User login
Cannabis use causes spike in ED visits
Cannabis users had a 22% increased risk of an emergency department (ED) visit or hospitalization compared to nonusers, as determined from data from more than 30,000 individuals.
Although cannabis contains compounds similar to tobacco, “data published on the association between cannabis smoking and airways health have been contradictory,” and whether smoking cannabis increases a user’s risk of developing acute respiratory illness remains unclear, wrote Nicholas T. Vozoris, MD, of the University of Toronto, and colleagues.
In a study published in BMJ Open Respiratory Research, the investigators reviewed national health records data from 35,114 individuals aged 12-65 years for the period January 2009 to December 2015. Of these persons, 4,807 of the 6,425 who reported cannabis use in the past year were matched with 10,395 never-users who served as controls. The mean age of the study population at the index date was 35 years, and 42% were women; demographics were similar between users and control persons.
Overall, the odds of respiratory-related emergency department visits or hospitalizations were not significantly different between the cannabis users and the control persons (3.6% vs. 3.9%; odds ratio, 0.91). However, cannabis users had significantly greater odds of all-cause ED visits or hospitalizations (30.0% vs. 26.0%; OR, 1.22). All-cause mortality was 0.2% for both groups.
Respiratory problems were the second-highest reason for all-cause visits, the researchers noted. The lack of a difference in respiratory-related visits between cannabis users and nonusers conflicts somewhat with previous studies on this topic, which were limited, the researchers noted in their discussion.
The negative results also might stem from factors for which the researchers could not adjust, including insufficient cannabis smoke exposure among users in the study population, noninhalational cannabis use, which is less likely to have a respiratory effect, and possible secondhand exposure among control persons.
“It is also possible that our analysis might have been insufficiently powered to detect a significant signal with respect to the primary outcome,” they noted.
However, after the researchers controlled for multiple variables, the risk of an equally important morbidity outcome, all-cause ED visits or hospitalizations, was significantly greater among cannabis users than among control individuals, and respiratory reasons were the second most common cause for ED visits and hospitalizations in the all-cause outcome, they emphasized.
The study findings were limited by several factors, including the retrospective and observational design and the inability to control for all confounding variables, the researchers noted. Other limitations include the use of self-reports and potential for bias, the inability to perform dose-response analysis, and the high number of infrequent cannabis users in the study population.
However, the results suggest that cannabis use is associated with an increased risk of serious health events and should be discouraged, although more research is needed to confirm the current study findings, they concluded.
Consider range of causes for cannabis emergency visits
“With growing numbers of states legalizing recreational use of cannabis, it’s important to understand whether cannabis use is associated with increased emergency department visits,” Robert D. Glatter, MD, an emergency medicine physician at Lenox Hill Hospital, New York, told this news organization.
Previous studies have shown an association between increased ED visits and cannabis use in states, especially with edibles, where cannabis is legal, and “the current study reinforces the elevated risk of ED visits along with hospitalizations,” he said.
“While the researchers found no increased risk of respiratory-related complaints among users compared to the general population, there was an associated increase in ED visits and hospitalizations, which is important to understand,” said Dr. Glatter, who was not involved in the study.
“While this observational study found that the incidence of respiratory complaints was not significantly different among frequent users of cannabis, the increased odds that cannabis users would require evaluation in the emergency room or even hospitalization was still apparent even after the investigators controlled for such factors as use of alcohol, tobacco, illicit drug use, or other mental health–related disorders,” Dr. Glatter noted.
“That said, it’s a bit surprising that with the continued popularity of vaping, especially among teens, there was still not any appreciable or significant increase in respiratory complaints observed. Beyond this finding, I was not surprised by the overall conclusions of the current study, as we continue to see an elevated number of patients presenting to the ED with adverse events related to cannabis use.”
Dr. Glatter noted that “the majority of patients we see in the ED are associated with use of edibles, since it takes longer for the person to feel the effects, leading the user to consume more of the product up front, with delayed effects lasting up to 12 hours. This is what gets people into trouble and leads to toxicity of cannabis, or ‘overdoses,’ “ he explained.
When consuming edible cannabis products, “[p]eople need to begin at low dosages and not take additional gummies up front, since it can take up to 2 or even 3 hours in some cases to feel the initial effects. With the drug’s effects lasting up to 12 hours, it’s especially important to avoid operating any motor vehicles, bicycles, or scooters, since reaction time is impaired, as well as overall judgment, balance, and fine motor skills,” Dr. Glatter said.
Cannabis can land users in the ED for a range of reasons, said Dr. Glatter. “According to the study, 15% of the emergency room visits and hospitalizations were due to acute trauma, 14% due to respiratory issues, and 13% to gastrointestinal illnesses. These effects were seen in first-time users but not those with chronic use, according to the study inclusion criteria.”
Cannabis use could result in physical injuries through “impaired judgment, coordination, combined with an altered state of consciousness or generalized drowsiness, that could contribute to an increase in motor vehicle collisions, along with an increased risk for falls leading to lacerations, fractures, contusions, or bruising,” said Dr. Glatter. “Cannabis may also lead to an altered sense of perception related to interactions with others, resulting in feelings of anxiety or restlessness culminating in physical altercations and other injuries.”
The current study indicates the need for understanding the potential physical and psychological effects of cannabis use, he said.
“Additional research is needed to better understand the relative percentage cases related to edibles vs. inhalation presenting to the ED,” he noted. “There is no question that edibles continue to present significant dangers for those who don’t read labels or remain poorly informed regarding their dosing as a result of delayed onset and longer duration,” he said. To help reduce risk of toxicity, the concept of a “high lasting 12-15 hours, as with edibles, as opposed to 3-4 hours from inhalation must be clearly stated on packaging and better communicated with users, as the toxicity with edibles is more often from lack of prior knowledge about onset of effects related to dosing.”
In addition, the “potential for psychosis to develop with more chronic cannabis use, along with cannabinoid hyperemesis syndrome should be on every clinician’s radar,” Dr. Glatter emphasized.
“The bottom line is that as more states legalize the use of cannabis, it’s vital to also implement comprehensive public education efforts to provide users with the reported risks associated with not only inhalation (vaping or flower) but also edibles, which account for an increasingly greater percentage of ED visits and associated adverse effects,” he said.
The study was supported by the Lung Association–Ontario, as well as by grants from the Ontario Ministry of Health and the Ministry of Long-Term Care. The researchers and Dr. Glatter have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cannabis users had a 22% increased risk of an emergency department (ED) visit or hospitalization compared to nonusers, as determined from data from more than 30,000 individuals.
Although cannabis contains compounds similar to tobacco, “data published on the association between cannabis smoking and airways health have been contradictory,” and whether smoking cannabis increases a user’s risk of developing acute respiratory illness remains unclear, wrote Nicholas T. Vozoris, MD, of the University of Toronto, and colleagues.
In a study published in BMJ Open Respiratory Research, the investigators reviewed national health records data from 35,114 individuals aged 12-65 years for the period January 2009 to December 2015. Of these persons, 4,807 of the 6,425 who reported cannabis use in the past year were matched with 10,395 never-users who served as controls. The mean age of the study population at the index date was 35 years, and 42% were women; demographics were similar between users and control persons.
Overall, the odds of respiratory-related emergency department visits or hospitalizations were not significantly different between the cannabis users and the control persons (3.6% vs. 3.9%; odds ratio, 0.91). However, cannabis users had significantly greater odds of all-cause ED visits or hospitalizations (30.0% vs. 26.0%; OR, 1.22). All-cause mortality was 0.2% for both groups.
Respiratory problems were the second-highest reason for all-cause visits, the researchers noted. The lack of a difference in respiratory-related visits between cannabis users and nonusers conflicts somewhat with previous studies on this topic, which were limited, the researchers noted in their discussion.
The negative results also might stem from factors for which the researchers could not adjust, including insufficient cannabis smoke exposure among users in the study population, noninhalational cannabis use, which is less likely to have a respiratory effect, and possible secondhand exposure among control persons.
“It is also possible that our analysis might have been insufficiently powered to detect a significant signal with respect to the primary outcome,” they noted.
However, after the researchers controlled for multiple variables, the risk of an equally important morbidity outcome, all-cause ED visits or hospitalizations, was significantly greater among cannabis users than among control individuals, and respiratory reasons were the second most common cause for ED visits and hospitalizations in the all-cause outcome, they emphasized.
The study findings were limited by several factors, including the retrospective and observational design and the inability to control for all confounding variables, the researchers noted. Other limitations include the use of self-reports and potential for bias, the inability to perform dose-response analysis, and the high number of infrequent cannabis users in the study population.
However, the results suggest that cannabis use is associated with an increased risk of serious health events and should be discouraged, although more research is needed to confirm the current study findings, they concluded.
Consider range of causes for cannabis emergency visits
“With growing numbers of states legalizing recreational use of cannabis, it’s important to understand whether cannabis use is associated with increased emergency department visits,” Robert D. Glatter, MD, an emergency medicine physician at Lenox Hill Hospital, New York, told this news organization.
Previous studies have shown an association between increased ED visits and cannabis use in states, especially with edibles, where cannabis is legal, and “the current study reinforces the elevated risk of ED visits along with hospitalizations,” he said.
“While the researchers found no increased risk of respiratory-related complaints among users compared to the general population, there was an associated increase in ED visits and hospitalizations, which is important to understand,” said Dr. Glatter, who was not involved in the study.
“While this observational study found that the incidence of respiratory complaints was not significantly different among frequent users of cannabis, the increased odds that cannabis users would require evaluation in the emergency room or even hospitalization was still apparent even after the investigators controlled for such factors as use of alcohol, tobacco, illicit drug use, or other mental health–related disorders,” Dr. Glatter noted.
“That said, it’s a bit surprising that with the continued popularity of vaping, especially among teens, there was still not any appreciable or significant increase in respiratory complaints observed. Beyond this finding, I was not surprised by the overall conclusions of the current study, as we continue to see an elevated number of patients presenting to the ED with adverse events related to cannabis use.”
Dr. Glatter noted that “the majority of patients we see in the ED are associated with use of edibles, since it takes longer for the person to feel the effects, leading the user to consume more of the product up front, with delayed effects lasting up to 12 hours. This is what gets people into trouble and leads to toxicity of cannabis, or ‘overdoses,’ “ he explained.
When consuming edible cannabis products, “[p]eople need to begin at low dosages and not take additional gummies up front, since it can take up to 2 or even 3 hours in some cases to feel the initial effects. With the drug’s effects lasting up to 12 hours, it’s especially important to avoid operating any motor vehicles, bicycles, or scooters, since reaction time is impaired, as well as overall judgment, balance, and fine motor skills,” Dr. Glatter said.
Cannabis can land users in the ED for a range of reasons, said Dr. Glatter. “According to the study, 15% of the emergency room visits and hospitalizations were due to acute trauma, 14% due to respiratory issues, and 13% to gastrointestinal illnesses. These effects were seen in first-time users but not those with chronic use, according to the study inclusion criteria.”
Cannabis use could result in physical injuries through “impaired judgment, coordination, combined with an altered state of consciousness or generalized drowsiness, that could contribute to an increase in motor vehicle collisions, along with an increased risk for falls leading to lacerations, fractures, contusions, or bruising,” said Dr. Glatter. “Cannabis may also lead to an altered sense of perception related to interactions with others, resulting in feelings of anxiety or restlessness culminating in physical altercations and other injuries.”
The current study indicates the need for understanding the potential physical and psychological effects of cannabis use, he said.
“Additional research is needed to better understand the relative percentage cases related to edibles vs. inhalation presenting to the ED,” he noted. “There is no question that edibles continue to present significant dangers for those who don’t read labels or remain poorly informed regarding their dosing as a result of delayed onset and longer duration,” he said. To help reduce risk of toxicity, the concept of a “high lasting 12-15 hours, as with edibles, as opposed to 3-4 hours from inhalation must be clearly stated on packaging and better communicated with users, as the toxicity with edibles is more often from lack of prior knowledge about onset of effects related to dosing.”
In addition, the “potential for psychosis to develop with more chronic cannabis use, along with cannabinoid hyperemesis syndrome should be on every clinician’s radar,” Dr. Glatter emphasized.
“The bottom line is that as more states legalize the use of cannabis, it’s vital to also implement comprehensive public education efforts to provide users with the reported risks associated with not only inhalation (vaping or flower) but also edibles, which account for an increasingly greater percentage of ED visits and associated adverse effects,” he said.
The study was supported by the Lung Association–Ontario, as well as by grants from the Ontario Ministry of Health and the Ministry of Long-Term Care. The researchers and Dr. Glatter have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cannabis users had a 22% increased risk of an emergency department (ED) visit or hospitalization compared to nonusers, as determined from data from more than 30,000 individuals.
Although cannabis contains compounds similar to tobacco, “data published on the association between cannabis smoking and airways health have been contradictory,” and whether smoking cannabis increases a user’s risk of developing acute respiratory illness remains unclear, wrote Nicholas T. Vozoris, MD, of the University of Toronto, and colleagues.
In a study published in BMJ Open Respiratory Research, the investigators reviewed national health records data from 35,114 individuals aged 12-65 years for the period January 2009 to December 2015. Of these persons, 4,807 of the 6,425 who reported cannabis use in the past year were matched with 10,395 never-users who served as controls. The mean age of the study population at the index date was 35 years, and 42% were women; demographics were similar between users and control persons.
Overall, the odds of respiratory-related emergency department visits or hospitalizations were not significantly different between the cannabis users and the control persons (3.6% vs. 3.9%; odds ratio, 0.91). However, cannabis users had significantly greater odds of all-cause ED visits or hospitalizations (30.0% vs. 26.0%; OR, 1.22). All-cause mortality was 0.2% for both groups.
Respiratory problems were the second-highest reason for all-cause visits, the researchers noted. The lack of a difference in respiratory-related visits between cannabis users and nonusers conflicts somewhat with previous studies on this topic, which were limited, the researchers noted in their discussion.
The negative results also might stem from factors for which the researchers could not adjust, including insufficient cannabis smoke exposure among users in the study population, noninhalational cannabis use, which is less likely to have a respiratory effect, and possible secondhand exposure among control persons.
“It is also possible that our analysis might have been insufficiently powered to detect a significant signal with respect to the primary outcome,” they noted.
However, after the researchers controlled for multiple variables, the risk of an equally important morbidity outcome, all-cause ED visits or hospitalizations, was significantly greater among cannabis users than among control individuals, and respiratory reasons were the second most common cause for ED visits and hospitalizations in the all-cause outcome, they emphasized.
The study findings were limited by several factors, including the retrospective and observational design and the inability to control for all confounding variables, the researchers noted. Other limitations include the use of self-reports and potential for bias, the inability to perform dose-response analysis, and the high number of infrequent cannabis users in the study population.
However, the results suggest that cannabis use is associated with an increased risk of serious health events and should be discouraged, although more research is needed to confirm the current study findings, they concluded.
Consider range of causes for cannabis emergency visits
“With growing numbers of states legalizing recreational use of cannabis, it’s important to understand whether cannabis use is associated with increased emergency department visits,” Robert D. Glatter, MD, an emergency medicine physician at Lenox Hill Hospital, New York, told this news organization.
Previous studies have shown an association between increased ED visits and cannabis use in states, especially with edibles, where cannabis is legal, and “the current study reinforces the elevated risk of ED visits along with hospitalizations,” he said.
“While the researchers found no increased risk of respiratory-related complaints among users compared to the general population, there was an associated increase in ED visits and hospitalizations, which is important to understand,” said Dr. Glatter, who was not involved in the study.
“While this observational study found that the incidence of respiratory complaints was not significantly different among frequent users of cannabis, the increased odds that cannabis users would require evaluation in the emergency room or even hospitalization was still apparent even after the investigators controlled for such factors as use of alcohol, tobacco, illicit drug use, or other mental health–related disorders,” Dr. Glatter noted.
“That said, it’s a bit surprising that with the continued popularity of vaping, especially among teens, there was still not any appreciable or significant increase in respiratory complaints observed. Beyond this finding, I was not surprised by the overall conclusions of the current study, as we continue to see an elevated number of patients presenting to the ED with adverse events related to cannabis use.”
Dr. Glatter noted that “the majority of patients we see in the ED are associated with use of edibles, since it takes longer for the person to feel the effects, leading the user to consume more of the product up front, with delayed effects lasting up to 12 hours. This is what gets people into trouble and leads to toxicity of cannabis, or ‘overdoses,’ “ he explained.
When consuming edible cannabis products, “[p]eople need to begin at low dosages and not take additional gummies up front, since it can take up to 2 or even 3 hours in some cases to feel the initial effects. With the drug’s effects lasting up to 12 hours, it’s especially important to avoid operating any motor vehicles, bicycles, or scooters, since reaction time is impaired, as well as overall judgment, balance, and fine motor skills,” Dr. Glatter said.
Cannabis can land users in the ED for a range of reasons, said Dr. Glatter. “According to the study, 15% of the emergency room visits and hospitalizations were due to acute trauma, 14% due to respiratory issues, and 13% to gastrointestinal illnesses. These effects were seen in first-time users but not those with chronic use, according to the study inclusion criteria.”
Cannabis use could result in physical injuries through “impaired judgment, coordination, combined with an altered state of consciousness or generalized drowsiness, that could contribute to an increase in motor vehicle collisions, along with an increased risk for falls leading to lacerations, fractures, contusions, or bruising,” said Dr. Glatter. “Cannabis may also lead to an altered sense of perception related to interactions with others, resulting in feelings of anxiety or restlessness culminating in physical altercations and other injuries.”
The current study indicates the need for understanding the potential physical and psychological effects of cannabis use, he said.
“Additional research is needed to better understand the relative percentage cases related to edibles vs. inhalation presenting to the ED,” he noted. “There is no question that edibles continue to present significant dangers for those who don’t read labels or remain poorly informed regarding their dosing as a result of delayed onset and longer duration,” he said. To help reduce risk of toxicity, the concept of a “high lasting 12-15 hours, as with edibles, as opposed to 3-4 hours from inhalation must be clearly stated on packaging and better communicated with users, as the toxicity with edibles is more often from lack of prior knowledge about onset of effects related to dosing.”
In addition, the “potential for psychosis to develop with more chronic cannabis use, along with cannabinoid hyperemesis syndrome should be on every clinician’s radar,” Dr. Glatter emphasized.
“The bottom line is that as more states legalize the use of cannabis, it’s vital to also implement comprehensive public education efforts to provide users with the reported risks associated with not only inhalation (vaping or flower) but also edibles, which account for an increasingly greater percentage of ED visits and associated adverse effects,” he said.
The study was supported by the Lung Association–Ontario, as well as by grants from the Ontario Ministry of Health and the Ministry of Long-Term Care. The researchers and Dr. Glatter have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
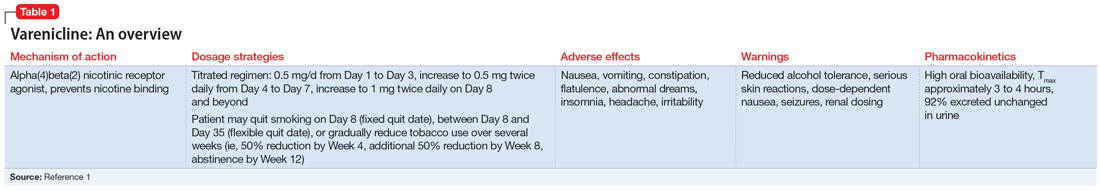
Smoking cessation: Varenicline and the risk of neuropsychiatric adverse events
Mr. T, age 34, is a veteran who recently returned to civilian life. He presents to his local Veteran Affairs facility for transition of care. During active duty, he had been diagnosed with obstructive sleep apnea, tobacco use disorder, posttraumatic stress disorder (PTSD) secondary to combat exposure, and insomnia. Mr. T says he wants to quit smoking; currently, he smokes 2 packs of cigarettes per day. The primary care clinician notes that Mr. T has uncontrolled PTSD symptoms and poor sleep, and refers him for an outpatient mental health appointment.
At the mental health appointment 3 weeks later, Mr. T asks about medications to quit smoking, specifically varenicline (Table 11). Mr. T’s PTSD Checklist for DSM-5 score is 52, which indicates severe PTSD symptomatology. He says he sees shadowy figures in his periphery every day, and worries they are spying on him. His wife reports Mr. T has had these symptoms for most of their 10-year marriage but has never been treated for them. After a discussion with the outpatient team, Mr. T says he is willing to engage in exposure therapy for PTSD, but he does not want to take any medications other than varenicline for smoking cessation.
Cigarette smoke is a known carcinogen and risk factor for the development of cardiovascular and respiratory diseases and other comorbidities. People with severe mental illness (SMI) are 3 to 5 times more likely to smoke, and they often face multiple barriers to cessation, including low socioeconomic status and lack of support.2 Even when patients with SMI are provided appropriate behavioral and pharmacologic interventions, they often require more frequent monitoring and counseling, receive a longer duration of drug therapy, and experience lower smoking cessation rates than the general population.2
Current guidelines recommend nicotine replacement therapy (NRT), bupropion, varenicline, and behavioral support as first-line therapies for smoking cessation in patients with and without SMI.2 Evidence suggests that varenicline is more effective than other pharmacologic options; however, in 2009 a black-box warning was added to both varenicline and bupropion to highlight an increased risk of neuropsychiatric events in individuals with SMI.2 This led some clinicians to hesitate to prescribe varenicline or bupropion to patients with psychiatric illness. However, in 2016, the EAGLES trial evaluated the safety of varenicline, bupropion, and NRT in smokers with and without psychiatric disorders, and based on the findings, the black-box warning was removed.2
This article reviews the evidence regarding the use of varenicline and the risk of neuropsychiatric adverse events in patients with psychiatric illness. Table 23-6 provides a summary of each varenicline trial we discuss.
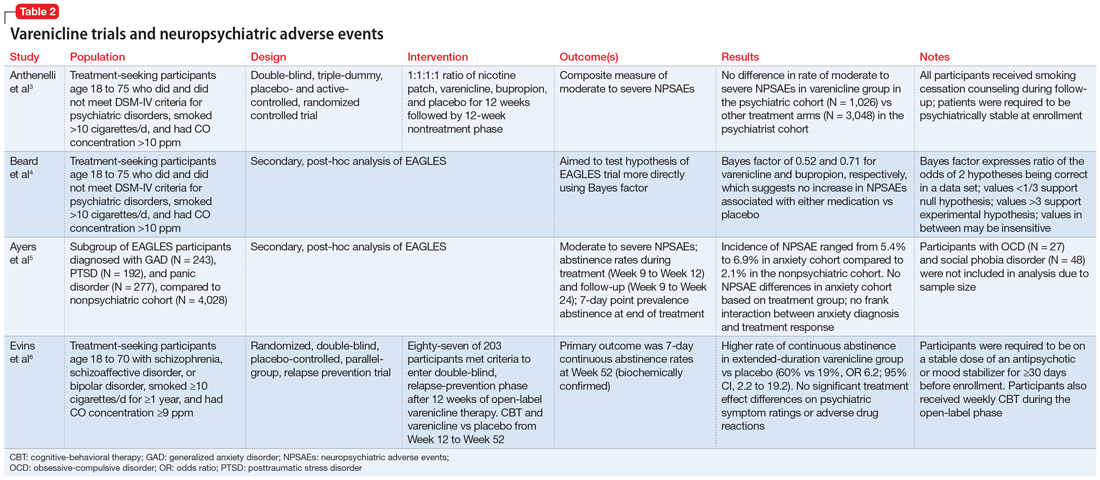
The EAGLES trial
EAGLES was a multicenter, multinational, randomized, double-blind, triple-dummy, placebo- and active-controlled trial of 8,144 individuals who received treatment for smoking cessation.3 The primary endpoint was the incidence of a composite measure of moderate to severe neuropsychiatric events (NPSAEs).3 Participants were split into psychiatric (N = 4,116) and nonpsychiatric (N = 4,028) cohorts and randomized into 4 treatment arms: varenicline 1 mg twice a day, bupropion 150 mg twice a day, nicotine patch 21 mg/d with taper, or placebo, all for 12 weeks with an additional 12 weeks of follow-up. All participants smoked ≥10 cigarettes per day. Individuals in the psychiatric cohort had to be psychiatrically stable (no exacerbations for 6 months and stable treatment for 3 months). Exclusionary diagnoses included psychotic disorders (except schizophrenia and schizoaffective disorder), dementia, substance use (except nicotine), and personality disorders (except borderline personality disorder).2
The rates of moderate to severe NPSAEs in the varenicline groups were 1.25% (95% CI, 0.60 to 1.90) in the nonpsychiatric cohort and 6.42% (95% CI, 4.91 to 7.93) in the psychiatric cohort.3 However, when comparing the varenicline group of the psychiatric cohort to the other arms of the psychiatric cohort, there were no differences (bupropion 6.62% [95% CI, 5.09 to 8.15], nicotine patch 5.20% [95% CI, 3.84 to 6.56], placebo 4.83% [95% CI, 3.51 to 6.16], respectively). The primary efficacy endpoint was continuous abstinence rates (CAR) for Week 9 through Week 12. In the psychiatric cohort, varenicline was superior compared to placebo (odds ratio [OR] 3.24; 95% CI, 2.56 to 4.11), bupropion (OR 1.74; 95% CI, 1.41 to 2.14), and nicotine patch (OR 1.62; 95% CI, 1.32 to 1.99).3
Continue to: Further analysis of EAGLES
Further analysis of EAGLES
Beard et al4 used Bayes factor testing for additional analysis of EAGLES data to determine whether the data were insensitive to neuropsychiatric effects secondary to a lack of statistical power. In the psychiatric cohort, the varenicline and bupropion groups exhibited suggestive but not conclusive data that there was no increase in NPSAEs compared to placebo (Bayes factor 0.52 and 0.71, respectively).4
Another EAGLES analysis by Ayers et al5 evaluated participants with anxiety disorders (N = 712), including PTSD (N = 192), generalized anxiety disorder (GAD) (N = 243), and panic disorder (N = 277).Of those with PTSD who received varenicline, there were no statistically significant differences in CAR from Week 9 to Week 12 vs placebo.5 However, there was a significant difference in individuals with GAD (OR 4.53; 95% CI, 1.20 to 17.10), and panic disorder (OR 8.49; 95% CI, 1.57 to 45.78).5 In contrast to CAR from Week 9 to Week 12, 7-day point prevalence abstinence at Week 12 for participants with PTSD was significant (OR 4.04; 95% CI, 1.39 to 11.74) when comparing varenicline to placebo. Within the anxiety disorder cohort, there were no significant differences in moderate to severe NPSAE rates based on treatment group. Calculated risk differences comparing varenicline to placebo were: PTSD group -7.73 (95% CI, -21.95 to 6.49), GAD group 2.80 (95% CI, -6.63 to 12.23), and panic disorder group -0.18 (95% CI, -9.57 to 9.21).5
Other studies
Evins et al6 conducted a randomized controlled trial to evaluate the safety of varenicline maintenance therapy in patients with schizophrenia or bipolar disorder. To be deemed clinically stable, participants in this study needed to be taking a stable dose of an antipsychotic or mood-stabilizing agent(s) for ≥30 days, compared to the 3-month requirement of the EAGLES trial.3,6 Participants received 12 weeks of open-label varenicline; those who achieved abstinence (N = 87) entered the relapse-prevention phase and were randomized to varenicline 1 mg twice a day or placebo for 40 weeks. Of those who entered relapse-prevention, 5 in the placebo group and 2 in the varenicline group were psychiatrically hospitalized (risk ratio 0.45; 95% CI, 0.04 to 2.9).6 These researchers concluded that varenicline maintenance therapy prolonged abstinence rates with no significant increase in neuropsychiatric events.6
Although treatment options for smoking cessation have advanced, individuals with SMI are still disproportionately affected by the negative outcomes of cigarette smoking. Current literature suggests that varenicline does not confer an appreciable risk of neuropsychiatric events in otherwise stable patients and is the preferred first-line treatment. However, there is a gap in understanding the impact of this medication on individuals with unstable psychiatric illness. Health care professionals should be encouraged to use varenicline with careful monitoring for appropriate patients with psychiatric disorders as a standard of care to help them quit smoking.
CASE CONTINUED
After consulting with the psychiatric pharmacist and discussing the risks and benefits of varenicline, Mr. T is started on the appropriate titration schedule (Table 11). A pharmacist provides varenicline education, including the possibility of psychiatric adverse effects, and tells Mr. T to report any worsening psychiatric symptoms. Mr. T is scheduled for frequent follow-up visits to monitor possible adverse effects and his tobacco use. He says he understands the potential adverse effects of varenicline and agrees to frequent follow-up appointments while taking it.
Related Resources
- Leone FT, Zhang Y, Evers-Casey S, et al. Initiating pharmacologic treatment in tobacco-dependent adults. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;202(2):e5-e31. doi:10.1164/rccm.202005.1982ST
- Cieslak K, Freudenreich O. 4 Ways to help your patients with schizophrenia quit smoking. Current Psychiatry. 2018; 17(2):28,33.
Drug Brand Names
Bupropion • Wellbutrin
Varenicline • Chantix
1. Chantix [package insert]. New York, NY: Pfizer Inc; 2019.
2. Sharma R, Alla K, Pfeffer D, et al. An appraisal of practice guidelines for smoking cessation in people with severe mental illness. Aust N Z J Psychiatry. 2017;51(11):1106-1120. doi:10.1177/0004867417726176
3. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520. doi:10.1016/s0140-6736(16)30272-0
4. Beard E, Jackson SE, Anthenelli RM, et al. Estimation of risk of neuropsychiatric adverse events from varenicline, bupropion and nicotine patch versus placebo: secondary analysis of results from the EAGLES trial using Bayes factors. Addiction. 2021;116(10):2816-2824. doi:10.1111/add.15440
5. Ayers CR, Heffner JL, Russ C, et al. Efficacy and safety of pharmacotherapies for smoking cessation in anxiety disorders: subgroup analysis of the randomized, active- and placebo-controlled EAGLES trial. Depress Anxiety. 2020;37(3)247-260. doi:10.1002/da.22982
6. Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311(2):145-154. doi:10.1001/jama.2013.285113
Mr. T, age 34, is a veteran who recently returned to civilian life. He presents to his local Veteran Affairs facility for transition of care. During active duty, he had been diagnosed with obstructive sleep apnea, tobacco use disorder, posttraumatic stress disorder (PTSD) secondary to combat exposure, and insomnia. Mr. T says he wants to quit smoking; currently, he smokes 2 packs of cigarettes per day. The primary care clinician notes that Mr. T has uncontrolled PTSD symptoms and poor sleep, and refers him for an outpatient mental health appointment.
At the mental health appointment 3 weeks later, Mr. T asks about medications to quit smoking, specifically varenicline (Table 11). Mr. T’s PTSD Checklist for DSM-5 score is 52, which indicates severe PTSD symptomatology. He says he sees shadowy figures in his periphery every day, and worries they are spying on him. His wife reports Mr. T has had these symptoms for most of their 10-year marriage but has never been treated for them. After a discussion with the outpatient team, Mr. T says he is willing to engage in exposure therapy for PTSD, but he does not want to take any medications other than varenicline for smoking cessation.

Cigarette smoke is a known carcinogen and risk factor for the development of cardiovascular and respiratory diseases and other comorbidities. People with severe mental illness (SMI) are 3 to 5 times more likely to smoke, and they often face multiple barriers to cessation, including low socioeconomic status and lack of support.2 Even when patients with SMI are provided appropriate behavioral and pharmacologic interventions, they often require more frequent monitoring and counseling, receive a longer duration of drug therapy, and experience lower smoking cessation rates than the general population.2
Current guidelines recommend nicotine replacement therapy (NRT), bupropion, varenicline, and behavioral support as first-line therapies for smoking cessation in patients with and without SMI.2 Evidence suggests that varenicline is more effective than other pharmacologic options; however, in 2009 a black-box warning was added to both varenicline and bupropion to highlight an increased risk of neuropsychiatric events in individuals with SMI.2 This led some clinicians to hesitate to prescribe varenicline or bupropion to patients with psychiatric illness. However, in 2016, the EAGLES trial evaluated the safety of varenicline, bupropion, and NRT in smokers with and without psychiatric disorders, and based on the findings, the black-box warning was removed.2
This article reviews the evidence regarding the use of varenicline and the risk of neuropsychiatric adverse events in patients with psychiatric illness. Table 23-6 provides a summary of each varenicline trial we discuss.

The EAGLES trial
EAGLES was a multicenter, multinational, randomized, double-blind, triple-dummy, placebo- and active-controlled trial of 8,144 individuals who received treatment for smoking cessation.3 The primary endpoint was the incidence of a composite measure of moderate to severe neuropsychiatric events (NPSAEs).3 Participants were split into psychiatric (N = 4,116) and nonpsychiatric (N = 4,028) cohorts and randomized into 4 treatment arms: varenicline 1 mg twice a day, bupropion 150 mg twice a day, nicotine patch 21 mg/d with taper, or placebo, all for 12 weeks with an additional 12 weeks of follow-up. All participants smoked ≥10 cigarettes per day. Individuals in the psychiatric cohort had to be psychiatrically stable (no exacerbations for 6 months and stable treatment for 3 months). Exclusionary diagnoses included psychotic disorders (except schizophrenia and schizoaffective disorder), dementia, substance use (except nicotine), and personality disorders (except borderline personality disorder).2
The rates of moderate to severe NPSAEs in the varenicline groups were 1.25% (95% CI, 0.60 to 1.90) in the nonpsychiatric cohort and 6.42% (95% CI, 4.91 to 7.93) in the psychiatric cohort.3 However, when comparing the varenicline group of the psychiatric cohort to the other arms of the psychiatric cohort, there were no differences (bupropion 6.62% [95% CI, 5.09 to 8.15], nicotine patch 5.20% [95% CI, 3.84 to 6.56], placebo 4.83% [95% CI, 3.51 to 6.16], respectively). The primary efficacy endpoint was continuous abstinence rates (CAR) for Week 9 through Week 12. In the psychiatric cohort, varenicline was superior compared to placebo (odds ratio [OR] 3.24; 95% CI, 2.56 to 4.11), bupropion (OR 1.74; 95% CI, 1.41 to 2.14), and nicotine patch (OR 1.62; 95% CI, 1.32 to 1.99).3
Continue to: Further analysis of EAGLES
Further analysis of EAGLES
Beard et al4 used Bayes factor testing for additional analysis of EAGLES data to determine whether the data were insensitive to neuropsychiatric effects secondary to a lack of statistical power. In the psychiatric cohort, the varenicline and bupropion groups exhibited suggestive but not conclusive data that there was no increase in NPSAEs compared to placebo (Bayes factor 0.52 and 0.71, respectively).4
Another EAGLES analysis by Ayers et al5 evaluated participants with anxiety disorders (N = 712), including PTSD (N = 192), generalized anxiety disorder (GAD) (N = 243), and panic disorder (N = 277).Of those with PTSD who received varenicline, there were no statistically significant differences in CAR from Week 9 to Week 12 vs placebo.5 However, there was a significant difference in individuals with GAD (OR 4.53; 95% CI, 1.20 to 17.10), and panic disorder (OR 8.49; 95% CI, 1.57 to 45.78).5 In contrast to CAR from Week 9 to Week 12, 7-day point prevalence abstinence at Week 12 for participants with PTSD was significant (OR 4.04; 95% CI, 1.39 to 11.74) when comparing varenicline to placebo. Within the anxiety disorder cohort, there were no significant differences in moderate to severe NPSAE rates based on treatment group. Calculated risk differences comparing varenicline to placebo were: PTSD group -7.73 (95% CI, -21.95 to 6.49), GAD group 2.80 (95% CI, -6.63 to 12.23), and panic disorder group -0.18 (95% CI, -9.57 to 9.21).5
Other studies
Evins et al6 conducted a randomized controlled trial to evaluate the safety of varenicline maintenance therapy in patients with schizophrenia or bipolar disorder. To be deemed clinically stable, participants in this study needed to be taking a stable dose of an antipsychotic or mood-stabilizing agent(s) for ≥30 days, compared to the 3-month requirement of the EAGLES trial.3,6 Participants received 12 weeks of open-label varenicline; those who achieved abstinence (N = 87) entered the relapse-prevention phase and were randomized to varenicline 1 mg twice a day or placebo for 40 weeks. Of those who entered relapse-prevention, 5 in the placebo group and 2 in the varenicline group were psychiatrically hospitalized (risk ratio 0.45; 95% CI, 0.04 to 2.9).6 These researchers concluded that varenicline maintenance therapy prolonged abstinence rates with no significant increase in neuropsychiatric events.6
Although treatment options for smoking cessation have advanced, individuals with SMI are still disproportionately affected by the negative outcomes of cigarette smoking. Current literature suggests that varenicline does not confer an appreciable risk of neuropsychiatric events in otherwise stable patients and is the preferred first-line treatment. However, there is a gap in understanding the impact of this medication on individuals with unstable psychiatric illness. Health care professionals should be encouraged to use varenicline with careful monitoring for appropriate patients with psychiatric disorders as a standard of care to help them quit smoking.
CASE CONTINUED
After consulting with the psychiatric pharmacist and discussing the risks and benefits of varenicline, Mr. T is started on the appropriate titration schedule (Table 11). A pharmacist provides varenicline education, including the possibility of psychiatric adverse effects, and tells Mr. T to report any worsening psychiatric symptoms. Mr. T is scheduled for frequent follow-up visits to monitor possible adverse effects and his tobacco use. He says he understands the potential adverse effects of varenicline and agrees to frequent follow-up appointments while taking it.
Related Resources
- Leone FT, Zhang Y, Evers-Casey S, et al. Initiating pharmacologic treatment in tobacco-dependent adults. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;202(2):e5-e31. doi:10.1164/rccm.202005.1982ST
- Cieslak K, Freudenreich O. 4 Ways to help your patients with schizophrenia quit smoking. Current Psychiatry. 2018; 17(2):28,33.
Drug Brand Names
Bupropion • Wellbutrin
Varenicline • Chantix
Mr. T, age 34, is a veteran who recently returned to civilian life. He presents to his local Veteran Affairs facility for transition of care. During active duty, he had been diagnosed with obstructive sleep apnea, tobacco use disorder, posttraumatic stress disorder (PTSD) secondary to combat exposure, and insomnia. Mr. T says he wants to quit smoking; currently, he smokes 2 packs of cigarettes per day. The primary care clinician notes that Mr. T has uncontrolled PTSD symptoms and poor sleep, and refers him for an outpatient mental health appointment.
At the mental health appointment 3 weeks later, Mr. T asks about medications to quit smoking, specifically varenicline (Table 11). Mr. T’s PTSD Checklist for DSM-5 score is 52, which indicates severe PTSD symptomatology. He says he sees shadowy figures in his periphery every day, and worries they are spying on him. His wife reports Mr. T has had these symptoms for most of their 10-year marriage but has never been treated for them. After a discussion with the outpatient team, Mr. T says he is willing to engage in exposure therapy for PTSD, but he does not want to take any medications other than varenicline for smoking cessation.

Cigarette smoke is a known carcinogen and risk factor for the development of cardiovascular and respiratory diseases and other comorbidities. People with severe mental illness (SMI) are 3 to 5 times more likely to smoke, and they often face multiple barriers to cessation, including low socioeconomic status and lack of support.2 Even when patients with SMI are provided appropriate behavioral and pharmacologic interventions, they often require more frequent monitoring and counseling, receive a longer duration of drug therapy, and experience lower smoking cessation rates than the general population.2
Current guidelines recommend nicotine replacement therapy (NRT), bupropion, varenicline, and behavioral support as first-line therapies for smoking cessation in patients with and without SMI.2 Evidence suggests that varenicline is more effective than other pharmacologic options; however, in 2009 a black-box warning was added to both varenicline and bupropion to highlight an increased risk of neuropsychiatric events in individuals with SMI.2 This led some clinicians to hesitate to prescribe varenicline or bupropion to patients with psychiatric illness. However, in 2016, the EAGLES trial evaluated the safety of varenicline, bupropion, and NRT in smokers with and without psychiatric disorders, and based on the findings, the black-box warning was removed.2
This article reviews the evidence regarding the use of varenicline and the risk of neuropsychiatric adverse events in patients with psychiatric illness. Table 23-6 provides a summary of each varenicline trial we discuss.

The EAGLES trial
EAGLES was a multicenter, multinational, randomized, double-blind, triple-dummy, placebo- and active-controlled trial of 8,144 individuals who received treatment for smoking cessation.3 The primary endpoint was the incidence of a composite measure of moderate to severe neuropsychiatric events (NPSAEs).3 Participants were split into psychiatric (N = 4,116) and nonpsychiatric (N = 4,028) cohorts and randomized into 4 treatment arms: varenicline 1 mg twice a day, bupropion 150 mg twice a day, nicotine patch 21 mg/d with taper, or placebo, all for 12 weeks with an additional 12 weeks of follow-up. All participants smoked ≥10 cigarettes per day. Individuals in the psychiatric cohort had to be psychiatrically stable (no exacerbations for 6 months and stable treatment for 3 months). Exclusionary diagnoses included psychotic disorders (except schizophrenia and schizoaffective disorder), dementia, substance use (except nicotine), and personality disorders (except borderline personality disorder).2
The rates of moderate to severe NPSAEs in the varenicline groups were 1.25% (95% CI, 0.60 to 1.90) in the nonpsychiatric cohort and 6.42% (95% CI, 4.91 to 7.93) in the psychiatric cohort.3 However, when comparing the varenicline group of the psychiatric cohort to the other arms of the psychiatric cohort, there were no differences (bupropion 6.62% [95% CI, 5.09 to 8.15], nicotine patch 5.20% [95% CI, 3.84 to 6.56], placebo 4.83% [95% CI, 3.51 to 6.16], respectively). The primary efficacy endpoint was continuous abstinence rates (CAR) for Week 9 through Week 12. In the psychiatric cohort, varenicline was superior compared to placebo (odds ratio [OR] 3.24; 95% CI, 2.56 to 4.11), bupropion (OR 1.74; 95% CI, 1.41 to 2.14), and nicotine patch (OR 1.62; 95% CI, 1.32 to 1.99).3
Continue to: Further analysis of EAGLES
Further analysis of EAGLES
Beard et al4 used Bayes factor testing for additional analysis of EAGLES data to determine whether the data were insensitive to neuropsychiatric effects secondary to a lack of statistical power. In the psychiatric cohort, the varenicline and bupropion groups exhibited suggestive but not conclusive data that there was no increase in NPSAEs compared to placebo (Bayes factor 0.52 and 0.71, respectively).4
Another EAGLES analysis by Ayers et al5 evaluated participants with anxiety disorders (N = 712), including PTSD (N = 192), generalized anxiety disorder (GAD) (N = 243), and panic disorder (N = 277).Of those with PTSD who received varenicline, there were no statistically significant differences in CAR from Week 9 to Week 12 vs placebo.5 However, there was a significant difference in individuals with GAD (OR 4.53; 95% CI, 1.20 to 17.10), and panic disorder (OR 8.49; 95% CI, 1.57 to 45.78).5 In contrast to CAR from Week 9 to Week 12, 7-day point prevalence abstinence at Week 12 for participants with PTSD was significant (OR 4.04; 95% CI, 1.39 to 11.74) when comparing varenicline to placebo. Within the anxiety disorder cohort, there were no significant differences in moderate to severe NPSAE rates based on treatment group. Calculated risk differences comparing varenicline to placebo were: PTSD group -7.73 (95% CI, -21.95 to 6.49), GAD group 2.80 (95% CI, -6.63 to 12.23), and panic disorder group -0.18 (95% CI, -9.57 to 9.21).5
Other studies
Evins et al6 conducted a randomized controlled trial to evaluate the safety of varenicline maintenance therapy in patients with schizophrenia or bipolar disorder. To be deemed clinically stable, participants in this study needed to be taking a stable dose of an antipsychotic or mood-stabilizing agent(s) for ≥30 days, compared to the 3-month requirement of the EAGLES trial.3,6 Participants received 12 weeks of open-label varenicline; those who achieved abstinence (N = 87) entered the relapse-prevention phase and were randomized to varenicline 1 mg twice a day or placebo for 40 weeks. Of those who entered relapse-prevention, 5 in the placebo group and 2 in the varenicline group were psychiatrically hospitalized (risk ratio 0.45; 95% CI, 0.04 to 2.9).6 These researchers concluded that varenicline maintenance therapy prolonged abstinence rates with no significant increase in neuropsychiatric events.6
Although treatment options for smoking cessation have advanced, individuals with SMI are still disproportionately affected by the negative outcomes of cigarette smoking. Current literature suggests that varenicline does not confer an appreciable risk of neuropsychiatric events in otherwise stable patients and is the preferred first-line treatment. However, there is a gap in understanding the impact of this medication on individuals with unstable psychiatric illness. Health care professionals should be encouraged to use varenicline with careful monitoring for appropriate patients with psychiatric disorders as a standard of care to help them quit smoking.
CASE CONTINUED
After consulting with the psychiatric pharmacist and discussing the risks and benefits of varenicline, Mr. T is started on the appropriate titration schedule (Table 11). A pharmacist provides varenicline education, including the possibility of psychiatric adverse effects, and tells Mr. T to report any worsening psychiatric symptoms. Mr. T is scheduled for frequent follow-up visits to monitor possible adverse effects and his tobacco use. He says he understands the potential adverse effects of varenicline and agrees to frequent follow-up appointments while taking it.
Related Resources
- Leone FT, Zhang Y, Evers-Casey S, et al. Initiating pharmacologic treatment in tobacco-dependent adults. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;202(2):e5-e31. doi:10.1164/rccm.202005.1982ST
- Cieslak K, Freudenreich O. 4 Ways to help your patients with schizophrenia quit smoking. Current Psychiatry. 2018; 17(2):28,33.
Drug Brand Names
Bupropion • Wellbutrin
Varenicline • Chantix
1. Chantix [package insert]. New York, NY: Pfizer Inc; 2019.
2. Sharma R, Alla K, Pfeffer D, et al. An appraisal of practice guidelines for smoking cessation in people with severe mental illness. Aust N Z J Psychiatry. 2017;51(11):1106-1120. doi:10.1177/0004867417726176
3. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520. doi:10.1016/s0140-6736(16)30272-0
4. Beard E, Jackson SE, Anthenelli RM, et al. Estimation of risk of neuropsychiatric adverse events from varenicline, bupropion and nicotine patch versus placebo: secondary analysis of results from the EAGLES trial using Bayes factors. Addiction. 2021;116(10):2816-2824. doi:10.1111/add.15440
5. Ayers CR, Heffner JL, Russ C, et al. Efficacy and safety of pharmacotherapies for smoking cessation in anxiety disorders: subgroup analysis of the randomized, active- and placebo-controlled EAGLES trial. Depress Anxiety. 2020;37(3)247-260. doi:10.1002/da.22982
6. Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311(2):145-154. doi:10.1001/jama.2013.285113
1. Chantix [package insert]. New York, NY: Pfizer Inc; 2019.
2. Sharma R, Alla K, Pfeffer D, et al. An appraisal of practice guidelines for smoking cessation in people with severe mental illness. Aust N Z J Psychiatry. 2017;51(11):1106-1120. doi:10.1177/0004867417726176
3. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520. doi:10.1016/s0140-6736(16)30272-0
4. Beard E, Jackson SE, Anthenelli RM, et al. Estimation of risk of neuropsychiatric adverse events from varenicline, bupropion and nicotine patch versus placebo: secondary analysis of results from the EAGLES trial using Bayes factors. Addiction. 2021;116(10):2816-2824. doi:10.1111/add.15440
5. Ayers CR, Heffner JL, Russ C, et al. Efficacy and safety of pharmacotherapies for smoking cessation in anxiety disorders: subgroup analysis of the randomized, active- and placebo-controlled EAGLES trial. Depress Anxiety. 2020;37(3)247-260. doi:10.1002/da.22982
6. Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311(2):145-154. doi:10.1001/jama.2013.285113
Mobile devices ‘addictive by design’: Obesity is one of many health effects
Wireless devices, like smart phones and tablets, appear to induce compulsive or even addictive use in many individuals, leading to adverse health consequences that are likely to be curtailed only through often difficult behavior modification, according to a pediatric endocrinologist’s take on the problem.
While the summary was based in part on the analysis of 234 published papers drawn from the medical literature, the lead author, Nidhi Gupta, MD, said the data reinforce her own clinical experience.
“As a pediatric endocrinologist, the trend in smartphone-associated health disorders, such as obesity, sleep, and behavior issues, worries me,” Dr. Gupta, director of KAP Pediatric Endocrinology, Nashville, Tenn., said at the annual meeting of the Endocrine Society.
Based on her search of the medical literature, the available data raise concern. In one study she cited, for example, each hour per day of screen time was found to translate into a body mass index increase of 0.5 to 0.7 kg/m2 (P < .001).
With this type of progressive rise in BMI comes prediabetes, dyslipidemia, and other metabolic disorders associated with major health risks, including cardiovascular disease. And there are others. Dr. Gupta cited data suggesting screen time before bed disturbs sleep, which has its own set of health risks.
“When I say health, it includes physical health, mental health, and emotional health,” said Dr. Gupta.
In the U.S. and other countries with a growing obesity epidemic, lack of physical activity and unhealthy eating are widely considered the major culprits. Excessive screen time contributes to both.
“When we are engaged with our devices, we are often snacking subconsciously and not very mindful that we are making unhealthy choices,” Dr. Gupta said.
The problem is that there is a vicious circle. Compulsive use of devices follows the same loop as other types of addictive behaviors, according to Dr. Gupta. She traced overuse of wireless devices to the dopaminergic system, which is a powerful neuroendocrine-mediated process of craving, response, and reward.
Like fat, sugar, and salt, which provoke a neuroendocrine reward signal, the chimes and buzzes of a cell phone provide their own cues for reward in the form of a dopamine surge. As a result, these become the “triggers of an irresistible and irrational urge to check our device that makes the dopamine go high in our brain,” Dr. Gupta explained.
Although the vicious cycle can be thwarted by turning off the device, Dr. Gupta characterized this as “impractical” when smartphones are so vital to daily communication. Rather, Dr. Gupta advocated a program of moderation, reserving the phone for useful tasks without succumbing to the siren song of apps that waste time.
The most conspicuous culprit is social media, which Dr. Gupta considers to be among the most Pavlovian triggers of cell phone addiction. However, she acknowledged that participation in social media has its justifications.
“I, myself, use social media for my own branding and marketing,” Dr. Gupta said.
The problem that users have is distinguishing between screen time that does and does not have value, according to Dr. Gupta. She indicated that many of those overusing their smart devices are being driven by the dopaminergic reward system, which is generally divorced from the real goals of life, such as personal satisfaction and activity that is rewarding monetarily or in other ways.
“I am not asking for these devices to be thrown out the window. I am advocating for moderation, balance, and real-life engagement,” Dr. Gupta said at the meeting, held in Atlanta and virtually.
She outlined a long list of practical suggestions, including turning off the alarms, chimes, and messages that engage the user into the vicious dopaminergic-reward system loop. She suggested mindfulness so that the user can distinguish between valuable device use and activity that is simply procrastination.
“The devices are designed to be addictive. They are designed to manipulate our brain,” she said. “Eliminate the reward. Let’s try to make our devices boring, unappealing, or enticing so that they only work as tools.”
The medical literature is filled with data that support the potential harms of excessive screen use, leading many others to make some of the same points. In 2017, Thomas N. Robinson, MD, professor of child health at Stanford (Calif.) University, reviewed data showing an association between screen media exposure and obesity in children and adolescents.
“This is an area crying out for more research,” Dr. Robinson said in an interview. The problem of screen time, sedentary behavior, and weight gain has been an issue since the television was invented, which was the point he made in his 2017 paper, but he agreed that the problem is only getting worse.
“Digital technology has become ubiquitous, touching nearly every aspect of people’s lives,” he said. Yet, as evidence grows that overuse of this technology can be harmful, it is creating a problem without a clear solution.
“There are few data about the efficacy of specific strategies to reduce harmful impacts of digital screen use,” he said.
While some of the solutions that Dr. Gupta described make sense, they are more easily described than executed. The dopaminergic reward system is strong and largely experienced subconsciously. Recruiting patients to recognize that dopaminergic rewards are not rewards in any true sense is already a challenge. Enlisting patients to take the difficult steps to avoid the behavioral cues might be even more difficult.
Dr. Gupta and Dr. Robinson report no potential conflicts of interest.
Wireless devices, like smart phones and tablets, appear to induce compulsive or even addictive use in many individuals, leading to adverse health consequences that are likely to be curtailed only through often difficult behavior modification, according to a pediatric endocrinologist’s take on the problem.
While the summary was based in part on the analysis of 234 published papers drawn from the medical literature, the lead author, Nidhi Gupta, MD, said the data reinforce her own clinical experience.
“As a pediatric endocrinologist, the trend in smartphone-associated health disorders, such as obesity, sleep, and behavior issues, worries me,” Dr. Gupta, director of KAP Pediatric Endocrinology, Nashville, Tenn., said at the annual meeting of the Endocrine Society.
Based on her search of the medical literature, the available data raise concern. In one study she cited, for example, each hour per day of screen time was found to translate into a body mass index increase of 0.5 to 0.7 kg/m2 (P < .001).
With this type of progressive rise in BMI comes prediabetes, dyslipidemia, and other metabolic disorders associated with major health risks, including cardiovascular disease. And there are others. Dr. Gupta cited data suggesting screen time before bed disturbs sleep, which has its own set of health risks.
“When I say health, it includes physical health, mental health, and emotional health,” said Dr. Gupta.
In the U.S. and other countries with a growing obesity epidemic, lack of physical activity and unhealthy eating are widely considered the major culprits. Excessive screen time contributes to both.
“When we are engaged with our devices, we are often snacking subconsciously and not very mindful that we are making unhealthy choices,” Dr. Gupta said.
The problem is that there is a vicious circle. Compulsive use of devices follows the same loop as other types of addictive behaviors, according to Dr. Gupta. She traced overuse of wireless devices to the dopaminergic system, which is a powerful neuroendocrine-mediated process of craving, response, and reward.
Like fat, sugar, and salt, which provoke a neuroendocrine reward signal, the chimes and buzzes of a cell phone provide their own cues for reward in the form of a dopamine surge. As a result, these become the “triggers of an irresistible and irrational urge to check our device that makes the dopamine go high in our brain,” Dr. Gupta explained.
Although the vicious cycle can be thwarted by turning off the device, Dr. Gupta characterized this as “impractical” when smartphones are so vital to daily communication. Rather, Dr. Gupta advocated a program of moderation, reserving the phone for useful tasks without succumbing to the siren song of apps that waste time.
The most conspicuous culprit is social media, which Dr. Gupta considers to be among the most Pavlovian triggers of cell phone addiction. However, she acknowledged that participation in social media has its justifications.
“I, myself, use social media for my own branding and marketing,” Dr. Gupta said.
The problem that users have is distinguishing between screen time that does and does not have value, according to Dr. Gupta. She indicated that many of those overusing their smart devices are being driven by the dopaminergic reward system, which is generally divorced from the real goals of life, such as personal satisfaction and activity that is rewarding monetarily or in other ways.
“I am not asking for these devices to be thrown out the window. I am advocating for moderation, balance, and real-life engagement,” Dr. Gupta said at the meeting, held in Atlanta and virtually.
She outlined a long list of practical suggestions, including turning off the alarms, chimes, and messages that engage the user into the vicious dopaminergic-reward system loop. She suggested mindfulness so that the user can distinguish between valuable device use and activity that is simply procrastination.
“The devices are designed to be addictive. They are designed to manipulate our brain,” she said. “Eliminate the reward. Let’s try to make our devices boring, unappealing, or enticing so that they only work as tools.”
The medical literature is filled with data that support the potential harms of excessive screen use, leading many others to make some of the same points. In 2017, Thomas N. Robinson, MD, professor of child health at Stanford (Calif.) University, reviewed data showing an association between screen media exposure and obesity in children and adolescents.
“This is an area crying out for more research,” Dr. Robinson said in an interview. The problem of screen time, sedentary behavior, and weight gain has been an issue since the television was invented, which was the point he made in his 2017 paper, but he agreed that the problem is only getting worse.
“Digital technology has become ubiquitous, touching nearly every aspect of people’s lives,” he said. Yet, as evidence grows that overuse of this technology can be harmful, it is creating a problem without a clear solution.
“There are few data about the efficacy of specific strategies to reduce harmful impacts of digital screen use,” he said.
While some of the solutions that Dr. Gupta described make sense, they are more easily described than executed. The dopaminergic reward system is strong and largely experienced subconsciously. Recruiting patients to recognize that dopaminergic rewards are not rewards in any true sense is already a challenge. Enlisting patients to take the difficult steps to avoid the behavioral cues might be even more difficult.
Dr. Gupta and Dr. Robinson report no potential conflicts of interest.
Wireless devices, like smart phones and tablets, appear to induce compulsive or even addictive use in many individuals, leading to adverse health consequences that are likely to be curtailed only through often difficult behavior modification, according to a pediatric endocrinologist’s take on the problem.
While the summary was based in part on the analysis of 234 published papers drawn from the medical literature, the lead author, Nidhi Gupta, MD, said the data reinforce her own clinical experience.
“As a pediatric endocrinologist, the trend in smartphone-associated health disorders, such as obesity, sleep, and behavior issues, worries me,” Dr. Gupta, director of KAP Pediatric Endocrinology, Nashville, Tenn., said at the annual meeting of the Endocrine Society.
Based on her search of the medical literature, the available data raise concern. In one study she cited, for example, each hour per day of screen time was found to translate into a body mass index increase of 0.5 to 0.7 kg/m2 (P < .001).
With this type of progressive rise in BMI comes prediabetes, dyslipidemia, and other metabolic disorders associated with major health risks, including cardiovascular disease. And there are others. Dr. Gupta cited data suggesting screen time before bed disturbs sleep, which has its own set of health risks.
“When I say health, it includes physical health, mental health, and emotional health,” said Dr. Gupta.
In the U.S. and other countries with a growing obesity epidemic, lack of physical activity and unhealthy eating are widely considered the major culprits. Excessive screen time contributes to both.
“When we are engaged with our devices, we are often snacking subconsciously and not very mindful that we are making unhealthy choices,” Dr. Gupta said.
The problem is that there is a vicious circle. Compulsive use of devices follows the same loop as other types of addictive behaviors, according to Dr. Gupta. She traced overuse of wireless devices to the dopaminergic system, which is a powerful neuroendocrine-mediated process of craving, response, and reward.
Like fat, sugar, and salt, which provoke a neuroendocrine reward signal, the chimes and buzzes of a cell phone provide their own cues for reward in the form of a dopamine surge. As a result, these become the “triggers of an irresistible and irrational urge to check our device that makes the dopamine go high in our brain,” Dr. Gupta explained.
Although the vicious cycle can be thwarted by turning off the device, Dr. Gupta characterized this as “impractical” when smartphones are so vital to daily communication. Rather, Dr. Gupta advocated a program of moderation, reserving the phone for useful tasks without succumbing to the siren song of apps that waste time.
The most conspicuous culprit is social media, which Dr. Gupta considers to be among the most Pavlovian triggers of cell phone addiction. However, she acknowledged that participation in social media has its justifications.
“I, myself, use social media for my own branding and marketing,” Dr. Gupta said.
The problem that users have is distinguishing between screen time that does and does not have value, according to Dr. Gupta. She indicated that many of those overusing their smart devices are being driven by the dopaminergic reward system, which is generally divorced from the real goals of life, such as personal satisfaction and activity that is rewarding monetarily or in other ways.
“I am not asking for these devices to be thrown out the window. I am advocating for moderation, balance, and real-life engagement,” Dr. Gupta said at the meeting, held in Atlanta and virtually.
She outlined a long list of practical suggestions, including turning off the alarms, chimes, and messages that engage the user into the vicious dopaminergic-reward system loop. She suggested mindfulness so that the user can distinguish between valuable device use and activity that is simply procrastination.
“The devices are designed to be addictive. They are designed to manipulate our brain,” she said. “Eliminate the reward. Let’s try to make our devices boring, unappealing, or enticing so that they only work as tools.”
The medical literature is filled with data that support the potential harms of excessive screen use, leading many others to make some of the same points. In 2017, Thomas N. Robinson, MD, professor of child health at Stanford (Calif.) University, reviewed data showing an association between screen media exposure and obesity in children and adolescents.
“This is an area crying out for more research,” Dr. Robinson said in an interview. The problem of screen time, sedentary behavior, and weight gain has been an issue since the television was invented, which was the point he made in his 2017 paper, but he agreed that the problem is only getting worse.
“Digital technology has become ubiquitous, touching nearly every aspect of people’s lives,” he said. Yet, as evidence grows that overuse of this technology can be harmful, it is creating a problem without a clear solution.
“There are few data about the efficacy of specific strategies to reduce harmful impacts of digital screen use,” he said.
While some of the solutions that Dr. Gupta described make sense, they are more easily described than executed. The dopaminergic reward system is strong and largely experienced subconsciously. Recruiting patients to recognize that dopaminergic rewards are not rewards in any true sense is already a challenge. Enlisting patients to take the difficult steps to avoid the behavioral cues might be even more difficult.
Dr. Gupta and Dr. Robinson report no potential conflicts of interest.
FROM ENDO 2022
FDA orders Juul to stop selling E-cigarettes
The marketing denial order covers all the company’s products in the United States, which means Juul must stop distributing the products and remove everything on the market. That includes the Juul device and flavor replacement pods in the tobacco and menthol flavors.
“Today’s action is further progress on the FDA’s commitment to ensuring that all e-cigarette and electronic nicotine delivery system products currently being marketed to consumers meet our public health standards,” Robert Califf, MD, the FDA commissioner, said in the announcement.
“The agency has dedicated significant resources to review products from the companies that account for most of the U.S. market,” he said. “We recognize these make up a significant part of the available products and many have played a disproportionate role in the rise in youth vaping.”
The marketing denial order covers only the commercial distribution and retail sale of Juul’s products and doesn’t restrict consumer possession or use. The FDA “cannot and will not” enforce actions against consumers, the agency said.
The order comes after a 2-year review of the company’s application seeking authorization to continue selling non–fruit-flavored products, such as menthol and tobacco. The FDA determined the application “lacked sufficient evidence regarding the toxicological profile of the products to demonstrate that marketing of the products would be appropriate for the protection of the public health.”
Some of Juul’s study findings raised concerns because of “insufficient and conflicting data,” the FDA said, including potentially harmful chemicals leaching from the Juul liquid replacement pods.
“To date, the FDA has not received clinical information to suggest an immediate hazard associated with the use of the JUUL device or JUUL pods,” the agency said. “However, the [orders] issued today reflect FDA’s determination that there is insufficient evidence to assess the potential toxicological risks of using the JUUL products.”
Juul is expected to appeal the FDA’s decision, according to The New York Times.
In recent years, the FDA has reviewed marketing applications from Juul and other e-cigarette companies as anti-tobacco groups have called for new rules to limit products that led to a surge in youth vaping during the past decade. At the same time, advocates of e-cigarettes and nicotine-delivery devices have said the products help adult smokers to quit cigarettes and other tobacco products.
Juul, in particular, has been blamed for fueling the surge in underage vaping due to fruity flavors and hip marketing, according to The Wall Street Journal. The company removed sweet and fruity flavors from shelves in 2019 and has been trying to repair its reputation by limiting its marketing and focusing on adult cigarette smokers.
In 2020, all e-cigarette manufacturers in the United States were required to submit their products for FDA review to stay on the market, the newspaper reported. The agency has been weighing the potential benefits for adult cigarette smokers against the harms for young people.
The FDA banned the sale of fruit- and mint-flavored cartridges and juice pods in 2020, but menthol and tobacco-flavored products were left on the market, according to USA Today. In September 2021, the agency also banned the sale of hundreds of thousands of vaping and e-cigarette products but didn’t rule on Juul.
Meanwhile, the FDA has cleared Reynolds American and NJOY Holdings – two of Juul’s biggest rivals – to keep tobacco-flavored products on the market. Industry experts expected Juul to receive similar clearance, the Journal reported.
Juul, which was at the top of the U.S. e-cigarette market in 2018, has moved to second place behind Reynolds’s Vuse brand, the newspaper reported. The United States represents most of the company’s revenue, though its products are also available in Canada, the United Kingdom, France, Italy, and the Philippines.
Underage vaping has fallen in the United States since federal restrictions raised the legal purchase age for tobacco products to 21 and banned the sale of sweet and fruity cartridges, according to the Journal. Juul’s popularity has also dropped among youth, with other products such as Puff Bar, Vuse, and Smok becoming more popular among e-cigarette users in high school.
In a separate decision announced this week, the FDA is also moving forward with a plan to reduce the amount of nicotine in cigarettes. The decision, which has been years in the making, is aimed at prompting millions of cigarette users to quit smoking or switch to alternatives such as e-cigarettes, as well as limit the number of users who pick up smoking at an early age.
A version of this article first appeared on WebMD.com .
The marketing denial order covers all the company’s products in the United States, which means Juul must stop distributing the products and remove everything on the market. That includes the Juul device and flavor replacement pods in the tobacco and menthol flavors.
“Today’s action is further progress on the FDA’s commitment to ensuring that all e-cigarette and electronic nicotine delivery system products currently being marketed to consumers meet our public health standards,” Robert Califf, MD, the FDA commissioner, said in the announcement.
“The agency has dedicated significant resources to review products from the companies that account for most of the U.S. market,” he said. “We recognize these make up a significant part of the available products and many have played a disproportionate role in the rise in youth vaping.”
The marketing denial order covers only the commercial distribution and retail sale of Juul’s products and doesn’t restrict consumer possession or use. The FDA “cannot and will not” enforce actions against consumers, the agency said.
The order comes after a 2-year review of the company’s application seeking authorization to continue selling non–fruit-flavored products, such as menthol and tobacco. The FDA determined the application “lacked sufficient evidence regarding the toxicological profile of the products to demonstrate that marketing of the products would be appropriate for the protection of the public health.”
Some of Juul’s study findings raised concerns because of “insufficient and conflicting data,” the FDA said, including potentially harmful chemicals leaching from the Juul liquid replacement pods.
“To date, the FDA has not received clinical information to suggest an immediate hazard associated with the use of the JUUL device or JUUL pods,” the agency said. “However, the [orders] issued today reflect FDA’s determination that there is insufficient evidence to assess the potential toxicological risks of using the JUUL products.”
Juul is expected to appeal the FDA’s decision, according to The New York Times.
In recent years, the FDA has reviewed marketing applications from Juul and other e-cigarette companies as anti-tobacco groups have called for new rules to limit products that led to a surge in youth vaping during the past decade. At the same time, advocates of e-cigarettes and nicotine-delivery devices have said the products help adult smokers to quit cigarettes and other tobacco products.
Juul, in particular, has been blamed for fueling the surge in underage vaping due to fruity flavors and hip marketing, according to The Wall Street Journal. The company removed sweet and fruity flavors from shelves in 2019 and has been trying to repair its reputation by limiting its marketing and focusing on adult cigarette smokers.
In 2020, all e-cigarette manufacturers in the United States were required to submit their products for FDA review to stay on the market, the newspaper reported. The agency has been weighing the potential benefits for adult cigarette smokers against the harms for young people.
The FDA banned the sale of fruit- and mint-flavored cartridges and juice pods in 2020, but menthol and tobacco-flavored products were left on the market, according to USA Today. In September 2021, the agency also banned the sale of hundreds of thousands of vaping and e-cigarette products but didn’t rule on Juul.
Meanwhile, the FDA has cleared Reynolds American and NJOY Holdings – two of Juul’s biggest rivals – to keep tobacco-flavored products on the market. Industry experts expected Juul to receive similar clearance, the Journal reported.
Juul, which was at the top of the U.S. e-cigarette market in 2018, has moved to second place behind Reynolds’s Vuse brand, the newspaper reported. The United States represents most of the company’s revenue, though its products are also available in Canada, the United Kingdom, France, Italy, and the Philippines.
Underage vaping has fallen in the United States since federal restrictions raised the legal purchase age for tobacco products to 21 and banned the sale of sweet and fruity cartridges, according to the Journal. Juul’s popularity has also dropped among youth, with other products such as Puff Bar, Vuse, and Smok becoming more popular among e-cigarette users in high school.
In a separate decision announced this week, the FDA is also moving forward with a plan to reduce the amount of nicotine in cigarettes. The decision, which has been years in the making, is aimed at prompting millions of cigarette users to quit smoking or switch to alternatives such as e-cigarettes, as well as limit the number of users who pick up smoking at an early age.
A version of this article first appeared on WebMD.com .
The marketing denial order covers all the company’s products in the United States, which means Juul must stop distributing the products and remove everything on the market. That includes the Juul device and flavor replacement pods in the tobacco and menthol flavors.
“Today’s action is further progress on the FDA’s commitment to ensuring that all e-cigarette and electronic nicotine delivery system products currently being marketed to consumers meet our public health standards,” Robert Califf, MD, the FDA commissioner, said in the announcement.
“The agency has dedicated significant resources to review products from the companies that account for most of the U.S. market,” he said. “We recognize these make up a significant part of the available products and many have played a disproportionate role in the rise in youth vaping.”
The marketing denial order covers only the commercial distribution and retail sale of Juul’s products and doesn’t restrict consumer possession or use. The FDA “cannot and will not” enforce actions against consumers, the agency said.
The order comes after a 2-year review of the company’s application seeking authorization to continue selling non–fruit-flavored products, such as menthol and tobacco. The FDA determined the application “lacked sufficient evidence regarding the toxicological profile of the products to demonstrate that marketing of the products would be appropriate for the protection of the public health.”
Some of Juul’s study findings raised concerns because of “insufficient and conflicting data,” the FDA said, including potentially harmful chemicals leaching from the Juul liquid replacement pods.
“To date, the FDA has not received clinical information to suggest an immediate hazard associated with the use of the JUUL device or JUUL pods,” the agency said. “However, the [orders] issued today reflect FDA’s determination that there is insufficient evidence to assess the potential toxicological risks of using the JUUL products.”
Juul is expected to appeal the FDA’s decision, according to The New York Times.
In recent years, the FDA has reviewed marketing applications from Juul and other e-cigarette companies as anti-tobacco groups have called for new rules to limit products that led to a surge in youth vaping during the past decade. At the same time, advocates of e-cigarettes and nicotine-delivery devices have said the products help adult smokers to quit cigarettes and other tobacco products.
Juul, in particular, has been blamed for fueling the surge in underage vaping due to fruity flavors and hip marketing, according to The Wall Street Journal. The company removed sweet and fruity flavors from shelves in 2019 and has been trying to repair its reputation by limiting its marketing and focusing on adult cigarette smokers.
In 2020, all e-cigarette manufacturers in the United States were required to submit their products for FDA review to stay on the market, the newspaper reported. The agency has been weighing the potential benefits for adult cigarette smokers against the harms for young people.
The FDA banned the sale of fruit- and mint-flavored cartridges and juice pods in 2020, but menthol and tobacco-flavored products were left on the market, according to USA Today. In September 2021, the agency also banned the sale of hundreds of thousands of vaping and e-cigarette products but didn’t rule on Juul.
Meanwhile, the FDA has cleared Reynolds American and NJOY Holdings – two of Juul’s biggest rivals – to keep tobacco-flavored products on the market. Industry experts expected Juul to receive similar clearance, the Journal reported.
Juul, which was at the top of the U.S. e-cigarette market in 2018, has moved to second place behind Reynolds’s Vuse brand, the newspaper reported. The United States represents most of the company’s revenue, though its products are also available in Canada, the United Kingdom, France, Italy, and the Philippines.
Underage vaping has fallen in the United States since federal restrictions raised the legal purchase age for tobacco products to 21 and banned the sale of sweet and fruity cartridges, according to the Journal. Juul’s popularity has also dropped among youth, with other products such as Puff Bar, Vuse, and Smok becoming more popular among e-cigarette users in high school.
In a separate decision announced this week, the FDA is also moving forward with a plan to reduce the amount of nicotine in cigarettes. The decision, which has been years in the making, is aimed at prompting millions of cigarette users to quit smoking or switch to alternatives such as e-cigarettes, as well as limit the number of users who pick up smoking at an early age.
A version of this article first appeared on WebMD.com .
Biden moves to limit nicotine levels in cigarettes
The Department of Health and Human Services posted a notice that details plans for a new rule to create a maximum allowed amount of nicotine in certain tobacco products. The Food and Drug Administration would take the action, the notice said, “to reduce addictiveness to certain tobacco products, thus giving addicted users a greater ability to quit.” The product standard would also help keep nonsmokers interested in trying tobacco, mainly youth, from starting to smoke and become regulars.
“Lowering nicotine levels to minimally addictive or non-addictive levels would decrease the likelihood that future generations of young people become addicted to cigarettes and help more currently addicted smokers to quit,” FDA Commissioner Robert Califf, MD, said in a statement.
The FDA, in charge of regulating cigarettes, issues a proposed rule when changes are discussed. That would be followed by a period for public comments before a final rule could be issued.
The proposed rule was first reported by The Washington Post.
The FDA in 2018 published a study in the New England Journal of Medicine that estimated that a potential limit on nicotine in cigarettes could, by the year 2100, prevent more than 33 million people from becoming regular smokers, and prevent the deaths of more than 8 million people from tobacco-related illnesses.
The action to reduce nicotine levels would fit in with President Joe Biden’s goal of reducing cancer death rates by half over 25 years. Each year, according to the American Cancer Society, about 480,000 deaths (about 1 in 5) are related to smoking. Currently, about 34 million American adults still smoke cigarettes.
Matthew Myers, president of the Campaign for Tobacco-Free Kids, called the proposed rule a “truly game-changing proposal.”
“There is no other single action our country can take that would prevent more young people from becoming addicted to tobacco or have a greater impact on reducing deaths from cancer, cardiovascular disease and respiratory disease,” Mr. Myers said in a statement.
However, he said, “these gains will only be realized if the administration and the FDA demonstrate a full-throated commitment to finalizing and implementing this proposal.”
The FDA proposed the nicotine reduction strategy in talks with the White House and the Department of Health and Human Services early in 2021, according to the Post.
Earlier this year, the FDA issued a proposed rule to ban menthol flavoring in cigarettes. The agency is accepting public comments though July 5.
The action of reducing nicotine levels would likely take years to complete, Mitch Zeller, JD, recently retired director of the FDA Center for Tobacco Products, told the Post.
In 2018, the FDA issued a proposed ruling to set a standard for maximum nicotine levels in cigarettes.
Advocates say the action of slashing nicotine, the active – and addictive – ingredient in cigarettes, would save millions of lives for generations to come. Opponents liken it to the prohibition of alcohol in the 1920s and predict the action will fail.
Others say that if limits are put on nicotine levels, adults should have greater access to noncombustible alternatives.
A version of this article first appeared on WebMD.com.
The Department of Health and Human Services posted a notice that details plans for a new rule to create a maximum allowed amount of nicotine in certain tobacco products. The Food and Drug Administration would take the action, the notice said, “to reduce addictiveness to certain tobacco products, thus giving addicted users a greater ability to quit.” The product standard would also help keep nonsmokers interested in trying tobacco, mainly youth, from starting to smoke and become regulars.
“Lowering nicotine levels to minimally addictive or non-addictive levels would decrease the likelihood that future generations of young people become addicted to cigarettes and help more currently addicted smokers to quit,” FDA Commissioner Robert Califf, MD, said in a statement.
The FDA, in charge of regulating cigarettes, issues a proposed rule when changes are discussed. That would be followed by a period for public comments before a final rule could be issued.
The proposed rule was first reported by The Washington Post.
The FDA in 2018 published a study in the New England Journal of Medicine that estimated that a potential limit on nicotine in cigarettes could, by the year 2100, prevent more than 33 million people from becoming regular smokers, and prevent the deaths of more than 8 million people from tobacco-related illnesses.
The action to reduce nicotine levels would fit in with President Joe Biden’s goal of reducing cancer death rates by half over 25 years. Each year, according to the American Cancer Society, about 480,000 deaths (about 1 in 5) are related to smoking. Currently, about 34 million American adults still smoke cigarettes.
Matthew Myers, president of the Campaign for Tobacco-Free Kids, called the proposed rule a “truly game-changing proposal.”
“There is no other single action our country can take that would prevent more young people from becoming addicted to tobacco or have a greater impact on reducing deaths from cancer, cardiovascular disease and respiratory disease,” Mr. Myers said in a statement.
However, he said, “these gains will only be realized if the administration and the FDA demonstrate a full-throated commitment to finalizing and implementing this proposal.”
The FDA proposed the nicotine reduction strategy in talks with the White House and the Department of Health and Human Services early in 2021, according to the Post.
Earlier this year, the FDA issued a proposed rule to ban menthol flavoring in cigarettes. The agency is accepting public comments though July 5.
The action of reducing nicotine levels would likely take years to complete, Mitch Zeller, JD, recently retired director of the FDA Center for Tobacco Products, told the Post.
In 2018, the FDA issued a proposed ruling to set a standard for maximum nicotine levels in cigarettes.
Advocates say the action of slashing nicotine, the active – and addictive – ingredient in cigarettes, would save millions of lives for generations to come. Opponents liken it to the prohibition of alcohol in the 1920s and predict the action will fail.
Others say that if limits are put on nicotine levels, adults should have greater access to noncombustible alternatives.
A version of this article first appeared on WebMD.com.
The Department of Health and Human Services posted a notice that details plans for a new rule to create a maximum allowed amount of nicotine in certain tobacco products. The Food and Drug Administration would take the action, the notice said, “to reduce addictiveness to certain tobacco products, thus giving addicted users a greater ability to quit.” The product standard would also help keep nonsmokers interested in trying tobacco, mainly youth, from starting to smoke and become regulars.
“Lowering nicotine levels to minimally addictive or non-addictive levels would decrease the likelihood that future generations of young people become addicted to cigarettes and help more currently addicted smokers to quit,” FDA Commissioner Robert Califf, MD, said in a statement.
The FDA, in charge of regulating cigarettes, issues a proposed rule when changes are discussed. That would be followed by a period for public comments before a final rule could be issued.
The proposed rule was first reported by The Washington Post.
The FDA in 2018 published a study in the New England Journal of Medicine that estimated that a potential limit on nicotine in cigarettes could, by the year 2100, prevent more than 33 million people from becoming regular smokers, and prevent the deaths of more than 8 million people from tobacco-related illnesses.
The action to reduce nicotine levels would fit in with President Joe Biden’s goal of reducing cancer death rates by half over 25 years. Each year, according to the American Cancer Society, about 480,000 deaths (about 1 in 5) are related to smoking. Currently, about 34 million American adults still smoke cigarettes.
Matthew Myers, president of the Campaign for Tobacco-Free Kids, called the proposed rule a “truly game-changing proposal.”
“There is no other single action our country can take that would prevent more young people from becoming addicted to tobacco or have a greater impact on reducing deaths from cancer, cardiovascular disease and respiratory disease,” Mr. Myers said in a statement.
However, he said, “these gains will only be realized if the administration and the FDA demonstrate a full-throated commitment to finalizing and implementing this proposal.”
The FDA proposed the nicotine reduction strategy in talks with the White House and the Department of Health and Human Services early in 2021, according to the Post.
Earlier this year, the FDA issued a proposed rule to ban menthol flavoring in cigarettes. The agency is accepting public comments though July 5.
The action of reducing nicotine levels would likely take years to complete, Mitch Zeller, JD, recently retired director of the FDA Center for Tobacco Products, told the Post.
In 2018, the FDA issued a proposed ruling to set a standard for maximum nicotine levels in cigarettes.
Advocates say the action of slashing nicotine, the active – and addictive – ingredient in cigarettes, would save millions of lives for generations to come. Opponents liken it to the prohibition of alcohol in the 1920s and predict the action will fail.
Others say that if limits are put on nicotine levels, adults should have greater access to noncombustible alternatives.
A version of this article first appeared on WebMD.com.
Alcohol, marijuana use declined among youth during pandemic
During the coronavirus pandemic, several substance use behaviors decreased among youths, namely drinking, smoking, vaping, and cannabis use, according to a recent study published in the journal Current Psychiatry Reports.
That likely happened because they had to spend more time at home and less time with their friends, the study authors wrote, adding that youth substance use should be monitored in the post-pandemic years.
“One of the driving factors for youth substance use is access to substances,” Hannah Layman, one of the co-authors and a social and behavioral sciences doctoral student at West Virginia University, said in a statement.
“With stay-at-home orders, virtual schooling, and social distancing, children have been spending more time with family and are more socially isolated from peers than before,” she said. “Although social isolation from peers may have a negative impact on their mental health, it may just be one of the desirable outcomes of the pandemic when considering substance use in children.”
Ms. Layman and colleagues analyzed 49 studies that followed substance use of alcohol, cannabis, tobacco, e-cigarettes/vaping, and other drugs among children, teens, and youths under age 24. The studies spanned across several countries, including 22 in North America and 19 in Europe.
The research team found that most studies across all categories reported reductions in prevalence, except for the category of “other drugs and unspecific drugs,” which included three studies that showed an increase in use and three studies that showed a decrease in use.
Teens and preteens tend to have easier access to alcohol, tobacco, cannabis products, and vaping products and see them as less serious than “hard drugs,” the authors said.
Future research should analyze the long-term effects of the pandemic on youth substance use, the study authors wrote, paying attention to differences by gender and those who face the highest risks for substance use. Previous studies have shown an increase in substance use among youths, particularly among those in low-income neighborhoods or in difficult family circumstances.
“Substance use can affect a young person’s body in many ways, such as the development of mental health issues (depression, anxiety, conduct problems, personality disorders, and suicidal thoughts), injuries due to accidents, decreased bone mineral density, preventing proper brain growth and function, delayed puberty, liver damage, and so much more,” Ms. Layman said.
Increased parent or caregiver supervision can help prevent substance use problems, she noted. Early intervention, open support in conversations, and ongoing education about the dangers of substance use can help as well.
“Our findings also identified the importance of improving youth mental health and the value of telemedicine to address young people’s needs during the pandemic,” she said.
A version of this article first appeared on WebMD.com.
During the coronavirus pandemic, several substance use behaviors decreased among youths, namely drinking, smoking, vaping, and cannabis use, according to a recent study published in the journal Current Psychiatry Reports.
That likely happened because they had to spend more time at home and less time with their friends, the study authors wrote, adding that youth substance use should be monitored in the post-pandemic years.
“One of the driving factors for youth substance use is access to substances,” Hannah Layman, one of the co-authors and a social and behavioral sciences doctoral student at West Virginia University, said in a statement.
“With stay-at-home orders, virtual schooling, and social distancing, children have been spending more time with family and are more socially isolated from peers than before,” she said. “Although social isolation from peers may have a negative impact on their mental health, it may just be one of the desirable outcomes of the pandemic when considering substance use in children.”
Ms. Layman and colleagues analyzed 49 studies that followed substance use of alcohol, cannabis, tobacco, e-cigarettes/vaping, and other drugs among children, teens, and youths under age 24. The studies spanned across several countries, including 22 in North America and 19 in Europe.
The research team found that most studies across all categories reported reductions in prevalence, except for the category of “other drugs and unspecific drugs,” which included three studies that showed an increase in use and three studies that showed a decrease in use.
Teens and preteens tend to have easier access to alcohol, tobacco, cannabis products, and vaping products and see them as less serious than “hard drugs,” the authors said.
Future research should analyze the long-term effects of the pandemic on youth substance use, the study authors wrote, paying attention to differences by gender and those who face the highest risks for substance use. Previous studies have shown an increase in substance use among youths, particularly among those in low-income neighborhoods or in difficult family circumstances.
“Substance use can affect a young person’s body in many ways, such as the development of mental health issues (depression, anxiety, conduct problems, personality disorders, and suicidal thoughts), injuries due to accidents, decreased bone mineral density, preventing proper brain growth and function, delayed puberty, liver damage, and so much more,” Ms. Layman said.
Increased parent or caregiver supervision can help prevent substance use problems, she noted. Early intervention, open support in conversations, and ongoing education about the dangers of substance use can help as well.
“Our findings also identified the importance of improving youth mental health and the value of telemedicine to address young people’s needs during the pandemic,” she said.
A version of this article first appeared on WebMD.com.
During the coronavirus pandemic, several substance use behaviors decreased among youths, namely drinking, smoking, vaping, and cannabis use, according to a recent study published in the journal Current Psychiatry Reports.
That likely happened because they had to spend more time at home and less time with their friends, the study authors wrote, adding that youth substance use should be monitored in the post-pandemic years.
“One of the driving factors for youth substance use is access to substances,” Hannah Layman, one of the co-authors and a social and behavioral sciences doctoral student at West Virginia University, said in a statement.
“With stay-at-home orders, virtual schooling, and social distancing, children have been spending more time with family and are more socially isolated from peers than before,” she said. “Although social isolation from peers may have a negative impact on their mental health, it may just be one of the desirable outcomes of the pandemic when considering substance use in children.”
Ms. Layman and colleagues analyzed 49 studies that followed substance use of alcohol, cannabis, tobacco, e-cigarettes/vaping, and other drugs among children, teens, and youths under age 24. The studies spanned across several countries, including 22 in North America and 19 in Europe.
The research team found that most studies across all categories reported reductions in prevalence, except for the category of “other drugs and unspecific drugs,” which included three studies that showed an increase in use and three studies that showed a decrease in use.
Teens and preteens tend to have easier access to alcohol, tobacco, cannabis products, and vaping products and see them as less serious than “hard drugs,” the authors said.
Future research should analyze the long-term effects of the pandemic on youth substance use, the study authors wrote, paying attention to differences by gender and those who face the highest risks for substance use. Previous studies have shown an increase in substance use among youths, particularly among those in low-income neighborhoods or in difficult family circumstances.
“Substance use can affect a young person’s body in many ways, such as the development of mental health issues (depression, anxiety, conduct problems, personality disorders, and suicidal thoughts), injuries due to accidents, decreased bone mineral density, preventing proper brain growth and function, delayed puberty, liver damage, and so much more,” Ms. Layman said.
Increased parent or caregiver supervision can help prevent substance use problems, she noted. Early intervention, open support in conversations, and ongoing education about the dangers of substance use can help as well.
“Our findings also identified the importance of improving youth mental health and the value of telemedicine to address young people’s needs during the pandemic,” she said.
A version of this article first appeared on WebMD.com.
Rhabdomyolysis Occurring After Use of Cocaine Contaminated With Fentanyl Causing Bilateral Brachial Plexopathy
The brachial plexus is a group of interwoven nerves arising from the cervical spinal cord and coursing through the neck, shoulder, and axilla with terminal branches extending to the distal arm.1 Disorders of the brachial plexus are more rare than other isolated peripheral nerve disorders, trauma being the most common etiology.1 Traction, neoplasms, radiation exposure, external compression, and inflammatory processes, such as Parsonage-Turner syndrome, have also been described as less common etiologies.2
Rhabdomyolysis, a condition in which muscle breakdown occurs, is an uncommon and perhaps underrecognized cause of brachial plexopathy. Rhabdomyolysis is often caused by muscle overuse, trauma, prolonged immobilization, drugs, or toxins. Substances indicated as precipitating factors include alcohol, opioids, cocaine, and amphetamines.3,4 As rhabdomyolysis progresses, swelling and edema can compress surrounding structures. Therefore, in cases of rhabdomyolysis involving the muscles of the neck and shoulder girdle, external compression of the brachial plexus can potentially cause brachial plexopathy. Rare cases of this phenomenon occurring as a sequela of substance use have been described.1,5-9 Few cases have been reported in the literature.
The following case report describes a patient who
Case Presentation
A 68-year-old male patient with a history of polysubstance use disorder presented to the emergency department with complete loss of sensory and motor function of both arms. He had fallen asleep on his couch the previous evening with his arms crossed over his chest in the prone position.
On admission, the patient presented with an agitated mental status. The patient presented with 0/5 strength bilaterally in the upper extremities (UEs) accompanied by numbness and tingling. Radial pulses were palpable in both arms. All UE reflexes were absent, but patellar reflex was intact bilaterally. On hospital day 2, the patient was awake, alert, and oriented to person, place, and time and could provide a full history. The patient’s cranial nerves were intact with shoulder shrug testing mildly weak at 4/5 strength.
Serum electrolytes and glucose levels were normal. The creatine phosphokinase (CPK) level was elevated at 21,292 IU/L. Creatinine and blood urea nitrogen levels were elevated at 1.7 mg/dL and 32 mg/dL, respectively. Serum B12, thyroid-stimulating hormone, and hemoglobin A1c levels were normal.
Due to the absence of evidence of spinal cord injury, presence of normal motor and sensory function of the lower extremities, an elevated CPK level, signal hyperintensities of the muscles of the shoulder girdle, and the patient’s history, the leading diagnosis at this time was brachial plexopathy secondary to focal rhabdomyolysis.
Over the next week, the patient regained some motor function of the left hand and some sensory function bilaterally. At 8 weeks postadmission, a nerve conduction study showed prolonged latencies in the median and ulnar nerves bilaterally. The following week, the patient reported pain in both shoulders (left greater than the right) as well as weakness of shoulder movement on the left greater than the right. There was pain in the right arm throughout. On examination, there was improved function of the arms distal to the elbow, which was better on the right side despite the associated pain (Table). There was atrophy of the left scapular muscles, hypothenar eminence, and deltoid muscle. There was weakness of the left triceps, with slight fourth and fifth finger flexion. The patient was unable to elevate or abduct the left shoulder but could elevate the right shoulder up to 45°. Sensation was decreased over the right outer arm and left posterior upper arm, with hypersensitivity in the right medial upper and lower arm. Deep tendon reflexes were absent in the upper arm aside from the biceps reflex (1+). All reflexes of the lower extremities were normal. It is interesting to note the relative greater improvement on the right despite the edema found on initial imaging being more prominent on the right.
Discussion
Rhabdomyolysis is a condition defined by myocyte necrosis that results in release of cellular contents and local edema. Inciting events may be traumatic, metabolic, ischemic, or substance induced. Common substances indicated include cocaine, amphetamines, acetaminophen, opioids, and alcohol.10 It classically presents with muscle pain and a marked elevation in serum CPK level, but other metabolic disturbances, acute kidney injury, or toxic hepatitis may also occur. A more uncommon sequela of rhabdomyolysis is plexopathy caused by edematous swelling and compression of the surrounding structures.
Rare cases of brachial plexopathy caused by rhabdomyolysis following substance use have been described. In many of these cases, rhabdomyolysis occurred after alcohol use with or without concurrent use of prescription opioids or heroin.7-9 One case following use of 3,4-methylenedioxy-N-methylamptamine (MDMA) and marijuana use was reported.1 Another case of concurrent brachial plexopathy and Horner syndrome in a 29-year-old male patient following ingestion of alcohol and opioids has also been described.5 The rate of occurrence of this phenomenon in the general population is unknown.
The pathophysiology of rhabdomyolysis caused by substance use has not been definitively identified, but it is hypothesized that the cause is 2-fold. The first insult is the direct toxicity of the substances to myocytes.8,9 The second factor is prolonged immobilization in a position that compresses the affected musculature and blood supply, causing both mechanical stress and ischemia to the muscles and brachial plexus. This prolonged immobilization can frequently follow use of substances, such as alcohol or opioids.9 Cases have been reported wherein rhabdomyolysis causing brachial plexopathy occurred despite relatively normal positioning of the arms and shoulders during sleep.9 In our case, the patient had fallen asleep with his arms crossed over his chest in the prone position with his head turned, though he could not recall to which side. Although he stated that he had slept in this position regularly, the effects of fentanyl may have prevented the patient from waking to adjust his posture. This position had potential to compress the musculature of the neck and shoulders and restrict blood flow, resulting in the focal rhabdomyolysis seen in this patient. In theory, the position could also cause a stretch injury of the brachial plexus, although a pure stretch injury would more likely present unilaterally and without evidence of rhabdomyolysis.
Chronic ethanol use may have been a major contributor by both sensitizing the muscles to toxicity of other substances and induction of CYP450 enzymes that are normally responsible for metabolizing other drugs.8 Alcohol also inhibits gluconeogenesis and leads to hyperpolarization of myocytes, further contributing to their susceptibility to damage.9 Our patient had a prior history of alcohol use years before this event, but not at the time of this event.
Our patient had other known risk factors for rhabdomyolysis, including his long-term statin therapy, but it is unclear whether these were contributing factors in his case.10 Of the medications that are known to cause rhabdomyolysis, statins are among the most commonly described, although the mechanism through which this process occurs is not clear. A case of rhabdomyolysis following use of cocaine and heroin in a patient on long-standing statin therapy has been described.13 Our review of the literature found no cases of statin-induced rhabdomyolysis associated with brachial plexopathy. It is possible that concurrent statin therapy has an additive effect to other substances in inducing rhabdomyolysis.
Parsonage-Turner syndrome, also known as neuralgic amyotrophy, should also be included in the differential diagnosis. While there have been multiple etiologies proposed for Parsonage-Turner syndrome, it is generally thought to begin as a primary inflammatory process targeting the brachial plexus. One case report describes Parsonage-Turner syndrome progressing to secondary rhabdomyolysis.6 In this case, no primary etiology was identified, so the Parsonage-Turner syndrome diagnosis was made with secondary rhabdomyolysis.6 We believe it is possible that this case and others may have been misdiagnosed as Parsonage-Turner syndrome.
Aside from physical rehabilitation programs, cases of plexopathy secondary to rhabdomyolysis similar to our patient have largely been treated with supportive therapy and symptom management. Pain management was the primary goal in this patient, which was achieved with moderate success using a combination of muscle relaxants, antiepileptics, tramadol, and serotonin-norepinephrine reuptake inhibitors. Some surgical approaches have been reported in the literature. One case of rhabdomyolysis of the shoulder girdle causing a similar process benefitted from fasciotomy and surgical decompression.7 This patient had a complete recovery of all motor functions aside from shoulder abduction at 8 weeks postoperation, but neuropathic pain persisted in both arms. It is possible our patient may have benefitted from a similar treatment. Further research is necessary to determine the utility of this type of procedure when treating such cases.
Conclusions
This case report adds to the literature describing focal rhabdomyolysis causing secondary bilateral brachial plexopathy after substance use. Further research is needed to establish a definitive pathophysiology as well as treatment guidelines. Evidence-based treatment could mean better outcomes and quicker recoveries for future patients with this condition.
1. Eker Büyüks¸ireci D, Polat M, Zinnurog˘lu M, Cengiz B, Kaymak Karatas¸ GK. Bilateral pan-plexus lesion after substance use: A case report. Turk J Phys Med Rehabil. 2019;65(4):411-414. doi:10.5606/tftrd.2019.3157
2. Rubin DI. Brachial and lumbosacral plexopathies: a review. Clin Neurophysiol Pract. 2020;5:173-193. doi:10.1016/j.cnp.2020.07.005
3. Oshima Y. Characteristics of drug-associated rhabdomyolysis: analysis of 8,610 cases reported to the US Food and Drug Administration. Intern Med. 2011;50(8):845-853. doi:10.2169/internalmedicine.50.4484
4. Waldman W, Kabata PM, Dines AM, et al. Rhabdomyolysis related to acute recreational drug toxicity-a euro-den study. PLoS One. 2021;16(3):e0246297. doi:10.1371/journal.pone.0246297
5. Lee SC, Geannette C, Wolfe SW, Feinberg JH, Sneag DB. Rhabdomyolysis resulting in concurrent Horner’s syndrome and brachial plexopathy: a case report. Skeletal Radiology. 2017;46(8):1131-1136. doi:10.1007/s00256-017-2634-5
6. Goetsch MR, Shen J, Jones JA, Memon A, Chatham W. Neuralgic amyotrophy presenting with multifocal myonecrosis and rhabdomyolysis. Cureus. 2020;12(3):e7382. doi:10.7759/cureus.7382
7. Tonetti DA, Tarkin IS, Bandi K, Moossy JJ. Complete bilateral brachial plexus injury from rhabdomyolysis and compartment syndrome: surgical case report. Oper Neurosurg (Hagerstown). 2019;17(2):E68-e72. doi:10.1093/ons/opy289
8. Riggs JE, Schochet SS Jr, Hogg JP. Focal rhabdomyolysis and brachial plexopathy: an association with heroin and chronic ethanol use. Mil Med. 1999;164(3):228-229.
9. Maddison P. Acute rhabdomyolysis and brachial plexopathy following alcohol ingestion. Muscle Nerve. 2002;25(2):283-285. doi:10.1002/mus.10021.abs
10. Giannoglou GD, Chatzizisis YS, Misirli G. The syndrome of rhabdomyolysis: pathophysiology and diagnosis. Eur J Intern Med. 2007;18(2):90-100. doi:10.1016/j.ejim.2006.09.020
11. Meacham MC, Lynch KL, Coffin PO, Wade A, Wheeler E, Riley ED. Addressing overdose risk among unstably housed women in San Francisco, California: an examination of potential fentanyl contamination of multiple substances. Harm Reduct J. 2020;17(1). doi:10.1186/s12954-020-00361-8
12. Klar SA, Brodkin E, Gibson E, et al. Notes from the field: furanyl-fentanyl overdose events caused by smoking contaminated crack cocaine - British Columbia, Canada, July 15-18, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(37):1015-1016. doi:10.15585/mmwr.mm6537a6
13. Mitaritonno M, Lupo M, Greco I, Mazza A, Cervellin G. Severe rhabdomyolysis induced by co-administration of cocaine and heroin in a 45 years old man treated with rosuvastatin: a case report. Acta Biomed. 2021;92(S1):e2021089. doi:10.23750/abm.v92iS1.8858
The brachial plexus is a group of interwoven nerves arising from the cervical spinal cord and coursing through the neck, shoulder, and axilla with terminal branches extending to the distal arm.1 Disorders of the brachial plexus are more rare than other isolated peripheral nerve disorders, trauma being the most common etiology.1 Traction, neoplasms, radiation exposure, external compression, and inflammatory processes, such as Parsonage-Turner syndrome, have also been described as less common etiologies.2
Rhabdomyolysis, a condition in which muscle breakdown occurs, is an uncommon and perhaps underrecognized cause of brachial plexopathy. Rhabdomyolysis is often caused by muscle overuse, trauma, prolonged immobilization, drugs, or toxins. Substances indicated as precipitating factors include alcohol, opioids, cocaine, and amphetamines.3,4 As rhabdomyolysis progresses, swelling and edema can compress surrounding structures. Therefore, in cases of rhabdomyolysis involving the muscles of the neck and shoulder girdle, external compression of the brachial plexus can potentially cause brachial plexopathy. Rare cases of this phenomenon occurring as a sequela of substance use have been described.1,5-9 Few cases have been reported in the literature.
The following case report describes a patient who
Case Presentation
A 68-year-old male patient with a history of polysubstance use disorder presented to the emergency department with complete loss of sensory and motor function of both arms. He had fallen asleep on his couch the previous evening with his arms crossed over his chest in the prone position.
On admission, the patient presented with an agitated mental status. The patient presented with 0/5 strength bilaterally in the upper extremities (UEs) accompanied by numbness and tingling. Radial pulses were palpable in both arms. All UE reflexes were absent, but patellar reflex was intact bilaterally. On hospital day 2, the patient was awake, alert, and oriented to person, place, and time and could provide a full history. The patient’s cranial nerves were intact with shoulder shrug testing mildly weak at 4/5 strength.
Serum electrolytes and glucose levels were normal. The creatine phosphokinase (CPK) level was elevated at 21,292 IU/L. Creatinine and blood urea nitrogen levels were elevated at 1.7 mg/dL and 32 mg/dL, respectively. Serum B12, thyroid-stimulating hormone, and hemoglobin A1c levels were normal.
Due to the absence of evidence of spinal cord injury, presence of normal motor and sensory function of the lower extremities, an elevated CPK level, signal hyperintensities of the muscles of the shoulder girdle, and the patient’s history, the leading diagnosis at this time was brachial plexopathy secondary to focal rhabdomyolysis.
Over the next week, the patient regained some motor function of the left hand and some sensory function bilaterally. At 8 weeks postadmission, a nerve conduction study showed prolonged latencies in the median and ulnar nerves bilaterally. The following week, the patient reported pain in both shoulders (left greater than the right) as well as weakness of shoulder movement on the left greater than the right. There was pain in the right arm throughout. On examination, there was improved function of the arms distal to the elbow, which was better on the right side despite the associated pain (Table). There was atrophy of the left scapular muscles, hypothenar eminence, and deltoid muscle. There was weakness of the left triceps, with slight fourth and fifth finger flexion. The patient was unable to elevate or abduct the left shoulder but could elevate the right shoulder up to 45°. Sensation was decreased over the right outer arm and left posterior upper arm, with hypersensitivity in the right medial upper and lower arm. Deep tendon reflexes were absent in the upper arm aside from the biceps reflex (1+). All reflexes of the lower extremities were normal. It is interesting to note the relative greater improvement on the right despite the edema found on initial imaging being more prominent on the right.
Discussion
Rhabdomyolysis is a condition defined by myocyte necrosis that results in release of cellular contents and local edema. Inciting events may be traumatic, metabolic, ischemic, or substance induced. Common substances indicated include cocaine, amphetamines, acetaminophen, opioids, and alcohol.10 It classically presents with muscle pain and a marked elevation in serum CPK level, but other metabolic disturbances, acute kidney injury, or toxic hepatitis may also occur. A more uncommon sequela of rhabdomyolysis is plexopathy caused by edematous swelling and compression of the surrounding structures.
Rare cases of brachial plexopathy caused by rhabdomyolysis following substance use have been described. In many of these cases, rhabdomyolysis occurred after alcohol use with or without concurrent use of prescription opioids or heroin.7-9 One case following use of 3,4-methylenedioxy-N-methylamptamine (MDMA) and marijuana use was reported.1 Another case of concurrent brachial plexopathy and Horner syndrome in a 29-year-old male patient following ingestion of alcohol and opioids has also been described.5 The rate of occurrence of this phenomenon in the general population is unknown.
The pathophysiology of rhabdomyolysis caused by substance use has not been definitively identified, but it is hypothesized that the cause is 2-fold. The first insult is the direct toxicity of the substances to myocytes.8,9 The second factor is prolonged immobilization in a position that compresses the affected musculature and blood supply, causing both mechanical stress and ischemia to the muscles and brachial plexus. This prolonged immobilization can frequently follow use of substances, such as alcohol or opioids.9 Cases have been reported wherein rhabdomyolysis causing brachial plexopathy occurred despite relatively normal positioning of the arms and shoulders during sleep.9 In our case, the patient had fallen asleep with his arms crossed over his chest in the prone position with his head turned, though he could not recall to which side. Although he stated that he had slept in this position regularly, the effects of fentanyl may have prevented the patient from waking to adjust his posture. This position had potential to compress the musculature of the neck and shoulders and restrict blood flow, resulting in the focal rhabdomyolysis seen in this patient. In theory, the position could also cause a stretch injury of the brachial plexus, although a pure stretch injury would more likely present unilaterally and without evidence of rhabdomyolysis.
Chronic ethanol use may have been a major contributor by both sensitizing the muscles to toxicity of other substances and induction of CYP450 enzymes that are normally responsible for metabolizing other drugs.8 Alcohol also inhibits gluconeogenesis and leads to hyperpolarization of myocytes, further contributing to their susceptibility to damage.9 Our patient had a prior history of alcohol use years before this event, but not at the time of this event.
Our patient had other known risk factors for rhabdomyolysis, including his long-term statin therapy, but it is unclear whether these were contributing factors in his case.10 Of the medications that are known to cause rhabdomyolysis, statins are among the most commonly described, although the mechanism through which this process occurs is not clear. A case of rhabdomyolysis following use of cocaine and heroin in a patient on long-standing statin therapy has been described.13 Our review of the literature found no cases of statin-induced rhabdomyolysis associated with brachial plexopathy. It is possible that concurrent statin therapy has an additive effect to other substances in inducing rhabdomyolysis.
Parsonage-Turner syndrome, also known as neuralgic amyotrophy, should also be included in the differential diagnosis. While there have been multiple etiologies proposed for Parsonage-Turner syndrome, it is generally thought to begin as a primary inflammatory process targeting the brachial plexus. One case report describes Parsonage-Turner syndrome progressing to secondary rhabdomyolysis.6 In this case, no primary etiology was identified, so the Parsonage-Turner syndrome diagnosis was made with secondary rhabdomyolysis.6 We believe it is possible that this case and others may have been misdiagnosed as Parsonage-Turner syndrome.
Aside from physical rehabilitation programs, cases of plexopathy secondary to rhabdomyolysis similar to our patient have largely been treated with supportive therapy and symptom management. Pain management was the primary goal in this patient, which was achieved with moderate success using a combination of muscle relaxants, antiepileptics, tramadol, and serotonin-norepinephrine reuptake inhibitors. Some surgical approaches have been reported in the literature. One case of rhabdomyolysis of the shoulder girdle causing a similar process benefitted from fasciotomy and surgical decompression.7 This patient had a complete recovery of all motor functions aside from shoulder abduction at 8 weeks postoperation, but neuropathic pain persisted in both arms. It is possible our patient may have benefitted from a similar treatment. Further research is necessary to determine the utility of this type of procedure when treating such cases.
Conclusions
This case report adds to the literature describing focal rhabdomyolysis causing secondary bilateral brachial plexopathy after substance use. Further research is needed to establish a definitive pathophysiology as well as treatment guidelines. Evidence-based treatment could mean better outcomes and quicker recoveries for future patients with this condition.
The brachial plexus is a group of interwoven nerves arising from the cervical spinal cord and coursing through the neck, shoulder, and axilla with terminal branches extending to the distal arm.1 Disorders of the brachial plexus are more rare than other isolated peripheral nerve disorders, trauma being the most common etiology.1 Traction, neoplasms, radiation exposure, external compression, and inflammatory processes, such as Parsonage-Turner syndrome, have also been described as less common etiologies.2
Rhabdomyolysis, a condition in which muscle breakdown occurs, is an uncommon and perhaps underrecognized cause of brachial plexopathy. Rhabdomyolysis is often caused by muscle overuse, trauma, prolonged immobilization, drugs, or toxins. Substances indicated as precipitating factors include alcohol, opioids, cocaine, and amphetamines.3,4 As rhabdomyolysis progresses, swelling and edema can compress surrounding structures. Therefore, in cases of rhabdomyolysis involving the muscles of the neck and shoulder girdle, external compression of the brachial plexus can potentially cause brachial plexopathy. Rare cases of this phenomenon occurring as a sequela of substance use have been described.1,5-9 Few cases have been reported in the literature.
The following case report describes a patient who
Case Presentation
A 68-year-old male patient with a history of polysubstance use disorder presented to the emergency department with complete loss of sensory and motor function of both arms. He had fallen asleep on his couch the previous evening with his arms crossed over his chest in the prone position.
On admission, the patient presented with an agitated mental status. The patient presented with 0/5 strength bilaterally in the upper extremities (UEs) accompanied by numbness and tingling. Radial pulses were palpable in both arms. All UE reflexes were absent, but patellar reflex was intact bilaterally. On hospital day 2, the patient was awake, alert, and oriented to person, place, and time and could provide a full history. The patient’s cranial nerves were intact with shoulder shrug testing mildly weak at 4/5 strength.
Serum electrolytes and glucose levels were normal. The creatine phosphokinase (CPK) level was elevated at 21,292 IU/L. Creatinine and blood urea nitrogen levels were elevated at 1.7 mg/dL and 32 mg/dL, respectively. Serum B12, thyroid-stimulating hormone, and hemoglobin A1c levels were normal.
Due to the absence of evidence of spinal cord injury, presence of normal motor and sensory function of the lower extremities, an elevated CPK level, signal hyperintensities of the muscles of the shoulder girdle, and the patient’s history, the leading diagnosis at this time was brachial plexopathy secondary to focal rhabdomyolysis.
Over the next week, the patient regained some motor function of the left hand and some sensory function bilaterally. At 8 weeks postadmission, a nerve conduction study showed prolonged latencies in the median and ulnar nerves bilaterally. The following week, the patient reported pain in both shoulders (left greater than the right) as well as weakness of shoulder movement on the left greater than the right. There was pain in the right arm throughout. On examination, there was improved function of the arms distal to the elbow, which was better on the right side despite the associated pain (Table). There was atrophy of the left scapular muscles, hypothenar eminence, and deltoid muscle. There was weakness of the left triceps, with slight fourth and fifth finger flexion. The patient was unable to elevate or abduct the left shoulder but could elevate the right shoulder up to 45°. Sensation was decreased over the right outer arm and left posterior upper arm, with hypersensitivity in the right medial upper and lower arm. Deep tendon reflexes were absent in the upper arm aside from the biceps reflex (1+). All reflexes of the lower extremities were normal. It is interesting to note the relative greater improvement on the right despite the edema found on initial imaging being more prominent on the right.
Discussion
Rhabdomyolysis is a condition defined by myocyte necrosis that results in release of cellular contents and local edema. Inciting events may be traumatic, metabolic, ischemic, or substance induced. Common substances indicated include cocaine, amphetamines, acetaminophen, opioids, and alcohol.10 It classically presents with muscle pain and a marked elevation in serum CPK level, but other metabolic disturbances, acute kidney injury, or toxic hepatitis may also occur. A more uncommon sequela of rhabdomyolysis is plexopathy caused by edematous swelling and compression of the surrounding structures.
Rare cases of brachial plexopathy caused by rhabdomyolysis following substance use have been described. In many of these cases, rhabdomyolysis occurred after alcohol use with or without concurrent use of prescription opioids or heroin.7-9 One case following use of 3,4-methylenedioxy-N-methylamptamine (MDMA) and marijuana use was reported.1 Another case of concurrent brachial plexopathy and Horner syndrome in a 29-year-old male patient following ingestion of alcohol and opioids has also been described.5 The rate of occurrence of this phenomenon in the general population is unknown.
The pathophysiology of rhabdomyolysis caused by substance use has not been definitively identified, but it is hypothesized that the cause is 2-fold. The first insult is the direct toxicity of the substances to myocytes.8,9 The second factor is prolonged immobilization in a position that compresses the affected musculature and blood supply, causing both mechanical stress and ischemia to the muscles and brachial plexus. This prolonged immobilization can frequently follow use of substances, such as alcohol or opioids.9 Cases have been reported wherein rhabdomyolysis causing brachial plexopathy occurred despite relatively normal positioning of the arms and shoulders during sleep.9 In our case, the patient had fallen asleep with his arms crossed over his chest in the prone position with his head turned, though he could not recall to which side. Although he stated that he had slept in this position regularly, the effects of fentanyl may have prevented the patient from waking to adjust his posture. This position had potential to compress the musculature of the neck and shoulders and restrict blood flow, resulting in the focal rhabdomyolysis seen in this patient. In theory, the position could also cause a stretch injury of the brachial plexus, although a pure stretch injury would more likely present unilaterally and without evidence of rhabdomyolysis.
Chronic ethanol use may have been a major contributor by both sensitizing the muscles to toxicity of other substances and induction of CYP450 enzymes that are normally responsible for metabolizing other drugs.8 Alcohol also inhibits gluconeogenesis and leads to hyperpolarization of myocytes, further contributing to their susceptibility to damage.9 Our patient had a prior history of alcohol use years before this event, but not at the time of this event.
Our patient had other known risk factors for rhabdomyolysis, including his long-term statin therapy, but it is unclear whether these were contributing factors in his case.10 Of the medications that are known to cause rhabdomyolysis, statins are among the most commonly described, although the mechanism through which this process occurs is not clear. A case of rhabdomyolysis following use of cocaine and heroin in a patient on long-standing statin therapy has been described.13 Our review of the literature found no cases of statin-induced rhabdomyolysis associated with brachial plexopathy. It is possible that concurrent statin therapy has an additive effect to other substances in inducing rhabdomyolysis.
Parsonage-Turner syndrome, also known as neuralgic amyotrophy, should also be included in the differential diagnosis. While there have been multiple etiologies proposed for Parsonage-Turner syndrome, it is generally thought to begin as a primary inflammatory process targeting the brachial plexus. One case report describes Parsonage-Turner syndrome progressing to secondary rhabdomyolysis.6 In this case, no primary etiology was identified, so the Parsonage-Turner syndrome diagnosis was made with secondary rhabdomyolysis.6 We believe it is possible that this case and others may have been misdiagnosed as Parsonage-Turner syndrome.
Aside from physical rehabilitation programs, cases of plexopathy secondary to rhabdomyolysis similar to our patient have largely been treated with supportive therapy and symptom management. Pain management was the primary goal in this patient, which was achieved with moderate success using a combination of muscle relaxants, antiepileptics, tramadol, and serotonin-norepinephrine reuptake inhibitors. Some surgical approaches have been reported in the literature. One case of rhabdomyolysis of the shoulder girdle causing a similar process benefitted from fasciotomy and surgical decompression.7 This patient had a complete recovery of all motor functions aside from shoulder abduction at 8 weeks postoperation, but neuropathic pain persisted in both arms. It is possible our patient may have benefitted from a similar treatment. Further research is necessary to determine the utility of this type of procedure when treating such cases.
Conclusions
This case report adds to the literature describing focal rhabdomyolysis causing secondary bilateral brachial plexopathy after substance use. Further research is needed to establish a definitive pathophysiology as well as treatment guidelines. Evidence-based treatment could mean better outcomes and quicker recoveries for future patients with this condition.
1. Eker Büyüks¸ireci D, Polat M, Zinnurog˘lu M, Cengiz B, Kaymak Karatas¸ GK. Bilateral pan-plexus lesion after substance use: A case report. Turk J Phys Med Rehabil. 2019;65(4):411-414. doi:10.5606/tftrd.2019.3157
2. Rubin DI. Brachial and lumbosacral plexopathies: a review. Clin Neurophysiol Pract. 2020;5:173-193. doi:10.1016/j.cnp.2020.07.005
3. Oshima Y. Characteristics of drug-associated rhabdomyolysis: analysis of 8,610 cases reported to the US Food and Drug Administration. Intern Med. 2011;50(8):845-853. doi:10.2169/internalmedicine.50.4484
4. Waldman W, Kabata PM, Dines AM, et al. Rhabdomyolysis related to acute recreational drug toxicity-a euro-den study. PLoS One. 2021;16(3):e0246297. doi:10.1371/journal.pone.0246297
5. Lee SC, Geannette C, Wolfe SW, Feinberg JH, Sneag DB. Rhabdomyolysis resulting in concurrent Horner’s syndrome and brachial plexopathy: a case report. Skeletal Radiology. 2017;46(8):1131-1136. doi:10.1007/s00256-017-2634-5
6. Goetsch MR, Shen J, Jones JA, Memon A, Chatham W. Neuralgic amyotrophy presenting with multifocal myonecrosis and rhabdomyolysis. Cureus. 2020;12(3):e7382. doi:10.7759/cureus.7382
7. Tonetti DA, Tarkin IS, Bandi K, Moossy JJ. Complete bilateral brachial plexus injury from rhabdomyolysis and compartment syndrome: surgical case report. Oper Neurosurg (Hagerstown). 2019;17(2):E68-e72. doi:10.1093/ons/opy289
8. Riggs JE, Schochet SS Jr, Hogg JP. Focal rhabdomyolysis and brachial plexopathy: an association with heroin and chronic ethanol use. Mil Med. 1999;164(3):228-229.
9. Maddison P. Acute rhabdomyolysis and brachial plexopathy following alcohol ingestion. Muscle Nerve. 2002;25(2):283-285. doi:10.1002/mus.10021.abs
10. Giannoglou GD, Chatzizisis YS, Misirli G. The syndrome of rhabdomyolysis: pathophysiology and diagnosis. Eur J Intern Med. 2007;18(2):90-100. doi:10.1016/j.ejim.2006.09.020
11. Meacham MC, Lynch KL, Coffin PO, Wade A, Wheeler E, Riley ED. Addressing overdose risk among unstably housed women in San Francisco, California: an examination of potential fentanyl contamination of multiple substances. Harm Reduct J. 2020;17(1). doi:10.1186/s12954-020-00361-8
12. Klar SA, Brodkin E, Gibson E, et al. Notes from the field: furanyl-fentanyl overdose events caused by smoking contaminated crack cocaine - British Columbia, Canada, July 15-18, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(37):1015-1016. doi:10.15585/mmwr.mm6537a6
13. Mitaritonno M, Lupo M, Greco I, Mazza A, Cervellin G. Severe rhabdomyolysis induced by co-administration of cocaine and heroin in a 45 years old man treated with rosuvastatin: a case report. Acta Biomed. 2021;92(S1):e2021089. doi:10.23750/abm.v92iS1.8858
1. Eker Büyüks¸ireci D, Polat M, Zinnurog˘lu M, Cengiz B, Kaymak Karatas¸ GK. Bilateral pan-plexus lesion after substance use: A case report. Turk J Phys Med Rehabil. 2019;65(4):411-414. doi:10.5606/tftrd.2019.3157
2. Rubin DI. Brachial and lumbosacral plexopathies: a review. Clin Neurophysiol Pract. 2020;5:173-193. doi:10.1016/j.cnp.2020.07.005
3. Oshima Y. Characteristics of drug-associated rhabdomyolysis: analysis of 8,610 cases reported to the US Food and Drug Administration. Intern Med. 2011;50(8):845-853. doi:10.2169/internalmedicine.50.4484
4. Waldman W, Kabata PM, Dines AM, et al. Rhabdomyolysis related to acute recreational drug toxicity-a euro-den study. PLoS One. 2021;16(3):e0246297. doi:10.1371/journal.pone.0246297
5. Lee SC, Geannette C, Wolfe SW, Feinberg JH, Sneag DB. Rhabdomyolysis resulting in concurrent Horner’s syndrome and brachial plexopathy: a case report. Skeletal Radiology. 2017;46(8):1131-1136. doi:10.1007/s00256-017-2634-5
6. Goetsch MR, Shen J, Jones JA, Memon A, Chatham W. Neuralgic amyotrophy presenting with multifocal myonecrosis and rhabdomyolysis. Cureus. 2020;12(3):e7382. doi:10.7759/cureus.7382
7. Tonetti DA, Tarkin IS, Bandi K, Moossy JJ. Complete bilateral brachial plexus injury from rhabdomyolysis and compartment syndrome: surgical case report. Oper Neurosurg (Hagerstown). 2019;17(2):E68-e72. doi:10.1093/ons/opy289
8. Riggs JE, Schochet SS Jr, Hogg JP. Focal rhabdomyolysis and brachial plexopathy: an association with heroin and chronic ethanol use. Mil Med. 1999;164(3):228-229.
9. Maddison P. Acute rhabdomyolysis and brachial plexopathy following alcohol ingestion. Muscle Nerve. 2002;25(2):283-285. doi:10.1002/mus.10021.abs
10. Giannoglou GD, Chatzizisis YS, Misirli G. The syndrome of rhabdomyolysis: pathophysiology and diagnosis. Eur J Intern Med. 2007;18(2):90-100. doi:10.1016/j.ejim.2006.09.020
11. Meacham MC, Lynch KL, Coffin PO, Wade A, Wheeler E, Riley ED. Addressing overdose risk among unstably housed women in San Francisco, California: an examination of potential fentanyl contamination of multiple substances. Harm Reduct J. 2020;17(1). doi:10.1186/s12954-020-00361-8
12. Klar SA, Brodkin E, Gibson E, et al. Notes from the field: furanyl-fentanyl overdose events caused by smoking contaminated crack cocaine - British Columbia, Canada, July 15-18, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(37):1015-1016. doi:10.15585/mmwr.mm6537a6
13. Mitaritonno M, Lupo M, Greco I, Mazza A, Cervellin G. Severe rhabdomyolysis induced by co-administration of cocaine and heroin in a 45 years old man treated with rosuvastatin: a case report. Acta Biomed. 2021;92(S1):e2021089. doi:10.23750/abm.v92iS1.8858
Synthetic opioid use up almost 800% nationwide
The results of a national urine drug test (UDT) study come as the United States is reporting a record-high number of drug overdose deaths – more than 80% of which involved fentanyl or other synthetic opioids and prompting a push for better surveillance models.
Researchers found that UDTs can be used to accurately identify which drugs are circulating in a community, revealing in just a matter of days critically important drug use trends that current surveillance methods take a month or longer to report.
The faster turnaround could potentially allow clinicians and public health officials to be more proactive with targeted overdose prevention and harm-reduction strategies such as distribution of naloxone and fentanyl test strips.
“We’re talking about trying to come up with an early-warning system,” study author Steven Passik, PhD, vice president for scientific affairs for Millennium Health, San Diego, Calif., told this news organization. “We’re trying to find out if we can let people in the harm reduction and treatment space know about what might be coming weeks or a month or more in advance so that some interventions could be marshaled.”
The study was published online in JAMA Network Open.
Call for better surveillance
More than 100,000 people in the United States died of an unintended drug overdose in 2021, a record high and a 15% increase over 2020 figures, which also set a record.
Part of the federal government’s plan to address the crisis includes strengthening epidemiologic efforts by better collection and mining of public health surveillance data.
Sources currently used to detect drug use trends include mortality data, poison control centers, emergency departments, electronic health records, and crime laboratories. But analysis of these sources can take weeks or more.
“One of the real challenges in addressing and reducing overdose deaths has been the relative lack of accessible real-time data that can support agile responses to deployment of resources in a specific geographic region,” study coauthor Rebecca Jackson, MD, professor and associate dean for clinical and translational research at Ohio State University in Columbus, said in an interview.
Ohio State researchers partnered with scientists at Millennium Health, one of the largest urine test labs in the United States, on a cross-sectional study to find out if UDTs could be an accurate and speedier tool for drug surveillance.
They analyzed 500,000 unique urine samples from patients in substance use disorder (SUD) treatment facilities in all 50 states from 2013 to 2020, comparing levels of cocaine, heroin, methamphetamine, synthetic opioids, and other opioids found in the samples to levels of the same drugs from overdose mortality data at the national, state, and county level from the National Vital Statistics System.
On a national level, synthetic opioids and methamphetamine were highly correlated with overdose mortality data (Spearman’s rho = .96 for both). When synthetic opioids were coinvolved, methamphetamine (rho = .98), heroin (rho = .78), cocaine (rho = .94), and other opioids (rho = .83) were also highly correlated with overdose mortality data.
Similar correlations were found when examining state-level data from 24 states and at the county level upon analysis of 19 counties in Ohio.
A changing landscape
Researchers said the strong correlation between overdose deaths and UDT results for synthetic opioids and methamphetamine are likely explained by the drugs’ availability and lethality.
“The most important thing that we found was just the strength of the correlation, which goes right to the heart of why we considered correlation to be so critical,” lead author Penn Whitley, senior director of bioinformatics for Millennium Health, told this news organization. “We needed to demonstrate that there was a strong correlation of just the UDT positivity rates with mortality – in this case, fatal drug overdose rates – as a steppingstone to build out tools that could utilize UDT as a real-time data source.”
While the main goal of the study was to establish correlation between UDT results and national mortality data, the study also offers a view of a changing landscape in the opioid epidemic.
Overall, UDT positivity for total synthetic opioids increased from 2.1% in 2013 to 19.1% in 2020 (a 792.5% increase). Positivity rates for all included drug categories increased when synthetic opioids were present.
However, in the absence of synthetic opioids, UDT positivity decreased for almost all drug categories from 2013 to 2020 (from 7.7% to 4.7% for cocaine; 3.9% to 1.6% for heroin; 20.5% to 6.9% for other opioids).
Only methamphetamine positivity increased with or without involvement of synthetic opioids. With synthetic opioids, meth positivity rose from 0.1% in 2013 to 7.9% in 2020. Without them, meth positivity rates still rose, from 2.1% in 2013 to 13.1% in 2020.
The findings track with an earlier study showing methamphetamine-involved overdose deaths rose sharply between 2011 and 2018.
“The data from this manuscript support that the opioid epidemic is transitioning from an opioid epidemic to a polysubstance epidemic where illicit synthetic opioids, largely fentanyl, in combination with other substances are now responsible for upwards of 80% of OD deaths,” Dr. Jackson said.
In an accompanying editorial Jeffrey Brent, MD, PhD, clinical professor in internal medicine at the University of Colorado at Denver, Aurora, and Stephanie T. Weiss, MD, PhD, staff clinician in the Translational Addiction Medicine Branch at the National Institute on Drug Abuse, Baltimore, note that as new agents emerge, different harm-reduction strategies will be needed, adding that having a real-time tool to identify the trends will be key to preventing deaths.
“Surveillance systems are an integral component of reducing morbidity and mortality associated with illicit drug use. On local, regional, and national levels, information of this type is needed to most efficiently allocate limited resources to maximize benefit and save lives,” Dr. Brent and Dr. Weiss write.
The study was funded by Millennium Health and the National Center for Advancing Translational Sciences. Full disclosures are included in the original articles, but no sources reported conflicts related to the study.
A version of this article first appeared on Medscape.com.
The results of a national urine drug test (UDT) study come as the United States is reporting a record-high number of drug overdose deaths – more than 80% of which involved fentanyl or other synthetic opioids and prompting a push for better surveillance models.
Researchers found that UDTs can be used to accurately identify which drugs are circulating in a community, revealing in just a matter of days critically important drug use trends that current surveillance methods take a month or longer to report.
The faster turnaround could potentially allow clinicians and public health officials to be more proactive with targeted overdose prevention and harm-reduction strategies such as distribution of naloxone and fentanyl test strips.
“We’re talking about trying to come up with an early-warning system,” study author Steven Passik, PhD, vice president for scientific affairs for Millennium Health, San Diego, Calif., told this news organization. “We’re trying to find out if we can let people in the harm reduction and treatment space know about what might be coming weeks or a month or more in advance so that some interventions could be marshaled.”
The study was published online in JAMA Network Open.
Call for better surveillance
More than 100,000 people in the United States died of an unintended drug overdose in 2021, a record high and a 15% increase over 2020 figures, which also set a record.
Part of the federal government’s plan to address the crisis includes strengthening epidemiologic efforts by better collection and mining of public health surveillance data.
Sources currently used to detect drug use trends include mortality data, poison control centers, emergency departments, electronic health records, and crime laboratories. But analysis of these sources can take weeks or more.
“One of the real challenges in addressing and reducing overdose deaths has been the relative lack of accessible real-time data that can support agile responses to deployment of resources in a specific geographic region,” study coauthor Rebecca Jackson, MD, professor and associate dean for clinical and translational research at Ohio State University in Columbus, said in an interview.
Ohio State researchers partnered with scientists at Millennium Health, one of the largest urine test labs in the United States, on a cross-sectional study to find out if UDTs could be an accurate and speedier tool for drug surveillance.
They analyzed 500,000 unique urine samples from patients in substance use disorder (SUD) treatment facilities in all 50 states from 2013 to 2020, comparing levels of cocaine, heroin, methamphetamine, synthetic opioids, and other opioids found in the samples to levels of the same drugs from overdose mortality data at the national, state, and county level from the National Vital Statistics System.
On a national level, synthetic opioids and methamphetamine were highly correlated with overdose mortality data (Spearman’s rho = .96 for both). When synthetic opioids were coinvolved, methamphetamine (rho = .98), heroin (rho = .78), cocaine (rho = .94), and other opioids (rho = .83) were also highly correlated with overdose mortality data.
Similar correlations were found when examining state-level data from 24 states and at the county level upon analysis of 19 counties in Ohio.
A changing landscape
Researchers said the strong correlation between overdose deaths and UDT results for synthetic opioids and methamphetamine are likely explained by the drugs’ availability and lethality.
“The most important thing that we found was just the strength of the correlation, which goes right to the heart of why we considered correlation to be so critical,” lead author Penn Whitley, senior director of bioinformatics for Millennium Health, told this news organization. “We needed to demonstrate that there was a strong correlation of just the UDT positivity rates with mortality – in this case, fatal drug overdose rates – as a steppingstone to build out tools that could utilize UDT as a real-time data source.”
While the main goal of the study was to establish correlation between UDT results and national mortality data, the study also offers a view of a changing landscape in the opioid epidemic.
Overall, UDT positivity for total synthetic opioids increased from 2.1% in 2013 to 19.1% in 2020 (a 792.5% increase). Positivity rates for all included drug categories increased when synthetic opioids were present.
However, in the absence of synthetic opioids, UDT positivity decreased for almost all drug categories from 2013 to 2020 (from 7.7% to 4.7% for cocaine; 3.9% to 1.6% for heroin; 20.5% to 6.9% for other opioids).
Only methamphetamine positivity increased with or without involvement of synthetic opioids. With synthetic opioids, meth positivity rose from 0.1% in 2013 to 7.9% in 2020. Without them, meth positivity rates still rose, from 2.1% in 2013 to 13.1% in 2020.
The findings track with an earlier study showing methamphetamine-involved overdose deaths rose sharply between 2011 and 2018.
“The data from this manuscript support that the opioid epidemic is transitioning from an opioid epidemic to a polysubstance epidemic where illicit synthetic opioids, largely fentanyl, in combination with other substances are now responsible for upwards of 80% of OD deaths,” Dr. Jackson said.
In an accompanying editorial Jeffrey Brent, MD, PhD, clinical professor in internal medicine at the University of Colorado at Denver, Aurora, and Stephanie T. Weiss, MD, PhD, staff clinician in the Translational Addiction Medicine Branch at the National Institute on Drug Abuse, Baltimore, note that as new agents emerge, different harm-reduction strategies will be needed, adding that having a real-time tool to identify the trends will be key to preventing deaths.
“Surveillance systems are an integral component of reducing morbidity and mortality associated with illicit drug use. On local, regional, and national levels, information of this type is needed to most efficiently allocate limited resources to maximize benefit and save lives,” Dr. Brent and Dr. Weiss write.
The study was funded by Millennium Health and the National Center for Advancing Translational Sciences. Full disclosures are included in the original articles, but no sources reported conflicts related to the study.
A version of this article first appeared on Medscape.com.
The results of a national urine drug test (UDT) study come as the United States is reporting a record-high number of drug overdose deaths – more than 80% of which involved fentanyl or other synthetic opioids and prompting a push for better surveillance models.
Researchers found that UDTs can be used to accurately identify which drugs are circulating in a community, revealing in just a matter of days critically important drug use trends that current surveillance methods take a month or longer to report.
The faster turnaround could potentially allow clinicians and public health officials to be more proactive with targeted overdose prevention and harm-reduction strategies such as distribution of naloxone and fentanyl test strips.
“We’re talking about trying to come up with an early-warning system,” study author Steven Passik, PhD, vice president for scientific affairs for Millennium Health, San Diego, Calif., told this news organization. “We’re trying to find out if we can let people in the harm reduction and treatment space know about what might be coming weeks or a month or more in advance so that some interventions could be marshaled.”
The study was published online in JAMA Network Open.
Call for better surveillance
More than 100,000 people in the United States died of an unintended drug overdose in 2021, a record high and a 15% increase over 2020 figures, which also set a record.
Part of the federal government’s plan to address the crisis includes strengthening epidemiologic efforts by better collection and mining of public health surveillance data.
Sources currently used to detect drug use trends include mortality data, poison control centers, emergency departments, electronic health records, and crime laboratories. But analysis of these sources can take weeks or more.
“One of the real challenges in addressing and reducing overdose deaths has been the relative lack of accessible real-time data that can support agile responses to deployment of resources in a specific geographic region,” study coauthor Rebecca Jackson, MD, professor and associate dean for clinical and translational research at Ohio State University in Columbus, said in an interview.
Ohio State researchers partnered with scientists at Millennium Health, one of the largest urine test labs in the United States, on a cross-sectional study to find out if UDTs could be an accurate and speedier tool for drug surveillance.
They analyzed 500,000 unique urine samples from patients in substance use disorder (SUD) treatment facilities in all 50 states from 2013 to 2020, comparing levels of cocaine, heroin, methamphetamine, synthetic opioids, and other opioids found in the samples to levels of the same drugs from overdose mortality data at the national, state, and county level from the National Vital Statistics System.
On a national level, synthetic opioids and methamphetamine were highly correlated with overdose mortality data (Spearman’s rho = .96 for both). When synthetic opioids were coinvolved, methamphetamine (rho = .98), heroin (rho = .78), cocaine (rho = .94), and other opioids (rho = .83) were also highly correlated with overdose mortality data.
Similar correlations were found when examining state-level data from 24 states and at the county level upon analysis of 19 counties in Ohio.
A changing landscape
Researchers said the strong correlation between overdose deaths and UDT results for synthetic opioids and methamphetamine are likely explained by the drugs’ availability and lethality.
“The most important thing that we found was just the strength of the correlation, which goes right to the heart of why we considered correlation to be so critical,” lead author Penn Whitley, senior director of bioinformatics for Millennium Health, told this news organization. “We needed to demonstrate that there was a strong correlation of just the UDT positivity rates with mortality – in this case, fatal drug overdose rates – as a steppingstone to build out tools that could utilize UDT as a real-time data source.”
While the main goal of the study was to establish correlation between UDT results and national mortality data, the study also offers a view of a changing landscape in the opioid epidemic.
Overall, UDT positivity for total synthetic opioids increased from 2.1% in 2013 to 19.1% in 2020 (a 792.5% increase). Positivity rates for all included drug categories increased when synthetic opioids were present.
However, in the absence of synthetic opioids, UDT positivity decreased for almost all drug categories from 2013 to 2020 (from 7.7% to 4.7% for cocaine; 3.9% to 1.6% for heroin; 20.5% to 6.9% for other opioids).
Only methamphetamine positivity increased with or without involvement of synthetic opioids. With synthetic opioids, meth positivity rose from 0.1% in 2013 to 7.9% in 2020. Without them, meth positivity rates still rose, from 2.1% in 2013 to 13.1% in 2020.
The findings track with an earlier study showing methamphetamine-involved overdose deaths rose sharply between 2011 and 2018.
“The data from this manuscript support that the opioid epidemic is transitioning from an opioid epidemic to a polysubstance epidemic where illicit synthetic opioids, largely fentanyl, in combination with other substances are now responsible for upwards of 80% of OD deaths,” Dr. Jackson said.
In an accompanying editorial Jeffrey Brent, MD, PhD, clinical professor in internal medicine at the University of Colorado at Denver, Aurora, and Stephanie T. Weiss, MD, PhD, staff clinician in the Translational Addiction Medicine Branch at the National Institute on Drug Abuse, Baltimore, note that as new agents emerge, different harm-reduction strategies will be needed, adding that having a real-time tool to identify the trends will be key to preventing deaths.
“Surveillance systems are an integral component of reducing morbidity and mortality associated with illicit drug use. On local, regional, and national levels, information of this type is needed to most efficiently allocate limited resources to maximize benefit and save lives,” Dr. Brent and Dr. Weiss write.
The study was funded by Millennium Health and the National Center for Advancing Translational Sciences. Full disclosures are included in the original articles, but no sources reported conflicts related to the study.
A version of this article first appeared on Medscape.com.
Opioid use in the elderly a dementia risk factor?
in new findings that suggest exposure to these drugs may be another modifiable risk factor for dementia.
“Clinicians and others may want to consider that opioid exposure in those aged 75-80 increases dementia risk, and to balance the potential benefits of opioid use in old age with adverse side effects,” said Stephen Z. Levine, PhD, professor, department of community mental health, University of Haifa (Israel).
The study was published online in the American Journal of Geriatric Psychiatry.
Widespread use
Evidence points to a relatively high rate of opioid prescriptions among older adults. A Morbidity and Mortality Weekly Report noted 19.2% of the U.S. adult population filled an opioid prescription in 2018, with the rate in those over 65 double that of adults aged 20-24 years (25% vs. 11.2%).
Disorders and illnesses for which opioids might be prescribed, including cancer and some pain conditions, “are far more prevalent in old age than at a younger age,” said Dr. Levine.
This high rate of opioid use underscores the need to consider the risks of opioid use in old age, said Dr. Levine. “Unfortunately, studies of the association between opioid use and dementia risk in old age are few, and their results are inconsistent.”
The study included 91,307 Israeli citizens aged 60 and over without dementia who were enrolled in the Meuhedet Healthcare Services, a nonprofit health maintenance organization (HMO) serving 14% of the country’s population. Meuhedet has maintained an up-to-date dementia registry since 2002.
The average age of the study sample was 68.29 years at the start of the study (in 2012).
In Israel, opioids are prescribed for a 30-day period. In this study, opioid exposure was defined as opioid medication fills covering 60 days (or two prescriptions) within a 120-day interval.
The primary outcome was incident dementia during follow-up from Jan. 1, 2013 to Oct. 30, 2017. The analysis controlled for a number of factors, including age, sex, smoking status, health conditions such as arthritis, depression, diabetes, osteoporosis, cognitive decline, vitamin deficiencies, cancer, cardiovascular conditions, and hospitalizations for falls.
Researchers also accounted for the competing risk of mortality.
During the study, 3.1% of subjects were exposed to opioids at a mean age of 73.94 years, and 5.8% of subjects developed dementia at an average age of 78.07 years.
Increased dementia risk
The risk of incident dementia was significantly increased in those exposed to opioids versus unexposed individuals in the 75- to 80-year age group (adjusted hazard ratio, 1.39; 95% confidence interval, 1.01-1.92; z statistic = 2.02; P < .05).
The authors noted the effect size for opioid exposure in this elderly age group is like other potentially modifiable risk factors for dementia, including body mass index and smoking.
The current study could not determine the biological explanation for the increased dementia risk among older opioid users. “Causal notions are challenging in observational studies and should be viewed with caution,” Dr. Levine noted.
However, a plausible mechanism highlighted in the literature is that opioids promote apoptosis of microglia and neurons that contribute to neurodegenerative diseases, he said.
The study included 14 sensitivity analyses, including those that looked at females, subjects older than 70, smokers, and groups with and without comorbid health conditions. The only sensitivity analysis that didn’t have similar findings to the primary analysis looked at dementia risk restricted to subjects without a vitamin deficiency.
“It’s reassuring that 13 or 14 sensitivity analyses found a significant association between opioid exposure and dementia risk,” said Dr. Levine.
Some prior studies did not show an association between opioid exposure and dementia risk. One possible reason for the discrepancy with the current findings is that the previous research didn’t account for age-specific opioid use effects, or the competing risk of mortality, said Dr. Levine.
Clinicians have a number of potential alternatives to opioids to treat various conditions including acetaminophen, non-steroidal anti-inflammatory drugs, amine reuptake inhibitors (ARIs), membrane stabilizers, muscle relaxants, topical capsaicin, botulinum toxin, cannabinoids, and steroids.
A limitation of the study was that it didn’t adjust for all possible comorbid health conditions, including vascular conditions, or for use of benzodiazepines, and surgical procedures.
In addition, since up to 50% of dementia cases are undetected, it’s possible some in the unexposed opioid group may actually have undiagnosed dementia, thereby reducing the effect sizes in the results.
Reverse causality is also a possibility as the neuropathological process associated with dementia could have started prior to opioid exposure. In addition, the results are limited to prolonged opioid exposure.
Interpret with caution
Commenting on the study, David Knopman, MD, a neurologist at Mayo Clinic in Rochester, Minn., whose research involves late-life cognitive disorders, was skeptical.
“On the face of it, the fact that an association was seen only in one narrow age range – 75+ to 80 years – ought to raise serious suspicion about the reliability and validity of the claim that opioid use is a risk factor for dementia, he said.
Although the researchers performed several sensitivity analyses, including accounting for mortality, “pharmacoepidemiological studies are terribly sensitive to residual biases” related to physician and patient choices related to medication use, added Dr. Knopman.
The claim that opioids are a dementia risk “should be viewed with great caution” and should not influence use of opioids where they’re truly indicated, he said.
“It would be a great pity if patients with pain requiring opioids avoid them because of fears about dementia based on the dubious relationship between age and opioid use.”
Dr. Levine and Dr. Knopman report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
in new findings that suggest exposure to these drugs may be another modifiable risk factor for dementia.
“Clinicians and others may want to consider that opioid exposure in those aged 75-80 increases dementia risk, and to balance the potential benefits of opioid use in old age with adverse side effects,” said Stephen Z. Levine, PhD, professor, department of community mental health, University of Haifa (Israel).
The study was published online in the American Journal of Geriatric Psychiatry.
Widespread use
Evidence points to a relatively high rate of opioid prescriptions among older adults. A Morbidity and Mortality Weekly Report noted 19.2% of the U.S. adult population filled an opioid prescription in 2018, with the rate in those over 65 double that of adults aged 20-24 years (25% vs. 11.2%).
Disorders and illnesses for which opioids might be prescribed, including cancer and some pain conditions, “are far more prevalent in old age than at a younger age,” said Dr. Levine.
This high rate of opioid use underscores the need to consider the risks of opioid use in old age, said Dr. Levine. “Unfortunately, studies of the association between opioid use and dementia risk in old age are few, and their results are inconsistent.”
The study included 91,307 Israeli citizens aged 60 and over without dementia who were enrolled in the Meuhedet Healthcare Services, a nonprofit health maintenance organization (HMO) serving 14% of the country’s population. Meuhedet has maintained an up-to-date dementia registry since 2002.
The average age of the study sample was 68.29 years at the start of the study (in 2012).
In Israel, opioids are prescribed for a 30-day period. In this study, opioid exposure was defined as opioid medication fills covering 60 days (or two prescriptions) within a 120-day interval.
The primary outcome was incident dementia during follow-up from Jan. 1, 2013 to Oct. 30, 2017. The analysis controlled for a number of factors, including age, sex, smoking status, health conditions such as arthritis, depression, diabetes, osteoporosis, cognitive decline, vitamin deficiencies, cancer, cardiovascular conditions, and hospitalizations for falls.
Researchers also accounted for the competing risk of mortality.
During the study, 3.1% of subjects were exposed to opioids at a mean age of 73.94 years, and 5.8% of subjects developed dementia at an average age of 78.07 years.
Increased dementia risk
The risk of incident dementia was significantly increased in those exposed to opioids versus unexposed individuals in the 75- to 80-year age group (adjusted hazard ratio, 1.39; 95% confidence interval, 1.01-1.92; z statistic = 2.02; P < .05).
The authors noted the effect size for opioid exposure in this elderly age group is like other potentially modifiable risk factors for dementia, including body mass index and smoking.
The current study could not determine the biological explanation for the increased dementia risk among older opioid users. “Causal notions are challenging in observational studies and should be viewed with caution,” Dr. Levine noted.
However, a plausible mechanism highlighted in the literature is that opioids promote apoptosis of microglia and neurons that contribute to neurodegenerative diseases, he said.
The study included 14 sensitivity analyses, including those that looked at females, subjects older than 70, smokers, and groups with and without comorbid health conditions. The only sensitivity analysis that didn’t have similar findings to the primary analysis looked at dementia risk restricted to subjects without a vitamin deficiency.
“It’s reassuring that 13 or 14 sensitivity analyses found a significant association between opioid exposure and dementia risk,” said Dr. Levine.
Some prior studies did not show an association between opioid exposure and dementia risk. One possible reason for the discrepancy with the current findings is that the previous research didn’t account for age-specific opioid use effects, or the competing risk of mortality, said Dr. Levine.
Clinicians have a number of potential alternatives to opioids to treat various conditions including acetaminophen, non-steroidal anti-inflammatory drugs, amine reuptake inhibitors (ARIs), membrane stabilizers, muscle relaxants, topical capsaicin, botulinum toxin, cannabinoids, and steroids.
A limitation of the study was that it didn’t adjust for all possible comorbid health conditions, including vascular conditions, or for use of benzodiazepines, and surgical procedures.
In addition, since up to 50% of dementia cases are undetected, it’s possible some in the unexposed opioid group may actually have undiagnosed dementia, thereby reducing the effect sizes in the results.
Reverse causality is also a possibility as the neuropathological process associated with dementia could have started prior to opioid exposure. In addition, the results are limited to prolonged opioid exposure.
Interpret with caution
Commenting on the study, David Knopman, MD, a neurologist at Mayo Clinic in Rochester, Minn., whose research involves late-life cognitive disorders, was skeptical.
“On the face of it, the fact that an association was seen only in one narrow age range – 75+ to 80 years – ought to raise serious suspicion about the reliability and validity of the claim that opioid use is a risk factor for dementia, he said.
Although the researchers performed several sensitivity analyses, including accounting for mortality, “pharmacoepidemiological studies are terribly sensitive to residual biases” related to physician and patient choices related to medication use, added Dr. Knopman.
The claim that opioids are a dementia risk “should be viewed with great caution” and should not influence use of opioids where they’re truly indicated, he said.
“It would be a great pity if patients with pain requiring opioids avoid them because of fears about dementia based on the dubious relationship between age and opioid use.”
Dr. Levine and Dr. Knopman report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
in new findings that suggest exposure to these drugs may be another modifiable risk factor for dementia.
“Clinicians and others may want to consider that opioid exposure in those aged 75-80 increases dementia risk, and to balance the potential benefits of opioid use in old age with adverse side effects,” said Stephen Z. Levine, PhD, professor, department of community mental health, University of Haifa (Israel).
The study was published online in the American Journal of Geriatric Psychiatry.
Widespread use
Evidence points to a relatively high rate of opioid prescriptions among older adults. A Morbidity and Mortality Weekly Report noted 19.2% of the U.S. adult population filled an opioid prescription in 2018, with the rate in those over 65 double that of adults aged 20-24 years (25% vs. 11.2%).
Disorders and illnesses for which opioids might be prescribed, including cancer and some pain conditions, “are far more prevalent in old age than at a younger age,” said Dr. Levine.
This high rate of opioid use underscores the need to consider the risks of opioid use in old age, said Dr. Levine. “Unfortunately, studies of the association between opioid use and dementia risk in old age are few, and their results are inconsistent.”
The study included 91,307 Israeli citizens aged 60 and over without dementia who were enrolled in the Meuhedet Healthcare Services, a nonprofit health maintenance organization (HMO) serving 14% of the country’s population. Meuhedet has maintained an up-to-date dementia registry since 2002.
The average age of the study sample was 68.29 years at the start of the study (in 2012).
In Israel, opioids are prescribed for a 30-day period. In this study, opioid exposure was defined as opioid medication fills covering 60 days (or two prescriptions) within a 120-day interval.
The primary outcome was incident dementia during follow-up from Jan. 1, 2013 to Oct. 30, 2017. The analysis controlled for a number of factors, including age, sex, smoking status, health conditions such as arthritis, depression, diabetes, osteoporosis, cognitive decline, vitamin deficiencies, cancer, cardiovascular conditions, and hospitalizations for falls.
Researchers also accounted for the competing risk of mortality.
During the study, 3.1% of subjects were exposed to opioids at a mean age of 73.94 years, and 5.8% of subjects developed dementia at an average age of 78.07 years.
Increased dementia risk
The risk of incident dementia was significantly increased in those exposed to opioids versus unexposed individuals in the 75- to 80-year age group (adjusted hazard ratio, 1.39; 95% confidence interval, 1.01-1.92; z statistic = 2.02; P < .05).
The authors noted the effect size for opioid exposure in this elderly age group is like other potentially modifiable risk factors for dementia, including body mass index and smoking.
The current study could not determine the biological explanation for the increased dementia risk among older opioid users. “Causal notions are challenging in observational studies and should be viewed with caution,” Dr. Levine noted.
However, a plausible mechanism highlighted in the literature is that opioids promote apoptosis of microglia and neurons that contribute to neurodegenerative diseases, he said.
The study included 14 sensitivity analyses, including those that looked at females, subjects older than 70, smokers, and groups with and without comorbid health conditions. The only sensitivity analysis that didn’t have similar findings to the primary analysis looked at dementia risk restricted to subjects without a vitamin deficiency.
“It’s reassuring that 13 or 14 sensitivity analyses found a significant association between opioid exposure and dementia risk,” said Dr. Levine.
Some prior studies did not show an association between opioid exposure and dementia risk. One possible reason for the discrepancy with the current findings is that the previous research didn’t account for age-specific opioid use effects, or the competing risk of mortality, said Dr. Levine.
Clinicians have a number of potential alternatives to opioids to treat various conditions including acetaminophen, non-steroidal anti-inflammatory drugs, amine reuptake inhibitors (ARIs), membrane stabilizers, muscle relaxants, topical capsaicin, botulinum toxin, cannabinoids, and steroids.
A limitation of the study was that it didn’t adjust for all possible comorbid health conditions, including vascular conditions, or for use of benzodiazepines, and surgical procedures.
In addition, since up to 50% of dementia cases are undetected, it’s possible some in the unexposed opioid group may actually have undiagnosed dementia, thereby reducing the effect sizes in the results.
Reverse causality is also a possibility as the neuropathological process associated with dementia could have started prior to opioid exposure. In addition, the results are limited to prolonged opioid exposure.
Interpret with caution
Commenting on the study, David Knopman, MD, a neurologist at Mayo Clinic in Rochester, Minn., whose research involves late-life cognitive disorders, was skeptical.
“On the face of it, the fact that an association was seen only in one narrow age range – 75+ to 80 years – ought to raise serious suspicion about the reliability and validity of the claim that opioid use is a risk factor for dementia, he said.
Although the researchers performed several sensitivity analyses, including accounting for mortality, “pharmacoepidemiological studies are terribly sensitive to residual biases” related to physician and patient choices related to medication use, added Dr. Knopman.
The claim that opioids are a dementia risk “should be viewed with great caution” and should not influence use of opioids where they’re truly indicated, he said.
“It would be a great pity if patients with pain requiring opioids avoid them because of fears about dementia based on the dubious relationship between age and opioid use.”
Dr. Levine and Dr. Knopman report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM AMERICAN JOURNAL OF GERIATRIC PSYCHIATRY
Substance use the main cause of physician license actions
Despite a sharp uptick in 2011, substance use–specific license actions taken against physicians dropped in frequency between 2004 and 2020.
More than three fourths (76.3%) of license actions taken against physicians were related to substance use, according to a recent study published in JAMA. Psychological impairment was the reason associated with more than 1 in 10 (11.5%) actions taken against physicians’ licenses, while physical impairment was the reason behind approximately 12% of such actions, per the study.
Researchers analyzed 5032 actions taken against the licenses of U.S. physicians. The actions were reported to the National Practitioner Data Bank and were related to substance use, psychological impairment, and physical impairment. The National Practitioner Data Bank is a web-based repository of reports with information on medical malpractice payments and certain adverse actions related to healthcare practitioners, providers, and suppliers. It is provided by the Department of Health & Human Services.
“While there has been increased attention [on] the mental health of physicians, we wanted to understand the extent to which changes in attitudes and practices were reflected in actions taken by hospitals or licensing boards, which are reported in the National Practitioner Data Bank,” Lisa Rotenstein, MD, a primary care physician at Boston’s Brigham and Women’s Hospital and lead author of the study, told this news organization.
Dr. Rotenstein, who is an assistant professor at Harvard Medical School, Boston, studies issues of mental health among physicians and trainees. Dr. Rotenstein was the lead author of a 2016 study that found that more than a quarter (27.2%) of medical students have depressive symptoms. She was also lead author of a 2018 study published in JAMA on the prevalence of burnout among attending physicians.
Actions against physicians trending downward
2011 marked the peak in actions taken against physicians’ licenses for substance use, per the study, but actions related to substance use have otherwise maintained a steady decline over the past 17 years. Researchers found that physicians with license actions as a result of substance use or psychological impairment were more likely to receive indefinite penalties, while also having emergency action taken against their license to practice.
In addition, physicians who had actions taken against their licenses because of substance use or psychological impairment were more likely to accrue a greater number of actions over the course of their careers, according to the study.
About 47% of physicians reported experiencing burnout per Medscape’s Physician Burnout and Depression Report 2022: Stress, Anxiety, and Anger report. Burnout among emergency physicians spiked from 43% in 2020 to 60% in 2021, according to the report.
More than one quarter (26%) of physicians reported drinking alcohol to cope with burnout in 2020, according to Medscape’s 2021 Physician Burnout and Suicide Report. Per the 2021 report, 48% of physicians chose exercise to deal with burnout, while 35% indulged in eating junk food.
Peter Grinspoon, MD, a Boston-based primary care physician, wrote in The Los Angeles Times in 2016 that the rate of substance abuse among physicians starts at 10% and can go as high as 15%; by comparison, rates of substance use among the general population are 8%-10%. “What appears to account for the difference is physician distress, and in the case of drug abuse, plentiful access,” he added.
Dr. Grinspoon wrote a 2016 book called “Free Refills: A Doctor Confronts His Addiction,” which chronicles his experience in recovery and relapse as a physician who was dependent on opioid painkillers.
The findings from the recent study in JAMA “suggest we have made some progress in addressing issues related to substance use in ways that don’t result in license actions or even in meeting physicians’ need for support related to substance use,” said Dr. Rotenstein.
Still, she insists that there’s “substantial opportunity to improve mental health and support offerings for physicians and to reduce stigma related to seeking and receiving mental health support, ideally averting the need for license actions.”
According to Dr. Rotenstein, the cases listed in the National Practitioner Data Bank represent the most severe cases; these reports have risen to a high level of attention or concern and are the result of adverse action reports submitted by healthcare institutions and state licensing boards.
“There are many, many more physicians whose cases are not represented here but who struggle with depression, anxiety, substance use, and more,” said Dr. Rotenstein.
A version of this article first appeared on Medscape.com.
Despite a sharp uptick in 2011, substance use–specific license actions taken against physicians dropped in frequency between 2004 and 2020.
More than three fourths (76.3%) of license actions taken against physicians were related to substance use, according to a recent study published in JAMA. Psychological impairment was the reason associated with more than 1 in 10 (11.5%) actions taken against physicians’ licenses, while physical impairment was the reason behind approximately 12% of such actions, per the study.
Researchers analyzed 5032 actions taken against the licenses of U.S. physicians. The actions were reported to the National Practitioner Data Bank and were related to substance use, psychological impairment, and physical impairment. The National Practitioner Data Bank is a web-based repository of reports with information on medical malpractice payments and certain adverse actions related to healthcare practitioners, providers, and suppliers. It is provided by the Department of Health & Human Services.
“While there has been increased attention [on] the mental health of physicians, we wanted to understand the extent to which changes in attitudes and practices were reflected in actions taken by hospitals or licensing boards, which are reported in the National Practitioner Data Bank,” Lisa Rotenstein, MD, a primary care physician at Boston’s Brigham and Women’s Hospital and lead author of the study, told this news organization.
Dr. Rotenstein, who is an assistant professor at Harvard Medical School, Boston, studies issues of mental health among physicians and trainees. Dr. Rotenstein was the lead author of a 2016 study that found that more than a quarter (27.2%) of medical students have depressive symptoms. She was also lead author of a 2018 study published in JAMA on the prevalence of burnout among attending physicians.
Actions against physicians trending downward
2011 marked the peak in actions taken against physicians’ licenses for substance use, per the study, but actions related to substance use have otherwise maintained a steady decline over the past 17 years. Researchers found that physicians with license actions as a result of substance use or psychological impairment were more likely to receive indefinite penalties, while also having emergency action taken against their license to practice.
In addition, physicians who had actions taken against their licenses because of substance use or psychological impairment were more likely to accrue a greater number of actions over the course of their careers, according to the study.
About 47% of physicians reported experiencing burnout per Medscape’s Physician Burnout and Depression Report 2022: Stress, Anxiety, and Anger report. Burnout among emergency physicians spiked from 43% in 2020 to 60% in 2021, according to the report.
More than one quarter (26%) of physicians reported drinking alcohol to cope with burnout in 2020, according to Medscape’s 2021 Physician Burnout and Suicide Report. Per the 2021 report, 48% of physicians chose exercise to deal with burnout, while 35% indulged in eating junk food.
Peter Grinspoon, MD, a Boston-based primary care physician, wrote in The Los Angeles Times in 2016 that the rate of substance abuse among physicians starts at 10% and can go as high as 15%; by comparison, rates of substance use among the general population are 8%-10%. “What appears to account for the difference is physician distress, and in the case of drug abuse, plentiful access,” he added.
Dr. Grinspoon wrote a 2016 book called “Free Refills: A Doctor Confronts His Addiction,” which chronicles his experience in recovery and relapse as a physician who was dependent on opioid painkillers.
The findings from the recent study in JAMA “suggest we have made some progress in addressing issues related to substance use in ways that don’t result in license actions or even in meeting physicians’ need for support related to substance use,” said Dr. Rotenstein.
Still, she insists that there’s “substantial opportunity to improve mental health and support offerings for physicians and to reduce stigma related to seeking and receiving mental health support, ideally averting the need for license actions.”
According to Dr. Rotenstein, the cases listed in the National Practitioner Data Bank represent the most severe cases; these reports have risen to a high level of attention or concern and are the result of adverse action reports submitted by healthcare institutions and state licensing boards.
“There are many, many more physicians whose cases are not represented here but who struggle with depression, anxiety, substance use, and more,” said Dr. Rotenstein.
A version of this article first appeared on Medscape.com.
Despite a sharp uptick in 2011, substance use–specific license actions taken against physicians dropped in frequency between 2004 and 2020.
More than three fourths (76.3%) of license actions taken against physicians were related to substance use, according to a recent study published in JAMA. Psychological impairment was the reason associated with more than 1 in 10 (11.5%) actions taken against physicians’ licenses, while physical impairment was the reason behind approximately 12% of such actions, per the study.
Researchers analyzed 5032 actions taken against the licenses of U.S. physicians. The actions were reported to the National Practitioner Data Bank and were related to substance use, psychological impairment, and physical impairment. The National Practitioner Data Bank is a web-based repository of reports with information on medical malpractice payments and certain adverse actions related to healthcare practitioners, providers, and suppliers. It is provided by the Department of Health & Human Services.
“While there has been increased attention [on] the mental health of physicians, we wanted to understand the extent to which changes in attitudes and practices were reflected in actions taken by hospitals or licensing boards, which are reported in the National Practitioner Data Bank,” Lisa Rotenstein, MD, a primary care physician at Boston’s Brigham and Women’s Hospital and lead author of the study, told this news organization.
Dr. Rotenstein, who is an assistant professor at Harvard Medical School, Boston, studies issues of mental health among physicians and trainees. Dr. Rotenstein was the lead author of a 2016 study that found that more than a quarter (27.2%) of medical students have depressive symptoms. She was also lead author of a 2018 study published in JAMA on the prevalence of burnout among attending physicians.
Actions against physicians trending downward
2011 marked the peak in actions taken against physicians’ licenses for substance use, per the study, but actions related to substance use have otherwise maintained a steady decline over the past 17 years. Researchers found that physicians with license actions as a result of substance use or psychological impairment were more likely to receive indefinite penalties, while also having emergency action taken against their license to practice.
In addition, physicians who had actions taken against their licenses because of substance use or psychological impairment were more likely to accrue a greater number of actions over the course of their careers, according to the study.
About 47% of physicians reported experiencing burnout per Medscape’s Physician Burnout and Depression Report 2022: Stress, Anxiety, and Anger report. Burnout among emergency physicians spiked from 43% in 2020 to 60% in 2021, according to the report.
More than one quarter (26%) of physicians reported drinking alcohol to cope with burnout in 2020, according to Medscape’s 2021 Physician Burnout and Suicide Report. Per the 2021 report, 48% of physicians chose exercise to deal with burnout, while 35% indulged in eating junk food.
Peter Grinspoon, MD, a Boston-based primary care physician, wrote in The Los Angeles Times in 2016 that the rate of substance abuse among physicians starts at 10% and can go as high as 15%; by comparison, rates of substance use among the general population are 8%-10%. “What appears to account for the difference is physician distress, and in the case of drug abuse, plentiful access,” he added.
Dr. Grinspoon wrote a 2016 book called “Free Refills: A Doctor Confronts His Addiction,” which chronicles his experience in recovery and relapse as a physician who was dependent on opioid painkillers.
The findings from the recent study in JAMA “suggest we have made some progress in addressing issues related to substance use in ways that don’t result in license actions or even in meeting physicians’ need for support related to substance use,” said Dr. Rotenstein.
Still, she insists that there’s “substantial opportunity to improve mental health and support offerings for physicians and to reduce stigma related to seeking and receiving mental health support, ideally averting the need for license actions.”
According to Dr. Rotenstein, the cases listed in the National Practitioner Data Bank represent the most severe cases; these reports have risen to a high level of attention or concern and are the result of adverse action reports submitted by healthcare institutions and state licensing boards.
“There are many, many more physicians whose cases are not represented here but who struggle with depression, anxiety, substance use, and more,” said Dr. Rotenstein.
A version of this article first appeared on Medscape.com.
FROM JAMA