User login
Parents driving the ‘talk’ supports healthy sexual behaviors in GBQ teens and young adults
When it comes to sexual health education in the United States, one thing is abundantly clear: It’s a messy patchwork of programs, topics, and criteria. Only 29 states and the District of Columbia currently mandate sexual health education. Sixteen states have an abstinence-only curriculum, whereas 13 do not require that instruction be age-appropriate, inclusive, medically accurate, or evidence-based/informed. And this is just the tip of the iceberg, according to a 2022 report issued by the Sex Ed for Social Change organization.
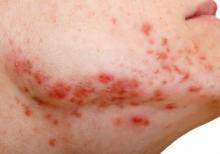
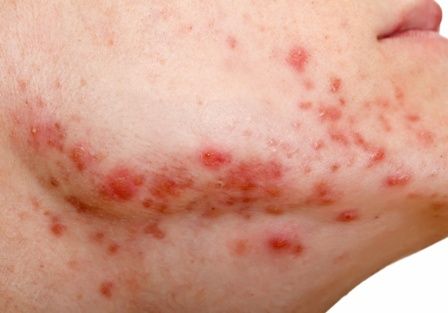
Parents should take an inclusive approach to sex communication and create a safe space for discussing sex and sexual orientation, said almost all (96.7%) of male young adults who participated in a qualitative study. This would help reinforce acceptance and parents could possibly serve as a proxy for children who’ve not yet disclosed their sexual orientation. Yet, few parents are equipped or prepared to have these meaningful conversations with gay, bisexual, queer, or gender-diverse children, despite the fact that they are especially vulnerable to poor sexual health outcomes, bullying, abuse, and mental health challenges, as well as high-risk sexual behaviors.
“Parents are sexual socialization agents,” Dalmacio Dennis Flores, PhD, ACRN, assistant professor of nursing at the University of Pennsylvania, Philadelphia, told this news organization. “It’s through the information that they convey, the way that they normalize rituals and expectations, that they inform young people of all of societal expectations or roles they’ll be fulfilling in the future.”
Dr. Flores is lead author of a study published in the Journal of Adolescent Health. He and his colleagues collected perspectives on comprehensive, inclusive, and age-appropriate parent-child sex communications from 30 GBQ adolescent males aged 15-20 years who were already “out” to their parents. Participants were asked to sort through 28 preprinted note cards containing broad sexual health topics (for instance, human anatomy, dating, sexually transmitted infections) as well as topics theoretically specific to GBQ individuals (for example, anal sex), and were asked to add additional topics that they felt were missing. They were then directed to recommend topics along with ideal timing (that is, elementary, middle, or high school) for these conversations.
Study findings also underscored the importance of initiating comprehensive sexuality talks as early as elementary school age – namely to start preparing GBQ children for inevitable adversities that they were likely to encounter later in life, as well as to form building blocks for more mature, in-depth discussions during high school.
Importantly, these recommendations generally align with those aimed at heterosexual youth.
“When we refer to topics for elementary school, they are general parameters of what kids might be interested in or want to hear more about; it’s not planting a seed,” explained Dr. Flores.
Eva Goldfarb, PhD, LHD, MA, professor of public health at Montclair (N.J.) State University, agreed. “We always talk about (in sex education) to follow young people’s lead. If your child is asking you a question, they deserve a response,” said Dr. Goldfarb, who wasn’t involved in the study. “It doesn’t mean you have to give a detailed- level explanation but if they’re asking about it, it means that they are thinking about it. But it’s really important for all young people to know all of this information.”
Along those lines, participants deemed that fundamental issues about bodies (for example, human anatomy, reproduction), different sexual orientations, and an introduction to foundational issues (like privacy, peer or social pressure, sexual abuse) would help elementary-aged children to normalize discussions about sex, anatomy, and sexual orientation.
Middle school conversations were ideally more in-depth to reflect the time when young people are beginning to explore and accept their social and sexual identities. Topics of discussion might include types of sexual intercourse (anal, oral, and vaginal), health promotion strategies (abstinence, condoms, and contraception), possible adverse outcomes of condomless intercourse (HIV, STIs), considerations about engaging in sexual intercourse (including readiness, negotiating boundaries, virginity), and interpersonal safety (for instance, sexting, alcohol/drugs/chemsex, sexual coercion, and partner abuse/violence).
Finally, high school age recommendations focused on socio-relational topics (such as hook-up culture, technology/online dating, and multiple or concurrent sex partners), which are most relevant during a time when adolescents are most prone to experimentation and risk-taking.
Acknowledging that the study approach was novel, Dr. Flores noted that hearing about these topics from the youth perspective allowed parents to prepare. “Communication is better when it’s anticipated vs. reactive,” he said.
Last but not least, clinicians also have an important role in supporting these conversations.
“We’ve always looked at sex communication as a dyadic process, as a parent bestowing wisdom on a child who doesn’t have that knowledge yet. But it can be a triadic model,” said Dr. Flores. “Providers can encourage parents to ask if a child is dating or is familiar with ways to protect themselves or provide consent, and act as a resource exclusively to troubleshoot emergent issues.”
This study was funded by the National Institutes of Health. The study also received supplementary funding from the Surgeon General C. Everett Koop HIV/AIDS Research Award. Dr. Flores and Dr. Goldfarb report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
When it comes to sexual health education in the United States, one thing is abundantly clear: It’s a messy patchwork of programs, topics, and criteria. Only 29 states and the District of Columbia currently mandate sexual health education. Sixteen states have an abstinence-only curriculum, whereas 13 do not require that instruction be age-appropriate, inclusive, medically accurate, or evidence-based/informed. And this is just the tip of the iceberg, according to a 2022 report issued by the Sex Ed for Social Change organization.
Parents should take an inclusive approach to sex communication and create a safe space for discussing sex and sexual orientation, said almost all (96.7%) of male young adults who participated in a qualitative study. This would help reinforce acceptance and parents could possibly serve as a proxy for children who’ve not yet disclosed their sexual orientation. Yet, few parents are equipped or prepared to have these meaningful conversations with gay, bisexual, queer, or gender-diverse children, despite the fact that they are especially vulnerable to poor sexual health outcomes, bullying, abuse, and mental health challenges, as well as high-risk sexual behaviors.
“Parents are sexual socialization agents,” Dalmacio Dennis Flores, PhD, ACRN, assistant professor of nursing at the University of Pennsylvania, Philadelphia, told this news organization. “It’s through the information that they convey, the way that they normalize rituals and expectations, that they inform young people of all of societal expectations or roles they’ll be fulfilling in the future.”
Dr. Flores is lead author of a study published in the Journal of Adolescent Health. He and his colleagues collected perspectives on comprehensive, inclusive, and age-appropriate parent-child sex communications from 30 GBQ adolescent males aged 15-20 years who were already “out” to their parents. Participants were asked to sort through 28 preprinted note cards containing broad sexual health topics (for instance, human anatomy, dating, sexually transmitted infections) as well as topics theoretically specific to GBQ individuals (for example, anal sex), and were asked to add additional topics that they felt were missing. They were then directed to recommend topics along with ideal timing (that is, elementary, middle, or high school) for these conversations.
Study findings also underscored the importance of initiating comprehensive sexuality talks as early as elementary school age – namely to start preparing GBQ children for inevitable adversities that they were likely to encounter later in life, as well as to form building blocks for more mature, in-depth discussions during high school.
Importantly, these recommendations generally align with those aimed at heterosexual youth.
“When we refer to topics for elementary school, they are general parameters of what kids might be interested in or want to hear more about; it’s not planting a seed,” explained Dr. Flores.
Eva Goldfarb, PhD, LHD, MA, professor of public health at Montclair (N.J.) State University, agreed. “We always talk about (in sex education) to follow young people’s lead. If your child is asking you a question, they deserve a response,” said Dr. Goldfarb, who wasn’t involved in the study. “It doesn’t mean you have to give a detailed- level explanation but if they’re asking about it, it means that they are thinking about it. But it’s really important for all young people to know all of this information.”
Along those lines, participants deemed that fundamental issues about bodies (for example, human anatomy, reproduction), different sexual orientations, and an introduction to foundational issues (like privacy, peer or social pressure, sexual abuse) would help elementary-aged children to normalize discussions about sex, anatomy, and sexual orientation.
Middle school conversations were ideally more in-depth to reflect the time when young people are beginning to explore and accept their social and sexual identities. Topics of discussion might include types of sexual intercourse (anal, oral, and vaginal), health promotion strategies (abstinence, condoms, and contraception), possible adverse outcomes of condomless intercourse (HIV, STIs), considerations about engaging in sexual intercourse (including readiness, negotiating boundaries, virginity), and interpersonal safety (for instance, sexting, alcohol/drugs/chemsex, sexual coercion, and partner abuse/violence).
Finally, high school age recommendations focused on socio-relational topics (such as hook-up culture, technology/online dating, and multiple or concurrent sex partners), which are most relevant during a time when adolescents are most prone to experimentation and risk-taking.
Acknowledging that the study approach was novel, Dr. Flores noted that hearing about these topics from the youth perspective allowed parents to prepare. “Communication is better when it’s anticipated vs. reactive,” he said.
Last but not least, clinicians also have an important role in supporting these conversations.
“We’ve always looked at sex communication as a dyadic process, as a parent bestowing wisdom on a child who doesn’t have that knowledge yet. But it can be a triadic model,” said Dr. Flores. “Providers can encourage parents to ask if a child is dating or is familiar with ways to protect themselves or provide consent, and act as a resource exclusively to troubleshoot emergent issues.”
This study was funded by the National Institutes of Health. The study also received supplementary funding from the Surgeon General C. Everett Koop HIV/AIDS Research Award. Dr. Flores and Dr. Goldfarb report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
When it comes to sexual health education in the United States, one thing is abundantly clear: It’s a messy patchwork of programs, topics, and criteria. Only 29 states and the District of Columbia currently mandate sexual health education. Sixteen states have an abstinence-only curriculum, whereas 13 do not require that instruction be age-appropriate, inclusive, medically accurate, or evidence-based/informed. And this is just the tip of the iceberg, according to a 2022 report issued by the Sex Ed for Social Change organization.
Parents should take an inclusive approach to sex communication and create a safe space for discussing sex and sexual orientation, said almost all (96.7%) of male young adults who participated in a qualitative study. This would help reinforce acceptance and parents could possibly serve as a proxy for children who’ve not yet disclosed their sexual orientation. Yet, few parents are equipped or prepared to have these meaningful conversations with gay, bisexual, queer, or gender-diverse children, despite the fact that they are especially vulnerable to poor sexual health outcomes, bullying, abuse, and mental health challenges, as well as high-risk sexual behaviors.
“Parents are sexual socialization agents,” Dalmacio Dennis Flores, PhD, ACRN, assistant professor of nursing at the University of Pennsylvania, Philadelphia, told this news organization. “It’s through the information that they convey, the way that they normalize rituals and expectations, that they inform young people of all of societal expectations or roles they’ll be fulfilling in the future.”
Dr. Flores is lead author of a study published in the Journal of Adolescent Health. He and his colleagues collected perspectives on comprehensive, inclusive, and age-appropriate parent-child sex communications from 30 GBQ adolescent males aged 15-20 years who were already “out” to their parents. Participants were asked to sort through 28 preprinted note cards containing broad sexual health topics (for instance, human anatomy, dating, sexually transmitted infections) as well as topics theoretically specific to GBQ individuals (for example, anal sex), and were asked to add additional topics that they felt were missing. They were then directed to recommend topics along with ideal timing (that is, elementary, middle, or high school) for these conversations.
Study findings also underscored the importance of initiating comprehensive sexuality talks as early as elementary school age – namely to start preparing GBQ children for inevitable adversities that they were likely to encounter later in life, as well as to form building blocks for more mature, in-depth discussions during high school.
Importantly, these recommendations generally align with those aimed at heterosexual youth.
“When we refer to topics for elementary school, they are general parameters of what kids might be interested in or want to hear more about; it’s not planting a seed,” explained Dr. Flores.
Eva Goldfarb, PhD, LHD, MA, professor of public health at Montclair (N.J.) State University, agreed. “We always talk about (in sex education) to follow young people’s lead. If your child is asking you a question, they deserve a response,” said Dr. Goldfarb, who wasn’t involved in the study. “It doesn’t mean you have to give a detailed- level explanation but if they’re asking about it, it means that they are thinking about it. But it’s really important for all young people to know all of this information.”
Along those lines, participants deemed that fundamental issues about bodies (for example, human anatomy, reproduction), different sexual orientations, and an introduction to foundational issues (like privacy, peer or social pressure, sexual abuse) would help elementary-aged children to normalize discussions about sex, anatomy, and sexual orientation.
Middle school conversations were ideally more in-depth to reflect the time when young people are beginning to explore and accept their social and sexual identities. Topics of discussion might include types of sexual intercourse (anal, oral, and vaginal), health promotion strategies (abstinence, condoms, and contraception), possible adverse outcomes of condomless intercourse (HIV, STIs), considerations about engaging in sexual intercourse (including readiness, negotiating boundaries, virginity), and interpersonal safety (for instance, sexting, alcohol/drugs/chemsex, sexual coercion, and partner abuse/violence).
Finally, high school age recommendations focused on socio-relational topics (such as hook-up culture, technology/online dating, and multiple or concurrent sex partners), which are most relevant during a time when adolescents are most prone to experimentation and risk-taking.
Acknowledging that the study approach was novel, Dr. Flores noted that hearing about these topics from the youth perspective allowed parents to prepare. “Communication is better when it’s anticipated vs. reactive,” he said.
Last but not least, clinicians also have an important role in supporting these conversations.
“We’ve always looked at sex communication as a dyadic process, as a parent bestowing wisdom on a child who doesn’t have that knowledge yet. But it can be a triadic model,” said Dr. Flores. “Providers can encourage parents to ask if a child is dating or is familiar with ways to protect themselves or provide consent, and act as a resource exclusively to troubleshoot emergent issues.”
This study was funded by the National Institutes of Health. The study also received supplementary funding from the Surgeon General C. Everett Koop HIV/AIDS Research Award. Dr. Flores and Dr. Goldfarb report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Large cohort study finds isotretinoin not associated with IBD
that also found no significant association of oral tetracycline-class antibiotics with IBD – and a small but statistically significant association of acne itself with the inflammatory disorders that make up IBD.
For the study, senior author John S. Barbieri, MD, MBA, of the department of dermatology, at Brigham and Women’s Hospital, Boston, and his colleagues used data from the TriNetX global research platform, which mines patient-level electronic medical record data from dozens of health care organizations, mainly in the United States. The network includes over 106 million patients. They looked at four cohorts: Patients without acne; those with acne but no current or prior use of systemic medications; those with acne managed with isotretinoin (and no prior use of oral tetracycline-class antibiotics); and those with acne managed with oral tetracycline-class antibiotics (and no exposure to isotretinoin).
For the acne cohorts, the investigators captured first encounters with a diagnosis of acne and first prescriptions of interest. And studywide, they used propensity score matching to balance cohorts for age, sex, race, ethnicity, and combined oral contraceptive use.
“These data should provide more reassurance to patients and prescribers that isotretinoin does not appear to result in a meaningfully increased risk of inflammatory bowel disease,” they wrote in the study, published online in the Journal of the American Academy of Dermatology.
“These are important findings as isotretinoin is a valuable treatment for acne that can result in a durable remission of disease activity, prevent acne scarring, and reduce our overreliance on oral antibiotics for acne,” they added.
Indeed, dermatologist Jonathan S. Weiss, MD, who was not involved in the research and was asked to comment on the study, said that the findings “are reassuring given the large numbers of patients evaluated and treated.” The smallest cohort – the isotretinoin group – had over 11,000 patients, and the other cohorts had over 100,000 patients each, he said in an interview.
“At this point, I’m not sure we need any other immediate information to feel comfortable using isotretinoin with respect to a potential to cause IBD, but it would be nice to see some longitudinal follow-up data for longer-term reassurance,” added Dr. Weiss, who practices in Snellville, Georgia, and is on the board of the directors of the American Acne and Rosacea Society.
The findings: Risk with acne
To assess the potential association between acne and IBD, the researchers identified more than 350,000 patients with acne managed without systemic medications, and propensity score matched them with patients who did not have acne. Altogether, their mean age was 22; 32.1% were male, and 59.6% were White.
Compared with the controls who did not have acne, they found a statistically significant association between acne and risk of incident IBD (odds ratio, 1.42; 95% confidence interval, 1.23-1.65) and an absolute risk difference of .04%. Separated into Crohn’s disease (CD) and ulcerative colitis (UC), ORs were 1.56 and 1.62, respectively.
Tetracyclines
To assess the association of oral tetracycline use and IBD, they compared more than 144,000 patients whose acne was managed with antibiotics with patients whose acne was managed without systemic medications. The patients had a mean age of 24.4; 34.7% were male, and 68.2% were White.
Compared with the patients who were not on systemic medications, there were no significant associations among those on oral tetracyclines, with an OR for incident IBD of 1 (95% CI, 0.82-1.22), an OR for incident CD of 1.09 (95% CI, 0.86-1.38), and an OR for UC of 0.78 (95% CI, 0.61-1.00).
Isotretinoin
To evaluate the association of isotretinoin and IBD, the researchers compared more than 11,000 patients treated with isotretinoin with two matched groups: patients with acne managed without systemic medications, and patients with acne managed with oral tetracyclines. The latter comparison was made to minimize potential confounding by acne severity. These patients had a mean age of 21.1; 49.5% were male, and 75.3% were White.
In the first comparison, compared with patients not treated with systemic medications, the OR for 1-year incidence of IBD among patients treated with isotretinoin was 1.29 (95% CI, 0.64-2.59), with an absolute risk difference of .036%. The ORs for CD and UC were 1.00 (95% CI, 0.45-2.23) and 1.27 (95% CI, .58-2.80), respectively.
And compared with the antibiotic-managed group, the OR for incident IBD among those on isotretinoin was 1.13 (95% CI, 0.57-2.21), with an absolute risk difference of .018%. The OR for CD was 1.00 (95% CI, 0.45-2.23). The OR for UC could not be accurately estimated because of an insufficient number of events in the tetracycline-treated group.
‘Challenging’ area of research
Researching acne treatments and the potential risk of IBD has been a methodologically “challenging topic to study” because of possible confounding and surveillance bias depending on study designs, Dr. Barbieri, director of the Brigham and Women’s Advanced Acne Therapeutics Clinic, said in an interview.
Studies that have identified a potential association between isotretinoin and IBD often have not adequately controlled for prior antibiotic exposure, for instance. And other studies, including a retrospective cohort study also published recently in JAAD using the same TriNetX database, have found 6-month isotretinoin-related risks of IBD but no increased risk at 1 year or more of follow-up – a finding that suggests a role of surveillance bias, Dr. Barbieri said.
The follow-up period of 1 year in their new study was chosen to minimize the risk of such bias. “Since patients on isotretinoin are seen more often, and since there are historical concerns about isotretinoin and IBD, patients on isotretinoin may be more likely to be screened earlier and thus could be diagnosed sooner than those not on [the medication],” he said.
He and his coauthors considered similar potential bias in designing the no-acne cohort, choosing patients who had routine primary care visits without abnormal findings in order to “reduce potential for bias due to frequency of interaction with the health care system,” they noted in their paper. (Patients had no prior encounters for acne and no history of acne treatments.)
Antibiotics, acne itself
Research on antibiotic use for acne and risk of IBD is scant, and the few studies that have been published show conflicting findings, Dr. Barbieri noted. In the meantime, studies and meta-analyses in the general medical literature – not involving acne – have identified an association between lifetime oral antibiotic exposure and IBD, he said.
While the results of the new study “are reassuring that oral tetracycline-class exposure for acne may not be associated with a significant absolute risk of inflammatory bowel disease, given the potential for antibiotic resistance and other antibiotic-associated complications, it remains important to be judicious” with their use in acne management, he and his coauthors wrote in the study.
The potential association between antibiotics for acne and IBD needs further study, preferably with longer follow-up duration, Dr. Barbieri said in the interview, but researchers are challenged by the lack of datasets with high-quality longitudinal data “beyond a few years of follow-up.”
The extent to which acne itself is associated with IBD is another area ripe for more research. Thus far, it seems that IBD and acne – and other chronic inflammatory skin diseases such as psoriasis – involve similar pathogenic pathways. “We know that in IBD Th17 and TNF immunologic pathways are important, so it’s not surprising that there may be associations,” he said.
In their paper, Dr. Barbieri and his coauthors emphasize, however, that the absolute risk difference between acne and IBD is small. It’s “unlikely that population level screening is warranted among patients with acne,” they wrote.
A second new study
The other study, also published recently in JAAD, used the same TriNetX research platform to identify approximately 77,000 patients with acne starting isotretinoin and matched them with patients starting oral antibiotics.
The investigators, Khalaf Kridin MD, PhD, and Ralf J. Ludwig, MD, of the Lübeck Institute of Experimental Dermatology, University of Lübeck (Germany), found that the lifetime risks (greater than 6 months) for patients on isotretinoin were not significantly elevated, compared with those on oral antibiotics for either CD (hazard ratio 1.05; 95% CI, 0.89-1.24, P = .583) or UC (HR, 1.13; 95% CI, 0.95-1.34; P = .162) They also looked at the risk of irritable bowel syndrome (IBS) and found a lower lifetime risk in the isotretinoin group.
In the short term, during the first 6 months after drug initiation, there was a significant, but slight increase in UC in the isotretinoin group. But this risk decreased to the level of the antibiotic group with longer follow up. “The absolute incidence rates [of IBD] and the risk difference of UC within the first 6 months are of limited clinical significance,” they wrote.
It may be, Dr. Weiss said in commenting on this study, “that isotretinoin unmasks an already-existing genetic tendency to UC early on in the course of treatment, but that it does not truly cause an increased incidence of any type of IBD.”
Both studies, said Dr. Barbieri, “add to an extensive body of literature that supports that isotretinoin is not associated with IBD.”
Dr. Barbieri had no disclosures for the study, for which Matthew T. Taylor served as first author. Coauthor Shawn Kwatra, MD, disclosed that he is an advisory board member/consultant for numerous pharmaceutical companies and has served as an investigator for several. Both are supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The other authors had no disclosures. Dr. Kridin and Dr. Ludwig had no disclosures for their study. Dr. Weiss had no disclosures.
that also found no significant association of oral tetracycline-class antibiotics with IBD – and a small but statistically significant association of acne itself with the inflammatory disorders that make up IBD.
For the study, senior author John S. Barbieri, MD, MBA, of the department of dermatology, at Brigham and Women’s Hospital, Boston, and his colleagues used data from the TriNetX global research platform, which mines patient-level electronic medical record data from dozens of health care organizations, mainly in the United States. The network includes over 106 million patients. They looked at four cohorts: Patients without acne; those with acne but no current or prior use of systemic medications; those with acne managed with isotretinoin (and no prior use of oral tetracycline-class antibiotics); and those with acne managed with oral tetracycline-class antibiotics (and no exposure to isotretinoin).
For the acne cohorts, the investigators captured first encounters with a diagnosis of acne and first prescriptions of interest. And studywide, they used propensity score matching to balance cohorts for age, sex, race, ethnicity, and combined oral contraceptive use.
“These data should provide more reassurance to patients and prescribers that isotretinoin does not appear to result in a meaningfully increased risk of inflammatory bowel disease,” they wrote in the study, published online in the Journal of the American Academy of Dermatology.
“These are important findings as isotretinoin is a valuable treatment for acne that can result in a durable remission of disease activity, prevent acne scarring, and reduce our overreliance on oral antibiotics for acne,” they added.
Indeed, dermatologist Jonathan S. Weiss, MD, who was not involved in the research and was asked to comment on the study, said that the findings “are reassuring given the large numbers of patients evaluated and treated.” The smallest cohort – the isotretinoin group – had over 11,000 patients, and the other cohorts had over 100,000 patients each, he said in an interview.
“At this point, I’m not sure we need any other immediate information to feel comfortable using isotretinoin with respect to a potential to cause IBD, but it would be nice to see some longitudinal follow-up data for longer-term reassurance,” added Dr. Weiss, who practices in Snellville, Georgia, and is on the board of the directors of the American Acne and Rosacea Society.
The findings: Risk with acne
To assess the potential association between acne and IBD, the researchers identified more than 350,000 patients with acne managed without systemic medications, and propensity score matched them with patients who did not have acne. Altogether, their mean age was 22; 32.1% were male, and 59.6% were White.
Compared with the controls who did not have acne, they found a statistically significant association between acne and risk of incident IBD (odds ratio, 1.42; 95% confidence interval, 1.23-1.65) and an absolute risk difference of .04%. Separated into Crohn’s disease (CD) and ulcerative colitis (UC), ORs were 1.56 and 1.62, respectively.
Tetracyclines
To assess the association of oral tetracycline use and IBD, they compared more than 144,000 patients whose acne was managed with antibiotics with patients whose acne was managed without systemic medications. The patients had a mean age of 24.4; 34.7% were male, and 68.2% were White.
Compared with the patients who were not on systemic medications, there were no significant associations among those on oral tetracyclines, with an OR for incident IBD of 1 (95% CI, 0.82-1.22), an OR for incident CD of 1.09 (95% CI, 0.86-1.38), and an OR for UC of 0.78 (95% CI, 0.61-1.00).
Isotretinoin
To evaluate the association of isotretinoin and IBD, the researchers compared more than 11,000 patients treated with isotretinoin with two matched groups: patients with acne managed without systemic medications, and patients with acne managed with oral tetracyclines. The latter comparison was made to minimize potential confounding by acne severity. These patients had a mean age of 21.1; 49.5% were male, and 75.3% were White.
In the first comparison, compared with patients not treated with systemic medications, the OR for 1-year incidence of IBD among patients treated with isotretinoin was 1.29 (95% CI, 0.64-2.59), with an absolute risk difference of .036%. The ORs for CD and UC were 1.00 (95% CI, 0.45-2.23) and 1.27 (95% CI, .58-2.80), respectively.
And compared with the antibiotic-managed group, the OR for incident IBD among those on isotretinoin was 1.13 (95% CI, 0.57-2.21), with an absolute risk difference of .018%. The OR for CD was 1.00 (95% CI, 0.45-2.23). The OR for UC could not be accurately estimated because of an insufficient number of events in the tetracycline-treated group.
‘Challenging’ area of research
Researching acne treatments and the potential risk of IBD has been a methodologically “challenging topic to study” because of possible confounding and surveillance bias depending on study designs, Dr. Barbieri, director of the Brigham and Women’s Advanced Acne Therapeutics Clinic, said in an interview.
Studies that have identified a potential association between isotretinoin and IBD often have not adequately controlled for prior antibiotic exposure, for instance. And other studies, including a retrospective cohort study also published recently in JAAD using the same TriNetX database, have found 6-month isotretinoin-related risks of IBD but no increased risk at 1 year or more of follow-up – a finding that suggests a role of surveillance bias, Dr. Barbieri said.
The follow-up period of 1 year in their new study was chosen to minimize the risk of such bias. “Since patients on isotretinoin are seen more often, and since there are historical concerns about isotretinoin and IBD, patients on isotretinoin may be more likely to be screened earlier and thus could be diagnosed sooner than those not on [the medication],” he said.
He and his coauthors considered similar potential bias in designing the no-acne cohort, choosing patients who had routine primary care visits without abnormal findings in order to “reduce potential for bias due to frequency of interaction with the health care system,” they noted in their paper. (Patients had no prior encounters for acne and no history of acne treatments.)
Antibiotics, acne itself
Research on antibiotic use for acne and risk of IBD is scant, and the few studies that have been published show conflicting findings, Dr. Barbieri noted. In the meantime, studies and meta-analyses in the general medical literature – not involving acne – have identified an association between lifetime oral antibiotic exposure and IBD, he said.
While the results of the new study “are reassuring that oral tetracycline-class exposure for acne may not be associated with a significant absolute risk of inflammatory bowel disease, given the potential for antibiotic resistance and other antibiotic-associated complications, it remains important to be judicious” with their use in acne management, he and his coauthors wrote in the study.
The potential association between antibiotics for acne and IBD needs further study, preferably with longer follow-up duration, Dr. Barbieri said in the interview, but researchers are challenged by the lack of datasets with high-quality longitudinal data “beyond a few years of follow-up.”
The extent to which acne itself is associated with IBD is another area ripe for more research. Thus far, it seems that IBD and acne – and other chronic inflammatory skin diseases such as psoriasis – involve similar pathogenic pathways. “We know that in IBD Th17 and TNF immunologic pathways are important, so it’s not surprising that there may be associations,” he said.
In their paper, Dr. Barbieri and his coauthors emphasize, however, that the absolute risk difference between acne and IBD is small. It’s “unlikely that population level screening is warranted among patients with acne,” they wrote.
A second new study
The other study, also published recently in JAAD, used the same TriNetX research platform to identify approximately 77,000 patients with acne starting isotretinoin and matched them with patients starting oral antibiotics.
The investigators, Khalaf Kridin MD, PhD, and Ralf J. Ludwig, MD, of the Lübeck Institute of Experimental Dermatology, University of Lübeck (Germany), found that the lifetime risks (greater than 6 months) for patients on isotretinoin were not significantly elevated, compared with those on oral antibiotics for either CD (hazard ratio 1.05; 95% CI, 0.89-1.24, P = .583) or UC (HR, 1.13; 95% CI, 0.95-1.34; P = .162) They also looked at the risk of irritable bowel syndrome (IBS) and found a lower lifetime risk in the isotretinoin group.
In the short term, during the first 6 months after drug initiation, there was a significant, but slight increase in UC in the isotretinoin group. But this risk decreased to the level of the antibiotic group with longer follow up. “The absolute incidence rates [of IBD] and the risk difference of UC within the first 6 months are of limited clinical significance,” they wrote.
It may be, Dr. Weiss said in commenting on this study, “that isotretinoin unmasks an already-existing genetic tendency to UC early on in the course of treatment, but that it does not truly cause an increased incidence of any type of IBD.”
Both studies, said Dr. Barbieri, “add to an extensive body of literature that supports that isotretinoin is not associated with IBD.”
Dr. Barbieri had no disclosures for the study, for which Matthew T. Taylor served as first author. Coauthor Shawn Kwatra, MD, disclosed that he is an advisory board member/consultant for numerous pharmaceutical companies and has served as an investigator for several. Both are supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The other authors had no disclosures. Dr. Kridin and Dr. Ludwig had no disclosures for their study. Dr. Weiss had no disclosures.
that also found no significant association of oral tetracycline-class antibiotics with IBD – and a small but statistically significant association of acne itself with the inflammatory disorders that make up IBD.
For the study, senior author John S. Barbieri, MD, MBA, of the department of dermatology, at Brigham and Women’s Hospital, Boston, and his colleagues used data from the TriNetX global research platform, which mines patient-level electronic medical record data from dozens of health care organizations, mainly in the United States. The network includes over 106 million patients. They looked at four cohorts: Patients without acne; those with acne but no current or prior use of systemic medications; those with acne managed with isotretinoin (and no prior use of oral tetracycline-class antibiotics); and those with acne managed with oral tetracycline-class antibiotics (and no exposure to isotretinoin).
For the acne cohorts, the investigators captured first encounters with a diagnosis of acne and first prescriptions of interest. And studywide, they used propensity score matching to balance cohorts for age, sex, race, ethnicity, and combined oral contraceptive use.
“These data should provide more reassurance to patients and prescribers that isotretinoin does not appear to result in a meaningfully increased risk of inflammatory bowel disease,” they wrote in the study, published online in the Journal of the American Academy of Dermatology.
“These are important findings as isotretinoin is a valuable treatment for acne that can result in a durable remission of disease activity, prevent acne scarring, and reduce our overreliance on oral antibiotics for acne,” they added.
Indeed, dermatologist Jonathan S. Weiss, MD, who was not involved in the research and was asked to comment on the study, said that the findings “are reassuring given the large numbers of patients evaluated and treated.” The smallest cohort – the isotretinoin group – had over 11,000 patients, and the other cohorts had over 100,000 patients each, he said in an interview.
“At this point, I’m not sure we need any other immediate information to feel comfortable using isotretinoin with respect to a potential to cause IBD, but it would be nice to see some longitudinal follow-up data for longer-term reassurance,” added Dr. Weiss, who practices in Snellville, Georgia, and is on the board of the directors of the American Acne and Rosacea Society.
The findings: Risk with acne
To assess the potential association between acne and IBD, the researchers identified more than 350,000 patients with acne managed without systemic medications, and propensity score matched them with patients who did not have acne. Altogether, their mean age was 22; 32.1% were male, and 59.6% were White.
Compared with the controls who did not have acne, they found a statistically significant association between acne and risk of incident IBD (odds ratio, 1.42; 95% confidence interval, 1.23-1.65) and an absolute risk difference of .04%. Separated into Crohn’s disease (CD) and ulcerative colitis (UC), ORs were 1.56 and 1.62, respectively.
Tetracyclines
To assess the association of oral tetracycline use and IBD, they compared more than 144,000 patients whose acne was managed with antibiotics with patients whose acne was managed without systemic medications. The patients had a mean age of 24.4; 34.7% were male, and 68.2% were White.
Compared with the patients who were not on systemic medications, there were no significant associations among those on oral tetracyclines, with an OR for incident IBD of 1 (95% CI, 0.82-1.22), an OR for incident CD of 1.09 (95% CI, 0.86-1.38), and an OR for UC of 0.78 (95% CI, 0.61-1.00).
Isotretinoin
To evaluate the association of isotretinoin and IBD, the researchers compared more than 11,000 patients treated with isotretinoin with two matched groups: patients with acne managed without systemic medications, and patients with acne managed with oral tetracyclines. The latter comparison was made to minimize potential confounding by acne severity. These patients had a mean age of 21.1; 49.5% were male, and 75.3% were White.
In the first comparison, compared with patients not treated with systemic medications, the OR for 1-year incidence of IBD among patients treated with isotretinoin was 1.29 (95% CI, 0.64-2.59), with an absolute risk difference of .036%. The ORs for CD and UC were 1.00 (95% CI, 0.45-2.23) and 1.27 (95% CI, .58-2.80), respectively.
And compared with the antibiotic-managed group, the OR for incident IBD among those on isotretinoin was 1.13 (95% CI, 0.57-2.21), with an absolute risk difference of .018%. The OR for CD was 1.00 (95% CI, 0.45-2.23). The OR for UC could not be accurately estimated because of an insufficient number of events in the tetracycline-treated group.
‘Challenging’ area of research
Researching acne treatments and the potential risk of IBD has been a methodologically “challenging topic to study” because of possible confounding and surveillance bias depending on study designs, Dr. Barbieri, director of the Brigham and Women’s Advanced Acne Therapeutics Clinic, said in an interview.
Studies that have identified a potential association between isotretinoin and IBD often have not adequately controlled for prior antibiotic exposure, for instance. And other studies, including a retrospective cohort study also published recently in JAAD using the same TriNetX database, have found 6-month isotretinoin-related risks of IBD but no increased risk at 1 year or more of follow-up – a finding that suggests a role of surveillance bias, Dr. Barbieri said.
The follow-up period of 1 year in their new study was chosen to minimize the risk of such bias. “Since patients on isotretinoin are seen more often, and since there are historical concerns about isotretinoin and IBD, patients on isotretinoin may be more likely to be screened earlier and thus could be diagnosed sooner than those not on [the medication],” he said.
He and his coauthors considered similar potential bias in designing the no-acne cohort, choosing patients who had routine primary care visits without abnormal findings in order to “reduce potential for bias due to frequency of interaction with the health care system,” they noted in their paper. (Patients had no prior encounters for acne and no history of acne treatments.)
Antibiotics, acne itself
Research on antibiotic use for acne and risk of IBD is scant, and the few studies that have been published show conflicting findings, Dr. Barbieri noted. In the meantime, studies and meta-analyses in the general medical literature – not involving acne – have identified an association between lifetime oral antibiotic exposure and IBD, he said.
While the results of the new study “are reassuring that oral tetracycline-class exposure for acne may not be associated with a significant absolute risk of inflammatory bowel disease, given the potential for antibiotic resistance and other antibiotic-associated complications, it remains important to be judicious” with their use in acne management, he and his coauthors wrote in the study.
The potential association between antibiotics for acne and IBD needs further study, preferably with longer follow-up duration, Dr. Barbieri said in the interview, but researchers are challenged by the lack of datasets with high-quality longitudinal data “beyond a few years of follow-up.”
The extent to which acne itself is associated with IBD is another area ripe for more research. Thus far, it seems that IBD and acne – and other chronic inflammatory skin diseases such as psoriasis – involve similar pathogenic pathways. “We know that in IBD Th17 and TNF immunologic pathways are important, so it’s not surprising that there may be associations,” he said.
In their paper, Dr. Barbieri and his coauthors emphasize, however, that the absolute risk difference between acne and IBD is small. It’s “unlikely that population level screening is warranted among patients with acne,” they wrote.
A second new study
The other study, also published recently in JAAD, used the same TriNetX research platform to identify approximately 77,000 patients with acne starting isotretinoin and matched them with patients starting oral antibiotics.
The investigators, Khalaf Kridin MD, PhD, and Ralf J. Ludwig, MD, of the Lübeck Institute of Experimental Dermatology, University of Lübeck (Germany), found that the lifetime risks (greater than 6 months) for patients on isotretinoin were not significantly elevated, compared with those on oral antibiotics for either CD (hazard ratio 1.05; 95% CI, 0.89-1.24, P = .583) or UC (HR, 1.13; 95% CI, 0.95-1.34; P = .162) They also looked at the risk of irritable bowel syndrome (IBS) and found a lower lifetime risk in the isotretinoin group.
In the short term, during the first 6 months after drug initiation, there was a significant, but slight increase in UC in the isotretinoin group. But this risk decreased to the level of the antibiotic group with longer follow up. “The absolute incidence rates [of IBD] and the risk difference of UC within the first 6 months are of limited clinical significance,” they wrote.
It may be, Dr. Weiss said in commenting on this study, “that isotretinoin unmasks an already-existing genetic tendency to UC early on in the course of treatment, but that it does not truly cause an increased incidence of any type of IBD.”
Both studies, said Dr. Barbieri, “add to an extensive body of literature that supports that isotretinoin is not associated with IBD.”
Dr. Barbieri had no disclosures for the study, for which Matthew T. Taylor served as first author. Coauthor Shawn Kwatra, MD, disclosed that he is an advisory board member/consultant for numerous pharmaceutical companies and has served as an investigator for several. Both are supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The other authors had no disclosures. Dr. Kridin and Dr. Ludwig had no disclosures for their study. Dr. Weiss had no disclosures.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Loneliness risk elevated among young cancer survivors
findings from a large retrospective study suggest.
Young cancer survivors were more than twice as likely to report loneliness at study baseline and follow-up. Loneliness at these times was associated with an almost 10-fold increased risk for anxiety and a nearly 18-fold increased risk for depression.
“We observed an elevated prevalence of loneliness in survivors, compared to sibling controls, and found that loneliness was associated with emotional, behavioral, and physical health morbidities,” lead study author Chiara Papini, PhD, of St. Jude Children’s Research Hospital, Memphis, and her colleagues write. “Our results highlight the importance of identifying and screening young adult survivors of childhood cancer for loneliness and the need for targeted interventions to reduce loneliness.”
The article was published online in the journal Cancer.
Most young cancer survivors in the United States reach adulthood and need to play catch-up: make up for missed school and work, become reacquainted with old friends, and develop new friendships, social networks, and intimate relationships. Meeting these needs may be hindered by adverse physical and psychosocial problems that linger or develop after treatment, which may leave cancer survivors feeling isolated.
“Young adult survivors of childhood cancer are navigating a developmental period marked by increased social expectations, during which loneliness may have significant impact on physical and mental health,” Dr. Papini and colleagues say.
To better understand the risks for loneliness among young cancer survivors, Dr. Papini and her colleagues analyzed data from the retrospective Childhood Cancer Survivor Study, which followed young survivors who had been diagnosed with a range of cancers before age 21 years. Study participants had been treated at one of 31 study sites in North America and had survived 5 years or longer after diagnosis.
The 9,664 survivors and 2,221 randomly sampled siblings ranged in age from 19 to 39 years at the time they completed a survey that assessed emotional distress at baseline and at follow‐up a median of 6.6 years. At baseline, the median age of the survivors was 27 years, and a median of 17.5 years had passed from the time of their diagnosis.
The most common diagnoses were leukemia (35%), Hodgkin lymphoma (15%), central nervous system (CNS) tumors (14%), and bone tumors (10%). More than half (56%) had received radiation therapy.
Using multivariable models, the researchers found that survivors were more likely than siblings to report moderate to extreme loneliness at either baseline or follow‐up (prevalence ratio, 1.04) and were more than two times more likely to report loneliness at both baseline and follow‐up (PR, 2.21).
Loneliness at baseline and follow‐up was associated with a much greater risk for anxiety (relative risk, 9.75) and depression (RR, 17.86). Loneliness at follow‐up was linked with increased risks for suicidal ideation (RR, 1.52), heavy or risky alcohol consumption (RR, 1.27), and any grade 2-4 new‐onset chronic health condition (RR, 1.29), especially those that were neurologic (RR, 4.37).
Survivors of CNS tumors (odds ratio, 2.59) and leukemia (OR, 2.52) were most likely to report loneliness at both baseline and follow‐up, though survivors of four other cancer types also faced an elevated risk for loneliness: neuroblastoma (OR, 2.32), bone tumor (OR, 2.12), soft tissue sarcoma (OR, 1.78), and Hodgkin lymphoma (OR, 1.69).
Treatment type appeared to matter as well. Survivors who underwent amputation (OR, 1.82) or were treated with cranial radiation greater than or equal to 20 Gy (OR, 1.56) or corticosteroids (OR, 1.31) were more likely to report loneliness at baseline and follow‐up, compared with those who reported no loneliness at both time points.
The authors acknowledge limitations to the study, including the fact that roughly 90% of survivors and siblings were White, which limits the applicability of their results to diverse groups. In addition, the responses were self-reported without external validation.
Overall, though, the findings provide a framework for clinicians to understand and identify loneliness among young cancer survivors and help them cope with their emotions.
“The Childhood Cancer Survivor Study provides the largest and the most comprehensive dataset on childhood cancer survivors and healthy-sibling comparisons, giving us powerful data on survivorship, late effects, and psychosocial and health outcomes,” Rachel M. Moore, PhD, child psychologist at Children’s Mercy Kansas City, Mo., said in an interview.
Asking a simple question – “Are you feeling lonely?” – can identify at-risk survivors and enable health care teams to provide timely interventions that address young patients’ physical and psychological needs, said Dr. Moore, who was not involved in the study.
Dr. Moore noted that within her clinical practice, “adolescent and young adult survivors frequently discuss loneliness in their daily lives. They feel different from their peers and misunderstood. Having a conversation early in survivorship care about the experience of loneliness as a product of cancer treatment can open the door to regular screening and destigmatizing mental health services.”
Supporting young people throughout their survivorship journey is important, said Rusha Bhandari, MD, medical director of the Childhood, Adolescent, and Young Adult Cancer Survivorship Program at City of Hope, Duarte, Calif. This study can help ensure that clinicians “provide comprehensive care, including psychosocial screening and support, to meet the unique needs of our young adult survivors,” said Dr. Bhandari, who also was not involved in the research.
The National Cancer Institute and the American Lebanese Syrian Associated Charities supported the study. One co-author reported receiving corporate consulting fees. Dr. Papini, the remaining co-authors, Dr. Moore, and Dr. Bhandari report no relevant financial involvements.
A version of this article first appeared on Medscape.com.
findings from a large retrospective study suggest.
Young cancer survivors were more than twice as likely to report loneliness at study baseline and follow-up. Loneliness at these times was associated with an almost 10-fold increased risk for anxiety and a nearly 18-fold increased risk for depression.
“We observed an elevated prevalence of loneliness in survivors, compared to sibling controls, and found that loneliness was associated with emotional, behavioral, and physical health morbidities,” lead study author Chiara Papini, PhD, of St. Jude Children’s Research Hospital, Memphis, and her colleagues write. “Our results highlight the importance of identifying and screening young adult survivors of childhood cancer for loneliness and the need for targeted interventions to reduce loneliness.”
The article was published online in the journal Cancer.
Most young cancer survivors in the United States reach adulthood and need to play catch-up: make up for missed school and work, become reacquainted with old friends, and develop new friendships, social networks, and intimate relationships. Meeting these needs may be hindered by adverse physical and psychosocial problems that linger or develop after treatment, which may leave cancer survivors feeling isolated.
“Young adult survivors of childhood cancer are navigating a developmental period marked by increased social expectations, during which loneliness may have significant impact on physical and mental health,” Dr. Papini and colleagues say.
To better understand the risks for loneliness among young cancer survivors, Dr. Papini and her colleagues analyzed data from the retrospective Childhood Cancer Survivor Study, which followed young survivors who had been diagnosed with a range of cancers before age 21 years. Study participants had been treated at one of 31 study sites in North America and had survived 5 years or longer after diagnosis.
The 9,664 survivors and 2,221 randomly sampled siblings ranged in age from 19 to 39 years at the time they completed a survey that assessed emotional distress at baseline and at follow‐up a median of 6.6 years. At baseline, the median age of the survivors was 27 years, and a median of 17.5 years had passed from the time of their diagnosis.
The most common diagnoses were leukemia (35%), Hodgkin lymphoma (15%), central nervous system (CNS) tumors (14%), and bone tumors (10%). More than half (56%) had received radiation therapy.
Using multivariable models, the researchers found that survivors were more likely than siblings to report moderate to extreme loneliness at either baseline or follow‐up (prevalence ratio, 1.04) and were more than two times more likely to report loneliness at both baseline and follow‐up (PR, 2.21).
Loneliness at baseline and follow‐up was associated with a much greater risk for anxiety (relative risk, 9.75) and depression (RR, 17.86). Loneliness at follow‐up was linked with increased risks for suicidal ideation (RR, 1.52), heavy or risky alcohol consumption (RR, 1.27), and any grade 2-4 new‐onset chronic health condition (RR, 1.29), especially those that were neurologic (RR, 4.37).
Survivors of CNS tumors (odds ratio, 2.59) and leukemia (OR, 2.52) were most likely to report loneliness at both baseline and follow‐up, though survivors of four other cancer types also faced an elevated risk for loneliness: neuroblastoma (OR, 2.32), bone tumor (OR, 2.12), soft tissue sarcoma (OR, 1.78), and Hodgkin lymphoma (OR, 1.69).
Treatment type appeared to matter as well. Survivors who underwent amputation (OR, 1.82) or were treated with cranial radiation greater than or equal to 20 Gy (OR, 1.56) or corticosteroids (OR, 1.31) were more likely to report loneliness at baseline and follow‐up, compared with those who reported no loneliness at both time points.
The authors acknowledge limitations to the study, including the fact that roughly 90% of survivors and siblings were White, which limits the applicability of their results to diverse groups. In addition, the responses were self-reported without external validation.
Overall, though, the findings provide a framework for clinicians to understand and identify loneliness among young cancer survivors and help them cope with their emotions.
“The Childhood Cancer Survivor Study provides the largest and the most comprehensive dataset on childhood cancer survivors and healthy-sibling comparisons, giving us powerful data on survivorship, late effects, and psychosocial and health outcomes,” Rachel M. Moore, PhD, child psychologist at Children’s Mercy Kansas City, Mo., said in an interview.
Asking a simple question – “Are you feeling lonely?” – can identify at-risk survivors and enable health care teams to provide timely interventions that address young patients’ physical and psychological needs, said Dr. Moore, who was not involved in the study.
Dr. Moore noted that within her clinical practice, “adolescent and young adult survivors frequently discuss loneliness in their daily lives. They feel different from their peers and misunderstood. Having a conversation early in survivorship care about the experience of loneliness as a product of cancer treatment can open the door to regular screening and destigmatizing mental health services.”
Supporting young people throughout their survivorship journey is important, said Rusha Bhandari, MD, medical director of the Childhood, Adolescent, and Young Adult Cancer Survivorship Program at City of Hope, Duarte, Calif. This study can help ensure that clinicians “provide comprehensive care, including psychosocial screening and support, to meet the unique needs of our young adult survivors,” said Dr. Bhandari, who also was not involved in the research.
The National Cancer Institute and the American Lebanese Syrian Associated Charities supported the study. One co-author reported receiving corporate consulting fees. Dr. Papini, the remaining co-authors, Dr. Moore, and Dr. Bhandari report no relevant financial involvements.
A version of this article first appeared on Medscape.com.
findings from a large retrospective study suggest.
Young cancer survivors were more than twice as likely to report loneliness at study baseline and follow-up. Loneliness at these times was associated with an almost 10-fold increased risk for anxiety and a nearly 18-fold increased risk for depression.
“We observed an elevated prevalence of loneliness in survivors, compared to sibling controls, and found that loneliness was associated with emotional, behavioral, and physical health morbidities,” lead study author Chiara Papini, PhD, of St. Jude Children’s Research Hospital, Memphis, and her colleagues write. “Our results highlight the importance of identifying and screening young adult survivors of childhood cancer for loneliness and the need for targeted interventions to reduce loneliness.”
The article was published online in the journal Cancer.
Most young cancer survivors in the United States reach adulthood and need to play catch-up: make up for missed school and work, become reacquainted with old friends, and develop new friendships, social networks, and intimate relationships. Meeting these needs may be hindered by adverse physical and psychosocial problems that linger or develop after treatment, which may leave cancer survivors feeling isolated.
“Young adult survivors of childhood cancer are navigating a developmental period marked by increased social expectations, during which loneliness may have significant impact on physical and mental health,” Dr. Papini and colleagues say.
To better understand the risks for loneliness among young cancer survivors, Dr. Papini and her colleagues analyzed data from the retrospective Childhood Cancer Survivor Study, which followed young survivors who had been diagnosed with a range of cancers before age 21 years. Study participants had been treated at one of 31 study sites in North America and had survived 5 years or longer after diagnosis.
The 9,664 survivors and 2,221 randomly sampled siblings ranged in age from 19 to 39 years at the time they completed a survey that assessed emotional distress at baseline and at follow‐up a median of 6.6 years. At baseline, the median age of the survivors was 27 years, and a median of 17.5 years had passed from the time of their diagnosis.
The most common diagnoses were leukemia (35%), Hodgkin lymphoma (15%), central nervous system (CNS) tumors (14%), and bone tumors (10%). More than half (56%) had received radiation therapy.
Using multivariable models, the researchers found that survivors were more likely than siblings to report moderate to extreme loneliness at either baseline or follow‐up (prevalence ratio, 1.04) and were more than two times more likely to report loneliness at both baseline and follow‐up (PR, 2.21).
Loneliness at baseline and follow‐up was associated with a much greater risk for anxiety (relative risk, 9.75) and depression (RR, 17.86). Loneliness at follow‐up was linked with increased risks for suicidal ideation (RR, 1.52), heavy or risky alcohol consumption (RR, 1.27), and any grade 2-4 new‐onset chronic health condition (RR, 1.29), especially those that were neurologic (RR, 4.37).
Survivors of CNS tumors (odds ratio, 2.59) and leukemia (OR, 2.52) were most likely to report loneliness at both baseline and follow‐up, though survivors of four other cancer types also faced an elevated risk for loneliness: neuroblastoma (OR, 2.32), bone tumor (OR, 2.12), soft tissue sarcoma (OR, 1.78), and Hodgkin lymphoma (OR, 1.69).
Treatment type appeared to matter as well. Survivors who underwent amputation (OR, 1.82) or were treated with cranial radiation greater than or equal to 20 Gy (OR, 1.56) or corticosteroids (OR, 1.31) were more likely to report loneliness at baseline and follow‐up, compared with those who reported no loneliness at both time points.
The authors acknowledge limitations to the study, including the fact that roughly 90% of survivors and siblings were White, which limits the applicability of their results to diverse groups. In addition, the responses were self-reported without external validation.
Overall, though, the findings provide a framework for clinicians to understand and identify loneliness among young cancer survivors and help them cope with their emotions.
“The Childhood Cancer Survivor Study provides the largest and the most comprehensive dataset on childhood cancer survivors and healthy-sibling comparisons, giving us powerful data on survivorship, late effects, and psychosocial and health outcomes,” Rachel M. Moore, PhD, child psychologist at Children’s Mercy Kansas City, Mo., said in an interview.
Asking a simple question – “Are you feeling lonely?” – can identify at-risk survivors and enable health care teams to provide timely interventions that address young patients’ physical and psychological needs, said Dr. Moore, who was not involved in the study.
Dr. Moore noted that within her clinical practice, “adolescent and young adult survivors frequently discuss loneliness in their daily lives. They feel different from their peers and misunderstood. Having a conversation early in survivorship care about the experience of loneliness as a product of cancer treatment can open the door to regular screening and destigmatizing mental health services.”
Supporting young people throughout their survivorship journey is important, said Rusha Bhandari, MD, medical director of the Childhood, Adolescent, and Young Adult Cancer Survivorship Program at City of Hope, Duarte, Calif. This study can help ensure that clinicians “provide comprehensive care, including psychosocial screening and support, to meet the unique needs of our young adult survivors,” said Dr. Bhandari, who also was not involved in the research.
The National Cancer Institute and the American Lebanese Syrian Associated Charities supported the study. One co-author reported receiving corporate consulting fees. Dr. Papini, the remaining co-authors, Dr. Moore, and Dr. Bhandari report no relevant financial involvements.
A version of this article first appeared on Medscape.com.
FROM CANCER
Surgeon General says 13-year-olds shouldn’t be on social media
The U.S. Surgeon General says 13 years old is too young to begin using social media.
Most social media platforms including TikTok, Snapchat, Instagram, and Facebook allow users to create accounts if they say they are at least 13 years old.
“I, personally, based on the data I’ve seen, believe that 13 is too early. ... It’s a time where it’s really important for us to be thoughtful about what’s going into how they think about their own self-worth and their relationships, and the skewed and often distorted environment of social media often does a disservice to many of those children,” U.S. Surgeon General Vivek Murthy, MD, told CNN.
Research has shown that teens are susceptible to cyberbullying and serious mental health impacts from social media usage and online activity during an era when the influence of the Internet has become everywhere for young people.
According to the Pew Research Center, 95% of teens age 13 and up have a smartphone, and 97% of teens say they use the Internet daily. Among 13- and 14-year-olds, 61% say they use TikTok and 51% say they use Snapchat. Older teens ages 15-17 use those social media platforms at higher rates, with 71% saying they use TikTok and 65% using Snapchat.
“If parents can band together and say you know, as a group, we’re not going to allow our kids to use social media until 16 or 17 or 18 or whatever age they choose, that’s a much more effective strategy in making sure your kids don’t get exposed to harm early,” Dr. Murthy said.
A version of this article originally appeared on WebMD.com.
The U.S. Surgeon General says 13 years old is too young to begin using social media.
Most social media platforms including TikTok, Snapchat, Instagram, and Facebook allow users to create accounts if they say they are at least 13 years old.
“I, personally, based on the data I’ve seen, believe that 13 is too early. ... It’s a time where it’s really important for us to be thoughtful about what’s going into how they think about their own self-worth and their relationships, and the skewed and often distorted environment of social media often does a disservice to many of those children,” U.S. Surgeon General Vivek Murthy, MD, told CNN.
Research has shown that teens are susceptible to cyberbullying and serious mental health impacts from social media usage and online activity during an era when the influence of the Internet has become everywhere for young people.
According to the Pew Research Center, 95% of teens age 13 and up have a smartphone, and 97% of teens say they use the Internet daily. Among 13- and 14-year-olds, 61% say they use TikTok and 51% say they use Snapchat. Older teens ages 15-17 use those social media platforms at higher rates, with 71% saying they use TikTok and 65% using Snapchat.
“If parents can band together and say you know, as a group, we’re not going to allow our kids to use social media until 16 or 17 or 18 or whatever age they choose, that’s a much more effective strategy in making sure your kids don’t get exposed to harm early,” Dr. Murthy said.
A version of this article originally appeared on WebMD.com.
The U.S. Surgeon General says 13 years old is too young to begin using social media.
Most social media platforms including TikTok, Snapchat, Instagram, and Facebook allow users to create accounts if they say they are at least 13 years old.
“I, personally, based on the data I’ve seen, believe that 13 is too early. ... It’s a time where it’s really important for us to be thoughtful about what’s going into how they think about their own self-worth and their relationships, and the skewed and often distorted environment of social media often does a disservice to many of those children,” U.S. Surgeon General Vivek Murthy, MD, told CNN.
Research has shown that teens are susceptible to cyberbullying and serious mental health impacts from social media usage and online activity during an era when the influence of the Internet has become everywhere for young people.
According to the Pew Research Center, 95% of teens age 13 and up have a smartphone, and 97% of teens say they use the Internet daily. Among 13- and 14-year-olds, 61% say they use TikTok and 51% say they use Snapchat. Older teens ages 15-17 use those social media platforms at higher rates, with 71% saying they use TikTok and 65% using Snapchat.
“If parents can band together and say you know, as a group, we’re not going to allow our kids to use social media until 16 or 17 or 18 or whatever age they choose, that’s a much more effective strategy in making sure your kids don’t get exposed to harm early,” Dr. Murthy said.
A version of this article originally appeared on WebMD.com.
Canadian guidance recommends reducing alcohol consumption
“Drinking less is better,” says the guidance, which replaces Canada’s 2011 Low-Risk Drinking Guidelines (LRDGs).
Developed in consultation with an executive committee from federal, provincial, and territorial governments; national organizations; three scientific expert panels; and an internal evidence review working group, the guidance presents the following findings:
- Consuming no drinks per week has benefits, such as better health and better sleep, and it’s the only safe option during pregnancy.
- Consuming one or two standard drinks weekly will likely not have alcohol-related consequences.
- Three to six drinks raise the risk of developing breast, colon, and other cancers.
- Seven or more increase the risk of heart disease or stroke.
- Each additional drink “radically increases” the risk of these health consequences.
“Alcohol is more harmful than was previously thought and is a key component of the health of your patients,” Adam Sherk, PhD, a scientist at the Canadian Institute for Substance Use Research at the University of Victoria (B.C.), and a member of the scientific expert panel that contributed to the guidance, said in an interview. “Display and discuss the new guidance with your patients with the main message that drinking less is better.”
Peter Butt, MD, a clinical associate professor at the University of Saskatchewan, Saskatoon, and cochair of the guidance project, said in an interview: “The World Health Organization has identified over 200 ICD-coded conditions associated with alcohol use. This creates many opportunities to inquire into quantity and frequency of alcohol use, relate it to the patient’s health and well-being, and provide advice on reduction.”
“Canada’s Guidance on Alcohol and Health: Final Report” and a related infographic were published online Jan. 17.
Continuum of risk
The impetus for the new guidance came from the fact that “our 2011 LRDGs were no longer current, and there was emerging evidence that people drinking within those levels were coming to harm,” said Dr. Butt.
That evidence indicates that alcohol causes at least seven types of cancer, mostly of the breast or colon; is a risk factor for most types of heart disease; and is a main cause of liver disease. Evidence also indicates that avoiding drinking to the point of intoxication will reduce people’s risk of perpetrating alcohol-related violence.
Responding to the need to accurately quantify the risk, the guidance defines a “standard” drink as 12 oz of beer, cooler, or cider (5% alcohol); 5 oz of wine (12% alcohol); and 1.5 oz of spirits such as whiskey, vodka, or gin (40% alcohol).
Using different mortality risk thresholds, the project’s experts developed the following continuum of risk:
- Low for individuals who consume two standard drinks or fewer per week
- Moderate for those who consume from three to six standard drinks per week
- Increasingly high for those who consume seven standard drinks or more per week
The guidance makes the following observations:
- Consuming more than two standard drinks per drinking occasion is associated with an increased risk of harms to self and others, including injuries and violence.
- When pregnant or trying to get pregnant, no amount of alcohol is safe.
- When breastfeeding, not drinking is safest.
- Above the upper limit of the moderate risk zone, health risks increase more steeply for females than males.
- Far more injuries, violence, and deaths result from men’s alcohol use, especially for per occasion drinking, than from women’s alcohol use.
- Young people should delay alcohol use for as long as possible.
- Individuals should not start to use alcohol or increase their alcohol use for health benefits.
- Any reduction in alcohol use is beneficial.
Other national guidelines
“Countries that haven’t updated their alcohol use guidelines recently should do so, as the evidence regarding alcohol and health has advanced considerably in the past 10 years,” said Dr. Sherk. He acknowledged that “any time health guidance changes substantially, it’s reasonable to expect a period of readjustment.”
“Some will be resistant,” Dr. Butt agreed. “Some professionals will need more education than others on the health effects of alcohol. Some patients will also be more invested in drinking than others. The harm-reduction, risk-zone approach should assist in the process of engaging patients and helping them reduce over time.
“Just as we benefited from the updates done in the United Kingdom, France, and especially Australia, so also researchers elsewhere will critique our work and our approach and make their own decisions on how best to communicate with their public,” Dr. Butt said. He noted that Canada’s contributions regarding the association between alcohol and violence, as well as their sex/gender approach to the evidence, “may influence the next country’s review.”
Commenting on whether the United States should consider changing its guidance, Timothy Brennan, MD, MPH, chief of clinical services for the Addiction Institute of Mount Sinai Health System in New York, said in an interview, “A lot of people will be surprised at the recommended limits on alcohol. Most think that they can have one or two glasses of alcohol per day and not have any increased risk to their health. I think the Canadians deserve credit for putting themselves out there.”
Dr. Brennan said there will “certainly be pushback by the drinking lobby, which is very strong both in the U.S. and in Canada.” In fact, the national trade group Beer Canada was recently quoted as stating that it still supports the 2011 guidelines and that the updating process lacked full transparency and expert technical peer review.
Nevertheless, Dr. Brennan said, “it’s overwhelmingly clear that alcohol affects a ton of different parts of our body, so limiting the amount of alcohol we take in is always going to be a good thing. The Canadian graphic is great because it color-codes the risk. I recommend that clinicians put it up in their offices and begin quantifying the units of alcohol that are going into a patient’s body each day.”
A version of this article originally appeared on Medscape.com.
“Drinking less is better,” says the guidance, which replaces Canada’s 2011 Low-Risk Drinking Guidelines (LRDGs).
Developed in consultation with an executive committee from federal, provincial, and territorial governments; national organizations; three scientific expert panels; and an internal evidence review working group, the guidance presents the following findings:
- Consuming no drinks per week has benefits, such as better health and better sleep, and it’s the only safe option during pregnancy.
- Consuming one or two standard drinks weekly will likely not have alcohol-related consequences.
- Three to six drinks raise the risk of developing breast, colon, and other cancers.
- Seven or more increase the risk of heart disease or stroke.
- Each additional drink “radically increases” the risk of these health consequences.
“Alcohol is more harmful than was previously thought and is a key component of the health of your patients,” Adam Sherk, PhD, a scientist at the Canadian Institute for Substance Use Research at the University of Victoria (B.C.), and a member of the scientific expert panel that contributed to the guidance, said in an interview. “Display and discuss the new guidance with your patients with the main message that drinking less is better.”
Peter Butt, MD, a clinical associate professor at the University of Saskatchewan, Saskatoon, and cochair of the guidance project, said in an interview: “The World Health Organization has identified over 200 ICD-coded conditions associated with alcohol use. This creates many opportunities to inquire into quantity and frequency of alcohol use, relate it to the patient’s health and well-being, and provide advice on reduction.”
“Canada’s Guidance on Alcohol and Health: Final Report” and a related infographic were published online Jan. 17.
Continuum of risk
The impetus for the new guidance came from the fact that “our 2011 LRDGs were no longer current, and there was emerging evidence that people drinking within those levels were coming to harm,” said Dr. Butt.
That evidence indicates that alcohol causes at least seven types of cancer, mostly of the breast or colon; is a risk factor for most types of heart disease; and is a main cause of liver disease. Evidence also indicates that avoiding drinking to the point of intoxication will reduce people’s risk of perpetrating alcohol-related violence.
Responding to the need to accurately quantify the risk, the guidance defines a “standard” drink as 12 oz of beer, cooler, or cider (5% alcohol); 5 oz of wine (12% alcohol); and 1.5 oz of spirits such as whiskey, vodka, or gin (40% alcohol).
Using different mortality risk thresholds, the project’s experts developed the following continuum of risk:
- Low for individuals who consume two standard drinks or fewer per week
- Moderate for those who consume from three to six standard drinks per week
- Increasingly high for those who consume seven standard drinks or more per week
The guidance makes the following observations:
- Consuming more than two standard drinks per drinking occasion is associated with an increased risk of harms to self and others, including injuries and violence.
- When pregnant or trying to get pregnant, no amount of alcohol is safe.
- When breastfeeding, not drinking is safest.
- Above the upper limit of the moderate risk zone, health risks increase more steeply for females than males.
- Far more injuries, violence, and deaths result from men’s alcohol use, especially for per occasion drinking, than from women’s alcohol use.
- Young people should delay alcohol use for as long as possible.
- Individuals should not start to use alcohol or increase their alcohol use for health benefits.
- Any reduction in alcohol use is beneficial.
Other national guidelines
“Countries that haven’t updated their alcohol use guidelines recently should do so, as the evidence regarding alcohol and health has advanced considerably in the past 10 years,” said Dr. Sherk. He acknowledged that “any time health guidance changes substantially, it’s reasonable to expect a period of readjustment.”
“Some will be resistant,” Dr. Butt agreed. “Some professionals will need more education than others on the health effects of alcohol. Some patients will also be more invested in drinking than others. The harm-reduction, risk-zone approach should assist in the process of engaging patients and helping them reduce over time.
“Just as we benefited from the updates done in the United Kingdom, France, and especially Australia, so also researchers elsewhere will critique our work and our approach and make their own decisions on how best to communicate with their public,” Dr. Butt said. He noted that Canada’s contributions regarding the association between alcohol and violence, as well as their sex/gender approach to the evidence, “may influence the next country’s review.”
Commenting on whether the United States should consider changing its guidance, Timothy Brennan, MD, MPH, chief of clinical services for the Addiction Institute of Mount Sinai Health System in New York, said in an interview, “A lot of people will be surprised at the recommended limits on alcohol. Most think that they can have one or two glasses of alcohol per day and not have any increased risk to their health. I think the Canadians deserve credit for putting themselves out there.”
Dr. Brennan said there will “certainly be pushback by the drinking lobby, which is very strong both in the U.S. and in Canada.” In fact, the national trade group Beer Canada was recently quoted as stating that it still supports the 2011 guidelines and that the updating process lacked full transparency and expert technical peer review.
Nevertheless, Dr. Brennan said, “it’s overwhelmingly clear that alcohol affects a ton of different parts of our body, so limiting the amount of alcohol we take in is always going to be a good thing. The Canadian graphic is great because it color-codes the risk. I recommend that clinicians put it up in their offices and begin quantifying the units of alcohol that are going into a patient’s body each day.”
A version of this article originally appeared on Medscape.com.
“Drinking less is better,” says the guidance, which replaces Canada’s 2011 Low-Risk Drinking Guidelines (LRDGs).
Developed in consultation with an executive committee from federal, provincial, and territorial governments; national organizations; three scientific expert panels; and an internal evidence review working group, the guidance presents the following findings:
- Consuming no drinks per week has benefits, such as better health and better sleep, and it’s the only safe option during pregnancy.
- Consuming one or two standard drinks weekly will likely not have alcohol-related consequences.
- Three to six drinks raise the risk of developing breast, colon, and other cancers.
- Seven or more increase the risk of heart disease or stroke.
- Each additional drink “radically increases” the risk of these health consequences.
“Alcohol is more harmful than was previously thought and is a key component of the health of your patients,” Adam Sherk, PhD, a scientist at the Canadian Institute for Substance Use Research at the University of Victoria (B.C.), and a member of the scientific expert panel that contributed to the guidance, said in an interview. “Display and discuss the new guidance with your patients with the main message that drinking less is better.”
Peter Butt, MD, a clinical associate professor at the University of Saskatchewan, Saskatoon, and cochair of the guidance project, said in an interview: “The World Health Organization has identified over 200 ICD-coded conditions associated with alcohol use. This creates many opportunities to inquire into quantity and frequency of alcohol use, relate it to the patient’s health and well-being, and provide advice on reduction.”
“Canada’s Guidance on Alcohol and Health: Final Report” and a related infographic were published online Jan. 17.
Continuum of risk
The impetus for the new guidance came from the fact that “our 2011 LRDGs were no longer current, and there was emerging evidence that people drinking within those levels were coming to harm,” said Dr. Butt.
That evidence indicates that alcohol causes at least seven types of cancer, mostly of the breast or colon; is a risk factor for most types of heart disease; and is a main cause of liver disease. Evidence also indicates that avoiding drinking to the point of intoxication will reduce people’s risk of perpetrating alcohol-related violence.
Responding to the need to accurately quantify the risk, the guidance defines a “standard” drink as 12 oz of beer, cooler, or cider (5% alcohol); 5 oz of wine (12% alcohol); and 1.5 oz of spirits such as whiskey, vodka, or gin (40% alcohol).
Using different mortality risk thresholds, the project’s experts developed the following continuum of risk:
- Low for individuals who consume two standard drinks or fewer per week
- Moderate for those who consume from three to six standard drinks per week
- Increasingly high for those who consume seven standard drinks or more per week
The guidance makes the following observations:
- Consuming more than two standard drinks per drinking occasion is associated with an increased risk of harms to self and others, including injuries and violence.
- When pregnant or trying to get pregnant, no amount of alcohol is safe.
- When breastfeeding, not drinking is safest.
- Above the upper limit of the moderate risk zone, health risks increase more steeply for females than males.
- Far more injuries, violence, and deaths result from men’s alcohol use, especially for per occasion drinking, than from women’s alcohol use.
- Young people should delay alcohol use for as long as possible.
- Individuals should not start to use alcohol or increase their alcohol use for health benefits.
- Any reduction in alcohol use is beneficial.
Other national guidelines
“Countries that haven’t updated their alcohol use guidelines recently should do so, as the evidence regarding alcohol and health has advanced considerably in the past 10 years,” said Dr. Sherk. He acknowledged that “any time health guidance changes substantially, it’s reasonable to expect a period of readjustment.”
“Some will be resistant,” Dr. Butt agreed. “Some professionals will need more education than others on the health effects of alcohol. Some patients will also be more invested in drinking than others. The harm-reduction, risk-zone approach should assist in the process of engaging patients and helping them reduce over time.
“Just as we benefited from the updates done in the United Kingdom, France, and especially Australia, so also researchers elsewhere will critique our work and our approach and make their own decisions on how best to communicate with their public,” Dr. Butt said. He noted that Canada’s contributions regarding the association between alcohol and violence, as well as their sex/gender approach to the evidence, “may influence the next country’s review.”
Commenting on whether the United States should consider changing its guidance, Timothy Brennan, MD, MPH, chief of clinical services for the Addiction Institute of Mount Sinai Health System in New York, said in an interview, “A lot of people will be surprised at the recommended limits on alcohol. Most think that they can have one or two glasses of alcohol per day and not have any increased risk to their health. I think the Canadians deserve credit for putting themselves out there.”
Dr. Brennan said there will “certainly be pushback by the drinking lobby, which is very strong both in the U.S. and in Canada.” In fact, the national trade group Beer Canada was recently quoted as stating that it still supports the 2011 guidelines and that the updating process lacked full transparency and expert technical peer review.
Nevertheless, Dr. Brennan said, “it’s overwhelmingly clear that alcohol affects a ton of different parts of our body, so limiting the amount of alcohol we take in is always going to be a good thing. The Canadian graphic is great because it color-codes the risk. I recommend that clinicians put it up in their offices and begin quantifying the units of alcohol that are going into a patient’s body each day.”
A version of this article originally appeared on Medscape.com.
Kids with concussions may benefit from early return to school
The timing for return to school after a concussion has been the subject of guidelines, but data on how the timing of school returns affects later symptom burdens are limited, Christopher G. Vaughan, PhD, of Children’s National Hospital, Rockville, Md., and colleagues wrote.
Examining how the timing of return to school (RTS) affects later symptoms is needed to inform early postinjury management, they said.
In the new study published in JAMA Network Open, the researchers identified 1,630 children and teens aged 5-18 years who were treated for concussions at nine Canadian pediatric EDs. The primary outcome was symptom burden at 14 days post concussion, based on the Post-Concussion Symptom Inventory (PCSI). Early RTS was defined as missing fewer than 3 days of school post concussion.
Overall, the mean number of missed school days was 3.74 (excluding weekends). When divided by age, the mean number of missed days was 2.61 for children aged 5-7 years, 3.26 for those aged 8-12 years, and 4.71 for those aged 13-18 years.
Slightly more than half (53.7%) of the participants had an early RTS of 2 missed days or fewer. Later RTS was most common in the oldest age group, followed by the middle and younger age groups.
The researchers used a propensity score–matched analysis to determine associations. At 14 days, an early RTS was associated with reduced symptoms among 8- to 12-year-olds and 13- to 18-year-olds, though not in the youngest patients aged 5-7 years. In addition, the researchers created quantiles based on initial symptom ratings.
For the youngest age group, the association between early RTS and reduced symptoms at day 14 was higher among those with lower initial symptoms.
For the two older groups, the association was higher for those with higher initial symptoms (based on the PCSI).
The findings that earlier RTS was associated with a lower symptom burden at day 14 for those with higher levels of symptoms at baseline was surprising, but the mechanisms of the timing and effect of RTS requires more study, the researchers wrote in their discussion.
The effect of early RTS on symptoms may be in part related to factors such as “the benefits of socialization, reduced stress from not missing too much school, maintaining or returning to a normal sleep-wake schedule, and returning to light to moderate physical activity (gym class and recreational activities),” the researchers noted.
Another study related to recovery and concussion recently appeared in Neurology. In that study, the authors found that those athletes who took a longer time to recover from a sports-related concussion could still return to play with additional time off, but the methods and populations differed from the current study, which focused on RTS rather than returning to play.
The current study findings were limited by several factors including the lack of randomization for RTS timing and a lack of data on the variety of potential supports and accommodations students received, the researchers noted.
However, the results were strengthened by the large size and diverse nature of the concussions, and the roughly equal representation of boys and girls, they said.
Although randomized trials are needed to determine the best timing for RTS, the current study suggests that RTS within 2 days of a concussion is associated with improved symptoms, “and may directly or indirectly promote faster recovery,” they concluded.
Early return remains feasible for most children and teens
“Return to school can be a complicated issue for children and teens with concussions,” said Caitlyn Mooney, MD, a pediatrician and specialist in sports medicine at the University of Texas Health Science Center, San Antonio, said in an interview. Although much research has focused on diagnosis and return to sport after a concussion, there has been less focus on returning to school and learning. Various issues post concussion can make schooling difficult, and students may experience trouble with vision, concentration, sleep, headaches, and more.
Despite this knowledge, studies that specifically address recommended school protocols are limited, Dr. Mooney said. “Additionally, all concussions are different; while some students will need minimal help to return and succeed in school, others may need individualized learning plans and accommodations for school.” A return to school ideally would be a team-based approach with input from the parent, patient, physician, and educators.
“The theory of cognitive rest stems from the idea that a concussion causes metabolic dysfunction in the brain, and that increasing the metabolic demands of the brain can result in symptoms and a delayed return to school,” said Dr. Mooney.
Evidence suggests that those who start resting early after a concussion improve more quickly, “but there has been ongoing discussion over the years of what is the correct balance of cognitive rest to returning to modified activity,” she said. “This has led to the current general recommendation of rest for 24-48 hours followed by a gradual return to school as tolerated.”
Although the current study is large, it is limited by the lack of randomization, Dr. Mooney noted, therefore conclusions cannot be made that the cause of the improved symptoms is a quicker return to school.
However, the results support data from previous studies, in that both of the older age groups showed less disease burden at 14 days after an earlier return to school, she said.
“With prolonged absences, adolescents get isolated at home away from friends, and they may have increased mood symptoms. Additionally, I have found a high number of my patients who do not go to school as quickly have more sleep disturbance, which seems to increase symptoms such as difficulty concentrating or headaches,” she said. “It seems like the students do benefit from a routine schedule even if they have to have some accommodations at school, especially older students who may have more stress about missing school and falling behind on schoolwork.”
The message for pediatricians is that return to school should be individualized, Dr. Mooney said.
Although the current study does not dictate the optimal return to school, the results support those of previous studies in showing that, after 1-2 days of rest, an early return does not harm children and teens and may improve symptoms in many cases, she said. “In my experience, sometimes schools find it easier to keep the student at home rather than manage rest or special accommodations,” but the current study suggests that delaying return to school may not be the right choice for many patients.
“I hope this study empowers clinicians to advocate for these students, that the right place for them is in the classroom even with rest, extra time, or other accommodations,” said Dr. Mooney.
“Each concussion should be evaluated and treated individually; there will likely be a few who may need to stay home for a longer period of time, but this study suggests that the majority of students will suffer no ill effects from returning to the normal routine after a 2-day rest,” she noted.
The study was supported by the Canadian Institutes for Health Research. Dr. Vaughan and several coauthors disclosed being authors of the Postconcussion Symptom Inventory outside of the current study. Dr. Mooney had no financial conflicts to disclose.
The timing for return to school after a concussion has been the subject of guidelines, but data on how the timing of school returns affects later symptom burdens are limited, Christopher G. Vaughan, PhD, of Children’s National Hospital, Rockville, Md., and colleagues wrote.
Examining how the timing of return to school (RTS) affects later symptoms is needed to inform early postinjury management, they said.
In the new study published in JAMA Network Open, the researchers identified 1,630 children and teens aged 5-18 years who were treated for concussions at nine Canadian pediatric EDs. The primary outcome was symptom burden at 14 days post concussion, based on the Post-Concussion Symptom Inventory (PCSI). Early RTS was defined as missing fewer than 3 days of school post concussion.
Overall, the mean number of missed school days was 3.74 (excluding weekends). When divided by age, the mean number of missed days was 2.61 for children aged 5-7 years, 3.26 for those aged 8-12 years, and 4.71 for those aged 13-18 years.
Slightly more than half (53.7%) of the participants had an early RTS of 2 missed days or fewer. Later RTS was most common in the oldest age group, followed by the middle and younger age groups.
The researchers used a propensity score–matched analysis to determine associations. At 14 days, an early RTS was associated with reduced symptoms among 8- to 12-year-olds and 13- to 18-year-olds, though not in the youngest patients aged 5-7 years. In addition, the researchers created quantiles based on initial symptom ratings.
For the youngest age group, the association between early RTS and reduced symptoms at day 14 was higher among those with lower initial symptoms.
For the two older groups, the association was higher for those with higher initial symptoms (based on the PCSI).
The findings that earlier RTS was associated with a lower symptom burden at day 14 for those with higher levels of symptoms at baseline was surprising, but the mechanisms of the timing and effect of RTS requires more study, the researchers wrote in their discussion.
The effect of early RTS on symptoms may be in part related to factors such as “the benefits of socialization, reduced stress from not missing too much school, maintaining or returning to a normal sleep-wake schedule, and returning to light to moderate physical activity (gym class and recreational activities),” the researchers noted.
Another study related to recovery and concussion recently appeared in Neurology. In that study, the authors found that those athletes who took a longer time to recover from a sports-related concussion could still return to play with additional time off, but the methods and populations differed from the current study, which focused on RTS rather than returning to play.
The current study findings were limited by several factors including the lack of randomization for RTS timing and a lack of data on the variety of potential supports and accommodations students received, the researchers noted.
However, the results were strengthened by the large size and diverse nature of the concussions, and the roughly equal representation of boys and girls, they said.
Although randomized trials are needed to determine the best timing for RTS, the current study suggests that RTS within 2 days of a concussion is associated with improved symptoms, “and may directly or indirectly promote faster recovery,” they concluded.
Early return remains feasible for most children and teens
“Return to school can be a complicated issue for children and teens with concussions,” said Caitlyn Mooney, MD, a pediatrician and specialist in sports medicine at the University of Texas Health Science Center, San Antonio, said in an interview. Although much research has focused on diagnosis and return to sport after a concussion, there has been less focus on returning to school and learning. Various issues post concussion can make schooling difficult, and students may experience trouble with vision, concentration, sleep, headaches, and more.
Despite this knowledge, studies that specifically address recommended school protocols are limited, Dr. Mooney said. “Additionally, all concussions are different; while some students will need minimal help to return and succeed in school, others may need individualized learning plans and accommodations for school.” A return to school ideally would be a team-based approach with input from the parent, patient, physician, and educators.
“The theory of cognitive rest stems from the idea that a concussion causes metabolic dysfunction in the brain, and that increasing the metabolic demands of the brain can result in symptoms and a delayed return to school,” said Dr. Mooney.
Evidence suggests that those who start resting early after a concussion improve more quickly, “but there has been ongoing discussion over the years of what is the correct balance of cognitive rest to returning to modified activity,” she said. “This has led to the current general recommendation of rest for 24-48 hours followed by a gradual return to school as tolerated.”
Although the current study is large, it is limited by the lack of randomization, Dr. Mooney noted, therefore conclusions cannot be made that the cause of the improved symptoms is a quicker return to school.
However, the results support data from previous studies, in that both of the older age groups showed less disease burden at 14 days after an earlier return to school, she said.
“With prolonged absences, adolescents get isolated at home away from friends, and they may have increased mood symptoms. Additionally, I have found a high number of my patients who do not go to school as quickly have more sleep disturbance, which seems to increase symptoms such as difficulty concentrating or headaches,” she said. “It seems like the students do benefit from a routine schedule even if they have to have some accommodations at school, especially older students who may have more stress about missing school and falling behind on schoolwork.”
The message for pediatricians is that return to school should be individualized, Dr. Mooney said.
Although the current study does not dictate the optimal return to school, the results support those of previous studies in showing that, after 1-2 days of rest, an early return does not harm children and teens and may improve symptoms in many cases, she said. “In my experience, sometimes schools find it easier to keep the student at home rather than manage rest or special accommodations,” but the current study suggests that delaying return to school may not be the right choice for many patients.
“I hope this study empowers clinicians to advocate for these students, that the right place for them is in the classroom even with rest, extra time, or other accommodations,” said Dr. Mooney.
“Each concussion should be evaluated and treated individually; there will likely be a few who may need to stay home for a longer period of time, but this study suggests that the majority of students will suffer no ill effects from returning to the normal routine after a 2-day rest,” she noted.
The study was supported by the Canadian Institutes for Health Research. Dr. Vaughan and several coauthors disclosed being authors of the Postconcussion Symptom Inventory outside of the current study. Dr. Mooney had no financial conflicts to disclose.
The timing for return to school after a concussion has been the subject of guidelines, but data on how the timing of school returns affects later symptom burdens are limited, Christopher G. Vaughan, PhD, of Children’s National Hospital, Rockville, Md., and colleagues wrote.
Examining how the timing of return to school (RTS) affects later symptoms is needed to inform early postinjury management, they said.
In the new study published in JAMA Network Open, the researchers identified 1,630 children and teens aged 5-18 years who were treated for concussions at nine Canadian pediatric EDs. The primary outcome was symptom burden at 14 days post concussion, based on the Post-Concussion Symptom Inventory (PCSI). Early RTS was defined as missing fewer than 3 days of school post concussion.
Overall, the mean number of missed school days was 3.74 (excluding weekends). When divided by age, the mean number of missed days was 2.61 for children aged 5-7 years, 3.26 for those aged 8-12 years, and 4.71 for those aged 13-18 years.
Slightly more than half (53.7%) of the participants had an early RTS of 2 missed days or fewer. Later RTS was most common in the oldest age group, followed by the middle and younger age groups.
The researchers used a propensity score–matched analysis to determine associations. At 14 days, an early RTS was associated with reduced symptoms among 8- to 12-year-olds and 13- to 18-year-olds, though not in the youngest patients aged 5-7 years. In addition, the researchers created quantiles based on initial symptom ratings.
For the youngest age group, the association between early RTS and reduced symptoms at day 14 was higher among those with lower initial symptoms.
For the two older groups, the association was higher for those with higher initial symptoms (based on the PCSI).
The findings that earlier RTS was associated with a lower symptom burden at day 14 for those with higher levels of symptoms at baseline was surprising, but the mechanisms of the timing and effect of RTS requires more study, the researchers wrote in their discussion.
The effect of early RTS on symptoms may be in part related to factors such as “the benefits of socialization, reduced stress from not missing too much school, maintaining or returning to a normal sleep-wake schedule, and returning to light to moderate physical activity (gym class and recreational activities),” the researchers noted.
Another study related to recovery and concussion recently appeared in Neurology. In that study, the authors found that those athletes who took a longer time to recover from a sports-related concussion could still return to play with additional time off, but the methods and populations differed from the current study, which focused on RTS rather than returning to play.
The current study findings were limited by several factors including the lack of randomization for RTS timing and a lack of data on the variety of potential supports and accommodations students received, the researchers noted.
However, the results were strengthened by the large size and diverse nature of the concussions, and the roughly equal representation of boys and girls, they said.
Although randomized trials are needed to determine the best timing for RTS, the current study suggests that RTS within 2 days of a concussion is associated with improved symptoms, “and may directly or indirectly promote faster recovery,” they concluded.
Early return remains feasible for most children and teens
“Return to school can be a complicated issue for children and teens with concussions,” said Caitlyn Mooney, MD, a pediatrician and specialist in sports medicine at the University of Texas Health Science Center, San Antonio, said in an interview. Although much research has focused on diagnosis and return to sport after a concussion, there has been less focus on returning to school and learning. Various issues post concussion can make schooling difficult, and students may experience trouble with vision, concentration, sleep, headaches, and more.
Despite this knowledge, studies that specifically address recommended school protocols are limited, Dr. Mooney said. “Additionally, all concussions are different; while some students will need minimal help to return and succeed in school, others may need individualized learning plans and accommodations for school.” A return to school ideally would be a team-based approach with input from the parent, patient, physician, and educators.
“The theory of cognitive rest stems from the idea that a concussion causes metabolic dysfunction in the brain, and that increasing the metabolic demands of the brain can result in symptoms and a delayed return to school,” said Dr. Mooney.
Evidence suggests that those who start resting early after a concussion improve more quickly, “but there has been ongoing discussion over the years of what is the correct balance of cognitive rest to returning to modified activity,” she said. “This has led to the current general recommendation of rest for 24-48 hours followed by a gradual return to school as tolerated.”
Although the current study is large, it is limited by the lack of randomization, Dr. Mooney noted, therefore conclusions cannot be made that the cause of the improved symptoms is a quicker return to school.
However, the results support data from previous studies, in that both of the older age groups showed less disease burden at 14 days after an earlier return to school, she said.
“With prolonged absences, adolescents get isolated at home away from friends, and they may have increased mood symptoms. Additionally, I have found a high number of my patients who do not go to school as quickly have more sleep disturbance, which seems to increase symptoms such as difficulty concentrating or headaches,” she said. “It seems like the students do benefit from a routine schedule even if they have to have some accommodations at school, especially older students who may have more stress about missing school and falling behind on schoolwork.”
The message for pediatricians is that return to school should be individualized, Dr. Mooney said.
Although the current study does not dictate the optimal return to school, the results support those of previous studies in showing that, after 1-2 days of rest, an early return does not harm children and teens and may improve symptoms in many cases, she said. “In my experience, sometimes schools find it easier to keep the student at home rather than manage rest or special accommodations,” but the current study suggests that delaying return to school may not be the right choice for many patients.
“I hope this study empowers clinicians to advocate for these students, that the right place for them is in the classroom even with rest, extra time, or other accommodations,” said Dr. Mooney.
“Each concussion should be evaluated and treated individually; there will likely be a few who may need to stay home for a longer period of time, but this study suggests that the majority of students will suffer no ill effects from returning to the normal routine after a 2-day rest,” she noted.
The study was supported by the Canadian Institutes for Health Research. Dr. Vaughan and several coauthors disclosed being authors of the Postconcussion Symptom Inventory outside of the current study. Dr. Mooney had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN
AD outcomes improved with lebrikizumab and topical steroids
, according to results of the 16-week phase 3 ADhere trial.
“Lebrikizumab, a monoclonal antibody inhibiting interleukin-13, combined with TCS was associated with reduced overall disease severity of moderate to severe AD in adolescents and adults, and had a safety profile consistent with previous lebrikizumab AD studies,” noted lead author Eric L. Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland, and coauthors in their article on the study, which was published in JAMA Dermatology.
The double-blind trial, conducted at 54 sites across Germany, Poland, Canada, and the United States, included 211 patients, mean age 37.2 years, of whom 48.8% were female and roughly 22% were adolescents. Almost 15% were Asian, and about 13% were Black.
At baseline, participants had a score of 16 or higher on the Eczema Area and Severity Index (EASI), a score of 3 or higher on the Investigator’s Global Assessment (IGA) scale, AD covering a body surface area of 10% or greater, and a history of inadequate response to treatment with topical medications.
After a minimum 1-week washout period from topical and systemic therapy, participants were randomized in a 2:1 ratio to receive lebrikizumab plus TCS (n = 145) or placebo plus TCS (n = 66) for 16 weeks.
Lebrikizumab or placebo was administered by subcutaneous injection every 2 weeks; the loading and week-2 doses of lebrikizumab were 500 mg, followed by 250 mg thereafter. All patients were instructed to use low- to mid-potency TCS at their own discretion. Study sites provided a mid-potency TCS (triamcinolone acetonide 0.1% cream) and a low-potency TCS (hydrocortisone 1% cream), with topical calcineurin inhibitors permitted for sensitive skin areas.
Primary outcomes at 16 weeks included a 2-point or more reduction in IGA score from baseline and EASI-75 response. Patients in the lebrikizumab arm had superior responses on both of these outcomes, with statistical significance achieved as early as week 8 and week 4, respectively, and maintained through week 16. Specifically, 41.2% of those treated with lebrikizumab had an IGA reduction of 2 points or more, compared with 22.1% of those receiving placebo plus TCS (P = .01), and the proportion of patients achieving EASI-75 responses was 69.5% vs. 42.2%, respectively (P < .001).
Patients treated with lebrikizumab also showed statistically significant improvements, compared with TCS alone in all key secondary endpoints, “including skin clearance, improvement in itch, itch interference on sleep, and enhanced QoL [quality of life],” noted the authors. “This study captured the clinical benefit of lebrikizumab through the combined end point of physician-assessed clinical sign of skin clearance (EASI-75) and patient-reported outcome of improvement in itch (Pruritus NRS).”
The percentage of patients who achieved the combined endpoint was more than double for the lebrikizumab plus TCS group vs. the group on TCS alone, indicating that patients treated with lebrikizumab plus TCS “were more likely to experience improvement in skin symptoms and itch,” the investigators added.
The authors noted that most treatment-emergent adverse events “were nonserious, mild, or moderate in severity, and did not lead to study discontinuation.” These included conjunctivitis (4.8%), headache (4.8%), hypertension (2.8%), injection-site reactions (2.8%), and herpes infection (3.4%) – all of which occurred in 1.5% or less of patients in the placebo group.
“The higher incidence of conjunctivitis has also been reported in other biologics inhibiting IL [interleukin]–13 and/or IL-4 signaling, as well as lebrikizumab monotherapy studies,” they noted. The 4.8% rate of conjunctivitis reported in the combination study, they added, is “compared with 7.5% frequency in 16-week data from the lebrikizumab monotherapy studies. Although the mechanism remains unclear, it has been reported that conjunctival goblet cell scarcity due to IL-13 and IL-4 inhibition, and subsequent effects on the homeostasis of the conjunctival mucosal surface, results in ocular AEs [adverse events].”
“This truly is a time of great hope and promise for our patients with AD,” commented Zelma Chiesa Fuxench, MD, who was not involved in the study. “The advent of newer, targeted therapeutic agents for AD continues to revolutionize the treatment experience for our patients, offering the possibility of greater AD disease control with a favorable risk profile and less need for blood work monitoring compared to traditional systemic agents.”
On the basis of the study results, Dr. Chiesa Fuxench, of the department of dermatology at the University of Pennsylvania, Philadelphia, said in an interview that “lebrikizumab represents an additional option in the treatment armamentarium for providers who care for patients with AD.” She added that, “while head-to-head trials comparing lebrikizumab to dupilumab, the first FDA-approved biologic for AD, would be beneficial, to the best of my knowledge this data is currently lacking. However, based on the results of this study, we would expect lebrikizumab to work at least similarly to dupilumab, based on the reported improvements in IGA and EASI score.”
Additionally, lebrikizumab showed a favorable safety profile, “with most treatment-emergent adverse effects reported as nonserious and not leading to drug discontinuation,” she said. “Of interest to clinicians may be the reported rates of conjunctivitis in this study. Rates of conjunctivitis for lebrikizumab appear to be lower than those reported in the LIBERTY AD CHRONOS study for dupilumab – a finding that merits further scrutiny in my opinion, as this one of the most frequent treatment-emergent adverse events that I encounter in my clinical practice.”
The study was funded by Dermira, a subsidiary of Eli Lilly. Dr. Simpson reported personal fees and grants from multiple sources, including Dermira and Eli Lilly, the companies developing lebrikizumab. Several authors were employees of Eli Lilly. Dr. Fuxench disclosed serving as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, Pfizer, AbbVie, and Incyte, for which she has received honoraria for AD-related work. She is the recipient of research grants through Regeneron, Sanofi, Tioga, Vanda, Menlo Therapeutics, Leo Pharma, and Eli Lilly for work related to AD as well as honoraria for continuing medical education work related to AD sponsored through educational grants from Regeneron/Sanofi and Pfizer.
A version of this article first appeared on Medscape.com.
, according to results of the 16-week phase 3 ADhere trial.
“Lebrikizumab, a monoclonal antibody inhibiting interleukin-13, combined with TCS was associated with reduced overall disease severity of moderate to severe AD in adolescents and adults, and had a safety profile consistent with previous lebrikizumab AD studies,” noted lead author Eric L. Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland, and coauthors in their article on the study, which was published in JAMA Dermatology.
The double-blind trial, conducted at 54 sites across Germany, Poland, Canada, and the United States, included 211 patients, mean age 37.2 years, of whom 48.8% were female and roughly 22% were adolescents. Almost 15% were Asian, and about 13% were Black.
At baseline, participants had a score of 16 or higher on the Eczema Area and Severity Index (EASI), a score of 3 or higher on the Investigator’s Global Assessment (IGA) scale, AD covering a body surface area of 10% or greater, and a history of inadequate response to treatment with topical medications.
After a minimum 1-week washout period from topical and systemic therapy, participants were randomized in a 2:1 ratio to receive lebrikizumab plus TCS (n = 145) or placebo plus TCS (n = 66) for 16 weeks.
Lebrikizumab or placebo was administered by subcutaneous injection every 2 weeks; the loading and week-2 doses of lebrikizumab were 500 mg, followed by 250 mg thereafter. All patients were instructed to use low- to mid-potency TCS at their own discretion. Study sites provided a mid-potency TCS (triamcinolone acetonide 0.1% cream) and a low-potency TCS (hydrocortisone 1% cream), with topical calcineurin inhibitors permitted for sensitive skin areas.
Primary outcomes at 16 weeks included a 2-point or more reduction in IGA score from baseline and EASI-75 response. Patients in the lebrikizumab arm had superior responses on both of these outcomes, with statistical significance achieved as early as week 8 and week 4, respectively, and maintained through week 16. Specifically, 41.2% of those treated with lebrikizumab had an IGA reduction of 2 points or more, compared with 22.1% of those receiving placebo plus TCS (P = .01), and the proportion of patients achieving EASI-75 responses was 69.5% vs. 42.2%, respectively (P < .001).
Patients treated with lebrikizumab also showed statistically significant improvements, compared with TCS alone in all key secondary endpoints, “including skin clearance, improvement in itch, itch interference on sleep, and enhanced QoL [quality of life],” noted the authors. “This study captured the clinical benefit of lebrikizumab through the combined end point of physician-assessed clinical sign of skin clearance (EASI-75) and patient-reported outcome of improvement in itch (Pruritus NRS).”
The percentage of patients who achieved the combined endpoint was more than double for the lebrikizumab plus TCS group vs. the group on TCS alone, indicating that patients treated with lebrikizumab plus TCS “were more likely to experience improvement in skin symptoms and itch,” the investigators added.
The authors noted that most treatment-emergent adverse events “were nonserious, mild, or moderate in severity, and did not lead to study discontinuation.” These included conjunctivitis (4.8%), headache (4.8%), hypertension (2.8%), injection-site reactions (2.8%), and herpes infection (3.4%) – all of which occurred in 1.5% or less of patients in the placebo group.
“The higher incidence of conjunctivitis has also been reported in other biologics inhibiting IL [interleukin]–13 and/or IL-4 signaling, as well as lebrikizumab monotherapy studies,” they noted. The 4.8% rate of conjunctivitis reported in the combination study, they added, is “compared with 7.5% frequency in 16-week data from the lebrikizumab monotherapy studies. Although the mechanism remains unclear, it has been reported that conjunctival goblet cell scarcity due to IL-13 and IL-4 inhibition, and subsequent effects on the homeostasis of the conjunctival mucosal surface, results in ocular AEs [adverse events].”
“This truly is a time of great hope and promise for our patients with AD,” commented Zelma Chiesa Fuxench, MD, who was not involved in the study. “The advent of newer, targeted therapeutic agents for AD continues to revolutionize the treatment experience for our patients, offering the possibility of greater AD disease control with a favorable risk profile and less need for blood work monitoring compared to traditional systemic agents.”
On the basis of the study results, Dr. Chiesa Fuxench, of the department of dermatology at the University of Pennsylvania, Philadelphia, said in an interview that “lebrikizumab represents an additional option in the treatment armamentarium for providers who care for patients with AD.” She added that, “while head-to-head trials comparing lebrikizumab to dupilumab, the first FDA-approved biologic for AD, would be beneficial, to the best of my knowledge this data is currently lacking. However, based on the results of this study, we would expect lebrikizumab to work at least similarly to dupilumab, based on the reported improvements in IGA and EASI score.”
Additionally, lebrikizumab showed a favorable safety profile, “with most treatment-emergent adverse effects reported as nonserious and not leading to drug discontinuation,” she said. “Of interest to clinicians may be the reported rates of conjunctivitis in this study. Rates of conjunctivitis for lebrikizumab appear to be lower than those reported in the LIBERTY AD CHRONOS study for dupilumab – a finding that merits further scrutiny in my opinion, as this one of the most frequent treatment-emergent adverse events that I encounter in my clinical practice.”
The study was funded by Dermira, a subsidiary of Eli Lilly. Dr. Simpson reported personal fees and grants from multiple sources, including Dermira and Eli Lilly, the companies developing lebrikizumab. Several authors were employees of Eli Lilly. Dr. Fuxench disclosed serving as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, Pfizer, AbbVie, and Incyte, for which she has received honoraria for AD-related work. She is the recipient of research grants through Regeneron, Sanofi, Tioga, Vanda, Menlo Therapeutics, Leo Pharma, and Eli Lilly for work related to AD as well as honoraria for continuing medical education work related to AD sponsored through educational grants from Regeneron/Sanofi and Pfizer.
A version of this article first appeared on Medscape.com.
, according to results of the 16-week phase 3 ADhere trial.
“Lebrikizumab, a monoclonal antibody inhibiting interleukin-13, combined with TCS was associated with reduced overall disease severity of moderate to severe AD in adolescents and adults, and had a safety profile consistent with previous lebrikizumab AD studies,” noted lead author Eric L. Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland, and coauthors in their article on the study, which was published in JAMA Dermatology.
The double-blind trial, conducted at 54 sites across Germany, Poland, Canada, and the United States, included 211 patients, mean age 37.2 years, of whom 48.8% were female and roughly 22% were adolescents. Almost 15% were Asian, and about 13% were Black.
At baseline, participants had a score of 16 or higher on the Eczema Area and Severity Index (EASI), a score of 3 or higher on the Investigator’s Global Assessment (IGA) scale, AD covering a body surface area of 10% or greater, and a history of inadequate response to treatment with topical medications.
After a minimum 1-week washout period from topical and systemic therapy, participants were randomized in a 2:1 ratio to receive lebrikizumab plus TCS (n = 145) or placebo plus TCS (n = 66) for 16 weeks.
Lebrikizumab or placebo was administered by subcutaneous injection every 2 weeks; the loading and week-2 doses of lebrikizumab were 500 mg, followed by 250 mg thereafter. All patients were instructed to use low- to mid-potency TCS at their own discretion. Study sites provided a mid-potency TCS (triamcinolone acetonide 0.1% cream) and a low-potency TCS (hydrocortisone 1% cream), with topical calcineurin inhibitors permitted for sensitive skin areas.
Primary outcomes at 16 weeks included a 2-point or more reduction in IGA score from baseline and EASI-75 response. Patients in the lebrikizumab arm had superior responses on both of these outcomes, with statistical significance achieved as early as week 8 and week 4, respectively, and maintained through week 16. Specifically, 41.2% of those treated with lebrikizumab had an IGA reduction of 2 points or more, compared with 22.1% of those receiving placebo plus TCS (P = .01), and the proportion of patients achieving EASI-75 responses was 69.5% vs. 42.2%, respectively (P < .001).
Patients treated with lebrikizumab also showed statistically significant improvements, compared with TCS alone in all key secondary endpoints, “including skin clearance, improvement in itch, itch interference on sleep, and enhanced QoL [quality of life],” noted the authors. “This study captured the clinical benefit of lebrikizumab through the combined end point of physician-assessed clinical sign of skin clearance (EASI-75) and patient-reported outcome of improvement in itch (Pruritus NRS).”
The percentage of patients who achieved the combined endpoint was more than double for the lebrikizumab plus TCS group vs. the group on TCS alone, indicating that patients treated with lebrikizumab plus TCS “were more likely to experience improvement in skin symptoms and itch,” the investigators added.
The authors noted that most treatment-emergent adverse events “were nonserious, mild, or moderate in severity, and did not lead to study discontinuation.” These included conjunctivitis (4.8%), headache (4.8%), hypertension (2.8%), injection-site reactions (2.8%), and herpes infection (3.4%) – all of which occurred in 1.5% or less of patients in the placebo group.
“The higher incidence of conjunctivitis has also been reported in other biologics inhibiting IL [interleukin]–13 and/or IL-4 signaling, as well as lebrikizumab monotherapy studies,” they noted. The 4.8% rate of conjunctivitis reported in the combination study, they added, is “compared with 7.5% frequency in 16-week data from the lebrikizumab monotherapy studies. Although the mechanism remains unclear, it has been reported that conjunctival goblet cell scarcity due to IL-13 and IL-4 inhibition, and subsequent effects on the homeostasis of the conjunctival mucosal surface, results in ocular AEs [adverse events].”
“This truly is a time of great hope and promise for our patients with AD,” commented Zelma Chiesa Fuxench, MD, who was not involved in the study. “The advent of newer, targeted therapeutic agents for AD continues to revolutionize the treatment experience for our patients, offering the possibility of greater AD disease control with a favorable risk profile and less need for blood work monitoring compared to traditional systemic agents.”
On the basis of the study results, Dr. Chiesa Fuxench, of the department of dermatology at the University of Pennsylvania, Philadelphia, said in an interview that “lebrikizumab represents an additional option in the treatment armamentarium for providers who care for patients with AD.” She added that, “while head-to-head trials comparing lebrikizumab to dupilumab, the first FDA-approved biologic for AD, would be beneficial, to the best of my knowledge this data is currently lacking. However, based on the results of this study, we would expect lebrikizumab to work at least similarly to dupilumab, based on the reported improvements in IGA and EASI score.”
Additionally, lebrikizumab showed a favorable safety profile, “with most treatment-emergent adverse effects reported as nonserious and not leading to drug discontinuation,” she said. “Of interest to clinicians may be the reported rates of conjunctivitis in this study. Rates of conjunctivitis for lebrikizumab appear to be lower than those reported in the LIBERTY AD CHRONOS study for dupilumab – a finding that merits further scrutiny in my opinion, as this one of the most frequent treatment-emergent adverse events that I encounter in my clinical practice.”
The study was funded by Dermira, a subsidiary of Eli Lilly. Dr. Simpson reported personal fees and grants from multiple sources, including Dermira and Eli Lilly, the companies developing lebrikizumab. Several authors were employees of Eli Lilly. Dr. Fuxench disclosed serving as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, Pfizer, AbbVie, and Incyte, for which she has received honoraria for AD-related work. She is the recipient of research grants through Regeneron, Sanofi, Tioga, Vanda, Menlo Therapeutics, Leo Pharma, and Eli Lilly for work related to AD as well as honoraria for continuing medical education work related to AD sponsored through educational grants from Regeneron/Sanofi and Pfizer.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Mood disorder? Assessment in primary care
The assessment and diagnosis of bipolar disorder in youth has a complicated and controversial history. I recall from my child and adolescent fellowship training that there was a thinly veiled faculty argument about the diagnosis itself with strong opinions on each side. To revisit this quandary, I reviewed the most up-to-date literature and outlined a case-based approach to the initial screening assessment. Certainly, the assessment by a child and adolescent psychiatrist would be the standard for diagnosis, but we do know that the pediatrician’s office may be the first setting for a child and parent to present with mood symptoms and concerns about bipolar disorder. What can you do to address this adolescent, Carrie, and her mother’s concerns?
Case
Carrie is a 17-year-old girl who has struggled through her childhood and adolescence with anxious and depressive symptoms which have ebbed and flowed with major life stressors, including her parent’s divorce. She has tried cognitive-behavioral therapy and selective serotonin reuptake inhibitors, but the SSRI seemed to cause feelings of anxiousness and agitation, so she stopped it within weeks.
Her mother presents to you concerned that Carrie has had a more persistently irritable mood toward her, often just wanting to be with her friends or otherwise isolate in her room when home to study.
Most concerning to her mother is that Carrie, as a straight A student, has also developed a pattern of staying up all night to study for tests and then “crashes” and sleeps through the weekend, avoiding her mother and only brightening with her friends.
To complicate matters, Carrie’s biological father had type 1 bipolar disorder and an addiction. Her mother comes to you with an initially nonparticipatory Carrie in tow and says: “My former husband began his manic episodes with a lack of sleep and Carrie is so irritable towards me. I feel like I am walking on eggshells all the time. Could this be bipolar disorder?”
Case discussion
First, it’s always useful to frame a visit stating that you will spend some time with the patient and some time with both the patient and parent. Emphasizing confidentiality about issues such as drug use, which can be comorbid with mood symptoms and go undetected in high-achieving students such as Carrie, is also important. Further emphasizing that information will not be reflexively shared with the parent unless the child presents a danger to herself or others is also paramount to receive an honest report of symptoms.
Second, there are many signs and symptoms of bipolar disorder that naturally overlap with other conditions such as distractibility with attention-deficit/hyperactivity disorder, or irritability in either a unipolar depression or disruptive mood dysregulation disorder.1 You are looking for an episodic (not chronic) course of symptoms with episodes that last over 5 days for hypomania and over the course of weeks for mania all while meeting all the classic criteria for bipolar disorder.
Note that the broadening of diagnostic criteria has been thought to contribute to an inflated sense of prevalence. The actual expert estimate of prevalence is around 0.8%-1.8% in pediatric populations, although there is a large published range depending on whether the criteria are modified or not.2 Use of the unmodified criteria from the DSM-5 is the recommended approach. Bipolar disorder is exceedingly rare in prepubertal children, and it would be more common for prodromal symptoms such as Carrie’s to emerge and escalate over the teenage years, culminating in a clearer diagnosis in the later teens or 20s.3
In my screening questions, I find the idea of an “infatiguable state” is the most pathognomonic one in considering mania in bipolar disorder.4 Carrie’s “crashing” after nights of studying shows that she clearly fatigues. Patients with bipolar disorder within episodes of hypomania or mania have a seismic shift in perceived energy and a matching lack of ability to sleep that can affect their thought processes, speech, and decision-making. At first blush, Carrie’s history does not indicate current symptoms of bipolar disorder.3
Case, continued
When you meet with Carrie alone she shares that she has been experimenting with prescribed stimulants from her older college-aged brother in order to study and ace her tests. She is also experimenting with alcohol and marijuana with her friends. You provide her the CRAFFT tool to deepen your screening of this issue.5
With her mother, you administer the Parent General Behavior Inventory6 and the and the Child Mania Rating Scale7. From these scales, you note that the irritability is more specific to Carrie’s family than pan-present in school and with friends. Her lack of sleep occurs at high-pressure and discreet times.
At this point, you reassure Carrie and her mother that Carrie does not present with symptoms of bipolar disorder but that certainly you will continue screening assessments over time, as they are a good means to track symptoms. You also recommend that Carrie consider mood tracking so she can develop insights into her mood and its relationship to sleep and other events as she prepares for college.8
Case discussion, continued
The strongest risk factor for bipolar disorder in youth is family history (specifically a parent) with bipolar disorder).9 If there is the chance to explore the parent’s illness with open-ended questions, you will want to hear about the parent’s age of symptom onset, course of treatment, any hospitalizations, and stabilizing medications because this has prognostic power for your patient. It is important to ensure that the parent indeed has a diagnosis of bipolar disorder and that it is not just being used colloquially to characterize an adult who has labile moods from hour to hour or day to day. This would give undue anticipatory anxiety to a youth about their risk, which is up to 8- to 10-fold greater with a parent with bipolar disorder.9
Even with a strong family history, we do not often see bipolar disorder emerge in prepubertal children.10,11 There may be still concerning prodromal symptoms in which a diagnosis of unipolar depression with more irritable features and mood lability seems more commonly complicated by substance use, as with Carrie.
Activation with an SSRI, as in Carrie’s case, even if not resulting in full mania or hypomania, can also be a soft sign of the serotonergic sensitivity present in bipolar disorder. However, if there are not additional symptoms of bipolar disorder and you are concerned based on family history alone, you do not want to withhold antidepressant treatment because fear of risk. You would want to consider a “dose low and go slow” titration process with more frequent monitoring.
A diagnostic interview with a child and adolescent psychiatrist and administration of scales such as the Young Mania Rating Scale and the Modified Child Depression Rating Scale are the standard means to assess for bipolar symptoms.12 Considering the dearth of child psychiatrists nationally, it would be useful to improve one’s screening in primary care so as to not inadvertently “refer out” all patients for whom mood dysregulation is a concern.
There is also a more expanded tool that includes several scales integrated with clinical information (parent’s age of mood disorder onset, child’s age) which can culminate in a risk score.13
Lastly, I provide my patients with a handout of the Young Mania Rating Scale to take home as a reference and to complete before our next visit.14
You can repeat scales to monitor for more striking bipolar disorder signs and symptoms that emerge over the course of one’s longitudinal treatment of a pediatric patient. This can be an ongoing, episodic assessment since the emergence of bipolar disorder has been shown to range from the teenage years and beyond into the 20s and sometimes 30s.
Case, continued
Carrie presents to you again while in her first semester of college at the age of 19. She is taking a leave of absence after she began experimenting with cocaine at college and had a manic episode characterized by a lack of sleep without fatigue, persistent unabating energy, rapid and pressured speech, and ultimately, concern from her college friends. She was admitted to a psychiatric unit and stabilized on a second-generation antipsychotic, risperidone, which has solid evidence for mania, but she and you are now concerned about longer-term metabolic effects.15,16
You discuss monitoring her lipid profile and hemoglobin A1c, in addition to weight gain and waist circumference. She has connected with a therapist and psychiatrist through the college counseling center and hopes to return next semester with a fresh start and commitment to sobriety and social rhythms therapy known to be helpful for patients with bipolar disorder.17
While it is challenging to manage a chronic illness at her age, she feels hopeful that she can make better choices for her overall health with your support and the support of her family and mental health team.
Dr. Pawlowski is a child and adolescent consulting psychiatrist. She is a division chief at the University of Vermont Medical Center, Burlington, where she focuses on primary care mental health integration within primary care pediatrics, internal medicine, and family medicine.
References
1. Bipolar Disord. 2016 Jan 9 doi: 10.1111/bdi.12358.
2. Int J Bipolar Disord. 2021 Jun 25. doi: 10.1186/s40345-021-00225-5.
3. Am J Psychiatry. 2018 Dec 11. doi: 10.1176/appi.ajp.2018.18040461.
4. DSM-5 Changes: Implications for Child Serious Emotional Disturbance. Rockville, Md.: Substance Abuse and Mental Health Services Administration, 2016.
5. The CRAFFT tool.
6. General Behavior Inventory. Parent Version (P-GBI) Short Form – H/B (Revised Version, 2008).
7. Child Mania Rating Scale, Parent Version (CMRS-P).
8. https://www.moodtracker.com.
9. J Clin Psychiatry. 2000 Sep. doi: 10.4088/jcp.v61n0906.
10. Int J Bipolar Disord. 2020 Apr 20. doi: 10.1186/s40345-020-00185-2.
11. Int J Bipolar Disord. 2021 Jun 25. doi: 10.1186/s40345-021-00225-5.
12. Bipolar Disord. 2017 Sep 25. doi: 10.1111/bdi.12556.
13. www.cabsresearch.pitt.edu/bpriskcalculator/.
14. Parent Version of the Young Mania Rating Scale (PYMRS).
15. Arch Gen Psychiatry. 2012 Jan 2. doi: 10.1001/archgenpsychiatry.2011.1508.
16. The Carlat Child Psychiatry Report. “Bipolar Disorder” Newburyport, Mass.: Carlat Publishing, 2012.
17. https://www.ipsrt.org/.
The assessment and diagnosis of bipolar disorder in youth has a complicated and controversial history. I recall from my child and adolescent fellowship training that there was a thinly veiled faculty argument about the diagnosis itself with strong opinions on each side. To revisit this quandary, I reviewed the most up-to-date literature and outlined a case-based approach to the initial screening assessment. Certainly, the assessment by a child and adolescent psychiatrist would be the standard for diagnosis, but we do know that the pediatrician’s office may be the first setting for a child and parent to present with mood symptoms and concerns about bipolar disorder. What can you do to address this adolescent, Carrie, and her mother’s concerns?
Case
Carrie is a 17-year-old girl who has struggled through her childhood and adolescence with anxious and depressive symptoms which have ebbed and flowed with major life stressors, including her parent’s divorce. She has tried cognitive-behavioral therapy and selective serotonin reuptake inhibitors, but the SSRI seemed to cause feelings of anxiousness and agitation, so she stopped it within weeks.
Her mother presents to you concerned that Carrie has had a more persistently irritable mood toward her, often just wanting to be with her friends or otherwise isolate in her room when home to study.
Most concerning to her mother is that Carrie, as a straight A student, has also developed a pattern of staying up all night to study for tests and then “crashes” and sleeps through the weekend, avoiding her mother and only brightening with her friends.
To complicate matters, Carrie’s biological father had type 1 bipolar disorder and an addiction. Her mother comes to you with an initially nonparticipatory Carrie in tow and says: “My former husband began his manic episodes with a lack of sleep and Carrie is so irritable towards me. I feel like I am walking on eggshells all the time. Could this be bipolar disorder?”
Case discussion
First, it’s always useful to frame a visit stating that you will spend some time with the patient and some time with both the patient and parent. Emphasizing confidentiality about issues such as drug use, which can be comorbid with mood symptoms and go undetected in high-achieving students such as Carrie, is also important. Further emphasizing that information will not be reflexively shared with the parent unless the child presents a danger to herself or others is also paramount to receive an honest report of symptoms.
Second, there are many signs and symptoms of bipolar disorder that naturally overlap with other conditions such as distractibility with attention-deficit/hyperactivity disorder, or irritability in either a unipolar depression or disruptive mood dysregulation disorder.1 You are looking for an episodic (not chronic) course of symptoms with episodes that last over 5 days for hypomania and over the course of weeks for mania all while meeting all the classic criteria for bipolar disorder.
Note that the broadening of diagnostic criteria has been thought to contribute to an inflated sense of prevalence. The actual expert estimate of prevalence is around 0.8%-1.8% in pediatric populations, although there is a large published range depending on whether the criteria are modified or not.2 Use of the unmodified criteria from the DSM-5 is the recommended approach. Bipolar disorder is exceedingly rare in prepubertal children, and it would be more common for prodromal symptoms such as Carrie’s to emerge and escalate over the teenage years, culminating in a clearer diagnosis in the later teens or 20s.3
In my screening questions, I find the idea of an “infatiguable state” is the most pathognomonic one in considering mania in bipolar disorder.4 Carrie’s “crashing” after nights of studying shows that she clearly fatigues. Patients with bipolar disorder within episodes of hypomania or mania have a seismic shift in perceived energy and a matching lack of ability to sleep that can affect their thought processes, speech, and decision-making. At first blush, Carrie’s history does not indicate current symptoms of bipolar disorder.3
Case, continued
When you meet with Carrie alone she shares that she has been experimenting with prescribed stimulants from her older college-aged brother in order to study and ace her tests. She is also experimenting with alcohol and marijuana with her friends. You provide her the CRAFFT tool to deepen your screening of this issue.5
With her mother, you administer the Parent General Behavior Inventory6 and the and the Child Mania Rating Scale7. From these scales, you note that the irritability is more specific to Carrie’s family than pan-present in school and with friends. Her lack of sleep occurs at high-pressure and discreet times.
At this point, you reassure Carrie and her mother that Carrie does not present with symptoms of bipolar disorder but that certainly you will continue screening assessments over time, as they are a good means to track symptoms. You also recommend that Carrie consider mood tracking so she can develop insights into her mood and its relationship to sleep and other events as she prepares for college.8
Case discussion, continued
The strongest risk factor for bipolar disorder in youth is family history (specifically a parent) with bipolar disorder).9 If there is the chance to explore the parent’s illness with open-ended questions, you will want to hear about the parent’s age of symptom onset, course of treatment, any hospitalizations, and stabilizing medications because this has prognostic power for your patient. It is important to ensure that the parent indeed has a diagnosis of bipolar disorder and that it is not just being used colloquially to characterize an adult who has labile moods from hour to hour or day to day. This would give undue anticipatory anxiety to a youth about their risk, which is up to 8- to 10-fold greater with a parent with bipolar disorder.9
Even with a strong family history, we do not often see bipolar disorder emerge in prepubertal children.10,11 There may be still concerning prodromal symptoms in which a diagnosis of unipolar depression with more irritable features and mood lability seems more commonly complicated by substance use, as with Carrie.
Activation with an SSRI, as in Carrie’s case, even if not resulting in full mania or hypomania, can also be a soft sign of the serotonergic sensitivity present in bipolar disorder. However, if there are not additional symptoms of bipolar disorder and you are concerned based on family history alone, you do not want to withhold antidepressant treatment because fear of risk. You would want to consider a “dose low and go slow” titration process with more frequent monitoring.
A diagnostic interview with a child and adolescent psychiatrist and administration of scales such as the Young Mania Rating Scale and the Modified Child Depression Rating Scale are the standard means to assess for bipolar symptoms.12 Considering the dearth of child psychiatrists nationally, it would be useful to improve one’s screening in primary care so as to not inadvertently “refer out” all patients for whom mood dysregulation is a concern.
There is also a more expanded tool that includes several scales integrated with clinical information (parent’s age of mood disorder onset, child’s age) which can culminate in a risk score.13
Lastly, I provide my patients with a handout of the Young Mania Rating Scale to take home as a reference and to complete before our next visit.14
You can repeat scales to monitor for more striking bipolar disorder signs and symptoms that emerge over the course of one’s longitudinal treatment of a pediatric patient. This can be an ongoing, episodic assessment since the emergence of bipolar disorder has been shown to range from the teenage years and beyond into the 20s and sometimes 30s.
Case, continued
Carrie presents to you again while in her first semester of college at the age of 19. She is taking a leave of absence after she began experimenting with cocaine at college and had a manic episode characterized by a lack of sleep without fatigue, persistent unabating energy, rapid and pressured speech, and ultimately, concern from her college friends. She was admitted to a psychiatric unit and stabilized on a second-generation antipsychotic, risperidone, which has solid evidence for mania, but she and you are now concerned about longer-term metabolic effects.15,16
You discuss monitoring her lipid profile and hemoglobin A1c, in addition to weight gain and waist circumference. She has connected with a therapist and psychiatrist through the college counseling center and hopes to return next semester with a fresh start and commitment to sobriety and social rhythms therapy known to be helpful for patients with bipolar disorder.17
While it is challenging to manage a chronic illness at her age, she feels hopeful that she can make better choices for her overall health with your support and the support of her family and mental health team.
Dr. Pawlowski is a child and adolescent consulting psychiatrist. She is a division chief at the University of Vermont Medical Center, Burlington, where she focuses on primary care mental health integration within primary care pediatrics, internal medicine, and family medicine.
References
1. Bipolar Disord. 2016 Jan 9 doi: 10.1111/bdi.12358.
2. Int J Bipolar Disord. 2021 Jun 25. doi: 10.1186/s40345-021-00225-5.
3. Am J Psychiatry. 2018 Dec 11. doi: 10.1176/appi.ajp.2018.18040461.
4. DSM-5 Changes: Implications for Child Serious Emotional Disturbance. Rockville, Md.: Substance Abuse and Mental Health Services Administration, 2016.
5. The CRAFFT tool.
6. General Behavior Inventory. Parent Version (P-GBI) Short Form – H/B (Revised Version, 2008).
7. Child Mania Rating Scale, Parent Version (CMRS-P).
8. https://www.moodtracker.com.
9. J Clin Psychiatry. 2000 Sep. doi: 10.4088/jcp.v61n0906.
10. Int J Bipolar Disord. 2020 Apr 20. doi: 10.1186/s40345-020-00185-2.
11. Int J Bipolar Disord. 2021 Jun 25. doi: 10.1186/s40345-021-00225-5.
12. Bipolar Disord. 2017 Sep 25. doi: 10.1111/bdi.12556.
13. www.cabsresearch.pitt.edu/bpriskcalculator/.
14. Parent Version of the Young Mania Rating Scale (PYMRS).
15. Arch Gen Psychiatry. 2012 Jan 2. doi: 10.1001/archgenpsychiatry.2011.1508.
16. The Carlat Child Psychiatry Report. “Bipolar Disorder” Newburyport, Mass.: Carlat Publishing, 2012.
17. https://www.ipsrt.org/.
The assessment and diagnosis of bipolar disorder in youth has a complicated and controversial history. I recall from my child and adolescent fellowship training that there was a thinly veiled faculty argument about the diagnosis itself with strong opinions on each side. To revisit this quandary, I reviewed the most up-to-date literature and outlined a case-based approach to the initial screening assessment. Certainly, the assessment by a child and adolescent psychiatrist would be the standard for diagnosis, but we do know that the pediatrician’s office may be the first setting for a child and parent to present with mood symptoms and concerns about bipolar disorder. What can you do to address this adolescent, Carrie, and her mother’s concerns?
Case
Carrie is a 17-year-old girl who has struggled through her childhood and adolescence with anxious and depressive symptoms which have ebbed and flowed with major life stressors, including her parent’s divorce. She has tried cognitive-behavioral therapy and selective serotonin reuptake inhibitors, but the SSRI seemed to cause feelings of anxiousness and agitation, so she stopped it within weeks.
Her mother presents to you concerned that Carrie has had a more persistently irritable mood toward her, often just wanting to be with her friends or otherwise isolate in her room when home to study.
Most concerning to her mother is that Carrie, as a straight A student, has also developed a pattern of staying up all night to study for tests and then “crashes” and sleeps through the weekend, avoiding her mother and only brightening with her friends.
To complicate matters, Carrie’s biological father had type 1 bipolar disorder and an addiction. Her mother comes to you with an initially nonparticipatory Carrie in tow and says: “My former husband began his manic episodes with a lack of sleep and Carrie is so irritable towards me. I feel like I am walking on eggshells all the time. Could this be bipolar disorder?”
Case discussion
First, it’s always useful to frame a visit stating that you will spend some time with the patient and some time with both the patient and parent. Emphasizing confidentiality about issues such as drug use, which can be comorbid with mood symptoms and go undetected in high-achieving students such as Carrie, is also important. Further emphasizing that information will not be reflexively shared with the parent unless the child presents a danger to herself or others is also paramount to receive an honest report of symptoms.
Second, there are many signs and symptoms of bipolar disorder that naturally overlap with other conditions such as distractibility with attention-deficit/hyperactivity disorder, or irritability in either a unipolar depression or disruptive mood dysregulation disorder.1 You are looking for an episodic (not chronic) course of symptoms with episodes that last over 5 days for hypomania and over the course of weeks for mania all while meeting all the classic criteria for bipolar disorder.
Note that the broadening of diagnostic criteria has been thought to contribute to an inflated sense of prevalence. The actual expert estimate of prevalence is around 0.8%-1.8% in pediatric populations, although there is a large published range depending on whether the criteria are modified or not.2 Use of the unmodified criteria from the DSM-5 is the recommended approach. Bipolar disorder is exceedingly rare in prepubertal children, and it would be more common for prodromal symptoms such as Carrie’s to emerge and escalate over the teenage years, culminating in a clearer diagnosis in the later teens or 20s.3
In my screening questions, I find the idea of an “infatiguable state” is the most pathognomonic one in considering mania in bipolar disorder.4 Carrie’s “crashing” after nights of studying shows that she clearly fatigues. Patients with bipolar disorder within episodes of hypomania or mania have a seismic shift in perceived energy and a matching lack of ability to sleep that can affect their thought processes, speech, and decision-making. At first blush, Carrie’s history does not indicate current symptoms of bipolar disorder.3
Case, continued
When you meet with Carrie alone she shares that she has been experimenting with prescribed stimulants from her older college-aged brother in order to study and ace her tests. She is also experimenting with alcohol and marijuana with her friends. You provide her the CRAFFT tool to deepen your screening of this issue.5
With her mother, you administer the Parent General Behavior Inventory6 and the and the Child Mania Rating Scale7. From these scales, you note that the irritability is more specific to Carrie’s family than pan-present in school and with friends. Her lack of sleep occurs at high-pressure and discreet times.
At this point, you reassure Carrie and her mother that Carrie does not present with symptoms of bipolar disorder but that certainly you will continue screening assessments over time, as they are a good means to track symptoms. You also recommend that Carrie consider mood tracking so she can develop insights into her mood and its relationship to sleep and other events as she prepares for college.8
Case discussion, continued
The strongest risk factor for bipolar disorder in youth is family history (specifically a parent) with bipolar disorder).9 If there is the chance to explore the parent’s illness with open-ended questions, you will want to hear about the parent’s age of symptom onset, course of treatment, any hospitalizations, and stabilizing medications because this has prognostic power for your patient. It is important to ensure that the parent indeed has a diagnosis of bipolar disorder and that it is not just being used colloquially to characterize an adult who has labile moods from hour to hour or day to day. This would give undue anticipatory anxiety to a youth about their risk, which is up to 8- to 10-fold greater with a parent with bipolar disorder.9
Even with a strong family history, we do not often see bipolar disorder emerge in prepubertal children.10,11 There may be still concerning prodromal symptoms in which a diagnosis of unipolar depression with more irritable features and mood lability seems more commonly complicated by substance use, as with Carrie.
Activation with an SSRI, as in Carrie’s case, even if not resulting in full mania or hypomania, can also be a soft sign of the serotonergic sensitivity present in bipolar disorder. However, if there are not additional symptoms of bipolar disorder and you are concerned based on family history alone, you do not want to withhold antidepressant treatment because fear of risk. You would want to consider a “dose low and go slow” titration process with more frequent monitoring.
A diagnostic interview with a child and adolescent psychiatrist and administration of scales such as the Young Mania Rating Scale and the Modified Child Depression Rating Scale are the standard means to assess for bipolar symptoms.12 Considering the dearth of child psychiatrists nationally, it would be useful to improve one’s screening in primary care so as to not inadvertently “refer out” all patients for whom mood dysregulation is a concern.
There is also a more expanded tool that includes several scales integrated with clinical information (parent’s age of mood disorder onset, child’s age) which can culminate in a risk score.13
Lastly, I provide my patients with a handout of the Young Mania Rating Scale to take home as a reference and to complete before our next visit.14
You can repeat scales to monitor for more striking bipolar disorder signs and symptoms that emerge over the course of one’s longitudinal treatment of a pediatric patient. This can be an ongoing, episodic assessment since the emergence of bipolar disorder has been shown to range from the teenage years and beyond into the 20s and sometimes 30s.
Case, continued
Carrie presents to you again while in her first semester of college at the age of 19. She is taking a leave of absence after she began experimenting with cocaine at college and had a manic episode characterized by a lack of sleep without fatigue, persistent unabating energy, rapid and pressured speech, and ultimately, concern from her college friends. She was admitted to a psychiatric unit and stabilized on a second-generation antipsychotic, risperidone, which has solid evidence for mania, but she and you are now concerned about longer-term metabolic effects.15,16
You discuss monitoring her lipid profile and hemoglobin A1c, in addition to weight gain and waist circumference. She has connected with a therapist and psychiatrist through the college counseling center and hopes to return next semester with a fresh start and commitment to sobriety and social rhythms therapy known to be helpful for patients with bipolar disorder.17
While it is challenging to manage a chronic illness at her age, she feels hopeful that she can make better choices for her overall health with your support and the support of her family and mental health team.
Dr. Pawlowski is a child and adolescent consulting psychiatrist. She is a division chief at the University of Vermont Medical Center, Burlington, where she focuses on primary care mental health integration within primary care pediatrics, internal medicine, and family medicine.
References
1. Bipolar Disord. 2016 Jan 9 doi: 10.1111/bdi.12358.
2. Int J Bipolar Disord. 2021 Jun 25. doi: 10.1186/s40345-021-00225-5.
3. Am J Psychiatry. 2018 Dec 11. doi: 10.1176/appi.ajp.2018.18040461.
4. DSM-5 Changes: Implications for Child Serious Emotional Disturbance. Rockville, Md.: Substance Abuse and Mental Health Services Administration, 2016.
5. The CRAFFT tool.
6. General Behavior Inventory. Parent Version (P-GBI) Short Form – H/B (Revised Version, 2008).
7. Child Mania Rating Scale, Parent Version (CMRS-P).
8. https://www.moodtracker.com.
9. J Clin Psychiatry. 2000 Sep. doi: 10.4088/jcp.v61n0906.
10. Int J Bipolar Disord. 2020 Apr 20. doi: 10.1186/s40345-020-00185-2.
11. Int J Bipolar Disord. 2021 Jun 25. doi: 10.1186/s40345-021-00225-5.
12. Bipolar Disord. 2017 Sep 25. doi: 10.1111/bdi.12556.
13. www.cabsresearch.pitt.edu/bpriskcalculator/.
14. Parent Version of the Young Mania Rating Scale (PYMRS).
15. Arch Gen Psychiatry. 2012 Jan 2. doi: 10.1001/archgenpsychiatry.2011.1508.
16. The Carlat Child Psychiatry Report. “Bipolar Disorder” Newburyport, Mass.: Carlat Publishing, 2012.
17. https://www.ipsrt.org/.
The pediatrician’s office may be the first setting for a child to present with mood symptoms.
Powering down cellphone use in middle schools
As vice principal of Pennsville Middle School in New Jersey, Adam J. Slusher knows he’s not always going to be Mr. Popularity.
Part of a vice principal’s job includes scheduling, enforcing policy, and discipline, so Dr. Slusher – who holds a doctorate in education from Wilmington University in Delaware – sometimes has to send emails or make phone calls that address unpleasant topics or unpopular new policies.
Or punishments.
But there was a much different reaction this past July, after he sent a message to the homes of Pennsville’s 450 students spanning grades 6 to 8. The email blast announced a new cellphone policy for the school. Starting in September, as he explained in the message – which also went out to the school’s 60 faculty and staff members – the use of cellphones by Pennsville students would be prohibited during school hours for any reason.
Phones, he emphasized, “are to be turned OFF” and stowed away in backpacks or handbags, not carried or tucked into back pockets.
The announcement of the new Away for the Day policy, which was decided upon by Dr. Slusher and Pennsville Principal Carolyn Carels, provoked a response different from those to his announcements on, say, test dates, emergency procedures, or new detention policies.
“It was one of the most popular emails I’ve ever sent,” chuckled Dr. Slusher, who has been an educator for 17 years. “We’ve gotten so many thanks from teachers for this.”
Ditto with the staff, who in conversations with Dr. Slusher and Ms. Carels, had reported on the rampant use of phones in the cafeteria and hallways – confirming what both of them had seen.
“They were telling us, ‘You’ve got to do something about the phones’ ” he recalled. “They were delighted that a clear policy was now going to be in place.”
The overwhelming majority of Pennsville parents have also supported the new policy, especially when presented with some of the sobering evidence about the extent of phone use among this population. One study Dr. Slusher cited in his email showed that the average middle school child is spending between 6 and 9 hours a day on screens.
“That’s like a full-time job,” he said.
The heavy cellphone use by kids – in school, out of school, anywhere and everywhere – was part of what prompted internal medicine doctor and filmmaker Delaney Ruston, MD, to create the “Away for the Day” initiative, which Pennsville has adopted.
She and collaborator Lisa Tabb were driven to do “Away for the Day” while working on Screenagers, their award-winning 2016 film examining the impact of social media, videos, and screen time on youngsters and their families that also offered tips for better navigating the digital world.
“Over 3 years of making the film, I was visiting schools all over the country,” Dr. Ruston said. “By the end, I was seeing devices all over the place, even in elementary schools. When I’d ask a student in the hall, ‘What’s the policy?’ they would shrug and say ‘I don’t know.’ When I got the same reaction from teachers – who in many cases were left to decide on their own, so that they had to be the bad guys – I realized there was a problem here.”
The result was what Dr. Ruston and Ms. Tabb describe on their website as a “movement,” designed to provide tools to parents, teachers, and administrators to help them make policies that put phones away during the school day.
The age of social centrality
As even a casual glance in the homeroom of every high school or college lecture hall will confirm, phone use is high in teenagers and young adults. But Dr. Ruston and Ms. Tabb decided to focus on middle schools.
“That’s the age where we know schools are facing the most challenges,” Dr. Ruston said. “This is also the age when social centrality becomes a major focus for youth. Thus, the pull to be on social media games, where their peers are, is incredibly enticing.”
A recent study in the journal JAMA Pediatrics found that middle schoolers who compulsively check social networks on their phones appear to have changes in areas of the brain linked to reward and punishment.
It was in middle schools, she concluded, “where effective policies on cellphones are most needed.”
As part of their research into the issue, she and ms. Tabb did a survey using email contacts collected by Dr. Ruston’s company, MyDoc Productions, during the making of the film, along with subscribers to her blog. In all, 1,200 parents – each of whom had at least one child in middle school at the time – were surveyed. The researchers found an interesting disconnect: Eighty-two percent of the parents surveyed did not want their children using phones in school. Yet 55% of middle schools allowed students to carry phones during the school day.
That survey was done in 2017. Since the COVID-19 pandemic, the use of cellphones by children, both in school and at home, has risen dramatically. A literature review of 46 studies, published in JAMA Pediatrics in November, found that average screen time among children and adolescents has increased by 52% – or 84 minutes a day – during the pandemic.
That trend has given many schools, including Pennsville, the drive to adopt an Away for the Day–type policy. As part of the program, Dr. Ruston’s website provides ammunition against the kinds of pushback they might expect to get. One of the most common is the idea that banning cellphone use among middle school children is a misguided, antitechnology measure.
“We’re not at all antitech,” Dr. Ruston asserts. Away for the Day, she explains, advocates the use of learning technologies in school that are monitored and supervised by teachers.
“The majority of students have access to learning devices in the school,” she said. “These have different kinds of blockers, making it harder for their kid to respond to their friend on TikTok when they’re supposed to be using technology for learning.”
Dr. Ruston estimates that about 10,000 middle schools are now using various pieces of the Away for the Day campaign, which includes videos, posters, fact sheets, and other materials. Other schools have adopted similar measures in the same spirit.
Predictable and calm? Not so much
When Katherine Holden was named principal of Oregon’s Talent Middle School in 2022, one of the first things she wanted to do was create some structure for the routines of students (and parents) who were frazzled after 2 years of remote learning, staggered schedules, and mask mandates.
“Predictable and calm,” she said, with a laugh. “I use those words every day.”
Achieving both is hard enough in a middle school without a pandemic – not to mention an epidemic of cellphone use. (Talent also endured a massive fire in 2020 that left many families homeless.)
For this school year, Ms. Holden is using a new and clearly articulated policy: “Devices are put away from the first bell to the last bell,” she said. “We want them to have a focus on other things. We want them to be socializing, interacting with their peers face to face, thinking about getting to class. We want them making eye contact, asking questions. Learning how to make a friend face to face. Those are important developmental social skills they should be practicing.”
Instead of scrolling through photos on Instagram, watching trending videos on TikTok, or texting their friends.
Like Dr. Slusher, she announced the new cellphone policy last summer, in a letter sent home to parents along with the list of school supplies their children would need.
“Students are welcome to use their cell phones and personal devices before entering the building prior to 8:30 a.m. and after exiting the school building at 3:10 p.m.,” she wrote. “However, during the school day students’ cellphones and personal devices need to be off and out of sight.
“I think parents generally understand the need for this,” Ms. Holden said. “They’ve watched their children getting distracted at home by these devices, so they have a sense of how a cellphone adds a layer of challenge to learning. And parents are aware of the unkind behavior that often happens online.”
As for the kids themselves? Safe to say the excitement that Dr. Slusher’s email got from Pennsville faculty, staff, and parents didn’t extend to students.
“They don’t like it all, to be honest,” he said. “But they understand it’s for their benefit. When we sold it to them at our beginning-of-the-year meeting, we presented our rationale. From the kids I speak to, I think the majority understand why we’re doing it.”
A version of this article first appeared on WebMD.com.
As vice principal of Pennsville Middle School in New Jersey, Adam J. Slusher knows he’s not always going to be Mr. Popularity.
Part of a vice principal’s job includes scheduling, enforcing policy, and discipline, so Dr. Slusher – who holds a doctorate in education from Wilmington University in Delaware – sometimes has to send emails or make phone calls that address unpleasant topics or unpopular new policies.
Or punishments.
But there was a much different reaction this past July, after he sent a message to the homes of Pennsville’s 450 students spanning grades 6 to 8. The email blast announced a new cellphone policy for the school. Starting in September, as he explained in the message – which also went out to the school’s 60 faculty and staff members – the use of cellphones by Pennsville students would be prohibited during school hours for any reason.
Phones, he emphasized, “are to be turned OFF” and stowed away in backpacks or handbags, not carried or tucked into back pockets.
The announcement of the new Away for the Day policy, which was decided upon by Dr. Slusher and Pennsville Principal Carolyn Carels, provoked a response different from those to his announcements on, say, test dates, emergency procedures, or new detention policies.
“It was one of the most popular emails I’ve ever sent,” chuckled Dr. Slusher, who has been an educator for 17 years. “We’ve gotten so many thanks from teachers for this.”
Ditto with the staff, who in conversations with Dr. Slusher and Ms. Carels, had reported on the rampant use of phones in the cafeteria and hallways – confirming what both of them had seen.
“They were telling us, ‘You’ve got to do something about the phones’ ” he recalled. “They were delighted that a clear policy was now going to be in place.”
The overwhelming majority of Pennsville parents have also supported the new policy, especially when presented with some of the sobering evidence about the extent of phone use among this population. One study Dr. Slusher cited in his email showed that the average middle school child is spending between 6 and 9 hours a day on screens.
“That’s like a full-time job,” he said.
The heavy cellphone use by kids – in school, out of school, anywhere and everywhere – was part of what prompted internal medicine doctor and filmmaker Delaney Ruston, MD, to create the “Away for the Day” initiative, which Pennsville has adopted.
She and collaborator Lisa Tabb were driven to do “Away for the Day” while working on Screenagers, their award-winning 2016 film examining the impact of social media, videos, and screen time on youngsters and their families that also offered tips for better navigating the digital world.
“Over 3 years of making the film, I was visiting schools all over the country,” Dr. Ruston said. “By the end, I was seeing devices all over the place, even in elementary schools. When I’d ask a student in the hall, ‘What’s the policy?’ they would shrug and say ‘I don’t know.’ When I got the same reaction from teachers – who in many cases were left to decide on their own, so that they had to be the bad guys – I realized there was a problem here.”
The result was what Dr. Ruston and Ms. Tabb describe on their website as a “movement,” designed to provide tools to parents, teachers, and administrators to help them make policies that put phones away during the school day.
The age of social centrality
As even a casual glance in the homeroom of every high school or college lecture hall will confirm, phone use is high in teenagers and young adults. But Dr. Ruston and Ms. Tabb decided to focus on middle schools.
“That’s the age where we know schools are facing the most challenges,” Dr. Ruston said. “This is also the age when social centrality becomes a major focus for youth. Thus, the pull to be on social media games, where their peers are, is incredibly enticing.”
A recent study in the journal JAMA Pediatrics found that middle schoolers who compulsively check social networks on their phones appear to have changes in areas of the brain linked to reward and punishment.
It was in middle schools, she concluded, “where effective policies on cellphones are most needed.”
As part of their research into the issue, she and ms. Tabb did a survey using email contacts collected by Dr. Ruston’s company, MyDoc Productions, during the making of the film, along with subscribers to her blog. In all, 1,200 parents – each of whom had at least one child in middle school at the time – were surveyed. The researchers found an interesting disconnect: Eighty-two percent of the parents surveyed did not want their children using phones in school. Yet 55% of middle schools allowed students to carry phones during the school day.
That survey was done in 2017. Since the COVID-19 pandemic, the use of cellphones by children, both in school and at home, has risen dramatically. A literature review of 46 studies, published in JAMA Pediatrics in November, found that average screen time among children and adolescents has increased by 52% – or 84 minutes a day – during the pandemic.
That trend has given many schools, including Pennsville, the drive to adopt an Away for the Day–type policy. As part of the program, Dr. Ruston’s website provides ammunition against the kinds of pushback they might expect to get. One of the most common is the idea that banning cellphone use among middle school children is a misguided, antitechnology measure.
“We’re not at all antitech,” Dr. Ruston asserts. Away for the Day, she explains, advocates the use of learning technologies in school that are monitored and supervised by teachers.
“The majority of students have access to learning devices in the school,” she said. “These have different kinds of blockers, making it harder for their kid to respond to their friend on TikTok when they’re supposed to be using technology for learning.”
Dr. Ruston estimates that about 10,000 middle schools are now using various pieces of the Away for the Day campaign, which includes videos, posters, fact sheets, and other materials. Other schools have adopted similar measures in the same spirit.
Predictable and calm? Not so much
When Katherine Holden was named principal of Oregon’s Talent Middle School in 2022, one of the first things she wanted to do was create some structure for the routines of students (and parents) who were frazzled after 2 years of remote learning, staggered schedules, and mask mandates.
“Predictable and calm,” she said, with a laugh. “I use those words every day.”
Achieving both is hard enough in a middle school without a pandemic – not to mention an epidemic of cellphone use. (Talent also endured a massive fire in 2020 that left many families homeless.)
For this school year, Ms. Holden is using a new and clearly articulated policy: “Devices are put away from the first bell to the last bell,” she said. “We want them to have a focus on other things. We want them to be socializing, interacting with their peers face to face, thinking about getting to class. We want them making eye contact, asking questions. Learning how to make a friend face to face. Those are important developmental social skills they should be practicing.”
Instead of scrolling through photos on Instagram, watching trending videos on TikTok, or texting their friends.
Like Dr. Slusher, she announced the new cellphone policy last summer, in a letter sent home to parents along with the list of school supplies their children would need.
“Students are welcome to use their cell phones and personal devices before entering the building prior to 8:30 a.m. and after exiting the school building at 3:10 p.m.,” she wrote. “However, during the school day students’ cellphones and personal devices need to be off and out of sight.
“I think parents generally understand the need for this,” Ms. Holden said. “They’ve watched their children getting distracted at home by these devices, so they have a sense of how a cellphone adds a layer of challenge to learning. And parents are aware of the unkind behavior that often happens online.”
As for the kids themselves? Safe to say the excitement that Dr. Slusher’s email got from Pennsville faculty, staff, and parents didn’t extend to students.
“They don’t like it all, to be honest,” he said. “But they understand it’s for their benefit. When we sold it to them at our beginning-of-the-year meeting, we presented our rationale. From the kids I speak to, I think the majority understand why we’re doing it.”
A version of this article first appeared on WebMD.com.
As vice principal of Pennsville Middle School in New Jersey, Adam J. Slusher knows he’s not always going to be Mr. Popularity.
Part of a vice principal’s job includes scheduling, enforcing policy, and discipline, so Dr. Slusher – who holds a doctorate in education from Wilmington University in Delaware – sometimes has to send emails or make phone calls that address unpleasant topics or unpopular new policies.
Or punishments.
But there was a much different reaction this past July, after he sent a message to the homes of Pennsville’s 450 students spanning grades 6 to 8. The email blast announced a new cellphone policy for the school. Starting in September, as he explained in the message – which also went out to the school’s 60 faculty and staff members – the use of cellphones by Pennsville students would be prohibited during school hours for any reason.
Phones, he emphasized, “are to be turned OFF” and stowed away in backpacks or handbags, not carried or tucked into back pockets.
The announcement of the new Away for the Day policy, which was decided upon by Dr. Slusher and Pennsville Principal Carolyn Carels, provoked a response different from those to his announcements on, say, test dates, emergency procedures, or new detention policies.
“It was one of the most popular emails I’ve ever sent,” chuckled Dr. Slusher, who has been an educator for 17 years. “We’ve gotten so many thanks from teachers for this.”
Ditto with the staff, who in conversations with Dr. Slusher and Ms. Carels, had reported on the rampant use of phones in the cafeteria and hallways – confirming what both of them had seen.
“They were telling us, ‘You’ve got to do something about the phones’ ” he recalled. “They were delighted that a clear policy was now going to be in place.”
The overwhelming majority of Pennsville parents have also supported the new policy, especially when presented with some of the sobering evidence about the extent of phone use among this population. One study Dr. Slusher cited in his email showed that the average middle school child is spending between 6 and 9 hours a day on screens.
“That’s like a full-time job,” he said.
The heavy cellphone use by kids – in school, out of school, anywhere and everywhere – was part of what prompted internal medicine doctor and filmmaker Delaney Ruston, MD, to create the “Away for the Day” initiative, which Pennsville has adopted.
She and collaborator Lisa Tabb were driven to do “Away for the Day” while working on Screenagers, their award-winning 2016 film examining the impact of social media, videos, and screen time on youngsters and their families that also offered tips for better navigating the digital world.
“Over 3 years of making the film, I was visiting schools all over the country,” Dr. Ruston said. “By the end, I was seeing devices all over the place, even in elementary schools. When I’d ask a student in the hall, ‘What’s the policy?’ they would shrug and say ‘I don’t know.’ When I got the same reaction from teachers – who in many cases were left to decide on their own, so that they had to be the bad guys – I realized there was a problem here.”
The result was what Dr. Ruston and Ms. Tabb describe on their website as a “movement,” designed to provide tools to parents, teachers, and administrators to help them make policies that put phones away during the school day.
The age of social centrality
As even a casual glance in the homeroom of every high school or college lecture hall will confirm, phone use is high in teenagers and young adults. But Dr. Ruston and Ms. Tabb decided to focus on middle schools.
“That’s the age where we know schools are facing the most challenges,” Dr. Ruston said. “This is also the age when social centrality becomes a major focus for youth. Thus, the pull to be on social media games, where their peers are, is incredibly enticing.”
A recent study in the journal JAMA Pediatrics found that middle schoolers who compulsively check social networks on their phones appear to have changes in areas of the brain linked to reward and punishment.
It was in middle schools, she concluded, “where effective policies on cellphones are most needed.”
As part of their research into the issue, she and ms. Tabb did a survey using email contacts collected by Dr. Ruston’s company, MyDoc Productions, during the making of the film, along with subscribers to her blog. In all, 1,200 parents – each of whom had at least one child in middle school at the time – were surveyed. The researchers found an interesting disconnect: Eighty-two percent of the parents surveyed did not want their children using phones in school. Yet 55% of middle schools allowed students to carry phones during the school day.
That survey was done in 2017. Since the COVID-19 pandemic, the use of cellphones by children, both in school and at home, has risen dramatically. A literature review of 46 studies, published in JAMA Pediatrics in November, found that average screen time among children and adolescents has increased by 52% – or 84 minutes a day – during the pandemic.
That trend has given many schools, including Pennsville, the drive to adopt an Away for the Day–type policy. As part of the program, Dr. Ruston’s website provides ammunition against the kinds of pushback they might expect to get. One of the most common is the idea that banning cellphone use among middle school children is a misguided, antitechnology measure.
“We’re not at all antitech,” Dr. Ruston asserts. Away for the Day, she explains, advocates the use of learning technologies in school that are monitored and supervised by teachers.
“The majority of students have access to learning devices in the school,” she said. “These have different kinds of blockers, making it harder for their kid to respond to their friend on TikTok when they’re supposed to be using technology for learning.”
Dr. Ruston estimates that about 10,000 middle schools are now using various pieces of the Away for the Day campaign, which includes videos, posters, fact sheets, and other materials. Other schools have adopted similar measures in the same spirit.
Predictable and calm? Not so much
When Katherine Holden was named principal of Oregon’s Talent Middle School in 2022, one of the first things she wanted to do was create some structure for the routines of students (and parents) who were frazzled after 2 years of remote learning, staggered schedules, and mask mandates.
“Predictable and calm,” she said, with a laugh. “I use those words every day.”
Achieving both is hard enough in a middle school without a pandemic – not to mention an epidemic of cellphone use. (Talent also endured a massive fire in 2020 that left many families homeless.)
For this school year, Ms. Holden is using a new and clearly articulated policy: “Devices are put away from the first bell to the last bell,” she said. “We want them to have a focus on other things. We want them to be socializing, interacting with their peers face to face, thinking about getting to class. We want them making eye contact, asking questions. Learning how to make a friend face to face. Those are important developmental social skills they should be practicing.”
Instead of scrolling through photos on Instagram, watching trending videos on TikTok, or texting their friends.
Like Dr. Slusher, she announced the new cellphone policy last summer, in a letter sent home to parents along with the list of school supplies their children would need.
“Students are welcome to use their cell phones and personal devices before entering the building prior to 8:30 a.m. and after exiting the school building at 3:10 p.m.,” she wrote. “However, during the school day students’ cellphones and personal devices need to be off and out of sight.
“I think parents generally understand the need for this,” Ms. Holden said. “They’ve watched their children getting distracted at home by these devices, so they have a sense of how a cellphone adds a layer of challenge to learning. And parents are aware of the unkind behavior that often happens online.”
As for the kids themselves? Safe to say the excitement that Dr. Slusher’s email got from Pennsville faculty, staff, and parents didn’t extend to students.
“They don’t like it all, to be honest,” he said. “But they understand it’s for their benefit. When we sold it to them at our beginning-of-the-year meeting, we presented our rationale. From the kids I speak to, I think the majority understand why we’re doing it.”
A version of this article first appeared on WebMD.com.
Marriage rates declining for Canadian adolescent mothers?
The decline in marriage rates in Canada is sharper among adolescent mothers, new data suggest.
An analysis of data from the Canadian Vital Statistics – Birth Database indicates that marriages declined by 80.5% among mothers younger than 18 years between 1989 and 2018.
“This study documents a decrease in marriage prevalence among mothers aged <18, 18-19, 20-24, and 25-49 years and suggests a larger relative decline in prevalence in younger mothers, especially those below age 18,” wrote study author Andrée-Anne Fafard St-Germain, PhD, a postdoctoral trainee at the University of Toronto, and colleagues.
The study was published online in the Canadian Journal of Public Health.
A general decline
The researchers estimated marriage rates among mothers in the four age groups mentioned above. They compared marriage rates in each group during the periods 1989-1990 and 2017-2018. The study included records of 10,399,250 mothers. In all, 1,118,630 records were used to identify socioeconomic and geographic patterns.
The researchers found an expected decline in marriage rates between 1989 and 2018, but the decline was steeper among younger women. Marriage rates decreased by 13.6% among women aged 25-49 years (16.0% relative decline), 29.3% among those aged 20-24 years (47.3% relative decline), 14.3% among those aged 18-19 years (60.2% relative decline), and 6.7% among those under 18 (80.5% relative decline).
Compared with Canadian-born mothers, foreign-born mothers were more likely to be married in all age groups (adjusted odds ratios [aORs], 5.18-6.36). Residence in rural locales or small towns was also associated with greater probability of marriage (aOR, 1.27-1.32).
Compared with women in the prairie provinces, those in Ontario were more likely to be married (aOR, 1.23-1.41), while the rate was lower in Quebec (aOR, 0.27-0.70), the Atlantic Provinces (aOR, 0.49-0.65), and the territories (aOR, 0.26-0.49). The researchers found no statistically significant association between neighborhood income quintiles and marriage rates in those younger than 18. They found a greater likelihood of marriage for mothers aged 18-19 years and those aged 20-24 years who resided in neighborhoods in higher income quintiles.
Human rights concern
Commenting on the study, Alissa Koski, PhD, assistant professor of epidemiology, biostatistics, and occupational health at McGill University, said, “Nearly all research on child marriage has focused on countries in South Asia and Africa. The authors acknowledge that child marriage remains legal across Canada. This draws attention to a domestic human rights concern that is largely unacknowledged.”
Overall, the study confirms trends that have been noted in Canada and have been studied by sociologists, Dr. Koski added. Traditional marriage rates have been declining for decades, while common-law marriages have been increasing. The mean age at the time of marriage increased from 30.0 years in 2016 to 30.7 years in 2020, according to Statistics Canada.
The study is limited by its failure to account for common-law marriages, which represent 98% of child marriages, said Dr. Koski. “The percentage of mothers in all age groups who were in legal or common-law marriage is almost certainly much higher than reported in this paper. However, because common-law unions are more common among younger people, the percentage of mothers in younger age groups, including those under 18, may be underestimated to a greater extent.”
The authors’ conclusion that the decline in marriages was larger among mothers younger than 18 years is not supported by the data in the article, said Dr. Koski. “That conclusion seems to overlook the fact that the absolute decline was lowest among those below age 18. The percentage decline in marriage was highest among mothers under 18, but this is at least in part because the percentage was so low to begin with. If we look instead at the absolute decline in marriage, it was lowest among mothers under age 18.”
The authors also suggest that the change in socioeconomic conditions associated with marriage may be different among mothers younger than 18 years, compared with mothers aged 18-19 years or 20-24 years. Higher socioeconomic status, as reflected in higher maternal neighborhood income quintiles, was associated with marriage among older adolescent mothers but not among mothers younger than 18 years. The socioeconomic distinction between married mothers younger than 18 years and older mothers could contribute to differences in the health advantages of marriage over time, the authors wrote.
But this conclusion as well is an “inappropriate interpretation ... not supported by the data,” said Dr. Koski. She noted that births to mothers younger than 18 years are rare in Canada, and births to married mothers in that age group are even rarer. That factor could have led the study to be underpowered in this group and yielded odds ratios that are not statistically significant. “The data are consistent with either an increase or a decrease in the odds of marriage. The data were too sparse to measure accurately,” said Dr. Koski.
The study was funded by the Canadian Institutes of Health Research Foundation. Dr. Fafard St-Germain and Dr. Koski reported having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
The decline in marriage rates in Canada is sharper among adolescent mothers, new data suggest.
An analysis of data from the Canadian Vital Statistics – Birth Database indicates that marriages declined by 80.5% among mothers younger than 18 years between 1989 and 2018.
“This study documents a decrease in marriage prevalence among mothers aged <18, 18-19, 20-24, and 25-49 years and suggests a larger relative decline in prevalence in younger mothers, especially those below age 18,” wrote study author Andrée-Anne Fafard St-Germain, PhD, a postdoctoral trainee at the University of Toronto, and colleagues.
The study was published online in the Canadian Journal of Public Health.
A general decline
The researchers estimated marriage rates among mothers in the four age groups mentioned above. They compared marriage rates in each group during the periods 1989-1990 and 2017-2018. The study included records of 10,399,250 mothers. In all, 1,118,630 records were used to identify socioeconomic and geographic patterns.
The researchers found an expected decline in marriage rates between 1989 and 2018, but the decline was steeper among younger women. Marriage rates decreased by 13.6% among women aged 25-49 years (16.0% relative decline), 29.3% among those aged 20-24 years (47.3% relative decline), 14.3% among those aged 18-19 years (60.2% relative decline), and 6.7% among those under 18 (80.5% relative decline).
Compared with Canadian-born mothers, foreign-born mothers were more likely to be married in all age groups (adjusted odds ratios [aORs], 5.18-6.36). Residence in rural locales or small towns was also associated with greater probability of marriage (aOR, 1.27-1.32).
Compared with women in the prairie provinces, those in Ontario were more likely to be married (aOR, 1.23-1.41), while the rate was lower in Quebec (aOR, 0.27-0.70), the Atlantic Provinces (aOR, 0.49-0.65), and the territories (aOR, 0.26-0.49). The researchers found no statistically significant association between neighborhood income quintiles and marriage rates in those younger than 18. They found a greater likelihood of marriage for mothers aged 18-19 years and those aged 20-24 years who resided in neighborhoods in higher income quintiles.
Human rights concern
Commenting on the study, Alissa Koski, PhD, assistant professor of epidemiology, biostatistics, and occupational health at McGill University, said, “Nearly all research on child marriage has focused on countries in South Asia and Africa. The authors acknowledge that child marriage remains legal across Canada. This draws attention to a domestic human rights concern that is largely unacknowledged.”
Overall, the study confirms trends that have been noted in Canada and have been studied by sociologists, Dr. Koski added. Traditional marriage rates have been declining for decades, while common-law marriages have been increasing. The mean age at the time of marriage increased from 30.0 years in 2016 to 30.7 years in 2020, according to Statistics Canada.
The study is limited by its failure to account for common-law marriages, which represent 98% of child marriages, said Dr. Koski. “The percentage of mothers in all age groups who were in legal or common-law marriage is almost certainly much higher than reported in this paper. However, because common-law unions are more common among younger people, the percentage of mothers in younger age groups, including those under 18, may be underestimated to a greater extent.”
The authors’ conclusion that the decline in marriages was larger among mothers younger than 18 years is not supported by the data in the article, said Dr. Koski. “That conclusion seems to overlook the fact that the absolute decline was lowest among those below age 18. The percentage decline in marriage was highest among mothers under 18, but this is at least in part because the percentage was so low to begin with. If we look instead at the absolute decline in marriage, it was lowest among mothers under age 18.”
The authors also suggest that the change in socioeconomic conditions associated with marriage may be different among mothers younger than 18 years, compared with mothers aged 18-19 years or 20-24 years. Higher socioeconomic status, as reflected in higher maternal neighborhood income quintiles, was associated with marriage among older adolescent mothers but not among mothers younger than 18 years. The socioeconomic distinction between married mothers younger than 18 years and older mothers could contribute to differences in the health advantages of marriage over time, the authors wrote.
But this conclusion as well is an “inappropriate interpretation ... not supported by the data,” said Dr. Koski. She noted that births to mothers younger than 18 years are rare in Canada, and births to married mothers in that age group are even rarer. That factor could have led the study to be underpowered in this group and yielded odds ratios that are not statistically significant. “The data are consistent with either an increase or a decrease in the odds of marriage. The data were too sparse to measure accurately,” said Dr. Koski.
The study was funded by the Canadian Institutes of Health Research Foundation. Dr. Fafard St-Germain and Dr. Koski reported having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
The decline in marriage rates in Canada is sharper among adolescent mothers, new data suggest.
An analysis of data from the Canadian Vital Statistics – Birth Database indicates that marriages declined by 80.5% among mothers younger than 18 years between 1989 and 2018.
“This study documents a decrease in marriage prevalence among mothers aged <18, 18-19, 20-24, and 25-49 years and suggests a larger relative decline in prevalence in younger mothers, especially those below age 18,” wrote study author Andrée-Anne Fafard St-Germain, PhD, a postdoctoral trainee at the University of Toronto, and colleagues.
The study was published online in the Canadian Journal of Public Health.
A general decline
The researchers estimated marriage rates among mothers in the four age groups mentioned above. They compared marriage rates in each group during the periods 1989-1990 and 2017-2018. The study included records of 10,399,250 mothers. In all, 1,118,630 records were used to identify socioeconomic and geographic patterns.
The researchers found an expected decline in marriage rates between 1989 and 2018, but the decline was steeper among younger women. Marriage rates decreased by 13.6% among women aged 25-49 years (16.0% relative decline), 29.3% among those aged 20-24 years (47.3% relative decline), 14.3% among those aged 18-19 years (60.2% relative decline), and 6.7% among those under 18 (80.5% relative decline).
Compared with Canadian-born mothers, foreign-born mothers were more likely to be married in all age groups (adjusted odds ratios [aORs], 5.18-6.36). Residence in rural locales or small towns was also associated with greater probability of marriage (aOR, 1.27-1.32).
Compared with women in the prairie provinces, those in Ontario were more likely to be married (aOR, 1.23-1.41), while the rate was lower in Quebec (aOR, 0.27-0.70), the Atlantic Provinces (aOR, 0.49-0.65), and the territories (aOR, 0.26-0.49). The researchers found no statistically significant association between neighborhood income quintiles and marriage rates in those younger than 18. They found a greater likelihood of marriage for mothers aged 18-19 years and those aged 20-24 years who resided in neighborhoods in higher income quintiles.
Human rights concern
Commenting on the study, Alissa Koski, PhD, assistant professor of epidemiology, biostatistics, and occupational health at McGill University, said, “Nearly all research on child marriage has focused on countries in South Asia and Africa. The authors acknowledge that child marriage remains legal across Canada. This draws attention to a domestic human rights concern that is largely unacknowledged.”
Overall, the study confirms trends that have been noted in Canada and have been studied by sociologists, Dr. Koski added. Traditional marriage rates have been declining for decades, while common-law marriages have been increasing. The mean age at the time of marriage increased from 30.0 years in 2016 to 30.7 years in 2020, according to Statistics Canada.
The study is limited by its failure to account for common-law marriages, which represent 98% of child marriages, said Dr. Koski. “The percentage of mothers in all age groups who were in legal or common-law marriage is almost certainly much higher than reported in this paper. However, because common-law unions are more common among younger people, the percentage of mothers in younger age groups, including those under 18, may be underestimated to a greater extent.”
The authors’ conclusion that the decline in marriages was larger among mothers younger than 18 years is not supported by the data in the article, said Dr. Koski. “That conclusion seems to overlook the fact that the absolute decline was lowest among those below age 18. The percentage decline in marriage was highest among mothers under 18, but this is at least in part because the percentage was so low to begin with. If we look instead at the absolute decline in marriage, it was lowest among mothers under age 18.”
The authors also suggest that the change in socioeconomic conditions associated with marriage may be different among mothers younger than 18 years, compared with mothers aged 18-19 years or 20-24 years. Higher socioeconomic status, as reflected in higher maternal neighborhood income quintiles, was associated with marriage among older adolescent mothers but not among mothers younger than 18 years. The socioeconomic distinction between married mothers younger than 18 years and older mothers could contribute to differences in the health advantages of marriage over time, the authors wrote.
But this conclusion as well is an “inappropriate interpretation ... not supported by the data,” said Dr. Koski. She noted that births to mothers younger than 18 years are rare in Canada, and births to married mothers in that age group are even rarer. That factor could have led the study to be underpowered in this group and yielded odds ratios that are not statistically significant. “The data are consistent with either an increase or a decrease in the odds of marriage. The data were too sparse to measure accurately,” said Dr. Koski.
The study was funded by the Canadian Institutes of Health Research Foundation. Dr. Fafard St-Germain and Dr. Koski reported having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM THE CANADIAN JOURNAL OF PUBLIC HEALTH