User login
Upadacitinib shows favorable long-term benefit-risk profile in moderate-to-severe atopic dermatitis
Key clinical point: Upadacitinib showed sustained efficacy through 52 weeks in adults and adolescents with moderate-to-severe atopic dermatitis (AD) along with an acceptable safety profile.
Major finding: At week 52, a 75% improvement in Eczema Area and Severity Index (EASI75) was achieved by 82.0% and 79.1% of patients continuing 15 mg upadacitinib and 84.9% and 84.3% of patients continuing 30 mg upadacitinib in Measure Up 1 and Measure Up 2, respectively. More than 80% of patients who switched from placebo to upadacitinib at week 16 achieved EASI75 at week 52. No new adverse events were reported.
Study details: Findings are from a 52-week analysis of two ongoing phase 3 trials, Measure Up 1 and Measure Up 2, including 1,609 adults and adolescents with moderate-to-severe AD who were randomly assigned to receive 15 mg upadacitinib once daily, 30 mg upadacitinib, or placebo.
Disclosures: This study was funded by AbbVie. Three authors reported ties with various sources, including AbbVie, with some receiving payments or personal fees and being employees or stockholders of AbbVie.
Source: Simpson EL et al. Efficacy and safety of upadacitinib in patients with moderate to severe atopic dermatitis: Analysis of follow-up data from the Measure Up 1 and Measure Up 2 randomized clinical trials. JAMA Dermatol. 2022 (Mar 9). Doi: 10.1001/jamadermatol.2022.0029
Key clinical point: Upadacitinib showed sustained efficacy through 52 weeks in adults and adolescents with moderate-to-severe atopic dermatitis (AD) along with an acceptable safety profile.
Major finding: At week 52, a 75% improvement in Eczema Area and Severity Index (EASI75) was achieved by 82.0% and 79.1% of patients continuing 15 mg upadacitinib and 84.9% and 84.3% of patients continuing 30 mg upadacitinib in Measure Up 1 and Measure Up 2, respectively. More than 80% of patients who switched from placebo to upadacitinib at week 16 achieved EASI75 at week 52. No new adverse events were reported.
Study details: Findings are from a 52-week analysis of two ongoing phase 3 trials, Measure Up 1 and Measure Up 2, including 1,609 adults and adolescents with moderate-to-severe AD who were randomly assigned to receive 15 mg upadacitinib once daily, 30 mg upadacitinib, or placebo.
Disclosures: This study was funded by AbbVie. Three authors reported ties with various sources, including AbbVie, with some receiving payments or personal fees and being employees or stockholders of AbbVie.
Source: Simpson EL et al. Efficacy and safety of upadacitinib in patients with moderate to severe atopic dermatitis: Analysis of follow-up data from the Measure Up 1 and Measure Up 2 randomized clinical trials. JAMA Dermatol. 2022 (Mar 9). Doi: 10.1001/jamadermatol.2022.0029
Key clinical point: Upadacitinib showed sustained efficacy through 52 weeks in adults and adolescents with moderate-to-severe atopic dermatitis (AD) along with an acceptable safety profile.
Major finding: At week 52, a 75% improvement in Eczema Area and Severity Index (EASI75) was achieved by 82.0% and 79.1% of patients continuing 15 mg upadacitinib and 84.9% and 84.3% of patients continuing 30 mg upadacitinib in Measure Up 1 and Measure Up 2, respectively. More than 80% of patients who switched from placebo to upadacitinib at week 16 achieved EASI75 at week 52. No new adverse events were reported.
Study details: Findings are from a 52-week analysis of two ongoing phase 3 trials, Measure Up 1 and Measure Up 2, including 1,609 adults and adolescents with moderate-to-severe AD who were randomly assigned to receive 15 mg upadacitinib once daily, 30 mg upadacitinib, or placebo.
Disclosures: This study was funded by AbbVie. Three authors reported ties with various sources, including AbbVie, with some receiving payments or personal fees and being employees or stockholders of AbbVie.
Source: Simpson EL et al. Efficacy and safety of upadacitinib in patients with moderate to severe atopic dermatitis: Analysis of follow-up data from the Measure Up 1 and Measure Up 2 randomized clinical trials. JAMA Dermatol. 2022 (Mar 9). Doi: 10.1001/jamadermatol.2022.0029
The Impact of Prenatal Nutrition on the Development of Atopic Dermatitis in Infancy and Childhood
Atopic dermatitis (AD) is an inflammatory skin disease characterized by skin barrier disruption, skin inflammation, and pruritus.1 It is a common and often chronic skin condition associated with the development of food allergies, asthma, and allergic rhinitis, known as the atopic march.2 Atopic dermatitis is estimated to affect 10% to 25% of children, most with onset before 5 years of age, and up to 7% of adults worldwide.3 Most patients improve with time, but multiple disease trajectories are possible. Several studies have demonstrated that fewer than 4% of children develop the classic atopic march—AD followed by food allergies, asthma, and finally allergic rhinitis—with recent evidence pointing to a more complex heterogeneous progression of disease and allergic comorbidities often occurring together.4,5 The prevalence of AD has been increasing globally over the last 30 years,6 with a marked increase in developed countries.6,7 It is well accepted that AD is based on an interplay between genetic predisposition and environmental factors,8 but many suspect that the rapid rise in prevalence cannot be attributed to genetic factors alone.9 The precipitant triggers for AD remain an area of intense investigation, with ongoing debate between the “inside out” and “outside in” hypotheses; these revolve around whether abnormalities in the immune system trigger barrier dysfunction or barrier dysfunction triggers immune programming to atopy.8 Ongoing research related to genetic predisposition of AD has identified candidate genes implicated in both impaired skin barrier function and altered immune system pathways, further supporting that both theories may contribute to disease pathogenesis.
The increasing prevalence of AD, with increasing disease burden within socioeconomically advantaged countries, raises the possibility of early modifiable environmental factors that may contribute to the disease process.10 Many studies point to the influence of the 21st century lifestyle and Western diet as primary contributing factors.9,11 However, it is not clear how these factors may influence the development of allergic atopic disease. Several studies have suggested that nonheritable influences in utero can alter fetus immune function and influence the subsequent development of allergic disease.12,13 Although many studies have examined environmental factors contributing to the development of AD in infancy and childhood, less is understood about the influence of prenatal factors. Currently, in utero exposure to tobacco smoke, phthalates, and maternal distress have been potentially implicated in the development of AD.14,15 Several studies have examined the role of maternal diet and nutrition on the development of AD in offspring; however, formal recommendations and robust trial data are lacking. In this article, we examine the existing literature surrounding maternal diet on the development of AD in infancy and childhood.
Allergen Avoidance
Extrapolating from the food allergy literature, it was once suggested that allergen avoidance in early childhood had a protective effect on the subsequent development of allergies; however, more recent research has found that early exposure to common food allergens, such as peanuts or eggs, may actually reduce a child’s risk for developing these allergies later in life.16 Among infants at high risk for food allergy, sustained consumption of peanut products beginning in the first 11 months of life resulted in an 81% lower rate of peanut allergy at 60 months of age than the rate among children who avoided peanuts.17 Given the results that antigen avoidance during infancy/childhood does not protect against the development of allergies and may actually be counterproductive, it is not surprising that research studying antigen avoidance during pregnancy on the development of AD also has demonstrated limited efficacy. A systematic review of 5 trials on maternal dietary antigen avoidance (N=952) suggested no protective effects of avoiding antigenic foods during pregnancy on the development of AD in the first 18 months of life.18 Another meta-analysis evaluating 12 intervention trials looked at the effects of maternal allergenic food avoidance during pregnancy or lactation and found no reduced risk for subsequent development of allergic disease, including AD.19 The American Academy of Pediatrics 2019 consensus statement does not support maternal dietary restrictions in pregnancy for the prevention of atopic disease and makes note that the data remain limited, which complicates drawing any firm conclusions.20
Probiotic Supplementation
One of the most investigated dietary supplements for the prevention of atopic disease is probiotics, with possible benefits noted in both the prenatal and postnatal periods. Baquerizo Nole et al21 examined several studies looking at the various benefits of probiotics in AD, which included inhibition of the helper T cell (TH2) response, stimulation of the TH1 response, upregulation of regulatory T cells, acceleration of skin and mucosal barrier function, increase in intestinal microflora diversity, suppression of toxic fermentation products in the intestinal lumen from increased production of short-chain fatty acids, and inhibition of Staphylococcus aureus attachment on epidermal keratinocytes. It is unclear how this may affect infants prenatally; however, transfer of maternal intestinal microflora during delivery and shortly thereafter has demonstrated that probiotic strains remain detectable in the infant’s stool up to 6 months after delivery, even if the mother has discontinued use.22 A 2008 meta-analysis of 10 double-bind, randomized, controlled trials (N=1880) looking at the use of maternal prenatal and postnatal probiotic supplementation in the prevention of pediatric AD found a relative risk (RR) ratio of 0.69 (95% CI, 0.57-0.83) using a fixed effects model and RR ratio of 0.66 (95% CI, 0.49-0.89) using a random effects model. After exclusion of one study that evaluated the effect of postnatal probiotic supplementation only, the RR ratio decreased to 0.61 for both the fixed effects and random effects models.23 A systematic review by Panduru et al24 noted similar findings with a subgroup meta-analysis of 11 studies of prenatal supplementation followed by postnatal supplementation of probiotics, which demonstrated a protective effect on the development of AD (odds ratio [OR]=0.61, P<.001). Postnatal supplementation alone (4 studies) did not have the same association (OR=0.95, P<.82).24 A 2012 meta-analysis by Doege et al25 evaluated 7 randomized, double-blinded, placebo-controlled trials that assessed probiotic supplementation during pregnancy (without incorporation of postnatal supplementation) and found a significant risk reduction of 5.7% (P=.022) for AD in children aged 2 to 7 years. Interestingly, this was only significant for Lactobacillus and not for other bacterial strains, even if a mixture of strains included Lactobacillus. However, Panduru et al24 found both maternal Lactobacillus supplementation alone (8 studies) and in combination with Bifidobacterium (9 studies) was protective against AD development in children (OR=0.70, P=.004; OR=0.62, P<.001). A more recent 2015 meta-analysis of 17 studies (N=4755) evaluating the use of maternal probiotic supplementation in pregnancy and/or through the infant’s first 3 months of life found a significantly lower RR (0.78 [95% CI, 0.69-0.89], P=.0003) for the development of AD in infants treated with probiotics and found this risk to be even further decreased when a mixture of probiotics including both Lactobacillus and Bifidobacterium was used (RR=0.54 [95% CI, 0.43-0.68], P<.00001).26
Antioxidants
The Westernization of many developing countries’ diets—diets high in saturated fats, protein, sucrose, salt, and processed foods and low in fresh fruits and green vegetables—has led to a reduced intake of antioxidants and an increase in susceptibility to oxidative damage.27,28 One hypothesis suggests that a reduction in nutritional antioxidants and subsequent oxidative damage leads to airway inflammation that may contribute to an increased prevalence of asthma.27 In vitro data suggest that antioxidant deficiency may influence the differentiation of helper T cells to a TH2 phenotype, which can increase susceptibility to the development of asthma and allergies.29 Vitamin E specifically has been shown to inhibit IL-4 gene expression, which drives type 2 immunity and decreases expression of multiple genes that regulate epidermal barrier function, subsequently increasing susceptibility to allergic inflammation and AD.29,30 Regardless of the proposed mechanisms for antioxidant deficiency increasing susceptibility to allergic disease, studies evaluating the benefits of antioxidant intake during pregnancy in relation to AD have not been promising. Several studies have found no association between prenatal vitamin E intake and the risk for AD development in infants and children.31,32 Another study found a statistically significant inverse relationship between vitamin E intake in mothers with a history of atopy and the development of AD in their children at 2 years of age but not at 1 year of age (P-trend=.024).33 It has been suggested that varying vitamin E isoforms may contribute to the discrepant results previously discussed, with the γ-tocopherol isoform (found frequently in Westernized diets)34 as a driver of inflammation in murine models.35 West et al31 noted an association between vitamin C intake and development of “any allergic disease”—AD, IgE-mediated food allergy, or asthma—with a crude OR of 0.48 (95% CI, 0.25-0.93). However, the P-trend and adjusted OR were not statistically significant. The investigators found no association between maternal intake of beta-carotene, vitamin E, or zinc, but they did find copper supplementation to be protective on the development of AD at 1 year of age (P-trend=0.03). Interestingly, when the data for total antioxidant intake—vitamin C, vitamin E, zinc, beta-carotene, and copper from both diet and supplementation—were combined and analyzed, no statistically significant associations for any of the antioxidants were found.31 Another study of 763 Japanese mother-child pairs found a reduced risk for AD at 16 to 24 months of age with high maternal intake of beta-carotene but found no statistically significant exposure-response associations with other antioxidants, including alpha-carotene, vitamin C, or zinc from dietary intake alone.32 These results were substantiated by 2 meta-analyses evaluating a total of 93 combined intervention trials and cohorts where no association was found between vitamin or mineral intake during pregnancy and/or during infancy and the development of AD.19,36
Fatty Acids
Other dietary changes that are associated with an increased prevalence of atopic diseases in children include excess consumption of omega-6 (n-6) long-chain polyunsaturated fatty acids (LC-PUFA) and insufficient omega-3 (n-3) LC-PUFA consumption.37 Given prior evidence that allergic immune responses in infants may be primed before birth,38 researchers have questioned whether the anti-inflammatory properties of n-3 LC-PUFA when supplemented during pregnancy may have immunomodulatory effects on infants that could alter their predisposition to develop allergic disease, including AD.39 A systematic review and meta-analysis of randomized controlled trials found a statistically significant RR of 0.53 (95% CI, 0.35-0.81; P=.004) for the incidence of AD at 12 months of age with maternal supplementation of n-3 LC-PUFA.9 Another trial of 145 pregnant women randomized to supplementation with fish oil vs placebo starting at gestational week 25 and continuing through 3.5 months of breastfeeding found a reduced cumulative incidence of AD in the intervention group compared to controls at 2 years of age, with a statistically significant crude OR of 0.33 (95% CI, 0.11-0.97; P=.04).40 However, the adjusted OR was not statistically significant. In addition, they found that mothers and infants with higher proportions of docosahexaenoic acid and eicosapentaenoic acid in plasma phospholipids have been noted to have a lower prevalence of IgE-associated disease in a dose-dependent manner (P<.05 and P<.05, respectively).40 In another trial of 98 pregnant women randomized to fish oil supplementation or placebo from 20 weeks’ gestation to delivery found no difference in the frequency of AD but did note that infants in the exposure group had significantly less severe AD compared to controls (OR=0.09 [95% CI, 0.1-0.94]; P=.045).39 A prospective birth cohort study of 2641 children evaluated dietary composition during the last 4 weeks of pregnancy and found that consumption of foods rich in n-6 LC-PUFAs (eg, margarine, vegetable oil) increased the risk for developing AD, while foods rich in n-3 LC-PUFAs (eg, fish) decreased the risk for developing AD in offspring at 2 years of age. All P values for margarine, vegetable oil, and fish were statistically significant on logistic regression at P<.05.41 A longitudinal analysis of follow-up data from a randomized controlled trial looking at maternal prenatal n-3 LC-PUFA intake and the development of allergic disease (including AD) found no differences in the development of disease at 1-, 3-, or 6-year follow-up.42 Despite several studies demonstrating a possible benefit of omega-3 fatty acid intake on the development of AD in offspring, the longitudinal analysis by Best et al42 reminds us that long-term follow-up is critical in establishing benefit of any intervention given the heterogeneous and progressive nature of the atopic march and AD.
Specific Diets
Several studies have evaluated the role of dietary patterns and their influence on atopic disease. Studies evaluating dietary patterns or supplement intake can be challenging, as data often are derived from questionnaires with bias in response to families with higher socioeconomic status.9 Further, analysis of any one food group does not account for the potential interplay between nutrients.43 Studies should focus more on dietary patterns vs individual foods to assess true risk.43,44 Given these limitations, study results on diet should be carefully scrutinized; however, there are still some positive findings that deserve further investigation. Chatzi et al44 followed 460 children for 6.5 years and found a protective effect for the development of atopy in the offspring of women who had high adherence to the Mediterranean diet (OR 0.55 [95% CI, 0.31-0.97]). Another cohort study evaluating the effects of the Mediterranean diet and risk for AD in the first year of life in 2516 mother-child pairs from Spain and Greece found no statistically significant association with consumption of the Mediterranean diet and AD. The investigators also evaluated intake of fruits, nuts, vegetables, meats, processed meats, dairy products, and cereal and found no statistically significant protective benefit.45 Another systematic review of more than 90 observational studies identified no significant relationship between prenatal dietary exposures of fruits, vegetables, nuts, fat, fatty acids, eggs, cereal, milk, alcohol, tea, or coffee and risk for allergic disease in offspring, including AD.19
A Chinese prospective cohort study evaluated the dietary protein patterns of 713 mother-child pairs and the incidence of infant AD at 6 months of age.46 Dietary protein patterns were characterized as predominantly poultry, plant based, dairy and eggs, and red meat and fish. The investigators found a statistically significant reduced risk for AD in mothers who consumed plant-based or dairy and eggs protein patterns when compared to a poultry protein pattern with an adjusted OR of 0.572 (95% CI, 0.330-0.992) and 0.478 (95% CI, 0.274-0.837), respectively. This protective effect was not seen with the red meat and fish protein patterns.46 Similar results were seen in a 2020 Canadian study that evaluated the effects of a Western (fats, meats, processed foods, and starchy vegetables), balanced (diverse sources of animal proteins [especially fish], fruits, vegetables, nuts, and seeds), or plant-based (dairy, legumes, vegetables, whole grains, and an aversion to meats) diet in more than 2000 mother-infant pairs from 24 to 28 weeks’ gestation to 1 year of age. The investigators found a lower OR of AD in mothers who followed a mostly plant-based diet compared to other dietary patterns (OR 0.65 [95% CI, 0.55-0.76]; P<.001).10 Another prospective Japanese study looking at healthy (high intake of green and yellow vegetables, seaweed, mushrooms, white vegetables, pulses, potatoes, fish, sea products, fruit, and shellfish, and low intake of confectioneries and soft drinks), Western (high intake of vegetable oil, salt-containing seasonings, beef, pork, processed meat, eggs, chicken, and white vegetables, and low intake of fruit, soft drinks, and confectioneries), or Japanese (high intake of rice, miso soup, sea products, and fish, and low intake of bread, confectioneries, and dairy products) dietary patterns in 763 mother-child pairs found no association between diet during pregnancy and development of AD in offspring at 16 to 24 months.47 Unfortunately, a longitudinal data analysis has not been performed for this study.
Final Thoughts
Atopic dermatitis is a complex, progressive, and heterogeneous disease with both genetic and environmental influences. Studying the effects of diet on the development, progression, or severity of disease can be very difficult due to the heterogeneity of study designs, lack of long-term follow-up, and high potential for residual confounding. Studies evaluating dietary patterns or supplement intake can be equally challenging, as data often are derived from questionnaires with bias in response to families with higher socioeconomic status.9 Very few studies have looked specifically at maternal dietary composition and the development of AD alone (without inclusion of asthma or food allergy). Ultimately, the inconsistency of the data makes it difficult to draw conclusions and make formal recommendations for this vulnerable population. Additional evidence from well-powered trials with comparable methodology and objective outcome measures will be imperative to make formal recommendations. In addition, longitudinal follow-up will be essential to determine long-term benefit and influence on the atopic march.
- Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66(suppl 1):8-16.
- Kapoor R, Menon C, Hoffstad O, et al. The prevalence of atopic triad in children with physician-confirmed atopic dermatitis. J Am Acad Dermatol. 2008;58:68-73.
- Abuabara K, Magyari A, McCulloch CE, et al. Prevalence of atopic eczema among patients seen in primary care: data from the Health Improvement Network. Ann Intern Med. 2019;170:354-356.
- Belgrave DC, Granell R, Simpson A, et al. Developmental profiles of eczema, wheeze, and rhinitis: two population-based birth cohort studies. PLoS Medicine. 2014;11:E1001748.
- Aguilar D, Pinart M, Koppelman GH, et al. Computational analysis of multimorbidity between asthma, eczema and rhinitis. PloS One. 2017;12:E0179125.
- Deckers IA, McLean S, Linssen S, et al. Investigating international time trends in the incidence and prevalence of atopic eczema 1990-2010: a systematic review of epidemiological studies. PloS One. 2012;7:E39803.
- Williams H, Stewart A, von Mutius E, et al. Is eczema really on the increase worldwide? J Allergy Clin Immunol. 2008;121:947-954.
- Sullivan M, Silverberg NB. Current and emerging concepts in atopic dermatitis pathogenesis. Clin Dermatol. 2017;35:349-353.
- Best KP, Gold M, Kennedy D, et al. Omega-3 long-chain PUFA intake during pregnancy and allergic disease outcomes in the offspring: a systematic review and meta-analysis of observational studies and randomized controlled trials. Am J Clin Nutr. 2016;103:128-143.
- Zulyniak MA, de Souza RJ, Shaikh M, et al. Ethnic differences in maternal diet in pregnancy and infant eczema. PloS One. 2020;15:E0232170.
- Jena PK, Sheng L, Mcneil K, et al. Long-term Western diet intake leads to dysregulated bile acid signaling and dermatitis with Th2 and Th17 pathway features in mice. J Dermatol Sci. 2019;95:13-20.
- Grieger JA, Clifton VL, Tuck AR, et al. In utero programming of allergic susceptibility. Int Arch Allergy Immunol. 2016;169:80-92. doi:10.1159/000443961
- Khan TK, Palmer DJ, Prescott SL. In-utero exposures and the evolving epidemiology of paediatric allergy. Curr Opin Allergy Clin Immunol. 2015;15:402-408. doi:10.1097/ACI.0000000000000209
- Bauer SM. Atopic eczema: genetic associations and potential links to developmental exposures. Int J Toxicol. 2017;36:187-198.
- Shinohara M, Saito H, Matsumoto K. Different timings of prenatal or postnatal tobacco smoke exposure have different effects on the development of atopic eczema/dermatitis syndrome (AEDS) during infancy. J Allergy Clin Immunol. 2012;129:AB40.
- Lerodiakonou D, Garcia-Larsen V, Logan A, et al. Timing of allergenic food introduction to the infant diet and risk of allergic or autoimmune disease: a systematic review and meta-analysis. JAMA. 2016;316:1181-1192.
- Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
- Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Evid Based Child Health. 2014;9:447-483.
- Garcia-Larsen V, Ierodiakonou D, Jarrold K, et al. Diet during pregnancy and infancy and risk of allergic or autoimmune disease: a systematic review and meta-analysis. PLoS Med. 2018;15:E1002507.
- Greer FR, Sicherer SH, Burks AW; Committee on Nutrition, Section on Allergy and Immunology. The effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. 2019;143:e20190281.
- Baquerizo Nole KL, Yim E, Keri JE. Probiotics and prebiotics in dermatology. J Am Acad Dermatol. 2014;71:814-821.
- Schultz M, Göttl C, Young RJ, et al. Administration of oral probiotic bacteria to pregnant women causes temporary infantile colonization. J Pediatr Gastroenterol Nutr. 2004;38:293-297.
- Lee J, Seto D, Bielory L. Meta-analysis of clinical trials of probiotics for prevention and treatment of pediatric atopic dermatitis. J Allergy Clin Immunol. 2008;121:116-121.
- Panduru M, Panduru NM, Sa˘la˘va˘stru CM, et al. Probiotics and primary prevention of atopic dermatitis: a meta‐analysis of randomized controlled studies. J Eur Acad Dermatol Venereol. 2015;29:232-242.
- Doege K, Grajecki D, Zyriax BC, et al. Impact of maternal supplementation with probiotics during pregnancy on atopic eczema in childhood—a meta-analysis. Br J Nutr. 2012;107:1-6.
- Zuccotti G, Meneghin F, Aceti A, et al. Probiotics for prevention of atopic diseases in infants: systematic review and meta‐analysis. Allergy. 2015;70:1356-1371.
- Seaton A, Godden DJ, Brown K. Increase in asthma: a more toxic environment or a more susceptible population? Thorax. 1994;49:171-174.
- Manzel A, Muller DN, Hafler DA, et al. Role of “Western diet” in inflammatory autoimmune diseases. Curr Allergy Asthma Rep. 2014;14:1-8.
- Li-Weber M, Giasisi M, Trieber MK, et al. Vitamin E inhibits IL-4 gene expression in peripheral blood T cells. Eur J Immunol. 2002;32:2401-2408.
- Sehra S, Yao Y, Howell MD, et al. IL-4 regulates skin homeostasis and the predisposition toward allergic skin inflammation. J Immunol. 2010;184:3186-3190.
- West CE, Dunstan J, McCarthy S, et al. Associations between maternal antioxidant intakes in pregnancy and infant allergic outcomes. Nutrients. 2012;4:1747-1758.
- Miyake Y, Sasaki S, Tanaka K, et al. Consumption of vegetables, fruit, and antioxidants during pregnancy and wheeze and eczema in infants. Allergy. 2010;65:758-765.
- Martindale S, McNeill G, Devereux G, et al. Antioxidant intake in pregnancy in relation to wheeze and eczema in the first two years of life. Am J Respir Crit Care Med. 2005;171:121-128.
- Robison R, Kumar R. The effect of prenatal and postnatal dietary exposures on childhood development of atopic disease. Curr Opin Allergy Clin Immunol. 2010;10:139-144.
- Berdnikovs S, Abdala-Valencia H, McCary C, et al. Isoforms of vitamin E have opposing immunoregulatory functions during inflammation by regulating leukocyte recruitment. J Immunol. 2009;182:4395-4405.
- Beckhaus AA, Garcia‐Marcos L, Forno E, et al. Maternal nutrition during pregnancy and risk of asthma, wheeze, and atopic diseases during childhood: a systematic review and meta‐analysis. Allergy. 2015;70:1588-1604.
- Calder PC, Miles EA. Fatty acids and atopic disease. Pediatr Allergy Immunol. 2000;11(suppl 13):29-36.
- Prescott S, Macaubas C, Holt B, et al. Transplacental priming of the human immune system to environmental allergens: universal skewing of initial T-cell responses towards Th-2 cytokine profile. J Immunol. 1998;160:4730-4737.
- Dunstan JA, Mori TA, Barden A, et al. Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: a randomized, controlled trial. J Allergy Clin Immunol. 2003;112:1178-1184.
- Furuhjelm C, Warstedt K, Fagerås M, et al. Allergic disease in infants up to 2 years of age in relation to plasma omega‐3 fatty acids and maternal fish oil supplementation in pregnancy and lactation. Pediatr Allergy Immunol. 2011;22:505-514.
- Sausenthaler S, Koletzko S, Schaaf B, et al; LISA Study Group. Maternal diet during pregnancy in relation to eczema and allergic sensitization in the offspring at 2 y of age. Am J Clin Nutr. 2007;85:530-537.
- Best KP, Sullivan TR, Palmer DJ, et al. Prenatal omega-3 LCPUFA and symptoms of allergic disease and sensitization throughout early childhood—a longitudinal analysis of long-term follow-up of a randomized controlled trial. World Allergy Organ J. 2018;11:10.
- Jacobs DR Jr, Steffen LM. Nutrients, foods, and dietary patterns as exposures in research: a framework for food synergy. Am J Clin Nutr. 2003;78:508-513.
- Chatzi L, Torrent M, Romieu I, et al. Mediterranean diet in pregnancy is protective for wheeze and atopy in childhood. Thorax. 2008;63:507-513.
- Chatzi L, Garcia R, Roumeliotaki T, et al. Mediterranean diet adherence during pregnancy and risk of wheeze and eczema in the first year of life: INMA (Spain) and RHEA (Greece) mother-child cohort studies. Br J Nutr. 2013;110:2058-2068.
- Zeng J, Wu W, Chen Y, et al. Maternal dietary protein patterns during pregnancy and the risk of infant eczema: a cohort study. Front Nutr. 2021;8:294.
- Miyake Y, Okubo H, Sasaki S, et al. Maternal dietary patterns during pregnancy and risk of wheeze and eczema in Japanese infants aged 16–24 months: the Osaka Maternal and Child Health Study. Pediatr Allergy Immunol. 2011;22:734-741.
Atopic dermatitis (AD) is an inflammatory skin disease characterized by skin barrier disruption, skin inflammation, and pruritus.1 It is a common and often chronic skin condition associated with the development of food allergies, asthma, and allergic rhinitis, known as the atopic march.2 Atopic dermatitis is estimated to affect 10% to 25% of children, most with onset before 5 years of age, and up to 7% of adults worldwide.3 Most patients improve with time, but multiple disease trajectories are possible. Several studies have demonstrated that fewer than 4% of children develop the classic atopic march—AD followed by food allergies, asthma, and finally allergic rhinitis—with recent evidence pointing to a more complex heterogeneous progression of disease and allergic comorbidities often occurring together.4,5 The prevalence of AD has been increasing globally over the last 30 years,6 with a marked increase in developed countries.6,7 It is well accepted that AD is based on an interplay between genetic predisposition and environmental factors,8 but many suspect that the rapid rise in prevalence cannot be attributed to genetic factors alone.9 The precipitant triggers for AD remain an area of intense investigation, with ongoing debate between the “inside out” and “outside in” hypotheses; these revolve around whether abnormalities in the immune system trigger barrier dysfunction or barrier dysfunction triggers immune programming to atopy.8 Ongoing research related to genetic predisposition of AD has identified candidate genes implicated in both impaired skin barrier function and altered immune system pathways, further supporting that both theories may contribute to disease pathogenesis.
The increasing prevalence of AD, with increasing disease burden within socioeconomically advantaged countries, raises the possibility of early modifiable environmental factors that may contribute to the disease process.10 Many studies point to the influence of the 21st century lifestyle and Western diet as primary contributing factors.9,11 However, it is not clear how these factors may influence the development of allergic atopic disease. Several studies have suggested that nonheritable influences in utero can alter fetus immune function and influence the subsequent development of allergic disease.12,13 Although many studies have examined environmental factors contributing to the development of AD in infancy and childhood, less is understood about the influence of prenatal factors. Currently, in utero exposure to tobacco smoke, phthalates, and maternal distress have been potentially implicated in the development of AD.14,15 Several studies have examined the role of maternal diet and nutrition on the development of AD in offspring; however, formal recommendations and robust trial data are lacking. In this article, we examine the existing literature surrounding maternal diet on the development of AD in infancy and childhood.
Allergen Avoidance
Extrapolating from the food allergy literature, it was once suggested that allergen avoidance in early childhood had a protective effect on the subsequent development of allergies; however, more recent research has found that early exposure to common food allergens, such as peanuts or eggs, may actually reduce a child’s risk for developing these allergies later in life.16 Among infants at high risk for food allergy, sustained consumption of peanut products beginning in the first 11 months of life resulted in an 81% lower rate of peanut allergy at 60 months of age than the rate among children who avoided peanuts.17 Given the results that antigen avoidance during infancy/childhood does not protect against the development of allergies and may actually be counterproductive, it is not surprising that research studying antigen avoidance during pregnancy on the development of AD also has demonstrated limited efficacy. A systematic review of 5 trials on maternal dietary antigen avoidance (N=952) suggested no protective effects of avoiding antigenic foods during pregnancy on the development of AD in the first 18 months of life.18 Another meta-analysis evaluating 12 intervention trials looked at the effects of maternal allergenic food avoidance during pregnancy or lactation and found no reduced risk for subsequent development of allergic disease, including AD.19 The American Academy of Pediatrics 2019 consensus statement does not support maternal dietary restrictions in pregnancy for the prevention of atopic disease and makes note that the data remain limited, which complicates drawing any firm conclusions.20
Probiotic Supplementation
One of the most investigated dietary supplements for the prevention of atopic disease is probiotics, with possible benefits noted in both the prenatal and postnatal periods. Baquerizo Nole et al21 examined several studies looking at the various benefits of probiotics in AD, which included inhibition of the helper T cell (TH2) response, stimulation of the TH1 response, upregulation of regulatory T cells, acceleration of skin and mucosal barrier function, increase in intestinal microflora diversity, suppression of toxic fermentation products in the intestinal lumen from increased production of short-chain fatty acids, and inhibition of Staphylococcus aureus attachment on epidermal keratinocytes. It is unclear how this may affect infants prenatally; however, transfer of maternal intestinal microflora during delivery and shortly thereafter has demonstrated that probiotic strains remain detectable in the infant’s stool up to 6 months after delivery, even if the mother has discontinued use.22 A 2008 meta-analysis of 10 double-bind, randomized, controlled trials (N=1880) looking at the use of maternal prenatal and postnatal probiotic supplementation in the prevention of pediatric AD found a relative risk (RR) ratio of 0.69 (95% CI, 0.57-0.83) using a fixed effects model and RR ratio of 0.66 (95% CI, 0.49-0.89) using a random effects model. After exclusion of one study that evaluated the effect of postnatal probiotic supplementation only, the RR ratio decreased to 0.61 for both the fixed effects and random effects models.23 A systematic review by Panduru et al24 noted similar findings with a subgroup meta-analysis of 11 studies of prenatal supplementation followed by postnatal supplementation of probiotics, which demonstrated a protective effect on the development of AD (odds ratio [OR]=0.61, P<.001). Postnatal supplementation alone (4 studies) did not have the same association (OR=0.95, P<.82).24 A 2012 meta-analysis by Doege et al25 evaluated 7 randomized, double-blinded, placebo-controlled trials that assessed probiotic supplementation during pregnancy (without incorporation of postnatal supplementation) and found a significant risk reduction of 5.7% (P=.022) for AD in children aged 2 to 7 years. Interestingly, this was only significant for Lactobacillus and not for other bacterial strains, even if a mixture of strains included Lactobacillus. However, Panduru et al24 found both maternal Lactobacillus supplementation alone (8 studies) and in combination with Bifidobacterium (9 studies) was protective against AD development in children (OR=0.70, P=.004; OR=0.62, P<.001). A more recent 2015 meta-analysis of 17 studies (N=4755) evaluating the use of maternal probiotic supplementation in pregnancy and/or through the infant’s first 3 months of life found a significantly lower RR (0.78 [95% CI, 0.69-0.89], P=.0003) for the development of AD in infants treated with probiotics and found this risk to be even further decreased when a mixture of probiotics including both Lactobacillus and Bifidobacterium was used (RR=0.54 [95% CI, 0.43-0.68], P<.00001).26
Antioxidants
The Westernization of many developing countries’ diets—diets high in saturated fats, protein, sucrose, salt, and processed foods and low in fresh fruits and green vegetables—has led to a reduced intake of antioxidants and an increase in susceptibility to oxidative damage.27,28 One hypothesis suggests that a reduction in nutritional antioxidants and subsequent oxidative damage leads to airway inflammation that may contribute to an increased prevalence of asthma.27 In vitro data suggest that antioxidant deficiency may influence the differentiation of helper T cells to a TH2 phenotype, which can increase susceptibility to the development of asthma and allergies.29 Vitamin E specifically has been shown to inhibit IL-4 gene expression, which drives type 2 immunity and decreases expression of multiple genes that regulate epidermal barrier function, subsequently increasing susceptibility to allergic inflammation and AD.29,30 Regardless of the proposed mechanisms for antioxidant deficiency increasing susceptibility to allergic disease, studies evaluating the benefits of antioxidant intake during pregnancy in relation to AD have not been promising. Several studies have found no association between prenatal vitamin E intake and the risk for AD development in infants and children.31,32 Another study found a statistically significant inverse relationship between vitamin E intake in mothers with a history of atopy and the development of AD in their children at 2 years of age but not at 1 year of age (P-trend=.024).33 It has been suggested that varying vitamin E isoforms may contribute to the discrepant results previously discussed, with the γ-tocopherol isoform (found frequently in Westernized diets)34 as a driver of inflammation in murine models.35 West et al31 noted an association between vitamin C intake and development of “any allergic disease”—AD, IgE-mediated food allergy, or asthma—with a crude OR of 0.48 (95% CI, 0.25-0.93). However, the P-trend and adjusted OR were not statistically significant. The investigators found no association between maternal intake of beta-carotene, vitamin E, or zinc, but they did find copper supplementation to be protective on the development of AD at 1 year of age (P-trend=0.03). Interestingly, when the data for total antioxidant intake—vitamin C, vitamin E, zinc, beta-carotene, and copper from both diet and supplementation—were combined and analyzed, no statistically significant associations for any of the antioxidants were found.31 Another study of 763 Japanese mother-child pairs found a reduced risk for AD at 16 to 24 months of age with high maternal intake of beta-carotene but found no statistically significant exposure-response associations with other antioxidants, including alpha-carotene, vitamin C, or zinc from dietary intake alone.32 These results were substantiated by 2 meta-analyses evaluating a total of 93 combined intervention trials and cohorts where no association was found between vitamin or mineral intake during pregnancy and/or during infancy and the development of AD.19,36
Fatty Acids
Other dietary changes that are associated with an increased prevalence of atopic diseases in children include excess consumption of omega-6 (n-6) long-chain polyunsaturated fatty acids (LC-PUFA) and insufficient omega-3 (n-3) LC-PUFA consumption.37 Given prior evidence that allergic immune responses in infants may be primed before birth,38 researchers have questioned whether the anti-inflammatory properties of n-3 LC-PUFA when supplemented during pregnancy may have immunomodulatory effects on infants that could alter their predisposition to develop allergic disease, including AD.39 A systematic review and meta-analysis of randomized controlled trials found a statistically significant RR of 0.53 (95% CI, 0.35-0.81; P=.004) for the incidence of AD at 12 months of age with maternal supplementation of n-3 LC-PUFA.9 Another trial of 145 pregnant women randomized to supplementation with fish oil vs placebo starting at gestational week 25 and continuing through 3.5 months of breastfeeding found a reduced cumulative incidence of AD in the intervention group compared to controls at 2 years of age, with a statistically significant crude OR of 0.33 (95% CI, 0.11-0.97; P=.04).40 However, the adjusted OR was not statistically significant. In addition, they found that mothers and infants with higher proportions of docosahexaenoic acid and eicosapentaenoic acid in plasma phospholipids have been noted to have a lower prevalence of IgE-associated disease in a dose-dependent manner (P<.05 and P<.05, respectively).40 In another trial of 98 pregnant women randomized to fish oil supplementation or placebo from 20 weeks’ gestation to delivery found no difference in the frequency of AD but did note that infants in the exposure group had significantly less severe AD compared to controls (OR=0.09 [95% CI, 0.1-0.94]; P=.045).39 A prospective birth cohort study of 2641 children evaluated dietary composition during the last 4 weeks of pregnancy and found that consumption of foods rich in n-6 LC-PUFAs (eg, margarine, vegetable oil) increased the risk for developing AD, while foods rich in n-3 LC-PUFAs (eg, fish) decreased the risk for developing AD in offspring at 2 years of age. All P values for margarine, vegetable oil, and fish were statistically significant on logistic regression at P<.05.41 A longitudinal analysis of follow-up data from a randomized controlled trial looking at maternal prenatal n-3 LC-PUFA intake and the development of allergic disease (including AD) found no differences in the development of disease at 1-, 3-, or 6-year follow-up.42 Despite several studies demonstrating a possible benefit of omega-3 fatty acid intake on the development of AD in offspring, the longitudinal analysis by Best et al42 reminds us that long-term follow-up is critical in establishing benefit of any intervention given the heterogeneous and progressive nature of the atopic march and AD.
Specific Diets
Several studies have evaluated the role of dietary patterns and their influence on atopic disease. Studies evaluating dietary patterns or supplement intake can be challenging, as data often are derived from questionnaires with bias in response to families with higher socioeconomic status.9 Further, analysis of any one food group does not account for the potential interplay between nutrients.43 Studies should focus more on dietary patterns vs individual foods to assess true risk.43,44 Given these limitations, study results on diet should be carefully scrutinized; however, there are still some positive findings that deserve further investigation. Chatzi et al44 followed 460 children for 6.5 years and found a protective effect for the development of atopy in the offspring of women who had high adherence to the Mediterranean diet (OR 0.55 [95% CI, 0.31-0.97]). Another cohort study evaluating the effects of the Mediterranean diet and risk for AD in the first year of life in 2516 mother-child pairs from Spain and Greece found no statistically significant association with consumption of the Mediterranean diet and AD. The investigators also evaluated intake of fruits, nuts, vegetables, meats, processed meats, dairy products, and cereal and found no statistically significant protective benefit.45 Another systematic review of more than 90 observational studies identified no significant relationship between prenatal dietary exposures of fruits, vegetables, nuts, fat, fatty acids, eggs, cereal, milk, alcohol, tea, or coffee and risk for allergic disease in offspring, including AD.19
A Chinese prospective cohort study evaluated the dietary protein patterns of 713 mother-child pairs and the incidence of infant AD at 6 months of age.46 Dietary protein patterns were characterized as predominantly poultry, plant based, dairy and eggs, and red meat and fish. The investigators found a statistically significant reduced risk for AD in mothers who consumed plant-based or dairy and eggs protein patterns when compared to a poultry protein pattern with an adjusted OR of 0.572 (95% CI, 0.330-0.992) and 0.478 (95% CI, 0.274-0.837), respectively. This protective effect was not seen with the red meat and fish protein patterns.46 Similar results were seen in a 2020 Canadian study that evaluated the effects of a Western (fats, meats, processed foods, and starchy vegetables), balanced (diverse sources of animal proteins [especially fish], fruits, vegetables, nuts, and seeds), or plant-based (dairy, legumes, vegetables, whole grains, and an aversion to meats) diet in more than 2000 mother-infant pairs from 24 to 28 weeks’ gestation to 1 year of age. The investigators found a lower OR of AD in mothers who followed a mostly plant-based diet compared to other dietary patterns (OR 0.65 [95% CI, 0.55-0.76]; P<.001).10 Another prospective Japanese study looking at healthy (high intake of green and yellow vegetables, seaweed, mushrooms, white vegetables, pulses, potatoes, fish, sea products, fruit, and shellfish, and low intake of confectioneries and soft drinks), Western (high intake of vegetable oil, salt-containing seasonings, beef, pork, processed meat, eggs, chicken, and white vegetables, and low intake of fruit, soft drinks, and confectioneries), or Japanese (high intake of rice, miso soup, sea products, and fish, and low intake of bread, confectioneries, and dairy products) dietary patterns in 763 mother-child pairs found no association between diet during pregnancy and development of AD in offspring at 16 to 24 months.47 Unfortunately, a longitudinal data analysis has not been performed for this study.
Final Thoughts
Atopic dermatitis is a complex, progressive, and heterogeneous disease with both genetic and environmental influences. Studying the effects of diet on the development, progression, or severity of disease can be very difficult due to the heterogeneity of study designs, lack of long-term follow-up, and high potential for residual confounding. Studies evaluating dietary patterns or supplement intake can be equally challenging, as data often are derived from questionnaires with bias in response to families with higher socioeconomic status.9 Very few studies have looked specifically at maternal dietary composition and the development of AD alone (without inclusion of asthma or food allergy). Ultimately, the inconsistency of the data makes it difficult to draw conclusions and make formal recommendations for this vulnerable population. Additional evidence from well-powered trials with comparable methodology and objective outcome measures will be imperative to make formal recommendations. In addition, longitudinal follow-up will be essential to determine long-term benefit and influence on the atopic march.
Atopic dermatitis (AD) is an inflammatory skin disease characterized by skin barrier disruption, skin inflammation, and pruritus.1 It is a common and often chronic skin condition associated with the development of food allergies, asthma, and allergic rhinitis, known as the atopic march.2 Atopic dermatitis is estimated to affect 10% to 25% of children, most with onset before 5 years of age, and up to 7% of adults worldwide.3 Most patients improve with time, but multiple disease trajectories are possible. Several studies have demonstrated that fewer than 4% of children develop the classic atopic march—AD followed by food allergies, asthma, and finally allergic rhinitis—with recent evidence pointing to a more complex heterogeneous progression of disease and allergic comorbidities often occurring together.4,5 The prevalence of AD has been increasing globally over the last 30 years,6 with a marked increase in developed countries.6,7 It is well accepted that AD is based on an interplay between genetic predisposition and environmental factors,8 but many suspect that the rapid rise in prevalence cannot be attributed to genetic factors alone.9 The precipitant triggers for AD remain an area of intense investigation, with ongoing debate between the “inside out” and “outside in” hypotheses; these revolve around whether abnormalities in the immune system trigger barrier dysfunction or barrier dysfunction triggers immune programming to atopy.8 Ongoing research related to genetic predisposition of AD has identified candidate genes implicated in both impaired skin barrier function and altered immune system pathways, further supporting that both theories may contribute to disease pathogenesis.
The increasing prevalence of AD, with increasing disease burden within socioeconomically advantaged countries, raises the possibility of early modifiable environmental factors that may contribute to the disease process.10 Many studies point to the influence of the 21st century lifestyle and Western diet as primary contributing factors.9,11 However, it is not clear how these factors may influence the development of allergic atopic disease. Several studies have suggested that nonheritable influences in utero can alter fetus immune function and influence the subsequent development of allergic disease.12,13 Although many studies have examined environmental factors contributing to the development of AD in infancy and childhood, less is understood about the influence of prenatal factors. Currently, in utero exposure to tobacco smoke, phthalates, and maternal distress have been potentially implicated in the development of AD.14,15 Several studies have examined the role of maternal diet and nutrition on the development of AD in offspring; however, formal recommendations and robust trial data are lacking. In this article, we examine the existing literature surrounding maternal diet on the development of AD in infancy and childhood.
Allergen Avoidance
Extrapolating from the food allergy literature, it was once suggested that allergen avoidance in early childhood had a protective effect on the subsequent development of allergies; however, more recent research has found that early exposure to common food allergens, such as peanuts or eggs, may actually reduce a child’s risk for developing these allergies later in life.16 Among infants at high risk for food allergy, sustained consumption of peanut products beginning in the first 11 months of life resulted in an 81% lower rate of peanut allergy at 60 months of age than the rate among children who avoided peanuts.17 Given the results that antigen avoidance during infancy/childhood does not protect against the development of allergies and may actually be counterproductive, it is not surprising that research studying antigen avoidance during pregnancy on the development of AD also has demonstrated limited efficacy. A systematic review of 5 trials on maternal dietary antigen avoidance (N=952) suggested no protective effects of avoiding antigenic foods during pregnancy on the development of AD in the first 18 months of life.18 Another meta-analysis evaluating 12 intervention trials looked at the effects of maternal allergenic food avoidance during pregnancy or lactation and found no reduced risk for subsequent development of allergic disease, including AD.19 The American Academy of Pediatrics 2019 consensus statement does not support maternal dietary restrictions in pregnancy for the prevention of atopic disease and makes note that the data remain limited, which complicates drawing any firm conclusions.20
Probiotic Supplementation
One of the most investigated dietary supplements for the prevention of atopic disease is probiotics, with possible benefits noted in both the prenatal and postnatal periods. Baquerizo Nole et al21 examined several studies looking at the various benefits of probiotics in AD, which included inhibition of the helper T cell (TH2) response, stimulation of the TH1 response, upregulation of regulatory T cells, acceleration of skin and mucosal barrier function, increase in intestinal microflora diversity, suppression of toxic fermentation products in the intestinal lumen from increased production of short-chain fatty acids, and inhibition of Staphylococcus aureus attachment on epidermal keratinocytes. It is unclear how this may affect infants prenatally; however, transfer of maternal intestinal microflora during delivery and shortly thereafter has demonstrated that probiotic strains remain detectable in the infant’s stool up to 6 months after delivery, even if the mother has discontinued use.22 A 2008 meta-analysis of 10 double-bind, randomized, controlled trials (N=1880) looking at the use of maternal prenatal and postnatal probiotic supplementation in the prevention of pediatric AD found a relative risk (RR) ratio of 0.69 (95% CI, 0.57-0.83) using a fixed effects model and RR ratio of 0.66 (95% CI, 0.49-0.89) using a random effects model. After exclusion of one study that evaluated the effect of postnatal probiotic supplementation only, the RR ratio decreased to 0.61 for both the fixed effects and random effects models.23 A systematic review by Panduru et al24 noted similar findings with a subgroup meta-analysis of 11 studies of prenatal supplementation followed by postnatal supplementation of probiotics, which demonstrated a protective effect on the development of AD (odds ratio [OR]=0.61, P<.001). Postnatal supplementation alone (4 studies) did not have the same association (OR=0.95, P<.82).24 A 2012 meta-analysis by Doege et al25 evaluated 7 randomized, double-blinded, placebo-controlled trials that assessed probiotic supplementation during pregnancy (without incorporation of postnatal supplementation) and found a significant risk reduction of 5.7% (P=.022) for AD in children aged 2 to 7 years. Interestingly, this was only significant for Lactobacillus and not for other bacterial strains, even if a mixture of strains included Lactobacillus. However, Panduru et al24 found both maternal Lactobacillus supplementation alone (8 studies) and in combination with Bifidobacterium (9 studies) was protective against AD development in children (OR=0.70, P=.004; OR=0.62, P<.001). A more recent 2015 meta-analysis of 17 studies (N=4755) evaluating the use of maternal probiotic supplementation in pregnancy and/or through the infant’s first 3 months of life found a significantly lower RR (0.78 [95% CI, 0.69-0.89], P=.0003) for the development of AD in infants treated with probiotics and found this risk to be even further decreased when a mixture of probiotics including both Lactobacillus and Bifidobacterium was used (RR=0.54 [95% CI, 0.43-0.68], P<.00001).26
Antioxidants
The Westernization of many developing countries’ diets—diets high in saturated fats, protein, sucrose, salt, and processed foods and low in fresh fruits and green vegetables—has led to a reduced intake of antioxidants and an increase in susceptibility to oxidative damage.27,28 One hypothesis suggests that a reduction in nutritional antioxidants and subsequent oxidative damage leads to airway inflammation that may contribute to an increased prevalence of asthma.27 In vitro data suggest that antioxidant deficiency may influence the differentiation of helper T cells to a TH2 phenotype, which can increase susceptibility to the development of asthma and allergies.29 Vitamin E specifically has been shown to inhibit IL-4 gene expression, which drives type 2 immunity and decreases expression of multiple genes that regulate epidermal barrier function, subsequently increasing susceptibility to allergic inflammation and AD.29,30 Regardless of the proposed mechanisms for antioxidant deficiency increasing susceptibility to allergic disease, studies evaluating the benefits of antioxidant intake during pregnancy in relation to AD have not been promising. Several studies have found no association between prenatal vitamin E intake and the risk for AD development in infants and children.31,32 Another study found a statistically significant inverse relationship between vitamin E intake in mothers with a history of atopy and the development of AD in their children at 2 years of age but not at 1 year of age (P-trend=.024).33 It has been suggested that varying vitamin E isoforms may contribute to the discrepant results previously discussed, with the γ-tocopherol isoform (found frequently in Westernized diets)34 as a driver of inflammation in murine models.35 West et al31 noted an association between vitamin C intake and development of “any allergic disease”—AD, IgE-mediated food allergy, or asthma—with a crude OR of 0.48 (95% CI, 0.25-0.93). However, the P-trend and adjusted OR were not statistically significant. The investigators found no association between maternal intake of beta-carotene, vitamin E, or zinc, but they did find copper supplementation to be protective on the development of AD at 1 year of age (P-trend=0.03). Interestingly, when the data for total antioxidant intake—vitamin C, vitamin E, zinc, beta-carotene, and copper from both diet and supplementation—were combined and analyzed, no statistically significant associations for any of the antioxidants were found.31 Another study of 763 Japanese mother-child pairs found a reduced risk for AD at 16 to 24 months of age with high maternal intake of beta-carotene but found no statistically significant exposure-response associations with other antioxidants, including alpha-carotene, vitamin C, or zinc from dietary intake alone.32 These results were substantiated by 2 meta-analyses evaluating a total of 93 combined intervention trials and cohorts where no association was found between vitamin or mineral intake during pregnancy and/or during infancy and the development of AD.19,36
Fatty Acids
Other dietary changes that are associated with an increased prevalence of atopic diseases in children include excess consumption of omega-6 (n-6) long-chain polyunsaturated fatty acids (LC-PUFA) and insufficient omega-3 (n-3) LC-PUFA consumption.37 Given prior evidence that allergic immune responses in infants may be primed before birth,38 researchers have questioned whether the anti-inflammatory properties of n-3 LC-PUFA when supplemented during pregnancy may have immunomodulatory effects on infants that could alter their predisposition to develop allergic disease, including AD.39 A systematic review and meta-analysis of randomized controlled trials found a statistically significant RR of 0.53 (95% CI, 0.35-0.81; P=.004) for the incidence of AD at 12 months of age with maternal supplementation of n-3 LC-PUFA.9 Another trial of 145 pregnant women randomized to supplementation with fish oil vs placebo starting at gestational week 25 and continuing through 3.5 months of breastfeeding found a reduced cumulative incidence of AD in the intervention group compared to controls at 2 years of age, with a statistically significant crude OR of 0.33 (95% CI, 0.11-0.97; P=.04).40 However, the adjusted OR was not statistically significant. In addition, they found that mothers and infants with higher proportions of docosahexaenoic acid and eicosapentaenoic acid in plasma phospholipids have been noted to have a lower prevalence of IgE-associated disease in a dose-dependent manner (P<.05 and P<.05, respectively).40 In another trial of 98 pregnant women randomized to fish oil supplementation or placebo from 20 weeks’ gestation to delivery found no difference in the frequency of AD but did note that infants in the exposure group had significantly less severe AD compared to controls (OR=0.09 [95% CI, 0.1-0.94]; P=.045).39 A prospective birth cohort study of 2641 children evaluated dietary composition during the last 4 weeks of pregnancy and found that consumption of foods rich in n-6 LC-PUFAs (eg, margarine, vegetable oil) increased the risk for developing AD, while foods rich in n-3 LC-PUFAs (eg, fish) decreased the risk for developing AD in offspring at 2 years of age. All P values for margarine, vegetable oil, and fish were statistically significant on logistic regression at P<.05.41 A longitudinal analysis of follow-up data from a randomized controlled trial looking at maternal prenatal n-3 LC-PUFA intake and the development of allergic disease (including AD) found no differences in the development of disease at 1-, 3-, or 6-year follow-up.42 Despite several studies demonstrating a possible benefit of omega-3 fatty acid intake on the development of AD in offspring, the longitudinal analysis by Best et al42 reminds us that long-term follow-up is critical in establishing benefit of any intervention given the heterogeneous and progressive nature of the atopic march and AD.
Specific Diets
Several studies have evaluated the role of dietary patterns and their influence on atopic disease. Studies evaluating dietary patterns or supplement intake can be challenging, as data often are derived from questionnaires with bias in response to families with higher socioeconomic status.9 Further, analysis of any one food group does not account for the potential interplay between nutrients.43 Studies should focus more on dietary patterns vs individual foods to assess true risk.43,44 Given these limitations, study results on diet should be carefully scrutinized; however, there are still some positive findings that deserve further investigation. Chatzi et al44 followed 460 children for 6.5 years and found a protective effect for the development of atopy in the offspring of women who had high adherence to the Mediterranean diet (OR 0.55 [95% CI, 0.31-0.97]). Another cohort study evaluating the effects of the Mediterranean diet and risk for AD in the first year of life in 2516 mother-child pairs from Spain and Greece found no statistically significant association with consumption of the Mediterranean diet and AD. The investigators also evaluated intake of fruits, nuts, vegetables, meats, processed meats, dairy products, and cereal and found no statistically significant protective benefit.45 Another systematic review of more than 90 observational studies identified no significant relationship between prenatal dietary exposures of fruits, vegetables, nuts, fat, fatty acids, eggs, cereal, milk, alcohol, tea, or coffee and risk for allergic disease in offspring, including AD.19
A Chinese prospective cohort study evaluated the dietary protein patterns of 713 mother-child pairs and the incidence of infant AD at 6 months of age.46 Dietary protein patterns were characterized as predominantly poultry, plant based, dairy and eggs, and red meat and fish. The investigators found a statistically significant reduced risk for AD in mothers who consumed plant-based or dairy and eggs protein patterns when compared to a poultry protein pattern with an adjusted OR of 0.572 (95% CI, 0.330-0.992) and 0.478 (95% CI, 0.274-0.837), respectively. This protective effect was not seen with the red meat and fish protein patterns.46 Similar results were seen in a 2020 Canadian study that evaluated the effects of a Western (fats, meats, processed foods, and starchy vegetables), balanced (diverse sources of animal proteins [especially fish], fruits, vegetables, nuts, and seeds), or plant-based (dairy, legumes, vegetables, whole grains, and an aversion to meats) diet in more than 2000 mother-infant pairs from 24 to 28 weeks’ gestation to 1 year of age. The investigators found a lower OR of AD in mothers who followed a mostly plant-based diet compared to other dietary patterns (OR 0.65 [95% CI, 0.55-0.76]; P<.001).10 Another prospective Japanese study looking at healthy (high intake of green and yellow vegetables, seaweed, mushrooms, white vegetables, pulses, potatoes, fish, sea products, fruit, and shellfish, and low intake of confectioneries and soft drinks), Western (high intake of vegetable oil, salt-containing seasonings, beef, pork, processed meat, eggs, chicken, and white vegetables, and low intake of fruit, soft drinks, and confectioneries), or Japanese (high intake of rice, miso soup, sea products, and fish, and low intake of bread, confectioneries, and dairy products) dietary patterns in 763 mother-child pairs found no association between diet during pregnancy and development of AD in offspring at 16 to 24 months.47 Unfortunately, a longitudinal data analysis has not been performed for this study.
Final Thoughts
Atopic dermatitis is a complex, progressive, and heterogeneous disease with both genetic and environmental influences. Studying the effects of diet on the development, progression, or severity of disease can be very difficult due to the heterogeneity of study designs, lack of long-term follow-up, and high potential for residual confounding. Studies evaluating dietary patterns or supplement intake can be equally challenging, as data often are derived from questionnaires with bias in response to families with higher socioeconomic status.9 Very few studies have looked specifically at maternal dietary composition and the development of AD alone (without inclusion of asthma or food allergy). Ultimately, the inconsistency of the data makes it difficult to draw conclusions and make formal recommendations for this vulnerable population. Additional evidence from well-powered trials with comparable methodology and objective outcome measures will be imperative to make formal recommendations. In addition, longitudinal follow-up will be essential to determine long-term benefit and influence on the atopic march.
- Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66(suppl 1):8-16.
- Kapoor R, Menon C, Hoffstad O, et al. The prevalence of atopic triad in children with physician-confirmed atopic dermatitis. J Am Acad Dermatol. 2008;58:68-73.
- Abuabara K, Magyari A, McCulloch CE, et al. Prevalence of atopic eczema among patients seen in primary care: data from the Health Improvement Network. Ann Intern Med. 2019;170:354-356.
- Belgrave DC, Granell R, Simpson A, et al. Developmental profiles of eczema, wheeze, and rhinitis: two population-based birth cohort studies. PLoS Medicine. 2014;11:E1001748.
- Aguilar D, Pinart M, Koppelman GH, et al. Computational analysis of multimorbidity between asthma, eczema and rhinitis. PloS One. 2017;12:E0179125.
- Deckers IA, McLean S, Linssen S, et al. Investigating international time trends in the incidence and prevalence of atopic eczema 1990-2010: a systematic review of epidemiological studies. PloS One. 2012;7:E39803.
- Williams H, Stewart A, von Mutius E, et al. Is eczema really on the increase worldwide? J Allergy Clin Immunol. 2008;121:947-954.
- Sullivan M, Silverberg NB. Current and emerging concepts in atopic dermatitis pathogenesis. Clin Dermatol. 2017;35:349-353.
- Best KP, Gold M, Kennedy D, et al. Omega-3 long-chain PUFA intake during pregnancy and allergic disease outcomes in the offspring: a systematic review and meta-analysis of observational studies and randomized controlled trials. Am J Clin Nutr. 2016;103:128-143.
- Zulyniak MA, de Souza RJ, Shaikh M, et al. Ethnic differences in maternal diet in pregnancy and infant eczema. PloS One. 2020;15:E0232170.
- Jena PK, Sheng L, Mcneil K, et al. Long-term Western diet intake leads to dysregulated bile acid signaling and dermatitis with Th2 and Th17 pathway features in mice. J Dermatol Sci. 2019;95:13-20.
- Grieger JA, Clifton VL, Tuck AR, et al. In utero programming of allergic susceptibility. Int Arch Allergy Immunol. 2016;169:80-92. doi:10.1159/000443961
- Khan TK, Palmer DJ, Prescott SL. In-utero exposures and the evolving epidemiology of paediatric allergy. Curr Opin Allergy Clin Immunol. 2015;15:402-408. doi:10.1097/ACI.0000000000000209
- Bauer SM. Atopic eczema: genetic associations and potential links to developmental exposures. Int J Toxicol. 2017;36:187-198.
- Shinohara M, Saito H, Matsumoto K. Different timings of prenatal or postnatal tobacco smoke exposure have different effects on the development of atopic eczema/dermatitis syndrome (AEDS) during infancy. J Allergy Clin Immunol. 2012;129:AB40.
- Lerodiakonou D, Garcia-Larsen V, Logan A, et al. Timing of allergenic food introduction to the infant diet and risk of allergic or autoimmune disease: a systematic review and meta-analysis. JAMA. 2016;316:1181-1192.
- Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
- Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Evid Based Child Health. 2014;9:447-483.
- Garcia-Larsen V, Ierodiakonou D, Jarrold K, et al. Diet during pregnancy and infancy and risk of allergic or autoimmune disease: a systematic review and meta-analysis. PLoS Med. 2018;15:E1002507.
- Greer FR, Sicherer SH, Burks AW; Committee on Nutrition, Section on Allergy and Immunology. The effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. 2019;143:e20190281.
- Baquerizo Nole KL, Yim E, Keri JE. Probiotics and prebiotics in dermatology. J Am Acad Dermatol. 2014;71:814-821.
- Schultz M, Göttl C, Young RJ, et al. Administration of oral probiotic bacteria to pregnant women causes temporary infantile colonization. J Pediatr Gastroenterol Nutr. 2004;38:293-297.
- Lee J, Seto D, Bielory L. Meta-analysis of clinical trials of probiotics for prevention and treatment of pediatric atopic dermatitis. J Allergy Clin Immunol. 2008;121:116-121.
- Panduru M, Panduru NM, Sa˘la˘va˘stru CM, et al. Probiotics and primary prevention of atopic dermatitis: a meta‐analysis of randomized controlled studies. J Eur Acad Dermatol Venereol. 2015;29:232-242.
- Doege K, Grajecki D, Zyriax BC, et al. Impact of maternal supplementation with probiotics during pregnancy on atopic eczema in childhood—a meta-analysis. Br J Nutr. 2012;107:1-6.
- Zuccotti G, Meneghin F, Aceti A, et al. Probiotics for prevention of atopic diseases in infants: systematic review and meta‐analysis. Allergy. 2015;70:1356-1371.
- Seaton A, Godden DJ, Brown K. Increase in asthma: a more toxic environment or a more susceptible population? Thorax. 1994;49:171-174.
- Manzel A, Muller DN, Hafler DA, et al. Role of “Western diet” in inflammatory autoimmune diseases. Curr Allergy Asthma Rep. 2014;14:1-8.
- Li-Weber M, Giasisi M, Trieber MK, et al. Vitamin E inhibits IL-4 gene expression in peripheral blood T cells. Eur J Immunol. 2002;32:2401-2408.
- Sehra S, Yao Y, Howell MD, et al. IL-4 regulates skin homeostasis and the predisposition toward allergic skin inflammation. J Immunol. 2010;184:3186-3190.
- West CE, Dunstan J, McCarthy S, et al. Associations between maternal antioxidant intakes in pregnancy and infant allergic outcomes. Nutrients. 2012;4:1747-1758.
- Miyake Y, Sasaki S, Tanaka K, et al. Consumption of vegetables, fruit, and antioxidants during pregnancy and wheeze and eczema in infants. Allergy. 2010;65:758-765.
- Martindale S, McNeill G, Devereux G, et al. Antioxidant intake in pregnancy in relation to wheeze and eczema in the first two years of life. Am J Respir Crit Care Med. 2005;171:121-128.
- Robison R, Kumar R. The effect of prenatal and postnatal dietary exposures on childhood development of atopic disease. Curr Opin Allergy Clin Immunol. 2010;10:139-144.
- Berdnikovs S, Abdala-Valencia H, McCary C, et al. Isoforms of vitamin E have opposing immunoregulatory functions during inflammation by regulating leukocyte recruitment. J Immunol. 2009;182:4395-4405.
- Beckhaus AA, Garcia‐Marcos L, Forno E, et al. Maternal nutrition during pregnancy and risk of asthma, wheeze, and atopic diseases during childhood: a systematic review and meta‐analysis. Allergy. 2015;70:1588-1604.
- Calder PC, Miles EA. Fatty acids and atopic disease. Pediatr Allergy Immunol. 2000;11(suppl 13):29-36.
- Prescott S, Macaubas C, Holt B, et al. Transplacental priming of the human immune system to environmental allergens: universal skewing of initial T-cell responses towards Th-2 cytokine profile. J Immunol. 1998;160:4730-4737.
- Dunstan JA, Mori TA, Barden A, et al. Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: a randomized, controlled trial. J Allergy Clin Immunol. 2003;112:1178-1184.
- Furuhjelm C, Warstedt K, Fagerås M, et al. Allergic disease in infants up to 2 years of age in relation to plasma omega‐3 fatty acids and maternal fish oil supplementation in pregnancy and lactation. Pediatr Allergy Immunol. 2011;22:505-514.
- Sausenthaler S, Koletzko S, Schaaf B, et al; LISA Study Group. Maternal diet during pregnancy in relation to eczema and allergic sensitization in the offspring at 2 y of age. Am J Clin Nutr. 2007;85:530-537.
- Best KP, Sullivan TR, Palmer DJ, et al. Prenatal omega-3 LCPUFA and symptoms of allergic disease and sensitization throughout early childhood—a longitudinal analysis of long-term follow-up of a randomized controlled trial. World Allergy Organ J. 2018;11:10.
- Jacobs DR Jr, Steffen LM. Nutrients, foods, and dietary patterns as exposures in research: a framework for food synergy. Am J Clin Nutr. 2003;78:508-513.
- Chatzi L, Torrent M, Romieu I, et al. Mediterranean diet in pregnancy is protective for wheeze and atopy in childhood. Thorax. 2008;63:507-513.
- Chatzi L, Garcia R, Roumeliotaki T, et al. Mediterranean diet adherence during pregnancy and risk of wheeze and eczema in the first year of life: INMA (Spain) and RHEA (Greece) mother-child cohort studies. Br J Nutr. 2013;110:2058-2068.
- Zeng J, Wu W, Chen Y, et al. Maternal dietary protein patterns during pregnancy and the risk of infant eczema: a cohort study. Front Nutr. 2021;8:294.
- Miyake Y, Okubo H, Sasaki S, et al. Maternal dietary patterns during pregnancy and risk of wheeze and eczema in Japanese infants aged 16–24 months: the Osaka Maternal and Child Health Study. Pediatr Allergy Immunol. 2011;22:734-741.
- Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66(suppl 1):8-16.
- Kapoor R, Menon C, Hoffstad O, et al. The prevalence of atopic triad in children with physician-confirmed atopic dermatitis. J Am Acad Dermatol. 2008;58:68-73.
- Abuabara K, Magyari A, McCulloch CE, et al. Prevalence of atopic eczema among patients seen in primary care: data from the Health Improvement Network. Ann Intern Med. 2019;170:354-356.
- Belgrave DC, Granell R, Simpson A, et al. Developmental profiles of eczema, wheeze, and rhinitis: two population-based birth cohort studies. PLoS Medicine. 2014;11:E1001748.
- Aguilar D, Pinart M, Koppelman GH, et al. Computational analysis of multimorbidity between asthma, eczema and rhinitis. PloS One. 2017;12:E0179125.
- Deckers IA, McLean S, Linssen S, et al. Investigating international time trends in the incidence and prevalence of atopic eczema 1990-2010: a systematic review of epidemiological studies. PloS One. 2012;7:E39803.
- Williams H, Stewart A, von Mutius E, et al. Is eczema really on the increase worldwide? J Allergy Clin Immunol. 2008;121:947-954.
- Sullivan M, Silverberg NB. Current and emerging concepts in atopic dermatitis pathogenesis. Clin Dermatol. 2017;35:349-353.
- Best KP, Gold M, Kennedy D, et al. Omega-3 long-chain PUFA intake during pregnancy and allergic disease outcomes in the offspring: a systematic review and meta-analysis of observational studies and randomized controlled trials. Am J Clin Nutr. 2016;103:128-143.
- Zulyniak MA, de Souza RJ, Shaikh M, et al. Ethnic differences in maternal diet in pregnancy and infant eczema. PloS One. 2020;15:E0232170.
- Jena PK, Sheng L, Mcneil K, et al. Long-term Western diet intake leads to dysregulated bile acid signaling and dermatitis with Th2 and Th17 pathway features in mice. J Dermatol Sci. 2019;95:13-20.
- Grieger JA, Clifton VL, Tuck AR, et al. In utero programming of allergic susceptibility. Int Arch Allergy Immunol. 2016;169:80-92. doi:10.1159/000443961
- Khan TK, Palmer DJ, Prescott SL. In-utero exposures and the evolving epidemiology of paediatric allergy. Curr Opin Allergy Clin Immunol. 2015;15:402-408. doi:10.1097/ACI.0000000000000209
- Bauer SM. Atopic eczema: genetic associations and potential links to developmental exposures. Int J Toxicol. 2017;36:187-198.
- Shinohara M, Saito H, Matsumoto K. Different timings of prenatal or postnatal tobacco smoke exposure have different effects on the development of atopic eczema/dermatitis syndrome (AEDS) during infancy. J Allergy Clin Immunol. 2012;129:AB40.
- Lerodiakonou D, Garcia-Larsen V, Logan A, et al. Timing of allergenic food introduction to the infant diet and risk of allergic or autoimmune disease: a systematic review and meta-analysis. JAMA. 2016;316:1181-1192.
- Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
- Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Evid Based Child Health. 2014;9:447-483.
- Garcia-Larsen V, Ierodiakonou D, Jarrold K, et al. Diet during pregnancy and infancy and risk of allergic or autoimmune disease: a systematic review and meta-analysis. PLoS Med. 2018;15:E1002507.
- Greer FR, Sicherer SH, Burks AW; Committee on Nutrition, Section on Allergy and Immunology. The effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. 2019;143:e20190281.
- Baquerizo Nole KL, Yim E, Keri JE. Probiotics and prebiotics in dermatology. J Am Acad Dermatol. 2014;71:814-821.
- Schultz M, Göttl C, Young RJ, et al. Administration of oral probiotic bacteria to pregnant women causes temporary infantile colonization. J Pediatr Gastroenterol Nutr. 2004;38:293-297.
- Lee J, Seto D, Bielory L. Meta-analysis of clinical trials of probiotics for prevention and treatment of pediatric atopic dermatitis. J Allergy Clin Immunol. 2008;121:116-121.
- Panduru M, Panduru NM, Sa˘la˘va˘stru CM, et al. Probiotics and primary prevention of atopic dermatitis: a meta‐analysis of randomized controlled studies. J Eur Acad Dermatol Venereol. 2015;29:232-242.
- Doege K, Grajecki D, Zyriax BC, et al. Impact of maternal supplementation with probiotics during pregnancy on atopic eczema in childhood—a meta-analysis. Br J Nutr. 2012;107:1-6.
- Zuccotti G, Meneghin F, Aceti A, et al. Probiotics for prevention of atopic diseases in infants: systematic review and meta‐analysis. Allergy. 2015;70:1356-1371.
- Seaton A, Godden DJ, Brown K. Increase in asthma: a more toxic environment or a more susceptible population? Thorax. 1994;49:171-174.
- Manzel A, Muller DN, Hafler DA, et al. Role of “Western diet” in inflammatory autoimmune diseases. Curr Allergy Asthma Rep. 2014;14:1-8.
- Li-Weber M, Giasisi M, Trieber MK, et al. Vitamin E inhibits IL-4 gene expression in peripheral blood T cells. Eur J Immunol. 2002;32:2401-2408.
- Sehra S, Yao Y, Howell MD, et al. IL-4 regulates skin homeostasis and the predisposition toward allergic skin inflammation. J Immunol. 2010;184:3186-3190.
- West CE, Dunstan J, McCarthy S, et al. Associations between maternal antioxidant intakes in pregnancy and infant allergic outcomes. Nutrients. 2012;4:1747-1758.
- Miyake Y, Sasaki S, Tanaka K, et al. Consumption of vegetables, fruit, and antioxidants during pregnancy and wheeze and eczema in infants. Allergy. 2010;65:758-765.
- Martindale S, McNeill G, Devereux G, et al. Antioxidant intake in pregnancy in relation to wheeze and eczema in the first two years of life. Am J Respir Crit Care Med. 2005;171:121-128.
- Robison R, Kumar R. The effect of prenatal and postnatal dietary exposures on childhood development of atopic disease. Curr Opin Allergy Clin Immunol. 2010;10:139-144.
- Berdnikovs S, Abdala-Valencia H, McCary C, et al. Isoforms of vitamin E have opposing immunoregulatory functions during inflammation by regulating leukocyte recruitment. J Immunol. 2009;182:4395-4405.
- Beckhaus AA, Garcia‐Marcos L, Forno E, et al. Maternal nutrition during pregnancy and risk of asthma, wheeze, and atopic diseases during childhood: a systematic review and meta‐analysis. Allergy. 2015;70:1588-1604.
- Calder PC, Miles EA. Fatty acids and atopic disease. Pediatr Allergy Immunol. 2000;11(suppl 13):29-36.
- Prescott S, Macaubas C, Holt B, et al. Transplacental priming of the human immune system to environmental allergens: universal skewing of initial T-cell responses towards Th-2 cytokine profile. J Immunol. 1998;160:4730-4737.
- Dunstan JA, Mori TA, Barden A, et al. Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: a randomized, controlled trial. J Allergy Clin Immunol. 2003;112:1178-1184.
- Furuhjelm C, Warstedt K, Fagerås M, et al. Allergic disease in infants up to 2 years of age in relation to plasma omega‐3 fatty acids and maternal fish oil supplementation in pregnancy and lactation. Pediatr Allergy Immunol. 2011;22:505-514.
- Sausenthaler S, Koletzko S, Schaaf B, et al; LISA Study Group. Maternal diet during pregnancy in relation to eczema and allergic sensitization in the offspring at 2 y of age. Am J Clin Nutr. 2007;85:530-537.
- Best KP, Sullivan TR, Palmer DJ, et al. Prenatal omega-3 LCPUFA and symptoms of allergic disease and sensitization throughout early childhood—a longitudinal analysis of long-term follow-up of a randomized controlled trial. World Allergy Organ J. 2018;11:10.
- Jacobs DR Jr, Steffen LM. Nutrients, foods, and dietary patterns as exposures in research: a framework for food synergy. Am J Clin Nutr. 2003;78:508-513.
- Chatzi L, Torrent M, Romieu I, et al. Mediterranean diet in pregnancy is protective for wheeze and atopy in childhood. Thorax. 2008;63:507-513.
- Chatzi L, Garcia R, Roumeliotaki T, et al. Mediterranean diet adherence during pregnancy and risk of wheeze and eczema in the first year of life: INMA (Spain) and RHEA (Greece) mother-child cohort studies. Br J Nutr. 2013;110:2058-2068.
- Zeng J, Wu W, Chen Y, et al. Maternal dietary protein patterns during pregnancy and the risk of infant eczema: a cohort study. Front Nutr. 2021;8:294.
- Miyake Y, Okubo H, Sasaki S, et al. Maternal dietary patterns during pregnancy and risk of wheeze and eczema in Japanese infants aged 16–24 months: the Osaka Maternal and Child Health Study. Pediatr Allergy Immunol. 2011;22:734-741.
Practice Points
- The prevalence of atopic dermatitis (AD) has been increasing globally, with a marked increase in developed countries.
- Maternal dietary restriction is not recommended in pregnancy for the prevention of atopic disease in infancy and childhood based on the existing literature.
- There is mixed evidence to support probiotic supplementation in the prenatal period.
- The recommendations supporting antioxidant and fatty acid supplementation as well as specific prenatal diets for the prevention of AD in infants and children are limited due to the heterogeneity of study designs.
Managing overuse of food IgE panels: Multiple approaches needed
PHOENIX – For at least a decade, professional allergy and pediatrics societies have urged against using food IgE tests unless the patient has a history consistent with potential IgE-mediated food allergies. Yet virtually every health system offers these blood tests, and their inappropriate use – especially of panels that measure many allergens at once – remains a huge problem.
Beyond wasteful spending, excessive food IgE testing can lead patients to worry needlessly and to avoid foods they aren’t allergic to. For babies and toddlers, avoidance can drive up the risk of developing allergies to those foods later in life – a consequence that was convincingly proven by the LEAP study but has still not translated to a widespread change in practice.
“I think we all know that there’s just a lot of system-wide resistance to making these changes, and we don’t completely understand why,” Nicholas Hartog, MD, an allergist with Spectrum Health in Grand Rapids, Mich., told this news organization.
At the American Academy of Allergy, Asthma & Immunology annual meeting, one of Dr. Hartog’s residents, Courtney Cotter, DO, presented a poster detailing their team’s retrospective review of food panel ordering practices across Spectrum Health, a large, multispecialty physician group in west Michigan.
The team combed Epic health records to evaluate food IgE ordering from January 2016 to December 2021. They tracked monthly figures for the number of patients who underwent food IgE tests, the percentage of tested patients for whom food panels were available, and the number of food panels and total number of food IgE tests ordered. They compared average rates from the final 3 months with rates from the first 3 months, which predated the August 2016 establishment of an academic pediatric allergy/immunology department.
Initially, Dr. Hartog and his colleagues focused on educating doctors on appropriate use of food IgE tests through informal conversations and lectures, but, he said, “It’s really difficult to change physician behavior, so sometimes we have to go about it by making it hard to do the wrong thing.”
To that end, the team tried to eliminate the food panels. However, some lab staff feared the possibility of losing revenue if physicians decided to order these tests elsewhere. After more negotiations, the laboratory agreed in December 2019 to restrict and rework food IgE testing by dropping the number of panels from nine to two and by restricting the number of foods in those panels. For example, in the basic panel, “we limited it to just four allergens, so even if you order a panel, you’re not getting 20 results,” Dr. Hartog told this news organization. “I finally found a friendly pathologist who was very on board with this positive change.”
In December 2020, the team implemented yet another strategy: Epic alerts. Each time doctors request a food panel, they receive a pop-up message stating that panel tests are not recommended and asking if they wish to proceed.
The multipronged effort had a modest impact on the number of food panels ordered per month, which dipped from 112.7 to 84.7 for the first and last 3 months of the study. Monthly totals of individual food IgE tests showed a steeper drop, decreasing from 2,379 to 1,180 in the initial and final 3-month periods – a change Dr. Hartog attributes to the revamped food panels. They estimated the cost savings at around $40 per patient, “and we were getting on average about 200 patients a month, so it adds up,” he said.
But the Epic alerts seemed to have little effect. Over the duration of the study, the monthly number of IgE tests ordered per clinician did not change. Neither did the percentage of patients evaluated with a food panel. “The alerts pop up, but people are still ordering,” Dr. Hartog said.
On the whole, the analysis shows that, “despite major efforts to educate providers and the public about these things, it is rampantly disregarded and is a huge problem for our specialty and is likely causing harm to patients,” said allergist-immunologist Gerald Lee, MD, of Emory University in Atlanta.
Dr. Lee said that a common scenario for inappropriate food IgE testing is severe eczema. Many parents request blood tests because they assume their child’s skin condition is driven by food allergies. When the child turns up positive to various foods on panel tests, which have high false-positive rates, the physician may recommend eliminating those foods to improve the skin rash – which “actually delays introduction of the food and potentially increases the risk for food allergy,” Dr. Lee said. “That was a common practice when I was in fellowship (2011) and is widely prevalent today.”
Edwin Kim, MD, director of the UNC Food Allergy Initiative at the University of North Carolina at Chapel Hill, agrees that food IgE panels are wasteful and harmful. However, he thinks the solution is not to tell primary care physicians and pediatricians to stop using the tests. “We’re insinuating that they’re being used inappropriately, but the problem is that these are people that are patient facing, the patients are asking a question, and the appropriate tests aren’t there,” Dr. Kim said. “A big part of that problem is that the tests we have available to us are not good enough.”
The Spectrum Health analysis did not examine ICD codes associated with the food IgE tests or track which physicians ordered the tests. A 2016 retrospective review published in Pediatrics did evaluate ordering practices by specialty and found that primary care providers ordered “significantly more food allergen panels, tests for uncommon causes of food allergy, and generate higher cost per patient compared with allergists.”
Given the immense challenges with implementing system-wide changes, sometimes it can help to educate parents and families. “When you sit down and take 2 or 3 minutes to explain why this is a bad test and that I care about your kid but just don’t want inappropriate testing, they’re okay with it. They understand,” Dr. Hartog said. “When I teach residents, I make sure to emphasize that we have these conversations all the time.”
Dr. Hartog reports financial relationships with Binding Site (speaker), Regeneron (advisory board), Genentech (advisory board), Horizon Pharmaceuticals (advisory board, consulting, speaker), Takeda (speaker, advisory board) and Pharming Healthcare (advisory board, scientific steering committee, consulting), though none related to food allergy. Dr. Lee has disclosed no relevant financial relationships. Dr. Kim reports consultancy with Aimmune Therapeutics, Allako, AllerGenis, Belhaven Pharma, DBV Technologies, Duke Clinical Research Institute, and Nutricia; advisory board membership with ALK, DBV Technologies, Kenota Health, and Ukko; and grant support from the National Institute of Allergy and Infectious Diseases and the Immune Tolerance Network; the National Center for Complementary and Integrative Health; Food Allergy Research and Education; and the Wallace Research Foundation.
A version of this article first appeared on Medscape.com.
PHOENIX – For at least a decade, professional allergy and pediatrics societies have urged against using food IgE tests unless the patient has a history consistent with potential IgE-mediated food allergies. Yet virtually every health system offers these blood tests, and their inappropriate use – especially of panels that measure many allergens at once – remains a huge problem.
Beyond wasteful spending, excessive food IgE testing can lead patients to worry needlessly and to avoid foods they aren’t allergic to. For babies and toddlers, avoidance can drive up the risk of developing allergies to those foods later in life – a consequence that was convincingly proven by the LEAP study but has still not translated to a widespread change in practice.
“I think we all know that there’s just a lot of system-wide resistance to making these changes, and we don’t completely understand why,” Nicholas Hartog, MD, an allergist with Spectrum Health in Grand Rapids, Mich., told this news organization.
At the American Academy of Allergy, Asthma & Immunology annual meeting, one of Dr. Hartog’s residents, Courtney Cotter, DO, presented a poster detailing their team’s retrospective review of food panel ordering practices across Spectrum Health, a large, multispecialty physician group in west Michigan.
The team combed Epic health records to evaluate food IgE ordering from January 2016 to December 2021. They tracked monthly figures for the number of patients who underwent food IgE tests, the percentage of tested patients for whom food panels were available, and the number of food panels and total number of food IgE tests ordered. They compared average rates from the final 3 months with rates from the first 3 months, which predated the August 2016 establishment of an academic pediatric allergy/immunology department.
Initially, Dr. Hartog and his colleagues focused on educating doctors on appropriate use of food IgE tests through informal conversations and lectures, but, he said, “It’s really difficult to change physician behavior, so sometimes we have to go about it by making it hard to do the wrong thing.”
To that end, the team tried to eliminate the food panels. However, some lab staff feared the possibility of losing revenue if physicians decided to order these tests elsewhere. After more negotiations, the laboratory agreed in December 2019 to restrict and rework food IgE testing by dropping the number of panels from nine to two and by restricting the number of foods in those panels. For example, in the basic panel, “we limited it to just four allergens, so even if you order a panel, you’re not getting 20 results,” Dr. Hartog told this news organization. “I finally found a friendly pathologist who was very on board with this positive change.”
In December 2020, the team implemented yet another strategy: Epic alerts. Each time doctors request a food panel, they receive a pop-up message stating that panel tests are not recommended and asking if they wish to proceed.
The multipronged effort had a modest impact on the number of food panels ordered per month, which dipped from 112.7 to 84.7 for the first and last 3 months of the study. Monthly totals of individual food IgE tests showed a steeper drop, decreasing from 2,379 to 1,180 in the initial and final 3-month periods – a change Dr. Hartog attributes to the revamped food panels. They estimated the cost savings at around $40 per patient, “and we were getting on average about 200 patients a month, so it adds up,” he said.
But the Epic alerts seemed to have little effect. Over the duration of the study, the monthly number of IgE tests ordered per clinician did not change. Neither did the percentage of patients evaluated with a food panel. “The alerts pop up, but people are still ordering,” Dr. Hartog said.
On the whole, the analysis shows that, “despite major efforts to educate providers and the public about these things, it is rampantly disregarded and is a huge problem for our specialty and is likely causing harm to patients,” said allergist-immunologist Gerald Lee, MD, of Emory University in Atlanta.
Dr. Lee said that a common scenario for inappropriate food IgE testing is severe eczema. Many parents request blood tests because they assume their child’s skin condition is driven by food allergies. When the child turns up positive to various foods on panel tests, which have high false-positive rates, the physician may recommend eliminating those foods to improve the skin rash – which “actually delays introduction of the food and potentially increases the risk for food allergy,” Dr. Lee said. “That was a common practice when I was in fellowship (2011) and is widely prevalent today.”
Edwin Kim, MD, director of the UNC Food Allergy Initiative at the University of North Carolina at Chapel Hill, agrees that food IgE panels are wasteful and harmful. However, he thinks the solution is not to tell primary care physicians and pediatricians to stop using the tests. “We’re insinuating that they’re being used inappropriately, but the problem is that these are people that are patient facing, the patients are asking a question, and the appropriate tests aren’t there,” Dr. Kim said. “A big part of that problem is that the tests we have available to us are not good enough.”
The Spectrum Health analysis did not examine ICD codes associated with the food IgE tests or track which physicians ordered the tests. A 2016 retrospective review published in Pediatrics did evaluate ordering practices by specialty and found that primary care providers ordered “significantly more food allergen panels, tests for uncommon causes of food allergy, and generate higher cost per patient compared with allergists.”
Given the immense challenges with implementing system-wide changes, sometimes it can help to educate parents and families. “When you sit down and take 2 or 3 minutes to explain why this is a bad test and that I care about your kid but just don’t want inappropriate testing, they’re okay with it. They understand,” Dr. Hartog said. “When I teach residents, I make sure to emphasize that we have these conversations all the time.”
Dr. Hartog reports financial relationships with Binding Site (speaker), Regeneron (advisory board), Genentech (advisory board), Horizon Pharmaceuticals (advisory board, consulting, speaker), Takeda (speaker, advisory board) and Pharming Healthcare (advisory board, scientific steering committee, consulting), though none related to food allergy. Dr. Lee has disclosed no relevant financial relationships. Dr. Kim reports consultancy with Aimmune Therapeutics, Allako, AllerGenis, Belhaven Pharma, DBV Technologies, Duke Clinical Research Institute, and Nutricia; advisory board membership with ALK, DBV Technologies, Kenota Health, and Ukko; and grant support from the National Institute of Allergy and Infectious Diseases and the Immune Tolerance Network; the National Center for Complementary and Integrative Health; Food Allergy Research and Education; and the Wallace Research Foundation.
A version of this article first appeared on Medscape.com.
PHOENIX – For at least a decade, professional allergy and pediatrics societies have urged against using food IgE tests unless the patient has a history consistent with potential IgE-mediated food allergies. Yet virtually every health system offers these blood tests, and their inappropriate use – especially of panels that measure many allergens at once – remains a huge problem.
Beyond wasteful spending, excessive food IgE testing can lead patients to worry needlessly and to avoid foods they aren’t allergic to. For babies and toddlers, avoidance can drive up the risk of developing allergies to those foods later in life – a consequence that was convincingly proven by the LEAP study but has still not translated to a widespread change in practice.
“I think we all know that there’s just a lot of system-wide resistance to making these changes, and we don’t completely understand why,” Nicholas Hartog, MD, an allergist with Spectrum Health in Grand Rapids, Mich., told this news organization.
At the American Academy of Allergy, Asthma & Immunology annual meeting, one of Dr. Hartog’s residents, Courtney Cotter, DO, presented a poster detailing their team’s retrospective review of food panel ordering practices across Spectrum Health, a large, multispecialty physician group in west Michigan.
The team combed Epic health records to evaluate food IgE ordering from January 2016 to December 2021. They tracked monthly figures for the number of patients who underwent food IgE tests, the percentage of tested patients for whom food panels were available, and the number of food panels and total number of food IgE tests ordered. They compared average rates from the final 3 months with rates from the first 3 months, which predated the August 2016 establishment of an academic pediatric allergy/immunology department.
Initially, Dr. Hartog and his colleagues focused on educating doctors on appropriate use of food IgE tests through informal conversations and lectures, but, he said, “It’s really difficult to change physician behavior, so sometimes we have to go about it by making it hard to do the wrong thing.”
To that end, the team tried to eliminate the food panels. However, some lab staff feared the possibility of losing revenue if physicians decided to order these tests elsewhere. After more negotiations, the laboratory agreed in December 2019 to restrict and rework food IgE testing by dropping the number of panels from nine to two and by restricting the number of foods in those panels. For example, in the basic panel, “we limited it to just four allergens, so even if you order a panel, you’re not getting 20 results,” Dr. Hartog told this news organization. “I finally found a friendly pathologist who was very on board with this positive change.”
In December 2020, the team implemented yet another strategy: Epic alerts. Each time doctors request a food panel, they receive a pop-up message stating that panel tests are not recommended and asking if they wish to proceed.
The multipronged effort had a modest impact on the number of food panels ordered per month, which dipped from 112.7 to 84.7 for the first and last 3 months of the study. Monthly totals of individual food IgE tests showed a steeper drop, decreasing from 2,379 to 1,180 in the initial and final 3-month periods – a change Dr. Hartog attributes to the revamped food panels. They estimated the cost savings at around $40 per patient, “and we were getting on average about 200 patients a month, so it adds up,” he said.
But the Epic alerts seemed to have little effect. Over the duration of the study, the monthly number of IgE tests ordered per clinician did not change. Neither did the percentage of patients evaluated with a food panel. “The alerts pop up, but people are still ordering,” Dr. Hartog said.
On the whole, the analysis shows that, “despite major efforts to educate providers and the public about these things, it is rampantly disregarded and is a huge problem for our specialty and is likely causing harm to patients,” said allergist-immunologist Gerald Lee, MD, of Emory University in Atlanta.
Dr. Lee said that a common scenario for inappropriate food IgE testing is severe eczema. Many parents request blood tests because they assume their child’s skin condition is driven by food allergies. When the child turns up positive to various foods on panel tests, which have high false-positive rates, the physician may recommend eliminating those foods to improve the skin rash – which “actually delays introduction of the food and potentially increases the risk for food allergy,” Dr. Lee said. “That was a common practice when I was in fellowship (2011) and is widely prevalent today.”
Edwin Kim, MD, director of the UNC Food Allergy Initiative at the University of North Carolina at Chapel Hill, agrees that food IgE panels are wasteful and harmful. However, he thinks the solution is not to tell primary care physicians and pediatricians to stop using the tests. “We’re insinuating that they’re being used inappropriately, but the problem is that these are people that are patient facing, the patients are asking a question, and the appropriate tests aren’t there,” Dr. Kim said. “A big part of that problem is that the tests we have available to us are not good enough.”
The Spectrum Health analysis did not examine ICD codes associated with the food IgE tests or track which physicians ordered the tests. A 2016 retrospective review published in Pediatrics did evaluate ordering practices by specialty and found that primary care providers ordered “significantly more food allergen panels, tests for uncommon causes of food allergy, and generate higher cost per patient compared with allergists.”
Given the immense challenges with implementing system-wide changes, sometimes it can help to educate parents and families. “When you sit down and take 2 or 3 minutes to explain why this is a bad test and that I care about your kid but just don’t want inappropriate testing, they’re okay with it. They understand,” Dr. Hartog said. “When I teach residents, I make sure to emphasize that we have these conversations all the time.”
Dr. Hartog reports financial relationships with Binding Site (speaker), Regeneron (advisory board), Genentech (advisory board), Horizon Pharmaceuticals (advisory board, consulting, speaker), Takeda (speaker, advisory board) and Pharming Healthcare (advisory board, scientific steering committee, consulting), though none related to food allergy. Dr. Lee has disclosed no relevant financial relationships. Dr. Kim reports consultancy with Aimmune Therapeutics, Allako, AllerGenis, Belhaven Pharma, DBV Technologies, Duke Clinical Research Institute, and Nutricia; advisory board membership with ALK, DBV Technologies, Kenota Health, and Ukko; and grant support from the National Institute of Allergy and Infectious Diseases and the Immune Tolerance Network; the National Center for Complementary and Integrative Health; Food Allergy Research and Education; and the Wallace Research Foundation.
A version of this article first appeared on Medscape.com.
Patch Testing on Dupilumab: Reliable or Not?
In patients with persistent atopic dermatitis (AD) who are taking dupilumab, is there benefit of patch testing to determine if allergic contact dermatitis (ACD) also is contributing to their disease? Results of patch testing are likely be influenced by the immunomodulatory effects of dupilumab. Similar to the recommendation for patients to refrain from using topical or systemic corticosteroids for 1 week or more prior to patch testing to eliminate false negatives, we reviewed the literature to create practice guidelines for dermatologists regarding patch testing while a patient is taking dupilumab.
Pathophysiology and Pathomechanism
Dupilumab functions through the blockade of T helper 2 (TH2) cells; ACD is propagated through the T helper 1 (TH1) cellular pathway. However, patients with ACD that is unresponsive to allergen avoidance and traditional therapies, such as topical and oral corticosteroids, have responded to dupilumab. The more common reports of this responsiveness are with fragrances; multiple case series described patients with ACD to fragrance mix I1 and balsam of Peru1,2 who improved on dupilumab when other treatments failed. There also are reports of response when ACD was secondary to nickel,2,3p-phenylenediamine,1 Compositae,4 and non–formaldehyde-releasing preservatives (non-FRPs).5 Therefore, not all ACD is propagated through the TH1 cellular pathway.
As noted in these cases, ACD can be a response to an allergen whose pathogenesis involves the TH2 pathway or when patient characteristics favor a TH2 response. It has been suggested that AD patients are more susceptible to TH2-mediated contact sensitization to less-potent allergens, such as fragrances.6
Patch Test Results
Positive patch test results for allergens have been reported while patients are on dupilumab therapy, including a few studies in which results prior to starting dupilumab were compared with those while patients were on dupilumab therapy. In a retrospective chart review of 48 patients on dupilumab for AD with persistent disease, 23 patients were patch tested before and during dupilumab therapy. In these patients, the majority of contact allergies were persistent and only 10% (13/125) of patch test–positive results resolved on dupilumab therapy.7 Contact allergies that resolved included those to emulsifiers (propylene glycol, Amerchol L101 [lanolin-containing products found in cosmetics and other goods], dimethylaminopropylamine), fragrances (fragrance mix I, balsam of Peru), sunscreens (sulisobenzone, phenylbenzimidazole-5-sulfonic acid), and metals (vanadium chloride, phenylmercuric acetate).7 The following results observed in individual cases demonstrated conflicting findings: persistence of allergy to non-FRPs (methylisothiazolinone [MI]) but resolution of allergy to formaldehyde8; persistence of allergy to corticosteroids (budesonide and alclometasone)9; persistence of allergy to an antibiotic (neomycin sulfate) but resolution of allergies to a different antibiotic (bacitracin), glues (ethyl acrylate), bleach, and glutaraldehyde9; persistence of nickel allergy but resolution of allergies to fragrances (cinnamic aldehyde, balsam of Peru) and non-FRPs (methylchloroisothiazolinone or MI)10; and persistence of allergies to non-FRPs (MI) and FRPs (bronopol) but resolution of allergies to nickel, fragrances (hydroperoxides of linalool), and Compositae.11 Additional case reports of positive patch test results while on dupilumab but with no pretreatment results for comparison include allergies to rubber additives,12-14 nickel,14 textile dyes,14 cosmetic and hair care additives,12,14,15 corticosteroids,15 FRPs,15 fragrances,15,16 emulsifiers,16 and non-FRPs.17
An evident theme in the dupilumab patch-testing literature has been that results are variable and case specific: a given patient with ACD to an allergen will respond to dupilumab treatment and have subsequent negative patch testing, while another patient will not respond to dupilumab treatment and have persistent positive patch testing. This is likely because, in certain individuals, the allergen-immune system combination shifts ACD pathogenesis from a purely TH1 response to at least a partial TH2 response, thus allowing for benefit from dupilumab therapy. T helper 1 cell–mediated ACD should not be affected by dupilumab; therefore, reliable results can be elucidated from patch testing despite the drug.
Final Thoughts
We propose that AD patients with residual disease after taking dupilumab undergo patch testing. Positive results indicate allergens that are not inhibited by the drug. Patients will need to follow strict allergen avoidance to resolve this component of their disease; failure to improve might suggest the result was a nonrelevant positive.
If patch testing is negative, an alternative cause for residual disease must be sought. We do not recommend stopping dupilumab prior to patch testing to avoid a disease flare from AD or possible TH2-mediated ACD.
- Chipalkatti N, Lee N, Zancanaro P, et al. Dupilumab as a treatment for allergic contact dermatitis. Dermatitis. 2018;29:347-348. doi:10.1097/DER.0000000000000414
- Jacob SE, Sung CT, Machler BC. Dupilumab for systemic allergy syndrome with dermatitis. Dermatitis. 2019;30:164-167. doi:10.1097/DER.0000000000000446
- Joshi SR, Khan DA. Effective use of dupilumab in managing systemic allergic contact dermatitis. Dermatitis. 2018;29:282-284. doi:10.1097/DER.0000000000000409
- Ruge IF, Skov L, Zachariae C, et al. Dupilumab treatment in two patients with severe allergic contact dermatitis caused by sesquiterpene lactones. Contact Dermatitis. 2020:83;137-139. doi:10.1111/cod.13545
- Goldminz AM, Scheinman PL. A case series of dupilumab-treated allergic contact dermatitis patients. Dermatol Ther. 2018;31:e12701. doi:10.1111/dth.12701
- Kohli N, Nedorost S. Inflamed skin predisposes to sensitization to less potent allergens. J Am Acad Dermatol. 2016;75:312-317. doi:10.1016/j.jaad.2016.03.010
- Raffi J, Suresh R, Botto N, et al. The impact of dupilumab on patch testing and the prevalence of comorbid allergic contact dermatitis in recalcitrant atopic dermatitis: a retrospective chart review. J Am Acad Dermatol. 2020;82:132-138. doi:10.1016/j.jaad.2019.09.028
- Puza CJ, Atwater AR. Positive patch test reaction in a patient taking dupilumab. Dermatitis. 2018;29:89. doi:10.1097/DER.0000000000000346
- Suresh R, Murase JE. The role of expanded series patch testing in identifying causality of residual facial dermatitis following initiation of dupilumab therapy. JAAD Case Rep. 2018;4:899-904. doi:10.1016/j.jdcr.2018.08.027
- Stout M, Silverberg JI. Variable impact of dupilumab on patch testing results and allergic contact dermatitis in adults with atopic dermatitis. J Am Acad Dermatol. 2019;81:157-162. doi:10.1016/j.jaad.2019.03.020
- Raffi J, Botto N. Patch testing and allergen-specific inhibition in a patient taking dupilumab. JAMA Dermatol. 2019;155:120-121. doi:10.1001/jamadermatol.2018.4098
- Hoot JW, Douglas JD, Falo LD Jr. Patch testing in a patient on dupilumab. Dermatitis. 2018;29:164. doi:10.1097/DER.0000000000000357
- Crepy M-N, Nosbaum A, Bensefa-Colas L. Blocking type 2 inflammation by dupilumab does not control classic (type 1-driven) allergic contact dermatitis in chronic hand eczema. Contact Dermatitis. 2019;81:145-147. doi:10.1111/cod.13266
- Raffi J, Chen R, Botto N. Wide dye reactors. JAAD Case Rep. 2019;5:877-879. doi:10.1016/j.jdcr.2019.08.005
- Koblinski JE, Hamann D. Mixed occupational and iatrogenic allergic contact dermatitis in a hairdresser. Occup Med (Lond). 2020;70:523-526. doi:10.1093/occmed/kqaa152
- Raffi J, Suresh R, Fishman H, et al. Investigating the role of allergic contact dermatitis in residual ocular surface disease on dupilumab (ROSDD). Int J Womens Dermatol. 2019;5:308-313. doi:10.1016/j.ijwd.2019.10.001
- Zhu GA, Chen JK, Chiou A, et al. Repeat patch testing in a patient with allergic contact dermatitis improved on dupilumab. JAAD Case Rep. 2019;5:336-338. doi:10.1016/j.jdcr.2019.01.023
In patients with persistent atopic dermatitis (AD) who are taking dupilumab, is there benefit of patch testing to determine if allergic contact dermatitis (ACD) also is contributing to their disease? Results of patch testing are likely be influenced by the immunomodulatory effects of dupilumab. Similar to the recommendation for patients to refrain from using topical or systemic corticosteroids for 1 week or more prior to patch testing to eliminate false negatives, we reviewed the literature to create practice guidelines for dermatologists regarding patch testing while a patient is taking dupilumab.
Pathophysiology and Pathomechanism
Dupilumab functions through the blockade of T helper 2 (TH2) cells; ACD is propagated through the T helper 1 (TH1) cellular pathway. However, patients with ACD that is unresponsive to allergen avoidance and traditional therapies, such as topical and oral corticosteroids, have responded to dupilumab. The more common reports of this responsiveness are with fragrances; multiple case series described patients with ACD to fragrance mix I1 and balsam of Peru1,2 who improved on dupilumab when other treatments failed. There also are reports of response when ACD was secondary to nickel,2,3p-phenylenediamine,1 Compositae,4 and non–formaldehyde-releasing preservatives (non-FRPs).5 Therefore, not all ACD is propagated through the TH1 cellular pathway.
As noted in these cases, ACD can be a response to an allergen whose pathogenesis involves the TH2 pathway or when patient characteristics favor a TH2 response. It has been suggested that AD patients are more susceptible to TH2-mediated contact sensitization to less-potent allergens, such as fragrances.6
Patch Test Results
Positive patch test results for allergens have been reported while patients are on dupilumab therapy, including a few studies in which results prior to starting dupilumab were compared with those while patients were on dupilumab therapy. In a retrospective chart review of 48 patients on dupilumab for AD with persistent disease, 23 patients were patch tested before and during dupilumab therapy. In these patients, the majority of contact allergies were persistent and only 10% (13/125) of patch test–positive results resolved on dupilumab therapy.7 Contact allergies that resolved included those to emulsifiers (propylene glycol, Amerchol L101 [lanolin-containing products found in cosmetics and other goods], dimethylaminopropylamine), fragrances (fragrance mix I, balsam of Peru), sunscreens (sulisobenzone, phenylbenzimidazole-5-sulfonic acid), and metals (vanadium chloride, phenylmercuric acetate).7 The following results observed in individual cases demonstrated conflicting findings: persistence of allergy to non-FRPs (methylisothiazolinone [MI]) but resolution of allergy to formaldehyde8; persistence of allergy to corticosteroids (budesonide and alclometasone)9; persistence of allergy to an antibiotic (neomycin sulfate) but resolution of allergies to a different antibiotic (bacitracin), glues (ethyl acrylate), bleach, and glutaraldehyde9; persistence of nickel allergy but resolution of allergies to fragrances (cinnamic aldehyde, balsam of Peru) and non-FRPs (methylchloroisothiazolinone or MI)10; and persistence of allergies to non-FRPs (MI) and FRPs (bronopol) but resolution of allergies to nickel, fragrances (hydroperoxides of linalool), and Compositae.11 Additional case reports of positive patch test results while on dupilumab but with no pretreatment results for comparison include allergies to rubber additives,12-14 nickel,14 textile dyes,14 cosmetic and hair care additives,12,14,15 corticosteroids,15 FRPs,15 fragrances,15,16 emulsifiers,16 and non-FRPs.17
An evident theme in the dupilumab patch-testing literature has been that results are variable and case specific: a given patient with ACD to an allergen will respond to dupilumab treatment and have subsequent negative patch testing, while another patient will not respond to dupilumab treatment and have persistent positive patch testing. This is likely because, in certain individuals, the allergen-immune system combination shifts ACD pathogenesis from a purely TH1 response to at least a partial TH2 response, thus allowing for benefit from dupilumab therapy. T helper 1 cell–mediated ACD should not be affected by dupilumab; therefore, reliable results can be elucidated from patch testing despite the drug.
Final Thoughts
We propose that AD patients with residual disease after taking dupilumab undergo patch testing. Positive results indicate allergens that are not inhibited by the drug. Patients will need to follow strict allergen avoidance to resolve this component of their disease; failure to improve might suggest the result was a nonrelevant positive.
If patch testing is negative, an alternative cause for residual disease must be sought. We do not recommend stopping dupilumab prior to patch testing to avoid a disease flare from AD or possible TH2-mediated ACD.
In patients with persistent atopic dermatitis (AD) who are taking dupilumab, is there benefit of patch testing to determine if allergic contact dermatitis (ACD) also is contributing to their disease? Results of patch testing are likely be influenced by the immunomodulatory effects of dupilumab. Similar to the recommendation for patients to refrain from using topical or systemic corticosteroids for 1 week or more prior to patch testing to eliminate false negatives, we reviewed the literature to create practice guidelines for dermatologists regarding patch testing while a patient is taking dupilumab.
Pathophysiology and Pathomechanism
Dupilumab functions through the blockade of T helper 2 (TH2) cells; ACD is propagated through the T helper 1 (TH1) cellular pathway. However, patients with ACD that is unresponsive to allergen avoidance and traditional therapies, such as topical and oral corticosteroids, have responded to dupilumab. The more common reports of this responsiveness are with fragrances; multiple case series described patients with ACD to fragrance mix I1 and balsam of Peru1,2 who improved on dupilumab when other treatments failed. There also are reports of response when ACD was secondary to nickel,2,3p-phenylenediamine,1 Compositae,4 and non–formaldehyde-releasing preservatives (non-FRPs).5 Therefore, not all ACD is propagated through the TH1 cellular pathway.
As noted in these cases, ACD can be a response to an allergen whose pathogenesis involves the TH2 pathway or when patient characteristics favor a TH2 response. It has been suggested that AD patients are more susceptible to TH2-mediated contact sensitization to less-potent allergens, such as fragrances.6
Patch Test Results
Positive patch test results for allergens have been reported while patients are on dupilumab therapy, including a few studies in which results prior to starting dupilumab were compared with those while patients were on dupilumab therapy. In a retrospective chart review of 48 patients on dupilumab for AD with persistent disease, 23 patients were patch tested before and during dupilumab therapy. In these patients, the majority of contact allergies were persistent and only 10% (13/125) of patch test–positive results resolved on dupilumab therapy.7 Contact allergies that resolved included those to emulsifiers (propylene glycol, Amerchol L101 [lanolin-containing products found in cosmetics and other goods], dimethylaminopropylamine), fragrances (fragrance mix I, balsam of Peru), sunscreens (sulisobenzone, phenylbenzimidazole-5-sulfonic acid), and metals (vanadium chloride, phenylmercuric acetate).7 The following results observed in individual cases demonstrated conflicting findings: persistence of allergy to non-FRPs (methylisothiazolinone [MI]) but resolution of allergy to formaldehyde8; persistence of allergy to corticosteroids (budesonide and alclometasone)9; persistence of allergy to an antibiotic (neomycin sulfate) but resolution of allergies to a different antibiotic (bacitracin), glues (ethyl acrylate), bleach, and glutaraldehyde9; persistence of nickel allergy but resolution of allergies to fragrances (cinnamic aldehyde, balsam of Peru) and non-FRPs (methylchloroisothiazolinone or MI)10; and persistence of allergies to non-FRPs (MI) and FRPs (bronopol) but resolution of allergies to nickel, fragrances (hydroperoxides of linalool), and Compositae.11 Additional case reports of positive patch test results while on dupilumab but with no pretreatment results for comparison include allergies to rubber additives,12-14 nickel,14 textile dyes,14 cosmetic and hair care additives,12,14,15 corticosteroids,15 FRPs,15 fragrances,15,16 emulsifiers,16 and non-FRPs.17
An evident theme in the dupilumab patch-testing literature has been that results are variable and case specific: a given patient with ACD to an allergen will respond to dupilumab treatment and have subsequent negative patch testing, while another patient will not respond to dupilumab treatment and have persistent positive patch testing. This is likely because, in certain individuals, the allergen-immune system combination shifts ACD pathogenesis from a purely TH1 response to at least a partial TH2 response, thus allowing for benefit from dupilumab therapy. T helper 1 cell–mediated ACD should not be affected by dupilumab; therefore, reliable results can be elucidated from patch testing despite the drug.
Final Thoughts
We propose that AD patients with residual disease after taking dupilumab undergo patch testing. Positive results indicate allergens that are not inhibited by the drug. Patients will need to follow strict allergen avoidance to resolve this component of their disease; failure to improve might suggest the result was a nonrelevant positive.
If patch testing is negative, an alternative cause for residual disease must be sought. We do not recommend stopping dupilumab prior to patch testing to avoid a disease flare from AD or possible TH2-mediated ACD.
- Chipalkatti N, Lee N, Zancanaro P, et al. Dupilumab as a treatment for allergic contact dermatitis. Dermatitis. 2018;29:347-348. doi:10.1097/DER.0000000000000414
- Jacob SE, Sung CT, Machler BC. Dupilumab for systemic allergy syndrome with dermatitis. Dermatitis. 2019;30:164-167. doi:10.1097/DER.0000000000000446
- Joshi SR, Khan DA. Effective use of dupilumab in managing systemic allergic contact dermatitis. Dermatitis. 2018;29:282-284. doi:10.1097/DER.0000000000000409
- Ruge IF, Skov L, Zachariae C, et al. Dupilumab treatment in two patients with severe allergic contact dermatitis caused by sesquiterpene lactones. Contact Dermatitis. 2020:83;137-139. doi:10.1111/cod.13545
- Goldminz AM, Scheinman PL. A case series of dupilumab-treated allergic contact dermatitis patients. Dermatol Ther. 2018;31:e12701. doi:10.1111/dth.12701
- Kohli N, Nedorost S. Inflamed skin predisposes to sensitization to less potent allergens. J Am Acad Dermatol. 2016;75:312-317. doi:10.1016/j.jaad.2016.03.010
- Raffi J, Suresh R, Botto N, et al. The impact of dupilumab on patch testing and the prevalence of comorbid allergic contact dermatitis in recalcitrant atopic dermatitis: a retrospective chart review. J Am Acad Dermatol. 2020;82:132-138. doi:10.1016/j.jaad.2019.09.028
- Puza CJ, Atwater AR. Positive patch test reaction in a patient taking dupilumab. Dermatitis. 2018;29:89. doi:10.1097/DER.0000000000000346
- Suresh R, Murase JE. The role of expanded series patch testing in identifying causality of residual facial dermatitis following initiation of dupilumab therapy. JAAD Case Rep. 2018;4:899-904. doi:10.1016/j.jdcr.2018.08.027
- Stout M, Silverberg JI. Variable impact of dupilumab on patch testing results and allergic contact dermatitis in adults with atopic dermatitis. J Am Acad Dermatol. 2019;81:157-162. doi:10.1016/j.jaad.2019.03.020
- Raffi J, Botto N. Patch testing and allergen-specific inhibition in a patient taking dupilumab. JAMA Dermatol. 2019;155:120-121. doi:10.1001/jamadermatol.2018.4098
- Hoot JW, Douglas JD, Falo LD Jr. Patch testing in a patient on dupilumab. Dermatitis. 2018;29:164. doi:10.1097/DER.0000000000000357
- Crepy M-N, Nosbaum A, Bensefa-Colas L. Blocking type 2 inflammation by dupilumab does not control classic (type 1-driven) allergic contact dermatitis in chronic hand eczema. Contact Dermatitis. 2019;81:145-147. doi:10.1111/cod.13266
- Raffi J, Chen R, Botto N. Wide dye reactors. JAAD Case Rep. 2019;5:877-879. doi:10.1016/j.jdcr.2019.08.005
- Koblinski JE, Hamann D. Mixed occupational and iatrogenic allergic contact dermatitis in a hairdresser. Occup Med (Lond). 2020;70:523-526. doi:10.1093/occmed/kqaa152
- Raffi J, Suresh R, Fishman H, et al. Investigating the role of allergic contact dermatitis in residual ocular surface disease on dupilumab (ROSDD). Int J Womens Dermatol. 2019;5:308-313. doi:10.1016/j.ijwd.2019.10.001
- Zhu GA, Chen JK, Chiou A, et al. Repeat patch testing in a patient with allergic contact dermatitis improved on dupilumab. JAAD Case Rep. 2019;5:336-338. doi:10.1016/j.jdcr.2019.01.023
- Chipalkatti N, Lee N, Zancanaro P, et al. Dupilumab as a treatment for allergic contact dermatitis. Dermatitis. 2018;29:347-348. doi:10.1097/DER.0000000000000414
- Jacob SE, Sung CT, Machler BC. Dupilumab for systemic allergy syndrome with dermatitis. Dermatitis. 2019;30:164-167. doi:10.1097/DER.0000000000000446
- Joshi SR, Khan DA. Effective use of dupilumab in managing systemic allergic contact dermatitis. Dermatitis. 2018;29:282-284. doi:10.1097/DER.0000000000000409
- Ruge IF, Skov L, Zachariae C, et al. Dupilumab treatment in two patients with severe allergic contact dermatitis caused by sesquiterpene lactones. Contact Dermatitis. 2020:83;137-139. doi:10.1111/cod.13545
- Goldminz AM, Scheinman PL. A case series of dupilumab-treated allergic contact dermatitis patients. Dermatol Ther. 2018;31:e12701. doi:10.1111/dth.12701
- Kohli N, Nedorost S. Inflamed skin predisposes to sensitization to less potent allergens. J Am Acad Dermatol. 2016;75:312-317. doi:10.1016/j.jaad.2016.03.010
- Raffi J, Suresh R, Botto N, et al. The impact of dupilumab on patch testing and the prevalence of comorbid allergic contact dermatitis in recalcitrant atopic dermatitis: a retrospective chart review. J Am Acad Dermatol. 2020;82:132-138. doi:10.1016/j.jaad.2019.09.028
- Puza CJ, Atwater AR. Positive patch test reaction in a patient taking dupilumab. Dermatitis. 2018;29:89. doi:10.1097/DER.0000000000000346
- Suresh R, Murase JE. The role of expanded series patch testing in identifying causality of residual facial dermatitis following initiation of dupilumab therapy. JAAD Case Rep. 2018;4:899-904. doi:10.1016/j.jdcr.2018.08.027
- Stout M, Silverberg JI. Variable impact of dupilumab on patch testing results and allergic contact dermatitis in adults with atopic dermatitis. J Am Acad Dermatol. 2019;81:157-162. doi:10.1016/j.jaad.2019.03.020
- Raffi J, Botto N. Patch testing and allergen-specific inhibition in a patient taking dupilumab. JAMA Dermatol. 2019;155:120-121. doi:10.1001/jamadermatol.2018.4098
- Hoot JW, Douglas JD, Falo LD Jr. Patch testing in a patient on dupilumab. Dermatitis. 2018;29:164. doi:10.1097/DER.0000000000000357
- Crepy M-N, Nosbaum A, Bensefa-Colas L. Blocking type 2 inflammation by dupilumab does not control classic (type 1-driven) allergic contact dermatitis in chronic hand eczema. Contact Dermatitis. 2019;81:145-147. doi:10.1111/cod.13266
- Raffi J, Chen R, Botto N. Wide dye reactors. JAAD Case Rep. 2019;5:877-879. doi:10.1016/j.jdcr.2019.08.005
- Koblinski JE, Hamann D. Mixed occupational and iatrogenic allergic contact dermatitis in a hairdresser. Occup Med (Lond). 2020;70:523-526. doi:10.1093/occmed/kqaa152
- Raffi J, Suresh R, Fishman H, et al. Investigating the role of allergic contact dermatitis in residual ocular surface disease on dupilumab (ROSDD). Int J Womens Dermatol. 2019;5:308-313. doi:10.1016/j.ijwd.2019.10.001
- Zhu GA, Chen JK, Chiou A, et al. Repeat patch testing in a patient with allergic contact dermatitis improved on dupilumab. JAAD Case Rep. 2019;5:336-338. doi:10.1016/j.jdcr.2019.01.023
Practice Points
- Allergic contact dermatitis is an important diagnostic consideration in patients with refractory or persistent dermatitis.
- Patch testing is important to help determine a possible allergic contactant, but there is confusion about its accuracy in patients taking dupilumab.
- Patients with residual dermatitis while on dupilumab are likely to benefit from patch testing.
Wet Your Whistles: Alcohol-Induced Flushing With Use of Topical Calcineurin Inhibitors
Practice Gap
The topical calcineurin inhibitors (TCIs) tacrolimus and pimecrolimus are US Food and Drug Administration approved for the treatment of atopic dermatitis.1 In addition, these 2 drugs are utilized off label for many other dermatologic conditions, including vitiligo, psoriasis, and periorificial dermatitis. They can be used safely for prolonged periods and on sensitive areas, including the face.
Treatment with a TCI provides advantages over topical steroids, which can cause atrophy, telangiectasia, dyspigmentation, ocular hypertension, cataracts, and tachyphylaxis after prolonged use. Adverse events resulting from use of a TCI most commonly include transient burning, warmth, and erythema in areas of application. Patients typically acclimate to these effects after a few consecutive days of use.
Localized flushing after alcohol ingestion is a known potential side effect of TCIs1; however, this association may be underappreciated and underreported to patients.
Counseling Patients Taking TCIs
Topical calcineurin inhibitors cause alcohol-induced flushing on areas of application (Figures 1 and 2) in approximately 3.4% to 6.9% of patients.1 The reaction has been reported with both topical TCIs but more often is noted with tacrolimus.2,3 Typically, flushing begins 2 to 4 weeks after treatment is initiated and within 5 to 20 minutes after alcohol intake.4 The phenomenon is self-limited; erythema typically resolves in 20 to 60 minutes.
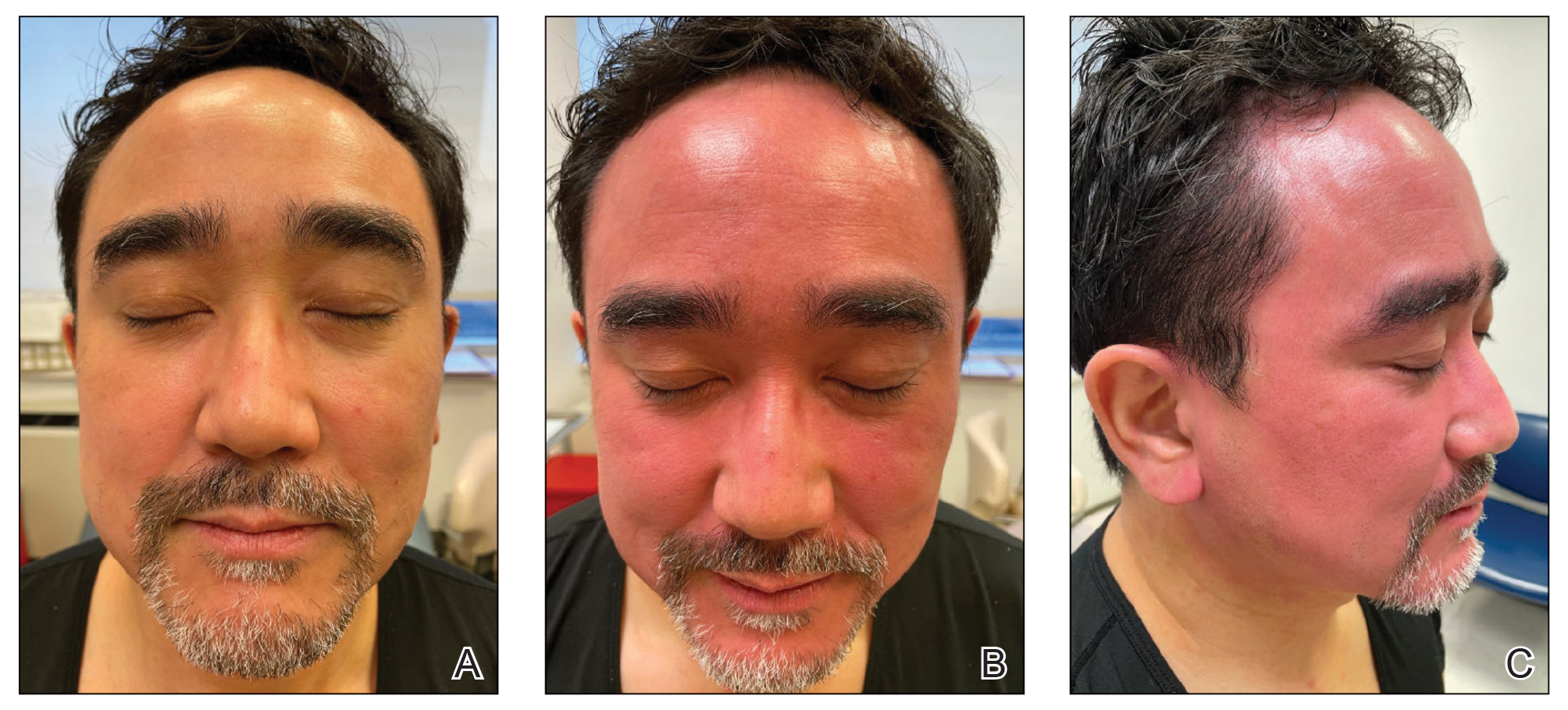
Topical calcineurin inhibitors are hypothesized to cause alcohol-induced flushing by locally inhibiting acetaldehyde dehydrogenase, an enzyme necessary for alcohol metabolism. This leads to accumulation of acetaldehyde, a by-product of alcohol metabolism, which indirectly causes concentrated vasodilation by means of prostaglandins, histamines, and other vasodilatory mediators. The combination of ethanol and a TCI also might induce release of neuropeptides, which could cause vasodilation.4
Alcohol-related flushing commonly is seen among individuals who are aldehyde dehydrogenase 2 (ALDH2) deficient; it is sometimes accompanied by nausea, headache, and tachycardia. The same pathway is implicated in disulfiram reactions, to a more intense and systemic degree, to discourage alcohol intake.
Oral calcineurin inhibitors are not reported to cause generalized flushing, perhaps because of differences in the relative dose. For example, topical tacrolimus 0.1% is 1 mg/g that is applied to a relatively small body surface area; oral calcineurin inhibitors are dosed at a range of 1 to 15 mg for an entire person.
Notably, erythema that develops after alcohol intake in a patient taking a topical TCI can mimic the dermatosis being treated—similar to one of our patients (Figure 2) whose flushing was mistaken for a flare of periorificial dermatitis—contact dermatitis or another flushing disorder such as rosacea. Uninformed patients might mistakenly self-diagnose the flushing as an allergic or anaphylactic reaction to foods, drugs, or other exposures contemporaneous with alcohol ingestion. The side effect can be frustrating owing to its appearance and discomfort, which often coincide with social interactions involving alcohol.
Techniques to Avoid Flushing
Discontinuing a TCI altogether leads to resolution of associated adverse effects, including flushing, typically within weeks to 1 month.5 Alternatively, oral aspirin (81 mg) might eliminate or attenuate symptoms, as documented in a double-blind, controlled trial in which relief of TCI-induced flushing after consuming wine was investigated.6
Another approach (albeit nontraditional) is for patients who experience this phenomenon to “wet their whistles” with an alcoholic drink before a social engagement. After flushing resolves in 20 to 60 minutes, subsequent drinks do not appear to elicit symptoms again in most patients. That said, we stop short of calling this tip “doctor’s orders.”
Practical Implication
Counseling patients who will be using a TCI—tacrolimus or pimecrolimus—about the potential for these drugs to produce localized flushing after alcohol ingestion as well as techniques for lessening or eliminating this adverse effect are important facets of their dermatologic care.
- Soter NA, Fleischer AB Jr, Webster GF, et al. Tacrolimus ointment for the treatment of atopic dermatitis in adult patients: part II, safety. J Am Acad Dermatol. 2001;44(suppl 1):S39-S46. doi:10.1067/mjd.2001.109817
- Milingou M, Antille C, Sorg O, et al. Alcohol intolerance and facial flushing in patients treated with topical tacrolimus. Arch Dermatol. 2004;140:1542-1544. doi:10.1001/archderm.140.12.1542-b
- Sabater-Abad J, Matellanes-Palacios M, Millán Parrilla F. Image gallery: interaction between alcohol and topical tacrolimus as a cause of facial flushing. Br J Dermatol. 2019;180:E144. doi:10.1111/bjd.17611
- Stinco G, Piccirillo F, Sallustio M, et al. Facial flush reaction after alcohol ingestion during topical pimecrolimus and tacrolimus treatment. Dermatology. 2009;218:71-72. doi:10.1159/000161123
- Lübbe J, Milingou M. Images in clinical medicine. tacrolimus ointment, alcohol, and facial flushing. N Engl J Med. 2004;351:2740. doi:10.1056/NEJMicm040139
- Ehst BD, Warshaw EM. Alcohol-induced application site erythema after topical immunomodulator use and its inhibition by aspirin. Arch Dermatol. 2004;140:1014-1015. doi:10.1001/archderm.140.8.1014
Practice Gap
The topical calcineurin inhibitors (TCIs) tacrolimus and pimecrolimus are US Food and Drug Administration approved for the treatment of atopic dermatitis.1 In addition, these 2 drugs are utilized off label for many other dermatologic conditions, including vitiligo, psoriasis, and periorificial dermatitis. They can be used safely for prolonged periods and on sensitive areas, including the face.
Treatment with a TCI provides advantages over topical steroids, which can cause atrophy, telangiectasia, dyspigmentation, ocular hypertension, cataracts, and tachyphylaxis after prolonged use. Adverse events resulting from use of a TCI most commonly include transient burning, warmth, and erythema in areas of application. Patients typically acclimate to these effects after a few consecutive days of use.
Localized flushing after alcohol ingestion is a known potential side effect of TCIs1; however, this association may be underappreciated and underreported to patients.
Counseling Patients Taking TCIs
Topical calcineurin inhibitors cause alcohol-induced flushing on areas of application (Figures 1 and 2) in approximately 3.4% to 6.9% of patients.1 The reaction has been reported with both topical TCIs but more often is noted with tacrolimus.2,3 Typically, flushing begins 2 to 4 weeks after treatment is initiated and within 5 to 20 minutes after alcohol intake.4 The phenomenon is self-limited; erythema typically resolves in 20 to 60 minutes.

Topical calcineurin inhibitors are hypothesized to cause alcohol-induced flushing by locally inhibiting acetaldehyde dehydrogenase, an enzyme necessary for alcohol metabolism. This leads to accumulation of acetaldehyde, a by-product of alcohol metabolism, which indirectly causes concentrated vasodilation by means of prostaglandins, histamines, and other vasodilatory mediators. The combination of ethanol and a TCI also might induce release of neuropeptides, which could cause vasodilation.4
Alcohol-related flushing commonly is seen among individuals who are aldehyde dehydrogenase 2 (ALDH2) deficient; it is sometimes accompanied by nausea, headache, and tachycardia. The same pathway is implicated in disulfiram reactions, to a more intense and systemic degree, to discourage alcohol intake.
Oral calcineurin inhibitors are not reported to cause generalized flushing, perhaps because of differences in the relative dose. For example, topical tacrolimus 0.1% is 1 mg/g that is applied to a relatively small body surface area; oral calcineurin inhibitors are dosed at a range of 1 to 15 mg for an entire person.
Notably, erythema that develops after alcohol intake in a patient taking a topical TCI can mimic the dermatosis being treated—similar to one of our patients (Figure 2) whose flushing was mistaken for a flare of periorificial dermatitis—contact dermatitis or another flushing disorder such as rosacea. Uninformed patients might mistakenly self-diagnose the flushing as an allergic or anaphylactic reaction to foods, drugs, or other exposures contemporaneous with alcohol ingestion. The side effect can be frustrating owing to its appearance and discomfort, which often coincide with social interactions involving alcohol.

Techniques to Avoid Flushing
Discontinuing a TCI altogether leads to resolution of associated adverse effects, including flushing, typically within weeks to 1 month.5 Alternatively, oral aspirin (81 mg) might eliminate or attenuate symptoms, as documented in a double-blind, controlled trial in which relief of TCI-induced flushing after consuming wine was investigated.6
Another approach (albeit nontraditional) is for patients who experience this phenomenon to “wet their whistles” with an alcoholic drink before a social engagement. After flushing resolves in 20 to 60 minutes, subsequent drinks do not appear to elicit symptoms again in most patients. That said, we stop short of calling this tip “doctor’s orders.”
Practical Implication
Counseling patients who will be using a TCI—tacrolimus or pimecrolimus—about the potential for these drugs to produce localized flushing after alcohol ingestion as well as techniques for lessening or eliminating this adverse effect are important facets of their dermatologic care.
Practice Gap
The topical calcineurin inhibitors (TCIs) tacrolimus and pimecrolimus are US Food and Drug Administration approved for the treatment of atopic dermatitis.1 In addition, these 2 drugs are utilized off label for many other dermatologic conditions, including vitiligo, psoriasis, and periorificial dermatitis. They can be used safely for prolonged periods and on sensitive areas, including the face.
Treatment with a TCI provides advantages over topical steroids, which can cause atrophy, telangiectasia, dyspigmentation, ocular hypertension, cataracts, and tachyphylaxis after prolonged use. Adverse events resulting from use of a TCI most commonly include transient burning, warmth, and erythema in areas of application. Patients typically acclimate to these effects after a few consecutive days of use.
Localized flushing after alcohol ingestion is a known potential side effect of TCIs1; however, this association may be underappreciated and underreported to patients.
Counseling Patients Taking TCIs
Topical calcineurin inhibitors cause alcohol-induced flushing on areas of application (Figures 1 and 2) in approximately 3.4% to 6.9% of patients.1 The reaction has been reported with both topical TCIs but more often is noted with tacrolimus.2,3 Typically, flushing begins 2 to 4 weeks after treatment is initiated and within 5 to 20 minutes after alcohol intake.4 The phenomenon is self-limited; erythema typically resolves in 20 to 60 minutes.

Topical calcineurin inhibitors are hypothesized to cause alcohol-induced flushing by locally inhibiting acetaldehyde dehydrogenase, an enzyme necessary for alcohol metabolism. This leads to accumulation of acetaldehyde, a by-product of alcohol metabolism, which indirectly causes concentrated vasodilation by means of prostaglandins, histamines, and other vasodilatory mediators. The combination of ethanol and a TCI also might induce release of neuropeptides, which could cause vasodilation.4
Alcohol-related flushing commonly is seen among individuals who are aldehyde dehydrogenase 2 (ALDH2) deficient; it is sometimes accompanied by nausea, headache, and tachycardia. The same pathway is implicated in disulfiram reactions, to a more intense and systemic degree, to discourage alcohol intake.
Oral calcineurin inhibitors are not reported to cause generalized flushing, perhaps because of differences in the relative dose. For example, topical tacrolimus 0.1% is 1 mg/g that is applied to a relatively small body surface area; oral calcineurin inhibitors are dosed at a range of 1 to 15 mg for an entire person.
Notably, erythema that develops after alcohol intake in a patient taking a topical TCI can mimic the dermatosis being treated—similar to one of our patients (Figure 2) whose flushing was mistaken for a flare of periorificial dermatitis—contact dermatitis or another flushing disorder such as rosacea. Uninformed patients might mistakenly self-diagnose the flushing as an allergic or anaphylactic reaction to foods, drugs, or other exposures contemporaneous with alcohol ingestion. The side effect can be frustrating owing to its appearance and discomfort, which often coincide with social interactions involving alcohol.

Techniques to Avoid Flushing
Discontinuing a TCI altogether leads to resolution of associated adverse effects, including flushing, typically within weeks to 1 month.5 Alternatively, oral aspirin (81 mg) might eliminate or attenuate symptoms, as documented in a double-blind, controlled trial in which relief of TCI-induced flushing after consuming wine was investigated.6
Another approach (albeit nontraditional) is for patients who experience this phenomenon to “wet their whistles” with an alcoholic drink before a social engagement. After flushing resolves in 20 to 60 minutes, subsequent drinks do not appear to elicit symptoms again in most patients. That said, we stop short of calling this tip “doctor’s orders.”
Practical Implication
Counseling patients who will be using a TCI—tacrolimus or pimecrolimus—about the potential for these drugs to produce localized flushing after alcohol ingestion as well as techniques for lessening or eliminating this adverse effect are important facets of their dermatologic care.
- Soter NA, Fleischer AB Jr, Webster GF, et al. Tacrolimus ointment for the treatment of atopic dermatitis in adult patients: part II, safety. J Am Acad Dermatol. 2001;44(suppl 1):S39-S46. doi:10.1067/mjd.2001.109817
- Milingou M, Antille C, Sorg O, et al. Alcohol intolerance and facial flushing in patients treated with topical tacrolimus. Arch Dermatol. 2004;140:1542-1544. doi:10.1001/archderm.140.12.1542-b
- Sabater-Abad J, Matellanes-Palacios M, Millán Parrilla F. Image gallery: interaction between alcohol and topical tacrolimus as a cause of facial flushing. Br J Dermatol. 2019;180:E144. doi:10.1111/bjd.17611
- Stinco G, Piccirillo F, Sallustio M, et al. Facial flush reaction after alcohol ingestion during topical pimecrolimus and tacrolimus treatment. Dermatology. 2009;218:71-72. doi:10.1159/000161123
- Lübbe J, Milingou M. Images in clinical medicine. tacrolimus ointment, alcohol, and facial flushing. N Engl J Med. 2004;351:2740. doi:10.1056/NEJMicm040139
- Ehst BD, Warshaw EM. Alcohol-induced application site erythema after topical immunomodulator use and its inhibition by aspirin. Arch Dermatol. 2004;140:1014-1015. doi:10.1001/archderm.140.8.1014
- Soter NA, Fleischer AB Jr, Webster GF, et al. Tacrolimus ointment for the treatment of atopic dermatitis in adult patients: part II, safety. J Am Acad Dermatol. 2001;44(suppl 1):S39-S46. doi:10.1067/mjd.2001.109817
- Milingou M, Antille C, Sorg O, et al. Alcohol intolerance and facial flushing in patients treated with topical tacrolimus. Arch Dermatol. 2004;140:1542-1544. doi:10.1001/archderm.140.12.1542-b
- Sabater-Abad J, Matellanes-Palacios M, Millán Parrilla F. Image gallery: interaction between alcohol and topical tacrolimus as a cause of facial flushing. Br J Dermatol. 2019;180:E144. doi:10.1111/bjd.17611
- Stinco G, Piccirillo F, Sallustio M, et al. Facial flush reaction after alcohol ingestion during topical pimecrolimus and tacrolimus treatment. Dermatology. 2009;218:71-72. doi:10.1159/000161123
- Lübbe J, Milingou M. Images in clinical medicine. tacrolimus ointment, alcohol, and facial flushing. N Engl J Med. 2004;351:2740. doi:10.1056/NEJMicm040139
- Ehst BD, Warshaw EM. Alcohol-induced application site erythema after topical immunomodulator use and its inhibition by aspirin. Arch Dermatol. 2004;140:1014-1015. doi:10.1001/archderm.140.8.1014
An Update on JAK Inhibitors in Skin Disease
Atopic dermatitis (AD) is a chronic inflammatory skin disorder affecting 7% of adults and 13% of children in the United States.1,2 Atopic dermatitis is characterized by pruritus, dry skin, and pain, all of which can negatively impact quality of life and put patients at higher risk for psychiatric comorbidities such as anxiety and depression.3 The pathogenesis of AD is multifactorial, involving genetics, epidermal barrier dysfunction, and immune dysregulation. Overactivation of helper T cell (TH2) pathway cytokines, including IL-4, IL-13, and IL-31, is thought to propagate both inflammation and pruritus, which are central to AD. The JAK-STAT signaling pathway plays a pivotal role in the immune system dysregulation and exaggeration of TH2 cell response, making JAK-STAT inhibitors (or JAK inhibitors) strong theoretical candidates for the treatment of AD.4 In humans, the Janus kinases are composed of 4 different members—JAK1, JAK2, JAK3, and tyrosine kinase 2—all of which can be targeted by JAK inhibitors.5
JAK inhibitors such as tofacitinib have already been approved by the US Food and Drug Administration (FDA) to treat various inflammatory conditions, including rheumatoid arthritis, ulcerative colitis, and psoriatic arthritis; other JAK inhibitors such as baricitinib are only approved for patients with rheumatoid arthritis.6,7 The success of these small molecule inhibitors in these immune-mediated conditions make them attractive candidates for the treatment of AD. Several JAK inhibitors are in phase 2 and phase 3 clinical trials as oral therapies (moderate to severe AD) or as topical treatments (mild to moderate AD). Currently, ruxolitinib (RUX) is the only topical JAK inhibitor that is FDA approved for the treatment of AD in the United States.8 In this editorial, we focus on recent trials of JAK inhibitors tested in patients with AD, including topical RUX, as well as oral abrocitinib, upadacitinib, and baricitinib.
Topical RUX in AD
Ruxolitinib is a topical JAK1/2 small molecule inhibitor approved by the FDA for the treatment of AD in 2021. In a randomized trial by Kim et al9 in 2020, all tested regimens of RUX demonstrated significant improvement in eczema area and severity index (EASI) scores vs vehicle; notably, RUX cream 1.5% applied twice daily achieved the greatest mean percentage change in baseline EASI score vs vehicle at 4 weeks (76.1% vs 15.5%; P<.0001). Ruxolitinib cream was well tolerated through week 8 of the trial, and all adverse events (AEs) were mild to moderate in severity and comparable to those in the vehicle group.9
Topical JAK inhibitors appear to be effective for mild to moderate AD and have had an acceptable safety profile in clinical trials thus far. Although topical corticosteroids and calcineurin inhibitors can have great clinical benefit in AD, they are recommended for short-term use given side effects such as thinning of the skin, burning, or telangiectasia formation.10,11 The hope is that topical JAK inhibitors may be an alternative to standard topical treatments for AD, as they can be used for longer periods due to a safer side-effect profile.
Oral JAK Inhibitors in AD
Several oral JAK inhibitors are undergoing investigation for the systemic treatment of moderate to severe AD. Abrocitinib is an oral JAK1 inhibitor that has demonstrated efficacy in several phase 3 trials in patients with moderate to severe AD. In a 2021 trial, patients were randomized in a 2:2:2:1 ratio to receive abrocitinib 200 mg daily, abrocitinib 100 mg daily, subcutaneous dupilumab 300 mg every other week, or placebo, respectively.12 Patients in both abrocitinib groups showed significant improvement in AD vs placebo, and EASI-75 response was achieved in 70.3%, 58.7%, 58.1%, and 27.1% of patients, respectively (P<.001 for both abrocitinib doses vs placebo). Adverse events occurred more frequently in the abrocitinib 200-mg group vs placebo. Nausea, acne, nasopharyngitis, and headache were the most frequently reported AEs with abrocitinib.12 Another phase 3 trial by Silverberg et al13 (N=391) had similar treatment results, with 38.1% of participants receiving abrocitinib 200 mg and 28.4% of participants receiving abrocitinib 100 mg achieving investigator global assessment scores of 0 (clear) or 1 (almost clear) vs 9.1% of participants receiving placebo (P<.001). Abrocitinib was well tolerated in this trial with few serious AEs (ie, herpangina [0.6%], pneumonia [0.6%]).13 In both trials, there were rare instances of laboratory values indicating thrombocytopenia with the 200-mg dose (0.9%12 and 3.2%13) without any clinical manifestations. Although a decrease in platelets was observed, no thrombocytopenia occurred in the abrocitinib 100-mg group in the latter trial.13
Baricitinib is another oral inhibitor of JAK1 and JAK2 with potential for the treatment of AD. One randomized trial (N=329) demonstrated its efficacy in combination with a topical corticosteroid (TCS). At 16 weeks, a higher number of participants treated with baricitinib and TCS achieved investigator global assessment scores of 0 (clear) or 1 (almost clear) compared to those who received placebo and TCS (31% with baricitinib 4 mg + TCS, 24% with baricitinib 2 mg + TCS, and 15% with placebo + TCS).14 Similarly, in BREEZE-AD5,another phase 3 trial (N=440), baricitinib monotherapy demonstrated a higher rate of treatment success vs placebo.15 Specifically, 13% of patients treated with baricitinib 1 mg and 30% of those treated with baricitinib 2 mg achieved 75% or greater reduction in EASI scores compared to 8% in the placebo group. The most common AEs associated with baricitinib were nasopharyngitis and headache. Adverse events occurred with similar frequency across both experimental and control groups.15 Reich et al14 demonstrated a higher overall rate of AEs—most commonly nasopharyngitis, upper respiratory tract infections, and folliculitis—in baricitinib-treated patients; however, serious AEs occurred with similar frequency across all groups, including the control group.
The selective JAK1 inhibitor upadacitinib also is undergoing testing in treating moderate to severe AD. In one trial, 167 patients were randomized to once daily oral upadacitinib 7.5 mg, 15 mg, or 30 mg or placebo.16 All doses of upadacitinib demonstrated considerably higher percentage improvements from baseline in EASI scores compared to placebo at 16 weeks with a clear dose-response relationship (39%, 62%, and 74% vs 23%, respectively). In this trial, there were no dose-limiting safety events. Serious AEs were infrequent, occurring in 4.8%, 2.4%, and 0% of upadacitinib groups vs 2.5% for placebo. The serious AEs observed with upadacitinib were 1 case of appendicitis, lower jaw pericoronitis in a patient with a history of repeated tooth infections, and an exacerbation of AD.16
Tofacitinib, another JAK inhibitor, has been shown to increase the risk for blood clots and death in a large trial in the treatment of rheumatoid arthritis. Following this study, the FDA is requiring black box warnings for tofacitinib and also for the 2 JAK inhibitors baricitinib and upadacitinib regarding the risks for heart-related events, cancer, blood clots, and death. Given that these medications share a similar mechanism of action to tofacitinib, they may have similar risks, though they have not yet been fully evaluated in large safety trials.17
With more recent investigation into novel therapeutics for AD, oral JAK inhibitors may play an important role in the future to treat patients with moderate to severe AD with inadequate response or contraindications to other systemic therapies. In trials thus far, oral JAK inhibitors have exhibited acceptable safety profiles and have demonstrated treatment success in AD. More randomized, controlled, phase 3 studies with larger patient populations are required to confirm their potential as effective treatments and elucidate their long-term safety.
Deucravacitinib in Psoriasis
Deucravacitinib is a first-in-class, oral, selective TYK2 inhibitor currently undergoing testing for the treatment of psoriasis. A randomized phase 2 trial (N=267) found that deucravacitinib was more effective than placebo in treating chronic plaque psoriasis at doses of 3 to 12 mg daily.18 The percentage of participants with a 75% or greater reduction from baseline in the psoriasis area and severity index score was 7% with placebo, 9% with deucravacitinib 3 mg every other day (P=.49 vs placebo), 39% with 3 mg once daily (P<.001 vs placebo), 69% with 3 mg twice daily (P<.001 vs placebo), 67% with 6 mg twice daily (P<.001 vs placebo), and 75% with 12 mg once daily (P<.001 vs placebo). The most commonly reported AEs were nasopharyngitis, headache, diarrhea, nausea, and upper respiratory tract infection. Adverse events occurred in 51% of participants in the control group and in 55% to 80% of those in the experimental groups. Additionally, there was 1 reported case of melanoma (stage 0) 96 days after the start of treatment in a patient in the 3-mg once-daily group. Serious AEs occurred in only 0% to 2% of participants who received deucravacitinib.18
Two phase 3 trials—POETYK PSO-1 and POETYK PSO-2 (N=1686)—found deucravacitinib to be notably more effective than both placebo and apremilast in treating psoriasis.19 Among participants receiving deucravacitinib 6 mg daily, 58.7% and 53.6% in the 2 respective trials achieved psoriasis area and severity index 75 response vs 12.7% and 9.4% receiving placebo and 35.1% and 40.2% receiving apremilast. Overall, the treatment was well tolerated, with a low rate of discontinuation of deucravacitinib due to AEs (2.4% of patients on deucravacitinib compared to 3.8% on placebo and 5.2% on apremilast). The most frequently observed AEs with deucravacitinib were nasopharyngitis and upper respiratory tract infection. The full results of these trials are expected to be published soon.19,20
Final Thoughts
Overall, JAK inhibitors are a novel class of therapeutics that may have further success in the treatment of other dermatologic conditions that negatively affect patients’ quality of life and productivity. We should look forward to additional successful trials with these promising medications.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Silverberg JI , Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis. 2014;25:107-114.
- Schonmann Y, Mansfield KE, Hayes JF, et al. Atopic eczema in adulthood and risk of depression and anxiety: a population-based cohort study. J Allergy Clin Immunol Pract. 2020;8:248-257.e16.
- Bao L, Zhang H, Chan LS. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAKSTAT. 2013;2:e24137.
- Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017;18:374-384.
- Xeljanz FDA approval history. Drugs.com website. Updated December 14, 2021. Accessed February 16, 2022. https://www.drugs.com/history/xeljanz.html
- Mullard A. FDA approves Eli Lilly’s baricitinib. Nat Rev Drug Discov. 2018;17:460.
- FDA approves Opzelura. Drugs.com website. Published September 2021. Accessed February 16, 2022. https://www.drugs.com/newdrugs/fda-approves-opzelura-ruxolitinib-cream-atopic-dermatitis-ad-5666.html
- Kim BS, Sun K, Papp K, et al. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: results from a phase 2, randomized, dose-ranging, vehicle- and active-controlled study.J Am Acad Dermatol. 2020;82:1305-1313.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2, management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32:657-682.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863-873.
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:1333-1343.
- Simpson EL, Forman S, Silverberg JI, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J Am Acad Dermatol. 2021;85:62-70.
- Guttman-Yassky E, Thaçi D, Pangan AL, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145:877-884.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Published September 1, 2022. Accessed February 16, 2022. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Papp K, Gordon K, Thaçi D, et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379:1313-1321.
- Bristol Myers Squibb presents positive data from two pivotal phase 3 psoriasis studies demonstrating superiority of deucravacitinib compared to placebo and Otezla® (apremilast). Press release. Bristol Meyers Squibb. April 23, 2021. Accessed February 16, 2022. https://news.bms.com/news/details/2021/Bristol-Myers-Squibb-Presents-Positive-Data-from-Two-Pivotal-Phase-3-Psoriasis-Studies-Demonstrating-Superiority-of-Deucravacitinib-Compared-to-Placebo-and-Otezla-apremilast/default.aspx
- Armstrong A, Gooderham M, Warren R, et al. Efficacy and safety of deucravacitinib, an oral, selective tyrosine kinase 2 (TYK2) inhibitor, compared with placebo and apremilast in moderate to severe plaque psoriasis: results from the POETYK PSO-1 study [abstract]. Abstract presented at: 2021 American Academy of Dermatology annual meeting; April 23-25, 2021; San Francisco, California.
Atopic dermatitis (AD) is a chronic inflammatory skin disorder affecting 7% of adults and 13% of children in the United States.1,2 Atopic dermatitis is characterized by pruritus, dry skin, and pain, all of which can negatively impact quality of life and put patients at higher risk for psychiatric comorbidities such as anxiety and depression.3 The pathogenesis of AD is multifactorial, involving genetics, epidermal barrier dysfunction, and immune dysregulation. Overactivation of helper T cell (TH2) pathway cytokines, including IL-4, IL-13, and IL-31, is thought to propagate both inflammation and pruritus, which are central to AD. The JAK-STAT signaling pathway plays a pivotal role in the immune system dysregulation and exaggeration of TH2 cell response, making JAK-STAT inhibitors (or JAK inhibitors) strong theoretical candidates for the treatment of AD.4 In humans, the Janus kinases are composed of 4 different members—JAK1, JAK2, JAK3, and tyrosine kinase 2—all of which can be targeted by JAK inhibitors.5
JAK inhibitors such as tofacitinib have already been approved by the US Food and Drug Administration (FDA) to treat various inflammatory conditions, including rheumatoid arthritis, ulcerative colitis, and psoriatic arthritis; other JAK inhibitors such as baricitinib are only approved for patients with rheumatoid arthritis.6,7 The success of these small molecule inhibitors in these immune-mediated conditions make them attractive candidates for the treatment of AD. Several JAK inhibitors are in phase 2 and phase 3 clinical trials as oral therapies (moderate to severe AD) or as topical treatments (mild to moderate AD). Currently, ruxolitinib (RUX) is the only topical JAK inhibitor that is FDA approved for the treatment of AD in the United States.8 In this editorial, we focus on recent trials of JAK inhibitors tested in patients with AD, including topical RUX, as well as oral abrocitinib, upadacitinib, and baricitinib.
Topical RUX in AD
Ruxolitinib is a topical JAK1/2 small molecule inhibitor approved by the FDA for the treatment of AD in 2021. In a randomized trial by Kim et al9 in 2020, all tested regimens of RUX demonstrated significant improvement in eczema area and severity index (EASI) scores vs vehicle; notably, RUX cream 1.5% applied twice daily achieved the greatest mean percentage change in baseline EASI score vs vehicle at 4 weeks (76.1% vs 15.5%; P<.0001). Ruxolitinib cream was well tolerated through week 8 of the trial, and all adverse events (AEs) were mild to moderate in severity and comparable to those in the vehicle group.9
Topical JAK inhibitors appear to be effective for mild to moderate AD and have had an acceptable safety profile in clinical trials thus far. Although topical corticosteroids and calcineurin inhibitors can have great clinical benefit in AD, they are recommended for short-term use given side effects such as thinning of the skin, burning, or telangiectasia formation.10,11 The hope is that topical JAK inhibitors may be an alternative to standard topical treatments for AD, as they can be used for longer periods due to a safer side-effect profile.
Oral JAK Inhibitors in AD
Several oral JAK inhibitors are undergoing investigation for the systemic treatment of moderate to severe AD. Abrocitinib is an oral JAK1 inhibitor that has demonstrated efficacy in several phase 3 trials in patients with moderate to severe AD. In a 2021 trial, patients were randomized in a 2:2:2:1 ratio to receive abrocitinib 200 mg daily, abrocitinib 100 mg daily, subcutaneous dupilumab 300 mg every other week, or placebo, respectively.12 Patients in both abrocitinib groups showed significant improvement in AD vs placebo, and EASI-75 response was achieved in 70.3%, 58.7%, 58.1%, and 27.1% of patients, respectively (P<.001 for both abrocitinib doses vs placebo). Adverse events occurred more frequently in the abrocitinib 200-mg group vs placebo. Nausea, acne, nasopharyngitis, and headache were the most frequently reported AEs with abrocitinib.12 Another phase 3 trial by Silverberg et al13 (N=391) had similar treatment results, with 38.1% of participants receiving abrocitinib 200 mg and 28.4% of participants receiving abrocitinib 100 mg achieving investigator global assessment scores of 0 (clear) or 1 (almost clear) vs 9.1% of participants receiving placebo (P<.001). Abrocitinib was well tolerated in this trial with few serious AEs (ie, herpangina [0.6%], pneumonia [0.6%]).13 In both trials, there were rare instances of laboratory values indicating thrombocytopenia with the 200-mg dose (0.9%12 and 3.2%13) without any clinical manifestations. Although a decrease in platelets was observed, no thrombocytopenia occurred in the abrocitinib 100-mg group in the latter trial.13
Baricitinib is another oral inhibitor of JAK1 and JAK2 with potential for the treatment of AD. One randomized trial (N=329) demonstrated its efficacy in combination with a topical corticosteroid (TCS). At 16 weeks, a higher number of participants treated with baricitinib and TCS achieved investigator global assessment scores of 0 (clear) or 1 (almost clear) compared to those who received placebo and TCS (31% with baricitinib 4 mg + TCS, 24% with baricitinib 2 mg + TCS, and 15% with placebo + TCS).14 Similarly, in BREEZE-AD5,another phase 3 trial (N=440), baricitinib monotherapy demonstrated a higher rate of treatment success vs placebo.15 Specifically, 13% of patients treated with baricitinib 1 mg and 30% of those treated with baricitinib 2 mg achieved 75% or greater reduction in EASI scores compared to 8% in the placebo group. The most common AEs associated with baricitinib were nasopharyngitis and headache. Adverse events occurred with similar frequency across both experimental and control groups.15 Reich et al14 demonstrated a higher overall rate of AEs—most commonly nasopharyngitis, upper respiratory tract infections, and folliculitis—in baricitinib-treated patients; however, serious AEs occurred with similar frequency across all groups, including the control group.
The selective JAK1 inhibitor upadacitinib also is undergoing testing in treating moderate to severe AD. In one trial, 167 patients were randomized to once daily oral upadacitinib 7.5 mg, 15 mg, or 30 mg or placebo.16 All doses of upadacitinib demonstrated considerably higher percentage improvements from baseline in EASI scores compared to placebo at 16 weeks with a clear dose-response relationship (39%, 62%, and 74% vs 23%, respectively). In this trial, there were no dose-limiting safety events. Serious AEs were infrequent, occurring in 4.8%, 2.4%, and 0% of upadacitinib groups vs 2.5% for placebo. The serious AEs observed with upadacitinib were 1 case of appendicitis, lower jaw pericoronitis in a patient with a history of repeated tooth infections, and an exacerbation of AD.16
Tofacitinib, another JAK inhibitor, has been shown to increase the risk for blood clots and death in a large trial in the treatment of rheumatoid arthritis. Following this study, the FDA is requiring black box warnings for tofacitinib and also for the 2 JAK inhibitors baricitinib and upadacitinib regarding the risks for heart-related events, cancer, blood clots, and death. Given that these medications share a similar mechanism of action to tofacitinib, they may have similar risks, though they have not yet been fully evaluated in large safety trials.17
With more recent investigation into novel therapeutics for AD, oral JAK inhibitors may play an important role in the future to treat patients with moderate to severe AD with inadequate response or contraindications to other systemic therapies. In trials thus far, oral JAK inhibitors have exhibited acceptable safety profiles and have demonstrated treatment success in AD. More randomized, controlled, phase 3 studies with larger patient populations are required to confirm their potential as effective treatments and elucidate their long-term safety.
Deucravacitinib in Psoriasis
Deucravacitinib is a first-in-class, oral, selective TYK2 inhibitor currently undergoing testing for the treatment of psoriasis. A randomized phase 2 trial (N=267) found that deucravacitinib was more effective than placebo in treating chronic plaque psoriasis at doses of 3 to 12 mg daily.18 The percentage of participants with a 75% or greater reduction from baseline in the psoriasis area and severity index score was 7% with placebo, 9% with deucravacitinib 3 mg every other day (P=.49 vs placebo), 39% with 3 mg once daily (P<.001 vs placebo), 69% with 3 mg twice daily (P<.001 vs placebo), 67% with 6 mg twice daily (P<.001 vs placebo), and 75% with 12 mg once daily (P<.001 vs placebo). The most commonly reported AEs were nasopharyngitis, headache, diarrhea, nausea, and upper respiratory tract infection. Adverse events occurred in 51% of participants in the control group and in 55% to 80% of those in the experimental groups. Additionally, there was 1 reported case of melanoma (stage 0) 96 days after the start of treatment in a patient in the 3-mg once-daily group. Serious AEs occurred in only 0% to 2% of participants who received deucravacitinib.18
Two phase 3 trials—POETYK PSO-1 and POETYK PSO-2 (N=1686)—found deucravacitinib to be notably more effective than both placebo and apremilast in treating psoriasis.19 Among participants receiving deucravacitinib 6 mg daily, 58.7% and 53.6% in the 2 respective trials achieved psoriasis area and severity index 75 response vs 12.7% and 9.4% receiving placebo and 35.1% and 40.2% receiving apremilast. Overall, the treatment was well tolerated, with a low rate of discontinuation of deucravacitinib due to AEs (2.4% of patients on deucravacitinib compared to 3.8% on placebo and 5.2% on apremilast). The most frequently observed AEs with deucravacitinib were nasopharyngitis and upper respiratory tract infection. The full results of these trials are expected to be published soon.19,20
Final Thoughts
Overall, JAK inhibitors are a novel class of therapeutics that may have further success in the treatment of other dermatologic conditions that negatively affect patients’ quality of life and productivity. We should look forward to additional successful trials with these promising medications.
Atopic dermatitis (AD) is a chronic inflammatory skin disorder affecting 7% of adults and 13% of children in the United States.1,2 Atopic dermatitis is characterized by pruritus, dry skin, and pain, all of which can negatively impact quality of life and put patients at higher risk for psychiatric comorbidities such as anxiety and depression.3 The pathogenesis of AD is multifactorial, involving genetics, epidermal barrier dysfunction, and immune dysregulation. Overactivation of helper T cell (TH2) pathway cytokines, including IL-4, IL-13, and IL-31, is thought to propagate both inflammation and pruritus, which are central to AD. The JAK-STAT signaling pathway plays a pivotal role in the immune system dysregulation and exaggeration of TH2 cell response, making JAK-STAT inhibitors (or JAK inhibitors) strong theoretical candidates for the treatment of AD.4 In humans, the Janus kinases are composed of 4 different members—JAK1, JAK2, JAK3, and tyrosine kinase 2—all of which can be targeted by JAK inhibitors.5
JAK inhibitors such as tofacitinib have already been approved by the US Food and Drug Administration (FDA) to treat various inflammatory conditions, including rheumatoid arthritis, ulcerative colitis, and psoriatic arthritis; other JAK inhibitors such as baricitinib are only approved for patients with rheumatoid arthritis.6,7 The success of these small molecule inhibitors in these immune-mediated conditions make them attractive candidates for the treatment of AD. Several JAK inhibitors are in phase 2 and phase 3 clinical trials as oral therapies (moderate to severe AD) or as topical treatments (mild to moderate AD). Currently, ruxolitinib (RUX) is the only topical JAK inhibitor that is FDA approved for the treatment of AD in the United States.8 In this editorial, we focus on recent trials of JAK inhibitors tested in patients with AD, including topical RUX, as well as oral abrocitinib, upadacitinib, and baricitinib.
Topical RUX in AD
Ruxolitinib is a topical JAK1/2 small molecule inhibitor approved by the FDA for the treatment of AD in 2021. In a randomized trial by Kim et al9 in 2020, all tested regimens of RUX demonstrated significant improvement in eczema area and severity index (EASI) scores vs vehicle; notably, RUX cream 1.5% applied twice daily achieved the greatest mean percentage change in baseline EASI score vs vehicle at 4 weeks (76.1% vs 15.5%; P<.0001). Ruxolitinib cream was well tolerated through week 8 of the trial, and all adverse events (AEs) were mild to moderate in severity and comparable to those in the vehicle group.9
Topical JAK inhibitors appear to be effective for mild to moderate AD and have had an acceptable safety profile in clinical trials thus far. Although topical corticosteroids and calcineurin inhibitors can have great clinical benefit in AD, they are recommended for short-term use given side effects such as thinning of the skin, burning, or telangiectasia formation.10,11 The hope is that topical JAK inhibitors may be an alternative to standard topical treatments for AD, as they can be used for longer periods due to a safer side-effect profile.
Oral JAK Inhibitors in AD
Several oral JAK inhibitors are undergoing investigation for the systemic treatment of moderate to severe AD. Abrocitinib is an oral JAK1 inhibitor that has demonstrated efficacy in several phase 3 trials in patients with moderate to severe AD. In a 2021 trial, patients were randomized in a 2:2:2:1 ratio to receive abrocitinib 200 mg daily, abrocitinib 100 mg daily, subcutaneous dupilumab 300 mg every other week, or placebo, respectively.12 Patients in both abrocitinib groups showed significant improvement in AD vs placebo, and EASI-75 response was achieved in 70.3%, 58.7%, 58.1%, and 27.1% of patients, respectively (P<.001 for both abrocitinib doses vs placebo). Adverse events occurred more frequently in the abrocitinib 200-mg group vs placebo. Nausea, acne, nasopharyngitis, and headache were the most frequently reported AEs with abrocitinib.12 Another phase 3 trial by Silverberg et al13 (N=391) had similar treatment results, with 38.1% of participants receiving abrocitinib 200 mg and 28.4% of participants receiving abrocitinib 100 mg achieving investigator global assessment scores of 0 (clear) or 1 (almost clear) vs 9.1% of participants receiving placebo (P<.001). Abrocitinib was well tolerated in this trial with few serious AEs (ie, herpangina [0.6%], pneumonia [0.6%]).13 In both trials, there were rare instances of laboratory values indicating thrombocytopenia with the 200-mg dose (0.9%12 and 3.2%13) without any clinical manifestations. Although a decrease in platelets was observed, no thrombocytopenia occurred in the abrocitinib 100-mg group in the latter trial.13
Baricitinib is another oral inhibitor of JAK1 and JAK2 with potential for the treatment of AD. One randomized trial (N=329) demonstrated its efficacy in combination with a topical corticosteroid (TCS). At 16 weeks, a higher number of participants treated with baricitinib and TCS achieved investigator global assessment scores of 0 (clear) or 1 (almost clear) compared to those who received placebo and TCS (31% with baricitinib 4 mg + TCS, 24% with baricitinib 2 mg + TCS, and 15% with placebo + TCS).14 Similarly, in BREEZE-AD5,another phase 3 trial (N=440), baricitinib monotherapy demonstrated a higher rate of treatment success vs placebo.15 Specifically, 13% of patients treated with baricitinib 1 mg and 30% of those treated with baricitinib 2 mg achieved 75% or greater reduction in EASI scores compared to 8% in the placebo group. The most common AEs associated with baricitinib were nasopharyngitis and headache. Adverse events occurred with similar frequency across both experimental and control groups.15 Reich et al14 demonstrated a higher overall rate of AEs—most commonly nasopharyngitis, upper respiratory tract infections, and folliculitis—in baricitinib-treated patients; however, serious AEs occurred with similar frequency across all groups, including the control group.
The selective JAK1 inhibitor upadacitinib also is undergoing testing in treating moderate to severe AD. In one trial, 167 patients were randomized to once daily oral upadacitinib 7.5 mg, 15 mg, or 30 mg or placebo.16 All doses of upadacitinib demonstrated considerably higher percentage improvements from baseline in EASI scores compared to placebo at 16 weeks with a clear dose-response relationship (39%, 62%, and 74% vs 23%, respectively). In this trial, there were no dose-limiting safety events. Serious AEs were infrequent, occurring in 4.8%, 2.4%, and 0% of upadacitinib groups vs 2.5% for placebo. The serious AEs observed with upadacitinib were 1 case of appendicitis, lower jaw pericoronitis in a patient with a history of repeated tooth infections, and an exacerbation of AD.16
Tofacitinib, another JAK inhibitor, has been shown to increase the risk for blood clots and death in a large trial in the treatment of rheumatoid arthritis. Following this study, the FDA is requiring black box warnings for tofacitinib and also for the 2 JAK inhibitors baricitinib and upadacitinib regarding the risks for heart-related events, cancer, blood clots, and death. Given that these medications share a similar mechanism of action to tofacitinib, they may have similar risks, though they have not yet been fully evaluated in large safety trials.17
With more recent investigation into novel therapeutics for AD, oral JAK inhibitors may play an important role in the future to treat patients with moderate to severe AD with inadequate response or contraindications to other systemic therapies. In trials thus far, oral JAK inhibitors have exhibited acceptable safety profiles and have demonstrated treatment success in AD. More randomized, controlled, phase 3 studies with larger patient populations are required to confirm their potential as effective treatments and elucidate their long-term safety.
Deucravacitinib in Psoriasis
Deucravacitinib is a first-in-class, oral, selective TYK2 inhibitor currently undergoing testing for the treatment of psoriasis. A randomized phase 2 trial (N=267) found that deucravacitinib was more effective than placebo in treating chronic plaque psoriasis at doses of 3 to 12 mg daily.18 The percentage of participants with a 75% or greater reduction from baseline in the psoriasis area and severity index score was 7% with placebo, 9% with deucravacitinib 3 mg every other day (P=.49 vs placebo), 39% with 3 mg once daily (P<.001 vs placebo), 69% with 3 mg twice daily (P<.001 vs placebo), 67% with 6 mg twice daily (P<.001 vs placebo), and 75% with 12 mg once daily (P<.001 vs placebo). The most commonly reported AEs were nasopharyngitis, headache, diarrhea, nausea, and upper respiratory tract infection. Adverse events occurred in 51% of participants in the control group and in 55% to 80% of those in the experimental groups. Additionally, there was 1 reported case of melanoma (stage 0) 96 days after the start of treatment in a patient in the 3-mg once-daily group. Serious AEs occurred in only 0% to 2% of participants who received deucravacitinib.18
Two phase 3 trials—POETYK PSO-1 and POETYK PSO-2 (N=1686)—found deucravacitinib to be notably more effective than both placebo and apremilast in treating psoriasis.19 Among participants receiving deucravacitinib 6 mg daily, 58.7% and 53.6% in the 2 respective trials achieved psoriasis area and severity index 75 response vs 12.7% and 9.4% receiving placebo and 35.1% and 40.2% receiving apremilast. Overall, the treatment was well tolerated, with a low rate of discontinuation of deucravacitinib due to AEs (2.4% of patients on deucravacitinib compared to 3.8% on placebo and 5.2% on apremilast). The most frequently observed AEs with deucravacitinib were nasopharyngitis and upper respiratory tract infection. The full results of these trials are expected to be published soon.19,20
Final Thoughts
Overall, JAK inhibitors are a novel class of therapeutics that may have further success in the treatment of other dermatologic conditions that negatively affect patients’ quality of life and productivity. We should look forward to additional successful trials with these promising medications.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Silverberg JI , Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis. 2014;25:107-114.
- Schonmann Y, Mansfield KE, Hayes JF, et al. Atopic eczema in adulthood and risk of depression and anxiety: a population-based cohort study. J Allergy Clin Immunol Pract. 2020;8:248-257.e16.
- Bao L, Zhang H, Chan LS. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAKSTAT. 2013;2:e24137.
- Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017;18:374-384.
- Xeljanz FDA approval history. Drugs.com website. Updated December 14, 2021. Accessed February 16, 2022. https://www.drugs.com/history/xeljanz.html
- Mullard A. FDA approves Eli Lilly’s baricitinib. Nat Rev Drug Discov. 2018;17:460.
- FDA approves Opzelura. Drugs.com website. Published September 2021. Accessed February 16, 2022. https://www.drugs.com/newdrugs/fda-approves-opzelura-ruxolitinib-cream-atopic-dermatitis-ad-5666.html
- Kim BS, Sun K, Papp K, et al. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: results from a phase 2, randomized, dose-ranging, vehicle- and active-controlled study.J Am Acad Dermatol. 2020;82:1305-1313.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2, management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32:657-682.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863-873.
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:1333-1343.
- Simpson EL, Forman S, Silverberg JI, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J Am Acad Dermatol. 2021;85:62-70.
- Guttman-Yassky E, Thaçi D, Pangan AL, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145:877-884.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Published September 1, 2022. Accessed February 16, 2022. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Papp K, Gordon K, Thaçi D, et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379:1313-1321.
- Bristol Myers Squibb presents positive data from two pivotal phase 3 psoriasis studies demonstrating superiority of deucravacitinib compared to placebo and Otezla® (apremilast). Press release. Bristol Meyers Squibb. April 23, 2021. Accessed February 16, 2022. https://news.bms.com/news/details/2021/Bristol-Myers-Squibb-Presents-Positive-Data-from-Two-Pivotal-Phase-3-Psoriasis-Studies-Demonstrating-Superiority-of-Deucravacitinib-Compared-to-Placebo-and-Otezla-apremilast/default.aspx
- Armstrong A, Gooderham M, Warren R, et al. Efficacy and safety of deucravacitinib, an oral, selective tyrosine kinase 2 (TYK2) inhibitor, compared with placebo and apremilast in moderate to severe plaque psoriasis: results from the POETYK PSO-1 study [abstract]. Abstract presented at: 2021 American Academy of Dermatology annual meeting; April 23-25, 2021; San Francisco, California.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Silverberg JI , Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis. 2014;25:107-114.
- Schonmann Y, Mansfield KE, Hayes JF, et al. Atopic eczema in adulthood and risk of depression and anxiety: a population-based cohort study. J Allergy Clin Immunol Pract. 2020;8:248-257.e16.
- Bao L, Zhang H, Chan LS. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAKSTAT. 2013;2:e24137.
- Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017;18:374-384.
- Xeljanz FDA approval history. Drugs.com website. Updated December 14, 2021. Accessed February 16, 2022. https://www.drugs.com/history/xeljanz.html
- Mullard A. FDA approves Eli Lilly’s baricitinib. Nat Rev Drug Discov. 2018;17:460.
- FDA approves Opzelura. Drugs.com website. Published September 2021. Accessed February 16, 2022. https://www.drugs.com/newdrugs/fda-approves-opzelura-ruxolitinib-cream-atopic-dermatitis-ad-5666.html
- Kim BS, Sun K, Papp K, et al. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: results from a phase 2, randomized, dose-ranging, vehicle- and active-controlled study.J Am Acad Dermatol. 2020;82:1305-1313.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2, management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32:657-682.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863-873.
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:1333-1343.
- Simpson EL, Forman S, Silverberg JI, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J Am Acad Dermatol. 2021;85:62-70.
- Guttman-Yassky E, Thaçi D, Pangan AL, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145:877-884.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Published September 1, 2022. Accessed February 16, 2022. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Papp K, Gordon K, Thaçi D, et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379:1313-1321.
- Bristol Myers Squibb presents positive data from two pivotal phase 3 psoriasis studies demonstrating superiority of deucravacitinib compared to placebo and Otezla® (apremilast). Press release. Bristol Meyers Squibb. April 23, 2021. Accessed February 16, 2022. https://news.bms.com/news/details/2021/Bristol-Myers-Squibb-Presents-Positive-Data-from-Two-Pivotal-Phase-3-Psoriasis-Studies-Demonstrating-Superiority-of-Deucravacitinib-Compared-to-Placebo-and-Otezla-apremilast/default.aspx
- Armstrong A, Gooderham M, Warren R, et al. Efficacy and safety of deucravacitinib, an oral, selective tyrosine kinase 2 (TYK2) inhibitor, compared with placebo and apremilast in moderate to severe plaque psoriasis: results from the POETYK PSO-1 study [abstract]. Abstract presented at: 2021 American Academy of Dermatology annual meeting; April 23-25, 2021; San Francisco, California.
Clinical Edge Journal Scan Commentary: Atopic Dermatitis March 2022
Recent insights into the epidemiology of atopic dermatitis
Atopic dermatitis (AD) has complex risk factors and effects on patients. AD patients experience itch, skin pain, sleep disturbances, and other symptoms that can profoundly impact their quality of life. Yet, little is known about the longitudinal epidemiology and burden of AD in children.
- Johansson et al1 reported on the ongoing BAMSE cohort study. BAMSE followed 4089 individuals regularly from birth regarding AD and atopic diseases with surveys and clinical examinations; 3055 individuals were assessed at year 24 of follow-up. At 24 years, the 12-month prevalence of AD was 17.8% and more common in women than men (20.5% vs. 14.8%). The point prevalence of AD on clinical examination was 8.0%. These high prevalence estimates are consistent with multiple other recent studies in the United States and globally.2-4 Prevalence measures a combination of both new-onset (incident) cases and persistence of childhood disease. Importantly, BAMSE found the proportion of adult-onset AD was 16.9%. These results are consistent with previous studies that found substantial rates of adult-onset AD.5 Additionally, men were more likely to have AD in the first year of life, but less likely than women to have AD in adolescence and young adulthood.
- Paller et al6 recently initiated PEDISTAT, an international, longitudinal 5-year registry of the disease course, comorbidities, treatment, and disease burden children age <12 years with moderate-severe AD. While the study is ongoing, the authors reported the baseline characteristics of the registry. They found that most of the enrolled children were not treated with a systemic therapy, had inadequately controlled disease and a high disease burden. These results emphasize the need for very safe and highly effective systemic therapies for moderate-severe AD in children.
I look forward to seeing the results of these ongoing study and how they will inform our understanding of the epidemiology and comorbidities of AD.
Numerous risk factors for AD have been examined. Infections have been explored as a potential risk factor for AD for more than 30 years.
- Lin et al7 conducted a population-based, nationwide case-control study including 5,454 children with AD matched with 16,362 healthy controls without AD. They found that prior to AD diagnosis, all infections including skin infection up to 2 years of age were more frequent in children who subsequently developed AD compared to healthy controls.
- Medeleanu et al8 reported findings from the Canadian Healthy Infant Longitudinal Development (CHILD) Cohort Study, which included a longitudinal birth cohort of 3,272 parents and infants recruited during pregnancy. They found that infants with moderate-severe vs. no or mild lower respiratory tract infections in the first 18 months of life had significantly higher rates of AD and type 1 allergen polysensitization at age 3 and 5 years. These associations remained significant after adjusting for sex, breastfeeding duration, and parental history of atopy or asthma.
Together, these studies suggest that prevention and expedient treatment of early life infections may lower risk for AD in childhood. Conversely, children at risk for AD who experience certain infections early in life may benefit from increased surveillance for AD and atopic disease.
References
- Johansson EK et al. Prevalence and characteristics of atopic dermatitis among young adult females and males-report from the Swedish population-based study BAMSE. J Eur Acad Dermatol Venereol. 2022 (Jan 15).
- Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin. 2017;35(3):283-289.
- Silverberg JI et al. Patient burden and quality of life in atopic dermatitis in US adults: A population-based cross-sectional study. Ann Allerg Asthma Immunol. 2018;121(3):340-347.
- Hua T, Silverberg JI. Atopic dermatitis in US adults: Epidemiology, association with marital status, and atopy. Ann Allerg Asthma Immunol. 2018;121(5):622-624.
- Lee HH et al. A systematic review and meta-analysis of the prevalence and phenotype of adult-onset atopic dermatitis. J Am Acad Dermatol. 2019;80(6):1526-1532.e7.
- Paller AS et al. Infections in children and adolescents treated with dupilumab in pediatric clinical trials for atopic dermatitis—A pooled analysis of trial data. Pediatr Dermatol. 2022 (Jan 26).
- Lin T-L et al. Early-life infections in association with the development of atopic dermatitis in infancy and early childhood: a nationwide nested case–control study. J Eur Acad Dermatol Venereol. 2022 (Jan 9).
- Medeleanu M et al. Moderate-to-severe lower respiratory tract infection in early life is associated with increased risk of polysensitization and atopic dermatitis: Findings from the CHILD Study. J Allergy Clin Immunol. 2022 (Jan 16).
Recent insights into the epidemiology of atopic dermatitis
Atopic dermatitis (AD) has complex risk factors and effects on patients. AD patients experience itch, skin pain, sleep disturbances, and other symptoms that can profoundly impact their quality of life. Yet, little is known about the longitudinal epidemiology and burden of AD in children.
- Johansson et al1 reported on the ongoing BAMSE cohort study. BAMSE followed 4089 individuals regularly from birth regarding AD and atopic diseases with surveys and clinical examinations; 3055 individuals were assessed at year 24 of follow-up. At 24 years, the 12-month prevalence of AD was 17.8% and more common in women than men (20.5% vs. 14.8%). The point prevalence of AD on clinical examination was 8.0%. These high prevalence estimates are consistent with multiple other recent studies in the United States and globally.2-4 Prevalence measures a combination of both new-onset (incident) cases and persistence of childhood disease. Importantly, BAMSE found the proportion of adult-onset AD was 16.9%. These results are consistent with previous studies that found substantial rates of adult-onset AD.5 Additionally, men were more likely to have AD in the first year of life, but less likely than women to have AD in adolescence and young adulthood.
- Paller et al6 recently initiated PEDISTAT, an international, longitudinal 5-year registry of the disease course, comorbidities, treatment, and disease burden children age <12 years with moderate-severe AD. While the study is ongoing, the authors reported the baseline characteristics of the registry. They found that most of the enrolled children were not treated with a systemic therapy, had inadequately controlled disease and a high disease burden. These results emphasize the need for very safe and highly effective systemic therapies for moderate-severe AD in children.
I look forward to seeing the results of these ongoing study and how they will inform our understanding of the epidemiology and comorbidities of AD.
Numerous risk factors for AD have been examined. Infections have been explored as a potential risk factor for AD for more than 30 years.
- Lin et al7 conducted a population-based, nationwide case-control study including 5,454 children with AD matched with 16,362 healthy controls without AD. They found that prior to AD diagnosis, all infections including skin infection up to 2 years of age were more frequent in children who subsequently developed AD compared to healthy controls.
- Medeleanu et al8 reported findings from the Canadian Healthy Infant Longitudinal Development (CHILD) Cohort Study, which included a longitudinal birth cohort of 3,272 parents and infants recruited during pregnancy. They found that infants with moderate-severe vs. no or mild lower respiratory tract infections in the first 18 months of life had significantly higher rates of AD and type 1 allergen polysensitization at age 3 and 5 years. These associations remained significant after adjusting for sex, breastfeeding duration, and parental history of atopy or asthma.
Together, these studies suggest that prevention and expedient treatment of early life infections may lower risk for AD in childhood. Conversely, children at risk for AD who experience certain infections early in life may benefit from increased surveillance for AD and atopic disease.
References
- Johansson EK et al. Prevalence and characteristics of atopic dermatitis among young adult females and males-report from the Swedish population-based study BAMSE. J Eur Acad Dermatol Venereol. 2022 (Jan 15).
- Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin. 2017;35(3):283-289.
- Silverberg JI et al. Patient burden and quality of life in atopic dermatitis in US adults: A population-based cross-sectional study. Ann Allerg Asthma Immunol. 2018;121(3):340-347.
- Hua T, Silverberg JI. Atopic dermatitis in US adults: Epidemiology, association with marital status, and atopy. Ann Allerg Asthma Immunol. 2018;121(5):622-624.
- Lee HH et al. A systematic review and meta-analysis of the prevalence and phenotype of adult-onset atopic dermatitis. J Am Acad Dermatol. 2019;80(6):1526-1532.e7.
- Paller AS et al. Infections in children and adolescents treated with dupilumab in pediatric clinical trials for atopic dermatitis—A pooled analysis of trial data. Pediatr Dermatol. 2022 (Jan 26).
- Lin T-L et al. Early-life infections in association with the development of atopic dermatitis in infancy and early childhood: a nationwide nested case–control study. J Eur Acad Dermatol Venereol. 2022 (Jan 9).
- Medeleanu M et al. Moderate-to-severe lower respiratory tract infection in early life is associated with increased risk of polysensitization and atopic dermatitis: Findings from the CHILD Study. J Allergy Clin Immunol. 2022 (Jan 16).
Recent insights into the epidemiology of atopic dermatitis
Atopic dermatitis (AD) has complex risk factors and effects on patients. AD patients experience itch, skin pain, sleep disturbances, and other symptoms that can profoundly impact their quality of life. Yet, little is known about the longitudinal epidemiology and burden of AD in children.
- Johansson et al1 reported on the ongoing BAMSE cohort study. BAMSE followed 4089 individuals regularly from birth regarding AD and atopic diseases with surveys and clinical examinations; 3055 individuals were assessed at year 24 of follow-up. At 24 years, the 12-month prevalence of AD was 17.8% and more common in women than men (20.5% vs. 14.8%). The point prevalence of AD on clinical examination was 8.0%. These high prevalence estimates are consistent with multiple other recent studies in the United States and globally.2-4 Prevalence measures a combination of both new-onset (incident) cases and persistence of childhood disease. Importantly, BAMSE found the proportion of adult-onset AD was 16.9%. These results are consistent with previous studies that found substantial rates of adult-onset AD.5 Additionally, men were more likely to have AD in the first year of life, but less likely than women to have AD in adolescence and young adulthood.
- Paller et al6 recently initiated PEDISTAT, an international, longitudinal 5-year registry of the disease course, comorbidities, treatment, and disease burden children age <12 years with moderate-severe AD. While the study is ongoing, the authors reported the baseline characteristics of the registry. They found that most of the enrolled children were not treated with a systemic therapy, had inadequately controlled disease and a high disease burden. These results emphasize the need for very safe and highly effective systemic therapies for moderate-severe AD in children.
I look forward to seeing the results of these ongoing study and how they will inform our understanding of the epidemiology and comorbidities of AD.
Numerous risk factors for AD have been examined. Infections have been explored as a potential risk factor for AD for more than 30 years.
- Lin et al7 conducted a population-based, nationwide case-control study including 5,454 children with AD matched with 16,362 healthy controls without AD. They found that prior to AD diagnosis, all infections including skin infection up to 2 years of age were more frequent in children who subsequently developed AD compared to healthy controls.
- Medeleanu et al8 reported findings from the Canadian Healthy Infant Longitudinal Development (CHILD) Cohort Study, which included a longitudinal birth cohort of 3,272 parents and infants recruited during pregnancy. They found that infants with moderate-severe vs. no or mild lower respiratory tract infections in the first 18 months of life had significantly higher rates of AD and type 1 allergen polysensitization at age 3 and 5 years. These associations remained significant after adjusting for sex, breastfeeding duration, and parental history of atopy or asthma.
Together, these studies suggest that prevention and expedient treatment of early life infections may lower risk for AD in childhood. Conversely, children at risk for AD who experience certain infections early in life may benefit from increased surveillance for AD and atopic disease.
References
- Johansson EK et al. Prevalence and characteristics of atopic dermatitis among young adult females and males-report from the Swedish population-based study BAMSE. J Eur Acad Dermatol Venereol. 2022 (Jan 15).
- Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin. 2017;35(3):283-289.
- Silverberg JI et al. Patient burden and quality of life in atopic dermatitis in US adults: A population-based cross-sectional study. Ann Allerg Asthma Immunol. 2018;121(3):340-347.
- Hua T, Silverberg JI. Atopic dermatitis in US adults: Epidemiology, association with marital status, and atopy. Ann Allerg Asthma Immunol. 2018;121(5):622-624.
- Lee HH et al. A systematic review and meta-analysis of the prevalence and phenotype of adult-onset atopic dermatitis. J Am Acad Dermatol. 2019;80(6):1526-1532.e7.
- Paller AS et al. Infections in children and adolescents treated with dupilumab in pediatric clinical trials for atopic dermatitis—A pooled analysis of trial data. Pediatr Dermatol. 2022 (Jan 26).
- Lin T-L et al. Early-life infections in association with the development of atopic dermatitis in infancy and early childhood: a nationwide nested case–control study. J Eur Acad Dermatol Venereol. 2022 (Jan 9).
- Medeleanu M et al. Moderate-to-severe lower respiratory tract infection in early life is associated with increased risk of polysensitization and atopic dermatitis: Findings from the CHILD Study. J Allergy Clin Immunol. 2022 (Jan 16).
Reduced hospitalization risk with dupilumab in moderate-to-severe atopic dermatitis
Key clinical point: The risk for all-cause and atopic dermatitis (AD)-related hospitalization was significantly lower in adult patients with moderate-to-severe AD who received dupilumab vs. placebo.
Major finding: Risk for all-cause hospitalizations was lower by 62% (risk ratio [RR] 0.38; P < .001) and AD-related hospitalizations by 79% (RR 0.21; P < .001) in patients who received dupilumab vs. placebo.
Study details: Findings are from a post hoc analysis of pooled data from 7 phase 2/3 trials including 2,932 patients with moderate-to-severe AD who were randomly assigned to 300 mg dupilumab (every 2 weeks or weekly) with or without topical corticosteroids or placebo for 12, 16, or 52 weeks.
Disclosures: The study was funded by Sanofi and Regeneron Pharmaceuticals. The authors declared serving as consultants or receiving grants and honoraria from several sources. Six authors declared being employees or shareholders of Sanofi or Regeneron or Sanofi Genzyme.
Source: Silverberg JI et al. J Allergy Clin Immunol Pract. 2022 (Jan 12). Doi: 10.1016/j.jaip.2021.11.034.
Key clinical point: The risk for all-cause and atopic dermatitis (AD)-related hospitalization was significantly lower in adult patients with moderate-to-severe AD who received dupilumab vs. placebo.
Major finding: Risk for all-cause hospitalizations was lower by 62% (risk ratio [RR] 0.38; P < .001) and AD-related hospitalizations by 79% (RR 0.21; P < .001) in patients who received dupilumab vs. placebo.
Study details: Findings are from a post hoc analysis of pooled data from 7 phase 2/3 trials including 2,932 patients with moderate-to-severe AD who were randomly assigned to 300 mg dupilumab (every 2 weeks or weekly) with or without topical corticosteroids or placebo for 12, 16, or 52 weeks.
Disclosures: The study was funded by Sanofi and Regeneron Pharmaceuticals. The authors declared serving as consultants or receiving grants and honoraria from several sources. Six authors declared being employees or shareholders of Sanofi or Regeneron or Sanofi Genzyme.
Source: Silverberg JI et al. J Allergy Clin Immunol Pract. 2022 (Jan 12). Doi: 10.1016/j.jaip.2021.11.034.
Key clinical point: The risk for all-cause and atopic dermatitis (AD)-related hospitalization was significantly lower in adult patients with moderate-to-severe AD who received dupilumab vs. placebo.
Major finding: Risk for all-cause hospitalizations was lower by 62% (risk ratio [RR] 0.38; P < .001) and AD-related hospitalizations by 79% (RR 0.21; P < .001) in patients who received dupilumab vs. placebo.
Study details: Findings are from a post hoc analysis of pooled data from 7 phase 2/3 trials including 2,932 patients with moderate-to-severe AD who were randomly assigned to 300 mg dupilumab (every 2 weeks or weekly) with or without topical corticosteroids or placebo for 12, 16, or 52 weeks.
Disclosures: The study was funded by Sanofi and Regeneron Pharmaceuticals. The authors declared serving as consultants or receiving grants and honoraria from several sources. Six authors declared being employees or shareholders of Sanofi or Regeneron or Sanofi Genzyme.
Source: Silverberg JI et al. J Allergy Clin Immunol Pract. 2022 (Jan 12). Doi: 10.1016/j.jaip.2021.11.034.
Atopic dermatitis: Dupilumab effective and safe in real world
Key clinical point: Under real-world settings, dupilumab was an effective and safe therapeutic option for adolescents and adults with moderate-to-severe atopic dermatitis (AD).
Major finding: At least 75% improvement in the Eczema Area and Severity Index was achieved by 53.3% and 79.4% of patients at weeks 12 and 48, respectively. Overall, mild adverse events were reported by 32% of patients, with the most frequent being conjunctivitis, persistent facial erythema, and arthritis/arthralgia.
Study details: Findings are from a nationwide, retrospective 48-week study including 169 patients aged 12 years or older with moderate-to-severe AD who received dupilumab.
Disclosures: This study did not report any source of funding. Some authors declared serving as a speakers and principal investigators or receiving consulting fees, research grants, and honoraria from several sources.
Source: Torres T et al. J Dermatolog Treat. 2022 (Jan 31). Doi: 10.1080/09546634.2022.2035309
Key clinical point: Under real-world settings, dupilumab was an effective and safe therapeutic option for adolescents and adults with moderate-to-severe atopic dermatitis (AD).
Major finding: At least 75% improvement in the Eczema Area and Severity Index was achieved by 53.3% and 79.4% of patients at weeks 12 and 48, respectively. Overall, mild adverse events were reported by 32% of patients, with the most frequent being conjunctivitis, persistent facial erythema, and arthritis/arthralgia.
Study details: Findings are from a nationwide, retrospective 48-week study including 169 patients aged 12 years or older with moderate-to-severe AD who received dupilumab.
Disclosures: This study did not report any source of funding. Some authors declared serving as a speakers and principal investigators or receiving consulting fees, research grants, and honoraria from several sources.
Source: Torres T et al. J Dermatolog Treat. 2022 (Jan 31). Doi: 10.1080/09546634.2022.2035309
Key clinical point: Under real-world settings, dupilumab was an effective and safe therapeutic option for adolescents and adults with moderate-to-severe atopic dermatitis (AD).
Major finding: At least 75% improvement in the Eczema Area and Severity Index was achieved by 53.3% and 79.4% of patients at weeks 12 and 48, respectively. Overall, mild adverse events were reported by 32% of patients, with the most frequent being conjunctivitis, persistent facial erythema, and arthritis/arthralgia.
Study details: Findings are from a nationwide, retrospective 48-week study including 169 patients aged 12 years or older with moderate-to-severe AD who received dupilumab.
Disclosures: This study did not report any source of funding. Some authors declared serving as a speakers and principal investigators or receiving consulting fees, research grants, and honoraria from several sources.
Source: Torres T et al. J Dermatolog Treat. 2022 (Jan 31). Doi: 10.1080/09546634.2022.2035309
Risk for infection in children and adolescents with atopic dermatitis treated with dupilumab
Key clinical point: The risk for overall infection was not higher in children and adolescents with moderate-to-severe or severe atopic dermatitis (AD) treated with dupilumab vs. placebo; however, the risk for skin infections was significantly lower with dupilumab.
Major finding: Dupilumab did not increase the risk for overall infections (risk ratio [RR] 0.76; P = .051) and was associated with a reduced risk for total skin infections (RR 0.45; P = .003) compared with placebo.
Study details: This was a pooled analysis of 2 phase 3 trials including 612 adolescents or children with moderate-to-severe/severe AD who received dupilumab either as monotherapy (LIBERTY AD ADOL) or with concomitant topical corticosteroids (LIBERTY AD PEDS).
Disclosures: This study was funded by Sanofi and Regeneron Pharmaceuticals. The authors declared serving as investigators, speakers, consultants, and scientific advisors or clinical study investigators, and advisory board members or receiving honoraria and grants from several sources. Six authors declared being employees or shareholders of Sanofi and Regeneron Pharmaceuticals.
Source: Paller AS et al. Pediatr Dermatol. 2022 (Jan 26). Doi: 10.1111/pde.14909
Key clinical point: The risk for overall infection was not higher in children and adolescents with moderate-to-severe or severe atopic dermatitis (AD) treated with dupilumab vs. placebo; however, the risk for skin infections was significantly lower with dupilumab.
Major finding: Dupilumab did not increase the risk for overall infections (risk ratio [RR] 0.76; P = .051) and was associated with a reduced risk for total skin infections (RR 0.45; P = .003) compared with placebo.
Study details: This was a pooled analysis of 2 phase 3 trials including 612 adolescents or children with moderate-to-severe/severe AD who received dupilumab either as monotherapy (LIBERTY AD ADOL) or with concomitant topical corticosteroids (LIBERTY AD PEDS).
Disclosures: This study was funded by Sanofi and Regeneron Pharmaceuticals. The authors declared serving as investigators, speakers, consultants, and scientific advisors or clinical study investigators, and advisory board members or receiving honoraria and grants from several sources. Six authors declared being employees or shareholders of Sanofi and Regeneron Pharmaceuticals.
Source: Paller AS et al. Pediatr Dermatol. 2022 (Jan 26). Doi: 10.1111/pde.14909
Key clinical point: The risk for overall infection was not higher in children and adolescents with moderate-to-severe or severe atopic dermatitis (AD) treated with dupilumab vs. placebo; however, the risk for skin infections was significantly lower with dupilumab.
Major finding: Dupilumab did not increase the risk for overall infections (risk ratio [RR] 0.76; P = .051) and was associated with a reduced risk for total skin infections (RR 0.45; P = .003) compared with placebo.
Study details: This was a pooled analysis of 2 phase 3 trials including 612 adolescents or children with moderate-to-severe/severe AD who received dupilumab either as monotherapy (LIBERTY AD ADOL) or with concomitant topical corticosteroids (LIBERTY AD PEDS).
Disclosures: This study was funded by Sanofi and Regeneron Pharmaceuticals. The authors declared serving as investigators, speakers, consultants, and scientific advisors or clinical study investigators, and advisory board members or receiving honoraria and grants from several sources. Six authors declared being employees or shareholders of Sanofi and Regeneron Pharmaceuticals.
Source: Paller AS et al. Pediatr Dermatol. 2022 (Jan 26). Doi: 10.1111/pde.14909