User login
Antipsychotic shows benefit for Alzheimer’s agitation
SAN FRANCISCO – In a widely anticipated report,
Members of a panel of dementia specialists here at the 15th Clinical Trials on Alzheimer’s Disease (CTAD) conference said that the results were encouraging. But they also noted that the available data make it difficult to understand the impact of the drug on the day-to-day life on patients.
“I’d like to be able to translate that into something else to understand the risk benefit calculus,” said neurologist and neuroscientist Alireza Atri, MD, PhD, of Banner Sun Health Research Institute in Phoenix. “How does it affect the patients themselves, their quality of life, and the family members and their burden?”
Currently, there’s no Food and Drug Administration–approved treatment for agitation in AD.
In 2015, the FDA approved brexpiprazole, an oral medication, as a treatment for schizophrenia and an adjunctive treatment for major depressive disorder (MDD). It is an expensive drug with an average retail price per GoodRx of $1,582 per month, and no generic is available.
Researchers released the results of a trio of phase 3 clinical trials at CTAD that examined various doses of brexpiprazole. The results of the first two trials had been released earlier in 2018.
Three trials
All trials were multicenter, 12-week, randomized, double-blind and placebo-controlled.
Study participants were aged 55-90 years, had probable AD diagnoses, and had agitation per various scales. The average age in the groups was 74 years, 56.0%-61.7% were women, and 94.3%-98.1% were White.
The first trial examined two fixed doses (1 mg/d, n = 137; and 2 mg/d, n = 140) or placebo (n = 136). “The study initially included a 0.5 mg/day arm,” the researchers reported, “which was removed in a protocol amendment, and patients randomized to that arm were not included in efficacy analyses.”
The second trial looked at a flexible dose (0.5-2 mg/d, n = 133) or placebo (n = 137).
In a CTAD presentation, Nanco Hefting of Lundbeck, a codeveloper of the drug, said that the researchers learned from the first two trials that 2 mg/d might be an appropriate dose, and the FDA recommended they also examine 3 mg/day. As a result, the third trial examined two fixed doses (2 mg/d, n = 75; 3 mg/d, n = 153; or placebo, n = 117).
In the third trial, both the placebo and drug groups improved per a measurement of agitation; those in the drug group improved somewhat more.
The mean change in baseline on the Cohen-Mansfield Agitation Inventory scale – the primary endpoint – was –5.32 for the 2-mg/d and 3-mg/d groups vs. placebo (P = .0026); the score in the placebo group fell by about 18 and by about 22 in the drug group.
The key secondary endpoint was an improvement from baseline to week 12 in the Clinical Global Impression–Severity (CGI-S) score related to agitation. Compared with the placebo group, this score was –0.27 in the drug group (P = .0078). Both scores hovered around –1.0.
Safety data show the percentage of treatment-emergent events ranged from 45.9% in the placebo group to 49.0%-56.8% for brexpiprazole in the three trials. The percentage of these events leading to discontinuation was 6.3% among those receiving the drug and 3.4% in the placebo group.
University of Exeter dementia researcher Clive Ballard, MD, MB ChB, one of the panelists who discussed the research after the CTAD presentation, praised the trials as “well-conducted” and said that he was pleased that subjects in institutions were included. “It’s not an easy environment to do trials in. They should be really commended for doing for doing that.”
But he echoed fellow panelist Dr. Atri by noting that more data are needed to understand how well the drug works. “I would like to see the effect sizes and a little bit more detail to understand the clinical meaningfulness of that level of benefit.”
What’s next? A spokeswoman for Otsuka, a codeveloper of brexpiprazole, said that it hopes to hear in 2023 about a supplemental new drug application that was filed in November 2022.
Otsuka and Lundbeck funded the research. Mr. Hefting is an employee of Lundbeck, and several other authors work for Lundbeck or Otsuka. The single non-employee author reports various disclosures. Disclosures for Dr. Atri and Dr. Ballard were not provided.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – In a widely anticipated report,
Members of a panel of dementia specialists here at the 15th Clinical Trials on Alzheimer’s Disease (CTAD) conference said that the results were encouraging. But they also noted that the available data make it difficult to understand the impact of the drug on the day-to-day life on patients.
“I’d like to be able to translate that into something else to understand the risk benefit calculus,” said neurologist and neuroscientist Alireza Atri, MD, PhD, of Banner Sun Health Research Institute in Phoenix. “How does it affect the patients themselves, their quality of life, and the family members and their burden?”
Currently, there’s no Food and Drug Administration–approved treatment for agitation in AD.
In 2015, the FDA approved brexpiprazole, an oral medication, as a treatment for schizophrenia and an adjunctive treatment for major depressive disorder (MDD). It is an expensive drug with an average retail price per GoodRx of $1,582 per month, and no generic is available.
Researchers released the results of a trio of phase 3 clinical trials at CTAD that examined various doses of brexpiprazole. The results of the first two trials had been released earlier in 2018.
Three trials
All trials were multicenter, 12-week, randomized, double-blind and placebo-controlled.
Study participants were aged 55-90 years, had probable AD diagnoses, and had agitation per various scales. The average age in the groups was 74 years, 56.0%-61.7% were women, and 94.3%-98.1% were White.
The first trial examined two fixed doses (1 mg/d, n = 137; and 2 mg/d, n = 140) or placebo (n = 136). “The study initially included a 0.5 mg/day arm,” the researchers reported, “which was removed in a protocol amendment, and patients randomized to that arm were not included in efficacy analyses.”
The second trial looked at a flexible dose (0.5-2 mg/d, n = 133) or placebo (n = 137).
In a CTAD presentation, Nanco Hefting of Lundbeck, a codeveloper of the drug, said that the researchers learned from the first two trials that 2 mg/d might be an appropriate dose, and the FDA recommended they also examine 3 mg/day. As a result, the third trial examined two fixed doses (2 mg/d, n = 75; 3 mg/d, n = 153; or placebo, n = 117).
In the third trial, both the placebo and drug groups improved per a measurement of agitation; those in the drug group improved somewhat more.
The mean change in baseline on the Cohen-Mansfield Agitation Inventory scale – the primary endpoint – was –5.32 for the 2-mg/d and 3-mg/d groups vs. placebo (P = .0026); the score in the placebo group fell by about 18 and by about 22 in the drug group.
The key secondary endpoint was an improvement from baseline to week 12 in the Clinical Global Impression–Severity (CGI-S) score related to agitation. Compared with the placebo group, this score was –0.27 in the drug group (P = .0078). Both scores hovered around –1.0.
Safety data show the percentage of treatment-emergent events ranged from 45.9% in the placebo group to 49.0%-56.8% for brexpiprazole in the three trials. The percentage of these events leading to discontinuation was 6.3% among those receiving the drug and 3.4% in the placebo group.
University of Exeter dementia researcher Clive Ballard, MD, MB ChB, one of the panelists who discussed the research after the CTAD presentation, praised the trials as “well-conducted” and said that he was pleased that subjects in institutions were included. “It’s not an easy environment to do trials in. They should be really commended for doing for doing that.”
But he echoed fellow panelist Dr. Atri by noting that more data are needed to understand how well the drug works. “I would like to see the effect sizes and a little bit more detail to understand the clinical meaningfulness of that level of benefit.”
What’s next? A spokeswoman for Otsuka, a codeveloper of brexpiprazole, said that it hopes to hear in 2023 about a supplemental new drug application that was filed in November 2022.
Otsuka and Lundbeck funded the research. Mr. Hefting is an employee of Lundbeck, and several other authors work for Lundbeck or Otsuka. The single non-employee author reports various disclosures. Disclosures for Dr. Atri and Dr. Ballard were not provided.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – In a widely anticipated report,
Members of a panel of dementia specialists here at the 15th Clinical Trials on Alzheimer’s Disease (CTAD) conference said that the results were encouraging. But they also noted that the available data make it difficult to understand the impact of the drug on the day-to-day life on patients.
“I’d like to be able to translate that into something else to understand the risk benefit calculus,” said neurologist and neuroscientist Alireza Atri, MD, PhD, of Banner Sun Health Research Institute in Phoenix. “How does it affect the patients themselves, their quality of life, and the family members and their burden?”
Currently, there’s no Food and Drug Administration–approved treatment for agitation in AD.
In 2015, the FDA approved brexpiprazole, an oral medication, as a treatment for schizophrenia and an adjunctive treatment for major depressive disorder (MDD). It is an expensive drug with an average retail price per GoodRx of $1,582 per month, and no generic is available.
Researchers released the results of a trio of phase 3 clinical trials at CTAD that examined various doses of brexpiprazole. The results of the first two trials had been released earlier in 2018.
Three trials
All trials were multicenter, 12-week, randomized, double-blind and placebo-controlled.
Study participants were aged 55-90 years, had probable AD diagnoses, and had agitation per various scales. The average age in the groups was 74 years, 56.0%-61.7% were women, and 94.3%-98.1% were White.
The first trial examined two fixed doses (1 mg/d, n = 137; and 2 mg/d, n = 140) or placebo (n = 136). “The study initially included a 0.5 mg/day arm,” the researchers reported, “which was removed in a protocol amendment, and patients randomized to that arm were not included in efficacy analyses.”
The second trial looked at a flexible dose (0.5-2 mg/d, n = 133) or placebo (n = 137).
In a CTAD presentation, Nanco Hefting of Lundbeck, a codeveloper of the drug, said that the researchers learned from the first two trials that 2 mg/d might be an appropriate dose, and the FDA recommended they also examine 3 mg/day. As a result, the third trial examined two fixed doses (2 mg/d, n = 75; 3 mg/d, n = 153; or placebo, n = 117).
In the third trial, both the placebo and drug groups improved per a measurement of agitation; those in the drug group improved somewhat more.
The mean change in baseline on the Cohen-Mansfield Agitation Inventory scale – the primary endpoint – was –5.32 for the 2-mg/d and 3-mg/d groups vs. placebo (P = .0026); the score in the placebo group fell by about 18 and by about 22 in the drug group.
The key secondary endpoint was an improvement from baseline to week 12 in the Clinical Global Impression–Severity (CGI-S) score related to agitation. Compared with the placebo group, this score was –0.27 in the drug group (P = .0078). Both scores hovered around –1.0.
Safety data show the percentage of treatment-emergent events ranged from 45.9% in the placebo group to 49.0%-56.8% for brexpiprazole in the three trials. The percentage of these events leading to discontinuation was 6.3% among those receiving the drug and 3.4% in the placebo group.
University of Exeter dementia researcher Clive Ballard, MD, MB ChB, one of the panelists who discussed the research after the CTAD presentation, praised the trials as “well-conducted” and said that he was pleased that subjects in institutions were included. “It’s not an easy environment to do trials in. They should be really commended for doing for doing that.”
But he echoed fellow panelist Dr. Atri by noting that more data are needed to understand how well the drug works. “I would like to see the effect sizes and a little bit more detail to understand the clinical meaningfulness of that level of benefit.”
What’s next? A spokeswoman for Otsuka, a codeveloper of brexpiprazole, said that it hopes to hear in 2023 about a supplemental new drug application that was filed in November 2022.
Otsuka and Lundbeck funded the research. Mr. Hefting is an employee of Lundbeck, and several other authors work for Lundbeck or Otsuka. The single non-employee author reports various disclosures. Disclosures for Dr. Atri and Dr. Ballard were not provided.
A version of this article first appeared on Medscape.com.
AT CTAD 2022
Mindfulness, exercise strike out in memory trial
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
We are coming to the end of the year, which always makes me think about getting older. Much like the search for the fountain of youth, many promising leads have ultimately led to dead ends. And yet, I had high hopes for a trial that focused on two cornerstones of wellness – exercise and mindfulness – to address the subjective loss of memory that comes with aging. Alas, meditation and exercise do not appear to be the fountain of youth.
I’m talking about this study, appearing in JAMA, known as the MEDEX trial.
It’s a clever design: a 2 x 2 factorial randomized trial where participants could be randomized to a mindfulness intervention, an exercise intervention, both, or neither.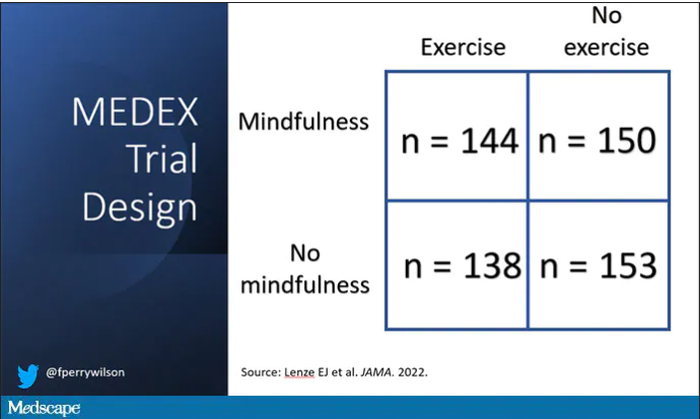
In this manner, you can test multiple hypotheses exploiting a shared control group. Or as a mentor of mine used to say, you get two trials for the price of one and a half.
The participants were older adults, aged 65-84, living in the community. They had to be relatively sedentary at baseline and not engaging in mindfulness practices. They had to subjectively report some memory or concentration issues but had to be cognitively intact, based on a standard dementia screening test. In other words, these are your average older people who are worried that they aren’t as sharp as they used to be.
The interventions themselves were fairly intense. The exercise group had instructor-led sessions for 90 minutes twice a week for the first 6 months of the study, once a week thereafter. And participants were encouraged to exercise at home such that they had a total of 300 minutes of weekly exercise.
The mindfulness program was characterized by eight weekly classes of 2.5 hours each as well as a half-day retreat to teach the tenets of mindfulness and meditation, with monthly refreshers thereafter. Participants were instructed to meditate for 60 minutes a day in addition to the classes.
For the 144 people who were randomized to both meditation and exercise, this trial amounted to something of a part-time job. So you might think that adherence to the interventions was low, but apparently that’s not the case. Attendance to the mindfulness classes was over 90%, and over 80% for the exercise classes. And diary-based reporting of home efforts was also pretty good.
The control group wasn’t left to their own devices. Recognizing that the community aspect of exercise or mindfulness classes might convey a benefit independent of the actual exercise or mindfulness, the control group met on a similar schedule to discuss health education, but no mention of exercise or mindfulness occurred in that setting.
The primary outcome was change in memory and executive function scores across a battery of neuropsychologic testing, but the story is told in just a few pictures.
Memory scores improved in all three groups – mindfulness, exercise, and health education – over time. Cognitive composite score improved in all three groups similarly. There was no synergistic effect of mindfulness and exercise either. Basically, everyone got a bit better.
But the study did way more than look at scores on tests. Researchers used MRI to measure brain anatomic outcomes as well. And the surprising thing is that virtually none of these outcomes were different between the groups either.
Hippocampal volume decreased a bit in all the groups. Dorsolateral prefrontal cortex volume was flat. There was no change in scores measuring tasks of daily living.
When you see negative results like this, right away you worry that the intervention wasn’t properly delivered. Were these people really exercising and meditating? Well, the authors showed that individuals randomized to exercise, at least, had less sleep latency, greater aerobic fitness, and greater strength. So we know something was happening.
They then asked, would the people in the exercise group with the greatest changes in those physiologic parameters show some improvement in cognitive parameters? In other words, we know you were exercising because you got stronger and are sleeping better; is your memory better? The answer? Surprisingly, still no. Even in that honestly somewhat cherry-picked group, the interventions had no effect.
Could it be that the control was inappropriate, that the “health education” intervention was actually so helpful that it obscured the benefits of exercise and meditation? After all, cognitive scores did improve in all groups. The authors doubt it. They say they think the improvement in cognitive scores reflects the fact that patients had learned a bit about how to take the tests. This is pretty common in the neuropsychiatric literature.
So here we are and I just want to say, well, shoot. This is not the result I wanted. And I think the reason I’m so disappointed is because aging and the loss of cognitive faculties that comes with aging are just sort of scary. We are all looking for some control over that fear, and how nice it would be to be able to tell ourselves not to worry – that we won’t have those problems as we get older because we exercise, or meditate, or drink red wine, or don’t drink wine, or whatever. And while I have no doubt that staying healthier physically will keep you healthier mentally, it may take more than one simple thing to move the needle.
Dr. Wilson is associate professor, department of medicine, and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
We are coming to the end of the year, which always makes me think about getting older. Much like the search for the fountain of youth, many promising leads have ultimately led to dead ends. And yet, I had high hopes for a trial that focused on two cornerstones of wellness – exercise and mindfulness – to address the subjective loss of memory that comes with aging. Alas, meditation and exercise do not appear to be the fountain of youth.
I’m talking about this study, appearing in JAMA, known as the MEDEX trial.
It’s a clever design: a 2 x 2 factorial randomized trial where participants could be randomized to a mindfulness intervention, an exercise intervention, both, or neither.
In this manner, you can test multiple hypotheses exploiting a shared control group. Or as a mentor of mine used to say, you get two trials for the price of one and a half.
The participants were older adults, aged 65-84, living in the community. They had to be relatively sedentary at baseline and not engaging in mindfulness practices. They had to subjectively report some memory or concentration issues but had to be cognitively intact, based on a standard dementia screening test. In other words, these are your average older people who are worried that they aren’t as sharp as they used to be.
The interventions themselves were fairly intense. The exercise group had instructor-led sessions for 90 minutes twice a week for the first 6 months of the study, once a week thereafter. And participants were encouraged to exercise at home such that they had a total of 300 minutes of weekly exercise.
The mindfulness program was characterized by eight weekly classes of 2.5 hours each as well as a half-day retreat to teach the tenets of mindfulness and meditation, with monthly refreshers thereafter. Participants were instructed to meditate for 60 minutes a day in addition to the classes.
For the 144 people who were randomized to both meditation and exercise, this trial amounted to something of a part-time job. So you might think that adherence to the interventions was low, but apparently that’s not the case. Attendance to the mindfulness classes was over 90%, and over 80% for the exercise classes. And diary-based reporting of home efforts was also pretty good.
The control group wasn’t left to their own devices. Recognizing that the community aspect of exercise or mindfulness classes might convey a benefit independent of the actual exercise or mindfulness, the control group met on a similar schedule to discuss health education, but no mention of exercise or mindfulness occurred in that setting.
The primary outcome was change in memory and executive function scores across a battery of neuropsychologic testing, but the story is told in just a few pictures.
Memory scores improved in all three groups – mindfulness, exercise, and health education – over time. Cognitive composite score improved in all three groups similarly. There was no synergistic effect of mindfulness and exercise either. Basically, everyone got a bit better.
But the study did way more than look at scores on tests. Researchers used MRI to measure brain anatomic outcomes as well. And the surprising thing is that virtually none of these outcomes were different between the groups either.
Hippocampal volume decreased a bit in all the groups. Dorsolateral prefrontal cortex volume was flat. There was no change in scores measuring tasks of daily living.
When you see negative results like this, right away you worry that the intervention wasn’t properly delivered. Were these people really exercising and meditating? Well, the authors showed that individuals randomized to exercise, at least, had less sleep latency, greater aerobic fitness, and greater strength. So we know something was happening.
They then asked, would the people in the exercise group with the greatest changes in those physiologic parameters show some improvement in cognitive parameters? In other words, we know you were exercising because you got stronger and are sleeping better; is your memory better? The answer? Surprisingly, still no. Even in that honestly somewhat cherry-picked group, the interventions had no effect.
Could it be that the control was inappropriate, that the “health education” intervention was actually so helpful that it obscured the benefits of exercise and meditation? After all, cognitive scores did improve in all groups. The authors doubt it. They say they think the improvement in cognitive scores reflects the fact that patients had learned a bit about how to take the tests. This is pretty common in the neuropsychiatric literature.
So here we are and I just want to say, well, shoot. This is not the result I wanted. And I think the reason I’m so disappointed is because aging and the loss of cognitive faculties that comes with aging are just sort of scary. We are all looking for some control over that fear, and how nice it would be to be able to tell ourselves not to worry – that we won’t have those problems as we get older because we exercise, or meditate, or drink red wine, or don’t drink wine, or whatever. And while I have no doubt that staying healthier physically will keep you healthier mentally, it may take more than one simple thing to move the needle.
Dr. Wilson is associate professor, department of medicine, and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
We are coming to the end of the year, which always makes me think about getting older. Much like the search for the fountain of youth, many promising leads have ultimately led to dead ends. And yet, I had high hopes for a trial that focused on two cornerstones of wellness – exercise and mindfulness – to address the subjective loss of memory that comes with aging. Alas, meditation and exercise do not appear to be the fountain of youth.
I’m talking about this study, appearing in JAMA, known as the MEDEX trial.
It’s a clever design: a 2 x 2 factorial randomized trial where participants could be randomized to a mindfulness intervention, an exercise intervention, both, or neither.
In this manner, you can test multiple hypotheses exploiting a shared control group. Or as a mentor of mine used to say, you get two trials for the price of one and a half.
The participants were older adults, aged 65-84, living in the community. They had to be relatively sedentary at baseline and not engaging in mindfulness practices. They had to subjectively report some memory or concentration issues but had to be cognitively intact, based on a standard dementia screening test. In other words, these are your average older people who are worried that they aren’t as sharp as they used to be.
The interventions themselves were fairly intense. The exercise group had instructor-led sessions for 90 minutes twice a week for the first 6 months of the study, once a week thereafter. And participants were encouraged to exercise at home such that they had a total of 300 minutes of weekly exercise.
The mindfulness program was characterized by eight weekly classes of 2.5 hours each as well as a half-day retreat to teach the tenets of mindfulness and meditation, with monthly refreshers thereafter. Participants were instructed to meditate for 60 minutes a day in addition to the classes.
For the 144 people who were randomized to both meditation and exercise, this trial amounted to something of a part-time job. So you might think that adherence to the interventions was low, but apparently that’s not the case. Attendance to the mindfulness classes was over 90%, and over 80% for the exercise classes. And diary-based reporting of home efforts was also pretty good.
The control group wasn’t left to their own devices. Recognizing that the community aspect of exercise or mindfulness classes might convey a benefit independent of the actual exercise or mindfulness, the control group met on a similar schedule to discuss health education, but no mention of exercise or mindfulness occurred in that setting.
The primary outcome was change in memory and executive function scores across a battery of neuropsychologic testing, but the story is told in just a few pictures.
Memory scores improved in all three groups – mindfulness, exercise, and health education – over time. Cognitive composite score improved in all three groups similarly. There was no synergistic effect of mindfulness and exercise either. Basically, everyone got a bit better.
But the study did way more than look at scores on tests. Researchers used MRI to measure brain anatomic outcomes as well. And the surprising thing is that virtually none of these outcomes were different between the groups either.
Hippocampal volume decreased a bit in all the groups. Dorsolateral prefrontal cortex volume was flat. There was no change in scores measuring tasks of daily living.
When you see negative results like this, right away you worry that the intervention wasn’t properly delivered. Were these people really exercising and meditating? Well, the authors showed that individuals randomized to exercise, at least, had less sleep latency, greater aerobic fitness, and greater strength. So we know something was happening.
They then asked, would the people in the exercise group with the greatest changes in those physiologic parameters show some improvement in cognitive parameters? In other words, we know you were exercising because you got stronger and are sleeping better; is your memory better? The answer? Surprisingly, still no. Even in that honestly somewhat cherry-picked group, the interventions had no effect.
Could it be that the control was inappropriate, that the “health education” intervention was actually so helpful that it obscured the benefits of exercise and meditation? After all, cognitive scores did improve in all groups. The authors doubt it. They say they think the improvement in cognitive scores reflects the fact that patients had learned a bit about how to take the tests. This is pretty common in the neuropsychiatric literature.
So here we are and I just want to say, well, shoot. This is not the result I wanted. And I think the reason I’m so disappointed is because aging and the loss of cognitive faculties that comes with aging are just sort of scary. We are all looking for some control over that fear, and how nice it would be to be able to tell ourselves not to worry – that we won’t have those problems as we get older because we exercise, or meditate, or drink red wine, or don’t drink wine, or whatever. And while I have no doubt that staying healthier physically will keep you healthier mentally, it may take more than one simple thing to move the needle.
Dr. Wilson is associate professor, department of medicine, and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Noninvasive laser therapy tied to improved short-term memory
Investigators compared the effect of 1,064 nm of tPBM delivered over a 12-minute session to the right PFC vs. three other treatment arms: delivery of the same intervention to the left PFC, delivery of the intervention at a lower frequency, and a sham intervention.
All participants were shown a series of items prior to the intervention and asked to recall them after the intervention. Those who received tPBM 1,064 nm to the right PFC showed a superior performance of up to 25% in the memory tasks compared with the other groups.
Patients with attention-related conditions, such as attention deficit hyperactivity disorder, “could benefit from this type of treatment, which is safe, simple, and noninvasive, with no side effects,” coinvestigator Dongwei Li, a visiting PhD student at the Centre for Human Brain Health, University of Birmingham, England, said in a news release.
The findings were published online in Science Advances.
Differing wavelengths
The researchers note that “in the past decades,” noninvasive brain stimulation technology using transcranial application of direct or alternating electrical or magnetic fields “has been proven to be useful” in the improvement of working memory (WM).
When applied to the right PFC, tPBM has been shown to improve accuracy and speed of reaction time in WM tasks and improvements in “high-order cognitive functions,” such as sustained attention, emotion, and executive functions.
The investigators wanted to assess the impact of tPBM applied to different parts of the brain and at different wavelengths. They conducted four double-blind, sham-controlled experiments encompassing 90 neurotypical college students (mean age, 22 years). Each student participated in only one of the four experiments.
All completed two different tPBM sessions, separated by a week, in which sham and active tPBM were compared. Two different types of change-detection memory tasks were given: one requiring participants to remember the orientation of a series of items before and after the intervention and one other requiring them to remember the color of the items (experiments 1 and 2).
A series of follow-up experiments focused on comparing different wavelengths (1,064 nm vs. 852 nm) and different stimulation sites (right vs. left PFC; experiments 3 and 4).
EEG recordings were obtained during the intervention and the memory tasks.
Each experiment consisted of one active tPBM session and one sham tPBM session, with sessions consisting of 12 minutes of laser light (or sham) intervention. These sessions were conducted on the first and the seventh day; then, on the eighth day, participants were asked to report (or guess) which session was the active tPBM session.
Stimulating astrocytes
Results showed that, compared with sham tPBM, there was an improvement in WM capacity and scores by the 1,064 nm intervention in the orientation as well as the color task.
Participants who received the targeted treatment were able to remember between four and five test objects, whereas those with the treatment variations were only able to remember between three and four objects.
“These results support the hypothesis that 1,064 nm tPBM on the right PFC enhances WM capacity,” the investigators wrote.
They also found improvements in WM in participants receiving tPBM vs. sham regardless of whether their performance in the WM task was at a low or high level. This finding held true in both the orientation and the color tasks.
“Therefore, participants with good and poor WM capacity improved after 1,064 nm tPBM,” the researchers noted.
In addition, participants were unable to guess or report whether they had received sham or active tPBM.
EEG monitoring showed changes in brain activity that predicted the improvements in memory performance. In particular, 1,064 tPBM applied to the right PFC increased occipitoparietal contralateral delay activity (CDA), with CDA mediating the WM improvement.
This is “consistent with previous research that CDA is indicative of the number of maintained objects in visual working memory,” the investigators wrote.
Pearson correlation analyses showed that the differences in CDA set-size effects between active and sham session “correlated positively” with the behavioral differences between these sessions. For the orientation task, the r was 0.446 (P < .04); and for the color task, the r was .563 (P < .02).
No similar improvements were found with the 852 nm tPBM.
“We need further research to understand exactly why the tPBM is having this positive effect,” coinvestigator Ole Jensen, PhD, professor in translational neuroscience and codirector of the Centre for Human Brain Health, said in the release.
“It’s possible that the light is stimulating the astrocytes – the powerplants – in the nerve cells within the PFC, and this has a positive effect on the cells’ efficiency,” he noted.
Dr. Jensen added that his team “will also be investigating how long the effects might last. Clearly, if these experiments are to lead to a clinical intervention, we will need to see long-lasting benefits.”
Beneficial cognitive, emotional effects
Commenting for this news organization, Francisco Gonzalez-Lima, PhD, professor in the department of psychology, University of Texas at Austin, called the study “well done.”
Dr. Gonzalez-Lima was one of the first researchers to demonstrate that 1,064 nm transcranial infrared laser stimulation “produces beneficial cognitive and emotional effects in humans, including improving visual working memory,” he said.
The current study “reported an additional brain effect linked to the improved visual working memory that consists of an EEG-derived response, which is a new finding,” noted Dr. Gonzales-Lima, who was not involved with the new research.
He added that the same laser method “has been found by the Gonzalez-Lima lab to be effective at improving cognition in older adults and depressed and bipolar patients.”
The study was supported by the National Natural Science Foundation of China, the Ministry of Science and Technology of the People’s Republic of China, and the National Defence Basic Scientific Research Program of China. The investigators and Dr. Gonzalez-Lima report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators compared the effect of 1,064 nm of tPBM delivered over a 12-minute session to the right PFC vs. three other treatment arms: delivery of the same intervention to the left PFC, delivery of the intervention at a lower frequency, and a sham intervention.
All participants were shown a series of items prior to the intervention and asked to recall them after the intervention. Those who received tPBM 1,064 nm to the right PFC showed a superior performance of up to 25% in the memory tasks compared with the other groups.
Patients with attention-related conditions, such as attention deficit hyperactivity disorder, “could benefit from this type of treatment, which is safe, simple, and noninvasive, with no side effects,” coinvestigator Dongwei Li, a visiting PhD student at the Centre for Human Brain Health, University of Birmingham, England, said in a news release.
The findings were published online in Science Advances.
Differing wavelengths
The researchers note that “in the past decades,” noninvasive brain stimulation technology using transcranial application of direct or alternating electrical or magnetic fields “has been proven to be useful” in the improvement of working memory (WM).
When applied to the right PFC, tPBM has been shown to improve accuracy and speed of reaction time in WM tasks and improvements in “high-order cognitive functions,” such as sustained attention, emotion, and executive functions.
The investigators wanted to assess the impact of tPBM applied to different parts of the brain and at different wavelengths. They conducted four double-blind, sham-controlled experiments encompassing 90 neurotypical college students (mean age, 22 years). Each student participated in only one of the four experiments.
All completed two different tPBM sessions, separated by a week, in which sham and active tPBM were compared. Two different types of change-detection memory tasks were given: one requiring participants to remember the orientation of a series of items before and after the intervention and one other requiring them to remember the color of the items (experiments 1 and 2).
A series of follow-up experiments focused on comparing different wavelengths (1,064 nm vs. 852 nm) and different stimulation sites (right vs. left PFC; experiments 3 and 4).
EEG recordings were obtained during the intervention and the memory tasks.
Each experiment consisted of one active tPBM session and one sham tPBM session, with sessions consisting of 12 minutes of laser light (or sham) intervention. These sessions were conducted on the first and the seventh day; then, on the eighth day, participants were asked to report (or guess) which session was the active tPBM session.
Stimulating astrocytes
Results showed that, compared with sham tPBM, there was an improvement in WM capacity and scores by the 1,064 nm intervention in the orientation as well as the color task.
Participants who received the targeted treatment were able to remember between four and five test objects, whereas those with the treatment variations were only able to remember between three and four objects.
“These results support the hypothesis that 1,064 nm tPBM on the right PFC enhances WM capacity,” the investigators wrote.
They also found improvements in WM in participants receiving tPBM vs. sham regardless of whether their performance in the WM task was at a low or high level. This finding held true in both the orientation and the color tasks.
“Therefore, participants with good and poor WM capacity improved after 1,064 nm tPBM,” the researchers noted.
In addition, participants were unable to guess or report whether they had received sham or active tPBM.
EEG monitoring showed changes in brain activity that predicted the improvements in memory performance. In particular, 1,064 tPBM applied to the right PFC increased occipitoparietal contralateral delay activity (CDA), with CDA mediating the WM improvement.
This is “consistent with previous research that CDA is indicative of the number of maintained objects in visual working memory,” the investigators wrote.
Pearson correlation analyses showed that the differences in CDA set-size effects between active and sham session “correlated positively” with the behavioral differences between these sessions. For the orientation task, the r was 0.446 (P < .04); and for the color task, the r was .563 (P < .02).
No similar improvements were found with the 852 nm tPBM.
“We need further research to understand exactly why the tPBM is having this positive effect,” coinvestigator Ole Jensen, PhD, professor in translational neuroscience and codirector of the Centre for Human Brain Health, said in the release.
“It’s possible that the light is stimulating the astrocytes – the powerplants – in the nerve cells within the PFC, and this has a positive effect on the cells’ efficiency,” he noted.
Dr. Jensen added that his team “will also be investigating how long the effects might last. Clearly, if these experiments are to lead to a clinical intervention, we will need to see long-lasting benefits.”
Beneficial cognitive, emotional effects
Commenting for this news organization, Francisco Gonzalez-Lima, PhD, professor in the department of psychology, University of Texas at Austin, called the study “well done.”
Dr. Gonzalez-Lima was one of the first researchers to demonstrate that 1,064 nm transcranial infrared laser stimulation “produces beneficial cognitive and emotional effects in humans, including improving visual working memory,” he said.
The current study “reported an additional brain effect linked to the improved visual working memory that consists of an EEG-derived response, which is a new finding,” noted Dr. Gonzales-Lima, who was not involved with the new research.
He added that the same laser method “has been found by the Gonzalez-Lima lab to be effective at improving cognition in older adults and depressed and bipolar patients.”
The study was supported by the National Natural Science Foundation of China, the Ministry of Science and Technology of the People’s Republic of China, and the National Defence Basic Scientific Research Program of China. The investigators and Dr. Gonzalez-Lima report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators compared the effect of 1,064 nm of tPBM delivered over a 12-minute session to the right PFC vs. three other treatment arms: delivery of the same intervention to the left PFC, delivery of the intervention at a lower frequency, and a sham intervention.
All participants were shown a series of items prior to the intervention and asked to recall them after the intervention. Those who received tPBM 1,064 nm to the right PFC showed a superior performance of up to 25% in the memory tasks compared with the other groups.
Patients with attention-related conditions, such as attention deficit hyperactivity disorder, “could benefit from this type of treatment, which is safe, simple, and noninvasive, with no side effects,” coinvestigator Dongwei Li, a visiting PhD student at the Centre for Human Brain Health, University of Birmingham, England, said in a news release.
The findings were published online in Science Advances.
Differing wavelengths
The researchers note that “in the past decades,” noninvasive brain stimulation technology using transcranial application of direct or alternating electrical or magnetic fields “has been proven to be useful” in the improvement of working memory (WM).
When applied to the right PFC, tPBM has been shown to improve accuracy and speed of reaction time in WM tasks and improvements in “high-order cognitive functions,” such as sustained attention, emotion, and executive functions.
The investigators wanted to assess the impact of tPBM applied to different parts of the brain and at different wavelengths. They conducted four double-blind, sham-controlled experiments encompassing 90 neurotypical college students (mean age, 22 years). Each student participated in only one of the four experiments.
All completed two different tPBM sessions, separated by a week, in which sham and active tPBM were compared. Two different types of change-detection memory tasks were given: one requiring participants to remember the orientation of a series of items before and after the intervention and one other requiring them to remember the color of the items (experiments 1 and 2).
A series of follow-up experiments focused on comparing different wavelengths (1,064 nm vs. 852 nm) and different stimulation sites (right vs. left PFC; experiments 3 and 4).
EEG recordings were obtained during the intervention and the memory tasks.
Each experiment consisted of one active tPBM session and one sham tPBM session, with sessions consisting of 12 minutes of laser light (or sham) intervention. These sessions were conducted on the first and the seventh day; then, on the eighth day, participants were asked to report (or guess) which session was the active tPBM session.
Stimulating astrocytes
Results showed that, compared with sham tPBM, there was an improvement in WM capacity and scores by the 1,064 nm intervention in the orientation as well as the color task.
Participants who received the targeted treatment were able to remember between four and five test objects, whereas those with the treatment variations were only able to remember between three and four objects.
“These results support the hypothesis that 1,064 nm tPBM on the right PFC enhances WM capacity,” the investigators wrote.
They also found improvements in WM in participants receiving tPBM vs. sham regardless of whether their performance in the WM task was at a low or high level. This finding held true in both the orientation and the color tasks.
“Therefore, participants with good and poor WM capacity improved after 1,064 nm tPBM,” the researchers noted.
In addition, participants were unable to guess or report whether they had received sham or active tPBM.
EEG monitoring showed changes in brain activity that predicted the improvements in memory performance. In particular, 1,064 tPBM applied to the right PFC increased occipitoparietal contralateral delay activity (CDA), with CDA mediating the WM improvement.
This is “consistent with previous research that CDA is indicative of the number of maintained objects in visual working memory,” the investigators wrote.
Pearson correlation analyses showed that the differences in CDA set-size effects between active and sham session “correlated positively” with the behavioral differences between these sessions. For the orientation task, the r was 0.446 (P < .04); and for the color task, the r was .563 (P < .02).
No similar improvements were found with the 852 nm tPBM.
“We need further research to understand exactly why the tPBM is having this positive effect,” coinvestigator Ole Jensen, PhD, professor in translational neuroscience and codirector of the Centre for Human Brain Health, said in the release.
“It’s possible that the light is stimulating the astrocytes – the powerplants – in the nerve cells within the PFC, and this has a positive effect on the cells’ efficiency,” he noted.
Dr. Jensen added that his team “will also be investigating how long the effects might last. Clearly, if these experiments are to lead to a clinical intervention, we will need to see long-lasting benefits.”
Beneficial cognitive, emotional effects
Commenting for this news organization, Francisco Gonzalez-Lima, PhD, professor in the department of psychology, University of Texas at Austin, called the study “well done.”
Dr. Gonzalez-Lima was one of the first researchers to demonstrate that 1,064 nm transcranial infrared laser stimulation “produces beneficial cognitive and emotional effects in humans, including improving visual working memory,” he said.
The current study “reported an additional brain effect linked to the improved visual working memory that consists of an EEG-derived response, which is a new finding,” noted Dr. Gonzales-Lima, who was not involved with the new research.
He added that the same laser method “has been found by the Gonzalez-Lima lab to be effective at improving cognition in older adults and depressed and bipolar patients.”
The study was supported by the National Natural Science Foundation of China, the Ministry of Science and Technology of the People’s Republic of China, and the National Defence Basic Scientific Research Program of China. The investigators and Dr. Gonzalez-Lima report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SCIENCE ADVANCES
Seizures in dementia hasten decline and death
NASHVILLE, TENN. – , according to a multicenter study presented at the 2022 annual meeting of the American Epilepsy Society.
“When we compared patients with seizures with those who did not have seizures, we found that patients with seizures were more likely to have more severe cognitive impairment; they were more likely to have physical dependence and so worse functional outcomes; and they also had higher mortality rates at a younger age,” lead study author Ifrah Zawar, MD, an assistant professor of neurology at the University of Virginia, Charlottesville, said in an interview.
“The average age of mortality for seizure patients was around 72 years and the average age of mortality for nonseizure patients was around 79 years, so there was a 7- to 8-year difference in mortality,” she said.
Seizures make matters worse
The study analyzed data on 26,425 patients with dementia, 374 (1.4%) of whom had seizures, collected from 2005 to 2021 at 39 Alzheimer’s disease centers in the United States. Patients who had seizures were significantly younger when cognitive decline began (ages 62.9 vs. 68.4 years, P < .001) and died younger (72.99 vs. 79.72 years, P < .001).
The study also found a number of factors associated with active seizures, including a history of dominant Alzheimer’s disease mutation (odds ratio, 5.55; P < .001), stroke (OR, 3.17; P < .001), transient ischemic attack (OR, 1.72; P = .003), traumatic brain injury (OR, 1.92; P < .001), Parkinson’s disease (OR, 1.79; P = .025), active depression (OR, 1.61; P < .001) and lower education (OR, 0.97; P =.043).
After the study made adjustments for sex and other associated factors, it found that patients with seizures were still at a 76% higher risk of dying younger (hazard ratio, 1.76; P < .001).
The study also determined that patients with seizures had worse functional assessment scores and were more likely to be physically dependent on others (OR, 2.52; P < .001). Seizure patients also performed worse on Mini-Mental Status Examination (18.50 vs. 22.88; P < .001) and Clinical Dementia Rating-Sum of boxes (7.95 vs. 4.28; P < .001) after adjusting for age and duration of cognitive decline.
A tip for caregivers
Dr. Zawar acknowledged that differentiating seizures from transient bouts of confusion in people with dementia can be difficult for family members and caregivers, but she offered advice to help them do so. “If they notice any unusual confusion or any altered mentation which is episodic in nature,” she said, “they should bring it to the neurologist’s attention as early as possible, because there are studies that have shown the diagnosis of seizures is delayed, and if they are treated in time they can be well-controlled.” Electroencephalography can also confirm the presence of seizures, she added.
Double whammy
One limitation of this study is the lack of details on the types of seizures the participants had along with the inconsistency of EEGs performed on the study population. “In future studies, I would like to have more EEG data on the types of seizures and the frequency of seizures to assess these factors further,” Dr. Zawar said.
Having more detailed information on the seizures would make the findings more valuable, Andrew J. Cole, MD, director of the epilepsy service at Massachusetts General Hospital in Boston said in an interview. “We know a lot about clinically apparent seizures, as witnessed by this paper, but we still don’t know a whole lot about clinically silent or cryptic or nighttime-only seizures that maybe no one would really recognize as such unless they were specifically looking for them, and this paper doesn’t address that issue,” he said.
While the finding that patients with other neurologic diseases have more seizures even if they also have Alzheimer’s disease isn’t “a huge surprise,” Dr. Cole added. “On the other hand, the paper is important because it shows us that in the course of having Alzheimer’s disease, having seizures also makes your outcome worse, the speed of progression faster, and it complicates the management and living with this disease, and they make that point quite clear.”
Dr. Zawar and Dr. Cole have no relevant disclosures.
NASHVILLE, TENN. – , according to a multicenter study presented at the 2022 annual meeting of the American Epilepsy Society.
“When we compared patients with seizures with those who did not have seizures, we found that patients with seizures were more likely to have more severe cognitive impairment; they were more likely to have physical dependence and so worse functional outcomes; and they also had higher mortality rates at a younger age,” lead study author Ifrah Zawar, MD, an assistant professor of neurology at the University of Virginia, Charlottesville, said in an interview.
“The average age of mortality for seizure patients was around 72 years and the average age of mortality for nonseizure patients was around 79 years, so there was a 7- to 8-year difference in mortality,” she said.
Seizures make matters worse
The study analyzed data on 26,425 patients with dementia, 374 (1.4%) of whom had seizures, collected from 2005 to 2021 at 39 Alzheimer’s disease centers in the United States. Patients who had seizures were significantly younger when cognitive decline began (ages 62.9 vs. 68.4 years, P < .001) and died younger (72.99 vs. 79.72 years, P < .001).
The study also found a number of factors associated with active seizures, including a history of dominant Alzheimer’s disease mutation (odds ratio, 5.55; P < .001), stroke (OR, 3.17; P < .001), transient ischemic attack (OR, 1.72; P = .003), traumatic brain injury (OR, 1.92; P < .001), Parkinson’s disease (OR, 1.79; P = .025), active depression (OR, 1.61; P < .001) and lower education (OR, 0.97; P =.043).
After the study made adjustments for sex and other associated factors, it found that patients with seizures were still at a 76% higher risk of dying younger (hazard ratio, 1.76; P < .001).
The study also determined that patients with seizures had worse functional assessment scores and were more likely to be physically dependent on others (OR, 2.52; P < .001). Seizure patients also performed worse on Mini-Mental Status Examination (18.50 vs. 22.88; P < .001) and Clinical Dementia Rating-Sum of boxes (7.95 vs. 4.28; P < .001) after adjusting for age and duration of cognitive decline.
A tip for caregivers
Dr. Zawar acknowledged that differentiating seizures from transient bouts of confusion in people with dementia can be difficult for family members and caregivers, but she offered advice to help them do so. “If they notice any unusual confusion or any altered mentation which is episodic in nature,” she said, “they should bring it to the neurologist’s attention as early as possible, because there are studies that have shown the diagnosis of seizures is delayed, and if they are treated in time they can be well-controlled.” Electroencephalography can also confirm the presence of seizures, she added.
Double whammy
One limitation of this study is the lack of details on the types of seizures the participants had along with the inconsistency of EEGs performed on the study population. “In future studies, I would like to have more EEG data on the types of seizures and the frequency of seizures to assess these factors further,” Dr. Zawar said.
Having more detailed information on the seizures would make the findings more valuable, Andrew J. Cole, MD, director of the epilepsy service at Massachusetts General Hospital in Boston said in an interview. “We know a lot about clinically apparent seizures, as witnessed by this paper, but we still don’t know a whole lot about clinically silent or cryptic or nighttime-only seizures that maybe no one would really recognize as such unless they were specifically looking for them, and this paper doesn’t address that issue,” he said.
While the finding that patients with other neurologic diseases have more seizures even if they also have Alzheimer’s disease isn’t “a huge surprise,” Dr. Cole added. “On the other hand, the paper is important because it shows us that in the course of having Alzheimer’s disease, having seizures also makes your outcome worse, the speed of progression faster, and it complicates the management and living with this disease, and they make that point quite clear.”
Dr. Zawar and Dr. Cole have no relevant disclosures.
NASHVILLE, TENN. – , according to a multicenter study presented at the 2022 annual meeting of the American Epilepsy Society.
“When we compared patients with seizures with those who did not have seizures, we found that patients with seizures were more likely to have more severe cognitive impairment; they were more likely to have physical dependence and so worse functional outcomes; and they also had higher mortality rates at a younger age,” lead study author Ifrah Zawar, MD, an assistant professor of neurology at the University of Virginia, Charlottesville, said in an interview.
“The average age of mortality for seizure patients was around 72 years and the average age of mortality for nonseizure patients was around 79 years, so there was a 7- to 8-year difference in mortality,” she said.
Seizures make matters worse
The study analyzed data on 26,425 patients with dementia, 374 (1.4%) of whom had seizures, collected from 2005 to 2021 at 39 Alzheimer’s disease centers in the United States. Patients who had seizures were significantly younger when cognitive decline began (ages 62.9 vs. 68.4 years, P < .001) and died younger (72.99 vs. 79.72 years, P < .001).
The study also found a number of factors associated with active seizures, including a history of dominant Alzheimer’s disease mutation (odds ratio, 5.55; P < .001), stroke (OR, 3.17; P < .001), transient ischemic attack (OR, 1.72; P = .003), traumatic brain injury (OR, 1.92; P < .001), Parkinson’s disease (OR, 1.79; P = .025), active depression (OR, 1.61; P < .001) and lower education (OR, 0.97; P =.043).
After the study made adjustments for sex and other associated factors, it found that patients with seizures were still at a 76% higher risk of dying younger (hazard ratio, 1.76; P < .001).
The study also determined that patients with seizures had worse functional assessment scores and were more likely to be physically dependent on others (OR, 2.52; P < .001). Seizure patients also performed worse on Mini-Mental Status Examination (18.50 vs. 22.88; P < .001) and Clinical Dementia Rating-Sum of boxes (7.95 vs. 4.28; P < .001) after adjusting for age and duration of cognitive decline.
A tip for caregivers
Dr. Zawar acknowledged that differentiating seizures from transient bouts of confusion in people with dementia can be difficult for family members and caregivers, but she offered advice to help them do so. “If they notice any unusual confusion or any altered mentation which is episodic in nature,” she said, “they should bring it to the neurologist’s attention as early as possible, because there are studies that have shown the diagnosis of seizures is delayed, and if they are treated in time they can be well-controlled.” Electroencephalography can also confirm the presence of seizures, she added.
Double whammy
One limitation of this study is the lack of details on the types of seizures the participants had along with the inconsistency of EEGs performed on the study population. “In future studies, I would like to have more EEG data on the types of seizures and the frequency of seizures to assess these factors further,” Dr. Zawar said.
Having more detailed information on the seizures would make the findings more valuable, Andrew J. Cole, MD, director of the epilepsy service at Massachusetts General Hospital in Boston said in an interview. “We know a lot about clinically apparent seizures, as witnessed by this paper, but we still don’t know a whole lot about clinically silent or cryptic or nighttime-only seizures that maybe no one would really recognize as such unless they were specifically looking for them, and this paper doesn’t address that issue,” he said.
While the finding that patients with other neurologic diseases have more seizures even if they also have Alzheimer’s disease isn’t “a huge surprise,” Dr. Cole added. “On the other hand, the paper is important because it shows us that in the course of having Alzheimer’s disease, having seizures also makes your outcome worse, the speed of progression faster, and it complicates the management and living with this disease, and they make that point quite clear.”
Dr. Zawar and Dr. Cole have no relevant disclosures.
AT AES 2022
SSRI tied to improved cognition in comorbid depression, dementia
The results of the 12-week open-label, single-group study are positive, study investigator Michael Cronquist Christensen, MPA, DrPH, a director with the Lundbeck pharmaceutical company, told this news organization before presenting the results in a poster at the 15th Clinical Trials on Alzheimer’s Disease conference.
“The study confirms earlier findings of improvement in both depressive symptoms and cognitive performance with vortioxetine in patients with depression and dementia and adds to this research that these clinical effects also extend to improvement in health-related quality of life and patients’ daily functioning,” Dr. Christensen said.
“It also demonstrates that patients with depression and comorbid dementia can be safely treated with 20 mg vortioxetine – starting dose of 5 mg for the first week and up-titration to 10 mg at day 8,” he added.
However, he reported that Lundbeck doesn’t plan to seek approval from the U.S. Food and Drug Administration for a new indication. Vortioxetine received FDA approval in 2013 to treat MDD, but 3 years later the agency rejected an expansion of its indication to include cognitive dysfunction.
“Vortioxetine is approved for MDD, but the product can be used in patients with MDD who have other diseases, including other mental illnesses,” Dr. Christensen said.
Potential neurotransmission modulator
Vortioxetine is a selective serotonin reuptake inhibitor and serotonin receptor modulator. According to Dr. Christensen, evidence suggests the drug’s receptor targets “have the potential to modulate neurotransmitter systems that are essential for regulation of cognitive function.”
The researchers recruited 83 individuals aged 55-85 with recurrent MDD that had started before the age of 55. All had MDD episodes within the previous 6 months and comorbid dementia for at least 6 months.
Of the participants, 65.9% were female. In addition, 42.7% had Alzheimer’s disease, 26.8% had mixed-type dementia, and the rest had other types of dementia.
The daily oral dose of vortioxetine started at 5 mg for up to week 1 and then was increased to 10 mg. It was then increased to 20 mg or decreased to 5 mg “based on investigator judgment and patient response.” The average daily dose was 12.3 mg.
In regard to the primary outcome, at week 12 (n = 70), scores on the Montgomery-Åsberg Depression Rating Scale (MADRS) fell by a mean of –12.4 (.78, P < .0001), which researchers deemed to be a significant reduction in severe symptoms.
“A significant and clinically meaningful effect was observed from week 1,” the researchers reported.
“As a basis for comparison, we typically see an improvement around 13-14 points during 8 weeks of antidepressant treatment in adults with MDD who do not have dementia,” Dr. Christensen added.
More than a third of patients (35.7%) saw a reduction in MADRS score by more than 50% at week 12, and 17.2% were considered to have reached MDD depression remission, defined as a MADRS score at or under 10.
For secondary outcomes, the total Digit Symbol Substitution test score grew by 0.65 (standardized effect size) by week 12, showing significant improvement (P < .0001). In addition, participants improved on some other cognitive measures, and Dr. Christensen noted that “significant improvement was also observed in the patients’ health-related quality of life and daily functioning.”
A third of patients had drug-related treatment-emergent adverse events.
Vortioxetine is one of the most expensive antidepressants: It has a list price of $444 a month, and no generic version is currently available.
Small trial, open-label design
In a comment, Claire Sexton, DPhil, senior director of scientific programs and outreach at the Alzheimer’s Association, said the study “reflects a valuable aspect of treatment research because of the close connection between depression and dementia. Depression is a known risk factor for dementia, including Alzheimer’s disease, and those who have dementia may experience depression.”
She cautioned, however, that the trial was small and had an open-label design instead of the “gold standard” of a double-blinded trial with a control group.
The study was funded by Lundbeck, where Dr. Christensen is an employee. Another author is a Lundbeck employee, and a third author reported various disclosures. Dr. Sexton reported no disclosures.
A version of this article first appeared on Medscape.com.
The results of the 12-week open-label, single-group study are positive, study investigator Michael Cronquist Christensen, MPA, DrPH, a director with the Lundbeck pharmaceutical company, told this news organization before presenting the results in a poster at the 15th Clinical Trials on Alzheimer’s Disease conference.
“The study confirms earlier findings of improvement in both depressive symptoms and cognitive performance with vortioxetine in patients with depression and dementia and adds to this research that these clinical effects also extend to improvement in health-related quality of life and patients’ daily functioning,” Dr. Christensen said.
“It also demonstrates that patients with depression and comorbid dementia can be safely treated with 20 mg vortioxetine – starting dose of 5 mg for the first week and up-titration to 10 mg at day 8,” he added.
However, he reported that Lundbeck doesn’t plan to seek approval from the U.S. Food and Drug Administration for a new indication. Vortioxetine received FDA approval in 2013 to treat MDD, but 3 years later the agency rejected an expansion of its indication to include cognitive dysfunction.
“Vortioxetine is approved for MDD, but the product can be used in patients with MDD who have other diseases, including other mental illnesses,” Dr. Christensen said.
Potential neurotransmission modulator
Vortioxetine is a selective serotonin reuptake inhibitor and serotonin receptor modulator. According to Dr. Christensen, evidence suggests the drug’s receptor targets “have the potential to modulate neurotransmitter systems that are essential for regulation of cognitive function.”
The researchers recruited 83 individuals aged 55-85 with recurrent MDD that had started before the age of 55. All had MDD episodes within the previous 6 months and comorbid dementia for at least 6 months.
Of the participants, 65.9% were female. In addition, 42.7% had Alzheimer’s disease, 26.8% had mixed-type dementia, and the rest had other types of dementia.
The daily oral dose of vortioxetine started at 5 mg for up to week 1 and then was increased to 10 mg. It was then increased to 20 mg or decreased to 5 mg “based on investigator judgment and patient response.” The average daily dose was 12.3 mg.
In regard to the primary outcome, at week 12 (n = 70), scores on the Montgomery-Åsberg Depression Rating Scale (MADRS) fell by a mean of –12.4 (.78, P < .0001), which researchers deemed to be a significant reduction in severe symptoms.
“A significant and clinically meaningful effect was observed from week 1,” the researchers reported.
“As a basis for comparison, we typically see an improvement around 13-14 points during 8 weeks of antidepressant treatment in adults with MDD who do not have dementia,” Dr. Christensen added.
More than a third of patients (35.7%) saw a reduction in MADRS score by more than 50% at week 12, and 17.2% were considered to have reached MDD depression remission, defined as a MADRS score at or under 10.
For secondary outcomes, the total Digit Symbol Substitution test score grew by 0.65 (standardized effect size) by week 12, showing significant improvement (P < .0001). In addition, participants improved on some other cognitive measures, and Dr. Christensen noted that “significant improvement was also observed in the patients’ health-related quality of life and daily functioning.”
A third of patients had drug-related treatment-emergent adverse events.
Vortioxetine is one of the most expensive antidepressants: It has a list price of $444 a month, and no generic version is currently available.
Small trial, open-label design
In a comment, Claire Sexton, DPhil, senior director of scientific programs and outreach at the Alzheimer’s Association, said the study “reflects a valuable aspect of treatment research because of the close connection between depression and dementia. Depression is a known risk factor for dementia, including Alzheimer’s disease, and those who have dementia may experience depression.”
She cautioned, however, that the trial was small and had an open-label design instead of the “gold standard” of a double-blinded trial with a control group.
The study was funded by Lundbeck, where Dr. Christensen is an employee. Another author is a Lundbeck employee, and a third author reported various disclosures. Dr. Sexton reported no disclosures.
A version of this article first appeared on Medscape.com.
The results of the 12-week open-label, single-group study are positive, study investigator Michael Cronquist Christensen, MPA, DrPH, a director with the Lundbeck pharmaceutical company, told this news organization before presenting the results in a poster at the 15th Clinical Trials on Alzheimer’s Disease conference.
“The study confirms earlier findings of improvement in both depressive symptoms and cognitive performance with vortioxetine in patients with depression and dementia and adds to this research that these clinical effects also extend to improvement in health-related quality of life and patients’ daily functioning,” Dr. Christensen said.
“It also demonstrates that patients with depression and comorbid dementia can be safely treated with 20 mg vortioxetine – starting dose of 5 mg for the first week and up-titration to 10 mg at day 8,” he added.
However, he reported that Lundbeck doesn’t plan to seek approval from the U.S. Food and Drug Administration for a new indication. Vortioxetine received FDA approval in 2013 to treat MDD, but 3 years later the agency rejected an expansion of its indication to include cognitive dysfunction.
“Vortioxetine is approved for MDD, but the product can be used in patients with MDD who have other diseases, including other mental illnesses,” Dr. Christensen said.
Potential neurotransmission modulator
Vortioxetine is a selective serotonin reuptake inhibitor and serotonin receptor modulator. According to Dr. Christensen, evidence suggests the drug’s receptor targets “have the potential to modulate neurotransmitter systems that are essential for regulation of cognitive function.”
The researchers recruited 83 individuals aged 55-85 with recurrent MDD that had started before the age of 55. All had MDD episodes within the previous 6 months and comorbid dementia for at least 6 months.
Of the participants, 65.9% were female. In addition, 42.7% had Alzheimer’s disease, 26.8% had mixed-type dementia, and the rest had other types of dementia.
The daily oral dose of vortioxetine started at 5 mg for up to week 1 and then was increased to 10 mg. It was then increased to 20 mg or decreased to 5 mg “based on investigator judgment and patient response.” The average daily dose was 12.3 mg.
In regard to the primary outcome, at week 12 (n = 70), scores on the Montgomery-Åsberg Depression Rating Scale (MADRS) fell by a mean of –12.4 (.78, P < .0001), which researchers deemed to be a significant reduction in severe symptoms.
“A significant and clinically meaningful effect was observed from week 1,” the researchers reported.
“As a basis for comparison, we typically see an improvement around 13-14 points during 8 weeks of antidepressant treatment in adults with MDD who do not have dementia,” Dr. Christensen added.
More than a third of patients (35.7%) saw a reduction in MADRS score by more than 50% at week 12, and 17.2% were considered to have reached MDD depression remission, defined as a MADRS score at or under 10.
For secondary outcomes, the total Digit Symbol Substitution test score grew by 0.65 (standardized effect size) by week 12, showing significant improvement (P < .0001). In addition, participants improved on some other cognitive measures, and Dr. Christensen noted that “significant improvement was also observed in the patients’ health-related quality of life and daily functioning.”
A third of patients had drug-related treatment-emergent adverse events.
Vortioxetine is one of the most expensive antidepressants: It has a list price of $444 a month, and no generic version is currently available.
Small trial, open-label design
In a comment, Claire Sexton, DPhil, senior director of scientific programs and outreach at the Alzheimer’s Association, said the study “reflects a valuable aspect of treatment research because of the close connection between depression and dementia. Depression is a known risk factor for dementia, including Alzheimer’s disease, and those who have dementia may experience depression.”
She cautioned, however, that the trial was small and had an open-label design instead of the “gold standard” of a double-blinded trial with a control group.
The study was funded by Lundbeck, where Dr. Christensen is an employee. Another author is a Lundbeck employee, and a third author reported various disclosures. Dr. Sexton reported no disclosures.
A version of this article first appeared on Medscape.com.
FROM CTAD 2022
How your voice could reveal hidden disease
: First during puberty, as the vocal cords thicken and the voice box migrates down the throat. Then a second time as aging causes structural changes that may weaken the voice.
But for some of us, there’s another voice shift, when a disease begins or when our mental health declines.
This is why more doctors are looking into voice as a biomarker – something that tells you that a disease is present.
Vital signs like blood pressure or heart rate “can give a general idea of how sick we are. But they’re not specific to certain diseases,” says Yael Bensoussan, MD, director of the University of South Florida, Tampa’s Health Voice Center and the coprincipal investigator for the National Institutes of Health’s Voice as a Biomarker of Health project.
“We’re learning that there are patterns” in voice changes that can indicate a range of conditions, including diseases of the nervous system and mental illnesses, she says.
Speaking is complicated, involving everything from the lungs and voice box to the mouth and brain. “A breakdown in any of those parts can affect the voice,” says Maria Powell, PhD, an assistant professor of otolaryngology (the study of diseases of the ear and throat) at Vanderbilt University, Nashville, Tenn., who is working on the NIH project.
You or those around you may not notice the changes. But researchers say voice analysis as a standard part of patient care – akin to blood pressure checks or cholesterol tests – could help identify those who need medical attention earlier.
Often, all it takes is a smartphone – “something that’s cheap, off-the-shelf, and that everyone can use,” says Ariana Anderson, PhD, director of the University of California, Los Angeles, Laboratory of Computational Neuropsychology.
“You can provide voice data in your pajamas, on your couch,” says Frank Rudzicz, PhD, a computer scientist for the NIH project. “It doesn’t require very complicated or expensive equipment, and it doesn’t require a lot of expertise to obtain.” Plus, multiple samples can be collected over time, giving a more accurate picture of health than a single snapshot from, say, a cognitive test.
Over the next 4 years, the Voice as a Biomarker team will receive nearly $18 million to gather a massive amount of voice data. The goal is 20,000-30,000 samples, along with health data about each person being studied. The result will be a sprawling database scientists can use to develop algorithms linking health conditions to the way we speak.
For the first 2 years, new data will be collected exclusively via universities and high-volume clinics to control quality and accuracy. Eventually, people will be invited to submit their own voice recordings, creating a crowdsourced dataset. “Google, Alexa, Amazon – they have access to tons of voice data,” says Dr. Bensoussan. “But it’s not usable in a clinical way, because they don’t have the health information.”
Dr. Bensoussan and her colleagues hope to fill that void with advance voice screening apps, which could prove especially valuable in remote communities that lack access to specialists or as a tool for telemedicine. Down the line, wearable devices with voice analysis could alert people with chronic conditions when they need to see a doctor.
“The watch says, ‘I’ve analyzed your breathing and coughing, and today, you’re really not doing well. You should go to the hospital,’ ” says Dr. Bensoussan, envisioning a wearable for patients with COPD. “It could tell people early that things are declining.”
Artificial intelligence may be better than a brain at pinpointing the right disease. For example, slurred speech could indicate Parkinson’s, a stroke, or ALS, among other things.
“We can hold approximately seven pieces of information in our head at one time,” says Dr. Rudzicz. “It’s really hard for us to get a holistic picture using dozens or hundreds of variables at once.” But a computer can consider a whole range of vocal markers at the same time, piecing them together for a more accurate assessment.
“The goal is not to outperform a ... clinician,” says Dr. Bensoussan. Yet the potential is unmistakably there: In a recent study of patients with cancer of the larynx, an automated voice analysis tool more accurately flagged the disease than laryngologists did.
“Algorithms have a larger training base,” says Dr. Anderson, who developed an app called ChatterBaby that analyzes infant cries. “We have a million samples at our disposal to train our algorithms. I don’t know if I’ve heard a million different babies crying in my life.”
So which health conditions show the most promise for voice analysis? The Voice as a Biomarker project will focus on five categories.
Voice disorders (cancers of the larynx, vocal fold paralysis, benign lesions on the larynx)
Obviously, vocal changes are a hallmark of these conditions, which cause things like breathiness or “roughness,” a type of vocal irregularity. Hoarseness that lasts at least 2 weeks is often one of the earliest signs of laryngeal cancer. Yet it can take months – one study found 16 weeks was the average – for patients to see a doctor after noticing the changes. Even then, laryngologists still misdiagnosed some cases of cancer when relying on vocal cues alone.
Now imagine a different scenario: The patient speaks into a smartphone app. An algorithm compares the vocal sample with the voices of laryngeal cancer patients. The app spits out the estimated odds of laryngeal cancer, helping providers decide whether to offer the patient specialist care.
Or consider spasmodic dysphonia, a neurological voice disorder that triggers spasms in the muscles of the voice box, causing a strained or breathy voice. Doctors who lack experience with vocal disorders may miss the condition. This is why diagnosis takes an average of nearly 4.5 years, according to a study in the Journal of Voice, and may include everything from allergy testing to psychiatric evaluation, says Dr. Powell. Artificial intelligence technology trained to recognize the disorder could help eliminate such unnecessary testing.
Neurological and neurodegenerative disorders (Alzheimer’s, Parkinson’s, stroke, ALS)
For Alzheimer’s and Parkinson’s, “one of the first changes that’s notable is voice,” usually appearing before a formal diagnosis, says Anais Rameau, MD, an assistant professor of laryngology at Weill Cornell Medicine, New York, and another member of the NIH project. Parkinson’s may soften the voice or make it sound monotone, while Alzheimer’s disease may change the content of speech, leading to an uptick in “umms” and a preference for pronouns over nouns.
With Parkinson’s, vocal changes can occur decades before movement is affected. If doctors could detect the disease at this stage, before tremor emerged, they might be able to flag patients for early intervention, says Max Little, PhD, project director for the Parkinson’s Voice Initiative. “That is the ‘holy grail’ for finding an eventual cure.”
Again, the smartphone shows potential. In a 2022 Australian study, an AI-powered app was able to identify people with Parkinson’s based on brief voice recordings, although the sample size was small. On a larger scale, the Parkinson’s Voice Initiative collected some 17,000 samples from people across the world. “The aim was to remotely detect those with the condition using a telephone call,” says Dr. Little. It did so with about 65% accuracy. “While this is not accurate enough for clinical use, it shows the potential of the idea,” he says.
Dr. Rudzicz worked on the team behind Winterlight, an iPad app that analyzes 550 features of speech to detect dementia and Alzheimer’s (as well as mental illness). “We deployed it in long-term care facilities,” he says, identifying patients who need further review of their mental skills. Stroke is another area of interest, because slurred speech is a highly subjective measure, says Dr. Anderson. AI technology could provide a more objective evaluation.
Mood and psychiatric disorders (depression, schizophrenia, bipolar disorders)
No established biomarkers exist for diagnosing depression. Yet if you’re feeling down, there’s a good chance your friends can tell – even over the phone.
“We carry a lot of our mood in our voice,” says Dr. Powell. Bipolar disorder can also alter voice, making it louder and faster during manic periods, then slower and quieter during depressive bouts. The catatonic stage of schizophrenia often comes with “a very monotone, robotic voice,” says Dr. Anderson. “These are all something an algorithm can measure.”
Apps are already being used – often in research settings – to monitor voices during phone calls, analyzing rate, rhythm, volume, and pitch, to predict mood changes. For example, the PRIORI project at the University of Michigan is working on a smartphone app to identify mood changes in people with bipolar disorder, especially shifts that could increase suicide risk.
The content of speech may also offer clues. In a University of California, Los Angeles, study published in the journal PLoS One, people with mental illnesses answered computer-programmed questions (like “How have you been over the past few days?”) over the phone. An app analyzed their word choices, paying attention to how they changed over time. The researchers found that AI analysis of mood aligned well with doctors’ assessments and that some people in the study actually felt more comfortable talking to a computer.
Respiratory disorders (pneumonia, COPD)
Beyond talking, respiratory sounds like gasping or coughing may point to specific conditions. “Emphysema cough is different, COPD cough is different,” says Dr. Bensoussan. Researchers are trying to find out if COVID-19 has a distinct cough.
Breathing sounds can also serve as signposts. “There are different sounds when we can’t breathe,” says Dr. Bensoussan. One is called stridor, a high-pitched wheezing often resulting from a blocked airway. “I see tons of people [with stridor] misdiagnosed for years – they’ve been told they have asthma, but they don’t,” says Dr. Bensoussan. AI analysis of these sounds could help doctors more quickly identify respiratory disorders.
Pediatric voice and speech disorders (speech and language delays, autism)
Babies who later have autism cry differently as early as 6 months of age, which means an app like ChatterBaby could help flag children for early intervention, says Dr. Anderson. Autism is linked to several other diagnoses, such as epilepsy and sleep disorders. So analyzing an infant’s cry could prompt pediatricians to screen for a range of conditions.
ChatterBaby has been “incredibly accurate” in identifying when babies are in pain, says Dr. Anderson, because pain increases muscle tension, resulting in a louder, more energetic cry. The next goal: “We’re collecting voices from babies around the world,” she says, and then tracking those children for 7 years, looking to see if early vocal signs could predict developmental disorders. Vocal samples from young children could serve a similar purpose.
And that’s only the beginning
Eventually, AI technology may pick up disease-related voice changes that we can’t even hear. In a new Mayo Clinic study, certain vocal features detectable by AI – but not by the human ear – were linked to a three-fold increase in the likelihood of having plaque buildup in the arteries.
“Voice is a huge spectrum of vibrations,” explains study author Amir Lerman, MD. “We hear a very narrow range.”
The researchers aren’t sure why heart disease alters voice, but the autonomic nervous system may play a role, because it regulates the voice box as well as blood pressure and heart rate. Dr. Lerman says other conditions, like diseases of the nerves and gut, may similarly alter the voice. Beyond patient screening, this discovery could help doctors adjust medication doses remotely, in line with these inaudible vocal signals.
“Hopefully, in the next few years, this is going to come to practice,” says Dr. Lerman.
Still, in the face of that hope, privacy concerns remain. Voice is an identifier that’s protected by the federal Health Insurance Portability and Accountability Act, which requires privacy of personal health information. That is a major reason why no large voice databases exist yet, says Dr. Bensoussan. (This makes collecting samples from children especially challenging.) Perhaps more concerning is the potential for diagnosing disease based on voice alone. “You could use that tool on anyone, including officials like the president,” says Dr. Rameau.
But the primary hurdle is the ethical sourcing of data to ensure a diversity of vocal samples. For the Voice as a Biomarker project, the researchers will establish voice quotas for different races and ethnicities, ensuring algorithms can accurately analyze a range of accents. Data from people with speech impediments will also be gathered.
Despite these challenges, researchers are optimistic. “Vocal analysis is going to be a great equalizer and improve health outcomes,” predicts Dr. Anderson. “I’m really happy that we are beginning to understand the strength of the voice.”
A version of this article first appeared on WebMD.com.
: First during puberty, as the vocal cords thicken and the voice box migrates down the throat. Then a second time as aging causes structural changes that may weaken the voice.
But for some of us, there’s another voice shift, when a disease begins or when our mental health declines.
This is why more doctors are looking into voice as a biomarker – something that tells you that a disease is present.
Vital signs like blood pressure or heart rate “can give a general idea of how sick we are. But they’re not specific to certain diseases,” says Yael Bensoussan, MD, director of the University of South Florida, Tampa’s Health Voice Center and the coprincipal investigator for the National Institutes of Health’s Voice as a Biomarker of Health project.
“We’re learning that there are patterns” in voice changes that can indicate a range of conditions, including diseases of the nervous system and mental illnesses, she says.
Speaking is complicated, involving everything from the lungs and voice box to the mouth and brain. “A breakdown in any of those parts can affect the voice,” says Maria Powell, PhD, an assistant professor of otolaryngology (the study of diseases of the ear and throat) at Vanderbilt University, Nashville, Tenn., who is working on the NIH project.
You or those around you may not notice the changes. But researchers say voice analysis as a standard part of patient care – akin to blood pressure checks or cholesterol tests – could help identify those who need medical attention earlier.
Often, all it takes is a smartphone – “something that’s cheap, off-the-shelf, and that everyone can use,” says Ariana Anderson, PhD, director of the University of California, Los Angeles, Laboratory of Computational Neuropsychology.
“You can provide voice data in your pajamas, on your couch,” says Frank Rudzicz, PhD, a computer scientist for the NIH project. “It doesn’t require very complicated or expensive equipment, and it doesn’t require a lot of expertise to obtain.” Plus, multiple samples can be collected over time, giving a more accurate picture of health than a single snapshot from, say, a cognitive test.
Over the next 4 years, the Voice as a Biomarker team will receive nearly $18 million to gather a massive amount of voice data. The goal is 20,000-30,000 samples, along with health data about each person being studied. The result will be a sprawling database scientists can use to develop algorithms linking health conditions to the way we speak.
For the first 2 years, new data will be collected exclusively via universities and high-volume clinics to control quality and accuracy. Eventually, people will be invited to submit their own voice recordings, creating a crowdsourced dataset. “Google, Alexa, Amazon – they have access to tons of voice data,” says Dr. Bensoussan. “But it’s not usable in a clinical way, because they don’t have the health information.”
Dr. Bensoussan and her colleagues hope to fill that void with advance voice screening apps, which could prove especially valuable in remote communities that lack access to specialists or as a tool for telemedicine. Down the line, wearable devices with voice analysis could alert people with chronic conditions when they need to see a doctor.
“The watch says, ‘I’ve analyzed your breathing and coughing, and today, you’re really not doing well. You should go to the hospital,’ ” says Dr. Bensoussan, envisioning a wearable for patients with COPD. “It could tell people early that things are declining.”
Artificial intelligence may be better than a brain at pinpointing the right disease. For example, slurred speech could indicate Parkinson’s, a stroke, or ALS, among other things.
“We can hold approximately seven pieces of information in our head at one time,” says Dr. Rudzicz. “It’s really hard for us to get a holistic picture using dozens or hundreds of variables at once.” But a computer can consider a whole range of vocal markers at the same time, piecing them together for a more accurate assessment.
“The goal is not to outperform a ... clinician,” says Dr. Bensoussan. Yet the potential is unmistakably there: In a recent study of patients with cancer of the larynx, an automated voice analysis tool more accurately flagged the disease than laryngologists did.
“Algorithms have a larger training base,” says Dr. Anderson, who developed an app called ChatterBaby that analyzes infant cries. “We have a million samples at our disposal to train our algorithms. I don’t know if I’ve heard a million different babies crying in my life.”
So which health conditions show the most promise for voice analysis? The Voice as a Biomarker project will focus on five categories.
Voice disorders (cancers of the larynx, vocal fold paralysis, benign lesions on the larynx)
Obviously, vocal changes are a hallmark of these conditions, which cause things like breathiness or “roughness,” a type of vocal irregularity. Hoarseness that lasts at least 2 weeks is often one of the earliest signs of laryngeal cancer. Yet it can take months – one study found 16 weeks was the average – for patients to see a doctor after noticing the changes. Even then, laryngologists still misdiagnosed some cases of cancer when relying on vocal cues alone.
Now imagine a different scenario: The patient speaks into a smartphone app. An algorithm compares the vocal sample with the voices of laryngeal cancer patients. The app spits out the estimated odds of laryngeal cancer, helping providers decide whether to offer the patient specialist care.
Or consider spasmodic dysphonia, a neurological voice disorder that triggers spasms in the muscles of the voice box, causing a strained or breathy voice. Doctors who lack experience with vocal disorders may miss the condition. This is why diagnosis takes an average of nearly 4.5 years, according to a study in the Journal of Voice, and may include everything from allergy testing to psychiatric evaluation, says Dr. Powell. Artificial intelligence technology trained to recognize the disorder could help eliminate such unnecessary testing.
Neurological and neurodegenerative disorders (Alzheimer’s, Parkinson’s, stroke, ALS)
For Alzheimer’s and Parkinson’s, “one of the first changes that’s notable is voice,” usually appearing before a formal diagnosis, says Anais Rameau, MD, an assistant professor of laryngology at Weill Cornell Medicine, New York, and another member of the NIH project. Parkinson’s may soften the voice or make it sound monotone, while Alzheimer’s disease may change the content of speech, leading to an uptick in “umms” and a preference for pronouns over nouns.
With Parkinson’s, vocal changes can occur decades before movement is affected. If doctors could detect the disease at this stage, before tremor emerged, they might be able to flag patients for early intervention, says Max Little, PhD, project director for the Parkinson’s Voice Initiative. “That is the ‘holy grail’ for finding an eventual cure.”
Again, the smartphone shows potential. In a 2022 Australian study, an AI-powered app was able to identify people with Parkinson’s based on brief voice recordings, although the sample size was small. On a larger scale, the Parkinson’s Voice Initiative collected some 17,000 samples from people across the world. “The aim was to remotely detect those with the condition using a telephone call,” says Dr. Little. It did so with about 65% accuracy. “While this is not accurate enough for clinical use, it shows the potential of the idea,” he says.
Dr. Rudzicz worked on the team behind Winterlight, an iPad app that analyzes 550 features of speech to detect dementia and Alzheimer’s (as well as mental illness). “We deployed it in long-term care facilities,” he says, identifying patients who need further review of their mental skills. Stroke is another area of interest, because slurred speech is a highly subjective measure, says Dr. Anderson. AI technology could provide a more objective evaluation.
Mood and psychiatric disorders (depression, schizophrenia, bipolar disorders)
No established biomarkers exist for diagnosing depression. Yet if you’re feeling down, there’s a good chance your friends can tell – even over the phone.
“We carry a lot of our mood in our voice,” says Dr. Powell. Bipolar disorder can also alter voice, making it louder and faster during manic periods, then slower and quieter during depressive bouts. The catatonic stage of schizophrenia often comes with “a very monotone, robotic voice,” says Dr. Anderson. “These are all something an algorithm can measure.”
Apps are already being used – often in research settings – to monitor voices during phone calls, analyzing rate, rhythm, volume, and pitch, to predict mood changes. For example, the PRIORI project at the University of Michigan is working on a smartphone app to identify mood changes in people with bipolar disorder, especially shifts that could increase suicide risk.
The content of speech may also offer clues. In a University of California, Los Angeles, study published in the journal PLoS One, people with mental illnesses answered computer-programmed questions (like “How have you been over the past few days?”) over the phone. An app analyzed their word choices, paying attention to how they changed over time. The researchers found that AI analysis of mood aligned well with doctors’ assessments and that some people in the study actually felt more comfortable talking to a computer.
Respiratory disorders (pneumonia, COPD)
Beyond talking, respiratory sounds like gasping or coughing may point to specific conditions. “Emphysema cough is different, COPD cough is different,” says Dr. Bensoussan. Researchers are trying to find out if COVID-19 has a distinct cough.
Breathing sounds can also serve as signposts. “There are different sounds when we can’t breathe,” says Dr. Bensoussan. One is called stridor, a high-pitched wheezing often resulting from a blocked airway. “I see tons of people [with stridor] misdiagnosed for years – they’ve been told they have asthma, but they don’t,” says Dr. Bensoussan. AI analysis of these sounds could help doctors more quickly identify respiratory disorders.
Pediatric voice and speech disorders (speech and language delays, autism)
Babies who later have autism cry differently as early as 6 months of age, which means an app like ChatterBaby could help flag children for early intervention, says Dr. Anderson. Autism is linked to several other diagnoses, such as epilepsy and sleep disorders. So analyzing an infant’s cry could prompt pediatricians to screen for a range of conditions.
ChatterBaby has been “incredibly accurate” in identifying when babies are in pain, says Dr. Anderson, because pain increases muscle tension, resulting in a louder, more energetic cry. The next goal: “We’re collecting voices from babies around the world,” she says, and then tracking those children for 7 years, looking to see if early vocal signs could predict developmental disorders. Vocal samples from young children could serve a similar purpose.
And that’s only the beginning
Eventually, AI technology may pick up disease-related voice changes that we can’t even hear. In a new Mayo Clinic study, certain vocal features detectable by AI – but not by the human ear – were linked to a three-fold increase in the likelihood of having plaque buildup in the arteries.
“Voice is a huge spectrum of vibrations,” explains study author Amir Lerman, MD. “We hear a very narrow range.”
The researchers aren’t sure why heart disease alters voice, but the autonomic nervous system may play a role, because it regulates the voice box as well as blood pressure and heart rate. Dr. Lerman says other conditions, like diseases of the nerves and gut, may similarly alter the voice. Beyond patient screening, this discovery could help doctors adjust medication doses remotely, in line with these inaudible vocal signals.
“Hopefully, in the next few years, this is going to come to practice,” says Dr. Lerman.
Still, in the face of that hope, privacy concerns remain. Voice is an identifier that’s protected by the federal Health Insurance Portability and Accountability Act, which requires privacy of personal health information. That is a major reason why no large voice databases exist yet, says Dr. Bensoussan. (This makes collecting samples from children especially challenging.) Perhaps more concerning is the potential for diagnosing disease based on voice alone. “You could use that tool on anyone, including officials like the president,” says Dr. Rameau.
But the primary hurdle is the ethical sourcing of data to ensure a diversity of vocal samples. For the Voice as a Biomarker project, the researchers will establish voice quotas for different races and ethnicities, ensuring algorithms can accurately analyze a range of accents. Data from people with speech impediments will also be gathered.
Despite these challenges, researchers are optimistic. “Vocal analysis is going to be a great equalizer and improve health outcomes,” predicts Dr. Anderson. “I’m really happy that we are beginning to understand the strength of the voice.”
A version of this article first appeared on WebMD.com.
: First during puberty, as the vocal cords thicken and the voice box migrates down the throat. Then a second time as aging causes structural changes that may weaken the voice.
But for some of us, there’s another voice shift, when a disease begins or when our mental health declines.
This is why more doctors are looking into voice as a biomarker – something that tells you that a disease is present.
Vital signs like blood pressure or heart rate “can give a general idea of how sick we are. But they’re not specific to certain diseases,” says Yael Bensoussan, MD, director of the University of South Florida, Tampa’s Health Voice Center and the coprincipal investigator for the National Institutes of Health’s Voice as a Biomarker of Health project.
“We’re learning that there are patterns” in voice changes that can indicate a range of conditions, including diseases of the nervous system and mental illnesses, she says.
Speaking is complicated, involving everything from the lungs and voice box to the mouth and brain. “A breakdown in any of those parts can affect the voice,” says Maria Powell, PhD, an assistant professor of otolaryngology (the study of diseases of the ear and throat) at Vanderbilt University, Nashville, Tenn., who is working on the NIH project.
You or those around you may not notice the changes. But researchers say voice analysis as a standard part of patient care – akin to blood pressure checks or cholesterol tests – could help identify those who need medical attention earlier.
Often, all it takes is a smartphone – “something that’s cheap, off-the-shelf, and that everyone can use,” says Ariana Anderson, PhD, director of the University of California, Los Angeles, Laboratory of Computational Neuropsychology.
“You can provide voice data in your pajamas, on your couch,” says Frank Rudzicz, PhD, a computer scientist for the NIH project. “It doesn’t require very complicated or expensive equipment, and it doesn’t require a lot of expertise to obtain.” Plus, multiple samples can be collected over time, giving a more accurate picture of health than a single snapshot from, say, a cognitive test.
Over the next 4 years, the Voice as a Biomarker team will receive nearly $18 million to gather a massive amount of voice data. The goal is 20,000-30,000 samples, along with health data about each person being studied. The result will be a sprawling database scientists can use to develop algorithms linking health conditions to the way we speak.
For the first 2 years, new data will be collected exclusively via universities and high-volume clinics to control quality and accuracy. Eventually, people will be invited to submit their own voice recordings, creating a crowdsourced dataset. “Google, Alexa, Amazon – they have access to tons of voice data,” says Dr. Bensoussan. “But it’s not usable in a clinical way, because they don’t have the health information.”
Dr. Bensoussan and her colleagues hope to fill that void with advance voice screening apps, which could prove especially valuable in remote communities that lack access to specialists or as a tool for telemedicine. Down the line, wearable devices with voice analysis could alert people with chronic conditions when they need to see a doctor.
“The watch says, ‘I’ve analyzed your breathing and coughing, and today, you’re really not doing well. You should go to the hospital,’ ” says Dr. Bensoussan, envisioning a wearable for patients with COPD. “It could tell people early that things are declining.”
Artificial intelligence may be better than a brain at pinpointing the right disease. For example, slurred speech could indicate Parkinson’s, a stroke, or ALS, among other things.
“We can hold approximately seven pieces of information in our head at one time,” says Dr. Rudzicz. “It’s really hard for us to get a holistic picture using dozens or hundreds of variables at once.” But a computer can consider a whole range of vocal markers at the same time, piecing them together for a more accurate assessment.
“The goal is not to outperform a ... clinician,” says Dr. Bensoussan. Yet the potential is unmistakably there: In a recent study of patients with cancer of the larynx, an automated voice analysis tool more accurately flagged the disease than laryngologists did.
“Algorithms have a larger training base,” says Dr. Anderson, who developed an app called ChatterBaby that analyzes infant cries. “We have a million samples at our disposal to train our algorithms. I don’t know if I’ve heard a million different babies crying in my life.”
So which health conditions show the most promise for voice analysis? The Voice as a Biomarker project will focus on five categories.
Voice disorders (cancers of the larynx, vocal fold paralysis, benign lesions on the larynx)
Obviously, vocal changes are a hallmark of these conditions, which cause things like breathiness or “roughness,” a type of vocal irregularity. Hoarseness that lasts at least 2 weeks is often one of the earliest signs of laryngeal cancer. Yet it can take months – one study found 16 weeks was the average – for patients to see a doctor after noticing the changes. Even then, laryngologists still misdiagnosed some cases of cancer when relying on vocal cues alone.
Now imagine a different scenario: The patient speaks into a smartphone app. An algorithm compares the vocal sample with the voices of laryngeal cancer patients. The app spits out the estimated odds of laryngeal cancer, helping providers decide whether to offer the patient specialist care.
Or consider spasmodic dysphonia, a neurological voice disorder that triggers spasms in the muscles of the voice box, causing a strained or breathy voice. Doctors who lack experience with vocal disorders may miss the condition. This is why diagnosis takes an average of nearly 4.5 years, according to a study in the Journal of Voice, and may include everything from allergy testing to psychiatric evaluation, says Dr. Powell. Artificial intelligence technology trained to recognize the disorder could help eliminate such unnecessary testing.
Neurological and neurodegenerative disorders (Alzheimer’s, Parkinson’s, stroke, ALS)
For Alzheimer’s and Parkinson’s, “one of the first changes that’s notable is voice,” usually appearing before a formal diagnosis, says Anais Rameau, MD, an assistant professor of laryngology at Weill Cornell Medicine, New York, and another member of the NIH project. Parkinson’s may soften the voice or make it sound monotone, while Alzheimer’s disease may change the content of speech, leading to an uptick in “umms” and a preference for pronouns over nouns.
With Parkinson’s, vocal changes can occur decades before movement is affected. If doctors could detect the disease at this stage, before tremor emerged, they might be able to flag patients for early intervention, says Max Little, PhD, project director for the Parkinson’s Voice Initiative. “That is the ‘holy grail’ for finding an eventual cure.”
Again, the smartphone shows potential. In a 2022 Australian study, an AI-powered app was able to identify people with Parkinson’s based on brief voice recordings, although the sample size was small. On a larger scale, the Parkinson’s Voice Initiative collected some 17,000 samples from people across the world. “The aim was to remotely detect those with the condition using a telephone call,” says Dr. Little. It did so with about 65% accuracy. “While this is not accurate enough for clinical use, it shows the potential of the idea,” he says.
Dr. Rudzicz worked on the team behind Winterlight, an iPad app that analyzes 550 features of speech to detect dementia and Alzheimer’s (as well as mental illness). “We deployed it in long-term care facilities,” he says, identifying patients who need further review of their mental skills. Stroke is another area of interest, because slurred speech is a highly subjective measure, says Dr. Anderson. AI technology could provide a more objective evaluation.
Mood and psychiatric disorders (depression, schizophrenia, bipolar disorders)
No established biomarkers exist for diagnosing depression. Yet if you’re feeling down, there’s a good chance your friends can tell – even over the phone.
“We carry a lot of our mood in our voice,” says Dr. Powell. Bipolar disorder can also alter voice, making it louder and faster during manic periods, then slower and quieter during depressive bouts. The catatonic stage of schizophrenia often comes with “a very monotone, robotic voice,” says Dr. Anderson. “These are all something an algorithm can measure.”
Apps are already being used – often in research settings – to monitor voices during phone calls, analyzing rate, rhythm, volume, and pitch, to predict mood changes. For example, the PRIORI project at the University of Michigan is working on a smartphone app to identify mood changes in people with bipolar disorder, especially shifts that could increase suicide risk.
The content of speech may also offer clues. In a University of California, Los Angeles, study published in the journal PLoS One, people with mental illnesses answered computer-programmed questions (like “How have you been over the past few days?”) over the phone. An app analyzed their word choices, paying attention to how they changed over time. The researchers found that AI analysis of mood aligned well with doctors’ assessments and that some people in the study actually felt more comfortable talking to a computer.
Respiratory disorders (pneumonia, COPD)
Beyond talking, respiratory sounds like gasping or coughing may point to specific conditions. “Emphysema cough is different, COPD cough is different,” says Dr. Bensoussan. Researchers are trying to find out if COVID-19 has a distinct cough.
Breathing sounds can also serve as signposts. “There are different sounds when we can’t breathe,” says Dr. Bensoussan. One is called stridor, a high-pitched wheezing often resulting from a blocked airway. “I see tons of people [with stridor] misdiagnosed for years – they’ve been told they have asthma, but they don’t,” says Dr. Bensoussan. AI analysis of these sounds could help doctors more quickly identify respiratory disorders.
Pediatric voice and speech disorders (speech and language delays, autism)
Babies who later have autism cry differently as early as 6 months of age, which means an app like ChatterBaby could help flag children for early intervention, says Dr. Anderson. Autism is linked to several other diagnoses, such as epilepsy and sleep disorders. So analyzing an infant’s cry could prompt pediatricians to screen for a range of conditions.
ChatterBaby has been “incredibly accurate” in identifying when babies are in pain, says Dr. Anderson, because pain increases muscle tension, resulting in a louder, more energetic cry. The next goal: “We’re collecting voices from babies around the world,” she says, and then tracking those children for 7 years, looking to see if early vocal signs could predict developmental disorders. Vocal samples from young children could serve a similar purpose.
And that’s only the beginning
Eventually, AI technology may pick up disease-related voice changes that we can’t even hear. In a new Mayo Clinic study, certain vocal features detectable by AI – but not by the human ear – were linked to a three-fold increase in the likelihood of having plaque buildup in the arteries.
“Voice is a huge spectrum of vibrations,” explains study author Amir Lerman, MD. “We hear a very narrow range.”
The researchers aren’t sure why heart disease alters voice, but the autonomic nervous system may play a role, because it regulates the voice box as well as blood pressure and heart rate. Dr. Lerman says other conditions, like diseases of the nerves and gut, may similarly alter the voice. Beyond patient screening, this discovery could help doctors adjust medication doses remotely, in line with these inaudible vocal signals.
“Hopefully, in the next few years, this is going to come to practice,” says Dr. Lerman.
Still, in the face of that hope, privacy concerns remain. Voice is an identifier that’s protected by the federal Health Insurance Portability and Accountability Act, which requires privacy of personal health information. That is a major reason why no large voice databases exist yet, says Dr. Bensoussan. (This makes collecting samples from children especially challenging.) Perhaps more concerning is the potential for diagnosing disease based on voice alone. “You could use that tool on anyone, including officials like the president,” says Dr. Rameau.
But the primary hurdle is the ethical sourcing of data to ensure a diversity of vocal samples. For the Voice as a Biomarker project, the researchers will establish voice quotas for different races and ethnicities, ensuring algorithms can accurately analyze a range of accents. Data from people with speech impediments will also be gathered.
Despite these challenges, researchers are optimistic. “Vocal analysis is going to be a great equalizer and improve health outcomes,” predicts Dr. Anderson. “I’m really happy that we are beginning to understand the strength of the voice.”
A version of this article first appeared on WebMD.com.
Mind the geriatrician gap
These should be the best of times for geriatric medicine.
The baby boom has become a senior surge, bringing in a rapidly growing pool of aging patients for geriatricians to treat. According to the U.S. Census Bureau, more than 56 million adults aged 65 and older live in the United States. They account for about 17% of the nation’s population. That number is expected to hit 73 million by 2030 and 86 million by 2050.
The American Geriatrics Society estimates that 30% of older people require the attention of geriatricians. These clinicians excel in managing complex cases – patients with multiple comorbidities, such as coronary artery disease, dementia, and osteoporosis, who are taking a half dozen, and often more, medications.
. In the 2010s, geriatricians called for “25,000 [such specialists] by 2025.” As of 2021, 7123 certified geriatricians were practicing in the United States, according to the American Board of Medical Specialties.
The Health Resources and Services Administration, a federal agency that addresses medical workforce shortages, estimates that there will be 6,230 geriatricians by 2025, or approximately 1 for every 3,000 older adults requiring geriatric care. HRSA projects a shortage of 27,000 geriatricians by 2025.
The specialty has faced an uphill battle to attract fellows. This year, only 43% of the nation’s 177 geriatrics fellowship slots were filled, according to November’s National Resident Match Program report. Family medicine–based geriatrics achieved only a 32% fill rate, while internal medicine–based programs saw a rate of 45%.
“Our numbers are shrinking so we need another approach to make sure older adults get the care they need and deserve,” said G. Michael Harper, MD, president of the 6,000-member AGS.
But Dr. Harper, who practices at the University of California, San Francisco, and the San Francisco VA Medical Center, added a positive note: “We may be struggling to increase the number of board-certified geriatricians, but the field itself has made a lot of progress in terms of improving clinical care through advancements in science and in the ways we deliver care.”
Dr. Harper cited the Hospital Elder Life Program, a hospital model developed at the Harvard-affiliated Marcus Institute for Aging Research, which uses an interprofessional team and trained volunteers to prevent delirium and functional decline. HELP has been adopted by more than 200 hospitals worldwide and has been successful at returning older adults to their homes or previous living situations with maintained or improved ability to function, he said.
Mark Supiano, MD, professor and chief of geriatrics at the University of Utah, Salt Lake City, said the specialty has been in shortage mode since ABMS recognized it in 1988. He was in the initial cohort of fellowship-trained geriatricians, sitting for the first certifying exam in geriatrics offered that year.
“Back then, the demographic imperative of the aging of our society was on the horizon. We’re living it now. I knew enough to recognize it was coming and saw an opportunity,” Dr. Supiano said in an interview. “There was so much then that we didn’t know about how to understand aging or how to care for older adults that there really was such a knowledge gap.”
Dr. Supiano is an associate editor of Hazzard’s Geriatric Medicine and Gerontology (McGraw-Hill Education), which has more than doubled in pages and word count during his career.
Unfavorable finances
Katherine Thompson, MD, director of the geriatrics fellowship program at the University of Chicago and codirector of UChicago’s Successful Aging and Frailty Evaluation Clinic, said money is a major reason for the struggle. “I think probably the biggest driver is financial,” she said. “A lot of people are graduating medical school with really astronomical amounts of medical school loans.”
Geriatricians, like other doctors, carry a large debt – $200,000, on average, not counting undergraduate debt, according to the Association of American Medical Colleges.
But the typical geriatrician earns less than an internist or family medicine doctor who doesn’t undergo the additional year of training, Dr. Thompson said. “There’s not a lot of financial motivation to do this fellowship,” she said.
The jobs website Zippia reports that geriatricians earned roughly $165,000 per year on average in 2022. The average annual incomes in 2022 were $191,000 for pediatricians, $215,000 for family physicians, and $223,000 for internists, according to the site.
In other words, Dr. Harper said, “geriatrics is one of the few professions where you can actually do additional training and make less money.”
The reason for the pay issue is simple: Geriatricians treat patients covered by Medicare, whose reimbursement schedules lag behind those of commercial insurers. The Kaiser Family Foundation reported in 2020 that private insurance paid 143% of Medicare rates on average for physician services.
Dr. Harper said overall compensation for geriatricians has “not gained a lot of traction,” but they can earn comfortable livings.
Still, representation of the specialty on the American Medical Association’s Relative Value Scale Update Committee has led to approval by the Centers for Medicare & Medicaid Services of billing codes that pay geriatricians “for what they do. Examples include chronic care management, advance care planning, and dementia evaluation,” he said.
But the geriatrician gap goes beyond money.
Ageism, too, may play a role in residents not choosing geriatrics.
“Our culture is ageist. It definitely focuses on youth and looks at aging as being loss rather than just a change in what works well and what doesn’t work well,” said Mary Tinetti, MD, a geriatrician and researcher at Yale University, New Haven, Conn. “Ageism happens among physicians, just because they’re part of the broader society.”
Time for a new goal?
Dr. Tinetti said she’s optimistic that new ideas about geriatricians teaching other primary care clinicians about the tenets of geriatric medicine, which offer a wholistic approach to comorbidities, such as diabetes, atrial fibrillation, dementia, hypertension, hyperlipidemia, and polypharmacy problems faced by this population, especially those 85 and older.
She has called on her profession to abandon the goal of increasing the numbers of board-certified geriatricians – whom she refers to as big “G” geriatricians. She instead wants to develop a “small, elite workforce” that discovers and tests geriatrics principles through research, teaches these principles to all healthcare professions and to the public, and disseminates and implements the policies.
“We need a cadre of geriatricians who train all other clinicians in the care of older adults,” Dr. Tinetti said. “The goal is not more geriatricians but rather the preparation of all clinicians in the care of older adults.”
Dr. Thompson said geriatricians are teaching primary care specialists, nurses, social workers, and other health care providers the principles of age-friendly care. AGS has for the past 20 years led a program called the Geriatrics for Specialists Initiative to increase geriatrics knowledge and expertise of surgical and medical specialists.
Some specialties have taken the cue and have added geriatrics-related hyphens through additional training: geriatric-emergency, geriatric-general surgery, geriatric-hospitalists, and more.
HRSA runs programs to encourage physicians to train as geriatricians and geriatrics faculty, and it encourages the geriatrics interdisciplinary team approach.
Richard Olague, director of public affairs for HRSA, said his agency has invested over $160 million over the past 4 years in the education and training of geriatricians and other health care professionals who care for the elderly through its Geriatrics Workforce Enhancement Program and Geriatrics Academic Career Awards Program. In the academic year 2020-2021, the two programs trained 109 geriatricians; 456 other geriatric/gerontology providers and students; 44,450 other healthcare workforce professionals and students; and served 17,666 patients and 5,409 caregivers.
Dr. Harper, like his fellow geriatricians, tells young doctors that geriatrics is a fulfilling specialty.
“I get to care for the whole person and sometimes their families, too, and in the process form rich and meaningful relationships. And while I’m rarely in the position to cure, I always have the ability to care,” he said. “Sometimes that can mean being an advocate trying to make sure my patients receive the care they need, and other times it might mean protecting them from burdensome care that is unlikely to lead to any meaningful benefit. There is great reward in all of that.”
Dr. Supiano said geriatric patients are being helped by the Age-Friendly Health System initiative of the John A. Hartford Foundation and the Institute for Healthcare Improvement in partnership with the American Hospital Association and the Catholic Health Association of the United States. This is sort of a seal of approval for facilities committed to age-friendly care.
“When you go to your hospital, if they don’t have this age-friendly health system banner on the front door ... you either ask why that is not there, or you vote with your feet and go to another health system that is age friendly,” he said. “Geriatricians are eternal optimists.”
A version of this article first appeared on Medscape.com.
These should be the best of times for geriatric medicine.
The baby boom has become a senior surge, bringing in a rapidly growing pool of aging patients for geriatricians to treat. According to the U.S. Census Bureau, more than 56 million adults aged 65 and older live in the United States. They account for about 17% of the nation’s population. That number is expected to hit 73 million by 2030 and 86 million by 2050.
The American Geriatrics Society estimates that 30% of older people require the attention of geriatricians. These clinicians excel in managing complex cases – patients with multiple comorbidities, such as coronary artery disease, dementia, and osteoporosis, who are taking a half dozen, and often more, medications.
. In the 2010s, geriatricians called for “25,000 [such specialists] by 2025.” As of 2021, 7123 certified geriatricians were practicing in the United States, according to the American Board of Medical Specialties.
The Health Resources and Services Administration, a federal agency that addresses medical workforce shortages, estimates that there will be 6,230 geriatricians by 2025, or approximately 1 for every 3,000 older adults requiring geriatric care. HRSA projects a shortage of 27,000 geriatricians by 2025.
The specialty has faced an uphill battle to attract fellows. This year, only 43% of the nation’s 177 geriatrics fellowship slots were filled, according to November’s National Resident Match Program report. Family medicine–based geriatrics achieved only a 32% fill rate, while internal medicine–based programs saw a rate of 45%.
“Our numbers are shrinking so we need another approach to make sure older adults get the care they need and deserve,” said G. Michael Harper, MD, president of the 6,000-member AGS.
But Dr. Harper, who practices at the University of California, San Francisco, and the San Francisco VA Medical Center, added a positive note: “We may be struggling to increase the number of board-certified geriatricians, but the field itself has made a lot of progress in terms of improving clinical care through advancements in science and in the ways we deliver care.”
Dr. Harper cited the Hospital Elder Life Program, a hospital model developed at the Harvard-affiliated Marcus Institute for Aging Research, which uses an interprofessional team and trained volunteers to prevent delirium and functional decline. HELP has been adopted by more than 200 hospitals worldwide and has been successful at returning older adults to their homes or previous living situations with maintained or improved ability to function, he said.
Mark Supiano, MD, professor and chief of geriatrics at the University of Utah, Salt Lake City, said the specialty has been in shortage mode since ABMS recognized it in 1988. He was in the initial cohort of fellowship-trained geriatricians, sitting for the first certifying exam in geriatrics offered that year.
“Back then, the demographic imperative of the aging of our society was on the horizon. We’re living it now. I knew enough to recognize it was coming and saw an opportunity,” Dr. Supiano said in an interview. “There was so much then that we didn’t know about how to understand aging or how to care for older adults that there really was such a knowledge gap.”
Dr. Supiano is an associate editor of Hazzard’s Geriatric Medicine and Gerontology (McGraw-Hill Education), which has more than doubled in pages and word count during his career.
Unfavorable finances
Katherine Thompson, MD, director of the geriatrics fellowship program at the University of Chicago and codirector of UChicago’s Successful Aging and Frailty Evaluation Clinic, said money is a major reason for the struggle. “I think probably the biggest driver is financial,” she said. “A lot of people are graduating medical school with really astronomical amounts of medical school loans.”
Geriatricians, like other doctors, carry a large debt – $200,000, on average, not counting undergraduate debt, according to the Association of American Medical Colleges.
But the typical geriatrician earns less than an internist or family medicine doctor who doesn’t undergo the additional year of training, Dr. Thompson said. “There’s not a lot of financial motivation to do this fellowship,” she said.
The jobs website Zippia reports that geriatricians earned roughly $165,000 per year on average in 2022. The average annual incomes in 2022 were $191,000 for pediatricians, $215,000 for family physicians, and $223,000 for internists, according to the site.
In other words, Dr. Harper said, “geriatrics is one of the few professions where you can actually do additional training and make less money.”
The reason for the pay issue is simple: Geriatricians treat patients covered by Medicare, whose reimbursement schedules lag behind those of commercial insurers. The Kaiser Family Foundation reported in 2020 that private insurance paid 143% of Medicare rates on average for physician services.
Dr. Harper said overall compensation for geriatricians has “not gained a lot of traction,” but they can earn comfortable livings.
Still, representation of the specialty on the American Medical Association’s Relative Value Scale Update Committee has led to approval by the Centers for Medicare & Medicaid Services of billing codes that pay geriatricians “for what they do. Examples include chronic care management, advance care planning, and dementia evaluation,” he said.
But the geriatrician gap goes beyond money.
Ageism, too, may play a role in residents not choosing geriatrics.
“Our culture is ageist. It definitely focuses on youth and looks at aging as being loss rather than just a change in what works well and what doesn’t work well,” said Mary Tinetti, MD, a geriatrician and researcher at Yale University, New Haven, Conn. “Ageism happens among physicians, just because they’re part of the broader society.”
Time for a new goal?
Dr. Tinetti said she’s optimistic that new ideas about geriatricians teaching other primary care clinicians about the tenets of geriatric medicine, which offer a wholistic approach to comorbidities, such as diabetes, atrial fibrillation, dementia, hypertension, hyperlipidemia, and polypharmacy problems faced by this population, especially those 85 and older.
She has called on her profession to abandon the goal of increasing the numbers of board-certified geriatricians – whom she refers to as big “G” geriatricians. She instead wants to develop a “small, elite workforce” that discovers and tests geriatrics principles through research, teaches these principles to all healthcare professions and to the public, and disseminates and implements the policies.
“We need a cadre of geriatricians who train all other clinicians in the care of older adults,” Dr. Tinetti said. “The goal is not more geriatricians but rather the preparation of all clinicians in the care of older adults.”
Dr. Thompson said geriatricians are teaching primary care specialists, nurses, social workers, and other health care providers the principles of age-friendly care. AGS has for the past 20 years led a program called the Geriatrics for Specialists Initiative to increase geriatrics knowledge and expertise of surgical and medical specialists.
Some specialties have taken the cue and have added geriatrics-related hyphens through additional training: geriatric-emergency, geriatric-general surgery, geriatric-hospitalists, and more.
HRSA runs programs to encourage physicians to train as geriatricians and geriatrics faculty, and it encourages the geriatrics interdisciplinary team approach.
Richard Olague, director of public affairs for HRSA, said his agency has invested over $160 million over the past 4 years in the education and training of geriatricians and other health care professionals who care for the elderly through its Geriatrics Workforce Enhancement Program and Geriatrics Academic Career Awards Program. In the academic year 2020-2021, the two programs trained 109 geriatricians; 456 other geriatric/gerontology providers and students; 44,450 other healthcare workforce professionals and students; and served 17,666 patients and 5,409 caregivers.
Dr. Harper, like his fellow geriatricians, tells young doctors that geriatrics is a fulfilling specialty.
“I get to care for the whole person and sometimes their families, too, and in the process form rich and meaningful relationships. And while I’m rarely in the position to cure, I always have the ability to care,” he said. “Sometimes that can mean being an advocate trying to make sure my patients receive the care they need, and other times it might mean protecting them from burdensome care that is unlikely to lead to any meaningful benefit. There is great reward in all of that.”
Dr. Supiano said geriatric patients are being helped by the Age-Friendly Health System initiative of the John A. Hartford Foundation and the Institute for Healthcare Improvement in partnership with the American Hospital Association and the Catholic Health Association of the United States. This is sort of a seal of approval for facilities committed to age-friendly care.
“When you go to your hospital, if they don’t have this age-friendly health system banner on the front door ... you either ask why that is not there, or you vote with your feet and go to another health system that is age friendly,” he said. “Geriatricians are eternal optimists.”
A version of this article first appeared on Medscape.com.
These should be the best of times for geriatric medicine.
The baby boom has become a senior surge, bringing in a rapidly growing pool of aging patients for geriatricians to treat. According to the U.S. Census Bureau, more than 56 million adults aged 65 and older live in the United States. They account for about 17% of the nation’s population. That number is expected to hit 73 million by 2030 and 86 million by 2050.
The American Geriatrics Society estimates that 30% of older people require the attention of geriatricians. These clinicians excel in managing complex cases – patients with multiple comorbidities, such as coronary artery disease, dementia, and osteoporosis, who are taking a half dozen, and often more, medications.
. In the 2010s, geriatricians called for “25,000 [such specialists] by 2025.” As of 2021, 7123 certified geriatricians were practicing in the United States, according to the American Board of Medical Specialties.
The Health Resources and Services Administration, a federal agency that addresses medical workforce shortages, estimates that there will be 6,230 geriatricians by 2025, or approximately 1 for every 3,000 older adults requiring geriatric care. HRSA projects a shortage of 27,000 geriatricians by 2025.
The specialty has faced an uphill battle to attract fellows. This year, only 43% of the nation’s 177 geriatrics fellowship slots were filled, according to November’s National Resident Match Program report. Family medicine–based geriatrics achieved only a 32% fill rate, while internal medicine–based programs saw a rate of 45%.
“Our numbers are shrinking so we need another approach to make sure older adults get the care they need and deserve,” said G. Michael Harper, MD, president of the 6,000-member AGS.
But Dr. Harper, who practices at the University of California, San Francisco, and the San Francisco VA Medical Center, added a positive note: “We may be struggling to increase the number of board-certified geriatricians, but the field itself has made a lot of progress in terms of improving clinical care through advancements in science and in the ways we deliver care.”
Dr. Harper cited the Hospital Elder Life Program, a hospital model developed at the Harvard-affiliated Marcus Institute for Aging Research, which uses an interprofessional team and trained volunteers to prevent delirium and functional decline. HELP has been adopted by more than 200 hospitals worldwide and has been successful at returning older adults to their homes or previous living situations with maintained or improved ability to function, he said.
Mark Supiano, MD, professor and chief of geriatrics at the University of Utah, Salt Lake City, said the specialty has been in shortage mode since ABMS recognized it in 1988. He was in the initial cohort of fellowship-trained geriatricians, sitting for the first certifying exam in geriatrics offered that year.
“Back then, the demographic imperative of the aging of our society was on the horizon. We’re living it now. I knew enough to recognize it was coming and saw an opportunity,” Dr. Supiano said in an interview. “There was so much then that we didn’t know about how to understand aging or how to care for older adults that there really was such a knowledge gap.”
Dr. Supiano is an associate editor of Hazzard’s Geriatric Medicine and Gerontology (McGraw-Hill Education), which has more than doubled in pages and word count during his career.
Unfavorable finances
Katherine Thompson, MD, director of the geriatrics fellowship program at the University of Chicago and codirector of UChicago’s Successful Aging and Frailty Evaluation Clinic, said money is a major reason for the struggle. “I think probably the biggest driver is financial,” she said. “A lot of people are graduating medical school with really astronomical amounts of medical school loans.”
Geriatricians, like other doctors, carry a large debt – $200,000, on average, not counting undergraduate debt, according to the Association of American Medical Colleges.
But the typical geriatrician earns less than an internist or family medicine doctor who doesn’t undergo the additional year of training, Dr. Thompson said. “There’s not a lot of financial motivation to do this fellowship,” she said.
The jobs website Zippia reports that geriatricians earned roughly $165,000 per year on average in 2022. The average annual incomes in 2022 were $191,000 for pediatricians, $215,000 for family physicians, and $223,000 for internists, according to the site.
In other words, Dr. Harper said, “geriatrics is one of the few professions where you can actually do additional training and make less money.”
The reason for the pay issue is simple: Geriatricians treat patients covered by Medicare, whose reimbursement schedules lag behind those of commercial insurers. The Kaiser Family Foundation reported in 2020 that private insurance paid 143% of Medicare rates on average for physician services.
Dr. Harper said overall compensation for geriatricians has “not gained a lot of traction,” but they can earn comfortable livings.
Still, representation of the specialty on the American Medical Association’s Relative Value Scale Update Committee has led to approval by the Centers for Medicare & Medicaid Services of billing codes that pay geriatricians “for what they do. Examples include chronic care management, advance care planning, and dementia evaluation,” he said.
But the geriatrician gap goes beyond money.
Ageism, too, may play a role in residents not choosing geriatrics.
“Our culture is ageist. It definitely focuses on youth and looks at aging as being loss rather than just a change in what works well and what doesn’t work well,” said Mary Tinetti, MD, a geriatrician and researcher at Yale University, New Haven, Conn. “Ageism happens among physicians, just because they’re part of the broader society.”
Time for a new goal?
Dr. Tinetti said she’s optimistic that new ideas about geriatricians teaching other primary care clinicians about the tenets of geriatric medicine, which offer a wholistic approach to comorbidities, such as diabetes, atrial fibrillation, dementia, hypertension, hyperlipidemia, and polypharmacy problems faced by this population, especially those 85 and older.
She has called on her profession to abandon the goal of increasing the numbers of board-certified geriatricians – whom she refers to as big “G” geriatricians. She instead wants to develop a “small, elite workforce” that discovers and tests geriatrics principles through research, teaches these principles to all healthcare professions and to the public, and disseminates and implements the policies.
“We need a cadre of geriatricians who train all other clinicians in the care of older adults,” Dr. Tinetti said. “The goal is not more geriatricians but rather the preparation of all clinicians in the care of older adults.”
Dr. Thompson said geriatricians are teaching primary care specialists, nurses, social workers, and other health care providers the principles of age-friendly care. AGS has for the past 20 years led a program called the Geriatrics for Specialists Initiative to increase geriatrics knowledge and expertise of surgical and medical specialists.
Some specialties have taken the cue and have added geriatrics-related hyphens through additional training: geriatric-emergency, geriatric-general surgery, geriatric-hospitalists, and more.
HRSA runs programs to encourage physicians to train as geriatricians and geriatrics faculty, and it encourages the geriatrics interdisciplinary team approach.
Richard Olague, director of public affairs for HRSA, said his agency has invested over $160 million over the past 4 years in the education and training of geriatricians and other health care professionals who care for the elderly through its Geriatrics Workforce Enhancement Program and Geriatrics Academic Career Awards Program. In the academic year 2020-2021, the two programs trained 109 geriatricians; 456 other geriatric/gerontology providers and students; 44,450 other healthcare workforce professionals and students; and served 17,666 patients and 5,409 caregivers.
Dr. Harper, like his fellow geriatricians, tells young doctors that geriatrics is a fulfilling specialty.
“I get to care for the whole person and sometimes their families, too, and in the process form rich and meaningful relationships. And while I’m rarely in the position to cure, I always have the ability to care,” he said. “Sometimes that can mean being an advocate trying to make sure my patients receive the care they need, and other times it might mean protecting them from burdensome care that is unlikely to lead to any meaningful benefit. There is great reward in all of that.”
Dr. Supiano said geriatric patients are being helped by the Age-Friendly Health System initiative of the John A. Hartford Foundation and the Institute for Healthcare Improvement in partnership with the American Hospital Association and the Catholic Health Association of the United States. This is sort of a seal of approval for facilities committed to age-friendly care.
“When you go to your hospital, if they don’t have this age-friendly health system banner on the front door ... you either ask why that is not there, or you vote with your feet and go to another health system that is age friendly,” he said. “Geriatricians are eternal optimists.”
A version of this article first appeared on Medscape.com.
Ultraprocessed foods tied to faster rate of cognitive decline
Results from the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil), which included more than 10,000 people aged 35 and older, showed that higher intake of UPF was significantly associated with a faster rate of decline in executive and global cognitive function.
“These findings show that lifestyle choices, particularly high intake of ultraprocessed foods, can influence our cognitive health many years later,” coinvestigator Natalia Goncalves, PhD, University of São Paulo, Brazil, said in an interview.
The study was published online in JAMA Neurology.
The study’s findings were presented in August at the Alzheimer’s Association International Conference (AAIC) 2022 and were reported by this news organization at that time.
High sugar, salt, fat
The new results align with another recent study linking a diet high in UPFs to an increased risk for dementia.
UPFs are highly manipulated, are packed with added ingredients, including sugar, fat, and salt, and are low in protein and fiber. Examples of UPFs are soft drinks, chips, chocolate, candy, ice cream, sweetened breakfast cereals, packaged soups, chicken nuggets, hot dogs, and fries.
The ELSA-Brasil study comprised 10,775 adults (mean age, 50.6 years at baseline; 55% women; 53% White) who were evaluated in three waves approximately 4 years apart from 2008 to 2017.
Information on diet was obtained via food frequency questionnaires and included details regarding consumption of unprocessed foods, minimally processed foods, and UPFs.
Participants were grouped according to UPF consumption quartiles (lowest to highest). Cognitive performance was evaluated by use of a standardized battery of tests.
During median follow-up of 8 years, people who consumed more than 20% of daily calories from UPFs (quartiles 2-4) experienced a 28% faster rate of decline in global cognition (beta = –0.004; 95% confidence interval [CI], –0.006 to –0.001; P = .003) and a 25% faster rate of decline in executive function (beta = –0.003, 95% CI, –0.005 to 0.000; P = .01) compared to peers in quartile 1 who consumed less than 20% of daily calories from UPFs.
The researchers did not investigate individual groups of UPFs.
However, Dr. Goncalves noted that some studies have linked the consumption of sugar-sweetened beverages with lower cognitive performance, lower brain volume, and poorer memory performance. Another group of ultraprocessed foods, processed meats, has been associated with increased all-cause dementia and Alzheimer’s disease.
Other limitations include the fact that self-reported diet habits were assessed only at baseline using a food frequency questionnaire that was not designed to assess the degree of processing.
While analyses were adjusted for several sociodemographic and clinical confounders, the researchers said they could not exclude the possibility of residual confounding.
Also, since neuroimaging is not available in the ELSA-Brasil study, they were not able to investigate potential mechanisms that could explain the association between higher UPF consumption and cognitive decline.
Despite these limitations, the researchers said their findings suggest that “limiting UPF consumption, particularly in middle-aged adults, may be an efficient form to prevent cognitive decline.”
Weighing the evidence
Several experts weighed in on the results in a statement from the UK nonprofit organization, Science Media Centre.
Kevin McConway, PhD, with Open University, Milton Keynes, England, said it’s important to note that the study suggests “an association, a correlation, and that doesn’t necessarily mean that the cognitive decline was caused by eating more ultra-processed foods.”
He also noted that some types of cognitive decline that are associated with aging occurred in participants in all four quartiles, which were defined by the percentage of their daily energy that came from consuming UPFs.
“That’s hardly surprising – it’s a sad fact of life that pretty well all of us gradually lose some of our cognitive functions as we go through middle and older age,” Dr. McConway said.
“The study doesn’t establish that differences in speed of cognitive decline are caused by ultra-processed food consumption anyway. That’s because it’s an observational study. If the consumption of ultra-processed food causes the differences in rate of cognitive decline, then eating less of it might slow cognitive decline, but if the cause is something else, then that won’t happen,” Dr. McConway added.
Gunter Kuhnle, PhD, professor of nutrition and food science, University of Reading, England, noted that UPFs have become a “fashionable term to explain associations between diet and ill health, and many studies have attempted to show associations.
“Most studies have been observational and had a key limitation: It is very difficult to determine ultra-processed food intake using methods that are not designed to do so, and so authors need to make a lot of assumptions. Bread and meat products are often classed as ‘ultra-processed,’ even though this is often wrong,” Dr. Kuhnle noted.
“The same applies to this study – the method used to measure ultra-processed food intake was not designed for that task and relied on assumptions. This makes it virtually impossible to draw any conclusions,” Dr. Kuhnle said.
Duane Mellor, PhD, RD, RNutr, registered dietitian and senior teaching fellow, Aston University, Birmingham, England, said the study does not change how we should try to eat to maintain good brain function and cognition.
“We should try to eat less foods which are high in added sugar, salt, and fat, which would include many of the foods classified as being ultra-processed, while eating more in terms of both quantity and variety of vegetables, fruit, nuts, seeds, and pulses, which are known to be beneficial for both our cognitive and overall health,” Dr. Mellor said.
The ELSA-Brasil study was supported by the Brazilian Ministry of Health, the Ministry of Science, Technology and Innovation, and the National Council for Scientific and Technological Development. The authors as well as Dr. McConway, Dr. Mellor, and Dr. Kuhnle have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil), which included more than 10,000 people aged 35 and older, showed that higher intake of UPF was significantly associated with a faster rate of decline in executive and global cognitive function.
“These findings show that lifestyle choices, particularly high intake of ultraprocessed foods, can influence our cognitive health many years later,” coinvestigator Natalia Goncalves, PhD, University of São Paulo, Brazil, said in an interview.
The study was published online in JAMA Neurology.
The study’s findings were presented in August at the Alzheimer’s Association International Conference (AAIC) 2022 and were reported by this news organization at that time.
High sugar, salt, fat
The new results align with another recent study linking a diet high in UPFs to an increased risk for dementia.
UPFs are highly manipulated, are packed with added ingredients, including sugar, fat, and salt, and are low in protein and fiber. Examples of UPFs are soft drinks, chips, chocolate, candy, ice cream, sweetened breakfast cereals, packaged soups, chicken nuggets, hot dogs, and fries.
The ELSA-Brasil study comprised 10,775 adults (mean age, 50.6 years at baseline; 55% women; 53% White) who were evaluated in three waves approximately 4 years apart from 2008 to 2017.
Information on diet was obtained via food frequency questionnaires and included details regarding consumption of unprocessed foods, minimally processed foods, and UPFs.
Participants were grouped according to UPF consumption quartiles (lowest to highest). Cognitive performance was evaluated by use of a standardized battery of tests.
During median follow-up of 8 years, people who consumed more than 20% of daily calories from UPFs (quartiles 2-4) experienced a 28% faster rate of decline in global cognition (beta = –0.004; 95% confidence interval [CI], –0.006 to –0.001; P = .003) and a 25% faster rate of decline in executive function (beta = –0.003, 95% CI, –0.005 to 0.000; P = .01) compared to peers in quartile 1 who consumed less than 20% of daily calories from UPFs.
The researchers did not investigate individual groups of UPFs.
However, Dr. Goncalves noted that some studies have linked the consumption of sugar-sweetened beverages with lower cognitive performance, lower brain volume, and poorer memory performance. Another group of ultraprocessed foods, processed meats, has been associated with increased all-cause dementia and Alzheimer’s disease.
Other limitations include the fact that self-reported diet habits were assessed only at baseline using a food frequency questionnaire that was not designed to assess the degree of processing.
While analyses were adjusted for several sociodemographic and clinical confounders, the researchers said they could not exclude the possibility of residual confounding.
Also, since neuroimaging is not available in the ELSA-Brasil study, they were not able to investigate potential mechanisms that could explain the association between higher UPF consumption and cognitive decline.
Despite these limitations, the researchers said their findings suggest that “limiting UPF consumption, particularly in middle-aged adults, may be an efficient form to prevent cognitive decline.”
Weighing the evidence
Several experts weighed in on the results in a statement from the UK nonprofit organization, Science Media Centre.
Kevin McConway, PhD, with Open University, Milton Keynes, England, said it’s important to note that the study suggests “an association, a correlation, and that doesn’t necessarily mean that the cognitive decline was caused by eating more ultra-processed foods.”
He also noted that some types of cognitive decline that are associated with aging occurred in participants in all four quartiles, which were defined by the percentage of their daily energy that came from consuming UPFs.
“That’s hardly surprising – it’s a sad fact of life that pretty well all of us gradually lose some of our cognitive functions as we go through middle and older age,” Dr. McConway said.
“The study doesn’t establish that differences in speed of cognitive decline are caused by ultra-processed food consumption anyway. That’s because it’s an observational study. If the consumption of ultra-processed food causes the differences in rate of cognitive decline, then eating less of it might slow cognitive decline, but if the cause is something else, then that won’t happen,” Dr. McConway added.
Gunter Kuhnle, PhD, professor of nutrition and food science, University of Reading, England, noted that UPFs have become a “fashionable term to explain associations between diet and ill health, and many studies have attempted to show associations.
“Most studies have been observational and had a key limitation: It is very difficult to determine ultra-processed food intake using methods that are not designed to do so, and so authors need to make a lot of assumptions. Bread and meat products are often classed as ‘ultra-processed,’ even though this is often wrong,” Dr. Kuhnle noted.
“The same applies to this study – the method used to measure ultra-processed food intake was not designed for that task and relied on assumptions. This makes it virtually impossible to draw any conclusions,” Dr. Kuhnle said.
Duane Mellor, PhD, RD, RNutr, registered dietitian and senior teaching fellow, Aston University, Birmingham, England, said the study does not change how we should try to eat to maintain good brain function and cognition.
“We should try to eat less foods which are high in added sugar, salt, and fat, which would include many of the foods classified as being ultra-processed, while eating more in terms of both quantity and variety of vegetables, fruit, nuts, seeds, and pulses, which are known to be beneficial for both our cognitive and overall health,” Dr. Mellor said.
The ELSA-Brasil study was supported by the Brazilian Ministry of Health, the Ministry of Science, Technology and Innovation, and the National Council for Scientific and Technological Development. The authors as well as Dr. McConway, Dr. Mellor, and Dr. Kuhnle have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil), which included more than 10,000 people aged 35 and older, showed that higher intake of UPF was significantly associated with a faster rate of decline in executive and global cognitive function.
“These findings show that lifestyle choices, particularly high intake of ultraprocessed foods, can influence our cognitive health many years later,” coinvestigator Natalia Goncalves, PhD, University of São Paulo, Brazil, said in an interview.
The study was published online in JAMA Neurology.
The study’s findings were presented in August at the Alzheimer’s Association International Conference (AAIC) 2022 and were reported by this news organization at that time.
High sugar, salt, fat
The new results align with another recent study linking a diet high in UPFs to an increased risk for dementia.
UPFs are highly manipulated, are packed with added ingredients, including sugar, fat, and salt, and are low in protein and fiber. Examples of UPFs are soft drinks, chips, chocolate, candy, ice cream, sweetened breakfast cereals, packaged soups, chicken nuggets, hot dogs, and fries.
The ELSA-Brasil study comprised 10,775 adults (mean age, 50.6 years at baseline; 55% women; 53% White) who were evaluated in three waves approximately 4 years apart from 2008 to 2017.
Information on diet was obtained via food frequency questionnaires and included details regarding consumption of unprocessed foods, minimally processed foods, and UPFs.
Participants were grouped according to UPF consumption quartiles (lowest to highest). Cognitive performance was evaluated by use of a standardized battery of tests.
During median follow-up of 8 years, people who consumed more than 20% of daily calories from UPFs (quartiles 2-4) experienced a 28% faster rate of decline in global cognition (beta = –0.004; 95% confidence interval [CI], –0.006 to –0.001; P = .003) and a 25% faster rate of decline in executive function (beta = –0.003, 95% CI, –0.005 to 0.000; P = .01) compared to peers in quartile 1 who consumed less than 20% of daily calories from UPFs.
The researchers did not investigate individual groups of UPFs.
However, Dr. Goncalves noted that some studies have linked the consumption of sugar-sweetened beverages with lower cognitive performance, lower brain volume, and poorer memory performance. Another group of ultraprocessed foods, processed meats, has been associated with increased all-cause dementia and Alzheimer’s disease.
Other limitations include the fact that self-reported diet habits were assessed only at baseline using a food frequency questionnaire that was not designed to assess the degree of processing.
While analyses were adjusted for several sociodemographic and clinical confounders, the researchers said they could not exclude the possibility of residual confounding.
Also, since neuroimaging is not available in the ELSA-Brasil study, they were not able to investigate potential mechanisms that could explain the association between higher UPF consumption and cognitive decline.
Despite these limitations, the researchers said their findings suggest that “limiting UPF consumption, particularly in middle-aged adults, may be an efficient form to prevent cognitive decline.”
Weighing the evidence
Several experts weighed in on the results in a statement from the UK nonprofit organization, Science Media Centre.
Kevin McConway, PhD, with Open University, Milton Keynes, England, said it’s important to note that the study suggests “an association, a correlation, and that doesn’t necessarily mean that the cognitive decline was caused by eating more ultra-processed foods.”
He also noted that some types of cognitive decline that are associated with aging occurred in participants in all four quartiles, which were defined by the percentage of their daily energy that came from consuming UPFs.
“That’s hardly surprising – it’s a sad fact of life that pretty well all of us gradually lose some of our cognitive functions as we go through middle and older age,” Dr. McConway said.
“The study doesn’t establish that differences in speed of cognitive decline are caused by ultra-processed food consumption anyway. That’s because it’s an observational study. If the consumption of ultra-processed food causes the differences in rate of cognitive decline, then eating less of it might slow cognitive decline, but if the cause is something else, then that won’t happen,” Dr. McConway added.
Gunter Kuhnle, PhD, professor of nutrition and food science, University of Reading, England, noted that UPFs have become a “fashionable term to explain associations between diet and ill health, and many studies have attempted to show associations.
“Most studies have been observational and had a key limitation: It is very difficult to determine ultra-processed food intake using methods that are not designed to do so, and so authors need to make a lot of assumptions. Bread and meat products are often classed as ‘ultra-processed,’ even though this is often wrong,” Dr. Kuhnle noted.
“The same applies to this study – the method used to measure ultra-processed food intake was not designed for that task and relied on assumptions. This makes it virtually impossible to draw any conclusions,” Dr. Kuhnle said.
Duane Mellor, PhD, RD, RNutr, registered dietitian and senior teaching fellow, Aston University, Birmingham, England, said the study does not change how we should try to eat to maintain good brain function and cognition.
“We should try to eat less foods which are high in added sugar, salt, and fat, which would include many of the foods classified as being ultra-processed, while eating more in terms of both quantity and variety of vegetables, fruit, nuts, seeds, and pulses, which are known to be beneficial for both our cognitive and overall health,” Dr. Mellor said.
The ELSA-Brasil study was supported by the Brazilian Ministry of Health, the Ministry of Science, Technology and Innovation, and the National Council for Scientific and Technological Development. The authors as well as Dr. McConway, Dr. Mellor, and Dr. Kuhnle have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
High drug costs exclude most neurology patients from cutting-edge treatment
, new research shows.
“Our study of people with neurologic conditions found that fewer than 20% were being treated with new medications,” study author Brian C. Callaghan, MD, with University of Michigan Health in Ann Arbor, said in a statement.
“For new, high-cost medications that have similar effectiveness to older drugs, limited use is likely appropriate. However, future studies are needed to look into whether the high costs are barriers to those new medications that can really make a difference for people living with neurologic disease,” Dr. Callaghan said.
The study was published online in Neurology.
Most expensive drugs
Using insurance claims data, the investigators compared the utilization and costs of new-to-market drugs from 2014 to 2018 with those for existing guideline-supported medications for treating 11 neurologic conditions.
The new drugs included:
- erenumab, fremanezumab, and galcanezumab for migraine.
- ocrelizumab and peginterferon beta-1a for multiple sclerosis (MS).
- pimavanserin and safinamide for Parkinson’s disease.
- droxidopa for orthostatic hypertension.
- eculizumab for myasthenia gravis (MG).
- edaravone for amyotrophic lateral sclerosis (ALS).
- deutetrabenazine and valbenazine for Huntington’s disease and tardive dyskinesia.
- patisiran and inotersen for transthyretin amyloidosis (ATTR).
- eteplirsen and deflazacort for Duchenne disease.
- nusinersen for spinal muscular atrophy (SMA).
Utilization of new drugs was modest – they accounted for one in five prescriptions for every condition except tardive dyskinesia (32% for valbenazine), the researchers noted.
Mean out-of-pocket costs were significantly higher for the new medications, although there was large variability among individual drugs.
The two most expensive drugs were edaravone, for ALS, with a mean out-of-pocket cost of $713 for a 30-day supply, and eculizumab, for MG, which costs $91 per month.
“For new-to-market medications, the distribution of out-of-pocket costs were highly variable and the trends over time were unpredictable compared with existing guideline-supported medications,” the authors reported.
They noted that potential reasons for low utilization of newer agents include delay in provider uptake and prescriber and/or patient avoidance because of high cost.
Given that most of the new neurologic agents offer little advantage compared with existing treatments – exceptions being new drugs for SMA and ATTR – drug costs should be a key consideration in prescribing decisions, Dr. Callaghan and colleagues concluded.
One limitation of the study is that follow-up time was short for some of the recently approved medications. Another limitation is that the number of people in the study who had rare diseases was small.
Revolution in neurotherapeutics
“We are living in a time when new treatments bring hope to people with neurologic diseases and disorders,” Orly Avitzur, MD, president of the American Academy of Neurology, said in a statement.
“However, even existing prescription medication can be expensive and drug prices continue to rise. In order for neurologists to provide people with the highest quality care, it is imperative that new drugs are accessible and affordable to the people who need them,” Dr. Avitzur added.
Writing in a linked editorial, A. Gordon Smith, MD, professor and chair, department of neurology, Virginia Commonwealth University, Richmond, said there is a revolution in neurotherapeutics, with particularly robust growth in new drug approvals for orphan diseases (those affecting < 200,000 Americans).
“This study adds to a growing literature indicating rising drug prices are a threat to the health care system. No matter how effective a disease-modifying therapy may be, if a patient cannot afford the cost, it doesn’t work,” Dr. Smith wrote.
He added that neurologists must be “diligent in assessing for financial toxicity and appropriately tailor individual treatment recommendations. We must insist on development of point-of-care tools to accurately estimate each patient’s potential financial toxicity including RTBT [real-time benefit tools].
“Neurologists’ primary obligation is to the individual patient, but we are also compelled to support access to high-quality care for all people, which requires advocacy for appropriate policy reforms to ensure value based and fair drug pricing and treatment success,” Dr. Smith added.
The study was funded by the American Academy of Neurology Health Services Research Subcommittee. Dr. Callaghan consults for a PCORI grant, DynaMed, receives research support from the American Academy of Neurology, and performs medical/legal consultations, including consultations for the Vaccine Injury Compensation Program. Dr. Smith has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows.
“Our study of people with neurologic conditions found that fewer than 20% were being treated with new medications,” study author Brian C. Callaghan, MD, with University of Michigan Health in Ann Arbor, said in a statement.
“For new, high-cost medications that have similar effectiveness to older drugs, limited use is likely appropriate. However, future studies are needed to look into whether the high costs are barriers to those new medications that can really make a difference for people living with neurologic disease,” Dr. Callaghan said.
The study was published online in Neurology.
Most expensive drugs
Using insurance claims data, the investigators compared the utilization and costs of new-to-market drugs from 2014 to 2018 with those for existing guideline-supported medications for treating 11 neurologic conditions.
The new drugs included:
- erenumab, fremanezumab, and galcanezumab for migraine.
- ocrelizumab and peginterferon beta-1a for multiple sclerosis (MS).
- pimavanserin and safinamide for Parkinson’s disease.
- droxidopa for orthostatic hypertension.
- eculizumab for myasthenia gravis (MG).
- edaravone for amyotrophic lateral sclerosis (ALS).
- deutetrabenazine and valbenazine for Huntington’s disease and tardive dyskinesia.
- patisiran and inotersen for transthyretin amyloidosis (ATTR).
- eteplirsen and deflazacort for Duchenne disease.
- nusinersen for spinal muscular atrophy (SMA).
Utilization of new drugs was modest – they accounted for one in five prescriptions for every condition except tardive dyskinesia (32% for valbenazine), the researchers noted.
Mean out-of-pocket costs were significantly higher for the new medications, although there was large variability among individual drugs.
The two most expensive drugs were edaravone, for ALS, with a mean out-of-pocket cost of $713 for a 30-day supply, and eculizumab, for MG, which costs $91 per month.
“For new-to-market medications, the distribution of out-of-pocket costs were highly variable and the trends over time were unpredictable compared with existing guideline-supported medications,” the authors reported.
They noted that potential reasons for low utilization of newer agents include delay in provider uptake and prescriber and/or patient avoidance because of high cost.
Given that most of the new neurologic agents offer little advantage compared with existing treatments – exceptions being new drugs for SMA and ATTR – drug costs should be a key consideration in prescribing decisions, Dr. Callaghan and colleagues concluded.
One limitation of the study is that follow-up time was short for some of the recently approved medications. Another limitation is that the number of people in the study who had rare diseases was small.
Revolution in neurotherapeutics
“We are living in a time when new treatments bring hope to people with neurologic diseases and disorders,” Orly Avitzur, MD, president of the American Academy of Neurology, said in a statement.
“However, even existing prescription medication can be expensive and drug prices continue to rise. In order for neurologists to provide people with the highest quality care, it is imperative that new drugs are accessible and affordable to the people who need them,” Dr. Avitzur added.
Writing in a linked editorial, A. Gordon Smith, MD, professor and chair, department of neurology, Virginia Commonwealth University, Richmond, said there is a revolution in neurotherapeutics, with particularly robust growth in new drug approvals for orphan diseases (those affecting < 200,000 Americans).
“This study adds to a growing literature indicating rising drug prices are a threat to the health care system. No matter how effective a disease-modifying therapy may be, if a patient cannot afford the cost, it doesn’t work,” Dr. Smith wrote.
He added that neurologists must be “diligent in assessing for financial toxicity and appropriately tailor individual treatment recommendations. We must insist on development of point-of-care tools to accurately estimate each patient’s potential financial toxicity including RTBT [real-time benefit tools].
“Neurologists’ primary obligation is to the individual patient, but we are also compelled to support access to high-quality care for all people, which requires advocacy for appropriate policy reforms to ensure value based and fair drug pricing and treatment success,” Dr. Smith added.
The study was funded by the American Academy of Neurology Health Services Research Subcommittee. Dr. Callaghan consults for a PCORI grant, DynaMed, receives research support from the American Academy of Neurology, and performs medical/legal consultations, including consultations for the Vaccine Injury Compensation Program. Dr. Smith has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows.
“Our study of people with neurologic conditions found that fewer than 20% were being treated with new medications,” study author Brian C. Callaghan, MD, with University of Michigan Health in Ann Arbor, said in a statement.
“For new, high-cost medications that have similar effectiveness to older drugs, limited use is likely appropriate. However, future studies are needed to look into whether the high costs are barriers to those new medications that can really make a difference for people living with neurologic disease,” Dr. Callaghan said.
The study was published online in Neurology.
Most expensive drugs
Using insurance claims data, the investigators compared the utilization and costs of new-to-market drugs from 2014 to 2018 with those for existing guideline-supported medications for treating 11 neurologic conditions.
The new drugs included:
- erenumab, fremanezumab, and galcanezumab for migraine.
- ocrelizumab and peginterferon beta-1a for multiple sclerosis (MS).
- pimavanserin and safinamide for Parkinson’s disease.
- droxidopa for orthostatic hypertension.
- eculizumab for myasthenia gravis (MG).
- edaravone for amyotrophic lateral sclerosis (ALS).
- deutetrabenazine and valbenazine for Huntington’s disease and tardive dyskinesia.
- patisiran and inotersen for transthyretin amyloidosis (ATTR).
- eteplirsen and deflazacort for Duchenne disease.
- nusinersen for spinal muscular atrophy (SMA).
Utilization of new drugs was modest – they accounted for one in five prescriptions for every condition except tardive dyskinesia (32% for valbenazine), the researchers noted.
Mean out-of-pocket costs were significantly higher for the new medications, although there was large variability among individual drugs.
The two most expensive drugs were edaravone, for ALS, with a mean out-of-pocket cost of $713 for a 30-day supply, and eculizumab, for MG, which costs $91 per month.
“For new-to-market medications, the distribution of out-of-pocket costs were highly variable and the trends over time were unpredictable compared with existing guideline-supported medications,” the authors reported.
They noted that potential reasons for low utilization of newer agents include delay in provider uptake and prescriber and/or patient avoidance because of high cost.
Given that most of the new neurologic agents offer little advantage compared with existing treatments – exceptions being new drugs for SMA and ATTR – drug costs should be a key consideration in prescribing decisions, Dr. Callaghan and colleagues concluded.
One limitation of the study is that follow-up time was short for some of the recently approved medications. Another limitation is that the number of people in the study who had rare diseases was small.
Revolution in neurotherapeutics
“We are living in a time when new treatments bring hope to people with neurologic diseases and disorders,” Orly Avitzur, MD, president of the American Academy of Neurology, said in a statement.
“However, even existing prescription medication can be expensive and drug prices continue to rise. In order for neurologists to provide people with the highest quality care, it is imperative that new drugs are accessible and affordable to the people who need them,” Dr. Avitzur added.
Writing in a linked editorial, A. Gordon Smith, MD, professor and chair, department of neurology, Virginia Commonwealth University, Richmond, said there is a revolution in neurotherapeutics, with particularly robust growth in new drug approvals for orphan diseases (those affecting < 200,000 Americans).
“This study adds to a growing literature indicating rising drug prices are a threat to the health care system. No matter how effective a disease-modifying therapy may be, if a patient cannot afford the cost, it doesn’t work,” Dr. Smith wrote.
He added that neurologists must be “diligent in assessing for financial toxicity and appropriately tailor individual treatment recommendations. We must insist on development of point-of-care tools to accurately estimate each patient’s potential financial toxicity including RTBT [real-time benefit tools].
“Neurologists’ primary obligation is to the individual patient, but we are also compelled to support access to high-quality care for all people, which requires advocacy for appropriate policy reforms to ensure value based and fair drug pricing and treatment success,” Dr. Smith added.
The study was funded by the American Academy of Neurology Health Services Research Subcommittee. Dr. Callaghan consults for a PCORI grant, DynaMed, receives research support from the American Academy of Neurology, and performs medical/legal consultations, including consultations for the Vaccine Injury Compensation Program. Dr. Smith has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Confirmed: Amyloid, tau levels rise years before Alzheimer’s onset
“Our results confirm accelerated biomarker changes during preclinical AD and highlight the important role of amyloid levels in tau accelerations,” the investigators note.
“These data may suggest that there is a short therapeutic window for slowing AD pathogenesis prior to the emergence of clinical symptoms – and that this window may occur after amyloid accumulation begins but before amyloid has substantial impacts on tau accumulation,” study investigator Corinne Pettigrew, PhD, department of neurology, Johns Hopkins University School of Medicine, Baltimore, told this news organization.
The study was published online in Alzheimer’s and Dementia.
Novel long-term CSF data
The study builds on previous research by examining changes in cerebrospinal fluid (CSF) biomarkers over longer periods than had been done previously, particularly among largely middle-aged and cognitively normal at baseline individuals.
The researchers examined changes in amyloid beta (Aβ) 42/Aβ40, phosphorylated tau181 (p-tau181), and total tau (t-tau) in CSF over an average of 10.7 years (and up to 23 years) among 278 individuals who were largely middle-aged persons who were cognitively normal at baseline.
“To our knowledge, no prior study among initially cognitively normal, primarily middle-aged individuals has described CSF AD biomarker changes over this duration of follow-up,” the researchers write.
During follow-up, 94 individuals who initially had normal cognition developed mild cognitive impairment (MCI).
Lower baseline levels of amyloid were associated with greater increases in tau (more strongly in men than women), while accelerations in tau were more closely linked to onset of MCI, the researchers report.
Among individuals who developed MCI, biomarker levels were more abnormal and tau increased to a greater extent prior to the onset of MCI symptoms, they found.
Clear impact of APOE4
The findings also suggest that among APOE4 carriers, amyloid onset occurs at an earlier age and rates of amyloid positivity are higher, but there are no differences in rates of change in amyloid over time.
“APOE4 genetic status was not related to changes in CSF beta-amyloid after accounting for the fact that APOE4 carriers have higher rates of amyloid positivity,” said Dr. Pettigrew.
“These findings suggest that APOE4 genetic status shifts the age of onset of amyloid accumulation (with APOE4 carriers having an earlier age of onset compared to non-carriers), but that APOE4 is not related to rates of change in CSF beta-amyloid over time,” she added.
“Thus, cognitively normal APOE4 carriers may be in more advanced preclinical AD stages at younger ages than individuals who are not APOE4 carriers, which is likely relevant for optimizing clinical trial recruitment strategies,” she said.
Funding for the study was provided by the National Institutes of Health. Dr. Pettigrew has disclosed no relevant financial relationships. The original article contains a complete list of author disclosures.
A version of this article first appeared on Medscape.com.
“Our results confirm accelerated biomarker changes during preclinical AD and highlight the important role of amyloid levels in tau accelerations,” the investigators note.
“These data may suggest that there is a short therapeutic window for slowing AD pathogenesis prior to the emergence of clinical symptoms – and that this window may occur after amyloid accumulation begins but before amyloid has substantial impacts on tau accumulation,” study investigator Corinne Pettigrew, PhD, department of neurology, Johns Hopkins University School of Medicine, Baltimore, told this news organization.
The study was published online in Alzheimer’s and Dementia.
Novel long-term CSF data
The study builds on previous research by examining changes in cerebrospinal fluid (CSF) biomarkers over longer periods than had been done previously, particularly among largely middle-aged and cognitively normal at baseline individuals.
The researchers examined changes in amyloid beta (Aβ) 42/Aβ40, phosphorylated tau181 (p-tau181), and total tau (t-tau) in CSF over an average of 10.7 years (and up to 23 years) among 278 individuals who were largely middle-aged persons who were cognitively normal at baseline.
“To our knowledge, no prior study among initially cognitively normal, primarily middle-aged individuals has described CSF AD biomarker changes over this duration of follow-up,” the researchers write.
During follow-up, 94 individuals who initially had normal cognition developed mild cognitive impairment (MCI).
Lower baseline levels of amyloid were associated with greater increases in tau (more strongly in men than women), while accelerations in tau were more closely linked to onset of MCI, the researchers report.
Among individuals who developed MCI, biomarker levels were more abnormal and tau increased to a greater extent prior to the onset of MCI symptoms, they found.
Clear impact of APOE4
The findings also suggest that among APOE4 carriers, amyloid onset occurs at an earlier age and rates of amyloid positivity are higher, but there are no differences in rates of change in amyloid over time.
“APOE4 genetic status was not related to changes in CSF beta-amyloid after accounting for the fact that APOE4 carriers have higher rates of amyloid positivity,” said Dr. Pettigrew.
“These findings suggest that APOE4 genetic status shifts the age of onset of amyloid accumulation (with APOE4 carriers having an earlier age of onset compared to non-carriers), but that APOE4 is not related to rates of change in CSF beta-amyloid over time,” she added.
“Thus, cognitively normal APOE4 carriers may be in more advanced preclinical AD stages at younger ages than individuals who are not APOE4 carriers, which is likely relevant for optimizing clinical trial recruitment strategies,” she said.
Funding for the study was provided by the National Institutes of Health. Dr. Pettigrew has disclosed no relevant financial relationships. The original article contains a complete list of author disclosures.
A version of this article first appeared on Medscape.com.
“Our results confirm accelerated biomarker changes during preclinical AD and highlight the important role of amyloid levels in tau accelerations,” the investigators note.
“These data may suggest that there is a short therapeutic window for slowing AD pathogenesis prior to the emergence of clinical symptoms – and that this window may occur after amyloid accumulation begins but before amyloid has substantial impacts on tau accumulation,” study investigator Corinne Pettigrew, PhD, department of neurology, Johns Hopkins University School of Medicine, Baltimore, told this news organization.
The study was published online in Alzheimer’s and Dementia.
Novel long-term CSF data
The study builds on previous research by examining changes in cerebrospinal fluid (CSF) biomarkers over longer periods than had been done previously, particularly among largely middle-aged and cognitively normal at baseline individuals.
The researchers examined changes in amyloid beta (Aβ) 42/Aβ40, phosphorylated tau181 (p-tau181), and total tau (t-tau) in CSF over an average of 10.7 years (and up to 23 years) among 278 individuals who were largely middle-aged persons who were cognitively normal at baseline.
“To our knowledge, no prior study among initially cognitively normal, primarily middle-aged individuals has described CSF AD biomarker changes over this duration of follow-up,” the researchers write.
During follow-up, 94 individuals who initially had normal cognition developed mild cognitive impairment (MCI).
Lower baseline levels of amyloid were associated with greater increases in tau (more strongly in men than women), while accelerations in tau were more closely linked to onset of MCI, the researchers report.
Among individuals who developed MCI, biomarker levels were more abnormal and tau increased to a greater extent prior to the onset of MCI symptoms, they found.
Clear impact of APOE4
The findings also suggest that among APOE4 carriers, amyloid onset occurs at an earlier age and rates of amyloid positivity are higher, but there are no differences in rates of change in amyloid over time.
“APOE4 genetic status was not related to changes in CSF beta-amyloid after accounting for the fact that APOE4 carriers have higher rates of amyloid positivity,” said Dr. Pettigrew.
“These findings suggest that APOE4 genetic status shifts the age of onset of amyloid accumulation (with APOE4 carriers having an earlier age of onset compared to non-carriers), but that APOE4 is not related to rates of change in CSF beta-amyloid over time,” she added.
“Thus, cognitively normal APOE4 carriers may be in more advanced preclinical AD stages at younger ages than individuals who are not APOE4 carriers, which is likely relevant for optimizing clinical trial recruitment strategies,” she said.
Funding for the study was provided by the National Institutes of Health. Dr. Pettigrew has disclosed no relevant financial relationships. The original article contains a complete list of author disclosures.
A version of this article first appeared on Medscape.com.
FROM ALZHEIMER’S AND DEMENTIA

