895-1967). Ann R Coll Surg Engl. 1967;41(5):438-439.
5. Benson LS, Bare AA, Nagle DJ, Harder VS, Williams CS, Visotsky JL. Complications of endoscopic and open carpal tunnel release. Arthroscopy. 2006;22(9):919-924, 924.e1-e2.
6. Jarvik JG, Comstock BA, Kliot M, et al. Surgery versus non-surgical therapy for carpal tunnel syndrome: a randomised parallel-group trial. Lancet. 2009;374(9695):1074-1081.
7. Verdugo RJ, Salinas RA, Castillo JL, et al. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane Database Syst Rev. 2008;(4):CD001552.
8. Garland H, Langworth EP, Taverner D, et al. Surgical treatment for the carpal tunnel syndrome. Lancet. 1964;1(7343):1129-1130.
9. Gerritsen AA, de Vet HC, Scholten RJ, et al. Splinting vs surgery in the treatment of carpal tunnel syndrome: a randomized controlled trial. JAMA. 2002;288(10):1245-1251.
10. Gelberman RH, Hergenroeder PT, Hargens AR, et al. The carpal tunnel syndrome. A study of carpal canal pressures. J Bone Joint Surg Am. 1981;63(3):380-383.
11. Sucher BM. Myofascial manipulative release of carpal tunnel syndrome: documentation with magnetic resonance imaging. J Am Osteopath Assoc. 1993;93(12):1273-1278.
12. Pereira EE, Miranda DA, Sere I, et al. Endoscopic release of the carpal tunnel: a 2-portal-modified technique. Tech Hand Up Extrem Surg. 2010;14(4):263-265.
13. Louis DS, Greene TL, Noellert RC. Complications of carpal tunnel surgery. J Neurosurg. 1985;62(3):352-356.
14. Mirza MA, King ET Jr, Tanveer S. Palmar uniportal extrabursal endoscopic carpal tunnel release. Arthroscopy. 1995;11(1):82-90.
15. Brown MG, Keyser B, Rothenberg ES. Endoscopic carpal tunnel release. J Hand Surg Am. 1992;17(6):1009-1011.
16. Agee JM, McCarroll HR Jr, Tortosa RD, et al. Endoscopic release of the carpal tunnel: a randomized prospective multicenter study. J Hand Surg Am. 1992;17(6):987-995.
17. Okutsu I, Ninomiya S, Takatori Y, et al. Endoscopic management of carpal tunnel syndrome. Arthroscopy. 1989;5(1):11-18.
18. Ghaly RF, Saban KL, Haley DA, et al. Endoscopic carpal tunnel release surgery: report of patient satisfaction. Neurol Res. 2000;22(6):551-555.
19. Lee WP, Plancher KD, Strickland JW. Carpal tunnel release with a small palmar incision. Hand Clin. 1996;12(2):271-284.
20. Biyani A, Downes EM. An open twin incision technique of carpal tunnel decompression with reduced incidence of scar tenderness. J Hand Surg Br. 1993;18(3):331-334.
21. Brown RA, Gelberman RH, Seiler JG 3rd, et al. Carpal tunnel release. A prospective, randomized assessment of open and endoscopic methods. J Bone Joint Surg Am. 1993;75(9):1265-1275.
22. Chow JC. Endoscopic release of the carpal ligament for carpal tunnel syndrome: 22-month clinical result. Arthroscopy. 1990;6(4):288-296.
23. Trumble TE, Diao E, Abrams RA, et al. Single-portal endoscopic carpal tunnel release compared with open release: a prospective, randomized trial. J Bone Joint Surg Am. 2002;84(7):1107-1115.
24. Gerritsen AA, Uitdehaag BM, van Geldere D, et al. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg. 2001;88(10):1285-1295.
25. Edgell SE, McCabe SJ, Breidenbach WC, et al. Predicting the outcome of carpal tunnel release. J Hand Surg Am. 2003;28(2):255-261.
26. Vella JC, Hartigan BJ, Stern PJ. Kaplan’s cardinal line. J Hand Surg Am. 2006;31(6):912-918.
27. Kwon JY, Kim JY, Hong JT, et al. Position change of the neurovascular structures around the carpal tunnel with dynamic wrist motion. J Korean Neurosurg Soc. 2011;50(4):377-380.
28. Netscher D, Polsen C, Thornby J, et al. Anatomic delineation of the ulnar nerve and ulnar artery in relation to the carpal tunnel by axial magnetic resonance imaging scanning. J Hand Surg Am. 1996;21(2):273-276.
29. Madhav TJ, To P, Stern PJ. The palmar fat pad is a reliable intraoperative landmark during carpal tunnel release. J Hand Surg Am. 2009;34(7):1204-1209.
30. Kulick MI, Gordillo G, Javidi T, et al. Long-term analysis of patients having surgical treatment for carpal tunnel syndrome. J Hand Surg Am. 1986;11(1):59-66.
31. Bland JD. Treatment of carpal tunnel syndrome. Muscle Nerve. 2007;36(2):167-171.
32. MacDonald RI, Lichtman DM, Hanlon JJ, et al. Complications of surgical release for carpal tunnel syndrome. J Hand Surg Am. 1978;3(1):70-76.
33. Atroshi I, Larsson GU, Ornstein E, Hofer M, Johnsson R, Ranstam J. Outcomes of endoscopic surgery compared with open surgery for carpal tunnel syndrome among employed patients: randomised controlled trial. BMJ. 2006;332(7556):1473.
34. Ferdinand RD, MacLean JG. Endoscopic versus open carpal tunnel release in bilateral carpal tunnel syndrome. A prospective, randomised, blinded assessment. J Bone Joint Surg Br. 2002;84(3):375-379.
35. Thoma A, Veltri K, Haines T, et al. A meta-analysis of randomized controlled trials comparing endoscopic and open carpal tunnel decompression. Plast Reconstr Surg. 2004;114(5):1137-1146.
36. Murphy RX Jr, Jennings JF, Wukich DK. Major neurovascular complications of endoscopic carpal tunnel release. J Hand Surg Am. 1994;19(1):114-118.
37. Palmer DH, Paulson JC, Lane-Larsen CL, et al. Endoscopic carpal tunnel release: a comparison of two techniques with open release. Arthroscopy. 1993;9(5):498-508.
A Novel Method of Skin Closure for Aging or Fragile Skin
Patients who have been on steroids, aspirin, or anticoagulants or who are elderly may have a fragile outer skin layer that is similar to parchment paper, which may be challenging for surgeons. In these patients, the epidermal layer is thin and translucent; when a surgeon cuts through this thin layer, the tissue beneath shows minimal dermis and poor-quality fat with weakened tissue support. When undergoing excisional surgery, there is no strong tissue to help the closure sutures remain intact. Surgeons may struggle with skin tears around the sutures and dehiscence on suture removal.
This article describes a novel approach to skin closure in patients with aging or thin skin using a polyethylene film with an acrylate adhesive in the excision area to aid in maintaining skin integrity throughout the healing process following surgery.
Closure Technique
First, the skin area is cleansed with a sterilizing soap preparation. A sterile marking pen then is used to outline the excision area. A 10×12-cm layer of polyethylene film is then attached to the excision site. Excision of the tumor is performed by cutting through the film in the marked area (Figure 1A), and closure is performed by suturing the wound edges through the polyethylene film while the area is still covered with the film (Figure 1B). The sutures can be left in for 2 weeks or longer if necessary. The patient should be instructed not to remove the film or perform any extensive cleansing of the treatment area. Antibiotics should be administered, as the polyethylene film maintains its sterile integrity for 7 days only. Because sutures are on the surface of the film, they are easily accessed for removal. Figure 1C shows the excision site after removal of the sutures and polyethylene film on the left tibia of a 95-year-old woman. Adhesive butterfly closures can be applied to strengthen the excision area after suture removal and prevent dehiscence.
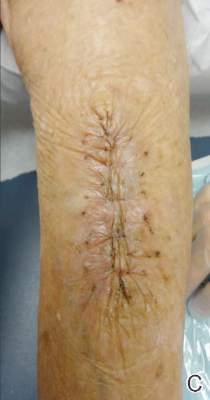
|
Figure 1. The excision site was marked after polyethylene adhesive film was applied to a squamous cell carcinoma on the left tibia of 95-year-old woman (A). Closure was performed by suturing the wound edges through the polyethylene film (B). The excision site appeared to have no dehiscence or signs of infection after removal of the sutures and polyethylene film (C). |
Case Reports
Twelve procedures for skin cancer excision were conducted in 10 patients using polyethylene adhesive film as a surgical aid due to extremely poor quality of the epidermis. The tumors were all squamous cell carcinomas and were located on the arms and legs. Patients were aged 73 to 95 years. Figure 2 demonstrates an example of excision of a squamous cell carcinoma on the left tibia of an 82-year-old man with prior dehiscence and infection after leg surgeries. Good results were achieved using the closure technique described here, along with prophylactic antibiotics.
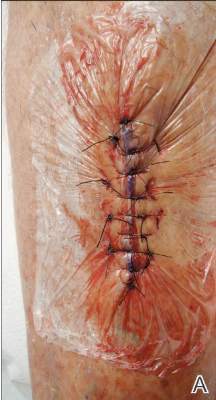
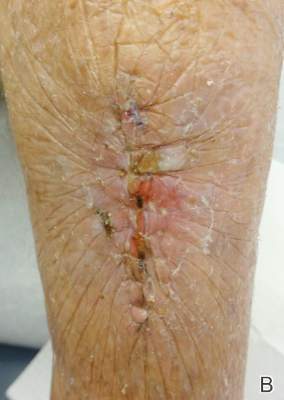
|
Figure 2. A squamous cell carcinoma excision site on the left tibia of an 82-year-old man that had been covered with polyethylene adhesive film prior to excision (A) and 17 days following removal of the sutures and film (B). |
One patient had complications from a Staphylococcus infection because antibiotics were not administered. The patient had prior infections with other surgeries. Antibiotics were given 4 days after surgery. The infection was cleared and the polyethylene film was retained for a total of 12 days.
Sutures were removed after 14 days for excision sites on the arms and 17 days for excision sites on the legs. All excision sites healed without dehiscence with a cosmetically acceptable scar. Figure 3A shows a completed excision on the left hand of a 92-year-old man, and Figure 3B is the result 5 weeks after excision.
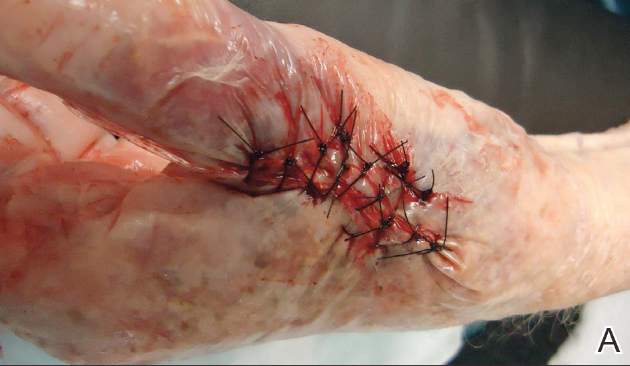
|
Figure 3. A squamous cell carcinoma excision site on the left thumb of a 92-year-old man that had been covered with polyethylene adhesive film prior to exci- sion (A). No visible scarring or dehiscence was noted 5 weeks after excision, following removal of the sutures and film (B). |
None of the patients reported discomfort from the polyethylene film remaining on the skin following surgery, though postoperative care required extra caution when dressing so as not to disturb or compromise the film. Patients were advised about postoperative care and were instructed not to remove the dressing. They were all given antibiotics as a necessary adjunct to maintain a lessened bacteria burden imposed by an impervious layer of acrylate adhesive. Complications resulted from failure to immediately provide antibiotics to 1 patient. The polyethylene film did not hinder healing or postoperative results.
Comment
Various techniques for handling fragile skin during surgery have been described in the literature. Fomon et al1 discussed aging skin as it relates to plastic surgery. Foster and Chan2 described a skin support technique for closing elliptical incisions in patients with fragile skin. Mazzurco and Krach3 discussed the use of a hydrocolloid dressing to aid in the closure of surgical wounds in patients with fragile skin.
The closure method described here was found to be particularly helpful when used as an adjunct to surgery in patients with fragile skin that lacked a suitable dermis. The polyethylene adhesive film helped to hold the sutures more securely. This method is cost-effective and is associated with a high level of patient satisfaction. For the surgeon, this technique may aid in dealing with difficult surgical situations and helps prevent wound complications in elderly patients or those with fragile skin.
1. Fomon S, Bell JW, Schattner A. Aging skin, a surgical challenge. AMA Arch Otolaryngol. 1955;61:554-562.
2. Foster RS, Chan J. The Fixomull skin support method for wound closure in patients with fragile skin. Australas J Dermatol. 2011;52:209-211.
3. Mazzurco JD, Krach KJ. Use of a hydrocolloid dressing to aid in the closure of surgical wounds in patients with fragile skin. J Am Acad Dermatol. 2012;66:335-336.
Patients who have been on steroids, aspirin, or anticoagulants or who are elderly may have a fragile outer skin layer that is similar to parchment paper, which may be challenging for surgeons. In these patients, the epidermal layer is thin and translucent; when a surgeon cuts through this thin layer, the tissue beneath shows minimal dermis and poor-quality fat with weakened tissue support. When undergoing excisional surgery, there is no strong tissue to help the closure sutures remain intact. Surgeons may struggle with skin tears around the sutures and dehiscence on suture removal.
This article describes a novel approach to skin closure in patients with aging or thin skin using a polyethylene film with an acrylate adhesive in the excision area to aid in maintaining skin integrity throughout the healing process following surgery.
Closure Technique
First, the skin area is cleansed with a sterilizing soap preparation. A sterile marking pen then is used to outline the excision area. A 10×12-cm layer of polyethylene film is then attached to the excision site. Excision of the tumor is performed by cutting through the film in the marked area (Figure 1A), and closure is performed by suturing the wound edges through the polyethylene film while the area is still covered with the film (Figure 1B). The sutures can be left in for 2 weeks or longer if necessary. The patient should be instructed not to remove the film or perform any extensive cleansing of the treatment area. Antibiotics should be administered, as the polyethylene film maintains its sterile integrity for 7 days only. Because sutures are on the surface of the film, they are easily accessed for removal. Figure 1C shows the excision site after removal of the sutures and polyethylene film on the left tibia of a 95-year-old woman. Adhesive butterfly closures can be applied to strengthen the excision area after suture removal and prevent dehiscence.



|
Figure 1. The excision site was marked after polyethylene adhesive film was applied to a squamous cell carcinoma on the left tibia of 95-year-old woman (A). Closure was performed by suturing the wound edges through the polyethylene film (B). The excision site appeared to have no dehiscence or signs of infection after removal of the sutures and polyethylene film (C). |
Case Reports
Twelve procedures for skin cancer excision were conducted in 10 patients using polyethylene adhesive film as a surgical aid due to extremely poor quality of the epidermis. The tumors were all squamous cell carcinomas and were located on the arms and legs. Patients were aged 73 to 95 years. Figure 2 demonstrates an example of excision of a squamous cell carcinoma on the left tibia of an 82-year-old man with prior dehiscence and infection after leg surgeries. Good results were achieved using the closure technique described here, along with prophylactic antibiotics.


|
Figure 2. A squamous cell carcinoma excision site on the left tibia of an 82-year-old man that had been covered with polyethylene adhesive film prior to excision (A) and 17 days following removal of the sutures and film (B). |
One patient had complications from a Staphylococcus infection because antibiotics were not administered. The patient had prior infections with other surgeries. Antibiotics were given 4 days after surgery. The infection was cleared and the polyethylene film was retained for a total of 12 days.
Sutures were removed after 14 days for excision sites on the arms and 17 days for excision sites on the legs. All excision sites healed without dehiscence with a cosmetically acceptable scar. Figure 3A shows a completed excision on the left hand of a 92-year-old man, and Figure 3B is the result 5 weeks after excision.


|
Figure 3. A squamous cell carcinoma excision site on the left thumb of a 92-year-old man that had been covered with polyethylene adhesive film prior to exci- sion (A). No visible scarring or dehiscence was noted 5 weeks after excision, following removal of the sutures and film (B). |
None of the patients reported discomfort from the polyethylene film remaining on the skin following surgery, though postoperative care required extra caution when dressing so as not to disturb or compromise the film. Patients were advised about postoperative care and were instructed not to remove the dressing. They were all given antibiotics as a necessary adjunct to maintain a lessened bacteria burden imposed by an impervious layer of acrylate adhesive. Complications resulted from failure to immediately provide antibiotics to 1 patient. The polyethylene film did not hinder healing or postoperative results.
Comment
Various techniques for handling fragile skin during surgery have been described in the literature. Fomon et al1 discussed aging skin as it relates to plastic surgery. Foster and Chan2 described a skin support technique for closing elliptical incisions in patients with fragile skin. Mazzurco and Krach3 discussed the use of a hydrocolloid dressing to aid in the closure of surgical wounds in patients with fragile skin.
The closure method described here was found to be particularly helpful when used as an adjunct to surgery in patients with fragile skin that lacked a suitable dermis. The polyethylene adhesive film helped to hold the sutures more securely. This method is cost-effective and is associated with a high level of patient satisfaction. For the surgeon, this technique may aid in dealing with difficult surgical situations and helps prevent wound complications in elderly patients or those with fragile skin.
Patients who have been on steroids, aspirin, or anticoagulants or who are elderly may have a fragile outer skin layer that is similar to parchment paper, which may be challenging for surgeons. In these patients, the epidermal layer is thin and translucent; when a surgeon cuts through this thin layer, the tissue beneath shows minimal dermis and poor-quality fat with weakened tissue support. When undergoing excisional surgery, there is no strong tissue to help the closure sutures remain intact. Surgeons may struggle with skin tears around the sutures and dehiscence on suture removal.
This article describes a novel approach to skin closure in patients with aging or thin skin using a polyethylene film with an acrylate adhesive in the excision area to aid in maintaining skin integrity throughout the healing process following surgery.
Closure Technique
First, the skin area is cleansed with a sterilizing soap preparation. A sterile marking pen then is used to outline the excision area. A 10×12-cm layer of polyethylene film is then attached to the excision site. Excision of the tumor is performed by cutting through the film in the marked area (Figure 1A), and closure is performed by suturing the wound edges through the polyethylene film while the area is still covered with the film (Figure 1B). The sutures can be left in for 2 weeks or longer if necessary. The patient should be instructed not to remove the film or perform any extensive cleansing of the treatment area. Antibiotics should be administered, as the polyethylene film maintains its sterile integrity for 7 days only. Because sutures are on the surface of the film, they are easily accessed for removal. Figure 1C shows the excision site after removal of the sutures and polyethylene film on the left tibia of a 95-year-old woman. Adhesive butterfly closures can be applied to strengthen the excision area after suture removal and prevent dehiscence.



|
Figure 1. The excision site was marked after polyethylene adhesive film was applied to a squamous cell carcinoma on the left tibia of 95-year-old woman (A). Closure was performed by suturing the wound edges through the polyethylene film (B). The excision site appeared to have no dehiscence or signs of infection after removal of the sutures and polyethylene film (C). |
Case Reports
Twelve procedures for skin cancer excision were conducted in 10 patients using polyethylene adhesive film as a surgical aid due to extremely poor quality of the epidermis. The tumors were all squamous cell carcinomas and were located on the arms and legs. Patients were aged 73 to 95 years. Figure 2 demonstrates an example of excision of a squamous cell carcinoma on the left tibia of an 82-year-old man with prior dehiscence and infection after leg surgeries. Good results were achieved using the closure technique described here, along with prophylactic antibiotics.


|
Figure 2. A squamous cell carcinoma excision site on the left tibia of an 82-year-old man that had been covered with polyethylene adhesive film prior to excision (A) and 17 days following removal of the sutures and film (B). |
One patient had complications from a Staphylococcus infection because antibiotics were not administered. The patient had prior infections with other surgeries. Antibiotics were given 4 days after surgery. The infection was cleared and the polyethylene film was retained for a total of 12 days.
Sutures were removed after 14 days for excision sites on the arms and 17 days for excision sites on the legs. All excision sites healed without dehiscence with a cosmetically acceptable scar. Figure 3A shows a completed excision on the left hand of a 92-year-old man, and Figure 3B is the result 5 weeks after excision.


|
Figure 3. A squamous cell carcinoma excision site on the left thumb of a 92-year-old man that had been covered with polyethylene adhesive film prior to exci- sion (A). No visible scarring or dehiscence was noted 5 weeks after excision, following removal of the sutures and film (B). |
None of the patients reported discomfort from the polyethylene film remaining on the skin following surgery, though postoperative care required extra caution when dressing so as not to disturb or compromise the film. Patients were advised about postoperative care and were instructed not to remove the dressing. They were all given antibiotics as a necessary adjunct to maintain a lessened bacteria burden imposed by an impervious layer of acrylate adhesive. Complications resulted from failure to immediately provide antibiotics to 1 patient. The polyethylene film did not hinder healing or postoperative results.
Comment
Various techniques for handling fragile skin during surgery have been described in the literature. Fomon et al1 discussed aging skin as it relates to plastic surgery. Foster and Chan2 described a skin support technique for closing elliptical incisions in patients with fragile skin. Mazzurco and Krach3 discussed the use of a hydrocolloid dressing to aid in the closure of surgical wounds in patients with fragile skin.
The closure method described here was found to be particularly helpful when used as an adjunct to surgery in patients with fragile skin that lacked a suitable dermis. The polyethylene adhesive film helped to hold the sutures more securely. This method is cost-effective and is associated with a high level of patient satisfaction. For the surgeon, this technique may aid in dealing with difficult surgical situations and helps prevent wound complications in elderly patients or those with fragile skin.
1. Fomon S, Bell JW, Schattner A. Aging skin, a surgical challenge. AMA Arch Otolaryngol. 1955;61:554-562.
2. Foster RS, Chan J. The Fixomull skin support method for wound closure in patients with fragile skin. Australas J Dermatol. 2011;52:209-211.
3. Mazzurco JD, Krach KJ. Use of a hydrocolloid dressing to aid in the closure of surgical wounds in patients with fragile skin. J Am Acad Dermatol. 2012;66:335-336.
1. Fomon S, Bell JW, Schattner A. Aging skin, a surgical challenge. AMA Arch Otolaryngol. 1955;61:554-562.
2. Foster RS, Chan J. The Fixomull skin support method for wound closure in patients with fragile skin. Australas J Dermatol. 2011;52:209-211.
3. Mazzurco JD, Krach KJ. Use of a hydrocolloid dressing to aid in the closure of surgical wounds in patients with fragile skin. J Am Acad Dermatol. 2012;66:335-336.
Practice Points
- A novel method of skin closure using a polyethylene film with an acrylate adhesive can aid in strengthening suture integrity and preventing skin tears.
- Dehiscence of excision sites in patients with aging or fragile skin can be prevented.
- This closure technique promotes healing and efficient scar formation.
The Supination-Pronation Test for Distal Biceps Tendon Rupture
Distal biceps tendon ruptures have been reported with increasing frequency, occurring 1.2 times per 100,000 patients per year, representing 3% of tendinous avulsions involving this muscle.1,2 This injury occurs most commonly in men between the ages of 40 and 60 years, and more often in the dominant extremity after an unexpected or violent eccentric contraction.2,3 Generally, the patient is performing a task that is more strenuous than usual and only performed occasionally; usually, it is a flexion task. The biceps muscle is the most superficial muscle in the anterior compartment of the arm with the distal tendon passing deep in the antecubital fossa to insert at the radial tuberosity (Figure 1). Pronation of the forearm rotates the radial tuberosity medially and posteriorly, drawing the biceps tendon distally with it (Figures 1-3). The biceps muscle is primarily responsible for supination of the forearm, although it is also important in elbow flexion.4,5 The bicipital aponeurosis (lacertus fibrosus) arises from the medial aspect of the muscle belly at the junction of the musculotendinous unit and the distal biceps tendon. This passes distally and medially across the antecubital fossa, blending with the fascia overlying the proximal flexor mass of the forearm, and inserts on the subcutaneous border of the ulna.3 A complete rupture of the distal biceps insertion can produce a 40% loss of supination strength, a 47% loss of supination endurance, and a 21% to 30% loss of flexion strength at the elbow when compared with the contralateral intact extremity.1,2,4
Background
Prompt diagnosis of a distal biceps tendon complete rupture increases the ability to perform a primary repair, and to restore motion and strength.3 Patients with acute ruptures of the distal biceps typically present with a history of experiencing a painful “pop” after a violent eccentric load force at the time of injury. Clinical examination of a patient with a distal biceps tendon rupture shows a loss of the normal upper arm contour, pain with flexion and supination of the forearm, ecchymosis, and an inability to palpate the distal biceps tendon in the antecubital fossa.5 It is important to note that a false-negative test can be elicited when examining the integrity of the muscle contour if the lacertus fibrosus remains intact when there is a complete rupture of the distal biceps tendon.6 This false negative also can occur with examination of the upper arm contour as the elbow flexes. Radiographic studies to evaluate the distal biceps tendon can aid in the diagnosis of ruptures but are not a substitute for a thorough history taking and physical examination.3 Plain radiographs may show hypertrophic bone formation at the radial tuberosity, although they are generally unrevealing.3,6 After a complete clinical examination of the distal biceps tendon, magnetic resonance imaging (MRI) can be an important tool for evaluation of the distal biceps tendon.3 This article introduces a special test used as a diagnostic tool during the physical examination to isolate the distal biceps tendon from the lacertus fibrosus and to evaluate the integrity of the distal biceps brachii tendon.
Test Description
To perform the supination-pronation test, the patient is positioned with both shoulders abducted to 90º and the elbows flexed to approximately 60º to 70º (Figures 4, 5). The examiner stands in front of the patient and observes the contour of the biceps muscle; the unaffected arm is used as a comparison. The examiner may either visually observe the contour of the muscle or may place a hand on the muscle belly throughout the test to feel for movement. The patient is asked to actively supinate and pronate the forearms by turning the hands. Through trial and error, we have found that the change in contour is most pronounced when placing the elbow in 60º to 70º of flexion. Additionally, through clinical experience, we have found testing the patient with both shoulders abducted to 90º provides the examiner with a reproducible examination that is easy to demonstrate to the patient; however, this shoulder position is not mandatory and can be modified if the patient struggles to get into testing position. Forearm position will maximize the size of the biceps, so the result is visually easier to appreciate. If the distal biceps tendon is intact, there is a substantial change in the shape of the biceps as the arm is supinated (the biceps moves proximally), then pronated (the biceps moves distally). Lack of migration of the biceps muscle during supination and pronation is considered a positive test, indicating rupture of the distal biceps tendon from its insertion on the radial tuberosity (Figure 6). We have found the anatomic correlations to a distal biceps injury may be clearly observed through the maneuver of the supination-pronation test and, therefore, provide a reliable clinical method to diagnose a complete distal biceps tendon rupture.
We have been using the supination-pronation test in our clinical practice for 2.5 years. In our experience, opportunities to use the supination-pronation test are very limited and specific. This type of tendon avulsion is rare, and the number of patients who warrant clinical examination using the supination-pronation test is small. We have had 5 positive supination-pronation tests in patients with suspected distal biceps tendon ruptures. To confirm if the supination-pronation test correctly demonstrated a full biceps tendon rupture in these 5 patients, we followed their clinical examination with MRI of the involved arm. Only 4 of the 5 patients were able to obtain MRI. Of these 4, all studies showed complete tearing of the distal biceps tendon from its attachment on the radial tuberosity. All 5 patients were taken into the operating room to confirm the clinical diagnosis and then repair it surgically. Through surgical exploration, we observed a full and complete tear of the distal biceps tendon in all patients, and the tears were repaired successfully. Postoperatively, all patients showed a full recovery with no complications, and all were able to regain full range of motion and strength in the involved arm. All 5 patients were discharged with no complaints.
Although we have not encountered false positive and false negatives using the supination-pronation test in clinical practice, we speculate that there would be a low rate of incidence for these outcomes. There is a possibility of a false-positive test in obese patients in whom the contours of the biceps are difficult to appreciate (although we have not observed this clinically). In these patients, the examiner may not see the migration of the biceps that is occurring. In practice, we have found that, if the contours of the bicep are difficult to appreciate, the test can be performed with the examiner placing his/her hand on the muscle belly during the test to actively feel for movement. This could decrease the risk of a false-positive supination-pronation test. A false negative may occur if the distal biceps tendon is almost completely torn. In this case, enough of the tendon fibers may remain intact to pull the biceps muscle belly distally as the hand is pronated. In our experience, this was not observed but should be noted as a potential risk for a false-negative test.
If the lacertus fibrosus is intact, and the distal biceps tendon is ruptured, the biceps will still change shape as the elbow is flexed and extended but will not change shape with supination and pronation. The biceps brachii muscle attaches distally to the radial tuberosity of the radius; contraction of the muscle pulls the tuberosity anteriorly, rotating the forearm into supination. When the forearm rotates into pronation, the tendon is pulled distally and the muscle lengthens, which causes the contour to be more elongated. Since the lacertus fibrosus attaches to the proximal ulna, it is not involved in forearm supination and pronation. It does, however, assist with elbow flexion.
It is very important to isolate the biceps brachii tendon from the lacertus fibrosus and the brachialis because the examiner may miss a distal tendon rupture by not isolating supination and pronation. The supination-pronation test is a novel clinical test that allows the examiner to isolate the biceps brachii tendon in supination and pronation to evaluate for distal biceps tendon rupture. It has been well established that early anatomic repair of distal biceps tendon rupture is advocated for optimal results in returning flexion and supination strength.3,4,6 Although some patients may choose nonoperative management of complete ruptures, prompt diagnosis of the injury is vital so that the option of surgical management at the time of presentation is not compromised by delay in diagnosis. Clinically, we have found that a delayed diagnosis results in more difficulty performing the surgery, and it may not be possible to obtain enough excursion for the biceps to be reattached with the passage of time. The literature suggests that patients with chronic ruptures (more than 4 weeks) often present with proximal retraction of the biceps muscles and scarring to the brachialis, which can make anatomic repair a difficult challenge.3,7
It is important to note the differences in treatment of proximal versus distal bicep tendon ruptures. Proximally, there are 2 tendon attachments. The tendon of the short head attaches to the coracoid process of the scapula. The tendon of the long head runs into the shoulder joint, attaching intra-articularly to the superior aspect of the glenoid. This tendon is often involved in degeneration concurrently with the adjacent rotator cuff and is vulnerable to rupture. Rupture of this tendon is usually treated nonoperatively. Because proximal rupture nearly always affects only the tendon to the long head, the muscle preserves 1 proximal attachment and continues to function, both as a supinator and as a flexor. Also, this type of rupture tends to occur in more elderly and less active patients who are less adversely affected by the modest loss of function associated with proximal ruptures.
Conclusion
The supination-pronation test properly isolates the distal biceps tendon and does not cause significant discomfort, which can be a problem with other physical examination tests for acute distal biceps ruptures. The squeeze test involves placing the patient in 60º to 80º of elbow flexion with the forearm pronated. The examiner places 1 hand at the distal myotendinous junction, and the other around the belly of the muscle and squeezes, looking for forearm supination.5 We have not found the squeeze test to be optimal because the amount of forearm supination obtained by performing this test can be subtle. Additionally, the patient commonly has significant ecchymosis and pain associated with this rupture, and it may be too painful to squeeze the muscle belly hard enough to have a reliable test. Another test is the hook test, which is performed by the examiner “hooking” an index finger under the intact biceps tendon from the lateral side.8 Clinically, we have found this test difficult to administer because it requires palpation of the tendon, which is often painful for the patient with an acute injury.
The supination-pronation test can easily be performed in the acute setting, and confirms attachment of the biceps tendon distally to the bicipital tuberosity of the radius. It will not show an incomplete tear, but in that case, the muscle retains its normal length, alleviating the urgency of surgical management. We have found the supination-pronation test to be a reliable and pain-free test that should be incorporated in the physical examination to evaluate patients for distal biceps injury.
1. Safran MR, Graham SM. Distal biceps tendon ruptures: incidence, demographics, and the effect of smoking. Clin Orthop Relat Res. 2002;(404):275-283.
2. McCarty III LP, Alpert JM, Bush-Joseph C. Reconstruction of a chronic distal biceps tendon rupture 4 years after initial injury. Am J Orthop. 2008;37(11):579-582.
3. Ramsey ML. Distal biceps tendon injuries: diagnosis and management. J Am Acad Orthop Surg. 1999;7(3):199-207.
4. Morrey BF, Askew L, An K, Dobyns J. Rupture of the distal tendon of the biceps brachii. A biomechanical study. J Bone Joint Surg Am. 1985;67(3):418-421.
5. Ruland RT, Dunbar RP, Bowen JD. The biceps squeeze test for diagnosis of distal biceps tendon ruptures. Clin Orthop Rel Res. 2005;(437):128-131.
6. Sutton KM, Dodds SD, Ahmad CS, Sethi PM. Surgical treatment of distal biceps rupture. J Am Acad Orthop Surg. 2010;18(3):139-148.
7. Leighton MM, Bush-Joseph CA, Bach BR Jr. Distal biceps brachii repair: results in dominant and nondominant extremities. Clin Orthop Relat Res. 1995;(317):114-121.
8. O’Driscoll SW, Goncalves LB, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1869.
Distal biceps tendon ruptures have been reported with increasing frequency, occurring 1.2 times per 100,000 patients per year, representing 3% of tendinous avulsions involving this muscle.1,2 This injury occurs most commonly in men between the ages of 40 and 60 years, and more often in the dominant extremity after an unexpected or violent eccentric contraction.2,3 Generally, the patient is performing a task that is more strenuous than usual and only performed occasionally; usually, it is a flexion task. The biceps muscle is the most superficial muscle in the anterior compartment of the arm with the distal tendon passing deep in the antecubital fossa to insert at the radial tuberosity (Figure 1). Pronation of the forearm rotates the radial tuberosity medially and posteriorly, drawing the biceps tendon distally with it (Figures 1-3). The biceps muscle is primarily responsible for supination of the forearm, although it is also important in elbow flexion.4,5 The bicipital aponeurosis (lacertus fibrosus) arises from the medial aspect of the muscle belly at the junction of the musculotendinous unit and the distal biceps tendon. This passes distally and medially across the antecubital fossa, blending with the fascia overlying the proximal flexor mass of the forearm, and inserts on the subcutaneous border of the ulna.3 A complete rupture of the distal biceps insertion can produce a 40% loss of supination strength, a 47% loss of supination endurance, and a 21% to 30% loss of flexion strength at the elbow when compared with the contralateral intact extremity.1,2,4
Background
Prompt diagnosis of a distal biceps tendon complete rupture increases the ability to perform a primary repair, and to restore motion and strength.3 Patients with acute ruptures of the distal biceps typically present with a history of experiencing a painful “pop” after a violent eccentric load force at the time of injury. Clinical examination of a patient with a distal biceps tendon rupture shows a loss of the normal upper arm contour, pain with flexion and supination of the forearm, ecchymosis, and an inability to palpate the distal biceps tendon in the antecubital fossa.5 It is important to note that a false-negative test can be elicited when examining the integrity of the muscle contour if the lacertus fibrosus remains intact when there is a complete rupture of the distal biceps tendon.6 This false negative also can occur with examination of the upper arm contour as the elbow flexes. Radiographic studies to evaluate the distal biceps tendon can aid in the diagnosis of ruptures but are not a substitute for a thorough history taking and physical examination.3 Plain radiographs may show hypertrophic bone formation at the radial tuberosity, although they are generally unrevealing.3,6 After a complete clinical examination of the distal biceps tendon, magnetic resonance imaging (MRI) can be an important tool for evaluation of the distal biceps tendon.3 This article introduces a special test used as a diagnostic tool during the physical examination to isolate the distal biceps tendon from the lacertus fibrosus and to evaluate the integrity of the distal biceps brachii tendon.
Test Description
To perform the supination-pronation test, the patient is positioned with both shoulders abducted to 90º and the elbows flexed to approximately 60º to 70º (Figures 4, 5). The examiner stands in front of the patient and observes the contour of the biceps muscle; the unaffected arm is used as a comparison. The examiner may either visually observe the contour of the muscle or may place a hand on the muscle belly throughout the test to feel for movement. The patient is asked to actively supinate and pronate the forearms by turning the hands. Through trial and error, we have found that the change in contour is most pronounced when placing the elbow in 60º to 70º of flexion. Additionally, through clinical experience, we have found testing the patient with both shoulders abducted to 90º provides the examiner with a reproducible examination that is easy to demonstrate to the patient; however, this shoulder position is not mandatory and can be modified if the patient struggles to get into testing position. Forearm position will maximize the size of the biceps, so the result is visually easier to appreciate. If the distal biceps tendon is intact, there is a substantial change in the shape of the biceps as the arm is supinated (the biceps moves proximally), then pronated (the biceps moves distally). Lack of migration of the biceps muscle during supination and pronation is considered a positive test, indicating rupture of the distal biceps tendon from its insertion on the radial tuberosity (Figure 6). We have found the anatomic correlations to a distal biceps injury may be clearly observed through the maneuver of the supination-pronation test and, therefore, provide a reliable clinical method to diagnose a complete distal biceps tendon rupture.
We have been using the supination-pronation test in our clinical practice for 2.5 years. In our experience, opportunities to use the supination-pronation test are very limited and specific. This type of tendon avulsion is rare, and the number of patients who warrant clinical examination using the supination-pronation test is small. We have had 5 positive supination-pronation tests in patients with suspected distal biceps tendon ruptures. To confirm if the supination-pronation test correctly demonstrated a full biceps tendon rupture in these 5 patients, we followed their clinical examination with MRI of the involved arm. Only 4 of the 5 patients were able to obtain MRI. Of these 4, all studies showed complete tearing of the distal biceps tendon from its attachment on the radial tuberosity. All 5 patients were taken into the operating room to confirm the clinical diagnosis and then repair it surgically. Through surgical exploration, we observed a full and complete tear of the distal biceps tendon in all patients, and the tears were repaired successfully. Postoperatively, all patients showed a full recovery with no complications, and all were able to regain full range of motion and strength in the involved arm. All 5 patients were discharged with no complaints.
Although we have not encountered false positive and false negatives using the supination-pronation test in clinical practice, we speculate that there would be a low rate of incidence for these outcomes. There is a possibility of a false-positive test in obese patients in whom the contours of the biceps are difficult to appreciate (although we have not observed this clinically). In these patients, the examiner may not see the migration of the biceps that is occurring. In practice, we have found that, if the contours of the bicep are difficult to appreciate, the test can be performed with the examiner placing his/her hand on the muscle belly during the test to actively feel for movement. This could decrease the risk of a false-positive supination-pronation test. A false negative may occur if the distal biceps tendon is almost completely torn. In this case, enough of the tendon fibers may remain intact to pull the biceps muscle belly distally as the hand is pronated. In our experience, this was not observed but should be noted as a potential risk for a false-negative test.
If the lacertus fibrosus is intact, and the distal biceps tendon is ruptured, the biceps will still change shape as the elbow is flexed and extended but will not change shape with supination and pronation. The biceps brachii muscle attaches distally to the radial tuberosity of the radius; contraction of the muscle pulls the tuberosity anteriorly, rotating the forearm into supination. When the forearm rotates into pronation, the tendon is pulled distally and the muscle lengthens, which causes the contour to be more elongated. Since the lacertus fibrosus attaches to the proximal ulna, it is not involved in forearm supination and pronation. It does, however, assist with elbow flexion.
It is very important to isolate the biceps brachii tendon from the lacertus fibrosus and the brachialis because the examiner may miss a distal tendon rupture by not isolating supination and pronation. The supination-pronation test is a novel clinical test that allows the examiner to isolate the biceps brachii tendon in supination and pronation to evaluate for distal biceps tendon rupture. It has been well established that early anatomic repair of distal biceps tendon rupture is advocated for optimal results in returning flexion and supination strength.3,4,6 Although some patients may choose nonoperative management of complete ruptures, prompt diagnosis of the injury is vital so that the option of surgical management at the time of presentation is not compromised by delay in diagnosis. Clinically, we have found that a delayed diagnosis results in more difficulty performing the surgery, and it may not be possible to obtain enough excursion for the biceps to be reattached with the passage of time. The literature suggests that patients with chronic ruptures (more than 4 weeks) often present with proximal retraction of the biceps muscles and scarring to the brachialis, which can make anatomic repair a difficult challenge.3,7
It is important to note the differences in treatment of proximal versus distal bicep tendon ruptures. Proximally, there are 2 tendon attachments. The tendon of the short head attaches to the coracoid process of the scapula. The tendon of the long head runs into the shoulder joint, attaching intra-articularly to the superior aspect of the glenoid. This tendon is often involved in degeneration concurrently with the adjacent rotator cuff and is vulnerable to rupture. Rupture of this tendon is usually treated nonoperatively. Because proximal rupture nearly always affects only the tendon to the long head, the muscle preserves 1 proximal attachment and continues to function, both as a supinator and as a flexor. Also, this type of rupture tends to occur in more elderly and less active patients who are less adversely affected by the modest loss of function associated with proximal ruptures.
Conclusion
The supination-pronation test properly isolates the distal biceps tendon and does not cause significant discomfort, which can be a problem with other physical examination tests for acute distal biceps ruptures. The squeeze test involves placing the patient in 60º to 80º of elbow flexion with the forearm pronated. The examiner places 1 hand at the distal myotendinous junction, and the other around the belly of the muscle and squeezes, looking for forearm supination.5 We have not found the squeeze test to be optimal because the amount of forearm supination obtained by performing this test can be subtle. Additionally, the patient commonly has significant ecchymosis and pain associated with this rupture, and it may be too painful to squeeze the muscle belly hard enough to have a reliable test. Another test is the hook test, which is performed by the examiner “hooking” an index finger under the intact biceps tendon from the lateral side.8 Clinically, we have found this test difficult to administer because it requires palpation of the tendon, which is often painful for the patient with an acute injury.
The supination-pronation test can easily be performed in the acute setting, and confirms attachment of the biceps tendon distally to the bicipital tuberosity of the radius. It will not show an incomplete tear, but in that case, the muscle retains its normal length, alleviating the urgency of surgical management. We have found the supination-pronation test to be a reliable and pain-free test that should be incorporated in the physical examination to evaluate patients for distal biceps injury.
Distal biceps tendon ruptures have been reported with increasing frequency, occurring 1.2 times per 100,000 patients per year, representing 3% of tendinous avulsions involving this muscle.1,2 This injury occurs most commonly in men between the ages of 40 and 60 years, and more often in the dominant extremity after an unexpected or violent eccentric contraction.2,3 Generally, the patient is performing a task that is more strenuous than usual and only performed occasionally; usually, it is a flexion task. The biceps muscle is the most superficial muscle in the anterior compartment of the arm with the distal tendon passing deep in the antecubital fossa to insert at the radial tuberosity (Figure 1). Pronation of the forearm rotates the radial tuberosity medially and posteriorly, drawing the biceps tendon distally with it (Figures 1-3). The biceps muscle is primarily responsible for supination of the forearm, although it is also important in elbow flexion.4,5 The bicipital aponeurosis (lacertus fibrosus) arises from the medial aspect of the muscle belly at the junction of the musculotendinous unit and the distal biceps tendon. This passes distally and medially across the antecubital fossa, blending with the fascia overlying the proximal flexor mass of the forearm, and inserts on the subcutaneous border of the ulna.3 A complete rupture of the distal biceps insertion can produce a 40% loss of supination strength, a 47% loss of supination endurance, and a 21% to 30% loss of flexion strength at the elbow when compared with the contralateral intact extremity.1,2,4
Background
Prompt diagnosis of a distal biceps tendon complete rupture increases the ability to perform a primary repair, and to restore motion and strength.3 Patients with acute ruptures of the distal biceps typically present with a history of experiencing a painful “pop” after a violent eccentric load force at the time of injury. Clinical examination of a patient with a distal biceps tendon rupture shows a loss of the normal upper arm contour, pain with flexion and supination of the forearm, ecchymosis, and an inability to palpate the distal biceps tendon in the antecubital fossa.5 It is important to note that a false-negative test can be elicited when examining the integrity of the muscle contour if the lacertus fibrosus remains intact when there is a complete rupture of the distal biceps tendon.6 This false negative also can occur with examination of the upper arm contour as the elbow flexes. Radiographic studies to evaluate the distal biceps tendon can aid in the diagnosis of ruptures but are not a substitute for a thorough history taking and physical examination.3 Plain radiographs may show hypertrophic bone formation at the radial tuberosity, although they are generally unrevealing.3,6 After a complete clinical examination of the distal biceps tendon, magnetic resonance imaging (MRI) can be an important tool for evaluation of the distal biceps tendon.3 This article introduces a special test used as a diagnostic tool during the physical examination to isolate the distal biceps tendon from the lacertus fibrosus and to evaluate the integrity of the distal biceps brachii tendon.
Test Description
To perform the supination-pronation test, the patient is positioned with both shoulders abducted to 90º and the elbows flexed to approximately 60º to 70º (Figures 4, 5). The examiner stands in front of the patient and observes the contour of the biceps muscle; the unaffected arm is used as a comparison. The examiner may either visually observe the contour of the muscle or may place a hand on the muscle belly throughout the test to feel for movement. The patient is asked to actively supinate and pronate the forearms by turning the hands. Through trial and error, we have found that the change in contour is most pronounced when placing the elbow in 60º to 70º of flexion. Additionally, through clinical experience, we have found testing the patient with both shoulders abducted to 90º provides the examiner with a reproducible examination that is easy to demonstrate to the patient; however, this shoulder position is not mandatory and can be modified if the patient struggles to get into testing position. Forearm position will maximize the size of the biceps, so the result is visually easier to appreciate. If the distal biceps tendon is intact, there is a substantial change in the shape of the biceps as the arm is supinated (the biceps moves proximally), then pronated (the biceps moves distally). Lack of migration of the biceps muscle during supination and pronation is considered a positive test, indicating rupture of the distal biceps tendon from its insertion on the radial tuberosity (Figure 6). We have found the anatomic correlations to a distal biceps injury may be clearly observed through the maneuver of the supination-pronation test and, therefore, provide a reliable clinical method to diagnose a complete distal biceps tendon rupture.
We have been using the supination-pronation test in our clinical practice for 2.5 years. In our experience, opportunities to use the supination-pronation test are very limited and specific. This type of tendon avulsion is rare, and the number of patients who warrant clinical examination using the supination-pronation test is small. We have had 5 positive supination-pronation tests in patients with suspected distal biceps tendon ruptures. To confirm if the supination-pronation test correctly demonstrated a full biceps tendon rupture in these 5 patients, we followed their clinical examination with MRI of the involved arm. Only 4 of the 5 patients were able to obtain MRI. Of these 4, all studies showed complete tearing of the distal biceps tendon from its attachment on the radial tuberosity. All 5 patients were taken into the operating room to confirm the clinical diagnosis and then repair it surgically. Through surgical exploration, we observed a full and complete tear of the distal biceps tendon in all patients, and the tears were repaired successfully. Postoperatively, all patients showed a full recovery with no complications, and all were able to regain full range of motion and strength in the involved arm. All 5 patients were discharged with no complaints.
Although we have not encountered false positive and false negatives using the supination-pronation test in clinical practice, we speculate that there would be a low rate of incidence for these outcomes. There is a possibility of a false-positive test in obese patients in whom the contours of the biceps are difficult to appreciate (although we have not observed this clinically). In these patients, the examiner may not see the migration of the biceps that is occurring. In practice, we have found that, if the contours of the bicep are difficult to appreciate, the test can be performed with the examiner placing his/her hand on the muscle belly during the test to actively feel for movement. This could decrease the risk of a false-positive supination-pronation test. A false negative may occur if the distal biceps tendon is almost completely torn. In this case, enough of the tendon fibers may remain intact to pull the biceps muscle belly distally as the hand is pronated. In our experience, this was not observed but should be noted as a potential risk for a false-negative test.
If the lacertus fibrosus is intact, and the distal biceps tendon is ruptured, the biceps will still change shape as the elbow is flexed and extended but will not change shape with supination and pronation. The biceps brachii muscle attaches distally to the radial tuberosity of the radius; contraction of the muscle pulls the tuberosity anteriorly, rotating the forearm into supination. When the forearm rotates into pronation, the tendon is pulled distally and the muscle lengthens, which causes the contour to be more elongated. Since the lacertus fibrosus attaches to the proximal ulna, it is not involved in forearm supination and pronation. It does, however, assist with elbow flexion.
It is very important to isolate the biceps brachii tendon from the lacertus fibrosus and the brachialis because the examiner may miss a distal tendon rupture by not isolating supination and pronation. The supination-pronation test is a novel clinical test that allows the examiner to isolate the biceps brachii tendon in supination and pronation to evaluate for distal biceps tendon rupture. It has been well established that early anatomic repair of distal biceps tendon rupture is advocated for optimal results in returning flexion and supination strength.3,4,6 Although some patients may choose nonoperative management of complete ruptures, prompt diagnosis of the injury is vital so that the option of surgical management at the time of presentation is not compromised by delay in diagnosis. Clinically, we have found that a delayed diagnosis results in more difficulty performing the surgery, and it may not be possible to obtain enough excursion for the biceps to be reattached with the passage of time. The literature suggests that patients with chronic ruptures (more than 4 weeks) often present with proximal retraction of the biceps muscles and scarring to the brachialis, which can make anatomic repair a difficult challenge.3,7
It is important to note the differences in treatment of proximal versus distal bicep tendon ruptures. Proximally, there are 2 tendon attachments. The tendon of the short head attaches to the coracoid process of the scapula. The tendon of the long head runs into the shoulder joint, attaching intra-articularly to the superior aspect of the glenoid. This tendon is often involved in degeneration concurrently with the adjacent rotator cuff and is vulnerable to rupture. Rupture of this tendon is usually treated nonoperatively. Because proximal rupture nearly always affects only the tendon to the long head, the muscle preserves 1 proximal attachment and continues to function, both as a supinator and as a flexor. Also, this type of rupture tends to occur in more elderly and less active patients who are less adversely affected by the modest loss of function associated with proximal ruptures.
Conclusion
The supination-pronation test properly isolates the distal biceps tendon and does not cause significant discomfort, which can be a problem with other physical examination tests for acute distal biceps ruptures. The squeeze test involves placing the patient in 60º to 80º of elbow flexion with the forearm pronated. The examiner places 1 hand at the distal myotendinous junction, and the other around the belly of the muscle and squeezes, looking for forearm supination.5 We have not found the squeeze test to be optimal because the amount of forearm supination obtained by performing this test can be subtle. Additionally, the patient commonly has significant ecchymosis and pain associated with this rupture, and it may be too painful to squeeze the muscle belly hard enough to have a reliable test. Another test is the hook test, which is performed by the examiner “hooking” an index finger under the intact biceps tendon from the lateral side.8 Clinically, we have found this test difficult to administer because it requires palpation of the tendon, which is often painful for the patient with an acute injury.
The supination-pronation test can easily be performed in the acute setting, and confirms attachment of the biceps tendon distally to the bicipital tuberosity of the radius. It will not show an incomplete tear, but in that case, the muscle retains its normal length, alleviating the urgency of surgical management. We have found the supination-pronation test to be a reliable and pain-free test that should be incorporated in the physical examination to evaluate patients for distal biceps injury.
1. Safran MR, Graham SM. Distal biceps tendon ruptures: incidence, demographics, and the effect of smoking. Clin Orthop Relat Res. 2002;(404):275-283.
2. McCarty III LP, Alpert JM, Bush-Joseph C. Reconstruction of a chronic distal biceps tendon rupture 4 years after initial injury. Am J Orthop. 2008;37(11):579-582.
3. Ramsey ML. Distal biceps tendon injuries: diagnosis and management. J Am Acad Orthop Surg. 1999;7(3):199-207.
4. Morrey BF, Askew L, An K, Dobyns J. Rupture of the distal tendon of the biceps brachii. A biomechanical study. J Bone Joint Surg Am. 1985;67(3):418-421.
5. Ruland RT, Dunbar RP, Bowen JD. The biceps squeeze test for diagnosis of distal biceps tendon ruptures. Clin Orthop Rel Res. 2005;(437):128-131.
6. Sutton KM, Dodds SD, Ahmad CS, Sethi PM. Surgical treatment of distal biceps rupture. J Am Acad Orthop Surg. 2010;18(3):139-148.
7. Leighton MM, Bush-Joseph CA, Bach BR Jr. Distal biceps brachii repair: results in dominant and nondominant extremities. Clin Orthop Relat Res. 1995;(317):114-121.
8. O’Driscoll SW, Goncalves LB, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1869.
1. Safran MR, Graham SM. Distal biceps tendon ruptures: incidence, demographics, and the effect of smoking. Clin Orthop Relat Res. 2002;(404):275-283.
2. McCarty III LP, Alpert JM, Bush-Joseph C. Reconstruction of a chronic distal biceps tendon rupture 4 years after initial injury. Am J Orthop. 2008;37(11):579-582.
3. Ramsey ML. Distal biceps tendon injuries: diagnosis and management. J Am Acad Orthop Surg. 1999;7(3):199-207.
4. Morrey BF, Askew L, An K, Dobyns J. Rupture of the distal tendon of the biceps brachii. A biomechanical study. J Bone Joint Surg Am. 1985;67(3):418-421.
5. Ruland RT, Dunbar RP, Bowen JD. The biceps squeeze test for diagnosis of distal biceps tendon ruptures. Clin Orthop Rel Res. 2005;(437):128-131.
6. Sutton KM, Dodds SD, Ahmad CS, Sethi PM. Surgical treatment of distal biceps rupture. J Am Acad Orthop Surg. 2010;18(3):139-148.
7. Leighton MM, Bush-Joseph CA, Bach BR Jr. Distal biceps brachii repair: results in dominant and nondominant extremities. Clin Orthop Relat Res. 1995;(317):114-121.
8. O’Driscoll SW, Goncalves LB, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1869.
Technique of Open Reduction and Internal Fixation of Comminuted Proximal Humerus Fractures With Allograft Femoral Head Metaphyseal Reconstruction
Proximal humerus fractures are exceedingly common and account for almost 5% of all fractures. As osteoporosis is a risk factor for these fractures, their incidence rises with patient age.1
In 1970, Neer2 described these type of fractures and classified them as having 2, 3, or 4 parts based on the amount of angulation and displacement of the humeral head and the greater and lesser tuberosities with respect to the shaft.
Three- and 4-part proximal humerus fractures can be treated either nonoperatively, or surgically with closed reduction and percutaneous fixation, intramedullary fixation, open reduction and internal fixation (ORIF), or arthroplasty. There remains controversy over the best treatment, but a key component of any surgical treatment is anatomical reduction, stable fixation, and then healing of the tuberosities. A current common form of treatment is augmentation with an allograft fibula placed in the medullary canal. Although not formally reported, anecdotal evidence demonstrates that revision to arthroplasty is very difficult in the setting of an ingrown graft in the medullary canal of the humerus.
In this article, we present a novel technique of using allograft femoral head to reconstruct the metaphysis in ORIF of comminuted proximal humerus fractures.
Technique
Presented in Figure 1 are preoperative images of a representative displaced 4-part proximal humerus fracture treated surgically using the technique described here. General anesthesia is used. After intubation on the operating table, the patient is placed in the beach-chair position with about 75° of hip flexion. All bony prominences are padded, and the head and trunk are well secured. A pneumatic arm positioner is used to alleviate the need for an assistant to manipulate the arm. An image intensifier is used before preparing to verify that appropriate images of the proximal humerus can be obtained. Once adequate images are confirmed, the floor can be marked at the position of the fluoroscopic unit’s wheels to allow easy reproduction of images once the arm is prepared and draped. The intensifier is then removed from the field, the shoulder is prepared and draped in usual fashion, and prophylactic antibiotics are administered.
A deltopectoral incision is used, and sharp dissection is made through the subcutaneous tissue to raise full-thickness subcutaneous flaps on each side. The deltopectoral interval is sharply dissected while protecting the cephalic vein. Subdeltoid adhesions are then released. Palpation of the axillary nerve in the quadrilateral space to identify its location is helpful to avoid injury during the procedure.
The fracture is then identified, and No. 5 permanent suture is placed through the posterior and superior rotator cuff and through the subscapularis insertion (Figure 2). The tuberosities are freed from the humeral head sharply. A blunt elevator is then used to gently elevate the humeral head upward, with care taken to avoid comminuting the metaphyseal bone while levering. Reduction is achieved by manipulating the sutures and levering the head with the elevator while placing the arm in extension and posterior translation. Fluoroscopic images are used to verify correct anatomical alignment. Generally, the metaphysis demonstrates comminution and impaction, with poor bone quality necessitating use of bone graft.
A frozen allograft femoral head is then obtained and split into 2 equal pieces using a saw (Figures 3–5). One piece is fashioned with a saw and a burr into a trapezoid such that the proximal portion is wider, and the distal, tapered portion is sized to fit the canal. The broad, proximal portion of the graft will serve as a pedestal to reduce the head to the shaft. Measuring the internal diameter of the humeral canal can be useful in estimating the necessary dimensions of the distal portion of the allograft. The graft often needs several small adjustments that necessitate attempting to place it in the intramedullary canal and then trimming as necessary to ensure proper fit distally within the shaft. For this reason, it is beneficial to perform the graft preparation near the surgical field. Once completed, the distal portion is then impacted into the humeral canal (Figure 6). Because of this impaction, there is no possibility for subsidence or pistoning of the graft within the canal, which can occur with a fibular graft. The humeral head is reduced onto the shaft with the already placed sutures; this is achieved by abducting the shoulder. The image intensifier is then used to confirm appropriate alignment and positioning of the fragments, making sure that both neck–shaft angle and medial calcar alignment have been restored (Figures 7, 8).
An appropriately sized proximal humerus plate is then selected based on the location of the fracture line. We have used standard lateral proximal humerus locking plates as well as laterality-specific anterolateral proximal humerus plates and found that both are suitable for incorporation of the screws through the graft and into the head. The plate is positioned on the humerus, and a guide pin is placed by hand through the proximal-most hole so that the appropriate height of the plate can be verified on fluoroscopy. The first screw is then a nonlocking bicortical screw placed through the oval hole in the shaft of the plate to allow further fine manipulation of the plate more proximally or distally as needed. The final height is confirmed, and the screw is firmly tightened (Figure 9). The locking-screw guide is fixed to the proximal portion of the plate, and 2 locking screws are then placed into the head. The arm is then rotated to an anteroposterior view by placing the arm in external rotation and neutral flexion and is then abducted and internally rotated to recreate a lateral view to perform final verification of the position of the plate on orthogonal images. If the surgeon is satisfied with the position of the plate, another nonlocking screw is placed distally, and then the proximal holes are used to place locking screws as needed. If the surgeon is not satisfied, the 2 proximal screws can be removed and the plate repositioned.
After each screw is placed, fluoroscopy is used to ensure there has been no breach of the articular surface. The number of proximal screws placed depends on fracture configuration and surgeon preference.
The sutures through the rotator cuff are then fixed to the plate, securing the tuberosities. Final intraoperative radiographs are used to confirm reduction, alignment, and final position of hardware (Figure 10). After copious irrigation, a surgical drain is placed as needed, and the wound is closed in layered fashion. Three years after surgery, follow-up examination revealed no radiographic change in alignment, no necrosis, and no varus collapse (Figure 11), and the patient was pain-free during activities.
Discussion
Surgical treatment of comminuted proximal humerus fractures usually consists of some type of plate fixation with screw fixation of the shaft, screws or smooth pegs to support the chondral surfaces, and screw fixation or suture cerclage of the tuberosities.
Fixed-angle locking-plate-and-screw constructs increased the biomechanical stability and pullout strength of proximal humerus plates.3,4 Nevertheless, avascular necrosis, malunion, and nonunion are still known complications of proximal humerus fractures, especially those with comminution, with up to 14% of patients still experiencing loss of fixation.5
For this reason, several authors have proposed using allograft bone and/or augmentation with calcium-containing cement to supplement fixation and provide an endosteal form of support for the head and tuberosities to decrease the risk for varus collapse. Osteobiologics (eg, calcium phosphate or sulfate cement) have been shown to decrease the risk for loss of reduction of proximal humerus fractures and decrease the risk for intra-articular screw penetration.6,7 Many calcium phosphate cements are commercially available. Cost and availability are 2 reasons that these supplements are not more widely used. Cancellous chips have also been used to aid in the reduction of proximal humerus fractures.8 No randomized study has been conducted to show a clinical advantage of this technique, though retrospective studies have shown that it is not as advantageous as using calcium phosphate cement with respect to loss of reduction or screw penetration.6 Certainly, cancellous chips are easily available in most hospitals and are less expensive than some alternatives. A recent review of these techniques in osteoporotic proximal humerus fractures found no clear indication for using one of these supplements over another.9
However, some fracture patterns require a structural graft to reduce the tuberosities and head component. Although described more than 30 years ago as a treatment for nonunions with an intramedullary “peg” of iliac crest graft,10 the graft most commonly reported today is allograft fibula.11-15 This technique consists of preparing the humeral shaft and often the fractured head segment with reaming to create a channel to receive the graft. Even with use of a small fibula, it is often time-consuming to use a saw, rasp, or burr to size the fibular segment to fit the medullary canal of the humerus. Once in place, the graft provides a strut on which the head fragment can be reduced and around which the tuberosities can be reduced. Although this technique is successful clinically and is biomechanically superior to plate-only constructs,16,17 concerns remain.
One such concern is keeping this graft in routine supply at most hospitals. Supply and pricing from vendors can differ significantly between hospitals, and a surgeon may need to request grafts in advance, which makes their use nonviable in a trauma case. Certain grafts are often kept in routine supply based on their overall utilization. At our institution, allograft femoral heads meet this criterion and are routinely stocked.
Of more importance are the ramifications of these procedures for future revision surgeries. The need for arthroplasty revision is common after ORIF of a proximal humerus fracture.18
Arthroplasty revision is an already challenging procedure that becomes more complex with the need to remove 6 to 8 cm of ingrown endosteal bone from a shell of outer osteoporotic cortical bone. Our experience with these complex revisions provided the impetus to search for an alternate graft type that still provides a strut for reducing the head and tuberosities but limits the amount of endosteal bone that would need to be removed in arthroplasty revision in order to place a stemmed component into the humeral canal.
Some currently available arthroplasty fracture systems modify the previous anatomy of the stem to provide a more anatomical platform to reduce the tuberosities to a broader metaphyseal construct that incorporates bone grafting to assist with healing.
Because of these concerns and factors, we adapted our technique to create an individual-specific pedestal with allograft femoral head that can be anatomically matched to each patient. This provides a strut to reduce the head and tuberosity fragments but still limits the amount of allograft bone needed to seat into the existing canal. The geometry of the allograft can also be customized to the fracture, with most 3- and 4-part fractures needing a trapezoidal strut that resembles the metaphyseal portion of a fracture-specific shoulder arthroplasty implant.
We have used this technique for comminuted 3- and 4-part fractures of the proximal humerus in 14 cases with at least 2-year follow-up and in several more cases that have not reached 2-year follow-up. All cases have gone on to radiographic union; none have had to be revised either with revision ORIF or to an arthroplasty. Formal measurements of final postoperative range of motion have not been tabulated in all cases, as some cases have been lost to follow-up after radiographic union was achieved. Medium- and long-term results are not yet available, but no short-term complications have been noted.
Disadvantages of this technique are that, while an individualized graft is created, proper shaping still takes time, and a moderate amount of the femoral head is not used. However, we have found that, if a graft is inadvertently undersized, there is still ample femoral head remaining to create another sized graft. Other disadvantages are the added cost and the (rare) risk of disease transmission, which come with use of any allograft, but the technique is used instead of another type of allograft, so these disadvantages are largely equivalent. At our hospital, differences in cost and availability between femoral head or fibular allografts are negligible.
This procedure, which is easily performed in a short amount of time, allows a stable base of bone graft to be used as an aid in the anatomical reduction of proximal humerus fractures, without the need for reaming and preparation of the medullary canal and without further increasing the difficulty associated with a future revision procedure.
1. Barrett JA, Baron JA, Karagas MR, Beach ML. Fracture risk in the U.S. Medicare population. J Clin Epidemiol. 1999;52(3):243-249.
2. Neer CS 2nd. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
3. Liew AS, Johnson JA, Patterson SD, King GJ, Chess DG. Effect of screw placement on fixation in the humeral head. J Shoulder Elbow Surg. 2000;9(5):423-426.
4. Weinstein DM, Bratton DR, Ciccone WJ 2nd, Elias JJ. Locking plates improve torsional resistance in the stabilization of three-part proximal humeral fractures. J Shoulder Elbow Surg. 2006;15(2):239-243.
5. Agudelo J, Schurmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
6. Egol KA, Sugi MT, Ong CC, Montero N, Davidovitch R, Zuckerman JD. Fracture site augmentation with calcium phosphate cement reduces screw penetration after open reduction-internal fixation of proximal humeral fractures. J Shoulder Elbow Surg. 2012;21(6):741-748.
7. Gradl G, Knobe M, Stoffel M, Prescher A, Dirrichs T, Pape HC. Biomechanical evaluation of locking plate fixation of proximal humeral fractures augmented with calcium phosphate cement. J Orthop Trauma. 2013;27(7):399-404.
8. Ong CC, Kwon YW, Walsh M, Davidovitch R, Zuckerman JD, Egol KA. Outcomes of open reduction and internal fixation of proximal humerus fractures managed with locking plates. Am J Orthop. 2012;41(9):407-412.
9. Namdari S, Voleti PB, Mehta S. Evaluation of the osteoporotic proximal humeral fracture and strategies for structural augmentation during surgical treatment. J Shoulder Elbow Surg. 2012;21(12):1787-1795.
10. Scheck M. Surgical treatment of nonunions of the surgical neck of the humerus. Clin Orthop Relat Res. 1982;(167):255-259.
11. Hettrich CM, Neviaser A, Beamer BS, Paul O, Helfet DL, Lorich DG. Locked plating of the proximal humerus using an endosteal implant. J Orthop Trauma. 2012;26(4):212-215.
12. Neviaser AS, Hettrich CM, Beamer BS, Dines JS, Lorich DG. Endosteal strut augment reduces complications associated with proximal humeral locking plates. Clin Orthop Relat Res. 2011;469(12):3300-3306.
13. Gardner MJ, Boraiah S, Helfet DL, Lorich DG. Indirect medial reduction and strut support of proximal humerus fractures using an endosteal implant. J Orthop Trauma. 2008;22(3):195-200.
14. Matassi F, Angeloni R, Carulli C, et al. Locking plate and fibular allograft augmentation in unstable fractures of proximal humerus. Injury. 2012;43(11):1939-1942.
15. Little MT, Berkes MB, Schottel PC, et al. The impact of preoperative coronal plane deformity on proximal humerus fixation with endosteal augmentation. J Orthop Trauma. 2014;28(6):338-347.
16. Mathison C, Chaudhary R, Beaupre L, Reynolds M, Adeeb S, Bouliane M. Biomechanical analysis of proximal humeral fixation using locking plate fixation with an intramedullary fibular allograft. Clin Biomech. 2010;25(7):642-646.
17. Chow RM, Begum F, Beaupre LA, Carey JP, Adeeb S, Bouliane MJ. Proximal humeral fracture fixation: locking plate construct +/- intramedullary fibular allograft. J Shoulder Elbow Surg. 2012;21(7):894-901.
18. Jost B, Spross C, Grehn H, Gerber C. Locking plate fixation of fractures of the proximal humerus: analysis of complications, revision strategies and outcome. J Shoulder Elbow Surg. 2013;22(4):542-549.
Proximal humerus fractures are exceedingly common and account for almost 5% of all fractures. As osteoporosis is a risk factor for these fractures, their incidence rises with patient age.1
In 1970, Neer2 described these type of fractures and classified them as having 2, 3, or 4 parts based on the amount of angulation and displacement of the humeral head and the greater and lesser tuberosities with respect to the shaft.
Three- and 4-part proximal humerus fractures can be treated either nonoperatively, or surgically with closed reduction and percutaneous fixation, intramedullary fixation, open reduction and internal fixation (ORIF), or arthroplasty. There remains controversy over the best treatment, but a key component of any surgical treatment is anatomical reduction, stable fixation, and then healing of the tuberosities. A current common form of treatment is augmentation with an allograft fibula placed in the medullary canal. Although not formally reported, anecdotal evidence demonstrates that revision to arthroplasty is very difficult in the setting of an ingrown graft in the medullary canal of the humerus.
In this article, we present a novel technique of using allograft femoral head to reconstruct the metaphysis in ORIF of comminuted proximal humerus fractures.
Technique
Presented in Figure 1 are preoperative images of a representative displaced 4-part proximal humerus fracture treated surgically using the technique described here. General anesthesia is used. After intubation on the operating table, the patient is placed in the beach-chair position with about 75° of hip flexion. All bony prominences are padded, and the head and trunk are well secured. A pneumatic arm positioner is used to alleviate the need for an assistant to manipulate the arm. An image intensifier is used before preparing to verify that appropriate images of the proximal humerus can be obtained. Once adequate images are confirmed, the floor can be marked at the position of the fluoroscopic unit’s wheels to allow easy reproduction of images once the arm is prepared and draped. The intensifier is then removed from the field, the shoulder is prepared and draped in usual fashion, and prophylactic antibiotics are administered.
A deltopectoral incision is used, and sharp dissection is made through the subcutaneous tissue to raise full-thickness subcutaneous flaps on each side. The deltopectoral interval is sharply dissected while protecting the cephalic vein. Subdeltoid adhesions are then released. Palpation of the axillary nerve in the quadrilateral space to identify its location is helpful to avoid injury during the procedure.
The fracture is then identified, and No. 5 permanent suture is placed through the posterior and superior rotator cuff and through the subscapularis insertion (Figure 2). The tuberosities are freed from the humeral head sharply. A blunt elevator is then used to gently elevate the humeral head upward, with care taken to avoid comminuting the metaphyseal bone while levering. Reduction is achieved by manipulating the sutures and levering the head with the elevator while placing the arm in extension and posterior translation. Fluoroscopic images are used to verify correct anatomical alignment. Generally, the metaphysis demonstrates comminution and impaction, with poor bone quality necessitating use of bone graft.
A frozen allograft femoral head is then obtained and split into 2 equal pieces using a saw (Figures 3–5). One piece is fashioned with a saw and a burr into a trapezoid such that the proximal portion is wider, and the distal, tapered portion is sized to fit the canal. The broad, proximal portion of the graft will serve as a pedestal to reduce the head to the shaft. Measuring the internal diameter of the humeral canal can be useful in estimating the necessary dimensions of the distal portion of the allograft. The graft often needs several small adjustments that necessitate attempting to place it in the intramedullary canal and then trimming as necessary to ensure proper fit distally within the shaft. For this reason, it is beneficial to perform the graft preparation near the surgical field. Once completed, the distal portion is then impacted into the humeral canal (Figure 6). Because of this impaction, there is no possibility for subsidence or pistoning of the graft within the canal, which can occur with a fibular graft. The humeral head is reduced onto the shaft with the already placed sutures; this is achieved by abducting the shoulder. The image intensifier is then used to confirm appropriate alignment and positioning of the fragments, making sure that both neck–shaft angle and medial calcar alignment have been restored (Figures 7, 8).
An appropriately sized proximal humerus plate is then selected based on the location of the fracture line. We have used standard lateral proximal humerus locking plates as well as laterality-specific anterolateral proximal humerus plates and found that both are suitable for incorporation of the screws through the graft and into the head. The plate is positioned on the humerus, and a guide pin is placed by hand through the proximal-most hole so that the appropriate height of the plate can be verified on fluoroscopy. The first screw is then a nonlocking bicortical screw placed through the oval hole in the shaft of the plate to allow further fine manipulation of the plate more proximally or distally as needed. The final height is confirmed, and the screw is firmly tightened (Figure 9). The locking-screw guide is fixed to the proximal portion of the plate, and 2 locking screws are then placed into the head. The arm is then rotated to an anteroposterior view by placing the arm in external rotation and neutral flexion and is then abducted and internally rotated to recreate a lateral view to perform final verification of the position of the plate on orthogonal images. If the surgeon is satisfied with the position of the plate, another nonlocking screw is placed distally, and then the proximal holes are used to place locking screws as needed. If the surgeon is not satisfied, the 2 proximal screws can be removed and the plate repositioned.
After each screw is placed, fluoroscopy is used to ensure there has been no breach of the articular surface. The number of proximal screws placed depends on fracture configuration and surgeon preference.
The sutures through the rotator cuff are then fixed to the plate, securing the tuberosities. Final intraoperative radiographs are used to confirm reduction, alignment, and final position of hardware (Figure 10). After copious irrigation, a surgical drain is placed as needed, and the wound is closed in layered fashion. Three years after surgery, follow-up examination revealed no radiographic change in alignment, no necrosis, and no varus collapse (Figure 11), and the patient was pain-free during activities.
Discussion
Surgical treatment of comminuted proximal humerus fractures usually consists of some type of plate fixation with screw fixation of the shaft, screws or smooth pegs to support the chondral surfaces, and screw fixation or suture cerclage of the tuberosities.
Fixed-angle locking-plate-and-screw constructs increased the biomechanical stability and pullout strength of proximal humerus plates.3,4 Nevertheless, avascular necrosis, malunion, and nonunion are still known complications of proximal humerus fractures, especially those with comminution, with up to 14% of patients still experiencing loss of fixation.5
For this reason, several authors have proposed using allograft bone and/or augmentation with calcium-containing cement to supplement fixation and provide an endosteal form of support for the head and tuberosities to decrease the risk for varus collapse. Osteobiologics (eg, calcium phosphate or sulfate cement) have been shown to decrease the risk for loss of reduction of proximal humerus fractures and decrease the risk for intra-articular screw penetration.6,7 Many calcium phosphate cements are commercially available. Cost and availability are 2 reasons that these supplements are not more widely used. Cancellous chips have also been used to aid in the reduction of proximal humerus fractures.8 No randomized study has been conducted to show a clinical advantage of this technique, though retrospective studies have shown that it is not as advantageous as using calcium phosphate cement with respect to loss of reduction or screw penetration.6 Certainly, cancellous chips are easily available in most hospitals and are less expensive than some alternatives. A recent review of these techniques in osteoporotic proximal humerus fractures found no clear indication for using one of these supplements over another.9
However, some fracture patterns require a structural graft to reduce the tuberosities and head component. Although described more than 30 years ago as a treatment for nonunions with an intramedullary “peg” of iliac crest graft,10 the graft most commonly reported today is allograft fibula.11-15 This technique consists of preparing the humeral shaft and often the fractured head segment with reaming to create a channel to receive the graft. Even with use of a small fibula, it is often time-consuming to use a saw, rasp, or burr to size the fibular segment to fit the medullary canal of the humerus. Once in place, the graft provides a strut on which the head fragment can be reduced and around which the tuberosities can be reduced. Although this technique is successful clinically and is biomechanically superior to plate-only constructs,16,17 concerns remain.
One such concern is keeping this graft in routine supply at most hospitals. Supply and pricing from vendors can differ significantly between hospitals, and a surgeon may need to request grafts in advance, which makes their use nonviable in a trauma case. Certain grafts are often kept in routine supply based on their overall utilization. At our institution, allograft femoral heads meet this criterion and are routinely stocked.
Of more importance are the ramifications of these procedures for future revision surgeries. The need for arthroplasty revision is common after ORIF of a proximal humerus fracture.18
Arthroplasty revision is an already challenging procedure that becomes more complex with the need to remove 6 to 8 cm of ingrown endosteal bone from a shell of outer osteoporotic cortical bone. Our experience with these complex revisions provided the impetus to search for an alternate graft type that still provides a strut for reducing the head and tuberosities but limits the amount of endosteal bone that would need to be removed in arthroplasty revision in order to place a stemmed component into the humeral canal.
Some currently available arthroplasty fracture systems modify the previous anatomy of the stem to provide a more anatomical platform to reduce the tuberosities to a broader metaphyseal construct that incorporates bone grafting to assist with healing.
Because of these concerns and factors, we adapted our technique to create an individual-specific pedestal with allograft femoral head that can be anatomically matched to each patient. This provides a strut to reduce the head and tuberosity fragments but still limits the amount of allograft bone needed to seat into the existing canal. The geometry of the allograft can also be customized to the fracture, with most 3- and 4-part fractures needing a trapezoidal strut that resembles the metaphyseal portion of a fracture-specific shoulder arthroplasty implant.
We have used this technique for comminuted 3- and 4-part fractures of the proximal humerus in 14 cases with at least 2-year follow-up and in several more cases that have not reached 2-year follow-up. All cases have gone on to radiographic union; none have had to be revised either with revision ORIF or to an arthroplasty. Formal measurements of final postoperative range of motion have not been tabulated in all cases, as some cases have been lost to follow-up after radiographic union was achieved. Medium- and long-term results are not yet available, but no short-term complications have been noted.
Disadvantages of this technique are that, while an individualized graft is created, proper shaping still takes time, and a moderate amount of the femoral head is not used. However, we have found that, if a graft is inadvertently undersized, there is still ample femoral head remaining to create another sized graft. Other disadvantages are the added cost and the (rare) risk of disease transmission, which come with use of any allograft, but the technique is used instead of another type of allograft, so these disadvantages are largely equivalent. At our hospital, differences in cost and availability between femoral head or fibular allografts are negligible.
This procedure, which is easily performed in a short amount of time, allows a stable base of bone graft to be used as an aid in the anatomical reduction of proximal humerus fractures, without the need for reaming and preparation of the medullary canal and without further increasing the difficulty associated with a future revision procedure.
Proximal humerus fractures are exceedingly common and account for almost 5% of all fractures. As osteoporosis is a risk factor for these fractures, their incidence rises with patient age.1
In 1970, Neer2 described these type of fractures and classified them as having 2, 3, or 4 parts based on the amount of angulation and displacement of the humeral head and the greater and lesser tuberosities with respect to the shaft.
Three- and 4-part proximal humerus fractures can be treated either nonoperatively, or surgically with closed reduction and percutaneous fixation, intramedullary fixation, open reduction and internal fixation (ORIF), or arthroplasty. There remains controversy over the best treatment, but a key component of any surgical treatment is anatomical reduction, stable fixation, and then healing of the tuberosities. A current common form of treatment is augmentation with an allograft fibula placed in the medullary canal. Although not formally reported, anecdotal evidence demonstrates that revision to arthroplasty is very difficult in the setting of an ingrown graft in the medullary canal of the humerus.
In this article, we present a novel technique of using allograft femoral head to reconstruct the metaphysis in ORIF of comminuted proximal humerus fractures.
Technique
Presented in Figure 1 are preoperative images of a representative displaced 4-part proximal humerus fracture treated surgically using the technique described here. General anesthesia is used. After intubation on the operating table, the patient is placed in the beach-chair position with about 75° of hip flexion. All bony prominences are padded, and the head and trunk are well secured. A pneumatic arm positioner is used to alleviate the need for an assistant to manipulate the arm. An image intensifier is used before preparing to verify that appropriate images of the proximal humerus can be obtained. Once adequate images are confirmed, the floor can be marked at the position of the fluoroscopic unit’s wheels to allow easy reproduction of images once the arm is prepared and draped. The intensifier is then removed from the field, the shoulder is prepared and draped in usual fashion, and prophylactic antibiotics are administered.
A deltopectoral incision is used, and sharp dissection is made through the subcutaneous tissue to raise full-thickness subcutaneous flaps on each side. The deltopectoral interval is sharply dissected while protecting the cephalic vein. Subdeltoid adhesions are then released. Palpation of the axillary nerve in the quadrilateral space to identify its location is helpful to avoid injury during the procedure.
The fracture is then identified, and No. 5 permanent suture is placed through the posterior and superior rotator cuff and through the subscapularis insertion (Figure 2). The tuberosities are freed from the humeral head sharply. A blunt elevator is then used to gently elevate the humeral head upward, with care taken to avoid comminuting the metaphyseal bone while levering. Reduction is achieved by manipulating the sutures and levering the head with the elevator while placing the arm in extension and posterior translation. Fluoroscopic images are used to verify correct anatomical alignment. Generally, the metaphysis demonstrates comminution and impaction, with poor bone quality necessitating use of bone graft.
A frozen allograft femoral head is then obtained and split into 2 equal pieces using a saw (Figures 3–5). One piece is fashioned with a saw and a burr into a trapezoid such that the proximal portion is wider, and the distal, tapered portion is sized to fit the canal. The broad, proximal portion of the graft will serve as a pedestal to reduce the head to the shaft. Measuring the internal diameter of the humeral canal can be useful in estimating the necessary dimensions of the distal portion of the allograft. The graft often needs several small adjustments that necessitate attempting to place it in the intramedullary canal and then trimming as necessary to ensure proper fit distally within the shaft. For this reason, it is beneficial to perform the graft preparation near the surgical field. Once completed, the distal portion is then impacted into the humeral canal (Figure 6). Because of this impaction, there is no possibility for subsidence or pistoning of the graft within the canal, which can occur with a fibular graft. The humeral head is reduced onto the shaft with the already placed sutures; this is achieved by abducting the shoulder. The image intensifier is then used to confirm appropriate alignment and positioning of the fragments, making sure that both neck–shaft angle and medial calcar alignment have been restored (Figures 7, 8).
An appropriately sized proximal humerus plate is then selected based on the location of the fracture line. We have used standard lateral proximal humerus locking plates as well as laterality-specific anterolateral proximal humerus plates and found that both are suitable for incorporation of the screws through the graft and into the head. The plate is positioned on the humerus, and a guide pin is placed by hand through the proximal-most hole so that the appropriate height of the plate can be verified on fluoroscopy. The first screw is then a nonlocking bicortical screw placed through the oval hole in the shaft of the plate to allow further fine manipulation of the plate more proximally or distally as needed. The final height is confirmed, and the screw is firmly tightened (Figure 9). The locking-screw guide is fixed to the proximal portion of the plate, and 2 locking screws are then placed into the head. The arm is then rotated to an anteroposterior view by placing the arm in external rotation and neutral flexion and is then abducted and internally rotated to recreate a lateral view to perform final verification of the position of the plate on orthogonal images. If the surgeon is satisfied with the position of the plate, another nonlocking screw is placed distally, and then the proximal holes are used to place locking screws as needed. If the surgeon is not satisfied, the 2 proximal screws can be removed and the plate repositioned.
After each screw is placed, fluoroscopy is used to ensure there has been no breach of the articular surface. The number of proximal screws placed depends on fracture configuration and surgeon preference.
The sutures through the rotator cuff are then fixed to the plate, securing the tuberosities. Final intraoperative radiographs are used to confirm reduction, alignment, and final position of hardware (Figure 10). After copious irrigation, a surgical drain is placed as needed, and the wound is closed in layered fashion. Three years after surgery, follow-up examination revealed no radiographic change in alignment, no necrosis, and no varus collapse (Figure 11), and the patient was pain-free during activities.
Discussion
Surgical treatment of comminuted proximal humerus fractures usually consists of some type of plate fixation with screw fixation of the shaft, screws or smooth pegs to support the chondral surfaces, and screw fixation or suture cerclage of the tuberosities.
Fixed-angle locking-plate-and-screw constructs increased the biomechanical stability and pullout strength of proximal humerus plates.3,4 Nevertheless, avascular necrosis, malunion, and nonunion are still known complications of proximal humerus fractures, especially those with comminution, with up to 14% of patients still experiencing loss of fixation.5
For this reason, several authors have proposed using allograft bone and/or augmentation with calcium-containing cement to supplement fixation and provide an endosteal form of support for the head and tuberosities to decrease the risk for varus collapse. Osteobiologics (eg, calcium phosphate or sulfate cement) have been shown to decrease the risk for loss of reduction of proximal humerus fractures and decrease the risk for intra-articular screw penetration.6,7 Many calcium phosphate cements are commercially available. Cost and availability are 2 reasons that these supplements are not more widely used. Cancellous chips have also been used to aid in the reduction of proximal humerus fractures.8 No randomized study has been conducted to show a clinical advantage of this technique, though retrospective studies have shown that it is not as advantageous as using calcium phosphate cement with respect to loss of reduction or screw penetration.6 Certainly, cancellous chips are easily available in most hospitals and are less expensive than some alternatives. A recent review of these techniques in osteoporotic proximal humerus fractures found no clear indication for using one of these supplements over another.9
However, some fracture patterns require a structural graft to reduce the tuberosities and head component. Although described more than 30 years ago as a treatment for nonunions with an intramedullary “peg” of iliac crest graft,10 the graft most commonly reported today is allograft fibula.11-15 This technique consists of preparing the humeral shaft and often the fractured head segment with reaming to create a channel to receive the graft. Even with use of a small fibula, it is often time-consuming to use a saw, rasp, or burr to size the fibular segment to fit the medullary canal of the humerus. Once in place, the graft provides a strut on which the head fragment can be reduced and around which the tuberosities can be reduced. Although this technique is successful clinically and is biomechanically superior to plate-only constructs,16,17 concerns remain.
One such concern is keeping this graft in routine supply at most hospitals. Supply and pricing from vendors can differ significantly between hospitals, and a surgeon may need to request grafts in advance, which makes their use nonviable in a trauma case. Certain grafts are often kept in routine supply based on their overall utilization. At our institution, allograft femoral heads meet this criterion and are routinely stocked.
Of more importance are the ramifications of these procedures for future revision surgeries. The need for arthroplasty revision is common after ORIF of a proximal humerus fracture.18
Arthroplasty revision is an already challenging procedure that becomes more complex with the need to remove 6 to 8 cm of ingrown endosteal bone from a shell of outer osteoporotic cortical bone. Our experience with these complex revisions provided the impetus to search for an alternate graft type that still provides a strut for reducing the head and tuberosities but limits the amount of endosteal bone that would need to be removed in arthroplasty revision in order to place a stemmed component into the humeral canal.
Some currently available arthroplasty fracture systems modify the previous anatomy of the stem to provide a more anatomical platform to reduce the tuberosities to a broader metaphyseal construct that incorporates bone grafting to assist with healing.
Because of these concerns and factors, we adapted our technique to create an individual-specific pedestal with allograft femoral head that can be anatomically matched to each patient. This provides a strut to reduce the head and tuberosity fragments but still limits the amount of allograft bone needed to seat into the existing canal. The geometry of the allograft can also be customized to the fracture, with most 3- and 4-part fractures needing a trapezoidal strut that resembles the metaphyseal portion of a fracture-specific shoulder arthroplasty implant.
We have used this technique for comminuted 3- and 4-part fractures of the proximal humerus in 14 cases with at least 2-year follow-up and in several more cases that have not reached 2-year follow-up. All cases have gone on to radiographic union; none have had to be revised either with revision ORIF or to an arthroplasty. Formal measurements of final postoperative range of motion have not been tabulated in all cases, as some cases have been lost to follow-up after radiographic union was achieved. Medium- and long-term results are not yet available, but no short-term complications have been noted.
Disadvantages of this technique are that, while an individualized graft is created, proper shaping still takes time, and a moderate amount of the femoral head is not used. However, we have found that, if a graft is inadvertently undersized, there is still ample femoral head remaining to create another sized graft. Other disadvantages are the added cost and the (rare) risk of disease transmission, which come with use of any allograft, but the technique is used instead of another type of allograft, so these disadvantages are largely equivalent. At our hospital, differences in cost and availability between femoral head or fibular allografts are negligible.
This procedure, which is easily performed in a short amount of time, allows a stable base of bone graft to be used as an aid in the anatomical reduction of proximal humerus fractures, without the need for reaming and preparation of the medullary canal and without further increasing the difficulty associated with a future revision procedure.
1. Barrett JA, Baron JA, Karagas MR, Beach ML. Fracture risk in the U.S. Medicare population. J Clin Epidemiol. 1999;52(3):243-249.
2. Neer CS 2nd. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
3. Liew AS, Johnson JA, Patterson SD, King GJ, Chess DG. Effect of screw placement on fixation in the humeral head. J Shoulder Elbow Surg. 2000;9(5):423-426.
4. Weinstein DM, Bratton DR, Ciccone WJ 2nd, Elias JJ. Locking plates improve torsional resistance in the stabilization of three-part proximal humeral fractures. J Shoulder Elbow Surg. 2006;15(2):239-243.
5. Agudelo J, Schurmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
6. Egol KA, Sugi MT, Ong CC, Montero N, Davidovitch R, Zuckerman JD. Fracture site augmentation with calcium phosphate cement reduces screw penetration after open reduction-internal fixation of proximal humeral fractures. J Shoulder Elbow Surg. 2012;21(6):741-748.
7. Gradl G, Knobe M, Stoffel M, Prescher A, Dirrichs T, Pape HC. Biomechanical evaluation of locking plate fixation of proximal humeral fractures augmented with calcium phosphate cement. J Orthop Trauma. 2013;27(7):399-404.
8. Ong CC, Kwon YW, Walsh M, Davidovitch R, Zuckerman JD, Egol KA. Outcomes of open reduction and internal fixation of proximal humerus fractures managed with locking plates. Am J Orthop. 2012;41(9):407-412.
9. Namdari S, Voleti PB, Mehta S. Evaluation of the osteoporotic proximal humeral fracture and strategies for structural augmentation during surgical treatment. J Shoulder Elbow Surg. 2012;21(12):1787-1795.
10. Scheck M. Surgical treatment of nonunions of the surgical neck of the humerus. Clin Orthop Relat Res. 1982;(167):255-259.
11. Hettrich CM, Neviaser A, Beamer BS, Paul O, Helfet DL, Lorich DG. Locked plating of the proximal humerus using an endosteal implant. J Orthop Trauma. 2012;26(4):212-215.
12. Neviaser AS, Hettrich CM, Beamer BS, Dines JS, Lorich DG. Endosteal strut augment reduces complications associated with proximal humeral locking plates. Clin Orthop Relat Res. 2011;469(12):3300-3306.
13. Gardner MJ, Boraiah S, Helfet DL, Lorich DG. Indirect medial reduction and strut support of proximal humerus fractures using an endosteal implant. J Orthop Trauma. 2008;22(3):195-200.
14. Matassi F, Angeloni R, Carulli C, et al. Locking plate and fibular allograft augmentation in unstable fractures of proximal humerus. Injury. 2012;43(11):1939-1942.
15. Little MT, Berkes MB, Schottel PC, et al. The impact of preoperative coronal plane deformity on proximal humerus fixation with endosteal augmentation. J Orthop Trauma. 2014;28(6):338-347.
16. Mathison C, Chaudhary R, Beaupre L, Reynolds M, Adeeb S, Bouliane M. Biomechanical analysis of proximal humeral fixation using locking plate fixation with an intramedullary fibular allograft. Clin Biomech. 2010;25(7):642-646.
17. Chow RM, Begum F, Beaupre LA, Carey JP, Adeeb S, Bouliane MJ. Proximal humeral fracture fixation: locking plate construct +/- intramedullary fibular allograft. J Shoulder Elbow Surg. 2012;21(7):894-901.
18. Jost B, Spross C, Grehn H, Gerber C. Locking plate fixation of fractures of the proximal humerus: analysis of complications, revision strategies and outcome. J Shoulder Elbow Surg. 2013;22(4):542-549.
1. Barrett JA, Baron JA, Karagas MR, Beach ML. Fracture risk in the U.S. Medicare population. J Clin Epidemiol. 1999;52(3):243-249.
2. Neer CS 2nd. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
3. Liew AS, Johnson JA, Patterson SD, King GJ, Chess DG. Effect of screw placement on fixation in the humeral head. J Shoulder Elbow Surg. 2000;9(5):423-426.
4. Weinstein DM, Bratton DR, Ciccone WJ 2nd, Elias JJ. Locking plates improve torsional resistance in the stabilization of three-part proximal humeral fractures. J Shoulder Elbow Surg. 2006;15(2):239-243.
5. Agudelo J, Schurmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
6. Egol KA, Sugi MT, Ong CC, Montero N, Davidovitch R, Zuckerman JD. Fracture site augmentation with calcium phosphate cement reduces screw penetration after open reduction-internal fixation of proximal humeral fractures. J Shoulder Elbow Surg. 2012;21(6):741-748.
7. Gradl G, Knobe M, Stoffel M, Prescher A, Dirrichs T, Pape HC. Biomechanical evaluation of locking plate fixation of proximal humeral fractures augmented with calcium phosphate cement. J Orthop Trauma. 2013;27(7):399-404.
8. Ong CC, Kwon YW, Walsh M, Davidovitch R, Zuckerman JD, Egol KA. Outcomes of open reduction and internal fixation of proximal humerus fractures managed with locking plates. Am J Orthop. 2012;41(9):407-412.
9. Namdari S, Voleti PB, Mehta S. Evaluation of the osteoporotic proximal humeral fracture and strategies for structural augmentation during surgical treatment. J Shoulder Elbow Surg. 2012;21(12):1787-1795.
10. Scheck M. Surgical treatment of nonunions of the surgical neck of the humerus. Clin Orthop Relat Res. 1982;(167):255-259.
11. Hettrich CM, Neviaser A, Beamer BS, Paul O, Helfet DL, Lorich DG. Locked plating of the proximal humerus using an endosteal implant. J Orthop Trauma. 2012;26(4):212-215.
12. Neviaser AS, Hettrich CM, Beamer BS, Dines JS, Lorich DG. Endosteal strut augment reduces complications associated with proximal humeral locking plates. Clin Orthop Relat Res. 2011;469(12):3300-3306.
13. Gardner MJ, Boraiah S, Helfet DL, Lorich DG. Indirect medial reduction and strut support of proximal humerus fractures using an endosteal implant. J Orthop Trauma. 2008;22(3):195-200.
14. Matassi F, Angeloni R, Carulli C, et al. Locking plate and fibular allograft augmentation in unstable fractures of proximal humerus. Injury. 2012;43(11):1939-1942.
15. Little MT, Berkes MB, Schottel PC, et al. The impact of preoperative coronal plane deformity on proximal humerus fixation with endosteal augmentation. J Orthop Trauma. 2014;28(6):338-347.
16. Mathison C, Chaudhary R, Beaupre L, Reynolds M, Adeeb S, Bouliane M. Biomechanical analysis of proximal humeral fixation using locking plate fixation with an intramedullary fibular allograft. Clin Biomech. 2010;25(7):642-646.
17. Chow RM, Begum F, Beaupre LA, Carey JP, Adeeb S, Bouliane MJ. Proximal humeral fracture fixation: locking plate construct +/- intramedullary fibular allograft. J Shoulder Elbow Surg. 2012;21(7):894-901.
18. Jost B, Spross C, Grehn H, Gerber C. Locking plate fixation of fractures of the proximal humerus: analysis of complications, revision strategies and outcome. J Shoulder Elbow Surg. 2013;22(4):542-549.