User login
Identifying Barriers in Germline Genetic Testing Referrals for Breast Cancer: A Single-Center Experience
Background
Purpose: to review the number of genetic testing referrals for breast cancer at the Stratton VA Medical Center and identify barriers that hinder testing, aiming to improve risk reduction strategies and therapeutic options for patients. National guidelines recommend genetic testing for breast cancer susceptibility genes in specific patient populations, such as those under 50, those with a high-risk family history, high-risk pathology, male breast cancer, or Ashkenazi Jewish ancestry. Despite efforts to adhere to these guidelines, several barriers persist that limit testing rates among eligible patients.
Methods
The medical oncology team selected breast cancer as the focus for reviewing adherence to germline genetic testing referrals in the Stratton VA Medical Center. With assistance from cancer registrars, a list of genetics referrals for breast cancer from January to December 2023 was compiled. Descriptive analysis was conducted to assess referral rates, evaluation visit completion rates, genetic testing outcomes, and reasons for non-completion of genetic testing.
Results
During the study period, 32 patients were referred for germline genetic testing for breast cancer. Of these, 26 (81%) completed the evaluation visit, and 11 (34%) underwent genetic testing. Of these, 7 patients had noteworthy results, and 2 patients (6%) were found to carry pathogenic variants: BRCA2 and CDH1. Reasons for non-completion included perceived irrelevance without biological children, need for additional time to consider testing, fear of exacerbating self-harm thoughts, and fear of losing service connection. Additionally, 2 patients did not meet the guidelines for testing per genetic counselor.
Conclusions
This project marks the initial step in identifying barriers to germline genetic testing for breast cancer based on an extensive review of patients diagnosed and treated at a single VA site. Despite the removal of the service connection clause from the consent form, some veterans still declined testing due to fear of losing their service connection. The findings emphasize the importance of educating providers on counseling techniques and education of veterans to enhance risk reduction strategies and patient care. Further research is essential to quantify the real-world outcomes and longterm impacts of improving genetic counseling rates on patient management and outcomes.
Background
Purpose: to review the number of genetic testing referrals for breast cancer at the Stratton VA Medical Center and identify barriers that hinder testing, aiming to improve risk reduction strategies and therapeutic options for patients. National guidelines recommend genetic testing for breast cancer susceptibility genes in specific patient populations, such as those under 50, those with a high-risk family history, high-risk pathology, male breast cancer, or Ashkenazi Jewish ancestry. Despite efforts to adhere to these guidelines, several barriers persist that limit testing rates among eligible patients.
Methods
The medical oncology team selected breast cancer as the focus for reviewing adherence to germline genetic testing referrals in the Stratton VA Medical Center. With assistance from cancer registrars, a list of genetics referrals for breast cancer from January to December 2023 was compiled. Descriptive analysis was conducted to assess referral rates, evaluation visit completion rates, genetic testing outcomes, and reasons for non-completion of genetic testing.
Results
During the study period, 32 patients were referred for germline genetic testing for breast cancer. Of these, 26 (81%) completed the evaluation visit, and 11 (34%) underwent genetic testing. Of these, 7 patients had noteworthy results, and 2 patients (6%) were found to carry pathogenic variants: BRCA2 and CDH1. Reasons for non-completion included perceived irrelevance without biological children, need for additional time to consider testing, fear of exacerbating self-harm thoughts, and fear of losing service connection. Additionally, 2 patients did not meet the guidelines for testing per genetic counselor.
Conclusions
This project marks the initial step in identifying barriers to germline genetic testing for breast cancer based on an extensive review of patients diagnosed and treated at a single VA site. Despite the removal of the service connection clause from the consent form, some veterans still declined testing due to fear of losing their service connection. The findings emphasize the importance of educating providers on counseling techniques and education of veterans to enhance risk reduction strategies and patient care. Further research is essential to quantify the real-world outcomes and longterm impacts of improving genetic counseling rates on patient management and outcomes.
Background
Purpose: to review the number of genetic testing referrals for breast cancer at the Stratton VA Medical Center and identify barriers that hinder testing, aiming to improve risk reduction strategies and therapeutic options for patients. National guidelines recommend genetic testing for breast cancer susceptibility genes in specific patient populations, such as those under 50, those with a high-risk family history, high-risk pathology, male breast cancer, or Ashkenazi Jewish ancestry. Despite efforts to adhere to these guidelines, several barriers persist that limit testing rates among eligible patients.
Methods
The medical oncology team selected breast cancer as the focus for reviewing adherence to germline genetic testing referrals in the Stratton VA Medical Center. With assistance from cancer registrars, a list of genetics referrals for breast cancer from January to December 2023 was compiled. Descriptive analysis was conducted to assess referral rates, evaluation visit completion rates, genetic testing outcomes, and reasons for non-completion of genetic testing.
Results
During the study period, 32 patients were referred for germline genetic testing for breast cancer. Of these, 26 (81%) completed the evaluation visit, and 11 (34%) underwent genetic testing. Of these, 7 patients had noteworthy results, and 2 patients (6%) were found to carry pathogenic variants: BRCA2 and CDH1. Reasons for non-completion included perceived irrelevance without biological children, need for additional time to consider testing, fear of exacerbating self-harm thoughts, and fear of losing service connection. Additionally, 2 patients did not meet the guidelines for testing per genetic counselor.
Conclusions
This project marks the initial step in identifying barriers to germline genetic testing for breast cancer based on an extensive review of patients diagnosed and treated at a single VA site. Despite the removal of the service connection clause from the consent form, some veterans still declined testing due to fear of losing their service connection. The findings emphasize the importance of educating providers on counseling techniques and education of veterans to enhance risk reduction strategies and patient care. Further research is essential to quantify the real-world outcomes and longterm impacts of improving genetic counseling rates on patient management and outcomes.
Asynchronous Bilateral Breast Cancer in a Male Patient
Background
Bilateral male breast cancer remains a rare occurrence with limited representation in published literature. Here we present a case of an 82-yearold male with asynchronous bilateral breast cancer.
Case Presentation
Our patient is an 82-year-old male past smoker initially diagnosed with left T1aN0M0 invasive lobular carcinoma in 2010 that was ER, PR positive and HER2 negative. He underwent a left mastectomy with sentinel node biopsy and was given tamoxifen therapy for 10 years. In 2020, the patient was also diagnosed with lung squamous cell carcinoma and was treated with stereotactic body radiotherapy. In September 2023, he started noticing discharge from his right nipple. A PET CT scan revealed hyper-metabolic activity in the bilateral upper lung lobes and slightly increased activity in the right breast. A biopsy of the left upper lobe showed atypical cells. He also underwent a right breast mastectomy and sentinel lymph node biopsy which showed grade 1-2 ductal carcinoma in situ and negative sentinel lymph nodes. The tumor board recommended no further treatment after his mastectomy and genetic testing which is currently pending.
Discussion
Male breast cancer comprises just 1% of breast cancer cases, with asynchronous bilateral occurrences being exceedingly rare. A review of PubMed literature yielded only 2 documented case reports. Male breast cancer usually diagnosed around ages 60 to 70 years. The predominant histopathological diagnosis is invasive ductal adenocarcinoma that more frequently expresses ER/PR over HER2. It often manifests as a painless lump, frequently diagnosed at an advanced stage, possibly due to factors such as lower screening rates in males and less breast parenchyma. Local treatment options include surgery and radiotherapy. Neoadjuvant tamoxifen therapy is appropriate for ER and PR expressing cancers and chemotherapy can be used for non-hormone expressing or metastatic tumors. Given its rarity, management and diagnostic strategies for male breast cancer are often adapted from research on female breast cancer
Conclusions
Our case is of a relatively uncommon incident of asynchronous bilateral male breast cancer, emphasizing the need for expanded research efforts in male breast cancer. An enhanced understanding could lead to improved diagnosis and management strategies, potentially enhancing survival outcomes.
Background
Bilateral male breast cancer remains a rare occurrence with limited representation in published literature. Here we present a case of an 82-yearold male with asynchronous bilateral breast cancer.
Case Presentation
Our patient is an 82-year-old male past smoker initially diagnosed with left T1aN0M0 invasive lobular carcinoma in 2010 that was ER, PR positive and HER2 negative. He underwent a left mastectomy with sentinel node biopsy and was given tamoxifen therapy for 10 years. In 2020, the patient was also diagnosed with lung squamous cell carcinoma and was treated with stereotactic body radiotherapy. In September 2023, he started noticing discharge from his right nipple. A PET CT scan revealed hyper-metabolic activity in the bilateral upper lung lobes and slightly increased activity in the right breast. A biopsy of the left upper lobe showed atypical cells. He also underwent a right breast mastectomy and sentinel lymph node biopsy which showed grade 1-2 ductal carcinoma in situ and negative sentinel lymph nodes. The tumor board recommended no further treatment after his mastectomy and genetic testing which is currently pending.
Discussion
Male breast cancer comprises just 1% of breast cancer cases, with asynchronous bilateral occurrences being exceedingly rare. A review of PubMed literature yielded only 2 documented case reports. Male breast cancer usually diagnosed around ages 60 to 70 years. The predominant histopathological diagnosis is invasive ductal adenocarcinoma that more frequently expresses ER/PR over HER2. It often manifests as a painless lump, frequently diagnosed at an advanced stage, possibly due to factors such as lower screening rates in males and less breast parenchyma. Local treatment options include surgery and radiotherapy. Neoadjuvant tamoxifen therapy is appropriate for ER and PR expressing cancers and chemotherapy can be used for non-hormone expressing or metastatic tumors. Given its rarity, management and diagnostic strategies for male breast cancer are often adapted from research on female breast cancer
Conclusions
Our case is of a relatively uncommon incident of asynchronous bilateral male breast cancer, emphasizing the need for expanded research efforts in male breast cancer. An enhanced understanding could lead to improved diagnosis and management strategies, potentially enhancing survival outcomes.
Background
Bilateral male breast cancer remains a rare occurrence with limited representation in published literature. Here we present a case of an 82-yearold male with asynchronous bilateral breast cancer.
Case Presentation
Our patient is an 82-year-old male past smoker initially diagnosed with left T1aN0M0 invasive lobular carcinoma in 2010 that was ER, PR positive and HER2 negative. He underwent a left mastectomy with sentinel node biopsy and was given tamoxifen therapy for 10 years. In 2020, the patient was also diagnosed with lung squamous cell carcinoma and was treated with stereotactic body radiotherapy. In September 2023, he started noticing discharge from his right nipple. A PET CT scan revealed hyper-metabolic activity in the bilateral upper lung lobes and slightly increased activity in the right breast. A biopsy of the left upper lobe showed atypical cells. He also underwent a right breast mastectomy and sentinel lymph node biopsy which showed grade 1-2 ductal carcinoma in situ and negative sentinel lymph nodes. The tumor board recommended no further treatment after his mastectomy and genetic testing which is currently pending.
Discussion
Male breast cancer comprises just 1% of breast cancer cases, with asynchronous bilateral occurrences being exceedingly rare. A review of PubMed literature yielded only 2 documented case reports. Male breast cancer usually diagnosed around ages 60 to 70 years. The predominant histopathological diagnosis is invasive ductal adenocarcinoma that more frequently expresses ER/PR over HER2. It often manifests as a painless lump, frequently diagnosed at an advanced stage, possibly due to factors such as lower screening rates in males and less breast parenchyma. Local treatment options include surgery and radiotherapy. Neoadjuvant tamoxifen therapy is appropriate for ER and PR expressing cancers and chemotherapy can be used for non-hormone expressing or metastatic tumors. Given its rarity, management and diagnostic strategies for male breast cancer are often adapted from research on female breast cancer
Conclusions
Our case is of a relatively uncommon incident of asynchronous bilateral male breast cancer, emphasizing the need for expanded research efforts in male breast cancer. An enhanced understanding could lead to improved diagnosis and management strategies, potentially enhancing survival outcomes.
National Tele-Oncology High-Risk Breast Clinic Program
Background
Assess implementation outcomes of the National Tele-Oncology’s first high-risk breast clinic program, part of the Breast and Gynecological System of Excellence (BGSOE). Women Veterans are the fastest-growing demographic in the Veteran population. Breast cancer (BC) is the most prevalent cancer among women. An estimated 15% of women will be considered high risk for BC at some point during their lifetime. For these reasons, the BGSOE high-risk breast clinic offers screening and risk reduction care to women with an increased risk for BC.
Methods
We described the patients seen in the BGSOE high-risk breast clinic since its implementation in 2023. We collected demographic and geographic information, genetic testing status, imaging, and risk-reducing agents (RRA) use. We reported percentages for categorical variables, followed by the total number of patients in parenthesis.
Results
There are a total of 124 patients served since 2023 (123 female, 1 male). The average age was 44.6 years. 61.3% (76) of patients lived in an urban setting, while 38.7% (48) lived in rural areas. Most patients were White at 63.7% (79), followed by African American 20.2%(25), Other 5.6% (7), and Unknown/declined 10.5%(13). Regarding ethnicity, 9% (12) were Hispanic. The most common reasons for referral to the clinic were a family history of breast cancer 89.2% (111), followed by high-risk genetic pathogenic variants 5.6% (7), mammary dysplasia 3.2% (4), inconclusive imaging 0.8% (1) and personal history of radiation 0.8%(1). 2 patients were started on RRAs. 56% (70) of patients had genetic testing discussions. The clinic coordinated 50 mammograms and 10 breast MRIs.
Conclusions
We demonstrated the successful implementation of the BGSOE high-risk breast program. We reached multiple historically underserved populations, including a high percentage of rural and African American patients. We also facilitated breast MRIs. Similar to other studies, there was a low uptake of RRA in our clinic. BGSOE is now working on a clinical pathway to standardize RRA and breast imaging recommendations for high-risk women. There are many more women Veterans at risk for BC and future expansion of the highrisk breast clinic could further raise awareness of lifetime breast cancer risk and risk-reducing and surveillance options in Veterans.
Background
Assess implementation outcomes of the National Tele-Oncology’s first high-risk breast clinic program, part of the Breast and Gynecological System of Excellence (BGSOE). Women Veterans are the fastest-growing demographic in the Veteran population. Breast cancer (BC) is the most prevalent cancer among women. An estimated 15% of women will be considered high risk for BC at some point during their lifetime. For these reasons, the BGSOE high-risk breast clinic offers screening and risk reduction care to women with an increased risk for BC.
Methods
We described the patients seen in the BGSOE high-risk breast clinic since its implementation in 2023. We collected demographic and geographic information, genetic testing status, imaging, and risk-reducing agents (RRA) use. We reported percentages for categorical variables, followed by the total number of patients in parenthesis.
Results
There are a total of 124 patients served since 2023 (123 female, 1 male). The average age was 44.6 years. 61.3% (76) of patients lived in an urban setting, while 38.7% (48) lived in rural areas. Most patients were White at 63.7% (79), followed by African American 20.2%(25), Other 5.6% (7), and Unknown/declined 10.5%(13). Regarding ethnicity, 9% (12) were Hispanic. The most common reasons for referral to the clinic were a family history of breast cancer 89.2% (111), followed by high-risk genetic pathogenic variants 5.6% (7), mammary dysplasia 3.2% (4), inconclusive imaging 0.8% (1) and personal history of radiation 0.8%(1). 2 patients were started on RRAs. 56% (70) of patients had genetic testing discussions. The clinic coordinated 50 mammograms and 10 breast MRIs.
Conclusions
We demonstrated the successful implementation of the BGSOE high-risk breast program. We reached multiple historically underserved populations, including a high percentage of rural and African American patients. We also facilitated breast MRIs. Similar to other studies, there was a low uptake of RRA in our clinic. BGSOE is now working on a clinical pathway to standardize RRA and breast imaging recommendations for high-risk women. There are many more women Veterans at risk for BC and future expansion of the highrisk breast clinic could further raise awareness of lifetime breast cancer risk and risk-reducing and surveillance options in Veterans.
Background
Assess implementation outcomes of the National Tele-Oncology’s first high-risk breast clinic program, part of the Breast and Gynecological System of Excellence (BGSOE). Women Veterans are the fastest-growing demographic in the Veteran population. Breast cancer (BC) is the most prevalent cancer among women. An estimated 15% of women will be considered high risk for BC at some point during their lifetime. For these reasons, the BGSOE high-risk breast clinic offers screening and risk reduction care to women with an increased risk for BC.
Methods
We described the patients seen in the BGSOE high-risk breast clinic since its implementation in 2023. We collected demographic and geographic information, genetic testing status, imaging, and risk-reducing agents (RRA) use. We reported percentages for categorical variables, followed by the total number of patients in parenthesis.
Results
There are a total of 124 patients served since 2023 (123 female, 1 male). The average age was 44.6 years. 61.3% (76) of patients lived in an urban setting, while 38.7% (48) lived in rural areas. Most patients were White at 63.7% (79), followed by African American 20.2%(25), Other 5.6% (7), and Unknown/declined 10.5%(13). Regarding ethnicity, 9% (12) were Hispanic. The most common reasons for referral to the clinic were a family history of breast cancer 89.2% (111), followed by high-risk genetic pathogenic variants 5.6% (7), mammary dysplasia 3.2% (4), inconclusive imaging 0.8% (1) and personal history of radiation 0.8%(1). 2 patients were started on RRAs. 56% (70) of patients had genetic testing discussions. The clinic coordinated 50 mammograms and 10 breast MRIs.
Conclusions
We demonstrated the successful implementation of the BGSOE high-risk breast program. We reached multiple historically underserved populations, including a high percentage of rural and African American patients. We also facilitated breast MRIs. Similar to other studies, there was a low uptake of RRA in our clinic. BGSOE is now working on a clinical pathway to standardize RRA and breast imaging recommendations for high-risk women. There are many more women Veterans at risk for BC and future expansion of the highrisk breast clinic could further raise awareness of lifetime breast cancer risk and risk-reducing and surveillance options in Veterans.
Cancer Cases, Deaths in Men Predicted to Surge by 2050
TOPLINE:
— with substantial disparities in cancer cases and deaths by age and region of the world, a recent analysis found.
METHODOLOGY:
- Overall, men have higher cancer incidence and mortality rates, which can be largely attributed to a higher prevalence of modifiable risk factors such as smoking, alcohol consumption, and occupational carcinogens, as well as the underuse of cancer prevention, screening, and treatment services.
- To assess the burden of cancer in men of different ages and from different regions of the world, researchers analyzed data from the 2022 Global Cancer Observatory (GLOBOCAN), which provides national-level estimates for cancer cases and deaths.
- Study outcomes included the incidence, mortality, and prevalence of cancer among men in 2022, along with projections for 2050. Estimates were stratified by several factors, including age; region; and Human Development Index (HDI), a composite score for health, education, and standard of living.
- Researchers also calculated mortality-to-incidence ratios (MIRs) for various cancer types, where higher values indicate worse survival.
TAKEAWAY:
- The researchers reported an estimated 10.3 million cancer cases and 5.4 million deaths globally in 2022, with almost two thirds of cases and deaths occurring in men aged 65 years or older.
- By 2050, cancer cases and deaths were projected to increase by 84.3% (to 19 million) and 93.2% (to 10.5 million), respectively. The increase from 2022 to 2050 was more than twofold higher for older men and countries with low and medium HDI.
- In 2022, the estimated global cancer MIR among men was nearly 55%, with variations by cancer types, age, and HDI. The MIR was lowest for thyroid cancer (7.6%) and highest for pancreatic cancer (90.9%); among World Health Organization regions, Africa had the highest MIR (72.6%), while the Americas had the lowest MIR (39.1%); countries with the lowest HDI had the highest MIR (73.5% vs 41.1% for very high HDI).
- Lung cancer was the leading cause for cases and deaths in 2022 and was projected to remain the leading cause in 2050.
IN PRACTICE:
“Disparities in cancer incidence and mortality among men were observed across age groups, countries/territories, and HDI in 2022, with these disparities projected to widen further by 2050,” according to the authors, who called for efforts to “reduce disparities in cancer burden and ensure equity in cancer prevention and care for men across the globe.”
SOURCE:
The study, led by Habtamu Mellie Bizuayehu, PhD, School of Public Health, Faculty of Medicine, The University of Queensland, Brisbane, Australia, was published online in Cancer.
LIMITATIONS:
The findings may be influenced by the quality of GLOBOCAN data. Interpretation should be cautious as MIR may not fully reflect cancer outcome inequalities. The study did not include other measures of cancer burden, such as years of life lost or years lived with disability, which were unavailable from the data source.
DISCLOSURES:
The authors did not disclose any funding information. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
— with substantial disparities in cancer cases and deaths by age and region of the world, a recent analysis found.
METHODOLOGY:
- Overall, men have higher cancer incidence and mortality rates, which can be largely attributed to a higher prevalence of modifiable risk factors such as smoking, alcohol consumption, and occupational carcinogens, as well as the underuse of cancer prevention, screening, and treatment services.
- To assess the burden of cancer in men of different ages and from different regions of the world, researchers analyzed data from the 2022 Global Cancer Observatory (GLOBOCAN), which provides national-level estimates for cancer cases and deaths.
- Study outcomes included the incidence, mortality, and prevalence of cancer among men in 2022, along with projections for 2050. Estimates were stratified by several factors, including age; region; and Human Development Index (HDI), a composite score for health, education, and standard of living.
- Researchers also calculated mortality-to-incidence ratios (MIRs) for various cancer types, where higher values indicate worse survival.
TAKEAWAY:
- The researchers reported an estimated 10.3 million cancer cases and 5.4 million deaths globally in 2022, with almost two thirds of cases and deaths occurring in men aged 65 years or older.
- By 2050, cancer cases and deaths were projected to increase by 84.3% (to 19 million) and 93.2% (to 10.5 million), respectively. The increase from 2022 to 2050 was more than twofold higher for older men and countries with low and medium HDI.
- In 2022, the estimated global cancer MIR among men was nearly 55%, with variations by cancer types, age, and HDI. The MIR was lowest for thyroid cancer (7.6%) and highest for pancreatic cancer (90.9%); among World Health Organization regions, Africa had the highest MIR (72.6%), while the Americas had the lowest MIR (39.1%); countries with the lowest HDI had the highest MIR (73.5% vs 41.1% for very high HDI).
- Lung cancer was the leading cause for cases and deaths in 2022 and was projected to remain the leading cause in 2050.
IN PRACTICE:
“Disparities in cancer incidence and mortality among men were observed across age groups, countries/territories, and HDI in 2022, with these disparities projected to widen further by 2050,” according to the authors, who called for efforts to “reduce disparities in cancer burden and ensure equity in cancer prevention and care for men across the globe.”
SOURCE:
The study, led by Habtamu Mellie Bizuayehu, PhD, School of Public Health, Faculty of Medicine, The University of Queensland, Brisbane, Australia, was published online in Cancer.
LIMITATIONS:
The findings may be influenced by the quality of GLOBOCAN data. Interpretation should be cautious as MIR may not fully reflect cancer outcome inequalities. The study did not include other measures of cancer burden, such as years of life lost or years lived with disability, which were unavailable from the data source.
DISCLOSURES:
The authors did not disclose any funding information. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
— with substantial disparities in cancer cases and deaths by age and region of the world, a recent analysis found.
METHODOLOGY:
- Overall, men have higher cancer incidence and mortality rates, which can be largely attributed to a higher prevalence of modifiable risk factors such as smoking, alcohol consumption, and occupational carcinogens, as well as the underuse of cancer prevention, screening, and treatment services.
- To assess the burden of cancer in men of different ages and from different regions of the world, researchers analyzed data from the 2022 Global Cancer Observatory (GLOBOCAN), which provides national-level estimates for cancer cases and deaths.
- Study outcomes included the incidence, mortality, and prevalence of cancer among men in 2022, along with projections for 2050. Estimates were stratified by several factors, including age; region; and Human Development Index (HDI), a composite score for health, education, and standard of living.
- Researchers also calculated mortality-to-incidence ratios (MIRs) for various cancer types, where higher values indicate worse survival.
TAKEAWAY:
- The researchers reported an estimated 10.3 million cancer cases and 5.4 million deaths globally in 2022, with almost two thirds of cases and deaths occurring in men aged 65 years or older.
- By 2050, cancer cases and deaths were projected to increase by 84.3% (to 19 million) and 93.2% (to 10.5 million), respectively. The increase from 2022 to 2050 was more than twofold higher for older men and countries with low and medium HDI.
- In 2022, the estimated global cancer MIR among men was nearly 55%, with variations by cancer types, age, and HDI. The MIR was lowest for thyroid cancer (7.6%) and highest for pancreatic cancer (90.9%); among World Health Organization regions, Africa had the highest MIR (72.6%), while the Americas had the lowest MIR (39.1%); countries with the lowest HDI had the highest MIR (73.5% vs 41.1% for very high HDI).
- Lung cancer was the leading cause for cases and deaths in 2022 and was projected to remain the leading cause in 2050.
IN PRACTICE:
“Disparities in cancer incidence and mortality among men were observed across age groups, countries/territories, and HDI in 2022, with these disparities projected to widen further by 2050,” according to the authors, who called for efforts to “reduce disparities in cancer burden and ensure equity in cancer prevention and care for men across the globe.”
SOURCE:
The study, led by Habtamu Mellie Bizuayehu, PhD, School of Public Health, Faculty of Medicine, The University of Queensland, Brisbane, Australia, was published online in Cancer.
LIMITATIONS:
The findings may be influenced by the quality of GLOBOCAN data. Interpretation should be cautious as MIR may not fully reflect cancer outcome inequalities. The study did not include other measures of cancer burden, such as years of life lost or years lived with disability, which were unavailable from the data source.
DISCLOSURES:
The authors did not disclose any funding information. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Cancer Treatment 101: A Primer for Non-Oncologists
The remaining 700,000 or so often proceed to chemotherapy either immediately or upon cancer recurrence, spread, or newly recognized metastases. “Cures” after that point are rare.
I’m speaking in generalities, understanding that each cancer and each patient is unique.
Chemotherapy
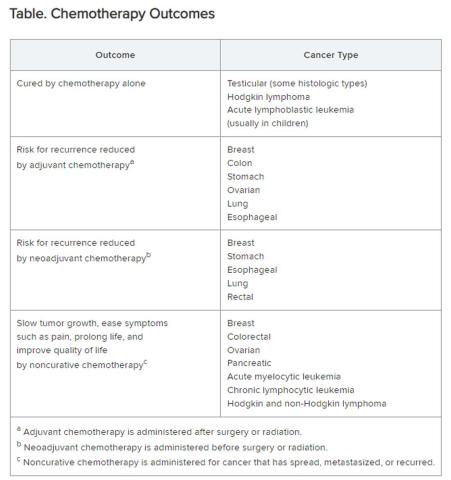
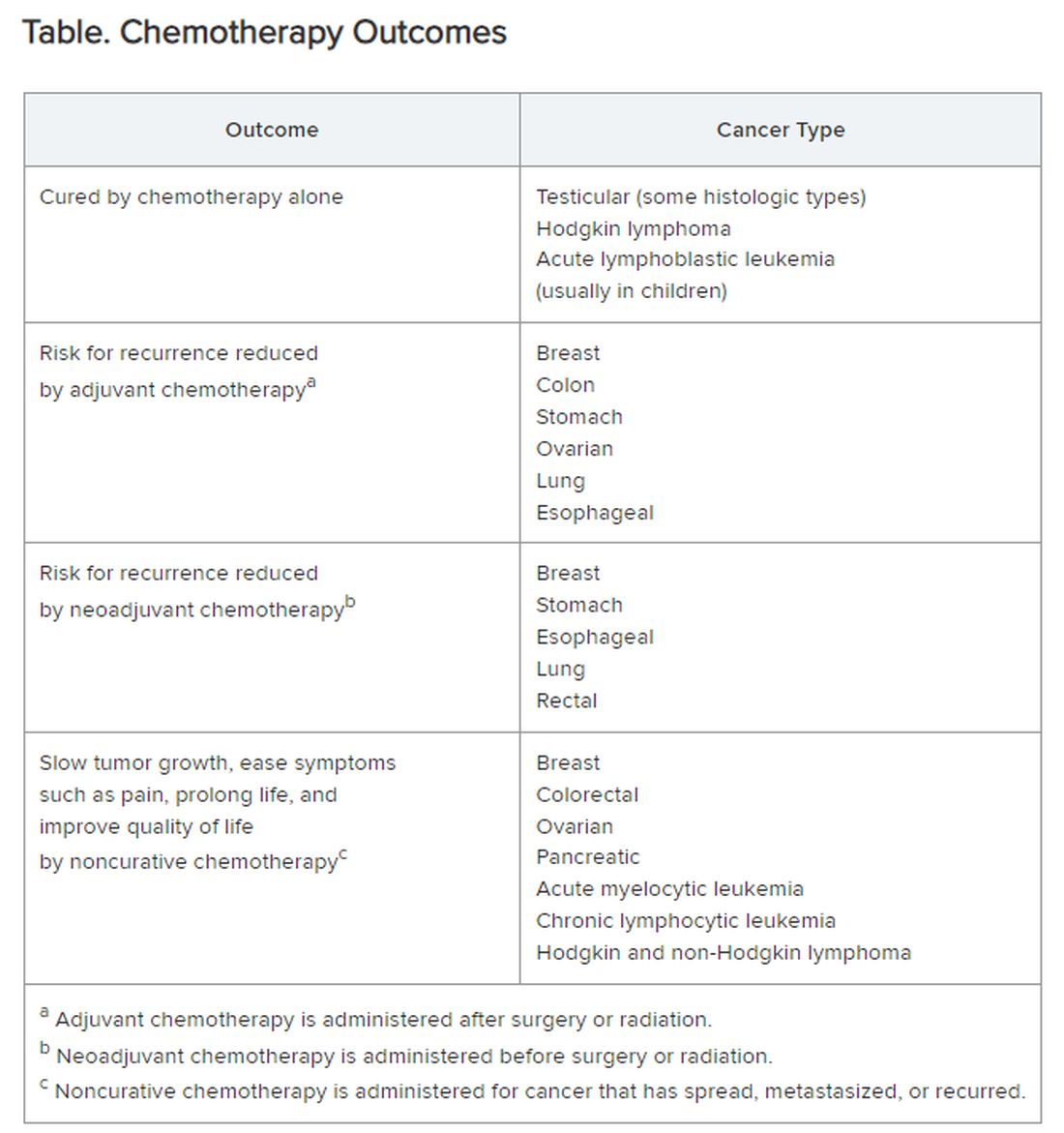
Chemotherapy alone can cure a small number of cancer types. When added to radiation or surgery, chemotherapy can help to cure a wider range of cancer types. As an add-on, chemotherapy can extend the length and quality of life for many patients with cancer. Since chemotherapy is by definition “toxic,” it can also shorten the duration or harm the quality of life and provide false hope. The Table summarizes what chemotherapy can and cannot achieve in selected cancer types.
Careful, compassionate communication between patient and physician is key. Goals and expectations must be clearly understood.
Organized chemotherapeutic efforts are further categorized as first line, second line, and third line.
First-line treatment. The initial round of recommended chemotherapy for a specific cancer. It is typically considered the most effective treatment for that type and stage of cancer on the basis of current research and clinical trials.
Second-line treatment. This is the treatment used if the first-line chemotherapy doesn’t work as desired. Reasons to switch to second-line chemo include:
- Lack of response (the tumor failed to shrink).
- Progression (the cancer may have grown or spread further).
- Adverse side effects were too severe to continue.
The drugs used in second-line chemo will typically be different from those used in first line, sometimes because cancer cells can develop resistance to chemotherapy drugs over time. Moreover, the goal of second-line chemo may differ from that of first-line therapy. Rather than chiefly aiming for a cure, second-line treatment might focus on slowing cancer growth, managing symptoms, or improving quality of life. Unfortunately, not every type of cancer has a readily available second-line option.
Third-line treatment. Third-line options come into play when both the initial course of chemo (first line) and the subsequent treatment (second line) have failed to achieve remission or control the cancer’s spread. Owing to the progressive nature of advanced cancers, patients might not be eligible or healthy enough for third-line therapy. Depending on cancer type, the patient’s general health, and response to previous treatments, third-line options could include:
- New or different chemotherapy drugs compared with prior lines.
- Surgery to debulk the tumor.
- Radiation for symptom control.
- Targeted therapy: drugs designed to target specific vulnerabilities in cancer cells.
- Immunotherapy: agents that help the body’s immune system fight cancer cells.
- Clinical trials testing new or investigational treatments, which may be applicable at any time, depending on the questions being addressed.
The goals of third-line therapy may shift from aiming for a cure to managing symptoms, improving quality of life, and potentially slowing cancer growth. The decision to pursue third-line therapy involves careful consideration by the doctor and patient, weighing the potential benefits and risks of treatment considering the individual’s overall health and specific situation.
It’s important to have realistic expectations about the potential outcomes of third-line therapy. Although remission may be unlikely, third-line therapy can still play a role in managing the disease.
Navigating advanced cancer treatment is very complex. The patient and physician must together consider detailed explanations and clarifications to set expectations and make informed decisions about care.
Interventions to Consider Earlier
In traditional clinical oncology practice, other interventions are possible, but these may not be offered until treatment has reached the third line:
- Molecular testing.
- Palliation.
- Clinical trials.
- Innovative testing to guide targeted therapy by ascertaining which agents are most likely (or not likely at all) to be effective.
I would argue that the patient’s interests are better served by considering and offering these other interventions much earlier, even before starting first-line chemotherapy.
Molecular testing. The best time for molecular testing of a new malignant tumor is typically at the time of diagnosis. Here’s why:
- Molecular testing helps identify specific genetic mutations in the cancer cells. This information can be crucial for selecting targeted therapies that are most effective against those specific mutations. Early detection allows for the most treatment options. For example, for non–small cell lung cancer, early is best because treatment and outcomes may well be changed by test results.
- Knowing the tumor’s molecular makeup can help determine whether a patient qualifies for clinical trials of new drugs designed for specific mutations.
- Some molecular markers can offer information about the tumor’s aggressiveness and potential for metastasis so that prognosis can be informed.
Molecular testing can be a valuable tool throughout a cancer patient’s journey. With genetically diverse tumors, the initial biopsy might not capture the full picture. Molecular testing of circulating tumor DNA can be used to monitor a patient’s response to treatment and detect potential mutations that might arise during treatment resistance. Retesting after metastasis can provide additional information that can aid in treatment decisions.
Palliative care. The ideal time to discuss palliative care with a patient with cancer is early in the diagnosis and treatment process. Palliative care is not the same as hospice care; it isn’t just about end-of-life. Palliative care focuses on improving a patient’s quality of life throughout cancer treatment. Palliative care specialists can address a wide range of symptoms a patient might experience from cancer or its treatment, including pain, fatigue, nausea, and anxiety.
Early discussions allow for a more comprehensive care plan. Open communication about all treatment options, including palliative care, empowers patients to make informed decisions about their care goals and preferences.
Specific situations where discussing palliative care might be appropriate are:
- Soon after a cancer diagnosis.
- If the patient experiences significant side effects from cancer treatment.
- When considering different treatment options, palliative care can complement those treatments.
- In advanced stages of cancer, to focus on comfort and quality of life.
Clinical trials. Participation in a clinical trial to explore new or investigational treatments should always be considered.
In theory, clinical trials should be an option at any time in the patient’s course. But the organized clinical trial experience may not be available or appropriate. Then, the individual becomes a de facto “clinical trial with an n of 1.” Read this brief open-access blog post at Cancer Commons to learn more about that circumstance.
Innovative testing. The best choice of chemotherapeutic or targeted therapies is often unclear. The clinician is likely to follow published guidelines, often from the National Comprehensive Cancer Network.
These are evidence based and driven by consensus of experts. But guideline-recommended therapy is not always effective, and weeks or months can pass before this ineffectiveness becomes apparent. Thus, many researchers and companies are seeking methods of testing each patient’s specific cancer to determine in advance, or very quickly, whether a particular drug is likely to be effective.
Read more about these leading innovations:
SAGE Oncotest: Entering the Next Generation of Tailored Cancer Treatment
Alibrex: A New Blood Test to Reveal Whether a Cancer Treatment is Working
PARIS Test Uses Lab-Grown Mini-Tumors to Find a Patient’s Best Treatment
Using Live Cells from Patients to Find the Right Cancer Drug
Other innovative therapies under investigation could even be agnostic to cancer type:
Treating Pancreatic Cancer: Could Metabolism — Not Genomics — Be the Key?
High-Energy Blue Light Powers a Promising New Treatment to Destroy Cancer Cells
All-Clear Follow-Up: Hydrogen Peroxide Appears to Treat Oral and Skin Lesions
Cancer is a tough nut to crack. Many people and organizations are trying very hard. So much is being learned. Some approaches will be effective. We can all hope.
Dr. Lundberg, editor in chief, Cancer Commons, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The remaining 700,000 or so often proceed to chemotherapy either immediately or upon cancer recurrence, spread, or newly recognized metastases. “Cures” after that point are rare.
I’m speaking in generalities, understanding that each cancer and each patient is unique.
Chemotherapy
Chemotherapy alone can cure a small number of cancer types. When added to radiation or surgery, chemotherapy can help to cure a wider range of cancer types. As an add-on, chemotherapy can extend the length and quality of life for many patients with cancer. Since chemotherapy is by definition “toxic,” it can also shorten the duration or harm the quality of life and provide false hope. The Table summarizes what chemotherapy can and cannot achieve in selected cancer types.
Careful, compassionate communication between patient and physician is key. Goals and expectations must be clearly understood.
Organized chemotherapeutic efforts are further categorized as first line, second line, and third line.
First-line treatment. The initial round of recommended chemotherapy for a specific cancer. It is typically considered the most effective treatment for that type and stage of cancer on the basis of current research and clinical trials.
Second-line treatment. This is the treatment used if the first-line chemotherapy doesn’t work as desired. Reasons to switch to second-line chemo include:
- Lack of response (the tumor failed to shrink).
- Progression (the cancer may have grown or spread further).
- Adverse side effects were too severe to continue.
The drugs used in second-line chemo will typically be different from those used in first line, sometimes because cancer cells can develop resistance to chemotherapy drugs over time. Moreover, the goal of second-line chemo may differ from that of first-line therapy. Rather than chiefly aiming for a cure, second-line treatment might focus on slowing cancer growth, managing symptoms, or improving quality of life. Unfortunately, not every type of cancer has a readily available second-line option.
Third-line treatment. Third-line options come into play when both the initial course of chemo (first line) and the subsequent treatment (second line) have failed to achieve remission or control the cancer’s spread. Owing to the progressive nature of advanced cancers, patients might not be eligible or healthy enough for third-line therapy. Depending on cancer type, the patient’s general health, and response to previous treatments, third-line options could include:
- New or different chemotherapy drugs compared with prior lines.
- Surgery to debulk the tumor.
- Radiation for symptom control.
- Targeted therapy: drugs designed to target specific vulnerabilities in cancer cells.
- Immunotherapy: agents that help the body’s immune system fight cancer cells.
- Clinical trials testing new or investigational treatments, which may be applicable at any time, depending on the questions being addressed.
The goals of third-line therapy may shift from aiming for a cure to managing symptoms, improving quality of life, and potentially slowing cancer growth. The decision to pursue third-line therapy involves careful consideration by the doctor and patient, weighing the potential benefits and risks of treatment considering the individual’s overall health and specific situation.
It’s important to have realistic expectations about the potential outcomes of third-line therapy. Although remission may be unlikely, third-line therapy can still play a role in managing the disease.
Navigating advanced cancer treatment is very complex. The patient and physician must together consider detailed explanations and clarifications to set expectations and make informed decisions about care.
Interventions to Consider Earlier
In traditional clinical oncology practice, other interventions are possible, but these may not be offered until treatment has reached the third line:
- Molecular testing.
- Palliation.
- Clinical trials.
- Innovative testing to guide targeted therapy by ascertaining which agents are most likely (or not likely at all) to be effective.
I would argue that the patient’s interests are better served by considering and offering these other interventions much earlier, even before starting first-line chemotherapy.
Molecular testing. The best time for molecular testing of a new malignant tumor is typically at the time of diagnosis. Here’s why:
- Molecular testing helps identify specific genetic mutations in the cancer cells. This information can be crucial for selecting targeted therapies that are most effective against those specific mutations. Early detection allows for the most treatment options. For example, for non–small cell lung cancer, early is best because treatment and outcomes may well be changed by test results.
- Knowing the tumor’s molecular makeup can help determine whether a patient qualifies for clinical trials of new drugs designed for specific mutations.
- Some molecular markers can offer information about the tumor’s aggressiveness and potential for metastasis so that prognosis can be informed.
Molecular testing can be a valuable tool throughout a cancer patient’s journey. With genetically diverse tumors, the initial biopsy might not capture the full picture. Molecular testing of circulating tumor DNA can be used to monitor a patient’s response to treatment and detect potential mutations that might arise during treatment resistance. Retesting after metastasis can provide additional information that can aid in treatment decisions.
Palliative care. The ideal time to discuss palliative care with a patient with cancer is early in the diagnosis and treatment process. Palliative care is not the same as hospice care; it isn’t just about end-of-life. Palliative care focuses on improving a patient’s quality of life throughout cancer treatment. Palliative care specialists can address a wide range of symptoms a patient might experience from cancer or its treatment, including pain, fatigue, nausea, and anxiety.
Early discussions allow for a more comprehensive care plan. Open communication about all treatment options, including palliative care, empowers patients to make informed decisions about their care goals and preferences.
Specific situations where discussing palliative care might be appropriate are:
- Soon after a cancer diagnosis.
- If the patient experiences significant side effects from cancer treatment.
- When considering different treatment options, palliative care can complement those treatments.
- In advanced stages of cancer, to focus on comfort and quality of life.
Clinical trials. Participation in a clinical trial to explore new or investigational treatments should always be considered.
In theory, clinical trials should be an option at any time in the patient’s course. But the organized clinical trial experience may not be available or appropriate. Then, the individual becomes a de facto “clinical trial with an n of 1.” Read this brief open-access blog post at Cancer Commons to learn more about that circumstance.
Innovative testing. The best choice of chemotherapeutic or targeted therapies is often unclear. The clinician is likely to follow published guidelines, often from the National Comprehensive Cancer Network.
These are evidence based and driven by consensus of experts. But guideline-recommended therapy is not always effective, and weeks or months can pass before this ineffectiveness becomes apparent. Thus, many researchers and companies are seeking methods of testing each patient’s specific cancer to determine in advance, or very quickly, whether a particular drug is likely to be effective.
Read more about these leading innovations:
SAGE Oncotest: Entering the Next Generation of Tailored Cancer Treatment
Alibrex: A New Blood Test to Reveal Whether a Cancer Treatment is Working
PARIS Test Uses Lab-Grown Mini-Tumors to Find a Patient’s Best Treatment
Using Live Cells from Patients to Find the Right Cancer Drug
Other innovative therapies under investigation could even be agnostic to cancer type:
Treating Pancreatic Cancer: Could Metabolism — Not Genomics — Be the Key?
High-Energy Blue Light Powers a Promising New Treatment to Destroy Cancer Cells
All-Clear Follow-Up: Hydrogen Peroxide Appears to Treat Oral and Skin Lesions
Cancer is a tough nut to crack. Many people and organizations are trying very hard. So much is being learned. Some approaches will be effective. We can all hope.
Dr. Lundberg, editor in chief, Cancer Commons, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The remaining 700,000 or so often proceed to chemotherapy either immediately or upon cancer recurrence, spread, or newly recognized metastases. “Cures” after that point are rare.
I’m speaking in generalities, understanding that each cancer and each patient is unique.
Chemotherapy
Chemotherapy alone can cure a small number of cancer types. When added to radiation or surgery, chemotherapy can help to cure a wider range of cancer types. As an add-on, chemotherapy can extend the length and quality of life for many patients with cancer. Since chemotherapy is by definition “toxic,” it can also shorten the duration or harm the quality of life and provide false hope. The Table summarizes what chemotherapy can and cannot achieve in selected cancer types.
Careful, compassionate communication between patient and physician is key. Goals and expectations must be clearly understood.
Organized chemotherapeutic efforts are further categorized as first line, second line, and third line.
First-line treatment. The initial round of recommended chemotherapy for a specific cancer. It is typically considered the most effective treatment for that type and stage of cancer on the basis of current research and clinical trials.
Second-line treatment. This is the treatment used if the first-line chemotherapy doesn’t work as desired. Reasons to switch to second-line chemo include:
- Lack of response (the tumor failed to shrink).
- Progression (the cancer may have grown or spread further).
- Adverse side effects were too severe to continue.
The drugs used in second-line chemo will typically be different from those used in first line, sometimes because cancer cells can develop resistance to chemotherapy drugs over time. Moreover, the goal of second-line chemo may differ from that of first-line therapy. Rather than chiefly aiming for a cure, second-line treatment might focus on slowing cancer growth, managing symptoms, or improving quality of life. Unfortunately, not every type of cancer has a readily available second-line option.
Third-line treatment. Third-line options come into play when both the initial course of chemo (first line) and the subsequent treatment (second line) have failed to achieve remission or control the cancer’s spread. Owing to the progressive nature of advanced cancers, patients might not be eligible or healthy enough for third-line therapy. Depending on cancer type, the patient’s general health, and response to previous treatments, third-line options could include:
- New or different chemotherapy drugs compared with prior lines.
- Surgery to debulk the tumor.
- Radiation for symptom control.
- Targeted therapy: drugs designed to target specific vulnerabilities in cancer cells.
- Immunotherapy: agents that help the body’s immune system fight cancer cells.
- Clinical trials testing new or investigational treatments, which may be applicable at any time, depending on the questions being addressed.
The goals of third-line therapy may shift from aiming for a cure to managing symptoms, improving quality of life, and potentially slowing cancer growth. The decision to pursue third-line therapy involves careful consideration by the doctor and patient, weighing the potential benefits and risks of treatment considering the individual’s overall health and specific situation.
It’s important to have realistic expectations about the potential outcomes of third-line therapy. Although remission may be unlikely, third-line therapy can still play a role in managing the disease.
Navigating advanced cancer treatment is very complex. The patient and physician must together consider detailed explanations and clarifications to set expectations and make informed decisions about care.
Interventions to Consider Earlier
In traditional clinical oncology practice, other interventions are possible, but these may not be offered until treatment has reached the third line:
- Molecular testing.
- Palliation.
- Clinical trials.
- Innovative testing to guide targeted therapy by ascertaining which agents are most likely (or not likely at all) to be effective.
I would argue that the patient’s interests are better served by considering and offering these other interventions much earlier, even before starting first-line chemotherapy.
Molecular testing. The best time for molecular testing of a new malignant tumor is typically at the time of diagnosis. Here’s why:
- Molecular testing helps identify specific genetic mutations in the cancer cells. This information can be crucial for selecting targeted therapies that are most effective against those specific mutations. Early detection allows for the most treatment options. For example, for non–small cell lung cancer, early is best because treatment and outcomes may well be changed by test results.
- Knowing the tumor’s molecular makeup can help determine whether a patient qualifies for clinical trials of new drugs designed for specific mutations.
- Some molecular markers can offer information about the tumor’s aggressiveness and potential for metastasis so that prognosis can be informed.
Molecular testing can be a valuable tool throughout a cancer patient’s journey. With genetically diverse tumors, the initial biopsy might not capture the full picture. Molecular testing of circulating tumor DNA can be used to monitor a patient’s response to treatment and detect potential mutations that might arise during treatment resistance. Retesting after metastasis can provide additional information that can aid in treatment decisions.
Palliative care. The ideal time to discuss palliative care with a patient with cancer is early in the diagnosis and treatment process. Palliative care is not the same as hospice care; it isn’t just about end-of-life. Palliative care focuses on improving a patient’s quality of life throughout cancer treatment. Palliative care specialists can address a wide range of symptoms a patient might experience from cancer or its treatment, including pain, fatigue, nausea, and anxiety.
Early discussions allow for a more comprehensive care plan. Open communication about all treatment options, including palliative care, empowers patients to make informed decisions about their care goals and preferences.
Specific situations where discussing palliative care might be appropriate are:
- Soon after a cancer diagnosis.
- If the patient experiences significant side effects from cancer treatment.
- When considering different treatment options, palliative care can complement those treatments.
- In advanced stages of cancer, to focus on comfort and quality of life.
Clinical trials. Participation in a clinical trial to explore new or investigational treatments should always be considered.
In theory, clinical trials should be an option at any time in the patient’s course. But the organized clinical trial experience may not be available or appropriate. Then, the individual becomes a de facto “clinical trial with an n of 1.” Read this brief open-access blog post at Cancer Commons to learn more about that circumstance.
Innovative testing. The best choice of chemotherapeutic or targeted therapies is often unclear. The clinician is likely to follow published guidelines, often from the National Comprehensive Cancer Network.
These are evidence based and driven by consensus of experts. But guideline-recommended therapy is not always effective, and weeks or months can pass before this ineffectiveness becomes apparent. Thus, many researchers and companies are seeking methods of testing each patient’s specific cancer to determine in advance, or very quickly, whether a particular drug is likely to be effective.
Read more about these leading innovations:
SAGE Oncotest: Entering the Next Generation of Tailored Cancer Treatment
Alibrex: A New Blood Test to Reveal Whether a Cancer Treatment is Working
PARIS Test Uses Lab-Grown Mini-Tumors to Find a Patient’s Best Treatment
Using Live Cells from Patients to Find the Right Cancer Drug
Other innovative therapies under investigation could even be agnostic to cancer type:
Treating Pancreatic Cancer: Could Metabolism — Not Genomics — Be the Key?
High-Energy Blue Light Powers a Promising New Treatment to Destroy Cancer Cells
All-Clear Follow-Up: Hydrogen Peroxide Appears to Treat Oral and Skin Lesions
Cancer is a tough nut to crack. Many people and organizations are trying very hard. So much is being learned. Some approaches will be effective. We can all hope.
Dr. Lundberg, editor in chief, Cancer Commons, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Few Severe Toxicities After SBRT in Oligometastatic Cancer
TOPLINE:
according to a large real-world analysis.
METHODOLOGY:
- Advances in cancer imaging have helped identify more patients with oligometastatic disease. Although the standard treatment approach typically involves systemic therapy such as chemotherapy and immunotherapy, SBRT has increasingly become an option for these patients. However, the toxicities associated with SBRT remain less clear.
- OligoCare, a European, prospective, registry-based, single-arm observational study, aims to provide real-world outcomes among patients with oligometastatic cancer who received SBRT. In this analysis, the researchers evaluated early toxicities among 1468 patients with different primary cancers — non–small cell lung cancer (NSCLC; 19.7%), colorectal cancer (20%), breast cancer (15.5%), and prostate cancer (44.8%).
- The primary outcome was acute toxicities, including new malignancies and deaths, within 6 months of initiating SBRT.
- Overall, 527 (35.9%) patients received concomitant systemic treatment and 828 (56%) had de novo oligometastatic disease.
TAKEAWAY:
- Overall, though, only eight patients (0.5%) experienced acute SBRT-related toxicity of grade 3 and above within 6 months; two events, however, were fatal (pneumonitis and cerebral hemorrhage), and both occurred in patients with NSCLC.
- The other six grade 3 events included one instance of each of the following: empyema, pneumonia, radiation pneumonitis, radiation skin injury, decreased appetite, and bone pain. Two of these events occurred in patients with NSCLC, two in patients with breast cancer, one in patients with colorectal cancer, and one in patients with prostate cancer.
- New primary malignancies were reported in 13 (0.9%) patients, which included bladder cancer (n = 3), nonmelanoma skin cancer (n = 3), and leukemia (n = 1).
- Overall, 43 (2.9%) patients died within 6 months, most from their primary cancer (58.1%).
IN PRACTICE:
Low rates of early acute toxicities reported in this real-world study help confirm the safety of SBRT in the treatment of oligometastases, the authors concluded. However, “some anatomical sites might be associated with an increased risk of even severe or fatal toxicities.”
SOURCE:
The study, led by Filippo Alongi, Advanced Radiation Oncology Department, IRCCS Sacro Cuore Don Calabria Hospital, Cancer Care Center, Negrar di Valpolicella, Italy, and University of Brescia, also in Italy, was published online in Radiotherapy & Oncology .
LIMITATIONS:
Some limitations of the study include the nonrandomized design and potential variability in patient selection criteria, treatment doses, and schedules.
DISCLOSURES:
The study did not receive any funding support. Two authors declared receiving speaker or lecture honoraria or consultation fees from various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
according to a large real-world analysis.
METHODOLOGY:
- Advances in cancer imaging have helped identify more patients with oligometastatic disease. Although the standard treatment approach typically involves systemic therapy such as chemotherapy and immunotherapy, SBRT has increasingly become an option for these patients. However, the toxicities associated with SBRT remain less clear.
- OligoCare, a European, prospective, registry-based, single-arm observational study, aims to provide real-world outcomes among patients with oligometastatic cancer who received SBRT. In this analysis, the researchers evaluated early toxicities among 1468 patients with different primary cancers — non–small cell lung cancer (NSCLC; 19.7%), colorectal cancer (20%), breast cancer (15.5%), and prostate cancer (44.8%).
- The primary outcome was acute toxicities, including new malignancies and deaths, within 6 months of initiating SBRT.
- Overall, 527 (35.9%) patients received concomitant systemic treatment and 828 (56%) had de novo oligometastatic disease.
TAKEAWAY:
- Overall, though, only eight patients (0.5%) experienced acute SBRT-related toxicity of grade 3 and above within 6 months; two events, however, were fatal (pneumonitis and cerebral hemorrhage), and both occurred in patients with NSCLC.
- The other six grade 3 events included one instance of each of the following: empyema, pneumonia, radiation pneumonitis, radiation skin injury, decreased appetite, and bone pain. Two of these events occurred in patients with NSCLC, two in patients with breast cancer, one in patients with colorectal cancer, and one in patients with prostate cancer.
- New primary malignancies were reported in 13 (0.9%) patients, which included bladder cancer (n = 3), nonmelanoma skin cancer (n = 3), and leukemia (n = 1).
- Overall, 43 (2.9%) patients died within 6 months, most from their primary cancer (58.1%).
IN PRACTICE:
Low rates of early acute toxicities reported in this real-world study help confirm the safety of SBRT in the treatment of oligometastases, the authors concluded. However, “some anatomical sites might be associated with an increased risk of even severe or fatal toxicities.”
SOURCE:
The study, led by Filippo Alongi, Advanced Radiation Oncology Department, IRCCS Sacro Cuore Don Calabria Hospital, Cancer Care Center, Negrar di Valpolicella, Italy, and University of Brescia, also in Italy, was published online in Radiotherapy & Oncology .
LIMITATIONS:
Some limitations of the study include the nonrandomized design and potential variability in patient selection criteria, treatment doses, and schedules.
DISCLOSURES:
The study did not receive any funding support. Two authors declared receiving speaker or lecture honoraria or consultation fees from various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
according to a large real-world analysis.
METHODOLOGY:
- Advances in cancer imaging have helped identify more patients with oligometastatic disease. Although the standard treatment approach typically involves systemic therapy such as chemotherapy and immunotherapy, SBRT has increasingly become an option for these patients. However, the toxicities associated with SBRT remain less clear.
- OligoCare, a European, prospective, registry-based, single-arm observational study, aims to provide real-world outcomes among patients with oligometastatic cancer who received SBRT. In this analysis, the researchers evaluated early toxicities among 1468 patients with different primary cancers — non–small cell lung cancer (NSCLC; 19.7%), colorectal cancer (20%), breast cancer (15.5%), and prostate cancer (44.8%).
- The primary outcome was acute toxicities, including new malignancies and deaths, within 6 months of initiating SBRT.
- Overall, 527 (35.9%) patients received concomitant systemic treatment and 828 (56%) had de novo oligometastatic disease.
TAKEAWAY:
- Overall, though, only eight patients (0.5%) experienced acute SBRT-related toxicity of grade 3 and above within 6 months; two events, however, were fatal (pneumonitis and cerebral hemorrhage), and both occurred in patients with NSCLC.
- The other six grade 3 events included one instance of each of the following: empyema, pneumonia, radiation pneumonitis, radiation skin injury, decreased appetite, and bone pain. Two of these events occurred in patients with NSCLC, two in patients with breast cancer, one in patients with colorectal cancer, and one in patients with prostate cancer.
- New primary malignancies were reported in 13 (0.9%) patients, which included bladder cancer (n = 3), nonmelanoma skin cancer (n = 3), and leukemia (n = 1).
- Overall, 43 (2.9%) patients died within 6 months, most from their primary cancer (58.1%).
IN PRACTICE:
Low rates of early acute toxicities reported in this real-world study help confirm the safety of SBRT in the treatment of oligometastases, the authors concluded. However, “some anatomical sites might be associated with an increased risk of even severe or fatal toxicities.”
SOURCE:
The study, led by Filippo Alongi, Advanced Radiation Oncology Department, IRCCS Sacro Cuore Don Calabria Hospital, Cancer Care Center, Negrar di Valpolicella, Italy, and University of Brescia, also in Italy, was published online in Radiotherapy & Oncology .
LIMITATIONS:
Some limitations of the study include the nonrandomized design and potential variability in patient selection criteria, treatment doses, and schedules.
DISCLOSURES:
The study did not receive any funding support. Two authors declared receiving speaker or lecture honoraria or consultation fees from various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Breast Cancer Index Predicts Benefit of Ovarian Function Suppression in Premenopausal Women
TOPLINE:
Women with BCI HOXB13/IL17BR ratio (BCI[H/I])–low tumors showed significant benefit from OFS, whereas those with BCI(H/I)-high tumors did not.
METHODOLOGY:
- Researchers conducted a prospective-retrospective translational study using tumor tissue samples from 1,718 premenopausal women with hormone receptor–positive early-stage breast cancer.
- Participants were randomly assigned to receive 5 years of tamoxifen alone, tamoxifen plus OFS, or exemestane plus OFS.
- BCI testing was performed on RNA extracted from formalin-fixed paraffin-embedded tumor specimens, blinded to clinical data and outcomes.
- The primary endpoints were breast cancer–free interval (BCFI) and distant recurrence-free interval (DRFI), with a median follow-up time of 12 years.
- Settings spanned multiple centers internationally, and data were collected from December 2003 to April 2021, analyzed from May 2022 to October 2022.
TAKEAWAY:
- According to the authors, patients with BCI(H/I)-low tumors exhibited a 12-year absolute benefit in BCFI of 11.6% from exemestane plus OFS (hazard ratio [HR], 0.48; 95% CI, 0.33-0.71) and 7.3% from tamoxifen plus OFS (HR, 0.69; 95% CI, 0.48-0.97), relative to tamoxifen alone.
- Patients with BCI(H/I)-high tumors did not derive significant benefit from either exemestane plus OFS (absolute benefit, -0.4%; HR, 1.03; 95% CI, 0.70-1.53) or tamoxifen plus OFS (absolute benefit, -1.2%; HR, 1.05; 95% CI, 0.72-1.54), compared with tamoxifen alone.
- In the ERBB2-negative subgroup, patients with BCI(H/I)-low tumors experienced a 12-year absolute benefit of 13.2% in BCFI from exemestane plus OFS (HR, 0.39; 95% CI, 0.25-0.60) and 7.4% from tamoxifen plus OFS (HR, 0.64; 95% CI, 0.44-0.93), compared with tamoxifen alone.
- BCI continuous index was significantly prognostic in the subgroup for DRFI (n = 1110; P =.004), with 12-year DRFI of 95.9%, 90.8%, and 86.3% in BCI low-risk, intermediate-risk, and high-risk cases of cancer than had not spread to nearly lymph nodes (N0 cancers), respectively.
IN PRACTICE:
“This investigation suggests a potential clinical use of BCI(H/I) results, adding to their use to identify patients most likely to benefit from extended endocrine therapy, as proven in multiple studies, although in the extended endocrine validation studies, it was the BCI(H/I)-high group that derived the greatest benefit,” wrote the authors of the study.
SOURCE:
The study was led by Ruth M. O’Regan, MD, University of Rochester Department of Medicine in Rochester, New York. It was published online on August 15, in JAMA Oncology.
LIMITATIONS:
The study’s retrospective nature may introduce biases despite the prospective statistical analysis plan. The sample size for certain clinical subgroups might be too small to definitively confirm the predictive value of BCI(H/I) for OFS benefit. The generalizability of the findings may be limited due to the specific population studied. Further validation in other patient cohorts is necessary to confirm these findings.
DISCLOSURES:
Dr. O’Regan disclosed receiving personal fees from Pfizer and Gilead DSMB, grants from Puma, and nonfinancial support from Novartis. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Women with BCI HOXB13/IL17BR ratio (BCI[H/I])–low tumors showed significant benefit from OFS, whereas those with BCI(H/I)-high tumors did not.
METHODOLOGY:
- Researchers conducted a prospective-retrospective translational study using tumor tissue samples from 1,718 premenopausal women with hormone receptor–positive early-stage breast cancer.
- Participants were randomly assigned to receive 5 years of tamoxifen alone, tamoxifen plus OFS, or exemestane plus OFS.
- BCI testing was performed on RNA extracted from formalin-fixed paraffin-embedded tumor specimens, blinded to clinical data and outcomes.
- The primary endpoints were breast cancer–free interval (BCFI) and distant recurrence-free interval (DRFI), with a median follow-up time of 12 years.
- Settings spanned multiple centers internationally, and data were collected from December 2003 to April 2021, analyzed from May 2022 to October 2022.
TAKEAWAY:
- According to the authors, patients with BCI(H/I)-low tumors exhibited a 12-year absolute benefit in BCFI of 11.6% from exemestane plus OFS (hazard ratio [HR], 0.48; 95% CI, 0.33-0.71) and 7.3% from tamoxifen plus OFS (HR, 0.69; 95% CI, 0.48-0.97), relative to tamoxifen alone.
- Patients with BCI(H/I)-high tumors did not derive significant benefit from either exemestane plus OFS (absolute benefit, -0.4%; HR, 1.03; 95% CI, 0.70-1.53) or tamoxifen plus OFS (absolute benefit, -1.2%; HR, 1.05; 95% CI, 0.72-1.54), compared with tamoxifen alone.
- In the ERBB2-negative subgroup, patients with BCI(H/I)-low tumors experienced a 12-year absolute benefit of 13.2% in BCFI from exemestane plus OFS (HR, 0.39; 95% CI, 0.25-0.60) and 7.4% from tamoxifen plus OFS (HR, 0.64; 95% CI, 0.44-0.93), compared with tamoxifen alone.
- BCI continuous index was significantly prognostic in the subgroup for DRFI (n = 1110; P =.004), with 12-year DRFI of 95.9%, 90.8%, and 86.3% in BCI low-risk, intermediate-risk, and high-risk cases of cancer than had not spread to nearly lymph nodes (N0 cancers), respectively.
IN PRACTICE:
“This investigation suggests a potential clinical use of BCI(H/I) results, adding to their use to identify patients most likely to benefit from extended endocrine therapy, as proven in multiple studies, although in the extended endocrine validation studies, it was the BCI(H/I)-high group that derived the greatest benefit,” wrote the authors of the study.
SOURCE:
The study was led by Ruth M. O’Regan, MD, University of Rochester Department of Medicine in Rochester, New York. It was published online on August 15, in JAMA Oncology.
LIMITATIONS:
The study’s retrospective nature may introduce biases despite the prospective statistical analysis plan. The sample size for certain clinical subgroups might be too small to definitively confirm the predictive value of BCI(H/I) for OFS benefit. The generalizability of the findings may be limited due to the specific population studied. Further validation in other patient cohorts is necessary to confirm these findings.
DISCLOSURES:
Dr. O’Regan disclosed receiving personal fees from Pfizer and Gilead DSMB, grants from Puma, and nonfinancial support from Novartis. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Women with BCI HOXB13/IL17BR ratio (BCI[H/I])–low tumors showed significant benefit from OFS, whereas those with BCI(H/I)-high tumors did not.
METHODOLOGY:
- Researchers conducted a prospective-retrospective translational study using tumor tissue samples from 1,718 premenopausal women with hormone receptor–positive early-stage breast cancer.
- Participants were randomly assigned to receive 5 years of tamoxifen alone, tamoxifen plus OFS, or exemestane plus OFS.
- BCI testing was performed on RNA extracted from formalin-fixed paraffin-embedded tumor specimens, blinded to clinical data and outcomes.
- The primary endpoints were breast cancer–free interval (BCFI) and distant recurrence-free interval (DRFI), with a median follow-up time of 12 years.
- Settings spanned multiple centers internationally, and data were collected from December 2003 to April 2021, analyzed from May 2022 to October 2022.
TAKEAWAY:
- According to the authors, patients with BCI(H/I)-low tumors exhibited a 12-year absolute benefit in BCFI of 11.6% from exemestane plus OFS (hazard ratio [HR], 0.48; 95% CI, 0.33-0.71) and 7.3% from tamoxifen plus OFS (HR, 0.69; 95% CI, 0.48-0.97), relative to tamoxifen alone.
- Patients with BCI(H/I)-high tumors did not derive significant benefit from either exemestane plus OFS (absolute benefit, -0.4%; HR, 1.03; 95% CI, 0.70-1.53) or tamoxifen plus OFS (absolute benefit, -1.2%; HR, 1.05; 95% CI, 0.72-1.54), compared with tamoxifen alone.
- In the ERBB2-negative subgroup, patients with BCI(H/I)-low tumors experienced a 12-year absolute benefit of 13.2% in BCFI from exemestane plus OFS (HR, 0.39; 95% CI, 0.25-0.60) and 7.4% from tamoxifen plus OFS (HR, 0.64; 95% CI, 0.44-0.93), compared with tamoxifen alone.
- BCI continuous index was significantly prognostic in the subgroup for DRFI (n = 1110; P =.004), with 12-year DRFI of 95.9%, 90.8%, and 86.3% in BCI low-risk, intermediate-risk, and high-risk cases of cancer than had not spread to nearly lymph nodes (N0 cancers), respectively.
IN PRACTICE:
“This investigation suggests a potential clinical use of BCI(H/I) results, adding to their use to identify patients most likely to benefit from extended endocrine therapy, as proven in multiple studies, although in the extended endocrine validation studies, it was the BCI(H/I)-high group that derived the greatest benefit,” wrote the authors of the study.
SOURCE:
The study was led by Ruth M. O’Regan, MD, University of Rochester Department of Medicine in Rochester, New York. It was published online on August 15, in JAMA Oncology.
LIMITATIONS:
The study’s retrospective nature may introduce biases despite the prospective statistical analysis plan. The sample size for certain clinical subgroups might be too small to definitively confirm the predictive value of BCI(H/I) for OFS benefit. The generalizability of the findings may be limited due to the specific population studied. Further validation in other patient cohorts is necessary to confirm these findings.
DISCLOSURES:
Dr. O’Regan disclosed receiving personal fees from Pfizer and Gilead DSMB, grants from Puma, and nonfinancial support from Novartis. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Radiation Therapy Underused After Nipple-Sparing Mastectomy
TOPLINE:
METHODOLOGY:
- Nipple-sparing mastectomy has become increasingly popular for treating early-stage breast cancer given the cosmetic and functional benefits of the procedure. However, appropriate use of adjuvant radiation therapy following nipple-sparing mastectomy has not been characterized.
- Researchers compared outcomes and appropriate uses of radiation therapy among 624,075 women diagnosed with cT1-3N0M0 invasive ductal or lobular breast cancer between 2004 and 2017 who underwent breast-conserving surgery (n = 611,907; median age, 63 years) or nipple-sparing mastectomy (n = 12,168; median age, 50 years).
- The researchers compared the rates of postoperative radiation therapy for two standard indications — positive margins and pathologic node involvement — in patients who had breast-conserving surgery or nipple-sparing mastectomy.
- The team also compared overall survival outcomes in patients with positive margins and node involvement.
TAKEAWAY:
- Patients who had nipple-sparing surgery had higher rates of positive margins (4.5% vs 3.7%; P < .001) and, on multivariable analysis, a 15% higher risk for positive margins compared with those who had breast-conserving surgery (odds ratio [OR], 1.15; P = .005).
- Similarly, patients who had nipple-sparing surgery had significantly higher rates of node involvement compared with those who had breast-conserving surgery (22.5% vs 13.5%) and, on multivariable analysis, an 8% higher risk for node involvement (OR, 1.08; P < .001).
- Despite higher rates of positive margins and node involvement in the nipple-sparing surgery group, these patients were significantly less likely than those in the breast-conserving surgery group to receive adjuvant radiation therapy (OR, 0.07). Overall, only 17.2% of patients who underwent nipple-sparing mastectomy received postoperative radiation therapy compared with 83.3% of those undergoing breast-conserving surgery — an almost fivefold difference (P < .001).
- In the overall study sample, overall survival in the two surgical groups did not differ significantly among patients with positive margins (OR, 0.62; 95% CI, 0.30-1.31; P = .21) and those with node involvement (OR, 1.01; 95% CI, 0.80-1.28; P = .93).
IN PRACTICE:
The researchers emphasized that although overall survival outcomes were comparable in the two surgery groups, the “current standard indications and guidelines for post-mastectomy radiation are not being appropriately” used after nipple-sparing mastectomy.
SOURCE:
The study, led by Wesley J. Talcott, MD, MBA, Department of Radiation Medicine, Northwell Health, New York City, was published online in Advances in Radiation Oncology.
LIMITATIONS:
Data on locoregional recurrence, cause-specific mortality, and all pathologic details were not available. The relatively short median follow-up period might not capture differences in the long-term survival outcomes.
DISCLOSURES:
The study did not receive any funding support. The authors disclosed no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Nipple-sparing mastectomy has become increasingly popular for treating early-stage breast cancer given the cosmetic and functional benefits of the procedure. However, appropriate use of adjuvant radiation therapy following nipple-sparing mastectomy has not been characterized.
- Researchers compared outcomes and appropriate uses of radiation therapy among 624,075 women diagnosed with cT1-3N0M0 invasive ductal or lobular breast cancer between 2004 and 2017 who underwent breast-conserving surgery (n = 611,907; median age, 63 years) or nipple-sparing mastectomy (n = 12,168; median age, 50 years).
- The researchers compared the rates of postoperative radiation therapy for two standard indications — positive margins and pathologic node involvement — in patients who had breast-conserving surgery or nipple-sparing mastectomy.
- The team also compared overall survival outcomes in patients with positive margins and node involvement.
TAKEAWAY:
- Patients who had nipple-sparing surgery had higher rates of positive margins (4.5% vs 3.7%; P < .001) and, on multivariable analysis, a 15% higher risk for positive margins compared with those who had breast-conserving surgery (odds ratio [OR], 1.15; P = .005).
- Similarly, patients who had nipple-sparing surgery had significantly higher rates of node involvement compared with those who had breast-conserving surgery (22.5% vs 13.5%) and, on multivariable analysis, an 8% higher risk for node involvement (OR, 1.08; P < .001).
- Despite higher rates of positive margins and node involvement in the nipple-sparing surgery group, these patients were significantly less likely than those in the breast-conserving surgery group to receive adjuvant radiation therapy (OR, 0.07). Overall, only 17.2% of patients who underwent nipple-sparing mastectomy received postoperative radiation therapy compared with 83.3% of those undergoing breast-conserving surgery — an almost fivefold difference (P < .001).
- In the overall study sample, overall survival in the two surgical groups did not differ significantly among patients with positive margins (OR, 0.62; 95% CI, 0.30-1.31; P = .21) and those with node involvement (OR, 1.01; 95% CI, 0.80-1.28; P = .93).
IN PRACTICE:
The researchers emphasized that although overall survival outcomes were comparable in the two surgery groups, the “current standard indications and guidelines for post-mastectomy radiation are not being appropriately” used after nipple-sparing mastectomy.
SOURCE:
The study, led by Wesley J. Talcott, MD, MBA, Department of Radiation Medicine, Northwell Health, New York City, was published online in Advances in Radiation Oncology.
LIMITATIONS:
Data on locoregional recurrence, cause-specific mortality, and all pathologic details were not available. The relatively short median follow-up period might not capture differences in the long-term survival outcomes.
DISCLOSURES:
The study did not receive any funding support. The authors disclosed no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Nipple-sparing mastectomy has become increasingly popular for treating early-stage breast cancer given the cosmetic and functional benefits of the procedure. However, appropriate use of adjuvant radiation therapy following nipple-sparing mastectomy has not been characterized.
- Researchers compared outcomes and appropriate uses of radiation therapy among 624,075 women diagnosed with cT1-3N0M0 invasive ductal or lobular breast cancer between 2004 and 2017 who underwent breast-conserving surgery (n = 611,907; median age, 63 years) or nipple-sparing mastectomy (n = 12,168; median age, 50 years).
- The researchers compared the rates of postoperative radiation therapy for two standard indications — positive margins and pathologic node involvement — in patients who had breast-conserving surgery or nipple-sparing mastectomy.
- The team also compared overall survival outcomes in patients with positive margins and node involvement.
TAKEAWAY:
- Patients who had nipple-sparing surgery had higher rates of positive margins (4.5% vs 3.7%; P < .001) and, on multivariable analysis, a 15% higher risk for positive margins compared with those who had breast-conserving surgery (odds ratio [OR], 1.15; P = .005).
- Similarly, patients who had nipple-sparing surgery had significantly higher rates of node involvement compared with those who had breast-conserving surgery (22.5% vs 13.5%) and, on multivariable analysis, an 8% higher risk for node involvement (OR, 1.08; P < .001).
- Despite higher rates of positive margins and node involvement in the nipple-sparing surgery group, these patients were significantly less likely than those in the breast-conserving surgery group to receive adjuvant radiation therapy (OR, 0.07). Overall, only 17.2% of patients who underwent nipple-sparing mastectomy received postoperative radiation therapy compared with 83.3% of those undergoing breast-conserving surgery — an almost fivefold difference (P < .001).
- In the overall study sample, overall survival in the two surgical groups did not differ significantly among patients with positive margins (OR, 0.62; 95% CI, 0.30-1.31; P = .21) and those with node involvement (OR, 1.01; 95% CI, 0.80-1.28; P = .93).
IN PRACTICE:
The researchers emphasized that although overall survival outcomes were comparable in the two surgery groups, the “current standard indications and guidelines for post-mastectomy radiation are not being appropriately” used after nipple-sparing mastectomy.
SOURCE:
The study, led by Wesley J. Talcott, MD, MBA, Department of Radiation Medicine, Northwell Health, New York City, was published online in Advances in Radiation Oncology.
LIMITATIONS:
Data on locoregional recurrence, cause-specific mortality, and all pathologic details were not available. The relatively short median follow-up period might not capture differences in the long-term survival outcomes.
DISCLOSURES:
The study did not receive any funding support. The authors disclosed no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
BRCA Mutations in Men: Important but Often Overlooked
BRCA1 and BRCA2 pathogenic variants carry well-known associations with breast and ovarian cancers in women, which has led to robust clinical guidelines for early genetic testing and risk-reduction strategies.
Male carriers of BRCA1/2 pathogenic variants also face an increased risk for cancer, particularly of the prostate, pancreas, and breast.
However, men often fly under the radar.
“Most people (including their clinicians) are unaware of their carrier status,” Heather Cheng, MD, PhD, with University of Washington, Seattle, and colleagues explained in a comprehensive review on the subject, published in JAMA Oncology. Most are also unaware of “the associated cancer risks, and management recommendations” for BRCA carriers.
The testing gap in males may exist, in part, because of a “general lack of awareness” that BRCA gene mutations can be passed down to children from both the mother and father, Elisa Port, MD, chief of breast surgery for the Mount Sinai Health System in New York City, told this news organization.
A daughter can inherit a mutated BRCA gene that puts her at risk for breast or ovarian cancer from her mother’s or father’s family and, similarly, a son can inherit a mutated BRCA gene from either side of the family that puts him at an increased risk for developing prostate and other cancers, explained Dr. Port, director of the Center of Excellence for Breast Cancer at The Tisch Cancer Institute at Mount Sinai.
Considering family history and genetics on both sides of the family is important when assessing cancer risk in men and women, Dr. Port said.
BRCA Mutations in Men: What’s the Risk?
Although fewer than 1% of all breast cancers occur in men, when men do carry a BRCA mutation, their risk for breast cancer can increase considerably. The lifetime risk for breast cancer can be as high as 9% in male BRCA2 carriers and up to 1.2% in BRCA1 carriers.
BRCA1/2 mutations also put men at increased risk for pancreatic and prostate cancers.
For pancreatic cancer, male BRCA1 carriers have a nearly twofold increased risk compared with the general population, with a lifetime risk of 3%. BRCA2 carriers have a three- to nearly eightfold increased risk, with a lifetime risk up to 7%.
Male BRCA1 carriers face a nearly fourfold increased risk of developing prostate cancer and an absolute lifetime risk of 15%-45%. Male BRCA2 carriers have a five- to ninefold increased risk for prostate cancer, with an absolute lifetime risk between 27% and 60%.
When to Test, When to Screen?
Despite the increased risk for several cancers associated with BRCA mutations, many men are not offered genetic testing.
BRCA1/2 genetic testing in men is “ultra-important but underutilized and is an evolving unmet need that the field needs to address,” Kai Tsao, MD MS, medical director of the Medical Oncology Prostate Cancer Program at Mount Sinai in New York City, told this news organization.
For men considering genetic testing, in Dr. Tsao’s experience, barriers may include fear that insurance may not cover the test and that a positive test may increase insurance premiums, as well as concerns about what the test result may mean for them and their family.
Even for confirmed BRCA carriers, cancer screening guidelines for men vary.
For breast screening in men, there’s limited data to inform guidelines. The National Cancer Center Network currently recommends breast awareness and teaching self-examination starting at age 35 and recommends men with BRCA variants consider yearly mammograms starting at age 50, or 10 years before the earliest male breast cancer diagnosis in the family.
Data show that screening mammography in men at high-risk for breast cancer yields similar cancer detection rates in men and women, “suggesting mammography screening may be valuable in male BRCA carriers,” the review authors noted. And, in a recent study of men with BRCA1/2 pathogenic variants, most (71%) recommended for screening mammography completed their screening.
The European Society for Medical Oncology (ESMO) has similar screening recommendations but focuses only on men with BRCA2 mutations and suggests breast ultrasonography as well as mammography as a screening option.
The larger “issue is the general population doesn’t think of breast cancer when they think of men, which may delay seeking medical attention,” said Melissa Fana, MD, of NYU Grossman Long Island School of Medicine and NYU Langone Health, who wasn’t involved in the review.
For pancreatic cancer, guidelines suggest BRCA1/2 carriers be screened for pancreatic cancer starting at age 50, or 10 years before the earliest known pancreatic cancer in the family, although the guidelines vary on the role family history should play.
And for prostate cancer, current guidelines recommend male BRCA carriers begin prostate-specific antigen screening between age 40 and 45 years, although recommendations on screening intervals and start age vary. ESMO recommendations are similar but only apply to BRCA2 carriers.
A male patient with a BRCA1/2 variant is typically referred for genetic counseling as well, Dr. Tsao explained. But “the challenge is that we don’t have a very good healthcare infrastructure right now” to follow through with that, he added. “Oftentimes a patient will wait many months or even more than a year for a genetic counseling appointment.”
To help improve these issues, Mount Sinai recently launched a comprehensive BRCA program for men and women that offers genetic testing and counseling for patients and family members.
Overall, identifying more male BRCA1/2 carriers will “maximize opportunities for cancer early detection, targeted risk management, and cancer treatment for males, along with facilitating opportunities for risk reduction and prevention in their family members, thereby decreasing the burden of hereditary cancer,” Dr. Cheng and colleagues concluded.
Support for the review was provided in part by BRCA Research and Cure Alliance and the Men & BRCA Program at the Basser Center for BRCA. Cheng reported grants from Promontory Pharmaceutics, Medivation, Sanofi, Janssen, royalties from UpToDate, nonfinancial support from Color Health, personal fees from AstraZeneca, BRCA Research and Cure Alliance (CureBRCA) outside the submitted work. Dr. Port, Dr. Tsao, and Dr. Fana had no conflicts of interest.
A version of this article first appeared on Medscape.com.
BRCA1 and BRCA2 pathogenic variants carry well-known associations with breast and ovarian cancers in women, which has led to robust clinical guidelines for early genetic testing and risk-reduction strategies.
Male carriers of BRCA1/2 pathogenic variants also face an increased risk for cancer, particularly of the prostate, pancreas, and breast.
However, men often fly under the radar.
“Most people (including their clinicians) are unaware of their carrier status,” Heather Cheng, MD, PhD, with University of Washington, Seattle, and colleagues explained in a comprehensive review on the subject, published in JAMA Oncology. Most are also unaware of “the associated cancer risks, and management recommendations” for BRCA carriers.
The testing gap in males may exist, in part, because of a “general lack of awareness” that BRCA gene mutations can be passed down to children from both the mother and father, Elisa Port, MD, chief of breast surgery for the Mount Sinai Health System in New York City, told this news organization.
A daughter can inherit a mutated BRCA gene that puts her at risk for breast or ovarian cancer from her mother’s or father’s family and, similarly, a son can inherit a mutated BRCA gene from either side of the family that puts him at an increased risk for developing prostate and other cancers, explained Dr. Port, director of the Center of Excellence for Breast Cancer at The Tisch Cancer Institute at Mount Sinai.
Considering family history and genetics on both sides of the family is important when assessing cancer risk in men and women, Dr. Port said.
BRCA Mutations in Men: What’s the Risk?
Although fewer than 1% of all breast cancers occur in men, when men do carry a BRCA mutation, their risk for breast cancer can increase considerably. The lifetime risk for breast cancer can be as high as 9% in male BRCA2 carriers and up to 1.2% in BRCA1 carriers.
BRCA1/2 mutations also put men at increased risk for pancreatic and prostate cancers.
For pancreatic cancer, male BRCA1 carriers have a nearly twofold increased risk compared with the general population, with a lifetime risk of 3%. BRCA2 carriers have a three- to nearly eightfold increased risk, with a lifetime risk up to 7%.
Male BRCA1 carriers face a nearly fourfold increased risk of developing prostate cancer and an absolute lifetime risk of 15%-45%. Male BRCA2 carriers have a five- to ninefold increased risk for prostate cancer, with an absolute lifetime risk between 27% and 60%.
When to Test, When to Screen?
Despite the increased risk for several cancers associated with BRCA mutations, many men are not offered genetic testing.
BRCA1/2 genetic testing in men is “ultra-important but underutilized and is an evolving unmet need that the field needs to address,” Kai Tsao, MD MS, medical director of the Medical Oncology Prostate Cancer Program at Mount Sinai in New York City, told this news organization.
For men considering genetic testing, in Dr. Tsao’s experience, barriers may include fear that insurance may not cover the test and that a positive test may increase insurance premiums, as well as concerns about what the test result may mean for them and their family.
Even for confirmed BRCA carriers, cancer screening guidelines for men vary.
For breast screening in men, there’s limited data to inform guidelines. The National Cancer Center Network currently recommends breast awareness and teaching self-examination starting at age 35 and recommends men with BRCA variants consider yearly mammograms starting at age 50, or 10 years before the earliest male breast cancer diagnosis in the family.
Data show that screening mammography in men at high-risk for breast cancer yields similar cancer detection rates in men and women, “suggesting mammography screening may be valuable in male BRCA carriers,” the review authors noted. And, in a recent study of men with BRCA1/2 pathogenic variants, most (71%) recommended for screening mammography completed their screening.
The European Society for Medical Oncology (ESMO) has similar screening recommendations but focuses only on men with BRCA2 mutations and suggests breast ultrasonography as well as mammography as a screening option.
The larger “issue is the general population doesn’t think of breast cancer when they think of men, which may delay seeking medical attention,” said Melissa Fana, MD, of NYU Grossman Long Island School of Medicine and NYU Langone Health, who wasn’t involved in the review.
For pancreatic cancer, guidelines suggest BRCA1/2 carriers be screened for pancreatic cancer starting at age 50, or 10 years before the earliest known pancreatic cancer in the family, although the guidelines vary on the role family history should play.
And for prostate cancer, current guidelines recommend male BRCA carriers begin prostate-specific antigen screening between age 40 and 45 years, although recommendations on screening intervals and start age vary. ESMO recommendations are similar but only apply to BRCA2 carriers.
A male patient with a BRCA1/2 variant is typically referred for genetic counseling as well, Dr. Tsao explained. But “the challenge is that we don’t have a very good healthcare infrastructure right now” to follow through with that, he added. “Oftentimes a patient will wait many months or even more than a year for a genetic counseling appointment.”
To help improve these issues, Mount Sinai recently launched a comprehensive BRCA program for men and women that offers genetic testing and counseling for patients and family members.
Overall, identifying more male BRCA1/2 carriers will “maximize opportunities for cancer early detection, targeted risk management, and cancer treatment for males, along with facilitating opportunities for risk reduction and prevention in their family members, thereby decreasing the burden of hereditary cancer,” Dr. Cheng and colleagues concluded.
Support for the review was provided in part by BRCA Research and Cure Alliance and the Men & BRCA Program at the Basser Center for BRCA. Cheng reported grants from Promontory Pharmaceutics, Medivation, Sanofi, Janssen, royalties from UpToDate, nonfinancial support from Color Health, personal fees from AstraZeneca, BRCA Research and Cure Alliance (CureBRCA) outside the submitted work. Dr. Port, Dr. Tsao, and Dr. Fana had no conflicts of interest.
A version of this article first appeared on Medscape.com.
BRCA1 and BRCA2 pathogenic variants carry well-known associations with breast and ovarian cancers in women, which has led to robust clinical guidelines for early genetic testing and risk-reduction strategies.
Male carriers of BRCA1/2 pathogenic variants also face an increased risk for cancer, particularly of the prostate, pancreas, and breast.
However, men often fly under the radar.
“Most people (including their clinicians) are unaware of their carrier status,” Heather Cheng, MD, PhD, with University of Washington, Seattle, and colleagues explained in a comprehensive review on the subject, published in JAMA Oncology. Most are also unaware of “the associated cancer risks, and management recommendations” for BRCA carriers.
The testing gap in males may exist, in part, because of a “general lack of awareness” that BRCA gene mutations can be passed down to children from both the mother and father, Elisa Port, MD, chief of breast surgery for the Mount Sinai Health System in New York City, told this news organization.
A daughter can inherit a mutated BRCA gene that puts her at risk for breast or ovarian cancer from her mother’s or father’s family and, similarly, a son can inherit a mutated BRCA gene from either side of the family that puts him at an increased risk for developing prostate and other cancers, explained Dr. Port, director of the Center of Excellence for Breast Cancer at The Tisch Cancer Institute at Mount Sinai.
Considering family history and genetics on both sides of the family is important when assessing cancer risk in men and women, Dr. Port said.
BRCA Mutations in Men: What’s the Risk?
Although fewer than 1% of all breast cancers occur in men, when men do carry a BRCA mutation, their risk for breast cancer can increase considerably. The lifetime risk for breast cancer can be as high as 9% in male BRCA2 carriers and up to 1.2% in BRCA1 carriers.
BRCA1/2 mutations also put men at increased risk for pancreatic and prostate cancers.
For pancreatic cancer, male BRCA1 carriers have a nearly twofold increased risk compared with the general population, with a lifetime risk of 3%. BRCA2 carriers have a three- to nearly eightfold increased risk, with a lifetime risk up to 7%.
Male BRCA1 carriers face a nearly fourfold increased risk of developing prostate cancer and an absolute lifetime risk of 15%-45%. Male BRCA2 carriers have a five- to ninefold increased risk for prostate cancer, with an absolute lifetime risk between 27% and 60%.
When to Test, When to Screen?
Despite the increased risk for several cancers associated with BRCA mutations, many men are not offered genetic testing.
BRCA1/2 genetic testing in men is “ultra-important but underutilized and is an evolving unmet need that the field needs to address,” Kai Tsao, MD MS, medical director of the Medical Oncology Prostate Cancer Program at Mount Sinai in New York City, told this news organization.
For men considering genetic testing, in Dr. Tsao’s experience, barriers may include fear that insurance may not cover the test and that a positive test may increase insurance premiums, as well as concerns about what the test result may mean for them and their family.
Even for confirmed BRCA carriers, cancer screening guidelines for men vary.
For breast screening in men, there’s limited data to inform guidelines. The National Cancer Center Network currently recommends breast awareness and teaching self-examination starting at age 35 and recommends men with BRCA variants consider yearly mammograms starting at age 50, or 10 years before the earliest male breast cancer diagnosis in the family.
Data show that screening mammography in men at high-risk for breast cancer yields similar cancer detection rates in men and women, “suggesting mammography screening may be valuable in male BRCA carriers,” the review authors noted. And, in a recent study of men with BRCA1/2 pathogenic variants, most (71%) recommended for screening mammography completed their screening.
The European Society for Medical Oncology (ESMO) has similar screening recommendations but focuses only on men with BRCA2 mutations and suggests breast ultrasonography as well as mammography as a screening option.
The larger “issue is the general population doesn’t think of breast cancer when they think of men, which may delay seeking medical attention,” said Melissa Fana, MD, of NYU Grossman Long Island School of Medicine and NYU Langone Health, who wasn’t involved in the review.
For pancreatic cancer, guidelines suggest BRCA1/2 carriers be screened for pancreatic cancer starting at age 50, or 10 years before the earliest known pancreatic cancer in the family, although the guidelines vary on the role family history should play.
And for prostate cancer, current guidelines recommend male BRCA carriers begin prostate-specific antigen screening between age 40 and 45 years, although recommendations on screening intervals and start age vary. ESMO recommendations are similar but only apply to BRCA2 carriers.
A male patient with a BRCA1/2 variant is typically referred for genetic counseling as well, Dr. Tsao explained. But “the challenge is that we don’t have a very good healthcare infrastructure right now” to follow through with that, he added. “Oftentimes a patient will wait many months or even more than a year for a genetic counseling appointment.”
To help improve these issues, Mount Sinai recently launched a comprehensive BRCA program for men and women that offers genetic testing and counseling for patients and family members.
Overall, identifying more male BRCA1/2 carriers will “maximize opportunities for cancer early detection, targeted risk management, and cancer treatment for males, along with facilitating opportunities for risk reduction and prevention in their family members, thereby decreasing the burden of hereditary cancer,” Dr. Cheng and colleagues concluded.
Support for the review was provided in part by BRCA Research and Cure Alliance and the Men & BRCA Program at the Basser Center for BRCA. Cheng reported grants from Promontory Pharmaceutics, Medivation, Sanofi, Janssen, royalties from UpToDate, nonfinancial support from Color Health, personal fees from AstraZeneca, BRCA Research and Cure Alliance (CureBRCA) outside the submitted work. Dr. Port, Dr. Tsao, and Dr. Fana had no conflicts of interest.
A version of this article first appeared on Medscape.com.
Pure Mucinous Breast Cancer Shows Better Survival Rates Than Other Subtypes
TOPLINE:
Patients with PMBC had a 5-year RFI of 96.1%, RFS of 94.9%, and OS of 98.1%.
METHODOLOGY:
- Researchers analyzed data from 23,102 women diagnosed with hormone receptor–positive HER2-negative stage I-III breast cancer, including 20,684 with IDC, 1475 with ILC, and 943 with PMBC.
- The multicenter cohort study included patients who underwent primary breast surgery at six academic institutions in Singapore, Taiwan, Korea, and Japan between January 2000 and December 2015.
- Current National Comprehensive Cancer Network Clinical Practice Guidelines “recommend consideration of adjuvant chemotherapy only for node-positive tumors,” whereas adjuvant endocrine therapy is recommended for estrogen receptor–positive and/or progesterone receptor–positive, node-positive tumors or tumors ≥ 3 cm. Previous studies have reported no significant association between adjuvant chemotherapy and breast cancer–specific survival or OS in patients with early-stage mucinous breast carcinoma.
- The study aimed to compare the recurrence and survival outcomes of PMBC against IDC and ILC, identify clinicopathologic prognostic factors of PMBC, and explore the association of adjuvant systemic therapy with outcomes across subgroups of PMBC.
- Extracted information included patient demographics, tumor characteristics, treatment administered, and staging according to the AJCC TNM classifications.
TAKEAWAY:
- Patients with PMBC had better RFI (hazard ratio [HR], 0.59; 95% CI, 0.43-0.80), RFS (HR, 0.70; 95% CI, 0.56-0.89), and OS (HR, 0.71; 95% CI, 0.53-0.96) than patients with IDC in multivariable Cox regression analyses.
- Fewer than half (48.7%) of the recurrences in patients with PMBC were distant, which was a lower rate than for patients with IDC (67.3%) and ILC (80.6%).
- Significant prognostic factors for RFI in PMBC included positive lymph node(s) (HR, 2.42; 95% CI, 1.08-5.40), radiotherapy (HR, 0.44; 95% CI, 0.23-0.85), and endocrine therapy (HR, 0.25; 95% CI, 0.09-0.70).
- No differential chemotherapy associations with outcomes were detected across PMBC subgroups by nodal stage, tumor size, and age.
IN PRACTICE:
“This international multicenter cohort study on PMBC evaluated one of the largest contemporary real-world datasets for clinical prognostic factors, which also includes valuable data on relapse events, associations of adjuvant systemic therapy, and a comparison with the SEER database,” wrote the authors of the study. “In our cohort, as anticipated, PMBC showed superior RFI, RFS, and OS compared with IDC and ILC, which both had comparatively similar survival outcomes.”
SOURCE:
Corresponding author, Yoon-Sim Yap, MBBS, PhD, of the National Cancer Centre Singapore in Singapore, designed the study. The paper was published online on May 14 in the Journal of the National Comprehensive Cancer Network.
LIMITATIONS:
The retrospective nature over a long period and lack of a central pathology review in this study are among its limitations. The high extent of missing values for tumor grade in PMBC in the multicenter cohort could impact the identified prognostic factors. The study’s findings may not be generalizable to all populations due to the specific geographic locations of the participating institutions.
DISCLOSURES:
Study author Yeon Hee Park, MD, PhD, disclosed serving on a data safety monitoring board and on an advisory board for AstraZeneca, Pfizer, Roche, Menarini, Novartis, and Daiichi Sankyo and serving as a consultant for AstraZeneca, Pfizer, Eli Lilly and Company, Gilead Sciences, Merck, Eisai, Roche, Daiichi Sankyo, Menarini, Everest Pharmaceuticals, and Novartis. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Patients with PMBC had a 5-year RFI of 96.1%, RFS of 94.9%, and OS of 98.1%.
METHODOLOGY:
- Researchers analyzed data from 23,102 women diagnosed with hormone receptor–positive HER2-negative stage I-III breast cancer, including 20,684 with IDC, 1475 with ILC, and 943 with PMBC.
- The multicenter cohort study included patients who underwent primary breast surgery at six academic institutions in Singapore, Taiwan, Korea, and Japan between January 2000 and December 2015.
- Current National Comprehensive Cancer Network Clinical Practice Guidelines “recommend consideration of adjuvant chemotherapy only for node-positive tumors,” whereas adjuvant endocrine therapy is recommended for estrogen receptor–positive and/or progesterone receptor–positive, node-positive tumors or tumors ≥ 3 cm. Previous studies have reported no significant association between adjuvant chemotherapy and breast cancer–specific survival or OS in patients with early-stage mucinous breast carcinoma.
- The study aimed to compare the recurrence and survival outcomes of PMBC against IDC and ILC, identify clinicopathologic prognostic factors of PMBC, and explore the association of adjuvant systemic therapy with outcomes across subgroups of PMBC.
- Extracted information included patient demographics, tumor characteristics, treatment administered, and staging according to the AJCC TNM classifications.
TAKEAWAY:
- Patients with PMBC had better RFI (hazard ratio [HR], 0.59; 95% CI, 0.43-0.80), RFS (HR, 0.70; 95% CI, 0.56-0.89), and OS (HR, 0.71; 95% CI, 0.53-0.96) than patients with IDC in multivariable Cox regression analyses.
- Fewer than half (48.7%) of the recurrences in patients with PMBC were distant, which was a lower rate than for patients with IDC (67.3%) and ILC (80.6%).
- Significant prognostic factors for RFI in PMBC included positive lymph node(s) (HR, 2.42; 95% CI, 1.08-5.40), radiotherapy (HR, 0.44; 95% CI, 0.23-0.85), and endocrine therapy (HR, 0.25; 95% CI, 0.09-0.70).
- No differential chemotherapy associations with outcomes were detected across PMBC subgroups by nodal stage, tumor size, and age.
IN PRACTICE:
“This international multicenter cohort study on PMBC evaluated one of the largest contemporary real-world datasets for clinical prognostic factors, which also includes valuable data on relapse events, associations of adjuvant systemic therapy, and a comparison with the SEER database,” wrote the authors of the study. “In our cohort, as anticipated, PMBC showed superior RFI, RFS, and OS compared with IDC and ILC, which both had comparatively similar survival outcomes.”
SOURCE:
Corresponding author, Yoon-Sim Yap, MBBS, PhD, of the National Cancer Centre Singapore in Singapore, designed the study. The paper was published online on May 14 in the Journal of the National Comprehensive Cancer Network.
LIMITATIONS:
The retrospective nature over a long period and lack of a central pathology review in this study are among its limitations. The high extent of missing values for tumor grade in PMBC in the multicenter cohort could impact the identified prognostic factors. The study’s findings may not be generalizable to all populations due to the specific geographic locations of the participating institutions.
DISCLOSURES:
Study author Yeon Hee Park, MD, PhD, disclosed serving on a data safety monitoring board and on an advisory board for AstraZeneca, Pfizer, Roche, Menarini, Novartis, and Daiichi Sankyo and serving as a consultant for AstraZeneca, Pfizer, Eli Lilly and Company, Gilead Sciences, Merck, Eisai, Roche, Daiichi Sankyo, Menarini, Everest Pharmaceuticals, and Novartis. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Patients with PMBC had a 5-year RFI of 96.1%, RFS of 94.9%, and OS of 98.1%.
METHODOLOGY:
- Researchers analyzed data from 23,102 women diagnosed with hormone receptor–positive HER2-negative stage I-III breast cancer, including 20,684 with IDC, 1475 with ILC, and 943 with PMBC.
- The multicenter cohort study included patients who underwent primary breast surgery at six academic institutions in Singapore, Taiwan, Korea, and Japan between January 2000 and December 2015.
- Current National Comprehensive Cancer Network Clinical Practice Guidelines “recommend consideration of adjuvant chemotherapy only for node-positive tumors,” whereas adjuvant endocrine therapy is recommended for estrogen receptor–positive and/or progesterone receptor–positive, node-positive tumors or tumors ≥ 3 cm. Previous studies have reported no significant association between adjuvant chemotherapy and breast cancer–specific survival or OS in patients with early-stage mucinous breast carcinoma.
- The study aimed to compare the recurrence and survival outcomes of PMBC against IDC and ILC, identify clinicopathologic prognostic factors of PMBC, and explore the association of adjuvant systemic therapy with outcomes across subgroups of PMBC.
- Extracted information included patient demographics, tumor characteristics, treatment administered, and staging according to the AJCC TNM classifications.
TAKEAWAY:
- Patients with PMBC had better RFI (hazard ratio [HR], 0.59; 95% CI, 0.43-0.80), RFS (HR, 0.70; 95% CI, 0.56-0.89), and OS (HR, 0.71; 95% CI, 0.53-0.96) than patients with IDC in multivariable Cox regression analyses.
- Fewer than half (48.7%) of the recurrences in patients with PMBC were distant, which was a lower rate than for patients with IDC (67.3%) and ILC (80.6%).
- Significant prognostic factors for RFI in PMBC included positive lymph node(s) (HR, 2.42; 95% CI, 1.08-5.40), radiotherapy (HR, 0.44; 95% CI, 0.23-0.85), and endocrine therapy (HR, 0.25; 95% CI, 0.09-0.70).
- No differential chemotherapy associations with outcomes were detected across PMBC subgroups by nodal stage, tumor size, and age.
IN PRACTICE:
“This international multicenter cohort study on PMBC evaluated one of the largest contemporary real-world datasets for clinical prognostic factors, which also includes valuable data on relapse events, associations of adjuvant systemic therapy, and a comparison with the SEER database,” wrote the authors of the study. “In our cohort, as anticipated, PMBC showed superior RFI, RFS, and OS compared with IDC and ILC, which both had comparatively similar survival outcomes.”
SOURCE:
Corresponding author, Yoon-Sim Yap, MBBS, PhD, of the National Cancer Centre Singapore in Singapore, designed the study. The paper was published online on May 14 in the Journal of the National Comprehensive Cancer Network.
LIMITATIONS:
The retrospective nature over a long period and lack of a central pathology review in this study are among its limitations. The high extent of missing values for tumor grade in PMBC in the multicenter cohort could impact the identified prognostic factors. The study’s findings may not be generalizable to all populations due to the specific geographic locations of the participating institutions.
DISCLOSURES:
Study author Yeon Hee Park, MD, PhD, disclosed serving on a data safety monitoring board and on an advisory board for AstraZeneca, Pfizer, Roche, Menarini, Novartis, and Daiichi Sankyo and serving as a consultant for AstraZeneca, Pfizer, Eli Lilly and Company, Gilead Sciences, Merck, Eisai, Roche, Daiichi Sankyo, Menarini, Everest Pharmaceuticals, and Novartis. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.