User login
Exercise program boosted physical, but not mental, health in young children with overweight
A defined exercise program significantly improved cardiometabolic health and body composition in children with overweight and obesity, but no effect was seen on mental health, based on data from 92 children.
Childhood obesity is associated with negative health outcomes including type 2 diabetes, cardiovascular disease, and mental health disorders, and exercise is considered essential to treatment, wrote Jairo H. Migueles, PhD, of the University of Granada, Spain, and colleagues. However, the effect on children with obesity and overweight of an exercise program on physical and mental health, including within-individual changes, has not been well studied, they said.
In a study published in JAMA Network Open, the researchers reviewed data from 36 girls and 56 boys with overweight or obesity who were randomized to a 20-week exercise program with aerobic and resistance elements, or waitlisted to serve as controls. The participants ranged in age from 8 to 11 years with a mean age of 10 years. The data were collected between Nov. 1, 2014, and June 30, 2016, as part of a parallel-group randomized clinical trial. The exercise program consisted of three to five 90-minute exercise sessions per week for 20 weeks, and the control children continued their usual routines.
The main cardiometabolic outcomes measured in the study were divided into three categories: body composition, physical fitness, and traditional risk factors (waist circumference, blood lipid levels, glucose levels, insulin levels, and blood pressure).
A cardiometabolic risk score was defined by z score. The researchers also added cardiorespiratory fitness (CRF) to the cardiometabolic risk score. Mental health was assessed using composite standardized scores for psychological well-being and poor mental health.
After 20 weeks, cardiometabolic risk scores decreased by approximately 0.38 standard deviations in the exercise group compared with the control group. In addition, specific measures of cardiometabolic health improved significantly from baseline in the exercise group compared with control children for low-density lipoprotein (change of –7.00 mg/dL), body mass index (–5.9 kg/m2), fat mass index (−0.67), and visceral adipose tissue (31.44 g).
Cardiorespiratory fitness improved by 2.75 laps in the exercise group compared with control children. In addition, significantly more children in the exercise group showed meaningful changes (defined as individual changes of at least 0.2 SDs) compared with control children in measures of fat mass index (37 vs. 17, P < .001) and CRF performance (30 vs. 17, P = .03).
However, no significant effects appeared on mental health outcomes in exercisers, the researchers noted.
The reduction in cardiometabolic score was attributable mainly to improvements in cardiovascular fitness, blood lipid levels, and total and visceral adiposity, the researchers wrote in their discussion. The lack of changes in mental health measures may be a result of the healthy mental state of the children at the study outset, they said. “The null effect on mental health outcomes needs to be further investigated, including, among other things, whether the instruments are sensitive enough to detect changes and whether there is a ceiling effect in young children who might be mentally healthy overall,” they wrote.
The findings were limited by several factors, including the relatively small sample size and lack of blinding for some evaluators. However, the results show the potential of exercise programs to affect meaningful change and improve cardiometabolic health in overweight and obese children, although more research is needed to explore the effects of larger-scale and longer-lasting public health interventions combining exercise and other health behaviors such as diet, the researchers concluded.
Bottom line: Exercise works
The increasing rates of overweight and obesity in children in the United States have “significant downstream consequences that include increased risk of metabolic disease, including diabetes and hypertension, as well as increased rates of anxiety and depression,” Neil Skolnik, MD, professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, said in an interview.
Therefore, the effect of interventions such as exercise training on outcomes is important, he said.
The current study findings are “what you would hope for and expect – improvement in cardiometabolic parameters and fitness,” said Dr. Skolnik. “It was encouraging to see the effect of this relatively short duration of intervention has a clear positive effect on weight, BMI, and cardiometabolic parameters,” he said. “The real benefit, of course, comes from sustaining these habits over a long period of time.”
The lack of improvement in mental health is not surprising given the small study population “who did not have a high rate of mental health problems to begin with,” Dr. Skolnik added.
Barriers to promoting exercise programs for obese and overweight children in primary care are many, Dr. Skolnik said, including “having the motivation and funding to create programs like this so they are readily available to youth.”
However, the key message from the current study is simple and straightforward, according to Dr. Skolnik. “Exercise works! It works to improve fitness, cardiometabolic parameters, and weight control,” he said.
“There is always room for more research,” Dr. Skolnik added. The questions now are not about whether exercise benefits health; they are about figuring out how to implement the known benefits of exercise into daily living for all children, athletes and nonathletes alike, he said. “We need to find nonjudgmental ways to encourage exercise as a part of routine daily healthy living, up there with brushing teeth every day,” he emphasized.
The study was supported by grants from the Spanish Ministry of Economy and Competitiveness and El Fondo Europeo de Desarrollo Regional (FEDER) and by the MCIN (Ministerio de Ciencia e Innovación) / AEI (Agencia Estatal de Investigación. The researchers and Dr. Skolnik had no financial conflicts to disclose. Dr. Skolnik serves on the editorial advisory board of Family Practice News.
A defined exercise program significantly improved cardiometabolic health and body composition in children with overweight and obesity, but no effect was seen on mental health, based on data from 92 children.
Childhood obesity is associated with negative health outcomes including type 2 diabetes, cardiovascular disease, and mental health disorders, and exercise is considered essential to treatment, wrote Jairo H. Migueles, PhD, of the University of Granada, Spain, and colleagues. However, the effect on children with obesity and overweight of an exercise program on physical and mental health, including within-individual changes, has not been well studied, they said.
In a study published in JAMA Network Open, the researchers reviewed data from 36 girls and 56 boys with overweight or obesity who were randomized to a 20-week exercise program with aerobic and resistance elements, or waitlisted to serve as controls. The participants ranged in age from 8 to 11 years with a mean age of 10 years. The data were collected between Nov. 1, 2014, and June 30, 2016, as part of a parallel-group randomized clinical trial. The exercise program consisted of three to five 90-minute exercise sessions per week for 20 weeks, and the control children continued their usual routines.
The main cardiometabolic outcomes measured in the study were divided into three categories: body composition, physical fitness, and traditional risk factors (waist circumference, blood lipid levels, glucose levels, insulin levels, and blood pressure).
A cardiometabolic risk score was defined by z score. The researchers also added cardiorespiratory fitness (CRF) to the cardiometabolic risk score. Mental health was assessed using composite standardized scores for psychological well-being and poor mental health.
After 20 weeks, cardiometabolic risk scores decreased by approximately 0.38 standard deviations in the exercise group compared with the control group. In addition, specific measures of cardiometabolic health improved significantly from baseline in the exercise group compared with control children for low-density lipoprotein (change of –7.00 mg/dL), body mass index (–5.9 kg/m2), fat mass index (−0.67), and visceral adipose tissue (31.44 g).
Cardiorespiratory fitness improved by 2.75 laps in the exercise group compared with control children. In addition, significantly more children in the exercise group showed meaningful changes (defined as individual changes of at least 0.2 SDs) compared with control children in measures of fat mass index (37 vs. 17, P < .001) and CRF performance (30 vs. 17, P = .03).
However, no significant effects appeared on mental health outcomes in exercisers, the researchers noted.
The reduction in cardiometabolic score was attributable mainly to improvements in cardiovascular fitness, blood lipid levels, and total and visceral adiposity, the researchers wrote in their discussion. The lack of changes in mental health measures may be a result of the healthy mental state of the children at the study outset, they said. “The null effect on mental health outcomes needs to be further investigated, including, among other things, whether the instruments are sensitive enough to detect changes and whether there is a ceiling effect in young children who might be mentally healthy overall,” they wrote.
The findings were limited by several factors, including the relatively small sample size and lack of blinding for some evaluators. However, the results show the potential of exercise programs to affect meaningful change and improve cardiometabolic health in overweight and obese children, although more research is needed to explore the effects of larger-scale and longer-lasting public health interventions combining exercise and other health behaviors such as diet, the researchers concluded.
Bottom line: Exercise works
The increasing rates of overweight and obesity in children in the United States have “significant downstream consequences that include increased risk of metabolic disease, including diabetes and hypertension, as well as increased rates of anxiety and depression,” Neil Skolnik, MD, professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, said in an interview.
Therefore, the effect of interventions such as exercise training on outcomes is important, he said.
The current study findings are “what you would hope for and expect – improvement in cardiometabolic parameters and fitness,” said Dr. Skolnik. “It was encouraging to see the effect of this relatively short duration of intervention has a clear positive effect on weight, BMI, and cardiometabolic parameters,” he said. “The real benefit, of course, comes from sustaining these habits over a long period of time.”
The lack of improvement in mental health is not surprising given the small study population “who did not have a high rate of mental health problems to begin with,” Dr. Skolnik added.
Barriers to promoting exercise programs for obese and overweight children in primary care are many, Dr. Skolnik said, including “having the motivation and funding to create programs like this so they are readily available to youth.”
However, the key message from the current study is simple and straightforward, according to Dr. Skolnik. “Exercise works! It works to improve fitness, cardiometabolic parameters, and weight control,” he said.
“There is always room for more research,” Dr. Skolnik added. The questions now are not about whether exercise benefits health; they are about figuring out how to implement the known benefits of exercise into daily living for all children, athletes and nonathletes alike, he said. “We need to find nonjudgmental ways to encourage exercise as a part of routine daily healthy living, up there with brushing teeth every day,” he emphasized.
The study was supported by grants from the Spanish Ministry of Economy and Competitiveness and El Fondo Europeo de Desarrollo Regional (FEDER) and by the MCIN (Ministerio de Ciencia e Innovación) / AEI (Agencia Estatal de Investigación. The researchers and Dr. Skolnik had no financial conflicts to disclose. Dr. Skolnik serves on the editorial advisory board of Family Practice News.
A defined exercise program significantly improved cardiometabolic health and body composition in children with overweight and obesity, but no effect was seen on mental health, based on data from 92 children.
Childhood obesity is associated with negative health outcomes including type 2 diabetes, cardiovascular disease, and mental health disorders, and exercise is considered essential to treatment, wrote Jairo H. Migueles, PhD, of the University of Granada, Spain, and colleagues. However, the effect on children with obesity and overweight of an exercise program on physical and mental health, including within-individual changes, has not been well studied, they said.
In a study published in JAMA Network Open, the researchers reviewed data from 36 girls and 56 boys with overweight or obesity who were randomized to a 20-week exercise program with aerobic and resistance elements, or waitlisted to serve as controls. The participants ranged in age from 8 to 11 years with a mean age of 10 years. The data were collected between Nov. 1, 2014, and June 30, 2016, as part of a parallel-group randomized clinical trial. The exercise program consisted of three to five 90-minute exercise sessions per week for 20 weeks, and the control children continued their usual routines.
The main cardiometabolic outcomes measured in the study were divided into three categories: body composition, physical fitness, and traditional risk factors (waist circumference, blood lipid levels, glucose levels, insulin levels, and blood pressure).
A cardiometabolic risk score was defined by z score. The researchers also added cardiorespiratory fitness (CRF) to the cardiometabolic risk score. Mental health was assessed using composite standardized scores for psychological well-being and poor mental health.
After 20 weeks, cardiometabolic risk scores decreased by approximately 0.38 standard deviations in the exercise group compared with the control group. In addition, specific measures of cardiometabolic health improved significantly from baseline in the exercise group compared with control children for low-density lipoprotein (change of –7.00 mg/dL), body mass index (–5.9 kg/m2), fat mass index (−0.67), and visceral adipose tissue (31.44 g).
Cardiorespiratory fitness improved by 2.75 laps in the exercise group compared with control children. In addition, significantly more children in the exercise group showed meaningful changes (defined as individual changes of at least 0.2 SDs) compared with control children in measures of fat mass index (37 vs. 17, P < .001) and CRF performance (30 vs. 17, P = .03).
However, no significant effects appeared on mental health outcomes in exercisers, the researchers noted.
The reduction in cardiometabolic score was attributable mainly to improvements in cardiovascular fitness, blood lipid levels, and total and visceral adiposity, the researchers wrote in their discussion. The lack of changes in mental health measures may be a result of the healthy mental state of the children at the study outset, they said. “The null effect on mental health outcomes needs to be further investigated, including, among other things, whether the instruments are sensitive enough to detect changes and whether there is a ceiling effect in young children who might be mentally healthy overall,” they wrote.
The findings were limited by several factors, including the relatively small sample size and lack of blinding for some evaluators. However, the results show the potential of exercise programs to affect meaningful change and improve cardiometabolic health in overweight and obese children, although more research is needed to explore the effects of larger-scale and longer-lasting public health interventions combining exercise and other health behaviors such as diet, the researchers concluded.
Bottom line: Exercise works
The increasing rates of overweight and obesity in children in the United States have “significant downstream consequences that include increased risk of metabolic disease, including diabetes and hypertension, as well as increased rates of anxiety and depression,” Neil Skolnik, MD, professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, said in an interview.
Therefore, the effect of interventions such as exercise training on outcomes is important, he said.
The current study findings are “what you would hope for and expect – improvement in cardiometabolic parameters and fitness,” said Dr. Skolnik. “It was encouraging to see the effect of this relatively short duration of intervention has a clear positive effect on weight, BMI, and cardiometabolic parameters,” he said. “The real benefit, of course, comes from sustaining these habits over a long period of time.”
The lack of improvement in mental health is not surprising given the small study population “who did not have a high rate of mental health problems to begin with,” Dr. Skolnik added.
Barriers to promoting exercise programs for obese and overweight children in primary care are many, Dr. Skolnik said, including “having the motivation and funding to create programs like this so they are readily available to youth.”
However, the key message from the current study is simple and straightforward, according to Dr. Skolnik. “Exercise works! It works to improve fitness, cardiometabolic parameters, and weight control,” he said.
“There is always room for more research,” Dr. Skolnik added. The questions now are not about whether exercise benefits health; they are about figuring out how to implement the known benefits of exercise into daily living for all children, athletes and nonathletes alike, he said. “We need to find nonjudgmental ways to encourage exercise as a part of routine daily healthy living, up there with brushing teeth every day,” he emphasized.
The study was supported by grants from the Spanish Ministry of Economy and Competitiveness and El Fondo Europeo de Desarrollo Regional (FEDER) and by the MCIN (Ministerio de Ciencia e Innovación) / AEI (Agencia Estatal de Investigación. The researchers and Dr. Skolnik had no financial conflicts to disclose. Dr. Skolnik serves on the editorial advisory board of Family Practice News.
FROM JAMA NETWORK OPEN
Research casts doubt on value of daily aspirin for healthy adults
Daily use of low-dose aspirin offers no significant protection against stroke and was linked to a higher rate of bleeding in the brain, according to new research published in JAMA.
The research matches other evidence advising that healthy older adults without a history of heart conditions or warning signs of stroke should not take low-dose aspirin.
The findings also support the recommendation from the U.S. Preventive Services Task Force that low-dose aspirin should not be prescribed for preventing a first heart attack or stroke in healthy older adults, The New York Times reported.
“We can be very emphatic that healthy people who are not on aspirin and do not have multiple risk factors should not be starting it now,” said Randall Stafford, MD, of Stanford (Calif.) University, who was not involved in the study, in the Times.
It’s not as clear for others, he said.
“The longer you’ve been on aspirin and the more risk factors you have for heart attacks and strokes, the murkier it gets,” he said.
Some cardiac and stroke experts say daily aspirin should remain part of the regimen for people who have had a heart attack or stroke.
The JAMA report was based on data from a randomized control trial of 19,000 people from Australia and America. Participants were over the age of 70 and did not have heart disease.
The data covered an average of almost 4.7 years and revealed that aspirin lowered the rate of ischemic stroke but not significantly. An ischemic stroke happens when a clot forms in a blood vessel that sends blood to the brain.
There was also a 38% higher rate of brain bleeds for people who took aspirin daily, compared with those who took a placebo.
The Times wrote, “In the past, some doctors regarded aspirin as something of a wonder drug, capable of protecting healthy patients against a future heart attack or stroke. But recent studies have shown that the powerful drug has limited protective power among people who have not yet had such an event, and it comes with dangerous side effects.”
A version of this article first appeared on WebMD.com.
Daily use of low-dose aspirin offers no significant protection against stroke and was linked to a higher rate of bleeding in the brain, according to new research published in JAMA.
The research matches other evidence advising that healthy older adults without a history of heart conditions or warning signs of stroke should not take low-dose aspirin.
The findings also support the recommendation from the U.S. Preventive Services Task Force that low-dose aspirin should not be prescribed for preventing a first heart attack or stroke in healthy older adults, The New York Times reported.
“We can be very emphatic that healthy people who are not on aspirin and do not have multiple risk factors should not be starting it now,” said Randall Stafford, MD, of Stanford (Calif.) University, who was not involved in the study, in the Times.
It’s not as clear for others, he said.
“The longer you’ve been on aspirin and the more risk factors you have for heart attacks and strokes, the murkier it gets,” he said.
Some cardiac and stroke experts say daily aspirin should remain part of the regimen for people who have had a heart attack or stroke.
The JAMA report was based on data from a randomized control trial of 19,000 people from Australia and America. Participants were over the age of 70 and did not have heart disease.
The data covered an average of almost 4.7 years and revealed that aspirin lowered the rate of ischemic stroke but not significantly. An ischemic stroke happens when a clot forms in a blood vessel that sends blood to the brain.
There was also a 38% higher rate of brain bleeds for people who took aspirin daily, compared with those who took a placebo.
The Times wrote, “In the past, some doctors regarded aspirin as something of a wonder drug, capable of protecting healthy patients against a future heart attack or stroke. But recent studies have shown that the powerful drug has limited protective power among people who have not yet had such an event, and it comes with dangerous side effects.”
A version of this article first appeared on WebMD.com.
Daily use of low-dose aspirin offers no significant protection against stroke and was linked to a higher rate of bleeding in the brain, according to new research published in JAMA.
The research matches other evidence advising that healthy older adults without a history of heart conditions or warning signs of stroke should not take low-dose aspirin.
The findings also support the recommendation from the U.S. Preventive Services Task Force that low-dose aspirin should not be prescribed for preventing a first heart attack or stroke in healthy older adults, The New York Times reported.
“We can be very emphatic that healthy people who are not on aspirin and do not have multiple risk factors should not be starting it now,” said Randall Stafford, MD, of Stanford (Calif.) University, who was not involved in the study, in the Times.
It’s not as clear for others, he said.
“The longer you’ve been on aspirin and the more risk factors you have for heart attacks and strokes, the murkier it gets,” he said.
Some cardiac and stroke experts say daily aspirin should remain part of the regimen for people who have had a heart attack or stroke.
The JAMA report was based on data from a randomized control trial of 19,000 people from Australia and America. Participants were over the age of 70 and did not have heart disease.
The data covered an average of almost 4.7 years and revealed that aspirin lowered the rate of ischemic stroke but not significantly. An ischemic stroke happens when a clot forms in a blood vessel that sends blood to the brain.
There was also a 38% higher rate of brain bleeds for people who took aspirin daily, compared with those who took a placebo.
The Times wrote, “In the past, some doctors regarded aspirin as something of a wonder drug, capable of protecting healthy patients against a future heart attack or stroke. But recent studies have shown that the powerful drug has limited protective power among people who have not yet had such an event, and it comes with dangerous side effects.”
A version of this article first appeared on WebMD.com.
FROM JAMA
Stiff arteries may cause metabolic syndrome
New research published in the American Journal of Physiology found that arterial stiffness occurred before the presence of metabolic syndrome. A progressive rise in stiffness was associated with a cumulative increase in risk for the condition among the 3,862 people studied over a 7-year period starting in late adolescence.
Results revealed a notable sex difference: Arterial stiffness increased the risk for metabolic syndrome by 9% for males but only by 1% for females. Males were also five times more likely than females to have metabolic syndrome.
“It seems metabolic syndrome has a new risk factor we haven’t thought about,” said author Andrew O. Agbaje, MD, clinical epidemiologist and researcher, University of Eastern Finland, Kuopio.
Arterial stiffness previously was associated with metabolic syndrome in numerous studies. But the new work is the first to find evidence for causality, Dr. Agbaje said in an interview.
“Interventions have focused on addressing the components of metabolic syndrome such as obesity, dyslipidemia, hyperglycemia, and hypertension,” Dr. Agbaje said. “But arterial stiffness may independently cause metabolic syndrome in 1 out of 10 male teens. I encourage clinicians to think about its role in preventing and managing metabolic syndrome, not just as a consequence but as a cause.”
The results have important implications for physicians, according to Sissi Cossio, MD, pediatric endocrinologist, Pediatrix Medical Group, Fort Lauderdale, Fla.
“The fact that arterial stiffness progression preceded metabolic syndrome is important because it could be used as an earlier detection marker of disease,” Dr. Cossio said.
To conduct the study, Dr. Agbaje and his research team used data collected by the Avon Longitudinal Study of Parents and Children at the University of Bristol in England. Arterial stiffness was measured using carotid-femoral pulse wave velocity, the speed of blood flow from the upper to the lower aorta. They assessed for metabolic syndrome by the presence of three or more risk factors, including high cholesterol, high triglycerides, and high trunk fat mass.
Participants were studied starting in gestation in the early 1990s, and were measured for arterial stiffness and metabolic syndrome starting at age 17 through age 24.
The overall risk for metabolic syndrome doubled within the 7-year study period of follow-up between 2009 and 2017, indicating that early intervention during adolescence is essential.
Dr. Agbaje recommended that physicians start treating arterial stiffness and other markers of metabolic syndrome as early as possible, noting that, “potentially irreversible cardiovascular health damage might occur after age 17.”
Arterial stiffness can be negated through physical activity and dietary changes that lower inflammation. Physicians should refer at-risk teens to a preventative clinic where they can be monitored and receive repeated measurements of arterial stiffness, lipid levels, blood pressure, glucose levels, and obesity every 3 months, Dr. Agbaje said.
“The health progress made after a year would be an indicator for physicians whether a more aggressive therapeutic approach is needed since it takes about 7 years for the risk of metabolic syndrome attributed to arterial stiffness to worsen remarkably in the young population,” he said.
Dr. Agbaje pointed to a few potential pathways through which arterial stiffness might create a disease cascade. Stiffer arteries disrupt blood flow to the liver and pancreas, which could adversely affect their functioning, he said. Damage to these organs may increase insulin and LDL cholesterol blood levels, increasing the risk for metabolic syndrome.
Arterial stiffness also can lead to higher blood pressure and insulin resistance, potentially inducing musculogenesis and vasculogenesis. The resulting excessive muscle mass may also increase the risk for the condition, he said.
Dr. Cossio acknowledged that treatments for metabolic syndrome become less effective with age, but emphasized that reversal is possible in adults with lifestyle changes and medications.
“Early detection will give patients the best chance at reversing the disease, and [primary care physicians] are a key factor in this process,” she said.
Dr. Cossio said that at-risk teens should receive treatment in a weight loss or endocrinology clinic. Treatment may include behavioral, surgical, and pharmacotherapeutic interventions.
“Teens with signs of insulin resistance and impaired fasting glucose, acanthosis, or prediabetes, should start metformin as the first line of therapy,” Dr. Cossio said.
For weight management, she recommends antiobesity medications such as liraglutide, semaglutide, and the combination of phentermine/topiramate in children aged 12 years or older. In teenagers 16 years or older, phentermine alone is another option.
The research group that conducted the study reported received funding from the Jenny and Antti Wihuri Foundation, the North Savo Regional Fund and Central Finnish Cultural Foundation, the Aarne Koskelo Foundation, the Foundation for Pediatric Research, and the Finnish Foundation for Cardiovascular Research, among others. The authors declared no conflicts of interest, financial or otherwise.
A version of this article appeared on Medscape.com.
New research published in the American Journal of Physiology found that arterial stiffness occurred before the presence of metabolic syndrome. A progressive rise in stiffness was associated with a cumulative increase in risk for the condition among the 3,862 people studied over a 7-year period starting in late adolescence.
Results revealed a notable sex difference: Arterial stiffness increased the risk for metabolic syndrome by 9% for males but only by 1% for females. Males were also five times more likely than females to have metabolic syndrome.
“It seems metabolic syndrome has a new risk factor we haven’t thought about,” said author Andrew O. Agbaje, MD, clinical epidemiologist and researcher, University of Eastern Finland, Kuopio.
Arterial stiffness previously was associated with metabolic syndrome in numerous studies. But the new work is the first to find evidence for causality, Dr. Agbaje said in an interview.
“Interventions have focused on addressing the components of metabolic syndrome such as obesity, dyslipidemia, hyperglycemia, and hypertension,” Dr. Agbaje said. “But arterial stiffness may independently cause metabolic syndrome in 1 out of 10 male teens. I encourage clinicians to think about its role in preventing and managing metabolic syndrome, not just as a consequence but as a cause.”
The results have important implications for physicians, according to Sissi Cossio, MD, pediatric endocrinologist, Pediatrix Medical Group, Fort Lauderdale, Fla.
“The fact that arterial stiffness progression preceded metabolic syndrome is important because it could be used as an earlier detection marker of disease,” Dr. Cossio said.
To conduct the study, Dr. Agbaje and his research team used data collected by the Avon Longitudinal Study of Parents and Children at the University of Bristol in England. Arterial stiffness was measured using carotid-femoral pulse wave velocity, the speed of blood flow from the upper to the lower aorta. They assessed for metabolic syndrome by the presence of three or more risk factors, including high cholesterol, high triglycerides, and high trunk fat mass.
Participants were studied starting in gestation in the early 1990s, and were measured for arterial stiffness and metabolic syndrome starting at age 17 through age 24.
The overall risk for metabolic syndrome doubled within the 7-year study period of follow-up between 2009 and 2017, indicating that early intervention during adolescence is essential.
Dr. Agbaje recommended that physicians start treating arterial stiffness and other markers of metabolic syndrome as early as possible, noting that, “potentially irreversible cardiovascular health damage might occur after age 17.”
Arterial stiffness can be negated through physical activity and dietary changes that lower inflammation. Physicians should refer at-risk teens to a preventative clinic where they can be monitored and receive repeated measurements of arterial stiffness, lipid levels, blood pressure, glucose levels, and obesity every 3 months, Dr. Agbaje said.
“The health progress made after a year would be an indicator for physicians whether a more aggressive therapeutic approach is needed since it takes about 7 years for the risk of metabolic syndrome attributed to arterial stiffness to worsen remarkably in the young population,” he said.
Dr. Agbaje pointed to a few potential pathways through which arterial stiffness might create a disease cascade. Stiffer arteries disrupt blood flow to the liver and pancreas, which could adversely affect their functioning, he said. Damage to these organs may increase insulin and LDL cholesterol blood levels, increasing the risk for metabolic syndrome.
Arterial stiffness also can lead to higher blood pressure and insulin resistance, potentially inducing musculogenesis and vasculogenesis. The resulting excessive muscle mass may also increase the risk for the condition, he said.
Dr. Cossio acknowledged that treatments for metabolic syndrome become less effective with age, but emphasized that reversal is possible in adults with lifestyle changes and medications.
“Early detection will give patients the best chance at reversing the disease, and [primary care physicians] are a key factor in this process,” she said.
Dr. Cossio said that at-risk teens should receive treatment in a weight loss or endocrinology clinic. Treatment may include behavioral, surgical, and pharmacotherapeutic interventions.
“Teens with signs of insulin resistance and impaired fasting glucose, acanthosis, or prediabetes, should start metformin as the first line of therapy,” Dr. Cossio said.
For weight management, she recommends antiobesity medications such as liraglutide, semaglutide, and the combination of phentermine/topiramate in children aged 12 years or older. In teenagers 16 years or older, phentermine alone is another option.
The research group that conducted the study reported received funding from the Jenny and Antti Wihuri Foundation, the North Savo Regional Fund and Central Finnish Cultural Foundation, the Aarne Koskelo Foundation, the Foundation for Pediatric Research, and the Finnish Foundation for Cardiovascular Research, among others. The authors declared no conflicts of interest, financial or otherwise.
A version of this article appeared on Medscape.com.
New research published in the American Journal of Physiology found that arterial stiffness occurred before the presence of metabolic syndrome. A progressive rise in stiffness was associated with a cumulative increase in risk for the condition among the 3,862 people studied over a 7-year period starting in late adolescence.
Results revealed a notable sex difference: Arterial stiffness increased the risk for metabolic syndrome by 9% for males but only by 1% for females. Males were also five times more likely than females to have metabolic syndrome.
“It seems metabolic syndrome has a new risk factor we haven’t thought about,” said author Andrew O. Agbaje, MD, clinical epidemiologist and researcher, University of Eastern Finland, Kuopio.
Arterial stiffness previously was associated with metabolic syndrome in numerous studies. But the new work is the first to find evidence for causality, Dr. Agbaje said in an interview.
“Interventions have focused on addressing the components of metabolic syndrome such as obesity, dyslipidemia, hyperglycemia, and hypertension,” Dr. Agbaje said. “But arterial stiffness may independently cause metabolic syndrome in 1 out of 10 male teens. I encourage clinicians to think about its role in preventing and managing metabolic syndrome, not just as a consequence but as a cause.”
The results have important implications for physicians, according to Sissi Cossio, MD, pediatric endocrinologist, Pediatrix Medical Group, Fort Lauderdale, Fla.
“The fact that arterial stiffness progression preceded metabolic syndrome is important because it could be used as an earlier detection marker of disease,” Dr. Cossio said.
To conduct the study, Dr. Agbaje and his research team used data collected by the Avon Longitudinal Study of Parents and Children at the University of Bristol in England. Arterial stiffness was measured using carotid-femoral pulse wave velocity, the speed of blood flow from the upper to the lower aorta. They assessed for metabolic syndrome by the presence of three or more risk factors, including high cholesterol, high triglycerides, and high trunk fat mass.
Participants were studied starting in gestation in the early 1990s, and were measured for arterial stiffness and metabolic syndrome starting at age 17 through age 24.
The overall risk for metabolic syndrome doubled within the 7-year study period of follow-up between 2009 and 2017, indicating that early intervention during adolescence is essential.
Dr. Agbaje recommended that physicians start treating arterial stiffness and other markers of metabolic syndrome as early as possible, noting that, “potentially irreversible cardiovascular health damage might occur after age 17.”
Arterial stiffness can be negated through physical activity and dietary changes that lower inflammation. Physicians should refer at-risk teens to a preventative clinic where they can be monitored and receive repeated measurements of arterial stiffness, lipid levels, blood pressure, glucose levels, and obesity every 3 months, Dr. Agbaje said.
“The health progress made after a year would be an indicator for physicians whether a more aggressive therapeutic approach is needed since it takes about 7 years for the risk of metabolic syndrome attributed to arterial stiffness to worsen remarkably in the young population,” he said.
Dr. Agbaje pointed to a few potential pathways through which arterial stiffness might create a disease cascade. Stiffer arteries disrupt blood flow to the liver and pancreas, which could adversely affect their functioning, he said. Damage to these organs may increase insulin and LDL cholesterol blood levels, increasing the risk for metabolic syndrome.
Arterial stiffness also can lead to higher blood pressure and insulin resistance, potentially inducing musculogenesis and vasculogenesis. The resulting excessive muscle mass may also increase the risk for the condition, he said.
Dr. Cossio acknowledged that treatments for metabolic syndrome become less effective with age, but emphasized that reversal is possible in adults with lifestyle changes and medications.
“Early detection will give patients the best chance at reversing the disease, and [primary care physicians] are a key factor in this process,” she said.
Dr. Cossio said that at-risk teens should receive treatment in a weight loss or endocrinology clinic. Treatment may include behavioral, surgical, and pharmacotherapeutic interventions.
“Teens with signs of insulin resistance and impaired fasting glucose, acanthosis, or prediabetes, should start metformin as the first line of therapy,” Dr. Cossio said.
For weight management, she recommends antiobesity medications such as liraglutide, semaglutide, and the combination of phentermine/topiramate in children aged 12 years or older. In teenagers 16 years or older, phentermine alone is another option.
The research group that conducted the study reported received funding from the Jenny and Antti Wihuri Foundation, the North Savo Regional Fund and Central Finnish Cultural Foundation, the Aarne Koskelo Foundation, the Foundation for Pediatric Research, and the Finnish Foundation for Cardiovascular Research, among others. The authors declared no conflicts of interest, financial or otherwise.
A version of this article appeared on Medscape.com.
FROM AMERICAN JOURNAL OF PHYSIOLOGY
Vegetarian diets can improve high-risk cardiovascular disease
, a meta-analysis of randomized controlled trials shows.
“To the best of our knowledge, this meta-analysis is the first that generates evidence from randomized controlled trials to assess the association of vegetarian diets with outcomes in people affected by cardiovascular diseases,” report the authors. The study was published online in JAMA Network Open.
“The greatest improvements in hemoglobin A1c and low-density lipoprotein cholesterol (LDL-C) were observed in individuals with type 2 diabetes and people at high risk of cardiovascular disease, highlighting the potential protective and synergistic effects of vegetarian diets for the primary prevention of cardiovascular disease,” they say.
Poor diet is well-established as increasing the morbidity and mortality associated with cardiovascular disease; however, although data has linked vegetarian diets to cardiovascular disease prevention in the general population, research on the effectiveness of such diets in people at high risk of cardiovascular disease is lacking.
“To the best of our knowledge, no meta-analysis of randomized controlled trials has been conducted to investigate the association of vegetarian diets with outcomes among people with CVD – indeed, research here has primarily focused on observational studies,” writes Tian Wang, RD, and colleagues at the University of Sydney.
Greater decreases in LDL-C, A1c, and body weight with vegetarian diets
For the meta-analysis, researchers identified 20 randomized controlled trials involving vegetarian diets that included 1,878 adults with or at a high risk of cardiovascular disease and included measurements of LDL-C, A1c, or systolic blood pressure.
The studies were conducted in the United States, Asia, Europe, and New Zealand between 1990 and 2021. Sample sizes ranged from 12 to 291 participants.
The mean range age of participants was 28-64 years. Studies included patients with cardiovascular disease (four studies), diabetes (seven studies), and those with at least two cardiovascular risk factors (nine studies).
The mean duration of the dietary intervention was 25.4 weeks (range 2-24 months). The most commonly prescribed diets were vegan (plant-based foods only), lacto-ovo-vegetarian (excluded meat, poultry, seafood, and dairy products, but allowed eggs), and lacto-vegetarian (same as previous but allowed dairy products).
Overall, those who consumed a vegetarian diet for an average of 6 months, versus comparison diets, had significantly greater decreases in LDL-C (6.6 mg/dL beyond the reduction achieved with standard therapy); A1c (0.24%); and body weight (3.4 kg), but the reduction in systolic blood pressure (0.1 mmHg) was not significantly greater.
Assessment of the overall certainty of evidence evaluated using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) tool showed a moderate level of evidence for reductions in LDL-C and A1c with the vegetarian diet.
Lacto-ovo vegetarian diets were associated with the greatest reduction in LDL-C (14.1 mg/dL); however, four out of the five trials restricted energy intake.
Of note, vegetarian diets were most effective for achieving glycemic control among people with type 2 diabetes and leading to improvements in weight among those at high risk of cardiovascular disease as well as those with type 2 diabetes.
The effects “suggest that vegetarian diets might have a synergistic [or at least nonantagonistic] use in potentiating the effects of optimal drug therapy in the prevention and treatment of a range of cardiometabolic diseases,” the authors write.
Although previous studies have shown similar improvements associated with a vegetarian diet, most studies did not stratify populations based on disease status, type of vegetarian diet, or comparison diet, the authors note.
The lack of improvement in systolic blood pressure is consistent with previous meta-analyses of vegetarian diets in general and suggests that salt intake may be the more important factor for those measures.
“[The meta-analysis] suggests that diet quality plays a major role in lowering blood pressure independent of animal food consumption, as the DASH [Dietary Approaches to Stop Hypertension] ... trial demonstrated,” the authors note.
Decreases in medication dose with vegetarian diet
Although most patients were taking medications to manage hypertension, hyperglycemia, and/or dyslipidemia at trial enrollment in as many as eight of the studies, the vegetarian diet intervention resulted in a decrease in medication dose.
In fact, medication use could obscure the favorable effects of vegetarian diets, which could have a larger effect size, the authors speculate.
“This hypothesis is supported by two randomized controlled trials in our meta-analysis that required patients not to take medication that could influence cardiometabolic outcomes, [and] these studies significantly improved systolic blood pressure and LDL-C,” they write.
Not all vegetarian diets are healthy
Although there are numerous variations in vegetarian diets, ranging from vegan diets that eliminate all animal food to pesco-vegetarian diets that allow fish or seafood, most that are well-balanced can provide health benefits including lower saturated fat, L-carnitine, and choline (precursors of the atherogenic TMAO), and other benefits that might explain the improvements seen in the meta-analysis.
The diets may also be high in dietary fiber, mono- and polyunsaturated fatty acids, potassium, magnesium, and phytochemical, and have lower glycemic index scores.
Of note, 12 studies in the meta-analysis emphasized low-fat content, which the authors speculate may have contributed to the improvements observed in LDC-C.
Specifically, lacto-ovo vegetarian diets were associated with the greatest reduction in LDL-C (–14.1 mg/dL); however, four out of five of the trials restricted energy intake, which could have also played a role in improvements.
Importantly, not all vegetarian diets are healthy, and the authors caution about some that allow, for instance, deep-fried foods rich in trans-fatty acids and salt, such as tempura vegetables, potentially increasing the risk of type 2 diabetes and coronary heart disease.
They note that “more than one-third of the studies included in our meta-analysis did not emphasize the importance of consuming minimally processed plant-based whole foods.”
Overall, however, the fact that the greatest improvements in A1c and LDL-C were seen in patients with type 2 diabetes and those at high risk of CVD “highlight[s] the potential protective and synergistic effects of vegetarian diets for the primary prevention of CVD.”
A version of this article first appeared on Medscape.com.
, a meta-analysis of randomized controlled trials shows.
“To the best of our knowledge, this meta-analysis is the first that generates evidence from randomized controlled trials to assess the association of vegetarian diets with outcomes in people affected by cardiovascular diseases,” report the authors. The study was published online in JAMA Network Open.
“The greatest improvements in hemoglobin A1c and low-density lipoprotein cholesterol (LDL-C) were observed in individuals with type 2 diabetes and people at high risk of cardiovascular disease, highlighting the potential protective and synergistic effects of vegetarian diets for the primary prevention of cardiovascular disease,” they say.
Poor diet is well-established as increasing the morbidity and mortality associated with cardiovascular disease; however, although data has linked vegetarian diets to cardiovascular disease prevention in the general population, research on the effectiveness of such diets in people at high risk of cardiovascular disease is lacking.
“To the best of our knowledge, no meta-analysis of randomized controlled trials has been conducted to investigate the association of vegetarian diets with outcomes among people with CVD – indeed, research here has primarily focused on observational studies,” writes Tian Wang, RD, and colleagues at the University of Sydney.
Greater decreases in LDL-C, A1c, and body weight with vegetarian diets
For the meta-analysis, researchers identified 20 randomized controlled trials involving vegetarian diets that included 1,878 adults with or at a high risk of cardiovascular disease and included measurements of LDL-C, A1c, or systolic blood pressure.
The studies were conducted in the United States, Asia, Europe, and New Zealand between 1990 and 2021. Sample sizes ranged from 12 to 291 participants.
The mean range age of participants was 28-64 years. Studies included patients with cardiovascular disease (four studies), diabetes (seven studies), and those with at least two cardiovascular risk factors (nine studies).
The mean duration of the dietary intervention was 25.4 weeks (range 2-24 months). The most commonly prescribed diets were vegan (plant-based foods only), lacto-ovo-vegetarian (excluded meat, poultry, seafood, and dairy products, but allowed eggs), and lacto-vegetarian (same as previous but allowed dairy products).
Overall, those who consumed a vegetarian diet for an average of 6 months, versus comparison diets, had significantly greater decreases in LDL-C (6.6 mg/dL beyond the reduction achieved with standard therapy); A1c (0.24%); and body weight (3.4 kg), but the reduction in systolic blood pressure (0.1 mmHg) was not significantly greater.
Assessment of the overall certainty of evidence evaluated using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) tool showed a moderate level of evidence for reductions in LDL-C and A1c with the vegetarian diet.
Lacto-ovo vegetarian diets were associated with the greatest reduction in LDL-C (14.1 mg/dL); however, four out of the five trials restricted energy intake.
Of note, vegetarian diets were most effective for achieving glycemic control among people with type 2 diabetes and leading to improvements in weight among those at high risk of cardiovascular disease as well as those with type 2 diabetes.
The effects “suggest that vegetarian diets might have a synergistic [or at least nonantagonistic] use in potentiating the effects of optimal drug therapy in the prevention and treatment of a range of cardiometabolic diseases,” the authors write.
Although previous studies have shown similar improvements associated with a vegetarian diet, most studies did not stratify populations based on disease status, type of vegetarian diet, or comparison diet, the authors note.
The lack of improvement in systolic blood pressure is consistent with previous meta-analyses of vegetarian diets in general and suggests that salt intake may be the more important factor for those measures.
“[The meta-analysis] suggests that diet quality plays a major role in lowering blood pressure independent of animal food consumption, as the DASH [Dietary Approaches to Stop Hypertension] ... trial demonstrated,” the authors note.
Decreases in medication dose with vegetarian diet
Although most patients were taking medications to manage hypertension, hyperglycemia, and/or dyslipidemia at trial enrollment in as many as eight of the studies, the vegetarian diet intervention resulted in a decrease in medication dose.
In fact, medication use could obscure the favorable effects of vegetarian diets, which could have a larger effect size, the authors speculate.
“This hypothesis is supported by two randomized controlled trials in our meta-analysis that required patients not to take medication that could influence cardiometabolic outcomes, [and] these studies significantly improved systolic blood pressure and LDL-C,” they write.
Not all vegetarian diets are healthy
Although there are numerous variations in vegetarian diets, ranging from vegan diets that eliminate all animal food to pesco-vegetarian diets that allow fish or seafood, most that are well-balanced can provide health benefits including lower saturated fat, L-carnitine, and choline (precursors of the atherogenic TMAO), and other benefits that might explain the improvements seen in the meta-analysis.
The diets may also be high in dietary fiber, mono- and polyunsaturated fatty acids, potassium, magnesium, and phytochemical, and have lower glycemic index scores.
Of note, 12 studies in the meta-analysis emphasized low-fat content, which the authors speculate may have contributed to the improvements observed in LDC-C.
Specifically, lacto-ovo vegetarian diets were associated with the greatest reduction in LDL-C (–14.1 mg/dL); however, four out of five of the trials restricted energy intake, which could have also played a role in improvements.
Importantly, not all vegetarian diets are healthy, and the authors caution about some that allow, for instance, deep-fried foods rich in trans-fatty acids and salt, such as tempura vegetables, potentially increasing the risk of type 2 diabetes and coronary heart disease.
They note that “more than one-third of the studies included in our meta-analysis did not emphasize the importance of consuming minimally processed plant-based whole foods.”
Overall, however, the fact that the greatest improvements in A1c and LDL-C were seen in patients with type 2 diabetes and those at high risk of CVD “highlight[s] the potential protective and synergistic effects of vegetarian diets for the primary prevention of CVD.”
A version of this article first appeared on Medscape.com.
, a meta-analysis of randomized controlled trials shows.
“To the best of our knowledge, this meta-analysis is the first that generates evidence from randomized controlled trials to assess the association of vegetarian diets with outcomes in people affected by cardiovascular diseases,” report the authors. The study was published online in JAMA Network Open.
“The greatest improvements in hemoglobin A1c and low-density lipoprotein cholesterol (LDL-C) were observed in individuals with type 2 diabetes and people at high risk of cardiovascular disease, highlighting the potential protective and synergistic effects of vegetarian diets for the primary prevention of cardiovascular disease,” they say.
Poor diet is well-established as increasing the morbidity and mortality associated with cardiovascular disease; however, although data has linked vegetarian diets to cardiovascular disease prevention in the general population, research on the effectiveness of such diets in people at high risk of cardiovascular disease is lacking.
“To the best of our knowledge, no meta-analysis of randomized controlled trials has been conducted to investigate the association of vegetarian diets with outcomes among people with CVD – indeed, research here has primarily focused on observational studies,” writes Tian Wang, RD, and colleagues at the University of Sydney.
Greater decreases in LDL-C, A1c, and body weight with vegetarian diets
For the meta-analysis, researchers identified 20 randomized controlled trials involving vegetarian diets that included 1,878 adults with or at a high risk of cardiovascular disease and included measurements of LDL-C, A1c, or systolic blood pressure.
The studies were conducted in the United States, Asia, Europe, and New Zealand between 1990 and 2021. Sample sizes ranged from 12 to 291 participants.
The mean range age of participants was 28-64 years. Studies included patients with cardiovascular disease (four studies), diabetes (seven studies), and those with at least two cardiovascular risk factors (nine studies).
The mean duration of the dietary intervention was 25.4 weeks (range 2-24 months). The most commonly prescribed diets were vegan (plant-based foods only), lacto-ovo-vegetarian (excluded meat, poultry, seafood, and dairy products, but allowed eggs), and lacto-vegetarian (same as previous but allowed dairy products).
Overall, those who consumed a vegetarian diet for an average of 6 months, versus comparison diets, had significantly greater decreases in LDL-C (6.6 mg/dL beyond the reduction achieved with standard therapy); A1c (0.24%); and body weight (3.4 kg), but the reduction in systolic blood pressure (0.1 mmHg) was not significantly greater.
Assessment of the overall certainty of evidence evaluated using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) tool showed a moderate level of evidence for reductions in LDL-C and A1c with the vegetarian diet.
Lacto-ovo vegetarian diets were associated with the greatest reduction in LDL-C (14.1 mg/dL); however, four out of the five trials restricted energy intake.
Of note, vegetarian diets were most effective for achieving glycemic control among people with type 2 diabetes and leading to improvements in weight among those at high risk of cardiovascular disease as well as those with type 2 diabetes.
The effects “suggest that vegetarian diets might have a synergistic [or at least nonantagonistic] use in potentiating the effects of optimal drug therapy in the prevention and treatment of a range of cardiometabolic diseases,” the authors write.
Although previous studies have shown similar improvements associated with a vegetarian diet, most studies did not stratify populations based on disease status, type of vegetarian diet, or comparison diet, the authors note.
The lack of improvement in systolic blood pressure is consistent with previous meta-analyses of vegetarian diets in general and suggests that salt intake may be the more important factor for those measures.
“[The meta-analysis] suggests that diet quality plays a major role in lowering blood pressure independent of animal food consumption, as the DASH [Dietary Approaches to Stop Hypertension] ... trial demonstrated,” the authors note.
Decreases in medication dose with vegetarian diet
Although most patients were taking medications to manage hypertension, hyperglycemia, and/or dyslipidemia at trial enrollment in as many as eight of the studies, the vegetarian diet intervention resulted in a decrease in medication dose.
In fact, medication use could obscure the favorable effects of vegetarian diets, which could have a larger effect size, the authors speculate.
“This hypothesis is supported by two randomized controlled trials in our meta-analysis that required patients not to take medication that could influence cardiometabolic outcomes, [and] these studies significantly improved systolic blood pressure and LDL-C,” they write.
Not all vegetarian diets are healthy
Although there are numerous variations in vegetarian diets, ranging from vegan diets that eliminate all animal food to pesco-vegetarian diets that allow fish or seafood, most that are well-balanced can provide health benefits including lower saturated fat, L-carnitine, and choline (precursors of the atherogenic TMAO), and other benefits that might explain the improvements seen in the meta-analysis.
The diets may also be high in dietary fiber, mono- and polyunsaturated fatty acids, potassium, magnesium, and phytochemical, and have lower glycemic index scores.
Of note, 12 studies in the meta-analysis emphasized low-fat content, which the authors speculate may have contributed to the improvements observed in LDC-C.
Specifically, lacto-ovo vegetarian diets were associated with the greatest reduction in LDL-C (–14.1 mg/dL); however, four out of five of the trials restricted energy intake, which could have also played a role in improvements.
Importantly, not all vegetarian diets are healthy, and the authors caution about some that allow, for instance, deep-fried foods rich in trans-fatty acids and salt, such as tempura vegetables, potentially increasing the risk of type 2 diabetes and coronary heart disease.
They note that “more than one-third of the studies included in our meta-analysis did not emphasize the importance of consuming minimally processed plant-based whole foods.”
Overall, however, the fact that the greatest improvements in A1c and LDL-C were seen in patients with type 2 diabetes and those at high risk of CVD “highlight[s] the potential protective and synergistic effects of vegetarian diets for the primary prevention of CVD.”
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Over-the-counter switches improve access but come with risks
On July 13, the Food and Drug Administration approved the first over-the-counter (OTC) norgestrel birth control pill (Opill). The daily oral contraceptive was approved for prescription use 5 decades ago, providing regulators with a half-century of data to show that the progestin-only drug can be used safely without a prescription.
The drug is the latest in a series of medications that have made the switch from behind the pharmacy counter to retail shelves.
Why switch?
When a drug manufacturer submits a proposal for a switch to OTC, the key question that the FDA considers is patient safety. Some risks can be mitigated by approving OTC drugs at lower doses than what is available as the prescription version.
“There is no drug that doesn’t have risks,” said Almut G. Winterstein, RPh, PhD, a distinguished professor in pharmaceutical outcomes and policy and director of the Center for Drug Evaluation and Safety at the University of Florida, Gainesville. “Risks are mitigated by putting specific constraints around access to those medications.”
Dr. Winterstein, a former chair of the FDA’s Drug Safety and Risk Management Advisory Committee, said that nonprescription drugs are unnecessary in a functional health care system.
Many patients may struggle with accessing health clinicians, so making medications available OTC fills gaps left by not being able to get a prescription, according to Dr. Winterstein.
A 2012 paper funded by the Consumer Healthcare Products Association (CHPA), the organization representing manufacturers and distributors of OTC medications, estimated that one quarter of people who bought OTC drugs would not otherwise seek treatment if these treatments were available only via prescription. The CHPA notes that the number of those who experience allergies who use nonprescription antihistamines and allergy-relief drugs increased by about 10% between 2009 and 2015.
Cholesterol drugs
Approximately 80 million U.S. adults are eligible for cholesterol-lowering medications, particularly statins, but nearly half don’t take them, according to the Centers for Disease Control and Prevention.
Fear of side effects is the most common reason people might avoid taking these drugs. But eliminating the need for a refill may encourage uptake of the statins.
“It’s refill, refill, refill,” said Allen J. Taylor, MD, chairman of cardiology at MedStar Heart and Vascular Institute, in Washington. “We spend a ton of time refilling statins and it’s a headache for patients, too.”
The need to secure regular prescriptions for the drug, “doesn’t put enough trust and faith in pharmacists and doesn’t put enough trust and faith in patients,” Dr. Taylor said.
Moving statins to the front end of a pharmacy might not be the best move given the potential for drug interactions, but a nonprescription behind-the-counter approach could work, according to Dr. Taylor.
“The concerns are modest at most, to where they can be monitored by a pharmacist,” he said. “There’s probably more people that would take a statin if they had that kind of access.”
Many statin manufacturers have attempted to make the prescription-to-OTC switch. In 2005, an FDA advisory panel rejected Merck’s proposal for OTC sales of lovastatin after reviewing a study that found only 55% of OTC purchases would have been medically appropriate.
In 2015, Pfizer pulled its application to make the cholesterol drug atorvastatin available to patients OTC because patients were not using the drug correctly. AstraZeneca is investigating an online platform that would allow patients to self-assess their eligibility for rosuvastatin.
Asthma inhalers
Inhalers are the main rescue therapy for asthma aside from a visit to the ED.
The only inhaler available OTC is epinephrine sold under the brand name Primatene Mist, but this type of medicine device is not recommended as a first-line therapy for acute asthma symptoms, according to the American Medical Association.
“It’s been around for a long time and has stayed over the counter even though newer, safer agents have come onto the market which aren’t available over the counter,” said William B. Feldman, MD, DPhil, MPH, a pulmonologist at Brigham and Women’s Hospital, Boston.
Patients who have a hard time getting to a doctor or patients who lack insurance often face barriers accessing albuterol inhalers and beta agonist–corticosteroid combinations, according to Dr. Feldman. A switch to OTC distribution would widen access.
“What we’re advocating is, if they’re going to have access to Primatene Mist, wouldn’t it be sensible to have access to a safer and more effective therapy?” Dr. Feldman said.
Triptans
Migraines affect an estimated 39 million people in the United States, according to the American Migraine Foundation. Several drugs to treat migraine are available OTC, including nonsteroidal anti-inflammatory drugs, aspirin, and acetaminophen. Triptans, drugs used for the short-term treatment of acute symptoms, are prescription-only in the United States.
But in the United Kingdom, triptans first became available in retail stores in 2006, leading to reduced costs for patients, employers, and the government. One study found that government health expenditures would be reduced by $84 million annually if the OTC switch were made in six European countries.
However, overuse of the drug and potential contraindications have been cited as concerns with OTC access.
For Dr. Winterstein, the decision to switch isn’t just about the freedom to buy a drug; it comes down to weighing potential risks and benefits.
“Drugs are only as good as if they’re used in the context of how they should be used,” Dr. Winterstein said. “It’s not candy.”
A version of this article first appeared on Medscape.com.
On July 13, the Food and Drug Administration approved the first over-the-counter (OTC) norgestrel birth control pill (Opill). The daily oral contraceptive was approved for prescription use 5 decades ago, providing regulators with a half-century of data to show that the progestin-only drug can be used safely without a prescription.
The drug is the latest in a series of medications that have made the switch from behind the pharmacy counter to retail shelves.
Why switch?
When a drug manufacturer submits a proposal for a switch to OTC, the key question that the FDA considers is patient safety. Some risks can be mitigated by approving OTC drugs at lower doses than what is available as the prescription version.
“There is no drug that doesn’t have risks,” said Almut G. Winterstein, RPh, PhD, a distinguished professor in pharmaceutical outcomes and policy and director of the Center for Drug Evaluation and Safety at the University of Florida, Gainesville. “Risks are mitigated by putting specific constraints around access to those medications.”
Dr. Winterstein, a former chair of the FDA’s Drug Safety and Risk Management Advisory Committee, said that nonprescription drugs are unnecessary in a functional health care system.
Many patients may struggle with accessing health clinicians, so making medications available OTC fills gaps left by not being able to get a prescription, according to Dr. Winterstein.
A 2012 paper funded by the Consumer Healthcare Products Association (CHPA), the organization representing manufacturers and distributors of OTC medications, estimated that one quarter of people who bought OTC drugs would not otherwise seek treatment if these treatments were available only via prescription. The CHPA notes that the number of those who experience allergies who use nonprescription antihistamines and allergy-relief drugs increased by about 10% between 2009 and 2015.
Cholesterol drugs
Approximately 80 million U.S. adults are eligible for cholesterol-lowering medications, particularly statins, but nearly half don’t take them, according to the Centers for Disease Control and Prevention.
Fear of side effects is the most common reason people might avoid taking these drugs. But eliminating the need for a refill may encourage uptake of the statins.
“It’s refill, refill, refill,” said Allen J. Taylor, MD, chairman of cardiology at MedStar Heart and Vascular Institute, in Washington. “We spend a ton of time refilling statins and it’s a headache for patients, too.”
The need to secure regular prescriptions for the drug, “doesn’t put enough trust and faith in pharmacists and doesn’t put enough trust and faith in patients,” Dr. Taylor said.
Moving statins to the front end of a pharmacy might not be the best move given the potential for drug interactions, but a nonprescription behind-the-counter approach could work, according to Dr. Taylor.
“The concerns are modest at most, to where they can be monitored by a pharmacist,” he said. “There’s probably more people that would take a statin if they had that kind of access.”
Many statin manufacturers have attempted to make the prescription-to-OTC switch. In 2005, an FDA advisory panel rejected Merck’s proposal for OTC sales of lovastatin after reviewing a study that found only 55% of OTC purchases would have been medically appropriate.
In 2015, Pfizer pulled its application to make the cholesterol drug atorvastatin available to patients OTC because patients were not using the drug correctly. AstraZeneca is investigating an online platform that would allow patients to self-assess their eligibility for rosuvastatin.
Asthma inhalers
Inhalers are the main rescue therapy for asthma aside from a visit to the ED.
The only inhaler available OTC is epinephrine sold under the brand name Primatene Mist, but this type of medicine device is not recommended as a first-line therapy for acute asthma symptoms, according to the American Medical Association.
“It’s been around for a long time and has stayed over the counter even though newer, safer agents have come onto the market which aren’t available over the counter,” said William B. Feldman, MD, DPhil, MPH, a pulmonologist at Brigham and Women’s Hospital, Boston.
Patients who have a hard time getting to a doctor or patients who lack insurance often face barriers accessing albuterol inhalers and beta agonist–corticosteroid combinations, according to Dr. Feldman. A switch to OTC distribution would widen access.
“What we’re advocating is, if they’re going to have access to Primatene Mist, wouldn’t it be sensible to have access to a safer and more effective therapy?” Dr. Feldman said.
Triptans
Migraines affect an estimated 39 million people in the United States, according to the American Migraine Foundation. Several drugs to treat migraine are available OTC, including nonsteroidal anti-inflammatory drugs, aspirin, and acetaminophen. Triptans, drugs used for the short-term treatment of acute symptoms, are prescription-only in the United States.
But in the United Kingdom, triptans first became available in retail stores in 2006, leading to reduced costs for patients, employers, and the government. One study found that government health expenditures would be reduced by $84 million annually if the OTC switch were made in six European countries.
However, overuse of the drug and potential contraindications have been cited as concerns with OTC access.
For Dr. Winterstein, the decision to switch isn’t just about the freedom to buy a drug; it comes down to weighing potential risks and benefits.
“Drugs are only as good as if they’re used in the context of how they should be used,” Dr. Winterstein said. “It’s not candy.”
A version of this article first appeared on Medscape.com.
On July 13, the Food and Drug Administration approved the first over-the-counter (OTC) norgestrel birth control pill (Opill). The daily oral contraceptive was approved for prescription use 5 decades ago, providing regulators with a half-century of data to show that the progestin-only drug can be used safely without a prescription.
The drug is the latest in a series of medications that have made the switch from behind the pharmacy counter to retail shelves.
Why switch?
When a drug manufacturer submits a proposal for a switch to OTC, the key question that the FDA considers is patient safety. Some risks can be mitigated by approving OTC drugs at lower doses than what is available as the prescription version.
“There is no drug that doesn’t have risks,” said Almut G. Winterstein, RPh, PhD, a distinguished professor in pharmaceutical outcomes and policy and director of the Center for Drug Evaluation and Safety at the University of Florida, Gainesville. “Risks are mitigated by putting specific constraints around access to those medications.”
Dr. Winterstein, a former chair of the FDA’s Drug Safety and Risk Management Advisory Committee, said that nonprescription drugs are unnecessary in a functional health care system.
Many patients may struggle with accessing health clinicians, so making medications available OTC fills gaps left by not being able to get a prescription, according to Dr. Winterstein.
A 2012 paper funded by the Consumer Healthcare Products Association (CHPA), the organization representing manufacturers and distributors of OTC medications, estimated that one quarter of people who bought OTC drugs would not otherwise seek treatment if these treatments were available only via prescription. The CHPA notes that the number of those who experience allergies who use nonprescription antihistamines and allergy-relief drugs increased by about 10% between 2009 and 2015.
Cholesterol drugs
Approximately 80 million U.S. adults are eligible for cholesterol-lowering medications, particularly statins, but nearly half don’t take them, according to the Centers for Disease Control and Prevention.
Fear of side effects is the most common reason people might avoid taking these drugs. But eliminating the need for a refill may encourage uptake of the statins.
“It’s refill, refill, refill,” said Allen J. Taylor, MD, chairman of cardiology at MedStar Heart and Vascular Institute, in Washington. “We spend a ton of time refilling statins and it’s a headache for patients, too.”
The need to secure regular prescriptions for the drug, “doesn’t put enough trust and faith in pharmacists and doesn’t put enough trust and faith in patients,” Dr. Taylor said.
Moving statins to the front end of a pharmacy might not be the best move given the potential for drug interactions, but a nonprescription behind-the-counter approach could work, according to Dr. Taylor.
“The concerns are modest at most, to where they can be monitored by a pharmacist,” he said. “There’s probably more people that would take a statin if they had that kind of access.”
Many statin manufacturers have attempted to make the prescription-to-OTC switch. In 2005, an FDA advisory panel rejected Merck’s proposal for OTC sales of lovastatin after reviewing a study that found only 55% of OTC purchases would have been medically appropriate.
In 2015, Pfizer pulled its application to make the cholesterol drug atorvastatin available to patients OTC because patients were not using the drug correctly. AstraZeneca is investigating an online platform that would allow patients to self-assess their eligibility for rosuvastatin.
Asthma inhalers
Inhalers are the main rescue therapy for asthma aside from a visit to the ED.
The only inhaler available OTC is epinephrine sold under the brand name Primatene Mist, but this type of medicine device is not recommended as a first-line therapy for acute asthma symptoms, according to the American Medical Association.
“It’s been around for a long time and has stayed over the counter even though newer, safer agents have come onto the market which aren’t available over the counter,” said William B. Feldman, MD, DPhil, MPH, a pulmonologist at Brigham and Women’s Hospital, Boston.
Patients who have a hard time getting to a doctor or patients who lack insurance often face barriers accessing albuterol inhalers and beta agonist–corticosteroid combinations, according to Dr. Feldman. A switch to OTC distribution would widen access.
“What we’re advocating is, if they’re going to have access to Primatene Mist, wouldn’t it be sensible to have access to a safer and more effective therapy?” Dr. Feldman said.
Triptans
Migraines affect an estimated 39 million people in the United States, according to the American Migraine Foundation. Several drugs to treat migraine are available OTC, including nonsteroidal anti-inflammatory drugs, aspirin, and acetaminophen. Triptans, drugs used for the short-term treatment of acute symptoms, are prescription-only in the United States.
But in the United Kingdom, triptans first became available in retail stores in 2006, leading to reduced costs for patients, employers, and the government. One study found that government health expenditures would be reduced by $84 million annually if the OTC switch were made in six European countries.
However, overuse of the drug and potential contraindications have been cited as concerns with OTC access.
For Dr. Winterstein, the decision to switch isn’t just about the freedom to buy a drug; it comes down to weighing potential risks and benefits.
“Drugs are only as good as if they’re used in the context of how they should be used,” Dr. Winterstein said. “It’s not candy.”
A version of this article first appeared on Medscape.com.
30-year-old woman • progressive dyspnea and peripheral edema • 35th week of gestation with a history of mild preeclampsia • Dx?
THE CASE
A 30-year-old woman sought care at her rural family physician’s office for progressive dyspnea and peripheral edema, which she had been experiencing for several weeks. She was G1P0 and in her 35th week of gestation.
Her medical history was remarkable for mild preeclampsia, which was being managed observantly by her obstetrician in consultation with a maternal-fetal medicine specialist. She had been evaluated by her local hospital’s labor and delivery department and her maternal-fetal medicine specialist earlier in the week and seen the previous day by her obstetrician for these signs and symptoms. They all reassured her and told her these symptoms were normal during pregnancy. No diagnostic studies were performed. However, she remained concerned and decided to see her family physician for another opinion.
Upon presentation to her family physician, the patient was afebrile. Her blood pressure was 135/98 mm Hg; heart rate, 96 beats/min; and respiration, 20 breaths/min and slightly labored. Edema of 2 to 3+ was noted in her lower extremities, hands, and face. Bibasilar breath sounds were diminished, and her abdomen was nontender.
The family physician suspected left ventricular systolic dysfunction. He worked in a small office that lacked access to a laboratory or radiographic studies. However, he did have an ultrasound machine available, and although he was not skilled in echocardiography to assess cardiac function, he was able to obtain a bedside lung ultrasound.
THE DIAGNOSIS
While no B-lines were seen on the lung ultrasound, bilateral plural effusions were noted (FIGURE). This finding, paired with the patient’s signs and symptoms, prompted the family physician to suspect a diagnosis of acute decompensated heart failure with presumptive peripartum cardiomyopathy. The patient was immediately driven to the hospital by her family physician for emergency admission with stat obstetric and cardiology consultations.
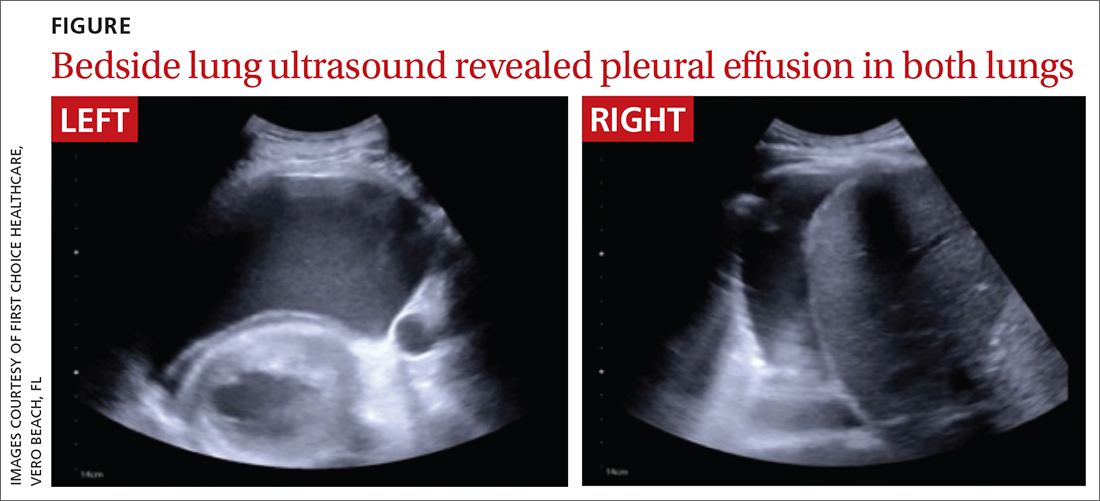
An in-hospital echocardiogram revealed severe global hypokinesia with a left ventricular ejection fraction of 25% to 30%, which confirmed the family physician’s suspicions. Laboratory studies were significant for elevated N-terminal pro-brain natriuretic peptide (43,449 pg/mL; normal, < 125 pg/mL), troponin (1.12 ng/mL; normal range, 0-0.10 ng/mL), and white blood cell count (27.6 x 103/µL). She also had evidence of acute renal injury, with blood urea nitrogen of 46 mg/dL (normal range, 7-18 mg/dL), creatinine of 2.0 mg/dL (normal range, 0.5-1.0 mg/dL), and potassium of 7.6 mmol/L (normal range, 3.5-5.1 mmol/L). Emergency delivery was induced by amniotomy, resulting in the birth of a baby girl weighing 5 lb 4 oz (Apgar scores 6, 8, and 9).
Following delivery, the patient was placed on a milrinone infusion and required dialysis. She was emergently transferred to a tertiary care hospital, where she was admitted to the cardiac intensive care unit by the cardiology/heart transplant service with nephrology and obstetric consultations. Hematology and infectious disease specialists were consulted to rule out HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome and sepsis, respectively. Her course of care remained complicated with further testing, including cardiac catheterization and biopsy, which was negative for additional pathology.
Continue to: One week after admission...
One week after admission, she was discharged home with a 24-hour wearable external cardiac defibrillator and a confirmed diagnosis of peripartum cardiomyopathy. Her medication regimen included digoxin (125 µg 3 times/wk), spironolactone (25 mg/d), carvedilol (3.125 mg twice daily), sacubitril/valsartan (24 mg/26 mg twice daily), furosemide (20 mg/d as needed for weight gain > 3-4 lb or leg swelling), magnesium oxide (400 mg twice daily), and ferrous sulfate (325 mg/d).
DISCUSSION
Peripartum cardiomyopathy is a rare, life-threatening, idiopathic cardiomyopathy that is responsible for one-half to two-thirds of cardiovascular disease–related maternal deaths in the United States.1,2 It manifests in late pregnancy or early in the postpartum period and is characterized by left ventricular systolic dysfunction with resultant heart failure and an ejection fraction of less than 45%.1,2
Recognized as early as the 1800s by Virchow,2,3 the incidence of peripartum cardiomyopathy in the United States ranges from 1 in 1000 to 4000 live births and is increasing worldwide.1,2 While the cause of peripartum cardiomyopathy remains unknown, risk factors include advanced maternal age, African descent, hypertension, preeclampsia, and multiple gestation pregnancy.1,2
Early diagnosis of peripartum cardiomyopathy is imperative for survival of both mother and baby.4 This may be difficult because the signs and symptoms of heart failure—such as dyspnea, edema, orthopnea, cough, and chest and abdominal pain—overlap with those of a typical pregnancy, resulting in it often being missed on evaluation.1,2
Dx with echocardiography; in a pinch, consider lung ultrasound
Usually a diagnosis of peripartum cardiomyopathy is established with echocardiography.1,2 Thus, this case is of significant importance because it illustrates the successful use of lung ultrasound—a simple and easy test—by a rural family doctor to identify this potentially fatal, elusive condition with no additional studies.
Continue to: Use of lung ultrasound...
Use of lung ultrasound in the detection of acute decompensated heart failure is accepted in the medical literature.5-7 Given clinical correlation, a positive scan is defined by the presence of at least 3 B-lines on a longitudinal plane between 2 ribs or, as seen in our case, by the presence of pleural effusion.5-8 Lung ultrasound is readily available worldwide, is completely safe in pregnancy, and is considered one of the easiest studies to perform.7-10
At the patient’s 9-month follow-up visit, she had made a full clinical recovery. Her ejection fraction was 59.8%, and she had stopped all medications. The patient and her child did not experience any continued complications.
THE TAKEAWAY
Family physicians should be aware of peripartum cardiomyopathy—one of the most elusive and life-threatening diseases of pregnancy. When managing a pregnant patient, it is imperative to follow up on complaints such as dyspnea, peripheral edema, and chest and/or abdominal pain. While these symptoms are not unusual during pregnancy, they should always prompt a more thorough evaluation. If peripartum cardiomyopathy is suspected, lung ultrasound is a valuable diagnostic tool for family physicians. Further research is needed before the findings of this case report can be universally applied in the routine prenatal care of women at risk for peripartum cardiomyopathy.
The authors thank their daughter, Nickel Cielo Abarbanell, for her help in the preparation of this manuscript.
CORRESPONDENCE
Neal Robert Abarbanell, MD, First Choice Healthcare, 1867 20th Avenue, Vero Beach, FL 32960; neal.abarbanell@ gmail.com
1. Honigberg MC, Givertz MM. Peripartum cardiomyopathy. BMJ. 2019;364:k5287. doi: 10.1136/bmj.k5287
2. Arany Z, Elkayam U. Peripartum cardiomyopathy. Circulation. 2016;133:1397-1409. doi: 10.1161/CIRCULATIONAHA.115.020491
3. Porak C. De L’influence reciproque de la grossesse et del maladies du Coceur [thesis]. Medical Faculty of Paris, France: 1880.
4. Lewey J, Levine LD, Elovitz MA, et al. Importance of early diagnosis in peripartum cardiomyopathy. Hypertension. 2020;75:91-97. doi: 10.1161/HYPERTENSIONAHA.119.13291
5. Volpicelli G, Caramello V, Cardinale L, et al. Bedside ultrasound of the lung for the monitoring of acute decompensated heart failure. Am J Emerg Med. 2008;26:585-591. doi: 10.1016/j.ajem.2007.09.014
6. Muniz RT, Mesquita ET, Souza CV Jr, et al. Pulmonary ultrasound in patients with heart failure-systematic review. Arq Bras Cardiol. 2018;110:577-584. doi: 10.5935/abc.20180097
7. Russell FM, Rutz M, Pang PS. Focused ultrasound in the emergency department for patients with acute heart failure. Card Fail Rev. 2015;1:83-86. doi: 10.15420/cfr.2015.1.2.83
8. Gustafsson M, Alehagen U, Johansson P. Imaging congestion with a pocket ultrasound device: prognostic implications in patients with chronic heart failure. J Card Fail. 2015;21:548-554. doi: 10.1016/j.cardfail.2015.02.004
9. Ntusi NA, Samuels P, Moosa S, et al. Diagnosing cardiac disease during pregnancy: imaging modalities. Cardiovasc J Afr. 2016;27:95-103. doi: 10.5830/CVJA-2016-022
10. Kimberly HH, Murray A, Mennicke M, et al. Focused maternal ultrasound by midwives in rural Zambia. Ultrasound Med Biol. 2010;36:1267-1272. doi: 10.1016/j.ultrasmedbio.2010.05.017
THE CASE
A 30-year-old woman sought care at her rural family physician’s office for progressive dyspnea and peripheral edema, which she had been experiencing for several weeks. She was G1P0 and in her 35th week of gestation.
Her medical history was remarkable for mild preeclampsia, which was being managed observantly by her obstetrician in consultation with a maternal-fetal medicine specialist. She had been evaluated by her local hospital’s labor and delivery department and her maternal-fetal medicine specialist earlier in the week and seen the previous day by her obstetrician for these signs and symptoms. They all reassured her and told her these symptoms were normal during pregnancy. No diagnostic studies were performed. However, she remained concerned and decided to see her family physician for another opinion.
Upon presentation to her family physician, the patient was afebrile. Her blood pressure was 135/98 mm Hg; heart rate, 96 beats/min; and respiration, 20 breaths/min and slightly labored. Edema of 2 to 3+ was noted in her lower extremities, hands, and face. Bibasilar breath sounds were diminished, and her abdomen was nontender.
The family physician suspected left ventricular systolic dysfunction. He worked in a small office that lacked access to a laboratory or radiographic studies. However, he did have an ultrasound machine available, and although he was not skilled in echocardiography to assess cardiac function, he was able to obtain a bedside lung ultrasound.
THE DIAGNOSIS
While no B-lines were seen on the lung ultrasound, bilateral plural effusions were noted (FIGURE). This finding, paired with the patient’s signs and symptoms, prompted the family physician to suspect a diagnosis of acute decompensated heart failure with presumptive peripartum cardiomyopathy. The patient was immediately driven to the hospital by her family physician for emergency admission with stat obstetric and cardiology consultations.

An in-hospital echocardiogram revealed severe global hypokinesia with a left ventricular ejection fraction of 25% to 30%, which confirmed the family physician’s suspicions. Laboratory studies were significant for elevated N-terminal pro-brain natriuretic peptide (43,449 pg/mL; normal, < 125 pg/mL), troponin (1.12 ng/mL; normal range, 0-0.10 ng/mL), and white blood cell count (27.6 x 103/µL). She also had evidence of acute renal injury, with blood urea nitrogen of 46 mg/dL (normal range, 7-18 mg/dL), creatinine of 2.0 mg/dL (normal range, 0.5-1.0 mg/dL), and potassium of 7.6 mmol/L (normal range, 3.5-5.1 mmol/L). Emergency delivery was induced by amniotomy, resulting in the birth of a baby girl weighing 5 lb 4 oz (Apgar scores 6, 8, and 9).
Following delivery, the patient was placed on a milrinone infusion and required dialysis. She was emergently transferred to a tertiary care hospital, where she was admitted to the cardiac intensive care unit by the cardiology/heart transplant service with nephrology and obstetric consultations. Hematology and infectious disease specialists were consulted to rule out HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome and sepsis, respectively. Her course of care remained complicated with further testing, including cardiac catheterization and biopsy, which was negative for additional pathology.
Continue to: One week after admission...
One week after admission, she was discharged home with a 24-hour wearable external cardiac defibrillator and a confirmed diagnosis of peripartum cardiomyopathy. Her medication regimen included digoxin (125 µg 3 times/wk), spironolactone (25 mg/d), carvedilol (3.125 mg twice daily), sacubitril/valsartan (24 mg/26 mg twice daily), furosemide (20 mg/d as needed for weight gain > 3-4 lb or leg swelling), magnesium oxide (400 mg twice daily), and ferrous sulfate (325 mg/d).
DISCUSSION
Peripartum cardiomyopathy is a rare, life-threatening, idiopathic cardiomyopathy that is responsible for one-half to two-thirds of cardiovascular disease–related maternal deaths in the United States.1,2 It manifests in late pregnancy or early in the postpartum period and is characterized by left ventricular systolic dysfunction with resultant heart failure and an ejection fraction of less than 45%.1,2
Recognized as early as the 1800s by Virchow,2,3 the incidence of peripartum cardiomyopathy in the United States ranges from 1 in 1000 to 4000 live births and is increasing worldwide.1,2 While the cause of peripartum cardiomyopathy remains unknown, risk factors include advanced maternal age, African descent, hypertension, preeclampsia, and multiple gestation pregnancy.1,2
Early diagnosis of peripartum cardiomyopathy is imperative for survival of both mother and baby.4 This may be difficult because the signs and symptoms of heart failure—such as dyspnea, edema, orthopnea, cough, and chest and abdominal pain—overlap with those of a typical pregnancy, resulting in it often being missed on evaluation.1,2
Dx with echocardiography; in a pinch, consider lung ultrasound
Usually a diagnosis of peripartum cardiomyopathy is established with echocardiography.1,2 Thus, this case is of significant importance because it illustrates the successful use of lung ultrasound—a simple and easy test—by a rural family doctor to identify this potentially fatal, elusive condition with no additional studies.
Continue to: Use of lung ultrasound...
Use of lung ultrasound in the detection of acute decompensated heart failure is accepted in the medical literature.5-7 Given clinical correlation, a positive scan is defined by the presence of at least 3 B-lines on a longitudinal plane between 2 ribs or, as seen in our case, by the presence of pleural effusion.5-8 Lung ultrasound is readily available worldwide, is completely safe in pregnancy, and is considered one of the easiest studies to perform.7-10
At the patient’s 9-month follow-up visit, she had made a full clinical recovery. Her ejection fraction was 59.8%, and she had stopped all medications. The patient and her child did not experience any continued complications.
THE TAKEAWAY
Family physicians should be aware of peripartum cardiomyopathy—one of the most elusive and life-threatening diseases of pregnancy. When managing a pregnant patient, it is imperative to follow up on complaints such as dyspnea, peripheral edema, and chest and/or abdominal pain. While these symptoms are not unusual during pregnancy, they should always prompt a more thorough evaluation. If peripartum cardiomyopathy is suspected, lung ultrasound is a valuable diagnostic tool for family physicians. Further research is needed before the findings of this case report can be universally applied in the routine prenatal care of women at risk for peripartum cardiomyopathy.
The authors thank their daughter, Nickel Cielo Abarbanell, for her help in the preparation of this manuscript.
CORRESPONDENCE
Neal Robert Abarbanell, MD, First Choice Healthcare, 1867 20th Avenue, Vero Beach, FL 32960; neal.abarbanell@ gmail.com
THE CASE
A 30-year-old woman sought care at her rural family physician’s office for progressive dyspnea and peripheral edema, which she had been experiencing for several weeks. She was G1P0 and in her 35th week of gestation.
Her medical history was remarkable for mild preeclampsia, which was being managed observantly by her obstetrician in consultation with a maternal-fetal medicine specialist. She had been evaluated by her local hospital’s labor and delivery department and her maternal-fetal medicine specialist earlier in the week and seen the previous day by her obstetrician for these signs and symptoms. They all reassured her and told her these symptoms were normal during pregnancy. No diagnostic studies were performed. However, she remained concerned and decided to see her family physician for another opinion.
Upon presentation to her family physician, the patient was afebrile. Her blood pressure was 135/98 mm Hg; heart rate, 96 beats/min; and respiration, 20 breaths/min and slightly labored. Edema of 2 to 3+ was noted in her lower extremities, hands, and face. Bibasilar breath sounds were diminished, and her abdomen was nontender.
The family physician suspected left ventricular systolic dysfunction. He worked in a small office that lacked access to a laboratory or radiographic studies. However, he did have an ultrasound machine available, and although he was not skilled in echocardiography to assess cardiac function, he was able to obtain a bedside lung ultrasound.
THE DIAGNOSIS
While no B-lines were seen on the lung ultrasound, bilateral plural effusions were noted (FIGURE). This finding, paired with the patient’s signs and symptoms, prompted the family physician to suspect a diagnosis of acute decompensated heart failure with presumptive peripartum cardiomyopathy. The patient was immediately driven to the hospital by her family physician for emergency admission with stat obstetric and cardiology consultations.

An in-hospital echocardiogram revealed severe global hypokinesia with a left ventricular ejection fraction of 25% to 30%, which confirmed the family physician’s suspicions. Laboratory studies were significant for elevated N-terminal pro-brain natriuretic peptide (43,449 pg/mL; normal, < 125 pg/mL), troponin (1.12 ng/mL; normal range, 0-0.10 ng/mL), and white blood cell count (27.6 x 103/µL). She also had evidence of acute renal injury, with blood urea nitrogen of 46 mg/dL (normal range, 7-18 mg/dL), creatinine of 2.0 mg/dL (normal range, 0.5-1.0 mg/dL), and potassium of 7.6 mmol/L (normal range, 3.5-5.1 mmol/L). Emergency delivery was induced by amniotomy, resulting in the birth of a baby girl weighing 5 lb 4 oz (Apgar scores 6, 8, and 9).
Following delivery, the patient was placed on a milrinone infusion and required dialysis. She was emergently transferred to a tertiary care hospital, where she was admitted to the cardiac intensive care unit by the cardiology/heart transplant service with nephrology and obstetric consultations. Hematology and infectious disease specialists were consulted to rule out HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome and sepsis, respectively. Her course of care remained complicated with further testing, including cardiac catheterization and biopsy, which was negative for additional pathology.
Continue to: One week after admission...
One week after admission, she was discharged home with a 24-hour wearable external cardiac defibrillator and a confirmed diagnosis of peripartum cardiomyopathy. Her medication regimen included digoxin (125 µg 3 times/wk), spironolactone (25 mg/d), carvedilol (3.125 mg twice daily), sacubitril/valsartan (24 mg/26 mg twice daily), furosemide (20 mg/d as needed for weight gain > 3-4 lb or leg swelling), magnesium oxide (400 mg twice daily), and ferrous sulfate (325 mg/d).
DISCUSSION
Peripartum cardiomyopathy is a rare, life-threatening, idiopathic cardiomyopathy that is responsible for one-half to two-thirds of cardiovascular disease–related maternal deaths in the United States.1,2 It manifests in late pregnancy or early in the postpartum period and is characterized by left ventricular systolic dysfunction with resultant heart failure and an ejection fraction of less than 45%.1,2
Recognized as early as the 1800s by Virchow,2,3 the incidence of peripartum cardiomyopathy in the United States ranges from 1 in 1000 to 4000 live births and is increasing worldwide.1,2 While the cause of peripartum cardiomyopathy remains unknown, risk factors include advanced maternal age, African descent, hypertension, preeclampsia, and multiple gestation pregnancy.1,2
Early diagnosis of peripartum cardiomyopathy is imperative for survival of both mother and baby.4 This may be difficult because the signs and symptoms of heart failure—such as dyspnea, edema, orthopnea, cough, and chest and abdominal pain—overlap with those of a typical pregnancy, resulting in it often being missed on evaluation.1,2
Dx with echocardiography; in a pinch, consider lung ultrasound
Usually a diagnosis of peripartum cardiomyopathy is established with echocardiography.1,2 Thus, this case is of significant importance because it illustrates the successful use of lung ultrasound—a simple and easy test—by a rural family doctor to identify this potentially fatal, elusive condition with no additional studies.
Continue to: Use of lung ultrasound...
Use of lung ultrasound in the detection of acute decompensated heart failure is accepted in the medical literature.5-7 Given clinical correlation, a positive scan is defined by the presence of at least 3 B-lines on a longitudinal plane between 2 ribs or, as seen in our case, by the presence of pleural effusion.5-8 Lung ultrasound is readily available worldwide, is completely safe in pregnancy, and is considered one of the easiest studies to perform.7-10
At the patient’s 9-month follow-up visit, she had made a full clinical recovery. Her ejection fraction was 59.8%, and she had stopped all medications. The patient and her child did not experience any continued complications.
THE TAKEAWAY
Family physicians should be aware of peripartum cardiomyopathy—one of the most elusive and life-threatening diseases of pregnancy. When managing a pregnant patient, it is imperative to follow up on complaints such as dyspnea, peripheral edema, and chest and/or abdominal pain. While these symptoms are not unusual during pregnancy, they should always prompt a more thorough evaluation. If peripartum cardiomyopathy is suspected, lung ultrasound is a valuable diagnostic tool for family physicians. Further research is needed before the findings of this case report can be universally applied in the routine prenatal care of women at risk for peripartum cardiomyopathy.
The authors thank their daughter, Nickel Cielo Abarbanell, for her help in the preparation of this manuscript.
CORRESPONDENCE
Neal Robert Abarbanell, MD, First Choice Healthcare, 1867 20th Avenue, Vero Beach, FL 32960; neal.abarbanell@ gmail.com
1. Honigberg MC, Givertz MM. Peripartum cardiomyopathy. BMJ. 2019;364:k5287. doi: 10.1136/bmj.k5287
2. Arany Z, Elkayam U. Peripartum cardiomyopathy. Circulation. 2016;133:1397-1409. doi: 10.1161/CIRCULATIONAHA.115.020491
3. Porak C. De L’influence reciproque de la grossesse et del maladies du Coceur [thesis]. Medical Faculty of Paris, France: 1880.
4. Lewey J, Levine LD, Elovitz MA, et al. Importance of early diagnosis in peripartum cardiomyopathy. Hypertension. 2020;75:91-97. doi: 10.1161/HYPERTENSIONAHA.119.13291
5. Volpicelli G, Caramello V, Cardinale L, et al. Bedside ultrasound of the lung for the monitoring of acute decompensated heart failure. Am J Emerg Med. 2008;26:585-591. doi: 10.1016/j.ajem.2007.09.014
6. Muniz RT, Mesquita ET, Souza CV Jr, et al. Pulmonary ultrasound in patients with heart failure-systematic review. Arq Bras Cardiol. 2018;110:577-584. doi: 10.5935/abc.20180097
7. Russell FM, Rutz M, Pang PS. Focused ultrasound in the emergency department for patients with acute heart failure. Card Fail Rev. 2015;1:83-86. doi: 10.15420/cfr.2015.1.2.83
8. Gustafsson M, Alehagen U, Johansson P. Imaging congestion with a pocket ultrasound device: prognostic implications in patients with chronic heart failure. J Card Fail. 2015;21:548-554. doi: 10.1016/j.cardfail.2015.02.004
9. Ntusi NA, Samuels P, Moosa S, et al. Diagnosing cardiac disease during pregnancy: imaging modalities. Cardiovasc J Afr. 2016;27:95-103. doi: 10.5830/CVJA-2016-022
10. Kimberly HH, Murray A, Mennicke M, et al. Focused maternal ultrasound by midwives in rural Zambia. Ultrasound Med Biol. 2010;36:1267-1272. doi: 10.1016/j.ultrasmedbio.2010.05.017
1. Honigberg MC, Givertz MM. Peripartum cardiomyopathy. BMJ. 2019;364:k5287. doi: 10.1136/bmj.k5287
2. Arany Z, Elkayam U. Peripartum cardiomyopathy. Circulation. 2016;133:1397-1409. doi: 10.1161/CIRCULATIONAHA.115.020491
3. Porak C. De L’influence reciproque de la grossesse et del maladies du Coceur [thesis]. Medical Faculty of Paris, France: 1880.
4. Lewey J, Levine LD, Elovitz MA, et al. Importance of early diagnosis in peripartum cardiomyopathy. Hypertension. 2020;75:91-97. doi: 10.1161/HYPERTENSIONAHA.119.13291
5. Volpicelli G, Caramello V, Cardinale L, et al. Bedside ultrasound of the lung for the monitoring of acute decompensated heart failure. Am J Emerg Med. 2008;26:585-591. doi: 10.1016/j.ajem.2007.09.014
6. Muniz RT, Mesquita ET, Souza CV Jr, et al. Pulmonary ultrasound in patients with heart failure-systematic review. Arq Bras Cardiol. 2018;110:577-584. doi: 10.5935/abc.20180097
7. Russell FM, Rutz M, Pang PS. Focused ultrasound in the emergency department for patients with acute heart failure. Card Fail Rev. 2015;1:83-86. doi: 10.15420/cfr.2015.1.2.83
8. Gustafsson M, Alehagen U, Johansson P. Imaging congestion with a pocket ultrasound device: prognostic implications in patients with chronic heart failure. J Card Fail. 2015;21:548-554. doi: 10.1016/j.cardfail.2015.02.004
9. Ntusi NA, Samuels P, Moosa S, et al. Diagnosing cardiac disease during pregnancy: imaging modalities. Cardiovasc J Afr. 2016;27:95-103. doi: 10.5830/CVJA-2016-022
10. Kimberly HH, Murray A, Mennicke M, et al. Focused maternal ultrasound by midwives in rural Zambia. Ultrasound Med Biol. 2010;36:1267-1272. doi: 10.1016/j.ultrasmedbio.2010.05.017
► Progressive dyspnea and peripheral edema
► 35th week of gestation with a history of mild preeclampsia
Even exercise by ‘weekend warriors’ can cut CV risk
Moderate to vigorous physical activity (MVPA) is a familiar and established approach to reducing cardiovascular (CV) risk, but it’s often believed that the exercise should be spread out across the week rather than concentrated within a couple of days.
A challenge to that view comes from an observational study of accelerometer-confirmed exercise in almost 90,000 people in their 60s. It suggests,
Researchers compared three patterns of MVPA in their subjects who wore accelerometers on their wrists for 1 week. Active WW subjects obtained at least 2.5 hours of exercise weekly, with at least half the amount completed over 1-2 days; “active regular” subjects achieved that exercise level but not mostly during 1 or 2 days; and those who were “inactive” fell short of 2.5 hours of exercise during the week. The group used a median exercise threshold of 3 hours, 50 minutes in a separate analysis.
The “active” groups, compared with inactive subjects, achieved similar and significant reductions in risk for incident atrial fibrillation (AF), myocardial infarction (MI), stroke, and heart failure (HF) over a median follow-up of 6.3 years at both weekly exercise thresholds, the group reported.
“The take-home [message] is that efforts to optimize activity, even if concentrated within just a day or 2 each week, should be expected to result in improved cardiovascular risk profiles,” lead author Shaan Khurshid, MD, MPH, Massachusetts General Hospital, Boston, said in an interview.
The study was published online in JAMA.
The research “provides novel data on patterns of physical activity accumulation and the risk of developing cardiovascular diseases,” observed Peter Katzmarzyk, PhD, Pennington Biomedical Research Center, Baton Rouge, La., in an interview. He was not involved with the research. Its “marked strengths,” he noted, include a large sample population and “use of accelerometers to measure physical activity levels and patterns.”
Moreover, Dr. Katzmarzyk said, its findings are “important” for showing that physical activity “can be accumulated throughout the week in different ways, which opens up more options for busy people to get their physical activity in.”
Current guidelines from the World Health Organization and the American Heart Association recommend at least 150 minutes of MVPA weekly to lower risk for cardiovascular disease and death, but do not specify an optimal exercise time frame. The U.K. National Health Service recommends MVPA daily or spread evenly over perhaps 4-5 days.
“The weekend warrior pattern has been studied previously, but typically relying on self-reported data, which may be biased, or [in studies] too small to look at specific cardiovascular outcomes,” Dr. Khurshid explained.
In the UK Biobank database, he said, “We saw the opportunity to leverage the largest sample of measured activity to date” to address the question of whether exercise time pattern “affects specific major cardiovascular diseases differently,” Dr. Khurshid said
The primary analysis assessed exercise amount in a week based on the guideline-recommended threshold of at least 2.5 hours; a 3-hour, 50-minutes threshold was used in a secondary analysis. The group assessed multiple thresholds because optimal MVPS levels derived from wrist-based accelerometers are “unclear,” he said.
The sample consisted of 89,573 participants with a mean age 62; slightly more than half (56%) were women. Based on the weekly MVPA threshold of 2.5 hours , the WW, active regular, and inactive groups made up 42.2%, 24%, and 33.7% of the population, respectively.
Compared with the inactive group, the two active groups both showed significant risk reductions for the four clinical outcomes, to similar degrees, in multivariate analysis. The results were similar at the 230-minute weekly exercise threshold for incident AF, MI, and HF but not for stroke.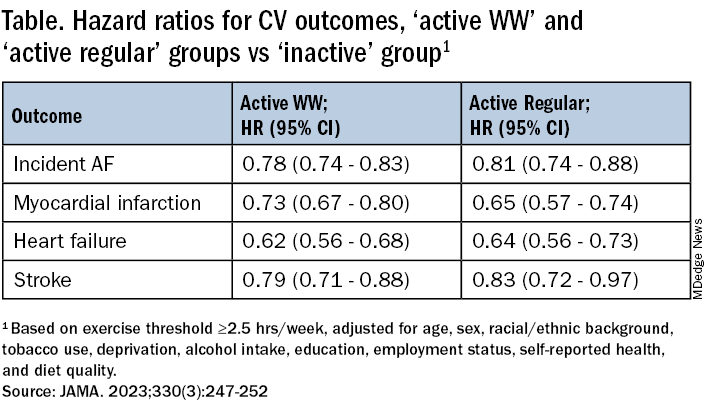
The findings were similarly consistent at the 3-hour, 50-minutes median threshold, although stroke differences were no longer significant.
Patients should be encouraged to exercise at recommended levels, “and should not be discouraged if, for whatever reasons, they are able to focus exercise within only 1 or a few days of the week,” said Dr. Khurshid. “Our findings suggest that it is the volume of activity, rather than the pattern, that matters most.”
The report notes several limitations of the study, including the exercise observation period limited to 1 week and that participants could have modified their behavior during the observation period. Also, the participants were almost all White, so the results may not be generalizable to other populations.
Clinicians should familiarize themselves with the “full range of recommendations” presented in the “Physical Activity Guidelines for Americans, 2nd Edition” “and personalize prescriptions by setting achievable physical activity goals” based on age, physical abilities, and activity levels, states an accompanying editorial from Dr. Katzmarzyk and John M. Jakicic, PhD, University of Kansas Medical Center, Kansas City.
Although MVPA at the recommended level of at least 2.5 hours per week will certainly be beneficial, they write, “the public health message should also clearly convey that every minute counts, especially among the three-quarters of U.S. adults who do not achieve that goal.”
Dr. Khurshid reported no relevant financial relationships; disclosures for the other authors are in the original article. Dr. Katzmarzyk reports no relevant financial relationships. Dr. Jakicic discloses receiving personal fees from Wondr Health, WW International (formerly Weight Watchers), and Educational Initiatives and grants from Epitomee Medical.
A version of this article appeared on Medscape.com.
Moderate to vigorous physical activity (MVPA) is a familiar and established approach to reducing cardiovascular (CV) risk, but it’s often believed that the exercise should be spread out across the week rather than concentrated within a couple of days.
A challenge to that view comes from an observational study of accelerometer-confirmed exercise in almost 90,000 people in their 60s. It suggests,
Researchers compared three patterns of MVPA in their subjects who wore accelerometers on their wrists for 1 week. Active WW subjects obtained at least 2.5 hours of exercise weekly, with at least half the amount completed over 1-2 days; “active regular” subjects achieved that exercise level but not mostly during 1 or 2 days; and those who were “inactive” fell short of 2.5 hours of exercise during the week. The group used a median exercise threshold of 3 hours, 50 minutes in a separate analysis.
The “active” groups, compared with inactive subjects, achieved similar and significant reductions in risk for incident atrial fibrillation (AF), myocardial infarction (MI), stroke, and heart failure (HF) over a median follow-up of 6.3 years at both weekly exercise thresholds, the group reported.
“The take-home [message] is that efforts to optimize activity, even if concentrated within just a day or 2 each week, should be expected to result in improved cardiovascular risk profiles,” lead author Shaan Khurshid, MD, MPH, Massachusetts General Hospital, Boston, said in an interview.
The study was published online in JAMA.
The research “provides novel data on patterns of physical activity accumulation and the risk of developing cardiovascular diseases,” observed Peter Katzmarzyk, PhD, Pennington Biomedical Research Center, Baton Rouge, La., in an interview. He was not involved with the research. Its “marked strengths,” he noted, include a large sample population and “use of accelerometers to measure physical activity levels and patterns.”
Moreover, Dr. Katzmarzyk said, its findings are “important” for showing that physical activity “can be accumulated throughout the week in different ways, which opens up more options for busy people to get their physical activity in.”
Current guidelines from the World Health Organization and the American Heart Association recommend at least 150 minutes of MVPA weekly to lower risk for cardiovascular disease and death, but do not specify an optimal exercise time frame. The U.K. National Health Service recommends MVPA daily or spread evenly over perhaps 4-5 days.
“The weekend warrior pattern has been studied previously, but typically relying on self-reported data, which may be biased, or [in studies] too small to look at specific cardiovascular outcomes,” Dr. Khurshid explained.
In the UK Biobank database, he said, “We saw the opportunity to leverage the largest sample of measured activity to date” to address the question of whether exercise time pattern “affects specific major cardiovascular diseases differently,” Dr. Khurshid said
The primary analysis assessed exercise amount in a week based on the guideline-recommended threshold of at least 2.5 hours; a 3-hour, 50-minutes threshold was used in a secondary analysis. The group assessed multiple thresholds because optimal MVPS levels derived from wrist-based accelerometers are “unclear,” he said.
The sample consisted of 89,573 participants with a mean age 62; slightly more than half (56%) were women. Based on the weekly MVPA threshold of 2.5 hours , the WW, active regular, and inactive groups made up 42.2%, 24%, and 33.7% of the population, respectively.
Compared with the inactive group, the two active groups both showed significant risk reductions for the four clinical outcomes, to similar degrees, in multivariate analysis. The results were similar at the 230-minute weekly exercise threshold for incident AF, MI, and HF but not for stroke.
The findings were similarly consistent at the 3-hour, 50-minutes median threshold, although stroke differences were no longer significant.
Patients should be encouraged to exercise at recommended levels, “and should not be discouraged if, for whatever reasons, they are able to focus exercise within only 1 or a few days of the week,” said Dr. Khurshid. “Our findings suggest that it is the volume of activity, rather than the pattern, that matters most.”
The report notes several limitations of the study, including the exercise observation period limited to 1 week and that participants could have modified their behavior during the observation period. Also, the participants were almost all White, so the results may not be generalizable to other populations.
Clinicians should familiarize themselves with the “full range of recommendations” presented in the “Physical Activity Guidelines for Americans, 2nd Edition” “and personalize prescriptions by setting achievable physical activity goals” based on age, physical abilities, and activity levels, states an accompanying editorial from Dr. Katzmarzyk and John M. Jakicic, PhD, University of Kansas Medical Center, Kansas City.
Although MVPA at the recommended level of at least 2.5 hours per week will certainly be beneficial, they write, “the public health message should also clearly convey that every minute counts, especially among the three-quarters of U.S. adults who do not achieve that goal.”
Dr. Khurshid reported no relevant financial relationships; disclosures for the other authors are in the original article. Dr. Katzmarzyk reports no relevant financial relationships. Dr. Jakicic discloses receiving personal fees from Wondr Health, WW International (formerly Weight Watchers), and Educational Initiatives and grants from Epitomee Medical.
A version of this article appeared on Medscape.com.
Moderate to vigorous physical activity (MVPA) is a familiar and established approach to reducing cardiovascular (CV) risk, but it’s often believed that the exercise should be spread out across the week rather than concentrated within a couple of days.
A challenge to that view comes from an observational study of accelerometer-confirmed exercise in almost 90,000 people in their 60s. It suggests,
Researchers compared three patterns of MVPA in their subjects who wore accelerometers on their wrists for 1 week. Active WW subjects obtained at least 2.5 hours of exercise weekly, with at least half the amount completed over 1-2 days; “active regular” subjects achieved that exercise level but not mostly during 1 or 2 days; and those who were “inactive” fell short of 2.5 hours of exercise during the week. The group used a median exercise threshold of 3 hours, 50 minutes in a separate analysis.
The “active” groups, compared with inactive subjects, achieved similar and significant reductions in risk for incident atrial fibrillation (AF), myocardial infarction (MI), stroke, and heart failure (HF) over a median follow-up of 6.3 years at both weekly exercise thresholds, the group reported.
“The take-home [message] is that efforts to optimize activity, even if concentrated within just a day or 2 each week, should be expected to result in improved cardiovascular risk profiles,” lead author Shaan Khurshid, MD, MPH, Massachusetts General Hospital, Boston, said in an interview.
The study was published online in JAMA.
The research “provides novel data on patterns of physical activity accumulation and the risk of developing cardiovascular diseases,” observed Peter Katzmarzyk, PhD, Pennington Biomedical Research Center, Baton Rouge, La., in an interview. He was not involved with the research. Its “marked strengths,” he noted, include a large sample population and “use of accelerometers to measure physical activity levels and patterns.”
Moreover, Dr. Katzmarzyk said, its findings are “important” for showing that physical activity “can be accumulated throughout the week in different ways, which opens up more options for busy people to get their physical activity in.”
Current guidelines from the World Health Organization and the American Heart Association recommend at least 150 minutes of MVPA weekly to lower risk for cardiovascular disease and death, but do not specify an optimal exercise time frame. The U.K. National Health Service recommends MVPA daily or spread evenly over perhaps 4-5 days.
“The weekend warrior pattern has been studied previously, but typically relying on self-reported data, which may be biased, or [in studies] too small to look at specific cardiovascular outcomes,” Dr. Khurshid explained.
In the UK Biobank database, he said, “We saw the opportunity to leverage the largest sample of measured activity to date” to address the question of whether exercise time pattern “affects specific major cardiovascular diseases differently,” Dr. Khurshid said
The primary analysis assessed exercise amount in a week based on the guideline-recommended threshold of at least 2.5 hours; a 3-hour, 50-minutes threshold was used in a secondary analysis. The group assessed multiple thresholds because optimal MVPS levels derived from wrist-based accelerometers are “unclear,” he said.
The sample consisted of 89,573 participants with a mean age 62; slightly more than half (56%) were women. Based on the weekly MVPA threshold of 2.5 hours , the WW, active regular, and inactive groups made up 42.2%, 24%, and 33.7% of the population, respectively.
Compared with the inactive group, the two active groups both showed significant risk reductions for the four clinical outcomes, to similar degrees, in multivariate analysis. The results were similar at the 230-minute weekly exercise threshold for incident AF, MI, and HF but not for stroke.
The findings were similarly consistent at the 3-hour, 50-minutes median threshold, although stroke differences were no longer significant.
Patients should be encouraged to exercise at recommended levels, “and should not be discouraged if, for whatever reasons, they are able to focus exercise within only 1 or a few days of the week,” said Dr. Khurshid. “Our findings suggest that it is the volume of activity, rather than the pattern, that matters most.”
The report notes several limitations of the study, including the exercise observation period limited to 1 week and that participants could have modified their behavior during the observation period. Also, the participants were almost all White, so the results may not be generalizable to other populations.
Clinicians should familiarize themselves with the “full range of recommendations” presented in the “Physical Activity Guidelines for Americans, 2nd Edition” “and personalize prescriptions by setting achievable physical activity goals” based on age, physical abilities, and activity levels, states an accompanying editorial from Dr. Katzmarzyk and John M. Jakicic, PhD, University of Kansas Medical Center, Kansas City.
Although MVPA at the recommended level of at least 2.5 hours per week will certainly be beneficial, they write, “the public health message should also clearly convey that every minute counts, especially among the three-quarters of U.S. adults who do not achieve that goal.”
Dr. Khurshid reported no relevant financial relationships; disclosures for the other authors are in the original article. Dr. Katzmarzyk reports no relevant financial relationships. Dr. Jakicic discloses receiving personal fees from Wondr Health, WW International (formerly Weight Watchers), and Educational Initiatives and grants from Epitomee Medical.
A version of this article appeared on Medscape.com.
FROM JAMA
High-intensity interval training before major surgery may boost postoperative outcomes
TOPLINE:
It cuts the risk of postoperative complications and may shorten hospital length of stay and improve postoperative quality of life.
METHODOLOGY:
Evidence suggests CRF – which improves physical and cognitive function and is associated with a reduction in cardiovascular risk – can be enhanced before major surgeries, but reported postoperative outcomes in previous reviews have been inconsistent.
In the study, HIIT involved repeated aerobic high-intensity exercise intervals at about 80% of maximum heart rate, followed by active recovery.
The meta-analysis included 12 studies with 832 patients (mean age, 67) that compared preoperative HIIT – supervised at hospitals, gyms, or community or physical therapy centers, or unsupervised at home – with standard care for patients slated for major surgery, including liver, lung, colorectal, urologic, and mixed major abdominal operations.
The primary outcome was change in CRF by peak VO2 or 6-minute walk test; other endpoints included change in endurance time and postoperative outcomes.
TAKEAWAY:
Preoperative HIIT (median total, 160 minutes; range, 80-240 minutes; intense exercise during 6-40 sessions) was associated with an increase in peak oxygen consumption (VO2 peak) by 2.59 mL/kg/min (95% confidence interval, 1.52-3.65 mL/kg/min; P < .001), compared with standard care, which represents about a 10% increase in CRF.
In eight studies that involved 770 patients, there was moderate evidence that preoperative HIIT cut the odds ratio for postoperative complications by more than half (OR, 0.44; 95% CI, 0.32-0.60; P < .001); there was a similar apparent benefit in an analysis that was limited to patients who were slated for abdominal surgery (OR, 0.45; 95% CI, 0.29-0.68; P < .001).
An analysis that was limited to studies that reported hospital length of stay showed a clinically relevant but nonsignificant 3-day reduction among patients in the HIIT groups.
Most quality of life assessments did not show post-HIIT improvements; some showed a significant benefit 6 weeks after surgery.
IN PRACTICE:
The results suggest preoperative HIIT may improve postoperative outcomes. By extension, it could be cost-effective and “should be included in prehabilitation programs,” the report states.
SOURCE:
The study was carried out by Kari Clifford, PhD, Otago Medical School, University of Otago, Dunedin, New Zealand, and colleagues. It was published online June 30, 2023, in JAMA Network Open.
LIMITATIONS:
Included studies were heterogeneous in methodology; for example, HIIT definitions and protocols varied across almost every study. Data reporting was incomplete, the samples sizes in the studies were limited, and patients could not be blinded to their intervention. The patients could not be stratified on the basis of frailty. There were limited HIIT data from patients who underwent orthopedic surgeries.
DISCLOSURES:
The study received funding from the University of Otago. The authors reported no conflicts.
A version of this article first appeared on Medscape.com.
TOPLINE:
It cuts the risk of postoperative complications and may shorten hospital length of stay and improve postoperative quality of life.
METHODOLOGY:
Evidence suggests CRF – which improves physical and cognitive function and is associated with a reduction in cardiovascular risk – can be enhanced before major surgeries, but reported postoperative outcomes in previous reviews have been inconsistent.
In the study, HIIT involved repeated aerobic high-intensity exercise intervals at about 80% of maximum heart rate, followed by active recovery.
The meta-analysis included 12 studies with 832 patients (mean age, 67) that compared preoperative HIIT – supervised at hospitals, gyms, or community or physical therapy centers, or unsupervised at home – with standard care for patients slated for major surgery, including liver, lung, colorectal, urologic, and mixed major abdominal operations.
The primary outcome was change in CRF by peak VO2 or 6-minute walk test; other endpoints included change in endurance time and postoperative outcomes.
TAKEAWAY:
Preoperative HIIT (median total, 160 minutes; range, 80-240 minutes; intense exercise during 6-40 sessions) was associated with an increase in peak oxygen consumption (VO2 peak) by 2.59 mL/kg/min (95% confidence interval, 1.52-3.65 mL/kg/min; P < .001), compared with standard care, which represents about a 10% increase in CRF.
In eight studies that involved 770 patients, there was moderate evidence that preoperative HIIT cut the odds ratio for postoperative complications by more than half (OR, 0.44; 95% CI, 0.32-0.60; P < .001); there was a similar apparent benefit in an analysis that was limited to patients who were slated for abdominal surgery (OR, 0.45; 95% CI, 0.29-0.68; P < .001).
An analysis that was limited to studies that reported hospital length of stay showed a clinically relevant but nonsignificant 3-day reduction among patients in the HIIT groups.
Most quality of life assessments did not show post-HIIT improvements; some showed a significant benefit 6 weeks after surgery.
IN PRACTICE:
The results suggest preoperative HIIT may improve postoperative outcomes. By extension, it could be cost-effective and “should be included in prehabilitation programs,” the report states.
SOURCE:
The study was carried out by Kari Clifford, PhD, Otago Medical School, University of Otago, Dunedin, New Zealand, and colleagues. It was published online June 30, 2023, in JAMA Network Open.
LIMITATIONS:
Included studies were heterogeneous in methodology; for example, HIIT definitions and protocols varied across almost every study. Data reporting was incomplete, the samples sizes in the studies were limited, and patients could not be blinded to their intervention. The patients could not be stratified on the basis of frailty. There were limited HIIT data from patients who underwent orthopedic surgeries.
DISCLOSURES:
The study received funding from the University of Otago. The authors reported no conflicts.
A version of this article first appeared on Medscape.com.
TOPLINE:
It cuts the risk of postoperative complications and may shorten hospital length of stay and improve postoperative quality of life.
METHODOLOGY:
Evidence suggests CRF – which improves physical and cognitive function and is associated with a reduction in cardiovascular risk – can be enhanced before major surgeries, but reported postoperative outcomes in previous reviews have been inconsistent.
In the study, HIIT involved repeated aerobic high-intensity exercise intervals at about 80% of maximum heart rate, followed by active recovery.
The meta-analysis included 12 studies with 832 patients (mean age, 67) that compared preoperative HIIT – supervised at hospitals, gyms, or community or physical therapy centers, or unsupervised at home – with standard care for patients slated for major surgery, including liver, lung, colorectal, urologic, and mixed major abdominal operations.
The primary outcome was change in CRF by peak VO2 or 6-minute walk test; other endpoints included change in endurance time and postoperative outcomes.
TAKEAWAY:
Preoperative HIIT (median total, 160 minutes; range, 80-240 minutes; intense exercise during 6-40 sessions) was associated with an increase in peak oxygen consumption (VO2 peak) by 2.59 mL/kg/min (95% confidence interval, 1.52-3.65 mL/kg/min; P < .001), compared with standard care, which represents about a 10% increase in CRF.
In eight studies that involved 770 patients, there was moderate evidence that preoperative HIIT cut the odds ratio for postoperative complications by more than half (OR, 0.44; 95% CI, 0.32-0.60; P < .001); there was a similar apparent benefit in an analysis that was limited to patients who were slated for abdominal surgery (OR, 0.45; 95% CI, 0.29-0.68; P < .001).
An analysis that was limited to studies that reported hospital length of stay showed a clinically relevant but nonsignificant 3-day reduction among patients in the HIIT groups.
Most quality of life assessments did not show post-HIIT improvements; some showed a significant benefit 6 weeks after surgery.
IN PRACTICE:
The results suggest preoperative HIIT may improve postoperative outcomes. By extension, it could be cost-effective and “should be included in prehabilitation programs,” the report states.
SOURCE:
The study was carried out by Kari Clifford, PhD, Otago Medical School, University of Otago, Dunedin, New Zealand, and colleagues. It was published online June 30, 2023, in JAMA Network Open.
LIMITATIONS:
Included studies were heterogeneous in methodology; for example, HIIT definitions and protocols varied across almost every study. Data reporting was incomplete, the samples sizes in the studies were limited, and patients could not be blinded to their intervention. The patients could not be stratified on the basis of frailty. There were limited HIIT data from patients who underwent orthopedic surgeries.
DISCLOSURES:
The study received funding from the University of Otago. The authors reported no conflicts.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Omega-3s and AFib: No added risk from eating fish but high-dose supplement questions persist
Regular consumption of fish and other foods rich in omega-3 fatty acids (FA) won’t raise an individual’s risk for developing atrial fibrillation (AFib), suggests a meta-analysis of population-based studies.
The finding may alleviate recent concerns about higher-dose omega-3 FA supplement intake in clinical-trial patients at elevated cardiovascular (CV) risk, researchers say.
Indeed, across the 17 cohort studies in the meta-analysis, risk for incident AFib was unaffected by elevated circulating and adipose tissue levels of eicosapentaenoic acid (EPA) from dietary intake. Moreover, the risk appeared to drop significantly with such levels of docosahexaenoic acid (DHA), docosapentaenoic acid (DPA), and EPA plus DHA.
The signals of AFib risk associated with high-dose omega-3 FA in supplements or prescription form in some clinical trials “may not necessarily be generalizable to lower-dose habitual dietary omega-3 intakes,” concludes the study’s report published in the Journal of the American College of Cardiology.
Other recent research suggests that any elevated AFib risk from omega-3 FA intake is dose-related and may be associated with omega-3 FA supplement or medication intake in high doses, such as 4 g/day.
“Coupled with the more consistent benefits of these fatty acids in the prevention of adverse coronary events, our study suggests that current dietary guidelines recommending fish/omega-3 fatty acid consumption should be maintained,” conclude the authors of the report, led by Frank Qian, MD, MPH, Harvard T.H. Chan School of Public Health and Beth Israel Deaconess Medical Center, Boston.
The current study is “important in showing that physiologic levels of omega-3s that would accumulate through diet don’t seem to increase the risk of arrhythmia,” preventive cardiologist Sean Heffron, MD, NYU Langone Health and Grossman School of Medicine, told this news organization.
“It also lends credence to the fact that the increased risk is specific to the high dose supplementation, because that’s the only instance in which we’ve seen increased atrial fibrillation in association with omega-3s,” said Dr. Heffron, who wasn’t involved in the meta-analysis.
An accompanying editorial agrees. “Based on present evidence, moderate dietary intake of fish and seafood is unlikely to achieve sufficiently high levels of omega-3-FAs in blood or tissue that would result in increased AFib risk as observed in clinical trials of fish oil supplements and high-dose prescriptions,” write Christie Ballantyne, MD, and Xiaoming Jia, MD, Baylor College of Medicine, Houston.
Therefore, they conclude, “fish should continue to be an important part of the menu of a heart-healthy diet.”
The meta-analysis comprised 54,799 participants from 21 countries worldwide in 17 prospective cohort studies that yielded data on incident AFib, in which there were 7,720 cases of the arrhythmia over a median follow-up of 13 years. It looked at associations between such cases and levels of omega-3 FA in blood and adipose tissue samples.
In multivariable analysis, EPA levels were not associated with incident AFib, with a hazard ratio of 1.00 (95% confidence interval, 0.95-1.05) per interquintile range, which the report describes as the difference between the 90th and 10th percentiles.
In contrast, levels of DPA, DHA, and EPA plus DHA were all associated with reduced AFib incidence at interquintile-range HRs of 0.89 (95% CI, 0.83-0.95) for DPA, 0.90 (95% CI, 0.85-0.96) for DHA and 0.93 (95% CI, 0.87-0.99) for EPA and DHA combined.
“We found little evidence that the associations significantly varied by age, sex, or global region, or across the various lipid compartments,” the report states. “Moreover, the relationship between omega-3 fatty acids and AFib did not significantly differ among individuals at higher CV risk.”
The authors observe that the prevalence of omega-3 FA supplement use in the cohorts was very low, suggesting that the omega-3 FA biomarker levels largely reflected habitual dietary intake.
Most of the meta-analysis population were free of CV disease or at relatively low CV risk, they write, and “it is conceivable that the effects of omega-3 fatty acids on atrial arrhythmias may differ in those with existing CV disease versus without.”
However, they note, in a prespecified subgroup analysis of participants mirroring the REDUCE-IT cohort of people with established CV disease or at elevated CV risk, no association with incident AFib was observed for EPA and inverse associations emerged for DPA, DHA, and EPA plus DHA.
In their editorial, Dr. Ballantyne and Dr. Jia say the meta-analysis “represents the largest epidemiological study assessing laboratory-measured omega-3 fatty acid concentrations and AFib risk in the general population.”
But significant heterogeneity across the studies and their populations is a major limitation of the analysis, they write, and made for differences in protocols, sample preparation, outcomes ascertainment, follow-up time, and other variables.
“Despite a rigorous approach to harmonize the data across cohorts and adjusting for multiple confounders,” note Dr. Ballantyne and Dr. Jia, “observational studies always have potential for residual confounders.”
The findings support fish consumption as heart-healthy, they write, but “clinicians should be aware of and discuss with patients the risks versus benefits when prescribing high-dose omega-3 FA therapies.”
The Fatty Acid Research Institute retrospectively provided a small honorarium to a subset of the analysts who participated in this study, but it had no role in its design, analysis, manuscript writing, or decision to submit for publication, the report states. Dr. Ballantyne received grant/research support through his institution from Akcea, Amgen, Arrowhead, Esperion, Ionis, Merck, Novartis, and Regeneron, as well as consulting fees from Alnylam Pharmaceuticals, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Esperion, Genentech, Gilead, Matinas BioPharma, Merck, New Amsterdam, Novartis, Pfizer, and Regeneron. Dr. Heffron and Dr. Jia have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Regular consumption of fish and other foods rich in omega-3 fatty acids (FA) won’t raise an individual’s risk for developing atrial fibrillation (AFib), suggests a meta-analysis of population-based studies.
The finding may alleviate recent concerns about higher-dose omega-3 FA supplement intake in clinical-trial patients at elevated cardiovascular (CV) risk, researchers say.
Indeed, across the 17 cohort studies in the meta-analysis, risk for incident AFib was unaffected by elevated circulating and adipose tissue levels of eicosapentaenoic acid (EPA) from dietary intake. Moreover, the risk appeared to drop significantly with such levels of docosahexaenoic acid (DHA), docosapentaenoic acid (DPA), and EPA plus DHA.
The signals of AFib risk associated with high-dose omega-3 FA in supplements or prescription form in some clinical trials “may not necessarily be generalizable to lower-dose habitual dietary omega-3 intakes,” concludes the study’s report published in the Journal of the American College of Cardiology.
Other recent research suggests that any elevated AFib risk from omega-3 FA intake is dose-related and may be associated with omega-3 FA supplement or medication intake in high doses, such as 4 g/day.
“Coupled with the more consistent benefits of these fatty acids in the prevention of adverse coronary events, our study suggests that current dietary guidelines recommending fish/omega-3 fatty acid consumption should be maintained,” conclude the authors of the report, led by Frank Qian, MD, MPH, Harvard T.H. Chan School of Public Health and Beth Israel Deaconess Medical Center, Boston.
The current study is “important in showing that physiologic levels of omega-3s that would accumulate through diet don’t seem to increase the risk of arrhythmia,” preventive cardiologist Sean Heffron, MD, NYU Langone Health and Grossman School of Medicine, told this news organization.
“It also lends credence to the fact that the increased risk is specific to the high dose supplementation, because that’s the only instance in which we’ve seen increased atrial fibrillation in association with omega-3s,” said Dr. Heffron, who wasn’t involved in the meta-analysis.
An accompanying editorial agrees. “Based on present evidence, moderate dietary intake of fish and seafood is unlikely to achieve sufficiently high levels of omega-3-FAs in blood or tissue that would result in increased AFib risk as observed in clinical trials of fish oil supplements and high-dose prescriptions,” write Christie Ballantyne, MD, and Xiaoming Jia, MD, Baylor College of Medicine, Houston.
Therefore, they conclude, “fish should continue to be an important part of the menu of a heart-healthy diet.”
The meta-analysis comprised 54,799 participants from 21 countries worldwide in 17 prospective cohort studies that yielded data on incident AFib, in which there were 7,720 cases of the arrhythmia over a median follow-up of 13 years. It looked at associations between such cases and levels of omega-3 FA in blood and adipose tissue samples.
In multivariable analysis, EPA levels were not associated with incident AFib, with a hazard ratio of 1.00 (95% confidence interval, 0.95-1.05) per interquintile range, which the report describes as the difference between the 90th and 10th percentiles.
In contrast, levels of DPA, DHA, and EPA plus DHA were all associated with reduced AFib incidence at interquintile-range HRs of 0.89 (95% CI, 0.83-0.95) for DPA, 0.90 (95% CI, 0.85-0.96) for DHA and 0.93 (95% CI, 0.87-0.99) for EPA and DHA combined.
“We found little evidence that the associations significantly varied by age, sex, or global region, or across the various lipid compartments,” the report states. “Moreover, the relationship between omega-3 fatty acids and AFib did not significantly differ among individuals at higher CV risk.”
The authors observe that the prevalence of omega-3 FA supplement use in the cohorts was very low, suggesting that the omega-3 FA biomarker levels largely reflected habitual dietary intake.
Most of the meta-analysis population were free of CV disease or at relatively low CV risk, they write, and “it is conceivable that the effects of omega-3 fatty acids on atrial arrhythmias may differ in those with existing CV disease versus without.”
However, they note, in a prespecified subgroup analysis of participants mirroring the REDUCE-IT cohort of people with established CV disease or at elevated CV risk, no association with incident AFib was observed for EPA and inverse associations emerged for DPA, DHA, and EPA plus DHA.
In their editorial, Dr. Ballantyne and Dr. Jia say the meta-analysis “represents the largest epidemiological study assessing laboratory-measured omega-3 fatty acid concentrations and AFib risk in the general population.”
But significant heterogeneity across the studies and their populations is a major limitation of the analysis, they write, and made for differences in protocols, sample preparation, outcomes ascertainment, follow-up time, and other variables.
“Despite a rigorous approach to harmonize the data across cohorts and adjusting for multiple confounders,” note Dr. Ballantyne and Dr. Jia, “observational studies always have potential for residual confounders.”
The findings support fish consumption as heart-healthy, they write, but “clinicians should be aware of and discuss with patients the risks versus benefits when prescribing high-dose omega-3 FA therapies.”
The Fatty Acid Research Institute retrospectively provided a small honorarium to a subset of the analysts who participated in this study, but it had no role in its design, analysis, manuscript writing, or decision to submit for publication, the report states. Dr. Ballantyne received grant/research support through his institution from Akcea, Amgen, Arrowhead, Esperion, Ionis, Merck, Novartis, and Regeneron, as well as consulting fees from Alnylam Pharmaceuticals, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Esperion, Genentech, Gilead, Matinas BioPharma, Merck, New Amsterdam, Novartis, Pfizer, and Regeneron. Dr. Heffron and Dr. Jia have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Regular consumption of fish and other foods rich in omega-3 fatty acids (FA) won’t raise an individual’s risk for developing atrial fibrillation (AFib), suggests a meta-analysis of population-based studies.
The finding may alleviate recent concerns about higher-dose omega-3 FA supplement intake in clinical-trial patients at elevated cardiovascular (CV) risk, researchers say.
Indeed, across the 17 cohort studies in the meta-analysis, risk for incident AFib was unaffected by elevated circulating and adipose tissue levels of eicosapentaenoic acid (EPA) from dietary intake. Moreover, the risk appeared to drop significantly with such levels of docosahexaenoic acid (DHA), docosapentaenoic acid (DPA), and EPA plus DHA.
The signals of AFib risk associated with high-dose omega-3 FA in supplements or prescription form in some clinical trials “may not necessarily be generalizable to lower-dose habitual dietary omega-3 intakes,” concludes the study’s report published in the Journal of the American College of Cardiology.
Other recent research suggests that any elevated AFib risk from omega-3 FA intake is dose-related and may be associated with omega-3 FA supplement or medication intake in high doses, such as 4 g/day.
“Coupled with the more consistent benefits of these fatty acids in the prevention of adverse coronary events, our study suggests that current dietary guidelines recommending fish/omega-3 fatty acid consumption should be maintained,” conclude the authors of the report, led by Frank Qian, MD, MPH, Harvard T.H. Chan School of Public Health and Beth Israel Deaconess Medical Center, Boston.
The current study is “important in showing that physiologic levels of omega-3s that would accumulate through diet don’t seem to increase the risk of arrhythmia,” preventive cardiologist Sean Heffron, MD, NYU Langone Health and Grossman School of Medicine, told this news organization.
“It also lends credence to the fact that the increased risk is specific to the high dose supplementation, because that’s the only instance in which we’ve seen increased atrial fibrillation in association with omega-3s,” said Dr. Heffron, who wasn’t involved in the meta-analysis.
An accompanying editorial agrees. “Based on present evidence, moderate dietary intake of fish and seafood is unlikely to achieve sufficiently high levels of omega-3-FAs in blood or tissue that would result in increased AFib risk as observed in clinical trials of fish oil supplements and high-dose prescriptions,” write Christie Ballantyne, MD, and Xiaoming Jia, MD, Baylor College of Medicine, Houston.
Therefore, they conclude, “fish should continue to be an important part of the menu of a heart-healthy diet.”
The meta-analysis comprised 54,799 participants from 21 countries worldwide in 17 prospective cohort studies that yielded data on incident AFib, in which there were 7,720 cases of the arrhythmia over a median follow-up of 13 years. It looked at associations between such cases and levels of omega-3 FA in blood and adipose tissue samples.
In multivariable analysis, EPA levels were not associated with incident AFib, with a hazard ratio of 1.00 (95% confidence interval, 0.95-1.05) per interquintile range, which the report describes as the difference between the 90th and 10th percentiles.
In contrast, levels of DPA, DHA, and EPA plus DHA were all associated with reduced AFib incidence at interquintile-range HRs of 0.89 (95% CI, 0.83-0.95) for DPA, 0.90 (95% CI, 0.85-0.96) for DHA and 0.93 (95% CI, 0.87-0.99) for EPA and DHA combined.
“We found little evidence that the associations significantly varied by age, sex, or global region, or across the various lipid compartments,” the report states. “Moreover, the relationship between omega-3 fatty acids and AFib did not significantly differ among individuals at higher CV risk.”
The authors observe that the prevalence of omega-3 FA supplement use in the cohorts was very low, suggesting that the omega-3 FA biomarker levels largely reflected habitual dietary intake.
Most of the meta-analysis population were free of CV disease or at relatively low CV risk, they write, and “it is conceivable that the effects of omega-3 fatty acids on atrial arrhythmias may differ in those with existing CV disease versus without.”
However, they note, in a prespecified subgroup analysis of participants mirroring the REDUCE-IT cohort of people with established CV disease or at elevated CV risk, no association with incident AFib was observed for EPA and inverse associations emerged for DPA, DHA, and EPA plus DHA.
In their editorial, Dr. Ballantyne and Dr. Jia say the meta-analysis “represents the largest epidemiological study assessing laboratory-measured omega-3 fatty acid concentrations and AFib risk in the general population.”
But significant heterogeneity across the studies and their populations is a major limitation of the analysis, they write, and made for differences in protocols, sample preparation, outcomes ascertainment, follow-up time, and other variables.
“Despite a rigorous approach to harmonize the data across cohorts and adjusting for multiple confounders,” note Dr. Ballantyne and Dr. Jia, “observational studies always have potential for residual confounders.”
The findings support fish consumption as heart-healthy, they write, but “clinicians should be aware of and discuss with patients the risks versus benefits when prescribing high-dose omega-3 FA therapies.”
The Fatty Acid Research Institute retrospectively provided a small honorarium to a subset of the analysts who participated in this study, but it had no role in its design, analysis, manuscript writing, or decision to submit for publication, the report states. Dr. Ballantyne received grant/research support through his institution from Akcea, Amgen, Arrowhead, Esperion, Ionis, Merck, Novartis, and Regeneron, as well as consulting fees from Alnylam Pharmaceuticals, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Esperion, Genentech, Gilead, Matinas BioPharma, Merck, New Amsterdam, Novartis, Pfizer, and Regeneron. Dr. Heffron and Dr. Jia have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Experts call for early screening for chronic kidney disease
MADRID – A late diagnosis of chronic kidney disease is cause for concern. Scientific societies are therefore advocating for screening at younger ages to reverse this trend and slow the progression of the disease. Nearly all patients seen in primary care are candidates for screening because of their risk factors for kidney disease.
During the 29th National Conference of General and Family Medicine of the Spanish Society for General and Family Physicians, Teresa Benedito, MD, family doctor and member of the society’s cardiovascular group, and Roberto Alcázar, MD, nephrologist at the Infanta Leonor University Hospital, Madrid, presented a clinical case encountered in primary care. They used this case to frame a strong argument for the importance of early screening for chronic kidney disease, and they discussed how to properly manage such screening.
The presentation followed the guidelines in the SEMG publication regarding the management and referral of patients with type 2 diabetes. Dr. Benedito explained that the first thing to ask oneself during a patient visit is “whether they present risk factors for kidney disease. If so, we can’t let them leave before we do a kidney screening.” She then listed the factors in question: age older than 60 years, African heritage, family history of chronic kidney disease, decreased kidney mass, weight loss at birth, hypertension, diabetes, smoking, obesity, and low socioeconomic status.
For his part, Dr. Alcázar mentioned how these factors are similar to cardiovascular risk factors, because “the kidneys are a ball of vessels with double capillarization for purifying blood. They’re the organs with the most arteries per unit of weight, so anything that can damage the arteries can damage the kidneys.”
Candidates for screening
“Chronic kidney disease develops in 15% of the adult population in Spain. So, it’s worth asking how many patients have been diagnosed and who should we should be screening.” To the factors listed above, Dr. Alcázar added treatment with nephrotoxic drugs (including nonsteroidal anti-inflammatory drugs) for patients with obstructive urinary tract disease, and a history of acute kidney injury for patients with chronic autoimmune disease or neoplasms. “Thus, nearly all patients seen in primary care would need to be screened.”
Another fundamental question raised was whether patients should be screened before age 60 years. “As a nephrologist, I feel that we have been diagnosing chronic kidney disease late, even though we’ve been doing everything by the book,” said Dr. Alcázar. In his opinion, “the answer to whether we should be screening earlier ... is yes, for two reasons: first, because it’s cost-effective, and second, because it’s very inexpensive.”
Dr. Benedito explained in detail the process for diagnosing this disease. She began by defining the disease as changes in kidney structure and function that last longer than 3 months. These changes are identified by use of two criteria: glomerular filtration rate less than 60 mL/min and kidney injury or lesions with or without reduced filtration rate (renal biopsy, albumin/creatinine ratio greater than 30 mg/g, proteinuria, alterations in urinary sediment or in imaging tests). Thus, “if one of these two criteria persists for more than 3 months, the diagnosis is chronic kidney disease. Also, high creatinine levels are not diagnostic for the disease,” she emphasized.
Two related parameters
Glomerular filtration and albuminuria “are highly relevant, because screening for chronic kidney disease is based on these two parameters,” said Dr. Benedito. Glomerular filtration rate varies with age, sex, ethnicity, and body mass. It is useful for identifying the stage of the disease and for monitoring disease progression. Albuminuria, on the other hand, is an indication of the severity of the disease. It’s an early marker for kidney injury and systemic disease and is more sensitive than proteinuria. Therefore, “this factor, together with glomerular filtration rate, allows us to detect, classify, and monitor the progression of chronic kidney disease.”
On this point, Dr. Alcázar emphasized the importance of trends, since variation in glomerular filtration depends on serum creatinine, which can vary by nearly 9%. He explained that glomerular filtration rate is related to the number of nephrons remaining. A glomerular filtration rate of less than 60 mL/min implies that more than half of the nephrons in each kidney have been lost. Albuminuria informs about structural damage (that is, the condition of the remaining nephrons). It’s therefore essential to test for both parameters. “We need to be actively monitoring and then making our decisions based on trends and not on isolated results. We need to be aware of albuminuria when we make our decisions,” said Dr. Alcázar. Some studies have shown the importance of testing for albuminuria whenever creatinine level is assessed. “We need to buy into this. If we don’t do this, we’ll only ever have half the information we need.”
Reducing late diagnosis
According to the IBERICAN study, 14% of patients seen in primary care in Spain have chronic kidney disease. “This statistic should make us stop and think, own our responsibility, and ask ourselves why this screening isn’t taking place [earlier],” said Dr. Benedito. She added, “We need to head off this trend toward late diagnosis. As the disease progresses, it significantly increases cardiovascular risk and leads to higher mortality, going on dialysis, transplants, et cetera.”
Dr. Alcázar noted that 80% of nephrology cases that are referred to him come from primary care. He explained the need to understand that “these patients have a sevenfold greater risk of suffering a serious cardiovascular event within the next year than people without kidney problems.” Most of these patients will experience an event, even if they don’t undergo dialysis (stage 3 and those near stage 4).
Correct staging
Also fundamental is having a detailed understanding of how staging is performed. Dr. Benedito explained that a chart that pairs glomerular filtration rate (six categories) with the level of albuminuria (three categories) should be used during the visit. For example, a case might be classified as G3a-A2. However, the simplified form of the chart may prove more practical. It classifies chronic kidney disease as being associated with mild, moderate, and severe risk, using different colors to aid comprehension.
Dr. Alcázar noted that the latest guidelines from the European Society of Hypertension for 2023 include albuminuria as an important parameter. The guidelines indicate that for a patient with moderate or severe risk, it is not necessary to calculate their score. “It’s considered high cardiovascular risk, and steps would need to be taken for intervention.”
He then listed the tools available for reversing albuminuria. The process begins by reducing salt consumption and involves the use of medications (angiotensin-converting enzyme inhibitors/angiotensin II receptor antagonists, aldosterone receptor antagonists, glucagon-like peptide-1 analogues, and sodium-glucose cotransporter-2 inhibitors, which slow kidney damage regardless of other measures) and strict management of cardiovascular risk factors (smoking, weight management, blood glucose, hypertension, and moderate physical activity).
Reducing cardiovascular risk
Dr. Alcázar highlighted important factors to keep in mind when managing each of the cardiovascular risk factors. For hypertension, the aim is to achieve levels less than 130/80 mm Hg, although recommendations vary, depending on the guidelines consulted. “KDIGO (Kidney Disease: Improving Global Outcomes) 2021 states that there is no evidence for monitoring diastolic blood pressure, only systolic blood pressure. If we measure it according to the standardized form, SBP should be less than 120 mm Hg, and if not, we would fall back on readings of 130/80 mm Hg.”
For lipid control (specifically, low-density lipoprotein cholesterol), the staging chart indicates that for patients at mild risk, levels should be less than 100 mg/dL; for those at moderate risk, less than 70 mg/dL; and for those at severe risk, less than 55 mg/dL. Hypertriglyceridemia “should only be treated with fibrates if it comes in over 1,000 mg/dL. Also, care must be taken, because these drugs interfere with creatinine excretion, increasing it,” said Dr. Alcázar.
Guidelines from the KDIGO and the American Diabetes Association state that anyone with diabetes and chronic kidney disease should receive a sodium-glucose cotransporter-2 inhibitor if their glomerular filtration rate exceeds 20 mL/min, “which may contradict slightly what it says on the label. Also, if they have hypertension, they should take an angiotensin-converting enzyme inhibitor,” said Dr. Alcázar. He added that “oral antidiabetics, including metformin, must be adjusted based on renal function if glomerular filtration rate is under 30 mL/min.”
Act immediately
When asked whether the course of chronic kidney disease can be changed, Dr. Alcázar responded with an emphatic yes and added that cardiovascular risk can also be substantially reduced. “As nephrologists, we don’t have access to patients in early stages. But family doctors do. Hence the importance of early screening, because going on dialysis at age 60 isn’t the same as at 80.” Currently, “scientific societies are encouraging authorities to screen for chronic kidney disease at earlier ages.”
Regarding drug-based therapy, Dr. Alcázar said that “empagliflozin is not currently indicated for chronic kidney disease in adults.” This sodium-glucose cotransporter-2 inhibitor delays kidney disease and reduces morbidity. Both benefits were highlighted in two recent studies (DAPA-CKD and CREDENCE). Published in January, EMPA-KIDNEY presents a new twist on nephroprotection for patients with chronic kidney disease (diabetic or not) whose glomerular filtration rates are between 20 and 40 mL/min without albuminuria or whose glomerular filtration rates are between 45 and 90 mL/min with albuminuria. For more than 6,000 patients, empagliflozin was observed “to clearly reduce kidney disease progression, cardiovascular mortality and all-cause mortality, and the need to go on dialysis,” stated Dr. Alcázar.
What professionals expect
Dr. Benedito also explained the criteria for referral to a specialist: glomerular filtration rate less than 30 mL/min (unless the patient is older than 80 years and does not have progressively worsening renal function), albumin/creatinine ratio greater than 300 mg/g, acute worsening of renal function, progressive worsening of renal function of greater than 5 mL/min/yr, chronic kidney disease, hypertension treated with triple therapy (including a diuretic) at maximum doses, anemia of less than 10 g/dL, and nonurologic hematuria, especially in combination with albuminuria.
Dr. Benedito explained what nephrologists expect from family doctors in the management of chronic kidney disease: “screening for early detection, identifying and treating risk factors for chronic kidney disease, detecting progression and complications, adjusting drugs based on glomerular filtration rate, and ensuring that our patients are benefiting from sodium-glucose cotransporter-2 inhibitors. These are among the most important steps to be taken.”
Dr. Alcázar mentioned what family doctors expect from nephrologists: “two-way communication, accessibility, coordination of actions to be taken, and using shared and mutually agreed-upon protocols.”
This article was translated from the Medscape Spanish Edition and a version appeared on Medscape.com.
MADRID – A late diagnosis of chronic kidney disease is cause for concern. Scientific societies are therefore advocating for screening at younger ages to reverse this trend and slow the progression of the disease. Nearly all patients seen in primary care are candidates for screening because of their risk factors for kidney disease.
During the 29th National Conference of General and Family Medicine of the Spanish Society for General and Family Physicians, Teresa Benedito, MD, family doctor and member of the society’s cardiovascular group, and Roberto Alcázar, MD, nephrologist at the Infanta Leonor University Hospital, Madrid, presented a clinical case encountered in primary care. They used this case to frame a strong argument for the importance of early screening for chronic kidney disease, and they discussed how to properly manage such screening.
The presentation followed the guidelines in the SEMG publication regarding the management and referral of patients with type 2 diabetes. Dr. Benedito explained that the first thing to ask oneself during a patient visit is “whether they present risk factors for kidney disease. If so, we can’t let them leave before we do a kidney screening.” She then listed the factors in question: age older than 60 years, African heritage, family history of chronic kidney disease, decreased kidney mass, weight loss at birth, hypertension, diabetes, smoking, obesity, and low socioeconomic status.
For his part, Dr. Alcázar mentioned how these factors are similar to cardiovascular risk factors, because “the kidneys are a ball of vessels with double capillarization for purifying blood. They’re the organs with the most arteries per unit of weight, so anything that can damage the arteries can damage the kidneys.”
Candidates for screening
“Chronic kidney disease develops in 15% of the adult population in Spain. So, it’s worth asking how many patients have been diagnosed and who should we should be screening.” To the factors listed above, Dr. Alcázar added treatment with nephrotoxic drugs (including nonsteroidal anti-inflammatory drugs) for patients with obstructive urinary tract disease, and a history of acute kidney injury for patients with chronic autoimmune disease or neoplasms. “Thus, nearly all patients seen in primary care would need to be screened.”
Another fundamental question raised was whether patients should be screened before age 60 years. “As a nephrologist, I feel that we have been diagnosing chronic kidney disease late, even though we’ve been doing everything by the book,” said Dr. Alcázar. In his opinion, “the answer to whether we should be screening earlier ... is yes, for two reasons: first, because it’s cost-effective, and second, because it’s very inexpensive.”
Dr. Benedito explained in detail the process for diagnosing this disease. She began by defining the disease as changes in kidney structure and function that last longer than 3 months. These changes are identified by use of two criteria: glomerular filtration rate less than 60 mL/min and kidney injury or lesions with or without reduced filtration rate (renal biopsy, albumin/creatinine ratio greater than 30 mg/g, proteinuria, alterations in urinary sediment or in imaging tests). Thus, “if one of these two criteria persists for more than 3 months, the diagnosis is chronic kidney disease. Also, high creatinine levels are not diagnostic for the disease,” she emphasized.
Two related parameters
Glomerular filtration and albuminuria “are highly relevant, because screening for chronic kidney disease is based on these two parameters,” said Dr. Benedito. Glomerular filtration rate varies with age, sex, ethnicity, and body mass. It is useful for identifying the stage of the disease and for monitoring disease progression. Albuminuria, on the other hand, is an indication of the severity of the disease. It’s an early marker for kidney injury and systemic disease and is more sensitive than proteinuria. Therefore, “this factor, together with glomerular filtration rate, allows us to detect, classify, and monitor the progression of chronic kidney disease.”
On this point, Dr. Alcázar emphasized the importance of trends, since variation in glomerular filtration depends on serum creatinine, which can vary by nearly 9%. He explained that glomerular filtration rate is related to the number of nephrons remaining. A glomerular filtration rate of less than 60 mL/min implies that more than half of the nephrons in each kidney have been lost. Albuminuria informs about structural damage (that is, the condition of the remaining nephrons). It’s therefore essential to test for both parameters. “We need to be actively monitoring and then making our decisions based on trends and not on isolated results. We need to be aware of albuminuria when we make our decisions,” said Dr. Alcázar. Some studies have shown the importance of testing for albuminuria whenever creatinine level is assessed. “We need to buy into this. If we don’t do this, we’ll only ever have half the information we need.”
Reducing late diagnosis
According to the IBERICAN study, 14% of patients seen in primary care in Spain have chronic kidney disease. “This statistic should make us stop and think, own our responsibility, and ask ourselves why this screening isn’t taking place [earlier],” said Dr. Benedito. She added, “We need to head off this trend toward late diagnosis. As the disease progresses, it significantly increases cardiovascular risk and leads to higher mortality, going on dialysis, transplants, et cetera.”
Dr. Alcázar noted that 80% of nephrology cases that are referred to him come from primary care. He explained the need to understand that “these patients have a sevenfold greater risk of suffering a serious cardiovascular event within the next year than people without kidney problems.” Most of these patients will experience an event, even if they don’t undergo dialysis (stage 3 and those near stage 4).
Correct staging
Also fundamental is having a detailed understanding of how staging is performed. Dr. Benedito explained that a chart that pairs glomerular filtration rate (six categories) with the level of albuminuria (three categories) should be used during the visit. For example, a case might be classified as G3a-A2. However, the simplified form of the chart may prove more practical. It classifies chronic kidney disease as being associated with mild, moderate, and severe risk, using different colors to aid comprehension.
Dr. Alcázar noted that the latest guidelines from the European Society of Hypertension for 2023 include albuminuria as an important parameter. The guidelines indicate that for a patient with moderate or severe risk, it is not necessary to calculate their score. “It’s considered high cardiovascular risk, and steps would need to be taken for intervention.”
He then listed the tools available for reversing albuminuria. The process begins by reducing salt consumption and involves the use of medications (angiotensin-converting enzyme inhibitors/angiotensin II receptor antagonists, aldosterone receptor antagonists, glucagon-like peptide-1 analogues, and sodium-glucose cotransporter-2 inhibitors, which slow kidney damage regardless of other measures) and strict management of cardiovascular risk factors (smoking, weight management, blood glucose, hypertension, and moderate physical activity).
Reducing cardiovascular risk
Dr. Alcázar highlighted important factors to keep in mind when managing each of the cardiovascular risk factors. For hypertension, the aim is to achieve levels less than 130/80 mm Hg, although recommendations vary, depending on the guidelines consulted. “KDIGO (Kidney Disease: Improving Global Outcomes) 2021 states that there is no evidence for monitoring diastolic blood pressure, only systolic blood pressure. If we measure it according to the standardized form, SBP should be less than 120 mm Hg, and if not, we would fall back on readings of 130/80 mm Hg.”
For lipid control (specifically, low-density lipoprotein cholesterol), the staging chart indicates that for patients at mild risk, levels should be less than 100 mg/dL; for those at moderate risk, less than 70 mg/dL; and for those at severe risk, less than 55 mg/dL. Hypertriglyceridemia “should only be treated with fibrates if it comes in over 1,000 mg/dL. Also, care must be taken, because these drugs interfere with creatinine excretion, increasing it,” said Dr. Alcázar.
Guidelines from the KDIGO and the American Diabetes Association state that anyone with diabetes and chronic kidney disease should receive a sodium-glucose cotransporter-2 inhibitor if their glomerular filtration rate exceeds 20 mL/min, “which may contradict slightly what it says on the label. Also, if they have hypertension, they should take an angiotensin-converting enzyme inhibitor,” said Dr. Alcázar. He added that “oral antidiabetics, including metformin, must be adjusted based on renal function if glomerular filtration rate is under 30 mL/min.”
Act immediately
When asked whether the course of chronic kidney disease can be changed, Dr. Alcázar responded with an emphatic yes and added that cardiovascular risk can also be substantially reduced. “As nephrologists, we don’t have access to patients in early stages. But family doctors do. Hence the importance of early screening, because going on dialysis at age 60 isn’t the same as at 80.” Currently, “scientific societies are encouraging authorities to screen for chronic kidney disease at earlier ages.”
Regarding drug-based therapy, Dr. Alcázar said that “empagliflozin is not currently indicated for chronic kidney disease in adults.” This sodium-glucose cotransporter-2 inhibitor delays kidney disease and reduces morbidity. Both benefits were highlighted in two recent studies (DAPA-CKD and CREDENCE). Published in January, EMPA-KIDNEY presents a new twist on nephroprotection for patients with chronic kidney disease (diabetic or not) whose glomerular filtration rates are between 20 and 40 mL/min without albuminuria or whose glomerular filtration rates are between 45 and 90 mL/min with albuminuria. For more than 6,000 patients, empagliflozin was observed “to clearly reduce kidney disease progression, cardiovascular mortality and all-cause mortality, and the need to go on dialysis,” stated Dr. Alcázar.
What professionals expect
Dr. Benedito also explained the criteria for referral to a specialist: glomerular filtration rate less than 30 mL/min (unless the patient is older than 80 years and does not have progressively worsening renal function), albumin/creatinine ratio greater than 300 mg/g, acute worsening of renal function, progressive worsening of renal function of greater than 5 mL/min/yr, chronic kidney disease, hypertension treated with triple therapy (including a diuretic) at maximum doses, anemia of less than 10 g/dL, and nonurologic hematuria, especially in combination with albuminuria.
Dr. Benedito explained what nephrologists expect from family doctors in the management of chronic kidney disease: “screening for early detection, identifying and treating risk factors for chronic kidney disease, detecting progression and complications, adjusting drugs based on glomerular filtration rate, and ensuring that our patients are benefiting from sodium-glucose cotransporter-2 inhibitors. These are among the most important steps to be taken.”
Dr. Alcázar mentioned what family doctors expect from nephrologists: “two-way communication, accessibility, coordination of actions to be taken, and using shared and mutually agreed-upon protocols.”
This article was translated from the Medscape Spanish Edition and a version appeared on Medscape.com.
MADRID – A late diagnosis of chronic kidney disease is cause for concern. Scientific societies are therefore advocating for screening at younger ages to reverse this trend and slow the progression of the disease. Nearly all patients seen in primary care are candidates for screening because of their risk factors for kidney disease.
During the 29th National Conference of General and Family Medicine of the Spanish Society for General and Family Physicians, Teresa Benedito, MD, family doctor and member of the society’s cardiovascular group, and Roberto Alcázar, MD, nephrologist at the Infanta Leonor University Hospital, Madrid, presented a clinical case encountered in primary care. They used this case to frame a strong argument for the importance of early screening for chronic kidney disease, and they discussed how to properly manage such screening.
The presentation followed the guidelines in the SEMG publication regarding the management and referral of patients with type 2 diabetes. Dr. Benedito explained that the first thing to ask oneself during a patient visit is “whether they present risk factors for kidney disease. If so, we can’t let them leave before we do a kidney screening.” She then listed the factors in question: age older than 60 years, African heritage, family history of chronic kidney disease, decreased kidney mass, weight loss at birth, hypertension, diabetes, smoking, obesity, and low socioeconomic status.
For his part, Dr. Alcázar mentioned how these factors are similar to cardiovascular risk factors, because “the kidneys are a ball of vessels with double capillarization for purifying blood. They’re the organs with the most arteries per unit of weight, so anything that can damage the arteries can damage the kidneys.”
Candidates for screening
“Chronic kidney disease develops in 15% of the adult population in Spain. So, it’s worth asking how many patients have been diagnosed and who should we should be screening.” To the factors listed above, Dr. Alcázar added treatment with nephrotoxic drugs (including nonsteroidal anti-inflammatory drugs) for patients with obstructive urinary tract disease, and a history of acute kidney injury for patients with chronic autoimmune disease or neoplasms. “Thus, nearly all patients seen in primary care would need to be screened.”
Another fundamental question raised was whether patients should be screened before age 60 years. “As a nephrologist, I feel that we have been diagnosing chronic kidney disease late, even though we’ve been doing everything by the book,” said Dr. Alcázar. In his opinion, “the answer to whether we should be screening earlier ... is yes, for two reasons: first, because it’s cost-effective, and second, because it’s very inexpensive.”
Dr. Benedito explained in detail the process for diagnosing this disease. She began by defining the disease as changes in kidney structure and function that last longer than 3 months. These changes are identified by use of two criteria: glomerular filtration rate less than 60 mL/min and kidney injury or lesions with or without reduced filtration rate (renal biopsy, albumin/creatinine ratio greater than 30 mg/g, proteinuria, alterations in urinary sediment or in imaging tests). Thus, “if one of these two criteria persists for more than 3 months, the diagnosis is chronic kidney disease. Also, high creatinine levels are not diagnostic for the disease,” she emphasized.
Two related parameters
Glomerular filtration and albuminuria “are highly relevant, because screening for chronic kidney disease is based on these two parameters,” said Dr. Benedito. Glomerular filtration rate varies with age, sex, ethnicity, and body mass. It is useful for identifying the stage of the disease and for monitoring disease progression. Albuminuria, on the other hand, is an indication of the severity of the disease. It’s an early marker for kidney injury and systemic disease and is more sensitive than proteinuria. Therefore, “this factor, together with glomerular filtration rate, allows us to detect, classify, and monitor the progression of chronic kidney disease.”
On this point, Dr. Alcázar emphasized the importance of trends, since variation in glomerular filtration depends on serum creatinine, which can vary by nearly 9%. He explained that glomerular filtration rate is related to the number of nephrons remaining. A glomerular filtration rate of less than 60 mL/min implies that more than half of the nephrons in each kidney have been lost. Albuminuria informs about structural damage (that is, the condition of the remaining nephrons). It’s therefore essential to test for both parameters. “We need to be actively monitoring and then making our decisions based on trends and not on isolated results. We need to be aware of albuminuria when we make our decisions,” said Dr. Alcázar. Some studies have shown the importance of testing for albuminuria whenever creatinine level is assessed. “We need to buy into this. If we don’t do this, we’ll only ever have half the information we need.”
Reducing late diagnosis
According to the IBERICAN study, 14% of patients seen in primary care in Spain have chronic kidney disease. “This statistic should make us stop and think, own our responsibility, and ask ourselves why this screening isn’t taking place [earlier],” said Dr. Benedito. She added, “We need to head off this trend toward late diagnosis. As the disease progresses, it significantly increases cardiovascular risk and leads to higher mortality, going on dialysis, transplants, et cetera.”
Dr. Alcázar noted that 80% of nephrology cases that are referred to him come from primary care. He explained the need to understand that “these patients have a sevenfold greater risk of suffering a serious cardiovascular event within the next year than people without kidney problems.” Most of these patients will experience an event, even if they don’t undergo dialysis (stage 3 and those near stage 4).
Correct staging
Also fundamental is having a detailed understanding of how staging is performed. Dr. Benedito explained that a chart that pairs glomerular filtration rate (six categories) with the level of albuminuria (three categories) should be used during the visit. For example, a case might be classified as G3a-A2. However, the simplified form of the chart may prove more practical. It classifies chronic kidney disease as being associated with mild, moderate, and severe risk, using different colors to aid comprehension.
Dr. Alcázar noted that the latest guidelines from the European Society of Hypertension for 2023 include albuminuria as an important parameter. The guidelines indicate that for a patient with moderate or severe risk, it is not necessary to calculate their score. “It’s considered high cardiovascular risk, and steps would need to be taken for intervention.”
He then listed the tools available for reversing albuminuria. The process begins by reducing salt consumption and involves the use of medications (angiotensin-converting enzyme inhibitors/angiotensin II receptor antagonists, aldosterone receptor antagonists, glucagon-like peptide-1 analogues, and sodium-glucose cotransporter-2 inhibitors, which slow kidney damage regardless of other measures) and strict management of cardiovascular risk factors (smoking, weight management, blood glucose, hypertension, and moderate physical activity).
Reducing cardiovascular risk
Dr. Alcázar highlighted important factors to keep in mind when managing each of the cardiovascular risk factors. For hypertension, the aim is to achieve levels less than 130/80 mm Hg, although recommendations vary, depending on the guidelines consulted. “KDIGO (Kidney Disease: Improving Global Outcomes) 2021 states that there is no evidence for monitoring diastolic blood pressure, only systolic blood pressure. If we measure it according to the standardized form, SBP should be less than 120 mm Hg, and if not, we would fall back on readings of 130/80 mm Hg.”
For lipid control (specifically, low-density lipoprotein cholesterol), the staging chart indicates that for patients at mild risk, levels should be less than 100 mg/dL; for those at moderate risk, less than 70 mg/dL; and for those at severe risk, less than 55 mg/dL. Hypertriglyceridemia “should only be treated with fibrates if it comes in over 1,000 mg/dL. Also, care must be taken, because these drugs interfere with creatinine excretion, increasing it,” said Dr. Alcázar.
Guidelines from the KDIGO and the American Diabetes Association state that anyone with diabetes and chronic kidney disease should receive a sodium-glucose cotransporter-2 inhibitor if their glomerular filtration rate exceeds 20 mL/min, “which may contradict slightly what it says on the label. Also, if they have hypertension, they should take an angiotensin-converting enzyme inhibitor,” said Dr. Alcázar. He added that “oral antidiabetics, including metformin, must be adjusted based on renal function if glomerular filtration rate is under 30 mL/min.”
Act immediately
When asked whether the course of chronic kidney disease can be changed, Dr. Alcázar responded with an emphatic yes and added that cardiovascular risk can also be substantially reduced. “As nephrologists, we don’t have access to patients in early stages. But family doctors do. Hence the importance of early screening, because going on dialysis at age 60 isn’t the same as at 80.” Currently, “scientific societies are encouraging authorities to screen for chronic kidney disease at earlier ages.”
Regarding drug-based therapy, Dr. Alcázar said that “empagliflozin is not currently indicated for chronic kidney disease in adults.” This sodium-glucose cotransporter-2 inhibitor delays kidney disease and reduces morbidity. Both benefits were highlighted in two recent studies (DAPA-CKD and CREDENCE). Published in January, EMPA-KIDNEY presents a new twist on nephroprotection for patients with chronic kidney disease (diabetic or not) whose glomerular filtration rates are between 20 and 40 mL/min without albuminuria or whose glomerular filtration rates are between 45 and 90 mL/min with albuminuria. For more than 6,000 patients, empagliflozin was observed “to clearly reduce kidney disease progression, cardiovascular mortality and all-cause mortality, and the need to go on dialysis,” stated Dr. Alcázar.
What professionals expect
Dr. Benedito also explained the criteria for referral to a specialist: glomerular filtration rate less than 30 mL/min (unless the patient is older than 80 years and does not have progressively worsening renal function), albumin/creatinine ratio greater than 300 mg/g, acute worsening of renal function, progressive worsening of renal function of greater than 5 mL/min/yr, chronic kidney disease, hypertension treated with triple therapy (including a diuretic) at maximum doses, anemia of less than 10 g/dL, and nonurologic hematuria, especially in combination with albuminuria.
Dr. Benedito explained what nephrologists expect from family doctors in the management of chronic kidney disease: “screening for early detection, identifying and treating risk factors for chronic kidney disease, detecting progression and complications, adjusting drugs based on glomerular filtration rate, and ensuring that our patients are benefiting from sodium-glucose cotransporter-2 inhibitors. These are among the most important steps to be taken.”
Dr. Alcázar mentioned what family doctors expect from nephrologists: “two-way communication, accessibility, coordination of actions to be taken, and using shared and mutually agreed-upon protocols.”
This article was translated from the Medscape Spanish Edition and a version appeared on Medscape.com.