User login
Who can sue docs for wrongful death? Some states are trying to expand that group
In addition, the types of emotional damage that physicians can be sued for is expanding in pockets across the nation. The latest effort to expand the capacity to sue, a bill in New York state, failed when it was not signed by the governor – but a toned-down bill is in the works.
The impact of New York’s proposed expansion of wrongful death lawsuits would have been widespread. The New York legislation would have expanded the definition of “close family members” to include spouses, domestic partners, children, parents, stepparents, siblings, grandparents, and perhaps more. Additionally, lawsuits could have allowed juries to determine “close family members” of the deceased patient on the basis of specific circumstances of the person’s relationship with the decedent.
Currently, every state allows a wrongful death claim to be filed by immediate family members. If the patient who died was married, a surviving spouse could bring the lawsuit. If the patient had been unmarried, an adult child could bring the lawsuit in some states. A parent typically brings a lawsuit if their minor child has died from alleged wrongful death. In some states, one member of a civil union or domestic partnership may bring a wrongful death lawsuit. And if a single adult has no children or spouse/partner, more distant family members, including aunts, uncles, siblings, or grandparents, may file the suit.
The New York bill would also have expanded compensable damages to include loss of affection and companionship, and it would have expanded emotional damages, which are not currently included in New York. It would also have extended the statute of limitations of a wrongful death claim from 2 years to 3.5 years.
In general, in states that allow emotional distress to be included in wrongful death lawsuits, attorneys must demonstrate that survivors have suffered mental harm, such as depression, loss of sleep, fear, and anger, says Russ Haven, JD, general counsel for the New York Public Interest Research Group. While mental harm is not particularly easy to prove, attorneys must show that survivors have ongoing distress that is the direct result of the loss of the loved one and that the distress is significant enough to severely affect their quality of life.
Mr. Haven gives an example of emotional distress: “We worked with a woman who lost her fiancé in a motor vehicle accident,” he says. “The funeral ended up on the day she had scheduled her wedding dress fitting. A situation like that causes a good deal of lasting emotional distress.”
Expanding family members who can bring the lawsuit
The fact that a fiancé could be included in a wrongful death settlement is another aspect of the New York bill that was central to arguments both for and against the expansion of family members who can make claims. “We think a modern society includes unmarried partners, grandparents, siblings, and others,” says Mr. Haven.
“The language of who is a close family member might seem clear, but to a defense attorney, it isn’t,” says Tom Stebbins, executive director of the Lawsuit Reform Alliance of New York. “This could end up being a situation where someone has 40 grandchildren, and all could be considered close family members.”
Many states currently allow damages for claims of grief and mental anguish resulting from a wrongful death.
In her recent veto of the Grieving Families Act, New York Gov. Kathy Hochul took fire for her choices. The bill represented years of effort by the state legislature to expand the qualifiers for wrongful death lawsuits. Those supporting what ultimately became Senate Bill S74A believed they finally had the law over the finish line. Those opposed breathed a sigh of relief when the bill was vetoed.
Had Gov. Hochul signed Bill 274A, the effect on costs would have been enormous for physicians. New York already has the highest cumulative medical liability payouts in the nation, according to the Medical Society of the State of New York.
The MSSNY was among many parties that fought against the law. The Greater New York Hospital Association, insurance companies, the Defense Association of New York, and the New York Conference of Mayors all joined in lobbying against the bill.
“Gov. Hochul, in her veto message, correctly noted that the proposed New York legislation represented an extraordinary departure from New York’s wrongful death jurisprudence,” says Remi Stone, director of government relations at The Doctors Company, part of the TDC Group. “I would add that while there are some other states that allow grief damages, none are as wide-ranging as the proposed legislation.”
The NYPIRG, the AARP, and the New York Immigration Coalition supported the bill. In a statement following the veto, the New York State Trial Lawyers Association said: “By vetoing the Grieving Families Act, Gov. Hochul has sided with insurance companies, the health care industry, big corporations, and anyone else who doesn’t want to be held accountable for the negligent killing of a person. This bill passed with overwhelming bipartisan support and would rectify over a century of injustice.”
Following Gov. Hochul’s veto, the bill’s proponents and the state legislature vowed to return to the drawing board and construct a bill that the governor would eventually approve. For now, however, the controversial legislation has been put to rest.
Mr. Haven and the NYPIRG argue that New York lags behind many other states in allowing survivors to claim loss for their emotional distress. “When there is relationship loss, it has a great impact on your life,” Mr. Haven says, “and this goes beyond simply the financial impact.”
“The bill was well intended but completely vague on who could bring lawsuits and would have increased medical malpractice insurance by far too much,” says MSSNY President Parag Mehta, MD. “For safety net hospitals, one lawsuit would halt their ability to provide many programs aimed at underserved populations.”
Peter Kolbert, JD, senior vice president of claim and litigation services at Healthcare Risk Advisors (part of the TDC Group), had this to say: “The current ‘recoverable’ damages in New York in a wrongful death case include loss of guidance and support for minor children of a decedent. Those damages have been sustained at $2 million per child. It is rationally very challenging, if not impossible, to distinguish between those damages and the proposed damages that the very same people would have been entitled to under the proposed statute.”
What will happen in the future?
While the veto has stalled New York’s wrongful death expansion for now, supporters in and out of the legislature remain determined to continue their fight. “Advocates argue that the bill would have brought the state in line with wrongful death law in others,” says Brian Whitelaw, JD, a partner at Michigan’s Foley, Baron, Metzger & Juip. “But if the bill had become law as written, the economic impact would have been substantial.”
Mr. Whitelaw says that such wide-ranging lawsuits can have consequences that extend far beyond physicians’ insurance premiums. “This could impact the average person on the street’s ability to obtain the medical care they need, because doctors will go elsewhere to practice,” he says. “Beyond impacting the health care system, it can hurt small businesses as well.”
Mr. Haven says supporters of the expansion are far from finished with their efforts. “New York’s current law dates back to 1847, and it was cutting edge then,” he says. “It was designed for an agrarian society where if the husband died, his widow and children wouldn’t become destitute. Now, 175 years later, we realize that the law has biases, and tort law has evolved. The state needs to evolve as well.”
For his part, Dr. Mehta is open to a dialogue with lawmakers to revise the law in a manner agreeable to all parties. “We want to work together to make the system right,” he says. “The liability system in New York needs an overall holistic change, and we are available at any time to have discussions. The vetoed bill was a Band-Aid and didn’t address the main, underlying issues in the state.”
Mr. Stebbins, too, says he would like to continue the debate over how an expansion should look. “We hope to go through a discussion on caps to these suits,” he explains. “We have already seen the cap of $10 million broken four times in the past few years through nuclear verdicts. That’s something we need to address.”
Given the legislature’s overwhelming support for the bill, some version of it will likely make another appearance in the coming session. Whether or not it can strike the middle ground that will make all parties happy – including the governor – is yet to be seen. “Is it wrong to seek compensation for pain and suffering from a wrongful death?” asks Mr. Whitelaw. “No. But there must be limits to such laws, or where does it end?”
A version of this article first appeared on Medscape.com.
In addition, the types of emotional damage that physicians can be sued for is expanding in pockets across the nation. The latest effort to expand the capacity to sue, a bill in New York state, failed when it was not signed by the governor – but a toned-down bill is in the works.
The impact of New York’s proposed expansion of wrongful death lawsuits would have been widespread. The New York legislation would have expanded the definition of “close family members” to include spouses, domestic partners, children, parents, stepparents, siblings, grandparents, and perhaps more. Additionally, lawsuits could have allowed juries to determine “close family members” of the deceased patient on the basis of specific circumstances of the person’s relationship with the decedent.
Currently, every state allows a wrongful death claim to be filed by immediate family members. If the patient who died was married, a surviving spouse could bring the lawsuit. If the patient had been unmarried, an adult child could bring the lawsuit in some states. A parent typically brings a lawsuit if their minor child has died from alleged wrongful death. In some states, one member of a civil union or domestic partnership may bring a wrongful death lawsuit. And if a single adult has no children or spouse/partner, more distant family members, including aunts, uncles, siblings, or grandparents, may file the suit.
The New York bill would also have expanded compensable damages to include loss of affection and companionship, and it would have expanded emotional damages, which are not currently included in New York. It would also have extended the statute of limitations of a wrongful death claim from 2 years to 3.5 years.
In general, in states that allow emotional distress to be included in wrongful death lawsuits, attorneys must demonstrate that survivors have suffered mental harm, such as depression, loss of sleep, fear, and anger, says Russ Haven, JD, general counsel for the New York Public Interest Research Group. While mental harm is not particularly easy to prove, attorneys must show that survivors have ongoing distress that is the direct result of the loss of the loved one and that the distress is significant enough to severely affect their quality of life.
Mr. Haven gives an example of emotional distress: “We worked with a woman who lost her fiancé in a motor vehicle accident,” he says. “The funeral ended up on the day she had scheduled her wedding dress fitting. A situation like that causes a good deal of lasting emotional distress.”
Expanding family members who can bring the lawsuit
The fact that a fiancé could be included in a wrongful death settlement is another aspect of the New York bill that was central to arguments both for and against the expansion of family members who can make claims. “We think a modern society includes unmarried partners, grandparents, siblings, and others,” says Mr. Haven.
“The language of who is a close family member might seem clear, but to a defense attorney, it isn’t,” says Tom Stebbins, executive director of the Lawsuit Reform Alliance of New York. “This could end up being a situation where someone has 40 grandchildren, and all could be considered close family members.”
Many states currently allow damages for claims of grief and mental anguish resulting from a wrongful death.
In her recent veto of the Grieving Families Act, New York Gov. Kathy Hochul took fire for her choices. The bill represented years of effort by the state legislature to expand the qualifiers for wrongful death lawsuits. Those supporting what ultimately became Senate Bill S74A believed they finally had the law over the finish line. Those opposed breathed a sigh of relief when the bill was vetoed.
Had Gov. Hochul signed Bill 274A, the effect on costs would have been enormous for physicians. New York already has the highest cumulative medical liability payouts in the nation, according to the Medical Society of the State of New York.
The MSSNY was among many parties that fought against the law. The Greater New York Hospital Association, insurance companies, the Defense Association of New York, and the New York Conference of Mayors all joined in lobbying against the bill.
“Gov. Hochul, in her veto message, correctly noted that the proposed New York legislation represented an extraordinary departure from New York’s wrongful death jurisprudence,” says Remi Stone, director of government relations at The Doctors Company, part of the TDC Group. “I would add that while there are some other states that allow grief damages, none are as wide-ranging as the proposed legislation.”
The NYPIRG, the AARP, and the New York Immigration Coalition supported the bill. In a statement following the veto, the New York State Trial Lawyers Association said: “By vetoing the Grieving Families Act, Gov. Hochul has sided with insurance companies, the health care industry, big corporations, and anyone else who doesn’t want to be held accountable for the negligent killing of a person. This bill passed with overwhelming bipartisan support and would rectify over a century of injustice.”
Following Gov. Hochul’s veto, the bill’s proponents and the state legislature vowed to return to the drawing board and construct a bill that the governor would eventually approve. For now, however, the controversial legislation has been put to rest.
Mr. Haven and the NYPIRG argue that New York lags behind many other states in allowing survivors to claim loss for their emotional distress. “When there is relationship loss, it has a great impact on your life,” Mr. Haven says, “and this goes beyond simply the financial impact.”
“The bill was well intended but completely vague on who could bring lawsuits and would have increased medical malpractice insurance by far too much,” says MSSNY President Parag Mehta, MD. “For safety net hospitals, one lawsuit would halt their ability to provide many programs aimed at underserved populations.”
Peter Kolbert, JD, senior vice president of claim and litigation services at Healthcare Risk Advisors (part of the TDC Group), had this to say: “The current ‘recoverable’ damages in New York in a wrongful death case include loss of guidance and support for minor children of a decedent. Those damages have been sustained at $2 million per child. It is rationally very challenging, if not impossible, to distinguish between those damages and the proposed damages that the very same people would have been entitled to under the proposed statute.”
What will happen in the future?
While the veto has stalled New York’s wrongful death expansion for now, supporters in and out of the legislature remain determined to continue their fight. “Advocates argue that the bill would have brought the state in line with wrongful death law in others,” says Brian Whitelaw, JD, a partner at Michigan’s Foley, Baron, Metzger & Juip. “But if the bill had become law as written, the economic impact would have been substantial.”
Mr. Whitelaw says that such wide-ranging lawsuits can have consequences that extend far beyond physicians’ insurance premiums. “This could impact the average person on the street’s ability to obtain the medical care they need, because doctors will go elsewhere to practice,” he says. “Beyond impacting the health care system, it can hurt small businesses as well.”
Mr. Haven says supporters of the expansion are far from finished with their efforts. “New York’s current law dates back to 1847, and it was cutting edge then,” he says. “It was designed for an agrarian society where if the husband died, his widow and children wouldn’t become destitute. Now, 175 years later, we realize that the law has biases, and tort law has evolved. The state needs to evolve as well.”
For his part, Dr. Mehta is open to a dialogue with lawmakers to revise the law in a manner agreeable to all parties. “We want to work together to make the system right,” he says. “The liability system in New York needs an overall holistic change, and we are available at any time to have discussions. The vetoed bill was a Band-Aid and didn’t address the main, underlying issues in the state.”
Mr. Stebbins, too, says he would like to continue the debate over how an expansion should look. “We hope to go through a discussion on caps to these suits,” he explains. “We have already seen the cap of $10 million broken four times in the past few years through nuclear verdicts. That’s something we need to address.”
Given the legislature’s overwhelming support for the bill, some version of it will likely make another appearance in the coming session. Whether or not it can strike the middle ground that will make all parties happy – including the governor – is yet to be seen. “Is it wrong to seek compensation for pain and suffering from a wrongful death?” asks Mr. Whitelaw. “No. But there must be limits to such laws, or where does it end?”
A version of this article first appeared on Medscape.com.
In addition, the types of emotional damage that physicians can be sued for is expanding in pockets across the nation. The latest effort to expand the capacity to sue, a bill in New York state, failed when it was not signed by the governor – but a toned-down bill is in the works.
The impact of New York’s proposed expansion of wrongful death lawsuits would have been widespread. The New York legislation would have expanded the definition of “close family members” to include spouses, domestic partners, children, parents, stepparents, siblings, grandparents, and perhaps more. Additionally, lawsuits could have allowed juries to determine “close family members” of the deceased patient on the basis of specific circumstances of the person’s relationship with the decedent.
Currently, every state allows a wrongful death claim to be filed by immediate family members. If the patient who died was married, a surviving spouse could bring the lawsuit. If the patient had been unmarried, an adult child could bring the lawsuit in some states. A parent typically brings a lawsuit if their minor child has died from alleged wrongful death. In some states, one member of a civil union or domestic partnership may bring a wrongful death lawsuit. And if a single adult has no children or spouse/partner, more distant family members, including aunts, uncles, siblings, or grandparents, may file the suit.
The New York bill would also have expanded compensable damages to include loss of affection and companionship, and it would have expanded emotional damages, which are not currently included in New York. It would also have extended the statute of limitations of a wrongful death claim from 2 years to 3.5 years.
In general, in states that allow emotional distress to be included in wrongful death lawsuits, attorneys must demonstrate that survivors have suffered mental harm, such as depression, loss of sleep, fear, and anger, says Russ Haven, JD, general counsel for the New York Public Interest Research Group. While mental harm is not particularly easy to prove, attorneys must show that survivors have ongoing distress that is the direct result of the loss of the loved one and that the distress is significant enough to severely affect their quality of life.
Mr. Haven gives an example of emotional distress: “We worked with a woman who lost her fiancé in a motor vehicle accident,” he says. “The funeral ended up on the day she had scheduled her wedding dress fitting. A situation like that causes a good deal of lasting emotional distress.”
Expanding family members who can bring the lawsuit
The fact that a fiancé could be included in a wrongful death settlement is another aspect of the New York bill that was central to arguments both for and against the expansion of family members who can make claims. “We think a modern society includes unmarried partners, grandparents, siblings, and others,” says Mr. Haven.
“The language of who is a close family member might seem clear, but to a defense attorney, it isn’t,” says Tom Stebbins, executive director of the Lawsuit Reform Alliance of New York. “This could end up being a situation where someone has 40 grandchildren, and all could be considered close family members.”
Many states currently allow damages for claims of grief and mental anguish resulting from a wrongful death.
In her recent veto of the Grieving Families Act, New York Gov. Kathy Hochul took fire for her choices. The bill represented years of effort by the state legislature to expand the qualifiers for wrongful death lawsuits. Those supporting what ultimately became Senate Bill S74A believed they finally had the law over the finish line. Those opposed breathed a sigh of relief when the bill was vetoed.
Had Gov. Hochul signed Bill 274A, the effect on costs would have been enormous for physicians. New York already has the highest cumulative medical liability payouts in the nation, according to the Medical Society of the State of New York.
The MSSNY was among many parties that fought against the law. The Greater New York Hospital Association, insurance companies, the Defense Association of New York, and the New York Conference of Mayors all joined in lobbying against the bill.
“Gov. Hochul, in her veto message, correctly noted that the proposed New York legislation represented an extraordinary departure from New York’s wrongful death jurisprudence,” says Remi Stone, director of government relations at The Doctors Company, part of the TDC Group. “I would add that while there are some other states that allow grief damages, none are as wide-ranging as the proposed legislation.”
The NYPIRG, the AARP, and the New York Immigration Coalition supported the bill. In a statement following the veto, the New York State Trial Lawyers Association said: “By vetoing the Grieving Families Act, Gov. Hochul has sided with insurance companies, the health care industry, big corporations, and anyone else who doesn’t want to be held accountable for the negligent killing of a person. This bill passed with overwhelming bipartisan support and would rectify over a century of injustice.”
Following Gov. Hochul’s veto, the bill’s proponents and the state legislature vowed to return to the drawing board and construct a bill that the governor would eventually approve. For now, however, the controversial legislation has been put to rest.
Mr. Haven and the NYPIRG argue that New York lags behind many other states in allowing survivors to claim loss for their emotional distress. “When there is relationship loss, it has a great impact on your life,” Mr. Haven says, “and this goes beyond simply the financial impact.”
“The bill was well intended but completely vague on who could bring lawsuits and would have increased medical malpractice insurance by far too much,” says MSSNY President Parag Mehta, MD. “For safety net hospitals, one lawsuit would halt their ability to provide many programs aimed at underserved populations.”
Peter Kolbert, JD, senior vice president of claim and litigation services at Healthcare Risk Advisors (part of the TDC Group), had this to say: “The current ‘recoverable’ damages in New York in a wrongful death case include loss of guidance and support for minor children of a decedent. Those damages have been sustained at $2 million per child. It is rationally very challenging, if not impossible, to distinguish between those damages and the proposed damages that the very same people would have been entitled to under the proposed statute.”
What will happen in the future?
While the veto has stalled New York’s wrongful death expansion for now, supporters in and out of the legislature remain determined to continue their fight. “Advocates argue that the bill would have brought the state in line with wrongful death law in others,” says Brian Whitelaw, JD, a partner at Michigan’s Foley, Baron, Metzger & Juip. “But if the bill had become law as written, the economic impact would have been substantial.”
Mr. Whitelaw says that such wide-ranging lawsuits can have consequences that extend far beyond physicians’ insurance premiums. “This could impact the average person on the street’s ability to obtain the medical care they need, because doctors will go elsewhere to practice,” he says. “Beyond impacting the health care system, it can hurt small businesses as well.”
Mr. Haven says supporters of the expansion are far from finished with their efforts. “New York’s current law dates back to 1847, and it was cutting edge then,” he says. “It was designed for an agrarian society where if the husband died, his widow and children wouldn’t become destitute. Now, 175 years later, we realize that the law has biases, and tort law has evolved. The state needs to evolve as well.”
For his part, Dr. Mehta is open to a dialogue with lawmakers to revise the law in a manner agreeable to all parties. “We want to work together to make the system right,” he says. “The liability system in New York needs an overall holistic change, and we are available at any time to have discussions. The vetoed bill was a Band-Aid and didn’t address the main, underlying issues in the state.”
Mr. Stebbins, too, says he would like to continue the debate over how an expansion should look. “We hope to go through a discussion on caps to these suits,” he explains. “We have already seen the cap of $10 million broken four times in the past few years through nuclear verdicts. That’s something we need to address.”
Given the legislature’s overwhelming support for the bill, some version of it will likely make another appearance in the coming session. Whether or not it can strike the middle ground that will make all parties happy – including the governor – is yet to be seen. “Is it wrong to seek compensation for pain and suffering from a wrongful death?” asks Mr. Whitelaw. “No. But there must be limits to such laws, or where does it end?”
A version of this article first appeared on Medscape.com.
FDA strengthens mammography regulations: Final rule
A final rule, updating the regulations issued under the Mammography Quality Standards Act of 1992, requires that mammography facilities notify patients about the density of their breasts, strengthens the FDA’s oversight of facilities, and provides guidance to help physicians better categorize and assess mammograms, according to a March 9 press release.
The rule requires implementation of the changes within 18 months.
According to the final rule document, the updates are “intended to improve the delivery of mammography services” in ways that reflect changes in mammography technology, quality standards, and the way results are categorized, reported, and communicated to patients and providers.
For instance, mammography reports must include an assessment of breast density to provide greater detail on the potential limitations of the mammogram results and allow patients and physicians to make more informed decisions, such as the possibility of additional imaging for women with dense breast tissue.
“Today’s action represents the agency’s broader commitment to support innovation to prevent, detect and treat cancer,” said Hilary Marston, MD, MPH, FDA’s chief medical officer, in the agency’s press release. The FDA remains “committed to advancing efforts to improve the health of women and strengthen the fight against breast cancer.”
A version of this article first appeared on Medscape.com.
A final rule, updating the regulations issued under the Mammography Quality Standards Act of 1992, requires that mammography facilities notify patients about the density of their breasts, strengthens the FDA’s oversight of facilities, and provides guidance to help physicians better categorize and assess mammograms, according to a March 9 press release.
The rule requires implementation of the changes within 18 months.
According to the final rule document, the updates are “intended to improve the delivery of mammography services” in ways that reflect changes in mammography technology, quality standards, and the way results are categorized, reported, and communicated to patients and providers.
For instance, mammography reports must include an assessment of breast density to provide greater detail on the potential limitations of the mammogram results and allow patients and physicians to make more informed decisions, such as the possibility of additional imaging for women with dense breast tissue.
“Today’s action represents the agency’s broader commitment to support innovation to prevent, detect and treat cancer,” said Hilary Marston, MD, MPH, FDA’s chief medical officer, in the agency’s press release. The FDA remains “committed to advancing efforts to improve the health of women and strengthen the fight against breast cancer.”
A version of this article first appeared on Medscape.com.
A final rule, updating the regulations issued under the Mammography Quality Standards Act of 1992, requires that mammography facilities notify patients about the density of their breasts, strengthens the FDA’s oversight of facilities, and provides guidance to help physicians better categorize and assess mammograms, according to a March 9 press release.
The rule requires implementation of the changes within 18 months.
According to the final rule document, the updates are “intended to improve the delivery of mammography services” in ways that reflect changes in mammography technology, quality standards, and the way results are categorized, reported, and communicated to patients and providers.
For instance, mammography reports must include an assessment of breast density to provide greater detail on the potential limitations of the mammogram results and allow patients and physicians to make more informed decisions, such as the possibility of additional imaging for women with dense breast tissue.
“Today’s action represents the agency’s broader commitment to support innovation to prevent, detect and treat cancer,” said Hilary Marston, MD, MPH, FDA’s chief medical officer, in the agency’s press release. The FDA remains “committed to advancing efforts to improve the health of women and strengthen the fight against breast cancer.”
A version of this article first appeared on Medscape.com.
Cancer clinical trials: Can industry stack the deck?
A year before the COVID-19 pandemic began, a team of clinical statisticians at the University of Texas MD Anderson Cancer Center sat together in small office for a year, painstakingly hand coding data from the U.S. clinical trials database, www.clinicaltrials.gov.
“We found marked disparities across different disease sites. ... The patients that are enrolling on studies are markedly younger than the average patient seen in the population with those same conditions,” said team leader Ethan Ludmir, MD, assistant professor, Division of Radiation Oncology at the University of Texas.
And this age disparity was significantly greater in industry-funded trials.
Researchers have known for 20 years that cancer trial participants are not representative of the wider cancer population, and numerous government guidance documents have been issued on the matter. However, this Texas team’s findings were the first unambiguous evidence that pharmaceutical companies seem to be selecting younger patients to test their drugs.
“If we’re being generous then perhaps the answer is: They’re looking for some element of homogeneity, which is to say they don’t want competing risks to make the signal-to-noise ratio uninterpretable,” said Dr. Ludmir.
Dr. Laura Bothwell, PhD, assistant professor, Yale School of Public Health, recently coauthored a 259-page consensus report for the National Academies of Sciences, Engineering and Medicine on how to increase the research involvement of under-represented groups.
Dr. Bothwell said, “The problem with industry funded research is that ... it’s an inevitable conflict of interest that exists. They want the research to show that their products work. And older populations ... have a lot more complications, which leads to potentially less favorable results.”
The MD Anderson findings were published in JAMA Oncology. “That was the starting point in our journey,” said Dr. Ludmir. For the next 3 years, the researchers mined their painstakingly constructed database to understand what was preventing greater numbers of older patients from enrollment in cancer trials.
Meanwhile, answers were coming from elsewhere. In parallel with the work at MD Anderson, a team in California led by Mina Sedrak, MD, a medical oncologist at the City of Hope National Medical Center, had also started investigating age disparities in clinical trials.
Dr. Sedrak, who also serves as deputy director of Clinical Trials at the Center for Cancer and Aging, said he had become increasingly concerned that he did not have adequate information on new cancer therapies for his older patients.
“I was caring for a large number of people who were ... older adults,” said Dr. Sedrak, “But the data that was being used to get the standard-of-care treatment for cancer did not include older adults. And so there was this lack of applicability.”
He summed up the challenges in a 2021 review paper: “Most of what we know about cancer therapeutics is based on clinical trials conducted in younger, healthier patients.”
By 2030, it is estimated that 70% of all new cancer diagnoses will be in patients 65 years old and older. By contrast, patients over age 65 still account for only 40% of patients in cancer trials registered with the FDA (2015 figures) and older adults make up only 44% of participants in practice-changing cancer trials, according to a 2022 study.
So what is going on? Are studies specifically designed to squeeze out older patients?
Surprisingly, patients are not being kept out of trials by formal age limits, according to Dr. Ludmir. His team found that only 10% of phase 3 trials over the past 30 years had an upper limit for age, and age restrictions have been dropping by 1% a year. (For example, 16% of trials that enrolled in 2002-2005 had an upper age limit, compared with just 8% of trials that started in 2010-2014.)
Dr. Sedrak’s team found that “clinician bias” may be a factor, a situation in which trial investigators – particularly academic oncologists – are subconsciously picking younger, healthier patients for trials and excluding older, sicker patients to protect them from drug toxicities.
Dr. Ludmir said this was understandable, especially in the case of industry-driven trials, which tend to have demanding endpoints and “an overall posture of more treatment aggressiveness.”
“These are typically not trials where they’re saying, `Hey, if we add acupuncture ... are we going to see improved patient reported outcomes?’” Dr. Ludmir explained. “You’re asking ... I’ve got this cocktail of two pretty rough chemos: I want to see what happens if I add an immunotherapy to that. If I’m the clinician in clinic, I might reasonably, subconsciously, say, is the 75-year-old really who I want on this?”
What about patient bias? Perhaps fewer older patients wish to join clinical trials?
Not so, at least not at community cancer centers, said Dr. Sedrak. His team’s analysis of the National Cancer Institute Community Oncology Research Program database for 2016-2019 revealed that older patients were just as keen as the younger patients to participate in trials (68% of patients aged 50-69 years and 65% of patients 70+; P = .28).
However, drug companies may be excluding older patients by more subtle means. One-fifth of patients over 65 have had a prior cancer. Dr. Ludmir and coauthor Roshal Patel, MD, used their hand-coded www.clinicaltrials.gov database to look at prior malignancy exclusion criteria (PMEC). The analysis found “pervasive utilization” of PMEC in phase 3 trials, cropping up in 41% of studies over the past 30 years.
PMEC was significantly associated with age disparities and was significantly more common in industry-funded trials.
When asked whether PMEC are “age restriction by stealth” on the part of drug companies, Dr. Ludmir was reluctant to assign blame, but stood by his data: “The wider you restrict people in terms of having a prior cancer, the wider the age disparities in the subsequent studies, which to me is about as strong, in terms of causal understanding of these phenomena, as you can reasonably get at this level.”
In March the FDA released a guidance document titled Inclusion of Older Adults in Cancer Clinical Trials. However, its recommendations are “nonbinding” and “do not have the force and effect of law.”
To fix the issues, said Dr. Sedrak, the FDA must be given teeth.
“Okay, you write guidelines,” he said. “But if you don’t actually hold people accountable to following the guidelines, how are we going to implement and make sure that we’re transforming policy into action?”
Dr. Bothwell of Yale’s School of Public Health agreed. “Accountability has been the weakest link for decades now.”
She concluded, “In medicine there’s a tendency to believe that a therapy, because it exists and it has been tested and it’s shown some efficacy, it’s useful. But we don’t know the answer to that question unless we have statistically valid research in the population that we’re using it in.”
Dr. Bothwell and Dr. Ludmir report no conflicts of interest. In his publications, Dr. Sedrak reports industry grants from Seattle Genetics, Eli Lilly, Novartis, and Pfizer Foundation.
A year before the COVID-19 pandemic began, a team of clinical statisticians at the University of Texas MD Anderson Cancer Center sat together in small office for a year, painstakingly hand coding data from the U.S. clinical trials database, www.clinicaltrials.gov.
“We found marked disparities across different disease sites. ... The patients that are enrolling on studies are markedly younger than the average patient seen in the population with those same conditions,” said team leader Ethan Ludmir, MD, assistant professor, Division of Radiation Oncology at the University of Texas.
And this age disparity was significantly greater in industry-funded trials.
Researchers have known for 20 years that cancer trial participants are not representative of the wider cancer population, and numerous government guidance documents have been issued on the matter. However, this Texas team’s findings were the first unambiguous evidence that pharmaceutical companies seem to be selecting younger patients to test their drugs.
“If we’re being generous then perhaps the answer is: They’re looking for some element of homogeneity, which is to say they don’t want competing risks to make the signal-to-noise ratio uninterpretable,” said Dr. Ludmir.
Dr. Laura Bothwell, PhD, assistant professor, Yale School of Public Health, recently coauthored a 259-page consensus report for the National Academies of Sciences, Engineering and Medicine on how to increase the research involvement of under-represented groups.
Dr. Bothwell said, “The problem with industry funded research is that ... it’s an inevitable conflict of interest that exists. They want the research to show that their products work. And older populations ... have a lot more complications, which leads to potentially less favorable results.”
The MD Anderson findings were published in JAMA Oncology. “That was the starting point in our journey,” said Dr. Ludmir. For the next 3 years, the researchers mined their painstakingly constructed database to understand what was preventing greater numbers of older patients from enrollment in cancer trials.
Meanwhile, answers were coming from elsewhere. In parallel with the work at MD Anderson, a team in California led by Mina Sedrak, MD, a medical oncologist at the City of Hope National Medical Center, had also started investigating age disparities in clinical trials.
Dr. Sedrak, who also serves as deputy director of Clinical Trials at the Center for Cancer and Aging, said he had become increasingly concerned that he did not have adequate information on new cancer therapies for his older patients.
“I was caring for a large number of people who were ... older adults,” said Dr. Sedrak, “But the data that was being used to get the standard-of-care treatment for cancer did not include older adults. And so there was this lack of applicability.”
He summed up the challenges in a 2021 review paper: “Most of what we know about cancer therapeutics is based on clinical trials conducted in younger, healthier patients.”
By 2030, it is estimated that 70% of all new cancer diagnoses will be in patients 65 years old and older. By contrast, patients over age 65 still account for only 40% of patients in cancer trials registered with the FDA (2015 figures) and older adults make up only 44% of participants in practice-changing cancer trials, according to a 2022 study.
So what is going on? Are studies specifically designed to squeeze out older patients?
Surprisingly, patients are not being kept out of trials by formal age limits, according to Dr. Ludmir. His team found that only 10% of phase 3 trials over the past 30 years had an upper limit for age, and age restrictions have been dropping by 1% a year. (For example, 16% of trials that enrolled in 2002-2005 had an upper age limit, compared with just 8% of trials that started in 2010-2014.)
Dr. Sedrak’s team found that “clinician bias” may be a factor, a situation in which trial investigators – particularly academic oncologists – are subconsciously picking younger, healthier patients for trials and excluding older, sicker patients to protect them from drug toxicities.
Dr. Ludmir said this was understandable, especially in the case of industry-driven trials, which tend to have demanding endpoints and “an overall posture of more treatment aggressiveness.”
“These are typically not trials where they’re saying, `Hey, if we add acupuncture ... are we going to see improved patient reported outcomes?’” Dr. Ludmir explained. “You’re asking ... I’ve got this cocktail of two pretty rough chemos: I want to see what happens if I add an immunotherapy to that. If I’m the clinician in clinic, I might reasonably, subconsciously, say, is the 75-year-old really who I want on this?”
What about patient bias? Perhaps fewer older patients wish to join clinical trials?
Not so, at least not at community cancer centers, said Dr. Sedrak. His team’s analysis of the National Cancer Institute Community Oncology Research Program database for 2016-2019 revealed that older patients were just as keen as the younger patients to participate in trials (68% of patients aged 50-69 years and 65% of patients 70+; P = .28).
However, drug companies may be excluding older patients by more subtle means. One-fifth of patients over 65 have had a prior cancer. Dr. Ludmir and coauthor Roshal Patel, MD, used their hand-coded www.clinicaltrials.gov database to look at prior malignancy exclusion criteria (PMEC). The analysis found “pervasive utilization” of PMEC in phase 3 trials, cropping up in 41% of studies over the past 30 years.
PMEC was significantly associated with age disparities and was significantly more common in industry-funded trials.
When asked whether PMEC are “age restriction by stealth” on the part of drug companies, Dr. Ludmir was reluctant to assign blame, but stood by his data: “The wider you restrict people in terms of having a prior cancer, the wider the age disparities in the subsequent studies, which to me is about as strong, in terms of causal understanding of these phenomena, as you can reasonably get at this level.”
In March the FDA released a guidance document titled Inclusion of Older Adults in Cancer Clinical Trials. However, its recommendations are “nonbinding” and “do not have the force and effect of law.”
To fix the issues, said Dr. Sedrak, the FDA must be given teeth.
“Okay, you write guidelines,” he said. “But if you don’t actually hold people accountable to following the guidelines, how are we going to implement and make sure that we’re transforming policy into action?”
Dr. Bothwell of Yale’s School of Public Health agreed. “Accountability has been the weakest link for decades now.”
She concluded, “In medicine there’s a tendency to believe that a therapy, because it exists and it has been tested and it’s shown some efficacy, it’s useful. But we don’t know the answer to that question unless we have statistically valid research in the population that we’re using it in.”
Dr. Bothwell and Dr. Ludmir report no conflicts of interest. In his publications, Dr. Sedrak reports industry grants from Seattle Genetics, Eli Lilly, Novartis, and Pfizer Foundation.
A year before the COVID-19 pandemic began, a team of clinical statisticians at the University of Texas MD Anderson Cancer Center sat together in small office for a year, painstakingly hand coding data from the U.S. clinical trials database, www.clinicaltrials.gov.
“We found marked disparities across different disease sites. ... The patients that are enrolling on studies are markedly younger than the average patient seen in the population with those same conditions,” said team leader Ethan Ludmir, MD, assistant professor, Division of Radiation Oncology at the University of Texas.
And this age disparity was significantly greater in industry-funded trials.
Researchers have known for 20 years that cancer trial participants are not representative of the wider cancer population, and numerous government guidance documents have been issued on the matter. However, this Texas team’s findings were the first unambiguous evidence that pharmaceutical companies seem to be selecting younger patients to test their drugs.
“If we’re being generous then perhaps the answer is: They’re looking for some element of homogeneity, which is to say they don’t want competing risks to make the signal-to-noise ratio uninterpretable,” said Dr. Ludmir.
Dr. Laura Bothwell, PhD, assistant professor, Yale School of Public Health, recently coauthored a 259-page consensus report for the National Academies of Sciences, Engineering and Medicine on how to increase the research involvement of under-represented groups.
Dr. Bothwell said, “The problem with industry funded research is that ... it’s an inevitable conflict of interest that exists. They want the research to show that their products work. And older populations ... have a lot more complications, which leads to potentially less favorable results.”
The MD Anderson findings were published in JAMA Oncology. “That was the starting point in our journey,” said Dr. Ludmir. For the next 3 years, the researchers mined their painstakingly constructed database to understand what was preventing greater numbers of older patients from enrollment in cancer trials.
Meanwhile, answers were coming from elsewhere. In parallel with the work at MD Anderson, a team in California led by Mina Sedrak, MD, a medical oncologist at the City of Hope National Medical Center, had also started investigating age disparities in clinical trials.
Dr. Sedrak, who also serves as deputy director of Clinical Trials at the Center for Cancer and Aging, said he had become increasingly concerned that he did not have adequate information on new cancer therapies for his older patients.
“I was caring for a large number of people who were ... older adults,” said Dr. Sedrak, “But the data that was being used to get the standard-of-care treatment for cancer did not include older adults. And so there was this lack of applicability.”
He summed up the challenges in a 2021 review paper: “Most of what we know about cancer therapeutics is based on clinical trials conducted in younger, healthier patients.”
By 2030, it is estimated that 70% of all new cancer diagnoses will be in patients 65 years old and older. By contrast, patients over age 65 still account for only 40% of patients in cancer trials registered with the FDA (2015 figures) and older adults make up only 44% of participants in practice-changing cancer trials, according to a 2022 study.
So what is going on? Are studies specifically designed to squeeze out older patients?
Surprisingly, patients are not being kept out of trials by formal age limits, according to Dr. Ludmir. His team found that only 10% of phase 3 trials over the past 30 years had an upper limit for age, and age restrictions have been dropping by 1% a year. (For example, 16% of trials that enrolled in 2002-2005 had an upper age limit, compared with just 8% of trials that started in 2010-2014.)
Dr. Sedrak’s team found that “clinician bias” may be a factor, a situation in which trial investigators – particularly academic oncologists – are subconsciously picking younger, healthier patients for trials and excluding older, sicker patients to protect them from drug toxicities.
Dr. Ludmir said this was understandable, especially in the case of industry-driven trials, which tend to have demanding endpoints and “an overall posture of more treatment aggressiveness.”
“These are typically not trials where they’re saying, `Hey, if we add acupuncture ... are we going to see improved patient reported outcomes?’” Dr. Ludmir explained. “You’re asking ... I’ve got this cocktail of two pretty rough chemos: I want to see what happens if I add an immunotherapy to that. If I’m the clinician in clinic, I might reasonably, subconsciously, say, is the 75-year-old really who I want on this?”
What about patient bias? Perhaps fewer older patients wish to join clinical trials?
Not so, at least not at community cancer centers, said Dr. Sedrak. His team’s analysis of the National Cancer Institute Community Oncology Research Program database for 2016-2019 revealed that older patients were just as keen as the younger patients to participate in trials (68% of patients aged 50-69 years and 65% of patients 70+; P = .28).
However, drug companies may be excluding older patients by more subtle means. One-fifth of patients over 65 have had a prior cancer. Dr. Ludmir and coauthor Roshal Patel, MD, used their hand-coded www.clinicaltrials.gov database to look at prior malignancy exclusion criteria (PMEC). The analysis found “pervasive utilization” of PMEC in phase 3 trials, cropping up in 41% of studies over the past 30 years.
PMEC was significantly associated with age disparities and was significantly more common in industry-funded trials.
When asked whether PMEC are “age restriction by stealth” on the part of drug companies, Dr. Ludmir was reluctant to assign blame, but stood by his data: “The wider you restrict people in terms of having a prior cancer, the wider the age disparities in the subsequent studies, which to me is about as strong, in terms of causal understanding of these phenomena, as you can reasonably get at this level.”
In March the FDA released a guidance document titled Inclusion of Older Adults in Cancer Clinical Trials. However, its recommendations are “nonbinding” and “do not have the force and effect of law.”
To fix the issues, said Dr. Sedrak, the FDA must be given teeth.
“Okay, you write guidelines,” he said. “But if you don’t actually hold people accountable to following the guidelines, how are we going to implement and make sure that we’re transforming policy into action?”
Dr. Bothwell of Yale’s School of Public Health agreed. “Accountability has been the weakest link for decades now.”
She concluded, “In medicine there’s a tendency to believe that a therapy, because it exists and it has been tested and it’s shown some efficacy, it’s useful. But we don’t know the answer to that question unless we have statistically valid research in the population that we’re using it in.”
Dr. Bothwell and Dr. Ludmir report no conflicts of interest. In his publications, Dr. Sedrak reports industry grants from Seattle Genetics, Eli Lilly, Novartis, and Pfizer Foundation.
Ob.gyns. reveal heavier suicide ideation burden than most specialists
Obstetricians and gynecologists are more likely than most specialists to have thoughts of suicide, and almost of quarter of physicians in general reported that they were depressed in a recent survey conducted by Medscape.
“Too much work with too little control is a recipe for depression in anyone,” Andrea Giedinghagen, MD, of Washington University, St. Louis, said in the Medscape Physician Suicide Report: Doctors’ Burden 2023. “Physicians are also still coping with a pandemic – the trauma from COVID-19 didn’t disappear just because the full ICUs did – and with a fractured health care system that virtually guarantees moral distress.”
About 23% of the almost 9,200 survey respondents said that they were depressed in 2022, compared with 21% the previous year. Suicide ideation was down in 2022, however, with 9% of all responding physicians reporting contemplation versus 13% in 2021, based on the results of the latest survey, which was conducted from June 28, 2022, to Oct. 2, 2022.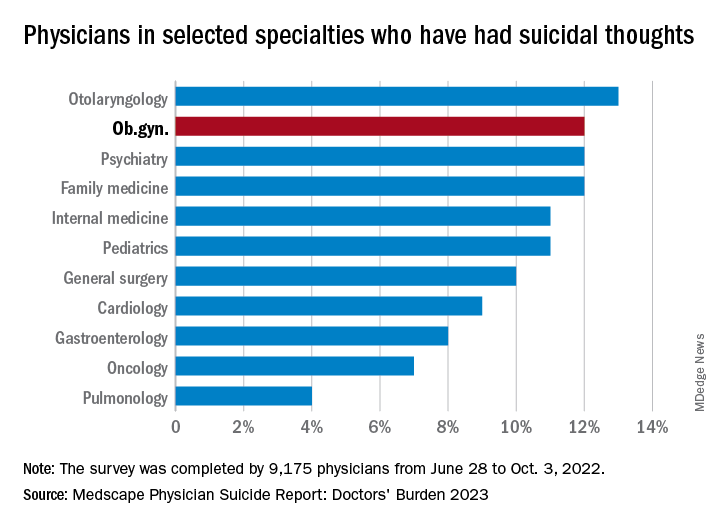
Ob.gyns. were above that average, with 12% reporting suicidal thoughts over the past year, equaling psychiatrists, family physicians, anesthesiologists, and emergency physicians and trailing only the otolaryngologists at 13%. The lowest rate among the 29 specialties included in the report was 4% for pulmonary medicine.
Differences between physicians, general population
Comparisons with the general U.S. population show that physicians are about twice as likely to report thoughts of suicide (9% vs. 4.9%) and to attempt it (1% vs. 0.5%). Among the overall population, however, “females are two to three times more likely to attempt suicide than males are,” noted Perry Lin, MD, national cochair of the American Association of Suicidology’s Physician Suicide Awareness Committee. That was not the case for survey respondents, as men and women both had an attempt rate of 1% and women were slightly ahead in ideation (11% to 9%).
There was a somewhat larger gap when age group was considered. Among physicians aged 57-75 years, 8% had thought about suicide, compared with 10% of those aged 42-56 years and 12% of respondents aged 27-41. This, again, runs counter to the general population, where older men typically deal with higher suicide rates, Michael F. Myers of the State University of New York, Brooklyn, said in the Medscape report.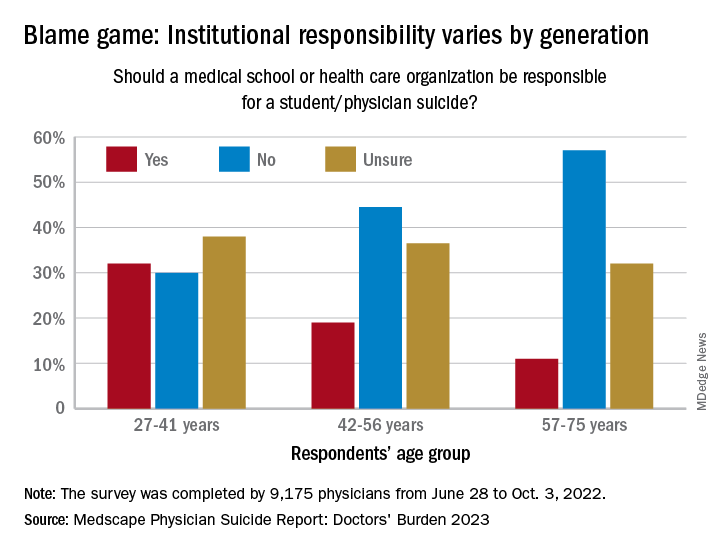
Age also was a factor when responsibility was brought into the equation. Over 30% of the youngest group of respondents (age 27-41) said that medical schools and health care organizations should be held responsible for an individual’s suicide, compared with 19% of those aged 42-56 and 11% of the 57- to 75-year-olds.
That trend was concerning to Dr. Myers: “Most suicides are multifactorial, many stressors coming together all at once in a person’s life, a so-called ‘perfect storm.’ ... But there are suicides each year involving medical students and physicians that have nothing to do with the medical school or place of work.”
Reasons to avoid professional help
Many of the survey respondents also were thinking about third parties when asked why they might not seek professional help for their suicidal thoughts. The most common response, cited by 52%, was that they didn’t need professional help, but 42% didn’t want to risk disclosure to a medical board, 33% were concerned about it being on their insurance record, and 25% were concerned about colleagues finding out.
“Doctors are willing and able to treat suicidal ideation among patients but appear fearful to seek such help themselves. We must do better,” Dr. Lin said in an interview.
Exact numbers of survey respondents were not given by specialty, but about 5% of the 9,175 total responses were completed by ob.gyns. The margin of error for the survey was ±1.02% at the 95% confidence interval.
Obstetricians and gynecologists are more likely than most specialists to have thoughts of suicide, and almost of quarter of physicians in general reported that they were depressed in a recent survey conducted by Medscape.
“Too much work with too little control is a recipe for depression in anyone,” Andrea Giedinghagen, MD, of Washington University, St. Louis, said in the Medscape Physician Suicide Report: Doctors’ Burden 2023. “Physicians are also still coping with a pandemic – the trauma from COVID-19 didn’t disappear just because the full ICUs did – and with a fractured health care system that virtually guarantees moral distress.”
About 23% of the almost 9,200 survey respondents said that they were depressed in 2022, compared with 21% the previous year. Suicide ideation was down in 2022, however, with 9% of all responding physicians reporting contemplation versus 13% in 2021, based on the results of the latest survey, which was conducted from June 28, 2022, to Oct. 2, 2022.
Ob.gyns. were above that average, with 12% reporting suicidal thoughts over the past year, equaling psychiatrists, family physicians, anesthesiologists, and emergency physicians and trailing only the otolaryngologists at 13%. The lowest rate among the 29 specialties included in the report was 4% for pulmonary medicine.
Differences between physicians, general population
Comparisons with the general U.S. population show that physicians are about twice as likely to report thoughts of suicide (9% vs. 4.9%) and to attempt it (1% vs. 0.5%). Among the overall population, however, “females are two to three times more likely to attempt suicide than males are,” noted Perry Lin, MD, national cochair of the American Association of Suicidology’s Physician Suicide Awareness Committee. That was not the case for survey respondents, as men and women both had an attempt rate of 1% and women were slightly ahead in ideation (11% to 9%).
There was a somewhat larger gap when age group was considered. Among physicians aged 57-75 years, 8% had thought about suicide, compared with 10% of those aged 42-56 years and 12% of respondents aged 27-41. This, again, runs counter to the general population, where older men typically deal with higher suicide rates, Michael F. Myers of the State University of New York, Brooklyn, said in the Medscape report.
Age also was a factor when responsibility was brought into the equation. Over 30% of the youngest group of respondents (age 27-41) said that medical schools and health care organizations should be held responsible for an individual’s suicide, compared with 19% of those aged 42-56 and 11% of the 57- to 75-year-olds.
That trend was concerning to Dr. Myers: “Most suicides are multifactorial, many stressors coming together all at once in a person’s life, a so-called ‘perfect storm.’ ... But there are suicides each year involving medical students and physicians that have nothing to do with the medical school or place of work.”
Reasons to avoid professional help
Many of the survey respondents also were thinking about third parties when asked why they might not seek professional help for their suicidal thoughts. The most common response, cited by 52%, was that they didn’t need professional help, but 42% didn’t want to risk disclosure to a medical board, 33% were concerned about it being on their insurance record, and 25% were concerned about colleagues finding out.
“Doctors are willing and able to treat suicidal ideation among patients but appear fearful to seek such help themselves. We must do better,” Dr. Lin said in an interview.
Exact numbers of survey respondents were not given by specialty, but about 5% of the 9,175 total responses were completed by ob.gyns. The margin of error for the survey was ±1.02% at the 95% confidence interval.
Obstetricians and gynecologists are more likely than most specialists to have thoughts of suicide, and almost of quarter of physicians in general reported that they were depressed in a recent survey conducted by Medscape.
“Too much work with too little control is a recipe for depression in anyone,” Andrea Giedinghagen, MD, of Washington University, St. Louis, said in the Medscape Physician Suicide Report: Doctors’ Burden 2023. “Physicians are also still coping with a pandemic – the trauma from COVID-19 didn’t disappear just because the full ICUs did – and with a fractured health care system that virtually guarantees moral distress.”
About 23% of the almost 9,200 survey respondents said that they were depressed in 2022, compared with 21% the previous year. Suicide ideation was down in 2022, however, with 9% of all responding physicians reporting contemplation versus 13% in 2021, based on the results of the latest survey, which was conducted from June 28, 2022, to Oct. 2, 2022.
Ob.gyns. were above that average, with 12% reporting suicidal thoughts over the past year, equaling psychiatrists, family physicians, anesthesiologists, and emergency physicians and trailing only the otolaryngologists at 13%. The lowest rate among the 29 specialties included in the report was 4% for pulmonary medicine.
Differences between physicians, general population
Comparisons with the general U.S. population show that physicians are about twice as likely to report thoughts of suicide (9% vs. 4.9%) and to attempt it (1% vs. 0.5%). Among the overall population, however, “females are two to three times more likely to attempt suicide than males are,” noted Perry Lin, MD, national cochair of the American Association of Suicidology’s Physician Suicide Awareness Committee. That was not the case for survey respondents, as men and women both had an attempt rate of 1% and women were slightly ahead in ideation (11% to 9%).
There was a somewhat larger gap when age group was considered. Among physicians aged 57-75 years, 8% had thought about suicide, compared with 10% of those aged 42-56 years and 12% of respondents aged 27-41. This, again, runs counter to the general population, where older men typically deal with higher suicide rates, Michael F. Myers of the State University of New York, Brooklyn, said in the Medscape report.
Age also was a factor when responsibility was brought into the equation. Over 30% of the youngest group of respondents (age 27-41) said that medical schools and health care organizations should be held responsible for an individual’s suicide, compared with 19% of those aged 42-56 and 11% of the 57- to 75-year-olds.
That trend was concerning to Dr. Myers: “Most suicides are multifactorial, many stressors coming together all at once in a person’s life, a so-called ‘perfect storm.’ ... But there are suicides each year involving medical students and physicians that have nothing to do with the medical school or place of work.”
Reasons to avoid professional help
Many of the survey respondents also were thinking about third parties when asked why they might not seek professional help for their suicidal thoughts. The most common response, cited by 52%, was that they didn’t need professional help, but 42% didn’t want to risk disclosure to a medical board, 33% were concerned about it being on their insurance record, and 25% were concerned about colleagues finding out.
“Doctors are willing and able to treat suicidal ideation among patients but appear fearful to seek such help themselves. We must do better,” Dr. Lin said in an interview.
Exact numbers of survey respondents were not given by specialty, but about 5% of the 9,175 total responses were completed by ob.gyns. The margin of error for the survey was ±1.02% at the 95% confidence interval.
Ob.gyn. loses PhD after committee finds he made up research
It was déjà vu last month when a university in Belgium stripped Egyptian physician Hatem Abu Hashim of his doctorate after he was found to have fabricated data in his thesis.
Just weeks earlier, another Egyptian doctor, Ahmed Badawy, lost the PhD degree he had earned at a Dutch university in 2008. Abu Hashim and Badawy are both professors in the department of obstetrics and gynecology at Mansoura University in Egypt.
According to an investigation by the Vrije Universeit Brussel (VUB), which awarded Abu Hashim his PhD in 2013, the researcher was in “serious violation of scientific integrity” based on “overwhelming evidence of fabrication of statistical outcomes” and “clear lack of statistical proficiency.”
Ben Mol of Monash University in Australia, a researcher turned data sleuth who alerted VUB and Utrecht University to problems with Abu Hashim and Badawy ‘s research in 2021 and 2020, respectively, told Retraction Watch by email, “The good news is obviously that there is a firm conclusion from both universities after a robust process independent of the complaint.”
Mol also laid out his concerns in a study published with then-PhD student Esmée Bordewijk and others in 2020, as Retraction Watch reported that year.
“Yes, it could have been a bit faster, but on the other hand we have this conversation because they took the right decision,” he added.
Abu Hashim’s PhD thesis is based on 11 randomized controlled trials, all of which have been published. Ostensibly, the studies were done at Mansoura University before Abu Hashim enrolled as an external PhD candidate at VUB.
A report from the Flemish Commission for Scientific Integrity, which gave a second opinion on the VUB findings following a request from Abu Hashim, offers a “credible” scenario for how the 11 papers came about, suggesting “that Abu Hashim had learned to write medical papers by reading others, that he made up all reported values and that he wrote more papers by adapting previous papers, copying results between articles and applying small alterations (+1 or -1 in some digits).”
The commission agreed with VUB that “complete (or virtually complete) fabrication is the only reasonable explanation for the findings.” It also noted that “strikingly,” the researcher did not address any of the allegations against him:
“To the contrary, his defence consists mainly of accusing those bringing forward the complaint of misconduct and questioning their work and methods.”
Neither Abu Hashim nor Mansoura University responded to requests for comment.
The school, however, has known about Abu Hashim’s fraudulent research for a decade. In an internal investigation from 2014, then-head of department Nasser El Lakany and five other professors found that one of the researcher’s trials had never been done; six trials included an impossibly large number of women with polycystic ovary syndrome; and two reported 366 ovarian-drilling procedures while records were found to exist only for 94. The latter two groups of studies formed part of Abu Hashim’s PhD thesis.
“There is no excuse for the researcher’ [sic] misconduct (fabricating imaginary data and studies not done at all, or studies with doubtful cases not in records),” the Mansoura professors wrote, according to an English translation of the original Arabic report.
In 2021, sleuth Nick Brown also began poring over the Egyptian researchers’ work after a Dutch journalist requested his opinion.
“People don’t read papers. They read the abstract. They say, congratulations, great paper. And then they go back to what they were doing the rest of their day because reading a paper is quite hard,” Brown told Retraction Watch. “I’m not very good at statistics, but I can read a table and things jump out at me.”
Brown quickly realized that Badawy and Abu Hashim’s publications were littered with “fatal flaws.” Virtually all of the P-values were wrong. In some cases, they exceeded 1 – a mathematical impossibility. In others, vastly different values were given for identical statistical tests that by definition should have yielded the same results.
“I assume the authors were just making up ‘likely-looking’ numbers in a hurry and didn’t realise that these needed to be identical,” Brown said in an email. “We often find that people who cheat are not very good at knowing what genuine numbers should look like.”
Brown, who himself has an external PhD from a Dutch university, noted that institutions receive the same amount of money from the government whether a PhD candidate is external or internal:
“So someone comes along with some papers already done. They need to write a top and tail of a thesis. They’re probably not going to need a whole lot of supervision. Exactly how many questions do you ask?”
A spokesperson for Utrecht University told Retraction Watch by email:
“We have asked ourselves the question how this could have happened. Why did the supervisor and the Doctoral Examination Committee not notice this? The articles that were the basis for the thesis, were published in peer reviewed journals. Only much later it came to light that the data underlying these articles had been compromised.”
She added that the rules for external PhD candidates have been tightened since 2008, when Badawy obtained his degree (the changes are described here).
Sam Jaspers, a VUB press officer, told us, “the Vrije Universiteit Brussel is updating its PhD regulations. External PhD students working with existing datasets created at a university other than the VUB and publications reviewed by scientific journals will soon (this spring) be fully audited by the VUB.”
Meanwhile, Mol, whose work on various cases recently featured in The Economist, worries about all the fake studies that have not yet been retracted, and the impact they might have on patient care.
“I cannot understand that ... three years after our publication of the Bordewijk study, still half of the Badawy and Abu Hashim studies are out there even without an expression of concern,” he said. “What ideally should happen is that there should be a mechanism that all the journals and publishers bundle their investigation.”
A version of this article first appeared on retractionwatch.com.
It was déjà vu last month when a university in Belgium stripped Egyptian physician Hatem Abu Hashim of his doctorate after he was found to have fabricated data in his thesis.
Just weeks earlier, another Egyptian doctor, Ahmed Badawy, lost the PhD degree he had earned at a Dutch university in 2008. Abu Hashim and Badawy are both professors in the department of obstetrics and gynecology at Mansoura University in Egypt.
According to an investigation by the Vrije Universeit Brussel (VUB), which awarded Abu Hashim his PhD in 2013, the researcher was in “serious violation of scientific integrity” based on “overwhelming evidence of fabrication of statistical outcomes” and “clear lack of statistical proficiency.”
Ben Mol of Monash University in Australia, a researcher turned data sleuth who alerted VUB and Utrecht University to problems with Abu Hashim and Badawy ‘s research in 2021 and 2020, respectively, told Retraction Watch by email, “The good news is obviously that there is a firm conclusion from both universities after a robust process independent of the complaint.”
Mol also laid out his concerns in a study published with then-PhD student Esmée Bordewijk and others in 2020, as Retraction Watch reported that year.
“Yes, it could have been a bit faster, but on the other hand we have this conversation because they took the right decision,” he added.
Abu Hashim’s PhD thesis is based on 11 randomized controlled trials, all of which have been published. Ostensibly, the studies were done at Mansoura University before Abu Hashim enrolled as an external PhD candidate at VUB.
A report from the Flemish Commission for Scientific Integrity, which gave a second opinion on the VUB findings following a request from Abu Hashim, offers a “credible” scenario for how the 11 papers came about, suggesting “that Abu Hashim had learned to write medical papers by reading others, that he made up all reported values and that he wrote more papers by adapting previous papers, copying results between articles and applying small alterations (+1 or -1 in some digits).”
The commission agreed with VUB that “complete (or virtually complete) fabrication is the only reasonable explanation for the findings.” It also noted that “strikingly,” the researcher did not address any of the allegations against him:
“To the contrary, his defence consists mainly of accusing those bringing forward the complaint of misconduct and questioning their work and methods.”
Neither Abu Hashim nor Mansoura University responded to requests for comment.
The school, however, has known about Abu Hashim’s fraudulent research for a decade. In an internal investigation from 2014, then-head of department Nasser El Lakany and five other professors found that one of the researcher’s trials had never been done; six trials included an impossibly large number of women with polycystic ovary syndrome; and two reported 366 ovarian-drilling procedures while records were found to exist only for 94. The latter two groups of studies formed part of Abu Hashim’s PhD thesis.
“There is no excuse for the researcher’ [sic] misconduct (fabricating imaginary data and studies not done at all, or studies with doubtful cases not in records),” the Mansoura professors wrote, according to an English translation of the original Arabic report.
In 2021, sleuth Nick Brown also began poring over the Egyptian researchers’ work after a Dutch journalist requested his opinion.
“People don’t read papers. They read the abstract. They say, congratulations, great paper. And then they go back to what they were doing the rest of their day because reading a paper is quite hard,” Brown told Retraction Watch. “I’m not very good at statistics, but I can read a table and things jump out at me.”
Brown quickly realized that Badawy and Abu Hashim’s publications were littered with “fatal flaws.” Virtually all of the P-values were wrong. In some cases, they exceeded 1 – a mathematical impossibility. In others, vastly different values were given for identical statistical tests that by definition should have yielded the same results.
“I assume the authors were just making up ‘likely-looking’ numbers in a hurry and didn’t realise that these needed to be identical,” Brown said in an email. “We often find that people who cheat are not very good at knowing what genuine numbers should look like.”
Brown, who himself has an external PhD from a Dutch university, noted that institutions receive the same amount of money from the government whether a PhD candidate is external or internal:
“So someone comes along with some papers already done. They need to write a top and tail of a thesis. They’re probably not going to need a whole lot of supervision. Exactly how many questions do you ask?”
A spokesperson for Utrecht University told Retraction Watch by email:
“We have asked ourselves the question how this could have happened. Why did the supervisor and the Doctoral Examination Committee not notice this? The articles that were the basis for the thesis, were published in peer reviewed journals. Only much later it came to light that the data underlying these articles had been compromised.”
She added that the rules for external PhD candidates have been tightened since 2008, when Badawy obtained his degree (the changes are described here).
Sam Jaspers, a VUB press officer, told us, “the Vrije Universiteit Brussel is updating its PhD regulations. External PhD students working with existing datasets created at a university other than the VUB and publications reviewed by scientific journals will soon (this spring) be fully audited by the VUB.”
Meanwhile, Mol, whose work on various cases recently featured in The Economist, worries about all the fake studies that have not yet been retracted, and the impact they might have on patient care.
“I cannot understand that ... three years after our publication of the Bordewijk study, still half of the Badawy and Abu Hashim studies are out there even without an expression of concern,” he said. “What ideally should happen is that there should be a mechanism that all the journals and publishers bundle their investigation.”
A version of this article first appeared on retractionwatch.com.
It was déjà vu last month when a university in Belgium stripped Egyptian physician Hatem Abu Hashim of his doctorate after he was found to have fabricated data in his thesis.
Just weeks earlier, another Egyptian doctor, Ahmed Badawy, lost the PhD degree he had earned at a Dutch university in 2008. Abu Hashim and Badawy are both professors in the department of obstetrics and gynecology at Mansoura University in Egypt.
According to an investigation by the Vrije Universeit Brussel (VUB), which awarded Abu Hashim his PhD in 2013, the researcher was in “serious violation of scientific integrity” based on “overwhelming evidence of fabrication of statistical outcomes” and “clear lack of statistical proficiency.”
Ben Mol of Monash University in Australia, a researcher turned data sleuth who alerted VUB and Utrecht University to problems with Abu Hashim and Badawy ‘s research in 2021 and 2020, respectively, told Retraction Watch by email, “The good news is obviously that there is a firm conclusion from both universities after a robust process independent of the complaint.”
Mol also laid out his concerns in a study published with then-PhD student Esmée Bordewijk and others in 2020, as Retraction Watch reported that year.
“Yes, it could have been a bit faster, but on the other hand we have this conversation because they took the right decision,” he added.
Abu Hashim’s PhD thesis is based on 11 randomized controlled trials, all of which have been published. Ostensibly, the studies were done at Mansoura University before Abu Hashim enrolled as an external PhD candidate at VUB.
A report from the Flemish Commission for Scientific Integrity, which gave a second opinion on the VUB findings following a request from Abu Hashim, offers a “credible” scenario for how the 11 papers came about, suggesting “that Abu Hashim had learned to write medical papers by reading others, that he made up all reported values and that he wrote more papers by adapting previous papers, copying results between articles and applying small alterations (+1 or -1 in some digits).”
The commission agreed with VUB that “complete (or virtually complete) fabrication is the only reasonable explanation for the findings.” It also noted that “strikingly,” the researcher did not address any of the allegations against him:
“To the contrary, his defence consists mainly of accusing those bringing forward the complaint of misconduct and questioning their work and methods.”
Neither Abu Hashim nor Mansoura University responded to requests for comment.
The school, however, has known about Abu Hashim’s fraudulent research for a decade. In an internal investigation from 2014, then-head of department Nasser El Lakany and five other professors found that one of the researcher’s trials had never been done; six trials included an impossibly large number of women with polycystic ovary syndrome; and two reported 366 ovarian-drilling procedures while records were found to exist only for 94. The latter two groups of studies formed part of Abu Hashim’s PhD thesis.
“There is no excuse for the researcher’ [sic] misconduct (fabricating imaginary data and studies not done at all, or studies with doubtful cases not in records),” the Mansoura professors wrote, according to an English translation of the original Arabic report.
In 2021, sleuth Nick Brown also began poring over the Egyptian researchers’ work after a Dutch journalist requested his opinion.
“People don’t read papers. They read the abstract. They say, congratulations, great paper. And then they go back to what they were doing the rest of their day because reading a paper is quite hard,” Brown told Retraction Watch. “I’m not very good at statistics, but I can read a table and things jump out at me.”
Brown quickly realized that Badawy and Abu Hashim’s publications were littered with “fatal flaws.” Virtually all of the P-values were wrong. In some cases, they exceeded 1 – a mathematical impossibility. In others, vastly different values were given for identical statistical tests that by definition should have yielded the same results.
“I assume the authors were just making up ‘likely-looking’ numbers in a hurry and didn’t realise that these needed to be identical,” Brown said in an email. “We often find that people who cheat are not very good at knowing what genuine numbers should look like.”
Brown, who himself has an external PhD from a Dutch university, noted that institutions receive the same amount of money from the government whether a PhD candidate is external or internal:
“So someone comes along with some papers already done. They need to write a top and tail of a thesis. They’re probably not going to need a whole lot of supervision. Exactly how many questions do you ask?”
A spokesperson for Utrecht University told Retraction Watch by email:
“We have asked ourselves the question how this could have happened. Why did the supervisor and the Doctoral Examination Committee not notice this? The articles that were the basis for the thesis, were published in peer reviewed journals. Only much later it came to light that the data underlying these articles had been compromised.”
She added that the rules for external PhD candidates have been tightened since 2008, when Badawy obtained his degree (the changes are described here).
Sam Jaspers, a VUB press officer, told us, “the Vrije Universiteit Brussel is updating its PhD regulations. External PhD students working with existing datasets created at a university other than the VUB and publications reviewed by scientific journals will soon (this spring) be fully audited by the VUB.”
Meanwhile, Mol, whose work on various cases recently featured in The Economist, worries about all the fake studies that have not yet been retracted, and the impact they might have on patient care.
“I cannot understand that ... three years after our publication of the Bordewijk study, still half of the Badawy and Abu Hashim studies are out there even without an expression of concern,” he said. “What ideally should happen is that there should be a mechanism that all the journals and publishers bundle their investigation.”
A version of this article first appeared on retractionwatch.com.
Telehealth doctor indicted on health care fraud, opioid distribution charges
Sangita Patel, MD, 50, practiced at Advance Medical Home Physicians in Troy.
According to court documents, between July 2020 and June 2022 Patel was responsible for submitting Medicare claims for improper telehealth visits she didn’t conduct herself.
Dr. Patel, who accepted patients who paid in cash as well as those with Medicare and Medicaid coverage, billed approximately $3.4 million to Medicare between 2018 and 2022, according to court documents. An unusual number of these visits were billed using complex codes, an indication of health care fraud. The investigation also found that on many days, Dr. Patel billed for more than 24 hours of services. During this period, according to the document, 76% of Dr. Patel’s Medicare reimbursements were for telehealth.
Prosecutors say that Dr. Patel prescribed Schedule II controlled substances to more than 90% of the patients in these telehealth visits. She delegated her prescription authority to an unlicensed medical assistant. Through undercover visits and cell site search warrant data, the investigation found that Dr. Patel directed patients to contact, via cell phone, this assistant, who then entered electronic prescriptions into the electronic medical records system. Dr. Patel then signed the prescriptions and sent them to the pharmacies without ever interacting with the patients. Prosecutors also used text messages, obtained by search warrant, between Dr. Patel and her assistant and between the assistant and undercover informers to build their case.
Dr. Patel is also accused of referring patients to other providers, who in turn billed Medicare for claims associated with those patients. Advance Medical received $143,000 from these providers, potentially in violation of anti-kickback laws, according to bank records obtained by subpoena.
If convicted, Dr. Patel could be sentenced to up to 10 years in federal prison.
A version of this article first appeared on Medscape.com.
Sangita Patel, MD, 50, practiced at Advance Medical Home Physicians in Troy.
According to court documents, between July 2020 and June 2022 Patel was responsible for submitting Medicare claims for improper telehealth visits she didn’t conduct herself.
Dr. Patel, who accepted patients who paid in cash as well as those with Medicare and Medicaid coverage, billed approximately $3.4 million to Medicare between 2018 and 2022, according to court documents. An unusual number of these visits were billed using complex codes, an indication of health care fraud. The investigation also found that on many days, Dr. Patel billed for more than 24 hours of services. During this period, according to the document, 76% of Dr. Patel’s Medicare reimbursements were for telehealth.
Prosecutors say that Dr. Patel prescribed Schedule II controlled substances to more than 90% of the patients in these telehealth visits. She delegated her prescription authority to an unlicensed medical assistant. Through undercover visits and cell site search warrant data, the investigation found that Dr. Patel directed patients to contact, via cell phone, this assistant, who then entered electronic prescriptions into the electronic medical records system. Dr. Patel then signed the prescriptions and sent them to the pharmacies without ever interacting with the patients. Prosecutors also used text messages, obtained by search warrant, between Dr. Patel and her assistant and between the assistant and undercover informers to build their case.
Dr. Patel is also accused of referring patients to other providers, who in turn billed Medicare for claims associated with those patients. Advance Medical received $143,000 from these providers, potentially in violation of anti-kickback laws, according to bank records obtained by subpoena.
If convicted, Dr. Patel could be sentenced to up to 10 years in federal prison.
A version of this article first appeared on Medscape.com.
Sangita Patel, MD, 50, practiced at Advance Medical Home Physicians in Troy.
According to court documents, between July 2020 and June 2022 Patel was responsible for submitting Medicare claims for improper telehealth visits she didn’t conduct herself.
Dr. Patel, who accepted patients who paid in cash as well as those with Medicare and Medicaid coverage, billed approximately $3.4 million to Medicare between 2018 and 2022, according to court documents. An unusual number of these visits were billed using complex codes, an indication of health care fraud. The investigation also found that on many days, Dr. Patel billed for more than 24 hours of services. During this period, according to the document, 76% of Dr. Patel’s Medicare reimbursements were for telehealth.
Prosecutors say that Dr. Patel prescribed Schedule II controlled substances to more than 90% of the patients in these telehealth visits. She delegated her prescription authority to an unlicensed medical assistant. Through undercover visits and cell site search warrant data, the investigation found that Dr. Patel directed patients to contact, via cell phone, this assistant, who then entered electronic prescriptions into the electronic medical records system. Dr. Patel then signed the prescriptions and sent them to the pharmacies without ever interacting with the patients. Prosecutors also used text messages, obtained by search warrant, between Dr. Patel and her assistant and between the assistant and undercover informers to build their case.
Dr. Patel is also accused of referring patients to other providers, who in turn billed Medicare for claims associated with those patients. Advance Medical received $143,000 from these providers, potentially in violation of anti-kickback laws, according to bank records obtained by subpoena.
If convicted, Dr. Patel could be sentenced to up to 10 years in federal prison.
A version of this article first appeared on Medscape.com.
What’s it like to take Ozempic? A doctor’s own story
With the rising popularity of weight-loss drug injections, I’ve received many questions from patients about the pros, cons, and costs. While Ozempic (semaglutide) is perhaps the best known, it’s technically an agent approved only for type 2 diabetes that has been used off label for obesity. The same substance, semaglutide, is approved for use in obesity, but at a higher dose, under the brand name Wegovy. Alternatives are available, and results will vary depending on the specific agent used and the individual.
Ultimately, I decided to try these new injections for myself. I am not a paid representative for, nor an advocate of, any of these medications; I’m here only to share my personal experience.
In my discussions with patients about weight, I sometimes felt like an imposter. While I was overweight by medical standards, I fortunately had none of the underlying health problems. I wasn’t on medications for blood pressure nor did I have diabetes, but I was counseling people to lose weight and eat better while not always following my own advice.
Since having children and turning 40, my metabolism, like many other women’s, seems to have plummeted. I tried a number of older weight-loss medications, like phentermine and phendimetrazine, under the supervision of medical professionals.
Each time, the efforts worked for a short while, particularly when I followed good portion control and practiced moderate exercise. Once the side effects (that is, tachycardia, palpitations, mood changes, constipation) became intolerable, or I became tired or fearful of being on the medications too long, I’d stop and I would regain some of the weight.
When the newer subcutaneous injectable medications arrived on the scene and I started to talk to my patients about them, I was intrigued by their novel mode of action and seeming benefits.
These medications, glucagonlike peptide–1 (GLP-1) receptor agonists, were first approved for type 2 diabetes, and it soon became apparent that patients were losing significant amounts of weight taking them, so manufacturers conducted further trials in obesity patients without type 2 diabetes.
The first of these, liraglutide, is injected daily and was first approved as Victoza for type 2 diabetes; it later received an additional approval for obesity, in December 2014, as Saxenda.
Semaglutide, another of the new GLP-1 agonists, was first approved for type 2 diabetes as Ozempic but again was found to lead to substantial weight loss, so a subsequent approval of the drug for obesity, as Wegovy, came in June 2021. Semaglutide is injected once a week.
Semaglutide was branded a “game changer” when it was licensed for obesity because the mean weight loss seen in trials was around 15%, more than for any other drug and approaching what could be achieved with bariatric surgery, some doctors said.
These medications work in a different way from the older weight loss drugs, which had focused on the use of amphetamines. The newer medications became very popular because treating obesity helps lower blood glucose, blood pressure, cholesterol, kidney disease risk, and other comorbidities that occur with diabetes. Plus, for most people, there were fewer side effects.
I first tried Saxenda when it arrived on the market, via some samples that our pharmaceutical representative brought, both out of curiosity and to see if it would help me lose the stubborn baby weight. I ended up stopping the daily injections after my second or third week because of nausea and vomiting. I took a break, got a prescription for antinausea medicine, and tried again because it did indeed decrease my appetite. However, when I took my prescription to the pharmacy, my insurance wouldn’t cover it. It happens to doctors, too.
Fast-forward to 2017-2018. The baby weight was still holding on despite lifestyle changes, diet, and exercising. The newer drug classes hit the market, and again we had samples from our reps.
When Ozempic was on backorder, I switched to a low dose of Mounjaro (tirzepatide), a new dual GLP-1 and glucose-dependent insulinotropic polypeptide agonist, approved for type 2 diabetes in May 2022, again using it off label as a weekly injection, as it isn’t currently approved for weight loss. However, it does produce significant weight loss and is awaiting approval for obesity.
With these new medications, I noticed that both my patients and I didn’t complain as much about nausea and vomiting, but I did experience stomach upset, constipation, and acid reflux.
The appetite suppression is effective. It slows down the emptying of the gut so I feel full longer. I’ve lost 30 lb with these weekly injections and would like to lose another 20 lb. I follow a routine of reasonable, portion-controlled eating and moderate exercise (30 minutes of cardiovascular activity at least two to three times a week).
Discontinuing the medications may cause rebound weight gain, especially if I’m no longer following a routine of healthy eating and/or moderate exercise. I deal with minimal constipation by taking stool softeners, and I take antacids for acid reflux.
Here’s what I recommend applying when working with patients who have obesity: First, explain how these medications work. Then conduct a health history to make sure these injections are right for them. Patients with a family history of pancreatic cancer can’t take these medications. You also want to monitor use in patients with a history of hypoglycemia so their blood sugar doesn’t drop too low. It’s also important to make sure your patients are able to afford the medication. My husband takes Ozempic for diabetes, and recently we were told that a refill would cost about $1,500 a month, even with insurance. “Covered” doesn’t necessarily mean affordable.
Take a baseline hemoglobin A1c and repeat it after the patient has been on the medication for 2-3 weeks. Also remind them that they can’t rely solely on the medication but need to practice portion control and healthier eating and to exercise more.
For myself, I want to lose those remaining 20 lb or so by eating healthy and being physically active without having to rely on medication for the rest of my life. Research on these medications is still early so we don’t know the long-term effects yet.
As clinicians, I feel it’s okay to be honest with our patients about our own personal struggles to help them understand that they are not alone and that losing weight is a challenge for everyone.
Dr. Swiner is a family physician in Durham, N.C. She reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
With the rising popularity of weight-loss drug injections, I’ve received many questions from patients about the pros, cons, and costs. While Ozempic (semaglutide) is perhaps the best known, it’s technically an agent approved only for type 2 diabetes that has been used off label for obesity. The same substance, semaglutide, is approved for use in obesity, but at a higher dose, under the brand name Wegovy. Alternatives are available, and results will vary depending on the specific agent used and the individual.
Ultimately, I decided to try these new injections for myself. I am not a paid representative for, nor an advocate of, any of these medications; I’m here only to share my personal experience.
In my discussions with patients about weight, I sometimes felt like an imposter. While I was overweight by medical standards, I fortunately had none of the underlying health problems. I wasn’t on medications for blood pressure nor did I have diabetes, but I was counseling people to lose weight and eat better while not always following my own advice.
Since having children and turning 40, my metabolism, like many other women’s, seems to have plummeted. I tried a number of older weight-loss medications, like phentermine and phendimetrazine, under the supervision of medical professionals.
Each time, the efforts worked for a short while, particularly when I followed good portion control and practiced moderate exercise. Once the side effects (that is, tachycardia, palpitations, mood changes, constipation) became intolerable, or I became tired or fearful of being on the medications too long, I’d stop and I would regain some of the weight.
When the newer subcutaneous injectable medications arrived on the scene and I started to talk to my patients about them, I was intrigued by their novel mode of action and seeming benefits.
These medications, glucagonlike peptide–1 (GLP-1) receptor agonists, were first approved for type 2 diabetes, and it soon became apparent that patients were losing significant amounts of weight taking them, so manufacturers conducted further trials in obesity patients without type 2 diabetes.
The first of these, liraglutide, is injected daily and was first approved as Victoza for type 2 diabetes; it later received an additional approval for obesity, in December 2014, as Saxenda.
Semaglutide, another of the new GLP-1 agonists, was first approved for type 2 diabetes as Ozempic but again was found to lead to substantial weight loss, so a subsequent approval of the drug for obesity, as Wegovy, came in June 2021. Semaglutide is injected once a week.
Semaglutide was branded a “game changer” when it was licensed for obesity because the mean weight loss seen in trials was around 15%, more than for any other drug and approaching what could be achieved with bariatric surgery, some doctors said.
These medications work in a different way from the older weight loss drugs, which had focused on the use of amphetamines. The newer medications became very popular because treating obesity helps lower blood glucose, blood pressure, cholesterol, kidney disease risk, and other comorbidities that occur with diabetes. Plus, for most people, there were fewer side effects.
I first tried Saxenda when it arrived on the market, via some samples that our pharmaceutical representative brought, both out of curiosity and to see if it would help me lose the stubborn baby weight. I ended up stopping the daily injections after my second or third week because of nausea and vomiting. I took a break, got a prescription for antinausea medicine, and tried again because it did indeed decrease my appetite. However, when I took my prescription to the pharmacy, my insurance wouldn’t cover it. It happens to doctors, too.
Fast-forward to 2017-2018. The baby weight was still holding on despite lifestyle changes, diet, and exercising. The newer drug classes hit the market, and again we had samples from our reps.
When Ozempic was on backorder, I switched to a low dose of Mounjaro (tirzepatide), a new dual GLP-1 and glucose-dependent insulinotropic polypeptide agonist, approved for type 2 diabetes in May 2022, again using it off label as a weekly injection, as it isn’t currently approved for weight loss. However, it does produce significant weight loss and is awaiting approval for obesity.
With these new medications, I noticed that both my patients and I didn’t complain as much about nausea and vomiting, but I did experience stomach upset, constipation, and acid reflux.
The appetite suppression is effective. It slows down the emptying of the gut so I feel full longer. I’ve lost 30 lb with these weekly injections and would like to lose another 20 lb. I follow a routine of reasonable, portion-controlled eating and moderate exercise (30 minutes of cardiovascular activity at least two to three times a week).
Discontinuing the medications may cause rebound weight gain, especially if I’m no longer following a routine of healthy eating and/or moderate exercise. I deal with minimal constipation by taking stool softeners, and I take antacids for acid reflux.
Here’s what I recommend applying when working with patients who have obesity: First, explain how these medications work. Then conduct a health history to make sure these injections are right for them. Patients with a family history of pancreatic cancer can’t take these medications. You also want to monitor use in patients with a history of hypoglycemia so their blood sugar doesn’t drop too low. It’s also important to make sure your patients are able to afford the medication. My husband takes Ozempic for diabetes, and recently we were told that a refill would cost about $1,500 a month, even with insurance. “Covered” doesn’t necessarily mean affordable.
Take a baseline hemoglobin A1c and repeat it after the patient has been on the medication for 2-3 weeks. Also remind them that they can’t rely solely on the medication but need to practice portion control and healthier eating and to exercise more.
For myself, I want to lose those remaining 20 lb or so by eating healthy and being physically active without having to rely on medication for the rest of my life. Research on these medications is still early so we don’t know the long-term effects yet.
As clinicians, I feel it’s okay to be honest with our patients about our own personal struggles to help them understand that they are not alone and that losing weight is a challenge for everyone.
Dr. Swiner is a family physician in Durham, N.C. She reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
With the rising popularity of weight-loss drug injections, I’ve received many questions from patients about the pros, cons, and costs. While Ozempic (semaglutide) is perhaps the best known, it’s technically an agent approved only for type 2 diabetes that has been used off label for obesity. The same substance, semaglutide, is approved for use in obesity, but at a higher dose, under the brand name Wegovy. Alternatives are available, and results will vary depending on the specific agent used and the individual.
Ultimately, I decided to try these new injections for myself. I am not a paid representative for, nor an advocate of, any of these medications; I’m here only to share my personal experience.
In my discussions with patients about weight, I sometimes felt like an imposter. While I was overweight by medical standards, I fortunately had none of the underlying health problems. I wasn’t on medications for blood pressure nor did I have diabetes, but I was counseling people to lose weight and eat better while not always following my own advice.
Since having children and turning 40, my metabolism, like many other women’s, seems to have plummeted. I tried a number of older weight-loss medications, like phentermine and phendimetrazine, under the supervision of medical professionals.
Each time, the efforts worked for a short while, particularly when I followed good portion control and practiced moderate exercise. Once the side effects (that is, tachycardia, palpitations, mood changes, constipation) became intolerable, or I became tired or fearful of being on the medications too long, I’d stop and I would regain some of the weight.
When the newer subcutaneous injectable medications arrived on the scene and I started to talk to my patients about them, I was intrigued by their novel mode of action and seeming benefits.
These medications, glucagonlike peptide–1 (GLP-1) receptor agonists, were first approved for type 2 diabetes, and it soon became apparent that patients were losing significant amounts of weight taking them, so manufacturers conducted further trials in obesity patients without type 2 diabetes.
The first of these, liraglutide, is injected daily and was first approved as Victoza for type 2 diabetes; it later received an additional approval for obesity, in December 2014, as Saxenda.
Semaglutide, another of the new GLP-1 agonists, was first approved for type 2 diabetes as Ozempic but again was found to lead to substantial weight loss, so a subsequent approval of the drug for obesity, as Wegovy, came in June 2021. Semaglutide is injected once a week.
Semaglutide was branded a “game changer” when it was licensed for obesity because the mean weight loss seen in trials was around 15%, more than for any other drug and approaching what could be achieved with bariatric surgery, some doctors said.
These medications work in a different way from the older weight loss drugs, which had focused on the use of amphetamines. The newer medications became very popular because treating obesity helps lower blood glucose, blood pressure, cholesterol, kidney disease risk, and other comorbidities that occur with diabetes. Plus, for most people, there were fewer side effects.
I first tried Saxenda when it arrived on the market, via some samples that our pharmaceutical representative brought, both out of curiosity and to see if it would help me lose the stubborn baby weight. I ended up stopping the daily injections after my second or third week because of nausea and vomiting. I took a break, got a prescription for antinausea medicine, and tried again because it did indeed decrease my appetite. However, when I took my prescription to the pharmacy, my insurance wouldn’t cover it. It happens to doctors, too.
Fast-forward to 2017-2018. The baby weight was still holding on despite lifestyle changes, diet, and exercising. The newer drug classes hit the market, and again we had samples from our reps.
When Ozempic was on backorder, I switched to a low dose of Mounjaro (tirzepatide), a new dual GLP-1 and glucose-dependent insulinotropic polypeptide agonist, approved for type 2 diabetes in May 2022, again using it off label as a weekly injection, as it isn’t currently approved for weight loss. However, it does produce significant weight loss and is awaiting approval for obesity.
With these new medications, I noticed that both my patients and I didn’t complain as much about nausea and vomiting, but I did experience stomach upset, constipation, and acid reflux.
The appetite suppression is effective. It slows down the emptying of the gut so I feel full longer. I’ve lost 30 lb with these weekly injections and would like to lose another 20 lb. I follow a routine of reasonable, portion-controlled eating and moderate exercise (30 minutes of cardiovascular activity at least two to three times a week).
Discontinuing the medications may cause rebound weight gain, especially if I’m no longer following a routine of healthy eating and/or moderate exercise. I deal with minimal constipation by taking stool softeners, and I take antacids for acid reflux.
Here’s what I recommend applying when working with patients who have obesity: First, explain how these medications work. Then conduct a health history to make sure these injections are right for them. Patients with a family history of pancreatic cancer can’t take these medications. You also want to monitor use in patients with a history of hypoglycemia so their blood sugar doesn’t drop too low. It’s also important to make sure your patients are able to afford the medication. My husband takes Ozempic for diabetes, and recently we were told that a refill would cost about $1,500 a month, even with insurance. “Covered” doesn’t necessarily mean affordable.
Take a baseline hemoglobin A1c and repeat it after the patient has been on the medication for 2-3 weeks. Also remind them that they can’t rely solely on the medication but need to practice portion control and healthier eating and to exercise more.
For myself, I want to lose those remaining 20 lb or so by eating healthy and being physically active without having to rely on medication for the rest of my life. Research on these medications is still early so we don’t know the long-term effects yet.
As clinicians, I feel it’s okay to be honest with our patients about our own personal struggles to help them understand that they are not alone and that losing weight is a challenge for everyone.
Dr. Swiner is a family physician in Durham, N.C. She reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
New documentary highlights human toll of high insulin cost
A new documentary premiering at the 2023 South by Southwest (SXSW) Festival illustrates the human consequences of insulin’s high cost in the United States. Its creators hope that it will help spur action toward overall prescription pricing reform.
Pay or Die: A Documentary is scheduled to premiere March 11. It will be shown twice more during the festival, which runs from March 10 to 19 in Austin, Texas. The documentary was co-created and directed by filmmaker and cinematographer Scott Alexander Ruderman, who has type 1 diabetes, and his partner, producer and journalist Rachael Dyer. One of the executive producers is Sarah Silverman, a comic, actor, producer, and health care reform advocate.
The 90-minute film follows three human stories: A mother and young daughter who both have type 1 diabetes and become homeless after spending their rent money on insulin, a young adult diagnosed during the COVID-19 pandemic, and a mother whose 26-year-old son died from diabetic ketoacidosis (DKA) after his insulin was rationed.
“As an Australian now living in the U.S. and seeing how the health care system works here, especially for people with type 1 diabetes like Scott, and how access to insulin is a life-or-death situation, has been very eye-opening for me. I’m also half Canadian, and both are countries where access to health care is a human right, not a business,” Ms. Dyer said in an interview.
In response to the March 1 announcement from Eli Lilly about its insulin price cut, the film’s team told this news organization: “While we commend Eli Lilly in taking this first step and hope that Novo Nordisk and Sanofi [the two other major insulin manufacturers] follow suit, it is important to remember that the key issue is not about these companies voluntarily slashing prices; it’s about changing laws so the insulin manufacturers do not have the ability to raise the prices again.
“This is the life-or-death issue that we focus on in our documentary Pay or Die. It’s also important to note that insulin is just one of the many expensive prescription drugs in the U.S., which is why we need to call for reform. Affordable medication needs to be a basic human right within reach for all Americans.”
Physician perspective: Good news on insulin, but broader issues
The film features four physicians. One, Mayo Clinic oncologist/hematologist S. Vincent Rajkumar, MD, has spoken and published widely on insulin prices specifically and U.S. drug costs more broadly.
The other three are Joslin Clinic endocrinologist Elizabeth Halprin, MD, Massachusetts General Hospital internist Leigh Simmons, MD, and New York University physician and essayist Danielle Ofri, MD, PhD.
In an interview after the Lilly announcement, Dr. Rajkumar said, “I think this is very, very good news for patients. ... The fact that they’re doing it means they’re listening to us and listening to patients, which is good. And I do hope that other insulin manufacturers do the same shortly.”
However, he added, “for prescription drug prices and particularly cancer drug prices, there’s more reform that’s needed, and that’s at the policy level. ... The goal of the film was to use insulin to highlight the prescription drug price problem in the U.S.”
‘Then life changed’
The filmmaker, Mr. Ruderman, was diagnosed at age 19, during his freshman year in college. He spent several days hospitalized with DKA, and “then life changed,” he said in an interview. He went into photography first and later filmmaking, always with the uneasy knowledge that he could lose access to insulin at any time.
The impetus for the film came after he and Ms. Dyer walked into a pharmacy while visiting Canada in 2018 and discovered how much cheaper insulin was compared to the United States – roughly $20 per vial, compared to $300 in the U.S.
“When Rachael [Dyer] and I came back to the U.S., we were actually quite shocked about how many people are struggling to afford their medication ... the uninsured, those aging off their parents’ health insurance. So that was really the kickoff to us going into the field for the last 4 years making this documentary.”
As a freelancer, Mr. Ruderman has been personally paying for expensive “premium” health insurance that covers the pump and glucose monitors he uses. He buys insulin overseas as often as possible.
“Fortunately, I haven’t been in a situation where I’ve had to ration my insulin, but the fear is instilled in me. What if there’s a month when I can’t afford it? What am I going to do?” (Note: The writer of this article is in the same situation, which could be alleviated by Lilly’s action.)
Timing is everything
To be sure, even before Lilly’s announcement, some progress had been made since work on the film began.
The issue of insulin pricing has received wide media attention. More than 20 states have passed copay caps on insulin, and a new law capping the cost of insulin for Medicare beneficiaries at $35/month went into effect in January 2023. President Biden mentioned insulin during his State of the Union address, and Georgia Senator Raphael Warnock made the issue a centerpiece of his campaign.
But there have also been losses, including the failure thus far to pass a nationwide copay cap.
These recent developments make this a good time for the film’s debut, producer Yael Melamede said in an interview. “There’s a lot happening in the space, but also a lot of incredible disappointments along the way, so we are really interested in getting this film out now.”
Ms. Melamede, who owns a film production company, said, “I’ve done a lot of films that have some issue advocacy side to them. I love this film because it’s grounded in the stories of real people. ... We feel this is a perfect catalyst to keep the energy going and for people to say this is super-important and not get distracted.”
While the film doesn’t advocate for specific policies, there is a “call to action” at the end that points viewers to resources on the website for writing to their members of Congress along with additional ways to become personally involved.
Ms. Dyer told this news organization, “This film is not only focusing on type 1 diabetes. That is obviously the crux of the issue, but it is a broader health care message for everyone wanting to make a change for health care in this country, the richest country in the world.”
At SXSW, Pay or Die will be competing with seven other films in the documentary feature competition, and it is eligible to win other awards.
Several other activities at the festival will address the topics of diabetes and U.S. health care costs, including a panel discussion titled Crushing: The Burden of Diabetes on Patients, featuring musician and actor Nick Jonas, who has type 1 diabetes, and a representative from the continuous glucose monitor manufacturer Dexcom.
Another panel, Young and Uninsured: Pay or Die, will include Dr. Rajkumar, Mr. Ruderman, Texas Representative James Talarico, who is advancing an insulin cap bill in that state, and Nicole Smith-Holt, the Minnesota mother of the young man who died because he couldn’t afford his insulin.
Mr. Ruderman, Ms. Dyer, Ms. Melamede, and Dr. Rajkumar have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new documentary premiering at the 2023 South by Southwest (SXSW) Festival illustrates the human consequences of insulin’s high cost in the United States. Its creators hope that it will help spur action toward overall prescription pricing reform.
Pay or Die: A Documentary is scheduled to premiere March 11. It will be shown twice more during the festival, which runs from March 10 to 19 in Austin, Texas. The documentary was co-created and directed by filmmaker and cinematographer Scott Alexander Ruderman, who has type 1 diabetes, and his partner, producer and journalist Rachael Dyer. One of the executive producers is Sarah Silverman, a comic, actor, producer, and health care reform advocate.
The 90-minute film follows three human stories: A mother and young daughter who both have type 1 diabetes and become homeless after spending their rent money on insulin, a young adult diagnosed during the COVID-19 pandemic, and a mother whose 26-year-old son died from diabetic ketoacidosis (DKA) after his insulin was rationed.
“As an Australian now living in the U.S. and seeing how the health care system works here, especially for people with type 1 diabetes like Scott, and how access to insulin is a life-or-death situation, has been very eye-opening for me. I’m also half Canadian, and both are countries where access to health care is a human right, not a business,” Ms. Dyer said in an interview.
In response to the March 1 announcement from Eli Lilly about its insulin price cut, the film’s team told this news organization: “While we commend Eli Lilly in taking this first step and hope that Novo Nordisk and Sanofi [the two other major insulin manufacturers] follow suit, it is important to remember that the key issue is not about these companies voluntarily slashing prices; it’s about changing laws so the insulin manufacturers do not have the ability to raise the prices again.
“This is the life-or-death issue that we focus on in our documentary Pay or Die. It’s also important to note that insulin is just one of the many expensive prescription drugs in the U.S., which is why we need to call for reform. Affordable medication needs to be a basic human right within reach for all Americans.”
Physician perspective: Good news on insulin, but broader issues
The film features four physicians. One, Mayo Clinic oncologist/hematologist S. Vincent Rajkumar, MD, has spoken and published widely on insulin prices specifically and U.S. drug costs more broadly.
The other three are Joslin Clinic endocrinologist Elizabeth Halprin, MD, Massachusetts General Hospital internist Leigh Simmons, MD, and New York University physician and essayist Danielle Ofri, MD, PhD.
In an interview after the Lilly announcement, Dr. Rajkumar said, “I think this is very, very good news for patients. ... The fact that they’re doing it means they’re listening to us and listening to patients, which is good. And I do hope that other insulin manufacturers do the same shortly.”
However, he added, “for prescription drug prices and particularly cancer drug prices, there’s more reform that’s needed, and that’s at the policy level. ... The goal of the film was to use insulin to highlight the prescription drug price problem in the U.S.”
‘Then life changed’
The filmmaker, Mr. Ruderman, was diagnosed at age 19, during his freshman year in college. He spent several days hospitalized with DKA, and “then life changed,” he said in an interview. He went into photography first and later filmmaking, always with the uneasy knowledge that he could lose access to insulin at any time.
The impetus for the film came after he and Ms. Dyer walked into a pharmacy while visiting Canada in 2018 and discovered how much cheaper insulin was compared to the United States – roughly $20 per vial, compared to $300 in the U.S.
“When Rachael [Dyer] and I came back to the U.S., we were actually quite shocked about how many people are struggling to afford their medication ... the uninsured, those aging off their parents’ health insurance. So that was really the kickoff to us going into the field for the last 4 years making this documentary.”
As a freelancer, Mr. Ruderman has been personally paying for expensive “premium” health insurance that covers the pump and glucose monitors he uses. He buys insulin overseas as often as possible.
“Fortunately, I haven’t been in a situation where I’ve had to ration my insulin, but the fear is instilled in me. What if there’s a month when I can’t afford it? What am I going to do?” (Note: The writer of this article is in the same situation, which could be alleviated by Lilly’s action.)
Timing is everything
To be sure, even before Lilly’s announcement, some progress had been made since work on the film began.
The issue of insulin pricing has received wide media attention. More than 20 states have passed copay caps on insulin, and a new law capping the cost of insulin for Medicare beneficiaries at $35/month went into effect in January 2023. President Biden mentioned insulin during his State of the Union address, and Georgia Senator Raphael Warnock made the issue a centerpiece of his campaign.
But there have also been losses, including the failure thus far to pass a nationwide copay cap.
These recent developments make this a good time for the film’s debut, producer Yael Melamede said in an interview. “There’s a lot happening in the space, but also a lot of incredible disappointments along the way, so we are really interested in getting this film out now.”
Ms. Melamede, who owns a film production company, said, “I’ve done a lot of films that have some issue advocacy side to them. I love this film because it’s grounded in the stories of real people. ... We feel this is a perfect catalyst to keep the energy going and for people to say this is super-important and not get distracted.”
While the film doesn’t advocate for specific policies, there is a “call to action” at the end that points viewers to resources on the website for writing to their members of Congress along with additional ways to become personally involved.
Ms. Dyer told this news organization, “This film is not only focusing on type 1 diabetes. That is obviously the crux of the issue, but it is a broader health care message for everyone wanting to make a change for health care in this country, the richest country in the world.”
At SXSW, Pay or Die will be competing with seven other films in the documentary feature competition, and it is eligible to win other awards.
Several other activities at the festival will address the topics of diabetes and U.S. health care costs, including a panel discussion titled Crushing: The Burden of Diabetes on Patients, featuring musician and actor Nick Jonas, who has type 1 diabetes, and a representative from the continuous glucose monitor manufacturer Dexcom.
Another panel, Young and Uninsured: Pay or Die, will include Dr. Rajkumar, Mr. Ruderman, Texas Representative James Talarico, who is advancing an insulin cap bill in that state, and Nicole Smith-Holt, the Minnesota mother of the young man who died because he couldn’t afford his insulin.
Mr. Ruderman, Ms. Dyer, Ms. Melamede, and Dr. Rajkumar have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new documentary premiering at the 2023 South by Southwest (SXSW) Festival illustrates the human consequences of insulin’s high cost in the United States. Its creators hope that it will help spur action toward overall prescription pricing reform.
Pay or Die: A Documentary is scheduled to premiere March 11. It will be shown twice more during the festival, which runs from March 10 to 19 in Austin, Texas. The documentary was co-created and directed by filmmaker and cinematographer Scott Alexander Ruderman, who has type 1 diabetes, and his partner, producer and journalist Rachael Dyer. One of the executive producers is Sarah Silverman, a comic, actor, producer, and health care reform advocate.
The 90-minute film follows three human stories: A mother and young daughter who both have type 1 diabetes and become homeless after spending their rent money on insulin, a young adult diagnosed during the COVID-19 pandemic, and a mother whose 26-year-old son died from diabetic ketoacidosis (DKA) after his insulin was rationed.
“As an Australian now living in the U.S. and seeing how the health care system works here, especially for people with type 1 diabetes like Scott, and how access to insulin is a life-or-death situation, has been very eye-opening for me. I’m also half Canadian, and both are countries where access to health care is a human right, not a business,” Ms. Dyer said in an interview.
In response to the March 1 announcement from Eli Lilly about its insulin price cut, the film’s team told this news organization: “While we commend Eli Lilly in taking this first step and hope that Novo Nordisk and Sanofi [the two other major insulin manufacturers] follow suit, it is important to remember that the key issue is not about these companies voluntarily slashing prices; it’s about changing laws so the insulin manufacturers do not have the ability to raise the prices again.
“This is the life-or-death issue that we focus on in our documentary Pay or Die. It’s also important to note that insulin is just one of the many expensive prescription drugs in the U.S., which is why we need to call for reform. Affordable medication needs to be a basic human right within reach for all Americans.”
Physician perspective: Good news on insulin, but broader issues
The film features four physicians. One, Mayo Clinic oncologist/hematologist S. Vincent Rajkumar, MD, has spoken and published widely on insulin prices specifically and U.S. drug costs more broadly.
The other three are Joslin Clinic endocrinologist Elizabeth Halprin, MD, Massachusetts General Hospital internist Leigh Simmons, MD, and New York University physician and essayist Danielle Ofri, MD, PhD.
In an interview after the Lilly announcement, Dr. Rajkumar said, “I think this is very, very good news for patients. ... The fact that they’re doing it means they’re listening to us and listening to patients, which is good. And I do hope that other insulin manufacturers do the same shortly.”
However, he added, “for prescription drug prices and particularly cancer drug prices, there’s more reform that’s needed, and that’s at the policy level. ... The goal of the film was to use insulin to highlight the prescription drug price problem in the U.S.”
‘Then life changed’
The filmmaker, Mr. Ruderman, was diagnosed at age 19, during his freshman year in college. He spent several days hospitalized with DKA, and “then life changed,” he said in an interview. He went into photography first and later filmmaking, always with the uneasy knowledge that he could lose access to insulin at any time.
The impetus for the film came after he and Ms. Dyer walked into a pharmacy while visiting Canada in 2018 and discovered how much cheaper insulin was compared to the United States – roughly $20 per vial, compared to $300 in the U.S.
“When Rachael [Dyer] and I came back to the U.S., we were actually quite shocked about how many people are struggling to afford their medication ... the uninsured, those aging off their parents’ health insurance. So that was really the kickoff to us going into the field for the last 4 years making this documentary.”
As a freelancer, Mr. Ruderman has been personally paying for expensive “premium” health insurance that covers the pump and glucose monitors he uses. He buys insulin overseas as often as possible.
“Fortunately, I haven’t been in a situation where I’ve had to ration my insulin, but the fear is instilled in me. What if there’s a month when I can’t afford it? What am I going to do?” (Note: The writer of this article is in the same situation, which could be alleviated by Lilly’s action.)
Timing is everything
To be sure, even before Lilly’s announcement, some progress had been made since work on the film began.
The issue of insulin pricing has received wide media attention. More than 20 states have passed copay caps on insulin, and a new law capping the cost of insulin for Medicare beneficiaries at $35/month went into effect in January 2023. President Biden mentioned insulin during his State of the Union address, and Georgia Senator Raphael Warnock made the issue a centerpiece of his campaign.
But there have also been losses, including the failure thus far to pass a nationwide copay cap.
These recent developments make this a good time for the film’s debut, producer Yael Melamede said in an interview. “There’s a lot happening in the space, but also a lot of incredible disappointments along the way, so we are really interested in getting this film out now.”
Ms. Melamede, who owns a film production company, said, “I’ve done a lot of films that have some issue advocacy side to them. I love this film because it’s grounded in the stories of real people. ... We feel this is a perfect catalyst to keep the energy going and for people to say this is super-important and not get distracted.”
While the film doesn’t advocate for specific policies, there is a “call to action” at the end that points viewers to resources on the website for writing to their members of Congress along with additional ways to become personally involved.
Ms. Dyer told this news organization, “This film is not only focusing on type 1 diabetes. That is obviously the crux of the issue, but it is a broader health care message for everyone wanting to make a change for health care in this country, the richest country in the world.”
At SXSW, Pay or Die will be competing with seven other films in the documentary feature competition, and it is eligible to win other awards.
Several other activities at the festival will address the topics of diabetes and U.S. health care costs, including a panel discussion titled Crushing: The Burden of Diabetes on Patients, featuring musician and actor Nick Jonas, who has type 1 diabetes, and a representative from the continuous glucose monitor manufacturer Dexcom.
Another panel, Young and Uninsured: Pay or Die, will include Dr. Rajkumar, Mr. Ruderman, Texas Representative James Talarico, who is advancing an insulin cap bill in that state, and Nicole Smith-Holt, the Minnesota mother of the young man who died because he couldn’t afford his insulin.
Mr. Ruderman, Ms. Dyer, Ms. Melamede, and Dr. Rajkumar have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Midwife-led care linked to positive outcomes across medical risk levels
Midwives provide safe primary care for pregnant women who are at various levels of medical risk in British Columbia, Canada, new data suggest.
In most cases, for midwifery clients, birth outcomes were similar to or were better than birth outcomes of patients who had physician-led or obstetrician-led care.
In addition, midwifery clients were less likely to experience preterm births or have low-birth-weight babies and to experience cesarean deliveries or births involving instruments.
“Based on previous research, we know that midwives provide safe care for healthy childbearing people or those with no or few risk factors that might complicate the pregnancy or birth,” lead author Kathrin Stoll, PhD, a research associate in the University of British Columbia’s department of family practice, told this news organization.
“What we didn’t know until now is whether midwives provide safe care to people with moderate and high medical risks and what proportion of B.C. [British Columbia] midwifery clients are low, moderate, and high risk,” she said. “This is important to know because of the misperception that midwives only look after low-risk people. This misperception is sometimes used against midwives to justify giving them fewer resources and supports.”
The study was published in the Canadian Medical Association Journal.
Increasing demand
Registered midwives have been part of the health care system in British Columbia since 1998, according to the study authors. The number of pregnant people who are attended by midwives during birth has steadily increased from 4.8% in 2004-2005 to 15.6% in 2019-2020.
The investigators analyzed 2008-2018 data from the British Columbia Perinatal Data Registry, which contains data for 99% of births, including home births. Their analysis included 425,056 births for which a family physician, an obstetrician, or a midwife was listed as the most responsible provider (MRP). The investigators assessed pregnancy risk status (low, moderate, or high), which was determined on the basis of an adapted perinatal risk scoring system used by the Alberta Perinatal Health Program. They estimated the differences in neonatal and maternal outcomes between MRP groups by calculating adjusted absolute and relative risks.
Among the 425,056 births, 63,151 (14.9%) had a midwife as the MRP, 189,679 (44.6%) had a family physician, and 172,226 (40.5%) had an obstetrician. The antenatal risk score ranged from 0 to 23 (median score, 2).
The proportion of births with midwife-led care increased from 9.2% to 19.8% from 2008-2018. In 2018, midwives were listed as the MRP for 24.3% of low-risk, 14.3% of moderate-risk, and 7.9% of high-risk births in the province. This represented an absolute increase of 9.1% for low-risk, 7.7% for moderate-risk, and 5.7% for high-risk births during the study period.
Among the 12,169 at-home births that took place during the study period, 9,776 (80.3%) were low-risk, 2,329 (19.1%) were moderate-risk, and 64 (0.5%) were high-risk births. As the risk score increased, so did the proportion of midwifery and family physician clients who were delivered by obstetricians. Across all risk strata, more family physician clients than midwifery clients underwent deliveries by obstetricians.
Overall, the risk of perinatal death for midwifery clients was similar to the risk for those under the care of family physicians across all risk levels. Low- and moderate-risk clients with midwife-led care were significantly less likely to experience a perinatal death, compared with those with obstetrician-led care, although the adjusted absolute risk differences were small. In the high-risk group, there was no significant difference in the rate of perinatal deaths between midwife-led and physician-led care.
In addition, clients with midwife-led care were significantly less likely to experience preterm birth and have a low-birth-weight baby regardless of medical risk level. The adjusted relative risk of an Apgar score of less than 7 at 5 minutes was significantly lower for midwife-led care than for physician-led care for nearly all comparisons.
The cesarean delivery rate among midwifery clients in the low-risk group was 7.2%, compared with 12.2% for family physicians and 42.3% for obstetrician clients. Cesarean delivery rates increased for midwifery clients as medical risk increased but were significantly lower than the physician rates across all medical risk levels.
Among low-risk clients, the absolute risk reduction for cesarean delivery was 34.4% with midwife-led care, compared with obstetrician-led care. The absolute risk difference increased to 55.3% for moderate-risk clients and to 42.2% for high-risk clients.
Labor induction varied
Although low-risk midwifery clients were significantly less likely to experience labor induction with oxytocin, high-risk midwifery clients were more than twice as likely to undergo induction with oxytocin than obstetrician clients (adjusted absolute difference, 11.3%).
For most risk levels, midwifery clients were less likely to have an assisted vaginal birth than physician clients, and they were significantly more likely to have a spontaneous vaginal birth. Low-risk clients who had a midwife as the MRP were nearly twice as likely to have a spontaneous vaginal birth than obstetricians’ clients, and moderate-risk clients were nearly four times as likely to have a spontaneous vaginal birth.
The rates of vaginal birth after cesarean delivery (VBAC) were significantly higher when a midwife was the MRP. In comparing midwifery clients with family physician clients, the relative and absolute differences were small, but they were larger when comparing midwifery clients with obstetrician clients. Among low-risk clients, the VBAC rate was 85.3% among midwifery clients, compared with 78.6% among family physician clients and 51.5% among obstetrician clients.
In general, the prevalence rates of adverse maternal outcomes (including blood transfusion, intensive care admissions, uterine rupture, and postpartum wound infection) were low for midwifery clients across all risk levels.
Breast- or chest-feeding at birth was significantly more common among midwifery clients across all risk levels as well.
Today, nearly 1 in 4 childbearing people in British Columbia receive care from a midwife at some point during pregnancy, birth, or the postpartum period, the study authors write. During the past 20 years, the profile of clients has evolved to include more moderate- and high-risk patients.
“Clients with more complex medical needs take more time and need more support,” said Dr. Stoll. “This means that midwives continue to stay on call, responding to pages and urgent medical concerns for their clients with no pay for being on call, no days off even for sick days, and unsafe working hours, often working more than 24 hours at a time. If we want to expand midwifery to communities where they are needed most, we need to provide an enabling environment.”
Additional studies are needed as to how different practice and remuneration models affect clinical outcomes, health care costs, and client and provider experiences, the study authors write. At the same time, there are several barriers to obtaining funding, conducting studies, and publishing research by and about midwives in Canada, Dr. Stoll said – barriers that she and her co-authors faced.
Seeking broader access
Alixandra Bacon, a registered midwife and president of the Canadian Association of Midwives, said, “These findings demonstrate that pregnant people at any level of medical risk can benefit from midwifery care. This is a testament both to the benefits of the Canadian midwifery model of care and to the seamless integration of midwifery into collaborative teams and the health system.” Ms. Bacon wasn’t involved with this study.
“If we can realize our goal of equitable access to midwifery care for all families in Canada, we can help to decrease rates of unnecessary medical intervention, preterm labor, and stillbirth,” she added.
“Midwifery is well established across most of Canada. This is yet one more piece of evidence that shows the clinical benefits of midwifery care,” Jasmin Tecson, a registered midwife and president of the Association of Ontario Midwives, said in an interview.
Ms. Tecson, who wasn’t involved with this study, noted the increasing number of clients with more complex health and social needs in Ontario. “It is time to think about how the skills and knowledge of midwives can be used with clients of different risk profiles and how the current scope of practice of midwives can be optimized and expanded,” she said. “For example, Ontario midwives are still required to prescribe medications from a limited list, despite the potential additional clinical risks and health system costs that this creates.”
The study received financial support from the University of British Columbia Stollery Fund and the University of British Columbia Work Learn Program. Dr. Stoll has an unpaid role with the Midwives Association Contract Negotiation Advisory Council. Ms. Bacon and Ms. Tecson disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Midwives provide safe primary care for pregnant women who are at various levels of medical risk in British Columbia, Canada, new data suggest.
In most cases, for midwifery clients, birth outcomes were similar to or were better than birth outcomes of patients who had physician-led or obstetrician-led care.
In addition, midwifery clients were less likely to experience preterm births or have low-birth-weight babies and to experience cesarean deliveries or births involving instruments.
“Based on previous research, we know that midwives provide safe care for healthy childbearing people or those with no or few risk factors that might complicate the pregnancy or birth,” lead author Kathrin Stoll, PhD, a research associate in the University of British Columbia’s department of family practice, told this news organization.
“What we didn’t know until now is whether midwives provide safe care to people with moderate and high medical risks and what proportion of B.C. [British Columbia] midwifery clients are low, moderate, and high risk,” she said. “This is important to know because of the misperception that midwives only look after low-risk people. This misperception is sometimes used against midwives to justify giving them fewer resources and supports.”
The study was published in the Canadian Medical Association Journal.
Increasing demand
Registered midwives have been part of the health care system in British Columbia since 1998, according to the study authors. The number of pregnant people who are attended by midwives during birth has steadily increased from 4.8% in 2004-2005 to 15.6% in 2019-2020.
The investigators analyzed 2008-2018 data from the British Columbia Perinatal Data Registry, which contains data for 99% of births, including home births. Their analysis included 425,056 births for which a family physician, an obstetrician, or a midwife was listed as the most responsible provider (MRP). The investigators assessed pregnancy risk status (low, moderate, or high), which was determined on the basis of an adapted perinatal risk scoring system used by the Alberta Perinatal Health Program. They estimated the differences in neonatal and maternal outcomes between MRP groups by calculating adjusted absolute and relative risks.
Among the 425,056 births, 63,151 (14.9%) had a midwife as the MRP, 189,679 (44.6%) had a family physician, and 172,226 (40.5%) had an obstetrician. The antenatal risk score ranged from 0 to 23 (median score, 2).
The proportion of births with midwife-led care increased from 9.2% to 19.8% from 2008-2018. In 2018, midwives were listed as the MRP for 24.3% of low-risk, 14.3% of moderate-risk, and 7.9% of high-risk births in the province. This represented an absolute increase of 9.1% for low-risk, 7.7% for moderate-risk, and 5.7% for high-risk births during the study period.
Among the 12,169 at-home births that took place during the study period, 9,776 (80.3%) were low-risk, 2,329 (19.1%) were moderate-risk, and 64 (0.5%) were high-risk births. As the risk score increased, so did the proportion of midwifery and family physician clients who were delivered by obstetricians. Across all risk strata, more family physician clients than midwifery clients underwent deliveries by obstetricians.
Overall, the risk of perinatal death for midwifery clients was similar to the risk for those under the care of family physicians across all risk levels. Low- and moderate-risk clients with midwife-led care were significantly less likely to experience a perinatal death, compared with those with obstetrician-led care, although the adjusted absolute risk differences were small. In the high-risk group, there was no significant difference in the rate of perinatal deaths between midwife-led and physician-led care.
In addition, clients with midwife-led care were significantly less likely to experience preterm birth and have a low-birth-weight baby regardless of medical risk level. The adjusted relative risk of an Apgar score of less than 7 at 5 minutes was significantly lower for midwife-led care than for physician-led care for nearly all comparisons.
The cesarean delivery rate among midwifery clients in the low-risk group was 7.2%, compared with 12.2% for family physicians and 42.3% for obstetrician clients. Cesarean delivery rates increased for midwifery clients as medical risk increased but were significantly lower than the physician rates across all medical risk levels.
Among low-risk clients, the absolute risk reduction for cesarean delivery was 34.4% with midwife-led care, compared with obstetrician-led care. The absolute risk difference increased to 55.3% for moderate-risk clients and to 42.2% for high-risk clients.
Labor induction varied
Although low-risk midwifery clients were significantly less likely to experience labor induction with oxytocin, high-risk midwifery clients were more than twice as likely to undergo induction with oxytocin than obstetrician clients (adjusted absolute difference, 11.3%).
For most risk levels, midwifery clients were less likely to have an assisted vaginal birth than physician clients, and they were significantly more likely to have a spontaneous vaginal birth. Low-risk clients who had a midwife as the MRP were nearly twice as likely to have a spontaneous vaginal birth than obstetricians’ clients, and moderate-risk clients were nearly four times as likely to have a spontaneous vaginal birth.
The rates of vaginal birth after cesarean delivery (VBAC) were significantly higher when a midwife was the MRP. In comparing midwifery clients with family physician clients, the relative and absolute differences were small, but they were larger when comparing midwifery clients with obstetrician clients. Among low-risk clients, the VBAC rate was 85.3% among midwifery clients, compared with 78.6% among family physician clients and 51.5% among obstetrician clients.
In general, the prevalence rates of adverse maternal outcomes (including blood transfusion, intensive care admissions, uterine rupture, and postpartum wound infection) were low for midwifery clients across all risk levels.
Breast- or chest-feeding at birth was significantly more common among midwifery clients across all risk levels as well.
Today, nearly 1 in 4 childbearing people in British Columbia receive care from a midwife at some point during pregnancy, birth, or the postpartum period, the study authors write. During the past 20 years, the profile of clients has evolved to include more moderate- and high-risk patients.
“Clients with more complex medical needs take more time and need more support,” said Dr. Stoll. “This means that midwives continue to stay on call, responding to pages and urgent medical concerns for their clients with no pay for being on call, no days off even for sick days, and unsafe working hours, often working more than 24 hours at a time. If we want to expand midwifery to communities where they are needed most, we need to provide an enabling environment.”
Additional studies are needed as to how different practice and remuneration models affect clinical outcomes, health care costs, and client and provider experiences, the study authors write. At the same time, there are several barriers to obtaining funding, conducting studies, and publishing research by and about midwives in Canada, Dr. Stoll said – barriers that she and her co-authors faced.
Seeking broader access
Alixandra Bacon, a registered midwife and president of the Canadian Association of Midwives, said, “These findings demonstrate that pregnant people at any level of medical risk can benefit from midwifery care. This is a testament both to the benefits of the Canadian midwifery model of care and to the seamless integration of midwifery into collaborative teams and the health system.” Ms. Bacon wasn’t involved with this study.
“If we can realize our goal of equitable access to midwifery care for all families in Canada, we can help to decrease rates of unnecessary medical intervention, preterm labor, and stillbirth,” she added.
“Midwifery is well established across most of Canada. This is yet one more piece of evidence that shows the clinical benefits of midwifery care,” Jasmin Tecson, a registered midwife and president of the Association of Ontario Midwives, said in an interview.
Ms. Tecson, who wasn’t involved with this study, noted the increasing number of clients with more complex health and social needs in Ontario. “It is time to think about how the skills and knowledge of midwives can be used with clients of different risk profiles and how the current scope of practice of midwives can be optimized and expanded,” she said. “For example, Ontario midwives are still required to prescribe medications from a limited list, despite the potential additional clinical risks and health system costs that this creates.”
The study received financial support from the University of British Columbia Stollery Fund and the University of British Columbia Work Learn Program. Dr. Stoll has an unpaid role with the Midwives Association Contract Negotiation Advisory Council. Ms. Bacon and Ms. Tecson disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Midwives provide safe primary care for pregnant women who are at various levels of medical risk in British Columbia, Canada, new data suggest.
In most cases, for midwifery clients, birth outcomes were similar to or were better than birth outcomes of patients who had physician-led or obstetrician-led care.
In addition, midwifery clients were less likely to experience preterm births or have low-birth-weight babies and to experience cesarean deliveries or births involving instruments.
“Based on previous research, we know that midwives provide safe care for healthy childbearing people or those with no or few risk factors that might complicate the pregnancy or birth,” lead author Kathrin Stoll, PhD, a research associate in the University of British Columbia’s department of family practice, told this news organization.
“What we didn’t know until now is whether midwives provide safe care to people with moderate and high medical risks and what proportion of B.C. [British Columbia] midwifery clients are low, moderate, and high risk,” she said. “This is important to know because of the misperception that midwives only look after low-risk people. This misperception is sometimes used against midwives to justify giving them fewer resources and supports.”
The study was published in the Canadian Medical Association Journal.
Increasing demand
Registered midwives have been part of the health care system in British Columbia since 1998, according to the study authors. The number of pregnant people who are attended by midwives during birth has steadily increased from 4.8% in 2004-2005 to 15.6% in 2019-2020.
The investigators analyzed 2008-2018 data from the British Columbia Perinatal Data Registry, which contains data for 99% of births, including home births. Their analysis included 425,056 births for which a family physician, an obstetrician, or a midwife was listed as the most responsible provider (MRP). The investigators assessed pregnancy risk status (low, moderate, or high), which was determined on the basis of an adapted perinatal risk scoring system used by the Alberta Perinatal Health Program. They estimated the differences in neonatal and maternal outcomes between MRP groups by calculating adjusted absolute and relative risks.
Among the 425,056 births, 63,151 (14.9%) had a midwife as the MRP, 189,679 (44.6%) had a family physician, and 172,226 (40.5%) had an obstetrician. The antenatal risk score ranged from 0 to 23 (median score, 2).
The proportion of births with midwife-led care increased from 9.2% to 19.8% from 2008-2018. In 2018, midwives were listed as the MRP for 24.3% of low-risk, 14.3% of moderate-risk, and 7.9% of high-risk births in the province. This represented an absolute increase of 9.1% for low-risk, 7.7% for moderate-risk, and 5.7% for high-risk births during the study period.
Among the 12,169 at-home births that took place during the study period, 9,776 (80.3%) were low-risk, 2,329 (19.1%) were moderate-risk, and 64 (0.5%) were high-risk births. As the risk score increased, so did the proportion of midwifery and family physician clients who were delivered by obstetricians. Across all risk strata, more family physician clients than midwifery clients underwent deliveries by obstetricians.
Overall, the risk of perinatal death for midwifery clients was similar to the risk for those under the care of family physicians across all risk levels. Low- and moderate-risk clients with midwife-led care were significantly less likely to experience a perinatal death, compared with those with obstetrician-led care, although the adjusted absolute risk differences were small. In the high-risk group, there was no significant difference in the rate of perinatal deaths between midwife-led and physician-led care.
In addition, clients with midwife-led care were significantly less likely to experience preterm birth and have a low-birth-weight baby regardless of medical risk level. The adjusted relative risk of an Apgar score of less than 7 at 5 minutes was significantly lower for midwife-led care than for physician-led care for nearly all comparisons.
The cesarean delivery rate among midwifery clients in the low-risk group was 7.2%, compared with 12.2% for family physicians and 42.3% for obstetrician clients. Cesarean delivery rates increased for midwifery clients as medical risk increased but were significantly lower than the physician rates across all medical risk levels.
Among low-risk clients, the absolute risk reduction for cesarean delivery was 34.4% with midwife-led care, compared with obstetrician-led care. The absolute risk difference increased to 55.3% for moderate-risk clients and to 42.2% for high-risk clients.
Labor induction varied
Although low-risk midwifery clients were significantly less likely to experience labor induction with oxytocin, high-risk midwifery clients were more than twice as likely to undergo induction with oxytocin than obstetrician clients (adjusted absolute difference, 11.3%).
For most risk levels, midwifery clients were less likely to have an assisted vaginal birth than physician clients, and they were significantly more likely to have a spontaneous vaginal birth. Low-risk clients who had a midwife as the MRP were nearly twice as likely to have a spontaneous vaginal birth than obstetricians’ clients, and moderate-risk clients were nearly four times as likely to have a spontaneous vaginal birth.
The rates of vaginal birth after cesarean delivery (VBAC) were significantly higher when a midwife was the MRP. In comparing midwifery clients with family physician clients, the relative and absolute differences were small, but they were larger when comparing midwifery clients with obstetrician clients. Among low-risk clients, the VBAC rate was 85.3% among midwifery clients, compared with 78.6% among family physician clients and 51.5% among obstetrician clients.
In general, the prevalence rates of adverse maternal outcomes (including blood transfusion, intensive care admissions, uterine rupture, and postpartum wound infection) were low for midwifery clients across all risk levels.
Breast- or chest-feeding at birth was significantly more common among midwifery clients across all risk levels as well.
Today, nearly 1 in 4 childbearing people in British Columbia receive care from a midwife at some point during pregnancy, birth, or the postpartum period, the study authors write. During the past 20 years, the profile of clients has evolved to include more moderate- and high-risk patients.
“Clients with more complex medical needs take more time and need more support,” said Dr. Stoll. “This means that midwives continue to stay on call, responding to pages and urgent medical concerns for their clients with no pay for being on call, no days off even for sick days, and unsafe working hours, often working more than 24 hours at a time. If we want to expand midwifery to communities where they are needed most, we need to provide an enabling environment.”
Additional studies are needed as to how different practice and remuneration models affect clinical outcomes, health care costs, and client and provider experiences, the study authors write. At the same time, there are several barriers to obtaining funding, conducting studies, and publishing research by and about midwives in Canada, Dr. Stoll said – barriers that she and her co-authors faced.
Seeking broader access
Alixandra Bacon, a registered midwife and president of the Canadian Association of Midwives, said, “These findings demonstrate that pregnant people at any level of medical risk can benefit from midwifery care. This is a testament both to the benefits of the Canadian midwifery model of care and to the seamless integration of midwifery into collaborative teams and the health system.” Ms. Bacon wasn’t involved with this study.
“If we can realize our goal of equitable access to midwifery care for all families in Canada, we can help to decrease rates of unnecessary medical intervention, preterm labor, and stillbirth,” she added.
“Midwifery is well established across most of Canada. This is yet one more piece of evidence that shows the clinical benefits of midwifery care,” Jasmin Tecson, a registered midwife and president of the Association of Ontario Midwives, said in an interview.
Ms. Tecson, who wasn’t involved with this study, noted the increasing number of clients with more complex health and social needs in Ontario. “It is time to think about how the skills and knowledge of midwives can be used with clients of different risk profiles and how the current scope of practice of midwives can be optimized and expanded,” she said. “For example, Ontario midwives are still required to prescribe medications from a limited list, despite the potential additional clinical risks and health system costs that this creates.”
The study received financial support from the University of British Columbia Stollery Fund and the University of British Columbia Work Learn Program. Dr. Stoll has an unpaid role with the Midwives Association Contract Negotiation Advisory Council. Ms. Bacon and Ms. Tecson disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Specialty and age may contribute to suicidal thoughts among physicians
A physician’s specialty can make a difference when it comes to having suicidal thoughts. Doctors who specialize in family medicine, obstetrics-gynecology, and psychiatry reported double the rates of suicidal thoughts than doctors in oncology, rheumatology, and pulmonary medicine, according to Doctors’ Burden: Medscape Physician Suicide Report 2023.
“The specialties with the highest reporting of physician suicidal thoughts are also those with the greatest physician shortages, based on the number of job openings posted by recruiting sites,” said Peter Yellowlees, MD, professor of psychiatry and chief wellness officer at UC Davis Health.
Doctors in those specialties are overworked, which can lead to burnout, he said.
There’s also a generational divide among physicians who reported suicidal thoughts. Millennials (age 27-41) and Gen-X physicians (age 42-56) were more likely to report these thoughts than were Baby Boomers (age 57-75) and the Silent Generation (age 76-95).
“Younger physicians are more burned out – they may have less control over their lives and less meaning than some older doctors who can do what they want,” said Dr. Yellowlees.
One millennial respondent commented that being on call and being required to chart detailed notes in the EHR has contributed to her burnout. “I’m more impatient and make less time and effort to see my friends and family.”
One Silent Generation respondent commented, “I am semi-retired, I take no call, I work no weekends, I provide anesthesia care in my area of special expertise, I work clinically about 46 days a year. Life is good, particularly compared to my younger colleagues who are working 60-plus hours a week with evening work, weekend work, and call. I feel really sorry for them.”
When young people enter medical school, they’re quite healthy, with low rates of depression and burnout, said Dr. Yellowlees. Yet, studies have shown that rates of burnout and suicidal thoughts increased within 2 years. “That reflects what happens when a group of idealistic young people hit a horrible system,” he said.
Who’s responsible?
Millennials were three times as likely as baby boomers to say that a medical school or health care organization should be responsible when a student or physician commits suicide.
“Young physicians may expect more of their employers than my generation did, which we see in residency programs that have unionized,” said Dr. Yellowlees, a Baby Boomer.
“As more young doctors are employed by health care organizations, they also may expect more resources to be available to them, such as wellness programs,” he added.
Younger doctors also focus more on work-life balance than older doctors, including time off and having hobbies, he said. “They are much more rational in terms of their overall beliefs and expectations than the older generation.”
Whom doctors confide in
Nearly 60% of physician-respondents with suicidal thoughts said they confided in a professional or someone they knew. Men were just as likely as women to reach out to a therapist (38%), whereas men were slightly more likely to confide in a family member and women were slightly more likely to confide in a colleague.
“It’s interesting that women are more active in seeking support at work – they often have developed a network of colleagues to support each other’s careers and whom they can confide in,” said Dr. Yellowlees.
He emphasized that 40% of physicians said they didn’t confide in anyone when they had suicidal thoughts. Of those, just over half said they could cope without professional help.
One respondent commented, “It’s just a thought; nothing I would actually do.” Another commented, “Mental health professionals can’t fix the underlying reason for the problem.”
Many doctors were concerned about risking disclosure to their medical boards (42%); that it would show up on their insurance records (33%); and that their colleagues would find out (25%), according to the report.
One respondent commented, “I don’t trust doctors to keep it to themselves.”
Another barrier doctors mentioned was a lack of time to seek help. One commented, “Time. I have none, when am I supposed to find an hour for counseling?”
A version of this article originally appeared on Medscape.com.
A physician’s specialty can make a difference when it comes to having suicidal thoughts. Doctors who specialize in family medicine, obstetrics-gynecology, and psychiatry reported double the rates of suicidal thoughts than doctors in oncology, rheumatology, and pulmonary medicine, according to Doctors’ Burden: Medscape Physician Suicide Report 2023.
“The specialties with the highest reporting of physician suicidal thoughts are also those with the greatest physician shortages, based on the number of job openings posted by recruiting sites,” said Peter Yellowlees, MD, professor of psychiatry and chief wellness officer at UC Davis Health.
Doctors in those specialties are overworked, which can lead to burnout, he said.
There’s also a generational divide among physicians who reported suicidal thoughts. Millennials (age 27-41) and Gen-X physicians (age 42-56) were more likely to report these thoughts than were Baby Boomers (age 57-75) and the Silent Generation (age 76-95).
“Younger physicians are more burned out – they may have less control over their lives and less meaning than some older doctors who can do what they want,” said Dr. Yellowlees.
One millennial respondent commented that being on call and being required to chart detailed notes in the EHR has contributed to her burnout. “I’m more impatient and make less time and effort to see my friends and family.”
One Silent Generation respondent commented, “I am semi-retired, I take no call, I work no weekends, I provide anesthesia care in my area of special expertise, I work clinically about 46 days a year. Life is good, particularly compared to my younger colleagues who are working 60-plus hours a week with evening work, weekend work, and call. I feel really sorry for them.”
When young people enter medical school, they’re quite healthy, with low rates of depression and burnout, said Dr. Yellowlees. Yet, studies have shown that rates of burnout and suicidal thoughts increased within 2 years. “That reflects what happens when a group of idealistic young people hit a horrible system,” he said.
Who’s responsible?
Millennials were three times as likely as baby boomers to say that a medical school or health care organization should be responsible when a student or physician commits suicide.
“Young physicians may expect more of their employers than my generation did, which we see in residency programs that have unionized,” said Dr. Yellowlees, a Baby Boomer.
“As more young doctors are employed by health care organizations, they also may expect more resources to be available to them, such as wellness programs,” he added.
Younger doctors also focus more on work-life balance than older doctors, including time off and having hobbies, he said. “They are much more rational in terms of their overall beliefs and expectations than the older generation.”
Whom doctors confide in
Nearly 60% of physician-respondents with suicidal thoughts said they confided in a professional or someone they knew. Men were just as likely as women to reach out to a therapist (38%), whereas men were slightly more likely to confide in a family member and women were slightly more likely to confide in a colleague.
“It’s interesting that women are more active in seeking support at work – they often have developed a network of colleagues to support each other’s careers and whom they can confide in,” said Dr. Yellowlees.
He emphasized that 40% of physicians said they didn’t confide in anyone when they had suicidal thoughts. Of those, just over half said they could cope without professional help.
One respondent commented, “It’s just a thought; nothing I would actually do.” Another commented, “Mental health professionals can’t fix the underlying reason for the problem.”
Many doctors were concerned about risking disclosure to their medical boards (42%); that it would show up on their insurance records (33%); and that their colleagues would find out (25%), according to the report.
One respondent commented, “I don’t trust doctors to keep it to themselves.”
Another barrier doctors mentioned was a lack of time to seek help. One commented, “Time. I have none, when am I supposed to find an hour for counseling?”
A version of this article originally appeared on Medscape.com.
A physician’s specialty can make a difference when it comes to having suicidal thoughts. Doctors who specialize in family medicine, obstetrics-gynecology, and psychiatry reported double the rates of suicidal thoughts than doctors in oncology, rheumatology, and pulmonary medicine, according to Doctors’ Burden: Medscape Physician Suicide Report 2023.
“The specialties with the highest reporting of physician suicidal thoughts are also those with the greatest physician shortages, based on the number of job openings posted by recruiting sites,” said Peter Yellowlees, MD, professor of psychiatry and chief wellness officer at UC Davis Health.
Doctors in those specialties are overworked, which can lead to burnout, he said.
There’s also a generational divide among physicians who reported suicidal thoughts. Millennials (age 27-41) and Gen-X physicians (age 42-56) were more likely to report these thoughts than were Baby Boomers (age 57-75) and the Silent Generation (age 76-95).
“Younger physicians are more burned out – they may have less control over their lives and less meaning than some older doctors who can do what they want,” said Dr. Yellowlees.
One millennial respondent commented that being on call and being required to chart detailed notes in the EHR has contributed to her burnout. “I’m more impatient and make less time and effort to see my friends and family.”
One Silent Generation respondent commented, “I am semi-retired, I take no call, I work no weekends, I provide anesthesia care in my area of special expertise, I work clinically about 46 days a year. Life is good, particularly compared to my younger colleagues who are working 60-plus hours a week with evening work, weekend work, and call. I feel really sorry for them.”
When young people enter medical school, they’re quite healthy, with low rates of depression and burnout, said Dr. Yellowlees. Yet, studies have shown that rates of burnout and suicidal thoughts increased within 2 years. “That reflects what happens when a group of idealistic young people hit a horrible system,” he said.
Who’s responsible?
Millennials were three times as likely as baby boomers to say that a medical school or health care organization should be responsible when a student or physician commits suicide.
“Young physicians may expect more of their employers than my generation did, which we see in residency programs that have unionized,” said Dr. Yellowlees, a Baby Boomer.
“As more young doctors are employed by health care organizations, they also may expect more resources to be available to them, such as wellness programs,” he added.
Younger doctors also focus more on work-life balance than older doctors, including time off and having hobbies, he said. “They are much more rational in terms of their overall beliefs and expectations than the older generation.”
Whom doctors confide in
Nearly 60% of physician-respondents with suicidal thoughts said they confided in a professional or someone they knew. Men were just as likely as women to reach out to a therapist (38%), whereas men were slightly more likely to confide in a family member and women were slightly more likely to confide in a colleague.
“It’s interesting that women are more active in seeking support at work – they often have developed a network of colleagues to support each other’s careers and whom they can confide in,” said Dr. Yellowlees.
He emphasized that 40% of physicians said they didn’t confide in anyone when they had suicidal thoughts. Of those, just over half said they could cope without professional help.
One respondent commented, “It’s just a thought; nothing I would actually do.” Another commented, “Mental health professionals can’t fix the underlying reason for the problem.”
Many doctors were concerned about risking disclosure to their medical boards (42%); that it would show up on their insurance records (33%); and that their colleagues would find out (25%), according to the report.
One respondent commented, “I don’t trust doctors to keep it to themselves.”
Another barrier doctors mentioned was a lack of time to seek help. One commented, “Time. I have none, when am I supposed to find an hour for counseling?”
A version of this article originally appeared on Medscape.com.


