User login
Cementing Multihole, Metal, Modular Acetabular Shells Into Cages in Revision Total Hip Arthroplasty
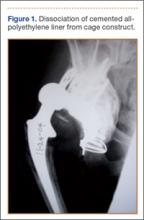
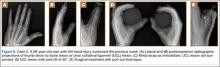
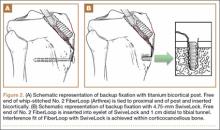
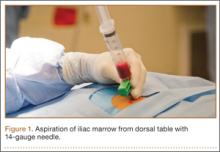
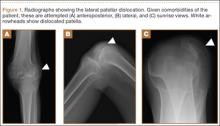
Although the number of total hip arthroplasties (THAs) being performed in the United States is increasing, revision THAs are more common.1 Many acetabular revisions can be successfully performed with standard or jumbo cementless acetabular cups, but major osseous deficiencies typically require reconstruction with a cage or cup/cage that bridges gaps in the pelvis and obtains fixation of the arthroplasty components.2,3 Cages and rings have been combined with all-polyethylene acetabular components (ie, all-polyethylene cups, or APCs) to reconstruct pelvic bone defects, but complications, including APC dissociation (Figure 1) and postoperative instability, can occur despite stable fixation of cage to pelvis.4 The incidence of dislocations with pelvic reconstruction rings using APCs has been reported to be 11%.4 If an APC has to be replaced because of wear, then major surgery may be required to extract the worn cup and cement a new cup in its place.
In this article, we describe a technique in which a metal, multihole acetabular shell is cemented into the cage or ring construct, avoiding some of the complications associated with traditional techniques by permitting use of a variety of liners.
Materials and Methods
We retrospectively reviewed the cases of all of Dr. Bolanos’ patients who underwent acetabular revision THA with cage reconstruction between February 1, 1998 and October 9, 2006. During this period, we were cementing a modular metal shell into the cage instead of an APC or polyethylene liner. All patients who underwent revision THA with cage reconstruction during the study period were included. Bone defects were treated with structural or morselized bone allograft. Every reconstruction involved use of an antiprotrusio cage or ring secured to the pelvis with screws, and a multihole acetabular shell cemented into place with a polyethylene liner applied. Elevated rims, lateralized liners, and constrained liners were used as needed to optimize stability. Femoral components were retained. Cage size was based on matching the osseous deficiencies. Shell size was determined by the inner diameter of the corresponding cage. Liner size was based on matching the shell and femoral head. During this period, none of the patients had other reconstructive techniques, such as trabecular metal augmentation, in combination with a modular acetabular shell, cup/cage reconstruction, or custom triflange components.
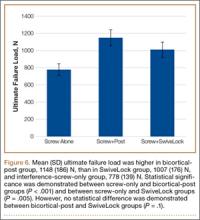
Patients engaged in protected weight-bearing ambulation for 3 months after surgery and were then permitted full, unrestricted activity. The primary outcome was mechanical failure of the reconstruction, or reoperation (Table). All reconstructions in this series consisted of acetabular revisions for aseptic loosening.
Surgical Technique
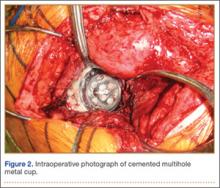
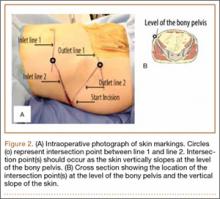
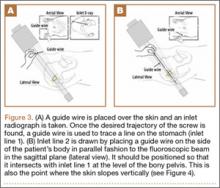
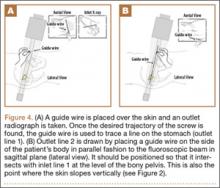
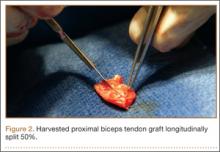
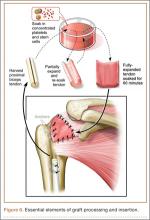
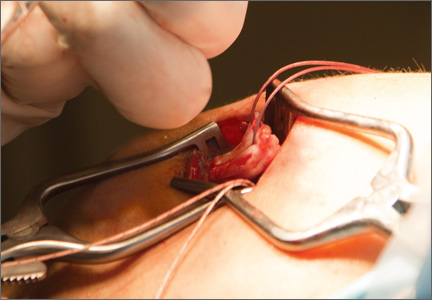
Six consecutive cases of pelvic discontinuity and 7 cases of segmental acetabular bone loss required use of cages or rings. Reconstruction cages were used to secure fixation to the ilium and ischium. With the technique described in this article, we used screws with rounded, prominent heads rather than flat heads between the cup and the cage or ring (Synthes, 6.5 mm) to ensure adequate cement mantle. The rounded screw heads were left prominent to approximate the function of cement pegs found on APCs. Screws were placed into the anterior, superior, medial, and posterior aspects of the cage to ensure adequate cement mantle between cup and cage. This was confirmed with trial placement of the cup into the cage before cementation and observation of the uniformity of the space between cup and cage. Trial placement also confirmed that the screws did not interfere with appropriate positioning of the cup. A multihole, metal acetabular cup was then cemented in the cage or ring such that cement extruded around the shell and into the holes of the cup and the cage, securing the cup to the cage. Use of a multihole, metal shell resulted in excellent cement fixation because the multiple holes created multiple circumferential cement pegs. Various liner options could then be used to optimize stability of the reconstruction. In some cases, excessive cement extruded into the interior aspect of the shell and hardened before curettage. If the excess cement could interfere with complete seating/locking of the liner, then a high-speed burr was used to easily remove cement (Figure 2). Polyethylene liners were then inserted into the shell. Femoral reconstruction was then performed, if needed, and stability of the arthroplasty checked. This technique allows the surgeon to then select from a variety of polyethylene liners as needed to optimize stability. Liners with elevated rims, lateralized liners, and constrained liners could be interchangeable options with this technique.
Results
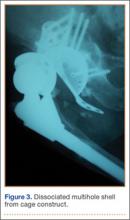
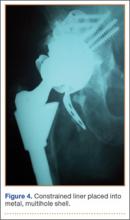
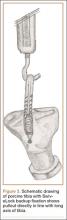
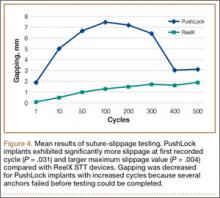
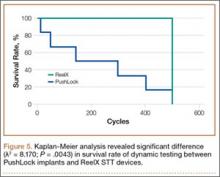
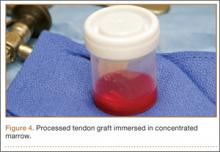
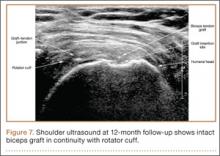
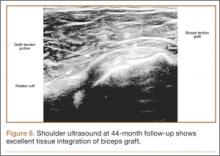
Thirteen patients with major osseous deficiencies of the pelvis were treated using this technique. At mean follow-up of 64.2 months (range, 3-133 months), 10 of the 13 patients had favorable outcomes without further surgery. One patient developed recurrent aseptic loosening that required re-revision, another patient developed recurrent instability that required acetabular liner and femoral head exchange, and a third patient with poor balance fell multiple times. This patient’s ninth fall resulted in dissociation of the acetabular shell from the cage (Figure 3), treated with placement of another cemented multihole metal shell with a standard liner. As dislocations recurred, the liner was changed to a constrained liner (Figure 4). The patient did not have any further dislocations or other hip-related problems. Integrity of cemented shell-cage fixation was maintained in 12 of the 13 patients at final follow-up.
Discussion
We have described a novel technique that facilitates reconstruction of major osseous deficiencies of the pelvis. The technique involves cementation of a multihole, metal acetabular shell into a cage or ring, permitting use of modular liners. The modularity in this approach to major hip reconstruction provides stability-optimization options that are not available with APCs. So far, the technique has demonstrated more advantages than disadvantages, so the indications for its use would be whenever a cage is used for pelvic reconstruction. Traditional techniques involve cementing an APC into the cage or ring. Use of multihole, metal shells for this purpose has several theoretical advantages. Multiple holes and the textured surface allow more interdigitation of cement with cup than APCs do; this interdigitation may improve the durability of the cemented interface. Cement also extrudes through the holes of the cage to secure the cup to the pelvis, as is done with cementation of APCs. Introduction of trabecular metal shells may also provide an even more secure bond to the shell, compared with APCs, though durability of a cemented trabecular metal interface has not been established. In addition, mechanical alignment guides cannot fasten as securely onto some APCs.
Nonmodular, cemented, metal-backed acetabular components, which were commonly used in hip arthroplasties at one time, were abandoned because of their relatively high loosening rate and because of advantages noted with modular components.5 The nonmodular components had been developed because of their theoretical advantages of improved distribution of forces into the cement mantle.5,6 However, those models had a relatively smooth metallic surface, which probably did not bond as well to cement as the shells used with the technique described in this article.
Dislocations can occur because of inadequately placed cups. Metallic cups can be improperly positioned, as can APCs. An advantage of the technique we have described over APCs is that liners with raised rims can be inserted with the apex placed wherever needed to best address instability. Dislocations can also occur because of factors such as inadequate offset and cognitive impairments. Our technique allows use of offset liners and constrained liners. Although these options may not prevent further dislocations, they often mitigate instability issues. Constrained liners and lateralized liners can be easily placed, and elevated rims can be swiveled as needed for stability. As use of cementless, metal-backed, modular acetabular components is common in primary THAs, most surgeons are familiar with the modular liner options available with use of the technique described in this article.
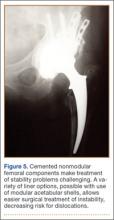
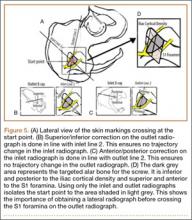
In this setting, modular, metal acetabular shells have the advantage of allowing surgeons to use the alignment guides they are accustomed to using. Modularity is another significant advantage over APCs. When an APC wears down, the component must be extracted to permit implantation of a new APC. With metal shells, a worn liner can be exchanged relatively easily. Modularity also gives surgeons many more options for addressing instability. Elevated rims can be moved, head sizes can be changed, and lateralized or constrained liners can be implanted easily. By comparison, with APCs, stability can be addressed only by modifying the femoral component or taking hip precautions which restrict range of motion of the hip. Modification of the femoral component is not possible with nonmodular femoral components in place (Figure 5). A potential disadvantage of this technique is increased cost associated with use of another component.
This small series of patients has had an excellent rate of success with cementation of multihole, metal-backed acetabular components into a cage or ring. These components may offer more secure fixation than APCs to cement extruded into the multiple holes, and improved metallurgy, such as trabecular metal. Surgeons who want to use modular components may prefer this technique because it allows them to select from various liner options. Surgeons should consider this technique for patients who need major pelvic reconstruction, though a larger study with longer follow-up is needed to determine its long-term durability.
Although the novel technique we have described has been helpful in our experience, this study had several limitations—small series, retrospective study, relatively short follow-up, lack of control group and functional data—that may have affected its conclusions. Further study and follow-up are needed to better determine the utility of this technique in clinical practice.
1. Kurtz SM, Ong KL, Schmier J, Zhao K, Mowat F, Lau E. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24(2):195-203.
2. Berry DJ, Lewallen DG, Hanssen AD, Cabanela ME. Pelvic discontinuity in revision total hip arthroplasty. J Bone Joint Surg Am. 1999;81(12):1692-1702.
3. Pieringer H, Auersperg V, Böhler N. Reconstruction of severe acetabular bone-deficiency: the Burch-Schneider antiprotrusio cage in primary and revision total hip arthroplasty. J Arthroplasty. 2006;21(4):489-496.
4. Goodman S, Saastamoinen H, Shasha N, Gross A. Complications of ilioischial reconstruction rings in revision total hip arthroplasty. J Arthroplasty. 2004;19(4):436-446.
5. Cates HE, Faris PM, Keating EM, Ritter MA. Polyethylene wear in cemented metal-backed acetabular cups. J Bone Joint Surg Br. 1993;75(2):249-253.
6. Vasu R, Carter DR, Harris WH. Stress distribution in the acetabular region—I. Before and after total joint replacement. J Biomech. 1982;15(3):155-164.
Although the number of total hip arthroplasties (THAs) being performed in the United States is increasing, revision THAs are more common.1 Many acetabular revisions can be successfully performed with standard or jumbo cementless acetabular cups, but major osseous deficiencies typically require reconstruction with a cage or cup/cage that bridges gaps in the pelvis and obtains fixation of the arthroplasty components.2,3 Cages and rings have been combined with all-polyethylene acetabular components (ie, all-polyethylene cups, or APCs) to reconstruct pelvic bone defects, but complications, including APC dissociation (Figure 1) and postoperative instability, can occur despite stable fixation of cage to pelvis.4 The incidence of dislocations with pelvic reconstruction rings using APCs has been reported to be 11%.4 If an APC has to be replaced because of wear, then major surgery may be required to extract the worn cup and cement a new cup in its place.
In this article, we describe a technique in which a metal, multihole acetabular shell is cemented into the cage or ring construct, avoiding some of the complications associated with traditional techniques by permitting use of a variety of liners.
Materials and Methods
We retrospectively reviewed the cases of all of Dr. Bolanos’ patients who underwent acetabular revision THA with cage reconstruction between February 1, 1998 and October 9, 2006. During this period, we were cementing a modular metal shell into the cage instead of an APC or polyethylene liner. All patients who underwent revision THA with cage reconstruction during the study period were included. Bone defects were treated with structural or morselized bone allograft. Every reconstruction involved use of an antiprotrusio cage or ring secured to the pelvis with screws, and a multihole acetabular shell cemented into place with a polyethylene liner applied. Elevated rims, lateralized liners, and constrained liners were used as needed to optimize stability. Femoral components were retained. Cage size was based on matching the osseous deficiencies. Shell size was determined by the inner diameter of the corresponding cage. Liner size was based on matching the shell and femoral head. During this period, none of the patients had other reconstructive techniques, such as trabecular metal augmentation, in combination with a modular acetabular shell, cup/cage reconstruction, or custom triflange components.
Patients engaged in protected weight-bearing ambulation for 3 months after surgery and were then permitted full, unrestricted activity. The primary outcome was mechanical failure of the reconstruction, or reoperation (Table). All reconstructions in this series consisted of acetabular revisions for aseptic loosening.
Surgical Technique
Six consecutive cases of pelvic discontinuity and 7 cases of segmental acetabular bone loss required use of cages or rings. Reconstruction cages were used to secure fixation to the ilium and ischium. With the technique described in this article, we used screws with rounded, prominent heads rather than flat heads between the cup and the cage or ring (Synthes, 6.5 mm) to ensure adequate cement mantle. The rounded screw heads were left prominent to approximate the function of cement pegs found on APCs. Screws were placed into the anterior, superior, medial, and posterior aspects of the cage to ensure adequate cement mantle between cup and cage. This was confirmed with trial placement of the cup into the cage before cementation and observation of the uniformity of the space between cup and cage. Trial placement also confirmed that the screws did not interfere with appropriate positioning of the cup. A multihole, metal acetabular cup was then cemented in the cage or ring such that cement extruded around the shell and into the holes of the cup and the cage, securing the cup to the cage. Use of a multihole, metal shell resulted in excellent cement fixation because the multiple holes created multiple circumferential cement pegs. Various liner options could then be used to optimize stability of the reconstruction. In some cases, excessive cement extruded into the interior aspect of the shell and hardened before curettage. If the excess cement could interfere with complete seating/locking of the liner, then a high-speed burr was used to easily remove cement (Figure 2). Polyethylene liners were then inserted into the shell. Femoral reconstruction was then performed, if needed, and stability of the arthroplasty checked. This technique allows the surgeon to then select from a variety of polyethylene liners as needed to optimize stability. Liners with elevated rims, lateralized liners, and constrained liners could be interchangeable options with this technique.
Results
Thirteen patients with major osseous deficiencies of the pelvis were treated using this technique. At mean follow-up of 64.2 months (range, 3-133 months), 10 of the 13 patients had favorable outcomes without further surgery. One patient developed recurrent aseptic loosening that required re-revision, another patient developed recurrent instability that required acetabular liner and femoral head exchange, and a third patient with poor balance fell multiple times. This patient’s ninth fall resulted in dissociation of the acetabular shell from the cage (Figure 3), treated with placement of another cemented multihole metal shell with a standard liner. As dislocations recurred, the liner was changed to a constrained liner (Figure 4). The patient did not have any further dislocations or other hip-related problems. Integrity of cemented shell-cage fixation was maintained in 12 of the 13 patients at final follow-up.
Discussion
We have described a novel technique that facilitates reconstruction of major osseous deficiencies of the pelvis. The technique involves cementation of a multihole, metal acetabular shell into a cage or ring, permitting use of modular liners. The modularity in this approach to major hip reconstruction provides stability-optimization options that are not available with APCs. So far, the technique has demonstrated more advantages than disadvantages, so the indications for its use would be whenever a cage is used for pelvic reconstruction. Traditional techniques involve cementing an APC into the cage or ring. Use of multihole, metal shells for this purpose has several theoretical advantages. Multiple holes and the textured surface allow more interdigitation of cement with cup than APCs do; this interdigitation may improve the durability of the cemented interface. Cement also extrudes through the holes of the cage to secure the cup to the pelvis, as is done with cementation of APCs. Introduction of trabecular metal shells may also provide an even more secure bond to the shell, compared with APCs, though durability of a cemented trabecular metal interface has not been established. In addition, mechanical alignment guides cannot fasten as securely onto some APCs.
Nonmodular, cemented, metal-backed acetabular components, which were commonly used in hip arthroplasties at one time, were abandoned because of their relatively high loosening rate and because of advantages noted with modular components.5 The nonmodular components had been developed because of their theoretical advantages of improved distribution of forces into the cement mantle.5,6 However, those models had a relatively smooth metallic surface, which probably did not bond as well to cement as the shells used with the technique described in this article.
Dislocations can occur because of inadequately placed cups. Metallic cups can be improperly positioned, as can APCs. An advantage of the technique we have described over APCs is that liners with raised rims can be inserted with the apex placed wherever needed to best address instability. Dislocations can also occur because of factors such as inadequate offset and cognitive impairments. Our technique allows use of offset liners and constrained liners. Although these options may not prevent further dislocations, they often mitigate instability issues. Constrained liners and lateralized liners can be easily placed, and elevated rims can be swiveled as needed for stability. As use of cementless, metal-backed, modular acetabular components is common in primary THAs, most surgeons are familiar with the modular liner options available with use of the technique described in this article.
In this setting, modular, metal acetabular shells have the advantage of allowing surgeons to use the alignment guides they are accustomed to using. Modularity is another significant advantage over APCs. When an APC wears down, the component must be extracted to permit implantation of a new APC. With metal shells, a worn liner can be exchanged relatively easily. Modularity also gives surgeons many more options for addressing instability. Elevated rims can be moved, head sizes can be changed, and lateralized or constrained liners can be implanted easily. By comparison, with APCs, stability can be addressed only by modifying the femoral component or taking hip precautions which restrict range of motion of the hip. Modification of the femoral component is not possible with nonmodular femoral components in place (Figure 5). A potential disadvantage of this technique is increased cost associated with use of another component.
This small series of patients has had an excellent rate of success with cementation of multihole, metal-backed acetabular components into a cage or ring. These components may offer more secure fixation than APCs to cement extruded into the multiple holes, and improved metallurgy, such as trabecular metal. Surgeons who want to use modular components may prefer this technique because it allows them to select from various liner options. Surgeons should consider this technique for patients who need major pelvic reconstruction, though a larger study with longer follow-up is needed to determine its long-term durability.
Although the novel technique we have described has been helpful in our experience, this study had several limitations—small series, retrospective study, relatively short follow-up, lack of control group and functional data—that may have affected its conclusions. Further study and follow-up are needed to better determine the utility of this technique in clinical practice.
Although the number of total hip arthroplasties (THAs) being performed in the United States is increasing, revision THAs are more common.1 Many acetabular revisions can be successfully performed with standard or jumbo cementless acetabular cups, but major osseous deficiencies typically require reconstruction with a cage or cup/cage that bridges gaps in the pelvis and obtains fixation of the arthroplasty components.2,3 Cages and rings have been combined with all-polyethylene acetabular components (ie, all-polyethylene cups, or APCs) to reconstruct pelvic bone defects, but complications, including APC dissociation (Figure 1) and postoperative instability, can occur despite stable fixation of cage to pelvis.4 The incidence of dislocations with pelvic reconstruction rings using APCs has been reported to be 11%.4 If an APC has to be replaced because of wear, then major surgery may be required to extract the worn cup and cement a new cup in its place.
In this article, we describe a technique in which a metal, multihole acetabular shell is cemented into the cage or ring construct, avoiding some of the complications associated with traditional techniques by permitting use of a variety of liners.
Materials and Methods
We retrospectively reviewed the cases of all of Dr. Bolanos’ patients who underwent acetabular revision THA with cage reconstruction between February 1, 1998 and October 9, 2006. During this period, we were cementing a modular metal shell into the cage instead of an APC or polyethylene liner. All patients who underwent revision THA with cage reconstruction during the study period were included. Bone defects were treated with structural or morselized bone allograft. Every reconstruction involved use of an antiprotrusio cage or ring secured to the pelvis with screws, and a multihole acetabular shell cemented into place with a polyethylene liner applied. Elevated rims, lateralized liners, and constrained liners were used as needed to optimize stability. Femoral components were retained. Cage size was based on matching the osseous deficiencies. Shell size was determined by the inner diameter of the corresponding cage. Liner size was based on matching the shell and femoral head. During this period, none of the patients had other reconstructive techniques, such as trabecular metal augmentation, in combination with a modular acetabular shell, cup/cage reconstruction, or custom triflange components.
Patients engaged in protected weight-bearing ambulation for 3 months after surgery and were then permitted full, unrestricted activity. The primary outcome was mechanical failure of the reconstruction, or reoperation (Table). All reconstructions in this series consisted of acetabular revisions for aseptic loosening.
Surgical Technique
Six consecutive cases of pelvic discontinuity and 7 cases of segmental acetabular bone loss required use of cages or rings. Reconstruction cages were used to secure fixation to the ilium and ischium. With the technique described in this article, we used screws with rounded, prominent heads rather than flat heads between the cup and the cage or ring (Synthes, 6.5 mm) to ensure adequate cement mantle. The rounded screw heads were left prominent to approximate the function of cement pegs found on APCs. Screws were placed into the anterior, superior, medial, and posterior aspects of the cage to ensure adequate cement mantle between cup and cage. This was confirmed with trial placement of the cup into the cage before cementation and observation of the uniformity of the space between cup and cage. Trial placement also confirmed that the screws did not interfere with appropriate positioning of the cup. A multihole, metal acetabular cup was then cemented in the cage or ring such that cement extruded around the shell and into the holes of the cup and the cage, securing the cup to the cage. Use of a multihole, metal shell resulted in excellent cement fixation because the multiple holes created multiple circumferential cement pegs. Various liner options could then be used to optimize stability of the reconstruction. In some cases, excessive cement extruded into the interior aspect of the shell and hardened before curettage. If the excess cement could interfere with complete seating/locking of the liner, then a high-speed burr was used to easily remove cement (Figure 2). Polyethylene liners were then inserted into the shell. Femoral reconstruction was then performed, if needed, and stability of the arthroplasty checked. This technique allows the surgeon to then select from a variety of polyethylene liners as needed to optimize stability. Liners with elevated rims, lateralized liners, and constrained liners could be interchangeable options with this technique.
Results
Thirteen patients with major osseous deficiencies of the pelvis were treated using this technique. At mean follow-up of 64.2 months (range, 3-133 months), 10 of the 13 patients had favorable outcomes without further surgery. One patient developed recurrent aseptic loosening that required re-revision, another patient developed recurrent instability that required acetabular liner and femoral head exchange, and a third patient with poor balance fell multiple times. This patient’s ninth fall resulted in dissociation of the acetabular shell from the cage (Figure 3), treated with placement of another cemented multihole metal shell with a standard liner. As dislocations recurred, the liner was changed to a constrained liner (Figure 4). The patient did not have any further dislocations or other hip-related problems. Integrity of cemented shell-cage fixation was maintained in 12 of the 13 patients at final follow-up.
Discussion
We have described a novel technique that facilitates reconstruction of major osseous deficiencies of the pelvis. The technique involves cementation of a multihole, metal acetabular shell into a cage or ring, permitting use of modular liners. The modularity in this approach to major hip reconstruction provides stability-optimization options that are not available with APCs. So far, the technique has demonstrated more advantages than disadvantages, so the indications for its use would be whenever a cage is used for pelvic reconstruction. Traditional techniques involve cementing an APC into the cage or ring. Use of multihole, metal shells for this purpose has several theoretical advantages. Multiple holes and the textured surface allow more interdigitation of cement with cup than APCs do; this interdigitation may improve the durability of the cemented interface. Cement also extrudes through the holes of the cage to secure the cup to the pelvis, as is done with cementation of APCs. Introduction of trabecular metal shells may also provide an even more secure bond to the shell, compared with APCs, though durability of a cemented trabecular metal interface has not been established. In addition, mechanical alignment guides cannot fasten as securely onto some APCs.
Nonmodular, cemented, metal-backed acetabular components, which were commonly used in hip arthroplasties at one time, were abandoned because of their relatively high loosening rate and because of advantages noted with modular components.5 The nonmodular components had been developed because of their theoretical advantages of improved distribution of forces into the cement mantle.5,6 However, those models had a relatively smooth metallic surface, which probably did not bond as well to cement as the shells used with the technique described in this article.
Dislocations can occur because of inadequately placed cups. Metallic cups can be improperly positioned, as can APCs. An advantage of the technique we have described over APCs is that liners with raised rims can be inserted with the apex placed wherever needed to best address instability. Dislocations can also occur because of factors such as inadequate offset and cognitive impairments. Our technique allows use of offset liners and constrained liners. Although these options may not prevent further dislocations, they often mitigate instability issues. Constrained liners and lateralized liners can be easily placed, and elevated rims can be swiveled as needed for stability. As use of cementless, metal-backed, modular acetabular components is common in primary THAs, most surgeons are familiar with the modular liner options available with use of the technique described in this article.
In this setting, modular, metal acetabular shells have the advantage of allowing surgeons to use the alignment guides they are accustomed to using. Modularity is another significant advantage over APCs. When an APC wears down, the component must be extracted to permit implantation of a new APC. With metal shells, a worn liner can be exchanged relatively easily. Modularity also gives surgeons many more options for addressing instability. Elevated rims can be moved, head sizes can be changed, and lateralized or constrained liners can be implanted easily. By comparison, with APCs, stability can be addressed only by modifying the femoral component or taking hip precautions which restrict range of motion of the hip. Modification of the femoral component is not possible with nonmodular femoral components in place (Figure 5). A potential disadvantage of this technique is increased cost associated with use of another component.
This small series of patients has had an excellent rate of success with cementation of multihole, metal-backed acetabular components into a cage or ring. These components may offer more secure fixation than APCs to cement extruded into the multiple holes, and improved metallurgy, such as trabecular metal. Surgeons who want to use modular components may prefer this technique because it allows them to select from various liner options. Surgeons should consider this technique for patients who need major pelvic reconstruction, though a larger study with longer follow-up is needed to determine its long-term durability.
Although the novel technique we have described has been helpful in our experience, this study had several limitations—small series, retrospective study, relatively short follow-up, lack of control group and functional data—that may have affected its conclusions. Further study and follow-up are needed to better determine the utility of this technique in clinical practice.
1. Kurtz SM, Ong KL, Schmier J, Zhao K, Mowat F, Lau E. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24(2):195-203.
2. Berry DJ, Lewallen DG, Hanssen AD, Cabanela ME. Pelvic discontinuity in revision total hip arthroplasty. J Bone Joint Surg Am. 1999;81(12):1692-1702.
3. Pieringer H, Auersperg V, Böhler N. Reconstruction of severe acetabular bone-deficiency: the Burch-Schneider antiprotrusio cage in primary and revision total hip arthroplasty. J Arthroplasty. 2006;21(4):489-496.
4. Goodman S, Saastamoinen H, Shasha N, Gross A. Complications of ilioischial reconstruction rings in revision total hip arthroplasty. J Arthroplasty. 2004;19(4):436-446.
5. Cates HE, Faris PM, Keating EM, Ritter MA. Polyethylene wear in cemented metal-backed acetabular cups. J Bone Joint Surg Br. 1993;75(2):249-253.
6. Vasu R, Carter DR, Harris WH. Stress distribution in the acetabular region—I. Before and after total joint replacement. J Biomech. 1982;15(3):155-164.
1. Kurtz SM, Ong KL, Schmier J, Zhao K, Mowat F, Lau E. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24(2):195-203.
2. Berry DJ, Lewallen DG, Hanssen AD, Cabanela ME. Pelvic discontinuity in revision total hip arthroplasty. J Bone Joint Surg Am. 1999;81(12):1692-1702.
3. Pieringer H, Auersperg V, Böhler N. Reconstruction of severe acetabular bone-deficiency: the Burch-Schneider antiprotrusio cage in primary and revision total hip arthroplasty. J Arthroplasty. 2006;21(4):489-496.
4. Goodman S, Saastamoinen H, Shasha N, Gross A. Complications of ilioischial reconstruction rings in revision total hip arthroplasty. J Arthroplasty. 2004;19(4):436-446.
5. Cates HE, Faris PM, Keating EM, Ritter MA. Polyethylene wear in cemented metal-backed acetabular cups. J Bone Joint Surg Br. 1993;75(2):249-253.
6. Vasu R, Carter DR, Harris WH. Stress distribution in the acetabular region—I. Before and after total joint replacement. J Biomech. 1982;15(3):155-164.
How to Teach the Potassium Hydroxide Preparation: A Disappearing Clinical Art Form
Potassium hydroxide (KOH) preparations remain an important bedside test for prompt and accurate diagnosis of superficial fungal infections known as dermatophytoses. This tool has been used for at least 100 years, with early terminology referring to it as potash; for the last century, it has largely been a technique passed down as a skill from master technician to learning apprentice. The original pioneer of the KOH preparation remains a mystery.1
Variations on techniques for performing the KOH preparation exist, and tips and tricks on the use of this test are a hot topic among dermatologists.2 Although primary care and dermatology-specific publications espouse the importance of the KOH preparation,3,4 it has unfortunately been identified and labeled as one of the forgotten diagnostic tools.5
It is incumbent on dermatologists to educate medical students and residents using a simple and specific method to ensure that this simple and effective technique, with sensitivity reported between 87% and 91% depending on the expertise of the examiner,6 remains part of the clinical armamentarium. One concern in the instruction of large groups of students and clinicians is the ready accessibility or availability of viable skin samples. This article describes a method of collecting and storing skin samples that will allow educators to train large groups of students on performing KOH preparations without having to repeatedly seek skin samples or patients with superficial skin infections. A detailed description of the pedagogy used to teach the preparation and interpretation of KOH slides to a large group of students also is reviewed.
Specimen Collection
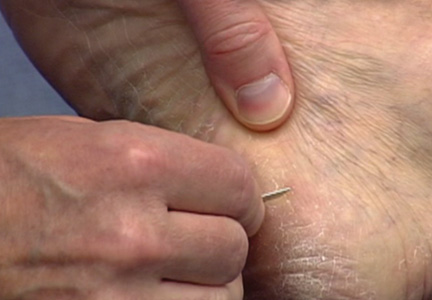
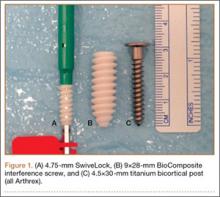
The first step in teaching the KOH preparation to a large group is the collection of a suitable number of skin scrapings from patients with a superficial fungal skin infection (eg, tinea corporis, tinea versicolor). A common technique for obtaining skin samples is to use a no. 15 scalpel blade (Figure 1) to scrape the scale of the lesion at its scaly border once the area is moistened with an alcohol pad or soap and water.7 The moisture from the alcohol pad allows the scale to stick to the no. 15 blade, facilitating collection. Once a suitable amount of scale is collected, it is placed on a glass microscope slide by smearing the scale from the blade onto the slide. This process has been modified to facilitate a larger quantity of specimen as follows: dermatophyte-infected plaques with scale are rubbed with the no. 15 blade and the free scale drops into a standard urine specimen cup. This process is repeated multiple times from different sites to capture the displaced scale with the dermatophyte. We have found that as long as the specimen cups are sealed tightly and stored in a relatively dry and cool environment (room temperature), the samples can be used to construct KOH teaching slides for at least 3 years. We have not used them beyond 3 years but suspect that they would continue to be viable after this time.
Preparation of Slides
Given that time for teaching often is limited, it is beneficial to fix many skin scrapings on a large number of glass slides prior to the session, which enables students to simply add KOH to the slides on the teaching day. To prepare the slides in advance, it is necessary to gather the following materials: a specimen cup with skin samples, glass slides, pickups or tweezers, a small pipette, a cup of water, protective gloves, and a pencil. After donning protective gloves, the pickups or tweezers are used to retrieve a few flakes of scale from the specimen cup and place them on the center of a glass slide. Using the pipette, 1 or 2 drops of water are added to the scale, and the slide is then allowed to dry. The slides are marked with the pencil to indicate the “up” side to prevent the students from applying KOH solution to the wrong side of the slide. The skin scale is fixed in place on the slide as the water evaporates and may be stored until needed for use in a standard slide box or folder.
Performing the KOH Preparation
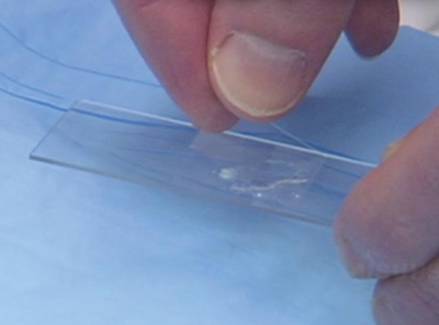
On the day of teaching, it is helpful to engage the entire group of students with an introductory lecture on the purpose and use of the KOH preparation. Upon completion, students move to a workstation with all of the materials needed to prepare the slide. Additional items needed at this time are 10% KOH solution, coverslips, and a heating device (eg, lighter, Bunsen burner, match)(optional). Students are instructed to place 1 or 2 skin scales onto a glass slide or retrieve a slide with skin scales already fixed, and then add 1 drop of 10% KOH solution directly to the sample (Figure 2). Next, they should place a slide coverslip onto the KOH drop and skin sample using a side-to-side technique that will move the scale into a thin layer within the KOH solution and push away any excess solution to the periphery (Figure 3). Large amounts of excess KOH solution should be cleared away with a paper towel, lens paper, or tissue. The heat source can be used to gently heat the underside of the glass slide (Figure 4), but it often is sufficient to simply wait 3 to 5 minutes for the KOH solution to take effect. The heat accelerates the maceration of the scale and makes it easier to see the hyphae among the keratinocytes. Some physicians advocate the use of dimethyl sulfoxide in lieu of heating,8 but this solution may not be available in all primary care settings.
 |  |
Microscopic Examination
Prior to examining the slides under the microscope, students may complete a self-guided tutorial (eg, digital or paper slide show) on the various features seen through the microscope that are indicative of dermatophytes, including branching hyphae and yeast buds. They also should be educated about the common appearance of artifacts that may resemble hyphae. Once the students have completed the tutorial, they may proceed to microscopic examination.
While the students are viewing their slides under the microscope, we find it helpful to have at least 1 experienced faculty member for every group of 10 students. This instructor should encourage the students to lower the microscope condenser all the way to facilitate better observation. Students should start with low power (×4 or red band) and scan for areas that are rich in skin scale. Once a collection of scale is found, the student can switch to higher power (×10 or yellow band) and start scanning for hyphae. Students should be reminded to search for filamentous and branching tubes that are refractile. The term refractile may be confusing to some students, so we explain that shifting the focus up or down will show the hyphae to change in brightness and may reveal a greenish tint. Another helpful indicator to point out is the feature that hyphae will cross the border of epidermal skin cells, whereas artifacts will not (Figure 5). Once the students have identified evidence of a dermatophyte infection, they must call the instructor to their station to verify the presence of hyphae or yeast buds, which helps confirm their understanding of the procedure. Once the student accurately identifies these items, the session is complete.
Comment
The use of a KOH preparation is a fast, simple, accurate, and cost-effective way to diagnose superficial fungal infections; however, because of insufficient familiarity with this tool, the technique often is replaced by initiation of empiric antifungal therapy in patients with suspected dermatophytosis. This empiric treatment has the potential to delay appropriate diagnosis and treatment (eg, in a patient with nummular dermatitis, which can clinically mimic tinea corporis). One way to encourage the use of the KOH preparation in the primary care and dermatologic setting is to educate large groups of next-generation physicians while in medical training. This article describes a teaching technique that allows for long-term storage of positive skin samples and a detailed description of the pedagogy used to train and educate a large group of students in a relatively short period of time.
All KOH preparations fall under the US federal government’s Clinical Laboratory Improvement Amendments and require proficiency testing.9 Although the teaching method presented here is designed for teaching medical students, it may be utilized to educate or refamiliarize experienced physicians with the procedure in an effort to improve proficiency in point-of-care testing programs used in many health care systems to comply with the Clinical Laboratories Improvement Amendments. Future analyses could assess whether the method described here improves provider performance on such proficiency measures and whether it ultimately helps ensure quality patient care.
1. Dasgupta T, Sahu J. Origins of the KOH technique. Clin Dermatol. 2012;2:238-242.
2. Stone S. Editor’s commentary. Clin Dermatol. 2012;2:241-242.
3. Monroe JR. The diagnostic value of a KOH. JAAPA. 2001;4:50-51.
4. Hainer BL. Dermatophyte infections. Am Fam Physician. 2003;1:101-109.
5. Ponka D, Baddar F. Microscopic potassium hydroxide preparation. Can Fam Physician. 2014;60:57.
6. Lilly KK, Koshnick RL, Grill JP, et al. Cost-effectiveness of diagnostic tests for toenail onychomycosis: a repeated-measure, single-blinded, cross-sectional evaluation of 7 diagnostic tests. J Am Acad Dermatol. 2006;4:620-626.
7. Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. New York, NY: Elsevier Saunders; 2012.
8. James WD, Berger T, Elston D. Andrew’s Diseases of the Skin: Clinical Dermatology. 11th ed. New York, NY: Elsevier Saunders; 2011.
9. Clinical Laboratory Improvement Amendments (CLIA). Centers for Medicare & Medicaid Services Web site. https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/index.html?redirect=/clia/. Updated June 6, 2015. Accessed July 21, 2015.
Potassium hydroxide (KOH) preparations remain an important bedside test for prompt and accurate diagnosis of superficial fungal infections known as dermatophytoses. This tool has been used for at least 100 years, with early terminology referring to it as potash; for the last century, it has largely been a technique passed down as a skill from master technician to learning apprentice. The original pioneer of the KOH preparation remains a mystery.1
Variations on techniques for performing the KOH preparation exist, and tips and tricks on the use of this test are a hot topic among dermatologists.2 Although primary care and dermatology-specific publications espouse the importance of the KOH preparation,3,4 it has unfortunately been identified and labeled as one of the forgotten diagnostic tools.5
It is incumbent on dermatologists to educate medical students and residents using a simple and specific method to ensure that this simple and effective technique, with sensitivity reported between 87% and 91% depending on the expertise of the examiner,6 remains part of the clinical armamentarium. One concern in the instruction of large groups of students and clinicians is the ready accessibility or availability of viable skin samples. This article describes a method of collecting and storing skin samples that will allow educators to train large groups of students on performing KOH preparations without having to repeatedly seek skin samples or patients with superficial skin infections. A detailed description of the pedagogy used to teach the preparation and interpretation of KOH slides to a large group of students also is reviewed.
Specimen Collection
The first step in teaching the KOH preparation to a large group is the collection of a suitable number of skin scrapings from patients with a superficial fungal skin infection (eg, tinea corporis, tinea versicolor). A common technique for obtaining skin samples is to use a no. 15 scalpel blade (Figure 1) to scrape the scale of the lesion at its scaly border once the area is moistened with an alcohol pad or soap and water.7 The moisture from the alcohol pad allows the scale to stick to the no. 15 blade, facilitating collection. Once a suitable amount of scale is collected, it is placed on a glass microscope slide by smearing the scale from the blade onto the slide. This process has been modified to facilitate a larger quantity of specimen as follows: dermatophyte-infected plaques with scale are rubbed with the no. 15 blade and the free scale drops into a standard urine specimen cup. This process is repeated multiple times from different sites to capture the displaced scale with the dermatophyte. We have found that as long as the specimen cups are sealed tightly and stored in a relatively dry and cool environment (room temperature), the samples can be used to construct KOH teaching slides for at least 3 years. We have not used them beyond 3 years but suspect that they would continue to be viable after this time.
Preparation of Slides
Given that time for teaching often is limited, it is beneficial to fix many skin scrapings on a large number of glass slides prior to the session, which enables students to simply add KOH to the slides on the teaching day. To prepare the slides in advance, it is necessary to gather the following materials: a specimen cup with skin samples, glass slides, pickups or tweezers, a small pipette, a cup of water, protective gloves, and a pencil. After donning protective gloves, the pickups or tweezers are used to retrieve a few flakes of scale from the specimen cup and place them on the center of a glass slide. Using the pipette, 1 or 2 drops of water are added to the scale, and the slide is then allowed to dry. The slides are marked with the pencil to indicate the “up” side to prevent the students from applying KOH solution to the wrong side of the slide. The skin scale is fixed in place on the slide as the water evaporates and may be stored until needed for use in a standard slide box or folder.
Performing the KOH Preparation
On the day of teaching, it is helpful to engage the entire group of students with an introductory lecture on the purpose and use of the KOH preparation. Upon completion, students move to a workstation with all of the materials needed to prepare the slide. Additional items needed at this time are 10% KOH solution, coverslips, and a heating device (eg, lighter, Bunsen burner, match)(optional). Students are instructed to place 1 or 2 skin scales onto a glass slide or retrieve a slide with skin scales already fixed, and then add 1 drop of 10% KOH solution directly to the sample (Figure 2). Next, they should place a slide coverslip onto the KOH drop and skin sample using a side-to-side technique that will move the scale into a thin layer within the KOH solution and push away any excess solution to the periphery (Figure 3). Large amounts of excess KOH solution should be cleared away with a paper towel, lens paper, or tissue. The heat source can be used to gently heat the underside of the glass slide (Figure 4), but it often is sufficient to simply wait 3 to 5 minutes for the KOH solution to take effect. The heat accelerates the maceration of the scale and makes it easier to see the hyphae among the keratinocytes. Some physicians advocate the use of dimethyl sulfoxide in lieu of heating,8 but this solution may not be available in all primary care settings.
 |  |
Microscopic Examination
Prior to examining the slides under the microscope, students may complete a self-guided tutorial (eg, digital or paper slide show) on the various features seen through the microscope that are indicative of dermatophytes, including branching hyphae and yeast buds. They also should be educated about the common appearance of artifacts that may resemble hyphae. Once the students have completed the tutorial, they may proceed to microscopic examination.
While the students are viewing their slides under the microscope, we find it helpful to have at least 1 experienced faculty member for every group of 10 students. This instructor should encourage the students to lower the microscope condenser all the way to facilitate better observation. Students should start with low power (×4 or red band) and scan for areas that are rich in skin scale. Once a collection of scale is found, the student can switch to higher power (×10 or yellow band) and start scanning for hyphae. Students should be reminded to search for filamentous and branching tubes that are refractile. The term refractile may be confusing to some students, so we explain that shifting the focus up or down will show the hyphae to change in brightness and may reveal a greenish tint. Another helpful indicator to point out is the feature that hyphae will cross the border of epidermal skin cells, whereas artifacts will not (Figure 5). Once the students have identified evidence of a dermatophyte infection, they must call the instructor to their station to verify the presence of hyphae or yeast buds, which helps confirm their understanding of the procedure. Once the student accurately identifies these items, the session is complete.
Comment
The use of a KOH preparation is a fast, simple, accurate, and cost-effective way to diagnose superficial fungal infections; however, because of insufficient familiarity with this tool, the technique often is replaced by initiation of empiric antifungal therapy in patients with suspected dermatophytosis. This empiric treatment has the potential to delay appropriate diagnosis and treatment (eg, in a patient with nummular dermatitis, which can clinically mimic tinea corporis). One way to encourage the use of the KOH preparation in the primary care and dermatologic setting is to educate large groups of next-generation physicians while in medical training. This article describes a teaching technique that allows for long-term storage of positive skin samples and a detailed description of the pedagogy used to train and educate a large group of students in a relatively short period of time.
All KOH preparations fall under the US federal government’s Clinical Laboratory Improvement Amendments and require proficiency testing.9 Although the teaching method presented here is designed for teaching medical students, it may be utilized to educate or refamiliarize experienced physicians with the procedure in an effort to improve proficiency in point-of-care testing programs used in many health care systems to comply with the Clinical Laboratories Improvement Amendments. Future analyses could assess whether the method described here improves provider performance on such proficiency measures and whether it ultimately helps ensure quality patient care.
Potassium hydroxide (KOH) preparations remain an important bedside test for prompt and accurate diagnosis of superficial fungal infections known as dermatophytoses. This tool has been used for at least 100 years, with early terminology referring to it as potash; for the last century, it has largely been a technique passed down as a skill from master technician to learning apprentice. The original pioneer of the KOH preparation remains a mystery.1
Variations on techniques for performing the KOH preparation exist, and tips and tricks on the use of this test are a hot topic among dermatologists.2 Although primary care and dermatology-specific publications espouse the importance of the KOH preparation,3,4 it has unfortunately been identified and labeled as one of the forgotten diagnostic tools.5
It is incumbent on dermatologists to educate medical students and residents using a simple and specific method to ensure that this simple and effective technique, with sensitivity reported between 87% and 91% depending on the expertise of the examiner,6 remains part of the clinical armamentarium. One concern in the instruction of large groups of students and clinicians is the ready accessibility or availability of viable skin samples. This article describes a method of collecting and storing skin samples that will allow educators to train large groups of students on performing KOH preparations without having to repeatedly seek skin samples or patients with superficial skin infections. A detailed description of the pedagogy used to teach the preparation and interpretation of KOH slides to a large group of students also is reviewed.
Specimen Collection
The first step in teaching the KOH preparation to a large group is the collection of a suitable number of skin scrapings from patients with a superficial fungal skin infection (eg, tinea corporis, tinea versicolor). A common technique for obtaining skin samples is to use a no. 15 scalpel blade (Figure 1) to scrape the scale of the lesion at its scaly border once the area is moistened with an alcohol pad or soap and water.7 The moisture from the alcohol pad allows the scale to stick to the no. 15 blade, facilitating collection. Once a suitable amount of scale is collected, it is placed on a glass microscope slide by smearing the scale from the blade onto the slide. This process has been modified to facilitate a larger quantity of specimen as follows: dermatophyte-infected plaques with scale are rubbed with the no. 15 blade and the free scale drops into a standard urine specimen cup. This process is repeated multiple times from different sites to capture the displaced scale with the dermatophyte. We have found that as long as the specimen cups are sealed tightly and stored in a relatively dry and cool environment (room temperature), the samples can be used to construct KOH teaching slides for at least 3 years. We have not used them beyond 3 years but suspect that they would continue to be viable after this time.
Preparation of Slides
Given that time for teaching often is limited, it is beneficial to fix many skin scrapings on a large number of glass slides prior to the session, which enables students to simply add KOH to the slides on the teaching day. To prepare the slides in advance, it is necessary to gather the following materials: a specimen cup with skin samples, glass slides, pickups or tweezers, a small pipette, a cup of water, protective gloves, and a pencil. After donning protective gloves, the pickups or tweezers are used to retrieve a few flakes of scale from the specimen cup and place them on the center of a glass slide. Using the pipette, 1 or 2 drops of water are added to the scale, and the slide is then allowed to dry. The slides are marked with the pencil to indicate the “up” side to prevent the students from applying KOH solution to the wrong side of the slide. The skin scale is fixed in place on the slide as the water evaporates and may be stored until needed for use in a standard slide box or folder.
Performing the KOH Preparation
On the day of teaching, it is helpful to engage the entire group of students with an introductory lecture on the purpose and use of the KOH preparation. Upon completion, students move to a workstation with all of the materials needed to prepare the slide. Additional items needed at this time are 10% KOH solution, coverslips, and a heating device (eg, lighter, Bunsen burner, match)(optional). Students are instructed to place 1 or 2 skin scales onto a glass slide or retrieve a slide with skin scales already fixed, and then add 1 drop of 10% KOH solution directly to the sample (Figure 2). Next, they should place a slide coverslip onto the KOH drop and skin sample using a side-to-side technique that will move the scale into a thin layer within the KOH solution and push away any excess solution to the periphery (Figure 3). Large amounts of excess KOH solution should be cleared away with a paper towel, lens paper, or tissue. The heat source can be used to gently heat the underside of the glass slide (Figure 4), but it often is sufficient to simply wait 3 to 5 minutes for the KOH solution to take effect. The heat accelerates the maceration of the scale and makes it easier to see the hyphae among the keratinocytes. Some physicians advocate the use of dimethyl sulfoxide in lieu of heating,8 but this solution may not be available in all primary care settings.
 |  |
Microscopic Examination
Prior to examining the slides under the microscope, students may complete a self-guided tutorial (eg, digital or paper slide show) on the various features seen through the microscope that are indicative of dermatophytes, including branching hyphae and yeast buds. They also should be educated about the common appearance of artifacts that may resemble hyphae. Once the students have completed the tutorial, they may proceed to microscopic examination.
While the students are viewing their slides under the microscope, we find it helpful to have at least 1 experienced faculty member for every group of 10 students. This instructor should encourage the students to lower the microscope condenser all the way to facilitate better observation. Students should start with low power (×4 or red band) and scan for areas that are rich in skin scale. Once a collection of scale is found, the student can switch to higher power (×10 or yellow band) and start scanning for hyphae. Students should be reminded to search for filamentous and branching tubes that are refractile. The term refractile may be confusing to some students, so we explain that shifting the focus up or down will show the hyphae to change in brightness and may reveal a greenish tint. Another helpful indicator to point out is the feature that hyphae will cross the border of epidermal skin cells, whereas artifacts will not (Figure 5). Once the students have identified evidence of a dermatophyte infection, they must call the instructor to their station to verify the presence of hyphae or yeast buds, which helps confirm their understanding of the procedure. Once the student accurately identifies these items, the session is complete.
Comment
The use of a KOH preparation is a fast, simple, accurate, and cost-effective way to diagnose superficial fungal infections; however, because of insufficient familiarity with this tool, the technique often is replaced by initiation of empiric antifungal therapy in patients with suspected dermatophytosis. This empiric treatment has the potential to delay appropriate diagnosis and treatment (eg, in a patient with nummular dermatitis, which can clinically mimic tinea corporis). One way to encourage the use of the KOH preparation in the primary care and dermatologic setting is to educate large groups of next-generation physicians while in medical training. This article describes a teaching technique that allows for long-term storage of positive skin samples and a detailed description of the pedagogy used to train and educate a large group of students in a relatively short period of time.
All KOH preparations fall under the US federal government’s Clinical Laboratory Improvement Amendments and require proficiency testing.9 Although the teaching method presented here is designed for teaching medical students, it may be utilized to educate or refamiliarize experienced physicians with the procedure in an effort to improve proficiency in point-of-care testing programs used in many health care systems to comply with the Clinical Laboratories Improvement Amendments. Future analyses could assess whether the method described here improves provider performance on such proficiency measures and whether it ultimately helps ensure quality patient care.
1. Dasgupta T, Sahu J. Origins of the KOH technique. Clin Dermatol. 2012;2:238-242.
2. Stone S. Editor’s commentary. Clin Dermatol. 2012;2:241-242.
3. Monroe JR. The diagnostic value of a KOH. JAAPA. 2001;4:50-51.
4. Hainer BL. Dermatophyte infections. Am Fam Physician. 2003;1:101-109.
5. Ponka D, Baddar F. Microscopic potassium hydroxide preparation. Can Fam Physician. 2014;60:57.
6. Lilly KK, Koshnick RL, Grill JP, et al. Cost-effectiveness of diagnostic tests for toenail onychomycosis: a repeated-measure, single-blinded, cross-sectional evaluation of 7 diagnostic tests. J Am Acad Dermatol. 2006;4:620-626.
7. Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. New York, NY: Elsevier Saunders; 2012.
8. James WD, Berger T, Elston D. Andrew’s Diseases of the Skin: Clinical Dermatology. 11th ed. New York, NY: Elsevier Saunders; 2011.
9. Clinical Laboratory Improvement Amendments (CLIA). Centers for Medicare & Medicaid Services Web site. https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/index.html?redirect=/clia/. Updated June 6, 2015. Accessed July 21, 2015.
1. Dasgupta T, Sahu J. Origins of the KOH technique. Clin Dermatol. 2012;2:238-242.
2. Stone S. Editor’s commentary. Clin Dermatol. 2012;2:241-242.
3. Monroe JR. The diagnostic value of a KOH. JAAPA. 2001;4:50-51.
4. Hainer BL. Dermatophyte infections. Am Fam Physician. 2003;1:101-109.
5. Ponka D, Baddar F. Microscopic potassium hydroxide preparation. Can Fam Physician. 2014;60:57.
6. Lilly KK, Koshnick RL, Grill JP, et al. Cost-effectiveness of diagnostic tests for toenail onychomycosis: a repeated-measure, single-blinded, cross-sectional evaluation of 7 diagnostic tests. J Am Acad Dermatol. 2006;4:620-626.
7. Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. New York, NY: Elsevier Saunders; 2012.
8. James WD, Berger T, Elston D. Andrew’s Diseases of the Skin: Clinical Dermatology. 11th ed. New York, NY: Elsevier Saunders; 2011.
9. Clinical Laboratory Improvement Amendments (CLIA). Centers for Medicare & Medicaid Services Web site. https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/index.html?redirect=/clia/. Updated June 6, 2015. Accessed July 21, 2015.
Practice Points
- Potassium hydroxide (KOH) preparations can lead to diagnostic confidence and direct appropriate therapy.
- Refreshing the basics of this simple technique can lead to better patient outcomes in the primary care setting and in the dermatology specialty clinic.
- Teaching the KOH preparation to the next generation of physicians will ensure its longevity and assure future benefit to patients.
Using Plate Osteosynthesis to Treat Isolated Greater Tuberosity Fractures
Proximal humerus fractures are the second most common fracture in the upper extremity, accounting for 4% to 5% of all fractures.1-4 The majority of these injuries can be treated without an operation. For fractures that require surgery, there are multiple options, including closed reduction, percutaneous pinning, open reduction and internal fixation (ORIF), hemiarthroplasty, and reverse total shoulder arthroplasty.3-9
Isolated greater tuberosity fractures (AO [Arbeitsgemeinschaft für Osteosynthesefragen] 11.A1) make up a small subset of proximal humerus fractures. In general, patients who sustain an isolated greater tuberosity fracture are younger and more active than those who sustain other proximal humerus fractures.2,10 As a result, in the treatment of greater tuberosity fractures, there is increased emphasis on return to high activity and function. Nondisplaced or minimally displaced fractures typically are treated nonoperatively with good success.11,12 Patients with fractures displaced more than 5 mm, and highly active patients with fractures displaced more than 3 mm, usually are recommended for surgical treatment.2,11-14 The many options for treating these difficult fractures include suture fixation, percutaneous techniques, screw fixation, and, more recently, arthroscopic suture techniques.2,5,13,15,16 The goal of any of these operative interventions is to restore normal function and minimize pain around the injured shoulder. Although most of the operative techniques for greater tuberosity fractures have predictable results, none has been established as the gold standard for the treatment of displaced greater tuberosity fractures.2,5,13,15-18 Use of plate osteosynthesis for displaced proximal humerus fractures not isolated to the greater tuberosity is becoming more widespread in the orthopedic community.1,4,19,20 However, the orthopedic literature includes very few reports of using this technique for isolated displaced greater tuberosity fractures.18 This surgical approach potentially provides increased stability, improved maintenance of reduction, and earlier range of motion (ROM) in the postoperative period. These outcomes in turn may allow for improved pain control and earlier return to normal activities than is the case with other operative interventions for these difficult injuries.
We conducted a study to determine the radiographic and clinical outcomes of plate osteosynthesis for displaced greater tuberosity fractures. We hypothesized that excellent clinical and radiographic outcomes could be achieved using this surgical technique.
Patients and Methods
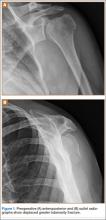
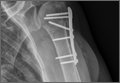
After obtaining institutional review board approval for this study, we retrospectively identified 11 consecutive patients with an isolated displaced greater tuberosity fracture (AO 11.A1) treated with plate osteosynthesis by Dr. Getz between December 2009 and May 2011 (Figures 1A, 1B). We collected data on age at time of surgery, sex, length of follow-up, worker’s compensation status, and complications. At a minimum of 21 months (mean, 27 months; SD, 8 months; range, 16-44 months), we assessed ROM and administered validated outcome scores, including the Single Assessment Numeric Evaluation (SANE)21,22 and the Penn Shoulder Score (PSS).23
Surgical Technique
The deltopectoral approach was used in all 11 patients. A standard incision was made over the deltopectoral interval starting at the coracoid and extending about 6 cm toward the deltoid insertion. After the internervous plane was entered between the deltoid and pectoralis major, the clavipectoral fascia was divided. The greater tuberosity fracture was identified with the leading edge of the fracture 1 cm posterior to the bicipital groove in all cases. Organized hematoma was removed from the fracture site to allow reduction. Three 1-mm braided polyester tapes were placed into the rotator cuff at the insertion onto the greater tuberosity fragment. The sutures thus captured the fragment and were used to obtain reduction and fixation. The fragment was provisionally pinned by placing a 2.0-mm Kirschner wire high on the fragment as to not block plate application. Fluoroscopic imaging was used to determine the appropriate position of the fracture reduction. A standard periarticular proximal humerus 3.5-mm locking compression plate (Zimmer) was used in all patients. The plate was contoured to achieve more compression in several cases in which plastic deformation or comminution was present. The sutures that were attached to the greater tuberosity were then brought through the plate. The plate was then slid down onto the humerus and pinned under fluoroscopic guidance. Three bicortical screws were used to affix the plate to the humeral shaft to compress the fracture into the fracture bed. Two to 4 locking screws were placed into the humeral head to improve the rotational stability of the construct. Last, the sutures through the plate were tied for added fixation.
Rehabilitation
In the immediate postoperative period, all patients were placed in a standard shoulder sling. The sling was worn for 6 weeks. At 2 weeks, patients started formal, standardized physical therapy, including passive ROM for elevation and external rotation. At 6 weeks, they began internal rotation stretching and active-assisted motion. Cuff strengthening began gently, as motion and pain allowed, after 8 weeks. Formal physical therapy continued until full or maximal improvement in motion and strength had been achieved.
Radiographic Measurements
Union/malunion was assessed by 2 orthopedic surgeons during their fellowship year in shoulder and elbow surgery. These surgeons were blinded to patients’ clinical outcomes. Each surgeon reviewed each patient’s radiographs twice to determine whether the reduction was anatomical. Anatomical reduction was achieved if the greater-tuberosity-to-head height was between 4 and 10 mm. Malunion was defined as loss of more than 3 mm of anatomical fracture reduction (from the original reduction) on any radiologic view at most recent follow-up. Loss of reduction was considered minimal if the fracture fragment was displaced less than 3 mm.
Statistical Analysis
A descriptive analysis of patient variables and outcomes was used for this small cohort of patients. Statistical significance was set at α = 0.05.
Results
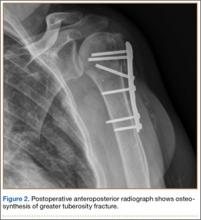
Eleven patients (7 women, 4 men) underwent plate osteosynthesis for an isolated greater tuberosity fracture (Figure 2). Mean age at surgery was 60 years (range, 37-71 years). All patients were right-hand–dominant; 7 of the 11 sustained the injury on the dominant side. For all 11 patients, final postoperative ROM and complications were recorded. No patient required additional surgery. Before injury, all patients felt their shoulder was 100% normal. Nine of the 11 patients were available for assessment of functional outcome and ROM at a mean (SD) of 27 (8) months (range, 16-44 months). At final follow-up, mean (SD) forward elevation was 147° (28°; range, 100°-180°), and mean (SD) external rotation was 25° (15°; range, 10°-60°). Mean (SD) SANE score was 72 (17; range, 50-90), and mean (SD) PSS was 79 (16; range 43-90). On a 1-to-10 scale, patients’ mean (SD) overall satisfaction was 8.6 (1.9; range, 4-10). Of the 9 patients who worked before injury, 8 returned to preoperative duty. Six patients reported stiffness (consistent with ROM). All patients said they would have the surgery again (Table).
All patients experienced radiographic union. Three of the 11 had minimal (<3 mm) loss of reduction. Mean (SD) time to union was 10.7 (4.2) weeks (range, 6.1-21.6 weeks). There were no wound complications and no need for any hardware removal.
Discussion
Isolated greater tuberosity fractures are less common than other types of proximal humerus fractures but often require surgical intervention for less displacement when compared with those fractures.2,14 Multiple techniques (eg, suture fixation, percutaneous pinning, arthroscopic techniques) have been used, but none has established itself as the gold standard for treatment of these difficult injuries.2,5,9,11,13-16 The results of the present study show that plate osteosynthesis can reliably be used to achieve anatomical reduction and good functional outcomes in isolated greater tuberosity fractures. Even with the added stability of the plate and suture construct, a small number of fractures still displaced. In addition, despite having achieved anatomical union, many patients in this study experienced stiffness and functional loss, which speaks to the challenges associated with management of these fractures.
Self-reported outcomes were less favorable for patients in our study (despite achieving mean forward elevation of 147°) than for patients who underwent greater tuberosity repair in other studies.2,5,10 In a study of 12 patients who underwent ORIF of a 2-part displaced fracture of the greater tuberosity of the proximal part of the humerus, Flatow and colleagues5 found half the patients had an excellent outcome, and the other half had a good outcome with active elevation averaging 170°. In another study, conducted over 11 years, 165 patients with a proximal humeral fracture were treated with transosseous suture fixation. Union occurred in all patients except the 2 patients with 3-part fractures, and 155 patients had excellent or very good fracture reduction.10 Therefore, final ROM for these patients may not be a good indicator of actual final function, and previous reports likely underestimated the functional loss experienced by these patients.
The incidence of isolated greater tuberosity fractures likely will increase as the population ages and becomes more active.2,14,16 Patients with isolated greater tuberosity fractures are more likely to be male, to be younger, and to have fewer medical problems than patients with other types of proximal humerus fractures.14 In addition, patient expectations regarding life after displaced greater tuberosity fractures are unique compared with expectations of patients who have other proximal humerus fractures; displaced greater tuberosity fractures usually occur in more active patients, who may expect to return to work and may place higher demands on themselves after treatment,2,14,16,24 possibly leading to lower subjective clinical outcomes.
Various operative treatment techniques for isolated greater tuberosity fractures have been described. Flatow and colleagues5 reported excellent return of forward elevation after ORIF with heavy suture, and half the patients reported excellent outcomes. Other techniques have had mixed results. Bhatia and colleagues11 reported on long-term outcomes of internal fixation using a double row of suture anchors in isolated, displaced greater tuberosity fractures in 21 patients. Outcomes were rated excellent in 8 patients, good in 10, satisfactory in 2, and unsatisfactory in 1. Braunstein and colleagues12 examined the biomechanical strength of various fixation constructs and found that tension band wiring or cancellous screws were superior to suture fixation. More recently, Ji and colleagues13 described encouraging outcomes of arthroscopic fixation of isolated displaced proximal humerus fractures in 16 patients. Mean postoperative American Shoulder and Elbow Surgeons (ASES) score was 88, and mean improvement in University of California, Los Angeles (UCLA) score was 31 points. In addition, mean forward elevation was 148.7° at most recent follow-up.
Our technique supplements the literature on greater tuberosity fracture fixation by using a plate as the point for suture fixation rather than suture anchors or screw fixation. As has been shown with 3- and 4-part fractures, plate osteosynthesis provides proximal suture fixation points and locking screws (often in poor-quality bone) that can prevent suture cut-out and isolated screw failure. In addition, compared with other techniques for greater tuberosity fixation, meta-diaphyseal cortical plate fixation bypasses the often poor bone quality of the greater tuberosity, preventing these modes of failure.18 Schoffl and colleagues18 reported on 10 patients who received a Bamberg plate; all 10 had excellent postoperative outcomes with no complications or secondary loss of reduction. Outcomes in the present study mirror those in the literature for operative fixation of displaced greater tuberosity fractures. Despite the near anatomical reduction in the majority of patients (mean forward elevation, 147°), functional results in this patient population remain guarded, with many patients reporting only good clinical outcomes.
This study had a few limitations. First is the inherent limitation of a retrospective study. Second, the small sample size limited the subgroup analysis. However, given the rarity of the injury and the single-surgeon series, we would have to have added considerable time to the study to increase its power. Third, there was no control group. This is a difficult situation with displaced fractures, as clinical outcomes are poorer with nonoperative management than with operative intervention.2,16,17 Compared with historical operative controls in the literature, our patients compare favorably over medium-term follow-up.2,5,15,16
Conclusion
Plate osteosynthesis is a novel technique in the treatment of displaced greater tuberosity fractures. It results in excellent fracture reduction, a 100% union rate, minimal fracture migration, and good return of ROM. However, self-reported functional assessment of the shoulder was about three-fourths of what is expected of normal or preinjury function.
1. Gardner MJ, Boraiah S, Helfet DL, Lorich DG. Indirect medial reduction and strut support of proximal humerus fractures using an endosteal implant.
J Orthop Trauma. 2008;22(3):195-200.
2. Green A, Izzi J Jr. Isolated fractures of the greater tuberosity of the proximal humerus. J Shoulder Elbow Surg. 2003;12(6):641-649.
3. Neer CS 2nd. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
4. Ricchetti ET, DeMola PM, Roman D, Abboud JA. The use of precontoured humeral locking plates in the management of displaced proximal humerus fracture. J Am Acad Orthop Surg. 2009;17(9):582-590.
5. Flatow EL, Cuomo F, Maday MG, Miller SR, McIlveen SJ, Bigliani LU. Open reduction and internal fixation of two-part displaced fractures of the greater tuberosity of the proximal part of the humerus. J Bone Joint Surg Am. 1991;73(8):1213-1218.
6. Lenarz C, Shishani Y, McCrum C, Nowinski RJ, Edwards TB, Gobezie R. Is reverse shoulder arthroplasty appropriate for the treatment of fractures in the older patient? Early observations. Clin Orthop Relat Res. 2011;469(12):3324-3331.
7. Park MC, Murthi AM, Roth NS, Blaine TA, Levine WN, Bigliani LU. Two-part and three-part fractures of the proximal humerus treated with suture fixation. J Orthop Trauma. 2003;17(5):319-325.
8. Young SW, Segal BS, Turner PC, Poon PC. Comparison of functional outcomes of reverse shoulder arthroplasty versus hemiarthroplasty in the primary treatment of acute proximal humerus fracture. ANZ J Surg. 2010;80(11):789-793.
9. Dimakopoulos P, Panagopoulos A, Kasimatis G. Transosseous suture fixation of proximal humeral fractures. J Bone Joint Surg Am. 2007;89(8):1700-1709.
10. Dimakopoulos P, Panagopoulos A, Kasimatis G. Transosseous suture fixation of proximal humeral fractures. Surgical technique. J Bone Joint Surg Am. 2009;91(suppl 2, pt 1):8-21.
11. Bhatia DN, van Rooyen KS, du Toit DF, de Beer JF. Surgical treatment of comminuted, displaced fractures of the greater tuberosity of the proximal humerus: a new technique of double-row suture-anchor fixation and long-term results. Injury. 2006;37(10):946-952.
12. Braunstein V, Wiedemann E, Plitz W, Muensterer OJ, Mutschler W, Hinterwimmer S. Operative treatment of greater tuberosity fractures of the humerus—a biomechanical analysis. Clin Biomech. 2007;22(6):652-657.
13. Ji JH, Shafi M, Song IS, Kim YY, McFarland EG, Moon CY. Arthroscopic fixation technique for comminuted, displaced greater tuberosity fracture. Arthroscopy. 2010;26(5):600-609.
14. Kim E, Shin HK, Kim CH. Characteristics of an isolated greater tuberosity fracture of the humerus. J Orthop Sci. 2005;10(5):441-444.
15. Lee SU, Jeong C, Park IJ. Arthroscopic fixation of displaced greater tuberosity fracture of the proximal humerus. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):378-380.
16. Mattyasovszky SG, Burkhart KJ, Ahlers C, et al. Isolated fractures of the greater tuberosity of the proximal humerus. Acta Orthop. 2011;82(6):714-720.
17. Platzer P, Thalhammer G, Oberleitner G, et al. Displaced fractures of the greater tuberosity: a comparison of operative and nonoperative treatment. J Trauma. 2008;65(4):843-848.
18. Schoffl V, Popp D, Strecker W. A simple and effective implant for displaced fractures of the greater tuberosity: the “Bamberg” plate. Arch Orthop Trauma Surg. 2011;131(4):509-512.
19. Gardner MJ, Boraiah S, Helfet DL, Lorich DG. The anterolateral acromial approach for fractures of the proximal humerus. J Orthop Trauma. 2008;22(2):132-137.
20. Ricchetti ET, Warrender WJ, Abboud JA. Use of locking plates in the treatment of proximal humerus fractures. J Shoulder Elbow Surg. 2010;19(2 suppl):66-75.
21. Williams GN, Gangel TJ, Arciero RA, Uhorchak JM, Taylor DC. Comparison of the Single Assessment Numeric Evaluation method and two shoulder rating scales. Outcomes measures after shoulder surgery. Am J Sports Med. 1999;27(2):214-221.
22. Williams GN, Taylor DC, Gangel TJ, Uhorchak JM, Arciero RA. Comparison of the Single Assessment Numeric Evaluation method and the Lysholm score. Clin Orthop Relat Res. 2000;(373):184-192.
23. Leggin BG, Michener LA, Shaffer MA, Brenneman SK, Iannotti JP, Williams GR Jr. The Penn Shoulder Score: reliability and validity. J Orthop Sports Phys Ther. 2006;36(3):138-151.
24. Gruson KI, Ruchelsman DE, Tejwani NC. Isolated tuberosity fractures of the proximal humeral: current concepts. Injury. 2008;39(3):284-298.
Proximal humerus fractures are the second most common fracture in the upper extremity, accounting for 4% to 5% of all fractures.1-4 The majority of these injuries can be treated without an operation. For fractures that require surgery, there are multiple options, including closed reduction, percutaneous pinning, open reduction and internal fixation (ORIF), hemiarthroplasty, and reverse total shoulder arthroplasty.3-9
Isolated greater tuberosity fractures (AO [Arbeitsgemeinschaft für Osteosynthesefragen] 11.A1) make up a small subset of proximal humerus fractures. In general, patients who sustain an isolated greater tuberosity fracture are younger and more active than those who sustain other proximal humerus fractures.2,10 As a result, in the treatment of greater tuberosity fractures, there is increased emphasis on return to high activity and function. Nondisplaced or minimally displaced fractures typically are treated nonoperatively with good success.11,12 Patients with fractures displaced more than 5 mm, and highly active patients with fractures displaced more than 3 mm, usually are recommended for surgical treatment.2,11-14 The many options for treating these difficult fractures include suture fixation, percutaneous techniques, screw fixation, and, more recently, arthroscopic suture techniques.2,5,13,15,16 The goal of any of these operative interventions is to restore normal function and minimize pain around the injured shoulder. Although most of the operative techniques for greater tuberosity fractures have predictable results, none has been established as the gold standard for the treatment of displaced greater tuberosity fractures.2,5,13,15-18 Use of plate osteosynthesis for displaced proximal humerus fractures not isolated to the greater tuberosity is becoming more widespread in the orthopedic community.1,4,19,20 However, the orthopedic literature includes very few reports of using this technique for isolated displaced greater tuberosity fractures.18 This surgical approach potentially provides increased stability, improved maintenance of reduction, and earlier range of motion (ROM) in the postoperative period. These outcomes in turn may allow for improved pain control and earlier return to normal activities than is the case with other operative interventions for these difficult injuries.
We conducted a study to determine the radiographic and clinical outcomes of plate osteosynthesis for displaced greater tuberosity fractures. We hypothesized that excellent clinical and radiographic outcomes could be achieved using this surgical technique.
Patients and Methods
After obtaining institutional review board approval for this study, we retrospectively identified 11 consecutive patients with an isolated displaced greater tuberosity fracture (AO 11.A1) treated with plate osteosynthesis by Dr. Getz between December 2009 and May 2011 (Figures 1A, 1B). We collected data on age at time of surgery, sex, length of follow-up, worker’s compensation status, and complications. At a minimum of 21 months (mean, 27 months; SD, 8 months; range, 16-44 months), we assessed ROM and administered validated outcome scores, including the Single Assessment Numeric Evaluation (SANE)21,22 and the Penn Shoulder Score (PSS).23
Surgical Technique
The deltopectoral approach was used in all 11 patients. A standard incision was made over the deltopectoral interval starting at the coracoid and extending about 6 cm toward the deltoid insertion. After the internervous plane was entered between the deltoid and pectoralis major, the clavipectoral fascia was divided. The greater tuberosity fracture was identified with the leading edge of the fracture 1 cm posterior to the bicipital groove in all cases. Organized hematoma was removed from the fracture site to allow reduction. Three 1-mm braided polyester tapes were placed into the rotator cuff at the insertion onto the greater tuberosity fragment. The sutures thus captured the fragment and were used to obtain reduction and fixation. The fragment was provisionally pinned by placing a 2.0-mm Kirschner wire high on the fragment as to not block plate application. Fluoroscopic imaging was used to determine the appropriate position of the fracture reduction. A standard periarticular proximal humerus 3.5-mm locking compression plate (Zimmer) was used in all patients. The plate was contoured to achieve more compression in several cases in which plastic deformation or comminution was present. The sutures that were attached to the greater tuberosity were then brought through the plate. The plate was then slid down onto the humerus and pinned under fluoroscopic guidance. Three bicortical screws were used to affix the plate to the humeral shaft to compress the fracture into the fracture bed. Two to 4 locking screws were placed into the humeral head to improve the rotational stability of the construct. Last, the sutures through the plate were tied for added fixation.
Rehabilitation
In the immediate postoperative period, all patients were placed in a standard shoulder sling. The sling was worn for 6 weeks. At 2 weeks, patients started formal, standardized physical therapy, including passive ROM for elevation and external rotation. At 6 weeks, they began internal rotation stretching and active-assisted motion. Cuff strengthening began gently, as motion and pain allowed, after 8 weeks. Formal physical therapy continued until full or maximal improvement in motion and strength had been achieved.
Radiographic Measurements
Union/malunion was assessed by 2 orthopedic surgeons during their fellowship year in shoulder and elbow surgery. These surgeons were blinded to patients’ clinical outcomes. Each surgeon reviewed each patient’s radiographs twice to determine whether the reduction was anatomical. Anatomical reduction was achieved if the greater-tuberosity-to-head height was between 4 and 10 mm. Malunion was defined as loss of more than 3 mm of anatomical fracture reduction (from the original reduction) on any radiologic view at most recent follow-up. Loss of reduction was considered minimal if the fracture fragment was displaced less than 3 mm.
Statistical Analysis
A descriptive analysis of patient variables and outcomes was used for this small cohort of patients. Statistical significance was set at α = 0.05.
Results
Eleven patients (7 women, 4 men) underwent plate osteosynthesis for an isolated greater tuberosity fracture (Figure 2). Mean age at surgery was 60 years (range, 37-71 years). All patients were right-hand–dominant; 7 of the 11 sustained the injury on the dominant side. For all 11 patients, final postoperative ROM and complications were recorded. No patient required additional surgery. Before injury, all patients felt their shoulder was 100% normal. Nine of the 11 patients were available for assessment of functional outcome and ROM at a mean (SD) of 27 (8) months (range, 16-44 months). At final follow-up, mean (SD) forward elevation was 147° (28°; range, 100°-180°), and mean (SD) external rotation was 25° (15°; range, 10°-60°). Mean (SD) SANE score was 72 (17; range, 50-90), and mean (SD) PSS was 79 (16; range 43-90). On a 1-to-10 scale, patients’ mean (SD) overall satisfaction was 8.6 (1.9; range, 4-10). Of the 9 patients who worked before injury, 8 returned to preoperative duty. Six patients reported stiffness (consistent with ROM). All patients said they would have the surgery again (Table).
All patients experienced radiographic union. Three of the 11 had minimal (<3 mm) loss of reduction. Mean (SD) time to union was 10.7 (4.2) weeks (range, 6.1-21.6 weeks). There were no wound complications and no need for any hardware removal.
Discussion
Isolated greater tuberosity fractures are less common than other types of proximal humerus fractures but often require surgical intervention for less displacement when compared with those fractures.2,14 Multiple techniques (eg, suture fixation, percutaneous pinning, arthroscopic techniques) have been used, but none has established itself as the gold standard for treatment of these difficult injuries.2,5,9,11,13-16 The results of the present study show that plate osteosynthesis can reliably be used to achieve anatomical reduction and good functional outcomes in isolated greater tuberosity fractures. Even with the added stability of the plate and suture construct, a small number of fractures still displaced. In addition, despite having achieved anatomical union, many patients in this study experienced stiffness and functional loss, which speaks to the challenges associated with management of these fractures.
Self-reported outcomes were less favorable for patients in our study (despite achieving mean forward elevation of 147°) than for patients who underwent greater tuberosity repair in other studies.2,5,10 In a study of 12 patients who underwent ORIF of a 2-part displaced fracture of the greater tuberosity of the proximal part of the humerus, Flatow and colleagues5 found half the patients had an excellent outcome, and the other half had a good outcome with active elevation averaging 170°. In another study, conducted over 11 years, 165 patients with a proximal humeral fracture were treated with transosseous suture fixation. Union occurred in all patients except the 2 patients with 3-part fractures, and 155 patients had excellent or very good fracture reduction.10 Therefore, final ROM for these patients may not be a good indicator of actual final function, and previous reports likely underestimated the functional loss experienced by these patients.
The incidence of isolated greater tuberosity fractures likely will increase as the population ages and becomes more active.2,14,16 Patients with isolated greater tuberosity fractures are more likely to be male, to be younger, and to have fewer medical problems than patients with other types of proximal humerus fractures.14 In addition, patient expectations regarding life after displaced greater tuberosity fractures are unique compared with expectations of patients who have other proximal humerus fractures; displaced greater tuberosity fractures usually occur in more active patients, who may expect to return to work and may place higher demands on themselves after treatment,2,14,16,24 possibly leading to lower subjective clinical outcomes.
Various operative treatment techniques for isolated greater tuberosity fractures have been described. Flatow and colleagues5 reported excellent return of forward elevation after ORIF with heavy suture, and half the patients reported excellent outcomes. Other techniques have had mixed results. Bhatia and colleagues11 reported on long-term outcomes of internal fixation using a double row of suture anchors in isolated, displaced greater tuberosity fractures in 21 patients. Outcomes were rated excellent in 8 patients, good in 10, satisfactory in 2, and unsatisfactory in 1. Braunstein and colleagues12 examined the biomechanical strength of various fixation constructs and found that tension band wiring or cancellous screws were superior to suture fixation. More recently, Ji and colleagues13 described encouraging outcomes of arthroscopic fixation of isolated displaced proximal humerus fractures in 16 patients. Mean postoperative American Shoulder and Elbow Surgeons (ASES) score was 88, and mean improvement in University of California, Los Angeles (UCLA) score was 31 points. In addition, mean forward elevation was 148.7° at most recent follow-up.
Our technique supplements the literature on greater tuberosity fracture fixation by using a plate as the point for suture fixation rather than suture anchors or screw fixation. As has been shown with 3- and 4-part fractures, plate osteosynthesis provides proximal suture fixation points and locking screws (often in poor-quality bone) that can prevent suture cut-out and isolated screw failure. In addition, compared with other techniques for greater tuberosity fixation, meta-diaphyseal cortical plate fixation bypasses the often poor bone quality of the greater tuberosity, preventing these modes of failure.18 Schoffl and colleagues18 reported on 10 patients who received a Bamberg plate; all 10 had excellent postoperative outcomes with no complications or secondary loss of reduction. Outcomes in the present study mirror those in the literature for operative fixation of displaced greater tuberosity fractures. Despite the near anatomical reduction in the majority of patients (mean forward elevation, 147°), functional results in this patient population remain guarded, with many patients reporting only good clinical outcomes.
This study had a few limitations. First is the inherent limitation of a retrospective study. Second, the small sample size limited the subgroup analysis. However, given the rarity of the injury and the single-surgeon series, we would have to have added considerable time to the study to increase its power. Third, there was no control group. This is a difficult situation with displaced fractures, as clinical outcomes are poorer with nonoperative management than with operative intervention.2,16,17 Compared with historical operative controls in the literature, our patients compare favorably over medium-term follow-up.2,5,15,16
Conclusion
Plate osteosynthesis is a novel technique in the treatment of displaced greater tuberosity fractures. It results in excellent fracture reduction, a 100% union rate, minimal fracture migration, and good return of ROM. However, self-reported functional assessment of the shoulder was about three-fourths of what is expected of normal or preinjury function.
Proximal humerus fractures are the second most common fracture in the upper extremity, accounting for 4% to 5% of all fractures.1-4 The majority of these injuries can be treated without an operation. For fractures that require surgery, there are multiple options, including closed reduction, percutaneous pinning, open reduction and internal fixation (ORIF), hemiarthroplasty, and reverse total shoulder arthroplasty.3-9
Isolated greater tuberosity fractures (AO [Arbeitsgemeinschaft für Osteosynthesefragen] 11.A1) make up a small subset of proximal humerus fractures. In general, patients who sustain an isolated greater tuberosity fracture are younger and more active than those who sustain other proximal humerus fractures.2,10 As a result, in the treatment of greater tuberosity fractures, there is increased emphasis on return to high activity and function. Nondisplaced or minimally displaced fractures typically are treated nonoperatively with good success.11,12 Patients with fractures displaced more than 5 mm, and highly active patients with fractures displaced more than 3 mm, usually are recommended for surgical treatment.2,11-14 The many options for treating these difficult fractures include suture fixation, percutaneous techniques, screw fixation, and, more recently, arthroscopic suture techniques.2,5,13,15,16 The goal of any of these operative interventions is to restore normal function and minimize pain around the injured shoulder. Although most of the operative techniques for greater tuberosity fractures have predictable results, none has been established as the gold standard for the treatment of displaced greater tuberosity fractures.2,5,13,15-18 Use of plate osteosynthesis for displaced proximal humerus fractures not isolated to the greater tuberosity is becoming more widespread in the orthopedic community.1,4,19,20 However, the orthopedic literature includes very few reports of using this technique for isolated displaced greater tuberosity fractures.18 This surgical approach potentially provides increased stability, improved maintenance of reduction, and earlier range of motion (ROM) in the postoperative period. These outcomes in turn may allow for improved pain control and earlier return to normal activities than is the case with other operative interventions for these difficult injuries.
We conducted a study to determine the radiographic and clinical outcomes of plate osteosynthesis for displaced greater tuberosity fractures. We hypothesized that excellent clinical and radiographic outcomes could be achieved using this surgical technique.
Patients and Methods
After obtaining institutional review board approval for this study, we retrospectively identified 11 consecutive patients with an isolated displaced greater tuberosity fracture (AO 11.A1) treated with plate osteosynthesis by Dr. Getz between December 2009 and May 2011 (Figures 1A, 1B). We collected data on age at time of surgery, sex, length of follow-up, worker’s compensation status, and complications. At a minimum of 21 months (mean, 27 months; SD, 8 months; range, 16-44 months), we assessed ROM and administered validated outcome scores, including the Single Assessment Numeric Evaluation (SANE)21,22 and the Penn Shoulder Score (PSS).23
Surgical Technique
The deltopectoral approach was used in all 11 patients. A standard incision was made over the deltopectoral interval starting at the coracoid and extending about 6 cm toward the deltoid insertion. After the internervous plane was entered between the deltoid and pectoralis major, the clavipectoral fascia was divided. The greater tuberosity fracture was identified with the leading edge of the fracture 1 cm posterior to the bicipital groove in all cases. Organized hematoma was removed from the fracture site to allow reduction. Three 1-mm braided polyester tapes were placed into the rotator cuff at the insertion onto the greater tuberosity fragment. The sutures thus captured the fragment and were used to obtain reduction and fixation. The fragment was provisionally pinned by placing a 2.0-mm Kirschner wire high on the fragment as to not block plate application. Fluoroscopic imaging was used to determine the appropriate position of the fracture reduction. A standard periarticular proximal humerus 3.5-mm locking compression plate (Zimmer) was used in all patients. The plate was contoured to achieve more compression in several cases in which plastic deformation or comminution was present. The sutures that were attached to the greater tuberosity were then brought through the plate. The plate was then slid down onto the humerus and pinned under fluoroscopic guidance. Three bicortical screws were used to affix the plate to the humeral shaft to compress the fracture into the fracture bed. Two to 4 locking screws were placed into the humeral head to improve the rotational stability of the construct. Last, the sutures through the plate were tied for added fixation.
Rehabilitation
In the immediate postoperative period, all patients were placed in a standard shoulder sling. The sling was worn for 6 weeks. At 2 weeks, patients started formal, standardized physical therapy, including passive ROM for elevation and external rotation. At 6 weeks, they began internal rotation stretching and active-assisted motion. Cuff strengthening began gently, as motion and pain allowed, after 8 weeks. Formal physical therapy continued until full or maximal improvement in motion and strength had been achieved.
Radiographic Measurements
Union/malunion was assessed by 2 orthopedic surgeons during their fellowship year in shoulder and elbow surgery. These surgeons were blinded to patients’ clinical outcomes. Each surgeon reviewed each patient’s radiographs twice to determine whether the reduction was anatomical. Anatomical reduction was achieved if the greater-tuberosity-to-head height was between 4 and 10 mm. Malunion was defined as loss of more than 3 mm of anatomical fracture reduction (from the original reduction) on any radiologic view at most recent follow-up. Loss of reduction was considered minimal if the fracture fragment was displaced less than 3 mm.
Statistical Analysis
A descriptive analysis of patient variables and outcomes was used for this small cohort of patients. Statistical significance was set at α = 0.05.
Results
Eleven patients (7 women, 4 men) underwent plate osteosynthesis for an isolated greater tuberosity fracture (Figure 2). Mean age at surgery was 60 years (range, 37-71 years). All patients were right-hand–dominant; 7 of the 11 sustained the injury on the dominant side. For all 11 patients, final postoperative ROM and complications were recorded. No patient required additional surgery. Before injury, all patients felt their shoulder was 100% normal. Nine of the 11 patients were available for assessment of functional outcome and ROM at a mean (SD) of 27 (8) months (range, 16-44 months). At final follow-up, mean (SD) forward elevation was 147° (28°; range, 100°-180°), and mean (SD) external rotation was 25° (15°; range, 10°-60°). Mean (SD) SANE score was 72 (17; range, 50-90), and mean (SD) PSS was 79 (16; range 43-90). On a 1-to-10 scale, patients’ mean (SD) overall satisfaction was 8.6 (1.9; range, 4-10). Of the 9 patients who worked before injury, 8 returned to preoperative duty. Six patients reported stiffness (consistent with ROM). All patients said they would have the surgery again (Table).
All patients experienced radiographic union. Three of the 11 had minimal (<3 mm) loss of reduction. Mean (SD) time to union was 10.7 (4.2) weeks (range, 6.1-21.6 weeks). There were no wound complications and no need for any hardware removal.
Discussion
Isolated greater tuberosity fractures are less common than other types of proximal humerus fractures but often require surgical intervention for less displacement when compared with those fractures.2,14 Multiple techniques (eg, suture fixation, percutaneous pinning, arthroscopic techniques) have been used, but none has established itself as the gold standard for treatment of these difficult injuries.2,5,9,11,13-16 The results of the present study show that plate osteosynthesis can reliably be used to achieve anatomical reduction and good functional outcomes in isolated greater tuberosity fractures. Even with the added stability of the plate and suture construct, a small number of fractures still displaced. In addition, despite having achieved anatomical union, many patients in this study experienced stiffness and functional loss, which speaks to the challenges associated with management of these fractures.
Self-reported outcomes were less favorable for patients in our study (despite achieving mean forward elevation of 147°) than for patients who underwent greater tuberosity repair in other studies.2,5,10 In a study of 12 patients who underwent ORIF of a 2-part displaced fracture of the greater tuberosity of the proximal part of the humerus, Flatow and colleagues5 found half the patients had an excellent outcome, and the other half had a good outcome with active elevation averaging 170°. In another study, conducted over 11 years, 165 patients with a proximal humeral fracture were treated with transosseous suture fixation. Union occurred in all patients except the 2 patients with 3-part fractures, and 155 patients had excellent or very good fracture reduction.10 Therefore, final ROM for these patients may not be a good indicator of actual final function, and previous reports likely underestimated the functional loss experienced by these patients.
The incidence of isolated greater tuberosity fractures likely will increase as the population ages and becomes more active.2,14,16 Patients with isolated greater tuberosity fractures are more likely to be male, to be younger, and to have fewer medical problems than patients with other types of proximal humerus fractures.14 In addition, patient expectations regarding life after displaced greater tuberosity fractures are unique compared with expectations of patients who have other proximal humerus fractures; displaced greater tuberosity fractures usually occur in more active patients, who may expect to return to work and may place higher demands on themselves after treatment,2,14,16,24 possibly leading to lower subjective clinical outcomes.
Various operative treatment techniques for isolated greater tuberosity fractures have been described. Flatow and colleagues5 reported excellent return of forward elevation after ORIF with heavy suture, and half the patients reported excellent outcomes. Other techniques have had mixed results. Bhatia and colleagues11 reported on long-term outcomes of internal fixation using a double row of suture anchors in isolated, displaced greater tuberosity fractures in 21 patients. Outcomes were rated excellent in 8 patients, good in 10, satisfactory in 2, and unsatisfactory in 1. Braunstein and colleagues12 examined the biomechanical strength of various fixation constructs and found that tension band wiring or cancellous screws were superior to suture fixation. More recently, Ji and colleagues13 described encouraging outcomes of arthroscopic fixation of isolated displaced proximal humerus fractures in 16 patients. Mean postoperative American Shoulder and Elbow Surgeons (ASES) score was 88, and mean improvement in University of California, Los Angeles (UCLA) score was 31 points. In addition, mean forward elevation was 148.7° at most recent follow-up.
Our technique supplements the literature on greater tuberosity fracture fixation by using a plate as the point for suture fixation rather than suture anchors or screw fixation. As has been shown with 3- and 4-part fractures, plate osteosynthesis provides proximal suture fixation points and locking screws (often in poor-quality bone) that can prevent suture cut-out and isolated screw failure. In addition, compared with other techniques for greater tuberosity fixation, meta-diaphyseal cortical plate fixation bypasses the often poor bone quality of the greater tuberosity, preventing these modes of failure.18 Schoffl and colleagues18 reported on 10 patients who received a Bamberg plate; all 10 had excellent postoperative outcomes with no complications or secondary loss of reduction. Outcomes in the present study mirror those in the literature for operative fixation of displaced greater tuberosity fractures. Despite the near anatomical reduction in the majority of patients (mean forward elevation, 147°), functional results in this patient population remain guarded, with many patients reporting only good clinical outcomes.
This study had a few limitations. First is the inherent limitation of a retrospective study. Second, the small sample size limited the subgroup analysis. However, given the rarity of the injury and the single-surgeon series, we would have to have added considerable time to the study to increase its power. Third, there was no control group. This is a difficult situation with displaced fractures, as clinical outcomes are poorer with nonoperative management than with operative intervention.2,16,17 Compared with historical operative controls in the literature, our patients compare favorably over medium-term follow-up.2,5,15,16
Conclusion
Plate osteosynthesis is a novel technique in the treatment of displaced greater tuberosity fractures. It results in excellent fracture reduction, a 100% union rate, minimal fracture migration, and good return of ROM. However, self-reported functional assessment of the shoulder was about three-fourths of what is expected of normal or preinjury function.
1. Gardner MJ, Boraiah S, Helfet DL, Lorich DG. Indirect medial reduction and strut support of proximal humerus fractures using an endosteal implant.
J Orthop Trauma. 2008;22(3):195-200.
2. Green A, Izzi J Jr. Isolated fractures of the greater tuberosity of the proximal humerus. J Shoulder Elbow Surg. 2003;12(6):641-649.
3. Neer CS 2nd. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
4. Ricchetti ET, DeMola PM, Roman D, Abboud JA. The use of precontoured humeral locking plates in the management of displaced proximal humerus fracture. J Am Acad Orthop Surg. 2009;17(9):582-590.
5. Flatow EL, Cuomo F, Maday MG, Miller SR, McIlveen SJ, Bigliani LU. Open reduction and internal fixation of two-part displaced fractures of the greater tuberosity of the proximal part of the humerus. J Bone Joint Surg Am. 1991;73(8):1213-1218.
6. Lenarz C, Shishani Y, McCrum C, Nowinski RJ, Edwards TB, Gobezie R. Is reverse shoulder arthroplasty appropriate for the treatment of fractures in the older patient? Early observations. Clin Orthop Relat Res. 2011;469(12):3324-3331.
7. Park MC, Murthi AM, Roth NS, Blaine TA, Levine WN, Bigliani LU. Two-part and three-part fractures of the proximal humerus treated with suture fixation. J Orthop Trauma. 2003;17(5):319-325.
8. Young SW, Segal BS, Turner PC, Poon PC. Comparison of functional outcomes of reverse shoulder arthroplasty versus hemiarthroplasty in the primary treatment of acute proximal humerus fracture. ANZ J Surg. 2010;80(11):789-793.
9. Dimakopoulos P, Panagopoulos A, Kasimatis G. Transosseous suture fixation of proximal humeral fractures. J Bone Joint Surg Am. 2007;89(8):1700-1709.
10. Dimakopoulos P, Panagopoulos A, Kasimatis G. Transosseous suture fixation of proximal humeral fractures. Surgical technique. J Bone Joint Surg Am. 2009;91(suppl 2, pt 1):8-21.
11. Bhatia DN, van Rooyen KS, du Toit DF, de Beer JF. Surgical treatment of comminuted, displaced fractures of the greater tuberosity of the proximal humerus: a new technique of double-row suture-anchor fixation and long-term results. Injury. 2006;37(10):946-952.
12. Braunstein V, Wiedemann E, Plitz W, Muensterer OJ, Mutschler W, Hinterwimmer S. Operative treatment of greater tuberosity fractures of the humerus—a biomechanical analysis. Clin Biomech. 2007;22(6):652-657.
13. Ji JH, Shafi M, Song IS, Kim YY, McFarland EG, Moon CY. Arthroscopic fixation technique for comminuted, displaced greater tuberosity fracture. Arthroscopy. 2010;26(5):600-609.
14. Kim E, Shin HK, Kim CH. Characteristics of an isolated greater tuberosity fracture of the humerus. J Orthop Sci. 2005;10(5):441-444.
15. Lee SU, Jeong C, Park IJ. Arthroscopic fixation of displaced greater tuberosity fracture of the proximal humerus. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):378-380.
16. Mattyasovszky SG, Burkhart KJ, Ahlers C, et al. Isolated fractures of the greater tuberosity of the proximal humerus. Acta Orthop. 2011;82(6):714-720.
17. Platzer P, Thalhammer G, Oberleitner G, et al. Displaced fractures of the greater tuberosity: a comparison of operative and nonoperative treatment. J Trauma. 2008;65(4):843-848.
18. Schoffl V, Popp D, Strecker W. A simple and effective implant for displaced fractures of the greater tuberosity: the “Bamberg” plate. Arch Orthop Trauma Surg. 2011;131(4):509-512.
19. Gardner MJ, Boraiah S, Helfet DL, Lorich DG. The anterolateral acromial approach for fractures of the proximal humerus. J Orthop Trauma. 2008;22(2):132-137.
20. Ricchetti ET, Warrender WJ, Abboud JA. Use of locking plates in the treatment of proximal humerus fractures. J Shoulder Elbow Surg. 2010;19(2 suppl):66-75.
21. Williams GN, Gangel TJ, Arciero RA, Uhorchak JM, Taylor DC. Comparison of the Single Assessment Numeric Evaluation method and two shoulder rating scales. Outcomes measures after shoulder surgery. Am J Sports Med. 1999;27(2):214-221.
22. Williams GN, Taylor DC, Gangel TJ, Uhorchak JM, Arciero RA. Comparison of the Single Assessment Numeric Evaluation method and the Lysholm score. Clin Orthop Relat Res. 2000;(373):184-192.
23. Leggin BG, Michener LA, Shaffer MA, Brenneman SK, Iannotti JP, Williams GR Jr. The Penn Shoulder Score: reliability and validity. J Orthop Sports Phys Ther. 2006;36(3):138-151.
24. Gruson KI, Ruchelsman DE, Tejwani NC. Isolated tuberosity fractures of the proximal humeral: current concepts. Injury. 2008;39(3):284-298.
1. Gardner MJ, Boraiah S, Helfet DL, Lorich DG. Indirect medial reduction and strut support of proximal humerus fractures using an endosteal implant.
J Orthop Trauma. 2008;22(3):195-200.
2. Green A, Izzi J Jr. Isolated fractures of the greater tuberosity of the proximal humerus. J Shoulder Elbow Surg. 2003;12(6):641-649.
3. Neer CS 2nd. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
4. Ricchetti ET, DeMola PM, Roman D, Abboud JA. The use of precontoured humeral locking plates in the management of displaced proximal humerus fracture. J Am Acad Orthop Surg. 2009;17(9):582-590.
5. Flatow EL, Cuomo F, Maday MG, Miller SR, McIlveen SJ, Bigliani LU. Open reduction and internal fixation of two-part displaced fractures of the greater tuberosity of the proximal part of the humerus. J Bone Joint Surg Am. 1991;73(8):1213-1218.
6. Lenarz C, Shishani Y, McCrum C, Nowinski RJ, Edwards TB, Gobezie R. Is reverse shoulder arthroplasty appropriate for the treatment of fractures in the older patient? Early observations. Clin Orthop Relat Res. 2011;469(12):3324-3331.
7. Park MC, Murthi AM, Roth NS, Blaine TA, Levine WN, Bigliani LU. Two-part and three-part fractures of the proximal humerus treated with suture fixation. J Orthop Trauma. 2003;17(5):319-325.
8. Young SW, Segal BS, Turner PC, Poon PC. Comparison of functional outcomes of reverse shoulder arthroplasty versus hemiarthroplasty in the primary treatment of acute proximal humerus fracture. ANZ J Surg. 2010;80(11):789-793.
9. Dimakopoulos P, Panagopoulos A, Kasimatis G. Transosseous suture fixation of proximal humeral fractures. J Bone Joint Surg Am. 2007;89(8):1700-1709.
10. Dimakopoulos P, Panagopoulos A, Kasimatis G. Transosseous suture fixation of proximal humeral fractures. Surgical technique. J Bone Joint Surg Am. 2009;91(suppl 2, pt 1):8-21.
11. Bhatia DN, van Rooyen KS, du Toit DF, de Beer JF. Surgical treatment of comminuted, displaced fractures of the greater tuberosity of the proximal humerus: a new technique of double-row suture-anchor fixation and long-term results. Injury. 2006;37(10):946-952.
12. Braunstein V, Wiedemann E, Plitz W, Muensterer OJ, Mutschler W, Hinterwimmer S. Operative treatment of greater tuberosity fractures of the humerus—a biomechanical analysis. Clin Biomech. 2007;22(6):652-657.
13. Ji JH, Shafi M, Song IS, Kim YY, McFarland EG, Moon CY. Arthroscopic fixation technique for comminuted, displaced greater tuberosity fracture. Arthroscopy. 2010;26(5):600-609.
14. Kim E, Shin HK, Kim CH. Characteristics of an isolated greater tuberosity fracture of the humerus. J Orthop Sci. 2005;10(5):441-444.
15. Lee SU, Jeong C, Park IJ. Arthroscopic fixation of displaced greater tuberosity fracture of the proximal humerus. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):378-380.
16. Mattyasovszky SG, Burkhart KJ, Ahlers C, et al. Isolated fractures of the greater tuberosity of the proximal humerus. Acta Orthop. 2011;82(6):714-720.
17. Platzer P, Thalhammer G, Oberleitner G, et al. Displaced fractures of the greater tuberosity: a comparison of operative and nonoperative treatment. J Trauma. 2008;65(4):843-848.
18. Schoffl V, Popp D, Strecker W. A simple and effective implant for displaced fractures of the greater tuberosity: the “Bamberg” plate. Arch Orthop Trauma Surg. 2011;131(4):509-512.
19. Gardner MJ, Boraiah S, Helfet DL, Lorich DG. The anterolateral acromial approach for fractures of the proximal humerus. J Orthop Trauma. 2008;22(2):132-137.
20. Ricchetti ET, Warrender WJ, Abboud JA. Use of locking plates in the treatment of proximal humerus fractures. J Shoulder Elbow Surg. 2010;19(2 suppl):66-75.
21. Williams GN, Gangel TJ, Arciero RA, Uhorchak JM, Taylor DC. Comparison of the Single Assessment Numeric Evaluation method and two shoulder rating scales. Outcomes measures after shoulder surgery. Am J Sports Med. 1999;27(2):214-221.
22. Williams GN, Taylor DC, Gangel TJ, Uhorchak JM, Arciero RA. Comparison of the Single Assessment Numeric Evaluation method and the Lysholm score. Clin Orthop Relat Res. 2000;(373):184-192.
23. Leggin BG, Michener LA, Shaffer MA, Brenneman SK, Iannotti JP, Williams GR Jr. The Penn Shoulder Score: reliability and validity. J Orthop Sports Phys Ther. 2006;36(3):138-151.
24. Gruson KI, Ruchelsman DE, Tejwani NC. Isolated tuberosity fractures of the proximal humeral: current concepts. Injury. 2008;39(3):284-298.
Commentary to "The Burden of Craft in Arthroscopic Rotator Cuff Repair: Where We Have Been and Where We Are Going"
“The Burden of Craft in Arthroscopic Rotator Cuff Repair” is a summary of the annual Neer Lecture that was delivered by Stephen S. Burkhart, MD, at the 2014 annual meeting of American Shoulder and Elbow Surgeons. It is a fascinating personal story of the 35-year evolution of arthroscopic rotator cuff surgery presented by one of the most respected arthroscopic innovators of our times. I especially enjoyed his apt citations of classic leaders—Churchill and Gandhi—but 3 points I believe deserve special comment.
First, Steve describes the challenges he faced bringing new products to market in the 1980s. How do we resolve the inherent conflict between innovation that introduces new technology and the “tried and true” standards of established practice? Do the hard work that Steve has done over the years: pose a hypothesis, design a study to answer the question, publish results in peer-reviewed journals, and embrace the techniques that demonstrate better outcomes for patients.
My second point relates to surgeon–device industry relationships, a subject of great interest to The American Journal of Orthopedics dating back to 2006.1-3 Dr. Burkhart learned early on that he could not fashion new arthroscopic instruments in his garage. Nor could a company develop useful instruments without a knowledgeable surgeon’s input. Hence, a partnership between the innovator-surgeon and the device industry is essential to bring new and effective “tools” to market. Dr. Burkhart’s partnership with Arthrex has benefited many thousands of patients.
The agreements announced in 2007 between the US Department of Justice and 5 orthopedic device manufacturers (interestingly, current presidential candidate and Governor of New Jersey Chris Christie was the lead US Attorney on the case!) dramatically altered the surgeon–industry interaction and established strict guidelines that governed these relationships.4 These were needed reforms. However, the changes did not preclude an entrepreneurial surgeon with great ideas and a device manufacturer from profiting from excellent products that advanced patient care, provided, quoting from my editorial of 2006, “that these partnerships comply with legal and ethical standards” and are transparent as well as fully disclosed.1
Finally, Steve’s last point focuses on the “burden of craft,” a topic dear to all orthopedic surgeons and our professional societies. All of us are committed to improving our surgical skills and, as a profession, we are consistently engaged in learning from our talented colleagues, who are only too willing to share their expertise. The burden of craft requires eager students and dedicated teachers, all committed to the same goal—better outcomes for our patients. We are indeed fortunate that, as orthopedic surgeons, we fundamentally support a culture of continued learning.
I thank Steve for his eloquent paper on this important principle.
1. McCann PD. Are surgeons accepting bribes? Am J Orthop. 2006;35(3):114.
2. Byrd AB, Tearney MB. Are you being bribed? Health care ethics and compliance in the AdvaMed Code era. Part I. Am J Orthop. 2006;35(3):117-120.
3. Byrd AB, Tearney MB. Are you being bribed? Health care ethics and compliance in the AdvaMed Code era. Part II. Am J Orthop. 2006;35(4):166-171.
4 Five companies in hip and knee replacement industry avoid prosecution by agreeing to compliance rules and monitoring [press release]. US Department of Justice website. http://www.justice.gov/usao/nj/Press/files/pdffiles/Older/hips0927.rel.pdf. Published September 27, 2007. Accessed July 14, 2015.
“The Burden of Craft in Arthroscopic Rotator Cuff Repair” is a summary of the annual Neer Lecture that was delivered by Stephen S. Burkhart, MD, at the 2014 annual meeting of American Shoulder and Elbow Surgeons. It is a fascinating personal story of the 35-year evolution of arthroscopic rotator cuff surgery presented by one of the most respected arthroscopic innovators of our times. I especially enjoyed his apt citations of classic leaders—Churchill and Gandhi—but 3 points I believe deserve special comment.
First, Steve describes the challenges he faced bringing new products to market in the 1980s. How do we resolve the inherent conflict between innovation that introduces new technology and the “tried and true” standards of established practice? Do the hard work that Steve has done over the years: pose a hypothesis, design a study to answer the question, publish results in peer-reviewed journals, and embrace the techniques that demonstrate better outcomes for patients.
My second point relates to surgeon–device industry relationships, a subject of great interest to The American Journal of Orthopedics dating back to 2006.1-3 Dr. Burkhart learned early on that he could not fashion new arthroscopic instruments in his garage. Nor could a company develop useful instruments without a knowledgeable surgeon’s input. Hence, a partnership between the innovator-surgeon and the device industry is essential to bring new and effective “tools” to market. Dr. Burkhart’s partnership with Arthrex has benefited many thousands of patients.
The agreements announced in 2007 between the US Department of Justice and 5 orthopedic device manufacturers (interestingly, current presidential candidate and Governor of New Jersey Chris Christie was the lead US Attorney on the case!) dramatically altered the surgeon–industry interaction and established strict guidelines that governed these relationships.4 These were needed reforms. However, the changes did not preclude an entrepreneurial surgeon with great ideas and a device manufacturer from profiting from excellent products that advanced patient care, provided, quoting from my editorial of 2006, “that these partnerships comply with legal and ethical standards” and are transparent as well as fully disclosed.1
Finally, Steve’s last point focuses on the “burden of craft,” a topic dear to all orthopedic surgeons and our professional societies. All of us are committed to improving our surgical skills and, as a profession, we are consistently engaged in learning from our talented colleagues, who are only too willing to share their expertise. The burden of craft requires eager students and dedicated teachers, all committed to the same goal—better outcomes for our patients. We are indeed fortunate that, as orthopedic surgeons, we fundamentally support a culture of continued learning.
I thank Steve for his eloquent paper on this important principle.
“The Burden of Craft in Arthroscopic Rotator Cuff Repair” is a summary of the annual Neer Lecture that was delivered by Stephen S. Burkhart, MD, at the 2014 annual meeting of American Shoulder and Elbow Surgeons. It is a fascinating personal story of the 35-year evolution of arthroscopic rotator cuff surgery presented by one of the most respected arthroscopic innovators of our times. I especially enjoyed his apt citations of classic leaders—Churchill and Gandhi—but 3 points I believe deserve special comment.
First, Steve describes the challenges he faced bringing new products to market in the 1980s. How do we resolve the inherent conflict between innovation that introduces new technology and the “tried and true” standards of established practice? Do the hard work that Steve has done over the years: pose a hypothesis, design a study to answer the question, publish results in peer-reviewed journals, and embrace the techniques that demonstrate better outcomes for patients.
My second point relates to surgeon–device industry relationships, a subject of great interest to The American Journal of Orthopedics dating back to 2006.1-3 Dr. Burkhart learned early on that he could not fashion new arthroscopic instruments in his garage. Nor could a company develop useful instruments without a knowledgeable surgeon’s input. Hence, a partnership between the innovator-surgeon and the device industry is essential to bring new and effective “tools” to market. Dr. Burkhart’s partnership with Arthrex has benefited many thousands of patients.
The agreements announced in 2007 between the US Department of Justice and 5 orthopedic device manufacturers (interestingly, current presidential candidate and Governor of New Jersey Chris Christie was the lead US Attorney on the case!) dramatically altered the surgeon–industry interaction and established strict guidelines that governed these relationships.4 These were needed reforms. However, the changes did not preclude an entrepreneurial surgeon with great ideas and a device manufacturer from profiting from excellent products that advanced patient care, provided, quoting from my editorial of 2006, “that these partnerships comply with legal and ethical standards” and are transparent as well as fully disclosed.1
Finally, Steve’s last point focuses on the “burden of craft,” a topic dear to all orthopedic surgeons and our professional societies. All of us are committed to improving our surgical skills and, as a profession, we are consistently engaged in learning from our talented colleagues, who are only too willing to share their expertise. The burden of craft requires eager students and dedicated teachers, all committed to the same goal—better outcomes for our patients. We are indeed fortunate that, as orthopedic surgeons, we fundamentally support a culture of continued learning.
I thank Steve for his eloquent paper on this important principle.
1. McCann PD. Are surgeons accepting bribes? Am J Orthop. 2006;35(3):114.
2. Byrd AB, Tearney MB. Are you being bribed? Health care ethics and compliance in the AdvaMed Code era. Part I. Am J Orthop. 2006;35(3):117-120.
3. Byrd AB, Tearney MB. Are you being bribed? Health care ethics and compliance in the AdvaMed Code era. Part II. Am J Orthop. 2006;35(4):166-171.
4 Five companies in hip and knee replacement industry avoid prosecution by agreeing to compliance rules and monitoring [press release]. US Department of Justice website. http://www.justice.gov/usao/nj/Press/files/pdffiles/Older/hips0927.rel.pdf. Published September 27, 2007. Accessed July 14, 2015.
1. McCann PD. Are surgeons accepting bribes? Am J Orthop. 2006;35(3):114.
2. Byrd AB, Tearney MB. Are you being bribed? Health care ethics and compliance in the AdvaMed Code era. Part I. Am J Orthop. 2006;35(3):117-120.
3. Byrd AB, Tearney MB. Are you being bribed? Health care ethics and compliance in the AdvaMed Code era. Part II. Am J Orthop. 2006;35(4):166-171.
4 Five companies in hip and knee replacement industry avoid prosecution by agreeing to compliance rules and monitoring [press release]. US Department of Justice website. http://www.justice.gov/usao/nj/Press/files/pdffiles/Older/hips0927.rel.pdf. Published September 27, 2007. Accessed July 14, 2015.
Simultaneous Bilateral Functional Radiography in Ulnar Collateral Ligament Lesion of the Thumb: An Original Technique
Gamekeeper’s or skier’s thumb is caused by an injury to the ulnar collateral ligament (UCL) of the metacarpophalangeal (MCP) joint of the thumb. The mechanism of injury is forced radial and palmar abduction and hyperextension.
This lesion was initially described in 1955 by Campbell.1 It occurred in gamekeepers who worked in preserves in Scotland. The UCL was injured because of the way they killed rabbits—hence, gamekeeper’s thumb. Now these injuries are more common in skiers—skier’s thumb. In skiers, the mechanism of injury is the force exerted by the ski pole strap on the thumb during a fall. This injury is also seen in breakdancers.1,2
Different lesions can result, the most common being that of the UCL. The UCL lesion may be partial, with no joint instability,3,4 or total, with instability and subdislocation of the proximal phalanx.5-9 Rupture of the thumb adductor aponeurosis and displacement of the long extensor have been described as the cause of thumb instability.6-8
UCL rupture can occur in its extension or can cause a fracture-tearing in the proximal phalanx.9-12 Intra-articular fractures are sometimes found. The essential problem in UCL injuries is the impossibility of spontaneous healing once the rupture is complete, because of the Stener effect. (When the UCL ruptures, its proximal part retracts and runs above the fibrous expansion of the adductor muscle, which is interposed between the 2 parts of the ruptured UCL and prevents healing, even if the thumb is immobilized.) In these cases, only surgery can repair the lesion.2
In any thumb injury, particularly one caused by hyperabduction, a UCL lesion should be considered. The main problem is diagnosing sprain severity, which is evidenced by the degree of joint hypermobility. Radiologic examination should be performed in all cases to rule out fracture with tear, posterior capsular tear, palmar plate tear, and palmar subdislocation of the proximal phalanx, all of which are associated with UCL tearing.7-9
If the diagnosis is suspected, and radiographs show no fracture, comparative radiographs should be obtained in forced valgus.
Technique
We report on a simple, reliable, reproducible method that allows the patient’s thumbs to be compared, under the same force application conditions, on a single radiograph. This technique reduces the patient’s and examiner’s exposure to x-rays and is well tolerated by the patient. Anesthesia for the thumb is usually not necessary.
In each hand, the patient holds a cylindrical object, such as a drinking glass (standard diameter, 7.5-8.5 cm). We use an elastic crepe bandage roll (diameter, 7.5 cm; width, 10 cm). This roll is common in emergency departments (EDs) and easily accessible. The patient holds the rolls in his or her hands with the thumbs in the posteroanterior position (Figures 1–3) and places himself or herself on a 18×24-cm frame or directly on the radiography table.
Both thumbs are captured on a single functional radiograph for comparison of forced valgus of the MCP joints, as in our example cases. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Control Case
The single functional radiograph of both thumbs showed no evidence of joint laxity on the valgus stress test (Figure 4).
Case 1
A 72-year-old woman landed on her left hand when she fell backward while supporting the hand on a piece of furniture. She presented to the ED with pain in the region of the thumb and thenar eminence. Posteroanterior and lateral radiograph projections showed no significant bone injury (Figure 5). Given the patient’s persistent pain, the traumatologist suspected damage to the thumb UCL, so a simultaneous bilateral functional radiographic projection was obtained. The projection showed joint laxity, implying damage to the thumb UCL. Repair and reinsertion of the UCL were performed using a bone harpoon suture.
Case 2
A 58-year-old man sustained a left hand injury when, using both hands, he tried to catch hold of a falling wooden plank. When he presented to the ED the following week, he was given a diagnosis of thumb contusion and forced hyperabduction and was wearing a metal strap for immobilization. Radiographs showed no bone damage (Figure 6). Thumb UCL injury was suspected on the basis of the physical examination findings and the mechanism of injury. A bilateral simultaneous functional radiographic projection showed significant joint laxity. Surgical treatment with the pull-out technique was performed.
Case 3
A 44-year-old woman experienced forced traction from a dog leash and presented to the ED with pain in the right thumb region. Radiographs showed no bone damage (Figure 7). Thumb UCL injury was suspected. A bilateral simultaneous functional radiographic projection showed slight joint laxity, a sprain was diagnosed, and plaster bandaging was applied. Figures 8A–8D show the accurate thumb positions for performing the functional radiograph in forced valgus. We call the technique J.J.’s thumb radiographic projection.
Discussion
Examination using the stress test to cause joint tilt is crucial in making an accurate diagnosis and deciding on the most appropriate therapeutic approach.10 Most authors accept that surgical management is required in joint tilts over 30º, as these involve complete UCL rupture.10-12
The MCP joint must be examined in flexion, when the main fascicle of the UCL is tight, and not in extension, when the main fascicle of the UCL is relaxed. If we examine the thumb in extension, radial deviations may occur that are not caused by joint instability. Tilt here must be compared with that of the healthy side.11
Early diagnosis and adequate management are essential, as unnoticed or undervalued injuries can progress to painful sequelae, associated with stiffness, instability, and osteoarthritis, with evident harm to the grip and pinch functions of the hand. In many cases, clinical evidence of MCP joint instability is difficult. The radiologic diagnosis is usually obtained with comparative radiographs in forced valgus of both thumbs.
The forced valgus maneuver typically is performed by the examiner, who must stay with the patient in the radiography room and wear radiologic protection. Incredibly, some patients must force the valgus themselves.
The maneuver we have described clearly has complications, as it is painful, and some patients are uncooperative. Usually the thumb is anesthetized, and the examiner assumes the exposure to x-rays. The valgus deviation force that can be applied during stability testing may lead to further disruption of a partially torn ligament or displacement of a ruptured ligament if the overforced maneuver is performed.13,14 That does not occur with our technique. On the other hand, the forces applied to the thumbs must be symmetrical for comparison purposes. The way to prevent these inconveniences is to perform the forced valgus maneuver over both thumbs simultaneously, under the same force application conditions and on a single radiograph, without requiring the examiner to remain with the patient in the radiography room.
Heim15 designed a system for simultaneous functional radiographs, but an apparatus must be built to adapt it to the frame of the radiography table, and the technique involves hyperpronating both hands and bandaging them to the forearm—which is uncomfortable and bothersome for patients and, in our opinion, has a poor application in high-volume EDs.
The technique of having the patient hold a bandage roll (J.J.’s thumb radiographic projection) offers several advantages:
1. The thumb can be placed in flexion, tightening the main fascicle of the UCL, which is how the UCL must be examined.
2. Forced valgus is allowed. Holding a water glass involves opening the thumb and the necessary stability of the MCP joint of the thumb (grip function of thumb); this radiographic technique is functional.
3. The examiner need not stay with the patient in the radiography room or be exposed to x-rays.
4. The bandage roll is thick enough to generate forced valgus in a patient with large hands. The nonrigid roll makes the examination more tolerable and avoids overforced valgus, eliminating the need for anesthetic blockade.
5. The technique is accessible and simple. In fact, there is no need to remove the roll from its wrapping.
1. Campbell CS. Gamekeeper’s thumb. J Bone Joint Surg Br. 1955;37(1):148-149.
2. Stener B. Displacement of the ruptured ulnar collateral ligament of the metacarpophalangeal joint of the thumb: a clinical and anatomic study. J Bone Joint Surg Br. 1962;44(4):869-879.
3. Stener B. Hyperextension injuries to the metacarpophalangeal joint of the thumb: rupture of ligaments, fracture of sesamoid bones, rupture of flexor pollicis brevis. An anatomical and clinical study. Acta Chir Scand. 1963;125:275-293.
4. Coonrad RW, Goldner JL. A study of the pathological findings and treatment in soft-tissue injury of the thumb metacarpophalangeal joint. With a clinical study of the normal range of motion in one thousand thumbs and a study of post mortem findings of ligamentous structures in relation to function. J Bone Joint Surg Am. 1968;50(3):439-451.
5. Parikh M, Nahigian S, Froimson A. Gamekeeper’s thumb. Plast Reconstr Surg. 1976;58(1):24-31.
6. Kaplan EB. The pathology and treatment of radial subluxation of the thumb with ulnar displacement of the head of the first metacarpal. J Bone Joint Surg Am. 1961;43:541-546.
7. Yamanaka K, Yoshida K, Inoue H, Inoue A, Miyagi T. Locking of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Am. 1985;67(5):782-787.
8. Sennwald G, Segmüller G, Egli A. The late reconstruction of the ligament of the metacarpo-phalangeal joint of the thumb [in English, French]. Ann Chir Main. 1987;6(1):15-24.
9. Smith RJ. Post-traumatic instability of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Am. 1977;59(1):14-21.
10. Louis DS, Huebner JJ Jr, Hankin FM. Rupture and displacement of the ulnar collateral ligament of the metacarpophalangeal joint of the thumb. Preoperative diagnosis. J Bone Joint Surg Am. 1986;68(9):1320-1326.
11. Heyman P, Gelberman RH, Duncan K, Hipp JA. Injuries of the ulnar collateral ligament of the thumb metacarpophalangeal joint. Biomechanical and prospective clinical studies on the usefulness of valgus stress testing. Clin Orthop Relat Res. 1993;(292):165-171.
12. Ritting AW, Baldwin PC, Rodner CM. Ulnar collateral ligament injury of the thumb metacarpophalangeal joint. Clin J Sport Med. 2010;20(2):106-112.
13. Cooper JG, Johnstone AJ, Hider P, Ardagh MW. Local anaesthetic infiltration increases the accuracy of assessment of ulnar collateral ligament injuries. Emerg Med Australas. 2005;17(2):132-136.
14. Noszian IM, Dinkhauser LM, Straub GM, Orthner E. Ulnar collateral ligament injuries of the thumb. Dislocation caused by stress radiography in 2 cases. Acta Orthop Scand. 1995;66(2):156-157.
15. Heim U. Simultaneous functional bilateral radiographies of the metacarpophalangeal joint of the thumb in hyper-pronation [in French]. Ann Chir Main. 1982;1(2):183-186.
Gamekeeper’s or skier’s thumb is caused by an injury to the ulnar collateral ligament (UCL) of the metacarpophalangeal (MCP) joint of the thumb. The mechanism of injury is forced radial and palmar abduction and hyperextension.
This lesion was initially described in 1955 by Campbell.1 It occurred in gamekeepers who worked in preserves in Scotland. The UCL was injured because of the way they killed rabbits—hence, gamekeeper’s thumb. Now these injuries are more common in skiers—skier’s thumb. In skiers, the mechanism of injury is the force exerted by the ski pole strap on the thumb during a fall. This injury is also seen in breakdancers.1,2
Different lesions can result, the most common being that of the UCL. The UCL lesion may be partial, with no joint instability,3,4 or total, with instability and subdislocation of the proximal phalanx.5-9 Rupture of the thumb adductor aponeurosis and displacement of the long extensor have been described as the cause of thumb instability.6-8
UCL rupture can occur in its extension or can cause a fracture-tearing in the proximal phalanx.9-12 Intra-articular fractures are sometimes found. The essential problem in UCL injuries is the impossibility of spontaneous healing once the rupture is complete, because of the Stener effect. (When the UCL ruptures, its proximal part retracts and runs above the fibrous expansion of the adductor muscle, which is interposed between the 2 parts of the ruptured UCL and prevents healing, even if the thumb is immobilized.) In these cases, only surgery can repair the lesion.2
In any thumb injury, particularly one caused by hyperabduction, a UCL lesion should be considered. The main problem is diagnosing sprain severity, which is evidenced by the degree of joint hypermobility. Radiologic examination should be performed in all cases to rule out fracture with tear, posterior capsular tear, palmar plate tear, and palmar subdislocation of the proximal phalanx, all of which are associated with UCL tearing.7-9
If the diagnosis is suspected, and radiographs show no fracture, comparative radiographs should be obtained in forced valgus.
Technique
We report on a simple, reliable, reproducible method that allows the patient’s thumbs to be compared, under the same force application conditions, on a single radiograph. This technique reduces the patient’s and examiner’s exposure to x-rays and is well tolerated by the patient. Anesthesia for the thumb is usually not necessary.
In each hand, the patient holds a cylindrical object, such as a drinking glass (standard diameter, 7.5-8.5 cm). We use an elastic crepe bandage roll (diameter, 7.5 cm; width, 10 cm). This roll is common in emergency departments (EDs) and easily accessible. The patient holds the rolls in his or her hands with the thumbs in the posteroanterior position (Figures 1–3) and places himself or herself on a 18×24-cm frame or directly on the radiography table.
Both thumbs are captured on a single functional radiograph for comparison of forced valgus of the MCP joints, as in our example cases. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Control Case
The single functional radiograph of both thumbs showed no evidence of joint laxity on the valgus stress test (Figure 4).
Case 1
A 72-year-old woman landed on her left hand when she fell backward while supporting the hand on a piece of furniture. She presented to the ED with pain in the region of the thumb and thenar eminence. Posteroanterior and lateral radiograph projections showed no significant bone injury (Figure 5). Given the patient’s persistent pain, the traumatologist suspected damage to the thumb UCL, so a simultaneous bilateral functional radiographic projection was obtained. The projection showed joint laxity, implying damage to the thumb UCL. Repair and reinsertion of the UCL were performed using a bone harpoon suture.
Case 2
A 58-year-old man sustained a left hand injury when, using both hands, he tried to catch hold of a falling wooden plank. When he presented to the ED the following week, he was given a diagnosis of thumb contusion and forced hyperabduction and was wearing a metal strap for immobilization. Radiographs showed no bone damage (Figure 6). Thumb UCL injury was suspected on the basis of the physical examination findings and the mechanism of injury. A bilateral simultaneous functional radiographic projection showed significant joint laxity. Surgical treatment with the pull-out technique was performed.
Case 3
A 44-year-old woman experienced forced traction from a dog leash and presented to the ED with pain in the right thumb region. Radiographs showed no bone damage (Figure 7). Thumb UCL injury was suspected. A bilateral simultaneous functional radiographic projection showed slight joint laxity, a sprain was diagnosed, and plaster bandaging was applied. Figures 8A–8D show the accurate thumb positions for performing the functional radiograph in forced valgus. We call the technique J.J.’s thumb radiographic projection.
Discussion
Examination using the stress test to cause joint tilt is crucial in making an accurate diagnosis and deciding on the most appropriate therapeutic approach.10 Most authors accept that surgical management is required in joint tilts over 30º, as these involve complete UCL rupture.10-12
The MCP joint must be examined in flexion, when the main fascicle of the UCL is tight, and not in extension, when the main fascicle of the UCL is relaxed. If we examine the thumb in extension, radial deviations may occur that are not caused by joint instability. Tilt here must be compared with that of the healthy side.11
Early diagnosis and adequate management are essential, as unnoticed or undervalued injuries can progress to painful sequelae, associated with stiffness, instability, and osteoarthritis, with evident harm to the grip and pinch functions of the hand. In many cases, clinical evidence of MCP joint instability is difficult. The radiologic diagnosis is usually obtained with comparative radiographs in forced valgus of both thumbs.
The forced valgus maneuver typically is performed by the examiner, who must stay with the patient in the radiography room and wear radiologic protection. Incredibly, some patients must force the valgus themselves.
The maneuver we have described clearly has complications, as it is painful, and some patients are uncooperative. Usually the thumb is anesthetized, and the examiner assumes the exposure to x-rays. The valgus deviation force that can be applied during stability testing may lead to further disruption of a partially torn ligament or displacement of a ruptured ligament if the overforced maneuver is performed.13,14 That does not occur with our technique. On the other hand, the forces applied to the thumbs must be symmetrical for comparison purposes. The way to prevent these inconveniences is to perform the forced valgus maneuver over both thumbs simultaneously, under the same force application conditions and on a single radiograph, without requiring the examiner to remain with the patient in the radiography room.
Heim15 designed a system for simultaneous functional radiographs, but an apparatus must be built to adapt it to the frame of the radiography table, and the technique involves hyperpronating both hands and bandaging them to the forearm—which is uncomfortable and bothersome for patients and, in our opinion, has a poor application in high-volume EDs.
The technique of having the patient hold a bandage roll (J.J.’s thumb radiographic projection) offers several advantages:
1. The thumb can be placed in flexion, tightening the main fascicle of the UCL, which is how the UCL must be examined.
2. Forced valgus is allowed. Holding a water glass involves opening the thumb and the necessary stability of the MCP joint of the thumb (grip function of thumb); this radiographic technique is functional.
3. The examiner need not stay with the patient in the radiography room or be exposed to x-rays.
4. The bandage roll is thick enough to generate forced valgus in a patient with large hands. The nonrigid roll makes the examination more tolerable and avoids overforced valgus, eliminating the need for anesthetic blockade.
5. The technique is accessible and simple. In fact, there is no need to remove the roll from its wrapping.
Gamekeeper’s or skier’s thumb is caused by an injury to the ulnar collateral ligament (UCL) of the metacarpophalangeal (MCP) joint of the thumb. The mechanism of injury is forced radial and palmar abduction and hyperextension.
This lesion was initially described in 1955 by Campbell.1 It occurred in gamekeepers who worked in preserves in Scotland. The UCL was injured because of the way they killed rabbits—hence, gamekeeper’s thumb. Now these injuries are more common in skiers—skier’s thumb. In skiers, the mechanism of injury is the force exerted by the ski pole strap on the thumb during a fall. This injury is also seen in breakdancers.1,2
Different lesions can result, the most common being that of the UCL. The UCL lesion may be partial, with no joint instability,3,4 or total, with instability and subdislocation of the proximal phalanx.5-9 Rupture of the thumb adductor aponeurosis and displacement of the long extensor have been described as the cause of thumb instability.6-8
UCL rupture can occur in its extension or can cause a fracture-tearing in the proximal phalanx.9-12 Intra-articular fractures are sometimes found. The essential problem in UCL injuries is the impossibility of spontaneous healing once the rupture is complete, because of the Stener effect. (When the UCL ruptures, its proximal part retracts and runs above the fibrous expansion of the adductor muscle, which is interposed between the 2 parts of the ruptured UCL and prevents healing, even if the thumb is immobilized.) In these cases, only surgery can repair the lesion.2
In any thumb injury, particularly one caused by hyperabduction, a UCL lesion should be considered. The main problem is diagnosing sprain severity, which is evidenced by the degree of joint hypermobility. Radiologic examination should be performed in all cases to rule out fracture with tear, posterior capsular tear, palmar plate tear, and palmar subdislocation of the proximal phalanx, all of which are associated with UCL tearing.7-9
If the diagnosis is suspected, and radiographs show no fracture, comparative radiographs should be obtained in forced valgus.
Technique
We report on a simple, reliable, reproducible method that allows the patient’s thumbs to be compared, under the same force application conditions, on a single radiograph. This technique reduces the patient’s and examiner’s exposure to x-rays and is well tolerated by the patient. Anesthesia for the thumb is usually not necessary.
In each hand, the patient holds a cylindrical object, such as a drinking glass (standard diameter, 7.5-8.5 cm). We use an elastic crepe bandage roll (diameter, 7.5 cm; width, 10 cm). This roll is common in emergency departments (EDs) and easily accessible. The patient holds the rolls in his or her hands with the thumbs in the posteroanterior position (Figures 1–3) and places himself or herself on a 18×24-cm frame or directly on the radiography table.
Both thumbs are captured on a single functional radiograph for comparison of forced valgus of the MCP joints, as in our example cases. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Control Case
The single functional radiograph of both thumbs showed no evidence of joint laxity on the valgus stress test (Figure 4).
Case 1
A 72-year-old woman landed on her left hand when she fell backward while supporting the hand on a piece of furniture. She presented to the ED with pain in the region of the thumb and thenar eminence. Posteroanterior and lateral radiograph projections showed no significant bone injury (Figure 5). Given the patient’s persistent pain, the traumatologist suspected damage to the thumb UCL, so a simultaneous bilateral functional radiographic projection was obtained. The projection showed joint laxity, implying damage to the thumb UCL. Repair and reinsertion of the UCL were performed using a bone harpoon suture.
Case 2
A 58-year-old man sustained a left hand injury when, using both hands, he tried to catch hold of a falling wooden plank. When he presented to the ED the following week, he was given a diagnosis of thumb contusion and forced hyperabduction and was wearing a metal strap for immobilization. Radiographs showed no bone damage (Figure 6). Thumb UCL injury was suspected on the basis of the physical examination findings and the mechanism of injury. A bilateral simultaneous functional radiographic projection showed significant joint laxity. Surgical treatment with the pull-out technique was performed.
Case 3
A 44-year-old woman experienced forced traction from a dog leash and presented to the ED with pain in the right thumb region. Radiographs showed no bone damage (Figure 7). Thumb UCL injury was suspected. A bilateral simultaneous functional radiographic projection showed slight joint laxity, a sprain was diagnosed, and plaster bandaging was applied. Figures 8A–8D show the accurate thumb positions for performing the functional radiograph in forced valgus. We call the technique J.J.’s thumb radiographic projection.
Discussion
Examination using the stress test to cause joint tilt is crucial in making an accurate diagnosis and deciding on the most appropriate therapeutic approach.10 Most authors accept that surgical management is required in joint tilts over 30º, as these involve complete UCL rupture.10-12
The MCP joint must be examined in flexion, when the main fascicle of the UCL is tight, and not in extension, when the main fascicle of the UCL is relaxed. If we examine the thumb in extension, radial deviations may occur that are not caused by joint instability. Tilt here must be compared with that of the healthy side.11
Early diagnosis and adequate management are essential, as unnoticed or undervalued injuries can progress to painful sequelae, associated with stiffness, instability, and osteoarthritis, with evident harm to the grip and pinch functions of the hand. In many cases, clinical evidence of MCP joint instability is difficult. The radiologic diagnosis is usually obtained with comparative radiographs in forced valgus of both thumbs.
The forced valgus maneuver typically is performed by the examiner, who must stay with the patient in the radiography room and wear radiologic protection. Incredibly, some patients must force the valgus themselves.
The maneuver we have described clearly has complications, as it is painful, and some patients are uncooperative. Usually the thumb is anesthetized, and the examiner assumes the exposure to x-rays. The valgus deviation force that can be applied during stability testing may lead to further disruption of a partially torn ligament or displacement of a ruptured ligament if the overforced maneuver is performed.13,14 That does not occur with our technique. On the other hand, the forces applied to the thumbs must be symmetrical for comparison purposes. The way to prevent these inconveniences is to perform the forced valgus maneuver over both thumbs simultaneously, under the same force application conditions and on a single radiograph, without requiring the examiner to remain with the patient in the radiography room.
Heim15 designed a system for simultaneous functional radiographs, but an apparatus must be built to adapt it to the frame of the radiography table, and the technique involves hyperpronating both hands and bandaging them to the forearm—which is uncomfortable and bothersome for patients and, in our opinion, has a poor application in high-volume EDs.
The technique of having the patient hold a bandage roll (J.J.’s thumb radiographic projection) offers several advantages:
1. The thumb can be placed in flexion, tightening the main fascicle of the UCL, which is how the UCL must be examined.
2. Forced valgus is allowed. Holding a water glass involves opening the thumb and the necessary stability of the MCP joint of the thumb (grip function of thumb); this radiographic technique is functional.
3. The examiner need not stay with the patient in the radiography room or be exposed to x-rays.
4. The bandage roll is thick enough to generate forced valgus in a patient with large hands. The nonrigid roll makes the examination more tolerable and avoids overforced valgus, eliminating the need for anesthetic blockade.
5. The technique is accessible and simple. In fact, there is no need to remove the roll from its wrapping.
1. Campbell CS. Gamekeeper’s thumb. J Bone Joint Surg Br. 1955;37(1):148-149.
2. Stener B. Displacement of the ruptured ulnar collateral ligament of the metacarpophalangeal joint of the thumb: a clinical and anatomic study. J Bone Joint Surg Br. 1962;44(4):869-879.
3. Stener B. Hyperextension injuries to the metacarpophalangeal joint of the thumb: rupture of ligaments, fracture of sesamoid bones, rupture of flexor pollicis brevis. An anatomical and clinical study. Acta Chir Scand. 1963;125:275-293.
4. Coonrad RW, Goldner JL. A study of the pathological findings and treatment in soft-tissue injury of the thumb metacarpophalangeal joint. With a clinical study of the normal range of motion in one thousand thumbs and a study of post mortem findings of ligamentous structures in relation to function. J Bone Joint Surg Am. 1968;50(3):439-451.
5. Parikh M, Nahigian S, Froimson A. Gamekeeper’s thumb. Plast Reconstr Surg. 1976;58(1):24-31.
6. Kaplan EB. The pathology and treatment of radial subluxation of the thumb with ulnar displacement of the head of the first metacarpal. J Bone Joint Surg Am. 1961;43:541-546.
7. Yamanaka K, Yoshida K, Inoue H, Inoue A, Miyagi T. Locking of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Am. 1985;67(5):782-787.
8. Sennwald G, Segmüller G, Egli A. The late reconstruction of the ligament of the metacarpo-phalangeal joint of the thumb [in English, French]. Ann Chir Main. 1987;6(1):15-24.
9. Smith RJ. Post-traumatic instability of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Am. 1977;59(1):14-21.
10. Louis DS, Huebner JJ Jr, Hankin FM. Rupture and displacement of the ulnar collateral ligament of the metacarpophalangeal joint of the thumb. Preoperative diagnosis. J Bone Joint Surg Am. 1986;68(9):1320-1326.
11. Heyman P, Gelberman RH, Duncan K, Hipp JA. Injuries of the ulnar collateral ligament of the thumb metacarpophalangeal joint. Biomechanical and prospective clinical studies on the usefulness of valgus stress testing. Clin Orthop Relat Res. 1993;(292):165-171.
12. Ritting AW, Baldwin PC, Rodner CM. Ulnar collateral ligament injury of the thumb metacarpophalangeal joint. Clin J Sport Med. 2010;20(2):106-112.
13. Cooper JG, Johnstone AJ, Hider P, Ardagh MW. Local anaesthetic infiltration increases the accuracy of assessment of ulnar collateral ligament injuries. Emerg Med Australas. 2005;17(2):132-136.
14. Noszian IM, Dinkhauser LM, Straub GM, Orthner E. Ulnar collateral ligament injuries of the thumb. Dislocation caused by stress radiography in 2 cases. Acta Orthop Scand. 1995;66(2):156-157.
15. Heim U. Simultaneous functional bilateral radiographies of the metacarpophalangeal joint of the thumb in hyper-pronation [in French]. Ann Chir Main. 1982;1(2):183-186.
1. Campbell CS. Gamekeeper’s thumb. J Bone Joint Surg Br. 1955;37(1):148-149.
2. Stener B. Displacement of the ruptured ulnar collateral ligament of the metacarpophalangeal joint of the thumb: a clinical and anatomic study. J Bone Joint Surg Br. 1962;44(4):869-879.
3. Stener B. Hyperextension injuries to the metacarpophalangeal joint of the thumb: rupture of ligaments, fracture of sesamoid bones, rupture of flexor pollicis brevis. An anatomical and clinical study. Acta Chir Scand. 1963;125:275-293.
4. Coonrad RW, Goldner JL. A study of the pathological findings and treatment in soft-tissue injury of the thumb metacarpophalangeal joint. With a clinical study of the normal range of motion in one thousand thumbs and a study of post mortem findings of ligamentous structures in relation to function. J Bone Joint Surg Am. 1968;50(3):439-451.
5. Parikh M, Nahigian S, Froimson A. Gamekeeper’s thumb. Plast Reconstr Surg. 1976;58(1):24-31.
6. Kaplan EB. The pathology and treatment of radial subluxation of the thumb with ulnar displacement of the head of the first metacarpal. J Bone Joint Surg Am. 1961;43:541-546.
7. Yamanaka K, Yoshida K, Inoue H, Inoue A, Miyagi T. Locking of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Am. 1985;67(5):782-787.
8. Sennwald G, Segmüller G, Egli A. The late reconstruction of the ligament of the metacarpo-phalangeal joint of the thumb [in English, French]. Ann Chir Main. 1987;6(1):15-24.
9. Smith RJ. Post-traumatic instability of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Am. 1977;59(1):14-21.
10. Louis DS, Huebner JJ Jr, Hankin FM. Rupture and displacement of the ulnar collateral ligament of the metacarpophalangeal joint of the thumb. Preoperative diagnosis. J Bone Joint Surg Am. 1986;68(9):1320-1326.
11. Heyman P, Gelberman RH, Duncan K, Hipp JA. Injuries of the ulnar collateral ligament of the thumb metacarpophalangeal joint. Biomechanical and prospective clinical studies on the usefulness of valgus stress testing. Clin Orthop Relat Res. 1993;(292):165-171.
12. Ritting AW, Baldwin PC, Rodner CM. Ulnar collateral ligament injury of the thumb metacarpophalangeal joint. Clin J Sport Med. 2010;20(2):106-112.
13. Cooper JG, Johnstone AJ, Hider P, Ardagh MW. Local anaesthetic infiltration increases the accuracy of assessment of ulnar collateral ligament injuries. Emerg Med Australas. 2005;17(2):132-136.
14. Noszian IM, Dinkhauser LM, Straub GM, Orthner E. Ulnar collateral ligament injuries of the thumb. Dislocation caused by stress radiography in 2 cases. Acta Orthop Scand. 1995;66(2):156-157.
15. Heim U. Simultaneous functional bilateral radiographies of the metacarpophalangeal joint of the thumb in hyper-pronation [in French]. Ann Chir Main. 1982;1(2):183-186.
Evaluation of 3 Fixation Devices for Tibial-Sided Anterior Cruciate Ligament Graft Backup Fixation
Restoration of stability with return to activity is generally expected after anterior cruciate ligament (ACL) reconstruction; long-term success rates range from 75% to 95%.1 However, graft failure occurs most frequently with soft-tissue grafts fixated only with interference screws.2,3 Fixation failure also occurs more frequently at the tibial site.2 This failure has been attributed to extensive graft slippage in cases of soft-tissue fixation with interference screws.2 Interference screw fixation alone, with a double-looped hamstring tendon graft, fails at 350 N in young human tibiae.4,5 However, failure is limited with use of a bone–tendon–bone graft or with backup fixation, particularly at the tibial site.3 The superiority of bicortical fixation has also been proven.5-7
In addition, as shown in a goat model, ACL graft fixation is a major cause of failure in the immediate postoperative period, before biological incorporation of the graft.8 Fixation techniques for soft-tissue grafts must withstand stresses during the healing period (grafts may take up to 12 weeks to incorporate).9 Failures may result from forces exerted on the graft—forces that may be as high as 450 to 700 N during daily activities.10,11 Within the tibial tunnel, various fixation devices are used, including interference screws, staples, pins, buttons, and interference screw/sheath constructs.12,13 Primary fixation is commonly achieved with interference screws because of their ease of insertion and greater stiffness. However, fixation of the soft-tissue graft is influenced by several variables, including bone density, insertion torque, thread geometry, and interference screw material.14-16 Many of these variables, which are a source of inconsistency and concern during the immediate postoperative period, have led surgeons to seek alternative methods of backup fixation at the tibial site. Nevertheless, good clinical and subjective results have been found after ACL reconstruction with a 4-stranded semitendinosus tendon at 10-year follow-up.17
An anchor used in rotator cuff repair is the SwiveLock system (Arthrex). Major advantages of this system include ease and speed of insertion, good strength, and reduced need for later hardware removal.
We conducted a study to biomechanically evaluate 3 methods of tibial-sided fixation for ACL reconstruction: fully threaded interference screw only, interference screw backed with 4.75-mm SwiveLock anchor, and fully threaded bio-interference screw backed with 4.5-mm bicortical screw. We hypothesized that a fully threaded bio-interference screw backed with a 4.75-mm SwiveLock anchor would provide mechanical strength no different from that provided by backup fixation with a bicortical post at the tibial site. We further hypothesized that SwiveLock backup fixation would provide more strength than fixation with bio-interference screw alone.
Materials and Methods
The design of this study was adapted from one used by Walsh and colleagues,3 who compared 3 fixation methods: retrograde interference screw, suture button, and combined fixation. Tibiae inspected before selection showed no signs of injury or abnormality. Bovine extensor tendons, which lacked any defects along their entire length, were stored in saline 0.9% solution. Both the tibiae and the extensor tendons were stored at –20°C before completion of the tibial-sided ACL reconstruction. Thirty fresh-frozen, skeletally mature porcine proximal tibiae were selected and thawed at 4°C before preparation. Specimens were prepared by potting the diaphysis in fiberglass resin, and a tunnel 9 mm in diameter was drilled through the anteromedial aspect of the tibia.
For consistency, one author (CAV) prepared all 30 specimens. Both tails of all 30 bovine extensor tendons were whip-stitched with No. 2 FiberLoop (Arthrex) 9 mm in diameter. Grafts and tibiae were randomly divided into 3 sample groups. The first group was prepared by antegrade graft fixation within the tibial tunnel using a fully threaded 9×28-mm BioComposite interference screw (Arthrex). The second and third groups used the same primary fixation within the tibial tunnel along with 2 types of secondary fixation. These backup fixation groups included a 4.5-mm titanium bicortical post (Arthrex) and a 4.75-mm BioComposite SwiveLock C anchor (Arthrex) (Figure 1). The FiberLoop at the ends of the distal graft tails for backup groups were fixated 1 cm distal to the tibial tunnel and tapped before insertion of backup devices (Figures 2A, 2B). Insertion was completed after 4.5-mm bicortical and 4.75-mm unicortical drilling and tapping of the anteromedial cortices for the titanium posts and SwiveLocks, respectively. The free ends of the whip-stitched No. 2 FiberLoop were tied to the proximal end of the titanium post with a single surgical knot followed by 5 square knots.3 The free ends of the No. 2 FiberLoop were inserted into the eyelet of the 4.75-mm SwiveLock and 1 cm directly inferior to the tibial tunnel. Interference fit of FiberLoop with SwiveLock was achieved within the corticocancellous bone of the tibiae. All samples retained a 30-mm tendon loop superior to the tibial plateau to simulate intra-articular ACL length. Specimens were then stored at –20°C and thawed at 4°C before biomechanical testing.
Each of the 30 tibiae was tested once. Each testing group consisted of 10 porcine tibiae. The tendons were kept moist during the entire testing procedure by spraying them thoroughly with saline 0.9% solution. Mechanical testing was performed with an Instron 8871 system with a 5-kN load cell secured to the crosshead. A fixed-angle aperture, attached to the testing surface, was adjusted so that the tendon would be pulled in line with the tibial tunnel. A hook fixture suspended from clevis and dowel was used to secure the tendon to the crosshead (Figure 3). A small preload of 5 N manually applied to each sample was followed by a precycling regimen of 10 cycles between 10 N and 50 N at 1 Hz. Precycling was performed to remove slack from the system. Mechanical testing consisted of 500 cycles between 50 N and 250 N at 1 Hz followed by pull to failure at 20 mm per minute. Load and displacement data were recorded at 500 Hz.
An a priori power analysis was not performed because 6 specimens per group in the study from which the testing protocol was adapted demonstrated sufficient power among 3 testing categories.3 In addition, other studies have demonstrated similar testing protocols using 10 specimens per testing group.7,12,13,18 The data for each sample were analyzed with OriginPro 8.0 software (OriginLab). Ultimate load, yield load, stiffness, and cyclic displacement of the 3 sample groups were compared with 1-way analysis of variance (α = 0.05). Holm-Sidak tests were used for post hoc analysis.19P < .05 was statistically significant.
Results
None of the 30 specimens failed during preloading. Modes of failure were consistent among groups. All 10 specimens in the interference-screw-only group failed by graft slippage past the screw in the tibial tunnel. Nineteen of the 20 specimens in the backup-fixation groups failed by graft slippage past the screw and suture cutout through the distal graft tail. In the bicortical-post backup group, 1 failure was attributed to tendon tearing proximal to whip-stitching. There were no instances of hardware breakage or failure of either titanium screw or SwiveLock anchor.
Mean (SD) cyclic displacement was higher in the interference-screw-only group, 3.5 (2.2) mm, than in the SwiveLock backup group, 2.6 (0.5) mm, and the bicortical-post backup group, 2.1 (0.6) mm; no statistical significance was demonstrated between any 2 of these groups alone (P = .12) (Figure 4). Mean (SD) pullout stiffness was higher in the bicortical-post backup group, 192 (48) N/mm, than in the SwiveLock backup group, 164 (53) N/mm, and the screw-only group, 163 (64) N/mm (P = .42) (Figure 5). Mean (SD) initial load at 5 mm of displacement was higher in the bicortical-post backup group, 482 (156) N, and the SwiveLock backup group, 423 (94) N, than in the screw-only group, 381 (169) N (P = .30).
Mean (SD) yield load was higher in the bicortical-post backup group, 829 (253) N, than in the SwiveLock backup group, 642 (172) N, and the interference-screw-only group, 496 (133) N (P = .003). Statistical significance was demonstrated between the screw-only and bicortical-post groups (P = .002) and between the screw-only and SwiveLock groups (P = .048). There was no statistical difference between the bicortical-post and SwiveLock groups (P = .07).
Mean (SD) ultimate load to failure was higher in the bicortical-post backup group, 1148 (186) N, than in the SwiveLock backup group, 1007 (176) N, and the interference-screw-only group, 778 (139) N (Figure 6). The difference was statistically significant, whereby the screw-only group failed at a lower load compared with the bicortical-post group (P < .001) and the SwiveLock group (P = .005). The 2 backup groups were not statistically different (P = .1).
Discussion
We investigated whether a fully threaded bio-interference screw backed with a 4.75-mm SwiveLock anchor would provide mechanically equivalent pullout strength within the tibial tunnel during ACL reconstruction with soft-tissue allografts in comparison either with a fully threaded bio-interference screw backed with a bicortical post or with a fully threaded bio-interference screw without backup fixation. The results of the study support this hypothesis. With SwiveLock used for backup fixation, there was no significant difference in stiffness or cyclic load displacement between the screw-only, SwiveLock, and bicortical-post groups. However, adding backup fixation could particularly help improve fixation consistency. Specifically, although after only 500 cycles there was no statistically significant difference in cyclic displacement, continued cycling may be clinically relevant if graft slippage exceeded limits to allow for healing within the tibial tunnel. Conversely, a significantly larger difference was found between the SwiveLock, bicortical-post, and screw-only groups in yield load and ultimate load to failure. However, there was no significant difference between the SwiveLock and bicortical-post groups.
In this study, interference screw with SwiveLock backup demonstrated a mean (SD) ultimate load to failure of 1007 (176) N, comparable to that found by Walsh and colleagues3 for retrograde bio-interference screw with suture button, 1027 (157.11) N. In a study comparing quadrupled hamstring tibial graft fixation, Intrafix (DePuy Mitek) and an 8×25-mm Bioscrew (Linvatec) demonstrated mean (SD) single-cycle yield loads of 1332 (304) N and 612 (176) N, respectively.13 These results are similar to the ultimate yield loads in the present study: bicortical-post group, 1148 (186) N; SwiveLock group, 1007 (176) N; screw-only group, 778 (139) N. Differences may be attributed to hamstring tendons used in a quadrupled manner in the aforementioned study.12,13 Last, mean (SD) ultimate load to failure in a study that used only a retrograde bio-interference screw (9×20 mm) was 679.00 (109.44) N,3 similar to the 778 (139) N found for interference-screw-only in the present study. The difference is likely attributable to the longer screw (9×28 mm) in our study. Using SwiveLock C in cortical bone, Barber and colleagues18 found mean (SD) loads to failure up to 711.9 (89.1) N.
Clinically, it has been shown that a statistically significant increase in anterior laxity occurred between 4 months and 2 years in 10.7% of patients who underwent hamstring ACL reconstruction.20 The knees were clinically categorized as unstable or severely abnormal. The authors concluded that the clinical outcome was more likely influenced by the methods used to fix the free ends of the graft, specifically with 2 staples or a washer. To simulate early postoperative rehabilitation in the present study, cyclic loading of the graft was performed. Ultimate load to failure was then determined in order to evaluate catastrophic failure strength of the backup fixation devices in comparison with the interference-screw-only group without supplementary fixation.
It has been shown in autologous hamstring ACL reconstruction that a centrally placed polyethylene screw with sheath (Intrafix) performed as well as a standard, eccentrically placed metal interference screw with staple.10 It is therefore logical that backup fixation with use of a similar device (eg, SwiveLock, bicortical post) is necessary to ensure comparable clinical outcomes in relation to a screw/sheath device that has been shown to endure the highest yield loads.2,9,12,13,21-23 Potential benefits of using SwiveLock anchors for backup fixation include a statistically significant increased mean (SD) ultimate yield load of 229 (176) N over interference screw only. These results are similar to those in comparable studies: 218.3 (59.7) N24 and 165 (24.15) N25 in healthy bone with a reported bone mineral density (BMD) of 1.39 g/cm2, similar to that of skeletally mature porcine tibia (1.220-1.675 g/cm²).3 In addition, ease of insertion of this device over a bicortical post was demonstrated. The titanium post required bicortical drilling as well as measurement with a depth gauge to ensure adequate screw length. This process appeared to require more time during specimen preparation and theoretically could prove to be more dangerous clinically.7 However, caution in using a SwiveLock anchor in osteoporotic bone is advised because of reduced pullout strength.26 In this case, bicortical-post backup fixation may be more suitable. Moreover, although not demonstrated in this study, hardware prominence and irritation with a post may cause postoperative morbidity necessitating future removal.20 Hardware removal was the most common reason for additional surgery using hamstring tendons as graft.20 A second surgery for hardware removal was required in 21% of these patients.20 This is unlikely to occur with a SwiveLock, as the anchor is buried within cortical bone.
Limitations
Regarding use of nonhuman tissues in a biomechanical model, porcine tibiae and bovine extensor tendons were used because of availability, consistency among specimens, and cost-effectiveness. However, bovine extensor tendons have been shown to exhibit stiffness and viscoelastic properties similar to those of a human hamstring graft.27 In addition, the BMD of the porcine tibiae used in this study was not tested because of time involved and cost-efficiency. However, it has been shown that average BMD of porcine tibiae, 1.220-1.675 g/cm², is similar to that in a young athletic population, 1.24-1.62 g/cm2.3,28-31 We therefore assumed similarity to a young athletic population and uniformity of BMD of the porcine tibiae used in this study.
In addition, the biomechanical testing protocol did not simulate physiologic loading within the tibial tunnel. Moreover, the testing protocol used loads of only 250 N during cyclic testing for 500 cycles. This simulates only the early rehabilitation period and not the healing period, which may last up to 12 weeks.9 In addition, as previously mentioned, forces on the graft may be as high as 450 to 700 N.11,32 Pullout testing in line with the long axis of the tibia was performed in order to compare mechanical testing results with those of similar studies.3,12,13 Last, the P of .07 for the comparison of ultimate load to failure between the 2 backup fixation groups suggests that this study may have been underpowered.
Conclusion
This study demonstrated an effective, alternative, and equivalent backup fixation device that can help prevent graft slippage within the tibial tunnel during soft-tissue ACL reconstruction. Potential benefits of using SwiveLock anchors for backup fixation include a significantly increased ultimate yield load (229 N) when supplementing an interference screw, ease of insertion compared with a bicortical post, and the improbable need for future hardware removal. We support using SwiveLock for supplementary fixation at the tibial tunnel site when using soft-tissue grafts in ACL reconstruction.
1. Wetzler MJ, Bartolozzi AR, Gillespie MJ, Rubenstein DL, Ciccotti MG, Miller LS. Revision anterior cruciate ligament reconstruction. Oper Tech Orthop. 1996;6(3):181-189.
2. Scheffler SU, Südkamp NP, Göckenjan A, Hoffmann RF, Weiler A. Biomechanical comparison of hamstring and patellar tendon graft anterior cruciate ligament reconstruction techniques: the impact of fixation level and fixation method under cyclic loading. Arthroscopy. 2002;18(3):304-315.
3. Walsh MP, Wijdicks CA, Parker JB, Hapa O, LaPrade RF. A comparison between a retrograde interference screw, suture button, and combined fixation on the tibial side in an all-inside anterior cruciate ligament reconstruction: a biomechanical study in a porcine model. Am J Sports Med. 2009;37(1):160-167.
4. Howell SM, Hull ML. Aggressive rehabilitation using hamstring tendons: graft construct, tibial tunnel placement, fixation properties, and clinical outcome. Am J Knee Surg. 1998;11(2):120-127.
5. Magen HE, Howell SM, Hull ML. Structural properties of six tibial fixation methods for anterior cruciate ligament soft tissue grafts. Am J Sports Med. 1999;27(1):35-43.
6. Beynnon BD, Meriam CM, Ryder SH, Fleming BC, Johnson RJ. The effect of screw insertion torque on tendons fixed with spiked washers. Am J Sports Med. 1998;26(4):536-539.
7. Post WR, King SS. Neurovascular risk of bicortical tibial drilling for screw and spiked washer fixation of soft-tissue anterior cruciate ligament graft. Arthroscopy. 2001;17(3):244-247.
8. Holden JP, Grood ES, Butler DL, et al. Biomechanics of fascia lata ligament replacements: early postoperative changes in the goat. J Orthop Res. 1988;6(5):639-647.
9. Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75(12):1795-1803.
10. Frank CB, Jackson DW. The science of reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. 1997;79(10):1556-1576.
11. Markolf KL, Willems MJ, Jackson SR, Finerman GA. In situ calibration of miniature sensors implanted into the anterior cruciate ligament. Part I: strain measurements. J Orthop Res. 1998;16(4):455-463.
12. Kousa P, Teppo LN, Jarvinen TL, Vihavainen M, Kannus P, Jarvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction: I. Femoral site. Am J Sports Med. 2003;3 (2)1:174-181.
13. Kousa P, Jarvinen TL, Vihavainen M, Kannus P, Jarvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction: II. Tibial site. Am J Sports Med. 2003;31(2):182-188.
14. Brand JC Jr, Pienkowski D, Steenlage E, Hamilton D, Johnson DL, Caborn DN. Interference screw fixation strength of a quadrupled hamstring tendon graft is directly related to bone mineral density and insertion torque. Am J Sports Med. 2000;28(5):705-710.
15. Weiler A, Hoffmann RF, Siepe CJ, Kolbeck SF, Südkamp NP. The influence of screw geometry on hamstring tendon interference fit fixation. Am J Sports Med. 2000;28(3):356-359.
16. Weiler A, Hoffmann RF, Stähelin AC, Bail HJ, Siepe CJ, Südkamp NP. Hamstring tendon fixation using interference screws: a biomechanical study in calf tibial bone. Arthroscopy. 1998;14(1):29-37.
17. Streich NA, Reichenbacher S, Barié A, Buchner M, Schmitt H. Long-term outcome of anterior cruciate ligament reconstruction with an autologous four-strand semitendinosus tendon autograft. Int Orthop. 2013;37(2):279-284.
18. Barber FA, Herbert MA, Beavis C, Barrera Oro F. Suture anchor materials, eyelets, and designs: update 2008. Arthroscopy. 2008;24(8):859-867.
19. Aickin M, Gensler H. Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. Am J Public Health. 1996;86(5):726-728.
20. Howell SM, Deutsch ML. Comparison of endoscopic and two-incision techniques for reconstructing a torn anterior cruciate ligament using hamstring tendons. Arthroscopy. 1999;15(6):594-606.
21. Gwynne-Jones DP, Draffin J, Vane A, Craig R, McMahon S. Failure strengths of concentric and eccentric implants for hamstring graft fixation. ANZ J Surg. 2008;78(3):177-181.
22. Hayes DA, Watts MC, Tevelen GA, Crawford RW. Central versus peripheral tibial screw placement in hamstring anterior cruciate ligament reconstruction: in vitro biomechanics. Arthroscopy. 2005;21(6):703-706.
23. Shino K, Pflaster DS. Comparison of eccentric and concentric screw placement for hamstring graft fixation in the tibial tunnel. Knee Surg Sports Traumatol Arthrosc. 2000;8(2):73-75.
24. Prevrhal S, Fuerst T, Fan B, et al. Quantitative ultrasound of the tibia depends on both cortical density and thickness. Osteoporosis Int. 2001;12(1):28-34.
25. Pietschmann MF, Gülecyüz MF, Fieseler S, et al. Biomechanical stability of knotless suture anchors used in rotator cuff repair in healthy and osteopenic bone. Arthroscopy. 2010;26(8):1035-1044.
26. Burns JP, Snyder SJ, Albritton M. Arthroscopic rotator cuff repair using triple-loaded anchors, suture shuttles, and suture savers. J Am Acad Orthop Surg. 2007;15(7):432-444.
27. Tetsumura S, Fujita A, Nakajima M, Abe M. Biomechanical comparison of different fixation methods on the tibial side in anterior cruciate ligament reconstruction: a biomechanical study in porcine tibial bone. J Orthop Sci. 2006;11(3):278-282.
28. Alfredson H, Nordstrom P, Lorentzon R. Total and regional bone mass in female soccer players. Calcif Tissue Int. 1996;59(6):438-442.
29. Nevill AM, Holder RL, Stewart AD. Modeling elite male athletes’ peripheral bone mass, assessed using regional dual x-ray absorptiometry. Bone. 2003;32(1):62-68.
30. Nordström P, Lorentzon R. Site-specific bone mass differences of the lower extremities in 17-year-old ice hockey players. Calcif Tissue Int. 1996;59(6):4443-4448.
31. Patzer T, Santo G, Olender GD, Wellmann M, Hurschler C, Schofer MD. Suprapectoral or subpectoral position for biceps tenodesis: biomechanical comparison of four different techniques in both positions. J Shoulder Elbow Surg. 2012;21(1):116-125.
32. De Wall M, Scholes CJ, Patel S, Coolican MR, Parker DA. Tibial fixation in anterior cruciate ligament reconstruction: a prospective randomized study comparing metal interference screw and staples with a centrally placed polyethylene screw and sheath. Am J Sports Med. 2011;39(9):1858-1864.
Restoration of stability with return to activity is generally expected after anterior cruciate ligament (ACL) reconstruction; long-term success rates range from 75% to 95%.1 However, graft failure occurs most frequently with soft-tissue grafts fixated only with interference screws.2,3 Fixation failure also occurs more frequently at the tibial site.2 This failure has been attributed to extensive graft slippage in cases of soft-tissue fixation with interference screws.2 Interference screw fixation alone, with a double-looped hamstring tendon graft, fails at 350 N in young human tibiae.4,5 However, failure is limited with use of a bone–tendon–bone graft or with backup fixation, particularly at the tibial site.3 The superiority of bicortical fixation has also been proven.5-7
In addition, as shown in a goat model, ACL graft fixation is a major cause of failure in the immediate postoperative period, before biological incorporation of the graft.8 Fixation techniques for soft-tissue grafts must withstand stresses during the healing period (grafts may take up to 12 weeks to incorporate).9 Failures may result from forces exerted on the graft—forces that may be as high as 450 to 700 N during daily activities.10,11 Within the tibial tunnel, various fixation devices are used, including interference screws, staples, pins, buttons, and interference screw/sheath constructs.12,13 Primary fixation is commonly achieved with interference screws because of their ease of insertion and greater stiffness. However, fixation of the soft-tissue graft is influenced by several variables, including bone density, insertion torque, thread geometry, and interference screw material.14-16 Many of these variables, which are a source of inconsistency and concern during the immediate postoperative period, have led surgeons to seek alternative methods of backup fixation at the tibial site. Nevertheless, good clinical and subjective results have been found after ACL reconstruction with a 4-stranded semitendinosus tendon at 10-year follow-up.17
An anchor used in rotator cuff repair is the SwiveLock system (Arthrex). Major advantages of this system include ease and speed of insertion, good strength, and reduced need for later hardware removal.
We conducted a study to biomechanically evaluate 3 methods of tibial-sided fixation for ACL reconstruction: fully threaded interference screw only, interference screw backed with 4.75-mm SwiveLock anchor, and fully threaded bio-interference screw backed with 4.5-mm bicortical screw. We hypothesized that a fully threaded bio-interference screw backed with a 4.75-mm SwiveLock anchor would provide mechanical strength no different from that provided by backup fixation with a bicortical post at the tibial site. We further hypothesized that SwiveLock backup fixation would provide more strength than fixation with bio-interference screw alone.
Materials and Methods
The design of this study was adapted from one used by Walsh and colleagues,3 who compared 3 fixation methods: retrograde interference screw, suture button, and combined fixation. Tibiae inspected before selection showed no signs of injury or abnormality. Bovine extensor tendons, which lacked any defects along their entire length, were stored in saline 0.9% solution. Both the tibiae and the extensor tendons were stored at –20°C before completion of the tibial-sided ACL reconstruction. Thirty fresh-frozen, skeletally mature porcine proximal tibiae were selected and thawed at 4°C before preparation. Specimens were prepared by potting the diaphysis in fiberglass resin, and a tunnel 9 mm in diameter was drilled through the anteromedial aspect of the tibia.
For consistency, one author (CAV) prepared all 30 specimens. Both tails of all 30 bovine extensor tendons were whip-stitched with No. 2 FiberLoop (Arthrex) 9 mm in diameter. Grafts and tibiae were randomly divided into 3 sample groups. The first group was prepared by antegrade graft fixation within the tibial tunnel using a fully threaded 9×28-mm BioComposite interference screw (Arthrex). The second and third groups used the same primary fixation within the tibial tunnel along with 2 types of secondary fixation. These backup fixation groups included a 4.5-mm titanium bicortical post (Arthrex) and a 4.75-mm BioComposite SwiveLock C anchor (Arthrex) (Figure 1). The FiberLoop at the ends of the distal graft tails for backup groups were fixated 1 cm distal to the tibial tunnel and tapped before insertion of backup devices (Figures 2A, 2B). Insertion was completed after 4.5-mm bicortical and 4.75-mm unicortical drilling and tapping of the anteromedial cortices for the titanium posts and SwiveLocks, respectively. The free ends of the whip-stitched No. 2 FiberLoop were tied to the proximal end of the titanium post with a single surgical knot followed by 5 square knots.3 The free ends of the No. 2 FiberLoop were inserted into the eyelet of the 4.75-mm SwiveLock and 1 cm directly inferior to the tibial tunnel. Interference fit of FiberLoop with SwiveLock was achieved within the corticocancellous bone of the tibiae. All samples retained a 30-mm tendon loop superior to the tibial plateau to simulate intra-articular ACL length. Specimens were then stored at –20°C and thawed at 4°C before biomechanical testing.
Each of the 30 tibiae was tested once. Each testing group consisted of 10 porcine tibiae. The tendons were kept moist during the entire testing procedure by spraying them thoroughly with saline 0.9% solution. Mechanical testing was performed with an Instron 8871 system with a 5-kN load cell secured to the crosshead. A fixed-angle aperture, attached to the testing surface, was adjusted so that the tendon would be pulled in line with the tibial tunnel. A hook fixture suspended from clevis and dowel was used to secure the tendon to the crosshead (Figure 3). A small preload of 5 N manually applied to each sample was followed by a precycling regimen of 10 cycles between 10 N and 50 N at 1 Hz. Precycling was performed to remove slack from the system. Mechanical testing consisted of 500 cycles between 50 N and 250 N at 1 Hz followed by pull to failure at 20 mm per minute. Load and displacement data were recorded at 500 Hz.
An a priori power analysis was not performed because 6 specimens per group in the study from which the testing protocol was adapted demonstrated sufficient power among 3 testing categories.3 In addition, other studies have demonstrated similar testing protocols using 10 specimens per testing group.7,12,13,18 The data for each sample were analyzed with OriginPro 8.0 software (OriginLab). Ultimate load, yield load, stiffness, and cyclic displacement of the 3 sample groups were compared with 1-way analysis of variance (α = 0.05). Holm-Sidak tests were used for post hoc analysis.19P < .05 was statistically significant.
Results
None of the 30 specimens failed during preloading. Modes of failure were consistent among groups. All 10 specimens in the interference-screw-only group failed by graft slippage past the screw in the tibial tunnel. Nineteen of the 20 specimens in the backup-fixation groups failed by graft slippage past the screw and suture cutout through the distal graft tail. In the bicortical-post backup group, 1 failure was attributed to tendon tearing proximal to whip-stitching. There were no instances of hardware breakage or failure of either titanium screw or SwiveLock anchor.
Mean (SD) cyclic displacement was higher in the interference-screw-only group, 3.5 (2.2) mm, than in the SwiveLock backup group, 2.6 (0.5) mm, and the bicortical-post backup group, 2.1 (0.6) mm; no statistical significance was demonstrated between any 2 of these groups alone (P = .12) (Figure 4). Mean (SD) pullout stiffness was higher in the bicortical-post backup group, 192 (48) N/mm, than in the SwiveLock backup group, 164 (53) N/mm, and the screw-only group, 163 (64) N/mm (P = .42) (Figure 5). Mean (SD) initial load at 5 mm of displacement was higher in the bicortical-post backup group, 482 (156) N, and the SwiveLock backup group, 423 (94) N, than in the screw-only group, 381 (169) N (P = .30).
Mean (SD) yield load was higher in the bicortical-post backup group, 829 (253) N, than in the SwiveLock backup group, 642 (172) N, and the interference-screw-only group, 496 (133) N (P = .003). Statistical significance was demonstrated between the screw-only and bicortical-post groups (P = .002) and between the screw-only and SwiveLock groups (P = .048). There was no statistical difference between the bicortical-post and SwiveLock groups (P = .07).
Mean (SD) ultimate load to failure was higher in the bicortical-post backup group, 1148 (186) N, than in the SwiveLock backup group, 1007 (176) N, and the interference-screw-only group, 778 (139) N (Figure 6). The difference was statistically significant, whereby the screw-only group failed at a lower load compared with the bicortical-post group (P < .001) and the SwiveLock group (P = .005). The 2 backup groups were not statistically different (P = .1).
Discussion
We investigated whether a fully threaded bio-interference screw backed with a 4.75-mm SwiveLock anchor would provide mechanically equivalent pullout strength within the tibial tunnel during ACL reconstruction with soft-tissue allografts in comparison either with a fully threaded bio-interference screw backed with a bicortical post or with a fully threaded bio-interference screw without backup fixation. The results of the study support this hypothesis. With SwiveLock used for backup fixation, there was no significant difference in stiffness or cyclic load displacement between the screw-only, SwiveLock, and bicortical-post groups. However, adding backup fixation could particularly help improve fixation consistency. Specifically, although after only 500 cycles there was no statistically significant difference in cyclic displacement, continued cycling may be clinically relevant if graft slippage exceeded limits to allow for healing within the tibial tunnel. Conversely, a significantly larger difference was found between the SwiveLock, bicortical-post, and screw-only groups in yield load and ultimate load to failure. However, there was no significant difference between the SwiveLock and bicortical-post groups.
In this study, interference screw with SwiveLock backup demonstrated a mean (SD) ultimate load to failure of 1007 (176) N, comparable to that found by Walsh and colleagues3 for retrograde bio-interference screw with suture button, 1027 (157.11) N. In a study comparing quadrupled hamstring tibial graft fixation, Intrafix (DePuy Mitek) and an 8×25-mm Bioscrew (Linvatec) demonstrated mean (SD) single-cycle yield loads of 1332 (304) N and 612 (176) N, respectively.13 These results are similar to the ultimate yield loads in the present study: bicortical-post group, 1148 (186) N; SwiveLock group, 1007 (176) N; screw-only group, 778 (139) N. Differences may be attributed to hamstring tendons used in a quadrupled manner in the aforementioned study.12,13 Last, mean (SD) ultimate load to failure in a study that used only a retrograde bio-interference screw (9×20 mm) was 679.00 (109.44) N,3 similar to the 778 (139) N found for interference-screw-only in the present study. The difference is likely attributable to the longer screw (9×28 mm) in our study. Using SwiveLock C in cortical bone, Barber and colleagues18 found mean (SD) loads to failure up to 711.9 (89.1) N.
Clinically, it has been shown that a statistically significant increase in anterior laxity occurred between 4 months and 2 years in 10.7% of patients who underwent hamstring ACL reconstruction.20 The knees were clinically categorized as unstable or severely abnormal. The authors concluded that the clinical outcome was more likely influenced by the methods used to fix the free ends of the graft, specifically with 2 staples or a washer. To simulate early postoperative rehabilitation in the present study, cyclic loading of the graft was performed. Ultimate load to failure was then determined in order to evaluate catastrophic failure strength of the backup fixation devices in comparison with the interference-screw-only group without supplementary fixation.
It has been shown in autologous hamstring ACL reconstruction that a centrally placed polyethylene screw with sheath (Intrafix) performed as well as a standard, eccentrically placed metal interference screw with staple.10 It is therefore logical that backup fixation with use of a similar device (eg, SwiveLock, bicortical post) is necessary to ensure comparable clinical outcomes in relation to a screw/sheath device that has been shown to endure the highest yield loads.2,9,12,13,21-23 Potential benefits of using SwiveLock anchors for backup fixation include a statistically significant increased mean (SD) ultimate yield load of 229 (176) N over interference screw only. These results are similar to those in comparable studies: 218.3 (59.7) N24 and 165 (24.15) N25 in healthy bone with a reported bone mineral density (BMD) of 1.39 g/cm2, similar to that of skeletally mature porcine tibia (1.220-1.675 g/cm²).3 In addition, ease of insertion of this device over a bicortical post was demonstrated. The titanium post required bicortical drilling as well as measurement with a depth gauge to ensure adequate screw length. This process appeared to require more time during specimen preparation and theoretically could prove to be more dangerous clinically.7 However, caution in using a SwiveLock anchor in osteoporotic bone is advised because of reduced pullout strength.26 In this case, bicortical-post backup fixation may be more suitable. Moreover, although not demonstrated in this study, hardware prominence and irritation with a post may cause postoperative morbidity necessitating future removal.20 Hardware removal was the most common reason for additional surgery using hamstring tendons as graft.20 A second surgery for hardware removal was required in 21% of these patients.20 This is unlikely to occur with a SwiveLock, as the anchor is buried within cortical bone.
Limitations
Regarding use of nonhuman tissues in a biomechanical model, porcine tibiae and bovine extensor tendons were used because of availability, consistency among specimens, and cost-effectiveness. However, bovine extensor tendons have been shown to exhibit stiffness and viscoelastic properties similar to those of a human hamstring graft.27 In addition, the BMD of the porcine tibiae used in this study was not tested because of time involved and cost-efficiency. However, it has been shown that average BMD of porcine tibiae, 1.220-1.675 g/cm², is similar to that in a young athletic population, 1.24-1.62 g/cm2.3,28-31 We therefore assumed similarity to a young athletic population and uniformity of BMD of the porcine tibiae used in this study.
In addition, the biomechanical testing protocol did not simulate physiologic loading within the tibial tunnel. Moreover, the testing protocol used loads of only 250 N during cyclic testing for 500 cycles. This simulates only the early rehabilitation period and not the healing period, which may last up to 12 weeks.9 In addition, as previously mentioned, forces on the graft may be as high as 450 to 700 N.11,32 Pullout testing in line with the long axis of the tibia was performed in order to compare mechanical testing results with those of similar studies.3,12,13 Last, the P of .07 for the comparison of ultimate load to failure between the 2 backup fixation groups suggests that this study may have been underpowered.
Conclusion
This study demonstrated an effective, alternative, and equivalent backup fixation device that can help prevent graft slippage within the tibial tunnel during soft-tissue ACL reconstruction. Potential benefits of using SwiveLock anchors for backup fixation include a significantly increased ultimate yield load (229 N) when supplementing an interference screw, ease of insertion compared with a bicortical post, and the improbable need for future hardware removal. We support using SwiveLock for supplementary fixation at the tibial tunnel site when using soft-tissue grafts in ACL reconstruction.
Restoration of stability with return to activity is generally expected after anterior cruciate ligament (ACL) reconstruction; long-term success rates range from 75% to 95%.1 However, graft failure occurs most frequently with soft-tissue grafts fixated only with interference screws.2,3 Fixation failure also occurs more frequently at the tibial site.2 This failure has been attributed to extensive graft slippage in cases of soft-tissue fixation with interference screws.2 Interference screw fixation alone, with a double-looped hamstring tendon graft, fails at 350 N in young human tibiae.4,5 However, failure is limited with use of a bone–tendon–bone graft or with backup fixation, particularly at the tibial site.3 The superiority of bicortical fixation has also been proven.5-7
In addition, as shown in a goat model, ACL graft fixation is a major cause of failure in the immediate postoperative period, before biological incorporation of the graft.8 Fixation techniques for soft-tissue grafts must withstand stresses during the healing period (grafts may take up to 12 weeks to incorporate).9 Failures may result from forces exerted on the graft—forces that may be as high as 450 to 700 N during daily activities.10,11 Within the tibial tunnel, various fixation devices are used, including interference screws, staples, pins, buttons, and interference screw/sheath constructs.12,13 Primary fixation is commonly achieved with interference screws because of their ease of insertion and greater stiffness. However, fixation of the soft-tissue graft is influenced by several variables, including bone density, insertion torque, thread geometry, and interference screw material.14-16 Many of these variables, which are a source of inconsistency and concern during the immediate postoperative period, have led surgeons to seek alternative methods of backup fixation at the tibial site. Nevertheless, good clinical and subjective results have been found after ACL reconstruction with a 4-stranded semitendinosus tendon at 10-year follow-up.17
An anchor used in rotator cuff repair is the SwiveLock system (Arthrex). Major advantages of this system include ease and speed of insertion, good strength, and reduced need for later hardware removal.
We conducted a study to biomechanically evaluate 3 methods of tibial-sided fixation for ACL reconstruction: fully threaded interference screw only, interference screw backed with 4.75-mm SwiveLock anchor, and fully threaded bio-interference screw backed with 4.5-mm bicortical screw. We hypothesized that a fully threaded bio-interference screw backed with a 4.75-mm SwiveLock anchor would provide mechanical strength no different from that provided by backup fixation with a bicortical post at the tibial site. We further hypothesized that SwiveLock backup fixation would provide more strength than fixation with bio-interference screw alone.
Materials and Methods
The design of this study was adapted from one used by Walsh and colleagues,3 who compared 3 fixation methods: retrograde interference screw, suture button, and combined fixation. Tibiae inspected before selection showed no signs of injury or abnormality. Bovine extensor tendons, which lacked any defects along their entire length, were stored in saline 0.9% solution. Both the tibiae and the extensor tendons were stored at –20°C before completion of the tibial-sided ACL reconstruction. Thirty fresh-frozen, skeletally mature porcine proximal tibiae were selected and thawed at 4°C before preparation. Specimens were prepared by potting the diaphysis in fiberglass resin, and a tunnel 9 mm in diameter was drilled through the anteromedial aspect of the tibia.
For consistency, one author (CAV) prepared all 30 specimens. Both tails of all 30 bovine extensor tendons were whip-stitched with No. 2 FiberLoop (Arthrex) 9 mm in diameter. Grafts and tibiae were randomly divided into 3 sample groups. The first group was prepared by antegrade graft fixation within the tibial tunnel using a fully threaded 9×28-mm BioComposite interference screw (Arthrex). The second and third groups used the same primary fixation within the tibial tunnel along with 2 types of secondary fixation. These backup fixation groups included a 4.5-mm titanium bicortical post (Arthrex) and a 4.75-mm BioComposite SwiveLock C anchor (Arthrex) (Figure 1). The FiberLoop at the ends of the distal graft tails for backup groups were fixated 1 cm distal to the tibial tunnel and tapped before insertion of backup devices (Figures 2A, 2B). Insertion was completed after 4.5-mm bicortical and 4.75-mm unicortical drilling and tapping of the anteromedial cortices for the titanium posts and SwiveLocks, respectively. The free ends of the whip-stitched No. 2 FiberLoop were tied to the proximal end of the titanium post with a single surgical knot followed by 5 square knots.3 The free ends of the No. 2 FiberLoop were inserted into the eyelet of the 4.75-mm SwiveLock and 1 cm directly inferior to the tibial tunnel. Interference fit of FiberLoop with SwiveLock was achieved within the corticocancellous bone of the tibiae. All samples retained a 30-mm tendon loop superior to the tibial plateau to simulate intra-articular ACL length. Specimens were then stored at –20°C and thawed at 4°C before biomechanical testing.
Each of the 30 tibiae was tested once. Each testing group consisted of 10 porcine tibiae. The tendons were kept moist during the entire testing procedure by spraying them thoroughly with saline 0.9% solution. Mechanical testing was performed with an Instron 8871 system with a 5-kN load cell secured to the crosshead. A fixed-angle aperture, attached to the testing surface, was adjusted so that the tendon would be pulled in line with the tibial tunnel. A hook fixture suspended from clevis and dowel was used to secure the tendon to the crosshead (Figure 3). A small preload of 5 N manually applied to each sample was followed by a precycling regimen of 10 cycles between 10 N and 50 N at 1 Hz. Precycling was performed to remove slack from the system. Mechanical testing consisted of 500 cycles between 50 N and 250 N at 1 Hz followed by pull to failure at 20 mm per minute. Load and displacement data were recorded at 500 Hz.
An a priori power analysis was not performed because 6 specimens per group in the study from which the testing protocol was adapted demonstrated sufficient power among 3 testing categories.3 In addition, other studies have demonstrated similar testing protocols using 10 specimens per testing group.7,12,13,18 The data for each sample were analyzed with OriginPro 8.0 software (OriginLab). Ultimate load, yield load, stiffness, and cyclic displacement of the 3 sample groups were compared with 1-way analysis of variance (α = 0.05). Holm-Sidak tests were used for post hoc analysis.19P < .05 was statistically significant.
Results
None of the 30 specimens failed during preloading. Modes of failure were consistent among groups. All 10 specimens in the interference-screw-only group failed by graft slippage past the screw in the tibial tunnel. Nineteen of the 20 specimens in the backup-fixation groups failed by graft slippage past the screw and suture cutout through the distal graft tail. In the bicortical-post backup group, 1 failure was attributed to tendon tearing proximal to whip-stitching. There were no instances of hardware breakage or failure of either titanium screw or SwiveLock anchor.
Mean (SD) cyclic displacement was higher in the interference-screw-only group, 3.5 (2.2) mm, than in the SwiveLock backup group, 2.6 (0.5) mm, and the bicortical-post backup group, 2.1 (0.6) mm; no statistical significance was demonstrated between any 2 of these groups alone (P = .12) (Figure 4). Mean (SD) pullout stiffness was higher in the bicortical-post backup group, 192 (48) N/mm, than in the SwiveLock backup group, 164 (53) N/mm, and the screw-only group, 163 (64) N/mm (P = .42) (Figure 5). Mean (SD) initial load at 5 mm of displacement was higher in the bicortical-post backup group, 482 (156) N, and the SwiveLock backup group, 423 (94) N, than in the screw-only group, 381 (169) N (P = .30).
Mean (SD) yield load was higher in the bicortical-post backup group, 829 (253) N, than in the SwiveLock backup group, 642 (172) N, and the interference-screw-only group, 496 (133) N (P = .003). Statistical significance was demonstrated between the screw-only and bicortical-post groups (P = .002) and between the screw-only and SwiveLock groups (P = .048). There was no statistical difference between the bicortical-post and SwiveLock groups (P = .07).
Mean (SD) ultimate load to failure was higher in the bicortical-post backup group, 1148 (186) N, than in the SwiveLock backup group, 1007 (176) N, and the interference-screw-only group, 778 (139) N (Figure 6). The difference was statistically significant, whereby the screw-only group failed at a lower load compared with the bicortical-post group (P < .001) and the SwiveLock group (P = .005). The 2 backup groups were not statistically different (P = .1).
Discussion
We investigated whether a fully threaded bio-interference screw backed with a 4.75-mm SwiveLock anchor would provide mechanically equivalent pullout strength within the tibial tunnel during ACL reconstruction with soft-tissue allografts in comparison either with a fully threaded bio-interference screw backed with a bicortical post or with a fully threaded bio-interference screw without backup fixation. The results of the study support this hypothesis. With SwiveLock used for backup fixation, there was no significant difference in stiffness or cyclic load displacement between the screw-only, SwiveLock, and bicortical-post groups. However, adding backup fixation could particularly help improve fixation consistency. Specifically, although after only 500 cycles there was no statistically significant difference in cyclic displacement, continued cycling may be clinically relevant if graft slippage exceeded limits to allow for healing within the tibial tunnel. Conversely, a significantly larger difference was found between the SwiveLock, bicortical-post, and screw-only groups in yield load and ultimate load to failure. However, there was no significant difference between the SwiveLock and bicortical-post groups.
In this study, interference screw with SwiveLock backup demonstrated a mean (SD) ultimate load to failure of 1007 (176) N, comparable to that found by Walsh and colleagues3 for retrograde bio-interference screw with suture button, 1027 (157.11) N. In a study comparing quadrupled hamstring tibial graft fixation, Intrafix (DePuy Mitek) and an 8×25-mm Bioscrew (Linvatec) demonstrated mean (SD) single-cycle yield loads of 1332 (304) N and 612 (176) N, respectively.13 These results are similar to the ultimate yield loads in the present study: bicortical-post group, 1148 (186) N; SwiveLock group, 1007 (176) N; screw-only group, 778 (139) N. Differences may be attributed to hamstring tendons used in a quadrupled manner in the aforementioned study.12,13 Last, mean (SD) ultimate load to failure in a study that used only a retrograde bio-interference screw (9×20 mm) was 679.00 (109.44) N,3 similar to the 778 (139) N found for interference-screw-only in the present study. The difference is likely attributable to the longer screw (9×28 mm) in our study. Using SwiveLock C in cortical bone, Barber and colleagues18 found mean (SD) loads to failure up to 711.9 (89.1) N.
Clinically, it has been shown that a statistically significant increase in anterior laxity occurred between 4 months and 2 years in 10.7% of patients who underwent hamstring ACL reconstruction.20 The knees were clinically categorized as unstable or severely abnormal. The authors concluded that the clinical outcome was more likely influenced by the methods used to fix the free ends of the graft, specifically with 2 staples or a washer. To simulate early postoperative rehabilitation in the present study, cyclic loading of the graft was performed. Ultimate load to failure was then determined in order to evaluate catastrophic failure strength of the backup fixation devices in comparison with the interference-screw-only group without supplementary fixation.
It has been shown in autologous hamstring ACL reconstruction that a centrally placed polyethylene screw with sheath (Intrafix) performed as well as a standard, eccentrically placed metal interference screw with staple.10 It is therefore logical that backup fixation with use of a similar device (eg, SwiveLock, bicortical post) is necessary to ensure comparable clinical outcomes in relation to a screw/sheath device that has been shown to endure the highest yield loads.2,9,12,13,21-23 Potential benefits of using SwiveLock anchors for backup fixation include a statistically significant increased mean (SD) ultimate yield load of 229 (176) N over interference screw only. These results are similar to those in comparable studies: 218.3 (59.7) N24 and 165 (24.15) N25 in healthy bone with a reported bone mineral density (BMD) of 1.39 g/cm2, similar to that of skeletally mature porcine tibia (1.220-1.675 g/cm²).3 In addition, ease of insertion of this device over a bicortical post was demonstrated. The titanium post required bicortical drilling as well as measurement with a depth gauge to ensure adequate screw length. This process appeared to require more time during specimen preparation and theoretically could prove to be more dangerous clinically.7 However, caution in using a SwiveLock anchor in osteoporotic bone is advised because of reduced pullout strength.26 In this case, bicortical-post backup fixation may be more suitable. Moreover, although not demonstrated in this study, hardware prominence and irritation with a post may cause postoperative morbidity necessitating future removal.20 Hardware removal was the most common reason for additional surgery using hamstring tendons as graft.20 A second surgery for hardware removal was required in 21% of these patients.20 This is unlikely to occur with a SwiveLock, as the anchor is buried within cortical bone.
Limitations
Regarding use of nonhuman tissues in a biomechanical model, porcine tibiae and bovine extensor tendons were used because of availability, consistency among specimens, and cost-effectiveness. However, bovine extensor tendons have been shown to exhibit stiffness and viscoelastic properties similar to those of a human hamstring graft.27 In addition, the BMD of the porcine tibiae used in this study was not tested because of time involved and cost-efficiency. However, it has been shown that average BMD of porcine tibiae, 1.220-1.675 g/cm², is similar to that in a young athletic population, 1.24-1.62 g/cm2.3,28-31 We therefore assumed similarity to a young athletic population and uniformity of BMD of the porcine tibiae used in this study.
In addition, the biomechanical testing protocol did not simulate physiologic loading within the tibial tunnel. Moreover, the testing protocol used loads of only 250 N during cyclic testing for 500 cycles. This simulates only the early rehabilitation period and not the healing period, which may last up to 12 weeks.9 In addition, as previously mentioned, forces on the graft may be as high as 450 to 700 N.11,32 Pullout testing in line with the long axis of the tibia was performed in order to compare mechanical testing results with those of similar studies.3,12,13 Last, the P of .07 for the comparison of ultimate load to failure between the 2 backup fixation groups suggests that this study may have been underpowered.
Conclusion
This study demonstrated an effective, alternative, and equivalent backup fixation device that can help prevent graft slippage within the tibial tunnel during soft-tissue ACL reconstruction. Potential benefits of using SwiveLock anchors for backup fixation include a significantly increased ultimate yield load (229 N) when supplementing an interference screw, ease of insertion compared with a bicortical post, and the improbable need for future hardware removal. We support using SwiveLock for supplementary fixation at the tibial tunnel site when using soft-tissue grafts in ACL reconstruction.
1. Wetzler MJ, Bartolozzi AR, Gillespie MJ, Rubenstein DL, Ciccotti MG, Miller LS. Revision anterior cruciate ligament reconstruction. Oper Tech Orthop. 1996;6(3):181-189.
2. Scheffler SU, Südkamp NP, Göckenjan A, Hoffmann RF, Weiler A. Biomechanical comparison of hamstring and patellar tendon graft anterior cruciate ligament reconstruction techniques: the impact of fixation level and fixation method under cyclic loading. Arthroscopy. 2002;18(3):304-315.
3. Walsh MP, Wijdicks CA, Parker JB, Hapa O, LaPrade RF. A comparison between a retrograde interference screw, suture button, and combined fixation on the tibial side in an all-inside anterior cruciate ligament reconstruction: a biomechanical study in a porcine model. Am J Sports Med. 2009;37(1):160-167.
4. Howell SM, Hull ML. Aggressive rehabilitation using hamstring tendons: graft construct, tibial tunnel placement, fixation properties, and clinical outcome. Am J Knee Surg. 1998;11(2):120-127.
5. Magen HE, Howell SM, Hull ML. Structural properties of six tibial fixation methods for anterior cruciate ligament soft tissue grafts. Am J Sports Med. 1999;27(1):35-43.
6. Beynnon BD, Meriam CM, Ryder SH, Fleming BC, Johnson RJ. The effect of screw insertion torque on tendons fixed with spiked washers. Am J Sports Med. 1998;26(4):536-539.
7. Post WR, King SS. Neurovascular risk of bicortical tibial drilling for screw and spiked washer fixation of soft-tissue anterior cruciate ligament graft. Arthroscopy. 2001;17(3):244-247.
8. Holden JP, Grood ES, Butler DL, et al. Biomechanics of fascia lata ligament replacements: early postoperative changes in the goat. J Orthop Res. 1988;6(5):639-647.
9. Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75(12):1795-1803.
10. Frank CB, Jackson DW. The science of reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. 1997;79(10):1556-1576.
11. Markolf KL, Willems MJ, Jackson SR, Finerman GA. In situ calibration of miniature sensors implanted into the anterior cruciate ligament. Part I: strain measurements. J Orthop Res. 1998;16(4):455-463.
12. Kousa P, Teppo LN, Jarvinen TL, Vihavainen M, Kannus P, Jarvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction: I. Femoral site. Am J Sports Med. 2003;3 (2)1:174-181.
13. Kousa P, Jarvinen TL, Vihavainen M, Kannus P, Jarvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction: II. Tibial site. Am J Sports Med. 2003;31(2):182-188.
14. Brand JC Jr, Pienkowski D, Steenlage E, Hamilton D, Johnson DL, Caborn DN. Interference screw fixation strength of a quadrupled hamstring tendon graft is directly related to bone mineral density and insertion torque. Am J Sports Med. 2000;28(5):705-710.
15. Weiler A, Hoffmann RF, Siepe CJ, Kolbeck SF, Südkamp NP. The influence of screw geometry on hamstring tendon interference fit fixation. Am J Sports Med. 2000;28(3):356-359.
16. Weiler A, Hoffmann RF, Stähelin AC, Bail HJ, Siepe CJ, Südkamp NP. Hamstring tendon fixation using interference screws: a biomechanical study in calf tibial bone. Arthroscopy. 1998;14(1):29-37.
17. Streich NA, Reichenbacher S, Barié A, Buchner M, Schmitt H. Long-term outcome of anterior cruciate ligament reconstruction with an autologous four-strand semitendinosus tendon autograft. Int Orthop. 2013;37(2):279-284.
18. Barber FA, Herbert MA, Beavis C, Barrera Oro F. Suture anchor materials, eyelets, and designs: update 2008. Arthroscopy. 2008;24(8):859-867.
19. Aickin M, Gensler H. Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. Am J Public Health. 1996;86(5):726-728.
20. Howell SM, Deutsch ML. Comparison of endoscopic and two-incision techniques for reconstructing a torn anterior cruciate ligament using hamstring tendons. Arthroscopy. 1999;15(6):594-606.
21. Gwynne-Jones DP, Draffin J, Vane A, Craig R, McMahon S. Failure strengths of concentric and eccentric implants for hamstring graft fixation. ANZ J Surg. 2008;78(3):177-181.
22. Hayes DA, Watts MC, Tevelen GA, Crawford RW. Central versus peripheral tibial screw placement in hamstring anterior cruciate ligament reconstruction: in vitro biomechanics. Arthroscopy. 2005;21(6):703-706.
23. Shino K, Pflaster DS. Comparison of eccentric and concentric screw placement for hamstring graft fixation in the tibial tunnel. Knee Surg Sports Traumatol Arthrosc. 2000;8(2):73-75.
24. Prevrhal S, Fuerst T, Fan B, et al. Quantitative ultrasound of the tibia depends on both cortical density and thickness. Osteoporosis Int. 2001;12(1):28-34.
25. Pietschmann MF, Gülecyüz MF, Fieseler S, et al. Biomechanical stability of knotless suture anchors used in rotator cuff repair in healthy and osteopenic bone. Arthroscopy. 2010;26(8):1035-1044.
26. Burns JP, Snyder SJ, Albritton M. Arthroscopic rotator cuff repair using triple-loaded anchors, suture shuttles, and suture savers. J Am Acad Orthop Surg. 2007;15(7):432-444.
27. Tetsumura S, Fujita A, Nakajima M, Abe M. Biomechanical comparison of different fixation methods on the tibial side in anterior cruciate ligament reconstruction: a biomechanical study in porcine tibial bone. J Orthop Sci. 2006;11(3):278-282.
28. Alfredson H, Nordstrom P, Lorentzon R. Total and regional bone mass in female soccer players. Calcif Tissue Int. 1996;59(6):438-442.
29. Nevill AM, Holder RL, Stewart AD. Modeling elite male athletes’ peripheral bone mass, assessed using regional dual x-ray absorptiometry. Bone. 2003;32(1):62-68.
30. Nordström P, Lorentzon R. Site-specific bone mass differences of the lower extremities in 17-year-old ice hockey players. Calcif Tissue Int. 1996;59(6):4443-4448.
31. Patzer T, Santo G, Olender GD, Wellmann M, Hurschler C, Schofer MD. Suprapectoral or subpectoral position for biceps tenodesis: biomechanical comparison of four different techniques in both positions. J Shoulder Elbow Surg. 2012;21(1):116-125.
32. De Wall M, Scholes CJ, Patel S, Coolican MR, Parker DA. Tibial fixation in anterior cruciate ligament reconstruction: a prospective randomized study comparing metal interference screw and staples with a centrally placed polyethylene screw and sheath. Am J Sports Med. 2011;39(9):1858-1864.
1. Wetzler MJ, Bartolozzi AR, Gillespie MJ, Rubenstein DL, Ciccotti MG, Miller LS. Revision anterior cruciate ligament reconstruction. Oper Tech Orthop. 1996;6(3):181-189.
2. Scheffler SU, Südkamp NP, Göckenjan A, Hoffmann RF, Weiler A. Biomechanical comparison of hamstring and patellar tendon graft anterior cruciate ligament reconstruction techniques: the impact of fixation level and fixation method under cyclic loading. Arthroscopy. 2002;18(3):304-315.
3. Walsh MP, Wijdicks CA, Parker JB, Hapa O, LaPrade RF. A comparison between a retrograde interference screw, suture button, and combined fixation on the tibial side in an all-inside anterior cruciate ligament reconstruction: a biomechanical study in a porcine model. Am J Sports Med. 2009;37(1):160-167.
4. Howell SM, Hull ML. Aggressive rehabilitation using hamstring tendons: graft construct, tibial tunnel placement, fixation properties, and clinical outcome. Am J Knee Surg. 1998;11(2):120-127.
5. Magen HE, Howell SM, Hull ML. Structural properties of six tibial fixation methods for anterior cruciate ligament soft tissue grafts. Am J Sports Med. 1999;27(1):35-43.
6. Beynnon BD, Meriam CM, Ryder SH, Fleming BC, Johnson RJ. The effect of screw insertion torque on tendons fixed with spiked washers. Am J Sports Med. 1998;26(4):536-539.
7. Post WR, King SS. Neurovascular risk of bicortical tibial drilling for screw and spiked washer fixation of soft-tissue anterior cruciate ligament graft. Arthroscopy. 2001;17(3):244-247.
8. Holden JP, Grood ES, Butler DL, et al. Biomechanics of fascia lata ligament replacements: early postoperative changes in the goat. J Orthop Res. 1988;6(5):639-647.
9. Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75(12):1795-1803.
10. Frank CB, Jackson DW. The science of reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. 1997;79(10):1556-1576.
11. Markolf KL, Willems MJ, Jackson SR, Finerman GA. In situ calibration of miniature sensors implanted into the anterior cruciate ligament. Part I: strain measurements. J Orthop Res. 1998;16(4):455-463.
12. Kousa P, Teppo LN, Jarvinen TL, Vihavainen M, Kannus P, Jarvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction: I. Femoral site. Am J Sports Med. 2003;3 (2)1:174-181.
13. Kousa P, Jarvinen TL, Vihavainen M, Kannus P, Jarvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction: II. Tibial site. Am J Sports Med. 2003;31(2):182-188.
14. Brand JC Jr, Pienkowski D, Steenlage E, Hamilton D, Johnson DL, Caborn DN. Interference screw fixation strength of a quadrupled hamstring tendon graft is directly related to bone mineral density and insertion torque. Am J Sports Med. 2000;28(5):705-710.
15. Weiler A, Hoffmann RF, Siepe CJ, Kolbeck SF, Südkamp NP. The influence of screw geometry on hamstring tendon interference fit fixation. Am J Sports Med. 2000;28(3):356-359.
16. Weiler A, Hoffmann RF, Stähelin AC, Bail HJ, Siepe CJ, Südkamp NP. Hamstring tendon fixation using interference screws: a biomechanical study in calf tibial bone. Arthroscopy. 1998;14(1):29-37.
17. Streich NA, Reichenbacher S, Barié A, Buchner M, Schmitt H. Long-term outcome of anterior cruciate ligament reconstruction with an autologous four-strand semitendinosus tendon autograft. Int Orthop. 2013;37(2):279-284.
18. Barber FA, Herbert MA, Beavis C, Barrera Oro F. Suture anchor materials, eyelets, and designs: update 2008. Arthroscopy. 2008;24(8):859-867.
19. Aickin M, Gensler H. Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. Am J Public Health. 1996;86(5):726-728.
20. Howell SM, Deutsch ML. Comparison of endoscopic and two-incision techniques for reconstructing a torn anterior cruciate ligament using hamstring tendons. Arthroscopy. 1999;15(6):594-606.
21. Gwynne-Jones DP, Draffin J, Vane A, Craig R, McMahon S. Failure strengths of concentric and eccentric implants for hamstring graft fixation. ANZ J Surg. 2008;78(3):177-181.
22. Hayes DA, Watts MC, Tevelen GA, Crawford RW. Central versus peripheral tibial screw placement in hamstring anterior cruciate ligament reconstruction: in vitro biomechanics. Arthroscopy. 2005;21(6):703-706.
23. Shino K, Pflaster DS. Comparison of eccentric and concentric screw placement for hamstring graft fixation in the tibial tunnel. Knee Surg Sports Traumatol Arthrosc. 2000;8(2):73-75.
24. Prevrhal S, Fuerst T, Fan B, et al. Quantitative ultrasound of the tibia depends on both cortical density and thickness. Osteoporosis Int. 2001;12(1):28-34.
25. Pietschmann MF, Gülecyüz MF, Fieseler S, et al. Biomechanical stability of knotless suture anchors used in rotator cuff repair in healthy and osteopenic bone. Arthroscopy. 2010;26(8):1035-1044.
26. Burns JP, Snyder SJ, Albritton M. Arthroscopic rotator cuff repair using triple-loaded anchors, suture shuttles, and suture savers. J Am Acad Orthop Surg. 2007;15(7):432-444.
27. Tetsumura S, Fujita A, Nakajima M, Abe M. Biomechanical comparison of different fixation methods on the tibial side in anterior cruciate ligament reconstruction: a biomechanical study in porcine tibial bone. J Orthop Sci. 2006;11(3):278-282.
28. Alfredson H, Nordstrom P, Lorentzon R. Total and regional bone mass in female soccer players. Calcif Tissue Int. 1996;59(6):438-442.
29. Nevill AM, Holder RL, Stewart AD. Modeling elite male athletes’ peripheral bone mass, assessed using regional dual x-ray absorptiometry. Bone. 2003;32(1):62-68.
30. Nordström P, Lorentzon R. Site-specific bone mass differences of the lower extremities in 17-year-old ice hockey players. Calcif Tissue Int. 1996;59(6):4443-4448.
31. Patzer T, Santo G, Olender GD, Wellmann M, Hurschler C, Schofer MD. Suprapectoral or subpectoral position for biceps tenodesis: biomechanical comparison of four different techniques in both positions. J Shoulder Elbow Surg. 2012;21(1):116-125.
32. De Wall M, Scholes CJ, Patel S, Coolican MR, Parker DA. Tibial fixation in anterior cruciate ligament reconstruction: a prospective randomized study comparing metal interference screw and staples with a centrally placed polyethylene screw and sheath. Am J Sports Med. 2011;39(9):1858-1864.
Isolating Suture Slippage During Cadaveric Testing of Knotless Anchors
Knotless suture anchor fixation techniques continue to evolve as efficient, low-profile options for arthroscopic rotator cuff repair (RCR).1,2 Excellent outcomes have been reported for constructs that use knotless fixation laterally, typically in suture bridge-type configurations.2-4 Early comparative biomechanical and clinical studies have also demonstrated equivalent results for all-knotless versus conventional constructs for arthroscopic RCR.5-10 Given the increased use and availability of multiple implant designs, it is important to supplement our clinical knowledge of these devices with laboratory studies delineating the biomechanical properties of the anchors that are used to help guide appropriate clinical use of the implants in specific patient populations.
Several biomechanical studies have shown suture slippage to be the weak but crucial link in the design of knotless anchors and the most likely mode of in vivo failure.11,12 Other studies have demonstrated frequent anchor dislodgement from bone, but these analyses involved use of elderly cadaveric specimens and relatively high-force testing protocols.12,13 Because suture-retention force may have exceeded anchor resistance to pullout (imparted by weak cadaveric bone in such biomechanical settings), the focus on suture-retention properties was limited.11 It is thought that, in clinical practice, the majority of patients who undergo RCR tend not to generate the high forces (relative to resistance to bone pullout) used to cause the anchor pullouts observed in biomechanical studies, particularly in the early postoperative setting.11-15 Cadaveric testing, however, often involves use of specimens with diminished bone mineral density (BMD), relative to age, because of the illness and other factors leading to death and donation.
Using a novel testing apparatus, we isolated, analyzed, and compared suture slippage in 2 anchor designs, one with entirely press-fit suture clamping and the other reliant on an intrinsic suture-locking mechanism.
Materials and Methods
Six human cadaveric proximal humeri specimens were used for this biomechanical study. Mean (SD) age was 53.3 (5.7) years (range, 46-59 years). Middle-aged specimens were used in order to best represent the quality of bone typically encountered in RCR surgery. To approximate tissue in clinical use, we used fresh-frozen cadaver tissue. Specimens were maintained at –20°C until about 24 hours before use and then were thawed to room temperature for testing. Specimens were included only if they had a completely intact humeral head and no prior surgery or hardware placement. Before instrumentation, dual-energy x-ray absorptiometry with a QDR-1000 scanner (Hologic) was used to determine BMD of all proximal humeri.
Two knotless suture anchors were compared: PushLock (4.5×18.5 mm; Arthrex) and ReelX STT (5.5×19.4 mm; Stryker). These anchors have multiple surgical indications (including RCR), allow patient-specific tissue tensioning, and use polyetheretherketone eyelets. The clamping force for PushLock depends entirely on the interference fit achieved for the suture between the outside of the anchor and the surrounding trabecular/cortical bone after device insertion, whereas the suture in ReelX is secured within the anchor shaft entirely by an internal ratchet-locking mechanism.
For anchor insertion, shoulders were dissected down to the greater tuberosity of the proximal humerus, and all implants were inserted (by a fellowship-trained surgeon in accordance with manufacturer guidelines) at a 25° insertion angle with manufacturer-supplied instruments. One anchor of each type (Figure 1) was inserted into the center of the rotator cuff footprint on the greater tuberosity of each specimen. Anterior and posterior positions were randomized, and an anchor from the other group was inserted into the matching location on the contralateral matched-pair specimen. In all instances, distance between the anterior and posterior anchors was 2 cm, and anchors were placed midway between the articular margin and the lateral edge of the greater tuberosity (Figure 2). Two strands of size 2 ultrahigh-molecular-weight–polyethylene Force Fiber (Stryker) were loaded into all anchors.
A custom urethane fixture was secured over the center of each anchor to allow testing to focus on suture slippage by minimizing anchor migration (Figure 3). The small aperture of this device allowed suture tails to pass freely through the center of the fixture but prevented disengagement and proximal migration of the suture anchor from the underlying bone through contact of the urethane fixture with the anchor perimeter. Any system deformation observed during testing was restricted to the suture and/or the anchor’s suture-locking mechanism. Testing fixtures also oriented the suture anchor coaxial with the axis of tension, creating a worst-case loading scenario (Figure 3).
PushLock implants were inserted with 5 pounds of tension, as indicated, using a manufacturer-supplied suture tensioner, and ReelX devices were inserted and locked with 2 full rotations, as specified by the manufacturer. After one end of each suture was cut, as would be done in vivo, the 2 other suture ends, which would have been part of the RCR in vivo, were tied together to form an 8-cm circumference loop that was brought through the urethane fixture. Humeri were then mounted in a materials testing system (MTS 810; MTS Systems) servohydraulic load frame, and the suture loop was passed around a cross-bar on the actuator of the testing device. A mechanical testing protocol consisting of modest repetitive forces was carefully chosen to simulate expected activity during rehabilitation after RCR.15 In this protocol, a 60-second preload of 10 N was followed by tensile loading between 10 N and 90 N at a frequency of 0.5 Hz for 500 cycles.15 Cycle duration at 3 mm and 5 mm of suture slippage (threshold for clinical failure) was recorded.12,16,17 In addition, suture slippage was measured after 1, 10, 50, 100, 200, 300, 400, and 500 cycles. The first 5 test cycles were not counted in the analysis to control for initial knot slippage. Finally, after completion of dynamic testing, samples were loaded at a displacement rate of 0.5 mm/s for tension-to-failure testing in the custom fixtures. Maximum failure load, stiffness, and failure mode were recorded. Ultimate failure was defined as suture breakage or gross suture slippage.
Paired Student t test was used to determine significant differences in suture slippage distance between the 2 groups at various cycle durations. In addition, Kaplan-Meier survival test was used to determine statistical differences in sample survival during the dynamic loading test.
Results
Mean (SD) BMD of the cadaveric shoulder specimens was 0.55 (0.13) g/cm2 (range, 0.29-0.68 g/cm2). The testing fixtures isolated suture slippage from anchor–bone disengagement. All 6 PushLock implants demonstrated slippage of more than 3 mm, and 5 of the 6 demonstrated slippage of more than 5 mm. All 6 ReelX devices exhibited slippage of less than 3 mm. In addition, PushLock demonstrated more suture slippage at cycles 1, 10, and 100 (P < .05) and more maximum slippage after 500 cycles (mean, 11.2 mm; SD, 4.7 mm) compared with ReelX (mean, 1.9 mm; SD, 0.5 mm) (P = .004). Figure 4 shows mean suture slippage at each cycle.
Kaplan-Meier analysis revealed significantly (λ2 = 8.170; P = .0043) decreased survival after dynamic testing for PushLock versus ReelX (Figure 5). Survival was defined as suture slippage of less than 5 mm after completion of dynamic testing. Only 1 of the 6 PushLock anchors completed dynamic testing; the other 5 failed via complete suture slippage from the anchor before testing could be completed. All 6 ReelX devices survived dynamic testing.
Therefore, 1 PushLock implant and all 6 ReelX devices were available for subsequent load-to-failure testing. Failure in this setting was defined as suture slippage of more than 10 mm or suture breakage. The PushLock implant failed at a maximum force of 171.8 N with a stiffness of 74.4 N/mm and eventually exhibited gross suture slippage. All 6 ReelX devices failed at a mean (SD) maximum of 273.5 (20.2) N, with a mean (SD) stiffness of 74.1 (17) N/mm. Mechanism of failure for all ReelX devices was suture breakage during the tensile load-to-failure test.
Discussion
We evaluated a new technique designed to isolate suture slippage in knotless anchors used for RCR. The impetus for developing this new method was to provide a means for better analyzing the ability of a knotless anchor to resist suture slippage in the cadaveric biomechanical testing setting. Suture slippage is an important mode of failure during such analyses.11,12 Significant slippage occurred in a range of implants before half the anchor–bone pullout strength was reached in a study using young bovine femoral heads.11 In another study, using young, high-BMD cadaveric humeral heads, initial slippage and maximum failure loads were equivalent among numerous devices using various suture-retention mechanisms, and suture slippage was the most common failure mode.12 Nevertheless, other biomechanical studies have demonstrated frequent failure caused by anchor pullout in elderly human cadaveric specimens with diminished BMD, often with high-force testing protocols.12,13 In the more modest-force, in vivo rehabilitative environment, suture slippage rather than anchor dislodgement may be the main failure mode.11-15
We compared the PushLock implant and its entirely press-fit suture clamping design with the ReelX device, which relies on an intrinsic suture-locking mechanism. Middle-aged (mean, 53.3 years; SD, 5.7 years) cadaveric humeri were tested under physiologically relevant biomechanical conditions to begin to help identify how relatively osteopenic bone may affect suture-retention properties for a given implant. The results showed that the study methodology prevented implant failure via anchor–bone pullout. To our knowledge, this was the first study to exclusively analyze suture slippage in knotless anchors. The findings indicated that implants that rely heavily on a tight interference fit of the suture between the anchor and the surrounding bone may exhibit early slippage and failure after RCR in middle-aged patients with relative osteopenia.11,12 However, this study also demonstrated that devices with intrinsic clamping mechanisms that do not depend on the quality of surrounding bone may better resist suture slippage. It is not clear that all knotless anchors with intrinsic locking mechanisms function equivalently. For instance, Pietschmann and colleagues12 found that 2 of 10 implants with a different internal clamping device were unable to resist failure via suture slippage, even in healthy bone. Similarly, in a study comparing ReelX devices with implants having a different internal suture-retention mechanism, ReelX failed at higher ultimate loads, and typically via anchor dislodgement, versus suture slippage in the other implants.18
It is important to note that, in the present study, the loads at which sutures broke in the intrinsic clamping anchors approached the maximum contractile force of the supraspinatus muscle (302 N).19,20 In addition, these loads were above the resistance of the rotator cuff tendon to cut out with modern suture material.21
This study’s limitations include use of an in vitro human cadaveric model that precluded analysis of the effects of postoperative healing. Biomechanical testing was also performed in a single row-type suture configuration with the rotator cuff tendon removed. Fixtures used during testing oriented the load coaxially with the axis of tension, creating a worst-case loading scenario. Although this form of testing may limit its clinical applicability, its purpose was to critically isolate how well a knotless anchor could resist suture slippage. The methods we used were also limited because the stability of the bone–anchor interface was not assessed. For patients with osteopenia, anchor pullout rather than suture slippage could be the most limiting factor for knotless anchor construct failure, and therefore further testing of both failure modes is needed. Future biomechanical studies should compare various knotless anchors’ suture-slippage characteristics in other constructs in physiologic testing orientations, including double-row and suture-bridge configurations, as well as with intact rotator cuff tendons. In addition, use of labral tape as a substitute for polyblend suture has been suggested to limit suture slippage, and this technique theoretically could have changed the results of this study.22
Conclusion
An implant with an internal ratcheting mechanism for suture retention demonstrated significantly less suture slippage in an axial tension evaluation protocol than a device reliant on interference fit of the suture between the anchor and surrounding bone. In the clinical setting, this may allow for less gap formation during the healing phase following RCR with a knotless anchor. There was also increased maximum load to failure, demonstrating an increased load until catastrophic failure using a device with a ratcheting internal locking mechanism.
1. Thal R. A knotless suture anchor. Design, function, and biomechanical testing. Am J Sports Med. 2001;29(5):646-649.
2. Cole BJ, ElAttrache NS, Anbari A. Arthroscopic rotator cuff repairs: an anatomic and biomechanical rationale for different suture-anchor repair configurations. Arthroscopy. 2007;23(6):662-669.
3. Kim KC, Shin HD, Cha SM, Lee WY. Comparison of repair integrity and functional outcomes for 3 arthroscopic suture bridge rotator cuff repair techniques. Am J Sports Med. 2013;41(2):271-277.
4. Choi CH, Kim SK, Cho MR, et al. Functional outcomes and structural integrity after double-pulley suture bridge rotator cuff repair using serial ultrasonographic examination. J Shoulder Elbow Surg. 2012;21(12):1753-1763.
5. Brown BS, Cooper AD, McIff TE, Key VH, Toby EB. Initial fixation and cyclic loading stability of knotless suture anchors for rotator cuff repair. J Shoulder Elbow Surg. 2008;17(2):313-318.
6. Burkhart SS, Adams CR, Burkhart SS, Schoolfield JD. A biomechanical comparison of 2 techniques of footprint reconstruction for rotator cuff repair: the SwiveLock-FiberChain construct versus standard double-row repair. Arthroscopy. 2009;25(3):274-281.
7. Hepp P, Osterhoff G, Engel T, Marquass B, Klink T, Josten C. Biomechanical evaluation of knotless anatomical double-layer double-row rotator cuff repair: a comparative ex vivo study. Am J Sports Med. 2009;37(7):1363-1369.
8. Maguire M, Goldberg J, Bokor D, et al. Biomechanical evaluation of four different transosseous-equivalent/suture bridge rotator cuff repairs. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1582-1587.
9. Millar NL, Wu X, Tantau R, Silverstone E, Murrell GA. Open versus two forms of arthroscopic rotator cuff repair. Clin Orthop Relat Res. 2009;467(4):966-978.
10. Rhee YG, Cho NS, Parke CS. Arthroscopic rotator cuff repair using modified Mason-Allen medial row stitch: knotless versus knot-tying suture bridge technique. Am J Sports Med. 2012;40(11):2440-2447.
11. Wieser K, Farshad M, Vlachopoulos L, Ruffieux K, Gerber C, Meyer DC. Suture slippage in knotless suture anchors as a potential failure mechanism in rotator cuff repair. Arthroscopy. 2012;28(11):1622-1627.
12. Pietschmann MF, Gülecyüz MF, Fieseler S, et al. Biomechanical stability of knotless suture anchors used in rotator cuff repair in healthy and osteopenic bone. Arthroscopy. 2010;26(8):1035-1044.
13. Barber FA, Hapa O, Bynum JA. Comparative testing by cyclic loading of rotator cuff suture anchors containing multiple high-strength sutures. Arthroscopy. 2010;26(9 suppl):S134-S141.
14. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing of biodegradable suture anchors containing 2 high-strength sutures. Arthroscopy. 2007;23(4):355-360.
15. Bynum CK, Lee S, Mahar A, Tasto J, Pedowitz R. Failure mode of suture anchors as a function of insertion depth. Am J Sports Med. 2005;33(7):1030-1034.
16. Gerber C, Schneeberger AG, Beck M, Schlegel U. Mechanical strength of repairs of the rotator cuff. J Bone Joint Surg Br. 1994;76(3):371-380.
17. Schneeberger AG, von Roll A, Kalberer F, Jacob HA, Gerber C. Mechanical strength of arthroscopic rotator cuff repair techniques: an in vitro study. J Bone Joint Surg Am. 2002;84(12):2152-2160.
18. Efird C, Traub S, Baldini T, et al. Knotless single-row rotator cuff repair: a comparative biomechanical study of 2 knotless suture anchors. Orthopedics. 2013;36(8):e1033-e1037.
19. Wright PB, Budoff JE, Yeh ML, Kelm ZS, Luo ZP. Strength of damaged suture: an in vitro study. Arthroscopy. 2006;22(12):1270-1275.
20. Burkhart SS. A stepwise approach to arthroscopic rotator cuff repair based on biomechanical principles. Arthroscopy. 2000;16(1):82-90.
21. Bisson LJ, Manohar LM. A biomechanical comparison of the pullout strength of No. 2 FiberWire suture and 2-mm FiberWire tape in bovine rotator cuff tendons. Arthroscopy. 2010;26(11):1463-1468.
22. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
Knotless suture anchor fixation techniques continue to evolve as efficient, low-profile options for arthroscopic rotator cuff repair (RCR).1,2 Excellent outcomes have been reported for constructs that use knotless fixation laterally, typically in suture bridge-type configurations.2-4 Early comparative biomechanical and clinical studies have also demonstrated equivalent results for all-knotless versus conventional constructs for arthroscopic RCR.5-10 Given the increased use and availability of multiple implant designs, it is important to supplement our clinical knowledge of these devices with laboratory studies delineating the biomechanical properties of the anchors that are used to help guide appropriate clinical use of the implants in specific patient populations.
Several biomechanical studies have shown suture slippage to be the weak but crucial link in the design of knotless anchors and the most likely mode of in vivo failure.11,12 Other studies have demonstrated frequent anchor dislodgement from bone, but these analyses involved use of elderly cadaveric specimens and relatively high-force testing protocols.12,13 Because suture-retention force may have exceeded anchor resistance to pullout (imparted by weak cadaveric bone in such biomechanical settings), the focus on suture-retention properties was limited.11 It is thought that, in clinical practice, the majority of patients who undergo RCR tend not to generate the high forces (relative to resistance to bone pullout) used to cause the anchor pullouts observed in biomechanical studies, particularly in the early postoperative setting.11-15 Cadaveric testing, however, often involves use of specimens with diminished bone mineral density (BMD), relative to age, because of the illness and other factors leading to death and donation.
Using a novel testing apparatus, we isolated, analyzed, and compared suture slippage in 2 anchor designs, one with entirely press-fit suture clamping and the other reliant on an intrinsic suture-locking mechanism.
Materials and Methods
Six human cadaveric proximal humeri specimens were used for this biomechanical study. Mean (SD) age was 53.3 (5.7) years (range, 46-59 years). Middle-aged specimens were used in order to best represent the quality of bone typically encountered in RCR surgery. To approximate tissue in clinical use, we used fresh-frozen cadaver tissue. Specimens were maintained at –20°C until about 24 hours before use and then were thawed to room temperature for testing. Specimens were included only if they had a completely intact humeral head and no prior surgery or hardware placement. Before instrumentation, dual-energy x-ray absorptiometry with a QDR-1000 scanner (Hologic) was used to determine BMD of all proximal humeri.
Two knotless suture anchors were compared: PushLock (4.5×18.5 mm; Arthrex) and ReelX STT (5.5×19.4 mm; Stryker). These anchors have multiple surgical indications (including RCR), allow patient-specific tissue tensioning, and use polyetheretherketone eyelets. The clamping force for PushLock depends entirely on the interference fit achieved for the suture between the outside of the anchor and the surrounding trabecular/cortical bone after device insertion, whereas the suture in ReelX is secured within the anchor shaft entirely by an internal ratchet-locking mechanism.
For anchor insertion, shoulders were dissected down to the greater tuberosity of the proximal humerus, and all implants were inserted (by a fellowship-trained surgeon in accordance with manufacturer guidelines) at a 25° insertion angle with manufacturer-supplied instruments. One anchor of each type (Figure 1) was inserted into the center of the rotator cuff footprint on the greater tuberosity of each specimen. Anterior and posterior positions were randomized, and an anchor from the other group was inserted into the matching location on the contralateral matched-pair specimen. In all instances, distance between the anterior and posterior anchors was 2 cm, and anchors were placed midway between the articular margin and the lateral edge of the greater tuberosity (Figure 2). Two strands of size 2 ultrahigh-molecular-weight–polyethylene Force Fiber (Stryker) were loaded into all anchors.
A custom urethane fixture was secured over the center of each anchor to allow testing to focus on suture slippage by minimizing anchor migration (Figure 3). The small aperture of this device allowed suture tails to pass freely through the center of the fixture but prevented disengagement and proximal migration of the suture anchor from the underlying bone through contact of the urethane fixture with the anchor perimeter. Any system deformation observed during testing was restricted to the suture and/or the anchor’s suture-locking mechanism. Testing fixtures also oriented the suture anchor coaxial with the axis of tension, creating a worst-case loading scenario (Figure 3).
PushLock implants were inserted with 5 pounds of tension, as indicated, using a manufacturer-supplied suture tensioner, and ReelX devices were inserted and locked with 2 full rotations, as specified by the manufacturer. After one end of each suture was cut, as would be done in vivo, the 2 other suture ends, which would have been part of the RCR in vivo, were tied together to form an 8-cm circumference loop that was brought through the urethane fixture. Humeri were then mounted in a materials testing system (MTS 810; MTS Systems) servohydraulic load frame, and the suture loop was passed around a cross-bar on the actuator of the testing device. A mechanical testing protocol consisting of modest repetitive forces was carefully chosen to simulate expected activity during rehabilitation after RCR.15 In this protocol, a 60-second preload of 10 N was followed by tensile loading between 10 N and 90 N at a frequency of 0.5 Hz for 500 cycles.15 Cycle duration at 3 mm and 5 mm of suture slippage (threshold for clinical failure) was recorded.12,16,17 In addition, suture slippage was measured after 1, 10, 50, 100, 200, 300, 400, and 500 cycles. The first 5 test cycles were not counted in the analysis to control for initial knot slippage. Finally, after completion of dynamic testing, samples were loaded at a displacement rate of 0.5 mm/s for tension-to-failure testing in the custom fixtures. Maximum failure load, stiffness, and failure mode were recorded. Ultimate failure was defined as suture breakage or gross suture slippage.
Paired Student t test was used to determine significant differences in suture slippage distance between the 2 groups at various cycle durations. In addition, Kaplan-Meier survival test was used to determine statistical differences in sample survival during the dynamic loading test.
Results
Mean (SD) BMD of the cadaveric shoulder specimens was 0.55 (0.13) g/cm2 (range, 0.29-0.68 g/cm2). The testing fixtures isolated suture slippage from anchor–bone disengagement. All 6 PushLock implants demonstrated slippage of more than 3 mm, and 5 of the 6 demonstrated slippage of more than 5 mm. All 6 ReelX devices exhibited slippage of less than 3 mm. In addition, PushLock demonstrated more suture slippage at cycles 1, 10, and 100 (P < .05) and more maximum slippage after 500 cycles (mean, 11.2 mm; SD, 4.7 mm) compared with ReelX (mean, 1.9 mm; SD, 0.5 mm) (P = .004). Figure 4 shows mean suture slippage at each cycle.
Kaplan-Meier analysis revealed significantly (λ2 = 8.170; P = .0043) decreased survival after dynamic testing for PushLock versus ReelX (Figure 5). Survival was defined as suture slippage of less than 5 mm after completion of dynamic testing. Only 1 of the 6 PushLock anchors completed dynamic testing; the other 5 failed via complete suture slippage from the anchor before testing could be completed. All 6 ReelX devices survived dynamic testing.
Therefore, 1 PushLock implant and all 6 ReelX devices were available for subsequent load-to-failure testing. Failure in this setting was defined as suture slippage of more than 10 mm or suture breakage. The PushLock implant failed at a maximum force of 171.8 N with a stiffness of 74.4 N/mm and eventually exhibited gross suture slippage. All 6 ReelX devices failed at a mean (SD) maximum of 273.5 (20.2) N, with a mean (SD) stiffness of 74.1 (17) N/mm. Mechanism of failure for all ReelX devices was suture breakage during the tensile load-to-failure test.
Discussion
We evaluated a new technique designed to isolate suture slippage in knotless anchors used for RCR. The impetus for developing this new method was to provide a means for better analyzing the ability of a knotless anchor to resist suture slippage in the cadaveric biomechanical testing setting. Suture slippage is an important mode of failure during such analyses.11,12 Significant slippage occurred in a range of implants before half the anchor–bone pullout strength was reached in a study using young bovine femoral heads.11 In another study, using young, high-BMD cadaveric humeral heads, initial slippage and maximum failure loads were equivalent among numerous devices using various suture-retention mechanisms, and suture slippage was the most common failure mode.12 Nevertheless, other biomechanical studies have demonstrated frequent failure caused by anchor pullout in elderly human cadaveric specimens with diminished BMD, often with high-force testing protocols.12,13 In the more modest-force, in vivo rehabilitative environment, suture slippage rather than anchor dislodgement may be the main failure mode.11-15
We compared the PushLock implant and its entirely press-fit suture clamping design with the ReelX device, which relies on an intrinsic suture-locking mechanism. Middle-aged (mean, 53.3 years; SD, 5.7 years) cadaveric humeri were tested under physiologically relevant biomechanical conditions to begin to help identify how relatively osteopenic bone may affect suture-retention properties for a given implant. The results showed that the study methodology prevented implant failure via anchor–bone pullout. To our knowledge, this was the first study to exclusively analyze suture slippage in knotless anchors. The findings indicated that implants that rely heavily on a tight interference fit of the suture between the anchor and the surrounding bone may exhibit early slippage and failure after RCR in middle-aged patients with relative osteopenia.11,12 However, this study also demonstrated that devices with intrinsic clamping mechanisms that do not depend on the quality of surrounding bone may better resist suture slippage. It is not clear that all knotless anchors with intrinsic locking mechanisms function equivalently. For instance, Pietschmann and colleagues12 found that 2 of 10 implants with a different internal clamping device were unable to resist failure via suture slippage, even in healthy bone. Similarly, in a study comparing ReelX devices with implants having a different internal suture-retention mechanism, ReelX failed at higher ultimate loads, and typically via anchor dislodgement, versus suture slippage in the other implants.18
It is important to note that, in the present study, the loads at which sutures broke in the intrinsic clamping anchors approached the maximum contractile force of the supraspinatus muscle (302 N).19,20 In addition, these loads were above the resistance of the rotator cuff tendon to cut out with modern suture material.21
This study’s limitations include use of an in vitro human cadaveric model that precluded analysis of the effects of postoperative healing. Biomechanical testing was also performed in a single row-type suture configuration with the rotator cuff tendon removed. Fixtures used during testing oriented the load coaxially with the axis of tension, creating a worst-case loading scenario. Although this form of testing may limit its clinical applicability, its purpose was to critically isolate how well a knotless anchor could resist suture slippage. The methods we used were also limited because the stability of the bone–anchor interface was not assessed. For patients with osteopenia, anchor pullout rather than suture slippage could be the most limiting factor for knotless anchor construct failure, and therefore further testing of both failure modes is needed. Future biomechanical studies should compare various knotless anchors’ suture-slippage characteristics in other constructs in physiologic testing orientations, including double-row and suture-bridge configurations, as well as with intact rotator cuff tendons. In addition, use of labral tape as a substitute for polyblend suture has been suggested to limit suture slippage, and this technique theoretically could have changed the results of this study.22
Conclusion
An implant with an internal ratcheting mechanism for suture retention demonstrated significantly less suture slippage in an axial tension evaluation protocol than a device reliant on interference fit of the suture between the anchor and surrounding bone. In the clinical setting, this may allow for less gap formation during the healing phase following RCR with a knotless anchor. There was also increased maximum load to failure, demonstrating an increased load until catastrophic failure using a device with a ratcheting internal locking mechanism.
Knotless suture anchor fixation techniques continue to evolve as efficient, low-profile options for arthroscopic rotator cuff repair (RCR).1,2 Excellent outcomes have been reported for constructs that use knotless fixation laterally, typically in suture bridge-type configurations.2-4 Early comparative biomechanical and clinical studies have also demonstrated equivalent results for all-knotless versus conventional constructs for arthroscopic RCR.5-10 Given the increased use and availability of multiple implant designs, it is important to supplement our clinical knowledge of these devices with laboratory studies delineating the biomechanical properties of the anchors that are used to help guide appropriate clinical use of the implants in specific patient populations.
Several biomechanical studies have shown suture slippage to be the weak but crucial link in the design of knotless anchors and the most likely mode of in vivo failure.11,12 Other studies have demonstrated frequent anchor dislodgement from bone, but these analyses involved use of elderly cadaveric specimens and relatively high-force testing protocols.12,13 Because suture-retention force may have exceeded anchor resistance to pullout (imparted by weak cadaveric bone in such biomechanical settings), the focus on suture-retention properties was limited.11 It is thought that, in clinical practice, the majority of patients who undergo RCR tend not to generate the high forces (relative to resistance to bone pullout) used to cause the anchor pullouts observed in biomechanical studies, particularly in the early postoperative setting.11-15 Cadaveric testing, however, often involves use of specimens with diminished bone mineral density (BMD), relative to age, because of the illness and other factors leading to death and donation.
Using a novel testing apparatus, we isolated, analyzed, and compared suture slippage in 2 anchor designs, one with entirely press-fit suture clamping and the other reliant on an intrinsic suture-locking mechanism.
Materials and Methods
Six human cadaveric proximal humeri specimens were used for this biomechanical study. Mean (SD) age was 53.3 (5.7) years (range, 46-59 years). Middle-aged specimens were used in order to best represent the quality of bone typically encountered in RCR surgery. To approximate tissue in clinical use, we used fresh-frozen cadaver tissue. Specimens were maintained at –20°C until about 24 hours before use and then were thawed to room temperature for testing. Specimens were included only if they had a completely intact humeral head and no prior surgery or hardware placement. Before instrumentation, dual-energy x-ray absorptiometry with a QDR-1000 scanner (Hologic) was used to determine BMD of all proximal humeri.
Two knotless suture anchors were compared: PushLock (4.5×18.5 mm; Arthrex) and ReelX STT (5.5×19.4 mm; Stryker). These anchors have multiple surgical indications (including RCR), allow patient-specific tissue tensioning, and use polyetheretherketone eyelets. The clamping force for PushLock depends entirely on the interference fit achieved for the suture between the outside of the anchor and the surrounding trabecular/cortical bone after device insertion, whereas the suture in ReelX is secured within the anchor shaft entirely by an internal ratchet-locking mechanism.
For anchor insertion, shoulders were dissected down to the greater tuberosity of the proximal humerus, and all implants were inserted (by a fellowship-trained surgeon in accordance with manufacturer guidelines) at a 25° insertion angle with manufacturer-supplied instruments. One anchor of each type (Figure 1) was inserted into the center of the rotator cuff footprint on the greater tuberosity of each specimen. Anterior and posterior positions were randomized, and an anchor from the other group was inserted into the matching location on the contralateral matched-pair specimen. In all instances, distance between the anterior and posterior anchors was 2 cm, and anchors were placed midway between the articular margin and the lateral edge of the greater tuberosity (Figure 2). Two strands of size 2 ultrahigh-molecular-weight–polyethylene Force Fiber (Stryker) were loaded into all anchors.
A custom urethane fixture was secured over the center of each anchor to allow testing to focus on suture slippage by minimizing anchor migration (Figure 3). The small aperture of this device allowed suture tails to pass freely through the center of the fixture but prevented disengagement and proximal migration of the suture anchor from the underlying bone through contact of the urethane fixture with the anchor perimeter. Any system deformation observed during testing was restricted to the suture and/or the anchor’s suture-locking mechanism. Testing fixtures also oriented the suture anchor coaxial with the axis of tension, creating a worst-case loading scenario (Figure 3).
PushLock implants were inserted with 5 pounds of tension, as indicated, using a manufacturer-supplied suture tensioner, and ReelX devices were inserted and locked with 2 full rotations, as specified by the manufacturer. After one end of each suture was cut, as would be done in vivo, the 2 other suture ends, which would have been part of the RCR in vivo, were tied together to form an 8-cm circumference loop that was brought through the urethane fixture. Humeri were then mounted in a materials testing system (MTS 810; MTS Systems) servohydraulic load frame, and the suture loop was passed around a cross-bar on the actuator of the testing device. A mechanical testing protocol consisting of modest repetitive forces was carefully chosen to simulate expected activity during rehabilitation after RCR.15 In this protocol, a 60-second preload of 10 N was followed by tensile loading between 10 N and 90 N at a frequency of 0.5 Hz for 500 cycles.15 Cycle duration at 3 mm and 5 mm of suture slippage (threshold for clinical failure) was recorded.12,16,17 In addition, suture slippage was measured after 1, 10, 50, 100, 200, 300, 400, and 500 cycles. The first 5 test cycles were not counted in the analysis to control for initial knot slippage. Finally, after completion of dynamic testing, samples were loaded at a displacement rate of 0.5 mm/s for tension-to-failure testing in the custom fixtures. Maximum failure load, stiffness, and failure mode were recorded. Ultimate failure was defined as suture breakage or gross suture slippage.
Paired Student t test was used to determine significant differences in suture slippage distance between the 2 groups at various cycle durations. In addition, Kaplan-Meier survival test was used to determine statistical differences in sample survival during the dynamic loading test.
Results
Mean (SD) BMD of the cadaveric shoulder specimens was 0.55 (0.13) g/cm2 (range, 0.29-0.68 g/cm2). The testing fixtures isolated suture slippage from anchor–bone disengagement. All 6 PushLock implants demonstrated slippage of more than 3 mm, and 5 of the 6 demonstrated slippage of more than 5 mm. All 6 ReelX devices exhibited slippage of less than 3 mm. In addition, PushLock demonstrated more suture slippage at cycles 1, 10, and 100 (P < .05) and more maximum slippage after 500 cycles (mean, 11.2 mm; SD, 4.7 mm) compared with ReelX (mean, 1.9 mm; SD, 0.5 mm) (P = .004). Figure 4 shows mean suture slippage at each cycle.
Kaplan-Meier analysis revealed significantly (λ2 = 8.170; P = .0043) decreased survival after dynamic testing for PushLock versus ReelX (Figure 5). Survival was defined as suture slippage of less than 5 mm after completion of dynamic testing. Only 1 of the 6 PushLock anchors completed dynamic testing; the other 5 failed via complete suture slippage from the anchor before testing could be completed. All 6 ReelX devices survived dynamic testing.
Therefore, 1 PushLock implant and all 6 ReelX devices were available for subsequent load-to-failure testing. Failure in this setting was defined as suture slippage of more than 10 mm or suture breakage. The PushLock implant failed at a maximum force of 171.8 N with a stiffness of 74.4 N/mm and eventually exhibited gross suture slippage. All 6 ReelX devices failed at a mean (SD) maximum of 273.5 (20.2) N, with a mean (SD) stiffness of 74.1 (17) N/mm. Mechanism of failure for all ReelX devices was suture breakage during the tensile load-to-failure test.
Discussion
We evaluated a new technique designed to isolate suture slippage in knotless anchors used for RCR. The impetus for developing this new method was to provide a means for better analyzing the ability of a knotless anchor to resist suture slippage in the cadaveric biomechanical testing setting. Suture slippage is an important mode of failure during such analyses.11,12 Significant slippage occurred in a range of implants before half the anchor–bone pullout strength was reached in a study using young bovine femoral heads.11 In another study, using young, high-BMD cadaveric humeral heads, initial slippage and maximum failure loads were equivalent among numerous devices using various suture-retention mechanisms, and suture slippage was the most common failure mode.12 Nevertheless, other biomechanical studies have demonstrated frequent failure caused by anchor pullout in elderly human cadaveric specimens with diminished BMD, often with high-force testing protocols.12,13 In the more modest-force, in vivo rehabilitative environment, suture slippage rather than anchor dislodgement may be the main failure mode.11-15
We compared the PushLock implant and its entirely press-fit suture clamping design with the ReelX device, which relies on an intrinsic suture-locking mechanism. Middle-aged (mean, 53.3 years; SD, 5.7 years) cadaveric humeri were tested under physiologically relevant biomechanical conditions to begin to help identify how relatively osteopenic bone may affect suture-retention properties for a given implant. The results showed that the study methodology prevented implant failure via anchor–bone pullout. To our knowledge, this was the first study to exclusively analyze suture slippage in knotless anchors. The findings indicated that implants that rely heavily on a tight interference fit of the suture between the anchor and the surrounding bone may exhibit early slippage and failure after RCR in middle-aged patients with relative osteopenia.11,12 However, this study also demonstrated that devices with intrinsic clamping mechanisms that do not depend on the quality of surrounding bone may better resist suture slippage. It is not clear that all knotless anchors with intrinsic locking mechanisms function equivalently. For instance, Pietschmann and colleagues12 found that 2 of 10 implants with a different internal clamping device were unable to resist failure via suture slippage, even in healthy bone. Similarly, in a study comparing ReelX devices with implants having a different internal suture-retention mechanism, ReelX failed at higher ultimate loads, and typically via anchor dislodgement, versus suture slippage in the other implants.18
It is important to note that, in the present study, the loads at which sutures broke in the intrinsic clamping anchors approached the maximum contractile force of the supraspinatus muscle (302 N).19,20 In addition, these loads were above the resistance of the rotator cuff tendon to cut out with modern suture material.21
This study’s limitations include use of an in vitro human cadaveric model that precluded analysis of the effects of postoperative healing. Biomechanical testing was also performed in a single row-type suture configuration with the rotator cuff tendon removed. Fixtures used during testing oriented the load coaxially with the axis of tension, creating a worst-case loading scenario. Although this form of testing may limit its clinical applicability, its purpose was to critically isolate how well a knotless anchor could resist suture slippage. The methods we used were also limited because the stability of the bone–anchor interface was not assessed. For patients with osteopenia, anchor pullout rather than suture slippage could be the most limiting factor for knotless anchor construct failure, and therefore further testing of both failure modes is needed. Future biomechanical studies should compare various knotless anchors’ suture-slippage characteristics in other constructs in physiologic testing orientations, including double-row and suture-bridge configurations, as well as with intact rotator cuff tendons. In addition, use of labral tape as a substitute for polyblend suture has been suggested to limit suture slippage, and this technique theoretically could have changed the results of this study.22
Conclusion
An implant with an internal ratcheting mechanism for suture retention demonstrated significantly less suture slippage in an axial tension evaluation protocol than a device reliant on interference fit of the suture between the anchor and surrounding bone. In the clinical setting, this may allow for less gap formation during the healing phase following RCR with a knotless anchor. There was also increased maximum load to failure, demonstrating an increased load until catastrophic failure using a device with a ratcheting internal locking mechanism.
1. Thal R. A knotless suture anchor. Design, function, and biomechanical testing. Am J Sports Med. 2001;29(5):646-649.
2. Cole BJ, ElAttrache NS, Anbari A. Arthroscopic rotator cuff repairs: an anatomic and biomechanical rationale for different suture-anchor repair configurations. Arthroscopy. 2007;23(6):662-669.
3. Kim KC, Shin HD, Cha SM, Lee WY. Comparison of repair integrity and functional outcomes for 3 arthroscopic suture bridge rotator cuff repair techniques. Am J Sports Med. 2013;41(2):271-277.
4. Choi CH, Kim SK, Cho MR, et al. Functional outcomes and structural integrity after double-pulley suture bridge rotator cuff repair using serial ultrasonographic examination. J Shoulder Elbow Surg. 2012;21(12):1753-1763.
5. Brown BS, Cooper AD, McIff TE, Key VH, Toby EB. Initial fixation and cyclic loading stability of knotless suture anchors for rotator cuff repair. J Shoulder Elbow Surg. 2008;17(2):313-318.
6. Burkhart SS, Adams CR, Burkhart SS, Schoolfield JD. A biomechanical comparison of 2 techniques of footprint reconstruction for rotator cuff repair: the SwiveLock-FiberChain construct versus standard double-row repair. Arthroscopy. 2009;25(3):274-281.
7. Hepp P, Osterhoff G, Engel T, Marquass B, Klink T, Josten C. Biomechanical evaluation of knotless anatomical double-layer double-row rotator cuff repair: a comparative ex vivo study. Am J Sports Med. 2009;37(7):1363-1369.
8. Maguire M, Goldberg J, Bokor D, et al. Biomechanical evaluation of four different transosseous-equivalent/suture bridge rotator cuff repairs. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1582-1587.
9. Millar NL, Wu X, Tantau R, Silverstone E, Murrell GA. Open versus two forms of arthroscopic rotator cuff repair. Clin Orthop Relat Res. 2009;467(4):966-978.
10. Rhee YG, Cho NS, Parke CS. Arthroscopic rotator cuff repair using modified Mason-Allen medial row stitch: knotless versus knot-tying suture bridge technique. Am J Sports Med. 2012;40(11):2440-2447.
11. Wieser K, Farshad M, Vlachopoulos L, Ruffieux K, Gerber C, Meyer DC. Suture slippage in knotless suture anchors as a potential failure mechanism in rotator cuff repair. Arthroscopy. 2012;28(11):1622-1627.
12. Pietschmann MF, Gülecyüz MF, Fieseler S, et al. Biomechanical stability of knotless suture anchors used in rotator cuff repair in healthy and osteopenic bone. Arthroscopy. 2010;26(8):1035-1044.
13. Barber FA, Hapa O, Bynum JA. Comparative testing by cyclic loading of rotator cuff suture anchors containing multiple high-strength sutures. Arthroscopy. 2010;26(9 suppl):S134-S141.
14. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing of biodegradable suture anchors containing 2 high-strength sutures. Arthroscopy. 2007;23(4):355-360.
15. Bynum CK, Lee S, Mahar A, Tasto J, Pedowitz R. Failure mode of suture anchors as a function of insertion depth. Am J Sports Med. 2005;33(7):1030-1034.
16. Gerber C, Schneeberger AG, Beck M, Schlegel U. Mechanical strength of repairs of the rotator cuff. J Bone Joint Surg Br. 1994;76(3):371-380.
17. Schneeberger AG, von Roll A, Kalberer F, Jacob HA, Gerber C. Mechanical strength of arthroscopic rotator cuff repair techniques: an in vitro study. J Bone Joint Surg Am. 2002;84(12):2152-2160.
18. Efird C, Traub S, Baldini T, et al. Knotless single-row rotator cuff repair: a comparative biomechanical study of 2 knotless suture anchors. Orthopedics. 2013;36(8):e1033-e1037.
19. Wright PB, Budoff JE, Yeh ML, Kelm ZS, Luo ZP. Strength of damaged suture: an in vitro study. Arthroscopy. 2006;22(12):1270-1275.
20. Burkhart SS. A stepwise approach to arthroscopic rotator cuff repair based on biomechanical principles. Arthroscopy. 2000;16(1):82-90.
21. Bisson LJ, Manohar LM. A biomechanical comparison of the pullout strength of No. 2 FiberWire suture and 2-mm FiberWire tape in bovine rotator cuff tendons. Arthroscopy. 2010;26(11):1463-1468.
22. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
1. Thal R. A knotless suture anchor. Design, function, and biomechanical testing. Am J Sports Med. 2001;29(5):646-649.
2. Cole BJ, ElAttrache NS, Anbari A. Arthroscopic rotator cuff repairs: an anatomic and biomechanical rationale for different suture-anchor repair configurations. Arthroscopy. 2007;23(6):662-669.
3. Kim KC, Shin HD, Cha SM, Lee WY. Comparison of repair integrity and functional outcomes for 3 arthroscopic suture bridge rotator cuff repair techniques. Am J Sports Med. 2013;41(2):271-277.
4. Choi CH, Kim SK, Cho MR, et al. Functional outcomes and structural integrity after double-pulley suture bridge rotator cuff repair using serial ultrasonographic examination. J Shoulder Elbow Surg. 2012;21(12):1753-1763.
5. Brown BS, Cooper AD, McIff TE, Key VH, Toby EB. Initial fixation and cyclic loading stability of knotless suture anchors for rotator cuff repair. J Shoulder Elbow Surg. 2008;17(2):313-318.
6. Burkhart SS, Adams CR, Burkhart SS, Schoolfield JD. A biomechanical comparison of 2 techniques of footprint reconstruction for rotator cuff repair: the SwiveLock-FiberChain construct versus standard double-row repair. Arthroscopy. 2009;25(3):274-281.
7. Hepp P, Osterhoff G, Engel T, Marquass B, Klink T, Josten C. Biomechanical evaluation of knotless anatomical double-layer double-row rotator cuff repair: a comparative ex vivo study. Am J Sports Med. 2009;37(7):1363-1369.
8. Maguire M, Goldberg J, Bokor D, et al. Biomechanical evaluation of four different transosseous-equivalent/suture bridge rotator cuff repairs. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1582-1587.
9. Millar NL, Wu X, Tantau R, Silverstone E, Murrell GA. Open versus two forms of arthroscopic rotator cuff repair. Clin Orthop Relat Res. 2009;467(4):966-978.
10. Rhee YG, Cho NS, Parke CS. Arthroscopic rotator cuff repair using modified Mason-Allen medial row stitch: knotless versus knot-tying suture bridge technique. Am J Sports Med. 2012;40(11):2440-2447.
11. Wieser K, Farshad M, Vlachopoulos L, Ruffieux K, Gerber C, Meyer DC. Suture slippage in knotless suture anchors as a potential failure mechanism in rotator cuff repair. Arthroscopy. 2012;28(11):1622-1627.
12. Pietschmann MF, Gülecyüz MF, Fieseler S, et al. Biomechanical stability of knotless suture anchors used in rotator cuff repair in healthy and osteopenic bone. Arthroscopy. 2010;26(8):1035-1044.
13. Barber FA, Hapa O, Bynum JA. Comparative testing by cyclic loading of rotator cuff suture anchors containing multiple high-strength sutures. Arthroscopy. 2010;26(9 suppl):S134-S141.
14. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing of biodegradable suture anchors containing 2 high-strength sutures. Arthroscopy. 2007;23(4):355-360.
15. Bynum CK, Lee S, Mahar A, Tasto J, Pedowitz R. Failure mode of suture anchors as a function of insertion depth. Am J Sports Med. 2005;33(7):1030-1034.
16. Gerber C, Schneeberger AG, Beck M, Schlegel U. Mechanical strength of repairs of the rotator cuff. J Bone Joint Surg Br. 1994;76(3):371-380.
17. Schneeberger AG, von Roll A, Kalberer F, Jacob HA, Gerber C. Mechanical strength of arthroscopic rotator cuff repair techniques: an in vitro study. J Bone Joint Surg Am. 2002;84(12):2152-2160.
18. Efird C, Traub S, Baldini T, et al. Knotless single-row rotator cuff repair: a comparative biomechanical study of 2 knotless suture anchors. Orthopedics. 2013;36(8):e1033-e1037.
19. Wright PB, Budoff JE, Yeh ML, Kelm ZS, Luo ZP. Strength of damaged suture: an in vitro study. Arthroscopy. 2006;22(12):1270-1275.
20. Burkhart SS. A stepwise approach to arthroscopic rotator cuff repair based on biomechanical principles. Arthroscopy. 2000;16(1):82-90.
21. Bisson LJ, Manohar LM. A biomechanical comparison of the pullout strength of No. 2 FiberWire suture and 2-mm FiberWire tape in bovine rotator cuff tendons. Arthroscopy. 2010;26(11):1463-1468.
22. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
Image-Based Techniques for Percutaneous Iliosacral Screw Start-Site Localization
Iliosacral (SI) screws remain the standard of care for the vast majority of posterior pelvic ring disruptions.1,2 However, despite their routine use, the procedure remains technically demanding with repeated cases of aberrant screw placement and complications.3,4 Sacral morphology is extremely variable within a patient population and affects accurate placement and trajectory of percutaneous screws.5 Classically, it is taught that the external starting position/landmark is at an intersection point of the greater trochanter and the anterior superior iliac spine (ASIS). While this “one size fits all” approach will certainly help to coordinate a start position, it is our experience that multiple stab incisions are necessary to find the optimal start site. To our knowledge, the most common image-based technique used to guide start-point localization and placement of SI screws begins with drawing a virtual sacrum on the patient’s side, guided by the lateral image.5 This article provides a novel image-based technique to be used with, or as a replacement for, the traditional technique.
Techniques
The patient is brought to the operating room and placed supine on a radiolucent operating table. If the closed reduction of the pelvic ring is successful or can be achieved via anterior manipulation/traction, posterior percutaneous pinning is planned. Either a rolled towel or a bag of saline is used as a bolster and placed midline underneath the sacrum and lumbar spine to help “bump” the pelvis and improve the range of motion for the surgeon’s drill. The patient is brought to the edge of the table when possible (ie, a posterior ring injury requiring fixation from only 1 side) to further enhance drill motion. If bilateral screws are planned, surgeons must be careful not to position 1 side at the expense of screw placement on the contralateral side. Nitrous-based anesthetic agents are avoided, because they may collect in the bowel and obscure good radiographic visualization. Arms are placed perpendicular to the body to facilitate the inlet view. Pre-preparation anteroposterior pelvis, inlet, and outlet views are obtained to assure ability to accurately and safely assess landmarks on all projections, and to mark the C-arm position and angles. This process helps decrease “useless” radiographs obtained during the procedure. Acceptable inlet radiographs show the anterior cortex of the S1 body superimposed on the S2 body. Acceptable outlet radiographs show the superior pubic symphysis at the level of the S2 foramen and visualization of the S1/S2 sacral foramen.6 The patient is then prepared in the standard fashion. Reduction maneuvers are performed and, if acceptable alignment is achieved, posterior percutaneous screw placement begins.
Technique 1
To our knowledge, the most common image-based technique used to guide start-point localization and placement of SI screws begins with drawing a virtual sacrum on the patient’s side using the lateral image. The fluoroscopic machine is set up in a lateral position.5 A free guide wire is superimposed upon the iliac cortical density and anterior sacral slope, which is marked on the skin (Figure 1). The superior portion of S1, as well as the posterior sacral slope, can be marked as well. This process has outlined the sacrum and provides an external landmark for the “safe zone” for screw placement. The operation proceeds in the standard fashion using inlet, outlet, and lateral radiographs. However, the externally drawn sacrum can aid as a reference during guide-pin placement.
Technique 2
This technique takes into account bone anatomy and soft-tissue coverage. It is helpful to think of the abdomen/pelvis as a box. The anterior abdomen represents the top of the box and the lateral buttock represents the side of the box. The corner of the imaginary box is where the abdomen begins to slope down and transitions laterally to become the buttock. This will be referenced as the “down-sloping point” and typically corresponds to the level of the iliac crest (Figure 2).
To begin, a standard cannulated screw guide wire is placed flush on the skin of the abdomen. An inlet fluoroscopy image is taken with the guide pin on the abdomen. Imagine that the resulting image represents the planned screw trajectory (Figure 3A). When the position of the guide wire is deemed adequate, a line is marked on the abdomen, using a pen, directly adjacent to the guide wire. This line represents inlet line 1 (Figure 2). The line must continue laterally until the down-sloping point. The sagittal angle of the imaginary inlet fluoroscopic beam is noted, and a guide wire is placed in the same sagittal orientation flush with the skin on the lateral buttock (Figure 3B). The guide wire must be placed so that it intersects with the first line at the down-sloping point. The skin on the lateral aspect of buttock is marked with a second line, which represents inlet line 2 (Figure 2).
The same process is repeated using an outlet view to create outlet lines 1 and 2 (Figures 4A, 4B). At this point there are 4 lines drawn on the patient (Figure 2). A stab incision is made at the intersection of the 2 lines drawn on the lateral buttock; this represents the skin start point, labeled “start incision” (Figure 2). The procedure continues in standard fashion.
The 4 external reference lines serve multiple purposes. First, the lines mark the true lateral start point for the pin at the level of the skin. This contrasts with the standard technique in which bony landmarks are marked on the skin and the surgeon must estimate a point on the skin that will provide an appropriate trajectory to the bony start point on the ilium. Further, the lines can also be used to reorient the cartesian plane so that adjustments can be isolated to a single plane, ensuring movements only alter the position on a single radiographic view (Figure 5).
Discussion
Despite the widespread use of percutaneous screw placement for posterior pelvic ring injuries, this remains a technically demanding surgery. Recent data suggest patient pelvic anatomy is extremely varied, especially the sacrum.7 Further, screw trajectories vary depending on surgical goals, fracture pattern, and number of screws. Taken together, this implies that there is no perfect universal starting site along the external ilium. Therefore, while classic teaching states to begin screw insertion within the vicinity of the intersection of the greater trochanter and the ASIS, it is our experience that this location is often not ideal.
The inlet, outlet, and lateral radiographs are all vital to assess correct trajectory of the guide pin and drill prior to final screw insertion, but the start site remains a critical step to assure a successful surgical outcome. We present 2 techniques, used together or separately, that allow the surgeon to place the initial guide pin more accurately for percutanous iliosacral screws. Though not specifically examined in this study, we think technique 2 has the potential to save operative time and use less fluoroscopic imaging because a lateral image is not required until later in the case. Technique 2 identifies the start point at the level of the skin. This is in contrast to technique 1, which identifies the desired sacral target and requires a surgeon to select a skin start site that will provide an optimal trajectory towards the desired target. Judging trajectory can be difficult, particularly in obese patients, and technique 2 eliminates this extra variable.
It is also important to consider that criteria-based nonorthogonal imaging is required for percutaneous screw placement. In these cases, it is more difficult to judge trajectory corrections because the fluoroscopic beam cannot guide perpendicular corrections as it can in operations that use orthogonal imaging. Adjustments made perpendicular to the fluoroscopic beam will change trajectory in multiple planes.8 Moreover, because the standard cartesian frame of reference is rotated, understanding the location of the sacrum in space can be especially challenging. When using the first technique, sacral landmarks are delineated, and a virtual sacrum drawn on the patient’s exterior helps with orientation. In the second technique, the ideal pin placement is mapped, and the external reference lines guide uniplanar changes. For example, the line drawn co-planar with the inlet view is essentially marking the sacral slope. Therefore, by following this line, uniplanar changes in the cranial and caudal direction are achieved on the outlet view (Figure 5). Because this line is also in reference to the already known ideal pin placement, ideal pin placement can be maintained in 1 radiographic projection while changing the start site in the appropriate direction. In a similar fashion, the co-planar line identified on the inlet view can be used on the outlet image to affect uniplanar changes in the anteroposterior direction. This technique effectively minimizes disorientation when placing percutaneous SI screws. This can be particularly beneficial when placing screws in the prone position.
Conclusion
We have shown 2 techniques that are routinely used at our institution to help identify an accurate starting position for percutaneous screw placement in posterior pelvic ring injuries. Even experienced traumatologists can more quickly and accurately identify the correct stab incisions leading to more confidently placed screws. Further, we believe understanding the usage of fluoroscopy and the concepts involved in drawing the lines enhance trainees’ comprehension of the complex anatomy of the sacrum.
1. Matta JM, Saucedo T. Internal fixation of pelvic ring fractures. Clin Orthop Relat Res. 1989;242:83-97.
2. Routt ML Jr, Kregor PJ, Simonian PT, Mayo KA. Early results of percutaneous iliosacral screws placed with the patient in the supine position. J Orthop Trauma. 1995;9(3):207-214.
3. Sagi HC, Lindvall EM. Inadvertent intraforaminal iliosacral screw placement despite apparent appropriate positioning on intraoperative fluoroscopy.
J Orthop Trauma. 2005;19(2):130-133.
4. Routt ML Jr, Simonian PT, Mills WJ. Iliosacral screw fixation: early complications of the percutaneous technique. J Orthop Trauma. 1997;11(8):584-589.
5. Routt ML Jr, Simonian PT, Agnew SG, Mann FA. Radiographic recognition of the sacral alar slope for optimal placement of iliosacral screws: a cadaveric and clinical study. J Orthop Trauma. 1996;10(3):171-177.
6. Gardner MJ, Ferrell ED, Nork SE, Segina DN, Routt ML Jr. Percutaneous placement of iliosacral screws without electrodiagnostic monitoring. J Trauma. 2009;66(5):1411-1415.
7. Miller AN, Routt ML Jr. Variations in sacral morphology and implications for iliosacral screw fixation. J Am Acad Orthop Surg. 2012;20(1):8-16.
8. Graves ML, Routt ML. Iliosacral screw placement: are uniplanar changes realistic based on standard fluoroscopic imaging? J Orthop Trauma. 2011;71(1):204-208.
Iliosacral (SI) screws remain the standard of care for the vast majority of posterior pelvic ring disruptions.1,2 However, despite their routine use, the procedure remains technically demanding with repeated cases of aberrant screw placement and complications.3,4 Sacral morphology is extremely variable within a patient population and affects accurate placement and trajectory of percutaneous screws.5 Classically, it is taught that the external starting position/landmark is at an intersection point of the greater trochanter and the anterior superior iliac spine (ASIS). While this “one size fits all” approach will certainly help to coordinate a start position, it is our experience that multiple stab incisions are necessary to find the optimal start site. To our knowledge, the most common image-based technique used to guide start-point localization and placement of SI screws begins with drawing a virtual sacrum on the patient’s side, guided by the lateral image.5 This article provides a novel image-based technique to be used with, or as a replacement for, the traditional technique.
Techniques
The patient is brought to the operating room and placed supine on a radiolucent operating table. If the closed reduction of the pelvic ring is successful or can be achieved via anterior manipulation/traction, posterior percutaneous pinning is planned. Either a rolled towel or a bag of saline is used as a bolster and placed midline underneath the sacrum and lumbar spine to help “bump” the pelvis and improve the range of motion for the surgeon’s drill. The patient is brought to the edge of the table when possible (ie, a posterior ring injury requiring fixation from only 1 side) to further enhance drill motion. If bilateral screws are planned, surgeons must be careful not to position 1 side at the expense of screw placement on the contralateral side. Nitrous-based anesthetic agents are avoided, because they may collect in the bowel and obscure good radiographic visualization. Arms are placed perpendicular to the body to facilitate the inlet view. Pre-preparation anteroposterior pelvis, inlet, and outlet views are obtained to assure ability to accurately and safely assess landmarks on all projections, and to mark the C-arm position and angles. This process helps decrease “useless” radiographs obtained during the procedure. Acceptable inlet radiographs show the anterior cortex of the S1 body superimposed on the S2 body. Acceptable outlet radiographs show the superior pubic symphysis at the level of the S2 foramen and visualization of the S1/S2 sacral foramen.6 The patient is then prepared in the standard fashion. Reduction maneuvers are performed and, if acceptable alignment is achieved, posterior percutaneous screw placement begins.
Technique 1
To our knowledge, the most common image-based technique used to guide start-point localization and placement of SI screws begins with drawing a virtual sacrum on the patient’s side using the lateral image. The fluoroscopic machine is set up in a lateral position.5 A free guide wire is superimposed upon the iliac cortical density and anterior sacral slope, which is marked on the skin (Figure 1). The superior portion of S1, as well as the posterior sacral slope, can be marked as well. This process has outlined the sacrum and provides an external landmark for the “safe zone” for screw placement. The operation proceeds in the standard fashion using inlet, outlet, and lateral radiographs. However, the externally drawn sacrum can aid as a reference during guide-pin placement.
Technique 2
This technique takes into account bone anatomy and soft-tissue coverage. It is helpful to think of the abdomen/pelvis as a box. The anterior abdomen represents the top of the box and the lateral buttock represents the side of the box. The corner of the imaginary box is where the abdomen begins to slope down and transitions laterally to become the buttock. This will be referenced as the “down-sloping point” and typically corresponds to the level of the iliac crest (Figure 2).
To begin, a standard cannulated screw guide wire is placed flush on the skin of the abdomen. An inlet fluoroscopy image is taken with the guide pin on the abdomen. Imagine that the resulting image represents the planned screw trajectory (Figure 3A). When the position of the guide wire is deemed adequate, a line is marked on the abdomen, using a pen, directly adjacent to the guide wire. This line represents inlet line 1 (Figure 2). The line must continue laterally until the down-sloping point. The sagittal angle of the imaginary inlet fluoroscopic beam is noted, and a guide wire is placed in the same sagittal orientation flush with the skin on the lateral buttock (Figure 3B). The guide wire must be placed so that it intersects with the first line at the down-sloping point. The skin on the lateral aspect of buttock is marked with a second line, which represents inlet line 2 (Figure 2).
The same process is repeated using an outlet view to create outlet lines 1 and 2 (Figures 4A, 4B). At this point there are 4 lines drawn on the patient (Figure 2). A stab incision is made at the intersection of the 2 lines drawn on the lateral buttock; this represents the skin start point, labeled “start incision” (Figure 2). The procedure continues in standard fashion.
The 4 external reference lines serve multiple purposes. First, the lines mark the true lateral start point for the pin at the level of the skin. This contrasts with the standard technique in which bony landmarks are marked on the skin and the surgeon must estimate a point on the skin that will provide an appropriate trajectory to the bony start point on the ilium. Further, the lines can also be used to reorient the cartesian plane so that adjustments can be isolated to a single plane, ensuring movements only alter the position on a single radiographic view (Figure 5).
Discussion
Despite the widespread use of percutaneous screw placement for posterior pelvic ring injuries, this remains a technically demanding surgery. Recent data suggest patient pelvic anatomy is extremely varied, especially the sacrum.7 Further, screw trajectories vary depending on surgical goals, fracture pattern, and number of screws. Taken together, this implies that there is no perfect universal starting site along the external ilium. Therefore, while classic teaching states to begin screw insertion within the vicinity of the intersection of the greater trochanter and the ASIS, it is our experience that this location is often not ideal.
The inlet, outlet, and lateral radiographs are all vital to assess correct trajectory of the guide pin and drill prior to final screw insertion, but the start site remains a critical step to assure a successful surgical outcome. We present 2 techniques, used together or separately, that allow the surgeon to place the initial guide pin more accurately for percutanous iliosacral screws. Though not specifically examined in this study, we think technique 2 has the potential to save operative time and use less fluoroscopic imaging because a lateral image is not required until later in the case. Technique 2 identifies the start point at the level of the skin. This is in contrast to technique 1, which identifies the desired sacral target and requires a surgeon to select a skin start site that will provide an optimal trajectory towards the desired target. Judging trajectory can be difficult, particularly in obese patients, and technique 2 eliminates this extra variable.
It is also important to consider that criteria-based nonorthogonal imaging is required for percutaneous screw placement. In these cases, it is more difficult to judge trajectory corrections because the fluoroscopic beam cannot guide perpendicular corrections as it can in operations that use orthogonal imaging. Adjustments made perpendicular to the fluoroscopic beam will change trajectory in multiple planes.8 Moreover, because the standard cartesian frame of reference is rotated, understanding the location of the sacrum in space can be especially challenging. When using the first technique, sacral landmarks are delineated, and a virtual sacrum drawn on the patient’s exterior helps with orientation. In the second technique, the ideal pin placement is mapped, and the external reference lines guide uniplanar changes. For example, the line drawn co-planar with the inlet view is essentially marking the sacral slope. Therefore, by following this line, uniplanar changes in the cranial and caudal direction are achieved on the outlet view (Figure 5). Because this line is also in reference to the already known ideal pin placement, ideal pin placement can be maintained in 1 radiographic projection while changing the start site in the appropriate direction. In a similar fashion, the co-planar line identified on the inlet view can be used on the outlet image to affect uniplanar changes in the anteroposterior direction. This technique effectively minimizes disorientation when placing percutaneous SI screws. This can be particularly beneficial when placing screws in the prone position.
Conclusion
We have shown 2 techniques that are routinely used at our institution to help identify an accurate starting position for percutaneous screw placement in posterior pelvic ring injuries. Even experienced traumatologists can more quickly and accurately identify the correct stab incisions leading to more confidently placed screws. Further, we believe understanding the usage of fluoroscopy and the concepts involved in drawing the lines enhance trainees’ comprehension of the complex anatomy of the sacrum.
Iliosacral (SI) screws remain the standard of care for the vast majority of posterior pelvic ring disruptions.1,2 However, despite their routine use, the procedure remains technically demanding with repeated cases of aberrant screw placement and complications.3,4 Sacral morphology is extremely variable within a patient population and affects accurate placement and trajectory of percutaneous screws.5 Classically, it is taught that the external starting position/landmark is at an intersection point of the greater trochanter and the anterior superior iliac spine (ASIS). While this “one size fits all” approach will certainly help to coordinate a start position, it is our experience that multiple stab incisions are necessary to find the optimal start site. To our knowledge, the most common image-based technique used to guide start-point localization and placement of SI screws begins with drawing a virtual sacrum on the patient’s side, guided by the lateral image.5 This article provides a novel image-based technique to be used with, or as a replacement for, the traditional technique.
Techniques
The patient is brought to the operating room and placed supine on a radiolucent operating table. If the closed reduction of the pelvic ring is successful or can be achieved via anterior manipulation/traction, posterior percutaneous pinning is planned. Either a rolled towel or a bag of saline is used as a bolster and placed midline underneath the sacrum and lumbar spine to help “bump” the pelvis and improve the range of motion for the surgeon’s drill. The patient is brought to the edge of the table when possible (ie, a posterior ring injury requiring fixation from only 1 side) to further enhance drill motion. If bilateral screws are planned, surgeons must be careful not to position 1 side at the expense of screw placement on the contralateral side. Nitrous-based anesthetic agents are avoided, because they may collect in the bowel and obscure good radiographic visualization. Arms are placed perpendicular to the body to facilitate the inlet view. Pre-preparation anteroposterior pelvis, inlet, and outlet views are obtained to assure ability to accurately and safely assess landmarks on all projections, and to mark the C-arm position and angles. This process helps decrease “useless” radiographs obtained during the procedure. Acceptable inlet radiographs show the anterior cortex of the S1 body superimposed on the S2 body. Acceptable outlet radiographs show the superior pubic symphysis at the level of the S2 foramen and visualization of the S1/S2 sacral foramen.6 The patient is then prepared in the standard fashion. Reduction maneuvers are performed and, if acceptable alignment is achieved, posterior percutaneous screw placement begins.
Technique 1
To our knowledge, the most common image-based technique used to guide start-point localization and placement of SI screws begins with drawing a virtual sacrum on the patient’s side using the lateral image. The fluoroscopic machine is set up in a lateral position.5 A free guide wire is superimposed upon the iliac cortical density and anterior sacral slope, which is marked on the skin (Figure 1). The superior portion of S1, as well as the posterior sacral slope, can be marked as well. This process has outlined the sacrum and provides an external landmark for the “safe zone” for screw placement. The operation proceeds in the standard fashion using inlet, outlet, and lateral radiographs. However, the externally drawn sacrum can aid as a reference during guide-pin placement.
Technique 2
This technique takes into account bone anatomy and soft-tissue coverage. It is helpful to think of the abdomen/pelvis as a box. The anterior abdomen represents the top of the box and the lateral buttock represents the side of the box. The corner of the imaginary box is where the abdomen begins to slope down and transitions laterally to become the buttock. This will be referenced as the “down-sloping point” and typically corresponds to the level of the iliac crest (Figure 2).
To begin, a standard cannulated screw guide wire is placed flush on the skin of the abdomen. An inlet fluoroscopy image is taken with the guide pin on the abdomen. Imagine that the resulting image represents the planned screw trajectory (Figure 3A). When the position of the guide wire is deemed adequate, a line is marked on the abdomen, using a pen, directly adjacent to the guide wire. This line represents inlet line 1 (Figure 2). The line must continue laterally until the down-sloping point. The sagittal angle of the imaginary inlet fluoroscopic beam is noted, and a guide wire is placed in the same sagittal orientation flush with the skin on the lateral buttock (Figure 3B). The guide wire must be placed so that it intersects with the first line at the down-sloping point. The skin on the lateral aspect of buttock is marked with a second line, which represents inlet line 2 (Figure 2).
The same process is repeated using an outlet view to create outlet lines 1 and 2 (Figures 4A, 4B). At this point there are 4 lines drawn on the patient (Figure 2). A stab incision is made at the intersection of the 2 lines drawn on the lateral buttock; this represents the skin start point, labeled “start incision” (Figure 2). The procedure continues in standard fashion.
The 4 external reference lines serve multiple purposes. First, the lines mark the true lateral start point for the pin at the level of the skin. This contrasts with the standard technique in which bony landmarks are marked on the skin and the surgeon must estimate a point on the skin that will provide an appropriate trajectory to the bony start point on the ilium. Further, the lines can also be used to reorient the cartesian plane so that adjustments can be isolated to a single plane, ensuring movements only alter the position on a single radiographic view (Figure 5).
Discussion
Despite the widespread use of percutaneous screw placement for posterior pelvic ring injuries, this remains a technically demanding surgery. Recent data suggest patient pelvic anatomy is extremely varied, especially the sacrum.7 Further, screw trajectories vary depending on surgical goals, fracture pattern, and number of screws. Taken together, this implies that there is no perfect universal starting site along the external ilium. Therefore, while classic teaching states to begin screw insertion within the vicinity of the intersection of the greater trochanter and the ASIS, it is our experience that this location is often not ideal.
The inlet, outlet, and lateral radiographs are all vital to assess correct trajectory of the guide pin and drill prior to final screw insertion, but the start site remains a critical step to assure a successful surgical outcome. We present 2 techniques, used together or separately, that allow the surgeon to place the initial guide pin more accurately for percutanous iliosacral screws. Though not specifically examined in this study, we think technique 2 has the potential to save operative time and use less fluoroscopic imaging because a lateral image is not required until later in the case. Technique 2 identifies the start point at the level of the skin. This is in contrast to technique 1, which identifies the desired sacral target and requires a surgeon to select a skin start site that will provide an optimal trajectory towards the desired target. Judging trajectory can be difficult, particularly in obese patients, and technique 2 eliminates this extra variable.
It is also important to consider that criteria-based nonorthogonal imaging is required for percutaneous screw placement. In these cases, it is more difficult to judge trajectory corrections because the fluoroscopic beam cannot guide perpendicular corrections as it can in operations that use orthogonal imaging. Adjustments made perpendicular to the fluoroscopic beam will change trajectory in multiple planes.8 Moreover, because the standard cartesian frame of reference is rotated, understanding the location of the sacrum in space can be especially challenging. When using the first technique, sacral landmarks are delineated, and a virtual sacrum drawn on the patient’s exterior helps with orientation. In the second technique, the ideal pin placement is mapped, and the external reference lines guide uniplanar changes. For example, the line drawn co-planar with the inlet view is essentially marking the sacral slope. Therefore, by following this line, uniplanar changes in the cranial and caudal direction are achieved on the outlet view (Figure 5). Because this line is also in reference to the already known ideal pin placement, ideal pin placement can be maintained in 1 radiographic projection while changing the start site in the appropriate direction. In a similar fashion, the co-planar line identified on the inlet view can be used on the outlet image to affect uniplanar changes in the anteroposterior direction. This technique effectively minimizes disorientation when placing percutaneous SI screws. This can be particularly beneficial when placing screws in the prone position.
Conclusion
We have shown 2 techniques that are routinely used at our institution to help identify an accurate starting position for percutaneous screw placement in posterior pelvic ring injuries. Even experienced traumatologists can more quickly and accurately identify the correct stab incisions leading to more confidently placed screws. Further, we believe understanding the usage of fluoroscopy and the concepts involved in drawing the lines enhance trainees’ comprehension of the complex anatomy of the sacrum.
1. Matta JM, Saucedo T. Internal fixation of pelvic ring fractures. Clin Orthop Relat Res. 1989;242:83-97.
2. Routt ML Jr, Kregor PJ, Simonian PT, Mayo KA. Early results of percutaneous iliosacral screws placed with the patient in the supine position. J Orthop Trauma. 1995;9(3):207-214.
3. Sagi HC, Lindvall EM. Inadvertent intraforaminal iliosacral screw placement despite apparent appropriate positioning on intraoperative fluoroscopy.
J Orthop Trauma. 2005;19(2):130-133.
4. Routt ML Jr, Simonian PT, Mills WJ. Iliosacral screw fixation: early complications of the percutaneous technique. J Orthop Trauma. 1997;11(8):584-589.
5. Routt ML Jr, Simonian PT, Agnew SG, Mann FA. Radiographic recognition of the sacral alar slope for optimal placement of iliosacral screws: a cadaveric and clinical study. J Orthop Trauma. 1996;10(3):171-177.
6. Gardner MJ, Ferrell ED, Nork SE, Segina DN, Routt ML Jr. Percutaneous placement of iliosacral screws without electrodiagnostic monitoring. J Trauma. 2009;66(5):1411-1415.
7. Miller AN, Routt ML Jr. Variations in sacral morphology and implications for iliosacral screw fixation. J Am Acad Orthop Surg. 2012;20(1):8-16.
8. Graves ML, Routt ML. Iliosacral screw placement: are uniplanar changes realistic based on standard fluoroscopic imaging? J Orthop Trauma. 2011;71(1):204-208.
1. Matta JM, Saucedo T. Internal fixation of pelvic ring fractures. Clin Orthop Relat Res. 1989;242:83-97.
2. Routt ML Jr, Kregor PJ, Simonian PT, Mayo KA. Early results of percutaneous iliosacral screws placed with the patient in the supine position. J Orthop Trauma. 1995;9(3):207-214.
3. Sagi HC, Lindvall EM. Inadvertent intraforaminal iliosacral screw placement despite apparent appropriate positioning on intraoperative fluoroscopy.
J Orthop Trauma. 2005;19(2):130-133.
4. Routt ML Jr, Simonian PT, Mills WJ. Iliosacral screw fixation: early complications of the percutaneous technique. J Orthop Trauma. 1997;11(8):584-589.
5. Routt ML Jr, Simonian PT, Agnew SG, Mann FA. Radiographic recognition of the sacral alar slope for optimal placement of iliosacral screws: a cadaveric and clinical study. J Orthop Trauma. 1996;10(3):171-177.
6. Gardner MJ, Ferrell ED, Nork SE, Segina DN, Routt ML Jr. Percutaneous placement of iliosacral screws without electrodiagnostic monitoring. J Trauma. 2009;66(5):1411-1415.
7. Miller AN, Routt ML Jr. Variations in sacral morphology and implications for iliosacral screw fixation. J Am Acad Orthop Surg. 2012;20(1):8-16.
8. Graves ML, Routt ML. Iliosacral screw placement: are uniplanar changes realistic based on standard fluoroscopic imaging? J Orthop Trauma. 2011;71(1):204-208.
Revision Rotator Cuff Reconstruction for Large Tears With Retraction: A Novel Technique Using Autogenous Tendon and Autologous Marrow
Primary rotator cuff repair is a common procedure that consistently yields favorable clinical results.1 Revision rotator cuff repair and reconstruction yield less consistent clinical results and are associated with a significant incidence of recurrent cuff tearing.2 Possible factors contributing to the loss of tissue continuity have included poor quality or frank loss of rotator cuff tissue, diminished biological potential of the rotator cuff tendon, and excessive mechanical stress on or strain of the reconstructive surgical construct.3
I conducted a pilot study involving a technique that addresses these potential factors, amalgamating several contemporary surgical methods with the addition of a novel step: an autogenous tendon graft incubated in autologous bone marrow concentrate.
Materials and Methods
Ten consecutive patients (7 men, 3 women) enrolled in this retrospective case series. Mean age at time of surgery was 58 years (range, 47-65 years). Mean follow-up was 24 months (range, 12-44 months), and no patients were lost to follow-up. Mean time between original primary repair and current reconstruction was 36 months (range, 6-120 months). Criteria for enrollment included unremitting shoulder pain, radiographs showing no significant degenerative joint disease, magnetic resonance imaging confirming a large (3-5 cm) full-thickness rotator cuff tear with retraction, and history of prior rotator cuff repair on the affected shoulder without associated biceps tenodesis. The intraoperative inclusion criterion was direct visualization of a 3- to 5-cm full-thickness rotator cuff tear with retraction of at least 3 cm. Validated Constant, American Shoulder and Elbow Surgeons (ASES), and University of California Los Angeles (UCLA) shoulder scoring systems were used to collect range-of-motion, pain, strength, daily function, and patient satisfaction data before and after surgery. Standard error was calculated. Two-sample t test was used for preoperative–postoperative comparisons. Postoperative integrity of the rotator cuff reconstruction was evaluated by an independent full-time academic musculoskeletal radiologist using dynamic diagnostic ultrasound (iU22 xMatrix Ultrasound System [Philips Healthcare] at L 9-3 MHz). Informed consent was obtained from each patient. The study was approved by institutional review board.
After induction of general anesthesia, each patient was placed in the lateral decubitus position. Bone marrow (60 mL) was aspirated through a 14-gauge needle from a dorsal iliac table, just inferior to the iliac crest (Figure 1). The patient was then placed into the beach-chair position on a surgical shoulder table. The aspirated marrow was centrifuged at 2800 and 3800 rpm for 14 to 17 minutes (Magellan Autologous Platelet Separator; Arteriocyte Medical Systems) to yield 10 mL of a concentrated (4- to 5-fold) mixture of platelet-rich plasma (PRP) and mesenchymal stem cells. Surgery was performed through a 3-cm oblique anterior mini-open incision between the anterior corner of the acromion and the coracoid process, as I previously described.4 The deltoid muscle was split, not detached. Acromioplasty and release of the coracoacromial ligament were performed. The rotator cuff was inspected under ×4.5 optical magnification. The cuff tissue was mobilized and débrided back to a healthy-appearing margin. The size and shape of the rotator cuff defect were then estimated. The long head of the biceps was harvested from its origin just distal to the superior glenoid labrum unto the intertubercular sulcus on the proximal humerus. The remainder of the biceps tendon was tenodesed to the surgical neck of the humerus. The biceps tendon graft was then manipulated and fashioned (by longitudinal partial-thickness incision and expansion) to fit the cuff defect (Figures 2, 3). The expanded graft was incubated in the concentrated marrow (10 mL) for 60 minutes (Figure 4). Débridement at the base of the greater tuberosity down to bleeding cancellous bone was followed by insertion of multiple bone anchors bearing several strands of No. 2 synthetic suture. These strands were then passed through the biceps tendon graft for secure fixation (Figure 5). The débrided end of the rotator cuff was then sewn to the biceps tendon graft using locking stitches under zero tissue tension with the arm in full adduction. The free end of the graft was sewn to the subscapularis tendon (Figure 6). The remaining marrow concentrate was injected both deep and superficial to the rotator cuff construct. No additional wound irrigation fluid was injected or suction drain inserted. After surgery, the patient was placed into an abduction pillow for 1 month and then engaged in passive motion for 1 month. Active-assisted motion began 3 months after surgery.
Results
Clinically, all patients improved with respect to pain, motion, strength, function, and satisfaction by virtue of the reconstructive surgery. After surgery, mean Constant score was increased, from 13 to 71 (P < .001). Mean ASES score increased from 18 to 75 (P < .001). Mean UCLA score increased from 4 to 28 (P < .001) (Table). Ultrasound showed 0% incidence of full-thickness retearing. Dynamic scanning during abduction showed maintained reduction of the humeral head within the glenoid socket; superior subluxation of the humeral head was not detected. The biceps tendon graft was continuous with the rotator cuff tendon, indicative of graft integration: tissue healing at the graft–bone and graft–tendon interfaces (Figures 7, 8). There were no intraoperative or postoperative patient-related complications.
Discussion
Primary rotator cuff surgery is beneficial.5 Irrespective of technique, open versus arthroscopic,6 single- versus double-row repair,7 the clinical results have been satisfactory.8 Nevertheless, the “tissue failure” rate of rotator cuff surgery (full-thickness discontinuity of rotator cuff) has been as high as 31% in primary repairs.9 In revision rotator cuff repair and reconstruction, the radiographic tissue failure rate has been even higher, particularly in the setting of chronic large tears with retraction, with tissue failure rates up to 91%.10 Although small to medium full-thickness tears and retears are well tolerated by patients with reduced activity levels,11 and pain symptoms do not necessarily correlate with rotator cuff tear size,12 large retracted full-thickness tears in active patients seldom result in optimal clinical outcomes or patient satisfaction.13,14 In addition, although recurrent tearing does not preclude a satisfactory clinical result, maintenance of cuff tissue integrity tends to produce a better objective clinical score and a more desirable clinical outcome.2
Few evidence-based restorative solutions exist for large recurrent rotator cuff tears with retraction in active nongeriatric patients.15 The no-treatment option in this context may result in gradual enlargement of the tear, chronic pain, weakness, and progressive degeneration of the glenohumeral joint and acromiohumeral confluence—so-called rotator cuff arthropathy, for which reverse total shoulder arthroplasty is required.16,17 Partial repair of a large rotator cuff tear by margin convergence, interval slide, split deltoid flap, or nonanatomical reinsertion may improve clinical outcome scores but may not alter or prevent the progressive degenerative changes associated with rotator cuff arthropathy.18,19 Synthetic scaffolds with and without biological enhancement have been used with varying degrees of success, particularly pain improvement and tissue integration.20 Nevertheless, the failure rate has been reported to be 17% to 51%,21 and no evidence exists that allograft augmentation improves functional outcomes.22 Tendon transfer using the latissimus dorsi has also proved to be a surgical alternative in younger, active patients.23 However, dissection in this procedure is a major undertaking for both surgeon and patient—compared with the minimally invasive technique used in the present study.24
I selected a cohort of active, symptomatic patients for application of a synthesis of accepted surgical techniques through a mini-open incision in order to improve the reliability of the surgical construct while minimizing surgical morbidity. Débridement of marginal tissue, safe mobilization of remaining cuff, and tension-free suture line using locking sutures maximized the mechanical strength of the construct.25,26 Biological enhancement with autogenous tissue (the patient’s own biceps tendon) as graft material (scaffolding), as well as autologous concentrated marrow delivering viable responding cells and chemokine/cytokine biofactors, increased the probability of reparative activity at the graft site.27 The net effect was consistent tissue healing at a biologically challenging locus. Nonenhanced biceps tendon grafting in the setting of “irreparable” primary rotator cuff repair has had a 40-year history of orthopedic utility and an excellent record of clinical success.28 Nevertheless, the retear rate has been 7% to 30%.29 There are no previous reports of biologically enhanced autogenous biceps tendon grafting for reconstruction of a torn rotator cuff, either primary or in the setting of chronic revision surgery.
Previous well-designed PRP enhancement studies in the context of primary rotator cuff repair failed to demonstrate a consistent benefit with concentrated platelet-only augmentation.30,31 The shared experimental design of these published studies used intra-articular injection as the sole delivery method without guarantee that the injected platelets would migrate, adhere to, and persist at the intended destination, the healing edge of the rotator cuff. In the present study, extended exposure of the splayed tendon graft by incubation in concentrated marrow was specifically designed to increase the probability that biologically active components would settle at the desired location by cellular seeding and plasmatic imbibition.32 Furthermore, use of PRP for growth factor (platelet-derived, PDGF; basic fibroblast, bFGF; transforming, TGF-β; epidermal, EGF; vascular endothelial, VEGF; connective tissue, CTGF) therapy, in addition to pluripotential mesenchymal cells for marrow-derived stem cell therapy, is in theory biologically superior to use of PRP alone.33,34
The recent expansion of information about biologics has generated much interest in augmentation of soft-tissue healing. Unfortunately, the optimal technique of using cellular processing to upregulate stem-cell capacity at the graft interface is yet to be defined.35 Clinical studies using PRP and related products to promote tendon healing have been both inconsistent and contradictory with respect to benefit of outcome. As we have been unable to harness the biological potential of this medium, application of biologics in contemporary clinical orthopedics remains narrow, random, and infrequent. The technique presented in this clinical series appears to be a small advancement in a positive direction. The described construct provides a starting point for study, combining mechanical as well as biological steps to promote rotator cuff healing. The consistency of the outcome in a clinical model in which retearing is an expectation rather than an exception is noteworthy. The zero tissue failure rate at 1 to 4 years, compared with the literature values in similar patient cohorts, is very promising.36 The clinical outcome as measured by validated shoulder scores is also comparable to literature outcome values.19 Also noteworthy is the dynamic stability the construct gives to the glenohumeral joint. Ideally, the reconstructed rotator cuff provides active force coupling with the deltoid, simulating normal shoulder biomechanics. At a minimum, the reconstructed cuff provides a viable passive barrier to superior migration of the humeral head—thus supporting the mechanical efficiency of the deltoid and preventing rotator cuff arthropathy.
This study’s small sample (10 patients) puts its conclusions at risk for type I statistical error, in that too few patients were examined over a long enough period to demonstrate failure. Nevertheless, retears typically occur within 6 months of repair.37,38 Therefore, minimum follow-up of 1 year was deemed sufficient. None of the 10 patients had diabetes or another chronic comorbidity. Nine of the 10 had either no or only mild preoperative fatty atrophy of the rotator cuff muscles. Eight of the 10 were nonsmokers. These factors, which suggest optimal surgical candidates, may prove to be significant as the clinical series expands over time. Incubation of the autogenous biceps graft in concentrated marrow for 60 minutes was arbitrarily chosen. In future in vitro examination, marrow cell viability as a function of incubation time will be assessed.
Conclusion
In active, middle-aged patients with chronic recurrent large retracted rotator cuff tears, the technique presented here, using autogenous biceps tendon and autologous concentrated marrow containing PRP and mesenchymal cells, consistently yielded satisfactory clinical results and promoted rotator cuff tissue healing without full-thickness retearing.
1. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
2. Kim HM, Caldwell JM, Buza JA, et al. Factors affecting satisfaction and shoulder function in patients with a recurrent rotator cuff tear. J Bone Joint Surg Am. 2014;96(2):106-112.
3. George MS, Khazzam M. Current concepts review: revision rotator cuff repair. J Shoulder Elbow Surg. 2012;21(4):431-440.
4. Skoff HD. Conservative open acromioplasty. J Bone Joint Surg Br. 1995;77(6):933-936.
5. Mather RC 3rd, Koenig L, Acevedo D, et al. The societal and economic value of rotator cuff repair. J Bone Joint Surg Am. 2013;95(22):1993-2000.
6. Sauerbrey AM, Getz CL, Piancastelli M, Iannotti JP, Ramsey ML, Williams GR. Arthroscopic versus mini-open rotator cuff repair: a comparison of clinical outcome. Arthroscopy. 2005;21(12):1415-1420.
7. Koh KH, Kang KC, Lim TK, Shon MS, Yoo JC. Prospective randomized clinical trial of single- versus double-row suture anchor repair in 2- to 4-cm rotator cuff tears: clinical and magnetic resonance imaging results. Arthroscopy. 2011;27(4):453-462.
8. Galatz LM, Griggs S, Cameron BD, Iannotti JP. Prospective longitudinal analysis of post-operative shoulder function: a ten-year follow-up study of full thickness rotator cuff tears. J Bone Joint Surg Am. 2001;83(7):1052-1056.
9. Oh JH, Kim SH, Kang JY, Oh CH, Gong HS. Effect of age on functional and structural outcome after rotator cuff repair. Am J Sports Med. 2010;38(4):672-678.
10. Kim JH, Kim SH, Lee SK, Seo JW, Chun YMC. Arthroscopic repair of massive contracted rotator cuff tears: aggressive release with anterior and posterior interval slides do not improve cuff healing and integrity. J Bone Joint Surg Am. 2014;95(16):1482-1488.
11. Moosmayer S, Lund G, Seljom US, et al. Tendon repair compared with physiotherapy in the treatment of rotator cuff tears. J Bone Joint Surg Am. 2014;96(18):1504-1514.
12. Dunn WR, Kuhn JE, Sanders R, et al. Symptoms of pain do not correlate with rotator cuff tear severity. J Bone Joint Surg Am. 2014;96(10):793-800.
13. Lubiatowski P, Kaczmarek P, Dzianach M, et al. Clinical and biomechanical performance of patients with failed rotator cuff repair. Int Orthop. 2013;37(12):2395-2401.
14. Holtby R, Razmjou H. Relationship between clinical and surgical findings and reparability of large and massive rotator cuff tears: a longitudinal study. BMC Musculoskelet Disord. 2014;15:180.
15. Nho SJ, Delos D, Yadav H, et al. Biomechanical and biological augmentation for the treatment of massive rotator cuff tears. Am J Sports Med. 2010;38(3):619-629.
16. Moosmayer S, Tariq R, Stiris M, Smith HJ. The natural history of asymptomatic rotator cuff tears: a three-year follow-up of fifty cases. J Bone Joint Surg Am. 2013;95(14):1249-1255.
17. Neer CS 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65(9):1232-1244.
18. Bartl C, Louloumentas P, Konstantin H, et al. Long-term outcome and structural integrity following open repair of massive rotator cuff tears. Int J Shoulder Surg. 2012;6(1):1-8.
19. Paxton ES, Teefey SA, Dahiya N, Keener JD, Yamaguchi K, Galatz LM. Clinical and radiographic outcomes of failed repairs of large or massive rotator cuff tears: minimum ten-year follow-up. J Bone Joint Surg Am. 2013;95(7):627-632.
20. Longo UG, Lamberti A, Maffulli N, Denaro V. Tendon augmentation grafts: a systematic review. Br Med Bull. 2010;94:165-188.
21. Ciampi P, Scotti C, Nonis A, et al. The benefit of synthetic versus biological patch augmentation in the repair of posterosuperior massive rotator cuff tears: a 3-year follow-up study. Am J Sports Med. 2014;42(5):1169-1175.
22. Murhi AM. Rotator cuff tears and cuff tear arthropathy. In: Boyer MI, ed. AAOS Comprehensive Orthopedic Review. Vol 2. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2014:921-929.
23. Namdari S, Voleti P, Baldwin K, Glaser D, Huffman GR. Latissimus dorsi tendon transfer for irreparable rotator cuff tears: a systematic review. J Bone Joint Surg Am. 2012;94(10):891-898.
24. Gerber C, Rahm SA, Catanzaro S, Farshad M, Moor BK. Latissimus dorsi tendon transfer for treatment of irreparable posterosuperior rotator cuff tears: long-term results at a minimum follow-up of ten years. J Bone Joint Surg Am. 2013;95(21):1920-1926.
25. Wagner JP, Krushall RJ, Masqueloet A, Gerber C. Anatomy and relationships of the suprascapular nerve: anatomical constraints to mobilization of the supraspinatus and infraspinatus muscles in the management of massive rotator cuff tears. J Bone Joint Surg Am. 1992;74(1):36-45.
26. Ponce BA, Hosemann CD, Reghava P, Tate JP, Sheppard ED, Ebenhardt AW. A biomechanical analysis of controllable intraoperative variables affecting the strength of rotator cuff repairs at the suture–tendon interface. Am J Sports Med. 2013;41(10):2256-2261.
27. Thomopoulos S. Tendon and ligaments. In: Boyer MI, ed. AAOS Comprehensive Orthopedic Review. Vol 2. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2014:105-111.
28. Sano H, Mineta M, Kitz A, Itoi E. Tendon patch grafting using the long head of the biceps for irreparable massive rotator cuff tears. J Orthop Sci. 2010;15(3):310-316.
29. Rhee YG, Cho NS, Lim CT, Yi JW, Vishvanathan T. Bridging the gap in immobile massive rotator cuff tears: augmentation using the tenotomized biceps. Am J Sports Med. 2008;36(8):1511-1518.
30. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258-265.
31. Rodeo SA, Delos, D, Williams, RJ, Adler RS, Pearle A, Warren RF. The effects of platelet-rich fibrin matrix on rotator cuff tendon healing: a prospective, randomized clinical study. Am J Sports Med. 2012;40(6):1234-1241.
32. Beitzel K, McCarthy MB, Cote MP, et al. Properties of biologic scaffolds and their response to mesenchymal stem cells. Arthroscopy. 2014;30(3):289-298.
33. Anz AW, Hackel JG, Nilssen ED, Andrews JR. Application of biologics in the treatment of rotator cuff, meniscus, cartilage and osteoarthritis. J Am Acad Orthop Surg. 2014;22(2):68-79.
34. Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014;38(9):1811-1818.
35. Hsu WK, Mishra A, Rodeo SR, et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21(12):739-748.
36. Kowalsky MS, Keener JD. Revision arthroscopic rotator cuff repair: repair integrity and clinical outcome: surgical technique. J Bone Joint Surg Am. 2011;93(suppl 1):62-74.
37. Iannotti JP, Deutsch A, Green A, et al. Time to failure after rotator cuff repair: a prospective imaging study. J Bone Joint Surg Am. 2013;95(11):965-971.
38. Le BT, Wu XL, Lam PH, Murrell GA. Factors predicting rotator cuff retears: an analysis of 1000 consecutive rotator cuff repairs. Am J Sports Med. 2014;42(5):1134-1142.
Primary rotator cuff repair is a common procedure that consistently yields favorable clinical results.1 Revision rotator cuff repair and reconstruction yield less consistent clinical results and are associated with a significant incidence of recurrent cuff tearing.2 Possible factors contributing to the loss of tissue continuity have included poor quality or frank loss of rotator cuff tissue, diminished biological potential of the rotator cuff tendon, and excessive mechanical stress on or strain of the reconstructive surgical construct.3
I conducted a pilot study involving a technique that addresses these potential factors, amalgamating several contemporary surgical methods with the addition of a novel step: an autogenous tendon graft incubated in autologous bone marrow concentrate.
Materials and Methods
Ten consecutive patients (7 men, 3 women) enrolled in this retrospective case series. Mean age at time of surgery was 58 years (range, 47-65 years). Mean follow-up was 24 months (range, 12-44 months), and no patients were lost to follow-up. Mean time between original primary repair and current reconstruction was 36 months (range, 6-120 months). Criteria for enrollment included unremitting shoulder pain, radiographs showing no significant degenerative joint disease, magnetic resonance imaging confirming a large (3-5 cm) full-thickness rotator cuff tear with retraction, and history of prior rotator cuff repair on the affected shoulder without associated biceps tenodesis. The intraoperative inclusion criterion was direct visualization of a 3- to 5-cm full-thickness rotator cuff tear with retraction of at least 3 cm. Validated Constant, American Shoulder and Elbow Surgeons (ASES), and University of California Los Angeles (UCLA) shoulder scoring systems were used to collect range-of-motion, pain, strength, daily function, and patient satisfaction data before and after surgery. Standard error was calculated. Two-sample t test was used for preoperative–postoperative comparisons. Postoperative integrity of the rotator cuff reconstruction was evaluated by an independent full-time academic musculoskeletal radiologist using dynamic diagnostic ultrasound (iU22 xMatrix Ultrasound System [Philips Healthcare] at L 9-3 MHz). Informed consent was obtained from each patient. The study was approved by institutional review board.
After induction of general anesthesia, each patient was placed in the lateral decubitus position. Bone marrow (60 mL) was aspirated through a 14-gauge needle from a dorsal iliac table, just inferior to the iliac crest (Figure 1). The patient was then placed into the beach-chair position on a surgical shoulder table. The aspirated marrow was centrifuged at 2800 and 3800 rpm for 14 to 17 minutes (Magellan Autologous Platelet Separator; Arteriocyte Medical Systems) to yield 10 mL of a concentrated (4- to 5-fold) mixture of platelet-rich plasma (PRP) and mesenchymal stem cells. Surgery was performed through a 3-cm oblique anterior mini-open incision between the anterior corner of the acromion and the coracoid process, as I previously described.4 The deltoid muscle was split, not detached. Acromioplasty and release of the coracoacromial ligament were performed. The rotator cuff was inspected under ×4.5 optical magnification. The cuff tissue was mobilized and débrided back to a healthy-appearing margin. The size and shape of the rotator cuff defect were then estimated. The long head of the biceps was harvested from its origin just distal to the superior glenoid labrum unto the intertubercular sulcus on the proximal humerus. The remainder of the biceps tendon was tenodesed to the surgical neck of the humerus. The biceps tendon graft was then manipulated and fashioned (by longitudinal partial-thickness incision and expansion) to fit the cuff defect (Figures 2, 3). The expanded graft was incubated in the concentrated marrow (10 mL) for 60 minutes (Figure 4). Débridement at the base of the greater tuberosity down to bleeding cancellous bone was followed by insertion of multiple bone anchors bearing several strands of No. 2 synthetic suture. These strands were then passed through the biceps tendon graft for secure fixation (Figure 5). The débrided end of the rotator cuff was then sewn to the biceps tendon graft using locking stitches under zero tissue tension with the arm in full adduction. The free end of the graft was sewn to the subscapularis tendon (Figure 6). The remaining marrow concentrate was injected both deep and superficial to the rotator cuff construct. No additional wound irrigation fluid was injected or suction drain inserted. After surgery, the patient was placed into an abduction pillow for 1 month and then engaged in passive motion for 1 month. Active-assisted motion began 3 months after surgery.
Results
Clinically, all patients improved with respect to pain, motion, strength, function, and satisfaction by virtue of the reconstructive surgery. After surgery, mean Constant score was increased, from 13 to 71 (P < .001). Mean ASES score increased from 18 to 75 (P < .001). Mean UCLA score increased from 4 to 28 (P < .001) (Table). Ultrasound showed 0% incidence of full-thickness retearing. Dynamic scanning during abduction showed maintained reduction of the humeral head within the glenoid socket; superior subluxation of the humeral head was not detected. The biceps tendon graft was continuous with the rotator cuff tendon, indicative of graft integration: tissue healing at the graft–bone and graft–tendon interfaces (Figures 7, 8). There were no intraoperative or postoperative patient-related complications.
Discussion
Primary rotator cuff surgery is beneficial.5 Irrespective of technique, open versus arthroscopic,6 single- versus double-row repair,7 the clinical results have been satisfactory.8 Nevertheless, the “tissue failure” rate of rotator cuff surgery (full-thickness discontinuity of rotator cuff) has been as high as 31% in primary repairs.9 In revision rotator cuff repair and reconstruction, the radiographic tissue failure rate has been even higher, particularly in the setting of chronic large tears with retraction, with tissue failure rates up to 91%.10 Although small to medium full-thickness tears and retears are well tolerated by patients with reduced activity levels,11 and pain symptoms do not necessarily correlate with rotator cuff tear size,12 large retracted full-thickness tears in active patients seldom result in optimal clinical outcomes or patient satisfaction.13,14 In addition, although recurrent tearing does not preclude a satisfactory clinical result, maintenance of cuff tissue integrity tends to produce a better objective clinical score and a more desirable clinical outcome.2
Few evidence-based restorative solutions exist for large recurrent rotator cuff tears with retraction in active nongeriatric patients.15 The no-treatment option in this context may result in gradual enlargement of the tear, chronic pain, weakness, and progressive degeneration of the glenohumeral joint and acromiohumeral confluence—so-called rotator cuff arthropathy, for which reverse total shoulder arthroplasty is required.16,17 Partial repair of a large rotator cuff tear by margin convergence, interval slide, split deltoid flap, or nonanatomical reinsertion may improve clinical outcome scores but may not alter or prevent the progressive degenerative changes associated with rotator cuff arthropathy.18,19 Synthetic scaffolds with and without biological enhancement have been used with varying degrees of success, particularly pain improvement and tissue integration.20 Nevertheless, the failure rate has been reported to be 17% to 51%,21 and no evidence exists that allograft augmentation improves functional outcomes.22 Tendon transfer using the latissimus dorsi has also proved to be a surgical alternative in younger, active patients.23 However, dissection in this procedure is a major undertaking for both surgeon and patient—compared with the minimally invasive technique used in the present study.24
I selected a cohort of active, symptomatic patients for application of a synthesis of accepted surgical techniques through a mini-open incision in order to improve the reliability of the surgical construct while minimizing surgical morbidity. Débridement of marginal tissue, safe mobilization of remaining cuff, and tension-free suture line using locking sutures maximized the mechanical strength of the construct.25,26 Biological enhancement with autogenous tissue (the patient’s own biceps tendon) as graft material (scaffolding), as well as autologous concentrated marrow delivering viable responding cells and chemokine/cytokine biofactors, increased the probability of reparative activity at the graft site.27 The net effect was consistent tissue healing at a biologically challenging locus. Nonenhanced biceps tendon grafting in the setting of “irreparable” primary rotator cuff repair has had a 40-year history of orthopedic utility and an excellent record of clinical success.28 Nevertheless, the retear rate has been 7% to 30%.29 There are no previous reports of biologically enhanced autogenous biceps tendon grafting for reconstruction of a torn rotator cuff, either primary or in the setting of chronic revision surgery.
Previous well-designed PRP enhancement studies in the context of primary rotator cuff repair failed to demonstrate a consistent benefit with concentrated platelet-only augmentation.30,31 The shared experimental design of these published studies used intra-articular injection as the sole delivery method without guarantee that the injected platelets would migrate, adhere to, and persist at the intended destination, the healing edge of the rotator cuff. In the present study, extended exposure of the splayed tendon graft by incubation in concentrated marrow was specifically designed to increase the probability that biologically active components would settle at the desired location by cellular seeding and plasmatic imbibition.32 Furthermore, use of PRP for growth factor (platelet-derived, PDGF; basic fibroblast, bFGF; transforming, TGF-β; epidermal, EGF; vascular endothelial, VEGF; connective tissue, CTGF) therapy, in addition to pluripotential mesenchymal cells for marrow-derived stem cell therapy, is in theory biologically superior to use of PRP alone.33,34
The recent expansion of information about biologics has generated much interest in augmentation of soft-tissue healing. Unfortunately, the optimal technique of using cellular processing to upregulate stem-cell capacity at the graft interface is yet to be defined.35 Clinical studies using PRP and related products to promote tendon healing have been both inconsistent and contradictory with respect to benefit of outcome. As we have been unable to harness the biological potential of this medium, application of biologics in contemporary clinical orthopedics remains narrow, random, and infrequent. The technique presented in this clinical series appears to be a small advancement in a positive direction. The described construct provides a starting point for study, combining mechanical as well as biological steps to promote rotator cuff healing. The consistency of the outcome in a clinical model in which retearing is an expectation rather than an exception is noteworthy. The zero tissue failure rate at 1 to 4 years, compared with the literature values in similar patient cohorts, is very promising.36 The clinical outcome as measured by validated shoulder scores is also comparable to literature outcome values.19 Also noteworthy is the dynamic stability the construct gives to the glenohumeral joint. Ideally, the reconstructed rotator cuff provides active force coupling with the deltoid, simulating normal shoulder biomechanics. At a minimum, the reconstructed cuff provides a viable passive barrier to superior migration of the humeral head—thus supporting the mechanical efficiency of the deltoid and preventing rotator cuff arthropathy.
This study’s small sample (10 patients) puts its conclusions at risk for type I statistical error, in that too few patients were examined over a long enough period to demonstrate failure. Nevertheless, retears typically occur within 6 months of repair.37,38 Therefore, minimum follow-up of 1 year was deemed sufficient. None of the 10 patients had diabetes or another chronic comorbidity. Nine of the 10 had either no or only mild preoperative fatty atrophy of the rotator cuff muscles. Eight of the 10 were nonsmokers. These factors, which suggest optimal surgical candidates, may prove to be significant as the clinical series expands over time. Incubation of the autogenous biceps graft in concentrated marrow for 60 minutes was arbitrarily chosen. In future in vitro examination, marrow cell viability as a function of incubation time will be assessed.
Conclusion
In active, middle-aged patients with chronic recurrent large retracted rotator cuff tears, the technique presented here, using autogenous biceps tendon and autologous concentrated marrow containing PRP and mesenchymal cells, consistently yielded satisfactory clinical results and promoted rotator cuff tissue healing without full-thickness retearing.
Primary rotator cuff repair is a common procedure that consistently yields favorable clinical results.1 Revision rotator cuff repair and reconstruction yield less consistent clinical results and are associated with a significant incidence of recurrent cuff tearing.2 Possible factors contributing to the loss of tissue continuity have included poor quality or frank loss of rotator cuff tissue, diminished biological potential of the rotator cuff tendon, and excessive mechanical stress on or strain of the reconstructive surgical construct.3
I conducted a pilot study involving a technique that addresses these potential factors, amalgamating several contemporary surgical methods with the addition of a novel step: an autogenous tendon graft incubated in autologous bone marrow concentrate.
Materials and Methods
Ten consecutive patients (7 men, 3 women) enrolled in this retrospective case series. Mean age at time of surgery was 58 years (range, 47-65 years). Mean follow-up was 24 months (range, 12-44 months), and no patients were lost to follow-up. Mean time between original primary repair and current reconstruction was 36 months (range, 6-120 months). Criteria for enrollment included unremitting shoulder pain, radiographs showing no significant degenerative joint disease, magnetic resonance imaging confirming a large (3-5 cm) full-thickness rotator cuff tear with retraction, and history of prior rotator cuff repair on the affected shoulder without associated biceps tenodesis. The intraoperative inclusion criterion was direct visualization of a 3- to 5-cm full-thickness rotator cuff tear with retraction of at least 3 cm. Validated Constant, American Shoulder and Elbow Surgeons (ASES), and University of California Los Angeles (UCLA) shoulder scoring systems were used to collect range-of-motion, pain, strength, daily function, and patient satisfaction data before and after surgery. Standard error was calculated. Two-sample t test was used for preoperative–postoperative comparisons. Postoperative integrity of the rotator cuff reconstruction was evaluated by an independent full-time academic musculoskeletal radiologist using dynamic diagnostic ultrasound (iU22 xMatrix Ultrasound System [Philips Healthcare] at L 9-3 MHz). Informed consent was obtained from each patient. The study was approved by institutional review board.
After induction of general anesthesia, each patient was placed in the lateral decubitus position. Bone marrow (60 mL) was aspirated through a 14-gauge needle from a dorsal iliac table, just inferior to the iliac crest (Figure 1). The patient was then placed into the beach-chair position on a surgical shoulder table. The aspirated marrow was centrifuged at 2800 and 3800 rpm for 14 to 17 minutes (Magellan Autologous Platelet Separator; Arteriocyte Medical Systems) to yield 10 mL of a concentrated (4- to 5-fold) mixture of platelet-rich plasma (PRP) and mesenchymal stem cells. Surgery was performed through a 3-cm oblique anterior mini-open incision between the anterior corner of the acromion and the coracoid process, as I previously described.4 The deltoid muscle was split, not detached. Acromioplasty and release of the coracoacromial ligament were performed. The rotator cuff was inspected under ×4.5 optical magnification. The cuff tissue was mobilized and débrided back to a healthy-appearing margin. The size and shape of the rotator cuff defect were then estimated. The long head of the biceps was harvested from its origin just distal to the superior glenoid labrum unto the intertubercular sulcus on the proximal humerus. The remainder of the biceps tendon was tenodesed to the surgical neck of the humerus. The biceps tendon graft was then manipulated and fashioned (by longitudinal partial-thickness incision and expansion) to fit the cuff defect (Figures 2, 3). The expanded graft was incubated in the concentrated marrow (10 mL) for 60 minutes (Figure 4). Débridement at the base of the greater tuberosity down to bleeding cancellous bone was followed by insertion of multiple bone anchors bearing several strands of No. 2 synthetic suture. These strands were then passed through the biceps tendon graft for secure fixation (Figure 5). The débrided end of the rotator cuff was then sewn to the biceps tendon graft using locking stitches under zero tissue tension with the arm in full adduction. The free end of the graft was sewn to the subscapularis tendon (Figure 6). The remaining marrow concentrate was injected both deep and superficial to the rotator cuff construct. No additional wound irrigation fluid was injected or suction drain inserted. After surgery, the patient was placed into an abduction pillow for 1 month and then engaged in passive motion for 1 month. Active-assisted motion began 3 months after surgery.
Results
Clinically, all patients improved with respect to pain, motion, strength, function, and satisfaction by virtue of the reconstructive surgery. After surgery, mean Constant score was increased, from 13 to 71 (P < .001). Mean ASES score increased from 18 to 75 (P < .001). Mean UCLA score increased from 4 to 28 (P < .001) (Table). Ultrasound showed 0% incidence of full-thickness retearing. Dynamic scanning during abduction showed maintained reduction of the humeral head within the glenoid socket; superior subluxation of the humeral head was not detected. The biceps tendon graft was continuous with the rotator cuff tendon, indicative of graft integration: tissue healing at the graft–bone and graft–tendon interfaces (Figures 7, 8). There were no intraoperative or postoperative patient-related complications.
Discussion
Primary rotator cuff surgery is beneficial.5 Irrespective of technique, open versus arthroscopic,6 single- versus double-row repair,7 the clinical results have been satisfactory.8 Nevertheless, the “tissue failure” rate of rotator cuff surgery (full-thickness discontinuity of rotator cuff) has been as high as 31% in primary repairs.9 In revision rotator cuff repair and reconstruction, the radiographic tissue failure rate has been even higher, particularly in the setting of chronic large tears with retraction, with tissue failure rates up to 91%.10 Although small to medium full-thickness tears and retears are well tolerated by patients with reduced activity levels,11 and pain symptoms do not necessarily correlate with rotator cuff tear size,12 large retracted full-thickness tears in active patients seldom result in optimal clinical outcomes or patient satisfaction.13,14 In addition, although recurrent tearing does not preclude a satisfactory clinical result, maintenance of cuff tissue integrity tends to produce a better objective clinical score and a more desirable clinical outcome.2
Few evidence-based restorative solutions exist for large recurrent rotator cuff tears with retraction in active nongeriatric patients.15 The no-treatment option in this context may result in gradual enlargement of the tear, chronic pain, weakness, and progressive degeneration of the glenohumeral joint and acromiohumeral confluence—so-called rotator cuff arthropathy, for which reverse total shoulder arthroplasty is required.16,17 Partial repair of a large rotator cuff tear by margin convergence, interval slide, split deltoid flap, or nonanatomical reinsertion may improve clinical outcome scores but may not alter or prevent the progressive degenerative changes associated with rotator cuff arthropathy.18,19 Synthetic scaffolds with and without biological enhancement have been used with varying degrees of success, particularly pain improvement and tissue integration.20 Nevertheless, the failure rate has been reported to be 17% to 51%,21 and no evidence exists that allograft augmentation improves functional outcomes.22 Tendon transfer using the latissimus dorsi has also proved to be a surgical alternative in younger, active patients.23 However, dissection in this procedure is a major undertaking for both surgeon and patient—compared with the minimally invasive technique used in the present study.24
I selected a cohort of active, symptomatic patients for application of a synthesis of accepted surgical techniques through a mini-open incision in order to improve the reliability of the surgical construct while minimizing surgical morbidity. Débridement of marginal tissue, safe mobilization of remaining cuff, and tension-free suture line using locking sutures maximized the mechanical strength of the construct.25,26 Biological enhancement with autogenous tissue (the patient’s own biceps tendon) as graft material (scaffolding), as well as autologous concentrated marrow delivering viable responding cells and chemokine/cytokine biofactors, increased the probability of reparative activity at the graft site.27 The net effect was consistent tissue healing at a biologically challenging locus. Nonenhanced biceps tendon grafting in the setting of “irreparable” primary rotator cuff repair has had a 40-year history of orthopedic utility and an excellent record of clinical success.28 Nevertheless, the retear rate has been 7% to 30%.29 There are no previous reports of biologically enhanced autogenous biceps tendon grafting for reconstruction of a torn rotator cuff, either primary or in the setting of chronic revision surgery.
Previous well-designed PRP enhancement studies in the context of primary rotator cuff repair failed to demonstrate a consistent benefit with concentrated platelet-only augmentation.30,31 The shared experimental design of these published studies used intra-articular injection as the sole delivery method without guarantee that the injected platelets would migrate, adhere to, and persist at the intended destination, the healing edge of the rotator cuff. In the present study, extended exposure of the splayed tendon graft by incubation in concentrated marrow was specifically designed to increase the probability that biologically active components would settle at the desired location by cellular seeding and plasmatic imbibition.32 Furthermore, use of PRP for growth factor (platelet-derived, PDGF; basic fibroblast, bFGF; transforming, TGF-β; epidermal, EGF; vascular endothelial, VEGF; connective tissue, CTGF) therapy, in addition to pluripotential mesenchymal cells for marrow-derived stem cell therapy, is in theory biologically superior to use of PRP alone.33,34
The recent expansion of information about biologics has generated much interest in augmentation of soft-tissue healing. Unfortunately, the optimal technique of using cellular processing to upregulate stem-cell capacity at the graft interface is yet to be defined.35 Clinical studies using PRP and related products to promote tendon healing have been both inconsistent and contradictory with respect to benefit of outcome. As we have been unable to harness the biological potential of this medium, application of biologics in contemporary clinical orthopedics remains narrow, random, and infrequent. The technique presented in this clinical series appears to be a small advancement in a positive direction. The described construct provides a starting point for study, combining mechanical as well as biological steps to promote rotator cuff healing. The consistency of the outcome in a clinical model in which retearing is an expectation rather than an exception is noteworthy. The zero tissue failure rate at 1 to 4 years, compared with the literature values in similar patient cohorts, is very promising.36 The clinical outcome as measured by validated shoulder scores is also comparable to literature outcome values.19 Also noteworthy is the dynamic stability the construct gives to the glenohumeral joint. Ideally, the reconstructed rotator cuff provides active force coupling with the deltoid, simulating normal shoulder biomechanics. At a minimum, the reconstructed cuff provides a viable passive barrier to superior migration of the humeral head—thus supporting the mechanical efficiency of the deltoid and preventing rotator cuff arthropathy.
This study’s small sample (10 patients) puts its conclusions at risk for type I statistical error, in that too few patients were examined over a long enough period to demonstrate failure. Nevertheless, retears typically occur within 6 months of repair.37,38 Therefore, minimum follow-up of 1 year was deemed sufficient. None of the 10 patients had diabetes or another chronic comorbidity. Nine of the 10 had either no or only mild preoperative fatty atrophy of the rotator cuff muscles. Eight of the 10 were nonsmokers. These factors, which suggest optimal surgical candidates, may prove to be significant as the clinical series expands over time. Incubation of the autogenous biceps graft in concentrated marrow for 60 minutes was arbitrarily chosen. In future in vitro examination, marrow cell viability as a function of incubation time will be assessed.
Conclusion
In active, middle-aged patients with chronic recurrent large retracted rotator cuff tears, the technique presented here, using autogenous biceps tendon and autologous concentrated marrow containing PRP and mesenchymal cells, consistently yielded satisfactory clinical results and promoted rotator cuff tissue healing without full-thickness retearing.
1. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
2. Kim HM, Caldwell JM, Buza JA, et al. Factors affecting satisfaction and shoulder function in patients with a recurrent rotator cuff tear. J Bone Joint Surg Am. 2014;96(2):106-112.
3. George MS, Khazzam M. Current concepts review: revision rotator cuff repair. J Shoulder Elbow Surg. 2012;21(4):431-440.
4. Skoff HD. Conservative open acromioplasty. J Bone Joint Surg Br. 1995;77(6):933-936.
5. Mather RC 3rd, Koenig L, Acevedo D, et al. The societal and economic value of rotator cuff repair. J Bone Joint Surg Am. 2013;95(22):1993-2000.
6. Sauerbrey AM, Getz CL, Piancastelli M, Iannotti JP, Ramsey ML, Williams GR. Arthroscopic versus mini-open rotator cuff repair: a comparison of clinical outcome. Arthroscopy. 2005;21(12):1415-1420.
7. Koh KH, Kang KC, Lim TK, Shon MS, Yoo JC. Prospective randomized clinical trial of single- versus double-row suture anchor repair in 2- to 4-cm rotator cuff tears: clinical and magnetic resonance imaging results. Arthroscopy. 2011;27(4):453-462.
8. Galatz LM, Griggs S, Cameron BD, Iannotti JP. Prospective longitudinal analysis of post-operative shoulder function: a ten-year follow-up study of full thickness rotator cuff tears. J Bone Joint Surg Am. 2001;83(7):1052-1056.
9. Oh JH, Kim SH, Kang JY, Oh CH, Gong HS. Effect of age on functional and structural outcome after rotator cuff repair. Am J Sports Med. 2010;38(4):672-678.
10. Kim JH, Kim SH, Lee SK, Seo JW, Chun YMC. Arthroscopic repair of massive contracted rotator cuff tears: aggressive release with anterior and posterior interval slides do not improve cuff healing and integrity. J Bone Joint Surg Am. 2014;95(16):1482-1488.
11. Moosmayer S, Lund G, Seljom US, et al. Tendon repair compared with physiotherapy in the treatment of rotator cuff tears. J Bone Joint Surg Am. 2014;96(18):1504-1514.
12. Dunn WR, Kuhn JE, Sanders R, et al. Symptoms of pain do not correlate with rotator cuff tear severity. J Bone Joint Surg Am. 2014;96(10):793-800.
13. Lubiatowski P, Kaczmarek P, Dzianach M, et al. Clinical and biomechanical performance of patients with failed rotator cuff repair. Int Orthop. 2013;37(12):2395-2401.
14. Holtby R, Razmjou H. Relationship between clinical and surgical findings and reparability of large and massive rotator cuff tears: a longitudinal study. BMC Musculoskelet Disord. 2014;15:180.
15. Nho SJ, Delos D, Yadav H, et al. Biomechanical and biological augmentation for the treatment of massive rotator cuff tears. Am J Sports Med. 2010;38(3):619-629.
16. Moosmayer S, Tariq R, Stiris M, Smith HJ. The natural history of asymptomatic rotator cuff tears: a three-year follow-up of fifty cases. J Bone Joint Surg Am. 2013;95(14):1249-1255.
17. Neer CS 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65(9):1232-1244.
18. Bartl C, Louloumentas P, Konstantin H, et al. Long-term outcome and structural integrity following open repair of massive rotator cuff tears. Int J Shoulder Surg. 2012;6(1):1-8.
19. Paxton ES, Teefey SA, Dahiya N, Keener JD, Yamaguchi K, Galatz LM. Clinical and radiographic outcomes of failed repairs of large or massive rotator cuff tears: minimum ten-year follow-up. J Bone Joint Surg Am. 2013;95(7):627-632.
20. Longo UG, Lamberti A, Maffulli N, Denaro V. Tendon augmentation grafts: a systematic review. Br Med Bull. 2010;94:165-188.
21. Ciampi P, Scotti C, Nonis A, et al. The benefit of synthetic versus biological patch augmentation in the repair of posterosuperior massive rotator cuff tears: a 3-year follow-up study. Am J Sports Med. 2014;42(5):1169-1175.
22. Murhi AM. Rotator cuff tears and cuff tear arthropathy. In: Boyer MI, ed. AAOS Comprehensive Orthopedic Review. Vol 2. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2014:921-929.
23. Namdari S, Voleti P, Baldwin K, Glaser D, Huffman GR. Latissimus dorsi tendon transfer for irreparable rotator cuff tears: a systematic review. J Bone Joint Surg Am. 2012;94(10):891-898.
24. Gerber C, Rahm SA, Catanzaro S, Farshad M, Moor BK. Latissimus dorsi tendon transfer for treatment of irreparable posterosuperior rotator cuff tears: long-term results at a minimum follow-up of ten years. J Bone Joint Surg Am. 2013;95(21):1920-1926.
25. Wagner JP, Krushall RJ, Masqueloet A, Gerber C. Anatomy and relationships of the suprascapular nerve: anatomical constraints to mobilization of the supraspinatus and infraspinatus muscles in the management of massive rotator cuff tears. J Bone Joint Surg Am. 1992;74(1):36-45.
26. Ponce BA, Hosemann CD, Reghava P, Tate JP, Sheppard ED, Ebenhardt AW. A biomechanical analysis of controllable intraoperative variables affecting the strength of rotator cuff repairs at the suture–tendon interface. Am J Sports Med. 2013;41(10):2256-2261.
27. Thomopoulos S. Tendon and ligaments. In: Boyer MI, ed. AAOS Comprehensive Orthopedic Review. Vol 2. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2014:105-111.
28. Sano H, Mineta M, Kitz A, Itoi E. Tendon patch grafting using the long head of the biceps for irreparable massive rotator cuff tears. J Orthop Sci. 2010;15(3):310-316.
29. Rhee YG, Cho NS, Lim CT, Yi JW, Vishvanathan T. Bridging the gap in immobile massive rotator cuff tears: augmentation using the tenotomized biceps. Am J Sports Med. 2008;36(8):1511-1518.
30. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258-265.
31. Rodeo SA, Delos, D, Williams, RJ, Adler RS, Pearle A, Warren RF. The effects of platelet-rich fibrin matrix on rotator cuff tendon healing: a prospective, randomized clinical study. Am J Sports Med. 2012;40(6):1234-1241.
32. Beitzel K, McCarthy MB, Cote MP, et al. Properties of biologic scaffolds and their response to mesenchymal stem cells. Arthroscopy. 2014;30(3):289-298.
33. Anz AW, Hackel JG, Nilssen ED, Andrews JR. Application of biologics in the treatment of rotator cuff, meniscus, cartilage and osteoarthritis. J Am Acad Orthop Surg. 2014;22(2):68-79.
34. Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014;38(9):1811-1818.
35. Hsu WK, Mishra A, Rodeo SR, et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21(12):739-748.
36. Kowalsky MS, Keener JD. Revision arthroscopic rotator cuff repair: repair integrity and clinical outcome: surgical technique. J Bone Joint Surg Am. 2011;93(suppl 1):62-74.
37. Iannotti JP, Deutsch A, Green A, et al. Time to failure after rotator cuff repair: a prospective imaging study. J Bone Joint Surg Am. 2013;95(11):965-971.
38. Le BT, Wu XL, Lam PH, Murrell GA. Factors predicting rotator cuff retears: an analysis of 1000 consecutive rotator cuff repairs. Am J Sports Med. 2014;42(5):1134-1142.
1. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
2. Kim HM, Caldwell JM, Buza JA, et al. Factors affecting satisfaction and shoulder function in patients with a recurrent rotator cuff tear. J Bone Joint Surg Am. 2014;96(2):106-112.
3. George MS, Khazzam M. Current concepts review: revision rotator cuff repair. J Shoulder Elbow Surg. 2012;21(4):431-440.
4. Skoff HD. Conservative open acromioplasty. J Bone Joint Surg Br. 1995;77(6):933-936.
5. Mather RC 3rd, Koenig L, Acevedo D, et al. The societal and economic value of rotator cuff repair. J Bone Joint Surg Am. 2013;95(22):1993-2000.
6. Sauerbrey AM, Getz CL, Piancastelli M, Iannotti JP, Ramsey ML, Williams GR. Arthroscopic versus mini-open rotator cuff repair: a comparison of clinical outcome. Arthroscopy. 2005;21(12):1415-1420.
7. Koh KH, Kang KC, Lim TK, Shon MS, Yoo JC. Prospective randomized clinical trial of single- versus double-row suture anchor repair in 2- to 4-cm rotator cuff tears: clinical and magnetic resonance imaging results. Arthroscopy. 2011;27(4):453-462.
8. Galatz LM, Griggs S, Cameron BD, Iannotti JP. Prospective longitudinal analysis of post-operative shoulder function: a ten-year follow-up study of full thickness rotator cuff tears. J Bone Joint Surg Am. 2001;83(7):1052-1056.
9. Oh JH, Kim SH, Kang JY, Oh CH, Gong HS. Effect of age on functional and structural outcome after rotator cuff repair. Am J Sports Med. 2010;38(4):672-678.
10. Kim JH, Kim SH, Lee SK, Seo JW, Chun YMC. Arthroscopic repair of massive contracted rotator cuff tears: aggressive release with anterior and posterior interval slides do not improve cuff healing and integrity. J Bone Joint Surg Am. 2014;95(16):1482-1488.
11. Moosmayer S, Lund G, Seljom US, et al. Tendon repair compared with physiotherapy in the treatment of rotator cuff tears. J Bone Joint Surg Am. 2014;96(18):1504-1514.
12. Dunn WR, Kuhn JE, Sanders R, et al. Symptoms of pain do not correlate with rotator cuff tear severity. J Bone Joint Surg Am. 2014;96(10):793-800.
13. Lubiatowski P, Kaczmarek P, Dzianach M, et al. Clinical and biomechanical performance of patients with failed rotator cuff repair. Int Orthop. 2013;37(12):2395-2401.
14. Holtby R, Razmjou H. Relationship between clinical and surgical findings and reparability of large and massive rotator cuff tears: a longitudinal study. BMC Musculoskelet Disord. 2014;15:180.
15. Nho SJ, Delos D, Yadav H, et al. Biomechanical and biological augmentation for the treatment of massive rotator cuff tears. Am J Sports Med. 2010;38(3):619-629.
16. Moosmayer S, Tariq R, Stiris M, Smith HJ. The natural history of asymptomatic rotator cuff tears: a three-year follow-up of fifty cases. J Bone Joint Surg Am. 2013;95(14):1249-1255.
17. Neer CS 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65(9):1232-1244.
18. Bartl C, Louloumentas P, Konstantin H, et al. Long-term outcome and structural integrity following open repair of massive rotator cuff tears. Int J Shoulder Surg. 2012;6(1):1-8.
19. Paxton ES, Teefey SA, Dahiya N, Keener JD, Yamaguchi K, Galatz LM. Clinical and radiographic outcomes of failed repairs of large or massive rotator cuff tears: minimum ten-year follow-up. J Bone Joint Surg Am. 2013;95(7):627-632.
20. Longo UG, Lamberti A, Maffulli N, Denaro V. Tendon augmentation grafts: a systematic review. Br Med Bull. 2010;94:165-188.
21. Ciampi P, Scotti C, Nonis A, et al. The benefit of synthetic versus biological patch augmentation in the repair of posterosuperior massive rotator cuff tears: a 3-year follow-up study. Am J Sports Med. 2014;42(5):1169-1175.
22. Murhi AM. Rotator cuff tears and cuff tear arthropathy. In: Boyer MI, ed. AAOS Comprehensive Orthopedic Review. Vol 2. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2014:921-929.
23. Namdari S, Voleti P, Baldwin K, Glaser D, Huffman GR. Latissimus dorsi tendon transfer for irreparable rotator cuff tears: a systematic review. J Bone Joint Surg Am. 2012;94(10):891-898.
24. Gerber C, Rahm SA, Catanzaro S, Farshad M, Moor BK. Latissimus dorsi tendon transfer for treatment of irreparable posterosuperior rotator cuff tears: long-term results at a minimum follow-up of ten years. J Bone Joint Surg Am. 2013;95(21):1920-1926.
25. Wagner JP, Krushall RJ, Masqueloet A, Gerber C. Anatomy and relationships of the suprascapular nerve: anatomical constraints to mobilization of the supraspinatus and infraspinatus muscles in the management of massive rotator cuff tears. J Bone Joint Surg Am. 1992;74(1):36-45.
26. Ponce BA, Hosemann CD, Reghava P, Tate JP, Sheppard ED, Ebenhardt AW. A biomechanical analysis of controllable intraoperative variables affecting the strength of rotator cuff repairs at the suture–tendon interface. Am J Sports Med. 2013;41(10):2256-2261.
27. Thomopoulos S. Tendon and ligaments. In: Boyer MI, ed. AAOS Comprehensive Orthopedic Review. Vol 2. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2014:105-111.
28. Sano H, Mineta M, Kitz A, Itoi E. Tendon patch grafting using the long head of the biceps for irreparable massive rotator cuff tears. J Orthop Sci. 2010;15(3):310-316.
29. Rhee YG, Cho NS, Lim CT, Yi JW, Vishvanathan T. Bridging the gap in immobile massive rotator cuff tears: augmentation using the tenotomized biceps. Am J Sports Med. 2008;36(8):1511-1518.
30. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258-265.
31. Rodeo SA, Delos, D, Williams, RJ, Adler RS, Pearle A, Warren RF. The effects of platelet-rich fibrin matrix on rotator cuff tendon healing: a prospective, randomized clinical study. Am J Sports Med. 2012;40(6):1234-1241.
32. Beitzel K, McCarthy MB, Cote MP, et al. Properties of biologic scaffolds and their response to mesenchymal stem cells. Arthroscopy. 2014;30(3):289-298.
33. Anz AW, Hackel JG, Nilssen ED, Andrews JR. Application of biologics in the treatment of rotator cuff, meniscus, cartilage and osteoarthritis. J Am Acad Orthop Surg. 2014;22(2):68-79.
34. Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014;38(9):1811-1818.
35. Hsu WK, Mishra A, Rodeo SR, et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21(12):739-748.
36. Kowalsky MS, Keener JD. Revision arthroscopic rotator cuff repair: repair integrity and clinical outcome: surgical technique. J Bone Joint Surg Am. 2011;93(suppl 1):62-74.
37. Iannotti JP, Deutsch A, Green A, et al. Time to failure after rotator cuff repair: a prospective imaging study. J Bone Joint Surg Am. 2013;95(11):965-971.
38. Le BT, Wu XL, Lam PH, Murrell GA. Factors predicting rotator cuff retears: an analysis of 1000 consecutive rotator cuff repairs. Am J Sports Med. 2014;42(5):1134-1142.
Closed Reduction of Subacute Patellar Dislocation Using Saline Joint Insufflation: A Technical Trick
As the largest sesamoid bone in the human body, the patella acts as a fulcrum to enhance the biomechanical advantage of the quadriceps in extension.1 It is subject to a variety of forces while improving distribution of forces along the extensor mechanism.2 With sufficient force, the patella can be dislocated. Acute patellar dislocations are the most common knee injury, encompassing 2% to 3% of all knee injuries3 and occurring in 5.8 per 100,000 individuals.4-5 These injuries are associated with acute trauma, frequently from sports and physical activities, occurring while in terminal extension with an axial-valgus stress on the knee during rotation.6
With acute patellar dislocations, patients are usually in significant discomfort. Often, the patella may spontaneously reduce; if not, closed reduction is usually successful with pressure applied anteromedially on the lateral patellar margin, while simultaneously attempting gentle extension of the leg.7 Closed reduction is almost universally successful, and there have only been case reports of irreducible, mainly fixed vertical axis patellar dislocations.8-11 No reports in the literature have described subacute patellar dislocations because of their rarity. Patients present immediately after dislocation, spontaneously reduce, or have a painless, chronically dislocated patella.
We present a case of an elderly man with dementia and a subacute fixed irreducible patellar dislocation, which was reduced using a technique not described in the literature. The patient and the patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 68-year-old nonambulatory man with a history of dementia and stroke presented to the emergency department with complaints of left knee pain and his knee locked in flexion. The patient’s knee had been in that fixed hyperflexed position for at least 10 days after he sustained a twisting injury to his knee while attempting to get out of bed. At baseline, the patient was mostly bedbound and could walk minimally with maximum support, but, given his dementia, he would often attempt to ambulate by himself. After the injury, the patient did not complain of much pain at rest, but attempts at his group home to straighten his leg had caused severe pain. As a result, the patient was brought to the emergency department to be evaluated for fractures.
Physical examination in the emergency department revealed atrophy of the lower extremity musculature and a left knee fixed at 120º in flexion. The skin was intact, and there was minimal effusion of the knee joint. The patella was noted to be laterally subluxated and tender to palpation over the lateral and medial facets. He was neurovascularly intact distally and had painless range of motion of his hips. His contralateral right knee had full range of motion with good patellar tracking.
Radiographs of the patient’s knee confirmed a lateral dislocation of the patella (Figures 1A-1C). After oral and intravenous administration of pain medication, a reduction was attempted without success. Next, an intra-articular knee injection of 10 mL of 1% lidocaine was given. After waiting 15 minutes, another reduction was tried. While the pain control was sufficient, the reduction was again unsuccessful. The knee was insufflated with 120 mL of sterile saline and reduction attempted again. By extending the knee and applying a medially directed force to the patella, reduction was successful. The patient was placed into a knee immobilizer and postreduction radiographs were taken (Figures 2A, 2B). Saline was extracted from the knee. The patient was admitted to the hospital where repeat examination of his knees during the next week revealed markedly less pain. The patient was lost to follow-up.
Discussion
Our patient presumably had a low-energy mechanism of injury, resulting in an undiagnosed patellar dislocation with delayed treatment. This subacute patellar dislocation was irreducible using the standard techniques. Alternatively, insufflation of the joint with saline provided the necessary impetus to allow for successful patellar reduction. The history of the patient reveals clues about the mechanism of injury. It is likely that the patient’s nonambulatory status resulted in a weak vastus medialis muscle that placed the patella at risk for dislocation. While the exact mechanism of dislocation is unknown, the patella was unable to be reduced spontaneously because our patient’s knee was maintained in a state of flexion secondary to pain and muscle contraction. The combination of weak quadriceps musculature, increased Q angle, and forced hyperflexion of the knee prevented closed reduction of the patella.
Fixed, irreducible patellar dislocations are rare and discussed infrequently in the literature.9,11-12 Reported mechanisms are mostly high energy, including blows during athletics and impacts from motor vehicle collisions.9,13 Vertical axis rotation, as first described by Cooper,14 is commonly implicated in irreducible patellar dislocations. This occurs when the patella internally rotates 180º on its vertical axis, associated with a large tear of the medial retinaculum but intact quadriceps tendon. The patella is fixed over the lateral femoral condyle with the articular surface pointing anterolaterally. Despite adequate sedation and analgesia, these are notoriously difficult to close-reduce and may necessitate open reduction.3 Our patient, while having a fixed dislocation, did not have a vertical axis component and, therefore, was amenable to our closed reduction attempt.
Our first reduction attempts were unsuccessful, likely because the patient continued to be tense, firing his quadriceps. Even after injecting the knee with lidocaine and eliminating the pain component, the patella was still impinging on the lateral femoral condyle (Figure 3A). By insufflating the knee with saline, we were able to increase the distance from the patella to the trochlea (Figure 3B). This is comparable to a knee arthroscopy, in which joint fluid pressure allows passage of arthroscopic instruments into the patellofemoral joint. We postulate that the farther the patella is anterior to the trochlea, the higher the likelihood that the patella can be reduced to its anatomic position.
Insufflation of the knee with sterile saline is a novel technique that involves minimal risk compared with the alternatives. Sometimes, for closed reduction to be successful, individuals need to be consciously sedated to relax their muscles and eliminate pain. While conscious sedation is generally considered low risk, complications have been noted, including hypotension, apnea, and retrograde amnesia.15 Manual closed reduction may also cause additional chondral damage when the medial patellar facet contacts the lateral femoral trochlea. When closed reduction of the patella fails, open reduction is required; this inherently includes all the risks of surgery, such as bleeding, infection, neurovascular injury, and wound complications.
Our insufflation technique does not require sedation and is minimally invasive. The saline creates space and provides lubrication to allow for easier manipulation of the patella. This theoretically protects the cartilage as the patella passes over the lateral trochlea. In addition to the intended effect of providing more space and lubrication for the reduction of the patella, insufflation of the joint may also relax the vastus musculature.16 In their study, Torry and colleagues16 injected 13 knees with 20 mL sterile saline and noted reduction in electromyography readings in the vastus medialis and lateralis muscles. This inhibition of vastus musculature may provide enough relaxation to aid in the successful reduction of the patella.
Our study is limited by our sample size of 1. Because acute patellar dislocations are often easily reduced, our technical trick is not frequently used. Additionally, while we were able to monitor his progress during his inpatient stay, our patient was lost to follow-up after his discharge from the hospital.
If successful, the insufflation technique eliminates the need for urgent open reduction in the operating room. As a result, we recommend attempting closed reduction using insufflation of the knee with sterile saline for irreducible patellar dislocations before proceeding with open reduction.
Conclusion
Saline insufflation of the knee can be safely and easily performed to aid in the reduction of subacute, difficult patellar dislocations.
1. Fu FH, Seel M, Berger RA. Patellofemoral biomechanics. In: Fox J, del Pizzo W, eds. The Patellofemoral Joint. New York, NY: McGraw-Hill; 1993:49.
2. Dye SF. Patellofemoral anatomy. In: Fox J, del Pizzo W, eds. The Patellofemoral Joint. New York, NY: McGraw-Hill; 1993:2-3.
3. Li X, Nielsen NM, Zhou H, Stein BS, Shelton YA, Busconi BD. Surgical treatment of a chronically fixed lateral patella dislocation in an adolescent patient. Orthop Rev (Pavia). 2013;5(2):45-47.
4. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121.
5. Colvin AC, West RV. Patellar instability. J Bone Joint Surg Am. 2008;90(12):2751-2762.
6. Panni AS, Vasso M, Cerciello S. Acute patellar dislocation. What to do? Knee Surg Sports Traumatol Arthrosc. 2013;21(2):275-278.
7. Lu DW, Wang EE, Self WH, Kharasch M. Patellar dislocation reduction. Acad Emerg Med. 2010;17(2):226.
8. Michels F, Pouliart N, Oosterlinck D. Locked patellar dislocation: a case report. J Med Case Rep. 2008;2:371.
9. ElMaraghy AW, Berry GK, Kreder HJ. Irreducible lateral patellar dislocation with vertical axis rotation: case report and review of the literature. J Trauma. 2002;53(1):131-132.
10. Wajid MA, Cheema MQ, Siddique MS. Vertical axis patellar dislocation with ipsilateral femoral fracture: use of a closed percutaneous technique for reduction of the dislocation. J Orthop Trauma. 2006;20(2):143-146.
11. Shetty S, Ramesh B, Gul A, Madhusudan TR, Altayeb T. Vertical dislocation of the patella: report of 2 cases. Orthopedics. 2009;32(10). doi: 10.3928/01477447-20090818-25.
12. Hackl W, Benedetto KP, Fink C, Sailer R, Rieger M. Locked lateral patellar dislocation: a rare case of irreducible patellar dislocation requiring open reduction. Knee Surg Sports Traumatol Arthrosc. 1999;7(6):352-355.
13. Gidden DJ, Bell KM. An unusual case of irreducible intra-articular patellar dislocation with vertical axis rotation. Injury. 1995;26(9):643-644.
14. Cooper A. Dislocation of the patella. In: Cooper A, ed. A Treatise on the Dislocations and Fractures of the Joints. Philadelphia, PA: Lea & Febiger; 1844:195.
15. Swanson ER, Seaberg DC, Mathias S. The use of propofol for sedation in the emergency department. Acad Emerg Med. 2008;3(3):234-238.
16. Torry MR, Decker MJ, Millett PJ, Steadman JR, Sterett WI. The effects of knee joint effusion on quadriceps electromyography during jogging. J Sports Sci Med. 2005;4(1):1-8.
As the largest sesamoid bone in the human body, the patella acts as a fulcrum to enhance the biomechanical advantage of the quadriceps in extension.1 It is subject to a variety of forces while improving distribution of forces along the extensor mechanism.2 With sufficient force, the patella can be dislocated. Acute patellar dislocations are the most common knee injury, encompassing 2% to 3% of all knee injuries3 and occurring in 5.8 per 100,000 individuals.4-5 These injuries are associated with acute trauma, frequently from sports and physical activities, occurring while in terminal extension with an axial-valgus stress on the knee during rotation.6
With acute patellar dislocations, patients are usually in significant discomfort. Often, the patella may spontaneously reduce; if not, closed reduction is usually successful with pressure applied anteromedially on the lateral patellar margin, while simultaneously attempting gentle extension of the leg.7 Closed reduction is almost universally successful, and there have only been case reports of irreducible, mainly fixed vertical axis patellar dislocations.8-11 No reports in the literature have described subacute patellar dislocations because of their rarity. Patients present immediately after dislocation, spontaneously reduce, or have a painless, chronically dislocated patella.
We present a case of an elderly man with dementia and a subacute fixed irreducible patellar dislocation, which was reduced using a technique not described in the literature. The patient and the patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 68-year-old nonambulatory man with a history of dementia and stroke presented to the emergency department with complaints of left knee pain and his knee locked in flexion. The patient’s knee had been in that fixed hyperflexed position for at least 10 days after he sustained a twisting injury to his knee while attempting to get out of bed. At baseline, the patient was mostly bedbound and could walk minimally with maximum support, but, given his dementia, he would often attempt to ambulate by himself. After the injury, the patient did not complain of much pain at rest, but attempts at his group home to straighten his leg had caused severe pain. As a result, the patient was brought to the emergency department to be evaluated for fractures.
Physical examination in the emergency department revealed atrophy of the lower extremity musculature and a left knee fixed at 120º in flexion. The skin was intact, and there was minimal effusion of the knee joint. The patella was noted to be laterally subluxated and tender to palpation over the lateral and medial facets. He was neurovascularly intact distally and had painless range of motion of his hips. His contralateral right knee had full range of motion with good patellar tracking.
Radiographs of the patient’s knee confirmed a lateral dislocation of the patella (Figures 1A-1C). After oral and intravenous administration of pain medication, a reduction was attempted without success. Next, an intra-articular knee injection of 10 mL of 1% lidocaine was given. After waiting 15 minutes, another reduction was tried. While the pain control was sufficient, the reduction was again unsuccessful. The knee was insufflated with 120 mL of sterile saline and reduction attempted again. By extending the knee and applying a medially directed force to the patella, reduction was successful. The patient was placed into a knee immobilizer and postreduction radiographs were taken (Figures 2A, 2B). Saline was extracted from the knee. The patient was admitted to the hospital where repeat examination of his knees during the next week revealed markedly less pain. The patient was lost to follow-up.
Discussion
Our patient presumably had a low-energy mechanism of injury, resulting in an undiagnosed patellar dislocation with delayed treatment. This subacute patellar dislocation was irreducible using the standard techniques. Alternatively, insufflation of the joint with saline provided the necessary impetus to allow for successful patellar reduction. The history of the patient reveals clues about the mechanism of injury. It is likely that the patient’s nonambulatory status resulted in a weak vastus medialis muscle that placed the patella at risk for dislocation. While the exact mechanism of dislocation is unknown, the patella was unable to be reduced spontaneously because our patient’s knee was maintained in a state of flexion secondary to pain and muscle contraction. The combination of weak quadriceps musculature, increased Q angle, and forced hyperflexion of the knee prevented closed reduction of the patella.
Fixed, irreducible patellar dislocations are rare and discussed infrequently in the literature.9,11-12 Reported mechanisms are mostly high energy, including blows during athletics and impacts from motor vehicle collisions.9,13 Vertical axis rotation, as first described by Cooper,14 is commonly implicated in irreducible patellar dislocations. This occurs when the patella internally rotates 180º on its vertical axis, associated with a large tear of the medial retinaculum but intact quadriceps tendon. The patella is fixed over the lateral femoral condyle with the articular surface pointing anterolaterally. Despite adequate sedation and analgesia, these are notoriously difficult to close-reduce and may necessitate open reduction.3 Our patient, while having a fixed dislocation, did not have a vertical axis component and, therefore, was amenable to our closed reduction attempt.
Our first reduction attempts were unsuccessful, likely because the patient continued to be tense, firing his quadriceps. Even after injecting the knee with lidocaine and eliminating the pain component, the patella was still impinging on the lateral femoral condyle (Figure 3A). By insufflating the knee with saline, we were able to increase the distance from the patella to the trochlea (Figure 3B). This is comparable to a knee arthroscopy, in which joint fluid pressure allows passage of arthroscopic instruments into the patellofemoral joint. We postulate that the farther the patella is anterior to the trochlea, the higher the likelihood that the patella can be reduced to its anatomic position.
Insufflation of the knee with sterile saline is a novel technique that involves minimal risk compared with the alternatives. Sometimes, for closed reduction to be successful, individuals need to be consciously sedated to relax their muscles and eliminate pain. While conscious sedation is generally considered low risk, complications have been noted, including hypotension, apnea, and retrograde amnesia.15 Manual closed reduction may also cause additional chondral damage when the medial patellar facet contacts the lateral femoral trochlea. When closed reduction of the patella fails, open reduction is required; this inherently includes all the risks of surgery, such as bleeding, infection, neurovascular injury, and wound complications.
Our insufflation technique does not require sedation and is minimally invasive. The saline creates space and provides lubrication to allow for easier manipulation of the patella. This theoretically protects the cartilage as the patella passes over the lateral trochlea. In addition to the intended effect of providing more space and lubrication for the reduction of the patella, insufflation of the joint may also relax the vastus musculature.16 In their study, Torry and colleagues16 injected 13 knees with 20 mL sterile saline and noted reduction in electromyography readings in the vastus medialis and lateralis muscles. This inhibition of vastus musculature may provide enough relaxation to aid in the successful reduction of the patella.
Our study is limited by our sample size of 1. Because acute patellar dislocations are often easily reduced, our technical trick is not frequently used. Additionally, while we were able to monitor his progress during his inpatient stay, our patient was lost to follow-up after his discharge from the hospital.
If successful, the insufflation technique eliminates the need for urgent open reduction in the operating room. As a result, we recommend attempting closed reduction using insufflation of the knee with sterile saline for irreducible patellar dislocations before proceeding with open reduction.
Conclusion
Saline insufflation of the knee can be safely and easily performed to aid in the reduction of subacute, difficult patellar dislocations.
As the largest sesamoid bone in the human body, the patella acts as a fulcrum to enhance the biomechanical advantage of the quadriceps in extension.1 It is subject to a variety of forces while improving distribution of forces along the extensor mechanism.2 With sufficient force, the patella can be dislocated. Acute patellar dislocations are the most common knee injury, encompassing 2% to 3% of all knee injuries3 and occurring in 5.8 per 100,000 individuals.4-5 These injuries are associated with acute trauma, frequently from sports and physical activities, occurring while in terminal extension with an axial-valgus stress on the knee during rotation.6
With acute patellar dislocations, patients are usually in significant discomfort. Often, the patella may spontaneously reduce; if not, closed reduction is usually successful with pressure applied anteromedially on the lateral patellar margin, while simultaneously attempting gentle extension of the leg.7 Closed reduction is almost universally successful, and there have only been case reports of irreducible, mainly fixed vertical axis patellar dislocations.8-11 No reports in the literature have described subacute patellar dislocations because of their rarity. Patients present immediately after dislocation, spontaneously reduce, or have a painless, chronically dislocated patella.
We present a case of an elderly man with dementia and a subacute fixed irreducible patellar dislocation, which was reduced using a technique not described in the literature. The patient and the patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 68-year-old nonambulatory man with a history of dementia and stroke presented to the emergency department with complaints of left knee pain and his knee locked in flexion. The patient’s knee had been in that fixed hyperflexed position for at least 10 days after he sustained a twisting injury to his knee while attempting to get out of bed. At baseline, the patient was mostly bedbound and could walk minimally with maximum support, but, given his dementia, he would often attempt to ambulate by himself. After the injury, the patient did not complain of much pain at rest, but attempts at his group home to straighten his leg had caused severe pain. As a result, the patient was brought to the emergency department to be evaluated for fractures.
Physical examination in the emergency department revealed atrophy of the lower extremity musculature and a left knee fixed at 120º in flexion. The skin was intact, and there was minimal effusion of the knee joint. The patella was noted to be laterally subluxated and tender to palpation over the lateral and medial facets. He was neurovascularly intact distally and had painless range of motion of his hips. His contralateral right knee had full range of motion with good patellar tracking.
Radiographs of the patient’s knee confirmed a lateral dislocation of the patella (Figures 1A-1C). After oral and intravenous administration of pain medication, a reduction was attempted without success. Next, an intra-articular knee injection of 10 mL of 1% lidocaine was given. After waiting 15 minutes, another reduction was tried. While the pain control was sufficient, the reduction was again unsuccessful. The knee was insufflated with 120 mL of sterile saline and reduction attempted again. By extending the knee and applying a medially directed force to the patella, reduction was successful. The patient was placed into a knee immobilizer and postreduction radiographs were taken (Figures 2A, 2B). Saline was extracted from the knee. The patient was admitted to the hospital where repeat examination of his knees during the next week revealed markedly less pain. The patient was lost to follow-up.
Discussion
Our patient presumably had a low-energy mechanism of injury, resulting in an undiagnosed patellar dislocation with delayed treatment. This subacute patellar dislocation was irreducible using the standard techniques. Alternatively, insufflation of the joint with saline provided the necessary impetus to allow for successful patellar reduction. The history of the patient reveals clues about the mechanism of injury. It is likely that the patient’s nonambulatory status resulted in a weak vastus medialis muscle that placed the patella at risk for dislocation. While the exact mechanism of dislocation is unknown, the patella was unable to be reduced spontaneously because our patient’s knee was maintained in a state of flexion secondary to pain and muscle contraction. The combination of weak quadriceps musculature, increased Q angle, and forced hyperflexion of the knee prevented closed reduction of the patella.
Fixed, irreducible patellar dislocations are rare and discussed infrequently in the literature.9,11-12 Reported mechanisms are mostly high energy, including blows during athletics and impacts from motor vehicle collisions.9,13 Vertical axis rotation, as first described by Cooper,14 is commonly implicated in irreducible patellar dislocations. This occurs when the patella internally rotates 180º on its vertical axis, associated with a large tear of the medial retinaculum but intact quadriceps tendon. The patella is fixed over the lateral femoral condyle with the articular surface pointing anterolaterally. Despite adequate sedation and analgesia, these are notoriously difficult to close-reduce and may necessitate open reduction.3 Our patient, while having a fixed dislocation, did not have a vertical axis component and, therefore, was amenable to our closed reduction attempt.
Our first reduction attempts were unsuccessful, likely because the patient continued to be tense, firing his quadriceps. Even after injecting the knee with lidocaine and eliminating the pain component, the patella was still impinging on the lateral femoral condyle (Figure 3A). By insufflating the knee with saline, we were able to increase the distance from the patella to the trochlea (Figure 3B). This is comparable to a knee arthroscopy, in which joint fluid pressure allows passage of arthroscopic instruments into the patellofemoral joint. We postulate that the farther the patella is anterior to the trochlea, the higher the likelihood that the patella can be reduced to its anatomic position.
Insufflation of the knee with sterile saline is a novel technique that involves minimal risk compared with the alternatives. Sometimes, for closed reduction to be successful, individuals need to be consciously sedated to relax their muscles and eliminate pain. While conscious sedation is generally considered low risk, complications have been noted, including hypotension, apnea, and retrograde amnesia.15 Manual closed reduction may also cause additional chondral damage when the medial patellar facet contacts the lateral femoral trochlea. When closed reduction of the patella fails, open reduction is required; this inherently includes all the risks of surgery, such as bleeding, infection, neurovascular injury, and wound complications.
Our insufflation technique does not require sedation and is minimally invasive. The saline creates space and provides lubrication to allow for easier manipulation of the patella. This theoretically protects the cartilage as the patella passes over the lateral trochlea. In addition to the intended effect of providing more space and lubrication for the reduction of the patella, insufflation of the joint may also relax the vastus musculature.16 In their study, Torry and colleagues16 injected 13 knees with 20 mL sterile saline and noted reduction in electromyography readings in the vastus medialis and lateralis muscles. This inhibition of vastus musculature may provide enough relaxation to aid in the successful reduction of the patella.
Our study is limited by our sample size of 1. Because acute patellar dislocations are often easily reduced, our technical trick is not frequently used. Additionally, while we were able to monitor his progress during his inpatient stay, our patient was lost to follow-up after his discharge from the hospital.
If successful, the insufflation technique eliminates the need for urgent open reduction in the operating room. As a result, we recommend attempting closed reduction using insufflation of the knee with sterile saline for irreducible patellar dislocations before proceeding with open reduction.
Conclusion
Saline insufflation of the knee can be safely and easily performed to aid in the reduction of subacute, difficult patellar dislocations.
1. Fu FH, Seel M, Berger RA. Patellofemoral biomechanics. In: Fox J, del Pizzo W, eds. The Patellofemoral Joint. New York, NY: McGraw-Hill; 1993:49.
2. Dye SF. Patellofemoral anatomy. In: Fox J, del Pizzo W, eds. The Patellofemoral Joint. New York, NY: McGraw-Hill; 1993:2-3.
3. Li X, Nielsen NM, Zhou H, Stein BS, Shelton YA, Busconi BD. Surgical treatment of a chronically fixed lateral patella dislocation in an adolescent patient. Orthop Rev (Pavia). 2013;5(2):45-47.
4. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121.
5. Colvin AC, West RV. Patellar instability. J Bone Joint Surg Am. 2008;90(12):2751-2762.
6. Panni AS, Vasso M, Cerciello S. Acute patellar dislocation. What to do? Knee Surg Sports Traumatol Arthrosc. 2013;21(2):275-278.
7. Lu DW, Wang EE, Self WH, Kharasch M. Patellar dislocation reduction. Acad Emerg Med. 2010;17(2):226.
8. Michels F, Pouliart N, Oosterlinck D. Locked patellar dislocation: a case report. J Med Case Rep. 2008;2:371.
9. ElMaraghy AW, Berry GK, Kreder HJ. Irreducible lateral patellar dislocation with vertical axis rotation: case report and review of the literature. J Trauma. 2002;53(1):131-132.
10. Wajid MA, Cheema MQ, Siddique MS. Vertical axis patellar dislocation with ipsilateral femoral fracture: use of a closed percutaneous technique for reduction of the dislocation. J Orthop Trauma. 2006;20(2):143-146.
11. Shetty S, Ramesh B, Gul A, Madhusudan TR, Altayeb T. Vertical dislocation of the patella: report of 2 cases. Orthopedics. 2009;32(10). doi: 10.3928/01477447-20090818-25.
12. Hackl W, Benedetto KP, Fink C, Sailer R, Rieger M. Locked lateral patellar dislocation: a rare case of irreducible patellar dislocation requiring open reduction. Knee Surg Sports Traumatol Arthrosc. 1999;7(6):352-355.
13. Gidden DJ, Bell KM. An unusual case of irreducible intra-articular patellar dislocation with vertical axis rotation. Injury. 1995;26(9):643-644.
14. Cooper A. Dislocation of the patella. In: Cooper A, ed. A Treatise on the Dislocations and Fractures of the Joints. Philadelphia, PA: Lea & Febiger; 1844:195.
15. Swanson ER, Seaberg DC, Mathias S. The use of propofol for sedation in the emergency department. Acad Emerg Med. 2008;3(3):234-238.
16. Torry MR, Decker MJ, Millett PJ, Steadman JR, Sterett WI. The effects of knee joint effusion on quadriceps electromyography during jogging. J Sports Sci Med. 2005;4(1):1-8.
1. Fu FH, Seel M, Berger RA. Patellofemoral biomechanics. In: Fox J, del Pizzo W, eds. The Patellofemoral Joint. New York, NY: McGraw-Hill; 1993:49.
2. Dye SF. Patellofemoral anatomy. In: Fox J, del Pizzo W, eds. The Patellofemoral Joint. New York, NY: McGraw-Hill; 1993:2-3.
3. Li X, Nielsen NM, Zhou H, Stein BS, Shelton YA, Busconi BD. Surgical treatment of a chronically fixed lateral patella dislocation in an adolescent patient. Orthop Rev (Pavia). 2013;5(2):45-47.
4. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121.
5. Colvin AC, West RV. Patellar instability. J Bone Joint Surg Am. 2008;90(12):2751-2762.
6. Panni AS, Vasso M, Cerciello S. Acute patellar dislocation. What to do? Knee Surg Sports Traumatol Arthrosc. 2013;21(2):275-278.
7. Lu DW, Wang EE, Self WH, Kharasch M. Patellar dislocation reduction. Acad Emerg Med. 2010;17(2):226.
8. Michels F, Pouliart N, Oosterlinck D. Locked patellar dislocation: a case report. J Med Case Rep. 2008;2:371.
9. ElMaraghy AW, Berry GK, Kreder HJ. Irreducible lateral patellar dislocation with vertical axis rotation: case report and review of the literature. J Trauma. 2002;53(1):131-132.
10. Wajid MA, Cheema MQ, Siddique MS. Vertical axis patellar dislocation with ipsilateral femoral fracture: use of a closed percutaneous technique for reduction of the dislocation. J Orthop Trauma. 2006;20(2):143-146.
11. Shetty S, Ramesh B, Gul A, Madhusudan TR, Altayeb T. Vertical dislocation of the patella: report of 2 cases. Orthopedics. 2009;32(10). doi: 10.3928/01477447-20090818-25.
12. Hackl W, Benedetto KP, Fink C, Sailer R, Rieger M. Locked lateral patellar dislocation: a rare case of irreducible patellar dislocation requiring open reduction. Knee Surg Sports Traumatol Arthrosc. 1999;7(6):352-355.
13. Gidden DJ, Bell KM. An unusual case of irreducible intra-articular patellar dislocation with vertical axis rotation. Injury. 1995;26(9):643-644.
14. Cooper A. Dislocation of the patella. In: Cooper A, ed. A Treatise on the Dislocations and Fractures of the Joints. Philadelphia, PA: Lea & Febiger; 1844:195.
15. Swanson ER, Seaberg DC, Mathias S. The use of propofol for sedation in the emergency department. Acad Emerg Med. 2008;3(3):234-238.
16. Torry MR, Decker MJ, Millett PJ, Steadman JR, Sterett WI. The effects of knee joint effusion on quadriceps electromyography during jogging. J Sports Sci Med. 2005;4(1):1-8.