User login
Long-Term Outcomes of Allograft Reconstruction of the Anterior Cruciate Ligament
Injuries of the anterior cruciate ligament (ACL) are common. Good to excellent long-term results are generally expected in more than 90% of ACL reconstructions.1,2 Although our knowledge of the biomechanics, kinematics, and long-term outcomes of ACL reconstruction is extensive, the ideal graft choice for ACL reconstruction is still up for debate.
Historically, both quadruple-stranded hamstring tendon and bone–patellar tendon–bone (BPTB) autografts have been the most popular graft options for operative reconstruction of the ACL.3 Recently, allograft tissues have become increasingly popular as a graft source. Proponents of allograft ACL reconstruction have cited several advantages over autograft reconstruction, including decreased donor-site morbidity, shorter operative times, and quicker postoperative recovery.4-7 Nevertheless, some authors have recently reported higher rates of both reoperation and graft failure after allograft ACL reconstruction.4,8-11 The 2 senior surgeons in the Sports Medicine Section of the Department of Orthopedic Surgery at the University of Arizona College of Medicine had not recognized such high failure and revision rates in their own clinical practices.
To evaluate the long-term outcomes of allograft ACL reconstruction, we retrospectively reviewed the cases of all patients who underwent allograft or autograft ACL reconstruction by 2 senior surgeons at a single institution over an 8-year period. We hypothesized that the reoperation and revision surgery rates for allograft ACL reconstruction would not be higher than those reported for autograft reconstruction. We also hypothesized that allograft ACL reconstruction failure rates would not be higher for patients younger than 25 years than for patients who are older and less active.
Materials and Methods
This study was approved by the Institutional Review Board at the University of Arizona College of Medicine. We retrospectively reviewed the cases of all patients who underwent primary endoscopic ACL reconstruction at the University of Arizona College of Medicine over an 8-year period (2000–2008). All ACL reconstructions were performed by 2 senior, fellowship-trained sports medicine specialists, including Dr. William A. Grana. Patients were identified from the Current Procedural Terminology (CPT) code for ACL reconstruction. Both autograft and allograft reconstructions were included in the study. Patients undergoing revision ACL reconstruction and patients with multi-ligamentous knee injuries were excluded. All available medical records were reviewed for patient demographics and any concomitant knee pathology. We included patients of all activity levels, patients with acute ACL tears, and patients with chronically ACL-deficient knees. We identified a separate cohort of Division I varsity athletes from the University of Arizona for evaluation. These patients were identified from the injury surveillance system in the athletic training facility of the University of Arizona.
ACL reconstructions at our institution during this 8-year period were performed with both allograft and autograft soft tissue. Allograft tendons were most commonly used. Tibialis anterior allograft was used in the majority of those knees. Tibialis posterior and semitendinosus allografts were used in a small subset of patients. Autograft reconstruction was performed with quadruple-stranded semitendinosus and gracilis tendons. We reviewed operative reports to determine type of graft used for reconstruction.
Patients were assessed clinically by telephone interview and/or mailed survey. They were specifically asked whether there had been any postoperative complications. We reviewed all operative and postoperative follow-up notes for postoperative complications. Objective clinical assessment involved use of the International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, the Tegner-Lysholm Knee Scoring Scale, and the Tegner Activity Scale.
Operative Technique
A standard, transtibial arthroscopically assisted ACL reconstruction was performed in all patients. For autograft reconstruction patients, both the semitendinosus and gracilis tendons were harvested through a small anteromedial incision and prepared to form a quadruple-stranded graft. All allograft tendons were obtained from the Musculoskeletal Transplant Foundation (MTF). Tibialis anterior and tibialis posterior allografts were folded in half to form a double-stranded graft. Alternatively, 2 semitendinosus allografts were prepared in the same fashion as that described for autograft hamstring tendons. The tibial tunnel was placed into the center of the ACL tibial footprint. With use of a transtibial approach, an endoscopic offset guide was used to place the femoral tunnel at the 10- and 2-o’clock positions in the right and left knees, respectively. In almost all cases, the graft was secured on the femoral side with a cortical fixation button. Tibial fixation was obtained with a bioabsorbable interference screw.
After ACL reconstruction, each patient participated in the standard accelerated rehabilitation outlined by Shelbourne and Gray.12 Guided rehabilitation was instituted within 1 week after surgery under the guidance of a physical therapist. Range-of-motion exercises and closed-chain strengthening exercises were begun at this time. The protocol emphasized early return of full terminal extension and normalization of gait patterns. Patients were allowed to return to play only after meeting specific criteria, about 6 months after surgery. Many athletes in our Division I university population are allowed to return to play 5 to 6 months after surgery, after meeting return-to-play criteria.
Statistical Analysis
We used Minitab 14 (Minitab, State College, Pennsylvania) to perform all statistical analyses, unpaired Student t tests to compare IKDC and Tegner-Lysholm results between allograft and autograft groups, and χ2 tests to compare revision and reoperation rates between groups. Significance was set at P = .05.
Results
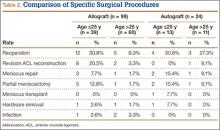
We identified 362 patients who underwent ACL reconstructions at our institution between 2000 and 2008. Of these patients, 302 met the study inclusion criteria. One-hundred twenty-three (40.7%) of the 302 were available for follow-up by telephone interview and/or mailed questionnaire. This follow-up group consisted of 67 males and 56 females. Mean age at surgery was 29 years (range, 17-53 years). Mean follow-up was 50.3 months (range, 11-111 months). Of the 123 patients, 99 underwent allograft ACL reconstruction, and 24 underwent autograft ACL reconstruction. Seventeen (17%) of the 99 allograft cases required additional surgery (Table 1). The reoperation rate for patients under age 25 years (30.8%) was higher than the rate for patients older than 25 years (Table 2). Regarding patients who underwent additional surgeries, mean scores were lower with allograft (Tegner-Lysholm, 59; IKDC, 54) than with autograft (Tegner-Lysholm, 83; IKDC, 79) (Ps = .0025 and .006, respectively).
Revision rates were 10.1% (allograft group) and 4.2% (autograft group) (Table 1). This difference was not statistically significant (P = .18). In the allograft group, the revision rate was higher for patients younger than 25 years (20.5%) than for patients older than 25 years (3.3%) (Table 2). In comparison, in the autograft group, the revision rate was only 4% for patients younger than 25 years. For younger patients, the higher rate of revision with allograft (vs autograft) was statistically significant (P = .038). For older patients, allograft and autograft revision rates did not differ significantly (P = .19). No patient younger than 25 years required revision reconstruction after autograft ACL reconstruction.
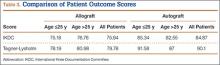
IKDC and Tegner-Lysholm outcome scores for allograft and autograft groups are shown in Table 3. In patients 25 years or younger, IKDC scores were 75.18 after allograft reconstruction and 85.34 after autograft reconstruction—a significant difference (P = .045). In addition, Tegner-Lysholm scores were significantly higher after autograft reconstruction (91.58) than allograft reconstruction (78.19) in these younger patients (P = .003) (Table 3). IKDC and Tegner-Lysholm scores were not significantly different for older patients (Ps = .241 and .211, respectively).
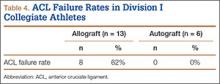
The study also included a subset of 19 primary ACL reconstructions (13 allograft, 6 autograft) performed on Division I athletes from the University of Arizona. (Nineteen [91%] of the 21 athletes in our Division I cohort were available for follow-up.) All these patients were younger than 25 years. All autograft reconstructions were performed with quadruple-stranded gracilis and semitendinosus tendons. ACL graft failure occurred in 8 (62%) of the 13 allograft cases; there were no failures in the autograft group (Table 4). One of the 5 allograft cases that did not fail required multiple surgical débridement procedures for infection, but the graft was ultimately retained. There were no infections among the 6 autograft cases.
Discussion
The ideal graft for ACL reconstruction is still a matter of intense debate. There are many graft options for ACL reconstruction. Both BPTB and hamstring autografts are associated with various graft-specific comorbidities. Anterior knee pain, knee extensor weakness, extension loss, patella fracture, patellofemoral crepitance, and infrapatellar nerve injury have been described with BPTB autografts.13-17 In a meta-analysis of 11 studies comparing BPTB autografts with hamstring autograft, Goldblatt and colleagues17 found more extension loss, kneeling pain, and patellofemoral crepitance in the BPTP group.
Knee flexion weakness, knee flexion loss, increased knee laxity, and saphenous nerve injury have all been described with use of hamstring autografts.16-19 Goldblatt and colleagues17 demonstrated a significant flexion loss in the hamstring group in their meta-analysis as well as increased laxity with both the Lachman test and the pivot shift test. They also found that the hamstring autograft group exhibited side-to-side differences of more than 3 mm on KT-1000 testing when compared with the BPTB autograft group.
Proposed advantages of allograft reconstruction include elimination of donor-site morbidity and/or pain from a less invasive procedure, faster initial recovery, more sizing options, and shorter operative times.4-7 In a 5-year follow-up of patients who had ACL reconstruction with either Achilles allograft or BPTB autograft, Poehling and colleagues7 demonstrated overall similar long-term outcomes between the groups. However, the allograft patients reported less pain 1 and 6 weeks after surgery; better function 1 week, 3 months, and 1 year after surgery; and fewer activity limitations throughout the follow-up period. Lamblin and colleagues20 also found no difference between nonirradiated allograft and autograft tissue in ACL reconstruction in a 2013 meta-analysis of ACL studies published over a 32-year period.
Despite the proposed advantages of allograft ACL reconstruction, several recent studies have demonstrated poorer outcomes in both younger patients and more active patients after allograft reconstruction.8-11,21 In a 2007 meta-analysis, Prodromos and colleagues11 compared a series of allograft reconstructions with previously published data sets of both BPTB and hamstring autografts. They found that allograft reconstructions had significantly lower stability rates than autograft reconstructions. In a case–control study by Borchers and colleagues,10 21 patients with ACL graft failure were identified over a 2-year period, and surgical outcomes were compared with those of 42 age- and sex-matched controls. The authors found higher activity level and allograft use to be risk factors for subsequent graft failure after ACL reconstruction. More important, they showed a multiplicative interaction between higher activity level after ACL reconstruction and allograft use—an interaction that greatly increased the odds for ACL graft failure. Last, in a retrospective review, Singhal and colleagues8 evaluated the outcomes of ACL reconstruction using tibialis anterior tendon allograft and reported a 23.1% revision rate. In addition, 37.7% of patients required repeat surgery. The failure/reoperation rate was 55% for patients 25 years or younger and 24% for patients older than 25 years. The authors recommended not using tibialis anterior allografts in patients 25 years or younger and in patients who frequently engage in level I ACL-dependent sports.
The poor outcomes reported by Singhal and colleagues8 may be related to use of irradiated soft-tissue allografts. In a comparison of nonirradiated BPTB allograft and BPTB autograft in patients 25 years or younger, Barber and colleagues22 found equivalent outcomes at 2-year follow-up. They actually found a higher rate of failure for autograft reconstruction (9.4%) than allograft reconstruction (7.1%). A potential critique of their study is the significant difference between the patient groups’ mean ages: 18.6 years (autograft) versus 20.1 years (allograft). Despite this selection bias, Barber and colleagues22 argued that nonirradiated BPTB allograft is equivalent to BPTB autograft for ACL reconstruction.
Our study is one of the largest allograft studies with a comparison group. The principal findings of this study demonstrate that overall reoperation and revision rates after irradiated soft-tissue allograft ACL reconstruction are higher than those historically quoted for autograft ACL reconstruction. Specifically, allograft patients younger than 25 years had a reoperation rate of 30.8% and a revision rate of 20.5%. (Allograft patients older than 25 years had lower rates of reoperation, 8.3%, and revision, 3.3%.) After revision surgery, autograft patients’ subjective outcomes (IKDC and Tegner-Lysholm scores) were significantly improved compared with those of allograft patients (Ps = .0017 and .0031, respectively). Most compelling, however, is the unexpected and quite concerning 62% failure rate in our high-level Division I intercollegiate athletes.
There are multiple hypotheses regarding the higher failure rates of allograft tissues versus autograft tissues in ACL reconstruction. Processing methods, exposure to ionizing radiation, and the incorporation/ligamentization process have all been cited as possible reasons for allograft failure. All the allograft tendons used in the present study were obtained from MTF, which uses a proprietary “aseptic” processing system that includes washing in buffered saline impregnated with antibiotics (imipenem/cilastatin, amphotericin B, gentamicin) followed by final rinsing in phosphate-buffered saline. The majority of grafts are subjected to low-level irradiation (<2 Mrad/20 kGy) based on the outcomes of MTF’s stringent donor-selection process. Although the washing process has not been shown to alter the structural integrity of donor grafts, multiple studies have outlined the detrimental effects of higher levels of gamma radiation on allograft tissues. Although lower levels are effective against potential bacterial contaminants, a radiation level of 4 Mrad is necessary to kill the human immunodeficiency virus (HIV). Thus, a dose of 4 Mrad or higher is needed to truly “sterilize” a graft. This higher dose is an issue, as it has been known for some time that higher levels of ionizing radiation can have adverse effects on the biomechanical strength of soft-tissue allografts. In fact, ionizing radiation has dose-dependent effects.23-26 Schwartz and colleagues27 showed in a caprine model that radiation exposure at 4 Mrad significantly decreased the biomechanical strength of ACL allografts at 6 months. Balsly and colleagues28 found in a biomechanical study that radiation doses of 18 to 22 Mrad did not significantly affect the mechanical integrity of soft-tissue allografts. Conversely, in an in vivo study, Rappe and colleagues29 showed that Achilles allografts irradiated at a dose of 2.0 to 2.5 Mrad had a failure rate (33%) much higher than that of nonirradiated allografts (2.4%). The radiation dose used by MTF is less than 2 Mrad. Although more than needed to kill bacterial contaminants, this dose is considered by MTF to be below the threshold for biomechanical alterations. Only a minority of grafts is treated without irradiation.
It is possible that any level of radiation affects ligamentization of allograft tissues. Multiple studies have outlined the ligamentization process of autograft tendons in vivo. Patellar tendon autografts undergo central degeneration 2 to 6 weeks after reconstruction, but, by 6 to 12 months, these tendons have structural properties similar to those of the native ACL.30-34 Findings are similar for hamstring autografts.35,36 Goradia and colleagues36 found that, by 52 weeks, semitendinosus autografts transform into a histologic structure similar to that of the normal ACL. Remodeling of allograft tendons has been described as occurring at a much slower rate.27,37-40 Bhatia and colleagues37 demonstrated faster remodeling in autograft tissues versus allograft tissues at early time points in an in vivo rabbit model. Ultimately, differences in graft incorporation and ligamentization may be a primary factor in the higher failure rates of allograft ACL reconstruction. Current rehabilitation protocols may not take into account the longer ligamentization process for allograft tissues. These protocols are largely based on our current understanding of the ligamentization process after autograft reconstruction. It is possible that the rehabilitation program and return-to-play schedule for allograft reconstruction need to be altered to help avoid higher failure rates. The return-to-play protocol at the authors’ institution scheduled most varsity athletes to return to play 6 months after surgery. In some cases, the timetable was shortened, and some athletes were returned to play 5 months after surgery, after meeting all return-to-play criteria. Based on the findings of the present study, this return-to-play schedule may be much too aggressive for high-level athletes after allograft reconstruction. It is possible these allografts have not reached “maturity,” as their autograft counterparts have, and thus are not ready for unrestricted return to play.
Our study had multiple strengths. All reconstructions were performed by 2 senior surgeons with extensive clinical experience. The autograft and allograft reconstructions used the same techniques and rehabilitation protocols. This is one of the largest studies of outcomes of allograft ACL reconstruction and one of the largest studies that used a comparison group of autograft reconstructions. Having a comparison group effectively allowed us to contrast the differences between allograft and autograft tissues. Last, this study evaluated a subgroup of high-level NCAA Division I athletes. Follow-up in the overall study was 40.7%, but follow-up in this subgroup was 91%. The very high follow-up rate in the university population helped us validate the overall results of the study. Study results reinforced the fact that irradiated soft-tissue allograft may not be indicated for ACL reconstruction in a younger, more active patient population and led to a change in approach to ACL reconstruction for Division I intercollegiate athletes at the University of Arizona. Allograft ACL reconstruction is no longer recommended for the intercollegiate athletes at the University of Arizona.
Our study had its limitations. First, it had the inherent biases of a retrospective study. Second, many patients were lost to follow-up. We contacted and surveyed 40.7% of the patients who met the inclusion criteria. We tried reaching them in multiple ways—through US mail, all listed phone numbers, family members, and so forth. Tucson, Arizona is a college town and has a larger transient population, which may have added to the difficulty in contacting patients.
Conclusion
Given the high rates of reoperation and revision surgery with allograft reconstruction in younger patients in this study, we recommend against routine use of irradiated soft-tissue allograft tissue for ACL reconstruction in patients 25 years or younger. In our clinical practices, we prefer using autograft tissue for ACL reconstruction in younger, more active individuals. Irradiated soft-tissue allograft ACL reconstruction is a viable option in the older, less active patient population. Although the overall reoperation rate in this cohort study is acceptable, the revision rate for patients younger than 25 years is concerning and should be taken into account when considering use of irradiated soft-tissue allograft for ACL reconstruction in these younger patients.
1. Schepsis AA, Busconi BD. Sports Medicine. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
2. Campbell WC, Canale ST, Beaty JH. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, PA: Mosby/Elsevier; 2008.
3. Sherman OH, Banffy MB. Anterior cruciate ligament reconstruction: which graft is best? Arthroscopy. 2004;20(9):974-980.
4. Lee JH, Bae DK, Song SJ, Cho SM, Yoon KH. Comparison of clinical results and second-look arthroscopy findings after arthroscopic anterior cruciate ligament reconstruction using 3 different types of grafts. Arthroscopy. 2010;26(1):41-49.
5. Sun K, Tian SQ, Zhang JH, Xia CS, Zhang CL, Yu TB. Anterior cruciate ligament reconstruction with bone-patellar tendon-bone autograft versus allograft. Arthroscopy. 2009;25(7):750-759.
6. Kuhn MA, Ross G. Allografts in the treatment of anterior cruciate ligament injuries. Sports Med Arthrosc Rev. 2007;15(3):133-138.
7. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. 2005;21(7):774-785.
8. Singhal MC, Gardiner JR, Johnson DL. Failure of primary anterior cruciate ligament surgery using anterior tibialis allograft. Arthroscopy. 2007;23(5):469-475.
9. Barrett GR, Luber K, Replogle WH, Manley JL. Allograft anterior cruciate ligament reconstruction in the young, active patient: Tegner activity level and failure rate. Arthroscopy. 2010;26(12):1593-1601.
10. Borchers JR, Pedroza A, Kaeding C. Activity level and graft type as risk factors for anterior cruciate ligament graft failure: a case–control study. Am J Sports Med. 2009;37(12):2362-2367.
11. Prodromos C, Joyce B, Shi K. A meta-analysis of stability of autografts compared to allografts after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):851-856.
12. Shelbourne KD, Gray T. Anterior cruciate ligament reconstruction with autogenous patellar tendon graft followed by accelerated rehabilitation. A two- to nine-year followup. Am J Sports Med. 1997;25(6):786-795.
13. Rosenberg TD, Franklin JL, Baldwin GN, Nelson KA. Extensor mechanism function after patellar tendon graft harvest for anterior cruciate ligament reconstruction. Am J Sports Med. 1992;20(5):519-525.
14. Piva SR, Childs JD, Klucinec BM, Irrgang JJ, Almeida GJ, Fitzgerald GK. Patella fracture during rehabilitation after bone–patellar tendon–bone anterior cruciate ligament reconstruction: 2 case reports. J Orthop Sports Phys Ther. 2009;39(4):278-286.
15. Lee GH, McCulloch P, Cole BJ, Bush-Joseph CA, Bach BR Jr. The incidence of acute patellar tendon harvest complications for anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(2):162-166.
16. Kartus J, Movin T, Karlsson J. Donor-site morbidity and anterior knee problems after anterior cruciate ligament reconstruction using autografts. Arthroscopy. 2001;17(9):971-980.
17. Goldblatt JP, Fitzsimmons SE, Balk E, Richmond JC. Reconstruction of the anterior cruciate ligament: meta-analysis of patellar tendon versus hamstring tendon autograft. Arthroscopy. 2005;21(7):791-803.
18. Freedman KB, D’Amato MJ, Nedeff DD, Kaz A, Bach BR Jr. Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med. 2003;31(1):2-11.
19. Yunes M, Richmond JC, Engels EA, Pinczewski LA. Patellar versus hamstring tendons in anterior cruciate ligament reconstruction: a meta-analysis. Arthroscopy. 2001;17(3):248-257.
20. Lamblin CJ, Waterman BR, Lubowitz JH. Anterior cruciate ligament reconstruction with autografts compared with non-irradiated, non-chemically treated allografts. Arthroscopy. 2013;29(6):1113-1122.
21. Pallis M, Svoboda SJ, Cameron KL, Owens BD. Survival comparison of allograft and autograft anterior cruciate ligament reconstruction at the United States Military Academy. Am J Sports Med. 2012;40(6):1242-1246.
22. Barber FA, Cowden CH 3rd, Sanders EJ. Revision rates after anterior cruciate ligament reconstruction using bone–patellar tendon–bone allograft or autograft in a population 25 years old and younger. Arthroscopy. 2014;30(4):483-491.
23. Salehpour A, Butler DL, Proch FS, et al. Dose-dependent response of gamma irradiation on mechanical properties and related biochemical composition of goat bone–patellar tendon–bone allografts. J Orthop Res. 1995;13(6):898-906.
24. Gibbons MJ, Butler DL, Grood ES, Bylski-Austrow DI, Levy MS, Noyes FR. Effects of gamma irradiation on the initial mechanical and material properties of goat bone–patellar tendon–bone allografts. J Orthop Res. 1991;9(2):209-218.
25. Fideler BM, Vangsness CT Jr, Lu B, Orlando C, Moore T. Gamma irradiation: effects on biomechanical properties of human bone–patellar tendon–bone allografts. Am J Sports Med. 1995;23(5):643-646.
26. De Deyne P, Haut RC. Some effects of gamma irradiation on patellar tendon allografts. Connect Tissue Res. 1991;27(1):51-62.
27. Schwartz HE, Matava MJ, Proch FS, et al. The effect of gamma irradiation on anterior cruciate ligament allograft biomechanical and biochemical properties in the caprine model at time zero and at 6 months after surgery. Am J Sports Med. 2006;34(11):1747-1755.
28. Balsly CR, Cotter AT, Williams LA, Gaskins BD, Moore MA, Wolfinbarger L Jr. Effect of low dose and moderate dose gamma irradiation on the mechanical properties of bone and soft tissue allografts. Cell Tissue Bank. 2008;9(4):289-298.
29. Rappe M, Horodyski M, Meister K, Indelicato PA. Nonirradiated versus irradiated Achilles allograft: in vivo failure comparison. Am J Sports Med. 2007;35(10):1653-1658.
30. Amiel D, Kleiner JB, Akeson WH. The natural history of the anterior cruciate ligament autograft of patellar tendon origin. Am J Sports Med. 1986;14(6):449-462.
31. Amiel D, Kleiner JB, Roux RD, Harwood FL, Akeson WH. The phenomenon of “ligamentization”: anterior cruciate ligament reconstruction with autogenous patellar tendon. J Orthop Res. 1986;4(2):162-172.
32. Arnoczky SP, Tarvin GB, Marshall JL. Anterior cruciate ligament replacement using patellar tendon. An evaluation of graft revascularization in the dog. J Bone Joint Surg Am. 1982;64(2):217-224.
33. Ballock RT, Woo SL, Lyon RM, Hollis JM, Akeson WH. Use of patellar tendon autograft for anterior cruciate ligament reconstruction in the rabbit: a long-term histologic and biomechanical study. J Orthop Res. 1989;7(4):474-485.
34. Clancy WG Jr, Narechania RG, Rosenberg TD, Gmeiner JG, Wisnefske DD, Lange TA. Anterior and posterior cruciate ligament reconstruction in rhesus monkeys. J Bone Joint Surg Am. 1981;63(8):1270-1284.
35. Blickenstaff KR, Grana WA, Egle D. Analysis of a semitendinosus autograft in a rabbit model. Am J Sports Med. 1997;25(4):554-559.
36. Goradia VK, Rochat MC, Kida M, Grana WA. Natural history of a hamstring tendon autograft used for anterior cruciate ligament reconstruction in a sheep model. Am J Sports Med. 2000;28(1):40-46.
37. Bhatia S, Bell R, Frank RM, et al. Bony incorporation of soft tissue anterior cruciate ligament grafts in an animal model: autograft versus allograft with low-dose gamma irradiation. Am J Sports Med. 2012;40(8):1789-1798.
38. Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21(2):176-185.
39. Goertzen MJ, Clahsen H, Schulitz KP. Anterior cruciate ligament reconstruction using cryopreserved irradiated bone-ACL-bone-allograft transplants. Knee Surg Sports Traumatol Arthrosc. 1994;2(3):150-157.
40. Mae T, Shino K, Maeda A, Toritsuka Y, Horibe S, Ochi T. Effect of gamma irradiation on remodeling process of tendon allograft. Clin Orthop. 2003;(414):305-314.
Injuries of the anterior cruciate ligament (ACL) are common. Good to excellent long-term results are generally expected in more than 90% of ACL reconstructions.1,2 Although our knowledge of the biomechanics, kinematics, and long-term outcomes of ACL reconstruction is extensive, the ideal graft choice for ACL reconstruction is still up for debate.
Historically, both quadruple-stranded hamstring tendon and bone–patellar tendon–bone (BPTB) autografts have been the most popular graft options for operative reconstruction of the ACL.3 Recently, allograft tissues have become increasingly popular as a graft source. Proponents of allograft ACL reconstruction have cited several advantages over autograft reconstruction, including decreased donor-site morbidity, shorter operative times, and quicker postoperative recovery.4-7 Nevertheless, some authors have recently reported higher rates of both reoperation and graft failure after allograft ACL reconstruction.4,8-11 The 2 senior surgeons in the Sports Medicine Section of the Department of Orthopedic Surgery at the University of Arizona College of Medicine had not recognized such high failure and revision rates in their own clinical practices.
To evaluate the long-term outcomes of allograft ACL reconstruction, we retrospectively reviewed the cases of all patients who underwent allograft or autograft ACL reconstruction by 2 senior surgeons at a single institution over an 8-year period. We hypothesized that the reoperation and revision surgery rates for allograft ACL reconstruction would not be higher than those reported for autograft reconstruction. We also hypothesized that allograft ACL reconstruction failure rates would not be higher for patients younger than 25 years than for patients who are older and less active.
Materials and Methods
This study was approved by the Institutional Review Board at the University of Arizona College of Medicine. We retrospectively reviewed the cases of all patients who underwent primary endoscopic ACL reconstruction at the University of Arizona College of Medicine over an 8-year period (2000–2008). All ACL reconstructions were performed by 2 senior, fellowship-trained sports medicine specialists, including Dr. William A. Grana. Patients were identified from the Current Procedural Terminology (CPT) code for ACL reconstruction. Both autograft and allograft reconstructions were included in the study. Patients undergoing revision ACL reconstruction and patients with multi-ligamentous knee injuries were excluded. All available medical records were reviewed for patient demographics and any concomitant knee pathology. We included patients of all activity levels, patients with acute ACL tears, and patients with chronically ACL-deficient knees. We identified a separate cohort of Division I varsity athletes from the University of Arizona for evaluation. These patients were identified from the injury surveillance system in the athletic training facility of the University of Arizona.
ACL reconstructions at our institution during this 8-year period were performed with both allograft and autograft soft tissue. Allograft tendons were most commonly used. Tibialis anterior allograft was used in the majority of those knees. Tibialis posterior and semitendinosus allografts were used in a small subset of patients. Autograft reconstruction was performed with quadruple-stranded semitendinosus and gracilis tendons. We reviewed operative reports to determine type of graft used for reconstruction.
Patients were assessed clinically by telephone interview and/or mailed survey. They were specifically asked whether there had been any postoperative complications. We reviewed all operative and postoperative follow-up notes for postoperative complications. Objective clinical assessment involved use of the International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, the Tegner-Lysholm Knee Scoring Scale, and the Tegner Activity Scale.
Operative Technique
A standard, transtibial arthroscopically assisted ACL reconstruction was performed in all patients. For autograft reconstruction patients, both the semitendinosus and gracilis tendons were harvested through a small anteromedial incision and prepared to form a quadruple-stranded graft. All allograft tendons were obtained from the Musculoskeletal Transplant Foundation (MTF). Tibialis anterior and tibialis posterior allografts were folded in half to form a double-stranded graft. Alternatively, 2 semitendinosus allografts were prepared in the same fashion as that described for autograft hamstring tendons. The tibial tunnel was placed into the center of the ACL tibial footprint. With use of a transtibial approach, an endoscopic offset guide was used to place the femoral tunnel at the 10- and 2-o’clock positions in the right and left knees, respectively. In almost all cases, the graft was secured on the femoral side with a cortical fixation button. Tibial fixation was obtained with a bioabsorbable interference screw.
After ACL reconstruction, each patient participated in the standard accelerated rehabilitation outlined by Shelbourne and Gray.12 Guided rehabilitation was instituted within 1 week after surgery under the guidance of a physical therapist. Range-of-motion exercises and closed-chain strengthening exercises were begun at this time. The protocol emphasized early return of full terminal extension and normalization of gait patterns. Patients were allowed to return to play only after meeting specific criteria, about 6 months after surgery. Many athletes in our Division I university population are allowed to return to play 5 to 6 months after surgery, after meeting return-to-play criteria.
Statistical Analysis
We used Minitab 14 (Minitab, State College, Pennsylvania) to perform all statistical analyses, unpaired Student t tests to compare IKDC and Tegner-Lysholm results between allograft and autograft groups, and χ2 tests to compare revision and reoperation rates between groups. Significance was set at P = .05.
Results
We identified 362 patients who underwent ACL reconstructions at our institution between 2000 and 2008. Of these patients, 302 met the study inclusion criteria. One-hundred twenty-three (40.7%) of the 302 were available for follow-up by telephone interview and/or mailed questionnaire. This follow-up group consisted of 67 males and 56 females. Mean age at surgery was 29 years (range, 17-53 years). Mean follow-up was 50.3 months (range, 11-111 months). Of the 123 patients, 99 underwent allograft ACL reconstruction, and 24 underwent autograft ACL reconstruction. Seventeen (17%) of the 99 allograft cases required additional surgery (Table 1). The reoperation rate for patients under age 25 years (30.8%) was higher than the rate for patients older than 25 years (Table 2). Regarding patients who underwent additional surgeries, mean scores were lower with allograft (Tegner-Lysholm, 59; IKDC, 54) than with autograft (Tegner-Lysholm, 83; IKDC, 79) (Ps = .0025 and .006, respectively).
Revision rates were 10.1% (allograft group) and 4.2% (autograft group) (Table 1). This difference was not statistically significant (P = .18). In the allograft group, the revision rate was higher for patients younger than 25 years (20.5%) than for patients older than 25 years (3.3%) (Table 2). In comparison, in the autograft group, the revision rate was only 4% for patients younger than 25 years. For younger patients, the higher rate of revision with allograft (vs autograft) was statistically significant (P = .038). For older patients, allograft and autograft revision rates did not differ significantly (P = .19). No patient younger than 25 years required revision reconstruction after autograft ACL reconstruction.
IKDC and Tegner-Lysholm outcome scores for allograft and autograft groups are shown in Table 3. In patients 25 years or younger, IKDC scores were 75.18 after allograft reconstruction and 85.34 after autograft reconstruction—a significant difference (P = .045). In addition, Tegner-Lysholm scores were significantly higher after autograft reconstruction (91.58) than allograft reconstruction (78.19) in these younger patients (P = .003) (Table 3). IKDC and Tegner-Lysholm scores were not significantly different for older patients (Ps = .241 and .211, respectively).
The study also included a subset of 19 primary ACL reconstructions (13 allograft, 6 autograft) performed on Division I athletes from the University of Arizona. (Nineteen [91%] of the 21 athletes in our Division I cohort were available for follow-up.) All these patients were younger than 25 years. All autograft reconstructions were performed with quadruple-stranded gracilis and semitendinosus tendons. ACL graft failure occurred in 8 (62%) of the 13 allograft cases; there were no failures in the autograft group (Table 4). One of the 5 allograft cases that did not fail required multiple surgical débridement procedures for infection, but the graft was ultimately retained. There were no infections among the 6 autograft cases.
Discussion
The ideal graft for ACL reconstruction is still a matter of intense debate. There are many graft options for ACL reconstruction. Both BPTB and hamstring autografts are associated with various graft-specific comorbidities. Anterior knee pain, knee extensor weakness, extension loss, patella fracture, patellofemoral crepitance, and infrapatellar nerve injury have been described with BPTB autografts.13-17 In a meta-analysis of 11 studies comparing BPTB autografts with hamstring autograft, Goldblatt and colleagues17 found more extension loss, kneeling pain, and patellofemoral crepitance in the BPTP group.
Knee flexion weakness, knee flexion loss, increased knee laxity, and saphenous nerve injury have all been described with use of hamstring autografts.16-19 Goldblatt and colleagues17 demonstrated a significant flexion loss in the hamstring group in their meta-analysis as well as increased laxity with both the Lachman test and the pivot shift test. They also found that the hamstring autograft group exhibited side-to-side differences of more than 3 mm on KT-1000 testing when compared with the BPTB autograft group.
Proposed advantages of allograft reconstruction include elimination of donor-site morbidity and/or pain from a less invasive procedure, faster initial recovery, more sizing options, and shorter operative times.4-7 In a 5-year follow-up of patients who had ACL reconstruction with either Achilles allograft or BPTB autograft, Poehling and colleagues7 demonstrated overall similar long-term outcomes between the groups. However, the allograft patients reported less pain 1 and 6 weeks after surgery; better function 1 week, 3 months, and 1 year after surgery; and fewer activity limitations throughout the follow-up period. Lamblin and colleagues20 also found no difference between nonirradiated allograft and autograft tissue in ACL reconstruction in a 2013 meta-analysis of ACL studies published over a 32-year period.
Despite the proposed advantages of allograft ACL reconstruction, several recent studies have demonstrated poorer outcomes in both younger patients and more active patients after allograft reconstruction.8-11,21 In a 2007 meta-analysis, Prodromos and colleagues11 compared a series of allograft reconstructions with previously published data sets of both BPTB and hamstring autografts. They found that allograft reconstructions had significantly lower stability rates than autograft reconstructions. In a case–control study by Borchers and colleagues,10 21 patients with ACL graft failure were identified over a 2-year period, and surgical outcomes were compared with those of 42 age- and sex-matched controls. The authors found higher activity level and allograft use to be risk factors for subsequent graft failure after ACL reconstruction. More important, they showed a multiplicative interaction between higher activity level after ACL reconstruction and allograft use—an interaction that greatly increased the odds for ACL graft failure. Last, in a retrospective review, Singhal and colleagues8 evaluated the outcomes of ACL reconstruction using tibialis anterior tendon allograft and reported a 23.1% revision rate. In addition, 37.7% of patients required repeat surgery. The failure/reoperation rate was 55% for patients 25 years or younger and 24% for patients older than 25 years. The authors recommended not using tibialis anterior allografts in patients 25 years or younger and in patients who frequently engage in level I ACL-dependent sports.
The poor outcomes reported by Singhal and colleagues8 may be related to use of irradiated soft-tissue allografts. In a comparison of nonirradiated BPTB allograft and BPTB autograft in patients 25 years or younger, Barber and colleagues22 found equivalent outcomes at 2-year follow-up. They actually found a higher rate of failure for autograft reconstruction (9.4%) than allograft reconstruction (7.1%). A potential critique of their study is the significant difference between the patient groups’ mean ages: 18.6 years (autograft) versus 20.1 years (allograft). Despite this selection bias, Barber and colleagues22 argued that nonirradiated BPTB allograft is equivalent to BPTB autograft for ACL reconstruction.
Our study is one of the largest allograft studies with a comparison group. The principal findings of this study demonstrate that overall reoperation and revision rates after irradiated soft-tissue allograft ACL reconstruction are higher than those historically quoted for autograft ACL reconstruction. Specifically, allograft patients younger than 25 years had a reoperation rate of 30.8% and a revision rate of 20.5%. (Allograft patients older than 25 years had lower rates of reoperation, 8.3%, and revision, 3.3%.) After revision surgery, autograft patients’ subjective outcomes (IKDC and Tegner-Lysholm scores) were significantly improved compared with those of allograft patients (Ps = .0017 and .0031, respectively). Most compelling, however, is the unexpected and quite concerning 62% failure rate in our high-level Division I intercollegiate athletes.
There are multiple hypotheses regarding the higher failure rates of allograft tissues versus autograft tissues in ACL reconstruction. Processing methods, exposure to ionizing radiation, and the incorporation/ligamentization process have all been cited as possible reasons for allograft failure. All the allograft tendons used in the present study were obtained from MTF, which uses a proprietary “aseptic” processing system that includes washing in buffered saline impregnated with antibiotics (imipenem/cilastatin, amphotericin B, gentamicin) followed by final rinsing in phosphate-buffered saline. The majority of grafts are subjected to low-level irradiation (<2 Mrad/20 kGy) based on the outcomes of MTF’s stringent donor-selection process. Although the washing process has not been shown to alter the structural integrity of donor grafts, multiple studies have outlined the detrimental effects of higher levels of gamma radiation on allograft tissues. Although lower levels are effective against potential bacterial contaminants, a radiation level of 4 Mrad is necessary to kill the human immunodeficiency virus (HIV). Thus, a dose of 4 Mrad or higher is needed to truly “sterilize” a graft. This higher dose is an issue, as it has been known for some time that higher levels of ionizing radiation can have adverse effects on the biomechanical strength of soft-tissue allografts. In fact, ionizing radiation has dose-dependent effects.23-26 Schwartz and colleagues27 showed in a caprine model that radiation exposure at 4 Mrad significantly decreased the biomechanical strength of ACL allografts at 6 months. Balsly and colleagues28 found in a biomechanical study that radiation doses of 18 to 22 Mrad did not significantly affect the mechanical integrity of soft-tissue allografts. Conversely, in an in vivo study, Rappe and colleagues29 showed that Achilles allografts irradiated at a dose of 2.0 to 2.5 Mrad had a failure rate (33%) much higher than that of nonirradiated allografts (2.4%). The radiation dose used by MTF is less than 2 Mrad. Although more than needed to kill bacterial contaminants, this dose is considered by MTF to be below the threshold for biomechanical alterations. Only a minority of grafts is treated without irradiation.
It is possible that any level of radiation affects ligamentization of allograft tissues. Multiple studies have outlined the ligamentization process of autograft tendons in vivo. Patellar tendon autografts undergo central degeneration 2 to 6 weeks after reconstruction, but, by 6 to 12 months, these tendons have structural properties similar to those of the native ACL.30-34 Findings are similar for hamstring autografts.35,36 Goradia and colleagues36 found that, by 52 weeks, semitendinosus autografts transform into a histologic structure similar to that of the normal ACL. Remodeling of allograft tendons has been described as occurring at a much slower rate.27,37-40 Bhatia and colleagues37 demonstrated faster remodeling in autograft tissues versus allograft tissues at early time points in an in vivo rabbit model. Ultimately, differences in graft incorporation and ligamentization may be a primary factor in the higher failure rates of allograft ACL reconstruction. Current rehabilitation protocols may not take into account the longer ligamentization process for allograft tissues. These protocols are largely based on our current understanding of the ligamentization process after autograft reconstruction. It is possible that the rehabilitation program and return-to-play schedule for allograft reconstruction need to be altered to help avoid higher failure rates. The return-to-play protocol at the authors’ institution scheduled most varsity athletes to return to play 6 months after surgery. In some cases, the timetable was shortened, and some athletes were returned to play 5 months after surgery, after meeting all return-to-play criteria. Based on the findings of the present study, this return-to-play schedule may be much too aggressive for high-level athletes after allograft reconstruction. It is possible these allografts have not reached “maturity,” as their autograft counterparts have, and thus are not ready for unrestricted return to play.
Our study had multiple strengths. All reconstructions were performed by 2 senior surgeons with extensive clinical experience. The autograft and allograft reconstructions used the same techniques and rehabilitation protocols. This is one of the largest studies of outcomes of allograft ACL reconstruction and one of the largest studies that used a comparison group of autograft reconstructions. Having a comparison group effectively allowed us to contrast the differences between allograft and autograft tissues. Last, this study evaluated a subgroup of high-level NCAA Division I athletes. Follow-up in the overall study was 40.7%, but follow-up in this subgroup was 91%. The very high follow-up rate in the university population helped us validate the overall results of the study. Study results reinforced the fact that irradiated soft-tissue allograft may not be indicated for ACL reconstruction in a younger, more active patient population and led to a change in approach to ACL reconstruction for Division I intercollegiate athletes at the University of Arizona. Allograft ACL reconstruction is no longer recommended for the intercollegiate athletes at the University of Arizona.
Our study had its limitations. First, it had the inherent biases of a retrospective study. Second, many patients were lost to follow-up. We contacted and surveyed 40.7% of the patients who met the inclusion criteria. We tried reaching them in multiple ways—through US mail, all listed phone numbers, family members, and so forth. Tucson, Arizona is a college town and has a larger transient population, which may have added to the difficulty in contacting patients.
Conclusion
Given the high rates of reoperation and revision surgery with allograft reconstruction in younger patients in this study, we recommend against routine use of irradiated soft-tissue allograft tissue for ACL reconstruction in patients 25 years or younger. In our clinical practices, we prefer using autograft tissue for ACL reconstruction in younger, more active individuals. Irradiated soft-tissue allograft ACL reconstruction is a viable option in the older, less active patient population. Although the overall reoperation rate in this cohort study is acceptable, the revision rate for patients younger than 25 years is concerning and should be taken into account when considering use of irradiated soft-tissue allograft for ACL reconstruction in these younger patients.
Injuries of the anterior cruciate ligament (ACL) are common. Good to excellent long-term results are generally expected in more than 90% of ACL reconstructions.1,2 Although our knowledge of the biomechanics, kinematics, and long-term outcomes of ACL reconstruction is extensive, the ideal graft choice for ACL reconstruction is still up for debate.
Historically, both quadruple-stranded hamstring tendon and bone–patellar tendon–bone (BPTB) autografts have been the most popular graft options for operative reconstruction of the ACL.3 Recently, allograft tissues have become increasingly popular as a graft source. Proponents of allograft ACL reconstruction have cited several advantages over autograft reconstruction, including decreased donor-site morbidity, shorter operative times, and quicker postoperative recovery.4-7 Nevertheless, some authors have recently reported higher rates of both reoperation and graft failure after allograft ACL reconstruction.4,8-11 The 2 senior surgeons in the Sports Medicine Section of the Department of Orthopedic Surgery at the University of Arizona College of Medicine had not recognized such high failure and revision rates in their own clinical practices.
To evaluate the long-term outcomes of allograft ACL reconstruction, we retrospectively reviewed the cases of all patients who underwent allograft or autograft ACL reconstruction by 2 senior surgeons at a single institution over an 8-year period. We hypothesized that the reoperation and revision surgery rates for allograft ACL reconstruction would not be higher than those reported for autograft reconstruction. We also hypothesized that allograft ACL reconstruction failure rates would not be higher for patients younger than 25 years than for patients who are older and less active.
Materials and Methods
This study was approved by the Institutional Review Board at the University of Arizona College of Medicine. We retrospectively reviewed the cases of all patients who underwent primary endoscopic ACL reconstruction at the University of Arizona College of Medicine over an 8-year period (2000–2008). All ACL reconstructions were performed by 2 senior, fellowship-trained sports medicine specialists, including Dr. William A. Grana. Patients were identified from the Current Procedural Terminology (CPT) code for ACL reconstruction. Both autograft and allograft reconstructions were included in the study. Patients undergoing revision ACL reconstruction and patients with multi-ligamentous knee injuries were excluded. All available medical records were reviewed for patient demographics and any concomitant knee pathology. We included patients of all activity levels, patients with acute ACL tears, and patients with chronically ACL-deficient knees. We identified a separate cohort of Division I varsity athletes from the University of Arizona for evaluation. These patients were identified from the injury surveillance system in the athletic training facility of the University of Arizona.
ACL reconstructions at our institution during this 8-year period were performed with both allograft and autograft soft tissue. Allograft tendons were most commonly used. Tibialis anterior allograft was used in the majority of those knees. Tibialis posterior and semitendinosus allografts were used in a small subset of patients. Autograft reconstruction was performed with quadruple-stranded semitendinosus and gracilis tendons. We reviewed operative reports to determine type of graft used for reconstruction.
Patients were assessed clinically by telephone interview and/or mailed survey. They were specifically asked whether there had been any postoperative complications. We reviewed all operative and postoperative follow-up notes for postoperative complications. Objective clinical assessment involved use of the International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, the Tegner-Lysholm Knee Scoring Scale, and the Tegner Activity Scale.
Operative Technique
A standard, transtibial arthroscopically assisted ACL reconstruction was performed in all patients. For autograft reconstruction patients, both the semitendinosus and gracilis tendons were harvested through a small anteromedial incision and prepared to form a quadruple-stranded graft. All allograft tendons were obtained from the Musculoskeletal Transplant Foundation (MTF). Tibialis anterior and tibialis posterior allografts were folded in half to form a double-stranded graft. Alternatively, 2 semitendinosus allografts were prepared in the same fashion as that described for autograft hamstring tendons. The tibial tunnel was placed into the center of the ACL tibial footprint. With use of a transtibial approach, an endoscopic offset guide was used to place the femoral tunnel at the 10- and 2-o’clock positions in the right and left knees, respectively. In almost all cases, the graft was secured on the femoral side with a cortical fixation button. Tibial fixation was obtained with a bioabsorbable interference screw.
After ACL reconstruction, each patient participated in the standard accelerated rehabilitation outlined by Shelbourne and Gray.12 Guided rehabilitation was instituted within 1 week after surgery under the guidance of a physical therapist. Range-of-motion exercises and closed-chain strengthening exercises were begun at this time. The protocol emphasized early return of full terminal extension and normalization of gait patterns. Patients were allowed to return to play only after meeting specific criteria, about 6 months after surgery. Many athletes in our Division I university population are allowed to return to play 5 to 6 months after surgery, after meeting return-to-play criteria.
Statistical Analysis
We used Minitab 14 (Minitab, State College, Pennsylvania) to perform all statistical analyses, unpaired Student t tests to compare IKDC and Tegner-Lysholm results between allograft and autograft groups, and χ2 tests to compare revision and reoperation rates between groups. Significance was set at P = .05.
Results
We identified 362 patients who underwent ACL reconstructions at our institution between 2000 and 2008. Of these patients, 302 met the study inclusion criteria. One-hundred twenty-three (40.7%) of the 302 were available for follow-up by telephone interview and/or mailed questionnaire. This follow-up group consisted of 67 males and 56 females. Mean age at surgery was 29 years (range, 17-53 years). Mean follow-up was 50.3 months (range, 11-111 months). Of the 123 patients, 99 underwent allograft ACL reconstruction, and 24 underwent autograft ACL reconstruction. Seventeen (17%) of the 99 allograft cases required additional surgery (Table 1). The reoperation rate for patients under age 25 years (30.8%) was higher than the rate for patients older than 25 years (Table 2). Regarding patients who underwent additional surgeries, mean scores were lower with allograft (Tegner-Lysholm, 59; IKDC, 54) than with autograft (Tegner-Lysholm, 83; IKDC, 79) (Ps = .0025 and .006, respectively).
Revision rates were 10.1% (allograft group) and 4.2% (autograft group) (Table 1). This difference was not statistically significant (P = .18). In the allograft group, the revision rate was higher for patients younger than 25 years (20.5%) than for patients older than 25 years (3.3%) (Table 2). In comparison, in the autograft group, the revision rate was only 4% for patients younger than 25 years. For younger patients, the higher rate of revision with allograft (vs autograft) was statistically significant (P = .038). For older patients, allograft and autograft revision rates did not differ significantly (P = .19). No patient younger than 25 years required revision reconstruction after autograft ACL reconstruction.
IKDC and Tegner-Lysholm outcome scores for allograft and autograft groups are shown in Table 3. In patients 25 years or younger, IKDC scores were 75.18 after allograft reconstruction and 85.34 after autograft reconstruction—a significant difference (P = .045). In addition, Tegner-Lysholm scores were significantly higher after autograft reconstruction (91.58) than allograft reconstruction (78.19) in these younger patients (P = .003) (Table 3). IKDC and Tegner-Lysholm scores were not significantly different for older patients (Ps = .241 and .211, respectively).
The study also included a subset of 19 primary ACL reconstructions (13 allograft, 6 autograft) performed on Division I athletes from the University of Arizona. (Nineteen [91%] of the 21 athletes in our Division I cohort were available for follow-up.) All these patients were younger than 25 years. All autograft reconstructions were performed with quadruple-stranded gracilis and semitendinosus tendons. ACL graft failure occurred in 8 (62%) of the 13 allograft cases; there were no failures in the autograft group (Table 4). One of the 5 allograft cases that did not fail required multiple surgical débridement procedures for infection, but the graft was ultimately retained. There were no infections among the 6 autograft cases.
Discussion
The ideal graft for ACL reconstruction is still a matter of intense debate. There are many graft options for ACL reconstruction. Both BPTB and hamstring autografts are associated with various graft-specific comorbidities. Anterior knee pain, knee extensor weakness, extension loss, patella fracture, patellofemoral crepitance, and infrapatellar nerve injury have been described with BPTB autografts.13-17 In a meta-analysis of 11 studies comparing BPTB autografts with hamstring autograft, Goldblatt and colleagues17 found more extension loss, kneeling pain, and patellofemoral crepitance in the BPTP group.
Knee flexion weakness, knee flexion loss, increased knee laxity, and saphenous nerve injury have all been described with use of hamstring autografts.16-19 Goldblatt and colleagues17 demonstrated a significant flexion loss in the hamstring group in their meta-analysis as well as increased laxity with both the Lachman test and the pivot shift test. They also found that the hamstring autograft group exhibited side-to-side differences of more than 3 mm on KT-1000 testing when compared with the BPTB autograft group.
Proposed advantages of allograft reconstruction include elimination of donor-site morbidity and/or pain from a less invasive procedure, faster initial recovery, more sizing options, and shorter operative times.4-7 In a 5-year follow-up of patients who had ACL reconstruction with either Achilles allograft or BPTB autograft, Poehling and colleagues7 demonstrated overall similar long-term outcomes between the groups. However, the allograft patients reported less pain 1 and 6 weeks after surgery; better function 1 week, 3 months, and 1 year after surgery; and fewer activity limitations throughout the follow-up period. Lamblin and colleagues20 also found no difference between nonirradiated allograft and autograft tissue in ACL reconstruction in a 2013 meta-analysis of ACL studies published over a 32-year period.
Despite the proposed advantages of allograft ACL reconstruction, several recent studies have demonstrated poorer outcomes in both younger patients and more active patients after allograft reconstruction.8-11,21 In a 2007 meta-analysis, Prodromos and colleagues11 compared a series of allograft reconstructions with previously published data sets of both BPTB and hamstring autografts. They found that allograft reconstructions had significantly lower stability rates than autograft reconstructions. In a case–control study by Borchers and colleagues,10 21 patients with ACL graft failure were identified over a 2-year period, and surgical outcomes were compared with those of 42 age- and sex-matched controls. The authors found higher activity level and allograft use to be risk factors for subsequent graft failure after ACL reconstruction. More important, they showed a multiplicative interaction between higher activity level after ACL reconstruction and allograft use—an interaction that greatly increased the odds for ACL graft failure. Last, in a retrospective review, Singhal and colleagues8 evaluated the outcomes of ACL reconstruction using tibialis anterior tendon allograft and reported a 23.1% revision rate. In addition, 37.7% of patients required repeat surgery. The failure/reoperation rate was 55% for patients 25 years or younger and 24% for patients older than 25 years. The authors recommended not using tibialis anterior allografts in patients 25 years or younger and in patients who frequently engage in level I ACL-dependent sports.
The poor outcomes reported by Singhal and colleagues8 may be related to use of irradiated soft-tissue allografts. In a comparison of nonirradiated BPTB allograft and BPTB autograft in patients 25 years or younger, Barber and colleagues22 found equivalent outcomes at 2-year follow-up. They actually found a higher rate of failure for autograft reconstruction (9.4%) than allograft reconstruction (7.1%). A potential critique of their study is the significant difference between the patient groups’ mean ages: 18.6 years (autograft) versus 20.1 years (allograft). Despite this selection bias, Barber and colleagues22 argued that nonirradiated BPTB allograft is equivalent to BPTB autograft for ACL reconstruction.
Our study is one of the largest allograft studies with a comparison group. The principal findings of this study demonstrate that overall reoperation and revision rates after irradiated soft-tissue allograft ACL reconstruction are higher than those historically quoted for autograft ACL reconstruction. Specifically, allograft patients younger than 25 years had a reoperation rate of 30.8% and a revision rate of 20.5%. (Allograft patients older than 25 years had lower rates of reoperation, 8.3%, and revision, 3.3%.) After revision surgery, autograft patients’ subjective outcomes (IKDC and Tegner-Lysholm scores) were significantly improved compared with those of allograft patients (Ps = .0017 and .0031, respectively). Most compelling, however, is the unexpected and quite concerning 62% failure rate in our high-level Division I intercollegiate athletes.
There are multiple hypotheses regarding the higher failure rates of allograft tissues versus autograft tissues in ACL reconstruction. Processing methods, exposure to ionizing radiation, and the incorporation/ligamentization process have all been cited as possible reasons for allograft failure. All the allograft tendons used in the present study were obtained from MTF, which uses a proprietary “aseptic” processing system that includes washing in buffered saline impregnated with antibiotics (imipenem/cilastatin, amphotericin B, gentamicin) followed by final rinsing in phosphate-buffered saline. The majority of grafts are subjected to low-level irradiation (<2 Mrad/20 kGy) based on the outcomes of MTF’s stringent donor-selection process. Although the washing process has not been shown to alter the structural integrity of donor grafts, multiple studies have outlined the detrimental effects of higher levels of gamma radiation on allograft tissues. Although lower levels are effective against potential bacterial contaminants, a radiation level of 4 Mrad is necessary to kill the human immunodeficiency virus (HIV). Thus, a dose of 4 Mrad or higher is needed to truly “sterilize” a graft. This higher dose is an issue, as it has been known for some time that higher levels of ionizing radiation can have adverse effects on the biomechanical strength of soft-tissue allografts. In fact, ionizing radiation has dose-dependent effects.23-26 Schwartz and colleagues27 showed in a caprine model that radiation exposure at 4 Mrad significantly decreased the biomechanical strength of ACL allografts at 6 months. Balsly and colleagues28 found in a biomechanical study that radiation doses of 18 to 22 Mrad did not significantly affect the mechanical integrity of soft-tissue allografts. Conversely, in an in vivo study, Rappe and colleagues29 showed that Achilles allografts irradiated at a dose of 2.0 to 2.5 Mrad had a failure rate (33%) much higher than that of nonirradiated allografts (2.4%). The radiation dose used by MTF is less than 2 Mrad. Although more than needed to kill bacterial contaminants, this dose is considered by MTF to be below the threshold for biomechanical alterations. Only a minority of grafts is treated without irradiation.
It is possible that any level of radiation affects ligamentization of allograft tissues. Multiple studies have outlined the ligamentization process of autograft tendons in vivo. Patellar tendon autografts undergo central degeneration 2 to 6 weeks after reconstruction, but, by 6 to 12 months, these tendons have structural properties similar to those of the native ACL.30-34 Findings are similar for hamstring autografts.35,36 Goradia and colleagues36 found that, by 52 weeks, semitendinosus autografts transform into a histologic structure similar to that of the normal ACL. Remodeling of allograft tendons has been described as occurring at a much slower rate.27,37-40 Bhatia and colleagues37 demonstrated faster remodeling in autograft tissues versus allograft tissues at early time points in an in vivo rabbit model. Ultimately, differences in graft incorporation and ligamentization may be a primary factor in the higher failure rates of allograft ACL reconstruction. Current rehabilitation protocols may not take into account the longer ligamentization process for allograft tissues. These protocols are largely based on our current understanding of the ligamentization process after autograft reconstruction. It is possible that the rehabilitation program and return-to-play schedule for allograft reconstruction need to be altered to help avoid higher failure rates. The return-to-play protocol at the authors’ institution scheduled most varsity athletes to return to play 6 months after surgery. In some cases, the timetable was shortened, and some athletes were returned to play 5 months after surgery, after meeting all return-to-play criteria. Based on the findings of the present study, this return-to-play schedule may be much too aggressive for high-level athletes after allograft reconstruction. It is possible these allografts have not reached “maturity,” as their autograft counterparts have, and thus are not ready for unrestricted return to play.
Our study had multiple strengths. All reconstructions were performed by 2 senior surgeons with extensive clinical experience. The autograft and allograft reconstructions used the same techniques and rehabilitation protocols. This is one of the largest studies of outcomes of allograft ACL reconstruction and one of the largest studies that used a comparison group of autograft reconstructions. Having a comparison group effectively allowed us to contrast the differences between allograft and autograft tissues. Last, this study evaluated a subgroup of high-level NCAA Division I athletes. Follow-up in the overall study was 40.7%, but follow-up in this subgroup was 91%. The very high follow-up rate in the university population helped us validate the overall results of the study. Study results reinforced the fact that irradiated soft-tissue allograft may not be indicated for ACL reconstruction in a younger, more active patient population and led to a change in approach to ACL reconstruction for Division I intercollegiate athletes at the University of Arizona. Allograft ACL reconstruction is no longer recommended for the intercollegiate athletes at the University of Arizona.
Our study had its limitations. First, it had the inherent biases of a retrospective study. Second, many patients were lost to follow-up. We contacted and surveyed 40.7% of the patients who met the inclusion criteria. We tried reaching them in multiple ways—through US mail, all listed phone numbers, family members, and so forth. Tucson, Arizona is a college town and has a larger transient population, which may have added to the difficulty in contacting patients.
Conclusion
Given the high rates of reoperation and revision surgery with allograft reconstruction in younger patients in this study, we recommend against routine use of irradiated soft-tissue allograft tissue for ACL reconstruction in patients 25 years or younger. In our clinical practices, we prefer using autograft tissue for ACL reconstruction in younger, more active individuals. Irradiated soft-tissue allograft ACL reconstruction is a viable option in the older, less active patient population. Although the overall reoperation rate in this cohort study is acceptable, the revision rate for patients younger than 25 years is concerning and should be taken into account when considering use of irradiated soft-tissue allograft for ACL reconstruction in these younger patients.
1. Schepsis AA, Busconi BD. Sports Medicine. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
2. Campbell WC, Canale ST, Beaty JH. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, PA: Mosby/Elsevier; 2008.
3. Sherman OH, Banffy MB. Anterior cruciate ligament reconstruction: which graft is best? Arthroscopy. 2004;20(9):974-980.
4. Lee JH, Bae DK, Song SJ, Cho SM, Yoon KH. Comparison of clinical results and second-look arthroscopy findings after arthroscopic anterior cruciate ligament reconstruction using 3 different types of grafts. Arthroscopy. 2010;26(1):41-49.
5. Sun K, Tian SQ, Zhang JH, Xia CS, Zhang CL, Yu TB. Anterior cruciate ligament reconstruction with bone-patellar tendon-bone autograft versus allograft. Arthroscopy. 2009;25(7):750-759.
6. Kuhn MA, Ross G. Allografts in the treatment of anterior cruciate ligament injuries. Sports Med Arthrosc Rev. 2007;15(3):133-138.
7. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. 2005;21(7):774-785.
8. Singhal MC, Gardiner JR, Johnson DL. Failure of primary anterior cruciate ligament surgery using anterior tibialis allograft. Arthroscopy. 2007;23(5):469-475.
9. Barrett GR, Luber K, Replogle WH, Manley JL. Allograft anterior cruciate ligament reconstruction in the young, active patient: Tegner activity level and failure rate. Arthroscopy. 2010;26(12):1593-1601.
10. Borchers JR, Pedroza A, Kaeding C. Activity level and graft type as risk factors for anterior cruciate ligament graft failure: a case–control study. Am J Sports Med. 2009;37(12):2362-2367.
11. Prodromos C, Joyce B, Shi K. A meta-analysis of stability of autografts compared to allografts after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):851-856.
12. Shelbourne KD, Gray T. Anterior cruciate ligament reconstruction with autogenous patellar tendon graft followed by accelerated rehabilitation. A two- to nine-year followup. Am J Sports Med. 1997;25(6):786-795.
13. Rosenberg TD, Franklin JL, Baldwin GN, Nelson KA. Extensor mechanism function after patellar tendon graft harvest for anterior cruciate ligament reconstruction. Am J Sports Med. 1992;20(5):519-525.
14. Piva SR, Childs JD, Klucinec BM, Irrgang JJ, Almeida GJ, Fitzgerald GK. Patella fracture during rehabilitation after bone–patellar tendon–bone anterior cruciate ligament reconstruction: 2 case reports. J Orthop Sports Phys Ther. 2009;39(4):278-286.
15. Lee GH, McCulloch P, Cole BJ, Bush-Joseph CA, Bach BR Jr. The incidence of acute patellar tendon harvest complications for anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(2):162-166.
16. Kartus J, Movin T, Karlsson J. Donor-site morbidity and anterior knee problems after anterior cruciate ligament reconstruction using autografts. Arthroscopy. 2001;17(9):971-980.
17. Goldblatt JP, Fitzsimmons SE, Balk E, Richmond JC. Reconstruction of the anterior cruciate ligament: meta-analysis of patellar tendon versus hamstring tendon autograft. Arthroscopy. 2005;21(7):791-803.
18. Freedman KB, D’Amato MJ, Nedeff DD, Kaz A, Bach BR Jr. Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med. 2003;31(1):2-11.
19. Yunes M, Richmond JC, Engels EA, Pinczewski LA. Patellar versus hamstring tendons in anterior cruciate ligament reconstruction: a meta-analysis. Arthroscopy. 2001;17(3):248-257.
20. Lamblin CJ, Waterman BR, Lubowitz JH. Anterior cruciate ligament reconstruction with autografts compared with non-irradiated, non-chemically treated allografts. Arthroscopy. 2013;29(6):1113-1122.
21. Pallis M, Svoboda SJ, Cameron KL, Owens BD. Survival comparison of allograft and autograft anterior cruciate ligament reconstruction at the United States Military Academy. Am J Sports Med. 2012;40(6):1242-1246.
22. Barber FA, Cowden CH 3rd, Sanders EJ. Revision rates after anterior cruciate ligament reconstruction using bone–patellar tendon–bone allograft or autograft in a population 25 years old and younger. Arthroscopy. 2014;30(4):483-491.
23. Salehpour A, Butler DL, Proch FS, et al. Dose-dependent response of gamma irradiation on mechanical properties and related biochemical composition of goat bone–patellar tendon–bone allografts. J Orthop Res. 1995;13(6):898-906.
24. Gibbons MJ, Butler DL, Grood ES, Bylski-Austrow DI, Levy MS, Noyes FR. Effects of gamma irradiation on the initial mechanical and material properties of goat bone–patellar tendon–bone allografts. J Orthop Res. 1991;9(2):209-218.
25. Fideler BM, Vangsness CT Jr, Lu B, Orlando C, Moore T. Gamma irradiation: effects on biomechanical properties of human bone–patellar tendon–bone allografts. Am J Sports Med. 1995;23(5):643-646.
26. De Deyne P, Haut RC. Some effects of gamma irradiation on patellar tendon allografts. Connect Tissue Res. 1991;27(1):51-62.
27. Schwartz HE, Matava MJ, Proch FS, et al. The effect of gamma irradiation on anterior cruciate ligament allograft biomechanical and biochemical properties in the caprine model at time zero and at 6 months after surgery. Am J Sports Med. 2006;34(11):1747-1755.
28. Balsly CR, Cotter AT, Williams LA, Gaskins BD, Moore MA, Wolfinbarger L Jr. Effect of low dose and moderate dose gamma irradiation on the mechanical properties of bone and soft tissue allografts. Cell Tissue Bank. 2008;9(4):289-298.
29. Rappe M, Horodyski M, Meister K, Indelicato PA. Nonirradiated versus irradiated Achilles allograft: in vivo failure comparison. Am J Sports Med. 2007;35(10):1653-1658.
30. Amiel D, Kleiner JB, Akeson WH. The natural history of the anterior cruciate ligament autograft of patellar tendon origin. Am J Sports Med. 1986;14(6):449-462.
31. Amiel D, Kleiner JB, Roux RD, Harwood FL, Akeson WH. The phenomenon of “ligamentization”: anterior cruciate ligament reconstruction with autogenous patellar tendon. J Orthop Res. 1986;4(2):162-172.
32. Arnoczky SP, Tarvin GB, Marshall JL. Anterior cruciate ligament replacement using patellar tendon. An evaluation of graft revascularization in the dog. J Bone Joint Surg Am. 1982;64(2):217-224.
33. Ballock RT, Woo SL, Lyon RM, Hollis JM, Akeson WH. Use of patellar tendon autograft for anterior cruciate ligament reconstruction in the rabbit: a long-term histologic and biomechanical study. J Orthop Res. 1989;7(4):474-485.
34. Clancy WG Jr, Narechania RG, Rosenberg TD, Gmeiner JG, Wisnefske DD, Lange TA. Anterior and posterior cruciate ligament reconstruction in rhesus monkeys. J Bone Joint Surg Am. 1981;63(8):1270-1284.
35. Blickenstaff KR, Grana WA, Egle D. Analysis of a semitendinosus autograft in a rabbit model. Am J Sports Med. 1997;25(4):554-559.
36. Goradia VK, Rochat MC, Kida M, Grana WA. Natural history of a hamstring tendon autograft used for anterior cruciate ligament reconstruction in a sheep model. Am J Sports Med. 2000;28(1):40-46.
37. Bhatia S, Bell R, Frank RM, et al. Bony incorporation of soft tissue anterior cruciate ligament grafts in an animal model: autograft versus allograft with low-dose gamma irradiation. Am J Sports Med. 2012;40(8):1789-1798.
38. Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21(2):176-185.
39. Goertzen MJ, Clahsen H, Schulitz KP. Anterior cruciate ligament reconstruction using cryopreserved irradiated bone-ACL-bone-allograft transplants. Knee Surg Sports Traumatol Arthrosc. 1994;2(3):150-157.
40. Mae T, Shino K, Maeda A, Toritsuka Y, Horibe S, Ochi T. Effect of gamma irradiation on remodeling process of tendon allograft. Clin Orthop. 2003;(414):305-314.
1. Schepsis AA, Busconi BD. Sports Medicine. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
2. Campbell WC, Canale ST, Beaty JH. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, PA: Mosby/Elsevier; 2008.
3. Sherman OH, Banffy MB. Anterior cruciate ligament reconstruction: which graft is best? Arthroscopy. 2004;20(9):974-980.
4. Lee JH, Bae DK, Song SJ, Cho SM, Yoon KH. Comparison of clinical results and second-look arthroscopy findings after arthroscopic anterior cruciate ligament reconstruction using 3 different types of grafts. Arthroscopy. 2010;26(1):41-49.
5. Sun K, Tian SQ, Zhang JH, Xia CS, Zhang CL, Yu TB. Anterior cruciate ligament reconstruction with bone-patellar tendon-bone autograft versus allograft. Arthroscopy. 2009;25(7):750-759.
6. Kuhn MA, Ross G. Allografts in the treatment of anterior cruciate ligament injuries. Sports Med Arthrosc Rev. 2007;15(3):133-138.
7. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. 2005;21(7):774-785.
8. Singhal MC, Gardiner JR, Johnson DL. Failure of primary anterior cruciate ligament surgery using anterior tibialis allograft. Arthroscopy. 2007;23(5):469-475.
9. Barrett GR, Luber K, Replogle WH, Manley JL. Allograft anterior cruciate ligament reconstruction in the young, active patient: Tegner activity level and failure rate. Arthroscopy. 2010;26(12):1593-1601.
10. Borchers JR, Pedroza A, Kaeding C. Activity level and graft type as risk factors for anterior cruciate ligament graft failure: a case–control study. Am J Sports Med. 2009;37(12):2362-2367.
11. Prodromos C, Joyce B, Shi K. A meta-analysis of stability of autografts compared to allografts after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):851-856.
12. Shelbourne KD, Gray T. Anterior cruciate ligament reconstruction with autogenous patellar tendon graft followed by accelerated rehabilitation. A two- to nine-year followup. Am J Sports Med. 1997;25(6):786-795.
13. Rosenberg TD, Franklin JL, Baldwin GN, Nelson KA. Extensor mechanism function after patellar tendon graft harvest for anterior cruciate ligament reconstruction. Am J Sports Med. 1992;20(5):519-525.
14. Piva SR, Childs JD, Klucinec BM, Irrgang JJ, Almeida GJ, Fitzgerald GK. Patella fracture during rehabilitation after bone–patellar tendon–bone anterior cruciate ligament reconstruction: 2 case reports. J Orthop Sports Phys Ther. 2009;39(4):278-286.
15. Lee GH, McCulloch P, Cole BJ, Bush-Joseph CA, Bach BR Jr. The incidence of acute patellar tendon harvest complications for anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(2):162-166.
16. Kartus J, Movin T, Karlsson J. Donor-site morbidity and anterior knee problems after anterior cruciate ligament reconstruction using autografts. Arthroscopy. 2001;17(9):971-980.
17. Goldblatt JP, Fitzsimmons SE, Balk E, Richmond JC. Reconstruction of the anterior cruciate ligament: meta-analysis of patellar tendon versus hamstring tendon autograft. Arthroscopy. 2005;21(7):791-803.
18. Freedman KB, D’Amato MJ, Nedeff DD, Kaz A, Bach BR Jr. Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med. 2003;31(1):2-11.
19. Yunes M, Richmond JC, Engels EA, Pinczewski LA. Patellar versus hamstring tendons in anterior cruciate ligament reconstruction: a meta-analysis. Arthroscopy. 2001;17(3):248-257.
20. Lamblin CJ, Waterman BR, Lubowitz JH. Anterior cruciate ligament reconstruction with autografts compared with non-irradiated, non-chemically treated allografts. Arthroscopy. 2013;29(6):1113-1122.
21. Pallis M, Svoboda SJ, Cameron KL, Owens BD. Survival comparison of allograft and autograft anterior cruciate ligament reconstruction at the United States Military Academy. Am J Sports Med. 2012;40(6):1242-1246.
22. Barber FA, Cowden CH 3rd, Sanders EJ. Revision rates after anterior cruciate ligament reconstruction using bone–patellar tendon–bone allograft or autograft in a population 25 years old and younger. Arthroscopy. 2014;30(4):483-491.
23. Salehpour A, Butler DL, Proch FS, et al. Dose-dependent response of gamma irradiation on mechanical properties and related biochemical composition of goat bone–patellar tendon–bone allografts. J Orthop Res. 1995;13(6):898-906.
24. Gibbons MJ, Butler DL, Grood ES, Bylski-Austrow DI, Levy MS, Noyes FR. Effects of gamma irradiation on the initial mechanical and material properties of goat bone–patellar tendon–bone allografts. J Orthop Res. 1991;9(2):209-218.
25. Fideler BM, Vangsness CT Jr, Lu B, Orlando C, Moore T. Gamma irradiation: effects on biomechanical properties of human bone–patellar tendon–bone allografts. Am J Sports Med. 1995;23(5):643-646.
26. De Deyne P, Haut RC. Some effects of gamma irradiation on patellar tendon allografts. Connect Tissue Res. 1991;27(1):51-62.
27. Schwartz HE, Matava MJ, Proch FS, et al. The effect of gamma irradiation on anterior cruciate ligament allograft biomechanical and biochemical properties in the caprine model at time zero and at 6 months after surgery. Am J Sports Med. 2006;34(11):1747-1755.
28. Balsly CR, Cotter AT, Williams LA, Gaskins BD, Moore MA, Wolfinbarger L Jr. Effect of low dose and moderate dose gamma irradiation on the mechanical properties of bone and soft tissue allografts. Cell Tissue Bank. 2008;9(4):289-298.
29. Rappe M, Horodyski M, Meister K, Indelicato PA. Nonirradiated versus irradiated Achilles allograft: in vivo failure comparison. Am J Sports Med. 2007;35(10):1653-1658.
30. Amiel D, Kleiner JB, Akeson WH. The natural history of the anterior cruciate ligament autograft of patellar tendon origin. Am J Sports Med. 1986;14(6):449-462.
31. Amiel D, Kleiner JB, Roux RD, Harwood FL, Akeson WH. The phenomenon of “ligamentization”: anterior cruciate ligament reconstruction with autogenous patellar tendon. J Orthop Res. 1986;4(2):162-172.
32. Arnoczky SP, Tarvin GB, Marshall JL. Anterior cruciate ligament replacement using patellar tendon. An evaluation of graft revascularization in the dog. J Bone Joint Surg Am. 1982;64(2):217-224.
33. Ballock RT, Woo SL, Lyon RM, Hollis JM, Akeson WH. Use of patellar tendon autograft for anterior cruciate ligament reconstruction in the rabbit: a long-term histologic and biomechanical study. J Orthop Res. 1989;7(4):474-485.
34. Clancy WG Jr, Narechania RG, Rosenberg TD, Gmeiner JG, Wisnefske DD, Lange TA. Anterior and posterior cruciate ligament reconstruction in rhesus monkeys. J Bone Joint Surg Am. 1981;63(8):1270-1284.
35. Blickenstaff KR, Grana WA, Egle D. Analysis of a semitendinosus autograft in a rabbit model. Am J Sports Med. 1997;25(4):554-559.
36. Goradia VK, Rochat MC, Kida M, Grana WA. Natural history of a hamstring tendon autograft used for anterior cruciate ligament reconstruction in a sheep model. Am J Sports Med. 2000;28(1):40-46.
37. Bhatia S, Bell R, Frank RM, et al. Bony incorporation of soft tissue anterior cruciate ligament grafts in an animal model: autograft versus allograft with low-dose gamma irradiation. Am J Sports Med. 2012;40(8):1789-1798.
38. Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21(2):176-185.
39. Goertzen MJ, Clahsen H, Schulitz KP. Anterior cruciate ligament reconstruction using cryopreserved irradiated bone-ACL-bone-allograft transplants. Knee Surg Sports Traumatol Arthrosc. 1994;2(3):150-157.
40. Mae T, Shino K, Maeda A, Toritsuka Y, Horibe S, Ochi T. Effect of gamma irradiation on remodeling process of tendon allograft. Clin Orthop. 2003;(414):305-314.
Enhancement of Acute Tendon Repair Using Chitosan Matrix
Rotator cuff tears (RCTs) are common tendon injuries that can cause chronic pain and severe functional disability. Massive RCTs do not heal spontaneously and, in many cases, result in poor clinical outcomes. Specifically, muscle atrophy and fatty infiltration correlate with poor outcomes after surgical repair.1 Fatty infiltration of the rotator cuff is a common phenomenon that can lead to permanent structural alterations within the tendon. It has been suggested that changes in muscle fiber orientation (the pennation angle) can cause mesenchymal stem cells to migrate to the interface between muscle fibers and the region of fatty infiltration of the muscle.2 Understanding the factors involved in muscle degeneration and atrophy, and in fatty infiltration, may lead to treatments that improve outcomes for patients with massive RCTs. One proposed treatment involves placing continuous mechanical traction on the ends of the torn tendon.2 Findings from this research have indicated that acute tears that become chronic tears are typified by inelasticity and poor function of the muscle–tendon unit. It is therefore important to develop a method that speeds tendon healing without causing the muscle fiber atrophy and pennation angle changes that lead to fatty atrophy, which appears to be an irreversible structural change.
On the basis of the theory that adding mesenchymal cells may improve tendon healing, investigators have studied use of transcription factors (eg, scleraxis) specific to tendogenesis in the embryonal stage.3,4 Nevertheless, certain transcription factors are associated with formation of fibrocartilage in higher concentrations.4 Moreover, decalcified bone matrix increases cartilage formation when added to the tendon repair site.5 Cartilage formation, however, is associated with poorer functional results.6 Thus, there is a need for a method that facilitates faster tendon healing with higher quality tissue formation and less muscle atrophy.
Chitosan, a linear polysaccharide, is associated with scarless healing of soft tissues and prevention of adhesion formation both intraperitoneally and during tendon healing after surgery.7,8 Chitosan tends to precipitate in physiologic pH, thereby mitigating its potency. Fortunately, a chitosan solution that does not precipitate in physiologic conditions was recently developed.9 The solution’s lack of precipitation, coupled with its in situ gelling, allows it to adhere to the repair site long enough to take effect. These characteristics could allow for intimate contact between gel and tendon, facilitating guided-tissue regeneration and preventing adhesion of the rotator cuff to surrounding tissue. By contrast, other biological agents (eg, platelet-rich plasma) are administered as fluid rather than gel and are therefore more susceptible to diffusing from the repair site, mitigating their effects. Thus, chitosan gel is fairly unique among agents.
In the study reported here, we histologically investigated whether a chitosan gel would help improve healing of rotator cuff tendon (acute supraspinatus) tears in a rat model.
Materials and Methods
Supraspinatus Surgical Model
Forty Wistar rats, each weighing between 300 and 400 g, were used in this study. All procedures were approved by the Institutional Animal Care and Use Committee at Rabin Medical Center in Petah Tikva, Israel. The rats were anesthetized with ketamine 90 mg/kg and xylazine 10 mg/kg, both administered intramuscularly, and anesthesia was prolonged as needed with 2% isoflurane, administered by nose cone. The skin was incised 5 cm along the upper back following the midline of the spine. The resulting skin flaps were retracted and the scapula exposed. Careful blunt dissection allowed visualization of the rotator cuff and the trans-scapular arch. A full-thickness incision of the supraspinatus tendon was then made 2 mm distal to the arch. This procedure was performed on both shoulders. For the right supraspinatus tendon, a bioabsorbable chitosan–hydrochloric acid solution (>70% de-acetylated chitosan, molecular weight of 600 kDa; Heppe Medical Chitosan GmbH, Halle, Germany) was sterilely applied to the ends of the tendon (total volume, 0.5 mL) and automatically gelled in situ by heating to about 37°C (rat’s internal body temperature). The tendon ends were subsequently approximated with a single 4-0 Prolene suture (Ethicon, Somerville, New Jersey). The left shoulder (tendon repaired with suture only) served as a control.
The rats were housed for a maximum of 12 weeks after surgery. They were sacrificed (in groups of 5 each) 2 hours, 3 days, 1 week, 2 weeks, 4 weeks, 6 weeks, 8 weeks, and 12 weeks after surgery. After each rat was sacrificed, both shoulder girdles were harvested, and the sutures were removed from the supraspinatus tendons.
Histologic Analysis
After routine fixation with 4% formalin for 48 hours and decalcification with 10% ethylenediaminetetraacetic acid (EDTA) for 3 weeks, the specimens were sectioned with a microtome blade. Care was taken to ensure the plane of the microtome blade was parallel with the longitudinal plane of the supraspinatus muscle and tendon to allow for evaluation of pennation angle. Hematoxylin-eosin staining and Masson trichrome staining were subsequently performed.
A variety of histologic measurements were obtained with use of ImageJ software (US National Institutes of Health). Percentage of fibrous tissue was determined by examining the slides at low magnification fields (×25) at the tendon healing site. Three such fields were evaluated per specimen. The fibrous tissue was circled manually, and percentage of tissue area was assessed and compared with total region of interest. Cellularity was carefully outlined and measured as percentage of total tendon area occupied by cells. Fatty atrophy was defined as either present or absent. Muscle fiber diameter was defined as average diameter of 10 muscle fibers measured within 2 mm of the tendon laceration site. Inflammatory cell collections were defined as either large (>100 µm in diameter) or small (<100 µm in diameter) and were dichotomized to either present or absent. Pennation angle was defined as average angle between muscle fibers and longitudinal axis of supraspinatus muscle and tendon unit. Ten fibers proximal to and within 2 mm of the laceration site were randomly selected, measured, and averaged.
Statistical Analysis
Statistical analysis was performed with Analyse-it 2.20 for Microsoft Excel 2010 (Analyse-it Software, Leeds, United Kingdom). Data were initially analyzed with the Kolmogorov-Smirnov test to assess for normality of distribution. The t test was used to compare continuous variables when the data were normally distributed and the Mann-Whitney test when the data were not normally distributed.
Results
All tendons (both groups) healed within 12 weeks. Generally, the tissue formed at the repair site exhibited a mixture of tenocyte-like cells (fibrotic tissue) and granulation tissue without clear orientation. As noted in Figure 1, the tendons treated with chitosan had more fibrotic tissue (overall mean, 21.5%) relative to the control group (mean, 12.3%), and the difference was significant (P = .003). The most notable differences were found at time points later than 1 week after surgery. In addition, amount of cellularity (Figure 2) was higher in chitosan-treated tendon and control tendon than in the normal, uninjured adjacent tendon at all time points (P < .001). Chitosan-treated tendons had significantly higher cellularity than untreated control tendons from 1 to 2 weeks (P < .001), and control tendons were significantly hypercellular compared with chitosan-treated tendons from 4 to 8 weeks (P < .001), but both groups exhibited similar cellularity by 12 weeks (P > .05). Fatty atrophy was found at significantly higher rates in control rats than in chitosan-treated rats (P = .001; Table). Furthermore, as noted in Figure 3, muscle fiber diameter decreased in both groups after injury (P < .001).
Figure 4 shows that the amount of inflammatory collections was significantly smaller in the chitosan-treated group than in the control group over the course of the study (P = .01). In addition, pennation angle steadily decreased in the control group throughout the study period, whereas it transiently decreased in the chitosan-treated group (until 2 weeks) before returning to its immediate postoperative level by 12 weeks (Figure 5). Overall, the chitosan-treated group maintained a higher pennation angle than the control group did (P < .001).
Discussion
RCTs affect more than 40% of patients over age 60 years and are a common cause of debilitating pain, reduced shoulder function, and weakness.10 Thirty thousand to 75,000 rotator cuff repairs are performed annually in the United States.11 Although the best treatment for this disorder remains a topic of debate, arthroscopic and (when necessary) open surgical repair is the accepted gold standard for the treatment of tears that do not improve with conservative management. Despite advances in the surgical treatment of these tears, the surgical failure rates are high (range, 20%-90%), with failures attributed to factors beyond patient age, tear size and chronicity, muscle atrophy and degeneration, tendon quality, repair technique, and postoperative rehabilitation.12,13 Repair strategies that biologically enhance the patient’s intrinsic healing potential are needed.
In tendon repair, choice of repair material (eg, graft) is crucial in determining the success of tissue engineering approaches. The ideal scaffold is biocompatible and does not elicit a host inflammatory response. The selected scaffold in its composition and fabricated form must be capable of holding and supporting cells. In addition, the scaffold should be biodegradable, serving as a temporary support for such cells and mechanically augmenting the repaired tendon while allowing for eventual replacement by matrix components. Moreover, the scaffold should have high porosity and a large surface area. Furthermore, the material should mimic the native tendon extracellular matrix (ECM) architecture to allow cells to be distributed throughout the scaffold and to facilitate diffusion of nutrients and factors that promote cellular proliferation and ECM production.
Given the importance of glycosaminoglycans (GAGs) in supporting the reticular structure of the matrix, use of GAGs or GAG-analogues as components of a tendon tissue scaffold for enhancing repair is well documented.14 One such candidate is chitosan, a partially de-acetylated derivative of chitin found in arthropod exoskeletons. Structurally, chitosan shares some characteristics with various GAGs and hyaluronic acid.15 More specifically, chitosan is a linear polysaccharide composed of glucosamine and N-acetyl glucosamine units linked by β-glycosidic bonds. Investigators have studied the properties of chitosan, including its biocompatibility, biodegradability, antibacterial activity, mucoadhesivity, and wound healing.16,17
One of the most promising features of chitosan is that it can be processed into porous structures for use in cell transplantation and tissue regeneration.18,19 Porous chitosan structures can be formed by freezing and lyophilizing chitosan-acetic acid solutions; chondrogenic cell adhesion and proliferation onto these structures have been reported.20,21 This chitosan scaffolding method has also been used to test different composites with collagens, gelatins, GAGs, and hyaluronic acid, all of which have also been proposed as useful 3-dimensional materials for tissue repair.22
In the present study, we used chitosan matrix in RCT repair. We hypothesized that chitosan matrix could enhance rotator cuff repair the same way it enhances repair in epidermal tissues.16 Histologic findings demonstrated that the percentage of fibrous tissue was significantly higher in the chitosan-treated group than in the control group. This improved fibroblastic response may be attributed to the ability of chitosan to enhance cell migration and serve as a scaffold for repair. Other studies have indicated that chitin, of which chitosan is the primary derivative, accelerated the healing of skin and subcutaneous tissues by increased cell migration.23 Moreover, Okamoto and colleagues24 reported that chitin implants stimulated abundant angiogenesis through the same mechanism.
Inadequate initial strength of a repair may lead to a recurrent cuff tear or a disability of rotator cuff function in the early healing stages. In our study, the chitosan matrix tended to be absorbed by 6 weeks after surgery. Its adherence to and ultimate absorption at the repair site may be challenged by the flow of irrigation fluid through the subacromial space in the setting of arthroscopic surgery. However, because the chitosan remains in a more robust gel form, it is better able to resist being washed from the repair site. For augmentation, it may be possible to apply a biocompatible patch over the gel to further protect it from being dislodged. In addition, histologic findings showed that the fibrous repair tissue gradually increased until reaching a peak 8 weeks after surgery—an indication that the absorption rate of the chitosan scaffold lags behind full recovery of the repair tissue. Given this relationship, further studies are needed to determine the mechanical strength of the repair between 6 and 8 weeks, which is important for avoiding recurrent tears.
This study had a few limitations. First, as with any animal model, the anatomy and function of the rat shoulder differ from those of the human shoulder. The acromial arch differs in quadruped animals, with less coverage of the supraspinatus and more of the subscapularis.25 These anatomical differences could yield altered stress mechanics that could affect tendon repair. Furthermore, rats and humans differ in their RCT healing rates. Thus, the pathophysiology of muscle atrophy and fat infiltration in rats may slightly differ from that in humans. In addition, no mechanical testing was performed to compare chitosan-treated and untreated rotator cuff repairs, and such testing is needed to clarify the biomechanical importance of augmentation. Furthermore, no immunohistochemical analysis was performed for collagen. In the repair of rotator cuff tendons, surgeons must consider not only the number of cells but also the production of ECM. Although not directly confirmed in this study, chitosan induced fibrous tissue proliferation that mirrored production of a large amount of collagen fibers. Last, we used an open RTC model. As an arthroscopic model was not used, no definitive conclusions can be drawn regarding use of chitosan in arthroscopy.
Conclusion
Use of chitosan as an acellular matrix improved formation of healing fibrous tissue, increased the number of cells, and prevented fatty atrophy and inflammatory aggregates inside repair sites while facilitating recovery of the natural pennation angle of the tissue. These results demonstrate that chitosan can enhance tendon healing in the setting of acute RCT. Further research, including biomechanical testing of repaired tendons, is needed to further delineate the utility of chitosan in regenerating irreparable RCTs.
1. Shen PH, Lien SB, Shen HC, Lee CH, Wu SS, Lin LC. Long-term functional outcomes after repair of rotator cuff tears correlated with atrophy of the supraspinatus muscles on magnetic resonance images. J Shoulder Elbow Surg. 2008;17(1 suppl):1S-7S.
2. Meyer DC, Hoppeler H, von Rechenberg B, Gerber C. A pathomechanical concept explains muscle loss and fatty muscular changes following surgical tendon release. J Orthop Res. 2004;22(5):1004-1007.
3. Gulotta LV, Kovacevic D, Packer JD, Deng XH, Rodeo SA. Bone marrow–derived mesenchymal stem cells transduced with scleraxis improve rotator cuff healing in a rat model. Am J Sports Med. 2011;39(6):1282-1289.
4. Gulotta LV, Rodeo SA. Emerging ideas: evaluation of stem cells genetically modified with scleraxis to improve rotator cuff healing. Clin Orthop. 2011;469(10):2977-2980.
5. Sundar S, Pendegrass CJ, Blunn GW. Tendon bone healing can be enhanced by demineralized bone matrix: a functional and histological study. J Biomed Mater Res B Appl Biomater. 2009;88(1):115-122.
6. Kumagai J, Sarkar K, Uhthoff HK. The collagen types in the attachment zone of rotator cuff tendons in the elderly: an immunohistochemical study. J Rheumatol. 1994;21(11):2096-2100.
7. Wang D, Mo J, Pan S, Chen H, Zhen H. Prevention of postoperative peritoneal adhesions by O-carboxymethyl chitosan in a rat cecal abrasion model. Clin Invest Med. 2010;33(4):E254-E260.
8. Zhang H, Sheng ZJ, Hou CL. Effect of chitosan membrane on tendon adhesion and healing [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 1999;13(6):382-385.
9. Cho MH, Kim KS, Ahn HH, et al. Chitosan gel as an in situ–forming scaffold for rat bone marrow mesenchymal stem cells in vivo. Tissue Eng Part A. 2008;14(6):1099-1108.
10. Yamaguchi K, Tetro AM, Blam O, Evanoff BA, Teefey SA, Middleton WD. Natural history of asymptomatic rotator cuff tears: a longitudinal analysis of asymptomatic tears detected sonographically. J Shoulder Elbow Surg. 2001;10(3):199-203.
11. Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16(2):181-187.
12. Accousti KJ, Flatow EL. Technical pearls on how to maximize healing of the rotator cuff. Instr Course Lect. 2007;56:3-12.
13. Bishop J, Klepps S, Lo IK, Bird J, Gladstone JN, Flatow EL. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. J Shoulder Elbow Surg. 2006;15(3):290-299.
14. Hunziker E, Spector M, Libera J, et al. Translation from research to applications. Tissue Eng. 2006;12(12):3341-3364.
15. Suh JK, Matthew HW. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 2000;21(24):2589-2598.
16. Kumar MN, Muzzarelli RA, Muzzarelli C, Sashiwa H, Domb AJ. Chitosan chemistry and pharmaceutical perspectives. Chem Rev. 2004;104(12):6017-6084.
17. Shi C, Zhu Y, Ran X, Wang M, Su Y, Cheng T. Therapeutic potential of chitosan and its derivatives in regenerative medicine. J Surg Res. 2006;133(2):185-192.
18. Hsieh WC, Chang CP, Lin SM. Morphology and characterization of 3D micro-porous structured chitosan scaffolds for tissue engineering. Colloids Surf B Biointerfaces. 2007;57(2):250-255.
19. Madihally SV, Matthew HW. Porous chitosan scaffolds for tissue engineering. Biomaterials. 1999;20(12):1133-1142.
20. Nettles DL, Elder SH, Gilbert JA. Potential use of chitosan as a cell scaffold material for cartilage tissue engineering. Tissue Eng. 2002;8(6):1009-1016.
21. Griffon DJ, Sedighi MR, Schaeffer DV, Eurell JA, Johnson AL. Chitosan scaffolds: interconnective pore size and cartilage engineering. Acta Biomater. 2006;2(3):313-320.
22. Manjubala I, Scheler S, Bossert J, Jandt KD. Mineralisation of chitosan scaffolds with nano-apatite formation by double diffusion technique. Acta Biomater. 2006;2(1):75-84.
23. Su CH, Sun CS, Juan SW, Ho HO, Hu CH, Sheu MT. Development of fungal mycelia as skin substitutes: effects on wound healing and fibroblast. Biomaterials. 1999;20(1):61-68.
24. Okamoto Y, Southwood L, Stashak TS. Effect of chitin on nonwoven fabric implant in tendon healing. Carbohydr Polym. 1997;33:33-38.
25. Gupta R, Lee TQ. Contributions of the different rabbit models to our understanding of rotator cuff pathology. J Shoulder Elbow Surg. 2007;16(5 suppl):S149-S157.
Rotator cuff tears (RCTs) are common tendon injuries that can cause chronic pain and severe functional disability. Massive RCTs do not heal spontaneously and, in many cases, result in poor clinical outcomes. Specifically, muscle atrophy and fatty infiltration correlate with poor outcomes after surgical repair.1 Fatty infiltration of the rotator cuff is a common phenomenon that can lead to permanent structural alterations within the tendon. It has been suggested that changes in muscle fiber orientation (the pennation angle) can cause mesenchymal stem cells to migrate to the interface between muscle fibers and the region of fatty infiltration of the muscle.2 Understanding the factors involved in muscle degeneration and atrophy, and in fatty infiltration, may lead to treatments that improve outcomes for patients with massive RCTs. One proposed treatment involves placing continuous mechanical traction on the ends of the torn tendon.2 Findings from this research have indicated that acute tears that become chronic tears are typified by inelasticity and poor function of the muscle–tendon unit. It is therefore important to develop a method that speeds tendon healing without causing the muscle fiber atrophy and pennation angle changes that lead to fatty atrophy, which appears to be an irreversible structural change.
On the basis of the theory that adding mesenchymal cells may improve tendon healing, investigators have studied use of transcription factors (eg, scleraxis) specific to tendogenesis in the embryonal stage.3,4 Nevertheless, certain transcription factors are associated with formation of fibrocartilage in higher concentrations.4 Moreover, decalcified bone matrix increases cartilage formation when added to the tendon repair site.5 Cartilage formation, however, is associated with poorer functional results.6 Thus, there is a need for a method that facilitates faster tendon healing with higher quality tissue formation and less muscle atrophy.
Chitosan, a linear polysaccharide, is associated with scarless healing of soft tissues and prevention of adhesion formation both intraperitoneally and during tendon healing after surgery.7,8 Chitosan tends to precipitate in physiologic pH, thereby mitigating its potency. Fortunately, a chitosan solution that does not precipitate in physiologic conditions was recently developed.9 The solution’s lack of precipitation, coupled with its in situ gelling, allows it to adhere to the repair site long enough to take effect. These characteristics could allow for intimate contact between gel and tendon, facilitating guided-tissue regeneration and preventing adhesion of the rotator cuff to surrounding tissue. By contrast, other biological agents (eg, platelet-rich plasma) are administered as fluid rather than gel and are therefore more susceptible to diffusing from the repair site, mitigating their effects. Thus, chitosan gel is fairly unique among agents.
In the study reported here, we histologically investigated whether a chitosan gel would help improve healing of rotator cuff tendon (acute supraspinatus) tears in a rat model.
Materials and Methods
Supraspinatus Surgical Model
Forty Wistar rats, each weighing between 300 and 400 g, were used in this study. All procedures were approved by the Institutional Animal Care and Use Committee at Rabin Medical Center in Petah Tikva, Israel. The rats were anesthetized with ketamine 90 mg/kg and xylazine 10 mg/kg, both administered intramuscularly, and anesthesia was prolonged as needed with 2% isoflurane, administered by nose cone. The skin was incised 5 cm along the upper back following the midline of the spine. The resulting skin flaps were retracted and the scapula exposed. Careful blunt dissection allowed visualization of the rotator cuff and the trans-scapular arch. A full-thickness incision of the supraspinatus tendon was then made 2 mm distal to the arch. This procedure was performed on both shoulders. For the right supraspinatus tendon, a bioabsorbable chitosan–hydrochloric acid solution (>70% de-acetylated chitosan, molecular weight of 600 kDa; Heppe Medical Chitosan GmbH, Halle, Germany) was sterilely applied to the ends of the tendon (total volume, 0.5 mL) and automatically gelled in situ by heating to about 37°C (rat’s internal body temperature). The tendon ends were subsequently approximated with a single 4-0 Prolene suture (Ethicon, Somerville, New Jersey). The left shoulder (tendon repaired with suture only) served as a control.
The rats were housed for a maximum of 12 weeks after surgery. They were sacrificed (in groups of 5 each) 2 hours, 3 days, 1 week, 2 weeks, 4 weeks, 6 weeks, 8 weeks, and 12 weeks after surgery. After each rat was sacrificed, both shoulder girdles were harvested, and the sutures were removed from the supraspinatus tendons.
Histologic Analysis
After routine fixation with 4% formalin for 48 hours and decalcification with 10% ethylenediaminetetraacetic acid (EDTA) for 3 weeks, the specimens were sectioned with a microtome blade. Care was taken to ensure the plane of the microtome blade was parallel with the longitudinal plane of the supraspinatus muscle and tendon to allow for evaluation of pennation angle. Hematoxylin-eosin staining and Masson trichrome staining were subsequently performed.
A variety of histologic measurements were obtained with use of ImageJ software (US National Institutes of Health). Percentage of fibrous tissue was determined by examining the slides at low magnification fields (×25) at the tendon healing site. Three such fields were evaluated per specimen. The fibrous tissue was circled manually, and percentage of tissue area was assessed and compared with total region of interest. Cellularity was carefully outlined and measured as percentage of total tendon area occupied by cells. Fatty atrophy was defined as either present or absent. Muscle fiber diameter was defined as average diameter of 10 muscle fibers measured within 2 mm of the tendon laceration site. Inflammatory cell collections were defined as either large (>100 µm in diameter) or small (<100 µm in diameter) and were dichotomized to either present or absent. Pennation angle was defined as average angle between muscle fibers and longitudinal axis of supraspinatus muscle and tendon unit. Ten fibers proximal to and within 2 mm of the laceration site were randomly selected, measured, and averaged.
Statistical Analysis
Statistical analysis was performed with Analyse-it 2.20 for Microsoft Excel 2010 (Analyse-it Software, Leeds, United Kingdom). Data were initially analyzed with the Kolmogorov-Smirnov test to assess for normality of distribution. The t test was used to compare continuous variables when the data were normally distributed and the Mann-Whitney test when the data were not normally distributed.
Results
All tendons (both groups) healed within 12 weeks. Generally, the tissue formed at the repair site exhibited a mixture of tenocyte-like cells (fibrotic tissue) and granulation tissue without clear orientation. As noted in Figure 1, the tendons treated with chitosan had more fibrotic tissue (overall mean, 21.5%) relative to the control group (mean, 12.3%), and the difference was significant (P = .003). The most notable differences were found at time points later than 1 week after surgery. In addition, amount of cellularity (Figure 2) was higher in chitosan-treated tendon and control tendon than in the normal, uninjured adjacent tendon at all time points (P < .001). Chitosan-treated tendons had significantly higher cellularity than untreated control tendons from 1 to 2 weeks (P < .001), and control tendons were significantly hypercellular compared with chitosan-treated tendons from 4 to 8 weeks (P < .001), but both groups exhibited similar cellularity by 12 weeks (P > .05). Fatty atrophy was found at significantly higher rates in control rats than in chitosan-treated rats (P = .001; Table). Furthermore, as noted in Figure 3, muscle fiber diameter decreased in both groups after injury (P < .001).
Figure 4 shows that the amount of inflammatory collections was significantly smaller in the chitosan-treated group than in the control group over the course of the study (P = .01). In addition, pennation angle steadily decreased in the control group throughout the study period, whereas it transiently decreased in the chitosan-treated group (until 2 weeks) before returning to its immediate postoperative level by 12 weeks (Figure 5). Overall, the chitosan-treated group maintained a higher pennation angle than the control group did (P < .001).
Discussion
RCTs affect more than 40% of patients over age 60 years and are a common cause of debilitating pain, reduced shoulder function, and weakness.10 Thirty thousand to 75,000 rotator cuff repairs are performed annually in the United States.11 Although the best treatment for this disorder remains a topic of debate, arthroscopic and (when necessary) open surgical repair is the accepted gold standard for the treatment of tears that do not improve with conservative management. Despite advances in the surgical treatment of these tears, the surgical failure rates are high (range, 20%-90%), with failures attributed to factors beyond patient age, tear size and chronicity, muscle atrophy and degeneration, tendon quality, repair technique, and postoperative rehabilitation.12,13 Repair strategies that biologically enhance the patient’s intrinsic healing potential are needed.
In tendon repair, choice of repair material (eg, graft) is crucial in determining the success of tissue engineering approaches. The ideal scaffold is biocompatible and does not elicit a host inflammatory response. The selected scaffold in its composition and fabricated form must be capable of holding and supporting cells. In addition, the scaffold should be biodegradable, serving as a temporary support for such cells and mechanically augmenting the repaired tendon while allowing for eventual replacement by matrix components. Moreover, the scaffold should have high porosity and a large surface area. Furthermore, the material should mimic the native tendon extracellular matrix (ECM) architecture to allow cells to be distributed throughout the scaffold and to facilitate diffusion of nutrients and factors that promote cellular proliferation and ECM production.
Given the importance of glycosaminoglycans (GAGs) in supporting the reticular structure of the matrix, use of GAGs or GAG-analogues as components of a tendon tissue scaffold for enhancing repair is well documented.14 One such candidate is chitosan, a partially de-acetylated derivative of chitin found in arthropod exoskeletons. Structurally, chitosan shares some characteristics with various GAGs and hyaluronic acid.15 More specifically, chitosan is a linear polysaccharide composed of glucosamine and N-acetyl glucosamine units linked by β-glycosidic bonds. Investigators have studied the properties of chitosan, including its biocompatibility, biodegradability, antibacterial activity, mucoadhesivity, and wound healing.16,17
One of the most promising features of chitosan is that it can be processed into porous structures for use in cell transplantation and tissue regeneration.18,19 Porous chitosan structures can be formed by freezing and lyophilizing chitosan-acetic acid solutions; chondrogenic cell adhesion and proliferation onto these structures have been reported.20,21 This chitosan scaffolding method has also been used to test different composites with collagens, gelatins, GAGs, and hyaluronic acid, all of which have also been proposed as useful 3-dimensional materials for tissue repair.22
In the present study, we used chitosan matrix in RCT repair. We hypothesized that chitosan matrix could enhance rotator cuff repair the same way it enhances repair in epidermal tissues.16 Histologic findings demonstrated that the percentage of fibrous tissue was significantly higher in the chitosan-treated group than in the control group. This improved fibroblastic response may be attributed to the ability of chitosan to enhance cell migration and serve as a scaffold for repair. Other studies have indicated that chitin, of which chitosan is the primary derivative, accelerated the healing of skin and subcutaneous tissues by increased cell migration.23 Moreover, Okamoto and colleagues24 reported that chitin implants stimulated abundant angiogenesis through the same mechanism.
Inadequate initial strength of a repair may lead to a recurrent cuff tear or a disability of rotator cuff function in the early healing stages. In our study, the chitosan matrix tended to be absorbed by 6 weeks after surgery. Its adherence to and ultimate absorption at the repair site may be challenged by the flow of irrigation fluid through the subacromial space in the setting of arthroscopic surgery. However, because the chitosan remains in a more robust gel form, it is better able to resist being washed from the repair site. For augmentation, it may be possible to apply a biocompatible patch over the gel to further protect it from being dislodged. In addition, histologic findings showed that the fibrous repair tissue gradually increased until reaching a peak 8 weeks after surgery—an indication that the absorption rate of the chitosan scaffold lags behind full recovery of the repair tissue. Given this relationship, further studies are needed to determine the mechanical strength of the repair between 6 and 8 weeks, which is important for avoiding recurrent tears.
This study had a few limitations. First, as with any animal model, the anatomy and function of the rat shoulder differ from those of the human shoulder. The acromial arch differs in quadruped animals, with less coverage of the supraspinatus and more of the subscapularis.25 These anatomical differences could yield altered stress mechanics that could affect tendon repair. Furthermore, rats and humans differ in their RCT healing rates. Thus, the pathophysiology of muscle atrophy and fat infiltration in rats may slightly differ from that in humans. In addition, no mechanical testing was performed to compare chitosan-treated and untreated rotator cuff repairs, and such testing is needed to clarify the biomechanical importance of augmentation. Furthermore, no immunohistochemical analysis was performed for collagen. In the repair of rotator cuff tendons, surgeons must consider not only the number of cells but also the production of ECM. Although not directly confirmed in this study, chitosan induced fibrous tissue proliferation that mirrored production of a large amount of collagen fibers. Last, we used an open RTC model. As an arthroscopic model was not used, no definitive conclusions can be drawn regarding use of chitosan in arthroscopy.
Conclusion
Use of chitosan as an acellular matrix improved formation of healing fibrous tissue, increased the number of cells, and prevented fatty atrophy and inflammatory aggregates inside repair sites while facilitating recovery of the natural pennation angle of the tissue. These results demonstrate that chitosan can enhance tendon healing in the setting of acute RCT. Further research, including biomechanical testing of repaired tendons, is needed to further delineate the utility of chitosan in regenerating irreparable RCTs.
Rotator cuff tears (RCTs) are common tendon injuries that can cause chronic pain and severe functional disability. Massive RCTs do not heal spontaneously and, in many cases, result in poor clinical outcomes. Specifically, muscle atrophy and fatty infiltration correlate with poor outcomes after surgical repair.1 Fatty infiltration of the rotator cuff is a common phenomenon that can lead to permanent structural alterations within the tendon. It has been suggested that changes in muscle fiber orientation (the pennation angle) can cause mesenchymal stem cells to migrate to the interface between muscle fibers and the region of fatty infiltration of the muscle.2 Understanding the factors involved in muscle degeneration and atrophy, and in fatty infiltration, may lead to treatments that improve outcomes for patients with massive RCTs. One proposed treatment involves placing continuous mechanical traction on the ends of the torn tendon.2 Findings from this research have indicated that acute tears that become chronic tears are typified by inelasticity and poor function of the muscle–tendon unit. It is therefore important to develop a method that speeds tendon healing without causing the muscle fiber atrophy and pennation angle changes that lead to fatty atrophy, which appears to be an irreversible structural change.
On the basis of the theory that adding mesenchymal cells may improve tendon healing, investigators have studied use of transcription factors (eg, scleraxis) specific to tendogenesis in the embryonal stage.3,4 Nevertheless, certain transcription factors are associated with formation of fibrocartilage in higher concentrations.4 Moreover, decalcified bone matrix increases cartilage formation when added to the tendon repair site.5 Cartilage formation, however, is associated with poorer functional results.6 Thus, there is a need for a method that facilitates faster tendon healing with higher quality tissue formation and less muscle atrophy.
Chitosan, a linear polysaccharide, is associated with scarless healing of soft tissues and prevention of adhesion formation both intraperitoneally and during tendon healing after surgery.7,8 Chitosan tends to precipitate in physiologic pH, thereby mitigating its potency. Fortunately, a chitosan solution that does not precipitate in physiologic conditions was recently developed.9 The solution’s lack of precipitation, coupled with its in situ gelling, allows it to adhere to the repair site long enough to take effect. These characteristics could allow for intimate contact between gel and tendon, facilitating guided-tissue regeneration and preventing adhesion of the rotator cuff to surrounding tissue. By contrast, other biological agents (eg, platelet-rich plasma) are administered as fluid rather than gel and are therefore more susceptible to diffusing from the repair site, mitigating their effects. Thus, chitosan gel is fairly unique among agents.
In the study reported here, we histologically investigated whether a chitosan gel would help improve healing of rotator cuff tendon (acute supraspinatus) tears in a rat model.
Materials and Methods
Supraspinatus Surgical Model
Forty Wistar rats, each weighing between 300 and 400 g, were used in this study. All procedures were approved by the Institutional Animal Care and Use Committee at Rabin Medical Center in Petah Tikva, Israel. The rats were anesthetized with ketamine 90 mg/kg and xylazine 10 mg/kg, both administered intramuscularly, and anesthesia was prolonged as needed with 2% isoflurane, administered by nose cone. The skin was incised 5 cm along the upper back following the midline of the spine. The resulting skin flaps were retracted and the scapula exposed. Careful blunt dissection allowed visualization of the rotator cuff and the trans-scapular arch. A full-thickness incision of the supraspinatus tendon was then made 2 mm distal to the arch. This procedure was performed on both shoulders. For the right supraspinatus tendon, a bioabsorbable chitosan–hydrochloric acid solution (>70% de-acetylated chitosan, molecular weight of 600 kDa; Heppe Medical Chitosan GmbH, Halle, Germany) was sterilely applied to the ends of the tendon (total volume, 0.5 mL) and automatically gelled in situ by heating to about 37°C (rat’s internal body temperature). The tendon ends were subsequently approximated with a single 4-0 Prolene suture (Ethicon, Somerville, New Jersey). The left shoulder (tendon repaired with suture only) served as a control.
The rats were housed for a maximum of 12 weeks after surgery. They were sacrificed (in groups of 5 each) 2 hours, 3 days, 1 week, 2 weeks, 4 weeks, 6 weeks, 8 weeks, and 12 weeks after surgery. After each rat was sacrificed, both shoulder girdles were harvested, and the sutures were removed from the supraspinatus tendons.
Histologic Analysis
After routine fixation with 4% formalin for 48 hours and decalcification with 10% ethylenediaminetetraacetic acid (EDTA) for 3 weeks, the specimens were sectioned with a microtome blade. Care was taken to ensure the plane of the microtome blade was parallel with the longitudinal plane of the supraspinatus muscle and tendon to allow for evaluation of pennation angle. Hematoxylin-eosin staining and Masson trichrome staining were subsequently performed.
A variety of histologic measurements were obtained with use of ImageJ software (US National Institutes of Health). Percentage of fibrous tissue was determined by examining the slides at low magnification fields (×25) at the tendon healing site. Three such fields were evaluated per specimen. The fibrous tissue was circled manually, and percentage of tissue area was assessed and compared with total region of interest. Cellularity was carefully outlined and measured as percentage of total tendon area occupied by cells. Fatty atrophy was defined as either present or absent. Muscle fiber diameter was defined as average diameter of 10 muscle fibers measured within 2 mm of the tendon laceration site. Inflammatory cell collections were defined as either large (>100 µm in diameter) or small (<100 µm in diameter) and were dichotomized to either present or absent. Pennation angle was defined as average angle between muscle fibers and longitudinal axis of supraspinatus muscle and tendon unit. Ten fibers proximal to and within 2 mm of the laceration site were randomly selected, measured, and averaged.
Statistical Analysis
Statistical analysis was performed with Analyse-it 2.20 for Microsoft Excel 2010 (Analyse-it Software, Leeds, United Kingdom). Data were initially analyzed with the Kolmogorov-Smirnov test to assess for normality of distribution. The t test was used to compare continuous variables when the data were normally distributed and the Mann-Whitney test when the data were not normally distributed.
Results
All tendons (both groups) healed within 12 weeks. Generally, the tissue formed at the repair site exhibited a mixture of tenocyte-like cells (fibrotic tissue) and granulation tissue without clear orientation. As noted in Figure 1, the tendons treated with chitosan had more fibrotic tissue (overall mean, 21.5%) relative to the control group (mean, 12.3%), and the difference was significant (P = .003). The most notable differences were found at time points later than 1 week after surgery. In addition, amount of cellularity (Figure 2) was higher in chitosan-treated tendon and control tendon than in the normal, uninjured adjacent tendon at all time points (P < .001). Chitosan-treated tendons had significantly higher cellularity than untreated control tendons from 1 to 2 weeks (P < .001), and control tendons were significantly hypercellular compared with chitosan-treated tendons from 4 to 8 weeks (P < .001), but both groups exhibited similar cellularity by 12 weeks (P > .05). Fatty atrophy was found at significantly higher rates in control rats than in chitosan-treated rats (P = .001; Table). Furthermore, as noted in Figure 3, muscle fiber diameter decreased in both groups after injury (P < .001).
Figure 4 shows that the amount of inflammatory collections was significantly smaller in the chitosan-treated group than in the control group over the course of the study (P = .01). In addition, pennation angle steadily decreased in the control group throughout the study period, whereas it transiently decreased in the chitosan-treated group (until 2 weeks) before returning to its immediate postoperative level by 12 weeks (Figure 5). Overall, the chitosan-treated group maintained a higher pennation angle than the control group did (P < .001).
Discussion
RCTs affect more than 40% of patients over age 60 years and are a common cause of debilitating pain, reduced shoulder function, and weakness.10 Thirty thousand to 75,000 rotator cuff repairs are performed annually in the United States.11 Although the best treatment for this disorder remains a topic of debate, arthroscopic and (when necessary) open surgical repair is the accepted gold standard for the treatment of tears that do not improve with conservative management. Despite advances in the surgical treatment of these tears, the surgical failure rates are high (range, 20%-90%), with failures attributed to factors beyond patient age, tear size and chronicity, muscle atrophy and degeneration, tendon quality, repair technique, and postoperative rehabilitation.12,13 Repair strategies that biologically enhance the patient’s intrinsic healing potential are needed.
In tendon repair, choice of repair material (eg, graft) is crucial in determining the success of tissue engineering approaches. The ideal scaffold is biocompatible and does not elicit a host inflammatory response. The selected scaffold in its composition and fabricated form must be capable of holding and supporting cells. In addition, the scaffold should be biodegradable, serving as a temporary support for such cells and mechanically augmenting the repaired tendon while allowing for eventual replacement by matrix components. Moreover, the scaffold should have high porosity and a large surface area. Furthermore, the material should mimic the native tendon extracellular matrix (ECM) architecture to allow cells to be distributed throughout the scaffold and to facilitate diffusion of nutrients and factors that promote cellular proliferation and ECM production.
Given the importance of glycosaminoglycans (GAGs) in supporting the reticular structure of the matrix, use of GAGs or GAG-analogues as components of a tendon tissue scaffold for enhancing repair is well documented.14 One such candidate is chitosan, a partially de-acetylated derivative of chitin found in arthropod exoskeletons. Structurally, chitosan shares some characteristics with various GAGs and hyaluronic acid.15 More specifically, chitosan is a linear polysaccharide composed of glucosamine and N-acetyl glucosamine units linked by β-glycosidic bonds. Investigators have studied the properties of chitosan, including its biocompatibility, biodegradability, antibacterial activity, mucoadhesivity, and wound healing.16,17
One of the most promising features of chitosan is that it can be processed into porous structures for use in cell transplantation and tissue regeneration.18,19 Porous chitosan structures can be formed by freezing and lyophilizing chitosan-acetic acid solutions; chondrogenic cell adhesion and proliferation onto these structures have been reported.20,21 This chitosan scaffolding method has also been used to test different composites with collagens, gelatins, GAGs, and hyaluronic acid, all of which have also been proposed as useful 3-dimensional materials for tissue repair.22
In the present study, we used chitosan matrix in RCT repair. We hypothesized that chitosan matrix could enhance rotator cuff repair the same way it enhances repair in epidermal tissues.16 Histologic findings demonstrated that the percentage of fibrous tissue was significantly higher in the chitosan-treated group than in the control group. This improved fibroblastic response may be attributed to the ability of chitosan to enhance cell migration and serve as a scaffold for repair. Other studies have indicated that chitin, of which chitosan is the primary derivative, accelerated the healing of skin and subcutaneous tissues by increased cell migration.23 Moreover, Okamoto and colleagues24 reported that chitin implants stimulated abundant angiogenesis through the same mechanism.
Inadequate initial strength of a repair may lead to a recurrent cuff tear or a disability of rotator cuff function in the early healing stages. In our study, the chitosan matrix tended to be absorbed by 6 weeks after surgery. Its adherence to and ultimate absorption at the repair site may be challenged by the flow of irrigation fluid through the subacromial space in the setting of arthroscopic surgery. However, because the chitosan remains in a more robust gel form, it is better able to resist being washed from the repair site. For augmentation, it may be possible to apply a biocompatible patch over the gel to further protect it from being dislodged. In addition, histologic findings showed that the fibrous repair tissue gradually increased until reaching a peak 8 weeks after surgery—an indication that the absorption rate of the chitosan scaffold lags behind full recovery of the repair tissue. Given this relationship, further studies are needed to determine the mechanical strength of the repair between 6 and 8 weeks, which is important for avoiding recurrent tears.
This study had a few limitations. First, as with any animal model, the anatomy and function of the rat shoulder differ from those of the human shoulder. The acromial arch differs in quadruped animals, with less coverage of the supraspinatus and more of the subscapularis.25 These anatomical differences could yield altered stress mechanics that could affect tendon repair. Furthermore, rats and humans differ in their RCT healing rates. Thus, the pathophysiology of muscle atrophy and fat infiltration in rats may slightly differ from that in humans. In addition, no mechanical testing was performed to compare chitosan-treated and untreated rotator cuff repairs, and such testing is needed to clarify the biomechanical importance of augmentation. Furthermore, no immunohistochemical analysis was performed for collagen. In the repair of rotator cuff tendons, surgeons must consider not only the number of cells but also the production of ECM. Although not directly confirmed in this study, chitosan induced fibrous tissue proliferation that mirrored production of a large amount of collagen fibers. Last, we used an open RTC model. As an arthroscopic model was not used, no definitive conclusions can be drawn regarding use of chitosan in arthroscopy.
Conclusion
Use of chitosan as an acellular matrix improved formation of healing fibrous tissue, increased the number of cells, and prevented fatty atrophy and inflammatory aggregates inside repair sites while facilitating recovery of the natural pennation angle of the tissue. These results demonstrate that chitosan can enhance tendon healing in the setting of acute RCT. Further research, including biomechanical testing of repaired tendons, is needed to further delineate the utility of chitosan in regenerating irreparable RCTs.
1. Shen PH, Lien SB, Shen HC, Lee CH, Wu SS, Lin LC. Long-term functional outcomes after repair of rotator cuff tears correlated with atrophy of the supraspinatus muscles on magnetic resonance images. J Shoulder Elbow Surg. 2008;17(1 suppl):1S-7S.
2. Meyer DC, Hoppeler H, von Rechenberg B, Gerber C. A pathomechanical concept explains muscle loss and fatty muscular changes following surgical tendon release. J Orthop Res. 2004;22(5):1004-1007.
3. Gulotta LV, Kovacevic D, Packer JD, Deng XH, Rodeo SA. Bone marrow–derived mesenchymal stem cells transduced with scleraxis improve rotator cuff healing in a rat model. Am J Sports Med. 2011;39(6):1282-1289.
4. Gulotta LV, Rodeo SA. Emerging ideas: evaluation of stem cells genetically modified with scleraxis to improve rotator cuff healing. Clin Orthop. 2011;469(10):2977-2980.
5. Sundar S, Pendegrass CJ, Blunn GW. Tendon bone healing can be enhanced by demineralized bone matrix: a functional and histological study. J Biomed Mater Res B Appl Biomater. 2009;88(1):115-122.
6. Kumagai J, Sarkar K, Uhthoff HK. The collagen types in the attachment zone of rotator cuff tendons in the elderly: an immunohistochemical study. J Rheumatol. 1994;21(11):2096-2100.
7. Wang D, Mo J, Pan S, Chen H, Zhen H. Prevention of postoperative peritoneal adhesions by O-carboxymethyl chitosan in a rat cecal abrasion model. Clin Invest Med. 2010;33(4):E254-E260.
8. Zhang H, Sheng ZJ, Hou CL. Effect of chitosan membrane on tendon adhesion and healing [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 1999;13(6):382-385.
9. Cho MH, Kim KS, Ahn HH, et al. Chitosan gel as an in situ–forming scaffold for rat bone marrow mesenchymal stem cells in vivo. Tissue Eng Part A. 2008;14(6):1099-1108.
10. Yamaguchi K, Tetro AM, Blam O, Evanoff BA, Teefey SA, Middleton WD. Natural history of asymptomatic rotator cuff tears: a longitudinal analysis of asymptomatic tears detected sonographically. J Shoulder Elbow Surg. 2001;10(3):199-203.
11. Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16(2):181-187.
12. Accousti KJ, Flatow EL. Technical pearls on how to maximize healing of the rotator cuff. Instr Course Lect. 2007;56:3-12.
13. Bishop J, Klepps S, Lo IK, Bird J, Gladstone JN, Flatow EL. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. J Shoulder Elbow Surg. 2006;15(3):290-299.
14. Hunziker E, Spector M, Libera J, et al. Translation from research to applications. Tissue Eng. 2006;12(12):3341-3364.
15. Suh JK, Matthew HW. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 2000;21(24):2589-2598.
16. Kumar MN, Muzzarelli RA, Muzzarelli C, Sashiwa H, Domb AJ. Chitosan chemistry and pharmaceutical perspectives. Chem Rev. 2004;104(12):6017-6084.
17. Shi C, Zhu Y, Ran X, Wang M, Su Y, Cheng T. Therapeutic potential of chitosan and its derivatives in regenerative medicine. J Surg Res. 2006;133(2):185-192.
18. Hsieh WC, Chang CP, Lin SM. Morphology and characterization of 3D micro-porous structured chitosan scaffolds for tissue engineering. Colloids Surf B Biointerfaces. 2007;57(2):250-255.
19. Madihally SV, Matthew HW. Porous chitosan scaffolds for tissue engineering. Biomaterials. 1999;20(12):1133-1142.
20. Nettles DL, Elder SH, Gilbert JA. Potential use of chitosan as a cell scaffold material for cartilage tissue engineering. Tissue Eng. 2002;8(6):1009-1016.
21. Griffon DJ, Sedighi MR, Schaeffer DV, Eurell JA, Johnson AL. Chitosan scaffolds: interconnective pore size and cartilage engineering. Acta Biomater. 2006;2(3):313-320.
22. Manjubala I, Scheler S, Bossert J, Jandt KD. Mineralisation of chitosan scaffolds with nano-apatite formation by double diffusion technique. Acta Biomater. 2006;2(1):75-84.
23. Su CH, Sun CS, Juan SW, Ho HO, Hu CH, Sheu MT. Development of fungal mycelia as skin substitutes: effects on wound healing and fibroblast. Biomaterials. 1999;20(1):61-68.
24. Okamoto Y, Southwood L, Stashak TS. Effect of chitin on nonwoven fabric implant in tendon healing. Carbohydr Polym. 1997;33:33-38.
25. Gupta R, Lee TQ. Contributions of the different rabbit models to our understanding of rotator cuff pathology. J Shoulder Elbow Surg. 2007;16(5 suppl):S149-S157.
1. Shen PH, Lien SB, Shen HC, Lee CH, Wu SS, Lin LC. Long-term functional outcomes after repair of rotator cuff tears correlated with atrophy of the supraspinatus muscles on magnetic resonance images. J Shoulder Elbow Surg. 2008;17(1 suppl):1S-7S.
2. Meyer DC, Hoppeler H, von Rechenberg B, Gerber C. A pathomechanical concept explains muscle loss and fatty muscular changes following surgical tendon release. J Orthop Res. 2004;22(5):1004-1007.
3. Gulotta LV, Kovacevic D, Packer JD, Deng XH, Rodeo SA. Bone marrow–derived mesenchymal stem cells transduced with scleraxis improve rotator cuff healing in a rat model. Am J Sports Med. 2011;39(6):1282-1289.
4. Gulotta LV, Rodeo SA. Emerging ideas: evaluation of stem cells genetically modified with scleraxis to improve rotator cuff healing. Clin Orthop. 2011;469(10):2977-2980.
5. Sundar S, Pendegrass CJ, Blunn GW. Tendon bone healing can be enhanced by demineralized bone matrix: a functional and histological study. J Biomed Mater Res B Appl Biomater. 2009;88(1):115-122.
6. Kumagai J, Sarkar K, Uhthoff HK. The collagen types in the attachment zone of rotator cuff tendons in the elderly: an immunohistochemical study. J Rheumatol. 1994;21(11):2096-2100.
7. Wang D, Mo J, Pan S, Chen H, Zhen H. Prevention of postoperative peritoneal adhesions by O-carboxymethyl chitosan in a rat cecal abrasion model. Clin Invest Med. 2010;33(4):E254-E260.
8. Zhang H, Sheng ZJ, Hou CL. Effect of chitosan membrane on tendon adhesion and healing [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 1999;13(6):382-385.
9. Cho MH, Kim KS, Ahn HH, et al. Chitosan gel as an in situ–forming scaffold for rat bone marrow mesenchymal stem cells in vivo. Tissue Eng Part A. 2008;14(6):1099-1108.
10. Yamaguchi K, Tetro AM, Blam O, Evanoff BA, Teefey SA, Middleton WD. Natural history of asymptomatic rotator cuff tears: a longitudinal analysis of asymptomatic tears detected sonographically. J Shoulder Elbow Surg. 2001;10(3):199-203.
11. Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16(2):181-187.
12. Accousti KJ, Flatow EL. Technical pearls on how to maximize healing of the rotator cuff. Instr Course Lect. 2007;56:3-12.
13. Bishop J, Klepps S, Lo IK, Bird J, Gladstone JN, Flatow EL. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. J Shoulder Elbow Surg. 2006;15(3):290-299.
14. Hunziker E, Spector M, Libera J, et al. Translation from research to applications. Tissue Eng. 2006;12(12):3341-3364.
15. Suh JK, Matthew HW. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 2000;21(24):2589-2598.
16. Kumar MN, Muzzarelli RA, Muzzarelli C, Sashiwa H, Domb AJ. Chitosan chemistry and pharmaceutical perspectives. Chem Rev. 2004;104(12):6017-6084.
17. Shi C, Zhu Y, Ran X, Wang M, Su Y, Cheng T. Therapeutic potential of chitosan and its derivatives in regenerative medicine. J Surg Res. 2006;133(2):185-192.
18. Hsieh WC, Chang CP, Lin SM. Morphology and characterization of 3D micro-porous structured chitosan scaffolds for tissue engineering. Colloids Surf B Biointerfaces. 2007;57(2):250-255.
19. Madihally SV, Matthew HW. Porous chitosan scaffolds for tissue engineering. Biomaterials. 1999;20(12):1133-1142.
20. Nettles DL, Elder SH, Gilbert JA. Potential use of chitosan as a cell scaffold material for cartilage tissue engineering. Tissue Eng. 2002;8(6):1009-1016.
21. Griffon DJ, Sedighi MR, Schaeffer DV, Eurell JA, Johnson AL. Chitosan scaffolds: interconnective pore size and cartilage engineering. Acta Biomater. 2006;2(3):313-320.
22. Manjubala I, Scheler S, Bossert J, Jandt KD. Mineralisation of chitosan scaffolds with nano-apatite formation by double diffusion technique. Acta Biomater. 2006;2(1):75-84.
23. Su CH, Sun CS, Juan SW, Ho HO, Hu CH, Sheu MT. Development of fungal mycelia as skin substitutes: effects on wound healing and fibroblast. Biomaterials. 1999;20(1):61-68.
24. Okamoto Y, Southwood L, Stashak TS. Effect of chitin on nonwoven fabric implant in tendon healing. Carbohydr Polym. 1997;33:33-38.
25. Gupta R, Lee TQ. Contributions of the different rabbit models to our understanding of rotator cuff pathology. J Shoulder Elbow Surg. 2007;16(5 suppl):S149-S157.
Retrograde Reamer/Irrigator/Aspirator Technique for Autologous Bone Graft Harvesting With the Patient in the Prone Position
The Reamer/Irrigator/Aspirator (RIA) system (Synthes, West Chester, Pennsylvania) has become a powerful tool for harvesting autologous bone graft from the intramedullary canal of the long bones of the lower extremity for the treatment of osseous defects, nonunions, and joint fusions.1,2 The RIA system provides satisfactory quality and quantity of bone graft (range, 40-90 mL)3-5 with osteogenic properties that rival those harvested from the iliac crest.6,7 Minimal donor-site morbidity and mortality have been reported in association with the RIA technique compared with iliac crest bone graft harvest.8
The RIA technique for the femur—with the antegrade approach and the supine position,8 with the antegrade approach and the prone position,9 and with the retrograde approach and the supine position4—has been described in the literature. To our knowledge, however, the RIA technique for the femur with the retrograde approach and the prone position has not been described. Antegrade harvesting uses the trochanteric entry point, and retrograde harvesting uses an entry at the intercondylar notch just anterior to the posterior cruciate ligament. In this article, we detail the technique for RIA harvesting of the femur with the patient in the prone position. Patient positioning is based on the diagnosis and the proposed procedure.
Advantages of a retrograde starting point include a more concentric trajectory (vs that of an antegrade starting point) and more efficient canal pressure reduction, which might decrease the risk of intraoperative fat embolization.10 This technique offers a more efficient solution to any procedure that requires the prone position, and it avoids the need to reposition, reprepare, or redrape the extremity. It is also very useful in treating obese patients.
After obtaining institutional review board (IRB) approval, we retrospectively reviewed patient files. Because the study was retrospective, the IRB waived the requirement for informed consent. The patients described here provided written informed consent for print and electronic publication of these case reports.
Surgical Technique
The patient is placed in a prone position on a radiolucent table with a bump under the thigh to allow access to the knee joint with full extension of the hip (Figures 1, 2A, 2B). The knee is then flexed to gain access to the intercondylar notch.
The anatomical axis of the femur is identified in the coronal and sagittal planes with the help of an image intensifier. Frequent intraoperative fluoroscopic imaging is required to prevent eccentric reaming and guide-wire movement from causing iatrogenic fractures and perforations, respectively.8 A 2-mm Steinmann pin is used to identify the point of entry into the femoral canal, which is located just above the posterior cruciate ligament insertion in the intercondylar notch, and care is taken not to ream this structure. A minimally invasive incision of about 15 mm is centered on this pin using a patellar tendon–splitting approach.
An 8-mm cannulated anterior cruciate ligament reamer is passed over the pin to enlarge the opening at the entry point, and a 2.5-mm ball-tipped guide wire is positioned in the femur. The image intensifier is used to confirm positioning of the guide in the trochanteric region and centered in the intramedullary canal. A radiolucent diving board facilitates fluoroscopic imaging.
The diameter (12.5 or 16.5 mm) of the reaming head is selected after the intramedullary guide is placed in the femoral canal. The isthmus of the femur is then identified radiographically, and a radiopaque ruler with increments in millimeters is used to measure the canal diameter (Figures 3A, 3B). Because the femoral canal is an ellipsoid, the canal diameter usually is much larger anteroposteriorly than laterally.8 We prefer to use a reaming head that overlaps the inner cortical diameter by 1 mm on each side. An alternative method includes measuring the outer diameter of the narrowest portion of the bone and using a reamer head no more than 45% of the outer diameter at the isthmus.8
The RIA system is prepared on the back table by attaching the reaming head to the irrigation and suction systems. As the reamer head enters the intramedullary canal, an approach–withdraw–pause technique is used to slowly advance the reamer through the femur. It is crucial to use the image intensifier to guide reaming in order to avoid overdrilling the anterior cortex and prevent eccentric reaming of the canal, which more commonly occurs in patients with large anterior femoral bows.11 When the collection filter becomes full, reaming is stopped. The bone graft in the filter is emptied into a specimen cup for measurement and storage until subsequent use (Figure 4). Suctioning is suspended when reaming is stopped because substantial blood loss can occur with prolonged suction and aspiration.12 When repeat reaming is required, care is taken not to overream the cortices, thereby avoiding the risk of iatrogenic fracture.10,12
The knee joint is irrigated to remove any intramedullary debris. Typically there is no debris, as it is captured by the RIA. The wound is closed in 2 layers. Dressing with Ace bandage (3M, St. Paul, Minnesota) is placed around the knee for comfort. Weight-bearing status is determined by the index procedure.
Case Reports
Case 1
A 68-year-old female smoker presented to our facility with right ankle pain after recent ankle arthrodesis for pilon fracture nonunion. Almost 3 years earlier, the patient sustained a Gustilo-Anderson type II open pilon fracture in a motorcycle accident. She underwent antibiotic therapy, irrigation and débridement of the fracture site, and external fixation before definitive treatment with repeat irrigation and débridement and open reduction and internal fixation of the tibial plafond. About 6 months after surgery, she presented to her surgeon with a draining abscess over the anteromedial surgical incision. Multiple débridement procedures were performed, the implant was removed, the ankle was stabilized with a bridging external fixator, and culture-specific antibiotic therapy was administered. Intraoperative cultures confirmed methicillin-resistant Staphylococcus aureus. Vancomycin was administered intravenously for 6 weeks. Once C-reactive protein level and erythrocyte sedimentation rate returned to normal, repeat débridement with a rectus abdominis free flap and ankle fusion were performed.
When the patient presented to our clinic, we saw atrophic nonunion of the ankle fusion on radiographs. Smoking cessation was encouraged but not required before surgery. The patient returned to the operating suite for tibiotalocalcaneal fusion with a retrograde intramedullary nail. With the patient in the prone position, retrograde femoral RIA reaming was performed to harvest 30 mL of autologous bone. After resection of the nonunion site using a trans-Achilles approach and insertion of the intramedullary nail, the autologous bone graft was mixed with recombinant human bone morphogenetic protein 2 (BMP-2), and the mixture was introduced into the fusion site. At final follow-up, 18 months after surgery, the patient was clinically asymptomatic and radiographically healed—without further intervention and despite continued smoking. She did not report any knee pain from the harvest site.
Case 2
A 59-year-old noncompliant woman with diabetes and Charcot neuropathy sustained a trimalleolar ankle fracture-dislocation that was initially treated with ankle and hindfoot arthrodesis. The postoperative course was uneventful, and she was discharged home. Less than a week later, she presented to the emergency department with a midshaft tibial fracture just proximal to the ankle and hindfoot fusion nail. She subsequently had the device removed and a long arthrodesis rod inserted to span the fracture site up to the proximal tibial metadiaphysis. About 9 months later, she returned to our office complaining of ankle pain. No signs of infection were clinically evident. Radiographs showed nonunion of the ankle and subtalar joint. Findings of the initial bone biopsy and pathologic examination were negative for infection. The patient returned to the operating room 4 weeks later for revision ankle fusion. With the patient in the prone position, autologous bone (~30 mL) was harvested using retrograde femoral RIA reaming. The nonunion site was resected, and a mixture of autologous bone graft and BMP-2 was applied. Through a posterior approach, an anterior ankle arthrodesis locking plate was applied to the posterior aspect of the calcaneus and tibia. The patient was kept non-weight-bearing for 3 months and progressed in weight-bearing for another 4 to 6 weeks. Ambulatory status was restored about 4 months after surgery. No harvest-site knee pain was reported.
Discussion
Given its osteogenic, osteoconductive, and osteoinductive properties, autologous cancellous bone graft is the gold standard for reconstruction and fusion procedures in foot and ankle surgery.13 Bone graft can be obtained from many potential donor sites, but the most common is the iliac crest.2 However, many comorbidities, such as residual donor-site pain, neurovascular injuries, infection, and increased surgical time, have been reported in the literature.14,15 The RIA system was initially developed for simultaneous reaming and aspiration to reduce intramedullary pressure, heat generation, operating time, and the systemic effects of reaming, such as the embolic phenomenon.16-22 The single-pass reamer has provided a minimally invasive strategy for procuring voluminous amounts of autologous cancellous bone from the intramedullary canal of lower extremity long bones. Schmidmaier and colleagues3 recently quantified the measurements of several growth factors, such as insulinlike growth factor 1, transforming growth factor β 1, and BMP-2—proving that RIA-derived aspirates have amounts comparable to if not larger than those of iliac crest autologous bone graft. Pratt and colleagues23 provided insight into the possibility of induction of mesenchymal stem cells using the previously unwanted supernatant reamings after filtration. Recently, the RIA technique of autologous tibial and hindfoot bone graft harvest was described for use in ankle or tibiotalocalcaneal arthrodesis.2 Although this technique is a useful surgical option, tibia size remains a limiting factor. Kovar and Wozasek24 reported harvesting significantly more bone graft in the femur than in the tibia. A tibia that cannot accommodate the 12-mm (smallest) reamer head in the RIA system would be a contraindication. In addition, concerns about the association between tibial stress fractures and reaming of the entire tibial canal and concerns about the overall donor-site morbidity of the tibial shaft remain.
Conclusion
With its retrograde approach and prone positioning, this RIA technique is an effective and efficient solution for harvesting autologous femoral bone graft. Although we have described its use in ankle and hindfoot arthrodesis, this technique can be applied to any prone-position surgical procedure, including spine surgery.
1. Kobbe P, Tarkin IS, Frink M, Pape HC. Voluminous bone graft harvesting of the femoral marrow cavity for autologous transplantation. An indication for the “reamer-irrigator-aspirator-” (RIA-)technique [in German]. Unfallchirurg. 2008;111(6):469-472.
2. Herscovici D Jr, Scaduto JM. Use of the reamer-irrigator-aspirator technique to obtain autograft for ankle and hindfoot arthrodesis. J Bone Joint Surg Br. 2012;94(1):75-79.
3. Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156-1163.
4. Qvick LM, Ritter CA, Mutty CE, Rohrbacher BJ, Buyea CM, Anders MJ. Donor site morbidity with reamer-irrigator-aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013;44(10):1263-1269.
5. Lehman AA, Irgit KS, Cush GJ. Harvest of autogenous bone graft using reamer-irrigator-aspirator in tibiotalocalcaneal arthrodesis: surgical technique and case series. Foot Ankle Int. 2012;33(12):1133-1138.
6. Wildemann B, Kadow-Romacker A, Haas NP, Schmidmaier G. Quantification of various growth factors in different demineralized bone matrix preparations. J Biomed Mater Res A. 2007;81(2):437-442.
7. Sagi HC, Young ML, Gerstenfeld L, Einhorn TA, Tornetta P. Qualitative and quantitative differences between bone graft obtained from the medullary canal (with a reamer/irrigator/aspirator) and the iliac crest of the same patient. J Bone Joint Surg Am. 2012;94(23):2128-2135.
8. Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop. 2008;466(12):2973-2980.
9. Nichols TA, Sagi HC, Weber TG, Guiot BH. An alternative source of autograft bone for spinal fusion: the femur: technical case report. Neurosurgery. 2008;62(3 suppl 1):E179.
10. Van Gorp CC, Falk JV, Kmiec SJ Jr, Siston RA. The reamer/irrigator/aspirator reduces femoral canal pressure in simulated TKA. Clin Orthop. 2009;467(3):805-809.
11. Quintero AJ, Tarkin IS, Pape HC. Technical tricks when using the reamer irrigator aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24(1):42-45.
12. Stafford PR, Norris B. Reamer-irrigator-aspirator as a bone graft harvester. Tech Foot Ankle Surg. 2007;6(2):100-107.
13. Whitehouse MR, Lankester BJ, Winson IG, Hepple S. Bone graft harvest from the proximal tibia in foot and ankle arthrodesis surgery. Foot Ankle Int. 2006;27(11):913-916.
14. Scharfenberger A, Weber T. RIA for bone graft harvest: applications for grafting large segmental defects in the tibia and femur. Presented at: 21st Annual Meeting of the Orthopaedic Trauma Association; 2005; Ottawa, Canada.
15. Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop. 1996;(329):300-309.
16. Bedi A, Karunakar MA. Physiologic effects of intramedullary reaming. Instr Course Lect. 2006;55:359-366.
17. Higgins TF, Casey V, Bachus K. Cortical heat generation using an irrigating/aspirating single-pass reaming vs conventional stepwise reaming. J Orthop Trauma. 2007;21(3):192-197.
18. Husebye EE, Lyberg T, Madsen JE, Eriksen M, Røise O. The influence of a one-step reamer-irrigator-aspirator technique on the intramedullary pressure in the pig femur. Injury. 2006;37(10):935-940.
19. Müller CA, Green J, Südkamp NP. Physical and technical aspects of intramedullary reaming. Injury. 2006;37(suppl 4):S39-S49.
20. Pape HC, Dwenger A, Grotz M, et al. Does the reamer type influence the degree of lung dysfunction after femoral nailing following severe trauma? An animal study. J Orthop Trauma. 1994;8(4):300-309.
21. Pape HC, Zelle BA, Hildebrand F, Giannoudis PV, Krettek C, van Griensven M. Reamed femoral nailing in sheep: does irrigation and aspiration of intramedullary contents alter the systemic response? J Bone Joint Surg Am. 2005;87(11):2515-2522.
22. Schult M, Küchle R, Hofmann A, et al. Pathophysiological advantages of rinsing-suction-reaming (RSR) in a pig model for intramedullary nailing. J Orthop Res. 2006;24(6):1186-1192.
23. Pratt DJ, Papagiannopoulos G, Rees PH, Quinnell R. The effects of medullary reaming on the torsional strength of the femur. Injury. 1987;18(3):177-179.
24. Kovar FM, Wozasek GE. Bone graft harvesting using the RIA (reamer irrigation aspirator) system—a quantitative assessment. Wien Klin Wochenschr. 2011;123(9-10):285-290.
The Reamer/Irrigator/Aspirator (RIA) system (Synthes, West Chester, Pennsylvania) has become a powerful tool for harvesting autologous bone graft from the intramedullary canal of the long bones of the lower extremity for the treatment of osseous defects, nonunions, and joint fusions.1,2 The RIA system provides satisfactory quality and quantity of bone graft (range, 40-90 mL)3-5 with osteogenic properties that rival those harvested from the iliac crest.6,7 Minimal donor-site morbidity and mortality have been reported in association with the RIA technique compared with iliac crest bone graft harvest.8
The RIA technique for the femur—with the antegrade approach and the supine position,8 with the antegrade approach and the prone position,9 and with the retrograde approach and the supine position4—has been described in the literature. To our knowledge, however, the RIA technique for the femur with the retrograde approach and the prone position has not been described. Antegrade harvesting uses the trochanteric entry point, and retrograde harvesting uses an entry at the intercondylar notch just anterior to the posterior cruciate ligament. In this article, we detail the technique for RIA harvesting of the femur with the patient in the prone position. Patient positioning is based on the diagnosis and the proposed procedure.
Advantages of a retrograde starting point include a more concentric trajectory (vs that of an antegrade starting point) and more efficient canal pressure reduction, which might decrease the risk of intraoperative fat embolization.10 This technique offers a more efficient solution to any procedure that requires the prone position, and it avoids the need to reposition, reprepare, or redrape the extremity. It is also very useful in treating obese patients.
After obtaining institutional review board (IRB) approval, we retrospectively reviewed patient files. Because the study was retrospective, the IRB waived the requirement for informed consent. The patients described here provided written informed consent for print and electronic publication of these case reports.
Surgical Technique
The patient is placed in a prone position on a radiolucent table with a bump under the thigh to allow access to the knee joint with full extension of the hip (Figures 1, 2A, 2B). The knee is then flexed to gain access to the intercondylar notch.
The anatomical axis of the femur is identified in the coronal and sagittal planes with the help of an image intensifier. Frequent intraoperative fluoroscopic imaging is required to prevent eccentric reaming and guide-wire movement from causing iatrogenic fractures and perforations, respectively.8 A 2-mm Steinmann pin is used to identify the point of entry into the femoral canal, which is located just above the posterior cruciate ligament insertion in the intercondylar notch, and care is taken not to ream this structure. A minimally invasive incision of about 15 mm is centered on this pin using a patellar tendon–splitting approach.
An 8-mm cannulated anterior cruciate ligament reamer is passed over the pin to enlarge the opening at the entry point, and a 2.5-mm ball-tipped guide wire is positioned in the femur. The image intensifier is used to confirm positioning of the guide in the trochanteric region and centered in the intramedullary canal. A radiolucent diving board facilitates fluoroscopic imaging.
The diameter (12.5 or 16.5 mm) of the reaming head is selected after the intramedullary guide is placed in the femoral canal. The isthmus of the femur is then identified radiographically, and a radiopaque ruler with increments in millimeters is used to measure the canal diameter (Figures 3A, 3B). Because the femoral canal is an ellipsoid, the canal diameter usually is much larger anteroposteriorly than laterally.8 We prefer to use a reaming head that overlaps the inner cortical diameter by 1 mm on each side. An alternative method includes measuring the outer diameter of the narrowest portion of the bone and using a reamer head no more than 45% of the outer diameter at the isthmus.8
The RIA system is prepared on the back table by attaching the reaming head to the irrigation and suction systems. As the reamer head enters the intramedullary canal, an approach–withdraw–pause technique is used to slowly advance the reamer through the femur. It is crucial to use the image intensifier to guide reaming in order to avoid overdrilling the anterior cortex and prevent eccentric reaming of the canal, which more commonly occurs in patients with large anterior femoral bows.11 When the collection filter becomes full, reaming is stopped. The bone graft in the filter is emptied into a specimen cup for measurement and storage until subsequent use (Figure 4). Suctioning is suspended when reaming is stopped because substantial blood loss can occur with prolonged suction and aspiration.12 When repeat reaming is required, care is taken not to overream the cortices, thereby avoiding the risk of iatrogenic fracture.10,12
The knee joint is irrigated to remove any intramedullary debris. Typically there is no debris, as it is captured by the RIA. The wound is closed in 2 layers. Dressing with Ace bandage (3M, St. Paul, Minnesota) is placed around the knee for comfort. Weight-bearing status is determined by the index procedure.
Case Reports
Case 1
A 68-year-old female smoker presented to our facility with right ankle pain after recent ankle arthrodesis for pilon fracture nonunion. Almost 3 years earlier, the patient sustained a Gustilo-Anderson type II open pilon fracture in a motorcycle accident. She underwent antibiotic therapy, irrigation and débridement of the fracture site, and external fixation before definitive treatment with repeat irrigation and débridement and open reduction and internal fixation of the tibial plafond. About 6 months after surgery, she presented to her surgeon with a draining abscess over the anteromedial surgical incision. Multiple débridement procedures were performed, the implant was removed, the ankle was stabilized with a bridging external fixator, and culture-specific antibiotic therapy was administered. Intraoperative cultures confirmed methicillin-resistant Staphylococcus aureus. Vancomycin was administered intravenously for 6 weeks. Once C-reactive protein level and erythrocyte sedimentation rate returned to normal, repeat débridement with a rectus abdominis free flap and ankle fusion were performed.
When the patient presented to our clinic, we saw atrophic nonunion of the ankle fusion on radiographs. Smoking cessation was encouraged but not required before surgery. The patient returned to the operating suite for tibiotalocalcaneal fusion with a retrograde intramedullary nail. With the patient in the prone position, retrograde femoral RIA reaming was performed to harvest 30 mL of autologous bone. After resection of the nonunion site using a trans-Achilles approach and insertion of the intramedullary nail, the autologous bone graft was mixed with recombinant human bone morphogenetic protein 2 (BMP-2), and the mixture was introduced into the fusion site. At final follow-up, 18 months after surgery, the patient was clinically asymptomatic and radiographically healed—without further intervention and despite continued smoking. She did not report any knee pain from the harvest site.
Case 2
A 59-year-old noncompliant woman with diabetes and Charcot neuropathy sustained a trimalleolar ankle fracture-dislocation that was initially treated with ankle and hindfoot arthrodesis. The postoperative course was uneventful, and she was discharged home. Less than a week later, she presented to the emergency department with a midshaft tibial fracture just proximal to the ankle and hindfoot fusion nail. She subsequently had the device removed and a long arthrodesis rod inserted to span the fracture site up to the proximal tibial metadiaphysis. About 9 months later, she returned to our office complaining of ankle pain. No signs of infection were clinically evident. Radiographs showed nonunion of the ankle and subtalar joint. Findings of the initial bone biopsy and pathologic examination were negative for infection. The patient returned to the operating room 4 weeks later for revision ankle fusion. With the patient in the prone position, autologous bone (~30 mL) was harvested using retrograde femoral RIA reaming. The nonunion site was resected, and a mixture of autologous bone graft and BMP-2 was applied. Through a posterior approach, an anterior ankle arthrodesis locking plate was applied to the posterior aspect of the calcaneus and tibia. The patient was kept non-weight-bearing for 3 months and progressed in weight-bearing for another 4 to 6 weeks. Ambulatory status was restored about 4 months after surgery. No harvest-site knee pain was reported.
Discussion
Given its osteogenic, osteoconductive, and osteoinductive properties, autologous cancellous bone graft is the gold standard for reconstruction and fusion procedures in foot and ankle surgery.13 Bone graft can be obtained from many potential donor sites, but the most common is the iliac crest.2 However, many comorbidities, such as residual donor-site pain, neurovascular injuries, infection, and increased surgical time, have been reported in the literature.14,15 The RIA system was initially developed for simultaneous reaming and aspiration to reduce intramedullary pressure, heat generation, operating time, and the systemic effects of reaming, such as the embolic phenomenon.16-22 The single-pass reamer has provided a minimally invasive strategy for procuring voluminous amounts of autologous cancellous bone from the intramedullary canal of lower extremity long bones. Schmidmaier and colleagues3 recently quantified the measurements of several growth factors, such as insulinlike growth factor 1, transforming growth factor β 1, and BMP-2—proving that RIA-derived aspirates have amounts comparable to if not larger than those of iliac crest autologous bone graft. Pratt and colleagues23 provided insight into the possibility of induction of mesenchymal stem cells using the previously unwanted supernatant reamings after filtration. Recently, the RIA technique of autologous tibial and hindfoot bone graft harvest was described for use in ankle or tibiotalocalcaneal arthrodesis.2 Although this technique is a useful surgical option, tibia size remains a limiting factor. Kovar and Wozasek24 reported harvesting significantly more bone graft in the femur than in the tibia. A tibia that cannot accommodate the 12-mm (smallest) reamer head in the RIA system would be a contraindication. In addition, concerns about the association between tibial stress fractures and reaming of the entire tibial canal and concerns about the overall donor-site morbidity of the tibial shaft remain.
Conclusion
With its retrograde approach and prone positioning, this RIA technique is an effective and efficient solution for harvesting autologous femoral bone graft. Although we have described its use in ankle and hindfoot arthrodesis, this technique can be applied to any prone-position surgical procedure, including spine surgery.
The Reamer/Irrigator/Aspirator (RIA) system (Synthes, West Chester, Pennsylvania) has become a powerful tool for harvesting autologous bone graft from the intramedullary canal of the long bones of the lower extremity for the treatment of osseous defects, nonunions, and joint fusions.1,2 The RIA system provides satisfactory quality and quantity of bone graft (range, 40-90 mL)3-5 with osteogenic properties that rival those harvested from the iliac crest.6,7 Minimal donor-site morbidity and mortality have been reported in association with the RIA technique compared with iliac crest bone graft harvest.8
The RIA technique for the femur—with the antegrade approach and the supine position,8 with the antegrade approach and the prone position,9 and with the retrograde approach and the supine position4—has been described in the literature. To our knowledge, however, the RIA technique for the femur with the retrograde approach and the prone position has not been described. Antegrade harvesting uses the trochanteric entry point, and retrograde harvesting uses an entry at the intercondylar notch just anterior to the posterior cruciate ligament. In this article, we detail the technique for RIA harvesting of the femur with the patient in the prone position. Patient positioning is based on the diagnosis and the proposed procedure.
Advantages of a retrograde starting point include a more concentric trajectory (vs that of an antegrade starting point) and more efficient canal pressure reduction, which might decrease the risk of intraoperative fat embolization.10 This technique offers a more efficient solution to any procedure that requires the prone position, and it avoids the need to reposition, reprepare, or redrape the extremity. It is also very useful in treating obese patients.
After obtaining institutional review board (IRB) approval, we retrospectively reviewed patient files. Because the study was retrospective, the IRB waived the requirement for informed consent. The patients described here provided written informed consent for print and electronic publication of these case reports.
Surgical Technique
The patient is placed in a prone position on a radiolucent table with a bump under the thigh to allow access to the knee joint with full extension of the hip (Figures 1, 2A, 2B). The knee is then flexed to gain access to the intercondylar notch.
The anatomical axis of the femur is identified in the coronal and sagittal planes with the help of an image intensifier. Frequent intraoperative fluoroscopic imaging is required to prevent eccentric reaming and guide-wire movement from causing iatrogenic fractures and perforations, respectively.8 A 2-mm Steinmann pin is used to identify the point of entry into the femoral canal, which is located just above the posterior cruciate ligament insertion in the intercondylar notch, and care is taken not to ream this structure. A minimally invasive incision of about 15 mm is centered on this pin using a patellar tendon–splitting approach.
An 8-mm cannulated anterior cruciate ligament reamer is passed over the pin to enlarge the opening at the entry point, and a 2.5-mm ball-tipped guide wire is positioned in the femur. The image intensifier is used to confirm positioning of the guide in the trochanteric region and centered in the intramedullary canal. A radiolucent diving board facilitates fluoroscopic imaging.
The diameter (12.5 or 16.5 mm) of the reaming head is selected after the intramedullary guide is placed in the femoral canal. The isthmus of the femur is then identified radiographically, and a radiopaque ruler with increments in millimeters is used to measure the canal diameter (Figures 3A, 3B). Because the femoral canal is an ellipsoid, the canal diameter usually is much larger anteroposteriorly than laterally.8 We prefer to use a reaming head that overlaps the inner cortical diameter by 1 mm on each side. An alternative method includes measuring the outer diameter of the narrowest portion of the bone and using a reamer head no more than 45% of the outer diameter at the isthmus.8
The RIA system is prepared on the back table by attaching the reaming head to the irrigation and suction systems. As the reamer head enters the intramedullary canal, an approach–withdraw–pause technique is used to slowly advance the reamer through the femur. It is crucial to use the image intensifier to guide reaming in order to avoid overdrilling the anterior cortex and prevent eccentric reaming of the canal, which more commonly occurs in patients with large anterior femoral bows.11 When the collection filter becomes full, reaming is stopped. The bone graft in the filter is emptied into a specimen cup for measurement and storage until subsequent use (Figure 4). Suctioning is suspended when reaming is stopped because substantial blood loss can occur with prolonged suction and aspiration.12 When repeat reaming is required, care is taken not to overream the cortices, thereby avoiding the risk of iatrogenic fracture.10,12
The knee joint is irrigated to remove any intramedullary debris. Typically there is no debris, as it is captured by the RIA. The wound is closed in 2 layers. Dressing with Ace bandage (3M, St. Paul, Minnesota) is placed around the knee for comfort. Weight-bearing status is determined by the index procedure.
Case Reports
Case 1
A 68-year-old female smoker presented to our facility with right ankle pain after recent ankle arthrodesis for pilon fracture nonunion. Almost 3 years earlier, the patient sustained a Gustilo-Anderson type II open pilon fracture in a motorcycle accident. She underwent antibiotic therapy, irrigation and débridement of the fracture site, and external fixation before definitive treatment with repeat irrigation and débridement and open reduction and internal fixation of the tibial plafond. About 6 months after surgery, she presented to her surgeon with a draining abscess over the anteromedial surgical incision. Multiple débridement procedures were performed, the implant was removed, the ankle was stabilized with a bridging external fixator, and culture-specific antibiotic therapy was administered. Intraoperative cultures confirmed methicillin-resistant Staphylococcus aureus. Vancomycin was administered intravenously for 6 weeks. Once C-reactive protein level and erythrocyte sedimentation rate returned to normal, repeat débridement with a rectus abdominis free flap and ankle fusion were performed.
When the patient presented to our clinic, we saw atrophic nonunion of the ankle fusion on radiographs. Smoking cessation was encouraged but not required before surgery. The patient returned to the operating suite for tibiotalocalcaneal fusion with a retrograde intramedullary nail. With the patient in the prone position, retrograde femoral RIA reaming was performed to harvest 30 mL of autologous bone. After resection of the nonunion site using a trans-Achilles approach and insertion of the intramedullary nail, the autologous bone graft was mixed with recombinant human bone morphogenetic protein 2 (BMP-2), and the mixture was introduced into the fusion site. At final follow-up, 18 months after surgery, the patient was clinically asymptomatic and radiographically healed—without further intervention and despite continued smoking. She did not report any knee pain from the harvest site.
Case 2
A 59-year-old noncompliant woman with diabetes and Charcot neuropathy sustained a trimalleolar ankle fracture-dislocation that was initially treated with ankle and hindfoot arthrodesis. The postoperative course was uneventful, and she was discharged home. Less than a week later, she presented to the emergency department with a midshaft tibial fracture just proximal to the ankle and hindfoot fusion nail. She subsequently had the device removed and a long arthrodesis rod inserted to span the fracture site up to the proximal tibial metadiaphysis. About 9 months later, she returned to our office complaining of ankle pain. No signs of infection were clinically evident. Radiographs showed nonunion of the ankle and subtalar joint. Findings of the initial bone biopsy and pathologic examination were negative for infection. The patient returned to the operating room 4 weeks later for revision ankle fusion. With the patient in the prone position, autologous bone (~30 mL) was harvested using retrograde femoral RIA reaming. The nonunion site was resected, and a mixture of autologous bone graft and BMP-2 was applied. Through a posterior approach, an anterior ankle arthrodesis locking plate was applied to the posterior aspect of the calcaneus and tibia. The patient was kept non-weight-bearing for 3 months and progressed in weight-bearing for another 4 to 6 weeks. Ambulatory status was restored about 4 months after surgery. No harvest-site knee pain was reported.
Discussion
Given its osteogenic, osteoconductive, and osteoinductive properties, autologous cancellous bone graft is the gold standard for reconstruction and fusion procedures in foot and ankle surgery.13 Bone graft can be obtained from many potential donor sites, but the most common is the iliac crest.2 However, many comorbidities, such as residual donor-site pain, neurovascular injuries, infection, and increased surgical time, have been reported in the literature.14,15 The RIA system was initially developed for simultaneous reaming and aspiration to reduce intramedullary pressure, heat generation, operating time, and the systemic effects of reaming, such as the embolic phenomenon.16-22 The single-pass reamer has provided a minimally invasive strategy for procuring voluminous amounts of autologous cancellous bone from the intramedullary canal of lower extremity long bones. Schmidmaier and colleagues3 recently quantified the measurements of several growth factors, such as insulinlike growth factor 1, transforming growth factor β 1, and BMP-2—proving that RIA-derived aspirates have amounts comparable to if not larger than those of iliac crest autologous bone graft. Pratt and colleagues23 provided insight into the possibility of induction of mesenchymal stem cells using the previously unwanted supernatant reamings after filtration. Recently, the RIA technique of autologous tibial and hindfoot bone graft harvest was described for use in ankle or tibiotalocalcaneal arthrodesis.2 Although this technique is a useful surgical option, tibia size remains a limiting factor. Kovar and Wozasek24 reported harvesting significantly more bone graft in the femur than in the tibia. A tibia that cannot accommodate the 12-mm (smallest) reamer head in the RIA system would be a contraindication. In addition, concerns about the association between tibial stress fractures and reaming of the entire tibial canal and concerns about the overall donor-site morbidity of the tibial shaft remain.
Conclusion
With its retrograde approach and prone positioning, this RIA technique is an effective and efficient solution for harvesting autologous femoral bone graft. Although we have described its use in ankle and hindfoot arthrodesis, this technique can be applied to any prone-position surgical procedure, including spine surgery.
1. Kobbe P, Tarkin IS, Frink M, Pape HC. Voluminous bone graft harvesting of the femoral marrow cavity for autologous transplantation. An indication for the “reamer-irrigator-aspirator-” (RIA-)technique [in German]. Unfallchirurg. 2008;111(6):469-472.
2. Herscovici D Jr, Scaduto JM. Use of the reamer-irrigator-aspirator technique to obtain autograft for ankle and hindfoot arthrodesis. J Bone Joint Surg Br. 2012;94(1):75-79.
3. Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156-1163.
4. Qvick LM, Ritter CA, Mutty CE, Rohrbacher BJ, Buyea CM, Anders MJ. Donor site morbidity with reamer-irrigator-aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013;44(10):1263-1269.
5. Lehman AA, Irgit KS, Cush GJ. Harvest of autogenous bone graft using reamer-irrigator-aspirator in tibiotalocalcaneal arthrodesis: surgical technique and case series. Foot Ankle Int. 2012;33(12):1133-1138.
6. Wildemann B, Kadow-Romacker A, Haas NP, Schmidmaier G. Quantification of various growth factors in different demineralized bone matrix preparations. J Biomed Mater Res A. 2007;81(2):437-442.
7. Sagi HC, Young ML, Gerstenfeld L, Einhorn TA, Tornetta P. Qualitative and quantitative differences between bone graft obtained from the medullary canal (with a reamer/irrigator/aspirator) and the iliac crest of the same patient. J Bone Joint Surg Am. 2012;94(23):2128-2135.
8. Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop. 2008;466(12):2973-2980.
9. Nichols TA, Sagi HC, Weber TG, Guiot BH. An alternative source of autograft bone for spinal fusion: the femur: technical case report. Neurosurgery. 2008;62(3 suppl 1):E179.
10. Van Gorp CC, Falk JV, Kmiec SJ Jr, Siston RA. The reamer/irrigator/aspirator reduces femoral canal pressure in simulated TKA. Clin Orthop. 2009;467(3):805-809.
11. Quintero AJ, Tarkin IS, Pape HC. Technical tricks when using the reamer irrigator aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24(1):42-45.
12. Stafford PR, Norris B. Reamer-irrigator-aspirator as a bone graft harvester. Tech Foot Ankle Surg. 2007;6(2):100-107.
13. Whitehouse MR, Lankester BJ, Winson IG, Hepple S. Bone graft harvest from the proximal tibia in foot and ankle arthrodesis surgery. Foot Ankle Int. 2006;27(11):913-916.
14. Scharfenberger A, Weber T. RIA for bone graft harvest: applications for grafting large segmental defects in the tibia and femur. Presented at: 21st Annual Meeting of the Orthopaedic Trauma Association; 2005; Ottawa, Canada.
15. Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop. 1996;(329):300-309.
16. Bedi A, Karunakar MA. Physiologic effects of intramedullary reaming. Instr Course Lect. 2006;55:359-366.
17. Higgins TF, Casey V, Bachus K. Cortical heat generation using an irrigating/aspirating single-pass reaming vs conventional stepwise reaming. J Orthop Trauma. 2007;21(3):192-197.
18. Husebye EE, Lyberg T, Madsen JE, Eriksen M, Røise O. The influence of a one-step reamer-irrigator-aspirator technique on the intramedullary pressure in the pig femur. Injury. 2006;37(10):935-940.
19. Müller CA, Green J, Südkamp NP. Physical and technical aspects of intramedullary reaming. Injury. 2006;37(suppl 4):S39-S49.
20. Pape HC, Dwenger A, Grotz M, et al. Does the reamer type influence the degree of lung dysfunction after femoral nailing following severe trauma? An animal study. J Orthop Trauma. 1994;8(4):300-309.
21. Pape HC, Zelle BA, Hildebrand F, Giannoudis PV, Krettek C, van Griensven M. Reamed femoral nailing in sheep: does irrigation and aspiration of intramedullary contents alter the systemic response? J Bone Joint Surg Am. 2005;87(11):2515-2522.
22. Schult M, Küchle R, Hofmann A, et al. Pathophysiological advantages of rinsing-suction-reaming (RSR) in a pig model for intramedullary nailing. J Orthop Res. 2006;24(6):1186-1192.
23. Pratt DJ, Papagiannopoulos G, Rees PH, Quinnell R. The effects of medullary reaming on the torsional strength of the femur. Injury. 1987;18(3):177-179.
24. Kovar FM, Wozasek GE. Bone graft harvesting using the RIA (reamer irrigation aspirator) system—a quantitative assessment. Wien Klin Wochenschr. 2011;123(9-10):285-290.
1. Kobbe P, Tarkin IS, Frink M, Pape HC. Voluminous bone graft harvesting of the femoral marrow cavity for autologous transplantation. An indication for the “reamer-irrigator-aspirator-” (RIA-)technique [in German]. Unfallchirurg. 2008;111(6):469-472.
2. Herscovici D Jr, Scaduto JM. Use of the reamer-irrigator-aspirator technique to obtain autograft for ankle and hindfoot arthrodesis. J Bone Joint Surg Br. 2012;94(1):75-79.
3. Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156-1163.
4. Qvick LM, Ritter CA, Mutty CE, Rohrbacher BJ, Buyea CM, Anders MJ. Donor site morbidity with reamer-irrigator-aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013;44(10):1263-1269.
5. Lehman AA, Irgit KS, Cush GJ. Harvest of autogenous bone graft using reamer-irrigator-aspirator in tibiotalocalcaneal arthrodesis: surgical technique and case series. Foot Ankle Int. 2012;33(12):1133-1138.
6. Wildemann B, Kadow-Romacker A, Haas NP, Schmidmaier G. Quantification of various growth factors in different demineralized bone matrix preparations. J Biomed Mater Res A. 2007;81(2):437-442.
7. Sagi HC, Young ML, Gerstenfeld L, Einhorn TA, Tornetta P. Qualitative and quantitative differences between bone graft obtained from the medullary canal (with a reamer/irrigator/aspirator) and the iliac crest of the same patient. J Bone Joint Surg Am. 2012;94(23):2128-2135.
8. Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop. 2008;466(12):2973-2980.
9. Nichols TA, Sagi HC, Weber TG, Guiot BH. An alternative source of autograft bone for spinal fusion: the femur: technical case report. Neurosurgery. 2008;62(3 suppl 1):E179.
10. Van Gorp CC, Falk JV, Kmiec SJ Jr, Siston RA. The reamer/irrigator/aspirator reduces femoral canal pressure in simulated TKA. Clin Orthop. 2009;467(3):805-809.
11. Quintero AJ, Tarkin IS, Pape HC. Technical tricks when using the reamer irrigator aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24(1):42-45.
12. Stafford PR, Norris B. Reamer-irrigator-aspirator as a bone graft harvester. Tech Foot Ankle Surg. 2007;6(2):100-107.
13. Whitehouse MR, Lankester BJ, Winson IG, Hepple S. Bone graft harvest from the proximal tibia in foot and ankle arthrodesis surgery. Foot Ankle Int. 2006;27(11):913-916.
14. Scharfenberger A, Weber T. RIA for bone graft harvest: applications for grafting large segmental defects in the tibia and femur. Presented at: 21st Annual Meeting of the Orthopaedic Trauma Association; 2005; Ottawa, Canada.
15. Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop. 1996;(329):300-309.
16. Bedi A, Karunakar MA. Physiologic effects of intramedullary reaming. Instr Course Lect. 2006;55:359-366.
17. Higgins TF, Casey V, Bachus K. Cortical heat generation using an irrigating/aspirating single-pass reaming vs conventional stepwise reaming. J Orthop Trauma. 2007;21(3):192-197.
18. Husebye EE, Lyberg T, Madsen JE, Eriksen M, Røise O. The influence of a one-step reamer-irrigator-aspirator technique on the intramedullary pressure in the pig femur. Injury. 2006;37(10):935-940.
19. Müller CA, Green J, Südkamp NP. Physical and technical aspects of intramedullary reaming. Injury. 2006;37(suppl 4):S39-S49.
20. Pape HC, Dwenger A, Grotz M, et al. Does the reamer type influence the degree of lung dysfunction after femoral nailing following severe trauma? An animal study. J Orthop Trauma. 1994;8(4):300-309.
21. Pape HC, Zelle BA, Hildebrand F, Giannoudis PV, Krettek C, van Griensven M. Reamed femoral nailing in sheep: does irrigation and aspiration of intramedullary contents alter the systemic response? J Bone Joint Surg Am. 2005;87(11):2515-2522.
22. Schult M, Küchle R, Hofmann A, et al. Pathophysiological advantages of rinsing-suction-reaming (RSR) in a pig model for intramedullary nailing. J Orthop Res. 2006;24(6):1186-1192.
23. Pratt DJ, Papagiannopoulos G, Rees PH, Quinnell R. The effects of medullary reaming on the torsional strength of the femur. Injury. 1987;18(3):177-179.
24. Kovar FM, Wozasek GE. Bone graft harvesting using the RIA (reamer irrigation aspirator) system—a quantitative assessment. Wien Klin Wochenschr. 2011;123(9-10):285-290.
Emerging Biologics in Orthopedics
The discipline of orthopedic medicine and surgery has dramatically advanced over the last several decades. Improved understanding of biomechanics, tissue healing, and the pathogenesis of musculoskeletal diseases has allowed us to make significant progress in the diagnosis, treatment, and rehabilitation of our patients. Despite these advancements, there is still much to be learned, especially in the field of orthobiologics and regenerative medicine. As our understanding of existing technologies, such as bone marrow aspirate, platelet-rich plasma, and adult stem cells, continues to evolve, even newer biologic treatment options are being developed. This issue of The American Journal of Orthopedics focuses on emerging biologics across the spectrum of orthopedic care.
In this issue, on pages 202-205, Mansour and Conway describe a new prone retrograde technique for obtaining bone graft using the Reamer/Irrigator/Aspirator (RIA) system (Synthes, West Chester, Pennsylvania). While iliac crest bone graft has been the gold standard for many years, use of the RIA system to obtain bone graft has been studied and has been shown to have decreased morbidity when compared with iliac crest harvest.1 Additionally, intramedullary bone graft from the femur appears to be just as concentrated with biologically active bone marrow as iliac crest harvest.2 This new technique allows increased efficiency, especially for surgeries that are done in the prone position.
Melamed and colleagues examine a new biologic to augment repair of rotator cuff tears (see pages 212-216). Chitosan, a linear polysaccharide, has been shown to help with soft-tissue healing. Although in the past its use has been limited secondary to problems with the compound precipitating at physiologic pH, new formulations mitigate that problem. In the authors’ animal model of acute supraspinatus repair, the use of chitosan gel increased the number of fibroblasts and the amount of repair tissue when compared with untreated controls. Additionally, the experimental group showed a decreased inflammatory response when compared with the control group. This is very exciting research as the biologic enhancement of rotator cuff tendon healing could potentially help decrease the rate of rotator cuff repair failure.
Lenehan and colleagues analyze the long-term outcomes of anterior cruciate ligament reconstruction in a cohort of patients studied over an 8-year period (see pages 217-222). During this period, 99 patients were reconstructed with allograft tissue and 24 with autograft. Their analysis, like other recently published work, shows that the rates of revision were much higher for patients under 25 years of age who were reconstructed using allograft tissue. The rate of revision for NCAA (National Collegiate Athletic Association) Division I athletes reconstructed with allograft tissue was found to be 62%, while the revision rate for all patients under the age of 25 years who received an allograft was found to be 20.5%. Clearly, there is still a great deal to learn about the biology of graft incorporation and healing, especially as it relates to allograft tissue.
These 3 articles exemplify the breadth of orthopedic biologics and their potential role in orthopedic surgery. Through efforts of investigators highlighted in this journal and in others, biologics will become better understood and more widely used when appropriate, leading to improved patient outcomes.
1. Calori GM, Colombo M, Mazza EL, Mazzola S, Malagoli E, Mineo GV. Incidence of donor site morbidity following harvesting from iliac crest or RIA graft. Injury. 2014;45 Suppl 6:S116-S120.
2. van der Bel R, Blokhuis TJ. Increased osteogenic capacity of Reamer/Irrigator/Aspirator derived mesenchymal stem cells. Injury. 2014;45(12):2060-2064.
The discipline of orthopedic medicine and surgery has dramatically advanced over the last several decades. Improved understanding of biomechanics, tissue healing, and the pathogenesis of musculoskeletal diseases has allowed us to make significant progress in the diagnosis, treatment, and rehabilitation of our patients. Despite these advancements, there is still much to be learned, especially in the field of orthobiologics and regenerative medicine. As our understanding of existing technologies, such as bone marrow aspirate, platelet-rich plasma, and adult stem cells, continues to evolve, even newer biologic treatment options are being developed. This issue of The American Journal of Orthopedics focuses on emerging biologics across the spectrum of orthopedic care.
In this issue, on pages 202-205, Mansour and Conway describe a new prone retrograde technique for obtaining bone graft using the Reamer/Irrigator/Aspirator (RIA) system (Synthes, West Chester, Pennsylvania). While iliac crest bone graft has been the gold standard for many years, use of the RIA system to obtain bone graft has been studied and has been shown to have decreased morbidity when compared with iliac crest harvest.1 Additionally, intramedullary bone graft from the femur appears to be just as concentrated with biologically active bone marrow as iliac crest harvest.2 This new technique allows increased efficiency, especially for surgeries that are done in the prone position.
Melamed and colleagues examine a new biologic to augment repair of rotator cuff tears (see pages 212-216). Chitosan, a linear polysaccharide, has been shown to help with soft-tissue healing. Although in the past its use has been limited secondary to problems with the compound precipitating at physiologic pH, new formulations mitigate that problem. In the authors’ animal model of acute supraspinatus repair, the use of chitosan gel increased the number of fibroblasts and the amount of repair tissue when compared with untreated controls. Additionally, the experimental group showed a decreased inflammatory response when compared with the control group. This is very exciting research as the biologic enhancement of rotator cuff tendon healing could potentially help decrease the rate of rotator cuff repair failure.
Lenehan and colleagues analyze the long-term outcomes of anterior cruciate ligament reconstruction in a cohort of patients studied over an 8-year period (see pages 217-222). During this period, 99 patients were reconstructed with allograft tissue and 24 with autograft. Their analysis, like other recently published work, shows that the rates of revision were much higher for patients under 25 years of age who were reconstructed using allograft tissue. The rate of revision for NCAA (National Collegiate Athletic Association) Division I athletes reconstructed with allograft tissue was found to be 62%, while the revision rate for all patients under the age of 25 years who received an allograft was found to be 20.5%. Clearly, there is still a great deal to learn about the biology of graft incorporation and healing, especially as it relates to allograft tissue.
These 3 articles exemplify the breadth of orthopedic biologics and their potential role in orthopedic surgery. Through efforts of investigators highlighted in this journal and in others, biologics will become better understood and more widely used when appropriate, leading to improved patient outcomes.
The discipline of orthopedic medicine and surgery has dramatically advanced over the last several decades. Improved understanding of biomechanics, tissue healing, and the pathogenesis of musculoskeletal diseases has allowed us to make significant progress in the diagnosis, treatment, and rehabilitation of our patients. Despite these advancements, there is still much to be learned, especially in the field of orthobiologics and regenerative medicine. As our understanding of existing technologies, such as bone marrow aspirate, platelet-rich plasma, and adult stem cells, continues to evolve, even newer biologic treatment options are being developed. This issue of The American Journal of Orthopedics focuses on emerging biologics across the spectrum of orthopedic care.
In this issue, on pages 202-205, Mansour and Conway describe a new prone retrograde technique for obtaining bone graft using the Reamer/Irrigator/Aspirator (RIA) system (Synthes, West Chester, Pennsylvania). While iliac crest bone graft has been the gold standard for many years, use of the RIA system to obtain bone graft has been studied and has been shown to have decreased morbidity when compared with iliac crest harvest.1 Additionally, intramedullary bone graft from the femur appears to be just as concentrated with biologically active bone marrow as iliac crest harvest.2 This new technique allows increased efficiency, especially for surgeries that are done in the prone position.
Melamed and colleagues examine a new biologic to augment repair of rotator cuff tears (see pages 212-216). Chitosan, a linear polysaccharide, has been shown to help with soft-tissue healing. Although in the past its use has been limited secondary to problems with the compound precipitating at physiologic pH, new formulations mitigate that problem. In the authors’ animal model of acute supraspinatus repair, the use of chitosan gel increased the number of fibroblasts and the amount of repair tissue when compared with untreated controls. Additionally, the experimental group showed a decreased inflammatory response when compared with the control group. This is very exciting research as the biologic enhancement of rotator cuff tendon healing could potentially help decrease the rate of rotator cuff repair failure.
Lenehan and colleagues analyze the long-term outcomes of anterior cruciate ligament reconstruction in a cohort of patients studied over an 8-year period (see pages 217-222). During this period, 99 patients were reconstructed with allograft tissue and 24 with autograft. Their analysis, like other recently published work, shows that the rates of revision were much higher for patients under 25 years of age who were reconstructed using allograft tissue. The rate of revision for NCAA (National Collegiate Athletic Association) Division I athletes reconstructed with allograft tissue was found to be 62%, while the revision rate for all patients under the age of 25 years who received an allograft was found to be 20.5%. Clearly, there is still a great deal to learn about the biology of graft incorporation and healing, especially as it relates to allograft tissue.
These 3 articles exemplify the breadth of orthopedic biologics and their potential role in orthopedic surgery. Through efforts of investigators highlighted in this journal and in others, biologics will become better understood and more widely used when appropriate, leading to improved patient outcomes.
1. Calori GM, Colombo M, Mazza EL, Mazzola S, Malagoli E, Mineo GV. Incidence of donor site morbidity following harvesting from iliac crest or RIA graft. Injury. 2014;45 Suppl 6:S116-S120.
2. van der Bel R, Blokhuis TJ. Increased osteogenic capacity of Reamer/Irrigator/Aspirator derived mesenchymal stem cells. Injury. 2014;45(12):2060-2064.
1. Calori GM, Colombo M, Mazza EL, Mazzola S, Malagoli E, Mineo GV. Incidence of donor site morbidity following harvesting from iliac crest or RIA graft. Injury. 2014;45 Suppl 6:S116-S120.
2. van der Bel R, Blokhuis TJ. Increased osteogenic capacity of Reamer/Irrigator/Aspirator derived mesenchymal stem cells. Injury. 2014;45(12):2060-2064.
Nanotechnology: Why Should We Care?
The orthopedic community is increasingly deluged with advancements in the basic sciences. With each step, we must evaluate the necessity of new information and the relevance of these topics for clinical practice. Since the late 1990s, the promise of nanotechnology to effect significant changes in the medical field has been heralded. However, in this coming decade, we as a profession will see unprecedented advances in the movement of this technology “from the bench to the bedside.” Not unlike many other basic science advancements in our field, nanotechnology is poorly understood among clinicians and residents. As the use of biologics and drug delivery systems expands in orthopedics, nanoparticle-based devices will become more prevalent and have a momentous impact on the way we treat and diagnose orthopedic patients.
A nanoparticle is generally defined as a particle in which at least 1 dimension is between 1 to 100 nanometers and has material properties consistent with quantum mechanics.1 Nanomaterials can be composed of organic and inorganic chemical elements that enable basic chemical processes to create more complex systems. Individual nanoparticle units can be synthesized to form nanostructures, including nanotubes, nanoscaffolds, nanofibers, and even nanodiamonds.2-4 Nanoparticles at this scale display unique optical, chemical, and physical properties that can be manipulated to create specific end-use applications. Such uses may include glass fabrication, optical probes, television screens, drug delivery, gene delivery, and multiplex diagnostic assays.5-7 By crossing disciplines of physics, engineering, and medical sciences, we can create novel technology that includes nanomanufacturing, targeted drug delivery, nanorobotics in conjunction with artificial intelligence, and point-of-care diagnostics.7-9
The field of orthopedics has benefited from nanotechnologic advances, such as new therapeutics and implant-related technology. Nanotubes are hollow nanosized cylinders that are commonly created from titania, silica, or carbon-based substrates. They have garnered significant interest for their high tensile and shear strength, favorable microstructure for bony ingrowth, and their capacity to hold antibiotics or growth factors, such as bone morphogenic proteins (BMPs).10 The current local delivery limitations of BMPs via a collagen sponge have the potential to be maximized and better controlled with a nanotechnology-based approach. The size, internal structure, and shape of the nanoparticle can be manipulated to control the release of these growth factors, and certain nanoparticles can be dual-layered, allowing for release of multiple growth factors at once or in succession.11,12 A more powerful and targeted delivery system of these types of growth factors may result in improved or more robust outcomes, and further research is warranted.
It is possible that carbon-based nanotubes can be categorized as a biomedical implant secondary to their mechanical properties.13 Their strength and ability to be augmented with osteogenic materials has made them an attractive area of research as alternative implant surfaces and stand-alone implants. Nanotubes are capable of acting as a scaffold for antibiotic-loaded, carbon-based nanodiamonds for localized treatment of periprosthetic infection, and research has been directed toward controlled release of the nanodiamond-antibiotic construct from these scaffolds or hydrogels.4,14 Technologies like this may allow the clinician to treat periprosthetic infections locally and minimize the use of systemic antibiotics. The perfection of this type of delivery system may augment the role of antibiotic-laden cement and improve our treatment success rates, even in traditionally hard-to-treat organisms.
Nanoscaffolds and nanofibers are created from nanosized polymers and rendered into a 3-dimensional structure that can be loaded with biologic particles or acting as a scaffold/template for tissue or bone ingrowth. Nanofibers created using biodegradable substrates such as poly(lactic-co-glycolic acid) (PLGA) and chitosan have been extensively studied for their delayed-release properties and biocompatibility.15 These scaffolds are often soaked or loaded with chondrogenic, osteogenic, or antibacterial agents, and have been evaluated in both in vitro and in vivo studies with promising results.15,16 They have been an exciting area of research in tissue engineering, and have been accepted as an adjunct in tendon-repair treatments and local bone regeneration.3,17 As this technology is perfected, the potential to treat more effectively massive rotator cuff tears or tears with poor tissue integrity will dramatically improve and expand the indications for rotator cuff repair.
Augmentation of implant surfaces with nanomaterials that improve osseointegration, or that act as antimicrobial agents have also been a focus of research in hopes of decreasing the rates of aseptic failure and periprosthetic infection in arthroplasty procedures. Nanocrystalline surfaces made of hydroxyapatite and cobalt chromium have been evaluated for their enhanced osteoconductive properties, and may replace standard surfaces.18-20 Recent work evaluating nanoparticle-antibiotic constructs that have been covalently bound to implant surfaces for delayed release of antibiotics during the perioperative period has shown promise, and may allow a more targeted and localized treatment strategy for periprosthetic infection.21,22
Major limitations regarding successful clinical implementation of nanotechnology include both cost and regulatory processes. Currently, pharmaceutical companies estimate that, on average, successful clinical trials from phase 1 to completion for new drugs can cost hundreds of millions of dollars.23 Such high costs result partially from the laborious and capital-intensive process of conducting clinical trials that meet US Food and Drug Administration (FDA) requirements. These regulations would apply to both surface-coated implants and nanoparticle-based drug delivery systems. These types of implants would not be expedited into the market secondary to their drug delivery component and would likely require lengthy clinical studies. Implant companies may be reluctant to invest millions of dollars in multiple FDA trials when they have lucrative implants on the market.
Other limitations include the particles’ complex 3-dimensional structure, which can present challenges for mass production. Producing large quantities of nanoparticles at a consistent quality may be a major limitation to the more unique and target-based nanotherapies. Recent concerns with the toxicity profile of nanotechnology-based medicines have resulted in more intense scrutiny of the nanotechnology safety profile.24,25 Currently, nanoparticle technology is evaluated case by case with each technology requiring its own toxicology and safety profile testing if it is intended for human use. These tests can be cost-prohibitive and require extensive private and government capital for successful market entry. Despite these limitations, nanotechnology will impact the next generation of orthopedic surgeons. Current estimates project the nanomedicine market to be worth $177.6 billion by 2019.26
Advances in nanobased orthopedic technologies have expanded dramatically in the past decade, and we, as the treating physicians, must make educated decisions on how and when to use nanoparticle-based therapies and treatment options. Nanotechnology’s basic science is confusing and often burdensome, but contemporary review articles may be helpful in keeping the orthopedic resident and clinician current with advancements.10,27,28 The more we educate ourselves about evolving nanotechnologies, the less reluctance we will have when evaluating new diagnostic and therapeutic treatment modalities.
1. Hewakuruppu YL, Dombrovsky LA, Chen C, et al. Plasmonic “pump-probe” method to study semi-transparent nanofluids. Appl Opt. 2013;52(24):6041-6050.
2. Balasundaram G, Webster TJ. An overview of nano-polymers for orthopedic applications. Macromol Biosci. 2007;7(5):635-642.
3. Zhang Z, Hu J, Ma PX. Nanofiber-based delivery of bioactive agents and stem cells to bone sites. Adv Drug Deliv Rev. 2012;64(12):1129-1141.
4. Mochalin VN, Shenderova O, Ho D, Gogotsi Y. The properties and applications of nanodiamonds. Nat Nanotechnol. 2012;7(1):11-23.
5. Kneipp J, Kneipp H, Rice WL, Kneipp K. Optical probes for biological applications based on surface-enhanced Raman scattering from indocyanine green on gold nanoparticles. Anal Chem. 2005;77(8):2381-2385.
6. Wang L, O’Donoghue MB, Tan W. Nanoparticles for multiplex diagnostics and imaging. Nanomedicine (Lond). 2006;1(4):413-426.
7. Krebs MD, Salter E, Chen E, Sutter KA, Alsberg E. Calcium phosphate-DNA nanoparticle gene delivery from alginate hydrogels induces in vivo osteogenesis. J Biomed Mater Res A. 2010;92(3):1131-1138.
8. Myers FB, Lee LP. Innovations in optical microfluidic technologies for point-of-care diagnostics. Lab Chip. 2008;8(12):2015-2031.
9. Sacha GM, Varona P. Artificial intelligence in nanotechnology. Nanotechnology. 2013;24(45):452002.
10. Ganguly DY, Shahbazian R, Shokuhfar T. Recent advances in nanotubes for orthopedic implants. J Nanotech Smart Mater. 2014;1:1-10.
11. Srivastava S, Kotov NA. Composite Layer-by-Layer (LBL) assembly with inorganic nanoparticles and nanowires. Acc Chem Res. 2008;41(12):1831-1841.
12. Panda HS, Srivastava R, Bahadur D. Shape and size control of nano dispersed Mg/Al layered double hydroxide. J Nanosci Nanotechnol. 2008;8(8):4218-4223.
13. Wang X, Li Q, Xie J, et al. Fabrication of ultralong and electrically uniform single-walled carbon nanotubes on clean substrates. Nano Lett. 2009;9(9):3137-3141.
14. Zhu Y, Li J, Li W, et al. The biocompatibility of nanodiamonds and their application in drug delivery systems. Theranostics. 2012;2(3):302-312.
15. Wu L, Ding J. In vitro degradation of three-dimensional porous poly(D,L-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2004;25(2):5821-5830.
16. Wu X, Rabkin-Aikawa E, Guleserian KJ, et al. Tissue-engineered microvessels on three-dimensional biodegradable scaffolds using human endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2004;287(2):H480-H487.
17. Xia W, Liu W, Cui L, et al. Tissue engineering of cartilage with the use of chitosan-gelatin complex scaffolds. J Biomed Mater Res B Appl Biomater. 2004;71(2):373-380.
18. Laurencin CT, Kumbar SG, Nukavarapu SP. Nanotechnology and orthopedics: a personal perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(1):6-10.
19. Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials. 2004;25(19):4731-4739.
20. Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000;21(17):1803-1810.
21. Stewart S, Barr S, Engiles J, et al. Vancomycin-modified implant surface inhibits biofilm formation and supports bone-healing in an infected osteotomy model in sheep: a proof-of-concept study. J Bone Joint Surg Am. 2012;94(15):1406-1415.
22. Hickok NJ, Shapiro IM. Immobilized antibiotics to prevent orthopaedic implant infections. Adv Drug Deliv Rev. 2012;64(12):1165-1176.
23. DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22(2):151-185.
24. Vines T, Faunce T. Assessing the safety and cost-effectiveness of early nanodrugs. J Law Med. 2009;16(5):822-845.
25. Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622-627.
26. Nanomedicine Market (Neurology, Cardiovascular, Anti-Inflammatory, Anti-Infective, and Oncology Applications): Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2013-2019. Transparency Market Research website. http://www.transparencymarketresearch.com/nanomedicine-market.html. Published August 1, 2014. Accessed January 20, 2015.
27. Sullivan MP, McHale KJ, Parvizi J, Mehta S. Nanotechnology: current concepts in orthopaedic surgery and future directions. Bone Joint J. 2014;96-B(5):569-573.
28. Pleshko N, Grande DA, Myers KR. Nanotechnology in orthopaedics. J Am Acad Orthop Surg. 2012;20(1):60-62.
The orthopedic community is increasingly deluged with advancements in the basic sciences. With each step, we must evaluate the necessity of new information and the relevance of these topics for clinical practice. Since the late 1990s, the promise of nanotechnology to effect significant changes in the medical field has been heralded. However, in this coming decade, we as a profession will see unprecedented advances in the movement of this technology “from the bench to the bedside.” Not unlike many other basic science advancements in our field, nanotechnology is poorly understood among clinicians and residents. As the use of biologics and drug delivery systems expands in orthopedics, nanoparticle-based devices will become more prevalent and have a momentous impact on the way we treat and diagnose orthopedic patients.
A nanoparticle is generally defined as a particle in which at least 1 dimension is between 1 to 100 nanometers and has material properties consistent with quantum mechanics.1 Nanomaterials can be composed of organic and inorganic chemical elements that enable basic chemical processes to create more complex systems. Individual nanoparticle units can be synthesized to form nanostructures, including nanotubes, nanoscaffolds, nanofibers, and even nanodiamonds.2-4 Nanoparticles at this scale display unique optical, chemical, and physical properties that can be manipulated to create specific end-use applications. Such uses may include glass fabrication, optical probes, television screens, drug delivery, gene delivery, and multiplex diagnostic assays.5-7 By crossing disciplines of physics, engineering, and medical sciences, we can create novel technology that includes nanomanufacturing, targeted drug delivery, nanorobotics in conjunction with artificial intelligence, and point-of-care diagnostics.7-9
The field of orthopedics has benefited from nanotechnologic advances, such as new therapeutics and implant-related technology. Nanotubes are hollow nanosized cylinders that are commonly created from titania, silica, or carbon-based substrates. They have garnered significant interest for their high tensile and shear strength, favorable microstructure for bony ingrowth, and their capacity to hold antibiotics or growth factors, such as bone morphogenic proteins (BMPs).10 The current local delivery limitations of BMPs via a collagen sponge have the potential to be maximized and better controlled with a nanotechnology-based approach. The size, internal structure, and shape of the nanoparticle can be manipulated to control the release of these growth factors, and certain nanoparticles can be dual-layered, allowing for release of multiple growth factors at once or in succession.11,12 A more powerful and targeted delivery system of these types of growth factors may result in improved or more robust outcomes, and further research is warranted.
It is possible that carbon-based nanotubes can be categorized as a biomedical implant secondary to their mechanical properties.13 Their strength and ability to be augmented with osteogenic materials has made them an attractive area of research as alternative implant surfaces and stand-alone implants. Nanotubes are capable of acting as a scaffold for antibiotic-loaded, carbon-based nanodiamonds for localized treatment of periprosthetic infection, and research has been directed toward controlled release of the nanodiamond-antibiotic construct from these scaffolds or hydrogels.4,14 Technologies like this may allow the clinician to treat periprosthetic infections locally and minimize the use of systemic antibiotics. The perfection of this type of delivery system may augment the role of antibiotic-laden cement and improve our treatment success rates, even in traditionally hard-to-treat organisms.
Nanoscaffolds and nanofibers are created from nanosized polymers and rendered into a 3-dimensional structure that can be loaded with biologic particles or acting as a scaffold/template for tissue or bone ingrowth. Nanofibers created using biodegradable substrates such as poly(lactic-co-glycolic acid) (PLGA) and chitosan have been extensively studied for their delayed-release properties and biocompatibility.15 These scaffolds are often soaked or loaded with chondrogenic, osteogenic, or antibacterial agents, and have been evaluated in both in vitro and in vivo studies with promising results.15,16 They have been an exciting area of research in tissue engineering, and have been accepted as an adjunct in tendon-repair treatments and local bone regeneration.3,17 As this technology is perfected, the potential to treat more effectively massive rotator cuff tears or tears with poor tissue integrity will dramatically improve and expand the indications for rotator cuff repair.
Augmentation of implant surfaces with nanomaterials that improve osseointegration, or that act as antimicrobial agents have also been a focus of research in hopes of decreasing the rates of aseptic failure and periprosthetic infection in arthroplasty procedures. Nanocrystalline surfaces made of hydroxyapatite and cobalt chromium have been evaluated for their enhanced osteoconductive properties, and may replace standard surfaces.18-20 Recent work evaluating nanoparticle-antibiotic constructs that have been covalently bound to implant surfaces for delayed release of antibiotics during the perioperative period has shown promise, and may allow a more targeted and localized treatment strategy for periprosthetic infection.21,22
Major limitations regarding successful clinical implementation of nanotechnology include both cost and regulatory processes. Currently, pharmaceutical companies estimate that, on average, successful clinical trials from phase 1 to completion for new drugs can cost hundreds of millions of dollars.23 Such high costs result partially from the laborious and capital-intensive process of conducting clinical trials that meet US Food and Drug Administration (FDA) requirements. These regulations would apply to both surface-coated implants and nanoparticle-based drug delivery systems. These types of implants would not be expedited into the market secondary to their drug delivery component and would likely require lengthy clinical studies. Implant companies may be reluctant to invest millions of dollars in multiple FDA trials when they have lucrative implants on the market.
Other limitations include the particles’ complex 3-dimensional structure, which can present challenges for mass production. Producing large quantities of nanoparticles at a consistent quality may be a major limitation to the more unique and target-based nanotherapies. Recent concerns with the toxicity profile of nanotechnology-based medicines have resulted in more intense scrutiny of the nanotechnology safety profile.24,25 Currently, nanoparticle technology is evaluated case by case with each technology requiring its own toxicology and safety profile testing if it is intended for human use. These tests can be cost-prohibitive and require extensive private and government capital for successful market entry. Despite these limitations, nanotechnology will impact the next generation of orthopedic surgeons. Current estimates project the nanomedicine market to be worth $177.6 billion by 2019.26
Advances in nanobased orthopedic technologies have expanded dramatically in the past decade, and we, as the treating physicians, must make educated decisions on how and when to use nanoparticle-based therapies and treatment options. Nanotechnology’s basic science is confusing and often burdensome, but contemporary review articles may be helpful in keeping the orthopedic resident and clinician current with advancements.10,27,28 The more we educate ourselves about evolving nanotechnologies, the less reluctance we will have when evaluating new diagnostic and therapeutic treatment modalities.
The orthopedic community is increasingly deluged with advancements in the basic sciences. With each step, we must evaluate the necessity of new information and the relevance of these topics for clinical practice. Since the late 1990s, the promise of nanotechnology to effect significant changes in the medical field has been heralded. However, in this coming decade, we as a profession will see unprecedented advances in the movement of this technology “from the bench to the bedside.” Not unlike many other basic science advancements in our field, nanotechnology is poorly understood among clinicians and residents. As the use of biologics and drug delivery systems expands in orthopedics, nanoparticle-based devices will become more prevalent and have a momentous impact on the way we treat and diagnose orthopedic patients.
A nanoparticle is generally defined as a particle in which at least 1 dimension is between 1 to 100 nanometers and has material properties consistent with quantum mechanics.1 Nanomaterials can be composed of organic and inorganic chemical elements that enable basic chemical processes to create more complex systems. Individual nanoparticle units can be synthesized to form nanostructures, including nanotubes, nanoscaffolds, nanofibers, and even nanodiamonds.2-4 Nanoparticles at this scale display unique optical, chemical, and physical properties that can be manipulated to create specific end-use applications. Such uses may include glass fabrication, optical probes, television screens, drug delivery, gene delivery, and multiplex diagnostic assays.5-7 By crossing disciplines of physics, engineering, and medical sciences, we can create novel technology that includes nanomanufacturing, targeted drug delivery, nanorobotics in conjunction with artificial intelligence, and point-of-care diagnostics.7-9
The field of orthopedics has benefited from nanotechnologic advances, such as new therapeutics and implant-related technology. Nanotubes are hollow nanosized cylinders that are commonly created from titania, silica, or carbon-based substrates. They have garnered significant interest for their high tensile and shear strength, favorable microstructure for bony ingrowth, and their capacity to hold antibiotics or growth factors, such as bone morphogenic proteins (BMPs).10 The current local delivery limitations of BMPs via a collagen sponge have the potential to be maximized and better controlled with a nanotechnology-based approach. The size, internal structure, and shape of the nanoparticle can be manipulated to control the release of these growth factors, and certain nanoparticles can be dual-layered, allowing for release of multiple growth factors at once or in succession.11,12 A more powerful and targeted delivery system of these types of growth factors may result in improved or more robust outcomes, and further research is warranted.
It is possible that carbon-based nanotubes can be categorized as a biomedical implant secondary to their mechanical properties.13 Their strength and ability to be augmented with osteogenic materials has made them an attractive area of research as alternative implant surfaces and stand-alone implants. Nanotubes are capable of acting as a scaffold for antibiotic-loaded, carbon-based nanodiamonds for localized treatment of periprosthetic infection, and research has been directed toward controlled release of the nanodiamond-antibiotic construct from these scaffolds or hydrogels.4,14 Technologies like this may allow the clinician to treat periprosthetic infections locally and minimize the use of systemic antibiotics. The perfection of this type of delivery system may augment the role of antibiotic-laden cement and improve our treatment success rates, even in traditionally hard-to-treat organisms.
Nanoscaffolds and nanofibers are created from nanosized polymers and rendered into a 3-dimensional structure that can be loaded with biologic particles or acting as a scaffold/template for tissue or bone ingrowth. Nanofibers created using biodegradable substrates such as poly(lactic-co-glycolic acid) (PLGA) and chitosan have been extensively studied for their delayed-release properties and biocompatibility.15 These scaffolds are often soaked or loaded with chondrogenic, osteogenic, or antibacterial agents, and have been evaluated in both in vitro and in vivo studies with promising results.15,16 They have been an exciting area of research in tissue engineering, and have been accepted as an adjunct in tendon-repair treatments and local bone regeneration.3,17 As this technology is perfected, the potential to treat more effectively massive rotator cuff tears or tears with poor tissue integrity will dramatically improve and expand the indications for rotator cuff repair.
Augmentation of implant surfaces with nanomaterials that improve osseointegration, or that act as antimicrobial agents have also been a focus of research in hopes of decreasing the rates of aseptic failure and periprosthetic infection in arthroplasty procedures. Nanocrystalline surfaces made of hydroxyapatite and cobalt chromium have been evaluated for their enhanced osteoconductive properties, and may replace standard surfaces.18-20 Recent work evaluating nanoparticle-antibiotic constructs that have been covalently bound to implant surfaces for delayed release of antibiotics during the perioperative period has shown promise, and may allow a more targeted and localized treatment strategy for periprosthetic infection.21,22
Major limitations regarding successful clinical implementation of nanotechnology include both cost and regulatory processes. Currently, pharmaceutical companies estimate that, on average, successful clinical trials from phase 1 to completion for new drugs can cost hundreds of millions of dollars.23 Such high costs result partially from the laborious and capital-intensive process of conducting clinical trials that meet US Food and Drug Administration (FDA) requirements. These regulations would apply to both surface-coated implants and nanoparticle-based drug delivery systems. These types of implants would not be expedited into the market secondary to their drug delivery component and would likely require lengthy clinical studies. Implant companies may be reluctant to invest millions of dollars in multiple FDA trials when they have lucrative implants on the market.
Other limitations include the particles’ complex 3-dimensional structure, which can present challenges for mass production. Producing large quantities of nanoparticles at a consistent quality may be a major limitation to the more unique and target-based nanotherapies. Recent concerns with the toxicity profile of nanotechnology-based medicines have resulted in more intense scrutiny of the nanotechnology safety profile.24,25 Currently, nanoparticle technology is evaluated case by case with each technology requiring its own toxicology and safety profile testing if it is intended for human use. These tests can be cost-prohibitive and require extensive private and government capital for successful market entry. Despite these limitations, nanotechnology will impact the next generation of orthopedic surgeons. Current estimates project the nanomedicine market to be worth $177.6 billion by 2019.26
Advances in nanobased orthopedic technologies have expanded dramatically in the past decade, and we, as the treating physicians, must make educated decisions on how and when to use nanoparticle-based therapies and treatment options. Nanotechnology’s basic science is confusing and often burdensome, but contemporary review articles may be helpful in keeping the orthopedic resident and clinician current with advancements.10,27,28 The more we educate ourselves about evolving nanotechnologies, the less reluctance we will have when evaluating new diagnostic and therapeutic treatment modalities.
1. Hewakuruppu YL, Dombrovsky LA, Chen C, et al. Plasmonic “pump-probe” method to study semi-transparent nanofluids. Appl Opt. 2013;52(24):6041-6050.
2. Balasundaram G, Webster TJ. An overview of nano-polymers for orthopedic applications. Macromol Biosci. 2007;7(5):635-642.
3. Zhang Z, Hu J, Ma PX. Nanofiber-based delivery of bioactive agents and stem cells to bone sites. Adv Drug Deliv Rev. 2012;64(12):1129-1141.
4. Mochalin VN, Shenderova O, Ho D, Gogotsi Y. The properties and applications of nanodiamonds. Nat Nanotechnol. 2012;7(1):11-23.
5. Kneipp J, Kneipp H, Rice WL, Kneipp K. Optical probes for biological applications based on surface-enhanced Raman scattering from indocyanine green on gold nanoparticles. Anal Chem. 2005;77(8):2381-2385.
6. Wang L, O’Donoghue MB, Tan W. Nanoparticles for multiplex diagnostics and imaging. Nanomedicine (Lond). 2006;1(4):413-426.
7. Krebs MD, Salter E, Chen E, Sutter KA, Alsberg E. Calcium phosphate-DNA nanoparticle gene delivery from alginate hydrogels induces in vivo osteogenesis. J Biomed Mater Res A. 2010;92(3):1131-1138.
8. Myers FB, Lee LP. Innovations in optical microfluidic technologies for point-of-care diagnostics. Lab Chip. 2008;8(12):2015-2031.
9. Sacha GM, Varona P. Artificial intelligence in nanotechnology. Nanotechnology. 2013;24(45):452002.
10. Ganguly DY, Shahbazian R, Shokuhfar T. Recent advances in nanotubes for orthopedic implants. J Nanotech Smart Mater. 2014;1:1-10.
11. Srivastava S, Kotov NA. Composite Layer-by-Layer (LBL) assembly with inorganic nanoparticles and nanowires. Acc Chem Res. 2008;41(12):1831-1841.
12. Panda HS, Srivastava R, Bahadur D. Shape and size control of nano dispersed Mg/Al layered double hydroxide. J Nanosci Nanotechnol. 2008;8(8):4218-4223.
13. Wang X, Li Q, Xie J, et al. Fabrication of ultralong and electrically uniform single-walled carbon nanotubes on clean substrates. Nano Lett. 2009;9(9):3137-3141.
14. Zhu Y, Li J, Li W, et al. The biocompatibility of nanodiamonds and their application in drug delivery systems. Theranostics. 2012;2(3):302-312.
15. Wu L, Ding J. In vitro degradation of three-dimensional porous poly(D,L-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2004;25(2):5821-5830.
16. Wu X, Rabkin-Aikawa E, Guleserian KJ, et al. Tissue-engineered microvessels on three-dimensional biodegradable scaffolds using human endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2004;287(2):H480-H487.
17. Xia W, Liu W, Cui L, et al. Tissue engineering of cartilage with the use of chitosan-gelatin complex scaffolds. J Biomed Mater Res B Appl Biomater. 2004;71(2):373-380.
18. Laurencin CT, Kumbar SG, Nukavarapu SP. Nanotechnology and orthopedics: a personal perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(1):6-10.
19. Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials. 2004;25(19):4731-4739.
20. Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000;21(17):1803-1810.
21. Stewart S, Barr S, Engiles J, et al. Vancomycin-modified implant surface inhibits biofilm formation and supports bone-healing in an infected osteotomy model in sheep: a proof-of-concept study. J Bone Joint Surg Am. 2012;94(15):1406-1415.
22. Hickok NJ, Shapiro IM. Immobilized antibiotics to prevent orthopaedic implant infections. Adv Drug Deliv Rev. 2012;64(12):1165-1176.
23. DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22(2):151-185.
24. Vines T, Faunce T. Assessing the safety and cost-effectiveness of early nanodrugs. J Law Med. 2009;16(5):822-845.
25. Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622-627.
26. Nanomedicine Market (Neurology, Cardiovascular, Anti-Inflammatory, Anti-Infective, and Oncology Applications): Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2013-2019. Transparency Market Research website. http://www.transparencymarketresearch.com/nanomedicine-market.html. Published August 1, 2014. Accessed January 20, 2015.
27. Sullivan MP, McHale KJ, Parvizi J, Mehta S. Nanotechnology: current concepts in orthopaedic surgery and future directions. Bone Joint J. 2014;96-B(5):569-573.
28. Pleshko N, Grande DA, Myers KR. Nanotechnology in orthopaedics. J Am Acad Orthop Surg. 2012;20(1):60-62.
1. Hewakuruppu YL, Dombrovsky LA, Chen C, et al. Plasmonic “pump-probe” method to study semi-transparent nanofluids. Appl Opt. 2013;52(24):6041-6050.
2. Balasundaram G, Webster TJ. An overview of nano-polymers for orthopedic applications. Macromol Biosci. 2007;7(5):635-642.
3. Zhang Z, Hu J, Ma PX. Nanofiber-based delivery of bioactive agents and stem cells to bone sites. Adv Drug Deliv Rev. 2012;64(12):1129-1141.
4. Mochalin VN, Shenderova O, Ho D, Gogotsi Y. The properties and applications of nanodiamonds. Nat Nanotechnol. 2012;7(1):11-23.
5. Kneipp J, Kneipp H, Rice WL, Kneipp K. Optical probes for biological applications based on surface-enhanced Raman scattering from indocyanine green on gold nanoparticles. Anal Chem. 2005;77(8):2381-2385.
6. Wang L, O’Donoghue MB, Tan W. Nanoparticles for multiplex diagnostics and imaging. Nanomedicine (Lond). 2006;1(4):413-426.
7. Krebs MD, Salter E, Chen E, Sutter KA, Alsberg E. Calcium phosphate-DNA nanoparticle gene delivery from alginate hydrogels induces in vivo osteogenesis. J Biomed Mater Res A. 2010;92(3):1131-1138.
8. Myers FB, Lee LP. Innovations in optical microfluidic technologies for point-of-care diagnostics. Lab Chip. 2008;8(12):2015-2031.
9. Sacha GM, Varona P. Artificial intelligence in nanotechnology. Nanotechnology. 2013;24(45):452002.
10. Ganguly DY, Shahbazian R, Shokuhfar T. Recent advances in nanotubes for orthopedic implants. J Nanotech Smart Mater. 2014;1:1-10.
11. Srivastava S, Kotov NA. Composite Layer-by-Layer (LBL) assembly with inorganic nanoparticles and nanowires. Acc Chem Res. 2008;41(12):1831-1841.
12. Panda HS, Srivastava R, Bahadur D. Shape and size control of nano dispersed Mg/Al layered double hydroxide. J Nanosci Nanotechnol. 2008;8(8):4218-4223.
13. Wang X, Li Q, Xie J, et al. Fabrication of ultralong and electrically uniform single-walled carbon nanotubes on clean substrates. Nano Lett. 2009;9(9):3137-3141.
14. Zhu Y, Li J, Li W, et al. The biocompatibility of nanodiamonds and their application in drug delivery systems. Theranostics. 2012;2(3):302-312.
15. Wu L, Ding J. In vitro degradation of three-dimensional porous poly(D,L-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2004;25(2):5821-5830.
16. Wu X, Rabkin-Aikawa E, Guleserian KJ, et al. Tissue-engineered microvessels on three-dimensional biodegradable scaffolds using human endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2004;287(2):H480-H487.
17. Xia W, Liu W, Cui L, et al. Tissue engineering of cartilage with the use of chitosan-gelatin complex scaffolds. J Biomed Mater Res B Appl Biomater. 2004;71(2):373-380.
18. Laurencin CT, Kumbar SG, Nukavarapu SP. Nanotechnology and orthopedics: a personal perspective. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(1):6-10.
19. Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials. 2004;25(19):4731-4739.
20. Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000;21(17):1803-1810.
21. Stewart S, Barr S, Engiles J, et al. Vancomycin-modified implant surface inhibits biofilm formation and supports bone-healing in an infected osteotomy model in sheep: a proof-of-concept study. J Bone Joint Surg Am. 2012;94(15):1406-1415.
22. Hickok NJ, Shapiro IM. Immobilized antibiotics to prevent orthopaedic implant infections. Adv Drug Deliv Rev. 2012;64(12):1165-1176.
23. DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22(2):151-185.
24. Vines T, Faunce T. Assessing the safety and cost-effectiveness of early nanodrugs. J Law Med. 2009;16(5):822-845.
25. Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622-627.
26. Nanomedicine Market (Neurology, Cardiovascular, Anti-Inflammatory, Anti-Infective, and Oncology Applications): Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2013-2019. Transparency Market Research website. http://www.transparencymarketresearch.com/nanomedicine-market.html. Published August 1, 2014. Accessed January 20, 2015.
27. Sullivan MP, McHale KJ, Parvizi J, Mehta S. Nanotechnology: current concepts in orthopaedic surgery and future directions. Bone Joint J. 2014;96-B(5):569-573.
28. Pleshko N, Grande DA, Myers KR. Nanotechnology in orthopaedics. J Am Acad Orthop Surg. 2012;20(1):60-62.
Glenoid Damage From Articular Protrusion of Metal Suture Anchor After Arthroscopic Rotator Cuff Repair
Complications with the use of anchor screws in shoulder surgery have been well-documented1,2 and can be divided into 3 categories: insertion (eg, incomplete seating, inadequate insertion, and migration), biologic (eg, large tacks producing synovitis and bone reaction), and, less commonly, mechanical (eg, intra- and extra-articular bone pull-out with migration) complications.
Prominent hardware, including suture anchors, as a cause of arthritis and joint damage has been well-documented in shoulder surgery.3,4 For example, anchors placed on the glenoid rim have been implicated in severe cartilage loss if they protrude above the level of the glenoid rim.3 However, to the authors’ knowledge, prominent anchor placement after rotator cuff repair has not been reported as a cause of arthritis unless the anchor dislodges into the glenohumeral joint. The authors present a case in which a suture anchor used for rotator cuff repair protruded through the humeral head, resulting in glenohumeral arthritis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 61-year-old woman presented with complaints of persistent right shoulder pain for 5 months after a fall from a bicycle. She had taken nonsteroidal anti-inflammatory medication without pain relief. On presentation, she had no atrophy or deformity, was neurologically intact for sensation and reflexes, and had full range of motion (ROM) but a painful arc. She had tenderness over the greater tuberosity and positive Neer and Hawkins-Kennedy impingement signs. She had pain but no weakness to resisted abduction or to resisted external rotation with the arms at the sides.
Preoperative conventional radiographs of the shoulder were normal. A gadolinium-enhanced magnetic resonance arthrogram showed a high-grade articular partial tear of the supraspinatus, which was judged to be at least two-thirds of the tendon width. Because nonoperative methods had failed, the patient elected operative intervention for this tear.
Diagnostic arthroscopy (with the patient in a lateral decubitus position) showed a normal joint except for a high-grade, 8×8-mm, greater than 6 mm deep, partial tear of the articular side of the supraspinatus tendon. The subacromial space had moderate to severe bursal tissue inflammation but no full-thickness component to the rotator cuff tear. A bursectomy, coracoacromial ligament release, and partial anterolateral acromioplasty were performed.
A transtendinous technique was used to repair this high-grade tear. For an anatomically rigid repair, we used 3 suture anchors with a straight configuration because each metal anchor has only 1 suture. According to the standard arthroscopic transtendinous repair technique, the suture anchors were placed through the rotator cuff tendon (at the lateral articular margin at the medial extent of the footprint) after localization of the angle with a spinal needle. A shuttle relay was used to pass the sutures, and the knot was pulled into the subacromial space, cinching the rotator cuff on top of the suture anchors and reestablishing the contact of the tendon to the footprint. We used two 2.4-mm FASTak suture anchors (Arthrex, Naples, Florida) and one 3.5-mm Corkscrew suture anchor (Arthrex). This process was repeated for the remaining suture limbs. The placement of the suture anchors adequately reduced the articular part of the cuff to the footprint.
After surgery, the patient had no complications, and radiographs taken the next day suggested no abnormalities (Figure 1A). The shoulder was immobilized for 4 weeks after surgery, and passive, gentle ROM exercise was supervised by a physical therapist twice a week during this period. After the first 4 weeks, an active ROM program was begun. However, shortly after initiating motion in the shoulder, the patient complained of a recurrence of pain that she described as a sharp and grinding sensation.
The patient was reevaluated 8 weeks after surgery. Her pain was worsening, and she was having difficulty regaining ROM. Conventional radiographs showed the tip of the metal anchor protruding through the articular cartilage of the humeral head (Figure 1B). The patient was informed of the findings, and immediate surgery was performed to remove the anchor.
Arthroscopic examination showed extensive damage to the glenoid cartilage (Figure 1C) and an intra-articularly intact rotator cuff repair. The cartilage damage was located in the posterior and inferior half of the glenoid, which is related to the forward flexion of the arm; the depth of the cartilage defect was approximately 2 mm. Under the image intensifier, an empty suture anchor driver was inserted into the previous screw insertion hole, and the anchor was screwed back out and removed.
After surgery, the patient’s arm was placed in a sling, and an ROM program began 4 weeks later. The sensation of grinding was eliminated, and her pain gradually improved. Three years after surgery, she had no pain, no weakness, and full ROM without limitations (Figure 2).
Discussion
Protrusion and migration of suture anchors in shoulder surgery has been documented extensively.3,4 Zuckerman and Matsen4 divided these complications into 4 groups: (1) incorrect placement, (2) migration after placement, (3) loosening, and (4) device breakage. These complications may be frequently related to surgical technique, and all these studies describe backward migration of the anchor out of the drill hole. In the current case, the anchor tip penetrated the articular surface of the humeral head, not because of anchor migration but because the anchor was inserted too far. To the authors’ knowledge, there is only 1 reported case of anchor protrusion through the humeral head; it involved a different type of anchor insertion system.5 In that case, there was only mild cartilage damage to the glenoid, and the patient recovered after removal of the anchors.
Several factors contributed to the improper insertion of the anchor in the current patient. First, repairing a high-grade articular side defect or partial articular supraspinatus tendon avulsion lesion can be technically challenging because rotator cuff tissue obscures the view when inserting the anchor. Second, the anchor was inserted too medially on the greater tuberosity, which made the distance from the tuberosity to the joint shorter. Wong and colleagues5 performed an analysis of the angle of insertion that would be safe using a PEEK PushLock SP system (Arthrex), but they emphasized that the angle depends on the configuration of the particular insertion system. The current case also shows that the surgeon should be cognizant of the fact that penetration of the humeral head by the anchor can occur if the surgeon is unaware of the distance from the anchor to the laser line on the insertion device or of the distance from the tuberosity to the articular surface of the humeral head.
The current case also shows that the type of anchor and delivery system may contribute to this complication. Double-loaded suture anchors can decrease the number of anchors needed for secure fixation. Bioabsorbable anchors can be used for this purpose, but they may be technically more difficult to use for repairing partial tears of the rotator cuff. Better visualization of the laser line on the anchor may be facilitated by using a probe from an anterior portal to hold the cuff up while the anchor is inserted.
This case has shown the importance of obtaining postoperative radiographic studies in patients who have metal anchors placed during shoulder surgery, especially if they complain of continued pain, new pain, crepitus, or grinding. When conventional radiography is insufficient for locating the anchor or its proximity to the joint line, computed tomography can be helpful.1
Conclusion
Removing failed suture anchors can be challenging, especially when they protrude into the joint on the humeral side.1,6 The best way to prevent this complication is through careful technique. The anchors should not be inserted beyond the depth of the laser line on the anchors, and every attempt should be made to make sure the laser line is visible at the time of anchor insertion. Postoperative radiographs should be considered for patients with metal anchors in the shoulder, especially if the patient continues to have symptoms or develops new symptoms in the shoulder after surgery.
1. Park HB, Keyurapan E, Gill HS, Selhi HS, McFarland EG. Suture anchors and tacks for shoulder surgery. Part II: The prevention and treatment of complications. Am J Sports Med. 2006;34(1):136-144.
2. McFarland EG, Park HB, Keyurapan E, Gill HS, Selhi HS. Suture anchors and tacks for shoulder surgery. Part I: Biology and biomechanics. Am J Sports Med. 2005;33(12):1918-1923.
3. Rhee YG, Lee DH, Chun IH, Bae SC. Glenohumeral arthropathy after arthroscopic anterior shoulder stabilization. Arthroscopy. 2004;20(4):402-406.
4. Zuckerman JD, Matsen FA III. Complications about the glenohumeral joint related to the use of screws and staples. J Bone Joint Surg Am. 1984;66(2):175-180.
5. Wong AS, Kokkalis ZT, Schmidt CC. Proper insertion angle is essential to prevent intra-articular protrusion of a knotless suture anchor in shoulder rotator cuff repair. Arthroscopy. 2010;26(2):286-290.
6. Grutter PW, McFarland EG, Zikria BA, Dai Z, Petersen SA. Techniques for suture anchor removal in shoulder surgery. Am J Sports Med. 2010;38(8):1706-1710.
Complications with the use of anchor screws in shoulder surgery have been well-documented1,2 and can be divided into 3 categories: insertion (eg, incomplete seating, inadequate insertion, and migration), biologic (eg, large tacks producing synovitis and bone reaction), and, less commonly, mechanical (eg, intra- and extra-articular bone pull-out with migration) complications.
Prominent hardware, including suture anchors, as a cause of arthritis and joint damage has been well-documented in shoulder surgery.3,4 For example, anchors placed on the glenoid rim have been implicated in severe cartilage loss if they protrude above the level of the glenoid rim.3 However, to the authors’ knowledge, prominent anchor placement after rotator cuff repair has not been reported as a cause of arthritis unless the anchor dislodges into the glenohumeral joint. The authors present a case in which a suture anchor used for rotator cuff repair protruded through the humeral head, resulting in glenohumeral arthritis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 61-year-old woman presented with complaints of persistent right shoulder pain for 5 months after a fall from a bicycle. She had taken nonsteroidal anti-inflammatory medication without pain relief. On presentation, she had no atrophy or deformity, was neurologically intact for sensation and reflexes, and had full range of motion (ROM) but a painful arc. She had tenderness over the greater tuberosity and positive Neer and Hawkins-Kennedy impingement signs. She had pain but no weakness to resisted abduction or to resisted external rotation with the arms at the sides.
Preoperative conventional radiographs of the shoulder were normal. A gadolinium-enhanced magnetic resonance arthrogram showed a high-grade articular partial tear of the supraspinatus, which was judged to be at least two-thirds of the tendon width. Because nonoperative methods had failed, the patient elected operative intervention for this tear.
Diagnostic arthroscopy (with the patient in a lateral decubitus position) showed a normal joint except for a high-grade, 8×8-mm, greater than 6 mm deep, partial tear of the articular side of the supraspinatus tendon. The subacromial space had moderate to severe bursal tissue inflammation but no full-thickness component to the rotator cuff tear. A bursectomy, coracoacromial ligament release, and partial anterolateral acromioplasty were performed.
A transtendinous technique was used to repair this high-grade tear. For an anatomically rigid repair, we used 3 suture anchors with a straight configuration because each metal anchor has only 1 suture. According to the standard arthroscopic transtendinous repair technique, the suture anchors were placed through the rotator cuff tendon (at the lateral articular margin at the medial extent of the footprint) after localization of the angle with a spinal needle. A shuttle relay was used to pass the sutures, and the knot was pulled into the subacromial space, cinching the rotator cuff on top of the suture anchors and reestablishing the contact of the tendon to the footprint. We used two 2.4-mm FASTak suture anchors (Arthrex, Naples, Florida) and one 3.5-mm Corkscrew suture anchor (Arthrex). This process was repeated for the remaining suture limbs. The placement of the suture anchors adequately reduced the articular part of the cuff to the footprint.
After surgery, the patient had no complications, and radiographs taken the next day suggested no abnormalities (Figure 1A). The shoulder was immobilized for 4 weeks after surgery, and passive, gentle ROM exercise was supervised by a physical therapist twice a week during this period. After the first 4 weeks, an active ROM program was begun. However, shortly after initiating motion in the shoulder, the patient complained of a recurrence of pain that she described as a sharp and grinding sensation.
The patient was reevaluated 8 weeks after surgery. Her pain was worsening, and she was having difficulty regaining ROM. Conventional radiographs showed the tip of the metal anchor protruding through the articular cartilage of the humeral head (Figure 1B). The patient was informed of the findings, and immediate surgery was performed to remove the anchor.
Arthroscopic examination showed extensive damage to the glenoid cartilage (Figure 1C) and an intra-articularly intact rotator cuff repair. The cartilage damage was located in the posterior and inferior half of the glenoid, which is related to the forward flexion of the arm; the depth of the cartilage defect was approximately 2 mm. Under the image intensifier, an empty suture anchor driver was inserted into the previous screw insertion hole, and the anchor was screwed back out and removed.
After surgery, the patient’s arm was placed in a sling, and an ROM program began 4 weeks later. The sensation of grinding was eliminated, and her pain gradually improved. Three years after surgery, she had no pain, no weakness, and full ROM without limitations (Figure 2).
Discussion
Protrusion and migration of suture anchors in shoulder surgery has been documented extensively.3,4 Zuckerman and Matsen4 divided these complications into 4 groups: (1) incorrect placement, (2) migration after placement, (3) loosening, and (4) device breakage. These complications may be frequently related to surgical technique, and all these studies describe backward migration of the anchor out of the drill hole. In the current case, the anchor tip penetrated the articular surface of the humeral head, not because of anchor migration but because the anchor was inserted too far. To the authors’ knowledge, there is only 1 reported case of anchor protrusion through the humeral head; it involved a different type of anchor insertion system.5 In that case, there was only mild cartilage damage to the glenoid, and the patient recovered after removal of the anchors.
Several factors contributed to the improper insertion of the anchor in the current patient. First, repairing a high-grade articular side defect or partial articular supraspinatus tendon avulsion lesion can be technically challenging because rotator cuff tissue obscures the view when inserting the anchor. Second, the anchor was inserted too medially on the greater tuberosity, which made the distance from the tuberosity to the joint shorter. Wong and colleagues5 performed an analysis of the angle of insertion that would be safe using a PEEK PushLock SP system (Arthrex), but they emphasized that the angle depends on the configuration of the particular insertion system. The current case also shows that the surgeon should be cognizant of the fact that penetration of the humeral head by the anchor can occur if the surgeon is unaware of the distance from the anchor to the laser line on the insertion device or of the distance from the tuberosity to the articular surface of the humeral head.
The current case also shows that the type of anchor and delivery system may contribute to this complication. Double-loaded suture anchors can decrease the number of anchors needed for secure fixation. Bioabsorbable anchors can be used for this purpose, but they may be technically more difficult to use for repairing partial tears of the rotator cuff. Better visualization of the laser line on the anchor may be facilitated by using a probe from an anterior portal to hold the cuff up while the anchor is inserted.
This case has shown the importance of obtaining postoperative radiographic studies in patients who have metal anchors placed during shoulder surgery, especially if they complain of continued pain, new pain, crepitus, or grinding. When conventional radiography is insufficient for locating the anchor or its proximity to the joint line, computed tomography can be helpful.1
Conclusion
Removing failed suture anchors can be challenging, especially when they protrude into the joint on the humeral side.1,6 The best way to prevent this complication is through careful technique. The anchors should not be inserted beyond the depth of the laser line on the anchors, and every attempt should be made to make sure the laser line is visible at the time of anchor insertion. Postoperative radiographs should be considered for patients with metal anchors in the shoulder, especially if the patient continues to have symptoms or develops new symptoms in the shoulder after surgery.
Complications with the use of anchor screws in shoulder surgery have been well-documented1,2 and can be divided into 3 categories: insertion (eg, incomplete seating, inadequate insertion, and migration), biologic (eg, large tacks producing synovitis and bone reaction), and, less commonly, mechanical (eg, intra- and extra-articular bone pull-out with migration) complications.
Prominent hardware, including suture anchors, as a cause of arthritis and joint damage has been well-documented in shoulder surgery.3,4 For example, anchors placed on the glenoid rim have been implicated in severe cartilage loss if they protrude above the level of the glenoid rim.3 However, to the authors’ knowledge, prominent anchor placement after rotator cuff repair has not been reported as a cause of arthritis unless the anchor dislodges into the glenohumeral joint. The authors present a case in which a suture anchor used for rotator cuff repair protruded through the humeral head, resulting in glenohumeral arthritis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 61-year-old woman presented with complaints of persistent right shoulder pain for 5 months after a fall from a bicycle. She had taken nonsteroidal anti-inflammatory medication without pain relief. On presentation, she had no atrophy or deformity, was neurologically intact for sensation and reflexes, and had full range of motion (ROM) but a painful arc. She had tenderness over the greater tuberosity and positive Neer and Hawkins-Kennedy impingement signs. She had pain but no weakness to resisted abduction or to resisted external rotation with the arms at the sides.
Preoperative conventional radiographs of the shoulder were normal. A gadolinium-enhanced magnetic resonance arthrogram showed a high-grade articular partial tear of the supraspinatus, which was judged to be at least two-thirds of the tendon width. Because nonoperative methods had failed, the patient elected operative intervention for this tear.
Diagnostic arthroscopy (with the patient in a lateral decubitus position) showed a normal joint except for a high-grade, 8×8-mm, greater than 6 mm deep, partial tear of the articular side of the supraspinatus tendon. The subacromial space had moderate to severe bursal tissue inflammation but no full-thickness component to the rotator cuff tear. A bursectomy, coracoacromial ligament release, and partial anterolateral acromioplasty were performed.
A transtendinous technique was used to repair this high-grade tear. For an anatomically rigid repair, we used 3 suture anchors with a straight configuration because each metal anchor has only 1 suture. According to the standard arthroscopic transtendinous repair technique, the suture anchors were placed through the rotator cuff tendon (at the lateral articular margin at the medial extent of the footprint) after localization of the angle with a spinal needle. A shuttle relay was used to pass the sutures, and the knot was pulled into the subacromial space, cinching the rotator cuff on top of the suture anchors and reestablishing the contact of the tendon to the footprint. We used two 2.4-mm FASTak suture anchors (Arthrex, Naples, Florida) and one 3.5-mm Corkscrew suture anchor (Arthrex). This process was repeated for the remaining suture limbs. The placement of the suture anchors adequately reduced the articular part of the cuff to the footprint.
After surgery, the patient had no complications, and radiographs taken the next day suggested no abnormalities (Figure 1A). The shoulder was immobilized for 4 weeks after surgery, and passive, gentle ROM exercise was supervised by a physical therapist twice a week during this period. After the first 4 weeks, an active ROM program was begun. However, shortly after initiating motion in the shoulder, the patient complained of a recurrence of pain that she described as a sharp and grinding sensation.
The patient was reevaluated 8 weeks after surgery. Her pain was worsening, and she was having difficulty regaining ROM. Conventional radiographs showed the tip of the metal anchor protruding through the articular cartilage of the humeral head (Figure 1B). The patient was informed of the findings, and immediate surgery was performed to remove the anchor.
Arthroscopic examination showed extensive damage to the glenoid cartilage (Figure 1C) and an intra-articularly intact rotator cuff repair. The cartilage damage was located in the posterior and inferior half of the glenoid, which is related to the forward flexion of the arm; the depth of the cartilage defect was approximately 2 mm. Under the image intensifier, an empty suture anchor driver was inserted into the previous screw insertion hole, and the anchor was screwed back out and removed.
After surgery, the patient’s arm was placed in a sling, and an ROM program began 4 weeks later. The sensation of grinding was eliminated, and her pain gradually improved. Three years after surgery, she had no pain, no weakness, and full ROM without limitations (Figure 2).
Discussion
Protrusion and migration of suture anchors in shoulder surgery has been documented extensively.3,4 Zuckerman and Matsen4 divided these complications into 4 groups: (1) incorrect placement, (2) migration after placement, (3) loosening, and (4) device breakage. These complications may be frequently related to surgical technique, and all these studies describe backward migration of the anchor out of the drill hole. In the current case, the anchor tip penetrated the articular surface of the humeral head, not because of anchor migration but because the anchor was inserted too far. To the authors’ knowledge, there is only 1 reported case of anchor protrusion through the humeral head; it involved a different type of anchor insertion system.5 In that case, there was only mild cartilage damage to the glenoid, and the patient recovered after removal of the anchors.
Several factors contributed to the improper insertion of the anchor in the current patient. First, repairing a high-grade articular side defect or partial articular supraspinatus tendon avulsion lesion can be technically challenging because rotator cuff tissue obscures the view when inserting the anchor. Second, the anchor was inserted too medially on the greater tuberosity, which made the distance from the tuberosity to the joint shorter. Wong and colleagues5 performed an analysis of the angle of insertion that would be safe using a PEEK PushLock SP system (Arthrex), but they emphasized that the angle depends on the configuration of the particular insertion system. The current case also shows that the surgeon should be cognizant of the fact that penetration of the humeral head by the anchor can occur if the surgeon is unaware of the distance from the anchor to the laser line on the insertion device or of the distance from the tuberosity to the articular surface of the humeral head.
The current case also shows that the type of anchor and delivery system may contribute to this complication. Double-loaded suture anchors can decrease the number of anchors needed for secure fixation. Bioabsorbable anchors can be used for this purpose, but they may be technically more difficult to use for repairing partial tears of the rotator cuff. Better visualization of the laser line on the anchor may be facilitated by using a probe from an anterior portal to hold the cuff up while the anchor is inserted.
This case has shown the importance of obtaining postoperative radiographic studies in patients who have metal anchors placed during shoulder surgery, especially if they complain of continued pain, new pain, crepitus, or grinding. When conventional radiography is insufficient for locating the anchor or its proximity to the joint line, computed tomography can be helpful.1
Conclusion
Removing failed suture anchors can be challenging, especially when they protrude into the joint on the humeral side.1,6 The best way to prevent this complication is through careful technique. The anchors should not be inserted beyond the depth of the laser line on the anchors, and every attempt should be made to make sure the laser line is visible at the time of anchor insertion. Postoperative radiographs should be considered for patients with metal anchors in the shoulder, especially if the patient continues to have symptoms or develops new symptoms in the shoulder after surgery.
1. Park HB, Keyurapan E, Gill HS, Selhi HS, McFarland EG. Suture anchors and tacks for shoulder surgery. Part II: The prevention and treatment of complications. Am J Sports Med. 2006;34(1):136-144.
2. McFarland EG, Park HB, Keyurapan E, Gill HS, Selhi HS. Suture anchors and tacks for shoulder surgery. Part I: Biology and biomechanics. Am J Sports Med. 2005;33(12):1918-1923.
3. Rhee YG, Lee DH, Chun IH, Bae SC. Glenohumeral arthropathy after arthroscopic anterior shoulder stabilization. Arthroscopy. 2004;20(4):402-406.
4. Zuckerman JD, Matsen FA III. Complications about the glenohumeral joint related to the use of screws and staples. J Bone Joint Surg Am. 1984;66(2):175-180.
5. Wong AS, Kokkalis ZT, Schmidt CC. Proper insertion angle is essential to prevent intra-articular protrusion of a knotless suture anchor in shoulder rotator cuff repair. Arthroscopy. 2010;26(2):286-290.
6. Grutter PW, McFarland EG, Zikria BA, Dai Z, Petersen SA. Techniques for suture anchor removal in shoulder surgery. Am J Sports Med. 2010;38(8):1706-1710.
1. Park HB, Keyurapan E, Gill HS, Selhi HS, McFarland EG. Suture anchors and tacks for shoulder surgery. Part II: The prevention and treatment of complications. Am J Sports Med. 2006;34(1):136-144.
2. McFarland EG, Park HB, Keyurapan E, Gill HS, Selhi HS. Suture anchors and tacks for shoulder surgery. Part I: Biology and biomechanics. Am J Sports Med. 2005;33(12):1918-1923.
3. Rhee YG, Lee DH, Chun IH, Bae SC. Glenohumeral arthropathy after arthroscopic anterior shoulder stabilization. Arthroscopy. 2004;20(4):402-406.
4. Zuckerman JD, Matsen FA III. Complications about the glenohumeral joint related to the use of screws and staples. J Bone Joint Surg Am. 1984;66(2):175-180.
5. Wong AS, Kokkalis ZT, Schmidt CC. Proper insertion angle is essential to prevent intra-articular protrusion of a knotless suture anchor in shoulder rotator cuff repair. Arthroscopy. 2010;26(2):286-290.
6. Grutter PW, McFarland EG, Zikria BA, Dai Z, Petersen SA. Techniques for suture anchor removal in shoulder surgery. Am J Sports Med. 2010;38(8):1706-1710.
Analysis of Intermediate Outcomes of Glenoid Bone Grafting in Revision Shoulder Arthroplasty
The Applications of Biologics in Orthopedic Surgery
As orthopedic surgeons, we have done a great job continually trying to improve the outcomes of our patients. During the first decade of the 21st century, many of these advances centered on strengthening the biomechanics of constructs used to repair patients’ pathologies. Trauma surgeons incorporated minimally invasive osteosynthesis with locked plates; shoulder surgeons began using double-row and transosseous-equivalent rotator cuff repairs. As a result of these shifts in treatment methods, healing rates and outcomes have improved. Unfortunately, to take rotator cuff repair as an example, healing rates have still not achieved 100%. To reach this goal in the future, biologic manipulation of the healing milieu will play a critical role.
This issue of The American Journal of Orthopedics features an article on the “Analysis of Intermediate Outcomes of Glenoid Bone Grafting in Revision Shoulder Arthroplasty” by Dr. Schubkegel and colleagues. While not as cutting edge or in vogue as growth factors or stem cells, bone graft is one of the original biologics used by orthopedic surgeons. The authors review the midterm results of glenoid bone grafting secondary to failed total shoulder arthroplasty and find that bone grafting resulted in good functional outcomes. Studies such as this one highlight the important role that biologics play, particularly in challenging or revision cases.
Platelet-rich plasma (PRP) is another biologic that is presently available for use. Reviewing its use as it pertains to orthopedics highlights both the potential benefits
as well as the difficulties associated with incorporating biologics into everyday practice. In 2006, Mishra and colleagues1 published one of the first studies that looked at the potential benefits of using PRP to treat lateral epicondylitis. While, from a purist’s standpoint, it wasn’t the best-designed study, it did provide cause for optimism with regard to a novel treatment option for an age-old problem. Since that time, hundreds of studies have been done on PRP looking at its potential treatment uses in everything from tennis elbow to rotator cuff repairs.
Study designs have improved, and with that, so have our indications for using PRP. Interestingly though, the more we study PRP (and other exogenous growth factors), it almost seems as if more questions are raised than answered. For instance, preparing PRP from a given patient will result in different concentrations of the PRP depending on what time of the day the patient’s blood is drawn. What is the ideal time to prepare the PRP? Additionally, PRP prepared using different companies’ systems results in different concentrations of growth factors. So, not only is a given patient’s PRP different at different times of day, but these differences get magnified by using different preparation systems.
One of the main issues with tendon healing is that the tissue heals via reactive scar formation instead of truly regenerating new tendon. In this scenario, it is possible that adding PRP or other growth factors to the repair construct may only increase scar formation. Along these lines, newer work is focusing on cellular solutions to healing problems. Stem cells, which are undifferentiated, unspecialized cells, have shown potential to improve healing when added to injury/repair sites. Thus far, unfortunately, there is very little clinical data pertaining to their use in orthopedic surgery. Compounding this problem are the US Food and Drug Administration’s regulations on manipulating stem cells.
In the future, it is likely that growth factors, cytokines, PRP, and cellular approaches will be used to enhance healing. For now, a significant amount of preclinical work is being done to figure out the most advantageous ways to use such adjuvants. This is an extremely exciting field with ample opportunities to
answer well-designed research questions. Future issues of this journal will likely highlight such studies. ◾
Reference
1. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered
platelet-rich plasma. Am J Sports Med. 2006;34(11):1774-1778.
As orthopedic surgeons, we have done a great job continually trying to improve the outcomes of our patients. During the first decade of the 21st century, many of these advances centered on strengthening the biomechanics of constructs used to repair patients’ pathologies. Trauma surgeons incorporated minimally invasive osteosynthesis with locked plates; shoulder surgeons began using double-row and transosseous-equivalent rotator cuff repairs. As a result of these shifts in treatment methods, healing rates and outcomes have improved. Unfortunately, to take rotator cuff repair as an example, healing rates have still not achieved 100%. To reach this goal in the future, biologic manipulation of the healing milieu will play a critical role.
This issue of The American Journal of Orthopedics features an article on the “Analysis of Intermediate Outcomes of Glenoid Bone Grafting in Revision Shoulder Arthroplasty” by Dr. Schubkegel and colleagues. While not as cutting edge or in vogue as growth factors or stem cells, bone graft is one of the original biologics used by orthopedic surgeons. The authors review the midterm results of glenoid bone grafting secondary to failed total shoulder arthroplasty and find that bone grafting resulted in good functional outcomes. Studies such as this one highlight the important role that biologics play, particularly in challenging or revision cases.
Platelet-rich plasma (PRP) is another biologic that is presently available for use. Reviewing its use as it pertains to orthopedics highlights both the potential benefits
as well as the difficulties associated with incorporating biologics into everyday practice. In 2006, Mishra and colleagues1 published one of the first studies that looked at the potential benefits of using PRP to treat lateral epicondylitis. While, from a purist’s standpoint, it wasn’t the best-designed study, it did provide cause for optimism with regard to a novel treatment option for an age-old problem. Since that time, hundreds of studies have been done on PRP looking at its potential treatment uses in everything from tennis elbow to rotator cuff repairs.
Study designs have improved, and with that, so have our indications for using PRP. Interestingly though, the more we study PRP (and other exogenous growth factors), it almost seems as if more questions are raised than answered. For instance, preparing PRP from a given patient will result in different concentrations of the PRP depending on what time of the day the patient’s blood is drawn. What is the ideal time to prepare the PRP? Additionally, PRP prepared using different companies’ systems results in different concentrations of growth factors. So, not only is a given patient’s PRP different at different times of day, but these differences get magnified by using different preparation systems.
One of the main issues with tendon healing is that the tissue heals via reactive scar formation instead of truly regenerating new tendon. In this scenario, it is possible that adding PRP or other growth factors to the repair construct may only increase scar formation. Along these lines, newer work is focusing on cellular solutions to healing problems. Stem cells, which are undifferentiated, unspecialized cells, have shown potential to improve healing when added to injury/repair sites. Thus far, unfortunately, there is very little clinical data pertaining to their use in orthopedic surgery. Compounding this problem are the US Food and Drug Administration’s regulations on manipulating stem cells.
In the future, it is likely that growth factors, cytokines, PRP, and cellular approaches will be used to enhance healing. For now, a significant amount of preclinical work is being done to figure out the most advantageous ways to use such adjuvants. This is an extremely exciting field with ample opportunities to
answer well-designed research questions. Future issues of this journal will likely highlight such studies. ◾
Reference
1. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered
platelet-rich plasma. Am J Sports Med. 2006;34(11):1774-1778.
As orthopedic surgeons, we have done a great job continually trying to improve the outcomes of our patients. During the first decade of the 21st century, many of these advances centered on strengthening the biomechanics of constructs used to repair patients’ pathologies. Trauma surgeons incorporated minimally invasive osteosynthesis with locked plates; shoulder surgeons began using double-row and transosseous-equivalent rotator cuff repairs. As a result of these shifts in treatment methods, healing rates and outcomes have improved. Unfortunately, to take rotator cuff repair as an example, healing rates have still not achieved 100%. To reach this goal in the future, biologic manipulation of the healing milieu will play a critical role.
This issue of The American Journal of Orthopedics features an article on the “Analysis of Intermediate Outcomes of Glenoid Bone Grafting in Revision Shoulder Arthroplasty” by Dr. Schubkegel and colleagues. While not as cutting edge or in vogue as growth factors or stem cells, bone graft is one of the original biologics used by orthopedic surgeons. The authors review the midterm results of glenoid bone grafting secondary to failed total shoulder arthroplasty and find that bone grafting resulted in good functional outcomes. Studies such as this one highlight the important role that biologics play, particularly in challenging or revision cases.
Platelet-rich plasma (PRP) is another biologic that is presently available for use. Reviewing its use as it pertains to orthopedics highlights both the potential benefits
as well as the difficulties associated with incorporating biologics into everyday practice. In 2006, Mishra and colleagues1 published one of the first studies that looked at the potential benefits of using PRP to treat lateral epicondylitis. While, from a purist’s standpoint, it wasn’t the best-designed study, it did provide cause for optimism with regard to a novel treatment option for an age-old problem. Since that time, hundreds of studies have been done on PRP looking at its potential treatment uses in everything from tennis elbow to rotator cuff repairs.
Study designs have improved, and with that, so have our indications for using PRP. Interestingly though, the more we study PRP (and other exogenous growth factors), it almost seems as if more questions are raised than answered. For instance, preparing PRP from a given patient will result in different concentrations of the PRP depending on what time of the day the patient’s blood is drawn. What is the ideal time to prepare the PRP? Additionally, PRP prepared using different companies’ systems results in different concentrations of growth factors. So, not only is a given patient’s PRP different at different times of day, but these differences get magnified by using different preparation systems.
One of the main issues with tendon healing is that the tissue heals via reactive scar formation instead of truly regenerating new tendon. In this scenario, it is possible that adding PRP or other growth factors to the repair construct may only increase scar formation. Along these lines, newer work is focusing on cellular solutions to healing problems. Stem cells, which are undifferentiated, unspecialized cells, have shown potential to improve healing when added to injury/repair sites. Thus far, unfortunately, there is very little clinical data pertaining to their use in orthopedic surgery. Compounding this problem are the US Food and Drug Administration’s regulations on manipulating stem cells.
In the future, it is likely that growth factors, cytokines, PRP, and cellular approaches will be used to enhance healing. For now, a significant amount of preclinical work is being done to figure out the most advantageous ways to use such adjuvants. This is an extremely exciting field with ample opportunities to
answer well-designed research questions. Future issues of this journal will likely highlight such studies. ◾
Reference
1. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered
platelet-rich plasma. Am J Sports Med. 2006;34(11):1774-1778.
Cost Estimates of Biologic Implants Among Orthopedic Surgeons
The Biologic Holy Grail: Will It Ever Be Found?
The problem is not new. A routine arthroscopic knee surgery is performed and an isolated Grade 4 cartilage is seen. So what is a surgeon to do? Certainly one could easily perform a microfracture but is the patient going to accept the often-prescribed 6 weeks of limited weight-bearing? Other options do exist, but once again, not all patients are accepting of a more invasive procedure with a prolonged rehabilitation period.
We thought we had an answer in the mid 1990s with the popularization of autologous chondrocyte transplantations (Carticel; Genzyme Corp, a Sanofi company, Cambridge, Massachusetts). There was a sense of excitement and theorthopedic community went biopsy crazy. Mandatory training was required, initially in Gothenburg, Sweden, and a new dawn of cartilage restoration was born. This excitement spilled over into other forms of cartilage treatments including Osteochondral Autograft Transfer Systems (OATS), with improved instrumentation and more options for the treatment of these cartilage lesions. This time period was the Renaissance Period of cartilage restoration: a period of excitement that led to the establishment of the International Cartilage Repair Society.
But as cartilage restoration became more popular, so did the amount of obstacles surgeons would encounter to be able to perform these procedures. Because of a paucity of literature describing the efficacy of these procedures, insurance companies were quick to describe the procedures as experimental, often refusing to approve the procedures or denying claims once performed.
While good results were eventually reported, some limitations remained. The procedure was expensive, two procedures, including an open arthrotomy was required, rehabilitation was slow and a high reoperation rate was reported. In addition, while this procedure is still being performed, it falls short of being the ultimate answer to isolated cartilage lesions of the knee.
What is the ideal method of cartilage repair? In a perfect world, all patients would be consented to routine arthroscopy and cartilage procedures as indicated (Figure 1). If an isolated lesion is seen, then the method of repair should be not only efficacious but should be performed arthroscopically, an off the shelf option, that can be performed at the same time as the diagnostic arthroscopy.
Over the last several years, we have seen a resurgence in cartilage restoration biologic options. DeNovo juvenile cartilage (Zimmer Inc, Warsaw, Indiana) has been introduced but does have its limitations. It is juvenile allograft cartilage that is prepared with a fibrin glue and placed currently as a second procedure. The lesion is seen at the time of diagnostic arthroscopy, lesion is sized, and how much of the cartilage to order is determined. Limits include not only the cost, but also the requirement of a second procedure, an arthrotomy, and lets not forget the need to bone graft the defect bed if significant subchondral bone loss has occurred.
Another recent advancement is the use of allograft cartilage plugs, Chondrofix, (Zimmer Inc) (Figures 2A, 2B). These are human allograft osteochondral plugs, irradiated for safety, have a long shelf life, and can be available as needed. Due to the radiation, the cartilage plugs may be disease-free, have been FDA approved, but there is a lack of long-term studies not only demonstrating efficacy but also long-term durability. Perhaps we are approaching the Holy Grail with biologic products such as this, but long-term acceptance will not occur until proper long-term studies are performed. Cost will remain an issue as well, since it is quite easy to place 3 to 4 plugs at one sitting and approach implant costs as high as a revision knee implant (Figure 3).
I am sad to say that the Holy Grail for biologic restoration of isolated cartilage lesions has yet to be found. We still do not have the perfect method for cartilage restoration at this time. While new attempts to restore cartilage remain in the pipeline, we must move away from pure animal studies, case reports, white papers, and small surgeon experience. Randomized controlled studies are needed to test these biologic advances, and finally find the ideal treatment for these isolated cartilage defects. We owe it to our patients to finally find the ideal treatment for these cartilage lesions.
Dr. Cushner is Editorial Review Board member of the journal; Chief of Orthopedics, Southside Hospital, Bay Shore, New York; and Director, Insall Scott Kelly, New York, New York.
Author’s Disclosure Statement: The author wishes to report that he will be a Speaker Bureau for Zimmer, Inc.
Am J Orthop. 2013;42(5):206-207. Copyright Frontline Medical Communications Inc. 2013. All rights reserved.
The problem is not new. A routine arthroscopic knee surgery is performed and an isolated Grade 4 cartilage is seen. So what is a surgeon to do? Certainly one could easily perform a microfracture but is the patient going to accept the often-prescribed 6 weeks of limited weight-bearing? Other options do exist, but once again, not all patients are accepting of a more invasive procedure with a prolonged rehabilitation period.
We thought we had an answer in the mid 1990s with the popularization of autologous chondrocyte transplantations (Carticel; Genzyme Corp, a Sanofi company, Cambridge, Massachusetts). There was a sense of excitement and theorthopedic community went biopsy crazy. Mandatory training was required, initially in Gothenburg, Sweden, and a new dawn of cartilage restoration was born. This excitement spilled over into other forms of cartilage treatments including Osteochondral Autograft Transfer Systems (OATS), with improved instrumentation and more options for the treatment of these cartilage lesions. This time period was the Renaissance Period of cartilage restoration: a period of excitement that led to the establishment of the International Cartilage Repair Society.
But as cartilage restoration became more popular, so did the amount of obstacles surgeons would encounter to be able to perform these procedures. Because of a paucity of literature describing the efficacy of these procedures, insurance companies were quick to describe the procedures as experimental, often refusing to approve the procedures or denying claims once performed.
While good results were eventually reported, some limitations remained. The procedure was expensive, two procedures, including an open arthrotomy was required, rehabilitation was slow and a high reoperation rate was reported. In addition, while this procedure is still being performed, it falls short of being the ultimate answer to isolated cartilage lesions of the knee.
What is the ideal method of cartilage repair? In a perfect world, all patients would be consented to routine arthroscopy and cartilage procedures as indicated (Figure 1). If an isolated lesion is seen, then the method of repair should be not only efficacious but should be performed arthroscopically, an off the shelf option, that can be performed at the same time as the diagnostic arthroscopy.
Over the last several years, we have seen a resurgence in cartilage restoration biologic options. DeNovo juvenile cartilage (Zimmer Inc, Warsaw, Indiana) has been introduced but does have its limitations. It is juvenile allograft cartilage that is prepared with a fibrin glue and placed currently as a second procedure. The lesion is seen at the time of diagnostic arthroscopy, lesion is sized, and how much of the cartilage to order is determined. Limits include not only the cost, but also the requirement of a second procedure, an arthrotomy, and lets not forget the need to bone graft the defect bed if significant subchondral bone loss has occurred.
Another recent advancement is the use of allograft cartilage plugs, Chondrofix, (Zimmer Inc) (Figures 2A, 2B). These are human allograft osteochondral plugs, irradiated for safety, have a long shelf life, and can be available as needed. Due to the radiation, the cartilage plugs may be disease-free, have been FDA approved, but there is a lack of long-term studies not only demonstrating efficacy but also long-term durability. Perhaps we are approaching the Holy Grail with biologic products such as this, but long-term acceptance will not occur until proper long-term studies are performed. Cost will remain an issue as well, since it is quite easy to place 3 to 4 plugs at one sitting and approach implant costs as high as a revision knee implant (Figure 3).
I am sad to say that the Holy Grail for biologic restoration of isolated cartilage lesions has yet to be found. We still do not have the perfect method for cartilage restoration at this time. While new attempts to restore cartilage remain in the pipeline, we must move away from pure animal studies, case reports, white papers, and small surgeon experience. Randomized controlled studies are needed to test these biologic advances, and finally find the ideal treatment for these isolated cartilage defects. We owe it to our patients to finally find the ideal treatment for these cartilage lesions.
Dr. Cushner is Editorial Review Board member of the journal; Chief of Orthopedics, Southside Hospital, Bay Shore, New York; and Director, Insall Scott Kelly, New York, New York.
Author’s Disclosure Statement: The author wishes to report that he will be a Speaker Bureau for Zimmer, Inc.
Am J Orthop. 2013;42(5):206-207. Copyright Frontline Medical Communications Inc. 2013. All rights reserved.
The problem is not new. A routine arthroscopic knee surgery is performed and an isolated Grade 4 cartilage is seen. So what is a surgeon to do? Certainly one could easily perform a microfracture but is the patient going to accept the often-prescribed 6 weeks of limited weight-bearing? Other options do exist, but once again, not all patients are accepting of a more invasive procedure with a prolonged rehabilitation period.
We thought we had an answer in the mid 1990s with the popularization of autologous chondrocyte transplantations (Carticel; Genzyme Corp, a Sanofi company, Cambridge, Massachusetts). There was a sense of excitement and theorthopedic community went biopsy crazy. Mandatory training was required, initially in Gothenburg, Sweden, and a new dawn of cartilage restoration was born. This excitement spilled over into other forms of cartilage treatments including Osteochondral Autograft Transfer Systems (OATS), with improved instrumentation and more options for the treatment of these cartilage lesions. This time period was the Renaissance Period of cartilage restoration: a period of excitement that led to the establishment of the International Cartilage Repair Society.
But as cartilage restoration became more popular, so did the amount of obstacles surgeons would encounter to be able to perform these procedures. Because of a paucity of literature describing the efficacy of these procedures, insurance companies were quick to describe the procedures as experimental, often refusing to approve the procedures or denying claims once performed.
While good results were eventually reported, some limitations remained. The procedure was expensive, two procedures, including an open arthrotomy was required, rehabilitation was slow and a high reoperation rate was reported. In addition, while this procedure is still being performed, it falls short of being the ultimate answer to isolated cartilage lesions of the knee.
What is the ideal method of cartilage repair? In a perfect world, all patients would be consented to routine arthroscopy and cartilage procedures as indicated (Figure 1). If an isolated lesion is seen, then the method of repair should be not only efficacious but should be performed arthroscopically, an off the shelf option, that can be performed at the same time as the diagnostic arthroscopy.
Over the last several years, we have seen a resurgence in cartilage restoration biologic options. DeNovo juvenile cartilage (Zimmer Inc, Warsaw, Indiana) has been introduced but does have its limitations. It is juvenile allograft cartilage that is prepared with a fibrin glue and placed currently as a second procedure. The lesion is seen at the time of diagnostic arthroscopy, lesion is sized, and how much of the cartilage to order is determined. Limits include not only the cost, but also the requirement of a second procedure, an arthrotomy, and lets not forget the need to bone graft the defect bed if significant subchondral bone loss has occurred.
Another recent advancement is the use of allograft cartilage plugs, Chondrofix, (Zimmer Inc) (Figures 2A, 2B). These are human allograft osteochondral plugs, irradiated for safety, have a long shelf life, and can be available as needed. Due to the radiation, the cartilage plugs may be disease-free, have been FDA approved, but there is a lack of long-term studies not only demonstrating efficacy but also long-term durability. Perhaps we are approaching the Holy Grail with biologic products such as this, but long-term acceptance will not occur until proper long-term studies are performed. Cost will remain an issue as well, since it is quite easy to place 3 to 4 plugs at one sitting and approach implant costs as high as a revision knee implant (Figure 3).
I am sad to say that the Holy Grail for biologic restoration of isolated cartilage lesions has yet to be found. We still do not have the perfect method for cartilage restoration at this time. While new attempts to restore cartilage remain in the pipeline, we must move away from pure animal studies, case reports, white papers, and small surgeon experience. Randomized controlled studies are needed to test these biologic advances, and finally find the ideal treatment for these isolated cartilage defects. We owe it to our patients to finally find the ideal treatment for these cartilage lesions.
Dr. Cushner is Editorial Review Board member of the journal; Chief of Orthopedics, Southside Hospital, Bay Shore, New York; and Director, Insall Scott Kelly, New York, New York.
Author’s Disclosure Statement: The author wishes to report that he will be a Speaker Bureau for Zimmer, Inc.
Am J Orthop. 2013;42(5):206-207. Copyright Frontline Medical Communications Inc. 2013. All rights reserved.