User login
Dapagliflozin reduces hospitalizations in patients with CKD
These findings add to a growing body of evidence supporting a range of positive benefits from dapagliflozin, including reduced risks of mortality, cardiovascular events, and kidney events, lead author Meir Schechter, MD, PhD, of the Hebrew University of Jerusalem and colleagues wrote in Annals of Internal Medicine.“Although cardiovascular and kidney outcomes with SGLT2 inhibitors have been studied extensively, there is a paucity of data evaluating the effects of SGLT2 inhibitors on hospitalizations for any cause.”
The findings are based on a post hoc analysis of the DAPA-CKD trial, which involved 4,304 patients with CKD in 21 countries. Patients were randomized in a 1:1 ratio to receive dapagliflozin 10 mg orally once a day or matching placebo. The present analysis quantified first hospitalizations for any cause, all hospitalizations, cause-specific hospitalizations, and several related outcomes.
After a median follow-up of 2.4 years, 28% of the population had been hospitalized a total of 2,072 times.
Compared with placebo, dapagliflozin significantly reduced risk of first hospitalization by 16% (hazard ratio, 0.84; 95% confidence interval, 0.75-0.94) and rate of all hospitalizations by 21% (rate ratio, 0.79; 95% CI, 0.70-0.89). These findings remained significant regardless of type 2 diabetes status, with significant benefits seen across reasons for admission, including renal/urinary disorders, cardiac disorders, neoplasms, and metabolism/nutrition disorders. In addition, dapagliflozin was associated with shorter mean time in hospital (2.3 vs. 2.8 days; P = .027) and longer time alive and out of hospital (354.9 vs. 351.7; P = .023).
“These findings highlight additional benefits of dapagliflozin beyond those seen for cardiovascular and kidney events, all-cause and cause-specific mortality, eGFR [estimated glomerular filtration rate] slope, and albuminuria and should be considered when evaluating the totality of evidence favoring provision of dapagliflozin to patients with CKD,” the investigators concluded.
Positive data, positive experiences
Shree Mulay, MD, a nephrologist in private practice in western Tennessee, said this study is “one of several other articles that already exist” demonstrating the broad benefits of SGLT2 inhibitors.
“The evidence is pretty substantial,” Dr. Mulay said in an interview. “I think SGLT2 inhibitors are the new statin of this era. ... I won’t be surprised if in the next year or 2 or 3 they truly become the standard of care.”
Dr. Mulay also speaks from experience working in both the chronic and acute setting, where he’s observed “some magical stuff happening” in patients started on SGLT2 inhibitors, especially those in heart failure who are fluid overloaded.
“It’s phenomenal stuff,” Dr. Mulay said. “You can really stabilize patients’ hemodynamics.”
In the private health care setting, he described widespread enthusiasm among nephrologists, although others still appear skeptical.
“It’s really our cardiology colleagues that I feel are underprescribing it,” Dr. Mulay said. “So, I’m kind of taking it on myself, when I see a heart failure patient, to go ahead and put them on this.”
It’s unclear why some cardiologists seem apprehensive, Dr. Mulay continued, although he suggested that unclear guidelines and a lack of first-hand experience may be to blame.
Nephrologists and cardiologists sometimes agree
In the academic arena, Leslie Gewin, MD, associate professor at Washington University in St. Louis and the John Cochran VA Hospital, also in St. Louis, has seen similar support for SGLT2 inhibitors among both nephrologists and cardiologists.
“We had a joint nephrology-cardiology medicine grand rounds at Wash U in St. Louis maybe 2 weeks ago,” Dr. Gewin said in an interview. “The cardiologists and nephrologists tag-teamed to present data about SGLT2 inhibitors, and we kind of joked that this was the one thing we both could get behind and support.”
Still, she has seen some reluctance among non-nephrology clinicians lacking SGLT2 experience, specifically when managing patients who have poor kidney function.
“There can be some hesitancy among physicians if the GFR is low,” Dr. Gewin said. “That’s where I’ve had to sort of push the envelope with non-nephrologists, saying: ‘Look, we feel pretty comfortable starting down to a GFR of about 20.’ ”
Early rises in creatinine may also spook providers, she noted.
“Sometimes, when we start SGLT2 inhibitors, the creatinine increases slightly, and the [primary care provider] gets concerned,” Dr. Gewin said. “We say: ‘No, this is expected. Don’t worry, hold the course, this is a good drug.’ ”
Like Dr. Mulay, Dr. Gewin said the present study offers further encouragement for the efficacy of this drug class. She also said sufficient data have been published to allay earlier concerns about potential safety signals, such as bone fractures and amputations.
“SGLT2 inhibitors seem to be a lot safer than what we initially had thought,” Dr. Gewin said. “That’s very encouraging.”
The study was funded by AstraZeneca. The investigators disclosed additional relationships with Bayer, Janssen, Gilead, and others. Dr. Gewin and Dr. Mulay disclosed no relevant conflicts of interest.
These findings add to a growing body of evidence supporting a range of positive benefits from dapagliflozin, including reduced risks of mortality, cardiovascular events, and kidney events, lead author Meir Schechter, MD, PhD, of the Hebrew University of Jerusalem and colleagues wrote in Annals of Internal Medicine.“Although cardiovascular and kidney outcomes with SGLT2 inhibitors have been studied extensively, there is a paucity of data evaluating the effects of SGLT2 inhibitors on hospitalizations for any cause.”
The findings are based on a post hoc analysis of the DAPA-CKD trial, which involved 4,304 patients with CKD in 21 countries. Patients were randomized in a 1:1 ratio to receive dapagliflozin 10 mg orally once a day or matching placebo. The present analysis quantified first hospitalizations for any cause, all hospitalizations, cause-specific hospitalizations, and several related outcomes.
After a median follow-up of 2.4 years, 28% of the population had been hospitalized a total of 2,072 times.
Compared with placebo, dapagliflozin significantly reduced risk of first hospitalization by 16% (hazard ratio, 0.84; 95% confidence interval, 0.75-0.94) and rate of all hospitalizations by 21% (rate ratio, 0.79; 95% CI, 0.70-0.89). These findings remained significant regardless of type 2 diabetes status, with significant benefits seen across reasons for admission, including renal/urinary disorders, cardiac disorders, neoplasms, and metabolism/nutrition disorders. In addition, dapagliflozin was associated with shorter mean time in hospital (2.3 vs. 2.8 days; P = .027) and longer time alive and out of hospital (354.9 vs. 351.7; P = .023).
“These findings highlight additional benefits of dapagliflozin beyond those seen for cardiovascular and kidney events, all-cause and cause-specific mortality, eGFR [estimated glomerular filtration rate] slope, and albuminuria and should be considered when evaluating the totality of evidence favoring provision of dapagliflozin to patients with CKD,” the investigators concluded.
Positive data, positive experiences
Shree Mulay, MD, a nephrologist in private practice in western Tennessee, said this study is “one of several other articles that already exist” demonstrating the broad benefits of SGLT2 inhibitors.
“The evidence is pretty substantial,” Dr. Mulay said in an interview. “I think SGLT2 inhibitors are the new statin of this era. ... I won’t be surprised if in the next year or 2 or 3 they truly become the standard of care.”
Dr. Mulay also speaks from experience working in both the chronic and acute setting, where he’s observed “some magical stuff happening” in patients started on SGLT2 inhibitors, especially those in heart failure who are fluid overloaded.
“It’s phenomenal stuff,” Dr. Mulay said. “You can really stabilize patients’ hemodynamics.”
In the private health care setting, he described widespread enthusiasm among nephrologists, although others still appear skeptical.
“It’s really our cardiology colleagues that I feel are underprescribing it,” Dr. Mulay said. “So, I’m kind of taking it on myself, when I see a heart failure patient, to go ahead and put them on this.”
It’s unclear why some cardiologists seem apprehensive, Dr. Mulay continued, although he suggested that unclear guidelines and a lack of first-hand experience may be to blame.
Nephrologists and cardiologists sometimes agree
In the academic arena, Leslie Gewin, MD, associate professor at Washington University in St. Louis and the John Cochran VA Hospital, also in St. Louis, has seen similar support for SGLT2 inhibitors among both nephrologists and cardiologists.
“We had a joint nephrology-cardiology medicine grand rounds at Wash U in St. Louis maybe 2 weeks ago,” Dr. Gewin said in an interview. “The cardiologists and nephrologists tag-teamed to present data about SGLT2 inhibitors, and we kind of joked that this was the one thing we both could get behind and support.”
Still, she has seen some reluctance among non-nephrology clinicians lacking SGLT2 experience, specifically when managing patients who have poor kidney function.
“There can be some hesitancy among physicians if the GFR is low,” Dr. Gewin said. “That’s where I’ve had to sort of push the envelope with non-nephrologists, saying: ‘Look, we feel pretty comfortable starting down to a GFR of about 20.’ ”
Early rises in creatinine may also spook providers, she noted.
“Sometimes, when we start SGLT2 inhibitors, the creatinine increases slightly, and the [primary care provider] gets concerned,” Dr. Gewin said. “We say: ‘No, this is expected. Don’t worry, hold the course, this is a good drug.’ ”
Like Dr. Mulay, Dr. Gewin said the present study offers further encouragement for the efficacy of this drug class. She also said sufficient data have been published to allay earlier concerns about potential safety signals, such as bone fractures and amputations.
“SGLT2 inhibitors seem to be a lot safer than what we initially had thought,” Dr. Gewin said. “That’s very encouraging.”
The study was funded by AstraZeneca. The investigators disclosed additional relationships with Bayer, Janssen, Gilead, and others. Dr. Gewin and Dr. Mulay disclosed no relevant conflicts of interest.
These findings add to a growing body of evidence supporting a range of positive benefits from dapagliflozin, including reduced risks of mortality, cardiovascular events, and kidney events, lead author Meir Schechter, MD, PhD, of the Hebrew University of Jerusalem and colleagues wrote in Annals of Internal Medicine.“Although cardiovascular and kidney outcomes with SGLT2 inhibitors have been studied extensively, there is a paucity of data evaluating the effects of SGLT2 inhibitors on hospitalizations for any cause.”
The findings are based on a post hoc analysis of the DAPA-CKD trial, which involved 4,304 patients with CKD in 21 countries. Patients were randomized in a 1:1 ratio to receive dapagliflozin 10 mg orally once a day or matching placebo. The present analysis quantified first hospitalizations for any cause, all hospitalizations, cause-specific hospitalizations, and several related outcomes.
After a median follow-up of 2.4 years, 28% of the population had been hospitalized a total of 2,072 times.
Compared with placebo, dapagliflozin significantly reduced risk of first hospitalization by 16% (hazard ratio, 0.84; 95% confidence interval, 0.75-0.94) and rate of all hospitalizations by 21% (rate ratio, 0.79; 95% CI, 0.70-0.89). These findings remained significant regardless of type 2 diabetes status, with significant benefits seen across reasons for admission, including renal/urinary disorders, cardiac disorders, neoplasms, and metabolism/nutrition disorders. In addition, dapagliflozin was associated with shorter mean time in hospital (2.3 vs. 2.8 days; P = .027) and longer time alive and out of hospital (354.9 vs. 351.7; P = .023).
“These findings highlight additional benefits of dapagliflozin beyond those seen for cardiovascular and kidney events, all-cause and cause-specific mortality, eGFR [estimated glomerular filtration rate] slope, and albuminuria and should be considered when evaluating the totality of evidence favoring provision of dapagliflozin to patients with CKD,” the investigators concluded.
Positive data, positive experiences
Shree Mulay, MD, a nephrologist in private practice in western Tennessee, said this study is “one of several other articles that already exist” demonstrating the broad benefits of SGLT2 inhibitors.
“The evidence is pretty substantial,” Dr. Mulay said in an interview. “I think SGLT2 inhibitors are the new statin of this era. ... I won’t be surprised if in the next year or 2 or 3 they truly become the standard of care.”
Dr. Mulay also speaks from experience working in both the chronic and acute setting, where he’s observed “some magical stuff happening” in patients started on SGLT2 inhibitors, especially those in heart failure who are fluid overloaded.
“It’s phenomenal stuff,” Dr. Mulay said. “You can really stabilize patients’ hemodynamics.”
In the private health care setting, he described widespread enthusiasm among nephrologists, although others still appear skeptical.
“It’s really our cardiology colleagues that I feel are underprescribing it,” Dr. Mulay said. “So, I’m kind of taking it on myself, when I see a heart failure patient, to go ahead and put them on this.”
It’s unclear why some cardiologists seem apprehensive, Dr. Mulay continued, although he suggested that unclear guidelines and a lack of first-hand experience may be to blame.
Nephrologists and cardiologists sometimes agree
In the academic arena, Leslie Gewin, MD, associate professor at Washington University in St. Louis and the John Cochran VA Hospital, also in St. Louis, has seen similar support for SGLT2 inhibitors among both nephrologists and cardiologists.
“We had a joint nephrology-cardiology medicine grand rounds at Wash U in St. Louis maybe 2 weeks ago,” Dr. Gewin said in an interview. “The cardiologists and nephrologists tag-teamed to present data about SGLT2 inhibitors, and we kind of joked that this was the one thing we both could get behind and support.”
Still, she has seen some reluctance among non-nephrology clinicians lacking SGLT2 experience, specifically when managing patients who have poor kidney function.
“There can be some hesitancy among physicians if the GFR is low,” Dr. Gewin said. “That’s where I’ve had to sort of push the envelope with non-nephrologists, saying: ‘Look, we feel pretty comfortable starting down to a GFR of about 20.’ ”
Early rises in creatinine may also spook providers, she noted.
“Sometimes, when we start SGLT2 inhibitors, the creatinine increases slightly, and the [primary care provider] gets concerned,” Dr. Gewin said. “We say: ‘No, this is expected. Don’t worry, hold the course, this is a good drug.’ ”
Like Dr. Mulay, Dr. Gewin said the present study offers further encouragement for the efficacy of this drug class. She also said sufficient data have been published to allay earlier concerns about potential safety signals, such as bone fractures and amputations.
“SGLT2 inhibitors seem to be a lot safer than what we initially had thought,” Dr. Gewin said. “That’s very encouraging.”
The study was funded by AstraZeneca. The investigators disclosed additional relationships with Bayer, Janssen, Gilead, and others. Dr. Gewin and Dr. Mulay disclosed no relevant conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE
Consider quality of life, comorbidities in hidradenitis suppurativa
LAS VEGAS – , Robert G. Micheletti, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
For patients with HS, “the quality-of-life impact is profound, greater than any other systematically studied dermatologic condition,” said Dr. Micheletti, associate professor of dermatology at the Hospital of the University of Pennsylavnia, and chief of hospital dermatology, and chief of dermatology at Pennsylvania Hospital, Philadelphia.
Two key aspects of quality of life that affect HS patients are sexual health and overall pain, he said. The female-to-male ratio of HS is approximately 3:1, and data show that approximately 40% of female HS patients experience fertility issues and have unaddressed questions about HS and pregnancy, said Dr. Micheletti. Additionally, data from a systematic review showed that 50%-60% of patients with HS reported sexual dysfunction. Impaired sexual function is also associated with both overall impaired quality of life ratings and the presence of mood disorders, he noted.
Pain also has a significant impact on quality of life for HS patients. When these patients present in an emergency department, 70% report severe pain, and approximately 60% receive opioids, said Dr. Micheletti.
Data from a 2021 study showed that HS patients are significantly more likely to receive opioids compared with controls, and also more likely to be diagnosed with opioid use disorder than controls, especially if they are seen by nondermatologists, he noted.
For acute pain, Dr. Micheletti recommended starting with acetaminophen 500 mg every 4 to 6 hours as needed, and topical nonsteroidal anti-inflammatory drugs (NSAIDs). “It still makes sense to do topical care,” said Dr. Micheletti, but he added that he also prescribes medications for anxiety for these patients.
Patients with increased pain severity or refractory disease may benefit from systemic NSAIDs, or intralesional triamcinolone, he noted. Incision and draining of abscesses may provide temporary symptomatic relief, but keep in mind that lesions will recur, he noted.
For the most severe cases, Dr. Micheletti advised adding tramadol as a first-line opioid, or another short-acting opioid for breakthrough pain.
To manage patients with HS who have chronic pain, Dr. Micheletti recommended starting with HS disease–directed therapy, but also screening for pain severity and psychological comorbidities.
His strategies in these cases include nonpharmacological pain management in the form of physical therapy, wound care, and behavioral health. His algorithm for nociceptive pain is NSAIDs with or without acetaminophen; duloxetine or nortriptyline are other options. For neuropathic pain, gabapentin and/or duloxetine are top choices, but pregabalin, venlafaxine, and nortriptyline are on the list as well.
Topical NSAIDs or topical lidocaine may serve as add-ons to systemic therapy in more severe cases, or as first-line therapy for milder chronic pain, Dr. Micheletti noted. Patients who have failed treatment with at least two pharmacologic agents, suffer medically refractory HS with debilitating pain, or use opioids on an ongoing basis should be referred to a pain management specialist, he said.
Don’t forget lifestyle
Although data on the impact of diet on patients with HS are limited, “we know anecdotally that dairy and refined carbohydrates are associated with exacerbations,” said Dr. Micheletti.
In addition, many patients use complementary medicine “and they aren’t always telling us,” he emphasized. Smoking is prevalent among patients with HS, and is a risk factor for the disease in general, and for more severe and refractory disease, he added. Consequently, screening for tobacco smoking is recommended for patients with HS not only because of the impact on disease, but because it is a potentially modifiable cardiovascular risk factor, he explained.
Consider comorbidities
Cardiovascular disease is among several comorbidities associated with HS, said Dr. Micheletti. HS foundations in the United States and Canada recently published evidence-based recommendations for comorbidity screening. The recommendations included screening for 19 specific comorbidities: acne, dissecting cellulitis, pilonidal disease, pyoderma gangrenosum, depression, anxiety, suicide, smoking, substance abuse, polycystic ovary syndrome, obesity, dyslipidemia, diabetes mellitus, metabolic syndrome, hypertension, cardiovascular disease, inflammatory bowel disease, spondyloarthritis, and sexual dysfunction.
Dr. Micheletti highlighted cardiovascular comorbidities, and noted the association between HS and modifiable cardiovascular risk factors: smoking, obesity, diabetes mellitus, and dyslipidemia. “HS is also independently associated with cardiovascular disease leading to myocardial infarction, stroke, cardiovascular-associated death, and all-cause mortality compared to controls,” he said. Studies show an incidence rate ratio of 1.53 for major adverse cardiovascular events in patients with HS compared with controls, with the highest relative risk among those aged 18-29 years, he added.
Medical management
Depending on the patient, medical management of HS may involve antibiotics, hormonal agents, and biologics, said Dr. Micheletti. Some of the most commonly used antibiotic regimens for HS are those recommended in treatment guidelines, including doxycycline and a clindamycin/rifampin combination, he said. However, the use of trimethoprim-sulfamethoxazole or ciprofloxacin has been associated with increased antibiotic resistance and is not supported by available evidence, he noted.
Hormonal therapies may help some women with HS, said Dr. Micheletti. Options include spironolactone, metformin, or estrogen-containing hormonal contraceptives, he said.
When it comes to biologics, only 33% of HS patients meet criteria for their use (Hurley stage II or III, moderate or severe HS), he noted. However, research suggests “a huge gap” in the use of anti-TNF therapy even among patients for whom it is recommended, he said.
Of the TNF-alpha inhibitors, data on adalimumab, which is FDA-approved for HS, are the most recent. Adalimumab “is our gold standard biologic and our gateway biologic, for HS at this time,” Dr. Micheletti said.
However, those who respond to adalimumab “can continue to do better, but they can wax and wane and flare,” he cautioned. Infliximab, while not approved for HS, has been studied in patients with HS and is prescribed by some providers. Although no comparative studies have been done for infliximab versus adalimumab, “anecdotally, response to infliximab tends to be better, and it is the most effective biologic in common use for severe HS,” he noted.
Dr. Micheletti’s top treatment recommendations for using biologics start with considering biosimilars. Most patients on biosimilars do fine, but some patients who previously responded to infliximab will unpredictably lose efficacy or have reactions when switched to a biosimilar, he said.
Patients on biologics also may experience waning efficacy in the wake of an immune response stimulated by foreign antibodies, said Dr. Micheletti. “Anti-drug antibody formation is more likely to occur when treatment is interrupted,” he noted. Minimize the risk of antibody formation by paying attention to adherence issues and dosing frequency, he advised.
If patients fail both adalimumab and infliximab, Dr. Micheletti tells them not to lose hope, and that treatment is a trial-and-error process that may involve more than one therapy. Other biologics in active use for HS include ustekinumab, anakinra, secukinumab, brodalumab, golimumab, and JAK inhibitors, any of which might be effective in any given patient, he said.
Surgical solutions
For HS patients with chronic, recurring inflammation and drainage associated with a sinus tract, surgical deroofing may the best treatment option, Dr. Micheletti said. “Deroofing involves the use of a probe to trace the extent of the subcutaneous tract, followed by incision and removal of the tract ‘roof,’ ’’ he explained. The deroofing procedure involves local anesthesia and has a low morbidity rate, as well as a low recurrence rate and high levels of patient satisfaction, he said.
“The acute role for surgery is to remove active foci of inflammation and relieve pain,” which is achieved more effectively with deroofing, said Dr. Micheletti. By contrast, incision and drainage is associated with an almost 100% recurrence rate, he added.
When planning elective surgery for HS, Dr. Micheletti noted that holding infliximab for less than 4 weeks does not affect postoperative infection rates in patients with rheumatoid arthritis, and a recent randomized, controlled trial showed that adalimumab can be continued safely through HS surgeries.
In fact, “continuing TNF inhibitors through elective surgery does not increase infection risk and results in better disease control,” and dermatologists should work with surgery to balance infection and disease flare concerns in HS patients, he said.
Dr. Micheletti disclosed serving as a consultant or advisor for Adaptimmune and Vertex, and research funding from Amgen and Cabaletta Bio. MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – , Robert G. Micheletti, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
For patients with HS, “the quality-of-life impact is profound, greater than any other systematically studied dermatologic condition,” said Dr. Micheletti, associate professor of dermatology at the Hospital of the University of Pennsylavnia, and chief of hospital dermatology, and chief of dermatology at Pennsylvania Hospital, Philadelphia.
Two key aspects of quality of life that affect HS patients are sexual health and overall pain, he said. The female-to-male ratio of HS is approximately 3:1, and data show that approximately 40% of female HS patients experience fertility issues and have unaddressed questions about HS and pregnancy, said Dr. Micheletti. Additionally, data from a systematic review showed that 50%-60% of patients with HS reported sexual dysfunction. Impaired sexual function is also associated with both overall impaired quality of life ratings and the presence of mood disorders, he noted.
Pain also has a significant impact on quality of life for HS patients. When these patients present in an emergency department, 70% report severe pain, and approximately 60% receive opioids, said Dr. Micheletti.
Data from a 2021 study showed that HS patients are significantly more likely to receive opioids compared with controls, and also more likely to be diagnosed with opioid use disorder than controls, especially if they are seen by nondermatologists, he noted.
For acute pain, Dr. Micheletti recommended starting with acetaminophen 500 mg every 4 to 6 hours as needed, and topical nonsteroidal anti-inflammatory drugs (NSAIDs). “It still makes sense to do topical care,” said Dr. Micheletti, but he added that he also prescribes medications for anxiety for these patients.
Patients with increased pain severity or refractory disease may benefit from systemic NSAIDs, or intralesional triamcinolone, he noted. Incision and draining of abscesses may provide temporary symptomatic relief, but keep in mind that lesions will recur, he noted.
For the most severe cases, Dr. Micheletti advised adding tramadol as a first-line opioid, or another short-acting opioid for breakthrough pain.
To manage patients with HS who have chronic pain, Dr. Micheletti recommended starting with HS disease–directed therapy, but also screening for pain severity and psychological comorbidities.
His strategies in these cases include nonpharmacological pain management in the form of physical therapy, wound care, and behavioral health. His algorithm for nociceptive pain is NSAIDs with or without acetaminophen; duloxetine or nortriptyline are other options. For neuropathic pain, gabapentin and/or duloxetine are top choices, but pregabalin, venlafaxine, and nortriptyline are on the list as well.
Topical NSAIDs or topical lidocaine may serve as add-ons to systemic therapy in more severe cases, or as first-line therapy for milder chronic pain, Dr. Micheletti noted. Patients who have failed treatment with at least two pharmacologic agents, suffer medically refractory HS with debilitating pain, or use opioids on an ongoing basis should be referred to a pain management specialist, he said.
Don’t forget lifestyle
Although data on the impact of diet on patients with HS are limited, “we know anecdotally that dairy and refined carbohydrates are associated with exacerbations,” said Dr. Micheletti.
In addition, many patients use complementary medicine “and they aren’t always telling us,” he emphasized. Smoking is prevalent among patients with HS, and is a risk factor for the disease in general, and for more severe and refractory disease, he added. Consequently, screening for tobacco smoking is recommended for patients with HS not only because of the impact on disease, but because it is a potentially modifiable cardiovascular risk factor, he explained.
Consider comorbidities
Cardiovascular disease is among several comorbidities associated with HS, said Dr. Micheletti. HS foundations in the United States and Canada recently published evidence-based recommendations for comorbidity screening. The recommendations included screening for 19 specific comorbidities: acne, dissecting cellulitis, pilonidal disease, pyoderma gangrenosum, depression, anxiety, suicide, smoking, substance abuse, polycystic ovary syndrome, obesity, dyslipidemia, diabetes mellitus, metabolic syndrome, hypertension, cardiovascular disease, inflammatory bowel disease, spondyloarthritis, and sexual dysfunction.
Dr. Micheletti highlighted cardiovascular comorbidities, and noted the association between HS and modifiable cardiovascular risk factors: smoking, obesity, diabetes mellitus, and dyslipidemia. “HS is also independently associated with cardiovascular disease leading to myocardial infarction, stroke, cardiovascular-associated death, and all-cause mortality compared to controls,” he said. Studies show an incidence rate ratio of 1.53 for major adverse cardiovascular events in patients with HS compared with controls, with the highest relative risk among those aged 18-29 years, he added.
Medical management
Depending on the patient, medical management of HS may involve antibiotics, hormonal agents, and biologics, said Dr. Micheletti. Some of the most commonly used antibiotic regimens for HS are those recommended in treatment guidelines, including doxycycline and a clindamycin/rifampin combination, he said. However, the use of trimethoprim-sulfamethoxazole or ciprofloxacin has been associated with increased antibiotic resistance and is not supported by available evidence, he noted.
Hormonal therapies may help some women with HS, said Dr. Micheletti. Options include spironolactone, metformin, or estrogen-containing hormonal contraceptives, he said.
When it comes to biologics, only 33% of HS patients meet criteria for their use (Hurley stage II or III, moderate or severe HS), he noted. However, research suggests “a huge gap” in the use of anti-TNF therapy even among patients for whom it is recommended, he said.
Of the TNF-alpha inhibitors, data on adalimumab, which is FDA-approved for HS, are the most recent. Adalimumab “is our gold standard biologic and our gateway biologic, for HS at this time,” Dr. Micheletti said.
However, those who respond to adalimumab “can continue to do better, but they can wax and wane and flare,” he cautioned. Infliximab, while not approved for HS, has been studied in patients with HS and is prescribed by some providers. Although no comparative studies have been done for infliximab versus adalimumab, “anecdotally, response to infliximab tends to be better, and it is the most effective biologic in common use for severe HS,” he noted.
Dr. Micheletti’s top treatment recommendations for using biologics start with considering biosimilars. Most patients on biosimilars do fine, but some patients who previously responded to infliximab will unpredictably lose efficacy or have reactions when switched to a biosimilar, he said.
Patients on biologics also may experience waning efficacy in the wake of an immune response stimulated by foreign antibodies, said Dr. Micheletti. “Anti-drug antibody formation is more likely to occur when treatment is interrupted,” he noted. Minimize the risk of antibody formation by paying attention to adherence issues and dosing frequency, he advised.
If patients fail both adalimumab and infliximab, Dr. Micheletti tells them not to lose hope, and that treatment is a trial-and-error process that may involve more than one therapy. Other biologics in active use for HS include ustekinumab, anakinra, secukinumab, brodalumab, golimumab, and JAK inhibitors, any of which might be effective in any given patient, he said.
Surgical solutions
For HS patients with chronic, recurring inflammation and drainage associated with a sinus tract, surgical deroofing may the best treatment option, Dr. Micheletti said. “Deroofing involves the use of a probe to trace the extent of the subcutaneous tract, followed by incision and removal of the tract ‘roof,’ ’’ he explained. The deroofing procedure involves local anesthesia and has a low morbidity rate, as well as a low recurrence rate and high levels of patient satisfaction, he said.
“The acute role for surgery is to remove active foci of inflammation and relieve pain,” which is achieved more effectively with deroofing, said Dr. Micheletti. By contrast, incision and drainage is associated with an almost 100% recurrence rate, he added.
When planning elective surgery for HS, Dr. Micheletti noted that holding infliximab for less than 4 weeks does not affect postoperative infection rates in patients with rheumatoid arthritis, and a recent randomized, controlled trial showed that adalimumab can be continued safely through HS surgeries.
In fact, “continuing TNF inhibitors through elective surgery does not increase infection risk and results in better disease control,” and dermatologists should work with surgery to balance infection and disease flare concerns in HS patients, he said.
Dr. Micheletti disclosed serving as a consultant or advisor for Adaptimmune and Vertex, and research funding from Amgen and Cabaletta Bio. MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – , Robert G. Micheletti, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
For patients with HS, “the quality-of-life impact is profound, greater than any other systematically studied dermatologic condition,” said Dr. Micheletti, associate professor of dermatology at the Hospital of the University of Pennsylavnia, and chief of hospital dermatology, and chief of dermatology at Pennsylvania Hospital, Philadelphia.
Two key aspects of quality of life that affect HS patients are sexual health and overall pain, he said. The female-to-male ratio of HS is approximately 3:1, and data show that approximately 40% of female HS patients experience fertility issues and have unaddressed questions about HS and pregnancy, said Dr. Micheletti. Additionally, data from a systematic review showed that 50%-60% of patients with HS reported sexual dysfunction. Impaired sexual function is also associated with both overall impaired quality of life ratings and the presence of mood disorders, he noted.
Pain also has a significant impact on quality of life for HS patients. When these patients present in an emergency department, 70% report severe pain, and approximately 60% receive opioids, said Dr. Micheletti.
Data from a 2021 study showed that HS patients are significantly more likely to receive opioids compared with controls, and also more likely to be diagnosed with opioid use disorder than controls, especially if they are seen by nondermatologists, he noted.
For acute pain, Dr. Micheletti recommended starting with acetaminophen 500 mg every 4 to 6 hours as needed, and topical nonsteroidal anti-inflammatory drugs (NSAIDs). “It still makes sense to do topical care,” said Dr. Micheletti, but he added that he also prescribes medications for anxiety for these patients.
Patients with increased pain severity or refractory disease may benefit from systemic NSAIDs, or intralesional triamcinolone, he noted. Incision and draining of abscesses may provide temporary symptomatic relief, but keep in mind that lesions will recur, he noted.
For the most severe cases, Dr. Micheletti advised adding tramadol as a first-line opioid, or another short-acting opioid for breakthrough pain.
To manage patients with HS who have chronic pain, Dr. Micheletti recommended starting with HS disease–directed therapy, but also screening for pain severity and psychological comorbidities.
His strategies in these cases include nonpharmacological pain management in the form of physical therapy, wound care, and behavioral health. His algorithm for nociceptive pain is NSAIDs with or without acetaminophen; duloxetine or nortriptyline are other options. For neuropathic pain, gabapentin and/or duloxetine are top choices, but pregabalin, venlafaxine, and nortriptyline are on the list as well.
Topical NSAIDs or topical lidocaine may serve as add-ons to systemic therapy in more severe cases, or as first-line therapy for milder chronic pain, Dr. Micheletti noted. Patients who have failed treatment with at least two pharmacologic agents, suffer medically refractory HS with debilitating pain, or use opioids on an ongoing basis should be referred to a pain management specialist, he said.
Don’t forget lifestyle
Although data on the impact of diet on patients with HS are limited, “we know anecdotally that dairy and refined carbohydrates are associated with exacerbations,” said Dr. Micheletti.
In addition, many patients use complementary medicine “and they aren’t always telling us,” he emphasized. Smoking is prevalent among patients with HS, and is a risk factor for the disease in general, and for more severe and refractory disease, he added. Consequently, screening for tobacco smoking is recommended for patients with HS not only because of the impact on disease, but because it is a potentially modifiable cardiovascular risk factor, he explained.
Consider comorbidities
Cardiovascular disease is among several comorbidities associated with HS, said Dr. Micheletti. HS foundations in the United States and Canada recently published evidence-based recommendations for comorbidity screening. The recommendations included screening for 19 specific comorbidities: acne, dissecting cellulitis, pilonidal disease, pyoderma gangrenosum, depression, anxiety, suicide, smoking, substance abuse, polycystic ovary syndrome, obesity, dyslipidemia, diabetes mellitus, metabolic syndrome, hypertension, cardiovascular disease, inflammatory bowel disease, spondyloarthritis, and sexual dysfunction.
Dr. Micheletti highlighted cardiovascular comorbidities, and noted the association between HS and modifiable cardiovascular risk factors: smoking, obesity, diabetes mellitus, and dyslipidemia. “HS is also independently associated with cardiovascular disease leading to myocardial infarction, stroke, cardiovascular-associated death, and all-cause mortality compared to controls,” he said. Studies show an incidence rate ratio of 1.53 for major adverse cardiovascular events in patients with HS compared with controls, with the highest relative risk among those aged 18-29 years, he added.
Medical management
Depending on the patient, medical management of HS may involve antibiotics, hormonal agents, and biologics, said Dr. Micheletti. Some of the most commonly used antibiotic regimens for HS are those recommended in treatment guidelines, including doxycycline and a clindamycin/rifampin combination, he said. However, the use of trimethoprim-sulfamethoxazole or ciprofloxacin has been associated with increased antibiotic resistance and is not supported by available evidence, he noted.
Hormonal therapies may help some women with HS, said Dr. Micheletti. Options include spironolactone, metformin, or estrogen-containing hormonal contraceptives, he said.
When it comes to biologics, only 33% of HS patients meet criteria for their use (Hurley stage II or III, moderate or severe HS), he noted. However, research suggests “a huge gap” in the use of anti-TNF therapy even among patients for whom it is recommended, he said.
Of the TNF-alpha inhibitors, data on adalimumab, which is FDA-approved for HS, are the most recent. Adalimumab “is our gold standard biologic and our gateway biologic, for HS at this time,” Dr. Micheletti said.
However, those who respond to adalimumab “can continue to do better, but they can wax and wane and flare,” he cautioned. Infliximab, while not approved for HS, has been studied in patients with HS and is prescribed by some providers. Although no comparative studies have been done for infliximab versus adalimumab, “anecdotally, response to infliximab tends to be better, and it is the most effective biologic in common use for severe HS,” he noted.
Dr. Micheletti’s top treatment recommendations for using biologics start with considering biosimilars. Most patients on biosimilars do fine, but some patients who previously responded to infliximab will unpredictably lose efficacy or have reactions when switched to a biosimilar, he said.
Patients on biologics also may experience waning efficacy in the wake of an immune response stimulated by foreign antibodies, said Dr. Micheletti. “Anti-drug antibody formation is more likely to occur when treatment is interrupted,” he noted. Minimize the risk of antibody formation by paying attention to adherence issues and dosing frequency, he advised.
If patients fail both adalimumab and infliximab, Dr. Micheletti tells them not to lose hope, and that treatment is a trial-and-error process that may involve more than one therapy. Other biologics in active use for HS include ustekinumab, anakinra, secukinumab, brodalumab, golimumab, and JAK inhibitors, any of which might be effective in any given patient, he said.
Surgical solutions
For HS patients with chronic, recurring inflammation and drainage associated with a sinus tract, surgical deroofing may the best treatment option, Dr. Micheletti said. “Deroofing involves the use of a probe to trace the extent of the subcutaneous tract, followed by incision and removal of the tract ‘roof,’ ’’ he explained. The deroofing procedure involves local anesthesia and has a low morbidity rate, as well as a low recurrence rate and high levels of patient satisfaction, he said.
“The acute role for surgery is to remove active foci of inflammation and relieve pain,” which is achieved more effectively with deroofing, said Dr. Micheletti. By contrast, incision and drainage is associated with an almost 100% recurrence rate, he added.
When planning elective surgery for HS, Dr. Micheletti noted that holding infliximab for less than 4 weeks does not affect postoperative infection rates in patients with rheumatoid arthritis, and a recent randomized, controlled trial showed that adalimumab can be continued safely through HS surgeries.
In fact, “continuing TNF inhibitors through elective surgery does not increase infection risk and results in better disease control,” and dermatologists should work with surgery to balance infection and disease flare concerns in HS patients, he said.
Dr. Micheletti disclosed serving as a consultant or advisor for Adaptimmune and Vertex, and research funding from Amgen and Cabaletta Bio. MedscapeLive and this news organization are owned by the same parent company.
AT INNOVATIONS IN DERMATOLOGY
Shorter fever prevention duration effective after cardiac arrest
a randomized trial shows.
“Since 2005, active fever prevention in comatose patients has been advocated by the guidelines for 72 hours after an out-of-hospital cardiac arrest,” Christian Hassager, MD, of the University of Copenhagen, told this news organization. “Our study is the first randomized trial ever on this subject – and it challenges the guidelines.”
At 90 days, a primary endpoint – a composite of death from any cause or hospital discharge with a high Cerebral Performance Category score – occurred in 32.4% of those in the 36-hour group and 33.6% of those in the 72-hour group; mortality was 29.5% versus 30.3%, respectively.
The study was published online in The New England Journal of Medicine. The results were also presented at the Resuscitation Science Symposium during the American Heart Association scientific sessions.
No significant differences
Assessment of the two device-based fever-prevention strategies for the duration was a predefined, additional randomly assigned open-label intervention in the Blood Pressure and Oxygenation Targets in Post Resuscitation Care (BOX) trial, which involved comatose adult patients who had been resuscitated after out-of-hospital cardiac arrest at two Danish cardiac arrest centers.
The main BOX analysis compared different primary strategies in these patients in a two-by-two factorial design: higher versus lower blood pressure targets and higher versus lower oxygenation targets. They found no difference between the various strategies in terms of death and discharge from hospital in a poor neurologic state. Those results were presented at the European Society of Cardiology Congress on Aug. 27, and simultaneously published in separate articles in The New England Journal of Medicine.
For this current analysis, a total of 789 comatose patients (mean age, 62; 80% men) received device-based temperature control targeting 36° C for 24 hours followed by 37° C for either 12 or 48 hours (total intervention times, 36 and 72 hours, respectively) or until the patient regained consciousness.
Patients were kept sedated and were receiving mechanical ventilation during the temperature control at 36° C, the authors note. Target core body temperature was controlled using commercially available surface cooling at one of the sites in 286 patients (Criticool and Allon, Belmont Medical Technologies) and using intravenous cooling in 503 patients at the other site (Thermogard XP, and Cool Line Catheter, Zoll).
Body temperature was maintained at 37° C with the same type of device that had been used for 36° C during the initial 24 hours. If the patient awakened, cooling was terminated.
Physicians in both groups were permitted to use non–device-based fever treatment (that is, for a body temperature > 37.5° C) with drugs such as paracetamol, by uncovering the patient’s body, or both, at the discretion of the treating physician. Ice packs or pads were not used.
The primary outcome was a composite of death from any cause or hospital discharge with a Cerebral Performance Category of 3 or 4 (range, 1 to 5, with higher scores indicating more severe disability) within 90 days after randomization.
Secondary outcomes at 90 days included death from any cause and the Montreal Cognitive Assessment score (range, 0 to 30, with higher scores indicating better cognitive ability).
A primary endpoint event occurred in 32.3% of patients in the 36-hour group and in 33.6% of those in the 72-hour group (hazard ratio, 0.99). Mortality was 29.5% in the 36-hour group and 30.3% in the 72-hour group.
The median Montreal Cognitive Assessment scores were 26 and 27, respectively. No significant between-group differences in the incidence of adverse events were observed.
The authors concluded that “active device-based fever prevention for 36 or 72 hours after cardiac arrest did not result in significantly different percentages of patients dying or having severe disability or coma.”
Dr. Hassager added, “We will continue with a new trial where we will randomize to treatment as usual or immediate wakeup call and no temperature intervention at all.”
Findings ‘very persuasive’
Intensivist Ken Parhar, MD, clinical associate professor, Critical Care Medicine at the University of Calgary (Alta.) and Alberta Health Services, Edmonton, and medical director, Cardiovascular Intensive Care Unit, commented on the study.
“The findings are very clear and very persuasive,” he said. “I think this should be incorporated into future guidelines, though it would be nice to see the trial repeated in another center.”
Dr. Parhar has kept comatose patients under temperature control for less than 72 hours, but mainly because those patients started to wake up. “This study provides clarity on the safety of that process – that we don’t have to unnecessarily keep somebody sedated just for an arbitrary timeline,” he said. “Beyond 36 hours, we need to continue to use our judgment.”
The study was supported by a grant from the Novo Nordisk Foundation, as was the work of one of the coauthors. Dr. Hassager’s work was funded by a grant from the Lundbeck Foundation; he also received an individual research grant from the Novo Nordisk Foundation, as well as honoraria from ABIOMED. No other disclosures were declared.
A version of this article first appeared on Medscape.com.
a randomized trial shows.
“Since 2005, active fever prevention in comatose patients has been advocated by the guidelines for 72 hours after an out-of-hospital cardiac arrest,” Christian Hassager, MD, of the University of Copenhagen, told this news organization. “Our study is the first randomized trial ever on this subject – and it challenges the guidelines.”
At 90 days, a primary endpoint – a composite of death from any cause or hospital discharge with a high Cerebral Performance Category score – occurred in 32.4% of those in the 36-hour group and 33.6% of those in the 72-hour group; mortality was 29.5% versus 30.3%, respectively.
The study was published online in The New England Journal of Medicine. The results were also presented at the Resuscitation Science Symposium during the American Heart Association scientific sessions.
No significant differences
Assessment of the two device-based fever-prevention strategies for the duration was a predefined, additional randomly assigned open-label intervention in the Blood Pressure and Oxygenation Targets in Post Resuscitation Care (BOX) trial, which involved comatose adult patients who had been resuscitated after out-of-hospital cardiac arrest at two Danish cardiac arrest centers.
The main BOX analysis compared different primary strategies in these patients in a two-by-two factorial design: higher versus lower blood pressure targets and higher versus lower oxygenation targets. They found no difference between the various strategies in terms of death and discharge from hospital in a poor neurologic state. Those results were presented at the European Society of Cardiology Congress on Aug. 27, and simultaneously published in separate articles in The New England Journal of Medicine.
For this current analysis, a total of 789 comatose patients (mean age, 62; 80% men) received device-based temperature control targeting 36° C for 24 hours followed by 37° C for either 12 or 48 hours (total intervention times, 36 and 72 hours, respectively) or until the patient regained consciousness.
Patients were kept sedated and were receiving mechanical ventilation during the temperature control at 36° C, the authors note. Target core body temperature was controlled using commercially available surface cooling at one of the sites in 286 patients (Criticool and Allon, Belmont Medical Technologies) and using intravenous cooling in 503 patients at the other site (Thermogard XP, and Cool Line Catheter, Zoll).
Body temperature was maintained at 37° C with the same type of device that had been used for 36° C during the initial 24 hours. If the patient awakened, cooling was terminated.
Physicians in both groups were permitted to use non–device-based fever treatment (that is, for a body temperature > 37.5° C) with drugs such as paracetamol, by uncovering the patient’s body, or both, at the discretion of the treating physician. Ice packs or pads were not used.
The primary outcome was a composite of death from any cause or hospital discharge with a Cerebral Performance Category of 3 or 4 (range, 1 to 5, with higher scores indicating more severe disability) within 90 days after randomization.
Secondary outcomes at 90 days included death from any cause and the Montreal Cognitive Assessment score (range, 0 to 30, with higher scores indicating better cognitive ability).
A primary endpoint event occurred in 32.3% of patients in the 36-hour group and in 33.6% of those in the 72-hour group (hazard ratio, 0.99). Mortality was 29.5% in the 36-hour group and 30.3% in the 72-hour group.
The median Montreal Cognitive Assessment scores were 26 and 27, respectively. No significant between-group differences in the incidence of adverse events were observed.
The authors concluded that “active device-based fever prevention for 36 or 72 hours after cardiac arrest did not result in significantly different percentages of patients dying or having severe disability or coma.”
Dr. Hassager added, “We will continue with a new trial where we will randomize to treatment as usual or immediate wakeup call and no temperature intervention at all.”
Findings ‘very persuasive’
Intensivist Ken Parhar, MD, clinical associate professor, Critical Care Medicine at the University of Calgary (Alta.) and Alberta Health Services, Edmonton, and medical director, Cardiovascular Intensive Care Unit, commented on the study.
“The findings are very clear and very persuasive,” he said. “I think this should be incorporated into future guidelines, though it would be nice to see the trial repeated in another center.”
Dr. Parhar has kept comatose patients under temperature control for less than 72 hours, but mainly because those patients started to wake up. “This study provides clarity on the safety of that process – that we don’t have to unnecessarily keep somebody sedated just for an arbitrary timeline,” he said. “Beyond 36 hours, we need to continue to use our judgment.”
The study was supported by a grant from the Novo Nordisk Foundation, as was the work of one of the coauthors. Dr. Hassager’s work was funded by a grant from the Lundbeck Foundation; he also received an individual research grant from the Novo Nordisk Foundation, as well as honoraria from ABIOMED. No other disclosures were declared.
A version of this article first appeared on Medscape.com.
a randomized trial shows.
“Since 2005, active fever prevention in comatose patients has been advocated by the guidelines for 72 hours after an out-of-hospital cardiac arrest,” Christian Hassager, MD, of the University of Copenhagen, told this news organization. “Our study is the first randomized trial ever on this subject – and it challenges the guidelines.”
At 90 days, a primary endpoint – a composite of death from any cause or hospital discharge with a high Cerebral Performance Category score – occurred in 32.4% of those in the 36-hour group and 33.6% of those in the 72-hour group; mortality was 29.5% versus 30.3%, respectively.
The study was published online in The New England Journal of Medicine. The results were also presented at the Resuscitation Science Symposium during the American Heart Association scientific sessions.
No significant differences
Assessment of the two device-based fever-prevention strategies for the duration was a predefined, additional randomly assigned open-label intervention in the Blood Pressure and Oxygenation Targets in Post Resuscitation Care (BOX) trial, which involved comatose adult patients who had been resuscitated after out-of-hospital cardiac arrest at two Danish cardiac arrest centers.
The main BOX analysis compared different primary strategies in these patients in a two-by-two factorial design: higher versus lower blood pressure targets and higher versus lower oxygenation targets. They found no difference between the various strategies in terms of death and discharge from hospital in a poor neurologic state. Those results were presented at the European Society of Cardiology Congress on Aug. 27, and simultaneously published in separate articles in The New England Journal of Medicine.
For this current analysis, a total of 789 comatose patients (mean age, 62; 80% men) received device-based temperature control targeting 36° C for 24 hours followed by 37° C for either 12 or 48 hours (total intervention times, 36 and 72 hours, respectively) or until the patient regained consciousness.
Patients were kept sedated and were receiving mechanical ventilation during the temperature control at 36° C, the authors note. Target core body temperature was controlled using commercially available surface cooling at one of the sites in 286 patients (Criticool and Allon, Belmont Medical Technologies) and using intravenous cooling in 503 patients at the other site (Thermogard XP, and Cool Line Catheter, Zoll).
Body temperature was maintained at 37° C with the same type of device that had been used for 36° C during the initial 24 hours. If the patient awakened, cooling was terminated.
Physicians in both groups were permitted to use non–device-based fever treatment (that is, for a body temperature > 37.5° C) with drugs such as paracetamol, by uncovering the patient’s body, or both, at the discretion of the treating physician. Ice packs or pads were not used.
The primary outcome was a composite of death from any cause or hospital discharge with a Cerebral Performance Category of 3 or 4 (range, 1 to 5, with higher scores indicating more severe disability) within 90 days after randomization.
Secondary outcomes at 90 days included death from any cause and the Montreal Cognitive Assessment score (range, 0 to 30, with higher scores indicating better cognitive ability).
A primary endpoint event occurred in 32.3% of patients in the 36-hour group and in 33.6% of those in the 72-hour group (hazard ratio, 0.99). Mortality was 29.5% in the 36-hour group and 30.3% in the 72-hour group.
The median Montreal Cognitive Assessment scores were 26 and 27, respectively. No significant between-group differences in the incidence of adverse events were observed.
The authors concluded that “active device-based fever prevention for 36 or 72 hours after cardiac arrest did not result in significantly different percentages of patients dying or having severe disability or coma.”
Dr. Hassager added, “We will continue with a new trial where we will randomize to treatment as usual or immediate wakeup call and no temperature intervention at all.”
Findings ‘very persuasive’
Intensivist Ken Parhar, MD, clinical associate professor, Critical Care Medicine at the University of Calgary (Alta.) and Alberta Health Services, Edmonton, and medical director, Cardiovascular Intensive Care Unit, commented on the study.
“The findings are very clear and very persuasive,” he said. “I think this should be incorporated into future guidelines, though it would be nice to see the trial repeated in another center.”
Dr. Parhar has kept comatose patients under temperature control for less than 72 hours, but mainly because those patients started to wake up. “This study provides clarity on the safety of that process – that we don’t have to unnecessarily keep somebody sedated just for an arbitrary timeline,” he said. “Beyond 36 hours, we need to continue to use our judgment.”
The study was supported by a grant from the Novo Nordisk Foundation, as was the work of one of the coauthors. Dr. Hassager’s work was funded by a grant from the Lundbeck Foundation; he also received an individual research grant from the Novo Nordisk Foundation, as well as honoraria from ABIOMED. No other disclosures were declared.
A version of this article first appeared on Medscape.com.
FROM NEJM
Single chest x-ray could predict 10-year CVD risk
who presented the results of their deep-learning model at the annual meeting of the Radiological Society of North America.
Current American College of Cardiologists and American Heart Association guidelines recommend estimating 10-year risk of major adverse cardiovascular events (MACE) to determine whether a patient should receive statins to help prevent atherosclerotic cardiovascular disease (ASCVD). Statins are recommended for patients with a 10-year risk of 7.5% or higher, the authors noted.
The current ASCVD risk score is determined with nine factors: age, sex, race, systolic blood pressure, hypertension treatment, smoking, type 2 diabetes, and a lipid panel.
Not all data points available in EHR
But not all of those data points may be available through the electronic health record, “which makes novel and easier approaches for population-wide screening desirable,” said lead researcher Jakob Weiss, MD, a radiologist affiliated with the Cardiovascular Imaging Research Center at Massachusetts General Hospital and the AI in medicine program at the Brigham and Women’s Hospital in Boston.
Chest x-ray images, on the other hand, are commonly available. The images carry rich information beyond diagnostic data but have not been used in this type of prediction model because AI models have been lacking, Dr. Weiss said.
The researchers trained a deep-learning model with single chest x-rays only.
They used 147,497 chest x-rays from 40,643 participants in the Prostate, Lung, Colorectal, and Ovarian Cancer (PLCO) Screening Trial, a multicenter, randomized controlled trial designed and sponsored by the National Cancer Institute.
Dr. Weiss acknowledged that the population used to train the model was heavily White and that should be a consideration in validating the model.
They compared their model’s ability to predict 10-year ASCVD risk with the standard ACC/AHA model.
“Based on a single chest radiograph image, deep learning can predict the risk of future cardiovascular events independent of cardiovascular risk factors and with similar performance to the established and guideline-recommended ASCVD risk score,” Dr. Weiss said.
Tested against independent group
They tested the model against an independent group of 11,430 outpatients (average age, 60 years; 42.9% male) who underwent a routine outpatient chest x-ray at Mass General Brigham and were potentially eligible to receive statins.
Of those 11,430 patients, 1,096 (9.6%) had a major adverse cardiac event over the median follow-up of 10.3 years.
There was a significant association of CXR-CVD risk and MACE among patients eligible to receive statins, the researchers found (hazard ratio, 2.03; 95% confidence interval, 1.81-2.30; P < .001), which remained significant after adjusting for cardiovascular risk factors (adjusted HR, 1.63; 95% CI, 1.43-1.86; P < .001).
Some of the variables were missing in the standard model, but in a subgroup of 2,401 patients, all the variables were available.
They calculated ASCVD risk in that subgroup using the standard model and the CXR model and found that the performance was similar (c-statistic, 0.64 vs. 0.65; P = .48) to the ASCVD risk score (aHR, 1.58; 95% CI, 1.20-2.09; P = .001).
Ritu R. Gill MD, MPH, associate professor of radiology at Harvard Medical School in Boston, who was not part of the study, said in an interview that “the predictive algorithm is promising and potentially translatable and could enhance the annual medical checkup in a select population.
“The algorithm was developed using the PLCO cohort with radiographs, which are likely subjects in the lung cancer screening arm,” she said. “This cohort would be at high risk of cardiovascular diseases, as smoking is a known risk factor for atherosclerotic disease, and therefore the results are expected.
“The algorithm needs to be validated in an independent database with inclusion of subjects with younger age groups and adjusted for gender and racial diversity,” Gill said.
David Cho, MD, a cardiologist at the University of California, Los Angeles, who also was not part of the study, said in an interview that “this work is a great example of AI being able to detect clinically relevant outcomes with a widely used and low-cost screening test.
“The volume of data needed to train these models is already out there,” Dr. Cho said. “It just needs to be mined.”
He noted that this tool, if validated in randomized trials, could help determine risk among patients living in places where access to specialized cardiac care is limited.
Dr. Weiss and Dr. Cho disclosed no relevant financial relationships. Dr. Gill has received research support from Cannon Inc and consultant fees from Imbio and WorldCare.
A version of this article first appeared on Medscape.com.
who presented the results of their deep-learning model at the annual meeting of the Radiological Society of North America.
Current American College of Cardiologists and American Heart Association guidelines recommend estimating 10-year risk of major adverse cardiovascular events (MACE) to determine whether a patient should receive statins to help prevent atherosclerotic cardiovascular disease (ASCVD). Statins are recommended for patients with a 10-year risk of 7.5% or higher, the authors noted.
The current ASCVD risk score is determined with nine factors: age, sex, race, systolic blood pressure, hypertension treatment, smoking, type 2 diabetes, and a lipid panel.
Not all data points available in EHR
But not all of those data points may be available through the electronic health record, “which makes novel and easier approaches for population-wide screening desirable,” said lead researcher Jakob Weiss, MD, a radiologist affiliated with the Cardiovascular Imaging Research Center at Massachusetts General Hospital and the AI in medicine program at the Brigham and Women’s Hospital in Boston.
Chest x-ray images, on the other hand, are commonly available. The images carry rich information beyond diagnostic data but have not been used in this type of prediction model because AI models have been lacking, Dr. Weiss said.
The researchers trained a deep-learning model with single chest x-rays only.
They used 147,497 chest x-rays from 40,643 participants in the Prostate, Lung, Colorectal, and Ovarian Cancer (PLCO) Screening Trial, a multicenter, randomized controlled trial designed and sponsored by the National Cancer Institute.
Dr. Weiss acknowledged that the population used to train the model was heavily White and that should be a consideration in validating the model.
They compared their model’s ability to predict 10-year ASCVD risk with the standard ACC/AHA model.
“Based on a single chest radiograph image, deep learning can predict the risk of future cardiovascular events independent of cardiovascular risk factors and with similar performance to the established and guideline-recommended ASCVD risk score,” Dr. Weiss said.
Tested against independent group
They tested the model against an independent group of 11,430 outpatients (average age, 60 years; 42.9% male) who underwent a routine outpatient chest x-ray at Mass General Brigham and were potentially eligible to receive statins.
Of those 11,430 patients, 1,096 (9.6%) had a major adverse cardiac event over the median follow-up of 10.3 years.
There was a significant association of CXR-CVD risk and MACE among patients eligible to receive statins, the researchers found (hazard ratio, 2.03; 95% confidence interval, 1.81-2.30; P < .001), which remained significant after adjusting for cardiovascular risk factors (adjusted HR, 1.63; 95% CI, 1.43-1.86; P < .001).
Some of the variables were missing in the standard model, but in a subgroup of 2,401 patients, all the variables were available.
They calculated ASCVD risk in that subgroup using the standard model and the CXR model and found that the performance was similar (c-statistic, 0.64 vs. 0.65; P = .48) to the ASCVD risk score (aHR, 1.58; 95% CI, 1.20-2.09; P = .001).
Ritu R. Gill MD, MPH, associate professor of radiology at Harvard Medical School in Boston, who was not part of the study, said in an interview that “the predictive algorithm is promising and potentially translatable and could enhance the annual medical checkup in a select population.
“The algorithm was developed using the PLCO cohort with radiographs, which are likely subjects in the lung cancer screening arm,” she said. “This cohort would be at high risk of cardiovascular diseases, as smoking is a known risk factor for atherosclerotic disease, and therefore the results are expected.
“The algorithm needs to be validated in an independent database with inclusion of subjects with younger age groups and adjusted for gender and racial diversity,” Gill said.
David Cho, MD, a cardiologist at the University of California, Los Angeles, who also was not part of the study, said in an interview that “this work is a great example of AI being able to detect clinically relevant outcomes with a widely used and low-cost screening test.
“The volume of data needed to train these models is already out there,” Dr. Cho said. “It just needs to be mined.”
He noted that this tool, if validated in randomized trials, could help determine risk among patients living in places where access to specialized cardiac care is limited.
Dr. Weiss and Dr. Cho disclosed no relevant financial relationships. Dr. Gill has received research support from Cannon Inc and consultant fees from Imbio and WorldCare.
A version of this article first appeared on Medscape.com.
who presented the results of their deep-learning model at the annual meeting of the Radiological Society of North America.
Current American College of Cardiologists and American Heart Association guidelines recommend estimating 10-year risk of major adverse cardiovascular events (MACE) to determine whether a patient should receive statins to help prevent atherosclerotic cardiovascular disease (ASCVD). Statins are recommended for patients with a 10-year risk of 7.5% or higher, the authors noted.
The current ASCVD risk score is determined with nine factors: age, sex, race, systolic blood pressure, hypertension treatment, smoking, type 2 diabetes, and a lipid panel.
Not all data points available in EHR
But not all of those data points may be available through the electronic health record, “which makes novel and easier approaches for population-wide screening desirable,” said lead researcher Jakob Weiss, MD, a radiologist affiliated with the Cardiovascular Imaging Research Center at Massachusetts General Hospital and the AI in medicine program at the Brigham and Women’s Hospital in Boston.
Chest x-ray images, on the other hand, are commonly available. The images carry rich information beyond diagnostic data but have not been used in this type of prediction model because AI models have been lacking, Dr. Weiss said.
The researchers trained a deep-learning model with single chest x-rays only.
They used 147,497 chest x-rays from 40,643 participants in the Prostate, Lung, Colorectal, and Ovarian Cancer (PLCO) Screening Trial, a multicenter, randomized controlled trial designed and sponsored by the National Cancer Institute.
Dr. Weiss acknowledged that the population used to train the model was heavily White and that should be a consideration in validating the model.
They compared their model’s ability to predict 10-year ASCVD risk with the standard ACC/AHA model.
“Based on a single chest radiograph image, deep learning can predict the risk of future cardiovascular events independent of cardiovascular risk factors and with similar performance to the established and guideline-recommended ASCVD risk score,” Dr. Weiss said.
Tested against independent group
They tested the model against an independent group of 11,430 outpatients (average age, 60 years; 42.9% male) who underwent a routine outpatient chest x-ray at Mass General Brigham and were potentially eligible to receive statins.
Of those 11,430 patients, 1,096 (9.6%) had a major adverse cardiac event over the median follow-up of 10.3 years.
There was a significant association of CXR-CVD risk and MACE among patients eligible to receive statins, the researchers found (hazard ratio, 2.03; 95% confidence interval, 1.81-2.30; P < .001), which remained significant after adjusting for cardiovascular risk factors (adjusted HR, 1.63; 95% CI, 1.43-1.86; P < .001).
Some of the variables were missing in the standard model, but in a subgroup of 2,401 patients, all the variables were available.
They calculated ASCVD risk in that subgroup using the standard model and the CXR model and found that the performance was similar (c-statistic, 0.64 vs. 0.65; P = .48) to the ASCVD risk score (aHR, 1.58; 95% CI, 1.20-2.09; P = .001).
Ritu R. Gill MD, MPH, associate professor of radiology at Harvard Medical School in Boston, who was not part of the study, said in an interview that “the predictive algorithm is promising and potentially translatable and could enhance the annual medical checkup in a select population.
“The algorithm was developed using the PLCO cohort with radiographs, which are likely subjects in the lung cancer screening arm,” she said. “This cohort would be at high risk of cardiovascular diseases, as smoking is a known risk factor for atherosclerotic disease, and therefore the results are expected.
“The algorithm needs to be validated in an independent database with inclusion of subjects with younger age groups and adjusted for gender and racial diversity,” Gill said.
David Cho, MD, a cardiologist at the University of California, Los Angeles, who also was not part of the study, said in an interview that “this work is a great example of AI being able to detect clinically relevant outcomes with a widely used and low-cost screening test.
“The volume of data needed to train these models is already out there,” Dr. Cho said. “It just needs to be mined.”
He noted that this tool, if validated in randomized trials, could help determine risk among patients living in places where access to specialized cardiac care is limited.
Dr. Weiss and Dr. Cho disclosed no relevant financial relationships. Dr. Gill has received research support from Cannon Inc and consultant fees from Imbio and WorldCare.
A version of this article first appeared on Medscape.com.
AT RSNA 2022
Gestational hypertension-diabetes combo signals CVD risk
Women who develop transient hypertensive disorders during their pregnancy are at risk for developing subsequent cardiovascular disease (CVD), particularly if this experienced at the same time as gestational diabetes.
In a large population-based study, the adjusted hazard ratios for developing CVD following a gestational hypertensive disorder (GHTD) alone were 1.90 (95% confidence interval, 1.151-2.25) within 5 years and 1.41 (95% CI, 1.12-1.76) after 5 years or more.
When gestational diabetes was added into the mix, however, the risk for CVD after 5 years more than doubled (aHR, 2.43; 95% CI, 1.60-3.67). Risk in the earlier postpartum period was also raised by the combination, but this was not significant (aHR, 1.42; 95% CI, 0.78-2.58).
Having gestational diabetes by itself did not seem to increase the risk for later CVD in the analysis, despite being linked to higher heart disease risk in other studies.
“These are women coming out of a pregnancy – young women of reproductive age – so this is not a group that typically has cardiovascular events,” said Ravi Retnakaran, MD, in an interview, an investigator in the new study, which is published in JAMA Network Open.
“If they are somebody who has both disorders concurrently in their pregnancy, they may be at even greater risk than a woman with one or the other disorder,” added Dr. Retnakaran, who is professor of medicine at the University of Toronto and an endocrinologist at the Leadership Sinai Centre for Diabetes, Mount Sinai Hospital, also in Toronto. “In other words, amongst already high-risk patients. This is identifying a subset at maybe an even higher risk.”
It doesn’t mean that there is a huge absolute risk, Dr. Retnakaran said, but it is showing that there is a heightened risk such that women and their clinicians need to be aware of and potentially the need for greater preventative care in the future.
“It is allowing you to identify future lifetime risk of cardiovascular disease,” he said.
Study rationale and design
GHTD is “a forerunner of hypertension,” and gestational diabetes is “a precursor of diabetes” – each associated with a high risk of developing CVD in the years after pregnancy, the investigators said. While studies have looked at their individual contributions to future CVD risk, not many had looked to see what risks having both may confer in the postpregnancy years.
For the analysis, data on 886,295 women with GHTD (43,861), gestational diabetes (54,061), both (4,975), or neither (783,398) were obtained from several Canadian administrative health databases.
The mean age was around 30 years across the groups, with those with both conditions or gestational diabetes alone more likely to be older than those with GTHD alone or neither condition (32 vs. 29 years, respectively, P < .001).
After a total follow-up period of 12 years, 1,999 CVD events were recorded, most of them (1,162) 5 years after the pregnancy.
Pregnancy is a stress test for the heart
“We know that what we call adverse pregnancy outcomes – things like gestational hypertension, and gestational diabetes, and preeclampsia – are on the rise globally,” Natalie A. Bello, MD, director of hypertension research at the Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, commented in an interview.
“People who are younger and of childbearing age who are going into pregnancy now are less healthy than they perhaps were in the past,” Dr. Bello suggested, with more hypertension, more obesity, and people being less physically active. “We think that’s translating into some of the pregnancy complications.”
That’s concerning for a number of reasons, said Dr. Bello, who is also the cochair of the American College of Cardiology’s Cardio-Obstetrics Workgroup, and the biggest one perhaps is the stress that these may conditions may be placing on the heart.
“We know that when individuals have an adverse pregnancy outcome like gestational hypertension, or gestational diabetes, their risk for heart disease is increased in the future compared to someone who has an uncomplicated pregnancy,” she said. “So, we sort of say pregnancy is like a stress test for your heart.”
Dr. Bello added that “these situations, these adverse pregnancy outcomes are an indicator for us as physicians, but also they should be for patients as well, to sort of make sure they’re talking to their doctor about their risk factors and modifying them whenever possible.”
The population studied came from quite a racially, ethnically, and economically diverse area of Canada, Dr. Bello pointed out, although because of the nature of an administrative database there wasn’t information on individual level risk factors.
“We don’t know things like smoking, or if individuals were obese when they were pregnant. So, there are some limitations that should be noted,” she said.
Also, the results don’t mean that isolated gestational diabetes “isn’t something we need to be concerned about,” Dr. Bello observed, adding that the study may have been underpowered to look at this association. “It may just be that it will take a longer time for individuals who have gestational diabetes who don’t make lifestyle changes to develop diabetes, and then develop heart disease.”
The main message is that the women who have a co-occurrence of gestational hypertension and gestational diabetes are at particularly high risk of cardiovascular disease in the future,” said Dr. Retnakaran.
“The way to look at it from a patient standpoint is that we are all on different tracks in terms of our cardiometabolic destiny,” and that these data give “some understanding of what kind of tracks they are on for future risk,” Dr. Retnakaran said.
“A history of either gestational hypertension, and/or gestational diabetes should be really a warning sign for physicians and for patients that they have a higher risk of heart disease,” said Dr. Bello.
She added that this is a signal “that we need to do things to modify their risk, because we know that about 80% of heart disease is modifiable and preventable with proper risk factor management.”
The study was funded by the Ontario Ministry of Health and Long-Term Care. Dr. Retnakaran has received grants and personal fees from Novo Nordisk and Merck, grants from Boehringer Ingelheim, and personal fees from Eli Lily Takeda, and Sanofi. Dr. Bello had no conflicts of interest to disclose.
Women who develop transient hypertensive disorders during their pregnancy are at risk for developing subsequent cardiovascular disease (CVD), particularly if this experienced at the same time as gestational diabetes.
In a large population-based study, the adjusted hazard ratios for developing CVD following a gestational hypertensive disorder (GHTD) alone were 1.90 (95% confidence interval, 1.151-2.25) within 5 years and 1.41 (95% CI, 1.12-1.76) after 5 years or more.
When gestational diabetes was added into the mix, however, the risk for CVD after 5 years more than doubled (aHR, 2.43; 95% CI, 1.60-3.67). Risk in the earlier postpartum period was also raised by the combination, but this was not significant (aHR, 1.42; 95% CI, 0.78-2.58).
Having gestational diabetes by itself did not seem to increase the risk for later CVD in the analysis, despite being linked to higher heart disease risk in other studies.
“These are women coming out of a pregnancy – young women of reproductive age – so this is not a group that typically has cardiovascular events,” said Ravi Retnakaran, MD, in an interview, an investigator in the new study, which is published in JAMA Network Open.
“If they are somebody who has both disorders concurrently in their pregnancy, they may be at even greater risk than a woman with one or the other disorder,” added Dr. Retnakaran, who is professor of medicine at the University of Toronto and an endocrinologist at the Leadership Sinai Centre for Diabetes, Mount Sinai Hospital, also in Toronto. “In other words, amongst already high-risk patients. This is identifying a subset at maybe an even higher risk.”
It doesn’t mean that there is a huge absolute risk, Dr. Retnakaran said, but it is showing that there is a heightened risk such that women and their clinicians need to be aware of and potentially the need for greater preventative care in the future.
“It is allowing you to identify future lifetime risk of cardiovascular disease,” he said.
Study rationale and design
GHTD is “a forerunner of hypertension,” and gestational diabetes is “a precursor of diabetes” – each associated with a high risk of developing CVD in the years after pregnancy, the investigators said. While studies have looked at their individual contributions to future CVD risk, not many had looked to see what risks having both may confer in the postpregnancy years.
For the analysis, data on 886,295 women with GHTD (43,861), gestational diabetes (54,061), both (4,975), or neither (783,398) were obtained from several Canadian administrative health databases.
The mean age was around 30 years across the groups, with those with both conditions or gestational diabetes alone more likely to be older than those with GTHD alone or neither condition (32 vs. 29 years, respectively, P < .001).
After a total follow-up period of 12 years, 1,999 CVD events were recorded, most of them (1,162) 5 years after the pregnancy.
Pregnancy is a stress test for the heart
“We know that what we call adverse pregnancy outcomes – things like gestational hypertension, and gestational diabetes, and preeclampsia – are on the rise globally,” Natalie A. Bello, MD, director of hypertension research at the Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, commented in an interview.
“People who are younger and of childbearing age who are going into pregnancy now are less healthy than they perhaps were in the past,” Dr. Bello suggested, with more hypertension, more obesity, and people being less physically active. “We think that’s translating into some of the pregnancy complications.”
That’s concerning for a number of reasons, said Dr. Bello, who is also the cochair of the American College of Cardiology’s Cardio-Obstetrics Workgroup, and the biggest one perhaps is the stress that these may conditions may be placing on the heart.
“We know that when individuals have an adverse pregnancy outcome like gestational hypertension, or gestational diabetes, their risk for heart disease is increased in the future compared to someone who has an uncomplicated pregnancy,” she said. “So, we sort of say pregnancy is like a stress test for your heart.”
Dr. Bello added that “these situations, these adverse pregnancy outcomes are an indicator for us as physicians, but also they should be for patients as well, to sort of make sure they’re talking to their doctor about their risk factors and modifying them whenever possible.”
The population studied came from quite a racially, ethnically, and economically diverse area of Canada, Dr. Bello pointed out, although because of the nature of an administrative database there wasn’t information on individual level risk factors.
“We don’t know things like smoking, or if individuals were obese when they were pregnant. So, there are some limitations that should be noted,” she said.
Also, the results don’t mean that isolated gestational diabetes “isn’t something we need to be concerned about,” Dr. Bello observed, adding that the study may have been underpowered to look at this association. “It may just be that it will take a longer time for individuals who have gestational diabetes who don’t make lifestyle changes to develop diabetes, and then develop heart disease.”
The main message is that the women who have a co-occurrence of gestational hypertension and gestational diabetes are at particularly high risk of cardiovascular disease in the future,” said Dr. Retnakaran.
“The way to look at it from a patient standpoint is that we are all on different tracks in terms of our cardiometabolic destiny,” and that these data give “some understanding of what kind of tracks they are on for future risk,” Dr. Retnakaran said.
“A history of either gestational hypertension, and/or gestational diabetes should be really a warning sign for physicians and for patients that they have a higher risk of heart disease,” said Dr. Bello.
She added that this is a signal “that we need to do things to modify their risk, because we know that about 80% of heart disease is modifiable and preventable with proper risk factor management.”
The study was funded by the Ontario Ministry of Health and Long-Term Care. Dr. Retnakaran has received grants and personal fees from Novo Nordisk and Merck, grants from Boehringer Ingelheim, and personal fees from Eli Lily Takeda, and Sanofi. Dr. Bello had no conflicts of interest to disclose.
Women who develop transient hypertensive disorders during their pregnancy are at risk for developing subsequent cardiovascular disease (CVD), particularly if this experienced at the same time as gestational diabetes.
In a large population-based study, the adjusted hazard ratios for developing CVD following a gestational hypertensive disorder (GHTD) alone were 1.90 (95% confidence interval, 1.151-2.25) within 5 years and 1.41 (95% CI, 1.12-1.76) after 5 years or more.
When gestational diabetes was added into the mix, however, the risk for CVD after 5 years more than doubled (aHR, 2.43; 95% CI, 1.60-3.67). Risk in the earlier postpartum period was also raised by the combination, but this was not significant (aHR, 1.42; 95% CI, 0.78-2.58).
Having gestational diabetes by itself did not seem to increase the risk for later CVD in the analysis, despite being linked to higher heart disease risk in other studies.
“These are women coming out of a pregnancy – young women of reproductive age – so this is not a group that typically has cardiovascular events,” said Ravi Retnakaran, MD, in an interview, an investigator in the new study, which is published in JAMA Network Open.
“If they are somebody who has both disorders concurrently in their pregnancy, they may be at even greater risk than a woman with one or the other disorder,” added Dr. Retnakaran, who is professor of medicine at the University of Toronto and an endocrinologist at the Leadership Sinai Centre for Diabetes, Mount Sinai Hospital, also in Toronto. “In other words, amongst already high-risk patients. This is identifying a subset at maybe an even higher risk.”
It doesn’t mean that there is a huge absolute risk, Dr. Retnakaran said, but it is showing that there is a heightened risk such that women and their clinicians need to be aware of and potentially the need for greater preventative care in the future.
“It is allowing you to identify future lifetime risk of cardiovascular disease,” he said.
Study rationale and design
GHTD is “a forerunner of hypertension,” and gestational diabetes is “a precursor of diabetes” – each associated with a high risk of developing CVD in the years after pregnancy, the investigators said. While studies have looked at their individual contributions to future CVD risk, not many had looked to see what risks having both may confer in the postpregnancy years.
For the analysis, data on 886,295 women with GHTD (43,861), gestational diabetes (54,061), both (4,975), or neither (783,398) were obtained from several Canadian administrative health databases.
The mean age was around 30 years across the groups, with those with both conditions or gestational diabetes alone more likely to be older than those with GTHD alone or neither condition (32 vs. 29 years, respectively, P < .001).
After a total follow-up period of 12 years, 1,999 CVD events were recorded, most of them (1,162) 5 years after the pregnancy.
Pregnancy is a stress test for the heart
“We know that what we call adverse pregnancy outcomes – things like gestational hypertension, and gestational diabetes, and preeclampsia – are on the rise globally,” Natalie A. Bello, MD, director of hypertension research at the Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, commented in an interview.
“People who are younger and of childbearing age who are going into pregnancy now are less healthy than they perhaps were in the past,” Dr. Bello suggested, with more hypertension, more obesity, and people being less physically active. “We think that’s translating into some of the pregnancy complications.”
That’s concerning for a number of reasons, said Dr. Bello, who is also the cochair of the American College of Cardiology’s Cardio-Obstetrics Workgroup, and the biggest one perhaps is the stress that these may conditions may be placing on the heart.
“We know that when individuals have an adverse pregnancy outcome like gestational hypertension, or gestational diabetes, their risk for heart disease is increased in the future compared to someone who has an uncomplicated pregnancy,” she said. “So, we sort of say pregnancy is like a stress test for your heart.”
Dr. Bello added that “these situations, these adverse pregnancy outcomes are an indicator for us as physicians, but also they should be for patients as well, to sort of make sure they’re talking to their doctor about their risk factors and modifying them whenever possible.”
The population studied came from quite a racially, ethnically, and economically diverse area of Canada, Dr. Bello pointed out, although because of the nature of an administrative database there wasn’t information on individual level risk factors.
“We don’t know things like smoking, or if individuals were obese when they were pregnant. So, there are some limitations that should be noted,” she said.
Also, the results don’t mean that isolated gestational diabetes “isn’t something we need to be concerned about,” Dr. Bello observed, adding that the study may have been underpowered to look at this association. “It may just be that it will take a longer time for individuals who have gestational diabetes who don’t make lifestyle changes to develop diabetes, and then develop heart disease.”
The main message is that the women who have a co-occurrence of gestational hypertension and gestational diabetes are at particularly high risk of cardiovascular disease in the future,” said Dr. Retnakaran.
“The way to look at it from a patient standpoint is that we are all on different tracks in terms of our cardiometabolic destiny,” and that these data give “some understanding of what kind of tracks they are on for future risk,” Dr. Retnakaran said.
“A history of either gestational hypertension, and/or gestational diabetes should be really a warning sign for physicians and for patients that they have a higher risk of heart disease,” said Dr. Bello.
She added that this is a signal “that we need to do things to modify their risk, because we know that about 80% of heart disease is modifiable and preventable with proper risk factor management.”
The study was funded by the Ontario Ministry of Health and Long-Term Care. Dr. Retnakaran has received grants and personal fees from Novo Nordisk and Merck, grants from Boehringer Ingelheim, and personal fees from Eli Lily Takeda, and Sanofi. Dr. Bello had no conflicts of interest to disclose.
FROM JAMA NETWORK OPEN
Move faster, live longer? A little more effort goes a long way
If there’s one public health message Americans have heard loud and clear, it’s this one:
Move more.
Take more steps.
Spend more time doing physical activity – at least 150 minutes a week, according to the latest guidelines.
But hearing the message doesn’t mean we act on it. A whopping 25% of Americans don’t get any physical activity beyond what they do in their job, according to a CDC survey.
Just do what you’re already doing, but with a little more effort.
The study, which was published in the European Heart Journal, builds on growing evidence that suggests exercise intensity matters just as much as the amount. So, something as simple as turning a leisurely stroll into a brisk walk can, over time, lead to significant reductions in your risk of cardiovascular disease. No additional moves, steps, or minutes needed.
Step it up
Researchers at Cambridge University and the University of Leicester in England looked at data from 88,000 middle-aged adults who wore an activity tracking device for 7 days.
The devices tracked both the total amount of activity they did and the intensity of that movement – that is, how fast they walked or how hard they pushed themselves.
The researchers then calculated their physical activity energy expenditure (the number of calories they burned when they were up and moving) and the percentage that came from moderate to vigorous physical activity.
What’s the difference?
- Physical activity means any and every movement you do throughout the day. Mostly it’s mundane tasks like shopping, walking to the mailbox, playing with your dog, or cooking.
- Moderate-intensity physical activity includes things you do at a faster pace. Maybe you’re walking for exercise, doing yard work or household chores, or running late and just trying to get somewhere faster. You’re breathing a little harder and possibly working up a sweat.
- Vigorous-intensity physical activity is usually an exercise session – a run, a strenuous hike, a tough workout in the gym. It can also be an exhausting chore like shoveling snow, which feels like a workout. You’re definitely breathing harder, and you’re probably working up a sweat, even in the middle of winter.
Over the next 6 to 7 years, there were 4,000 new cases of cardiovascular disease among the people in the study.
Those who got at least 20% of their physical activity energy expenditure from moderate to vigorous activities had significantly less risk of heart disease, compared with those whose higher-effort activities were about 10%.
That was true even for those whose total activity was relatively low. As long as higher-effort activities reached 20% of their total, they were 14% less likely to be diagnosed with a heart condition.
And for those with relatively high activity levels, there was little extra benefit if their moderate and vigorous activities remained around 10%.
That finding surprised Paddy Dempsey, PhD, a medical research scientist at Cambridge and the study’s lead author. But it also makes sense.
“People can improve their cardiorespiratory fitness to a greater degree with higher-intensity activity,” he says. “More intensity will stress the system and lead to greater adaptation.”
The key is an increase in the amount of oxygen your heart and lungs can provide your muscles during exercise, a measure known as VO2max.
Raising your VO2max is the best way to reduce your risk of early death, especially death from heart disease. Simply moving up from the lowest conditioning category to a higher one will cut your risk of dying in any given year by as much as 60%.
Making strides
The study builds on previous research that shows the benefits of moving faster.
Walking faster will naturally increase your stride length, another predictor of longevity and future health. A review study published in 2021 found that older adults who took shorter steps were 26% more likely to have a disability, 34% more likely to have a major adverse event (like an injury that leads to a loss of independence), and 69% more likely to die over the next several years.
Quality versus quantity
We’ve focused so far on the quality of your physical activity – moving faster, taking longer strides.
But there’s still a lot to be said for movement quantity.
“It would be a mistake to say volume doesn’t matter,” Dr. Dempsey cautions.
A 2022 study in the journal The Lancet found that the risk of dying during a given period decreases with each increase in daily steps. The protective effect peaks at about 6,000 to 8,000 steps a day for adults 60 and over, and at 8,000 to 10,000 steps for those under 60.
“The relative value of the quality and quantity of exercise are very specific to a person’s goals,” says Chhanda Dutta, PhD, chief of the Clinical Gerontology Branch at the National Institute on Aging. “If performance is the goal, quality matters at least as much as quantity.”
Dr. Dempsey agrees that it’s not a cage match between two. Every step you take is a step in the right direction.
“People can choose or gravitate to an approach that works best for them,” he says. “It’s also helpful to think about where some everyday activities can be punctuated with intensity,” which could be as simple as walking faster when possible.
What matters most is that you choose something, Dr. Dutta says. “You have more to risk by not exercising.”
A version of this article first appeared on WebMD.com.
If there’s one public health message Americans have heard loud and clear, it’s this one:
Move more.
Take more steps.
Spend more time doing physical activity – at least 150 minutes a week, according to the latest guidelines.
But hearing the message doesn’t mean we act on it. A whopping 25% of Americans don’t get any physical activity beyond what they do in their job, according to a CDC survey.
Just do what you’re already doing, but with a little more effort.
The study, which was published in the European Heart Journal, builds on growing evidence that suggests exercise intensity matters just as much as the amount. So, something as simple as turning a leisurely stroll into a brisk walk can, over time, lead to significant reductions in your risk of cardiovascular disease. No additional moves, steps, or minutes needed.
Step it up
Researchers at Cambridge University and the University of Leicester in England looked at data from 88,000 middle-aged adults who wore an activity tracking device for 7 days.
The devices tracked both the total amount of activity they did and the intensity of that movement – that is, how fast they walked or how hard they pushed themselves.
The researchers then calculated their physical activity energy expenditure (the number of calories they burned when they were up and moving) and the percentage that came from moderate to vigorous physical activity.
What’s the difference?
- Physical activity means any and every movement you do throughout the day. Mostly it’s mundane tasks like shopping, walking to the mailbox, playing with your dog, or cooking.
- Moderate-intensity physical activity includes things you do at a faster pace. Maybe you’re walking for exercise, doing yard work or household chores, or running late and just trying to get somewhere faster. You’re breathing a little harder and possibly working up a sweat.
- Vigorous-intensity physical activity is usually an exercise session – a run, a strenuous hike, a tough workout in the gym. It can also be an exhausting chore like shoveling snow, which feels like a workout. You’re definitely breathing harder, and you’re probably working up a sweat, even in the middle of winter.
Over the next 6 to 7 years, there were 4,000 new cases of cardiovascular disease among the people in the study.
Those who got at least 20% of their physical activity energy expenditure from moderate to vigorous activities had significantly less risk of heart disease, compared with those whose higher-effort activities were about 10%.
That was true even for those whose total activity was relatively low. As long as higher-effort activities reached 20% of their total, they were 14% less likely to be diagnosed with a heart condition.
And for those with relatively high activity levels, there was little extra benefit if their moderate and vigorous activities remained around 10%.
That finding surprised Paddy Dempsey, PhD, a medical research scientist at Cambridge and the study’s lead author. But it also makes sense.
“People can improve their cardiorespiratory fitness to a greater degree with higher-intensity activity,” he says. “More intensity will stress the system and lead to greater adaptation.”
The key is an increase in the amount of oxygen your heart and lungs can provide your muscles during exercise, a measure known as VO2max.
Raising your VO2max is the best way to reduce your risk of early death, especially death from heart disease. Simply moving up from the lowest conditioning category to a higher one will cut your risk of dying in any given year by as much as 60%.
Making strides
The study builds on previous research that shows the benefits of moving faster.
Walking faster will naturally increase your stride length, another predictor of longevity and future health. A review study published in 2021 found that older adults who took shorter steps were 26% more likely to have a disability, 34% more likely to have a major adverse event (like an injury that leads to a loss of independence), and 69% more likely to die over the next several years.
Quality versus quantity
We’ve focused so far on the quality of your physical activity – moving faster, taking longer strides.
But there’s still a lot to be said for movement quantity.
“It would be a mistake to say volume doesn’t matter,” Dr. Dempsey cautions.
A 2022 study in the journal The Lancet found that the risk of dying during a given period decreases with each increase in daily steps. The protective effect peaks at about 6,000 to 8,000 steps a day for adults 60 and over, and at 8,000 to 10,000 steps for those under 60.
“The relative value of the quality and quantity of exercise are very specific to a person’s goals,” says Chhanda Dutta, PhD, chief of the Clinical Gerontology Branch at the National Institute on Aging. “If performance is the goal, quality matters at least as much as quantity.”
Dr. Dempsey agrees that it’s not a cage match between two. Every step you take is a step in the right direction.
“People can choose or gravitate to an approach that works best for them,” he says. “It’s also helpful to think about where some everyday activities can be punctuated with intensity,” which could be as simple as walking faster when possible.
What matters most is that you choose something, Dr. Dutta says. “You have more to risk by not exercising.”
A version of this article first appeared on WebMD.com.
If there’s one public health message Americans have heard loud and clear, it’s this one:
Move more.
Take more steps.
Spend more time doing physical activity – at least 150 minutes a week, according to the latest guidelines.
But hearing the message doesn’t mean we act on it. A whopping 25% of Americans don’t get any physical activity beyond what they do in their job, according to a CDC survey.
Just do what you’re already doing, but with a little more effort.
The study, which was published in the European Heart Journal, builds on growing evidence that suggests exercise intensity matters just as much as the amount. So, something as simple as turning a leisurely stroll into a brisk walk can, over time, lead to significant reductions in your risk of cardiovascular disease. No additional moves, steps, or minutes needed.
Step it up
Researchers at Cambridge University and the University of Leicester in England looked at data from 88,000 middle-aged adults who wore an activity tracking device for 7 days.
The devices tracked both the total amount of activity they did and the intensity of that movement – that is, how fast they walked or how hard they pushed themselves.
The researchers then calculated their physical activity energy expenditure (the number of calories they burned when they were up and moving) and the percentage that came from moderate to vigorous physical activity.
What’s the difference?
- Physical activity means any and every movement you do throughout the day. Mostly it’s mundane tasks like shopping, walking to the mailbox, playing with your dog, or cooking.
- Moderate-intensity physical activity includes things you do at a faster pace. Maybe you’re walking for exercise, doing yard work or household chores, or running late and just trying to get somewhere faster. You’re breathing a little harder and possibly working up a sweat.
- Vigorous-intensity physical activity is usually an exercise session – a run, a strenuous hike, a tough workout in the gym. It can also be an exhausting chore like shoveling snow, which feels like a workout. You’re definitely breathing harder, and you’re probably working up a sweat, even in the middle of winter.
Over the next 6 to 7 years, there were 4,000 new cases of cardiovascular disease among the people in the study.
Those who got at least 20% of their physical activity energy expenditure from moderate to vigorous activities had significantly less risk of heart disease, compared with those whose higher-effort activities were about 10%.
That was true even for those whose total activity was relatively low. As long as higher-effort activities reached 20% of their total, they were 14% less likely to be diagnosed with a heart condition.
And for those with relatively high activity levels, there was little extra benefit if their moderate and vigorous activities remained around 10%.
That finding surprised Paddy Dempsey, PhD, a medical research scientist at Cambridge and the study’s lead author. But it also makes sense.
“People can improve their cardiorespiratory fitness to a greater degree with higher-intensity activity,” he says. “More intensity will stress the system and lead to greater adaptation.”
The key is an increase in the amount of oxygen your heart and lungs can provide your muscles during exercise, a measure known as VO2max.
Raising your VO2max is the best way to reduce your risk of early death, especially death from heart disease. Simply moving up from the lowest conditioning category to a higher one will cut your risk of dying in any given year by as much as 60%.
Making strides
The study builds on previous research that shows the benefits of moving faster.
Walking faster will naturally increase your stride length, another predictor of longevity and future health. A review study published in 2021 found that older adults who took shorter steps were 26% more likely to have a disability, 34% more likely to have a major adverse event (like an injury that leads to a loss of independence), and 69% more likely to die over the next several years.
Quality versus quantity
We’ve focused so far on the quality of your physical activity – moving faster, taking longer strides.
But there’s still a lot to be said for movement quantity.
“It would be a mistake to say volume doesn’t matter,” Dr. Dempsey cautions.
A 2022 study in the journal The Lancet found that the risk of dying during a given period decreases with each increase in daily steps. The protective effect peaks at about 6,000 to 8,000 steps a day for adults 60 and over, and at 8,000 to 10,000 steps for those under 60.
“The relative value of the quality and quantity of exercise are very specific to a person’s goals,” says Chhanda Dutta, PhD, chief of the Clinical Gerontology Branch at the National Institute on Aging. “If performance is the goal, quality matters at least as much as quantity.”
Dr. Dempsey agrees that it’s not a cage match between two. Every step you take is a step in the right direction.
“People can choose or gravitate to an approach that works best for them,” he says. “It’s also helpful to think about where some everyday activities can be punctuated with intensity,” which could be as simple as walking faster when possible.
What matters most is that you choose something, Dr. Dutta says. “You have more to risk by not exercising.”
A version of this article first appeared on WebMD.com.
FROM EUROPEAN HEART JOURNAL
Lp(a) tied to more early CV events than familial hypercholesterolemia
Many more people are at risk for early cardiovascular events because of raised lipoprotein(a) levels than from having familial hypercholesterolemia (FH), a new study suggests.
The Danish study set out to try and establish a level of Lp(a) that would be associated with a cardiovascular risk similar to that seen with FH. As there are many different definitions of FH, results showed a large range of Lp(a) values that corresponded to risk levels of the different FH definitions.
However, if considering one of the broadest FH definitions (from MEDPED – Make Early Diagnoses, Prevent Early Deaths), which is the one most commonly used in the United States, results showed that the level of cardiovascular risk in patients with this definition of FH is similar to that associated with Lp(a) levels of around 70 mg/dL (0.7 g/L).
“While FH is fairly unusual, occurring in less than 1% of the population, levels of Lp(a) of 70 mg/dL or above are much more common, occurring in around 10% of the White population,” Børge Nordestgaard, MD, Copenhagen University Hospital, said in an interview. Around 20% of the Black population have such high levels, while levels in Hispanics are in between.
“Our results suggest that there will be many more individuals at risk of premature MI or cardiovascular death because of raised Lp(a) levels than because of FH,” added Dr. Nordestgaard, the senior author of the current study.
Dr. Nordestgaard explained that FH is well established to be a serious condition. “We consider FH to be the genetic disease that causes the most cases of early heart disease and early death worldwide.”
“But we know now that raised levels of Lp(a), which is also genetically determined, can also lead to an increased risk of cardiovascular events relatively early in life, and when you look into the numbers, it seems like high levels of Lp(a) could be more common than FH. We wanted to try and find the levels of Lp(a) that corresponded to similar cardiovascular risk as FH.”
The Danish study was published in the Journal of the American College of Cardiology.
The authors note that the 2019 joint European Society of Cardiology and European Atherosclerosis Society guidelines suggested that an Lp(a) level greater than 180 mg/dL (0.8 g/L) may confer a lifetime risk for heart disease equivalent to the risk associated with heterozygous FH, but they point out that this value was speculative and not based on a direct comparison of risk associated with the two conditions in the same population.
For their study, Dr. Nordestgaard and colleagues analyzed information from a large database of the Danish population, the Copenhagen General Population Study, including 69,644 individuals for whom data on FH and Lp(a) levels were available. As these conditions are genetically determined, and the study held records on individuals going back several decades, the researchers were able to analyze event rates over a median follow up time of 42 years. During this time, there were 4,166 cases of myocardial infarction and 11,464 cases of atherosclerotic cardiovascular disease (ASCVD).
Results showed that Lp(a) levels associated with MI risk equivalent to that of clinical FH ranged from 67 to 402 mg/dL depending on the definition used for FH. The Lp(a) level corresponding to the MI risk of genetically determined FH was 180 mg/dL.
In terms of risk of ASCVD events, the levels of Lp(a) corresponding to the risk associated with clinical FH ranged from 130 to 391 mg/dL, and the Lp(a) level corresponding to the ASCVD risk of genetically determined FH was 175 mg/dL.
“All these different definitions of FH may cause some confusion, but basically we are saying that if an individual is found to have an Lp(a) above 70 mg/dL, then they have a similar level of cardiovascular risk as that associated with the broadest definition of FH, and they should be taken as seriously as a patient diagnosed with FH,” Dr. Nordestgaard said.
He estimated that these individuals have approximately a doubling of cardiovascular risk, compared with the general population, and risk increases further with rising Lp(a) levels.
The researchers also found that if an individual has both FH and raised Lp(a) they are at very high risk, as these two conditions are independent of each other.
Although a specific treatment for lowering Lp(a) levels is not yet available, Dr. Nordestgaard stresses that it is still worth identifying individuals with raised Lp(a) as efforts can be made to address other cardiovascular risk factors.
“We know raised Lp(a) increases cardiovascular risk, but there are also many other factors that likewise increase this risk, and they are all additive. So, it is very important that individuals with raised Lp(a) levels address these other risk factors,” he said. “These include stopping smoking, being at healthy weight, exercising regularly, eating a heart-healthy diet, and aggressive treatment of raised LDL, hypertension, and diabetes. All these things will lower their overall risk of cardiovascular disease.”
And there is the promise of new drugs to lower Lp(a) on the horizon, with several such products now in clinical development.
Dr. Nordestgaard also points out that as Lp(a) is genetically determined, cascade screening of close relatives of the individual with raised Lp(a) should also take place to detect others who may be at risk.
Although a level of Lp(a) of around 70 mg/dL confers similar cardiovascular risk than some definitions of FH, Dr. Nordestgaard says lower levels than this should also be a signal for concern.
“We usually say Lp(a) levels of 50 mg/dL are when we need to start to take this seriously. And it’s estimated that about 20% of the White population will have levels of 50 mg/dL or over and even more in the Black population,” he added.
‘Screen for both conditions’
In an accompanying editorial, Pamela Morris, MD, Medical University of South Carolina, Charleston; Jagat Narula, MD, Icahn School of Medicine, New York; and Sotirios Tsimikas, MD, University of California, San Diego, say “the weight of evidence strongly supports that both genetic lipid disorders, elevated Lp(a) levels and FH, are causally associated with an increased risk of premature ASCVD and should be carefully considered in risk assessment and management for ASCVD risk reduction.”
Dr. Morris told this news organization that the current study found a very large range of Lp(a) levels that conferred a similar cardiovascular risk to FH, because of the many different definitions of FH in use.
“But this should not take away the importance of screening for raised Lp(a) levels,” she stressed.
“We know that increased Lp(a) levels signal a high risk of cardiovascular disease. A diagnosis of FH is also a high-risk condition,” she said. “Both are important, and we need to screen for both, but it is difficult to directly compare the two conditions because the different definitions of FH get in the way.”
Dr. Morris agrees with Dr. Nordestgaard that raised levels of Lp(a) may actually be more important for the population risk of cardiovascular disease than FH, as the prevalence of increased Lp(a) levels is higher.
“Because raised Lp(a) levels are more prevalent than confirmed FH, the risk to the population is greater,” she said.
Dr. Morris points out that cardiovascular risk starts to increase at Lp(a) levels of 30 mg/dL (75 nmol/L).
The editorialists recommend that “in addition to performing a lipid panel periodically according to evidence-based guidelines, measurement of Lp(a) levels should also be performed at least once in an individual’s lifetime for ASCVD risk assessment.”
They conclude that “it is vital to continue to raise awareness among clinicians and patients of these high-risk genetic lipid disorders. Our understanding of both disorders is rapidly expanding, and promising novel therapeutics may offer hope for prevention of cardiovascular disease in patients with elevated Lp(a) levels in the future.”
This work was supported by Copenhagen University Hospital – Herlev Gentofte, Denmark, and the Danish Beckett-Foundation. The Copenhagen General Population Study is supported by the Copenhagen County Foundation and Copenhagen University Hospital – Herlev Gentofte. Dr. Nordestgaard has been a consultant and a speaker for AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Kowa, Denka, Amarin, Novartis, Novo Nordisk, Silence Therapeutics, Abbott, and Esperion.
A version of this article first appeared on Medscape.com.
Many more people are at risk for early cardiovascular events because of raised lipoprotein(a) levels than from having familial hypercholesterolemia (FH), a new study suggests.
The Danish study set out to try and establish a level of Lp(a) that would be associated with a cardiovascular risk similar to that seen with FH. As there are many different definitions of FH, results showed a large range of Lp(a) values that corresponded to risk levels of the different FH definitions.
However, if considering one of the broadest FH definitions (from MEDPED – Make Early Diagnoses, Prevent Early Deaths), which is the one most commonly used in the United States, results showed that the level of cardiovascular risk in patients with this definition of FH is similar to that associated with Lp(a) levels of around 70 mg/dL (0.7 g/L).
“While FH is fairly unusual, occurring in less than 1% of the population, levels of Lp(a) of 70 mg/dL or above are much more common, occurring in around 10% of the White population,” Børge Nordestgaard, MD, Copenhagen University Hospital, said in an interview. Around 20% of the Black population have such high levels, while levels in Hispanics are in between.
“Our results suggest that there will be many more individuals at risk of premature MI or cardiovascular death because of raised Lp(a) levels than because of FH,” added Dr. Nordestgaard, the senior author of the current study.
Dr. Nordestgaard explained that FH is well established to be a serious condition. “We consider FH to be the genetic disease that causes the most cases of early heart disease and early death worldwide.”
“But we know now that raised levels of Lp(a), which is also genetically determined, can also lead to an increased risk of cardiovascular events relatively early in life, and when you look into the numbers, it seems like high levels of Lp(a) could be more common than FH. We wanted to try and find the levels of Lp(a) that corresponded to similar cardiovascular risk as FH.”
The Danish study was published in the Journal of the American College of Cardiology.
The authors note that the 2019 joint European Society of Cardiology and European Atherosclerosis Society guidelines suggested that an Lp(a) level greater than 180 mg/dL (0.8 g/L) may confer a lifetime risk for heart disease equivalent to the risk associated with heterozygous FH, but they point out that this value was speculative and not based on a direct comparison of risk associated with the two conditions in the same population.
For their study, Dr. Nordestgaard and colleagues analyzed information from a large database of the Danish population, the Copenhagen General Population Study, including 69,644 individuals for whom data on FH and Lp(a) levels were available. As these conditions are genetically determined, and the study held records on individuals going back several decades, the researchers were able to analyze event rates over a median follow up time of 42 years. During this time, there were 4,166 cases of myocardial infarction and 11,464 cases of atherosclerotic cardiovascular disease (ASCVD).
Results showed that Lp(a) levels associated with MI risk equivalent to that of clinical FH ranged from 67 to 402 mg/dL depending on the definition used for FH. The Lp(a) level corresponding to the MI risk of genetically determined FH was 180 mg/dL.
In terms of risk of ASCVD events, the levels of Lp(a) corresponding to the risk associated with clinical FH ranged from 130 to 391 mg/dL, and the Lp(a) level corresponding to the ASCVD risk of genetically determined FH was 175 mg/dL.
“All these different definitions of FH may cause some confusion, but basically we are saying that if an individual is found to have an Lp(a) above 70 mg/dL, then they have a similar level of cardiovascular risk as that associated with the broadest definition of FH, and they should be taken as seriously as a patient diagnosed with FH,” Dr. Nordestgaard said.
He estimated that these individuals have approximately a doubling of cardiovascular risk, compared with the general population, and risk increases further with rising Lp(a) levels.
The researchers also found that if an individual has both FH and raised Lp(a) they are at very high risk, as these two conditions are independent of each other.
Although a specific treatment for lowering Lp(a) levels is not yet available, Dr. Nordestgaard stresses that it is still worth identifying individuals with raised Lp(a) as efforts can be made to address other cardiovascular risk factors.
“We know raised Lp(a) increases cardiovascular risk, but there are also many other factors that likewise increase this risk, and they are all additive. So, it is very important that individuals with raised Lp(a) levels address these other risk factors,” he said. “These include stopping smoking, being at healthy weight, exercising regularly, eating a heart-healthy diet, and aggressive treatment of raised LDL, hypertension, and diabetes. All these things will lower their overall risk of cardiovascular disease.”
And there is the promise of new drugs to lower Lp(a) on the horizon, with several such products now in clinical development.
Dr. Nordestgaard also points out that as Lp(a) is genetically determined, cascade screening of close relatives of the individual with raised Lp(a) should also take place to detect others who may be at risk.
Although a level of Lp(a) of around 70 mg/dL confers similar cardiovascular risk than some definitions of FH, Dr. Nordestgaard says lower levels than this should also be a signal for concern.
“We usually say Lp(a) levels of 50 mg/dL are when we need to start to take this seriously. And it’s estimated that about 20% of the White population will have levels of 50 mg/dL or over and even more in the Black population,” he added.
‘Screen for both conditions’
In an accompanying editorial, Pamela Morris, MD, Medical University of South Carolina, Charleston; Jagat Narula, MD, Icahn School of Medicine, New York; and Sotirios Tsimikas, MD, University of California, San Diego, say “the weight of evidence strongly supports that both genetic lipid disorders, elevated Lp(a) levels and FH, are causally associated with an increased risk of premature ASCVD and should be carefully considered in risk assessment and management for ASCVD risk reduction.”
Dr. Morris told this news organization that the current study found a very large range of Lp(a) levels that conferred a similar cardiovascular risk to FH, because of the many different definitions of FH in use.
“But this should not take away the importance of screening for raised Lp(a) levels,” she stressed.
“We know that increased Lp(a) levels signal a high risk of cardiovascular disease. A diagnosis of FH is also a high-risk condition,” she said. “Both are important, and we need to screen for both, but it is difficult to directly compare the two conditions because the different definitions of FH get in the way.”
Dr. Morris agrees with Dr. Nordestgaard that raised levels of Lp(a) may actually be more important for the population risk of cardiovascular disease than FH, as the prevalence of increased Lp(a) levels is higher.
“Because raised Lp(a) levels are more prevalent than confirmed FH, the risk to the population is greater,” she said.
Dr. Morris points out that cardiovascular risk starts to increase at Lp(a) levels of 30 mg/dL (75 nmol/L).
The editorialists recommend that “in addition to performing a lipid panel periodically according to evidence-based guidelines, measurement of Lp(a) levels should also be performed at least once in an individual’s lifetime for ASCVD risk assessment.”
They conclude that “it is vital to continue to raise awareness among clinicians and patients of these high-risk genetic lipid disorders. Our understanding of both disorders is rapidly expanding, and promising novel therapeutics may offer hope for prevention of cardiovascular disease in patients with elevated Lp(a) levels in the future.”
This work was supported by Copenhagen University Hospital – Herlev Gentofte, Denmark, and the Danish Beckett-Foundation. The Copenhagen General Population Study is supported by the Copenhagen County Foundation and Copenhagen University Hospital – Herlev Gentofte. Dr. Nordestgaard has been a consultant and a speaker for AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Kowa, Denka, Amarin, Novartis, Novo Nordisk, Silence Therapeutics, Abbott, and Esperion.
A version of this article first appeared on Medscape.com.
Many more people are at risk for early cardiovascular events because of raised lipoprotein(a) levels than from having familial hypercholesterolemia (FH), a new study suggests.
The Danish study set out to try and establish a level of Lp(a) that would be associated with a cardiovascular risk similar to that seen with FH. As there are many different definitions of FH, results showed a large range of Lp(a) values that corresponded to risk levels of the different FH definitions.
However, if considering one of the broadest FH definitions (from MEDPED – Make Early Diagnoses, Prevent Early Deaths), which is the one most commonly used in the United States, results showed that the level of cardiovascular risk in patients with this definition of FH is similar to that associated with Lp(a) levels of around 70 mg/dL (0.7 g/L).
“While FH is fairly unusual, occurring in less than 1% of the population, levels of Lp(a) of 70 mg/dL or above are much more common, occurring in around 10% of the White population,” Børge Nordestgaard, MD, Copenhagen University Hospital, said in an interview. Around 20% of the Black population have such high levels, while levels in Hispanics are in between.
“Our results suggest that there will be many more individuals at risk of premature MI or cardiovascular death because of raised Lp(a) levels than because of FH,” added Dr. Nordestgaard, the senior author of the current study.
Dr. Nordestgaard explained that FH is well established to be a serious condition. “We consider FH to be the genetic disease that causes the most cases of early heart disease and early death worldwide.”
“But we know now that raised levels of Lp(a), which is also genetically determined, can also lead to an increased risk of cardiovascular events relatively early in life, and when you look into the numbers, it seems like high levels of Lp(a) could be more common than FH. We wanted to try and find the levels of Lp(a) that corresponded to similar cardiovascular risk as FH.”
The Danish study was published in the Journal of the American College of Cardiology.
The authors note that the 2019 joint European Society of Cardiology and European Atherosclerosis Society guidelines suggested that an Lp(a) level greater than 180 mg/dL (0.8 g/L) may confer a lifetime risk for heart disease equivalent to the risk associated with heterozygous FH, but they point out that this value was speculative and not based on a direct comparison of risk associated with the two conditions in the same population.
For their study, Dr. Nordestgaard and colleagues analyzed information from a large database of the Danish population, the Copenhagen General Population Study, including 69,644 individuals for whom data on FH and Lp(a) levels were available. As these conditions are genetically determined, and the study held records on individuals going back several decades, the researchers were able to analyze event rates over a median follow up time of 42 years. During this time, there were 4,166 cases of myocardial infarction and 11,464 cases of atherosclerotic cardiovascular disease (ASCVD).
Results showed that Lp(a) levels associated with MI risk equivalent to that of clinical FH ranged from 67 to 402 mg/dL depending on the definition used for FH. The Lp(a) level corresponding to the MI risk of genetically determined FH was 180 mg/dL.
In terms of risk of ASCVD events, the levels of Lp(a) corresponding to the risk associated with clinical FH ranged from 130 to 391 mg/dL, and the Lp(a) level corresponding to the ASCVD risk of genetically determined FH was 175 mg/dL.
“All these different definitions of FH may cause some confusion, but basically we are saying that if an individual is found to have an Lp(a) above 70 mg/dL, then they have a similar level of cardiovascular risk as that associated with the broadest definition of FH, and they should be taken as seriously as a patient diagnosed with FH,” Dr. Nordestgaard said.
He estimated that these individuals have approximately a doubling of cardiovascular risk, compared with the general population, and risk increases further with rising Lp(a) levels.
The researchers also found that if an individual has both FH and raised Lp(a) they are at very high risk, as these two conditions are independent of each other.
Although a specific treatment for lowering Lp(a) levels is not yet available, Dr. Nordestgaard stresses that it is still worth identifying individuals with raised Lp(a) as efforts can be made to address other cardiovascular risk factors.
“We know raised Lp(a) increases cardiovascular risk, but there are also many other factors that likewise increase this risk, and they are all additive. So, it is very important that individuals with raised Lp(a) levels address these other risk factors,” he said. “These include stopping smoking, being at healthy weight, exercising regularly, eating a heart-healthy diet, and aggressive treatment of raised LDL, hypertension, and diabetes. All these things will lower their overall risk of cardiovascular disease.”
And there is the promise of new drugs to lower Lp(a) on the horizon, with several such products now in clinical development.
Dr. Nordestgaard also points out that as Lp(a) is genetically determined, cascade screening of close relatives of the individual with raised Lp(a) should also take place to detect others who may be at risk.
Although a level of Lp(a) of around 70 mg/dL confers similar cardiovascular risk than some definitions of FH, Dr. Nordestgaard says lower levels than this should also be a signal for concern.
“We usually say Lp(a) levels of 50 mg/dL are when we need to start to take this seriously. And it’s estimated that about 20% of the White population will have levels of 50 mg/dL or over and even more in the Black population,” he added.
‘Screen for both conditions’
In an accompanying editorial, Pamela Morris, MD, Medical University of South Carolina, Charleston; Jagat Narula, MD, Icahn School of Medicine, New York; and Sotirios Tsimikas, MD, University of California, San Diego, say “the weight of evidence strongly supports that both genetic lipid disorders, elevated Lp(a) levels and FH, are causally associated with an increased risk of premature ASCVD and should be carefully considered in risk assessment and management for ASCVD risk reduction.”
Dr. Morris told this news organization that the current study found a very large range of Lp(a) levels that conferred a similar cardiovascular risk to FH, because of the many different definitions of FH in use.
“But this should not take away the importance of screening for raised Lp(a) levels,” she stressed.
“We know that increased Lp(a) levels signal a high risk of cardiovascular disease. A diagnosis of FH is also a high-risk condition,” she said. “Both are important, and we need to screen for both, but it is difficult to directly compare the two conditions because the different definitions of FH get in the way.”
Dr. Morris agrees with Dr. Nordestgaard that raised levels of Lp(a) may actually be more important for the population risk of cardiovascular disease than FH, as the prevalence of increased Lp(a) levels is higher.
“Because raised Lp(a) levels are more prevalent than confirmed FH, the risk to the population is greater,” she said.
Dr. Morris points out that cardiovascular risk starts to increase at Lp(a) levels of 30 mg/dL (75 nmol/L).
The editorialists recommend that “in addition to performing a lipid panel periodically according to evidence-based guidelines, measurement of Lp(a) levels should also be performed at least once in an individual’s lifetime for ASCVD risk assessment.”
They conclude that “it is vital to continue to raise awareness among clinicians and patients of these high-risk genetic lipid disorders. Our understanding of both disorders is rapidly expanding, and promising novel therapeutics may offer hope for prevention of cardiovascular disease in patients with elevated Lp(a) levels in the future.”
This work was supported by Copenhagen University Hospital – Herlev Gentofte, Denmark, and the Danish Beckett-Foundation. The Copenhagen General Population Study is supported by the Copenhagen County Foundation and Copenhagen University Hospital – Herlev Gentofte. Dr. Nordestgaard has been a consultant and a speaker for AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Kowa, Denka, Amarin, Novartis, Novo Nordisk, Silence Therapeutics, Abbott, and Esperion.
A version of this article first appeared on Medscape.com.
New studies change beliefs about cardiovascular disease
This transcript has been edited for clarity.
I’m going to review a few of these.
The first is the TIME study. The TIME study looked at whether it matters if you give antihypertensive agents in the morning or the evening. This was a prospective, pragmatic, parallel-group study that was performed in the U.K. and published in The Lancet.
Their question was whether evening dosing of antihypertensives has benefit in cardiovascular outcomes in adults. They enrolled over 21,000 people with hypertension who were taking at least one antihypertensive medication. Patients were randomized to morning or evening dosing.
The primary outcome was death or hospitalization due to myocardial infarction or stroke. There was no difference. It doesn’t matter if you take your antihypertensive agent in the morning or the evening. I think this is important because, clinically, the simpler the regimen for the patient, the greater the adherence, leading to better outcomes.
I know I can safely ask a patient when they would rather take their medicine. For many people, that may be the morning because they’re brushing their teeth and they remember. If they want to take it in the evening, that’s fine, too. We’re no longer slave to telling a patient to take their antihypertensive medications in the evening.
At the meeting of the American Society of Nephrology, results from a study on the use of renin-angiotensin system (RAS) inhibitors in advanced CKD was presented, called the STOP ACEi trial. Again, another interesting trial asking a simple question. This was a randomized controlled trial (RCT) in patients who had an estimated glomerular filtration rate (eGFR) less than 30, and they were randomized to stop or continue therapy with their RAS inhibitors.
The primary outcome was the eGFR at 3 years. They enrolled 411 patients with a median baseline eGFR of 18. At 3 years, there was no difference in the eGFR between the groups. In the discontinuation group, the eGFR was 12.6 versus 13.3 in the continuation group. There were no differences in complications or anything else. Their conclusion was that among patients with advanced and progressive CKD, the discontinuation of a RAS inhibitor was not associated with a significant difference in the long-term rate of decrease in eGFR.
I think this is important because it changes our paradigm a bit. You can stop the RAS inhibitor; reduce the need for excessive medication in these patients; and, hopefully, focus on some newer medications that have been shown to prevent the decline in eGFR that are now available.
Next is from a letter published in JAMA, which asks the following question: Is diabetes itself an equivalent cardiovascular risk factor to those who have had a prior cardiovascular event?
We used to put having diabetes in that same high-risk category as people who’d already had a cardiovascular disease event. Well, have we made that any different? These authors are from Canada, and they did a retrospective population-based study looking at administrative health claims from Ontario, Canada, to assess the association of diabetes and prior cardiovascular disease with cardiovascular events from 1994 to 2014.
What I think is kind of cool, because I’m a diabetologist, is that over time the magnitude of the association between diabetes and cardiovascular event rates decreased. In somebody with diabetes, they don’t have the same high risk that a person who’s already had a cardiovascular event rate does. Diabetes is less of a risk factor for cardiovascular disease than having established cardiovascular disease, which means we’re treating diabetes better and reducing the risk for cardiovascular disease.
If you look at people with diabetes and a prior cardiovascular event, that’s still the very highest risk. The risk of people having another event who have established cardiovascular disease is pretty flat. Those people didn’t get better and the people with preexisting diabetes and cardiovascular events at baseline didn’t get much better, but those who had diabetes alone did improve in terms of looking at cardiovascular event rates.
I think this is good news because diabetes itself isn’t as high a cardiovascular risk factor as we once thought. It doesn’t mean that it isn’t a cardiovascular risk factor, but I think we’ve done better at mitigating the risk.
Finally, there is a relatively small study that was presented at the American Heart Association and published in the Journal of the American College of Cardiology, which asks whether supplements that are often used to lower LDL cholesterol are equivalent to a statin.
They compared six supplements with a placebo and with rosuvastatin, and looked to see what happened. This is not an outcome study, but a very short study, at 28 days, that used a placebo. They included 190 people with no history of cardiovascular disease but an increased 10-year risk for sclerotic cardiovascular disease.
The agents studied were rosuvastatin, placebo, fish oil, cinnamon, garlic, turmeric, plant sterols, and red yeast rice. Well, not surprisingly, rosuvastatin worked. It showed a 35% reduction in LDL cholesterol, and there was no significant impact on cholesterol levels with any of the other agents. The supplements yielded a similar response, as did the placebo. Side effects were similar, but they were most common with plant sterols and red yeast rice.
Clearly, a statin is better if you want to lower cholesterol levels. My approach, when patients want to take supplements, is to tell them what I know factually, which basically is that they don’t really cause much in the way of LDL cholesterol lowering. If I think the supplement isn’t going to hurt someone, I don’t tell them not to use it. I certainly tell them that they need to use agents that we know can actually reduce cardiovascular risk.
I think these studies really go through the gamut of asking questions. When can we stop an agent? What time of day do we need to give an agent? What, really, is the risk for type 2 diabetes with regard to cardiovascular events? What’s the value of supplements?
I think this is interesting, because I really encourage researchers to ask and answer these kinds of questions because it helps us clinically decide what’s best for treating our patients.
Thank you.
Dr. Peters is a professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She reported conflicts of interest with numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m going to review a few of these.
The first is the TIME study. The TIME study looked at whether it matters if you give antihypertensive agents in the morning or the evening. This was a prospective, pragmatic, parallel-group study that was performed in the U.K. and published in The Lancet.
Their question was whether evening dosing of antihypertensives has benefit in cardiovascular outcomes in adults. They enrolled over 21,000 people with hypertension who were taking at least one antihypertensive medication. Patients were randomized to morning or evening dosing.
The primary outcome was death or hospitalization due to myocardial infarction or stroke. There was no difference. It doesn’t matter if you take your antihypertensive agent in the morning or the evening. I think this is important because, clinically, the simpler the regimen for the patient, the greater the adherence, leading to better outcomes.
I know I can safely ask a patient when they would rather take their medicine. For many people, that may be the morning because they’re brushing their teeth and they remember. If they want to take it in the evening, that’s fine, too. We’re no longer slave to telling a patient to take their antihypertensive medications in the evening.
At the meeting of the American Society of Nephrology, results from a study on the use of renin-angiotensin system (RAS) inhibitors in advanced CKD was presented, called the STOP ACEi trial. Again, another interesting trial asking a simple question. This was a randomized controlled trial (RCT) in patients who had an estimated glomerular filtration rate (eGFR) less than 30, and they were randomized to stop or continue therapy with their RAS inhibitors.
The primary outcome was the eGFR at 3 years. They enrolled 411 patients with a median baseline eGFR of 18. At 3 years, there was no difference in the eGFR between the groups. In the discontinuation group, the eGFR was 12.6 versus 13.3 in the continuation group. There were no differences in complications or anything else. Their conclusion was that among patients with advanced and progressive CKD, the discontinuation of a RAS inhibitor was not associated with a significant difference in the long-term rate of decrease in eGFR.
I think this is important because it changes our paradigm a bit. You can stop the RAS inhibitor; reduce the need for excessive medication in these patients; and, hopefully, focus on some newer medications that have been shown to prevent the decline in eGFR that are now available.
Next is from a letter published in JAMA, which asks the following question: Is diabetes itself an equivalent cardiovascular risk factor to those who have had a prior cardiovascular event?
We used to put having diabetes in that same high-risk category as people who’d already had a cardiovascular disease event. Well, have we made that any different? These authors are from Canada, and they did a retrospective population-based study looking at administrative health claims from Ontario, Canada, to assess the association of diabetes and prior cardiovascular disease with cardiovascular events from 1994 to 2014.
What I think is kind of cool, because I’m a diabetologist, is that over time the magnitude of the association between diabetes and cardiovascular event rates decreased. In somebody with diabetes, they don’t have the same high risk that a person who’s already had a cardiovascular event rate does. Diabetes is less of a risk factor for cardiovascular disease than having established cardiovascular disease, which means we’re treating diabetes better and reducing the risk for cardiovascular disease.
If you look at people with diabetes and a prior cardiovascular event, that’s still the very highest risk. The risk of people having another event who have established cardiovascular disease is pretty flat. Those people didn’t get better and the people with preexisting diabetes and cardiovascular events at baseline didn’t get much better, but those who had diabetes alone did improve in terms of looking at cardiovascular event rates.
I think this is good news because diabetes itself isn’t as high a cardiovascular risk factor as we once thought. It doesn’t mean that it isn’t a cardiovascular risk factor, but I think we’ve done better at mitigating the risk.
Finally, there is a relatively small study that was presented at the American Heart Association and published in the Journal of the American College of Cardiology, which asks whether supplements that are often used to lower LDL cholesterol are equivalent to a statin.
They compared six supplements with a placebo and with rosuvastatin, and looked to see what happened. This is not an outcome study, but a very short study, at 28 days, that used a placebo. They included 190 people with no history of cardiovascular disease but an increased 10-year risk for sclerotic cardiovascular disease.
The agents studied were rosuvastatin, placebo, fish oil, cinnamon, garlic, turmeric, plant sterols, and red yeast rice. Well, not surprisingly, rosuvastatin worked. It showed a 35% reduction in LDL cholesterol, and there was no significant impact on cholesterol levels with any of the other agents. The supplements yielded a similar response, as did the placebo. Side effects were similar, but they were most common with plant sterols and red yeast rice.
Clearly, a statin is better if you want to lower cholesterol levels. My approach, when patients want to take supplements, is to tell them what I know factually, which basically is that they don’t really cause much in the way of LDL cholesterol lowering. If I think the supplement isn’t going to hurt someone, I don’t tell them not to use it. I certainly tell them that they need to use agents that we know can actually reduce cardiovascular risk.
I think these studies really go through the gamut of asking questions. When can we stop an agent? What time of day do we need to give an agent? What, really, is the risk for type 2 diabetes with regard to cardiovascular events? What’s the value of supplements?
I think this is interesting, because I really encourage researchers to ask and answer these kinds of questions because it helps us clinically decide what’s best for treating our patients.
Thank you.
Dr. Peters is a professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She reported conflicts of interest with numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m going to review a few of these.
The first is the TIME study. The TIME study looked at whether it matters if you give antihypertensive agents in the morning or the evening. This was a prospective, pragmatic, parallel-group study that was performed in the U.K. and published in The Lancet.
Their question was whether evening dosing of antihypertensives has benefit in cardiovascular outcomes in adults. They enrolled over 21,000 people with hypertension who were taking at least one antihypertensive medication. Patients were randomized to morning or evening dosing.
The primary outcome was death or hospitalization due to myocardial infarction or stroke. There was no difference. It doesn’t matter if you take your antihypertensive agent in the morning or the evening. I think this is important because, clinically, the simpler the regimen for the patient, the greater the adherence, leading to better outcomes.
I know I can safely ask a patient when they would rather take their medicine. For many people, that may be the morning because they’re brushing their teeth and they remember. If they want to take it in the evening, that’s fine, too. We’re no longer slave to telling a patient to take their antihypertensive medications in the evening.
At the meeting of the American Society of Nephrology, results from a study on the use of renin-angiotensin system (RAS) inhibitors in advanced CKD was presented, called the STOP ACEi trial. Again, another interesting trial asking a simple question. This was a randomized controlled trial (RCT) in patients who had an estimated glomerular filtration rate (eGFR) less than 30, and they were randomized to stop or continue therapy with their RAS inhibitors.
The primary outcome was the eGFR at 3 years. They enrolled 411 patients with a median baseline eGFR of 18. At 3 years, there was no difference in the eGFR between the groups. In the discontinuation group, the eGFR was 12.6 versus 13.3 in the continuation group. There were no differences in complications or anything else. Their conclusion was that among patients with advanced and progressive CKD, the discontinuation of a RAS inhibitor was not associated with a significant difference in the long-term rate of decrease in eGFR.
I think this is important because it changes our paradigm a bit. You can stop the RAS inhibitor; reduce the need for excessive medication in these patients; and, hopefully, focus on some newer medications that have been shown to prevent the decline in eGFR that are now available.
Next is from a letter published in JAMA, which asks the following question: Is diabetes itself an equivalent cardiovascular risk factor to those who have had a prior cardiovascular event?
We used to put having diabetes in that same high-risk category as people who’d already had a cardiovascular disease event. Well, have we made that any different? These authors are from Canada, and they did a retrospective population-based study looking at administrative health claims from Ontario, Canada, to assess the association of diabetes and prior cardiovascular disease with cardiovascular events from 1994 to 2014.
What I think is kind of cool, because I’m a diabetologist, is that over time the magnitude of the association between diabetes and cardiovascular event rates decreased. In somebody with diabetes, they don’t have the same high risk that a person who’s already had a cardiovascular event rate does. Diabetes is less of a risk factor for cardiovascular disease than having established cardiovascular disease, which means we’re treating diabetes better and reducing the risk for cardiovascular disease.
If you look at people with diabetes and a prior cardiovascular event, that’s still the very highest risk. The risk of people having another event who have established cardiovascular disease is pretty flat. Those people didn’t get better and the people with preexisting diabetes and cardiovascular events at baseline didn’t get much better, but those who had diabetes alone did improve in terms of looking at cardiovascular event rates.
I think this is good news because diabetes itself isn’t as high a cardiovascular risk factor as we once thought. It doesn’t mean that it isn’t a cardiovascular risk factor, but I think we’ve done better at mitigating the risk.
Finally, there is a relatively small study that was presented at the American Heart Association and published in the Journal of the American College of Cardiology, which asks whether supplements that are often used to lower LDL cholesterol are equivalent to a statin.
They compared six supplements with a placebo and with rosuvastatin, and looked to see what happened. This is not an outcome study, but a very short study, at 28 days, that used a placebo. They included 190 people with no history of cardiovascular disease but an increased 10-year risk for sclerotic cardiovascular disease.
The agents studied were rosuvastatin, placebo, fish oil, cinnamon, garlic, turmeric, plant sterols, and red yeast rice. Well, not surprisingly, rosuvastatin worked. It showed a 35% reduction in LDL cholesterol, and there was no significant impact on cholesterol levels with any of the other agents. The supplements yielded a similar response, as did the placebo. Side effects were similar, but they were most common with plant sterols and red yeast rice.
Clearly, a statin is better if you want to lower cholesterol levels. My approach, when patients want to take supplements, is to tell them what I know factually, which basically is that they don’t really cause much in the way of LDL cholesterol lowering. If I think the supplement isn’t going to hurt someone, I don’t tell them not to use it. I certainly tell them that they need to use agents that we know can actually reduce cardiovascular risk.
I think these studies really go through the gamut of asking questions. When can we stop an agent? What time of day do we need to give an agent? What, really, is the risk for type 2 diabetes with regard to cardiovascular events? What’s the value of supplements?
I think this is interesting, because I really encourage researchers to ask and answer these kinds of questions because it helps us clinically decide what’s best for treating our patients.
Thank you.
Dr. Peters is a professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She reported conflicts of interest with numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
Persistent asthma linked to higher carotid plaque burden
Persistent asthma is associated with increased carotid plaque burden and higher levels of inflammation, putting these patients at risk for atherosclerotic cardiovascular disease (ASCVD) events, new research suggests.
Using data from the MESA study, investigators analyzed more than 5,000 individuals, comparing carotid plaque and inflammatory markers in those with and without asthma.
They found that carotid plaque was present in half of participants without asthma and half of those with intermittent asthma but in close to 70% of participants with persistent asthma.
.
“The take-home message is that the current study, paired with prior studies, highlights that individuals with more significant forms of asthma may be at higher cardiovascular risk and makes it imperative to address modifiable risk factors among patients with asthma,” lead author Matthew Tattersall, DO, MS, assistant professor of cardiovascular medicine, University of Wisconsin School of Medicine and Public Health, Madison, told this news organization.
The study was published online in the Journal of the American Heart Association.
Limited data
Asthma and ASCVD are “highly prevalent inflammatory diseases,” the authors write. Carotid artery plaque detected by B-mode ultrasound “represents advanced, typically subclinical atherosclerosis that is a strong independent predictor of incident ASCVD events,” with inflammation playing a “key role” in precipitating these events, they note.
Serum inflammatory markers such as C-reactive protein (CRP) and IL-6 are associated with increased ASCVD events, and in asthma, CRP and other inflammatory biomarkers are elevated and tend to further increase during exacerbations.
Currently, there are limited data looking at the associations of asthma, asthma severity, and atherosclerotic plaque burden, they note, so the researchers turned to the MESA study – a multiethnic population of individuals free of prevalent ASCVD at baseline. They hypothesized that persistent asthma would be associated with higher carotid plaque presence and burden.
They also wanted to explore “whether these associations would be attenuated after adjustment for baseline inflammatory biomarkers.”
Dr. Tattersall said the current study “links our previous work studying the manifestations of asthma,” in which he and his colleagues demonstrated increased cardiovascular events among MESA participants with persistent asthma, as well as late-onset asthma participants in the Wisconsin Sleep Cohort. His group also showed that early arterial injury occurs in adolescents with asthma.
However, there are also few data looking at the association with carotid plaque, “a late manifestation of arterial injury and a strong predictor of future cardiovascular events and asthma,” Dr. Tattersall added.
He and his group therefore “wanted to explore the entire spectrum of arterial injury, from the initial increase in the carotid media thickness to plaque formation to cardiovascular events.”
To do so, they studied participants in MESA, a study of close to 7,000 adults that began in the year 2000 and continues to follow participants today. At the time of enrollment, all were free from CVD.
The current analysis looked at 5,029 MESA participants (mean age 61.6 years, 53% female, 26% Black, 23% Hispanic, 12% Asian), comparing those with persistent asthma, defined as “asthma requiring use of controller medications,” intermittent asthma, defined as “asthma without controller medications,” and no asthma.
Participants underwent B-mode carotid ultrasound to detect carotid plaques, with a total plaque score (TPS) ranging from 0-12. The researchers used multivariable regression modeling to evaluate the association of asthma subtype and carotid plaque burden.
Interpret cautiously
Participants with persistent asthma were more likely to be female, have higher body mass index (BMI), and higher high-density lipoprotein (HDL) cholesterol levels, compared with those without asthma.
Participants with persistent asthma had the highest burden of carotid plaque (P ≤ .003 for comparison of proportions and .002 for comparison of means).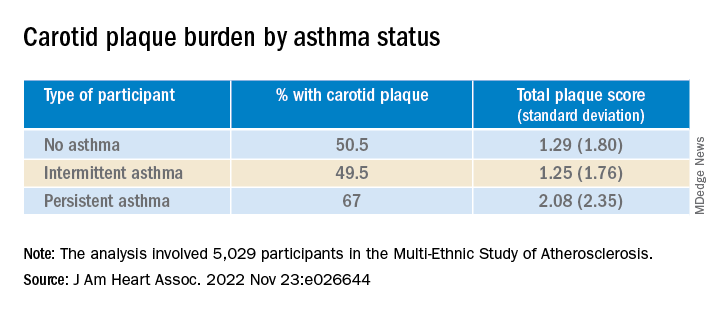
Moreover, participants with persistent asthma also had the highest systemic inflammatory marker levels – both CRP and IL-6 – compared with those without asthma. While participants with intermittent asthma also had higher average CRP, compared with those without asthma, their IL-6 levels were comparable.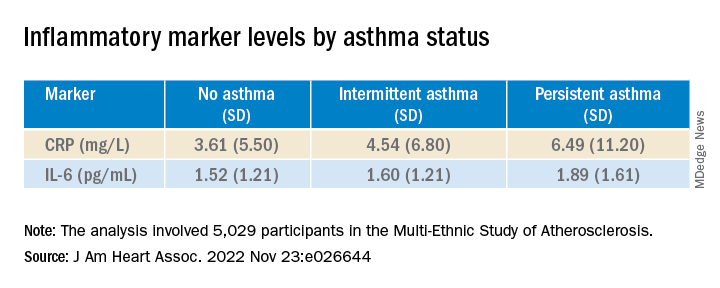
In unadjusted models, persistent asthma was associated with higher odds of carotid plaque presence (odds ratio, 1.97; 95% confidence interval, 1.32-2.95) – an association that persisted even in models that adjusted for biologic confounders (both P < .01). There also was an association between persistent asthma and higher carotid TPS (P < .001).
In further adjusted models, IL-6 was independently associated with presence of carotid plaque (P = .0001 per 1-SD increment of 1.53), as well as TPS (P < .001). CRP was “slightly associated” with carotid TPS (P = .04) but not carotid plaque presence (P = .07).
There was no attenuation after the researchers evaluated the associations of asthma subtype and carotid plaque presence or TPS and fully adjusted for baseline IL-6 or CRP (P = .02 and P = .01, respectively).
“Since this study is observational, we cannot confirm causation, but the study adds to the growing literature exploring the systemic effects of asthma,” Dr. Tattersall commented.
“Our initial hypothesis was that it was driven by inflammation, as both asthma and CVD are inflammatory conditions,” he continued. “We did adjust for inflammatory biomarkers in this analysis, but there was no change in the association.”
Nevertheless, Dr. Tattersall and colleagues are “cautious in the interpretation,” since the inflammatory biomarkers “were only collected at one point, and these measures can be dynamic, thus adjustment may not tell the whole story.”
Heightened awareness
Robert Brook, MD, professor and director of cardiovascular disease prevention, Wayne State University, Detroit, said the “main contribution of this study is the novel demonstration of a significant association between persistent (but not intermittent) asthma with carotid atherosclerosis in the MESA cohort, a large multi-ethnic population.”
These findings “support the biological plausibility of the growing epidemiological evidence that asthma independently increases the risk for cardiovascular morbidity and mortality,” added Dr. Brook, who was not involved with the study.
“The main take-home message for clinicians is that, just like in COPD (which is well-established), asthma is often a systemic condition in that the inflammation and disease process can impact the whole body,” he said.
“Health care providers should have a heightened awareness of the potentially increased cardiovascular risk of their patients with asthma and pay special attention to controlling their heart disease risk factors (for example, hyperlipidemia, hypertension),” Dr. Brook stated.
Dr. Tattersall was supported by an American Heart Association Career Development Award. The Multi-Ethnic Study of Atherosclerosis was supported by the National Heart, Lung, and Blood Institute and the National Center for Research Resources. Dr. Tattersall and co-authors and Dr. Brook declare no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Persistent asthma is associated with increased carotid plaque burden and higher levels of inflammation, putting these patients at risk for atherosclerotic cardiovascular disease (ASCVD) events, new research suggests.
Using data from the MESA study, investigators analyzed more than 5,000 individuals, comparing carotid plaque and inflammatory markers in those with and without asthma.
They found that carotid plaque was present in half of participants without asthma and half of those with intermittent asthma but in close to 70% of participants with persistent asthma.
.
“The take-home message is that the current study, paired with prior studies, highlights that individuals with more significant forms of asthma may be at higher cardiovascular risk and makes it imperative to address modifiable risk factors among patients with asthma,” lead author Matthew Tattersall, DO, MS, assistant professor of cardiovascular medicine, University of Wisconsin School of Medicine and Public Health, Madison, told this news organization.
The study was published online in the Journal of the American Heart Association.
Limited data
Asthma and ASCVD are “highly prevalent inflammatory diseases,” the authors write. Carotid artery plaque detected by B-mode ultrasound “represents advanced, typically subclinical atherosclerosis that is a strong independent predictor of incident ASCVD events,” with inflammation playing a “key role” in precipitating these events, they note.
Serum inflammatory markers such as C-reactive protein (CRP) and IL-6 are associated with increased ASCVD events, and in asthma, CRP and other inflammatory biomarkers are elevated and tend to further increase during exacerbations.
Currently, there are limited data looking at the associations of asthma, asthma severity, and atherosclerotic plaque burden, they note, so the researchers turned to the MESA study – a multiethnic population of individuals free of prevalent ASCVD at baseline. They hypothesized that persistent asthma would be associated with higher carotid plaque presence and burden.
They also wanted to explore “whether these associations would be attenuated after adjustment for baseline inflammatory biomarkers.”
Dr. Tattersall said the current study “links our previous work studying the manifestations of asthma,” in which he and his colleagues demonstrated increased cardiovascular events among MESA participants with persistent asthma, as well as late-onset asthma participants in the Wisconsin Sleep Cohort. His group also showed that early arterial injury occurs in adolescents with asthma.
However, there are also few data looking at the association with carotid plaque, “a late manifestation of arterial injury and a strong predictor of future cardiovascular events and asthma,” Dr. Tattersall added.
He and his group therefore “wanted to explore the entire spectrum of arterial injury, from the initial increase in the carotid media thickness to plaque formation to cardiovascular events.”
To do so, they studied participants in MESA, a study of close to 7,000 adults that began in the year 2000 and continues to follow participants today. At the time of enrollment, all were free from CVD.
The current analysis looked at 5,029 MESA participants (mean age 61.6 years, 53% female, 26% Black, 23% Hispanic, 12% Asian), comparing those with persistent asthma, defined as “asthma requiring use of controller medications,” intermittent asthma, defined as “asthma without controller medications,” and no asthma.
Participants underwent B-mode carotid ultrasound to detect carotid plaques, with a total plaque score (TPS) ranging from 0-12. The researchers used multivariable regression modeling to evaluate the association of asthma subtype and carotid plaque burden.
Interpret cautiously
Participants with persistent asthma were more likely to be female, have higher body mass index (BMI), and higher high-density lipoprotein (HDL) cholesterol levels, compared with those without asthma.
Participants with persistent asthma had the highest burden of carotid plaque (P ≤ .003 for comparison of proportions and .002 for comparison of means).
Moreover, participants with persistent asthma also had the highest systemic inflammatory marker levels – both CRP and IL-6 – compared with those without asthma. While participants with intermittent asthma also had higher average CRP, compared with those without asthma, their IL-6 levels were comparable.
In unadjusted models, persistent asthma was associated with higher odds of carotid plaque presence (odds ratio, 1.97; 95% confidence interval, 1.32-2.95) – an association that persisted even in models that adjusted for biologic confounders (both P < .01). There also was an association between persistent asthma and higher carotid TPS (P < .001).
In further adjusted models, IL-6 was independently associated with presence of carotid plaque (P = .0001 per 1-SD increment of 1.53), as well as TPS (P < .001). CRP was “slightly associated” with carotid TPS (P = .04) but not carotid plaque presence (P = .07).
There was no attenuation after the researchers evaluated the associations of asthma subtype and carotid plaque presence or TPS and fully adjusted for baseline IL-6 or CRP (P = .02 and P = .01, respectively).
“Since this study is observational, we cannot confirm causation, but the study adds to the growing literature exploring the systemic effects of asthma,” Dr. Tattersall commented.
“Our initial hypothesis was that it was driven by inflammation, as both asthma and CVD are inflammatory conditions,” he continued. “We did adjust for inflammatory biomarkers in this analysis, but there was no change in the association.”
Nevertheless, Dr. Tattersall and colleagues are “cautious in the interpretation,” since the inflammatory biomarkers “were only collected at one point, and these measures can be dynamic, thus adjustment may not tell the whole story.”
Heightened awareness
Robert Brook, MD, professor and director of cardiovascular disease prevention, Wayne State University, Detroit, said the “main contribution of this study is the novel demonstration of a significant association between persistent (but not intermittent) asthma with carotid atherosclerosis in the MESA cohort, a large multi-ethnic population.”
These findings “support the biological plausibility of the growing epidemiological evidence that asthma independently increases the risk for cardiovascular morbidity and mortality,” added Dr. Brook, who was not involved with the study.
“The main take-home message for clinicians is that, just like in COPD (which is well-established), asthma is often a systemic condition in that the inflammation and disease process can impact the whole body,” he said.
“Health care providers should have a heightened awareness of the potentially increased cardiovascular risk of their patients with asthma and pay special attention to controlling their heart disease risk factors (for example, hyperlipidemia, hypertension),” Dr. Brook stated.
Dr. Tattersall was supported by an American Heart Association Career Development Award. The Multi-Ethnic Study of Atherosclerosis was supported by the National Heart, Lung, and Blood Institute and the National Center for Research Resources. Dr. Tattersall and co-authors and Dr. Brook declare no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Persistent asthma is associated with increased carotid plaque burden and higher levels of inflammation, putting these patients at risk for atherosclerotic cardiovascular disease (ASCVD) events, new research suggests.
Using data from the MESA study, investigators analyzed more than 5,000 individuals, comparing carotid plaque and inflammatory markers in those with and without asthma.
They found that carotid plaque was present in half of participants without asthma and half of those with intermittent asthma but in close to 70% of participants with persistent asthma.
.
“The take-home message is that the current study, paired with prior studies, highlights that individuals with more significant forms of asthma may be at higher cardiovascular risk and makes it imperative to address modifiable risk factors among patients with asthma,” lead author Matthew Tattersall, DO, MS, assistant professor of cardiovascular medicine, University of Wisconsin School of Medicine and Public Health, Madison, told this news organization.
The study was published online in the Journal of the American Heart Association.
Limited data
Asthma and ASCVD are “highly prevalent inflammatory diseases,” the authors write. Carotid artery plaque detected by B-mode ultrasound “represents advanced, typically subclinical atherosclerosis that is a strong independent predictor of incident ASCVD events,” with inflammation playing a “key role” in precipitating these events, they note.
Serum inflammatory markers such as C-reactive protein (CRP) and IL-6 are associated with increased ASCVD events, and in asthma, CRP and other inflammatory biomarkers are elevated and tend to further increase during exacerbations.
Currently, there are limited data looking at the associations of asthma, asthma severity, and atherosclerotic plaque burden, they note, so the researchers turned to the MESA study – a multiethnic population of individuals free of prevalent ASCVD at baseline. They hypothesized that persistent asthma would be associated with higher carotid plaque presence and burden.
They also wanted to explore “whether these associations would be attenuated after adjustment for baseline inflammatory biomarkers.”
Dr. Tattersall said the current study “links our previous work studying the manifestations of asthma,” in which he and his colleagues demonstrated increased cardiovascular events among MESA participants with persistent asthma, as well as late-onset asthma participants in the Wisconsin Sleep Cohort. His group also showed that early arterial injury occurs in adolescents with asthma.
However, there are also few data looking at the association with carotid plaque, “a late manifestation of arterial injury and a strong predictor of future cardiovascular events and asthma,” Dr. Tattersall added.
He and his group therefore “wanted to explore the entire spectrum of arterial injury, from the initial increase in the carotid media thickness to plaque formation to cardiovascular events.”
To do so, they studied participants in MESA, a study of close to 7,000 adults that began in the year 2000 and continues to follow participants today. At the time of enrollment, all were free from CVD.
The current analysis looked at 5,029 MESA participants (mean age 61.6 years, 53% female, 26% Black, 23% Hispanic, 12% Asian), comparing those with persistent asthma, defined as “asthma requiring use of controller medications,” intermittent asthma, defined as “asthma without controller medications,” and no asthma.
Participants underwent B-mode carotid ultrasound to detect carotid plaques, with a total plaque score (TPS) ranging from 0-12. The researchers used multivariable regression modeling to evaluate the association of asthma subtype and carotid plaque burden.
Interpret cautiously
Participants with persistent asthma were more likely to be female, have higher body mass index (BMI), and higher high-density lipoprotein (HDL) cholesterol levels, compared with those without asthma.
Participants with persistent asthma had the highest burden of carotid plaque (P ≤ .003 for comparison of proportions and .002 for comparison of means).
Moreover, participants with persistent asthma also had the highest systemic inflammatory marker levels – both CRP and IL-6 – compared with those without asthma. While participants with intermittent asthma also had higher average CRP, compared with those without asthma, their IL-6 levels were comparable.
In unadjusted models, persistent asthma was associated with higher odds of carotid plaque presence (odds ratio, 1.97; 95% confidence interval, 1.32-2.95) – an association that persisted even in models that adjusted for biologic confounders (both P < .01). There also was an association between persistent asthma and higher carotid TPS (P < .001).
In further adjusted models, IL-6 was independently associated with presence of carotid plaque (P = .0001 per 1-SD increment of 1.53), as well as TPS (P < .001). CRP was “slightly associated” with carotid TPS (P = .04) but not carotid plaque presence (P = .07).
There was no attenuation after the researchers evaluated the associations of asthma subtype and carotid plaque presence or TPS and fully adjusted for baseline IL-6 or CRP (P = .02 and P = .01, respectively).
“Since this study is observational, we cannot confirm causation, but the study adds to the growing literature exploring the systemic effects of asthma,” Dr. Tattersall commented.
“Our initial hypothesis was that it was driven by inflammation, as both asthma and CVD are inflammatory conditions,” he continued. “We did adjust for inflammatory biomarkers in this analysis, but there was no change in the association.”
Nevertheless, Dr. Tattersall and colleagues are “cautious in the interpretation,” since the inflammatory biomarkers “were only collected at one point, and these measures can be dynamic, thus adjustment may not tell the whole story.”
Heightened awareness
Robert Brook, MD, professor and director of cardiovascular disease prevention, Wayne State University, Detroit, said the “main contribution of this study is the novel demonstration of a significant association between persistent (but not intermittent) asthma with carotid atherosclerosis in the MESA cohort, a large multi-ethnic population.”
These findings “support the biological plausibility of the growing epidemiological evidence that asthma independently increases the risk for cardiovascular morbidity and mortality,” added Dr. Brook, who was not involved with the study.
“The main take-home message for clinicians is that, just like in COPD (which is well-established), asthma is often a systemic condition in that the inflammation and disease process can impact the whole body,” he said.
“Health care providers should have a heightened awareness of the potentially increased cardiovascular risk of their patients with asthma and pay special attention to controlling their heart disease risk factors (for example, hyperlipidemia, hypertension),” Dr. Brook stated.
Dr. Tattersall was supported by an American Heart Association Career Development Award. The Multi-Ethnic Study of Atherosclerosis was supported by the National Heart, Lung, and Blood Institute and the National Center for Research Resources. Dr. Tattersall and co-authors and Dr. Brook declare no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Just 8 minutes of exercise a day is all you need
according to a new study in the European Heart Journal.
Just 54 minutes of vigorous exercise per week provides the most bang for your buck, researchers found, lowering the risk of early death from any cause by 36%, and your chances of getting heart disease by 35%.
Scientists examined data from fitness trackers worn by more than 71,000 people studied in the United Kingdom, then analyzed their health over the next several years.
While more time spent exercising unsurprisingly led to better health, the protective effects of exercise start to plateau after a certain point, according to the study.
A tough, short workout improves blood pressure, shrinks artery-clogging plaques, and boosts your overall fitness.
Vigorous exercise helps your body adapt better than moderate exercise does, leading to more notable benefits, says study author Matthew Ahmadi, PhD, a postdoctoral research fellow at the University of Sydney.
“Collectively, these will lower a person’s risk of cardiovascular disease. Exercise can also lower body inflammation, which will in turn lower the risk for certain cancers,” he says.
The CDC recommends at least 150 minutes of “moderate intensity” exercise each week, such as walking at a brisk pace. Or you could spend 75 minutes each week doing vigorous exercise, like running, it says. The CDC also recommends muscle strengthening activities, like lifting weights, at least 2 days per week.
But only 54% of Americans actually manage to get their 150 minutes of aerobic activity in each week, according to the most recent data from the National Center for Health Statistics. Even fewer – just 24% – also squeeze in the two recommended strength workouts.
So 8 minutes a day instead of 30 minutes could persuade busy people to get the exercise they need.
“Lack of time is one of the main reasons people have reported for not engaging in exercise,” says Dr. Ahmadi.
Vigorous exercise doesn’t mean you have to run, bike, or lift weights. Scientists consider a physical activity “vigorous” if it’s greater than 6 times your resting metabolic rate, or MET. That includes all kinds of strenuous movement, including dancing in a nightclub or carrying groceries upstairs.
“All of these activities are equally beneficial,” says Dr. Ahmadi.
He recommends aiming for 2-minute bouts of a heart-pumping activity, spread throughout the day for the most benefit in the least amount of time. If you wear a smartwatch or other device that tracks your heart rate, you’ll be above the threshold if your heart is pumping at 77% or more of your max heart rate (which most fitness trackers help you calculate).
No smartwatch? “The easiest way a person can infer if they are doing vigorous activity is if they are breathing hard enough that it’s difficult to have a conversation or speak in a full sentence while doing the activity,” Dr. Ahmadi says. In other words, if you’re huffing and puffing, then you’re in the zone.
A version of this article first appeared on WebMD.com.
according to a new study in the European Heart Journal.
Just 54 minutes of vigorous exercise per week provides the most bang for your buck, researchers found, lowering the risk of early death from any cause by 36%, and your chances of getting heart disease by 35%.
Scientists examined data from fitness trackers worn by more than 71,000 people studied in the United Kingdom, then analyzed their health over the next several years.
While more time spent exercising unsurprisingly led to better health, the protective effects of exercise start to plateau after a certain point, according to the study.
A tough, short workout improves blood pressure, shrinks artery-clogging plaques, and boosts your overall fitness.
Vigorous exercise helps your body adapt better than moderate exercise does, leading to more notable benefits, says study author Matthew Ahmadi, PhD, a postdoctoral research fellow at the University of Sydney.
“Collectively, these will lower a person’s risk of cardiovascular disease. Exercise can also lower body inflammation, which will in turn lower the risk for certain cancers,” he says.
The CDC recommends at least 150 minutes of “moderate intensity” exercise each week, such as walking at a brisk pace. Or you could spend 75 minutes each week doing vigorous exercise, like running, it says. The CDC also recommends muscle strengthening activities, like lifting weights, at least 2 days per week.
But only 54% of Americans actually manage to get their 150 minutes of aerobic activity in each week, according to the most recent data from the National Center for Health Statistics. Even fewer – just 24% – also squeeze in the two recommended strength workouts.
So 8 minutes a day instead of 30 minutes could persuade busy people to get the exercise they need.
“Lack of time is one of the main reasons people have reported for not engaging in exercise,” says Dr. Ahmadi.
Vigorous exercise doesn’t mean you have to run, bike, or lift weights. Scientists consider a physical activity “vigorous” if it’s greater than 6 times your resting metabolic rate, or MET. That includes all kinds of strenuous movement, including dancing in a nightclub or carrying groceries upstairs.
“All of these activities are equally beneficial,” says Dr. Ahmadi.
He recommends aiming for 2-minute bouts of a heart-pumping activity, spread throughout the day for the most benefit in the least amount of time. If you wear a smartwatch or other device that tracks your heart rate, you’ll be above the threshold if your heart is pumping at 77% or more of your max heart rate (which most fitness trackers help you calculate).
No smartwatch? “The easiest way a person can infer if they are doing vigorous activity is if they are breathing hard enough that it’s difficult to have a conversation or speak in a full sentence while doing the activity,” Dr. Ahmadi says. In other words, if you’re huffing and puffing, then you’re in the zone.
A version of this article first appeared on WebMD.com.
according to a new study in the European Heart Journal.
Just 54 minutes of vigorous exercise per week provides the most bang for your buck, researchers found, lowering the risk of early death from any cause by 36%, and your chances of getting heart disease by 35%.
Scientists examined data from fitness trackers worn by more than 71,000 people studied in the United Kingdom, then analyzed their health over the next several years.
While more time spent exercising unsurprisingly led to better health, the protective effects of exercise start to plateau after a certain point, according to the study.
A tough, short workout improves blood pressure, shrinks artery-clogging plaques, and boosts your overall fitness.
Vigorous exercise helps your body adapt better than moderate exercise does, leading to more notable benefits, says study author Matthew Ahmadi, PhD, a postdoctoral research fellow at the University of Sydney.
“Collectively, these will lower a person’s risk of cardiovascular disease. Exercise can also lower body inflammation, which will in turn lower the risk for certain cancers,” he says.
The CDC recommends at least 150 minutes of “moderate intensity” exercise each week, such as walking at a brisk pace. Or you could spend 75 minutes each week doing vigorous exercise, like running, it says. The CDC also recommends muscle strengthening activities, like lifting weights, at least 2 days per week.
But only 54% of Americans actually manage to get their 150 minutes of aerobic activity in each week, according to the most recent data from the National Center for Health Statistics. Even fewer – just 24% – also squeeze in the two recommended strength workouts.
So 8 minutes a day instead of 30 minutes could persuade busy people to get the exercise they need.
“Lack of time is one of the main reasons people have reported for not engaging in exercise,” says Dr. Ahmadi.
Vigorous exercise doesn’t mean you have to run, bike, or lift weights. Scientists consider a physical activity “vigorous” if it’s greater than 6 times your resting metabolic rate, or MET. That includes all kinds of strenuous movement, including dancing in a nightclub or carrying groceries upstairs.
“All of these activities are equally beneficial,” says Dr. Ahmadi.
He recommends aiming for 2-minute bouts of a heart-pumping activity, spread throughout the day for the most benefit in the least amount of time. If you wear a smartwatch or other device that tracks your heart rate, you’ll be above the threshold if your heart is pumping at 77% or more of your max heart rate (which most fitness trackers help you calculate).
No smartwatch? “The easiest way a person can infer if they are doing vigorous activity is if they are breathing hard enough that it’s difficult to have a conversation or speak in a full sentence while doing the activity,” Dr. Ahmadi says. In other words, if you’re huffing and puffing, then you’re in the zone.
A version of this article first appeared on WebMD.com.
FROM EUROPEAN HEART JOURNAL