User login
MACRA Monday: Try this measure
If you haven’t started reporting quality data for the Merit-based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Measure #112: Breast Cancer Screening
This measure is aimed at capturing the percentage of women 50-74 years old who had a mammogram to screen for breast cancer.
What you need to do: The patient should either be screened for breast cancer on the date of service or there should be documentation that the patient was screened for breast cancer at least once within 27 months prior to the date of service.
Eligible cases include patients 51-74 years of age on the date of encounter and a patient encounter during the performance period. Applicable codes (CPT or HCPCS) include 99201, 99202, 99203, 99204, 99205, 99212, 99213, 99214, 99215, 99341, 99342, 99343, 99344, 99345, 99347, 99348, 99349, 99350, G0402, G0438, G0439.
To get credit under MIPS, be sure to include a QDC that shows that you successfully performed the measure or had a good reason for not doing so. For instance, CPT II 3014F indicates that screening mammography results were documented and reviewed. Code G9708 is an exclusion code for women who had a bilateral mastectomy or evidence of a right or left unilateral mastectomy.
CMS has a full list measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
• Those who enrolled in Medicare for the first time during a performance period.
• Those who have Medicare Part B allowed charges of $30,000 or less.
• Those who have 100 or fewer Medicare Part B patients.
• Those who are significantly participating in an Advanced Alternative Payment Model (APM).
If you haven’t started reporting quality data for the Merit-based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Measure #112: Breast Cancer Screening
This measure is aimed at capturing the percentage of women 50-74 years old who had a mammogram to screen for breast cancer.
What you need to do: The patient should either be screened for breast cancer on the date of service or there should be documentation that the patient was screened for breast cancer at least once within 27 months prior to the date of service.
Eligible cases include patients 51-74 years of age on the date of encounter and a patient encounter during the performance period. Applicable codes (CPT or HCPCS) include 99201, 99202, 99203, 99204, 99205, 99212, 99213, 99214, 99215, 99341, 99342, 99343, 99344, 99345, 99347, 99348, 99349, 99350, G0402, G0438, G0439.
To get credit under MIPS, be sure to include a QDC that shows that you successfully performed the measure or had a good reason for not doing so. For instance, CPT II 3014F indicates that screening mammography results were documented and reviewed. Code G9708 is an exclusion code for women who had a bilateral mastectomy or evidence of a right or left unilateral mastectomy.
CMS has a full list measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
• Those who enrolled in Medicare for the first time during a performance period.
• Those who have Medicare Part B allowed charges of $30,000 or less.
• Those who have 100 or fewer Medicare Part B patients.
• Those who are significantly participating in an Advanced Alternative Payment Model (APM).
If you haven’t started reporting quality data for the Merit-based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Measure #112: Breast Cancer Screening
This measure is aimed at capturing the percentage of women 50-74 years old who had a mammogram to screen for breast cancer.
What you need to do: The patient should either be screened for breast cancer on the date of service or there should be documentation that the patient was screened for breast cancer at least once within 27 months prior to the date of service.
Eligible cases include patients 51-74 years of age on the date of encounter and a patient encounter during the performance period. Applicable codes (CPT or HCPCS) include 99201, 99202, 99203, 99204, 99205, 99212, 99213, 99214, 99215, 99341, 99342, 99343, 99344, 99345, 99347, 99348, 99349, 99350, G0402, G0438, G0439.
To get credit under MIPS, be sure to include a QDC that shows that you successfully performed the measure or had a good reason for not doing so. For instance, CPT II 3014F indicates that screening mammography results were documented and reviewed. Code G9708 is an exclusion code for women who had a bilateral mastectomy or evidence of a right or left unilateral mastectomy.
CMS has a full list measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
• Those who enrolled in Medicare for the first time during a performance period.
• Those who have Medicare Part B allowed charges of $30,000 or less.
• Those who have 100 or fewer Medicare Part B patients.
• Those who are significantly participating in an Advanced Alternative Payment Model (APM).
MIPS: It’s time to get started
David O. Barbe, MD, is urging physicians to participate in the Medicare Quality Payment Program, even if the business case isn’t quite there.
QPP is the value-based payment system created by the Medicare Access and CHIP Reauthorization Act (MACRA). It promotes high-value care through Medicare payment increases. But for some practices, the investment in personnel and technology needed to earn those increases may be more than the increases themselves, leading doctors to do just enough to avoid being penalized.
“I think that many physicians don’t feel they are ever going to get a bonus but sure would like to avoid a penalty,” Dr. Barbe, president of the American Medical Association, said in an exclusive interview. “I am afraid many will simply perform at the lowest level that keeps them out of the penalty. Because many of them find that making the investment it takes to perform highly, there is not a business case for that.”
Full participation in QPP’s Merit-based Incentive Payment System (MIPS) could run small practices an additional $10,000 to $30,000 a year, he said. “If you’ve got $200,000 in Medicare receipts, if you get adjusted even the maximum of 4%, that is $8,000. You can’t cover $20,000 with $8,000. The math doesn’t work. There is not a business case there for it.”
That said, Dr. Barbe still spoke in favor of QPP and noted that the AMA is working with the Centers for Medicare & Medicaid Services as well as Congress to make the program more valuable and meaningful for physicians.
“We understand where we need to go as a profession, as an industry,” he said. “How we get there is the key, it’s the challenge and it requires flexibility. ... CMS has been accommodating but there are limits to how long they can go.”
The AMA is urging doctors who have missed the 90-day window for full participation – which effectively closed for most on Oct. 2 – to consider the Pick Your Pace option offered by the CMS.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Pick Your Pace allows physicians and practices to submit data on one measure for one patient to avoid a reduction in Medicare pay, even though they would not be eligible for a bonus.
“AMA has put out a lot of tools to help physicians assess their readiness, assess the gap between what they are able to do in their practice now and what they need to do to be successful under [the MIPS] primarily down to and including a video that would walk a physician step-by-step through the one patient, one measure, no penalty,” Dr. Barbe said.
He also encouraged doctors to pick a measure that is meaningful to their practice if only to get the ball rolling and get their feet wet in the QPP pool.
“What I tell physicians is pick something that is relevant for your practice,” he said.”If I see a lot of diabetes patients in my practice but I don’t see many people on anticoagulants, it doesn’t make sense for me to pick an anticoagulant measure.”
And if all a practice can do this year is one patient, one measure, Dr. Barbe urged physicians to look toward the next reporting year with an eye to do more, as that will ultimately lead to better quality of care delivered.
“Report on one patient and one measure this year ... but look at next year to say ‘that’s going to be a 90-day project for me,’ and get in on that. There is a pretty long laundry list of conditions and metrics that you can report on.”
And if practices start capturing relevant data, it opens the door to improving their practice if they also take the time to analyze what they are collecting.
“That is the purpose,” Dr. Barbe said. “As you measure yourself along the way, if the threshold for performance is here, and you find yourself working at this [lower] level for the first 30 days or whatever, then you stop and take stock of that” and react accordingly, whether its providing patients with a little more information about their condition or perhaps being more diligent in terms of monitoring high risk patients.
David O. Barbe, MD, is urging physicians to participate in the Medicare Quality Payment Program, even if the business case isn’t quite there.
QPP is the value-based payment system created by the Medicare Access and CHIP Reauthorization Act (MACRA). It promotes high-value care through Medicare payment increases. But for some practices, the investment in personnel and technology needed to earn those increases may be more than the increases themselves, leading doctors to do just enough to avoid being penalized.
“I think that many physicians don’t feel they are ever going to get a bonus but sure would like to avoid a penalty,” Dr. Barbe, president of the American Medical Association, said in an exclusive interview. “I am afraid many will simply perform at the lowest level that keeps them out of the penalty. Because many of them find that making the investment it takes to perform highly, there is not a business case for that.”
Full participation in QPP’s Merit-based Incentive Payment System (MIPS) could run small practices an additional $10,000 to $30,000 a year, he said. “If you’ve got $200,000 in Medicare receipts, if you get adjusted even the maximum of 4%, that is $8,000. You can’t cover $20,000 with $8,000. The math doesn’t work. There is not a business case there for it.”
That said, Dr. Barbe still spoke in favor of QPP and noted that the AMA is working with the Centers for Medicare & Medicaid Services as well as Congress to make the program more valuable and meaningful for physicians.
“We understand where we need to go as a profession, as an industry,” he said. “How we get there is the key, it’s the challenge and it requires flexibility. ... CMS has been accommodating but there are limits to how long they can go.”
The AMA is urging doctors who have missed the 90-day window for full participation – which effectively closed for most on Oct. 2 – to consider the Pick Your Pace option offered by the CMS.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Pick Your Pace allows physicians and practices to submit data on one measure for one patient to avoid a reduction in Medicare pay, even though they would not be eligible for a bonus.
“AMA has put out a lot of tools to help physicians assess their readiness, assess the gap between what they are able to do in their practice now and what they need to do to be successful under [the MIPS] primarily down to and including a video that would walk a physician step-by-step through the one patient, one measure, no penalty,” Dr. Barbe said.
He also encouraged doctors to pick a measure that is meaningful to their practice if only to get the ball rolling and get their feet wet in the QPP pool.
“What I tell physicians is pick something that is relevant for your practice,” he said.”If I see a lot of diabetes patients in my practice but I don’t see many people on anticoagulants, it doesn’t make sense for me to pick an anticoagulant measure.”
And if all a practice can do this year is one patient, one measure, Dr. Barbe urged physicians to look toward the next reporting year with an eye to do more, as that will ultimately lead to better quality of care delivered.
“Report on one patient and one measure this year ... but look at next year to say ‘that’s going to be a 90-day project for me,’ and get in on that. There is a pretty long laundry list of conditions and metrics that you can report on.”
And if practices start capturing relevant data, it opens the door to improving their practice if they also take the time to analyze what they are collecting.
“That is the purpose,” Dr. Barbe said. “As you measure yourself along the way, if the threshold for performance is here, and you find yourself working at this [lower] level for the first 30 days or whatever, then you stop and take stock of that” and react accordingly, whether its providing patients with a little more information about their condition or perhaps being more diligent in terms of monitoring high risk patients.
David O. Barbe, MD, is urging physicians to participate in the Medicare Quality Payment Program, even if the business case isn’t quite there.
QPP is the value-based payment system created by the Medicare Access and CHIP Reauthorization Act (MACRA). It promotes high-value care through Medicare payment increases. But for some practices, the investment in personnel and technology needed to earn those increases may be more than the increases themselves, leading doctors to do just enough to avoid being penalized.
“I think that many physicians don’t feel they are ever going to get a bonus but sure would like to avoid a penalty,” Dr. Barbe, president of the American Medical Association, said in an exclusive interview. “I am afraid many will simply perform at the lowest level that keeps them out of the penalty. Because many of them find that making the investment it takes to perform highly, there is not a business case for that.”
Full participation in QPP’s Merit-based Incentive Payment System (MIPS) could run small practices an additional $10,000 to $30,000 a year, he said. “If you’ve got $200,000 in Medicare receipts, if you get adjusted even the maximum of 4%, that is $8,000. You can’t cover $20,000 with $8,000. The math doesn’t work. There is not a business case there for it.”
That said, Dr. Barbe still spoke in favor of QPP and noted that the AMA is working with the Centers for Medicare & Medicaid Services as well as Congress to make the program more valuable and meaningful for physicians.
“We understand where we need to go as a profession, as an industry,” he said. “How we get there is the key, it’s the challenge and it requires flexibility. ... CMS has been accommodating but there are limits to how long they can go.”
The AMA is urging doctors who have missed the 90-day window for full participation – which effectively closed for most on Oct. 2 – to consider the Pick Your Pace option offered by the CMS.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Pick Your Pace allows physicians and practices to submit data on one measure for one patient to avoid a reduction in Medicare pay, even though they would not be eligible for a bonus.
“AMA has put out a lot of tools to help physicians assess their readiness, assess the gap between what they are able to do in their practice now and what they need to do to be successful under [the MIPS] primarily down to and including a video that would walk a physician step-by-step through the one patient, one measure, no penalty,” Dr. Barbe said.
He also encouraged doctors to pick a measure that is meaningful to their practice if only to get the ball rolling and get their feet wet in the QPP pool.
“What I tell physicians is pick something that is relevant for your practice,” he said.”If I see a lot of diabetes patients in my practice but I don’t see many people on anticoagulants, it doesn’t make sense for me to pick an anticoagulant measure.”
And if all a practice can do this year is one patient, one measure, Dr. Barbe urged physicians to look toward the next reporting year with an eye to do more, as that will ultimately lead to better quality of care delivered.
“Report on one patient and one measure this year ... but look at next year to say ‘that’s going to be a 90-day project for me,’ and get in on that. There is a pretty long laundry list of conditions and metrics that you can report on.”
And if practices start capturing relevant data, it opens the door to improving their practice if they also take the time to analyze what they are collecting.
“That is the purpose,” Dr. Barbe said. “As you measure yourself along the way, if the threshold for performance is here, and you find yourself working at this [lower] level for the first 30 days or whatever, then you stop and take stock of that” and react accordingly, whether its providing patients with a little more information about their condition or perhaps being more diligent in terms of monitoring high risk patients.
VIDEO: How a public health approach can cut opioid abuse, suicide risks
Reaching people at risk of opioid abuse and suicide will require a shift beyond a focus on treating individuals to a more comprehensive public health approach.
“The majority of people aren’t showing up at our treatment doors, so we have to have a very different approach if we’re going to reach everyone,” explained Arthur C. Evans Jr. PhD, chief executive officer of the American Psychological Association.
In an interview at an event sponsored by the Education Development Center and the National Action Alliance for Suicide Prevention, Dr. Evans discussed effective strategies for taking a population-health treatment perspective.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Reaching people at risk of opioid abuse and suicide will require a shift beyond a focus on treating individuals to a more comprehensive public health approach.
“The majority of people aren’t showing up at our treatment doors, so we have to have a very different approach if we’re going to reach everyone,” explained Arthur C. Evans Jr. PhD, chief executive officer of the American Psychological Association.
In an interview at an event sponsored by the Education Development Center and the National Action Alliance for Suicide Prevention, Dr. Evans discussed effective strategies for taking a population-health treatment perspective.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Reaching people at risk of opioid abuse and suicide will require a shift beyond a focus on treating individuals to a more comprehensive public health approach.
“The majority of people aren’t showing up at our treatment doors, so we have to have a very different approach if we’re going to reach everyone,” explained Arthur C. Evans Jr. PhD, chief executive officer of the American Psychological Association.
In an interview at an event sponsored by the Education Development Center and the National Action Alliance for Suicide Prevention, Dr. Evans discussed effective strategies for taking a population-health treatment perspective.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
VIDEO: When to consider systemic exposure in patients with contact dermatitis
SAN FRANCISCO – When patients with contact dermatitis who have had a patch test positive to an allergen and are not improving despite avoiding cutaneous exposure, it’s important to consider the possibility of systemic exposure, according to Nina Botto, MD, of the department of dermatology, at the University of California, San Francisco.
“Theoretically, any allergen can cause a systemic contact dermatitis. The ones that we think about and encounter more frequently are earth metals like nickel and balsam of Peru, which is a component of many fragrances and flavorings,” she said in a video interview at the annual meeting of the Pacific Dermatologic Association.
In the interview, Dr. Botto, who is codirector of the Occupational and Contact Dermatitis Clinic at UCSF, provides recommendations on how to approach patients with systemic contact dermatitis, including dietary avoidance. But following these diets can be challenging. She recommends starting with avoiding cutaneous exposure to the suspected allergen. For patients not improving after two months of avoidance, “it may be reasonable to consider a diet,”she advised.
Dr. Botto cited the following two publications with tables and guidelines for diets as helpful resources for patients: Dermatitis. 2013 Jul-Aug;24(4):153-60 (for a diet low in balsam of Peru); and Dermatitis. 2013 Jul-Aug; 24(4):190-5 (for a diet low in nickel).
Another useful resource is the American Contact Dermatitis Society website, which produces a customized list of safe products for patients after they enter the allergen into the system.
Dr. Botto had no disclosures.
SAN FRANCISCO – When patients with contact dermatitis who have had a patch test positive to an allergen and are not improving despite avoiding cutaneous exposure, it’s important to consider the possibility of systemic exposure, according to Nina Botto, MD, of the department of dermatology, at the University of California, San Francisco.
“Theoretically, any allergen can cause a systemic contact dermatitis. The ones that we think about and encounter more frequently are earth metals like nickel and balsam of Peru, which is a component of many fragrances and flavorings,” she said in a video interview at the annual meeting of the Pacific Dermatologic Association.
In the interview, Dr. Botto, who is codirector of the Occupational and Contact Dermatitis Clinic at UCSF, provides recommendations on how to approach patients with systemic contact dermatitis, including dietary avoidance. But following these diets can be challenging. She recommends starting with avoiding cutaneous exposure to the suspected allergen. For patients not improving after two months of avoidance, “it may be reasonable to consider a diet,”she advised.
Dr. Botto cited the following two publications with tables and guidelines for diets as helpful resources for patients: Dermatitis. 2013 Jul-Aug;24(4):153-60 (for a diet low in balsam of Peru); and Dermatitis. 2013 Jul-Aug; 24(4):190-5 (for a diet low in nickel).
Another useful resource is the American Contact Dermatitis Society website, which produces a customized list of safe products for patients after they enter the allergen into the system.
Dr. Botto had no disclosures.
SAN FRANCISCO – When patients with contact dermatitis who have had a patch test positive to an allergen and are not improving despite avoiding cutaneous exposure, it’s important to consider the possibility of systemic exposure, according to Nina Botto, MD, of the department of dermatology, at the University of California, San Francisco.
“Theoretically, any allergen can cause a systemic contact dermatitis. The ones that we think about and encounter more frequently are earth metals like nickel and balsam of Peru, which is a component of many fragrances and flavorings,” she said in a video interview at the annual meeting of the Pacific Dermatologic Association.
In the interview, Dr. Botto, who is codirector of the Occupational and Contact Dermatitis Clinic at UCSF, provides recommendations on how to approach patients with systemic contact dermatitis, including dietary avoidance. But following these diets can be challenging. She recommends starting with avoiding cutaneous exposure to the suspected allergen. For patients not improving after two months of avoidance, “it may be reasonable to consider a diet,”she advised.
Dr. Botto cited the following two publications with tables and guidelines for diets as helpful resources for patients: Dermatitis. 2013 Jul-Aug;24(4):153-60 (for a diet low in balsam of Peru); and Dermatitis. 2013 Jul-Aug; 24(4):190-5 (for a diet low in nickel).
Another useful resource is the American Contact Dermatitis Society website, which produces a customized list of safe products for patients after they enter the allergen into the system.
Dr. Botto had no disclosures.
AT THE ANNUAL MEETING OF THE PACIFIC DERMATOLOGIC ASSOCIATION
A multidisciplinary approach to diaphragmatic endometriosis
Endometriosis affects approximately 11% of women; the disease can be categorized as pelvic endometriosis and extrapelvic endometriosis, based on anatomic presentation. It is estimated that about 12% of extrapelvic disease involves the diaphragm or thoracic cavity.
While diaphragmatic endometriosis often is asymptomatic, patients who are symptomatic can experience progressive and incapacitating pain. because of a traditional focus on the lower pelvic region. Some cases are misdiagnosed as other conditions involving the gastrointestinal tract or of cardiothoracic origin, because of the propensity of diaphragmatic disease to occur posteriorly and hide behind the liver. The variable appearance of endometriotic lesions and the lack of reliable diagnostic or imaging tests also can contribute to delayed diagnosis.
Symptoms usually occur cyclically with the onset of menses, but sometimes are unrelated to menses. Most diaphragmatic lesions occur on the abdominal side and right hemidiaphragm, which may offer evidence for the theory that retrograde menstruation drives the development of endometriosis because of the clockwise flow of peritoneal fluid. However, lesions have been found on all parts of the diaphragm, including the left side only, the thoracic and visceral sides of the diaphragm, and the phrenic nerve. There is no correlation between the size/number of lesions and either pneumothorax or hemothorax, nor pain.
The best diagnostic method is thorough surveillance intraoperatively. In our practice, we routinely inspect the diaphragm for endometriosis at the time of video laparoscopy.
In women who have symptoms, it is important to ensure the best exposure of the diaphragm by properly considering the patient’s positioning and port placement, and by using an atraumatic liver retractor or grasping forceps to gently push the liver down and away from the visual/operative field. Posterior diaphragm viewing can also be enhanced by utilizing a 30-degree laparoscope angled toward the back. At times, it is helpful to cut the falciform ligament near the liver to expose the right side of the diaphragm completely while the patient is in steep reverse Trendelenburg position.
Most lesions in symptomatic patients can be successfully removed with hydrodissection and vaporization or excision. For asymptomatic patients with an incidental finding of diaphragmatic endometriosis, the suggestion is not to treat lesions in order to avoid the potential risk of injury to the diaphragm, phrenic nerve, lungs, or heart – especially when an adequate multidisciplinary team is not available.
Pathophysiology
In addition to retrograde menstruation, there are two other common theories regarding the pathophysiology of thoracic endometriosis. First, high prostaglandin F2-alpha at ovulation may result in vasospasm and ischemia of the lungs (resulting, in turn, in alveolar rupture and subsequent pneumothorax). Second, the loss of a mucus plug during menses may result in communication between the environment and peritoneal cavity.
What is clear is that patients who have symptoms consistent with pelvic endometriosis and chest complaints should be evaluated for both diaphragmatic and pelvic endometriosis. It’s also increasing clear that a multidisciplinary approach utilizing combined laparoscopy and thoracoscopy is a safe and effective method for addressing pelvic, diaphragmatic, and other thoracic endometriosis when other treatments have failed.
A multidisciplinary approach
Since the introduction of video laparoscopy and ease of evaluation of the upper abdomen, more extrapelvic endometriosis – including disease in the upper abdomen and diaphragm – is being diagnosed. The thoracic and visceral diaphragm are the most commonly described sites of thoracic endometriosis, and disease is often right sided, with parenchymal involvement less commonly reported.
Abdominopelvic and visceral diaphragmatic endometriosis are treated endoscopically with hydrodissection followed by excision or ablation. Superficial lesions away from the central diaphragm can be coagulated using bipolar current.
Thoracoscopic treatment varies, involving ablation or excision of smaller diaphragmatic lesions, pulmonary wedge resection of deep parenchymal nodules (using a stapling device), diaphragm resection of deep diaphragmatic lesions using a stapling device, or by excision and manual suturing.
Endoscopic diagnosis and treatment begins by introducing a 10-mm port at the umbilicus and placing three additional ports in the upper quadrant (right or left, depending on implant location). The arrangement (similar to that of a laparoscopic cholecystectomy or splenectomy) allows for examination of the posterior portion of the right hemidiaphragm and almost the entire left hemidiaphragm in addition to routine abdominopelvic exploration.
For better laparoscopic visualization, the patient is repositioned in steep reverse Trendelenburg, and the liver is gently pushed caudally to view the adjacent diaphragm. The upper abdominal walls and the liver also may be evaluated while in this position.
Bluish pigmented lesions are the most commonly reported form of diaphragmatic endometriosis, followed by lesions with a reddish-purple appearance. However, lesions can present with various colors and morphologic appearances, such as fibrotic white lesions or adhesions to the liver.
In our practice, we recommend using the CO2 laser (set at 20-25 watts) with hydrodissection for superficial lesions. The CO2 laser is much more precise and has a smaller depth of penetration and less thermal spread, compared with electrocautery. The CO2 laser beam also reaches otherwise hard-to-access areas behind the liver and has proven to be safe for vaporizing and/or excising many types of diaphragmatic lesions. We have successfully treated diaphragmatic endometriosis in the vicinity of the phrenic nerve and directly in line with the left ventricle.
Watch a video from Dr. Ceana Nezhat demonstrating a step wise vaporization and excision of diaphragmatic endometriosis utilizing different techniques.
(Courtesy Dr. Ceana Nezhat)
Plasma jet energy and ultrasonic energy are good alternatives when a CO2 laser is not available and are preferable to the use of cold scissors because of subsequent bleeding, which requires bipolar hemostasis.
Monopolar electrocautery is not as good a choice for treating diaphragmatic endometriosis because of higher depth of penetration, which may cause tissue necrosis and subsequent delayed diaphragmatic fenestrations. It also may cause unpredictable diaphragmatic muscular contractions and electrical conduction transmitted to the heart, inducing arrhythmia.
For patients treated via combined VALS and VATS procedures, endometriotic lesions involving the entire thickness of the diaphragm should be completely resected, and the defect can be repaired with either sutures or staples.
In all cases, special anesthesia considerations must be made given the inability to completely ventilate the lung. In our practice, we use a double-lumen endotracheal tube for single lung ventilation, if needed. A bronchial blocker is used to isolate the lung when the double-lumen endotracheal tube cannot be inserted.
It is important to note that we do not recommend VATS with VALS in all suspicious cases. We reserve VATS only for patients with catamenial pneumothorax, catamenial hemothorax, hemoptysis, and pulmonary nodules, defined as Thoracic Endometriosis Syndrome. We usually start with medical management first, then proceed to VALS, and finally, VATS, with the intention to treat if the patient fails nonsurgical treatments. It is better to avoid VATS, if possible, because it is associated with longer recovery and more pain; it should be done if all else fails.
If the patient has completed childbearing or passed reproductive age, bilateral salpingectomy, or hysterectomy with or without bilateral salpingo-oophorectomy, may be considered as the first step prior to more aggressive excisional procedures. This is especially true for widespread lesions, as branches of the phrenic nerve are difficult to see and injury could result in paralysis of the diaphragm. It’s important to appreciate that if estrogen stimulation to the diaphragmatic lesions is to cease for the long term, hormonal suppression or surgical treatment including bilateral oophorectomy should be utilized.
My colleagues and I have reported on our experience with a multidisciplinary approach in the treatment of diaphragmatic endometriosis in 25 patients. All had both pelvic and thoracic symptoms, and the majority had endometrial implants on both the thoracic and visceral sides of the diaphragm.
There were two postoperative complications: a diaphragmatic hernia and a vaginal cuff hematoma. Over a follow-up period of 3-18 months, all 25 patients had significant improvement or resolution of their chest complaints, and most remained asymptomatic for more than 6 months (JSLS. 2014 Jul-Sep;18[3]. pii: e2014.00312. doi: 10.4293/JSLS.2014.00312).
Dr. Ceana Nezhat is the fellowship director of Nezhat Medical Center, the medical director of training and education at Northside Hospital, and an adjunct clinical professor of gynecology and obstetrics at Emory University, all in Atlanta. He is president of SRS (Society of Reproductive Surgeons) and past president of AAGL (American Association of Gynecologic Laparoscopists). Dr. Nezhat is a consultant for Novuson Surgical, Karl Storz Endoscopy, Lumenis, and AbbVie; a medical advisor for Plasma Surgical, and a member of the scientific advisory board for SurgiQuest.
Suggested readings
1. Nezhat C, Nezhat F, Nezhat C. Nezhat’s Operative Gynecologic Laparoscopy with Hysteroscopy. Fourth Edition. Cambridge University Press. 2013.
2. Am J Med. 1996 Feb;100(2):164-70.
3. Fertil Steril. 1998 Jun;69(6):1048-55.
4. Clin Obstet Gynecol. 1999 Sep;42(3):699-711.
5. JSLS. 2012 Jan-Mar; 16(1):140-2.
Endometriosis affects approximately 11% of women; the disease can be categorized as pelvic endometriosis and extrapelvic endometriosis, based on anatomic presentation. It is estimated that about 12% of extrapelvic disease involves the diaphragm or thoracic cavity.
While diaphragmatic endometriosis often is asymptomatic, patients who are symptomatic can experience progressive and incapacitating pain. because of a traditional focus on the lower pelvic region. Some cases are misdiagnosed as other conditions involving the gastrointestinal tract or of cardiothoracic origin, because of the propensity of diaphragmatic disease to occur posteriorly and hide behind the liver. The variable appearance of endometriotic lesions and the lack of reliable diagnostic or imaging tests also can contribute to delayed diagnosis.
Symptoms usually occur cyclically with the onset of menses, but sometimes are unrelated to menses. Most diaphragmatic lesions occur on the abdominal side and right hemidiaphragm, which may offer evidence for the theory that retrograde menstruation drives the development of endometriosis because of the clockwise flow of peritoneal fluid. However, lesions have been found on all parts of the diaphragm, including the left side only, the thoracic and visceral sides of the diaphragm, and the phrenic nerve. There is no correlation between the size/number of lesions and either pneumothorax or hemothorax, nor pain.
The best diagnostic method is thorough surveillance intraoperatively. In our practice, we routinely inspect the diaphragm for endometriosis at the time of video laparoscopy.
In women who have symptoms, it is important to ensure the best exposure of the diaphragm by properly considering the patient’s positioning and port placement, and by using an atraumatic liver retractor or grasping forceps to gently push the liver down and away from the visual/operative field. Posterior diaphragm viewing can also be enhanced by utilizing a 30-degree laparoscope angled toward the back. At times, it is helpful to cut the falciform ligament near the liver to expose the right side of the diaphragm completely while the patient is in steep reverse Trendelenburg position.
Most lesions in symptomatic patients can be successfully removed with hydrodissection and vaporization or excision. For asymptomatic patients with an incidental finding of diaphragmatic endometriosis, the suggestion is not to treat lesions in order to avoid the potential risk of injury to the diaphragm, phrenic nerve, lungs, or heart – especially when an adequate multidisciplinary team is not available.
Pathophysiology
In addition to retrograde menstruation, there are two other common theories regarding the pathophysiology of thoracic endometriosis. First, high prostaglandin F2-alpha at ovulation may result in vasospasm and ischemia of the lungs (resulting, in turn, in alveolar rupture and subsequent pneumothorax). Second, the loss of a mucus plug during menses may result in communication between the environment and peritoneal cavity.
What is clear is that patients who have symptoms consistent with pelvic endometriosis and chest complaints should be evaluated for both diaphragmatic and pelvic endometriosis. It’s also increasing clear that a multidisciplinary approach utilizing combined laparoscopy and thoracoscopy is a safe and effective method for addressing pelvic, diaphragmatic, and other thoracic endometriosis when other treatments have failed.
A multidisciplinary approach
Since the introduction of video laparoscopy and ease of evaluation of the upper abdomen, more extrapelvic endometriosis – including disease in the upper abdomen and diaphragm – is being diagnosed. The thoracic and visceral diaphragm are the most commonly described sites of thoracic endometriosis, and disease is often right sided, with parenchymal involvement less commonly reported.
Abdominopelvic and visceral diaphragmatic endometriosis are treated endoscopically with hydrodissection followed by excision or ablation. Superficial lesions away from the central diaphragm can be coagulated using bipolar current.
Thoracoscopic treatment varies, involving ablation or excision of smaller diaphragmatic lesions, pulmonary wedge resection of deep parenchymal nodules (using a stapling device), diaphragm resection of deep diaphragmatic lesions using a stapling device, or by excision and manual suturing.
Endoscopic diagnosis and treatment begins by introducing a 10-mm port at the umbilicus and placing three additional ports in the upper quadrant (right or left, depending on implant location). The arrangement (similar to that of a laparoscopic cholecystectomy or splenectomy) allows for examination of the posterior portion of the right hemidiaphragm and almost the entire left hemidiaphragm in addition to routine abdominopelvic exploration.
For better laparoscopic visualization, the patient is repositioned in steep reverse Trendelenburg, and the liver is gently pushed caudally to view the adjacent diaphragm. The upper abdominal walls and the liver also may be evaluated while in this position.
Bluish pigmented lesions are the most commonly reported form of diaphragmatic endometriosis, followed by lesions with a reddish-purple appearance. However, lesions can present with various colors and morphologic appearances, such as fibrotic white lesions or adhesions to the liver.
In our practice, we recommend using the CO2 laser (set at 20-25 watts) with hydrodissection for superficial lesions. The CO2 laser is much more precise and has a smaller depth of penetration and less thermal spread, compared with electrocautery. The CO2 laser beam also reaches otherwise hard-to-access areas behind the liver and has proven to be safe for vaporizing and/or excising many types of diaphragmatic lesions. We have successfully treated diaphragmatic endometriosis in the vicinity of the phrenic nerve and directly in line with the left ventricle.
Watch a video from Dr. Ceana Nezhat demonstrating a step wise vaporization and excision of diaphragmatic endometriosis utilizing different techniques.
(Courtesy Dr. Ceana Nezhat)
Plasma jet energy and ultrasonic energy are good alternatives when a CO2 laser is not available and are preferable to the use of cold scissors because of subsequent bleeding, which requires bipolar hemostasis.
Monopolar electrocautery is not as good a choice for treating diaphragmatic endometriosis because of higher depth of penetration, which may cause tissue necrosis and subsequent delayed diaphragmatic fenestrations. It also may cause unpredictable diaphragmatic muscular contractions and electrical conduction transmitted to the heart, inducing arrhythmia.
For patients treated via combined VALS and VATS procedures, endometriotic lesions involving the entire thickness of the diaphragm should be completely resected, and the defect can be repaired with either sutures or staples.
In all cases, special anesthesia considerations must be made given the inability to completely ventilate the lung. In our practice, we use a double-lumen endotracheal tube for single lung ventilation, if needed. A bronchial blocker is used to isolate the lung when the double-lumen endotracheal tube cannot be inserted.
It is important to note that we do not recommend VATS with VALS in all suspicious cases. We reserve VATS only for patients with catamenial pneumothorax, catamenial hemothorax, hemoptysis, and pulmonary nodules, defined as Thoracic Endometriosis Syndrome. We usually start with medical management first, then proceed to VALS, and finally, VATS, with the intention to treat if the patient fails nonsurgical treatments. It is better to avoid VATS, if possible, because it is associated with longer recovery and more pain; it should be done if all else fails.
If the patient has completed childbearing or passed reproductive age, bilateral salpingectomy, or hysterectomy with or without bilateral salpingo-oophorectomy, may be considered as the first step prior to more aggressive excisional procedures. This is especially true for widespread lesions, as branches of the phrenic nerve are difficult to see and injury could result in paralysis of the diaphragm. It’s important to appreciate that if estrogen stimulation to the diaphragmatic lesions is to cease for the long term, hormonal suppression or surgical treatment including bilateral oophorectomy should be utilized.
My colleagues and I have reported on our experience with a multidisciplinary approach in the treatment of diaphragmatic endometriosis in 25 patients. All had both pelvic and thoracic symptoms, and the majority had endometrial implants on both the thoracic and visceral sides of the diaphragm.
There were two postoperative complications: a diaphragmatic hernia and a vaginal cuff hematoma. Over a follow-up period of 3-18 months, all 25 patients had significant improvement or resolution of their chest complaints, and most remained asymptomatic for more than 6 months (JSLS. 2014 Jul-Sep;18[3]. pii: e2014.00312. doi: 10.4293/JSLS.2014.00312).
Dr. Ceana Nezhat is the fellowship director of Nezhat Medical Center, the medical director of training and education at Northside Hospital, and an adjunct clinical professor of gynecology and obstetrics at Emory University, all in Atlanta. He is president of SRS (Society of Reproductive Surgeons) and past president of AAGL (American Association of Gynecologic Laparoscopists). Dr. Nezhat is a consultant for Novuson Surgical, Karl Storz Endoscopy, Lumenis, and AbbVie; a medical advisor for Plasma Surgical, and a member of the scientific advisory board for SurgiQuest.
Suggested readings
1. Nezhat C, Nezhat F, Nezhat C. Nezhat’s Operative Gynecologic Laparoscopy with Hysteroscopy. Fourth Edition. Cambridge University Press. 2013.
2. Am J Med. 1996 Feb;100(2):164-70.
3. Fertil Steril. 1998 Jun;69(6):1048-55.
4. Clin Obstet Gynecol. 1999 Sep;42(3):699-711.
5. JSLS. 2012 Jan-Mar; 16(1):140-2.
Endometriosis affects approximately 11% of women; the disease can be categorized as pelvic endometriosis and extrapelvic endometriosis, based on anatomic presentation. It is estimated that about 12% of extrapelvic disease involves the diaphragm or thoracic cavity.
While diaphragmatic endometriosis often is asymptomatic, patients who are symptomatic can experience progressive and incapacitating pain. because of a traditional focus on the lower pelvic region. Some cases are misdiagnosed as other conditions involving the gastrointestinal tract or of cardiothoracic origin, because of the propensity of diaphragmatic disease to occur posteriorly and hide behind the liver. The variable appearance of endometriotic lesions and the lack of reliable diagnostic or imaging tests also can contribute to delayed diagnosis.
Symptoms usually occur cyclically with the onset of menses, but sometimes are unrelated to menses. Most diaphragmatic lesions occur on the abdominal side and right hemidiaphragm, which may offer evidence for the theory that retrograde menstruation drives the development of endometriosis because of the clockwise flow of peritoneal fluid. However, lesions have been found on all parts of the diaphragm, including the left side only, the thoracic and visceral sides of the diaphragm, and the phrenic nerve. There is no correlation between the size/number of lesions and either pneumothorax or hemothorax, nor pain.
The best diagnostic method is thorough surveillance intraoperatively. In our practice, we routinely inspect the diaphragm for endometriosis at the time of video laparoscopy.
In women who have symptoms, it is important to ensure the best exposure of the diaphragm by properly considering the patient’s positioning and port placement, and by using an atraumatic liver retractor or grasping forceps to gently push the liver down and away from the visual/operative field. Posterior diaphragm viewing can also be enhanced by utilizing a 30-degree laparoscope angled toward the back. At times, it is helpful to cut the falciform ligament near the liver to expose the right side of the diaphragm completely while the patient is in steep reverse Trendelenburg position.
Most lesions in symptomatic patients can be successfully removed with hydrodissection and vaporization or excision. For asymptomatic patients with an incidental finding of diaphragmatic endometriosis, the suggestion is not to treat lesions in order to avoid the potential risk of injury to the diaphragm, phrenic nerve, lungs, or heart – especially when an adequate multidisciplinary team is not available.
Pathophysiology
In addition to retrograde menstruation, there are two other common theories regarding the pathophysiology of thoracic endometriosis. First, high prostaglandin F2-alpha at ovulation may result in vasospasm and ischemia of the lungs (resulting, in turn, in alveolar rupture and subsequent pneumothorax). Second, the loss of a mucus plug during menses may result in communication between the environment and peritoneal cavity.
What is clear is that patients who have symptoms consistent with pelvic endometriosis and chest complaints should be evaluated for both diaphragmatic and pelvic endometriosis. It’s also increasing clear that a multidisciplinary approach utilizing combined laparoscopy and thoracoscopy is a safe and effective method for addressing pelvic, diaphragmatic, and other thoracic endometriosis when other treatments have failed.
A multidisciplinary approach
Since the introduction of video laparoscopy and ease of evaluation of the upper abdomen, more extrapelvic endometriosis – including disease in the upper abdomen and diaphragm – is being diagnosed. The thoracic and visceral diaphragm are the most commonly described sites of thoracic endometriosis, and disease is often right sided, with parenchymal involvement less commonly reported.
Abdominopelvic and visceral diaphragmatic endometriosis are treated endoscopically with hydrodissection followed by excision or ablation. Superficial lesions away from the central diaphragm can be coagulated using bipolar current.
Thoracoscopic treatment varies, involving ablation or excision of smaller diaphragmatic lesions, pulmonary wedge resection of deep parenchymal nodules (using a stapling device), diaphragm resection of deep diaphragmatic lesions using a stapling device, or by excision and manual suturing.
Endoscopic diagnosis and treatment begins by introducing a 10-mm port at the umbilicus and placing three additional ports in the upper quadrant (right or left, depending on implant location). The arrangement (similar to that of a laparoscopic cholecystectomy or splenectomy) allows for examination of the posterior portion of the right hemidiaphragm and almost the entire left hemidiaphragm in addition to routine abdominopelvic exploration.
For better laparoscopic visualization, the patient is repositioned in steep reverse Trendelenburg, and the liver is gently pushed caudally to view the adjacent diaphragm. The upper abdominal walls and the liver also may be evaluated while in this position.
Bluish pigmented lesions are the most commonly reported form of diaphragmatic endometriosis, followed by lesions with a reddish-purple appearance. However, lesions can present with various colors and morphologic appearances, such as fibrotic white lesions or adhesions to the liver.
In our practice, we recommend using the CO2 laser (set at 20-25 watts) with hydrodissection for superficial lesions. The CO2 laser is much more precise and has a smaller depth of penetration and less thermal spread, compared with electrocautery. The CO2 laser beam also reaches otherwise hard-to-access areas behind the liver and has proven to be safe for vaporizing and/or excising many types of diaphragmatic lesions. We have successfully treated diaphragmatic endometriosis in the vicinity of the phrenic nerve and directly in line with the left ventricle.
Watch a video from Dr. Ceana Nezhat demonstrating a step wise vaporization and excision of diaphragmatic endometriosis utilizing different techniques.
(Courtesy Dr. Ceana Nezhat)
Plasma jet energy and ultrasonic energy are good alternatives when a CO2 laser is not available and are preferable to the use of cold scissors because of subsequent bleeding, which requires bipolar hemostasis.
Monopolar electrocautery is not as good a choice for treating diaphragmatic endometriosis because of higher depth of penetration, which may cause tissue necrosis and subsequent delayed diaphragmatic fenestrations. It also may cause unpredictable diaphragmatic muscular contractions and electrical conduction transmitted to the heart, inducing arrhythmia.
For patients treated via combined VALS and VATS procedures, endometriotic lesions involving the entire thickness of the diaphragm should be completely resected, and the defect can be repaired with either sutures or staples.
In all cases, special anesthesia considerations must be made given the inability to completely ventilate the lung. In our practice, we use a double-lumen endotracheal tube for single lung ventilation, if needed. A bronchial blocker is used to isolate the lung when the double-lumen endotracheal tube cannot be inserted.
It is important to note that we do not recommend VATS with VALS in all suspicious cases. We reserve VATS only for patients with catamenial pneumothorax, catamenial hemothorax, hemoptysis, and pulmonary nodules, defined as Thoracic Endometriosis Syndrome. We usually start with medical management first, then proceed to VALS, and finally, VATS, with the intention to treat if the patient fails nonsurgical treatments. It is better to avoid VATS, if possible, because it is associated with longer recovery and more pain; it should be done if all else fails.
If the patient has completed childbearing or passed reproductive age, bilateral salpingectomy, or hysterectomy with or without bilateral salpingo-oophorectomy, may be considered as the first step prior to more aggressive excisional procedures. This is especially true for widespread lesions, as branches of the phrenic nerve are difficult to see and injury could result in paralysis of the diaphragm. It’s important to appreciate that if estrogen stimulation to the diaphragmatic lesions is to cease for the long term, hormonal suppression or surgical treatment including bilateral oophorectomy should be utilized.
My colleagues and I have reported on our experience with a multidisciplinary approach in the treatment of diaphragmatic endometriosis in 25 patients. All had both pelvic and thoracic symptoms, and the majority had endometrial implants on both the thoracic and visceral sides of the diaphragm.
There were two postoperative complications: a diaphragmatic hernia and a vaginal cuff hematoma. Over a follow-up period of 3-18 months, all 25 patients had significant improvement or resolution of their chest complaints, and most remained asymptomatic for more than 6 months (JSLS. 2014 Jul-Sep;18[3]. pii: e2014.00312. doi: 10.4293/JSLS.2014.00312).
Dr. Ceana Nezhat is the fellowship director of Nezhat Medical Center, the medical director of training and education at Northside Hospital, and an adjunct clinical professor of gynecology and obstetrics at Emory University, all in Atlanta. He is president of SRS (Society of Reproductive Surgeons) and past president of AAGL (American Association of Gynecologic Laparoscopists). Dr. Nezhat is a consultant for Novuson Surgical, Karl Storz Endoscopy, Lumenis, and AbbVie; a medical advisor for Plasma Surgical, and a member of the scientific advisory board for SurgiQuest.
Suggested readings
1. Nezhat C, Nezhat F, Nezhat C. Nezhat’s Operative Gynecologic Laparoscopy with Hysteroscopy. Fourth Edition. Cambridge University Press. 2013.
2. Am J Med. 1996 Feb;100(2):164-70.
3. Fertil Steril. 1998 Jun;69(6):1048-55.
4. Clin Obstet Gynecol. 1999 Sep;42(3):699-711.
5. JSLS. 2012 Jan-Mar; 16(1):140-2.
Alcohol showed no cardiovascular benefits in nonalcoholic fatty liver disease
Alcohol consumption produced no apparent cardiovascular benefits among individuals with nonalcoholic fatty liver disease, according to a study of 570 white and black adults from the Coronary Artery Risk Development in Young Adults (CARDIA) longitudinal cohort.
After researchers controlled for multiple demographic and clinical confounders, alcohol use was not associated with cardiovascular risk factors such as diabetes, hypertension, or hyperlipidemia, nor with homeostatic model assessment of insulin resistance, C-reactive protein level, total cholesterol, systolic or diastolic blood pressure, coronary artery calcification, E/A ratio, or global longitudinal strain among individuals with nonalcoholic fatty liver disease (NAFLD), reported Lisa B. VanWagner, MD, of Northwestern University, Chicago, and her associates. “[A] recommendation of cardiovascular disease risk benefit of alcohol use in persons with NAFLD cannot be made based on the current findings,” they wrote. They advocated for prospective, long-term studies to better understand how various types and doses of alcohol affect hard cardiovascular endpoints in patients with NAFLD. Their study was published in Gastroenterology.
CARDIA enrolled 5,115 black and white adults aged 18-30 years from four cities in the United States, and followed them long term. Participants were asked about alcohol consumption at study entry and again at 15, 20, and 25 years of follow-up. At year 25, participants underwent computed tomography (CT) examinations of the thorax and abdomen and tissue Doppler echocardiography with myocardial strain measured by speckle tracking (Gastroenterology. 2017 Aug 9. doi: 10.1053/j.gastro.2017.08.012).
The 570 participants with NAFLD averaged 50 years of age, 54% were black, 46% were female, and 58% consumed at least one alcoholic drink per week, said the researchers. Compared with nondrinkers, drinkers had attained significantly higher education levels, were significantly more likely to be white and male, and had a significantly lower average body mass index (34.4 kg/m2 vs. 37.3 kg/m2) and C-reactive protein level (4.2 vs. 6.1 mg per L), and a significantly lower prevalence of diabetes (23% vs. 37%), impaired glucose tolerance (42% vs. 49%), obesity (75% vs. 83%) and metabolic syndrome (55% vs. 66%) (P less than .05 for all comparisons). Drinkers and nondrinkers resembled each other in terms of lipid profiles, use of lipid-lowering medications, liver attenuation scores, and systolic and diastolic blood pressures, although significantly more nondrinkers used antihypertensive medications (46% vs.35%; P = .005).
Drinkers had a higher prevalence of coronary artery calcification, defined as Agatston score above 0 (42% vs. 34%), and the difference approached statistical significance (P = .05). However, after they adjusted for multiple potential confounders, the researchers found no link between alcohol consumption and risk factors for cardiovascular disease or between alcohol consumption and measures of subclinical cardiovascular disease. This finding persisted in sensitivity analyses that examined alcohol dose, binge drinking, history of cardiovascular events, and former heavy alcohol use.
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
Taken together, the findings “challenge the belief that alcohol use may reduce cardiovascular disease risk in persons with nonalcoholic fatty liver disease,” the investigators concluded. Clinical heart failure was too rare to reliably assess, but “we failed to observe an association between alcohol use and multiple markers of subclinical changes in cardiac structure and function that may be precursors of incident heart failure in NAFLD,” they wrote. More longitudinal studies would be needed to clarify how moderate alcohol use in NAFLD affects coronary artery calcification or changes in myocardial structure and function, they cautioned.
The National Institutes of Health supported the work. The investigators reported having no relevant conflicts of interest.
Alcohol consumption produced no apparent cardiovascular benefits among individuals with nonalcoholic fatty liver disease, according to a study of 570 white and black adults from the Coronary Artery Risk Development in Young Adults (CARDIA) longitudinal cohort.
After researchers controlled for multiple demographic and clinical confounders, alcohol use was not associated with cardiovascular risk factors such as diabetes, hypertension, or hyperlipidemia, nor with homeostatic model assessment of insulin resistance, C-reactive protein level, total cholesterol, systolic or diastolic blood pressure, coronary artery calcification, E/A ratio, or global longitudinal strain among individuals with nonalcoholic fatty liver disease (NAFLD), reported Lisa B. VanWagner, MD, of Northwestern University, Chicago, and her associates. “[A] recommendation of cardiovascular disease risk benefit of alcohol use in persons with NAFLD cannot be made based on the current findings,” they wrote. They advocated for prospective, long-term studies to better understand how various types and doses of alcohol affect hard cardiovascular endpoints in patients with NAFLD. Their study was published in Gastroenterology.
CARDIA enrolled 5,115 black and white adults aged 18-30 years from four cities in the United States, and followed them long term. Participants were asked about alcohol consumption at study entry and again at 15, 20, and 25 years of follow-up. At year 25, participants underwent computed tomography (CT) examinations of the thorax and abdomen and tissue Doppler echocardiography with myocardial strain measured by speckle tracking (Gastroenterology. 2017 Aug 9. doi: 10.1053/j.gastro.2017.08.012).
The 570 participants with NAFLD averaged 50 years of age, 54% were black, 46% were female, and 58% consumed at least one alcoholic drink per week, said the researchers. Compared with nondrinkers, drinkers had attained significantly higher education levels, were significantly more likely to be white and male, and had a significantly lower average body mass index (34.4 kg/m2 vs. 37.3 kg/m2) and C-reactive protein level (4.2 vs. 6.1 mg per L), and a significantly lower prevalence of diabetes (23% vs. 37%), impaired glucose tolerance (42% vs. 49%), obesity (75% vs. 83%) and metabolic syndrome (55% vs. 66%) (P less than .05 for all comparisons). Drinkers and nondrinkers resembled each other in terms of lipid profiles, use of lipid-lowering medications, liver attenuation scores, and systolic and diastolic blood pressures, although significantly more nondrinkers used antihypertensive medications (46% vs.35%; P = .005).
Drinkers had a higher prevalence of coronary artery calcification, defined as Agatston score above 0 (42% vs. 34%), and the difference approached statistical significance (P = .05). However, after they adjusted for multiple potential confounders, the researchers found no link between alcohol consumption and risk factors for cardiovascular disease or between alcohol consumption and measures of subclinical cardiovascular disease. This finding persisted in sensitivity analyses that examined alcohol dose, binge drinking, history of cardiovascular events, and former heavy alcohol use.
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
Taken together, the findings “challenge the belief that alcohol use may reduce cardiovascular disease risk in persons with nonalcoholic fatty liver disease,” the investigators concluded. Clinical heart failure was too rare to reliably assess, but “we failed to observe an association between alcohol use and multiple markers of subclinical changes in cardiac structure and function that may be precursors of incident heart failure in NAFLD,” they wrote. More longitudinal studies would be needed to clarify how moderate alcohol use in NAFLD affects coronary artery calcification or changes in myocardial structure and function, they cautioned.
The National Institutes of Health supported the work. The investigators reported having no relevant conflicts of interest.
Alcohol consumption produced no apparent cardiovascular benefits among individuals with nonalcoholic fatty liver disease, according to a study of 570 white and black adults from the Coronary Artery Risk Development in Young Adults (CARDIA) longitudinal cohort.
After researchers controlled for multiple demographic and clinical confounders, alcohol use was not associated with cardiovascular risk factors such as diabetes, hypertension, or hyperlipidemia, nor with homeostatic model assessment of insulin resistance, C-reactive protein level, total cholesterol, systolic or diastolic blood pressure, coronary artery calcification, E/A ratio, or global longitudinal strain among individuals with nonalcoholic fatty liver disease (NAFLD), reported Lisa B. VanWagner, MD, of Northwestern University, Chicago, and her associates. “[A] recommendation of cardiovascular disease risk benefit of alcohol use in persons with NAFLD cannot be made based on the current findings,” they wrote. They advocated for prospective, long-term studies to better understand how various types and doses of alcohol affect hard cardiovascular endpoints in patients with NAFLD. Their study was published in Gastroenterology.
CARDIA enrolled 5,115 black and white adults aged 18-30 years from four cities in the United States, and followed them long term. Participants were asked about alcohol consumption at study entry and again at 15, 20, and 25 years of follow-up. At year 25, participants underwent computed tomography (CT) examinations of the thorax and abdomen and tissue Doppler echocardiography with myocardial strain measured by speckle tracking (Gastroenterology. 2017 Aug 9. doi: 10.1053/j.gastro.2017.08.012).
The 570 participants with NAFLD averaged 50 years of age, 54% were black, 46% were female, and 58% consumed at least one alcoholic drink per week, said the researchers. Compared with nondrinkers, drinkers had attained significantly higher education levels, were significantly more likely to be white and male, and had a significantly lower average body mass index (34.4 kg/m2 vs. 37.3 kg/m2) and C-reactive protein level (4.2 vs. 6.1 mg per L), and a significantly lower prevalence of diabetes (23% vs. 37%), impaired glucose tolerance (42% vs. 49%), obesity (75% vs. 83%) and metabolic syndrome (55% vs. 66%) (P less than .05 for all comparisons). Drinkers and nondrinkers resembled each other in terms of lipid profiles, use of lipid-lowering medications, liver attenuation scores, and systolic and diastolic blood pressures, although significantly more nondrinkers used antihypertensive medications (46% vs.35%; P = .005).
Drinkers had a higher prevalence of coronary artery calcification, defined as Agatston score above 0 (42% vs. 34%), and the difference approached statistical significance (P = .05). However, after they adjusted for multiple potential confounders, the researchers found no link between alcohol consumption and risk factors for cardiovascular disease or between alcohol consumption and measures of subclinical cardiovascular disease. This finding persisted in sensitivity analyses that examined alcohol dose, binge drinking, history of cardiovascular events, and former heavy alcohol use.
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
Taken together, the findings “challenge the belief that alcohol use may reduce cardiovascular disease risk in persons with nonalcoholic fatty liver disease,” the investigators concluded. Clinical heart failure was too rare to reliably assess, but “we failed to observe an association between alcohol use and multiple markers of subclinical changes in cardiac structure and function that may be precursors of incident heart failure in NAFLD,” they wrote. More longitudinal studies would be needed to clarify how moderate alcohol use in NAFLD affects coronary artery calcification or changes in myocardial structure and function, they cautioned.
The National Institutes of Health supported the work. The investigators reported having no relevant conflicts of interest.
FROM GASTROENTEROLOGY
Key clinical point: No cardioprotective effects were shown with alcohol consumption in adults with nonalcoholic fatty liver disease.
Major finding: After researchers adjusted for multiple confounders, alcohol use was not associated with risk factors for cardiovascular disease or with indicators of subclinical cardiovascular disease.
Data source: A longitudinal, population-based study of 570 individuals with nonalcoholic fatty liver disease.
Disclosures: The National Institutes of Health supported the work. The investigators reported having no relevant conflicts of interest.
Cannabis use disorder
Clinicians: Be clear about flu vaccine’s value
WASHINGTON – Flu vaccination rates remain below the 70% Healthy People 2020 goal for most of the U.S. population, but data show that a recommendation from a clinician can encourage individuals to get vaccinated and to vaccinate their children, according to a panel of experts who spoke at a press briefing sponsored by the National Foundation for Infectious Diseases.
“Annual vaccination is our first line of defense against the flu,” William Schaffner, MD, of Vanderbilt University, Nashville, Tenn., said at the briefing. The unpredictable nature of the flu makes annual vaccination even more important – and the earlier, the better, said Dr. Schaffner. “If you have seen one flu season, you have seen ... one flu season.”
In a video interview at the briefing, experts emphasized the safety and effectiveness of the flu vaccine for a range of populations, including children, pregnant women, and older adults. And they offered tips to convince patients of the importance of vaccination, as well as the need to make sure health care staff are protected.
Briefing participants included former Department of Health and Human Services Secretary Thomas A. Price, MD; Patricia A. Stinchfield, RN, MS, CPNP, CIC of Children’s Hospitals and Clinics of Minnesota, St. Paul; Kathleen M. Neuzil, MD, of the University of Maryland; and Daniel B. Jernigan, MD, of the Centers for Disease Control and Prevention.
The clinicians interviewed had no financial conflicts to disclose.
WASHINGTON – Flu vaccination rates remain below the 70% Healthy People 2020 goal for most of the U.S. population, but data show that a recommendation from a clinician can encourage individuals to get vaccinated and to vaccinate their children, according to a panel of experts who spoke at a press briefing sponsored by the National Foundation for Infectious Diseases.
“Annual vaccination is our first line of defense against the flu,” William Schaffner, MD, of Vanderbilt University, Nashville, Tenn., said at the briefing. The unpredictable nature of the flu makes annual vaccination even more important – and the earlier, the better, said Dr. Schaffner. “If you have seen one flu season, you have seen ... one flu season.”
In a video interview at the briefing, experts emphasized the safety and effectiveness of the flu vaccine for a range of populations, including children, pregnant women, and older adults. And they offered tips to convince patients of the importance of vaccination, as well as the need to make sure health care staff are protected.
Briefing participants included former Department of Health and Human Services Secretary Thomas A. Price, MD; Patricia A. Stinchfield, RN, MS, CPNP, CIC of Children’s Hospitals and Clinics of Minnesota, St. Paul; Kathleen M. Neuzil, MD, of the University of Maryland; and Daniel B. Jernigan, MD, of the Centers for Disease Control and Prevention.
The clinicians interviewed had no financial conflicts to disclose.
WASHINGTON – Flu vaccination rates remain below the 70% Healthy People 2020 goal for most of the U.S. population, but data show that a recommendation from a clinician can encourage individuals to get vaccinated and to vaccinate their children, according to a panel of experts who spoke at a press briefing sponsored by the National Foundation for Infectious Diseases.
“Annual vaccination is our first line of defense against the flu,” William Schaffner, MD, of Vanderbilt University, Nashville, Tenn., said at the briefing. The unpredictable nature of the flu makes annual vaccination even more important – and the earlier, the better, said Dr. Schaffner. “If you have seen one flu season, you have seen ... one flu season.”
In a video interview at the briefing, experts emphasized the safety and effectiveness of the flu vaccine for a range of populations, including children, pregnant women, and older adults. And they offered tips to convince patients of the importance of vaccination, as well as the need to make sure health care staff are protected.
Briefing participants included former Department of Health and Human Services Secretary Thomas A. Price, MD; Patricia A. Stinchfield, RN, MS, CPNP, CIC of Children’s Hospitals and Clinics of Minnesota, St. Paul; Kathleen M. Neuzil, MD, of the University of Maryland; and Daniel B. Jernigan, MD, of the Centers for Disease Control and Prevention.
The clinicians interviewed had no financial conflicts to disclose.
AT A PRESS BRIEFING BY THE NATIONAL FOUNDATION FOR INFECTIOUS DISEASES
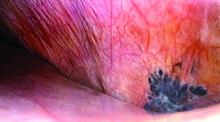
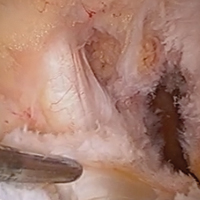
Medial Oblique Meniscomeniscal Ligament
Arthroscopic identification and evaluation of the meniscomeniscal ligament.
Arthroscopic identification and evaluation of the meniscomeniscal ligament.
Arthroscopic identification and evaluation of the meniscomeniscal ligament.