User login
Impact of the COVID-19 Pandemic on Care for Patients With Skin Cancer
To the Editor:
The most common malignancy in the United States is skin cancer, with melanoma accounting for the majority of skin cancer deaths.1 Despite the lack of established guidelines for routine total-body skin examinations, many patients regularly visit their dermatologist for assessment of pigmented skin lesions.2 During the COVID-19 pandemic, many patients were unable to attend in-person dermatology visits, which resulted in many high-risk individuals not receiving care or alternatively seeking virtual care for cutaneous lesions.3 There has been a lack of research in the United States exploring the utilization of teledermatology during the pandemic and its overall impact on the care of patients with a history of skin cancer. We explored the impact of the COVID-19 pandemic on care for patients with skin cancer in a large US population.

Using anonymous survey data from the 2020-2021 National Health Interview Survey,4 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with a self-reported history of skin cancer—melanoma, nonmelanoma skin cancer, or unknown skin cancer. The 3 outcome variables included having a virtual medical appointment in the past 12 months (yes/no), delaying medical care due to the COVID-19 pandemic (yes/no), and not receiving care due to the COVID-19 pandemic (yes/no). Multivariable logistic regression models evaluating the relationship between a history of skin cancer and access to care were constructed using Stata/MP 17.0 (StataCorp LLC). We controlled for patient age; education; race/ethnicity; received public assistance or welfare payments; sex; region; US citizenship status; health insurance status; comorbidities including history of hypertension, diabetes, and hypercholesterolemia; and birthplace in the United States in the logistic regression models.
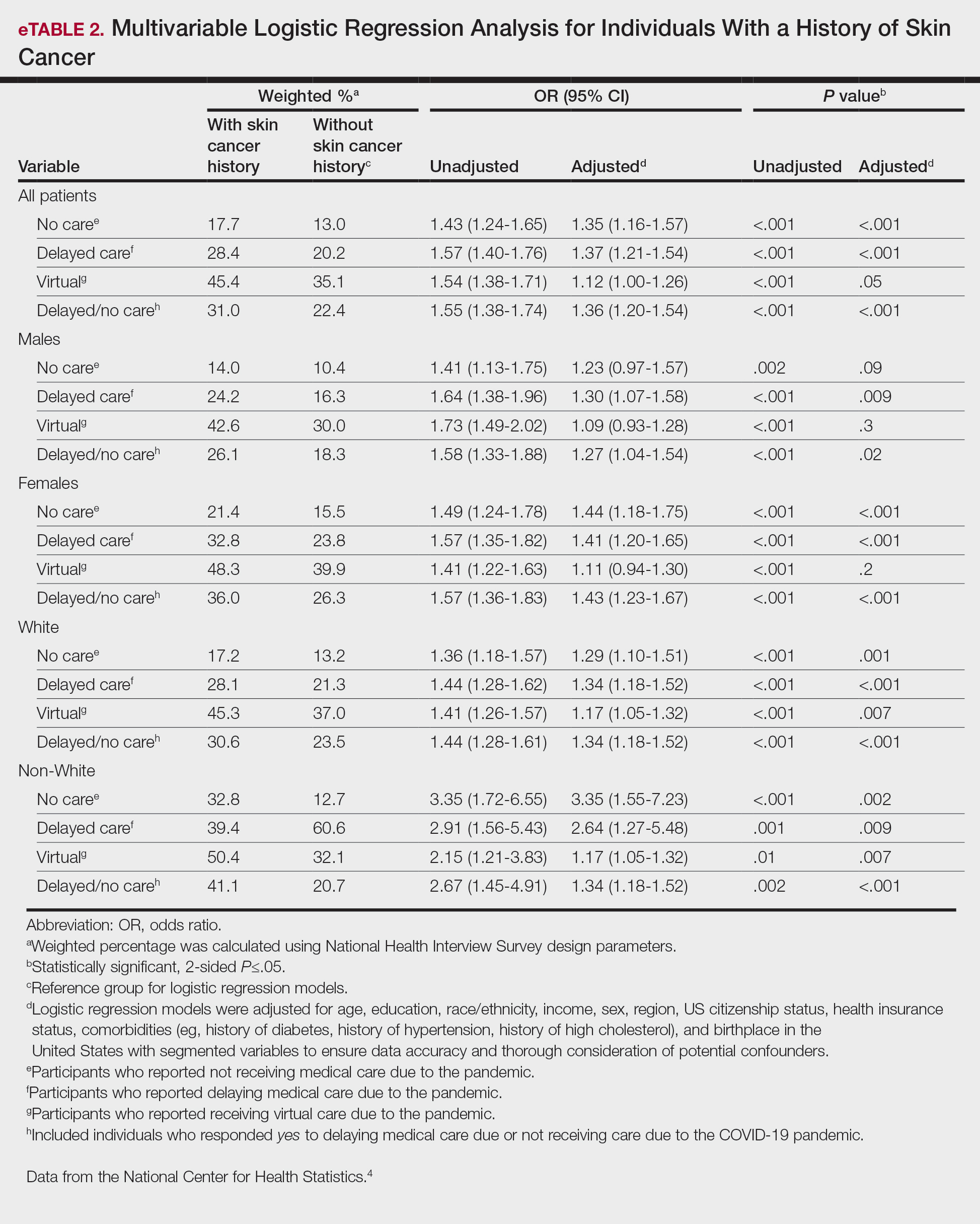
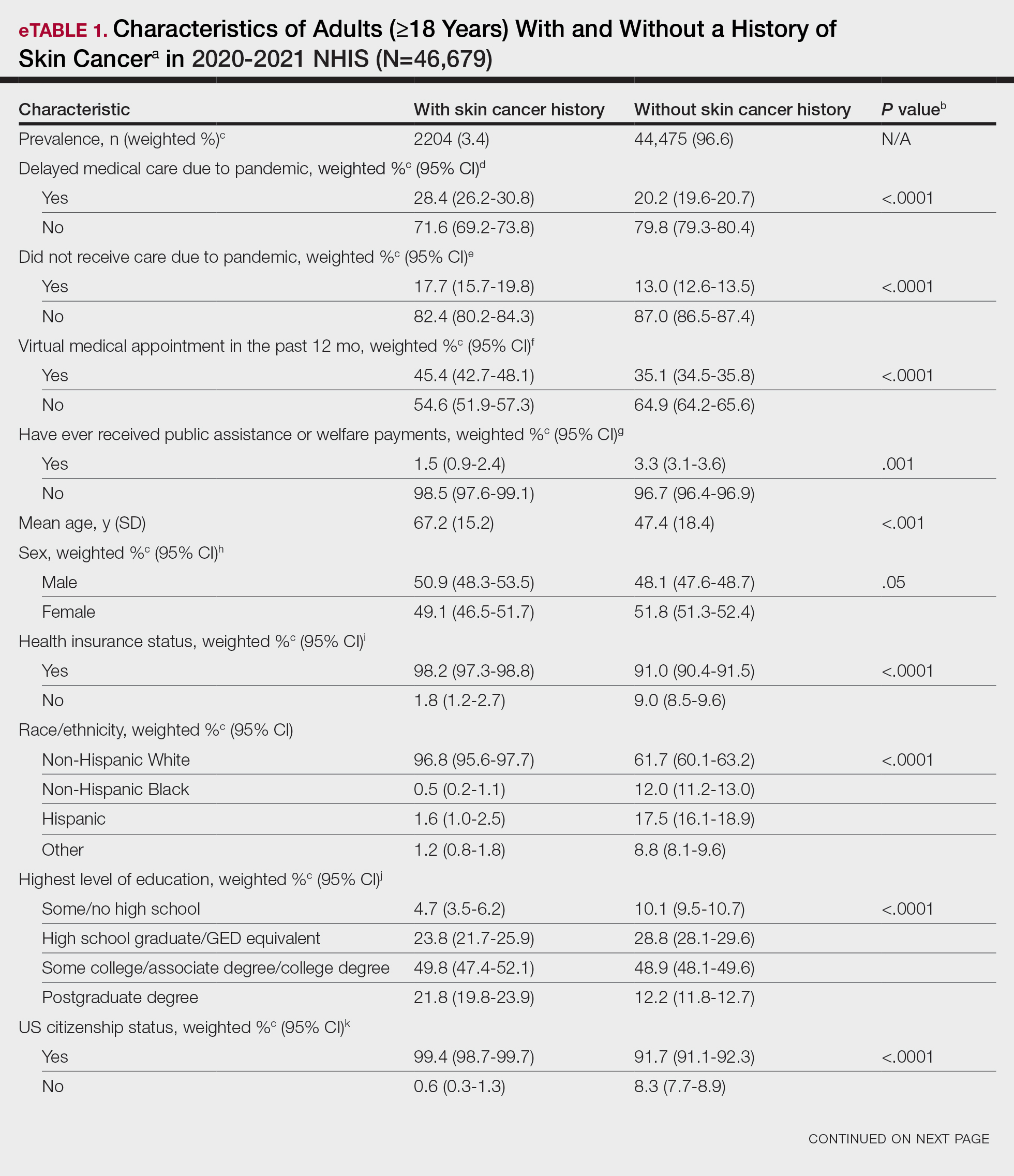
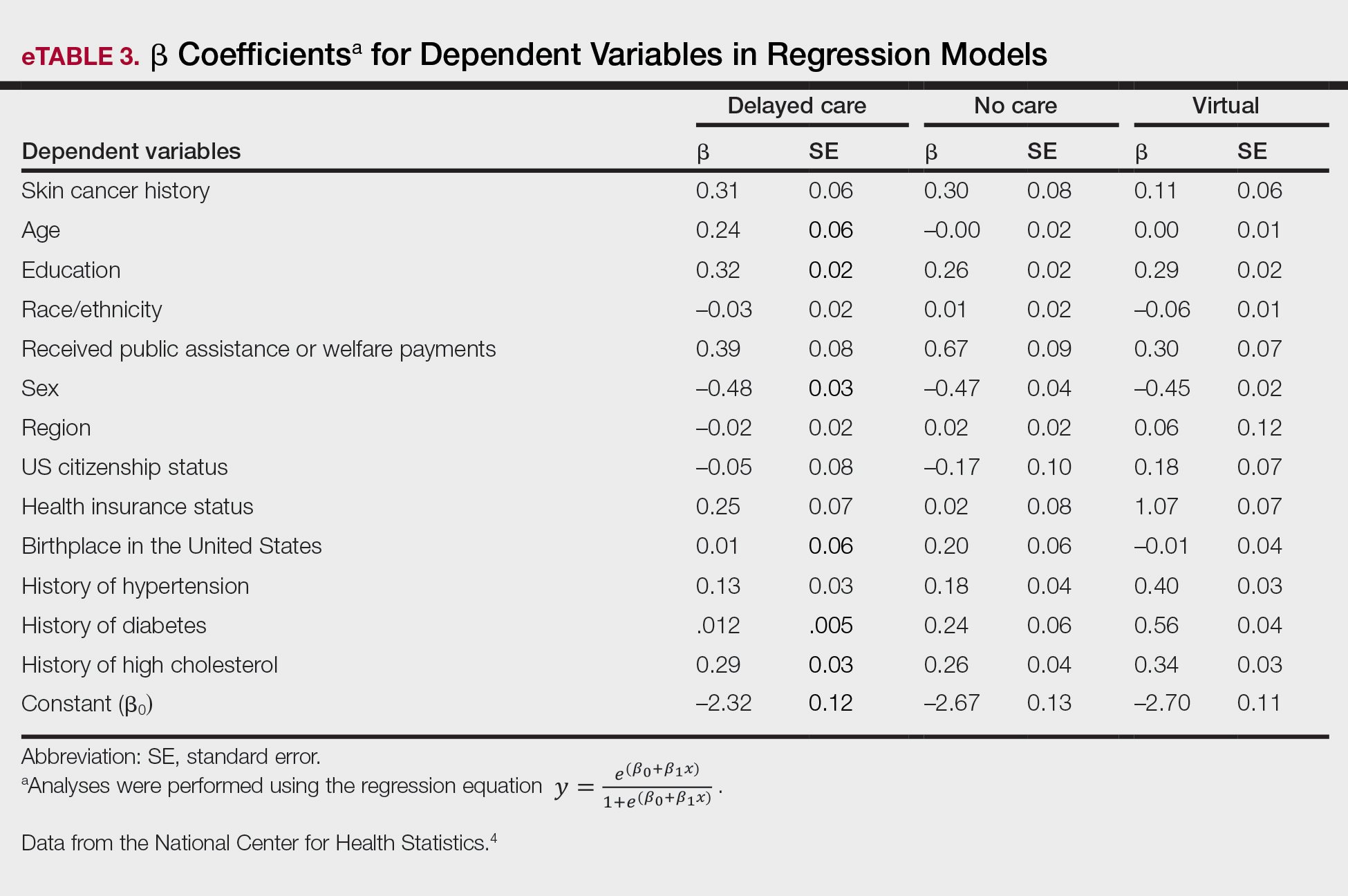
Our analysis included 46,679 patients aged 18 years or older, of whom 3.4% (weighted)(n=2204) reported a history of skin cancer (eTable 1). The weighted percentage was calculated using National Health Interview Survey design parameters (accounting for the multistage sampling design) to represent the general US population. Compared with those with no history of skin cancer, patients with a history of skin cancer were significantly more likely to delay medical care (adjusted odds ratio [AOR], 1.37; 95% CI, 1.21-1.54; P<.001) or not receive care (AOR, 1.35; 95% CI, 1.16-1.57; P<.001) due to the pandemic and were more likely to have had a virtual medical visit in the past 12 months (AOR, 1.12; 95% CI, 1.00-1.26; P=.05). Additionally, subgroup analysis revealed that females were more likely than males to forego medical care (eTable 2). β Coefficients for independent and dependent variables were further analyzed using logistic regression (eTable 3).
After adjusting for various potential confounders including comorbidities, our results revealed that patients with a history of skin cancer reported that they were less likely to receive in-person medical care due to the COVID-19 pandemic, as high-risk individuals with a history of skin cancer may have stopped receiving total-body skin examinations and dermatology care during the pandemic. Our findings showed that patients with a history of skin cancer were more likely than those without skin cancer to delay or forego care due to the pandemic, which may contribute to a higher incidence of advanced-stage melanomas postpandemic. Trepanowski et al5 reported an increased incidence of patients presenting with more advanced melanomas during the pandemic. Telemedicine was more commonly utilized by patients with a history of skin cancer during the pandemic.
In the future, virtual care may help limit advanced stages of skin cancer by serving as a viable alternative to in-person care.6 It has been reported that telemedicine can serve as a useful triage service reducing patient wait times.7 Teledermatology should not replace in-person care, as there is no evidence of the diagnostic accuracy of this service and many patients still will need to be seen in-person for confirmation of their diagnosis and potential biopsy. Further studies are needed to assess for missed skin cancer diagnoses due to the utilization of telemedicine.
Limitations of this study included a self-reported history of skin cancer, β coefficients that may suggest a high degree of collinearity, and lack of specific survey questions regarding dermatologic care during the COVID-19 pandemic. Further long-term studies exploring the clinical applicability and diagnostic accuracy of virtual medicine visits for cutaneous malignancies are vital, as teledermatology may play an essential role in curbing rising skin cancer rates even beyond the pandemic.
- Guy GP Jr, Thomas CC, Thompson T, et al. Vital signs: melanoma incidence and mortality trends and projections—United States, 1982-2030. MMWR Morb Mortal Wkly Rep. 2015;64:591-596.
- Whiteman DC, Olsen CM, MacGregor S, et al; QSkin Study. The effect of screening on melanoma incidence and biopsy rates. Br J Dermatol. 2022;187:515-522. doi:10.1111/bjd.21649
- Jobbágy A, Kiss N, Meznerics FA, et al. Emergency use and efficacy of an asynchronous teledermatology system as a novel tool for early diagnosis of skin cancer during the first wave of COVID-19 pandemic. Int J Environ Res Public Health. 2022;19:2699. doi:10.3390/ijerph19052699
- National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed April 19, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
- Trepanowski N, Chang MS, Zhou G, et al. Delays in melanoma presentation during the COVID-19 pandemic: a nationwide multi-institutional cohort study. J Am Acad Dermatol. 2022;87:1217-1219. doi:10.1016/j.jaad.2022.06.031
- Chiru MR, Hindocha S, Burova E, et al. Management of the two-week wait pathway for skin cancer patients, before and during the pandemic: is virtual consultation an option? J Pers Med. 2022;12:1258. doi:10.3390/jpm12081258
- Finnane A Dallest K Janda M et al. Teledermatology for the diagnosis and management of skin cancer: a systematic review. JAMA Dermatol. 2017;153:319-327. doi:10.1001/jamadermatol.2016.4361
To the Editor:
The most common malignancy in the United States is skin cancer, with melanoma accounting for the majority of skin cancer deaths.1 Despite the lack of established guidelines for routine total-body skin examinations, many patients regularly visit their dermatologist for assessment of pigmented skin lesions.2 During the COVID-19 pandemic, many patients were unable to attend in-person dermatology visits, which resulted in many high-risk individuals not receiving care or alternatively seeking virtual care for cutaneous lesions.3 There has been a lack of research in the United States exploring the utilization of teledermatology during the pandemic and its overall impact on the care of patients with a history of skin cancer. We explored the impact of the COVID-19 pandemic on care for patients with skin cancer in a large US population.


Using anonymous survey data from the 2020-2021 National Health Interview Survey,4 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with a self-reported history of skin cancer—melanoma, nonmelanoma skin cancer, or unknown skin cancer. The 3 outcome variables included having a virtual medical appointment in the past 12 months (yes/no), delaying medical care due to the COVID-19 pandemic (yes/no), and not receiving care due to the COVID-19 pandemic (yes/no). Multivariable logistic regression models evaluating the relationship between a history of skin cancer and access to care were constructed using Stata/MP 17.0 (StataCorp LLC). We controlled for patient age; education; race/ethnicity; received public assistance or welfare payments; sex; region; US citizenship status; health insurance status; comorbidities including history of hypertension, diabetes, and hypercholesterolemia; and birthplace in the United States in the logistic regression models.

Our analysis included 46,679 patients aged 18 years or older, of whom 3.4% (weighted)(n=2204) reported a history of skin cancer (eTable 1). The weighted percentage was calculated using National Health Interview Survey design parameters (accounting for the multistage sampling design) to represent the general US population. Compared with those with no history of skin cancer, patients with a history of skin cancer were significantly more likely to delay medical care (adjusted odds ratio [AOR], 1.37; 95% CI, 1.21-1.54; P<.001) or not receive care (AOR, 1.35; 95% CI, 1.16-1.57; P<.001) due to the pandemic and were more likely to have had a virtual medical visit in the past 12 months (AOR, 1.12; 95% CI, 1.00-1.26; P=.05). Additionally, subgroup analysis revealed that females were more likely than males to forego medical care (eTable 2). β Coefficients for independent and dependent variables were further analyzed using logistic regression (eTable 3).

After adjusting for various potential confounders including comorbidities, our results revealed that patients with a history of skin cancer reported that they were less likely to receive in-person medical care due to the COVID-19 pandemic, as high-risk individuals with a history of skin cancer may have stopped receiving total-body skin examinations and dermatology care during the pandemic. Our findings showed that patients with a history of skin cancer were more likely than those without skin cancer to delay or forego care due to the pandemic, which may contribute to a higher incidence of advanced-stage melanomas postpandemic. Trepanowski et al5 reported an increased incidence of patients presenting with more advanced melanomas during the pandemic. Telemedicine was more commonly utilized by patients with a history of skin cancer during the pandemic.
In the future, virtual care may help limit advanced stages of skin cancer by serving as a viable alternative to in-person care.6 It has been reported that telemedicine can serve as a useful triage service reducing patient wait times.7 Teledermatology should not replace in-person care, as there is no evidence of the diagnostic accuracy of this service and many patients still will need to be seen in-person for confirmation of their diagnosis and potential biopsy. Further studies are needed to assess for missed skin cancer diagnoses due to the utilization of telemedicine.
Limitations of this study included a self-reported history of skin cancer, β coefficients that may suggest a high degree of collinearity, and lack of specific survey questions regarding dermatologic care during the COVID-19 pandemic. Further long-term studies exploring the clinical applicability and diagnostic accuracy of virtual medicine visits for cutaneous malignancies are vital, as teledermatology may play an essential role in curbing rising skin cancer rates even beyond the pandemic.
To the Editor:
The most common malignancy in the United States is skin cancer, with melanoma accounting for the majority of skin cancer deaths.1 Despite the lack of established guidelines for routine total-body skin examinations, many patients regularly visit their dermatologist for assessment of pigmented skin lesions.2 During the COVID-19 pandemic, many patients were unable to attend in-person dermatology visits, which resulted in many high-risk individuals not receiving care or alternatively seeking virtual care for cutaneous lesions.3 There has been a lack of research in the United States exploring the utilization of teledermatology during the pandemic and its overall impact on the care of patients with a history of skin cancer. We explored the impact of the COVID-19 pandemic on care for patients with skin cancer in a large US population.


Using anonymous survey data from the 2020-2021 National Health Interview Survey,4 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with a self-reported history of skin cancer—melanoma, nonmelanoma skin cancer, or unknown skin cancer. The 3 outcome variables included having a virtual medical appointment in the past 12 months (yes/no), delaying medical care due to the COVID-19 pandemic (yes/no), and not receiving care due to the COVID-19 pandemic (yes/no). Multivariable logistic regression models evaluating the relationship between a history of skin cancer and access to care were constructed using Stata/MP 17.0 (StataCorp LLC). We controlled for patient age; education; race/ethnicity; received public assistance or welfare payments; sex; region; US citizenship status; health insurance status; comorbidities including history of hypertension, diabetes, and hypercholesterolemia; and birthplace in the United States in the logistic regression models.

Our analysis included 46,679 patients aged 18 years or older, of whom 3.4% (weighted)(n=2204) reported a history of skin cancer (eTable 1). The weighted percentage was calculated using National Health Interview Survey design parameters (accounting for the multistage sampling design) to represent the general US population. Compared with those with no history of skin cancer, patients with a history of skin cancer were significantly more likely to delay medical care (adjusted odds ratio [AOR], 1.37; 95% CI, 1.21-1.54; P<.001) or not receive care (AOR, 1.35; 95% CI, 1.16-1.57; P<.001) due to the pandemic and were more likely to have had a virtual medical visit in the past 12 months (AOR, 1.12; 95% CI, 1.00-1.26; P=.05). Additionally, subgroup analysis revealed that females were more likely than males to forego medical care (eTable 2). β Coefficients for independent and dependent variables were further analyzed using logistic regression (eTable 3).

After adjusting for various potential confounders including comorbidities, our results revealed that patients with a history of skin cancer reported that they were less likely to receive in-person medical care due to the COVID-19 pandemic, as high-risk individuals with a history of skin cancer may have stopped receiving total-body skin examinations and dermatology care during the pandemic. Our findings showed that patients with a history of skin cancer were more likely than those without skin cancer to delay or forego care due to the pandemic, which may contribute to a higher incidence of advanced-stage melanomas postpandemic. Trepanowski et al5 reported an increased incidence of patients presenting with more advanced melanomas during the pandemic. Telemedicine was more commonly utilized by patients with a history of skin cancer during the pandemic.
In the future, virtual care may help limit advanced stages of skin cancer by serving as a viable alternative to in-person care.6 It has been reported that telemedicine can serve as a useful triage service reducing patient wait times.7 Teledermatology should not replace in-person care, as there is no evidence of the diagnostic accuracy of this service and many patients still will need to be seen in-person for confirmation of their diagnosis and potential biopsy. Further studies are needed to assess for missed skin cancer diagnoses due to the utilization of telemedicine.
Limitations of this study included a self-reported history of skin cancer, β coefficients that may suggest a high degree of collinearity, and lack of specific survey questions regarding dermatologic care during the COVID-19 pandemic. Further long-term studies exploring the clinical applicability and diagnostic accuracy of virtual medicine visits for cutaneous malignancies are vital, as teledermatology may play an essential role in curbing rising skin cancer rates even beyond the pandemic.
- Guy GP Jr, Thomas CC, Thompson T, et al. Vital signs: melanoma incidence and mortality trends and projections—United States, 1982-2030. MMWR Morb Mortal Wkly Rep. 2015;64:591-596.
- Whiteman DC, Olsen CM, MacGregor S, et al; QSkin Study. The effect of screening on melanoma incidence and biopsy rates. Br J Dermatol. 2022;187:515-522. doi:10.1111/bjd.21649
- Jobbágy A, Kiss N, Meznerics FA, et al. Emergency use and efficacy of an asynchronous teledermatology system as a novel tool for early diagnosis of skin cancer during the first wave of COVID-19 pandemic. Int J Environ Res Public Health. 2022;19:2699. doi:10.3390/ijerph19052699
- National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed April 19, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
- Trepanowski N, Chang MS, Zhou G, et al. Delays in melanoma presentation during the COVID-19 pandemic: a nationwide multi-institutional cohort study. J Am Acad Dermatol. 2022;87:1217-1219. doi:10.1016/j.jaad.2022.06.031
- Chiru MR, Hindocha S, Burova E, et al. Management of the two-week wait pathway for skin cancer patients, before and during the pandemic: is virtual consultation an option? J Pers Med. 2022;12:1258. doi:10.3390/jpm12081258
- Finnane A Dallest K Janda M et al. Teledermatology for the diagnosis and management of skin cancer: a systematic review. JAMA Dermatol. 2017;153:319-327. doi:10.1001/jamadermatol.2016.4361
- Guy GP Jr, Thomas CC, Thompson T, et al. Vital signs: melanoma incidence and mortality trends and projections—United States, 1982-2030. MMWR Morb Mortal Wkly Rep. 2015;64:591-596.
- Whiteman DC, Olsen CM, MacGregor S, et al; QSkin Study. The effect of screening on melanoma incidence and biopsy rates. Br J Dermatol. 2022;187:515-522. doi:10.1111/bjd.21649
- Jobbágy A, Kiss N, Meznerics FA, et al. Emergency use and efficacy of an asynchronous teledermatology system as a novel tool for early diagnosis of skin cancer during the first wave of COVID-19 pandemic. Int J Environ Res Public Health. 2022;19:2699. doi:10.3390/ijerph19052699
- National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed April 19, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
- Trepanowski N, Chang MS, Zhou G, et al. Delays in melanoma presentation during the COVID-19 pandemic: a nationwide multi-institutional cohort study. J Am Acad Dermatol. 2022;87:1217-1219. doi:10.1016/j.jaad.2022.06.031
- Chiru MR, Hindocha S, Burova E, et al. Management of the two-week wait pathway for skin cancer patients, before and during the pandemic: is virtual consultation an option? J Pers Med. 2022;12:1258. doi:10.3390/jpm12081258
- Finnane A Dallest K Janda M et al. Teledermatology for the diagnosis and management of skin cancer: a systematic review. JAMA Dermatol. 2017;153:319-327. doi:10.1001/jamadermatol.2016.4361
PRACTICE POINTS
- The COVID-19 pandemic has altered the landscape of medicine, as many individuals are now utilizing telemedicine to receive care.
- Many individuals will continue to receive telemedicine moving forward, making it crucial to understand access to care.
Association Between Atopic Dermatitis and Chronic Obstructive Pulmonary Disease Among US Adults in the 1999-2006 NHANES Survey
To the Editor:
Atopic dermatitis (AD) is an inflammatory skin condition that affects approximately 16.5 million adults in the United States.1 Atopic dermatitis is associated with skin barrier dysfunction and the activation of type 2 inflammatory cytokines. Multiorgan involvement of AD has been demonstrated, as patients with AD are more prone to asthma, allergic rhinitis, and other systemic diseases.2 In 2020, Smirnova et al3 reported a significant association (adjusted odds ratio [AOR], 1.58; 95% CI, 1.30-1.92) between AD and chronic obstructive pulmonary disease (COPD) in a large Swedish population. Currently, there is a lack of research evaluating the association between AD and COPD in a population of US adults. Therefore, we explored the association between AD and COPD (chronic bronchitis or emphysema) in a population of US adults utilizing the 1999-2006 National Health and Nutrition Examination Survey (NHANES), as these were the latest data for AD available in NHANES.4
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 1999-2006 NHANES database. Three outcome variables—emphysema, chronic bronchitis, and COPD—and numerous confounding variables for each participant were extracted from the NHANES database. The original cohort consisted of 13,134 participants, and 43 patients were excluded from our analysis owing to the lack of response to survey questions regarding AD and COPD status. The relationship between AD and COPD was evaluated by multivariable logistic regression analyses utilizing Stata/MP 17 (StataCorp LLC). In our logistic regression models, we controlled for age, sex, race/ethnicity, education, income, tobacco usage, diabetes mellitus and asthma status, and body mass index (eTable).
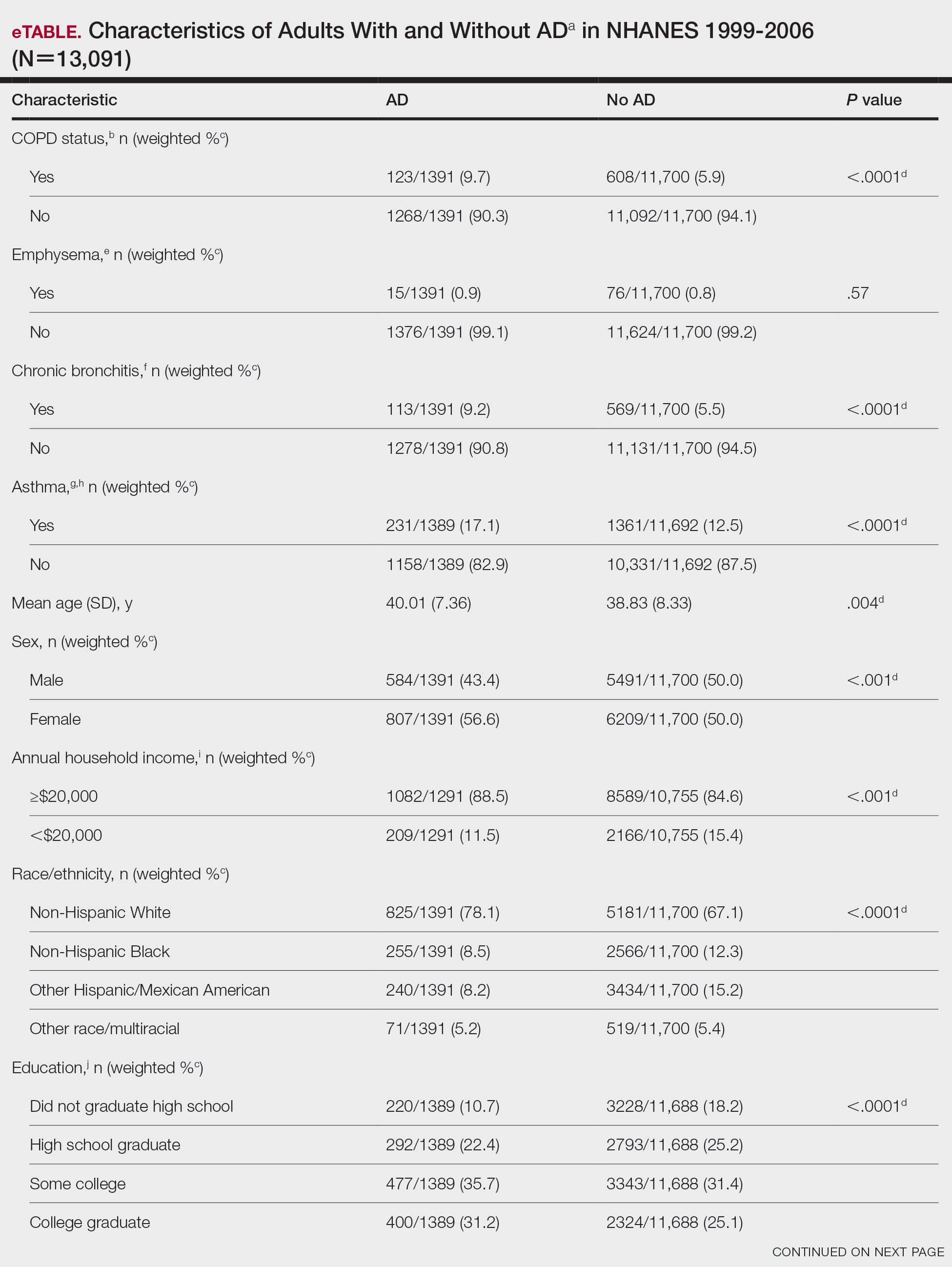
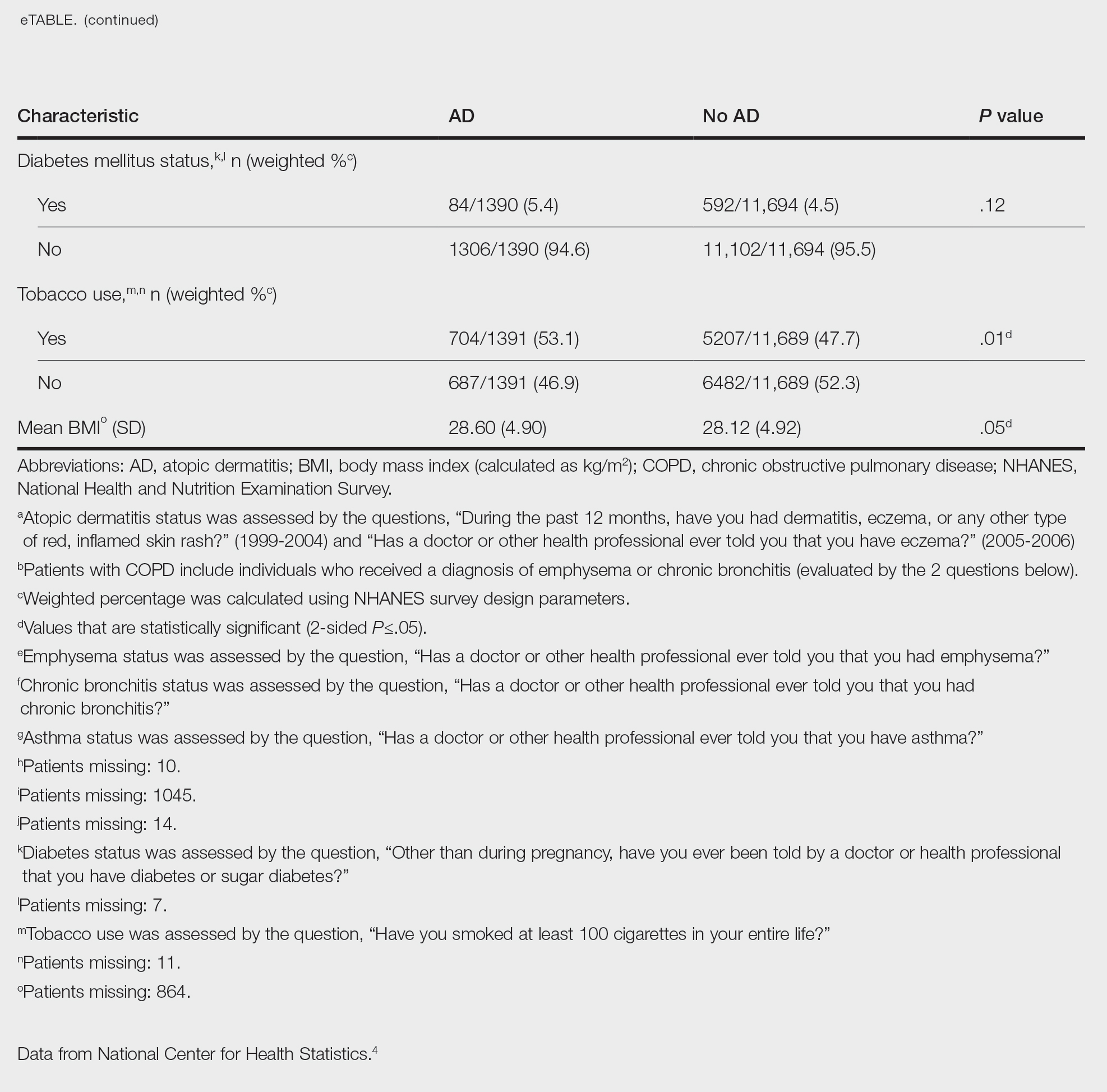
Our study consisted of 13,091 participants. Multivariable logistic regressions were utilized to examine the association between AD and COPD (Table). Approximately 12.5% (weighted) of the patients in our analysis had AD. Additionally, 9.7% (weighted) of patients with AD had received a diagnosis of COPD; conversely, 5.9% (weighted) of patients without AD had received a diagnosis of COPD. More patients with AD reported a diagnosis of chronic bronchitis (9.2%) rather than emphysema (0.9%). Our analysis revealed a significant association between AD and COPD among adults aged 20 to 59 years (AOR, 1.43; 95% CI, 1.13-1.80; P=.003) after controlling for potential confounding variables. Subsequently, we performed subgroup analyses, including exclusion of patients with an asthma diagnosis, to further explore the association between AD and COPD. After excluding participants with asthma, there was still a significant association between AD and COPD (AOR, 1.57; 95% CI, 1.14-2.16; P=.007). Moreover, the odds of receiving a COPD diagnosis were significantly higher among male patients with AD (AOR, 1.54; 95% CI, 1.06-2.25; P=.03).
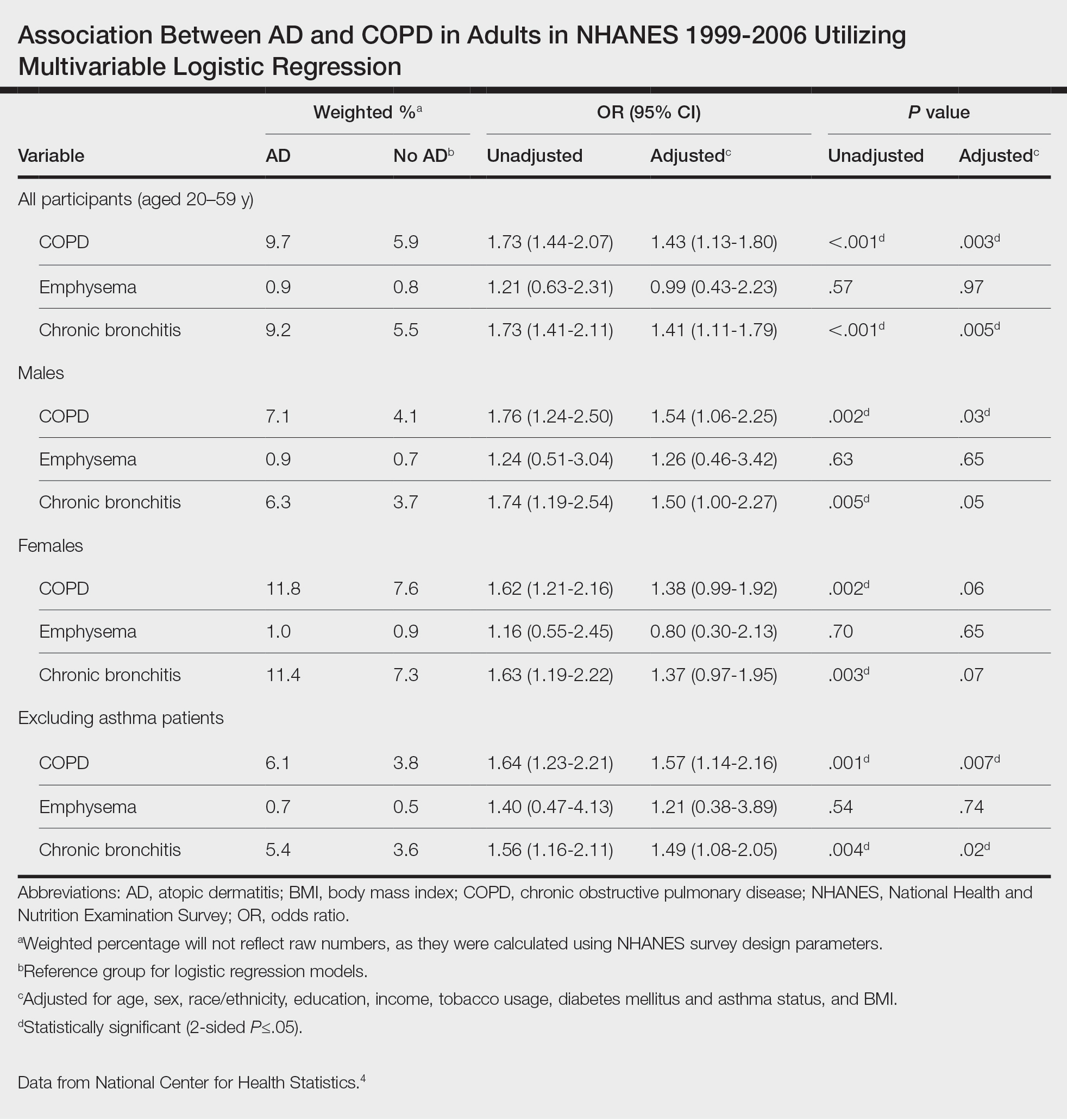
Our results support the association between AD and COPD, more specifically chronic bronchitis. This finding may be due to similar pathogenic mechanisms in both conditions, including overlapping cytokine production and immune pathways.5 Additionally, Harazin et al6 discussed the role of a novel gene, collagen 29A1 (COL29A1), in the pathogenesis of AD, COPD, and asthma. Variations in this gene may predispose patients to not only atopic diseases but also COPD.6
Limitations of our study include self-reported diagnoses and lack of patients older than 59 years. Self-reported diagnoses could have resulted in some misclassification of COPD, as some individuals may have reported a diagnosis of COPD rather than their true diagnosis of asthma. We mitigated this limitation by constructing a subpopulation model with exclusion of individuals with asthma. Further studies with spirometry-diagnosed COPD are needed to explore this relationship and the potential contributory pathophysiologic mechanisms. Understanding this association may increase awareness of potential comorbidities and assist clinicians with adequate management of patients with AD.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic Dermatitis in America Study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590. doi:10.1016/j.jid.2018.08.028
- Darlenski R, Kazandjieva J, Hristakieva E, et al. Atopic dermatitis as a systemic disease. Clin Dermatol. 2014;32:409-413. doi:10.1016/j.clindermatol.2013.11.007
- Smirnova J, Montgomery S, Lindberg M, et al. Associations of self-reported atopic dermatitis with comorbid conditions in adults: a population-based cross-sectional study. BMC Dermatol. 2020;20:23. doi:10.1186/s12895-020-00117-8
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://wwwn.cdc.gov/nchs/nhanes/
- Kawayama T, Okamoto M, Imaoka H, et al. Interleukin-18 in pulmonary inflammatory diseases. J Interferon Cytokine Res. 2012;32:443-449. doi:10.1089/jir.2012.0029
- Harazin M, Parwez Q, Petrasch-Parwez E, et al. Variation in the COL29A1 gene in German patients with atopic dermatitis, asthma and chronic obstructive pulmonary disease. J Dermatol. 2010;37:740-742. doi:10.1111/j.1346-8138.2010.00923.x
To the Editor:
Atopic dermatitis (AD) is an inflammatory skin condition that affects approximately 16.5 million adults in the United States.1 Atopic dermatitis is associated with skin barrier dysfunction and the activation of type 2 inflammatory cytokines. Multiorgan involvement of AD has been demonstrated, as patients with AD are more prone to asthma, allergic rhinitis, and other systemic diseases.2 In 2020, Smirnova et al3 reported a significant association (adjusted odds ratio [AOR], 1.58; 95% CI, 1.30-1.92) between AD and chronic obstructive pulmonary disease (COPD) in a large Swedish population. Currently, there is a lack of research evaluating the association between AD and COPD in a population of US adults. Therefore, we explored the association between AD and COPD (chronic bronchitis or emphysema) in a population of US adults utilizing the 1999-2006 National Health and Nutrition Examination Survey (NHANES), as these were the latest data for AD available in NHANES.4
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 1999-2006 NHANES database. Three outcome variables—emphysema, chronic bronchitis, and COPD—and numerous confounding variables for each participant were extracted from the NHANES database. The original cohort consisted of 13,134 participants, and 43 patients were excluded from our analysis owing to the lack of response to survey questions regarding AD and COPD status. The relationship between AD and COPD was evaluated by multivariable logistic regression analyses utilizing Stata/MP 17 (StataCorp LLC). In our logistic regression models, we controlled for age, sex, race/ethnicity, education, income, tobacco usage, diabetes mellitus and asthma status, and body mass index (eTable).


Our study consisted of 13,091 participants. Multivariable logistic regressions were utilized to examine the association between AD and COPD (Table). Approximately 12.5% (weighted) of the patients in our analysis had AD. Additionally, 9.7% (weighted) of patients with AD had received a diagnosis of COPD; conversely, 5.9% (weighted) of patients without AD had received a diagnosis of COPD. More patients with AD reported a diagnosis of chronic bronchitis (9.2%) rather than emphysema (0.9%). Our analysis revealed a significant association between AD and COPD among adults aged 20 to 59 years (AOR, 1.43; 95% CI, 1.13-1.80; P=.003) after controlling for potential confounding variables. Subsequently, we performed subgroup analyses, including exclusion of patients with an asthma diagnosis, to further explore the association between AD and COPD. After excluding participants with asthma, there was still a significant association between AD and COPD (AOR, 1.57; 95% CI, 1.14-2.16; P=.007). Moreover, the odds of receiving a COPD diagnosis were significantly higher among male patients with AD (AOR, 1.54; 95% CI, 1.06-2.25; P=.03).

Our results support the association between AD and COPD, more specifically chronic bronchitis. This finding may be due to similar pathogenic mechanisms in both conditions, including overlapping cytokine production and immune pathways.5 Additionally, Harazin et al6 discussed the role of a novel gene, collagen 29A1 (COL29A1), in the pathogenesis of AD, COPD, and asthma. Variations in this gene may predispose patients to not only atopic diseases but also COPD.6
Limitations of our study include self-reported diagnoses and lack of patients older than 59 years. Self-reported diagnoses could have resulted in some misclassification of COPD, as some individuals may have reported a diagnosis of COPD rather than their true diagnosis of asthma. We mitigated this limitation by constructing a subpopulation model with exclusion of individuals with asthma. Further studies with spirometry-diagnosed COPD are needed to explore this relationship and the potential contributory pathophysiologic mechanisms. Understanding this association may increase awareness of potential comorbidities and assist clinicians with adequate management of patients with AD.
To the Editor:
Atopic dermatitis (AD) is an inflammatory skin condition that affects approximately 16.5 million adults in the United States.1 Atopic dermatitis is associated with skin barrier dysfunction and the activation of type 2 inflammatory cytokines. Multiorgan involvement of AD has been demonstrated, as patients with AD are more prone to asthma, allergic rhinitis, and other systemic diseases.2 In 2020, Smirnova et al3 reported a significant association (adjusted odds ratio [AOR], 1.58; 95% CI, 1.30-1.92) between AD and chronic obstructive pulmonary disease (COPD) in a large Swedish population. Currently, there is a lack of research evaluating the association between AD and COPD in a population of US adults. Therefore, we explored the association between AD and COPD (chronic bronchitis or emphysema) in a population of US adults utilizing the 1999-2006 National Health and Nutrition Examination Survey (NHANES), as these were the latest data for AD available in NHANES.4
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 1999-2006 NHANES database. Three outcome variables—emphysema, chronic bronchitis, and COPD—and numerous confounding variables for each participant were extracted from the NHANES database. The original cohort consisted of 13,134 participants, and 43 patients were excluded from our analysis owing to the lack of response to survey questions regarding AD and COPD status. The relationship between AD and COPD was evaluated by multivariable logistic regression analyses utilizing Stata/MP 17 (StataCorp LLC). In our logistic regression models, we controlled for age, sex, race/ethnicity, education, income, tobacco usage, diabetes mellitus and asthma status, and body mass index (eTable).


Our study consisted of 13,091 participants. Multivariable logistic regressions were utilized to examine the association between AD and COPD (Table). Approximately 12.5% (weighted) of the patients in our analysis had AD. Additionally, 9.7% (weighted) of patients with AD had received a diagnosis of COPD; conversely, 5.9% (weighted) of patients without AD had received a diagnosis of COPD. More patients with AD reported a diagnosis of chronic bronchitis (9.2%) rather than emphysema (0.9%). Our analysis revealed a significant association between AD and COPD among adults aged 20 to 59 years (AOR, 1.43; 95% CI, 1.13-1.80; P=.003) after controlling for potential confounding variables. Subsequently, we performed subgroup analyses, including exclusion of patients with an asthma diagnosis, to further explore the association between AD and COPD. After excluding participants with asthma, there was still a significant association between AD and COPD (AOR, 1.57; 95% CI, 1.14-2.16; P=.007). Moreover, the odds of receiving a COPD diagnosis were significantly higher among male patients with AD (AOR, 1.54; 95% CI, 1.06-2.25; P=.03).

Our results support the association between AD and COPD, more specifically chronic bronchitis. This finding may be due to similar pathogenic mechanisms in both conditions, including overlapping cytokine production and immune pathways.5 Additionally, Harazin et al6 discussed the role of a novel gene, collagen 29A1 (COL29A1), in the pathogenesis of AD, COPD, and asthma. Variations in this gene may predispose patients to not only atopic diseases but also COPD.6
Limitations of our study include self-reported diagnoses and lack of patients older than 59 years. Self-reported diagnoses could have resulted in some misclassification of COPD, as some individuals may have reported a diagnosis of COPD rather than their true diagnosis of asthma. We mitigated this limitation by constructing a subpopulation model with exclusion of individuals with asthma. Further studies with spirometry-diagnosed COPD are needed to explore this relationship and the potential contributory pathophysiologic mechanisms. Understanding this association may increase awareness of potential comorbidities and assist clinicians with adequate management of patients with AD.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic Dermatitis in America Study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590. doi:10.1016/j.jid.2018.08.028
- Darlenski R, Kazandjieva J, Hristakieva E, et al. Atopic dermatitis as a systemic disease. Clin Dermatol. 2014;32:409-413. doi:10.1016/j.clindermatol.2013.11.007
- Smirnova J, Montgomery S, Lindberg M, et al. Associations of self-reported atopic dermatitis with comorbid conditions in adults: a population-based cross-sectional study. BMC Dermatol. 2020;20:23. doi:10.1186/s12895-020-00117-8
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://wwwn.cdc.gov/nchs/nhanes/
- Kawayama T, Okamoto M, Imaoka H, et al. Interleukin-18 in pulmonary inflammatory diseases. J Interferon Cytokine Res. 2012;32:443-449. doi:10.1089/jir.2012.0029
- Harazin M, Parwez Q, Petrasch-Parwez E, et al. Variation in the COL29A1 gene in German patients with atopic dermatitis, asthma and chronic obstructive pulmonary disease. J Dermatol. 2010;37:740-742. doi:10.1111/j.1346-8138.2010.00923.x
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic Dermatitis in America Study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590. doi:10.1016/j.jid.2018.08.028
- Darlenski R, Kazandjieva J, Hristakieva E, et al. Atopic dermatitis as a systemic disease. Clin Dermatol. 2014;32:409-413. doi:10.1016/j.clindermatol.2013.11.007
- Smirnova J, Montgomery S, Lindberg M, et al. Associations of self-reported atopic dermatitis with comorbid conditions in adults: a population-based cross-sectional study. BMC Dermatol. 2020;20:23. doi:10.1186/s12895-020-00117-8
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://wwwn.cdc.gov/nchs/nhanes/
- Kawayama T, Okamoto M, Imaoka H, et al. Interleukin-18 in pulmonary inflammatory diseases. J Interferon Cytokine Res. 2012;32:443-449. doi:10.1089/jir.2012.0029
- Harazin M, Parwez Q, Petrasch-Parwez E, et al. Variation in the COL29A1 gene in German patients with atopic dermatitis, asthma and chronic obstructive pulmonary disease. J Dermatol. 2010;37:740-742. doi:10.1111/j.1346-8138.2010.00923.x
Practice Points
- Various comorbidities are associated with atopic dermatitis (AD). Currently, research exploring the association between AD and chronic obstructive pulmonary disease is limited.
- Understanding the systemic diseases associated with inflammatory skin diseases can assist with adequate patient management.
Impact of the COVID-19 Pandemic on Care for Patients With Atopic Dermatitis
To the Editor:
Atopic dermatitis (AD) is a widely prevalent dermatologic condition that can severely impact a patient’s quality of life.1 Individuals with AD have been substantially affected during the COVID-19 pandemic due to the increased use of irritants, decreased access to care, and rise in psychological stress.1,2 These factors have resulted in lower quality of life and worsening dermatologic symptoms for many AD patients over the last few years.1 One major potential contributory component of these findings is decreased accessibility to in-office care during the pandemic, with a shift to telemedicine instead. Accessibility to care during the COVID-19 pandemic for AD patients compared to those without AD remains unknown. Therefore, we explored the impact of the COVID-19 pandemic on care for patients with AD in a large US population.
Using anonymous survey data from the 2021 National Health Interview Survey,3 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with AD compared to those without AD. We assigned the following 3 survey questions as outcome variables to assess access to care: delayed medical care due to COVID-19 pandemic (yes/no), did not get care due to COVID-19 pandemic (yes/no), and virtual medical appointment in the last 12 months (yes/no). In Table 1, numerous categorical survey variables, including sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region, were analyzed using χ2 testing to evaluate for differences among individuals with and without AD. Multivariable logistic regression models evaluating the relationship between AD and access to care were constructed using Stata/MP 17 (StataCorp LLC). In our analysis we controlled for age, sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region.
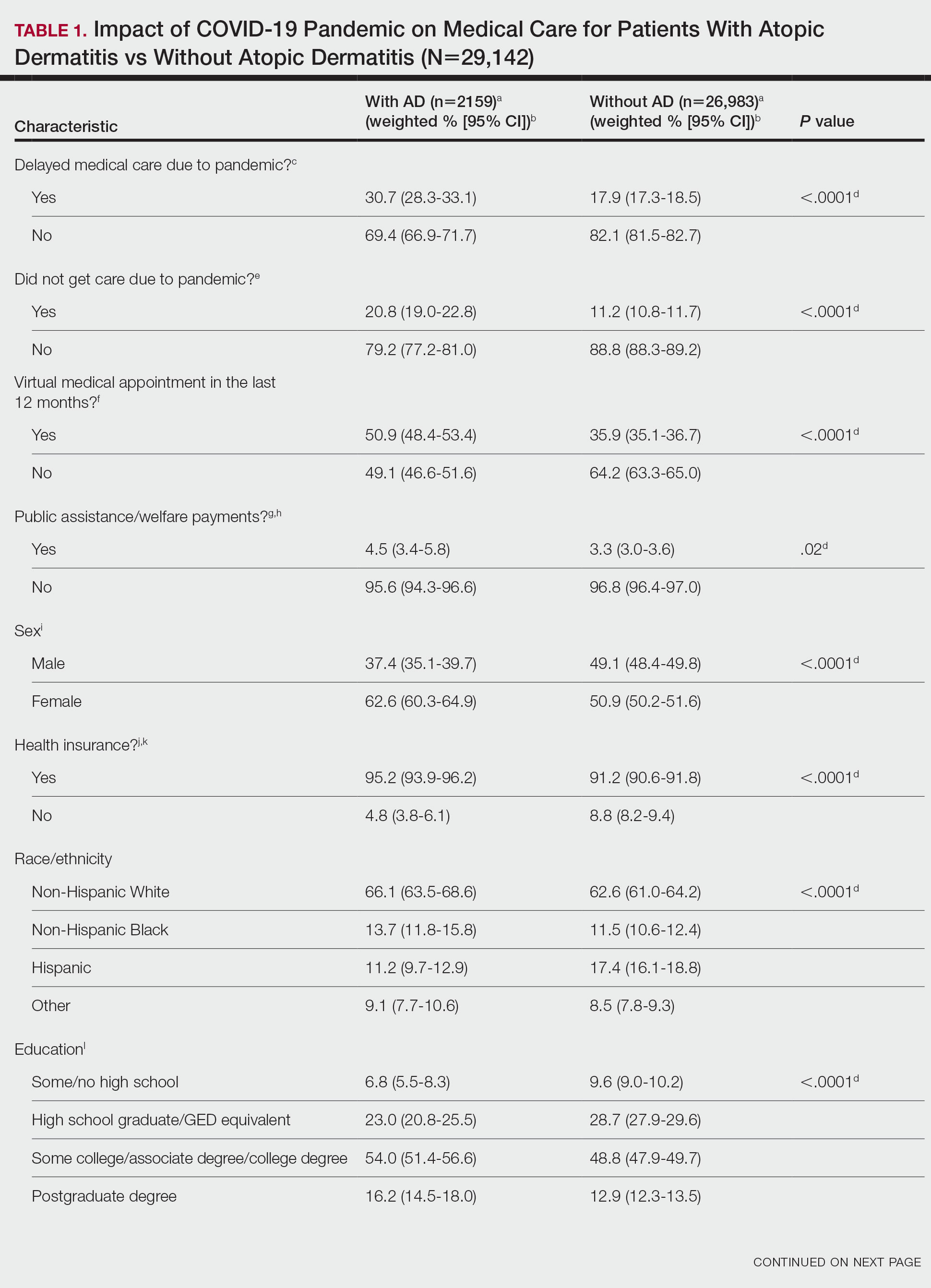
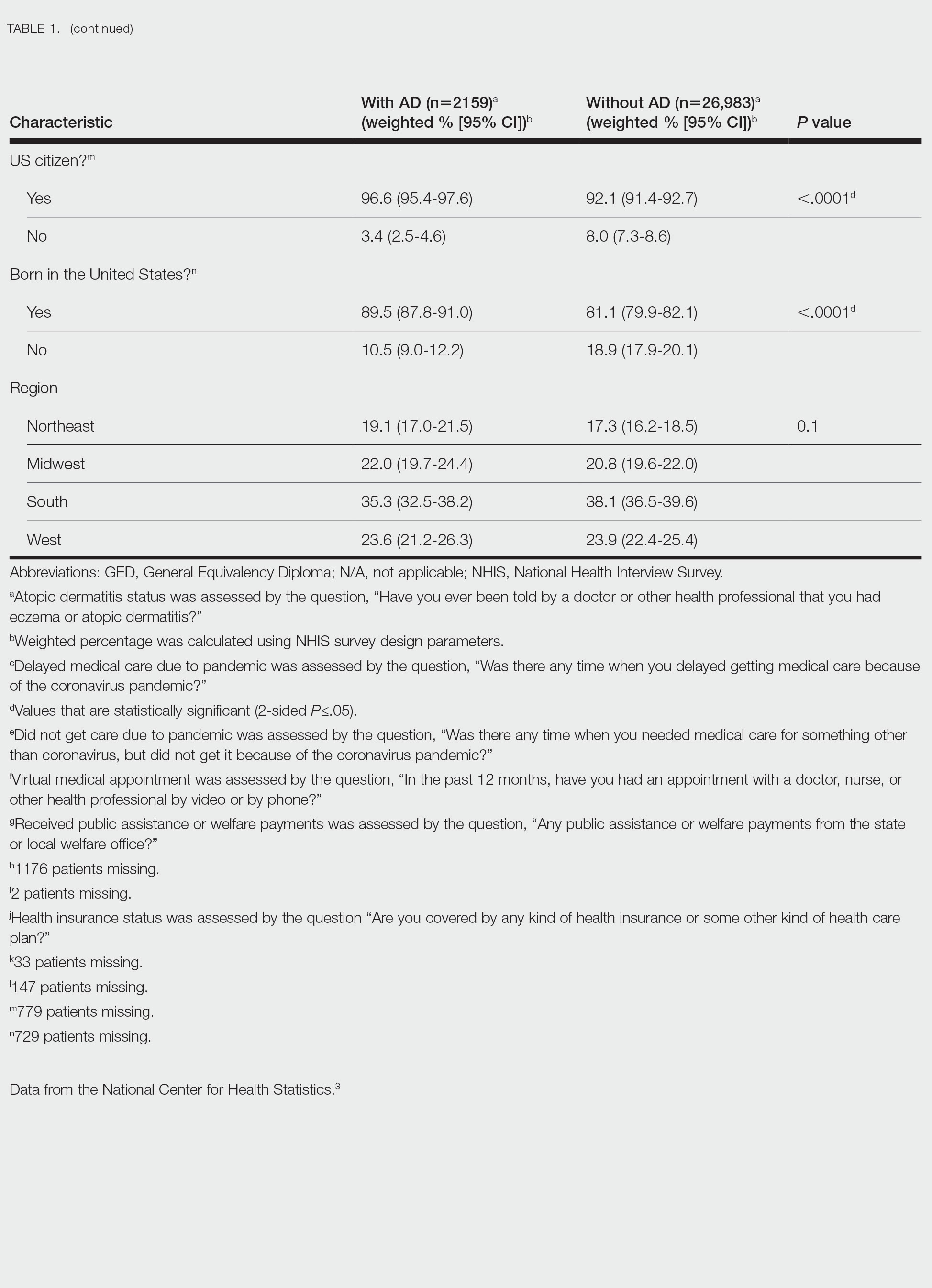
There were 29,142 adult patients (aged ≥18 years) included in our analysis. Approximately 7.4% (weighted) of individuals had AD (Table 1). After adjusting for confounding variables, patients with AD had a higher odds of delaying medical care due to the COVID-19 pandemic (adjusted odds ratio [AOR], 1.91; 95% CI, 1.69-2.16; P<.001), not receiving care due to the COVID-19 pandemic (AOR, 1.94; 95% CI, 1.71-2.22; P<.001), and having a virtual medical visit in the last 12 months (AOR, 1.72; 95% CI, 1.54-1.93; P<.001)(Table 2) compared with patients without AD.
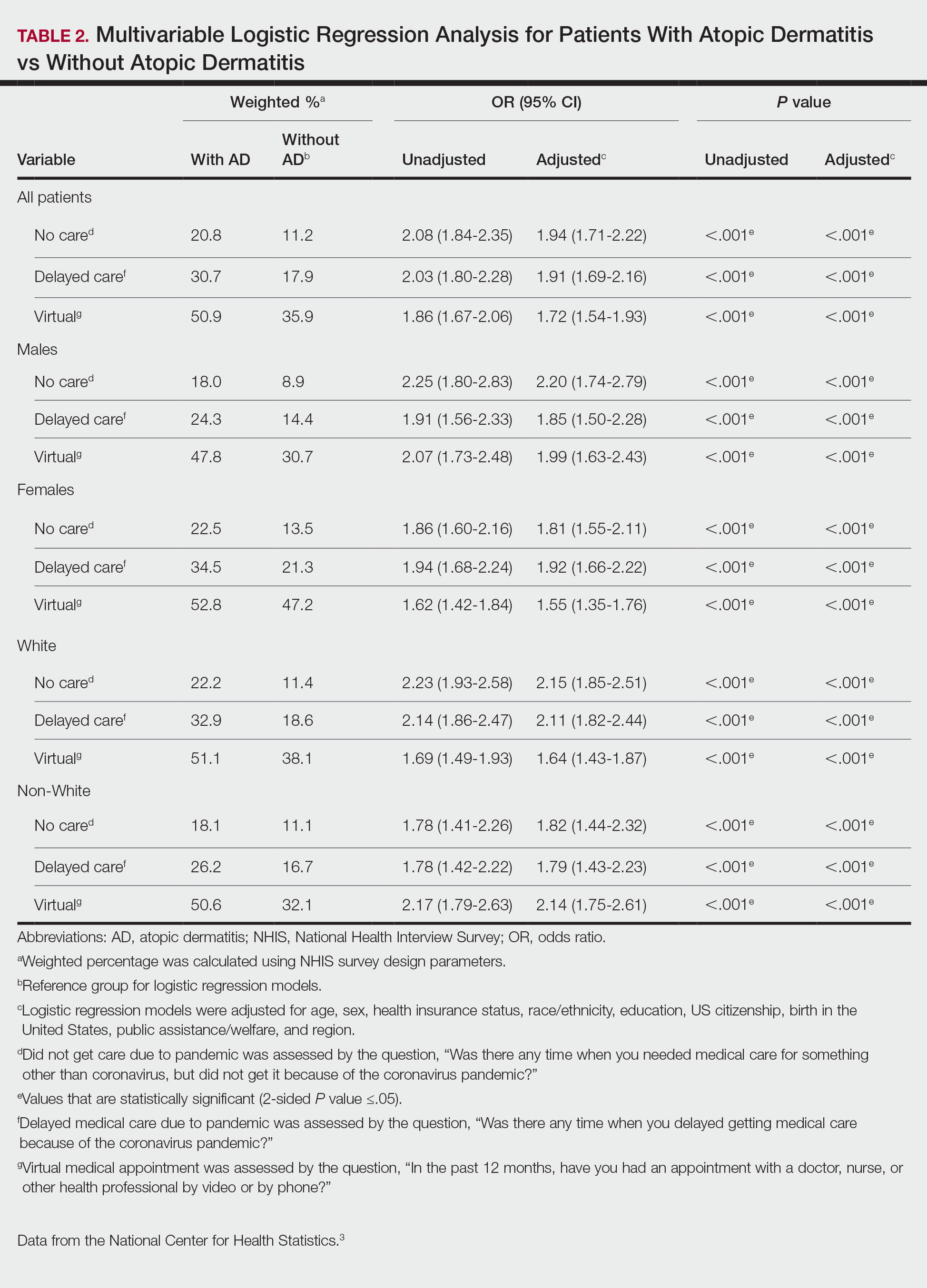
Our findings support the association between AD and decreased access to in-person care due to the COVID-19 pandemic. Moreover, telemedicine was utilized more among individuals with AD, possibly due to the accessibility of diagnostic tools for dermatologic diagnoses, such as high-quality photographs.4 According to Trinidad et al,4 telemedicine became an invaluable tool for dermatology hospitalists during the COVID-19 pandemic, as many physicians were able to comfortably diagnose patients with cutaneous diseases without an in-person visit. Utilizing telemedicine for patient care can help reduce the risk for COVID-19 transmission while also providing quality care for individuals living in rural areas.5 Chiricozzi et al6 discussed the importance of telemedicine in Italy during the pandemic, as many AD patients were able to maintain control of their disease while on systemic treatments.
Limitations of this study include self-reported measures; inability to compare patients with AD to individuals with other cutaneous diseases; and additional potential confounders, such as chronic comorbidities. Future studies should evaluate the use of telemedicine and access to care among individuals with other common skin diseases and help determine why such discrepancies exist. Understanding the difficulties in access to care and the viable alternatives in place may increase awareness and assist clinicians with adequate management of patients with AD.
1. Sieniawska J, Lesiak A, Cia˛z˙yn´ski K, et al. Impact of the COVID-19 pandemic on atopic dermatitis patients. Int J Environ Res Public Health. 2022;19:1734. doi:10.3390/ijerph19031734
2. Pourani MR, Ganji R, Dashti T, et al. Impact of COVID-19 pandemic on patients with atopic dermatitis [in Spanish]. Actas Dermosifiliogr. 2022;113:T286-T293. doi:10.1016/j.ad.2021.08.004
3. National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
4. Trinidad J, Gabel CK, Han JJ, et al. Telemedicine and dermatology hospital consultations during the COVID-19 pandemic: a multi-centre observational study on resource utilization and conversion to in-person consultations during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. 2022;36:E323-E325. doi:10.1111/jdv.17898
5. Marasca C, Annunziata MC, Camela E, et al. Teledermatology and inflammatory skin conditions during COVID-19 era: new perspectives and applications. J Clin Med. 2022;11:1511. doi:10.3390/jcm11061511
6. Chiricozzi A, Talamonti M, De Simone C, et al. Management of patients with atopic dermatitis undergoing systemic therapy during COVID-19 pandemic in Italy: data from the DA-COVID-19 registry. Allergy. 2021;76:1813-1824. doi:10.1111/all.14767
To the Editor:
Atopic dermatitis (AD) is a widely prevalent dermatologic condition that can severely impact a patient’s quality of life.1 Individuals with AD have been substantially affected during the COVID-19 pandemic due to the increased use of irritants, decreased access to care, and rise in psychological stress.1,2 These factors have resulted in lower quality of life and worsening dermatologic symptoms for many AD patients over the last few years.1 One major potential contributory component of these findings is decreased accessibility to in-office care during the pandemic, with a shift to telemedicine instead. Accessibility to care during the COVID-19 pandemic for AD patients compared to those without AD remains unknown. Therefore, we explored the impact of the COVID-19 pandemic on care for patients with AD in a large US population.
Using anonymous survey data from the 2021 National Health Interview Survey,3 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with AD compared to those without AD. We assigned the following 3 survey questions as outcome variables to assess access to care: delayed medical care due to COVID-19 pandemic (yes/no), did not get care due to COVID-19 pandemic (yes/no), and virtual medical appointment in the last 12 months (yes/no). In Table 1, numerous categorical survey variables, including sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region, were analyzed using χ2 testing to evaluate for differences among individuals with and without AD. Multivariable logistic regression models evaluating the relationship between AD and access to care were constructed using Stata/MP 17 (StataCorp LLC). In our analysis we controlled for age, sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region.


There were 29,142 adult patients (aged ≥18 years) included in our analysis. Approximately 7.4% (weighted) of individuals had AD (Table 1). After adjusting for confounding variables, patients with AD had a higher odds of delaying medical care due to the COVID-19 pandemic (adjusted odds ratio [AOR], 1.91; 95% CI, 1.69-2.16; P<.001), not receiving care due to the COVID-19 pandemic (AOR, 1.94; 95% CI, 1.71-2.22; P<.001), and having a virtual medical visit in the last 12 months (AOR, 1.72; 95% CI, 1.54-1.93; P<.001)(Table 2) compared with patients without AD.

Our findings support the association between AD and decreased access to in-person care due to the COVID-19 pandemic. Moreover, telemedicine was utilized more among individuals with AD, possibly due to the accessibility of diagnostic tools for dermatologic diagnoses, such as high-quality photographs.4 According to Trinidad et al,4 telemedicine became an invaluable tool for dermatology hospitalists during the COVID-19 pandemic, as many physicians were able to comfortably diagnose patients with cutaneous diseases without an in-person visit. Utilizing telemedicine for patient care can help reduce the risk for COVID-19 transmission while also providing quality care for individuals living in rural areas.5 Chiricozzi et al6 discussed the importance of telemedicine in Italy during the pandemic, as many AD patients were able to maintain control of their disease while on systemic treatments.
Limitations of this study include self-reported measures; inability to compare patients with AD to individuals with other cutaneous diseases; and additional potential confounders, such as chronic comorbidities. Future studies should evaluate the use of telemedicine and access to care among individuals with other common skin diseases and help determine why such discrepancies exist. Understanding the difficulties in access to care and the viable alternatives in place may increase awareness and assist clinicians with adequate management of patients with AD.
To the Editor:
Atopic dermatitis (AD) is a widely prevalent dermatologic condition that can severely impact a patient’s quality of life.1 Individuals with AD have been substantially affected during the COVID-19 pandemic due to the increased use of irritants, decreased access to care, and rise in psychological stress.1,2 These factors have resulted in lower quality of life and worsening dermatologic symptoms for many AD patients over the last few years.1 One major potential contributory component of these findings is decreased accessibility to in-office care during the pandemic, with a shift to telemedicine instead. Accessibility to care during the COVID-19 pandemic for AD patients compared to those without AD remains unknown. Therefore, we explored the impact of the COVID-19 pandemic on care for patients with AD in a large US population.
Using anonymous survey data from the 2021 National Health Interview Survey,3 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with AD compared to those without AD. We assigned the following 3 survey questions as outcome variables to assess access to care: delayed medical care due to COVID-19 pandemic (yes/no), did not get care due to COVID-19 pandemic (yes/no), and virtual medical appointment in the last 12 months (yes/no). In Table 1, numerous categorical survey variables, including sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region, were analyzed using χ2 testing to evaluate for differences among individuals with and without AD. Multivariable logistic regression models evaluating the relationship between AD and access to care were constructed using Stata/MP 17 (StataCorp LLC). In our analysis we controlled for age, sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region.


There were 29,142 adult patients (aged ≥18 years) included in our analysis. Approximately 7.4% (weighted) of individuals had AD (Table 1). After adjusting for confounding variables, patients with AD had a higher odds of delaying medical care due to the COVID-19 pandemic (adjusted odds ratio [AOR], 1.91; 95% CI, 1.69-2.16; P<.001), not receiving care due to the COVID-19 pandemic (AOR, 1.94; 95% CI, 1.71-2.22; P<.001), and having a virtual medical visit in the last 12 months (AOR, 1.72; 95% CI, 1.54-1.93; P<.001)(Table 2) compared with patients without AD.

Our findings support the association between AD and decreased access to in-person care due to the COVID-19 pandemic. Moreover, telemedicine was utilized more among individuals with AD, possibly due to the accessibility of diagnostic tools for dermatologic diagnoses, such as high-quality photographs.4 According to Trinidad et al,4 telemedicine became an invaluable tool for dermatology hospitalists during the COVID-19 pandemic, as many physicians were able to comfortably diagnose patients with cutaneous diseases without an in-person visit. Utilizing telemedicine for patient care can help reduce the risk for COVID-19 transmission while also providing quality care for individuals living in rural areas.5 Chiricozzi et al6 discussed the importance of telemedicine in Italy during the pandemic, as many AD patients were able to maintain control of their disease while on systemic treatments.
Limitations of this study include self-reported measures; inability to compare patients with AD to individuals with other cutaneous diseases; and additional potential confounders, such as chronic comorbidities. Future studies should evaluate the use of telemedicine and access to care among individuals with other common skin diseases and help determine why such discrepancies exist. Understanding the difficulties in access to care and the viable alternatives in place may increase awareness and assist clinicians with adequate management of patients with AD.
1. Sieniawska J, Lesiak A, Cia˛z˙yn´ski K, et al. Impact of the COVID-19 pandemic on atopic dermatitis patients. Int J Environ Res Public Health. 2022;19:1734. doi:10.3390/ijerph19031734
2. Pourani MR, Ganji R, Dashti T, et al. Impact of COVID-19 pandemic on patients with atopic dermatitis [in Spanish]. Actas Dermosifiliogr. 2022;113:T286-T293. doi:10.1016/j.ad.2021.08.004
3. National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
4. Trinidad J, Gabel CK, Han JJ, et al. Telemedicine and dermatology hospital consultations during the COVID-19 pandemic: a multi-centre observational study on resource utilization and conversion to in-person consultations during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. 2022;36:E323-E325. doi:10.1111/jdv.17898
5. Marasca C, Annunziata MC, Camela E, et al. Teledermatology and inflammatory skin conditions during COVID-19 era: new perspectives and applications. J Clin Med. 2022;11:1511. doi:10.3390/jcm11061511
6. Chiricozzi A, Talamonti M, De Simone C, et al. Management of patients with atopic dermatitis undergoing systemic therapy during COVID-19 pandemic in Italy: data from the DA-COVID-19 registry. Allergy. 2021;76:1813-1824. doi:10.1111/all.14767
1. Sieniawska J, Lesiak A, Cia˛z˙yn´ski K, et al. Impact of the COVID-19 pandemic on atopic dermatitis patients. Int J Environ Res Public Health. 2022;19:1734. doi:10.3390/ijerph19031734
2. Pourani MR, Ganji R, Dashti T, et al. Impact of COVID-19 pandemic on patients with atopic dermatitis [in Spanish]. Actas Dermosifiliogr. 2022;113:T286-T293. doi:10.1016/j.ad.2021.08.004
3. National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
4. Trinidad J, Gabel CK, Han JJ, et al. Telemedicine and dermatology hospital consultations during the COVID-19 pandemic: a multi-centre observational study on resource utilization and conversion to in-person consultations during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. 2022;36:E323-E325. doi:10.1111/jdv.17898
5. Marasca C, Annunziata MC, Camela E, et al. Teledermatology and inflammatory skin conditions during COVID-19 era: new perspectives and applications. J Clin Med. 2022;11:1511. doi:10.3390/jcm11061511
6. Chiricozzi A, Talamonti M, De Simone C, et al. Management of patients with atopic dermatitis undergoing systemic therapy during COVID-19 pandemic in Italy: data from the DA-COVID-19 registry. Allergy. 2021;76:1813-1824. doi:10.1111/all.14767
Practice Points
- The landscape of dermatology has seen major changes due to the COVID-19 pandemic, as many patients now utilize telemedicine to receive care.
- Understanding accessibility to in-person care for patients with atopic dermatitis during the COVID-19 pandemic can assist with the development of methods to enhance management.
An Update on JAK Inhibitors in Skin Disease
Atopic dermatitis (AD) is a chronic inflammatory skin disorder affecting 7% of adults and 13% of children in the United States.1,2 Atopic dermatitis is characterized by pruritus, dry skin, and pain, all of which can negatively impact quality of life and put patients at higher risk for psychiatric comorbidities such as anxiety and depression.3 The pathogenesis of AD is multifactorial, involving genetics, epidermal barrier dysfunction, and immune dysregulation. Overactivation of helper T cell (TH2) pathway cytokines, including IL-4, IL-13, and IL-31, is thought to propagate both inflammation and pruritus, which are central to AD. The JAK-STAT signaling pathway plays a pivotal role in the immune system dysregulation and exaggeration of TH2 cell response, making JAK-STAT inhibitors (or JAK inhibitors) strong theoretical candidates for the treatment of AD.4 In humans, the Janus kinases are composed of 4 different members—JAK1, JAK2, JAK3, and tyrosine kinase 2—all of which can be targeted by JAK inhibitors.5
JAK inhibitors such as tofacitinib have already been approved by the US Food and Drug Administration (FDA) to treat various inflammatory conditions, including rheumatoid arthritis, ulcerative colitis, and psoriatic arthritis; other JAK inhibitors such as baricitinib are only approved for patients with rheumatoid arthritis.6,7 The success of these small molecule inhibitors in these immune-mediated conditions make them attractive candidates for the treatment of AD. Several JAK inhibitors are in phase 2 and phase 3 clinical trials as oral therapies (moderate to severe AD) or as topical treatments (mild to moderate AD). Currently, ruxolitinib (RUX) is the only topical JAK inhibitor that is FDA approved for the treatment of AD in the United States.8 In this editorial, we focus on recent trials of JAK inhibitors tested in patients with AD, including topical RUX, as well as oral abrocitinib, upadacitinib, and baricitinib.
Topical RUX in AD
Ruxolitinib is a topical JAK1/2 small molecule inhibitor approved by the FDA for the treatment of AD in 2021. In a randomized trial by Kim et al9 in 2020, all tested regimens of RUX demonstrated significant improvement in eczema area and severity index (EASI) scores vs vehicle; notably, RUX cream 1.5% applied twice daily achieved the greatest mean percentage change in baseline EASI score vs vehicle at 4 weeks (76.1% vs 15.5%; P<.0001). Ruxolitinib cream was well tolerated through week 8 of the trial, and all adverse events (AEs) were mild to moderate in severity and comparable to those in the vehicle group.9
Topical JAK inhibitors appear to be effective for mild to moderate AD and have had an acceptable safety profile in clinical trials thus far. Although topical corticosteroids and calcineurin inhibitors can have great clinical benefit in AD, they are recommended for short-term use given side effects such as thinning of the skin, burning, or telangiectasia formation.10,11 The hope is that topical JAK inhibitors may be an alternative to standard topical treatments for AD, as they can be used for longer periods due to a safer side-effect profile.
Oral JAK Inhibitors in AD
Several oral JAK inhibitors are undergoing investigation for the systemic treatment of moderate to severe AD. Abrocitinib is an oral JAK1 inhibitor that has demonstrated efficacy in several phase 3 trials in patients with moderate to severe AD. In a 2021 trial, patients were randomized in a 2:2:2:1 ratio to receive abrocitinib 200 mg daily, abrocitinib 100 mg daily, subcutaneous dupilumab 300 mg every other week, or placebo, respectively.12 Patients in both abrocitinib groups showed significant improvement in AD vs placebo, and EASI-75 response was achieved in 70.3%, 58.7%, 58.1%, and 27.1% of patients, respectively (P<.001 for both abrocitinib doses vs placebo). Adverse events occurred more frequently in the abrocitinib 200-mg group vs placebo. Nausea, acne, nasopharyngitis, and headache were the most frequently reported AEs with abrocitinib.12 Another phase 3 trial by Silverberg et al13 (N=391) had similar treatment results, with 38.1% of participants receiving abrocitinib 200 mg and 28.4% of participants receiving abrocitinib 100 mg achieving investigator global assessment scores of 0 (clear) or 1 (almost clear) vs 9.1% of participants receiving placebo (P<.001). Abrocitinib was well tolerated in this trial with few serious AEs (ie, herpangina [0.6%], pneumonia [0.6%]).13 In both trials, there were rare instances of laboratory values indicating thrombocytopenia with the 200-mg dose (0.9%12 and 3.2%13) without any clinical manifestations. Although a decrease in platelets was observed, no thrombocytopenia occurred in the abrocitinib 100-mg group in the latter trial.13
Baricitinib is another oral inhibitor of JAK1 and JAK2 with potential for the treatment of AD. One randomized trial (N=329) demonstrated its efficacy in combination with a topical corticosteroid (TCS). At 16 weeks, a higher number of participants treated with baricitinib and TCS achieved investigator global assessment scores of 0 (clear) or 1 (almost clear) compared to those who received placebo and TCS (31% with baricitinib 4 mg + TCS, 24% with baricitinib 2 mg + TCS, and 15% with placebo + TCS).14 Similarly, in BREEZE-AD5,another phase 3 trial (N=440), baricitinib monotherapy demonstrated a higher rate of treatment success vs placebo.15 Specifically, 13% of patients treated with baricitinib 1 mg and 30% of those treated with baricitinib 2 mg achieved 75% or greater reduction in EASI scores compared to 8% in the placebo group. The most common AEs associated with baricitinib were nasopharyngitis and headache. Adverse events occurred with similar frequency across both experimental and control groups.15 Reich et al14 demonstrated a higher overall rate of AEs—most commonly nasopharyngitis, upper respiratory tract infections, and folliculitis—in baricitinib-treated patients; however, serious AEs occurred with similar frequency across all groups, including the control group.
The selective JAK1 inhibitor upadacitinib also is undergoing testing in treating moderate to severe AD. In one trial, 167 patients were randomized to once daily oral upadacitinib 7.5 mg, 15 mg, or 30 mg or placebo.16 All doses of upadacitinib demonstrated considerably higher percentage improvements from baseline in EASI scores compared to placebo at 16 weeks with a clear dose-response relationship (39%, 62%, and 74% vs 23%, respectively). In this trial, there were no dose-limiting safety events. Serious AEs were infrequent, occurring in 4.8%, 2.4%, and 0% of upadacitinib groups vs 2.5% for placebo. The serious AEs observed with upadacitinib were 1 case of appendicitis, lower jaw pericoronitis in a patient with a history of repeated tooth infections, and an exacerbation of AD.16
Tofacitinib, another JAK inhibitor, has been shown to increase the risk for blood clots and death in a large trial in the treatment of rheumatoid arthritis. Following this study, the FDA is requiring black box warnings for tofacitinib and also for the 2 JAK inhibitors baricitinib and upadacitinib regarding the risks for heart-related events, cancer, blood clots, and death. Given that these medications share a similar mechanism of action to tofacitinib, they may have similar risks, though they have not yet been fully evaluated in large safety trials.17
With more recent investigation into novel therapeutics for AD, oral JAK inhibitors may play an important role in the future to treat patients with moderate to severe AD with inadequate response or contraindications to other systemic therapies. In trials thus far, oral JAK inhibitors have exhibited acceptable safety profiles and have demonstrated treatment success in AD. More randomized, controlled, phase 3 studies with larger patient populations are required to confirm their potential as effective treatments and elucidate their long-term safety.
Deucravacitinib in Psoriasis
Deucravacitinib is a first-in-class, oral, selective TYK2 inhibitor currently undergoing testing for the treatment of psoriasis. A randomized phase 2 trial (N=267) found that deucravacitinib was more effective than placebo in treating chronic plaque psoriasis at doses of 3 to 12 mg daily.18 The percentage of participants with a 75% or greater reduction from baseline in the psoriasis area and severity index score was 7% with placebo, 9% with deucravacitinib 3 mg every other day (P=.49 vs placebo), 39% with 3 mg once daily (P<.001 vs placebo), 69% with 3 mg twice daily (P<.001 vs placebo), 67% with 6 mg twice daily (P<.001 vs placebo), and 75% with 12 mg once daily (P<.001 vs placebo). The most commonly reported AEs were nasopharyngitis, headache, diarrhea, nausea, and upper respiratory tract infection. Adverse events occurred in 51% of participants in the control group and in 55% to 80% of those in the experimental groups. Additionally, there was 1 reported case of melanoma (stage 0) 96 days after the start of treatment in a patient in the 3-mg once-daily group. Serious AEs occurred in only 0% to 2% of participants who received deucravacitinib.18
Two phase 3 trials—POETYK PSO-1 and POETYK PSO-2 (N=1686)—found deucravacitinib to be notably more effective than both placebo and apremilast in treating psoriasis.19 Among participants receiving deucravacitinib 6 mg daily, 58.7% and 53.6% in the 2 respective trials achieved psoriasis area and severity index 75 response vs 12.7% and 9.4% receiving placebo and 35.1% and 40.2% receiving apremilast. Overall, the treatment was well tolerated, with a low rate of discontinuation of deucravacitinib due to AEs (2.4% of patients on deucravacitinib compared to 3.8% on placebo and 5.2% on apremilast). The most frequently observed AEs with deucravacitinib were nasopharyngitis and upper respiratory tract infection. The full results of these trials are expected to be published soon.19,20
Final Thoughts
Overall, JAK inhibitors are a novel class of therapeutics that may have further success in the treatment of other dermatologic conditions that negatively affect patients’ quality of life and productivity. We should look forward to additional successful trials with these promising medications.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Silverberg JI , Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis. 2014;25:107-114.
- Schonmann Y, Mansfield KE, Hayes JF, et al. Atopic eczema in adulthood and risk of depression and anxiety: a population-based cohort study. J Allergy Clin Immunol Pract. 2020;8:248-257.e16.
- Bao L, Zhang H, Chan LS. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAKSTAT. 2013;2:e24137.
- Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017;18:374-384.
- Xeljanz FDA approval history. Drugs.com website. Updated December 14, 2021. Accessed February 16, 2022. https://www.drugs.com/history/xeljanz.html
- Mullard A. FDA approves Eli Lilly’s baricitinib. Nat Rev Drug Discov. 2018;17:460.
- FDA approves Opzelura. Drugs.com website. Published September 2021. Accessed February 16, 2022. https://www.drugs.com/newdrugs/fda-approves-opzelura-ruxolitinib-cream-atopic-dermatitis-ad-5666.html
- Kim BS, Sun K, Papp K, et al. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: results from a phase 2, randomized, dose-ranging, vehicle- and active-controlled study.J Am Acad Dermatol. 2020;82:1305-1313.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2, management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32:657-682.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863-873.
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:1333-1343.
- Simpson EL, Forman S, Silverberg JI, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J Am Acad Dermatol. 2021;85:62-70.
- Guttman-Yassky E, Thaçi D, Pangan AL, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145:877-884.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Published September 1, 2022. Accessed February 16, 2022. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Papp K, Gordon K, Thaçi D, et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379:1313-1321.
- Bristol Myers Squibb presents positive data from two pivotal phase 3 psoriasis studies demonstrating superiority of deucravacitinib compared to placebo and Otezla® (apremilast). Press release. Bristol Meyers Squibb. April 23, 2021. Accessed February 16, 2022. https://news.bms.com/news/details/2021/Bristol-Myers-Squibb-Presents-Positive-Data-from-Two-Pivotal-Phase-3-Psoriasis-Studies-Demonstrating-Superiority-of-Deucravacitinib-Compared-to-Placebo-and-Otezla-apremilast/default.aspx
- Armstrong A, Gooderham M, Warren R, et al. Efficacy and safety of deucravacitinib, an oral, selective tyrosine kinase 2 (TYK2) inhibitor, compared with placebo and apremilast in moderate to severe plaque psoriasis: results from the POETYK PSO-1 study [abstract]. Abstract presented at: 2021 American Academy of Dermatology annual meeting; April 23-25, 2021; San Francisco, California.
Atopic dermatitis (AD) is a chronic inflammatory skin disorder affecting 7% of adults and 13% of children in the United States.1,2 Atopic dermatitis is characterized by pruritus, dry skin, and pain, all of which can negatively impact quality of life and put patients at higher risk for psychiatric comorbidities such as anxiety and depression.3 The pathogenesis of AD is multifactorial, involving genetics, epidermal barrier dysfunction, and immune dysregulation. Overactivation of helper T cell (TH2) pathway cytokines, including IL-4, IL-13, and IL-31, is thought to propagate both inflammation and pruritus, which are central to AD. The JAK-STAT signaling pathway plays a pivotal role in the immune system dysregulation and exaggeration of TH2 cell response, making JAK-STAT inhibitors (or JAK inhibitors) strong theoretical candidates for the treatment of AD.4 In humans, the Janus kinases are composed of 4 different members—JAK1, JAK2, JAK3, and tyrosine kinase 2—all of which can be targeted by JAK inhibitors.5
JAK inhibitors such as tofacitinib have already been approved by the US Food and Drug Administration (FDA) to treat various inflammatory conditions, including rheumatoid arthritis, ulcerative colitis, and psoriatic arthritis; other JAK inhibitors such as baricitinib are only approved for patients with rheumatoid arthritis.6,7 The success of these small molecule inhibitors in these immune-mediated conditions make them attractive candidates for the treatment of AD. Several JAK inhibitors are in phase 2 and phase 3 clinical trials as oral therapies (moderate to severe AD) or as topical treatments (mild to moderate AD). Currently, ruxolitinib (RUX) is the only topical JAK inhibitor that is FDA approved for the treatment of AD in the United States.8 In this editorial, we focus on recent trials of JAK inhibitors tested in patients with AD, including topical RUX, as well as oral abrocitinib, upadacitinib, and baricitinib.
Topical RUX in AD
Ruxolitinib is a topical JAK1/2 small molecule inhibitor approved by the FDA for the treatment of AD in 2021. In a randomized trial by Kim et al9 in 2020, all tested regimens of RUX demonstrated significant improvement in eczema area and severity index (EASI) scores vs vehicle; notably, RUX cream 1.5% applied twice daily achieved the greatest mean percentage change in baseline EASI score vs vehicle at 4 weeks (76.1% vs 15.5%; P<.0001). Ruxolitinib cream was well tolerated through week 8 of the trial, and all adverse events (AEs) were mild to moderate in severity and comparable to those in the vehicle group.9
Topical JAK inhibitors appear to be effective for mild to moderate AD and have had an acceptable safety profile in clinical trials thus far. Although topical corticosteroids and calcineurin inhibitors can have great clinical benefit in AD, they are recommended for short-term use given side effects such as thinning of the skin, burning, or telangiectasia formation.10,11 The hope is that topical JAK inhibitors may be an alternative to standard topical treatments for AD, as they can be used for longer periods due to a safer side-effect profile.
Oral JAK Inhibitors in AD
Several oral JAK inhibitors are undergoing investigation for the systemic treatment of moderate to severe AD. Abrocitinib is an oral JAK1 inhibitor that has demonstrated efficacy in several phase 3 trials in patients with moderate to severe AD. In a 2021 trial, patients were randomized in a 2:2:2:1 ratio to receive abrocitinib 200 mg daily, abrocitinib 100 mg daily, subcutaneous dupilumab 300 mg every other week, or placebo, respectively.12 Patients in both abrocitinib groups showed significant improvement in AD vs placebo, and EASI-75 response was achieved in 70.3%, 58.7%, 58.1%, and 27.1% of patients, respectively (P<.001 for both abrocitinib doses vs placebo). Adverse events occurred more frequently in the abrocitinib 200-mg group vs placebo. Nausea, acne, nasopharyngitis, and headache were the most frequently reported AEs with abrocitinib.12 Another phase 3 trial by Silverberg et al13 (N=391) had similar treatment results, with 38.1% of participants receiving abrocitinib 200 mg and 28.4% of participants receiving abrocitinib 100 mg achieving investigator global assessment scores of 0 (clear) or 1 (almost clear) vs 9.1% of participants receiving placebo (P<.001). Abrocitinib was well tolerated in this trial with few serious AEs (ie, herpangina [0.6%], pneumonia [0.6%]).13 In both trials, there were rare instances of laboratory values indicating thrombocytopenia with the 200-mg dose (0.9%12 and 3.2%13) without any clinical manifestations. Although a decrease in platelets was observed, no thrombocytopenia occurred in the abrocitinib 100-mg group in the latter trial.13
Baricitinib is another oral inhibitor of JAK1 and JAK2 with potential for the treatment of AD. One randomized trial (N=329) demonstrated its efficacy in combination with a topical corticosteroid (TCS). At 16 weeks, a higher number of participants treated with baricitinib and TCS achieved investigator global assessment scores of 0 (clear) or 1 (almost clear) compared to those who received placebo and TCS (31% with baricitinib 4 mg + TCS, 24% with baricitinib 2 mg + TCS, and 15% with placebo + TCS).14 Similarly, in BREEZE-AD5,another phase 3 trial (N=440), baricitinib monotherapy demonstrated a higher rate of treatment success vs placebo.15 Specifically, 13% of patients treated with baricitinib 1 mg and 30% of those treated with baricitinib 2 mg achieved 75% or greater reduction in EASI scores compared to 8% in the placebo group. The most common AEs associated with baricitinib were nasopharyngitis and headache. Adverse events occurred with similar frequency across both experimental and control groups.15 Reich et al14 demonstrated a higher overall rate of AEs—most commonly nasopharyngitis, upper respiratory tract infections, and folliculitis—in baricitinib-treated patients; however, serious AEs occurred with similar frequency across all groups, including the control group.
The selective JAK1 inhibitor upadacitinib also is undergoing testing in treating moderate to severe AD. In one trial, 167 patients were randomized to once daily oral upadacitinib 7.5 mg, 15 mg, or 30 mg or placebo.16 All doses of upadacitinib demonstrated considerably higher percentage improvements from baseline in EASI scores compared to placebo at 16 weeks with a clear dose-response relationship (39%, 62%, and 74% vs 23%, respectively). In this trial, there were no dose-limiting safety events. Serious AEs were infrequent, occurring in 4.8%, 2.4%, and 0% of upadacitinib groups vs 2.5% for placebo. The serious AEs observed with upadacitinib were 1 case of appendicitis, lower jaw pericoronitis in a patient with a history of repeated tooth infections, and an exacerbation of AD.16
Tofacitinib, another JAK inhibitor, has been shown to increase the risk for blood clots and death in a large trial in the treatment of rheumatoid arthritis. Following this study, the FDA is requiring black box warnings for tofacitinib and also for the 2 JAK inhibitors baricitinib and upadacitinib regarding the risks for heart-related events, cancer, blood clots, and death. Given that these medications share a similar mechanism of action to tofacitinib, they may have similar risks, though they have not yet been fully evaluated in large safety trials.17
With more recent investigation into novel therapeutics for AD, oral JAK inhibitors may play an important role in the future to treat patients with moderate to severe AD with inadequate response or contraindications to other systemic therapies. In trials thus far, oral JAK inhibitors have exhibited acceptable safety profiles and have demonstrated treatment success in AD. More randomized, controlled, phase 3 studies with larger patient populations are required to confirm their potential as effective treatments and elucidate their long-term safety.
Deucravacitinib in Psoriasis
Deucravacitinib is a first-in-class, oral, selective TYK2 inhibitor currently undergoing testing for the treatment of psoriasis. A randomized phase 2 trial (N=267) found that deucravacitinib was more effective than placebo in treating chronic plaque psoriasis at doses of 3 to 12 mg daily.18 The percentage of participants with a 75% or greater reduction from baseline in the psoriasis area and severity index score was 7% with placebo, 9% with deucravacitinib 3 mg every other day (P=.49 vs placebo), 39% with 3 mg once daily (P<.001 vs placebo), 69% with 3 mg twice daily (P<.001 vs placebo), 67% with 6 mg twice daily (P<.001 vs placebo), and 75% with 12 mg once daily (P<.001 vs placebo). The most commonly reported AEs were nasopharyngitis, headache, diarrhea, nausea, and upper respiratory tract infection. Adverse events occurred in 51% of participants in the control group and in 55% to 80% of those in the experimental groups. Additionally, there was 1 reported case of melanoma (stage 0) 96 days after the start of treatment in a patient in the 3-mg once-daily group. Serious AEs occurred in only 0% to 2% of participants who received deucravacitinib.18
Two phase 3 trials—POETYK PSO-1 and POETYK PSO-2 (N=1686)—found deucravacitinib to be notably more effective than both placebo and apremilast in treating psoriasis.19 Among participants receiving deucravacitinib 6 mg daily, 58.7% and 53.6% in the 2 respective trials achieved psoriasis area and severity index 75 response vs 12.7% and 9.4% receiving placebo and 35.1% and 40.2% receiving apremilast. Overall, the treatment was well tolerated, with a low rate of discontinuation of deucravacitinib due to AEs (2.4% of patients on deucravacitinib compared to 3.8% on placebo and 5.2% on apremilast). The most frequently observed AEs with deucravacitinib were nasopharyngitis and upper respiratory tract infection. The full results of these trials are expected to be published soon.19,20
Final Thoughts
Overall, JAK inhibitors are a novel class of therapeutics that may have further success in the treatment of other dermatologic conditions that negatively affect patients’ quality of life and productivity. We should look forward to additional successful trials with these promising medications.
Atopic dermatitis (AD) is a chronic inflammatory skin disorder affecting 7% of adults and 13% of children in the United States.1,2 Atopic dermatitis is characterized by pruritus, dry skin, and pain, all of which can negatively impact quality of life and put patients at higher risk for psychiatric comorbidities such as anxiety and depression.3 The pathogenesis of AD is multifactorial, involving genetics, epidermal barrier dysfunction, and immune dysregulation. Overactivation of helper T cell (TH2) pathway cytokines, including IL-4, IL-13, and IL-31, is thought to propagate both inflammation and pruritus, which are central to AD. The JAK-STAT signaling pathway plays a pivotal role in the immune system dysregulation and exaggeration of TH2 cell response, making JAK-STAT inhibitors (or JAK inhibitors) strong theoretical candidates for the treatment of AD.4 In humans, the Janus kinases are composed of 4 different members—JAK1, JAK2, JAK3, and tyrosine kinase 2—all of which can be targeted by JAK inhibitors.5
JAK inhibitors such as tofacitinib have already been approved by the US Food and Drug Administration (FDA) to treat various inflammatory conditions, including rheumatoid arthritis, ulcerative colitis, and psoriatic arthritis; other JAK inhibitors such as baricitinib are only approved for patients with rheumatoid arthritis.6,7 The success of these small molecule inhibitors in these immune-mediated conditions make them attractive candidates for the treatment of AD. Several JAK inhibitors are in phase 2 and phase 3 clinical trials as oral therapies (moderate to severe AD) or as topical treatments (mild to moderate AD). Currently, ruxolitinib (RUX) is the only topical JAK inhibitor that is FDA approved for the treatment of AD in the United States.8 In this editorial, we focus on recent trials of JAK inhibitors tested in patients with AD, including topical RUX, as well as oral abrocitinib, upadacitinib, and baricitinib.
Topical RUX in AD
Ruxolitinib is a topical JAK1/2 small molecule inhibitor approved by the FDA for the treatment of AD in 2021. In a randomized trial by Kim et al9 in 2020, all tested regimens of RUX demonstrated significant improvement in eczema area and severity index (EASI) scores vs vehicle; notably, RUX cream 1.5% applied twice daily achieved the greatest mean percentage change in baseline EASI score vs vehicle at 4 weeks (76.1% vs 15.5%; P<.0001). Ruxolitinib cream was well tolerated through week 8 of the trial, and all adverse events (AEs) were mild to moderate in severity and comparable to those in the vehicle group.9
Topical JAK inhibitors appear to be effective for mild to moderate AD and have had an acceptable safety profile in clinical trials thus far. Although topical corticosteroids and calcineurin inhibitors can have great clinical benefit in AD, they are recommended for short-term use given side effects such as thinning of the skin, burning, or telangiectasia formation.10,11 The hope is that topical JAK inhibitors may be an alternative to standard topical treatments for AD, as they can be used for longer periods due to a safer side-effect profile.
Oral JAK Inhibitors in AD
Several oral JAK inhibitors are undergoing investigation for the systemic treatment of moderate to severe AD. Abrocitinib is an oral JAK1 inhibitor that has demonstrated efficacy in several phase 3 trials in patients with moderate to severe AD. In a 2021 trial, patients were randomized in a 2:2:2:1 ratio to receive abrocitinib 200 mg daily, abrocitinib 100 mg daily, subcutaneous dupilumab 300 mg every other week, or placebo, respectively.12 Patients in both abrocitinib groups showed significant improvement in AD vs placebo, and EASI-75 response was achieved in 70.3%, 58.7%, 58.1%, and 27.1% of patients, respectively (P<.001 for both abrocitinib doses vs placebo). Adverse events occurred more frequently in the abrocitinib 200-mg group vs placebo. Nausea, acne, nasopharyngitis, and headache were the most frequently reported AEs with abrocitinib.12 Another phase 3 trial by Silverberg et al13 (N=391) had similar treatment results, with 38.1% of participants receiving abrocitinib 200 mg and 28.4% of participants receiving abrocitinib 100 mg achieving investigator global assessment scores of 0 (clear) or 1 (almost clear) vs 9.1% of participants receiving placebo (P<.001). Abrocitinib was well tolerated in this trial with few serious AEs (ie, herpangina [0.6%], pneumonia [0.6%]).13 In both trials, there were rare instances of laboratory values indicating thrombocytopenia with the 200-mg dose (0.9%12 and 3.2%13) without any clinical manifestations. Although a decrease in platelets was observed, no thrombocytopenia occurred in the abrocitinib 100-mg group in the latter trial.13
Baricitinib is another oral inhibitor of JAK1 and JAK2 with potential for the treatment of AD. One randomized trial (N=329) demonstrated its efficacy in combination with a topical corticosteroid (TCS). At 16 weeks, a higher number of participants treated with baricitinib and TCS achieved investigator global assessment scores of 0 (clear) or 1 (almost clear) compared to those who received placebo and TCS (31% with baricitinib 4 mg + TCS, 24% with baricitinib 2 mg + TCS, and 15% with placebo + TCS).14 Similarly, in BREEZE-AD5,another phase 3 trial (N=440), baricitinib monotherapy demonstrated a higher rate of treatment success vs placebo.15 Specifically, 13% of patients treated with baricitinib 1 mg and 30% of those treated with baricitinib 2 mg achieved 75% or greater reduction in EASI scores compared to 8% in the placebo group. The most common AEs associated with baricitinib were nasopharyngitis and headache. Adverse events occurred with similar frequency across both experimental and control groups.15 Reich et al14 demonstrated a higher overall rate of AEs—most commonly nasopharyngitis, upper respiratory tract infections, and folliculitis—in baricitinib-treated patients; however, serious AEs occurred with similar frequency across all groups, including the control group.
The selective JAK1 inhibitor upadacitinib also is undergoing testing in treating moderate to severe AD. In one trial, 167 patients were randomized to once daily oral upadacitinib 7.5 mg, 15 mg, or 30 mg or placebo.16 All doses of upadacitinib demonstrated considerably higher percentage improvements from baseline in EASI scores compared to placebo at 16 weeks with a clear dose-response relationship (39%, 62%, and 74% vs 23%, respectively). In this trial, there were no dose-limiting safety events. Serious AEs were infrequent, occurring in 4.8%, 2.4%, and 0% of upadacitinib groups vs 2.5% for placebo. The serious AEs observed with upadacitinib were 1 case of appendicitis, lower jaw pericoronitis in a patient with a history of repeated tooth infections, and an exacerbation of AD.16
Tofacitinib, another JAK inhibitor, has been shown to increase the risk for blood clots and death in a large trial in the treatment of rheumatoid arthritis. Following this study, the FDA is requiring black box warnings for tofacitinib and also for the 2 JAK inhibitors baricitinib and upadacitinib regarding the risks for heart-related events, cancer, blood clots, and death. Given that these medications share a similar mechanism of action to tofacitinib, they may have similar risks, though they have not yet been fully evaluated in large safety trials.17
With more recent investigation into novel therapeutics for AD, oral JAK inhibitors may play an important role in the future to treat patients with moderate to severe AD with inadequate response or contraindications to other systemic therapies. In trials thus far, oral JAK inhibitors have exhibited acceptable safety profiles and have demonstrated treatment success in AD. More randomized, controlled, phase 3 studies with larger patient populations are required to confirm their potential as effective treatments and elucidate their long-term safety.
Deucravacitinib in Psoriasis
Deucravacitinib is a first-in-class, oral, selective TYK2 inhibitor currently undergoing testing for the treatment of psoriasis. A randomized phase 2 trial (N=267) found that deucravacitinib was more effective than placebo in treating chronic plaque psoriasis at doses of 3 to 12 mg daily.18 The percentage of participants with a 75% or greater reduction from baseline in the psoriasis area and severity index score was 7% with placebo, 9% with deucravacitinib 3 mg every other day (P=.49 vs placebo), 39% with 3 mg once daily (P<.001 vs placebo), 69% with 3 mg twice daily (P<.001 vs placebo), 67% with 6 mg twice daily (P<.001 vs placebo), and 75% with 12 mg once daily (P<.001 vs placebo). The most commonly reported AEs were nasopharyngitis, headache, diarrhea, nausea, and upper respiratory tract infection. Adverse events occurred in 51% of participants in the control group and in 55% to 80% of those in the experimental groups. Additionally, there was 1 reported case of melanoma (stage 0) 96 days after the start of treatment in a patient in the 3-mg once-daily group. Serious AEs occurred in only 0% to 2% of participants who received deucravacitinib.18
Two phase 3 trials—POETYK PSO-1 and POETYK PSO-2 (N=1686)—found deucravacitinib to be notably more effective than both placebo and apremilast in treating psoriasis.19 Among participants receiving deucravacitinib 6 mg daily, 58.7% and 53.6% in the 2 respective trials achieved psoriasis area and severity index 75 response vs 12.7% and 9.4% receiving placebo and 35.1% and 40.2% receiving apremilast. Overall, the treatment was well tolerated, with a low rate of discontinuation of deucravacitinib due to AEs (2.4% of patients on deucravacitinib compared to 3.8% on placebo and 5.2% on apremilast). The most frequently observed AEs with deucravacitinib were nasopharyngitis and upper respiratory tract infection. The full results of these trials are expected to be published soon.19,20
Final Thoughts
Overall, JAK inhibitors are a novel class of therapeutics that may have further success in the treatment of other dermatologic conditions that negatively affect patients’ quality of life and productivity. We should look forward to additional successful trials with these promising medications.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Silverberg JI , Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis. 2014;25:107-114.
- Schonmann Y, Mansfield KE, Hayes JF, et al. Atopic eczema in adulthood and risk of depression and anxiety: a population-based cohort study. J Allergy Clin Immunol Pract. 2020;8:248-257.e16.
- Bao L, Zhang H, Chan LS. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAKSTAT. 2013;2:e24137.
- Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017;18:374-384.
- Xeljanz FDA approval history. Drugs.com website. Updated December 14, 2021. Accessed February 16, 2022. https://www.drugs.com/history/xeljanz.html
- Mullard A. FDA approves Eli Lilly’s baricitinib. Nat Rev Drug Discov. 2018;17:460.
- FDA approves Opzelura. Drugs.com website. Published September 2021. Accessed February 16, 2022. https://www.drugs.com/newdrugs/fda-approves-opzelura-ruxolitinib-cream-atopic-dermatitis-ad-5666.html
- Kim BS, Sun K, Papp K, et al. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: results from a phase 2, randomized, dose-ranging, vehicle- and active-controlled study.J Am Acad Dermatol. 2020;82:1305-1313.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2, management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32:657-682.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863-873.
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:1333-1343.
- Simpson EL, Forman S, Silverberg JI, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J Am Acad Dermatol. 2021;85:62-70.
- Guttman-Yassky E, Thaçi D, Pangan AL, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145:877-884.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Published September 1, 2022. Accessed February 16, 2022. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Papp K, Gordon K, Thaçi D, et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379:1313-1321.
- Bristol Myers Squibb presents positive data from two pivotal phase 3 psoriasis studies demonstrating superiority of deucravacitinib compared to placebo and Otezla® (apremilast). Press release. Bristol Meyers Squibb. April 23, 2021. Accessed February 16, 2022. https://news.bms.com/news/details/2021/Bristol-Myers-Squibb-Presents-Positive-Data-from-Two-Pivotal-Phase-3-Psoriasis-Studies-Demonstrating-Superiority-of-Deucravacitinib-Compared-to-Placebo-and-Otezla-apremilast/default.aspx
- Armstrong A, Gooderham M, Warren R, et al. Efficacy and safety of deucravacitinib, an oral, selective tyrosine kinase 2 (TYK2) inhibitor, compared with placebo and apremilast in moderate to severe plaque psoriasis: results from the POETYK PSO-1 study [abstract]. Abstract presented at: 2021 American Academy of Dermatology annual meeting; April 23-25, 2021; San Francisco, California.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Silverberg JI , Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis. 2014;25:107-114.
- Schonmann Y, Mansfield KE, Hayes JF, et al. Atopic eczema in adulthood and risk of depression and anxiety: a population-based cohort study. J Allergy Clin Immunol Pract. 2020;8:248-257.e16.
- Bao L, Zhang H, Chan LS. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAKSTAT. 2013;2:e24137.
- Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017;18:374-384.
- Xeljanz FDA approval history. Drugs.com website. Updated December 14, 2021. Accessed February 16, 2022. https://www.drugs.com/history/xeljanz.html
- Mullard A. FDA approves Eli Lilly’s baricitinib. Nat Rev Drug Discov. 2018;17:460.
- FDA approves Opzelura. Drugs.com website. Published September 2021. Accessed February 16, 2022. https://www.drugs.com/newdrugs/fda-approves-opzelura-ruxolitinib-cream-atopic-dermatitis-ad-5666.html
- Kim BS, Sun K, Papp K, et al. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: results from a phase 2, randomized, dose-ranging, vehicle- and active-controlled study.J Am Acad Dermatol. 2020;82:1305-1313.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2, management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32:657-682.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863-873.
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:1333-1343.
- Simpson EL, Forman S, Silverberg JI, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J Am Acad Dermatol. 2021;85:62-70.
- Guttman-Yassky E, Thaçi D, Pangan AL, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145:877-884.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Published September 1, 2022. Accessed February 16, 2022. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Papp K, Gordon K, Thaçi D, et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379:1313-1321.
- Bristol Myers Squibb presents positive data from two pivotal phase 3 psoriasis studies demonstrating superiority of deucravacitinib compared to placebo and Otezla® (apremilast). Press release. Bristol Meyers Squibb. April 23, 2021. Accessed February 16, 2022. https://news.bms.com/news/details/2021/Bristol-Myers-Squibb-Presents-Positive-Data-from-Two-Pivotal-Phase-3-Psoriasis-Studies-Demonstrating-Superiority-of-Deucravacitinib-Compared-to-Placebo-and-Otezla-apremilast/default.aspx
- Armstrong A, Gooderham M, Warren R, et al. Efficacy and safety of deucravacitinib, an oral, selective tyrosine kinase 2 (TYK2) inhibitor, compared with placebo and apremilast in moderate to severe plaque psoriasis: results from the POETYK PSO-1 study [abstract]. Abstract presented at: 2021 American Academy of Dermatology annual meeting; April 23-25, 2021; San Francisco, California.