User login
Inside the 2024 AAD Acne Guidelines: New Therapies Join Old Standbys
SAN DIEGO — Just weeks after the American Academy of Dermatology (AAD) published its updated acne management guidelines, a dermatologist who helped write the recommendations provided colleagues with insight into recently approved topical therapies, the importance of multimodal therapy, and a controversial report linking benzoyl peroxide (BP) to the carcinogen benzene.
In regard to topical treatments, the guidelines make a “strong” recommendation for topical retinoids based on “moderate” evidence, Andrea L. Zaenglein, MD, professor of dermatology and pediatrics, Penn State University, Hershey, Pennsylvania, said at the annual meeting of the American Academy of Dermatology. The recommendation was based on a pooled analysis of four randomized controlled trials that found patients with acne who used the medications were more likely to have improvement via the Investigator Global Assessment (IGA) scale at 12 weeks than were those treated with a vehicle (risk ratio [RR], 1.57; 1.21-2.04).
The updated guidelines were published on January 30 in the Journal of the American Academy of Dermatology. The previous guidelines were issued in 2016.
“We have four current retinoids that we use: adapalene, tretinoin, tazarotene, and trifarotene,” Dr. Zaenglein said. “Typically, when we think about retinoids, we think of adapalene as being more tolerable and tazarotene as being more effective. But we also know that they can work to prevent and treat scarring, and they work against comedonal lesions and inflammatory lesions.”
Newer concentrations include tretinoin 0.05% lotion, tazarotene 0.045% lotion, and trifarotene 0.005% cream. She noted that this trifarotene concentration can be helpful for moderate truncal acne and also referred to evidence that whey protein appears to exacerbate that condition. “I always ask teenage kids about that: Are they using those protein powders?”
Recommendations for ‘Multimodal Therapy,’ Especially With Antibiotics
Dr. Zaenglein highlighted a “good practice statement” in the new guidelines that says, “when managing acne with topical medications, we recommend multimodal therapy combining multiple mechanisms of action.”
Topical antibiotics are effective treatments on their own and include erythromycin, clindamycin, and minocycline (Minocin), she said. But the guidelines, which refer to evidence supporting them as “moderate,” do not recommend them as monotherapy because of the risk for antibiotic resistance.
The oral retinoid isotretinoin may be appropriate in conjunction with topical medications, she said, “and we also recommend fixed combination products because they’re associated with increased adherence.”
Dermatologists are familiar with several of these products because “we’ve been using them for years and years,” she said. The guidelines note that “compared to vehicle at 12 weeks, a greater proportion of patients treated with combined BP and topical retinoid achieved IGA success in three RCTs (RR, 2.19; 1.77-2.72).”
Dr. Zaenglein noted that the guidelines recommend that patients taking antibiotics also use benzoyl peroxide, which has “moderate” evidence regarding preventing the development of antibiotic resistance. “Lower strengths tend to be less irritating, and over-the-counter formulations are readily available,” she said, adding that colleagues should make sure to warn patients about the risk of bleaching clothes and towels with BP.
Now, there’s a newly approved treatment, the first fixed-dose triple combination therapy for acne, she said. It combines 1.2% clindamycin, 3.1% benzoyl peroxide, and 0.15% adapalene (Cabtreo) and is Food and Drug Administration (FDA)-approved for treating acne in patients ages 12 and up.
The new AAD guidelines note that “potential adverse effect profiles of the fixed-dose combinations generally reflect those of the individual agents in summation. Some fixed-dose combination products may be less expensive than prescribing their individual components separately.” The evidence supporting fixed-dose combinations in conjunction with benzoyl peroxide is considered “moderate.”
Dapsone gel, 7.5% (Aczone) is another option for acne. “It’s a topical so you don’t need to do G6PD [glucose-6-phosphate dehydrogenase] testing,” Dr. Zaenglein said. “It’s well tolerated, and mean total lesions fell by 48.9% vs 43.2% for vehicle,” in a 2018 study, which she said also found that females benefited more than males from this treatment.
Clascoterone 1% cream (Winlevi), approved in 2020, is appropriate for males and females aged 12 and up, Dr. Zaenglein said. She noted that it’s the only topical anti-androgen that can be used in males. However, while it has a “high” level of evidence because of phase 3 clinical trials showing benefits in moderate to severe acne, the AAD guidelines only conditionally recommend this option because the high price of clascoterone “may impact equitable acne treatment access.” The price listed on the website GoodRx (accessed on March 12) lists drugstore prices for a single 60-gram tube as ranging from $590 to $671.
“One of the harder things is trying to figure out where clascoterone fits in our kind of standard combination therapy,” she said. “Much like other hormonal therapies, it works better over the long term.”
Two more topical options per the AAD guidelines are salicylic acid, based on one randomized controlled trial, and azelaic acid (Azelex, Finacea), based on three randomized controlled trials. Both of these recommendations are conditional because of limited evidence: Evidence is considered “low” for salicylic acid and “moderate” for azelaic acid, the guidelines say, and azelaic acid “may be particularly helpful for patients with sensitive skin or darker skin types due to its lightening effect on dyspigmentation.”
As for risk for topical treatments during pregnancy/lactation, the guidelines note that topical therapies other than topical retinoids are “preferred” during pregnancy. Tazarotene is contraindicated during pregnancy, and salicylic acid should be used only in limited areas of exposure. There are no data for dapsone and clascoterone during pregnancy/lactation, and minocycline is “not recommended.”
The guideline authors noted that “available evidence is insufficient to develop a recommendation on the use of topical glycolic acid, sulfur, sodium sulfacetamide, and resorcinol for acne treatment or to make recommendations that compare topical BP, retinoids, antibiotics, and their combinations directly against each other.”
Could BP Post a Risk From Benzene?
Dr. Zaenglein highlighted a recently released report by Valisure, an independent laboratory, which reported finding high levels of the cancer-causing chemical benzene in several acne treatments, including brands such as Clearasil. “They didn’t release all of the ones that they evaluated, but there were a lot ... that we commonly recommend for our patients,” she said.
On March 6, CBS News reported that Valisure “ran tests at various temperatures over 18 days and found some products ‘can form over 800 times the conditionally restricted FDA concentration limit of two parts per million (ppm) for benzene’ in 2 weeks at 50° C (122° F),” but that benzene levels “at room temperature were more modest, ranging from about one to 24 parts per million.”
Dr. Zaenglein said she’s not ready to urge patients to discontinue BP, although in light of the findings, “I will tell them to store it at room temperature or lower.”
For now, it’s important to wait for independent verification of the results, she said. “And then it’s up to the manufacturers to reevaluate the stability of their benzoyl peroxide products with heat.”
Dr. Zaenglein disclosed relationships with AbbVie, Arcutis, Biofrontera, Galderma, and Incyte (grants/research funding), Church & Dwight (consulting fees), and UCB (consulting honoraria).
A version of this article appeared on Medscape.com.
SAN DIEGO — Just weeks after the American Academy of Dermatology (AAD) published its updated acne management guidelines, a dermatologist who helped write the recommendations provided colleagues with insight into recently approved topical therapies, the importance of multimodal therapy, and a controversial report linking benzoyl peroxide (BP) to the carcinogen benzene.
In regard to topical treatments, the guidelines make a “strong” recommendation for topical retinoids based on “moderate” evidence, Andrea L. Zaenglein, MD, professor of dermatology and pediatrics, Penn State University, Hershey, Pennsylvania, said at the annual meeting of the American Academy of Dermatology. The recommendation was based on a pooled analysis of four randomized controlled trials that found patients with acne who used the medications were more likely to have improvement via the Investigator Global Assessment (IGA) scale at 12 weeks than were those treated with a vehicle (risk ratio [RR], 1.57; 1.21-2.04).
The updated guidelines were published on January 30 in the Journal of the American Academy of Dermatology. The previous guidelines were issued in 2016.
“We have four current retinoids that we use: adapalene, tretinoin, tazarotene, and trifarotene,” Dr. Zaenglein said. “Typically, when we think about retinoids, we think of adapalene as being more tolerable and tazarotene as being more effective. But we also know that they can work to prevent and treat scarring, and they work against comedonal lesions and inflammatory lesions.”
Newer concentrations include tretinoin 0.05% lotion, tazarotene 0.045% lotion, and trifarotene 0.005% cream. She noted that this trifarotene concentration can be helpful for moderate truncal acne and also referred to evidence that whey protein appears to exacerbate that condition. “I always ask teenage kids about that: Are they using those protein powders?”
Recommendations for ‘Multimodal Therapy,’ Especially With Antibiotics
Dr. Zaenglein highlighted a “good practice statement” in the new guidelines that says, “when managing acne with topical medications, we recommend multimodal therapy combining multiple mechanisms of action.”
Topical antibiotics are effective treatments on their own and include erythromycin, clindamycin, and minocycline (Minocin), she said. But the guidelines, which refer to evidence supporting them as “moderate,” do not recommend them as monotherapy because of the risk for antibiotic resistance.
The oral retinoid isotretinoin may be appropriate in conjunction with topical medications, she said, “and we also recommend fixed combination products because they’re associated with increased adherence.”
Dermatologists are familiar with several of these products because “we’ve been using them for years and years,” she said. The guidelines note that “compared to vehicle at 12 weeks, a greater proportion of patients treated with combined BP and topical retinoid achieved IGA success in three RCTs (RR, 2.19; 1.77-2.72).”
Dr. Zaenglein noted that the guidelines recommend that patients taking antibiotics also use benzoyl peroxide, which has “moderate” evidence regarding preventing the development of antibiotic resistance. “Lower strengths tend to be less irritating, and over-the-counter formulations are readily available,” she said, adding that colleagues should make sure to warn patients about the risk of bleaching clothes and towels with BP.
Now, there’s a newly approved treatment, the first fixed-dose triple combination therapy for acne, she said. It combines 1.2% clindamycin, 3.1% benzoyl peroxide, and 0.15% adapalene (Cabtreo) and is Food and Drug Administration (FDA)-approved for treating acne in patients ages 12 and up.
The new AAD guidelines note that “potential adverse effect profiles of the fixed-dose combinations generally reflect those of the individual agents in summation. Some fixed-dose combination products may be less expensive than prescribing their individual components separately.” The evidence supporting fixed-dose combinations in conjunction with benzoyl peroxide is considered “moderate.”
Dapsone gel, 7.5% (Aczone) is another option for acne. “It’s a topical so you don’t need to do G6PD [glucose-6-phosphate dehydrogenase] testing,” Dr. Zaenglein said. “It’s well tolerated, and mean total lesions fell by 48.9% vs 43.2% for vehicle,” in a 2018 study, which she said also found that females benefited more than males from this treatment.
Clascoterone 1% cream (Winlevi), approved in 2020, is appropriate for males and females aged 12 and up, Dr. Zaenglein said. She noted that it’s the only topical anti-androgen that can be used in males. However, while it has a “high” level of evidence because of phase 3 clinical trials showing benefits in moderate to severe acne, the AAD guidelines only conditionally recommend this option because the high price of clascoterone “may impact equitable acne treatment access.” The price listed on the website GoodRx (accessed on March 12) lists drugstore prices for a single 60-gram tube as ranging from $590 to $671.
“One of the harder things is trying to figure out where clascoterone fits in our kind of standard combination therapy,” she said. “Much like other hormonal therapies, it works better over the long term.”
Two more topical options per the AAD guidelines are salicylic acid, based on one randomized controlled trial, and azelaic acid (Azelex, Finacea), based on three randomized controlled trials. Both of these recommendations are conditional because of limited evidence: Evidence is considered “low” for salicylic acid and “moderate” for azelaic acid, the guidelines say, and azelaic acid “may be particularly helpful for patients with sensitive skin or darker skin types due to its lightening effect on dyspigmentation.”
As for risk for topical treatments during pregnancy/lactation, the guidelines note that topical therapies other than topical retinoids are “preferred” during pregnancy. Tazarotene is contraindicated during pregnancy, and salicylic acid should be used only in limited areas of exposure. There are no data for dapsone and clascoterone during pregnancy/lactation, and minocycline is “not recommended.”
The guideline authors noted that “available evidence is insufficient to develop a recommendation on the use of topical glycolic acid, sulfur, sodium sulfacetamide, and resorcinol for acne treatment or to make recommendations that compare topical BP, retinoids, antibiotics, and their combinations directly against each other.”
Could BP Post a Risk From Benzene?
Dr. Zaenglein highlighted a recently released report by Valisure, an independent laboratory, which reported finding high levels of the cancer-causing chemical benzene in several acne treatments, including brands such as Clearasil. “They didn’t release all of the ones that they evaluated, but there were a lot ... that we commonly recommend for our patients,” she said.
On March 6, CBS News reported that Valisure “ran tests at various temperatures over 18 days and found some products ‘can form over 800 times the conditionally restricted FDA concentration limit of two parts per million (ppm) for benzene’ in 2 weeks at 50° C (122° F),” but that benzene levels “at room temperature were more modest, ranging from about one to 24 parts per million.”
Dr. Zaenglein said she’s not ready to urge patients to discontinue BP, although in light of the findings, “I will tell them to store it at room temperature or lower.”
For now, it’s important to wait for independent verification of the results, she said. “And then it’s up to the manufacturers to reevaluate the stability of their benzoyl peroxide products with heat.”
Dr. Zaenglein disclosed relationships with AbbVie, Arcutis, Biofrontera, Galderma, and Incyte (grants/research funding), Church & Dwight (consulting fees), and UCB (consulting honoraria).
A version of this article appeared on Medscape.com.
SAN DIEGO — Just weeks after the American Academy of Dermatology (AAD) published its updated acne management guidelines, a dermatologist who helped write the recommendations provided colleagues with insight into recently approved topical therapies, the importance of multimodal therapy, and a controversial report linking benzoyl peroxide (BP) to the carcinogen benzene.
In regard to topical treatments, the guidelines make a “strong” recommendation for topical retinoids based on “moderate” evidence, Andrea L. Zaenglein, MD, professor of dermatology and pediatrics, Penn State University, Hershey, Pennsylvania, said at the annual meeting of the American Academy of Dermatology. The recommendation was based on a pooled analysis of four randomized controlled trials that found patients with acne who used the medications were more likely to have improvement via the Investigator Global Assessment (IGA) scale at 12 weeks than were those treated with a vehicle (risk ratio [RR], 1.57; 1.21-2.04).
The updated guidelines were published on January 30 in the Journal of the American Academy of Dermatology. The previous guidelines were issued in 2016.
“We have four current retinoids that we use: adapalene, tretinoin, tazarotene, and trifarotene,” Dr. Zaenglein said. “Typically, when we think about retinoids, we think of adapalene as being more tolerable and tazarotene as being more effective. But we also know that they can work to prevent and treat scarring, and they work against comedonal lesions and inflammatory lesions.”
Newer concentrations include tretinoin 0.05% lotion, tazarotene 0.045% lotion, and trifarotene 0.005% cream. She noted that this trifarotene concentration can be helpful for moderate truncal acne and also referred to evidence that whey protein appears to exacerbate that condition. “I always ask teenage kids about that: Are they using those protein powders?”
Recommendations for ‘Multimodal Therapy,’ Especially With Antibiotics
Dr. Zaenglein highlighted a “good practice statement” in the new guidelines that says, “when managing acne with topical medications, we recommend multimodal therapy combining multiple mechanisms of action.”
Topical antibiotics are effective treatments on their own and include erythromycin, clindamycin, and minocycline (Minocin), she said. But the guidelines, which refer to evidence supporting them as “moderate,” do not recommend them as monotherapy because of the risk for antibiotic resistance.
The oral retinoid isotretinoin may be appropriate in conjunction with topical medications, she said, “and we also recommend fixed combination products because they’re associated with increased adherence.”
Dermatologists are familiar with several of these products because “we’ve been using them for years and years,” she said. The guidelines note that “compared to vehicle at 12 weeks, a greater proportion of patients treated with combined BP and topical retinoid achieved IGA success in three RCTs (RR, 2.19; 1.77-2.72).”
Dr. Zaenglein noted that the guidelines recommend that patients taking antibiotics also use benzoyl peroxide, which has “moderate” evidence regarding preventing the development of antibiotic resistance. “Lower strengths tend to be less irritating, and over-the-counter formulations are readily available,” she said, adding that colleagues should make sure to warn patients about the risk of bleaching clothes and towels with BP.
Now, there’s a newly approved treatment, the first fixed-dose triple combination therapy for acne, she said. It combines 1.2% clindamycin, 3.1% benzoyl peroxide, and 0.15% adapalene (Cabtreo) and is Food and Drug Administration (FDA)-approved for treating acne in patients ages 12 and up.
The new AAD guidelines note that “potential adverse effect profiles of the fixed-dose combinations generally reflect those of the individual agents in summation. Some fixed-dose combination products may be less expensive than prescribing their individual components separately.” The evidence supporting fixed-dose combinations in conjunction with benzoyl peroxide is considered “moderate.”
Dapsone gel, 7.5% (Aczone) is another option for acne. “It’s a topical so you don’t need to do G6PD [glucose-6-phosphate dehydrogenase] testing,” Dr. Zaenglein said. “It’s well tolerated, and mean total lesions fell by 48.9% vs 43.2% for vehicle,” in a 2018 study, which she said also found that females benefited more than males from this treatment.
Clascoterone 1% cream (Winlevi), approved in 2020, is appropriate for males and females aged 12 and up, Dr. Zaenglein said. She noted that it’s the only topical anti-androgen that can be used in males. However, while it has a “high” level of evidence because of phase 3 clinical trials showing benefits in moderate to severe acne, the AAD guidelines only conditionally recommend this option because the high price of clascoterone “may impact equitable acne treatment access.” The price listed on the website GoodRx (accessed on March 12) lists drugstore prices for a single 60-gram tube as ranging from $590 to $671.
“One of the harder things is trying to figure out where clascoterone fits in our kind of standard combination therapy,” she said. “Much like other hormonal therapies, it works better over the long term.”
Two more topical options per the AAD guidelines are salicylic acid, based on one randomized controlled trial, and azelaic acid (Azelex, Finacea), based on three randomized controlled trials. Both of these recommendations are conditional because of limited evidence: Evidence is considered “low” for salicylic acid and “moderate” for azelaic acid, the guidelines say, and azelaic acid “may be particularly helpful for patients with sensitive skin or darker skin types due to its lightening effect on dyspigmentation.”
As for risk for topical treatments during pregnancy/lactation, the guidelines note that topical therapies other than topical retinoids are “preferred” during pregnancy. Tazarotene is contraindicated during pregnancy, and salicylic acid should be used only in limited areas of exposure. There are no data for dapsone and clascoterone during pregnancy/lactation, and minocycline is “not recommended.”
The guideline authors noted that “available evidence is insufficient to develop a recommendation on the use of topical glycolic acid, sulfur, sodium sulfacetamide, and resorcinol for acne treatment or to make recommendations that compare topical BP, retinoids, antibiotics, and their combinations directly against each other.”
Could BP Post a Risk From Benzene?
Dr. Zaenglein highlighted a recently released report by Valisure, an independent laboratory, which reported finding high levels of the cancer-causing chemical benzene in several acne treatments, including brands such as Clearasil. “They didn’t release all of the ones that they evaluated, but there were a lot ... that we commonly recommend for our patients,” she said.
On March 6, CBS News reported that Valisure “ran tests at various temperatures over 18 days and found some products ‘can form over 800 times the conditionally restricted FDA concentration limit of two parts per million (ppm) for benzene’ in 2 weeks at 50° C (122° F),” but that benzene levels “at room temperature were more modest, ranging from about one to 24 parts per million.”
Dr. Zaenglein said she’s not ready to urge patients to discontinue BP, although in light of the findings, “I will tell them to store it at room temperature or lower.”
For now, it’s important to wait for independent verification of the results, she said. “And then it’s up to the manufacturers to reevaluate the stability of their benzoyl peroxide products with heat.”
Dr. Zaenglein disclosed relationships with AbbVie, Arcutis, Biofrontera, Galderma, and Incyte (grants/research funding), Church & Dwight (consulting fees), and UCB (consulting honoraria).
A version of this article appeared on Medscape.com.
FROM AAD 2024
Study Finds No Increased Cancer Risk With Spironolactone
TOPLINE:
than that of unexposed women.
METHODOLOGY:
- Spironolactone, used off-label for several skin conditions in women, carries a warning about an increased tumor risk associated with high doses in rat models, and its antiandrogen properties have prompted hypotheses about a possible increased risk for breast or gynecologic cancers.
- The researchers reviewed data on 420 women with a history of spironolactone use for acne, hair loss, and hirsutism and 3272 women with no spironolactone use at the authors› institution. Their mean age ranged from 42 to 63 years; the majority were White, and 38% were non-White.
- Median spironolactone doses ranged from 25 mg to 225 mg; chart reviews included 5-year follow-up data from the first spironolactone exposure to allow time for tumor development.
TAKEAWAY:
- A total of 37 of the 420 women exposed to spironolactone developed any tumors, as did 546 of the 3272 with no spironolactone exposure.
- After the researchers controlled for age and race, women exposed to spironolactone were no more likely to develop a malignant tumor than a benign tumor, compared with unexposed women (odds ratio [OR], 0.48, P = .2).
- The risk for breast or uterine cancer was not significantly different in the spironolactone and non-spironolactone groups (OR, 0.95, P > .9).
IN PRACTICE:
“Women taking spironolactone for acne, hair loss, and hirsutism and who are at low risk of breast or gynecologic cancers may be counseled to have regular gynecology follow-up, but no more frequently than the general population,” but more studies are needed to evaluate risk over longer periods of time, the researchers wrote.
SOURCE:
The lead author of the study was Rachel C. Hill, BS, a student at Weill Cornell Medical College, New York City, and Shari R. Lipner, MD, PhD, of the department of dermatology at Weill Cornell Medical College, was the corresponding author. The study was published online in The Journal of the American Academy of Dermatology.
LIMITATIONS:
The findings were limited by the retrospective design, as well as the small number of spironolactone patients analyzed, the short follow-up period, the lack of information about spironolactone courses, and the inability to control for family history of malignancy.
DISCLOSURES:
The study was supported by the National Center for Advancing Translational Sciences and a grant from the Clinical and Translational Science Center at Weill Cornell Medical College awarded to Ms. Hill. None of the authors had relevant disclosures; Dr. Lipner disclosed serving as a consultant for Ortho-Dermatologics, Eli Lilly, Moberg Pharmaceuticals, and BelleTorus Corporation.
A version of this article appeared on Medscape.com.
TOPLINE:
than that of unexposed women.
METHODOLOGY:
- Spironolactone, used off-label for several skin conditions in women, carries a warning about an increased tumor risk associated with high doses in rat models, and its antiandrogen properties have prompted hypotheses about a possible increased risk for breast or gynecologic cancers.
- The researchers reviewed data on 420 women with a history of spironolactone use for acne, hair loss, and hirsutism and 3272 women with no spironolactone use at the authors› institution. Their mean age ranged from 42 to 63 years; the majority were White, and 38% were non-White.
- Median spironolactone doses ranged from 25 mg to 225 mg; chart reviews included 5-year follow-up data from the first spironolactone exposure to allow time for tumor development.
TAKEAWAY:
- A total of 37 of the 420 women exposed to spironolactone developed any tumors, as did 546 of the 3272 with no spironolactone exposure.
- After the researchers controlled for age and race, women exposed to spironolactone were no more likely to develop a malignant tumor than a benign tumor, compared with unexposed women (odds ratio [OR], 0.48, P = .2).
- The risk for breast or uterine cancer was not significantly different in the spironolactone and non-spironolactone groups (OR, 0.95, P > .9).
IN PRACTICE:
“Women taking spironolactone for acne, hair loss, and hirsutism and who are at low risk of breast or gynecologic cancers may be counseled to have regular gynecology follow-up, but no more frequently than the general population,” but more studies are needed to evaluate risk over longer periods of time, the researchers wrote.
SOURCE:
The lead author of the study was Rachel C. Hill, BS, a student at Weill Cornell Medical College, New York City, and Shari R. Lipner, MD, PhD, of the department of dermatology at Weill Cornell Medical College, was the corresponding author. The study was published online in The Journal of the American Academy of Dermatology.
LIMITATIONS:
The findings were limited by the retrospective design, as well as the small number of spironolactone patients analyzed, the short follow-up period, the lack of information about spironolactone courses, and the inability to control for family history of malignancy.
DISCLOSURES:
The study was supported by the National Center for Advancing Translational Sciences and a grant from the Clinical and Translational Science Center at Weill Cornell Medical College awarded to Ms. Hill. None of the authors had relevant disclosures; Dr. Lipner disclosed serving as a consultant for Ortho-Dermatologics, Eli Lilly, Moberg Pharmaceuticals, and BelleTorus Corporation.
A version of this article appeared on Medscape.com.
TOPLINE:
than that of unexposed women.
METHODOLOGY:
- Spironolactone, used off-label for several skin conditions in women, carries a warning about an increased tumor risk associated with high doses in rat models, and its antiandrogen properties have prompted hypotheses about a possible increased risk for breast or gynecologic cancers.
- The researchers reviewed data on 420 women with a history of spironolactone use for acne, hair loss, and hirsutism and 3272 women with no spironolactone use at the authors› institution. Their mean age ranged from 42 to 63 years; the majority were White, and 38% were non-White.
- Median spironolactone doses ranged from 25 mg to 225 mg; chart reviews included 5-year follow-up data from the first spironolactone exposure to allow time for tumor development.
TAKEAWAY:
- A total of 37 of the 420 women exposed to spironolactone developed any tumors, as did 546 of the 3272 with no spironolactone exposure.
- After the researchers controlled for age and race, women exposed to spironolactone were no more likely to develop a malignant tumor than a benign tumor, compared with unexposed women (odds ratio [OR], 0.48, P = .2).
- The risk for breast or uterine cancer was not significantly different in the spironolactone and non-spironolactone groups (OR, 0.95, P > .9).
IN PRACTICE:
“Women taking spironolactone for acne, hair loss, and hirsutism and who are at low risk of breast or gynecologic cancers may be counseled to have regular gynecology follow-up, but no more frequently than the general population,” but more studies are needed to evaluate risk over longer periods of time, the researchers wrote.
SOURCE:
The lead author of the study was Rachel C. Hill, BS, a student at Weill Cornell Medical College, New York City, and Shari R. Lipner, MD, PhD, of the department of dermatology at Weill Cornell Medical College, was the corresponding author. The study was published online in The Journal of the American Academy of Dermatology.
LIMITATIONS:
The findings were limited by the retrospective design, as well as the small number of spironolactone patients analyzed, the short follow-up period, the lack of information about spironolactone courses, and the inability to control for family history of malignancy.
DISCLOSURES:
The study was supported by the National Center for Advancing Translational Sciences and a grant from the Clinical and Translational Science Center at Weill Cornell Medical College awarded to Ms. Hill. None of the authors had relevant disclosures; Dr. Lipner disclosed serving as a consultant for Ortho-Dermatologics, Eli Lilly, Moberg Pharmaceuticals, and BelleTorus Corporation.
A version of this article appeared on Medscape.com.
What Skin Manifestations Are Associated With Pediatric IBD?
TOPLINE:
Skin conditions burden many children with inflammatory bowel disease (IBD), according to the authors of a single-center study.
METHODOLOGY:
- Researchers retrospectively reviewed the medical charts of 425 children and adolescents with (CD) or ulcerative (UC) at one or more dermatologic diagnoses who were seen at Mayo Clinic, Rochester, Minnesota, between 1999 and 2017.
- Of the children studied, 53% were male, 64.9% had CD, and 42.8% had one or more cutaneous infections.
- They used the chi-square/Fischer’s exact test to compare categorical outcomes between patients with CD and UC and to detect differences in IBD/CD/UC disease severity and skin conditions.
- Researchers retrospectively reviewed the medical charts of 425 children and adolescents with Crohn’s disease (CD) or ulcerative colitis (UC) at one or more dermatologic diagnoses who were seen at Mayo Clinic, Rochester, Minnesota, between 1999 and 2017.
- Of the children studied, 53% were male, 64.9% had CD, and 42.8% had one or more cutaneous infections.
- They used the chi-square/Fischer’s exact test to compare categorical outcomes between patients with CD and UC and to detect differences in IBD/CD/UC disease severity and skin conditions.
TAKEAWAY:
- The most common noninfectious dermatologic condition among the 425 children and adolescents was (30.8%), followed by eczema (15.8%) and perianal skin tags (14.6%).
- Angular cheilitis was more common among those with CD than those with UC (7.2% vs 2%, respectively; P = .024) as was keratosis pilaris (6.9% vs 0.7%; P = .003), and perianal skin complications such as skin tags (20.3% vs 4%), fistulas (13.4% vs 2.7%), and abscesses (13.4% vs 2%; P < .001 for all associations).
- Fungal skin infections were more frequently diagnosed in children with UC than those with CD (15.4% vs 8%; P = .017).
- The researchers observed that the severity of IBD correlated with a higher prevalence of perianal fistula (P = .003), perianal region abscess (P = .041), psoriasis (P < .001), and pyoderma gangrenosum (P = .003).
IN PRACTICE:
“Early identification of common dermatologic conditions in children and adolescents with IBD and recognizing their characteristic associations may alter management and improve skin-related outcomes in this patient population,” the authors wrote.
SOURCE:
Corresponding author Megha M. Tollefson, MD, of the Department of Dermatology at Mayo Clinic, Rochester, Minnesota, and colleagues conducted the research, which was published in Pediatric Dermatology.
LIMITATIONS:
The single-center design and the fact that database studies are subject to extraction error. There was no age- and sex-matched cohort to determine whether the prevalence of cutaneous infections, acne, eczema, and other inflammatory disorders was truly increased in IBD.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Skin conditions burden many children with inflammatory bowel disease (IBD), according to the authors of a single-center study.
METHODOLOGY:
- Researchers retrospectively reviewed the medical charts of 425 children and adolescents with (CD) or ulcerative (UC) at one or more dermatologic diagnoses who were seen at Mayo Clinic, Rochester, Minnesota, between 1999 and 2017.
- Of the children studied, 53% were male, 64.9% had CD, and 42.8% had one or more cutaneous infections.
- They used the chi-square/Fischer’s exact test to compare categorical outcomes between patients with CD and UC and to detect differences in IBD/CD/UC disease severity and skin conditions.
- Researchers retrospectively reviewed the medical charts of 425 children and adolescents with Crohn’s disease (CD) or ulcerative colitis (UC) at one or more dermatologic diagnoses who were seen at Mayo Clinic, Rochester, Minnesota, between 1999 and 2017.
- Of the children studied, 53% were male, 64.9% had CD, and 42.8% had one or more cutaneous infections.
- They used the chi-square/Fischer’s exact test to compare categorical outcomes between patients with CD and UC and to detect differences in IBD/CD/UC disease severity and skin conditions.
TAKEAWAY:
- The most common noninfectious dermatologic condition among the 425 children and adolescents was (30.8%), followed by eczema (15.8%) and perianal skin tags (14.6%).
- Angular cheilitis was more common among those with CD than those with UC (7.2% vs 2%, respectively; P = .024) as was keratosis pilaris (6.9% vs 0.7%; P = .003), and perianal skin complications such as skin tags (20.3% vs 4%), fistulas (13.4% vs 2.7%), and abscesses (13.4% vs 2%; P < .001 for all associations).
- Fungal skin infections were more frequently diagnosed in children with UC than those with CD (15.4% vs 8%; P = .017).
- The researchers observed that the severity of IBD correlated with a higher prevalence of perianal fistula (P = .003), perianal region abscess (P = .041), psoriasis (P < .001), and pyoderma gangrenosum (P = .003).
IN PRACTICE:
“Early identification of common dermatologic conditions in children and adolescents with IBD and recognizing their characteristic associations may alter management and improve skin-related outcomes in this patient population,” the authors wrote.
SOURCE:
Corresponding author Megha M. Tollefson, MD, of the Department of Dermatology at Mayo Clinic, Rochester, Minnesota, and colleagues conducted the research, which was published in Pediatric Dermatology.
LIMITATIONS:
The single-center design and the fact that database studies are subject to extraction error. There was no age- and sex-matched cohort to determine whether the prevalence of cutaneous infections, acne, eczema, and other inflammatory disorders was truly increased in IBD.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Skin conditions burden many children with inflammatory bowel disease (IBD), according to the authors of a single-center study.
METHODOLOGY:
- Researchers retrospectively reviewed the medical charts of 425 children and adolescents with (CD) or ulcerative (UC) at one or more dermatologic diagnoses who were seen at Mayo Clinic, Rochester, Minnesota, between 1999 and 2017.
- Of the children studied, 53% were male, 64.9% had CD, and 42.8% had one or more cutaneous infections.
- They used the chi-square/Fischer’s exact test to compare categorical outcomes between patients with CD and UC and to detect differences in IBD/CD/UC disease severity and skin conditions.
- Researchers retrospectively reviewed the medical charts of 425 children and adolescents with Crohn’s disease (CD) or ulcerative colitis (UC) at one or more dermatologic diagnoses who were seen at Mayo Clinic, Rochester, Minnesota, between 1999 and 2017.
- Of the children studied, 53% were male, 64.9% had CD, and 42.8% had one or more cutaneous infections.
- They used the chi-square/Fischer’s exact test to compare categorical outcomes between patients with CD and UC and to detect differences in IBD/CD/UC disease severity and skin conditions.
TAKEAWAY:
- The most common noninfectious dermatologic condition among the 425 children and adolescents was (30.8%), followed by eczema (15.8%) and perianal skin tags (14.6%).
- Angular cheilitis was more common among those with CD than those with UC (7.2% vs 2%, respectively; P = .024) as was keratosis pilaris (6.9% vs 0.7%; P = .003), and perianal skin complications such as skin tags (20.3% vs 4%), fistulas (13.4% vs 2.7%), and abscesses (13.4% vs 2%; P < .001 for all associations).
- Fungal skin infections were more frequently diagnosed in children with UC than those with CD (15.4% vs 8%; P = .017).
- The researchers observed that the severity of IBD correlated with a higher prevalence of perianal fistula (P = .003), perianal region abscess (P = .041), psoriasis (P < .001), and pyoderma gangrenosum (P = .003).
IN PRACTICE:
“Early identification of common dermatologic conditions in children and adolescents with IBD and recognizing their characteristic associations may alter management and improve skin-related outcomes in this patient population,” the authors wrote.
SOURCE:
Corresponding author Megha M. Tollefson, MD, of the Department of Dermatology at Mayo Clinic, Rochester, Minnesota, and colleagues conducted the research, which was published in Pediatric Dermatology.
LIMITATIONS:
The single-center design and the fact that database studies are subject to extraction error. There was no age- and sex-matched cohort to determine whether the prevalence of cutaneous infections, acne, eczema, and other inflammatory disorders was truly increased in IBD.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.
AAD Updates Guidelines for Managing Acne
. The guidelines also conditionally recommend the use of topical clascoterone, salicylic acid, azelaic acid, oral minocycline, sarecycline, combined oral contraceptives, and spironolactone.
The development updates the AAD’s 2016 guidelines for managing acne. “Since there have been several important new treatments introduced since the prior guidelines, it was determined that there was a need to update these guidelines,” John S. Barbieri, MD, MBA, who cochaired a 16-member multidisciplinary work group that assembled the guidelines, told this news organization.
For the new guidelines, which were published online January 30, 2023, in the Journal of the American Academy of Dermatology, Dr. Barbieri, a dermatologist who directs the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital, Boston, guidelines cochair Rachel V. Reynolds, MD, a dermatologist at Beth Israel Deaconess Medical Center, Boston, and colleagues conducted a systematic review of evidence regarding the management of acne. Next, the work group applied the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach for assessing the certainty of the evidence and formulating and grading clinical recommendations based on relevant randomized trials in the medical literature.
In all, the work group made 18 recommendations and 5 good practice statements. They ranked 7 of the recommendations as “strong” based on the evidence reviewed, and the rest as “conditional.” The “strong” recommendations include the use of benzoyl peroxide, the use of topical retinoids, the use of topical antibiotics, a fixed dose of a combination topical antibiotic with benzoyl peroxide, a fixed dose of a combination topical retinoid with topical antibiotic, a fixed dose combination of a topical retinoid with benzoyl peroxide, and the use of doxycycline.
“Conditional” recommendations include those for the use of clascoterone, salicylic acid, azelaic acid, minocycline, sarecycline, doxycycline over azithromycin; combined oral contraceptive pills, spironolactone, and, for patients with severe acne, traditional daily dosing of isotretinoin over intermittent dosing of isotretinoin.
Meanwhile, good clinical practice statements contained in the document include using topical therapies combining multiple mechanisms of action, limiting systemic antibiotic use, combining topical and systemic antibiotics with benzoyl peroxide and other topical therapies, and adjuvant intralesional corticosteroid injections.
In Dr. Barbieri’s opinion, the recommendations regarding clascoterone and sarecycline represent important developments. “Clascoterone is the first FDA-approved treatment that can address hormonal causes of acne in both men and women,” he told this news organization. “Sarecycline is a narrow-spectrum tetracycline that might have some advantages over other tetracyclines such as doxycycline and minocycline. It will be important to payers to provide coverage to ensure that patients have access to these valuable new treatments.”
Dr. Barbieri added that while no evidence exists to suggest that minocycline is more effective than doxycycline, minocycline can be associated with rare but serious side effects, such as vestibular dysfunction, autoimmune hepatitis, drug-induced lupus, and drug reaction with eosinophilia and systemic symptoms (DRESS). “We should consider whether reducing use of minocycline might be beneficial to our overall care of patients with acne,” he said. “In addition, we discuss that use of trimethoprim-sulfamethoxazole should be limited due to risk of severe adverse reactions such as Stevens-Johnson syndrome/toxic epidermal necrolysis, and acute respiratory failure.”
Another highlight of the guidelines, he continued, are specific recommendations for young, healthy patients on isotretinoin or spironolactone, which “can help clinicians and patients who are interested in less frequent monitoring feel more comfortable with these approaches,” he said.
Many discussions among work group members dealt with how to best implement the GRADE approach to the project “while ensuring the guidelines were as clinically relevant and actionable as possible,” according to Dr. Barbieri. “I think an important issue going forward will be to consider how to update and modify the GRADE approach to fit the unique needs of creating evidence-based guidelines for the management of skin disease.”
The work group acknowledged limitations of the guidelines, including identification of “important evidence gaps on the use of microbiology and endocrinology testing in acne, the use of systemic antibiotics beyond tetracycline-class antibiotics, physical modalities, complementary and alternative therapies, dietary interventions for the treatment of acne, and cost-effectiveness of acne treatments,” they wrote. “RCTs with long-term follow-up and comparative effectiveness research are necessary to examine and compare patient-centered acne treatment outcomes.”
The AAD funded the project. Dr. Barbieri disclosed that he serves as investigator for the National Institutes of Health and the National Psoriasis Foundation. Many coauthors reported being a speaker for and/or a consultant and advisory board member to many pharmaceutical companies.
. The guidelines also conditionally recommend the use of topical clascoterone, salicylic acid, azelaic acid, oral minocycline, sarecycline, combined oral contraceptives, and spironolactone.
The development updates the AAD’s 2016 guidelines for managing acne. “Since there have been several important new treatments introduced since the prior guidelines, it was determined that there was a need to update these guidelines,” John S. Barbieri, MD, MBA, who cochaired a 16-member multidisciplinary work group that assembled the guidelines, told this news organization.
For the new guidelines, which were published online January 30, 2023, in the Journal of the American Academy of Dermatology, Dr. Barbieri, a dermatologist who directs the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital, Boston, guidelines cochair Rachel V. Reynolds, MD, a dermatologist at Beth Israel Deaconess Medical Center, Boston, and colleagues conducted a systematic review of evidence regarding the management of acne. Next, the work group applied the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach for assessing the certainty of the evidence and formulating and grading clinical recommendations based on relevant randomized trials in the medical literature.
In all, the work group made 18 recommendations and 5 good practice statements. They ranked 7 of the recommendations as “strong” based on the evidence reviewed, and the rest as “conditional.” The “strong” recommendations include the use of benzoyl peroxide, the use of topical retinoids, the use of topical antibiotics, a fixed dose of a combination topical antibiotic with benzoyl peroxide, a fixed dose of a combination topical retinoid with topical antibiotic, a fixed dose combination of a topical retinoid with benzoyl peroxide, and the use of doxycycline.
“Conditional” recommendations include those for the use of clascoterone, salicylic acid, azelaic acid, minocycline, sarecycline, doxycycline over azithromycin; combined oral contraceptive pills, spironolactone, and, for patients with severe acne, traditional daily dosing of isotretinoin over intermittent dosing of isotretinoin.
Meanwhile, good clinical practice statements contained in the document include using topical therapies combining multiple mechanisms of action, limiting systemic antibiotic use, combining topical and systemic antibiotics with benzoyl peroxide and other topical therapies, and adjuvant intralesional corticosteroid injections.
In Dr. Barbieri’s opinion, the recommendations regarding clascoterone and sarecycline represent important developments. “Clascoterone is the first FDA-approved treatment that can address hormonal causes of acne in both men and women,” he told this news organization. “Sarecycline is a narrow-spectrum tetracycline that might have some advantages over other tetracyclines such as doxycycline and minocycline. It will be important to payers to provide coverage to ensure that patients have access to these valuable new treatments.”
Dr. Barbieri added that while no evidence exists to suggest that minocycline is more effective than doxycycline, minocycline can be associated with rare but serious side effects, such as vestibular dysfunction, autoimmune hepatitis, drug-induced lupus, and drug reaction with eosinophilia and systemic symptoms (DRESS). “We should consider whether reducing use of minocycline might be beneficial to our overall care of patients with acne,” he said. “In addition, we discuss that use of trimethoprim-sulfamethoxazole should be limited due to risk of severe adverse reactions such as Stevens-Johnson syndrome/toxic epidermal necrolysis, and acute respiratory failure.”
Another highlight of the guidelines, he continued, are specific recommendations for young, healthy patients on isotretinoin or spironolactone, which “can help clinicians and patients who are interested in less frequent monitoring feel more comfortable with these approaches,” he said.
Many discussions among work group members dealt with how to best implement the GRADE approach to the project “while ensuring the guidelines were as clinically relevant and actionable as possible,” according to Dr. Barbieri. “I think an important issue going forward will be to consider how to update and modify the GRADE approach to fit the unique needs of creating evidence-based guidelines for the management of skin disease.”
The work group acknowledged limitations of the guidelines, including identification of “important evidence gaps on the use of microbiology and endocrinology testing in acne, the use of systemic antibiotics beyond tetracycline-class antibiotics, physical modalities, complementary and alternative therapies, dietary interventions for the treatment of acne, and cost-effectiveness of acne treatments,” they wrote. “RCTs with long-term follow-up and comparative effectiveness research are necessary to examine and compare patient-centered acne treatment outcomes.”
The AAD funded the project. Dr. Barbieri disclosed that he serves as investigator for the National Institutes of Health and the National Psoriasis Foundation. Many coauthors reported being a speaker for and/or a consultant and advisory board member to many pharmaceutical companies.
. The guidelines also conditionally recommend the use of topical clascoterone, salicylic acid, azelaic acid, oral minocycline, sarecycline, combined oral contraceptives, and spironolactone.
The development updates the AAD’s 2016 guidelines for managing acne. “Since there have been several important new treatments introduced since the prior guidelines, it was determined that there was a need to update these guidelines,” John S. Barbieri, MD, MBA, who cochaired a 16-member multidisciplinary work group that assembled the guidelines, told this news organization.
For the new guidelines, which were published online January 30, 2023, in the Journal of the American Academy of Dermatology, Dr. Barbieri, a dermatologist who directs the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital, Boston, guidelines cochair Rachel V. Reynolds, MD, a dermatologist at Beth Israel Deaconess Medical Center, Boston, and colleagues conducted a systematic review of evidence regarding the management of acne. Next, the work group applied the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach for assessing the certainty of the evidence and formulating and grading clinical recommendations based on relevant randomized trials in the medical literature.
In all, the work group made 18 recommendations and 5 good practice statements. They ranked 7 of the recommendations as “strong” based on the evidence reviewed, and the rest as “conditional.” The “strong” recommendations include the use of benzoyl peroxide, the use of topical retinoids, the use of topical antibiotics, a fixed dose of a combination topical antibiotic with benzoyl peroxide, a fixed dose of a combination topical retinoid with topical antibiotic, a fixed dose combination of a topical retinoid with benzoyl peroxide, and the use of doxycycline.
“Conditional” recommendations include those for the use of clascoterone, salicylic acid, azelaic acid, minocycline, sarecycline, doxycycline over azithromycin; combined oral contraceptive pills, spironolactone, and, for patients with severe acne, traditional daily dosing of isotretinoin over intermittent dosing of isotretinoin.
Meanwhile, good clinical practice statements contained in the document include using topical therapies combining multiple mechanisms of action, limiting systemic antibiotic use, combining topical and systemic antibiotics with benzoyl peroxide and other topical therapies, and adjuvant intralesional corticosteroid injections.
In Dr. Barbieri’s opinion, the recommendations regarding clascoterone and sarecycline represent important developments. “Clascoterone is the first FDA-approved treatment that can address hormonal causes of acne in both men and women,” he told this news organization. “Sarecycline is a narrow-spectrum tetracycline that might have some advantages over other tetracyclines such as doxycycline and minocycline. It will be important to payers to provide coverage to ensure that patients have access to these valuable new treatments.”
Dr. Barbieri added that while no evidence exists to suggest that minocycline is more effective than doxycycline, minocycline can be associated with rare but serious side effects, such as vestibular dysfunction, autoimmune hepatitis, drug-induced lupus, and drug reaction with eosinophilia and systemic symptoms (DRESS). “We should consider whether reducing use of minocycline might be beneficial to our overall care of patients with acne,” he said. “In addition, we discuss that use of trimethoprim-sulfamethoxazole should be limited due to risk of severe adverse reactions such as Stevens-Johnson syndrome/toxic epidermal necrolysis, and acute respiratory failure.”
Another highlight of the guidelines, he continued, are specific recommendations for young, healthy patients on isotretinoin or spironolactone, which “can help clinicians and patients who are interested in less frequent monitoring feel more comfortable with these approaches,” he said.
Many discussions among work group members dealt with how to best implement the GRADE approach to the project “while ensuring the guidelines were as clinically relevant and actionable as possible,” according to Dr. Barbieri. “I think an important issue going forward will be to consider how to update and modify the GRADE approach to fit the unique needs of creating evidence-based guidelines for the management of skin disease.”
The work group acknowledged limitations of the guidelines, including identification of “important evidence gaps on the use of microbiology and endocrinology testing in acne, the use of systemic antibiotics beyond tetracycline-class antibiotics, physical modalities, complementary and alternative therapies, dietary interventions for the treatment of acne, and cost-effectiveness of acne treatments,” they wrote. “RCTs with long-term follow-up and comparative effectiveness research are necessary to examine and compare patient-centered acne treatment outcomes.”
The AAD funded the project. Dr. Barbieri disclosed that he serves as investigator for the National Institutes of Health and the National Psoriasis Foundation. Many coauthors reported being a speaker for and/or a consultant and advisory board member to many pharmaceutical companies.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Treating Acne Scars Can Improve Aesthetics, Quality of Life
ORLANDO, FLORIDA — For some people, acne carries a one-two punch. First, they experience acne that is significant enough to decrease their quality of life, followed by scarring that can last a lifetime. For those patients, dermatologists have several options: Subcision to lift the depression of the scar, laser treatment to lower the height of scar tissue, and injections to fill scars.
“In my practice, I find that ,” Robyn Siperstein, MD, said at the annual ODAC Dermatology, Aesthetic & Surgical Conference.
Dr. Siperstein starts by identifying the type of acne scar — rolling scars, boxcar scars, or ice pick scars. Rolling scars tend to be shallower with no sharp edges; boxcar scars are deeper, more defined round or oval depressions; and ice pick scars, as the name suggests, look like someone stuck tiny ice picks into the skin, leaving a sunken or pitted appearance.
“It’s really important to categorize so that we know which ones are going to be effectively treated with different modalities and which ones aren’t, so that we can give our patients realistic expectations,” said Dr. Siperstein, a cosmetic dermatologist in private practice in Boca Raton, Florida, and a clinical affiliate associate professor of dermatology at Florida Atlantic University, Boca Raton.
“There’s not going to be one treatment that’s right for everything,” she said. Different approaches may be required even for the same patient because some people present with all three types of acne scars, she added.
Combining Treatments
When it comes to injecting dermal fillers into acne scars to lift the depressed areas, the US Food and Drug Administration approved a filler with polymethyl methacrylate filler and bovine collagen (Bellafill) for this indication (moderate to severe, atrophic, distensible facial acne scars on the cheek in patients over age 21) in 2015. “And off-label, I use hyaluronic acid in my practice,” Dr. Siperstein said. Each filler “probably works a little bit better or differently on different types of scars.”
For rolling scars, she recommends hyaluronic acid (HA) dermal filler for everyone. “Of course, this is my opinion.” She was also a lead investigator in a randomized, placebo-controlled split-face study comparing HA filler with saline for correcting atrophic facial scars in 15 patients. The HA filler emerged superior, although there were some improvements with saline.
In her clinical experience, patients are happy with the results and ask, “Why didn’t the last four doctors do this?”
Boxcar scars are more challenging to fill with HA. In some cases, Dr. Siperstein is able to raise the depressed portion of the scar, but some of the vertical edges remain. In this scenario, she might combine treatments. Laser resurfacing, for example, might help convert boxcar scars into rolling scars, which then can be filled more successfully.
“Ice pick scars are tough,” Dr. Siperstein said. A punch removal technique can work in some cases, or she might try the “cross technique.” This involves placing acetic acid inside the scar using a Q-tip. “You have to be really careful,” she added, “because if you get it around the edges, it’s actually going to make the scar bigger.”
Choosing the Right Candidates
Selecting the right candidate for HA treatment of acne scars is essential. Dr. Siperstein shared the example of a lifeguard who had prominent acne scarring down the center of his chest. “He was embarrassed to go to the beach and take off his shirt. He said he felt like he had bullet holes in his chest.”
One month following treatment, “he had a really nice improvement, and now he feels really comfortable,” she said.
Some dermatologists might be reluctant to consider HA fillers for acne scarring because there is a misconception that HA is short-acting, lasting 6 months to 1 year before the effect wears off. That impression can persist from company-sponsored studies that limit follow-up to 6 months or 1 year “to get their drug to market,” she noted.
Also adding to this impression is that HA fillers in wrinkles may not last as long. Dr. Siperstein explained that wrinkles on the face are dynamic and constantly moving. In contrast, acne scars experience less movement, which helps the HA last longer. There is MRI evidence that shows HA fillers last over 2 years in the face, she added.
One tip to predict how well an acne scar might respond to filler injections is to squeeze it and look for the “dimple sign.” If the floor of the scar lifts up when squeezed, “we know that they’ll be a good candidate for hyaluronic acid filler.” Another tip is to inject HA in a retrograde technique high up in the skin. Inject tiny amounts — microdroplets — of the HA filler high on the dermis, she advised.
Deeper injections run the risk of raising the entire scar instead of filling it, she added.
Like many dermatologic procedures, before and after photos are essential to demonstrate improvements, Dr. Siperstein pointed out. Patients are often skeptical. “This happens a lot with acne scar patients. They’ve been to a million places that have promised results, they have not gotten them, and they are frustrated.”
Acne scars can result from picking, inflammation, or treatment. “This is what we see all day in clinic,” Dr. Siperstein said. “Somebody who had to undergo Accutane treatment but unfortunately is left with holes. This is a huge psychological burden on our patients,” she said, describing a younger patient who had scarring, which “led to depression — it was ruining his life.”
“His mom was willing to do whatever it took. And I said, You know what, I think filler will be enough,” Dr. Siperstein said. She counseled them that treatment would not make the scars disappear completely. But patients used to 10% improvements are very happy when their acne scars look 80% or 90% better, she added.
Dr. Siperstein received grant or research support and is a member of the speakers bureau for Allergan and Galderma. She is also a consultant/advisory board member for Allergan.
A version of this article appeared on Medscape.com.
ORLANDO, FLORIDA — For some people, acne carries a one-two punch. First, they experience acne that is significant enough to decrease their quality of life, followed by scarring that can last a lifetime. For those patients, dermatologists have several options: Subcision to lift the depression of the scar, laser treatment to lower the height of scar tissue, and injections to fill scars.
“In my practice, I find that ,” Robyn Siperstein, MD, said at the annual ODAC Dermatology, Aesthetic & Surgical Conference.
Dr. Siperstein starts by identifying the type of acne scar — rolling scars, boxcar scars, or ice pick scars. Rolling scars tend to be shallower with no sharp edges; boxcar scars are deeper, more defined round or oval depressions; and ice pick scars, as the name suggests, look like someone stuck tiny ice picks into the skin, leaving a sunken or pitted appearance.
“It’s really important to categorize so that we know which ones are going to be effectively treated with different modalities and which ones aren’t, so that we can give our patients realistic expectations,” said Dr. Siperstein, a cosmetic dermatologist in private practice in Boca Raton, Florida, and a clinical affiliate associate professor of dermatology at Florida Atlantic University, Boca Raton.
“There’s not going to be one treatment that’s right for everything,” she said. Different approaches may be required even for the same patient because some people present with all three types of acne scars, she added.
Combining Treatments
When it comes to injecting dermal fillers into acne scars to lift the depressed areas, the US Food and Drug Administration approved a filler with polymethyl methacrylate filler and bovine collagen (Bellafill) for this indication (moderate to severe, atrophic, distensible facial acne scars on the cheek in patients over age 21) in 2015. “And off-label, I use hyaluronic acid in my practice,” Dr. Siperstein said. Each filler “probably works a little bit better or differently on different types of scars.”
For rolling scars, she recommends hyaluronic acid (HA) dermal filler for everyone. “Of course, this is my opinion.” She was also a lead investigator in a randomized, placebo-controlled split-face study comparing HA filler with saline for correcting atrophic facial scars in 15 patients. The HA filler emerged superior, although there were some improvements with saline.
In her clinical experience, patients are happy with the results and ask, “Why didn’t the last four doctors do this?”
Boxcar scars are more challenging to fill with HA. In some cases, Dr. Siperstein is able to raise the depressed portion of the scar, but some of the vertical edges remain. In this scenario, she might combine treatments. Laser resurfacing, for example, might help convert boxcar scars into rolling scars, which then can be filled more successfully.
“Ice pick scars are tough,” Dr. Siperstein said. A punch removal technique can work in some cases, or she might try the “cross technique.” This involves placing acetic acid inside the scar using a Q-tip. “You have to be really careful,” she added, “because if you get it around the edges, it’s actually going to make the scar bigger.”
Choosing the Right Candidates
Selecting the right candidate for HA treatment of acne scars is essential. Dr. Siperstein shared the example of a lifeguard who had prominent acne scarring down the center of his chest. “He was embarrassed to go to the beach and take off his shirt. He said he felt like he had bullet holes in his chest.”
One month following treatment, “he had a really nice improvement, and now he feels really comfortable,” she said.
Some dermatologists might be reluctant to consider HA fillers for acne scarring because there is a misconception that HA is short-acting, lasting 6 months to 1 year before the effect wears off. That impression can persist from company-sponsored studies that limit follow-up to 6 months or 1 year “to get their drug to market,” she noted.
Also adding to this impression is that HA fillers in wrinkles may not last as long. Dr. Siperstein explained that wrinkles on the face are dynamic and constantly moving. In contrast, acne scars experience less movement, which helps the HA last longer. There is MRI evidence that shows HA fillers last over 2 years in the face, she added.
One tip to predict how well an acne scar might respond to filler injections is to squeeze it and look for the “dimple sign.” If the floor of the scar lifts up when squeezed, “we know that they’ll be a good candidate for hyaluronic acid filler.” Another tip is to inject HA in a retrograde technique high up in the skin. Inject tiny amounts — microdroplets — of the HA filler high on the dermis, she advised.
Deeper injections run the risk of raising the entire scar instead of filling it, she added.
Like many dermatologic procedures, before and after photos are essential to demonstrate improvements, Dr. Siperstein pointed out. Patients are often skeptical. “This happens a lot with acne scar patients. They’ve been to a million places that have promised results, they have not gotten them, and they are frustrated.”
Acne scars can result from picking, inflammation, or treatment. “This is what we see all day in clinic,” Dr. Siperstein said. “Somebody who had to undergo Accutane treatment but unfortunately is left with holes. This is a huge psychological burden on our patients,” she said, describing a younger patient who had scarring, which “led to depression — it was ruining his life.”
“His mom was willing to do whatever it took. And I said, You know what, I think filler will be enough,” Dr. Siperstein said. She counseled them that treatment would not make the scars disappear completely. But patients used to 10% improvements are very happy when their acne scars look 80% or 90% better, she added.
Dr. Siperstein received grant or research support and is a member of the speakers bureau for Allergan and Galderma. She is also a consultant/advisory board member for Allergan.
A version of this article appeared on Medscape.com.
ORLANDO, FLORIDA — For some people, acne carries a one-two punch. First, they experience acne that is significant enough to decrease their quality of life, followed by scarring that can last a lifetime. For those patients, dermatologists have several options: Subcision to lift the depression of the scar, laser treatment to lower the height of scar tissue, and injections to fill scars.
“In my practice, I find that ,” Robyn Siperstein, MD, said at the annual ODAC Dermatology, Aesthetic & Surgical Conference.
Dr. Siperstein starts by identifying the type of acne scar — rolling scars, boxcar scars, or ice pick scars. Rolling scars tend to be shallower with no sharp edges; boxcar scars are deeper, more defined round or oval depressions; and ice pick scars, as the name suggests, look like someone stuck tiny ice picks into the skin, leaving a sunken or pitted appearance.
“It’s really important to categorize so that we know which ones are going to be effectively treated with different modalities and which ones aren’t, so that we can give our patients realistic expectations,” said Dr. Siperstein, a cosmetic dermatologist in private practice in Boca Raton, Florida, and a clinical affiliate associate professor of dermatology at Florida Atlantic University, Boca Raton.
“There’s not going to be one treatment that’s right for everything,” she said. Different approaches may be required even for the same patient because some people present with all three types of acne scars, she added.
Combining Treatments
When it comes to injecting dermal fillers into acne scars to lift the depressed areas, the US Food and Drug Administration approved a filler with polymethyl methacrylate filler and bovine collagen (Bellafill) for this indication (moderate to severe, atrophic, distensible facial acne scars on the cheek in patients over age 21) in 2015. “And off-label, I use hyaluronic acid in my practice,” Dr. Siperstein said. Each filler “probably works a little bit better or differently on different types of scars.”
For rolling scars, she recommends hyaluronic acid (HA) dermal filler for everyone. “Of course, this is my opinion.” She was also a lead investigator in a randomized, placebo-controlled split-face study comparing HA filler with saline for correcting atrophic facial scars in 15 patients. The HA filler emerged superior, although there were some improvements with saline.
In her clinical experience, patients are happy with the results and ask, “Why didn’t the last four doctors do this?”
Boxcar scars are more challenging to fill with HA. In some cases, Dr. Siperstein is able to raise the depressed portion of the scar, but some of the vertical edges remain. In this scenario, she might combine treatments. Laser resurfacing, for example, might help convert boxcar scars into rolling scars, which then can be filled more successfully.
“Ice pick scars are tough,” Dr. Siperstein said. A punch removal technique can work in some cases, or she might try the “cross technique.” This involves placing acetic acid inside the scar using a Q-tip. “You have to be really careful,” she added, “because if you get it around the edges, it’s actually going to make the scar bigger.”
Choosing the Right Candidates
Selecting the right candidate for HA treatment of acne scars is essential. Dr. Siperstein shared the example of a lifeguard who had prominent acne scarring down the center of his chest. “He was embarrassed to go to the beach and take off his shirt. He said he felt like he had bullet holes in his chest.”
One month following treatment, “he had a really nice improvement, and now he feels really comfortable,” she said.
Some dermatologists might be reluctant to consider HA fillers for acne scarring because there is a misconception that HA is short-acting, lasting 6 months to 1 year before the effect wears off. That impression can persist from company-sponsored studies that limit follow-up to 6 months or 1 year “to get their drug to market,” she noted.
Also adding to this impression is that HA fillers in wrinkles may not last as long. Dr. Siperstein explained that wrinkles on the face are dynamic and constantly moving. In contrast, acne scars experience less movement, which helps the HA last longer. There is MRI evidence that shows HA fillers last over 2 years in the face, she added.
One tip to predict how well an acne scar might respond to filler injections is to squeeze it and look for the “dimple sign.” If the floor of the scar lifts up when squeezed, “we know that they’ll be a good candidate for hyaluronic acid filler.” Another tip is to inject HA in a retrograde technique high up in the skin. Inject tiny amounts — microdroplets — of the HA filler high on the dermis, she advised.
Deeper injections run the risk of raising the entire scar instead of filling it, she added.
Like many dermatologic procedures, before and after photos are essential to demonstrate improvements, Dr. Siperstein pointed out. Patients are often skeptical. “This happens a lot with acne scar patients. They’ve been to a million places that have promised results, they have not gotten them, and they are frustrated.”
Acne scars can result from picking, inflammation, or treatment. “This is what we see all day in clinic,” Dr. Siperstein said. “Somebody who had to undergo Accutane treatment but unfortunately is left with holes. This is a huge psychological burden on our patients,” she said, describing a younger patient who had scarring, which “led to depression — it was ruining his life.”
“His mom was willing to do whatever it took. And I said, You know what, I think filler will be enough,” Dr. Siperstein said. She counseled them that treatment would not make the scars disappear completely. But patients used to 10% improvements are very happy when their acne scars look 80% or 90% better, she added.
Dr. Siperstein received grant or research support and is a member of the speakers bureau for Allergan and Galderma. She is also a consultant/advisory board member for Allergan.
A version of this article appeared on Medscape.com.
FROM ODAC 2024
Impact of Ketogenic and Low-Glycemic Diets on Inflammatory Skin Conditions
Inflammatory skin conditions often have a relapsing and remitting course and represent a large proportion of chronic skin diseases. Common inflammatory skin disorders include acne, psoriasis, hidradenitis suppurativa (HS), atopic dermatitis (AD), and seborrheic dermatitis (SD).1 Although each of these conditions has a unique pathogenesis, they all are driven by a background of chronic inflammation. It has been reported that diets with high levels of refined carbohydrates and saturated or trans-fatty acids may exacerbate existing inflammation.2 Consequently, dietary interventions, such as the ketogenic and low-glycemic diets, have potential anti-inflammatory and metabolic effects that are being assessed as stand-alone or adjunctive therapies for dermatologic diseases.
Diet may partially influence systemic inflammation through its effect on weight. Higher body mass index and obesity are linked to a low-grade inflammatory state and higher levels of circulating inflammatory markers. Therefore, weight loss leads to decreases in inflammatory cytokines, including C-reactive protein, tumor necrosis factor α, and IL-6.3 These cytokines and metabolic effects overlap with inflammatory skin condition pathways. It also is posited that decreased insulin release associated with weight loss results in decreased sebaceous lipogenesis and androgens, which drive keratinocyte proliferation and acne development.4,5 For instance, in a 2015 meta-analysis of 5 randomized controlled trials on psoriasis, patients in the weight loss intervention group had more substantial reductions in psoriasis area and severity index (PASI) scores compared with controls receiving usual care (P=.004).6 However, in a systematic review of 35 studies on acne vulgaris, overweight and obese patients (defined by a body mass index of ≥23 kg/m2) had similar odds of having acne compared with normal-weight individuals (P=.671).7
Similar to weight loss, ketogenesis acts as a negative feedback mechanism to reduce insulin release, leading to decreased inflammation and androgens that often exacerbate inflammatory skin diseases.8 Ketogenesis ensues when daily carbohydrate intake is limited to less than 50 g, and long-term adherence to a ketogenic diet results in metabolic reliance on ketone bodies such as acetoacetate, β-hydroxybutyrate, and acetone.9 These metabolites may decrease free radical damage and consequently improve signs and symptoms of acne, psoriasis, and other inflammatory skin diseases.10-12 Similarly, increased ketones also may decrease activation of the NLRP3 (NOD-, LRR-, and Pyrin domain-containing protein 3) inflammasome and therefore reduce inflammatory markers such as IL-1β and IL-1.4,13 Several proposed mechanisms are outlined in the Table.

Collectively, low-glycemic and ketogenic diets have been proposed as potential interventions for reducing inflammatory skin conditions. These dietary approaches are hypothesized to exert their effects by facilitating weight loss, elevating ketone levels, and reducing systemic inflammation. The current review summarizes the existing evidence on ketogenic and low-glycemic diets as treatments for inflammatory skin conditions and evaluates the potential benefits of these dietary interventions in managing and improving outcomes for individuals with inflammatory skin conditions.
Methods
Using PubMed for articles indexed for MEDLINE and Google Scholar, a review of the literature was conducted with a combination of the following search terms: low-glycemic diet, inflammatory, dermatologic, ketogenic diet, inflammation, dermatology, acne, psoriasis, eczema, seborrheic dermatitis, and hidradenitis suppurativa. Reference citations in identified works also were reviewed. Interventional (experimental studies or clinical trials), survey-based, and observational studies that investigated the effects of low-glycemic or ketogenic diets for the treatment of inflammatory skin conditions were included. Inclusion criteria were studies assessing acne, psoriasis, SD, AD, and HS. Exclusion criteria were studies published before 1965; those written in languages other than English; and those analyzing other diets, such as the Mediterranean or low-fat diets. The search yielded a total of 11 observational studies and 4 controlled studies published between 1966 and January 2023. Because this analysis utilized publicly available data and did not qualify as human subject research, institutional review board approval was not required.
Results
Acne Vulgaris—Acne vulgaris is a disease of chronic pilosebaceous inflammation and follicular epithelial proliferation associated with Propionibacterium acnes. The association between acne and low-glycemic diets has been examined in several studies. Diet quality is measured and assessed using the glycemic index (GI), which is the effect of a single food on postprandial blood glucose, and the glycemic load, which is the GI adjusted for carbohydrates per serving.14 High levels of GI and glycemic load are associated with hyperinsulinemia and an increase in insulinlike growth factor 1 concentration that promotes
Six survey-based studies evaluated sugar intake in patients with acne compared to healthy matched controls (eTable). Among these studies, 5 reported higher glycemic loads or daily sugar intake in acne patients compared to individuals without acne.12,19,20,26,28 The remaining study was conducted in 1967 and enrolled 16 acne patients and 32 matched controls. It reported no significant difference in sugar intake between the groups (P>.05).17
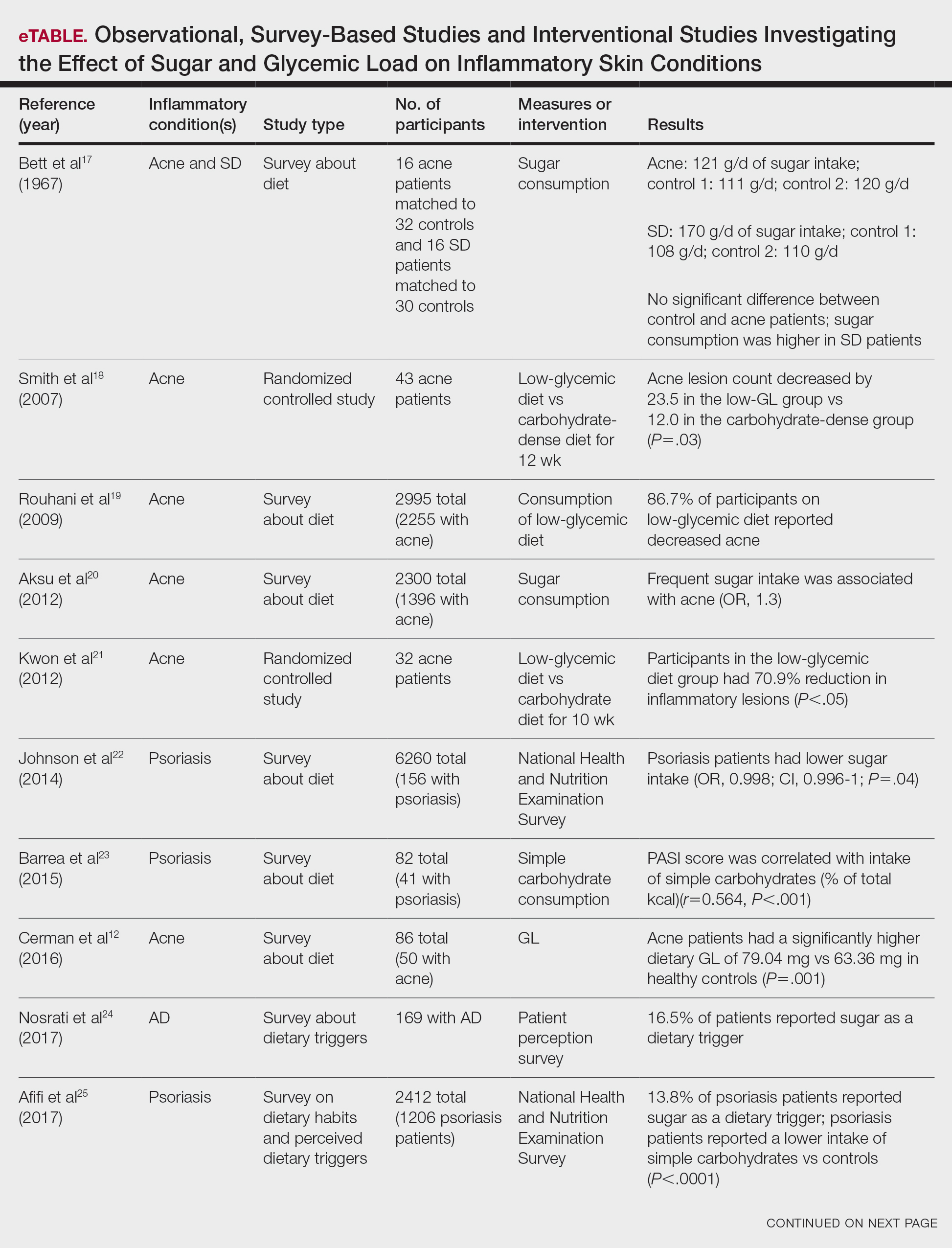

Smith et al18 randomized 43 male patients aged 15 to 25 years with facial acne into 2 cohorts for 12 weeks, each consuming either a low-glycemic diet (25% protein, 45% low-glycemic food [fruits, whole grains], and 30% fat) or a carbohydrate-dense diet of foods with medium to high GI based on prior documentation of the original diet. Patients were instructed to use a noncomedogenic cleanser as their only acne treatment. At 12 weeks, patients consuming the low-glycemic diet had an average of 23.5 fewer inflammatory lesions, while those in the intervention group had 12.0 fewer lesions (P=.03).18
In another controlled study by Kwon et al,21 32 male and female acne patients were randomized to a low-glycemic diet (25% protein, 45% low-glycemic food, and 30% fat) or a standard diet for 10 weeks. Patients on the low-glycemic diet experienced a 70.9% reduction in inflammatory lesions (P<.05). Hematoxylin and eosin staining and image analysis were performed to measure sebaceous gland surface area in the low-glycemic diet group, which decreased from 0.32 to 0.24 mm2 (P=.03). The sebaceous gland surface area in the control group was not reported. Moreover, patients on the low-glycemic diet had reduced IL-8 immunohistochemical staining (decreasing from 2.9 to 1.7 [P=.03]) and sterol regulatory element-binding protein 1 levels (decreasing from 2.6 to 1.3 [P=.03]), suggesting suppression of ongoing inflammation. Patients on the low-glycemic diet had no significant difference in transforming growth factor β1(P=.83). In the control group, there was no difference in IL-8, sterol regulatory element binding protein 1, or transforming growth factor β1 (P>.05) on immunohistochemical staining.21
Psoriasis—Psoriasis is a systemic inflammatory disease characterized by hyperproliferation and aberrant keratinocyte plaque formation. The innate immune response of keratinocytes in response to epidermal damage or infection begins with neutrophil recruitment and dendritic cell activation. Dendritic cell secretion of IL-23 promotes T-cell differentiation into helper T cells (TH1) that subsequently secrete IL-17 and IL-22, thereby stimulating keratinocyte proliferation and eventual plaque formation. The relationship between diet and psoriasis is poorly understood; however, hyperinsulinemia is associated with greater severity of psoriasis.31
Four observational studies examined sugar intake in psoriasis patients. Barrea et al23 conducted a survey-based study of 82 male participants (41 with psoriasis and 41 healthy controls), reporting that PASI score was correlated with intake of simple carbohydrates (percentage of total kilocalorie)(r=0.564, P<.001). Another study by Yamashita et al27 found higher sugar intake in psoriasis patients than controls (P=.003) based on surveys from 70 patients with psoriasis and 70 matched healthy controls.
These findings contrast with 2 survey-based studies by Johnson et al22 and Afifi et al25 of sugar intake in psoriasis patients using the National Health and Nutrition Examination Survey. Johnson et al22 reported reduced sugar intake among 156 psoriasis patients compared with 6104 unmatched controls (odds ratio, 0.998; CI, 0.996-1 [P=.04]) from 2003 to 2006. Similarly, Afifi et al25 reported decreased sugar intake in 1206 psoriasis patients compared with sex- and age-matched controls (P<.0001) in 2009 and 2010. When patients were asked about dietary triggers, 13.8% of psoriasis patients reported sugar as the most common trigger, which was more frequent than alcohol (13.6%), gluten (7.2%), and dairy (6%).25
Castaldo et al29,30 published 2 nonrandomized clinical intervention studies in 2020 and 2021 evaluating the impact of the ketogenic diet on psoriasis. In the first study, 37 psoriasis patients followed a 10-week diet consisting of 4 weeks on a ketogenic diet (500 kcal/d) followed by 6 weeks on a low-caloric Mediterranean diet.29 At the end of the intervention, there was a 17.4% reduction in PASI score, a 33.2-point reduction in itch severity score, and a 13.4-point reduction in the dermatology life quality index score; however, this study did not include a control diet group for comparison.29 The second study included 30 psoriasis patients on a ketogenic diet and 30 control patients without psoriasis on a regular diet.30 The ketogenic diet consisted of 400 to 500 g of vegetables, 20 to 30 g of fat, and a proportion of protein based on body weight with at least 12 g of whey protein and various amino acids. Patients on the ketogenic diet had significant reduction in PASI scores (value relative to clinical features, 1.4916 [P=.007]). Furthermore, concentrations of cytokines IL-2 (P=.04) and IL-1β (P=.006) decreased following the ketogenic diet but were not measured in the control group.30
Seborrheic Dermatitis—Seborrheic dermatitis is associated with overcolonization of Malassezia species near lipid-rich sebaceous glands. Malassezia hydrolyzes free fatty acids, yielding oleic acids and leading to T-cell release of IL-8 and IL-17.32 Literature is sparse regarding how dietary modifications may play a role in disease severity. In a survey study, Bett et al17 compared 16 SD patients to 1:2 matched controls (N=29) to investigate the relationship between sugar consumption and presence of disease. Two control cohorts were selected, 1 from clinic patients diagnosed with verruca and 1 matched by age and sex from a survey-based study at a facility in London, England. Sugar intake was measured both in total grams per day and in “beverage sugar” per day, defined as sugar taken in tea and coffee. There was higher total sugar and higher beverage sugar intake among the SD group compared with both control groups (P<.05).17
Atopic Dermatitis—Atopic dermatitis is a disease of epidermal barrier dysfunction and IgE-mediated allergic sensitization.33 There are several mechanisms by which skin structure may be disrupted. It is well established that filaggrin mutations inhibit stratum corneum maturation and lamellar matrix deposition.34 Upregulation of IL-4–, IL-13–, and IL-17–secreting TH2 cells also is associated with disruption of tight junctions and reduction of filaggrin.35,36 Given that a T cell–mediated inflammatory response is involved in disease pathogenesis, glycemic control is hypothesized to have therapeutic potential.
Nosrati et al24 surveyed 169 AD patients about their perceived dietary triggers through a 61-question survey based on the National Health and Nutrition Examination Survey. Respondents were queried about their perceptions and dietary changes, such as removal or addition of specific food groups and trial of specific diets. Overall, 16.5% of patients reported sugar being a trigger, making it the fourth most common among those surveyed and less common than dairy (24.8%), gluten (18.3%), and alcohol (17.1%).24
Hidradenitis Suppurativa—Hidradenitis suppurativa is driven by hyperkeratosis, dilatation, and occlusion of pilosebaceous follicular ducts, whose eventual rupture evokes a local acute inflammatory response.37 The inciting event for both acne and HS involves mTOR complex–mediated follicular hyperproliferation andinsulinlike growth factor 1 stimulation of androgen receptors in pilosebaceous glands. Given the similarities between the pathogenesis of acne and HS, it is hypothesized that lifestyle changes, including diet modification, may have a beneficial effect on HS.38-40
Comment
Acne—Overall, there is strong evidence supporting the efficacy of a low-glycemic diet in the treatment of acne. Notably, among the 6 observational studies identified, there was 1 conflicting study by Bett et al17 that did not find a statistically significant difference in glucose intake between acne and control patients. However, this study included only 16 acne patients, whereas the other 5 observational studies included 32 to 2255 patients.17 The strongest evidence supporting low-glycemic dietary interventions in acne treatment is from 2 rigorous randomized clinical trials by Kwon et al21 and Smith et al.18 These trials used intention-to-treat models and maintained consistency in gender, age, and acne treatment protocols across both control and treatment groups. To ensure compliance with dietary interventions, daily telephone calls, food logs, and 24-hour urea sampling were utilized. Acne outcomes were assessed by a dermatologist who remained blinded with well-defined outcome measures. An important limitation of these studies is the difficulty in attributing the observed results solely to reduced glucose intake, as low-glycemic diets often lead to other dietary changes, including reduced fat intake and increased nutrient consumption.18,21
A 2022 systematic review of acne by Meixiong et al41 further reinforced the beneficial effects of low-glycemic diets in the management of acne patients. The group reviewed 6 interventional studies and 28 observational studies to investigate the relationship among acne, dairy, and glycemic content and found an association between decreased glucose and dairy on reduction of acne.41
It is likely that the ketogenic diet, which limits glucose, would be beneficial for acne patients. There may be added benefit through elevated ketone bodies and substantially reduced insulin secretion. However, because there are no observational or interventional studies, further research is needed to draw firm conclusions regarding diet for acne treatment. A randomized clinical trial investigating the effects of the ketogenic diet compared to the low-glycemic diet compared to a regular diet would be valuable.
Psoriasis—Among psoriasis studies, there was a lack of consensus regarding glucose intake and correlation with disease. Among the 4 observational studies, 2 reported increased glucose intake among psoriasis patients and 2 reported decreased glucose intake. It is plausible that the variability in studies is due to differences in sample size and diet heterogeneity among study populations. More specifically, Johnson et al22 and Afifi et al25 analyzed large sample sizes of 6260 and 2412 US participants, respectively, and found decreased sugar intake among psoriasis patients compared to controls. In comparison, Barrea et al23 and Yamashita et al27 analyzed substantially smaller and more specific populations consisting of 82 Italian and 140 Japanese participants, respectively; both reported increased glucose intake among psoriasis patients compared to controls. These seemingly antithetical results may be explained by regional dietary differences, with varying proportions of meats, vegetables, antioxidants, and vitamins.
Moreover, the variation among studies may be further explained by the high prevalence of comorbidities among psoriasis patients. In the study by Barrea et al,23 psoriasis patients had higher fasting glucose (P=.004) and insulin (P=.022) levels than healthy patients. After adjusting for body mass index and metabolic syndrome, the correlation coefficient measuring the relationship between the PASI score and intake of simple carbohydrates changed from r=0.564 (P<.001) to r=0.352 (P=.028). The confounding impact of these comorbidities was further highlighted by Yamashita et al,27 who found statistically significant differences in glucose intake between psoriasis and healthy patients (P=.003). However, they reported diminished significance on additional subgroup analysis accounting for potential comorbidities (P=.994).27 Johnson et al22 and Afifi et al25 did not account for comorbidities; therefore, the 4 observational study results must be interpreted cautiously.
The 2 randomized clinical trials by Castaldo et al29,30 weakly suggest that a ketogenic diet may be beneficial for psoriasis patients. The studies have several notable limitations, including insufficient sample sizes and control groups. Thus, the decreased PASI scores reported in psoriasis patients on the ketogenic diets are challenging to interpret. Additionally, both studies placed patients on highly restrictive diets of 500 kcal/d for 4 weeks. The feasibility of recommending such a diet to patients in clinical practice is questionable. Diets of less than 500 kcal/d may be dangerous for patients with underlying comorbidities and are unlikely to serve as long-term solutions.23 To contextualize our findings, a 2022 review by Chung et al42 examined the impact of various diets—low-caloric, gluten-free, Mediterranean, Western, and ketogenic—on psoriasis and reported insufficient evidence to suggest a benefit to the ketogenic diet for psoriasis patients, though the Mediterranean diet may be well suited for psoriasis patients because of improved cardiovascular health and reduced mortality.
Seborrheic Dermatitis—Sanders et al43 found that patients with a high-fruit diet had lower odds of having SD, while those on a Western diet had higher odds of having SD. Although the study did not measure glycemic load, it is conceivable that the high glycemic load characteristic of the Western diet contributed to these findings.43 However, no studies have investigated the direct link between low-glycemic or ketogenic diets and SD, leaving this area open for further study.
Atopic Dermatitis—It has been hypothesized that mitigating T cell–mediated inflammation via glucose control may contribute to the improvement in AD.35,36 However, in one study, 16.5% of AD patients self-identified sugar as a dietary trigger, ranking fourth among other dietary triggers.24 Thus, the connection between glucose levels and AD warrants further exploration.
Hidradenitis Suppurativa—Given the role of metabolic and hormonal influence in HS as well as the overlapping pathophysiology with acne, it is possible that low-glycemic and ketogenic diets may have a role in improving HS.38-40 However, there is a gap in observation and controlled studies investigating the link between low-glycemic or ketogenic diets and HS.
Conclusion
Our analysis focused on interventional and observational research exploring the effects of low-glycemic and ketogenic diets on associations and treatment of inflammatory skin conditions. There is sufficient evidence to counsel acne patients on the benefits of a low-glycemic diet as an adjunctive treatment for acne. Currently, there is insufficient evidence to recommend a low-glycemic or ketogenic diet as a treatment for patients with any other inflammatory skin disease. Prospective and controlled clinical trials are needed to clarify the utility of dietary interventions for treating inflammatory skin conditions.
- Pickett K, Loveman E, Kalita N, et al. Educational interventions to improve quality of life in people with chronic inflammatory skin diseases: systematic reviews of clinical effectiveness and cost-effectiveness. Health Technol Assess. 2015;19:1-176, v-vi.
- Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48:677-685.
- Dowlatshahi EA, van der Voort EA, Arends LR, et al. Markers of systemic inflammation in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2013;169:266-282.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Melnik BC. Acne vulgaris: the metabolic syndrome of the pilosebaceous follicle. Clin Dermatol. 2018;36:29-40.
- Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond). 2015;39:1197-1202.
- Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10:5754.
- Paoli A, Grimaldi K, Toniolo L, et al. Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- Masood W, Annamaraju P, Khan Suheb MZ, et al. Ketogenic diet. StatPearls. StatPearls Publishing; 2023.
- Fomin DA, McDaniel B, Crane J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatolog Treat. 2017;28:484-487.
- Zhang D, Jin W, Wu R, et al. High glucose intake exacerbates autoimmunity through reactive-oxygen-species-mediated TGF-β cytokine activation. Immunity. 2019;51:671-681.e5.
- Cerman AA, Aktas E, Altunay IK, et al. Dietary glycemic factors, insulin resistance, and adiponectin levels in acne vulgaris. J Am Acad Dermatol. 2016;75:155-162.
- Ferrere G, Tidjani Alou M, Liu P, et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight. 2021;6:e145207.
- Burris J, Shikany JM, Rietkerk W, et al. A Low glycemic index and glycemic load diet decreases insulin-like growth factor-1 among adults with moderate and severe acne: a short-duration, 2-week randomized controlled trial. J Acad Nutr Diet. 2018;118:1874-1885.
- Tan JKL, Stein Gold LF, Alexis AF, et al. Current concepts in acne pathogenesis: pathways to inflammation. Semin Cutan Med Surg. 2018;37(3S):S60-S62.
- Kim J, Ochoa MT, Krutzik SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535-1541.
- Bett DG, Morland J, Yudkin J. Sugar consumption in acne vulgaris and seborrhoeic dermatitis. Br Med J. 1967;3:153-155.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Rouhani P, Berman B, Rouhani G. Acne improves with a popular, low glycemic diet from South Beach. J Am Acad Dermatol. 2009;60(Suppl 1):AB14.
- Aksu AE, Metintas S, Saracoglu ZN, et al. Acne: prevalence and relationship with dietary habits in Eskisehir, Turkey. J Eur Acad Dermatol Venereol. 2012;26:1503-1509.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Johnson JA, Ma C, Kanada KN, et al. Diet and nutrition in psoriasis: analysis of the National Health and Nutrition Examination Survey (NHANES) in the United States. J Eur Acad Dermatol Venereol. 2014;28:327-332.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Nosrati A, Afifi L, Danesh MJ, et al. Dietary modifications in atopic dermatitis: patient-reported outcomes. J Dermatolog Treat. 2017;28:523-538.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Burris J, Rietkerk W, Shikany JM, et al. Differences in dietary glycemic load and hormones in New York City adults with no and moderate/severe acne. J Acad Nutr Diet. 2017;117:1375-1383.
- Yamashita H, Morita T, Ito M, et al. Dietary habits in Japanese patients with psoriasis and psoriatic arthritis: low intake of meat in psoriasis and high intake of vitamin A in psoriatic arthritis. J Dermatol. 2019;46:759-769.
- Marson J, Baldwin HE. 12761 Acne, twins, and glycemic index: a sweet pilot study of diet and dietary beliefs. J Am Acad Dermatol. 2020;83(Suppl):AB110.
- Castaldo G, Rastrelli L, Galdo G, et al. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: a proof-of-concept, single-arm, open-label clinical trial. Nutrition. 2020;74:110757.
- Castaldo G, Pagano I, Grimaldi M, et al. Effect of very-low-calorie ketogenic diet on psoriasis patients: a nuclear magnetic resonance-based metabolomic study. J Proteome Res. 2021;20:1509-1521.
- Ip W, Kirchhof MG. Glycemic control in the treatment of psoriasis. Dermatology. 2017;233:23-29.
- Vijaya Chandra SH, Srinivas R, Dawson TL Jr, et al. Cutaneous Malassezia: commensal, pathogen, or protector? Front Cell Infect Microbiol. 2020;10:614446.
- David Boothe W, Tarbox JA, Tarbox MB. Atopic dermatitis: pathophysiology. Adv Exp Med Biol. 2017;1027:21-37.
- Guttman-Yassky E, Hanifin JM, Boguniewicz M, et al. The role of phosphodiesterase 4 in the pathophysiology of atopic dermatitis and the perspective for its inhibition. Exp Dermatol. 2019;28:3-10.
- Furue K, Ito T, Tsuji G, et al. The IL-13–OVOL1–FLG axis in atopic dermatitis. Immunology. 2019;158:281-286.
- Renert-Yuval Y, Guttman-Yassky E. New treatments for atopic dermatitis targeting beyond IL-4/IL-13 cytokines. Ann Allergy Asthma Immunol. 2020;124:28-35.
- Sellheyer K, Krahl D. “Hidradenitis suppurativa” is acne inversa! An appeal to (finally) abandon a misnomer. Int J Dermatol. 2005;44:535-540.
- Danby FW, Margesson LJ. Hidradenitis suppurativa. Dermatol Clin. 2010;28:779-793.
- Fernandez JM, Marr KD, Hendricks AJ, et al. Alleviating and exacerbating foods in hidradenitis suppurativa. Dermatol Ther. 2020;33:E14246.
- Yamanaka-Takaichi M, Revankar R, Shih T, et al. Expert consensus on priority research gaps in dietary and lifestyle factors in hidradenitis suppurativa: a Delphi consensus study. Arch Dermatol Res. 2023;315:2129-2136.
- Meixiong J, Ricco C, Vasavda C, et al. Diet and acne: a systematic review. JAAD Int. 2022;7:95-112.
- Chung M, Bartholomew E, Yeroushalmi S, et al. Dietary intervention and supplements in the management of psoriasis: current perspectives. Psoriasis (Auckland). 2022;12:151-176. doi:10.2147/PTT.S328581
- Sanders MGH, Pardo LM, Ginger RS, et al. Association between diet and seborrheic dermatitis: a cross-sectional study. J Invest Dermatol. 2019;139:108-114.
Inflammatory skin conditions often have a relapsing and remitting course and represent a large proportion of chronic skin diseases. Common inflammatory skin disorders include acne, psoriasis, hidradenitis suppurativa (HS), atopic dermatitis (AD), and seborrheic dermatitis (SD).1 Although each of these conditions has a unique pathogenesis, they all are driven by a background of chronic inflammation. It has been reported that diets with high levels of refined carbohydrates and saturated or trans-fatty acids may exacerbate existing inflammation.2 Consequently, dietary interventions, such as the ketogenic and low-glycemic diets, have potential anti-inflammatory and metabolic effects that are being assessed as stand-alone or adjunctive therapies for dermatologic diseases.
Diet may partially influence systemic inflammation through its effect on weight. Higher body mass index and obesity are linked to a low-grade inflammatory state and higher levels of circulating inflammatory markers. Therefore, weight loss leads to decreases in inflammatory cytokines, including C-reactive protein, tumor necrosis factor α, and IL-6.3 These cytokines and metabolic effects overlap with inflammatory skin condition pathways. It also is posited that decreased insulin release associated with weight loss results in decreased sebaceous lipogenesis and androgens, which drive keratinocyte proliferation and acne development.4,5 For instance, in a 2015 meta-analysis of 5 randomized controlled trials on psoriasis, patients in the weight loss intervention group had more substantial reductions in psoriasis area and severity index (PASI) scores compared with controls receiving usual care (P=.004).6 However, in a systematic review of 35 studies on acne vulgaris, overweight and obese patients (defined by a body mass index of ≥23 kg/m2) had similar odds of having acne compared with normal-weight individuals (P=.671).7
Similar to weight loss, ketogenesis acts as a negative feedback mechanism to reduce insulin release, leading to decreased inflammation and androgens that often exacerbate inflammatory skin diseases.8 Ketogenesis ensues when daily carbohydrate intake is limited to less than 50 g, and long-term adherence to a ketogenic diet results in metabolic reliance on ketone bodies such as acetoacetate, β-hydroxybutyrate, and acetone.9 These metabolites may decrease free radical damage and consequently improve signs and symptoms of acne, psoriasis, and other inflammatory skin diseases.10-12 Similarly, increased ketones also may decrease activation of the NLRP3 (NOD-, LRR-, and Pyrin domain-containing protein 3) inflammasome and therefore reduce inflammatory markers such as IL-1β and IL-1.4,13 Several proposed mechanisms are outlined in the Table.

Collectively, low-glycemic and ketogenic diets have been proposed as potential interventions for reducing inflammatory skin conditions. These dietary approaches are hypothesized to exert their effects by facilitating weight loss, elevating ketone levels, and reducing systemic inflammation. The current review summarizes the existing evidence on ketogenic and low-glycemic diets as treatments for inflammatory skin conditions and evaluates the potential benefits of these dietary interventions in managing and improving outcomes for individuals with inflammatory skin conditions.
Methods
Using PubMed for articles indexed for MEDLINE and Google Scholar, a review of the literature was conducted with a combination of the following search terms: low-glycemic diet, inflammatory, dermatologic, ketogenic diet, inflammation, dermatology, acne, psoriasis, eczema, seborrheic dermatitis, and hidradenitis suppurativa. Reference citations in identified works also were reviewed. Interventional (experimental studies or clinical trials), survey-based, and observational studies that investigated the effects of low-glycemic or ketogenic diets for the treatment of inflammatory skin conditions were included. Inclusion criteria were studies assessing acne, psoriasis, SD, AD, and HS. Exclusion criteria were studies published before 1965; those written in languages other than English; and those analyzing other diets, such as the Mediterranean or low-fat diets. The search yielded a total of 11 observational studies and 4 controlled studies published between 1966 and January 2023. Because this analysis utilized publicly available data and did not qualify as human subject research, institutional review board approval was not required.
Results
Acne Vulgaris—Acne vulgaris is a disease of chronic pilosebaceous inflammation and follicular epithelial proliferation associated with Propionibacterium acnes. The association between acne and low-glycemic diets has been examined in several studies. Diet quality is measured and assessed using the glycemic index (GI), which is the effect of a single food on postprandial blood glucose, and the glycemic load, which is the GI adjusted for carbohydrates per serving.14 High levels of GI and glycemic load are associated with hyperinsulinemia and an increase in insulinlike growth factor 1 concentration that promotes
Six survey-based studies evaluated sugar intake in patients with acne compared to healthy matched controls (eTable). Among these studies, 5 reported higher glycemic loads or daily sugar intake in acne patients compared to individuals without acne.12,19,20,26,28 The remaining study was conducted in 1967 and enrolled 16 acne patients and 32 matched controls. It reported no significant difference in sugar intake between the groups (P>.05).17


Smith et al18 randomized 43 male patients aged 15 to 25 years with facial acne into 2 cohorts for 12 weeks, each consuming either a low-glycemic diet (25% protein, 45% low-glycemic food [fruits, whole grains], and 30% fat) or a carbohydrate-dense diet of foods with medium to high GI based on prior documentation of the original diet. Patients were instructed to use a noncomedogenic cleanser as their only acne treatment. At 12 weeks, patients consuming the low-glycemic diet had an average of 23.5 fewer inflammatory lesions, while those in the intervention group had 12.0 fewer lesions (P=.03).18
In another controlled study by Kwon et al,21 32 male and female acne patients were randomized to a low-glycemic diet (25% protein, 45% low-glycemic food, and 30% fat) or a standard diet for 10 weeks. Patients on the low-glycemic diet experienced a 70.9% reduction in inflammatory lesions (P<.05). Hematoxylin and eosin staining and image analysis were performed to measure sebaceous gland surface area in the low-glycemic diet group, which decreased from 0.32 to 0.24 mm2 (P=.03). The sebaceous gland surface area in the control group was not reported. Moreover, patients on the low-glycemic diet had reduced IL-8 immunohistochemical staining (decreasing from 2.9 to 1.7 [P=.03]) and sterol regulatory element-binding protein 1 levels (decreasing from 2.6 to 1.3 [P=.03]), suggesting suppression of ongoing inflammation. Patients on the low-glycemic diet had no significant difference in transforming growth factor β1(P=.83). In the control group, there was no difference in IL-8, sterol regulatory element binding protein 1, or transforming growth factor β1 (P>.05) on immunohistochemical staining.21
Psoriasis—Psoriasis is a systemic inflammatory disease characterized by hyperproliferation and aberrant keratinocyte plaque formation. The innate immune response of keratinocytes in response to epidermal damage or infection begins with neutrophil recruitment and dendritic cell activation. Dendritic cell secretion of IL-23 promotes T-cell differentiation into helper T cells (TH1) that subsequently secrete IL-17 and IL-22, thereby stimulating keratinocyte proliferation and eventual plaque formation. The relationship between diet and psoriasis is poorly understood; however, hyperinsulinemia is associated with greater severity of psoriasis.31
Four observational studies examined sugar intake in psoriasis patients. Barrea et al23 conducted a survey-based study of 82 male participants (41 with psoriasis and 41 healthy controls), reporting that PASI score was correlated with intake of simple carbohydrates (percentage of total kilocalorie)(r=0.564, P<.001). Another study by Yamashita et al27 found higher sugar intake in psoriasis patients than controls (P=.003) based on surveys from 70 patients with psoriasis and 70 matched healthy controls.
These findings contrast with 2 survey-based studies by Johnson et al22 and Afifi et al25 of sugar intake in psoriasis patients using the National Health and Nutrition Examination Survey. Johnson et al22 reported reduced sugar intake among 156 psoriasis patients compared with 6104 unmatched controls (odds ratio, 0.998; CI, 0.996-1 [P=.04]) from 2003 to 2006. Similarly, Afifi et al25 reported decreased sugar intake in 1206 psoriasis patients compared with sex- and age-matched controls (P<.0001) in 2009 and 2010. When patients were asked about dietary triggers, 13.8% of psoriasis patients reported sugar as the most common trigger, which was more frequent than alcohol (13.6%), gluten (7.2%), and dairy (6%).25
Castaldo et al29,30 published 2 nonrandomized clinical intervention studies in 2020 and 2021 evaluating the impact of the ketogenic diet on psoriasis. In the first study, 37 psoriasis patients followed a 10-week diet consisting of 4 weeks on a ketogenic diet (500 kcal/d) followed by 6 weeks on a low-caloric Mediterranean diet.29 At the end of the intervention, there was a 17.4% reduction in PASI score, a 33.2-point reduction in itch severity score, and a 13.4-point reduction in the dermatology life quality index score; however, this study did not include a control diet group for comparison.29 The second study included 30 psoriasis patients on a ketogenic diet and 30 control patients without psoriasis on a regular diet.30 The ketogenic diet consisted of 400 to 500 g of vegetables, 20 to 30 g of fat, and a proportion of protein based on body weight with at least 12 g of whey protein and various amino acids. Patients on the ketogenic diet had significant reduction in PASI scores (value relative to clinical features, 1.4916 [P=.007]). Furthermore, concentrations of cytokines IL-2 (P=.04) and IL-1β (P=.006) decreased following the ketogenic diet but were not measured in the control group.30
Seborrheic Dermatitis—Seborrheic dermatitis is associated with overcolonization of Malassezia species near lipid-rich sebaceous glands. Malassezia hydrolyzes free fatty acids, yielding oleic acids and leading to T-cell release of IL-8 and IL-17.32 Literature is sparse regarding how dietary modifications may play a role in disease severity. In a survey study, Bett et al17 compared 16 SD patients to 1:2 matched controls (N=29) to investigate the relationship between sugar consumption and presence of disease. Two control cohorts were selected, 1 from clinic patients diagnosed with verruca and 1 matched by age and sex from a survey-based study at a facility in London, England. Sugar intake was measured both in total grams per day and in “beverage sugar” per day, defined as sugar taken in tea and coffee. There was higher total sugar and higher beverage sugar intake among the SD group compared with both control groups (P<.05).17
Atopic Dermatitis—Atopic dermatitis is a disease of epidermal barrier dysfunction and IgE-mediated allergic sensitization.33 There are several mechanisms by which skin structure may be disrupted. It is well established that filaggrin mutations inhibit stratum corneum maturation and lamellar matrix deposition.34 Upregulation of IL-4–, IL-13–, and IL-17–secreting TH2 cells also is associated with disruption of tight junctions and reduction of filaggrin.35,36 Given that a T cell–mediated inflammatory response is involved in disease pathogenesis, glycemic control is hypothesized to have therapeutic potential.
Nosrati et al24 surveyed 169 AD patients about their perceived dietary triggers through a 61-question survey based on the National Health and Nutrition Examination Survey. Respondents were queried about their perceptions and dietary changes, such as removal or addition of specific food groups and trial of specific diets. Overall, 16.5% of patients reported sugar being a trigger, making it the fourth most common among those surveyed and less common than dairy (24.8%), gluten (18.3%), and alcohol (17.1%).24
Hidradenitis Suppurativa—Hidradenitis suppurativa is driven by hyperkeratosis, dilatation, and occlusion of pilosebaceous follicular ducts, whose eventual rupture evokes a local acute inflammatory response.37 The inciting event for both acne and HS involves mTOR complex–mediated follicular hyperproliferation andinsulinlike growth factor 1 stimulation of androgen receptors in pilosebaceous glands. Given the similarities between the pathogenesis of acne and HS, it is hypothesized that lifestyle changes, including diet modification, may have a beneficial effect on HS.38-40
Comment
Acne—Overall, there is strong evidence supporting the efficacy of a low-glycemic diet in the treatment of acne. Notably, among the 6 observational studies identified, there was 1 conflicting study by Bett et al17 that did not find a statistically significant difference in glucose intake between acne and control patients. However, this study included only 16 acne patients, whereas the other 5 observational studies included 32 to 2255 patients.17 The strongest evidence supporting low-glycemic dietary interventions in acne treatment is from 2 rigorous randomized clinical trials by Kwon et al21 and Smith et al.18 These trials used intention-to-treat models and maintained consistency in gender, age, and acne treatment protocols across both control and treatment groups. To ensure compliance with dietary interventions, daily telephone calls, food logs, and 24-hour urea sampling were utilized. Acne outcomes were assessed by a dermatologist who remained blinded with well-defined outcome measures. An important limitation of these studies is the difficulty in attributing the observed results solely to reduced glucose intake, as low-glycemic diets often lead to other dietary changes, including reduced fat intake and increased nutrient consumption.18,21
A 2022 systematic review of acne by Meixiong et al41 further reinforced the beneficial effects of low-glycemic diets in the management of acne patients. The group reviewed 6 interventional studies and 28 observational studies to investigate the relationship among acne, dairy, and glycemic content and found an association between decreased glucose and dairy on reduction of acne.41
It is likely that the ketogenic diet, which limits glucose, would be beneficial for acne patients. There may be added benefit through elevated ketone bodies and substantially reduced insulin secretion. However, because there are no observational or interventional studies, further research is needed to draw firm conclusions regarding diet for acne treatment. A randomized clinical trial investigating the effects of the ketogenic diet compared to the low-glycemic diet compared to a regular diet would be valuable.
Psoriasis—Among psoriasis studies, there was a lack of consensus regarding glucose intake and correlation with disease. Among the 4 observational studies, 2 reported increased glucose intake among psoriasis patients and 2 reported decreased glucose intake. It is plausible that the variability in studies is due to differences in sample size and diet heterogeneity among study populations. More specifically, Johnson et al22 and Afifi et al25 analyzed large sample sizes of 6260 and 2412 US participants, respectively, and found decreased sugar intake among psoriasis patients compared to controls. In comparison, Barrea et al23 and Yamashita et al27 analyzed substantially smaller and more specific populations consisting of 82 Italian and 140 Japanese participants, respectively; both reported increased glucose intake among psoriasis patients compared to controls. These seemingly antithetical results may be explained by regional dietary differences, with varying proportions of meats, vegetables, antioxidants, and vitamins.
Moreover, the variation among studies may be further explained by the high prevalence of comorbidities among psoriasis patients. In the study by Barrea et al,23 psoriasis patients had higher fasting glucose (P=.004) and insulin (P=.022) levels than healthy patients. After adjusting for body mass index and metabolic syndrome, the correlation coefficient measuring the relationship between the PASI score and intake of simple carbohydrates changed from r=0.564 (P<.001) to r=0.352 (P=.028). The confounding impact of these comorbidities was further highlighted by Yamashita et al,27 who found statistically significant differences in glucose intake between psoriasis and healthy patients (P=.003). However, they reported diminished significance on additional subgroup analysis accounting for potential comorbidities (P=.994).27 Johnson et al22 and Afifi et al25 did not account for comorbidities; therefore, the 4 observational study results must be interpreted cautiously.
The 2 randomized clinical trials by Castaldo et al29,30 weakly suggest that a ketogenic diet may be beneficial for psoriasis patients. The studies have several notable limitations, including insufficient sample sizes and control groups. Thus, the decreased PASI scores reported in psoriasis patients on the ketogenic diets are challenging to interpret. Additionally, both studies placed patients on highly restrictive diets of 500 kcal/d for 4 weeks. The feasibility of recommending such a diet to patients in clinical practice is questionable. Diets of less than 500 kcal/d may be dangerous for patients with underlying comorbidities and are unlikely to serve as long-term solutions.23 To contextualize our findings, a 2022 review by Chung et al42 examined the impact of various diets—low-caloric, gluten-free, Mediterranean, Western, and ketogenic—on psoriasis and reported insufficient evidence to suggest a benefit to the ketogenic diet for psoriasis patients, though the Mediterranean diet may be well suited for psoriasis patients because of improved cardiovascular health and reduced mortality.
Seborrheic Dermatitis—Sanders et al43 found that patients with a high-fruit diet had lower odds of having SD, while those on a Western diet had higher odds of having SD. Although the study did not measure glycemic load, it is conceivable that the high glycemic load characteristic of the Western diet contributed to these findings.43 However, no studies have investigated the direct link between low-glycemic or ketogenic diets and SD, leaving this area open for further study.
Atopic Dermatitis—It has been hypothesized that mitigating T cell–mediated inflammation via glucose control may contribute to the improvement in AD.35,36 However, in one study, 16.5% of AD patients self-identified sugar as a dietary trigger, ranking fourth among other dietary triggers.24 Thus, the connection between glucose levels and AD warrants further exploration.
Hidradenitis Suppurativa—Given the role of metabolic and hormonal influence in HS as well as the overlapping pathophysiology with acne, it is possible that low-glycemic and ketogenic diets may have a role in improving HS.38-40 However, there is a gap in observation and controlled studies investigating the link between low-glycemic or ketogenic diets and HS.
Conclusion
Our analysis focused on interventional and observational research exploring the effects of low-glycemic and ketogenic diets on associations and treatment of inflammatory skin conditions. There is sufficient evidence to counsel acne patients on the benefits of a low-glycemic diet as an adjunctive treatment for acne. Currently, there is insufficient evidence to recommend a low-glycemic or ketogenic diet as a treatment for patients with any other inflammatory skin disease. Prospective and controlled clinical trials are needed to clarify the utility of dietary interventions for treating inflammatory skin conditions.
Inflammatory skin conditions often have a relapsing and remitting course and represent a large proportion of chronic skin diseases. Common inflammatory skin disorders include acne, psoriasis, hidradenitis suppurativa (HS), atopic dermatitis (AD), and seborrheic dermatitis (SD).1 Although each of these conditions has a unique pathogenesis, they all are driven by a background of chronic inflammation. It has been reported that diets with high levels of refined carbohydrates and saturated or trans-fatty acids may exacerbate existing inflammation.2 Consequently, dietary interventions, such as the ketogenic and low-glycemic diets, have potential anti-inflammatory and metabolic effects that are being assessed as stand-alone or adjunctive therapies for dermatologic diseases.
Diet may partially influence systemic inflammation through its effect on weight. Higher body mass index and obesity are linked to a low-grade inflammatory state and higher levels of circulating inflammatory markers. Therefore, weight loss leads to decreases in inflammatory cytokines, including C-reactive protein, tumor necrosis factor α, and IL-6.3 These cytokines and metabolic effects overlap with inflammatory skin condition pathways. It also is posited that decreased insulin release associated with weight loss results in decreased sebaceous lipogenesis and androgens, which drive keratinocyte proliferation and acne development.4,5 For instance, in a 2015 meta-analysis of 5 randomized controlled trials on psoriasis, patients in the weight loss intervention group had more substantial reductions in psoriasis area and severity index (PASI) scores compared with controls receiving usual care (P=.004).6 However, in a systematic review of 35 studies on acne vulgaris, overweight and obese patients (defined by a body mass index of ≥23 kg/m2) had similar odds of having acne compared with normal-weight individuals (P=.671).7
Similar to weight loss, ketogenesis acts as a negative feedback mechanism to reduce insulin release, leading to decreased inflammation and androgens that often exacerbate inflammatory skin diseases.8 Ketogenesis ensues when daily carbohydrate intake is limited to less than 50 g, and long-term adherence to a ketogenic diet results in metabolic reliance on ketone bodies such as acetoacetate, β-hydroxybutyrate, and acetone.9 These metabolites may decrease free radical damage and consequently improve signs and symptoms of acne, psoriasis, and other inflammatory skin diseases.10-12 Similarly, increased ketones also may decrease activation of the NLRP3 (NOD-, LRR-, and Pyrin domain-containing protein 3) inflammasome and therefore reduce inflammatory markers such as IL-1β and IL-1.4,13 Several proposed mechanisms are outlined in the Table.

Collectively, low-glycemic and ketogenic diets have been proposed as potential interventions for reducing inflammatory skin conditions. These dietary approaches are hypothesized to exert their effects by facilitating weight loss, elevating ketone levels, and reducing systemic inflammation. The current review summarizes the existing evidence on ketogenic and low-glycemic diets as treatments for inflammatory skin conditions and evaluates the potential benefits of these dietary interventions in managing and improving outcomes for individuals with inflammatory skin conditions.
Methods
Using PubMed for articles indexed for MEDLINE and Google Scholar, a review of the literature was conducted with a combination of the following search terms: low-glycemic diet, inflammatory, dermatologic, ketogenic diet, inflammation, dermatology, acne, psoriasis, eczema, seborrheic dermatitis, and hidradenitis suppurativa. Reference citations in identified works also were reviewed. Interventional (experimental studies or clinical trials), survey-based, and observational studies that investigated the effects of low-glycemic or ketogenic diets for the treatment of inflammatory skin conditions were included. Inclusion criteria were studies assessing acne, psoriasis, SD, AD, and HS. Exclusion criteria were studies published before 1965; those written in languages other than English; and those analyzing other diets, such as the Mediterranean or low-fat diets. The search yielded a total of 11 observational studies and 4 controlled studies published between 1966 and January 2023. Because this analysis utilized publicly available data and did not qualify as human subject research, institutional review board approval was not required.
Results
Acne Vulgaris—Acne vulgaris is a disease of chronic pilosebaceous inflammation and follicular epithelial proliferation associated with Propionibacterium acnes. The association between acne and low-glycemic diets has been examined in several studies. Diet quality is measured and assessed using the glycemic index (GI), which is the effect of a single food on postprandial blood glucose, and the glycemic load, which is the GI adjusted for carbohydrates per serving.14 High levels of GI and glycemic load are associated with hyperinsulinemia and an increase in insulinlike growth factor 1 concentration that promotes
Six survey-based studies evaluated sugar intake in patients with acne compared to healthy matched controls (eTable). Among these studies, 5 reported higher glycemic loads or daily sugar intake in acne patients compared to individuals without acne.12,19,20,26,28 The remaining study was conducted in 1967 and enrolled 16 acne patients and 32 matched controls. It reported no significant difference in sugar intake between the groups (P>.05).17


Smith et al18 randomized 43 male patients aged 15 to 25 years with facial acne into 2 cohorts for 12 weeks, each consuming either a low-glycemic diet (25% protein, 45% low-glycemic food [fruits, whole grains], and 30% fat) or a carbohydrate-dense diet of foods with medium to high GI based on prior documentation of the original diet. Patients were instructed to use a noncomedogenic cleanser as their only acne treatment. At 12 weeks, patients consuming the low-glycemic diet had an average of 23.5 fewer inflammatory lesions, while those in the intervention group had 12.0 fewer lesions (P=.03).18
In another controlled study by Kwon et al,21 32 male and female acne patients were randomized to a low-glycemic diet (25% protein, 45% low-glycemic food, and 30% fat) or a standard diet for 10 weeks. Patients on the low-glycemic diet experienced a 70.9% reduction in inflammatory lesions (P<.05). Hematoxylin and eosin staining and image analysis were performed to measure sebaceous gland surface area in the low-glycemic diet group, which decreased from 0.32 to 0.24 mm2 (P=.03). The sebaceous gland surface area in the control group was not reported. Moreover, patients on the low-glycemic diet had reduced IL-8 immunohistochemical staining (decreasing from 2.9 to 1.7 [P=.03]) and sterol regulatory element-binding protein 1 levels (decreasing from 2.6 to 1.3 [P=.03]), suggesting suppression of ongoing inflammation. Patients on the low-glycemic diet had no significant difference in transforming growth factor β1(P=.83). In the control group, there was no difference in IL-8, sterol regulatory element binding protein 1, or transforming growth factor β1 (P>.05) on immunohistochemical staining.21
Psoriasis—Psoriasis is a systemic inflammatory disease characterized by hyperproliferation and aberrant keratinocyte plaque formation. The innate immune response of keratinocytes in response to epidermal damage or infection begins with neutrophil recruitment and dendritic cell activation. Dendritic cell secretion of IL-23 promotes T-cell differentiation into helper T cells (TH1) that subsequently secrete IL-17 and IL-22, thereby stimulating keratinocyte proliferation and eventual plaque formation. The relationship between diet and psoriasis is poorly understood; however, hyperinsulinemia is associated with greater severity of psoriasis.31
Four observational studies examined sugar intake in psoriasis patients. Barrea et al23 conducted a survey-based study of 82 male participants (41 with psoriasis and 41 healthy controls), reporting that PASI score was correlated with intake of simple carbohydrates (percentage of total kilocalorie)(r=0.564, P<.001). Another study by Yamashita et al27 found higher sugar intake in psoriasis patients than controls (P=.003) based on surveys from 70 patients with psoriasis and 70 matched healthy controls.
These findings contrast with 2 survey-based studies by Johnson et al22 and Afifi et al25 of sugar intake in psoriasis patients using the National Health and Nutrition Examination Survey. Johnson et al22 reported reduced sugar intake among 156 psoriasis patients compared with 6104 unmatched controls (odds ratio, 0.998; CI, 0.996-1 [P=.04]) from 2003 to 2006. Similarly, Afifi et al25 reported decreased sugar intake in 1206 psoriasis patients compared with sex- and age-matched controls (P<.0001) in 2009 and 2010. When patients were asked about dietary triggers, 13.8% of psoriasis patients reported sugar as the most common trigger, which was more frequent than alcohol (13.6%), gluten (7.2%), and dairy (6%).25
Castaldo et al29,30 published 2 nonrandomized clinical intervention studies in 2020 and 2021 evaluating the impact of the ketogenic diet on psoriasis. In the first study, 37 psoriasis patients followed a 10-week diet consisting of 4 weeks on a ketogenic diet (500 kcal/d) followed by 6 weeks on a low-caloric Mediterranean diet.29 At the end of the intervention, there was a 17.4% reduction in PASI score, a 33.2-point reduction in itch severity score, and a 13.4-point reduction in the dermatology life quality index score; however, this study did not include a control diet group for comparison.29 The second study included 30 psoriasis patients on a ketogenic diet and 30 control patients without psoriasis on a regular diet.30 The ketogenic diet consisted of 400 to 500 g of vegetables, 20 to 30 g of fat, and a proportion of protein based on body weight with at least 12 g of whey protein and various amino acids. Patients on the ketogenic diet had significant reduction in PASI scores (value relative to clinical features, 1.4916 [P=.007]). Furthermore, concentrations of cytokines IL-2 (P=.04) and IL-1β (P=.006) decreased following the ketogenic diet but were not measured in the control group.30
Seborrheic Dermatitis—Seborrheic dermatitis is associated with overcolonization of Malassezia species near lipid-rich sebaceous glands. Malassezia hydrolyzes free fatty acids, yielding oleic acids and leading to T-cell release of IL-8 and IL-17.32 Literature is sparse regarding how dietary modifications may play a role in disease severity. In a survey study, Bett et al17 compared 16 SD patients to 1:2 matched controls (N=29) to investigate the relationship between sugar consumption and presence of disease. Two control cohorts were selected, 1 from clinic patients diagnosed with verruca and 1 matched by age and sex from a survey-based study at a facility in London, England. Sugar intake was measured both in total grams per day and in “beverage sugar” per day, defined as sugar taken in tea and coffee. There was higher total sugar and higher beverage sugar intake among the SD group compared with both control groups (P<.05).17
Atopic Dermatitis—Atopic dermatitis is a disease of epidermal barrier dysfunction and IgE-mediated allergic sensitization.33 There are several mechanisms by which skin structure may be disrupted. It is well established that filaggrin mutations inhibit stratum corneum maturation and lamellar matrix deposition.34 Upregulation of IL-4–, IL-13–, and IL-17–secreting TH2 cells also is associated with disruption of tight junctions and reduction of filaggrin.35,36 Given that a T cell–mediated inflammatory response is involved in disease pathogenesis, glycemic control is hypothesized to have therapeutic potential.
Nosrati et al24 surveyed 169 AD patients about their perceived dietary triggers through a 61-question survey based on the National Health and Nutrition Examination Survey. Respondents were queried about their perceptions and dietary changes, such as removal or addition of specific food groups and trial of specific diets. Overall, 16.5% of patients reported sugar being a trigger, making it the fourth most common among those surveyed and less common than dairy (24.8%), gluten (18.3%), and alcohol (17.1%).24
Hidradenitis Suppurativa—Hidradenitis suppurativa is driven by hyperkeratosis, dilatation, and occlusion of pilosebaceous follicular ducts, whose eventual rupture evokes a local acute inflammatory response.37 The inciting event for both acne and HS involves mTOR complex–mediated follicular hyperproliferation andinsulinlike growth factor 1 stimulation of androgen receptors in pilosebaceous glands. Given the similarities between the pathogenesis of acne and HS, it is hypothesized that lifestyle changes, including diet modification, may have a beneficial effect on HS.38-40
Comment
Acne—Overall, there is strong evidence supporting the efficacy of a low-glycemic diet in the treatment of acne. Notably, among the 6 observational studies identified, there was 1 conflicting study by Bett et al17 that did not find a statistically significant difference in glucose intake between acne and control patients. However, this study included only 16 acne patients, whereas the other 5 observational studies included 32 to 2255 patients.17 The strongest evidence supporting low-glycemic dietary interventions in acne treatment is from 2 rigorous randomized clinical trials by Kwon et al21 and Smith et al.18 These trials used intention-to-treat models and maintained consistency in gender, age, and acne treatment protocols across both control and treatment groups. To ensure compliance with dietary interventions, daily telephone calls, food logs, and 24-hour urea sampling were utilized. Acne outcomes were assessed by a dermatologist who remained blinded with well-defined outcome measures. An important limitation of these studies is the difficulty in attributing the observed results solely to reduced glucose intake, as low-glycemic diets often lead to other dietary changes, including reduced fat intake and increased nutrient consumption.18,21
A 2022 systematic review of acne by Meixiong et al41 further reinforced the beneficial effects of low-glycemic diets in the management of acne patients. The group reviewed 6 interventional studies and 28 observational studies to investigate the relationship among acne, dairy, and glycemic content and found an association between decreased glucose and dairy on reduction of acne.41
It is likely that the ketogenic diet, which limits glucose, would be beneficial for acne patients. There may be added benefit through elevated ketone bodies and substantially reduced insulin secretion. However, because there are no observational or interventional studies, further research is needed to draw firm conclusions regarding diet for acne treatment. A randomized clinical trial investigating the effects of the ketogenic diet compared to the low-glycemic diet compared to a regular diet would be valuable.
Psoriasis—Among psoriasis studies, there was a lack of consensus regarding glucose intake and correlation with disease. Among the 4 observational studies, 2 reported increased glucose intake among psoriasis patients and 2 reported decreased glucose intake. It is plausible that the variability in studies is due to differences in sample size and diet heterogeneity among study populations. More specifically, Johnson et al22 and Afifi et al25 analyzed large sample sizes of 6260 and 2412 US participants, respectively, and found decreased sugar intake among psoriasis patients compared to controls. In comparison, Barrea et al23 and Yamashita et al27 analyzed substantially smaller and more specific populations consisting of 82 Italian and 140 Japanese participants, respectively; both reported increased glucose intake among psoriasis patients compared to controls. These seemingly antithetical results may be explained by regional dietary differences, with varying proportions of meats, vegetables, antioxidants, and vitamins.
Moreover, the variation among studies may be further explained by the high prevalence of comorbidities among psoriasis patients. In the study by Barrea et al,23 psoriasis patients had higher fasting glucose (P=.004) and insulin (P=.022) levels than healthy patients. After adjusting for body mass index and metabolic syndrome, the correlation coefficient measuring the relationship between the PASI score and intake of simple carbohydrates changed from r=0.564 (P<.001) to r=0.352 (P=.028). The confounding impact of these comorbidities was further highlighted by Yamashita et al,27 who found statistically significant differences in glucose intake between psoriasis and healthy patients (P=.003). However, they reported diminished significance on additional subgroup analysis accounting for potential comorbidities (P=.994).27 Johnson et al22 and Afifi et al25 did not account for comorbidities; therefore, the 4 observational study results must be interpreted cautiously.
The 2 randomized clinical trials by Castaldo et al29,30 weakly suggest that a ketogenic diet may be beneficial for psoriasis patients. The studies have several notable limitations, including insufficient sample sizes and control groups. Thus, the decreased PASI scores reported in psoriasis patients on the ketogenic diets are challenging to interpret. Additionally, both studies placed patients on highly restrictive diets of 500 kcal/d for 4 weeks. The feasibility of recommending such a diet to patients in clinical practice is questionable. Diets of less than 500 kcal/d may be dangerous for patients with underlying comorbidities and are unlikely to serve as long-term solutions.23 To contextualize our findings, a 2022 review by Chung et al42 examined the impact of various diets—low-caloric, gluten-free, Mediterranean, Western, and ketogenic—on psoriasis and reported insufficient evidence to suggest a benefit to the ketogenic diet for psoriasis patients, though the Mediterranean diet may be well suited for psoriasis patients because of improved cardiovascular health and reduced mortality.
Seborrheic Dermatitis—Sanders et al43 found that patients with a high-fruit diet had lower odds of having SD, while those on a Western diet had higher odds of having SD. Although the study did not measure glycemic load, it is conceivable that the high glycemic load characteristic of the Western diet contributed to these findings.43 However, no studies have investigated the direct link between low-glycemic or ketogenic diets and SD, leaving this area open for further study.
Atopic Dermatitis—It has been hypothesized that mitigating T cell–mediated inflammation via glucose control may contribute to the improvement in AD.35,36 However, in one study, 16.5% of AD patients self-identified sugar as a dietary trigger, ranking fourth among other dietary triggers.24 Thus, the connection between glucose levels and AD warrants further exploration.
Hidradenitis Suppurativa—Given the role of metabolic and hormonal influence in HS as well as the overlapping pathophysiology with acne, it is possible that low-glycemic and ketogenic diets may have a role in improving HS.38-40 However, there is a gap in observation and controlled studies investigating the link between low-glycemic or ketogenic diets and HS.
Conclusion
Our analysis focused on interventional and observational research exploring the effects of low-glycemic and ketogenic diets on associations and treatment of inflammatory skin conditions. There is sufficient evidence to counsel acne patients on the benefits of a low-glycemic diet as an adjunctive treatment for acne. Currently, there is insufficient evidence to recommend a low-glycemic or ketogenic diet as a treatment for patients with any other inflammatory skin disease. Prospective and controlled clinical trials are needed to clarify the utility of dietary interventions for treating inflammatory skin conditions.
- Pickett K, Loveman E, Kalita N, et al. Educational interventions to improve quality of life in people with chronic inflammatory skin diseases: systematic reviews of clinical effectiveness and cost-effectiveness. Health Technol Assess. 2015;19:1-176, v-vi.
- Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48:677-685.
- Dowlatshahi EA, van der Voort EA, Arends LR, et al. Markers of systemic inflammation in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2013;169:266-282.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Melnik BC. Acne vulgaris: the metabolic syndrome of the pilosebaceous follicle. Clin Dermatol. 2018;36:29-40.
- Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond). 2015;39:1197-1202.
- Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10:5754.
- Paoli A, Grimaldi K, Toniolo L, et al. Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- Masood W, Annamaraju P, Khan Suheb MZ, et al. Ketogenic diet. StatPearls. StatPearls Publishing; 2023.
- Fomin DA, McDaniel B, Crane J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatolog Treat. 2017;28:484-487.
- Zhang D, Jin W, Wu R, et al. High glucose intake exacerbates autoimmunity through reactive-oxygen-species-mediated TGF-β cytokine activation. Immunity. 2019;51:671-681.e5.
- Cerman AA, Aktas E, Altunay IK, et al. Dietary glycemic factors, insulin resistance, and adiponectin levels in acne vulgaris. J Am Acad Dermatol. 2016;75:155-162.
- Ferrere G, Tidjani Alou M, Liu P, et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight. 2021;6:e145207.
- Burris J, Shikany JM, Rietkerk W, et al. A Low glycemic index and glycemic load diet decreases insulin-like growth factor-1 among adults with moderate and severe acne: a short-duration, 2-week randomized controlled trial. J Acad Nutr Diet. 2018;118:1874-1885.
- Tan JKL, Stein Gold LF, Alexis AF, et al. Current concepts in acne pathogenesis: pathways to inflammation. Semin Cutan Med Surg. 2018;37(3S):S60-S62.
- Kim J, Ochoa MT, Krutzik SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535-1541.
- Bett DG, Morland J, Yudkin J. Sugar consumption in acne vulgaris and seborrhoeic dermatitis. Br Med J. 1967;3:153-155.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Rouhani P, Berman B, Rouhani G. Acne improves with a popular, low glycemic diet from South Beach. J Am Acad Dermatol. 2009;60(Suppl 1):AB14.
- Aksu AE, Metintas S, Saracoglu ZN, et al. Acne: prevalence and relationship with dietary habits in Eskisehir, Turkey. J Eur Acad Dermatol Venereol. 2012;26:1503-1509.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Johnson JA, Ma C, Kanada KN, et al. Diet and nutrition in psoriasis: analysis of the National Health and Nutrition Examination Survey (NHANES) in the United States. J Eur Acad Dermatol Venereol. 2014;28:327-332.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Nosrati A, Afifi L, Danesh MJ, et al. Dietary modifications in atopic dermatitis: patient-reported outcomes. J Dermatolog Treat. 2017;28:523-538.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Burris J, Rietkerk W, Shikany JM, et al. Differences in dietary glycemic load and hormones in New York City adults with no and moderate/severe acne. J Acad Nutr Diet. 2017;117:1375-1383.
- Yamashita H, Morita T, Ito M, et al. Dietary habits in Japanese patients with psoriasis and psoriatic arthritis: low intake of meat in psoriasis and high intake of vitamin A in psoriatic arthritis. J Dermatol. 2019;46:759-769.
- Marson J, Baldwin HE. 12761 Acne, twins, and glycemic index: a sweet pilot study of diet and dietary beliefs. J Am Acad Dermatol. 2020;83(Suppl):AB110.
- Castaldo G, Rastrelli L, Galdo G, et al. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: a proof-of-concept, single-arm, open-label clinical trial. Nutrition. 2020;74:110757.
- Castaldo G, Pagano I, Grimaldi M, et al. Effect of very-low-calorie ketogenic diet on psoriasis patients: a nuclear magnetic resonance-based metabolomic study. J Proteome Res. 2021;20:1509-1521.
- Ip W, Kirchhof MG. Glycemic control in the treatment of psoriasis. Dermatology. 2017;233:23-29.
- Vijaya Chandra SH, Srinivas R, Dawson TL Jr, et al. Cutaneous Malassezia: commensal, pathogen, or protector? Front Cell Infect Microbiol. 2020;10:614446.
- David Boothe W, Tarbox JA, Tarbox MB. Atopic dermatitis: pathophysiology. Adv Exp Med Biol. 2017;1027:21-37.
- Guttman-Yassky E, Hanifin JM, Boguniewicz M, et al. The role of phosphodiesterase 4 in the pathophysiology of atopic dermatitis and the perspective for its inhibition. Exp Dermatol. 2019;28:3-10.
- Furue K, Ito T, Tsuji G, et al. The IL-13–OVOL1–FLG axis in atopic dermatitis. Immunology. 2019;158:281-286.
- Renert-Yuval Y, Guttman-Yassky E. New treatments for atopic dermatitis targeting beyond IL-4/IL-13 cytokines. Ann Allergy Asthma Immunol. 2020;124:28-35.
- Sellheyer K, Krahl D. “Hidradenitis suppurativa” is acne inversa! An appeal to (finally) abandon a misnomer. Int J Dermatol. 2005;44:535-540.
- Danby FW, Margesson LJ. Hidradenitis suppurativa. Dermatol Clin. 2010;28:779-793.
- Fernandez JM, Marr KD, Hendricks AJ, et al. Alleviating and exacerbating foods in hidradenitis suppurativa. Dermatol Ther. 2020;33:E14246.
- Yamanaka-Takaichi M, Revankar R, Shih T, et al. Expert consensus on priority research gaps in dietary and lifestyle factors in hidradenitis suppurativa: a Delphi consensus study. Arch Dermatol Res. 2023;315:2129-2136.
- Meixiong J, Ricco C, Vasavda C, et al. Diet and acne: a systematic review. JAAD Int. 2022;7:95-112.
- Chung M, Bartholomew E, Yeroushalmi S, et al. Dietary intervention and supplements in the management of psoriasis: current perspectives. Psoriasis (Auckland). 2022;12:151-176. doi:10.2147/PTT.S328581
- Sanders MGH, Pardo LM, Ginger RS, et al. Association between diet and seborrheic dermatitis: a cross-sectional study. J Invest Dermatol. 2019;139:108-114.
- Pickett K, Loveman E, Kalita N, et al. Educational interventions to improve quality of life in people with chronic inflammatory skin diseases: systematic reviews of clinical effectiveness and cost-effectiveness. Health Technol Assess. 2015;19:1-176, v-vi.
- Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48:677-685.
- Dowlatshahi EA, van der Voort EA, Arends LR, et al. Markers of systemic inflammation in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2013;169:266-282.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Melnik BC. Acne vulgaris: the metabolic syndrome of the pilosebaceous follicle. Clin Dermatol. 2018;36:29-40.
- Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond). 2015;39:1197-1202.
- Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10:5754.
- Paoli A, Grimaldi K, Toniolo L, et al. Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- Masood W, Annamaraju P, Khan Suheb MZ, et al. Ketogenic diet. StatPearls. StatPearls Publishing; 2023.
- Fomin DA, McDaniel B, Crane J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatolog Treat. 2017;28:484-487.
- Zhang D, Jin W, Wu R, et al. High glucose intake exacerbates autoimmunity through reactive-oxygen-species-mediated TGF-β cytokine activation. Immunity. 2019;51:671-681.e5.
- Cerman AA, Aktas E, Altunay IK, et al. Dietary glycemic factors, insulin resistance, and adiponectin levels in acne vulgaris. J Am Acad Dermatol. 2016;75:155-162.
- Ferrere G, Tidjani Alou M, Liu P, et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight. 2021;6:e145207.
- Burris J, Shikany JM, Rietkerk W, et al. A Low glycemic index and glycemic load diet decreases insulin-like growth factor-1 among adults with moderate and severe acne: a short-duration, 2-week randomized controlled trial. J Acad Nutr Diet. 2018;118:1874-1885.
- Tan JKL, Stein Gold LF, Alexis AF, et al. Current concepts in acne pathogenesis: pathways to inflammation. Semin Cutan Med Surg. 2018;37(3S):S60-S62.
- Kim J, Ochoa MT, Krutzik SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535-1541.
- Bett DG, Morland J, Yudkin J. Sugar consumption in acne vulgaris and seborrhoeic dermatitis. Br Med J. 1967;3:153-155.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Rouhani P, Berman B, Rouhani G. Acne improves with a popular, low glycemic diet from South Beach. J Am Acad Dermatol. 2009;60(Suppl 1):AB14.
- Aksu AE, Metintas S, Saracoglu ZN, et al. Acne: prevalence and relationship with dietary habits in Eskisehir, Turkey. J Eur Acad Dermatol Venereol. 2012;26:1503-1509.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Johnson JA, Ma C, Kanada KN, et al. Diet and nutrition in psoriasis: analysis of the National Health and Nutrition Examination Survey (NHANES) in the United States. J Eur Acad Dermatol Venereol. 2014;28:327-332.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Nosrati A, Afifi L, Danesh MJ, et al. Dietary modifications in atopic dermatitis: patient-reported outcomes. J Dermatolog Treat. 2017;28:523-538.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Burris J, Rietkerk W, Shikany JM, et al. Differences in dietary glycemic load and hormones in New York City adults with no and moderate/severe acne. J Acad Nutr Diet. 2017;117:1375-1383.
- Yamashita H, Morita T, Ito M, et al. Dietary habits in Japanese patients with psoriasis and psoriatic arthritis: low intake of meat in psoriasis and high intake of vitamin A in psoriatic arthritis. J Dermatol. 2019;46:759-769.
- Marson J, Baldwin HE. 12761 Acne, twins, and glycemic index: a sweet pilot study of diet and dietary beliefs. J Am Acad Dermatol. 2020;83(Suppl):AB110.
- Castaldo G, Rastrelli L, Galdo G, et al. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: a proof-of-concept, single-arm, open-label clinical trial. Nutrition. 2020;74:110757.
- Castaldo G, Pagano I, Grimaldi M, et al. Effect of very-low-calorie ketogenic diet on psoriasis patients: a nuclear magnetic resonance-based metabolomic study. J Proteome Res. 2021;20:1509-1521.
- Ip W, Kirchhof MG. Glycemic control in the treatment of psoriasis. Dermatology. 2017;233:23-29.
- Vijaya Chandra SH, Srinivas R, Dawson TL Jr, et al. Cutaneous Malassezia: commensal, pathogen, or protector? Front Cell Infect Microbiol. 2020;10:614446.
- David Boothe W, Tarbox JA, Tarbox MB. Atopic dermatitis: pathophysiology. Adv Exp Med Biol. 2017;1027:21-37.
- Guttman-Yassky E, Hanifin JM, Boguniewicz M, et al. The role of phosphodiesterase 4 in the pathophysiology of atopic dermatitis and the perspective for its inhibition. Exp Dermatol. 2019;28:3-10.
- Furue K, Ito T, Tsuji G, et al. The IL-13–OVOL1–FLG axis in atopic dermatitis. Immunology. 2019;158:281-286.
- Renert-Yuval Y, Guttman-Yassky E. New treatments for atopic dermatitis targeting beyond IL-4/IL-13 cytokines. Ann Allergy Asthma Immunol. 2020;124:28-35.
- Sellheyer K, Krahl D. “Hidradenitis suppurativa” is acne inversa! An appeal to (finally) abandon a misnomer. Int J Dermatol. 2005;44:535-540.
- Danby FW, Margesson LJ. Hidradenitis suppurativa. Dermatol Clin. 2010;28:779-793.
- Fernandez JM, Marr KD, Hendricks AJ, et al. Alleviating and exacerbating foods in hidradenitis suppurativa. Dermatol Ther. 2020;33:E14246.
- Yamanaka-Takaichi M, Revankar R, Shih T, et al. Expert consensus on priority research gaps in dietary and lifestyle factors in hidradenitis suppurativa: a Delphi consensus study. Arch Dermatol Res. 2023;315:2129-2136.
- Meixiong J, Ricco C, Vasavda C, et al. Diet and acne: a systematic review. JAAD Int. 2022;7:95-112.
- Chung M, Bartholomew E, Yeroushalmi S, et al. Dietary intervention and supplements in the management of psoriasis: current perspectives. Psoriasis (Auckland). 2022;12:151-176. doi:10.2147/PTT.S328581
- Sanders MGH, Pardo LM, Ginger RS, et al. Association between diet and seborrheic dermatitis: a cross-sectional study. J Invest Dermatol. 2019;139:108-114.
Practice Points
- As the ketogenic diet gains in popularity, dermatologists may inform patients that there is emerging evidence supporting the idea that low-glycemic diets may contribute to improvement in inflammatory skin conditions.
- Dermatologists may educate patients about the potential benefits of a low-glycemic diet as a supplementary treatment for acne based on existing evidence.
- Current evidence is insufficient to endorse a ketogenic diet as superior to other dietary approaches in treating inflammatory skin conditions.
Acne and Pregnancy: A Clinical Review and Practice Pearls
Acne vulgaris, or acne, is a highly common inflammatory skin disorder affecting up to 85% of the population, and it constitutes the most commonly presenting chief concern in routine dermatology practice.1 Older teenagers and young adults are most often affected by acne.2 Although acne generally is more common in males, adult-onset acne occurs more frequently in women.2,3 Black and Hispanic women are at higher risk for acne compared to those of Asian, White, or Continental Indian descent.4 As such, acne is a common concern in all women of childbearing age.
Concerns for maternal and fetal safety are important therapeutic considerations, especially because hormonal and physiologic changes in pregnancy can lead to onset of inflammatory acne lesions, particularly during the second and third trimesters.5 Female patients younger than 25 years; with a higher body mass index, prior irregular menstruation, or polycystic ovary syndrome; or those experiencing their first pregnancy are thought to be more commonly affected.5-7 In fact, acne affects up to 43% of pregnant women, and lesions typically extend beyond the face to involve the trunk.6,8-10 Importantly, one-third of women with a history of acne experience symptom relapse after disease-free periods, while two-thirds of those with ongoing disease experience symptom deterioration during pregnancy.10 Although acne is not a life-threatening condition, it has a well-documented, detrimental impact on social, emotional, and psychological well-being, namely self-perception, social interactions, quality-of-life scores, depression, and anxiety.11
Therefore, safe and effective treatment of pregnant women is of paramount importance. Because pregnant women are not included in clinical trials, there is a paucity of medication safety data, further augmented by inefficient access to available information. The US Food and Drug Administration (FDA) pregnancy safety categories were updated in 2015, letting go of the traditional A, B, C, D, and X categories.12 The Table reviews the current pregnancy classification system. In this narrative review, we summarize the most recent available data and recommendations on the safety and efficacy of acne treatment during pregnancy.

Topical Treatments for Acne
Benzoyl Peroxide—Benzoyl peroxide commonly is used as first-line therapy alone or in combination with other agents for the treatment of mild to moderate acne.13 It is safe for use during pregnancy.14 Although the medication is systemically absorbed, it undergoes complete metabolism to benzoic acid, a commonly used food additive.15,16 Benzoic acid has low bioavailability, as it gets rapidly metabolized by the kidneys; therefore, benzoyl peroxide is unlikely to reach clinically significant levels in the maternal circulation and consequently the fetal circulation. Additionally, it has a low risk for causing congenital malformations.17
Salicylic Acid—For mild to moderate acne, salicylic acid is a second-line agent that likely is safe for use by pregnant women at low concentrations and over limited body surface areas.14,18,19 There is minimal systemic absorption of the drug.20 Additionally, aspirin, which is broken down in the body into salicylic acid, is used in low doses for the treatment of pre-eclampsia during pregnancy.21
Dapsone—The use of dapsone gel 5% as a second-line agent has shown efficacy for mild to moderate acne.22 The oral formulation, commonly used for malaria and leprosy prophylaxis, has failed to show associated fetal toxicity or congenital anomalies.23,24 It also has been used as a first-line treatment for dermatitis herpetiformis in pregnancy.25 Although the medication likely is safe, it is better to minimize its use during the third trimester to reduce the theoretical risk for hyperbilirubinemia in the neonate.17,26-29
Azelaic Acid—Azelaic acid effectively targets noninflammatory and inflammatory acne and generally is well tolerated, harboring a good safety profile.30 Topical 20% azelaic acid has localized antibacterial and comedolytic effects and is safe for use during pregnancy.31,32
Glycolic Acid—Limited data exist on the safety of glycolic acid during pregnancy. In vitro studies have shown up to 27% systemic absorption depending on pH, concentration, and duration of application.33 Animal reproductive studies involving rats have shown fetal multisystem malformations and developmental abnormalities with oral administration of glycolic acid at doses far exceeding those used in humans.34 Although no human reproductive studies exist, topical glycolic acid is unlikely to reach the developing fetus in notable amounts, and the medication is likely safe for use.17,35
Clindamycin—Topical clindamycin phosphate is an effective and well-tolerated agent for the treatment of mild to moderate acne.36 Its systemic absorption is minimal, and it is considered safe for use during all trimesters of pregnancy.14,17,26,27,35,37
Erythromycin—Topical erythromycin is another commonly prescribed topical antibiotic used to target mild to moderate acne. However, its use recently has been associated with a decrease in efficacy secondary to the rise of antibacterial resistance in the community.38-40 Nevertheless, it remains a safe treatment for use during all trimesters of pregnancy.14,17,26,27,35,37
Topical Retinoids—Vitamin A derivatives (also known as retinoids) are the mainstay for the treatment of mild to moderate acne. Limited data exist regarding pregnancy outcomes after in utero exposure.41 A rare case report suggested topical tretinoin has been associated with fetal otocerebral anomalies.42 For tazarotene, teratogenic effects were seen in animal reproductive studies at doses exceeding maximum recommended human doses.41,43 However, a large meta-analysis failed to find a clear risk for increased congenital malformations, spontaneous abortions, stillbirth, elective termination of pregnancy, low birthweight, or prematurity following first-trimester exposure to topical retinoids.44 As the level of exposure that could lead to teratogenicity in humans is unknown, avoidance of both tretinoin and tazarotene is recommended in pregnant women.41,45 Nevertheless, women inadvertently exposed should be reassured.44
Conversely, adapalene has been associated with 1 case of anophthalmia and agenesis of the optic chiasma in a fetus following exposure until 13 weeks’ gestation.46 However, a large, open-label trial prior to the patient transitioning from adapalene to over-the-counter treatment showed that the drug harbors a large and reassuring margin of safety and no risk for teratogenicity in a maximal usage trial and Pregnancy Safety Review.47 Therefore, adapalene gel 0.1% is a safe and effective medication for the treatment of acne in a nonprescription environment and does not pose harm to the fetus.
Clascoterone—Clascoterone is a novel topical antiandrogenic drug approved for the treatment of hormonal and inflammatory moderate to severe acne.48-51 Human reproductive data are limited to 1 case of pregnancy that occurred during phase 3 trial investigations, and no adverse outcomes were reported.51 Minimal systemic absorption follows topical use.52 Nonetheless, dose-independent malformations were reported in animal reproductive studies.53 As such, it remains better to avoid the use of clascoterone during pregnancy pending further safety data.
Minocycline Foam—Minocycline foam 4% is approved to treat inflammatory lesions of nonnodular moderate to severe acne in patients 9 years and older.54 Systemic absorption is minimal, and the drug has limited bioavailability with minimal systemic accumulation in the patient’s serum.55 Given this information, it is unlikely that topical minocycline will reach notable levels in the fetal serum or harbor teratogenic effects, as seen with the oral formulation.56 However, it may be best to avoid its use during the second and third trimesters given the potential risk for tooth discoloration in the fetus.57,58
Systemic Treatments for Acne
Isotretinoin—Isotretinoin is the most effective treatment for moderate to severe acne with a well-documented potential for long-term clearance.59 Its use during pregnancy is absolutely contraindicated, as the medication is a well-known teratogen. Associated congenital malformations include numerous craniofacial defects, cardiovascular and neurologic malformations, or thymic disorders that are estimated to affect 20% to 35% of infants exposed in utero.60 Furthermore, strict contraception use during treatment is mandated for patients who can become pregnant. It is recommended to wait at least 1 month and 1 menstrual cycle after medication discontinuation before attempting to conceive.17 Pregnancy termination is recommended if conception occurs during treatment with isotretinoin.
Spironolactone—Spironolactone is an androgen-receptor antagonist commonly prescribed off label for mild to severe acne in females.61,62 Spironolactone promotes the feminization of male fetuses and should be avoided in pregnancy.63
Doxycycline/Minocycline—Tetracyclines are the most commonly prescribed oral antibiotics for moderate to severe acne.64 Although highly effective at treating acne, tetracyclines generally should be avoided in pregnancy. First-trimester use of doxycycline is not absolutely contraindicated but should be reserved for severe illness and not employed for the treatment of acne. However, accidental exposure to doxycycline has not been associated with congenital malformations.65 Nevertheless, after the 15th week of gestation, permanent tooth discoloration and bone growth inhibition in the fetus are serious and well-documented risks.14,17 Additional adverse events following in utero exposure include infantile inguinal hernia, hypospadias, and limb hypoplasia.63
Sarecycline—Sarecycline is a novel tetracycline-class antibiotic for the treatment of moderate to severe inflammatory acne. It has a narrower spectrum of activity compared to its counterparts within its class, which translates to an improved safety profile, namely when it comes to gastrointestinal tract microbiome disruption and potentially decreased likelihood of developing bacterial resistance.66 Data on human reproductive studies are limited, but it is advisable to avoid sarecycline in pregnancy, as it may cause adverse developmental effects in the fetus, such as reduced bone growth, in addition to the well-known tetracycline-associated risk for permanent discoloration of the teeth if used during the second and third trimesters.67,68
Erythromycin—Oral erythromycin targets moderate to severe inflammatory acne and is considered safe for use during pregnancy.69,70 There has been 1 study reporting an increased risk for atrial and ventricular septal defects (1.8%) and pyloric stenosis (0.2%), but these risks are still uncertain, and erythromycin is considered compatible with pregnancy.71 However, erythromycin estolate formulations should be avoided given the associated 10% to 15% risk for reversible cholestatic liver injury.72 Erythromycin base or erythromycin ethylsuccinate formulations should be favored.
Systemic Steroids—Prednisone is indicated for severe acne with scarring and should only be used during pregnancy after clearance from the patient’s obstetrician. Doses of 0.5 mg/kg or less should be prescribed in combination with systemic antibiotics as well as agents for bone and gastrointestinal tract prophylaxis.29
Zinc—The exact mechanism by which zinc exerts its effects to improve acne remains largely obscure. It has been found effective against inflammatory lesions of mild to moderate acne.73 Generally recommended dosages range from 30 to 200 mg/d but may be associated with gastrointestinal tract disturbances. Dosages of 75 mg/d have shown no harm to the fetus.74 When taking this supplement, patients should not exceed the recommended doses given the risk for hypocupremia associated with high-dose zinc supplementation.
Light-Based Therapies
Phototherapy—Narrowband UVB phototherapy is effective for the treatment of mild to moderate acne.75 It has been proven to be a safe treatment option during pregnancy, but its use has been associated with decreased folic acid levels.76-79 Therefore, in addition to attaining baseline folic acid serum levels, supplementation with folic acid prior to treatment, as per routine prenatal guidelines, should be sought.80
AviClear—The AviClear (Cutera) laser is the first device cleared by the FDA for mild to severe acne in March 2022.81 The FDA clearance for the Accure (Accure Acne Inc) laser, also targeting mild to severe acne, followed soon after (November 2022). Both lasers harbor a wavelength of 1726 nm and target sebaceous glands with electrothermolysis.82,83 Further research and long-term safety data are required before using them in pregnancy.
Other Therapies
Cosmetic Peels—Glycolic acid peels induce epidermolysis and desquamation.84 Although data on use during pregnancy are limited, these peels have limited dermal penetration and are considered safe for use in pregnancy.33,85,86 Similarly, keratolytic lactic acid peels harbor limited dermal penetration and can be safely used in pregnant women.87-89 Salicylic acid peels also work through epidermolysis and desquamation84; however, they tend to penetrate deeper into the skin, reaching down to the basal layer, if large areas are treated or when applied under occlusion.86,90 Although their use is not contraindicated in pregnancy, they should be limited to small areas of coverage.91
Intralesional Triamcinolone—Acne cysts and inflammatory papules can be treated with intralesional triamcinolone injections to relieve acute symptoms such as pain.92 Low doses at concentrations of 2.5 mg/mL are considered compatible with pregnancy when indicated.29
Approaching the Patient Clinical Encounter
In patients seeking treatment prior to conception, a few recommendations can be made to minimize the risk for acne recurrence or flares during pregnancy. For instance, because data show an association between increased acne severity in those with a higher body mass index and in pregnancy, weight loss may be recommended prior to pregnancy to help mitigate symptoms after conception.7 The Figure summarizes our recommendations for approaching and treating acne in pregnancy.
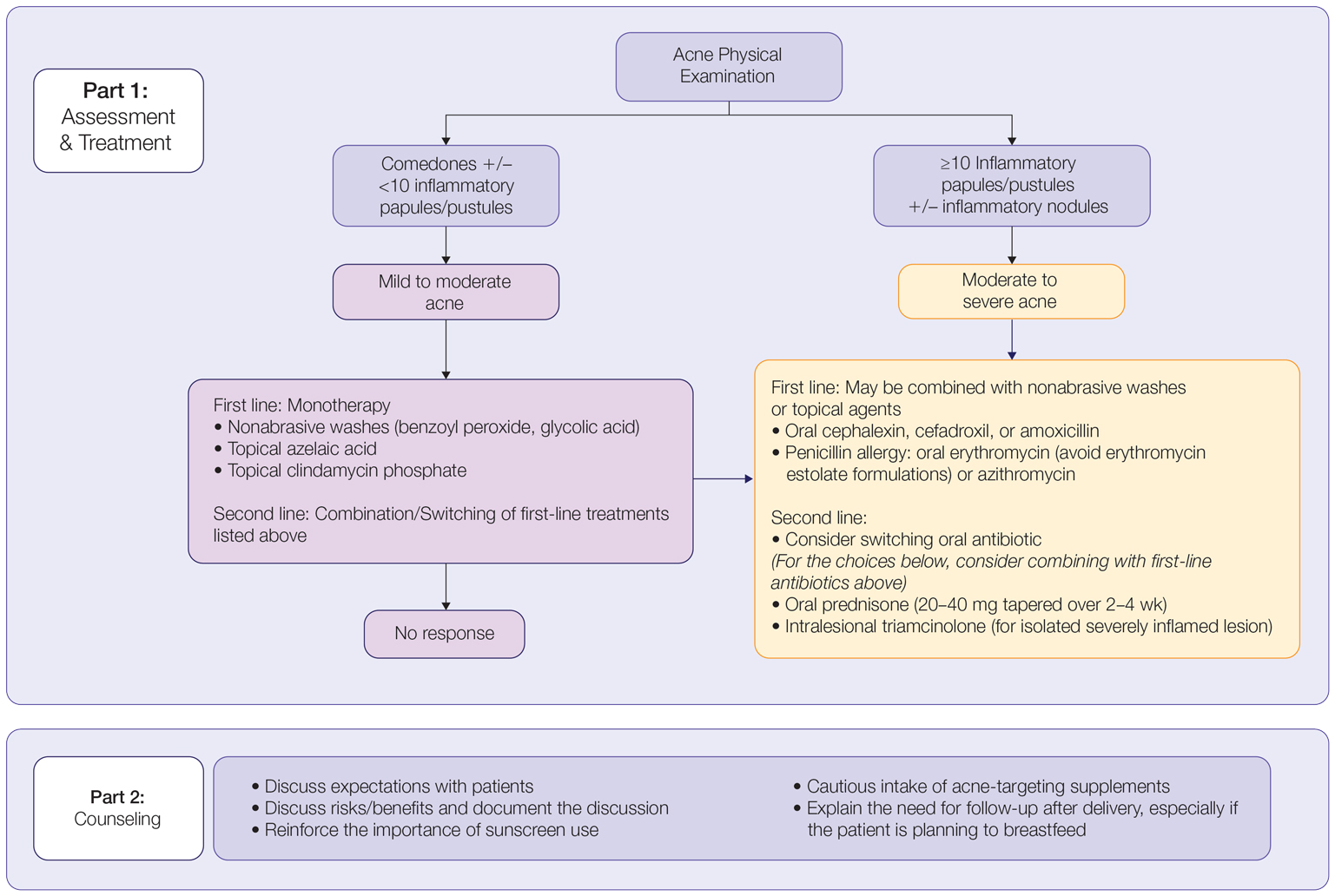
In all patients, grading the severity of the patient’s acne as mild, moderate, or severe is the first step. The presence of scarring is an additional consideration during the physical examination and should be documented. A careful discussion of treatment expectations and prognosis should be the focus before treatment initiation. Meticulous documentation of the physical examination and discussion with the patient should be prioritized.
To minimize toxicity and risks to the developing fetus, monotherapy is favored. Topical therapy should be considered first line. Safe regimens include mild nonabrasive washes, such as those containing benzoyl peroxide or glycolic acid, or topical azelaic acid or clindamycin phosphate for mild to moderate acne. More severe cases warrant the consideration of systemic medications as second line, as more severe acne is better treated with oral antibiotics such as the macrolides erythromycin or clindamycin or systemic corticosteroids when concern exists for severe scarring. The additional use of physical sunscreen also is recommended.
An important topic to address during the clinical encounter is cautious intake of oral supplements for acne during pregnancy, as they may contain harmful and teratogenic ingredients. A recent search focusing on acne supplements available online between March and May 2020 uncovered 49 different supplements, 26 (53%) of which contained vitamin A.93 Importantly, 3 (6%) of these 49 supplements were likely teratogenic, 4 (8%) contained vitamin A doses exceeding the recommended daily nutritional intake level, and 15 (31%) harbored an unknown teratogenic risk. Furthermore, among the 6 (12%) supplements with vitamin A levels exceeding 10,000 IU, 2 lacked any mention of pregnancy warning, including the supplement with the highest vitamin A dose found in this study.93 Because dietary supplements are not subject to the same stringent regulations by the FDA as drugs, inadvertent use by unaware patients ought to be prevented by careful counseling and education.
Finally, patients should be counseled to seek care following delivery for potentially updated medication management of acne, especially if they are breastfeeding. Co-management with a pediatrician may be indicated during lactation, particularly when newborns are born preterm or with other health conditions that may warrant additional caution with the use of certain agents.
- Bhate K, Williams H. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485.
- Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10:5754.
- Fisk WA, Lev-Tov HA, Sivamani RK. Epidemiology and management of acne in adult women. Curr Dermatol Rep. 2014;3:29-39.
- Perkins A, Cheng C, Hillebrand G, et al. Comparison of the epidemiology of acne vulgaris among Caucasian, Asian, Continental Indian and African American women. J Eur Acad Dermatol Venereol. 2011;25:1054-1060.
- Yang CC, Huang YT, Yu CH, et al. Inflammatory facial acne during uncomplicated pregnancy and post‐partum in adult women: a preliminary hospital‐based prospective observational study of 35 cases from Taiwan. J Eur Acad Dermatol Venereol. 2016;30:1787-1789.
- Dréno B, Blouin E, Moyse D, et al. Acne in pregnant women: a French survey. Acta Derm Venereol. 2014;94:82-83.
- Kutlu Ö, Karadag˘ AS, Ünal E, et al. Acne in pregnancy: a prospective multicenter, cross‐sectional study of 295 patients in Turkey. Int J Dermatol. 2020;59:1098-1105.
- Hoefel IDR, Weber MB, Manzoni APD, et al. Striae gravidarum, acne, facial spots, and hair disorders: risk factors in a study with 1284 puerperal patients. J Pregnancy. 2020;2020:8036109.
- Ayanlowo OO, Otrofanowei E, Shorunmu TO, et al. Pregnancy dermatoses: a study of patients attending the antenatal clinic at two tertiary care centers in south west Nigeria. PAMJ Clin Med. 2020;3.
- Bechstein S, Ochsendorf F. Acne and rosacea in pregnancy. Hautarzt. 2017;68:111-119.
- Habeshian KA, Cohen BA. Current issues in the treatment of acne vulgaris. Pediatrics. 2020;145(suppl 2):S225-S230.
- Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling (21 CFR 201). Fed Regist. 2014;79:72064-72103.
- Sagransky M, Yentzer BA, Feldman SR. Benzoyl peroxide: a review of its current use in the treatment of acne vulgaris. Expert Opin Pharmacother. 2009;10:2555-2562.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part I. Pregnancy. J Am Acad Dermatol. 2014;70:401.e1-401.e14; quiz 415.
- Wolverton SE. Systemic corticosteroids. Comprehensive Dermatol Drug Ther. 2012;3:143-168.
- Kirtschig G, Schaefer C. Dermatological medications and local therapeutics. In: Schaefer C, Peters P, Miller RK, eds. Drugs During Pregnancy and Lactation. 3rd edition. Elsevier; 2015:467-492.
- Pugashetti R, Shinkai K. Treatment of acne vulgaris in pregnant patients. Dermatol Ther. 2013;26:302-311.
- Touitou E, Godin B, Shumilov M, et al. Efficacy and tolerability of clindamycin phosphate and salicylic acid gel in the treatment of mild to moderate acne vulgaris. J Eur Acad Dermatol Venereol. 2008;22:629-631.
- Schaefer C, Peters PW, Miller RK, eds. Drugs During Pregnancy and Lactation: Treatment Options and Risk Assessment. 2nd ed. Academic Press; 2014.
- Birmingham B, Greene D, Rhodes C. Systemic absorption of topical salicylic acid. Int J Dermatol. 1979;18:228-231.
- Trivedi NA. A meta-analysis of low-dose aspirin for prevention of preeclampsia. J Postgrad Med. 2011;57:91-95.
- Lucky AW, Maloney JM, Roberts J, et al. Dapsone gel 5% for the treatment of acne vulgaris: safety and efficacy of long-term (1 year) treatment. J Drugs Dermatol. 2007;6:981-987.
- Nosten F, McGready R, d’Alessandro U, et al. Antimalarial drugs in pregnancy: a review. Curr Drug Saf. 2006;1:1-15.
- Brabin BJ, Eggelte TA, Parise M, et al. Dapsone therapy for malaria during pregnancy: maternal and fetal outcomes. Drug Saf. 2004;27:633-648.
- Tuffanelli DL. Successful pregnancy in a patient with dermatitis herpetiformis treated with low-dose dapsone. Arch Dermatol. 1982;118:876.
- Meredith FM, Ormerod AD. The management of acne vulgaris in pregnancy. Am J Clin Dermatol. 2013;14:351-358.
- Kong Y, Tey H. Treatment of acne vulgaris during pregnancy and lactation. Drugs. 2013;73:779-787.
- Leachman SA, Reed BR. The use of dermatologic drugs in pregnancy and lactation. Dermatol Clin. 2006;24:167-197.
- Ly S, Kamal K, Manjaly P, et al. Treatment of acne vulgaris during pregnancy and lactation: a narrative review. Dermatol Ther. 2023;13:115-130.
- Webster G. Combination azelaic acid therapy for acne vulgaris. J Am Acad Dermatol. 2000;43:S47-S50.
- Archer CB, Cohen SN, Baron SE. Guidance on the diagnosis and clinical management of acne. Clin Exp Dermatol. 2012;37(suppl 1):1-6.
- Graupe K, Cunliffe W, Gollnick H, et al. Efficacy and safety of topical azelaic acid (20 percent cream): an overview of results from European clinical trials and experimental reports. Cutis. 1996;57(1 suppl):20-35.
- Bozzo P, Chua-Gocheco A, Einarson A. Safety of skin care products during pregnancy. Can Fam Physician. 2011;57:665-667.
- Munley SM, Kennedy GL, Hurtt ME. Developmental toxicity study of glycolic acid in rats. Drug Chem Toxicol. 1999;22:569-582.
- Chien AL, Qi J, Rainer B, et al. Treatment of acne in pregnancy. J Am Board Fam Med. 2016;29:254-262.
- Stuart B, Maund E, Wilcox C, et al. Topical preparations for the treatment of mild‐to‐moderate acne vulgaris: systematic review and network meta‐analysis. Br J Dermatol. 2021;185:512-525.
- van Hoogdalem EJ, Baven TL, Spiegel‐Melsen I, et al. Transdermal absorption of clindamycin and tretinoin from topically applied anti‐acne formulations in man. Biopharm Drug Dispos. 1998;19:563-569.
- Austin BA, Fleischer AB Jr. The extinction of topical erythromycin therapy for acne vulgaris and concern for the future of topical clindamycin. J Dermatolog Treat. 2017;28:145-148.
- Eady EA, Cove J, Holland K, et al. Erythromycin resistant propionibacteria in antibiotic treated acne patients: association with therapeutic failure. Br J. Dermatol. 1989;121:51-57.
- Alkhawaja E, Hammadi S, Abdelmalek M, et al. Antibiotic resistant Cutibacterium acnes among acne patients in Jordan: a cross sectional study. BMC Dermatol. 2020;20:1-9.
- Han G, Wu JJ, Del Rosso JQ. Use of topical tazarotene for the treatment of acne vulgaris in pregnancy: a literature review. J Clin Aesthet Dermatol. 2020;13:E59-E65.
- Selcen D, Seidman S, Nigro MA. Otocerebral anomalies associated with topical tretinoin use. Brain Dev. 2000;22:218-220.
- Moretz D. Drug Class Update with New Drug Evaluations: Topical Products for Inflammatory Skin Conditions. Oregon State University Drug Use & Research Management Program; December 2022. Accessed January 8, 2024. https://www.orpdl.org/durm/meetings/meetingdocs/2022_12_01/archives/2022_12_01_Inflammatory_Skin_Dz_ClassUpdate.pdf
- Kaplan YC, Ozsarfati J, Etwel F, et al. Pregnancy outcomes following first‐trimester exposure to topical retinoids: a systematic review and meta‐analysis. Br J Dermatol. 2015;173:1132-1141.
- Menter A. Pharmacokinetics and safety of tazarotene. J Am Acad Dermatol. 2000;43(2, pt 3):S31-S35.
- Autret E, Berjot M, Jonville-Béra A-P, et al. Anophthalmia and agenesis of optic chiasma associated with adapalene gel in early pregnancy. Lancet. 1997;350:339.
- Weiss J, Mallavalli S, Meckfessel M, et al. Safe use of adapalene 0.1% gel in a non-prescription environment. J Drugs Dermatol. 2021;20:1330-1335.
- Alessandro Mazzetti M. A phase 2b, randomized, double-blind vehicle controlled, dose escalation study evaluating clascoterone 0.1%, 0.5%, and 1% topical cream in subjects with facial acne. J Drugs Dermatol. 2019;18:570-575.
- Eichenfield L, Hebert A, Gold LS, et al. Open-label, long-term extension study to evaluate the safety of clascoterone (CB-03-01) cream, 1% twice daily, in patients with acne vulgaris. J Am Acad Dermatol. 2020;83:477-485.
- Trifu V, Tiplica GS, Naumescu E, et al. Cortexolone 17α‐propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. a pilot randomized, double‐blind comparative study vs. placebo and tretinoin 0.05% cream. Br J Dermatol. 2011;165:177-183.
- Hebert A, Thiboutot D, Gold LS, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630.
- Alkhodaidi ST, Al Hawsawi KA, Alkhudaidi IT, et al. Efficacy and safety of topical clascoterone cream for treatment of acne vulgaris: a systematic review and meta‐analysis of randomized placebo‐controlled trials. Dermatol Ther. 2021;34:e14609.
- Clasoterone. Package insert. Cassiopea Inc; 2020.
- Paik J. Topical minocycline foam 4%: a review in acne vulgaris. Am J Clin Dermatol. 2020;21:449-456.
- Jones TM, Ellman H. Pharmacokinetic comparison of once-daily topical minocycline foam 4% vs oral minocycline for moderate-to-severe acne. J Drugs Dermatol. 2017;16:1022-1028.
- Minocycline hydrochloride extended-release tablets. Package insert. JG Pharma; July 2020. Accessed January 8, 2024. https://www.jgpharmainc.com/assets/pdf/minocycline-hydrochloride.pdf
- Dinnendahl V, Fricke U (eds). Arzneistoff-Profile: Basisinformation über arzneiliche Wirkstoffe. Govi Pharmazeutischer Verlag; 2010.
- Martins AM, Marto JM, Johnson JL, et al. A review of systemic minocycline side effects and topical minocycline as a safer alternative for treating acne and rosacea. Antibiotics. 2021;10:757.
- Landis MN. Optimizing isotretinoin treatment of acne: update on current recommendations for monitoring, dosing, safety, adverse effects, compliance, and outcomes. Am J Clin Dermatol. 2020;21:411-419.
- Draghici C-C, Miulescu R-G, Petca R-C, et al. Teratogenic effect of isotretinoin in both fertile females and males. Exp Ther Med. 2021;21:1-5.
- Barker RA, Wilcox C, Layton AM. Oral spironolactone for acne vulgaris in adult females: an update of the literature. Am J Clin Dermatol. 2020;21:303-305.
- Han JJ, Faletsky A, Barbieri JS, et al. New acne therapies and updates on use of spironolactone and isotretinoin: a narrative review. Dermatol Ther (Heidelb). 2021;11:79-91.
- Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. Lippincott Williams & Wilkins; 2012.
- Patel DJ, Bhatia N. Oral antibiotics for acne. Am J Clin Dermatol. 2021;22:193-204.
- Jick H, Holmes LB, Hunter JR, et al. First-trimester drug use and congenital disorders. JAMA. 1981;246:343-346.
- Valente Duarte de Sousa IC. An overview of sarecycline for the treatment of moderate-to-severe acne vulgaris. Exp Opin Pharmacother. 2021;22:145-154.
- Hussar DA, Chahine EB. Omadacycline tosylate, sarecycline hydrochloride, rifamycin sodium, and moxidectin. J Am Pharm Assoc. 2019;59:756-760.
- Haidari W, Bruinsma R, Cardenas-de la Garza JA, et al. Sarecycline review. Ann Pharmacother. 2020;54:164-170.
- Feldman S, Careccia RE, Barham KL, et al. Diagnosis and treatment of acne. Am Fam Physician. 2004;69:2123-2130.
- Gammon WR, Meyer C, Lantis S, et al. Comparative efficacy of oral erythromycin versus oral tetracycline in the treatment of acne vulgaris: a double-blind study. J Am Acad Dermatol. 1986;14:183-186.
- Källén BA, Olausson PO, Danielsson BR. Is erythromycin therapy teratogenic in humans? Reprod Toxicol. 2005;20:209-214.
- McCormack WM, George H, Donner A, et al. Hepatotoxicity of erythromycin estolate during pregnancy. Antimicrob Agents Chemother. 1977;12:630-635.
- Cervantes J, Eber AE, Perper M, et al. The role of zinc in the treatment of acne: a review of the literature. Dermatolog Ther. 2018;31:e12576.
- Dréno B, Blouin E. Acne, pregnant women and zinc salts: a literature review [in French]. Ann Dermatol Venereol. 2008;135:27-33.
- Eid MM, Saleh MS, Allam NM, et al. Narrow band ultraviolet B versus red light-emitting diodes in the treatment of facial acne vulgaris: a randomized controlled trial. Photobiomodul Photomed Laser Surg. 2021;39:418-424.
- Zeichner JA. Narrowband UV-B phototherapy for the treatment of acne vulgaris during pregnancy. Arch Dermatol. 2011;147:537-539.
- El-Saie LT, Rabie AR, Kamel MI, et al. Effect of narrowband ultraviolet B phototherapy on serum folic acid levels in patients with psoriasis. Lasers Med Sci. 2011;26:481-485.
- Park KK, Murase JE. Narrowband UV-B phototherapy during pregnancy and folic acid depletion. Arch Dermatol. 2012;148:132-133.
- Jablonski NG. A possible link between neural tube defects and ultraviolet light exposure. Med Hypotheses. 1999;52:581-582.
- Zhang M, Goyert G, Lim HW. Folate and phototherapy: what should we inform our patients? J Am Acad Dermatol. 2017;77:958-964.
- AviClear. Cutera website. Accessed January 8, 2024. https://www.cutera.com/solutions/aviclear/
- Wu X, Yang Y, Wang Y, et al. Treatment of refractory acne using selective sebaceous gland electro-thermolysis combined with non-thermal plasma. J Cosmet Laser Ther. 2021;23:188-194.
- Ahn GR, Kim JM, Park SJ, et al. Selective sebaceous gland electrothermolysis using a single microneedle radiofrequency device for acne patients: a prospective randomized controlled study. Lasers Surg Med. 2020;52:396-401.
- Fabbrocini G, De Padova MP, Tosti A. Chemical peels: what’s new and what isn’t new but still works well. Facial Plast Surg. 2009;25:329-336.
- Andersen FA. Final report on the safety assessment of glycolic acid, ammonium, calcium, potassium, and sodium glycolates, methyl, ethyl, propyl, and butyl glycolates, and lactic acid, ammonium, calcium, potassium, sodium, and TEA-lactates, methyl, ethyl, isopropyl, and butyl lactates, and lauryl, myristyl, and cetyl lactates. Int J Toxicol. 1998;17(1_suppl):1-241.
- Lee KC, Korgavkar K, Dufresne RG Jr, et al. Safety of cosmetic dermatologic procedures during pregnancy. Dermatol Surg. 2013;39:1573-1586.
- James AH, Brancazio LR, Price T. Aspirin and reproductive outcomes. Obstet Gynecol Surv. 2008;63:49-57.
- Zhou W-S, Xu L, Xie S-H, et al. Decreased birth weight in relation to maternal urinary trichloroacetic acid levels. Sci Total Environ. 2012;416:105-110.
- Schwartz DB, Greenberg MD, Daoud Y, et al. Genital condylomas in pregnancy: use of trichloroacetic acid and laser therapy. Am J Obstet Gynecol. 1988;158:1407-1416.
- Starkman SJ, Mangat DS. Chemical peel (deep, medium, light). Facial Plast Surg Clin North Am. 2020;28:45-57.
- Trivedi M, Kroumpouzos G, Murase J. A review of the safety of cosmetic procedures during pregnancy and lactation. Int J Womens Dermatol. 2017;3:6-10.
- Gallagher T, Taliercio M, Nia JK, et al. Dermatologist use of intralesional triamcinolone in the treatment of acne. J Clin Aesthet Dermatol. 2020;13:41-43.
- Zamil DH, Burns EK, Perez-Sanchez A, et al. Risk of birth defects from vitamin A “acne supplements” sold online. Dermatol Pract Concept. 2021;11:e2021075.
Acne vulgaris, or acne, is a highly common inflammatory skin disorder affecting up to 85% of the population, and it constitutes the most commonly presenting chief concern in routine dermatology practice.1 Older teenagers and young adults are most often affected by acne.2 Although acne generally is more common in males, adult-onset acne occurs more frequently in women.2,3 Black and Hispanic women are at higher risk for acne compared to those of Asian, White, or Continental Indian descent.4 As such, acne is a common concern in all women of childbearing age.
Concerns for maternal and fetal safety are important therapeutic considerations, especially because hormonal and physiologic changes in pregnancy can lead to onset of inflammatory acne lesions, particularly during the second and third trimesters.5 Female patients younger than 25 years; with a higher body mass index, prior irregular menstruation, or polycystic ovary syndrome; or those experiencing their first pregnancy are thought to be more commonly affected.5-7 In fact, acne affects up to 43% of pregnant women, and lesions typically extend beyond the face to involve the trunk.6,8-10 Importantly, one-third of women with a history of acne experience symptom relapse after disease-free periods, while two-thirds of those with ongoing disease experience symptom deterioration during pregnancy.10 Although acne is not a life-threatening condition, it has a well-documented, detrimental impact on social, emotional, and psychological well-being, namely self-perception, social interactions, quality-of-life scores, depression, and anxiety.11
Therefore, safe and effective treatment of pregnant women is of paramount importance. Because pregnant women are not included in clinical trials, there is a paucity of medication safety data, further augmented by inefficient access to available information. The US Food and Drug Administration (FDA) pregnancy safety categories were updated in 2015, letting go of the traditional A, B, C, D, and X categories.12 The Table reviews the current pregnancy classification system. In this narrative review, we summarize the most recent available data and recommendations on the safety and efficacy of acne treatment during pregnancy.

Topical Treatments for Acne
Benzoyl Peroxide—Benzoyl peroxide commonly is used as first-line therapy alone or in combination with other agents for the treatment of mild to moderate acne.13 It is safe for use during pregnancy.14 Although the medication is systemically absorbed, it undergoes complete metabolism to benzoic acid, a commonly used food additive.15,16 Benzoic acid has low bioavailability, as it gets rapidly metabolized by the kidneys; therefore, benzoyl peroxide is unlikely to reach clinically significant levels in the maternal circulation and consequently the fetal circulation. Additionally, it has a low risk for causing congenital malformations.17
Salicylic Acid—For mild to moderate acne, salicylic acid is a second-line agent that likely is safe for use by pregnant women at low concentrations and over limited body surface areas.14,18,19 There is minimal systemic absorption of the drug.20 Additionally, aspirin, which is broken down in the body into salicylic acid, is used in low doses for the treatment of pre-eclampsia during pregnancy.21
Dapsone—The use of dapsone gel 5% as a second-line agent has shown efficacy for mild to moderate acne.22 The oral formulation, commonly used for malaria and leprosy prophylaxis, has failed to show associated fetal toxicity or congenital anomalies.23,24 It also has been used as a first-line treatment for dermatitis herpetiformis in pregnancy.25 Although the medication likely is safe, it is better to minimize its use during the third trimester to reduce the theoretical risk for hyperbilirubinemia in the neonate.17,26-29
Azelaic Acid—Azelaic acid effectively targets noninflammatory and inflammatory acne and generally is well tolerated, harboring a good safety profile.30 Topical 20% azelaic acid has localized antibacterial and comedolytic effects and is safe for use during pregnancy.31,32
Glycolic Acid—Limited data exist on the safety of glycolic acid during pregnancy. In vitro studies have shown up to 27% systemic absorption depending on pH, concentration, and duration of application.33 Animal reproductive studies involving rats have shown fetal multisystem malformations and developmental abnormalities with oral administration of glycolic acid at doses far exceeding those used in humans.34 Although no human reproductive studies exist, topical glycolic acid is unlikely to reach the developing fetus in notable amounts, and the medication is likely safe for use.17,35
Clindamycin—Topical clindamycin phosphate is an effective and well-tolerated agent for the treatment of mild to moderate acne.36 Its systemic absorption is minimal, and it is considered safe for use during all trimesters of pregnancy.14,17,26,27,35,37
Erythromycin—Topical erythromycin is another commonly prescribed topical antibiotic used to target mild to moderate acne. However, its use recently has been associated with a decrease in efficacy secondary to the rise of antibacterial resistance in the community.38-40 Nevertheless, it remains a safe treatment for use during all trimesters of pregnancy.14,17,26,27,35,37
Topical Retinoids—Vitamin A derivatives (also known as retinoids) are the mainstay for the treatment of mild to moderate acne. Limited data exist regarding pregnancy outcomes after in utero exposure.41 A rare case report suggested topical tretinoin has been associated with fetal otocerebral anomalies.42 For tazarotene, teratogenic effects were seen in animal reproductive studies at doses exceeding maximum recommended human doses.41,43 However, a large meta-analysis failed to find a clear risk for increased congenital malformations, spontaneous abortions, stillbirth, elective termination of pregnancy, low birthweight, or prematurity following first-trimester exposure to topical retinoids.44 As the level of exposure that could lead to teratogenicity in humans is unknown, avoidance of both tretinoin and tazarotene is recommended in pregnant women.41,45 Nevertheless, women inadvertently exposed should be reassured.44
Conversely, adapalene has been associated with 1 case of anophthalmia and agenesis of the optic chiasma in a fetus following exposure until 13 weeks’ gestation.46 However, a large, open-label trial prior to the patient transitioning from adapalene to over-the-counter treatment showed that the drug harbors a large and reassuring margin of safety and no risk for teratogenicity in a maximal usage trial and Pregnancy Safety Review.47 Therefore, adapalene gel 0.1% is a safe and effective medication for the treatment of acne in a nonprescription environment and does not pose harm to the fetus.
Clascoterone—Clascoterone is a novel topical antiandrogenic drug approved for the treatment of hormonal and inflammatory moderate to severe acne.48-51 Human reproductive data are limited to 1 case of pregnancy that occurred during phase 3 trial investigations, and no adverse outcomes were reported.51 Minimal systemic absorption follows topical use.52 Nonetheless, dose-independent malformations were reported in animal reproductive studies.53 As such, it remains better to avoid the use of clascoterone during pregnancy pending further safety data.
Minocycline Foam—Minocycline foam 4% is approved to treat inflammatory lesions of nonnodular moderate to severe acne in patients 9 years and older.54 Systemic absorption is minimal, and the drug has limited bioavailability with minimal systemic accumulation in the patient’s serum.55 Given this information, it is unlikely that topical minocycline will reach notable levels in the fetal serum or harbor teratogenic effects, as seen with the oral formulation.56 However, it may be best to avoid its use during the second and third trimesters given the potential risk for tooth discoloration in the fetus.57,58
Systemic Treatments for Acne
Isotretinoin—Isotretinoin is the most effective treatment for moderate to severe acne with a well-documented potential for long-term clearance.59 Its use during pregnancy is absolutely contraindicated, as the medication is a well-known teratogen. Associated congenital malformations include numerous craniofacial defects, cardiovascular and neurologic malformations, or thymic disorders that are estimated to affect 20% to 35% of infants exposed in utero.60 Furthermore, strict contraception use during treatment is mandated for patients who can become pregnant. It is recommended to wait at least 1 month and 1 menstrual cycle after medication discontinuation before attempting to conceive.17 Pregnancy termination is recommended if conception occurs during treatment with isotretinoin.
Spironolactone—Spironolactone is an androgen-receptor antagonist commonly prescribed off label for mild to severe acne in females.61,62 Spironolactone promotes the feminization of male fetuses and should be avoided in pregnancy.63
Doxycycline/Minocycline—Tetracyclines are the most commonly prescribed oral antibiotics for moderate to severe acne.64 Although highly effective at treating acne, tetracyclines generally should be avoided in pregnancy. First-trimester use of doxycycline is not absolutely contraindicated but should be reserved for severe illness and not employed for the treatment of acne. However, accidental exposure to doxycycline has not been associated with congenital malformations.65 Nevertheless, after the 15th week of gestation, permanent tooth discoloration and bone growth inhibition in the fetus are serious and well-documented risks.14,17 Additional adverse events following in utero exposure include infantile inguinal hernia, hypospadias, and limb hypoplasia.63
Sarecycline—Sarecycline is a novel tetracycline-class antibiotic for the treatment of moderate to severe inflammatory acne. It has a narrower spectrum of activity compared to its counterparts within its class, which translates to an improved safety profile, namely when it comes to gastrointestinal tract microbiome disruption and potentially decreased likelihood of developing bacterial resistance.66 Data on human reproductive studies are limited, but it is advisable to avoid sarecycline in pregnancy, as it may cause adverse developmental effects in the fetus, such as reduced bone growth, in addition to the well-known tetracycline-associated risk for permanent discoloration of the teeth if used during the second and third trimesters.67,68
Erythromycin—Oral erythromycin targets moderate to severe inflammatory acne and is considered safe for use during pregnancy.69,70 There has been 1 study reporting an increased risk for atrial and ventricular septal defects (1.8%) and pyloric stenosis (0.2%), but these risks are still uncertain, and erythromycin is considered compatible with pregnancy.71 However, erythromycin estolate formulations should be avoided given the associated 10% to 15% risk for reversible cholestatic liver injury.72 Erythromycin base or erythromycin ethylsuccinate formulations should be favored.
Systemic Steroids—Prednisone is indicated for severe acne with scarring and should only be used during pregnancy after clearance from the patient’s obstetrician. Doses of 0.5 mg/kg or less should be prescribed in combination with systemic antibiotics as well as agents for bone and gastrointestinal tract prophylaxis.29
Zinc—The exact mechanism by which zinc exerts its effects to improve acne remains largely obscure. It has been found effective against inflammatory lesions of mild to moderate acne.73 Generally recommended dosages range from 30 to 200 mg/d but may be associated with gastrointestinal tract disturbances. Dosages of 75 mg/d have shown no harm to the fetus.74 When taking this supplement, patients should not exceed the recommended doses given the risk for hypocupremia associated with high-dose zinc supplementation.
Light-Based Therapies
Phototherapy—Narrowband UVB phototherapy is effective for the treatment of mild to moderate acne.75 It has been proven to be a safe treatment option during pregnancy, but its use has been associated with decreased folic acid levels.76-79 Therefore, in addition to attaining baseline folic acid serum levels, supplementation with folic acid prior to treatment, as per routine prenatal guidelines, should be sought.80
AviClear—The AviClear (Cutera) laser is the first device cleared by the FDA for mild to severe acne in March 2022.81 The FDA clearance for the Accure (Accure Acne Inc) laser, also targeting mild to severe acne, followed soon after (November 2022). Both lasers harbor a wavelength of 1726 nm and target sebaceous glands with electrothermolysis.82,83 Further research and long-term safety data are required before using them in pregnancy.
Other Therapies
Cosmetic Peels—Glycolic acid peels induce epidermolysis and desquamation.84 Although data on use during pregnancy are limited, these peels have limited dermal penetration and are considered safe for use in pregnancy.33,85,86 Similarly, keratolytic lactic acid peels harbor limited dermal penetration and can be safely used in pregnant women.87-89 Salicylic acid peels also work through epidermolysis and desquamation84; however, they tend to penetrate deeper into the skin, reaching down to the basal layer, if large areas are treated or when applied under occlusion.86,90 Although their use is not contraindicated in pregnancy, they should be limited to small areas of coverage.91
Intralesional Triamcinolone—Acne cysts and inflammatory papules can be treated with intralesional triamcinolone injections to relieve acute symptoms such as pain.92 Low doses at concentrations of 2.5 mg/mL are considered compatible with pregnancy when indicated.29
Approaching the Patient Clinical Encounter
In patients seeking treatment prior to conception, a few recommendations can be made to minimize the risk for acne recurrence or flares during pregnancy. For instance, because data show an association between increased acne severity in those with a higher body mass index and in pregnancy, weight loss may be recommended prior to pregnancy to help mitigate symptoms after conception.7 The Figure summarizes our recommendations for approaching and treating acne in pregnancy.

In all patients, grading the severity of the patient’s acne as mild, moderate, or severe is the first step. The presence of scarring is an additional consideration during the physical examination and should be documented. A careful discussion of treatment expectations and prognosis should be the focus before treatment initiation. Meticulous documentation of the physical examination and discussion with the patient should be prioritized.
To minimize toxicity and risks to the developing fetus, monotherapy is favored. Topical therapy should be considered first line. Safe regimens include mild nonabrasive washes, such as those containing benzoyl peroxide or glycolic acid, or topical azelaic acid or clindamycin phosphate for mild to moderate acne. More severe cases warrant the consideration of systemic medications as second line, as more severe acne is better treated with oral antibiotics such as the macrolides erythromycin or clindamycin or systemic corticosteroids when concern exists for severe scarring. The additional use of physical sunscreen also is recommended.
An important topic to address during the clinical encounter is cautious intake of oral supplements for acne during pregnancy, as they may contain harmful and teratogenic ingredients. A recent search focusing on acne supplements available online between March and May 2020 uncovered 49 different supplements, 26 (53%) of which contained vitamin A.93 Importantly, 3 (6%) of these 49 supplements were likely teratogenic, 4 (8%) contained vitamin A doses exceeding the recommended daily nutritional intake level, and 15 (31%) harbored an unknown teratogenic risk. Furthermore, among the 6 (12%) supplements with vitamin A levels exceeding 10,000 IU, 2 lacked any mention of pregnancy warning, including the supplement with the highest vitamin A dose found in this study.93 Because dietary supplements are not subject to the same stringent regulations by the FDA as drugs, inadvertent use by unaware patients ought to be prevented by careful counseling and education.
Finally, patients should be counseled to seek care following delivery for potentially updated medication management of acne, especially if they are breastfeeding. Co-management with a pediatrician may be indicated during lactation, particularly when newborns are born preterm or with other health conditions that may warrant additional caution with the use of certain agents.
Acne vulgaris, or acne, is a highly common inflammatory skin disorder affecting up to 85% of the population, and it constitutes the most commonly presenting chief concern in routine dermatology practice.1 Older teenagers and young adults are most often affected by acne.2 Although acne generally is more common in males, adult-onset acne occurs more frequently in women.2,3 Black and Hispanic women are at higher risk for acne compared to those of Asian, White, or Continental Indian descent.4 As such, acne is a common concern in all women of childbearing age.
Concerns for maternal and fetal safety are important therapeutic considerations, especially because hormonal and physiologic changes in pregnancy can lead to onset of inflammatory acne lesions, particularly during the second and third trimesters.5 Female patients younger than 25 years; with a higher body mass index, prior irregular menstruation, or polycystic ovary syndrome; or those experiencing their first pregnancy are thought to be more commonly affected.5-7 In fact, acne affects up to 43% of pregnant women, and lesions typically extend beyond the face to involve the trunk.6,8-10 Importantly, one-third of women with a history of acne experience symptom relapse after disease-free periods, while two-thirds of those with ongoing disease experience symptom deterioration during pregnancy.10 Although acne is not a life-threatening condition, it has a well-documented, detrimental impact on social, emotional, and psychological well-being, namely self-perception, social interactions, quality-of-life scores, depression, and anxiety.11
Therefore, safe and effective treatment of pregnant women is of paramount importance. Because pregnant women are not included in clinical trials, there is a paucity of medication safety data, further augmented by inefficient access to available information. The US Food and Drug Administration (FDA) pregnancy safety categories were updated in 2015, letting go of the traditional A, B, C, D, and X categories.12 The Table reviews the current pregnancy classification system. In this narrative review, we summarize the most recent available data and recommendations on the safety and efficacy of acne treatment during pregnancy.

Topical Treatments for Acne
Benzoyl Peroxide—Benzoyl peroxide commonly is used as first-line therapy alone or in combination with other agents for the treatment of mild to moderate acne.13 It is safe for use during pregnancy.14 Although the medication is systemically absorbed, it undergoes complete metabolism to benzoic acid, a commonly used food additive.15,16 Benzoic acid has low bioavailability, as it gets rapidly metabolized by the kidneys; therefore, benzoyl peroxide is unlikely to reach clinically significant levels in the maternal circulation and consequently the fetal circulation. Additionally, it has a low risk for causing congenital malformations.17
Salicylic Acid—For mild to moderate acne, salicylic acid is a second-line agent that likely is safe for use by pregnant women at low concentrations and over limited body surface areas.14,18,19 There is minimal systemic absorption of the drug.20 Additionally, aspirin, which is broken down in the body into salicylic acid, is used in low doses for the treatment of pre-eclampsia during pregnancy.21
Dapsone—The use of dapsone gel 5% as a second-line agent has shown efficacy for mild to moderate acne.22 The oral formulation, commonly used for malaria and leprosy prophylaxis, has failed to show associated fetal toxicity or congenital anomalies.23,24 It also has been used as a first-line treatment for dermatitis herpetiformis in pregnancy.25 Although the medication likely is safe, it is better to minimize its use during the third trimester to reduce the theoretical risk for hyperbilirubinemia in the neonate.17,26-29
Azelaic Acid—Azelaic acid effectively targets noninflammatory and inflammatory acne and generally is well tolerated, harboring a good safety profile.30 Topical 20% azelaic acid has localized antibacterial and comedolytic effects and is safe for use during pregnancy.31,32
Glycolic Acid—Limited data exist on the safety of glycolic acid during pregnancy. In vitro studies have shown up to 27% systemic absorption depending on pH, concentration, and duration of application.33 Animal reproductive studies involving rats have shown fetal multisystem malformations and developmental abnormalities with oral administration of glycolic acid at doses far exceeding those used in humans.34 Although no human reproductive studies exist, topical glycolic acid is unlikely to reach the developing fetus in notable amounts, and the medication is likely safe for use.17,35
Clindamycin—Topical clindamycin phosphate is an effective and well-tolerated agent for the treatment of mild to moderate acne.36 Its systemic absorption is minimal, and it is considered safe for use during all trimesters of pregnancy.14,17,26,27,35,37
Erythromycin—Topical erythromycin is another commonly prescribed topical antibiotic used to target mild to moderate acne. However, its use recently has been associated with a decrease in efficacy secondary to the rise of antibacterial resistance in the community.38-40 Nevertheless, it remains a safe treatment for use during all trimesters of pregnancy.14,17,26,27,35,37
Topical Retinoids—Vitamin A derivatives (also known as retinoids) are the mainstay for the treatment of mild to moderate acne. Limited data exist regarding pregnancy outcomes after in utero exposure.41 A rare case report suggested topical tretinoin has been associated with fetal otocerebral anomalies.42 For tazarotene, teratogenic effects were seen in animal reproductive studies at doses exceeding maximum recommended human doses.41,43 However, a large meta-analysis failed to find a clear risk for increased congenital malformations, spontaneous abortions, stillbirth, elective termination of pregnancy, low birthweight, or prematurity following first-trimester exposure to topical retinoids.44 As the level of exposure that could lead to teratogenicity in humans is unknown, avoidance of both tretinoin and tazarotene is recommended in pregnant women.41,45 Nevertheless, women inadvertently exposed should be reassured.44
Conversely, adapalene has been associated with 1 case of anophthalmia and agenesis of the optic chiasma in a fetus following exposure until 13 weeks’ gestation.46 However, a large, open-label trial prior to the patient transitioning from adapalene to over-the-counter treatment showed that the drug harbors a large and reassuring margin of safety and no risk for teratogenicity in a maximal usage trial and Pregnancy Safety Review.47 Therefore, adapalene gel 0.1% is a safe and effective medication for the treatment of acne in a nonprescription environment and does not pose harm to the fetus.
Clascoterone—Clascoterone is a novel topical antiandrogenic drug approved for the treatment of hormonal and inflammatory moderate to severe acne.48-51 Human reproductive data are limited to 1 case of pregnancy that occurred during phase 3 trial investigations, and no adverse outcomes were reported.51 Minimal systemic absorption follows topical use.52 Nonetheless, dose-independent malformations were reported in animal reproductive studies.53 As such, it remains better to avoid the use of clascoterone during pregnancy pending further safety data.
Minocycline Foam—Minocycline foam 4% is approved to treat inflammatory lesions of nonnodular moderate to severe acne in patients 9 years and older.54 Systemic absorption is minimal, and the drug has limited bioavailability with minimal systemic accumulation in the patient’s serum.55 Given this information, it is unlikely that topical minocycline will reach notable levels in the fetal serum or harbor teratogenic effects, as seen with the oral formulation.56 However, it may be best to avoid its use during the second and third trimesters given the potential risk for tooth discoloration in the fetus.57,58
Systemic Treatments for Acne
Isotretinoin—Isotretinoin is the most effective treatment for moderate to severe acne with a well-documented potential for long-term clearance.59 Its use during pregnancy is absolutely contraindicated, as the medication is a well-known teratogen. Associated congenital malformations include numerous craniofacial defects, cardiovascular and neurologic malformations, or thymic disorders that are estimated to affect 20% to 35% of infants exposed in utero.60 Furthermore, strict contraception use during treatment is mandated for patients who can become pregnant. It is recommended to wait at least 1 month and 1 menstrual cycle after medication discontinuation before attempting to conceive.17 Pregnancy termination is recommended if conception occurs during treatment with isotretinoin.
Spironolactone—Spironolactone is an androgen-receptor antagonist commonly prescribed off label for mild to severe acne in females.61,62 Spironolactone promotes the feminization of male fetuses and should be avoided in pregnancy.63
Doxycycline/Minocycline—Tetracyclines are the most commonly prescribed oral antibiotics for moderate to severe acne.64 Although highly effective at treating acne, tetracyclines generally should be avoided in pregnancy. First-trimester use of doxycycline is not absolutely contraindicated but should be reserved for severe illness and not employed for the treatment of acne. However, accidental exposure to doxycycline has not been associated with congenital malformations.65 Nevertheless, after the 15th week of gestation, permanent tooth discoloration and bone growth inhibition in the fetus are serious and well-documented risks.14,17 Additional adverse events following in utero exposure include infantile inguinal hernia, hypospadias, and limb hypoplasia.63
Sarecycline—Sarecycline is a novel tetracycline-class antibiotic for the treatment of moderate to severe inflammatory acne. It has a narrower spectrum of activity compared to its counterparts within its class, which translates to an improved safety profile, namely when it comes to gastrointestinal tract microbiome disruption and potentially decreased likelihood of developing bacterial resistance.66 Data on human reproductive studies are limited, but it is advisable to avoid sarecycline in pregnancy, as it may cause adverse developmental effects in the fetus, such as reduced bone growth, in addition to the well-known tetracycline-associated risk for permanent discoloration of the teeth if used during the second and third trimesters.67,68
Erythromycin—Oral erythromycin targets moderate to severe inflammatory acne and is considered safe for use during pregnancy.69,70 There has been 1 study reporting an increased risk for atrial and ventricular septal defects (1.8%) and pyloric stenosis (0.2%), but these risks are still uncertain, and erythromycin is considered compatible with pregnancy.71 However, erythromycin estolate formulations should be avoided given the associated 10% to 15% risk for reversible cholestatic liver injury.72 Erythromycin base or erythromycin ethylsuccinate formulations should be favored.
Systemic Steroids—Prednisone is indicated for severe acne with scarring and should only be used during pregnancy after clearance from the patient’s obstetrician. Doses of 0.5 mg/kg or less should be prescribed in combination with systemic antibiotics as well as agents for bone and gastrointestinal tract prophylaxis.29
Zinc—The exact mechanism by which zinc exerts its effects to improve acne remains largely obscure. It has been found effective against inflammatory lesions of mild to moderate acne.73 Generally recommended dosages range from 30 to 200 mg/d but may be associated with gastrointestinal tract disturbances. Dosages of 75 mg/d have shown no harm to the fetus.74 When taking this supplement, patients should not exceed the recommended doses given the risk for hypocupremia associated with high-dose zinc supplementation.
Light-Based Therapies
Phototherapy—Narrowband UVB phototherapy is effective for the treatment of mild to moderate acne.75 It has been proven to be a safe treatment option during pregnancy, but its use has been associated with decreased folic acid levels.76-79 Therefore, in addition to attaining baseline folic acid serum levels, supplementation with folic acid prior to treatment, as per routine prenatal guidelines, should be sought.80
AviClear—The AviClear (Cutera) laser is the first device cleared by the FDA for mild to severe acne in March 2022.81 The FDA clearance for the Accure (Accure Acne Inc) laser, also targeting mild to severe acne, followed soon after (November 2022). Both lasers harbor a wavelength of 1726 nm and target sebaceous glands with electrothermolysis.82,83 Further research and long-term safety data are required before using them in pregnancy.
Other Therapies
Cosmetic Peels—Glycolic acid peels induce epidermolysis and desquamation.84 Although data on use during pregnancy are limited, these peels have limited dermal penetration and are considered safe for use in pregnancy.33,85,86 Similarly, keratolytic lactic acid peels harbor limited dermal penetration and can be safely used in pregnant women.87-89 Salicylic acid peels also work through epidermolysis and desquamation84; however, they tend to penetrate deeper into the skin, reaching down to the basal layer, if large areas are treated or when applied under occlusion.86,90 Although their use is not contraindicated in pregnancy, they should be limited to small areas of coverage.91
Intralesional Triamcinolone—Acne cysts and inflammatory papules can be treated with intralesional triamcinolone injections to relieve acute symptoms such as pain.92 Low doses at concentrations of 2.5 mg/mL are considered compatible with pregnancy when indicated.29
Approaching the Patient Clinical Encounter
In patients seeking treatment prior to conception, a few recommendations can be made to minimize the risk for acne recurrence or flares during pregnancy. For instance, because data show an association between increased acne severity in those with a higher body mass index and in pregnancy, weight loss may be recommended prior to pregnancy to help mitigate symptoms after conception.7 The Figure summarizes our recommendations for approaching and treating acne in pregnancy.

In all patients, grading the severity of the patient’s acne as mild, moderate, or severe is the first step. The presence of scarring is an additional consideration during the physical examination and should be documented. A careful discussion of treatment expectations and prognosis should be the focus before treatment initiation. Meticulous documentation of the physical examination and discussion with the patient should be prioritized.
To minimize toxicity and risks to the developing fetus, monotherapy is favored. Topical therapy should be considered first line. Safe regimens include mild nonabrasive washes, such as those containing benzoyl peroxide or glycolic acid, or topical azelaic acid or clindamycin phosphate for mild to moderate acne. More severe cases warrant the consideration of systemic medications as second line, as more severe acne is better treated with oral antibiotics such as the macrolides erythromycin or clindamycin or systemic corticosteroids when concern exists for severe scarring. The additional use of physical sunscreen also is recommended.
An important topic to address during the clinical encounter is cautious intake of oral supplements for acne during pregnancy, as they may contain harmful and teratogenic ingredients. A recent search focusing on acne supplements available online between March and May 2020 uncovered 49 different supplements, 26 (53%) of which contained vitamin A.93 Importantly, 3 (6%) of these 49 supplements were likely teratogenic, 4 (8%) contained vitamin A doses exceeding the recommended daily nutritional intake level, and 15 (31%) harbored an unknown teratogenic risk. Furthermore, among the 6 (12%) supplements with vitamin A levels exceeding 10,000 IU, 2 lacked any mention of pregnancy warning, including the supplement with the highest vitamin A dose found in this study.93 Because dietary supplements are not subject to the same stringent regulations by the FDA as drugs, inadvertent use by unaware patients ought to be prevented by careful counseling and education.
Finally, patients should be counseled to seek care following delivery for potentially updated medication management of acne, especially if they are breastfeeding. Co-management with a pediatrician may be indicated during lactation, particularly when newborns are born preterm or with other health conditions that may warrant additional caution with the use of certain agents.
- Bhate K, Williams H. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485.
- Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10:5754.
- Fisk WA, Lev-Tov HA, Sivamani RK. Epidemiology and management of acne in adult women. Curr Dermatol Rep. 2014;3:29-39.
- Perkins A, Cheng C, Hillebrand G, et al. Comparison of the epidemiology of acne vulgaris among Caucasian, Asian, Continental Indian and African American women. J Eur Acad Dermatol Venereol. 2011;25:1054-1060.
- Yang CC, Huang YT, Yu CH, et al. Inflammatory facial acne during uncomplicated pregnancy and post‐partum in adult women: a preliminary hospital‐based prospective observational study of 35 cases from Taiwan. J Eur Acad Dermatol Venereol. 2016;30:1787-1789.
- Dréno B, Blouin E, Moyse D, et al. Acne in pregnant women: a French survey. Acta Derm Venereol. 2014;94:82-83.
- Kutlu Ö, Karadag˘ AS, Ünal E, et al. Acne in pregnancy: a prospective multicenter, cross‐sectional study of 295 patients in Turkey. Int J Dermatol. 2020;59:1098-1105.
- Hoefel IDR, Weber MB, Manzoni APD, et al. Striae gravidarum, acne, facial spots, and hair disorders: risk factors in a study with 1284 puerperal patients. J Pregnancy. 2020;2020:8036109.
- Ayanlowo OO, Otrofanowei E, Shorunmu TO, et al. Pregnancy dermatoses: a study of patients attending the antenatal clinic at two tertiary care centers in south west Nigeria. PAMJ Clin Med. 2020;3.
- Bechstein S, Ochsendorf F. Acne and rosacea in pregnancy. Hautarzt. 2017;68:111-119.
- Habeshian KA, Cohen BA. Current issues in the treatment of acne vulgaris. Pediatrics. 2020;145(suppl 2):S225-S230.
- Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling (21 CFR 201). Fed Regist. 2014;79:72064-72103.
- Sagransky M, Yentzer BA, Feldman SR. Benzoyl peroxide: a review of its current use in the treatment of acne vulgaris. Expert Opin Pharmacother. 2009;10:2555-2562.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part I. Pregnancy. J Am Acad Dermatol. 2014;70:401.e1-401.e14; quiz 415.
- Wolverton SE. Systemic corticosteroids. Comprehensive Dermatol Drug Ther. 2012;3:143-168.
- Kirtschig G, Schaefer C. Dermatological medications and local therapeutics. In: Schaefer C, Peters P, Miller RK, eds. Drugs During Pregnancy and Lactation. 3rd edition. Elsevier; 2015:467-492.
- Pugashetti R, Shinkai K. Treatment of acne vulgaris in pregnant patients. Dermatol Ther. 2013;26:302-311.
- Touitou E, Godin B, Shumilov M, et al. Efficacy and tolerability of clindamycin phosphate and salicylic acid gel in the treatment of mild to moderate acne vulgaris. J Eur Acad Dermatol Venereol. 2008;22:629-631.
- Schaefer C, Peters PW, Miller RK, eds. Drugs During Pregnancy and Lactation: Treatment Options and Risk Assessment. 2nd ed. Academic Press; 2014.
- Birmingham B, Greene D, Rhodes C. Systemic absorption of topical salicylic acid. Int J Dermatol. 1979;18:228-231.
- Trivedi NA. A meta-analysis of low-dose aspirin for prevention of preeclampsia. J Postgrad Med. 2011;57:91-95.
- Lucky AW, Maloney JM, Roberts J, et al. Dapsone gel 5% for the treatment of acne vulgaris: safety and efficacy of long-term (1 year) treatment. J Drugs Dermatol. 2007;6:981-987.
- Nosten F, McGready R, d’Alessandro U, et al. Antimalarial drugs in pregnancy: a review. Curr Drug Saf. 2006;1:1-15.
- Brabin BJ, Eggelte TA, Parise M, et al. Dapsone therapy for malaria during pregnancy: maternal and fetal outcomes. Drug Saf. 2004;27:633-648.
- Tuffanelli DL. Successful pregnancy in a patient with dermatitis herpetiformis treated with low-dose dapsone. Arch Dermatol. 1982;118:876.
- Meredith FM, Ormerod AD. The management of acne vulgaris in pregnancy. Am J Clin Dermatol. 2013;14:351-358.
- Kong Y, Tey H. Treatment of acne vulgaris during pregnancy and lactation. Drugs. 2013;73:779-787.
- Leachman SA, Reed BR. The use of dermatologic drugs in pregnancy and lactation. Dermatol Clin. 2006;24:167-197.
- Ly S, Kamal K, Manjaly P, et al. Treatment of acne vulgaris during pregnancy and lactation: a narrative review. Dermatol Ther. 2023;13:115-130.
- Webster G. Combination azelaic acid therapy for acne vulgaris. J Am Acad Dermatol. 2000;43:S47-S50.
- Archer CB, Cohen SN, Baron SE. Guidance on the diagnosis and clinical management of acne. Clin Exp Dermatol. 2012;37(suppl 1):1-6.
- Graupe K, Cunliffe W, Gollnick H, et al. Efficacy and safety of topical azelaic acid (20 percent cream): an overview of results from European clinical trials and experimental reports. Cutis. 1996;57(1 suppl):20-35.
- Bozzo P, Chua-Gocheco A, Einarson A. Safety of skin care products during pregnancy. Can Fam Physician. 2011;57:665-667.
- Munley SM, Kennedy GL, Hurtt ME. Developmental toxicity study of glycolic acid in rats. Drug Chem Toxicol. 1999;22:569-582.
- Chien AL, Qi J, Rainer B, et al. Treatment of acne in pregnancy. J Am Board Fam Med. 2016;29:254-262.
- Stuart B, Maund E, Wilcox C, et al. Topical preparations for the treatment of mild‐to‐moderate acne vulgaris: systematic review and network meta‐analysis. Br J Dermatol. 2021;185:512-525.
- van Hoogdalem EJ, Baven TL, Spiegel‐Melsen I, et al. Transdermal absorption of clindamycin and tretinoin from topically applied anti‐acne formulations in man. Biopharm Drug Dispos. 1998;19:563-569.
- Austin BA, Fleischer AB Jr. The extinction of topical erythromycin therapy for acne vulgaris and concern for the future of topical clindamycin. J Dermatolog Treat. 2017;28:145-148.
- Eady EA, Cove J, Holland K, et al. Erythromycin resistant propionibacteria in antibiotic treated acne patients: association with therapeutic failure. Br J. Dermatol. 1989;121:51-57.
- Alkhawaja E, Hammadi S, Abdelmalek M, et al. Antibiotic resistant Cutibacterium acnes among acne patients in Jordan: a cross sectional study. BMC Dermatol. 2020;20:1-9.
- Han G, Wu JJ, Del Rosso JQ. Use of topical tazarotene for the treatment of acne vulgaris in pregnancy: a literature review. J Clin Aesthet Dermatol. 2020;13:E59-E65.
- Selcen D, Seidman S, Nigro MA. Otocerebral anomalies associated with topical tretinoin use. Brain Dev. 2000;22:218-220.
- Moretz D. Drug Class Update with New Drug Evaluations: Topical Products for Inflammatory Skin Conditions. Oregon State University Drug Use & Research Management Program; December 2022. Accessed January 8, 2024. https://www.orpdl.org/durm/meetings/meetingdocs/2022_12_01/archives/2022_12_01_Inflammatory_Skin_Dz_ClassUpdate.pdf
- Kaplan YC, Ozsarfati J, Etwel F, et al. Pregnancy outcomes following first‐trimester exposure to topical retinoids: a systematic review and meta‐analysis. Br J Dermatol. 2015;173:1132-1141.
- Menter A. Pharmacokinetics and safety of tazarotene. J Am Acad Dermatol. 2000;43(2, pt 3):S31-S35.
- Autret E, Berjot M, Jonville-Béra A-P, et al. Anophthalmia and agenesis of optic chiasma associated with adapalene gel in early pregnancy. Lancet. 1997;350:339.
- Weiss J, Mallavalli S, Meckfessel M, et al. Safe use of adapalene 0.1% gel in a non-prescription environment. J Drugs Dermatol. 2021;20:1330-1335.
- Alessandro Mazzetti M. A phase 2b, randomized, double-blind vehicle controlled, dose escalation study evaluating clascoterone 0.1%, 0.5%, and 1% topical cream in subjects with facial acne. J Drugs Dermatol. 2019;18:570-575.
- Eichenfield L, Hebert A, Gold LS, et al. Open-label, long-term extension study to evaluate the safety of clascoterone (CB-03-01) cream, 1% twice daily, in patients with acne vulgaris. J Am Acad Dermatol. 2020;83:477-485.
- Trifu V, Tiplica GS, Naumescu E, et al. Cortexolone 17α‐propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. a pilot randomized, double‐blind comparative study vs. placebo and tretinoin 0.05% cream. Br J Dermatol. 2011;165:177-183.
- Hebert A, Thiboutot D, Gold LS, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630.
- Alkhodaidi ST, Al Hawsawi KA, Alkhudaidi IT, et al. Efficacy and safety of topical clascoterone cream for treatment of acne vulgaris: a systematic review and meta‐analysis of randomized placebo‐controlled trials. Dermatol Ther. 2021;34:e14609.
- Clasoterone. Package insert. Cassiopea Inc; 2020.
- Paik J. Topical minocycline foam 4%: a review in acne vulgaris. Am J Clin Dermatol. 2020;21:449-456.
- Jones TM, Ellman H. Pharmacokinetic comparison of once-daily topical minocycline foam 4% vs oral minocycline for moderate-to-severe acne. J Drugs Dermatol. 2017;16:1022-1028.
- Minocycline hydrochloride extended-release tablets. Package insert. JG Pharma; July 2020. Accessed January 8, 2024. https://www.jgpharmainc.com/assets/pdf/minocycline-hydrochloride.pdf
- Dinnendahl V, Fricke U (eds). Arzneistoff-Profile: Basisinformation über arzneiliche Wirkstoffe. Govi Pharmazeutischer Verlag; 2010.
- Martins AM, Marto JM, Johnson JL, et al. A review of systemic minocycline side effects and topical minocycline as a safer alternative for treating acne and rosacea. Antibiotics. 2021;10:757.
- Landis MN. Optimizing isotretinoin treatment of acne: update on current recommendations for monitoring, dosing, safety, adverse effects, compliance, and outcomes. Am J Clin Dermatol. 2020;21:411-419.
- Draghici C-C, Miulescu R-G, Petca R-C, et al. Teratogenic effect of isotretinoin in both fertile females and males. Exp Ther Med. 2021;21:1-5.
- Barker RA, Wilcox C, Layton AM. Oral spironolactone for acne vulgaris in adult females: an update of the literature. Am J Clin Dermatol. 2020;21:303-305.
- Han JJ, Faletsky A, Barbieri JS, et al. New acne therapies and updates on use of spironolactone and isotretinoin: a narrative review. Dermatol Ther (Heidelb). 2021;11:79-91.
- Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. Lippincott Williams & Wilkins; 2012.
- Patel DJ, Bhatia N. Oral antibiotics for acne. Am J Clin Dermatol. 2021;22:193-204.
- Jick H, Holmes LB, Hunter JR, et al. First-trimester drug use and congenital disorders. JAMA. 1981;246:343-346.
- Valente Duarte de Sousa IC. An overview of sarecycline for the treatment of moderate-to-severe acne vulgaris. Exp Opin Pharmacother. 2021;22:145-154.
- Hussar DA, Chahine EB. Omadacycline tosylate, sarecycline hydrochloride, rifamycin sodium, and moxidectin. J Am Pharm Assoc. 2019;59:756-760.
- Haidari W, Bruinsma R, Cardenas-de la Garza JA, et al. Sarecycline review. Ann Pharmacother. 2020;54:164-170.
- Feldman S, Careccia RE, Barham KL, et al. Diagnosis and treatment of acne. Am Fam Physician. 2004;69:2123-2130.
- Gammon WR, Meyer C, Lantis S, et al. Comparative efficacy of oral erythromycin versus oral tetracycline in the treatment of acne vulgaris: a double-blind study. J Am Acad Dermatol. 1986;14:183-186.
- Källén BA, Olausson PO, Danielsson BR. Is erythromycin therapy teratogenic in humans? Reprod Toxicol. 2005;20:209-214.
- McCormack WM, George H, Donner A, et al. Hepatotoxicity of erythromycin estolate during pregnancy. Antimicrob Agents Chemother. 1977;12:630-635.
- Cervantes J, Eber AE, Perper M, et al. The role of zinc in the treatment of acne: a review of the literature. Dermatolog Ther. 2018;31:e12576.
- Dréno B, Blouin E. Acne, pregnant women and zinc salts: a literature review [in French]. Ann Dermatol Venereol. 2008;135:27-33.
- Eid MM, Saleh MS, Allam NM, et al. Narrow band ultraviolet B versus red light-emitting diodes in the treatment of facial acne vulgaris: a randomized controlled trial. Photobiomodul Photomed Laser Surg. 2021;39:418-424.
- Zeichner JA. Narrowband UV-B phototherapy for the treatment of acne vulgaris during pregnancy. Arch Dermatol. 2011;147:537-539.
- El-Saie LT, Rabie AR, Kamel MI, et al. Effect of narrowband ultraviolet B phototherapy on serum folic acid levels in patients with psoriasis. Lasers Med Sci. 2011;26:481-485.
- Park KK, Murase JE. Narrowband UV-B phototherapy during pregnancy and folic acid depletion. Arch Dermatol. 2012;148:132-133.
- Jablonski NG. A possible link between neural tube defects and ultraviolet light exposure. Med Hypotheses. 1999;52:581-582.
- Zhang M, Goyert G, Lim HW. Folate and phototherapy: what should we inform our patients? J Am Acad Dermatol. 2017;77:958-964.
- AviClear. Cutera website. Accessed January 8, 2024. https://www.cutera.com/solutions/aviclear/
- Wu X, Yang Y, Wang Y, et al. Treatment of refractory acne using selective sebaceous gland electro-thermolysis combined with non-thermal plasma. J Cosmet Laser Ther. 2021;23:188-194.
- Ahn GR, Kim JM, Park SJ, et al. Selective sebaceous gland electrothermolysis using a single microneedle radiofrequency device for acne patients: a prospective randomized controlled study. Lasers Surg Med. 2020;52:396-401.
- Fabbrocini G, De Padova MP, Tosti A. Chemical peels: what’s new and what isn’t new but still works well. Facial Plast Surg. 2009;25:329-336.
- Andersen FA. Final report on the safety assessment of glycolic acid, ammonium, calcium, potassium, and sodium glycolates, methyl, ethyl, propyl, and butyl glycolates, and lactic acid, ammonium, calcium, potassium, sodium, and TEA-lactates, methyl, ethyl, isopropyl, and butyl lactates, and lauryl, myristyl, and cetyl lactates. Int J Toxicol. 1998;17(1_suppl):1-241.
- Lee KC, Korgavkar K, Dufresne RG Jr, et al. Safety of cosmetic dermatologic procedures during pregnancy. Dermatol Surg. 2013;39:1573-1586.
- James AH, Brancazio LR, Price T. Aspirin and reproductive outcomes. Obstet Gynecol Surv. 2008;63:49-57.
- Zhou W-S, Xu L, Xie S-H, et al. Decreased birth weight in relation to maternal urinary trichloroacetic acid levels. Sci Total Environ. 2012;416:105-110.
- Schwartz DB, Greenberg MD, Daoud Y, et al. Genital condylomas in pregnancy: use of trichloroacetic acid and laser therapy. Am J Obstet Gynecol. 1988;158:1407-1416.
- Starkman SJ, Mangat DS. Chemical peel (deep, medium, light). Facial Plast Surg Clin North Am. 2020;28:45-57.
- Trivedi M, Kroumpouzos G, Murase J. A review of the safety of cosmetic procedures during pregnancy and lactation. Int J Womens Dermatol. 2017;3:6-10.
- Gallagher T, Taliercio M, Nia JK, et al. Dermatologist use of intralesional triamcinolone in the treatment of acne. J Clin Aesthet Dermatol. 2020;13:41-43.
- Zamil DH, Burns EK, Perez-Sanchez A, et al. Risk of birth defects from vitamin A “acne supplements” sold online. Dermatol Pract Concept. 2021;11:e2021075.
- Bhate K, Williams H. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485.
- Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10:5754.
- Fisk WA, Lev-Tov HA, Sivamani RK. Epidemiology and management of acne in adult women. Curr Dermatol Rep. 2014;3:29-39.
- Perkins A, Cheng C, Hillebrand G, et al. Comparison of the epidemiology of acne vulgaris among Caucasian, Asian, Continental Indian and African American women. J Eur Acad Dermatol Venereol. 2011;25:1054-1060.
- Yang CC, Huang YT, Yu CH, et al. Inflammatory facial acne during uncomplicated pregnancy and post‐partum in adult women: a preliminary hospital‐based prospective observational study of 35 cases from Taiwan. J Eur Acad Dermatol Venereol. 2016;30:1787-1789.
- Dréno B, Blouin E, Moyse D, et al. Acne in pregnant women: a French survey. Acta Derm Venereol. 2014;94:82-83.
- Kutlu Ö, Karadag˘ AS, Ünal E, et al. Acne in pregnancy: a prospective multicenter, cross‐sectional study of 295 patients in Turkey. Int J Dermatol. 2020;59:1098-1105.
- Hoefel IDR, Weber MB, Manzoni APD, et al. Striae gravidarum, acne, facial spots, and hair disorders: risk factors in a study with 1284 puerperal patients. J Pregnancy. 2020;2020:8036109.
- Ayanlowo OO, Otrofanowei E, Shorunmu TO, et al. Pregnancy dermatoses: a study of patients attending the antenatal clinic at two tertiary care centers in south west Nigeria. PAMJ Clin Med. 2020;3.
- Bechstein S, Ochsendorf F. Acne and rosacea in pregnancy. Hautarzt. 2017;68:111-119.
- Habeshian KA, Cohen BA. Current issues in the treatment of acne vulgaris. Pediatrics. 2020;145(suppl 2):S225-S230.
- Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling (21 CFR 201). Fed Regist. 2014;79:72064-72103.
- Sagransky M, Yentzer BA, Feldman SR. Benzoyl peroxide: a review of its current use in the treatment of acne vulgaris. Expert Opin Pharmacother. 2009;10:2555-2562.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part I. Pregnancy. J Am Acad Dermatol. 2014;70:401.e1-401.e14; quiz 415.
- Wolverton SE. Systemic corticosteroids. Comprehensive Dermatol Drug Ther. 2012;3:143-168.
- Kirtschig G, Schaefer C. Dermatological medications and local therapeutics. In: Schaefer C, Peters P, Miller RK, eds. Drugs During Pregnancy and Lactation. 3rd edition. Elsevier; 2015:467-492.
- Pugashetti R, Shinkai K. Treatment of acne vulgaris in pregnant patients. Dermatol Ther. 2013;26:302-311.
- Touitou E, Godin B, Shumilov M, et al. Efficacy and tolerability of clindamycin phosphate and salicylic acid gel in the treatment of mild to moderate acne vulgaris. J Eur Acad Dermatol Venereol. 2008;22:629-631.
- Schaefer C, Peters PW, Miller RK, eds. Drugs During Pregnancy and Lactation: Treatment Options and Risk Assessment. 2nd ed. Academic Press; 2014.
- Birmingham B, Greene D, Rhodes C. Systemic absorption of topical salicylic acid. Int J Dermatol. 1979;18:228-231.
- Trivedi NA. A meta-analysis of low-dose aspirin for prevention of preeclampsia. J Postgrad Med. 2011;57:91-95.
- Lucky AW, Maloney JM, Roberts J, et al. Dapsone gel 5% for the treatment of acne vulgaris: safety and efficacy of long-term (1 year) treatment. J Drugs Dermatol. 2007;6:981-987.
- Nosten F, McGready R, d’Alessandro U, et al. Antimalarial drugs in pregnancy: a review. Curr Drug Saf. 2006;1:1-15.
- Brabin BJ, Eggelte TA, Parise M, et al. Dapsone therapy for malaria during pregnancy: maternal and fetal outcomes. Drug Saf. 2004;27:633-648.
- Tuffanelli DL. Successful pregnancy in a patient with dermatitis herpetiformis treated with low-dose dapsone. Arch Dermatol. 1982;118:876.
- Meredith FM, Ormerod AD. The management of acne vulgaris in pregnancy. Am J Clin Dermatol. 2013;14:351-358.
- Kong Y, Tey H. Treatment of acne vulgaris during pregnancy and lactation. Drugs. 2013;73:779-787.
- Leachman SA, Reed BR. The use of dermatologic drugs in pregnancy and lactation. Dermatol Clin. 2006;24:167-197.
- Ly S, Kamal K, Manjaly P, et al. Treatment of acne vulgaris during pregnancy and lactation: a narrative review. Dermatol Ther. 2023;13:115-130.
- Webster G. Combination azelaic acid therapy for acne vulgaris. J Am Acad Dermatol. 2000;43:S47-S50.
- Archer CB, Cohen SN, Baron SE. Guidance on the diagnosis and clinical management of acne. Clin Exp Dermatol. 2012;37(suppl 1):1-6.
- Graupe K, Cunliffe W, Gollnick H, et al. Efficacy and safety of topical azelaic acid (20 percent cream): an overview of results from European clinical trials and experimental reports. Cutis. 1996;57(1 suppl):20-35.
- Bozzo P, Chua-Gocheco A, Einarson A. Safety of skin care products during pregnancy. Can Fam Physician. 2011;57:665-667.
- Munley SM, Kennedy GL, Hurtt ME. Developmental toxicity study of glycolic acid in rats. Drug Chem Toxicol. 1999;22:569-582.
- Chien AL, Qi J, Rainer B, et al. Treatment of acne in pregnancy. J Am Board Fam Med. 2016;29:254-262.
- Stuart B, Maund E, Wilcox C, et al. Topical preparations for the treatment of mild‐to‐moderate acne vulgaris: systematic review and network meta‐analysis. Br J Dermatol. 2021;185:512-525.
- van Hoogdalem EJ, Baven TL, Spiegel‐Melsen I, et al. Transdermal absorption of clindamycin and tretinoin from topically applied anti‐acne formulations in man. Biopharm Drug Dispos. 1998;19:563-569.
- Austin BA, Fleischer AB Jr. The extinction of topical erythromycin therapy for acne vulgaris and concern for the future of topical clindamycin. J Dermatolog Treat. 2017;28:145-148.
- Eady EA, Cove J, Holland K, et al. Erythromycin resistant propionibacteria in antibiotic treated acne patients: association with therapeutic failure. Br J. Dermatol. 1989;121:51-57.
- Alkhawaja E, Hammadi S, Abdelmalek M, et al. Antibiotic resistant Cutibacterium acnes among acne patients in Jordan: a cross sectional study. BMC Dermatol. 2020;20:1-9.
- Han G, Wu JJ, Del Rosso JQ. Use of topical tazarotene for the treatment of acne vulgaris in pregnancy: a literature review. J Clin Aesthet Dermatol. 2020;13:E59-E65.
- Selcen D, Seidman S, Nigro MA. Otocerebral anomalies associated with topical tretinoin use. Brain Dev. 2000;22:218-220.
- Moretz D. Drug Class Update with New Drug Evaluations: Topical Products for Inflammatory Skin Conditions. Oregon State University Drug Use & Research Management Program; December 2022. Accessed January 8, 2024. https://www.orpdl.org/durm/meetings/meetingdocs/2022_12_01/archives/2022_12_01_Inflammatory_Skin_Dz_ClassUpdate.pdf
- Kaplan YC, Ozsarfati J, Etwel F, et al. Pregnancy outcomes following first‐trimester exposure to topical retinoids: a systematic review and meta‐analysis. Br J Dermatol. 2015;173:1132-1141.
- Menter A. Pharmacokinetics and safety of tazarotene. J Am Acad Dermatol. 2000;43(2, pt 3):S31-S35.
- Autret E, Berjot M, Jonville-Béra A-P, et al. Anophthalmia and agenesis of optic chiasma associated with adapalene gel in early pregnancy. Lancet. 1997;350:339.
- Weiss J, Mallavalli S, Meckfessel M, et al. Safe use of adapalene 0.1% gel in a non-prescription environment. J Drugs Dermatol. 2021;20:1330-1335.
- Alessandro Mazzetti M. A phase 2b, randomized, double-blind vehicle controlled, dose escalation study evaluating clascoterone 0.1%, 0.5%, and 1% topical cream in subjects with facial acne. J Drugs Dermatol. 2019;18:570-575.
- Eichenfield L, Hebert A, Gold LS, et al. Open-label, long-term extension study to evaluate the safety of clascoterone (CB-03-01) cream, 1% twice daily, in patients with acne vulgaris. J Am Acad Dermatol. 2020;83:477-485.
- Trifu V, Tiplica GS, Naumescu E, et al. Cortexolone 17α‐propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. a pilot randomized, double‐blind comparative study vs. placebo and tretinoin 0.05% cream. Br J Dermatol. 2011;165:177-183.
- Hebert A, Thiboutot D, Gold LS, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630.
- Alkhodaidi ST, Al Hawsawi KA, Alkhudaidi IT, et al. Efficacy and safety of topical clascoterone cream for treatment of acne vulgaris: a systematic review and meta‐analysis of randomized placebo‐controlled trials. Dermatol Ther. 2021;34:e14609.
- Clasoterone. Package insert. Cassiopea Inc; 2020.
- Paik J. Topical minocycline foam 4%: a review in acne vulgaris. Am J Clin Dermatol. 2020;21:449-456.
- Jones TM, Ellman H. Pharmacokinetic comparison of once-daily topical minocycline foam 4% vs oral minocycline for moderate-to-severe acne. J Drugs Dermatol. 2017;16:1022-1028.
- Minocycline hydrochloride extended-release tablets. Package insert. JG Pharma; July 2020. Accessed January 8, 2024. https://www.jgpharmainc.com/assets/pdf/minocycline-hydrochloride.pdf
- Dinnendahl V, Fricke U (eds). Arzneistoff-Profile: Basisinformation über arzneiliche Wirkstoffe. Govi Pharmazeutischer Verlag; 2010.
- Martins AM, Marto JM, Johnson JL, et al. A review of systemic minocycline side effects and topical minocycline as a safer alternative for treating acne and rosacea. Antibiotics. 2021;10:757.
- Landis MN. Optimizing isotretinoin treatment of acne: update on current recommendations for monitoring, dosing, safety, adverse effects, compliance, and outcomes. Am J Clin Dermatol. 2020;21:411-419.
- Draghici C-C, Miulescu R-G, Petca R-C, et al. Teratogenic effect of isotretinoin in both fertile females and males. Exp Ther Med. 2021;21:1-5.
- Barker RA, Wilcox C, Layton AM. Oral spironolactone for acne vulgaris in adult females: an update of the literature. Am J Clin Dermatol. 2020;21:303-305.
- Han JJ, Faletsky A, Barbieri JS, et al. New acne therapies and updates on use of spironolactone and isotretinoin: a narrative review. Dermatol Ther (Heidelb). 2021;11:79-91.
- Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. Lippincott Williams & Wilkins; 2012.
- Patel DJ, Bhatia N. Oral antibiotics for acne. Am J Clin Dermatol. 2021;22:193-204.
- Jick H, Holmes LB, Hunter JR, et al. First-trimester drug use and congenital disorders. JAMA. 1981;246:343-346.
- Valente Duarte de Sousa IC. An overview of sarecycline for the treatment of moderate-to-severe acne vulgaris. Exp Opin Pharmacother. 2021;22:145-154.
- Hussar DA, Chahine EB. Omadacycline tosylate, sarecycline hydrochloride, rifamycin sodium, and moxidectin. J Am Pharm Assoc. 2019;59:756-760.
- Haidari W, Bruinsma R, Cardenas-de la Garza JA, et al. Sarecycline review. Ann Pharmacother. 2020;54:164-170.
- Feldman S, Careccia RE, Barham KL, et al. Diagnosis and treatment of acne. Am Fam Physician. 2004;69:2123-2130.
- Gammon WR, Meyer C, Lantis S, et al. Comparative efficacy of oral erythromycin versus oral tetracycline in the treatment of acne vulgaris: a double-blind study. J Am Acad Dermatol. 1986;14:183-186.
- Källén BA, Olausson PO, Danielsson BR. Is erythromycin therapy teratogenic in humans? Reprod Toxicol. 2005;20:209-214.
- McCormack WM, George H, Donner A, et al. Hepatotoxicity of erythromycin estolate during pregnancy. Antimicrob Agents Chemother. 1977;12:630-635.
- Cervantes J, Eber AE, Perper M, et al. The role of zinc in the treatment of acne: a review of the literature. Dermatolog Ther. 2018;31:e12576.
- Dréno B, Blouin E. Acne, pregnant women and zinc salts: a literature review [in French]. Ann Dermatol Venereol. 2008;135:27-33.
- Eid MM, Saleh MS, Allam NM, et al. Narrow band ultraviolet B versus red light-emitting diodes in the treatment of facial acne vulgaris: a randomized controlled trial. Photobiomodul Photomed Laser Surg. 2021;39:418-424.
- Zeichner JA. Narrowband UV-B phototherapy for the treatment of acne vulgaris during pregnancy. Arch Dermatol. 2011;147:537-539.
- El-Saie LT, Rabie AR, Kamel MI, et al. Effect of narrowband ultraviolet B phototherapy on serum folic acid levels in patients with psoriasis. Lasers Med Sci. 2011;26:481-485.
- Park KK, Murase JE. Narrowband UV-B phototherapy during pregnancy and folic acid depletion. Arch Dermatol. 2012;148:132-133.
- Jablonski NG. A possible link between neural tube defects and ultraviolet light exposure. Med Hypotheses. 1999;52:581-582.
- Zhang M, Goyert G, Lim HW. Folate and phototherapy: what should we inform our patients? J Am Acad Dermatol. 2017;77:958-964.
- AviClear. Cutera website. Accessed January 8, 2024. https://www.cutera.com/solutions/aviclear/
- Wu X, Yang Y, Wang Y, et al. Treatment of refractory acne using selective sebaceous gland electro-thermolysis combined with non-thermal plasma. J Cosmet Laser Ther. 2021;23:188-194.
- Ahn GR, Kim JM, Park SJ, et al. Selective sebaceous gland electrothermolysis using a single microneedle radiofrequency device for acne patients: a prospective randomized controlled study. Lasers Surg Med. 2020;52:396-401.
- Fabbrocini G, De Padova MP, Tosti A. Chemical peels: what’s new and what isn’t new but still works well. Facial Plast Surg. 2009;25:329-336.
- Andersen FA. Final report on the safety assessment of glycolic acid, ammonium, calcium, potassium, and sodium glycolates, methyl, ethyl, propyl, and butyl glycolates, and lactic acid, ammonium, calcium, potassium, sodium, and TEA-lactates, methyl, ethyl, isopropyl, and butyl lactates, and lauryl, myristyl, and cetyl lactates. Int J Toxicol. 1998;17(1_suppl):1-241.
- Lee KC, Korgavkar K, Dufresne RG Jr, et al. Safety of cosmetic dermatologic procedures during pregnancy. Dermatol Surg. 2013;39:1573-1586.
- James AH, Brancazio LR, Price T. Aspirin and reproductive outcomes. Obstet Gynecol Surv. 2008;63:49-57.
- Zhou W-S, Xu L, Xie S-H, et al. Decreased birth weight in relation to maternal urinary trichloroacetic acid levels. Sci Total Environ. 2012;416:105-110.
- Schwartz DB, Greenberg MD, Daoud Y, et al. Genital condylomas in pregnancy: use of trichloroacetic acid and laser therapy. Am J Obstet Gynecol. 1988;158:1407-1416.
- Starkman SJ, Mangat DS. Chemical peel (deep, medium, light). Facial Plast Surg Clin North Am. 2020;28:45-57.
- Trivedi M, Kroumpouzos G, Murase J. A review of the safety of cosmetic procedures during pregnancy and lactation. Int J Womens Dermatol. 2017;3:6-10.
- Gallagher T, Taliercio M, Nia JK, et al. Dermatologist use of intralesional triamcinolone in the treatment of acne. J Clin Aesthet Dermatol. 2020;13:41-43.
- Zamil DH, Burns EK, Perez-Sanchez A, et al. Risk of birth defects from vitamin A “acne supplements” sold online. Dermatol Pract Concept. 2021;11:e2021075.
Practice Points
- The management of acne in pregnancy requires careful consideration of therapeutic choices to guarantee the safety of both the mother and the developing fetus.
- The use of topicals should be observed as first-line therapy, but consideration for systemic therapy in cases of treatment failure or more severe disease is warranted.
- Discussion of patient expectations and involving them in decision-making for therapeutic choice is crucial.
A Look at the Evidence Linking Diet to Skin Conditions
ORLANDO, FLORIDA — Amid all the hype, claims, and confusion, there is evidence linking some foods and drinks to an increased risk for acne, psoriasis, atopic dermatitis, rosacea, and other common skin conditions. So, what is the connection in each case? And how can people with any of these skin conditions potentially improve their health and quality of life with dietary changes?
What is clear is that there has been an explosion of interest in learning which foods can improve or worsen skin issues in recent years. It’s a good idea to familiarize yourself with the research and also to Google ‘diet’ and ‘skin’, said Vivian Shi, MD, associate professor of dermatology at the University of Arkansas for Medical Sciences, Little Rock. “As practitioners, we should be well prepared to talk about what patients want to talk about.”
Acne
One of the major areas of interest is diet and acne. “We’ve all heard sugar and dairy are bad, and the Western diet is high in sugar and dairy,” Dr. Shi said at the ODAC Dermatology, Aesthetic & Surgical Conference.
Dairy, red meat, and carbohydrates can break down into leucine, an essential amino acid found in protein. Leucine and sugar together, in turn, can produce insulin and insulin-like growth factor 1 (IGF-1), which, through different pathways, can reach the androgen receptors throughout the body, including the skin. This results in sebogenesis, lipogenesis, and keratinization, which triggers follicular inflammation and results in more of the acne-causing bacteria Cutibacterium acnes.
Milk and other dairy products also can increase IGF-1 levels, which can alter hormonal mediators and increase acne.
Not all types of dairy milk are created equal, however, when it comes to acne. Dr. Shi wondered why 2% milk has overall color and nutritional content very similar to that of whole milk. “I looked into this.” She discovered that when milk manufacturers remove the fat, they often add whey proteins to restore some nutrients. Whey protein can increase acne, Dr. Shi added.
“So, if you’re going to choose any milk to drink, I think from an acne perspective, it’s better to use whole milk. If you can get it organic, even better.” Skim milk is the most acnegenic, she said.
Psoriasis
A systematic review of 55 studies evaluating diet and psoriasis found obesity can be an exacerbating factor. The strongest evidence for dietary weight reduction points to a hypocaloric diet in people with overweight or obesity, according to the review. Other evidence suggests alcohol can lower response to treatment and is linked with more severe psoriasis. Furthermore, a gluten-free diet or vitamin D supplements can help some subpopulations of people with psoriasis.
“An overwhelming majority of our psoriasis patients are vitamin D deficient,” Dr. Shi said.
The National Psoriasis Foundation (NPF) publishes dietary modification guidelines, updated as recently as November 2023. The NPF states that “there is no diet that will cure psoriatic disease, but there are many ways in which eating healthful food may lessen the severity of symptoms and play a role in lowering the likelihood of developing comorbidities.”
Healthier choices include fruits, vegetables, whole grains, and fat-free or low-fat dairy products. Include lean meats, poultry, fish, beans, eggs, and nuts. Adherence to a Mediterranean diet has been linked to a lower severity of psoriasis.
Atopic Dermatitis
Atopic dermatitis (AD) is “one of the prototypical diseases related to diet,” Dr. Shi said. A different meta-analysis looked at randomized controlled trials of synbiotics (a combination of prebiotics and probiotics) for treatment of AD.
These researchers found that synbiotics do not prevent AD, but they can help treat it in adults and children older than 1 year. In addition, synbiotics are more beneficial than probiotics in treating the condition, although there are no head-to-head comparison studies. In addition, the meta-analysis found that prebiotics alone can lower AD severity.
However, Dr. Shi said, there are no recommendations from the American Academy of Dermatology (AAD) on prebiotics or probiotics for AD, and the AAD does not recommend any supplement or essential oil for AD.
In a 2022 review, investigators ranked the efficacy of different supplements for AD based on available evidence. They found the greatest benefit associated with vitamin D supplementation, followed by vitamin E, probiotics, hemp seed oil, histidine, and oolong tea. They also noted the ‘Six Food Elimination Diet and Autoimmune Protocol’ featured the least amount of evidence to back it up.
Rosacea
Rosacea appears to be caused by “all the fun things in life” like sunlight, alcohol, chocolate, spicy foods, and caffeine, Dr. Shi said. In people with rosacea, they can cause facial flushing, edema, burning, and an inflammatory response.
Certain foods can activate skin receptors and sensory neurons, which can release neuropeptides that act on mast cells in blood that lead to flushing. The skin-gut axis may also be involved, evidence suggests. “And that is why food has a pretty profound impact on rosacea,” Dr. Shi said.
Dr. Shi reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
ORLANDO, FLORIDA — Amid all the hype, claims, and confusion, there is evidence linking some foods and drinks to an increased risk for acne, psoriasis, atopic dermatitis, rosacea, and other common skin conditions. So, what is the connection in each case? And how can people with any of these skin conditions potentially improve their health and quality of life with dietary changes?
What is clear is that there has been an explosion of interest in learning which foods can improve or worsen skin issues in recent years. It’s a good idea to familiarize yourself with the research and also to Google ‘diet’ and ‘skin’, said Vivian Shi, MD, associate professor of dermatology at the University of Arkansas for Medical Sciences, Little Rock. “As practitioners, we should be well prepared to talk about what patients want to talk about.”
Acne
One of the major areas of interest is diet and acne. “We’ve all heard sugar and dairy are bad, and the Western diet is high in sugar and dairy,” Dr. Shi said at the ODAC Dermatology, Aesthetic & Surgical Conference.
Dairy, red meat, and carbohydrates can break down into leucine, an essential amino acid found in protein. Leucine and sugar together, in turn, can produce insulin and insulin-like growth factor 1 (IGF-1), which, through different pathways, can reach the androgen receptors throughout the body, including the skin. This results in sebogenesis, lipogenesis, and keratinization, which triggers follicular inflammation and results in more of the acne-causing bacteria Cutibacterium acnes.
Milk and other dairy products also can increase IGF-1 levels, which can alter hormonal mediators and increase acne.
Not all types of dairy milk are created equal, however, when it comes to acne. Dr. Shi wondered why 2% milk has overall color and nutritional content very similar to that of whole milk. “I looked into this.” She discovered that when milk manufacturers remove the fat, they often add whey proteins to restore some nutrients. Whey protein can increase acne, Dr. Shi added.
“So, if you’re going to choose any milk to drink, I think from an acne perspective, it’s better to use whole milk. If you can get it organic, even better.” Skim milk is the most acnegenic, she said.
Psoriasis
A systematic review of 55 studies evaluating diet and psoriasis found obesity can be an exacerbating factor. The strongest evidence for dietary weight reduction points to a hypocaloric diet in people with overweight or obesity, according to the review. Other evidence suggests alcohol can lower response to treatment and is linked with more severe psoriasis. Furthermore, a gluten-free diet or vitamin D supplements can help some subpopulations of people with psoriasis.
“An overwhelming majority of our psoriasis patients are vitamin D deficient,” Dr. Shi said.
The National Psoriasis Foundation (NPF) publishes dietary modification guidelines, updated as recently as November 2023. The NPF states that “there is no diet that will cure psoriatic disease, but there are many ways in which eating healthful food may lessen the severity of symptoms and play a role in lowering the likelihood of developing comorbidities.”
Healthier choices include fruits, vegetables, whole grains, and fat-free or low-fat dairy products. Include lean meats, poultry, fish, beans, eggs, and nuts. Adherence to a Mediterranean diet has been linked to a lower severity of psoriasis.
Atopic Dermatitis
Atopic dermatitis (AD) is “one of the prototypical diseases related to diet,” Dr. Shi said. A different meta-analysis looked at randomized controlled trials of synbiotics (a combination of prebiotics and probiotics) for treatment of AD.
These researchers found that synbiotics do not prevent AD, but they can help treat it in adults and children older than 1 year. In addition, synbiotics are more beneficial than probiotics in treating the condition, although there are no head-to-head comparison studies. In addition, the meta-analysis found that prebiotics alone can lower AD severity.
However, Dr. Shi said, there are no recommendations from the American Academy of Dermatology (AAD) on prebiotics or probiotics for AD, and the AAD does not recommend any supplement or essential oil for AD.
In a 2022 review, investigators ranked the efficacy of different supplements for AD based on available evidence. They found the greatest benefit associated with vitamin D supplementation, followed by vitamin E, probiotics, hemp seed oil, histidine, and oolong tea. They also noted the ‘Six Food Elimination Diet and Autoimmune Protocol’ featured the least amount of evidence to back it up.
Rosacea
Rosacea appears to be caused by “all the fun things in life” like sunlight, alcohol, chocolate, spicy foods, and caffeine, Dr. Shi said. In people with rosacea, they can cause facial flushing, edema, burning, and an inflammatory response.
Certain foods can activate skin receptors and sensory neurons, which can release neuropeptides that act on mast cells in blood that lead to flushing. The skin-gut axis may also be involved, evidence suggests. “And that is why food has a pretty profound impact on rosacea,” Dr. Shi said.
Dr. Shi reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
ORLANDO, FLORIDA — Amid all the hype, claims, and confusion, there is evidence linking some foods and drinks to an increased risk for acne, psoriasis, atopic dermatitis, rosacea, and other common skin conditions. So, what is the connection in each case? And how can people with any of these skin conditions potentially improve their health and quality of life with dietary changes?
What is clear is that there has been an explosion of interest in learning which foods can improve or worsen skin issues in recent years. It’s a good idea to familiarize yourself with the research and also to Google ‘diet’ and ‘skin’, said Vivian Shi, MD, associate professor of dermatology at the University of Arkansas for Medical Sciences, Little Rock. “As practitioners, we should be well prepared to talk about what patients want to talk about.”
Acne
One of the major areas of interest is diet and acne. “We’ve all heard sugar and dairy are bad, and the Western diet is high in sugar and dairy,” Dr. Shi said at the ODAC Dermatology, Aesthetic & Surgical Conference.
Dairy, red meat, and carbohydrates can break down into leucine, an essential amino acid found in protein. Leucine and sugar together, in turn, can produce insulin and insulin-like growth factor 1 (IGF-1), which, through different pathways, can reach the androgen receptors throughout the body, including the skin. This results in sebogenesis, lipogenesis, and keratinization, which triggers follicular inflammation and results in more of the acne-causing bacteria Cutibacterium acnes.
Milk and other dairy products also can increase IGF-1 levels, which can alter hormonal mediators and increase acne.
Not all types of dairy milk are created equal, however, when it comes to acne. Dr. Shi wondered why 2% milk has overall color and nutritional content very similar to that of whole milk. “I looked into this.” She discovered that when milk manufacturers remove the fat, they often add whey proteins to restore some nutrients. Whey protein can increase acne, Dr. Shi added.
“So, if you’re going to choose any milk to drink, I think from an acne perspective, it’s better to use whole milk. If you can get it organic, even better.” Skim milk is the most acnegenic, she said.
Psoriasis
A systematic review of 55 studies evaluating diet and psoriasis found obesity can be an exacerbating factor. The strongest evidence for dietary weight reduction points to a hypocaloric diet in people with overweight or obesity, according to the review. Other evidence suggests alcohol can lower response to treatment and is linked with more severe psoriasis. Furthermore, a gluten-free diet or vitamin D supplements can help some subpopulations of people with psoriasis.
“An overwhelming majority of our psoriasis patients are vitamin D deficient,” Dr. Shi said.
The National Psoriasis Foundation (NPF) publishes dietary modification guidelines, updated as recently as November 2023. The NPF states that “there is no diet that will cure psoriatic disease, but there are many ways in which eating healthful food may lessen the severity of symptoms and play a role in lowering the likelihood of developing comorbidities.”
Healthier choices include fruits, vegetables, whole grains, and fat-free or low-fat dairy products. Include lean meats, poultry, fish, beans, eggs, and nuts. Adherence to a Mediterranean diet has been linked to a lower severity of psoriasis.
Atopic Dermatitis
Atopic dermatitis (AD) is “one of the prototypical diseases related to diet,” Dr. Shi said. A different meta-analysis looked at randomized controlled trials of synbiotics (a combination of prebiotics and probiotics) for treatment of AD.
These researchers found that synbiotics do not prevent AD, but they can help treat it in adults and children older than 1 year. In addition, synbiotics are more beneficial than probiotics in treating the condition, although there are no head-to-head comparison studies. In addition, the meta-analysis found that prebiotics alone can lower AD severity.
However, Dr. Shi said, there are no recommendations from the American Academy of Dermatology (AAD) on prebiotics or probiotics for AD, and the AAD does not recommend any supplement or essential oil for AD.
In a 2022 review, investigators ranked the efficacy of different supplements for AD based on available evidence. They found the greatest benefit associated with vitamin D supplementation, followed by vitamin E, probiotics, hemp seed oil, histidine, and oolong tea. They also noted the ‘Six Food Elimination Diet and Autoimmune Protocol’ featured the least amount of evidence to back it up.
Rosacea
Rosacea appears to be caused by “all the fun things in life” like sunlight, alcohol, chocolate, spicy foods, and caffeine, Dr. Shi said. In people with rosacea, they can cause facial flushing, edema, burning, and an inflammatory response.
Certain foods can activate skin receptors and sensory neurons, which can release neuropeptides that act on mast cells in blood that lead to flushing. The skin-gut axis may also be involved, evidence suggests. “And that is why food has a pretty profound impact on rosacea,” Dr. Shi said.
Dr. Shi reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Efficacy of Topical Clascoterone for Acne Increased Over Time, Analysis Shows
TOPLINE:
METHODOLOGY:
- A 1% cream formulation of clascoterone, a topical androgen receptor inhibitor, is approved for the treatment of acne vulgaris in patients aged 12 years and older based on results from two identical phase 3 12-week trials, NCT02608450 and NCT02608476, and a long-term extension (LTE) study.
- The purpose of the current study was to evaluate the integrated efficacy of clascoterone cream 1% (Winlevi) in the intention-to-treat population of patients from all three trials.
- In the pivotal trials, investigators randomized patients with acne 1:1 to receive clascoterone cream 1% or vehicle twice daily for 12 weeks. Participants were eligible to enter the LTE study, in which patients applied clascoterone to the face, and if they wanted to, the trunk for up to 9 more months.
- To assess combined efficacy, researchers evaluated the proportion of patients who achieved an Investigator’s Global Assessment (IGA) of 0 or 1.
TAKEAWAY:
- Of the 1143 patients from the pivotal trials who completed 12 weeks of treatment, 576 were in the clascoterone group and 567 were in the vehicle group. Of the 600 patients who entered the LTE study, 311 were in the clascoterone group and 289 were in the vehicle group. Of these, 343 completed the LTE study.
- At week 12, the proportion of patients who achieved treatment success was higher in the clascoterone group than in the vehicle group (19.9% vs 7.7%, respectively; P < .0001).
- In the LTE study, the proportion of patients previously treated with clascoterone who achieved a facial IGA of 0/1 increased from 13.5% at extension day 0 to 29.9% at extension day 274, while the proportion of patients previously treated with vehicle and switched to clascoterone who achieved a facial IGA of 0/1 increased from 6.2% at extension day 0 to 30.4% at extension day 274.
- Similarly, the proportion of patients in the LTE study with a truncal IGA of 0/1 increased from 4.9% at extension day 0 to 31.7% on extension day 274.
IN PRACTICE:
“Clinicians may consider counseling patients that treatment persistence is required to maximize the efficacy of clascoterone treatment,” the authors concluded.
SOURCE:
Lawrence F. Eichenfield, MD, of the departments of dermatology and pediatrics at the University of California and Rady Children’s Hospital, San Diego, California, led the research. The study was published in the January 2024 issue of the Journal of Drugs in Dermatology.
LIMITATIONS:
There was a high patient discontinuation rate before and during the LET study. Also, no assessment was made as to how clascoterone affected patients’ quality of life.
DISCLOSURES:
Clascoterone manufacturer Cassiopea funded the studies. Dr. Eichenfield and fellow investigators Adelaide A. Hebert, MD, and Linda Stein Gold, MD, received compensation from Cassiopea as advisers and disclosed ties to many other pharmaceutical companies.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- A 1% cream formulation of clascoterone, a topical androgen receptor inhibitor, is approved for the treatment of acne vulgaris in patients aged 12 years and older based on results from two identical phase 3 12-week trials, NCT02608450 and NCT02608476, and a long-term extension (LTE) study.
- The purpose of the current study was to evaluate the integrated efficacy of clascoterone cream 1% (Winlevi) in the intention-to-treat population of patients from all three trials.
- In the pivotal trials, investigators randomized patients with acne 1:1 to receive clascoterone cream 1% or vehicle twice daily for 12 weeks. Participants were eligible to enter the LTE study, in which patients applied clascoterone to the face, and if they wanted to, the trunk for up to 9 more months.
- To assess combined efficacy, researchers evaluated the proportion of patients who achieved an Investigator’s Global Assessment (IGA) of 0 or 1.
TAKEAWAY:
- Of the 1143 patients from the pivotal trials who completed 12 weeks of treatment, 576 were in the clascoterone group and 567 were in the vehicle group. Of the 600 patients who entered the LTE study, 311 were in the clascoterone group and 289 were in the vehicle group. Of these, 343 completed the LTE study.
- At week 12, the proportion of patients who achieved treatment success was higher in the clascoterone group than in the vehicle group (19.9% vs 7.7%, respectively; P < .0001).
- In the LTE study, the proportion of patients previously treated with clascoterone who achieved a facial IGA of 0/1 increased from 13.5% at extension day 0 to 29.9% at extension day 274, while the proportion of patients previously treated with vehicle and switched to clascoterone who achieved a facial IGA of 0/1 increased from 6.2% at extension day 0 to 30.4% at extension day 274.
- Similarly, the proportion of patients in the LTE study with a truncal IGA of 0/1 increased from 4.9% at extension day 0 to 31.7% on extension day 274.
IN PRACTICE:
“Clinicians may consider counseling patients that treatment persistence is required to maximize the efficacy of clascoterone treatment,” the authors concluded.
SOURCE:
Lawrence F. Eichenfield, MD, of the departments of dermatology and pediatrics at the University of California and Rady Children’s Hospital, San Diego, California, led the research. The study was published in the January 2024 issue of the Journal of Drugs in Dermatology.
LIMITATIONS:
There was a high patient discontinuation rate before and during the LET study. Also, no assessment was made as to how clascoterone affected patients’ quality of life.
DISCLOSURES:
Clascoterone manufacturer Cassiopea funded the studies. Dr. Eichenfield and fellow investigators Adelaide A. Hebert, MD, and Linda Stein Gold, MD, received compensation from Cassiopea as advisers and disclosed ties to many other pharmaceutical companies.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- A 1% cream formulation of clascoterone, a topical androgen receptor inhibitor, is approved for the treatment of acne vulgaris in patients aged 12 years and older based on results from two identical phase 3 12-week trials, NCT02608450 and NCT02608476, and a long-term extension (LTE) study.
- The purpose of the current study was to evaluate the integrated efficacy of clascoterone cream 1% (Winlevi) in the intention-to-treat population of patients from all three trials.
- In the pivotal trials, investigators randomized patients with acne 1:1 to receive clascoterone cream 1% or vehicle twice daily for 12 weeks. Participants were eligible to enter the LTE study, in which patients applied clascoterone to the face, and if they wanted to, the trunk for up to 9 more months.
- To assess combined efficacy, researchers evaluated the proportion of patients who achieved an Investigator’s Global Assessment (IGA) of 0 or 1.
TAKEAWAY:
- Of the 1143 patients from the pivotal trials who completed 12 weeks of treatment, 576 were in the clascoterone group and 567 were in the vehicle group. Of the 600 patients who entered the LTE study, 311 were in the clascoterone group and 289 were in the vehicle group. Of these, 343 completed the LTE study.
- At week 12, the proportion of patients who achieved treatment success was higher in the clascoterone group than in the vehicle group (19.9% vs 7.7%, respectively; P < .0001).
- In the LTE study, the proportion of patients previously treated with clascoterone who achieved a facial IGA of 0/1 increased from 13.5% at extension day 0 to 29.9% at extension day 274, while the proportion of patients previously treated with vehicle and switched to clascoterone who achieved a facial IGA of 0/1 increased from 6.2% at extension day 0 to 30.4% at extension day 274.
- Similarly, the proportion of patients in the LTE study with a truncal IGA of 0/1 increased from 4.9% at extension day 0 to 31.7% on extension day 274.
IN PRACTICE:
“Clinicians may consider counseling patients that treatment persistence is required to maximize the efficacy of clascoterone treatment,” the authors concluded.
SOURCE:
Lawrence F. Eichenfield, MD, of the departments of dermatology and pediatrics at the University of California and Rady Children’s Hospital, San Diego, California, led the research. The study was published in the January 2024 issue of the Journal of Drugs in Dermatology.
LIMITATIONS:
There was a high patient discontinuation rate before and during the LET study. Also, no assessment was made as to how clascoterone affected patients’ quality of life.
DISCLOSURES:
Clascoterone manufacturer Cassiopea funded the studies. Dr. Eichenfield and fellow investigators Adelaide A. Hebert, MD, and Linda Stein Gold, MD, received compensation from Cassiopea as advisers and disclosed ties to many other pharmaceutical companies.
A version of this article appeared on Medscape.com.
Myo-inositol is one of the components of an integrative approach to acne
, Jonette Elizabeth Keri, MD, PhD, professor of dermatology at the University of Miami, said during a presentation on therapies for acne at the annual Integrative Dermatology Symposium.
Probiotics and omega-3 fatty acids are among the other complementary therapies that have a role in acne treatment, she and others said during the meeting.
Myo-inositol has been well-studied in the gynecology-endocrinology community in patients with polycystic ovary syndrome (PCOS), demonstrating an ability to improve the metabolic profile and reduce acne and hirsutism, Dr. Keri said.
A study of 137 young, overweight women with PCOS and moderate acne, for example, found that compared with placebo, 6 months of myo-inositol or D-chiro-inositol, another isoform of inositol, significantly improved the acne score, endocrine and metabolic parameters, insulin resistance, and regularity of the menstrual cycle, Dr. Keri said. Both isoforms of inositol are second messengers in the signal transduction of insulin.
During a panel discussion, asked about a case of an adult female with acne, Dr. Keri said that many of her adult female patients “don’t want to do isotretinoin or antibiotics, and they don’t want to do any kind of hormonal treatment,” the options she would recommend. But for patients who do not want these treatments, she said, “I go down the route of supplements,” and myo-inositol is her “favorite” option. It’s safe to use during pregnancy, she emphasized, noting that myo-inositol is being studied for the prevention of preterm birth.
Dr. Keri, who described herself as “more of a traditionalist,” prescribes myo-inositol 2 gm twice a day in pill form. In Europe, she noted in her presentation, myo-inositol is also compounded for topical use.
Diet, probiotics, other nutraceuticals
A low-glycemic-load diet was among several complementary therapies reported in a 2015 Cochrane Database Systematic Review to have some evidence (though low-quality) of reducing total skin lesions in acne (along with tea tree oil and bee venom) and today, it is the most evidence-based dietary recommendation for acne, Dr. Keri said.
Omega-3 fatty acids and increased fruit and vegetable intake have also been reported to be acne-protective — and hyperglycemia, carbohydrates, milk and dairy products, and saturated fats and trans fats have been reported to be acne-promoting, she noted.
But, the low-glycemic-load data “is the strongest,” she said. The best advice for patients, she added, is to consume less sugar and fewer sugary drinks and “avoid white foods” such as white bread, rice, and pasta.
Probiotics can also be recommended, especially for patients on antibiotic therapy, Dr. Keri said. For “basic science evidence,” she pointed to a randomized, double-blinded, placebo-controlled study of 20 adults with acne, which evaluated the impact of a probiotic on improvement in acne and skin expression of genes involved with insulin signaling. Participants took either a liquid supplement containing Lactobacillus rhamnosus SP1 (LSP1) or placebo over a 12-week period. The investigators performed paired skin biopsies before and after 12 weeks of treatment and analyzed them for insulin-like growth factor 1 (IGF1) and forkhead box protein O1 (FOXO1) gene expression.
They found that compared with baseline, the probiotic group showed a 32% reduction in IGF1 and a 65% increase in FOXO1 gene expression (P < .0001 for both), with no such differences observed in the placebo group.
Clinically, patients in the probiotic group had an adjusted odds ratio of 28.4 (95% confidence interval, 2.2-411.1, P < .05) of acne being rated as improved or markedly improved compared with those on placebo.
Dr. Keri and others at the meeting also referenced a 2013 prospective, open-label trial that randomized 45 women with acne, ages 18-35 years, to one of three arms: Probiotic supplementation only, minocycline only, and both probiotic and minocycline. The probiotic used was a product containing Lactobacillus acidophilus, Lactobacillus bulgaricus, and Bifidobacterium bifidum. At 8 and 12 weeks, the combination group “did the best with the lowest total lesion count” compared with the probiotic group and the minocycline group, differences that were significant (P < .001 and P <.01, respectively), she said. “And they also had less candidiasis when using a probiotic than when using an antibiotic alone,” she said. Two patients in the minocycline-only group failed to complete the study because they developed vaginal candidiasis.
In addition to reducing potential adverse events secondary to chronic antibiotic use, probiotics can have synergistic anti-inflammatory effects, she said.
Dr. Keri said she recommends probiotics for patients taking antibiotics and encourages them “to get a branded probiotic,” such as Culturelle, “or if they prefer a food source, soy or almond milk–based yogurt.” As with other elements of a holistic approach to acne, she urged clinicians to consider the cost of treatment.
Probiotics (Lactobacillus plantarum) were one of four nutraceuticals determined in a 2023 systematic review to have “good-quality” evidence for potential efficacy, Dr. Keri noted, along with vitamin D, green tea extract, and cheongsangbangpoong-tang, the latter of which is an herbal therapeutic formula approved by the Korean Food and Drug Administration for use in acne.
“There were really no bad systemic effects for any of these,” she said. “The tricky part of this review is that each of the four have only one study” deemed to be a good-quality study. Omega-3 fatty acids were among several other nutraceuticals deemed to have “fair-quality” evidence for efficacy. Zinc was reported to be the most studied nutraceutical in acne, but didn’t rate as highly in the review. Dr. Keri said she likes to include zinc in her armamentarium because “it can be used in pregnant women,” noting that reviews and guidelines “are just that, a guide ... to combine with experience.”
Omega-3 fatty acids with isotretinoin
Several speakers at the meeting, including Steven Daveluy, MD, associate professor and residency program director in the department of dermatology, Wayne State University, Dearborn, Michigan, spoke about the value of omega-3 fatty acids in reducing side effects of isotretinoin. “In the FDA trials [of isotretinoin] they had patients take 50 grams of fat,” he said. “You can use the good fats to help you out.”
Research has shown that 1 gm per day of oral omega-3 reduces dryness of the lips, nose, eyes, and skin, “which are the big side effects we see with isotretinoin,” he said. An impact on triglyceride levels has also been demonstrated, Dr. Daveluy said, pointing to a longitudinal survey study of 39 patients treated with isotretinoin that showed a mean increase in triglyceride levels of 49% during treatment in patients who did not use omega-3 supplementation, compared with a mean increase of 13.9% (P =.04) in patients who used the supplements.“There is also evidence that omega-3 can decrease depression, which may or may not be a side effect of isotretinoin ... but it’s something we consider in our [acne] patients,” Dr. Daveluy said.
During a panel discussion at the meeting, Apple A. Bodemer, MD, associate professor of dermatology at the University of Wisconsin, Madison, said she usually prescribes 2 g of docosahexaenoic acid eicosapentaenoic acid combined in patients on isotretinoin because “at that dose, omega-3s have been found to be anti-inflammatory.”
Dr. Keri reported being an investigator and speaker for Galderma, and an advisory board member for Ortho Dermatologics and for Almirall. Dr. Daveluy reported no relevant disclosures.
, Jonette Elizabeth Keri, MD, PhD, professor of dermatology at the University of Miami, said during a presentation on therapies for acne at the annual Integrative Dermatology Symposium.
Probiotics and omega-3 fatty acids are among the other complementary therapies that have a role in acne treatment, she and others said during the meeting.
Myo-inositol has been well-studied in the gynecology-endocrinology community in patients with polycystic ovary syndrome (PCOS), demonstrating an ability to improve the metabolic profile and reduce acne and hirsutism, Dr. Keri said.
A study of 137 young, overweight women with PCOS and moderate acne, for example, found that compared with placebo, 6 months of myo-inositol or D-chiro-inositol, another isoform of inositol, significantly improved the acne score, endocrine and metabolic parameters, insulin resistance, and regularity of the menstrual cycle, Dr. Keri said. Both isoforms of inositol are second messengers in the signal transduction of insulin.
During a panel discussion, asked about a case of an adult female with acne, Dr. Keri said that many of her adult female patients “don’t want to do isotretinoin or antibiotics, and they don’t want to do any kind of hormonal treatment,” the options she would recommend. But for patients who do not want these treatments, she said, “I go down the route of supplements,” and myo-inositol is her “favorite” option. It’s safe to use during pregnancy, she emphasized, noting that myo-inositol is being studied for the prevention of preterm birth.
Dr. Keri, who described herself as “more of a traditionalist,” prescribes myo-inositol 2 gm twice a day in pill form. In Europe, she noted in her presentation, myo-inositol is also compounded for topical use.
Diet, probiotics, other nutraceuticals
A low-glycemic-load diet was among several complementary therapies reported in a 2015 Cochrane Database Systematic Review to have some evidence (though low-quality) of reducing total skin lesions in acne (along with tea tree oil and bee venom) and today, it is the most evidence-based dietary recommendation for acne, Dr. Keri said.
Omega-3 fatty acids and increased fruit and vegetable intake have also been reported to be acne-protective — and hyperglycemia, carbohydrates, milk and dairy products, and saturated fats and trans fats have been reported to be acne-promoting, she noted.
But, the low-glycemic-load data “is the strongest,” she said. The best advice for patients, she added, is to consume less sugar and fewer sugary drinks and “avoid white foods” such as white bread, rice, and pasta.
Probiotics can also be recommended, especially for patients on antibiotic therapy, Dr. Keri said. For “basic science evidence,” she pointed to a randomized, double-blinded, placebo-controlled study of 20 adults with acne, which evaluated the impact of a probiotic on improvement in acne and skin expression of genes involved with insulin signaling. Participants took either a liquid supplement containing Lactobacillus rhamnosus SP1 (LSP1) or placebo over a 12-week period. The investigators performed paired skin biopsies before and after 12 weeks of treatment and analyzed them for insulin-like growth factor 1 (IGF1) and forkhead box protein O1 (FOXO1) gene expression.
They found that compared with baseline, the probiotic group showed a 32% reduction in IGF1 and a 65% increase in FOXO1 gene expression (P < .0001 for both), with no such differences observed in the placebo group.
Clinically, patients in the probiotic group had an adjusted odds ratio of 28.4 (95% confidence interval, 2.2-411.1, P < .05) of acne being rated as improved or markedly improved compared with those on placebo.
Dr. Keri and others at the meeting also referenced a 2013 prospective, open-label trial that randomized 45 women with acne, ages 18-35 years, to one of three arms: Probiotic supplementation only, minocycline only, and both probiotic and minocycline. The probiotic used was a product containing Lactobacillus acidophilus, Lactobacillus bulgaricus, and Bifidobacterium bifidum. At 8 and 12 weeks, the combination group “did the best with the lowest total lesion count” compared with the probiotic group and the minocycline group, differences that were significant (P < .001 and P <.01, respectively), she said. “And they also had less candidiasis when using a probiotic than when using an antibiotic alone,” she said. Two patients in the minocycline-only group failed to complete the study because they developed vaginal candidiasis.
In addition to reducing potential adverse events secondary to chronic antibiotic use, probiotics can have synergistic anti-inflammatory effects, she said.
Dr. Keri said she recommends probiotics for patients taking antibiotics and encourages them “to get a branded probiotic,” such as Culturelle, “or if they prefer a food source, soy or almond milk–based yogurt.” As with other elements of a holistic approach to acne, she urged clinicians to consider the cost of treatment.
Probiotics (Lactobacillus plantarum) were one of four nutraceuticals determined in a 2023 systematic review to have “good-quality” evidence for potential efficacy, Dr. Keri noted, along with vitamin D, green tea extract, and cheongsangbangpoong-tang, the latter of which is an herbal therapeutic formula approved by the Korean Food and Drug Administration for use in acne.
“There were really no bad systemic effects for any of these,” she said. “The tricky part of this review is that each of the four have only one study” deemed to be a good-quality study. Omega-3 fatty acids were among several other nutraceuticals deemed to have “fair-quality” evidence for efficacy. Zinc was reported to be the most studied nutraceutical in acne, but didn’t rate as highly in the review. Dr. Keri said she likes to include zinc in her armamentarium because “it can be used in pregnant women,” noting that reviews and guidelines “are just that, a guide ... to combine with experience.”
Omega-3 fatty acids with isotretinoin
Several speakers at the meeting, including Steven Daveluy, MD, associate professor and residency program director in the department of dermatology, Wayne State University, Dearborn, Michigan, spoke about the value of omega-3 fatty acids in reducing side effects of isotretinoin. “In the FDA trials [of isotretinoin] they had patients take 50 grams of fat,” he said. “You can use the good fats to help you out.”
Research has shown that 1 gm per day of oral omega-3 reduces dryness of the lips, nose, eyes, and skin, “which are the big side effects we see with isotretinoin,” he said. An impact on triglyceride levels has also been demonstrated, Dr. Daveluy said, pointing to a longitudinal survey study of 39 patients treated with isotretinoin that showed a mean increase in triglyceride levels of 49% during treatment in patients who did not use omega-3 supplementation, compared with a mean increase of 13.9% (P =.04) in patients who used the supplements.“There is also evidence that omega-3 can decrease depression, which may or may not be a side effect of isotretinoin ... but it’s something we consider in our [acne] patients,” Dr. Daveluy said.
During a panel discussion at the meeting, Apple A. Bodemer, MD, associate professor of dermatology at the University of Wisconsin, Madison, said she usually prescribes 2 g of docosahexaenoic acid eicosapentaenoic acid combined in patients on isotretinoin because “at that dose, omega-3s have been found to be anti-inflammatory.”
Dr. Keri reported being an investigator and speaker for Galderma, and an advisory board member for Ortho Dermatologics and for Almirall. Dr. Daveluy reported no relevant disclosures.
, Jonette Elizabeth Keri, MD, PhD, professor of dermatology at the University of Miami, said during a presentation on therapies for acne at the annual Integrative Dermatology Symposium.
Probiotics and omega-3 fatty acids are among the other complementary therapies that have a role in acne treatment, she and others said during the meeting.
Myo-inositol has been well-studied in the gynecology-endocrinology community in patients with polycystic ovary syndrome (PCOS), demonstrating an ability to improve the metabolic profile and reduce acne and hirsutism, Dr. Keri said.
A study of 137 young, overweight women with PCOS and moderate acne, for example, found that compared with placebo, 6 months of myo-inositol or D-chiro-inositol, another isoform of inositol, significantly improved the acne score, endocrine and metabolic parameters, insulin resistance, and regularity of the menstrual cycle, Dr. Keri said. Both isoforms of inositol are second messengers in the signal transduction of insulin.
During a panel discussion, asked about a case of an adult female with acne, Dr. Keri said that many of her adult female patients “don’t want to do isotretinoin or antibiotics, and they don’t want to do any kind of hormonal treatment,” the options she would recommend. But for patients who do not want these treatments, she said, “I go down the route of supplements,” and myo-inositol is her “favorite” option. It’s safe to use during pregnancy, she emphasized, noting that myo-inositol is being studied for the prevention of preterm birth.
Dr. Keri, who described herself as “more of a traditionalist,” prescribes myo-inositol 2 gm twice a day in pill form. In Europe, she noted in her presentation, myo-inositol is also compounded for topical use.
Diet, probiotics, other nutraceuticals
A low-glycemic-load diet was among several complementary therapies reported in a 2015 Cochrane Database Systematic Review to have some evidence (though low-quality) of reducing total skin lesions in acne (along with tea tree oil and bee venom) and today, it is the most evidence-based dietary recommendation for acne, Dr. Keri said.
Omega-3 fatty acids and increased fruit and vegetable intake have also been reported to be acne-protective — and hyperglycemia, carbohydrates, milk and dairy products, and saturated fats and trans fats have been reported to be acne-promoting, she noted.
But, the low-glycemic-load data “is the strongest,” she said. The best advice for patients, she added, is to consume less sugar and fewer sugary drinks and “avoid white foods” such as white bread, rice, and pasta.
Probiotics can also be recommended, especially for patients on antibiotic therapy, Dr. Keri said. For “basic science evidence,” she pointed to a randomized, double-blinded, placebo-controlled study of 20 adults with acne, which evaluated the impact of a probiotic on improvement in acne and skin expression of genes involved with insulin signaling. Participants took either a liquid supplement containing Lactobacillus rhamnosus SP1 (LSP1) or placebo over a 12-week period. The investigators performed paired skin biopsies before and after 12 weeks of treatment and analyzed them for insulin-like growth factor 1 (IGF1) and forkhead box protein O1 (FOXO1) gene expression.
They found that compared with baseline, the probiotic group showed a 32% reduction in IGF1 and a 65% increase in FOXO1 gene expression (P < .0001 for both), with no such differences observed in the placebo group.
Clinically, patients in the probiotic group had an adjusted odds ratio of 28.4 (95% confidence interval, 2.2-411.1, P < .05) of acne being rated as improved or markedly improved compared with those on placebo.
Dr. Keri and others at the meeting also referenced a 2013 prospective, open-label trial that randomized 45 women with acne, ages 18-35 years, to one of three arms: Probiotic supplementation only, minocycline only, and both probiotic and minocycline. The probiotic used was a product containing Lactobacillus acidophilus, Lactobacillus bulgaricus, and Bifidobacterium bifidum. At 8 and 12 weeks, the combination group “did the best with the lowest total lesion count” compared with the probiotic group and the minocycline group, differences that were significant (P < .001 and P <.01, respectively), she said. “And they also had less candidiasis when using a probiotic than when using an antibiotic alone,” she said. Two patients in the minocycline-only group failed to complete the study because they developed vaginal candidiasis.
In addition to reducing potential adverse events secondary to chronic antibiotic use, probiotics can have synergistic anti-inflammatory effects, she said.
Dr. Keri said she recommends probiotics for patients taking antibiotics and encourages them “to get a branded probiotic,” such as Culturelle, “or if they prefer a food source, soy or almond milk–based yogurt.” As with other elements of a holistic approach to acne, she urged clinicians to consider the cost of treatment.
Probiotics (Lactobacillus plantarum) were one of four nutraceuticals determined in a 2023 systematic review to have “good-quality” evidence for potential efficacy, Dr. Keri noted, along with vitamin D, green tea extract, and cheongsangbangpoong-tang, the latter of which is an herbal therapeutic formula approved by the Korean Food and Drug Administration for use in acne.
“There were really no bad systemic effects for any of these,” she said. “The tricky part of this review is that each of the four have only one study” deemed to be a good-quality study. Omega-3 fatty acids were among several other nutraceuticals deemed to have “fair-quality” evidence for efficacy. Zinc was reported to be the most studied nutraceutical in acne, but didn’t rate as highly in the review. Dr. Keri said she likes to include zinc in her armamentarium because “it can be used in pregnant women,” noting that reviews and guidelines “are just that, a guide ... to combine with experience.”
Omega-3 fatty acids with isotretinoin
Several speakers at the meeting, including Steven Daveluy, MD, associate professor and residency program director in the department of dermatology, Wayne State University, Dearborn, Michigan, spoke about the value of omega-3 fatty acids in reducing side effects of isotretinoin. “In the FDA trials [of isotretinoin] they had patients take 50 grams of fat,” he said. “You can use the good fats to help you out.”
Research has shown that 1 gm per day of oral omega-3 reduces dryness of the lips, nose, eyes, and skin, “which are the big side effects we see with isotretinoin,” he said. An impact on triglyceride levels has also been demonstrated, Dr. Daveluy said, pointing to a longitudinal survey study of 39 patients treated with isotretinoin that showed a mean increase in triglyceride levels of 49% during treatment in patients who did not use omega-3 supplementation, compared with a mean increase of 13.9% (P =.04) in patients who used the supplements.“There is also evidence that omega-3 can decrease depression, which may or may not be a side effect of isotretinoin ... but it’s something we consider in our [acne] patients,” Dr. Daveluy said.
During a panel discussion at the meeting, Apple A. Bodemer, MD, associate professor of dermatology at the University of Wisconsin, Madison, said she usually prescribes 2 g of docosahexaenoic acid eicosapentaenoic acid combined in patients on isotretinoin because “at that dose, omega-3s have been found to be anti-inflammatory.”
Dr. Keri reported being an investigator and speaker for Galderma, and an advisory board member for Ortho Dermatologics and for Almirall. Dr. Daveluy reported no relevant disclosures.
FROM IDS 2023