User login
Sublingual buprenorphine plus buprenorphine XR for opioid use disorder
Mr. L, age 31, presents to the emergency department (ED) with somnolence after sustaining an arm laceration at work. While in the ED, Mr. L explains he has opioid use disorder (OUD) and last week received an initial 300 mg injection of extended-release buprenorphine (BUP-XR). Due to ongoing opioid cravings, he took nonprescribed fentanyl and alprazolam before work.
The ED clinicians address Mr. L’s arm injury and transfer him to the hospital’s low-threshold outpatient addiction clinic for further assessment and management. There, he is prescribed sublingual buprenorphine/naloxone (SL-BUP) 8 mg/2 mg daily as needed for 1 week to address ongoing opioid cravings, and is encouraged to return for another visit the following week.
The United States continues to struggle with the overdose crisis, largely fueled by illicitly manufactured opioids such as fentanyl.1 Opioid agonist and partial agonist treatments such as methadone and buprenorphine decrease the risk of death in individuals with OUD by up to 50%.2 While methadone has a history of proven effectiveness for OUD, accessibility is fraught with barriers (eg, patients must attend an opioid treatment program daily to receive a dose, pharmacies are unable to dispense methadone for OUD).
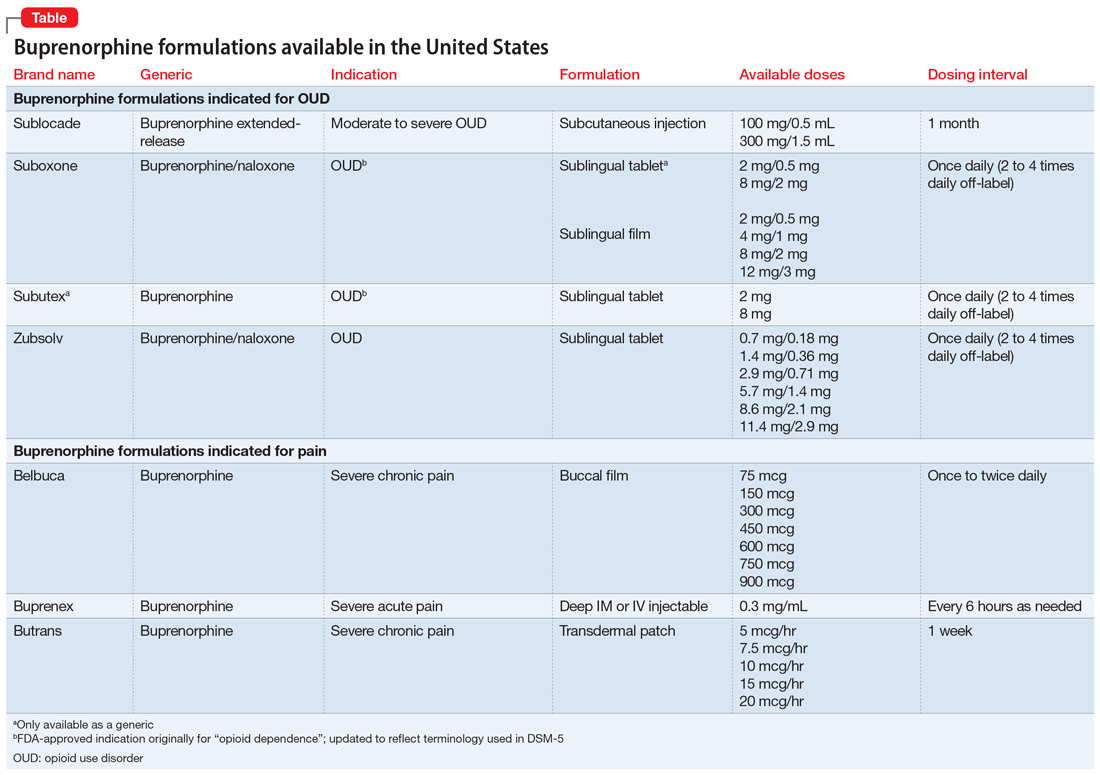
Buprenorphine has been shown to decrease opioid cravings while limiting euphoria due to its partial—as opposed to full—agonist activity.3 Several buprenorphine formulations are available (Table). Buprenorphine presents an opportunity to treat OUD like other chronic illnesses. In accordance with the US Department of Health and Human Services Practice Guideline (2021), any clinician can obtain a waiver to prescribe buprenorphine in any treatment setting, and patients can receive the medication at a pharmacy.4
However, many patients have barriers to consistent daily dosing of buprenorphine due to strict clinic/prescriber requirements, transportation difficulties, continued cravings, and other factors. BUP-XR, a buprenorphine injection administered once a month, may address several of these concerns, most notably the potential for better suppression of cravings by delivering a consistent level of buprenorphine over the course of 28 days.5 Since BUP-XR was FDA-approved in 2017, questions remain whether it can adequately quell opioid cravings in early treatment months prior to steady-state concentration.
This article addresses whether clinicians should consider supplemental SL-BUP in addition to BUP-XR during early treatment months and/or prior to steady-state.
Pharmacokinetics of BUP-XR
BUP-XR is administered by subcutaneous injection via an ATRIGEL delivery system (BUP-XR; Albany Molecular Research, Burlington, Massachusetts).6 Upon injection, approximately 7% of the buprenorphine dose dissipates with the solvent, leading to maximum concentration approximately 24 hours post-dose. The remaining dose hardens to create a depot that elutes buprenorphine gradually over 28 days.7
Continue to: Buprenorphine requires...
Buprenorphine requires ≥70% mu-opioid receptor (MOR) occupancy to effectively suppress symptoms of craving and withdrawal in patients with OUD. Buprenorphine serum concentration correlates significantly with MOR occupancy, such that concentrations of 2 to 3 ng/mL are acknowledged as baseline minimums for clinical efficacy.8
BUP-XR is administered in 1 of 2 dosing regimens. In both, 2 separate 300 mg doses are administered 28 days apart during Month 1 and Month 2, followed by maintenance doses of either 300 mg (300/300 mg dosing regimen) or 100 mg (300/100 mg dosing regimen) every 28 days thereafter. Combined Phase II and Phase III data analyzing serum concentrations of BUP-XR across both dosing regimens revealed that, for most patients, there is a noticeable period during Month 1 and Month 2 when serum concentrations fall below 2 ng/mL.7 Steady-state concentrations of both regimens develop after 4 to 6 appropriately timed injections, providing average steady-state serum concentrations in Phase II and Phase III trials of 6.54 ng/mL for the 300/300 mg dosing regimen and 3.00 ng/mL for 300/100 mg dosing regimen.7
Real-world experiences with BUP-XR
The theoretical need for supplementation has been voiced in practice. A case series by Peckham et al9 noted that 55% (n = 22) of patients required SL-BUP supplementation for up to 120 days after the first BUP-XR injection to quell cravings and reduce nonprescribed opioid use.
The RECOVER trial by Ling et al10 demonstrated the importance of the first 2 months of BUP-XR therapy in the overall treatment success for patients with OUD. In this analysis, patients maintained on BUP-XR for 12 months reported a 75% likelihood of abstinence, compared to 24% for patients receiving 0 to 2 months of BUP-XR treatment. Other benefits included improved employment status and reduced depression rates. This trial did not specifically discuss supplemental SL-BUP or subthreshold concentrations of buprenorphine during early months.10
Individualized treatment should be based on OUD symptoms
While BUP-XR was designed to continuously deliver at least 2 ng/mL of buprenorphine, serum concentrations are labile during the first 2 months of treatment. This may result in breakthrough OUD symptoms, particularly withdrawal or opioid cravings. Additionally, due to individual variability, some patients may still experience serum concentrations below 2 ng/mL after Month 2 and until steady-state is achieved between Month 4 and Month 6.7
Continue to: Beyond a theoretical...
Beyond a theoretical need for supplementation with SL-BUP, there is limited information regarding optimal dosing, dosage intervals, or length of supplementation. Therefore, clear guidance is not available at this time, and treatment should be individualized based on subjective and objective OUD symptoms.
What also remains unknown are potential barriers patients may face in receiving 2 concurrent buprenorphine prescriptions. BUP-XR, administered in a health care setting, can be obtained 2 ways. A clinician can directly order the medication from the distributor to be administered via buy-and-bill. An alternate option requires the clinician to send a prescription to an appropriately credentialed pharmacy that will ship patient-specific orders directly to the clinic. Despite this, most SL-BUP prescriptions are billed and dispensed from community pharmacies. At the insurance level, there is risk the prescription claim will be rejected for duplication of therapy, which may require additional collaboration between the prescribing clinician, pharmacist, and insurance representative to ensure patients have access to the medication.
Pending studies and approvals may also provide greater guidance and flexibility in decision-making for patients with OUD. The CoLAB study currently underway in Australia is examining the efficacy and outcomes of an intermediate dose (200 mg) of BUP-XR and will also allow for supplemental SL-BUP doses.11 Additionally, an alternative BUP-XR formulation, Brixadi, currently in use in the European Union as Buvidal, has submitted an application for FDA approval in the United States. The application indicates that Brixadi will be available with a wider range of doses and at both weekly and monthly intervals. Approval has been delayed due to deficiencies in the United States–based third-party production facilities. It is unclear how the FDA and manufacturer plan to proceed.12
Short-term supplementation with SL-BUP during early the months of treatment with BUP-XR should be considered to control OUD symptoms and assist with patient retention. Once steady-state is achieved, trough concentrations of buprenorphine are not expected to drop below 2 ng/mL with continued on-time maintenance doses and thus, supplementation can likely cease.
CASE CONTINUED
Mr. L is seen in the low-threshold outpatient clinic 1 week after his ED visit. His arm laceration is healing well, and he is noticeably more alert and engaged. Each morning this week, he awakes with cravings, sweating, and anxiety. These symptoms alleviate after he takes SL-BUP. Mr. L’s clinician gives him a copy of the Subjective Opioid Withdrawal Scale so he can assess his withdrawal symptoms each morning and provide this data at follow-up appointments. Mr. L and his clinician decide to meet weekly until his next injection to continue assessing his current supplemental dose, symptoms, and whether there should be additional adjustments to his treatment plan.
Related Resources
- Cho J, Bhimani J, Patel M, et al. Substance abuse among older adults: a growing problem. Current Psychiatry. 2018;17(3):14-20.
- Verma S. Opioid use disorder in adolescents: an overview. Current Psychiatry. 2020;19(2):12-14,16-21.
Drug Brand Names
Alprazolam • Xanax
Buprenorphine • Sublocade, Subutex
Buprenorphine/naloxone • Suboxone, Zubsolv
Methadone • Methadose
1. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths - United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202-207. doi:10.15585/mmwr.mm7006a4
2. Ma J, Bao YP, Wang RJ, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(12):1868-1883. doi:10.1038/s41380-018-0094-5
3. Coe MA, Lofwall MR, Walsh SL. Buprenorphine pharmacology review: update on transmucosal and long-acting formulations. J Addict Med. 2019;13(2):93-103. doi:10.1097/ADM.0000000000000457
4. Becerra X. Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. US Dept of Health and Human Services; 2021:22439-22440. FR Document 2021-08961. Accessed April 5, 2021. https://www.federalregister.gov/documents/2021/04/28/2021-08961/practice-guidelines-for-the-administration-of-buprenorphine-for-treating-opioid-use-disorder
5. Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2019;393(10173):778-790. doi:10.1016/S0140-6736(18)32259-1
6. Sublocade [package insert]. North Chesterfield, VA: Indivior Inc; 2021.
7. Jones AK, Ngaimisi E, Gopalakrishnan M, et al. Population pharmacokinetics of a monthly buprenorphine depot injection for the treatment of opioid use disorder: a combined analysis of phase II and phase III trials. Clin Pharmacokinet. 2021;60(4):527-540. doi:10.1007/s40262-020-00957-0
8. Greenwald MK, Comer SD, Fiellin DA. Buprenorphine maintenance and mu-opioid receptor availability in the treatment of opioid use disorder: implications for clinical use and policy. Drug Alcohol Depend. 2014;144:1-11. doi:10.1016/j.drugalcdep.2014.07.035
9. Peckham AM, Kehoe LG, Gray JR, et al. Real-world outcomes with extended-release buprenorphine (XR-BUP) in a low threshold bridge clinic: a retrospective case series. J Subst Abuse Treat. 2021;126:108316. doi:10.1016/j.jsat.2021.108316
10. Ling W, Nadipelli VR, Aldridge AP, et al. Recovery from opioid use disorder (OUD) after monthly long-acting buprenorphine treatment: 12-month longitudinal outcomes from RECOVER, an observational study. J Addict Med. 2020;14(5):e233-e240. doi:10.1097/ADM.0000000000000647
11. Larance B, Byrne M, Lintzeris N, et al. Open-label, multicentre, single-arm trial of monthly injections of depot buprenorphine in people with opioid dependence: protocol for the CoLAB study. BMJ Open. 2020;10(7):e034389. doi:10.1136/bmjopen-2019-034389
12. Braeburn receives new Complete Response Letter for Brixadi in the US. News release. News Powered by Cision. December 15, 2021. Accessed April 13, 2022. https://news.cision.com/camurus-ab/r/braeburn-receives-new-complete-response-letter-for-brixadi-in-the-us,c3473281
Mr. L, age 31, presents to the emergency department (ED) with somnolence after sustaining an arm laceration at work. While in the ED, Mr. L explains he has opioid use disorder (OUD) and last week received an initial 300 mg injection of extended-release buprenorphine (BUP-XR). Due to ongoing opioid cravings, he took nonprescribed fentanyl and alprazolam before work.
The ED clinicians address Mr. L’s arm injury and transfer him to the hospital’s low-threshold outpatient addiction clinic for further assessment and management. There, he is prescribed sublingual buprenorphine/naloxone (SL-BUP) 8 mg/2 mg daily as needed for 1 week to address ongoing opioid cravings, and is encouraged to return for another visit the following week.
The United States continues to struggle with the overdose crisis, largely fueled by illicitly manufactured opioids such as fentanyl.1 Opioid agonist and partial agonist treatments such as methadone and buprenorphine decrease the risk of death in individuals with OUD by up to 50%.2 While methadone has a history of proven effectiveness for OUD, accessibility is fraught with barriers (eg, patients must attend an opioid treatment program daily to receive a dose, pharmacies are unable to dispense methadone for OUD).
Buprenorphine has been shown to decrease opioid cravings while limiting euphoria due to its partial—as opposed to full—agonist activity.3 Several buprenorphine formulations are available (Table). Buprenorphine presents an opportunity to treat OUD like other chronic illnesses. In accordance with the US Department of Health and Human Services Practice Guideline (2021), any clinician can obtain a waiver to prescribe buprenorphine in any treatment setting, and patients can receive the medication at a pharmacy.4

However, many patients have barriers to consistent daily dosing of buprenorphine due to strict clinic/prescriber requirements, transportation difficulties, continued cravings, and other factors. BUP-XR, a buprenorphine injection administered once a month, may address several of these concerns, most notably the potential for better suppression of cravings by delivering a consistent level of buprenorphine over the course of 28 days.5 Since BUP-XR was FDA-approved in 2017, questions remain whether it can adequately quell opioid cravings in early treatment months prior to steady-state concentration.
This article addresses whether clinicians should consider supplemental SL-BUP in addition to BUP-XR during early treatment months and/or prior to steady-state.
Pharmacokinetics of BUP-XR
BUP-XR is administered by subcutaneous injection via an ATRIGEL delivery system (BUP-XR; Albany Molecular Research, Burlington, Massachusetts).6 Upon injection, approximately 7% of the buprenorphine dose dissipates with the solvent, leading to maximum concentration approximately 24 hours post-dose. The remaining dose hardens to create a depot that elutes buprenorphine gradually over 28 days.7
Continue to: Buprenorphine requires...
Buprenorphine requires ≥70% mu-opioid receptor (MOR) occupancy to effectively suppress symptoms of craving and withdrawal in patients with OUD. Buprenorphine serum concentration correlates significantly with MOR occupancy, such that concentrations of 2 to 3 ng/mL are acknowledged as baseline minimums for clinical efficacy.8
BUP-XR is administered in 1 of 2 dosing regimens. In both, 2 separate 300 mg doses are administered 28 days apart during Month 1 and Month 2, followed by maintenance doses of either 300 mg (300/300 mg dosing regimen) or 100 mg (300/100 mg dosing regimen) every 28 days thereafter. Combined Phase II and Phase III data analyzing serum concentrations of BUP-XR across both dosing regimens revealed that, for most patients, there is a noticeable period during Month 1 and Month 2 when serum concentrations fall below 2 ng/mL.7 Steady-state concentrations of both regimens develop after 4 to 6 appropriately timed injections, providing average steady-state serum concentrations in Phase II and Phase III trials of 6.54 ng/mL for the 300/300 mg dosing regimen and 3.00 ng/mL for 300/100 mg dosing regimen.7
Real-world experiences with BUP-XR
The theoretical need for supplementation has been voiced in practice. A case series by Peckham et al9 noted that 55% (n = 22) of patients required SL-BUP supplementation for up to 120 days after the first BUP-XR injection to quell cravings and reduce nonprescribed opioid use.
The RECOVER trial by Ling et al10 demonstrated the importance of the first 2 months of BUP-XR therapy in the overall treatment success for patients with OUD. In this analysis, patients maintained on BUP-XR for 12 months reported a 75% likelihood of abstinence, compared to 24% for patients receiving 0 to 2 months of BUP-XR treatment. Other benefits included improved employment status and reduced depression rates. This trial did not specifically discuss supplemental SL-BUP or subthreshold concentrations of buprenorphine during early months.10
Individualized treatment should be based on OUD symptoms
While BUP-XR was designed to continuously deliver at least 2 ng/mL of buprenorphine, serum concentrations are labile during the first 2 months of treatment. This may result in breakthrough OUD symptoms, particularly withdrawal or opioid cravings. Additionally, due to individual variability, some patients may still experience serum concentrations below 2 ng/mL after Month 2 and until steady-state is achieved between Month 4 and Month 6.7
Continue to: Beyond a theoretical...
Beyond a theoretical need for supplementation with SL-BUP, there is limited information regarding optimal dosing, dosage intervals, or length of supplementation. Therefore, clear guidance is not available at this time, and treatment should be individualized based on subjective and objective OUD symptoms.
What also remains unknown are potential barriers patients may face in receiving 2 concurrent buprenorphine prescriptions. BUP-XR, administered in a health care setting, can be obtained 2 ways. A clinician can directly order the medication from the distributor to be administered via buy-and-bill. An alternate option requires the clinician to send a prescription to an appropriately credentialed pharmacy that will ship patient-specific orders directly to the clinic. Despite this, most SL-BUP prescriptions are billed and dispensed from community pharmacies. At the insurance level, there is risk the prescription claim will be rejected for duplication of therapy, which may require additional collaboration between the prescribing clinician, pharmacist, and insurance representative to ensure patients have access to the medication.
Pending studies and approvals may also provide greater guidance and flexibility in decision-making for patients with OUD. The CoLAB study currently underway in Australia is examining the efficacy and outcomes of an intermediate dose (200 mg) of BUP-XR and will also allow for supplemental SL-BUP doses.11 Additionally, an alternative BUP-XR formulation, Brixadi, currently in use in the European Union as Buvidal, has submitted an application for FDA approval in the United States. The application indicates that Brixadi will be available with a wider range of doses and at both weekly and monthly intervals. Approval has been delayed due to deficiencies in the United States–based third-party production facilities. It is unclear how the FDA and manufacturer plan to proceed.12
Short-term supplementation with SL-BUP during early the months of treatment with BUP-XR should be considered to control OUD symptoms and assist with patient retention. Once steady-state is achieved, trough concentrations of buprenorphine are not expected to drop below 2 ng/mL with continued on-time maintenance doses and thus, supplementation can likely cease.
CASE CONTINUED
Mr. L is seen in the low-threshold outpatient clinic 1 week after his ED visit. His arm laceration is healing well, and he is noticeably more alert and engaged. Each morning this week, he awakes with cravings, sweating, and anxiety. These symptoms alleviate after he takes SL-BUP. Mr. L’s clinician gives him a copy of the Subjective Opioid Withdrawal Scale so he can assess his withdrawal symptoms each morning and provide this data at follow-up appointments. Mr. L and his clinician decide to meet weekly until his next injection to continue assessing his current supplemental dose, symptoms, and whether there should be additional adjustments to his treatment plan.
Related Resources
- Cho J, Bhimani J, Patel M, et al. Substance abuse among older adults: a growing problem. Current Psychiatry. 2018;17(3):14-20.
- Verma S. Opioid use disorder in adolescents: an overview. Current Psychiatry. 2020;19(2):12-14,16-21.
Drug Brand Names
Alprazolam • Xanax
Buprenorphine • Sublocade, Subutex
Buprenorphine/naloxone • Suboxone, Zubsolv
Methadone • Methadose
Mr. L, age 31, presents to the emergency department (ED) with somnolence after sustaining an arm laceration at work. While in the ED, Mr. L explains he has opioid use disorder (OUD) and last week received an initial 300 mg injection of extended-release buprenorphine (BUP-XR). Due to ongoing opioid cravings, he took nonprescribed fentanyl and alprazolam before work.
The ED clinicians address Mr. L’s arm injury and transfer him to the hospital’s low-threshold outpatient addiction clinic for further assessment and management. There, he is prescribed sublingual buprenorphine/naloxone (SL-BUP) 8 mg/2 mg daily as needed for 1 week to address ongoing opioid cravings, and is encouraged to return for another visit the following week.
The United States continues to struggle with the overdose crisis, largely fueled by illicitly manufactured opioids such as fentanyl.1 Opioid agonist and partial agonist treatments such as methadone and buprenorphine decrease the risk of death in individuals with OUD by up to 50%.2 While methadone has a history of proven effectiveness for OUD, accessibility is fraught with barriers (eg, patients must attend an opioid treatment program daily to receive a dose, pharmacies are unable to dispense methadone for OUD).
Buprenorphine has been shown to decrease opioid cravings while limiting euphoria due to its partial—as opposed to full—agonist activity.3 Several buprenorphine formulations are available (Table). Buprenorphine presents an opportunity to treat OUD like other chronic illnesses. In accordance with the US Department of Health and Human Services Practice Guideline (2021), any clinician can obtain a waiver to prescribe buprenorphine in any treatment setting, and patients can receive the medication at a pharmacy.4

However, many patients have barriers to consistent daily dosing of buprenorphine due to strict clinic/prescriber requirements, transportation difficulties, continued cravings, and other factors. BUP-XR, a buprenorphine injection administered once a month, may address several of these concerns, most notably the potential for better suppression of cravings by delivering a consistent level of buprenorphine over the course of 28 days.5 Since BUP-XR was FDA-approved in 2017, questions remain whether it can adequately quell opioid cravings in early treatment months prior to steady-state concentration.
This article addresses whether clinicians should consider supplemental SL-BUP in addition to BUP-XR during early treatment months and/or prior to steady-state.
Pharmacokinetics of BUP-XR
BUP-XR is administered by subcutaneous injection via an ATRIGEL delivery system (BUP-XR; Albany Molecular Research, Burlington, Massachusetts).6 Upon injection, approximately 7% of the buprenorphine dose dissipates with the solvent, leading to maximum concentration approximately 24 hours post-dose. The remaining dose hardens to create a depot that elutes buprenorphine gradually over 28 days.7
Continue to: Buprenorphine requires...
Buprenorphine requires ≥70% mu-opioid receptor (MOR) occupancy to effectively suppress symptoms of craving and withdrawal in patients with OUD. Buprenorphine serum concentration correlates significantly with MOR occupancy, such that concentrations of 2 to 3 ng/mL are acknowledged as baseline minimums for clinical efficacy.8
BUP-XR is administered in 1 of 2 dosing regimens. In both, 2 separate 300 mg doses are administered 28 days apart during Month 1 and Month 2, followed by maintenance doses of either 300 mg (300/300 mg dosing regimen) or 100 mg (300/100 mg dosing regimen) every 28 days thereafter. Combined Phase II and Phase III data analyzing serum concentrations of BUP-XR across both dosing regimens revealed that, for most patients, there is a noticeable period during Month 1 and Month 2 when serum concentrations fall below 2 ng/mL.7 Steady-state concentrations of both regimens develop after 4 to 6 appropriately timed injections, providing average steady-state serum concentrations in Phase II and Phase III trials of 6.54 ng/mL for the 300/300 mg dosing regimen and 3.00 ng/mL for 300/100 mg dosing regimen.7
Real-world experiences with BUP-XR
The theoretical need for supplementation has been voiced in practice. A case series by Peckham et al9 noted that 55% (n = 22) of patients required SL-BUP supplementation for up to 120 days after the first BUP-XR injection to quell cravings and reduce nonprescribed opioid use.
The RECOVER trial by Ling et al10 demonstrated the importance of the first 2 months of BUP-XR therapy in the overall treatment success for patients with OUD. In this analysis, patients maintained on BUP-XR for 12 months reported a 75% likelihood of abstinence, compared to 24% for patients receiving 0 to 2 months of BUP-XR treatment. Other benefits included improved employment status and reduced depression rates. This trial did not specifically discuss supplemental SL-BUP or subthreshold concentrations of buprenorphine during early months.10
Individualized treatment should be based on OUD symptoms
While BUP-XR was designed to continuously deliver at least 2 ng/mL of buprenorphine, serum concentrations are labile during the first 2 months of treatment. This may result in breakthrough OUD symptoms, particularly withdrawal or opioid cravings. Additionally, due to individual variability, some patients may still experience serum concentrations below 2 ng/mL after Month 2 and until steady-state is achieved between Month 4 and Month 6.7
Continue to: Beyond a theoretical...
Beyond a theoretical need for supplementation with SL-BUP, there is limited information regarding optimal dosing, dosage intervals, or length of supplementation. Therefore, clear guidance is not available at this time, and treatment should be individualized based on subjective and objective OUD symptoms.
What also remains unknown are potential barriers patients may face in receiving 2 concurrent buprenorphine prescriptions. BUP-XR, administered in a health care setting, can be obtained 2 ways. A clinician can directly order the medication from the distributor to be administered via buy-and-bill. An alternate option requires the clinician to send a prescription to an appropriately credentialed pharmacy that will ship patient-specific orders directly to the clinic. Despite this, most SL-BUP prescriptions are billed and dispensed from community pharmacies. At the insurance level, there is risk the prescription claim will be rejected for duplication of therapy, which may require additional collaboration between the prescribing clinician, pharmacist, and insurance representative to ensure patients have access to the medication.
Pending studies and approvals may also provide greater guidance and flexibility in decision-making for patients with OUD. The CoLAB study currently underway in Australia is examining the efficacy and outcomes of an intermediate dose (200 mg) of BUP-XR and will also allow for supplemental SL-BUP doses.11 Additionally, an alternative BUP-XR formulation, Brixadi, currently in use in the European Union as Buvidal, has submitted an application for FDA approval in the United States. The application indicates that Brixadi will be available with a wider range of doses and at both weekly and monthly intervals. Approval has been delayed due to deficiencies in the United States–based third-party production facilities. It is unclear how the FDA and manufacturer plan to proceed.12
Short-term supplementation with SL-BUP during early the months of treatment with BUP-XR should be considered to control OUD symptoms and assist with patient retention. Once steady-state is achieved, trough concentrations of buprenorphine are not expected to drop below 2 ng/mL with continued on-time maintenance doses and thus, supplementation can likely cease.
CASE CONTINUED
Mr. L is seen in the low-threshold outpatient clinic 1 week after his ED visit. His arm laceration is healing well, and he is noticeably more alert and engaged. Each morning this week, he awakes with cravings, sweating, and anxiety. These symptoms alleviate after he takes SL-BUP. Mr. L’s clinician gives him a copy of the Subjective Opioid Withdrawal Scale so he can assess his withdrawal symptoms each morning and provide this data at follow-up appointments. Mr. L and his clinician decide to meet weekly until his next injection to continue assessing his current supplemental dose, symptoms, and whether there should be additional adjustments to his treatment plan.
Related Resources
- Cho J, Bhimani J, Patel M, et al. Substance abuse among older adults: a growing problem. Current Psychiatry. 2018;17(3):14-20.
- Verma S. Opioid use disorder in adolescents: an overview. Current Psychiatry. 2020;19(2):12-14,16-21.
Drug Brand Names
Alprazolam • Xanax
Buprenorphine • Sublocade, Subutex
Buprenorphine/naloxone • Suboxone, Zubsolv
Methadone • Methadose
1. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths - United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202-207. doi:10.15585/mmwr.mm7006a4
2. Ma J, Bao YP, Wang RJ, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(12):1868-1883. doi:10.1038/s41380-018-0094-5
3. Coe MA, Lofwall MR, Walsh SL. Buprenorphine pharmacology review: update on transmucosal and long-acting formulations. J Addict Med. 2019;13(2):93-103. doi:10.1097/ADM.0000000000000457
4. Becerra X. Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. US Dept of Health and Human Services; 2021:22439-22440. FR Document 2021-08961. Accessed April 5, 2021. https://www.federalregister.gov/documents/2021/04/28/2021-08961/practice-guidelines-for-the-administration-of-buprenorphine-for-treating-opioid-use-disorder
5. Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2019;393(10173):778-790. doi:10.1016/S0140-6736(18)32259-1
6. Sublocade [package insert]. North Chesterfield, VA: Indivior Inc; 2021.
7. Jones AK, Ngaimisi E, Gopalakrishnan M, et al. Population pharmacokinetics of a monthly buprenorphine depot injection for the treatment of opioid use disorder: a combined analysis of phase II and phase III trials. Clin Pharmacokinet. 2021;60(4):527-540. doi:10.1007/s40262-020-00957-0
8. Greenwald MK, Comer SD, Fiellin DA. Buprenorphine maintenance and mu-opioid receptor availability in the treatment of opioid use disorder: implications for clinical use and policy. Drug Alcohol Depend. 2014;144:1-11. doi:10.1016/j.drugalcdep.2014.07.035
9. Peckham AM, Kehoe LG, Gray JR, et al. Real-world outcomes with extended-release buprenorphine (XR-BUP) in a low threshold bridge clinic: a retrospective case series. J Subst Abuse Treat. 2021;126:108316. doi:10.1016/j.jsat.2021.108316
10. Ling W, Nadipelli VR, Aldridge AP, et al. Recovery from opioid use disorder (OUD) after monthly long-acting buprenorphine treatment: 12-month longitudinal outcomes from RECOVER, an observational study. J Addict Med. 2020;14(5):e233-e240. doi:10.1097/ADM.0000000000000647
11. Larance B, Byrne M, Lintzeris N, et al. Open-label, multicentre, single-arm trial of monthly injections of depot buprenorphine in people with opioid dependence: protocol for the CoLAB study. BMJ Open. 2020;10(7):e034389. doi:10.1136/bmjopen-2019-034389
12. Braeburn receives new Complete Response Letter for Brixadi in the US. News release. News Powered by Cision. December 15, 2021. Accessed April 13, 2022. https://news.cision.com/camurus-ab/r/braeburn-receives-new-complete-response-letter-for-brixadi-in-the-us,c3473281
1. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths - United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202-207. doi:10.15585/mmwr.mm7006a4
2. Ma J, Bao YP, Wang RJ, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(12):1868-1883. doi:10.1038/s41380-018-0094-5
3. Coe MA, Lofwall MR, Walsh SL. Buprenorphine pharmacology review: update on transmucosal and long-acting formulations. J Addict Med. 2019;13(2):93-103. doi:10.1097/ADM.0000000000000457
4. Becerra X. Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. US Dept of Health and Human Services; 2021:22439-22440. FR Document 2021-08961. Accessed April 5, 2021. https://www.federalregister.gov/documents/2021/04/28/2021-08961/practice-guidelines-for-the-administration-of-buprenorphine-for-treating-opioid-use-disorder
5. Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2019;393(10173):778-790. doi:10.1016/S0140-6736(18)32259-1
6. Sublocade [package insert]. North Chesterfield, VA: Indivior Inc; 2021.
7. Jones AK, Ngaimisi E, Gopalakrishnan M, et al. Population pharmacokinetics of a monthly buprenorphine depot injection for the treatment of opioid use disorder: a combined analysis of phase II and phase III trials. Clin Pharmacokinet. 2021;60(4):527-540. doi:10.1007/s40262-020-00957-0
8. Greenwald MK, Comer SD, Fiellin DA. Buprenorphine maintenance and mu-opioid receptor availability in the treatment of opioid use disorder: implications for clinical use and policy. Drug Alcohol Depend. 2014;144:1-11. doi:10.1016/j.drugalcdep.2014.07.035
9. Peckham AM, Kehoe LG, Gray JR, et al. Real-world outcomes with extended-release buprenorphine (XR-BUP) in a low threshold bridge clinic: a retrospective case series. J Subst Abuse Treat. 2021;126:108316. doi:10.1016/j.jsat.2021.108316
10. Ling W, Nadipelli VR, Aldridge AP, et al. Recovery from opioid use disorder (OUD) after monthly long-acting buprenorphine treatment: 12-month longitudinal outcomes from RECOVER, an observational study. J Addict Med. 2020;14(5):e233-e240. doi:10.1097/ADM.0000000000000647
11. Larance B, Byrne M, Lintzeris N, et al. Open-label, multicentre, single-arm trial of monthly injections of depot buprenorphine in people with opioid dependence: protocol for the CoLAB study. BMJ Open. 2020;10(7):e034389. doi:10.1136/bmjopen-2019-034389
12. Braeburn receives new Complete Response Letter for Brixadi in the US. News release. News Powered by Cision. December 15, 2021. Accessed April 13, 2022. https://news.cision.com/camurus-ab/r/braeburn-receives-new-complete-response-letter-for-brixadi-in-the-us,c3473281
Safe supply programs aim to reduce drug overdose deaths
The Safer Alternatives for Emergency Response (SAFER) program provides a safe supply of substances to prevent drug overdose deaths, according to a new report.
The program has been operating in Vancouver, British Columbia, since April 2021. So far, the program has enrolled 58 participants who have reported benefits from having new options when other forms of treatment or harm reduction didn’t work. In addition, doctors who work with the program have reported increased medication adherence among the participants, as well as better chronic disease management.
Similar safe supply programs are being implemented or considered in other places across Canada. Since 2019, Health Canada has funded 18 safe supply pilot programs.
“When we look at the number of overdose deaths, it should be zero. These are preventable deaths,” author Christy Sutherland, MD, medical director at the PHS Community Services Society, Vancouver, which operates the SAFER program, told this news organization.
“As clinicians, we can see that the tools we have are working less because of prohibition. It drives the market to provide more potent and more dangerous options,” she said. “It’s critical that we disrupt the illicit market and provide medical solutions to keep people safe.”
The report was published in the Canadian Medical Association Journal.
Safe supply programs
Between January 2016 and June 2021, more than 24,000 people died from opioid toxicity in Canada, according to the authors. A key driver of the ongoing public health crisis has been the introduction of illicit fentanyl and other dangerous substances into the unregulated drug supply.
In recent years, several harm-reduction options and substance use disorder treatment programs have been introduced in Canada to stem overdose deaths. However, they haven’t been sufficient, and the number of deaths continues to rise.
“In 2010, methadone worked, but now even high doses don’t keep people out of withdrawal due to the infiltration of fentanyl,” Dr. Sutherland said. “It’s clinically not working anymore. People are now going through benzodiazepine withdrawal and opiate withdrawal at the same time.”
The changes have led doctors to call for programs that provide legal and regulated sources of psychoactive substances, also known as “safe supply” programs. In particular, low-barrier and flexible options are necessary to meet the needs of various people in the community.
In Vancouver, the SAFER program provides medications that are prescribed off-label as substitutes to the illicit drug supply. A multidisciplinary team oversees the program, including doctors, nurses, pharmacists, social workers, and people who have experience living with substance use.
The program’s approach is akin to the use of medications as treatments for substance use disorder, such as opioid-agonist therapy. However,
Enrolled participants can access medications, including opioids such as hydromorphone and fentanyl, as a substitute for the unregulated substances that they consume. A notable aspect of SAFER is the offer of fentanyl – with a known potency and without dangerous adulterants found in the local drug supply.
Promoting participant autonomy
Given the increasing rate of overdose deaths involving stimulants in Canada, the program also offers prescribed psychostimulants, such as methylphenidate and dextroamphetamine.
The program focuses on harm reduction and promoting participant autonomy. SAFER doesn’t have a predetermined schedule for medication access, which allows participants to return as they need.
“Creating this program has required patience to change our practices,” Dr. Sutherland said. “As you learn more and do more, you’re always growing because you care about your patients and want to help them, especially vulnerable people with a high risk for death.”
The SAFER program is integrated into health care and social services, and participants have access to on-site primary care from clinicians trained in addiction medicine. The program is located alongside a low-barrier prevention site, where supplies such as syringes, take-home naloxone kits, and drug-checking services are available.
The SAFER program will undergo a scientific evaluation, led by two of the co-authors, which will include about 200 participants. During a 2-year period, the evaluation will assess whether the program reduces the risk for overdose deaths and supports access to primary care, harm reduction, and substance use disorder treatment. In addition, the researchers will analyze other key outcomes, such as fatal versus nonfatal overdoses, medication adherence, and the qualitative lived experience of participants.
The end of prohibition?
“We’ve had the same challenges with people buying illegal drugs on the street for almost 30 years, but about 5 years ago, that all changed when fentanyl became a prominent drug, and overdose deaths skyrocketed,” Mark Tyndall, MD, a public health professor at the University of British Columbia, Vancouver, said in an interview.
Dr. Tyndall is also executive director of the British Columbia Centre for Disease Control and executive director of MySafe Society, a safe supply program in Canada for those with opioid addiction. He is not involved in the SAFER program.
SAFER and MySafe Society are positioned as low-barrier programs, he said, meaning that the public health response is primarily focused on preventing deaths and helping people to get access to medication that won’t kill them. The idea is to meet people where they are today.
However, these programs still face major barriers, such as limitations from federal regulators and stigmas around illicit drugs and harm-reduction programs.
“These beliefs are entrenched, and it takes a long time to help people understand that prohibition means that dangerous drugs are on the street,” he said. “I don’t think way more people are using than 10 years ago, but there was a supply of heroin that was stable in potency back then, and people weren’t dying.”
Ultimately, Dr. Tyndall said, drug policy experts would like to create a regulated supply, similar to the supply of cannabis. The political and regulatory process may take much longer to catch up, but he believes that it’s the most ethical way to reduce overdose deaths and the unregulated drug supply.
“The harshest critics of harm reduction often go to the liquor store every weekend,” he said. “It’s going to be a long process before people think this way, but having fentanyl and other dangerous drugs on the street has signaled the end stage of prohibition.”
The SAFER program is operated by PHS Community Services Society in partnership with Vancouver Coastal Health and funded through Health Canada’s Substance Use and Addiction Program. Dr. Tyndall reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
The Safer Alternatives for Emergency Response (SAFER) program provides a safe supply of substances to prevent drug overdose deaths, according to a new report.
The program has been operating in Vancouver, British Columbia, since April 2021. So far, the program has enrolled 58 participants who have reported benefits from having new options when other forms of treatment or harm reduction didn’t work. In addition, doctors who work with the program have reported increased medication adherence among the participants, as well as better chronic disease management.
Similar safe supply programs are being implemented or considered in other places across Canada. Since 2019, Health Canada has funded 18 safe supply pilot programs.
“When we look at the number of overdose deaths, it should be zero. These are preventable deaths,” author Christy Sutherland, MD, medical director at the PHS Community Services Society, Vancouver, which operates the SAFER program, told this news organization.
“As clinicians, we can see that the tools we have are working less because of prohibition. It drives the market to provide more potent and more dangerous options,” she said. “It’s critical that we disrupt the illicit market and provide medical solutions to keep people safe.”
The report was published in the Canadian Medical Association Journal.
Safe supply programs
Between January 2016 and June 2021, more than 24,000 people died from opioid toxicity in Canada, according to the authors. A key driver of the ongoing public health crisis has been the introduction of illicit fentanyl and other dangerous substances into the unregulated drug supply.
In recent years, several harm-reduction options and substance use disorder treatment programs have been introduced in Canada to stem overdose deaths. However, they haven’t been sufficient, and the number of deaths continues to rise.
“In 2010, methadone worked, but now even high doses don’t keep people out of withdrawal due to the infiltration of fentanyl,” Dr. Sutherland said. “It’s clinically not working anymore. People are now going through benzodiazepine withdrawal and opiate withdrawal at the same time.”
The changes have led doctors to call for programs that provide legal and regulated sources of psychoactive substances, also known as “safe supply” programs. In particular, low-barrier and flexible options are necessary to meet the needs of various people in the community.
In Vancouver, the SAFER program provides medications that are prescribed off-label as substitutes to the illicit drug supply. A multidisciplinary team oversees the program, including doctors, nurses, pharmacists, social workers, and people who have experience living with substance use.
The program’s approach is akin to the use of medications as treatments for substance use disorder, such as opioid-agonist therapy. However,
Enrolled participants can access medications, including opioids such as hydromorphone and fentanyl, as a substitute for the unregulated substances that they consume. A notable aspect of SAFER is the offer of fentanyl – with a known potency and without dangerous adulterants found in the local drug supply.
Promoting participant autonomy
Given the increasing rate of overdose deaths involving stimulants in Canada, the program also offers prescribed psychostimulants, such as methylphenidate and dextroamphetamine.
The program focuses on harm reduction and promoting participant autonomy. SAFER doesn’t have a predetermined schedule for medication access, which allows participants to return as they need.
“Creating this program has required patience to change our practices,” Dr. Sutherland said. “As you learn more and do more, you’re always growing because you care about your patients and want to help them, especially vulnerable people with a high risk for death.”
The SAFER program is integrated into health care and social services, and participants have access to on-site primary care from clinicians trained in addiction medicine. The program is located alongside a low-barrier prevention site, where supplies such as syringes, take-home naloxone kits, and drug-checking services are available.
The SAFER program will undergo a scientific evaluation, led by two of the co-authors, which will include about 200 participants. During a 2-year period, the evaluation will assess whether the program reduces the risk for overdose deaths and supports access to primary care, harm reduction, and substance use disorder treatment. In addition, the researchers will analyze other key outcomes, such as fatal versus nonfatal overdoses, medication adherence, and the qualitative lived experience of participants.
The end of prohibition?
“We’ve had the same challenges with people buying illegal drugs on the street for almost 30 years, but about 5 years ago, that all changed when fentanyl became a prominent drug, and overdose deaths skyrocketed,” Mark Tyndall, MD, a public health professor at the University of British Columbia, Vancouver, said in an interview.
Dr. Tyndall is also executive director of the British Columbia Centre for Disease Control and executive director of MySafe Society, a safe supply program in Canada for those with opioid addiction. He is not involved in the SAFER program.
SAFER and MySafe Society are positioned as low-barrier programs, he said, meaning that the public health response is primarily focused on preventing deaths and helping people to get access to medication that won’t kill them. The idea is to meet people where they are today.
However, these programs still face major barriers, such as limitations from federal regulators and stigmas around illicit drugs and harm-reduction programs.
“These beliefs are entrenched, and it takes a long time to help people understand that prohibition means that dangerous drugs are on the street,” he said. “I don’t think way more people are using than 10 years ago, but there was a supply of heroin that was stable in potency back then, and people weren’t dying.”
Ultimately, Dr. Tyndall said, drug policy experts would like to create a regulated supply, similar to the supply of cannabis. The political and regulatory process may take much longer to catch up, but he believes that it’s the most ethical way to reduce overdose deaths and the unregulated drug supply.
“The harshest critics of harm reduction often go to the liquor store every weekend,” he said. “It’s going to be a long process before people think this way, but having fentanyl and other dangerous drugs on the street has signaled the end stage of prohibition.”
The SAFER program is operated by PHS Community Services Society in partnership with Vancouver Coastal Health and funded through Health Canada’s Substance Use and Addiction Program. Dr. Tyndall reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
The Safer Alternatives for Emergency Response (SAFER) program provides a safe supply of substances to prevent drug overdose deaths, according to a new report.
The program has been operating in Vancouver, British Columbia, since April 2021. So far, the program has enrolled 58 participants who have reported benefits from having new options when other forms of treatment or harm reduction didn’t work. In addition, doctors who work with the program have reported increased medication adherence among the participants, as well as better chronic disease management.
Similar safe supply programs are being implemented or considered in other places across Canada. Since 2019, Health Canada has funded 18 safe supply pilot programs.
“When we look at the number of overdose deaths, it should be zero. These are preventable deaths,” author Christy Sutherland, MD, medical director at the PHS Community Services Society, Vancouver, which operates the SAFER program, told this news organization.
“As clinicians, we can see that the tools we have are working less because of prohibition. It drives the market to provide more potent and more dangerous options,” she said. “It’s critical that we disrupt the illicit market and provide medical solutions to keep people safe.”
The report was published in the Canadian Medical Association Journal.
Safe supply programs
Between January 2016 and June 2021, more than 24,000 people died from opioid toxicity in Canada, according to the authors. A key driver of the ongoing public health crisis has been the introduction of illicit fentanyl and other dangerous substances into the unregulated drug supply.
In recent years, several harm-reduction options and substance use disorder treatment programs have been introduced in Canada to stem overdose deaths. However, they haven’t been sufficient, and the number of deaths continues to rise.
“In 2010, methadone worked, but now even high doses don’t keep people out of withdrawal due to the infiltration of fentanyl,” Dr. Sutherland said. “It’s clinically not working anymore. People are now going through benzodiazepine withdrawal and opiate withdrawal at the same time.”
The changes have led doctors to call for programs that provide legal and regulated sources of psychoactive substances, also known as “safe supply” programs. In particular, low-barrier and flexible options are necessary to meet the needs of various people in the community.
In Vancouver, the SAFER program provides medications that are prescribed off-label as substitutes to the illicit drug supply. A multidisciplinary team oversees the program, including doctors, nurses, pharmacists, social workers, and people who have experience living with substance use.
The program’s approach is akin to the use of medications as treatments for substance use disorder, such as opioid-agonist therapy. However,
Enrolled participants can access medications, including opioids such as hydromorphone and fentanyl, as a substitute for the unregulated substances that they consume. A notable aspect of SAFER is the offer of fentanyl – with a known potency and without dangerous adulterants found in the local drug supply.
Promoting participant autonomy
Given the increasing rate of overdose deaths involving stimulants in Canada, the program also offers prescribed psychostimulants, such as methylphenidate and dextroamphetamine.
The program focuses on harm reduction and promoting participant autonomy. SAFER doesn’t have a predetermined schedule for medication access, which allows participants to return as they need.
“Creating this program has required patience to change our practices,” Dr. Sutherland said. “As you learn more and do more, you’re always growing because you care about your patients and want to help them, especially vulnerable people with a high risk for death.”
The SAFER program is integrated into health care and social services, and participants have access to on-site primary care from clinicians trained in addiction medicine. The program is located alongside a low-barrier prevention site, where supplies such as syringes, take-home naloxone kits, and drug-checking services are available.
The SAFER program will undergo a scientific evaluation, led by two of the co-authors, which will include about 200 participants. During a 2-year period, the evaluation will assess whether the program reduces the risk for overdose deaths and supports access to primary care, harm reduction, and substance use disorder treatment. In addition, the researchers will analyze other key outcomes, such as fatal versus nonfatal overdoses, medication adherence, and the qualitative lived experience of participants.
The end of prohibition?
“We’ve had the same challenges with people buying illegal drugs on the street for almost 30 years, but about 5 years ago, that all changed when fentanyl became a prominent drug, and overdose deaths skyrocketed,” Mark Tyndall, MD, a public health professor at the University of British Columbia, Vancouver, said in an interview.
Dr. Tyndall is also executive director of the British Columbia Centre for Disease Control and executive director of MySafe Society, a safe supply program in Canada for those with opioid addiction. He is not involved in the SAFER program.
SAFER and MySafe Society are positioned as low-barrier programs, he said, meaning that the public health response is primarily focused on preventing deaths and helping people to get access to medication that won’t kill them. The idea is to meet people where they are today.
However, these programs still face major barriers, such as limitations from federal regulators and stigmas around illicit drugs and harm-reduction programs.
“These beliefs are entrenched, and it takes a long time to help people understand that prohibition means that dangerous drugs are on the street,” he said. “I don’t think way more people are using than 10 years ago, but there was a supply of heroin that was stable in potency back then, and people weren’t dying.”
Ultimately, Dr. Tyndall said, drug policy experts would like to create a regulated supply, similar to the supply of cannabis. The political and regulatory process may take much longer to catch up, but he believes that it’s the most ethical way to reduce overdose deaths and the unregulated drug supply.
“The harshest critics of harm reduction often go to the liquor store every weekend,” he said. “It’s going to be a long process before people think this way, but having fentanyl and other dangerous drugs on the street has signaled the end stage of prohibition.”
The SAFER program is operated by PHS Community Services Society in partnership with Vancouver Coastal Health and funded through Health Canada’s Substance Use and Addiction Program. Dr. Tyndall reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL
Video game obsession: Definitions and best treatments remain elusive
NEW ORLEANS – Research into video game addiction is turning up new insights, and some treatments seem to make a difference, according to addiction psychiatry experts speaking at the annual meeting of the American Psychiatric Association. Still, understanding remains limited amid a general lack of clarity about definitions, measurements, and the most effective treatment strategies.
“Video games have the potential to be uniquely addictive, and it’s difficult to come up with treatment modalities that you can use for kids who have access to these things 24/7 on their mobile phones or laptops,” psychiatrist James C. Sherer, MD, of NYU Langone Health, said during the May 22 session, “Internet Gaming Disorder: From Harmless Fun to Dependence,” at the meeting. “It makes treating this a really complicated endeavor.”
The number of people with so-called Internet gaming disorder is unknown, but video games remain wildly popular among adults and children of all genders. According to a 2021 survey by Common Sense Media, U.S. individuals aged 8-12 and 13-18 spent an average of 1:27 hours and 1:46 hours per day, respectively, playing video games.
“Video games are an extremely important part of normal social networking among kids, and there’s a huge amount of social pressure to be good,” Dr. Sherer said. “If you’re in a particularly affluent neighborhood, it’s not unheard of for a parent to hire a coach to make their kid good at a game like Fortnite so they impress the other kids.”
The 2013 edition of the DSM-5 doesn’t list Internet gaming disorder as a mental illness but suggests that the topic warrants more research and evaluation, Dr. Sherer said.
Why are video games so addicting? According to Dr. Sherer, they’re simply designed that way. Game manufacturers “employ psychologists and behaviorists whose only job is to look at the game and determine what colors and what sounds are most likely to make you spend a little bit extra.” And with the help of the Internet, video games have evolved over the past 40 years to encourage users to make multiple purchases on single games such as Candy Crush instead of simply buying, say, a single 1980s-style Atari cartridge.
According to Dr. Sherer, research suggests that video games place users into something called the “flow state,” which a recent review article published in Frontiers in Psychology describes as “a state of full task engagement that is accompanied with low-levels of self-referential thinking” and “highly relevant for human performance and well-being.”
Diagnosing gaming addiction
How can psychiatrists diagnose video gaming addiction? Dr. Sherer, who is himself a devoted gamer, advised against focusing too much on time spent gaming in determining whether a patient has a problem. Instead, keep in mind that excessive gaming can displace exercise and normal socialization, he said, and lead to worsening mood.
Rober Aziz, MD, also of NYU Langone Health, suggested asking these questions: What types of games do you play? How long do you spend playing? What’s your reason for playing? What’s the meaning of your character choices? Does this game interfere with school or work? Have you neglected your self-care to play more?
He recommends other questions, too: Have you tried to limit your play time without success? How uncomfortable do you get if you must stop in the middle of playing? Do you get agitated if servers go down unexpectedly?
“There’s actually a lot of parallel here to other addictions that we’re very familiar with,” he said.
According to Dr. Sherer, it’s helpful to know that children who have attention-deficit/hyperactivity disorder tend to struggle with gaming addiction the most. He highlighted a brain-scan study in the Journal of Attention Disorders that found that patients with gaming addiction and ADHD had less functional connectivity from the cortex to the subcortex compared to matched controls. But treatment helped increase connectivity in those with good prognoses.
The findings are “heartening,” he said. “Basically, if you’re treating ADHD, you’re treating Internet gaming disorder. And if you’re treating Internet gaming disorder, you’re treating ADHD.”
As for treatments, the speakers agreed that there is little research to point in the right direction regarding gaming addiction specifically.
According to Dr. Aziz, research has suggested that bupropion, methylphenidate, and escitalopram can be helpful. In terms of nondrug approaches, he recommends directing patients toward games that have distinct beginnings, middles, and ends instead of endlessly providing rewards. One such game is “Legend of Zelda: Breath of the Wild” on the Nintendo Switch platform, he said.
On the psychotherapy front, Dr. Aziz said, “reducing use rather than abstinence should be the treatment goal.” Research suggests that cognitive behavioral therapy may not help patients in the long term, he said. Other strategies, he said, include specific approaches known as “CBT for Internet addiction” and “motivational interviewing for Internet gaming disorder.”
Gaming addiction treatment centers have also popped up in the U.S., he said, and there’s now an organization called Gaming Addicts Anonymous.
The good news is that “there is a lot of active research that’s being done” into treating video game addiction, said psychiatrist Anil Thomas, MD, program director of the addiction psychiatry fellowship at NYU Langone Health and moderator of the APA session. “We just have to wait to see what the results are.”
NEW ORLEANS – Research into video game addiction is turning up new insights, and some treatments seem to make a difference, according to addiction psychiatry experts speaking at the annual meeting of the American Psychiatric Association. Still, understanding remains limited amid a general lack of clarity about definitions, measurements, and the most effective treatment strategies.
“Video games have the potential to be uniquely addictive, and it’s difficult to come up with treatment modalities that you can use for kids who have access to these things 24/7 on their mobile phones or laptops,” psychiatrist James C. Sherer, MD, of NYU Langone Health, said during the May 22 session, “Internet Gaming Disorder: From Harmless Fun to Dependence,” at the meeting. “It makes treating this a really complicated endeavor.”
The number of people with so-called Internet gaming disorder is unknown, but video games remain wildly popular among adults and children of all genders. According to a 2021 survey by Common Sense Media, U.S. individuals aged 8-12 and 13-18 spent an average of 1:27 hours and 1:46 hours per day, respectively, playing video games.
“Video games are an extremely important part of normal social networking among kids, and there’s a huge amount of social pressure to be good,” Dr. Sherer said. “If you’re in a particularly affluent neighborhood, it’s not unheard of for a parent to hire a coach to make their kid good at a game like Fortnite so they impress the other kids.”
The 2013 edition of the DSM-5 doesn’t list Internet gaming disorder as a mental illness but suggests that the topic warrants more research and evaluation, Dr. Sherer said.
Why are video games so addicting? According to Dr. Sherer, they’re simply designed that way. Game manufacturers “employ psychologists and behaviorists whose only job is to look at the game and determine what colors and what sounds are most likely to make you spend a little bit extra.” And with the help of the Internet, video games have evolved over the past 40 years to encourage users to make multiple purchases on single games such as Candy Crush instead of simply buying, say, a single 1980s-style Atari cartridge.
According to Dr. Sherer, research suggests that video games place users into something called the “flow state,” which a recent review article published in Frontiers in Psychology describes as “a state of full task engagement that is accompanied with low-levels of self-referential thinking” and “highly relevant for human performance and well-being.”
Diagnosing gaming addiction
How can psychiatrists diagnose video gaming addiction? Dr. Sherer, who is himself a devoted gamer, advised against focusing too much on time spent gaming in determining whether a patient has a problem. Instead, keep in mind that excessive gaming can displace exercise and normal socialization, he said, and lead to worsening mood.
Rober Aziz, MD, also of NYU Langone Health, suggested asking these questions: What types of games do you play? How long do you spend playing? What’s your reason for playing? What’s the meaning of your character choices? Does this game interfere with school or work? Have you neglected your self-care to play more?
He recommends other questions, too: Have you tried to limit your play time without success? How uncomfortable do you get if you must stop in the middle of playing? Do you get agitated if servers go down unexpectedly?
“There’s actually a lot of parallel here to other addictions that we’re very familiar with,” he said.
According to Dr. Sherer, it’s helpful to know that children who have attention-deficit/hyperactivity disorder tend to struggle with gaming addiction the most. He highlighted a brain-scan study in the Journal of Attention Disorders that found that patients with gaming addiction and ADHD had less functional connectivity from the cortex to the subcortex compared to matched controls. But treatment helped increase connectivity in those with good prognoses.
The findings are “heartening,” he said. “Basically, if you’re treating ADHD, you’re treating Internet gaming disorder. And if you’re treating Internet gaming disorder, you’re treating ADHD.”
As for treatments, the speakers agreed that there is little research to point in the right direction regarding gaming addiction specifically.
According to Dr. Aziz, research has suggested that bupropion, methylphenidate, and escitalopram can be helpful. In terms of nondrug approaches, he recommends directing patients toward games that have distinct beginnings, middles, and ends instead of endlessly providing rewards. One such game is “Legend of Zelda: Breath of the Wild” on the Nintendo Switch platform, he said.
On the psychotherapy front, Dr. Aziz said, “reducing use rather than abstinence should be the treatment goal.” Research suggests that cognitive behavioral therapy may not help patients in the long term, he said. Other strategies, he said, include specific approaches known as “CBT for Internet addiction” and “motivational interviewing for Internet gaming disorder.”
Gaming addiction treatment centers have also popped up in the U.S., he said, and there’s now an organization called Gaming Addicts Anonymous.
The good news is that “there is a lot of active research that’s being done” into treating video game addiction, said psychiatrist Anil Thomas, MD, program director of the addiction psychiatry fellowship at NYU Langone Health and moderator of the APA session. “We just have to wait to see what the results are.”
NEW ORLEANS – Research into video game addiction is turning up new insights, and some treatments seem to make a difference, according to addiction psychiatry experts speaking at the annual meeting of the American Psychiatric Association. Still, understanding remains limited amid a general lack of clarity about definitions, measurements, and the most effective treatment strategies.
“Video games have the potential to be uniquely addictive, and it’s difficult to come up with treatment modalities that you can use for kids who have access to these things 24/7 on their mobile phones or laptops,” psychiatrist James C. Sherer, MD, of NYU Langone Health, said during the May 22 session, “Internet Gaming Disorder: From Harmless Fun to Dependence,” at the meeting. “It makes treating this a really complicated endeavor.”
The number of people with so-called Internet gaming disorder is unknown, but video games remain wildly popular among adults and children of all genders. According to a 2021 survey by Common Sense Media, U.S. individuals aged 8-12 and 13-18 spent an average of 1:27 hours and 1:46 hours per day, respectively, playing video games.
“Video games are an extremely important part of normal social networking among kids, and there’s a huge amount of social pressure to be good,” Dr. Sherer said. “If you’re in a particularly affluent neighborhood, it’s not unheard of for a parent to hire a coach to make their kid good at a game like Fortnite so they impress the other kids.”
The 2013 edition of the DSM-5 doesn’t list Internet gaming disorder as a mental illness but suggests that the topic warrants more research and evaluation, Dr. Sherer said.
Why are video games so addicting? According to Dr. Sherer, they’re simply designed that way. Game manufacturers “employ psychologists and behaviorists whose only job is to look at the game and determine what colors and what sounds are most likely to make you spend a little bit extra.” And with the help of the Internet, video games have evolved over the past 40 years to encourage users to make multiple purchases on single games such as Candy Crush instead of simply buying, say, a single 1980s-style Atari cartridge.
According to Dr. Sherer, research suggests that video games place users into something called the “flow state,” which a recent review article published in Frontiers in Psychology describes as “a state of full task engagement that is accompanied with low-levels of self-referential thinking” and “highly relevant for human performance and well-being.”
Diagnosing gaming addiction
How can psychiatrists diagnose video gaming addiction? Dr. Sherer, who is himself a devoted gamer, advised against focusing too much on time spent gaming in determining whether a patient has a problem. Instead, keep in mind that excessive gaming can displace exercise and normal socialization, he said, and lead to worsening mood.
Rober Aziz, MD, also of NYU Langone Health, suggested asking these questions: What types of games do you play? How long do you spend playing? What’s your reason for playing? What’s the meaning of your character choices? Does this game interfere with school or work? Have you neglected your self-care to play more?
He recommends other questions, too: Have you tried to limit your play time without success? How uncomfortable do you get if you must stop in the middle of playing? Do you get agitated if servers go down unexpectedly?
“There’s actually a lot of parallel here to other addictions that we’re very familiar with,” he said.
According to Dr. Sherer, it’s helpful to know that children who have attention-deficit/hyperactivity disorder tend to struggle with gaming addiction the most. He highlighted a brain-scan study in the Journal of Attention Disorders that found that patients with gaming addiction and ADHD had less functional connectivity from the cortex to the subcortex compared to matched controls. But treatment helped increase connectivity in those with good prognoses.
The findings are “heartening,” he said. “Basically, if you’re treating ADHD, you’re treating Internet gaming disorder. And if you’re treating Internet gaming disorder, you’re treating ADHD.”
As for treatments, the speakers agreed that there is little research to point in the right direction regarding gaming addiction specifically.
According to Dr. Aziz, research has suggested that bupropion, methylphenidate, and escitalopram can be helpful. In terms of nondrug approaches, he recommends directing patients toward games that have distinct beginnings, middles, and ends instead of endlessly providing rewards. One such game is “Legend of Zelda: Breath of the Wild” on the Nintendo Switch platform, he said.
On the psychotherapy front, Dr. Aziz said, “reducing use rather than abstinence should be the treatment goal.” Research suggests that cognitive behavioral therapy may not help patients in the long term, he said. Other strategies, he said, include specific approaches known as “CBT for Internet addiction” and “motivational interviewing for Internet gaming disorder.”
Gaming addiction treatment centers have also popped up in the U.S., he said, and there’s now an organization called Gaming Addicts Anonymous.
The good news is that “there is a lot of active research that’s being done” into treating video game addiction, said psychiatrist Anil Thomas, MD, program director of the addiction psychiatry fellowship at NYU Langone Health and moderator of the APA session. “We just have to wait to see what the results are.”
AT APA 2022
Innovative med school curriculum could help curb the opioid epidemic
, new research suggests.
“Our study showed that implementing training for medical students about opioid use disorder and its treatment improves knowledge and understanding of clinical principles and may better prepare students to treat patients with this disorder,” study investigator Kimberly Hu, MD, psychiatry resident, Ohio State University, Columbus, told this news organization.
The findings were presented at the annual meeting of the American Psychiatric Association.
The U.S. opioid epidemic claims thousands of lives every year, and there’s evidence it’s getting worse, said Dr. Hu. U.S. data from December 2020 to December 2021 show opioid-related deaths increased by almost 15%.
In 2019, about 70% of the nearly 71,000 drug overdose deaths in the United States involved opioids and now it exceeds 100,000 per year, said Dr. Hu. She noted 80% of heroin users report their addiction started with prescription opioids, data that she described as “pretty staggering.”
Although treatments such as buprenorphine are available for OUD, “insufficient access to medications for opioid use disorder remains a significant barrier for patients,” said Dr. Hu.
“Training the next generation of physicians across all specialties is one way that we can work to improve access to care and improve the health and well-being of our patients.”
The study, which is ongoing, included 405 3rd-year medical students at Ohio State. Researchers provided these students with in-person or virtual (during the pandemic) training in buprenorphine prescribing and in-person clinical experience.
Dr. Hu and her colleagues tested the students before and after the intervention and estimated improvement in knowledge (score 0-23) and approach to clinical management principles (1-5).
The investigators found a statistically significant increase in overall knowledge (from a mean total score of 18.34 to 19.32; P < .001). There was also a statistically significant increase in self-reported understanding of clinical management principles related to screening for and treating OUDs (from a mean of 3.12 to a mean of 4.02; P < .001).
An additional evaluation survey was completed by 162 students at the end of the program. About 83% of these students said they knew how to manage acute pain, 62% felt they knew how to manage chronic pain, and 77% agreed they knew how to screen a patient for OUD.
Dr. Hu noted 3rd-year medical students are a little over halfway through medical school, after which they will go into residency in various specialties. Providing them with this knowledge early on allows them to incorporate it as they continue their training, she said.
“If they are able to screen their patients in any specialty they eventually choose to go into, then they can help link these patients to resources early and make sure there aren’t patients who are slipping through the cracks.”
Worthwhile, important research
Howard Liu, MD, chair of the department of psychiatry at the University of Nebraska Medical Center, Omaha, and incoming chair of the APA’s Council on Communications, applauded the study.
The proposed curriculum, he said, instills confidence in students and teaches important lessons they can apply no matter what field they choose.
Dr. Liu, who moderated a press briefing highlighting the study, noted every state is affected differently by the opioid epidemic, but the shortage of appropriate treatments for OUD is nationwide.
Commenting on the study, addiction specialist Elie G. Aoun, MD, of the division of law, medicine, and psychiatry at Columbia University, New York, said this research is “very worthwhile and important.”
He noted that attitudes about addiction need to change. When he taught medical students about substance use disorders, he was struck by some of their negative beliefs about addiction. For example, considering addicts as “junkies” who are “taking resources away” from what they perceive as more deserving patients.
Addiction has been ignored in medicine for too long, added Dr. Aoun. He noted the requirement for addiction training for psychiatry residents is 2 months while they spend 4 months learning internal medicine. “That makes no sense,” he said.
“And now with the opioid epidemic, we’re faced with the consequences of dismissing addiction for such a long time.”
A lack of understanding about addiction, and the “very limited number” of experienced people treating addictions, has contributed to the “huge problem” experts now face in treating addictions, said Dr. Aoun.
“So you want to approach this problem from as many different angles as you can.”
He praised the study for presenting “a framework to ‘medicalize’ the addiction model” for students. This, he said, will help them build empathy and see those with a substance use disorder as no different from other patients with medical conditions.
A curriculum such as the one presented by Dr. Hu and colleagues may spur more medical students into the addiction field, he said. “It may make them more willing to treat patients with addiction using evidence-based medicine rather than dismissing them.”
The study was supported by the Substance Abuse and Mental Health Services Administration.
A version of this article first appeared on Medscape.com.
, new research suggests.
“Our study showed that implementing training for medical students about opioid use disorder and its treatment improves knowledge and understanding of clinical principles and may better prepare students to treat patients with this disorder,” study investigator Kimberly Hu, MD, psychiatry resident, Ohio State University, Columbus, told this news organization.
The findings were presented at the annual meeting of the American Psychiatric Association.
The U.S. opioid epidemic claims thousands of lives every year, and there’s evidence it’s getting worse, said Dr. Hu. U.S. data from December 2020 to December 2021 show opioid-related deaths increased by almost 15%.
In 2019, about 70% of the nearly 71,000 drug overdose deaths in the United States involved opioids and now it exceeds 100,000 per year, said Dr. Hu. She noted 80% of heroin users report their addiction started with prescription opioids, data that she described as “pretty staggering.”
Although treatments such as buprenorphine are available for OUD, “insufficient access to medications for opioid use disorder remains a significant barrier for patients,” said Dr. Hu.
“Training the next generation of physicians across all specialties is one way that we can work to improve access to care and improve the health and well-being of our patients.”
The study, which is ongoing, included 405 3rd-year medical students at Ohio State. Researchers provided these students with in-person or virtual (during the pandemic) training in buprenorphine prescribing and in-person clinical experience.
Dr. Hu and her colleagues tested the students before and after the intervention and estimated improvement in knowledge (score 0-23) and approach to clinical management principles (1-5).
The investigators found a statistically significant increase in overall knowledge (from a mean total score of 18.34 to 19.32; P < .001). There was also a statistically significant increase in self-reported understanding of clinical management principles related to screening for and treating OUDs (from a mean of 3.12 to a mean of 4.02; P < .001).
An additional evaluation survey was completed by 162 students at the end of the program. About 83% of these students said they knew how to manage acute pain, 62% felt they knew how to manage chronic pain, and 77% agreed they knew how to screen a patient for OUD.
Dr. Hu noted 3rd-year medical students are a little over halfway through medical school, after which they will go into residency in various specialties. Providing them with this knowledge early on allows them to incorporate it as they continue their training, she said.
“If they are able to screen their patients in any specialty they eventually choose to go into, then they can help link these patients to resources early and make sure there aren’t patients who are slipping through the cracks.”
Worthwhile, important research
Howard Liu, MD, chair of the department of psychiatry at the University of Nebraska Medical Center, Omaha, and incoming chair of the APA’s Council on Communications, applauded the study.
The proposed curriculum, he said, instills confidence in students and teaches important lessons they can apply no matter what field they choose.
Dr. Liu, who moderated a press briefing highlighting the study, noted every state is affected differently by the opioid epidemic, but the shortage of appropriate treatments for OUD is nationwide.
Commenting on the study, addiction specialist Elie G. Aoun, MD, of the division of law, medicine, and psychiatry at Columbia University, New York, said this research is “very worthwhile and important.”
He noted that attitudes about addiction need to change. When he taught medical students about substance use disorders, he was struck by some of their negative beliefs about addiction. For example, considering addicts as “junkies” who are “taking resources away” from what they perceive as more deserving patients.
Addiction has been ignored in medicine for too long, added Dr. Aoun. He noted the requirement for addiction training for psychiatry residents is 2 months while they spend 4 months learning internal medicine. “That makes no sense,” he said.
“And now with the opioid epidemic, we’re faced with the consequences of dismissing addiction for such a long time.”
A lack of understanding about addiction, and the “very limited number” of experienced people treating addictions, has contributed to the “huge problem” experts now face in treating addictions, said Dr. Aoun.
“So you want to approach this problem from as many different angles as you can.”
He praised the study for presenting “a framework to ‘medicalize’ the addiction model” for students. This, he said, will help them build empathy and see those with a substance use disorder as no different from other patients with medical conditions.
A curriculum such as the one presented by Dr. Hu and colleagues may spur more medical students into the addiction field, he said. “It may make them more willing to treat patients with addiction using evidence-based medicine rather than dismissing them.”
The study was supported by the Substance Abuse and Mental Health Services Administration.
A version of this article first appeared on Medscape.com.
, new research suggests.
“Our study showed that implementing training for medical students about opioid use disorder and its treatment improves knowledge and understanding of clinical principles and may better prepare students to treat patients with this disorder,” study investigator Kimberly Hu, MD, psychiatry resident, Ohio State University, Columbus, told this news organization.
The findings were presented at the annual meeting of the American Psychiatric Association.
The U.S. opioid epidemic claims thousands of lives every year, and there’s evidence it’s getting worse, said Dr. Hu. U.S. data from December 2020 to December 2021 show opioid-related deaths increased by almost 15%.
In 2019, about 70% of the nearly 71,000 drug overdose deaths in the United States involved opioids and now it exceeds 100,000 per year, said Dr. Hu. She noted 80% of heroin users report their addiction started with prescription opioids, data that she described as “pretty staggering.”
Although treatments such as buprenorphine are available for OUD, “insufficient access to medications for opioid use disorder remains a significant barrier for patients,” said Dr. Hu.
“Training the next generation of physicians across all specialties is one way that we can work to improve access to care and improve the health and well-being of our patients.”
The study, which is ongoing, included 405 3rd-year medical students at Ohio State. Researchers provided these students with in-person or virtual (during the pandemic) training in buprenorphine prescribing and in-person clinical experience.
Dr. Hu and her colleagues tested the students before and after the intervention and estimated improvement in knowledge (score 0-23) and approach to clinical management principles (1-5).
The investigators found a statistically significant increase in overall knowledge (from a mean total score of 18.34 to 19.32; P < .001). There was also a statistically significant increase in self-reported understanding of clinical management principles related to screening for and treating OUDs (from a mean of 3.12 to a mean of 4.02; P < .001).
An additional evaluation survey was completed by 162 students at the end of the program. About 83% of these students said they knew how to manage acute pain, 62% felt they knew how to manage chronic pain, and 77% agreed they knew how to screen a patient for OUD.
Dr. Hu noted 3rd-year medical students are a little over halfway through medical school, after which they will go into residency in various specialties. Providing them with this knowledge early on allows them to incorporate it as they continue their training, she said.
“If they are able to screen their patients in any specialty they eventually choose to go into, then they can help link these patients to resources early and make sure there aren’t patients who are slipping through the cracks.”
Worthwhile, important research
Howard Liu, MD, chair of the department of psychiatry at the University of Nebraska Medical Center, Omaha, and incoming chair of the APA’s Council on Communications, applauded the study.
The proposed curriculum, he said, instills confidence in students and teaches important lessons they can apply no matter what field they choose.
Dr. Liu, who moderated a press briefing highlighting the study, noted every state is affected differently by the opioid epidemic, but the shortage of appropriate treatments for OUD is nationwide.
Commenting on the study, addiction specialist Elie G. Aoun, MD, of the division of law, medicine, and psychiatry at Columbia University, New York, said this research is “very worthwhile and important.”
He noted that attitudes about addiction need to change. When he taught medical students about substance use disorders, he was struck by some of their negative beliefs about addiction. For example, considering addicts as “junkies” who are “taking resources away” from what they perceive as more deserving patients.
Addiction has been ignored in medicine for too long, added Dr. Aoun. He noted the requirement for addiction training for psychiatry residents is 2 months while they spend 4 months learning internal medicine. “That makes no sense,” he said.
“And now with the opioid epidemic, we’re faced with the consequences of dismissing addiction for such a long time.”
A lack of understanding about addiction, and the “very limited number” of experienced people treating addictions, has contributed to the “huge problem” experts now face in treating addictions, said Dr. Aoun.
“So you want to approach this problem from as many different angles as you can.”
He praised the study for presenting “a framework to ‘medicalize’ the addiction model” for students. This, he said, will help them build empathy and see those with a substance use disorder as no different from other patients with medical conditions.
A curriculum such as the one presented by Dr. Hu and colleagues may spur more medical students into the addiction field, he said. “It may make them more willing to treat patients with addiction using evidence-based medicine rather than dismissing them.”
The study was supported by the Substance Abuse and Mental Health Services Administration.
A version of this article first appeared on Medscape.com.
FROM APA 2022
Multiple mental health woes? Blame it on genetics
Investigators conducted a genetic analysis of 11 major psychiatric disorders, including schizophrenia and bipolar disorder.
“Our findings confirm that high comorbidity across some disorders in part reflects overlapping pathways of genetic risk,” lead author Andrew Grotzinger, PhD, department of psychology and neuroscience, University of Colorado at Boulder, said in a press release.
The results could lead to the development of treatments that address multiple psychiatric disorders at once and help reshape the way diagnoses are established, the researchers note.
The findings were published online in Nature Genetics.
Common genetic patterns
Using the massive UK Biobank and the Psychiatric Genomics Consortium, the researchers applied novel statistical genetic methods to identify common patterns across 11 major psychiatric disorders: schizophrenia, bipolar disorder, major depressive disorder, anxiety disorder, anorexia nervosa, obsessive-compulsive disorder (OCD), Tourette syndrome, post traumatic stress disorder, problematic alcohol use, attention deficit hyperactive disorder, and autism.
The average total sample size per disorder was 156,771 participants, with a range of 9,725 to 802,939 participants.
In all, the investigators identified 152 genetic variants shared across multiple disorders, including those already known to influence certain types of brain cells.
For example, they found that 70% of the genetic signal associated with schizophrenia was also associated with bipolar disorder.
Results also showed that anorexia nervosa and OCD have a strong, shared genetic architecture and that individuals with a genetic predisposition to low body mass index also tend to have a genetic predisposition to these two disorders.
Not surprisingly, the researchers note, there was a large genetic overlap between anxiety disorder and major depressive disorder.
They also observed that psychiatric disorders that tend to cluster together also tend to share genes that influence how and when individuals are physically active during the day.
For example, patients with internalizing disorders such as anxiety and depression tend to have a genetic architecture associated with low movement throughout the day. On the other hand, those with OCD and anorexia tend to have genes associated with higher movement throughout the day.
“When you think about it, it makes sense,” said Dr. Grotzinger. Depressed individuals often experience fatigue or low energy while those with compulsive disorders may have a tough time sitting still, he noted.
One treatment for multiple disorders?
“Collectively, these results offer key insights into the shared and disorder-specific mechanisms of genetic risk for psychiatric disease,” the investigators write.
Their research is also a first step toward developing therapies that can address multiple disorders with one treatment, they add.
“People are more likely today to be prescribed multiple medications intended to treat multiple diagnoses, and in some instances those medicines can have side effects,” Dr. Grotzinger said.
“By identifying what is shared across these issues, we can hopefully come up with ways to target them in a different way that doesn’t require four separate pills or four separate psychotherapy interventions,” he added.
Dr. Grotzinger noted that, for now, the knowledge that genetics are underlying their disorders may provide comfort to some patients.
“It’s important for people to know that they didn’t just get a terrible roll of the dice in life – that they are not facing multiple different issues but rather one set of risk factors bleeding into them all,” he said.
This research had no commercial funding. Dr. Grotzinger reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Investigators conducted a genetic analysis of 11 major psychiatric disorders, including schizophrenia and bipolar disorder.
“Our findings confirm that high comorbidity across some disorders in part reflects overlapping pathways of genetic risk,” lead author Andrew Grotzinger, PhD, department of psychology and neuroscience, University of Colorado at Boulder, said in a press release.
The results could lead to the development of treatments that address multiple psychiatric disorders at once and help reshape the way diagnoses are established, the researchers note.
The findings were published online in Nature Genetics.
Common genetic patterns
Using the massive UK Biobank and the Psychiatric Genomics Consortium, the researchers applied novel statistical genetic methods to identify common patterns across 11 major psychiatric disorders: schizophrenia, bipolar disorder, major depressive disorder, anxiety disorder, anorexia nervosa, obsessive-compulsive disorder (OCD), Tourette syndrome, post traumatic stress disorder, problematic alcohol use, attention deficit hyperactive disorder, and autism.
The average total sample size per disorder was 156,771 participants, with a range of 9,725 to 802,939 participants.
In all, the investigators identified 152 genetic variants shared across multiple disorders, including those already known to influence certain types of brain cells.
For example, they found that 70% of the genetic signal associated with schizophrenia was also associated with bipolar disorder.
Results also showed that anorexia nervosa and OCD have a strong, shared genetic architecture and that individuals with a genetic predisposition to low body mass index also tend to have a genetic predisposition to these two disorders.
Not surprisingly, the researchers note, there was a large genetic overlap between anxiety disorder and major depressive disorder.
They also observed that psychiatric disorders that tend to cluster together also tend to share genes that influence how and when individuals are physically active during the day.
For example, patients with internalizing disorders such as anxiety and depression tend to have a genetic architecture associated with low movement throughout the day. On the other hand, those with OCD and anorexia tend to have genes associated with higher movement throughout the day.
“When you think about it, it makes sense,” said Dr. Grotzinger. Depressed individuals often experience fatigue or low energy while those with compulsive disorders may have a tough time sitting still, he noted.
One treatment for multiple disorders?
“Collectively, these results offer key insights into the shared and disorder-specific mechanisms of genetic risk for psychiatric disease,” the investigators write.
Their research is also a first step toward developing therapies that can address multiple disorders with one treatment, they add.
“People are more likely today to be prescribed multiple medications intended to treat multiple diagnoses, and in some instances those medicines can have side effects,” Dr. Grotzinger said.
“By identifying what is shared across these issues, we can hopefully come up with ways to target them in a different way that doesn’t require four separate pills or four separate psychotherapy interventions,” he added.
Dr. Grotzinger noted that, for now, the knowledge that genetics are underlying their disorders may provide comfort to some patients.
“It’s important for people to know that they didn’t just get a terrible roll of the dice in life – that they are not facing multiple different issues but rather one set of risk factors bleeding into them all,” he said.
This research had no commercial funding. Dr. Grotzinger reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Investigators conducted a genetic analysis of 11 major psychiatric disorders, including schizophrenia and bipolar disorder.
“Our findings confirm that high comorbidity across some disorders in part reflects overlapping pathways of genetic risk,” lead author Andrew Grotzinger, PhD, department of psychology and neuroscience, University of Colorado at Boulder, said in a press release.
The results could lead to the development of treatments that address multiple psychiatric disorders at once and help reshape the way diagnoses are established, the researchers note.
The findings were published online in Nature Genetics.
Common genetic patterns
Using the massive UK Biobank and the Psychiatric Genomics Consortium, the researchers applied novel statistical genetic methods to identify common patterns across 11 major psychiatric disorders: schizophrenia, bipolar disorder, major depressive disorder, anxiety disorder, anorexia nervosa, obsessive-compulsive disorder (OCD), Tourette syndrome, post traumatic stress disorder, problematic alcohol use, attention deficit hyperactive disorder, and autism.
The average total sample size per disorder was 156,771 participants, with a range of 9,725 to 802,939 participants.
In all, the investigators identified 152 genetic variants shared across multiple disorders, including those already known to influence certain types of brain cells.
For example, they found that 70% of the genetic signal associated with schizophrenia was also associated with bipolar disorder.
Results also showed that anorexia nervosa and OCD have a strong, shared genetic architecture and that individuals with a genetic predisposition to low body mass index also tend to have a genetic predisposition to these two disorders.
Not surprisingly, the researchers note, there was a large genetic overlap between anxiety disorder and major depressive disorder.
They also observed that psychiatric disorders that tend to cluster together also tend to share genes that influence how and when individuals are physically active during the day.
For example, patients with internalizing disorders such as anxiety and depression tend to have a genetic architecture associated with low movement throughout the day. On the other hand, those with OCD and anorexia tend to have genes associated with higher movement throughout the day.
“When you think about it, it makes sense,” said Dr. Grotzinger. Depressed individuals often experience fatigue or low energy while those with compulsive disorders may have a tough time sitting still, he noted.
One treatment for multiple disorders?
“Collectively, these results offer key insights into the shared and disorder-specific mechanisms of genetic risk for psychiatric disease,” the investigators write.
Their research is also a first step toward developing therapies that can address multiple disorders with one treatment, they add.
“People are more likely today to be prescribed multiple medications intended to treat multiple diagnoses, and in some instances those medicines can have side effects,” Dr. Grotzinger said.
“By identifying what is shared across these issues, we can hopefully come up with ways to target them in a different way that doesn’t require four separate pills or four separate psychotherapy interventions,” he added.
Dr. Grotzinger noted that, for now, the knowledge that genetics are underlying their disorders may provide comfort to some patients.
“It’s important for people to know that they didn’t just get a terrible roll of the dice in life – that they are not facing multiple different issues but rather one set of risk factors bleeding into them all,” he said.
This research had no commercial funding. Dr. Grotzinger reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM NATURE GENETICS
Exploding e-cigarettes cause traumatic injuries in teens
A study shows that, over a 4-year period, 15 teenagers were injured from exploding e-cigarettes, according to surgeons who have treated young people at nine hospitals in the United States.
“It definitely was an injury we were seeing frequently,” Shannon Acker, MD, an assistant professor of pediatric surgery at the University of Colorado at Denver, Aurora, and a pediatric surgeon at Children’s Hospital Colorado, said in a statement.
Reporting in the Journal of Surgical Research, doctors detail injuries from e-cigarette explosions from January 2016 through December 2019. Ten teens were hospitalized, including three who were admitted to ICUs.
“When we think about e-cigarettes, vaping, and the problems of marketing cigarettes to teenagers, it usually has to do with addiction and lung injury,” said Dr. Acker, a coauthor of the new study. “Whereas we, as trauma surgeons, were seeing these other traumatic injuries.”
Six of the teens had facial burns, five of them lost multiple teeth, five had burns around the thighs and groin, four burned their hands, and four burned their eyes. One teen injured their radial nerve, which runs through the arm. Another cut their face, and one fractured their jaw.
Overall, six teens needed surgery, including one who needed multiple operations for a severe hand injury.
Three of the teenagers had never used e-cigarettes before the day they were hurt.
Vaping has become far more common than smoking traditional cigarettes among U.S. teens in recent years. More than 2 million of them currently use e-cigarettes, according to the Food and Drug Administration, including more than 11% of high school students and almost 3% of middle schoolers.
Most e-cigarettes contain nicotine, which is highly addictive and can impair healthy brain development in adolescents, according to the CDC. Other chemicals and flavorings in the liquids that are heated during vaping can also damage the lungs. Fires and explosions, while rare, are also a risk that’s been previously documented by the FDA, the Centers for Disease Control and Prevention, and the Federal Emergency Management Agency.
Nationwide, there were 195 reported explosions and fires involving e-cigarettes in all ages between 2009 and 2016, according to a FEMA report. While no deaths were reported, 29% of these cases involved severe injuries.
“The shape and construction of electronic cigarettes” can make them behave like “flaming rockets when a battery fails,” according to FEMA.
Vaping devices typically use a rechargeable lithium-ion battery that vaporizes the liquid nicotine solution, Dr. Acker said. “They are not highly regulated, and the batteries may be of inferior quality and prone to explosion.”
A version of this article first appeared on WebMD.com.
A study shows that, over a 4-year period, 15 teenagers were injured from exploding e-cigarettes, according to surgeons who have treated young people at nine hospitals in the United States.
“It definitely was an injury we were seeing frequently,” Shannon Acker, MD, an assistant professor of pediatric surgery at the University of Colorado at Denver, Aurora, and a pediatric surgeon at Children’s Hospital Colorado, said in a statement.
Reporting in the Journal of Surgical Research, doctors detail injuries from e-cigarette explosions from January 2016 through December 2019. Ten teens were hospitalized, including three who were admitted to ICUs.
“When we think about e-cigarettes, vaping, and the problems of marketing cigarettes to teenagers, it usually has to do with addiction and lung injury,” said Dr. Acker, a coauthor of the new study. “Whereas we, as trauma surgeons, were seeing these other traumatic injuries.”
Six of the teens had facial burns, five of them lost multiple teeth, five had burns around the thighs and groin, four burned their hands, and four burned their eyes. One teen injured their radial nerve, which runs through the arm. Another cut their face, and one fractured their jaw.
Overall, six teens needed surgery, including one who needed multiple operations for a severe hand injury.
Three of the teenagers had never used e-cigarettes before the day they were hurt.
Vaping has become far more common than smoking traditional cigarettes among U.S. teens in recent years. More than 2 million of them currently use e-cigarettes, according to the Food and Drug Administration, including more than 11% of high school students and almost 3% of middle schoolers.
Most e-cigarettes contain nicotine, which is highly addictive and can impair healthy brain development in adolescents, according to the CDC. Other chemicals and flavorings in the liquids that are heated during vaping can also damage the lungs. Fires and explosions, while rare, are also a risk that’s been previously documented by the FDA, the Centers for Disease Control and Prevention, and the Federal Emergency Management Agency.
Nationwide, there were 195 reported explosions and fires involving e-cigarettes in all ages between 2009 and 2016, according to a FEMA report. While no deaths were reported, 29% of these cases involved severe injuries.
“The shape and construction of electronic cigarettes” can make them behave like “flaming rockets when a battery fails,” according to FEMA.
Vaping devices typically use a rechargeable lithium-ion battery that vaporizes the liquid nicotine solution, Dr. Acker said. “They are not highly regulated, and the batteries may be of inferior quality and prone to explosion.”
A version of this article first appeared on WebMD.com.
A study shows that, over a 4-year period, 15 teenagers were injured from exploding e-cigarettes, according to surgeons who have treated young people at nine hospitals in the United States.
“It definitely was an injury we were seeing frequently,” Shannon Acker, MD, an assistant professor of pediatric surgery at the University of Colorado at Denver, Aurora, and a pediatric surgeon at Children’s Hospital Colorado, said in a statement.
Reporting in the Journal of Surgical Research, doctors detail injuries from e-cigarette explosions from January 2016 through December 2019. Ten teens were hospitalized, including three who were admitted to ICUs.
“When we think about e-cigarettes, vaping, and the problems of marketing cigarettes to teenagers, it usually has to do with addiction and lung injury,” said Dr. Acker, a coauthor of the new study. “Whereas we, as trauma surgeons, were seeing these other traumatic injuries.”
Six of the teens had facial burns, five of them lost multiple teeth, five had burns around the thighs and groin, four burned their hands, and four burned their eyes. One teen injured their radial nerve, which runs through the arm. Another cut their face, and one fractured their jaw.
Overall, six teens needed surgery, including one who needed multiple operations for a severe hand injury.
Three of the teenagers had never used e-cigarettes before the day they were hurt.
Vaping has become far more common than smoking traditional cigarettes among U.S. teens in recent years. More than 2 million of them currently use e-cigarettes, according to the Food and Drug Administration, including more than 11% of high school students and almost 3% of middle schoolers.
Most e-cigarettes contain nicotine, which is highly addictive and can impair healthy brain development in adolescents, according to the CDC. Other chemicals and flavorings in the liquids that are heated during vaping can also damage the lungs. Fires and explosions, while rare, are also a risk that’s been previously documented by the FDA, the Centers for Disease Control and Prevention, and the Federal Emergency Management Agency.
Nationwide, there were 195 reported explosions and fires involving e-cigarettes in all ages between 2009 and 2016, according to a FEMA report. While no deaths were reported, 29% of these cases involved severe injuries.
“The shape and construction of electronic cigarettes” can make them behave like “flaming rockets when a battery fails,” according to FEMA.
Vaping devices typically use a rechargeable lithium-ion battery that vaporizes the liquid nicotine solution, Dr. Acker said. “They are not highly regulated, and the batteries may be of inferior quality and prone to explosion.”
A version of this article first appeared on WebMD.com.
FROM THE JOURNAL OF SURGICAL RESEARCH
Treatment for alcohol abuse reduces hepatitis readmission
SAN DIEGO – Treating people with alcoholic hepatitis for alcohol abuse may reduce their risk of hospital readmission, researchers reported.
In a retrospective analysis of nationwide data, 7.83% of those patients who received psychotherapy, counseling, or drug treatment for alcohol abuse were readmitted within 30 days, versus 11.67% of those who did not receive these kinds of treatment.
The finding lends support to the argument that hospitals should invest more in the treatments, despite the complexities involved.
“It takes a multidisciplinary approach, starting from the physician or the health care provider along with the pharmacists, the behavioral health specialists, or a psychiatrist or psychologist, along with case management as well,” said Harleen Chela, MD, a third-year resident at the University of Missouri in Columbia. She presented the findings at the annual Digestive Disease Week® (DDW).
The researchers started with the premise that patients with alcoholic hepatitis can prevent the condition from worsening by abstaining from alcohol. To see whether interventions aimed at encouraging that abstention could prevent readmissions, Dr. Chela and colleagues analyzed data on readmissions for the first 11 months of the year 2018.
They included patients who were at least 18 years of age and who had a nonelective admission with a principal diagnosis of alcohol abuse.
Using procedure codes, they compared those patients given psychotherapy (including cognitive behavioral therapy), formal inpatient counseling, and drug treatment for alcohol abuse to those who didn’t. Then they counted how many patients were readmitted within 30 days.
They found records of 45,617 patients admitted for alcoholic hepatitis of whom 1,552 received treatment for alcohol abuse and 44,065 did not.
They did not find any significant difference between the two groups in demographics, income, or insurance status.
Adjusting for such factors, the researchers found that people who received alcohol abuse treatment were 64% as likely to be readmitted as were those who did not (hazard ratio, 0.64; 95% confidence interval, 0.46-0.91; P = 0.01).
If alcohol abuse treatment is so effective, why isn’t it routine? “It’s not always feasible to implement this, on the inpatient side, because it takes more than a day or two just to get some of these things put in place,” Dr. Chela told this news organization.
They did find that people were more likely to get treatment for alcohol abuse if they were admitted to a hospital in a big city rather than a small town and if their hospital was owned by private investors rather than by a not-for-profit organization or the government.
“Larger hospitals and private sector institutions have more access to resources and money to have those kinds of systems in place for the patients,” said Dr. Chela.
She became interested in the issue at her hospital when she noticed that patients with alcoholic hepatitis were not getting behavioral counseling. “The inpatient load in the behavioral health side is so much that they don’t have time for these kinds of consults,” she said. “That’s one of the challenges: A shortage of behavioral specialists like psychiatrists.”
And hospitals tend to focus on treating conditions that threaten their patients’ lives in the short term. “Someone who has a heart attack or a gastrointestinal bleed – there’s more focus on resources for those kinds of patients,” she said.
Virginia Commonwealth University in Richmond provides alcohol abuse treatment to patients with alcoholic hepatitis partly using telehealth, said Richard Sterling, MD, MSc, chief of hepatology, who was not involved in the study. “For people who live too far away, don’t have transportation, or have other health disparities, we now have technology and mechanisms to keep them engaged in care,” he told this news organization. “We’re doing a lot of Zoom visits.”
Dr. Chela and colleagues also found that those who got alcohol abuse treatment were less likely to be discharged to a skilled nursing facility or to home health. The data couldn’t give the researchers a definitive reason for this, but Dr. Chela speculated that the patients who received treatment for alcohol abuse stayed longer in the hospital and may have been in better shape when they were discharged.
The U.S. health care system doesn’t necessarily provide incentives to keep patients healthy, Dr. Sterling said. “Hospital systems make money off of filling beds, and providing a lot of inpatient care and hospital days,” he said. “That may be not necessarily congruent with a health system that is supposed to provide health for these covered lives.”
Neither Dr. Chela nor Dr. Sterling reported any relevant financial relationships.
SAN DIEGO – Treating people with alcoholic hepatitis for alcohol abuse may reduce their risk of hospital readmission, researchers reported.
In a retrospective analysis of nationwide data, 7.83% of those patients who received psychotherapy, counseling, or drug treatment for alcohol abuse were readmitted within 30 days, versus 11.67% of those who did not receive these kinds of treatment.
The finding lends support to the argument that hospitals should invest more in the treatments, despite the complexities involved.
“It takes a multidisciplinary approach, starting from the physician or the health care provider along with the pharmacists, the behavioral health specialists, or a psychiatrist or psychologist, along with case management as well,” said Harleen Chela, MD, a third-year resident at the University of Missouri in Columbia. She presented the findings at the annual Digestive Disease Week® (DDW).
The researchers started with the premise that patients with alcoholic hepatitis can prevent the condition from worsening by abstaining from alcohol. To see whether interventions aimed at encouraging that abstention could prevent readmissions, Dr. Chela and colleagues analyzed data on readmissions for the first 11 months of the year 2018.
They included patients who were at least 18 years of age and who had a nonelective admission with a principal diagnosis of alcohol abuse.
Using procedure codes, they compared those patients given psychotherapy (including cognitive behavioral therapy), formal inpatient counseling, and drug treatment for alcohol abuse to those who didn’t. Then they counted how many patients were readmitted within 30 days.
They found records of 45,617 patients admitted for alcoholic hepatitis of whom 1,552 received treatment for alcohol abuse and 44,065 did not.
They did not find any significant difference between the two groups in demographics, income, or insurance status.
Adjusting for such factors, the researchers found that people who received alcohol abuse treatment were 64% as likely to be readmitted as were those who did not (hazard ratio, 0.64; 95% confidence interval, 0.46-0.91; P = 0.01).
If alcohol abuse treatment is so effective, why isn’t it routine? “It’s not always feasible to implement this, on the inpatient side, because it takes more than a day or two just to get some of these things put in place,” Dr. Chela told this news organization.
They did find that people were more likely to get treatment for alcohol abuse if they were admitted to a hospital in a big city rather than a small town and if their hospital was owned by private investors rather than by a not-for-profit organization or the government.
“Larger hospitals and private sector institutions have more access to resources and money to have those kinds of systems in place for the patients,” said Dr. Chela.
She became interested in the issue at her hospital when she noticed that patients with alcoholic hepatitis were not getting behavioral counseling. “The inpatient load in the behavioral health side is so much that they don’t have time for these kinds of consults,” she said. “That’s one of the challenges: A shortage of behavioral specialists like psychiatrists.”
And hospitals tend to focus on treating conditions that threaten their patients’ lives in the short term. “Someone who has a heart attack or a gastrointestinal bleed – there’s more focus on resources for those kinds of patients,” she said.
Virginia Commonwealth University in Richmond provides alcohol abuse treatment to patients with alcoholic hepatitis partly using telehealth, said Richard Sterling, MD, MSc, chief of hepatology, who was not involved in the study. “For people who live too far away, don’t have transportation, or have other health disparities, we now have technology and mechanisms to keep them engaged in care,” he told this news organization. “We’re doing a lot of Zoom visits.”
Dr. Chela and colleagues also found that those who got alcohol abuse treatment were less likely to be discharged to a skilled nursing facility or to home health. The data couldn’t give the researchers a definitive reason for this, but Dr. Chela speculated that the patients who received treatment for alcohol abuse stayed longer in the hospital and may have been in better shape when they were discharged.
The U.S. health care system doesn’t necessarily provide incentives to keep patients healthy, Dr. Sterling said. “Hospital systems make money off of filling beds, and providing a lot of inpatient care and hospital days,” he said. “That may be not necessarily congruent with a health system that is supposed to provide health for these covered lives.”
Neither Dr. Chela nor Dr. Sterling reported any relevant financial relationships.
SAN DIEGO – Treating people with alcoholic hepatitis for alcohol abuse may reduce their risk of hospital readmission, researchers reported.
In a retrospective analysis of nationwide data, 7.83% of those patients who received psychotherapy, counseling, or drug treatment for alcohol abuse were readmitted within 30 days, versus 11.67% of those who did not receive these kinds of treatment.
The finding lends support to the argument that hospitals should invest more in the treatments, despite the complexities involved.
“It takes a multidisciplinary approach, starting from the physician or the health care provider along with the pharmacists, the behavioral health specialists, or a psychiatrist or psychologist, along with case management as well,” said Harleen Chela, MD, a third-year resident at the University of Missouri in Columbia. She presented the findings at the annual Digestive Disease Week® (DDW).
The researchers started with the premise that patients with alcoholic hepatitis can prevent the condition from worsening by abstaining from alcohol. To see whether interventions aimed at encouraging that abstention could prevent readmissions, Dr. Chela and colleagues analyzed data on readmissions for the first 11 months of the year 2018.
They included patients who were at least 18 years of age and who had a nonelective admission with a principal diagnosis of alcohol abuse.
Using procedure codes, they compared those patients given psychotherapy (including cognitive behavioral therapy), formal inpatient counseling, and drug treatment for alcohol abuse to those who didn’t. Then they counted how many patients were readmitted within 30 days.
They found records of 45,617 patients admitted for alcoholic hepatitis of whom 1,552 received treatment for alcohol abuse and 44,065 did not.
They did not find any significant difference between the two groups in demographics, income, or insurance status.
Adjusting for such factors, the researchers found that people who received alcohol abuse treatment were 64% as likely to be readmitted as were those who did not (hazard ratio, 0.64; 95% confidence interval, 0.46-0.91; P = 0.01).
If alcohol abuse treatment is so effective, why isn’t it routine? “It’s not always feasible to implement this, on the inpatient side, because it takes more than a day or two just to get some of these things put in place,” Dr. Chela told this news organization.
They did find that people were more likely to get treatment for alcohol abuse if they were admitted to a hospital in a big city rather than a small town and if their hospital was owned by private investors rather than by a not-for-profit organization or the government.
“Larger hospitals and private sector institutions have more access to resources and money to have those kinds of systems in place for the patients,” said Dr. Chela.
She became interested in the issue at her hospital when she noticed that patients with alcoholic hepatitis were not getting behavioral counseling. “The inpatient load in the behavioral health side is so much that they don’t have time for these kinds of consults,” she said. “That’s one of the challenges: A shortage of behavioral specialists like psychiatrists.”
And hospitals tend to focus on treating conditions that threaten their patients’ lives in the short term. “Someone who has a heart attack or a gastrointestinal bleed – there’s more focus on resources for those kinds of patients,” she said.
Virginia Commonwealth University in Richmond provides alcohol abuse treatment to patients with alcoholic hepatitis partly using telehealth, said Richard Sterling, MD, MSc, chief of hepatology, who was not involved in the study. “For people who live too far away, don’t have transportation, or have other health disparities, we now have technology and mechanisms to keep them engaged in care,” he told this news organization. “We’re doing a lot of Zoom visits.”
Dr. Chela and colleagues also found that those who got alcohol abuse treatment were less likely to be discharged to a skilled nursing facility or to home health. The data couldn’t give the researchers a definitive reason for this, but Dr. Chela speculated that the patients who received treatment for alcohol abuse stayed longer in the hospital and may have been in better shape when they were discharged.
The U.S. health care system doesn’t necessarily provide incentives to keep patients healthy, Dr. Sterling said. “Hospital systems make money off of filling beds, and providing a lot of inpatient care and hospital days,” he said. “That may be not necessarily congruent with a health system that is supposed to provide health for these covered lives.”
Neither Dr. Chela nor Dr. Sterling reported any relevant financial relationships.
AT DDW 2022
Cannabis vaping continues its rise in teens
More teenagers in the United States reported cannabis use with vaping in 2019, compared with 2017, while cannabis use without vaping declined, based on annual survey data from more than 50,000 teens.
“With vaping prevalence rising so quickly among teens, getting a clearer picture of how cannabis use is shifting helps inform prevention and cessation efforts,” corresponding author Noah T. Kreski, MPH, of Columbia University, New York, said in an interview.
“In just 2 years, the most common cannabis use pattern changed from ‘occasional use without vaping’ to ‘frequent use with vaping,’ said Mx. Kreski, who uses the honorific Mx. and the pronouns they/them. “Knowing that, as well as the high overlap of cannabis vaping with nicotine use and binge drinking, adds to the urgency of reducing adolescent vaping.”
To quantify the trends in cannabis vaping, the researchers reviewed data from Monitoring the Future, an annual survey of high school students across the United States. The study population included 51,052 individuals; approximately 49% were male and 49% were non-Hispanic White. The researchers examined frequency of cannabis use, trends across demographic groups, and concurrent use of cannabis and other substances such as alcohol and tobacco. The findings were published in the journal Addiction.
Frequent cannabis use was defined as six or more times in the past 30 days; occasional use was defined as one to five times in the past 30 days.
Frequent cannabis use with vaping increased from 2.1% in 2017 to 5.4% in 2019. Occasional cannabis use with vaping also increased, though less dramatically, from less than 2% in 2017 to approximately 3.5% in 2019.
By contrast, both frequent and occasional cannabis use without vaping declined from 2017 to 2019 (from 3.8% to 2.1% and from 6.9% to 4.4%, respectively).
Overall, the prevalence of any level of cannabis use increased from 13.9% in 2017 to 15.4% in 2019. Both males and females showed a similar increase in reported frequent cannabis use with vaping of approximately 3%.
The results document that vaping cannabis has become more common than smoking alone among U.S. teens across almost all demographic groups, and across sex, race, urbanicity, and level of parent education; however, the increased was especially marked among Hispanic/Latinx teens and those of lower socioeconomic status, the researchers wrote.
The researchers also examined the associations between cannabis use with and without vaping and concurrent nicotine and alcohol use. Overall, the strongest association was between smoking or vaping nicotine and vaping cannabis; teens who smoked or vaped nicotine were 42 times more likely than nonnicotine users to report vaping cannabis in the past 30 days (adjusted odds ratio, 42.28). In addition, more occasions of binge drinking were more strongly associated with cannabis use with vaping (up to 10 times more likely), compared with cannabis use without vaping, (aORs, 4.48-10.09).
The study findings were limited by several factors, including the lack of questions on tetrahydrocannabinol (THC) or cannabidiol content of the cannabis products used, although evidence suggests that the potency of cannabis products in the United States is increasing, the researchers noted. Other limitations included the cross-sectional design, which prevents making associations about causality, and lack of data on the quantity of cannabis used; only data on frequency of use were recorded.
However, the results reflect a rise in cannabis use with vaping among teens in the United States, along with an increased risk of tobacco use, e-cigarette use, and binge drinking, the researchers said.
As cannabis legalization expands across the United States, policies are needed to deter use among adolescents, the researchers wrote. “These policies should be crafted to reduce an emphasis on criminalization in preference for public health promotion given the history of unequal application of punitive consequences of drug use for racialized minorities in the United States. As products, delivery systems, potency, and marketing proliferate within a for-profit industry, increased attention to youth trends, including investment in sustained and evidence-based prevention and intervention, is increasingly necessary.”
The take-home message for clinicians is to ask whether your patients are vaping, because the prevalence is not only up, but fairly universal, Mx. Kreski said. “Have a discussion that covers a broad range of substance use topics and informs teens of the potential risks of vaping, while avoiding stigma.”
The message for parents is “to talk to your kids about the risks of vaping,” said Mx. Kreski. “Prioritize open communication rather than punishment, and work together with your teens to prevent or reduce vaping.” The message for teens: “Understand that vaping has risks. You should feel empowered to talk to your parents or doctor about those risks. While it may seem like everyone’s vaping, the majority don’t. Keeping communication open between parents/caregivers, teens, and health care providers is one of the best ways to address these trends in vaping.”
Beware more powerful cannabis products
“While drug use in general is declining in adolescents, marijuana use remains very common,” Kelly A. Curran, MD, of the University of Oklahoma Health Sciences Center, Oklahoma City, said in an interview.
“There is growing evidence that marijuana is now the first drug used by adolescents – replacing alcohol and nicotine – and frequent use can lead to substance abuse,” said Dr. Curran, who specializes in adolescent medicine but was involved in the study. “Cannabis use patterns have evolved over time. As I frequently tell my patients and their families, new strains and hybrids of marijuana have higher potencies of THC. Many adolescents are eschewing smoking and in its place using marijuana concentrates (wax, oil, shatter) via vape, dab pen, or rig. Use of these methods puts adolescents at high risk of social and health complications such as [e-cigarette or vaping use-associated lung injury], cannabis hyperemesis syndrome, and psychosis – and understanding these patterns and associated drug use helps health care professionals and parents keep adolescents safe.”
The take-home message for clinicians is that marijuana use via vaping continues to rise and to become more common than “traditional” marijuana smoking, Dr. Curran said. “This increase is across genders, in nearly all race/ethnicities (especially in Latinx youth), and in youth from lower socioeconomic status.” Vaping marijuana is associated with other substance abuse, so health care professionals should include questions about different forms of marijuana use, such as vape, dab pen, or rig, when working with patients, and counsel patients and families about the risks associated with use of any of these products.
The study was supported by the National Center for Injury Prevention and Control and by the National Institute on Drug Abuse. The researchers had no financial conflicts to disclose. Dr. Curran had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
More teenagers in the United States reported cannabis use with vaping in 2019, compared with 2017, while cannabis use without vaping declined, based on annual survey data from more than 50,000 teens.
“With vaping prevalence rising so quickly among teens, getting a clearer picture of how cannabis use is shifting helps inform prevention and cessation efforts,” corresponding author Noah T. Kreski, MPH, of Columbia University, New York, said in an interview.
“In just 2 years, the most common cannabis use pattern changed from ‘occasional use without vaping’ to ‘frequent use with vaping,’ said Mx. Kreski, who uses the honorific Mx. and the pronouns they/them. “Knowing that, as well as the high overlap of cannabis vaping with nicotine use and binge drinking, adds to the urgency of reducing adolescent vaping.”
To quantify the trends in cannabis vaping, the researchers reviewed data from Monitoring the Future, an annual survey of high school students across the United States. The study population included 51,052 individuals; approximately 49% were male and 49% were non-Hispanic White. The researchers examined frequency of cannabis use, trends across demographic groups, and concurrent use of cannabis and other substances such as alcohol and tobacco. The findings were published in the journal Addiction.
Frequent cannabis use was defined as six or more times in the past 30 days; occasional use was defined as one to five times in the past 30 days.
Frequent cannabis use with vaping increased from 2.1% in 2017 to 5.4% in 2019. Occasional cannabis use with vaping also increased, though less dramatically, from less than 2% in 2017 to approximately 3.5% in 2019.
By contrast, both frequent and occasional cannabis use without vaping declined from 2017 to 2019 (from 3.8% to 2.1% and from 6.9% to 4.4%, respectively).
Overall, the prevalence of any level of cannabis use increased from 13.9% in 2017 to 15.4% in 2019. Both males and females showed a similar increase in reported frequent cannabis use with vaping of approximately 3%.
The results document that vaping cannabis has become more common than smoking alone among U.S. teens across almost all demographic groups, and across sex, race, urbanicity, and level of parent education; however, the increased was especially marked among Hispanic/Latinx teens and those of lower socioeconomic status, the researchers wrote.
The researchers also examined the associations between cannabis use with and without vaping and concurrent nicotine and alcohol use. Overall, the strongest association was between smoking or vaping nicotine and vaping cannabis; teens who smoked or vaped nicotine were 42 times more likely than nonnicotine users to report vaping cannabis in the past 30 days (adjusted odds ratio, 42.28). In addition, more occasions of binge drinking were more strongly associated with cannabis use with vaping (up to 10 times more likely), compared with cannabis use without vaping, (aORs, 4.48-10.09).
The study findings were limited by several factors, including the lack of questions on tetrahydrocannabinol (THC) or cannabidiol content of the cannabis products used, although evidence suggests that the potency of cannabis products in the United States is increasing, the researchers noted. Other limitations included the cross-sectional design, which prevents making associations about causality, and lack of data on the quantity of cannabis used; only data on frequency of use were recorded.
However, the results reflect a rise in cannabis use with vaping among teens in the United States, along with an increased risk of tobacco use, e-cigarette use, and binge drinking, the researchers said.
As cannabis legalization expands across the United States, policies are needed to deter use among adolescents, the researchers wrote. “These policies should be crafted to reduce an emphasis on criminalization in preference for public health promotion given the history of unequal application of punitive consequences of drug use for racialized minorities in the United States. As products, delivery systems, potency, and marketing proliferate within a for-profit industry, increased attention to youth trends, including investment in sustained and evidence-based prevention and intervention, is increasingly necessary.”
The take-home message for clinicians is to ask whether your patients are vaping, because the prevalence is not only up, but fairly universal, Mx. Kreski said. “Have a discussion that covers a broad range of substance use topics and informs teens of the potential risks of vaping, while avoiding stigma.”
The message for parents is “to talk to your kids about the risks of vaping,” said Mx. Kreski. “Prioritize open communication rather than punishment, and work together with your teens to prevent or reduce vaping.” The message for teens: “Understand that vaping has risks. You should feel empowered to talk to your parents or doctor about those risks. While it may seem like everyone’s vaping, the majority don’t. Keeping communication open between parents/caregivers, teens, and health care providers is one of the best ways to address these trends in vaping.”
Beware more powerful cannabis products
“While drug use in general is declining in adolescents, marijuana use remains very common,” Kelly A. Curran, MD, of the University of Oklahoma Health Sciences Center, Oklahoma City, said in an interview.
“There is growing evidence that marijuana is now the first drug used by adolescents – replacing alcohol and nicotine – and frequent use can lead to substance abuse,” said Dr. Curran, who specializes in adolescent medicine but was involved in the study. “Cannabis use patterns have evolved over time. As I frequently tell my patients and their families, new strains and hybrids of marijuana have higher potencies of THC. Many adolescents are eschewing smoking and in its place using marijuana concentrates (wax, oil, shatter) via vape, dab pen, or rig. Use of these methods puts adolescents at high risk of social and health complications such as [e-cigarette or vaping use-associated lung injury], cannabis hyperemesis syndrome, and psychosis – and understanding these patterns and associated drug use helps health care professionals and parents keep adolescents safe.”
The take-home message for clinicians is that marijuana use via vaping continues to rise and to become more common than “traditional” marijuana smoking, Dr. Curran said. “This increase is across genders, in nearly all race/ethnicities (especially in Latinx youth), and in youth from lower socioeconomic status.” Vaping marijuana is associated with other substance abuse, so health care professionals should include questions about different forms of marijuana use, such as vape, dab pen, or rig, when working with patients, and counsel patients and families about the risks associated with use of any of these products.
The study was supported by the National Center for Injury Prevention and Control and by the National Institute on Drug Abuse. The researchers had no financial conflicts to disclose. Dr. Curran had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
More teenagers in the United States reported cannabis use with vaping in 2019, compared with 2017, while cannabis use without vaping declined, based on annual survey data from more than 50,000 teens.
“With vaping prevalence rising so quickly among teens, getting a clearer picture of how cannabis use is shifting helps inform prevention and cessation efforts,” corresponding author Noah T. Kreski, MPH, of Columbia University, New York, said in an interview.
“In just 2 years, the most common cannabis use pattern changed from ‘occasional use without vaping’ to ‘frequent use with vaping,’ said Mx. Kreski, who uses the honorific Mx. and the pronouns they/them. “Knowing that, as well as the high overlap of cannabis vaping with nicotine use and binge drinking, adds to the urgency of reducing adolescent vaping.”
To quantify the trends in cannabis vaping, the researchers reviewed data from Monitoring the Future, an annual survey of high school students across the United States. The study population included 51,052 individuals; approximately 49% were male and 49% were non-Hispanic White. The researchers examined frequency of cannabis use, trends across demographic groups, and concurrent use of cannabis and other substances such as alcohol and tobacco. The findings were published in the journal Addiction.
Frequent cannabis use was defined as six or more times in the past 30 days; occasional use was defined as one to five times in the past 30 days.
Frequent cannabis use with vaping increased from 2.1% in 2017 to 5.4% in 2019. Occasional cannabis use with vaping also increased, though less dramatically, from less than 2% in 2017 to approximately 3.5% in 2019.
By contrast, both frequent and occasional cannabis use without vaping declined from 2017 to 2019 (from 3.8% to 2.1% and from 6.9% to 4.4%, respectively).
Overall, the prevalence of any level of cannabis use increased from 13.9% in 2017 to 15.4% in 2019. Both males and females showed a similar increase in reported frequent cannabis use with vaping of approximately 3%.
The results document that vaping cannabis has become more common than smoking alone among U.S. teens across almost all demographic groups, and across sex, race, urbanicity, and level of parent education; however, the increased was especially marked among Hispanic/Latinx teens and those of lower socioeconomic status, the researchers wrote.
The researchers also examined the associations between cannabis use with and without vaping and concurrent nicotine and alcohol use. Overall, the strongest association was between smoking or vaping nicotine and vaping cannabis; teens who smoked or vaped nicotine were 42 times more likely than nonnicotine users to report vaping cannabis in the past 30 days (adjusted odds ratio, 42.28). In addition, more occasions of binge drinking were more strongly associated with cannabis use with vaping (up to 10 times more likely), compared with cannabis use without vaping, (aORs, 4.48-10.09).
The study findings were limited by several factors, including the lack of questions on tetrahydrocannabinol (THC) or cannabidiol content of the cannabis products used, although evidence suggests that the potency of cannabis products in the United States is increasing, the researchers noted. Other limitations included the cross-sectional design, which prevents making associations about causality, and lack of data on the quantity of cannabis used; only data on frequency of use were recorded.
However, the results reflect a rise in cannabis use with vaping among teens in the United States, along with an increased risk of tobacco use, e-cigarette use, and binge drinking, the researchers said.
As cannabis legalization expands across the United States, policies are needed to deter use among adolescents, the researchers wrote. “These policies should be crafted to reduce an emphasis on criminalization in preference for public health promotion given the history of unequal application of punitive consequences of drug use for racialized minorities in the United States. As products, delivery systems, potency, and marketing proliferate within a for-profit industry, increased attention to youth trends, including investment in sustained and evidence-based prevention and intervention, is increasingly necessary.”
The take-home message for clinicians is to ask whether your patients are vaping, because the prevalence is not only up, but fairly universal, Mx. Kreski said. “Have a discussion that covers a broad range of substance use topics and informs teens of the potential risks of vaping, while avoiding stigma.”
The message for parents is “to talk to your kids about the risks of vaping,” said Mx. Kreski. “Prioritize open communication rather than punishment, and work together with your teens to prevent or reduce vaping.” The message for teens: “Understand that vaping has risks. You should feel empowered to talk to your parents or doctor about those risks. While it may seem like everyone’s vaping, the majority don’t. Keeping communication open between parents/caregivers, teens, and health care providers is one of the best ways to address these trends in vaping.”
Beware more powerful cannabis products
“While drug use in general is declining in adolescents, marijuana use remains very common,” Kelly A. Curran, MD, of the University of Oklahoma Health Sciences Center, Oklahoma City, said in an interview.
“There is growing evidence that marijuana is now the first drug used by adolescents – replacing alcohol and nicotine – and frequent use can lead to substance abuse,” said Dr. Curran, who specializes in adolescent medicine but was involved in the study. “Cannabis use patterns have evolved over time. As I frequently tell my patients and their families, new strains and hybrids of marijuana have higher potencies of THC. Many adolescents are eschewing smoking and in its place using marijuana concentrates (wax, oil, shatter) via vape, dab pen, or rig. Use of these methods puts adolescents at high risk of social and health complications such as [e-cigarette or vaping use-associated lung injury], cannabis hyperemesis syndrome, and psychosis – and understanding these patterns and associated drug use helps health care professionals and parents keep adolescents safe.”
The take-home message for clinicians is that marijuana use via vaping continues to rise and to become more common than “traditional” marijuana smoking, Dr. Curran said. “This increase is across genders, in nearly all race/ethnicities (especially in Latinx youth), and in youth from lower socioeconomic status.” Vaping marijuana is associated with other substance abuse, so health care professionals should include questions about different forms of marijuana use, such as vape, dab pen, or rig, when working with patients, and counsel patients and families about the risks associated with use of any of these products.
The study was supported by the National Center for Injury Prevention and Control and by the National Institute on Drug Abuse. The researchers had no financial conflicts to disclose. Dr. Curran had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
FROM ADDICTION
Up in smoke: Cannabis-related ED visits increased 18-fold for older Californians
Researchers in California found an 18-fold increase in the rate of cannabis-related trips to the ED visits among adults over age 65 in the state from 2005 to 2019.
Addressing potential harms of cannabis use among older adults, who face heightened risk for adverse reactions to the substance, “is urgently required,” the researchers reported at the annual meeting of the American Geriatrics Society.
The researchers advised doctors to discuss cannabis use with older patients and screen older adults for cannabis use. Those living with multiple chronic conditions and taking multiple medications are especially likely to be at risk for harm, coinvestigator Benjamin Han, MD, MPH, a geriatrician at the University of California, San Diego, said in an interview.
Dr. Han added that “very little” is understood about the risks and benefits of cannabis use in the elderly, and more studies are needed “so that clinicians can have data-informed discussions with their patients.”
California legalized medical marijuana in 1996 and recreational marijuana in 2016.
The researchers used diagnostic code data from California’s nonmilitary acute care hospitals, collected by the state’s Department of Healthcare Access and Information, to calculate annual rates of cannabis-related visits per 10,000 ED visits.
ED trips up sharply among older adults
Rates of cannabis-related visits increased significantly for all older adult age ranges (P < .001), according to the researchers. Among those aged 65-74 years, the rate increased about 15-fold, from 44.9 per 10,000 visits in 2005 to 714.5 per 100,000 in 2019; for ages 75-84, the rate increased about 22-fold, from 8.4 to 193.9 per 10,000; and for those 85 and older the rate jumped nearly 18-fold, from 2.1 to 39.2 per 10,000.
The greatest increase occurred in visits categorized in diagnostic codes as cannabis abuse and unspecified use. Cannabis dependence and cannabis poisoning accounted for only a small fraction of cases, the investigators found.
The researchers did not have data on specific reasons for a visit, or whether patients had smoked or ingested marijuana products. They also could not discern whether patients had used delta-9-tetrahydrocannabinol, which has psychoactive properties, or cannabidiol, which typically does not have the same mind-altering effects.
Dr. Han said the data may not present a full picture of marijuana-related ED visits. “It is important to recognize that older adults have lived through the very putative language around drug use – including cannabis – as part of the racist war on drugs,” which could lead them to omit having used drugs during the intake process.
A 2017 study linked cannabis use among older adults with more injuries, which in turn led to greater emergency department use. Brian Kaskie, PhD, associate professor in health management and policy at the University of Iowa, Iowa City, said in an interview that the new findings show a state-specific, but alarming trend, and that more research is needed.
“Were these first-time users who were not familiar with anxiety-inducing aspects of cannabis use and took high potency products? Did they complete any education about how to use cannabis?” said Dr. Kaskie, who was not involved in the new study. “Were the ER visits for relatively benign, nonemergent reasons or were these ... visits an outcome of a tragic, harmful event like a car accident or overdose?”
Dr. Han and Dr. Kaskie disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers in California found an 18-fold increase in the rate of cannabis-related trips to the ED visits among adults over age 65 in the state from 2005 to 2019.
Addressing potential harms of cannabis use among older adults, who face heightened risk for adverse reactions to the substance, “is urgently required,” the researchers reported at the annual meeting of the American Geriatrics Society.
The researchers advised doctors to discuss cannabis use with older patients and screen older adults for cannabis use. Those living with multiple chronic conditions and taking multiple medications are especially likely to be at risk for harm, coinvestigator Benjamin Han, MD, MPH, a geriatrician at the University of California, San Diego, said in an interview.
Dr. Han added that “very little” is understood about the risks and benefits of cannabis use in the elderly, and more studies are needed “so that clinicians can have data-informed discussions with their patients.”
California legalized medical marijuana in 1996 and recreational marijuana in 2016.
The researchers used diagnostic code data from California’s nonmilitary acute care hospitals, collected by the state’s Department of Healthcare Access and Information, to calculate annual rates of cannabis-related visits per 10,000 ED visits.
ED trips up sharply among older adults
Rates of cannabis-related visits increased significantly for all older adult age ranges (P < .001), according to the researchers. Among those aged 65-74 years, the rate increased about 15-fold, from 44.9 per 10,000 visits in 2005 to 714.5 per 100,000 in 2019; for ages 75-84, the rate increased about 22-fold, from 8.4 to 193.9 per 10,000; and for those 85 and older the rate jumped nearly 18-fold, from 2.1 to 39.2 per 10,000.
The greatest increase occurred in visits categorized in diagnostic codes as cannabis abuse and unspecified use. Cannabis dependence and cannabis poisoning accounted for only a small fraction of cases, the investigators found.
The researchers did not have data on specific reasons for a visit, or whether patients had smoked or ingested marijuana products. They also could not discern whether patients had used delta-9-tetrahydrocannabinol, which has psychoactive properties, or cannabidiol, which typically does not have the same mind-altering effects.
Dr. Han said the data may not present a full picture of marijuana-related ED visits. “It is important to recognize that older adults have lived through the very putative language around drug use – including cannabis – as part of the racist war on drugs,” which could lead them to omit having used drugs during the intake process.
A 2017 study linked cannabis use among older adults with more injuries, which in turn led to greater emergency department use. Brian Kaskie, PhD, associate professor in health management and policy at the University of Iowa, Iowa City, said in an interview that the new findings show a state-specific, but alarming trend, and that more research is needed.
“Were these first-time users who were not familiar with anxiety-inducing aspects of cannabis use and took high potency products? Did they complete any education about how to use cannabis?” said Dr. Kaskie, who was not involved in the new study. “Were the ER visits for relatively benign, nonemergent reasons or were these ... visits an outcome of a tragic, harmful event like a car accident or overdose?”
Dr. Han and Dr. Kaskie disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers in California found an 18-fold increase in the rate of cannabis-related trips to the ED visits among adults over age 65 in the state from 2005 to 2019.
Addressing potential harms of cannabis use among older adults, who face heightened risk for adverse reactions to the substance, “is urgently required,” the researchers reported at the annual meeting of the American Geriatrics Society.
The researchers advised doctors to discuss cannabis use with older patients and screen older adults for cannabis use. Those living with multiple chronic conditions and taking multiple medications are especially likely to be at risk for harm, coinvestigator Benjamin Han, MD, MPH, a geriatrician at the University of California, San Diego, said in an interview.
Dr. Han added that “very little” is understood about the risks and benefits of cannabis use in the elderly, and more studies are needed “so that clinicians can have data-informed discussions with their patients.”
California legalized medical marijuana in 1996 and recreational marijuana in 2016.
The researchers used diagnostic code data from California’s nonmilitary acute care hospitals, collected by the state’s Department of Healthcare Access and Information, to calculate annual rates of cannabis-related visits per 10,000 ED visits.
ED trips up sharply among older adults
Rates of cannabis-related visits increased significantly for all older adult age ranges (P < .001), according to the researchers. Among those aged 65-74 years, the rate increased about 15-fold, from 44.9 per 10,000 visits in 2005 to 714.5 per 100,000 in 2019; for ages 75-84, the rate increased about 22-fold, from 8.4 to 193.9 per 10,000; and for those 85 and older the rate jumped nearly 18-fold, from 2.1 to 39.2 per 10,000.
The greatest increase occurred in visits categorized in diagnostic codes as cannabis abuse and unspecified use. Cannabis dependence and cannabis poisoning accounted for only a small fraction of cases, the investigators found.
The researchers did not have data on specific reasons for a visit, or whether patients had smoked or ingested marijuana products. They also could not discern whether patients had used delta-9-tetrahydrocannabinol, which has psychoactive properties, or cannabidiol, which typically does not have the same mind-altering effects.
Dr. Han said the data may not present a full picture of marijuana-related ED visits. “It is important to recognize that older adults have lived through the very putative language around drug use – including cannabis – as part of the racist war on drugs,” which could lead them to omit having used drugs during the intake process.
A 2017 study linked cannabis use among older adults with more injuries, which in turn led to greater emergency department use. Brian Kaskie, PhD, associate professor in health management and policy at the University of Iowa, Iowa City, said in an interview that the new findings show a state-specific, but alarming trend, and that more research is needed.
“Were these first-time users who were not familiar with anxiety-inducing aspects of cannabis use and took high potency products? Did they complete any education about how to use cannabis?” said Dr. Kaskie, who was not involved in the new study. “Were the ER visits for relatively benign, nonemergent reasons or were these ... visits an outcome of a tragic, harmful event like a car accident or overdose?”
Dr. Han and Dr. Kaskie disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AGS 2022
Race difference seen in prenatal pot screens
Black patients and those with public insurance are more likely than their White, wealthier counterparts to be screened for marijuana use during pregnancy, researchers have found.
The data build on a growing body of evidence that disparities in age, insurance type, and race affect which women undergo drug testing during pregnancy and come under scrutiny from state social service agencies.
Many states require health care facilities to notify child protective services or law enforcement of a positive drug screening, but the consequences for women vary greatly from state to state. Twenty-four states and the District of Columbia consider prenatal drug use to be child abuse. But recent evidence suggests that urine drug screenings may not be reliable but can lead to separation of parents and babies.
“In many ways, the health system is better equipped to address these concerns than the criminal justice system,” Rebecca Stone, PhD, associate professor of sociology and criminal justice at Suffolk University, Boston, told this news organization. “They shouldn’t be criminal justice problems in many cases,” added Dr. Stone, who was not involved with the study.
The researchers analyzed data from the 2,045 patients who gave birth between January and July 2020. Of those, roughly one-fourth (24%) underwent a urine drug screening. The most common reason for a screen was that clinicians either suspected or patients self-reported use of marijuana during or shortly before pregnancy, according to the researchers, who presented their findings at the American College of Obstetricians and Gynecologists (ACOG) 2022 Annual Meeting.
Nearly 80% of the 209 patients who underwent drug testing because of suspected marijuana use were Black, and nearly 61% had public insurance. The median age of persons who underwent drug testing was 25 years; the overall median age of pregnant patients was 29 years.
Of the 1,561 patients who didn’t undergo drug screening, 43% were Black, and 37% had public insurance coverage.
Clinicians reported that nearly all patients (117/125; 94%) who tested positive for marijuana were reported to the Missouri child abuse/neglect hotline. Only four women who tested positive for marijuana use also tested positive for at least one other illegal drug.
“Marijuana did not predict other drug exposure; thus, we suggest that a history of marijuana use should not be used as a criteria for sending a urine drug screen on patients [who are admitted to the labor unit],” said Jeannie Kelly, MD, medical director of maternal-fetal transport and labor and delivery at the Washington University School of Medicine, St. Louis, who is the senior author of the study. “In our experience, this is a policy that increases inequitable screening without improving our ability to identify families who need extra support or monitoring.”
All patients in the study verbally agreed to a urine drug screening. Hospitals around the country have faced lawsuits for failing to gain consent from women undergoing such tests. A 2001 ruling from the U.S. Supreme Court made informed consent mandatory in the absence of a warrant.
Legal consequences of a positive test
Children exposed to marijuana in the womb are at heightened risk for impaired cognition and learning disabilities, according to a 2015 report from ACOG’s Committee on Obstetric Practice. However, a lack of care before birth can be harmful to infants and result in low birth weight and severe neurologic and other problems.
In a 2015 study, Dr. Stone found that women were less likely to seek prenatal care if they worried about the legal consequences of a positive test.
Dr. Kelly said the threat of interference from child protective services is often the top worry of pregnant women with substance use disorders. She argued that clinicians should treat marijuana the same way they do tobacco: discourage its use without reporting patients to law enforcement.
“Our suggestion is that this history you elicit of someone using marijuana probably shouldn’t be used [as a trigger for drug screening],” Dr. Kelly said.
She added that doctors can use discretion in choosing to screen for drugs, and she urged clinicians and health care institutions to reevaluate their drug screening practices to reduce harm and increase equitable care.
“We can only work the system in the places that we have control over,” she said. “I can’t control the downward cascade, but I can definitely control who I send a urine drug screen on.”
Dr. Kelly and Dr. Stone reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Black patients and those with public insurance are more likely than their White, wealthier counterparts to be screened for marijuana use during pregnancy, researchers have found.
The data build on a growing body of evidence that disparities in age, insurance type, and race affect which women undergo drug testing during pregnancy and come under scrutiny from state social service agencies.
Many states require health care facilities to notify child protective services or law enforcement of a positive drug screening, but the consequences for women vary greatly from state to state. Twenty-four states and the District of Columbia consider prenatal drug use to be child abuse. But recent evidence suggests that urine drug screenings may not be reliable but can lead to separation of parents and babies.
“In many ways, the health system is better equipped to address these concerns than the criminal justice system,” Rebecca Stone, PhD, associate professor of sociology and criminal justice at Suffolk University, Boston, told this news organization. “They shouldn’t be criminal justice problems in many cases,” added Dr. Stone, who was not involved with the study.
The researchers analyzed data from the 2,045 patients who gave birth between January and July 2020. Of those, roughly one-fourth (24%) underwent a urine drug screening. The most common reason for a screen was that clinicians either suspected or patients self-reported use of marijuana during or shortly before pregnancy, according to the researchers, who presented their findings at the American College of Obstetricians and Gynecologists (ACOG) 2022 Annual Meeting.
Nearly 80% of the 209 patients who underwent drug testing because of suspected marijuana use were Black, and nearly 61% had public insurance. The median age of persons who underwent drug testing was 25 years; the overall median age of pregnant patients was 29 years.
Of the 1,561 patients who didn’t undergo drug screening, 43% were Black, and 37% had public insurance coverage.
Clinicians reported that nearly all patients (117/125; 94%) who tested positive for marijuana were reported to the Missouri child abuse/neglect hotline. Only four women who tested positive for marijuana use also tested positive for at least one other illegal drug.
“Marijuana did not predict other drug exposure; thus, we suggest that a history of marijuana use should not be used as a criteria for sending a urine drug screen on patients [who are admitted to the labor unit],” said Jeannie Kelly, MD, medical director of maternal-fetal transport and labor and delivery at the Washington University School of Medicine, St. Louis, who is the senior author of the study. “In our experience, this is a policy that increases inequitable screening without improving our ability to identify families who need extra support or monitoring.”
All patients in the study verbally agreed to a urine drug screening. Hospitals around the country have faced lawsuits for failing to gain consent from women undergoing such tests. A 2001 ruling from the U.S. Supreme Court made informed consent mandatory in the absence of a warrant.
Legal consequences of a positive test
Children exposed to marijuana in the womb are at heightened risk for impaired cognition and learning disabilities, according to a 2015 report from ACOG’s Committee on Obstetric Practice. However, a lack of care before birth can be harmful to infants and result in low birth weight and severe neurologic and other problems.
In a 2015 study, Dr. Stone found that women were less likely to seek prenatal care if they worried about the legal consequences of a positive test.
Dr. Kelly said the threat of interference from child protective services is often the top worry of pregnant women with substance use disorders. She argued that clinicians should treat marijuana the same way they do tobacco: discourage its use without reporting patients to law enforcement.
“Our suggestion is that this history you elicit of someone using marijuana probably shouldn’t be used [as a trigger for drug screening],” Dr. Kelly said.
She added that doctors can use discretion in choosing to screen for drugs, and she urged clinicians and health care institutions to reevaluate their drug screening practices to reduce harm and increase equitable care.
“We can only work the system in the places that we have control over,” she said. “I can’t control the downward cascade, but I can definitely control who I send a urine drug screen on.”
Dr. Kelly and Dr. Stone reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Black patients and those with public insurance are more likely than their White, wealthier counterparts to be screened for marijuana use during pregnancy, researchers have found.
The data build on a growing body of evidence that disparities in age, insurance type, and race affect which women undergo drug testing during pregnancy and come under scrutiny from state social service agencies.
Many states require health care facilities to notify child protective services or law enforcement of a positive drug screening, but the consequences for women vary greatly from state to state. Twenty-four states and the District of Columbia consider prenatal drug use to be child abuse. But recent evidence suggests that urine drug screenings may not be reliable but can lead to separation of parents and babies.
“In many ways, the health system is better equipped to address these concerns than the criminal justice system,” Rebecca Stone, PhD, associate professor of sociology and criminal justice at Suffolk University, Boston, told this news organization. “They shouldn’t be criminal justice problems in many cases,” added Dr. Stone, who was not involved with the study.
The researchers analyzed data from the 2,045 patients who gave birth between January and July 2020. Of those, roughly one-fourth (24%) underwent a urine drug screening. The most common reason for a screen was that clinicians either suspected or patients self-reported use of marijuana during or shortly before pregnancy, according to the researchers, who presented their findings at the American College of Obstetricians and Gynecologists (ACOG) 2022 Annual Meeting.
Nearly 80% of the 209 patients who underwent drug testing because of suspected marijuana use were Black, and nearly 61% had public insurance. The median age of persons who underwent drug testing was 25 years; the overall median age of pregnant patients was 29 years.
Of the 1,561 patients who didn’t undergo drug screening, 43% were Black, and 37% had public insurance coverage.
Clinicians reported that nearly all patients (117/125; 94%) who tested positive for marijuana were reported to the Missouri child abuse/neglect hotline. Only four women who tested positive for marijuana use also tested positive for at least one other illegal drug.
“Marijuana did not predict other drug exposure; thus, we suggest that a history of marijuana use should not be used as a criteria for sending a urine drug screen on patients [who are admitted to the labor unit],” said Jeannie Kelly, MD, medical director of maternal-fetal transport and labor and delivery at the Washington University School of Medicine, St. Louis, who is the senior author of the study. “In our experience, this is a policy that increases inequitable screening without improving our ability to identify families who need extra support or monitoring.”
All patients in the study verbally agreed to a urine drug screening. Hospitals around the country have faced lawsuits for failing to gain consent from women undergoing such tests. A 2001 ruling from the U.S. Supreme Court made informed consent mandatory in the absence of a warrant.
Legal consequences of a positive test
Children exposed to marijuana in the womb are at heightened risk for impaired cognition and learning disabilities, according to a 2015 report from ACOG’s Committee on Obstetric Practice. However, a lack of care before birth can be harmful to infants and result in low birth weight and severe neurologic and other problems.
In a 2015 study, Dr. Stone found that women were less likely to seek prenatal care if they worried about the legal consequences of a positive test.
Dr. Kelly said the threat of interference from child protective services is often the top worry of pregnant women with substance use disorders. She argued that clinicians should treat marijuana the same way they do tobacco: discourage its use without reporting patients to law enforcement.
“Our suggestion is that this history you elicit of someone using marijuana probably shouldn’t be used [as a trigger for drug screening],” Dr. Kelly said.
She added that doctors can use discretion in choosing to screen for drugs, and she urged clinicians and health care institutions to reevaluate their drug screening practices to reduce harm and increase equitable care.
“We can only work the system in the places that we have control over,” she said. “I can’t control the downward cascade, but I can definitely control who I send a urine drug screen on.”
Dr. Kelly and Dr. Stone reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACOG 2022