User login
Emerging research shows link between suicidality, ‘high-potency’ cannabis products
Number of suicides positive for marijuana on rise soared among Colorado youth
In the days since recreational sales of marijuana became legal in Colorado in January 2014, concerning trends have emerged among the state’s young cannabis users.
According to a report from the Rocky Mountain High Intensity Drug Trafficking Area, between 2014 and 2017, the number of suicides positive for marijuana increased 250% among those aged 10-19 years (from 4 to 14) and 22% among those aged 20 and older (from 118 to 144). “Other states are seeing something similar, and there is an emerging research showing a relationship between suicidality and the use of marijuana, especially high-potency products that are available in legalized markets,” Paula D. Riggs, MD, reported during an annual psychopharmacology update held by the Nevada Psychiatric Association.
During that same 3-year time span, the proportion of Colorado youth aged 12 years and older who used marijuana in the past month jumped by 45%, which is more than 85% above the national average. “Similarly, among college-age students, we’ve seen an 18% increase in past-month marijuana use, which is 60% above the national average,” said Dr. Riggs, professor and vice chair of psychiatry at the University of Colorado at Denver, Aurora.
Among adolescents, state health officials have observed a 5% increase in the proportion of those who used marijuana in the past month, which is more than 54% above the national average. “But a concerning trend is that we’re seeing an increase in the use of concentrates such as dabs and waxes,” she said. “That’s worrisome in terms of exposure to high-potency products.”
In other findings, 48% of young marijuana users reported going to work high (40% at least once per week), and there has been a 170% increase in youth ED urgent care visits for marijuana-related illnesses such as cannabinoid hyperemesis syndrome or first-episode psychosis. State health officials have also observed a 148% increase in marijuana-related hospitalizations.
According to Dr. Riggs, who also directs the University of Colorado’s division of addiction science, prevention, and treatment, the average marijuana joint in the 1960s contained about 3% tetrahydrocannabinol (THC), a level that crept up to the 4%-6% range in 2002. In today’s postlegalization era, the average joint now contains 13%-23% THC. “What’s concerning is that the concentrates – the dabs, waxes, shatter, and butane hash oils – contain upward of 70%-95% THC,” Dr. Riggs said. “Those are highly potent products that represent about 25% of the market share now. That’s a very big concern because the higher the potency the cannabis product used, the greater the abuse liability and addictive potential.”
The use of high-potency products also doubles the risk of developing generalized anxiety disorder, triples the risk of tobacco dependence, doubles the risk of other illicit substance disorders, and it at least quadruples the risk of developing first-episode psychosis in young people. “So, when you’re taking a cannabis use history, it’s important to ask patients about the potency of the products being used,” she said.
In the 2019 Monitoring the Future survey, 12% of U.S. 8th graders self-reported marijuana use in the past year and 7% in the past month, compared with 29% and 18% of 10th graders, respectively. Self-reported use by 12th graders was even more elevated (36% in the past year and 29% in the past month). “The concern is, this survey doesn’t really capture what’s happening with marijuana concentrates,” Dr. Riggs said.
A survey of Colorado youth conducted by the state’s Department of Public Health and Environment found that the percentage of students who reported using concentrated forms of marijuana has risen steadily in recent years and now stands at roughly 34%. “The use of edibles has also crept up,” said Dr. Riggs, who noted that marijuana dispensaries in Colorado outnumber Starbucks locations and McDonald’s restaurants. “You might not think that’s particularly concerning, except that the use of edibles is even more associated with onset of psychosis than other forms. This is probably because when you eat a marijuana product, you can’t control the exposure or the dose that you’re ingesting. We need to be concerned about these trends.”
European studies report that 30%-50% of new cases of first-onset psychosis are attributed to high-potency cannabis. “There is a dose-response relationship between cannabis and psychosis,” Dr. Riggs said. “That is, the frequency and duration of cannabis use, or the use of high-potency products, and the age of onset, are strongly associated with the risk of first-episode psychosis.
Researchers have known for some time that alterations in the endocannabinoid system are associated with psychosis independent of cannabis exposure. “Dysregulation of that endocannabinoid system occurs in patients at all stages of the psychosis continuum,” she continued. “It also means that the endocannabinoid system is a potential therapeutic target for psychosis.”
According to Dr. Riggs, THC exposure acutely increases dopamine in the ventral striatum and it can produce transient psychotomimetic effects in clinical and nonclinical populations. Genetic differences in the dopaminergic system can also interact with cannabis use to increase the risk of psychosis.
“For example, the COMT (catechol-O-methyltransferase) breaks down catecholamines such as dopamine in the prefrontal cortex,” she explained. “If you have a COMT gene polymorphism, that increases your risk of developing psychosis due to increased levels of dopamine signaling.”
She emphasized the importance of clinicians to understand that the age of cannabis use onset, the duration, frequency, and THC potency is related to the psychosis risk and worse prognosis. The earlier the initiation of marijuana use, the greater potential for first-episode psychosis. “Those who continue using cannabis after a first-episode psychosis have greater severity of psychotic illness and more treatment resistance, and they’re less likely to engage or be compliant with treatment recommendations,” Dr. Riggs said. “So, Because if they resume cannabis use, this can turn into a more chronic psychotic disorder.”
She added that, while insufficient evidence exists to determine whether cannabis plays a causal role in the development of schizophrenia or not, mounting evidence suggests that cannabis use may precipitate earlier onset of schizophrenia in those with other risk factors for the disorder. “There is considerable evidence that cannabis use increases the risk of psychosis in a dose-related manner, especially with an onset before age 16,” Dr. Riggs said. “However, this does not mean that cannabis is safe for young adults. Cannabis-induced psychotic symptoms often develop during young adulthood and may become chronic.”
Dr. Riggs disclosed that she had received grant funding from the National Institute on Drug Abuse. She is also executive director for Encompass, which provides integrated treatment for adolescents and young adults.
Number of suicides positive for marijuana on rise soared among Colorado youth
Number of suicides positive for marijuana on rise soared among Colorado youth
In the days since recreational sales of marijuana became legal in Colorado in January 2014, concerning trends have emerged among the state’s young cannabis users.
According to a report from the Rocky Mountain High Intensity Drug Trafficking Area, between 2014 and 2017, the number of suicides positive for marijuana increased 250% among those aged 10-19 years (from 4 to 14) and 22% among those aged 20 and older (from 118 to 144). “Other states are seeing something similar, and there is an emerging research showing a relationship between suicidality and the use of marijuana, especially high-potency products that are available in legalized markets,” Paula D. Riggs, MD, reported during an annual psychopharmacology update held by the Nevada Psychiatric Association.
During that same 3-year time span, the proportion of Colorado youth aged 12 years and older who used marijuana in the past month jumped by 45%, which is more than 85% above the national average. “Similarly, among college-age students, we’ve seen an 18% increase in past-month marijuana use, which is 60% above the national average,” said Dr. Riggs, professor and vice chair of psychiatry at the University of Colorado at Denver, Aurora.
Among adolescents, state health officials have observed a 5% increase in the proportion of those who used marijuana in the past month, which is more than 54% above the national average. “But a concerning trend is that we’re seeing an increase in the use of concentrates such as dabs and waxes,” she said. “That’s worrisome in terms of exposure to high-potency products.”
In other findings, 48% of young marijuana users reported going to work high (40% at least once per week), and there has been a 170% increase in youth ED urgent care visits for marijuana-related illnesses such as cannabinoid hyperemesis syndrome or first-episode psychosis. State health officials have also observed a 148% increase in marijuana-related hospitalizations.
According to Dr. Riggs, who also directs the University of Colorado’s division of addiction science, prevention, and treatment, the average marijuana joint in the 1960s contained about 3% tetrahydrocannabinol (THC), a level that crept up to the 4%-6% range in 2002. In today’s postlegalization era, the average joint now contains 13%-23% THC. “What’s concerning is that the concentrates – the dabs, waxes, shatter, and butane hash oils – contain upward of 70%-95% THC,” Dr. Riggs said. “Those are highly potent products that represent about 25% of the market share now. That’s a very big concern because the higher the potency the cannabis product used, the greater the abuse liability and addictive potential.”
The use of high-potency products also doubles the risk of developing generalized anxiety disorder, triples the risk of tobacco dependence, doubles the risk of other illicit substance disorders, and it at least quadruples the risk of developing first-episode psychosis in young people. “So, when you’re taking a cannabis use history, it’s important to ask patients about the potency of the products being used,” she said.
In the 2019 Monitoring the Future survey, 12% of U.S. 8th graders self-reported marijuana use in the past year and 7% in the past month, compared with 29% and 18% of 10th graders, respectively. Self-reported use by 12th graders was even more elevated (36% in the past year and 29% in the past month). “The concern is, this survey doesn’t really capture what’s happening with marijuana concentrates,” Dr. Riggs said.
A survey of Colorado youth conducted by the state’s Department of Public Health and Environment found that the percentage of students who reported using concentrated forms of marijuana has risen steadily in recent years and now stands at roughly 34%. “The use of edibles has also crept up,” said Dr. Riggs, who noted that marijuana dispensaries in Colorado outnumber Starbucks locations and McDonald’s restaurants. “You might not think that’s particularly concerning, except that the use of edibles is even more associated with onset of psychosis than other forms. This is probably because when you eat a marijuana product, you can’t control the exposure or the dose that you’re ingesting. We need to be concerned about these trends.”
European studies report that 30%-50% of new cases of first-onset psychosis are attributed to high-potency cannabis. “There is a dose-response relationship between cannabis and psychosis,” Dr. Riggs said. “That is, the frequency and duration of cannabis use, or the use of high-potency products, and the age of onset, are strongly associated with the risk of first-episode psychosis.
Researchers have known for some time that alterations in the endocannabinoid system are associated with psychosis independent of cannabis exposure. “Dysregulation of that endocannabinoid system occurs in patients at all stages of the psychosis continuum,” she continued. “It also means that the endocannabinoid system is a potential therapeutic target for psychosis.”
According to Dr. Riggs, THC exposure acutely increases dopamine in the ventral striatum and it can produce transient psychotomimetic effects in clinical and nonclinical populations. Genetic differences in the dopaminergic system can also interact with cannabis use to increase the risk of psychosis.
“For example, the COMT (catechol-O-methyltransferase) breaks down catecholamines such as dopamine in the prefrontal cortex,” she explained. “If you have a COMT gene polymorphism, that increases your risk of developing psychosis due to increased levels of dopamine signaling.”
She emphasized the importance of clinicians to understand that the age of cannabis use onset, the duration, frequency, and THC potency is related to the psychosis risk and worse prognosis. The earlier the initiation of marijuana use, the greater potential for first-episode psychosis. “Those who continue using cannabis after a first-episode psychosis have greater severity of psychotic illness and more treatment resistance, and they’re less likely to engage or be compliant with treatment recommendations,” Dr. Riggs said. “So, Because if they resume cannabis use, this can turn into a more chronic psychotic disorder.”
She added that, while insufficient evidence exists to determine whether cannabis plays a causal role in the development of schizophrenia or not, mounting evidence suggests that cannabis use may precipitate earlier onset of schizophrenia in those with other risk factors for the disorder. “There is considerable evidence that cannabis use increases the risk of psychosis in a dose-related manner, especially with an onset before age 16,” Dr. Riggs said. “However, this does not mean that cannabis is safe for young adults. Cannabis-induced psychotic symptoms often develop during young adulthood and may become chronic.”
Dr. Riggs disclosed that she had received grant funding from the National Institute on Drug Abuse. She is also executive director for Encompass, which provides integrated treatment for adolescents and young adults.
In the days since recreational sales of marijuana became legal in Colorado in January 2014, concerning trends have emerged among the state’s young cannabis users.
According to a report from the Rocky Mountain High Intensity Drug Trafficking Area, between 2014 and 2017, the number of suicides positive for marijuana increased 250% among those aged 10-19 years (from 4 to 14) and 22% among those aged 20 and older (from 118 to 144). “Other states are seeing something similar, and there is an emerging research showing a relationship between suicidality and the use of marijuana, especially high-potency products that are available in legalized markets,” Paula D. Riggs, MD, reported during an annual psychopharmacology update held by the Nevada Psychiatric Association.
During that same 3-year time span, the proportion of Colorado youth aged 12 years and older who used marijuana in the past month jumped by 45%, which is more than 85% above the national average. “Similarly, among college-age students, we’ve seen an 18% increase in past-month marijuana use, which is 60% above the national average,” said Dr. Riggs, professor and vice chair of psychiatry at the University of Colorado at Denver, Aurora.
Among adolescents, state health officials have observed a 5% increase in the proportion of those who used marijuana in the past month, which is more than 54% above the national average. “But a concerning trend is that we’re seeing an increase in the use of concentrates such as dabs and waxes,” she said. “That’s worrisome in terms of exposure to high-potency products.”
In other findings, 48% of young marijuana users reported going to work high (40% at least once per week), and there has been a 170% increase in youth ED urgent care visits for marijuana-related illnesses such as cannabinoid hyperemesis syndrome or first-episode psychosis. State health officials have also observed a 148% increase in marijuana-related hospitalizations.
According to Dr. Riggs, who also directs the University of Colorado’s division of addiction science, prevention, and treatment, the average marijuana joint in the 1960s contained about 3% tetrahydrocannabinol (THC), a level that crept up to the 4%-6% range in 2002. In today’s postlegalization era, the average joint now contains 13%-23% THC. “What’s concerning is that the concentrates – the dabs, waxes, shatter, and butane hash oils – contain upward of 70%-95% THC,” Dr. Riggs said. “Those are highly potent products that represent about 25% of the market share now. That’s a very big concern because the higher the potency the cannabis product used, the greater the abuse liability and addictive potential.”
The use of high-potency products also doubles the risk of developing generalized anxiety disorder, triples the risk of tobacco dependence, doubles the risk of other illicit substance disorders, and it at least quadruples the risk of developing first-episode psychosis in young people. “So, when you’re taking a cannabis use history, it’s important to ask patients about the potency of the products being used,” she said.
In the 2019 Monitoring the Future survey, 12% of U.S. 8th graders self-reported marijuana use in the past year and 7% in the past month, compared with 29% and 18% of 10th graders, respectively. Self-reported use by 12th graders was even more elevated (36% in the past year and 29% in the past month). “The concern is, this survey doesn’t really capture what’s happening with marijuana concentrates,” Dr. Riggs said.
A survey of Colorado youth conducted by the state’s Department of Public Health and Environment found that the percentage of students who reported using concentrated forms of marijuana has risen steadily in recent years and now stands at roughly 34%. “The use of edibles has also crept up,” said Dr. Riggs, who noted that marijuana dispensaries in Colorado outnumber Starbucks locations and McDonald’s restaurants. “You might not think that’s particularly concerning, except that the use of edibles is even more associated with onset of psychosis than other forms. This is probably because when you eat a marijuana product, you can’t control the exposure or the dose that you’re ingesting. We need to be concerned about these trends.”
European studies report that 30%-50% of new cases of first-onset psychosis are attributed to high-potency cannabis. “There is a dose-response relationship between cannabis and psychosis,” Dr. Riggs said. “That is, the frequency and duration of cannabis use, or the use of high-potency products, and the age of onset, are strongly associated with the risk of first-episode psychosis.
Researchers have known for some time that alterations in the endocannabinoid system are associated with psychosis independent of cannabis exposure. “Dysregulation of that endocannabinoid system occurs in patients at all stages of the psychosis continuum,” she continued. “It also means that the endocannabinoid system is a potential therapeutic target for psychosis.”
According to Dr. Riggs, THC exposure acutely increases dopamine in the ventral striatum and it can produce transient psychotomimetic effects in clinical and nonclinical populations. Genetic differences in the dopaminergic system can also interact with cannabis use to increase the risk of psychosis.
“For example, the COMT (catechol-O-methyltransferase) breaks down catecholamines such as dopamine in the prefrontal cortex,” she explained. “If you have a COMT gene polymorphism, that increases your risk of developing psychosis due to increased levels of dopamine signaling.”
She emphasized the importance of clinicians to understand that the age of cannabis use onset, the duration, frequency, and THC potency is related to the psychosis risk and worse prognosis. The earlier the initiation of marijuana use, the greater potential for first-episode psychosis. “Those who continue using cannabis after a first-episode psychosis have greater severity of psychotic illness and more treatment resistance, and they’re less likely to engage or be compliant with treatment recommendations,” Dr. Riggs said. “So, Because if they resume cannabis use, this can turn into a more chronic psychotic disorder.”
She added that, while insufficient evidence exists to determine whether cannabis plays a causal role in the development of schizophrenia or not, mounting evidence suggests that cannabis use may precipitate earlier onset of schizophrenia in those with other risk factors for the disorder. “There is considerable evidence that cannabis use increases the risk of psychosis in a dose-related manner, especially with an onset before age 16,” Dr. Riggs said. “However, this does not mean that cannabis is safe for young adults. Cannabis-induced psychotic symptoms often develop during young adulthood and may become chronic.”
Dr. Riggs disclosed that she had received grant funding from the National Institute on Drug Abuse. She is also executive director for Encompass, which provides integrated treatment for adolescents and young adults.
FROM NPA 2021
Vaccine may blunt effects of deadly synthetic opioids
New experimental vaccines could stop the worst effects of synthetic fentanyl and carfentanil, two drugs that have been major drivers of the opioid epidemic in the United States, according to a new study published in ACS Chemical Biology on Feb. 3, 2021.
During several experiments in mice, the vaccines prevented respiratory depression, which is the main cause of overdose deaths. The vaccines also reduced the amount of drug that was distributed to the brain. Once in the brain, synthetic opioids prompt the body to slow down breathing, and when too much of the drug is consumed, breathing can stop.
“Synthetic opioids are not only extremely deadly but also addictive and easy to manufacture, making them a formidable public health threat, especially when the coronavirus crisis is negatively impacting mental health,” Kim Janda, PhD, a chemist at Scripps Research Institute in La Jolla, Calif., who developed the vaccines, said in a statement.
Fentanyl is up to 100 times stronger than morphine, and carfentanil, which is often used by veterinarians to sedate large animals such as elephants, is up to 10,000 times stronger than morphine. Carfentanil isn’t as well-known as a street drug, but it’s being used more often as an additive in heroin and cocaine.
“We’ve shown it is possible to prevent these unnecessary deaths by eliciting antibodies that stop the drug from reaching the brain,” he said.
The vaccines could be used in emergency situations to treat overdoses and as a therapy for those with substance abuse disorders, Dr. Janda said. In addition, the vaccines could protect military officers who are exposed to opioids as chemical weapons, and they may also help opioid-sniffing police dogs to train for the job.
The vaccines are still in the early stages of testing, but looking at the latest data “brings us hope that this approach will work to treat a number of opioid-related maladies,” Dr. Janda said.
In December, the CDC reported that more than 81,000 drug overdose deaths happened in the United States between May 2019 and May 2020, which was the highest number ever recorded in a 12-month period. Synthetic opioids, particularly illegally created fentanyl, were to blame.
“Unfortunately, currently battling a pandemic,” Dr. Janda said. “We look forward to continuing our vaccine research and translating it to the clinic, where we can begin to make an impact on the opioid crisis.”
A version of this article first appeared on Medscape.com.
New experimental vaccines could stop the worst effects of synthetic fentanyl and carfentanil, two drugs that have been major drivers of the opioid epidemic in the United States, according to a new study published in ACS Chemical Biology on Feb. 3, 2021.
During several experiments in mice, the vaccines prevented respiratory depression, which is the main cause of overdose deaths. The vaccines also reduced the amount of drug that was distributed to the brain. Once in the brain, synthetic opioids prompt the body to slow down breathing, and when too much of the drug is consumed, breathing can stop.
“Synthetic opioids are not only extremely deadly but also addictive and easy to manufacture, making them a formidable public health threat, especially when the coronavirus crisis is negatively impacting mental health,” Kim Janda, PhD, a chemist at Scripps Research Institute in La Jolla, Calif., who developed the vaccines, said in a statement.
Fentanyl is up to 100 times stronger than morphine, and carfentanil, which is often used by veterinarians to sedate large animals such as elephants, is up to 10,000 times stronger than morphine. Carfentanil isn’t as well-known as a street drug, but it’s being used more often as an additive in heroin and cocaine.
“We’ve shown it is possible to prevent these unnecessary deaths by eliciting antibodies that stop the drug from reaching the brain,” he said.
The vaccines could be used in emergency situations to treat overdoses and as a therapy for those with substance abuse disorders, Dr. Janda said. In addition, the vaccines could protect military officers who are exposed to opioids as chemical weapons, and they may also help opioid-sniffing police dogs to train for the job.
The vaccines are still in the early stages of testing, but looking at the latest data “brings us hope that this approach will work to treat a number of opioid-related maladies,” Dr. Janda said.
In December, the CDC reported that more than 81,000 drug overdose deaths happened in the United States between May 2019 and May 2020, which was the highest number ever recorded in a 12-month period. Synthetic opioids, particularly illegally created fentanyl, were to blame.
“Unfortunately, currently battling a pandemic,” Dr. Janda said. “We look forward to continuing our vaccine research and translating it to the clinic, where we can begin to make an impact on the opioid crisis.”
A version of this article first appeared on Medscape.com.
New experimental vaccines could stop the worst effects of synthetic fentanyl and carfentanil, two drugs that have been major drivers of the opioid epidemic in the United States, according to a new study published in ACS Chemical Biology on Feb. 3, 2021.
During several experiments in mice, the vaccines prevented respiratory depression, which is the main cause of overdose deaths. The vaccines also reduced the amount of drug that was distributed to the brain. Once in the brain, synthetic opioids prompt the body to slow down breathing, and when too much of the drug is consumed, breathing can stop.
“Synthetic opioids are not only extremely deadly but also addictive and easy to manufacture, making them a formidable public health threat, especially when the coronavirus crisis is negatively impacting mental health,” Kim Janda, PhD, a chemist at Scripps Research Institute in La Jolla, Calif., who developed the vaccines, said in a statement.
Fentanyl is up to 100 times stronger than morphine, and carfentanil, which is often used by veterinarians to sedate large animals such as elephants, is up to 10,000 times stronger than morphine. Carfentanil isn’t as well-known as a street drug, but it’s being used more often as an additive in heroin and cocaine.
“We’ve shown it is possible to prevent these unnecessary deaths by eliciting antibodies that stop the drug from reaching the brain,” he said.
The vaccines could be used in emergency situations to treat overdoses and as a therapy for those with substance abuse disorders, Dr. Janda said. In addition, the vaccines could protect military officers who are exposed to opioids as chemical weapons, and they may also help opioid-sniffing police dogs to train for the job.
The vaccines are still in the early stages of testing, but looking at the latest data “brings us hope that this approach will work to treat a number of opioid-related maladies,” Dr. Janda said.
In December, the CDC reported that more than 81,000 drug overdose deaths happened in the United States between May 2019 and May 2020, which was the highest number ever recorded in a 12-month period. Synthetic opioids, particularly illegally created fentanyl, were to blame.
“Unfortunately, currently battling a pandemic,” Dr. Janda said. “We look forward to continuing our vaccine research and translating it to the clinic, where we can begin to make an impact on the opioid crisis.”
A version of this article first appeared on Medscape.com.
As demand for mental health care spikes, budget ax set to strike
When the pandemic hit, health officials in Montana’s Beaverhead County had barely begun to fill a hole left by the 2017 closure of the local public assistance office, mental health clinic, chemical dependency center and job placement office after the state’s last budget shortfall.
Now, those health officials worry more cuts are coming, even as they brace for a spike in demand for substance abuse and mental health services. That would be no small challenge in a poor farming and ranching region where stigma often prevents people from admitting they need help, said Katherine Buckley-Patton, who chairs the county’s Mental Health Local Advisory Council.
“I find it very challenging to find the words that will not make one of my hard-nosed cowboys turn around and walk away,” Ms. Buckley-Patton said.
States across the U.S. are still stinging after businesses closed and millions of people lost jobs because of COVID-related shutdowns and restrictions. Meanwhile, the pandemic has led to a dramatic increase in the number of people who say their mental health has suffered, rising from one in three people in March to more than half of people polled by KFF in July. (KHN is an editorially independent program of KFF.)
The full extent of the mental health crisis and the demand for behavioral health services may not be known until after the pandemic is over, mental health experts said. That could add costs that budget writers haven’t anticipated.
“It usually takes a while before people feel comfortable seeking care from a specialty behavioral health organization,” said Chuck Ingoglia, president and CEO of the nonprofit National Council for Behavioral Health in Washington, D.C. “We are not likely to see the results of that either in terms of people seeking care – or suicide rates going up – until we’re on the other side of the pandemic.”
Last year, states slashed agency budgets, froze pay, furloughed workers, borrowed money, and tapped into rainy-day funds to make ends meet. Health programs, often among the most expensive part of a state’s budget, were targeted for cuts in several states even as health officials led efforts to stem the spread of the coronavirus.
This year, the outlook doesn’t seem quite so bleak, partly because of relief packages passed by Congress last spring and in December that buoyed state economies. Another major advantage was that income increased or held steady for people with well-paying jobs and investment income, which boosted states’ tax revenues even as millions of lower-income workers were laid off.
“It has turned out to be not as bad as it might have been in terms of state budgets,” said Mike Leachman, vice president for state fiscal policy for the nonpartisan Center on Budget and Policy Priorities.
But many states still face cash shortfalls that will be made worse if additional federal aid doesn’t come, Mr. Leachman said. President Joe Biden has pledged to push through Congress a $1.9 billion relief package that includes aid to states, while congressional Republicans are proposing a package worth about a third of that amount. States are banking on federal help.
New York Gov. Andrew Cuomo, a Democrat, predicted his state would have to plug a $15 billion deficit with spending cuts and tax increases if a fresh round of aid doesn’t materialize. Some states, such as New Jersey, borrowed to make their budgets whole, and they’re going to have to start paying that money back. Tourism states such as Hawaii and energy-producing states such as Alaska and Wyoming continue to face grim economic outlooks with oil, gas, and coal prices down and tourists cutting back on travel, Mr. Leachman said.
Even states with a relatively rosy economic outlook are being cautious. In Colorado, for example, Democratic Gov. Jared Polis proposed a budget that restores the cuts made last year to Medicaid and substance abuse programs. But health providers are doubtful the legislature will approve any significant spending increases in this economy.
“Everybody right now is just trying to protect and make sure we don’t have additional cuts,” said Doyle Forrestal, CEO of the Colorado Behavioral Healthcare Council.
That’s also what Ms. Buckley-Patton wants for Montana’s Beaverhead County, where most of the 9,400 residents live in poverty or earn low incomes.
She led the county’s effort to recover from the loss in 2017 of a wide range of behavioral health services, along with offices to help poor people receive Medicaid health services, plus cash and food assistance.
Through persuasive grant writing and donations coaxed from elected officials, Ms. Buckley-Patton and her team secured office space, equipment, and a part-time employee for a resource center that’s open once a week in the county in the southwestern corner of the state, she said. They also convinced the state health department to send two people every other week on a 120-mile round trip from the Butte office to help county residents with their Medicaid and public assistance applications.
But now Ms. Buckley-Patton worries even those modest gains will be threatened in this year’s budget. Montana is one of the few states with a budget on a 2-year cycle, so this is the first time lawmakers have had to craft a spending plan since the pandemic began.
Revenue forecasts predict healthy tax collections over the next 2 years.
In January, at the start of the legislative session, the panel in charge of building the state health department’s budget proposed starting with nearly $1 billion in cuts. The panel’s chairperson, Republican Rep. Matt Regier, pledged to add back programs and services on their merits during the months-long budget process.
It’s a strategy Ms. Buckley-Patton worries will lead to a net loss of funding for Beaverhead County, which covers more land than Connecticut.
“I have grave concerns about this legislative session,” she said. “We’re not digging out of the hole; we’re only going deeper.”
Republicans, who are in control of the Montana House, Senate, and governor’s office for the first time in 16 years, are considering reducing the income tax level for the state’s top earners. Such a measure that could affect state revenue in an uncertain economy has some observers concerned, particularly when an increased need for health services is expected.
“Are legislators committed to building back up that budget in a way that works for communities and for health providers, or are we going to see tax cuts that reduce revenue that put us yet again in another really tight budget?” asked Heather O’Loughlin, codirector of the Montana Budget and Policy Center.
Mary Windecker, executive director of the Behavioral Health Alliance of Montana, said that health providers across the state are still clawing back from more than $100 million in budget cuts in 2017, and that she worries more cuts are on the horizon.
But one bright spot, she said, is a proposal by new Gov. Greg Gianforte to create a fund that would put $23 million a year toward community substance abuse prevention and treatment programs. It would be partially funded by tax revenue the state will receive from recreational marijuana, which voters approved in November, with sales to begin next year.
Ms. Windecker cautioned, though, that mental health and substance use are linked, and the governor and lawmakers should plan with that in mind.
“In the public’s mind, there’s drug addicts and there’s the mentally ill,” she said. “Quite often, the same people who have a substance use disorder are using it to treat a mental health issue that is underlying that substance use. So, you can never split the two out.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
When the pandemic hit, health officials in Montana’s Beaverhead County had barely begun to fill a hole left by the 2017 closure of the local public assistance office, mental health clinic, chemical dependency center and job placement office after the state’s last budget shortfall.
Now, those health officials worry more cuts are coming, even as they brace for a spike in demand for substance abuse and mental health services. That would be no small challenge in a poor farming and ranching region where stigma often prevents people from admitting they need help, said Katherine Buckley-Patton, who chairs the county’s Mental Health Local Advisory Council.
“I find it very challenging to find the words that will not make one of my hard-nosed cowboys turn around and walk away,” Ms. Buckley-Patton said.
States across the U.S. are still stinging after businesses closed and millions of people lost jobs because of COVID-related shutdowns and restrictions. Meanwhile, the pandemic has led to a dramatic increase in the number of people who say their mental health has suffered, rising from one in three people in March to more than half of people polled by KFF in July. (KHN is an editorially independent program of KFF.)
The full extent of the mental health crisis and the demand for behavioral health services may not be known until after the pandemic is over, mental health experts said. That could add costs that budget writers haven’t anticipated.
“It usually takes a while before people feel comfortable seeking care from a specialty behavioral health organization,” said Chuck Ingoglia, president and CEO of the nonprofit National Council for Behavioral Health in Washington, D.C. “We are not likely to see the results of that either in terms of people seeking care – or suicide rates going up – until we’re on the other side of the pandemic.”
Last year, states slashed agency budgets, froze pay, furloughed workers, borrowed money, and tapped into rainy-day funds to make ends meet. Health programs, often among the most expensive part of a state’s budget, were targeted for cuts in several states even as health officials led efforts to stem the spread of the coronavirus.
This year, the outlook doesn’t seem quite so bleak, partly because of relief packages passed by Congress last spring and in December that buoyed state economies. Another major advantage was that income increased or held steady for people with well-paying jobs and investment income, which boosted states’ tax revenues even as millions of lower-income workers were laid off.
“It has turned out to be not as bad as it might have been in terms of state budgets,” said Mike Leachman, vice president for state fiscal policy for the nonpartisan Center on Budget and Policy Priorities.
But many states still face cash shortfalls that will be made worse if additional federal aid doesn’t come, Mr. Leachman said. President Joe Biden has pledged to push through Congress a $1.9 billion relief package that includes aid to states, while congressional Republicans are proposing a package worth about a third of that amount. States are banking on federal help.
New York Gov. Andrew Cuomo, a Democrat, predicted his state would have to plug a $15 billion deficit with spending cuts and tax increases if a fresh round of aid doesn’t materialize. Some states, such as New Jersey, borrowed to make their budgets whole, and they’re going to have to start paying that money back. Tourism states such as Hawaii and energy-producing states such as Alaska and Wyoming continue to face grim economic outlooks with oil, gas, and coal prices down and tourists cutting back on travel, Mr. Leachman said.
Even states with a relatively rosy economic outlook are being cautious. In Colorado, for example, Democratic Gov. Jared Polis proposed a budget that restores the cuts made last year to Medicaid and substance abuse programs. But health providers are doubtful the legislature will approve any significant spending increases in this economy.
“Everybody right now is just trying to protect and make sure we don’t have additional cuts,” said Doyle Forrestal, CEO of the Colorado Behavioral Healthcare Council.
That’s also what Ms. Buckley-Patton wants for Montana’s Beaverhead County, where most of the 9,400 residents live in poverty or earn low incomes.
She led the county’s effort to recover from the loss in 2017 of a wide range of behavioral health services, along with offices to help poor people receive Medicaid health services, plus cash and food assistance.
Through persuasive grant writing and donations coaxed from elected officials, Ms. Buckley-Patton and her team secured office space, equipment, and a part-time employee for a resource center that’s open once a week in the county in the southwestern corner of the state, she said. They also convinced the state health department to send two people every other week on a 120-mile round trip from the Butte office to help county residents with their Medicaid and public assistance applications.
But now Ms. Buckley-Patton worries even those modest gains will be threatened in this year’s budget. Montana is one of the few states with a budget on a 2-year cycle, so this is the first time lawmakers have had to craft a spending plan since the pandemic began.
Revenue forecasts predict healthy tax collections over the next 2 years.
In January, at the start of the legislative session, the panel in charge of building the state health department’s budget proposed starting with nearly $1 billion in cuts. The panel’s chairperson, Republican Rep. Matt Regier, pledged to add back programs and services on their merits during the months-long budget process.
It’s a strategy Ms. Buckley-Patton worries will lead to a net loss of funding for Beaverhead County, which covers more land than Connecticut.
“I have grave concerns about this legislative session,” she said. “We’re not digging out of the hole; we’re only going deeper.”
Republicans, who are in control of the Montana House, Senate, and governor’s office for the first time in 16 years, are considering reducing the income tax level for the state’s top earners. Such a measure that could affect state revenue in an uncertain economy has some observers concerned, particularly when an increased need for health services is expected.
“Are legislators committed to building back up that budget in a way that works for communities and for health providers, or are we going to see tax cuts that reduce revenue that put us yet again in another really tight budget?” asked Heather O’Loughlin, codirector of the Montana Budget and Policy Center.
Mary Windecker, executive director of the Behavioral Health Alliance of Montana, said that health providers across the state are still clawing back from more than $100 million in budget cuts in 2017, and that she worries more cuts are on the horizon.
But one bright spot, she said, is a proposal by new Gov. Greg Gianforte to create a fund that would put $23 million a year toward community substance abuse prevention and treatment programs. It would be partially funded by tax revenue the state will receive from recreational marijuana, which voters approved in November, with sales to begin next year.
Ms. Windecker cautioned, though, that mental health and substance use are linked, and the governor and lawmakers should plan with that in mind.
“In the public’s mind, there’s drug addicts and there’s the mentally ill,” she said. “Quite often, the same people who have a substance use disorder are using it to treat a mental health issue that is underlying that substance use. So, you can never split the two out.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
When the pandemic hit, health officials in Montana’s Beaverhead County had barely begun to fill a hole left by the 2017 closure of the local public assistance office, mental health clinic, chemical dependency center and job placement office after the state’s last budget shortfall.
Now, those health officials worry more cuts are coming, even as they brace for a spike in demand for substance abuse and mental health services. That would be no small challenge in a poor farming and ranching region where stigma often prevents people from admitting they need help, said Katherine Buckley-Patton, who chairs the county’s Mental Health Local Advisory Council.
“I find it very challenging to find the words that will not make one of my hard-nosed cowboys turn around and walk away,” Ms. Buckley-Patton said.
States across the U.S. are still stinging after businesses closed and millions of people lost jobs because of COVID-related shutdowns and restrictions. Meanwhile, the pandemic has led to a dramatic increase in the number of people who say their mental health has suffered, rising from one in three people in March to more than half of people polled by KFF in July. (KHN is an editorially independent program of KFF.)
The full extent of the mental health crisis and the demand for behavioral health services may not be known until after the pandemic is over, mental health experts said. That could add costs that budget writers haven’t anticipated.
“It usually takes a while before people feel comfortable seeking care from a specialty behavioral health organization,” said Chuck Ingoglia, president and CEO of the nonprofit National Council for Behavioral Health in Washington, D.C. “We are not likely to see the results of that either in terms of people seeking care – or suicide rates going up – until we’re on the other side of the pandemic.”
Last year, states slashed agency budgets, froze pay, furloughed workers, borrowed money, and tapped into rainy-day funds to make ends meet. Health programs, often among the most expensive part of a state’s budget, were targeted for cuts in several states even as health officials led efforts to stem the spread of the coronavirus.
This year, the outlook doesn’t seem quite so bleak, partly because of relief packages passed by Congress last spring and in December that buoyed state economies. Another major advantage was that income increased or held steady for people with well-paying jobs and investment income, which boosted states’ tax revenues even as millions of lower-income workers were laid off.
“It has turned out to be not as bad as it might have been in terms of state budgets,” said Mike Leachman, vice president for state fiscal policy for the nonpartisan Center on Budget and Policy Priorities.
But many states still face cash shortfalls that will be made worse if additional federal aid doesn’t come, Mr. Leachman said. President Joe Biden has pledged to push through Congress a $1.9 billion relief package that includes aid to states, while congressional Republicans are proposing a package worth about a third of that amount. States are banking on federal help.
New York Gov. Andrew Cuomo, a Democrat, predicted his state would have to plug a $15 billion deficit with spending cuts and tax increases if a fresh round of aid doesn’t materialize. Some states, such as New Jersey, borrowed to make their budgets whole, and they’re going to have to start paying that money back. Tourism states such as Hawaii and energy-producing states such as Alaska and Wyoming continue to face grim economic outlooks with oil, gas, and coal prices down and tourists cutting back on travel, Mr. Leachman said.
Even states with a relatively rosy economic outlook are being cautious. In Colorado, for example, Democratic Gov. Jared Polis proposed a budget that restores the cuts made last year to Medicaid and substance abuse programs. But health providers are doubtful the legislature will approve any significant spending increases in this economy.
“Everybody right now is just trying to protect and make sure we don’t have additional cuts,” said Doyle Forrestal, CEO of the Colorado Behavioral Healthcare Council.
That’s also what Ms. Buckley-Patton wants for Montana’s Beaverhead County, where most of the 9,400 residents live in poverty or earn low incomes.
She led the county’s effort to recover from the loss in 2017 of a wide range of behavioral health services, along with offices to help poor people receive Medicaid health services, plus cash and food assistance.
Through persuasive grant writing and donations coaxed from elected officials, Ms. Buckley-Patton and her team secured office space, equipment, and a part-time employee for a resource center that’s open once a week in the county in the southwestern corner of the state, she said. They also convinced the state health department to send two people every other week on a 120-mile round trip from the Butte office to help county residents with their Medicaid and public assistance applications.
But now Ms. Buckley-Patton worries even those modest gains will be threatened in this year’s budget. Montana is one of the few states with a budget on a 2-year cycle, so this is the first time lawmakers have had to craft a spending plan since the pandemic began.
Revenue forecasts predict healthy tax collections over the next 2 years.
In January, at the start of the legislative session, the panel in charge of building the state health department’s budget proposed starting with nearly $1 billion in cuts. The panel’s chairperson, Republican Rep. Matt Regier, pledged to add back programs and services on their merits during the months-long budget process.
It’s a strategy Ms. Buckley-Patton worries will lead to a net loss of funding for Beaverhead County, which covers more land than Connecticut.
“I have grave concerns about this legislative session,” she said. “We’re not digging out of the hole; we’re only going deeper.”
Republicans, who are in control of the Montana House, Senate, and governor’s office for the first time in 16 years, are considering reducing the income tax level for the state’s top earners. Such a measure that could affect state revenue in an uncertain economy has some observers concerned, particularly when an increased need for health services is expected.
“Are legislators committed to building back up that budget in a way that works for communities and for health providers, or are we going to see tax cuts that reduce revenue that put us yet again in another really tight budget?” asked Heather O’Loughlin, codirector of the Montana Budget and Policy Center.
Mary Windecker, executive director of the Behavioral Health Alliance of Montana, said that health providers across the state are still clawing back from more than $100 million in budget cuts in 2017, and that she worries more cuts are on the horizon.
But one bright spot, she said, is a proposal by new Gov. Greg Gianforte to create a fund that would put $23 million a year toward community substance abuse prevention and treatment programs. It would be partially funded by tax revenue the state will receive from recreational marijuana, which voters approved in November, with sales to begin next year.
Ms. Windecker cautioned, though, that mental health and substance use are linked, and the governor and lawmakers should plan with that in mind.
“In the public’s mind, there’s drug addicts and there’s the mentally ill,” she said. “Quite often, the same people who have a substance use disorder are using it to treat a mental health issue that is underlying that substance use. So, you can never split the two out.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Minimizing Opioids After Joint Operation: Protocol to Decrease Postoperative Opioid Use After Primary Total Knee Arthroplasty
For decades, opioids have been a mainstay in the management of pain after total joint arthroplasty. In the past 10 years, however, opioid prescribing has come under increased scrutiny due to a rise in rates of opioid abuse, pill diversion, and opioid-related deaths.1,2 Opioids are associated with adverse effects, including nausea, vomiting, constipation, apathy, and respiratory depression, all of which influence arthroplasty outcomes and affect the patient experience. Although primary care groups account for nearly half of prescriptions written, orthopedic surgeons have the third highest per capita rate of opioid prescribing of all medical specialties.3,4 This puts orthopedic surgeons, particularly those who perform routine procedures, in an opportune but challenging position to confront this problem through novel pain management strategies.
Approximately 1 million total knee arthroplasties (TKAs) are performed in the US every year, and the US Department of Veterans Affairs (VA) health system performs about 10,000 hip and knee joint replacements.5,6 There is no standardization of opioid prescribing in the postoperative period following these procedures, and studies have reported a wide variation in prescribing habits even within a single institution for a specific surgery.7 Patients who undergo TKA are at particularly high risk of long-term opioid use if they are on continuous opioids at the time of surgery; this is problematic in a VA patient population in which at least 16% of patients are prescribed opioids in a given year.8 Furthermore, veterans are twice as likely as nonveterans to die of an accidental overdose.9 Despite these risks, opioids remain a cornerstone of postoperative pain management both within and outside of the VA.10
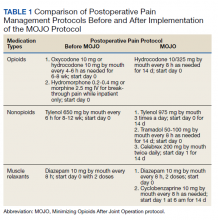
In 2018, to limit unnecessary prescribing of opioid pain medication, the total joint service at the VA Portland Health Care System (VAPHCS) in Oregon implemented the Minimizing Opioids after Joint Operation (MOJO) postoperative pain protocol. The goal of the protocol was to reduce opioid use following TKA. The objectives were to provide safe, appropriate analgesia while allowing early mobilization and discharge without a concomitant increase in readmissions or emergency department (ED) visits. The purpose of this retrospective chart review was to compare the efficacy of the MOJO protocol with our historical experience and report our preliminary results.
Methods
Institutional review board approval was obtained to retrospectively review the medical records of patients who had undergone TKA surgery during 2018 at VAPHCS. The MOJO protocol was composed of several simultaneous changes. The centerpiece of the new protocol was a drastic decrease in routine prescription of postoperative opioids (Table 1). Other changes included instructing patients to reduce the use of preoperative opioid pain medication 6 weeks before surgery with a goal of no opioid consumption, perform daily sets of preoperative exercises, and attend a preoperative consultation/education session with a nurse coordinator to emphasize early recovery and discharge. In patients with chronic use of opioid pain medication (particularly those for whom the medication had been prescribed for other sources of pain, such as lumbar back pain), the goal was daily opioid use of ≤ 30 morphine equivalent doses (MEDs). During the inpatient stay, we stopped prescribing prophylactic pain medication prior to physical therapy (PT).
We encouraged preoperative optimization of muscle strength by giving instructions for 4 to 8 weeks of daily exercises (Appendix). We introduced perioperative adductor canal blocks (at the discretion of the anesthesia team) and transitioned to surgery without a tourniquet. Patients in both groups received intraoperative antibiotics and IV tranexamic acid (TXA); the MOJO group also received topical TXA.
Further patient care optimization included providing patients with a team-based approach, which consisted of nurse coordinators, physician assistants and nurse practitioners, residents, and the attending surgeon. Our team reviews the planned pain management protocol, perioperative expectations, criteria for discharge, and anticipated surgical outcomes with the patient during their preoperative visits. On postoperative day 1, these members round as a team to encourage patients in their immediate postoperative recovery and rehabilitation. During rounds, the team assesses whether the patient meets the criteria for discharge, adjusting the pain management protocol if necessary.
Changes in surgical technique included arthrotomy with electrocautery, minimizing traumatic dissection or resection of the synovial tissue, and intra-articular injection of a cocktail of ropivacaine 5 mg/mL 40 mL, epinephrine 1:1,000 0.5 mL, and methylprednisolone sodium 40 mg diluted with normal saline to a total volume of 120 mL.
The new routine was gradually implemented beginning January 2017 and fully implemented by July 2018. This study compared the first 20 consecutive patients undergoing primary TKA after July 2018 to the last 20 consecutive patients undergoing primary TKA prior to January 2017. Exclusion criteria included bilateral TKA, death before 90 days, and revision as the indication for surgery. The senior attending surgeon performed all surgeries using a standard midline approach. The majority of surgeries were performed using a cemented Vanguard total knee system (Zimmer Biomet); 4 patients in the historical group had a NexGen knee system, cementless monoblock tibial components (Zimmer Biomet); and 1 patient had a Logic knee system (Exactech). Surgical selection criteria for patients did not differ between groups.
Electronic health records were reviewed and data were abstracted. The data included demographic information (age, gender, body mass index [BMI], diagnosis, and procedure), surgical factors (American Society of Anesthesiologists score, Risk Assessment and Predictive Tool score, operative time, tourniquet time, estimated blood loss), hospital factors (length of stay [LOS], discharge location), postoperative pain scores (measured on postoperative day 1 and on day of discharge), and postdischarge events (90-day complications, telephone calls reporting pain, reoperations, returns to the ED, 90-day readmissions).
The primary outcome was the mean postoperative daily MED during the inpatient stay. Secondary outcomes included pain on postoperative day 1, pain at the time of discharge, LOS, hospital readmissions, and ED visits within 90 days of surgery. Because different opioid pain medications were used by patients postoperatively, all opioids were converted to MED prior to the final analysis. Collected patient data were de-identified prior to analysis.
Power analysis was conducted to determine whether the study had sufficient population size to reject the null hypothesis for the primary outcome measure. Because practitioners controlled postoperative opioid use, a Cohen’s d of 1.0 was used so that a very large effect size was needed to reach clinical significance. Statistical significance was set to 0.05, and patient groups were set at 20 patients each. This yielded an appropriate power of 0.87. Population characteristics were compared between groups using t tests and χ2 tests as appropriate. To analyze the primary outcome, comparisons were made between the 2 cohorts using 2-tailed t tests. Secondary outcomes were compared between groups using t tests or χ2 tests. All statistics were performed using R version 3.5.2. Power analysis was conducted using the package pwr.11 Statistical significance was set at
Results
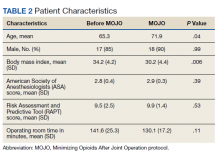
Forty patients met the inclusion criteria, evenly divided between those undergoing TKA before and after instituting the MOJO protocol (Table 2). A single patient in the MOJO group died and was excluded. A patient who underwent bilateral TKA also was excluded. Both groups reflected the male predominance of the VA patient population. MOJO patients tended to have lower BMIs (34 vs 30, P < .01). All patients indicated for surgery with preoperative opioid use were able to titrate down to their preoperative goal as verified by prescriptions filled at VA pharmacies. Twelve of the patients in the MOJO group received adductor canal blocks.
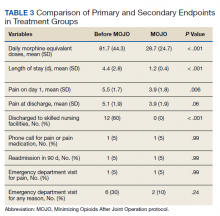
Results of t tests and χ2 tests comparing primary and secondary endpoints are listed in Table 3. Differences between the daily MEDs given in the historical and MOJO groups are shown. There were significant differences between the pre-MOJO and MOJO groups with regard to daily inpatient MEDs (82 mg vs 29 mg, P < .01) and total inpatient MEDs (306 mg vs 32 mg, P < .01). There was less self-reported pain on postoperative day 1 in the MOJO group (5.5 vs 3.9, P < .01), decreased LOS (4.4 days vs 1.2 days, P < .01), a trend toward fewer total ED visits (6 vs 2, P = .24), and fewer discharges to skilled nursing facilities (12 vs 0, P < .01). There were no blood transfusions in either group.
There were no readmissions due to uncontrolled pain. There was 1 readmission for shortness of breath in the MOJO group. The patient was discharged home the following day after ruling out thromboembolic and cardiovascular events. One patient from the control group was readmitted after missing a step on a staircase and falling. The patient sustained a quadriceps tendon rupture and underwent primary suture repair.
Discussion
Our results demonstrate that a multimodal approach to significantly reduce postoperative opioid use in patients with TKA is possible without increasing readmissions or ED visits for pain control. The patients in the MOJO group had a faster recovery, earlier discharge, and less use of postoperative opioid medication. Our approach to postoperative pain management was divided into 2 main categories: patient optimization and surgical optimization.
Patient Selection
Besides the standard evaluation and optimization of patients’ medical conditions, identifying and optimizing at-risk patients before surgery was a critical component of our protocol. Managing postoperative pain in patients with prior opioid use is an intractable challenge in orthopedic surgery. Patients with a history of chronic pain and preoperative use of opioid medications remain at higher risk of postoperative chronic pain and persistent use of opioid medication despite no obvious surgical complications.8 In a sample of > 6,000 veterans who underwent TKA at VA hospitals in 2014, 57% of the patients with daily use of opioids in the 90 days before surgery remained on opioids 1 year after surgery (vs 2 % in patients not on long-term opioids).8 This relationship between pre- and postoperative opioid use also was dose dependent.12
Furthermore, those with high preoperative use may experience worse outcomes relative to the opioid naive population as measured by arthritis-specific pain indices.13 In a well-powered retrospective study of patients who underwent elective orthopedic procedures, preoperative opioid abuse or dependence (determined by the International Classification of Diseases, Ninth Revision diagnosis) increased inpatient mortality, aggregate morbidity, surgical site infection, myocardial infarction, and LOS.14 Preoperative opioid use also has been associated with increased risk of ED visits, readmission, infection, stiffness, and aseptic revision.15 In patients with TKA in the VA specifically, preoperative opioid use (> 3 months in the prior year) was associated with increased revision rates that were even higher than those for patients with diabetes mellitus.16
Patient Education
Based on this evidence, we instruct patients to reduce their preoperative opioid dosing to zero (for patients with joint pain) or < 30 MED (for patients using opioids for other reasons). Although preoperative reduction of opioid use has been shown to improve outcomes after TKA, pain subspecialty recommendations for patients with chronic opioid use recommend considering adjunctive therapies, including transcutaneous electrical nerve stimulation, cognitive behavioral therapy, gabapentin, or ketamine.17,18 Through patient education our team has been successful in decreasing preoperative opioid use without adding other drugs or modalities.
Patient Optimization
Preoperative patient optimization included 4 to 8 weeks of daily sets of physical activity instructions (prehab) to improve the musculoskeletal function. These instructions are given to patients 4 to 8 weeks before surgery and aim to improve the patient’s balance, mobility, and functional ability (Appendix). Meta-analysis has shown that patients who undergo preoperative PT have a small but statistically significant decrease in postoperative pain at 4 weeks, though this does not persist beyond that period.19
We did note a lower BMI in patients in the MOJO group. Though this has the potential to be a confounder, a study of BMI in > 4,000 patients who underwent joint replacement surgery has shown that BMI is not associated with differences in postoperative pain.20
Surgeon and Surgical-Related Variables
Patients in the MOJO group had increased use of adductor canal blocks. A 2017 meta-analysis of 12,530 patients comparing analgesic modalities found that peripheral nerve blocks targeting multiple nerves (eg, femoral/sciatic) decreased pain at rest, decreased opioid consumption, and improved range of motion postoperatively.21 Also, these were found to be superior to single nerve blocks, periarticular infiltration, and epidural blocks.21 However, major nerve and epidural blocks affecting the lower extremity may increase the risk of falls and prolong LOS.22,23 The preferred peripheral block at VAPHCS is a single shot ultrasound-guided adductor canal block before the induction of general or spinal anesthesia. A randomized controlled trial has demonstrated superiority of this block to the femoral nerve block with regard to postoperative quadriceps strength, conferring the theoretical advantage of decreased fall risk and ability to participate in immediate PT.24 Although we are unable to confirm an association between anesthetic modalities and opioid burden, our clinical impression is that blocks were effective at reducing immediate postoperative pain. However, among MOJO patients there were no differences in patients with and without blocks for either pain (4.2 vs 3.8, P = .69) or opioid consumption (28.8 vs 33.0, P = .72) after surgery, though our study was not powered to detect a difference in this restricted subgroup.
Patients who frequently had reported postoperative thigh pain prompted us to make changes in our surgical technique, performing TKA without use of a tourniquet. Tourniquet use has been associated with an increased risk of thigh pain after TKA by multiple authors.25,26 Postoperative thigh pain also is pressure dependent.27 In addition, its use may be associated with a slightly increased risk of thromboembolic events and delayed functional recovery.28,29
Because postoperative hemarthrosis is associated with more pain and reduced joint recovery function, we used topical TXA to reduce postoperative surgical site and joint hematoma. TXA (either oral, IV, or topical) during TKA is used to control postoperative bleeding primarily and decrease the need for transfusion without concomitant increase in thromboembolic events.30,31 Topical TXA may be more effective than IV, particularly in the immediate postoperative period.32 Although pain typically is not an endpoint in studies of TXA, a prospective study of 48 patients showed evidence that its use may be associated with decreased postoperative pain in the first 24 hours after surgery (though not after).33 Finally, the use of intra-articular injection has evolved in our clinical practice, but literature is lacking with regard to its efficacy; more studies are needed to determine its effect relative to no injection. We have not seen any benefits to using
Limitations
This is a nonrandomized retrospective single-institution study. Our study population is composed of mostly males with military experience and is not necessarily a representative sample of the general population eligible for joint arthroplasty. Our primary endpoint (reduction of opioid use postoperatively) also was a cornerstone of our intervention. To account for this, we set a very large effect size in our power analysis and evaluated multiple secondary endpoints to determine whether postoperative pain remained well controlled and complications/readmission minimized with our interventions. Because our intervention was multimodal, our study cannot make conclusions about the effect of a particular component of our treatment strategy. We did not measure or compare functional outcomes between both groups, which offers an opportunity for further research.
These limitations are balanced by several strengths. Our cohort was well controlled with respect to the dose and type of drug used. There is staff dedicated to postoperative telephone follow-up after discharge, and veterans are apt to seek care within the VA health care system, which improves case finding for complications and ED visits. No patients were lost to follow-up. Moreover, our drastic reduction in opioid use is promising enough to warrant reporting, while the broader orthopedic literature explores the relative impact of each variable.
Conclusions
The MOJO protocol has been effective for reducing postoperative opioid use after TKA without compromising effective pain management. The drastic reduction in the postoperative use of opioid pain medications and LOS have contributed to a cultural shift within our department, comprehensive team approach, multimodal pain management, and preoperative patient optimization. Further investigations are required to assess the impact of each intervention on observed outcomes. However, the framework and routines are applicable to other institutions and surgical specialties.
Acknowledgments
The authors recognize Derek Bond, MD, for his help in creating the MOJO acronym.
1. Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999-2017. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics Data Brief No. 329. Published November 2018. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db329-h.pdf
2. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999-2016. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics NCHS data brief No. 294. Published December 2017. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db294.pdf
3. Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic–prescribing rates by specialty, U.S., 2007-2012. Am J Prev Med. 2015;49(3):409-413. doi:10.1016/j.amepre.2015.02.020
4. Guy GP, Zhang K. Opioid prescribing by specialty and volume in the U.S. Am J Prev Med. 2018;55(5):e153-155. doi:10.1016/j.amepre.2018.06.008
5. Kremers HM, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surgery Am. 2015;17:1386-1397. doi:10.2106/JBJS.N.01141
6. Giori NJ, Amanatullah DF, Gupta S, Bowe T, Harris AHS. Risk reduction compared with access to care: quantifying the trade-off of enforcing a body mass index eligibility criterion for joint replacement. J Bone Joint Surg Am. 2018; 4(100):539-545. doi:10.2106/JBJS.17.00120
7. Sabatino MJ, Kunkel ST, Ramkumar DB, Keeney BJ, Jevsevar DS. Excess opioid medication and variation in prescribing patterns following common orthopaedic procedures. J Bone Joint Surg Am. 2018;100(3):180-188. doi:10.2106/JBJS.17.00672
8. Hadlandsmyth K, Vander Weg MW, McCoy KD, Mosher HJ, Vaughan-Sarrazin MS, Lund BC. Risk for prolonged opioid use following total knee arthroplasty in veterans. J Arthroplasty. 2018;33(1):119-123. doi:10.1016/j.arth.2017.08.022
9. Bohnert ASB, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321. doi:10.1001/jama.2011.370
10. Hall MJ, Schwartzman A, Zhang J, Liu X. Ambulatory surgery data from hospitals and ambulatory surgery centers: United States, 2010. Natl Health Stat Report. 2017(102):1-15.
11. Champely S. pwr: basic functions for power analysis. R package version 1.2-2; 2018. Accessed January 13, 2021. https://rdrr.io/cran/pwr/
12. Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157(6):1259-1265. doi:10.1097/j.pain.0000000000000516
13. Smith SR, Bido J, Collins JE, Yang H, Katz JN, Losina E. Impact of preoperative opioid use on total knee arthroplasty outcomes. J Bone Joint Surg Am. 2017;99(10):803-808. doi:10.2106/JBJS.16.01200
14. Menendez ME, Ring D, Bateman BT. Preoperative opioid misuse is associated with increased morbidity and mortality after elective orthopaedic surgery. Clin Orthop Relat Res. 2015;473(7):2402-412. doi:10.1007/s11999-015-4173-5
15. Cancienne JM, Patel KJ, Browne JA, Werner BC. Narcotic use and total knee arthroplasty. J Arthroplasty. 2018;33(1):113-118. doi:10.1016/j.arth.2017.08.006
16. Ben-Ari A, Chansky H, Rozet I. Preoperative opioid use is associated with early revision after total knee arthroplasty: a study of male patients treated in the Veterans Affairs System. J Bone Joint Surg Am. 2017;99(1):1-9. doi:10.2106/JBJS.16.00167
17. Nguyen L-CL, Sing DC, Bozic KJ. Preoperative reduction of opioid use before total joint arthroplasty. J Arthroplasty. 2016;31(suppl 9):282-287. doi:10.1016/j.arth.2016.01.068
18. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157. doi:10.1016/j.jpain.2015.12.008
19. Wang L, Lee M, Zhang Z, Moodie J, Cheng D, Martin J. Does preoperative rehabilitation for patients planning to undergo joint replacement surgery improve outcomes? A systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2016;6(2):e009857. doi:10.1136/bmjopen-2015-009857
20. Li W, Ayers DC, Lewis CG, Bowen TR, Allison JJ, Franklin PD. Functional gain and pain relief after total joint replacement according to obesity status. J Bone Joint Surg. 2017;99(14):1183-1189. doi:10.2106/JBJS.16.00960
21. Terkawi AS, Mavridis D, Sessler DI, et al. Pain management modalities after total knee arthroplasty: a network meta-analysis of 170 randomized controlled trials. Anesthesiology. 2017;126(5):923-937. doi:10.1097/ALN.0000000000001607
22. Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg. 2010;111(6):1552-1554. doi:10.1213/ANE.0b013e3181fb9507
23. Elkassabany NM, Antosh S, Ahmed M, et al. The risk of falls after total knee arthroplasty with the use of a femoral nerve block versus an adductor canal block. Anest Analg. 2016;122(5):1696-1703. doi:10.1213/ane.0000000000001237
24. Wang D, Yang Y, Li Q, et al. Adductor canal block versus femoral nerve block for total knee arthroplasty: a meta-analysis of randomized controlled trials. Sci Rep. 2017;7:40721. doi:10.1038/srep40721
25. Liu D, Graham D, Gillies K, Gillies RM. Effects of tourniquet use on quadriceps function and pain in total knee arthroplasty. Knee Surg Relat Res. 2014;26(4):207-213. doi:10.5792/ksrr.2014.26.4.207
26. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253.
27. Worland RL, Arredondo J, Angles F, Lopez-Jimenez F, Jessup DE. Thigh pain following tourniquet application in simultaneous bilateral total knee replacement arthroplasty. J Arthroplasty. 1997;12(8):848-852. doi:10.1016/s0883-5403(97)90153-4
28. Tai T-W, Lin C-J, Jou I-M, Chang C-W, Lai K-A, Yang C-Y. Tourniquet use in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol, Arthrosc. 2011;19(7):1121-1130. doi:10.1007/s00167-010-1342-7
29. Jiang F-Z, Zhong H-M, Hong Y-C, Zhao G-F. Use of a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Orthop Sci. 2015;20(21):110-123. doi:10.1007/s00776-014-0664-6
30. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585. doi:10.1302/0301-620X.93B12.26989
31. Panteli M, Papakostidis C, Dahabreh Z, Giannoudis PV. Topical tranexamic acid in total knee replacement: a systematic review and meta-analysis. Knee. 2013;20(5):300-309. doi:10.1016/j.knee.2013.05.014
32. Wang J, Wang Q, Zhang X, Wang Q. Intra-articular application is more effective than intravenous application of tranexamic acid in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2017;32(11):3385-3389. doi:10.1016/j.arth.2017.06.024
33. Guerreiro JPF, Badaro BS, Balbino JRM, Danieli MV, Queiroz AO, Cataneo DC. Application of tranexamic acid in total knee arthroplasty – prospective randomized trial. J Open Orthop J. 2017;11:1049-1057. doi:10.2174/1874325001711011049
For decades, opioids have been a mainstay in the management of pain after total joint arthroplasty. In the past 10 years, however, opioid prescribing has come under increased scrutiny due to a rise in rates of opioid abuse, pill diversion, and opioid-related deaths.1,2 Opioids are associated with adverse effects, including nausea, vomiting, constipation, apathy, and respiratory depression, all of which influence arthroplasty outcomes and affect the patient experience. Although primary care groups account for nearly half of prescriptions written, orthopedic surgeons have the third highest per capita rate of opioid prescribing of all medical specialties.3,4 This puts orthopedic surgeons, particularly those who perform routine procedures, in an opportune but challenging position to confront this problem through novel pain management strategies.
Approximately 1 million total knee arthroplasties (TKAs) are performed in the US every year, and the US Department of Veterans Affairs (VA) health system performs about 10,000 hip and knee joint replacements.5,6 There is no standardization of opioid prescribing in the postoperative period following these procedures, and studies have reported a wide variation in prescribing habits even within a single institution for a specific surgery.7 Patients who undergo TKA are at particularly high risk of long-term opioid use if they are on continuous opioids at the time of surgery; this is problematic in a VA patient population in which at least 16% of patients are prescribed opioids in a given year.8 Furthermore, veterans are twice as likely as nonveterans to die of an accidental overdose.9 Despite these risks, opioids remain a cornerstone of postoperative pain management both within and outside of the VA.10
In 2018, to limit unnecessary prescribing of opioid pain medication, the total joint service at the VA Portland Health Care System (VAPHCS) in Oregon implemented the Minimizing Opioids after Joint Operation (MOJO) postoperative pain protocol. The goal of the protocol was to reduce opioid use following TKA. The objectives were to provide safe, appropriate analgesia while allowing early mobilization and discharge without a concomitant increase in readmissions or emergency department (ED) visits. The purpose of this retrospective chart review was to compare the efficacy of the MOJO protocol with our historical experience and report our preliminary results.
Methods
Institutional review board approval was obtained to retrospectively review the medical records of patients who had undergone TKA surgery during 2018 at VAPHCS. The MOJO protocol was composed of several simultaneous changes. The centerpiece of the new protocol was a drastic decrease in routine prescription of postoperative opioids (Table 1). Other changes included instructing patients to reduce the use of preoperative opioid pain medication 6 weeks before surgery with a goal of no opioid consumption, perform daily sets of preoperative exercises, and attend a preoperative consultation/education session with a nurse coordinator to emphasize early recovery and discharge. In patients with chronic use of opioid pain medication (particularly those for whom the medication had been prescribed for other sources of pain, such as lumbar back pain), the goal was daily opioid use of ≤ 30 morphine equivalent doses (MEDs). During the inpatient stay, we stopped prescribing prophylactic pain medication prior to physical therapy (PT).
We encouraged preoperative optimization of muscle strength by giving instructions for 4 to 8 weeks of daily exercises (Appendix). We introduced perioperative adductor canal blocks (at the discretion of the anesthesia team) and transitioned to surgery without a tourniquet. Patients in both groups received intraoperative antibiotics and IV tranexamic acid (TXA); the MOJO group also received topical TXA.
Further patient care optimization included providing patients with a team-based approach, which consisted of nurse coordinators, physician assistants and nurse practitioners, residents, and the attending surgeon. Our team reviews the planned pain management protocol, perioperative expectations, criteria for discharge, and anticipated surgical outcomes with the patient during their preoperative visits. On postoperative day 1, these members round as a team to encourage patients in their immediate postoperative recovery and rehabilitation. During rounds, the team assesses whether the patient meets the criteria for discharge, adjusting the pain management protocol if necessary.
Changes in surgical technique included arthrotomy with electrocautery, minimizing traumatic dissection or resection of the synovial tissue, and intra-articular injection of a cocktail of ropivacaine 5 mg/mL 40 mL, epinephrine 1:1,000 0.5 mL, and methylprednisolone sodium 40 mg diluted with normal saline to a total volume of 120 mL.
The new routine was gradually implemented beginning January 2017 and fully implemented by July 2018. This study compared the first 20 consecutive patients undergoing primary TKA after July 2018 to the last 20 consecutive patients undergoing primary TKA prior to January 2017. Exclusion criteria included bilateral TKA, death before 90 days, and revision as the indication for surgery. The senior attending surgeon performed all surgeries using a standard midline approach. The majority of surgeries were performed using a cemented Vanguard total knee system (Zimmer Biomet); 4 patients in the historical group had a NexGen knee system, cementless monoblock tibial components (Zimmer Biomet); and 1 patient had a Logic knee system (Exactech). Surgical selection criteria for patients did not differ between groups.
Electronic health records were reviewed and data were abstracted. The data included demographic information (age, gender, body mass index [BMI], diagnosis, and procedure), surgical factors (American Society of Anesthesiologists score, Risk Assessment and Predictive Tool score, operative time, tourniquet time, estimated blood loss), hospital factors (length of stay [LOS], discharge location), postoperative pain scores (measured on postoperative day 1 and on day of discharge), and postdischarge events (90-day complications, telephone calls reporting pain, reoperations, returns to the ED, 90-day readmissions).
The primary outcome was the mean postoperative daily MED during the inpatient stay. Secondary outcomes included pain on postoperative day 1, pain at the time of discharge, LOS, hospital readmissions, and ED visits within 90 days of surgery. Because different opioid pain medications were used by patients postoperatively, all opioids were converted to MED prior to the final analysis. Collected patient data were de-identified prior to analysis.
Power analysis was conducted to determine whether the study had sufficient population size to reject the null hypothesis for the primary outcome measure. Because practitioners controlled postoperative opioid use, a Cohen’s d of 1.0 was used so that a very large effect size was needed to reach clinical significance. Statistical significance was set to 0.05, and patient groups were set at 20 patients each. This yielded an appropriate power of 0.87. Population characteristics were compared between groups using t tests and χ2 tests as appropriate. To analyze the primary outcome, comparisons were made between the 2 cohorts using 2-tailed t tests. Secondary outcomes were compared between groups using t tests or χ2 tests. All statistics were performed using R version 3.5.2. Power analysis was conducted using the package pwr.11 Statistical significance was set at
Results
Forty patients met the inclusion criteria, evenly divided between those undergoing TKA before and after instituting the MOJO protocol (Table 2). A single patient in the MOJO group died and was excluded. A patient who underwent bilateral TKA also was excluded. Both groups reflected the male predominance of the VA patient population. MOJO patients tended to have lower BMIs (34 vs 30, P < .01). All patients indicated for surgery with preoperative opioid use were able to titrate down to their preoperative goal as verified by prescriptions filled at VA pharmacies. Twelve of the patients in the MOJO group received adductor canal blocks.
Results of t tests and χ2 tests comparing primary and secondary endpoints are listed in Table 3. Differences between the daily MEDs given in the historical and MOJO groups are shown. There were significant differences between the pre-MOJO and MOJO groups with regard to daily inpatient MEDs (82 mg vs 29 mg, P < .01) and total inpatient MEDs (306 mg vs 32 mg, P < .01). There was less self-reported pain on postoperative day 1 in the MOJO group (5.5 vs 3.9, P < .01), decreased LOS (4.4 days vs 1.2 days, P < .01), a trend toward fewer total ED visits (6 vs 2, P = .24), and fewer discharges to skilled nursing facilities (12 vs 0, P < .01). There were no blood transfusions in either group.
There were no readmissions due to uncontrolled pain. There was 1 readmission for shortness of breath in the MOJO group. The patient was discharged home the following day after ruling out thromboembolic and cardiovascular events. One patient from the control group was readmitted after missing a step on a staircase and falling. The patient sustained a quadriceps tendon rupture and underwent primary suture repair.
Discussion
Our results demonstrate that a multimodal approach to significantly reduce postoperative opioid use in patients with TKA is possible without increasing readmissions or ED visits for pain control. The patients in the MOJO group had a faster recovery, earlier discharge, and less use of postoperative opioid medication. Our approach to postoperative pain management was divided into 2 main categories: patient optimization and surgical optimization.
Patient Selection
Besides the standard evaluation and optimization of patients’ medical conditions, identifying and optimizing at-risk patients before surgery was a critical component of our protocol. Managing postoperative pain in patients with prior opioid use is an intractable challenge in orthopedic surgery. Patients with a history of chronic pain and preoperative use of opioid medications remain at higher risk of postoperative chronic pain and persistent use of opioid medication despite no obvious surgical complications.8 In a sample of > 6,000 veterans who underwent TKA at VA hospitals in 2014, 57% of the patients with daily use of opioids in the 90 days before surgery remained on opioids 1 year after surgery (vs 2 % in patients not on long-term opioids).8 This relationship between pre- and postoperative opioid use also was dose dependent.12
Furthermore, those with high preoperative use may experience worse outcomes relative to the opioid naive population as measured by arthritis-specific pain indices.13 In a well-powered retrospective study of patients who underwent elective orthopedic procedures, preoperative opioid abuse or dependence (determined by the International Classification of Diseases, Ninth Revision diagnosis) increased inpatient mortality, aggregate morbidity, surgical site infection, myocardial infarction, and LOS.14 Preoperative opioid use also has been associated with increased risk of ED visits, readmission, infection, stiffness, and aseptic revision.15 In patients with TKA in the VA specifically, preoperative opioid use (> 3 months in the prior year) was associated with increased revision rates that were even higher than those for patients with diabetes mellitus.16
Patient Education
Based on this evidence, we instruct patients to reduce their preoperative opioid dosing to zero (for patients with joint pain) or < 30 MED (for patients using opioids for other reasons). Although preoperative reduction of opioid use has been shown to improve outcomes after TKA, pain subspecialty recommendations for patients with chronic opioid use recommend considering adjunctive therapies, including transcutaneous electrical nerve stimulation, cognitive behavioral therapy, gabapentin, or ketamine.17,18 Through patient education our team has been successful in decreasing preoperative opioid use without adding other drugs or modalities.
Patient Optimization
Preoperative patient optimization included 4 to 8 weeks of daily sets of physical activity instructions (prehab) to improve the musculoskeletal function. These instructions are given to patients 4 to 8 weeks before surgery and aim to improve the patient’s balance, mobility, and functional ability (Appendix). Meta-analysis has shown that patients who undergo preoperative PT have a small but statistically significant decrease in postoperative pain at 4 weeks, though this does not persist beyond that period.19
We did note a lower BMI in patients in the MOJO group. Though this has the potential to be a confounder, a study of BMI in > 4,000 patients who underwent joint replacement surgery has shown that BMI is not associated with differences in postoperative pain.20
Surgeon and Surgical-Related Variables
Patients in the MOJO group had increased use of adductor canal blocks. A 2017 meta-analysis of 12,530 patients comparing analgesic modalities found that peripheral nerve blocks targeting multiple nerves (eg, femoral/sciatic) decreased pain at rest, decreased opioid consumption, and improved range of motion postoperatively.21 Also, these were found to be superior to single nerve blocks, periarticular infiltration, and epidural blocks.21 However, major nerve and epidural blocks affecting the lower extremity may increase the risk of falls and prolong LOS.22,23 The preferred peripheral block at VAPHCS is a single shot ultrasound-guided adductor canal block before the induction of general or spinal anesthesia. A randomized controlled trial has demonstrated superiority of this block to the femoral nerve block with regard to postoperative quadriceps strength, conferring the theoretical advantage of decreased fall risk and ability to participate in immediate PT.24 Although we are unable to confirm an association between anesthetic modalities and opioid burden, our clinical impression is that blocks were effective at reducing immediate postoperative pain. However, among MOJO patients there were no differences in patients with and without blocks for either pain (4.2 vs 3.8, P = .69) or opioid consumption (28.8 vs 33.0, P = .72) after surgery, though our study was not powered to detect a difference in this restricted subgroup.
Patients who frequently had reported postoperative thigh pain prompted us to make changes in our surgical technique, performing TKA without use of a tourniquet. Tourniquet use has been associated with an increased risk of thigh pain after TKA by multiple authors.25,26 Postoperative thigh pain also is pressure dependent.27 In addition, its use may be associated with a slightly increased risk of thromboembolic events and delayed functional recovery.28,29
Because postoperative hemarthrosis is associated with more pain and reduced joint recovery function, we used topical TXA to reduce postoperative surgical site and joint hematoma. TXA (either oral, IV, or topical) during TKA is used to control postoperative bleeding primarily and decrease the need for transfusion without concomitant increase in thromboembolic events.30,31 Topical TXA may be more effective than IV, particularly in the immediate postoperative period.32 Although pain typically is not an endpoint in studies of TXA, a prospective study of 48 patients showed evidence that its use may be associated with decreased postoperative pain in the first 24 hours after surgery (though not after).33 Finally, the use of intra-articular injection has evolved in our clinical practice, but literature is lacking with regard to its efficacy; more studies are needed to determine its effect relative to no injection. We have not seen any benefits to using
Limitations
This is a nonrandomized retrospective single-institution study. Our study population is composed of mostly males with military experience and is not necessarily a representative sample of the general population eligible for joint arthroplasty. Our primary endpoint (reduction of opioid use postoperatively) also was a cornerstone of our intervention. To account for this, we set a very large effect size in our power analysis and evaluated multiple secondary endpoints to determine whether postoperative pain remained well controlled and complications/readmission minimized with our interventions. Because our intervention was multimodal, our study cannot make conclusions about the effect of a particular component of our treatment strategy. We did not measure or compare functional outcomes between both groups, which offers an opportunity for further research.
These limitations are balanced by several strengths. Our cohort was well controlled with respect to the dose and type of drug used. There is staff dedicated to postoperative telephone follow-up after discharge, and veterans are apt to seek care within the VA health care system, which improves case finding for complications and ED visits. No patients were lost to follow-up. Moreover, our drastic reduction in opioid use is promising enough to warrant reporting, while the broader orthopedic literature explores the relative impact of each variable.
Conclusions
The MOJO protocol has been effective for reducing postoperative opioid use after TKA without compromising effective pain management. The drastic reduction in the postoperative use of opioid pain medications and LOS have contributed to a cultural shift within our department, comprehensive team approach, multimodal pain management, and preoperative patient optimization. Further investigations are required to assess the impact of each intervention on observed outcomes. However, the framework and routines are applicable to other institutions and surgical specialties.
Acknowledgments
The authors recognize Derek Bond, MD, for his help in creating the MOJO acronym.
For decades, opioids have been a mainstay in the management of pain after total joint arthroplasty. In the past 10 years, however, opioid prescribing has come under increased scrutiny due to a rise in rates of opioid abuse, pill diversion, and opioid-related deaths.1,2 Opioids are associated with adverse effects, including nausea, vomiting, constipation, apathy, and respiratory depression, all of which influence arthroplasty outcomes and affect the patient experience. Although primary care groups account for nearly half of prescriptions written, orthopedic surgeons have the third highest per capita rate of opioid prescribing of all medical specialties.3,4 This puts orthopedic surgeons, particularly those who perform routine procedures, in an opportune but challenging position to confront this problem through novel pain management strategies.
Approximately 1 million total knee arthroplasties (TKAs) are performed in the US every year, and the US Department of Veterans Affairs (VA) health system performs about 10,000 hip and knee joint replacements.5,6 There is no standardization of opioid prescribing in the postoperative period following these procedures, and studies have reported a wide variation in prescribing habits even within a single institution for a specific surgery.7 Patients who undergo TKA are at particularly high risk of long-term opioid use if they are on continuous opioids at the time of surgery; this is problematic in a VA patient population in which at least 16% of patients are prescribed opioids in a given year.8 Furthermore, veterans are twice as likely as nonveterans to die of an accidental overdose.9 Despite these risks, opioids remain a cornerstone of postoperative pain management both within and outside of the VA.10
In 2018, to limit unnecessary prescribing of opioid pain medication, the total joint service at the VA Portland Health Care System (VAPHCS) in Oregon implemented the Minimizing Opioids after Joint Operation (MOJO) postoperative pain protocol. The goal of the protocol was to reduce opioid use following TKA. The objectives were to provide safe, appropriate analgesia while allowing early mobilization and discharge without a concomitant increase in readmissions or emergency department (ED) visits. The purpose of this retrospective chart review was to compare the efficacy of the MOJO protocol with our historical experience and report our preliminary results.
Methods
Institutional review board approval was obtained to retrospectively review the medical records of patients who had undergone TKA surgery during 2018 at VAPHCS. The MOJO protocol was composed of several simultaneous changes. The centerpiece of the new protocol was a drastic decrease in routine prescription of postoperative opioids (Table 1). Other changes included instructing patients to reduce the use of preoperative opioid pain medication 6 weeks before surgery with a goal of no opioid consumption, perform daily sets of preoperative exercises, and attend a preoperative consultation/education session with a nurse coordinator to emphasize early recovery and discharge. In patients with chronic use of opioid pain medication (particularly those for whom the medication had been prescribed for other sources of pain, such as lumbar back pain), the goal was daily opioid use of ≤ 30 morphine equivalent doses (MEDs). During the inpatient stay, we stopped prescribing prophylactic pain medication prior to physical therapy (PT).
We encouraged preoperative optimization of muscle strength by giving instructions for 4 to 8 weeks of daily exercises (Appendix). We introduced perioperative adductor canal blocks (at the discretion of the anesthesia team) and transitioned to surgery without a tourniquet. Patients in both groups received intraoperative antibiotics and IV tranexamic acid (TXA); the MOJO group also received topical TXA.
Further patient care optimization included providing patients with a team-based approach, which consisted of nurse coordinators, physician assistants and nurse practitioners, residents, and the attending surgeon. Our team reviews the planned pain management protocol, perioperative expectations, criteria for discharge, and anticipated surgical outcomes with the patient during their preoperative visits. On postoperative day 1, these members round as a team to encourage patients in their immediate postoperative recovery and rehabilitation. During rounds, the team assesses whether the patient meets the criteria for discharge, adjusting the pain management protocol if necessary.
Changes in surgical technique included arthrotomy with electrocautery, minimizing traumatic dissection or resection of the synovial tissue, and intra-articular injection of a cocktail of ropivacaine 5 mg/mL 40 mL, epinephrine 1:1,000 0.5 mL, and methylprednisolone sodium 40 mg diluted with normal saline to a total volume of 120 mL.
The new routine was gradually implemented beginning January 2017 and fully implemented by July 2018. This study compared the first 20 consecutive patients undergoing primary TKA after July 2018 to the last 20 consecutive patients undergoing primary TKA prior to January 2017. Exclusion criteria included bilateral TKA, death before 90 days, and revision as the indication for surgery. The senior attending surgeon performed all surgeries using a standard midline approach. The majority of surgeries were performed using a cemented Vanguard total knee system (Zimmer Biomet); 4 patients in the historical group had a NexGen knee system, cementless monoblock tibial components (Zimmer Biomet); and 1 patient had a Logic knee system (Exactech). Surgical selection criteria for patients did not differ between groups.
Electronic health records were reviewed and data were abstracted. The data included demographic information (age, gender, body mass index [BMI], diagnosis, and procedure), surgical factors (American Society of Anesthesiologists score, Risk Assessment and Predictive Tool score, operative time, tourniquet time, estimated blood loss), hospital factors (length of stay [LOS], discharge location), postoperative pain scores (measured on postoperative day 1 and on day of discharge), and postdischarge events (90-day complications, telephone calls reporting pain, reoperations, returns to the ED, 90-day readmissions).
The primary outcome was the mean postoperative daily MED during the inpatient stay. Secondary outcomes included pain on postoperative day 1, pain at the time of discharge, LOS, hospital readmissions, and ED visits within 90 days of surgery. Because different opioid pain medications were used by patients postoperatively, all opioids were converted to MED prior to the final analysis. Collected patient data were de-identified prior to analysis.
Power analysis was conducted to determine whether the study had sufficient population size to reject the null hypothesis for the primary outcome measure. Because practitioners controlled postoperative opioid use, a Cohen’s d of 1.0 was used so that a very large effect size was needed to reach clinical significance. Statistical significance was set to 0.05, and patient groups were set at 20 patients each. This yielded an appropriate power of 0.87. Population characteristics were compared between groups using t tests and χ2 tests as appropriate. To analyze the primary outcome, comparisons were made between the 2 cohorts using 2-tailed t tests. Secondary outcomes were compared between groups using t tests or χ2 tests. All statistics were performed using R version 3.5.2. Power analysis was conducted using the package pwr.11 Statistical significance was set at
Results
Forty patients met the inclusion criteria, evenly divided between those undergoing TKA before and after instituting the MOJO protocol (Table 2). A single patient in the MOJO group died and was excluded. A patient who underwent bilateral TKA also was excluded. Both groups reflected the male predominance of the VA patient population. MOJO patients tended to have lower BMIs (34 vs 30, P < .01). All patients indicated for surgery with preoperative opioid use were able to titrate down to their preoperative goal as verified by prescriptions filled at VA pharmacies. Twelve of the patients in the MOJO group received adductor canal blocks.
Results of t tests and χ2 tests comparing primary and secondary endpoints are listed in Table 3. Differences between the daily MEDs given in the historical and MOJO groups are shown. There were significant differences between the pre-MOJO and MOJO groups with regard to daily inpatient MEDs (82 mg vs 29 mg, P < .01) and total inpatient MEDs (306 mg vs 32 mg, P < .01). There was less self-reported pain on postoperative day 1 in the MOJO group (5.5 vs 3.9, P < .01), decreased LOS (4.4 days vs 1.2 days, P < .01), a trend toward fewer total ED visits (6 vs 2, P = .24), and fewer discharges to skilled nursing facilities (12 vs 0, P < .01). There were no blood transfusions in either group.
There were no readmissions due to uncontrolled pain. There was 1 readmission for shortness of breath in the MOJO group. The patient was discharged home the following day after ruling out thromboembolic and cardiovascular events. One patient from the control group was readmitted after missing a step on a staircase and falling. The patient sustained a quadriceps tendon rupture and underwent primary suture repair.
Discussion
Our results demonstrate that a multimodal approach to significantly reduce postoperative opioid use in patients with TKA is possible without increasing readmissions or ED visits for pain control. The patients in the MOJO group had a faster recovery, earlier discharge, and less use of postoperative opioid medication. Our approach to postoperative pain management was divided into 2 main categories: patient optimization and surgical optimization.
Patient Selection
Besides the standard evaluation and optimization of patients’ medical conditions, identifying and optimizing at-risk patients before surgery was a critical component of our protocol. Managing postoperative pain in patients with prior opioid use is an intractable challenge in orthopedic surgery. Patients with a history of chronic pain and preoperative use of opioid medications remain at higher risk of postoperative chronic pain and persistent use of opioid medication despite no obvious surgical complications.8 In a sample of > 6,000 veterans who underwent TKA at VA hospitals in 2014, 57% of the patients with daily use of opioids in the 90 days before surgery remained on opioids 1 year after surgery (vs 2 % in patients not on long-term opioids).8 This relationship between pre- and postoperative opioid use also was dose dependent.12
Furthermore, those with high preoperative use may experience worse outcomes relative to the opioid naive population as measured by arthritis-specific pain indices.13 In a well-powered retrospective study of patients who underwent elective orthopedic procedures, preoperative opioid abuse or dependence (determined by the International Classification of Diseases, Ninth Revision diagnosis) increased inpatient mortality, aggregate morbidity, surgical site infection, myocardial infarction, and LOS.14 Preoperative opioid use also has been associated with increased risk of ED visits, readmission, infection, stiffness, and aseptic revision.15 In patients with TKA in the VA specifically, preoperative opioid use (> 3 months in the prior year) was associated with increased revision rates that were even higher than those for patients with diabetes mellitus.16
Patient Education
Based on this evidence, we instruct patients to reduce their preoperative opioid dosing to zero (for patients with joint pain) or < 30 MED (for patients using opioids for other reasons). Although preoperative reduction of opioid use has been shown to improve outcomes after TKA, pain subspecialty recommendations for patients with chronic opioid use recommend considering adjunctive therapies, including transcutaneous electrical nerve stimulation, cognitive behavioral therapy, gabapentin, or ketamine.17,18 Through patient education our team has been successful in decreasing preoperative opioid use without adding other drugs or modalities.
Patient Optimization
Preoperative patient optimization included 4 to 8 weeks of daily sets of physical activity instructions (prehab) to improve the musculoskeletal function. These instructions are given to patients 4 to 8 weeks before surgery and aim to improve the patient’s balance, mobility, and functional ability (Appendix). Meta-analysis has shown that patients who undergo preoperative PT have a small but statistically significant decrease in postoperative pain at 4 weeks, though this does not persist beyond that period.19
We did note a lower BMI in patients in the MOJO group. Though this has the potential to be a confounder, a study of BMI in > 4,000 patients who underwent joint replacement surgery has shown that BMI is not associated with differences in postoperative pain.20
Surgeon and Surgical-Related Variables
Patients in the MOJO group had increased use of adductor canal blocks. A 2017 meta-analysis of 12,530 patients comparing analgesic modalities found that peripheral nerve blocks targeting multiple nerves (eg, femoral/sciatic) decreased pain at rest, decreased opioid consumption, and improved range of motion postoperatively.21 Also, these were found to be superior to single nerve blocks, periarticular infiltration, and epidural blocks.21 However, major nerve and epidural blocks affecting the lower extremity may increase the risk of falls and prolong LOS.22,23 The preferred peripheral block at VAPHCS is a single shot ultrasound-guided adductor canal block before the induction of general or spinal anesthesia. A randomized controlled trial has demonstrated superiority of this block to the femoral nerve block with regard to postoperative quadriceps strength, conferring the theoretical advantage of decreased fall risk and ability to participate in immediate PT.24 Although we are unable to confirm an association between anesthetic modalities and opioid burden, our clinical impression is that blocks were effective at reducing immediate postoperative pain. However, among MOJO patients there were no differences in patients with and without blocks for either pain (4.2 vs 3.8, P = .69) or opioid consumption (28.8 vs 33.0, P = .72) after surgery, though our study was not powered to detect a difference in this restricted subgroup.
Patients who frequently had reported postoperative thigh pain prompted us to make changes in our surgical technique, performing TKA without use of a tourniquet. Tourniquet use has been associated with an increased risk of thigh pain after TKA by multiple authors.25,26 Postoperative thigh pain also is pressure dependent.27 In addition, its use may be associated with a slightly increased risk of thromboembolic events and delayed functional recovery.28,29
Because postoperative hemarthrosis is associated with more pain and reduced joint recovery function, we used topical TXA to reduce postoperative surgical site and joint hematoma. TXA (either oral, IV, or topical) during TKA is used to control postoperative bleeding primarily and decrease the need for transfusion without concomitant increase in thromboembolic events.30,31 Topical TXA may be more effective than IV, particularly in the immediate postoperative period.32 Although pain typically is not an endpoint in studies of TXA, a prospective study of 48 patients showed evidence that its use may be associated with decreased postoperative pain in the first 24 hours after surgery (though not after).33 Finally, the use of intra-articular injection has evolved in our clinical practice, but literature is lacking with regard to its efficacy; more studies are needed to determine its effect relative to no injection. We have not seen any benefits to using
Limitations
This is a nonrandomized retrospective single-institution study. Our study population is composed of mostly males with military experience and is not necessarily a representative sample of the general population eligible for joint arthroplasty. Our primary endpoint (reduction of opioid use postoperatively) also was a cornerstone of our intervention. To account for this, we set a very large effect size in our power analysis and evaluated multiple secondary endpoints to determine whether postoperative pain remained well controlled and complications/readmission minimized with our interventions. Because our intervention was multimodal, our study cannot make conclusions about the effect of a particular component of our treatment strategy. We did not measure or compare functional outcomes between both groups, which offers an opportunity for further research.
These limitations are balanced by several strengths. Our cohort was well controlled with respect to the dose and type of drug used. There is staff dedicated to postoperative telephone follow-up after discharge, and veterans are apt to seek care within the VA health care system, which improves case finding for complications and ED visits. No patients were lost to follow-up. Moreover, our drastic reduction in opioid use is promising enough to warrant reporting, while the broader orthopedic literature explores the relative impact of each variable.
Conclusions
The MOJO protocol has been effective for reducing postoperative opioid use after TKA without compromising effective pain management. The drastic reduction in the postoperative use of opioid pain medications and LOS have contributed to a cultural shift within our department, comprehensive team approach, multimodal pain management, and preoperative patient optimization. Further investigations are required to assess the impact of each intervention on observed outcomes. However, the framework and routines are applicable to other institutions and surgical specialties.
Acknowledgments
The authors recognize Derek Bond, MD, for his help in creating the MOJO acronym.
1. Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999-2017. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics Data Brief No. 329. Published November 2018. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db329-h.pdf
2. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999-2016. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics NCHS data brief No. 294. Published December 2017. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db294.pdf
3. Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic–prescribing rates by specialty, U.S., 2007-2012. Am J Prev Med. 2015;49(3):409-413. doi:10.1016/j.amepre.2015.02.020
4. Guy GP, Zhang K. Opioid prescribing by specialty and volume in the U.S. Am J Prev Med. 2018;55(5):e153-155. doi:10.1016/j.amepre.2018.06.008
5. Kremers HM, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surgery Am. 2015;17:1386-1397. doi:10.2106/JBJS.N.01141
6. Giori NJ, Amanatullah DF, Gupta S, Bowe T, Harris AHS. Risk reduction compared with access to care: quantifying the trade-off of enforcing a body mass index eligibility criterion for joint replacement. J Bone Joint Surg Am. 2018; 4(100):539-545. doi:10.2106/JBJS.17.00120
7. Sabatino MJ, Kunkel ST, Ramkumar DB, Keeney BJ, Jevsevar DS. Excess opioid medication and variation in prescribing patterns following common orthopaedic procedures. J Bone Joint Surg Am. 2018;100(3):180-188. doi:10.2106/JBJS.17.00672
8. Hadlandsmyth K, Vander Weg MW, McCoy KD, Mosher HJ, Vaughan-Sarrazin MS, Lund BC. Risk for prolonged opioid use following total knee arthroplasty in veterans. J Arthroplasty. 2018;33(1):119-123. doi:10.1016/j.arth.2017.08.022
9. Bohnert ASB, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321. doi:10.1001/jama.2011.370
10. Hall MJ, Schwartzman A, Zhang J, Liu X. Ambulatory surgery data from hospitals and ambulatory surgery centers: United States, 2010. Natl Health Stat Report. 2017(102):1-15.
11. Champely S. pwr: basic functions for power analysis. R package version 1.2-2; 2018. Accessed January 13, 2021. https://rdrr.io/cran/pwr/
12. Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157(6):1259-1265. doi:10.1097/j.pain.0000000000000516
13. Smith SR, Bido J, Collins JE, Yang H, Katz JN, Losina E. Impact of preoperative opioid use on total knee arthroplasty outcomes. J Bone Joint Surg Am. 2017;99(10):803-808. doi:10.2106/JBJS.16.01200
14. Menendez ME, Ring D, Bateman BT. Preoperative opioid misuse is associated with increased morbidity and mortality after elective orthopaedic surgery. Clin Orthop Relat Res. 2015;473(7):2402-412. doi:10.1007/s11999-015-4173-5
15. Cancienne JM, Patel KJ, Browne JA, Werner BC. Narcotic use and total knee arthroplasty. J Arthroplasty. 2018;33(1):113-118. doi:10.1016/j.arth.2017.08.006
16. Ben-Ari A, Chansky H, Rozet I. Preoperative opioid use is associated with early revision after total knee arthroplasty: a study of male patients treated in the Veterans Affairs System. J Bone Joint Surg Am. 2017;99(1):1-9. doi:10.2106/JBJS.16.00167
17. Nguyen L-CL, Sing DC, Bozic KJ. Preoperative reduction of opioid use before total joint arthroplasty. J Arthroplasty. 2016;31(suppl 9):282-287. doi:10.1016/j.arth.2016.01.068
18. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157. doi:10.1016/j.jpain.2015.12.008
19. Wang L, Lee M, Zhang Z, Moodie J, Cheng D, Martin J. Does preoperative rehabilitation for patients planning to undergo joint replacement surgery improve outcomes? A systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2016;6(2):e009857. doi:10.1136/bmjopen-2015-009857
20. Li W, Ayers DC, Lewis CG, Bowen TR, Allison JJ, Franklin PD. Functional gain and pain relief after total joint replacement according to obesity status. J Bone Joint Surg. 2017;99(14):1183-1189. doi:10.2106/JBJS.16.00960
21. Terkawi AS, Mavridis D, Sessler DI, et al. Pain management modalities after total knee arthroplasty: a network meta-analysis of 170 randomized controlled trials. Anesthesiology. 2017;126(5):923-937. doi:10.1097/ALN.0000000000001607
22. Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg. 2010;111(6):1552-1554. doi:10.1213/ANE.0b013e3181fb9507
23. Elkassabany NM, Antosh S, Ahmed M, et al. The risk of falls after total knee arthroplasty with the use of a femoral nerve block versus an adductor canal block. Anest Analg. 2016;122(5):1696-1703. doi:10.1213/ane.0000000000001237
24. Wang D, Yang Y, Li Q, et al. Adductor canal block versus femoral nerve block for total knee arthroplasty: a meta-analysis of randomized controlled trials. Sci Rep. 2017;7:40721. doi:10.1038/srep40721
25. Liu D, Graham D, Gillies K, Gillies RM. Effects of tourniquet use on quadriceps function and pain in total knee arthroplasty. Knee Surg Relat Res. 2014;26(4):207-213. doi:10.5792/ksrr.2014.26.4.207
26. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253.
27. Worland RL, Arredondo J, Angles F, Lopez-Jimenez F, Jessup DE. Thigh pain following tourniquet application in simultaneous bilateral total knee replacement arthroplasty. J Arthroplasty. 1997;12(8):848-852. doi:10.1016/s0883-5403(97)90153-4
28. Tai T-W, Lin C-J, Jou I-M, Chang C-W, Lai K-A, Yang C-Y. Tourniquet use in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol, Arthrosc. 2011;19(7):1121-1130. doi:10.1007/s00167-010-1342-7
29. Jiang F-Z, Zhong H-M, Hong Y-C, Zhao G-F. Use of a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Orthop Sci. 2015;20(21):110-123. doi:10.1007/s00776-014-0664-6
30. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585. doi:10.1302/0301-620X.93B12.26989
31. Panteli M, Papakostidis C, Dahabreh Z, Giannoudis PV. Topical tranexamic acid in total knee replacement: a systematic review and meta-analysis. Knee. 2013;20(5):300-309. doi:10.1016/j.knee.2013.05.014
32. Wang J, Wang Q, Zhang X, Wang Q. Intra-articular application is more effective than intravenous application of tranexamic acid in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2017;32(11):3385-3389. doi:10.1016/j.arth.2017.06.024
33. Guerreiro JPF, Badaro BS, Balbino JRM, Danieli MV, Queiroz AO, Cataneo DC. Application of tranexamic acid in total knee arthroplasty – prospective randomized trial. J Open Orthop J. 2017;11:1049-1057. doi:10.2174/1874325001711011049
1. Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999-2017. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics Data Brief No. 329. Published November 2018. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db329-h.pdf
2. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999-2016. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics NCHS data brief No. 294. Published December 2017. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db294.pdf
3. Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic–prescribing rates by specialty, U.S., 2007-2012. Am J Prev Med. 2015;49(3):409-413. doi:10.1016/j.amepre.2015.02.020
4. Guy GP, Zhang K. Opioid prescribing by specialty and volume in the U.S. Am J Prev Med. 2018;55(5):e153-155. doi:10.1016/j.amepre.2018.06.008
5. Kremers HM, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surgery Am. 2015;17:1386-1397. doi:10.2106/JBJS.N.01141
6. Giori NJ, Amanatullah DF, Gupta S, Bowe T, Harris AHS. Risk reduction compared with access to care: quantifying the trade-off of enforcing a body mass index eligibility criterion for joint replacement. J Bone Joint Surg Am. 2018; 4(100):539-545. doi:10.2106/JBJS.17.00120
7. Sabatino MJ, Kunkel ST, Ramkumar DB, Keeney BJ, Jevsevar DS. Excess opioid medication and variation in prescribing patterns following common orthopaedic procedures. J Bone Joint Surg Am. 2018;100(3):180-188. doi:10.2106/JBJS.17.00672
8. Hadlandsmyth K, Vander Weg MW, McCoy KD, Mosher HJ, Vaughan-Sarrazin MS, Lund BC. Risk for prolonged opioid use following total knee arthroplasty in veterans. J Arthroplasty. 2018;33(1):119-123. doi:10.1016/j.arth.2017.08.022
9. Bohnert ASB, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321. doi:10.1001/jama.2011.370
10. Hall MJ, Schwartzman A, Zhang J, Liu X. Ambulatory surgery data from hospitals and ambulatory surgery centers: United States, 2010. Natl Health Stat Report. 2017(102):1-15.
11. Champely S. pwr: basic functions for power analysis. R package version 1.2-2; 2018. Accessed January 13, 2021. https://rdrr.io/cran/pwr/
12. Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157(6):1259-1265. doi:10.1097/j.pain.0000000000000516
13. Smith SR, Bido J, Collins JE, Yang H, Katz JN, Losina E. Impact of preoperative opioid use on total knee arthroplasty outcomes. J Bone Joint Surg Am. 2017;99(10):803-808. doi:10.2106/JBJS.16.01200
14. Menendez ME, Ring D, Bateman BT. Preoperative opioid misuse is associated with increased morbidity and mortality after elective orthopaedic surgery. Clin Orthop Relat Res. 2015;473(7):2402-412. doi:10.1007/s11999-015-4173-5
15. Cancienne JM, Patel KJ, Browne JA, Werner BC. Narcotic use and total knee arthroplasty. J Arthroplasty. 2018;33(1):113-118. doi:10.1016/j.arth.2017.08.006
16. Ben-Ari A, Chansky H, Rozet I. Preoperative opioid use is associated with early revision after total knee arthroplasty: a study of male patients treated in the Veterans Affairs System. J Bone Joint Surg Am. 2017;99(1):1-9. doi:10.2106/JBJS.16.00167
17. Nguyen L-CL, Sing DC, Bozic KJ. Preoperative reduction of opioid use before total joint arthroplasty. J Arthroplasty. 2016;31(suppl 9):282-287. doi:10.1016/j.arth.2016.01.068
18. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157. doi:10.1016/j.jpain.2015.12.008
19. Wang L, Lee M, Zhang Z, Moodie J, Cheng D, Martin J. Does preoperative rehabilitation for patients planning to undergo joint replacement surgery improve outcomes? A systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2016;6(2):e009857. doi:10.1136/bmjopen-2015-009857
20. Li W, Ayers DC, Lewis CG, Bowen TR, Allison JJ, Franklin PD. Functional gain and pain relief after total joint replacement according to obesity status. J Bone Joint Surg. 2017;99(14):1183-1189. doi:10.2106/JBJS.16.00960
21. Terkawi AS, Mavridis D, Sessler DI, et al. Pain management modalities after total knee arthroplasty: a network meta-analysis of 170 randomized controlled trials. Anesthesiology. 2017;126(5):923-937. doi:10.1097/ALN.0000000000001607
22. Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg. 2010;111(6):1552-1554. doi:10.1213/ANE.0b013e3181fb9507
23. Elkassabany NM, Antosh S, Ahmed M, et al. The risk of falls after total knee arthroplasty with the use of a femoral nerve block versus an adductor canal block. Anest Analg. 2016;122(5):1696-1703. doi:10.1213/ane.0000000000001237
24. Wang D, Yang Y, Li Q, et al. Adductor canal block versus femoral nerve block for total knee arthroplasty: a meta-analysis of randomized controlled trials. Sci Rep. 2017;7:40721. doi:10.1038/srep40721
25. Liu D, Graham D, Gillies K, Gillies RM. Effects of tourniquet use on quadriceps function and pain in total knee arthroplasty. Knee Surg Relat Res. 2014;26(4):207-213. doi:10.5792/ksrr.2014.26.4.207
26. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253.
27. Worland RL, Arredondo J, Angles F, Lopez-Jimenez F, Jessup DE. Thigh pain following tourniquet application in simultaneous bilateral total knee replacement arthroplasty. J Arthroplasty. 1997;12(8):848-852. doi:10.1016/s0883-5403(97)90153-4
28. Tai T-W, Lin C-J, Jou I-M, Chang C-W, Lai K-A, Yang C-Y. Tourniquet use in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol, Arthrosc. 2011;19(7):1121-1130. doi:10.1007/s00167-010-1342-7
29. Jiang F-Z, Zhong H-M, Hong Y-C, Zhao G-F. Use of a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Orthop Sci. 2015;20(21):110-123. doi:10.1007/s00776-014-0664-6
30. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585. doi:10.1302/0301-620X.93B12.26989
31. Panteli M, Papakostidis C, Dahabreh Z, Giannoudis PV. Topical tranexamic acid in total knee replacement: a systematic review and meta-analysis. Knee. 2013;20(5):300-309. doi:10.1016/j.knee.2013.05.014
32. Wang J, Wang Q, Zhang X, Wang Q. Intra-articular application is more effective than intravenous application of tranexamic acid in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2017;32(11):3385-3389. doi:10.1016/j.arth.2017.06.024
33. Guerreiro JPF, Badaro BS, Balbino JRM, Danieli MV, Queiroz AO, Cataneo DC. Application of tranexamic acid in total knee arthroplasty – prospective randomized trial. J Open Orthop J. 2017;11:1049-1057. doi:10.2174/1874325001711011049
Cannabis tied to self-harm, death in youth with mood disorders
Adolescents and young adults with mood disorders and cannabis use disorder (CUD) are at significantly increased risk for self-harm, all-cause mortality, homicide, and death by unintentional overdose, new research suggests.
Investigators found the risk for self-harm was three times higher, all-cause mortality was 59% higher, unintentional overdose was 2.5 times higher, and homicide was more than three times higher in those with versus without CUD.
“The take-home message of these findings is that we need to be aware of the perception that cannabis use is harmless, when it’s actually not,” lead author Cynthia Fontanella, PhD, associate professor of psychiatry, Ohio State University Wexner Medical Center, Columbus, said in an interview.
“We need to educate parents and clinicians that there are risks associated with cannabis, including increased risk for self-harm and death, and we need to effectively treat both cannabis use disorder and mood disorders,” she said.
The study was published online Jan. 19, 2021, in JAMA Pediatrics.
Little research in youth
“There has been very little research conducted on CUD in the adolescent population, and most studies have been conducted with adults,” Dr. Fontanella said.
Research on adults has shown that, even in people without mood disorders, cannabis use is associated with the early onset of mood disorders, psychosis, and anxiety disorders and has also been linked with suicidal behavior and increased risk for motor vehicle accidents, Dr. Fontanella said.
“We were motivated to conduct this study because we treat kids with depression and bipolar disorder and we noticed a high prevalence of CUD in this population, so we were curious about what its negative effects might be,” Dr. Fontanella recounted.
The researchers analyzed 7-year data drawn from Ohio Medicaid claims and linked to data from death certificates in 204,780 youths between the ages of 10 and 24 years (mean age was 17.2 years at the time of mood disorder diagnosis). Most were female, non-Hispanic White, enrolled in Medicaid because of poverty, and living in a metropolitan area (65.0%, 66.9%, 87.6%, and 77.1%, respectively).
Participants were followed up to 1 year from diagnosis until the end of enrollment, a self-harm event, or death.
Researchers included demographic, clinical, and treatment factors as covariates.
Close to three-quarters (72.7%) of the cohort had a depressive disorder, followed by unspecified/persistent mood disorder and bipolar disorder (14.9% and 12.4%, respectively). Comorbidities included ADHD (12.4%), anxiety disorder (12.3%), and other mental disorders (13.1%).
One -tenth of the cohort (10.3%) were diagnosed with CUD.
CUD treatment referrals
“Although CUD was associated with suicide in the unadjusted model, it was not significantly associated in adjusted models,” the authors reported.
Dr. Fontanella noted that the risk for these adverse outcomes is greater among those who engage in heavy, frequent use or who use cannabis that has higher-potency tetrahydrocannabinol (THC) content.
Reasons why CUD might be associated with these adverse outcomes are that it can increase impulsivity, poor judgment, and clouded thinking, which may in turn increase the risk for self-harm behaviors, she said.
She recommended that clinicians refer youth with CUD for “effective treatments,” including family-based models and individual approaches, such as cognitive behavioral therapy and motivational enhancement therapy.
Open dialogue
In a comment, Wilfrid Noel Raby, MD, PhD, adjunct clinical professor, Albert Einstein College of Medicine, New York, noted that psychosis can occur in patients with CUD and mood disorders – especially bipolar disorder – but was not included as a study outcome. “I would have liked to see more data about that,” he said.
However, “The trend is that cannabis use is starting at younger and younger ages, which has all kinds of ramifications in terms of cerebral development.”
Christopher Hammond, MD, PhD, assistant professor of psychiatry, Johns Hopkins University, Baltimore, said: “Three major strengths of the study are the size of the sample, its longitudinal analysis, and that the authors controlled for a number of potential confounding variables.”
In light of the findings, Dr. Hammond recommended clinicians and other health professionals who work with young people “should screen for cannabis-related problems in youth with mood disorders.”
Dr. Hammond, who is the director of the Co-occurring Disorders in Adolescents and Young Adults Clinical and Research Program, Johns Hopkins Bayview Medical Center, Baltimore, and was not involved with the study, recommended counseling youth with mood disorders and their parents and families “regarding the potential adverse health effects related to cannabis use.”
He also recommended “open dialogue with youth with and without mental health conditions about misleading reports in the national media and advertising about cannabis’ health benefits.”
The study was funded by the National Institute of Mental Health. Dr. Fontanella reported receiving grants from the National Institute of Mental Health during the conduct of the study. Dr. Raby reported no relevant financial relationships. Dr. Hammond reported receiving research grant funding from the National Institutes of Health, the American Academy of Child & Adolescent Psychiatry, Substance Abuse Mental Health Services Administration, the National Network of Depression Centers, and the Armstrong Institute at Johns Hopkins Bayview and serves as a scientific adviser for the National Courts and Science Institute and as a subject matter expert for SAMHSA related to co-occurring substance use disorders and severe emotional disturbance in youth.
A version of this article first appeared on Medscape.com.
Adolescents and young adults with mood disorders and cannabis use disorder (CUD) are at significantly increased risk for self-harm, all-cause mortality, homicide, and death by unintentional overdose, new research suggests.
Investigators found the risk for self-harm was three times higher, all-cause mortality was 59% higher, unintentional overdose was 2.5 times higher, and homicide was more than three times higher in those with versus without CUD.
“The take-home message of these findings is that we need to be aware of the perception that cannabis use is harmless, when it’s actually not,” lead author Cynthia Fontanella, PhD, associate professor of psychiatry, Ohio State University Wexner Medical Center, Columbus, said in an interview.
“We need to educate parents and clinicians that there are risks associated with cannabis, including increased risk for self-harm and death, and we need to effectively treat both cannabis use disorder and mood disorders,” she said.
The study was published online Jan. 19, 2021, in JAMA Pediatrics.
Little research in youth
“There has been very little research conducted on CUD in the adolescent population, and most studies have been conducted with adults,” Dr. Fontanella said.
Research on adults has shown that, even in people without mood disorders, cannabis use is associated with the early onset of mood disorders, psychosis, and anxiety disorders and has also been linked with suicidal behavior and increased risk for motor vehicle accidents, Dr. Fontanella said.
“We were motivated to conduct this study because we treat kids with depression and bipolar disorder and we noticed a high prevalence of CUD in this population, so we were curious about what its negative effects might be,” Dr. Fontanella recounted.
The researchers analyzed 7-year data drawn from Ohio Medicaid claims and linked to data from death certificates in 204,780 youths between the ages of 10 and 24 years (mean age was 17.2 years at the time of mood disorder diagnosis). Most were female, non-Hispanic White, enrolled in Medicaid because of poverty, and living in a metropolitan area (65.0%, 66.9%, 87.6%, and 77.1%, respectively).
Participants were followed up to 1 year from diagnosis until the end of enrollment, a self-harm event, or death.
Researchers included demographic, clinical, and treatment factors as covariates.
Close to three-quarters (72.7%) of the cohort had a depressive disorder, followed by unspecified/persistent mood disorder and bipolar disorder (14.9% and 12.4%, respectively). Comorbidities included ADHD (12.4%), anxiety disorder (12.3%), and other mental disorders (13.1%).
One -tenth of the cohort (10.3%) were diagnosed with CUD.
CUD treatment referrals
“Although CUD was associated with suicide in the unadjusted model, it was not significantly associated in adjusted models,” the authors reported.
Dr. Fontanella noted that the risk for these adverse outcomes is greater among those who engage in heavy, frequent use or who use cannabis that has higher-potency tetrahydrocannabinol (THC) content.
Reasons why CUD might be associated with these adverse outcomes are that it can increase impulsivity, poor judgment, and clouded thinking, which may in turn increase the risk for self-harm behaviors, she said.
She recommended that clinicians refer youth with CUD for “effective treatments,” including family-based models and individual approaches, such as cognitive behavioral therapy and motivational enhancement therapy.
Open dialogue
In a comment, Wilfrid Noel Raby, MD, PhD, adjunct clinical professor, Albert Einstein College of Medicine, New York, noted that psychosis can occur in patients with CUD and mood disorders – especially bipolar disorder – but was not included as a study outcome. “I would have liked to see more data about that,” he said.
However, “The trend is that cannabis use is starting at younger and younger ages, which has all kinds of ramifications in terms of cerebral development.”
Christopher Hammond, MD, PhD, assistant professor of psychiatry, Johns Hopkins University, Baltimore, said: “Three major strengths of the study are the size of the sample, its longitudinal analysis, and that the authors controlled for a number of potential confounding variables.”
In light of the findings, Dr. Hammond recommended clinicians and other health professionals who work with young people “should screen for cannabis-related problems in youth with mood disorders.”
Dr. Hammond, who is the director of the Co-occurring Disorders in Adolescents and Young Adults Clinical and Research Program, Johns Hopkins Bayview Medical Center, Baltimore, and was not involved with the study, recommended counseling youth with mood disorders and their parents and families “regarding the potential adverse health effects related to cannabis use.”
He also recommended “open dialogue with youth with and without mental health conditions about misleading reports in the national media and advertising about cannabis’ health benefits.”
The study was funded by the National Institute of Mental Health. Dr. Fontanella reported receiving grants from the National Institute of Mental Health during the conduct of the study. Dr. Raby reported no relevant financial relationships. Dr. Hammond reported receiving research grant funding from the National Institutes of Health, the American Academy of Child & Adolescent Psychiatry, Substance Abuse Mental Health Services Administration, the National Network of Depression Centers, and the Armstrong Institute at Johns Hopkins Bayview and serves as a scientific adviser for the National Courts and Science Institute and as a subject matter expert for SAMHSA related to co-occurring substance use disorders and severe emotional disturbance in youth.
A version of this article first appeared on Medscape.com.
Adolescents and young adults with mood disorders and cannabis use disorder (CUD) are at significantly increased risk for self-harm, all-cause mortality, homicide, and death by unintentional overdose, new research suggests.
Investigators found the risk for self-harm was three times higher, all-cause mortality was 59% higher, unintentional overdose was 2.5 times higher, and homicide was more than three times higher in those with versus without CUD.
“The take-home message of these findings is that we need to be aware of the perception that cannabis use is harmless, when it’s actually not,” lead author Cynthia Fontanella, PhD, associate professor of psychiatry, Ohio State University Wexner Medical Center, Columbus, said in an interview.
“We need to educate parents and clinicians that there are risks associated with cannabis, including increased risk for self-harm and death, and we need to effectively treat both cannabis use disorder and mood disorders,” she said.
The study was published online Jan. 19, 2021, in JAMA Pediatrics.
Little research in youth
“There has been very little research conducted on CUD in the adolescent population, and most studies have been conducted with adults,” Dr. Fontanella said.
Research on adults has shown that, even in people without mood disorders, cannabis use is associated with the early onset of mood disorders, psychosis, and anxiety disorders and has also been linked with suicidal behavior and increased risk for motor vehicle accidents, Dr. Fontanella said.
“We were motivated to conduct this study because we treat kids with depression and bipolar disorder and we noticed a high prevalence of CUD in this population, so we were curious about what its negative effects might be,” Dr. Fontanella recounted.
The researchers analyzed 7-year data drawn from Ohio Medicaid claims and linked to data from death certificates in 204,780 youths between the ages of 10 and 24 years (mean age was 17.2 years at the time of mood disorder diagnosis). Most were female, non-Hispanic White, enrolled in Medicaid because of poverty, and living in a metropolitan area (65.0%, 66.9%, 87.6%, and 77.1%, respectively).
Participants were followed up to 1 year from diagnosis until the end of enrollment, a self-harm event, or death.
Researchers included demographic, clinical, and treatment factors as covariates.
Close to three-quarters (72.7%) of the cohort had a depressive disorder, followed by unspecified/persistent mood disorder and bipolar disorder (14.9% and 12.4%, respectively). Comorbidities included ADHD (12.4%), anxiety disorder (12.3%), and other mental disorders (13.1%).
One -tenth of the cohort (10.3%) were diagnosed with CUD.
CUD treatment referrals
“Although CUD was associated with suicide in the unadjusted model, it was not significantly associated in adjusted models,” the authors reported.
Dr. Fontanella noted that the risk for these adverse outcomes is greater among those who engage in heavy, frequent use or who use cannabis that has higher-potency tetrahydrocannabinol (THC) content.
Reasons why CUD might be associated with these adverse outcomes are that it can increase impulsivity, poor judgment, and clouded thinking, which may in turn increase the risk for self-harm behaviors, she said.
She recommended that clinicians refer youth with CUD for “effective treatments,” including family-based models and individual approaches, such as cognitive behavioral therapy and motivational enhancement therapy.
Open dialogue
In a comment, Wilfrid Noel Raby, MD, PhD, adjunct clinical professor, Albert Einstein College of Medicine, New York, noted that psychosis can occur in patients with CUD and mood disorders – especially bipolar disorder – but was not included as a study outcome. “I would have liked to see more data about that,” he said.
However, “The trend is that cannabis use is starting at younger and younger ages, which has all kinds of ramifications in terms of cerebral development.”
Christopher Hammond, MD, PhD, assistant professor of psychiatry, Johns Hopkins University, Baltimore, said: “Three major strengths of the study are the size of the sample, its longitudinal analysis, and that the authors controlled for a number of potential confounding variables.”
In light of the findings, Dr. Hammond recommended clinicians and other health professionals who work with young people “should screen for cannabis-related problems in youth with mood disorders.”
Dr. Hammond, who is the director of the Co-occurring Disorders in Adolescents and Young Adults Clinical and Research Program, Johns Hopkins Bayview Medical Center, Baltimore, and was not involved with the study, recommended counseling youth with mood disorders and their parents and families “regarding the potential adverse health effects related to cannabis use.”
He also recommended “open dialogue with youth with and without mental health conditions about misleading reports in the national media and advertising about cannabis’ health benefits.”
The study was funded by the National Institute of Mental Health. Dr. Fontanella reported receiving grants from the National Institute of Mental Health during the conduct of the study. Dr. Raby reported no relevant financial relationships. Dr. Hammond reported receiving research grant funding from the National Institutes of Health, the American Academy of Child & Adolescent Psychiatry, Substance Abuse Mental Health Services Administration, the National Network of Depression Centers, and the Armstrong Institute at Johns Hopkins Bayview and serves as a scientific adviser for the National Courts and Science Institute and as a subject matter expert for SAMHSA related to co-occurring substance use disorders and severe emotional disturbance in youth.
A version of this article first appeared on Medscape.com.
Opioid-related deaths lower in counties with active cannabis dispensaries
Areas with active cannabis dispensaries have seen a decrease in opioid-related mortalities, recent research has shown.
“Our findings suggest that higher storefront cannabis dispensary counts are associated with reduced opioid related mortality rates at the county level,” wrote Greta Hsu, PhD, professor of management, University of California, Davis, and Balázs Kovács, PhD, associate professor of organizational behavior, Yale University, New Haven, Conn. “, which include the highly potent synthetic opioid fentanyl and its analogs.”
In the study, published in BMJ, the researchers evaluated the prevalence of medical and recreational cannabis dispensaries in 812 U.S. counties within 23 states with some degree of cannabis legalization between 2014 and 2018. Overall, dispensaries located in counties in eight U.S. states and the District of Columbia that sold cannabis recreationally and an additional 15 states that contained medical cannabis dispensaries were included.
Dr. Hsu and Dr. Kovács performed their analysis by examining dispensaries that were operating storefronts by the end of 2017 at the county level using panel-regression methods, combining data obtained from the consumer-facing website Weedmaps.com, Centers for Disease Control and Prevention U.S. mortality data, and data from the U.S. Census Bureau.
To measure opioid-related mortality, the researchers measured ICD-10 codes specific to natural opioid analgesics and semisynthetic opioids, methadone, heroin, nonmethadone synthetic opioid analgesics, and fentanyl-related deaths.
The analysis showed a negative association between the number of cannabis dispensaries at the county level and overall opioid-related mortality rates (95% confidence interval, −0.23 to −0.11), with an increase from one to two dispensaries in a county resulting in a 17% decrease in opioid-related mortality rates and an increase from two to three dispensaries resulting in another decrease in opioid-related mortality of 8.5%.
When evaluating mortality by specific opioid type, the researchers found a negative association between the number of dispensaries and synthetic nonmethadone opioids, with an increase from one to two dispensaries resulting in a 21% decrease in mortality attributable to synthetic nonmethadone opioids (95% CI, −0.27 to −0.14; P = .002). There were also negative associations between the number of dispensaries and prescription opioid-related mortality rates (95% CI, −0.13 to −0.03) and heroin-related mortality rates (95% CI, −0.13 to −0.02). The negative association was similar in comparisons between synthetic nonmethadone opioid-related mortality and the number of dispensaries for medical cannabis (95% CI, −0.21 to −0.09; P = .002) and recreational cannabis (95% CI, −0.17 to −0.04; P = .01).
Evidence of a negative association between legalization of medical or recreational cannabis and opioid-related mortality has been mixed in the literature, with some studies also showing a “spurious or nonsignificant” association, according to Dr. Hsu and Dr. Kovács.
While previous studies have looked at the legalization of cannabis for medical or recreational use, legalization on its own is an “incomplete picture,” they said, which might offer one explanation for these mixed findings. Some states that legalize medical cannabis, for example, might not allow dispensaries to legally sell cannabis, and there may be a delay of 1-2 years between the time a state legalizes cannabis for recreational use and when dispensaries are open and available to the public.
“These results were obtained after controlling for county level population characteristics, yearly effects, whether recreational dispensaries were legal or not in the focal county’s state, and opioid-related state policies,” the authors wrote.
Results ‘may be even stronger’ than reported
Christopher G. Fichtner, MD, clinical professor of psychiatry and neuroscience at the University of California, Riverside, said in an interview that the evidence for using cannabis as an opioid substitution for pain management has not been balanced, but noted “the bulk of it suggests that there is some harm reduction benefit by having liberalized access to cannabis.”
One strength of the study by Dr. Hsu and Dr. Kovács was how they were able to examine implementation of legalization of medical or recreational cannabis, rather than simply a change in the law, he said.
“By looking at dispensary count, it’s actually looking at a better measure of on-the-ground implementation than just change in policy,” Dr. Fichtner explained. “You’re looking at what was actually accomplished in terms of making cannabis legally available.”
The choice to evaluate storefront dispensaries only and not include delivery services in their data, “probably makes it a relatively conservative estimate. I think that would be a strength, that their findings may be even stronger than what it is they’re reporting,” Dr. Fichtner said.
“I do think, if anything, the paper is relatively tentative about advancing its conclusions, which I think is a weakness in a lot of these studies,” he added. In 2017, the National Academy of Sciences released a report that found evidence cannabis or cannabinoids can significantly reduce pain symptoms. In that report, “one of their strongest conclusions is that there’s conclusive or substantial evidence that cannabis or cannabinoids are effective management of chronic pain,” Dr. Fichtner said.
He said that digging deeper into what kinds of pain cannabis can treat is one area for future research. “Certainly, it seems that it’s unlikely that cannabis is going to be good for every kind of pain,” he said. “What kinds of pain is it better for than others? Is it some benefit for many kinds of pain, or only a few types of pain?”
The authors reported no relevant financial disclosures. Dr. Fichtner is the author of a book on cannabis policy in the United States, but reported no other financial disclosures.
Areas with active cannabis dispensaries have seen a decrease in opioid-related mortalities, recent research has shown.
“Our findings suggest that higher storefront cannabis dispensary counts are associated with reduced opioid related mortality rates at the county level,” wrote Greta Hsu, PhD, professor of management, University of California, Davis, and Balázs Kovács, PhD, associate professor of organizational behavior, Yale University, New Haven, Conn. “, which include the highly potent synthetic opioid fentanyl and its analogs.”
In the study, published in BMJ, the researchers evaluated the prevalence of medical and recreational cannabis dispensaries in 812 U.S. counties within 23 states with some degree of cannabis legalization between 2014 and 2018. Overall, dispensaries located in counties in eight U.S. states and the District of Columbia that sold cannabis recreationally and an additional 15 states that contained medical cannabis dispensaries were included.
Dr. Hsu and Dr. Kovács performed their analysis by examining dispensaries that were operating storefronts by the end of 2017 at the county level using panel-regression methods, combining data obtained from the consumer-facing website Weedmaps.com, Centers for Disease Control and Prevention U.S. mortality data, and data from the U.S. Census Bureau.
To measure opioid-related mortality, the researchers measured ICD-10 codes specific to natural opioid analgesics and semisynthetic opioids, methadone, heroin, nonmethadone synthetic opioid analgesics, and fentanyl-related deaths.
The analysis showed a negative association between the number of cannabis dispensaries at the county level and overall opioid-related mortality rates (95% confidence interval, −0.23 to −0.11), with an increase from one to two dispensaries in a county resulting in a 17% decrease in opioid-related mortality rates and an increase from two to three dispensaries resulting in another decrease in opioid-related mortality of 8.5%.
When evaluating mortality by specific opioid type, the researchers found a negative association between the number of dispensaries and synthetic nonmethadone opioids, with an increase from one to two dispensaries resulting in a 21% decrease in mortality attributable to synthetic nonmethadone opioids (95% CI, −0.27 to −0.14; P = .002). There were also negative associations between the number of dispensaries and prescription opioid-related mortality rates (95% CI, −0.13 to −0.03) and heroin-related mortality rates (95% CI, −0.13 to −0.02). The negative association was similar in comparisons between synthetic nonmethadone opioid-related mortality and the number of dispensaries for medical cannabis (95% CI, −0.21 to −0.09; P = .002) and recreational cannabis (95% CI, −0.17 to −0.04; P = .01).
Evidence of a negative association between legalization of medical or recreational cannabis and opioid-related mortality has been mixed in the literature, with some studies also showing a “spurious or nonsignificant” association, according to Dr. Hsu and Dr. Kovács.
While previous studies have looked at the legalization of cannabis for medical or recreational use, legalization on its own is an “incomplete picture,” they said, which might offer one explanation for these mixed findings. Some states that legalize medical cannabis, for example, might not allow dispensaries to legally sell cannabis, and there may be a delay of 1-2 years between the time a state legalizes cannabis for recreational use and when dispensaries are open and available to the public.
“These results were obtained after controlling for county level population characteristics, yearly effects, whether recreational dispensaries were legal or not in the focal county’s state, and opioid-related state policies,” the authors wrote.
Results ‘may be even stronger’ than reported
Christopher G. Fichtner, MD, clinical professor of psychiatry and neuroscience at the University of California, Riverside, said in an interview that the evidence for using cannabis as an opioid substitution for pain management has not been balanced, but noted “the bulk of it suggests that there is some harm reduction benefit by having liberalized access to cannabis.”
One strength of the study by Dr. Hsu and Dr. Kovács was how they were able to examine implementation of legalization of medical or recreational cannabis, rather than simply a change in the law, he said.
“By looking at dispensary count, it’s actually looking at a better measure of on-the-ground implementation than just change in policy,” Dr. Fichtner explained. “You’re looking at what was actually accomplished in terms of making cannabis legally available.”
The choice to evaluate storefront dispensaries only and not include delivery services in their data, “probably makes it a relatively conservative estimate. I think that would be a strength, that their findings may be even stronger than what it is they’re reporting,” Dr. Fichtner said.
“I do think, if anything, the paper is relatively tentative about advancing its conclusions, which I think is a weakness in a lot of these studies,” he added. In 2017, the National Academy of Sciences released a report that found evidence cannabis or cannabinoids can significantly reduce pain symptoms. In that report, “one of their strongest conclusions is that there’s conclusive or substantial evidence that cannabis or cannabinoids are effective management of chronic pain,” Dr. Fichtner said.
He said that digging deeper into what kinds of pain cannabis can treat is one area for future research. “Certainly, it seems that it’s unlikely that cannabis is going to be good for every kind of pain,” he said. “What kinds of pain is it better for than others? Is it some benefit for many kinds of pain, or only a few types of pain?”
The authors reported no relevant financial disclosures. Dr. Fichtner is the author of a book on cannabis policy in the United States, but reported no other financial disclosures.
Areas with active cannabis dispensaries have seen a decrease in opioid-related mortalities, recent research has shown.
“Our findings suggest that higher storefront cannabis dispensary counts are associated with reduced opioid related mortality rates at the county level,” wrote Greta Hsu, PhD, professor of management, University of California, Davis, and Balázs Kovács, PhD, associate professor of organizational behavior, Yale University, New Haven, Conn. “, which include the highly potent synthetic opioid fentanyl and its analogs.”
In the study, published in BMJ, the researchers evaluated the prevalence of medical and recreational cannabis dispensaries in 812 U.S. counties within 23 states with some degree of cannabis legalization between 2014 and 2018. Overall, dispensaries located in counties in eight U.S. states and the District of Columbia that sold cannabis recreationally and an additional 15 states that contained medical cannabis dispensaries were included.
Dr. Hsu and Dr. Kovács performed their analysis by examining dispensaries that were operating storefronts by the end of 2017 at the county level using panel-regression methods, combining data obtained from the consumer-facing website Weedmaps.com, Centers for Disease Control and Prevention U.S. mortality data, and data from the U.S. Census Bureau.
To measure opioid-related mortality, the researchers measured ICD-10 codes specific to natural opioid analgesics and semisynthetic opioids, methadone, heroin, nonmethadone synthetic opioid analgesics, and fentanyl-related deaths.
The analysis showed a negative association between the number of cannabis dispensaries at the county level and overall opioid-related mortality rates (95% confidence interval, −0.23 to −0.11), with an increase from one to two dispensaries in a county resulting in a 17% decrease in opioid-related mortality rates and an increase from two to three dispensaries resulting in another decrease in opioid-related mortality of 8.5%.
When evaluating mortality by specific opioid type, the researchers found a negative association between the number of dispensaries and synthetic nonmethadone opioids, with an increase from one to two dispensaries resulting in a 21% decrease in mortality attributable to synthetic nonmethadone opioids (95% CI, −0.27 to −0.14; P = .002). There were also negative associations between the number of dispensaries and prescription opioid-related mortality rates (95% CI, −0.13 to −0.03) and heroin-related mortality rates (95% CI, −0.13 to −0.02). The negative association was similar in comparisons between synthetic nonmethadone opioid-related mortality and the number of dispensaries for medical cannabis (95% CI, −0.21 to −0.09; P = .002) and recreational cannabis (95% CI, −0.17 to −0.04; P = .01).
Evidence of a negative association between legalization of medical or recreational cannabis and opioid-related mortality has been mixed in the literature, with some studies also showing a “spurious or nonsignificant” association, according to Dr. Hsu and Dr. Kovács.
While previous studies have looked at the legalization of cannabis for medical or recreational use, legalization on its own is an “incomplete picture,” they said, which might offer one explanation for these mixed findings. Some states that legalize medical cannabis, for example, might not allow dispensaries to legally sell cannabis, and there may be a delay of 1-2 years between the time a state legalizes cannabis for recreational use and when dispensaries are open and available to the public.
“These results were obtained after controlling for county level population characteristics, yearly effects, whether recreational dispensaries were legal or not in the focal county’s state, and opioid-related state policies,” the authors wrote.
Results ‘may be even stronger’ than reported
Christopher G. Fichtner, MD, clinical professor of psychiatry and neuroscience at the University of California, Riverside, said in an interview that the evidence for using cannabis as an opioid substitution for pain management has not been balanced, but noted “the bulk of it suggests that there is some harm reduction benefit by having liberalized access to cannabis.”
One strength of the study by Dr. Hsu and Dr. Kovács was how they were able to examine implementation of legalization of medical or recreational cannabis, rather than simply a change in the law, he said.
“By looking at dispensary count, it’s actually looking at a better measure of on-the-ground implementation than just change in policy,” Dr. Fichtner explained. “You’re looking at what was actually accomplished in terms of making cannabis legally available.”
The choice to evaluate storefront dispensaries only and not include delivery services in their data, “probably makes it a relatively conservative estimate. I think that would be a strength, that their findings may be even stronger than what it is they’re reporting,” Dr. Fichtner said.
“I do think, if anything, the paper is relatively tentative about advancing its conclusions, which I think is a weakness in a lot of these studies,” he added. In 2017, the National Academy of Sciences released a report that found evidence cannabis or cannabinoids can significantly reduce pain symptoms. In that report, “one of their strongest conclusions is that there’s conclusive or substantial evidence that cannabis or cannabinoids are effective management of chronic pain,” Dr. Fichtner said.
He said that digging deeper into what kinds of pain cannabis can treat is one area for future research. “Certainly, it seems that it’s unlikely that cannabis is going to be good for every kind of pain,” he said. “What kinds of pain is it better for than others? Is it some benefit for many kinds of pain, or only a few types of pain?”
The authors reported no relevant financial disclosures. Dr. Fichtner is the author of a book on cannabis policy in the United States, but reported no other financial disclosures.
FROM BMJ
Biden administration nixes buprenorphine waiver, docs disappointed
The Biden administration has halted a Trump administration initiative that would have allowed more physicians to prescribe buprenorphine for opioid use disorder (OUD).
Under the Trump administration’s plan, many doctors would be exempt from taking a day’s training before they could prescribe buprenorphine for OUD.
On Jan. 25, 2021, citing anonymous sources, the Washington Post reported that this action by the Biden administration was likely. At the time, there were concerns about whether the Department of Health & Human Services had the legal authority to make this policy change, the Post reported. The Substance Abuse and Mental Health Services Administration subsequently announced the derailment of the buprenorphine proposal on its website.
In SAMHSA’s view, the proposal was made “prematurely.” The SAMHSA statement did not detail the reasons for abandoning the Jan. 14 proposal. It had been scheduled to take effect upon publication in the Federal Register.
Instead of finalizing it in this way, the HHS said it would work with other federal agencies to “increase access to buprenorphine, reduce overdose rates and save lives.”
The HHS decision to scupper the proposal disappointed many physician groups. In a letter dated Jan. 27, several physician groups called on the Biden administration to proceed with the Trump proposal.
Under current federal law, physicians who wish to prescribe buprenorphine outside of opioid treatment programs must take an 8-hour course and receive a waiver from the Drug Enforcement Administration, the letter noted. It was signed by the American College of Emergency Physicians, the American Medical Association, and other organizations.
Treatment barrier
After taking the training course, it can take 60-90 days for physicians to receive the waiver. The license application can then be submitted. Physician groups argue that this so-called X-waiver requirement creates a barrier to providing medication-assisted treatment.
“Due to the stigma, some clinicians are not willing to pursue this DEA license or even engage in treatment of patients with [OUD],” the letter said.
The Trump administration’s proposal would have limited most physicians to treating no more than 30 patients with buprenorphine for OUD at any one time. This cap would not have applied to hospital-based physicians, such as those practicing emergency medicine, the HHS noted in a statement. The policy would have applied to only physicians who already have registered with the DEA.
Patrice A. Harris, MD, the immediate past president of the AMA and chair of the organization’s Opioid Task Force, was among the many physicians who supported the Trump administration proposal.
“It is estimated that more than 2 million Americans need treatment for opioid use disorder, but only a small percentage actually receive treatment,” Dr. Harris said in statement. Dr. Harris also noted that overdose deaths have reportedly accelerated during the COVID-19 pandemic.
Centers for Disease Control and Prevention data show there were more than 83,000 drug overdose deaths in the United States in the 12 months ending in June 2020. That is the highest number of overdose deaths ever recorded in a 12-month period and is an increase of more than 21%, compared with the previous year.
A ‘disappointment’
On Jan. 28, Dr. Harris said the decision to drop the plan was a disappointment.
“We encourage the current administration to quickly develop a path forward that removes the burdensome waiver requirement, thus allowing more physicians to prescribe this lifesaving medication,” she said in a statement sent to this news organization.
In a Jan. 26 statement, the American Society of Addiction Medicine urged Congress to eliminate the X-waiver and called for more education and training in the treatment of patients who struggle with opioids.
In the 116th session of Congress, which ended on Jan. 3, there was bipartisan support for proposed legislation to ease requirements for buprenorphine prescribing. A House bill had more than 90 Democratic and 21 Republican sponsors. A companion Senate bill had three Democratic and three Republican Sponsors, including Sen. Maggie Hassan (D-N.H.). On Jan. 25, Dr. Hassan tweeted that she would be seeking an explanation from the Biden administration if it halted the plan to ease the waiver restriction.
“Medication-assisted treatment can save lives, and the buprenorphine waiver requirement should be eliminated so that physicians can more easily prescribe it to those who need it,” she said.
Many clinicians and policy experts turned to Twitter to urge an easing of buprenorphine prescribing, using the hashtag “Xthexwaiver.”
Among them was the official who put forward the Jan. 14 proposal, Brett Giroir, MD. He served as assistant secretary for health during the Trump administration.
Objections
In its Jan. 25 article, the Washington Post referred to an article in Alcoholism and Drug Abuse Weekly in which a top federal official in the Trump administration objected to Dr. Giroir’s plan.
Elinore F. McCance-Katz, MD, PhD, who served as the assistant secretary of HHS for SAMHSA, had earlier proposed raising the cap for addiction experts. Alcoholism and Drug Abuse Weekly quotes Dr. McCance-Katz as saying the Trump buprenorphine proposal was “unfair to the incoming administration.”
“The Biden administration has so much work to do to get their programs and policies into place, and to do something like this at the 11th hour that could get doctors into trouble – it’s heinous,” she said in the article.
Dr. McCance-Katz had resigned before the Trump administration proposal was unveiled. On Jan. 7, she issued a public notice announcing she would resign, citing concerns about the previous day’s attack on the U.S. Capitol.
“It had been my plan to stay until the change in administration occurred, but my plans abruptly changed last evening when, on my way back from visiting an excellent residential treatment program in New York, I saw the violent takeover of the Capitol building,” she said.
On Twitter, Roland Flores, MD, an anesthesiologist and pain specialist, urged his colleagues to consider the need for more education among clinicians who treat OUD. He jousted a bit with those favoring a swift drive to “XtheXwaiver” and questioned their arguments about the burden of the current rules.
“I think ‘all this red tape’ is a little bit of an exaggeration – it’s an 8-hour online course, and an application,” Dr. Flores tweeted in one exchange. “But #XtheXwaiver is fine – it’s probably rooted in stigma. It’s unlikely to make much difference tho. The waiver wasn’t the thing keeping docs from prescribing.”
A version of this article first appeared on Medscape.com.
The Biden administration has halted a Trump administration initiative that would have allowed more physicians to prescribe buprenorphine for opioid use disorder (OUD).
Under the Trump administration’s plan, many doctors would be exempt from taking a day’s training before they could prescribe buprenorphine for OUD.
On Jan. 25, 2021, citing anonymous sources, the Washington Post reported that this action by the Biden administration was likely. At the time, there were concerns about whether the Department of Health & Human Services had the legal authority to make this policy change, the Post reported. The Substance Abuse and Mental Health Services Administration subsequently announced the derailment of the buprenorphine proposal on its website.
In SAMHSA’s view, the proposal was made “prematurely.” The SAMHSA statement did not detail the reasons for abandoning the Jan. 14 proposal. It had been scheduled to take effect upon publication in the Federal Register.
Instead of finalizing it in this way, the HHS said it would work with other federal agencies to “increase access to buprenorphine, reduce overdose rates and save lives.”
The HHS decision to scupper the proposal disappointed many physician groups. In a letter dated Jan. 27, several physician groups called on the Biden administration to proceed with the Trump proposal.
Under current federal law, physicians who wish to prescribe buprenorphine outside of opioid treatment programs must take an 8-hour course and receive a waiver from the Drug Enforcement Administration, the letter noted. It was signed by the American College of Emergency Physicians, the American Medical Association, and other organizations.
Treatment barrier
After taking the training course, it can take 60-90 days for physicians to receive the waiver. The license application can then be submitted. Physician groups argue that this so-called X-waiver requirement creates a barrier to providing medication-assisted treatment.
“Due to the stigma, some clinicians are not willing to pursue this DEA license or even engage in treatment of patients with [OUD],” the letter said.
The Trump administration’s proposal would have limited most physicians to treating no more than 30 patients with buprenorphine for OUD at any one time. This cap would not have applied to hospital-based physicians, such as those practicing emergency medicine, the HHS noted in a statement. The policy would have applied to only physicians who already have registered with the DEA.
Patrice A. Harris, MD, the immediate past president of the AMA and chair of the organization’s Opioid Task Force, was among the many physicians who supported the Trump administration proposal.
“It is estimated that more than 2 million Americans need treatment for opioid use disorder, but only a small percentage actually receive treatment,” Dr. Harris said in statement. Dr. Harris also noted that overdose deaths have reportedly accelerated during the COVID-19 pandemic.
Centers for Disease Control and Prevention data show there were more than 83,000 drug overdose deaths in the United States in the 12 months ending in June 2020. That is the highest number of overdose deaths ever recorded in a 12-month period and is an increase of more than 21%, compared with the previous year.
A ‘disappointment’
On Jan. 28, Dr. Harris said the decision to drop the plan was a disappointment.
“We encourage the current administration to quickly develop a path forward that removes the burdensome waiver requirement, thus allowing more physicians to prescribe this lifesaving medication,” she said in a statement sent to this news organization.
In a Jan. 26 statement, the American Society of Addiction Medicine urged Congress to eliminate the X-waiver and called for more education and training in the treatment of patients who struggle with opioids.
In the 116th session of Congress, which ended on Jan. 3, there was bipartisan support for proposed legislation to ease requirements for buprenorphine prescribing. A House bill had more than 90 Democratic and 21 Republican sponsors. A companion Senate bill had three Democratic and three Republican Sponsors, including Sen. Maggie Hassan (D-N.H.). On Jan. 25, Dr. Hassan tweeted that she would be seeking an explanation from the Biden administration if it halted the plan to ease the waiver restriction.
“Medication-assisted treatment can save lives, and the buprenorphine waiver requirement should be eliminated so that physicians can more easily prescribe it to those who need it,” she said.
Many clinicians and policy experts turned to Twitter to urge an easing of buprenorphine prescribing, using the hashtag “Xthexwaiver.”
Among them was the official who put forward the Jan. 14 proposal, Brett Giroir, MD. He served as assistant secretary for health during the Trump administration.
Objections
In its Jan. 25 article, the Washington Post referred to an article in Alcoholism and Drug Abuse Weekly in which a top federal official in the Trump administration objected to Dr. Giroir’s plan.
Elinore F. McCance-Katz, MD, PhD, who served as the assistant secretary of HHS for SAMHSA, had earlier proposed raising the cap for addiction experts. Alcoholism and Drug Abuse Weekly quotes Dr. McCance-Katz as saying the Trump buprenorphine proposal was “unfair to the incoming administration.”
“The Biden administration has so much work to do to get their programs and policies into place, and to do something like this at the 11th hour that could get doctors into trouble – it’s heinous,” she said in the article.
Dr. McCance-Katz had resigned before the Trump administration proposal was unveiled. On Jan. 7, she issued a public notice announcing she would resign, citing concerns about the previous day’s attack on the U.S. Capitol.
“It had been my plan to stay until the change in administration occurred, but my plans abruptly changed last evening when, on my way back from visiting an excellent residential treatment program in New York, I saw the violent takeover of the Capitol building,” she said.
On Twitter, Roland Flores, MD, an anesthesiologist and pain specialist, urged his colleagues to consider the need for more education among clinicians who treat OUD. He jousted a bit with those favoring a swift drive to “XtheXwaiver” and questioned their arguments about the burden of the current rules.
“I think ‘all this red tape’ is a little bit of an exaggeration – it’s an 8-hour online course, and an application,” Dr. Flores tweeted in one exchange. “But #XtheXwaiver is fine – it’s probably rooted in stigma. It’s unlikely to make much difference tho. The waiver wasn’t the thing keeping docs from prescribing.”
A version of this article first appeared on Medscape.com.
The Biden administration has halted a Trump administration initiative that would have allowed more physicians to prescribe buprenorphine for opioid use disorder (OUD).
Under the Trump administration’s plan, many doctors would be exempt from taking a day’s training before they could prescribe buprenorphine for OUD.
On Jan. 25, 2021, citing anonymous sources, the Washington Post reported that this action by the Biden administration was likely. At the time, there were concerns about whether the Department of Health & Human Services had the legal authority to make this policy change, the Post reported. The Substance Abuse and Mental Health Services Administration subsequently announced the derailment of the buprenorphine proposal on its website.
In SAMHSA’s view, the proposal was made “prematurely.” The SAMHSA statement did not detail the reasons for abandoning the Jan. 14 proposal. It had been scheduled to take effect upon publication in the Federal Register.
Instead of finalizing it in this way, the HHS said it would work with other federal agencies to “increase access to buprenorphine, reduce overdose rates and save lives.”
The HHS decision to scupper the proposal disappointed many physician groups. In a letter dated Jan. 27, several physician groups called on the Biden administration to proceed with the Trump proposal.
Under current federal law, physicians who wish to prescribe buprenorphine outside of opioid treatment programs must take an 8-hour course and receive a waiver from the Drug Enforcement Administration, the letter noted. It was signed by the American College of Emergency Physicians, the American Medical Association, and other organizations.
Treatment barrier
After taking the training course, it can take 60-90 days for physicians to receive the waiver. The license application can then be submitted. Physician groups argue that this so-called X-waiver requirement creates a barrier to providing medication-assisted treatment.
“Due to the stigma, some clinicians are not willing to pursue this DEA license or even engage in treatment of patients with [OUD],” the letter said.
The Trump administration’s proposal would have limited most physicians to treating no more than 30 patients with buprenorphine for OUD at any one time. This cap would not have applied to hospital-based physicians, such as those practicing emergency medicine, the HHS noted in a statement. The policy would have applied to only physicians who already have registered with the DEA.
Patrice A. Harris, MD, the immediate past president of the AMA and chair of the organization’s Opioid Task Force, was among the many physicians who supported the Trump administration proposal.
“It is estimated that more than 2 million Americans need treatment for opioid use disorder, but only a small percentage actually receive treatment,” Dr. Harris said in statement. Dr. Harris also noted that overdose deaths have reportedly accelerated during the COVID-19 pandemic.
Centers for Disease Control and Prevention data show there were more than 83,000 drug overdose deaths in the United States in the 12 months ending in June 2020. That is the highest number of overdose deaths ever recorded in a 12-month period and is an increase of more than 21%, compared with the previous year.
A ‘disappointment’
On Jan. 28, Dr. Harris said the decision to drop the plan was a disappointment.
“We encourage the current administration to quickly develop a path forward that removes the burdensome waiver requirement, thus allowing more physicians to prescribe this lifesaving medication,” she said in a statement sent to this news organization.
In a Jan. 26 statement, the American Society of Addiction Medicine urged Congress to eliminate the X-waiver and called for more education and training in the treatment of patients who struggle with opioids.
In the 116th session of Congress, which ended on Jan. 3, there was bipartisan support for proposed legislation to ease requirements for buprenorphine prescribing. A House bill had more than 90 Democratic and 21 Republican sponsors. A companion Senate bill had three Democratic and three Republican Sponsors, including Sen. Maggie Hassan (D-N.H.). On Jan. 25, Dr. Hassan tweeted that she would be seeking an explanation from the Biden administration if it halted the plan to ease the waiver restriction.
“Medication-assisted treatment can save lives, and the buprenorphine waiver requirement should be eliminated so that physicians can more easily prescribe it to those who need it,” she said.
Many clinicians and policy experts turned to Twitter to urge an easing of buprenorphine prescribing, using the hashtag “Xthexwaiver.”
Among them was the official who put forward the Jan. 14 proposal, Brett Giroir, MD. He served as assistant secretary for health during the Trump administration.
Objections
In its Jan. 25 article, the Washington Post referred to an article in Alcoholism and Drug Abuse Weekly in which a top federal official in the Trump administration objected to Dr. Giroir’s plan.
Elinore F. McCance-Katz, MD, PhD, who served as the assistant secretary of HHS for SAMHSA, had earlier proposed raising the cap for addiction experts. Alcoholism and Drug Abuse Weekly quotes Dr. McCance-Katz as saying the Trump buprenorphine proposal was “unfair to the incoming administration.”
“The Biden administration has so much work to do to get their programs and policies into place, and to do something like this at the 11th hour that could get doctors into trouble – it’s heinous,” she said in the article.
Dr. McCance-Katz had resigned before the Trump administration proposal was unveiled. On Jan. 7, she issued a public notice announcing she would resign, citing concerns about the previous day’s attack on the U.S. Capitol.
“It had been my plan to stay until the change in administration occurred, but my plans abruptly changed last evening when, on my way back from visiting an excellent residential treatment program in New York, I saw the violent takeover of the Capitol building,” she said.
On Twitter, Roland Flores, MD, an anesthesiologist and pain specialist, urged his colleagues to consider the need for more education among clinicians who treat OUD. He jousted a bit with those favoring a swift drive to “XtheXwaiver” and questioned their arguments about the burden of the current rules.
“I think ‘all this red tape’ is a little bit of an exaggeration – it’s an 8-hour online course, and an application,” Dr. Flores tweeted in one exchange. “But #XtheXwaiver is fine – it’s probably rooted in stigma. It’s unlikely to make much difference tho. The waiver wasn’t the thing keeping docs from prescribing.”
A version of this article first appeared on Medscape.com.
Anticonvulsants for alcohol withdrawal: A review of the evidence
Abrupt cessation or reduction of alcohol consumption may result in alcohol withdrawal syndrome (AWS), which is a medical emergency that can lead to serious complications when unrecognized or treatment is delayed. Symptoms of AWS include tremors, anxiety attacks, cognitive impairment, hallucinations, seizures, delirium tremens (DT), and in severe, untreated cases, death.1 Low to moderate alcohol consumption produces euphoria and excitation via activation of glutamatergic neurotransmission, while higher concentrations produce severe intoxication via GABAergic mechanisms. Acute withdrawal unmasks the hyper-excitatory state of the brain, causing anxiety, agitation, and autonomic activation characteristic of AWS, which typically begins 1 to 3 days after the last drink.2 In the 2012-2013 National Epidemiologic Survey on Alcohol and Related Conditions conducted by the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the 12-month and lifetime prevalences of AWS were 13.9% and 29.1%, respectively.3 Within the general inpatient population, AWS can be present in nearly 30% of patients; if left untreated, AWS has a 15% mortality rate, although when AWS is recognized early and treated, the mortality rate falls dramatically to 2%.4
AWS has most commonly been treated with benzodiazepines.5 However, benzodiazepines have the potential for significant adverse effects when used in older adults and in individuals with complicated medical issues, such as obstructive lung disease and sleep apnea.6 Anticonvulsants have been increasingly used to treat alcohol withdrawal, and their use is supported by several retrospective and prospective studies. In this article, we review the data from randomized control trials (RCTs) on the use of anticonvulsants for the treatment of AWS to see if we can make any recommendations for the use of anticonvulsants for treating AWS.
Our literature search
We searched 5 databases (PubMed, Cochrane, Medline, PsycInfo, and Embase) using the following terms: “alcohol withdrawal syndrome treatment”, “anticonvulsants”, “anti-epileptic”, “gabapentin”, “carbamazepine”, “sodium valproate”, “oxcarbazepine”, “phenytoin”, “levetiracetam”, and “lamotrigine.” We included only double-blind RCTs published between January 1, 1976 and September 30, 2016 in English-language journals or that had an official English translation. There were no restrictions on patient age or location of treatment (inpatient vs outpatient). All RCTs that compared anticonvulsants or a combination of an anticonvulsant and an active pharmacotherapeutic agent with either placebo or gold standard treatment for AWS were included. Database reviews, systematic reviews, and meta-analyses were excluded.
We identified 662 articles that met these criteria. However, most were duplicates, review articles, systematic reviews, meta-analyses, case reports, or open-label or non-randomized trials. Only 16 articles met our inclusion criteria. In the following sections, we discuss these 16 studies by medication type and in chronological order.
Gabapentin
The characteristics of the gabapentin studies included in this review are summarized in Table 1.7-13
Bonnet et al7 (2003) examined 61 adults who met the clinical criteria for alcohol dependence and displayed moderate or severe AWS according to their Mainz Alcohol Withdrawal Score (MAWS ≥4). They were randomized to receive placebo or gabapentin, 400 mg 4 times a day, along with clomethiazole. The attrition rate was not significantly different between the 2 groups (P = .66). The difference in the number of clomethiazole capsules taken during the first 24 hours between the groups was small and not significant (P = .96). Analysis of MAWS over time revealed no significant main effect for group (P = .26) and a significant effect for the time variable (P < .001). The interaction between group and time was not significant (P =.4). This means that there was a significant decrease in MAWS from baseline over 48 hours, and this decrease in MAWS was considered equal for both study groups. Adverse clinical events were observed in both groups, and there was no significant difference (P = .74) between the groups. Nausea and ataxia, which are specific to gabapentin, were observed more frequently in this group.
Conclusion: The authors concluded that gabapentin, 400 mg 4 times a day, is no better than placebo in reducing the amount of clomethiazole required to treat acute AWS.7
Continue to: Bonnet et al
Bonnet et al8 (2007) also conducted a study examining 59 patients with alcohol dependence who displayed moderate or severe AWS. Participants received placebo or gabapentin, 400 mg, and a rescue medication, clomethiazole, if needed. Subsequently, a capsule of study medication was administered every 6 hours for 2 days and then tapered. During the study, mood was measured by Profile of Mood States (POMS), and subjective complaints of withdrawal were measured using the Essen Self-Assessment of Alcohol Withdrawal Scale (ESA). Of the 59 patients, only 46 were analyzed; 5 patients dropped out, and 8 patients were missing data. Compared with the placebo group, the gabapentin group displayed less dejection, fatigue, and anger, and more vigor. Analysis of variance (ANOVA) measures revealed significant overall changes over time on all 4 scales (all P < .001). A significant (F = 3.62, df 2;43, P = .035) group × time interaction resulted exclusively for vigor. Analysis was repeated using rank-transformed data, resulting in a significant (P = .046) interaction effect. The significant increase in vigor was not apparent after tapering off gabapentin, which suggests gabapentin has a reversible effect on vigor. There was a significant (P < .001) overall decline of subjective withdrawal symptoms complaints, but no group × time interaction (P = .62). Analysis of 11 patients with comorbid mild depression revealed no significant time × group interaction for dejection, fatigue, anger, or subjective withdrawal (all P > .05). However, for vigor, the group × time interaction was significant (P = .022). Throughout the treatment, vigor scores of those mild depressive patients who received gabapentin increased to a level comparable to that of patients without a mood disorder.
Conclusion: The authors authors concluded that gabapentin was markedly more efficacious in improving vigor in the small subgroup of patients with mild depression.8
Myrick et al9 (2007) evaluated the safety and tolerability of gabapentin in patients who abused alcohol, as well as the ability of gabapentin to reduce alcohol craving and consumption. This study included 35 participants randomly assigned to receive gabapentin (n = 17) or placebo (n = 18) for 7 days. All medications were administered in standard gel caps with riboflavin, 25 mg, to assess for compliance via a laboratory-based urinary fluorescence assay. Urine samples were assessed for riboflavin at baseline and Day 6, and a reading of 1,500 ng/mL of riboflavin on Day 6 was interpreted as being compliant. Participants were required to abstain completely from drinking alcohol on Day 6 and the morning of Day 7. At the first session, the following measures were completed: demographic form, alcohol and drug section of the Structured Clinical Interview (SCID), Obsessive-Compulsive Drinking Scale, Self-Administered Alcohol Screening Test (SAAST), and Alcohol Dependence Scale (ADS); there also was collection of a urine sample for detection of abused drugs and a blood sample for liver function and general health screening.
At the second session, patients completed the psychiatric sections of the SCID and the Alcohol Craving Questionnaire, and received a physical exam. To assess the negative clinical effects of gabapentin and alcohol on the CNS, the Epworth Sleepiness Scale (ESS) and POMS were administered at baseline and on Day 6. Also, several other scales were used to identify any impact of gabapentin on acute alcohol effects and craving: the Clinical Institute Withdrawal Assessment of Alcohol, Revised (CIWA-Ar), Biphasic Alcohol Effects Scale (BAES), Subjective High Assessment Scale (SHAS), and Alcohol Urge Questionnaire (AUQ).
Conclusion: Gabapentin was well tolerated, but compared with placebo, gabapentin had no effect on alcohol stimulation (P = .75) or sedation (P = .99) as measured by the BAES. The difference in SHAS scores was also not significant (P = .19). There was also no significant reduction in craving for alcohol as measured on the AUQ scale
Continue to: Malcolm et al
Malcolm et al10 conducted an outpatient treatment study. Patients were men and women age 21 to 70 years from multiple ethnic groups. They were randomized to receive gabapentin or lorazepam; 449 patients were screened and 68 completed the follow-up. Scales used included the CIWA-Ar, Beck Depression Inventory (BDI), and ESS.
Patients receiving lorazepam reported less insomnia and more sleepiness early in treatment than patients receiving gabapentin. However, upon completing treatment and discontinuing medication administration, patients previously treated with lorazepam reported increased insomnia and daytime sleepiness, while patients previously treated with gabapentin continued to report improvements in these self-reported sleep measures. The differences between lorazepam and gabapentin were further evidenced in BDI scores at Day 5, Day 7, and Day 12 in patients who had previously experienced multiple withdrawals. Gabapentin was superior to lorazepam in reducing insomnia as assessed by BDI score, an effect that was sustained throughout the post-treatment week. Participants’ ESS scores indicated less daytime sleepiness in the gabapentin group than in the lorazepam group.
Conclusion: Among patients who abused alcohol and had a history of multiple withdrawals, lorazepam is less effective than gabapentin in reducing insomnia.10 However, this study had several limitations: <25% of individuals who were initially screened were enrolled in the study, and it used subjective tests such as BDI. Objective electrophysiologic measures of sleep and daytime sleepiness would have been very helpful.
Myrick et al11 (2009) also compared gabapentin and lorazepam for treating alcohol withdrawal. One hundred patients were randomized to receive 4 days of fixed-dose taper of gabapentin or lorazepam. Patients could receive 1 of 3 gabapentin dosing regimens (600 mg/d, 900 mg/d, or 1,200 mg/d) for 3 days. Participants who were randomized to receive lorazepam were given 6 mg/d for 3 days and then tapered to 4 mg/d. Also, blinded supplemental medications (rescue packs) were given to each patient on Days 1 to 4 to treat subjective feelings of alcohol withdrawal. All patients also received thiamine for 12 days. Assessment of severity of alcohol withdrawal was measured by the CIWA-Ar. To quantify the severity of alcohol dependence and alcohol use, patients were asked to complete the ADS and Time-Line Follow-Back (TLFB) scales, respectively. Other scales administered included the BDI, Zung Anxiety Scale (ZAS), ESS, and visual analogue scales that assessed craving, ability to perform work, and need for additional medication.
There was a decrease in CIWA-Ar scores over time in all groups. High-dose gabapentin was found to be statistically superior but clinically similar to lorazepam (P = .009). Researchers also found that compared with patients who were treated with lorazepam, patients who were treated with gabapentin experienced reduced craving and anxiety/depressive symptoms, and complained of less subjective discomfort. Compared to patients who were treated with gabapentin, patients who were treated with lorazepam had higher probabilities of drinking on the first day of dose decrease (Day 2) and the second day off medication (Day 6) (P = .0002). During post-treatment, patients who were treated with gabapentin had less probability of drinking during the follow-up post-treatment period (P = .2 for 900 mg/d and P = .3 for 1,200 mg/d) compared with patients who were treated with lorazepam (P = .55).
Continue to: Conclusion
Conclusion: The researchers concluded that gabapentin was well tolerated and effectively diminished the symptoms of alcohol withdrawal, especially at the higher target dose (1,200 mg/d), and that compared with lorazepam, gabapentin decreased the probability of drinking during alcohol withdrawal and in the immediate post-withdrawal week.11
Stock et al12 randomized 26 patients who met criteria for AWS to receive gabapentin or chlordiazepoxide. Gabapentin doses were 1,200 mg/d orally for 3 days, followed by 900 mg/d, 600 mg/d, and 300 mg/d for 1 day each. Chlordiazepoxide doses were 100 mg/d orally for 3 days, followed by 75 mg/d, 50 mg/d, and 25 mg/d for 1 day each. The ESS, Penn Alcohol Craving Scale (PACS), ataxia rating, and CIWA-Ar were administered daily. Thirty-five percent of participants dropped out at the end of the 7-day treatment period. Days 1 to 4 were considered the early treatment period, and Days 5 to 7 were considered the late treatment period. The adjusted mean ESS score did not differ significantly between the randomized groups during the early stage (P = .61) vs the late stage, in which the adjusted mean ESS score was significantly lower with gabapentin compared with chlordiazepoxide (P = .04). The differences in PACS scores between the groups were not statistically significant in either stage (early stage P = .59 vs late stage P = .08), but a trend of lower PACS scores was noted with gabapentin in the later stage. No participant in either group had ataxia during the study. In both groups, CIWA-Ar scores were reduced similarly.
Conclusion: The researchers concluded that gabapentin treatment resulted in a significantly greater reduction in sedation (ESS) and a trend toward reduced alcohol craving (PACS) by the end of treatment compared with chlordiazepoxide treatment.12
Schacht et al13 analyzed functional magnetic resonance imaging data from 48 patients who were alcohol-dependent in a 6-week RCT. Patients were randomized to receive gabapentin up to 1,200 mg/d for 39 days plus flumazenil for 2 days (GP/FMZ group) or an oral placebo and placebo infusions on the same time course. Evaluations included the SCID, ADS, and Obsessive-Compulsive Drinking Scale (OCDS). On Day 1, the CIWA-Ar was administered; it was used to ensure equal distribution of individuals with higher alcohol withdrawal symptoms between medication groups. There were no significant effects of initial alcohol withdrawal symptom status or medication. However, there was a significant interaction between these factors: patients with higher alcohol withdrawal symptoms who received GP/FMZ and those with lower alcohol withdrawal symptoms who received placebo demonstrated greater cue-elicited activation, relative to the other groups, and had less subsequent drinking, which reflected differences in deactivation between alcohol and beverage stimuli, in a cluster that encompassed the dorsal ACC (dACC) (family-wise error-corrected cluster probability of P = .012; 99 voxels; local maxima at [-3, 39, 18] and [6, 33, 9]). In the GP/FMZ group, patients with higher alcohol withdrawal symptoms had significantly greater activation, while in the placebo group, patients with lower alcohol withdrawal symptoms had greater activation.
Conclusion: The researchers concluded that alterations in task-related deactivation of dACC, a component of the default mode network, may predict better alcohol treatment response, while activation of DLPFC, an area associated with selective attention, may predict relapse drinking.13
Continue to: Carbamazepine
Carbamazepine
The characteristics of the carbamazepine studies included in this review are summarized in Table 2.14-19
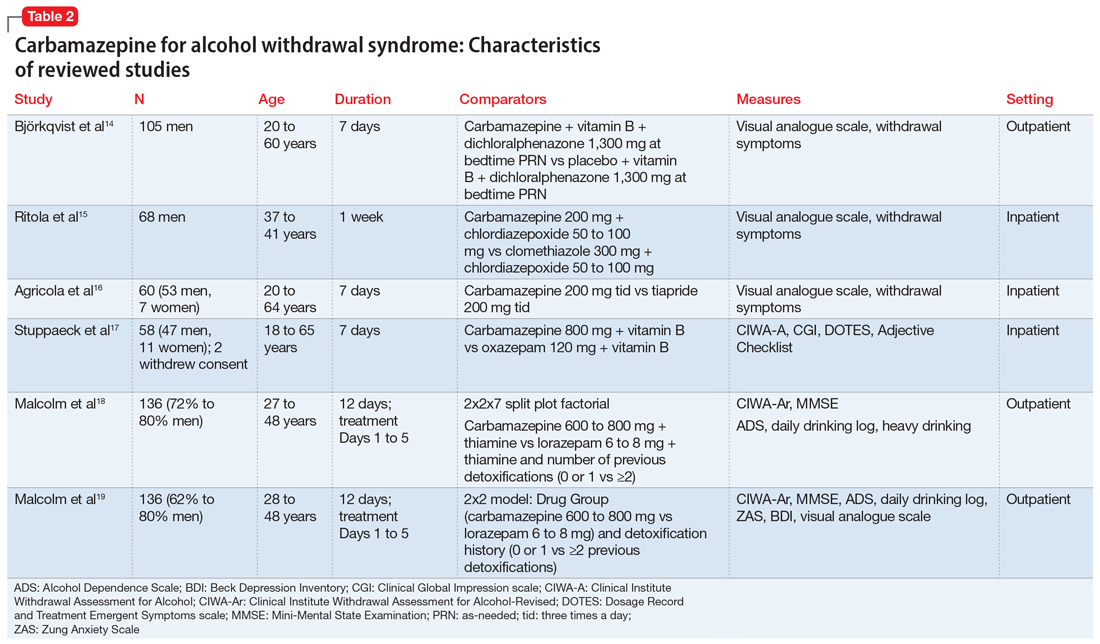
Björkqvist et al14 randomized 105 men with AWS to placebo or carbamazepine. On initial assessment, history, physical examination, relevant labs, and intoxication assessments were recorded. On subsequent visits, nursing staff recorded withdrawal symptoms for patients as 0 to 2 (0 = no specific symptoms, 1 = patient only complained when asked about specific symptoms, 2 = patient complained of withdrawal symptoms without being asked, or if symptoms were severe or obvious to others). Along with the above, vital signs and a visual analogue scale of 0 to 10 (0 = feeling could not be worse, 10 = feeling could not be better) were recorded at each visit. The dose was weight-dependent and administered as follows: on Days 1 and 2, 1+1+2 tablets of carbamazepine, 200 mg, or placebo; Days 3 and 4, 1+1+1 tablets; and Days 5 and 6, 1+0+1 tablets. Every patient received dichloralphenazone as needed. All patients were given vitamin B 3 times a day. Most withdrawal symptoms decreased faster in the carbamazepine group on Day 2 (P = .01) and on Day 4 (P = .1). On the visual analogue scale, scores varied between patients. On Day 1, the mean score was 2.5 times higher in the carbamazepine group compared with the placebo group, and this difference increased to 3 times by Day 7 (P < .01). The patient’s estimated ability to work improved significantly faster in the carbamazepine group than in the placebo group (P < .01).
Conclusion: The authors concluded that compared with placebo, carbamazepine was able to more quickly decrease withdrawal symptoms, especially insomnia and subjective recovery.14
Ritola et al15 randomized 68 hospitalized men with AWS to carbamazepine, 200 mg/d, or clomethiazole, 300 mg/d, for 1 week. The target withdrawal symptoms included gastrointestinal and sleep disturbances; anxiety; aggressiveness; and cardiovascular, depressive, psychotic, and neurologic symptoms. A 4-point rating scale was used for individual symptoms (0 = no symptom, 1 = mild symptom, 2 = moderate symptom, and 3 = severe symptom). On the day of admission (Day 0), all patients were given 50 to 100 mg of chlordiazepoxide IM and 2 tablets and 4 capsules of the trial preparations (either the tablets or capsules were active, and the others were placebos) in the evening. Five patients dropped out of the clomethiazole group and 1 from the carbamazepine group. No significant difference between the 2 treatments were found by the patient, nurse, or physician.
Conclusion: The authors concluded that carbamazepine seemed to be as effective as clomethiazole in the treatment of milder alcohol withdrawal symptoms. Final treatment results were equally good in both groups. Sleep disturbance resolved faster in the carbamazepine group.15
Continue to: Agricola et al
Agricola et al16 compared carbamazepine to tiapride for treatment of acute AWS. In this study, 60 patients were randomized to carbamazepine, 200 mg 3 times a day, or tiapride, 200 mg 3 times a day. All patients were hospitalized with severe AWS preceding DT. The patients were evaluated for withdrawal symptoms (gastrointestinal and cardiovascular symptoms, sleep disturbances, anxiety, aggression, fear, depression, psychotic symptoms, and certain neurologic symptoms). The severity of these symptoms was scored as follows: 0 = no symptoms; 1 = moderate symptoms; and 2 = severe symptoms. At each visit, an overall evaluation of the patient’s clinical condition was made according to a visual analogue scale (100 = worst condition, 0 = best condition). On Day 7, both the doctor and patient evaluated treatment efficacy according to a 4-point scale (1 = no efficacy, 4 = excellent efficacy). There was no significant difference between carbamazepine and tiapride in terms of total symptoms score and visual analogue scale assessment. Carbamazepine was found to have faster relief of symptoms and a significantly greater reduction in symptom score on Day 2 (P < .01). Carbamazepine had a preferential action on fear, nightmares, and hallucinations. The proportion of patients in whom anxiety improved after treatment was 96.2% for carbamazepine and 71.4% for tiapride (P < .05). Aggressiveness and gastrointestinal discomfort resolved faster in the tiapride group. No cases of DT were observed.
Conclusion: The researchers concluded that either carbamazepine or tiapride provides an appropriate alternative in the treatment of inpatients with severe AWS.16
Stuppaeck et al17 compared the efficacy of carbamazepine to oxazepam in 60 inpatients who had symptoms of alcohol withdrawal. Alcohol withdrawal was measured with the CIWA-A, and patients with scores >20 were enrolled in the study. The Clinical Global Impression (CGI) scale and self-rated Adjective Checklist (ACL) were also used. On Days 1 to 3, patients received oxazepam, 120 mg/d, or carbamazepine, 800 mg/d. From Day 4 to 7, doses were decreased to 90 mg/d and 600 mg/d, respectively. After the 7-day trial, all patients were treated with carbamazepine, 200 mg twice a day on Day 8 and 200 mg at night on Day 9. Two patients withdrew consent and 6 dropped out due to adverse effects. During the 7-day trial, when comparing all improvements on CIWA-A, ACL, and CGI scales, carbamazepine was equivalent to oxazepam up to Day 5, and then superior on Days 6 and 7 (P ≤ .05). No decrease in white blood cell count was found in the carbamazepine group.
Conclusion: The authors concluded that carbamazepine is as effective as oxazepam and may be a viable alternative that does not interact with alcohol or cause delirium.17
Malcolm et al18 compared the effects of carbamazepine and lorazepam in patients in an outpatient setting who had single vs multiple previous alcohol withdrawals. The study included 136 patients who satisfied DSM-IV criteria for alcohol dependence and alcohol withdrawal, with a blood alcohol level ≤0.1 g/dL, a Mini-Mental State Examination (MMSE) score ≤26, and a CIWA-Ar score ≤10 on admission. Patients also completed the ADS to quantify the severity of alcohol dependence. Daily drinking was measured by patient report using a daily drinking log and blood alcohol level. Heavy drinking was defined as ≥4 standard drinks per day for women and ≥5 drinks per day for men. On Day 1, patients were randomized to receive carbamazepine, 600 to 800 mg/d,or lorazepam, 6 to 8 mg/d, in divided doses, which was tapered to carbamazepine, 200 mg/d, or lorazepam, 2 mg/d, on Day 5. All patients received thiamine for 12 days. In the immediate post-detoxification period, carbamazepine-treated patients were less likely to relapse, and if they did drink, they drank less than those treated with lorazepam (P = .003). Even in patients who had multiple previous detoxifications, those randomized to carbamazepine drank less than those in lorazepam group (P = .004). Patients in the lorazepam group had significant higher rebound withdrawal symptoms (P = .007).
Continue to: Conclusion
Conclusion: The researchers concluded that carbamazepine and lorazepam were both effective in reducing alcohol withdrawal symptoms. They also concluded that carbamazepine was less likely to cause rebound withdrawal and more likely to reduce post-treatment drinking; among those who did drink, there was less heavy drinking.18
Malcolm et al19 conducted a 5-day double-blind RCT with 136 outpatients who met DSM-IV criteria for alcohol withdrawal. Patients were evaluated by CIWA before getting medications and then daily for 5 days. Patients were randomized to receive carbamazepine, 600 to 800 mg/d on Day 1, 200 mg 3 times a day on Day 2, 200 mg twice a day on Days 3 and 4, and 200 mg once on Day 5. Participants were randomized to receive lorazepam, 6 to 8 mg/d in divided doses on Day 1, 2 mg 3 times a day on Day 2, 2 mg twice a day on Days 3 and 4, and 2 mg once on Day 5. Ability to return to work was self-rated on a 100-mm visual analogue scale, with 0 being “totally unable to return to work’’ and 100 representing “being fully able to return to work.’’ Self-report measures of sleep quality were made using a 100-mm visual analogue scale, with 0 = “the very worst night’s sleep I’ve ever had’’ and 100 = “the very best night’s sleep I’ve ever had.’’ Carbamazepine significantly reduced anxiety (P = .0007). Visual analogue measures of sleep quality indicated a statistically significant main effect of medication on sleep that favored carbamazepine (P = .0186).
Conclusion: The authors concluded that when treating patients with mild to moderate alcohol withdrawal symptoms, carbamazepine was superior to lorazepam in reducing anxiety and improving sleep.19
Sodium valproate
The characteristics of the sodium valproate studies included in this review are summarized in Table 3.20,21
Lambie et al20 evaluated the use of sodium valproate in the treatment of AWS. A total of 49 patients were randomized to a sodium valproate group (n = 22) or a control group (n = 27). All participants were inpatients receiving treatment for alcohol use disorder and substance use disorder for 7 days. Patients in the sodium valproate group received 800 mg every 8 hours for 7 days. Patients were observed daily for occurrence of withdrawal symptoms. Nurses who were blinded to the group assignment graded the degree and severity of symptoms. The trial was initially designed so that chlormethiazole and/or tranquilizers were added to sodium valproate when withdrawal symptoms occurred. However, after treating the first few patients, it became evident that additional medications were not needed. In the treatment group, 13 participants received only sodium valproate, 4 patients needed a tranquilizer, 4 needed chlormethiazole, and 1 needed both. In the control group, 1 received only sodium valproate, 4 received a tranquilizer, 14 received chlormethiazole, and 8 needed both. One patient, who entered the study twice, had a withdrawal seizure when in control group and no seizure on second admission in the sodium valproate group. Physical symptoms disappeared quickly in the sodium valproate group (mean of 2 days vs 2.6 days in the control group). Fourteen patients in the control group received chlormethiazole, compared with only 4 patients in sodium valproate group.
Continue to: Conclusion
Conclusion: The researchers concluded that physical symptoms disappeared quicker in the sodium valproate group than in the control group.20
Hillbom et al21 evaluated the efficacy of sodium valproate vs carbamazepine vs placebo to prevent alcohol withdrawal seizures. A total of 138 participants were studied. Forty-three were assigned to the carbamazepine group, 46 to the sodium valproate group, and 49 to the placebo group. The RCT lasted 4 days. The initial medication doses were 1,200 mg/d. Participants in the carbamazepine group experienced more adverse effects than those in the sodium valproate or placebo groups (P < .001). As a result, approximately one-half of the participants in the carbamazepine group stopped taking the medication. This finding was dependent on the dose of carbamazepine; >800 mg/d resulted in poor tolerance to adverse effects. Seizures occurred among patients in all 3 arms of the study; in the sodium valproate group, 1 participant had a seizure vs 2 participants in the carbamazepine group and 3 in the placebo group. On the other hand, DT occurred only in the sodium valproate and placebo groups.
Conclusion: Researchers concluded that when using sodium valproate or carbamazepine to prevent alcohol withdrawal seizures in an outpatient setting, the adverse effects may outweigh the benefits.21
Lamotrigine
The characteristics of the lamotrigine study included in this review are summarized in Table 3.22
Djokić et al22 evaluated the efficiency of lamotrigine in the treatment of DT. A total of 240 participants who met International Classification of Diseases-10 criteria for DT were randomized to a control group that was treated with anticonvulsants according to an NIAAA protocol (2004), or to an experimental group that was treated with lamotrigine. The CIWA-Ar and the Memorial Delirium Assessment Scale (MDAS) were administered for objective assessment of clinical symptoms, superimposed medical complications, general condition of the patient, adverse effects, and mortality rate. Statistically significant differences between the experimental and control groups were apparent after the third day of therapy, when a drop in the average CIWA-Ar score was observed in the experimental group, while the control group still had high scores (P < .01). After the fifth day of treatment, the differences in scores were more apparent, with the experimental group showing CIWA-Ar scores equal to those of persons with mild/moderate DT, while those in the control group still had high scores. After the tenth day, participants in the experimental group did not have any alcohol withdrawal symptoms, while control group participants were just beginning to get out of life-threatening danger. Death occurred in 4.1% of control group participants and 3.4% of experimental group participants; this difference in mortality rate was not statistically significant.
Continue to: Conclusion
Conclusion: Researchers concluded that lamotrigine is significantly efficacious in the treatment of DT, but does not decrease the mortality rate.22
What to know before you prescribe
AWS is a medical emergency that if left untreated leads to several complications and possibly death. Although benzodiazepines are considered the gold standard for treating AWS, the adverse effects associated with their use advocates for finding alternatives. Anticonvulsants can be an effective alternative for treating AWS. In our literature review, we found 16 double-blind RCTs that used an anticonvulsant medication for the treatment of AWS. Of these, 7 involved gabapentin, 6 involved carbamazepine, 1 involved sodium valproate, 1 involved sodium valproate vs carbamazepine, and 1 involved lamotrigine. Overall, the use of anticonvulsants resulted in significant improvement of mild to moderate symptoms of AWS.
There were more studies of carbamazepine and gabapentin than of other anticonvulsants. All the anticonvulsants offered potential benefits. They decreased the probability of a withdrawal seizure and other complications and effectively reduced alcohol cravings. Anticonvulsants were useful for preventing rebound withdrawal symptoms and reducing post-treatment alcohol consumption, especially in patients who had multiple previous withdrawals. Anticonvulsants were particularly helpful for patients with mood disorders such as depression. In the studies we reviewed, anticonvulsants caused less sedation compared with benzodiazepines, and also decreased the occurrence of relapse.
Dosing recommendations. In the studies included in our review, gabapentin was effective at a dosage of 1,600 mg/d (given as 400 mg 4 times a day). This was tapered as follows: 400 mg 4 times a day on Days 1 to 3, 400 mg 3 times a day on Day 4, 400 mg twice a day on Day 5, and 400 mg once a day on Day 6. Carbamazepine was effective at 600 to 800 mg/d, and was tapered by decreasing by 200 mg as follows: 800 mg/d on Days 1 to 3, 600 mg/d on Day 4, 400 mg on Day 5, and 200 mg/d on Day 6. In the reviewed study, the maximum dose of lamotrigine never exceeded 200 mg/d and was administered for 28 days; the exact dosing and taper plan were not described. The dosing of sodium valproate ranged from 1,200 mg/d to 1600 mg/d for 7 days, followed by decreasing by 200 mg each day. The recommended duration of treatment varied; on average for all anticonvulsants, it was 7 to 12 days, followed by a taper. Carbamazepine was shown to be superior to oxazepam in ameliorating the symptoms of AWS.
Adverse effects. When considering the tolerability, adverse effect profile, duration of action, and effectiveness of the anticonvulsants included in our review, gabapentin appears to be the safest agent to choose. For the other anticonvulsants, the risks might outweigh the benefits. Specifically, in a comparison of sodium valproate and carbamazepine, Hillbom et al21 concluded that in doses >800 mg/d, carbamazepine has potential to cause more adverse effects than benefits. However, Agricola et al16 found that carbamazepine had a preferential action on fear, nightmares, and hallucinations.
Continue to: A few caveats
A few caveats
Our review focused a large collection of data from multiple databases and RCTs only. However, its limitations include:
- there was no measure of heterogeneity
- the studies had short treatment duration
- most studies evaluated predominantly male participants
- some studies were underpowered.
Our review laid a groundwork for future research that includes more well-designed RCTs and/or meta-analyses of recent studies that evaluated the use anticonvulsants for treating AWS.
Bottom Line
Evidence suggests certain anticonvulsants may be an effective alternative to benzodiazepines for the treatment of mild to moderate alcohol withdrawal syndrome. Gabapentin may be the safest anticonvulsant to prescribe. Other anticonvulsants to consider include carbamazepine, sodium valproate, and lamotrigine, but for these agents, the risks might outweigh the benefits.
Related Resources
- Myrick H, Anton RF. Treatment of alcohol withdrawal. Alcohol Health Res World. 1998;22(1):38-43. https://pubs.niaaa.nih.gov/publications/arh22-1/38-43.pdf
- World Health Organization. Management of alcohol withdrawal. Published 2012. https://www.who.int/mental_health/mhgap/evidence/alcohol/q2/en/
Drug Brand Names
Carbamazepine • Tegretol
Gabapentin • Neurontin
Lamotrigine • Lamictal
Levetiracetam • Keppra
Lorazepam • Ativan
Oxcarbazepine • Trileptal
Phenytoin • Dilantin
Sodium valproate • Depakote
Acknowledgments
The authors thank Geetha Manikkara, MD, Madhuri Jakkam Setty, MD, and Elizabeth DeOreo, MD, for their efforts with the systematic review research.
1. Trevisan LA, Boutros N, Petrakis IL, et al. Complications of alcohol withdrawal: pathophysiological insights. Alcohol Health Res World. 1998;22(1):61-66.
2. Borghesani P. Alcohol withdrawal. In: Nordstrom KD, Wilson MP, eds. Quick guide to psychiatric emergencies. Springer: 2018;209-215.
3. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757-766.
4. Ungur LA, Neuner B, John S, et al. Prevention and therapy of alcohol withdrawal on intensive care units: systematic review of controlled trials. Alcohol Clin Exp Res. 2013;37(4):675-686.
5. Sachdeva A, Choudhary M, Chandra M. Alcohol withdrawal syndrome: benzodiazepines and beyond. J Clin Diagn Res. 2015;9(9):VE01-VE07.
6. Ashton H. Toxicity and adverse consequences of benzodiazepine use. Psychiatr Ann. 1995;25:158-165.
7. Bonnet U, Banger M, Leweke FM, et al. Treatment of acute alcohol withdrawal with gabapentin: results from a controlled two-center trial. J Clin Psychopharmacol. 2003;23(5):514-519.
8. Bonnet U, Specka M, Leweke FM, et al. Gabapentin’s acute effect on mood profile--a controlled study on patients with alcohol withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):434-438.
9. Myrick H, Anton R, Voronin K, et al. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res. 2007;31(2):221-227.
10. Malcolm R, Myrick L, Veatch L, et al. Self-reported sleep, sleepiness, and repeated alcohol withdrawals: a randomized, double blind, controlled comparison of lorazepam vs gabapentin. J Clin Sleep Med. 2007;3(1):24-32.
11. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
12. Stock CJ, Carpenter L, Ying J, et al. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother. 2013;47(7-8):961-969.
13. Schacht JP, Anton RF, Randall PK, et al. Effects of a GABA-ergic medication combination and initial alcohol withdrawal severity on cue-elicited brain activation among treatment-seeking alcoholics. Psychopharmacol. 2013;227(4):627-637.
14. Björkqvist SE, Isohanni M, Mäkelä R, et al. Ambulant treatment of alcohol withdrawal symptoms with carbamazepine: a formal multicenter double blind comparison with placebo. Acta Psychiatr Scand. 1976;53(5):333-342.
15. Ritola E, Malinen L. A double-blind comparison of carbamazepine and clomethiazole in the treatment of alcohol withdrawal syndrome. Acta Psychiatr Scand. 1981;64(3):254-259.
16. Agricola R, Mazzarino M, Urani R, et al. Treatment of acute alcohol withdrawal syndrome with carbamazepine: a double-blind comparison with tiapride. J Int Med Res. 1982;10(3):160-165.
17. Stuppaeck CH, Pycha R, Miller C, et al. Carbamazepine versus oxazepam in the treatment of alcohol withdrawal: a double-blind study. Alcohol Alcohol. 1992;27(2):153-158.
18. Malcolm R, Myrick H, Roberts J, et al. The effects of carbamazepine and lorazepam on single vs multiple previous withdrawals in an outpatient randomized trial. J Gen Intern Med. 2002;17(5):349-355.
19. Malcolm R, Myrick H, Roberts J, et al. The differential effects of medications on mood, sleep disturbance, and work ability in outpatient alcohol detoxification. Am J Addict. 2002;11(2):141-150.
20. Lambie D, Johnson R, Vijayasenan M, et al. Sodium valproate in the treatment of the alcohol withdrawal syndrome. Aust N Z J. 1980;14(3):213-215.
21. Hillbom M, Tokola R, Kuusela V, et al. Prevention of alcohol withdrawal seizures with carbamazepine and valproic acid. Alcohol. 1989;6(3):223-226.
22. Djokic
Abrupt cessation or reduction of alcohol consumption may result in alcohol withdrawal syndrome (AWS), which is a medical emergency that can lead to serious complications when unrecognized or treatment is delayed. Symptoms of AWS include tremors, anxiety attacks, cognitive impairment, hallucinations, seizures, delirium tremens (DT), and in severe, untreated cases, death.1 Low to moderate alcohol consumption produces euphoria and excitation via activation of glutamatergic neurotransmission, while higher concentrations produce severe intoxication via GABAergic mechanisms. Acute withdrawal unmasks the hyper-excitatory state of the brain, causing anxiety, agitation, and autonomic activation characteristic of AWS, which typically begins 1 to 3 days after the last drink.2 In the 2012-2013 National Epidemiologic Survey on Alcohol and Related Conditions conducted by the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the 12-month and lifetime prevalences of AWS were 13.9% and 29.1%, respectively.3 Within the general inpatient population, AWS can be present in nearly 30% of patients; if left untreated, AWS has a 15% mortality rate, although when AWS is recognized early and treated, the mortality rate falls dramatically to 2%.4
AWS has most commonly been treated with benzodiazepines.5 However, benzodiazepines have the potential for significant adverse effects when used in older adults and in individuals with complicated medical issues, such as obstructive lung disease and sleep apnea.6 Anticonvulsants have been increasingly used to treat alcohol withdrawal, and their use is supported by several retrospective and prospective studies. In this article, we review the data from randomized control trials (RCTs) on the use of anticonvulsants for the treatment of AWS to see if we can make any recommendations for the use of anticonvulsants for treating AWS.
Our literature search
We searched 5 databases (PubMed, Cochrane, Medline, PsycInfo, and Embase) using the following terms: “alcohol withdrawal syndrome treatment”, “anticonvulsants”, “anti-epileptic”, “gabapentin”, “carbamazepine”, “sodium valproate”, “oxcarbazepine”, “phenytoin”, “levetiracetam”, and “lamotrigine.” We included only double-blind RCTs published between January 1, 1976 and September 30, 2016 in English-language journals or that had an official English translation. There were no restrictions on patient age or location of treatment (inpatient vs outpatient). All RCTs that compared anticonvulsants or a combination of an anticonvulsant and an active pharmacotherapeutic agent with either placebo or gold standard treatment for AWS were included. Database reviews, systematic reviews, and meta-analyses were excluded.
We identified 662 articles that met these criteria. However, most were duplicates, review articles, systematic reviews, meta-analyses, case reports, or open-label or non-randomized trials. Only 16 articles met our inclusion criteria. In the following sections, we discuss these 16 studies by medication type and in chronological order.
Gabapentin
The characteristics of the gabapentin studies included in this review are summarized in Table 1.7-13
Bonnet et al7 (2003) examined 61 adults who met the clinical criteria for alcohol dependence and displayed moderate or severe AWS according to their Mainz Alcohol Withdrawal Score (MAWS ≥4). They were randomized to receive placebo or gabapentin, 400 mg 4 times a day, along with clomethiazole. The attrition rate was not significantly different between the 2 groups (P = .66). The difference in the number of clomethiazole capsules taken during the first 24 hours between the groups was small and not significant (P = .96). Analysis of MAWS over time revealed no significant main effect for group (P = .26) and a significant effect for the time variable (P < .001). The interaction between group and time was not significant (P =.4). This means that there was a significant decrease in MAWS from baseline over 48 hours, and this decrease in MAWS was considered equal for both study groups. Adverse clinical events were observed in both groups, and there was no significant difference (P = .74) between the groups. Nausea and ataxia, which are specific to gabapentin, were observed more frequently in this group.
Conclusion: The authors concluded that gabapentin, 400 mg 4 times a day, is no better than placebo in reducing the amount of clomethiazole required to treat acute AWS.7
Continue to: Bonnet et al
Bonnet et al8 (2007) also conducted a study examining 59 patients with alcohol dependence who displayed moderate or severe AWS. Participants received placebo or gabapentin, 400 mg, and a rescue medication, clomethiazole, if needed. Subsequently, a capsule of study medication was administered every 6 hours for 2 days and then tapered. During the study, mood was measured by Profile of Mood States (POMS), and subjective complaints of withdrawal were measured using the Essen Self-Assessment of Alcohol Withdrawal Scale (ESA). Of the 59 patients, only 46 were analyzed; 5 patients dropped out, and 8 patients were missing data. Compared with the placebo group, the gabapentin group displayed less dejection, fatigue, and anger, and more vigor. Analysis of variance (ANOVA) measures revealed significant overall changes over time on all 4 scales (all P < .001). A significant (F = 3.62, df 2;43, P = .035) group × time interaction resulted exclusively for vigor. Analysis was repeated using rank-transformed data, resulting in a significant (P = .046) interaction effect. The significant increase in vigor was not apparent after tapering off gabapentin, which suggests gabapentin has a reversible effect on vigor. There was a significant (P < .001) overall decline of subjective withdrawal symptoms complaints, but no group × time interaction (P = .62). Analysis of 11 patients with comorbid mild depression revealed no significant time × group interaction for dejection, fatigue, anger, or subjective withdrawal (all P > .05). However, for vigor, the group × time interaction was significant (P = .022). Throughout the treatment, vigor scores of those mild depressive patients who received gabapentin increased to a level comparable to that of patients without a mood disorder.
Conclusion: The authors authors concluded that gabapentin was markedly more efficacious in improving vigor in the small subgroup of patients with mild depression.8
Myrick et al9 (2007) evaluated the safety and tolerability of gabapentin in patients who abused alcohol, as well as the ability of gabapentin to reduce alcohol craving and consumption. This study included 35 participants randomly assigned to receive gabapentin (n = 17) or placebo (n = 18) for 7 days. All medications were administered in standard gel caps with riboflavin, 25 mg, to assess for compliance via a laboratory-based urinary fluorescence assay. Urine samples were assessed for riboflavin at baseline and Day 6, and a reading of 1,500 ng/mL of riboflavin on Day 6 was interpreted as being compliant. Participants were required to abstain completely from drinking alcohol on Day 6 and the morning of Day 7. At the first session, the following measures were completed: demographic form, alcohol and drug section of the Structured Clinical Interview (SCID), Obsessive-Compulsive Drinking Scale, Self-Administered Alcohol Screening Test (SAAST), and Alcohol Dependence Scale (ADS); there also was collection of a urine sample for detection of abused drugs and a blood sample for liver function and general health screening.
At the second session, patients completed the psychiatric sections of the SCID and the Alcohol Craving Questionnaire, and received a physical exam. To assess the negative clinical effects of gabapentin and alcohol on the CNS, the Epworth Sleepiness Scale (ESS) and POMS were administered at baseline and on Day 6. Also, several other scales were used to identify any impact of gabapentin on acute alcohol effects and craving: the Clinical Institute Withdrawal Assessment of Alcohol, Revised (CIWA-Ar), Biphasic Alcohol Effects Scale (BAES), Subjective High Assessment Scale (SHAS), and Alcohol Urge Questionnaire (AUQ).
Conclusion: Gabapentin was well tolerated, but compared with placebo, gabapentin had no effect on alcohol stimulation (P = .75) or sedation (P = .99) as measured by the BAES. The difference in SHAS scores was also not significant (P = .19). There was also no significant reduction in craving for alcohol as measured on the AUQ scale
Continue to: Malcolm et al
Malcolm et al10 conducted an outpatient treatment study. Patients were men and women age 21 to 70 years from multiple ethnic groups. They were randomized to receive gabapentin or lorazepam; 449 patients were screened and 68 completed the follow-up. Scales used included the CIWA-Ar, Beck Depression Inventory (BDI), and ESS.
Patients receiving lorazepam reported less insomnia and more sleepiness early in treatment than patients receiving gabapentin. However, upon completing treatment and discontinuing medication administration, patients previously treated with lorazepam reported increased insomnia and daytime sleepiness, while patients previously treated with gabapentin continued to report improvements in these self-reported sleep measures. The differences between lorazepam and gabapentin were further evidenced in BDI scores at Day 5, Day 7, and Day 12 in patients who had previously experienced multiple withdrawals. Gabapentin was superior to lorazepam in reducing insomnia as assessed by BDI score, an effect that was sustained throughout the post-treatment week. Participants’ ESS scores indicated less daytime sleepiness in the gabapentin group than in the lorazepam group.
Conclusion: Among patients who abused alcohol and had a history of multiple withdrawals, lorazepam is less effective than gabapentin in reducing insomnia.10 However, this study had several limitations: <25% of individuals who were initially screened were enrolled in the study, and it used subjective tests such as BDI. Objective electrophysiologic measures of sleep and daytime sleepiness would have been very helpful.
Myrick et al11 (2009) also compared gabapentin and lorazepam for treating alcohol withdrawal. One hundred patients were randomized to receive 4 days of fixed-dose taper of gabapentin or lorazepam. Patients could receive 1 of 3 gabapentin dosing regimens (600 mg/d, 900 mg/d, or 1,200 mg/d) for 3 days. Participants who were randomized to receive lorazepam were given 6 mg/d for 3 days and then tapered to 4 mg/d. Also, blinded supplemental medications (rescue packs) were given to each patient on Days 1 to 4 to treat subjective feelings of alcohol withdrawal. All patients also received thiamine for 12 days. Assessment of severity of alcohol withdrawal was measured by the CIWA-Ar. To quantify the severity of alcohol dependence and alcohol use, patients were asked to complete the ADS and Time-Line Follow-Back (TLFB) scales, respectively. Other scales administered included the BDI, Zung Anxiety Scale (ZAS), ESS, and visual analogue scales that assessed craving, ability to perform work, and need for additional medication.
There was a decrease in CIWA-Ar scores over time in all groups. High-dose gabapentin was found to be statistically superior but clinically similar to lorazepam (P = .009). Researchers also found that compared with patients who were treated with lorazepam, patients who were treated with gabapentin experienced reduced craving and anxiety/depressive symptoms, and complained of less subjective discomfort. Compared to patients who were treated with gabapentin, patients who were treated with lorazepam had higher probabilities of drinking on the first day of dose decrease (Day 2) and the second day off medication (Day 6) (P = .0002). During post-treatment, patients who were treated with gabapentin had less probability of drinking during the follow-up post-treatment period (P = .2 for 900 mg/d and P = .3 for 1,200 mg/d) compared with patients who were treated with lorazepam (P = .55).
Continue to: Conclusion
Conclusion: The researchers concluded that gabapentin was well tolerated and effectively diminished the symptoms of alcohol withdrawal, especially at the higher target dose (1,200 mg/d), and that compared with lorazepam, gabapentin decreased the probability of drinking during alcohol withdrawal and in the immediate post-withdrawal week.11
Stock et al12 randomized 26 patients who met criteria for AWS to receive gabapentin or chlordiazepoxide. Gabapentin doses were 1,200 mg/d orally for 3 days, followed by 900 mg/d, 600 mg/d, and 300 mg/d for 1 day each. Chlordiazepoxide doses were 100 mg/d orally for 3 days, followed by 75 mg/d, 50 mg/d, and 25 mg/d for 1 day each. The ESS, Penn Alcohol Craving Scale (PACS), ataxia rating, and CIWA-Ar were administered daily. Thirty-five percent of participants dropped out at the end of the 7-day treatment period. Days 1 to 4 were considered the early treatment period, and Days 5 to 7 were considered the late treatment period. The adjusted mean ESS score did not differ significantly between the randomized groups during the early stage (P = .61) vs the late stage, in which the adjusted mean ESS score was significantly lower with gabapentin compared with chlordiazepoxide (P = .04). The differences in PACS scores between the groups were not statistically significant in either stage (early stage P = .59 vs late stage P = .08), but a trend of lower PACS scores was noted with gabapentin in the later stage. No participant in either group had ataxia during the study. In both groups, CIWA-Ar scores were reduced similarly.
Conclusion: The researchers concluded that gabapentin treatment resulted in a significantly greater reduction in sedation (ESS) and a trend toward reduced alcohol craving (PACS) by the end of treatment compared with chlordiazepoxide treatment.12
Schacht et al13 analyzed functional magnetic resonance imaging data from 48 patients who were alcohol-dependent in a 6-week RCT. Patients were randomized to receive gabapentin up to 1,200 mg/d for 39 days plus flumazenil for 2 days (GP/FMZ group) or an oral placebo and placebo infusions on the same time course. Evaluations included the SCID, ADS, and Obsessive-Compulsive Drinking Scale (OCDS). On Day 1, the CIWA-Ar was administered; it was used to ensure equal distribution of individuals with higher alcohol withdrawal symptoms between medication groups. There were no significant effects of initial alcohol withdrawal symptom status or medication. However, there was a significant interaction between these factors: patients with higher alcohol withdrawal symptoms who received GP/FMZ and those with lower alcohol withdrawal symptoms who received placebo demonstrated greater cue-elicited activation, relative to the other groups, and had less subsequent drinking, which reflected differences in deactivation between alcohol and beverage stimuli, in a cluster that encompassed the dorsal ACC (dACC) (family-wise error-corrected cluster probability of P = .012; 99 voxels; local maxima at [-3, 39, 18] and [6, 33, 9]). In the GP/FMZ group, patients with higher alcohol withdrawal symptoms had significantly greater activation, while in the placebo group, patients with lower alcohol withdrawal symptoms had greater activation.
Conclusion: The researchers concluded that alterations in task-related deactivation of dACC, a component of the default mode network, may predict better alcohol treatment response, while activation of DLPFC, an area associated with selective attention, may predict relapse drinking.13
Continue to: Carbamazepine
Carbamazepine
The characteristics of the carbamazepine studies included in this review are summarized in Table 2.14-19

Björkqvist et al14 randomized 105 men with AWS to placebo or carbamazepine. On initial assessment, history, physical examination, relevant labs, and intoxication assessments were recorded. On subsequent visits, nursing staff recorded withdrawal symptoms for patients as 0 to 2 (0 = no specific symptoms, 1 = patient only complained when asked about specific symptoms, 2 = patient complained of withdrawal symptoms without being asked, or if symptoms were severe or obvious to others). Along with the above, vital signs and a visual analogue scale of 0 to 10 (0 = feeling could not be worse, 10 = feeling could not be better) were recorded at each visit. The dose was weight-dependent and administered as follows: on Days 1 and 2, 1+1+2 tablets of carbamazepine, 200 mg, or placebo; Days 3 and 4, 1+1+1 tablets; and Days 5 and 6, 1+0+1 tablets. Every patient received dichloralphenazone as needed. All patients were given vitamin B 3 times a day. Most withdrawal symptoms decreased faster in the carbamazepine group on Day 2 (P = .01) and on Day 4 (P = .1). On the visual analogue scale, scores varied between patients. On Day 1, the mean score was 2.5 times higher in the carbamazepine group compared with the placebo group, and this difference increased to 3 times by Day 7 (P < .01). The patient’s estimated ability to work improved significantly faster in the carbamazepine group than in the placebo group (P < .01).
Conclusion: The authors concluded that compared with placebo, carbamazepine was able to more quickly decrease withdrawal symptoms, especially insomnia and subjective recovery.14
Ritola et al15 randomized 68 hospitalized men with AWS to carbamazepine, 200 mg/d, or clomethiazole, 300 mg/d, for 1 week. The target withdrawal symptoms included gastrointestinal and sleep disturbances; anxiety; aggressiveness; and cardiovascular, depressive, psychotic, and neurologic symptoms. A 4-point rating scale was used for individual symptoms (0 = no symptom, 1 = mild symptom, 2 = moderate symptom, and 3 = severe symptom). On the day of admission (Day 0), all patients were given 50 to 100 mg of chlordiazepoxide IM and 2 tablets and 4 capsules of the trial preparations (either the tablets or capsules were active, and the others were placebos) in the evening. Five patients dropped out of the clomethiazole group and 1 from the carbamazepine group. No significant difference between the 2 treatments were found by the patient, nurse, or physician.
Conclusion: The authors concluded that carbamazepine seemed to be as effective as clomethiazole in the treatment of milder alcohol withdrawal symptoms. Final treatment results were equally good in both groups. Sleep disturbance resolved faster in the carbamazepine group.15
Continue to: Agricola et al
Agricola et al16 compared carbamazepine to tiapride for treatment of acute AWS. In this study, 60 patients were randomized to carbamazepine, 200 mg 3 times a day, or tiapride, 200 mg 3 times a day. All patients were hospitalized with severe AWS preceding DT. The patients were evaluated for withdrawal symptoms (gastrointestinal and cardiovascular symptoms, sleep disturbances, anxiety, aggression, fear, depression, psychotic symptoms, and certain neurologic symptoms). The severity of these symptoms was scored as follows: 0 = no symptoms; 1 = moderate symptoms; and 2 = severe symptoms. At each visit, an overall evaluation of the patient’s clinical condition was made according to a visual analogue scale (100 = worst condition, 0 = best condition). On Day 7, both the doctor and patient evaluated treatment efficacy according to a 4-point scale (1 = no efficacy, 4 = excellent efficacy). There was no significant difference between carbamazepine and tiapride in terms of total symptoms score and visual analogue scale assessment. Carbamazepine was found to have faster relief of symptoms and a significantly greater reduction in symptom score on Day 2 (P < .01). Carbamazepine had a preferential action on fear, nightmares, and hallucinations. The proportion of patients in whom anxiety improved after treatment was 96.2% for carbamazepine and 71.4% for tiapride (P < .05). Aggressiveness and gastrointestinal discomfort resolved faster in the tiapride group. No cases of DT were observed.
Conclusion: The researchers concluded that either carbamazepine or tiapride provides an appropriate alternative in the treatment of inpatients with severe AWS.16
Stuppaeck et al17 compared the efficacy of carbamazepine to oxazepam in 60 inpatients who had symptoms of alcohol withdrawal. Alcohol withdrawal was measured with the CIWA-A, and patients with scores >20 were enrolled in the study. The Clinical Global Impression (CGI) scale and self-rated Adjective Checklist (ACL) were also used. On Days 1 to 3, patients received oxazepam, 120 mg/d, or carbamazepine, 800 mg/d. From Day 4 to 7, doses were decreased to 90 mg/d and 600 mg/d, respectively. After the 7-day trial, all patients were treated with carbamazepine, 200 mg twice a day on Day 8 and 200 mg at night on Day 9. Two patients withdrew consent and 6 dropped out due to adverse effects. During the 7-day trial, when comparing all improvements on CIWA-A, ACL, and CGI scales, carbamazepine was equivalent to oxazepam up to Day 5, and then superior on Days 6 and 7 (P ≤ .05). No decrease in white blood cell count was found in the carbamazepine group.
Conclusion: The authors concluded that carbamazepine is as effective as oxazepam and may be a viable alternative that does not interact with alcohol or cause delirium.17
Malcolm et al18 compared the effects of carbamazepine and lorazepam in patients in an outpatient setting who had single vs multiple previous alcohol withdrawals. The study included 136 patients who satisfied DSM-IV criteria for alcohol dependence and alcohol withdrawal, with a blood alcohol level ≤0.1 g/dL, a Mini-Mental State Examination (MMSE) score ≤26, and a CIWA-Ar score ≤10 on admission. Patients also completed the ADS to quantify the severity of alcohol dependence. Daily drinking was measured by patient report using a daily drinking log and blood alcohol level. Heavy drinking was defined as ≥4 standard drinks per day for women and ≥5 drinks per day for men. On Day 1, patients were randomized to receive carbamazepine, 600 to 800 mg/d,or lorazepam, 6 to 8 mg/d, in divided doses, which was tapered to carbamazepine, 200 mg/d, or lorazepam, 2 mg/d, on Day 5. All patients received thiamine for 12 days. In the immediate post-detoxification period, carbamazepine-treated patients were less likely to relapse, and if they did drink, they drank less than those treated with lorazepam (P = .003). Even in patients who had multiple previous detoxifications, those randomized to carbamazepine drank less than those in lorazepam group (P = .004). Patients in the lorazepam group had significant higher rebound withdrawal symptoms (P = .007).
Continue to: Conclusion
Conclusion: The researchers concluded that carbamazepine and lorazepam were both effective in reducing alcohol withdrawal symptoms. They also concluded that carbamazepine was less likely to cause rebound withdrawal and more likely to reduce post-treatment drinking; among those who did drink, there was less heavy drinking.18
Malcolm et al19 conducted a 5-day double-blind RCT with 136 outpatients who met DSM-IV criteria for alcohol withdrawal. Patients were evaluated by CIWA before getting medications and then daily for 5 days. Patients were randomized to receive carbamazepine, 600 to 800 mg/d on Day 1, 200 mg 3 times a day on Day 2, 200 mg twice a day on Days 3 and 4, and 200 mg once on Day 5. Participants were randomized to receive lorazepam, 6 to 8 mg/d in divided doses on Day 1, 2 mg 3 times a day on Day 2, 2 mg twice a day on Days 3 and 4, and 2 mg once on Day 5. Ability to return to work was self-rated on a 100-mm visual analogue scale, with 0 being “totally unable to return to work’’ and 100 representing “being fully able to return to work.’’ Self-report measures of sleep quality were made using a 100-mm visual analogue scale, with 0 = “the very worst night’s sleep I’ve ever had’’ and 100 = “the very best night’s sleep I’ve ever had.’’ Carbamazepine significantly reduced anxiety (P = .0007). Visual analogue measures of sleep quality indicated a statistically significant main effect of medication on sleep that favored carbamazepine (P = .0186).
Conclusion: The authors concluded that when treating patients with mild to moderate alcohol withdrawal symptoms, carbamazepine was superior to lorazepam in reducing anxiety and improving sleep.19
Sodium valproate
The characteristics of the sodium valproate studies included in this review are summarized in Table 3.20,21
Lambie et al20 evaluated the use of sodium valproate in the treatment of AWS. A total of 49 patients were randomized to a sodium valproate group (n = 22) or a control group (n = 27). All participants were inpatients receiving treatment for alcohol use disorder and substance use disorder for 7 days. Patients in the sodium valproate group received 800 mg every 8 hours for 7 days. Patients were observed daily for occurrence of withdrawal symptoms. Nurses who were blinded to the group assignment graded the degree and severity of symptoms. The trial was initially designed so that chlormethiazole and/or tranquilizers were added to sodium valproate when withdrawal symptoms occurred. However, after treating the first few patients, it became evident that additional medications were not needed. In the treatment group, 13 participants received only sodium valproate, 4 patients needed a tranquilizer, 4 needed chlormethiazole, and 1 needed both. In the control group, 1 received only sodium valproate, 4 received a tranquilizer, 14 received chlormethiazole, and 8 needed both. One patient, who entered the study twice, had a withdrawal seizure when in control group and no seizure on second admission in the sodium valproate group. Physical symptoms disappeared quickly in the sodium valproate group (mean of 2 days vs 2.6 days in the control group). Fourteen patients in the control group received chlormethiazole, compared with only 4 patients in sodium valproate group.
Continue to: Conclusion
Conclusion: The researchers concluded that physical symptoms disappeared quicker in the sodium valproate group than in the control group.20
Hillbom et al21 evaluated the efficacy of sodium valproate vs carbamazepine vs placebo to prevent alcohol withdrawal seizures. A total of 138 participants were studied. Forty-three were assigned to the carbamazepine group, 46 to the sodium valproate group, and 49 to the placebo group. The RCT lasted 4 days. The initial medication doses were 1,200 mg/d. Participants in the carbamazepine group experienced more adverse effects than those in the sodium valproate or placebo groups (P < .001). As a result, approximately one-half of the participants in the carbamazepine group stopped taking the medication. This finding was dependent on the dose of carbamazepine; >800 mg/d resulted in poor tolerance to adverse effects. Seizures occurred among patients in all 3 arms of the study; in the sodium valproate group, 1 participant had a seizure vs 2 participants in the carbamazepine group and 3 in the placebo group. On the other hand, DT occurred only in the sodium valproate and placebo groups.
Conclusion: Researchers concluded that when using sodium valproate or carbamazepine to prevent alcohol withdrawal seizures in an outpatient setting, the adverse effects may outweigh the benefits.21
Lamotrigine
The characteristics of the lamotrigine study included in this review are summarized in Table 3.22
Djokić et al22 evaluated the efficiency of lamotrigine in the treatment of DT. A total of 240 participants who met International Classification of Diseases-10 criteria for DT were randomized to a control group that was treated with anticonvulsants according to an NIAAA protocol (2004), or to an experimental group that was treated with lamotrigine. The CIWA-Ar and the Memorial Delirium Assessment Scale (MDAS) were administered for objective assessment of clinical symptoms, superimposed medical complications, general condition of the patient, adverse effects, and mortality rate. Statistically significant differences between the experimental and control groups were apparent after the third day of therapy, when a drop in the average CIWA-Ar score was observed in the experimental group, while the control group still had high scores (P < .01). After the fifth day of treatment, the differences in scores were more apparent, with the experimental group showing CIWA-Ar scores equal to those of persons with mild/moderate DT, while those in the control group still had high scores. After the tenth day, participants in the experimental group did not have any alcohol withdrawal symptoms, while control group participants were just beginning to get out of life-threatening danger. Death occurred in 4.1% of control group participants and 3.4% of experimental group participants; this difference in mortality rate was not statistically significant.
Continue to: Conclusion
Conclusion: Researchers concluded that lamotrigine is significantly efficacious in the treatment of DT, but does not decrease the mortality rate.22
What to know before you prescribe
AWS is a medical emergency that if left untreated leads to several complications and possibly death. Although benzodiazepines are considered the gold standard for treating AWS, the adverse effects associated with their use advocates for finding alternatives. Anticonvulsants can be an effective alternative for treating AWS. In our literature review, we found 16 double-blind RCTs that used an anticonvulsant medication for the treatment of AWS. Of these, 7 involved gabapentin, 6 involved carbamazepine, 1 involved sodium valproate, 1 involved sodium valproate vs carbamazepine, and 1 involved lamotrigine. Overall, the use of anticonvulsants resulted in significant improvement of mild to moderate symptoms of AWS.
There were more studies of carbamazepine and gabapentin than of other anticonvulsants. All the anticonvulsants offered potential benefits. They decreased the probability of a withdrawal seizure and other complications and effectively reduced alcohol cravings. Anticonvulsants were useful for preventing rebound withdrawal symptoms and reducing post-treatment alcohol consumption, especially in patients who had multiple previous withdrawals. Anticonvulsants were particularly helpful for patients with mood disorders such as depression. In the studies we reviewed, anticonvulsants caused less sedation compared with benzodiazepines, and also decreased the occurrence of relapse.
Dosing recommendations. In the studies included in our review, gabapentin was effective at a dosage of 1,600 mg/d (given as 400 mg 4 times a day). This was tapered as follows: 400 mg 4 times a day on Days 1 to 3, 400 mg 3 times a day on Day 4, 400 mg twice a day on Day 5, and 400 mg once a day on Day 6. Carbamazepine was effective at 600 to 800 mg/d, and was tapered by decreasing by 200 mg as follows: 800 mg/d on Days 1 to 3, 600 mg/d on Day 4, 400 mg on Day 5, and 200 mg/d on Day 6. In the reviewed study, the maximum dose of lamotrigine never exceeded 200 mg/d and was administered for 28 days; the exact dosing and taper plan were not described. The dosing of sodium valproate ranged from 1,200 mg/d to 1600 mg/d for 7 days, followed by decreasing by 200 mg each day. The recommended duration of treatment varied; on average for all anticonvulsants, it was 7 to 12 days, followed by a taper. Carbamazepine was shown to be superior to oxazepam in ameliorating the symptoms of AWS.
Adverse effects. When considering the tolerability, adverse effect profile, duration of action, and effectiveness of the anticonvulsants included in our review, gabapentin appears to be the safest agent to choose. For the other anticonvulsants, the risks might outweigh the benefits. Specifically, in a comparison of sodium valproate and carbamazepine, Hillbom et al21 concluded that in doses >800 mg/d, carbamazepine has potential to cause more adverse effects than benefits. However, Agricola et al16 found that carbamazepine had a preferential action on fear, nightmares, and hallucinations.
Continue to: A few caveats
A few caveats
Our review focused a large collection of data from multiple databases and RCTs only. However, its limitations include:
- there was no measure of heterogeneity
- the studies had short treatment duration
- most studies evaluated predominantly male participants
- some studies were underpowered.
Our review laid a groundwork for future research that includes more well-designed RCTs and/or meta-analyses of recent studies that evaluated the use anticonvulsants for treating AWS.
Bottom Line
Evidence suggests certain anticonvulsants may be an effective alternative to benzodiazepines for the treatment of mild to moderate alcohol withdrawal syndrome. Gabapentin may be the safest anticonvulsant to prescribe. Other anticonvulsants to consider include carbamazepine, sodium valproate, and lamotrigine, but for these agents, the risks might outweigh the benefits.
Related Resources
- Myrick H, Anton RF. Treatment of alcohol withdrawal. Alcohol Health Res World. 1998;22(1):38-43. https://pubs.niaaa.nih.gov/publications/arh22-1/38-43.pdf
- World Health Organization. Management of alcohol withdrawal. Published 2012. https://www.who.int/mental_health/mhgap/evidence/alcohol/q2/en/
Drug Brand Names
Carbamazepine • Tegretol
Gabapentin • Neurontin
Lamotrigine • Lamictal
Levetiracetam • Keppra
Lorazepam • Ativan
Oxcarbazepine • Trileptal
Phenytoin • Dilantin
Sodium valproate • Depakote
Acknowledgments
The authors thank Geetha Manikkara, MD, Madhuri Jakkam Setty, MD, and Elizabeth DeOreo, MD, for their efforts with the systematic review research.
Abrupt cessation or reduction of alcohol consumption may result in alcohol withdrawal syndrome (AWS), which is a medical emergency that can lead to serious complications when unrecognized or treatment is delayed. Symptoms of AWS include tremors, anxiety attacks, cognitive impairment, hallucinations, seizures, delirium tremens (DT), and in severe, untreated cases, death.1 Low to moderate alcohol consumption produces euphoria and excitation via activation of glutamatergic neurotransmission, while higher concentrations produce severe intoxication via GABAergic mechanisms. Acute withdrawal unmasks the hyper-excitatory state of the brain, causing anxiety, agitation, and autonomic activation characteristic of AWS, which typically begins 1 to 3 days after the last drink.2 In the 2012-2013 National Epidemiologic Survey on Alcohol and Related Conditions conducted by the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the 12-month and lifetime prevalences of AWS were 13.9% and 29.1%, respectively.3 Within the general inpatient population, AWS can be present in nearly 30% of patients; if left untreated, AWS has a 15% mortality rate, although when AWS is recognized early and treated, the mortality rate falls dramatically to 2%.4
AWS has most commonly been treated with benzodiazepines.5 However, benzodiazepines have the potential for significant adverse effects when used in older adults and in individuals with complicated medical issues, such as obstructive lung disease and sleep apnea.6 Anticonvulsants have been increasingly used to treat alcohol withdrawal, and their use is supported by several retrospective and prospective studies. In this article, we review the data from randomized control trials (RCTs) on the use of anticonvulsants for the treatment of AWS to see if we can make any recommendations for the use of anticonvulsants for treating AWS.
Our literature search
We searched 5 databases (PubMed, Cochrane, Medline, PsycInfo, and Embase) using the following terms: “alcohol withdrawal syndrome treatment”, “anticonvulsants”, “anti-epileptic”, “gabapentin”, “carbamazepine”, “sodium valproate”, “oxcarbazepine”, “phenytoin”, “levetiracetam”, and “lamotrigine.” We included only double-blind RCTs published between January 1, 1976 and September 30, 2016 in English-language journals or that had an official English translation. There were no restrictions on patient age or location of treatment (inpatient vs outpatient). All RCTs that compared anticonvulsants or a combination of an anticonvulsant and an active pharmacotherapeutic agent with either placebo or gold standard treatment for AWS were included. Database reviews, systematic reviews, and meta-analyses were excluded.
We identified 662 articles that met these criteria. However, most were duplicates, review articles, systematic reviews, meta-analyses, case reports, or open-label or non-randomized trials. Only 16 articles met our inclusion criteria. In the following sections, we discuss these 16 studies by medication type and in chronological order.
Gabapentin
The characteristics of the gabapentin studies included in this review are summarized in Table 1.7-13
Bonnet et al7 (2003) examined 61 adults who met the clinical criteria for alcohol dependence and displayed moderate or severe AWS according to their Mainz Alcohol Withdrawal Score (MAWS ≥4). They were randomized to receive placebo or gabapentin, 400 mg 4 times a day, along with clomethiazole. The attrition rate was not significantly different between the 2 groups (P = .66). The difference in the number of clomethiazole capsules taken during the first 24 hours between the groups was small and not significant (P = .96). Analysis of MAWS over time revealed no significant main effect for group (P = .26) and a significant effect for the time variable (P < .001). The interaction between group and time was not significant (P =.4). This means that there was a significant decrease in MAWS from baseline over 48 hours, and this decrease in MAWS was considered equal for both study groups. Adverse clinical events were observed in both groups, and there was no significant difference (P = .74) between the groups. Nausea and ataxia, which are specific to gabapentin, were observed more frequently in this group.
Conclusion: The authors concluded that gabapentin, 400 mg 4 times a day, is no better than placebo in reducing the amount of clomethiazole required to treat acute AWS.7
Continue to: Bonnet et al
Bonnet et al8 (2007) also conducted a study examining 59 patients with alcohol dependence who displayed moderate or severe AWS. Participants received placebo or gabapentin, 400 mg, and a rescue medication, clomethiazole, if needed. Subsequently, a capsule of study medication was administered every 6 hours for 2 days and then tapered. During the study, mood was measured by Profile of Mood States (POMS), and subjective complaints of withdrawal were measured using the Essen Self-Assessment of Alcohol Withdrawal Scale (ESA). Of the 59 patients, only 46 were analyzed; 5 patients dropped out, and 8 patients were missing data. Compared with the placebo group, the gabapentin group displayed less dejection, fatigue, and anger, and more vigor. Analysis of variance (ANOVA) measures revealed significant overall changes over time on all 4 scales (all P < .001). A significant (F = 3.62, df 2;43, P = .035) group × time interaction resulted exclusively for vigor. Analysis was repeated using rank-transformed data, resulting in a significant (P = .046) interaction effect. The significant increase in vigor was not apparent after tapering off gabapentin, which suggests gabapentin has a reversible effect on vigor. There was a significant (P < .001) overall decline of subjective withdrawal symptoms complaints, but no group × time interaction (P = .62). Analysis of 11 patients with comorbid mild depression revealed no significant time × group interaction for dejection, fatigue, anger, or subjective withdrawal (all P > .05). However, for vigor, the group × time interaction was significant (P = .022). Throughout the treatment, vigor scores of those mild depressive patients who received gabapentin increased to a level comparable to that of patients without a mood disorder.
Conclusion: The authors authors concluded that gabapentin was markedly more efficacious in improving vigor in the small subgroup of patients with mild depression.8
Myrick et al9 (2007) evaluated the safety and tolerability of gabapentin in patients who abused alcohol, as well as the ability of gabapentin to reduce alcohol craving and consumption. This study included 35 participants randomly assigned to receive gabapentin (n = 17) or placebo (n = 18) for 7 days. All medications were administered in standard gel caps with riboflavin, 25 mg, to assess for compliance via a laboratory-based urinary fluorescence assay. Urine samples were assessed for riboflavin at baseline and Day 6, and a reading of 1,500 ng/mL of riboflavin on Day 6 was interpreted as being compliant. Participants were required to abstain completely from drinking alcohol on Day 6 and the morning of Day 7. At the first session, the following measures were completed: demographic form, alcohol and drug section of the Structured Clinical Interview (SCID), Obsessive-Compulsive Drinking Scale, Self-Administered Alcohol Screening Test (SAAST), and Alcohol Dependence Scale (ADS); there also was collection of a urine sample for detection of abused drugs and a blood sample for liver function and general health screening.
At the second session, patients completed the psychiatric sections of the SCID and the Alcohol Craving Questionnaire, and received a physical exam. To assess the negative clinical effects of gabapentin and alcohol on the CNS, the Epworth Sleepiness Scale (ESS) and POMS were administered at baseline and on Day 6. Also, several other scales were used to identify any impact of gabapentin on acute alcohol effects and craving: the Clinical Institute Withdrawal Assessment of Alcohol, Revised (CIWA-Ar), Biphasic Alcohol Effects Scale (BAES), Subjective High Assessment Scale (SHAS), and Alcohol Urge Questionnaire (AUQ).
Conclusion: Gabapentin was well tolerated, but compared with placebo, gabapentin had no effect on alcohol stimulation (P = .75) or sedation (P = .99) as measured by the BAES. The difference in SHAS scores was also not significant (P = .19). There was also no significant reduction in craving for alcohol as measured on the AUQ scale
Continue to: Malcolm et al
Malcolm et al10 conducted an outpatient treatment study. Patients were men and women age 21 to 70 years from multiple ethnic groups. They were randomized to receive gabapentin or lorazepam; 449 patients were screened and 68 completed the follow-up. Scales used included the CIWA-Ar, Beck Depression Inventory (BDI), and ESS.
Patients receiving lorazepam reported less insomnia and more sleepiness early in treatment than patients receiving gabapentin. However, upon completing treatment and discontinuing medication administration, patients previously treated with lorazepam reported increased insomnia and daytime sleepiness, while patients previously treated with gabapentin continued to report improvements in these self-reported sleep measures. The differences between lorazepam and gabapentin were further evidenced in BDI scores at Day 5, Day 7, and Day 12 in patients who had previously experienced multiple withdrawals. Gabapentin was superior to lorazepam in reducing insomnia as assessed by BDI score, an effect that was sustained throughout the post-treatment week. Participants’ ESS scores indicated less daytime sleepiness in the gabapentin group than in the lorazepam group.
Conclusion: Among patients who abused alcohol and had a history of multiple withdrawals, lorazepam is less effective than gabapentin in reducing insomnia.10 However, this study had several limitations: <25% of individuals who were initially screened were enrolled in the study, and it used subjective tests such as BDI. Objective electrophysiologic measures of sleep and daytime sleepiness would have been very helpful.
Myrick et al11 (2009) also compared gabapentin and lorazepam for treating alcohol withdrawal. One hundred patients were randomized to receive 4 days of fixed-dose taper of gabapentin or lorazepam. Patients could receive 1 of 3 gabapentin dosing regimens (600 mg/d, 900 mg/d, or 1,200 mg/d) for 3 days. Participants who were randomized to receive lorazepam were given 6 mg/d for 3 days and then tapered to 4 mg/d. Also, blinded supplemental medications (rescue packs) were given to each patient on Days 1 to 4 to treat subjective feelings of alcohol withdrawal. All patients also received thiamine for 12 days. Assessment of severity of alcohol withdrawal was measured by the CIWA-Ar. To quantify the severity of alcohol dependence and alcohol use, patients were asked to complete the ADS and Time-Line Follow-Back (TLFB) scales, respectively. Other scales administered included the BDI, Zung Anxiety Scale (ZAS), ESS, and visual analogue scales that assessed craving, ability to perform work, and need for additional medication.
There was a decrease in CIWA-Ar scores over time in all groups. High-dose gabapentin was found to be statistically superior but clinically similar to lorazepam (P = .009). Researchers also found that compared with patients who were treated with lorazepam, patients who were treated with gabapentin experienced reduced craving and anxiety/depressive symptoms, and complained of less subjective discomfort. Compared to patients who were treated with gabapentin, patients who were treated with lorazepam had higher probabilities of drinking on the first day of dose decrease (Day 2) and the second day off medication (Day 6) (P = .0002). During post-treatment, patients who were treated with gabapentin had less probability of drinking during the follow-up post-treatment period (P = .2 for 900 mg/d and P = .3 for 1,200 mg/d) compared with patients who were treated with lorazepam (P = .55).
Continue to: Conclusion
Conclusion: The researchers concluded that gabapentin was well tolerated and effectively diminished the symptoms of alcohol withdrawal, especially at the higher target dose (1,200 mg/d), and that compared with lorazepam, gabapentin decreased the probability of drinking during alcohol withdrawal and in the immediate post-withdrawal week.11
Stock et al12 randomized 26 patients who met criteria for AWS to receive gabapentin or chlordiazepoxide. Gabapentin doses were 1,200 mg/d orally for 3 days, followed by 900 mg/d, 600 mg/d, and 300 mg/d for 1 day each. Chlordiazepoxide doses were 100 mg/d orally for 3 days, followed by 75 mg/d, 50 mg/d, and 25 mg/d for 1 day each. The ESS, Penn Alcohol Craving Scale (PACS), ataxia rating, and CIWA-Ar were administered daily. Thirty-five percent of participants dropped out at the end of the 7-day treatment period. Days 1 to 4 were considered the early treatment period, and Days 5 to 7 were considered the late treatment period. The adjusted mean ESS score did not differ significantly between the randomized groups during the early stage (P = .61) vs the late stage, in which the adjusted mean ESS score was significantly lower with gabapentin compared with chlordiazepoxide (P = .04). The differences in PACS scores between the groups were not statistically significant in either stage (early stage P = .59 vs late stage P = .08), but a trend of lower PACS scores was noted with gabapentin in the later stage. No participant in either group had ataxia during the study. In both groups, CIWA-Ar scores were reduced similarly.
Conclusion: The researchers concluded that gabapentin treatment resulted in a significantly greater reduction in sedation (ESS) and a trend toward reduced alcohol craving (PACS) by the end of treatment compared with chlordiazepoxide treatment.12
Schacht et al13 analyzed functional magnetic resonance imaging data from 48 patients who were alcohol-dependent in a 6-week RCT. Patients were randomized to receive gabapentin up to 1,200 mg/d for 39 days plus flumazenil for 2 days (GP/FMZ group) or an oral placebo and placebo infusions on the same time course. Evaluations included the SCID, ADS, and Obsessive-Compulsive Drinking Scale (OCDS). On Day 1, the CIWA-Ar was administered; it was used to ensure equal distribution of individuals with higher alcohol withdrawal symptoms between medication groups. There were no significant effects of initial alcohol withdrawal symptom status or medication. However, there was a significant interaction between these factors: patients with higher alcohol withdrawal symptoms who received GP/FMZ and those with lower alcohol withdrawal symptoms who received placebo demonstrated greater cue-elicited activation, relative to the other groups, and had less subsequent drinking, which reflected differences in deactivation between alcohol and beverage stimuli, in a cluster that encompassed the dorsal ACC (dACC) (family-wise error-corrected cluster probability of P = .012; 99 voxels; local maxima at [-3, 39, 18] and [6, 33, 9]). In the GP/FMZ group, patients with higher alcohol withdrawal symptoms had significantly greater activation, while in the placebo group, patients with lower alcohol withdrawal symptoms had greater activation.
Conclusion: The researchers concluded that alterations in task-related deactivation of dACC, a component of the default mode network, may predict better alcohol treatment response, while activation of DLPFC, an area associated with selective attention, may predict relapse drinking.13
Continue to: Carbamazepine
Carbamazepine
The characteristics of the carbamazepine studies included in this review are summarized in Table 2.14-19

Björkqvist et al14 randomized 105 men with AWS to placebo or carbamazepine. On initial assessment, history, physical examination, relevant labs, and intoxication assessments were recorded. On subsequent visits, nursing staff recorded withdrawal symptoms for patients as 0 to 2 (0 = no specific symptoms, 1 = patient only complained when asked about specific symptoms, 2 = patient complained of withdrawal symptoms without being asked, or if symptoms were severe or obvious to others). Along with the above, vital signs and a visual analogue scale of 0 to 10 (0 = feeling could not be worse, 10 = feeling could not be better) were recorded at each visit. The dose was weight-dependent and administered as follows: on Days 1 and 2, 1+1+2 tablets of carbamazepine, 200 mg, or placebo; Days 3 and 4, 1+1+1 tablets; and Days 5 and 6, 1+0+1 tablets. Every patient received dichloralphenazone as needed. All patients were given vitamin B 3 times a day. Most withdrawal symptoms decreased faster in the carbamazepine group on Day 2 (P = .01) and on Day 4 (P = .1). On the visual analogue scale, scores varied between patients. On Day 1, the mean score was 2.5 times higher in the carbamazepine group compared with the placebo group, and this difference increased to 3 times by Day 7 (P < .01). The patient’s estimated ability to work improved significantly faster in the carbamazepine group than in the placebo group (P < .01).
Conclusion: The authors concluded that compared with placebo, carbamazepine was able to more quickly decrease withdrawal symptoms, especially insomnia and subjective recovery.14
Ritola et al15 randomized 68 hospitalized men with AWS to carbamazepine, 200 mg/d, or clomethiazole, 300 mg/d, for 1 week. The target withdrawal symptoms included gastrointestinal and sleep disturbances; anxiety; aggressiveness; and cardiovascular, depressive, psychotic, and neurologic symptoms. A 4-point rating scale was used for individual symptoms (0 = no symptom, 1 = mild symptom, 2 = moderate symptom, and 3 = severe symptom). On the day of admission (Day 0), all patients were given 50 to 100 mg of chlordiazepoxide IM and 2 tablets and 4 capsules of the trial preparations (either the tablets or capsules were active, and the others were placebos) in the evening. Five patients dropped out of the clomethiazole group and 1 from the carbamazepine group. No significant difference between the 2 treatments were found by the patient, nurse, or physician.
Conclusion: The authors concluded that carbamazepine seemed to be as effective as clomethiazole in the treatment of milder alcohol withdrawal symptoms. Final treatment results were equally good in both groups. Sleep disturbance resolved faster in the carbamazepine group.15
Continue to: Agricola et al
Agricola et al16 compared carbamazepine to tiapride for treatment of acute AWS. In this study, 60 patients were randomized to carbamazepine, 200 mg 3 times a day, or tiapride, 200 mg 3 times a day. All patients were hospitalized with severe AWS preceding DT. The patients were evaluated for withdrawal symptoms (gastrointestinal and cardiovascular symptoms, sleep disturbances, anxiety, aggression, fear, depression, psychotic symptoms, and certain neurologic symptoms). The severity of these symptoms was scored as follows: 0 = no symptoms; 1 = moderate symptoms; and 2 = severe symptoms. At each visit, an overall evaluation of the patient’s clinical condition was made according to a visual analogue scale (100 = worst condition, 0 = best condition). On Day 7, both the doctor and patient evaluated treatment efficacy according to a 4-point scale (1 = no efficacy, 4 = excellent efficacy). There was no significant difference between carbamazepine and tiapride in terms of total symptoms score and visual analogue scale assessment. Carbamazepine was found to have faster relief of symptoms and a significantly greater reduction in symptom score on Day 2 (P < .01). Carbamazepine had a preferential action on fear, nightmares, and hallucinations. The proportion of patients in whom anxiety improved after treatment was 96.2% for carbamazepine and 71.4% for tiapride (P < .05). Aggressiveness and gastrointestinal discomfort resolved faster in the tiapride group. No cases of DT were observed.
Conclusion: The researchers concluded that either carbamazepine or tiapride provides an appropriate alternative in the treatment of inpatients with severe AWS.16
Stuppaeck et al17 compared the efficacy of carbamazepine to oxazepam in 60 inpatients who had symptoms of alcohol withdrawal. Alcohol withdrawal was measured with the CIWA-A, and patients with scores >20 were enrolled in the study. The Clinical Global Impression (CGI) scale and self-rated Adjective Checklist (ACL) were also used. On Days 1 to 3, patients received oxazepam, 120 mg/d, or carbamazepine, 800 mg/d. From Day 4 to 7, doses were decreased to 90 mg/d and 600 mg/d, respectively. After the 7-day trial, all patients were treated with carbamazepine, 200 mg twice a day on Day 8 and 200 mg at night on Day 9. Two patients withdrew consent and 6 dropped out due to adverse effects. During the 7-day trial, when comparing all improvements on CIWA-A, ACL, and CGI scales, carbamazepine was equivalent to oxazepam up to Day 5, and then superior on Days 6 and 7 (P ≤ .05). No decrease in white blood cell count was found in the carbamazepine group.
Conclusion: The authors concluded that carbamazepine is as effective as oxazepam and may be a viable alternative that does not interact with alcohol or cause delirium.17
Malcolm et al18 compared the effects of carbamazepine and lorazepam in patients in an outpatient setting who had single vs multiple previous alcohol withdrawals. The study included 136 patients who satisfied DSM-IV criteria for alcohol dependence and alcohol withdrawal, with a blood alcohol level ≤0.1 g/dL, a Mini-Mental State Examination (MMSE) score ≤26, and a CIWA-Ar score ≤10 on admission. Patients also completed the ADS to quantify the severity of alcohol dependence. Daily drinking was measured by patient report using a daily drinking log and blood alcohol level. Heavy drinking was defined as ≥4 standard drinks per day for women and ≥5 drinks per day for men. On Day 1, patients were randomized to receive carbamazepine, 600 to 800 mg/d,or lorazepam, 6 to 8 mg/d, in divided doses, which was tapered to carbamazepine, 200 mg/d, or lorazepam, 2 mg/d, on Day 5. All patients received thiamine for 12 days. In the immediate post-detoxification period, carbamazepine-treated patients were less likely to relapse, and if they did drink, they drank less than those treated with lorazepam (P = .003). Even in patients who had multiple previous detoxifications, those randomized to carbamazepine drank less than those in lorazepam group (P = .004). Patients in the lorazepam group had significant higher rebound withdrawal symptoms (P = .007).
Continue to: Conclusion
Conclusion: The researchers concluded that carbamazepine and lorazepam were both effective in reducing alcohol withdrawal symptoms. They also concluded that carbamazepine was less likely to cause rebound withdrawal and more likely to reduce post-treatment drinking; among those who did drink, there was less heavy drinking.18
Malcolm et al19 conducted a 5-day double-blind RCT with 136 outpatients who met DSM-IV criteria for alcohol withdrawal. Patients were evaluated by CIWA before getting medications and then daily for 5 days. Patients were randomized to receive carbamazepine, 600 to 800 mg/d on Day 1, 200 mg 3 times a day on Day 2, 200 mg twice a day on Days 3 and 4, and 200 mg once on Day 5. Participants were randomized to receive lorazepam, 6 to 8 mg/d in divided doses on Day 1, 2 mg 3 times a day on Day 2, 2 mg twice a day on Days 3 and 4, and 2 mg once on Day 5. Ability to return to work was self-rated on a 100-mm visual analogue scale, with 0 being “totally unable to return to work’’ and 100 representing “being fully able to return to work.’’ Self-report measures of sleep quality were made using a 100-mm visual analogue scale, with 0 = “the very worst night’s sleep I’ve ever had’’ and 100 = “the very best night’s sleep I’ve ever had.’’ Carbamazepine significantly reduced anxiety (P = .0007). Visual analogue measures of sleep quality indicated a statistically significant main effect of medication on sleep that favored carbamazepine (P = .0186).
Conclusion: The authors concluded that when treating patients with mild to moderate alcohol withdrawal symptoms, carbamazepine was superior to lorazepam in reducing anxiety and improving sleep.19
Sodium valproate
The characteristics of the sodium valproate studies included in this review are summarized in Table 3.20,21
Lambie et al20 evaluated the use of sodium valproate in the treatment of AWS. A total of 49 patients were randomized to a sodium valproate group (n = 22) or a control group (n = 27). All participants were inpatients receiving treatment for alcohol use disorder and substance use disorder for 7 days. Patients in the sodium valproate group received 800 mg every 8 hours for 7 days. Patients were observed daily for occurrence of withdrawal symptoms. Nurses who were blinded to the group assignment graded the degree and severity of symptoms. The trial was initially designed so that chlormethiazole and/or tranquilizers were added to sodium valproate when withdrawal symptoms occurred. However, after treating the first few patients, it became evident that additional medications were not needed. In the treatment group, 13 participants received only sodium valproate, 4 patients needed a tranquilizer, 4 needed chlormethiazole, and 1 needed both. In the control group, 1 received only sodium valproate, 4 received a tranquilizer, 14 received chlormethiazole, and 8 needed both. One patient, who entered the study twice, had a withdrawal seizure when in control group and no seizure on second admission in the sodium valproate group. Physical symptoms disappeared quickly in the sodium valproate group (mean of 2 days vs 2.6 days in the control group). Fourteen patients in the control group received chlormethiazole, compared with only 4 patients in sodium valproate group.
Continue to: Conclusion
Conclusion: The researchers concluded that physical symptoms disappeared quicker in the sodium valproate group than in the control group.20
Hillbom et al21 evaluated the efficacy of sodium valproate vs carbamazepine vs placebo to prevent alcohol withdrawal seizures. A total of 138 participants were studied. Forty-three were assigned to the carbamazepine group, 46 to the sodium valproate group, and 49 to the placebo group. The RCT lasted 4 days. The initial medication doses were 1,200 mg/d. Participants in the carbamazepine group experienced more adverse effects than those in the sodium valproate or placebo groups (P < .001). As a result, approximately one-half of the participants in the carbamazepine group stopped taking the medication. This finding was dependent on the dose of carbamazepine; >800 mg/d resulted in poor tolerance to adverse effects. Seizures occurred among patients in all 3 arms of the study; in the sodium valproate group, 1 participant had a seizure vs 2 participants in the carbamazepine group and 3 in the placebo group. On the other hand, DT occurred only in the sodium valproate and placebo groups.
Conclusion: Researchers concluded that when using sodium valproate or carbamazepine to prevent alcohol withdrawal seizures in an outpatient setting, the adverse effects may outweigh the benefits.21
Lamotrigine
The characteristics of the lamotrigine study included in this review are summarized in Table 3.22
Djokić et al22 evaluated the efficiency of lamotrigine in the treatment of DT. A total of 240 participants who met International Classification of Diseases-10 criteria for DT were randomized to a control group that was treated with anticonvulsants according to an NIAAA protocol (2004), or to an experimental group that was treated with lamotrigine. The CIWA-Ar and the Memorial Delirium Assessment Scale (MDAS) were administered for objective assessment of clinical symptoms, superimposed medical complications, general condition of the patient, adverse effects, and mortality rate. Statistically significant differences between the experimental and control groups were apparent after the third day of therapy, when a drop in the average CIWA-Ar score was observed in the experimental group, while the control group still had high scores (P < .01). After the fifth day of treatment, the differences in scores were more apparent, with the experimental group showing CIWA-Ar scores equal to those of persons with mild/moderate DT, while those in the control group still had high scores. After the tenth day, participants in the experimental group did not have any alcohol withdrawal symptoms, while control group participants were just beginning to get out of life-threatening danger. Death occurred in 4.1% of control group participants and 3.4% of experimental group participants; this difference in mortality rate was not statistically significant.
Continue to: Conclusion
Conclusion: Researchers concluded that lamotrigine is significantly efficacious in the treatment of DT, but does not decrease the mortality rate.22
What to know before you prescribe
AWS is a medical emergency that if left untreated leads to several complications and possibly death. Although benzodiazepines are considered the gold standard for treating AWS, the adverse effects associated with their use advocates for finding alternatives. Anticonvulsants can be an effective alternative for treating AWS. In our literature review, we found 16 double-blind RCTs that used an anticonvulsant medication for the treatment of AWS. Of these, 7 involved gabapentin, 6 involved carbamazepine, 1 involved sodium valproate, 1 involved sodium valproate vs carbamazepine, and 1 involved lamotrigine. Overall, the use of anticonvulsants resulted in significant improvement of mild to moderate symptoms of AWS.
There were more studies of carbamazepine and gabapentin than of other anticonvulsants. All the anticonvulsants offered potential benefits. They decreased the probability of a withdrawal seizure and other complications and effectively reduced alcohol cravings. Anticonvulsants were useful for preventing rebound withdrawal symptoms and reducing post-treatment alcohol consumption, especially in patients who had multiple previous withdrawals. Anticonvulsants were particularly helpful for patients with mood disorders such as depression. In the studies we reviewed, anticonvulsants caused less sedation compared with benzodiazepines, and also decreased the occurrence of relapse.
Dosing recommendations. In the studies included in our review, gabapentin was effective at a dosage of 1,600 mg/d (given as 400 mg 4 times a day). This was tapered as follows: 400 mg 4 times a day on Days 1 to 3, 400 mg 3 times a day on Day 4, 400 mg twice a day on Day 5, and 400 mg once a day on Day 6. Carbamazepine was effective at 600 to 800 mg/d, and was tapered by decreasing by 200 mg as follows: 800 mg/d on Days 1 to 3, 600 mg/d on Day 4, 400 mg on Day 5, and 200 mg/d on Day 6. In the reviewed study, the maximum dose of lamotrigine never exceeded 200 mg/d and was administered for 28 days; the exact dosing and taper plan were not described. The dosing of sodium valproate ranged from 1,200 mg/d to 1600 mg/d for 7 days, followed by decreasing by 200 mg each day. The recommended duration of treatment varied; on average for all anticonvulsants, it was 7 to 12 days, followed by a taper. Carbamazepine was shown to be superior to oxazepam in ameliorating the symptoms of AWS.
Adverse effects. When considering the tolerability, adverse effect profile, duration of action, and effectiveness of the anticonvulsants included in our review, gabapentin appears to be the safest agent to choose. For the other anticonvulsants, the risks might outweigh the benefits. Specifically, in a comparison of sodium valproate and carbamazepine, Hillbom et al21 concluded that in doses >800 mg/d, carbamazepine has potential to cause more adverse effects than benefits. However, Agricola et al16 found that carbamazepine had a preferential action on fear, nightmares, and hallucinations.
Continue to: A few caveats
A few caveats
Our review focused a large collection of data from multiple databases and RCTs only. However, its limitations include:
- there was no measure of heterogeneity
- the studies had short treatment duration
- most studies evaluated predominantly male participants
- some studies were underpowered.
Our review laid a groundwork for future research that includes more well-designed RCTs and/or meta-analyses of recent studies that evaluated the use anticonvulsants for treating AWS.
Bottom Line
Evidence suggests certain anticonvulsants may be an effective alternative to benzodiazepines for the treatment of mild to moderate alcohol withdrawal syndrome. Gabapentin may be the safest anticonvulsant to prescribe. Other anticonvulsants to consider include carbamazepine, sodium valproate, and lamotrigine, but for these agents, the risks might outweigh the benefits.
Related Resources
- Myrick H, Anton RF. Treatment of alcohol withdrawal. Alcohol Health Res World. 1998;22(1):38-43. https://pubs.niaaa.nih.gov/publications/arh22-1/38-43.pdf
- World Health Organization. Management of alcohol withdrawal. Published 2012. https://www.who.int/mental_health/mhgap/evidence/alcohol/q2/en/
Drug Brand Names
Carbamazepine • Tegretol
Gabapentin • Neurontin
Lamotrigine • Lamictal
Levetiracetam • Keppra
Lorazepam • Ativan
Oxcarbazepine • Trileptal
Phenytoin • Dilantin
Sodium valproate • Depakote
Acknowledgments
The authors thank Geetha Manikkara, MD, Madhuri Jakkam Setty, MD, and Elizabeth DeOreo, MD, for their efforts with the systematic review research.
1. Trevisan LA, Boutros N, Petrakis IL, et al. Complications of alcohol withdrawal: pathophysiological insights. Alcohol Health Res World. 1998;22(1):61-66.
2. Borghesani P. Alcohol withdrawal. In: Nordstrom KD, Wilson MP, eds. Quick guide to psychiatric emergencies. Springer: 2018;209-215.
3. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757-766.
4. Ungur LA, Neuner B, John S, et al. Prevention and therapy of alcohol withdrawal on intensive care units: systematic review of controlled trials. Alcohol Clin Exp Res. 2013;37(4):675-686.
5. Sachdeva A, Choudhary M, Chandra M. Alcohol withdrawal syndrome: benzodiazepines and beyond. J Clin Diagn Res. 2015;9(9):VE01-VE07.
6. Ashton H. Toxicity and adverse consequences of benzodiazepine use. Psychiatr Ann. 1995;25:158-165.
7. Bonnet U, Banger M, Leweke FM, et al. Treatment of acute alcohol withdrawal with gabapentin: results from a controlled two-center trial. J Clin Psychopharmacol. 2003;23(5):514-519.
8. Bonnet U, Specka M, Leweke FM, et al. Gabapentin’s acute effect on mood profile--a controlled study on patients with alcohol withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):434-438.
9. Myrick H, Anton R, Voronin K, et al. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res. 2007;31(2):221-227.
10. Malcolm R, Myrick L, Veatch L, et al. Self-reported sleep, sleepiness, and repeated alcohol withdrawals: a randomized, double blind, controlled comparison of lorazepam vs gabapentin. J Clin Sleep Med. 2007;3(1):24-32.
11. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
12. Stock CJ, Carpenter L, Ying J, et al. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother. 2013;47(7-8):961-969.
13. Schacht JP, Anton RF, Randall PK, et al. Effects of a GABA-ergic medication combination and initial alcohol withdrawal severity on cue-elicited brain activation among treatment-seeking alcoholics. Psychopharmacol. 2013;227(4):627-637.
14. Björkqvist SE, Isohanni M, Mäkelä R, et al. Ambulant treatment of alcohol withdrawal symptoms with carbamazepine: a formal multicenter double blind comparison with placebo. Acta Psychiatr Scand. 1976;53(5):333-342.
15. Ritola E, Malinen L. A double-blind comparison of carbamazepine and clomethiazole in the treatment of alcohol withdrawal syndrome. Acta Psychiatr Scand. 1981;64(3):254-259.
16. Agricola R, Mazzarino M, Urani R, et al. Treatment of acute alcohol withdrawal syndrome with carbamazepine: a double-blind comparison with tiapride. J Int Med Res. 1982;10(3):160-165.
17. Stuppaeck CH, Pycha R, Miller C, et al. Carbamazepine versus oxazepam in the treatment of alcohol withdrawal: a double-blind study. Alcohol Alcohol. 1992;27(2):153-158.
18. Malcolm R, Myrick H, Roberts J, et al. The effects of carbamazepine and lorazepam on single vs multiple previous withdrawals in an outpatient randomized trial. J Gen Intern Med. 2002;17(5):349-355.
19. Malcolm R, Myrick H, Roberts J, et al. The differential effects of medications on mood, sleep disturbance, and work ability in outpatient alcohol detoxification. Am J Addict. 2002;11(2):141-150.
20. Lambie D, Johnson R, Vijayasenan M, et al. Sodium valproate in the treatment of the alcohol withdrawal syndrome. Aust N Z J. 1980;14(3):213-215.
21. Hillbom M, Tokola R, Kuusela V, et al. Prevention of alcohol withdrawal seizures with carbamazepine and valproic acid. Alcohol. 1989;6(3):223-226.
22. Djokic
1. Trevisan LA, Boutros N, Petrakis IL, et al. Complications of alcohol withdrawal: pathophysiological insights. Alcohol Health Res World. 1998;22(1):61-66.
2. Borghesani P. Alcohol withdrawal. In: Nordstrom KD, Wilson MP, eds. Quick guide to psychiatric emergencies. Springer: 2018;209-215.
3. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757-766.
4. Ungur LA, Neuner B, John S, et al. Prevention and therapy of alcohol withdrawal on intensive care units: systematic review of controlled trials. Alcohol Clin Exp Res. 2013;37(4):675-686.
5. Sachdeva A, Choudhary M, Chandra M. Alcohol withdrawal syndrome: benzodiazepines and beyond. J Clin Diagn Res. 2015;9(9):VE01-VE07.
6. Ashton H. Toxicity and adverse consequences of benzodiazepine use. Psychiatr Ann. 1995;25:158-165.
7. Bonnet U, Banger M, Leweke FM, et al. Treatment of acute alcohol withdrawal with gabapentin: results from a controlled two-center trial. J Clin Psychopharmacol. 2003;23(5):514-519.
8. Bonnet U, Specka M, Leweke FM, et al. Gabapentin’s acute effect on mood profile--a controlled study on patients with alcohol withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):434-438.
9. Myrick H, Anton R, Voronin K, et al. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res. 2007;31(2):221-227.
10. Malcolm R, Myrick L, Veatch L, et al. Self-reported sleep, sleepiness, and repeated alcohol withdrawals: a randomized, double blind, controlled comparison of lorazepam vs gabapentin. J Clin Sleep Med. 2007;3(1):24-32.
11. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
12. Stock CJ, Carpenter L, Ying J, et al. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother. 2013;47(7-8):961-969.
13. Schacht JP, Anton RF, Randall PK, et al. Effects of a GABA-ergic medication combination and initial alcohol withdrawal severity on cue-elicited brain activation among treatment-seeking alcoholics. Psychopharmacol. 2013;227(4):627-637.
14. Björkqvist SE, Isohanni M, Mäkelä R, et al. Ambulant treatment of alcohol withdrawal symptoms with carbamazepine: a formal multicenter double blind comparison with placebo. Acta Psychiatr Scand. 1976;53(5):333-342.
15. Ritola E, Malinen L. A double-blind comparison of carbamazepine and clomethiazole in the treatment of alcohol withdrawal syndrome. Acta Psychiatr Scand. 1981;64(3):254-259.
16. Agricola R, Mazzarino M, Urani R, et al. Treatment of acute alcohol withdrawal syndrome with carbamazepine: a double-blind comparison with tiapride. J Int Med Res. 1982;10(3):160-165.
17. Stuppaeck CH, Pycha R, Miller C, et al. Carbamazepine versus oxazepam in the treatment of alcohol withdrawal: a double-blind study. Alcohol Alcohol. 1992;27(2):153-158.
18. Malcolm R, Myrick H, Roberts J, et al. The effects of carbamazepine and lorazepam on single vs multiple previous withdrawals in an outpatient randomized trial. J Gen Intern Med. 2002;17(5):349-355.
19. Malcolm R, Myrick H, Roberts J, et al. The differential effects of medications on mood, sleep disturbance, and work ability in outpatient alcohol detoxification. Am J Addict. 2002;11(2):141-150.
20. Lambie D, Johnson R, Vijayasenan M, et al. Sodium valproate in the treatment of the alcohol withdrawal syndrome. Aust N Z J. 1980;14(3):213-215.
21. Hillbom M, Tokola R, Kuusela V, et al. Prevention of alcohol withdrawal seizures with carbamazepine and valproic acid. Alcohol. 1989;6(3):223-226.
22. Djokic
Psychiatric consequences of nitrous oxide abuse
We would like to describe the case of a patient we treated who developed neuropsychiatric symptoms secondary to recreational use of nitrous oxide (N2O).
Mr. N, a 24-year-old military veteran, presented to the emergency department (ED) with symptoms of numbness, tingling of his entire body, and difficulty walking for the past 3 days. His family recently became concerned when they noted changes in his personality and behavior, including increased irritability, verbal aggression, and paranoia. The family reported that before the recent changes, Mr. N had typically been calm and had a pleasant temperament. When Mr. N’s symptoms progressed to difficulty ambulating, his family brought him to the ED for evaluation.
During his interview, Mr. N reported that he started using N2O 2 years ago for recreational purposes because he learned it is legal to purchase and undetectable on a urine drug screen. He said he had been using >100 N2O canisters per day and had spent approximately $15,000 over the past few months. His use had increasingly escalated up to 3 days before his visit to the ED, which was the last day he used N2O.
Mr. N was admitted to the inpatient medical service. Laboratory testing revealed a low-normal vitamin B12 level of 254 pg/mL (normal range: 200 to 900 pg/mL), an elevated methylmalonic acid blood level of 2,491 nmol/L (normal range: 73 to 376 nmol/L), and an elevated homocysteine blood level of 22.4 μmol/L (normal range: 0 to 15 μmol/L). Magnetic resonance imaging studies showed hyperintensity regions on his cervical spine from the C1 to C6 levels. These changes suggested demyelination due to vitamin B12 deficiency from N2O abuse.
Mr. N was started on vitamin B12 injections and physical therapy, which led to the resolution of his concerning neurologic symptoms. A few weeks after admission, he was discharged with outpatient follow-up services. Unfortunately, he was lost to follow-up.
Approximately 1 year later, Mr. N returned to the ED with anxiety and paranoid ideation. Medical workup at the time was normal (including vitamin B12 and methylmalonic acid blood levels). He denied any recent substance use and was admitted voluntarily to the psychiatric unit. He declined the recommended treatment of risperidone. Because he showed no signs or symptoms that warranted involuntary retention, he was discharged. Over the next few months, he had 4 visits to the ED with similar concerns and poor adherence to outpatient treatment.
On Mr. N’s fourth admission, he agreed to a course of long-acting injectable paliperidone and escitalopram to target his psychotic and anxious symptoms. These treatments stabilized him, and he was discharged. Neuropsychological testing later showed impairment across several cognitive domains, including memory, processing speed, attention, and executive functioning.
Continue to: Identifying N2O use
Identifying N2O use. N2O is not detected on routine drug screen panels. Obtaining a careful psychiatric and substance use history, as well as conducting a neurologic assessment, are helpful to identify N2O use. Both acute and chronic inhalation of N2O can result in vitamin B12 deficiency with hematologic (megaloblastic anemia), neurologic (subacute combined degeneration of spinal cord, motor-sensory polyneuropathy), and psychiatric sequelae (memory loss, depression, hypomania, transient psychosis).1 Patients who exhibit these changes warrant workup for vitamin B12 deficiency, which includes testing for B12, homocysteine, and methylmalonic acid blood levels. Magnetic resonance imaging should be considered for patients who exhibit neurologic symptoms.
The means by which N2O causes neuropsychiatric changes have been explored in the literature. There is general consensus that part of N2O’s deleterious effects is due to the inactivation of vitamin B12 by the irreversible oxidation of Cob(I)alamin to Cob(III)alamin.1
Treatment. The recommended treatment is high-dose oral or parenteral vitamin B12.1 Repletion of vitamin B12 is believed to reverse the course of illness. However, our patient’s symptoms of paranoia and delusions persisted despite resolution of his neurologic symptoms after the underlying vitamin B12 deficiency was corrected.
Due to N2O’s wide availability and growing recreational use, it is important for clinicians to ask their patients about their use of this substance. The abuse of N2O remains an important topic that requires further research, particularly in adolescents, who are still undergoing significant brain development.2,3
Daniel Roberts, MD, MSW
PGY-3 Psychiatric Resident
Department of Psychiatry
New York University Grossman School of Medicine
New York, New York
Pantea Farahmand, MA, MD
Assistant Professor
Department of Psychiatry
New York University Grossman School of Medicine
Inpatient Psychiatrist
Veterans Administration New York Harbor Healthcare System
New York, New York
Adam Wolkin, MD
Associate Professor and Vice Chair
New York University Grossman School of Medicine
Associate Chief of Staff for Mental Health
Veterans Administration New York Harbor Healthcare System
New York, New York
Disclosures: The authors report no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
1. Thompson AG, Leite MI, Lunn MP, et al. Whippits, nitrous oxide and the dangers of legal highs. Pract Neurol. 2015;15(3):207-209.
2. Global Drug Survey 2017. Global Drug Survey. Published May 24, 2017. Accessed January 12, 2021. https://www.globaldrugsurvey.com/past-findings/gds2017-launch/results-released/
3. Kaar SJ, Ferris J, Waldron J, et al. Up: the rise of nitrous oxide abuse. An international survey of contemporary nitrous oxide use. J Psychopharmacol. 2016;30(4):395-401.
We would like to describe the case of a patient we treated who developed neuropsychiatric symptoms secondary to recreational use of nitrous oxide (N2O).
Mr. N, a 24-year-old military veteran, presented to the emergency department (ED) with symptoms of numbness, tingling of his entire body, and difficulty walking for the past 3 days. His family recently became concerned when they noted changes in his personality and behavior, including increased irritability, verbal aggression, and paranoia. The family reported that before the recent changes, Mr. N had typically been calm and had a pleasant temperament. When Mr. N’s symptoms progressed to difficulty ambulating, his family brought him to the ED for evaluation.
During his interview, Mr. N reported that he started using N2O 2 years ago for recreational purposes because he learned it is legal to purchase and undetectable on a urine drug screen. He said he had been using >100 N2O canisters per day and had spent approximately $15,000 over the past few months. His use had increasingly escalated up to 3 days before his visit to the ED, which was the last day he used N2O.
Mr. N was admitted to the inpatient medical service. Laboratory testing revealed a low-normal vitamin B12 level of 254 pg/mL (normal range: 200 to 900 pg/mL), an elevated methylmalonic acid blood level of 2,491 nmol/L (normal range: 73 to 376 nmol/L), and an elevated homocysteine blood level of 22.4 μmol/L (normal range: 0 to 15 μmol/L). Magnetic resonance imaging studies showed hyperintensity regions on his cervical spine from the C1 to C6 levels. These changes suggested demyelination due to vitamin B12 deficiency from N2O abuse.
Mr. N was started on vitamin B12 injections and physical therapy, which led to the resolution of his concerning neurologic symptoms. A few weeks after admission, he was discharged with outpatient follow-up services. Unfortunately, he was lost to follow-up.
Approximately 1 year later, Mr. N returned to the ED with anxiety and paranoid ideation. Medical workup at the time was normal (including vitamin B12 and methylmalonic acid blood levels). He denied any recent substance use and was admitted voluntarily to the psychiatric unit. He declined the recommended treatment of risperidone. Because he showed no signs or symptoms that warranted involuntary retention, he was discharged. Over the next few months, he had 4 visits to the ED with similar concerns and poor adherence to outpatient treatment.
On Mr. N’s fourth admission, he agreed to a course of long-acting injectable paliperidone and escitalopram to target his psychotic and anxious symptoms. These treatments stabilized him, and he was discharged. Neuropsychological testing later showed impairment across several cognitive domains, including memory, processing speed, attention, and executive functioning.
Continue to: Identifying N2O use
Identifying N2O use. N2O is not detected on routine drug screen panels. Obtaining a careful psychiatric and substance use history, as well as conducting a neurologic assessment, are helpful to identify N2O use. Both acute and chronic inhalation of N2O can result in vitamin B12 deficiency with hematologic (megaloblastic anemia), neurologic (subacute combined degeneration of spinal cord, motor-sensory polyneuropathy), and psychiatric sequelae (memory loss, depression, hypomania, transient psychosis).1 Patients who exhibit these changes warrant workup for vitamin B12 deficiency, which includes testing for B12, homocysteine, and methylmalonic acid blood levels. Magnetic resonance imaging should be considered for patients who exhibit neurologic symptoms.
The means by which N2O causes neuropsychiatric changes have been explored in the literature. There is general consensus that part of N2O’s deleterious effects is due to the inactivation of vitamin B12 by the irreversible oxidation of Cob(I)alamin to Cob(III)alamin.1
Treatment. The recommended treatment is high-dose oral or parenteral vitamin B12.1 Repletion of vitamin B12 is believed to reverse the course of illness. However, our patient’s symptoms of paranoia and delusions persisted despite resolution of his neurologic symptoms after the underlying vitamin B12 deficiency was corrected.
Due to N2O’s wide availability and growing recreational use, it is important for clinicians to ask their patients about their use of this substance. The abuse of N2O remains an important topic that requires further research, particularly in adolescents, who are still undergoing significant brain development.2,3
Daniel Roberts, MD, MSW
PGY-3 Psychiatric Resident
Department of Psychiatry
New York University Grossman School of Medicine
New York, New York
Pantea Farahmand, MA, MD
Assistant Professor
Department of Psychiatry
New York University Grossman School of Medicine
Inpatient Psychiatrist
Veterans Administration New York Harbor Healthcare System
New York, New York
Adam Wolkin, MD
Associate Professor and Vice Chair
New York University Grossman School of Medicine
Associate Chief of Staff for Mental Health
Veterans Administration New York Harbor Healthcare System
New York, New York
Disclosures: The authors report no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
We would like to describe the case of a patient we treated who developed neuropsychiatric symptoms secondary to recreational use of nitrous oxide (N2O).
Mr. N, a 24-year-old military veteran, presented to the emergency department (ED) with symptoms of numbness, tingling of his entire body, and difficulty walking for the past 3 days. His family recently became concerned when they noted changes in his personality and behavior, including increased irritability, verbal aggression, and paranoia. The family reported that before the recent changes, Mr. N had typically been calm and had a pleasant temperament. When Mr. N’s symptoms progressed to difficulty ambulating, his family brought him to the ED for evaluation.
During his interview, Mr. N reported that he started using N2O 2 years ago for recreational purposes because he learned it is legal to purchase and undetectable on a urine drug screen. He said he had been using >100 N2O canisters per day and had spent approximately $15,000 over the past few months. His use had increasingly escalated up to 3 days before his visit to the ED, which was the last day he used N2O.
Mr. N was admitted to the inpatient medical service. Laboratory testing revealed a low-normal vitamin B12 level of 254 pg/mL (normal range: 200 to 900 pg/mL), an elevated methylmalonic acid blood level of 2,491 nmol/L (normal range: 73 to 376 nmol/L), and an elevated homocysteine blood level of 22.4 μmol/L (normal range: 0 to 15 μmol/L). Magnetic resonance imaging studies showed hyperintensity regions on his cervical spine from the C1 to C6 levels. These changes suggested demyelination due to vitamin B12 deficiency from N2O abuse.
Mr. N was started on vitamin B12 injections and physical therapy, which led to the resolution of his concerning neurologic symptoms. A few weeks after admission, he was discharged with outpatient follow-up services. Unfortunately, he was lost to follow-up.
Approximately 1 year later, Mr. N returned to the ED with anxiety and paranoid ideation. Medical workup at the time was normal (including vitamin B12 and methylmalonic acid blood levels). He denied any recent substance use and was admitted voluntarily to the psychiatric unit. He declined the recommended treatment of risperidone. Because he showed no signs or symptoms that warranted involuntary retention, he was discharged. Over the next few months, he had 4 visits to the ED with similar concerns and poor adherence to outpatient treatment.
On Mr. N’s fourth admission, he agreed to a course of long-acting injectable paliperidone and escitalopram to target his psychotic and anxious symptoms. These treatments stabilized him, and he was discharged. Neuropsychological testing later showed impairment across several cognitive domains, including memory, processing speed, attention, and executive functioning.
Continue to: Identifying N2O use
Identifying N2O use. N2O is not detected on routine drug screen panels. Obtaining a careful psychiatric and substance use history, as well as conducting a neurologic assessment, are helpful to identify N2O use. Both acute and chronic inhalation of N2O can result in vitamin B12 deficiency with hematologic (megaloblastic anemia), neurologic (subacute combined degeneration of spinal cord, motor-sensory polyneuropathy), and psychiatric sequelae (memory loss, depression, hypomania, transient psychosis).1 Patients who exhibit these changes warrant workup for vitamin B12 deficiency, which includes testing for B12, homocysteine, and methylmalonic acid blood levels. Magnetic resonance imaging should be considered for patients who exhibit neurologic symptoms.
The means by which N2O causes neuropsychiatric changes have been explored in the literature. There is general consensus that part of N2O’s deleterious effects is due to the inactivation of vitamin B12 by the irreversible oxidation of Cob(I)alamin to Cob(III)alamin.1
Treatment. The recommended treatment is high-dose oral or parenteral vitamin B12.1 Repletion of vitamin B12 is believed to reverse the course of illness. However, our patient’s symptoms of paranoia and delusions persisted despite resolution of his neurologic symptoms after the underlying vitamin B12 deficiency was corrected.
Due to N2O’s wide availability and growing recreational use, it is important for clinicians to ask their patients about their use of this substance. The abuse of N2O remains an important topic that requires further research, particularly in adolescents, who are still undergoing significant brain development.2,3
Daniel Roberts, MD, MSW
PGY-3 Psychiatric Resident
Department of Psychiatry
New York University Grossman School of Medicine
New York, New York
Pantea Farahmand, MA, MD
Assistant Professor
Department of Psychiatry
New York University Grossman School of Medicine
Inpatient Psychiatrist
Veterans Administration New York Harbor Healthcare System
New York, New York
Adam Wolkin, MD
Associate Professor and Vice Chair
New York University Grossman School of Medicine
Associate Chief of Staff for Mental Health
Veterans Administration New York Harbor Healthcare System
New York, New York
Disclosures: The authors report no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
1. Thompson AG, Leite MI, Lunn MP, et al. Whippits, nitrous oxide and the dangers of legal highs. Pract Neurol. 2015;15(3):207-209.
2. Global Drug Survey 2017. Global Drug Survey. Published May 24, 2017. Accessed January 12, 2021. https://www.globaldrugsurvey.com/past-findings/gds2017-launch/results-released/
3. Kaar SJ, Ferris J, Waldron J, et al. Up: the rise of nitrous oxide abuse. An international survey of contemporary nitrous oxide use. J Psychopharmacol. 2016;30(4):395-401.
1. Thompson AG, Leite MI, Lunn MP, et al. Whippits, nitrous oxide and the dangers of legal highs. Pract Neurol. 2015;15(3):207-209.
2. Global Drug Survey 2017. Global Drug Survey. Published May 24, 2017. Accessed January 12, 2021. https://www.globaldrugsurvey.com/past-findings/gds2017-launch/results-released/
3. Kaar SJ, Ferris J, Waldron J, et al. Up: the rise of nitrous oxide abuse. An international survey of contemporary nitrous oxide use. J Psychopharmacol. 2016;30(4):395-401.
Naltrexone cuts hospitalization, deaths in alcohol use disorder
Naltrexone reduces the risk for hospitalization for alcohol use disorder (AUD), regardless of whether it is used alone or in conjunction with disulfiram or acamprosate, research suggests.
Investigators analyzed 10-year data on more than 125,000 Swedish residents with AUD and found that naltrexone, used as monotherapy or combined with acamprosate or disulfiram, was associated with significantly lower risk for AUD hospitalization or all-cause hospitalization in comparison with patients who did not use AUD medication. The patients ranged in age from 16 to 64 years.
By contrast, benzodiazepines and acamprosate monotherapy were associated with increased risk for AUD hospitalization.
“The take-home message for practicing clinicians would be that especially naltrexone use is associated with favorable treatment outcomes and should be utilized as part of the treatment protocol for AUD,” study investigator Milja Heikkinen, MD, specialist in forensic psychiatry and addiction medicine, University of Eastern Finland, Kuopio, told this news organization.
On the other hand, “benzodiazepines should be avoided and should not be administered other than for alcohol withdrawal symptoms,” she said.
The study was published online Jan. 4 in Addiction.
Real-world data
Previous research has shown that disulfiram, acamprosate, naltrexone, and nalmefene are efficacious in treating AUD, but most studies have been randomized controlled trials or meta-analyses, the authors write.
“Very little is known about overall health outcomes (such as risks of hospitalization and mortality) associated with specific treatments in real-world circumstances,” they write.
“The study was motivated by the fact that, although AUD is a significant public health concern, very little is known, especially about the comparative effectiveness of medications indicated in AUD,” said Dr. Heikkinen.
who had been diagnosed with AUD (62.5% men; mean [standard deviation] age, 38.1 [15.9] years). They followed the cohort over a median of 4.6 years (interquartile range, 2.1-.2 years).
During the follow-up period, roughly one-fourth of patients (25.6) underwent treatment with one or more drugs.

The main outcome measure was AUD-related hospitalization. Secondary outcomes were hospitalization for any cause and for alcohol-related somatic causes; all-cause mortality; and work disability.
Two types of analyses were conducted. The within-individual analyses, designed to eliminate selection bias, compared the use of a medication to periods during which the same individual was not using the medication.
Between-individual analyses (adjusted for sex, age, educational level, number of previous AUD-related hospitalizations, time since first AUD diagnosis, comorbidities, and use of other medications) utilized a “traditional” multivariate-adjusted Cox hazards regression model.
AUD pharmacotherapy ‘underutilized’
Close to one-fourth of patients (23.9%) experienced the main outcome event (AUD-related hospitalization) during the follow-up period.
The within-individual analysis showed that naltrexone – whether used as monotherapy or adjunctively with disulfiram or acamprosate – “was associated with a significantly lower risk of AUD-related hospitalization, compared to those time periods in which the same individual did not use any AUD medication,” the authors report.
By contrast, they state, acamprosate monotherapy and benzodiazepines were associated with a significantly higher risk for AUD-related hospitalization.
Similar results were obtained in the between-individual analysis. Longer duration of naltrexone use was associated with lower risk for AUD-related hospitalization.
The pattern was also found when the outcome was hospitalization for any cause. However, unlike the findings of the within-individual model, the second model found that acamprosate monotherapy was not associated with a higher risk for any-cause hospitalization.
Polytherapy, including combinations of the four AUD medications, as well as disulfiram monotherapy were similarly associated with lower risk for hospitalization for alcohol-related somatic causes.
Of the overall cohort, 6.2% died during the follow-up period. No association was found between disulfiram, acamprosate, nalmefene, and naltrexone use and all-cause mortality. By contrast, benzodiazepine use was associated with a significantly higher mortality rate (hazard ratio, 1.11; 95% confidence interval, 1.04-1.19).
“AUD drugs are underutilized, despite AUD being a significant public health concern,” Dr. Heikkinen noted. On the other hand, benzodiazepine use is “very common.”
‘Ravages’ of benzodiazepines
Commenting on the study in an interview, John Krystal, MD, professor and chair of psychiatry and director of the Center for the Translational Neuroscience of Alcoholism, Yale University, New Haven, Conn., said, “The main message from the study for practicing clinicians is that treatment works.”
Dr. Krystal, who was not involved with the study, noted that “many practicing clinicians are discouraged by the course of their patients with AUD, and this study highlights that naltrexone, perhaps in combination with other medications, may be effective in preventing hospitalization and, presumably, other hospitalization-related complications of AUD.”
Also commenting on the study, Raymond Anton, MD, professor, department of psychiatry and behavioral sciences, Medical University of South Carolina, Charleston, suggested that the “clinical knowledge of the harm of benzodiazepines in those with AUD is reinforced by these findings.”
In fact, the harm of benzodiazepines might be the study’s “most important message ... [a message that was] recently highlighted by the Netflix series “The Queen’s Gambit”, which shows the ravages of using both together, or how one leads to potential addiction with the other,” said Dr. Anton, who was not involved with the study.
The other “big take-home message is that naltrexone should be used more frequently,” said Dr. Anton, distinguished professor of psychiatry at the university and scientific director of the Charleston Alcohol Research Center. He noted that there are “recent data suggesting some clinical and genetic indicators that predict responsiveness to these medications, improving efficacy.”
The study was funded by the Finnish Ministry of Social Affairs and Health. Dr. Heikkinen reports no relevant financial relationships. The other authors’ disclosures are listed on the original article. Dr. Krystal consults for companies currently developing other treatments for AUDs and receives medications to test from AstraZeneca and Novartis for NIAAA-funded research programs. Dr. Anton has consulted for Alkermes, Lipha, and Lundbeck in the past. He is also chair of the Alcohol Clinical Trials Initiative, which is a public-private partnership partially sponsored by several companies and has received grant funding from the National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism to study pharmacotherapies, including naltrexone, nalmefene, and acamprosate.
A version of this article first appeared on Medscape.com.
Naltrexone reduces the risk for hospitalization for alcohol use disorder (AUD), regardless of whether it is used alone or in conjunction with disulfiram or acamprosate, research suggests.
Investigators analyzed 10-year data on more than 125,000 Swedish residents with AUD and found that naltrexone, used as monotherapy or combined with acamprosate or disulfiram, was associated with significantly lower risk for AUD hospitalization or all-cause hospitalization in comparison with patients who did not use AUD medication. The patients ranged in age from 16 to 64 years.
By contrast, benzodiazepines and acamprosate monotherapy were associated with increased risk for AUD hospitalization.
“The take-home message for practicing clinicians would be that especially naltrexone use is associated with favorable treatment outcomes and should be utilized as part of the treatment protocol for AUD,” study investigator Milja Heikkinen, MD, specialist in forensic psychiatry and addiction medicine, University of Eastern Finland, Kuopio, told this news organization.
On the other hand, “benzodiazepines should be avoided and should not be administered other than for alcohol withdrawal symptoms,” she said.
The study was published online Jan. 4 in Addiction.
Real-world data
Previous research has shown that disulfiram, acamprosate, naltrexone, and nalmefene are efficacious in treating AUD, but most studies have been randomized controlled trials or meta-analyses, the authors write.
“Very little is known about overall health outcomes (such as risks of hospitalization and mortality) associated with specific treatments in real-world circumstances,” they write.
“The study was motivated by the fact that, although AUD is a significant public health concern, very little is known, especially about the comparative effectiveness of medications indicated in AUD,” said Dr. Heikkinen.
who had been diagnosed with AUD (62.5% men; mean [standard deviation] age, 38.1 [15.9] years). They followed the cohort over a median of 4.6 years (interquartile range, 2.1-.2 years).
During the follow-up period, roughly one-fourth of patients (25.6) underwent treatment with one or more drugs.

The main outcome measure was AUD-related hospitalization. Secondary outcomes were hospitalization for any cause and for alcohol-related somatic causes; all-cause mortality; and work disability.
Two types of analyses were conducted. The within-individual analyses, designed to eliminate selection bias, compared the use of a medication to periods during which the same individual was not using the medication.
Between-individual analyses (adjusted for sex, age, educational level, number of previous AUD-related hospitalizations, time since first AUD diagnosis, comorbidities, and use of other medications) utilized a “traditional” multivariate-adjusted Cox hazards regression model.
AUD pharmacotherapy ‘underutilized’
Close to one-fourth of patients (23.9%) experienced the main outcome event (AUD-related hospitalization) during the follow-up period.
The within-individual analysis showed that naltrexone – whether used as monotherapy or adjunctively with disulfiram or acamprosate – “was associated with a significantly lower risk of AUD-related hospitalization, compared to those time periods in which the same individual did not use any AUD medication,” the authors report.
By contrast, they state, acamprosate monotherapy and benzodiazepines were associated with a significantly higher risk for AUD-related hospitalization.
Similar results were obtained in the between-individual analysis. Longer duration of naltrexone use was associated with lower risk for AUD-related hospitalization.
The pattern was also found when the outcome was hospitalization for any cause. However, unlike the findings of the within-individual model, the second model found that acamprosate monotherapy was not associated with a higher risk for any-cause hospitalization.
Polytherapy, including combinations of the four AUD medications, as well as disulfiram monotherapy were similarly associated with lower risk for hospitalization for alcohol-related somatic causes.
Of the overall cohort, 6.2% died during the follow-up period. No association was found between disulfiram, acamprosate, nalmefene, and naltrexone use and all-cause mortality. By contrast, benzodiazepine use was associated with a significantly higher mortality rate (hazard ratio, 1.11; 95% confidence interval, 1.04-1.19).
“AUD drugs are underutilized, despite AUD being a significant public health concern,” Dr. Heikkinen noted. On the other hand, benzodiazepine use is “very common.”
‘Ravages’ of benzodiazepines
Commenting on the study in an interview, John Krystal, MD, professor and chair of psychiatry and director of the Center for the Translational Neuroscience of Alcoholism, Yale University, New Haven, Conn., said, “The main message from the study for practicing clinicians is that treatment works.”
Dr. Krystal, who was not involved with the study, noted that “many practicing clinicians are discouraged by the course of their patients with AUD, and this study highlights that naltrexone, perhaps in combination with other medications, may be effective in preventing hospitalization and, presumably, other hospitalization-related complications of AUD.”
Also commenting on the study, Raymond Anton, MD, professor, department of psychiatry and behavioral sciences, Medical University of South Carolina, Charleston, suggested that the “clinical knowledge of the harm of benzodiazepines in those with AUD is reinforced by these findings.”
In fact, the harm of benzodiazepines might be the study’s “most important message ... [a message that was] recently highlighted by the Netflix series “The Queen’s Gambit”, which shows the ravages of using both together, or how one leads to potential addiction with the other,” said Dr. Anton, who was not involved with the study.
The other “big take-home message is that naltrexone should be used more frequently,” said Dr. Anton, distinguished professor of psychiatry at the university and scientific director of the Charleston Alcohol Research Center. He noted that there are “recent data suggesting some clinical and genetic indicators that predict responsiveness to these medications, improving efficacy.”
The study was funded by the Finnish Ministry of Social Affairs and Health. Dr. Heikkinen reports no relevant financial relationships. The other authors’ disclosures are listed on the original article. Dr. Krystal consults for companies currently developing other treatments for AUDs and receives medications to test from AstraZeneca and Novartis for NIAAA-funded research programs. Dr. Anton has consulted for Alkermes, Lipha, and Lundbeck in the past. He is also chair of the Alcohol Clinical Trials Initiative, which is a public-private partnership partially sponsored by several companies and has received grant funding from the National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism to study pharmacotherapies, including naltrexone, nalmefene, and acamprosate.
A version of this article first appeared on Medscape.com.
Naltrexone reduces the risk for hospitalization for alcohol use disorder (AUD), regardless of whether it is used alone or in conjunction with disulfiram or acamprosate, research suggests.
Investigators analyzed 10-year data on more than 125,000 Swedish residents with AUD and found that naltrexone, used as monotherapy or combined with acamprosate or disulfiram, was associated with significantly lower risk for AUD hospitalization or all-cause hospitalization in comparison with patients who did not use AUD medication. The patients ranged in age from 16 to 64 years.
By contrast, benzodiazepines and acamprosate monotherapy were associated with increased risk for AUD hospitalization.
“The take-home message for practicing clinicians would be that especially naltrexone use is associated with favorable treatment outcomes and should be utilized as part of the treatment protocol for AUD,” study investigator Milja Heikkinen, MD, specialist in forensic psychiatry and addiction medicine, University of Eastern Finland, Kuopio, told this news organization.
On the other hand, “benzodiazepines should be avoided and should not be administered other than for alcohol withdrawal symptoms,” she said.
The study was published online Jan. 4 in Addiction.
Real-world data
Previous research has shown that disulfiram, acamprosate, naltrexone, and nalmefene are efficacious in treating AUD, but most studies have been randomized controlled trials or meta-analyses, the authors write.
“Very little is known about overall health outcomes (such as risks of hospitalization and mortality) associated with specific treatments in real-world circumstances,” they write.
“The study was motivated by the fact that, although AUD is a significant public health concern, very little is known, especially about the comparative effectiveness of medications indicated in AUD,” said Dr. Heikkinen.
who had been diagnosed with AUD (62.5% men; mean [standard deviation] age, 38.1 [15.9] years). They followed the cohort over a median of 4.6 years (interquartile range, 2.1-.2 years).
During the follow-up period, roughly one-fourth of patients (25.6) underwent treatment with one or more drugs.

The main outcome measure was AUD-related hospitalization. Secondary outcomes were hospitalization for any cause and for alcohol-related somatic causes; all-cause mortality; and work disability.
Two types of analyses were conducted. The within-individual analyses, designed to eliminate selection bias, compared the use of a medication to periods during which the same individual was not using the medication.
Between-individual analyses (adjusted for sex, age, educational level, number of previous AUD-related hospitalizations, time since first AUD diagnosis, comorbidities, and use of other medications) utilized a “traditional” multivariate-adjusted Cox hazards regression model.
AUD pharmacotherapy ‘underutilized’
Close to one-fourth of patients (23.9%) experienced the main outcome event (AUD-related hospitalization) during the follow-up period.
The within-individual analysis showed that naltrexone – whether used as monotherapy or adjunctively with disulfiram or acamprosate – “was associated with a significantly lower risk of AUD-related hospitalization, compared to those time periods in which the same individual did not use any AUD medication,” the authors report.
By contrast, they state, acamprosate monotherapy and benzodiazepines were associated with a significantly higher risk for AUD-related hospitalization.
Similar results were obtained in the between-individual analysis. Longer duration of naltrexone use was associated with lower risk for AUD-related hospitalization.
The pattern was also found when the outcome was hospitalization for any cause. However, unlike the findings of the within-individual model, the second model found that acamprosate monotherapy was not associated with a higher risk for any-cause hospitalization.
Polytherapy, including combinations of the four AUD medications, as well as disulfiram monotherapy were similarly associated with lower risk for hospitalization for alcohol-related somatic causes.
Of the overall cohort, 6.2% died during the follow-up period. No association was found between disulfiram, acamprosate, nalmefene, and naltrexone use and all-cause mortality. By contrast, benzodiazepine use was associated with a significantly higher mortality rate (hazard ratio, 1.11; 95% confidence interval, 1.04-1.19).
“AUD drugs are underutilized, despite AUD being a significant public health concern,” Dr. Heikkinen noted. On the other hand, benzodiazepine use is “very common.”
‘Ravages’ of benzodiazepines
Commenting on the study in an interview, John Krystal, MD, professor and chair of psychiatry and director of the Center for the Translational Neuroscience of Alcoholism, Yale University, New Haven, Conn., said, “The main message from the study for practicing clinicians is that treatment works.”
Dr. Krystal, who was not involved with the study, noted that “many practicing clinicians are discouraged by the course of their patients with AUD, and this study highlights that naltrexone, perhaps in combination with other medications, may be effective in preventing hospitalization and, presumably, other hospitalization-related complications of AUD.”
Also commenting on the study, Raymond Anton, MD, professor, department of psychiatry and behavioral sciences, Medical University of South Carolina, Charleston, suggested that the “clinical knowledge of the harm of benzodiazepines in those with AUD is reinforced by these findings.”
In fact, the harm of benzodiazepines might be the study’s “most important message ... [a message that was] recently highlighted by the Netflix series “The Queen’s Gambit”, which shows the ravages of using both together, or how one leads to potential addiction with the other,” said Dr. Anton, who was not involved with the study.
The other “big take-home message is that naltrexone should be used more frequently,” said Dr. Anton, distinguished professor of psychiatry at the university and scientific director of the Charleston Alcohol Research Center. He noted that there are “recent data suggesting some clinical and genetic indicators that predict responsiveness to these medications, improving efficacy.”
The study was funded by the Finnish Ministry of Social Affairs and Health. Dr. Heikkinen reports no relevant financial relationships. The other authors’ disclosures are listed on the original article. Dr. Krystal consults for companies currently developing other treatments for AUDs and receives medications to test from AstraZeneca and Novartis for NIAAA-funded research programs. Dr. Anton has consulted for Alkermes, Lipha, and Lundbeck in the past. He is also chair of the Alcohol Clinical Trials Initiative, which is a public-private partnership partially sponsored by several companies and has received grant funding from the National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism to study pharmacotherapies, including naltrexone, nalmefene, and acamprosate.
A version of this article first appeared on Medscape.com.