User login
Neutrophils Take Center Stage in Growing Understanding of Colchicine’s Role in Treating Atherosclerotic Cardiovascular Disease
NEW YORK — New insights into colchicine’s disruption of the pathway that contributes to arterial inflammation and new clinical studies of the drug could pave the way toward greater use of the anti-inflammatory drug in patients with or at risk for atherosclerotic cardiovascular disease (ASCVD), researchers said at the 4th Annual Cardiometabolic Risk in Inflammatory Conditions conference.
Colchicine was approved by the US Food and Drug Administration (FDA) in June 2023 in a once-daily 0.5-mg formulation under the brand name Lodoco to reduce the risk for major adverse cardiovascular events (MACE) in patients with established atherosclerotic disease or with multiple risk factors for CVD. The Lodoco formulation is slightly smaller than the 0.6-mg formulation that’s taken twice daily for the prophylaxis and treatment of acute gout flares.
In a presentation at the conference, Binita Shah, MD, one of the principal investigators in trials of Lodoco, explained how the inflammatory pathway contributes to atherosclerosis and provided an update on how colchicine disrupts the pathway. Dr. Shah is an associate professor of medicine at New York University in New York City and director of research at NYU Langone Health Interventional Cardiology.
“Colchicine dampens inflammatory markers on neutrophils so that they are less likely to be attracted to inflamed or injured endothelium, which would be the site of where plaque is building up or where the plaque has ruptured in the setting of a heart attack,” Shah told this news organization after her presentation.
The Inflammatory Pathway
Dr. Shah explained that normal coronary endothelium resists adhesion by circulating leukocytes, but inflamed or injured coronary endothelium attracts those neutrophils via two types of selectins: L-selectins on neutrophils and E-selectins on endothelial cells. Those neutrophils then release inflammatory cytokines including interleukin-1 beta (IL-1ß), which then triggers production of IL-6 and, subsequently, high-sensitivity C-reactive protein (hsCRP), which contributes to plaque formation, she said.
“Colchicine affects these pathways with a balance for safety and effect on clinical outcomes, particularly to reduce recurrent myocardial infarction [MI],” Dr. Shah said during her presentation.
Results from the CIRT trial demonstrated that methotrexate is ineffective in blocking the adenosine-mediated anti-inflammatory pathway, Dr. Shah said, so focusing on the IL-1ß–IL-6–hsCRP pathway, which is known to work based on the results of the CANTOS trial, could pay dividends.
“This is where colchicine can potentially play a role,” she said.
Dr. Shah cited a secondary analysis of the CANTOS trial in which the magnitude of hsCRP reduction correlated with a reduction in MI, stroke, or cardiovascular death. The secondary analysis showed that patients who received canakinumab and achieved hsCRP ≥ 2 mg/L had a nonsignificant 5% lower risk and those who reached < 2 mg/L had a statistically significant 25% lower risk than those who received placebo.
The COPE-PCI Pilot trial demonstrated the benefit of targeting the interleukin pathways, she noted.
Further clarification of the role of colchicine in managing patients with acute coronary syndrome may come from two other randomized trials now underway, Dr. Shah said: POPCORN is evaluating colchicine to reduce MACE after noncardiac surgery, and CLEAR SYNERGY is evaluating the best timing for colchicine therapy after an acute MI.
Dr. Shah presented preliminary data from her group from a neutrophil biomarker substudy of CLEAR SYNERGY that isolated neutrophils from patients who had an acute MI. “We treated them with various doses of colchicine and showed that the interaction between those treated neutrophils [and] the endothelial cells were a lot lower; they were less sticky to endothelial cells as colchicine was administered,” she said in her presentation. She added that colchicine also reduced neutrophil chemotaxis and neutrophil activation and potentially inhibited inflammasomes, decreasing IL-1ß production.
What’s more, colchicine has been shown to not affect platelets alone but rather platelets at the site of inflammation or plaque rupture, Dr. Shah added. “At currently used doses, colchicine does not inhibit platelet activity [by] itself, so we’ve never seen increased bleeding events, but it will dampen neutrophils’ ability to latch onto a platelet that could contribute to a clot,” she later told this news organization.
“There are multiple studies, both retrospective studies in gout cohorts as well as prospective studies in the cardiovascular cohort, that all show consistently one thing, which is that colchicine continues to reduce the risk of having a recurrent MI in patients who either have cardiovascular disease or are at high risk of having cardiovascular disease,” she said.
“I think that’s very helpful to know that it’s not just one study — it’s not just a fluke, potentially a play of chance — but multiple studies consistently showing the same thing: That there’s a reduced risk of acute MI.”
Slow to Embrace Colchicine
Despite this evidence, cardiologists and rheumatologists have been slow to embrace colchicine for patients at risk for cardiovascular events, said Michael S. Garshick, MD, who attended the conference and is head of the Cardio-Rheumatology Program at NYU Langone. “What [Shah] really highlighted was that for a number of years now, we’ve had several clinical trials showing the benefit of low-dose colchicine to prevent atherosclerotic cardiovascular events, and yet despite these and that there’s now an indication to use low-dose colchicine to reduce cardiovascular disease, we’re still struggling for this medication to be taken up by the general cardiology community to treat high-risk patients.
“There’s still some work to do to prove that we need to break those barriers,” Dr. Garshick added. Some of the confusion surrounding the use of colchicine for ASCVD may be attributed to the 0.5-mg dose approved for CVD as opposed to the long-approved 0.6-mg dose for gout, he said. “People are generally confused: Is it OK to use the 0.6-mg dose?” Dr. Garshick said.
Potential gastrointestinal side effects may be another concerning factor, although, he added, “we didn’t see any major complications.” Another issue could be polypharmacy in many of these patients, he said.
Dr. Garshick concurred with Shah that the existing evidence supporting the use of colchicine to reduce risk for cardiovascular events is strong, but more will come out. “I think there’s going to be evolving data supporting it,” he said.
Dr. Shah disclosed financial relationships with Philips Volcano and Novo Nordisk. She is a principal investigator of the CLEAR SYNERGY biomarker substudy and the POPCORN trial. Dr. Garshick disclosed relationships with Kiniksa Pharmaceuticals, Agepha Pharma, Bristol Myers Squibb, and Horizon Therapeutics.
A version of this article appeared on Medscape.com .
NEW YORK — New insights into colchicine’s disruption of the pathway that contributes to arterial inflammation and new clinical studies of the drug could pave the way toward greater use of the anti-inflammatory drug in patients with or at risk for atherosclerotic cardiovascular disease (ASCVD), researchers said at the 4th Annual Cardiometabolic Risk in Inflammatory Conditions conference.
Colchicine was approved by the US Food and Drug Administration (FDA) in June 2023 in a once-daily 0.5-mg formulation under the brand name Lodoco to reduce the risk for major adverse cardiovascular events (MACE) in patients with established atherosclerotic disease or with multiple risk factors for CVD. The Lodoco formulation is slightly smaller than the 0.6-mg formulation that’s taken twice daily for the prophylaxis and treatment of acute gout flares.
In a presentation at the conference, Binita Shah, MD, one of the principal investigators in trials of Lodoco, explained how the inflammatory pathway contributes to atherosclerosis and provided an update on how colchicine disrupts the pathway. Dr. Shah is an associate professor of medicine at New York University in New York City and director of research at NYU Langone Health Interventional Cardiology.
“Colchicine dampens inflammatory markers on neutrophils so that they are less likely to be attracted to inflamed or injured endothelium, which would be the site of where plaque is building up or where the plaque has ruptured in the setting of a heart attack,” Shah told this news organization after her presentation.
The Inflammatory Pathway
Dr. Shah explained that normal coronary endothelium resists adhesion by circulating leukocytes, but inflamed or injured coronary endothelium attracts those neutrophils via two types of selectins: L-selectins on neutrophils and E-selectins on endothelial cells. Those neutrophils then release inflammatory cytokines including interleukin-1 beta (IL-1ß), which then triggers production of IL-6 and, subsequently, high-sensitivity C-reactive protein (hsCRP), which contributes to plaque formation, she said.
“Colchicine affects these pathways with a balance for safety and effect on clinical outcomes, particularly to reduce recurrent myocardial infarction [MI],” Dr. Shah said during her presentation.
Results from the CIRT trial demonstrated that methotrexate is ineffective in blocking the adenosine-mediated anti-inflammatory pathway, Dr. Shah said, so focusing on the IL-1ß–IL-6–hsCRP pathway, which is known to work based on the results of the CANTOS trial, could pay dividends.
“This is where colchicine can potentially play a role,” she said.
Dr. Shah cited a secondary analysis of the CANTOS trial in which the magnitude of hsCRP reduction correlated with a reduction in MI, stroke, or cardiovascular death. The secondary analysis showed that patients who received canakinumab and achieved hsCRP ≥ 2 mg/L had a nonsignificant 5% lower risk and those who reached < 2 mg/L had a statistically significant 25% lower risk than those who received placebo.
The COPE-PCI Pilot trial demonstrated the benefit of targeting the interleukin pathways, she noted.
Further clarification of the role of colchicine in managing patients with acute coronary syndrome may come from two other randomized trials now underway, Dr. Shah said: POPCORN is evaluating colchicine to reduce MACE after noncardiac surgery, and CLEAR SYNERGY is evaluating the best timing for colchicine therapy after an acute MI.
Dr. Shah presented preliminary data from her group from a neutrophil biomarker substudy of CLEAR SYNERGY that isolated neutrophils from patients who had an acute MI. “We treated them with various doses of colchicine and showed that the interaction between those treated neutrophils [and] the endothelial cells were a lot lower; they were less sticky to endothelial cells as colchicine was administered,” she said in her presentation. She added that colchicine also reduced neutrophil chemotaxis and neutrophil activation and potentially inhibited inflammasomes, decreasing IL-1ß production.
What’s more, colchicine has been shown to not affect platelets alone but rather platelets at the site of inflammation or plaque rupture, Dr. Shah added. “At currently used doses, colchicine does not inhibit platelet activity [by] itself, so we’ve never seen increased bleeding events, but it will dampen neutrophils’ ability to latch onto a platelet that could contribute to a clot,” she later told this news organization.
“There are multiple studies, both retrospective studies in gout cohorts as well as prospective studies in the cardiovascular cohort, that all show consistently one thing, which is that colchicine continues to reduce the risk of having a recurrent MI in patients who either have cardiovascular disease or are at high risk of having cardiovascular disease,” she said.
“I think that’s very helpful to know that it’s not just one study — it’s not just a fluke, potentially a play of chance — but multiple studies consistently showing the same thing: That there’s a reduced risk of acute MI.”
Slow to Embrace Colchicine
Despite this evidence, cardiologists and rheumatologists have been slow to embrace colchicine for patients at risk for cardiovascular events, said Michael S. Garshick, MD, who attended the conference and is head of the Cardio-Rheumatology Program at NYU Langone. “What [Shah] really highlighted was that for a number of years now, we’ve had several clinical trials showing the benefit of low-dose colchicine to prevent atherosclerotic cardiovascular events, and yet despite these and that there’s now an indication to use low-dose colchicine to reduce cardiovascular disease, we’re still struggling for this medication to be taken up by the general cardiology community to treat high-risk patients.
“There’s still some work to do to prove that we need to break those barriers,” Dr. Garshick added. Some of the confusion surrounding the use of colchicine for ASCVD may be attributed to the 0.5-mg dose approved for CVD as opposed to the long-approved 0.6-mg dose for gout, he said. “People are generally confused: Is it OK to use the 0.6-mg dose?” Dr. Garshick said.
Potential gastrointestinal side effects may be another concerning factor, although, he added, “we didn’t see any major complications.” Another issue could be polypharmacy in many of these patients, he said.
Dr. Garshick concurred with Shah that the existing evidence supporting the use of colchicine to reduce risk for cardiovascular events is strong, but more will come out. “I think there’s going to be evolving data supporting it,” he said.
Dr. Shah disclosed financial relationships with Philips Volcano and Novo Nordisk. She is a principal investigator of the CLEAR SYNERGY biomarker substudy and the POPCORN trial. Dr. Garshick disclosed relationships with Kiniksa Pharmaceuticals, Agepha Pharma, Bristol Myers Squibb, and Horizon Therapeutics.
A version of this article appeared on Medscape.com .
NEW YORK — New insights into colchicine’s disruption of the pathway that contributes to arterial inflammation and new clinical studies of the drug could pave the way toward greater use of the anti-inflammatory drug in patients with or at risk for atherosclerotic cardiovascular disease (ASCVD), researchers said at the 4th Annual Cardiometabolic Risk in Inflammatory Conditions conference.
Colchicine was approved by the US Food and Drug Administration (FDA) in June 2023 in a once-daily 0.5-mg formulation under the brand name Lodoco to reduce the risk for major adverse cardiovascular events (MACE) in patients with established atherosclerotic disease or with multiple risk factors for CVD. The Lodoco formulation is slightly smaller than the 0.6-mg formulation that’s taken twice daily for the prophylaxis and treatment of acute gout flares.
In a presentation at the conference, Binita Shah, MD, one of the principal investigators in trials of Lodoco, explained how the inflammatory pathway contributes to atherosclerosis and provided an update on how colchicine disrupts the pathway. Dr. Shah is an associate professor of medicine at New York University in New York City and director of research at NYU Langone Health Interventional Cardiology.
“Colchicine dampens inflammatory markers on neutrophils so that they are less likely to be attracted to inflamed or injured endothelium, which would be the site of where plaque is building up or where the plaque has ruptured in the setting of a heart attack,” Shah told this news organization after her presentation.
The Inflammatory Pathway
Dr. Shah explained that normal coronary endothelium resists adhesion by circulating leukocytes, but inflamed or injured coronary endothelium attracts those neutrophils via two types of selectins: L-selectins on neutrophils and E-selectins on endothelial cells. Those neutrophils then release inflammatory cytokines including interleukin-1 beta (IL-1ß), which then triggers production of IL-6 and, subsequently, high-sensitivity C-reactive protein (hsCRP), which contributes to plaque formation, she said.
“Colchicine affects these pathways with a balance for safety and effect on clinical outcomes, particularly to reduce recurrent myocardial infarction [MI],” Dr. Shah said during her presentation.
Results from the CIRT trial demonstrated that methotrexate is ineffective in blocking the adenosine-mediated anti-inflammatory pathway, Dr. Shah said, so focusing on the IL-1ß–IL-6–hsCRP pathway, which is known to work based on the results of the CANTOS trial, could pay dividends.
“This is where colchicine can potentially play a role,” she said.
Dr. Shah cited a secondary analysis of the CANTOS trial in which the magnitude of hsCRP reduction correlated with a reduction in MI, stroke, or cardiovascular death. The secondary analysis showed that patients who received canakinumab and achieved hsCRP ≥ 2 mg/L had a nonsignificant 5% lower risk and those who reached < 2 mg/L had a statistically significant 25% lower risk than those who received placebo.
The COPE-PCI Pilot trial demonstrated the benefit of targeting the interleukin pathways, she noted.
Further clarification of the role of colchicine in managing patients with acute coronary syndrome may come from two other randomized trials now underway, Dr. Shah said: POPCORN is evaluating colchicine to reduce MACE after noncardiac surgery, and CLEAR SYNERGY is evaluating the best timing for colchicine therapy after an acute MI.
Dr. Shah presented preliminary data from her group from a neutrophil biomarker substudy of CLEAR SYNERGY that isolated neutrophils from patients who had an acute MI. “We treated them with various doses of colchicine and showed that the interaction between those treated neutrophils [and] the endothelial cells were a lot lower; they were less sticky to endothelial cells as colchicine was administered,” she said in her presentation. She added that colchicine also reduced neutrophil chemotaxis and neutrophil activation and potentially inhibited inflammasomes, decreasing IL-1ß production.
What’s more, colchicine has been shown to not affect platelets alone but rather platelets at the site of inflammation or plaque rupture, Dr. Shah added. “At currently used doses, colchicine does not inhibit platelet activity [by] itself, so we’ve never seen increased bleeding events, but it will dampen neutrophils’ ability to latch onto a platelet that could contribute to a clot,” she later told this news organization.
“There are multiple studies, both retrospective studies in gout cohorts as well as prospective studies in the cardiovascular cohort, that all show consistently one thing, which is that colchicine continues to reduce the risk of having a recurrent MI in patients who either have cardiovascular disease or are at high risk of having cardiovascular disease,” she said.
“I think that’s very helpful to know that it’s not just one study — it’s not just a fluke, potentially a play of chance — but multiple studies consistently showing the same thing: That there’s a reduced risk of acute MI.”
Slow to Embrace Colchicine
Despite this evidence, cardiologists and rheumatologists have been slow to embrace colchicine for patients at risk for cardiovascular events, said Michael S. Garshick, MD, who attended the conference and is head of the Cardio-Rheumatology Program at NYU Langone. “What [Shah] really highlighted was that for a number of years now, we’ve had several clinical trials showing the benefit of low-dose colchicine to prevent atherosclerotic cardiovascular events, and yet despite these and that there’s now an indication to use low-dose colchicine to reduce cardiovascular disease, we’re still struggling for this medication to be taken up by the general cardiology community to treat high-risk patients.
“There’s still some work to do to prove that we need to break those barriers,” Dr. Garshick added. Some of the confusion surrounding the use of colchicine for ASCVD may be attributed to the 0.5-mg dose approved for CVD as opposed to the long-approved 0.6-mg dose for gout, he said. “People are generally confused: Is it OK to use the 0.6-mg dose?” Dr. Garshick said.
Potential gastrointestinal side effects may be another concerning factor, although, he added, “we didn’t see any major complications.” Another issue could be polypharmacy in many of these patients, he said.
Dr. Garshick concurred with Shah that the existing evidence supporting the use of colchicine to reduce risk for cardiovascular events is strong, but more will come out. “I think there’s going to be evolving data supporting it,” he said.
Dr. Shah disclosed financial relationships with Philips Volcano and Novo Nordisk. She is a principal investigator of the CLEAR SYNERGY biomarker substudy and the POPCORN trial. Dr. Garshick disclosed relationships with Kiniksa Pharmaceuticals, Agepha Pharma, Bristol Myers Squibb, and Horizon Therapeutics.
A version of this article appeared on Medscape.com .
What Underlies Sex Differences in CKD Cardiovascular Risk?
Older men with chronic kidney disease (CKD) show higher resting muscle sympathetic nerve activity, but not vascular stiffness, compared with older women, offering clues to the underlying reasons why men with CKD have a higher cardiovascular risk than do women with the disease.
“Although it is well established that sympathetic nerve system activity is chronically elevated in patients with impaired kidney function, we show for the first time that males with CKD have higher resting muscle sympathetic nerve activity compared with females with CKD,” report the authors on research published in the American Journal of Physiology-Renal Physiology.
“For clinicians, the key takeaway is the importance of recognizing sex-specific differences in sympathetic activity and vascular function when assessing cardiovascular risk in CKD patients,” first author Matias G. Zanuzzi, MD, of the Division of Renal Medicine, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, told this news organization.
In the general population, cardiovascular risk is lower in younger women vs men, but their risks converge in older age as women develop similar levels of sympathetic overactivity, vascular stiffness, and cardiovascular risk.
However, an exception to that pattern is seen in the CKD population, where men continue to have a higher cardiovascular mortality risk vs women even in older age.
Studies evaluating the reasons for that have been conflicting, with some reporting a tendency of higher muscle sympathetic nerve activity in older women compared with men and others suggest the opposite finding — lower activity vs men.
To further investigate, Dr. Zanuzzi and colleagues enrolled 129 participants, including 96 men and 33 women with stage III or IV CKD.
The mean age of the study participants was 64 years for men and65 years for women. Most had obesity, and importantly, more than 80% of participants in each group was Black. There were no significant differences between the groups in terms of body mass index or comorbidities, including smoking, diabetes, or hypertension.
At two separate study visits, vascular stiffness was assessed with carotid-femoral pulse wave velocity measurement, and resting muscle sympathetic nerve activity was measured using microneurography.
The results showed that men with CKD had significantly higher resting muscle sympathetic nerve activity compared with women with CKD (68 vs 55 bursts per 100 heartbeats; P = .005), whereas no differences in vascular stiffness were observed between the genders (P = .248).
“The findings suggest that the higher cardiovascular disease risk observed in older males with CKD may be influenced by elevated sympathetic activity,” Dr. Zanuzzi explained.
“However, the lack of significant differences in vascular stiffness between genders implies that additional factors beyond vascular remodeling may contribute to the observed sex-specific differences in cardiovascular risk,” he said.
Of note, resting vascular stiffness was not associated with muscular sympathetic nerve activity in either men or women, which was surprising to the authors, Dr. Zanuzzi noted.
“This underscores the multifactorial nature of vascular pathophysiology in CKD and underscores the need for further research to unravel the underlying mechanisms.”
In other findings, although prior studies have shown a positive correlation between age and resting muscle sympathetic nerve activity in White, healthy women and men without obesity,, no similar relationship was observed in men or women with CKD.
“These findings suggest that the protective effect of younger age on sympathetic function may not be present in the setting of decreased kidney function in both males and females,” the authors note.
In addition, whereas previous research has shown a clear association between sympathetic overactivity and a wide variety of measures of obesity, in the current study, that association was only observed in men with CKD.
Important limitations of the study include the cross-sectional design and that the population was predominantly Black, Dr. Zanuzzi noted.
“Generalizability to other demographic groups may be limited, and future longitudinal studies are needed to validate these findings and explore potential causal relationships,” he said.
The findings underscore “the need for novel therapeutic approaches targeting sympathetic overactivity and vascular stiffness in CKD patients, especially considering the observed sex-specific differences,” Dr. Zanuzzi added.
“Potential interventions may include pharmacological agents that modulate sympathetic tone or vascular remodeling pathways,” he said.
“Lifestyle modifications focusing on stress reduction and cardiovascular health promotion could also play a crucial role in mitigating cardiovascular risk.”
Dr. Zanuzzi concluded that “tailoring treatment strategies to address these differences may lead to more personalized and effective management approaches, ultimately improving clinical outcomes in this high-risk population.”
The authors had no disclosures to report.
A version of this article first appeared on Medscape.com.
Older men with chronic kidney disease (CKD) show higher resting muscle sympathetic nerve activity, but not vascular stiffness, compared with older women, offering clues to the underlying reasons why men with CKD have a higher cardiovascular risk than do women with the disease.
“Although it is well established that sympathetic nerve system activity is chronically elevated in patients with impaired kidney function, we show for the first time that males with CKD have higher resting muscle sympathetic nerve activity compared with females with CKD,” report the authors on research published in the American Journal of Physiology-Renal Physiology.
“For clinicians, the key takeaway is the importance of recognizing sex-specific differences in sympathetic activity and vascular function when assessing cardiovascular risk in CKD patients,” first author Matias G. Zanuzzi, MD, of the Division of Renal Medicine, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, told this news organization.
In the general population, cardiovascular risk is lower in younger women vs men, but their risks converge in older age as women develop similar levels of sympathetic overactivity, vascular stiffness, and cardiovascular risk.
However, an exception to that pattern is seen in the CKD population, where men continue to have a higher cardiovascular mortality risk vs women even in older age.
Studies evaluating the reasons for that have been conflicting, with some reporting a tendency of higher muscle sympathetic nerve activity in older women compared with men and others suggest the opposite finding — lower activity vs men.
To further investigate, Dr. Zanuzzi and colleagues enrolled 129 participants, including 96 men and 33 women with stage III or IV CKD.
The mean age of the study participants was 64 years for men and65 years for women. Most had obesity, and importantly, more than 80% of participants in each group was Black. There were no significant differences between the groups in terms of body mass index or comorbidities, including smoking, diabetes, or hypertension.
At two separate study visits, vascular stiffness was assessed with carotid-femoral pulse wave velocity measurement, and resting muscle sympathetic nerve activity was measured using microneurography.
The results showed that men with CKD had significantly higher resting muscle sympathetic nerve activity compared with women with CKD (68 vs 55 bursts per 100 heartbeats; P = .005), whereas no differences in vascular stiffness were observed between the genders (P = .248).
“The findings suggest that the higher cardiovascular disease risk observed in older males with CKD may be influenced by elevated sympathetic activity,” Dr. Zanuzzi explained.
“However, the lack of significant differences in vascular stiffness between genders implies that additional factors beyond vascular remodeling may contribute to the observed sex-specific differences in cardiovascular risk,” he said.
Of note, resting vascular stiffness was not associated with muscular sympathetic nerve activity in either men or women, which was surprising to the authors, Dr. Zanuzzi noted.
“This underscores the multifactorial nature of vascular pathophysiology in CKD and underscores the need for further research to unravel the underlying mechanisms.”
In other findings, although prior studies have shown a positive correlation between age and resting muscle sympathetic nerve activity in White, healthy women and men without obesity,, no similar relationship was observed in men or women with CKD.
“These findings suggest that the protective effect of younger age on sympathetic function may not be present in the setting of decreased kidney function in both males and females,” the authors note.
In addition, whereas previous research has shown a clear association between sympathetic overactivity and a wide variety of measures of obesity, in the current study, that association was only observed in men with CKD.
Important limitations of the study include the cross-sectional design and that the population was predominantly Black, Dr. Zanuzzi noted.
“Generalizability to other demographic groups may be limited, and future longitudinal studies are needed to validate these findings and explore potential causal relationships,” he said.
The findings underscore “the need for novel therapeutic approaches targeting sympathetic overactivity and vascular stiffness in CKD patients, especially considering the observed sex-specific differences,” Dr. Zanuzzi added.
“Potential interventions may include pharmacological agents that modulate sympathetic tone or vascular remodeling pathways,” he said.
“Lifestyle modifications focusing on stress reduction and cardiovascular health promotion could also play a crucial role in mitigating cardiovascular risk.”
Dr. Zanuzzi concluded that “tailoring treatment strategies to address these differences may lead to more personalized and effective management approaches, ultimately improving clinical outcomes in this high-risk population.”
The authors had no disclosures to report.
A version of this article first appeared on Medscape.com.
Older men with chronic kidney disease (CKD) show higher resting muscle sympathetic nerve activity, but not vascular stiffness, compared with older women, offering clues to the underlying reasons why men with CKD have a higher cardiovascular risk than do women with the disease.
“Although it is well established that sympathetic nerve system activity is chronically elevated in patients with impaired kidney function, we show for the first time that males with CKD have higher resting muscle sympathetic nerve activity compared with females with CKD,” report the authors on research published in the American Journal of Physiology-Renal Physiology.
“For clinicians, the key takeaway is the importance of recognizing sex-specific differences in sympathetic activity and vascular function when assessing cardiovascular risk in CKD patients,” first author Matias G. Zanuzzi, MD, of the Division of Renal Medicine, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, told this news organization.
In the general population, cardiovascular risk is lower in younger women vs men, but their risks converge in older age as women develop similar levels of sympathetic overactivity, vascular stiffness, and cardiovascular risk.
However, an exception to that pattern is seen in the CKD population, where men continue to have a higher cardiovascular mortality risk vs women even in older age.
Studies evaluating the reasons for that have been conflicting, with some reporting a tendency of higher muscle sympathetic nerve activity in older women compared with men and others suggest the opposite finding — lower activity vs men.
To further investigate, Dr. Zanuzzi and colleagues enrolled 129 participants, including 96 men and 33 women with stage III or IV CKD.
The mean age of the study participants was 64 years for men and65 years for women. Most had obesity, and importantly, more than 80% of participants in each group was Black. There were no significant differences between the groups in terms of body mass index or comorbidities, including smoking, diabetes, or hypertension.
At two separate study visits, vascular stiffness was assessed with carotid-femoral pulse wave velocity measurement, and resting muscle sympathetic nerve activity was measured using microneurography.
The results showed that men with CKD had significantly higher resting muscle sympathetic nerve activity compared with women with CKD (68 vs 55 bursts per 100 heartbeats; P = .005), whereas no differences in vascular stiffness were observed between the genders (P = .248).
“The findings suggest that the higher cardiovascular disease risk observed in older males with CKD may be influenced by elevated sympathetic activity,” Dr. Zanuzzi explained.
“However, the lack of significant differences in vascular stiffness between genders implies that additional factors beyond vascular remodeling may contribute to the observed sex-specific differences in cardiovascular risk,” he said.
Of note, resting vascular stiffness was not associated with muscular sympathetic nerve activity in either men or women, which was surprising to the authors, Dr. Zanuzzi noted.
“This underscores the multifactorial nature of vascular pathophysiology in CKD and underscores the need for further research to unravel the underlying mechanisms.”
In other findings, although prior studies have shown a positive correlation between age and resting muscle sympathetic nerve activity in White, healthy women and men without obesity,, no similar relationship was observed in men or women with CKD.
“These findings suggest that the protective effect of younger age on sympathetic function may not be present in the setting of decreased kidney function in both males and females,” the authors note.
In addition, whereas previous research has shown a clear association between sympathetic overactivity and a wide variety of measures of obesity, in the current study, that association was only observed in men with CKD.
Important limitations of the study include the cross-sectional design and that the population was predominantly Black, Dr. Zanuzzi noted.
“Generalizability to other demographic groups may be limited, and future longitudinal studies are needed to validate these findings and explore potential causal relationships,” he said.
The findings underscore “the need for novel therapeutic approaches targeting sympathetic overactivity and vascular stiffness in CKD patients, especially considering the observed sex-specific differences,” Dr. Zanuzzi added.
“Potential interventions may include pharmacological agents that modulate sympathetic tone or vascular remodeling pathways,” he said.
“Lifestyle modifications focusing on stress reduction and cardiovascular health promotion could also play a crucial role in mitigating cardiovascular risk.”
Dr. Zanuzzi concluded that “tailoring treatment strategies to address these differences may lead to more personalized and effective management approaches, ultimately improving clinical outcomes in this high-risk population.”
The authors had no disclosures to report.
A version of this article first appeared on Medscape.com.
Metabolic Dysfunction–Associated Steatotic Liver Disease Plus HIV Ups Risk for CVD but Not Liver Disease
TOPLINE:
Metabolic dysfunction-associated steatotic liver disease (MASLD) co-occurring with HIV infection does not appear to increase the risk for cirrhosis or hepatocellular carcinoma (HCC) compared with MASLD alone. However, the incidence of major adverse cardiovascular events (MACE) is significantly increased among patients with MASLD and HIV, a large study suggested.
METHODOLOGY:
- MASLD is highly prevalent in people living with HIV, but the impact of HIV on liver and cardiovascular disease (CVD) outcomes in people with MASLD remains unclear.
- To investigate, researchers created a propensity score-matched cohort of veterans with noncirrhotic MASLD, with and without HIV (920 patients in each group).
- They evaluated the incidence of cirrhosis, HCC, and MACE, as well as overall survival, among the two groups. They also assessed these outcomes in MASLD patients with HIV on the basis of whether they were on antiretroviral therapy (ART).
TAKEAWAY:
- During a median follow-up of 10.4 years in the MASLD with HIV group and 11.8 years in the MASLD-only group, the overall incidence of cirrhosis and HCC was similar in MASLD with vs without HIV (cirrhosis: 0.97 vs 1.06 per 100 person-years, P = .54; HCC: 0.26 vs 0.17 per 100,000 person-years, P = .23), regardless of ART use.
- In contrast, the incidence of MACE was significantly higher in MASLD with vs without HIV (5.18 vs 4.48 per 100 person-years, P = .03). The incidence also was higher in patients with MASLD and HIV who were not on ART compared with those on ART (5.83 vs 4.7 per 100 person-years, P = .07).
- Compared with MASLD without HIV, the overall 5-year survival was significantly lower in MASLD with HIV (91.3% vs 85.7%). In MASLD with HIV, receipt of ART was associated with a significantly higher 5-year survival than no ART (87.4% vs 81.6%).
IN PRACTICE:
“Ensuring timely and appropriate initiation of HIV treatment is critical in patients with MASLD who have concurrent HIV infection, as well as optimizing metabolic comorbidities that may also contribute to increased risks of CVD and increased mortality,” the authors wrote.
SOURCE:
The study, led by Robert J. Wong, MD, Division of Gastroenterology and Hepatology, Stanford University School of Medicine, Palo Alto, California, was published online in the American Journal of Gastroenterology.
LIMITATIONS:
The study cohort consisted predominantly of older men, which may limit generalizability to women and younger populations. Metabolic comorbidities are more common in veterans compared with the general population, potentially affecting the generalizability of the CVD risk findings.
DISCLOSURES:
The study was supported by an investigator-initiated research grant from Theratechnologies. Wong has received funding for his institution from Gilead Sciences, Exact Sciences, and Durect Corporation and has served as a consultant for Gilead Sciences.
A version of this article appeared on Medscape.com.
TOPLINE:
Metabolic dysfunction-associated steatotic liver disease (MASLD) co-occurring with HIV infection does not appear to increase the risk for cirrhosis or hepatocellular carcinoma (HCC) compared with MASLD alone. However, the incidence of major adverse cardiovascular events (MACE) is significantly increased among patients with MASLD and HIV, a large study suggested.
METHODOLOGY:
- MASLD is highly prevalent in people living with HIV, but the impact of HIV on liver and cardiovascular disease (CVD) outcomes in people with MASLD remains unclear.
- To investigate, researchers created a propensity score-matched cohort of veterans with noncirrhotic MASLD, with and without HIV (920 patients in each group).
- They evaluated the incidence of cirrhosis, HCC, and MACE, as well as overall survival, among the two groups. They also assessed these outcomes in MASLD patients with HIV on the basis of whether they were on antiretroviral therapy (ART).
TAKEAWAY:
- During a median follow-up of 10.4 years in the MASLD with HIV group and 11.8 years in the MASLD-only group, the overall incidence of cirrhosis and HCC was similar in MASLD with vs without HIV (cirrhosis: 0.97 vs 1.06 per 100 person-years, P = .54; HCC: 0.26 vs 0.17 per 100,000 person-years, P = .23), regardless of ART use.
- In contrast, the incidence of MACE was significantly higher in MASLD with vs without HIV (5.18 vs 4.48 per 100 person-years, P = .03). The incidence also was higher in patients with MASLD and HIV who were not on ART compared with those on ART (5.83 vs 4.7 per 100 person-years, P = .07).
- Compared with MASLD without HIV, the overall 5-year survival was significantly lower in MASLD with HIV (91.3% vs 85.7%). In MASLD with HIV, receipt of ART was associated with a significantly higher 5-year survival than no ART (87.4% vs 81.6%).
IN PRACTICE:
“Ensuring timely and appropriate initiation of HIV treatment is critical in patients with MASLD who have concurrent HIV infection, as well as optimizing metabolic comorbidities that may also contribute to increased risks of CVD and increased mortality,” the authors wrote.
SOURCE:
The study, led by Robert J. Wong, MD, Division of Gastroenterology and Hepatology, Stanford University School of Medicine, Palo Alto, California, was published online in the American Journal of Gastroenterology.
LIMITATIONS:
The study cohort consisted predominantly of older men, which may limit generalizability to women and younger populations. Metabolic comorbidities are more common in veterans compared with the general population, potentially affecting the generalizability of the CVD risk findings.
DISCLOSURES:
The study was supported by an investigator-initiated research grant from Theratechnologies. Wong has received funding for his institution from Gilead Sciences, Exact Sciences, and Durect Corporation and has served as a consultant for Gilead Sciences.
A version of this article appeared on Medscape.com.
TOPLINE:
Metabolic dysfunction-associated steatotic liver disease (MASLD) co-occurring with HIV infection does not appear to increase the risk for cirrhosis or hepatocellular carcinoma (HCC) compared with MASLD alone. However, the incidence of major adverse cardiovascular events (MACE) is significantly increased among patients with MASLD and HIV, a large study suggested.
METHODOLOGY:
- MASLD is highly prevalent in people living with HIV, but the impact of HIV on liver and cardiovascular disease (CVD) outcomes in people with MASLD remains unclear.
- To investigate, researchers created a propensity score-matched cohort of veterans with noncirrhotic MASLD, with and without HIV (920 patients in each group).
- They evaluated the incidence of cirrhosis, HCC, and MACE, as well as overall survival, among the two groups. They also assessed these outcomes in MASLD patients with HIV on the basis of whether they were on antiretroviral therapy (ART).
TAKEAWAY:
- During a median follow-up of 10.4 years in the MASLD with HIV group and 11.8 years in the MASLD-only group, the overall incidence of cirrhosis and HCC was similar in MASLD with vs without HIV (cirrhosis: 0.97 vs 1.06 per 100 person-years, P = .54; HCC: 0.26 vs 0.17 per 100,000 person-years, P = .23), regardless of ART use.
- In contrast, the incidence of MACE was significantly higher in MASLD with vs without HIV (5.18 vs 4.48 per 100 person-years, P = .03). The incidence also was higher in patients with MASLD and HIV who were not on ART compared with those on ART (5.83 vs 4.7 per 100 person-years, P = .07).
- Compared with MASLD without HIV, the overall 5-year survival was significantly lower in MASLD with HIV (91.3% vs 85.7%). In MASLD with HIV, receipt of ART was associated with a significantly higher 5-year survival than no ART (87.4% vs 81.6%).
IN PRACTICE:
“Ensuring timely and appropriate initiation of HIV treatment is critical in patients with MASLD who have concurrent HIV infection, as well as optimizing metabolic comorbidities that may also contribute to increased risks of CVD and increased mortality,” the authors wrote.
SOURCE:
The study, led by Robert J. Wong, MD, Division of Gastroenterology and Hepatology, Stanford University School of Medicine, Palo Alto, California, was published online in the American Journal of Gastroenterology.
LIMITATIONS:
The study cohort consisted predominantly of older men, which may limit generalizability to women and younger populations. Metabolic comorbidities are more common in veterans compared with the general population, potentially affecting the generalizability of the CVD risk findings.
DISCLOSURES:
The study was supported by an investigator-initiated research grant from Theratechnologies. Wong has received funding for his institution from Gilead Sciences, Exact Sciences, and Durect Corporation and has served as a consultant for Gilead Sciences.
A version of this article appeared on Medscape.com.
Higher BMI More CVD Protective in Older Adults With T2D?
Among adults with type 2 diabetes (T2D) older than 65 years, a body mass index (BMI) in the moderate overweight category (26-28) appears to offer better protection from cardiovascular death than does a BMI in the “normal” range, new data suggested.
On the other hand, the study findings also suggest that the “normal” range of 23-25 is optimal for middle-aged adults with T2D.
The findings reflect a previously demonstrated phenomenon called the “obesity paradox,” in which older people with overweight may have better outcomes than leaner people due to factors such as bone loss, frailty, and nutritional deficits, study lead author Shaoyong Xu, of Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Xiangyang, China, told this news organization.
“In this era of population growth and aging, the question arises as to whether obesity or overweight can be beneficial in improving survival rates for older individuals with diabetes. This topic holds significant relevance due to the potential implications it has on weight management strategies for older adults. If overweight does not pose an increased risk of cardiovascular mortality, it may suggest that older individuals are not necessarily required to strive for weight loss to achieve so-called normal values.”
Moreover, Dr. Xu added, “inappropriate weight loss and being underweight could potentially elevate the risk of cardiovascular events, myocardial infarction, cerebral infarction, and all-cause mortality.”
Thus, he said, “while there are general guidelines recommending a BMI below 25, our findings suggest that personalized BMI targets may be more beneficial, particularly for different age groups and individuals with specific health conditions.”
Asked to comment, Ian J. Neeland, MD, director of cardiovascular prevention, University Hospitals Harrington Heart & Vascular Institute, Case Western Reserve University, Cleveland, Ohio, pointed out that older people who are underweight or in lower weight categories may be more likely to smoke or have undiagnosed cancer, or that “their BMI is not so much reflective of fat mass as of low muscle mass, or sarcopenia, and that is definitely a risk factor for adverse outcomes and risks. ... And those who have slightly higher BMIs may be maintaining muscle mass, even though they’re older, and therefore they have less risk.”
However, Dr. Neeland disagreed with the authors’ conclusions regarding “optimal” BMI. “Just because you have different risk categories based on BMI doesn’t mean that’s ‘optimal’ BMI. The way I would interpret this paper is that there’s an association of mildly overweight with better outcomes in adults who are over 65 with type 2 diabetes. We need to try to understand the mechanisms underlying that observation.”
Dr. Neeland advised that for an older person with T2D who has low muscle mass and frailty, “I wouldn’t recommend necessarily targeted weight loss in that person. But I would potentially recommend weight loss in addition to resistance training, muscle building, and endurance training, and therefore reducing fat mass. The goal would be not so much weight loss but reduction of body fat and maintaining and improving muscle health.”
U-Shaped Relationship Found Between Age, BMI, and Cardiovascular Disease (CVD) Risk
The data come from the UK Biobank, a population-based prospective cohort study of adults in the United Kingdom. A total of 22,874 participants with baseline T2D were included in the current study. Baseline surveys were conducted between 2006 and 2010, and follow-up was a median of 12.52 years. During that time, 891 people died of CVD.
Hazard ratios were adjusted for baseline variables including age, sex, smoking history, alcohol consumption, level of physical exercise, and history of CVDs.
Compared with people with BMI a < 25 in the group who were aged 65 years or younger, those with a BMI of 25.0-29.9 had a 13% higher risk for cardiovascular death. However, among those older than 65 years, a BMI between 25.0 and 29.9 was associated with an 18% lower risk.
A U-shaped relationship was found between BMI and the risk for cardiovascular death, with an optimal BMI cutoff of 24.0 in the under-65 group and a 27.0 cutoff in the older group. Ranges of 23.0-25.0 in the under-65 group and 26.0-28 in the older group were associated with the lowest cardiovascular risk.
In contrast, there was a linear relationship between both waist circumference and waist-to-height ratio and the risk for cardiovascular death, making those more direct measures of adiposity, Dr. Xu told this news organization.
“For clinicians, our data underscores the importance of considering age when assessing BMI targets for cardiovascular health. Personalized treatment plans that account for age-specific BMI cutoffs and other risk factors may enhance patient outcomes and reduce CVD mortality,” Dr. Xu said.
However, he added, “while these findings suggest an optimal BMI range, it is crucial to acknowledge that these cutoff points may vary based on gender, race, and other factors. Our future studies will validate these findings in different populations and attempt to explain the mechanism by which the optimal nodal values exist in people with diabetes at different ages.”
Dr. Neeland cautioned, “I think more work needs to be done in terms of not just identifying the risk differences but understanding why and how to better risk stratify individuals and do personalized medicine. I think that’s important, but you have to have good data to support the strategies you’re going to use. These data are observational, and they’re a good start, but they wouldn’t directly impact practice at this point.”
The data will be presented at the European Congress on Obesity taking place May 12-15 in Venice, Italy.
The authors declared no competing interests. Study funding came from several sources, including the Young Talents Project of Hubei Provincial Health Commission, China, Hubei Provincial Natural Science Foundation of China, the Science and Technology Research Key Project of the Education Department of Hubei Province China, and the Sanuo Diabetes Charity Foundation, China, and the Xiangyang Science and Technology Plan Project, China. Dr. Neeland is a speaker and/or consultant for Boehringer Ingelheim, Novo Nordisk, Bayer, and Eli Lilly and Company.
A version of this article appeared on Medscape.com.
Among adults with type 2 diabetes (T2D) older than 65 years, a body mass index (BMI) in the moderate overweight category (26-28) appears to offer better protection from cardiovascular death than does a BMI in the “normal” range, new data suggested.
On the other hand, the study findings also suggest that the “normal” range of 23-25 is optimal for middle-aged adults with T2D.
The findings reflect a previously demonstrated phenomenon called the “obesity paradox,” in which older people with overweight may have better outcomes than leaner people due to factors such as bone loss, frailty, and nutritional deficits, study lead author Shaoyong Xu, of Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Xiangyang, China, told this news organization.
“In this era of population growth and aging, the question arises as to whether obesity or overweight can be beneficial in improving survival rates for older individuals with diabetes. This topic holds significant relevance due to the potential implications it has on weight management strategies for older adults. If overweight does not pose an increased risk of cardiovascular mortality, it may suggest that older individuals are not necessarily required to strive for weight loss to achieve so-called normal values.”
Moreover, Dr. Xu added, “inappropriate weight loss and being underweight could potentially elevate the risk of cardiovascular events, myocardial infarction, cerebral infarction, and all-cause mortality.”
Thus, he said, “while there are general guidelines recommending a BMI below 25, our findings suggest that personalized BMI targets may be more beneficial, particularly for different age groups and individuals with specific health conditions.”
Asked to comment, Ian J. Neeland, MD, director of cardiovascular prevention, University Hospitals Harrington Heart & Vascular Institute, Case Western Reserve University, Cleveland, Ohio, pointed out that older people who are underweight or in lower weight categories may be more likely to smoke or have undiagnosed cancer, or that “their BMI is not so much reflective of fat mass as of low muscle mass, or sarcopenia, and that is definitely a risk factor for adverse outcomes and risks. ... And those who have slightly higher BMIs may be maintaining muscle mass, even though they’re older, and therefore they have less risk.”
However, Dr. Neeland disagreed with the authors’ conclusions regarding “optimal” BMI. “Just because you have different risk categories based on BMI doesn’t mean that’s ‘optimal’ BMI. The way I would interpret this paper is that there’s an association of mildly overweight with better outcomes in adults who are over 65 with type 2 diabetes. We need to try to understand the mechanisms underlying that observation.”
Dr. Neeland advised that for an older person with T2D who has low muscle mass and frailty, “I wouldn’t recommend necessarily targeted weight loss in that person. But I would potentially recommend weight loss in addition to resistance training, muscle building, and endurance training, and therefore reducing fat mass. The goal would be not so much weight loss but reduction of body fat and maintaining and improving muscle health.”
U-Shaped Relationship Found Between Age, BMI, and Cardiovascular Disease (CVD) Risk
The data come from the UK Biobank, a population-based prospective cohort study of adults in the United Kingdom. A total of 22,874 participants with baseline T2D were included in the current study. Baseline surveys were conducted between 2006 and 2010, and follow-up was a median of 12.52 years. During that time, 891 people died of CVD.
Hazard ratios were adjusted for baseline variables including age, sex, smoking history, alcohol consumption, level of physical exercise, and history of CVDs.
Compared with people with BMI a < 25 in the group who were aged 65 years or younger, those with a BMI of 25.0-29.9 had a 13% higher risk for cardiovascular death. However, among those older than 65 years, a BMI between 25.0 and 29.9 was associated with an 18% lower risk.
A U-shaped relationship was found between BMI and the risk for cardiovascular death, with an optimal BMI cutoff of 24.0 in the under-65 group and a 27.0 cutoff in the older group. Ranges of 23.0-25.0 in the under-65 group and 26.0-28 in the older group were associated with the lowest cardiovascular risk.
In contrast, there was a linear relationship between both waist circumference and waist-to-height ratio and the risk for cardiovascular death, making those more direct measures of adiposity, Dr. Xu told this news organization.
“For clinicians, our data underscores the importance of considering age when assessing BMI targets for cardiovascular health. Personalized treatment plans that account for age-specific BMI cutoffs and other risk factors may enhance patient outcomes and reduce CVD mortality,” Dr. Xu said.
However, he added, “while these findings suggest an optimal BMI range, it is crucial to acknowledge that these cutoff points may vary based on gender, race, and other factors. Our future studies will validate these findings in different populations and attempt to explain the mechanism by which the optimal nodal values exist in people with diabetes at different ages.”
Dr. Neeland cautioned, “I think more work needs to be done in terms of not just identifying the risk differences but understanding why and how to better risk stratify individuals and do personalized medicine. I think that’s important, but you have to have good data to support the strategies you’re going to use. These data are observational, and they’re a good start, but they wouldn’t directly impact practice at this point.”
The data will be presented at the European Congress on Obesity taking place May 12-15 in Venice, Italy.
The authors declared no competing interests. Study funding came from several sources, including the Young Talents Project of Hubei Provincial Health Commission, China, Hubei Provincial Natural Science Foundation of China, the Science and Technology Research Key Project of the Education Department of Hubei Province China, and the Sanuo Diabetes Charity Foundation, China, and the Xiangyang Science and Technology Plan Project, China. Dr. Neeland is a speaker and/or consultant for Boehringer Ingelheim, Novo Nordisk, Bayer, and Eli Lilly and Company.
A version of this article appeared on Medscape.com.
Among adults with type 2 diabetes (T2D) older than 65 years, a body mass index (BMI) in the moderate overweight category (26-28) appears to offer better protection from cardiovascular death than does a BMI in the “normal” range, new data suggested.
On the other hand, the study findings also suggest that the “normal” range of 23-25 is optimal for middle-aged adults with T2D.
The findings reflect a previously demonstrated phenomenon called the “obesity paradox,” in which older people with overweight may have better outcomes than leaner people due to factors such as bone loss, frailty, and nutritional deficits, study lead author Shaoyong Xu, of Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Xiangyang, China, told this news organization.
“In this era of population growth and aging, the question arises as to whether obesity or overweight can be beneficial in improving survival rates for older individuals with diabetes. This topic holds significant relevance due to the potential implications it has on weight management strategies for older adults. If overweight does not pose an increased risk of cardiovascular mortality, it may suggest that older individuals are not necessarily required to strive for weight loss to achieve so-called normal values.”
Moreover, Dr. Xu added, “inappropriate weight loss and being underweight could potentially elevate the risk of cardiovascular events, myocardial infarction, cerebral infarction, and all-cause mortality.”
Thus, he said, “while there are general guidelines recommending a BMI below 25, our findings suggest that personalized BMI targets may be more beneficial, particularly for different age groups and individuals with specific health conditions.”
Asked to comment, Ian J. Neeland, MD, director of cardiovascular prevention, University Hospitals Harrington Heart & Vascular Institute, Case Western Reserve University, Cleveland, Ohio, pointed out that older people who are underweight or in lower weight categories may be more likely to smoke or have undiagnosed cancer, or that “their BMI is not so much reflective of fat mass as of low muscle mass, or sarcopenia, and that is definitely a risk factor for adverse outcomes and risks. ... And those who have slightly higher BMIs may be maintaining muscle mass, even though they’re older, and therefore they have less risk.”
However, Dr. Neeland disagreed with the authors’ conclusions regarding “optimal” BMI. “Just because you have different risk categories based on BMI doesn’t mean that’s ‘optimal’ BMI. The way I would interpret this paper is that there’s an association of mildly overweight with better outcomes in adults who are over 65 with type 2 diabetes. We need to try to understand the mechanisms underlying that observation.”
Dr. Neeland advised that for an older person with T2D who has low muscle mass and frailty, “I wouldn’t recommend necessarily targeted weight loss in that person. But I would potentially recommend weight loss in addition to resistance training, muscle building, and endurance training, and therefore reducing fat mass. The goal would be not so much weight loss but reduction of body fat and maintaining and improving muscle health.”
U-Shaped Relationship Found Between Age, BMI, and Cardiovascular Disease (CVD) Risk
The data come from the UK Biobank, a population-based prospective cohort study of adults in the United Kingdom. A total of 22,874 participants with baseline T2D were included in the current study. Baseline surveys were conducted between 2006 and 2010, and follow-up was a median of 12.52 years. During that time, 891 people died of CVD.
Hazard ratios were adjusted for baseline variables including age, sex, smoking history, alcohol consumption, level of physical exercise, and history of CVDs.
Compared with people with BMI a < 25 in the group who were aged 65 years or younger, those with a BMI of 25.0-29.9 had a 13% higher risk for cardiovascular death. However, among those older than 65 years, a BMI between 25.0 and 29.9 was associated with an 18% lower risk.
A U-shaped relationship was found between BMI and the risk for cardiovascular death, with an optimal BMI cutoff of 24.0 in the under-65 group and a 27.0 cutoff in the older group. Ranges of 23.0-25.0 in the under-65 group and 26.0-28 in the older group were associated with the lowest cardiovascular risk.
In contrast, there was a linear relationship between both waist circumference and waist-to-height ratio and the risk for cardiovascular death, making those more direct measures of adiposity, Dr. Xu told this news organization.
“For clinicians, our data underscores the importance of considering age when assessing BMI targets for cardiovascular health. Personalized treatment plans that account for age-specific BMI cutoffs and other risk factors may enhance patient outcomes and reduce CVD mortality,” Dr. Xu said.
However, he added, “while these findings suggest an optimal BMI range, it is crucial to acknowledge that these cutoff points may vary based on gender, race, and other factors. Our future studies will validate these findings in different populations and attempt to explain the mechanism by which the optimal nodal values exist in people with diabetes at different ages.”
Dr. Neeland cautioned, “I think more work needs to be done in terms of not just identifying the risk differences but understanding why and how to better risk stratify individuals and do personalized medicine. I think that’s important, but you have to have good data to support the strategies you’re going to use. These data are observational, and they’re a good start, but they wouldn’t directly impact practice at this point.”
The data will be presented at the European Congress on Obesity taking place May 12-15 in Venice, Italy.
The authors declared no competing interests. Study funding came from several sources, including the Young Talents Project of Hubei Provincial Health Commission, China, Hubei Provincial Natural Science Foundation of China, the Science and Technology Research Key Project of the Education Department of Hubei Province China, and the Sanuo Diabetes Charity Foundation, China, and the Xiangyang Science and Technology Plan Project, China. Dr. Neeland is a speaker and/or consultant for Boehringer Ingelheim, Novo Nordisk, Bayer, and Eli Lilly and Company.
A version of this article appeared on Medscape.com.
Maternal Lifestyle Interventions Boost Babies’ Heart Health
Infants born to women with obesity showed improved measures of cardiovascular health when their mothers adopted healthier lifestyles before and during pregnancy, based on data from a systematic review presented at the annual meeting of the Society for Reproductive Investigation.
Previous research has shown that children born to mothers with a high body mass index (BMI) are more likely to die from cardiovascular disease in later life, said presenting author Samuel J. Burden, PhD, in an interview.
“Surprisingly, early signs of these heart issues can start before birth and continue into childhood,” said Dr. Burden, a research associate in the Department of Women & Children’s Health, School of Life Course & Population Sciences, King’s College London, London, United Kingdom.
To examine the effect of interventions such as a healthy diet and exercise in pregnant women with obesity on the heart health of their infants, Dr. Burden and colleagues reviewed data from eight randomized, controlled trials involving diet and exercise for pregnant women with obesity. Of these, two used antenatal exercise, two used diet and physical activity, and one used preconception diet and physical activity. The studies ranged in size from 18 to 404 participants. Two studies included infants younger than 2 months of age, and four studies included children aged 3-7 years.
Overall, lifestyle interventions before conception and before birth were associated with significant changes in cardiac remodeling, specifically reduced interventricular septal wall thickness.
In addition, one of three studies of cardiac diastolic function and four of five studies of systolic function showed significant improvements. The five studies of cardiac systolic function and three studies of diastolic function also showed improvement in systolic and diastolic blood pressure in infants of mothers who took part in the interventions. The studies were limited mainly by large attrition rates, the researchers wrote in their presentation. However, more studies in larger populations that also include older children could confirm the findings and inform public health strategies to promote healthy lifestyles for pregnant women, they noted.
Encourage Healthy Lifestyle Before and During Pregnancy
The evidence supports the findings from animal studies showing that an offspring’s health is influenced by maternal lifestyle before and during pregnancy, Dr. Burden said in an interview. The data suggest that healthcare providers should encourage women with a high BMI who want to become pregnant to eat healthfully and become more active as a way to enhance the future cardiovascular health of their children, he said.
The full results of the current study are soon to be published, but more work is needed, said Dr. Burden. “While we observed a protective effect from these lifestyle programs, there is a need for more extensive studies involving a larger number of women (and their children) who were part of the initial research,” he said. “Additionally, it will be crucial to track these children into adulthood to determine whether these antenatal lifestyle interventions persist in lowering the risk of future cardiovascular disease.”
Beginning healthy lifestyle programs prior to pregnancy might yield the best results for promoting infant cardiovascular health, and more prepregnancy interventions for women with obesity are needed, Dr. Burden added.
The current study adds to the growing body of evidence that the in utero environment can have lifelong effects on offspring, Joseph R. Biggio Jr, MD, system chair of maternal fetal medicine at Ochsner Health, New Orleans, Louisiana, said in an interview.
“Several studies have previously shown that the children of mothers with diabetes, hypertension, or obesity are at increased risk for developing signs of metabolic syndrome and cardiovascular changes during childhood or adolescence,” said Dr. Biggio.
The data from this systematic review support the potential value of interventions aimed at improving maternal weight gain and cardiovascular performance before and during pregnancy that may result in reduced cardiovascular remodeling and myocardial thickening in infants, he said.
The study was supported by a British Heart Foundation Special Project Grant. The researchers had no financial conflicts to disclose. Dr. Biggio had no financial conflicts to disclose.
Infants born to women with obesity showed improved measures of cardiovascular health when their mothers adopted healthier lifestyles before and during pregnancy, based on data from a systematic review presented at the annual meeting of the Society for Reproductive Investigation.
Previous research has shown that children born to mothers with a high body mass index (BMI) are more likely to die from cardiovascular disease in later life, said presenting author Samuel J. Burden, PhD, in an interview.
“Surprisingly, early signs of these heart issues can start before birth and continue into childhood,” said Dr. Burden, a research associate in the Department of Women & Children’s Health, School of Life Course & Population Sciences, King’s College London, London, United Kingdom.
To examine the effect of interventions such as a healthy diet and exercise in pregnant women with obesity on the heart health of their infants, Dr. Burden and colleagues reviewed data from eight randomized, controlled trials involving diet and exercise for pregnant women with obesity. Of these, two used antenatal exercise, two used diet and physical activity, and one used preconception diet and physical activity. The studies ranged in size from 18 to 404 participants. Two studies included infants younger than 2 months of age, and four studies included children aged 3-7 years.
Overall, lifestyle interventions before conception and before birth were associated with significant changes in cardiac remodeling, specifically reduced interventricular septal wall thickness.
In addition, one of three studies of cardiac diastolic function and four of five studies of systolic function showed significant improvements. The five studies of cardiac systolic function and three studies of diastolic function also showed improvement in systolic and diastolic blood pressure in infants of mothers who took part in the interventions. The studies were limited mainly by large attrition rates, the researchers wrote in their presentation. However, more studies in larger populations that also include older children could confirm the findings and inform public health strategies to promote healthy lifestyles for pregnant women, they noted.
Encourage Healthy Lifestyle Before and During Pregnancy
The evidence supports the findings from animal studies showing that an offspring’s health is influenced by maternal lifestyle before and during pregnancy, Dr. Burden said in an interview. The data suggest that healthcare providers should encourage women with a high BMI who want to become pregnant to eat healthfully and become more active as a way to enhance the future cardiovascular health of their children, he said.
The full results of the current study are soon to be published, but more work is needed, said Dr. Burden. “While we observed a protective effect from these lifestyle programs, there is a need for more extensive studies involving a larger number of women (and their children) who were part of the initial research,” he said. “Additionally, it will be crucial to track these children into adulthood to determine whether these antenatal lifestyle interventions persist in lowering the risk of future cardiovascular disease.”
Beginning healthy lifestyle programs prior to pregnancy might yield the best results for promoting infant cardiovascular health, and more prepregnancy interventions for women with obesity are needed, Dr. Burden added.
The current study adds to the growing body of evidence that the in utero environment can have lifelong effects on offspring, Joseph R. Biggio Jr, MD, system chair of maternal fetal medicine at Ochsner Health, New Orleans, Louisiana, said in an interview.
“Several studies have previously shown that the children of mothers with diabetes, hypertension, or obesity are at increased risk for developing signs of metabolic syndrome and cardiovascular changes during childhood or adolescence,” said Dr. Biggio.
The data from this systematic review support the potential value of interventions aimed at improving maternal weight gain and cardiovascular performance before and during pregnancy that may result in reduced cardiovascular remodeling and myocardial thickening in infants, he said.
The study was supported by a British Heart Foundation Special Project Grant. The researchers had no financial conflicts to disclose. Dr. Biggio had no financial conflicts to disclose.
Infants born to women with obesity showed improved measures of cardiovascular health when their mothers adopted healthier lifestyles before and during pregnancy, based on data from a systematic review presented at the annual meeting of the Society for Reproductive Investigation.
Previous research has shown that children born to mothers with a high body mass index (BMI) are more likely to die from cardiovascular disease in later life, said presenting author Samuel J. Burden, PhD, in an interview.
“Surprisingly, early signs of these heart issues can start before birth and continue into childhood,” said Dr. Burden, a research associate in the Department of Women & Children’s Health, School of Life Course & Population Sciences, King’s College London, London, United Kingdom.
To examine the effect of interventions such as a healthy diet and exercise in pregnant women with obesity on the heart health of their infants, Dr. Burden and colleagues reviewed data from eight randomized, controlled trials involving diet and exercise for pregnant women with obesity. Of these, two used antenatal exercise, two used diet and physical activity, and one used preconception diet and physical activity. The studies ranged in size from 18 to 404 participants. Two studies included infants younger than 2 months of age, and four studies included children aged 3-7 years.
Overall, lifestyle interventions before conception and before birth were associated with significant changes in cardiac remodeling, specifically reduced interventricular septal wall thickness.
In addition, one of three studies of cardiac diastolic function and four of five studies of systolic function showed significant improvements. The five studies of cardiac systolic function and three studies of diastolic function also showed improvement in systolic and diastolic blood pressure in infants of mothers who took part in the interventions. The studies were limited mainly by large attrition rates, the researchers wrote in their presentation. However, more studies in larger populations that also include older children could confirm the findings and inform public health strategies to promote healthy lifestyles for pregnant women, they noted.
Encourage Healthy Lifestyle Before and During Pregnancy
The evidence supports the findings from animal studies showing that an offspring’s health is influenced by maternal lifestyle before and during pregnancy, Dr. Burden said in an interview. The data suggest that healthcare providers should encourage women with a high BMI who want to become pregnant to eat healthfully and become more active as a way to enhance the future cardiovascular health of their children, he said.
The full results of the current study are soon to be published, but more work is needed, said Dr. Burden. “While we observed a protective effect from these lifestyle programs, there is a need for more extensive studies involving a larger number of women (and their children) who were part of the initial research,” he said. “Additionally, it will be crucial to track these children into adulthood to determine whether these antenatal lifestyle interventions persist in lowering the risk of future cardiovascular disease.”
Beginning healthy lifestyle programs prior to pregnancy might yield the best results for promoting infant cardiovascular health, and more prepregnancy interventions for women with obesity are needed, Dr. Burden added.
The current study adds to the growing body of evidence that the in utero environment can have lifelong effects on offspring, Joseph R. Biggio Jr, MD, system chair of maternal fetal medicine at Ochsner Health, New Orleans, Louisiana, said in an interview.
“Several studies have previously shown that the children of mothers with diabetes, hypertension, or obesity are at increased risk for developing signs of metabolic syndrome and cardiovascular changes during childhood or adolescence,” said Dr. Biggio.
The data from this systematic review support the potential value of interventions aimed at improving maternal weight gain and cardiovascular performance before and during pregnancy that may result in reduced cardiovascular remodeling and myocardial thickening in infants, he said.
The study was supported by a British Heart Foundation Special Project Grant. The researchers had no financial conflicts to disclose. Dr. Biggio had no financial conflicts to disclose.
Gaps Found in Appropriate SGLT2, GLP-1 Prescribing
Sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are often not prescribed or accessible to people who could benefit from them, a trio of new studies suggested.
First approved for the treatment of type 2 diabetes, the indications for SGLT2 inhibitors and GLP-1 RA medications have now been extended to people with obesity, heart failure, and chronic kidney disease.
The papers were presented at the American Heart Association (AHA) Epidemiology and Prevention | Lifestyle and Cardiometabolic Scientific Sessions 2024.
The new data show there is “work to be done in terms of access and equity to these treatments,” Robert H. Eckel, MD, who was not involved in the research, said in a conference statement.
“There is no question that the cost of these medications is high, yet when issues go beyond coverage and include sociodemographic and racial differences that influence treatment, these major issues need to be evaluated and addressed,” said Dr. Eckel, professor emeritus of medicine, Division of Endocrinology, Metabolism, and Diabetes, University of Colorado Anschutz Medical Campus, Denver, and a past president of the AHA.
Low Prescription Rates
In one study, researchers analyzed health records for 18,164 adults with obesity (mean age, 51 years; 64% women; mean body mass index [BMI], 36 kg/m2) who had health insurance covering semaglutide and liraglutide (GLP-1 RAs) and tirzepatide (GLP-1/glucose-dependent insulinotropic polypeptide RA). The cohort was 54% White, 35% Black, and 5% Asian.
Only about 3% of eligible adults were prescribed one of these medications, reported Meron Haile, BS, a second-year medical student at Johns Hopkins University School of Medicine in Baltimore, and colleagues.
The likelihood of prescription was lower among Black patients (odds ratio [OR], 0.76) and men (OR, 0.54) and higher in people with higher BMI (OR, 1.06 per 1-unit higher BMI).
Living in a neighborhood with a higher area deprivation index or lower income was not independently associated with the likelihood of prescription.
Individuals with diabetes or hypertension were more likely to be prescribed one of these medications (OR, 3.52 and 1.36, respectively).
“While prescription rates for new obesity therapies are low among the overall population, we saw pronounced lower accessibility among Black adults, who exhibit a higher burden of severe obesity, hypertension, and type 2 diabetes,” Haile said in a conference statement.
“There is a crucial need for understanding prescription practices for obesity medications and to facilitate similar access among people in all races and ethnic groups,” Haile added.
Similar findings emerged in a separate study, in which researchers analyzed the health records of 687,165 adults with type 2 diabetes treated at six large health systems from 2014 to 2022.
The rate of annual pharmacy dispensing of SGLT2 inhibitors and GLP-1 RA medications rose during the study period, but there were clear racial and ethnic differences in prescribing.
In fully adjusted models, SGLT2 inhibitors dispensing was lower for American Indian/Alaska Native (AI/AN; OR, 0.80), Black (OR, 0.89), and Hispanic (OR, 0.87) individuals than for White patients.
Likewise, GLP-1 RA dispensing was also lower for AI/AN (OR, 0.78), Asian (OR, 0.50), Black (OR, 0.86), Hawaiian/Pacific Islander (OR, 0.52), and Hispanic (OR, 0.69) patients than for White patients.
“It’s possible that not all patients have equal access to information about these medications or that not all patients are equally comfortable asking their doctors about them,” lead author Luis A. Rodriguez, PhD, research scientist at Kaiser Permanente’s Northern California Division of Research, Oakland, told this news organization.
“We also don’t know if the cost of the new medications contributed to what we found or if some patients prefer to keep taking a pill rather than switch to some of the GLP-1 receptor agonists that are self-injectable medications. We need to learn more about why this is happening,” Dr. Rodriguez said.
‘Concerning’ Data Raise Key Questions
The third study explored how often prescribing recommendations for SGLT2 inhibitors are followed.
“Our study revealed a significant gap between the recommendations for prescribing SGLT2 inhibitors and the actual prescription rates among patients who could benefit from them,” Jung-Im Shin, MD, PhD, with the Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins University, Baltimore, told this news organization.
“This could have important implications for patient care and outcomes, as SGLT2 inhibitors have been shown to be effective for heart and kidney protection in people with high-risk type 2 diabetes, chronic kidney disease, or heart failure,” Dr. Shin said.
Dr. Shin and colleagues analyzed the health records for more than 700,000 adults with type 2 diabetes and 2.5 million people without type 2 diabetes, who received care in 28 US health systems from 2022 to 2023.
Among people with type 2 diabetes recommended for first-line SGLT2 inhibitors treatment, only 12% received a prescription for a SGLT2 inhibitor, and there was no significant difference in prescription between people who met the criteria for first-line SGLT2 inhibitors treatment vs people who did not meet the criteria.
Among people without type 2 diabetes, SGLT2 inhibitor prescription was substantially lower, with only about 3% of people with conditions that are guideline-recommended for SGLT2 inhibitors receiving a prescription.
SGLT2 inhibitor prescription rates varied across health systems; however, less than 30% of people who met guideline criteria received a SGLT2 inhibitors prescription across all health systems in the study.
“Barriers to SGLT2 inhibitor prescription include limited insurance coverage, prohibitive out-of-pocket costs, formulary restrictions, and lack of physicians’ awareness or familiarity regarding benefits and appropriate indications for SGLT2 inhibitors,” Dr. Shin said.
“Efforts to improve access and affordability of SGLT2 inhibitors along with strategies to educate both patients and providers on the updated guidelines for SGLT2 inhibitors use may increase adoption,” Dr. Shin added.
In a conference recording, Dr. Eckel said he found it “particularly concerning” that among patients with insurance to help pay for these medications, “there were still discrepancies” between prescriptions to Asian and Black vs White patients, “who are being prescribed these important medications.”
“Why these medications are not being offered more regularly by healthcare providers” needs to be addressed, Dr. Eckel said. “I think part of it is ignorance and inadequate education as to their new indications for treatment of diseases that go beyond type 2 diabetes,” he noted.
None of the studies had commercial funding. The authors had no relevant disclosures.
A version of this article appeared on Medscape.com.
Sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are often not prescribed or accessible to people who could benefit from them, a trio of new studies suggested.
First approved for the treatment of type 2 diabetes, the indications for SGLT2 inhibitors and GLP-1 RA medications have now been extended to people with obesity, heart failure, and chronic kidney disease.
The papers were presented at the American Heart Association (AHA) Epidemiology and Prevention | Lifestyle and Cardiometabolic Scientific Sessions 2024.
The new data show there is “work to be done in terms of access and equity to these treatments,” Robert H. Eckel, MD, who was not involved in the research, said in a conference statement.
“There is no question that the cost of these medications is high, yet when issues go beyond coverage and include sociodemographic and racial differences that influence treatment, these major issues need to be evaluated and addressed,” said Dr. Eckel, professor emeritus of medicine, Division of Endocrinology, Metabolism, and Diabetes, University of Colorado Anschutz Medical Campus, Denver, and a past president of the AHA.
Low Prescription Rates
In one study, researchers analyzed health records for 18,164 adults with obesity (mean age, 51 years; 64% women; mean body mass index [BMI], 36 kg/m2) who had health insurance covering semaglutide and liraglutide (GLP-1 RAs) and tirzepatide (GLP-1/glucose-dependent insulinotropic polypeptide RA). The cohort was 54% White, 35% Black, and 5% Asian.
Only about 3% of eligible adults were prescribed one of these medications, reported Meron Haile, BS, a second-year medical student at Johns Hopkins University School of Medicine in Baltimore, and colleagues.
The likelihood of prescription was lower among Black patients (odds ratio [OR], 0.76) and men (OR, 0.54) and higher in people with higher BMI (OR, 1.06 per 1-unit higher BMI).
Living in a neighborhood with a higher area deprivation index or lower income was not independently associated with the likelihood of prescription.
Individuals with diabetes or hypertension were more likely to be prescribed one of these medications (OR, 3.52 and 1.36, respectively).
“While prescription rates for new obesity therapies are low among the overall population, we saw pronounced lower accessibility among Black adults, who exhibit a higher burden of severe obesity, hypertension, and type 2 diabetes,” Haile said in a conference statement.
“There is a crucial need for understanding prescription practices for obesity medications and to facilitate similar access among people in all races and ethnic groups,” Haile added.
Similar findings emerged in a separate study, in which researchers analyzed the health records of 687,165 adults with type 2 diabetes treated at six large health systems from 2014 to 2022.
The rate of annual pharmacy dispensing of SGLT2 inhibitors and GLP-1 RA medications rose during the study period, but there were clear racial and ethnic differences in prescribing.
In fully adjusted models, SGLT2 inhibitors dispensing was lower for American Indian/Alaska Native (AI/AN; OR, 0.80), Black (OR, 0.89), and Hispanic (OR, 0.87) individuals than for White patients.
Likewise, GLP-1 RA dispensing was also lower for AI/AN (OR, 0.78), Asian (OR, 0.50), Black (OR, 0.86), Hawaiian/Pacific Islander (OR, 0.52), and Hispanic (OR, 0.69) patients than for White patients.
“It’s possible that not all patients have equal access to information about these medications or that not all patients are equally comfortable asking their doctors about them,” lead author Luis A. Rodriguez, PhD, research scientist at Kaiser Permanente’s Northern California Division of Research, Oakland, told this news organization.
“We also don’t know if the cost of the new medications contributed to what we found or if some patients prefer to keep taking a pill rather than switch to some of the GLP-1 receptor agonists that are self-injectable medications. We need to learn more about why this is happening,” Dr. Rodriguez said.
‘Concerning’ Data Raise Key Questions
The third study explored how often prescribing recommendations for SGLT2 inhibitors are followed.
“Our study revealed a significant gap between the recommendations for prescribing SGLT2 inhibitors and the actual prescription rates among patients who could benefit from them,” Jung-Im Shin, MD, PhD, with the Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins University, Baltimore, told this news organization.
“This could have important implications for patient care and outcomes, as SGLT2 inhibitors have been shown to be effective for heart and kidney protection in people with high-risk type 2 diabetes, chronic kidney disease, or heart failure,” Dr. Shin said.
Dr. Shin and colleagues analyzed the health records for more than 700,000 adults with type 2 diabetes and 2.5 million people without type 2 diabetes, who received care in 28 US health systems from 2022 to 2023.
Among people with type 2 diabetes recommended for first-line SGLT2 inhibitors treatment, only 12% received a prescription for a SGLT2 inhibitor, and there was no significant difference in prescription between people who met the criteria for first-line SGLT2 inhibitors treatment vs people who did not meet the criteria.
Among people without type 2 diabetes, SGLT2 inhibitor prescription was substantially lower, with only about 3% of people with conditions that are guideline-recommended for SGLT2 inhibitors receiving a prescription.
SGLT2 inhibitor prescription rates varied across health systems; however, less than 30% of people who met guideline criteria received a SGLT2 inhibitors prescription across all health systems in the study.
“Barriers to SGLT2 inhibitor prescription include limited insurance coverage, prohibitive out-of-pocket costs, formulary restrictions, and lack of physicians’ awareness or familiarity regarding benefits and appropriate indications for SGLT2 inhibitors,” Dr. Shin said.
“Efforts to improve access and affordability of SGLT2 inhibitors along with strategies to educate both patients and providers on the updated guidelines for SGLT2 inhibitors use may increase adoption,” Dr. Shin added.
In a conference recording, Dr. Eckel said he found it “particularly concerning” that among patients with insurance to help pay for these medications, “there were still discrepancies” between prescriptions to Asian and Black vs White patients, “who are being prescribed these important medications.”
“Why these medications are not being offered more regularly by healthcare providers” needs to be addressed, Dr. Eckel said. “I think part of it is ignorance and inadequate education as to their new indications for treatment of diseases that go beyond type 2 diabetes,” he noted.
None of the studies had commercial funding. The authors had no relevant disclosures.
A version of this article appeared on Medscape.com.
Sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are often not prescribed or accessible to people who could benefit from them, a trio of new studies suggested.
First approved for the treatment of type 2 diabetes, the indications for SGLT2 inhibitors and GLP-1 RA medications have now been extended to people with obesity, heart failure, and chronic kidney disease.
The papers were presented at the American Heart Association (AHA) Epidemiology and Prevention | Lifestyle and Cardiometabolic Scientific Sessions 2024.
The new data show there is “work to be done in terms of access and equity to these treatments,” Robert H. Eckel, MD, who was not involved in the research, said in a conference statement.
“There is no question that the cost of these medications is high, yet when issues go beyond coverage and include sociodemographic and racial differences that influence treatment, these major issues need to be evaluated and addressed,” said Dr. Eckel, professor emeritus of medicine, Division of Endocrinology, Metabolism, and Diabetes, University of Colorado Anschutz Medical Campus, Denver, and a past president of the AHA.
Low Prescription Rates
In one study, researchers analyzed health records for 18,164 adults with obesity (mean age, 51 years; 64% women; mean body mass index [BMI], 36 kg/m2) who had health insurance covering semaglutide and liraglutide (GLP-1 RAs) and tirzepatide (GLP-1/glucose-dependent insulinotropic polypeptide RA). The cohort was 54% White, 35% Black, and 5% Asian.
Only about 3% of eligible adults were prescribed one of these medications, reported Meron Haile, BS, a second-year medical student at Johns Hopkins University School of Medicine in Baltimore, and colleagues.
The likelihood of prescription was lower among Black patients (odds ratio [OR], 0.76) and men (OR, 0.54) and higher in people with higher BMI (OR, 1.06 per 1-unit higher BMI).
Living in a neighborhood with a higher area deprivation index or lower income was not independently associated with the likelihood of prescription.
Individuals with diabetes or hypertension were more likely to be prescribed one of these medications (OR, 3.52 and 1.36, respectively).
“While prescription rates for new obesity therapies are low among the overall population, we saw pronounced lower accessibility among Black adults, who exhibit a higher burden of severe obesity, hypertension, and type 2 diabetes,” Haile said in a conference statement.
“There is a crucial need for understanding prescription practices for obesity medications and to facilitate similar access among people in all races and ethnic groups,” Haile added.
Similar findings emerged in a separate study, in which researchers analyzed the health records of 687,165 adults with type 2 diabetes treated at six large health systems from 2014 to 2022.
The rate of annual pharmacy dispensing of SGLT2 inhibitors and GLP-1 RA medications rose during the study period, but there were clear racial and ethnic differences in prescribing.
In fully adjusted models, SGLT2 inhibitors dispensing was lower for American Indian/Alaska Native (AI/AN; OR, 0.80), Black (OR, 0.89), and Hispanic (OR, 0.87) individuals than for White patients.
Likewise, GLP-1 RA dispensing was also lower for AI/AN (OR, 0.78), Asian (OR, 0.50), Black (OR, 0.86), Hawaiian/Pacific Islander (OR, 0.52), and Hispanic (OR, 0.69) patients than for White patients.
“It’s possible that not all patients have equal access to information about these medications or that not all patients are equally comfortable asking their doctors about them,” lead author Luis A. Rodriguez, PhD, research scientist at Kaiser Permanente’s Northern California Division of Research, Oakland, told this news organization.
“We also don’t know if the cost of the new medications contributed to what we found or if some patients prefer to keep taking a pill rather than switch to some of the GLP-1 receptor agonists that are self-injectable medications. We need to learn more about why this is happening,” Dr. Rodriguez said.
‘Concerning’ Data Raise Key Questions
The third study explored how often prescribing recommendations for SGLT2 inhibitors are followed.
“Our study revealed a significant gap between the recommendations for prescribing SGLT2 inhibitors and the actual prescription rates among patients who could benefit from them,” Jung-Im Shin, MD, PhD, with the Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins University, Baltimore, told this news organization.
“This could have important implications for patient care and outcomes, as SGLT2 inhibitors have been shown to be effective for heart and kidney protection in people with high-risk type 2 diabetes, chronic kidney disease, or heart failure,” Dr. Shin said.
Dr. Shin and colleagues analyzed the health records for more than 700,000 adults with type 2 diabetes and 2.5 million people without type 2 diabetes, who received care in 28 US health systems from 2022 to 2023.
Among people with type 2 diabetes recommended for first-line SGLT2 inhibitors treatment, only 12% received a prescription for a SGLT2 inhibitor, and there was no significant difference in prescription between people who met the criteria for first-line SGLT2 inhibitors treatment vs people who did not meet the criteria.
Among people without type 2 diabetes, SGLT2 inhibitor prescription was substantially lower, with only about 3% of people with conditions that are guideline-recommended for SGLT2 inhibitors receiving a prescription.
SGLT2 inhibitor prescription rates varied across health systems; however, less than 30% of people who met guideline criteria received a SGLT2 inhibitors prescription across all health systems in the study.
“Barriers to SGLT2 inhibitor prescription include limited insurance coverage, prohibitive out-of-pocket costs, formulary restrictions, and lack of physicians’ awareness or familiarity regarding benefits and appropriate indications for SGLT2 inhibitors,” Dr. Shin said.
“Efforts to improve access and affordability of SGLT2 inhibitors along with strategies to educate both patients and providers on the updated guidelines for SGLT2 inhibitors use may increase adoption,” Dr. Shin added.
In a conference recording, Dr. Eckel said he found it “particularly concerning” that among patients with insurance to help pay for these medications, “there were still discrepancies” between prescriptions to Asian and Black vs White patients, “who are being prescribed these important medications.”
“Why these medications are not being offered more regularly by healthcare providers” needs to be addressed, Dr. Eckel said. “I think part of it is ignorance and inadequate education as to their new indications for treatment of diseases that go beyond type 2 diabetes,” he noted.
None of the studies had commercial funding. The authors had no relevant disclosures.
A version of this article appeared on Medscape.com.
Statins Tied to Lower Mortality, Even With Comorbid Dementia
Use of statin drugs was associated with improved mortality in older nursing home residents, regardless of dementia status, a new study showed.
The study is among the first to explore whether statin use in older nursing home residents offers a mortality benefit, especially among individuals with dementia, a group largely excluded from earlier statin trials.
Investigators’ analysis of 4 years of data on nearly 300,000 nursing home residents revealed that statin use was associated with a 40% lower risk for all-cause mortality than statin nonuse in those without dementia and a 20% lower risk in those with dementia.
“These findings may provide evidence that supports the continued use of statins in older nursing home patients with multiple medical conditions,” wrote lead author Julie Lorraine O’Sullivan, PhD, of the Charité–Universitätsmedizin Berlin, Freie Universität Berlin, German Center for Mental Health, Berlin, and colleagues.
The study was published online on February 27 in Neurology.
Understudied Population
Statins are the first-line treatment for preventing atherosclerotic cardiovascular disease (ASCVD), but they are also known to carry risks to patients who are frail or care-dependent. Many prior clinical trials excluded older participants with multiple comorbidities, especially those with dementia. So, evidence regarding the drugs’ efficacy in this population was lacking.
Investigators retrospectively examined 5 years of claims data from a German health and long-term care insurance provider on 282,693 nursing home residents (mean age, 83 years) who had used statins consecutively for ≥ 6 months.
Researchers used propensity score matching in 96,162 individuals to adjust for potential imbalances in the distribution of covariates (eg, age, sex, atrial fibrillation, ASCVD, and other conditions, as well as medications) and to reduce bias. Cox regression models were similarly adjusted for these factors, as well as care level. Residents were followed for an average of 2 years.
There were 54,269 recorded deaths during the study period, with most patients requiring a high level of care and 65% with dementia.
Statin use was associated with lower all-cause mortality in residents with dementia (hazard ratio [HR], 0.80, P < .001) and those without dementia (HR, 0.73; P < .001) compared with nonusers. The benefits remained consistent even after excluding participants with a history of ASCVD and across subgroups stratified by age sex, care level, and dementia type.
Limitations included the potential for unknown confounders and a lack of information about previous statin use, smoking and sedentary behavior, and the cause of mortality.
“Although our findings suggest the benefits of statins ... it is vital to acknowledge the need for further research to understand the underlying mechanism and the need for replication of our results to understand the potential risks before making recommendations to clinicians and families regarding statin therapy,” investigators wrote.
‘First Step’
In an accompanying editorial, Ariela R. Orkaby, MD, MPH, assistant professor of medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, called the study a “first step” to a better understanding of statin use in an understudied population.
“These findings build on a limited body of observational evidence for statin use in high-risk older adults, which has generally demonstrated protective associations for statins and mortality, including those with dementia and frailty, although nursing home status has not been specifically explored,” Dr. Orkaby wrote.
Perhaps more important than gaining information about statins’ effect on mortality risk in older people with dementia may be a better understanding of how the drugs might improve quality of life by reducing the risk for stroke or other cardiovascular events.
“It may be time to reconsider the broad recommendations to avoid or deprescribe statins in nursing home residents and rather invest in high-quality evidence to guide the care of this vulnerable population. After all, a lack of evidence does not imply benefit or harm, rather a need for more data,” Dr. Orkaby added.
The research was funded by Stiftung Charité; Dr. O’Sullivan and coauthors reported no relevant financial relationships. Dr. Orkaby received funding from a VA CSR&D CDA-2 award.
A version of this article appeared on Medscape.com.
Use of statin drugs was associated with improved mortality in older nursing home residents, regardless of dementia status, a new study showed.
The study is among the first to explore whether statin use in older nursing home residents offers a mortality benefit, especially among individuals with dementia, a group largely excluded from earlier statin trials.
Investigators’ analysis of 4 years of data on nearly 300,000 nursing home residents revealed that statin use was associated with a 40% lower risk for all-cause mortality than statin nonuse in those without dementia and a 20% lower risk in those with dementia.
“These findings may provide evidence that supports the continued use of statins in older nursing home patients with multiple medical conditions,” wrote lead author Julie Lorraine O’Sullivan, PhD, of the Charité–Universitätsmedizin Berlin, Freie Universität Berlin, German Center for Mental Health, Berlin, and colleagues.
The study was published online on February 27 in Neurology.
Understudied Population
Statins are the first-line treatment for preventing atherosclerotic cardiovascular disease (ASCVD), but they are also known to carry risks to patients who are frail or care-dependent. Many prior clinical trials excluded older participants with multiple comorbidities, especially those with dementia. So, evidence regarding the drugs’ efficacy in this population was lacking.
Investigators retrospectively examined 5 years of claims data from a German health and long-term care insurance provider on 282,693 nursing home residents (mean age, 83 years) who had used statins consecutively for ≥ 6 months.
Researchers used propensity score matching in 96,162 individuals to adjust for potential imbalances in the distribution of covariates (eg, age, sex, atrial fibrillation, ASCVD, and other conditions, as well as medications) and to reduce bias. Cox regression models were similarly adjusted for these factors, as well as care level. Residents were followed for an average of 2 years.
There were 54,269 recorded deaths during the study period, with most patients requiring a high level of care and 65% with dementia.
Statin use was associated with lower all-cause mortality in residents with dementia (hazard ratio [HR], 0.80, P < .001) and those without dementia (HR, 0.73; P < .001) compared with nonusers. The benefits remained consistent even after excluding participants with a history of ASCVD and across subgroups stratified by age sex, care level, and dementia type.
Limitations included the potential for unknown confounders and a lack of information about previous statin use, smoking and sedentary behavior, and the cause of mortality.
“Although our findings suggest the benefits of statins ... it is vital to acknowledge the need for further research to understand the underlying mechanism and the need for replication of our results to understand the potential risks before making recommendations to clinicians and families regarding statin therapy,” investigators wrote.
‘First Step’
In an accompanying editorial, Ariela R. Orkaby, MD, MPH, assistant professor of medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, called the study a “first step” to a better understanding of statin use in an understudied population.
“These findings build on a limited body of observational evidence for statin use in high-risk older adults, which has generally demonstrated protective associations for statins and mortality, including those with dementia and frailty, although nursing home status has not been specifically explored,” Dr. Orkaby wrote.
Perhaps more important than gaining information about statins’ effect on mortality risk in older people with dementia may be a better understanding of how the drugs might improve quality of life by reducing the risk for stroke or other cardiovascular events.
“It may be time to reconsider the broad recommendations to avoid or deprescribe statins in nursing home residents and rather invest in high-quality evidence to guide the care of this vulnerable population. After all, a lack of evidence does not imply benefit or harm, rather a need for more data,” Dr. Orkaby added.
The research was funded by Stiftung Charité; Dr. O’Sullivan and coauthors reported no relevant financial relationships. Dr. Orkaby received funding from a VA CSR&D CDA-2 award.
A version of this article appeared on Medscape.com.
Use of statin drugs was associated with improved mortality in older nursing home residents, regardless of dementia status, a new study showed.
The study is among the first to explore whether statin use in older nursing home residents offers a mortality benefit, especially among individuals with dementia, a group largely excluded from earlier statin trials.
Investigators’ analysis of 4 years of data on nearly 300,000 nursing home residents revealed that statin use was associated with a 40% lower risk for all-cause mortality than statin nonuse in those without dementia and a 20% lower risk in those with dementia.
“These findings may provide evidence that supports the continued use of statins in older nursing home patients with multiple medical conditions,” wrote lead author Julie Lorraine O’Sullivan, PhD, of the Charité–Universitätsmedizin Berlin, Freie Universität Berlin, German Center for Mental Health, Berlin, and colleagues.
The study was published online on February 27 in Neurology.
Understudied Population
Statins are the first-line treatment for preventing atherosclerotic cardiovascular disease (ASCVD), but they are also known to carry risks to patients who are frail or care-dependent. Many prior clinical trials excluded older participants with multiple comorbidities, especially those with dementia. So, evidence regarding the drugs’ efficacy in this population was lacking.
Investigators retrospectively examined 5 years of claims data from a German health and long-term care insurance provider on 282,693 nursing home residents (mean age, 83 years) who had used statins consecutively for ≥ 6 months.
Researchers used propensity score matching in 96,162 individuals to adjust for potential imbalances in the distribution of covariates (eg, age, sex, atrial fibrillation, ASCVD, and other conditions, as well as medications) and to reduce bias. Cox regression models were similarly adjusted for these factors, as well as care level. Residents were followed for an average of 2 years.
There were 54,269 recorded deaths during the study period, with most patients requiring a high level of care and 65% with dementia.
Statin use was associated with lower all-cause mortality in residents with dementia (hazard ratio [HR], 0.80, P < .001) and those without dementia (HR, 0.73; P < .001) compared with nonusers. The benefits remained consistent even after excluding participants with a history of ASCVD and across subgroups stratified by age sex, care level, and dementia type.
Limitations included the potential for unknown confounders and a lack of information about previous statin use, smoking and sedentary behavior, and the cause of mortality.
“Although our findings suggest the benefits of statins ... it is vital to acknowledge the need for further research to understand the underlying mechanism and the need for replication of our results to understand the potential risks before making recommendations to clinicians and families regarding statin therapy,” investigators wrote.
‘First Step’
In an accompanying editorial, Ariela R. Orkaby, MD, MPH, assistant professor of medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, called the study a “first step” to a better understanding of statin use in an understudied population.
“These findings build on a limited body of observational evidence for statin use in high-risk older adults, which has generally demonstrated protective associations for statins and mortality, including those with dementia and frailty, although nursing home status has not been specifically explored,” Dr. Orkaby wrote.
Perhaps more important than gaining information about statins’ effect on mortality risk in older people with dementia may be a better understanding of how the drugs might improve quality of life by reducing the risk for stroke or other cardiovascular events.
“It may be time to reconsider the broad recommendations to avoid or deprescribe statins in nursing home residents and rather invest in high-quality evidence to guide the care of this vulnerable population. After all, a lack of evidence does not imply benefit or harm, rather a need for more data,” Dr. Orkaby added.
The research was funded by Stiftung Charité; Dr. O’Sullivan and coauthors reported no relevant financial relationships. Dr. Orkaby received funding from a VA CSR&D CDA-2 award.
A version of this article appeared on Medscape.com.
Lung Cancer Screening Unveils Hidden Health Risks
The reason is because the low-dose CT scans used for screening cover the lower neck down to the upper abdomen, revealing far more anatomy than simply the lungs.
In fact, lung cancer screening can provide information on three of the top 10 causes of death worldwide: ischemic heart disease, chronic obstructive pulmonary disease, and, of course, lung cancer.
With lung cancer screening, “we are basically targeting many birds with one low-dose stone,” explained Jelena Spasic MD, PhD, at the European Lung Cancer Congress (ELCC) 2024.
Dr. Spasic, a medical oncologist at the Institute for Oncology and Radiology of Serbia in Belgrade, was the discussant on a study that gave an indication on just how useful screening can be for other diseases.
The study, dubbed 4-IN-THE-LUNG-RUN trial (4ITLR), is an ongoing prospective trial in six European countries that is using lung cancer screening scans to also look for coronary artery calcifications, a marker of atherosclerosis.
Usually, coronary calcifications are considered incidental findings on lung cancer screenings and reported to subjects’ physicians for heart disease risk assessment.
The difference in 4ITLR is that investigators are actively looking for the lesions and quantifying the extent of calcifications.
It’s made possible by the artificial intelligence-based software being used to read the scans. In addition to generating reports on lung nodules, it also automatically calculates an Agatston score, a quantification of the degree of coronary artery calcification for each subject.
At the meeting, which was organized by the European Society for Clinical Oncology, 4ITLR investigator Daiwei Han, MD, PhD, a research associate at the Institute for Diagnostic Accuracy in Groningen, the Netherlands, reported outcomes in the first 2487 of the 24,000 planned subjects.
To be eligible for screening, participants had to be 60-79 years old and either current smokers, past smokers who had quit within 10 years, or people with a 35 or more pack-year history. The median age in the study was 68.1 years.
Overall, 53% of subjects had Agatston scores of 100 or more, indicating the need for treatment to prevent active coronary artery disease, Dr. Han said.
Fifteen percent were at high risk for heart disease with scores of 400-999, indicating extensive coronary artery calcification, and 16.2% were at very high risk, with scores of 1000 or higher. The information is being shared with participants’ physicians.
The risk of heart disease was far higher in men, who made up 56% of the study population. While women had a median Agatston score of 61, the median score for men was 211.1.
The findings illustrate the potential of dedicated cardiovascular screening within lung cancer screening programs, Dr. Han said, noting that 4ITLR will also incorporate COPD risk assessment.
The study also shows the increased impact lung cancer screening programs could have if greater use were made of the CT images to look for other diseases, Dr. Spasic said.
4ITLR is funded by the European Union’s Horizon 2020 Program. Dr. Spasic and Dr. Han didn’t have any relevant disclosures.
The reason is because the low-dose CT scans used for screening cover the lower neck down to the upper abdomen, revealing far more anatomy than simply the lungs.
In fact, lung cancer screening can provide information on three of the top 10 causes of death worldwide: ischemic heart disease, chronic obstructive pulmonary disease, and, of course, lung cancer.
With lung cancer screening, “we are basically targeting many birds with one low-dose stone,” explained Jelena Spasic MD, PhD, at the European Lung Cancer Congress (ELCC) 2024.
Dr. Spasic, a medical oncologist at the Institute for Oncology and Radiology of Serbia in Belgrade, was the discussant on a study that gave an indication on just how useful screening can be for other diseases.
The study, dubbed 4-IN-THE-LUNG-RUN trial (4ITLR), is an ongoing prospective trial in six European countries that is using lung cancer screening scans to also look for coronary artery calcifications, a marker of atherosclerosis.
Usually, coronary calcifications are considered incidental findings on lung cancer screenings and reported to subjects’ physicians for heart disease risk assessment.
The difference in 4ITLR is that investigators are actively looking for the lesions and quantifying the extent of calcifications.
It’s made possible by the artificial intelligence-based software being used to read the scans. In addition to generating reports on lung nodules, it also automatically calculates an Agatston score, a quantification of the degree of coronary artery calcification for each subject.
At the meeting, which was organized by the European Society for Clinical Oncology, 4ITLR investigator Daiwei Han, MD, PhD, a research associate at the Institute for Diagnostic Accuracy in Groningen, the Netherlands, reported outcomes in the first 2487 of the 24,000 planned subjects.
To be eligible for screening, participants had to be 60-79 years old and either current smokers, past smokers who had quit within 10 years, or people with a 35 or more pack-year history. The median age in the study was 68.1 years.
Overall, 53% of subjects had Agatston scores of 100 or more, indicating the need for treatment to prevent active coronary artery disease, Dr. Han said.
Fifteen percent were at high risk for heart disease with scores of 400-999, indicating extensive coronary artery calcification, and 16.2% were at very high risk, with scores of 1000 or higher. The information is being shared with participants’ physicians.
The risk of heart disease was far higher in men, who made up 56% of the study population. While women had a median Agatston score of 61, the median score for men was 211.1.
The findings illustrate the potential of dedicated cardiovascular screening within lung cancer screening programs, Dr. Han said, noting that 4ITLR will also incorporate COPD risk assessment.
The study also shows the increased impact lung cancer screening programs could have if greater use were made of the CT images to look for other diseases, Dr. Spasic said.
4ITLR is funded by the European Union’s Horizon 2020 Program. Dr. Spasic and Dr. Han didn’t have any relevant disclosures.
The reason is because the low-dose CT scans used for screening cover the lower neck down to the upper abdomen, revealing far more anatomy than simply the lungs.
In fact, lung cancer screening can provide information on three of the top 10 causes of death worldwide: ischemic heart disease, chronic obstructive pulmonary disease, and, of course, lung cancer.
With lung cancer screening, “we are basically targeting many birds with one low-dose stone,” explained Jelena Spasic MD, PhD, at the European Lung Cancer Congress (ELCC) 2024.
Dr. Spasic, a medical oncologist at the Institute for Oncology and Radiology of Serbia in Belgrade, was the discussant on a study that gave an indication on just how useful screening can be for other diseases.
The study, dubbed 4-IN-THE-LUNG-RUN trial (4ITLR), is an ongoing prospective trial in six European countries that is using lung cancer screening scans to also look for coronary artery calcifications, a marker of atherosclerosis.
Usually, coronary calcifications are considered incidental findings on lung cancer screenings and reported to subjects’ physicians for heart disease risk assessment.
The difference in 4ITLR is that investigators are actively looking for the lesions and quantifying the extent of calcifications.
It’s made possible by the artificial intelligence-based software being used to read the scans. In addition to generating reports on lung nodules, it also automatically calculates an Agatston score, a quantification of the degree of coronary artery calcification for each subject.
At the meeting, which was organized by the European Society for Clinical Oncology, 4ITLR investigator Daiwei Han, MD, PhD, a research associate at the Institute for Diagnostic Accuracy in Groningen, the Netherlands, reported outcomes in the first 2487 of the 24,000 planned subjects.
To be eligible for screening, participants had to be 60-79 years old and either current smokers, past smokers who had quit within 10 years, or people with a 35 or more pack-year history. The median age in the study was 68.1 years.
Overall, 53% of subjects had Agatston scores of 100 or more, indicating the need for treatment to prevent active coronary artery disease, Dr. Han said.
Fifteen percent were at high risk for heart disease with scores of 400-999, indicating extensive coronary artery calcification, and 16.2% were at very high risk, with scores of 1000 or higher. The information is being shared with participants’ physicians.
The risk of heart disease was far higher in men, who made up 56% of the study population. While women had a median Agatston score of 61, the median score for men was 211.1.
The findings illustrate the potential of dedicated cardiovascular screening within lung cancer screening programs, Dr. Han said, noting that 4ITLR will also incorporate COPD risk assessment.
The study also shows the increased impact lung cancer screening programs could have if greater use were made of the CT images to look for other diseases, Dr. Spasic said.
4ITLR is funded by the European Union’s Horizon 2020 Program. Dr. Spasic and Dr. Han didn’t have any relevant disclosures.
FROM ELCC 2024
Niacin and CV Risk: Should Advice on Intake Change?
A recent study linking a niacin derivative to an increased risk for cardiovascular events has raised questions about the safety of this B vitamin, which is added to many food staples in the Western diet and taken in the form of supplements.
The findings, which were published in Nature Medicine, may also help explain why taking niacin, which lowers low-density lipoprotein cholesterol and raises high-density lipoprotein cholesterol, did not lead to a reduction in cardiovascular events in major clinical trials.
But could this essential micronutrient really have an adverse effect on cardiovascular risk, and what are the implications for niacin intake?
Senior author of the new study Stanley Hazen, MD, believes some prudence on excessive niacin intake may be justified.
“I’m not suggesting we should completely avoid niacin — it is an essential nutrient, but our results suggest that too much may be harmful,” Dr. Hazen said.
Niacin supplements are also sold with claims of antiaging effects, arthritis relief, and boosting brain function, although none of these claims have been proven. And the related compound, nicotinamide, is recommended to prevent skin cancer in high-risk patients; however, a recent study questioned that guidance.
“I would say to the general public that avoiding supplements containing niacin or related compounds could be a sensible approach at present, while these findings are investigated further.”
Other experts are unsure if such action is justified on the basis of this single study.
Residual Cardiovascular Risk
Dr. Hazen, who is chair of the Department of Cardiovascular & Metabolic Sciences, at the Lerner Research Institute, Cleveland Clinic, Cleveland, Ohio, explained to this news organization that they did not set out to study niacin.
“It began as a study to look for novel pathways involved in residual cardiovascular disease risk — the risk for cardiovascular events after adjusting for traditional risk factors such as cholesterol, blood pressure, and diabetes.”
The researchers began looking for compounds in plasma that predicted future adverse cardiovascular events in individuals undergoing elective diagnostic cardiac evaluation. Two of the leading candidates identified were niacin derivatives — 2PY and 4PY — that are only formed in the presence of excess niacin.
They then developed assays to measure 2PY and 4PY and conducted further studies in two validation cohorts — 2331 US individuals and a European cohort of 832 individuals. In both cohorts, elevated plasma levels of 2PY and 4PY predicted future adverse cardiovascular events, with a doubling in cardiovascular risk seen in those with levels in the highest vs the lowest quartile.
To move beyond these observational studies and to explore a potentially causal relationship, Dr. Hazen’s team went on to perform genome-wide association studies and found that genetic variants that tracked with higher levels of 4PY also linked to levels of the inflammatory marker, vascular cell adhesion molecule 1 (VCAM-1).
And in cell culture and animal studies, they found that 4PY was a driver of inflammation, upregulating VCAM-1 and eliciting vascular inflammation responses.
“So, we have shown in several different ways that the niacin derivative, 4PY, is linked to increased cardiovascular risk,” Dr. Hazen commented.
Significant Health Implications?
Dr. Hazen believed these findings could have significant health implications.
He noted that Western populations have been consuming large amounts of niacin ever since World War 2 when we began to fortify many foods with essential vitamins to avoid diseases caused by deficiencies. Niacin was added to foods to prevent pellagra — a disease characterized by inflamed skin, diarrhea, and dementia, that was often fatal.
“While we may have eliminated pellagra, have we, as a consequence, increased the prevalence of cardiovascular disease many years later?” Dr. Hazen asked.
This may be a clue to why niacin does not lower cardiovascular risk as much as would be expected from the degree of cholesterol lowering it brings about. “This is the niacin paradox and has led to the thought that there could be some kind of adverse effect that niacin is promoting. I think we may have found something that contributes to the niacin paradox,” he said.
However, the niacin pathway is complicated. Niacin is the major source of nicotinamide adenine dinucleotide (NAD), an integral molecule that allows cells to create energy. “Because it is so important, our bodies are designed to salvage and retain NADs, but once storage capacity is exceeded, then these 4PY and 2PY derivatives are generated,” Dr. Hazen explained. “But you have to really eat a lot of niacin-rich foods for this to happen.”
He is not claiming that niacin causes cardiovascular disease. “It is 4PY that appears to be the driver of vascular inflammation. And 4PY is a breakdown product of niacin. But there is more than one pathway that could lead to 4PY generation. There is a whole interconnecting network of compounds that interchange with each other — known as the niacin pool — any one or more of these compounds can be ingested and raise pool levels and ultimately 4PY levels. However, by far and away, niacin is one of the major sources,” Dr. Hazen commented.
Are High-Protein Diets Also Implicated?
Other sources of NADs include tryptophan, present in protein. And one of the genetic variants linked to changes in 4PY levels is connected to how dietary protein is directed into the niacin pool, raising the possibility that a high-protein diet may also raise cardiovascular risk in some people, Dr. Hazen noted.
Dr. Hazen estimated that about 3% of the niacin pool in a normal diet comes from protein intake, but that the percentage could increase substantially in very high–protein diets.
“Our data support the concept that if we lower our 4PY level long-term, then that would result in a reduction in cardiovascular disease. But this is still just a hypothesis. If we lower niacin intake, we will lower 4PY,” Dr. Hazen stated.
He said that this research is at too early a stage to give firm recommendations in what this means for the consumer.
“Based on these findings, I would advise people to avoid taking niacin or nicotinic acid or nicotinamide supplements and to eat a sensible balanced diet — maybe not to overdo the high protein–type diets. That’s all we can really say at the moment.”
Noting that niacin can also be one of the major components in energy drinks, he suggested it may be prudent to limit consumption of these products.
What Is the Optimum Niacin Intake?
Dr. Hazen noted that the recommended dietary allowance (RDA) for niacin is well known — between 14 and 18 mg, but he said the average American ingests four times that amount, and some people have substantially higher intakes — up to 50 times the RDA if taking supplements.
While food fortification with niacin may have been useful in the past, Dr. Hazen questioned whether it should still be mandated.
“In the US, you cannot buy flour or cereal or rice that is not fortified. And if you look closely, some products have much higher levels than those that are mandated. The food companies advertise this as a benefit, but there is no good data in support of that. What if several decades of eating excessive amounts of niacin has led to an increase in cardiovascular disease?”
He does not propose stopping all niacin fortification, “but maybe, we could have the choice of selecting an unfortified option,” he said.
Causal Link Not Proven
Commenting for this news organization, John Guyton, MD, Professor Emeritus of Medicine, Duke University Medical Center, Durham, North Carolina, who has been involved in niacin research for many years, said the Nature Medicine study showed “interesting and important results,” but they do not at this point prove a causal link between niacin intake and risk for cardiovascular disease.
“These findings need to be investigated further, and more studies are certainly justified, but I don’t think that this study alone makes an adequate case for restricting niacin intake, or thinking about stopping niacin fortification of foodstuffs,” Dr. Guyton said.
Noting that niacin is present in large quantities in many fast foods, he suggested the researchers may have just picked up the consequences of eating an unhealthy diet.
“If you look at foods that contain high quantities of niacin, red meat is at the top of the list. And if you think of a hamburger, niacin is present in relatively large quantities both the burger and the bun. So, these findings may just be a reflection of an overall unhealthy diet,” he commented.
Dr. Guyton also pointed out that major clinical trials with niacin have shown mixed results, and its effect on cardiovascular risk is still not completely understood. While the HPS2-THRIVE and AIM-HIGH trials did not show benefits in reducing cardiovascular events, an earlier study, the Coronary Drug Project in which the agent was given with food, did show some positive effects with substantial reductions in myocardial infarction and stroke, and there was the suggestion of a reduction in long-term mortality in the niacin group several years after the trial had ended.
Nicotinamide in Skin Cancer Prevention
What about the use of nicotinamide in skin cancer prevention?
Addressing this question, Kristin Bibee, MD, assistant professor of dermatology at Johns Hopkins University School of Medicine, Baltimore, pointed out that nicotinamide, although closely related to niacin, may have different effects. “This study does not specifically address nicotinamide supplementation and 4PY levels,” she said.
Diona Damian, MD, professor of dermatology at the University of Sydney, Camperdown, Australia, told this news organization that it was hard to extrapolate these findings on basal levels of niacin in a cardiac cohort to the administration of supra-physiological doses of nicotinamide for skin cancer prevention.
There may be different effects of supplemental niacin compared to nicotinamide, which lacks the vasodilatory effects seen with niacin, Dr. Damian said, adding that it would be interesting to see the results from higher, therapeutic nicotinamide doses in patients with and without cardiac disease.
She pointed out that high vs low levels of nicotinamide supplementation can have different and even opposite effects on cellular processes, such as upregulating or inhibiting DNA repair enzymes. At high doses, nicotinamide is anti-inflammatory in skin.
Dr. Damian noted that two phase 3 studies (ONTRAC and ONTRANS) of nicotinamide 500 mg twice daily for skin cancer prevention did not find a significant increase in cardiovascular events compared to placebo over 12 months.
“Oral nicotinamide has been shown to reduce nonmelanoma skin cancer by about a quarter in patients with normal immunity and multiple skin cancers. The doses used for skin cancer prevention are well above daily dietary levels, and treatment needs to be ongoing for the protective effects to continue. Nicotinamide should not be recommended as a preventive agent for people who have not had multiple skin cancers but should be reserved for those with a heavy burden of skin cancers,” she commented.
“For now, it would be reasonable to balance the benefits of skin cancer reduction against possible effects on inflammatory markers in patients with cardiac risk factors, when helping patients to decide whether or not nicotinamide therapy is appropriate for them,” she added.
Meanwhile, Dr. Hazen said the most exciting part of this new research is the discovery of a new pathway that contributes to cardiovascular disease and potentially a new target to treat residual cardiovascular risk.
“I believe our results show that we should be measuring 4PY levels and individuals with high levels need to be extra vigilant about lowering their cardiovascular risk.”
The next step will be to confirm these results in other populations and then to develop a diagnostic test to identify people with a high 4PY level, he said.
A version of this article appeared on Medscape.com.
A recent study linking a niacin derivative to an increased risk for cardiovascular events has raised questions about the safety of this B vitamin, which is added to many food staples in the Western diet and taken in the form of supplements.
The findings, which were published in Nature Medicine, may also help explain why taking niacin, which lowers low-density lipoprotein cholesterol and raises high-density lipoprotein cholesterol, did not lead to a reduction in cardiovascular events in major clinical trials.
But could this essential micronutrient really have an adverse effect on cardiovascular risk, and what are the implications for niacin intake?
Senior author of the new study Stanley Hazen, MD, believes some prudence on excessive niacin intake may be justified.
“I’m not suggesting we should completely avoid niacin — it is an essential nutrient, but our results suggest that too much may be harmful,” Dr. Hazen said.
Niacin supplements are also sold with claims of antiaging effects, arthritis relief, and boosting brain function, although none of these claims have been proven. And the related compound, nicotinamide, is recommended to prevent skin cancer in high-risk patients; however, a recent study questioned that guidance.
“I would say to the general public that avoiding supplements containing niacin or related compounds could be a sensible approach at present, while these findings are investigated further.”
Other experts are unsure if such action is justified on the basis of this single study.
Residual Cardiovascular Risk
Dr. Hazen, who is chair of the Department of Cardiovascular & Metabolic Sciences, at the Lerner Research Institute, Cleveland Clinic, Cleveland, Ohio, explained to this news organization that they did not set out to study niacin.
“It began as a study to look for novel pathways involved in residual cardiovascular disease risk — the risk for cardiovascular events after adjusting for traditional risk factors such as cholesterol, blood pressure, and diabetes.”
The researchers began looking for compounds in plasma that predicted future adverse cardiovascular events in individuals undergoing elective diagnostic cardiac evaluation. Two of the leading candidates identified were niacin derivatives — 2PY and 4PY — that are only formed in the presence of excess niacin.
They then developed assays to measure 2PY and 4PY and conducted further studies in two validation cohorts — 2331 US individuals and a European cohort of 832 individuals. In both cohorts, elevated plasma levels of 2PY and 4PY predicted future adverse cardiovascular events, with a doubling in cardiovascular risk seen in those with levels in the highest vs the lowest quartile.
To move beyond these observational studies and to explore a potentially causal relationship, Dr. Hazen’s team went on to perform genome-wide association studies and found that genetic variants that tracked with higher levels of 4PY also linked to levels of the inflammatory marker, vascular cell adhesion molecule 1 (VCAM-1).
And in cell culture and animal studies, they found that 4PY was a driver of inflammation, upregulating VCAM-1 and eliciting vascular inflammation responses.
“So, we have shown in several different ways that the niacin derivative, 4PY, is linked to increased cardiovascular risk,” Dr. Hazen commented.
Significant Health Implications?
Dr. Hazen believed these findings could have significant health implications.
He noted that Western populations have been consuming large amounts of niacin ever since World War 2 when we began to fortify many foods with essential vitamins to avoid diseases caused by deficiencies. Niacin was added to foods to prevent pellagra — a disease characterized by inflamed skin, diarrhea, and dementia, that was often fatal.
“While we may have eliminated pellagra, have we, as a consequence, increased the prevalence of cardiovascular disease many years later?” Dr. Hazen asked.
This may be a clue to why niacin does not lower cardiovascular risk as much as would be expected from the degree of cholesterol lowering it brings about. “This is the niacin paradox and has led to the thought that there could be some kind of adverse effect that niacin is promoting. I think we may have found something that contributes to the niacin paradox,” he said.
However, the niacin pathway is complicated. Niacin is the major source of nicotinamide adenine dinucleotide (NAD), an integral molecule that allows cells to create energy. “Because it is so important, our bodies are designed to salvage and retain NADs, but once storage capacity is exceeded, then these 4PY and 2PY derivatives are generated,” Dr. Hazen explained. “But you have to really eat a lot of niacin-rich foods for this to happen.”
He is not claiming that niacin causes cardiovascular disease. “It is 4PY that appears to be the driver of vascular inflammation. And 4PY is a breakdown product of niacin. But there is more than one pathway that could lead to 4PY generation. There is a whole interconnecting network of compounds that interchange with each other — known as the niacin pool — any one or more of these compounds can be ingested and raise pool levels and ultimately 4PY levels. However, by far and away, niacin is one of the major sources,” Dr. Hazen commented.
Are High-Protein Diets Also Implicated?
Other sources of NADs include tryptophan, present in protein. And one of the genetic variants linked to changes in 4PY levels is connected to how dietary protein is directed into the niacin pool, raising the possibility that a high-protein diet may also raise cardiovascular risk in some people, Dr. Hazen noted.
Dr. Hazen estimated that about 3% of the niacin pool in a normal diet comes from protein intake, but that the percentage could increase substantially in very high–protein diets.
“Our data support the concept that if we lower our 4PY level long-term, then that would result in a reduction in cardiovascular disease. But this is still just a hypothesis. If we lower niacin intake, we will lower 4PY,” Dr. Hazen stated.
He said that this research is at too early a stage to give firm recommendations in what this means for the consumer.
“Based on these findings, I would advise people to avoid taking niacin or nicotinic acid or nicotinamide supplements and to eat a sensible balanced diet — maybe not to overdo the high protein–type diets. That’s all we can really say at the moment.”
Noting that niacin can also be one of the major components in energy drinks, he suggested it may be prudent to limit consumption of these products.
What Is the Optimum Niacin Intake?
Dr. Hazen noted that the recommended dietary allowance (RDA) for niacin is well known — between 14 and 18 mg, but he said the average American ingests four times that amount, and some people have substantially higher intakes — up to 50 times the RDA if taking supplements.
While food fortification with niacin may have been useful in the past, Dr. Hazen questioned whether it should still be mandated.
“In the US, you cannot buy flour or cereal or rice that is not fortified. And if you look closely, some products have much higher levels than those that are mandated. The food companies advertise this as a benefit, but there is no good data in support of that. What if several decades of eating excessive amounts of niacin has led to an increase in cardiovascular disease?”
He does not propose stopping all niacin fortification, “but maybe, we could have the choice of selecting an unfortified option,” he said.
Causal Link Not Proven
Commenting for this news organization, John Guyton, MD, Professor Emeritus of Medicine, Duke University Medical Center, Durham, North Carolina, who has been involved in niacin research for many years, said the Nature Medicine study showed “interesting and important results,” but they do not at this point prove a causal link between niacin intake and risk for cardiovascular disease.
“These findings need to be investigated further, and more studies are certainly justified, but I don’t think that this study alone makes an adequate case for restricting niacin intake, or thinking about stopping niacin fortification of foodstuffs,” Dr. Guyton said.
Noting that niacin is present in large quantities in many fast foods, he suggested the researchers may have just picked up the consequences of eating an unhealthy diet.
“If you look at foods that contain high quantities of niacin, red meat is at the top of the list. And if you think of a hamburger, niacin is present in relatively large quantities both the burger and the bun. So, these findings may just be a reflection of an overall unhealthy diet,” he commented.
Dr. Guyton also pointed out that major clinical trials with niacin have shown mixed results, and its effect on cardiovascular risk is still not completely understood. While the HPS2-THRIVE and AIM-HIGH trials did not show benefits in reducing cardiovascular events, an earlier study, the Coronary Drug Project in which the agent was given with food, did show some positive effects with substantial reductions in myocardial infarction and stroke, and there was the suggestion of a reduction in long-term mortality in the niacin group several years after the trial had ended.
Nicotinamide in Skin Cancer Prevention
What about the use of nicotinamide in skin cancer prevention?
Addressing this question, Kristin Bibee, MD, assistant professor of dermatology at Johns Hopkins University School of Medicine, Baltimore, pointed out that nicotinamide, although closely related to niacin, may have different effects. “This study does not specifically address nicotinamide supplementation and 4PY levels,” she said.
Diona Damian, MD, professor of dermatology at the University of Sydney, Camperdown, Australia, told this news organization that it was hard to extrapolate these findings on basal levels of niacin in a cardiac cohort to the administration of supra-physiological doses of nicotinamide for skin cancer prevention.
There may be different effects of supplemental niacin compared to nicotinamide, which lacks the vasodilatory effects seen with niacin, Dr. Damian said, adding that it would be interesting to see the results from higher, therapeutic nicotinamide doses in patients with and without cardiac disease.
She pointed out that high vs low levels of nicotinamide supplementation can have different and even opposite effects on cellular processes, such as upregulating or inhibiting DNA repair enzymes. At high doses, nicotinamide is anti-inflammatory in skin.
Dr. Damian noted that two phase 3 studies (ONTRAC and ONTRANS) of nicotinamide 500 mg twice daily for skin cancer prevention did not find a significant increase in cardiovascular events compared to placebo over 12 months.
“Oral nicotinamide has been shown to reduce nonmelanoma skin cancer by about a quarter in patients with normal immunity and multiple skin cancers. The doses used for skin cancer prevention are well above daily dietary levels, and treatment needs to be ongoing for the protective effects to continue. Nicotinamide should not be recommended as a preventive agent for people who have not had multiple skin cancers but should be reserved for those with a heavy burden of skin cancers,” she commented.
“For now, it would be reasonable to balance the benefits of skin cancer reduction against possible effects on inflammatory markers in patients with cardiac risk factors, when helping patients to decide whether or not nicotinamide therapy is appropriate for them,” she added.
Meanwhile, Dr. Hazen said the most exciting part of this new research is the discovery of a new pathway that contributes to cardiovascular disease and potentially a new target to treat residual cardiovascular risk.
“I believe our results show that we should be measuring 4PY levels and individuals with high levels need to be extra vigilant about lowering their cardiovascular risk.”
The next step will be to confirm these results in other populations and then to develop a diagnostic test to identify people with a high 4PY level, he said.
A version of this article appeared on Medscape.com.
A recent study linking a niacin derivative to an increased risk for cardiovascular events has raised questions about the safety of this B vitamin, which is added to many food staples in the Western diet and taken in the form of supplements.
The findings, which were published in Nature Medicine, may also help explain why taking niacin, which lowers low-density lipoprotein cholesterol and raises high-density lipoprotein cholesterol, did not lead to a reduction in cardiovascular events in major clinical trials.
But could this essential micronutrient really have an adverse effect on cardiovascular risk, and what are the implications for niacin intake?
Senior author of the new study Stanley Hazen, MD, believes some prudence on excessive niacin intake may be justified.
“I’m not suggesting we should completely avoid niacin — it is an essential nutrient, but our results suggest that too much may be harmful,” Dr. Hazen said.
Niacin supplements are also sold with claims of antiaging effects, arthritis relief, and boosting brain function, although none of these claims have been proven. And the related compound, nicotinamide, is recommended to prevent skin cancer in high-risk patients; however, a recent study questioned that guidance.
“I would say to the general public that avoiding supplements containing niacin or related compounds could be a sensible approach at present, while these findings are investigated further.”
Other experts are unsure if such action is justified on the basis of this single study.
Residual Cardiovascular Risk
Dr. Hazen, who is chair of the Department of Cardiovascular & Metabolic Sciences, at the Lerner Research Institute, Cleveland Clinic, Cleveland, Ohio, explained to this news organization that they did not set out to study niacin.
“It began as a study to look for novel pathways involved in residual cardiovascular disease risk — the risk for cardiovascular events after adjusting for traditional risk factors such as cholesterol, blood pressure, and diabetes.”
The researchers began looking for compounds in plasma that predicted future adverse cardiovascular events in individuals undergoing elective diagnostic cardiac evaluation. Two of the leading candidates identified were niacin derivatives — 2PY and 4PY — that are only formed in the presence of excess niacin.
They then developed assays to measure 2PY and 4PY and conducted further studies in two validation cohorts — 2331 US individuals and a European cohort of 832 individuals. In both cohorts, elevated plasma levels of 2PY and 4PY predicted future adverse cardiovascular events, with a doubling in cardiovascular risk seen in those with levels in the highest vs the lowest quartile.
To move beyond these observational studies and to explore a potentially causal relationship, Dr. Hazen’s team went on to perform genome-wide association studies and found that genetic variants that tracked with higher levels of 4PY also linked to levels of the inflammatory marker, vascular cell adhesion molecule 1 (VCAM-1).
And in cell culture and animal studies, they found that 4PY was a driver of inflammation, upregulating VCAM-1 and eliciting vascular inflammation responses.
“So, we have shown in several different ways that the niacin derivative, 4PY, is linked to increased cardiovascular risk,” Dr. Hazen commented.
Significant Health Implications?
Dr. Hazen believed these findings could have significant health implications.
He noted that Western populations have been consuming large amounts of niacin ever since World War 2 when we began to fortify many foods with essential vitamins to avoid diseases caused by deficiencies. Niacin was added to foods to prevent pellagra — a disease characterized by inflamed skin, diarrhea, and dementia, that was often fatal.
“While we may have eliminated pellagra, have we, as a consequence, increased the prevalence of cardiovascular disease many years later?” Dr. Hazen asked.
This may be a clue to why niacin does not lower cardiovascular risk as much as would be expected from the degree of cholesterol lowering it brings about. “This is the niacin paradox and has led to the thought that there could be some kind of adverse effect that niacin is promoting. I think we may have found something that contributes to the niacin paradox,” he said.
However, the niacin pathway is complicated. Niacin is the major source of nicotinamide adenine dinucleotide (NAD), an integral molecule that allows cells to create energy. “Because it is so important, our bodies are designed to salvage and retain NADs, but once storage capacity is exceeded, then these 4PY and 2PY derivatives are generated,” Dr. Hazen explained. “But you have to really eat a lot of niacin-rich foods for this to happen.”
He is not claiming that niacin causes cardiovascular disease. “It is 4PY that appears to be the driver of vascular inflammation. And 4PY is a breakdown product of niacin. But there is more than one pathway that could lead to 4PY generation. There is a whole interconnecting network of compounds that interchange with each other — known as the niacin pool — any one or more of these compounds can be ingested and raise pool levels and ultimately 4PY levels. However, by far and away, niacin is one of the major sources,” Dr. Hazen commented.
Are High-Protein Diets Also Implicated?
Other sources of NADs include tryptophan, present in protein. And one of the genetic variants linked to changes in 4PY levels is connected to how dietary protein is directed into the niacin pool, raising the possibility that a high-protein diet may also raise cardiovascular risk in some people, Dr. Hazen noted.
Dr. Hazen estimated that about 3% of the niacin pool in a normal diet comes from protein intake, but that the percentage could increase substantially in very high–protein diets.
“Our data support the concept that if we lower our 4PY level long-term, then that would result in a reduction in cardiovascular disease. But this is still just a hypothesis. If we lower niacin intake, we will lower 4PY,” Dr. Hazen stated.
He said that this research is at too early a stage to give firm recommendations in what this means for the consumer.
“Based on these findings, I would advise people to avoid taking niacin or nicotinic acid or nicotinamide supplements and to eat a sensible balanced diet — maybe not to overdo the high protein–type diets. That’s all we can really say at the moment.”
Noting that niacin can also be one of the major components in energy drinks, he suggested it may be prudent to limit consumption of these products.
What Is the Optimum Niacin Intake?
Dr. Hazen noted that the recommended dietary allowance (RDA) for niacin is well known — between 14 and 18 mg, but he said the average American ingests four times that amount, and some people have substantially higher intakes — up to 50 times the RDA if taking supplements.
While food fortification with niacin may have been useful in the past, Dr. Hazen questioned whether it should still be mandated.
“In the US, you cannot buy flour or cereal or rice that is not fortified. And if you look closely, some products have much higher levels than those that are mandated. The food companies advertise this as a benefit, but there is no good data in support of that. What if several decades of eating excessive amounts of niacin has led to an increase in cardiovascular disease?”
He does not propose stopping all niacin fortification, “but maybe, we could have the choice of selecting an unfortified option,” he said.
Causal Link Not Proven
Commenting for this news organization, John Guyton, MD, Professor Emeritus of Medicine, Duke University Medical Center, Durham, North Carolina, who has been involved in niacin research for many years, said the Nature Medicine study showed “interesting and important results,” but they do not at this point prove a causal link between niacin intake and risk for cardiovascular disease.
“These findings need to be investigated further, and more studies are certainly justified, but I don’t think that this study alone makes an adequate case for restricting niacin intake, or thinking about stopping niacin fortification of foodstuffs,” Dr. Guyton said.
Noting that niacin is present in large quantities in many fast foods, he suggested the researchers may have just picked up the consequences of eating an unhealthy diet.
“If you look at foods that contain high quantities of niacin, red meat is at the top of the list. And if you think of a hamburger, niacin is present in relatively large quantities both the burger and the bun. So, these findings may just be a reflection of an overall unhealthy diet,” he commented.
Dr. Guyton also pointed out that major clinical trials with niacin have shown mixed results, and its effect on cardiovascular risk is still not completely understood. While the HPS2-THRIVE and AIM-HIGH trials did not show benefits in reducing cardiovascular events, an earlier study, the Coronary Drug Project in which the agent was given with food, did show some positive effects with substantial reductions in myocardial infarction and stroke, and there was the suggestion of a reduction in long-term mortality in the niacin group several years after the trial had ended.
Nicotinamide in Skin Cancer Prevention
What about the use of nicotinamide in skin cancer prevention?
Addressing this question, Kristin Bibee, MD, assistant professor of dermatology at Johns Hopkins University School of Medicine, Baltimore, pointed out that nicotinamide, although closely related to niacin, may have different effects. “This study does not specifically address nicotinamide supplementation and 4PY levels,” she said.
Diona Damian, MD, professor of dermatology at the University of Sydney, Camperdown, Australia, told this news organization that it was hard to extrapolate these findings on basal levels of niacin in a cardiac cohort to the administration of supra-physiological doses of nicotinamide for skin cancer prevention.
There may be different effects of supplemental niacin compared to nicotinamide, which lacks the vasodilatory effects seen with niacin, Dr. Damian said, adding that it would be interesting to see the results from higher, therapeutic nicotinamide doses in patients with and without cardiac disease.
She pointed out that high vs low levels of nicotinamide supplementation can have different and even opposite effects on cellular processes, such as upregulating or inhibiting DNA repair enzymes. At high doses, nicotinamide is anti-inflammatory in skin.
Dr. Damian noted that two phase 3 studies (ONTRAC and ONTRANS) of nicotinamide 500 mg twice daily for skin cancer prevention did not find a significant increase in cardiovascular events compared to placebo over 12 months.
“Oral nicotinamide has been shown to reduce nonmelanoma skin cancer by about a quarter in patients with normal immunity and multiple skin cancers. The doses used for skin cancer prevention are well above daily dietary levels, and treatment needs to be ongoing for the protective effects to continue. Nicotinamide should not be recommended as a preventive agent for people who have not had multiple skin cancers but should be reserved for those with a heavy burden of skin cancers,” she commented.
“For now, it would be reasonable to balance the benefits of skin cancer reduction against possible effects on inflammatory markers in patients with cardiac risk factors, when helping patients to decide whether or not nicotinamide therapy is appropriate for them,” she added.
Meanwhile, Dr. Hazen said the most exciting part of this new research is the discovery of a new pathway that contributes to cardiovascular disease and potentially a new target to treat residual cardiovascular risk.
“I believe our results show that we should be measuring 4PY levels and individuals with high levels need to be extra vigilant about lowering their cardiovascular risk.”
The next step will be to confirm these results in other populations and then to develop a diagnostic test to identify people with a high 4PY level, he said.
A version of this article appeared on Medscape.com.
Vitamin D Supplements May Be a Double-Edged Sword
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Imagine, if you will, the great Cathedral of Our Lady of Correlation. You walk through the majestic oak doors depicting the link between ice cream sales and shark attacks, past the rose window depicting the cardiovascular benefits of red wine, and down the aisles frescoed in dramatic images showing how Facebook usage is associated with less life satisfaction. And then you reach the altar, the holy of holies where, emblazoned in shimmering pyrite, you see the patron saint of this church: vitamin D.
Yes, if you’ve watched this space, then you know that I have little truck with the wildly popular supplement. In all of clinical research, I believe that there is no molecule with stronger data for correlation and weaker data for causation.
Low serum vitamin D levels have been linked to higher risks for heart disease, cancer, falls, COVID, dementia, C diff, and others. And yet, when we do randomized trials of vitamin D supplementation — the thing that can prove that the low level was causally linked to the outcome of interest — we get negative results.
Trials aren’t perfect, of course, and we’ll talk in a moment about a big one that had some issues. But we are at a point where we need to either be vitamin D apologists, saying, “Forget what those lying RCTs tell you and buy this supplement” — an $800 million-a-year industry, by the way — or conclude that vitamin D levels are a convenient marker of various lifestyle factors that are associated with better outcomes: markers of exercise, getting outside, eating a varied diet.
Or perhaps vitamin D supplements have real effects. It’s just that the beneficial effects are matched by the harmful ones. Stay tuned.
The Women’s Health Initiative remains among the largest randomized trials of vitamin D and calcium supplementation ever conducted — and a major contributor to the negative outcomes of vitamin D trials.
But if you dig into the inclusion and exclusion criteria for this trial, you’ll find that individuals were allowed to continue taking vitamins and supplements while they were in the trial, regardless of their randomization status. In fact, the majority took supplements at baseline, and more took supplements over time.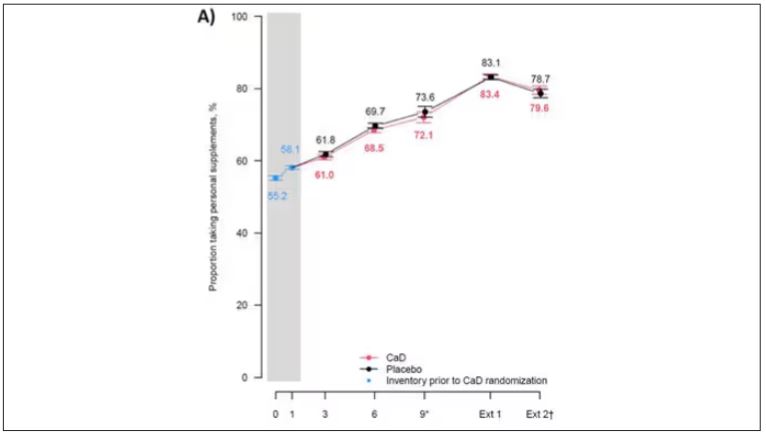
That means, of course, that people in the placebo group, who were getting sugar pills instead of vitamin D and calcium, may have been taking vitamin D and calcium on the side. That would certainly bias the results of the trial toward the null, which is what the primary analyses showed. To wit, the original analysis of the Women’s Health Initiative trial showed no effect of randomization to vitamin D supplementation on improving cancer or cardiovascular outcomes.
But the Women’s Health Initiative trial started 30 years ago. Today, with the benefit of decades of follow-up, we can re-investigate — and perhaps re-litigate — those findings, courtesy of this study, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women” appearing in Annals of Internal Medicine.
Dr Cynthia Thomson, of the Mel and Enid Zuckerman College of Public Health at the University of Arizona, and colleagues led this updated analysis focused on two findings that had been hinted at, but not statistically confirmed, in other vitamin D studies: a potential for the supplement to reduce the risk for cancer, and a potential for it to increase the risk for heart disease.
The randomized trial itself only lasted 7 years. What we are seeing in this analysis of 36,282 women is outcomes that happened at any time from randomization to the end of 2023 — around 20 years after the randomization to supplementation stopped. But, the researchers would argue, that’s probably okay. Cancer and heart disease take time to develop; we see lung cancer long after people stop smoking. So a history of consistent vitamin D supplementation may indeed be protective — or harmful.
Here are the top-line results. Those randomized to vitamin D and calcium supplementation had a 7% reduction in the rate of death from cancer, driven primarily by a reduction in colorectal cancer. This was statistically significant. Also statistically significant? Those randomized to supplementation had a 6% increase in the rate of death from cardiovascular disease. Put those findings together and what do you get? Stone-cold nothing, in terms of overall mortality.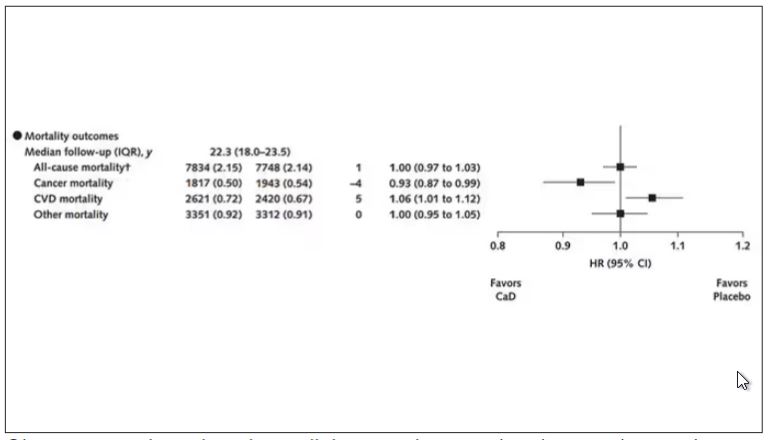
Okay, you say, but what about all that supplementation that was happening outside of the context of the trial, biasing our results toward the null?
The researchers finally clue us in.
First of all, I’ll tell you that, yes, people who were supplementing outside of the trial had higher baseline vitamin D levels — a median of 54.5 nmol/L vs 32.8 nmol/L. This may be because they were supplementing with vitamin D, but it could also be because people who take supplements tend to do other healthy things — another correlation to add to the great cathedral.
To get a better view of the real effects of randomization, the authors restricted the analysis to just those who did not use outside supplements. If vitamin D supplements help, then these are the people they should help. This group had about a 11% reduction in the incidence of cancer — statistically significant — and a 7% reduction in cancer mortality that did not meet the bar for statistical significance.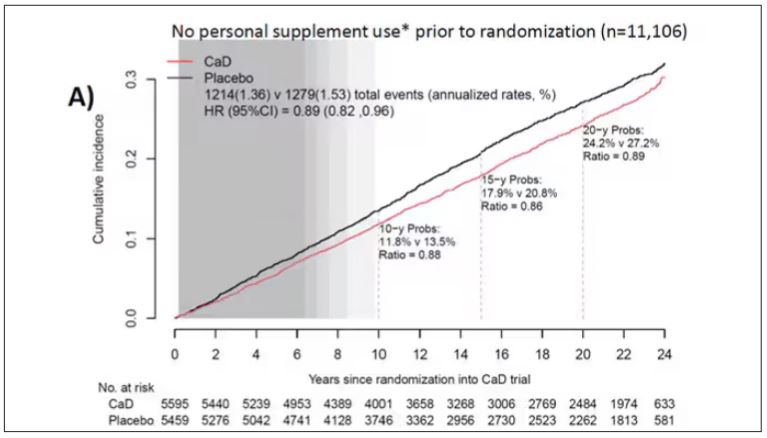
There was no increase in cardiovascular disease among this group. But this small effect on cancer was nowhere near enough to significantly reduce the rate of all-cause mortality.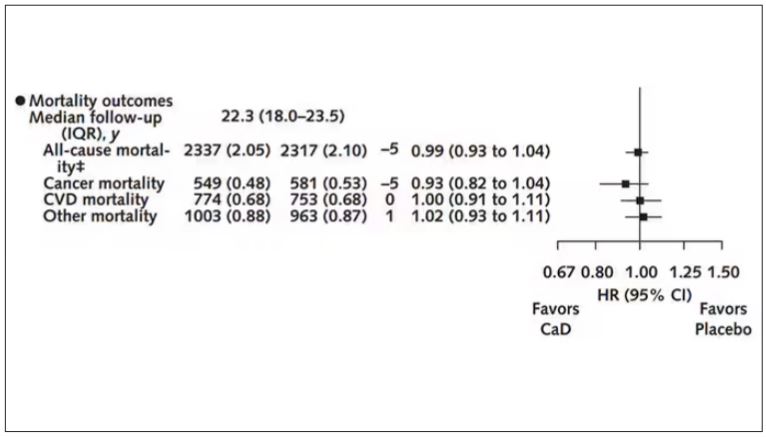
Among those using supplements, vitamin D supplementation didn’t really move the needle on any outcome.
I know what you’re thinking: How many of these women were vitamin D deficient when we got started? These results may simply be telling us that people who have normal vitamin D levels are fine to go without supplementation.
Nearly three fourths of women who were not taking supplements entered the trial with vitamin D levels below the 50 nmol/L cutoff that the authors suggest would qualify for deficiency. Around half of those who used supplements were deficient. And yet, frustratingly, I could not find data on the effect of randomization to supplementation stratified by baseline vitamin D level. I even reached out to Dr Thomson to ask about this. She replied, “We did not stratify on baseline values because the numbers are too small statistically to test this.” Sorry.
In the meantime, I can tell you that for your “average woman,” vitamin D supplementation likely has no effect on mortality. It might modestly reduce the risk for certain cancers while increasing the risk for heart disease (probably through coronary calcification). So, there might be some room for personalization here. Perhaps women with a strong family history of cancer or other risk factors would do better with supplements, and those with a high risk for heart disease would do worse. Seems like a strategy that could be tested in a clinical trial. But maybe we could ask the participants to give up their extracurricular supplement use before they enter the trial. F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his book, How Medicine Works and When It Doesn’t, is available now.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Imagine, if you will, the great Cathedral of Our Lady of Correlation. You walk through the majestic oak doors depicting the link between ice cream sales and shark attacks, past the rose window depicting the cardiovascular benefits of red wine, and down the aisles frescoed in dramatic images showing how Facebook usage is associated with less life satisfaction. And then you reach the altar, the holy of holies where, emblazoned in shimmering pyrite, you see the patron saint of this church: vitamin D.
Yes, if you’ve watched this space, then you know that I have little truck with the wildly popular supplement. In all of clinical research, I believe that there is no molecule with stronger data for correlation and weaker data for causation.
Low serum vitamin D levels have been linked to higher risks for heart disease, cancer, falls, COVID, dementia, C diff, and others. And yet, when we do randomized trials of vitamin D supplementation — the thing that can prove that the low level was causally linked to the outcome of interest — we get negative results.
Trials aren’t perfect, of course, and we’ll talk in a moment about a big one that had some issues. But we are at a point where we need to either be vitamin D apologists, saying, “Forget what those lying RCTs tell you and buy this supplement” — an $800 million-a-year industry, by the way — or conclude that vitamin D levels are a convenient marker of various lifestyle factors that are associated with better outcomes: markers of exercise, getting outside, eating a varied diet.
Or perhaps vitamin D supplements have real effects. It’s just that the beneficial effects are matched by the harmful ones. Stay tuned.
The Women’s Health Initiative remains among the largest randomized trials of vitamin D and calcium supplementation ever conducted — and a major contributor to the negative outcomes of vitamin D trials.
But if you dig into the inclusion and exclusion criteria for this trial, you’ll find that individuals were allowed to continue taking vitamins and supplements while they were in the trial, regardless of their randomization status. In fact, the majority took supplements at baseline, and more took supplements over time.
That means, of course, that people in the placebo group, who were getting sugar pills instead of vitamin D and calcium, may have been taking vitamin D and calcium on the side. That would certainly bias the results of the trial toward the null, which is what the primary analyses showed. To wit, the original analysis of the Women’s Health Initiative trial showed no effect of randomization to vitamin D supplementation on improving cancer or cardiovascular outcomes.
But the Women’s Health Initiative trial started 30 years ago. Today, with the benefit of decades of follow-up, we can re-investigate — and perhaps re-litigate — those findings, courtesy of this study, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women” appearing in Annals of Internal Medicine.
Dr Cynthia Thomson, of the Mel and Enid Zuckerman College of Public Health at the University of Arizona, and colleagues led this updated analysis focused on two findings that had been hinted at, but not statistically confirmed, in other vitamin D studies: a potential for the supplement to reduce the risk for cancer, and a potential for it to increase the risk for heart disease.
The randomized trial itself only lasted 7 years. What we are seeing in this analysis of 36,282 women is outcomes that happened at any time from randomization to the end of 2023 — around 20 years after the randomization to supplementation stopped. But, the researchers would argue, that’s probably okay. Cancer and heart disease take time to develop; we see lung cancer long after people stop smoking. So a history of consistent vitamin D supplementation may indeed be protective — or harmful.
Here are the top-line results. Those randomized to vitamin D and calcium supplementation had a 7% reduction in the rate of death from cancer, driven primarily by a reduction in colorectal cancer. This was statistically significant. Also statistically significant? Those randomized to supplementation had a 6% increase in the rate of death from cardiovascular disease. Put those findings together and what do you get? Stone-cold nothing, in terms of overall mortality.
Okay, you say, but what about all that supplementation that was happening outside of the context of the trial, biasing our results toward the null?
The researchers finally clue us in.
First of all, I’ll tell you that, yes, people who were supplementing outside of the trial had higher baseline vitamin D levels — a median of 54.5 nmol/L vs 32.8 nmol/L. This may be because they were supplementing with vitamin D, but it could also be because people who take supplements tend to do other healthy things — another correlation to add to the great cathedral.
To get a better view of the real effects of randomization, the authors restricted the analysis to just those who did not use outside supplements. If vitamin D supplements help, then these are the people they should help. This group had about a 11% reduction in the incidence of cancer — statistically significant — and a 7% reduction in cancer mortality that did not meet the bar for statistical significance.
There was no increase in cardiovascular disease among this group. But this small effect on cancer was nowhere near enough to significantly reduce the rate of all-cause mortality.
Among those using supplements, vitamin D supplementation didn’t really move the needle on any outcome.
I know what you’re thinking: How many of these women were vitamin D deficient when we got started? These results may simply be telling us that people who have normal vitamin D levels are fine to go without supplementation.
Nearly three fourths of women who were not taking supplements entered the trial with vitamin D levels below the 50 nmol/L cutoff that the authors suggest would qualify for deficiency. Around half of those who used supplements were deficient. And yet, frustratingly, I could not find data on the effect of randomization to supplementation stratified by baseline vitamin D level. I even reached out to Dr Thomson to ask about this. She replied, “We did not stratify on baseline values because the numbers are too small statistically to test this.” Sorry.
In the meantime, I can tell you that for your “average woman,” vitamin D supplementation likely has no effect on mortality. It might modestly reduce the risk for certain cancers while increasing the risk for heart disease (probably through coronary calcification). So, there might be some room for personalization here. Perhaps women with a strong family history of cancer or other risk factors would do better with supplements, and those with a high risk for heart disease would do worse. Seems like a strategy that could be tested in a clinical trial. But maybe we could ask the participants to give up their extracurricular supplement use before they enter the trial. F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his book, How Medicine Works and When It Doesn’t, is available now.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Imagine, if you will, the great Cathedral of Our Lady of Correlation. You walk through the majestic oak doors depicting the link between ice cream sales and shark attacks, past the rose window depicting the cardiovascular benefits of red wine, and down the aisles frescoed in dramatic images showing how Facebook usage is associated with less life satisfaction. And then you reach the altar, the holy of holies where, emblazoned in shimmering pyrite, you see the patron saint of this church: vitamin D.
Yes, if you’ve watched this space, then you know that I have little truck with the wildly popular supplement. In all of clinical research, I believe that there is no molecule with stronger data for correlation and weaker data for causation.
Low serum vitamin D levels have been linked to higher risks for heart disease, cancer, falls, COVID, dementia, C diff, and others. And yet, when we do randomized trials of vitamin D supplementation — the thing that can prove that the low level was causally linked to the outcome of interest — we get negative results.
Trials aren’t perfect, of course, and we’ll talk in a moment about a big one that had some issues. But we are at a point where we need to either be vitamin D apologists, saying, “Forget what those lying RCTs tell you and buy this supplement” — an $800 million-a-year industry, by the way — or conclude that vitamin D levels are a convenient marker of various lifestyle factors that are associated with better outcomes: markers of exercise, getting outside, eating a varied diet.
Or perhaps vitamin D supplements have real effects. It’s just that the beneficial effects are matched by the harmful ones. Stay tuned.
The Women’s Health Initiative remains among the largest randomized trials of vitamin D and calcium supplementation ever conducted — and a major contributor to the negative outcomes of vitamin D trials.
But if you dig into the inclusion and exclusion criteria for this trial, you’ll find that individuals were allowed to continue taking vitamins and supplements while they were in the trial, regardless of their randomization status. In fact, the majority took supplements at baseline, and more took supplements over time.
That means, of course, that people in the placebo group, who were getting sugar pills instead of vitamin D and calcium, may have been taking vitamin D and calcium on the side. That would certainly bias the results of the trial toward the null, which is what the primary analyses showed. To wit, the original analysis of the Women’s Health Initiative trial showed no effect of randomization to vitamin D supplementation on improving cancer or cardiovascular outcomes.
But the Women’s Health Initiative trial started 30 years ago. Today, with the benefit of decades of follow-up, we can re-investigate — and perhaps re-litigate — those findings, courtesy of this study, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women” appearing in Annals of Internal Medicine.
Dr Cynthia Thomson, of the Mel and Enid Zuckerman College of Public Health at the University of Arizona, and colleagues led this updated analysis focused on two findings that had been hinted at, but not statistically confirmed, in other vitamin D studies: a potential for the supplement to reduce the risk for cancer, and a potential for it to increase the risk for heart disease.
The randomized trial itself only lasted 7 years. What we are seeing in this analysis of 36,282 women is outcomes that happened at any time from randomization to the end of 2023 — around 20 years after the randomization to supplementation stopped. But, the researchers would argue, that’s probably okay. Cancer and heart disease take time to develop; we see lung cancer long after people stop smoking. So a history of consistent vitamin D supplementation may indeed be protective — or harmful.
Here are the top-line results. Those randomized to vitamin D and calcium supplementation had a 7% reduction in the rate of death from cancer, driven primarily by a reduction in colorectal cancer. This was statistically significant. Also statistically significant? Those randomized to supplementation had a 6% increase in the rate of death from cardiovascular disease. Put those findings together and what do you get? Stone-cold nothing, in terms of overall mortality.
Okay, you say, but what about all that supplementation that was happening outside of the context of the trial, biasing our results toward the null?
The researchers finally clue us in.
First of all, I’ll tell you that, yes, people who were supplementing outside of the trial had higher baseline vitamin D levels — a median of 54.5 nmol/L vs 32.8 nmol/L. This may be because they were supplementing with vitamin D, but it could also be because people who take supplements tend to do other healthy things — another correlation to add to the great cathedral.
To get a better view of the real effects of randomization, the authors restricted the analysis to just those who did not use outside supplements. If vitamin D supplements help, then these are the people they should help. This group had about a 11% reduction in the incidence of cancer — statistically significant — and a 7% reduction in cancer mortality that did not meet the bar for statistical significance.
There was no increase in cardiovascular disease among this group. But this small effect on cancer was nowhere near enough to significantly reduce the rate of all-cause mortality.
Among those using supplements, vitamin D supplementation didn’t really move the needle on any outcome.
I know what you’re thinking: How many of these women were vitamin D deficient when we got started? These results may simply be telling us that people who have normal vitamin D levels are fine to go without supplementation.
Nearly three fourths of women who were not taking supplements entered the trial with vitamin D levels below the 50 nmol/L cutoff that the authors suggest would qualify for deficiency. Around half of those who used supplements were deficient. And yet, frustratingly, I could not find data on the effect of randomization to supplementation stratified by baseline vitamin D level. I even reached out to Dr Thomson to ask about this. She replied, “We did not stratify on baseline values because the numbers are too small statistically to test this.” Sorry.
In the meantime, I can tell you that for your “average woman,” vitamin D supplementation likely has no effect on mortality. It might modestly reduce the risk for certain cancers while increasing the risk for heart disease (probably through coronary calcification). So, there might be some room for personalization here. Perhaps women with a strong family history of cancer or other risk factors would do better with supplements, and those with a high risk for heart disease would do worse. Seems like a strategy that could be tested in a clinical trial. But maybe we could ask the participants to give up their extracurricular supplement use before they enter the trial. F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his book, How Medicine Works and When It Doesn’t, is available now.