User login
New global initiative aims to reform cancer trials and care
After 15 years of researching what works well in oncology – and where the field has gone awry – Christopher Booth, MD, had a career moment.
“As I approached mid-career, I realized publishing and describing problems wasn’t fulfilling. It wasn’t doing enough,” recalled Dr. Booth, an oncologist and professor at Queen’s University, Kingston, Ont. “I wanted to change mindsets and change systems so that things actually improved for the better for patients.”
His colleague, Bishal Gyawali, MD, PhD, described a similar epiphany. As a trainee, he noticed that the real-world effects of some so-called blockbuster cancer drugs too often failed to measure up to the hype.
“I realized we were lacking common sense in oncology,” said Dr. Gyawali, a medical oncologist and assistant professor at Queen’s University.
In 2019, Dr. Gyawali launched a Medscape column addressing what he considers to be that lack of common sense, and in 2022, he and Dr. Booth published a similarly titled opinion piece in Nature Medicine. The core idea: The cancer community needs to prioritize cancer treatments that benefit patients, treatments that meaningfully improve survival and quality of life.
Aaron Goodman, MD, a hematologist and associate professor at UC San Diego Health, was on the same page. He’d been interested in the evidence-based medicine movement since his time as a hematology fellow when that movement was “a bit of a counterculture,” he explained.
Dr. Goodman and Dr. Booth connected through their common interests and collaborated on a 2021 paper exploring the discomfort clinicians might feel when a patient’s needs fall on the “edge of oncology”: that is, when the guideline-recommended standard of care offers marginal benefit, at best, and could, at worst, cause patient harm.
“We said, ‘Now is the time to make change,’ ” he recalled. It was time to stop talking and do something.
Common sense and a common purpose
Dr. Booth, Dr. Gyawali, and Dr. Goodman joined forces and, with the backing of a philanthropist who had experience as a patient with cancer, convened an organizing committee of more than 30 like-minded oncologists and patient advocates from across the globe.
The group convened for a 3-day “meeting of the minds” in Kingston in April and laid out their intentions in a position paper published online in The Lancet Oncology.
In their paper, the committee outline the vision for Common Sense Oncology. The mission: prioritize patient-centered and equitable care by focusing on treatments that improve survival and quality of life, communication that promotes informed decision-making, and systems that ensure access to all patients.
However, increasingly, the cancer community faces a “troubling paradox,” the team wrote in The Lancet. In some instance, treatments that bring minimal benefit are overused while those that can make a meaningful difference in patients’ lives are not accessible to most worldwide.
One reason for this shift: Commercial interests, rather than patient interests, appear to be driving cancer research and care. The team explained, for instance, that over the past few decades, clinical trials have largely pivoted from publicly funded efforts to industry funded ones “designed to achieve regulatory approval or commercial advantage, [often] at the expense of investigating new approaches to surgery, radiotherapy, palliative care, and prevention.”
But “patients deserve better,” the group wrote.
The team outlined three pillars for the initiative: evidence generation, evidence interpretation, and evidence communication.
The evidence generation pillar will aim to improve trial design and reporting to prioritize outcomes that matter to patients.
“One concern is that over the last 10 years or so, most of our new treatments have had very, very small benefits, and we think the bar has dropped too low,” Dr. Booth said, explaining that many trials have moved away from focusing on improving survival and quality of life and toward detecting small differences between treatments on other endpoints – namely progression-free survival. “Those small benefits need to be balanced against the very real risks to our patients.”
The evidence interpretation pillar will aim to foster critical thinking so that clinicians can better identify poorly designed or reported trials and help patients make more informed decisions.
Lastly, the evidence communication pillar will focus on fostering better communication about treatment options among patients, the public, and policymakers. Without clear and thoughtful communication, patients may have unrealistic expectations about the effectiveness of treatments that offer only marginal clinical benefits.
The team also emphasized a need to focus on improving global equity and access to affordable treatments so all patients can benefit from care that extends survival or quality of life.
It’s an ambitious undertaking, especially for a group of full-time clinicians, researchers, and patient advocates “volunteering their time for societal good,” said Dr. Gyawali, but the project teams intend to hit the ground running.
The team has established short-term targets, such as identifying deficiencies in data interpretation within education programs within 6 months and developing educational materials that begin to correct those deficiencies within 12 months, Dr. Booth explained. In the longer term, the team will also aim to design clinical trials that focus on patient outcomes, such as overall survival and quality of life.
Breast cancer survivor and patient advocate Michelle Tregear, PhD, who was recruited to help with Common Sense Oncology, also hopes the initiative will lead to better regulatory control that requires trial sponsors to “focus on what matters to patients, not on surrogate endpoints.”
When it comes to clinical trials, “more, more, more is not always better,” said Dr. Tregear, director of Education and Training Programs for patient advocates at the National Breast Cancer Coalition, Washington, D.C. “Industry interests are not always aligned with patient interests,” and “the system, by and large, is not addressing questions that really matter to patients and their families.”
Although “it’s a tall order to change the direction that we’re going in,” Dr. Tregear is up to the challenge of helping raise awareness, which will hopefully spur patients to demand change.
When Dr. Goodman announced the Common Sense Oncology initiative on Twitter, the news brought excitement, with many oncologists asking to join.
With its sweeping, ambitious goals, the Common Sense Oncology initiative has a long road ahead. Figuring out how to implement some of its aims in practice will take time, Dr. Booth acknowledges, and the initial launch marks the first steps, which will continue to evolve over time.
“We’re not proposing we have all the answers or that we know what every patient would want – we’re saying we’ve not done a good job of communicating to patients the relative benefits and risks of different treatments,” Dr. Booth explained. “We want to celebrate and promote what helps and speak out about what’s not in the best interest of patients.”
Dr. Goodman reported consulting fees from Seattle Genetics and speaking honoraria from Curio. Dr. Booth, Dr. Gyawali, and Dr. Tregear reported having no financial conflicts of interest.
A version of this article appeared on Medscape.com.
After 15 years of researching what works well in oncology – and where the field has gone awry – Christopher Booth, MD, had a career moment.
“As I approached mid-career, I realized publishing and describing problems wasn’t fulfilling. It wasn’t doing enough,” recalled Dr. Booth, an oncologist and professor at Queen’s University, Kingston, Ont. “I wanted to change mindsets and change systems so that things actually improved for the better for patients.”
His colleague, Bishal Gyawali, MD, PhD, described a similar epiphany. As a trainee, he noticed that the real-world effects of some so-called blockbuster cancer drugs too often failed to measure up to the hype.
“I realized we were lacking common sense in oncology,” said Dr. Gyawali, a medical oncologist and assistant professor at Queen’s University.
In 2019, Dr. Gyawali launched a Medscape column addressing what he considers to be that lack of common sense, and in 2022, he and Dr. Booth published a similarly titled opinion piece in Nature Medicine. The core idea: The cancer community needs to prioritize cancer treatments that benefit patients, treatments that meaningfully improve survival and quality of life.
Aaron Goodman, MD, a hematologist and associate professor at UC San Diego Health, was on the same page. He’d been interested in the evidence-based medicine movement since his time as a hematology fellow when that movement was “a bit of a counterculture,” he explained.
Dr. Goodman and Dr. Booth connected through their common interests and collaborated on a 2021 paper exploring the discomfort clinicians might feel when a patient’s needs fall on the “edge of oncology”: that is, when the guideline-recommended standard of care offers marginal benefit, at best, and could, at worst, cause patient harm.
“We said, ‘Now is the time to make change,’ ” he recalled. It was time to stop talking and do something.
Common sense and a common purpose
Dr. Booth, Dr. Gyawali, and Dr. Goodman joined forces and, with the backing of a philanthropist who had experience as a patient with cancer, convened an organizing committee of more than 30 like-minded oncologists and patient advocates from across the globe.
The group convened for a 3-day “meeting of the minds” in Kingston in April and laid out their intentions in a position paper published online in The Lancet Oncology.
In their paper, the committee outline the vision for Common Sense Oncology. The mission: prioritize patient-centered and equitable care by focusing on treatments that improve survival and quality of life, communication that promotes informed decision-making, and systems that ensure access to all patients.
However, increasingly, the cancer community faces a “troubling paradox,” the team wrote in The Lancet. In some instance, treatments that bring minimal benefit are overused while those that can make a meaningful difference in patients’ lives are not accessible to most worldwide.
One reason for this shift: Commercial interests, rather than patient interests, appear to be driving cancer research and care. The team explained, for instance, that over the past few decades, clinical trials have largely pivoted from publicly funded efforts to industry funded ones “designed to achieve regulatory approval or commercial advantage, [often] at the expense of investigating new approaches to surgery, radiotherapy, palliative care, and prevention.”
But “patients deserve better,” the group wrote.
The team outlined three pillars for the initiative: evidence generation, evidence interpretation, and evidence communication.
The evidence generation pillar will aim to improve trial design and reporting to prioritize outcomes that matter to patients.
“One concern is that over the last 10 years or so, most of our new treatments have had very, very small benefits, and we think the bar has dropped too low,” Dr. Booth said, explaining that many trials have moved away from focusing on improving survival and quality of life and toward detecting small differences between treatments on other endpoints – namely progression-free survival. “Those small benefits need to be balanced against the very real risks to our patients.”
The evidence interpretation pillar will aim to foster critical thinking so that clinicians can better identify poorly designed or reported trials and help patients make more informed decisions.
Lastly, the evidence communication pillar will focus on fostering better communication about treatment options among patients, the public, and policymakers. Without clear and thoughtful communication, patients may have unrealistic expectations about the effectiveness of treatments that offer only marginal clinical benefits.
The team also emphasized a need to focus on improving global equity and access to affordable treatments so all patients can benefit from care that extends survival or quality of life.
It’s an ambitious undertaking, especially for a group of full-time clinicians, researchers, and patient advocates “volunteering their time for societal good,” said Dr. Gyawali, but the project teams intend to hit the ground running.
The team has established short-term targets, such as identifying deficiencies in data interpretation within education programs within 6 months and developing educational materials that begin to correct those deficiencies within 12 months, Dr. Booth explained. In the longer term, the team will also aim to design clinical trials that focus on patient outcomes, such as overall survival and quality of life.
Breast cancer survivor and patient advocate Michelle Tregear, PhD, who was recruited to help with Common Sense Oncology, also hopes the initiative will lead to better regulatory control that requires trial sponsors to “focus on what matters to patients, not on surrogate endpoints.”
When it comes to clinical trials, “more, more, more is not always better,” said Dr. Tregear, director of Education and Training Programs for patient advocates at the National Breast Cancer Coalition, Washington, D.C. “Industry interests are not always aligned with patient interests,” and “the system, by and large, is not addressing questions that really matter to patients and their families.”
Although “it’s a tall order to change the direction that we’re going in,” Dr. Tregear is up to the challenge of helping raise awareness, which will hopefully spur patients to demand change.
When Dr. Goodman announced the Common Sense Oncology initiative on Twitter, the news brought excitement, with many oncologists asking to join.
With its sweeping, ambitious goals, the Common Sense Oncology initiative has a long road ahead. Figuring out how to implement some of its aims in practice will take time, Dr. Booth acknowledges, and the initial launch marks the first steps, which will continue to evolve over time.
“We’re not proposing we have all the answers or that we know what every patient would want – we’re saying we’ve not done a good job of communicating to patients the relative benefits and risks of different treatments,” Dr. Booth explained. “We want to celebrate and promote what helps and speak out about what’s not in the best interest of patients.”
Dr. Goodman reported consulting fees from Seattle Genetics and speaking honoraria from Curio. Dr. Booth, Dr. Gyawali, and Dr. Tregear reported having no financial conflicts of interest.
A version of this article appeared on Medscape.com.
After 15 years of researching what works well in oncology – and where the field has gone awry – Christopher Booth, MD, had a career moment.
“As I approached mid-career, I realized publishing and describing problems wasn’t fulfilling. It wasn’t doing enough,” recalled Dr. Booth, an oncologist and professor at Queen’s University, Kingston, Ont. “I wanted to change mindsets and change systems so that things actually improved for the better for patients.”
His colleague, Bishal Gyawali, MD, PhD, described a similar epiphany. As a trainee, he noticed that the real-world effects of some so-called blockbuster cancer drugs too often failed to measure up to the hype.
“I realized we were lacking common sense in oncology,” said Dr. Gyawali, a medical oncologist and assistant professor at Queen’s University.
In 2019, Dr. Gyawali launched a Medscape column addressing what he considers to be that lack of common sense, and in 2022, he and Dr. Booth published a similarly titled opinion piece in Nature Medicine. The core idea: The cancer community needs to prioritize cancer treatments that benefit patients, treatments that meaningfully improve survival and quality of life.
Aaron Goodman, MD, a hematologist and associate professor at UC San Diego Health, was on the same page. He’d been interested in the evidence-based medicine movement since his time as a hematology fellow when that movement was “a bit of a counterculture,” he explained.
Dr. Goodman and Dr. Booth connected through their common interests and collaborated on a 2021 paper exploring the discomfort clinicians might feel when a patient’s needs fall on the “edge of oncology”: that is, when the guideline-recommended standard of care offers marginal benefit, at best, and could, at worst, cause patient harm.
“We said, ‘Now is the time to make change,’ ” he recalled. It was time to stop talking and do something.
Common sense and a common purpose
Dr. Booth, Dr. Gyawali, and Dr. Goodman joined forces and, with the backing of a philanthropist who had experience as a patient with cancer, convened an organizing committee of more than 30 like-minded oncologists and patient advocates from across the globe.
The group convened for a 3-day “meeting of the minds” in Kingston in April and laid out their intentions in a position paper published online in The Lancet Oncology.
In their paper, the committee outline the vision for Common Sense Oncology. The mission: prioritize patient-centered and equitable care by focusing on treatments that improve survival and quality of life, communication that promotes informed decision-making, and systems that ensure access to all patients.
However, increasingly, the cancer community faces a “troubling paradox,” the team wrote in The Lancet. In some instance, treatments that bring minimal benefit are overused while those that can make a meaningful difference in patients’ lives are not accessible to most worldwide.
One reason for this shift: Commercial interests, rather than patient interests, appear to be driving cancer research and care. The team explained, for instance, that over the past few decades, clinical trials have largely pivoted from publicly funded efforts to industry funded ones “designed to achieve regulatory approval or commercial advantage, [often] at the expense of investigating new approaches to surgery, radiotherapy, palliative care, and prevention.”
But “patients deserve better,” the group wrote.
The team outlined three pillars for the initiative: evidence generation, evidence interpretation, and evidence communication.
The evidence generation pillar will aim to improve trial design and reporting to prioritize outcomes that matter to patients.
“One concern is that over the last 10 years or so, most of our new treatments have had very, very small benefits, and we think the bar has dropped too low,” Dr. Booth said, explaining that many trials have moved away from focusing on improving survival and quality of life and toward detecting small differences between treatments on other endpoints – namely progression-free survival. “Those small benefits need to be balanced against the very real risks to our patients.”
The evidence interpretation pillar will aim to foster critical thinking so that clinicians can better identify poorly designed or reported trials and help patients make more informed decisions.
Lastly, the evidence communication pillar will focus on fostering better communication about treatment options among patients, the public, and policymakers. Without clear and thoughtful communication, patients may have unrealistic expectations about the effectiveness of treatments that offer only marginal clinical benefits.
The team also emphasized a need to focus on improving global equity and access to affordable treatments so all patients can benefit from care that extends survival or quality of life.
It’s an ambitious undertaking, especially for a group of full-time clinicians, researchers, and patient advocates “volunteering their time for societal good,” said Dr. Gyawali, but the project teams intend to hit the ground running.
The team has established short-term targets, such as identifying deficiencies in data interpretation within education programs within 6 months and developing educational materials that begin to correct those deficiencies within 12 months, Dr. Booth explained. In the longer term, the team will also aim to design clinical trials that focus on patient outcomes, such as overall survival and quality of life.
Breast cancer survivor and patient advocate Michelle Tregear, PhD, who was recruited to help with Common Sense Oncology, also hopes the initiative will lead to better regulatory control that requires trial sponsors to “focus on what matters to patients, not on surrogate endpoints.”
When it comes to clinical trials, “more, more, more is not always better,” said Dr. Tregear, director of Education and Training Programs for patient advocates at the National Breast Cancer Coalition, Washington, D.C. “Industry interests are not always aligned with patient interests,” and “the system, by and large, is not addressing questions that really matter to patients and their families.”
Although “it’s a tall order to change the direction that we’re going in,” Dr. Tregear is up to the challenge of helping raise awareness, which will hopefully spur patients to demand change.
When Dr. Goodman announced the Common Sense Oncology initiative on Twitter, the news brought excitement, with many oncologists asking to join.
With its sweeping, ambitious goals, the Common Sense Oncology initiative has a long road ahead. Figuring out how to implement some of its aims in practice will take time, Dr. Booth acknowledges, and the initial launch marks the first steps, which will continue to evolve over time.
“We’re not proposing we have all the answers or that we know what every patient would want – we’re saying we’ve not done a good job of communicating to patients the relative benefits and risks of different treatments,” Dr. Booth explained. “We want to celebrate and promote what helps and speak out about what’s not in the best interest of patients.”
Dr. Goodman reported consulting fees from Seattle Genetics and speaking honoraria from Curio. Dr. Booth, Dr. Gyawali, and Dr. Tregear reported having no financial conflicts of interest.
A version of this article appeared on Medscape.com.
Interrupting radiotherapy for TNBC linked to worse survival
Topline
Methodology
- Clinicians sometimes give women with TNBC a break between radiation sessions so that their skin can heal.
- To gauge the impact, investigators reviewed data from the National Cancer Database on 35,845 patients with TNBC who were treated between 2010 and 2014.
- The researchers determined the number of interrupted radiation treatment days as the difference between the number of days women received radiotherapy versus the number of expected treatment days.
- The team then correlated treatment interruptions with overall survival.
Takeaway
- Longer duration of treatment was associated with worse overall survival (hazard ratio, 1.023).
- Compared with no days or just 1 day off, 2-5 interrupted days (HR, 1.069), 6-10 interrupted days (HR, 1.239), and 11-15 interrupted days (HR, 1.265) increased the likelihood of death in a stepwise fashion.
- More days between diagnosis and first cancer treatment of any kind (HR, 1.001) were associated with worse overall survival.
- Older age (HR, 1.014), Black race (HR, 1.278), race than other Black or White (HR, 1.337), grade II or III/IV tumors (HR, 1.471 and 1.743, respectively), and clinical N1-N3 stage (HR, 2.534, 3.729, 4.992, respectively) were also associated with worse overall survival.
In practice
“All reasonable efforts should be made to prevent any treatment interruptions,” including “prophylactic measures to reduce the severity of radiation dermatitis,” and consideration should be given to the use of hypofractionated regimens to shorten radiation schedules.
Source
The study was led by Ronald Chow, MS, of the Memorial Sloan Kettering Cancer Center, New York, and was published in the Journal of the National Cancer Institute.
Limitations
- The findings may not be applicable to less aggressive forms of breast cancer.
- Treatment interruptions may have been caused by poor performance status and other confounders that shorten survival.
Disclosures
The study was funded by the National Cancer Institute. The investigators had no disclosures.
A version of this article appeared on Medscape.com.
Topline
Methodology
- Clinicians sometimes give women with TNBC a break between radiation sessions so that their skin can heal.
- To gauge the impact, investigators reviewed data from the National Cancer Database on 35,845 patients with TNBC who were treated between 2010 and 2014.
- The researchers determined the number of interrupted radiation treatment days as the difference between the number of days women received radiotherapy versus the number of expected treatment days.
- The team then correlated treatment interruptions with overall survival.
Takeaway
- Longer duration of treatment was associated with worse overall survival (hazard ratio, 1.023).
- Compared with no days or just 1 day off, 2-5 interrupted days (HR, 1.069), 6-10 interrupted days (HR, 1.239), and 11-15 interrupted days (HR, 1.265) increased the likelihood of death in a stepwise fashion.
- More days between diagnosis and first cancer treatment of any kind (HR, 1.001) were associated with worse overall survival.
- Older age (HR, 1.014), Black race (HR, 1.278), race than other Black or White (HR, 1.337), grade II or III/IV tumors (HR, 1.471 and 1.743, respectively), and clinical N1-N3 stage (HR, 2.534, 3.729, 4.992, respectively) were also associated with worse overall survival.
In practice
“All reasonable efforts should be made to prevent any treatment interruptions,” including “prophylactic measures to reduce the severity of radiation dermatitis,” and consideration should be given to the use of hypofractionated regimens to shorten radiation schedules.
Source
The study was led by Ronald Chow, MS, of the Memorial Sloan Kettering Cancer Center, New York, and was published in the Journal of the National Cancer Institute.
Limitations
- The findings may not be applicable to less aggressive forms of breast cancer.
- Treatment interruptions may have been caused by poor performance status and other confounders that shorten survival.
Disclosures
The study was funded by the National Cancer Institute. The investigators had no disclosures.
A version of this article appeared on Medscape.com.
Topline
Methodology
- Clinicians sometimes give women with TNBC a break between radiation sessions so that their skin can heal.
- To gauge the impact, investigators reviewed data from the National Cancer Database on 35,845 patients with TNBC who were treated between 2010 and 2014.
- The researchers determined the number of interrupted radiation treatment days as the difference between the number of days women received radiotherapy versus the number of expected treatment days.
- The team then correlated treatment interruptions with overall survival.
Takeaway
- Longer duration of treatment was associated with worse overall survival (hazard ratio, 1.023).
- Compared with no days or just 1 day off, 2-5 interrupted days (HR, 1.069), 6-10 interrupted days (HR, 1.239), and 11-15 interrupted days (HR, 1.265) increased the likelihood of death in a stepwise fashion.
- More days between diagnosis and first cancer treatment of any kind (HR, 1.001) were associated with worse overall survival.
- Older age (HR, 1.014), Black race (HR, 1.278), race than other Black or White (HR, 1.337), grade II or III/IV tumors (HR, 1.471 and 1.743, respectively), and clinical N1-N3 stage (HR, 2.534, 3.729, 4.992, respectively) were also associated with worse overall survival.
In practice
“All reasonable efforts should be made to prevent any treatment interruptions,” including “prophylactic measures to reduce the severity of radiation dermatitis,” and consideration should be given to the use of hypofractionated regimens to shorten radiation schedules.
Source
The study was led by Ronald Chow, MS, of the Memorial Sloan Kettering Cancer Center, New York, and was published in the Journal of the National Cancer Institute.
Limitations
- The findings may not be applicable to less aggressive forms of breast cancer.
- Treatment interruptions may have been caused by poor performance status and other confounders that shorten survival.
Disclosures
The study was funded by the National Cancer Institute. The investigators had no disclosures.
A version of this article appeared on Medscape.com.
Fatigue after breast cancer radiotherapy: Who’s most at risk?
Topline
Many patients with breast cancer who receive radiotherapy can still experience fatigue years after treatment; risk factors, including pain, insomnia, depression, baseline fatigue, and endocrine therapy were associated with long-term fatigue, new data show.
Methodology
- Overall, 1,443 patients with breast cancer from the REQUITE study responded to the Multidimensional Fatigue Inventory 20 (MFI-20) tool to assess five dimensions of fatigue: general, physical, and mental fatigue as well as reduced activity and motivation.
- Patients from France, Spain, Germany, Italy, the United Kingdom, and United States were assessed for characteristics, including age, body mass index (BMI), smoking, depression, pain, insomnia, fatigue, and therapy type, at baseline and at 24 months.
- Investigators identified factors associated with fatigue at 2 years post-radiotherapy among a total of 664 patients without chemotherapy and 324 with chemotherapy.
- General fatigue trajectories were classified as low, moderate, high, or decreasing.
Takeaways
- In general, levels of fatigue increased significantly from baseline to the end of radiotherapy for all fatigue dimensions (P < .05) and returned close to baseline levels after 1-2 years.
- About 24% of patients had high general fatigue trajectories and 25% had moderate, while 46% had low and 5% had decreasing fatigue trajectories.
- Factors such as age, BMI, global health status, insomnia, pain, dyspnea, depression, and baseline fatigue were each associated with multiple fatigue dimensions at 2 years; for instance, fatigue at baseline was associated with all five MFI-20 dimensions at 2 years regardless of chemotherapy status.
- Those with a combination of factors such as pain, insomnia, depression, younger age, and endocrine therapy were especially likely to develop high fatigue early and have it persist years after treatment.
In practice
the authors concluded.
Source
The study was led by Juan C. Rosas, with the German Cancer Research Center, Heidelberg. It was published online July 5 in the International Journal of Cancer.
Limitations
About one-quarter of patients did not complete the 2-year follow-up. Some variables identified in the literature as possible fatigue predictors such as socioeconomic status, physical activity, and social support were not included.
Disclosures
The study had no commercial funding. The authors reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Topline
Many patients with breast cancer who receive radiotherapy can still experience fatigue years after treatment; risk factors, including pain, insomnia, depression, baseline fatigue, and endocrine therapy were associated with long-term fatigue, new data show.
Methodology
- Overall, 1,443 patients with breast cancer from the REQUITE study responded to the Multidimensional Fatigue Inventory 20 (MFI-20) tool to assess five dimensions of fatigue: general, physical, and mental fatigue as well as reduced activity and motivation.
- Patients from France, Spain, Germany, Italy, the United Kingdom, and United States were assessed for characteristics, including age, body mass index (BMI), smoking, depression, pain, insomnia, fatigue, and therapy type, at baseline and at 24 months.
- Investigators identified factors associated with fatigue at 2 years post-radiotherapy among a total of 664 patients without chemotherapy and 324 with chemotherapy.
- General fatigue trajectories were classified as low, moderate, high, or decreasing.
Takeaways
- In general, levels of fatigue increased significantly from baseline to the end of radiotherapy for all fatigue dimensions (P < .05) and returned close to baseline levels after 1-2 years.
- About 24% of patients had high general fatigue trajectories and 25% had moderate, while 46% had low and 5% had decreasing fatigue trajectories.
- Factors such as age, BMI, global health status, insomnia, pain, dyspnea, depression, and baseline fatigue were each associated with multiple fatigue dimensions at 2 years; for instance, fatigue at baseline was associated with all five MFI-20 dimensions at 2 years regardless of chemotherapy status.
- Those with a combination of factors such as pain, insomnia, depression, younger age, and endocrine therapy were especially likely to develop high fatigue early and have it persist years after treatment.
In practice
the authors concluded.
Source
The study was led by Juan C. Rosas, with the German Cancer Research Center, Heidelberg. It was published online July 5 in the International Journal of Cancer.
Limitations
About one-quarter of patients did not complete the 2-year follow-up. Some variables identified in the literature as possible fatigue predictors such as socioeconomic status, physical activity, and social support were not included.
Disclosures
The study had no commercial funding. The authors reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Topline
Many patients with breast cancer who receive radiotherapy can still experience fatigue years after treatment; risk factors, including pain, insomnia, depression, baseline fatigue, and endocrine therapy were associated with long-term fatigue, new data show.
Methodology
- Overall, 1,443 patients with breast cancer from the REQUITE study responded to the Multidimensional Fatigue Inventory 20 (MFI-20) tool to assess five dimensions of fatigue: general, physical, and mental fatigue as well as reduced activity and motivation.
- Patients from France, Spain, Germany, Italy, the United Kingdom, and United States were assessed for characteristics, including age, body mass index (BMI), smoking, depression, pain, insomnia, fatigue, and therapy type, at baseline and at 24 months.
- Investigators identified factors associated with fatigue at 2 years post-radiotherapy among a total of 664 patients without chemotherapy and 324 with chemotherapy.
- General fatigue trajectories were classified as low, moderate, high, or decreasing.
Takeaways
- In general, levels of fatigue increased significantly from baseline to the end of radiotherapy for all fatigue dimensions (P < .05) and returned close to baseline levels after 1-2 years.
- About 24% of patients had high general fatigue trajectories and 25% had moderate, while 46% had low and 5% had decreasing fatigue trajectories.
- Factors such as age, BMI, global health status, insomnia, pain, dyspnea, depression, and baseline fatigue were each associated with multiple fatigue dimensions at 2 years; for instance, fatigue at baseline was associated with all five MFI-20 dimensions at 2 years regardless of chemotherapy status.
- Those with a combination of factors such as pain, insomnia, depression, younger age, and endocrine therapy were especially likely to develop high fatigue early and have it persist years after treatment.
In practice
the authors concluded.
Source
The study was led by Juan C. Rosas, with the German Cancer Research Center, Heidelberg. It was published online July 5 in the International Journal of Cancer.
Limitations
About one-quarter of patients did not complete the 2-year follow-up. Some variables identified in the literature as possible fatigue predictors such as socioeconomic status, physical activity, and social support were not included.
Disclosures
The study had no commercial funding. The authors reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Higher risk of death with endocrine therapy nonadherence
TOPLINE:
a new systematic review found.
METHODOLOGY:
- The investigators conducted a systematic literature search of five databases, looking for studies involving patients with nonmetastatic hormone receptor–positive breast cancer that were published between 2010 and 2020.
- Adequate adherence was defined as a medical possession ratio – the percentage of days the prescribed treatment dose of adjuvant endocrine therapy was available to the patient – of at least 80%.
- Medication nonpersistence was defined as a period in which no new adjuvant endocrine therapy prescriptions were filled before the scheduled end of treatment of 90-180 days, depending on the study.
- The impact of both parameters on event-free survival, which included breast cancer recurrence, disease-free survival, breast cancer–specific survival, and overall survival cancer was calculated.
- Of 2,026 articles retrieved, 14 studies, with sample sizes ranging from 857 to 30,573 patients, met the eligibility and quality criteria; 11 examined patient adherence, and 6 examined patient persistence.
TAKEAWAY:
- Of 10 studies that assessed event-free survival, 7 showed significantly worse survival for nonadherent or nonpersistent patients, at hazard ratios of 1.39-2.44.
- Of nine studies that examined overall survival, seven demonstrated a significantly higher risk for mortality in the groups with nonadherence and nonpersistence, at HRs of 1.26-2.18.
- The largest study, which included data on more than 30,000 patients in Taiwan, found that nonadherence and nonpersistence were associated with a significantly increased risk for mortality, at HRs of 1.98 and 2.18, respectively.
IN PRACTICE:
“The available data highlight the dangers of nonadherence and nonpersistence, showing an up to twofold higher risk of relapse or death for patients who do not use endocrine treatment as prescribed,” the researchers said. “Importantly, improving adherence and persistence represents a low-hanging fruit for increasing survival in luminal breast cancer.”
SOURCE:
The study, led by Finn Magnus Eliassen, MD, department of surgery, Stavanger (Norway) University Hospital, was published online on July 4 in BMC Cancer.
LIMITATIONS:
- The review is limited by the relatively small number of studies that met the eligibility criteria and by their heterogeneity, which ruled out a meta-analysis.
- There are no gold-standard definitions of adherence and persistence.
DISCLOSURES:
- No funding was declared. No relevant financial relationships were declared.
- A version of this article first appeared on Medscape.com.
TOPLINE:
a new systematic review found.
METHODOLOGY:
- The investigators conducted a systematic literature search of five databases, looking for studies involving patients with nonmetastatic hormone receptor–positive breast cancer that were published between 2010 and 2020.
- Adequate adherence was defined as a medical possession ratio – the percentage of days the prescribed treatment dose of adjuvant endocrine therapy was available to the patient – of at least 80%.
- Medication nonpersistence was defined as a period in which no new adjuvant endocrine therapy prescriptions were filled before the scheduled end of treatment of 90-180 days, depending on the study.
- The impact of both parameters on event-free survival, which included breast cancer recurrence, disease-free survival, breast cancer–specific survival, and overall survival cancer was calculated.
- Of 2,026 articles retrieved, 14 studies, with sample sizes ranging from 857 to 30,573 patients, met the eligibility and quality criteria; 11 examined patient adherence, and 6 examined patient persistence.
TAKEAWAY:
- Of 10 studies that assessed event-free survival, 7 showed significantly worse survival for nonadherent or nonpersistent patients, at hazard ratios of 1.39-2.44.
- Of nine studies that examined overall survival, seven demonstrated a significantly higher risk for mortality in the groups with nonadherence and nonpersistence, at HRs of 1.26-2.18.
- The largest study, which included data on more than 30,000 patients in Taiwan, found that nonadherence and nonpersistence were associated with a significantly increased risk for mortality, at HRs of 1.98 and 2.18, respectively.
IN PRACTICE:
“The available data highlight the dangers of nonadherence and nonpersistence, showing an up to twofold higher risk of relapse or death for patients who do not use endocrine treatment as prescribed,” the researchers said. “Importantly, improving adherence and persistence represents a low-hanging fruit for increasing survival in luminal breast cancer.”
SOURCE:
The study, led by Finn Magnus Eliassen, MD, department of surgery, Stavanger (Norway) University Hospital, was published online on July 4 in BMC Cancer.
LIMITATIONS:
- The review is limited by the relatively small number of studies that met the eligibility criteria and by their heterogeneity, which ruled out a meta-analysis.
- There are no gold-standard definitions of adherence and persistence.
DISCLOSURES:
- No funding was declared. No relevant financial relationships were declared.
- A version of this article first appeared on Medscape.com.
TOPLINE:
a new systematic review found.
METHODOLOGY:
- The investigators conducted a systematic literature search of five databases, looking for studies involving patients with nonmetastatic hormone receptor–positive breast cancer that were published between 2010 and 2020.
- Adequate adherence was defined as a medical possession ratio – the percentage of days the prescribed treatment dose of adjuvant endocrine therapy was available to the patient – of at least 80%.
- Medication nonpersistence was defined as a period in which no new adjuvant endocrine therapy prescriptions were filled before the scheduled end of treatment of 90-180 days, depending on the study.
- The impact of both parameters on event-free survival, which included breast cancer recurrence, disease-free survival, breast cancer–specific survival, and overall survival cancer was calculated.
- Of 2,026 articles retrieved, 14 studies, with sample sizes ranging from 857 to 30,573 patients, met the eligibility and quality criteria; 11 examined patient adherence, and 6 examined patient persistence.
TAKEAWAY:
- Of 10 studies that assessed event-free survival, 7 showed significantly worse survival for nonadherent or nonpersistent patients, at hazard ratios of 1.39-2.44.
- Of nine studies that examined overall survival, seven demonstrated a significantly higher risk for mortality in the groups with nonadherence and nonpersistence, at HRs of 1.26-2.18.
- The largest study, which included data on more than 30,000 patients in Taiwan, found that nonadherence and nonpersistence were associated with a significantly increased risk for mortality, at HRs of 1.98 and 2.18, respectively.
IN PRACTICE:
“The available data highlight the dangers of nonadherence and nonpersistence, showing an up to twofold higher risk of relapse or death for patients who do not use endocrine treatment as prescribed,” the researchers said. “Importantly, improving adherence and persistence represents a low-hanging fruit for increasing survival in luminal breast cancer.”
SOURCE:
The study, led by Finn Magnus Eliassen, MD, department of surgery, Stavanger (Norway) University Hospital, was published online on July 4 in BMC Cancer.
LIMITATIONS:
- The review is limited by the relatively small number of studies that met the eligibility criteria and by their heterogeneity, which ruled out a meta-analysis.
- There are no gold-standard definitions of adherence and persistence.
DISCLOSURES:
- No funding was declared. No relevant financial relationships were declared.
- A version of this article first appeared on Medscape.com.
Circulating Tumor DNA Testing and Liquid Biopsy: The Future for Precision Medicine and Guided Targeted Therapy for Breast Cancer?
The current standard for breast cancer screening (for non–high-risk patients) is an annual or semiannual mammogram for women aged 40 and older.1 However, mammography-based screening can give false-positive or false-negative results. This can lead to excessive use of invasive tissue biopsies and unnecessary exposure to ionizing radiation—which can also become expensive and time-consuming for patients.2
Both normal and cancerous cells shed cell-free DNA (cfDNA) into the blood circulation.3 Circulating tumor DNA (ctDNA) are fragments of DNA derived from tumor cells that circulate in the blood together with cfDNA. The ctDNA originates directly from a tumor or from circulating tumor cells (and carries information from the tumor cell genome), whereas cfDNA enters the bloodstream after apoptosis or necrosis and carries genome-wide DNA information. The amount of ctDNA in the blood has been shown to be elevated in patients with cancer.3 Different cancers release varying levels of ctDNA; the amount of ctDNA released depends on the number of tumor cells that are in senescence vs undergoing apoptosis.4
The possibility of incorporating this biomarker obtained from a “liquid biopsy” is currently being studied and will hopefully become a standard of care for breast cancer screening and monitoring. The liquid biopsy detects ctDNA that has been released into the bloodstream from tumor regions and helps identify intratumoral heterogeneity and clonal evolution.5 Additionally, sequencing tumor DNA has opened new possibilities for precision oncology.6 Detection of somatic gene mutations, amplifications, and gene fusions helps to deliver targeted therapies.6 Analysis of potential somatic mutations in ctDNA, in combination with cfDNA levels, can help capture clinically relevant information beyond single genetic alterations and tumor fraction, potentially improving the accuracy of early detection and screening for breast cancer.
Recent advances in ctDNA testing technology have made it more accurate and reliable. ctDNA testing has several benefits, including early detection of cancer (detecting ctDNA at low levels)7; monitoring of tumor dynamics, therapeutic response, and residual disease8; as well as analysis of the evolution of genetic or epigenetic alterations characterizing the tumor.9 Its noninvasiveness, rapidity, and low cost allow for longitudinal monitoring of cancer in real time, potentially capturing tumor heterogeneity.10,11
The liquid biopsy potentially can give more options for therapeutic monitoring for breast cancer and may mirror clinically relevant genetic alterations that occur in all tumor tissues. Liquid biopsy offers many advantages. It allows for the detection of minimal residual disease and micrometastatic disease that may be difficult to detect with a traditional tissue biopsy.12 Liquid biopsy detects ctDNA that has been released into the bloodstream from multiple tumor regions and allows the possibility of identifying intratumoral heterogeneity and clonal evolution.5 It can also detect small quantitative variations within the blood, enabling real-time surveillance.
The liquid biopsy can offer earlier and easier access to some tumor-based genetic information at any given timepoint and can replace a tumor tissue biopsy in some cases, helping to avoid delays and complications of a solid tumor invasive biopsy procedure. This is especially relevant in the metastatic setting, in which ctDNA might be the only available genetic material from tumors.13 Tissue biopsy can only provide a static and spatially limited view of the disease at the time of sampling; ctDNA analysis could potentially reflect the genetic alterations that occur in all metastatic breast cancer sites over time.14,15 Furthermore, machine learning of multi-gene signatures, obtained from ctDNA, can possibly identify complex biological features, including measures of tumor proliferation and estrogen receptor signaling, similar to direct tumor tissue DNA or RNA profiling.16
ctDNA testing is currently being studied to monitor patients who have been diagnosed with breast cancer. Small retrospective studies have shown that detection of ctDNA in plasma, after patients have completed therapy for early-stage breast cancer, is associated with a very high risk of relapse.17
Ongoing studies are examining the tailoring of adjuvant treatment based on ctDNA. If these trials are successful, certain aspects of adjuvant treatment could be lessened, or omitted, for patients who have undetectable ctDNA or intensified for patients who have detectable ctDNA after definitive treatments. This could personalize treatment specifically to the patient.
The detection and persistence of ctDNA in the middle of neoadjuvant systemic therapy may have the potential to negatively predict response to treatment and identify patients who will not achieve pathologic complete response. This may have the potential to aid in clinical decision-making for treatment escalation in these nonresponders.18
Despite these distinct characteristics, the low levels of ctDNA found in early-stage disease, along with the lack of ctDNA shedding from some tumors, can further complicate or impede detection of recurrence in early-stage breast cancer. Testing is further complicated by hematologic genetic alterations.5 The limitation of ctDNA approaches is that these techniques only detect known mutations in certain genes, so patients without these mutations could be overlooked, limiting the application of this technology.19
Overall, ctDNA testing represents a promising area of research for the diagnosis, treatment, and monitoring of breast cancer. While more research is needed to fully understand its potential, the advances in this technology are certainly exciting and could lead to significant improvements in patient outcomes. It is hopeful that in the near future, ctDNA testing from liquid biopsy could become a standard of care in breast cancer screening, ultimately helping clinicians to personalize treatment therapies and improve patient outcomes when treating patients with breast cancer.
1. Oeffinger KC, Fontham ETH, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599-1614.
2. Zubor P, Kubatka P, Kajo K, et al. Why the gold standard approach by mammography demands extension by multiomics? Application of liquid biopsy miRNA profiles to breast cancer disease management. Int J Mol Sci. 2019;20(12):E2878.
3. Thierry AR, El Messaoudi S, Gahan PB, Anker P, Stroun M. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev. 2016;35(3):347-376.
4. Rostami A, Lambie M, Yu CW, Stambolic V, Waldron JN, Bratman SV. Senescence, necrosis, and apoptosis govern circulating cell-free DNA release kinetics. Cell Rep. 2020;31(13):107830.
5. De Rubis G, Rajeev Krishnan S, Bebawy M. Liquid biopsies in cancer diagnosis, monitoring, and prognosis. Trends Pharmacol Sci. 2019;40(3):172-186.
6. Mateo J, Chakravarty D, Dienstmann R, et al. A framework to rank genomic alterations as targets for cancer precision medicine: the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann Oncol. 2018;29:1895-1902.
7. Wang J, Han X, Sun Y. DNA methylation signatures in circulating cell-free DNA as biomarkers for the early detection of cancer. Sci China Life Sci. 2017;60(4):356-362.
8. Dawson S-J, Tsui DWY, Murtaza M, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368(13):1199-1209.
9. Diaz Jr LA, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32(6):579-586.
10. Oxnard GR, Paweletz CP, Kuang Y, et al. Noninvasive detection of response and resistance in EGFR-mutant lung cancer using quantitative next-generation genotyping of cell-free plasma DNA. Clin Cancer Res. 2014;20(6):1698-1705.
11. Jamal-Hanjani M, Wilson GA, Horswell S, et al. Detection of ubiquitous and heterogeneous mutations in cell-free DNA from patients with early-stage non-small-cell lung cancer. Ann Oncol. 2016;27(5):862-867.
12. Fiala C, Diamandis EP. Utility of circulating tumor DNA in cancer diagnostics with
13. Xia Y, Fan C, Hoadley KA, Parker JS, Perou CM. Genetic determinants of the molecular portraits of epithelial cancers. Nat Commun. 2019;10(1):5666.
14. Wan JCM, Massie C, Garcia-Corbacho J, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17(4):223-238.
15. Boldrin E, Nardo G, Zulato E, et al. Detection of loss of heterozygosity in cfDNA of advanced EGFR- or KRAS-mutated non-small-cell lung cancer patients. Int J Mol Sci. 2019;21(1):66.
16. Prat A, Brasó-Maristany F, Martínez-Sáez O, et al. Circulating tumor DNA reveals complex biological features with clinical relevance in metastatic breast cancer. Nat Commun. 2023;14(1):1157.
17. Coombes RC, Page K, Salari R, et al. Personalized detection of circulating tumor DNA antedates breast cancer metastatic recurrence. Clin Cancer Res. 2019;25(14):4255-4263.
18. Zhou Q, Gampenrieder SP, Frantal S, et al. Persistence of ctDNA in patients with breast cancer during neoadjuvant treatment is a significant predictor of poor tumor response. Clin Cancer Res. 2022;28(4):697-707.
19. Lin C, Liu X, Zheng B, Ke R, Tzeng C-M. Liquid biopsy, ctDNA diagnosis through NGS. Life (Basel). 2021;11(9):890.
The current standard for breast cancer screening (for non–high-risk patients) is an annual or semiannual mammogram for women aged 40 and older.1 However, mammography-based screening can give false-positive or false-negative results. This can lead to excessive use of invasive tissue biopsies and unnecessary exposure to ionizing radiation—which can also become expensive and time-consuming for patients.2
Both normal and cancerous cells shed cell-free DNA (cfDNA) into the blood circulation.3 Circulating tumor DNA (ctDNA) are fragments of DNA derived from tumor cells that circulate in the blood together with cfDNA. The ctDNA originates directly from a tumor or from circulating tumor cells (and carries information from the tumor cell genome), whereas cfDNA enters the bloodstream after apoptosis or necrosis and carries genome-wide DNA information. The amount of ctDNA in the blood has been shown to be elevated in patients with cancer.3 Different cancers release varying levels of ctDNA; the amount of ctDNA released depends on the number of tumor cells that are in senescence vs undergoing apoptosis.4
The possibility of incorporating this biomarker obtained from a “liquid biopsy” is currently being studied and will hopefully become a standard of care for breast cancer screening and monitoring. The liquid biopsy detects ctDNA that has been released into the bloodstream from tumor regions and helps identify intratumoral heterogeneity and clonal evolution.5 Additionally, sequencing tumor DNA has opened new possibilities for precision oncology.6 Detection of somatic gene mutations, amplifications, and gene fusions helps to deliver targeted therapies.6 Analysis of potential somatic mutations in ctDNA, in combination with cfDNA levels, can help capture clinically relevant information beyond single genetic alterations and tumor fraction, potentially improving the accuracy of early detection and screening for breast cancer.
Recent advances in ctDNA testing technology have made it more accurate and reliable. ctDNA testing has several benefits, including early detection of cancer (detecting ctDNA at low levels)7; monitoring of tumor dynamics, therapeutic response, and residual disease8; as well as analysis of the evolution of genetic or epigenetic alterations characterizing the tumor.9 Its noninvasiveness, rapidity, and low cost allow for longitudinal monitoring of cancer in real time, potentially capturing tumor heterogeneity.10,11
The liquid biopsy potentially can give more options for therapeutic monitoring for breast cancer and may mirror clinically relevant genetic alterations that occur in all tumor tissues. Liquid biopsy offers many advantages. It allows for the detection of minimal residual disease and micrometastatic disease that may be difficult to detect with a traditional tissue biopsy.12 Liquid biopsy detects ctDNA that has been released into the bloodstream from multiple tumor regions and allows the possibility of identifying intratumoral heterogeneity and clonal evolution.5 It can also detect small quantitative variations within the blood, enabling real-time surveillance.
The liquid biopsy can offer earlier and easier access to some tumor-based genetic information at any given timepoint and can replace a tumor tissue biopsy in some cases, helping to avoid delays and complications of a solid tumor invasive biopsy procedure. This is especially relevant in the metastatic setting, in which ctDNA might be the only available genetic material from tumors.13 Tissue biopsy can only provide a static and spatially limited view of the disease at the time of sampling; ctDNA analysis could potentially reflect the genetic alterations that occur in all metastatic breast cancer sites over time.14,15 Furthermore, machine learning of multi-gene signatures, obtained from ctDNA, can possibly identify complex biological features, including measures of tumor proliferation and estrogen receptor signaling, similar to direct tumor tissue DNA or RNA profiling.16
ctDNA testing is currently being studied to monitor patients who have been diagnosed with breast cancer. Small retrospective studies have shown that detection of ctDNA in plasma, after patients have completed therapy for early-stage breast cancer, is associated with a very high risk of relapse.17
Ongoing studies are examining the tailoring of adjuvant treatment based on ctDNA. If these trials are successful, certain aspects of adjuvant treatment could be lessened, or omitted, for patients who have undetectable ctDNA or intensified for patients who have detectable ctDNA after definitive treatments. This could personalize treatment specifically to the patient.
The detection and persistence of ctDNA in the middle of neoadjuvant systemic therapy may have the potential to negatively predict response to treatment and identify patients who will not achieve pathologic complete response. This may have the potential to aid in clinical decision-making for treatment escalation in these nonresponders.18
Despite these distinct characteristics, the low levels of ctDNA found in early-stage disease, along with the lack of ctDNA shedding from some tumors, can further complicate or impede detection of recurrence in early-stage breast cancer. Testing is further complicated by hematologic genetic alterations.5 The limitation of ctDNA approaches is that these techniques only detect known mutations in certain genes, so patients without these mutations could be overlooked, limiting the application of this technology.19
Overall, ctDNA testing represents a promising area of research for the diagnosis, treatment, and monitoring of breast cancer. While more research is needed to fully understand its potential, the advances in this technology are certainly exciting and could lead to significant improvements in patient outcomes. It is hopeful that in the near future, ctDNA testing from liquid biopsy could become a standard of care in breast cancer screening, ultimately helping clinicians to personalize treatment therapies and improve patient outcomes when treating patients with breast cancer.
The current standard for breast cancer screening (for non–high-risk patients) is an annual or semiannual mammogram for women aged 40 and older.1 However, mammography-based screening can give false-positive or false-negative results. This can lead to excessive use of invasive tissue biopsies and unnecessary exposure to ionizing radiation—which can also become expensive and time-consuming for patients.2
Both normal and cancerous cells shed cell-free DNA (cfDNA) into the blood circulation.3 Circulating tumor DNA (ctDNA) are fragments of DNA derived from tumor cells that circulate in the blood together with cfDNA. The ctDNA originates directly from a tumor or from circulating tumor cells (and carries information from the tumor cell genome), whereas cfDNA enters the bloodstream after apoptosis or necrosis and carries genome-wide DNA information. The amount of ctDNA in the blood has been shown to be elevated in patients with cancer.3 Different cancers release varying levels of ctDNA; the amount of ctDNA released depends on the number of tumor cells that are in senescence vs undergoing apoptosis.4
The possibility of incorporating this biomarker obtained from a “liquid biopsy” is currently being studied and will hopefully become a standard of care for breast cancer screening and monitoring. The liquid biopsy detects ctDNA that has been released into the bloodstream from tumor regions and helps identify intratumoral heterogeneity and clonal evolution.5 Additionally, sequencing tumor DNA has opened new possibilities for precision oncology.6 Detection of somatic gene mutations, amplifications, and gene fusions helps to deliver targeted therapies.6 Analysis of potential somatic mutations in ctDNA, in combination with cfDNA levels, can help capture clinically relevant information beyond single genetic alterations and tumor fraction, potentially improving the accuracy of early detection and screening for breast cancer.
Recent advances in ctDNA testing technology have made it more accurate and reliable. ctDNA testing has several benefits, including early detection of cancer (detecting ctDNA at low levels)7; monitoring of tumor dynamics, therapeutic response, and residual disease8; as well as analysis of the evolution of genetic or epigenetic alterations characterizing the tumor.9 Its noninvasiveness, rapidity, and low cost allow for longitudinal monitoring of cancer in real time, potentially capturing tumor heterogeneity.10,11
The liquid biopsy potentially can give more options for therapeutic monitoring for breast cancer and may mirror clinically relevant genetic alterations that occur in all tumor tissues. Liquid biopsy offers many advantages. It allows for the detection of minimal residual disease and micrometastatic disease that may be difficult to detect with a traditional tissue biopsy.12 Liquid biopsy detects ctDNA that has been released into the bloodstream from multiple tumor regions and allows the possibility of identifying intratumoral heterogeneity and clonal evolution.5 It can also detect small quantitative variations within the blood, enabling real-time surveillance.
The liquid biopsy can offer earlier and easier access to some tumor-based genetic information at any given timepoint and can replace a tumor tissue biopsy in some cases, helping to avoid delays and complications of a solid tumor invasive biopsy procedure. This is especially relevant in the metastatic setting, in which ctDNA might be the only available genetic material from tumors.13 Tissue biopsy can only provide a static and spatially limited view of the disease at the time of sampling; ctDNA analysis could potentially reflect the genetic alterations that occur in all metastatic breast cancer sites over time.14,15 Furthermore, machine learning of multi-gene signatures, obtained from ctDNA, can possibly identify complex biological features, including measures of tumor proliferation and estrogen receptor signaling, similar to direct tumor tissue DNA or RNA profiling.16
ctDNA testing is currently being studied to monitor patients who have been diagnosed with breast cancer. Small retrospective studies have shown that detection of ctDNA in plasma, after patients have completed therapy for early-stage breast cancer, is associated with a very high risk of relapse.17
Ongoing studies are examining the tailoring of adjuvant treatment based on ctDNA. If these trials are successful, certain aspects of adjuvant treatment could be lessened, or omitted, for patients who have undetectable ctDNA or intensified for patients who have detectable ctDNA after definitive treatments. This could personalize treatment specifically to the patient.
The detection and persistence of ctDNA in the middle of neoadjuvant systemic therapy may have the potential to negatively predict response to treatment and identify patients who will not achieve pathologic complete response. This may have the potential to aid in clinical decision-making for treatment escalation in these nonresponders.18
Despite these distinct characteristics, the low levels of ctDNA found in early-stage disease, along with the lack of ctDNA shedding from some tumors, can further complicate or impede detection of recurrence in early-stage breast cancer. Testing is further complicated by hematologic genetic alterations.5 The limitation of ctDNA approaches is that these techniques only detect known mutations in certain genes, so patients without these mutations could be overlooked, limiting the application of this technology.19
Overall, ctDNA testing represents a promising area of research for the diagnosis, treatment, and monitoring of breast cancer. While more research is needed to fully understand its potential, the advances in this technology are certainly exciting and could lead to significant improvements in patient outcomes. It is hopeful that in the near future, ctDNA testing from liquid biopsy could become a standard of care in breast cancer screening, ultimately helping clinicians to personalize treatment therapies and improve patient outcomes when treating patients with breast cancer.
1. Oeffinger KC, Fontham ETH, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599-1614.
2. Zubor P, Kubatka P, Kajo K, et al. Why the gold standard approach by mammography demands extension by multiomics? Application of liquid biopsy miRNA profiles to breast cancer disease management. Int J Mol Sci. 2019;20(12):E2878.
3. Thierry AR, El Messaoudi S, Gahan PB, Anker P, Stroun M. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev. 2016;35(3):347-376.
4. Rostami A, Lambie M, Yu CW, Stambolic V, Waldron JN, Bratman SV. Senescence, necrosis, and apoptosis govern circulating cell-free DNA release kinetics. Cell Rep. 2020;31(13):107830.
5. De Rubis G, Rajeev Krishnan S, Bebawy M. Liquid biopsies in cancer diagnosis, monitoring, and prognosis. Trends Pharmacol Sci. 2019;40(3):172-186.
6. Mateo J, Chakravarty D, Dienstmann R, et al. A framework to rank genomic alterations as targets for cancer precision medicine: the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann Oncol. 2018;29:1895-1902.
7. Wang J, Han X, Sun Y. DNA methylation signatures in circulating cell-free DNA as biomarkers for the early detection of cancer. Sci China Life Sci. 2017;60(4):356-362.
8. Dawson S-J, Tsui DWY, Murtaza M, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368(13):1199-1209.
9. Diaz Jr LA, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32(6):579-586.
10. Oxnard GR, Paweletz CP, Kuang Y, et al. Noninvasive detection of response and resistance in EGFR-mutant lung cancer using quantitative next-generation genotyping of cell-free plasma DNA. Clin Cancer Res. 2014;20(6):1698-1705.
11. Jamal-Hanjani M, Wilson GA, Horswell S, et al. Detection of ubiquitous and heterogeneous mutations in cell-free DNA from patients with early-stage non-small-cell lung cancer. Ann Oncol. 2016;27(5):862-867.
12. Fiala C, Diamandis EP. Utility of circulating tumor DNA in cancer diagnostics with
13. Xia Y, Fan C, Hoadley KA, Parker JS, Perou CM. Genetic determinants of the molecular portraits of epithelial cancers. Nat Commun. 2019;10(1):5666.
14. Wan JCM, Massie C, Garcia-Corbacho J, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17(4):223-238.
15. Boldrin E, Nardo G, Zulato E, et al. Detection of loss of heterozygosity in cfDNA of advanced EGFR- or KRAS-mutated non-small-cell lung cancer patients. Int J Mol Sci. 2019;21(1):66.
16. Prat A, Brasó-Maristany F, Martínez-Sáez O, et al. Circulating tumor DNA reveals complex biological features with clinical relevance in metastatic breast cancer. Nat Commun. 2023;14(1):1157.
17. Coombes RC, Page K, Salari R, et al. Personalized detection of circulating tumor DNA antedates breast cancer metastatic recurrence. Clin Cancer Res. 2019;25(14):4255-4263.
18. Zhou Q, Gampenrieder SP, Frantal S, et al. Persistence of ctDNA in patients with breast cancer during neoadjuvant treatment is a significant predictor of poor tumor response. Clin Cancer Res. 2022;28(4):697-707.
19. Lin C, Liu X, Zheng B, Ke R, Tzeng C-M. Liquid biopsy, ctDNA diagnosis through NGS. Life (Basel). 2021;11(9):890.
1. Oeffinger KC, Fontham ETH, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599-1614.
2. Zubor P, Kubatka P, Kajo K, et al. Why the gold standard approach by mammography demands extension by multiomics? Application of liquid biopsy miRNA profiles to breast cancer disease management. Int J Mol Sci. 2019;20(12):E2878.
3. Thierry AR, El Messaoudi S, Gahan PB, Anker P, Stroun M. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev. 2016;35(3):347-376.
4. Rostami A, Lambie M, Yu CW, Stambolic V, Waldron JN, Bratman SV. Senescence, necrosis, and apoptosis govern circulating cell-free DNA release kinetics. Cell Rep. 2020;31(13):107830.
5. De Rubis G, Rajeev Krishnan S, Bebawy M. Liquid biopsies in cancer diagnosis, monitoring, and prognosis. Trends Pharmacol Sci. 2019;40(3):172-186.
6. Mateo J, Chakravarty D, Dienstmann R, et al. A framework to rank genomic alterations as targets for cancer precision medicine: the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann Oncol. 2018;29:1895-1902.
7. Wang J, Han X, Sun Y. DNA methylation signatures in circulating cell-free DNA as biomarkers for the early detection of cancer. Sci China Life Sci. 2017;60(4):356-362.
8. Dawson S-J, Tsui DWY, Murtaza M, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368(13):1199-1209.
9. Diaz Jr LA, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32(6):579-586.
10. Oxnard GR, Paweletz CP, Kuang Y, et al. Noninvasive detection of response and resistance in EGFR-mutant lung cancer using quantitative next-generation genotyping of cell-free plasma DNA. Clin Cancer Res. 2014;20(6):1698-1705.
11. Jamal-Hanjani M, Wilson GA, Horswell S, et al. Detection of ubiquitous and heterogeneous mutations in cell-free DNA from patients with early-stage non-small-cell lung cancer. Ann Oncol. 2016;27(5):862-867.
12. Fiala C, Diamandis EP. Utility of circulating tumor DNA in cancer diagnostics with
13. Xia Y, Fan C, Hoadley KA, Parker JS, Perou CM. Genetic determinants of the molecular portraits of epithelial cancers. Nat Commun. 2019;10(1):5666.
14. Wan JCM, Massie C, Garcia-Corbacho J, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17(4):223-238.
15. Boldrin E, Nardo G, Zulato E, et al. Detection of loss of heterozygosity in cfDNA of advanced EGFR- or KRAS-mutated non-small-cell lung cancer patients. Int J Mol Sci. 2019;21(1):66.
16. Prat A, Brasó-Maristany F, Martínez-Sáez O, et al. Circulating tumor DNA reveals complex biological features with clinical relevance in metastatic breast cancer. Nat Commun. 2023;14(1):1157.
17. Coombes RC, Page K, Salari R, et al. Personalized detection of circulating tumor DNA antedates breast cancer metastatic recurrence. Clin Cancer Res. 2019;25(14):4255-4263.
18. Zhou Q, Gampenrieder SP, Frantal S, et al. Persistence of ctDNA in patients with breast cancer during neoadjuvant treatment is a significant predictor of poor tumor response. Clin Cancer Res. 2022;28(4):697-707.
19. Lin C, Liu X, Zheng B, Ke R, Tzeng C-M. Liquid biopsy, ctDNA diagnosis through NGS. Life (Basel). 2021;11(9):890.
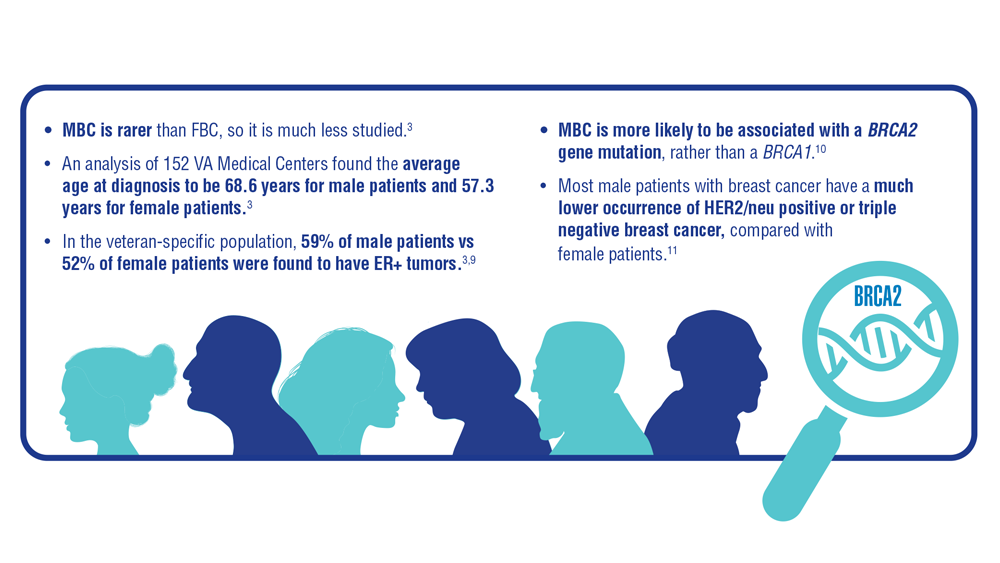
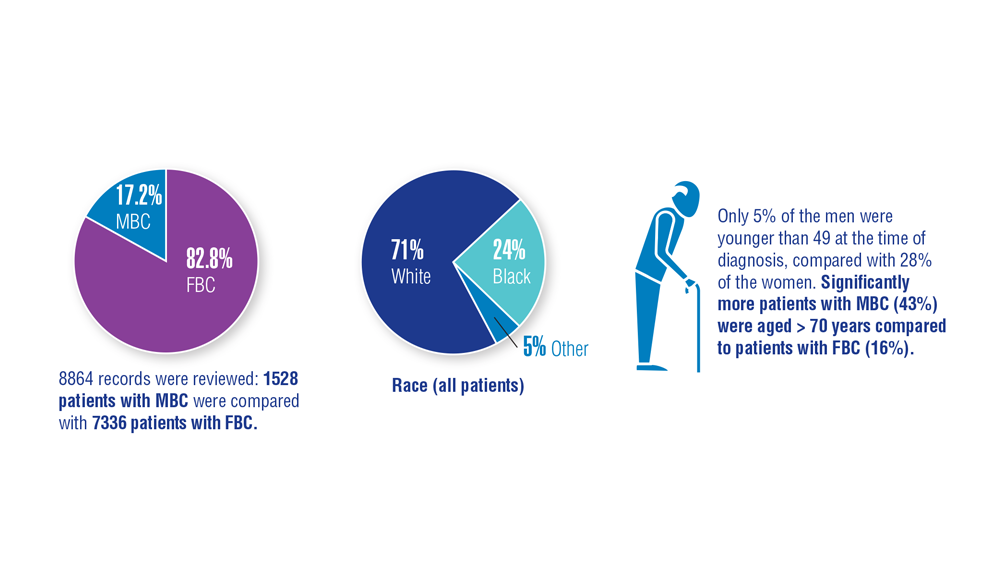
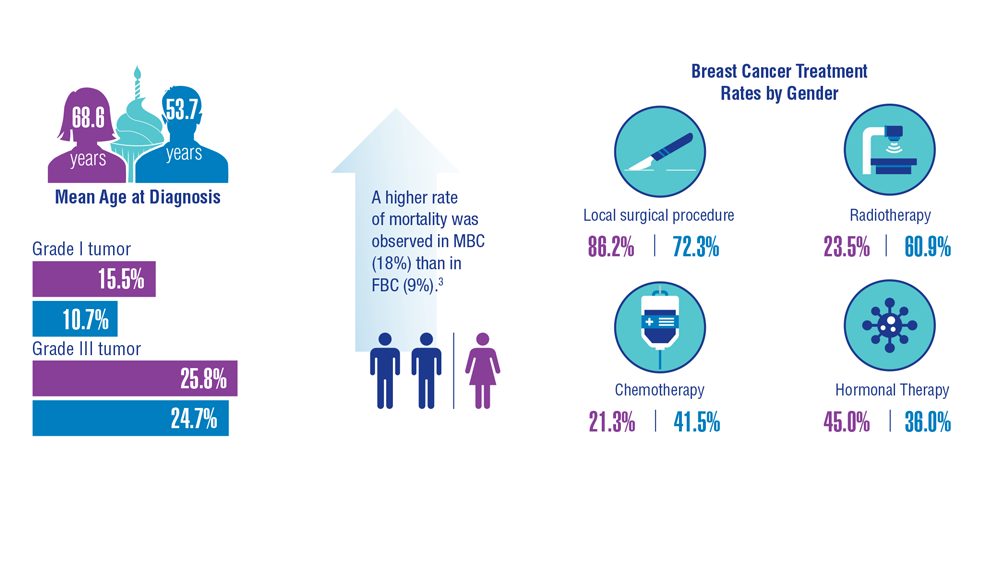
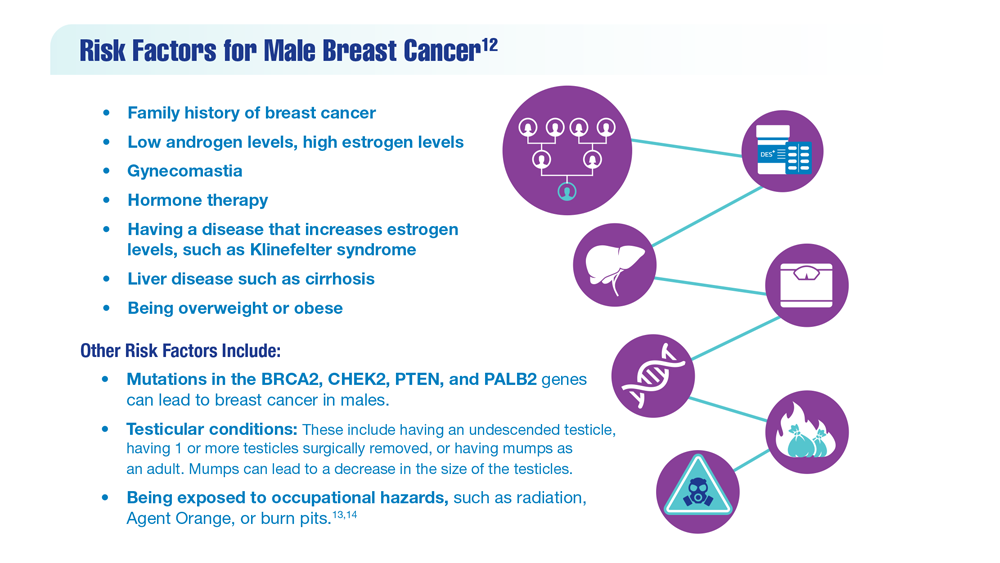
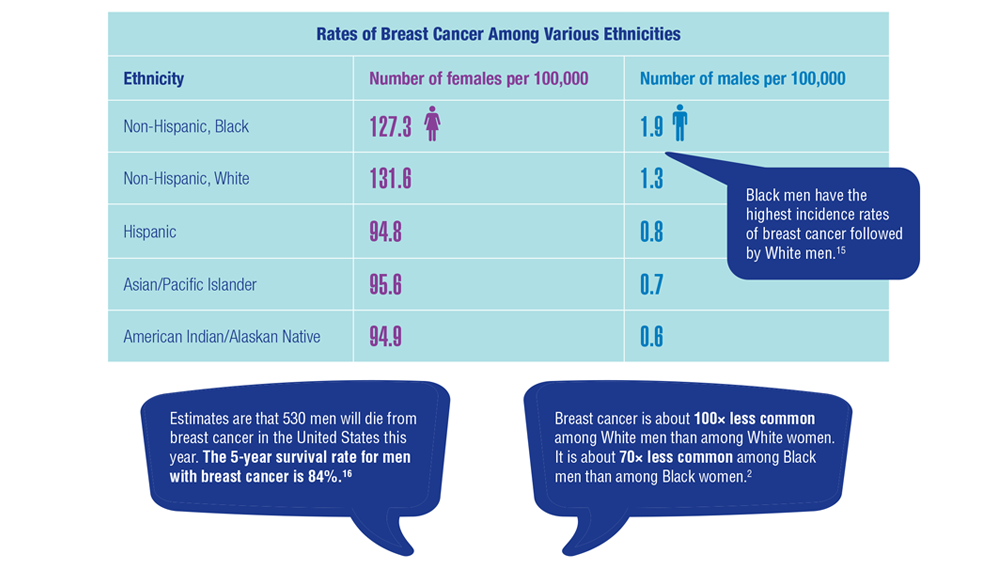
Gender Disparity in Breast Cancer Among US Veterans
1. Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyi GN. Breast carcinoma in men: a population-based study. Cancer. 2004;101(1):51-57. doi:10.1002/cncr.20312
2. Key statistics for breast cancer in men. American Cancer Society. Updated January 12, 2022. Accessed December 14, 2022. https://www.cancer.org/cancer/breast-cancer-in-men/about/key-statistics.html
3. Aggarwal A, Adepoju B, Yacur M, Maron D, Sharma MH. Gender disparity in breast cancer: a veteran population-based comparison. Clin Breast Cancer. 2021;21(4):e471-e478. doi:10.1016/j.clbc.2021.01.013
4. Ravandi-Kashani F, Hayes TG. Male breast cancer: a review of the literature. Eur J Cancer. 1998;34(9):1341-1347. doi:10.1016/s0959-8049(98)00028-8
5. Giordano SH. A review of diagnosis and management of male breast cancer. Oncologist. 2005;10(7):471-479. doi:10.1634/theoncologist.10-7-471
6. Midding E, Halbach SM, Kowalski C, Weber R, Würstlein R, Ernstmann N. Men with a “woman's disease”: stigmatization of male breast cancer patients—a mixed methods analysis. Am J Mens Health. 2018;12(6):2194-2207. doi:10.1177/1557988318799025
7. Key statistics for breast cancer. American Cancer Society. Updated October 6, 2022. Accessed December 14, 2022. https://www.cancer.org/cancer/breast-cancer/about/how-common-is-breast-cancer.html
8. Male breast cancer incidence and mortality, United States—2013-2017. Centers for Disease Control and Prevention. Updated October 1, 2020. Accessed December 14, 2022. https://www.cdc.gov/cancer/uscs/about/data-briefs/no19-male-breast-cancer-incidence-mortality-UnitedStates-2013-2017.htm
9. Anderson WF, Althuis MD, Brinton LA, Devesa SS. Is male breast cancer similar or different than female breast cancer? Breast Cancer Res Treat. 2004;83(1):77-86. doi:10.1023/B:BREA.0000010701.08825.2d 10. Pritzlaff M, Summerour P, McFarland R, et al. Male breast cancer in a multi-gene panel testing cohort: insights and unexpected results. Breast Cancer Res Treat. 2017;161(3):575-586. doi:10.1007/s10549-016-4085-4
11. Ottini L, Capalbo C, Rizzolo P, et al. HER2-positive male breast cancer: an update. Breast Cancer (Dove Med Press). 2010;2:45-58. doi:10.2147/BCTT.S6519
12. Risk factors for breast cancer in men. American Cancer Society. Updated April 27, 2018. Accessed December 14, 2022. https://www.cancer.org/cancer/breast-cancer-in-men/causes-risks-prevention/risk-factors.html
13. Palli D, Masala G, Mariani-Constantini R, et al. A gene–environment interaction between occupation and BRCA1/BRCA2 mutations in male breast cancer? Eur J Cancer. 2004;40(16):2472-2479. doi:10.1016/j.ejca.2004.07.012
14. Hansen J. Elevated risk for male breast cancer after occupational exposure to gasoline and vehicular combustion products. Am J Ind Med. 2000;37(4):349-352. doi:10.1002/(sici)1097-0274(200004)37:4<349::aid-ajim4>3.0.co;2-l
15. Sung H, DeSantis C, Jemal A. Subtype-specific breast cancer incidence rates in Black versus White men in the United States. JNCI Cancer Spectr. 2020;4(1):pkz091. doi:10.1093/jncics/pkz091
16. Breast cancer, male: statistics. Cancer.net. January 2022. Accessed December 14, 2022. https://www.cancer.net/cancer-types/breast-cancer-male/statistics
1. Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyi GN. Breast carcinoma in men: a population-based study. Cancer. 2004;101(1):51-57. doi:10.1002/cncr.20312
2. Key statistics for breast cancer in men. American Cancer Society. Updated January 12, 2022. Accessed December 14, 2022. https://www.cancer.org/cancer/breast-cancer-in-men/about/key-statistics.html
3. Aggarwal A, Adepoju B, Yacur M, Maron D, Sharma MH. Gender disparity in breast cancer: a veteran population-based comparison. Clin Breast Cancer. 2021;21(4):e471-e478. doi:10.1016/j.clbc.2021.01.013
4. Ravandi-Kashani F, Hayes TG. Male breast cancer: a review of the literature. Eur J Cancer. 1998;34(9):1341-1347. doi:10.1016/s0959-8049(98)00028-8
5. Giordano SH. A review of diagnosis and management of male breast cancer. Oncologist. 2005;10(7):471-479. doi:10.1634/theoncologist.10-7-471
6. Midding E, Halbach SM, Kowalski C, Weber R, Würstlein R, Ernstmann N. Men with a “woman's disease”: stigmatization of male breast cancer patients—a mixed methods analysis. Am J Mens Health. 2018;12(6):2194-2207. doi:10.1177/1557988318799025
7. Key statistics for breast cancer. American Cancer Society. Updated October 6, 2022. Accessed December 14, 2022. https://www.cancer.org/cancer/breast-cancer/about/how-common-is-breast-cancer.html
8. Male breast cancer incidence and mortality, United States—2013-2017. Centers for Disease Control and Prevention. Updated October 1, 2020. Accessed December 14, 2022. https://www.cdc.gov/cancer/uscs/about/data-briefs/no19-male-breast-cancer-incidence-mortality-UnitedStates-2013-2017.htm
9. Anderson WF, Althuis MD, Brinton LA, Devesa SS. Is male breast cancer similar or different than female breast cancer? Breast Cancer Res Treat. 2004;83(1):77-86. doi:10.1023/B:BREA.0000010701.08825.2d 10. Pritzlaff M, Summerour P, McFarland R, et al. Male breast cancer in a multi-gene panel testing cohort: insights and unexpected results. Breast Cancer Res Treat. 2017;161(3):575-586. doi:10.1007/s10549-016-4085-4
11. Ottini L, Capalbo C, Rizzolo P, et al. HER2-positive male breast cancer: an update. Breast Cancer (Dove Med Press). 2010;2:45-58. doi:10.2147/BCTT.S6519
12. Risk factors for breast cancer in men. American Cancer Society. Updated April 27, 2018. Accessed December 14, 2022. https://www.cancer.org/cancer/breast-cancer-in-men/causes-risks-prevention/risk-factors.html
13. Palli D, Masala G, Mariani-Constantini R, et al. A gene–environment interaction between occupation and BRCA1/BRCA2 mutations in male breast cancer? Eur J Cancer. 2004;40(16):2472-2479. doi:10.1016/j.ejca.2004.07.012
14. Hansen J. Elevated risk for male breast cancer after occupational exposure to gasoline and vehicular combustion products. Am J Ind Med. 2000;37(4):349-352. doi:10.1002/(sici)1097-0274(200004)37:4<349::aid-ajim4>3.0.co;2-l
15. Sung H, DeSantis C, Jemal A. Subtype-specific breast cancer incidence rates in Black versus White men in the United States. JNCI Cancer Spectr. 2020;4(1):pkz091. doi:10.1093/jncics/pkz091
16. Breast cancer, male: statistics. Cancer.net. January 2022. Accessed December 14, 2022. https://www.cancer.net/cancer-types/breast-cancer-male/statistics
1. Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyi GN. Breast carcinoma in men: a population-based study. Cancer. 2004;101(1):51-57. doi:10.1002/cncr.20312
2. Key statistics for breast cancer in men. American Cancer Society. Updated January 12, 2022. Accessed December 14, 2022. https://www.cancer.org/cancer/breast-cancer-in-men/about/key-statistics.html
3. Aggarwal A, Adepoju B, Yacur M, Maron D, Sharma MH. Gender disparity in breast cancer: a veteran population-based comparison. Clin Breast Cancer. 2021;21(4):e471-e478. doi:10.1016/j.clbc.2021.01.013
4. Ravandi-Kashani F, Hayes TG. Male breast cancer: a review of the literature. Eur J Cancer. 1998;34(9):1341-1347. doi:10.1016/s0959-8049(98)00028-8
5. Giordano SH. A review of diagnosis and management of male breast cancer. Oncologist. 2005;10(7):471-479. doi:10.1634/theoncologist.10-7-471
6. Midding E, Halbach SM, Kowalski C, Weber R, Würstlein R, Ernstmann N. Men with a “woman's disease”: stigmatization of male breast cancer patients—a mixed methods analysis. Am J Mens Health. 2018;12(6):2194-2207. doi:10.1177/1557988318799025
7. Key statistics for breast cancer. American Cancer Society. Updated October 6, 2022. Accessed December 14, 2022. https://www.cancer.org/cancer/breast-cancer/about/how-common-is-breast-cancer.html
8. Male breast cancer incidence and mortality, United States—2013-2017. Centers for Disease Control and Prevention. Updated October 1, 2020. Accessed December 14, 2022. https://www.cdc.gov/cancer/uscs/about/data-briefs/no19-male-breast-cancer-incidence-mortality-UnitedStates-2013-2017.htm
9. Anderson WF, Althuis MD, Brinton LA, Devesa SS. Is male breast cancer similar or different than female breast cancer? Breast Cancer Res Treat. 2004;83(1):77-86. doi:10.1023/B:BREA.0000010701.08825.2d 10. Pritzlaff M, Summerour P, McFarland R, et al. Male breast cancer in a multi-gene panel testing cohort: insights and unexpected results. Breast Cancer Res Treat. 2017;161(3):575-586. doi:10.1007/s10549-016-4085-4
11. Ottini L, Capalbo C, Rizzolo P, et al. HER2-positive male breast cancer: an update. Breast Cancer (Dove Med Press). 2010;2:45-58. doi:10.2147/BCTT.S6519
12. Risk factors for breast cancer in men. American Cancer Society. Updated April 27, 2018. Accessed December 14, 2022. https://www.cancer.org/cancer/breast-cancer-in-men/causes-risks-prevention/risk-factors.html
13. Palli D, Masala G, Mariani-Constantini R, et al. A gene–environment interaction between occupation and BRCA1/BRCA2 mutations in male breast cancer? Eur J Cancer. 2004;40(16):2472-2479. doi:10.1016/j.ejca.2004.07.012
14. Hansen J. Elevated risk for male breast cancer after occupational exposure to gasoline and vehicular combustion products. Am J Ind Med. 2000;37(4):349-352. doi:10.1002/(sici)1097-0274(200004)37:4<349::aid-ajim4>3.0.co;2-l
15. Sung H, DeSantis C, Jemal A. Subtype-specific breast cancer incidence rates in Black versus White men in the United States. JNCI Cancer Spectr. 2020;4(1):pkz091. doi:10.1093/jncics/pkz091
16. Breast cancer, male: statistics. Cancer.net. January 2022. Accessed December 14, 2022. https://www.cancer.net/cancer-types/breast-cancer-male/statistics
Cancer Data Trends 2023
Federal Practitioner and the Association of VA Hematology/Oncology (AVAHO) present the 2023 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- COVID-19 Outcomes in Veterans With Hematologic Cancers
- Promising New Approaches for Testicular and Prostate Cancer
- Screening Guideline Updates and New Treatments in Colon Cancer
- Exposure-Related Cancers: A Look at the PACT Act
- New Classifications and Emerging Treatments in Brain Cancer
- Gender Disparity in Breast Cancer Among US Veterans
- Lung Cancer Screening in Veterans
- Necessary Updates to Skin Cancer Risk Stratification
- Innovation in Cancer Treatment
Federal Practitioner and the Association of VA Hematology/Oncology (AVAHO) present the 2023 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- COVID-19 Outcomes in Veterans With Hematologic Cancers
- Promising New Approaches for Testicular and Prostate Cancer
- Screening Guideline Updates and New Treatments in Colon Cancer
- Exposure-Related Cancers: A Look at the PACT Act
- New Classifications and Emerging Treatments in Brain Cancer
- Gender Disparity in Breast Cancer Among US Veterans
- Lung Cancer Screening in Veterans
- Necessary Updates to Skin Cancer Risk Stratification
- Innovation in Cancer Treatment
Federal Practitioner and the Association of VA Hematology/Oncology (AVAHO) present the 2023 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- COVID-19 Outcomes in Veterans With Hematologic Cancers
- Promising New Approaches for Testicular and Prostate Cancer
- Screening Guideline Updates and New Treatments in Colon Cancer
- Exposure-Related Cancers: A Look at the PACT Act
- New Classifications and Emerging Treatments in Brain Cancer
- Gender Disparity in Breast Cancer Among US Veterans
- Lung Cancer Screening in Veterans
- Necessary Updates to Skin Cancer Risk Stratification
- Innovation in Cancer Treatment
PET-CT scans move more women with LABC up to stage IV
In women who have locally advanced breast cancer (LABC), staging defines the extent of the disease and guides therapy.
Researchers have found in the first large, randomized, controlled study on the subject that 18 F-labeled fluorodeoxyglucose positron emission tomography–computed tomography (PET-CT) finds more distant metastases and allows more accurate staging than usual staging, which is determined by a bone scan and computed tomography (CT) of the thorax/abdomen and pelvis.
Findings of the study, led by Ian S. Dayes, MD, MSc, with the department of oncology at McMaster University in Hamilton, Ont., were published online in the Journal of Clinical Oncology.
Scans indicate less aggressive treatment strategy
The authors of the new study wrote that women with LABC, who are at high risk of metastatic disease, have large tumors that “can involve the chest wall or skin, clinically fixed axillary lymph nodes, or infraclavicular, supraclavicular, or internal mammary lymphadenopathy.”
If staging does not detect metastases, treatment is centered on combined modality therapy with curative intent (neoadjuvant chemotherapy and surgery, followed by regional radiation). If metastases are found, the treatment goal changes to controlling the disease.
In this study, twice as many women saw their stage increase from stage IIB or III to stage IV when PET-CT was used instead of conventional staging, guiding their treatment toward less aggressive care to control, rather than attempt to cure, the disease.
The women included in this study had histological evidence of invasive ductal carcinoma of the breast and TNM stage III or IIb (T3N0, but not T2N1).
Methods and results
Between December 2016 and April 2022, consenting patients from six regional cancer centers in Ontario were randomly assigned to one of two groups: 184 patients were randomly assigned to whole-body PET-CT and 185 patients to conventional staging.
Overall, the authors wrote, 43 (23%) of PET-CT patients “were upstaged” to stage IV compared with 21 (11%) of the conventionally staged patients (relative risk, 2.4; 95% confidence interval [CI], 1.4-4.2, P = .002).
There were 33 patients in a subset with inflammatory breast cancer and, among them, 4 of 16 (25%) PET-CT patients were upstaged to stage IV compared with 4 of 17 (24%) conventionally staged patients.
In the patients who did not have inflammatory breast cancer, 39 of 168 (23%) PET-CT patients were upstaged compared with 17 (10%) of 168 in the conventionally staged group.
Journal of Clinical Oncology (JCO) Senior Deputy Editor Kathy D. Miller, MD, said that, “PET/CT staging identifies distant disease in more patients and changes goals of therapy. Further research is needed to determine the impact on patient outcome.”
Findings have already changed practice
Senior author, Mark Levine, MD, MSc, also with McMaster, said in an interview that the results of this study have already changed practice in Canada, and he expects the United States to follow suit.
Dr. Levine said the study is important “in terms of helping plan therapy and being very open and honest with patients as to their prognosis.”
The findings constitute level 1 evidence in favor of PET-CT. Already, in Canada, “because of the results of the study, people with stage III breast cancer can get a PET scan,” he said.
Dr. Levine said he expects this evidence also to clarify “wishy-washy” National Comprehensive Cancer Network guidelines on using PET scans for LABC in the United States when the guidelines are next updated.
“That will make it easier for payers in the United States,” he added.
Cost effectiveness, Dr. Levine said, is complicated, because on one hand PET scans are quite costly. But its use would lead to more women getting less aggressive and expensive therapy and surgery.
Dr. Levine noted that his team will be analyzing cost-effectiveness over the next year.
New questions with more in stage IV
In an editorial, Lajos Pusztai, MD, DPhil, scientific codirector of the breast center at Yale University in New Haven, Conn., noted that, “all good studies raise new questions” and this one is no exception.
He pointed out that the number of women with stage IV metastatic breast cancer (MBC) has been increasing over the past 2 decades because of more sensitive staging methods. At the same time the number of women with recurrent metastatic disease is decreasing, because adjuvant therapies have improved.
Findings highlight need for stage IV treatment studies
Dr. Pusztai noted that the patients who have de novo oligometastatic stage IV disease “are a unique subset among patients with MBC,” and the best treatment [for them] has not been established in randomized, controlled trials.
“Almost all randomized trials that targeted oligometastatic patients accrued mostly recurrent metastatic cancers; many included various cancer types, and none have tested the value of systemic multidrug regimens administered with curative intent,” he wrote.
If the health care systems adopt PET-CT for routine staging of locally advanced breast cancer, that will increase the diagnosis of de novo oligometastatic stage IV breast cancer, Dr. Pusztai said. That “underlines the importance of conducting studies for this unique subset of patients to establish level 1 evidence-based treatment strategies.”
Dr. Dayes has received honoraria from Verity Pharmaceuticals. One coauthor is employed by Point Biopharma. Other coauthors reported ties with AbbVie, Agendia, Genomic Health, InMode and Lutronic. Dr. Pusztai’s institution has received research funding from Merck, Genentech, Seagen, AstraZeneca, Bristol Myers Squibb, and Pfizer. He has received honoraria and travel expenses and has served in a consulting role for several pharmaceutical companies. Full disclosures are available on Open Payments.
In women who have locally advanced breast cancer (LABC), staging defines the extent of the disease and guides therapy.
Researchers have found in the first large, randomized, controlled study on the subject that 18 F-labeled fluorodeoxyglucose positron emission tomography–computed tomography (PET-CT) finds more distant metastases and allows more accurate staging than usual staging, which is determined by a bone scan and computed tomography (CT) of the thorax/abdomen and pelvis.
Findings of the study, led by Ian S. Dayes, MD, MSc, with the department of oncology at McMaster University in Hamilton, Ont., were published online in the Journal of Clinical Oncology.
Scans indicate less aggressive treatment strategy
The authors of the new study wrote that women with LABC, who are at high risk of metastatic disease, have large tumors that “can involve the chest wall or skin, clinically fixed axillary lymph nodes, or infraclavicular, supraclavicular, or internal mammary lymphadenopathy.”
If staging does not detect metastases, treatment is centered on combined modality therapy with curative intent (neoadjuvant chemotherapy and surgery, followed by regional radiation). If metastases are found, the treatment goal changes to controlling the disease.
In this study, twice as many women saw their stage increase from stage IIB or III to stage IV when PET-CT was used instead of conventional staging, guiding their treatment toward less aggressive care to control, rather than attempt to cure, the disease.
The women included in this study had histological evidence of invasive ductal carcinoma of the breast and TNM stage III or IIb (T3N0, but not T2N1).
Methods and results
Between December 2016 and April 2022, consenting patients from six regional cancer centers in Ontario were randomly assigned to one of two groups: 184 patients were randomly assigned to whole-body PET-CT and 185 patients to conventional staging.
Overall, the authors wrote, 43 (23%) of PET-CT patients “were upstaged” to stage IV compared with 21 (11%) of the conventionally staged patients (relative risk, 2.4; 95% confidence interval [CI], 1.4-4.2, P = .002).
There were 33 patients in a subset with inflammatory breast cancer and, among them, 4 of 16 (25%) PET-CT patients were upstaged to stage IV compared with 4 of 17 (24%) conventionally staged patients.
In the patients who did not have inflammatory breast cancer, 39 of 168 (23%) PET-CT patients were upstaged compared with 17 (10%) of 168 in the conventionally staged group.
Journal of Clinical Oncology (JCO) Senior Deputy Editor Kathy D. Miller, MD, said that, “PET/CT staging identifies distant disease in more patients and changes goals of therapy. Further research is needed to determine the impact on patient outcome.”
Findings have already changed practice
Senior author, Mark Levine, MD, MSc, also with McMaster, said in an interview that the results of this study have already changed practice in Canada, and he expects the United States to follow suit.
Dr. Levine said the study is important “in terms of helping plan therapy and being very open and honest with patients as to their prognosis.”
The findings constitute level 1 evidence in favor of PET-CT. Already, in Canada, “because of the results of the study, people with stage III breast cancer can get a PET scan,” he said.
Dr. Levine said he expects this evidence also to clarify “wishy-washy” National Comprehensive Cancer Network guidelines on using PET scans for LABC in the United States when the guidelines are next updated.
“That will make it easier for payers in the United States,” he added.
Cost effectiveness, Dr. Levine said, is complicated, because on one hand PET scans are quite costly. But its use would lead to more women getting less aggressive and expensive therapy and surgery.
Dr. Levine noted that his team will be analyzing cost-effectiveness over the next year.
New questions with more in stage IV
In an editorial, Lajos Pusztai, MD, DPhil, scientific codirector of the breast center at Yale University in New Haven, Conn., noted that, “all good studies raise new questions” and this one is no exception.
He pointed out that the number of women with stage IV metastatic breast cancer (MBC) has been increasing over the past 2 decades because of more sensitive staging methods. At the same time the number of women with recurrent metastatic disease is decreasing, because adjuvant therapies have improved.
Findings highlight need for stage IV treatment studies
Dr. Pusztai noted that the patients who have de novo oligometastatic stage IV disease “are a unique subset among patients with MBC,” and the best treatment [for them] has not been established in randomized, controlled trials.
“Almost all randomized trials that targeted oligometastatic patients accrued mostly recurrent metastatic cancers; many included various cancer types, and none have tested the value of systemic multidrug regimens administered with curative intent,” he wrote.
If the health care systems adopt PET-CT for routine staging of locally advanced breast cancer, that will increase the diagnosis of de novo oligometastatic stage IV breast cancer, Dr. Pusztai said. That “underlines the importance of conducting studies for this unique subset of patients to establish level 1 evidence-based treatment strategies.”
Dr. Dayes has received honoraria from Verity Pharmaceuticals. One coauthor is employed by Point Biopharma. Other coauthors reported ties with AbbVie, Agendia, Genomic Health, InMode and Lutronic. Dr. Pusztai’s institution has received research funding from Merck, Genentech, Seagen, AstraZeneca, Bristol Myers Squibb, and Pfizer. He has received honoraria and travel expenses and has served in a consulting role for several pharmaceutical companies. Full disclosures are available on Open Payments.
In women who have locally advanced breast cancer (LABC), staging defines the extent of the disease and guides therapy.
Researchers have found in the first large, randomized, controlled study on the subject that 18 F-labeled fluorodeoxyglucose positron emission tomography–computed tomography (PET-CT) finds more distant metastases and allows more accurate staging than usual staging, which is determined by a bone scan and computed tomography (CT) of the thorax/abdomen and pelvis.
Findings of the study, led by Ian S. Dayes, MD, MSc, with the department of oncology at McMaster University in Hamilton, Ont., were published online in the Journal of Clinical Oncology.
Scans indicate less aggressive treatment strategy
The authors of the new study wrote that women with LABC, who are at high risk of metastatic disease, have large tumors that “can involve the chest wall or skin, clinically fixed axillary lymph nodes, or infraclavicular, supraclavicular, or internal mammary lymphadenopathy.”
If staging does not detect metastases, treatment is centered on combined modality therapy with curative intent (neoadjuvant chemotherapy and surgery, followed by regional radiation). If metastases are found, the treatment goal changes to controlling the disease.
In this study, twice as many women saw their stage increase from stage IIB or III to stage IV when PET-CT was used instead of conventional staging, guiding their treatment toward less aggressive care to control, rather than attempt to cure, the disease.
The women included in this study had histological evidence of invasive ductal carcinoma of the breast and TNM stage III or IIb (T3N0, but not T2N1).
Methods and results
Between December 2016 and April 2022, consenting patients from six regional cancer centers in Ontario were randomly assigned to one of two groups: 184 patients were randomly assigned to whole-body PET-CT and 185 patients to conventional staging.
Overall, the authors wrote, 43 (23%) of PET-CT patients “were upstaged” to stage IV compared with 21 (11%) of the conventionally staged patients (relative risk, 2.4; 95% confidence interval [CI], 1.4-4.2, P = .002).
There were 33 patients in a subset with inflammatory breast cancer and, among them, 4 of 16 (25%) PET-CT patients were upstaged to stage IV compared with 4 of 17 (24%) conventionally staged patients.
In the patients who did not have inflammatory breast cancer, 39 of 168 (23%) PET-CT patients were upstaged compared with 17 (10%) of 168 in the conventionally staged group.
Journal of Clinical Oncology (JCO) Senior Deputy Editor Kathy D. Miller, MD, said that, “PET/CT staging identifies distant disease in more patients and changes goals of therapy. Further research is needed to determine the impact on patient outcome.”
Findings have already changed practice
Senior author, Mark Levine, MD, MSc, also with McMaster, said in an interview that the results of this study have already changed practice in Canada, and he expects the United States to follow suit.
Dr. Levine said the study is important “in terms of helping plan therapy and being very open and honest with patients as to their prognosis.”
The findings constitute level 1 evidence in favor of PET-CT. Already, in Canada, “because of the results of the study, people with stage III breast cancer can get a PET scan,” he said.
Dr. Levine said he expects this evidence also to clarify “wishy-washy” National Comprehensive Cancer Network guidelines on using PET scans for LABC in the United States when the guidelines are next updated.
“That will make it easier for payers in the United States,” he added.
Cost effectiveness, Dr. Levine said, is complicated, because on one hand PET scans are quite costly. But its use would lead to more women getting less aggressive and expensive therapy and surgery.
Dr. Levine noted that his team will be analyzing cost-effectiveness over the next year.
New questions with more in stage IV
In an editorial, Lajos Pusztai, MD, DPhil, scientific codirector of the breast center at Yale University in New Haven, Conn., noted that, “all good studies raise new questions” and this one is no exception.
He pointed out that the number of women with stage IV metastatic breast cancer (MBC) has been increasing over the past 2 decades because of more sensitive staging methods. At the same time the number of women with recurrent metastatic disease is decreasing, because adjuvant therapies have improved.
Findings highlight need for stage IV treatment studies
Dr. Pusztai noted that the patients who have de novo oligometastatic stage IV disease “are a unique subset among patients with MBC,” and the best treatment [for them] has not been established in randomized, controlled trials.
“Almost all randomized trials that targeted oligometastatic patients accrued mostly recurrent metastatic cancers; many included various cancer types, and none have tested the value of systemic multidrug regimens administered with curative intent,” he wrote.
If the health care systems adopt PET-CT for routine staging of locally advanced breast cancer, that will increase the diagnosis of de novo oligometastatic stage IV breast cancer, Dr. Pusztai said. That “underlines the importance of conducting studies for this unique subset of patients to establish level 1 evidence-based treatment strategies.”
Dr. Dayes has received honoraria from Verity Pharmaceuticals. One coauthor is employed by Point Biopharma. Other coauthors reported ties with AbbVie, Agendia, Genomic Health, InMode and Lutronic. Dr. Pusztai’s institution has received research funding from Merck, Genentech, Seagen, AstraZeneca, Bristol Myers Squibb, and Pfizer. He has received honoraria and travel expenses and has served in a consulting role for several pharmaceutical companies. Full disclosures are available on Open Payments.
FROM JOURNAL OF CLINICAL ONCOLOGY
Commentary: CDK4/6 Inhibitors, Breast Irradiation, and Aromatase Inhibitors in Breast Cancer Treatment, July 2023
After a median follow-up of 21.6 mo, the dalpiciclib group demonstrated a significantly longer median progression-free survival (PFS) compared with the placebo group (30.6 mo vs 18.2 mo; stratified hazard ratio [HR] 0.51; 95% CI 0.38-0.69; P < .0001). Overall, the dalpiciclib group demonstrated a manageable safety profile, although a higher percentage of grade 3/4 adverse events was noted with dalpiciclib than with placebo (90% vs 12%), as expected. Overall survival data for this CDK4/6 inhibitor are yet to come. These results suggest that dalpiciclib in combination with endocrine therapy is an alternative treatment for this group of patients, especially in countries where the traditionally approved CDK4/6 inhibitors (palbociclib, ribociclib, and abemaciclib) are not available.
The optimal sequencing of endocrine therapy (ET) after progression on CDK4/6 inhibitor–based therapy remains a challenge. In the phase 2 MAINTAIN trial, 119 patients (all of whom had HR+/HER2- metastatic breast cancer and who progressed on ET and CDK4/6 inhibitors) were randomly assigned to receive a different ET (fulvestrant or exemestane) from the previous ET they had received plus either the CDK4/6 inhibitor ribociclib or placebo. In the study by Kalinksky and colleagues, at a median follow-up of 18.2 mo, a significant improvement in PFS was observed in the switched ET-plus-ribociclib group compared with the switched ET-plus-placebo group (HR 0.57; P = .006). The phase 2 MAINTAIN trial is the first randomized trial to show the benefit of a CDK4/6 inhibitor after progression on another CDK4/6 inhibitor. It is important to note that the majority of patients in the MAINTAIN study previously received palbociclib in the first-line setting, which in recent studies has been demonstrated to be inferior to other CDK4/6 inhibitors. Therefore, it is important to confirm whether this will hold true upon progression from ribociclib or abemaciclib in the first-line setting. In addition, more data are needed to compare this approach with other ET treatment options, such as phosphoinositide 3-kinases inhibitors and oral selective estrogen receptor degraders.
There are several options for adjuvant radiation therapy for early-stage breast cancer. A meta-analysis of 14 randomized controlled trials and six comparative observational studies assessed the efficacy of whole breast irradiation (WBI) compared with partial breast irradiation (PBI) in 17,234 adults with early-stage breast cancer. Results of this meta-analysis showed that PBI was not significantly different from WBI, with similar rates of ipsilateral breast recurrence at 5 years (relative risk [RR] 1.34; 95% CI 0.83-2.18) and 10 years (RR 1.29; 95% CI 0.87-1.91), although patients undergoing PBI reported fewer acute adverse events compared with patients undergoing WBI (incidence rate ratio [IRR] 0.53; 95% CI 0.31-0.92) and acute grade ≥2 adverse events (IRR 0.21; 95% CI 0.07-0.62). These findings support using PBI as the adjuvant radiotherapy modality for select patients with favorable-risk early-stage breast cancer.
Another meta-analysis looked at assessing the survival benefit of adding CDK4/6 inhibitors to standard ET in older patients with advanced breast cancer. The study included 10 trials with 1985 older patients with advanced ER+ breast cancer who received ET with or without CDK4/6 inhibitors. The findings showed that adding CDK4/6 inhibitors to ET (letrozole or fulvestrant) significantly reduced the mortality risk by 21% (HR 0.79; 95% CI 0.69-0.91) and progression risk by 41% (HR 0.59; 95% CI 0.51-0.69) in older patients (age ≥ 65 years) with advanced breast cancer. Grade 3-4 neutropenia and diarrhea were similar in older patients. This study supports the use of CDK4/6 inhibitors as a reasonable treatment modality for older patients. More studies dedicated to the geriatric population are needed to help elaborate on the efficacy and tolerability of such agents in this population.
The phase 3 National Surgical Adjuvant Breast and Bowel Project B-42 (NSABP B-42) trial evaluated the role of extended letrozole therapy in postmenopausal breast cancer patients who were disease-free after 5 years of aromatase inhibitor–based therapy. The study included 3966 postmenopausal women with stage I-IIIA HR+ breast cancer who were randomly assigned to receive letrozole or placebo for 5 more years. After a median follow-up of 10.3 years, letrozole significantly improved disease-free survival (10-year absolute benefit 3.4%; HR 0.85; P = .01) compared with placebo, although there were no differences noted in overall survival between the groups (HR 0.97, P = .74). Furthermore, letrozole significantly reduced the breast cancer–free interval (HR 0.75, ,P = .003) and distant recurrence (HR 0.72, P = .01). There were no notable differences in toxicity, particularly rates of osteoporotic fractures and arterial thrombotic events, between the groups. Extended therapy with aromatase inhibitors beyond 5 years can be considered for select patients with early-stage breast cancer. Careful consideration of risks and benefits is needed to make these recommendations.
After a median follow-up of 21.6 mo, the dalpiciclib group demonstrated a significantly longer median progression-free survival (PFS) compared with the placebo group (30.6 mo vs 18.2 mo; stratified hazard ratio [HR] 0.51; 95% CI 0.38-0.69; P < .0001). Overall, the dalpiciclib group demonstrated a manageable safety profile, although a higher percentage of grade 3/4 adverse events was noted with dalpiciclib than with placebo (90% vs 12%), as expected. Overall survival data for this CDK4/6 inhibitor are yet to come. These results suggest that dalpiciclib in combination with endocrine therapy is an alternative treatment for this group of patients, especially in countries where the traditionally approved CDK4/6 inhibitors (palbociclib, ribociclib, and abemaciclib) are not available.
The optimal sequencing of endocrine therapy (ET) after progression on CDK4/6 inhibitor–based therapy remains a challenge. In the phase 2 MAINTAIN trial, 119 patients (all of whom had HR+/HER2- metastatic breast cancer and who progressed on ET and CDK4/6 inhibitors) were randomly assigned to receive a different ET (fulvestrant or exemestane) from the previous ET they had received plus either the CDK4/6 inhibitor ribociclib or placebo. In the study by Kalinksky and colleagues, at a median follow-up of 18.2 mo, a significant improvement in PFS was observed in the switched ET-plus-ribociclib group compared with the switched ET-plus-placebo group (HR 0.57; P = .006). The phase 2 MAINTAIN trial is the first randomized trial to show the benefit of a CDK4/6 inhibitor after progression on another CDK4/6 inhibitor. It is important to note that the majority of patients in the MAINTAIN study previously received palbociclib in the first-line setting, which in recent studies has been demonstrated to be inferior to other CDK4/6 inhibitors. Therefore, it is important to confirm whether this will hold true upon progression from ribociclib or abemaciclib in the first-line setting. In addition, more data are needed to compare this approach with other ET treatment options, such as phosphoinositide 3-kinases inhibitors and oral selective estrogen receptor degraders.
There are several options for adjuvant radiation therapy for early-stage breast cancer. A meta-analysis of 14 randomized controlled trials and six comparative observational studies assessed the efficacy of whole breast irradiation (WBI) compared with partial breast irradiation (PBI) in 17,234 adults with early-stage breast cancer. Results of this meta-analysis showed that PBI was not significantly different from WBI, with similar rates of ipsilateral breast recurrence at 5 years (relative risk [RR] 1.34; 95% CI 0.83-2.18) and 10 years (RR 1.29; 95% CI 0.87-1.91), although patients undergoing PBI reported fewer acute adverse events compared with patients undergoing WBI (incidence rate ratio [IRR] 0.53; 95% CI 0.31-0.92) and acute grade ≥2 adverse events (IRR 0.21; 95% CI 0.07-0.62). These findings support using PBI as the adjuvant radiotherapy modality for select patients with favorable-risk early-stage breast cancer.
Another meta-analysis looked at assessing the survival benefit of adding CDK4/6 inhibitors to standard ET in older patients with advanced breast cancer. The study included 10 trials with 1985 older patients with advanced ER+ breast cancer who received ET with or without CDK4/6 inhibitors. The findings showed that adding CDK4/6 inhibitors to ET (letrozole or fulvestrant) significantly reduced the mortality risk by 21% (HR 0.79; 95% CI 0.69-0.91) and progression risk by 41% (HR 0.59; 95% CI 0.51-0.69) in older patients (age ≥ 65 years) with advanced breast cancer. Grade 3-4 neutropenia and diarrhea were similar in older patients. This study supports the use of CDK4/6 inhibitors as a reasonable treatment modality for older patients. More studies dedicated to the geriatric population are needed to help elaborate on the efficacy and tolerability of such agents in this population.
The phase 3 National Surgical Adjuvant Breast and Bowel Project B-42 (NSABP B-42) trial evaluated the role of extended letrozole therapy in postmenopausal breast cancer patients who were disease-free after 5 years of aromatase inhibitor–based therapy. The study included 3966 postmenopausal women with stage I-IIIA HR+ breast cancer who were randomly assigned to receive letrozole or placebo for 5 more years. After a median follow-up of 10.3 years, letrozole significantly improved disease-free survival (10-year absolute benefit 3.4%; HR 0.85; P = .01) compared with placebo, although there were no differences noted in overall survival between the groups (HR 0.97, P = .74). Furthermore, letrozole significantly reduced the breast cancer–free interval (HR 0.75, ,P = .003) and distant recurrence (HR 0.72, P = .01). There were no notable differences in toxicity, particularly rates of osteoporotic fractures and arterial thrombotic events, between the groups. Extended therapy with aromatase inhibitors beyond 5 years can be considered for select patients with early-stage breast cancer. Careful consideration of risks and benefits is needed to make these recommendations.
After a median follow-up of 21.6 mo, the dalpiciclib group demonstrated a significantly longer median progression-free survival (PFS) compared with the placebo group (30.6 mo vs 18.2 mo; stratified hazard ratio [HR] 0.51; 95% CI 0.38-0.69; P < .0001). Overall, the dalpiciclib group demonstrated a manageable safety profile, although a higher percentage of grade 3/4 adverse events was noted with dalpiciclib than with placebo (90% vs 12%), as expected. Overall survival data for this CDK4/6 inhibitor are yet to come. These results suggest that dalpiciclib in combination with endocrine therapy is an alternative treatment for this group of patients, especially in countries where the traditionally approved CDK4/6 inhibitors (palbociclib, ribociclib, and abemaciclib) are not available.
The optimal sequencing of endocrine therapy (ET) after progression on CDK4/6 inhibitor–based therapy remains a challenge. In the phase 2 MAINTAIN trial, 119 patients (all of whom had HR+/HER2- metastatic breast cancer and who progressed on ET and CDK4/6 inhibitors) were randomly assigned to receive a different ET (fulvestrant or exemestane) from the previous ET they had received plus either the CDK4/6 inhibitor ribociclib or placebo. In the study by Kalinksky and colleagues, at a median follow-up of 18.2 mo, a significant improvement in PFS was observed in the switched ET-plus-ribociclib group compared with the switched ET-plus-placebo group (HR 0.57; P = .006). The phase 2 MAINTAIN trial is the first randomized trial to show the benefit of a CDK4/6 inhibitor after progression on another CDK4/6 inhibitor. It is important to note that the majority of patients in the MAINTAIN study previously received palbociclib in the first-line setting, which in recent studies has been demonstrated to be inferior to other CDK4/6 inhibitors. Therefore, it is important to confirm whether this will hold true upon progression from ribociclib or abemaciclib in the first-line setting. In addition, more data are needed to compare this approach with other ET treatment options, such as phosphoinositide 3-kinases inhibitors and oral selective estrogen receptor degraders.
There are several options for adjuvant radiation therapy for early-stage breast cancer. A meta-analysis of 14 randomized controlled trials and six comparative observational studies assessed the efficacy of whole breast irradiation (WBI) compared with partial breast irradiation (PBI) in 17,234 adults with early-stage breast cancer. Results of this meta-analysis showed that PBI was not significantly different from WBI, with similar rates of ipsilateral breast recurrence at 5 years (relative risk [RR] 1.34; 95% CI 0.83-2.18) and 10 years (RR 1.29; 95% CI 0.87-1.91), although patients undergoing PBI reported fewer acute adverse events compared with patients undergoing WBI (incidence rate ratio [IRR] 0.53; 95% CI 0.31-0.92) and acute grade ≥2 adverse events (IRR 0.21; 95% CI 0.07-0.62). These findings support using PBI as the adjuvant radiotherapy modality for select patients with favorable-risk early-stage breast cancer.
Another meta-analysis looked at assessing the survival benefit of adding CDK4/6 inhibitors to standard ET in older patients with advanced breast cancer. The study included 10 trials with 1985 older patients with advanced ER+ breast cancer who received ET with or without CDK4/6 inhibitors. The findings showed that adding CDK4/6 inhibitors to ET (letrozole or fulvestrant) significantly reduced the mortality risk by 21% (HR 0.79; 95% CI 0.69-0.91) and progression risk by 41% (HR 0.59; 95% CI 0.51-0.69) in older patients (age ≥ 65 years) with advanced breast cancer. Grade 3-4 neutropenia and diarrhea were similar in older patients. This study supports the use of CDK4/6 inhibitors as a reasonable treatment modality for older patients. More studies dedicated to the geriatric population are needed to help elaborate on the efficacy and tolerability of such agents in this population.
The phase 3 National Surgical Adjuvant Breast and Bowel Project B-42 (NSABP B-42) trial evaluated the role of extended letrozole therapy in postmenopausal breast cancer patients who were disease-free after 5 years of aromatase inhibitor–based therapy. The study included 3966 postmenopausal women with stage I-IIIA HR+ breast cancer who were randomly assigned to receive letrozole or placebo for 5 more years. After a median follow-up of 10.3 years, letrozole significantly improved disease-free survival (10-year absolute benefit 3.4%; HR 0.85; P = .01) compared with placebo, although there were no differences noted in overall survival between the groups (HR 0.97, P = .74). Furthermore, letrozole significantly reduced the breast cancer–free interval (HR 0.75, ,P = .003) and distant recurrence (HR 0.72, P = .01). There were no notable differences in toxicity, particularly rates of osteoporotic fractures and arterial thrombotic events, between the groups. Extended therapy with aromatase inhibitors beyond 5 years can be considered for select patients with early-stage breast cancer. Careful consideration of risks and benefits is needed to make these recommendations.
Commentary: Advances in HER2 advanced breast cancer, July 2023
The neoadjuvant setting provides a favorable environment to study de-escalation approaches as treatment response (via pathologic complete response [pCR] assessment) can be used as a surrogate marker for outcome. Studies have shown the effect of HER2-enriched subtype and high ERBB2 expression on pCR rates after receipt of a chemotherapy-free, dual HER2-targeted regimen.2 The prospective, multicenter, neoadjuvant phase 2 WSG-TP-II trial randomly assigned 207 patients with HR+/HER2+ early breast cancer to 12 weeks of endocrine therapy (ET)–trastuzumab-pertuzumab vs paclitaxel-trastuzumab-pertuzumab. The pCR rate was inferior in the ET arm compared with the paclitaxel arm (23.7% vs 56.4%; odds ratio 0.24; 95% CI 0.12-0.46; P < .001). In addition, an immunohistochemistry ERBB2 score of 3 or higher and ERBB2-enriched subtype were predictors of higher pCR rates in both arms (Gluz et al). This study not only supports a deescalated chemotherapy neoadjuvant strategy of paclitaxel + dual HER2 blockade but also suggests that a portion of patients may potentially be spared chemotherapy with very good results. The role of biomarkers is integral to patient selection for these approaches, and the evaluation of response in real-time will allow for the tailoring of therapy to achieve the best outcome.
Systemic staging for locally advanced breast cancer (LABC) is important for informing prognosis as well as aiding in development of an appropriate treatment plan for patients. The PETABC study included 369 patients with LABC (TNM stage III or IIB [T3N0]) with random assignment to 18F-labeled fluorodeoxyglucose PET-CT or conventional staging (bone scan, CT of chest/abdomen/pelvis), and was designed to assess the rate of upstaging with each imaging modality and effect on treatment (Dayes et al). In the PET-CT group, 23% (N = 43) of patients were upstaged to stage IV compared with 11% (N = 21) in the conventional-staging group (absolute difference 12.3%; 95% CI 3.9-19.9; P = .002). Fewer patients in the PET-CT group received combined modality treatment vs those patients in the conventional staging group (81% vs 89.2%; P = .03). These results support the consideration of PET-CT as a staging tool for LABC, and this is reflected in various clinical guidelines. Furthermore, the evolving role of other imaging techniques such as 18F-fluoroestradiol (18F-FES) PET-CT in detection of metastatic lesions related to estrogen receptor–positive breast cancer3 will continue to advance the field of imaging.
Additional References
- Rugo HS, Lerebours F, Ciruelos E, et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): One cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 2021;22:489-498. doi: 10.1016/S1470-2045(21)00034-6. Erratum in: Lancet Oncol. 2021;22(5):e184. doi: 10.1016/S1470-2045(21)00194-7
- Prat A, Pascual T, De Angelis C, et al. HER2-enriched subtype and ERBB2 expression in HER2-positive breast cancer treated with dual HER2 blockade. J Natl Cancer Inst. 2020;112:46-54. doi: 10.1093/jnci/djz042
- Ulaner GA, Jhaveri K, Chandarlapaty S, et al. Head-to-head evaluation of 18F-FES and 18F-FDG PET/CT in metastatic invasive lobular breast cancer. J Nucl Med. 2021;62:326-331. doi: 10.2967/jnumed.120.247882
The neoadjuvant setting provides a favorable environment to study de-escalation approaches as treatment response (via pathologic complete response [pCR] assessment) can be used as a surrogate marker for outcome. Studies have shown the effect of HER2-enriched subtype and high ERBB2 expression on pCR rates after receipt of a chemotherapy-free, dual HER2-targeted regimen.2 The prospective, multicenter, neoadjuvant phase 2 WSG-TP-II trial randomly assigned 207 patients with HR+/HER2+ early breast cancer to 12 weeks of endocrine therapy (ET)–trastuzumab-pertuzumab vs paclitaxel-trastuzumab-pertuzumab. The pCR rate was inferior in the ET arm compared with the paclitaxel arm (23.7% vs 56.4%; odds ratio 0.24; 95% CI 0.12-0.46; P < .001). In addition, an immunohistochemistry ERBB2 score of 3 or higher and ERBB2-enriched subtype were predictors of higher pCR rates in both arms (Gluz et al). This study not only supports a deescalated chemotherapy neoadjuvant strategy of paclitaxel + dual HER2 blockade but also suggests that a portion of patients may potentially be spared chemotherapy with very good results. The role of biomarkers is integral to patient selection for these approaches, and the evaluation of response in real-time will allow for the tailoring of therapy to achieve the best outcome.
Systemic staging for locally advanced breast cancer (LABC) is important for informing prognosis as well as aiding in development of an appropriate treatment plan for patients. The PETABC study included 369 patients with LABC (TNM stage III or IIB [T3N0]) with random assignment to 18F-labeled fluorodeoxyglucose PET-CT or conventional staging (bone scan, CT of chest/abdomen/pelvis), and was designed to assess the rate of upstaging with each imaging modality and effect on treatment (Dayes et al). In the PET-CT group, 23% (N = 43) of patients were upstaged to stage IV compared with 11% (N = 21) in the conventional-staging group (absolute difference 12.3%; 95% CI 3.9-19.9; P = .002). Fewer patients in the PET-CT group received combined modality treatment vs those patients in the conventional staging group (81% vs 89.2%; P = .03). These results support the consideration of PET-CT as a staging tool for LABC, and this is reflected in various clinical guidelines. Furthermore, the evolving role of other imaging techniques such as 18F-fluoroestradiol (18F-FES) PET-CT in detection of metastatic lesions related to estrogen receptor–positive breast cancer3 will continue to advance the field of imaging.
Additional References
- Rugo HS, Lerebours F, Ciruelos E, et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): One cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 2021;22:489-498. doi: 10.1016/S1470-2045(21)00034-6. Erratum in: Lancet Oncol. 2021;22(5):e184. doi: 10.1016/S1470-2045(21)00194-7
- Prat A, Pascual T, De Angelis C, et al. HER2-enriched subtype and ERBB2 expression in HER2-positive breast cancer treated with dual HER2 blockade. J Natl Cancer Inst. 2020;112:46-54. doi: 10.1093/jnci/djz042
- Ulaner GA, Jhaveri K, Chandarlapaty S, et al. Head-to-head evaluation of 18F-FES and 18F-FDG PET/CT in metastatic invasive lobular breast cancer. J Nucl Med. 2021;62:326-331. doi: 10.2967/jnumed.120.247882
The neoadjuvant setting provides a favorable environment to study de-escalation approaches as treatment response (via pathologic complete response [pCR] assessment) can be used as a surrogate marker for outcome. Studies have shown the effect of HER2-enriched subtype and high ERBB2 expression on pCR rates after receipt of a chemotherapy-free, dual HER2-targeted regimen.2 The prospective, multicenter, neoadjuvant phase 2 WSG-TP-II trial randomly assigned 207 patients with HR+/HER2+ early breast cancer to 12 weeks of endocrine therapy (ET)–trastuzumab-pertuzumab vs paclitaxel-trastuzumab-pertuzumab. The pCR rate was inferior in the ET arm compared with the paclitaxel arm (23.7% vs 56.4%; odds ratio 0.24; 95% CI 0.12-0.46; P < .001). In addition, an immunohistochemistry ERBB2 score of 3 or higher and ERBB2-enriched subtype were predictors of higher pCR rates in both arms (Gluz et al). This study not only supports a deescalated chemotherapy neoadjuvant strategy of paclitaxel + dual HER2 blockade but also suggests that a portion of patients may potentially be spared chemotherapy with very good results. The role of biomarkers is integral to patient selection for these approaches, and the evaluation of response in real-time will allow for the tailoring of therapy to achieve the best outcome.
Systemic staging for locally advanced breast cancer (LABC) is important for informing prognosis as well as aiding in development of an appropriate treatment plan for patients. The PETABC study included 369 patients with LABC (TNM stage III or IIB [T3N0]) with random assignment to 18F-labeled fluorodeoxyglucose PET-CT or conventional staging (bone scan, CT of chest/abdomen/pelvis), and was designed to assess the rate of upstaging with each imaging modality and effect on treatment (Dayes et al). In the PET-CT group, 23% (N = 43) of patients were upstaged to stage IV compared with 11% (N = 21) in the conventional-staging group (absolute difference 12.3%; 95% CI 3.9-19.9; P = .002). Fewer patients in the PET-CT group received combined modality treatment vs those patients in the conventional staging group (81% vs 89.2%; P = .03). These results support the consideration of PET-CT as a staging tool for LABC, and this is reflected in various clinical guidelines. Furthermore, the evolving role of other imaging techniques such as 18F-fluoroestradiol (18F-FES) PET-CT in detection of metastatic lesions related to estrogen receptor–positive breast cancer3 will continue to advance the field of imaging.
Additional References
- Rugo HS, Lerebours F, Ciruelos E, et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): One cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 2021;22:489-498. doi: 10.1016/S1470-2045(21)00034-6. Erratum in: Lancet Oncol. 2021;22(5):e184. doi: 10.1016/S1470-2045(21)00194-7
- Prat A, Pascual T, De Angelis C, et al. HER2-enriched subtype and ERBB2 expression in HER2-positive breast cancer treated with dual HER2 blockade. J Natl Cancer Inst. 2020;112:46-54. doi: 10.1093/jnci/djz042
- Ulaner GA, Jhaveri K, Chandarlapaty S, et al. Head-to-head evaluation of 18F-FES and 18F-FDG PET/CT in metastatic invasive lobular breast cancer. J Nucl Med. 2021;62:326-331. doi: 10.2967/jnumed.120.247882