User login
Alzheimer's Disease Workup
Anxiety and panic attacks
Given the patient's insidious cognitive decline, as well as increased agitation, irritability, anxiety, social isolation, inability to fully manage finances, loss of routine hygienic practices, and loss of interest in regular meals, this patient is diagnosed with probable Alzheimer's disease (AD) dementia and is referred to a specialist for further testing.
AD is one of the most common forms of dementia. More than 6 million people in the United States have clinical AD or mild cognitive impairment because of AD. By 2060, the incidence of AD is expected to grow to 15 million people. AD is classified into four stages: preclinical, mild, moderate, and severe. Patients with preclinical AD — a relatively new classification currently only used for research — do not yet show abnormal results on physical exam or mental status testing, but areas of the brain are undergoing pathologic changes. Mild AD signs and symptoms include memory loss, compromised judgment, trouble handling money and paying bills, mood and personality changes, and increased anxiety. People with moderate AD show increasing signs of memory loss and confusion, problems with recognizing family and friends, and difficulty with organizing thoughts and thinking logically, and they repeat themselves in conversation, among other symptoms. Severe AD is generally described as a complete loss of self, with the inability to recognize family and friends, inability to communicate effectively, and complete dependence on others for care.
Diagnosing AD currently relies on a clinical approach. A complete physical examination, with a detailed neurologic examination and a mental status examination, is used to evaluate disease stage and rule out comorbid conditions. Initial mental status testing should evaluate attention and concentration, recent and remote memory, language, praxis, executive function, and visuospatial function. Imaging studies may be performed to rule out other treatable causes of cognitive decline. In addition, volumetric studies of the hippocampus and 2-[18F]fluoro-2-deoxy-D-glucose PET with or without amyloid imaging can be used for early detection and differentiating dementia etiologies. Lumbar puncture as a diagnostic measure for levels of tau (which is often elevated in AD) and amyloid (which is often reduced in AD) is currently reserved for research settings.
Although the cause of AD is unknown, experts believe that environmental and genetic risk factors trigger a pathophysiologic cascade that, over decades, leads to Alzheimer's pathology and dementia. Universally accepted pathologic hallmarks of AD are beta-amyloid plaques and neurofibrillary tangles (NFTs). NFTs result from changes in the tau protein, a key chemical in neuronal support structures, and are associated with malfunctions in communication between neurons as well as cell death. Beta-amyloid plaques are dense, mostly insoluble deposits that develop around neurons in the hippocampus and other regions in the cerebral cortex used for decision-making, disrupting function and leading to brain atrophy. Risk factors for AD include advancing age, family history, APOE e4 genotype, insulin resistance, hypertension, depression, and traumatic brain injury.
After an AD diagnosis, physicians should encourage the involvement of family and friends who agree to become more involved in the patient's care as the disease progresses. These individuals need to understand the patient's wishes around care, especially for the future, when the patient is no longer able to make decisions. The patient may also consider establishing medical advance directives and durable power of attorney for medical and financial decision-making. Caregivers supporting the patient are encouraged to help balance the physical needs of the patient while maintaining respect for them as a competent adult to the extent allowed by the progression of their disease.
Currently, AD treatments are focused on symptomatic therapies that modulate neurotransmitters — either acetylcholine or glutamate. The standard medical treatment includes cholinesterase inhibitors and a partial N-methyl-D-aspartate antagonist. Two amyloid-directed antibodies (aducanumab, lecanemab) are currently available in the US for individuals with AD exhibiting mild cognitive impairment or mild dementia. A third agent currently in clinical trials (donanemab) has shown significantly slowed clinical progression after 1.5 years among clinical trial participants with early symptomatic AD and amyloid and tau pathology.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Given the patient's insidious cognitive decline, as well as increased agitation, irritability, anxiety, social isolation, inability to fully manage finances, loss of routine hygienic practices, and loss of interest in regular meals, this patient is diagnosed with probable Alzheimer's disease (AD) dementia and is referred to a specialist for further testing.
AD is one of the most common forms of dementia. More than 6 million people in the United States have clinical AD or mild cognitive impairment because of AD. By 2060, the incidence of AD is expected to grow to 15 million people. AD is classified into four stages: preclinical, mild, moderate, and severe. Patients with preclinical AD — a relatively new classification currently only used for research — do not yet show abnormal results on physical exam or mental status testing, but areas of the brain are undergoing pathologic changes. Mild AD signs and symptoms include memory loss, compromised judgment, trouble handling money and paying bills, mood and personality changes, and increased anxiety. People with moderate AD show increasing signs of memory loss and confusion, problems with recognizing family and friends, and difficulty with organizing thoughts and thinking logically, and they repeat themselves in conversation, among other symptoms. Severe AD is generally described as a complete loss of self, with the inability to recognize family and friends, inability to communicate effectively, and complete dependence on others for care.
Diagnosing AD currently relies on a clinical approach. A complete physical examination, with a detailed neurologic examination and a mental status examination, is used to evaluate disease stage and rule out comorbid conditions. Initial mental status testing should evaluate attention and concentration, recent and remote memory, language, praxis, executive function, and visuospatial function. Imaging studies may be performed to rule out other treatable causes of cognitive decline. In addition, volumetric studies of the hippocampus and 2-[18F]fluoro-2-deoxy-D-glucose PET with or without amyloid imaging can be used for early detection and differentiating dementia etiologies. Lumbar puncture as a diagnostic measure for levels of tau (which is often elevated in AD) and amyloid (which is often reduced in AD) is currently reserved for research settings.
Although the cause of AD is unknown, experts believe that environmental and genetic risk factors trigger a pathophysiologic cascade that, over decades, leads to Alzheimer's pathology and dementia. Universally accepted pathologic hallmarks of AD are beta-amyloid plaques and neurofibrillary tangles (NFTs). NFTs result from changes in the tau protein, a key chemical in neuronal support structures, and are associated with malfunctions in communication between neurons as well as cell death. Beta-amyloid plaques are dense, mostly insoluble deposits that develop around neurons in the hippocampus and other regions in the cerebral cortex used for decision-making, disrupting function and leading to brain atrophy. Risk factors for AD include advancing age, family history, APOE e4 genotype, insulin resistance, hypertension, depression, and traumatic brain injury.
After an AD diagnosis, physicians should encourage the involvement of family and friends who agree to become more involved in the patient's care as the disease progresses. These individuals need to understand the patient's wishes around care, especially for the future, when the patient is no longer able to make decisions. The patient may also consider establishing medical advance directives and durable power of attorney for medical and financial decision-making. Caregivers supporting the patient are encouraged to help balance the physical needs of the patient while maintaining respect for them as a competent adult to the extent allowed by the progression of their disease.
Currently, AD treatments are focused on symptomatic therapies that modulate neurotransmitters — either acetylcholine or glutamate. The standard medical treatment includes cholinesterase inhibitors and a partial N-methyl-D-aspartate antagonist. Two amyloid-directed antibodies (aducanumab, lecanemab) are currently available in the US for individuals with AD exhibiting mild cognitive impairment or mild dementia. A third agent currently in clinical trials (donanemab) has shown significantly slowed clinical progression after 1.5 years among clinical trial participants with early symptomatic AD and amyloid and tau pathology.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Given the patient's insidious cognitive decline, as well as increased agitation, irritability, anxiety, social isolation, inability to fully manage finances, loss of routine hygienic practices, and loss of interest in regular meals, this patient is diagnosed with probable Alzheimer's disease (AD) dementia and is referred to a specialist for further testing.
AD is one of the most common forms of dementia. More than 6 million people in the United States have clinical AD or mild cognitive impairment because of AD. By 2060, the incidence of AD is expected to grow to 15 million people. AD is classified into four stages: preclinical, mild, moderate, and severe. Patients with preclinical AD — a relatively new classification currently only used for research — do not yet show abnormal results on physical exam or mental status testing, but areas of the brain are undergoing pathologic changes. Mild AD signs and symptoms include memory loss, compromised judgment, trouble handling money and paying bills, mood and personality changes, and increased anxiety. People with moderate AD show increasing signs of memory loss and confusion, problems with recognizing family and friends, and difficulty with organizing thoughts and thinking logically, and they repeat themselves in conversation, among other symptoms. Severe AD is generally described as a complete loss of self, with the inability to recognize family and friends, inability to communicate effectively, and complete dependence on others for care.
Diagnosing AD currently relies on a clinical approach. A complete physical examination, with a detailed neurologic examination and a mental status examination, is used to evaluate disease stage and rule out comorbid conditions. Initial mental status testing should evaluate attention and concentration, recent and remote memory, language, praxis, executive function, and visuospatial function. Imaging studies may be performed to rule out other treatable causes of cognitive decline. In addition, volumetric studies of the hippocampus and 2-[18F]fluoro-2-deoxy-D-glucose PET with or without amyloid imaging can be used for early detection and differentiating dementia etiologies. Lumbar puncture as a diagnostic measure for levels of tau (which is often elevated in AD) and amyloid (which is often reduced in AD) is currently reserved for research settings.
Although the cause of AD is unknown, experts believe that environmental and genetic risk factors trigger a pathophysiologic cascade that, over decades, leads to Alzheimer's pathology and dementia. Universally accepted pathologic hallmarks of AD are beta-amyloid plaques and neurofibrillary tangles (NFTs). NFTs result from changes in the tau protein, a key chemical in neuronal support structures, and are associated with malfunctions in communication between neurons as well as cell death. Beta-amyloid plaques are dense, mostly insoluble deposits that develop around neurons in the hippocampus and other regions in the cerebral cortex used for decision-making, disrupting function and leading to brain atrophy. Risk factors for AD include advancing age, family history, APOE e4 genotype, insulin resistance, hypertension, depression, and traumatic brain injury.
After an AD diagnosis, physicians should encourage the involvement of family and friends who agree to become more involved in the patient's care as the disease progresses. These individuals need to understand the patient's wishes around care, especially for the future, when the patient is no longer able to make decisions. The patient may also consider establishing medical advance directives and durable power of attorney for medical and financial decision-making. Caregivers supporting the patient are encouraged to help balance the physical needs of the patient while maintaining respect for them as a competent adult to the extent allowed by the progression of their disease.
Currently, AD treatments are focused on symptomatic therapies that modulate neurotransmitters — either acetylcholine or glutamate. The standard medical treatment includes cholinesterase inhibitors and a partial N-methyl-D-aspartate antagonist. Two amyloid-directed antibodies (aducanumab, lecanemab) are currently available in the US for individuals with AD exhibiting mild cognitive impairment or mild dementia. A third agent currently in clinical trials (donanemab) has shown significantly slowed clinical progression after 1.5 years among clinical trial participants with early symptomatic AD and amyloid and tau pathology.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 73-year-old man who lives independently presents to his primary care physician (PCP) with irritability, anxiety, and panic attacks. Last year, he saw his PCP at the urging of his brother, who noticed that the patient was becoming more forgetful and agitated. At that time, the brother reported concerns that the patient, who normally enjoyed spending time with his extended family, was beginning to regularly forget to show up at family functions. When asked why he hadn't attended, the patient would become irate, saying it was his family who failed to invite him. The patient wouldn't have agreed to seeing the PCP except he was having issues with insomnia that he wanted to address. During last year's visit, the physician conducted a complete physical examination, as well as detailed neurologic and mental status examinations; all came back normal.
At today's visit, in addition to patient-reported mood fluctuations, the brother tells the physician that the patient has become reclusive, skipping nearly all family functions as well as daily walks with friends. His daily hygiene has suffered, and he has stopped eating regularly. The brother also mentions to the doctor that the patient has received some late-payment notices for utilities that he normally meticulously paid on time. The PCP orders another round of cognitive, behavioral, and functional assessments, which reveal a decline in all areas from last year's results, as well as a complete neurologic examination that reveals mild hyposmia.
Unique twin study sheds new light on TBI and risk of cognitive decline
The research, which included almost 9,000 individuals, showed that twins who had experienced a TBI were more likely to have lower cognitive function at age 70 versus their twin who did not experience a TBI, especially if they had lost consciousness or were older than age 24 at the time of injury. In addition, their cognitive decline occurred at a more rapid rate.
“We know that TBI increases the risk of developing Alzheimer’s disease and other dementias in later life, but we haven’t known about TBI’s effect on cognitive decline that does not quite meet the threshold for dementia,” study investigator Marianne Chanti-Ketterl, PhD, Duke University, Durham, N.C., said in an interview.
“We know that TBI increases the risk of dementia in later life, but we haven’t known if TBI affects cognitive function, causes cognitive decline that has not progressed to the point of severity with Alzheimer’s or dementia,” she added.
Being able to study the impact of TBI in monozygotic twins gives this study a unique strength, she noted.
“The important thing about this is that they are monozygotic twins, and we know they shared a lot of early life exposure, and almost 100% genetics,” Dr. Chanti-Ketterl said.
The study was published online in Neurology.
For the study, the investigators assessed 8,662 participants born between 1917 and 1927 who were part of the National Academy of Sciences National Research Council’s Twin Registry. The registry is composed of male veterans of World War II with a history of TBI, as reported by themselves or a caregiver.
The men were followed up for many years as part of the registry, but cognitive assessment only began in the 1990s. They were followed up at four different time points, at which time the Telephone Interview for Cognitive Status (TICS-m), an alternative to the Mini-Mental State Examination that must be given in person, was administered.
A total of 25% of participants had experienced concussion in their lifetime. Of this cohort, there were 589 pairs of monozygotic twins who were discordant (one twin had TBI and the other had not).
Among the monozygotic twin cohort, a history of any TBI and being older than age 24 at the time of TBI were associated with lower TICS-m scores.
A twin who experienced TBI after age 24 scored 0.59 points lower on the TICS-m at age 70 than his twin with no TBI, and cognitive function declined faster, by 0.05 points per year.
First study of its kind
Holly Elser, MD, PhD, MPH, an epidemiologist and resident physician in neurology at the University of Pennsylvania, Philadelphia, and coauthor of an accompanying editorial, said in an interview that the study’s twin design was a definite strength.
“There are lots of papers that have remarked on the apparent association between head injury and subsequent dementia or cognitive decline, but to my knowledge, this is one of the first, if not the first, to use a twin study design, which has the unique advantage of having better control over early life and genetic factors than would ever typically be possible in a dataset of unrelated adults,” said Dr. Elser.
She added that the study findings “strengthen our understanding of the relationship between TBI and later cognitive decline, so I think there is an etiologic value to the study.”
However, Dr. Elser noted that the composition of the study population may limit the extent to which the results apply to contemporary populations.
“This was a population of White male twins born between 1917 and 1927,” she noted. “However, does the experience of people who were in the military generalize to civilian populations? Are twins representative of the general population or are they unique in terms of their risk factors?”
It is always important to emphasize inclusivity in clinical research, and in dementia research in particular, Dr. Elser added.
“There are many examples of instances where racialized and otherwise economically marginalized groups have been excluded from analysis, which is problematic because there are already economically and socially marginalized groups who disproportionately bear the brunt of dementia.
“This is not a criticism of the authors’ work, that their data didn’t include a more diverse patient base, but I think it is an important reminder that we should always interpret study findings within the limitations of the data. It’s a reminder to be thoughtful about taking explicit steps to include more diverse groups in future research,” she said.
The study was funded by the National Institute on Aging/National Institutes of Health and the Department of Defense. Dr. Chanti-Ketterl and Dr. Elser have reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
The research, which included almost 9,000 individuals, showed that twins who had experienced a TBI were more likely to have lower cognitive function at age 70 versus their twin who did not experience a TBI, especially if they had lost consciousness or were older than age 24 at the time of injury. In addition, their cognitive decline occurred at a more rapid rate.
“We know that TBI increases the risk of developing Alzheimer’s disease and other dementias in later life, but we haven’t known about TBI’s effect on cognitive decline that does not quite meet the threshold for dementia,” study investigator Marianne Chanti-Ketterl, PhD, Duke University, Durham, N.C., said in an interview.
“We know that TBI increases the risk of dementia in later life, but we haven’t known if TBI affects cognitive function, causes cognitive decline that has not progressed to the point of severity with Alzheimer’s or dementia,” she added.
Being able to study the impact of TBI in monozygotic twins gives this study a unique strength, she noted.
“The important thing about this is that they are monozygotic twins, and we know they shared a lot of early life exposure, and almost 100% genetics,” Dr. Chanti-Ketterl said.
The study was published online in Neurology.
For the study, the investigators assessed 8,662 participants born between 1917 and 1927 who were part of the National Academy of Sciences National Research Council’s Twin Registry. The registry is composed of male veterans of World War II with a history of TBI, as reported by themselves or a caregiver.
The men were followed up for many years as part of the registry, but cognitive assessment only began in the 1990s. They were followed up at four different time points, at which time the Telephone Interview for Cognitive Status (TICS-m), an alternative to the Mini-Mental State Examination that must be given in person, was administered.
A total of 25% of participants had experienced concussion in their lifetime. Of this cohort, there were 589 pairs of monozygotic twins who were discordant (one twin had TBI and the other had not).
Among the monozygotic twin cohort, a history of any TBI and being older than age 24 at the time of TBI were associated with lower TICS-m scores.
A twin who experienced TBI after age 24 scored 0.59 points lower on the TICS-m at age 70 than his twin with no TBI, and cognitive function declined faster, by 0.05 points per year.
First study of its kind
Holly Elser, MD, PhD, MPH, an epidemiologist and resident physician in neurology at the University of Pennsylvania, Philadelphia, and coauthor of an accompanying editorial, said in an interview that the study’s twin design was a definite strength.
“There are lots of papers that have remarked on the apparent association between head injury and subsequent dementia or cognitive decline, but to my knowledge, this is one of the first, if not the first, to use a twin study design, which has the unique advantage of having better control over early life and genetic factors than would ever typically be possible in a dataset of unrelated adults,” said Dr. Elser.
She added that the study findings “strengthen our understanding of the relationship between TBI and later cognitive decline, so I think there is an etiologic value to the study.”
However, Dr. Elser noted that the composition of the study population may limit the extent to which the results apply to contemporary populations.
“This was a population of White male twins born between 1917 and 1927,” she noted. “However, does the experience of people who were in the military generalize to civilian populations? Are twins representative of the general population or are they unique in terms of their risk factors?”
It is always important to emphasize inclusivity in clinical research, and in dementia research in particular, Dr. Elser added.
“There are many examples of instances where racialized and otherwise economically marginalized groups have been excluded from analysis, which is problematic because there are already economically and socially marginalized groups who disproportionately bear the brunt of dementia.
“This is not a criticism of the authors’ work, that their data didn’t include a more diverse patient base, but I think it is an important reminder that we should always interpret study findings within the limitations of the data. It’s a reminder to be thoughtful about taking explicit steps to include more diverse groups in future research,” she said.
The study was funded by the National Institute on Aging/National Institutes of Health and the Department of Defense. Dr. Chanti-Ketterl and Dr. Elser have reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
The research, which included almost 9,000 individuals, showed that twins who had experienced a TBI were more likely to have lower cognitive function at age 70 versus their twin who did not experience a TBI, especially if they had lost consciousness or were older than age 24 at the time of injury. In addition, their cognitive decline occurred at a more rapid rate.
“We know that TBI increases the risk of developing Alzheimer’s disease and other dementias in later life, but we haven’t known about TBI’s effect on cognitive decline that does not quite meet the threshold for dementia,” study investigator Marianne Chanti-Ketterl, PhD, Duke University, Durham, N.C., said in an interview.
“We know that TBI increases the risk of dementia in later life, but we haven’t known if TBI affects cognitive function, causes cognitive decline that has not progressed to the point of severity with Alzheimer’s or dementia,” she added.
Being able to study the impact of TBI in monozygotic twins gives this study a unique strength, she noted.
“The important thing about this is that they are monozygotic twins, and we know they shared a lot of early life exposure, and almost 100% genetics,” Dr. Chanti-Ketterl said.
The study was published online in Neurology.
For the study, the investigators assessed 8,662 participants born between 1917 and 1927 who were part of the National Academy of Sciences National Research Council’s Twin Registry. The registry is composed of male veterans of World War II with a history of TBI, as reported by themselves or a caregiver.
The men were followed up for many years as part of the registry, but cognitive assessment only began in the 1990s. They were followed up at four different time points, at which time the Telephone Interview for Cognitive Status (TICS-m), an alternative to the Mini-Mental State Examination that must be given in person, was administered.
A total of 25% of participants had experienced concussion in their lifetime. Of this cohort, there were 589 pairs of monozygotic twins who were discordant (one twin had TBI and the other had not).
Among the monozygotic twin cohort, a history of any TBI and being older than age 24 at the time of TBI were associated with lower TICS-m scores.
A twin who experienced TBI after age 24 scored 0.59 points lower on the TICS-m at age 70 than his twin with no TBI, and cognitive function declined faster, by 0.05 points per year.
First study of its kind
Holly Elser, MD, PhD, MPH, an epidemiologist and resident physician in neurology at the University of Pennsylvania, Philadelphia, and coauthor of an accompanying editorial, said in an interview that the study’s twin design was a definite strength.
“There are lots of papers that have remarked on the apparent association between head injury and subsequent dementia or cognitive decline, but to my knowledge, this is one of the first, if not the first, to use a twin study design, which has the unique advantage of having better control over early life and genetic factors than would ever typically be possible in a dataset of unrelated adults,” said Dr. Elser.
She added that the study findings “strengthen our understanding of the relationship between TBI and later cognitive decline, so I think there is an etiologic value to the study.”
However, Dr. Elser noted that the composition of the study population may limit the extent to which the results apply to contemporary populations.
“This was a population of White male twins born between 1917 and 1927,” she noted. “However, does the experience of people who were in the military generalize to civilian populations? Are twins representative of the general population or are they unique in terms of their risk factors?”
It is always important to emphasize inclusivity in clinical research, and in dementia research in particular, Dr. Elser added.
“There are many examples of instances where racialized and otherwise economically marginalized groups have been excluded from analysis, which is problematic because there are already economically and socially marginalized groups who disproportionately bear the brunt of dementia.
“This is not a criticism of the authors’ work, that their data didn’t include a more diverse patient base, but I think it is an important reminder that we should always interpret study findings within the limitations of the data. It’s a reminder to be thoughtful about taking explicit steps to include more diverse groups in future research,” she said.
The study was funded by the National Institute on Aging/National Institutes of Health and the Department of Defense. Dr. Chanti-Ketterl and Dr. Elser have reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM NEUROLOGY
AHA reviews impact of aggressive LDL lowering on the brain
“The brain is the body’s most cholesterol-rich organ, and some have questioned whether aggressive LDL-C lowering induces abnormal structural and functional changes,” the writing group, led by Larry Goldstein, MD, chair, department of neurology, University of Kentucky, Lexington, points out.
The 39-page AHA scientific statement, titled “Aggressive LDL-C Lowering and the Brain: Impact on Risk for Dementia and Hemorrhagic Stroke,” was published online in the journal Arteriosclerosis, Thrombosis, and Vascular Biology.
The objective was to evaluate contemporary evidence that either supports or refutes the conclusion that aggressive LDL-C lowering or lipid lowering exerts toxic effects on the brain, leading to cognitive impairment or dementia or hemorrhagic stroke.
The eight-member writing group used literature reviews, references to published clinical and epidemiology studies, clinical and public health guidelines, authoritative statements, and expert opinion to summarize the latest evidence and identify gaps in current knowledge.
They reached four main conclusions:
- First, the available data “consistently” show that LDL-C lowering reduces the risk of atherosclerotic cardiovascular disease-related events in high-risk groups.
- Second, although some older retrospective, case-control, and prospective longitudinal studies suggest that statins and LDL-C lowering are associated with cognitive impairment or dementia, the “preponderance” of observational studies and data from randomized trials do not support this conclusion, at least among trials with median follow-up of up to 6 years. The group says additional studies are needed to ensure cognitive safety over longer periods of time. For now, contemporary guidelines recommending the risk-stratified attainment of lipid-lowering goals are “reasonable,” they conclude.
- Third, the risk for hemorrhagic stroke associated with statin therapy in patients without a history of cerebrovascular disease is “small and consistently nonsignificant.” They found no evidence that PCSK9 inhibitors or ezetimibe (Zetia) increases bleeding risk. Further, there is “no indication” that patients or populations with lifelong low LDL-C have enhanced vulnerability to hemorrhagic stroke, and there is “little evidence” that achieving very low levels of LDL-C increases that risk. What is clear, the writing group says, is that lower LDL-C levels correlate with lower risk of overall stroke and stroke recurrence, mostly related to a reduction in ischemic stroke. “Concern about hemorrhagic stroke risk should not deter a clinician from treating LDL-C to guideline-recommended risk-stratified targets,” the writing group says.
- Fourth, the group notes that data reflecting the risk of hemorrhagic stroke with statin therapy among patients with a history of hemorrhagic stroke are not robust. PCSK9 inhibitors have not been adequately tested in patients with prior intracerebral hemorrhage. Lipid lowering in these populations requires more focused study.
The research had no commercial funding. A list of disclosures for the writing group is available with the original article.
A version of this article appeared on Medscape.com.
“The brain is the body’s most cholesterol-rich organ, and some have questioned whether aggressive LDL-C lowering induces abnormal structural and functional changes,” the writing group, led by Larry Goldstein, MD, chair, department of neurology, University of Kentucky, Lexington, points out.
The 39-page AHA scientific statement, titled “Aggressive LDL-C Lowering and the Brain: Impact on Risk for Dementia and Hemorrhagic Stroke,” was published online in the journal Arteriosclerosis, Thrombosis, and Vascular Biology.
The objective was to evaluate contemporary evidence that either supports or refutes the conclusion that aggressive LDL-C lowering or lipid lowering exerts toxic effects on the brain, leading to cognitive impairment or dementia or hemorrhagic stroke.
The eight-member writing group used literature reviews, references to published clinical and epidemiology studies, clinical and public health guidelines, authoritative statements, and expert opinion to summarize the latest evidence and identify gaps in current knowledge.
They reached four main conclusions:
- First, the available data “consistently” show that LDL-C lowering reduces the risk of atherosclerotic cardiovascular disease-related events in high-risk groups.
- Second, although some older retrospective, case-control, and prospective longitudinal studies suggest that statins and LDL-C lowering are associated with cognitive impairment or dementia, the “preponderance” of observational studies and data from randomized trials do not support this conclusion, at least among trials with median follow-up of up to 6 years. The group says additional studies are needed to ensure cognitive safety over longer periods of time. For now, contemporary guidelines recommending the risk-stratified attainment of lipid-lowering goals are “reasonable,” they conclude.
- Third, the risk for hemorrhagic stroke associated with statin therapy in patients without a history of cerebrovascular disease is “small and consistently nonsignificant.” They found no evidence that PCSK9 inhibitors or ezetimibe (Zetia) increases bleeding risk. Further, there is “no indication” that patients or populations with lifelong low LDL-C have enhanced vulnerability to hemorrhagic stroke, and there is “little evidence” that achieving very low levels of LDL-C increases that risk. What is clear, the writing group says, is that lower LDL-C levels correlate with lower risk of overall stroke and stroke recurrence, mostly related to a reduction in ischemic stroke. “Concern about hemorrhagic stroke risk should not deter a clinician from treating LDL-C to guideline-recommended risk-stratified targets,” the writing group says.
- Fourth, the group notes that data reflecting the risk of hemorrhagic stroke with statin therapy among patients with a history of hemorrhagic stroke are not robust. PCSK9 inhibitors have not been adequately tested in patients with prior intracerebral hemorrhage. Lipid lowering in these populations requires more focused study.
The research had no commercial funding. A list of disclosures for the writing group is available with the original article.
A version of this article appeared on Medscape.com.
“The brain is the body’s most cholesterol-rich organ, and some have questioned whether aggressive LDL-C lowering induces abnormal structural and functional changes,” the writing group, led by Larry Goldstein, MD, chair, department of neurology, University of Kentucky, Lexington, points out.
The 39-page AHA scientific statement, titled “Aggressive LDL-C Lowering and the Brain: Impact on Risk for Dementia and Hemorrhagic Stroke,” was published online in the journal Arteriosclerosis, Thrombosis, and Vascular Biology.
The objective was to evaluate contemporary evidence that either supports or refutes the conclusion that aggressive LDL-C lowering or lipid lowering exerts toxic effects on the brain, leading to cognitive impairment or dementia or hemorrhagic stroke.
The eight-member writing group used literature reviews, references to published clinical and epidemiology studies, clinical and public health guidelines, authoritative statements, and expert opinion to summarize the latest evidence and identify gaps in current knowledge.
They reached four main conclusions:
- First, the available data “consistently” show that LDL-C lowering reduces the risk of atherosclerotic cardiovascular disease-related events in high-risk groups.
- Second, although some older retrospective, case-control, and prospective longitudinal studies suggest that statins and LDL-C lowering are associated with cognitive impairment or dementia, the “preponderance” of observational studies and data from randomized trials do not support this conclusion, at least among trials with median follow-up of up to 6 years. The group says additional studies are needed to ensure cognitive safety over longer periods of time. For now, contemporary guidelines recommending the risk-stratified attainment of lipid-lowering goals are “reasonable,” they conclude.
- Third, the risk for hemorrhagic stroke associated with statin therapy in patients without a history of cerebrovascular disease is “small and consistently nonsignificant.” They found no evidence that PCSK9 inhibitors or ezetimibe (Zetia) increases bleeding risk. Further, there is “no indication” that patients or populations with lifelong low LDL-C have enhanced vulnerability to hemorrhagic stroke, and there is “little evidence” that achieving very low levels of LDL-C increases that risk. What is clear, the writing group says, is that lower LDL-C levels correlate with lower risk of overall stroke and stroke recurrence, mostly related to a reduction in ischemic stroke. “Concern about hemorrhagic stroke risk should not deter a clinician from treating LDL-C to guideline-recommended risk-stratified targets,” the writing group says.
- Fourth, the group notes that data reflecting the risk of hemorrhagic stroke with statin therapy among patients with a history of hemorrhagic stroke are not robust. PCSK9 inhibitors have not been adequately tested in patients with prior intracerebral hemorrhage. Lipid lowering in these populations requires more focused study.
The research had no commercial funding. A list of disclosures for the writing group is available with the original article.
A version of this article appeared on Medscape.com.
FROM ARTERIOSCLEROSIS, THROMBOSIS, AND VASCULAR BIOLOGY
How does lecanemab work in Alzheimer’s?
Lecanemab (Lequembi, Esai), an amyloid-beta–directed antibody therapy, is approved by the Food and Drug Administration for the treatment of Alzheimer’s disease (AD). But exactly how the drug clears amyloid-beta wasn’t clear.
The investigators tested the effectiveness of various forms of amyloid-beta in activating the plasma contact system and found that amyloid-beta protofibrils, known to be the most toxic form of amyloid-beta, promoted the activation of this molecular cascade and that lecanemab inhibited pathway activation.
“In our study, we looked at lecanemab and found it can block the activation of the contact system, which could be one of the reasons that it works so well for AD,” study coinvestigator Erin Norris, PhD, research associate professor, Rockefeller University, New York, said in an interview.
The study was published online in the Proceedings of the National Academy of Science.
Unknown mechanism
“Many years ago, we started looking at the involvement of vascular dysfunction in AD,” Dr. Norris said. “We wanted to see whether or not irregular blood clotting or problems with blood flow was problematic in Alzheimer’s patients.”
The researchers found that fibrin, a major component involved in blood clotting, can extravasate into the brain.
“The blood-brain barrier can break down in Alzheimer’s, so things from the blood can move into the brain and deposit there,” she added. Fibrin then interacts with amyloid-beta, the major pathogenic protein in AD.
Dr. Norris explained that fibrin clots can form in two different ways. One is through the normal process that occurs when there’s an injury and bleeding. The second is through intrinsic clotting, which takes place through the contact system.
“We started looking into this system and found that the plasma of Alzheimer’s patients showed irregular levels of these enzymes and proteins that are part of the intrinsic clotting system compared to those of normal controls,” said Dr. Norris.
“This paper was an extension of years studying this pathway and these mechanisms. It was also inspired by the approval of lecanemab and its release for use in Alzheimer’s patients,” she added.
In previous research, the same researchers found that amyloid-beta has different forms. “It’s normally soluble, and it’s a very tiny molecule,” Dr. Norris said. “But over time, and in different situations, it can start to aggregate, becoming bigger and bigger.”
Implications beyond Alzheimer’s
Postmortem tissue analysis has found fibrillar plaques that are “clumped together.” These are insoluble and hard to get rid of, she said. “Protofibrils are the step before amyloid-beta forms fibrils and are considered to be the most toxic form, although the mechanism behind why it’s so toxic is not understood.”
Previous research has already shown that amyloid-beta can activate the contact system. The contact system has two “arms,” the first of which is involved with clotting, and the second with inflammation, Dr. Norris said. In fact, it’s the plasma contact system that links vascular and inflammatory pathways.
The plasma contact system leads to the clotting of fibrin, Dr. Norris continued. It activates factor XII, which leads to blood clotting by binding to coagulation factor XI.
The contact system also causes inflammation – the second “arm.” Bradykinin, a potent inflammatory molecule, is released by binding to high-molecular-weight kininogen (HK). In addition to inflammation, bradykinin can cause edema and blood-brain barrier permeability.
Although it’s been known that amyloid-beta can activate the contact system, the particular form of amyloid-beta implicated in this cascade has not been identified. And so, the researchers incubated amyloid-beta42 with human plasma, testing various types of amyloid-beta – monomers, oligomers, protofibrils, and fibrils – to see which would activate the contact system.
Amyloid-beta protofibrils promoted the activation of the contact system, as evidenced by several reactions, including activation of factor XII, while other forms of amyloid-beta did not. HK also “bound tightly” to amyloid-beta protofibrils, with “weaker” binding to other amyloid-beta species, the authors reported, confirming that amyloid-beta protofibrils bind to HK and factor XII.
Bradykinin levels were increased by amyloid-beta protofibrils, which also induced faster clotting, compared with other forms of amyloid-beta.
The researchers introduced lecanemab into the picture and found it “dramatically inhibited” contact system activation induced by amyloid-beta protofibrils. For example, it blocked the binding of factor XII to amyloid-beta. By contrast, human IgG (which the researchers used as a control) had no effect.
Additionally, lecanemab also prevented accelerated intrinsic coagulation in normal human plasma mediated by amyloid-beta protofibril.
Senior author Sidney Strickland, PhD, the Zachary and Elizabeth M. Fisher professor in Alzheimer’s and neurodegenerative disease, Rockefeller University, said in an interview: “One of the strong motivators for conducting this study was the fact that this drug, which is effective in AD, targets this specific form of amyloid-beta; but no one knows why it›s more toxic. We thought we could see if we could tie it to what we›re working on, and we found it ties in beautifully.”
The findings have implications that go beyond AD, Dr. Strickland said. “The contact system is implicated in lots of different pathologies, including sickle cell anemia, sepsis, inflammatory bowel disease, and so on.” Blocking the contact system might be a helpful approach in these conditions too.
Innovative, plausible, but still preliminary
In a comment, Heather M. Snyder, PhD, vice president of medical and scientific relations at the Alzheimer’s Association, called the investigation “innovative,” with ideas that are “certainly plausible.” However, “at this time, the work is preliminary and not conclusive.”
The hypothesized mechanisms for why amyloid (lecanemab’s target) is toxic to the brain “does incorporate important AD-related brain changes that have been observed in other studies, including inflammatory/immune changes and vascular-related changes,” said Dr. Snyder, who was not involved with the current study.
However, “additional studies that look both in model systems and in humans are needed to further illuminate these relationships,” Dr. Snyder said.
The study was supported by grants from the National Institutes of Health as well as the Robertson Therapeutic Development Fund, Samuel Newhouse Foundation, John A. Herrmann, and the May and Samuel Rudin Family Foundation. Dr. Norris, Dr. Strickland, and Dr. Snyder declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Lecanemab (Lequembi, Esai), an amyloid-beta–directed antibody therapy, is approved by the Food and Drug Administration for the treatment of Alzheimer’s disease (AD). But exactly how the drug clears amyloid-beta wasn’t clear.
The investigators tested the effectiveness of various forms of amyloid-beta in activating the plasma contact system and found that amyloid-beta protofibrils, known to be the most toxic form of amyloid-beta, promoted the activation of this molecular cascade and that lecanemab inhibited pathway activation.
“In our study, we looked at lecanemab and found it can block the activation of the contact system, which could be one of the reasons that it works so well for AD,” study coinvestigator Erin Norris, PhD, research associate professor, Rockefeller University, New York, said in an interview.
The study was published online in the Proceedings of the National Academy of Science.
Unknown mechanism
“Many years ago, we started looking at the involvement of vascular dysfunction in AD,” Dr. Norris said. “We wanted to see whether or not irregular blood clotting or problems with blood flow was problematic in Alzheimer’s patients.”
The researchers found that fibrin, a major component involved in blood clotting, can extravasate into the brain.
“The blood-brain barrier can break down in Alzheimer’s, so things from the blood can move into the brain and deposit there,” she added. Fibrin then interacts with amyloid-beta, the major pathogenic protein in AD.
Dr. Norris explained that fibrin clots can form in two different ways. One is through the normal process that occurs when there’s an injury and bleeding. The second is through intrinsic clotting, which takes place through the contact system.
“We started looking into this system and found that the plasma of Alzheimer’s patients showed irregular levels of these enzymes and proteins that are part of the intrinsic clotting system compared to those of normal controls,” said Dr. Norris.
“This paper was an extension of years studying this pathway and these mechanisms. It was also inspired by the approval of lecanemab and its release for use in Alzheimer’s patients,” she added.
In previous research, the same researchers found that amyloid-beta has different forms. “It’s normally soluble, and it’s a very tiny molecule,” Dr. Norris said. “But over time, and in different situations, it can start to aggregate, becoming bigger and bigger.”
Implications beyond Alzheimer’s
Postmortem tissue analysis has found fibrillar plaques that are “clumped together.” These are insoluble and hard to get rid of, she said. “Protofibrils are the step before amyloid-beta forms fibrils and are considered to be the most toxic form, although the mechanism behind why it’s so toxic is not understood.”
Previous research has already shown that amyloid-beta can activate the contact system. The contact system has two “arms,” the first of which is involved with clotting, and the second with inflammation, Dr. Norris said. In fact, it’s the plasma contact system that links vascular and inflammatory pathways.
The plasma contact system leads to the clotting of fibrin, Dr. Norris continued. It activates factor XII, which leads to blood clotting by binding to coagulation factor XI.
The contact system also causes inflammation – the second “arm.” Bradykinin, a potent inflammatory molecule, is released by binding to high-molecular-weight kininogen (HK). In addition to inflammation, bradykinin can cause edema and blood-brain barrier permeability.
Although it’s been known that amyloid-beta can activate the contact system, the particular form of amyloid-beta implicated in this cascade has not been identified. And so, the researchers incubated amyloid-beta42 with human plasma, testing various types of amyloid-beta – monomers, oligomers, protofibrils, and fibrils – to see which would activate the contact system.
Amyloid-beta protofibrils promoted the activation of the contact system, as evidenced by several reactions, including activation of factor XII, while other forms of amyloid-beta did not. HK also “bound tightly” to amyloid-beta protofibrils, with “weaker” binding to other amyloid-beta species, the authors reported, confirming that amyloid-beta protofibrils bind to HK and factor XII.
Bradykinin levels were increased by amyloid-beta protofibrils, which also induced faster clotting, compared with other forms of amyloid-beta.
The researchers introduced lecanemab into the picture and found it “dramatically inhibited” contact system activation induced by amyloid-beta protofibrils. For example, it blocked the binding of factor XII to amyloid-beta. By contrast, human IgG (which the researchers used as a control) had no effect.
Additionally, lecanemab also prevented accelerated intrinsic coagulation in normal human plasma mediated by amyloid-beta protofibril.
Senior author Sidney Strickland, PhD, the Zachary and Elizabeth M. Fisher professor in Alzheimer’s and neurodegenerative disease, Rockefeller University, said in an interview: “One of the strong motivators for conducting this study was the fact that this drug, which is effective in AD, targets this specific form of amyloid-beta; but no one knows why it›s more toxic. We thought we could see if we could tie it to what we›re working on, and we found it ties in beautifully.”
The findings have implications that go beyond AD, Dr. Strickland said. “The contact system is implicated in lots of different pathologies, including sickle cell anemia, sepsis, inflammatory bowel disease, and so on.” Blocking the contact system might be a helpful approach in these conditions too.
Innovative, plausible, but still preliminary
In a comment, Heather M. Snyder, PhD, vice president of medical and scientific relations at the Alzheimer’s Association, called the investigation “innovative,” with ideas that are “certainly plausible.” However, “at this time, the work is preliminary and not conclusive.”
The hypothesized mechanisms for why amyloid (lecanemab’s target) is toxic to the brain “does incorporate important AD-related brain changes that have been observed in other studies, including inflammatory/immune changes and vascular-related changes,” said Dr. Snyder, who was not involved with the current study.
However, “additional studies that look both in model systems and in humans are needed to further illuminate these relationships,” Dr. Snyder said.
The study was supported by grants from the National Institutes of Health as well as the Robertson Therapeutic Development Fund, Samuel Newhouse Foundation, John A. Herrmann, and the May and Samuel Rudin Family Foundation. Dr. Norris, Dr. Strickland, and Dr. Snyder declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Lecanemab (Lequembi, Esai), an amyloid-beta–directed antibody therapy, is approved by the Food and Drug Administration for the treatment of Alzheimer’s disease (AD). But exactly how the drug clears amyloid-beta wasn’t clear.
The investigators tested the effectiveness of various forms of amyloid-beta in activating the plasma contact system and found that amyloid-beta protofibrils, known to be the most toxic form of amyloid-beta, promoted the activation of this molecular cascade and that lecanemab inhibited pathway activation.
“In our study, we looked at lecanemab and found it can block the activation of the contact system, which could be one of the reasons that it works so well for AD,” study coinvestigator Erin Norris, PhD, research associate professor, Rockefeller University, New York, said in an interview.
The study was published online in the Proceedings of the National Academy of Science.
Unknown mechanism
“Many years ago, we started looking at the involvement of vascular dysfunction in AD,” Dr. Norris said. “We wanted to see whether or not irregular blood clotting or problems with blood flow was problematic in Alzheimer’s patients.”
The researchers found that fibrin, a major component involved in blood clotting, can extravasate into the brain.
“The blood-brain barrier can break down in Alzheimer’s, so things from the blood can move into the brain and deposit there,” she added. Fibrin then interacts with amyloid-beta, the major pathogenic protein in AD.
Dr. Norris explained that fibrin clots can form in two different ways. One is through the normal process that occurs when there’s an injury and bleeding. The second is through intrinsic clotting, which takes place through the contact system.
“We started looking into this system and found that the plasma of Alzheimer’s patients showed irregular levels of these enzymes and proteins that are part of the intrinsic clotting system compared to those of normal controls,” said Dr. Norris.
“This paper was an extension of years studying this pathway and these mechanisms. It was also inspired by the approval of lecanemab and its release for use in Alzheimer’s patients,” she added.
In previous research, the same researchers found that amyloid-beta has different forms. “It’s normally soluble, and it’s a very tiny molecule,” Dr. Norris said. “But over time, and in different situations, it can start to aggregate, becoming bigger and bigger.”
Implications beyond Alzheimer’s
Postmortem tissue analysis has found fibrillar plaques that are “clumped together.” These are insoluble and hard to get rid of, she said. “Protofibrils are the step before amyloid-beta forms fibrils and are considered to be the most toxic form, although the mechanism behind why it’s so toxic is not understood.”
Previous research has already shown that amyloid-beta can activate the contact system. The contact system has two “arms,” the first of which is involved with clotting, and the second with inflammation, Dr. Norris said. In fact, it’s the plasma contact system that links vascular and inflammatory pathways.
The plasma contact system leads to the clotting of fibrin, Dr. Norris continued. It activates factor XII, which leads to blood clotting by binding to coagulation factor XI.
The contact system also causes inflammation – the second “arm.” Bradykinin, a potent inflammatory molecule, is released by binding to high-molecular-weight kininogen (HK). In addition to inflammation, bradykinin can cause edema and blood-brain barrier permeability.
Although it’s been known that amyloid-beta can activate the contact system, the particular form of amyloid-beta implicated in this cascade has not been identified. And so, the researchers incubated amyloid-beta42 with human plasma, testing various types of amyloid-beta – monomers, oligomers, protofibrils, and fibrils – to see which would activate the contact system.
Amyloid-beta protofibrils promoted the activation of the contact system, as evidenced by several reactions, including activation of factor XII, while other forms of amyloid-beta did not. HK also “bound tightly” to amyloid-beta protofibrils, with “weaker” binding to other amyloid-beta species, the authors reported, confirming that amyloid-beta protofibrils bind to HK and factor XII.
Bradykinin levels were increased by amyloid-beta protofibrils, which also induced faster clotting, compared with other forms of amyloid-beta.
The researchers introduced lecanemab into the picture and found it “dramatically inhibited” contact system activation induced by amyloid-beta protofibrils. For example, it blocked the binding of factor XII to amyloid-beta. By contrast, human IgG (which the researchers used as a control) had no effect.
Additionally, lecanemab also prevented accelerated intrinsic coagulation in normal human plasma mediated by amyloid-beta protofibril.
Senior author Sidney Strickland, PhD, the Zachary and Elizabeth M. Fisher professor in Alzheimer’s and neurodegenerative disease, Rockefeller University, said in an interview: “One of the strong motivators for conducting this study was the fact that this drug, which is effective in AD, targets this specific form of amyloid-beta; but no one knows why it›s more toxic. We thought we could see if we could tie it to what we›re working on, and we found it ties in beautifully.”
The findings have implications that go beyond AD, Dr. Strickland said. “The contact system is implicated in lots of different pathologies, including sickle cell anemia, sepsis, inflammatory bowel disease, and so on.” Blocking the contact system might be a helpful approach in these conditions too.
Innovative, plausible, but still preliminary
In a comment, Heather M. Snyder, PhD, vice president of medical and scientific relations at the Alzheimer’s Association, called the investigation “innovative,” with ideas that are “certainly plausible.” However, “at this time, the work is preliminary and not conclusive.”
The hypothesized mechanisms for why amyloid (lecanemab’s target) is toxic to the brain “does incorporate important AD-related brain changes that have been observed in other studies, including inflammatory/immune changes and vascular-related changes,” said Dr. Snyder, who was not involved with the current study.
However, “additional studies that look both in model systems and in humans are needed to further illuminate these relationships,” Dr. Snyder said.
The study was supported by grants from the National Institutes of Health as well as the Robertson Therapeutic Development Fund, Samuel Newhouse Foundation, John A. Herrmann, and the May and Samuel Rudin Family Foundation. Dr. Norris, Dr. Strickland, and Dr. Snyder declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCE
Sedentary lifestyle tied to increased dementia risk
The study of nearly 50,000 adults in the UK Biobank shows that dementia risk increased 8% with 10 hours of sedentary time and 63% with 12 hours. That’s particularly concerning because Americans spend an average of 9.5 hours a day sitting.
Sleep wasn’t factored into the sedentary time and how someone accumulated the 10 hours – either in one continuous block or broken up throughout the day – was irrelevant.
“Our analysis cannot determine whether there is a causal link, so prescriptive conclusions are not really possible; however. I think it is very reasonable to conclude that sitting less and moving more may help reduce risk of dementia,” lead investigator David Raichlen, PhD, professor of biological sciences and anthropology, University of Southern California, Los Angeles, said in an interview.
The findings were published online in JAMA.
A surprising find?
The study is a retrospective analysis of prospectively collected data from the UK Biobank of 49,841 adults aged 60 years or older who wore an accelerometer on their wrists 24 hours a day for a week. Participants had no history of dementia when they wore the movement monitoring device.
Investigators used machine-based learning to determine sedentary time based on readings from the accelerometers. Sleep was not included as sedentary behavior.
Over a mean follow-up of 6.72 years, 414 participants were diagnosed with dementia.
Investigators found that dementia risk rises by 8% at 10 hours a day (adjusted hazard ratio, 1.08; P < .001) and 63% at 12 hours a day (aHR, 1.63; P < .001), compared with 9.27 hours a day. Those who logged 15 hours of sedentary behavior a day had more than triple the dementia risk (aHR, 3.21; P < .001).
Although previous studies had found that breaking up sedentary periods with short bursts of activity help offset some negative health effects of sitting, that wasn’t the case here. Dementia risk was elevated whether participants were sedentary for 10 uninterrupted hours or multiple sedentary periods that totaled 10 hours over the whole day.
“This was surprising,” Dr. Raichlen said. “We expected to find that patterns of sedentary behavior would play a role in risk of dementia, but once you take into account the daily volume of time spent sedentary, how you get there doesn’t seem to matter as much.”
The study did not examine how participants spent sedentary time, but an earlier study by Dr. Raichlen found that watching TV was associated with a greater risk of dementia in older adults, compared with working on a computer.
More research welcome
Dr. Raichlen noted that the number of dementia cases in the study is low and that the view of sedentary behavior is based on 1 week of accelerometer readings. A longitudinal study is needed to determine if the findings last over a longer time period.
In a comment, Claire Sexton, DPhil, senior director of scientific programs and outreach for the Alzheimer’s Association, says that earlier studies reported an association between sedentary time and dementia, so these results aren’t “particularly surprising.”
“However, reports that did not find an association have also been published, so additional research on possible associations is welcome,” she said.
It’s also important to note that this observational study doesn’t establish a causal relationship between inactivity and cognitive function, which Dr. Sexton said means the influence of other dementia risk factors that are also exacerbated by sedentary behavior can’t be ruled out.
“Although results remained significant after adjusting for several of these factors, further research is required to better understand the various elements that may influence the observed relationship,” noted Dr. Sexton, who was not part of the study. “Reverse causality – that changes in the brain related to dementia are causing the sedentary behavior – cannot be ruled out.”
The study was funded by the National Institutes of Health, the state of Arizona, the Arizona Department of Health Services, and the McKnight Brain Research Foundation. Dr. Raichlen and Dr. Sexton report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The study of nearly 50,000 adults in the UK Biobank shows that dementia risk increased 8% with 10 hours of sedentary time and 63% with 12 hours. That’s particularly concerning because Americans spend an average of 9.5 hours a day sitting.
Sleep wasn’t factored into the sedentary time and how someone accumulated the 10 hours – either in one continuous block or broken up throughout the day – was irrelevant.
“Our analysis cannot determine whether there is a causal link, so prescriptive conclusions are not really possible; however. I think it is very reasonable to conclude that sitting less and moving more may help reduce risk of dementia,” lead investigator David Raichlen, PhD, professor of biological sciences and anthropology, University of Southern California, Los Angeles, said in an interview.
The findings were published online in JAMA.
A surprising find?
The study is a retrospective analysis of prospectively collected data from the UK Biobank of 49,841 adults aged 60 years or older who wore an accelerometer on their wrists 24 hours a day for a week. Participants had no history of dementia when they wore the movement monitoring device.
Investigators used machine-based learning to determine sedentary time based on readings from the accelerometers. Sleep was not included as sedentary behavior.
Over a mean follow-up of 6.72 years, 414 participants were diagnosed with dementia.
Investigators found that dementia risk rises by 8% at 10 hours a day (adjusted hazard ratio, 1.08; P < .001) and 63% at 12 hours a day (aHR, 1.63; P < .001), compared with 9.27 hours a day. Those who logged 15 hours of sedentary behavior a day had more than triple the dementia risk (aHR, 3.21; P < .001).
Although previous studies had found that breaking up sedentary periods with short bursts of activity help offset some negative health effects of sitting, that wasn’t the case here. Dementia risk was elevated whether participants were sedentary for 10 uninterrupted hours or multiple sedentary periods that totaled 10 hours over the whole day.
“This was surprising,” Dr. Raichlen said. “We expected to find that patterns of sedentary behavior would play a role in risk of dementia, but once you take into account the daily volume of time spent sedentary, how you get there doesn’t seem to matter as much.”
The study did not examine how participants spent sedentary time, but an earlier study by Dr. Raichlen found that watching TV was associated with a greater risk of dementia in older adults, compared with working on a computer.
More research welcome
Dr. Raichlen noted that the number of dementia cases in the study is low and that the view of sedentary behavior is based on 1 week of accelerometer readings. A longitudinal study is needed to determine if the findings last over a longer time period.
In a comment, Claire Sexton, DPhil, senior director of scientific programs and outreach for the Alzheimer’s Association, says that earlier studies reported an association between sedentary time and dementia, so these results aren’t “particularly surprising.”
“However, reports that did not find an association have also been published, so additional research on possible associations is welcome,” she said.
It’s also important to note that this observational study doesn’t establish a causal relationship between inactivity and cognitive function, which Dr. Sexton said means the influence of other dementia risk factors that are also exacerbated by sedentary behavior can’t be ruled out.
“Although results remained significant after adjusting for several of these factors, further research is required to better understand the various elements that may influence the observed relationship,” noted Dr. Sexton, who was not part of the study. “Reverse causality – that changes in the brain related to dementia are causing the sedentary behavior – cannot be ruled out.”
The study was funded by the National Institutes of Health, the state of Arizona, the Arizona Department of Health Services, and the McKnight Brain Research Foundation. Dr. Raichlen and Dr. Sexton report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The study of nearly 50,000 adults in the UK Biobank shows that dementia risk increased 8% with 10 hours of sedentary time and 63% with 12 hours. That’s particularly concerning because Americans spend an average of 9.5 hours a day sitting.
Sleep wasn’t factored into the sedentary time and how someone accumulated the 10 hours – either in one continuous block or broken up throughout the day – was irrelevant.
“Our analysis cannot determine whether there is a causal link, so prescriptive conclusions are not really possible; however. I think it is very reasonable to conclude that sitting less and moving more may help reduce risk of dementia,” lead investigator David Raichlen, PhD, professor of biological sciences and anthropology, University of Southern California, Los Angeles, said in an interview.
The findings were published online in JAMA.
A surprising find?
The study is a retrospective analysis of prospectively collected data from the UK Biobank of 49,841 adults aged 60 years or older who wore an accelerometer on their wrists 24 hours a day for a week. Participants had no history of dementia when they wore the movement monitoring device.
Investigators used machine-based learning to determine sedentary time based on readings from the accelerometers. Sleep was not included as sedentary behavior.
Over a mean follow-up of 6.72 years, 414 participants were diagnosed with dementia.
Investigators found that dementia risk rises by 8% at 10 hours a day (adjusted hazard ratio, 1.08; P < .001) and 63% at 12 hours a day (aHR, 1.63; P < .001), compared with 9.27 hours a day. Those who logged 15 hours of sedentary behavior a day had more than triple the dementia risk (aHR, 3.21; P < .001).
Although previous studies had found that breaking up sedentary periods with short bursts of activity help offset some negative health effects of sitting, that wasn’t the case here. Dementia risk was elevated whether participants were sedentary for 10 uninterrupted hours or multiple sedentary periods that totaled 10 hours over the whole day.
“This was surprising,” Dr. Raichlen said. “We expected to find that patterns of sedentary behavior would play a role in risk of dementia, but once you take into account the daily volume of time spent sedentary, how you get there doesn’t seem to matter as much.”
The study did not examine how participants spent sedentary time, but an earlier study by Dr. Raichlen found that watching TV was associated with a greater risk of dementia in older adults, compared with working on a computer.
More research welcome
Dr. Raichlen noted that the number of dementia cases in the study is low and that the view of sedentary behavior is based on 1 week of accelerometer readings. A longitudinal study is needed to determine if the findings last over a longer time period.
In a comment, Claire Sexton, DPhil, senior director of scientific programs and outreach for the Alzheimer’s Association, says that earlier studies reported an association between sedentary time and dementia, so these results aren’t “particularly surprising.”
“However, reports that did not find an association have also been published, so additional research on possible associations is welcome,” she said.
It’s also important to note that this observational study doesn’t establish a causal relationship between inactivity and cognitive function, which Dr. Sexton said means the influence of other dementia risk factors that are also exacerbated by sedentary behavior can’t be ruled out.
“Although results remained significant after adjusting for several of these factors, further research is required to better understand the various elements that may influence the observed relationship,” noted Dr. Sexton, who was not part of the study. “Reverse causality – that changes in the brain related to dementia are causing the sedentary behavior – cannot be ruled out.”
The study was funded by the National Institutes of Health, the state of Arizona, the Arizona Department of Health Services, and the McKnight Brain Research Foundation. Dr. Raichlen and Dr. Sexton report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA
Abdominal fat linked to lower brain volume in midlife
In a large study of healthy middle-aged adults, greater visceral and subcutaneous abdominal fat on abdominal MRI predicted brain atrophy on imaging, especially in women.
“The study shows that excess fat is bad for the brain and worse in women, including in Alzheimer’s disease risk regions,” lead author Cyrus Raji, MD, PhD, with the Mallinckrodt Institute of Radiology, Washington University, St. Louis, Mo., said in an interview.
The study was published online in the journal Aging and Disease
Modifiable risk factor
Multiple studies have suggested a connection between body fat accumulation and increased dementia risk. But few have examined the relationship between types of fat (visceral and subcutaneous) and brain volume.
For the new study, 10,000 healthy adults aged 20-80 years (mean age, 52.9 years; 53% men) underwent a short whole-body MRI protocol. Regression analyses of abdominal fat types and normalized brain volumes were evaluated, controlling for age and sex.
The research team found that higher amounts of both visceral and subcutaneous abdominal fat predicted lower total gray and white matter volume, as well as lower volume in the hippocampus, frontal cortex, and temporal, parietal, and occipital lobes.
“The findings are quite dramatic,” Dr. Raji told this news organization. “Overall, we found that both subcutaneous and visceral fat has similar levels of negative relationships with brain volumes.”
Women had a higher burden of brain atrophy with increased visceral fat than men. However, it’s difficult to place the sex differences in context because of the lack of prior work specifically investigating visceral fat, brain volume loss, and sex differences, the researchers caution.
They also note that while statistically significant relationships were observed between visceral fat levels and gray matter volume changes, their effect sizes were generally small.
“Thus, the statistical significance of this work is influenced by the large sample size and less so by large effect size in any given set of regions,” the investigators write.
Other limitations include the cross-sectional nature of the study, which precludes conclusions about causality. The analysis also did not account for other lifestyle factors such as physical activity, diet, and genetic variables.
The researchers call for further investigation “to better elucidate underlying mechanisms and discover possible interventions targeting abdominal fat reduction as a strategy to maintain brain health.”
‘Helpful addition to the literature’
In a comment, Claire Sexton, DPhil, Alzheimer’s Association senior director of scientific programs and outreach, noted that “previous studies have linked obesity with cognitive decline and increased risk of dementia. Rather than using BMI as a proxy for body fat, the current study examined visceral and subcutaneous fat directly using imaging techniques.”
Dr. Sexton, who was not associated with this study, said the finding that increased body fat was associated with reduced brain volumes suggests “a possible mechanism to explain the previously reported associations between obesity and cognition.”
“Though some degree of atrophy and brain shrinkage is common with old age, awareness of this association is important because reduced brain volume may be associated with problems with thinking, memory, and performing everyday tasks, and because rates of obesity continue to rise in the United States, along with obesity-related conditions including heart disease, stroke, type 2 diabetes and certain types of cancer,” she added.
“While a helpful addition to the literature, the study does have important limitations. As an observational study, it cannot establish whether higher levels of body fat directly causes reduced brain volumes,” Dr. Sexton cautioned.
In addition, the study did not take into account important related factors like physical activity and diet, which may influence any relationship between body fat and brain volumes, she noted. “Overall, it is not just one factor that is important to consider when considering risk for cognitive decline and dementia, but multiple factors.
“Obesity and the location of body fat must be considered in combination with one’s total lived experience and habits, including physical activity, education, head injury, sleep, mental health, and the health of your heart/cardiovascular system and other key bodily systems,” Dr. Sexton said.
The Alzheimer’s Association is leading a 2-year clinical trial known as U.S. POINTER to see whether combining physical activity, healthy nutrition, social and intellectual challenges, and improved self-management of medical conditions can protect cognitive function in older adults who are at increased risk for cognitive decline.
This work was supported in part by Providence St. Joseph Health in Seattle; Saint John’s Health Center Foundation; Pacific Neuroscience Institute and Foundation; Will and Cary Singleton; and the McLoughlin family. Dr. Raji is a consultant for Brainreader, Apollo Health, Voxelwise, Neurevolution, Pacific Neuroscience Institute Foundation, and Icometrix. Dr. Sexton reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a large study of healthy middle-aged adults, greater visceral and subcutaneous abdominal fat on abdominal MRI predicted brain atrophy on imaging, especially in women.
“The study shows that excess fat is bad for the brain and worse in women, including in Alzheimer’s disease risk regions,” lead author Cyrus Raji, MD, PhD, with the Mallinckrodt Institute of Radiology, Washington University, St. Louis, Mo., said in an interview.
The study was published online in the journal Aging and Disease
Modifiable risk factor
Multiple studies have suggested a connection between body fat accumulation and increased dementia risk. But few have examined the relationship between types of fat (visceral and subcutaneous) and brain volume.
For the new study, 10,000 healthy adults aged 20-80 years (mean age, 52.9 years; 53% men) underwent a short whole-body MRI protocol. Regression analyses of abdominal fat types and normalized brain volumes were evaluated, controlling for age and sex.
The research team found that higher amounts of both visceral and subcutaneous abdominal fat predicted lower total gray and white matter volume, as well as lower volume in the hippocampus, frontal cortex, and temporal, parietal, and occipital lobes.
“The findings are quite dramatic,” Dr. Raji told this news organization. “Overall, we found that both subcutaneous and visceral fat has similar levels of negative relationships with brain volumes.”
Women had a higher burden of brain atrophy with increased visceral fat than men. However, it’s difficult to place the sex differences in context because of the lack of prior work specifically investigating visceral fat, brain volume loss, and sex differences, the researchers caution.
They also note that while statistically significant relationships were observed between visceral fat levels and gray matter volume changes, their effect sizes were generally small.
“Thus, the statistical significance of this work is influenced by the large sample size and less so by large effect size in any given set of regions,” the investigators write.
Other limitations include the cross-sectional nature of the study, which precludes conclusions about causality. The analysis also did not account for other lifestyle factors such as physical activity, diet, and genetic variables.
The researchers call for further investigation “to better elucidate underlying mechanisms and discover possible interventions targeting abdominal fat reduction as a strategy to maintain brain health.”
‘Helpful addition to the literature’
In a comment, Claire Sexton, DPhil, Alzheimer’s Association senior director of scientific programs and outreach, noted that “previous studies have linked obesity with cognitive decline and increased risk of dementia. Rather than using BMI as a proxy for body fat, the current study examined visceral and subcutaneous fat directly using imaging techniques.”
Dr. Sexton, who was not associated with this study, said the finding that increased body fat was associated with reduced brain volumes suggests “a possible mechanism to explain the previously reported associations between obesity and cognition.”
“Though some degree of atrophy and brain shrinkage is common with old age, awareness of this association is important because reduced brain volume may be associated with problems with thinking, memory, and performing everyday tasks, and because rates of obesity continue to rise in the United States, along with obesity-related conditions including heart disease, stroke, type 2 diabetes and certain types of cancer,” she added.
“While a helpful addition to the literature, the study does have important limitations. As an observational study, it cannot establish whether higher levels of body fat directly causes reduced brain volumes,” Dr. Sexton cautioned.
In addition, the study did not take into account important related factors like physical activity and diet, which may influence any relationship between body fat and brain volumes, she noted. “Overall, it is not just one factor that is important to consider when considering risk for cognitive decline and dementia, but multiple factors.
“Obesity and the location of body fat must be considered in combination with one’s total lived experience and habits, including physical activity, education, head injury, sleep, mental health, and the health of your heart/cardiovascular system and other key bodily systems,” Dr. Sexton said.
The Alzheimer’s Association is leading a 2-year clinical trial known as U.S. POINTER to see whether combining physical activity, healthy nutrition, social and intellectual challenges, and improved self-management of medical conditions can protect cognitive function in older adults who are at increased risk for cognitive decline.
This work was supported in part by Providence St. Joseph Health in Seattle; Saint John’s Health Center Foundation; Pacific Neuroscience Institute and Foundation; Will and Cary Singleton; and the McLoughlin family. Dr. Raji is a consultant for Brainreader, Apollo Health, Voxelwise, Neurevolution, Pacific Neuroscience Institute Foundation, and Icometrix. Dr. Sexton reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a large study of healthy middle-aged adults, greater visceral and subcutaneous abdominal fat on abdominal MRI predicted brain atrophy on imaging, especially in women.
“The study shows that excess fat is bad for the brain and worse in women, including in Alzheimer’s disease risk regions,” lead author Cyrus Raji, MD, PhD, with the Mallinckrodt Institute of Radiology, Washington University, St. Louis, Mo., said in an interview.
The study was published online in the journal Aging and Disease
Modifiable risk factor
Multiple studies have suggested a connection between body fat accumulation and increased dementia risk. But few have examined the relationship between types of fat (visceral and subcutaneous) and brain volume.
For the new study, 10,000 healthy adults aged 20-80 years (mean age, 52.9 years; 53% men) underwent a short whole-body MRI protocol. Regression analyses of abdominal fat types and normalized brain volumes were evaluated, controlling for age and sex.
The research team found that higher amounts of both visceral and subcutaneous abdominal fat predicted lower total gray and white matter volume, as well as lower volume in the hippocampus, frontal cortex, and temporal, parietal, and occipital lobes.
“The findings are quite dramatic,” Dr. Raji told this news organization. “Overall, we found that both subcutaneous and visceral fat has similar levels of negative relationships with brain volumes.”
Women had a higher burden of brain atrophy with increased visceral fat than men. However, it’s difficult to place the sex differences in context because of the lack of prior work specifically investigating visceral fat, brain volume loss, and sex differences, the researchers caution.
They also note that while statistically significant relationships were observed between visceral fat levels and gray matter volume changes, their effect sizes were generally small.
“Thus, the statistical significance of this work is influenced by the large sample size and less so by large effect size in any given set of regions,” the investigators write.
Other limitations include the cross-sectional nature of the study, which precludes conclusions about causality. The analysis also did not account for other lifestyle factors such as physical activity, diet, and genetic variables.
The researchers call for further investigation “to better elucidate underlying mechanisms and discover possible interventions targeting abdominal fat reduction as a strategy to maintain brain health.”
‘Helpful addition to the literature’
In a comment, Claire Sexton, DPhil, Alzheimer’s Association senior director of scientific programs and outreach, noted that “previous studies have linked obesity with cognitive decline and increased risk of dementia. Rather than using BMI as a proxy for body fat, the current study examined visceral and subcutaneous fat directly using imaging techniques.”
Dr. Sexton, who was not associated with this study, said the finding that increased body fat was associated with reduced brain volumes suggests “a possible mechanism to explain the previously reported associations between obesity and cognition.”
“Though some degree of atrophy and brain shrinkage is common with old age, awareness of this association is important because reduced brain volume may be associated with problems with thinking, memory, and performing everyday tasks, and because rates of obesity continue to rise in the United States, along with obesity-related conditions including heart disease, stroke, type 2 diabetes and certain types of cancer,” she added.
“While a helpful addition to the literature, the study does have important limitations. As an observational study, it cannot establish whether higher levels of body fat directly causes reduced brain volumes,” Dr. Sexton cautioned.
In addition, the study did not take into account important related factors like physical activity and diet, which may influence any relationship between body fat and brain volumes, she noted. “Overall, it is not just one factor that is important to consider when considering risk for cognitive decline and dementia, but multiple factors.
“Obesity and the location of body fat must be considered in combination with one’s total lived experience and habits, including physical activity, education, head injury, sleep, mental health, and the health of your heart/cardiovascular system and other key bodily systems,” Dr. Sexton said.
The Alzheimer’s Association is leading a 2-year clinical trial known as U.S. POINTER to see whether combining physical activity, healthy nutrition, social and intellectual challenges, and improved self-management of medical conditions can protect cognitive function in older adults who are at increased risk for cognitive decline.
This work was supported in part by Providence St. Joseph Health in Seattle; Saint John’s Health Center Foundation; Pacific Neuroscience Institute and Foundation; Will and Cary Singleton; and the McLoughlin family. Dr. Raji is a consultant for Brainreader, Apollo Health, Voxelwise, Neurevolution, Pacific Neuroscience Institute Foundation, and Icometrix. Dr. Sexton reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AGING AND DISEASES
The magic of music
I’m really going to miss Jimmy Buffett.
I’ve liked his music as far back as I can remember, and was lucky enough to see him in person in the mid-90s.
I’ve written about music before, but its affect on us never fails to amaze me. Songs can be background noise conducive to getting things done. They can also be in the foreground, serving as a mental vacation (or accompanying a real one). They can transport you to another place, briefly clearing your head from the daily goings-on around you. Even if it’s just during the drive home, it’s a welcome escape to a virtual beach and tropical drink.
Songs can bring back memories of certain events or people that we link them to. My dad loved anything by Neil Diamond, and nothing brings back thoughts of Dad more than when my iTunes randomly picks “I Am ... I Said.” Or John Williams’ Star Wars theme, taking me back to the summer of 1977 when I sat, spellbound, by this incredible movie whose magic is still going strong two generations later.
It’s amazing how our brain tries to make music out of nothing. Even in silence we have ear worms, the songs stuck in our head for hours to days (recently I’ve had “I Sing the Body Electric” from the 1980 movie Fame playing in there).
My office is over an MRI scanner, so I can always hear the chiller pumps softly running in the background. Sometimes my brain will turn their rhythmic chirping into a song, altering the pace of the song to fit them. The soft clicking of the ceiling fan, in my home office, does the same thing (for some reason my brain usually tries to fit “Yellow Submarine” to that one, no idea why).
Music is a part of that mysterious essence that makes us human. It touches all of us in some way, which varies between people, songs, and artists.
Jimmy Buffet’s music has a vacation vibe. Songs of the Caribbean & Keys, beaches, bars, boats, and tropical drinks. The 4:12 running time of his most well-known song, “Margaritaville,” gives a brief respite from my day when it comes on.
He passes into the beyond, to the sadness of his family, friends, and fans. But, unlike people, music can be immortal, and so he lives on through his creations. Like, Bach, Lennon, Bowie, Joplin, Sousa, and too many others to count, his work – and the enjoyment we get from it – are a gift left behind for the future.
Tight lines, Jimmy.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’m really going to miss Jimmy Buffett.
I’ve liked his music as far back as I can remember, and was lucky enough to see him in person in the mid-90s.
I’ve written about music before, but its affect on us never fails to amaze me. Songs can be background noise conducive to getting things done. They can also be in the foreground, serving as a mental vacation (or accompanying a real one). They can transport you to another place, briefly clearing your head from the daily goings-on around you. Even if it’s just during the drive home, it’s a welcome escape to a virtual beach and tropical drink.
Songs can bring back memories of certain events or people that we link them to. My dad loved anything by Neil Diamond, and nothing brings back thoughts of Dad more than when my iTunes randomly picks “I Am ... I Said.” Or John Williams’ Star Wars theme, taking me back to the summer of 1977 when I sat, spellbound, by this incredible movie whose magic is still going strong two generations later.
It’s amazing how our brain tries to make music out of nothing. Even in silence we have ear worms, the songs stuck in our head for hours to days (recently I’ve had “I Sing the Body Electric” from the 1980 movie Fame playing in there).
My office is over an MRI scanner, so I can always hear the chiller pumps softly running in the background. Sometimes my brain will turn their rhythmic chirping into a song, altering the pace of the song to fit them. The soft clicking of the ceiling fan, in my home office, does the same thing (for some reason my brain usually tries to fit “Yellow Submarine” to that one, no idea why).
Music is a part of that mysterious essence that makes us human. It touches all of us in some way, which varies between people, songs, and artists.
Jimmy Buffet’s music has a vacation vibe. Songs of the Caribbean & Keys, beaches, bars, boats, and tropical drinks. The 4:12 running time of his most well-known song, “Margaritaville,” gives a brief respite from my day when it comes on.
He passes into the beyond, to the sadness of his family, friends, and fans. But, unlike people, music can be immortal, and so he lives on through his creations. Like, Bach, Lennon, Bowie, Joplin, Sousa, and too many others to count, his work – and the enjoyment we get from it – are a gift left behind for the future.
Tight lines, Jimmy.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’m really going to miss Jimmy Buffett.
I’ve liked his music as far back as I can remember, and was lucky enough to see him in person in the mid-90s.
I’ve written about music before, but its affect on us never fails to amaze me. Songs can be background noise conducive to getting things done. They can also be in the foreground, serving as a mental vacation (or accompanying a real one). They can transport you to another place, briefly clearing your head from the daily goings-on around you. Even if it’s just during the drive home, it’s a welcome escape to a virtual beach and tropical drink.
Songs can bring back memories of certain events or people that we link them to. My dad loved anything by Neil Diamond, and nothing brings back thoughts of Dad more than when my iTunes randomly picks “I Am ... I Said.” Or John Williams’ Star Wars theme, taking me back to the summer of 1977 when I sat, spellbound, by this incredible movie whose magic is still going strong two generations later.
It’s amazing how our brain tries to make music out of nothing. Even in silence we have ear worms, the songs stuck in our head for hours to days (recently I’ve had “I Sing the Body Electric” from the 1980 movie Fame playing in there).
My office is over an MRI scanner, so I can always hear the chiller pumps softly running in the background. Sometimes my brain will turn their rhythmic chirping into a song, altering the pace of the song to fit them. The soft clicking of the ceiling fan, in my home office, does the same thing (for some reason my brain usually tries to fit “Yellow Submarine” to that one, no idea why).
Music is a part of that mysterious essence that makes us human. It touches all of us in some way, which varies between people, songs, and artists.
Jimmy Buffet’s music has a vacation vibe. Songs of the Caribbean & Keys, beaches, bars, boats, and tropical drinks. The 4:12 running time of his most well-known song, “Margaritaville,” gives a brief respite from my day when it comes on.
He passes into the beyond, to the sadness of his family, friends, and fans. But, unlike people, music can be immortal, and so he lives on through his creations. Like, Bach, Lennon, Bowie, Joplin, Sousa, and too many others to count, his work – and the enjoyment we get from it – are a gift left behind for the future.
Tight lines, Jimmy.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Abnormal sexual behaviors in frontotemporal dementia
Mr. S, age 77, is admitted to a long-term care facility due to progressive cognitive impairment and sexually inappropriate behavior. He has a history of sexual assault of medical staff. His medical history includes significant frontotemporal dementia (FTD) with behavioral disturbances, abnormal sexual behaviors, subclinical hypothyroidism, schizoid personality disorder, Parkinson disease, posttraumatic stress disorder, and hyperammonemia.
Upon admission, Mr. S’s vital signs are within normal limits except for an elevated thyroid-stimulating hormone (4.54 mIU/L; reference range 0.40 to 4.50 mIU/L). Prior cognitive testing results and updated ammonia levels are unavailable. Mr. S’s current medications include acetaminophen 650 mg every 4 hours as needed for pain, calcium carbonate/vitamin D twice daily for bone health, carbidopa/levodopa 25/100 mg twice daily for Parkinson disease, melatonin 3 mg/d at bedtime for insomnia, quetiapine 25 mg twice daily for psychosis with disturbance of behavior and 12.5 mg every 4 hours as needed for agitation, and trazodone 50 mg/d at bedtime for insomnia. Before Mr. S was admitted, previous therapy with selective serotonin reuptake inhibitors (SSRIs) had been tapered and discontinued. Mr. S had also started antipsychotic therapy at another facility due to worsening behaviors.
In patients with dementia, the brain is experiencing neurodegeneration. Progressively, neurons may stop functioning, lose connections with other neurons, and ultimately face cell death. The specific dementia diagnosis and its clinical features depend on the type of neurons and region of the brain affected.1,2
FTD occurs in response to damage to the frontal and temporal lobes. The frontal lobe correlates to executive functioning, while the temporal lobe plays a role in speech and comprehension. Damage to these areas may result in loss of movement, trouble speaking, difficulty solving complex problems, and problems with social behavior. Specifically, damage to the orbital frontal cortex may cause disinhibition and abnormal behaviors, including emotional lability, vulgarity, and indifference to social nuances.1 Within an FTD diagnosis, there are 3 disorders: behavioral-variant FTD (bvFTD), semantic dementia, and progressive nonfluent aphasia.1 Specifically, bvFTD can result in abnormal sexual behaviors such as making sexually inappropriate statements, masturbating in public, undressing in public, inappropriately or aggressively touching others, or confusing another individual as an intimate partner. In addition to cognitive impairment, these neurobehavioral symptoms can significantly impact an individual’s quality of life while increasing caregiver burden.2
Occurring at a similar frequency to Alzheimer’s disease in patients age <65, FTD is one of the more common causes of early-onset dementia. The mean age of onset is 58 and onset after age 75 is particularly unusual. Memory may not be affected early in the course of the disease, but social changes are likely. As FTD progresses, symptoms will resemble those of Alzheimer’s disease and patients will require assistance with activities of daily living. In later stages of FTD, patients will exhibit language and behavior symptoms. Due to its unique progression, FTD can be commonly misdiagnosed as other mental illnesses or neurocognitive disorders.1
Approaches to treatment: What to consider
Both nonpharmacologic and pharmacologic interventions are appropriate for addressing FTD. Because nonpharmacologic options improve patient safety and overall physical health, they should be used whenever practical. These interventions include safe driving measures, exercise, speech therapy, redirection, offering simple choices when making decisions, and managing environmental cues for behaviors that should be encouraged or discouraged.3
There are no FDA-approved medications to cure or slow the progression of FTD. Therefore, treatment is focused on alleviating neurobehavioral symptoms. The symptoms depend on the type of FTD the patient has; they include cognitive impairment, anxiety, insomnia or sleep disturbances, compulsive behaviors, speech and language problems, and agitation. While many medications have been commonly used for symptomatic relief, evidence for the efficacy of these treatments in FTD is limited.2
Continue to: A review of the literature...
A review of the literature on potential treatments for cognitive impairment and behavioral symptoms of FTD identified 2 trials and 1 case series (Table 14-6) in addition to a 2014 review article7 of current pharmacologic treatments. These trials evaluated cognitive improvement with rivastigmine, memantine, galantamine, and donepezil. None of the trials found a significant benefit from any of these medications for cognitive improvement in FTD. Data were conflicting on whether these medications improved or worsened behavioral symptoms. For example, the case series of 3 patients by Swanberg6 suggested improvement in behavior with memantine, while an open-label study analyzed in a 2014 review article7 found that donepezil may have worsened behaviors. Use of cholinesterase inhibitors or memantine in FTD is not recommended unless it is not certain if the patient has FTD or Alzheimer’s disease.7
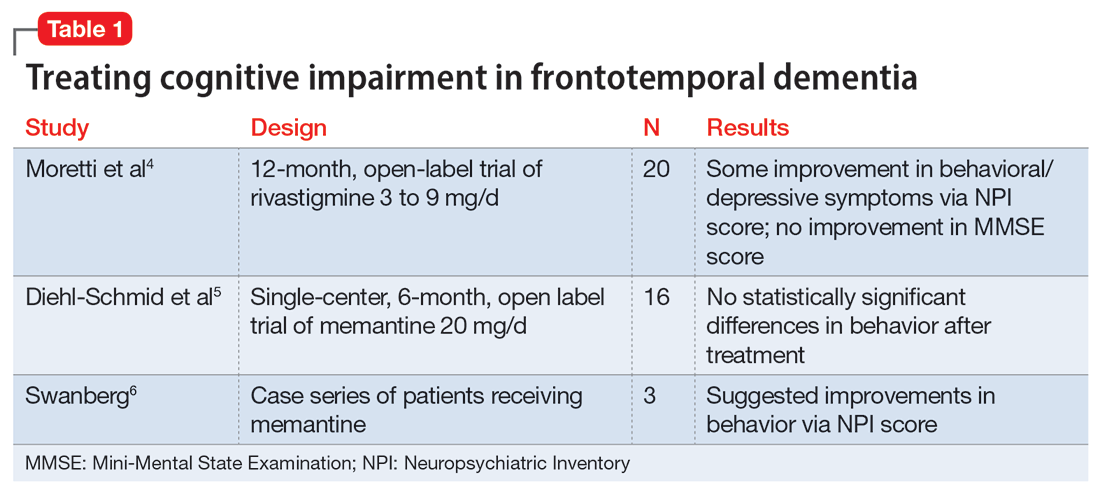
Addressing sexual behaviors. Creating a treatment regimen for FTD behavioral symptoms—specifically for abnormal sexual behaviors—can be challenging. Before starting pharmacotherapy directed at behavioral symptoms secondary to FTD, other causes of symptoms such as delirium, pain, or discomfort should be excluded. Nonpharmacologic approaches should be aimed at the type of sexual behavior and likely underlying environmental cause. For example, patients may inappropriately disrobe themselves. To address this behavior, hospital staff or caregivers should first eliminate environmental causes by ensuring the room is at a comfortable temperature, dressing the patient in light, breathable clothing, or checking if the patient needs to use the bathroom. If no environmental causes are found, a one-piece jumpsuit with closures on the back of the garment could be utilized to increase the difficulty of undressing.
Other nonpharmacologic methods include providing private areas for patients who are behaving inappropriately or removing potentially stimulating television or media from the environment. Another option is to increase the use of positive, pleasant stimuli. One approach that has shown benefit is music therapy, utilizing popular music genres from the patient’s youth.3
Evidence for pharmacotherapy is limited and largely from case reports and case series. A 2020 meta-analysis by Trieu et al8 reviewed 23 studies to expand on current clinical guidance for patients with bvFTD. These studies showed improvements in behavioral symptoms and reductions in caregiver fatigue with citalopram, trazodone, paroxetine, and fluvoxamine. Six of the trials included in this meta-analysis that evaluated these 4 medications are summarized in Table 2.9-14
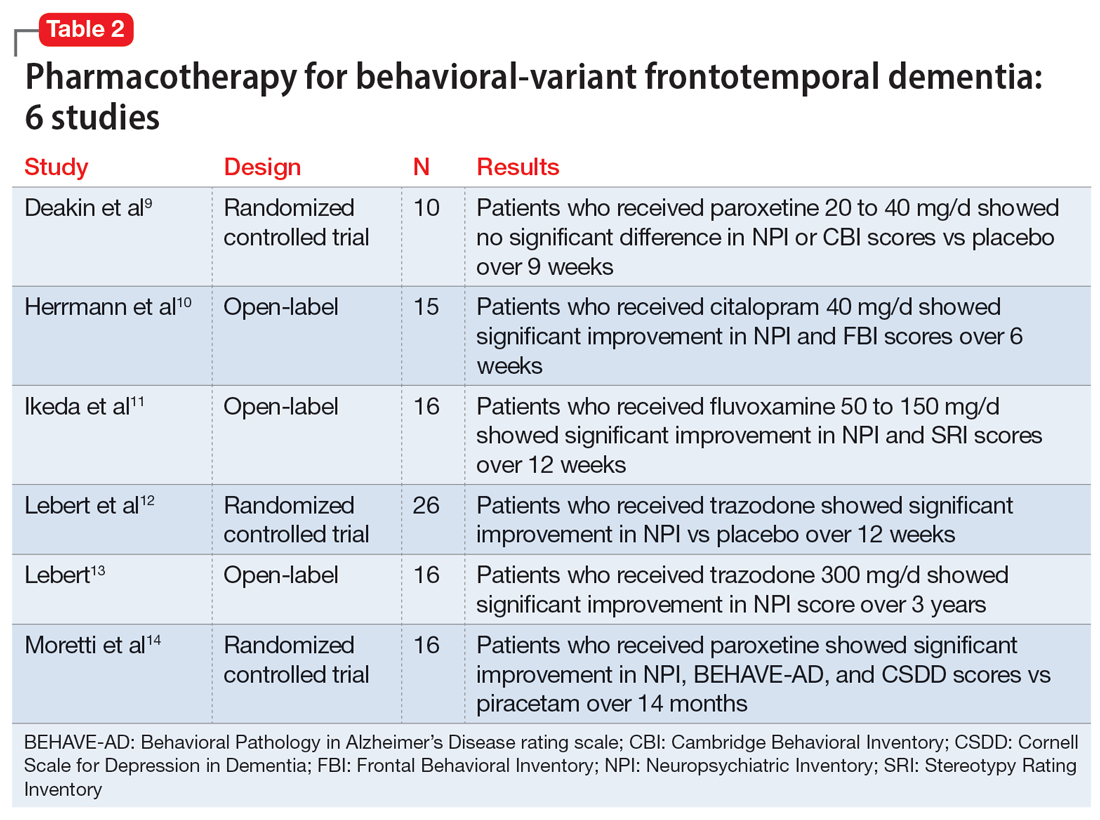
Due to the lower risk of adverse effects and favorable safety profiles, SSRIs and trazodone are considered first-line treatment options. Benefit from these medications is theorized to be a result of their serotonergic effects, because serotonin abnormalities and dysfunction have been linked to FTD symptoms. For example, in a patient experiencing hypersexuality, the common adverse effect of low libido associated with SSRIs can be particularly beneficial.8
Continue to: Other medication classes studied in patients...
Other medication classes studied in patients with FTD include antipsychotics, stimulants, anticonvulsants, benzodiazepines, and hormonal therapies. In addition to a black box warning for increased mortality in older patients with dementia-related psychosis, antipsychotics are associated with other serious adverse effects and should be used with caution.7
FTD is a debilitating disease that has a major impact on quality of life, particularly when behavioral symptoms accompany cognitive decline. Though some therapies may possibly improve behavioral symptoms, their routine use remains controversial due to a lack of clear evidence of benefit. In caring for patients with FTD and behavioral symptoms, a multimodal, team-based approach is vital.1
CASE CONTINUED
The treatment team starts Mr. S on several of the modalities discussed in this article over the span of 2 years, with limited efficacy. Nonpharmacologic methods do not provide much benefit because Mr. S is extremely difficult to redirect. Given Mr. S’s past trials of SSRIs prior to admission, sertraline was retrialed and titrated over 2 years. The highest dose utilized during his admission was 200 mg/d. The team starts estrogen therapy but tapers and discontinues it due to ineffectiveness. Mr. S’s use of carbidopa/levodopa is thought to be contributing to his behavioral abnormalities, so the team tapers it to discontinuation; however, Mr. S’s sexually inappropriate behaviors and agitation continue. The team initiates a plan to reduce the dose of quetiapine and switch to gabapentin, but Mr. S fails gradual dose reduction due to his worsening behaviors. He starts gabapentin. The team gradually increases the dose of gabapentin to decrease libido and agitation, respectively. The increase in sertraline dose and use of nonpharmacologic modalities causes Mr. S’s use of as-needed antipsychotics to decrease.
Related Resources
- Ellison JM. What are the stages of frontotemporal dementia? BrightFocus Foundation. July 5, 2021. Accessed July 7, 2023. https://www.brightfocus.org/alzheimers/article/what-are-stages-frontotemporal-dementia
- Dementia and sexually inappropriate behavior. ReaDementia. January 31, 2022. Accessed July 7, 2023. https://readementia.com/dementia-and-sexually-inappropriate-behavior/
Drug Brand Names
Carbidopa/levodopa • Sinemet
Citalopram • Celexa
Donepezil • Aricept
Fluvoxamine • Luvox
Gabapentin • Neurontin
Galantamine • Razadyne
Memantine • Namenda
Paroxetine • Paxil
Quetiapine • Seroquel
Rivastigmine • Exelon
Sertraline • Zoloft
Trazodone • Desyrel
1. Grossman M. Frontotemporal dementia: a review. J Int Neuropsychol Soc. 2002;8(4):566-583. doi:10.1017/s1355617702814357
2. The Johns Hopkins University. Frontotemporal dementia. Johns Hopkins Medicine. Accessed September 12, 2021. https://www.hopkinsmedicine.org/health/conditions-and-diseases/dementia/frontotemporal-dementia
3. Shinagawa S, Nakajima S, Plitman E, et al. Non-pharmacological management for patients with frontotemporal dementia: a systematic review. J Alzheimers Dis. 2015;45(1):283-293. doi:10.3233/JAD-142109
4. Moretti R, Torre P, Antonello RM, et al. Rivastigmine in frontotemporal dementia: an open-label study. Drugs Aging. 2004;21(14):931-937. doi:10.2165/00002512-200421140-00003
5. Diehl-Schmid J, Förstl H, Perneczky R, et al. A 6-month, open-label study for memantine in patients with frontotemporal dementia. In J Geriatr Psychiatry. 2008;23(7):754-759. doi:10.1002/gps.1973
6. Swanberg MM. Memantine for behavioral disturbances in frontotemporal dementia: a case series. Alzheimer Dis Assoc Disord. 2007;21(2):164-166. doi:10.1097/WAD.0b013e318047df5d
7. Tsai RM, Boxer AL. Treatment of frontotemporal dementia. Curr Treat Options Neurol. 2014;16(11):319. doi:10.1007/s11940-014-0319-0
8. Trieu C, Gossink F, Stek ML, et al. Effectiveness of pharmacological interventions for symptoms of behavioral variant frontotemporal dementia: a systematic review. Cogn Behav Neurol. 2020;33(1):1-15. doi:10.1097/WNN.0000000000000217
9. Deakin JB, Rahman S, Nestor PJ, et al. Paroxetine does not improve symptoms and impairs cognition in frontotemporal dementia: a double-blind randomized controlled trial. Psychopharmacology (Berl). 2004;172(4):400-408. doi:10.1007/s00213-003-1686-5
10. Herrmann N, Black SE, Chow T, et al. Serotonergic function and treatment of behavioral and psychological symptoms of frontotemporal dementia. Am J Geriatr Psychiatry. 2012;20(9):789-797. doi:10.1097/JGP.0b013e31823033f3
11. Ikeda M, Shigenobu K, Fukuhara R, et al. Efficacy of fluvoxamine as a treatment for behavioral symptoms in frontotemporal lobar degeneration patients. Dement Geriatr Cogn Disord. 2004;17(3):117-121. doi:10.1159/000076343
12. Lebert F, Stekke W, Hasenbroekx C, et al. Frontotemporal dementia: a randomised, controlled trial with trazodone. Dement Geriatr Cogn Disord. 2004;17(4):355-359. doi:10.1159/000077171
13. Lebert F. Behavioral benefits of trazodone are sustained for the long term in frontotemporal dementia. Therapy. 2006;3(1):93-96. doi:10.1586/14750708.3.1.93
14. Moretti R, Torre P, Antonello RM, et al. Frontotemporal dementia: paroxetine as a possible treatment of behavior symptoms. A randomized, controlled, open 14-month study. Eur Neurol. 2003;49(1):13-19. doi:10.1159/000067021
Mr. S, age 77, is admitted to a long-term care facility due to progressive cognitive impairment and sexually inappropriate behavior. He has a history of sexual assault of medical staff. His medical history includes significant frontotemporal dementia (FTD) with behavioral disturbances, abnormal sexual behaviors, subclinical hypothyroidism, schizoid personality disorder, Parkinson disease, posttraumatic stress disorder, and hyperammonemia.
Upon admission, Mr. S’s vital signs are within normal limits except for an elevated thyroid-stimulating hormone (4.54 mIU/L; reference range 0.40 to 4.50 mIU/L). Prior cognitive testing results and updated ammonia levels are unavailable. Mr. S’s current medications include acetaminophen 650 mg every 4 hours as needed for pain, calcium carbonate/vitamin D twice daily for bone health, carbidopa/levodopa 25/100 mg twice daily for Parkinson disease, melatonin 3 mg/d at bedtime for insomnia, quetiapine 25 mg twice daily for psychosis with disturbance of behavior and 12.5 mg every 4 hours as needed for agitation, and trazodone 50 mg/d at bedtime for insomnia. Before Mr. S was admitted, previous therapy with selective serotonin reuptake inhibitors (SSRIs) had been tapered and discontinued. Mr. S had also started antipsychotic therapy at another facility due to worsening behaviors.
In patients with dementia, the brain is experiencing neurodegeneration. Progressively, neurons may stop functioning, lose connections with other neurons, and ultimately face cell death. The specific dementia diagnosis and its clinical features depend on the type of neurons and region of the brain affected.1,2
FTD occurs in response to damage to the frontal and temporal lobes. The frontal lobe correlates to executive functioning, while the temporal lobe plays a role in speech and comprehension. Damage to these areas may result in loss of movement, trouble speaking, difficulty solving complex problems, and problems with social behavior. Specifically, damage to the orbital frontal cortex may cause disinhibition and abnormal behaviors, including emotional lability, vulgarity, and indifference to social nuances.1 Within an FTD diagnosis, there are 3 disorders: behavioral-variant FTD (bvFTD), semantic dementia, and progressive nonfluent aphasia.1 Specifically, bvFTD can result in abnormal sexual behaviors such as making sexually inappropriate statements, masturbating in public, undressing in public, inappropriately or aggressively touching others, or confusing another individual as an intimate partner. In addition to cognitive impairment, these neurobehavioral symptoms can significantly impact an individual’s quality of life while increasing caregiver burden.2
Occurring at a similar frequency to Alzheimer’s disease in patients age <65, FTD is one of the more common causes of early-onset dementia. The mean age of onset is 58 and onset after age 75 is particularly unusual. Memory may not be affected early in the course of the disease, but social changes are likely. As FTD progresses, symptoms will resemble those of Alzheimer’s disease and patients will require assistance with activities of daily living. In later stages of FTD, patients will exhibit language and behavior symptoms. Due to its unique progression, FTD can be commonly misdiagnosed as other mental illnesses or neurocognitive disorders.1
Approaches to treatment: What to consider
Both nonpharmacologic and pharmacologic interventions are appropriate for addressing FTD. Because nonpharmacologic options improve patient safety and overall physical health, they should be used whenever practical. These interventions include safe driving measures, exercise, speech therapy, redirection, offering simple choices when making decisions, and managing environmental cues for behaviors that should be encouraged or discouraged.3
There are no FDA-approved medications to cure or slow the progression of FTD. Therefore, treatment is focused on alleviating neurobehavioral symptoms. The symptoms depend on the type of FTD the patient has; they include cognitive impairment, anxiety, insomnia or sleep disturbances, compulsive behaviors, speech and language problems, and agitation. While many medications have been commonly used for symptomatic relief, evidence for the efficacy of these treatments in FTD is limited.2
Continue to: A review of the literature...
A review of the literature on potential treatments for cognitive impairment and behavioral symptoms of FTD identified 2 trials and 1 case series (Table 14-6) in addition to a 2014 review article7 of current pharmacologic treatments. These trials evaluated cognitive improvement with rivastigmine, memantine, galantamine, and donepezil. None of the trials found a significant benefit from any of these medications for cognitive improvement in FTD. Data were conflicting on whether these medications improved or worsened behavioral symptoms. For example, the case series of 3 patients by Swanberg6 suggested improvement in behavior with memantine, while an open-label study analyzed in a 2014 review article7 found that donepezil may have worsened behaviors. Use of cholinesterase inhibitors or memantine in FTD is not recommended unless it is not certain if the patient has FTD or Alzheimer’s disease.7

Addressing sexual behaviors. Creating a treatment regimen for FTD behavioral symptoms—specifically for abnormal sexual behaviors—can be challenging. Before starting pharmacotherapy directed at behavioral symptoms secondary to FTD, other causes of symptoms such as delirium, pain, or discomfort should be excluded. Nonpharmacologic approaches should be aimed at the type of sexual behavior and likely underlying environmental cause. For example, patients may inappropriately disrobe themselves. To address this behavior, hospital staff or caregivers should first eliminate environmental causes by ensuring the room is at a comfortable temperature, dressing the patient in light, breathable clothing, or checking if the patient needs to use the bathroom. If no environmental causes are found, a one-piece jumpsuit with closures on the back of the garment could be utilized to increase the difficulty of undressing.
Other nonpharmacologic methods include providing private areas for patients who are behaving inappropriately or removing potentially stimulating television or media from the environment. Another option is to increase the use of positive, pleasant stimuli. One approach that has shown benefit is music therapy, utilizing popular music genres from the patient’s youth.3
Evidence for pharmacotherapy is limited and largely from case reports and case series. A 2020 meta-analysis by Trieu et al8 reviewed 23 studies to expand on current clinical guidance for patients with bvFTD. These studies showed improvements in behavioral symptoms and reductions in caregiver fatigue with citalopram, trazodone, paroxetine, and fluvoxamine. Six of the trials included in this meta-analysis that evaluated these 4 medications are summarized in Table 2.9-14

Due to the lower risk of adverse effects and favorable safety profiles, SSRIs and trazodone are considered first-line treatment options. Benefit from these medications is theorized to be a result of their serotonergic effects, because serotonin abnormalities and dysfunction have been linked to FTD symptoms. For example, in a patient experiencing hypersexuality, the common adverse effect of low libido associated with SSRIs can be particularly beneficial.8
Continue to: Other medication classes studied in patients...
Other medication classes studied in patients with FTD include antipsychotics, stimulants, anticonvulsants, benzodiazepines, and hormonal therapies. In addition to a black box warning for increased mortality in older patients with dementia-related psychosis, antipsychotics are associated with other serious adverse effects and should be used with caution.7
FTD is a debilitating disease that has a major impact on quality of life, particularly when behavioral symptoms accompany cognitive decline. Though some therapies may possibly improve behavioral symptoms, their routine use remains controversial due to a lack of clear evidence of benefit. In caring for patients with FTD and behavioral symptoms, a multimodal, team-based approach is vital.1
CASE CONTINUED
The treatment team starts Mr. S on several of the modalities discussed in this article over the span of 2 years, with limited efficacy. Nonpharmacologic methods do not provide much benefit because Mr. S is extremely difficult to redirect. Given Mr. S’s past trials of SSRIs prior to admission, sertraline was retrialed and titrated over 2 years. The highest dose utilized during his admission was 200 mg/d. The team starts estrogen therapy but tapers and discontinues it due to ineffectiveness. Mr. S’s use of carbidopa/levodopa is thought to be contributing to his behavioral abnormalities, so the team tapers it to discontinuation; however, Mr. S’s sexually inappropriate behaviors and agitation continue. The team initiates a plan to reduce the dose of quetiapine and switch to gabapentin, but Mr. S fails gradual dose reduction due to his worsening behaviors. He starts gabapentin. The team gradually increases the dose of gabapentin to decrease libido and agitation, respectively. The increase in sertraline dose and use of nonpharmacologic modalities causes Mr. S’s use of as-needed antipsychotics to decrease.
Related Resources
- Ellison JM. What are the stages of frontotemporal dementia? BrightFocus Foundation. July 5, 2021. Accessed July 7, 2023. https://www.brightfocus.org/alzheimers/article/what-are-stages-frontotemporal-dementia
- Dementia and sexually inappropriate behavior. ReaDementia. January 31, 2022. Accessed July 7, 2023. https://readementia.com/dementia-and-sexually-inappropriate-behavior/
Drug Brand Names
Carbidopa/levodopa • Sinemet
Citalopram • Celexa
Donepezil • Aricept
Fluvoxamine • Luvox
Gabapentin • Neurontin
Galantamine • Razadyne
Memantine • Namenda
Paroxetine • Paxil
Quetiapine • Seroquel
Rivastigmine • Exelon
Sertraline • Zoloft
Trazodone • Desyrel
Mr. S, age 77, is admitted to a long-term care facility due to progressive cognitive impairment and sexually inappropriate behavior. He has a history of sexual assault of medical staff. His medical history includes significant frontotemporal dementia (FTD) with behavioral disturbances, abnormal sexual behaviors, subclinical hypothyroidism, schizoid personality disorder, Parkinson disease, posttraumatic stress disorder, and hyperammonemia.
Upon admission, Mr. S’s vital signs are within normal limits except for an elevated thyroid-stimulating hormone (4.54 mIU/L; reference range 0.40 to 4.50 mIU/L). Prior cognitive testing results and updated ammonia levels are unavailable. Mr. S’s current medications include acetaminophen 650 mg every 4 hours as needed for pain, calcium carbonate/vitamin D twice daily for bone health, carbidopa/levodopa 25/100 mg twice daily for Parkinson disease, melatonin 3 mg/d at bedtime for insomnia, quetiapine 25 mg twice daily for psychosis with disturbance of behavior and 12.5 mg every 4 hours as needed for agitation, and trazodone 50 mg/d at bedtime for insomnia. Before Mr. S was admitted, previous therapy with selective serotonin reuptake inhibitors (SSRIs) had been tapered and discontinued. Mr. S had also started antipsychotic therapy at another facility due to worsening behaviors.
In patients with dementia, the brain is experiencing neurodegeneration. Progressively, neurons may stop functioning, lose connections with other neurons, and ultimately face cell death. The specific dementia diagnosis and its clinical features depend on the type of neurons and region of the brain affected.1,2
FTD occurs in response to damage to the frontal and temporal lobes. The frontal lobe correlates to executive functioning, while the temporal lobe plays a role in speech and comprehension. Damage to these areas may result in loss of movement, trouble speaking, difficulty solving complex problems, and problems with social behavior. Specifically, damage to the orbital frontal cortex may cause disinhibition and abnormal behaviors, including emotional lability, vulgarity, and indifference to social nuances.1 Within an FTD diagnosis, there are 3 disorders: behavioral-variant FTD (bvFTD), semantic dementia, and progressive nonfluent aphasia.1 Specifically, bvFTD can result in abnormal sexual behaviors such as making sexually inappropriate statements, masturbating in public, undressing in public, inappropriately or aggressively touching others, or confusing another individual as an intimate partner. In addition to cognitive impairment, these neurobehavioral symptoms can significantly impact an individual’s quality of life while increasing caregiver burden.2
Occurring at a similar frequency to Alzheimer’s disease in patients age <65, FTD is one of the more common causes of early-onset dementia. The mean age of onset is 58 and onset after age 75 is particularly unusual. Memory may not be affected early in the course of the disease, but social changes are likely. As FTD progresses, symptoms will resemble those of Alzheimer’s disease and patients will require assistance with activities of daily living. In later stages of FTD, patients will exhibit language and behavior symptoms. Due to its unique progression, FTD can be commonly misdiagnosed as other mental illnesses or neurocognitive disorders.1
Approaches to treatment: What to consider
Both nonpharmacologic and pharmacologic interventions are appropriate for addressing FTD. Because nonpharmacologic options improve patient safety and overall physical health, they should be used whenever practical. These interventions include safe driving measures, exercise, speech therapy, redirection, offering simple choices when making decisions, and managing environmental cues for behaviors that should be encouraged or discouraged.3
There are no FDA-approved medications to cure or slow the progression of FTD. Therefore, treatment is focused on alleviating neurobehavioral symptoms. The symptoms depend on the type of FTD the patient has; they include cognitive impairment, anxiety, insomnia or sleep disturbances, compulsive behaviors, speech and language problems, and agitation. While many medications have been commonly used for symptomatic relief, evidence for the efficacy of these treatments in FTD is limited.2
Continue to: A review of the literature...
A review of the literature on potential treatments for cognitive impairment and behavioral symptoms of FTD identified 2 trials and 1 case series (Table 14-6) in addition to a 2014 review article7 of current pharmacologic treatments. These trials evaluated cognitive improvement with rivastigmine, memantine, galantamine, and donepezil. None of the trials found a significant benefit from any of these medications for cognitive improvement in FTD. Data were conflicting on whether these medications improved or worsened behavioral symptoms. For example, the case series of 3 patients by Swanberg6 suggested improvement in behavior with memantine, while an open-label study analyzed in a 2014 review article7 found that donepezil may have worsened behaviors. Use of cholinesterase inhibitors or memantine in FTD is not recommended unless it is not certain if the patient has FTD or Alzheimer’s disease.7

Addressing sexual behaviors. Creating a treatment regimen for FTD behavioral symptoms—specifically for abnormal sexual behaviors—can be challenging. Before starting pharmacotherapy directed at behavioral symptoms secondary to FTD, other causes of symptoms such as delirium, pain, or discomfort should be excluded. Nonpharmacologic approaches should be aimed at the type of sexual behavior and likely underlying environmental cause. For example, patients may inappropriately disrobe themselves. To address this behavior, hospital staff or caregivers should first eliminate environmental causes by ensuring the room is at a comfortable temperature, dressing the patient in light, breathable clothing, or checking if the patient needs to use the bathroom. If no environmental causes are found, a one-piece jumpsuit with closures on the back of the garment could be utilized to increase the difficulty of undressing.
Other nonpharmacologic methods include providing private areas for patients who are behaving inappropriately or removing potentially stimulating television or media from the environment. Another option is to increase the use of positive, pleasant stimuli. One approach that has shown benefit is music therapy, utilizing popular music genres from the patient’s youth.3
Evidence for pharmacotherapy is limited and largely from case reports and case series. A 2020 meta-analysis by Trieu et al8 reviewed 23 studies to expand on current clinical guidance for patients with bvFTD. These studies showed improvements in behavioral symptoms and reductions in caregiver fatigue with citalopram, trazodone, paroxetine, and fluvoxamine. Six of the trials included in this meta-analysis that evaluated these 4 medications are summarized in Table 2.9-14

Due to the lower risk of adverse effects and favorable safety profiles, SSRIs and trazodone are considered first-line treatment options. Benefit from these medications is theorized to be a result of their serotonergic effects, because serotonin abnormalities and dysfunction have been linked to FTD symptoms. For example, in a patient experiencing hypersexuality, the common adverse effect of low libido associated with SSRIs can be particularly beneficial.8
Continue to: Other medication classes studied in patients...
Other medication classes studied in patients with FTD include antipsychotics, stimulants, anticonvulsants, benzodiazepines, and hormonal therapies. In addition to a black box warning for increased mortality in older patients with dementia-related psychosis, antipsychotics are associated with other serious adverse effects and should be used with caution.7
FTD is a debilitating disease that has a major impact on quality of life, particularly when behavioral symptoms accompany cognitive decline. Though some therapies may possibly improve behavioral symptoms, their routine use remains controversial due to a lack of clear evidence of benefit. In caring for patients with FTD and behavioral symptoms, a multimodal, team-based approach is vital.1
CASE CONTINUED
The treatment team starts Mr. S on several of the modalities discussed in this article over the span of 2 years, with limited efficacy. Nonpharmacologic methods do not provide much benefit because Mr. S is extremely difficult to redirect. Given Mr. S’s past trials of SSRIs prior to admission, sertraline was retrialed and titrated over 2 years. The highest dose utilized during his admission was 200 mg/d. The team starts estrogen therapy but tapers and discontinues it due to ineffectiveness. Mr. S’s use of carbidopa/levodopa is thought to be contributing to his behavioral abnormalities, so the team tapers it to discontinuation; however, Mr. S’s sexually inappropriate behaviors and agitation continue. The team initiates a plan to reduce the dose of quetiapine and switch to gabapentin, but Mr. S fails gradual dose reduction due to his worsening behaviors. He starts gabapentin. The team gradually increases the dose of gabapentin to decrease libido and agitation, respectively. The increase in sertraline dose and use of nonpharmacologic modalities causes Mr. S’s use of as-needed antipsychotics to decrease.
Related Resources
- Ellison JM. What are the stages of frontotemporal dementia? BrightFocus Foundation. July 5, 2021. Accessed July 7, 2023. https://www.brightfocus.org/alzheimers/article/what-are-stages-frontotemporal-dementia
- Dementia and sexually inappropriate behavior. ReaDementia. January 31, 2022. Accessed July 7, 2023. https://readementia.com/dementia-and-sexually-inappropriate-behavior/
Drug Brand Names
Carbidopa/levodopa • Sinemet
Citalopram • Celexa
Donepezil • Aricept
Fluvoxamine • Luvox
Gabapentin • Neurontin
Galantamine • Razadyne
Memantine • Namenda
Paroxetine • Paxil
Quetiapine • Seroquel
Rivastigmine • Exelon
Sertraline • Zoloft
Trazodone • Desyrel
1. Grossman M. Frontotemporal dementia: a review. J Int Neuropsychol Soc. 2002;8(4):566-583. doi:10.1017/s1355617702814357
2. The Johns Hopkins University. Frontotemporal dementia. Johns Hopkins Medicine. Accessed September 12, 2021. https://www.hopkinsmedicine.org/health/conditions-and-diseases/dementia/frontotemporal-dementia
3. Shinagawa S, Nakajima S, Plitman E, et al. Non-pharmacological management for patients with frontotemporal dementia: a systematic review. J Alzheimers Dis. 2015;45(1):283-293. doi:10.3233/JAD-142109
4. Moretti R, Torre P, Antonello RM, et al. Rivastigmine in frontotemporal dementia: an open-label study. Drugs Aging. 2004;21(14):931-937. doi:10.2165/00002512-200421140-00003
5. Diehl-Schmid J, Förstl H, Perneczky R, et al. A 6-month, open-label study for memantine in patients with frontotemporal dementia. In J Geriatr Psychiatry. 2008;23(7):754-759. doi:10.1002/gps.1973
6. Swanberg MM. Memantine for behavioral disturbances in frontotemporal dementia: a case series. Alzheimer Dis Assoc Disord. 2007;21(2):164-166. doi:10.1097/WAD.0b013e318047df5d
7. Tsai RM, Boxer AL. Treatment of frontotemporal dementia. Curr Treat Options Neurol. 2014;16(11):319. doi:10.1007/s11940-014-0319-0
8. Trieu C, Gossink F, Stek ML, et al. Effectiveness of pharmacological interventions for symptoms of behavioral variant frontotemporal dementia: a systematic review. Cogn Behav Neurol. 2020;33(1):1-15. doi:10.1097/WNN.0000000000000217
9. Deakin JB, Rahman S, Nestor PJ, et al. Paroxetine does not improve symptoms and impairs cognition in frontotemporal dementia: a double-blind randomized controlled trial. Psychopharmacology (Berl). 2004;172(4):400-408. doi:10.1007/s00213-003-1686-5
10. Herrmann N, Black SE, Chow T, et al. Serotonergic function and treatment of behavioral and psychological symptoms of frontotemporal dementia. Am J Geriatr Psychiatry. 2012;20(9):789-797. doi:10.1097/JGP.0b013e31823033f3
11. Ikeda M, Shigenobu K, Fukuhara R, et al. Efficacy of fluvoxamine as a treatment for behavioral symptoms in frontotemporal lobar degeneration patients. Dement Geriatr Cogn Disord. 2004;17(3):117-121. doi:10.1159/000076343
12. Lebert F, Stekke W, Hasenbroekx C, et al. Frontotemporal dementia: a randomised, controlled trial with trazodone. Dement Geriatr Cogn Disord. 2004;17(4):355-359. doi:10.1159/000077171
13. Lebert F. Behavioral benefits of trazodone are sustained for the long term in frontotemporal dementia. Therapy. 2006;3(1):93-96. doi:10.1586/14750708.3.1.93
14. Moretti R, Torre P, Antonello RM, et al. Frontotemporal dementia: paroxetine as a possible treatment of behavior symptoms. A randomized, controlled, open 14-month study. Eur Neurol. 2003;49(1):13-19. doi:10.1159/000067021
1. Grossman M. Frontotemporal dementia: a review. J Int Neuropsychol Soc. 2002;8(4):566-583. doi:10.1017/s1355617702814357
2. The Johns Hopkins University. Frontotemporal dementia. Johns Hopkins Medicine. Accessed September 12, 2021. https://www.hopkinsmedicine.org/health/conditions-and-diseases/dementia/frontotemporal-dementia
3. Shinagawa S, Nakajima S, Plitman E, et al. Non-pharmacological management for patients with frontotemporal dementia: a systematic review. J Alzheimers Dis. 2015;45(1):283-293. doi:10.3233/JAD-142109
4. Moretti R, Torre P, Antonello RM, et al. Rivastigmine in frontotemporal dementia: an open-label study. Drugs Aging. 2004;21(14):931-937. doi:10.2165/00002512-200421140-00003
5. Diehl-Schmid J, Förstl H, Perneczky R, et al. A 6-month, open-label study for memantine in patients with frontotemporal dementia. In J Geriatr Psychiatry. 2008;23(7):754-759. doi:10.1002/gps.1973
6. Swanberg MM. Memantine for behavioral disturbances in frontotemporal dementia: a case series. Alzheimer Dis Assoc Disord. 2007;21(2):164-166. doi:10.1097/WAD.0b013e318047df5d
7. Tsai RM, Boxer AL. Treatment of frontotemporal dementia. Curr Treat Options Neurol. 2014;16(11):319. doi:10.1007/s11940-014-0319-0
8. Trieu C, Gossink F, Stek ML, et al. Effectiveness of pharmacological interventions for symptoms of behavioral variant frontotemporal dementia: a systematic review. Cogn Behav Neurol. 2020;33(1):1-15. doi:10.1097/WNN.0000000000000217
9. Deakin JB, Rahman S, Nestor PJ, et al. Paroxetine does not improve symptoms and impairs cognition in frontotemporal dementia: a double-blind randomized controlled trial. Psychopharmacology (Berl). 2004;172(4):400-408. doi:10.1007/s00213-003-1686-5
10. Herrmann N, Black SE, Chow T, et al. Serotonergic function and treatment of behavioral and psychological symptoms of frontotemporal dementia. Am J Geriatr Psychiatry. 2012;20(9):789-797. doi:10.1097/JGP.0b013e31823033f3
11. Ikeda M, Shigenobu K, Fukuhara R, et al. Efficacy of fluvoxamine as a treatment for behavioral symptoms in frontotemporal lobar degeneration patients. Dement Geriatr Cogn Disord. 2004;17(3):117-121. doi:10.1159/000076343
12. Lebert F, Stekke W, Hasenbroekx C, et al. Frontotemporal dementia: a randomised, controlled trial with trazodone. Dement Geriatr Cogn Disord. 2004;17(4):355-359. doi:10.1159/000077171
13. Lebert F. Behavioral benefits of trazodone are sustained for the long term in frontotemporal dementia. Therapy. 2006;3(1):93-96. doi:10.1586/14750708.3.1.93
14. Moretti R, Torre P, Antonello RM, et al. Frontotemporal dementia: paroxetine as a possible treatment of behavior symptoms. A randomized, controlled, open 14-month study. Eur Neurol. 2003;49(1):13-19. doi:10.1159/000067021
Dementia diagnosis a good time to reduce polypharmacy
Physicians may be missing opportunities to reduce harmful polypharmacy in elderly patients with newly diagnosed dementia, investigators for a large study of Medicare beneficiaries reported.
They found that those with an incident dementia diagnosis were somewhat more likely to initiate central nervous system–active medications and slightly more likely to discontinue cardiometabolic and anticholinergic medications, compared with controls.
According to the authors, time of diagnosis can be a potential inflexion point for deprescribing long-term medications with high safety risks, limited likelihood of benefit, or possible association with impaired cognition.
“Understanding the chronology of medication changes following a first dementia diagnosis may identify targets for deprescribing interventions to reduce preventable medication-related harms, said Timothy S. Anderson, MD, MAS, of the division of general medicine at Beth Israel Deaconess Medical Center, Boston, and colleagues in JAMA Internal Medicine.
“Our results provide a baseline to inform efforts to rethink the clinical approach to medication use at the time of a new dementia diagnosis.”
Hundreds of thousands of Americans are diagnosed annually with Alzheimer’s and related dementias, the authors pointed out, and the majority have multiple other chronic conditions. Worsening cognitive impairment may alter the risk-benefit balance of medications taken for these conditions.
Matched cohort study
The sample consisted of adults 67 years or older enrolled in traditional Medicare and Medicare Part D. Patients with an initial incident dementia diagnosis between January 2012 and December 2018 were matched with controls (as of last doctor’s office visit) based on demographics, geographic location, and baseline medication count. Data were analyzed from 2021 to June 2023.
The study included 266,675 adults with incident dementia and 266,675 controls. In both groups, 65.1% were 80 years or older (mean age, 82.2) and 67.8% were female. At baseline, patients with incident dementia were more likely than controls to use CNS-active medications (54.32% vs. 48.39%) and anticholinergic medications (17.79% vs. 15.96%) and less likely to use most cardiometabolic medications (for example, antidiabetics, 31.19% vs. 36.45%).
Immediately following the index diagnosis, the dementia cohort had greater increases in the mean number of medications used: 0.41 vs. –0.06 (95% confidence interval, 0.27-0.66) and in the proportion using CNS-active medications (absolute change, 3.44% vs. 0.79%; 95% CI, 0.85%-4.45%). The rise was because of an increased use of antipsychotics, antidepressants, and antiepileptics.
The affected cohort showed a modestly greater decline in anticholinergic medications: quarterly change in use: −0.53% vs. −0.21% (95% CI, −0.55% to −0.08%); and in most cardiometabolic medications: for example, quarterly change in antihypertensive use: –0.84% vs. –0.40% (95% CI, –0.64% to –0.25%). Still, a year post diagnosis, 75.2% of dementia patients were using five or more medications, for a 2.8% increase.
The drug classes with the steepest rate of discontinuation – such as lipid-lowering and antihypertensive medications – had low risks for adverse drug events, while higher-risk classes – such as insulins and antiplatelet and anticoagulant agents – had smaller or no reductions in use.
While the findings point to opportunities to reduce polypharmacy by deprescribing long-term medications of dubious benefit, interventions to reduce polypharmacy and inappropriate medications have been modestly successful for patients without dementia, the authors said. But the recent OPTIMIZE trial, an educational effort aimed at primary care clinicians and patients with cognitive impairment, reduced neither polypharmacy nor potentially inappropriate medications.
Luke D. Kim, MD, a geriatrician at the Cleveland Clinic in Ohio, agreed that seniors with dementia can benefit from reassessment of their pharmacologic therapies. “Older adults in general are more prone to have side effects from medications as their renal and hepatic clearance and metabolism are different and lower than those of younger individuals. But they tend to take multiple medications owing to more comorbidities,” said Dr. Kim, who was not involved in the study. “While all older adults need to be more careful about medication management, those with dementia need an even more careful approach as they have diminished cognitive reserve and risk more potential harm from medications.”
The authors noted that since decision-making models aligned with patient priorities for older adults without dementia led to reductions in overall medication use, that may be a path forward in populations with dementia.
The study was supported by grants from the National Institute on Aging, National Institutes of Health. The authors had no competing interests to disclose. Dr. Kim disclosed no competing interests relevant to his comments.
Physicians may be missing opportunities to reduce harmful polypharmacy in elderly patients with newly diagnosed dementia, investigators for a large study of Medicare beneficiaries reported.
They found that those with an incident dementia diagnosis were somewhat more likely to initiate central nervous system–active medications and slightly more likely to discontinue cardiometabolic and anticholinergic medications, compared with controls.
According to the authors, time of diagnosis can be a potential inflexion point for deprescribing long-term medications with high safety risks, limited likelihood of benefit, or possible association with impaired cognition.
“Understanding the chronology of medication changes following a first dementia diagnosis may identify targets for deprescribing interventions to reduce preventable medication-related harms, said Timothy S. Anderson, MD, MAS, of the division of general medicine at Beth Israel Deaconess Medical Center, Boston, and colleagues in JAMA Internal Medicine.
“Our results provide a baseline to inform efforts to rethink the clinical approach to medication use at the time of a new dementia diagnosis.”
Hundreds of thousands of Americans are diagnosed annually with Alzheimer’s and related dementias, the authors pointed out, and the majority have multiple other chronic conditions. Worsening cognitive impairment may alter the risk-benefit balance of medications taken for these conditions.
Matched cohort study
The sample consisted of adults 67 years or older enrolled in traditional Medicare and Medicare Part D. Patients with an initial incident dementia diagnosis between January 2012 and December 2018 were matched with controls (as of last doctor’s office visit) based on demographics, geographic location, and baseline medication count. Data were analyzed from 2021 to June 2023.
The study included 266,675 adults with incident dementia and 266,675 controls. In both groups, 65.1% were 80 years or older (mean age, 82.2) and 67.8% were female. At baseline, patients with incident dementia were more likely than controls to use CNS-active medications (54.32% vs. 48.39%) and anticholinergic medications (17.79% vs. 15.96%) and less likely to use most cardiometabolic medications (for example, antidiabetics, 31.19% vs. 36.45%).
Immediately following the index diagnosis, the dementia cohort had greater increases in the mean number of medications used: 0.41 vs. –0.06 (95% confidence interval, 0.27-0.66) and in the proportion using CNS-active medications (absolute change, 3.44% vs. 0.79%; 95% CI, 0.85%-4.45%). The rise was because of an increased use of antipsychotics, antidepressants, and antiepileptics.
The affected cohort showed a modestly greater decline in anticholinergic medications: quarterly change in use: −0.53% vs. −0.21% (95% CI, −0.55% to −0.08%); and in most cardiometabolic medications: for example, quarterly change in antihypertensive use: –0.84% vs. –0.40% (95% CI, –0.64% to –0.25%). Still, a year post diagnosis, 75.2% of dementia patients were using five or more medications, for a 2.8% increase.
The drug classes with the steepest rate of discontinuation – such as lipid-lowering and antihypertensive medications – had low risks for adverse drug events, while higher-risk classes – such as insulins and antiplatelet and anticoagulant agents – had smaller or no reductions in use.
While the findings point to opportunities to reduce polypharmacy by deprescribing long-term medications of dubious benefit, interventions to reduce polypharmacy and inappropriate medications have been modestly successful for patients without dementia, the authors said. But the recent OPTIMIZE trial, an educational effort aimed at primary care clinicians and patients with cognitive impairment, reduced neither polypharmacy nor potentially inappropriate medications.
Luke D. Kim, MD, a geriatrician at the Cleveland Clinic in Ohio, agreed that seniors with dementia can benefit from reassessment of their pharmacologic therapies. “Older adults in general are more prone to have side effects from medications as their renal and hepatic clearance and metabolism are different and lower than those of younger individuals. But they tend to take multiple medications owing to more comorbidities,” said Dr. Kim, who was not involved in the study. “While all older adults need to be more careful about medication management, those with dementia need an even more careful approach as they have diminished cognitive reserve and risk more potential harm from medications.”
The authors noted that since decision-making models aligned with patient priorities for older adults without dementia led to reductions in overall medication use, that may be a path forward in populations with dementia.
The study was supported by grants from the National Institute on Aging, National Institutes of Health. The authors had no competing interests to disclose. Dr. Kim disclosed no competing interests relevant to his comments.
Physicians may be missing opportunities to reduce harmful polypharmacy in elderly patients with newly diagnosed dementia, investigators for a large study of Medicare beneficiaries reported.
They found that those with an incident dementia diagnosis were somewhat more likely to initiate central nervous system–active medications and slightly more likely to discontinue cardiometabolic and anticholinergic medications, compared with controls.
According to the authors, time of diagnosis can be a potential inflexion point for deprescribing long-term medications with high safety risks, limited likelihood of benefit, or possible association with impaired cognition.
“Understanding the chronology of medication changes following a first dementia diagnosis may identify targets for deprescribing interventions to reduce preventable medication-related harms, said Timothy S. Anderson, MD, MAS, of the division of general medicine at Beth Israel Deaconess Medical Center, Boston, and colleagues in JAMA Internal Medicine.
“Our results provide a baseline to inform efforts to rethink the clinical approach to medication use at the time of a new dementia diagnosis.”
Hundreds of thousands of Americans are diagnosed annually with Alzheimer’s and related dementias, the authors pointed out, and the majority have multiple other chronic conditions. Worsening cognitive impairment may alter the risk-benefit balance of medications taken for these conditions.
Matched cohort study
The sample consisted of adults 67 years or older enrolled in traditional Medicare and Medicare Part D. Patients with an initial incident dementia diagnosis between January 2012 and December 2018 were matched with controls (as of last doctor’s office visit) based on demographics, geographic location, and baseline medication count. Data were analyzed from 2021 to June 2023.
The study included 266,675 adults with incident dementia and 266,675 controls. In both groups, 65.1% were 80 years or older (mean age, 82.2) and 67.8% were female. At baseline, patients with incident dementia were more likely than controls to use CNS-active medications (54.32% vs. 48.39%) and anticholinergic medications (17.79% vs. 15.96%) and less likely to use most cardiometabolic medications (for example, antidiabetics, 31.19% vs. 36.45%).
Immediately following the index diagnosis, the dementia cohort had greater increases in the mean number of medications used: 0.41 vs. –0.06 (95% confidence interval, 0.27-0.66) and in the proportion using CNS-active medications (absolute change, 3.44% vs. 0.79%; 95% CI, 0.85%-4.45%). The rise was because of an increased use of antipsychotics, antidepressants, and antiepileptics.
The affected cohort showed a modestly greater decline in anticholinergic medications: quarterly change in use: −0.53% vs. −0.21% (95% CI, −0.55% to −0.08%); and in most cardiometabolic medications: for example, quarterly change in antihypertensive use: –0.84% vs. –0.40% (95% CI, –0.64% to –0.25%). Still, a year post diagnosis, 75.2% of dementia patients were using five or more medications, for a 2.8% increase.
The drug classes with the steepest rate of discontinuation – such as lipid-lowering and antihypertensive medications – had low risks for adverse drug events, while higher-risk classes – such as insulins and antiplatelet and anticoagulant agents – had smaller or no reductions in use.
While the findings point to opportunities to reduce polypharmacy by deprescribing long-term medications of dubious benefit, interventions to reduce polypharmacy and inappropriate medications have been modestly successful for patients without dementia, the authors said. But the recent OPTIMIZE trial, an educational effort aimed at primary care clinicians and patients with cognitive impairment, reduced neither polypharmacy nor potentially inappropriate medications.
Luke D. Kim, MD, a geriatrician at the Cleveland Clinic in Ohio, agreed that seniors with dementia can benefit from reassessment of their pharmacologic therapies. “Older adults in general are more prone to have side effects from medications as their renal and hepatic clearance and metabolism are different and lower than those of younger individuals. But they tend to take multiple medications owing to more comorbidities,” said Dr. Kim, who was not involved in the study. “While all older adults need to be more careful about medication management, those with dementia need an even more careful approach as they have diminished cognitive reserve and risk more potential harm from medications.”
The authors noted that since decision-making models aligned with patient priorities for older adults without dementia led to reductions in overall medication use, that may be a path forward in populations with dementia.
The study was supported by grants from the National Institute on Aging, National Institutes of Health. The authors had no competing interests to disclose. Dr. Kim disclosed no competing interests relevant to his comments.
FROM JAMA INTERNAL MEDICINE