User login
Oncologists’ income and satisfaction are up
Oncologists continue to rank above the middle range for all specialties in annual compensation for physicians, according to findings from the newly released Medscape Oncologist Compensation Report 2020.
The average earnings for oncologists who participated in the survey was $377,000, which was a 5% increase from the $359,000 reported for 2018.
Just over two-thirds (67%) of oncologists reported that they felt that they were fairly compensated, which is quite a jump from 53% last year.
In addition, oncologists appear to be very satisfied with their profession. Similar to last year’s findings, 84% said they would choose medicine again, and 96% said they would choose the specialty of oncology again.
Earning in top third of all specialties
The average annual earnings reported by oncologists put this specialty in eleventh place among 29 specialties. Orthopedic specialists remain at the head of the list, with estimated earnings of $511,000, followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000), according to Medscape’s compensation report, which included responses from 17,461 physicians in over 30 specialties.

At the bottom of the estimated earnings list were public health and preventive medicine doctors and pediatricians. For both specialties, the reported annual earnings was $232,000. Family medicine specialists were only marginally higher at $234,000.
Radiologists ($427,000), gastroenterologists ($419,000), and urologists ($417,000) all reported higher earnings than oncologists, whereas neurologists, at $280,000, rheumatologists, at $262,000, and internal medicine physicians, at $251,000, earned less.
The report also found that gender disparities in income persist, with male oncologists earning 17% more than their female colleagues. The gender gap in oncology is somewhat less than that seen for all specialties combined, in which men earned 31% more than women, similar to last year’s figure of 33%.
Male oncologists reported spending 38.8 hours per week seeing patients, compared with 34.9 hours reported by female oncologists. This could be a factor contributing to the gender pay disparity. Overall, the average amount of time seeing patients was 37.9 hours per week.
Frustrations with paperwork and denied claims
Surveyed oncologists cited some of the frustrations they are facing, such as spending nearly 17 hours a week on paperwork and administrative tasks. They reported that 16% of claims are denied or have to be resubmitted. As for the most challenging part of the job, oncologists (22%), similar to physicians overall (26%), found that having so many rules and regulations takes first place, followed by working with electronic health record systems (20%), difficulties getting fair reimbursement (19%), having to work long hours (12%), and dealing with difficult patients (8%). Few oncologists were concerned about lawsuits (4%), and 4% reported that there were no challenges.
Oncologists reported that the most rewarding part of their job was gratitude/relationships with patients (31%), followed by knowing that they are making the world a better place (27%). After that, oncologists agreed with statements about being very good at what they do/finding answers/diagnoses (22%), having pride in being a doctor (9%), and making good money at a job they like (8%).
Other key findings
Other key findings from the Medscape Oncologist Compensation Report 2020 included the following:
- Regarding payment models, 80% take insurance, 41% are in fee-for-service arrangements, and 18% are in accountable care organizations (21%). Only 3% are in direct primary care, and 1% are cash-only practices or have a concierge practice.
- 65% of oncologists state that they will continue taking new and current Medicare/Medicaid patients. None said that they would not take on new Medicare/Medicaid patients, and 35% remain undecided. These numbers differed from physicians overall; 73% of all physicians surveyed said they would continue taking new/current Medicare/Medicaid patients, 6% said that will not take on new Medicare patients, and 4% said they will not take new Medicaid patients. In addition, 3% and 2% said that they would stop treating some or all of their Medicare and Medicaid patients, respectively.
- About half (51%) of oncologists use nurse practitioners, about a third (34%) use physician assistants, and 37% use neither. This was about the same as physicians overall.
- A larger percentage of oncologists (38%) expect to participate in MIPS (merit-based incentive payment system), and only 8% expect to participate in APMs (alternative payment models). This was similar to the findings for physicians overall, with more than one-third (37%) expecting to participate in MIPS and 9% planning to take part in APMs.
Impact of COVID-19 pandemic
The Medscape compensation reports also gives a glimpse of the impact the COVID-19 pandemic is having on physician compensation.
Since the beginning of the pandemic, practices have reported a 55% decrease in revenue and a 60% drop in patient volume. Physician practices and hospitals have laid off or furloughed personnel and have cut pay, and 9% of practices have closed their doors, at least for the time being.
A total of 43,000 health care workers were laid off in March, the report notes.
The findings tie in with those reported elsewhere. For example, a survey conducted by the Medical Group Management Association, which was reported by Medscape Medical News, found that 97% of physician practices have experienced negative financial effects directly or indirectly related to COVID-19.
Specialties were hard hit, especially those that rely on elective procedures, such as dermatology and cardiology. Oncology care has also been disrupted. For example, a survey conducted by the American Cancer Society Cancer Action Network found that half of the cancer patients and survivors who responded reported changes, delays, or disruptions to the care they were receiving.
This article first appeared on Medscape.com.
Oncologists continue to rank above the middle range for all specialties in annual compensation for physicians, according to findings from the newly released Medscape Oncologist Compensation Report 2020.
The average earnings for oncologists who participated in the survey was $377,000, which was a 5% increase from the $359,000 reported for 2018.
Just over two-thirds (67%) of oncologists reported that they felt that they were fairly compensated, which is quite a jump from 53% last year.
In addition, oncologists appear to be very satisfied with their profession. Similar to last year’s findings, 84% said they would choose medicine again, and 96% said they would choose the specialty of oncology again.
Earning in top third of all specialties
The average annual earnings reported by oncologists put this specialty in eleventh place among 29 specialties. Orthopedic specialists remain at the head of the list, with estimated earnings of $511,000, followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000), according to Medscape’s compensation report, which included responses from 17,461 physicians in over 30 specialties.

At the bottom of the estimated earnings list were public health and preventive medicine doctors and pediatricians. For both specialties, the reported annual earnings was $232,000. Family medicine specialists were only marginally higher at $234,000.
Radiologists ($427,000), gastroenterologists ($419,000), and urologists ($417,000) all reported higher earnings than oncologists, whereas neurologists, at $280,000, rheumatologists, at $262,000, and internal medicine physicians, at $251,000, earned less.
The report also found that gender disparities in income persist, with male oncologists earning 17% more than their female colleagues. The gender gap in oncology is somewhat less than that seen for all specialties combined, in which men earned 31% more than women, similar to last year’s figure of 33%.
Male oncologists reported spending 38.8 hours per week seeing patients, compared with 34.9 hours reported by female oncologists. This could be a factor contributing to the gender pay disparity. Overall, the average amount of time seeing patients was 37.9 hours per week.
Frustrations with paperwork and denied claims
Surveyed oncologists cited some of the frustrations they are facing, such as spending nearly 17 hours a week on paperwork and administrative tasks. They reported that 16% of claims are denied or have to be resubmitted. As for the most challenging part of the job, oncologists (22%), similar to physicians overall (26%), found that having so many rules and regulations takes first place, followed by working with electronic health record systems (20%), difficulties getting fair reimbursement (19%), having to work long hours (12%), and dealing with difficult patients (8%). Few oncologists were concerned about lawsuits (4%), and 4% reported that there were no challenges.
Oncologists reported that the most rewarding part of their job was gratitude/relationships with patients (31%), followed by knowing that they are making the world a better place (27%). After that, oncologists agreed with statements about being very good at what they do/finding answers/diagnoses (22%), having pride in being a doctor (9%), and making good money at a job they like (8%).
Other key findings
Other key findings from the Medscape Oncologist Compensation Report 2020 included the following:
- Regarding payment models, 80% take insurance, 41% are in fee-for-service arrangements, and 18% are in accountable care organizations (21%). Only 3% are in direct primary care, and 1% are cash-only practices or have a concierge practice.
- 65% of oncologists state that they will continue taking new and current Medicare/Medicaid patients. None said that they would not take on new Medicare/Medicaid patients, and 35% remain undecided. These numbers differed from physicians overall; 73% of all physicians surveyed said they would continue taking new/current Medicare/Medicaid patients, 6% said that will not take on new Medicare patients, and 4% said they will not take new Medicaid patients. In addition, 3% and 2% said that they would stop treating some or all of their Medicare and Medicaid patients, respectively.
- About half (51%) of oncologists use nurse practitioners, about a third (34%) use physician assistants, and 37% use neither. This was about the same as physicians overall.
- A larger percentage of oncologists (38%) expect to participate in MIPS (merit-based incentive payment system), and only 8% expect to participate in APMs (alternative payment models). This was similar to the findings for physicians overall, with more than one-third (37%) expecting to participate in MIPS and 9% planning to take part in APMs.
Impact of COVID-19 pandemic
The Medscape compensation reports also gives a glimpse of the impact the COVID-19 pandemic is having on physician compensation.
Since the beginning of the pandemic, practices have reported a 55% decrease in revenue and a 60% drop in patient volume. Physician practices and hospitals have laid off or furloughed personnel and have cut pay, and 9% of practices have closed their doors, at least for the time being.
A total of 43,000 health care workers were laid off in March, the report notes.
The findings tie in with those reported elsewhere. For example, a survey conducted by the Medical Group Management Association, which was reported by Medscape Medical News, found that 97% of physician practices have experienced negative financial effects directly or indirectly related to COVID-19.
Specialties were hard hit, especially those that rely on elective procedures, such as dermatology and cardiology. Oncology care has also been disrupted. For example, a survey conducted by the American Cancer Society Cancer Action Network found that half of the cancer patients and survivors who responded reported changes, delays, or disruptions to the care they were receiving.
This article first appeared on Medscape.com.
Oncologists continue to rank above the middle range for all specialties in annual compensation for physicians, according to findings from the newly released Medscape Oncologist Compensation Report 2020.
The average earnings for oncologists who participated in the survey was $377,000, which was a 5% increase from the $359,000 reported for 2018.
Just over two-thirds (67%) of oncologists reported that they felt that they were fairly compensated, which is quite a jump from 53% last year.
In addition, oncologists appear to be very satisfied with their profession. Similar to last year’s findings, 84% said they would choose medicine again, and 96% said they would choose the specialty of oncology again.
Earning in top third of all specialties
The average annual earnings reported by oncologists put this specialty in eleventh place among 29 specialties. Orthopedic specialists remain at the head of the list, with estimated earnings of $511,000, followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000), according to Medscape’s compensation report, which included responses from 17,461 physicians in over 30 specialties.

At the bottom of the estimated earnings list were public health and preventive medicine doctors and pediatricians. For both specialties, the reported annual earnings was $232,000. Family medicine specialists were only marginally higher at $234,000.
Radiologists ($427,000), gastroenterologists ($419,000), and urologists ($417,000) all reported higher earnings than oncologists, whereas neurologists, at $280,000, rheumatologists, at $262,000, and internal medicine physicians, at $251,000, earned less.
The report also found that gender disparities in income persist, with male oncologists earning 17% more than their female colleagues. The gender gap in oncology is somewhat less than that seen for all specialties combined, in which men earned 31% more than women, similar to last year’s figure of 33%.
Male oncologists reported spending 38.8 hours per week seeing patients, compared with 34.9 hours reported by female oncologists. This could be a factor contributing to the gender pay disparity. Overall, the average amount of time seeing patients was 37.9 hours per week.
Frustrations with paperwork and denied claims
Surveyed oncologists cited some of the frustrations they are facing, such as spending nearly 17 hours a week on paperwork and administrative tasks. They reported that 16% of claims are denied or have to be resubmitted. As for the most challenging part of the job, oncologists (22%), similar to physicians overall (26%), found that having so many rules and regulations takes first place, followed by working with electronic health record systems (20%), difficulties getting fair reimbursement (19%), having to work long hours (12%), and dealing with difficult patients (8%). Few oncologists were concerned about lawsuits (4%), and 4% reported that there were no challenges.
Oncologists reported that the most rewarding part of their job was gratitude/relationships with patients (31%), followed by knowing that they are making the world a better place (27%). After that, oncologists agreed with statements about being very good at what they do/finding answers/diagnoses (22%), having pride in being a doctor (9%), and making good money at a job they like (8%).
Other key findings
Other key findings from the Medscape Oncologist Compensation Report 2020 included the following:
- Regarding payment models, 80% take insurance, 41% are in fee-for-service arrangements, and 18% are in accountable care organizations (21%). Only 3% are in direct primary care, and 1% are cash-only practices or have a concierge practice.
- 65% of oncologists state that they will continue taking new and current Medicare/Medicaid patients. None said that they would not take on new Medicare/Medicaid patients, and 35% remain undecided. These numbers differed from physicians overall; 73% of all physicians surveyed said they would continue taking new/current Medicare/Medicaid patients, 6% said that will not take on new Medicare patients, and 4% said they will not take new Medicaid patients. In addition, 3% and 2% said that they would stop treating some or all of their Medicare and Medicaid patients, respectively.
- About half (51%) of oncologists use nurse practitioners, about a third (34%) use physician assistants, and 37% use neither. This was about the same as physicians overall.
- A larger percentage of oncologists (38%) expect to participate in MIPS (merit-based incentive payment system), and only 8% expect to participate in APMs (alternative payment models). This was similar to the findings for physicians overall, with more than one-third (37%) expecting to participate in MIPS and 9% planning to take part in APMs.
Impact of COVID-19 pandemic
The Medscape compensation reports also gives a glimpse of the impact the COVID-19 pandemic is having on physician compensation.
Since the beginning of the pandemic, practices have reported a 55% decrease in revenue and a 60% drop in patient volume. Physician practices and hospitals have laid off or furloughed personnel and have cut pay, and 9% of practices have closed their doors, at least for the time being.
A total of 43,000 health care workers were laid off in March, the report notes.
The findings tie in with those reported elsewhere. For example, a survey conducted by the Medical Group Management Association, which was reported by Medscape Medical News, found that 97% of physician practices have experienced negative financial effects directly or indirectly related to COVID-19.
Specialties were hard hit, especially those that rely on elective procedures, such as dermatology and cardiology. Oncology care has also been disrupted. For example, a survey conducted by the American Cancer Society Cancer Action Network found that half of the cancer patients and survivors who responded reported changes, delays, or disruptions to the care they were receiving.
This article first appeared on Medscape.com.
Tumor molecular profiling may help identify ‘exceptional responders’
Virtually all oncologists, at one time or another, have treated a patient who defied the odds and achieved an unexpectedly long-lasting response. These “exceptional responders” are patients who experience a unique response to therapies that have largely failed to be effective for others with similar cancers.
Genetic and molecular mechanisms may partly account for these responses and may offer clues about why the treatment works for only a few and not for others. To delve more deeply into that area of research, the National Cancer Institute (NCI) began the Exceptional Responders Initiative (ERI) with the goal of identifying potential biological processes that may be responsible, at least in part, for these unusual responses.
NCI researchers have now successfully completed a pilot study that analyzed tumor specimens from more than 100 cases, and the study has affirmed the feasibility of this approach.
Of these cases, six were identified as involving potentially clinically actionable germline mutations.
The findings were published online ahead of print in the Journal of the National Cancer Institute.
“Clearly, the analysis and validation of these results will prove critical to determining the success of this approach,” write James M. Ford, MD, and Beverly S. Mitchell, MD, both of Stanford University School of Medicine, California, in an accompanying editorial. “Ultimately, prospective studies of tumors from exceptional responders, particularly to novel, genomically-targeted agents, may provide a powerful approach to cancer treatment discoveries.”
A special case
Molecular profiling technology, including next-generation sequencing, has significantly changed the landscape of the development of cancer therapies, and clinical trials in early drug development are increasingly selecting patients on the basis of molecular alterations.
The ERI grew out of several meetings held by the NCI in 2013 and 2014. It was built on the ability to profile archived tumor material, explained study author S. Percy Ivy, PhD, associate chief of the Investigational Drug Branch in the Division of Cancer Treatment and Diagnosis of the NCI. “This made it possible to collect cases from participating clinicians from all over the country.
“Published cases included patients treated with a targeted therapy but not treated with knowledge of their tumor’s genomics, who then later turned out to have genomic changes that made their tumor exquisitely sensitive to inhibition of a driving pathway,” she said. “There have been published cases as well as cases in the experience of practicing oncologists that seem to do much better than expected.
“We wondered if we could find molecular reasons why tumors respond not only to targeted therapies but also to standard chemotherapy,” said Percy. “If so, we could refine our choice of therapy to patients who are most likely to respond to it.”’
On its website, the NCI writes that there was a particular case that triggered the interest in going ahead with this initiative. Mutations in the TSC1 and NF2 genes, which result in a loss of gene function, were detected in a patient with metastatic bladder cancer. In a clinical trial, the patient was treated with everolimus (Afinitor, Novartis), an inhibitor of the mammalian target of rapamycin (mTORC1), and achieved a complete response with a duration of more than 2 years.
In a separate analysis, researchers sequenced tumors from 96 other individuals with high-grade bladder cancer and identified five TSC1 gene mutations. Tumors were sequenced from 13 patients with bladder cancer who had received everolimus. Results showed that 3 of 4 patients with TSC1 gene mutations experienced some degree of tumor shrinkage after treatment; 8 of 9 patients who did not have the mutation experienced disease progression.
The NCI notes that in “subsequent workshops and discussions, it became obvious that all clinicians have seen a few exceptional responders.”
Testing for feasibility
The aim of the current study was to assess the feasibility and potential usefulness of sequencing DNA and RNA from clinical tumor specimens from patients who had experienced unusually profound or durable responses to systemic therapy.
Its main feasibility goal was to identify at least 100 cases involving exceptional responders whose cases could be analyzed in less than 3 years.
An exceptional patient was defined as one who had experienced a complete response to one or more drugs in which complete responses were seen in fewer than 10% of patients who received similar treatment; or a partial response lasting at least 6 months in which such a response is seen in fewer than 10% of patients who receive similar treatment; or a complete or partial response of a duration that is three times the median response duration represented in the literature for the treatment.
Studying exceptional responders presents many challenges, the first being to define what an exceptional response is and what it is not, explained Ivy. “This definition relies on the existence of data that a particular therapy will produce particular responses in groups of patients with similar tumors, as defined by organ of origin,” she said.
Other challenges include obtaining tumor tissue and all the relevant clinical data, such as the number of prior treatments and the patient’s response, as well as any known molecular characteristics (eg, HER2/NEU amplification, estrogen-receptor expression, germline mutations). “We also do not have data on other exposures, such as smoking or chemical exposure,” she said. “In addition, when patients are not on clinical trial, the data are not uniformly obtained ― such as that scans may not be performed at particular intervals.”
Importantly, the molecular tools used to analyze tumors were not available in the past, so many trials did not collect tumor tissue for subsequent research. “Even now, we are learning that there are characteristics beyond DNA and RNA that are potentially important to the ability of a tumor to respond, such as the immune system or epigenetic changes,” she said.
From August 2014 to July 2017, a total of 520 cases were proposed by clinicians as possibly involving exceptional responders, and 222 cases met the criteria.
Analyzable tissue was available for 117 patients. Most of the responders (n = 80, 68.4%) had been treated with combination chemotherapy regimens; 34 patients (29.0%) had received one or more antiangiogenesis agents. In addition, six patients had an exceptional response following treatment with immune checkpoint inhibitors. The final analysis included 109 cases.
One exceptional responder was a woman with metastatic squamous lung cancer that was treated with paclitaxel and carboplatin. The patient achieved a 41-month complete response (expected rate, <10%). Another patient with esophageal adenocarcinoma who was treated with docetaxel and cisplatin experienced a partial response that lasted 128 months (reported median response duration, 24 months). After the patient’s tumor recurred, he experienced for the second time a response to concurrent chemoradiation with the same drug regimen.
Overall, potentially clinically relevant germline mutations were identified in six tumors. Pathogenic BRCA1 or BRCA2 mutations were found in two breast cancer patients, one patient with non–small cell lung cancer, and one patient with rectal cancer. A breast cancer patient had a pathogenic BRCA1 germline mutation, and another had a likely germline mutation in CHEK2. A patient with poorly differentiated lung cancer and a history of breast cancer had a PALB2 mutation.
Future steps
Molecular mechanisms are important, but other factors could also play a role in eliciting a response. One is the presence of comorbidities, which was not assessed in the study. Ivy noted that comorbidities could be very important to responses, along with medications that the patient is using for different types of ailments. In addition, the use of complementary and alternative medicines may also have an impact.
“As the field matures, we hope that others will collect these and other characteristics, so that all the data could be used to develop hypotheses about molecular and other factors that can better predict response or resistance,” she said.
The results from this pilot study demonstrated feasibility. Ivy noted that “additional collaboration in similar studies would be welcome, as would methods to use data from various sources to improve our ability to correlate patient characteristics, tumor characteristics and response.
“We envision a larger national and international effort to collect more exceptional responder cases, including more from patients treated with targeted therapies,” she added. “The NCI has been meeting with an interest group that focuses on ER cases in the UK, France, Italy, Canada, and Australia, and this collaborative effort is maturing, albeit slowly.”
The project has been funded in whole or in part with federal funds from the NCI and NIH. Ivy has disclosed no relevant financial relationships. Several coauthors report relationships with industry. The editorialists have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Virtually all oncologists, at one time or another, have treated a patient who defied the odds and achieved an unexpectedly long-lasting response. These “exceptional responders” are patients who experience a unique response to therapies that have largely failed to be effective for others with similar cancers.
Genetic and molecular mechanisms may partly account for these responses and may offer clues about why the treatment works for only a few and not for others. To delve more deeply into that area of research, the National Cancer Institute (NCI) began the Exceptional Responders Initiative (ERI) with the goal of identifying potential biological processes that may be responsible, at least in part, for these unusual responses.
NCI researchers have now successfully completed a pilot study that analyzed tumor specimens from more than 100 cases, and the study has affirmed the feasibility of this approach.
Of these cases, six were identified as involving potentially clinically actionable germline mutations.
The findings were published online ahead of print in the Journal of the National Cancer Institute.
“Clearly, the analysis and validation of these results will prove critical to determining the success of this approach,” write James M. Ford, MD, and Beverly S. Mitchell, MD, both of Stanford University School of Medicine, California, in an accompanying editorial. “Ultimately, prospective studies of tumors from exceptional responders, particularly to novel, genomically-targeted agents, may provide a powerful approach to cancer treatment discoveries.”
A special case
Molecular profiling technology, including next-generation sequencing, has significantly changed the landscape of the development of cancer therapies, and clinical trials in early drug development are increasingly selecting patients on the basis of molecular alterations.
The ERI grew out of several meetings held by the NCI in 2013 and 2014. It was built on the ability to profile archived tumor material, explained study author S. Percy Ivy, PhD, associate chief of the Investigational Drug Branch in the Division of Cancer Treatment and Diagnosis of the NCI. “This made it possible to collect cases from participating clinicians from all over the country.
“Published cases included patients treated with a targeted therapy but not treated with knowledge of their tumor’s genomics, who then later turned out to have genomic changes that made their tumor exquisitely sensitive to inhibition of a driving pathway,” she said. “There have been published cases as well as cases in the experience of practicing oncologists that seem to do much better than expected.
“We wondered if we could find molecular reasons why tumors respond not only to targeted therapies but also to standard chemotherapy,” said Percy. “If so, we could refine our choice of therapy to patients who are most likely to respond to it.”’
On its website, the NCI writes that there was a particular case that triggered the interest in going ahead with this initiative. Mutations in the TSC1 and NF2 genes, which result in a loss of gene function, were detected in a patient with metastatic bladder cancer. In a clinical trial, the patient was treated with everolimus (Afinitor, Novartis), an inhibitor of the mammalian target of rapamycin (mTORC1), and achieved a complete response with a duration of more than 2 years.
In a separate analysis, researchers sequenced tumors from 96 other individuals with high-grade bladder cancer and identified five TSC1 gene mutations. Tumors were sequenced from 13 patients with bladder cancer who had received everolimus. Results showed that 3 of 4 patients with TSC1 gene mutations experienced some degree of tumor shrinkage after treatment; 8 of 9 patients who did not have the mutation experienced disease progression.
The NCI notes that in “subsequent workshops and discussions, it became obvious that all clinicians have seen a few exceptional responders.”
Testing for feasibility
The aim of the current study was to assess the feasibility and potential usefulness of sequencing DNA and RNA from clinical tumor specimens from patients who had experienced unusually profound or durable responses to systemic therapy.
Its main feasibility goal was to identify at least 100 cases involving exceptional responders whose cases could be analyzed in less than 3 years.
An exceptional patient was defined as one who had experienced a complete response to one or more drugs in which complete responses were seen in fewer than 10% of patients who received similar treatment; or a partial response lasting at least 6 months in which such a response is seen in fewer than 10% of patients who receive similar treatment; or a complete or partial response of a duration that is three times the median response duration represented in the literature for the treatment.
Studying exceptional responders presents many challenges, the first being to define what an exceptional response is and what it is not, explained Ivy. “This definition relies on the existence of data that a particular therapy will produce particular responses in groups of patients with similar tumors, as defined by organ of origin,” she said.
Other challenges include obtaining tumor tissue and all the relevant clinical data, such as the number of prior treatments and the patient’s response, as well as any known molecular characteristics (eg, HER2/NEU amplification, estrogen-receptor expression, germline mutations). “We also do not have data on other exposures, such as smoking or chemical exposure,” she said. “In addition, when patients are not on clinical trial, the data are not uniformly obtained ― such as that scans may not be performed at particular intervals.”
Importantly, the molecular tools used to analyze tumors were not available in the past, so many trials did not collect tumor tissue for subsequent research. “Even now, we are learning that there are characteristics beyond DNA and RNA that are potentially important to the ability of a tumor to respond, such as the immune system or epigenetic changes,” she said.
From August 2014 to July 2017, a total of 520 cases were proposed by clinicians as possibly involving exceptional responders, and 222 cases met the criteria.
Analyzable tissue was available for 117 patients. Most of the responders (n = 80, 68.4%) had been treated with combination chemotherapy regimens; 34 patients (29.0%) had received one or more antiangiogenesis agents. In addition, six patients had an exceptional response following treatment with immune checkpoint inhibitors. The final analysis included 109 cases.
One exceptional responder was a woman with metastatic squamous lung cancer that was treated with paclitaxel and carboplatin. The patient achieved a 41-month complete response (expected rate, <10%). Another patient with esophageal adenocarcinoma who was treated with docetaxel and cisplatin experienced a partial response that lasted 128 months (reported median response duration, 24 months). After the patient’s tumor recurred, he experienced for the second time a response to concurrent chemoradiation with the same drug regimen.
Overall, potentially clinically relevant germline mutations were identified in six tumors. Pathogenic BRCA1 or BRCA2 mutations were found in two breast cancer patients, one patient with non–small cell lung cancer, and one patient with rectal cancer. A breast cancer patient had a pathogenic BRCA1 germline mutation, and another had a likely germline mutation in CHEK2. A patient with poorly differentiated lung cancer and a history of breast cancer had a PALB2 mutation.
Future steps
Molecular mechanisms are important, but other factors could also play a role in eliciting a response. One is the presence of comorbidities, which was not assessed in the study. Ivy noted that comorbidities could be very important to responses, along with medications that the patient is using for different types of ailments. In addition, the use of complementary and alternative medicines may also have an impact.
“As the field matures, we hope that others will collect these and other characteristics, so that all the data could be used to develop hypotheses about molecular and other factors that can better predict response or resistance,” she said.
The results from this pilot study demonstrated feasibility. Ivy noted that “additional collaboration in similar studies would be welcome, as would methods to use data from various sources to improve our ability to correlate patient characteristics, tumor characteristics and response.
“We envision a larger national and international effort to collect more exceptional responder cases, including more from patients treated with targeted therapies,” she added. “The NCI has been meeting with an interest group that focuses on ER cases in the UK, France, Italy, Canada, and Australia, and this collaborative effort is maturing, albeit slowly.”
The project has been funded in whole or in part with federal funds from the NCI and NIH. Ivy has disclosed no relevant financial relationships. Several coauthors report relationships with industry. The editorialists have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Virtually all oncologists, at one time or another, have treated a patient who defied the odds and achieved an unexpectedly long-lasting response. These “exceptional responders” are patients who experience a unique response to therapies that have largely failed to be effective for others with similar cancers.
Genetic and molecular mechanisms may partly account for these responses and may offer clues about why the treatment works for only a few and not for others. To delve more deeply into that area of research, the National Cancer Institute (NCI) began the Exceptional Responders Initiative (ERI) with the goal of identifying potential biological processes that may be responsible, at least in part, for these unusual responses.
NCI researchers have now successfully completed a pilot study that analyzed tumor specimens from more than 100 cases, and the study has affirmed the feasibility of this approach.
Of these cases, six were identified as involving potentially clinically actionable germline mutations.
The findings were published online ahead of print in the Journal of the National Cancer Institute.
“Clearly, the analysis and validation of these results will prove critical to determining the success of this approach,” write James M. Ford, MD, and Beverly S. Mitchell, MD, both of Stanford University School of Medicine, California, in an accompanying editorial. “Ultimately, prospective studies of tumors from exceptional responders, particularly to novel, genomically-targeted agents, may provide a powerful approach to cancer treatment discoveries.”
A special case
Molecular profiling technology, including next-generation sequencing, has significantly changed the landscape of the development of cancer therapies, and clinical trials in early drug development are increasingly selecting patients on the basis of molecular alterations.
The ERI grew out of several meetings held by the NCI in 2013 and 2014. It was built on the ability to profile archived tumor material, explained study author S. Percy Ivy, PhD, associate chief of the Investigational Drug Branch in the Division of Cancer Treatment and Diagnosis of the NCI. “This made it possible to collect cases from participating clinicians from all over the country.
“Published cases included patients treated with a targeted therapy but not treated with knowledge of their tumor’s genomics, who then later turned out to have genomic changes that made their tumor exquisitely sensitive to inhibition of a driving pathway,” she said. “There have been published cases as well as cases in the experience of practicing oncologists that seem to do much better than expected.
“We wondered if we could find molecular reasons why tumors respond not only to targeted therapies but also to standard chemotherapy,” said Percy. “If so, we could refine our choice of therapy to patients who are most likely to respond to it.”’
On its website, the NCI writes that there was a particular case that triggered the interest in going ahead with this initiative. Mutations in the TSC1 and NF2 genes, which result in a loss of gene function, were detected in a patient with metastatic bladder cancer. In a clinical trial, the patient was treated with everolimus (Afinitor, Novartis), an inhibitor of the mammalian target of rapamycin (mTORC1), and achieved a complete response with a duration of more than 2 years.
In a separate analysis, researchers sequenced tumors from 96 other individuals with high-grade bladder cancer and identified five TSC1 gene mutations. Tumors were sequenced from 13 patients with bladder cancer who had received everolimus. Results showed that 3 of 4 patients with TSC1 gene mutations experienced some degree of tumor shrinkage after treatment; 8 of 9 patients who did not have the mutation experienced disease progression.
The NCI notes that in “subsequent workshops and discussions, it became obvious that all clinicians have seen a few exceptional responders.”
Testing for feasibility
The aim of the current study was to assess the feasibility and potential usefulness of sequencing DNA and RNA from clinical tumor specimens from patients who had experienced unusually profound or durable responses to systemic therapy.
Its main feasibility goal was to identify at least 100 cases involving exceptional responders whose cases could be analyzed in less than 3 years.
An exceptional patient was defined as one who had experienced a complete response to one or more drugs in which complete responses were seen in fewer than 10% of patients who received similar treatment; or a partial response lasting at least 6 months in which such a response is seen in fewer than 10% of patients who receive similar treatment; or a complete or partial response of a duration that is three times the median response duration represented in the literature for the treatment.
Studying exceptional responders presents many challenges, the first being to define what an exceptional response is and what it is not, explained Ivy. “This definition relies on the existence of data that a particular therapy will produce particular responses in groups of patients with similar tumors, as defined by organ of origin,” she said.
Other challenges include obtaining tumor tissue and all the relevant clinical data, such as the number of prior treatments and the patient’s response, as well as any known molecular characteristics (eg, HER2/NEU amplification, estrogen-receptor expression, germline mutations). “We also do not have data on other exposures, such as smoking or chemical exposure,” she said. “In addition, when patients are not on clinical trial, the data are not uniformly obtained ― such as that scans may not be performed at particular intervals.”
Importantly, the molecular tools used to analyze tumors were not available in the past, so many trials did not collect tumor tissue for subsequent research. “Even now, we are learning that there are characteristics beyond DNA and RNA that are potentially important to the ability of a tumor to respond, such as the immune system or epigenetic changes,” she said.
From August 2014 to July 2017, a total of 520 cases were proposed by clinicians as possibly involving exceptional responders, and 222 cases met the criteria.
Analyzable tissue was available for 117 patients. Most of the responders (n = 80, 68.4%) had been treated with combination chemotherapy regimens; 34 patients (29.0%) had received one or more antiangiogenesis agents. In addition, six patients had an exceptional response following treatment with immune checkpoint inhibitors. The final analysis included 109 cases.
One exceptional responder was a woman with metastatic squamous lung cancer that was treated with paclitaxel and carboplatin. The patient achieved a 41-month complete response (expected rate, <10%). Another patient with esophageal adenocarcinoma who was treated with docetaxel and cisplatin experienced a partial response that lasted 128 months (reported median response duration, 24 months). After the patient’s tumor recurred, he experienced for the second time a response to concurrent chemoradiation with the same drug regimen.
Overall, potentially clinically relevant germline mutations were identified in six tumors. Pathogenic BRCA1 or BRCA2 mutations were found in two breast cancer patients, one patient with non–small cell lung cancer, and one patient with rectal cancer. A breast cancer patient had a pathogenic BRCA1 germline mutation, and another had a likely germline mutation in CHEK2. A patient with poorly differentiated lung cancer and a history of breast cancer had a PALB2 mutation.
Future steps
Molecular mechanisms are important, but other factors could also play a role in eliciting a response. One is the presence of comorbidities, which was not assessed in the study. Ivy noted that comorbidities could be very important to responses, along with medications that the patient is using for different types of ailments. In addition, the use of complementary and alternative medicines may also have an impact.
“As the field matures, we hope that others will collect these and other characteristics, so that all the data could be used to develop hypotheses about molecular and other factors that can better predict response or resistance,” she said.
The results from this pilot study demonstrated feasibility. Ivy noted that “additional collaboration in similar studies would be welcome, as would methods to use data from various sources to improve our ability to correlate patient characteristics, tumor characteristics and response.
“We envision a larger national and international effort to collect more exceptional responder cases, including more from patients treated with targeted therapies,” she added. “The NCI has been meeting with an interest group that focuses on ER cases in the UK, France, Italy, Canada, and Australia, and this collaborative effort is maturing, albeit slowly.”
The project has been funded in whole or in part with federal funds from the NCI and NIH. Ivy has disclosed no relevant financial relationships. Several coauthors report relationships with industry. The editorialists have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Video coaching may relieve anxiety and distress for long-distance cancer caregivers
Anxiety and distress related to caring for a cancer patient who lives far away may be alleviated through an intervention that includes video-based coaching sessions with a nurse practitioner or social worker, a randomized study suggests.
About 20% of long-distance caregivers had a significant reduction in anxiety and 25% had a significant reduction in distress when they received video coaching sessions, attended oncologist visits via video, and had access to a website specifically designed for their needs.
Adding the caregiver to oncologist office visits made the patients feel better supported and didn’t add a significant amount of time to the encounter, said Sara L. Douglas, PhD, RN, of Case Western Reserve University, Cleveland.
Taken together, these results suggest that fairly simple technologies can be leveraged to help caregivers cope with psychological strains related to supporting a patient who doesn’t live nearby, Dr. Douglas said.
Distance caregivers, defined as those who live an hour or more away from the patient, can experience high rates of distress and anxiety because they lack first-hand information or may have uncertainty about the patient’s current condition, according to Dr. Douglas and colleagues.
“Caregivers’ high rates of anxiety and distress have been found to have a negative impact not only upon their own health but upon their ability to provide high quality care to the patient,” Dr. Douglas said.
With this in mind, she and her colleagues conducted a 4-month study of distance caregivers. Dr. Douglas presented results from the study at the American Society of Clinical Oncology virtual scientific program during a press briefing in advance of the meeting. This year, ASCO’s annual meeting is split into two parts. The virtual scientific program will be presented online on May 29-31, and the virtual education program will be available Aug. 8-10.
Study details
The study enrolled 441 distance caregivers of cancer patients, and Dr. Douglas presented results in 311 of those caregivers. (Data in the presentation differ from the abstract.) The caregivers were, on average, 47 years of age. Most were female (72%), white (67%), the child of the patient (63%), currently employed (81%), and new to the distance caregiver role (89%).
The caregivers were randomized to one of three study arms.
One arm received the full intervention, which consisted of four video-coaching sessions with an advanced practice nurse or social worker, videoconference office visits with the physician and patient, and access to a website with information for cancer distance caregivers. A second arm received no video coaching but had access to the website and participated in video visits with the physician and patient. The third arm, which only received access to the website, served as the study’s control group.
Results
Dr. Douglas said that the full intervention had the biggest impact on caregivers’ distress and anxiety.
Among distance caregivers who received the full intervention, 19.2% had a significant reduction in anxiety (P = .03), as measured in online surveys before and after the intervention using the PROMIS Anxiety instrument. Furthermore, 24.8% of these caregivers had a significant reduction in distress (P = .02) from preintervention to post intervention, as measured by the National Comprehensive Cancer Network Distress Thermometer. Overall, distress and anxiety scores decreased in this arm.
Distance caregivers who only had physician-patient video visits and website access had a “moderate” reduction in distress and anxiety, Dr. Douglas said. Among these caregivers, 17.3% had an improvement in anxiety from baseline, and 19.8% had an improvement in distress. Overall, distress scores decreased, but anxiety scores increased slightly in this arm.
In the control arm, 13.1% of caregivers had an improvement in anxiety from baseline, and 18% had an improvement in distress. Overall, both anxiety and distress scores increased in this arm.
“While the full intervention yielded the best results for distance caregivers, we recognize that not all health care systems have the resources to provide individualized coaching sessions to distance caregivers,” Dr. Douglas said. “Therefore, it is worth noting that videoconference office visits alone are found to be of some benefit in improving distress and anxiety in this group of cancer caregivers.”
The study results suggest videoconferencing interventions can improve the emotional well-being of remote caregivers who provide “critical support” for cancer patients, said ASCO President Howard A. “Skip” Burris III, MD.
“As COVID-19 forces separation from loved ones and increases anxiety for people with cancer and their caregivers, providing emotional support virtually is more important than ever,” Dr. Burris said in a news release highlighting the study.
This study was funded by the National Institutes of Health and Case Comprehensive Cancer Center. Dr. Douglas reported having no disclosures. Other researchers involved in the study disclosed relationships with BridgeBio Pharma, Cardinal Health, Apexigen, Roche/Genentech, Seattle Genetics, Tesaro, Array BioPharma, Abbvie, Bristol-Myers Squibb, and Celgene. A full list of Dr. Burris’s financial disclosures is available on the ASCO website.
SOURCE: Douglas SL et al. ASCO 2020, Abstract 12123.
Anxiety and distress related to caring for a cancer patient who lives far away may be alleviated through an intervention that includes video-based coaching sessions with a nurse practitioner or social worker, a randomized study suggests.
About 20% of long-distance caregivers had a significant reduction in anxiety and 25% had a significant reduction in distress when they received video coaching sessions, attended oncologist visits via video, and had access to a website specifically designed for their needs.
Adding the caregiver to oncologist office visits made the patients feel better supported and didn’t add a significant amount of time to the encounter, said Sara L. Douglas, PhD, RN, of Case Western Reserve University, Cleveland.
Taken together, these results suggest that fairly simple technologies can be leveraged to help caregivers cope with psychological strains related to supporting a patient who doesn’t live nearby, Dr. Douglas said.
Distance caregivers, defined as those who live an hour or more away from the patient, can experience high rates of distress and anxiety because they lack first-hand information or may have uncertainty about the patient’s current condition, according to Dr. Douglas and colleagues.
“Caregivers’ high rates of anxiety and distress have been found to have a negative impact not only upon their own health but upon their ability to provide high quality care to the patient,” Dr. Douglas said.
With this in mind, she and her colleagues conducted a 4-month study of distance caregivers. Dr. Douglas presented results from the study at the American Society of Clinical Oncology virtual scientific program during a press briefing in advance of the meeting. This year, ASCO’s annual meeting is split into two parts. The virtual scientific program will be presented online on May 29-31, and the virtual education program will be available Aug. 8-10.
Study details
The study enrolled 441 distance caregivers of cancer patients, and Dr. Douglas presented results in 311 of those caregivers. (Data in the presentation differ from the abstract.) The caregivers were, on average, 47 years of age. Most were female (72%), white (67%), the child of the patient (63%), currently employed (81%), and new to the distance caregiver role (89%).
The caregivers were randomized to one of three study arms.
One arm received the full intervention, which consisted of four video-coaching sessions with an advanced practice nurse or social worker, videoconference office visits with the physician and patient, and access to a website with information for cancer distance caregivers. A second arm received no video coaching but had access to the website and participated in video visits with the physician and patient. The third arm, which only received access to the website, served as the study’s control group.
Results
Dr. Douglas said that the full intervention had the biggest impact on caregivers’ distress and anxiety.
Among distance caregivers who received the full intervention, 19.2% had a significant reduction in anxiety (P = .03), as measured in online surveys before and after the intervention using the PROMIS Anxiety instrument. Furthermore, 24.8% of these caregivers had a significant reduction in distress (P = .02) from preintervention to post intervention, as measured by the National Comprehensive Cancer Network Distress Thermometer. Overall, distress and anxiety scores decreased in this arm.
Distance caregivers who only had physician-patient video visits and website access had a “moderate” reduction in distress and anxiety, Dr. Douglas said. Among these caregivers, 17.3% had an improvement in anxiety from baseline, and 19.8% had an improvement in distress. Overall, distress scores decreased, but anxiety scores increased slightly in this arm.
In the control arm, 13.1% of caregivers had an improvement in anxiety from baseline, and 18% had an improvement in distress. Overall, both anxiety and distress scores increased in this arm.
“While the full intervention yielded the best results for distance caregivers, we recognize that not all health care systems have the resources to provide individualized coaching sessions to distance caregivers,” Dr. Douglas said. “Therefore, it is worth noting that videoconference office visits alone are found to be of some benefit in improving distress and anxiety in this group of cancer caregivers.”
The study results suggest videoconferencing interventions can improve the emotional well-being of remote caregivers who provide “critical support” for cancer patients, said ASCO President Howard A. “Skip” Burris III, MD.
“As COVID-19 forces separation from loved ones and increases anxiety for people with cancer and their caregivers, providing emotional support virtually is more important than ever,” Dr. Burris said in a news release highlighting the study.
This study was funded by the National Institutes of Health and Case Comprehensive Cancer Center. Dr. Douglas reported having no disclosures. Other researchers involved in the study disclosed relationships with BridgeBio Pharma, Cardinal Health, Apexigen, Roche/Genentech, Seattle Genetics, Tesaro, Array BioPharma, Abbvie, Bristol-Myers Squibb, and Celgene. A full list of Dr. Burris’s financial disclosures is available on the ASCO website.
SOURCE: Douglas SL et al. ASCO 2020, Abstract 12123.
Anxiety and distress related to caring for a cancer patient who lives far away may be alleviated through an intervention that includes video-based coaching sessions with a nurse practitioner or social worker, a randomized study suggests.
About 20% of long-distance caregivers had a significant reduction in anxiety and 25% had a significant reduction in distress when they received video coaching sessions, attended oncologist visits via video, and had access to a website specifically designed for their needs.
Adding the caregiver to oncologist office visits made the patients feel better supported and didn’t add a significant amount of time to the encounter, said Sara L. Douglas, PhD, RN, of Case Western Reserve University, Cleveland.
Taken together, these results suggest that fairly simple technologies can be leveraged to help caregivers cope with psychological strains related to supporting a patient who doesn’t live nearby, Dr. Douglas said.
Distance caregivers, defined as those who live an hour or more away from the patient, can experience high rates of distress and anxiety because they lack first-hand information or may have uncertainty about the patient’s current condition, according to Dr. Douglas and colleagues.
“Caregivers’ high rates of anxiety and distress have been found to have a negative impact not only upon their own health but upon their ability to provide high quality care to the patient,” Dr. Douglas said.
With this in mind, she and her colleagues conducted a 4-month study of distance caregivers. Dr. Douglas presented results from the study at the American Society of Clinical Oncology virtual scientific program during a press briefing in advance of the meeting. This year, ASCO’s annual meeting is split into two parts. The virtual scientific program will be presented online on May 29-31, and the virtual education program will be available Aug. 8-10.
Study details
The study enrolled 441 distance caregivers of cancer patients, and Dr. Douglas presented results in 311 of those caregivers. (Data in the presentation differ from the abstract.) The caregivers were, on average, 47 years of age. Most were female (72%), white (67%), the child of the patient (63%), currently employed (81%), and new to the distance caregiver role (89%).
The caregivers were randomized to one of three study arms.
One arm received the full intervention, which consisted of four video-coaching sessions with an advanced practice nurse or social worker, videoconference office visits with the physician and patient, and access to a website with information for cancer distance caregivers. A second arm received no video coaching but had access to the website and participated in video visits with the physician and patient. The third arm, which only received access to the website, served as the study’s control group.
Results
Dr. Douglas said that the full intervention had the biggest impact on caregivers’ distress and anxiety.
Among distance caregivers who received the full intervention, 19.2% had a significant reduction in anxiety (P = .03), as measured in online surveys before and after the intervention using the PROMIS Anxiety instrument. Furthermore, 24.8% of these caregivers had a significant reduction in distress (P = .02) from preintervention to post intervention, as measured by the National Comprehensive Cancer Network Distress Thermometer. Overall, distress and anxiety scores decreased in this arm.
Distance caregivers who only had physician-patient video visits and website access had a “moderate” reduction in distress and anxiety, Dr. Douglas said. Among these caregivers, 17.3% had an improvement in anxiety from baseline, and 19.8% had an improvement in distress. Overall, distress scores decreased, but anxiety scores increased slightly in this arm.
In the control arm, 13.1% of caregivers had an improvement in anxiety from baseline, and 18% had an improvement in distress. Overall, both anxiety and distress scores increased in this arm.
“While the full intervention yielded the best results for distance caregivers, we recognize that not all health care systems have the resources to provide individualized coaching sessions to distance caregivers,” Dr. Douglas said. “Therefore, it is worth noting that videoconference office visits alone are found to be of some benefit in improving distress and anxiety in this group of cancer caregivers.”
The study results suggest videoconferencing interventions can improve the emotional well-being of remote caregivers who provide “critical support” for cancer patients, said ASCO President Howard A. “Skip” Burris III, MD.
“As COVID-19 forces separation from loved ones and increases anxiety for people with cancer and their caregivers, providing emotional support virtually is more important than ever,” Dr. Burris said in a news release highlighting the study.
This study was funded by the National Institutes of Health and Case Comprehensive Cancer Center. Dr. Douglas reported having no disclosures. Other researchers involved in the study disclosed relationships with BridgeBio Pharma, Cardinal Health, Apexigen, Roche/Genentech, Seattle Genetics, Tesaro, Array BioPharma, Abbvie, Bristol-Myers Squibb, and Celgene. A full list of Dr. Burris’s financial disclosures is available on the ASCO website.
SOURCE: Douglas SL et al. ASCO 2020, Abstract 12123.
FROM ASCO 2020
Mammography cuts risk for fatal breast cancers: New data
Three experts who were approached by Medscape Medical News say this is further evidence that regular screening mammography significantly reduces the risk of dying from breast cancer, but one expert questioned the methodology used in the study.
The primary goal of cancer screening is to detect tumors at an early stage, when they are most treatable. The hope is that this will reduce the number of advanced cancers associated with poor prognosis and hence the risk of dying from that cancer.
So far, for mammography, the data have been somewhat conflicting. For example, some evidence suggests that widespread breast cancer screening may catch more small, slow-growing tumors that are unlikely to be fatal but will not curb the number of cancers that are diagnosed at a late stage.
The new study, published online in Cancer, refutes this view.
It followed a Swedish cohort of 549,091 women (covering approximately 30% of the Swedish screening-eligible population) who underwent regular mammography.
For the women in this cohort, there was a statistically significant 41% reduction in the risk of dying of breast cancer within 10 years and a 25% reduction in the incidence of advanced disease, compared to women who did not undergo screening. “Even in this age of effective treatments, early detection confers a substantial and significant additional reduction in risk of dying from breast cancer,” said lead author Stephen W. Duffy, MSc, from the Center for Cancer Prevention at Queen Mary University, London, United Kingdom.
The current study confirms the findings of a smaller earlier study (Cancer. 2019;125:515-23) from the same investigators. “It finds the same result with an extremely large evidence base, with more than half a million women, and it also adds further to the evidence that screening achieves this reduction in the context of routine healthcare, not only in the research context,” Duffy commented. “The results are generalizable to other populations, particularly in North America, Western Europe, and Australasia, where the epidemiology and demographics of breast cancer are similar,” said Duffy. “Clearly, more intensive screening is likely to achieve a greater benefit, but a trade-off between costs, both financial and human, and benefits always has to be made specific to each societal and healthcare environment.”
In Sweden, the policy regarding breast cancer screening is to screen women aged 40 to 54 years every 18 months. For those aged 55 to 69 years, screening is recommended every 24 months.
“The use of the incidence-based endpoints means that there is accurate classification of both the breast cancer cases and the whole study population in terms of exposure to screening and avoids a number of biases seen in other studies of service screening,” Duffy told Medscape Medical News.
“I have never seen persuasive evidence for the assertion that breast cancer screening does not reduce deaths from metastatic disease – indeed, the randomized trials seem to show the opposite,” said Duffy. “This may have arisen from a misunderstanding about the mechanism whereby screening works. It primarily works by diagnosing cancer early so that treatment is successful and recurrence with distant metastases, followed by death, does not occur some years later. I suspect some colleagues have confused this with distant metastases at initial diagnosis,” he added.
One expert questions methodology
One of the experts who was approached by Medscape Medical News to comment on the new study, Philippe Autier, MD, MPH, PhD, University of Strathclyde Institute of Global Public Health at the International Prevention Research Institute, Dardilly, France, questioned the methodology of the study. “This method is incorrect simply because women attending screening are different from women not attending screening,” he said. “The former are more health aware and have healthier behaviors than the latter, and this is a well-known fact and supported by the literature.”
Autier emphasized that it is practically impossible to control for that bias, which is known as confounding by indication.
“The statistical methods used for attenuating the so-called self-selection are very approximate and based on unverified assumptions,” he said. “For this reason, the Handbook on Breast Cancer Screening produced by the International Agency for Research on Cancer [IARC] clearly stated that ‘observational studies based on individual screening history, no matter how well designed and conducted, should not be regarded as providing evidence for an effect of screening,’ and the methodology in this paper has never been recommended by the IARC.”
A better way of conducting this type of study would have been to show the incidence trends of advanced-stage breast cancer in Sweden for the entire female population aged 40 years and older, he asserts. Autier used that methodology in his own study in the Netherlands, as previously reported by Medscape Medical News. That study found that in the Netherlands, screening mammography over a period of 24 years among women aged 50 to 74 years had little effect on reducing rates of advanced breast cancer or mortality from the disease.
Experts applaud the new findings
Three of the experts who were approached by Medscape Medical News to comment on the new findings applauded the efforts of Duffy and colleagues in providing evidence that mammography can reduce breast cancer–related mortality.
Marie Quinn, MD, director of diagnostic radiology at Roswell Park Comprehensive Cancer Center, Buffalo, New York, said this study adds to the growing body of scientific evidence that confirms that women who undergo regular screening mammography significantly reduce their risk of dying from breast cancer.
“Women who underwent regular screening also had a 25% reduction in the incidence of advanced-stage breast cancer,” she said. “This is important, because breast cancers are less fatal and often require less treatment when picked up at an earlier stage. We know the risk reduction benefit detected in this well-designed study can be attributed to screening mammography and not advances in cancer treatment, due to the long-term follow-up and outcome of cancer death within 10 years.”
The findings from this study support the guidelines recommending routine screening mammography in the United States, Quinn continued, but she pointed out that some aspects of screening (e.g., the age at which to begin screening and how often to screen) can vary. “This can be confusing for patients and providers,” she said. “Overall, research has shown us that women who undergo regular screening mammograms reduce their risk of dying from breast cancer. For women of average risk, the benefit of mammography is maximized with annual screening beginning at age 40,” she said.
Jay A. Baker, MD, FACR, FSBI, chief of the Division of Breast Imaging at Duke University Medical Center, Durham, North Carolina, emphasized that this is yet another study that confirms that the improvement in breast cancer mortality is not the result of improved treatments alone, as some have speculated. “Others have tried to model the benefit of screening vs treatment, but this study is a more direct measurement,” he said. “This conclusion is important for both patients and physicians to hear.”
Although the study strongly supports regular screening for all women, it does not specifically address which set of screening guidelines is optimal, Baker commented. “Fortunately, even though some organizations in the US curiously suggest a delayed start to screening, all organizations and professional societies agree that the most lives and the most years of life are saved by yearly screening beginning at age 40,” he added. “This new study tells us that new treatments alone aren’t enough and confirms that screening saves at least one-third more lives.”
Another expert, Bonnie N. Joe, MD, PhD, professor in residence and chief of breast imaging in the Department of Radiology and Biomedical Imaging at the University of California, San Francisco, agreed that the study shows the mortality benefits of regular screening mammography. “Notably, these benefits were related to participation in mammography screening and independent of any advances in treatment,” she said, “And these findings in this study support regular screening mammography to reduce advanced-stage breast cancers and to reduce a woman’s risk of dying from breast cancer.”
Joe noted that overall, this was a “well-done, large-scale screening study with long-term outcomes and should be applicable to other populations. In the US, we know that peak cancer incidence is in the 40s for minority women, and the results of this study support regular screening starting at 40.”
The study was supported by the American Cancer Society through a gift from the Longaberger Company’s Horizon of Hope Campaign. Additional financial support was provided by Brostcancerförbundet, Sweden. Duffy, Autier, Quinn, Joe, and Baker have disclosed no relevant financial relationships. One coauthor of the study has disclosed relationships with industry, as noted in the original article.
This article first appeared on Medscape.com.
Three experts who were approached by Medscape Medical News say this is further evidence that regular screening mammography significantly reduces the risk of dying from breast cancer, but one expert questioned the methodology used in the study.
The primary goal of cancer screening is to detect tumors at an early stage, when they are most treatable. The hope is that this will reduce the number of advanced cancers associated with poor prognosis and hence the risk of dying from that cancer.
So far, for mammography, the data have been somewhat conflicting. For example, some evidence suggests that widespread breast cancer screening may catch more small, slow-growing tumors that are unlikely to be fatal but will not curb the number of cancers that are diagnosed at a late stage.
The new study, published online in Cancer, refutes this view.
It followed a Swedish cohort of 549,091 women (covering approximately 30% of the Swedish screening-eligible population) who underwent regular mammography.
For the women in this cohort, there was a statistically significant 41% reduction in the risk of dying of breast cancer within 10 years and a 25% reduction in the incidence of advanced disease, compared to women who did not undergo screening. “Even in this age of effective treatments, early detection confers a substantial and significant additional reduction in risk of dying from breast cancer,” said lead author Stephen W. Duffy, MSc, from the Center for Cancer Prevention at Queen Mary University, London, United Kingdom.
The current study confirms the findings of a smaller earlier study (Cancer. 2019;125:515-23) from the same investigators. “It finds the same result with an extremely large evidence base, with more than half a million women, and it also adds further to the evidence that screening achieves this reduction in the context of routine healthcare, not only in the research context,” Duffy commented. “The results are generalizable to other populations, particularly in North America, Western Europe, and Australasia, where the epidemiology and demographics of breast cancer are similar,” said Duffy. “Clearly, more intensive screening is likely to achieve a greater benefit, but a trade-off between costs, both financial and human, and benefits always has to be made specific to each societal and healthcare environment.”
In Sweden, the policy regarding breast cancer screening is to screen women aged 40 to 54 years every 18 months. For those aged 55 to 69 years, screening is recommended every 24 months.
“The use of the incidence-based endpoints means that there is accurate classification of both the breast cancer cases and the whole study population in terms of exposure to screening and avoids a number of biases seen in other studies of service screening,” Duffy told Medscape Medical News.
“I have never seen persuasive evidence for the assertion that breast cancer screening does not reduce deaths from metastatic disease – indeed, the randomized trials seem to show the opposite,” said Duffy. “This may have arisen from a misunderstanding about the mechanism whereby screening works. It primarily works by diagnosing cancer early so that treatment is successful and recurrence with distant metastases, followed by death, does not occur some years later. I suspect some colleagues have confused this with distant metastases at initial diagnosis,” he added.
One expert questions methodology
One of the experts who was approached by Medscape Medical News to comment on the new study, Philippe Autier, MD, MPH, PhD, University of Strathclyde Institute of Global Public Health at the International Prevention Research Institute, Dardilly, France, questioned the methodology of the study. “This method is incorrect simply because women attending screening are different from women not attending screening,” he said. “The former are more health aware and have healthier behaviors than the latter, and this is a well-known fact and supported by the literature.”
Autier emphasized that it is practically impossible to control for that bias, which is known as confounding by indication.
“The statistical methods used for attenuating the so-called self-selection are very approximate and based on unverified assumptions,” he said. “For this reason, the Handbook on Breast Cancer Screening produced by the International Agency for Research on Cancer [IARC] clearly stated that ‘observational studies based on individual screening history, no matter how well designed and conducted, should not be regarded as providing evidence for an effect of screening,’ and the methodology in this paper has never been recommended by the IARC.”
A better way of conducting this type of study would have been to show the incidence trends of advanced-stage breast cancer in Sweden for the entire female population aged 40 years and older, he asserts. Autier used that methodology in his own study in the Netherlands, as previously reported by Medscape Medical News. That study found that in the Netherlands, screening mammography over a period of 24 years among women aged 50 to 74 years had little effect on reducing rates of advanced breast cancer or mortality from the disease.
Experts applaud the new findings
Three of the experts who were approached by Medscape Medical News to comment on the new findings applauded the efforts of Duffy and colleagues in providing evidence that mammography can reduce breast cancer–related mortality.
Marie Quinn, MD, director of diagnostic radiology at Roswell Park Comprehensive Cancer Center, Buffalo, New York, said this study adds to the growing body of scientific evidence that confirms that women who undergo regular screening mammography significantly reduce their risk of dying from breast cancer.
“Women who underwent regular screening also had a 25% reduction in the incidence of advanced-stage breast cancer,” she said. “This is important, because breast cancers are less fatal and often require less treatment when picked up at an earlier stage. We know the risk reduction benefit detected in this well-designed study can be attributed to screening mammography and not advances in cancer treatment, due to the long-term follow-up and outcome of cancer death within 10 years.”
The findings from this study support the guidelines recommending routine screening mammography in the United States, Quinn continued, but she pointed out that some aspects of screening (e.g., the age at which to begin screening and how often to screen) can vary. “This can be confusing for patients and providers,” she said. “Overall, research has shown us that women who undergo regular screening mammograms reduce their risk of dying from breast cancer. For women of average risk, the benefit of mammography is maximized with annual screening beginning at age 40,” she said.
Jay A. Baker, MD, FACR, FSBI, chief of the Division of Breast Imaging at Duke University Medical Center, Durham, North Carolina, emphasized that this is yet another study that confirms that the improvement in breast cancer mortality is not the result of improved treatments alone, as some have speculated. “Others have tried to model the benefit of screening vs treatment, but this study is a more direct measurement,” he said. “This conclusion is important for both patients and physicians to hear.”
Although the study strongly supports regular screening for all women, it does not specifically address which set of screening guidelines is optimal, Baker commented. “Fortunately, even though some organizations in the US curiously suggest a delayed start to screening, all organizations and professional societies agree that the most lives and the most years of life are saved by yearly screening beginning at age 40,” he added. “This new study tells us that new treatments alone aren’t enough and confirms that screening saves at least one-third more lives.”
Another expert, Bonnie N. Joe, MD, PhD, professor in residence and chief of breast imaging in the Department of Radiology and Biomedical Imaging at the University of California, San Francisco, agreed that the study shows the mortality benefits of regular screening mammography. “Notably, these benefits were related to participation in mammography screening and independent of any advances in treatment,” she said, “And these findings in this study support regular screening mammography to reduce advanced-stage breast cancers and to reduce a woman’s risk of dying from breast cancer.”
Joe noted that overall, this was a “well-done, large-scale screening study with long-term outcomes and should be applicable to other populations. In the US, we know that peak cancer incidence is in the 40s for minority women, and the results of this study support regular screening starting at 40.”
The study was supported by the American Cancer Society through a gift from the Longaberger Company’s Horizon of Hope Campaign. Additional financial support was provided by Brostcancerförbundet, Sweden. Duffy, Autier, Quinn, Joe, and Baker have disclosed no relevant financial relationships. One coauthor of the study has disclosed relationships with industry, as noted in the original article.
This article first appeared on Medscape.com.
Three experts who were approached by Medscape Medical News say this is further evidence that regular screening mammography significantly reduces the risk of dying from breast cancer, but one expert questioned the methodology used in the study.
The primary goal of cancer screening is to detect tumors at an early stage, when they are most treatable. The hope is that this will reduce the number of advanced cancers associated with poor prognosis and hence the risk of dying from that cancer.
So far, for mammography, the data have been somewhat conflicting. For example, some evidence suggests that widespread breast cancer screening may catch more small, slow-growing tumors that are unlikely to be fatal but will not curb the number of cancers that are diagnosed at a late stage.
The new study, published online in Cancer, refutes this view.
It followed a Swedish cohort of 549,091 women (covering approximately 30% of the Swedish screening-eligible population) who underwent regular mammography.
For the women in this cohort, there was a statistically significant 41% reduction in the risk of dying of breast cancer within 10 years and a 25% reduction in the incidence of advanced disease, compared to women who did not undergo screening. “Even in this age of effective treatments, early detection confers a substantial and significant additional reduction in risk of dying from breast cancer,” said lead author Stephen W. Duffy, MSc, from the Center for Cancer Prevention at Queen Mary University, London, United Kingdom.
The current study confirms the findings of a smaller earlier study (Cancer. 2019;125:515-23) from the same investigators. “It finds the same result with an extremely large evidence base, with more than half a million women, and it also adds further to the evidence that screening achieves this reduction in the context of routine healthcare, not only in the research context,” Duffy commented. “The results are generalizable to other populations, particularly in North America, Western Europe, and Australasia, where the epidemiology and demographics of breast cancer are similar,” said Duffy. “Clearly, more intensive screening is likely to achieve a greater benefit, but a trade-off between costs, both financial and human, and benefits always has to be made specific to each societal and healthcare environment.”
In Sweden, the policy regarding breast cancer screening is to screen women aged 40 to 54 years every 18 months. For those aged 55 to 69 years, screening is recommended every 24 months.
“The use of the incidence-based endpoints means that there is accurate classification of both the breast cancer cases and the whole study population in terms of exposure to screening and avoids a number of biases seen in other studies of service screening,” Duffy told Medscape Medical News.
“I have never seen persuasive evidence for the assertion that breast cancer screening does not reduce deaths from metastatic disease – indeed, the randomized trials seem to show the opposite,” said Duffy. “This may have arisen from a misunderstanding about the mechanism whereby screening works. It primarily works by diagnosing cancer early so that treatment is successful and recurrence with distant metastases, followed by death, does not occur some years later. I suspect some colleagues have confused this with distant metastases at initial diagnosis,” he added.
One expert questions methodology
One of the experts who was approached by Medscape Medical News to comment on the new study, Philippe Autier, MD, MPH, PhD, University of Strathclyde Institute of Global Public Health at the International Prevention Research Institute, Dardilly, France, questioned the methodology of the study. “This method is incorrect simply because women attending screening are different from women not attending screening,” he said. “The former are more health aware and have healthier behaviors than the latter, and this is a well-known fact and supported by the literature.”
Autier emphasized that it is practically impossible to control for that bias, which is known as confounding by indication.
“The statistical methods used for attenuating the so-called self-selection are very approximate and based on unverified assumptions,” he said. “For this reason, the Handbook on Breast Cancer Screening produced by the International Agency for Research on Cancer [IARC] clearly stated that ‘observational studies based on individual screening history, no matter how well designed and conducted, should not be regarded as providing evidence for an effect of screening,’ and the methodology in this paper has never been recommended by the IARC.”
A better way of conducting this type of study would have been to show the incidence trends of advanced-stage breast cancer in Sweden for the entire female population aged 40 years and older, he asserts. Autier used that methodology in his own study in the Netherlands, as previously reported by Medscape Medical News. That study found that in the Netherlands, screening mammography over a period of 24 years among women aged 50 to 74 years had little effect on reducing rates of advanced breast cancer or mortality from the disease.
Experts applaud the new findings
Three of the experts who were approached by Medscape Medical News to comment on the new findings applauded the efforts of Duffy and colleagues in providing evidence that mammography can reduce breast cancer–related mortality.
Marie Quinn, MD, director of diagnostic radiology at Roswell Park Comprehensive Cancer Center, Buffalo, New York, said this study adds to the growing body of scientific evidence that confirms that women who undergo regular screening mammography significantly reduce their risk of dying from breast cancer.
“Women who underwent regular screening also had a 25% reduction in the incidence of advanced-stage breast cancer,” she said. “This is important, because breast cancers are less fatal and often require less treatment when picked up at an earlier stage. We know the risk reduction benefit detected in this well-designed study can be attributed to screening mammography and not advances in cancer treatment, due to the long-term follow-up and outcome of cancer death within 10 years.”
The findings from this study support the guidelines recommending routine screening mammography in the United States, Quinn continued, but she pointed out that some aspects of screening (e.g., the age at which to begin screening and how often to screen) can vary. “This can be confusing for patients and providers,” she said. “Overall, research has shown us that women who undergo regular screening mammograms reduce their risk of dying from breast cancer. For women of average risk, the benefit of mammography is maximized with annual screening beginning at age 40,” she said.
Jay A. Baker, MD, FACR, FSBI, chief of the Division of Breast Imaging at Duke University Medical Center, Durham, North Carolina, emphasized that this is yet another study that confirms that the improvement in breast cancer mortality is not the result of improved treatments alone, as some have speculated. “Others have tried to model the benefit of screening vs treatment, but this study is a more direct measurement,” he said. “This conclusion is important for both patients and physicians to hear.”
Although the study strongly supports regular screening for all women, it does not specifically address which set of screening guidelines is optimal, Baker commented. “Fortunately, even though some organizations in the US curiously suggest a delayed start to screening, all organizations and professional societies agree that the most lives and the most years of life are saved by yearly screening beginning at age 40,” he added. “This new study tells us that new treatments alone aren’t enough and confirms that screening saves at least one-third more lives.”
Another expert, Bonnie N. Joe, MD, PhD, professor in residence and chief of breast imaging in the Department of Radiology and Biomedical Imaging at the University of California, San Francisco, agreed that the study shows the mortality benefits of regular screening mammography. “Notably, these benefits were related to participation in mammography screening and independent of any advances in treatment,” she said, “And these findings in this study support regular screening mammography to reduce advanced-stage breast cancers and to reduce a woman’s risk of dying from breast cancer.”
Joe noted that overall, this was a “well-done, large-scale screening study with long-term outcomes and should be applicable to other populations. In the US, we know that peak cancer incidence is in the 40s for minority women, and the results of this study support regular screening starting at 40.”
The study was supported by the American Cancer Society through a gift from the Longaberger Company’s Horizon of Hope Campaign. Additional financial support was provided by Brostcancerförbundet, Sweden. Duffy, Autier, Quinn, Joe, and Baker have disclosed no relevant financial relationships. One coauthor of the study has disclosed relationships with industry, as noted in the original article.
This article first appeared on Medscape.com.
ASCO goes ahead online, as conference center is used as hospital
Traditionally at this time of year, everyone working in cancer turns their attention toward Chicago, and 40,000 or so travel to the city for the annual meeting of the American Society of Clinical Oncology (ASCO).
Not this year.
The McCormick Place convention center has been converted to a field hospital to cope with the ongoing COVID-19 pandemic. The cavernous meeting halls have been filled with makeshift wards with 750 acute care beds, as shown in a tweet from Toni Choueiri, MD, chief of genitourinary oncology at the Dana Farber Cancer Center in Boston.
But the annual meeting is still going ahead, having been transferred online.
“We have to remember that even though there’s a pandemic going on and people are dying every day from coronavirus, people are still dying every day from cancer,” Richard Schilsky, MD, PhD, chief medical officer at ASCO, told Medscape Medical News.
“This pandemic will end, but cancer will continue, and we need to be able to continue to get the most cutting edge scientific results out there to our members and our constituents so they can act on those results on behalf of their patients,” he said.
The ASCO Virtual Scientific Program will take place over the weekend of May 30-31.
“We’re certainly hoping that we’re going to deliver a program that features all of the most important science that would have been presented in person in Chicago,” Schilsky commented in an interview.
Most of the presentations will be prerecorded and then streamed, which “we hope will mitigate any of the technical glitches that could come from trying to do a live broadcast of the meeting,” he said.
There will be 250 oral and 2500 poster presentations in 24 disease-based and specialty tracks.
The majority of the abstracts will be released online on May 13. The majority of the on-demand content will be released on May 29. Some of the abstracts will be highlighted at ASCO press briefings and released on those two dates.
But some of the material will be made available only on the weekend of the meeting. The opening session, plenaries featuring late-breaking abstracts, special highlights sessions, and other clinical science symposia will be broadcast on Saturday, May 30, and Sunday, May 31 (the schedule for the weekend program is available on the ASCO meeting website).
Among the plenary presentations are some clinical results that are likely to change practice immediately, Schilsky predicted. These include data to be presented in the following abstracts:
- Abstract LBA4 on the KEYNOTE-177 study comparing immunotherapy using pembrolizumab (Keytruda, Merck & Co) with chemotherapy in patients with metastatic colorectal cancer whose tumors show microsatellite instability or mismatch repair deficiency;
- Abstract LBA5 on the ADAURA study exploring osimertinib (Tagrisso, AstraZeneca) as adjuvant therapy after complete tumor reseaction in patients with early-stage non–small cell lung cancer whose tumors are EGFR mutation positive;
- Abstract LBA1 on the JAVELIN Bladder 100 study exploring maintenance avelumab (Bavencio, Merck and Pfizer) with best supportive care after platinum-based first-line chemotherapy in patients with advanced urothelial carcinoma.
However, some of the material that would have been part of the annual meeting, which includes mostly educational sessions and invited talks, has been moved to another event, the ASCO Educational Program, to be held in August 2020.
“So I suppose, in the grand scheme of things, the meeting is going to be compressed a little bit,” Schilsky commented. “Obviously, we can’t deliver all the interactions that happen in the hallways and everywhere else at the meeting that really gives so much energy to the meeting, but, at this moment in our history, probably getting the science out there is what’s most important.”
Virtual exhibition hall
There will also be a virtual exhibition hall, which will open on May 29.
“Just as there is a typical exhibit hall in the convention center,” Schilsky commented, most of the companies that were planning to be in Chicago have “now transitioned to creating a virtual booth that people who are participating in the virtual meeting can visit.
“I don’t know exactly how each company is going to use their time and their virtual space, and that’s part of the whole learning process here to see how this whole experiment is going to work out,” he added.
Unlike some of the other conferences that have gone virtual, in which access has been made available to everyone for free, registration is still required for the ASCO meeting. But the society notes that the registration fee has been discounted for nonmembers and has been waived for ASCO members. Also, the fee covers both the Virtual Scientific Program in May and the ASCO Educational Program in August.
Registrants will have access to video and slide presentations, as well as discussant commentaries, for 180 days.
The article first appeared on Medscape.com.
Traditionally at this time of year, everyone working in cancer turns their attention toward Chicago, and 40,000 or so travel to the city for the annual meeting of the American Society of Clinical Oncology (ASCO).
Not this year.
The McCormick Place convention center has been converted to a field hospital to cope with the ongoing COVID-19 pandemic. The cavernous meeting halls have been filled with makeshift wards with 750 acute care beds, as shown in a tweet from Toni Choueiri, MD, chief of genitourinary oncology at the Dana Farber Cancer Center in Boston.
But the annual meeting is still going ahead, having been transferred online.
“We have to remember that even though there’s a pandemic going on and people are dying every day from coronavirus, people are still dying every day from cancer,” Richard Schilsky, MD, PhD, chief medical officer at ASCO, told Medscape Medical News.
“This pandemic will end, but cancer will continue, and we need to be able to continue to get the most cutting edge scientific results out there to our members and our constituents so they can act on those results on behalf of their patients,” he said.
The ASCO Virtual Scientific Program will take place over the weekend of May 30-31.
“We’re certainly hoping that we’re going to deliver a program that features all of the most important science that would have been presented in person in Chicago,” Schilsky commented in an interview.
Most of the presentations will be prerecorded and then streamed, which “we hope will mitigate any of the technical glitches that could come from trying to do a live broadcast of the meeting,” he said.
There will be 250 oral and 2500 poster presentations in 24 disease-based and specialty tracks.
The majority of the abstracts will be released online on May 13. The majority of the on-demand content will be released on May 29. Some of the abstracts will be highlighted at ASCO press briefings and released on those two dates.
But some of the material will be made available only on the weekend of the meeting. The opening session, plenaries featuring late-breaking abstracts, special highlights sessions, and other clinical science symposia will be broadcast on Saturday, May 30, and Sunday, May 31 (the schedule for the weekend program is available on the ASCO meeting website).
Among the plenary presentations are some clinical results that are likely to change practice immediately, Schilsky predicted. These include data to be presented in the following abstracts:
- Abstract LBA4 on the KEYNOTE-177 study comparing immunotherapy using pembrolizumab (Keytruda, Merck & Co) with chemotherapy in patients with metastatic colorectal cancer whose tumors show microsatellite instability or mismatch repair deficiency;
- Abstract LBA5 on the ADAURA study exploring osimertinib (Tagrisso, AstraZeneca) as adjuvant therapy after complete tumor reseaction in patients with early-stage non–small cell lung cancer whose tumors are EGFR mutation positive;
- Abstract LBA1 on the JAVELIN Bladder 100 study exploring maintenance avelumab (Bavencio, Merck and Pfizer) with best supportive care after platinum-based first-line chemotherapy in patients with advanced urothelial carcinoma.
However, some of the material that would have been part of the annual meeting, which includes mostly educational sessions and invited talks, has been moved to another event, the ASCO Educational Program, to be held in August 2020.
“So I suppose, in the grand scheme of things, the meeting is going to be compressed a little bit,” Schilsky commented. “Obviously, we can’t deliver all the interactions that happen in the hallways and everywhere else at the meeting that really gives so much energy to the meeting, but, at this moment in our history, probably getting the science out there is what’s most important.”
Virtual exhibition hall
There will also be a virtual exhibition hall, which will open on May 29.
“Just as there is a typical exhibit hall in the convention center,” Schilsky commented, most of the companies that were planning to be in Chicago have “now transitioned to creating a virtual booth that people who are participating in the virtual meeting can visit.
“I don’t know exactly how each company is going to use their time and their virtual space, and that’s part of the whole learning process here to see how this whole experiment is going to work out,” he added.
Unlike some of the other conferences that have gone virtual, in which access has been made available to everyone for free, registration is still required for the ASCO meeting. But the society notes that the registration fee has been discounted for nonmembers and has been waived for ASCO members. Also, the fee covers both the Virtual Scientific Program in May and the ASCO Educational Program in August.
Registrants will have access to video and slide presentations, as well as discussant commentaries, for 180 days.
The article first appeared on Medscape.com.
Traditionally at this time of year, everyone working in cancer turns their attention toward Chicago, and 40,000 or so travel to the city for the annual meeting of the American Society of Clinical Oncology (ASCO).
Not this year.
The McCormick Place convention center has been converted to a field hospital to cope with the ongoing COVID-19 pandemic. The cavernous meeting halls have been filled with makeshift wards with 750 acute care beds, as shown in a tweet from Toni Choueiri, MD, chief of genitourinary oncology at the Dana Farber Cancer Center in Boston.
But the annual meeting is still going ahead, having been transferred online.
“We have to remember that even though there’s a pandemic going on and people are dying every day from coronavirus, people are still dying every day from cancer,” Richard Schilsky, MD, PhD, chief medical officer at ASCO, told Medscape Medical News.
“This pandemic will end, but cancer will continue, and we need to be able to continue to get the most cutting edge scientific results out there to our members and our constituents so they can act on those results on behalf of their patients,” he said.
The ASCO Virtual Scientific Program will take place over the weekend of May 30-31.
“We’re certainly hoping that we’re going to deliver a program that features all of the most important science that would have been presented in person in Chicago,” Schilsky commented in an interview.
Most of the presentations will be prerecorded and then streamed, which “we hope will mitigate any of the technical glitches that could come from trying to do a live broadcast of the meeting,” he said.
There will be 250 oral and 2500 poster presentations in 24 disease-based and specialty tracks.
The majority of the abstracts will be released online on May 13. The majority of the on-demand content will be released on May 29. Some of the abstracts will be highlighted at ASCO press briefings and released on those two dates.
But some of the material will be made available only on the weekend of the meeting. The opening session, plenaries featuring late-breaking abstracts, special highlights sessions, and other clinical science symposia will be broadcast on Saturday, May 30, and Sunday, May 31 (the schedule for the weekend program is available on the ASCO meeting website).
Among the plenary presentations are some clinical results that are likely to change practice immediately, Schilsky predicted. These include data to be presented in the following abstracts:
- Abstract LBA4 on the KEYNOTE-177 study comparing immunotherapy using pembrolizumab (Keytruda, Merck & Co) with chemotherapy in patients with metastatic colorectal cancer whose tumors show microsatellite instability or mismatch repair deficiency;
- Abstract LBA5 on the ADAURA study exploring osimertinib (Tagrisso, AstraZeneca) as adjuvant therapy after complete tumor reseaction in patients with early-stage non–small cell lung cancer whose tumors are EGFR mutation positive;
- Abstract LBA1 on the JAVELIN Bladder 100 study exploring maintenance avelumab (Bavencio, Merck and Pfizer) with best supportive care after platinum-based first-line chemotherapy in patients with advanced urothelial carcinoma.
However, some of the material that would have been part of the annual meeting, which includes mostly educational sessions and invited talks, has been moved to another event, the ASCO Educational Program, to be held in August 2020.
“So I suppose, in the grand scheme of things, the meeting is going to be compressed a little bit,” Schilsky commented. “Obviously, we can’t deliver all the interactions that happen in the hallways and everywhere else at the meeting that really gives so much energy to the meeting, but, at this moment in our history, probably getting the science out there is what’s most important.”
Virtual exhibition hall
There will also be a virtual exhibition hall, which will open on May 29.
“Just as there is a typical exhibit hall in the convention center,” Schilsky commented, most of the companies that were planning to be in Chicago have “now transitioned to creating a virtual booth that people who are participating in the virtual meeting can visit.
“I don’t know exactly how each company is going to use their time and their virtual space, and that’s part of the whole learning process here to see how this whole experiment is going to work out,” he added.
Unlike some of the other conferences that have gone virtual, in which access has been made available to everyone for free, registration is still required for the ASCO meeting. But the society notes that the registration fee has been discounted for nonmembers and has been waived for ASCO members. Also, the fee covers both the Virtual Scientific Program in May and the ASCO Educational Program in August.
Registrants will have access to video and slide presentations, as well as discussant commentaries, for 180 days.
The article first appeared on Medscape.com.
COVID-19 death rate was twice as high in cancer patients in NYC study
COVID-19 patients with cancer had double the fatality rate of COVID-19 patients without cancer treated in an urban New York hospital system, according to data from a retrospective study.
with COVID-19 treated during the same time period in the same hospital system.
Vikas Mehta, MD, of Montefiore Medical Center, New York, and colleagues reported these results in Cancer Discovery.
“As New York has emerged as the current epicenter of the pandemic, we sought to investigate the risk posed by COVID-19 to our cancer population,” the authors wrote.
They identified 218 cancer patients treated for COVID-19 in the Montefiore Health System between March 18 and April 8, 2020. Three-quarters of patients had solid tumors, and 25% had hematologic malignancies. Most patients were adults (98.6%), their median age was 69 years (range, 10-92 years), and 58% were men.
In all, 28% of the cancer patients (61/218) died from COVID-19, including 25% (41/164) of those with solid tumors and 37% (20/54) of those with hematologic malignancies.
Deaths by cancer type
Among the 164 patients with solid tumors, case fatality rates were as follows:
- Pancreatic – 67% (2/3)
- Lung – 55% (6/11)
- Colorectal – 38% (8/21)
- Upper gastrointestinal – 38% (3/8)
- Gynecologic – 38% (5/13)
- Skin – 33% (1/3)
- Hepatobiliary – 29% (2/7)
- Bone/soft tissue – 20% (1/5)
- Genitourinary – 15% (7/46)
- Breast – 14% (4/28)
- Neurologic – 13% (1/8)
- Head and neck – 13% (1/8).
None of the three patients with neuroendocrine tumors died.
Among the 54 patients with hematologic malignancies, case fatality rates were as follows:
- Chronic myeloid leukemia – 100% (1/1)
- Hodgkin lymphoma – 60% (3/5)
- Myelodysplastic syndromes – 60% (3/5)
- Multiple myeloma – 38% (5/13)
- Non-Hodgkin lymphoma – 33% (5/15)
- Chronic lymphocytic leukemia – 33% (1/3)
- Myeloproliferative neoplasms – 29% (2/7).
None of the four patients with acute lymphoblastic leukemia died, and there was one patient with acute myeloid leukemia who did not die.
Factors associated with increased mortality
The researchers compared the 218 cancer patients with COVID-19 with 1,090 age- and sex-matched noncancer patients with COVID-19 treated in the Montefiore Health System between March 18 and April 8, 2020.
Case fatality rates in cancer patients with COVID-19 were significantly increased in all age groups, but older age was associated with higher mortality.
“We observed case fatality rates were elevated in all age cohorts in cancer patients and achieved statistical significance in the age groups 45-64 and in patients older than 75 years of age,” the authors reported.
Other factors significantly associated with higher mortality in a multivariable analysis included the presence of multiple comorbidities; the need for ICU support; and increased levels of d-dimer, lactate, and lactate dehydrogenase.
Additional factors, such as socioeconomic and health disparities, may also be significant predictors of mortality, according to the authors. They noted that this cohort largely consisted of patients from a socioeconomically underprivileged community where mortality because of COVID-19 is reportedly higher.
Proactive strategies moving forward
“We have been addressing the significant burden of the COVID-19 pandemic on our vulnerable cancer patients through a variety of ways,” said study author Balazs Halmos, MD, of Montefiore Medical Center.
The center set up a separate infusion unit exclusively for COVID-positive patients and established separate inpatient areas. Dr. Halmos and colleagues are also providing telemedicine, virtual supportive care services, telephonic counseling, and bilingual peer-support programs.
“Many questions remain as we continue to establish new practices for our cancer patients,” Dr. Halmos said. “We will find answers to these questions as we continue to focus on adaptation and not acceptance in response to the COVID crisis. Our patients deserve nothing less.”
The Albert Einstein Cancer Center supported this study. The authors reported having no conflicts of interest.
SOURCE: Mehta V et al. Cancer Discov. 2020 May 1. doi: 10.1158/2159-8290.CD-20-0516.
COVID-19 patients with cancer had double the fatality rate of COVID-19 patients without cancer treated in an urban New York hospital system, according to data from a retrospective study.
with COVID-19 treated during the same time period in the same hospital system.
Vikas Mehta, MD, of Montefiore Medical Center, New York, and colleagues reported these results in Cancer Discovery.
“As New York has emerged as the current epicenter of the pandemic, we sought to investigate the risk posed by COVID-19 to our cancer population,” the authors wrote.
They identified 218 cancer patients treated for COVID-19 in the Montefiore Health System between March 18 and April 8, 2020. Three-quarters of patients had solid tumors, and 25% had hematologic malignancies. Most patients were adults (98.6%), their median age was 69 years (range, 10-92 years), and 58% were men.
In all, 28% of the cancer patients (61/218) died from COVID-19, including 25% (41/164) of those with solid tumors and 37% (20/54) of those with hematologic malignancies.
Deaths by cancer type
Among the 164 patients with solid tumors, case fatality rates were as follows:
- Pancreatic – 67% (2/3)
- Lung – 55% (6/11)
- Colorectal – 38% (8/21)
- Upper gastrointestinal – 38% (3/8)
- Gynecologic – 38% (5/13)
- Skin – 33% (1/3)
- Hepatobiliary – 29% (2/7)
- Bone/soft tissue – 20% (1/5)
- Genitourinary – 15% (7/46)
- Breast – 14% (4/28)
- Neurologic – 13% (1/8)
- Head and neck – 13% (1/8).
None of the three patients with neuroendocrine tumors died.
Among the 54 patients with hematologic malignancies, case fatality rates were as follows:
- Chronic myeloid leukemia – 100% (1/1)
- Hodgkin lymphoma – 60% (3/5)
- Myelodysplastic syndromes – 60% (3/5)
- Multiple myeloma – 38% (5/13)
- Non-Hodgkin lymphoma – 33% (5/15)
- Chronic lymphocytic leukemia – 33% (1/3)
- Myeloproliferative neoplasms – 29% (2/7).
None of the four patients with acute lymphoblastic leukemia died, and there was one patient with acute myeloid leukemia who did not die.
Factors associated with increased mortality
The researchers compared the 218 cancer patients with COVID-19 with 1,090 age- and sex-matched noncancer patients with COVID-19 treated in the Montefiore Health System between March 18 and April 8, 2020.
Case fatality rates in cancer patients with COVID-19 were significantly increased in all age groups, but older age was associated with higher mortality.
“We observed case fatality rates were elevated in all age cohorts in cancer patients and achieved statistical significance in the age groups 45-64 and in patients older than 75 years of age,” the authors reported.
Other factors significantly associated with higher mortality in a multivariable analysis included the presence of multiple comorbidities; the need for ICU support; and increased levels of d-dimer, lactate, and lactate dehydrogenase.
Additional factors, such as socioeconomic and health disparities, may also be significant predictors of mortality, according to the authors. They noted that this cohort largely consisted of patients from a socioeconomically underprivileged community where mortality because of COVID-19 is reportedly higher.
Proactive strategies moving forward
“We have been addressing the significant burden of the COVID-19 pandemic on our vulnerable cancer patients through a variety of ways,” said study author Balazs Halmos, MD, of Montefiore Medical Center.
The center set up a separate infusion unit exclusively for COVID-positive patients and established separate inpatient areas. Dr. Halmos and colleagues are also providing telemedicine, virtual supportive care services, telephonic counseling, and bilingual peer-support programs.
“Many questions remain as we continue to establish new practices for our cancer patients,” Dr. Halmos said. “We will find answers to these questions as we continue to focus on adaptation and not acceptance in response to the COVID crisis. Our patients deserve nothing less.”
The Albert Einstein Cancer Center supported this study. The authors reported having no conflicts of interest.
SOURCE: Mehta V et al. Cancer Discov. 2020 May 1. doi: 10.1158/2159-8290.CD-20-0516.
COVID-19 patients with cancer had double the fatality rate of COVID-19 patients without cancer treated in an urban New York hospital system, according to data from a retrospective study.
with COVID-19 treated during the same time period in the same hospital system.
Vikas Mehta, MD, of Montefiore Medical Center, New York, and colleagues reported these results in Cancer Discovery.
“As New York has emerged as the current epicenter of the pandemic, we sought to investigate the risk posed by COVID-19 to our cancer population,” the authors wrote.
They identified 218 cancer patients treated for COVID-19 in the Montefiore Health System between March 18 and April 8, 2020. Three-quarters of patients had solid tumors, and 25% had hematologic malignancies. Most patients were adults (98.6%), their median age was 69 years (range, 10-92 years), and 58% were men.
In all, 28% of the cancer patients (61/218) died from COVID-19, including 25% (41/164) of those with solid tumors and 37% (20/54) of those with hematologic malignancies.
Deaths by cancer type
Among the 164 patients with solid tumors, case fatality rates were as follows:
- Pancreatic – 67% (2/3)
- Lung – 55% (6/11)
- Colorectal – 38% (8/21)
- Upper gastrointestinal – 38% (3/8)
- Gynecologic – 38% (5/13)
- Skin – 33% (1/3)
- Hepatobiliary – 29% (2/7)
- Bone/soft tissue – 20% (1/5)
- Genitourinary – 15% (7/46)
- Breast – 14% (4/28)
- Neurologic – 13% (1/8)
- Head and neck – 13% (1/8).
None of the three patients with neuroendocrine tumors died.
Among the 54 patients with hematologic malignancies, case fatality rates were as follows:
- Chronic myeloid leukemia – 100% (1/1)
- Hodgkin lymphoma – 60% (3/5)
- Myelodysplastic syndromes – 60% (3/5)
- Multiple myeloma – 38% (5/13)
- Non-Hodgkin lymphoma – 33% (5/15)
- Chronic lymphocytic leukemia – 33% (1/3)
- Myeloproliferative neoplasms – 29% (2/7).
None of the four patients with acute lymphoblastic leukemia died, and there was one patient with acute myeloid leukemia who did not die.
Factors associated with increased mortality
The researchers compared the 218 cancer patients with COVID-19 with 1,090 age- and sex-matched noncancer patients with COVID-19 treated in the Montefiore Health System between March 18 and April 8, 2020.
Case fatality rates in cancer patients with COVID-19 were significantly increased in all age groups, but older age was associated with higher mortality.
“We observed case fatality rates were elevated in all age cohorts in cancer patients and achieved statistical significance in the age groups 45-64 and in patients older than 75 years of age,” the authors reported.
Other factors significantly associated with higher mortality in a multivariable analysis included the presence of multiple comorbidities; the need for ICU support; and increased levels of d-dimer, lactate, and lactate dehydrogenase.
Additional factors, such as socioeconomic and health disparities, may also be significant predictors of mortality, according to the authors. They noted that this cohort largely consisted of patients from a socioeconomically underprivileged community where mortality because of COVID-19 is reportedly higher.
Proactive strategies moving forward
“We have been addressing the significant burden of the COVID-19 pandemic on our vulnerable cancer patients through a variety of ways,” said study author Balazs Halmos, MD, of Montefiore Medical Center.
The center set up a separate infusion unit exclusively for COVID-positive patients and established separate inpatient areas. Dr. Halmos and colleagues are also providing telemedicine, virtual supportive care services, telephonic counseling, and bilingual peer-support programs.
“Many questions remain as we continue to establish new practices for our cancer patients,” Dr. Halmos said. “We will find answers to these questions as we continue to focus on adaptation and not acceptance in response to the COVID crisis. Our patients deserve nothing less.”
The Albert Einstein Cancer Center supported this study. The authors reported having no conflicts of interest.
SOURCE: Mehta V et al. Cancer Discov. 2020 May 1. doi: 10.1158/2159-8290.CD-20-0516.
FROM CANCER DISCOVERY
Three months of COVID-19 may mean 80,000 missed cancer diagnoses
, according to a report by the IQVIA Institute for Human Data Science looking at trends in the United States.
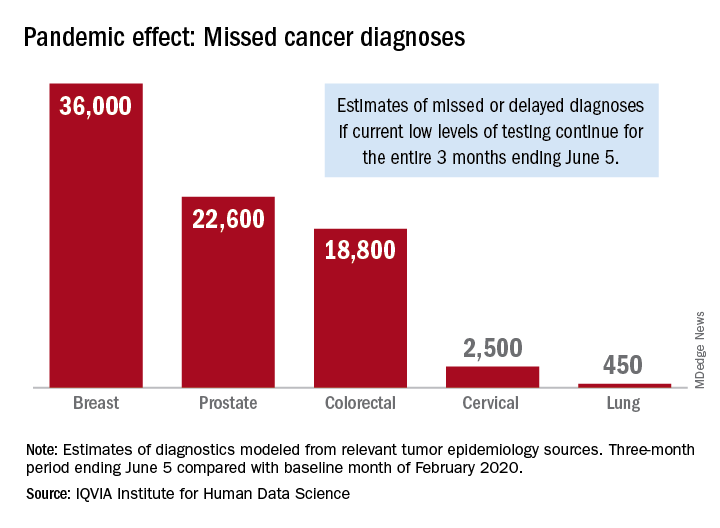
Screening and monitoring tests for breast, prostate, colorectal, cervical, and lung cancer were down 39%-90% in early April, compared with the baseline month of February, according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
These findings are based on data from IQVIA’s medical claims database, which includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data suggest that, at current positivity rates, there could be 36,000 missed or delayed diagnoses of breast cancer during the 3-month period from early March through early June. Estimates for missed diagnoses of the four other cancers analyzed include 450 for lung cancer, 2,500 for cervical cancer, 18,800 for colorectal cancer, and 22,600 for prostate cancer.
The authors project a total of 22 million canceled or delayed tests for the five cancers over the 3-month period ending June 5, based on a comparison of claims data for early April with the February baseline. Catching up on this backlog will be problematic, according to the authors.
“Current excess health care capacity ... would require providers to shift priorities to make time and space in schedules and facilities as well as the cooperation of patients to return to health care providers,” the authors wrote. “Both of these could be further disrupted by economic factors or reintroduction of social distancing in a reemergence of the outbreak.”
The report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
, according to a report by the IQVIA Institute for Human Data Science looking at trends in the United States.

Screening and monitoring tests for breast, prostate, colorectal, cervical, and lung cancer were down 39%-90% in early April, compared with the baseline month of February, according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
These findings are based on data from IQVIA’s medical claims database, which includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data suggest that, at current positivity rates, there could be 36,000 missed or delayed diagnoses of breast cancer during the 3-month period from early March through early June. Estimates for missed diagnoses of the four other cancers analyzed include 450 for lung cancer, 2,500 for cervical cancer, 18,800 for colorectal cancer, and 22,600 for prostate cancer.
The authors project a total of 22 million canceled or delayed tests for the five cancers over the 3-month period ending June 5, based on a comparison of claims data for early April with the February baseline. Catching up on this backlog will be problematic, according to the authors.
“Current excess health care capacity ... would require providers to shift priorities to make time and space in schedules and facilities as well as the cooperation of patients to return to health care providers,” the authors wrote. “Both of these could be further disrupted by economic factors or reintroduction of social distancing in a reemergence of the outbreak.”
The report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
, according to a report by the IQVIA Institute for Human Data Science looking at trends in the United States.

Screening and monitoring tests for breast, prostate, colorectal, cervical, and lung cancer were down 39%-90% in early April, compared with the baseline month of February, according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
These findings are based on data from IQVIA’s medical claims database, which includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data suggest that, at current positivity rates, there could be 36,000 missed or delayed diagnoses of breast cancer during the 3-month period from early March through early June. Estimates for missed diagnoses of the four other cancers analyzed include 450 for lung cancer, 2,500 for cervical cancer, 18,800 for colorectal cancer, and 22,600 for prostate cancer.
The authors project a total of 22 million canceled or delayed tests for the five cancers over the 3-month period ending June 5, based on a comparison of claims data for early April with the February baseline. Catching up on this backlog will be problematic, according to the authors.
“Current excess health care capacity ... would require providers to shift priorities to make time and space in schedules and facilities as well as the cooperation of patients to return to health care providers,” the authors wrote. “Both of these could be further disrupted by economic factors or reintroduction of social distancing in a reemergence of the outbreak.”
The report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
Cancer screening, monitoring down during pandemic
according to a report by the IQVIA Institute for Human Data Science.
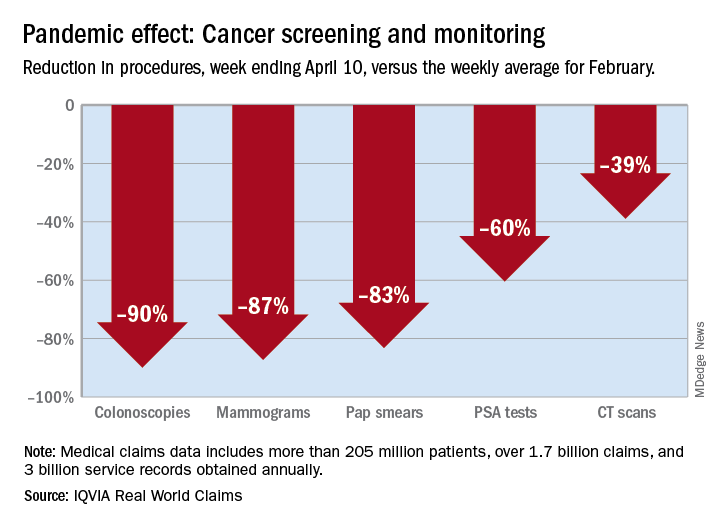
There were 90% fewer colonoscopies ordered during the week ending April 10, compared with the weekly average for Feb. 1-28, based on claims data analyzed by IQVIA.
IQVIA’s medical claims database includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data also showed an 87% reduction in mammograms and an 83% reduction in Pap smears during the week ending April 10. Prostate-specific antigen tests for prostate cancer decreased by 60%, and CT scans for lung cancer decreased by 39%.
The smaller decrease in CT scans for lung cancer “may reflect the generally more serious nature of those tumors or be due to concerns about ruling out COVID-related issues in some patients,” according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
The report also showed that overall patient interactions with oncologists were down by 20% through April 3, based on medical and pharmacy claims processed since February, but there was variation by tumor type.
The authors noted “little or no disruption” in oncologist visits in March for patients with aggressive tumors or those diagnosed at advanced stages, compared with February. However, for patients with skin cancer or prostate cancer, visit rates were down by 20%-50% in March.
“This may reflect that oncologists who are providing care across multiple tumor types are prioritizing their time and efforts to those patients with more advanced or aggressive tumors,” the authors wrote.
This report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
according to a report by the IQVIA Institute for Human Data Science.

There were 90% fewer colonoscopies ordered during the week ending April 10, compared with the weekly average for Feb. 1-28, based on claims data analyzed by IQVIA.
IQVIA’s medical claims database includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data also showed an 87% reduction in mammograms and an 83% reduction in Pap smears during the week ending April 10. Prostate-specific antigen tests for prostate cancer decreased by 60%, and CT scans for lung cancer decreased by 39%.
The smaller decrease in CT scans for lung cancer “may reflect the generally more serious nature of those tumors or be due to concerns about ruling out COVID-related issues in some patients,” according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
The report also showed that overall patient interactions with oncologists were down by 20% through April 3, based on medical and pharmacy claims processed since February, but there was variation by tumor type.
The authors noted “little or no disruption” in oncologist visits in March for patients with aggressive tumors or those diagnosed at advanced stages, compared with February. However, for patients with skin cancer or prostate cancer, visit rates were down by 20%-50% in March.
“This may reflect that oncologists who are providing care across multiple tumor types are prioritizing their time and efforts to those patients with more advanced or aggressive tumors,” the authors wrote.
This report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
according to a report by the IQVIA Institute for Human Data Science.

There were 90% fewer colonoscopies ordered during the week ending April 10, compared with the weekly average for Feb. 1-28, based on claims data analyzed by IQVIA.
IQVIA’s medical claims database includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data also showed an 87% reduction in mammograms and an 83% reduction in Pap smears during the week ending April 10. Prostate-specific antigen tests for prostate cancer decreased by 60%, and CT scans for lung cancer decreased by 39%.
The smaller decrease in CT scans for lung cancer “may reflect the generally more serious nature of those tumors or be due to concerns about ruling out COVID-related issues in some patients,” according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
The report also showed that overall patient interactions with oncologists were down by 20% through April 3, based on medical and pharmacy claims processed since February, but there was variation by tumor type.
The authors noted “little or no disruption” in oncologist visits in March for patients with aggressive tumors or those diagnosed at advanced stages, compared with February. However, for patients with skin cancer or prostate cancer, visit rates were down by 20%-50% in March.
“This may reflect that oncologists who are providing care across multiple tumor types are prioritizing their time and efforts to those patients with more advanced or aggressive tumors,” the authors wrote.
This report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
Adding a blood test to standard screening may improve early cancer detection
A minimally invasive multicancer blood test used with standard-of-care screening is safe, effective, and feasible for use in routine clinical care, according to interim findings from a large, prospective study.
The DETECT-A blood test, an early version of the CancerSEEK test currently in development, effectively guided patient management in real time, in some cases leading to diagnosis of early cancer and potentially curative surgery in asymptomatic women with no history of cancer.
Nickolas Papadopoulos, PhD, of Johns Hopkins Medicine in Baltimore, reported these findings at the AACR virtual meeting I. The findings were simultaneously published in Science.
The study enrolled 10,006 women, aged 65-75 years, with no prior cancer diagnosis. After exclusion and loss to follow-up, 9,911 women remained.
There were 26 patients who had cancer detected by the DETECT-A blood test, 15 of whom underwent follow-up PET-CT imaging and 9 of whom underwent surgical excision. An additional 24 cancers were detected by standard screening, and 46 were detected by other means.
The positive predictive value of the blood test was 19%. When the blood test was combined with imaging, the positive predictive value was 41%.
Improving upon standard screening
“Standard-of-care screening [was used] for three different organs: breast, lung, and colon. It was more sensitive for breast cancer,” Dr. Papadopoulos noted. “Blood testing, though, identified cancer in 10 different organs.”
In fact, the DETECT-A blood test detected 14 of 45 cancers in 7 organs for which no standard screening test is available.
In addition, 12 cancers in 3 organs (breast, lung, and colon) were first detected by DETECT-A rather than by standard screening. This increased the sensitivity of cancer detection from 47% with standard screening alone to 71% with standard screening plus blood testing.
“More important, 65% [of the cancers detected by blood test] were localized or regional, which have higher chance of successful treatment with intent to cure,” Dr. Papadopoulos said.
DETECT-A covers regions of 16 commonly mutated genes and 9 proteins known to be associated with cancer. In this study, 57% of cancers were detected by mutations.
Safety and additional screening
DETECT-A also proved safe, “without incurring a large number of futile invasive follow-up tests,” Dr. Papadopoulos said.
In fact, only 1% of patients without cancer underwent PET-CT imaging, and only 0.22% underwent a “futile” invasive follow-up procedure.
Three surgeries occurred in patients who were counted as false-positives, but the surgeries were determined to be indicated, Dr. Papadopoulos said. He explained that one was for large colonic polyps with high-grade dysplasia that could not be removed endoscopically, one was for an in situ carcinoma of the appendix, and one was for a 10-cm ovarian lesion that was found to be a mucinous cystadenoma.
The investigators also analyzed whether the availability of a “liquid biopsy” test like DETECT-A would inadvertently reduce patients’ use of standard screening and found that it did not. Mammography screening habits after receiving the baseline DETECT-A blood test did not differ significantly from those prior to study enrollment.
These findings are important because early detection is a key factor in reducing cancer-specific morbidity and mortality, and although minimally invasive screening tests, including liquid biopsies like DETECT-A, hold great promise, prospective clinical studies of these new methods are needed to ensure that the anticipated benefits outweigh the potential risks, Dr. Papadopoulos explained.
“The problem is that most cancers are detected at advanced stages when they are difficult to treat,” he said. “The earlier cancer is detected, the greater the chance of successful treatment.”
Unanswered questions and future studies
This study demonstrates that it is feasible for a minimally invasive blood test to safely detect multiple cancer types in patients without a history of cancer and to enable treatment with curative intent, at least in a subset of individuals, Dr. Papadopoulos said. He added that the findings also inform the design of future randomized trials “to establish clinical utility, cost-effectiveness, and benefit-to-risk ratio of future tests.”
Further studies will also be required to determine the clinical validity and utility of the strategy of using liquid biopsy as a complement to standard-of-care screening, Dr. Papadopoulos said.
Invited discussant David G. Huntsman, MD, of the University of British Columbia in Vancouver, applauded the investigators, saying this study serves to “move the field forward.” However, it still isn’t clear how sensitivity and negative predictive value will be determined and what the optimal testing schedule is.
“This is a prospective study that will provide the data on how this assay will be used [and] whether it should be used going forward,” Dr. Huntsman said, noting that the “much bigger and more important question” is whether it improves survival.
Cost-effectiveness will also be critical, he said.
This research was supported by The Marcus Foundation, Lustgarten Foundation for Pancreatic Cancer Research, The Virginia and D.K. Ludwig Fund for Cancer Research, The Sol Goldman Center for Pancreatic Cancer Research, Susan Wojcicki and Dennis Troper, the Rolfe Foundation, The Conrad R. Hilton Foundation, The John Templeton Foundation, Burroughs Wellcome Career Award For Medical Scientists, and grants/contracts from the National Institutes of Health.
Dr. Papadopoulos disclosed relationships with Thrive Earlier Detection Inc., PGDx Inc., NeoPhore, Cage Pharma, and other companies. Dr. Huntsman is a founder, shareholder, and chief medical officer for Contextual Genomics.
SOURCE: Papadopoulos N et al. AACR 2020, Abstract CT022; Lennon AM et al. Science. 2020 Apr 28. pii: eabb9601. doi: 10.1126/science.abb9601.
A minimally invasive multicancer blood test used with standard-of-care screening is safe, effective, and feasible for use in routine clinical care, according to interim findings from a large, prospective study.
The DETECT-A blood test, an early version of the CancerSEEK test currently in development, effectively guided patient management in real time, in some cases leading to diagnosis of early cancer and potentially curative surgery in asymptomatic women with no history of cancer.
Nickolas Papadopoulos, PhD, of Johns Hopkins Medicine in Baltimore, reported these findings at the AACR virtual meeting I. The findings were simultaneously published in Science.
The study enrolled 10,006 women, aged 65-75 years, with no prior cancer diagnosis. After exclusion and loss to follow-up, 9,911 women remained.
There were 26 patients who had cancer detected by the DETECT-A blood test, 15 of whom underwent follow-up PET-CT imaging and 9 of whom underwent surgical excision. An additional 24 cancers were detected by standard screening, and 46 were detected by other means.
The positive predictive value of the blood test was 19%. When the blood test was combined with imaging, the positive predictive value was 41%.
Improving upon standard screening
“Standard-of-care screening [was used] for three different organs: breast, lung, and colon. It was more sensitive for breast cancer,” Dr. Papadopoulos noted. “Blood testing, though, identified cancer in 10 different organs.”
In fact, the DETECT-A blood test detected 14 of 45 cancers in 7 organs for which no standard screening test is available.
In addition, 12 cancers in 3 organs (breast, lung, and colon) were first detected by DETECT-A rather than by standard screening. This increased the sensitivity of cancer detection from 47% with standard screening alone to 71% with standard screening plus blood testing.
“More important, 65% [of the cancers detected by blood test] were localized or regional, which have higher chance of successful treatment with intent to cure,” Dr. Papadopoulos said.
DETECT-A covers regions of 16 commonly mutated genes and 9 proteins known to be associated with cancer. In this study, 57% of cancers were detected by mutations.
Safety and additional screening
DETECT-A also proved safe, “without incurring a large number of futile invasive follow-up tests,” Dr. Papadopoulos said.
In fact, only 1% of patients without cancer underwent PET-CT imaging, and only 0.22% underwent a “futile” invasive follow-up procedure.
Three surgeries occurred in patients who were counted as false-positives, but the surgeries were determined to be indicated, Dr. Papadopoulos said. He explained that one was for large colonic polyps with high-grade dysplasia that could not be removed endoscopically, one was for an in situ carcinoma of the appendix, and one was for a 10-cm ovarian lesion that was found to be a mucinous cystadenoma.
The investigators also analyzed whether the availability of a “liquid biopsy” test like DETECT-A would inadvertently reduce patients’ use of standard screening and found that it did not. Mammography screening habits after receiving the baseline DETECT-A blood test did not differ significantly from those prior to study enrollment.
These findings are important because early detection is a key factor in reducing cancer-specific morbidity and mortality, and although minimally invasive screening tests, including liquid biopsies like DETECT-A, hold great promise, prospective clinical studies of these new methods are needed to ensure that the anticipated benefits outweigh the potential risks, Dr. Papadopoulos explained.
“The problem is that most cancers are detected at advanced stages when they are difficult to treat,” he said. “The earlier cancer is detected, the greater the chance of successful treatment.”
Unanswered questions and future studies
This study demonstrates that it is feasible for a minimally invasive blood test to safely detect multiple cancer types in patients without a history of cancer and to enable treatment with curative intent, at least in a subset of individuals, Dr. Papadopoulos said. He added that the findings also inform the design of future randomized trials “to establish clinical utility, cost-effectiveness, and benefit-to-risk ratio of future tests.”
Further studies will also be required to determine the clinical validity and utility of the strategy of using liquid biopsy as a complement to standard-of-care screening, Dr. Papadopoulos said.
Invited discussant David G. Huntsman, MD, of the University of British Columbia in Vancouver, applauded the investigators, saying this study serves to “move the field forward.” However, it still isn’t clear how sensitivity and negative predictive value will be determined and what the optimal testing schedule is.
“This is a prospective study that will provide the data on how this assay will be used [and] whether it should be used going forward,” Dr. Huntsman said, noting that the “much bigger and more important question” is whether it improves survival.
Cost-effectiveness will also be critical, he said.
This research was supported by The Marcus Foundation, Lustgarten Foundation for Pancreatic Cancer Research, The Virginia and D.K. Ludwig Fund for Cancer Research, The Sol Goldman Center for Pancreatic Cancer Research, Susan Wojcicki and Dennis Troper, the Rolfe Foundation, The Conrad R. Hilton Foundation, The John Templeton Foundation, Burroughs Wellcome Career Award For Medical Scientists, and grants/contracts from the National Institutes of Health.
Dr. Papadopoulos disclosed relationships with Thrive Earlier Detection Inc., PGDx Inc., NeoPhore, Cage Pharma, and other companies. Dr. Huntsman is a founder, shareholder, and chief medical officer for Contextual Genomics.
SOURCE: Papadopoulos N et al. AACR 2020, Abstract CT022; Lennon AM et al. Science. 2020 Apr 28. pii: eabb9601. doi: 10.1126/science.abb9601.
A minimally invasive multicancer blood test used with standard-of-care screening is safe, effective, and feasible for use in routine clinical care, according to interim findings from a large, prospective study.
The DETECT-A blood test, an early version of the CancerSEEK test currently in development, effectively guided patient management in real time, in some cases leading to diagnosis of early cancer and potentially curative surgery in asymptomatic women with no history of cancer.
Nickolas Papadopoulos, PhD, of Johns Hopkins Medicine in Baltimore, reported these findings at the AACR virtual meeting I. The findings were simultaneously published in Science.
The study enrolled 10,006 women, aged 65-75 years, with no prior cancer diagnosis. After exclusion and loss to follow-up, 9,911 women remained.
There were 26 patients who had cancer detected by the DETECT-A blood test, 15 of whom underwent follow-up PET-CT imaging and 9 of whom underwent surgical excision. An additional 24 cancers were detected by standard screening, and 46 were detected by other means.
The positive predictive value of the blood test was 19%. When the blood test was combined with imaging, the positive predictive value was 41%.
Improving upon standard screening
“Standard-of-care screening [was used] for three different organs: breast, lung, and colon. It was more sensitive for breast cancer,” Dr. Papadopoulos noted. “Blood testing, though, identified cancer in 10 different organs.”
In fact, the DETECT-A blood test detected 14 of 45 cancers in 7 organs for which no standard screening test is available.
In addition, 12 cancers in 3 organs (breast, lung, and colon) were first detected by DETECT-A rather than by standard screening. This increased the sensitivity of cancer detection from 47% with standard screening alone to 71% with standard screening plus blood testing.
“More important, 65% [of the cancers detected by blood test] were localized or regional, which have higher chance of successful treatment with intent to cure,” Dr. Papadopoulos said.
DETECT-A covers regions of 16 commonly mutated genes and 9 proteins known to be associated with cancer. In this study, 57% of cancers were detected by mutations.
Safety and additional screening
DETECT-A also proved safe, “without incurring a large number of futile invasive follow-up tests,” Dr. Papadopoulos said.
In fact, only 1% of patients without cancer underwent PET-CT imaging, and only 0.22% underwent a “futile” invasive follow-up procedure.
Three surgeries occurred in patients who were counted as false-positives, but the surgeries were determined to be indicated, Dr. Papadopoulos said. He explained that one was for large colonic polyps with high-grade dysplasia that could not be removed endoscopically, one was for an in situ carcinoma of the appendix, and one was for a 10-cm ovarian lesion that was found to be a mucinous cystadenoma.
The investigators also analyzed whether the availability of a “liquid biopsy” test like DETECT-A would inadvertently reduce patients’ use of standard screening and found that it did not. Mammography screening habits after receiving the baseline DETECT-A blood test did not differ significantly from those prior to study enrollment.
These findings are important because early detection is a key factor in reducing cancer-specific morbidity and mortality, and although minimally invasive screening tests, including liquid biopsies like DETECT-A, hold great promise, prospective clinical studies of these new methods are needed to ensure that the anticipated benefits outweigh the potential risks, Dr. Papadopoulos explained.
“The problem is that most cancers are detected at advanced stages when they are difficult to treat,” he said. “The earlier cancer is detected, the greater the chance of successful treatment.”
Unanswered questions and future studies
This study demonstrates that it is feasible for a minimally invasive blood test to safely detect multiple cancer types in patients without a history of cancer and to enable treatment with curative intent, at least in a subset of individuals, Dr. Papadopoulos said. He added that the findings also inform the design of future randomized trials “to establish clinical utility, cost-effectiveness, and benefit-to-risk ratio of future tests.”
Further studies will also be required to determine the clinical validity and utility of the strategy of using liquid biopsy as a complement to standard-of-care screening, Dr. Papadopoulos said.
Invited discussant David G. Huntsman, MD, of the University of British Columbia in Vancouver, applauded the investigators, saying this study serves to “move the field forward.” However, it still isn’t clear how sensitivity and negative predictive value will be determined and what the optimal testing schedule is.
“This is a prospective study that will provide the data on how this assay will be used [and] whether it should be used going forward,” Dr. Huntsman said, noting that the “much bigger and more important question” is whether it improves survival.
Cost-effectiveness will also be critical, he said.
This research was supported by The Marcus Foundation, Lustgarten Foundation for Pancreatic Cancer Research, The Virginia and D.K. Ludwig Fund for Cancer Research, The Sol Goldman Center for Pancreatic Cancer Research, Susan Wojcicki and Dennis Troper, the Rolfe Foundation, The Conrad R. Hilton Foundation, The John Templeton Foundation, Burroughs Wellcome Career Award For Medical Scientists, and grants/contracts from the National Institutes of Health.
Dr. Papadopoulos disclosed relationships with Thrive Earlier Detection Inc., PGDx Inc., NeoPhore, Cage Pharma, and other companies. Dr. Huntsman is a founder, shareholder, and chief medical officer for Contextual Genomics.
SOURCE: Papadopoulos N et al. AACR 2020, Abstract CT022; Lennon AM et al. Science. 2020 Apr 28. pii: eabb9601. doi: 10.1126/science.abb9601.
FROM AACR 2020
Novel immune activator boosts immunotherapy benefit in TNBC
Triple-negative breast cancer (TNBC) is a particularly aggressive form of this disease, with a poor prognosis, so there is great interest in any new treatment approach. Immunotherapy has raised hopes in TNBC, but more recently, studies have produced conflicting results.
New results show that adding a novel immune activator, Imprime PGG (Biothera), to immunotherapy with pembrolizumab (Keytruda, Merck) appears to improve the clinical benefit. The overall survival seen with the combination was twice that seen in a separate trial with pembrolizumab alone.
The new results were presented during the American Association for Cancer Research (AACR) virtual annual meeting I.
They come from the IMPRIME 1 trial, conducted in 44 women with metastatic TNBC who had anti-glucan antibodies.
“These were patients who had had prior chemotherapy and had extensive disease, including the majority with visceral disease and even liver metastasis,” said investigator Steven O’Day, MD, from the John Wayne Cancer Institute in Santa Monica, California.
All patients were treated with the combination. “We see encouraging clinical benefit evidence across all of our clinical measurements: response, durable response, and median and overall survival compared to historical single-agent [anti] PD-1 in a similar metastatic triple-negative breast cancer population,” he said.
At a median follow-up of 22.5 months, median overall survival with the combination among the 44 patients treated was 16.4 months.
In contrast, in the Keynote-086 trial of pembrolizumab monotherapy in patients with TNBC, median overall survival was 9 months, O’Day said.
He emphasized, however, that the IMPRIME 1 trial was not designed or powered to directly compare the combination therapy with pembrolizumab monotherapy.
Clinical benefit with the combination was particularly pronounced for patients who were so-called TNBC “converters” — that is, they originally had estrogen receptor (ER)-positive tumors that had progressed on endocrine therapy and, prior to starting treatment with Imprime PGG and pembrolizumab, they had biopsy results confirming TNBC, O’Day said.
The overall response rate (ORR) for all 44 patients included in the efficacy analysis was 15.9%. But among the 12 patients whose disease converted from ER-positive to TNBC after endocrine therapy, six had a response, for an ORR of 50% and a median overall survival of 17.1 months.
“It is not clear whether hormone resistance may have led to the increased responses versus secondary triple-negative status, but it is of great interest to us,” O’Day said.
Why This Special Benefit?
Invited discussant Ben Ho Park, MD, PhD, from Vanderbilt University Medical Center in Nashville, Tennessee, commented that the finding of special benefit among TNBC converters raises the question of biomarkers to determine which patients might most benefit from the combination.
“We already know that anti-beta-glucan antibodies were required to be actually eligible for this study, but is it that, in combination with immune activation, or prior ER-positive disease?” he said. “What about the role of PD-L1 staining? Can we actually combine all this data to come up with some sort of predictive score for whether or not a patient is more or less likely to respond, and more or less likely to have toxicities?”
Yeast-Derived Compound
Imprime PGG is a novel beta-glucan isolated from the cell walls of saccharomyces yeast that binds to endogenous anti-beta-glucan antibodies to form an immune complex.
The immune complex, which is the active drug, binds to a receptor known as dectin-1 to activate innate immunity and reprogram the immunosuppressive tumor microenvironment, enhance antigen presentation, and trigger T-cell activation to improve the efficacy of immune checkpoint inhibitor therapy, O’Day explained.
The complex has been administered to date to approximately 600 healthy volunteers and patients. In these studies, it was administered intravenously at doses of 2 mg/kg to 6 mg/kg weekly as monotherapy or in combination with anti-angiogenic antibodies or tumor-targeting antibodies, with or without chemotherapy.
Studies in volunteers showed that the complex activated innate immunity. Patients have tolerated it well, with no significant safety signals in either monotherapy or combination, with grade 1 or 2 infusion-related reactions being the most common adverse events to date, O’Day reported.
Study Details
Imprime 1 was a single-arm phase 2 trial enrolling 44 women with TNBC who had received at least one prior line of treatment, but not with an immune checkpoint inhibitor. They were all required to have anti-beta-glucan antibody levels of at least 20 mcg/mL.
All patients received the combination, which comprised Imprime PGG 4 mg/kg weekly plus pembrolizumab 200 mg IV every 3 weeks.
Twenty one patients were under age 50 years, and 23 were 50 years old and older. Seventeen patients were premenopausal, and 27 were postmenopausal. In all, 15 patients had more than three prior lines of therapy for metastatic disease, 30 had visceral disease, and 12 had liver metastases; only four had metastases confined to lymph nodes.
As noted above, median overall survival for all patients was 16.4 months. The ORR was 15.9%, and the disease control rate (a combination of complete and partial responses plus stable disease) was 25%. The median progression-free survival was 2.7 months (vs 2 months in Keynote-086).
In all, 39 of the 44 patients had treatment-related adverse events, with the most common being nausea, back pain, chills, fatigue, diarrhea, arthralgia, and headache. Four patients had grade 3 or 4 events, which included an infusion-related reaction, hyperglycemia, pericarditis, and pancreatitis.
Infusion-related reactions were seen in 27 patients, but only one of these reactions was grade 3 or 4.
The most common immune-mediated events were grade 1 or 2 thyroid dysfunction, which is commonly seen with PD-1 inhibitors, and there were single low-grade events of pancreatitis, pneumonitis, and pericarditis “most likely related to PD-1 inhibitor therapy,” O’Day said.
Translational data showed that innate and adaptive immunity in peripheral blood correlates with clinical benefit, with longer overall survival among patients with either monocyte activation (P = .0045) or T-cell activation (P = .012) compared with patients without activation of those components.
Taken together, the findings suggest that larger controlled studies of the combination are warranted, O’Day said.
The study was sponsored by Biothera and Merck. O’Day disclosed advisory board activities and research funding from both companies and others, and consulting for Biothera. Park disclosed royalties and consulting activities from several companies, not including the Imprime 1 sponsors.
This article first appeared on Medscape.com.
Triple-negative breast cancer (TNBC) is a particularly aggressive form of this disease, with a poor prognosis, so there is great interest in any new treatment approach. Immunotherapy has raised hopes in TNBC, but more recently, studies have produced conflicting results.
New results show that adding a novel immune activator, Imprime PGG (Biothera), to immunotherapy with pembrolizumab (Keytruda, Merck) appears to improve the clinical benefit. The overall survival seen with the combination was twice that seen in a separate trial with pembrolizumab alone.
The new results were presented during the American Association for Cancer Research (AACR) virtual annual meeting I.
They come from the IMPRIME 1 trial, conducted in 44 women with metastatic TNBC who had anti-glucan antibodies.
“These were patients who had had prior chemotherapy and had extensive disease, including the majority with visceral disease and even liver metastasis,” said investigator Steven O’Day, MD, from the John Wayne Cancer Institute in Santa Monica, California.
All patients were treated with the combination. “We see encouraging clinical benefit evidence across all of our clinical measurements: response, durable response, and median and overall survival compared to historical single-agent [anti] PD-1 in a similar metastatic triple-negative breast cancer population,” he said.
At a median follow-up of 22.5 months, median overall survival with the combination among the 44 patients treated was 16.4 months.
In contrast, in the Keynote-086 trial of pembrolizumab monotherapy in patients with TNBC, median overall survival was 9 months, O’Day said.
He emphasized, however, that the IMPRIME 1 trial was not designed or powered to directly compare the combination therapy with pembrolizumab monotherapy.
Clinical benefit with the combination was particularly pronounced for patients who were so-called TNBC “converters” — that is, they originally had estrogen receptor (ER)-positive tumors that had progressed on endocrine therapy and, prior to starting treatment with Imprime PGG and pembrolizumab, they had biopsy results confirming TNBC, O’Day said.
The overall response rate (ORR) for all 44 patients included in the efficacy analysis was 15.9%. But among the 12 patients whose disease converted from ER-positive to TNBC after endocrine therapy, six had a response, for an ORR of 50% and a median overall survival of 17.1 months.
“It is not clear whether hormone resistance may have led to the increased responses versus secondary triple-negative status, but it is of great interest to us,” O’Day said.
Why This Special Benefit?
Invited discussant Ben Ho Park, MD, PhD, from Vanderbilt University Medical Center in Nashville, Tennessee, commented that the finding of special benefit among TNBC converters raises the question of biomarkers to determine which patients might most benefit from the combination.
“We already know that anti-beta-glucan antibodies were required to be actually eligible for this study, but is it that, in combination with immune activation, or prior ER-positive disease?” he said. “What about the role of PD-L1 staining? Can we actually combine all this data to come up with some sort of predictive score for whether or not a patient is more or less likely to respond, and more or less likely to have toxicities?”
Yeast-Derived Compound
Imprime PGG is a novel beta-glucan isolated from the cell walls of saccharomyces yeast that binds to endogenous anti-beta-glucan antibodies to form an immune complex.
The immune complex, which is the active drug, binds to a receptor known as dectin-1 to activate innate immunity and reprogram the immunosuppressive tumor microenvironment, enhance antigen presentation, and trigger T-cell activation to improve the efficacy of immune checkpoint inhibitor therapy, O’Day explained.
The complex has been administered to date to approximately 600 healthy volunteers and patients. In these studies, it was administered intravenously at doses of 2 mg/kg to 6 mg/kg weekly as monotherapy or in combination with anti-angiogenic antibodies or tumor-targeting antibodies, with or without chemotherapy.
Studies in volunteers showed that the complex activated innate immunity. Patients have tolerated it well, with no significant safety signals in either monotherapy or combination, with grade 1 or 2 infusion-related reactions being the most common adverse events to date, O’Day reported.
Study Details
Imprime 1 was a single-arm phase 2 trial enrolling 44 women with TNBC who had received at least one prior line of treatment, but not with an immune checkpoint inhibitor. They were all required to have anti-beta-glucan antibody levels of at least 20 mcg/mL.
All patients received the combination, which comprised Imprime PGG 4 mg/kg weekly plus pembrolizumab 200 mg IV every 3 weeks.
Twenty one patients were under age 50 years, and 23 were 50 years old and older. Seventeen patients were premenopausal, and 27 were postmenopausal. In all, 15 patients had more than three prior lines of therapy for metastatic disease, 30 had visceral disease, and 12 had liver metastases; only four had metastases confined to lymph nodes.
As noted above, median overall survival for all patients was 16.4 months. The ORR was 15.9%, and the disease control rate (a combination of complete and partial responses plus stable disease) was 25%. The median progression-free survival was 2.7 months (vs 2 months in Keynote-086).
In all, 39 of the 44 patients had treatment-related adverse events, with the most common being nausea, back pain, chills, fatigue, diarrhea, arthralgia, and headache. Four patients had grade 3 or 4 events, which included an infusion-related reaction, hyperglycemia, pericarditis, and pancreatitis.
Infusion-related reactions were seen in 27 patients, but only one of these reactions was grade 3 or 4.
The most common immune-mediated events were grade 1 or 2 thyroid dysfunction, which is commonly seen with PD-1 inhibitors, and there were single low-grade events of pancreatitis, pneumonitis, and pericarditis “most likely related to PD-1 inhibitor therapy,” O’Day said.
Translational data showed that innate and adaptive immunity in peripheral blood correlates with clinical benefit, with longer overall survival among patients with either monocyte activation (P = .0045) or T-cell activation (P = .012) compared with patients without activation of those components.
Taken together, the findings suggest that larger controlled studies of the combination are warranted, O’Day said.
The study was sponsored by Biothera and Merck. O’Day disclosed advisory board activities and research funding from both companies and others, and consulting for Biothera. Park disclosed royalties and consulting activities from several companies, not including the Imprime 1 sponsors.
This article first appeared on Medscape.com.
Triple-negative breast cancer (TNBC) is a particularly aggressive form of this disease, with a poor prognosis, so there is great interest in any new treatment approach. Immunotherapy has raised hopes in TNBC, but more recently, studies have produced conflicting results.
New results show that adding a novel immune activator, Imprime PGG (Biothera), to immunotherapy with pembrolizumab (Keytruda, Merck) appears to improve the clinical benefit. The overall survival seen with the combination was twice that seen in a separate trial with pembrolizumab alone.
The new results were presented during the American Association for Cancer Research (AACR) virtual annual meeting I.
They come from the IMPRIME 1 trial, conducted in 44 women with metastatic TNBC who had anti-glucan antibodies.
“These were patients who had had prior chemotherapy and had extensive disease, including the majority with visceral disease and even liver metastasis,” said investigator Steven O’Day, MD, from the John Wayne Cancer Institute in Santa Monica, California.
All patients were treated with the combination. “We see encouraging clinical benefit evidence across all of our clinical measurements: response, durable response, and median and overall survival compared to historical single-agent [anti] PD-1 in a similar metastatic triple-negative breast cancer population,” he said.
At a median follow-up of 22.5 months, median overall survival with the combination among the 44 patients treated was 16.4 months.
In contrast, in the Keynote-086 trial of pembrolizumab monotherapy in patients with TNBC, median overall survival was 9 months, O’Day said.
He emphasized, however, that the IMPRIME 1 trial was not designed or powered to directly compare the combination therapy with pembrolizumab monotherapy.
Clinical benefit with the combination was particularly pronounced for patients who were so-called TNBC “converters” — that is, they originally had estrogen receptor (ER)-positive tumors that had progressed on endocrine therapy and, prior to starting treatment with Imprime PGG and pembrolizumab, they had biopsy results confirming TNBC, O’Day said.
The overall response rate (ORR) for all 44 patients included in the efficacy analysis was 15.9%. But among the 12 patients whose disease converted from ER-positive to TNBC after endocrine therapy, six had a response, for an ORR of 50% and a median overall survival of 17.1 months.
“It is not clear whether hormone resistance may have led to the increased responses versus secondary triple-negative status, but it is of great interest to us,” O’Day said.
Why This Special Benefit?
Invited discussant Ben Ho Park, MD, PhD, from Vanderbilt University Medical Center in Nashville, Tennessee, commented that the finding of special benefit among TNBC converters raises the question of biomarkers to determine which patients might most benefit from the combination.
“We already know that anti-beta-glucan antibodies were required to be actually eligible for this study, but is it that, in combination with immune activation, or prior ER-positive disease?” he said. “What about the role of PD-L1 staining? Can we actually combine all this data to come up with some sort of predictive score for whether or not a patient is more or less likely to respond, and more or less likely to have toxicities?”
Yeast-Derived Compound
Imprime PGG is a novel beta-glucan isolated from the cell walls of saccharomyces yeast that binds to endogenous anti-beta-glucan antibodies to form an immune complex.
The immune complex, which is the active drug, binds to a receptor known as dectin-1 to activate innate immunity and reprogram the immunosuppressive tumor microenvironment, enhance antigen presentation, and trigger T-cell activation to improve the efficacy of immune checkpoint inhibitor therapy, O’Day explained.
The complex has been administered to date to approximately 600 healthy volunteers and patients. In these studies, it was administered intravenously at doses of 2 mg/kg to 6 mg/kg weekly as monotherapy or in combination with anti-angiogenic antibodies or tumor-targeting antibodies, with or without chemotherapy.
Studies in volunteers showed that the complex activated innate immunity. Patients have tolerated it well, with no significant safety signals in either monotherapy or combination, with grade 1 or 2 infusion-related reactions being the most common adverse events to date, O’Day reported.
Study Details
Imprime 1 was a single-arm phase 2 trial enrolling 44 women with TNBC who had received at least one prior line of treatment, but not with an immune checkpoint inhibitor. They were all required to have anti-beta-glucan antibody levels of at least 20 mcg/mL.
All patients received the combination, which comprised Imprime PGG 4 mg/kg weekly plus pembrolizumab 200 mg IV every 3 weeks.
Twenty one patients were under age 50 years, and 23 were 50 years old and older. Seventeen patients were premenopausal, and 27 were postmenopausal. In all, 15 patients had more than three prior lines of therapy for metastatic disease, 30 had visceral disease, and 12 had liver metastases; only four had metastases confined to lymph nodes.
As noted above, median overall survival for all patients was 16.4 months. The ORR was 15.9%, and the disease control rate (a combination of complete and partial responses plus stable disease) was 25%. The median progression-free survival was 2.7 months (vs 2 months in Keynote-086).
In all, 39 of the 44 patients had treatment-related adverse events, with the most common being nausea, back pain, chills, fatigue, diarrhea, arthralgia, and headache. Four patients had grade 3 or 4 events, which included an infusion-related reaction, hyperglycemia, pericarditis, and pancreatitis.
Infusion-related reactions were seen in 27 patients, but only one of these reactions was grade 3 or 4.
The most common immune-mediated events were grade 1 or 2 thyroid dysfunction, which is commonly seen with PD-1 inhibitors, and there were single low-grade events of pancreatitis, pneumonitis, and pericarditis “most likely related to PD-1 inhibitor therapy,” O’Day said.
Translational data showed that innate and adaptive immunity in peripheral blood correlates with clinical benefit, with longer overall survival among patients with either monocyte activation (P = .0045) or T-cell activation (P = .012) compared with patients without activation of those components.
Taken together, the findings suggest that larger controlled studies of the combination are warranted, O’Day said.
The study was sponsored by Biothera and Merck. O’Day disclosed advisory board activities and research funding from both companies and others, and consulting for Biothera. Park disclosed royalties and consulting activities from several companies, not including the Imprime 1 sponsors.
This article first appeared on Medscape.com.
FROM AACR 20

