User login
Five thoughts on the Damar Hamlin collapse
The obvious first statement is that it’s neither wise nor appropriate to speculate on the specifics of Damar Hamlin’s cardiac event during a football game on Jan. 2 (including the possibility of commotio cordis) or his ongoing care. The public nature of his collapse induces intense curiosity but people with illness deserve privacy. Privacy in health care is in short supply. I disagree strongly with those who say his doctors ought to be giving public updates. That’s up to the family.
But there are important general concepts to consider about this incident. These include ...
Cardiac arrest can happen to anyone
People with structural heart disease or other chronic illnesses have a higher risk of arrhythmia, but the notion that athletes are immune from cardiac arrest is wrong. This sentence almost seems too obvious to write, but to this day, I hear clinicians express surprise that an athletic person has heart disease.
Survival turns on rapid and effective intervention
In the old days of electrophysiology, we used to test implantable cardioverter-defibrillators during an implant procedure by inducing ventricular fibrillation (VF) and watching the device convert it. Thankfully, trials have shown that this is no longer necessary for most implants.
When you induce VF In the EP lab, you learn quickly that a) it causes loss of consciousness in a matter of seconds, b) rapid defibrillation restores consciousness, often without the patients knowing or remembering they passed out, and c) the failure of the shock to terminate VF results in deterioration in a matter of 1-2 minutes. Even 1 minute in VF feels so long.
Need is an appropriate word in VF treatment
Clinicians often use the verb need. As in, this patient needs this pill or this procedure. It’s rarely appropriate.
But in the case of treating VF, patients truly need rapid defibrillation. Survival of out-of-hospital cardiac arrest is low because there just aren’t enough automated external defibrillators (AEDs) or people trained to use them. A study of patients who had out-of-hospital cardiac arrest in Denmark found that 30-day survival almost doubled (28.8% vs. 16.4%), when the nearest AED was accessible.
Bystanders must act
The public messages are simple: If a person loses consciousness in front of you, and is not breathing normally, assume it is a cardiac arrest, call 911 to get professional help, and start hands-only chest compressions. Don’t spend time checking for a pulse or trying to wake the person. If this is not a cardiac arrest, they will soon tell you to stop compressing their chest. Seconds matter.
Chest compressions are important but what is really needed is defibrillation. A crucial step in CPR is to send someone to get an AED and get the pads attached. If this is a shockable rhythm, deliver the shock. Hamlin’s collapse emphasizes the importance of the AED; without it, his survival to the hospital would have been unlikely.
Widespread preparticipation screening of young athletes remains a bad idea
Whenever cardiac arrest occurs in an athlete, in such a public way, people think about prevention. Surely it is better to prevent such an event than react to it, goes the thinking. The argument against this idea has four prongs:
The incidence of cardiac disease in a young athlete is extremely low, which sets up a situation where most “positive” tests are false positive. A false positive screening ECG or echocardiogram can create harm in multiple ways. One is the risk from downstream procedures, but worse is the inappropriate disqualification from sport. Healthwise, few harms could be greater than creating long-term fear of exercise in someone.
There is also the problem of false-negative screening tests. An ECG may be normal in the setting of hypertrophic cardiomyopathy. And a normal echocardiogram does not exclude arrhythmogenic right ventricular cardiomyopathy or other genetic causes of cardiac arrest. In a 2018 study from a major sports cardiology center in London, 6 of the 8 sudden cardiac deaths in their series were in athletes who had no detectable abnormalities on screening.
Even when disease is found, it’s not clear that prohibiting participation in sports prevents sudden death. Many previous class III recommendations against participation in sport now carry class II – may be considered – designations.
Finally, screening for any disease loses value as treatments improve. Public education regarding rapid intervention with CPR and AED use is the best treatment option. A great example is the case of Christian Erikson, a Danish soccer player who suffered cardiac arrest during a match at the European Championships in 2021 and was rapidly defibrillated on the field. Therapy was so effective that he was conscious and able to wave to fans on his way out of the stadium. He has now returned to elite competition.
Proponents of screening might oppose my take by saying that National Football League players are intensely screened. But this is different from widespread screening of high school and college athletes. It might sound harsh to say, but professional teams have dualities of interests in the health of their athletes given the million-dollar contracts.
What’s more, professional teams can afford to hire expert cardiologists to perform the testing. This would likely reduce the rate of false-positive findings, compared with screening in the community setting. I often have young people referred to me because of asymptomatic bradycardia found during athletic screening – an obviously normal finding.
Conclusions
As long as there are sports, there will be athletes who suffer cardiac arrest.
We can both hope for Hamlin’s full recovery and learn lessons to help reduce the rate of death from out-of-hospital cardiac arrest. This mostly involves education on how to help fellow humans and a public health commitment to access to AEDs.
John Mandrola, MD, practices cardiac electrophysiology in Louisville, Ky. and is a writer and podcaster for Medscape. He has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
The obvious first statement is that it’s neither wise nor appropriate to speculate on the specifics of Damar Hamlin’s cardiac event during a football game on Jan. 2 (including the possibility of commotio cordis) or his ongoing care. The public nature of his collapse induces intense curiosity but people with illness deserve privacy. Privacy in health care is in short supply. I disagree strongly with those who say his doctors ought to be giving public updates. That’s up to the family.
But there are important general concepts to consider about this incident. These include ...
Cardiac arrest can happen to anyone
People with structural heart disease or other chronic illnesses have a higher risk of arrhythmia, but the notion that athletes are immune from cardiac arrest is wrong. This sentence almost seems too obvious to write, but to this day, I hear clinicians express surprise that an athletic person has heart disease.
Survival turns on rapid and effective intervention
In the old days of electrophysiology, we used to test implantable cardioverter-defibrillators during an implant procedure by inducing ventricular fibrillation (VF) and watching the device convert it. Thankfully, trials have shown that this is no longer necessary for most implants.
When you induce VF In the EP lab, you learn quickly that a) it causes loss of consciousness in a matter of seconds, b) rapid defibrillation restores consciousness, often without the patients knowing or remembering they passed out, and c) the failure of the shock to terminate VF results in deterioration in a matter of 1-2 minutes. Even 1 minute in VF feels so long.
Need is an appropriate word in VF treatment
Clinicians often use the verb need. As in, this patient needs this pill or this procedure. It’s rarely appropriate.
But in the case of treating VF, patients truly need rapid defibrillation. Survival of out-of-hospital cardiac arrest is low because there just aren’t enough automated external defibrillators (AEDs) or people trained to use them. A study of patients who had out-of-hospital cardiac arrest in Denmark found that 30-day survival almost doubled (28.8% vs. 16.4%), when the nearest AED was accessible.
Bystanders must act
The public messages are simple: If a person loses consciousness in front of you, and is not breathing normally, assume it is a cardiac arrest, call 911 to get professional help, and start hands-only chest compressions. Don’t spend time checking for a pulse or trying to wake the person. If this is not a cardiac arrest, they will soon tell you to stop compressing their chest. Seconds matter.
Chest compressions are important but what is really needed is defibrillation. A crucial step in CPR is to send someone to get an AED and get the pads attached. If this is a shockable rhythm, deliver the shock. Hamlin’s collapse emphasizes the importance of the AED; without it, his survival to the hospital would have been unlikely.
Widespread preparticipation screening of young athletes remains a bad idea
Whenever cardiac arrest occurs in an athlete, in such a public way, people think about prevention. Surely it is better to prevent such an event than react to it, goes the thinking. The argument against this idea has four prongs:
The incidence of cardiac disease in a young athlete is extremely low, which sets up a situation where most “positive” tests are false positive. A false positive screening ECG or echocardiogram can create harm in multiple ways. One is the risk from downstream procedures, but worse is the inappropriate disqualification from sport. Healthwise, few harms could be greater than creating long-term fear of exercise in someone.
There is also the problem of false-negative screening tests. An ECG may be normal in the setting of hypertrophic cardiomyopathy. And a normal echocardiogram does not exclude arrhythmogenic right ventricular cardiomyopathy or other genetic causes of cardiac arrest. In a 2018 study from a major sports cardiology center in London, 6 of the 8 sudden cardiac deaths in their series were in athletes who had no detectable abnormalities on screening.
Even when disease is found, it’s not clear that prohibiting participation in sports prevents sudden death. Many previous class III recommendations against participation in sport now carry class II – may be considered – designations.
Finally, screening for any disease loses value as treatments improve. Public education regarding rapid intervention with CPR and AED use is the best treatment option. A great example is the case of Christian Erikson, a Danish soccer player who suffered cardiac arrest during a match at the European Championships in 2021 and was rapidly defibrillated on the field. Therapy was so effective that he was conscious and able to wave to fans on his way out of the stadium. He has now returned to elite competition.
Proponents of screening might oppose my take by saying that National Football League players are intensely screened. But this is different from widespread screening of high school and college athletes. It might sound harsh to say, but professional teams have dualities of interests in the health of their athletes given the million-dollar contracts.
What’s more, professional teams can afford to hire expert cardiologists to perform the testing. This would likely reduce the rate of false-positive findings, compared with screening in the community setting. I often have young people referred to me because of asymptomatic bradycardia found during athletic screening – an obviously normal finding.
Conclusions
As long as there are sports, there will be athletes who suffer cardiac arrest.
We can both hope for Hamlin’s full recovery and learn lessons to help reduce the rate of death from out-of-hospital cardiac arrest. This mostly involves education on how to help fellow humans and a public health commitment to access to AEDs.
John Mandrola, MD, practices cardiac electrophysiology in Louisville, Ky. and is a writer and podcaster for Medscape. He has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
The obvious first statement is that it’s neither wise nor appropriate to speculate on the specifics of Damar Hamlin’s cardiac event during a football game on Jan. 2 (including the possibility of commotio cordis) or his ongoing care. The public nature of his collapse induces intense curiosity but people with illness deserve privacy. Privacy in health care is in short supply. I disagree strongly with those who say his doctors ought to be giving public updates. That’s up to the family.
But there are important general concepts to consider about this incident. These include ...
Cardiac arrest can happen to anyone
People with structural heart disease or other chronic illnesses have a higher risk of arrhythmia, but the notion that athletes are immune from cardiac arrest is wrong. This sentence almost seems too obvious to write, but to this day, I hear clinicians express surprise that an athletic person has heart disease.
Survival turns on rapid and effective intervention
In the old days of electrophysiology, we used to test implantable cardioverter-defibrillators during an implant procedure by inducing ventricular fibrillation (VF) and watching the device convert it. Thankfully, trials have shown that this is no longer necessary for most implants.
When you induce VF In the EP lab, you learn quickly that a) it causes loss of consciousness in a matter of seconds, b) rapid defibrillation restores consciousness, often without the patients knowing or remembering they passed out, and c) the failure of the shock to terminate VF results in deterioration in a matter of 1-2 minutes. Even 1 minute in VF feels so long.
Need is an appropriate word in VF treatment
Clinicians often use the verb need. As in, this patient needs this pill or this procedure. It’s rarely appropriate.
But in the case of treating VF, patients truly need rapid defibrillation. Survival of out-of-hospital cardiac arrest is low because there just aren’t enough automated external defibrillators (AEDs) or people trained to use them. A study of patients who had out-of-hospital cardiac arrest in Denmark found that 30-day survival almost doubled (28.8% vs. 16.4%), when the nearest AED was accessible.
Bystanders must act
The public messages are simple: If a person loses consciousness in front of you, and is not breathing normally, assume it is a cardiac arrest, call 911 to get professional help, and start hands-only chest compressions. Don’t spend time checking for a pulse or trying to wake the person. If this is not a cardiac arrest, they will soon tell you to stop compressing their chest. Seconds matter.
Chest compressions are important but what is really needed is defibrillation. A crucial step in CPR is to send someone to get an AED and get the pads attached. If this is a shockable rhythm, deliver the shock. Hamlin’s collapse emphasizes the importance of the AED; without it, his survival to the hospital would have been unlikely.
Widespread preparticipation screening of young athletes remains a bad idea
Whenever cardiac arrest occurs in an athlete, in such a public way, people think about prevention. Surely it is better to prevent such an event than react to it, goes the thinking. The argument against this idea has four prongs:
The incidence of cardiac disease in a young athlete is extremely low, which sets up a situation where most “positive” tests are false positive. A false positive screening ECG or echocardiogram can create harm in multiple ways. One is the risk from downstream procedures, but worse is the inappropriate disqualification from sport. Healthwise, few harms could be greater than creating long-term fear of exercise in someone.
There is also the problem of false-negative screening tests. An ECG may be normal in the setting of hypertrophic cardiomyopathy. And a normal echocardiogram does not exclude arrhythmogenic right ventricular cardiomyopathy or other genetic causes of cardiac arrest. In a 2018 study from a major sports cardiology center in London, 6 of the 8 sudden cardiac deaths in their series were in athletes who had no detectable abnormalities on screening.
Even when disease is found, it’s not clear that prohibiting participation in sports prevents sudden death. Many previous class III recommendations against participation in sport now carry class II – may be considered – designations.
Finally, screening for any disease loses value as treatments improve. Public education regarding rapid intervention with CPR and AED use is the best treatment option. A great example is the case of Christian Erikson, a Danish soccer player who suffered cardiac arrest during a match at the European Championships in 2021 and was rapidly defibrillated on the field. Therapy was so effective that he was conscious and able to wave to fans on his way out of the stadium. He has now returned to elite competition.
Proponents of screening might oppose my take by saying that National Football League players are intensely screened. But this is different from widespread screening of high school and college athletes. It might sound harsh to say, but professional teams have dualities of interests in the health of their athletes given the million-dollar contracts.
What’s more, professional teams can afford to hire expert cardiologists to perform the testing. This would likely reduce the rate of false-positive findings, compared with screening in the community setting. I often have young people referred to me because of asymptomatic bradycardia found during athletic screening – an obviously normal finding.
Conclusions
As long as there are sports, there will be athletes who suffer cardiac arrest.
We can both hope for Hamlin’s full recovery and learn lessons to help reduce the rate of death from out-of-hospital cardiac arrest. This mostly involves education on how to help fellow humans and a public health commitment to access to AEDs.
John Mandrola, MD, practices cardiac electrophysiology in Louisville, Ky. and is a writer and podcaster for Medscape. He has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
STEMI times to treatment usually miss established goals
Therapy initiated within national treatment-time goals set a decade ago for patients with ST-segment elevation myocardial infarction (STEMI) remains associated with improved survival in recent years. But for many such patients, time from first symptoms to initiation of reperfusion therapy still fails to meet those goals, suggests a cross-sectional registry analysis.
For example, patients initially transported to centers with percutaneous coronary intervention (PCI) capability had a median treatment time of 148 minutes, in the analysis spanning the second quarter (Q2) of 2018 to the third quarter (Q3) of 2021. But the goal for centers called for treatment initiation within 90 minutes for at least 75% of such STEMI patients.
Moreover, overall STEMI treatment times and in-hospital mortality rose in tandem significantly from Q2 2018 through the first quarter (Q1) of 2021, which included the first year of the COVID-19 pandemic. Median time to treatment went from 86 minutes to 91 minutes during that period. Meanwhile, in-hospital mortality went from 5.6% to 8.7%, report the study authors led by James G. Jollis, MD, Duke University, Durham, N.C.
Their report, based on 114,871 STEMI patients at 601 US hospitals contributing to the Get With The Guidelines – Coronary Artery Disease registry, was published online in JAMA.
Of those patients, 25,085 had been transferred from non-PCI hospitals, 32,483 were walk-ins, and 57,303 arrived via emergency medical services (EMS). Their median times from symptom onset to PCI were 240, 195, and 148 minutes, respectively.
In-hospital mortality was significantly reduced in an adjusted analysis for patients treated within target times, compared with those whose treatment missed the time goals, regardless of whether they were transported by EMS, walked into a hospital with on-site PCI, or were transferred from a non-PCI center (Table 1).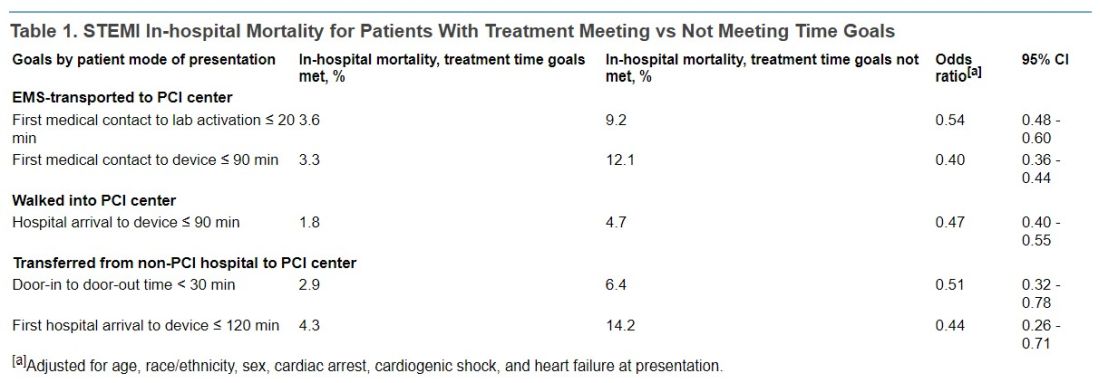
Regardless of mode of patient presentation, treatment time goals were not met most of the time, the group reports. Patients who required interhospital transfer experienced the longest system delays; only 17% were treated within 120 minutes.
Among patients who received primary PCI, 20% had a registry-defined hospital-specified reason for delay, including cardiac arrest and/or need for intubation in 6.8%, “difficulty crossing the culprit lesion” in 3.8%, and “other reasons” in 5.8%, the group reports.
“In 2020, a new reason for delay was added to the registry, ‘need for additional personal protective equipment for suspected/confirmed infectious disease.’ This reason was most commonly used in the second quarter of 2020 (6%) and then declined over time to 1% in the final 2 quarters,” they write.
“Thus, active SARS-CoV-2 infection appeared to have a smaller direct role in longer treatment times or worse outcomes.” Rather, they continue, “the pandemic potentially had a significant indirect role as hospitals were overwhelmed with patients, EMS and hospitals were challenged in maintaining paramedic and nurse staffing and intensive care bed availability, and patients experienced delayed care due to barriers to access or perceived fear of becoming entangled in an overwhelmed medical system.”
Still an important quality metric
STEMI treatment times remain an important quality metric to which hospitals should continue to pay attention because shorter times improve patient care, Deepak Bhatt, MD, MPH, told this news organization.
“Having said that, as with all metrics, one needs to be thoughtful and realize that a difference of a couple of minutes is probably not a crucial thing,” said Dr. Bhatt, Brigham and Women’s Hospital and Harvard Medical School, Boston, who was not involved with the current study.
Interhospital transfers indeed involve longer delays, he observed, suggesting that regional integrated health systems should develop methods for optimizing STEMI care – even, for example, if they involve bypassing non-PCI centers or stopping patients briefly for stabilization followed by rapid transport to a PCI-capable facility.
“That, of course, requires cooperation among hospitals. Sometimes that requires hospitals putting aside economic considerations and just focusing on doing the right thing for that individual patient,” Dr. Bhatt said.
Transfer delays are common for patients presenting with STEMI at hospitals without PCI capability, he noted. “Having clear protocols in place that expedite that type of transfer, I think, could go a long way in reducing the time to treatment in patients that are presenting to the hospital without cath labs. That’s an important message that these data provide.”
The onset of COVID-19 led to widespread delays in STEMI time to treatment early in the pandemic. There were concerns about exposing cath lab personnel to SARS-CoV-2 and potential adverse consequences of sick personnel being unable to provide patient care in the subsequent weeks and months, Dr. Bhatt observed.
However, “All of that seems to have quieted down, and I don’t think COVID is impacting time to treatment right now.”
‘Suboptimal compliance’ with standards
The current findings of “suboptimal compliance with national targets underscore why reassessing quality metrics, in light of changing practice patterns and other secular trends, is critical,” write Andrew S. Oseran, MD, MBA, and Robert W. Yeh, MD, both of Harvard Medical School, in an accompanying editorial.
“While the importance of coordinated and expeditious care for this high-risk patient population is undeniable, the specific actions that hospitals can – or should – take to further improve overall STEMI outcomes are less clear,” they say.
“As physicians contemplate the optimal path forward in managing the care of STEMI patients, they must recognize the clinical and operational nuance that exists in caring for this diverse population and acknowledge the trade-offs associated with uniform quality metrics,” write the editorialists.
“Global reductions in time to treatment for STEMI patients has been one of health care’s great success stories. As we move forward, it may be time to consider whether efforts to achieve additional improvement in target treatment times will result in substantive benefits, or whether we have reached the point of diminishing returns.”
A version of this article first appeared on Medscape.com.
Therapy initiated within national treatment-time goals set a decade ago for patients with ST-segment elevation myocardial infarction (STEMI) remains associated with improved survival in recent years. But for many such patients, time from first symptoms to initiation of reperfusion therapy still fails to meet those goals, suggests a cross-sectional registry analysis.
For example, patients initially transported to centers with percutaneous coronary intervention (PCI) capability had a median treatment time of 148 minutes, in the analysis spanning the second quarter (Q2) of 2018 to the third quarter (Q3) of 2021. But the goal for centers called for treatment initiation within 90 minutes for at least 75% of such STEMI patients.
Moreover, overall STEMI treatment times and in-hospital mortality rose in tandem significantly from Q2 2018 through the first quarter (Q1) of 2021, which included the first year of the COVID-19 pandemic. Median time to treatment went from 86 minutes to 91 minutes during that period. Meanwhile, in-hospital mortality went from 5.6% to 8.7%, report the study authors led by James G. Jollis, MD, Duke University, Durham, N.C.
Their report, based on 114,871 STEMI patients at 601 US hospitals contributing to the Get With The Guidelines – Coronary Artery Disease registry, was published online in JAMA.
Of those patients, 25,085 had been transferred from non-PCI hospitals, 32,483 were walk-ins, and 57,303 arrived via emergency medical services (EMS). Their median times from symptom onset to PCI were 240, 195, and 148 minutes, respectively.
In-hospital mortality was significantly reduced in an adjusted analysis for patients treated within target times, compared with those whose treatment missed the time goals, regardless of whether they were transported by EMS, walked into a hospital with on-site PCI, or were transferred from a non-PCI center (Table 1).
Regardless of mode of patient presentation, treatment time goals were not met most of the time, the group reports. Patients who required interhospital transfer experienced the longest system delays; only 17% were treated within 120 minutes.
Among patients who received primary PCI, 20% had a registry-defined hospital-specified reason for delay, including cardiac arrest and/or need for intubation in 6.8%, “difficulty crossing the culprit lesion” in 3.8%, and “other reasons” in 5.8%, the group reports.
“In 2020, a new reason for delay was added to the registry, ‘need for additional personal protective equipment for suspected/confirmed infectious disease.’ This reason was most commonly used in the second quarter of 2020 (6%) and then declined over time to 1% in the final 2 quarters,” they write.
“Thus, active SARS-CoV-2 infection appeared to have a smaller direct role in longer treatment times or worse outcomes.” Rather, they continue, “the pandemic potentially had a significant indirect role as hospitals were overwhelmed with patients, EMS and hospitals were challenged in maintaining paramedic and nurse staffing and intensive care bed availability, and patients experienced delayed care due to barriers to access or perceived fear of becoming entangled in an overwhelmed medical system.”
Still an important quality metric
STEMI treatment times remain an important quality metric to which hospitals should continue to pay attention because shorter times improve patient care, Deepak Bhatt, MD, MPH, told this news organization.
“Having said that, as with all metrics, one needs to be thoughtful and realize that a difference of a couple of minutes is probably not a crucial thing,” said Dr. Bhatt, Brigham and Women’s Hospital and Harvard Medical School, Boston, who was not involved with the current study.
Interhospital transfers indeed involve longer delays, he observed, suggesting that regional integrated health systems should develop methods for optimizing STEMI care – even, for example, if they involve bypassing non-PCI centers or stopping patients briefly for stabilization followed by rapid transport to a PCI-capable facility.
“That, of course, requires cooperation among hospitals. Sometimes that requires hospitals putting aside economic considerations and just focusing on doing the right thing for that individual patient,” Dr. Bhatt said.
Transfer delays are common for patients presenting with STEMI at hospitals without PCI capability, he noted. “Having clear protocols in place that expedite that type of transfer, I think, could go a long way in reducing the time to treatment in patients that are presenting to the hospital without cath labs. That’s an important message that these data provide.”
The onset of COVID-19 led to widespread delays in STEMI time to treatment early in the pandemic. There were concerns about exposing cath lab personnel to SARS-CoV-2 and potential adverse consequences of sick personnel being unable to provide patient care in the subsequent weeks and months, Dr. Bhatt observed.
However, “All of that seems to have quieted down, and I don’t think COVID is impacting time to treatment right now.”
‘Suboptimal compliance’ with standards
The current findings of “suboptimal compliance with national targets underscore why reassessing quality metrics, in light of changing practice patterns and other secular trends, is critical,” write Andrew S. Oseran, MD, MBA, and Robert W. Yeh, MD, both of Harvard Medical School, in an accompanying editorial.
“While the importance of coordinated and expeditious care for this high-risk patient population is undeniable, the specific actions that hospitals can – or should – take to further improve overall STEMI outcomes are less clear,” they say.
“As physicians contemplate the optimal path forward in managing the care of STEMI patients, they must recognize the clinical and operational nuance that exists in caring for this diverse population and acknowledge the trade-offs associated with uniform quality metrics,” write the editorialists.
“Global reductions in time to treatment for STEMI patients has been one of health care’s great success stories. As we move forward, it may be time to consider whether efforts to achieve additional improvement in target treatment times will result in substantive benefits, or whether we have reached the point of diminishing returns.”
A version of this article first appeared on Medscape.com.
Therapy initiated within national treatment-time goals set a decade ago for patients with ST-segment elevation myocardial infarction (STEMI) remains associated with improved survival in recent years. But for many such patients, time from first symptoms to initiation of reperfusion therapy still fails to meet those goals, suggests a cross-sectional registry analysis.
For example, patients initially transported to centers with percutaneous coronary intervention (PCI) capability had a median treatment time of 148 minutes, in the analysis spanning the second quarter (Q2) of 2018 to the third quarter (Q3) of 2021. But the goal for centers called for treatment initiation within 90 minutes for at least 75% of such STEMI patients.
Moreover, overall STEMI treatment times and in-hospital mortality rose in tandem significantly from Q2 2018 through the first quarter (Q1) of 2021, which included the first year of the COVID-19 pandemic. Median time to treatment went from 86 minutes to 91 minutes during that period. Meanwhile, in-hospital mortality went from 5.6% to 8.7%, report the study authors led by James G. Jollis, MD, Duke University, Durham, N.C.
Their report, based on 114,871 STEMI patients at 601 US hospitals contributing to the Get With The Guidelines – Coronary Artery Disease registry, was published online in JAMA.
Of those patients, 25,085 had been transferred from non-PCI hospitals, 32,483 were walk-ins, and 57,303 arrived via emergency medical services (EMS). Their median times from symptom onset to PCI were 240, 195, and 148 minutes, respectively.
In-hospital mortality was significantly reduced in an adjusted analysis for patients treated within target times, compared with those whose treatment missed the time goals, regardless of whether they were transported by EMS, walked into a hospital with on-site PCI, or were transferred from a non-PCI center (Table 1).
Regardless of mode of patient presentation, treatment time goals were not met most of the time, the group reports. Patients who required interhospital transfer experienced the longest system delays; only 17% were treated within 120 minutes.
Among patients who received primary PCI, 20% had a registry-defined hospital-specified reason for delay, including cardiac arrest and/or need for intubation in 6.8%, “difficulty crossing the culprit lesion” in 3.8%, and “other reasons” in 5.8%, the group reports.
“In 2020, a new reason for delay was added to the registry, ‘need for additional personal protective equipment for suspected/confirmed infectious disease.’ This reason was most commonly used in the second quarter of 2020 (6%) and then declined over time to 1% in the final 2 quarters,” they write.
“Thus, active SARS-CoV-2 infection appeared to have a smaller direct role in longer treatment times or worse outcomes.” Rather, they continue, “the pandemic potentially had a significant indirect role as hospitals were overwhelmed with patients, EMS and hospitals were challenged in maintaining paramedic and nurse staffing and intensive care bed availability, and patients experienced delayed care due to barriers to access or perceived fear of becoming entangled in an overwhelmed medical system.”
Still an important quality metric
STEMI treatment times remain an important quality metric to which hospitals should continue to pay attention because shorter times improve patient care, Deepak Bhatt, MD, MPH, told this news organization.
“Having said that, as with all metrics, one needs to be thoughtful and realize that a difference of a couple of minutes is probably not a crucial thing,” said Dr. Bhatt, Brigham and Women’s Hospital and Harvard Medical School, Boston, who was not involved with the current study.
Interhospital transfers indeed involve longer delays, he observed, suggesting that regional integrated health systems should develop methods for optimizing STEMI care – even, for example, if they involve bypassing non-PCI centers or stopping patients briefly for stabilization followed by rapid transport to a PCI-capable facility.
“That, of course, requires cooperation among hospitals. Sometimes that requires hospitals putting aside economic considerations and just focusing on doing the right thing for that individual patient,” Dr. Bhatt said.
Transfer delays are common for patients presenting with STEMI at hospitals without PCI capability, he noted. “Having clear protocols in place that expedite that type of transfer, I think, could go a long way in reducing the time to treatment in patients that are presenting to the hospital without cath labs. That’s an important message that these data provide.”
The onset of COVID-19 led to widespread delays in STEMI time to treatment early in the pandemic. There were concerns about exposing cath lab personnel to SARS-CoV-2 and potential adverse consequences of sick personnel being unable to provide patient care in the subsequent weeks and months, Dr. Bhatt observed.
However, “All of that seems to have quieted down, and I don’t think COVID is impacting time to treatment right now.”
‘Suboptimal compliance’ with standards
The current findings of “suboptimal compliance with national targets underscore why reassessing quality metrics, in light of changing practice patterns and other secular trends, is critical,” write Andrew S. Oseran, MD, MBA, and Robert W. Yeh, MD, both of Harvard Medical School, in an accompanying editorial.
“While the importance of coordinated and expeditious care for this high-risk patient population is undeniable, the specific actions that hospitals can – or should – take to further improve overall STEMI outcomes are less clear,” they say.
“As physicians contemplate the optimal path forward in managing the care of STEMI patients, they must recognize the clinical and operational nuance that exists in caring for this diverse population and acknowledge the trade-offs associated with uniform quality metrics,” write the editorialists.
“Global reductions in time to treatment for STEMI patients has been one of health care’s great success stories. As we move forward, it may be time to consider whether efforts to achieve additional improvement in target treatment times will result in substantive benefits, or whether we have reached the point of diminishing returns.”
A version of this article first appeared on Medscape.com.
FROM JAMA
Heart benefits begin at well under 10,000 daily steps
– and the benefits accrue at well below the widely promoted threshold of 10,000 steps per day, new research shows.
Among adults aged 60 and older, those who took roughly 6,000 to 9,000 steps per day had a 40% to 50% lower risk of CVD, compared with peers logging just 2,000 steps per day.
“We hope this study will contribute evidence to future public health and clinical guidance on how many steps we need for health,” Amanda Paluch, PhD, with University of Massachusetts Amherst, told this news organization.
Getting in more steps per day can lower an individual’s risk for heart disease – but it’s not an “all or nothing” situation, Dr. Paluch said.
“The heart health benefits begin at lower than 10,000 steps per day. So, for the many adults that may find 10,000 steps a bit out of reach, it is important to promote that even small increases in steps can be beneficial for health,” Dr. Paluch said.
The study was published online in Circulation.
Attainable step goals
As part of the Steps for Health Collaborative, Dr. Paluch and colleagues examined the dose-response relationship between steps per day and CVD in a meta-analysis of eight prospective studies involving 20,152 adults (mean age 63, 52% women).
Steps were measured in each study using one of five different commercially available step-measuring devices. Adults aged 60 years and older took a median of 4,323 steps per day (interquartile range, 2,760-6,924), while younger adults walked a bit more (median 6,911 daily steps; IQR, 4,783-9,794).
During follow-up lasting an average of 6.2 years, a total of 1,523 CVD events were reported.
In the final adjusted model, for older adults, compared with those in quartile 1 who got the fewest steps per day (median 1,811), the risk of CVD was 20% lower in those in quartile 2, who got a median of 3,823 steps per day (hazard ratio, 0.80; 95% confidence interval, 0.69-0.93).
CVD risk was 38% lower in older adults in quartile 3 who got a median of 5,520 steps per day (HR, 0.62; 95% CI, 0.52-0.74) and 49% lower in those in quartile 4 who walked the most (a median of 9,259 steps per day; HR, 0.51; 95% CI, 0.41-0.63).
Restricting the analysis to individuals without known CVD at baseline showed similar results.
Among six studies that excluded adults with a history of CVD at baseline, compared with the lowest quartile, the HR for incident CVD events was 0.74 (95% CI, 0.60-0.91) in the second quartile, 0.60 (95% CI, 0.47-0.77) in the third quartile, and 0.55 (95% CI, 0.40-0.76) in the fourth quartile.
Despite the inverse association of steps with CVD in older adults, there was no association in younger adults. The researchers caution, however, that CVD is a disease of aging, and the follow-up period in these studies may not have been long enough to capture CVD incidence in younger adults.
Stepping rate (pace or cadence) was not associated with CVD risk beyond that of total steps per day. However, only four of the eight studies reported data on stepping rate, so this finding should be viewed as preliminary, Dr. Paluch and colleagues say.
Start small and go from there
Dr. Paluch said the take-home message from this study and numerous others is simple.
“Move more and sit less! Being physically active, by getting in your steps, is an important part of keeping your heart healthy,” she said in an interview.
For adults who are currently inactive, Dr. Paluch suggests finding small ways to get in a few more steps per day. “It does not need to be drastic changes. Consider a brief 5- to 10-minute walking break at lunch, taking the stairs, or playing a game of hide and seek with the grandchildren,” Dr. Paluch advised.
“For adults starting at 3,000 steps a day, set a goal of 4,000, and then 5,000. Each improvement can lead to better heart health,” Dr. Paluch said. “And for those who are already active, keep it up, as there are benefits with higher volumes of steps per day as well.”
Support for this research was provided by the Intergovernmental Personnel Act Agreement through the Centers for Disease Control and Prevention. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
– and the benefits accrue at well below the widely promoted threshold of 10,000 steps per day, new research shows.
Among adults aged 60 and older, those who took roughly 6,000 to 9,000 steps per day had a 40% to 50% lower risk of CVD, compared with peers logging just 2,000 steps per day.
“We hope this study will contribute evidence to future public health and clinical guidance on how many steps we need for health,” Amanda Paluch, PhD, with University of Massachusetts Amherst, told this news organization.
Getting in more steps per day can lower an individual’s risk for heart disease – but it’s not an “all or nothing” situation, Dr. Paluch said.
“The heart health benefits begin at lower than 10,000 steps per day. So, for the many adults that may find 10,000 steps a bit out of reach, it is important to promote that even small increases in steps can be beneficial for health,” Dr. Paluch said.
The study was published online in Circulation.
Attainable step goals
As part of the Steps for Health Collaborative, Dr. Paluch and colleagues examined the dose-response relationship between steps per day and CVD in a meta-analysis of eight prospective studies involving 20,152 adults (mean age 63, 52% women).
Steps were measured in each study using one of five different commercially available step-measuring devices. Adults aged 60 years and older took a median of 4,323 steps per day (interquartile range, 2,760-6,924), while younger adults walked a bit more (median 6,911 daily steps; IQR, 4,783-9,794).
During follow-up lasting an average of 6.2 years, a total of 1,523 CVD events were reported.
In the final adjusted model, for older adults, compared with those in quartile 1 who got the fewest steps per day (median 1,811), the risk of CVD was 20% lower in those in quartile 2, who got a median of 3,823 steps per day (hazard ratio, 0.80; 95% confidence interval, 0.69-0.93).
CVD risk was 38% lower in older adults in quartile 3 who got a median of 5,520 steps per day (HR, 0.62; 95% CI, 0.52-0.74) and 49% lower in those in quartile 4 who walked the most (a median of 9,259 steps per day; HR, 0.51; 95% CI, 0.41-0.63).
Restricting the analysis to individuals without known CVD at baseline showed similar results.
Among six studies that excluded adults with a history of CVD at baseline, compared with the lowest quartile, the HR for incident CVD events was 0.74 (95% CI, 0.60-0.91) in the second quartile, 0.60 (95% CI, 0.47-0.77) in the third quartile, and 0.55 (95% CI, 0.40-0.76) in the fourth quartile.
Despite the inverse association of steps with CVD in older adults, there was no association in younger adults. The researchers caution, however, that CVD is a disease of aging, and the follow-up period in these studies may not have been long enough to capture CVD incidence in younger adults.
Stepping rate (pace or cadence) was not associated with CVD risk beyond that of total steps per day. However, only four of the eight studies reported data on stepping rate, so this finding should be viewed as preliminary, Dr. Paluch and colleagues say.
Start small and go from there
Dr. Paluch said the take-home message from this study and numerous others is simple.
“Move more and sit less! Being physically active, by getting in your steps, is an important part of keeping your heart healthy,” she said in an interview.
For adults who are currently inactive, Dr. Paluch suggests finding small ways to get in a few more steps per day. “It does not need to be drastic changes. Consider a brief 5- to 10-minute walking break at lunch, taking the stairs, or playing a game of hide and seek with the grandchildren,” Dr. Paluch advised.
“For adults starting at 3,000 steps a day, set a goal of 4,000, and then 5,000. Each improvement can lead to better heart health,” Dr. Paluch said. “And for those who are already active, keep it up, as there are benefits with higher volumes of steps per day as well.”
Support for this research was provided by the Intergovernmental Personnel Act Agreement through the Centers for Disease Control and Prevention. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
– and the benefits accrue at well below the widely promoted threshold of 10,000 steps per day, new research shows.
Among adults aged 60 and older, those who took roughly 6,000 to 9,000 steps per day had a 40% to 50% lower risk of CVD, compared with peers logging just 2,000 steps per day.
“We hope this study will contribute evidence to future public health and clinical guidance on how many steps we need for health,” Amanda Paluch, PhD, with University of Massachusetts Amherst, told this news organization.
Getting in more steps per day can lower an individual’s risk for heart disease – but it’s not an “all or nothing” situation, Dr. Paluch said.
“The heart health benefits begin at lower than 10,000 steps per day. So, for the many adults that may find 10,000 steps a bit out of reach, it is important to promote that even small increases in steps can be beneficial for health,” Dr. Paluch said.
The study was published online in Circulation.
Attainable step goals
As part of the Steps for Health Collaborative, Dr. Paluch and colleagues examined the dose-response relationship between steps per day and CVD in a meta-analysis of eight prospective studies involving 20,152 adults (mean age 63, 52% women).
Steps were measured in each study using one of five different commercially available step-measuring devices. Adults aged 60 years and older took a median of 4,323 steps per day (interquartile range, 2,760-6,924), while younger adults walked a bit more (median 6,911 daily steps; IQR, 4,783-9,794).
During follow-up lasting an average of 6.2 years, a total of 1,523 CVD events were reported.
In the final adjusted model, for older adults, compared with those in quartile 1 who got the fewest steps per day (median 1,811), the risk of CVD was 20% lower in those in quartile 2, who got a median of 3,823 steps per day (hazard ratio, 0.80; 95% confidence interval, 0.69-0.93).
CVD risk was 38% lower in older adults in quartile 3 who got a median of 5,520 steps per day (HR, 0.62; 95% CI, 0.52-0.74) and 49% lower in those in quartile 4 who walked the most (a median of 9,259 steps per day; HR, 0.51; 95% CI, 0.41-0.63).
Restricting the analysis to individuals without known CVD at baseline showed similar results.
Among six studies that excluded adults with a history of CVD at baseline, compared with the lowest quartile, the HR for incident CVD events was 0.74 (95% CI, 0.60-0.91) in the second quartile, 0.60 (95% CI, 0.47-0.77) in the third quartile, and 0.55 (95% CI, 0.40-0.76) in the fourth quartile.
Despite the inverse association of steps with CVD in older adults, there was no association in younger adults. The researchers caution, however, that CVD is a disease of aging, and the follow-up period in these studies may not have been long enough to capture CVD incidence in younger adults.
Stepping rate (pace or cadence) was not associated with CVD risk beyond that of total steps per day. However, only four of the eight studies reported data on stepping rate, so this finding should be viewed as preliminary, Dr. Paluch and colleagues say.
Start small and go from there
Dr. Paluch said the take-home message from this study and numerous others is simple.
“Move more and sit less! Being physically active, by getting in your steps, is an important part of keeping your heart healthy,” she said in an interview.
For adults who are currently inactive, Dr. Paluch suggests finding small ways to get in a few more steps per day. “It does not need to be drastic changes. Consider a brief 5- to 10-minute walking break at lunch, taking the stairs, or playing a game of hide and seek with the grandchildren,” Dr. Paluch advised.
“For adults starting at 3,000 steps a day, set a goal of 4,000, and then 5,000. Each improvement can lead to better heart health,” Dr. Paluch said. “And for those who are already active, keep it up, as there are benefits with higher volumes of steps per day as well.”
Support for this research was provided by the Intergovernmental Personnel Act Agreement through the Centers for Disease Control and Prevention. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION
Women with cycle disorders across their life span may be at increased risk of cardiovascular disease
This finding is demonstrated in a new analysis of the Nurses’ Health Study II.
“To date, several studies have reported increased risks of cardiovascular risk factors or cardiovascular disease in connection with cycle disorders,” Yi-Xin Wang, MD, PhD, a research fellow in nutrition, and associates from the Harvard School of Public Health, Boston, wrote in an article published in JAMA Network Open.
Ute Seeland, MD, speaker of the Gender Medicine in Cardiology Working Group of the German Cardiology Society, said in an interview“We know that women who have indicated in their medical history that they have irregular menstrual cycles, invariably in connection with polycystic ovary syndrome (PCOS), more commonly develop diabetes and other metabolic disorders, as well as cardiovascular diseases.”
Cycle disorders’ role
However, the role that irregular or especially long cycles play at different points of a woman’s reproductive life span was unclear. Therefore, the research group investigated the associations in the Nurses’ Health Study II between cycle irregularity and cycle length in women of different age groups who later experienced cardiovascular events.
At the end of this study in 1989, the participants also provided information regarding the length and irregularity of their menstrual cycle from ages 14 to 17 years and again from ages 18 to 22 years. The information was updated in 1993 when the participants were aged 29-46 years. The data from 2019 to 2022 were analyzed.
“This kind of long-term cohort study is extremely rare and therefore something special,” said Dr. Seeland, who conducts research at the Institute for Social Medicine, Epidemiology, and Health Economics at the Charité – University Hospital Berlin.
The investigators used the following cycle classifications: very regular (no more than 3 or 4 days before or after the expected date), regular (within 5-7 days), usually irregular, always irregular, or no periods.
The cycle lengths were divided into the following categories: less than 21 days, 21-25 days, 26-31 days, 32-39 days, 40-50 days, more than 50 days, and too irregular to estimate the length.
The onset of cardiovascular diseases was determined using information from the participants and was confirmed by reviewing the medical files. Relevant to the study were lethal and nonlethal coronary heart diseases (such as myocardial infarction or coronary artery revascularization), as well as strokes.
Significant in adulthood
The data from 80,630 study participants were included in the analysis. At study inclusion, the average age of the participants was 37.7 years, and the average body mass index (BMI) was 25.1. “Since it was predominantly White nurses who took part in the study, the data are not transferable to other, more diverse populations,” said Dr. Seeland.
Over 24 years, 1,816 women (2.4%) had a cardiovascular event. “We observed an increased rate of cardiovascular events in women with an irregular cycle and longer cycle, both in early an in mid-adulthood,” wrote Dr. Wang and associates. “Similar trends were also observed for cycle disorders when younger, but this association was weaker than in adulthood.”
Compared with women with very regular cycles, women with irregular cycles or without periods who were aged 14-17 years, 18-22 years, or 29-49 years exhibited a 15%, 36%, and 40% higher risk of a cardiovascular event, respectively.
Similarly, women aged 18-22 years or 29-46 years with long cycles of 40 days or more had a 44% or 30% higher risk of cardiovascular disease, respectively, compared with women with cycle lengths of 26-31 days.
“The coronary heart diseases were decisive for the increase, and less so, the strokes,” wrote the researchers.
Classic risk factors?
Dr. Seeland praised the fact that the study authors tried to determine the role that classic cardiovascular risk factors played. “Compared with women with a regular cycle, women with an irregular cycle had a higher BMI, more frequently increased cholesterol levels, and an elevated blood pressure,” she said. Women with a long cycle displayed a similar pattern.
It can be assumed from this that over a woman’s life span, BMI affects the risk of cardiovascular disease. Therefore, Dr. Wang and coauthors adjusted the results on the basis of BMI, which varies over time.
Regarding other classic risk factors that may have played a role, “hypercholesterolemia, chronic high blood pressure, and type 2 diabetes were only responsible in 5.4%-13.5% of the associations,” wrote the researchers.
“Our results suggest that certain characteristics of the menstrual cycle across a woman’s reproductive lifespan may constitute additional risk markers for cardiovascular disease,” according to the authors.
The highest rates of cardiovascular disease were among women with permanently irregular or long cycles in early to mid adulthood, as well as women who had regular cycles when younger but had irregular cycles in mid adulthood. “This indicates that the change from one cycle phenotype to another could be a surrogate marker for metabolic changes, which in turn contribute to the formation of cardiovascular diseases,” wrote the authors.
The study was observational and so conclusions cannot be drawn regarding causal relationships. But Dr. Wang and associates indicate that the most common cause of an irregular menstrual cycle may be PCOS. “Roughly 90% of women with cycle disorders or oligomenorrhea have signs of PCOS. And it was shown that women with PCOS have an increased risk of cardiovascular disease.”
They concluded that “the associations observed between irregular and long cycles in early to mid-adulthood and cardiovascular diseases are likely attributable to underlying PCOS.”
For Dr. Seeland, however, this conclusion is “too monocausal. At no point in time did there seem to be any direct information regarding the frequency of PCOS during the data collection by the respondents.”
For now, we can only speculate about the mechanisms. “The association between a very irregular and long cycle and the increased risk of cardiovascular diseases has now only been described. More research should be done on the causes,” said Dr. Seeland.
This article was translated from the Medscape German edition. A version of this article first appeared on Medscape.com.
This finding is demonstrated in a new analysis of the Nurses’ Health Study II.
“To date, several studies have reported increased risks of cardiovascular risk factors or cardiovascular disease in connection with cycle disorders,” Yi-Xin Wang, MD, PhD, a research fellow in nutrition, and associates from the Harvard School of Public Health, Boston, wrote in an article published in JAMA Network Open.
Ute Seeland, MD, speaker of the Gender Medicine in Cardiology Working Group of the German Cardiology Society, said in an interview“We know that women who have indicated in their medical history that they have irregular menstrual cycles, invariably in connection with polycystic ovary syndrome (PCOS), more commonly develop diabetes and other metabolic disorders, as well as cardiovascular diseases.”
Cycle disorders’ role
However, the role that irregular or especially long cycles play at different points of a woman’s reproductive life span was unclear. Therefore, the research group investigated the associations in the Nurses’ Health Study II between cycle irregularity and cycle length in women of different age groups who later experienced cardiovascular events.
At the end of this study in 1989, the participants also provided information regarding the length and irregularity of their menstrual cycle from ages 14 to 17 years and again from ages 18 to 22 years. The information was updated in 1993 when the participants were aged 29-46 years. The data from 2019 to 2022 were analyzed.
“This kind of long-term cohort study is extremely rare and therefore something special,” said Dr. Seeland, who conducts research at the Institute for Social Medicine, Epidemiology, and Health Economics at the Charité – University Hospital Berlin.
The investigators used the following cycle classifications: very regular (no more than 3 or 4 days before or after the expected date), regular (within 5-7 days), usually irregular, always irregular, or no periods.
The cycle lengths were divided into the following categories: less than 21 days, 21-25 days, 26-31 days, 32-39 days, 40-50 days, more than 50 days, and too irregular to estimate the length.
The onset of cardiovascular diseases was determined using information from the participants and was confirmed by reviewing the medical files. Relevant to the study were lethal and nonlethal coronary heart diseases (such as myocardial infarction or coronary artery revascularization), as well as strokes.
Significant in adulthood
The data from 80,630 study participants were included in the analysis. At study inclusion, the average age of the participants was 37.7 years, and the average body mass index (BMI) was 25.1. “Since it was predominantly White nurses who took part in the study, the data are not transferable to other, more diverse populations,” said Dr. Seeland.
Over 24 years, 1,816 women (2.4%) had a cardiovascular event. “We observed an increased rate of cardiovascular events in women with an irregular cycle and longer cycle, both in early an in mid-adulthood,” wrote Dr. Wang and associates. “Similar trends were also observed for cycle disorders when younger, but this association was weaker than in adulthood.”
Compared with women with very regular cycles, women with irregular cycles or without periods who were aged 14-17 years, 18-22 years, or 29-49 years exhibited a 15%, 36%, and 40% higher risk of a cardiovascular event, respectively.
Similarly, women aged 18-22 years or 29-46 years with long cycles of 40 days or more had a 44% or 30% higher risk of cardiovascular disease, respectively, compared with women with cycle lengths of 26-31 days.
“The coronary heart diseases were decisive for the increase, and less so, the strokes,” wrote the researchers.
Classic risk factors?
Dr. Seeland praised the fact that the study authors tried to determine the role that classic cardiovascular risk factors played. “Compared with women with a regular cycle, women with an irregular cycle had a higher BMI, more frequently increased cholesterol levels, and an elevated blood pressure,” she said. Women with a long cycle displayed a similar pattern.
It can be assumed from this that over a woman’s life span, BMI affects the risk of cardiovascular disease. Therefore, Dr. Wang and coauthors adjusted the results on the basis of BMI, which varies over time.
Regarding other classic risk factors that may have played a role, “hypercholesterolemia, chronic high blood pressure, and type 2 diabetes were only responsible in 5.4%-13.5% of the associations,” wrote the researchers.
“Our results suggest that certain characteristics of the menstrual cycle across a woman’s reproductive lifespan may constitute additional risk markers for cardiovascular disease,” according to the authors.
The highest rates of cardiovascular disease were among women with permanently irregular or long cycles in early to mid adulthood, as well as women who had regular cycles when younger but had irregular cycles in mid adulthood. “This indicates that the change from one cycle phenotype to another could be a surrogate marker for metabolic changes, which in turn contribute to the formation of cardiovascular diseases,” wrote the authors.
The study was observational and so conclusions cannot be drawn regarding causal relationships. But Dr. Wang and associates indicate that the most common cause of an irregular menstrual cycle may be PCOS. “Roughly 90% of women with cycle disorders or oligomenorrhea have signs of PCOS. And it was shown that women with PCOS have an increased risk of cardiovascular disease.”
They concluded that “the associations observed between irregular and long cycles in early to mid-adulthood and cardiovascular diseases are likely attributable to underlying PCOS.”
For Dr. Seeland, however, this conclusion is “too monocausal. At no point in time did there seem to be any direct information regarding the frequency of PCOS during the data collection by the respondents.”
For now, we can only speculate about the mechanisms. “The association between a very irregular and long cycle and the increased risk of cardiovascular diseases has now only been described. More research should be done on the causes,” said Dr. Seeland.
This article was translated from the Medscape German edition. A version of this article first appeared on Medscape.com.
This finding is demonstrated in a new analysis of the Nurses’ Health Study II.
“To date, several studies have reported increased risks of cardiovascular risk factors or cardiovascular disease in connection with cycle disorders,” Yi-Xin Wang, MD, PhD, a research fellow in nutrition, and associates from the Harvard School of Public Health, Boston, wrote in an article published in JAMA Network Open.
Ute Seeland, MD, speaker of the Gender Medicine in Cardiology Working Group of the German Cardiology Society, said in an interview“We know that women who have indicated in their medical history that they have irregular menstrual cycles, invariably in connection with polycystic ovary syndrome (PCOS), more commonly develop diabetes and other metabolic disorders, as well as cardiovascular diseases.”
Cycle disorders’ role
However, the role that irregular or especially long cycles play at different points of a woman’s reproductive life span was unclear. Therefore, the research group investigated the associations in the Nurses’ Health Study II between cycle irregularity and cycle length in women of different age groups who later experienced cardiovascular events.
At the end of this study in 1989, the participants also provided information regarding the length and irregularity of their menstrual cycle from ages 14 to 17 years and again from ages 18 to 22 years. The information was updated in 1993 when the participants were aged 29-46 years. The data from 2019 to 2022 were analyzed.
“This kind of long-term cohort study is extremely rare and therefore something special,” said Dr. Seeland, who conducts research at the Institute for Social Medicine, Epidemiology, and Health Economics at the Charité – University Hospital Berlin.
The investigators used the following cycle classifications: very regular (no more than 3 or 4 days before or after the expected date), regular (within 5-7 days), usually irregular, always irregular, or no periods.
The cycle lengths were divided into the following categories: less than 21 days, 21-25 days, 26-31 days, 32-39 days, 40-50 days, more than 50 days, and too irregular to estimate the length.
The onset of cardiovascular diseases was determined using information from the participants and was confirmed by reviewing the medical files. Relevant to the study were lethal and nonlethal coronary heart diseases (such as myocardial infarction or coronary artery revascularization), as well as strokes.
Significant in adulthood
The data from 80,630 study participants were included in the analysis. At study inclusion, the average age of the participants was 37.7 years, and the average body mass index (BMI) was 25.1. “Since it was predominantly White nurses who took part in the study, the data are not transferable to other, more diverse populations,” said Dr. Seeland.
Over 24 years, 1,816 women (2.4%) had a cardiovascular event. “We observed an increased rate of cardiovascular events in women with an irregular cycle and longer cycle, both in early an in mid-adulthood,” wrote Dr. Wang and associates. “Similar trends were also observed for cycle disorders when younger, but this association was weaker than in adulthood.”
Compared with women with very regular cycles, women with irregular cycles or without periods who were aged 14-17 years, 18-22 years, or 29-49 years exhibited a 15%, 36%, and 40% higher risk of a cardiovascular event, respectively.
Similarly, women aged 18-22 years or 29-46 years with long cycles of 40 days or more had a 44% or 30% higher risk of cardiovascular disease, respectively, compared with women with cycle lengths of 26-31 days.
“The coronary heart diseases were decisive for the increase, and less so, the strokes,” wrote the researchers.
Classic risk factors?
Dr. Seeland praised the fact that the study authors tried to determine the role that classic cardiovascular risk factors played. “Compared with women with a regular cycle, women with an irregular cycle had a higher BMI, more frequently increased cholesterol levels, and an elevated blood pressure,” she said. Women with a long cycle displayed a similar pattern.
It can be assumed from this that over a woman’s life span, BMI affects the risk of cardiovascular disease. Therefore, Dr. Wang and coauthors adjusted the results on the basis of BMI, which varies over time.
Regarding other classic risk factors that may have played a role, “hypercholesterolemia, chronic high blood pressure, and type 2 diabetes were only responsible in 5.4%-13.5% of the associations,” wrote the researchers.
“Our results suggest that certain characteristics of the menstrual cycle across a woman’s reproductive lifespan may constitute additional risk markers for cardiovascular disease,” according to the authors.
The highest rates of cardiovascular disease were among women with permanently irregular or long cycles in early to mid adulthood, as well as women who had regular cycles when younger but had irregular cycles in mid adulthood. “This indicates that the change from one cycle phenotype to another could be a surrogate marker for metabolic changes, which in turn contribute to the formation of cardiovascular diseases,” wrote the authors.
The study was observational and so conclusions cannot be drawn regarding causal relationships. But Dr. Wang and associates indicate that the most common cause of an irregular menstrual cycle may be PCOS. “Roughly 90% of women with cycle disorders or oligomenorrhea have signs of PCOS. And it was shown that women with PCOS have an increased risk of cardiovascular disease.”
They concluded that “the associations observed between irregular and long cycles in early to mid-adulthood and cardiovascular diseases are likely attributable to underlying PCOS.”
For Dr. Seeland, however, this conclusion is “too monocausal. At no point in time did there seem to be any direct information regarding the frequency of PCOS during the data collection by the respondents.”
For now, we can only speculate about the mechanisms. “The association between a very irregular and long cycle and the increased risk of cardiovascular diseases has now only been described. More research should be done on the causes,” said Dr. Seeland.
This article was translated from the Medscape German edition. A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Top cardiology societies call for revamp of clinical trials
Leading cardiology societies have issued a “call for action” on a global scale to reinvent randomized clinical trials fit for the 21st century.
“Randomized trials are an essential tool for reliably assessing the effects of treatments, but they have become too costly and too burdensome,” first author Louise Bowman, University of Oxford, England, told this news organization. “We urgently need to modernize our approach to clinical trials in order to continue to improve patient care.”
The joint opinion is from the European Society of Cardiology, the American Heart Association, the American College of Cardiology, and the World Heart Federation. It was simultaneously published online in the European Heart Journal, Circulation, Journal of the American College of Cardiology, and Global Heart.
The authors note that the availability of large-scale “real-world” data is increasingly being touted as a way to bypass the challenges of conducting randomized trials. Yet, observational analyses of real-world data “are not a suitable alternative to randomization,” Prof. Bowman said.
Cardiology has historically led the way in transforming clinical practice with groundbreaking “mega-trials,” such as the International Study of Infarct Survival (ISIS), Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto (GISSI), and Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO).
But over the past 25 years, there has been a huge increase in the rules and related bureaucracy governing clinical trials, which hinders the ability to conduct trials swiftly and affordably, the authors point out.
The COVID-19 pandemic has shown that important clinical trials can be performed quickly and efficiently in busy hospitals, they note.
“The RECOVERY trial in COVID-19 has been an excellent example of this, with results that are estimated to have saved around 1 million lives worldwide within just 1 year,” Prof. Bowman told this news organization.
A Good Clinical Trials Collaborative made up of key stakeholders recently developed new guidelines designed to promote better, more efficient randomized controlled trials.
“If widely adopted and used alongside valuable 21st century electronic health records, we could transform the clinical trials landscape and do many more high-quality trials very cost-effectively,” Prof. Bowman said.
“Widespread adoption and implementation of the revised guidelines will require collaboration with a wide range of national and international organizations, including patient, professional, academic, and industry groups, funders and government organizations, and ethics, health policy, and regulatory bodies,” Prof. Bowman acknowledged.
“This is work that the Good Clinical Trials Collaborative is leading. It is hoped that this endorsement by the joint cardiovascular societies will increase awareness and provide valuable support to his important work,” she added.
No commercial funding was received. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Leading cardiology societies have issued a “call for action” on a global scale to reinvent randomized clinical trials fit for the 21st century.
“Randomized trials are an essential tool for reliably assessing the effects of treatments, but they have become too costly and too burdensome,” first author Louise Bowman, University of Oxford, England, told this news organization. “We urgently need to modernize our approach to clinical trials in order to continue to improve patient care.”
The joint opinion is from the European Society of Cardiology, the American Heart Association, the American College of Cardiology, and the World Heart Federation. It was simultaneously published online in the European Heart Journal, Circulation, Journal of the American College of Cardiology, and Global Heart.
The authors note that the availability of large-scale “real-world” data is increasingly being touted as a way to bypass the challenges of conducting randomized trials. Yet, observational analyses of real-world data “are not a suitable alternative to randomization,” Prof. Bowman said.
Cardiology has historically led the way in transforming clinical practice with groundbreaking “mega-trials,” such as the International Study of Infarct Survival (ISIS), Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto (GISSI), and Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO).
But over the past 25 years, there has been a huge increase in the rules and related bureaucracy governing clinical trials, which hinders the ability to conduct trials swiftly and affordably, the authors point out.
The COVID-19 pandemic has shown that important clinical trials can be performed quickly and efficiently in busy hospitals, they note.
“The RECOVERY trial in COVID-19 has been an excellent example of this, with results that are estimated to have saved around 1 million lives worldwide within just 1 year,” Prof. Bowman told this news organization.
A Good Clinical Trials Collaborative made up of key stakeholders recently developed new guidelines designed to promote better, more efficient randomized controlled trials.
“If widely adopted and used alongside valuable 21st century electronic health records, we could transform the clinical trials landscape and do many more high-quality trials very cost-effectively,” Prof. Bowman said.
“Widespread adoption and implementation of the revised guidelines will require collaboration with a wide range of national and international organizations, including patient, professional, academic, and industry groups, funders and government organizations, and ethics, health policy, and regulatory bodies,” Prof. Bowman acknowledged.
“This is work that the Good Clinical Trials Collaborative is leading. It is hoped that this endorsement by the joint cardiovascular societies will increase awareness and provide valuable support to his important work,” she added.
No commercial funding was received. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Leading cardiology societies have issued a “call for action” on a global scale to reinvent randomized clinical trials fit for the 21st century.
“Randomized trials are an essential tool for reliably assessing the effects of treatments, but they have become too costly and too burdensome,” first author Louise Bowman, University of Oxford, England, told this news organization. “We urgently need to modernize our approach to clinical trials in order to continue to improve patient care.”
The joint opinion is from the European Society of Cardiology, the American Heart Association, the American College of Cardiology, and the World Heart Federation. It was simultaneously published online in the European Heart Journal, Circulation, Journal of the American College of Cardiology, and Global Heart.
The authors note that the availability of large-scale “real-world” data is increasingly being touted as a way to bypass the challenges of conducting randomized trials. Yet, observational analyses of real-world data “are not a suitable alternative to randomization,” Prof. Bowman said.
Cardiology has historically led the way in transforming clinical practice with groundbreaking “mega-trials,” such as the International Study of Infarct Survival (ISIS), Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto (GISSI), and Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO).
But over the past 25 years, there has been a huge increase in the rules and related bureaucracy governing clinical trials, which hinders the ability to conduct trials swiftly and affordably, the authors point out.
The COVID-19 pandemic has shown that important clinical trials can be performed quickly and efficiently in busy hospitals, they note.
“The RECOVERY trial in COVID-19 has been an excellent example of this, with results that are estimated to have saved around 1 million lives worldwide within just 1 year,” Prof. Bowman told this news organization.
A Good Clinical Trials Collaborative made up of key stakeholders recently developed new guidelines designed to promote better, more efficient randomized controlled trials.
“If widely adopted and used alongside valuable 21st century electronic health records, we could transform the clinical trials landscape and do many more high-quality trials very cost-effectively,” Prof. Bowman said.
“Widespread adoption and implementation of the revised guidelines will require collaboration with a wide range of national and international organizations, including patient, professional, academic, and industry groups, funders and government organizations, and ethics, health policy, and regulatory bodies,” Prof. Bowman acknowledged.
“This is work that the Good Clinical Trials Collaborative is leading. It is hoped that this endorsement by the joint cardiovascular societies will increase awareness and provide valuable support to his important work,” she added.
No commercial funding was received. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
High lipoprotein(a) levels plus hypertension add to CVD risk
High levels of lipoprotein(a) increase the risk for incident cardiovascular disease (CVD) for hypertensive individuals but not for those without hypertension, a new MESA analysis suggests.
There are ways to test for statistical interaction, “in this case, multiplicative interaction between Lp(a) and hypertension, which suggests that Lp(a) is actually modifying the effect between blood pressure and cardiovascular disease. It’s not simply additive,” senior author Michael D. Shapiro, DO, Wake Forest University, Winston-Salem, N.C., told this news organization.
“So that’s new and I don’t think anybody’s looked at that before.”
Although Lp(a) is recognized as an independent cause of atherosclerotic CVD (ASCVD), the significance of Lp(a) in hypertension has been “virtually untapped,” he noted. A recent prospective study reported that elevated CVD risk was present only in individuals with Lp(a) ≥ 30 mg/dL and hypertension but it included only Chinese participants with stable coronary artery disease.
The current analysis, published online in the journal Hypertension, included 6,674 participants in the ongoing Multi-Ethnic Study of Atherosclerosis (MESA), all free of baseline ASCVD, who were recruited from six communities in the United States and had measured baseline Lp(a), blood pressure, and CVD events data over follow-up from 2000 to 2018.
Participants were stratified into four groups based on the presence or absence of hypertension (defined as 140/90 mm Hg or higher or the use of antihypertensive drugs) and an Lp(a) threshold of 50 mg/dL, as recommended by the American College of Cardiology/American Heart Association cholesterol guideline for consideration as an ASCVD risk-enhancing factor.
Slightly more than half of participants were female (52.8%), 38.6% were White, 27.5% were African American, 22.1% were Hispanic, and 11.9% were Chinese American.
According to the researchers, 809 participants had a CVD event over an average follow-up of 13.9 years, including 7.7% of group 1 with Lp(a) < 50 mg/dL and no hypertension, 8.0% of group 2 with Lp(a) ≥ 50 mg/dL and no hypertension, 16.2% of group 3 with Lp(a) < 50 mg/dL and hypertension, and 18.8% of group 4 with Lp(a) ≥ 50 mg/dL and hypertension.
When compared with group 1 in a fully adjusted Cox proportional model, participants with elevated Lp(a) and no hypertension (group 2) did not have an increased risk of CVD events (hazard ratio [HR], 1.09; 95% confidence interval [CI], 0.79-1.50).
CVD risk, however, was significantly higher in group 3 with normal Lp(a) and hypertension (HR, 1.66; 95% CI, 1.39-1.98) and group 4 with elevated Lp(a) and hypertension (HR, 2.07, 95% CI, 1.63-2.62).
Among all participants with hypertension (groups 3 and 4), Lp(a) was associated with a significant increase in CVD risk (HR, 1.24, 95% CI, 1.01-1.53).
“What I think is interesting here is that in the absence of hypertension, we didn’t really see an increased risk despite having an elevated Lp(a),” said Dr. Shapiro. “What it may indicate is that really for Lp(a) to be associated with risk, there may already need to be some kind of arterial damage that allows the Lp(a) to have its atherogenic impact.
“In other words, in individuals who have totally normal arterial walls, potentially, maybe that is protective enough against Lp(a) that in the absence of any other injurious factor, maybe it’s not an issue,” he said. “That’s a big hypothesis-generating [statement], but hypertension is certainly one of those risk factors that’s known to cause endothelial injury and endothelial dysfunction.”
Dr. Shapiro pointed out that when first measured in MESA, Lp(a) was measured in 4,600 participants who were not on statins, which is important because statins can increase Lp(a) levels.
“When you look just at those participants, those 4,600, you actually do see a relationship between Lp(a) and cardiovascular disease,” he said. “When you look at the whole population, including the 17% who are baseline populations, even when you adjust for statin therapy, we fail to see that, at least in the long-term follow up.”
Nevertheless, he cautioned that hypertension is just one of many traditional cardiovascular risk factors that could affect the relationship between Lp(a) and CVD risk. “I don’t want to suggest that we believe there’s something specifically magical about hypertension and Lp(a). If we chose, say, diabetes or smoking or another traditional risk factor, we may or may not have seen kind of similar results.”
When the investigators stratified the analyses by sex and race/ethnicity, they found that Lp(a) was not associated with CVD risk, regardless of hypertension status. In Black participants, however, greater CVD risk was seen when both elevated Lp(a) and hypertension were present (HR, 2.07, 95% CI, 1.34-3.21; P = .001).
Asked whether the results support one-time universal screening for Lp(a), which is almost exclusively genetically determined, Dr. Shapiro said he supports screening but that this was a secondary analysis and its numbers were modest. He added that median Lp(a) level is higher in African Americans than any other racial/ethnic group but the “most recent data has clarified that, per any absolute level of Lp(a), it appears to confer the same absolute risk in any racial or ethnic group.”
The authors acknowledge that differential loss to follow-up could have resulted in selection bias in the study and that there were relatively few CVD events in group 2, which may have limited the ability to detect differences in groups without hypertension, particularly in the subgroup analyses. Other limitations are the potential for residual confounding and participants may have developed hypertension during follow-up, resulting in misclassification bias.
Further research is needed to better understand the mechanistic link between Lp(a), hypertension, and CVD, Dr. Shapiro said. Further insights also should be provided by the ongoing phase 3 Lp(a) HORIZON trial evaluating the effect of Lp(a) lowering with the investigational antisense drug, pelacarsen, on cardiovascular events in 8,324 patients with established CVD and elevated Lp(a). The study is expected to be completed in May 2025.
The study was supported by contracts from the National Heart, Lung, and Blood Institute and by grants from the National Center for Advanced Translational Sciences. Dr. Shapiro reports participating in scientific advisory boards with Amgen, Novartis, and Novo Nordisk, and consulting for Regeneron.
A version of this article first appeared on Medscape.com.
High levels of lipoprotein(a) increase the risk for incident cardiovascular disease (CVD) for hypertensive individuals but not for those without hypertension, a new MESA analysis suggests.
There are ways to test for statistical interaction, “in this case, multiplicative interaction between Lp(a) and hypertension, which suggests that Lp(a) is actually modifying the effect between blood pressure and cardiovascular disease. It’s not simply additive,” senior author Michael D. Shapiro, DO, Wake Forest University, Winston-Salem, N.C., told this news organization.
“So that’s new and I don’t think anybody’s looked at that before.”
Although Lp(a) is recognized as an independent cause of atherosclerotic CVD (ASCVD), the significance of Lp(a) in hypertension has been “virtually untapped,” he noted. A recent prospective study reported that elevated CVD risk was present only in individuals with Lp(a) ≥ 30 mg/dL and hypertension but it included only Chinese participants with stable coronary artery disease.
The current analysis, published online in the journal Hypertension, included 6,674 participants in the ongoing Multi-Ethnic Study of Atherosclerosis (MESA), all free of baseline ASCVD, who were recruited from six communities in the United States and had measured baseline Lp(a), blood pressure, and CVD events data over follow-up from 2000 to 2018.
Participants were stratified into four groups based on the presence or absence of hypertension (defined as 140/90 mm Hg or higher or the use of antihypertensive drugs) and an Lp(a) threshold of 50 mg/dL, as recommended by the American College of Cardiology/American Heart Association cholesterol guideline for consideration as an ASCVD risk-enhancing factor.
Slightly more than half of participants were female (52.8%), 38.6% were White, 27.5% were African American, 22.1% were Hispanic, and 11.9% were Chinese American.
According to the researchers, 809 participants had a CVD event over an average follow-up of 13.9 years, including 7.7% of group 1 with Lp(a) < 50 mg/dL and no hypertension, 8.0% of group 2 with Lp(a) ≥ 50 mg/dL and no hypertension, 16.2% of group 3 with Lp(a) < 50 mg/dL and hypertension, and 18.8% of group 4 with Lp(a) ≥ 50 mg/dL and hypertension.
When compared with group 1 in a fully adjusted Cox proportional model, participants with elevated Lp(a) and no hypertension (group 2) did not have an increased risk of CVD events (hazard ratio [HR], 1.09; 95% confidence interval [CI], 0.79-1.50).
CVD risk, however, was significantly higher in group 3 with normal Lp(a) and hypertension (HR, 1.66; 95% CI, 1.39-1.98) and group 4 with elevated Lp(a) and hypertension (HR, 2.07, 95% CI, 1.63-2.62).
Among all participants with hypertension (groups 3 and 4), Lp(a) was associated with a significant increase in CVD risk (HR, 1.24, 95% CI, 1.01-1.53).
“What I think is interesting here is that in the absence of hypertension, we didn’t really see an increased risk despite having an elevated Lp(a),” said Dr. Shapiro. “What it may indicate is that really for Lp(a) to be associated with risk, there may already need to be some kind of arterial damage that allows the Lp(a) to have its atherogenic impact.
“In other words, in individuals who have totally normal arterial walls, potentially, maybe that is protective enough against Lp(a) that in the absence of any other injurious factor, maybe it’s not an issue,” he said. “That’s a big hypothesis-generating [statement], but hypertension is certainly one of those risk factors that’s known to cause endothelial injury and endothelial dysfunction.”
Dr. Shapiro pointed out that when first measured in MESA, Lp(a) was measured in 4,600 participants who were not on statins, which is important because statins can increase Lp(a) levels.
“When you look just at those participants, those 4,600, you actually do see a relationship between Lp(a) and cardiovascular disease,” he said. “When you look at the whole population, including the 17% who are baseline populations, even when you adjust for statin therapy, we fail to see that, at least in the long-term follow up.”
Nevertheless, he cautioned that hypertension is just one of many traditional cardiovascular risk factors that could affect the relationship between Lp(a) and CVD risk. “I don’t want to suggest that we believe there’s something specifically magical about hypertension and Lp(a). If we chose, say, diabetes or smoking or another traditional risk factor, we may or may not have seen kind of similar results.”
When the investigators stratified the analyses by sex and race/ethnicity, they found that Lp(a) was not associated with CVD risk, regardless of hypertension status. In Black participants, however, greater CVD risk was seen when both elevated Lp(a) and hypertension were present (HR, 2.07, 95% CI, 1.34-3.21; P = .001).
Asked whether the results support one-time universal screening for Lp(a), which is almost exclusively genetically determined, Dr. Shapiro said he supports screening but that this was a secondary analysis and its numbers were modest. He added that median Lp(a) level is higher in African Americans than any other racial/ethnic group but the “most recent data has clarified that, per any absolute level of Lp(a), it appears to confer the same absolute risk in any racial or ethnic group.”
The authors acknowledge that differential loss to follow-up could have resulted in selection bias in the study and that there were relatively few CVD events in group 2, which may have limited the ability to detect differences in groups without hypertension, particularly in the subgroup analyses. Other limitations are the potential for residual confounding and participants may have developed hypertension during follow-up, resulting in misclassification bias.
Further research is needed to better understand the mechanistic link between Lp(a), hypertension, and CVD, Dr. Shapiro said. Further insights also should be provided by the ongoing phase 3 Lp(a) HORIZON trial evaluating the effect of Lp(a) lowering with the investigational antisense drug, pelacarsen, on cardiovascular events in 8,324 patients with established CVD and elevated Lp(a). The study is expected to be completed in May 2025.
The study was supported by contracts from the National Heart, Lung, and Blood Institute and by grants from the National Center for Advanced Translational Sciences. Dr. Shapiro reports participating in scientific advisory boards with Amgen, Novartis, and Novo Nordisk, and consulting for Regeneron.
A version of this article first appeared on Medscape.com.
High levels of lipoprotein(a) increase the risk for incident cardiovascular disease (CVD) for hypertensive individuals but not for those without hypertension, a new MESA analysis suggests.
There are ways to test for statistical interaction, “in this case, multiplicative interaction between Lp(a) and hypertension, which suggests that Lp(a) is actually modifying the effect between blood pressure and cardiovascular disease. It’s not simply additive,” senior author Michael D. Shapiro, DO, Wake Forest University, Winston-Salem, N.C., told this news organization.
“So that’s new and I don’t think anybody’s looked at that before.”
Although Lp(a) is recognized as an independent cause of atherosclerotic CVD (ASCVD), the significance of Lp(a) in hypertension has been “virtually untapped,” he noted. A recent prospective study reported that elevated CVD risk was present only in individuals with Lp(a) ≥ 30 mg/dL and hypertension but it included only Chinese participants with stable coronary artery disease.
The current analysis, published online in the journal Hypertension, included 6,674 participants in the ongoing Multi-Ethnic Study of Atherosclerosis (MESA), all free of baseline ASCVD, who were recruited from six communities in the United States and had measured baseline Lp(a), blood pressure, and CVD events data over follow-up from 2000 to 2018.
Participants were stratified into four groups based on the presence or absence of hypertension (defined as 140/90 mm Hg or higher or the use of antihypertensive drugs) and an Lp(a) threshold of 50 mg/dL, as recommended by the American College of Cardiology/American Heart Association cholesterol guideline for consideration as an ASCVD risk-enhancing factor.
Slightly more than half of participants were female (52.8%), 38.6% were White, 27.5% were African American, 22.1% were Hispanic, and 11.9% were Chinese American.
According to the researchers, 809 participants had a CVD event over an average follow-up of 13.9 years, including 7.7% of group 1 with Lp(a) < 50 mg/dL and no hypertension, 8.0% of group 2 with Lp(a) ≥ 50 mg/dL and no hypertension, 16.2% of group 3 with Lp(a) < 50 mg/dL and hypertension, and 18.8% of group 4 with Lp(a) ≥ 50 mg/dL and hypertension.
When compared with group 1 in a fully adjusted Cox proportional model, participants with elevated Lp(a) and no hypertension (group 2) did not have an increased risk of CVD events (hazard ratio [HR], 1.09; 95% confidence interval [CI], 0.79-1.50).
CVD risk, however, was significantly higher in group 3 with normal Lp(a) and hypertension (HR, 1.66; 95% CI, 1.39-1.98) and group 4 with elevated Lp(a) and hypertension (HR, 2.07, 95% CI, 1.63-2.62).
Among all participants with hypertension (groups 3 and 4), Lp(a) was associated with a significant increase in CVD risk (HR, 1.24, 95% CI, 1.01-1.53).
“What I think is interesting here is that in the absence of hypertension, we didn’t really see an increased risk despite having an elevated Lp(a),” said Dr. Shapiro. “What it may indicate is that really for Lp(a) to be associated with risk, there may already need to be some kind of arterial damage that allows the Lp(a) to have its atherogenic impact.
“In other words, in individuals who have totally normal arterial walls, potentially, maybe that is protective enough against Lp(a) that in the absence of any other injurious factor, maybe it’s not an issue,” he said. “That’s a big hypothesis-generating [statement], but hypertension is certainly one of those risk factors that’s known to cause endothelial injury and endothelial dysfunction.”
Dr. Shapiro pointed out that when first measured in MESA, Lp(a) was measured in 4,600 participants who were not on statins, which is important because statins can increase Lp(a) levels.
“When you look just at those participants, those 4,600, you actually do see a relationship between Lp(a) and cardiovascular disease,” he said. “When you look at the whole population, including the 17% who are baseline populations, even when you adjust for statin therapy, we fail to see that, at least in the long-term follow up.”
Nevertheless, he cautioned that hypertension is just one of many traditional cardiovascular risk factors that could affect the relationship between Lp(a) and CVD risk. “I don’t want to suggest that we believe there’s something specifically magical about hypertension and Lp(a). If we chose, say, diabetes or smoking or another traditional risk factor, we may or may not have seen kind of similar results.”
When the investigators stratified the analyses by sex and race/ethnicity, they found that Lp(a) was not associated with CVD risk, regardless of hypertension status. In Black participants, however, greater CVD risk was seen when both elevated Lp(a) and hypertension were present (HR, 2.07, 95% CI, 1.34-3.21; P = .001).
Asked whether the results support one-time universal screening for Lp(a), which is almost exclusively genetically determined, Dr. Shapiro said he supports screening but that this was a secondary analysis and its numbers were modest. He added that median Lp(a) level is higher in African Americans than any other racial/ethnic group but the “most recent data has clarified that, per any absolute level of Lp(a), it appears to confer the same absolute risk in any racial or ethnic group.”
The authors acknowledge that differential loss to follow-up could have resulted in selection bias in the study and that there were relatively few CVD events in group 2, which may have limited the ability to detect differences in groups without hypertension, particularly in the subgroup analyses. Other limitations are the potential for residual confounding and participants may have developed hypertension during follow-up, resulting in misclassification bias.
Further research is needed to better understand the mechanistic link between Lp(a), hypertension, and CVD, Dr. Shapiro said. Further insights also should be provided by the ongoing phase 3 Lp(a) HORIZON trial evaluating the effect of Lp(a) lowering with the investigational antisense drug, pelacarsen, on cardiovascular events in 8,324 patients with established CVD and elevated Lp(a). The study is expected to be completed in May 2025.
The study was supported by contracts from the National Heart, Lung, and Blood Institute and by grants from the National Center for Advanced Translational Sciences. Dr. Shapiro reports participating in scientific advisory boards with Amgen, Novartis, and Novo Nordisk, and consulting for Regeneron.
A version of this article first appeared on Medscape.com.
FROM HYPERTENSION
‘Reassuring’ data on pregnancy with ischemic heart disease
Women with preexisting ischemic heart disease without another cardiac diagnosis have a higher risk of severe maternal morbidity and mortality than women with no cardiac disease, a new study suggests.
However, after adjustment for other comorbidities, the risk associated with isolated preexisting ischemic heart disease without additional evidence of cardiomyopathy was relatively similar to that of other low-risk cardiac diseases.
“These are reassuring findings,” lead author of the study, Anna E. Denoble, MD, Yale University, New Haven, Conn., told this news organization. “The risk is not zero. Women with preexisting ischemic heart disease are at a small increased risk compared to women without preexisting cardiac disease. But with good control of cardiovascular risk factors, these women have a good chance of a positive outcome.”
The study was published online in JACC: Advances.
“To our knowledge, this study provides the largest analysis to date examining the risk of severe morbidity and mortality among pregnant people with pre-existing ischemic heart disease,” the authors noted.
Dr. Denoble, a maternal and fetal medicine specialist, explained that in recent years, there has been an increase in the number of patients with preexisting ischemic heart disease who are considering pregnancy or who are pregnant when they present, but there is little information on outcomes for these patients.
The diagnosis of ischemic heart disease is not included in the main classification used for heart disease in pregnancy – the modified World Health Organization classification, Dr. Denoble noted. “This classification includes information on pregnancy outcomes in women with many cardiac conditions, including arrhythmias, congenital heart disease, heart failure, and aortic aneurysm, but ischemic heart disease is missing.”
She suggested this is probably because ischemic heart disease is regarded as a condition that occurs mainly in older people. “But we are seeing more and more women with ischemic heart disease who are pregnant or considering pregnancy. This could be because women are now often older when considering pregnancy, and also risk factors for ischemic heart disease, such as obesity and diabetes, are becoming more frequent in younger women.”
The researchers conducted the current study to investigate pregnancy outcomes for these women.
The retrospective cohort study analyzed data from the Nationwide Readmissions Database on women who had experienced a delivery hospitalization from Oct. 1, 2015, to Dec. 31, 2018. They compared outcomes for women with isolated preexisting ischemic heart disease with those of women who had no apparent cardiac condition and to those with mild or more severe cardiac conditions included in the mWHO classification after controlling for other comorbidities.
The primary outcome was severe maternal morbidity or death. Dr. Denoble explained that severe maternal morbidity includes mechanical ventilation, blood transfusion, and hysterectomy – the more severe maternal adverse outcomes of pregnancy.
Results showed that, of 11,556,136 delivery hospitalizations, 65,331 patients had another cardiac diagnosis, and 3,009 had ischemic heart disease alone. Patients with ischemic heart disease were older, and rates of diabetes and hypertension were higher.
In unadjusted analyses, adverse outcomes were more common among patients with ischemic heart disease alone than among patients with no cardiac disease and mild cardiac conditions (mWHO class I-II cardiac disease).
Of those with preexisting ischemic heart disease, 6.6% experienced severe maternal morbidity or death, compared with 1.5% of those without a cardiac disease (unadjusted relative risk vs. no cardiac disease, 4.3; 95% confidence interval, 3.5-5.2).
In comparison, 4.2% of women with mWHO I-II cardiac diseases and 23.1% of those with more severe mWHO II/III-IV cardiac diseases experienced severe maternal morbidity or death.
Similar differences were noted for nontransfusion severe maternal morbidity and mortality, as well as cardiac severe maternal morbidity and mortality.
After adjustment, ischemic heart disease alone was associated with a higher risk of severe maternal morbidity or death compared to no cardiac disease (adjusted RR, 1.51; 95% CI, 1.19-1.92).
In comparison, the aRR was 1.90 for WHO class I-II diseases and 5.87 (95% CI, 5.49-6.27) for more severe cardiac conditions defined as WHO II/III-IV diseases.
Risk for nontransfusion severe maternal morbidity or death (aRR, 1.60) and cardiac severe maternal morbidity or death (aRR, 2.98) were also higher for those with ischemic heart disease than for women without any cardiac disease.
There were no significant differences in preterm birth for those with preexisting ischemic heart disease compared to those with no cardiac disease after adjustment.
The risk of severe maternal morbidity and mortality, nontransfusion severe maternal morbidity and mortality, and cardiac severe maternal morbidity and mortality for ischemic heart disease alone most closely approximated that of mWHO class I or II cardiac diseases, the researchers said.
“We found that individuals with preexisting ischemic heart disease had a rate of severe maternal morbidity/mortality in the same range as those with other cardiac diagnoses in the mild cardiac disease classification (class I or II),” Dr. Denoble commented.
“This prognosis suggests it is very reasonable for these women to consider pregnancy. The risk of adverse outcomes is not so high that pregnancy is contraindicated,” she added.
Dr. Denoble said this information will be very helpful when counseling women with preexisting ischemic heart disease who are considering pregnancy. “These patients may need some extra monitoring, but in general, they have a high chance of a good outcome,” she noted.
“I would still advise these women to register with a high-risk obstetrics provider to have a baseline cardiovascular pregnancy evaluation. As long as that is reassuring, then further frequent intensive supervision may not be necessary,” she said.
However, the authors pointed out, “it is important to communicate to patients that while pregnancy may be considered low risk in the setting of pre-existing ischemic heart disease, 6.6% of patients with pre-existing ischemic heart disease alone did experience severe maternal morbidity or death during the delivery hospitalization.”
They added that other medical comorbidities should be factored into discussions regarding the risks of pregnancy.
The researchers also noted that the study was limited to evaluation of maternal outcomes occurring during the delivery hospitalization and that additional research that assesses rates of maternal adverse cardiac events and maternal morbidity occurring prior to or after the delivery hospitalization would be beneficial.
Future studies examining the potential gradation in risk associated with additional cardiac comorbidities in individuals with preexisting ischemic heart disease would also be worthwhile, they added.
The study was supported by funding from the National Institutes of Health and the Foundation for Women and Girls with Blood Disorders. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women with preexisting ischemic heart disease without another cardiac diagnosis have a higher risk of severe maternal morbidity and mortality than women with no cardiac disease, a new study suggests.
However, after adjustment for other comorbidities, the risk associated with isolated preexisting ischemic heart disease without additional evidence of cardiomyopathy was relatively similar to that of other low-risk cardiac diseases.
“These are reassuring findings,” lead author of the study, Anna E. Denoble, MD, Yale University, New Haven, Conn., told this news organization. “The risk is not zero. Women with preexisting ischemic heart disease are at a small increased risk compared to women without preexisting cardiac disease. But with good control of cardiovascular risk factors, these women have a good chance of a positive outcome.”
The study was published online in JACC: Advances.
“To our knowledge, this study provides the largest analysis to date examining the risk of severe morbidity and mortality among pregnant people with pre-existing ischemic heart disease,” the authors noted.
Dr. Denoble, a maternal and fetal medicine specialist, explained that in recent years, there has been an increase in the number of patients with preexisting ischemic heart disease who are considering pregnancy or who are pregnant when they present, but there is little information on outcomes for these patients.
The diagnosis of ischemic heart disease is not included in the main classification used for heart disease in pregnancy – the modified World Health Organization classification, Dr. Denoble noted. “This classification includes information on pregnancy outcomes in women with many cardiac conditions, including arrhythmias, congenital heart disease, heart failure, and aortic aneurysm, but ischemic heart disease is missing.”
She suggested this is probably because ischemic heart disease is regarded as a condition that occurs mainly in older people. “But we are seeing more and more women with ischemic heart disease who are pregnant or considering pregnancy. This could be because women are now often older when considering pregnancy, and also risk factors for ischemic heart disease, such as obesity and diabetes, are becoming more frequent in younger women.”
The researchers conducted the current study to investigate pregnancy outcomes for these women.
The retrospective cohort study analyzed data from the Nationwide Readmissions Database on women who had experienced a delivery hospitalization from Oct. 1, 2015, to Dec. 31, 2018. They compared outcomes for women with isolated preexisting ischemic heart disease with those of women who had no apparent cardiac condition and to those with mild or more severe cardiac conditions included in the mWHO classification after controlling for other comorbidities.
The primary outcome was severe maternal morbidity or death. Dr. Denoble explained that severe maternal morbidity includes mechanical ventilation, blood transfusion, and hysterectomy – the more severe maternal adverse outcomes of pregnancy.
Results showed that, of 11,556,136 delivery hospitalizations, 65,331 patients had another cardiac diagnosis, and 3,009 had ischemic heart disease alone. Patients with ischemic heart disease were older, and rates of diabetes and hypertension were higher.
In unadjusted analyses, adverse outcomes were more common among patients with ischemic heart disease alone than among patients with no cardiac disease and mild cardiac conditions (mWHO class I-II cardiac disease).
Of those with preexisting ischemic heart disease, 6.6% experienced severe maternal morbidity or death, compared with 1.5% of those without a cardiac disease (unadjusted relative risk vs. no cardiac disease, 4.3; 95% confidence interval, 3.5-5.2).
In comparison, 4.2% of women with mWHO I-II cardiac diseases and 23.1% of those with more severe mWHO II/III-IV cardiac diseases experienced severe maternal morbidity or death.
Similar differences were noted for nontransfusion severe maternal morbidity and mortality, as well as cardiac severe maternal morbidity and mortality.
After adjustment, ischemic heart disease alone was associated with a higher risk of severe maternal morbidity or death compared to no cardiac disease (adjusted RR, 1.51; 95% CI, 1.19-1.92).
In comparison, the aRR was 1.90 for WHO class I-II diseases and 5.87 (95% CI, 5.49-6.27) for more severe cardiac conditions defined as WHO II/III-IV diseases.
Risk for nontransfusion severe maternal morbidity or death (aRR, 1.60) and cardiac severe maternal morbidity or death (aRR, 2.98) were also higher for those with ischemic heart disease than for women without any cardiac disease.
There were no significant differences in preterm birth for those with preexisting ischemic heart disease compared to those with no cardiac disease after adjustment.
The risk of severe maternal morbidity and mortality, nontransfusion severe maternal morbidity and mortality, and cardiac severe maternal morbidity and mortality for ischemic heart disease alone most closely approximated that of mWHO class I or II cardiac diseases, the researchers said.
“We found that individuals with preexisting ischemic heart disease had a rate of severe maternal morbidity/mortality in the same range as those with other cardiac diagnoses in the mild cardiac disease classification (class I or II),” Dr. Denoble commented.
“This prognosis suggests it is very reasonable for these women to consider pregnancy. The risk of adverse outcomes is not so high that pregnancy is contraindicated,” she added.
Dr. Denoble said this information will be very helpful when counseling women with preexisting ischemic heart disease who are considering pregnancy. “These patients may need some extra monitoring, but in general, they have a high chance of a good outcome,” she noted.
“I would still advise these women to register with a high-risk obstetrics provider to have a baseline cardiovascular pregnancy evaluation. As long as that is reassuring, then further frequent intensive supervision may not be necessary,” she said.
However, the authors pointed out, “it is important to communicate to patients that while pregnancy may be considered low risk in the setting of pre-existing ischemic heart disease, 6.6% of patients with pre-existing ischemic heart disease alone did experience severe maternal morbidity or death during the delivery hospitalization.”
They added that other medical comorbidities should be factored into discussions regarding the risks of pregnancy.
The researchers also noted that the study was limited to evaluation of maternal outcomes occurring during the delivery hospitalization and that additional research that assesses rates of maternal adverse cardiac events and maternal morbidity occurring prior to or after the delivery hospitalization would be beneficial.
Future studies examining the potential gradation in risk associated with additional cardiac comorbidities in individuals with preexisting ischemic heart disease would also be worthwhile, they added.
The study was supported by funding from the National Institutes of Health and the Foundation for Women and Girls with Blood Disorders. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women with preexisting ischemic heart disease without another cardiac diagnosis have a higher risk of severe maternal morbidity and mortality than women with no cardiac disease, a new study suggests.
However, after adjustment for other comorbidities, the risk associated with isolated preexisting ischemic heart disease without additional evidence of cardiomyopathy was relatively similar to that of other low-risk cardiac diseases.
“These are reassuring findings,” lead author of the study, Anna E. Denoble, MD, Yale University, New Haven, Conn., told this news organization. “The risk is not zero. Women with preexisting ischemic heart disease are at a small increased risk compared to women without preexisting cardiac disease. But with good control of cardiovascular risk factors, these women have a good chance of a positive outcome.”
The study was published online in JACC: Advances.
“To our knowledge, this study provides the largest analysis to date examining the risk of severe morbidity and mortality among pregnant people with pre-existing ischemic heart disease,” the authors noted.
Dr. Denoble, a maternal and fetal medicine specialist, explained that in recent years, there has been an increase in the number of patients with preexisting ischemic heart disease who are considering pregnancy or who are pregnant when they present, but there is little information on outcomes for these patients.
The diagnosis of ischemic heart disease is not included in the main classification used for heart disease in pregnancy – the modified World Health Organization classification, Dr. Denoble noted. “This classification includes information on pregnancy outcomes in women with many cardiac conditions, including arrhythmias, congenital heart disease, heart failure, and aortic aneurysm, but ischemic heart disease is missing.”
She suggested this is probably because ischemic heart disease is regarded as a condition that occurs mainly in older people. “But we are seeing more and more women with ischemic heart disease who are pregnant or considering pregnancy. This could be because women are now often older when considering pregnancy, and also risk factors for ischemic heart disease, such as obesity and diabetes, are becoming more frequent in younger women.”
The researchers conducted the current study to investigate pregnancy outcomes for these women.
The retrospective cohort study analyzed data from the Nationwide Readmissions Database on women who had experienced a delivery hospitalization from Oct. 1, 2015, to Dec. 31, 2018. They compared outcomes for women with isolated preexisting ischemic heart disease with those of women who had no apparent cardiac condition and to those with mild or more severe cardiac conditions included in the mWHO classification after controlling for other comorbidities.
The primary outcome was severe maternal morbidity or death. Dr. Denoble explained that severe maternal morbidity includes mechanical ventilation, blood transfusion, and hysterectomy – the more severe maternal adverse outcomes of pregnancy.
Results showed that, of 11,556,136 delivery hospitalizations, 65,331 patients had another cardiac diagnosis, and 3,009 had ischemic heart disease alone. Patients with ischemic heart disease were older, and rates of diabetes and hypertension were higher.
In unadjusted analyses, adverse outcomes were more common among patients with ischemic heart disease alone than among patients with no cardiac disease and mild cardiac conditions (mWHO class I-II cardiac disease).
Of those with preexisting ischemic heart disease, 6.6% experienced severe maternal morbidity or death, compared with 1.5% of those without a cardiac disease (unadjusted relative risk vs. no cardiac disease, 4.3; 95% confidence interval, 3.5-5.2).
In comparison, 4.2% of women with mWHO I-II cardiac diseases and 23.1% of those with more severe mWHO II/III-IV cardiac diseases experienced severe maternal morbidity or death.
Similar differences were noted for nontransfusion severe maternal morbidity and mortality, as well as cardiac severe maternal morbidity and mortality.
After adjustment, ischemic heart disease alone was associated with a higher risk of severe maternal morbidity or death compared to no cardiac disease (adjusted RR, 1.51; 95% CI, 1.19-1.92).
In comparison, the aRR was 1.90 for WHO class I-II diseases and 5.87 (95% CI, 5.49-6.27) for more severe cardiac conditions defined as WHO II/III-IV diseases.
Risk for nontransfusion severe maternal morbidity or death (aRR, 1.60) and cardiac severe maternal morbidity or death (aRR, 2.98) were also higher for those with ischemic heart disease than for women without any cardiac disease.
There were no significant differences in preterm birth for those with preexisting ischemic heart disease compared to those with no cardiac disease after adjustment.
The risk of severe maternal morbidity and mortality, nontransfusion severe maternal morbidity and mortality, and cardiac severe maternal morbidity and mortality for ischemic heart disease alone most closely approximated that of mWHO class I or II cardiac diseases, the researchers said.
“We found that individuals with preexisting ischemic heart disease had a rate of severe maternal morbidity/mortality in the same range as those with other cardiac diagnoses in the mild cardiac disease classification (class I or II),” Dr. Denoble commented.
“This prognosis suggests it is very reasonable for these women to consider pregnancy. The risk of adverse outcomes is not so high that pregnancy is contraindicated,” she added.
Dr. Denoble said this information will be very helpful when counseling women with preexisting ischemic heart disease who are considering pregnancy. “These patients may need some extra monitoring, but in general, they have a high chance of a good outcome,” she noted.
“I would still advise these women to register with a high-risk obstetrics provider to have a baseline cardiovascular pregnancy evaluation. As long as that is reassuring, then further frequent intensive supervision may not be necessary,” she said.
However, the authors pointed out, “it is important to communicate to patients that while pregnancy may be considered low risk in the setting of pre-existing ischemic heart disease, 6.6% of patients with pre-existing ischemic heart disease alone did experience severe maternal morbidity or death during the delivery hospitalization.”
They added that other medical comorbidities should be factored into discussions regarding the risks of pregnancy.
The researchers also noted that the study was limited to evaluation of maternal outcomes occurring during the delivery hospitalization and that additional research that assesses rates of maternal adverse cardiac events and maternal morbidity occurring prior to or after the delivery hospitalization would be beneficial.
Future studies examining the potential gradation in risk associated with additional cardiac comorbidities in individuals with preexisting ischemic heart disease would also be worthwhile, they added.
The study was supported by funding from the National Institutes of Health and the Foundation for Women and Girls with Blood Disorders. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JACC: ADVANCES
The 'Plaque Hypothesis': Focus on vulnerable lesions to cut events
A new strategy for the management of atherosclerotic plaque as a source of major adverse cardiac events is needed with the focus shifting from the flow-limiting coronary artery luminal lesions to the overall atherosclerotic burden, be it obstructive or nonobstructive, according to a review article.
The article, by Peter H. Stone, MD, and Peter Libby, MD, Brigham and Women’s Hospital, Boston, and William E. Boden, MD, Boston University School of Medicine, was published online in JAMA Cardiology.
The review explored new data from vascular biology, atherosclerosis imaging, natural history outcome studies, and large-scale clinical trials that support what the authors refer to as “The Plaque Hypothesis” – the idea that major adverse cardiac events such as myocardial infarction and cardiac death are triggered by destabilization of vulnerable plaque, which may be obstructive or nonobstructive.
“We need to consider embracing a new management strategy that directs our diagnostic and management focus to evaluate the entire length of the atheromatous coronary artery and broaden the target of our therapeutic intervention to include all regions of the plaque (both flow-limiting and non–flow-limiting), even those that are distant from the presumed ischemia-producing obstruction,” the authors concluded.
Dr. Stone explained to this news organization that, for several decades, the medical community has focused on plaques causing severe obstruction of coronary arteries as being responsible for major adverse cardiac events. This approach – known as the Ischemia Hypothesis – has been the accepted strategy for many years, with all guidelines advising the identification of the stenoses that cause the most obstruction for treatment with stenting.
However, the authors pointed out that a number of studies have now suggested that, while these severe obstructive stenoses cause angina, they do not seem to be responsible for the hard events of MI, acute coronary syndrome (ACS), and cardiac death.
Several studies including the COURAGE trial and BARI-2D, and the recent ISCHEMIA trial have all failed to show a reduction in these hard endpoints by intervening on these severe obstructive lesions, Dr. Stone noted.
“We present evidence for a new approach – that it is the composition and vascular biology of the atherosclerotic plaques that cause MI, ACS, and cardiac death, rather than simply how obstructive they are,” he said.
Dr. Stone pointed out that plaque seen on a coronary angiogram looks at only the lumen of the artery, but plaque is primarily based in the wall of the artery, and if that plaque is inflamed it can easily be the culprit responsible for adverse events even without encroaching into the lumen.
“Our paper describes many factors which can cause plaques to destabilize and cause an ACS. These include anatomical, biochemical, and biomechanical features that together cause plaque rupture or erosion and precipitate a clinical event. It is not sufficient to just look for obstructive plaques on a coronary angiogram,” he said. “We are barking up the wrong tree. We need to look for inflamed plaque in the whole wall of the coronary arteries.”
The authors described different factors that identify a plaque at high risk of destabilization. These include a large area of vulnerable plaque, a thin-cap atheroma, a severe inflamed core, macrocalcifications, a large plaque burden, and a physical profile that would encourage a thrombus to become trapped.
“Atherosclerotic plaques are very heterogeneous and complex structures and it is not just the mountain peaks but also the lower foothills that can precipitate a flow-limiting obstruction,” Dr. Stone noted.
“The slope of the mountain is probably very important in the ability for a thrombus to form. If the slope is gradual there isn’t a problem. But if the slope is jagged with sharp edges this can cause a thrombus to become trapped. We need to look at the entirety of plaque and all its risk features to identify the culprit areas that could cause MI or cardiac death. These are typically not the obstructive plaques we have all been fixated on for many years,” he added.
“We need to focus on plaque heterogeneity. Once plaque is old and just made up of scar tissue which is not inflamed it does not cause much [of] a problem – we can probably just leave it alone. Some of these obstructive plaques may cause some angina but many do not cause major cardiac events unless they have other high-risk features,” he said.
“Cardiac events are still caused by obstruction of blood flow but that can be an abrupt process where a thrombus attaches itself to an area of destabilized plaque. These areas of plaque were not necessarily obstructing to start with. We believe that this is the explanation behind the observation that 50% of all people who have an MI (half of which are fatal) do not have symptoms beforehand,” Dr. Stone commented.
Because these areas of destabilized plaque do not cause symptoms, he believes that vast populations of people with established cardiovascular risk factors should undergo screening. “At the moment we wait for people to experience chest pain or to have an MI – that is far too little too late.”
To identify these areas of high-risk plaques, imaging techniques looking inside the artery wall are needed such as intravascular ultrasound. However, this is an invasive procedure, and the noninvasive coronary CT angiography also gives a good picture, so it is probably the best way to begin as a wider screening modality, with more invasive screening methods then used in those found to be at risk, Dr. Stone suggested.
Plaques that are identified as likely to destabilize can be treated with percutaneous coronary intervention and stenting.
While systemic therapies are useful, those currently available are not sufficient, Dr. Stone noted. For example, there are still high levels of major cardiac events in patients treated with the PCSK9 inhibitors, which bring about very large reductions in LDL cholesterol. “These therapies are beneficial, but they are not enough on their own. So, these areas of unstable plaque would need to be treated with stenting or something similar. We believe that the intervention of stenting is good but at present it is targeted at the wrong areas,” he stated.
“Clearly what we’ve been doing – stenting only obstructive lesions – does not reduce hard clinical events. Imaging methods have improved so much in recent years that we can now identify high-risk areas of plaque. This whole field of studying the vulnerable plaque has been ongoing for many years, but it is only recently that imaging methods have become good enough to identify plaques at risk. This field is now coming of age,” he added.
The next steps are to start identifying these plaques in larger populations, more accurately characterizing those at the highest risk, and then performing randomized trials of preemptive intervention in those believed to be at highest risk, and follow up for clinical events, Dr. Stone explained.
Advances in detecting unstable plaque may also permit early evaluation of novel therapeutics and gauge the intensity of lifestyle and disease-modifying pharmacotherapy, the authors suggested.
This work was supported in part by the National Heart, Lung, and Blood Institute, the American Heart Association, the RRM Charitable Fund, the Simard Fund, and the Schaubert Family. Dr. Libby is an unpaid consultant to or involved in clinical trials with Amgen, AstraZeneca, Baim Institute, Beren Therapeutics, Esperion Therapeutics, Genentech, Kancera, Kowa Pharmaceuticals, MedImmune, Merck, Norvo Nordisk, Novartis, Pfizer, and Sanofi-Regeneron; and is a member of the scientific advisory board for Amgen, Caristo Diagnostics, Cartesian Therapeutics, CSL Behring, DalCor Pharmaceuticals, Dewpoint Therapeutics, Elucid Bioimaging, Kancera, Kowa Pharmaceuticals, Olatec Therapeutics, MedImmune, Moderna, Novartis, PlaqueTec, TenSixteen Bio, Soley Thereapeutics, and XBiotech.
A version of this article first appeared on Medscape.com.
A new strategy for the management of atherosclerotic plaque as a source of major adverse cardiac events is needed with the focus shifting from the flow-limiting coronary artery luminal lesions to the overall atherosclerotic burden, be it obstructive or nonobstructive, according to a review article.
The article, by Peter H. Stone, MD, and Peter Libby, MD, Brigham and Women’s Hospital, Boston, and William E. Boden, MD, Boston University School of Medicine, was published online in JAMA Cardiology.
The review explored new data from vascular biology, atherosclerosis imaging, natural history outcome studies, and large-scale clinical trials that support what the authors refer to as “The Plaque Hypothesis” – the idea that major adverse cardiac events such as myocardial infarction and cardiac death are triggered by destabilization of vulnerable plaque, which may be obstructive or nonobstructive.
“We need to consider embracing a new management strategy that directs our diagnostic and management focus to evaluate the entire length of the atheromatous coronary artery and broaden the target of our therapeutic intervention to include all regions of the plaque (both flow-limiting and non–flow-limiting), even those that are distant from the presumed ischemia-producing obstruction,” the authors concluded.
Dr. Stone explained to this news organization that, for several decades, the medical community has focused on plaques causing severe obstruction of coronary arteries as being responsible for major adverse cardiac events. This approach – known as the Ischemia Hypothesis – has been the accepted strategy for many years, with all guidelines advising the identification of the stenoses that cause the most obstruction for treatment with stenting.
However, the authors pointed out that a number of studies have now suggested that, while these severe obstructive stenoses cause angina, they do not seem to be responsible for the hard events of MI, acute coronary syndrome (ACS), and cardiac death.
Several studies including the COURAGE trial and BARI-2D, and the recent ISCHEMIA trial have all failed to show a reduction in these hard endpoints by intervening on these severe obstructive lesions, Dr. Stone noted.
“We present evidence for a new approach – that it is the composition and vascular biology of the atherosclerotic plaques that cause MI, ACS, and cardiac death, rather than simply how obstructive they are,” he said.
Dr. Stone pointed out that plaque seen on a coronary angiogram looks at only the lumen of the artery, but plaque is primarily based in the wall of the artery, and if that plaque is inflamed it can easily be the culprit responsible for adverse events even without encroaching into the lumen.
“Our paper describes many factors which can cause plaques to destabilize and cause an ACS. These include anatomical, biochemical, and biomechanical features that together cause plaque rupture or erosion and precipitate a clinical event. It is not sufficient to just look for obstructive plaques on a coronary angiogram,” he said. “We are barking up the wrong tree. We need to look for inflamed plaque in the whole wall of the coronary arteries.”
The authors described different factors that identify a plaque at high risk of destabilization. These include a large area of vulnerable plaque, a thin-cap atheroma, a severe inflamed core, macrocalcifications, a large plaque burden, and a physical profile that would encourage a thrombus to become trapped.
“Atherosclerotic plaques are very heterogeneous and complex structures and it is not just the mountain peaks but also the lower foothills that can precipitate a flow-limiting obstruction,” Dr. Stone noted.
“The slope of the mountain is probably very important in the ability for a thrombus to form. If the slope is gradual there isn’t a problem. But if the slope is jagged with sharp edges this can cause a thrombus to become trapped. We need to look at the entirety of plaque and all its risk features to identify the culprit areas that could cause MI or cardiac death. These are typically not the obstructive plaques we have all been fixated on for many years,” he added.
“We need to focus on plaque heterogeneity. Once plaque is old and just made up of scar tissue which is not inflamed it does not cause much [of] a problem – we can probably just leave it alone. Some of these obstructive plaques may cause some angina but many do not cause major cardiac events unless they have other high-risk features,” he said.
“Cardiac events are still caused by obstruction of blood flow but that can be an abrupt process where a thrombus attaches itself to an area of destabilized plaque. These areas of plaque were not necessarily obstructing to start with. We believe that this is the explanation behind the observation that 50% of all people who have an MI (half of which are fatal) do not have symptoms beforehand,” Dr. Stone commented.
Because these areas of destabilized plaque do not cause symptoms, he believes that vast populations of people with established cardiovascular risk factors should undergo screening. “At the moment we wait for people to experience chest pain or to have an MI – that is far too little too late.”
To identify these areas of high-risk plaques, imaging techniques looking inside the artery wall are needed such as intravascular ultrasound. However, this is an invasive procedure, and the noninvasive coronary CT angiography also gives a good picture, so it is probably the best way to begin as a wider screening modality, with more invasive screening methods then used in those found to be at risk, Dr. Stone suggested.
Plaques that are identified as likely to destabilize can be treated with percutaneous coronary intervention and stenting.
While systemic therapies are useful, those currently available are not sufficient, Dr. Stone noted. For example, there are still high levels of major cardiac events in patients treated with the PCSK9 inhibitors, which bring about very large reductions in LDL cholesterol. “These therapies are beneficial, but they are not enough on their own. So, these areas of unstable plaque would need to be treated with stenting or something similar. We believe that the intervention of stenting is good but at present it is targeted at the wrong areas,” he stated.
“Clearly what we’ve been doing – stenting only obstructive lesions – does not reduce hard clinical events. Imaging methods have improved so much in recent years that we can now identify high-risk areas of plaque. This whole field of studying the vulnerable plaque has been ongoing for many years, but it is only recently that imaging methods have become good enough to identify plaques at risk. This field is now coming of age,” he added.
The next steps are to start identifying these plaques in larger populations, more accurately characterizing those at the highest risk, and then performing randomized trials of preemptive intervention in those believed to be at highest risk, and follow up for clinical events, Dr. Stone explained.
Advances in detecting unstable plaque may also permit early evaluation of novel therapeutics and gauge the intensity of lifestyle and disease-modifying pharmacotherapy, the authors suggested.
This work was supported in part by the National Heart, Lung, and Blood Institute, the American Heart Association, the RRM Charitable Fund, the Simard Fund, and the Schaubert Family. Dr. Libby is an unpaid consultant to or involved in clinical trials with Amgen, AstraZeneca, Baim Institute, Beren Therapeutics, Esperion Therapeutics, Genentech, Kancera, Kowa Pharmaceuticals, MedImmune, Merck, Norvo Nordisk, Novartis, Pfizer, and Sanofi-Regeneron; and is a member of the scientific advisory board for Amgen, Caristo Diagnostics, Cartesian Therapeutics, CSL Behring, DalCor Pharmaceuticals, Dewpoint Therapeutics, Elucid Bioimaging, Kancera, Kowa Pharmaceuticals, Olatec Therapeutics, MedImmune, Moderna, Novartis, PlaqueTec, TenSixteen Bio, Soley Thereapeutics, and XBiotech.
A version of this article first appeared on Medscape.com.
A new strategy for the management of atherosclerotic plaque as a source of major adverse cardiac events is needed with the focus shifting from the flow-limiting coronary artery luminal lesions to the overall atherosclerotic burden, be it obstructive or nonobstructive, according to a review article.
The article, by Peter H. Stone, MD, and Peter Libby, MD, Brigham and Women’s Hospital, Boston, and William E. Boden, MD, Boston University School of Medicine, was published online in JAMA Cardiology.
The review explored new data from vascular biology, atherosclerosis imaging, natural history outcome studies, and large-scale clinical trials that support what the authors refer to as “The Plaque Hypothesis” – the idea that major adverse cardiac events such as myocardial infarction and cardiac death are triggered by destabilization of vulnerable plaque, which may be obstructive or nonobstructive.
“We need to consider embracing a new management strategy that directs our diagnostic and management focus to evaluate the entire length of the atheromatous coronary artery and broaden the target of our therapeutic intervention to include all regions of the plaque (both flow-limiting and non–flow-limiting), even those that are distant from the presumed ischemia-producing obstruction,” the authors concluded.
Dr. Stone explained to this news organization that, for several decades, the medical community has focused on plaques causing severe obstruction of coronary arteries as being responsible for major adverse cardiac events. This approach – known as the Ischemia Hypothesis – has been the accepted strategy for many years, with all guidelines advising the identification of the stenoses that cause the most obstruction for treatment with stenting.
However, the authors pointed out that a number of studies have now suggested that, while these severe obstructive stenoses cause angina, they do not seem to be responsible for the hard events of MI, acute coronary syndrome (ACS), and cardiac death.
Several studies including the COURAGE trial and BARI-2D, and the recent ISCHEMIA trial have all failed to show a reduction in these hard endpoints by intervening on these severe obstructive lesions, Dr. Stone noted.
“We present evidence for a new approach – that it is the composition and vascular biology of the atherosclerotic plaques that cause MI, ACS, and cardiac death, rather than simply how obstructive they are,” he said.
Dr. Stone pointed out that plaque seen on a coronary angiogram looks at only the lumen of the artery, but plaque is primarily based in the wall of the artery, and if that plaque is inflamed it can easily be the culprit responsible for adverse events even without encroaching into the lumen.
“Our paper describes many factors which can cause plaques to destabilize and cause an ACS. These include anatomical, biochemical, and biomechanical features that together cause plaque rupture or erosion and precipitate a clinical event. It is not sufficient to just look for obstructive plaques on a coronary angiogram,” he said. “We are barking up the wrong tree. We need to look for inflamed plaque in the whole wall of the coronary arteries.”
The authors described different factors that identify a plaque at high risk of destabilization. These include a large area of vulnerable plaque, a thin-cap atheroma, a severe inflamed core, macrocalcifications, a large plaque burden, and a physical profile that would encourage a thrombus to become trapped.
“Atherosclerotic plaques are very heterogeneous and complex structures and it is not just the mountain peaks but also the lower foothills that can precipitate a flow-limiting obstruction,” Dr. Stone noted.
“The slope of the mountain is probably very important in the ability for a thrombus to form. If the slope is gradual there isn’t a problem. But if the slope is jagged with sharp edges this can cause a thrombus to become trapped. We need to look at the entirety of plaque and all its risk features to identify the culprit areas that could cause MI or cardiac death. These are typically not the obstructive plaques we have all been fixated on for many years,” he added.
“We need to focus on plaque heterogeneity. Once plaque is old and just made up of scar tissue which is not inflamed it does not cause much [of] a problem – we can probably just leave it alone. Some of these obstructive plaques may cause some angina but many do not cause major cardiac events unless they have other high-risk features,” he said.
“Cardiac events are still caused by obstruction of blood flow but that can be an abrupt process where a thrombus attaches itself to an area of destabilized plaque. These areas of plaque were not necessarily obstructing to start with. We believe that this is the explanation behind the observation that 50% of all people who have an MI (half of which are fatal) do not have symptoms beforehand,” Dr. Stone commented.
Because these areas of destabilized plaque do not cause symptoms, he believes that vast populations of people with established cardiovascular risk factors should undergo screening. “At the moment we wait for people to experience chest pain or to have an MI – that is far too little too late.”
To identify these areas of high-risk plaques, imaging techniques looking inside the artery wall are needed such as intravascular ultrasound. However, this is an invasive procedure, and the noninvasive coronary CT angiography also gives a good picture, so it is probably the best way to begin as a wider screening modality, with more invasive screening methods then used in those found to be at risk, Dr. Stone suggested.
Plaques that are identified as likely to destabilize can be treated with percutaneous coronary intervention and stenting.
While systemic therapies are useful, those currently available are not sufficient, Dr. Stone noted. For example, there are still high levels of major cardiac events in patients treated with the PCSK9 inhibitors, which bring about very large reductions in LDL cholesterol. “These therapies are beneficial, but they are not enough on their own. So, these areas of unstable plaque would need to be treated with stenting or something similar. We believe that the intervention of stenting is good but at present it is targeted at the wrong areas,” he stated.
“Clearly what we’ve been doing – stenting only obstructive lesions – does not reduce hard clinical events. Imaging methods have improved so much in recent years that we can now identify high-risk areas of plaque. This whole field of studying the vulnerable plaque has been ongoing for many years, but it is only recently that imaging methods have become good enough to identify plaques at risk. This field is now coming of age,” he added.
The next steps are to start identifying these plaques in larger populations, more accurately characterizing those at the highest risk, and then performing randomized trials of preemptive intervention in those believed to be at highest risk, and follow up for clinical events, Dr. Stone explained.
Advances in detecting unstable plaque may also permit early evaluation of novel therapeutics and gauge the intensity of lifestyle and disease-modifying pharmacotherapy, the authors suggested.
This work was supported in part by the National Heart, Lung, and Blood Institute, the American Heart Association, the RRM Charitable Fund, the Simard Fund, and the Schaubert Family. Dr. Libby is an unpaid consultant to or involved in clinical trials with Amgen, AstraZeneca, Baim Institute, Beren Therapeutics, Esperion Therapeutics, Genentech, Kancera, Kowa Pharmaceuticals, MedImmune, Merck, Norvo Nordisk, Novartis, Pfizer, and Sanofi-Regeneron; and is a member of the scientific advisory board for Amgen, Caristo Diagnostics, Cartesian Therapeutics, CSL Behring, DalCor Pharmaceuticals, Dewpoint Therapeutics, Elucid Bioimaging, Kancera, Kowa Pharmaceuticals, Olatec Therapeutics, MedImmune, Moderna, Novartis, PlaqueTec, TenSixteen Bio, Soley Thereapeutics, and XBiotech.
A version of this article first appeared on Medscape.com.
New AHA statement on managing ACS in older adults
Age-related changes in general and cardiovascular health likely require modifications in how acute coronary syndrome (ACS) is diagnosed and managed in adults aged 75 and older, the American Heart Association says in a new scientific statement.
The statement outlines a framework to integrate geriatric risks into the management of ACS, including the diagnostic approach, pharmacotherapy, revascularization strategies, prevention of adverse events, and transition care planning.
The 31-page statement was published online in the AHA journal Circulation (2022 Dec 12. doi: 10.1161/CIR.0000000000001112). It updates a 2007 AHA statement on treatment of ACS in the elderly.
Complex patient group
Adults aged 75 and older make up roughly 30%-40% of all hospitalized patients with ACS and the majority of ACS-related deaths occur in this group, the writing group notes.
“Older patients have more pronounced anatomical changes and more severe functional impairment, and they are more likely to have additional health conditions,” writing group chair Abdulla A. Damluji, MD, PhD, director of the Inova Center of Outcomes Research in Fairfax, Va., notes in a news release.
“These include frailty, other chronic disorders (treated with multiple medications), physical dysfunction, cognitive decline and/or urinary incontinence – and these are not regularly studied in the context of ACS,” Dr. Damluji explained.
The writing group notes that the presence of one or more geriatric syndromes may substantially affect ACS clinical presentation, clinical course and prognosis, therapeutic decision-making, and response to treatment.
“It is therefore fundamental that clinicians caring for older patients with ACS be alert to the presence of geriatric syndromes and be able to integrate them into the care plan when appropriate,” they say.
They recommend a holistic, individualized, and patient-centered approach to ACS care in the elderly, taking into consideration coexisting and overlapping health issues.
Considerations for clinical care
The AHA statement offers several “considerations for clinical practice” with regard to ACS diagnosis and management in elderly adults. They include:
- ACS presentations without chest pain, such as shortness of breath, syncope, or sudden confusion, are more common in older adults.
- Many older adults have persistent elevations in cardiac troponin levels from myocardial fibrosis and kidney disease that diminish the positive predictive value of high-sensitivity cardiac troponin (hs-cTn) assays for identifying acute and chronic myocardial injury. For this reason, evaluating patterns of rise and fall is essential.
- Age-related changes in metabolism, weight, and muscle mass may require different choices in anticoagulant medications to lower bleeding risk.
- Clopidogrel (Plavix) is the preferred P2Y12 inhibitor because of a significantly lower bleeding profile than ticagrelor (Brilinta) or prasugrel (Effient). For patients with ST-segment myocardial infarction (STEMI) or complex anatomy, the use of ticagrelor is “reasonable.”
- Poor kidney function can increase the risk for contrast-induced acute kidney injury.
- Although the risks are greater, percutaneous coronary intervention or bypass surgery are beneficial in select older adults with ACS.
- Post-MI care should include cardiac rehabilitation tailored to address each patient’s circumstances and personal goals of care.
- For patients with cognitive difficulties and limited mobility, consider simplified medication plans with fewer doses per day and 90-day supplies to prevent the need for frequent refills.
- Patient care plans should be individualized, with input from a multidisciplinary team that may include cardiologists, surgeons, geriatricians, primary care clinicians, nutritionists, social workers, and family members.
- Determine a priori goals of care in older patients to help avoid an unwanted or futile intervention.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Cardiovascular Diseases in Older Populations Committee of the Council on Clinical Cardiology; the Council on Cardiovascular and Stroke Nursing; the Council on Cardiovascular Radiology and Intervention; and the Council on Lifestyle and Cardiometabolic Health.
A version of this article first appeared on Medscape.com.
Age-related changes in general and cardiovascular health likely require modifications in how acute coronary syndrome (ACS) is diagnosed and managed in adults aged 75 and older, the American Heart Association says in a new scientific statement.
The statement outlines a framework to integrate geriatric risks into the management of ACS, including the diagnostic approach, pharmacotherapy, revascularization strategies, prevention of adverse events, and transition care planning.
The 31-page statement was published online in the AHA journal Circulation (2022 Dec 12. doi: 10.1161/CIR.0000000000001112). It updates a 2007 AHA statement on treatment of ACS in the elderly.
Complex patient group
Adults aged 75 and older make up roughly 30%-40% of all hospitalized patients with ACS and the majority of ACS-related deaths occur in this group, the writing group notes.
“Older patients have more pronounced anatomical changes and more severe functional impairment, and they are more likely to have additional health conditions,” writing group chair Abdulla A. Damluji, MD, PhD, director of the Inova Center of Outcomes Research in Fairfax, Va., notes in a news release.
“These include frailty, other chronic disorders (treated with multiple medications), physical dysfunction, cognitive decline and/or urinary incontinence – and these are not regularly studied in the context of ACS,” Dr. Damluji explained.
The writing group notes that the presence of one or more geriatric syndromes may substantially affect ACS clinical presentation, clinical course and prognosis, therapeutic decision-making, and response to treatment.
“It is therefore fundamental that clinicians caring for older patients with ACS be alert to the presence of geriatric syndromes and be able to integrate them into the care plan when appropriate,” they say.
They recommend a holistic, individualized, and patient-centered approach to ACS care in the elderly, taking into consideration coexisting and overlapping health issues.
Considerations for clinical care
The AHA statement offers several “considerations for clinical practice” with regard to ACS diagnosis and management in elderly adults. They include:
- ACS presentations without chest pain, such as shortness of breath, syncope, or sudden confusion, are more common in older adults.
- Many older adults have persistent elevations in cardiac troponin levels from myocardial fibrosis and kidney disease that diminish the positive predictive value of high-sensitivity cardiac troponin (hs-cTn) assays for identifying acute and chronic myocardial injury. For this reason, evaluating patterns of rise and fall is essential.
- Age-related changes in metabolism, weight, and muscle mass may require different choices in anticoagulant medications to lower bleeding risk.
- Clopidogrel (Plavix) is the preferred P2Y12 inhibitor because of a significantly lower bleeding profile than ticagrelor (Brilinta) or prasugrel (Effient). For patients with ST-segment myocardial infarction (STEMI) or complex anatomy, the use of ticagrelor is “reasonable.”
- Poor kidney function can increase the risk for contrast-induced acute kidney injury.
- Although the risks are greater, percutaneous coronary intervention or bypass surgery are beneficial in select older adults with ACS.
- Post-MI care should include cardiac rehabilitation tailored to address each patient’s circumstances and personal goals of care.
- For patients with cognitive difficulties and limited mobility, consider simplified medication plans with fewer doses per day and 90-day supplies to prevent the need for frequent refills.
- Patient care plans should be individualized, with input from a multidisciplinary team that may include cardiologists, surgeons, geriatricians, primary care clinicians, nutritionists, social workers, and family members.
- Determine a priori goals of care in older patients to help avoid an unwanted or futile intervention.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Cardiovascular Diseases in Older Populations Committee of the Council on Clinical Cardiology; the Council on Cardiovascular and Stroke Nursing; the Council on Cardiovascular Radiology and Intervention; and the Council on Lifestyle and Cardiometabolic Health.
A version of this article first appeared on Medscape.com.
Age-related changes in general and cardiovascular health likely require modifications in how acute coronary syndrome (ACS) is diagnosed and managed in adults aged 75 and older, the American Heart Association says in a new scientific statement.
The statement outlines a framework to integrate geriatric risks into the management of ACS, including the diagnostic approach, pharmacotherapy, revascularization strategies, prevention of adverse events, and transition care planning.
The 31-page statement was published online in the AHA journal Circulation (2022 Dec 12. doi: 10.1161/CIR.0000000000001112). It updates a 2007 AHA statement on treatment of ACS in the elderly.
Complex patient group
Adults aged 75 and older make up roughly 30%-40% of all hospitalized patients with ACS and the majority of ACS-related deaths occur in this group, the writing group notes.
“Older patients have more pronounced anatomical changes and more severe functional impairment, and they are more likely to have additional health conditions,” writing group chair Abdulla A. Damluji, MD, PhD, director of the Inova Center of Outcomes Research in Fairfax, Va., notes in a news release.
“These include frailty, other chronic disorders (treated with multiple medications), physical dysfunction, cognitive decline and/or urinary incontinence – and these are not regularly studied in the context of ACS,” Dr. Damluji explained.
The writing group notes that the presence of one or more geriatric syndromes may substantially affect ACS clinical presentation, clinical course and prognosis, therapeutic decision-making, and response to treatment.
“It is therefore fundamental that clinicians caring for older patients with ACS be alert to the presence of geriatric syndromes and be able to integrate them into the care plan when appropriate,” they say.
They recommend a holistic, individualized, and patient-centered approach to ACS care in the elderly, taking into consideration coexisting and overlapping health issues.
Considerations for clinical care
The AHA statement offers several “considerations for clinical practice” with regard to ACS diagnosis and management in elderly adults. They include:
- ACS presentations without chest pain, such as shortness of breath, syncope, or sudden confusion, are more common in older adults.
- Many older adults have persistent elevations in cardiac troponin levels from myocardial fibrosis and kidney disease that diminish the positive predictive value of high-sensitivity cardiac troponin (hs-cTn) assays for identifying acute and chronic myocardial injury. For this reason, evaluating patterns of rise and fall is essential.
- Age-related changes in metabolism, weight, and muscle mass may require different choices in anticoagulant medications to lower bleeding risk.
- Clopidogrel (Plavix) is the preferred P2Y12 inhibitor because of a significantly lower bleeding profile than ticagrelor (Brilinta) or prasugrel (Effient). For patients with ST-segment myocardial infarction (STEMI) or complex anatomy, the use of ticagrelor is “reasonable.”
- Poor kidney function can increase the risk for contrast-induced acute kidney injury.
- Although the risks are greater, percutaneous coronary intervention or bypass surgery are beneficial in select older adults with ACS.
- Post-MI care should include cardiac rehabilitation tailored to address each patient’s circumstances and personal goals of care.
- For patients with cognitive difficulties and limited mobility, consider simplified medication plans with fewer doses per day and 90-day supplies to prevent the need for frequent refills.
- Patient care plans should be individualized, with input from a multidisciplinary team that may include cardiologists, surgeons, geriatricians, primary care clinicians, nutritionists, social workers, and family members.
- Determine a priori goals of care in older patients to help avoid an unwanted or futile intervention.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Cardiovascular Diseases in Older Populations Committee of the Council on Clinical Cardiology; the Council on Cardiovascular and Stroke Nursing; the Council on Cardiovascular Radiology and Intervention; and the Council on Lifestyle and Cardiometabolic Health.
A version of this article first appeared on Medscape.com.
Nitroglycerin’s safety and value examined
He has stable angina, having chest pain with exercise. He uses sublingual nitroglycerin (SL NTG prn) about three times a month. His blood pressure is 140/70 mm Hg. His pulse is 60 beats per minute. His current medications are lisinopril, atorvastatin, aspirin, and SL NTG tablets as needed.
What would you recommend?
A. No sildenafil; refer to urologist for other ED options.
B. Okay to use sildenafil if greater than 6 hours from NTG use.
C. Recommend tadalafil.
Is coprescribing nitrates and phosphodiesterase inhibitors safe?
The FDA warns against the use of phosphodiesterase inhibitors in patients taking nitrates. Combining nitrates with phosphodiesterase type 5 (PDE5) inhibitors is contraindicated because of a synergistic blood pressure lowering effect.1 This warning/contraindication was based on theoretical concerns, as well as concern that of the first 130 deaths reported in patients who took sildenafil, 16 of the patients also were taking nitrates.2
Parker and colleagues studied the safety of giving IV nitroglycerin to patients with coronary artery disease (CAD) who have taken sildenafil.3 The study was a randomized, placebo-controlled, crossover trial. Participants received sildenafil 100 mg or placebo, then received intravenous NTG. Patients who received sildenafil had a 4-6 mm Hg systolic BP drop compared with those who took the placebo. There was no difference in severe events between the sildenafil and placebo groups. The blood levels of nitroglycerin in this study were very likely much higher than the levels that occur with SL NTG.
A recent study by Holt et al. looked at overall cardiovascular outcomes with coprescribing nitrates and phosphodiesterase inhibitors.4 The study was a case crossover design, using a nationwide Danish health registry over the period of 2000-2018. In 2000, the rate of coprescribing of phosphodiesterase inhibitors in ischemic heart disease patients on nitrates was .9 per 100 persons/year and rose to 19.5 prescriptions per 100 persons/year in 2018. During this same time, no statistically significant association was found between the coprescription of nitrates with PDE5 inhibitors and the risk for MI, cardiac arrest, syncope, stroke, or an adverse drug event.
Does nitroglycerin response help determine cause of chest pain?
Nitroglycerin response has long been used as a clinical indicator on whether a patient’s chest pain is cardiac or not. Eric A. Shry, MD, and his colleagues looked at the usefulness of nitroglycerin response in the treatment of chest pain as a predictor of ischemic chest pain in an emergency department setting.5
The study was a retrospective review of 223 patients who presented to the emergency department over a 5-month period with ongoing chest pain. They looked at patients who had ongoing chest pain in the emergency department, received nitroglycerin, and did not receive any therapy other than aspirin within 10 minutes of receiving nitroglycerin. Response to the drug was compared with the final diagnosis of cardiac versus noncardiac chest pain.
Of the patients with a final determination of cardiac chest pain, 88% had a nitroglycerin response, whereas 92% of the patients with noncardiac chest pain had a nitroglycerin response (P = .50).
Deborah B. Diercks, MD, and her colleagues looked at improvement in chest pain scores in the emergency department in patients treated with nitroglycerin and whether it correlated with a cardiac etiology of chest pain.6 The study was a prospective, observational study of 664 patients in an urban tertiary care emergency department over a 16-month period. An 11-point numeric chest pain scale was assessed and recorded by research assistants before and 5 minutes after receiving nitroglycerin. The scale ranged from 0 (no pain) to 10 (worst pain imaginable).
A final diagnosis of a cardiac etiology for chest pain was found in 18% of the patients in the study. Of the patients who had cardiac-related chest pain, 20% had no reduction in pain with nitroglycerin, compared with 19% of the patients without cardiac-related chest pain.
A complete or significant reduction in chest pain occurred with nitroglycerin in 31% of patients with cardiac chest pain and 27% of the patients without cardiac chest pain (P = .76).
Nitroglycerin response does not appear to be helpful in distinguishing cardiac from noncardiac chest pain, but a study by His and colleagues offers an interesting twist.7
The authors of this research studied 118 patients looking to see if the side effect of headache with nitroglycerin was more common in patients who did not have CAD than in those who did. All the patients had a varying degree of relief of chest pain with NTG administration within 10 minutes. In patients with normal coronary arteries or minimal CAD, 73% had headache caused by NTG, whereas in patients with obstructive CAD, only 23% had headache after NTG use.
Take-home messages
- Short acting nitroglycerin may not be a contraindication for phosphodiesterase inhibitor use.
- More data are still needed.
- Nitroglycerin response does not help distinguish chest pain from CAD from noncardiac causes.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Schwartz BG, Kloner RA. Drug interactions with phosphodiesterase-5 inhibitors used for the treatment of erectile dysfunction or pulmonary hypertension. Circulation. 2010;122:88-95.
2. Kloner RA, Zusman RM. Cardiovascular effects of sildenafil citrate and recommendations for its use. Am J Cardiol. 1999 Sep 9;84(5B):11N-17N.
3. Parker JD et al. Safety of intravenous nitroglycerin after administration of sildenafil citrate to men with coronary artery disease: A double-blind, placebo-controlled, randomized, crossover trial. Crit Care Med. 2007;35:1863-8.
4. Holt A et al. Adverse events associated with coprescription of phosphodiesterase type inhibitors and oral organic nitrates in male patients with ischemic heart disease. Ann Intern Med. 2022 Jun;175(6):774-82.
5. Shry EA et al. Usefulness of the response to sublingual nitroglycerin as a predictor of ischemic chest pain in the emergency department. Am J Cardiol. 2002 Dec 1;90(11):1264-6.
6. Diercks DB et al. Changes in the numeric descriptive scale for pain after sublingual nitroglycerin do not predict cardiac etiology of chest pain. Ann Emerg Med. 2005 Jun;45(6):581-5.
7. His DH et al. Headache response to glyceryl trinitrate in patients with and without obstructive coronary artery disease. Heart 2005;91:1164-6.
He has stable angina, having chest pain with exercise. He uses sublingual nitroglycerin (SL NTG prn) about three times a month. His blood pressure is 140/70 mm Hg. His pulse is 60 beats per minute. His current medications are lisinopril, atorvastatin, aspirin, and SL NTG tablets as needed.
What would you recommend?
A. No sildenafil; refer to urologist for other ED options.
B. Okay to use sildenafil if greater than 6 hours from NTG use.
C. Recommend tadalafil.
Is coprescribing nitrates and phosphodiesterase inhibitors safe?
The FDA warns against the use of phosphodiesterase inhibitors in patients taking nitrates. Combining nitrates with phosphodiesterase type 5 (PDE5) inhibitors is contraindicated because of a synergistic blood pressure lowering effect.1 This warning/contraindication was based on theoretical concerns, as well as concern that of the first 130 deaths reported in patients who took sildenafil, 16 of the patients also were taking nitrates.2
Parker and colleagues studied the safety of giving IV nitroglycerin to patients with coronary artery disease (CAD) who have taken sildenafil.3 The study was a randomized, placebo-controlled, crossover trial. Participants received sildenafil 100 mg or placebo, then received intravenous NTG. Patients who received sildenafil had a 4-6 mm Hg systolic BP drop compared with those who took the placebo. There was no difference in severe events between the sildenafil and placebo groups. The blood levels of nitroglycerin in this study were very likely much higher than the levels that occur with SL NTG.
A recent study by Holt et al. looked at overall cardiovascular outcomes with coprescribing nitrates and phosphodiesterase inhibitors.4 The study was a case crossover design, using a nationwide Danish health registry over the period of 2000-2018. In 2000, the rate of coprescribing of phosphodiesterase inhibitors in ischemic heart disease patients on nitrates was .9 per 100 persons/year and rose to 19.5 prescriptions per 100 persons/year in 2018. During this same time, no statistically significant association was found between the coprescription of nitrates with PDE5 inhibitors and the risk for MI, cardiac arrest, syncope, stroke, or an adverse drug event.
Does nitroglycerin response help determine cause of chest pain?
Nitroglycerin response has long been used as a clinical indicator on whether a patient’s chest pain is cardiac or not. Eric A. Shry, MD, and his colleagues looked at the usefulness of nitroglycerin response in the treatment of chest pain as a predictor of ischemic chest pain in an emergency department setting.5
The study was a retrospective review of 223 patients who presented to the emergency department over a 5-month period with ongoing chest pain. They looked at patients who had ongoing chest pain in the emergency department, received nitroglycerin, and did not receive any therapy other than aspirin within 10 minutes of receiving nitroglycerin. Response to the drug was compared with the final diagnosis of cardiac versus noncardiac chest pain.
Of the patients with a final determination of cardiac chest pain, 88% had a nitroglycerin response, whereas 92% of the patients with noncardiac chest pain had a nitroglycerin response (P = .50).
Deborah B. Diercks, MD, and her colleagues looked at improvement in chest pain scores in the emergency department in patients treated with nitroglycerin and whether it correlated with a cardiac etiology of chest pain.6 The study was a prospective, observational study of 664 patients in an urban tertiary care emergency department over a 16-month period. An 11-point numeric chest pain scale was assessed and recorded by research assistants before and 5 minutes after receiving nitroglycerin. The scale ranged from 0 (no pain) to 10 (worst pain imaginable).
A final diagnosis of a cardiac etiology for chest pain was found in 18% of the patients in the study. Of the patients who had cardiac-related chest pain, 20% had no reduction in pain with nitroglycerin, compared with 19% of the patients without cardiac-related chest pain.
A complete or significant reduction in chest pain occurred with nitroglycerin in 31% of patients with cardiac chest pain and 27% of the patients without cardiac chest pain (P = .76).
Nitroglycerin response does not appear to be helpful in distinguishing cardiac from noncardiac chest pain, but a study by His and colleagues offers an interesting twist.7
The authors of this research studied 118 patients looking to see if the side effect of headache with nitroglycerin was more common in patients who did not have CAD than in those who did. All the patients had a varying degree of relief of chest pain with NTG administration within 10 minutes. In patients with normal coronary arteries or minimal CAD, 73% had headache caused by NTG, whereas in patients with obstructive CAD, only 23% had headache after NTG use.
Take-home messages
- Short acting nitroglycerin may not be a contraindication for phosphodiesterase inhibitor use.
- More data are still needed.
- Nitroglycerin response does not help distinguish chest pain from CAD from noncardiac causes.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Schwartz BG, Kloner RA. Drug interactions with phosphodiesterase-5 inhibitors used for the treatment of erectile dysfunction or pulmonary hypertension. Circulation. 2010;122:88-95.
2. Kloner RA, Zusman RM. Cardiovascular effects of sildenafil citrate and recommendations for its use. Am J Cardiol. 1999 Sep 9;84(5B):11N-17N.
3. Parker JD et al. Safety of intravenous nitroglycerin after administration of sildenafil citrate to men with coronary artery disease: A double-blind, placebo-controlled, randomized, crossover trial. Crit Care Med. 2007;35:1863-8.
4. Holt A et al. Adverse events associated with coprescription of phosphodiesterase type inhibitors and oral organic nitrates in male patients with ischemic heart disease. Ann Intern Med. 2022 Jun;175(6):774-82.
5. Shry EA et al. Usefulness of the response to sublingual nitroglycerin as a predictor of ischemic chest pain in the emergency department. Am J Cardiol. 2002 Dec 1;90(11):1264-6.
6. Diercks DB et al. Changes in the numeric descriptive scale for pain after sublingual nitroglycerin do not predict cardiac etiology of chest pain. Ann Emerg Med. 2005 Jun;45(6):581-5.
7. His DH et al. Headache response to glyceryl trinitrate in patients with and without obstructive coronary artery disease. Heart 2005;91:1164-6.
He has stable angina, having chest pain with exercise. He uses sublingual nitroglycerin (SL NTG prn) about three times a month. His blood pressure is 140/70 mm Hg. His pulse is 60 beats per minute. His current medications are lisinopril, atorvastatin, aspirin, and SL NTG tablets as needed.
What would you recommend?
A. No sildenafil; refer to urologist for other ED options.
B. Okay to use sildenafil if greater than 6 hours from NTG use.
C. Recommend tadalafil.
Is coprescribing nitrates and phosphodiesterase inhibitors safe?
The FDA warns against the use of phosphodiesterase inhibitors in patients taking nitrates. Combining nitrates with phosphodiesterase type 5 (PDE5) inhibitors is contraindicated because of a synergistic blood pressure lowering effect.1 This warning/contraindication was based on theoretical concerns, as well as concern that of the first 130 deaths reported in patients who took sildenafil, 16 of the patients also were taking nitrates.2
Parker and colleagues studied the safety of giving IV nitroglycerin to patients with coronary artery disease (CAD) who have taken sildenafil.3 The study was a randomized, placebo-controlled, crossover trial. Participants received sildenafil 100 mg or placebo, then received intravenous NTG. Patients who received sildenafil had a 4-6 mm Hg systolic BP drop compared with those who took the placebo. There was no difference in severe events between the sildenafil and placebo groups. The blood levels of nitroglycerin in this study were very likely much higher than the levels that occur with SL NTG.
A recent study by Holt et al. looked at overall cardiovascular outcomes with coprescribing nitrates and phosphodiesterase inhibitors.4 The study was a case crossover design, using a nationwide Danish health registry over the period of 2000-2018. In 2000, the rate of coprescribing of phosphodiesterase inhibitors in ischemic heart disease patients on nitrates was .9 per 100 persons/year and rose to 19.5 prescriptions per 100 persons/year in 2018. During this same time, no statistically significant association was found between the coprescription of nitrates with PDE5 inhibitors and the risk for MI, cardiac arrest, syncope, stroke, or an adverse drug event.
Does nitroglycerin response help determine cause of chest pain?
Nitroglycerin response has long been used as a clinical indicator on whether a patient’s chest pain is cardiac or not. Eric A. Shry, MD, and his colleagues looked at the usefulness of nitroglycerin response in the treatment of chest pain as a predictor of ischemic chest pain in an emergency department setting.5
The study was a retrospective review of 223 patients who presented to the emergency department over a 5-month period with ongoing chest pain. They looked at patients who had ongoing chest pain in the emergency department, received nitroglycerin, and did not receive any therapy other than aspirin within 10 minutes of receiving nitroglycerin. Response to the drug was compared with the final diagnosis of cardiac versus noncardiac chest pain.
Of the patients with a final determination of cardiac chest pain, 88% had a nitroglycerin response, whereas 92% of the patients with noncardiac chest pain had a nitroglycerin response (P = .50).
Deborah B. Diercks, MD, and her colleagues looked at improvement in chest pain scores in the emergency department in patients treated with nitroglycerin and whether it correlated with a cardiac etiology of chest pain.6 The study was a prospective, observational study of 664 patients in an urban tertiary care emergency department over a 16-month period. An 11-point numeric chest pain scale was assessed and recorded by research assistants before and 5 minutes after receiving nitroglycerin. The scale ranged from 0 (no pain) to 10 (worst pain imaginable).
A final diagnosis of a cardiac etiology for chest pain was found in 18% of the patients in the study. Of the patients who had cardiac-related chest pain, 20% had no reduction in pain with nitroglycerin, compared with 19% of the patients without cardiac-related chest pain.
A complete or significant reduction in chest pain occurred with nitroglycerin in 31% of patients with cardiac chest pain and 27% of the patients without cardiac chest pain (P = .76).
Nitroglycerin response does not appear to be helpful in distinguishing cardiac from noncardiac chest pain, but a study by His and colleagues offers an interesting twist.7
The authors of this research studied 118 patients looking to see if the side effect of headache with nitroglycerin was more common in patients who did not have CAD than in those who did. All the patients had a varying degree of relief of chest pain with NTG administration within 10 minutes. In patients with normal coronary arteries or minimal CAD, 73% had headache caused by NTG, whereas in patients with obstructive CAD, only 23% had headache after NTG use.
Take-home messages
- Short acting nitroglycerin may not be a contraindication for phosphodiesterase inhibitor use.
- More data are still needed.
- Nitroglycerin response does not help distinguish chest pain from CAD from noncardiac causes.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Schwartz BG, Kloner RA. Drug interactions with phosphodiesterase-5 inhibitors used for the treatment of erectile dysfunction or pulmonary hypertension. Circulation. 2010;122:88-95.
2. Kloner RA, Zusman RM. Cardiovascular effects of sildenafil citrate and recommendations for its use. Am J Cardiol. 1999 Sep 9;84(5B):11N-17N.
3. Parker JD et al. Safety of intravenous nitroglycerin after administration of sildenafil citrate to men with coronary artery disease: A double-blind, placebo-controlled, randomized, crossover trial. Crit Care Med. 2007;35:1863-8.
4. Holt A et al. Adverse events associated with coprescription of phosphodiesterase type inhibitors and oral organic nitrates in male patients with ischemic heart disease. Ann Intern Med. 2022 Jun;175(6):774-82.
5. Shry EA et al. Usefulness of the response to sublingual nitroglycerin as a predictor of ischemic chest pain in the emergency department. Am J Cardiol. 2002 Dec 1;90(11):1264-6.
6. Diercks DB et al. Changes in the numeric descriptive scale for pain after sublingual nitroglycerin do not predict cardiac etiology of chest pain. Ann Emerg Med. 2005 Jun;45(6):581-5.
7. His DH et al. Headache response to glyceryl trinitrate in patients with and without obstructive coronary artery disease. Heart 2005;91:1164-6.



