User login
Vitamin D fails to stave off statin-related muscle symptoms
Vitamin D supplements do not prevent muscle symptoms in new statin users or affect the likelihood of discontinuing a statin due to muscle pain and discomfort, a substudy of the VITAL trial indicates.
Among more than 2,000 randomized participants, statin-associated muscle symptoms (SAMS) were reported by 31% assigned to vitamin D and 31% assigned to placebo.
The two groups were equally likely to stop taking a statin due to muscle symptoms, at 13%.
No significant difference was observed in SAMS (odds ratio [OR], 0.97; 95% confidence interval [CI], 0.80-1.18) or statin discontinuations (OR, 1.04; 95% CI, 0.80-1.35) after adjustment for baseline variables and other characteristics, namely age, sex, and African-American race, previously found to be associated with SAMS in VITAL.
“We actually thought when we started out that maybe we were going to show something, that maybe it was going to be that the people who got the vitamin D were least likely to have a problem with a statin than all those who didn’t get vitamin D, but that is not what we showed,” senior author Neil J. Stone, MD, Northwestern University, Chicago, told this news organization.
He noted that patients in the clinic with low levels of vitamin D often have muscle pain and discomfort and that previous unblinded studies suggested vitamin D might benefit patients with SAMS and reduce statin intolerance.
As previously reported, the double-blind VITAL trial showed no difference in the primary prevention of cardiovascular disease or cancer at 5 years among 25,871 middle-aged adults randomized to vitamin D3 at 2000 IU/d or placebo, regardless of their baseline vitamin D level.
Unlike previous studies showing a benefit with vitamin D on SAMS, importantly, VITAL participants were unaware of whether they were taking vitamin D or placebo and were not expecting any help with their muscle symptoms, first author Mark A. Hlatky, MD, Stanford (Calif.) University, pointed out in an interview.
As to how many statin users turn to the popular supplement for SAMS, he said that number couldn’t be pinned down, despite a lengthy search. “But I think it’s very common, because up to half of people stop taking their statins within a year and many of these do so because of statin-associated muscle symptoms, and we found it in about 30% of people who have them. I have them myself and was motivated to study it because I thought this was an interesting question.”
The results were published online in JAMA Cardiology.
SAMS by baseline 25-OHD
The substudy included 2,083 patients who initiated statin therapy after randomization and were surveyed in early 2016 about their statin use and muscle symptoms.
Two-thirds, or 1,397 patients, had 25-hydroxy vitamin D (25-OHD) measured at baseline, with 47% having levels < 30 ng/mL and 13% levels < 20 ng/mL.
Serum 25-OHD levels were virtually identical in the two treatment groups (mean, 30.4 ng/mL; median, 30.0 ng/mL). The frequency of SAMS did not differ between those assigned to vitamin D or placebo (28% vs. 31%).
The odds ratios for the association with vitamin D on SAMS were:
- 0.86 in all respondents with 25-OHD measured (95% CI, 0.69-1.09).
- 0.87 in those with levels ≥ 30 ng/mL (95% CI, 0.64-1.19).
- 0.85 with levels of 20-30 ng/mL (95% CI, 0.56-1.28).
- 0.93 with levels < 20 ng/mL (95% CI, 0.50-1.74).
The test for treatment effect modification by baseline serum 25-OHD level was not significant (P for interaction = .83).
In addition, the rate of muscle symptoms was similar between participants randomized to vitamin D and placebo when researchers used a cutpoint to define low 25-OHD of < 30 ng/mL (27% vs. 30%) or < 20 ng/mL (33% vs. 35%).
“We didn’t find any evidence at all that the people who came into the study with low levels of vitamin D did better with the supplement in this case,” Dr. Hlatky said. “So that wasn’t the reason we didn’t see anything.”
Critics may suggest the trial didn’t use a high enough dose of vitamin D, but both Dr. Hlatky and Dr. Stone say that’s unlikely to be a factor in the results because 2,000 IU/d is a substantial dose and well above the recommended adult daily dose of 600-800 IU.
They caution that the substudy wasn’t prespecified, was smaller than the parent trial, and did not have a protocol in place to detail SAMS. They also can’t rule out the possibility that vitamin D may have an effect in patients who have confirmed intolerance to multiple statins, especially after adjustment for the statin type and dose.
“If you’re taking vitamin D to keep from having statin-associated muscle symptoms, this very carefully done substudy with the various caveats doesn’t support that and that’s not something I would give my patients,” Dr. Stone said.
“The most important thing from a negative study is that it allows you to focus your attention on things that may be much more productive rather than assuming that just giving everybody vitamin D will take care of the statin issue,” he added. “Maybe the answer is going to be somewhere else, and there’ll be a lot of people I’m sure who will offer their advice as what the answer is but, I would argue, we want to see more studies to pin it down. So people can get some science behind what they do to try to reduce statin-associated muscle symptoms.”
Paul D. Thompson, MD, chief of cardiology emeritus at Hartford (Conn.) Hospital, and a SAMS expert who was not involved with the research, said, “This is a useful publication, and it’s smart in that it took advantage of a study that was already done.”
He acknowledged being skeptical of a beneficial effect of vitamin D supplementation on SAMS, because some previous data have been retracted, but said that potential treatments are best tested in patients with confirmed statin myalgia, as was the case in his team’s negative trial of CoQ10 supplementation.
That said, the present “study was able to at least give some of the best evidence so far that vitamin D doesn’t do anything to improve symptoms,” Dr. Thompson said. “So maybe it will cut down on so many vitamin D levels [being measured] and use of vitamin D when you don’t really need it.”
The study was sponsored by the Hyperlipidemia Research Fund at Northwestern University. The VITAL trial was supported by grants from the National Institutes of Health, and Quest Diagnostics performed the laboratory measurements at no additional costs. Dr. Hlatky reports no relevant financial relationships. Dr. Stone reports a grant from the Hyperlipidemia Research Fund at Northwestern and honorarium for educational activity for Knowledge to Practice. Dr. Thompson is on the executive committee for a study examining bempedoic acid in patients with statin-associated muscle symptoms.
A version of this article first appeared on Medscape.com.
Vitamin D supplements do not prevent muscle symptoms in new statin users or affect the likelihood of discontinuing a statin due to muscle pain and discomfort, a substudy of the VITAL trial indicates.
Among more than 2,000 randomized participants, statin-associated muscle symptoms (SAMS) were reported by 31% assigned to vitamin D and 31% assigned to placebo.
The two groups were equally likely to stop taking a statin due to muscle symptoms, at 13%.
No significant difference was observed in SAMS (odds ratio [OR], 0.97; 95% confidence interval [CI], 0.80-1.18) or statin discontinuations (OR, 1.04; 95% CI, 0.80-1.35) after adjustment for baseline variables and other characteristics, namely age, sex, and African-American race, previously found to be associated with SAMS in VITAL.
“We actually thought when we started out that maybe we were going to show something, that maybe it was going to be that the people who got the vitamin D were least likely to have a problem with a statin than all those who didn’t get vitamin D, but that is not what we showed,” senior author Neil J. Stone, MD, Northwestern University, Chicago, told this news organization.
He noted that patients in the clinic with low levels of vitamin D often have muscle pain and discomfort and that previous unblinded studies suggested vitamin D might benefit patients with SAMS and reduce statin intolerance.
As previously reported, the double-blind VITAL trial showed no difference in the primary prevention of cardiovascular disease or cancer at 5 years among 25,871 middle-aged adults randomized to vitamin D3 at 2000 IU/d or placebo, regardless of their baseline vitamin D level.
Unlike previous studies showing a benefit with vitamin D on SAMS, importantly, VITAL participants were unaware of whether they were taking vitamin D or placebo and were not expecting any help with their muscle symptoms, first author Mark A. Hlatky, MD, Stanford (Calif.) University, pointed out in an interview.
As to how many statin users turn to the popular supplement for SAMS, he said that number couldn’t be pinned down, despite a lengthy search. “But I think it’s very common, because up to half of people stop taking their statins within a year and many of these do so because of statin-associated muscle symptoms, and we found it in about 30% of people who have them. I have them myself and was motivated to study it because I thought this was an interesting question.”
The results were published online in JAMA Cardiology.
SAMS by baseline 25-OHD
The substudy included 2,083 patients who initiated statin therapy after randomization and were surveyed in early 2016 about their statin use and muscle symptoms.
Two-thirds, or 1,397 patients, had 25-hydroxy vitamin D (25-OHD) measured at baseline, with 47% having levels < 30 ng/mL and 13% levels < 20 ng/mL.
Serum 25-OHD levels were virtually identical in the two treatment groups (mean, 30.4 ng/mL; median, 30.0 ng/mL). The frequency of SAMS did not differ between those assigned to vitamin D or placebo (28% vs. 31%).
The odds ratios for the association with vitamin D on SAMS were:
- 0.86 in all respondents with 25-OHD measured (95% CI, 0.69-1.09).
- 0.87 in those with levels ≥ 30 ng/mL (95% CI, 0.64-1.19).
- 0.85 with levels of 20-30 ng/mL (95% CI, 0.56-1.28).
- 0.93 with levels < 20 ng/mL (95% CI, 0.50-1.74).
The test for treatment effect modification by baseline serum 25-OHD level was not significant (P for interaction = .83).
In addition, the rate of muscle symptoms was similar between participants randomized to vitamin D and placebo when researchers used a cutpoint to define low 25-OHD of < 30 ng/mL (27% vs. 30%) or < 20 ng/mL (33% vs. 35%).
“We didn’t find any evidence at all that the people who came into the study with low levels of vitamin D did better with the supplement in this case,” Dr. Hlatky said. “So that wasn’t the reason we didn’t see anything.”
Critics may suggest the trial didn’t use a high enough dose of vitamin D, but both Dr. Hlatky and Dr. Stone say that’s unlikely to be a factor in the results because 2,000 IU/d is a substantial dose and well above the recommended adult daily dose of 600-800 IU.
They caution that the substudy wasn’t prespecified, was smaller than the parent trial, and did not have a protocol in place to detail SAMS. They also can’t rule out the possibility that vitamin D may have an effect in patients who have confirmed intolerance to multiple statins, especially after adjustment for the statin type and dose.
“If you’re taking vitamin D to keep from having statin-associated muscle symptoms, this very carefully done substudy with the various caveats doesn’t support that and that’s not something I would give my patients,” Dr. Stone said.
“The most important thing from a negative study is that it allows you to focus your attention on things that may be much more productive rather than assuming that just giving everybody vitamin D will take care of the statin issue,” he added. “Maybe the answer is going to be somewhere else, and there’ll be a lot of people I’m sure who will offer their advice as what the answer is but, I would argue, we want to see more studies to pin it down. So people can get some science behind what they do to try to reduce statin-associated muscle symptoms.”
Paul D. Thompson, MD, chief of cardiology emeritus at Hartford (Conn.) Hospital, and a SAMS expert who was not involved with the research, said, “This is a useful publication, and it’s smart in that it took advantage of a study that was already done.”
He acknowledged being skeptical of a beneficial effect of vitamin D supplementation on SAMS, because some previous data have been retracted, but said that potential treatments are best tested in patients with confirmed statin myalgia, as was the case in his team’s negative trial of CoQ10 supplementation.
That said, the present “study was able to at least give some of the best evidence so far that vitamin D doesn’t do anything to improve symptoms,” Dr. Thompson said. “So maybe it will cut down on so many vitamin D levels [being measured] and use of vitamin D when you don’t really need it.”
The study was sponsored by the Hyperlipidemia Research Fund at Northwestern University. The VITAL trial was supported by grants from the National Institutes of Health, and Quest Diagnostics performed the laboratory measurements at no additional costs. Dr. Hlatky reports no relevant financial relationships. Dr. Stone reports a grant from the Hyperlipidemia Research Fund at Northwestern and honorarium for educational activity for Knowledge to Practice. Dr. Thompson is on the executive committee for a study examining bempedoic acid in patients with statin-associated muscle symptoms.
A version of this article first appeared on Medscape.com.
Vitamin D supplements do not prevent muscle symptoms in new statin users or affect the likelihood of discontinuing a statin due to muscle pain and discomfort, a substudy of the VITAL trial indicates.
Among more than 2,000 randomized participants, statin-associated muscle symptoms (SAMS) were reported by 31% assigned to vitamin D and 31% assigned to placebo.
The two groups were equally likely to stop taking a statin due to muscle symptoms, at 13%.
No significant difference was observed in SAMS (odds ratio [OR], 0.97; 95% confidence interval [CI], 0.80-1.18) or statin discontinuations (OR, 1.04; 95% CI, 0.80-1.35) after adjustment for baseline variables and other characteristics, namely age, sex, and African-American race, previously found to be associated with SAMS in VITAL.
“We actually thought when we started out that maybe we were going to show something, that maybe it was going to be that the people who got the vitamin D were least likely to have a problem with a statin than all those who didn’t get vitamin D, but that is not what we showed,” senior author Neil J. Stone, MD, Northwestern University, Chicago, told this news organization.
He noted that patients in the clinic with low levels of vitamin D often have muscle pain and discomfort and that previous unblinded studies suggested vitamin D might benefit patients with SAMS and reduce statin intolerance.
As previously reported, the double-blind VITAL trial showed no difference in the primary prevention of cardiovascular disease or cancer at 5 years among 25,871 middle-aged adults randomized to vitamin D3 at 2000 IU/d or placebo, regardless of their baseline vitamin D level.
Unlike previous studies showing a benefit with vitamin D on SAMS, importantly, VITAL participants were unaware of whether they were taking vitamin D or placebo and were not expecting any help with their muscle symptoms, first author Mark A. Hlatky, MD, Stanford (Calif.) University, pointed out in an interview.
As to how many statin users turn to the popular supplement for SAMS, he said that number couldn’t be pinned down, despite a lengthy search. “But I think it’s very common, because up to half of people stop taking their statins within a year and many of these do so because of statin-associated muscle symptoms, and we found it in about 30% of people who have them. I have them myself and was motivated to study it because I thought this was an interesting question.”
The results were published online in JAMA Cardiology.
SAMS by baseline 25-OHD
The substudy included 2,083 patients who initiated statin therapy after randomization and were surveyed in early 2016 about their statin use and muscle symptoms.
Two-thirds, or 1,397 patients, had 25-hydroxy vitamin D (25-OHD) measured at baseline, with 47% having levels < 30 ng/mL and 13% levels < 20 ng/mL.
Serum 25-OHD levels were virtually identical in the two treatment groups (mean, 30.4 ng/mL; median, 30.0 ng/mL). The frequency of SAMS did not differ between those assigned to vitamin D or placebo (28% vs. 31%).
The odds ratios for the association with vitamin D on SAMS were:
- 0.86 in all respondents with 25-OHD measured (95% CI, 0.69-1.09).
- 0.87 in those with levels ≥ 30 ng/mL (95% CI, 0.64-1.19).
- 0.85 with levels of 20-30 ng/mL (95% CI, 0.56-1.28).
- 0.93 with levels < 20 ng/mL (95% CI, 0.50-1.74).
The test for treatment effect modification by baseline serum 25-OHD level was not significant (P for interaction = .83).
In addition, the rate of muscle symptoms was similar between participants randomized to vitamin D and placebo when researchers used a cutpoint to define low 25-OHD of < 30 ng/mL (27% vs. 30%) or < 20 ng/mL (33% vs. 35%).
“We didn’t find any evidence at all that the people who came into the study with low levels of vitamin D did better with the supplement in this case,” Dr. Hlatky said. “So that wasn’t the reason we didn’t see anything.”
Critics may suggest the trial didn’t use a high enough dose of vitamin D, but both Dr. Hlatky and Dr. Stone say that’s unlikely to be a factor in the results because 2,000 IU/d is a substantial dose and well above the recommended adult daily dose of 600-800 IU.
They caution that the substudy wasn’t prespecified, was smaller than the parent trial, and did not have a protocol in place to detail SAMS. They also can’t rule out the possibility that vitamin D may have an effect in patients who have confirmed intolerance to multiple statins, especially after adjustment for the statin type and dose.
“If you’re taking vitamin D to keep from having statin-associated muscle symptoms, this very carefully done substudy with the various caveats doesn’t support that and that’s not something I would give my patients,” Dr. Stone said.
“The most important thing from a negative study is that it allows you to focus your attention on things that may be much more productive rather than assuming that just giving everybody vitamin D will take care of the statin issue,” he added. “Maybe the answer is going to be somewhere else, and there’ll be a lot of people I’m sure who will offer their advice as what the answer is but, I would argue, we want to see more studies to pin it down. So people can get some science behind what they do to try to reduce statin-associated muscle symptoms.”
Paul D. Thompson, MD, chief of cardiology emeritus at Hartford (Conn.) Hospital, and a SAMS expert who was not involved with the research, said, “This is a useful publication, and it’s smart in that it took advantage of a study that was already done.”
He acknowledged being skeptical of a beneficial effect of vitamin D supplementation on SAMS, because some previous data have been retracted, but said that potential treatments are best tested in patients with confirmed statin myalgia, as was the case in his team’s negative trial of CoQ10 supplementation.
That said, the present “study was able to at least give some of the best evidence so far that vitamin D doesn’t do anything to improve symptoms,” Dr. Thompson said. “So maybe it will cut down on so many vitamin D levels [being measured] and use of vitamin D when you don’t really need it.”
The study was sponsored by the Hyperlipidemia Research Fund at Northwestern University. The VITAL trial was supported by grants from the National Institutes of Health, and Quest Diagnostics performed the laboratory measurements at no additional costs. Dr. Hlatky reports no relevant financial relationships. Dr. Stone reports a grant from the Hyperlipidemia Research Fund at Northwestern and honorarium for educational activity for Knowledge to Practice. Dr. Thompson is on the executive committee for a study examining bempedoic acid in patients with statin-associated muscle symptoms.
A version of this article first appeared on Medscape.com.
HDL cholesterol not linked to CHD risk in Blacks: REGARDS
High-density lipoprotein cholesterol may not be as effective a biomarker of cardiovascular disease risk as once thought, particularly in Black adults, according to results from a large biracial cohort study that also raised questions about the validity of high HDL cholesterol as a potentially protective factor in White and Black adults alike.
“I think this opens the door to suggest that every biomarker we use might have a race-specific association with disease outcome,” Nathalie Pamir, PhD, an associate professor at Oregon Health & Science University in Portland, said in an interview. “So, something as basic as HDL cholesterol – we’ve known about it since 1970 – has a race signature.”
Dr. Pamir and colleagues reported their findings from the REGARDS (Reasons for Geographic and Racial Differences in Stroke) cohort study that recruited 30,239 Black and White individuals aged 45 years and older from the contiguous United States from 2003 to 2007.
The study found that LDL cholesterol “modestly” predicted coronary heart disease (CHD) risk in Black and White adults. However, low HDL cholesterol, while associated with an increased risk in White patients (hazard ratio, 1.22; 95% confidence interval, 1.05-1.43), did not have a similar association in Blacks (HR, 0.94; 95% CI: 0.78-1.14). And high HDL cholesterol wasn’t found to be predictive in either group (HR, 0.96; 95% CI, 0.79-1.16 for White participants: HR, 0.91; 95% CI, 0.74-1.12 for Black participants).
Among 23,901 study participants who were CHD-risk free over a 10-year follow-up, 664 and 951 CHD events occurred in Black and White participants, respectively. The study cohort was 57.8% White and 58.4% women, with a mean age of 65 years.
The study noted that LDL cholesterol and triglycerides conferred similar risks for CHD in both White and Black participants.
Acknowledging that this study focused on Blacks, Dr. Pamir added that “we need to know about Asian Americans; we need to know about Hispanic Americans.”
Change of approach to lipid management called for
Dr. Pamir noted that the current understanding about HDL cholesterol and CHD risk comes from the Framingham heart study in the 1970s, whose population was 100% White.
Care algorithms derived from the Framingham study as well as the Multi-Ethnic Study of Atherosclerosis incorporate that association between HDL cholesterol and CHD risk, she noted, but these findings from REGARDS should change how cardiologists approach lipid management in Black and White patients.
“The conversation would go something like: High HDL cholesterol levels put you in a higher risk [bracket] but HDL cholesterol levels are not something we treat; we have no drugs for that,” Dr. Pamir said.
“The conversation would continue along the lines that: ‘You need to do more exercise, you need to change your diet, incorporate healthy fats, walnuts, and omega 3s.’
“But what might the conversation be for Black patients? ‘We don’t see the association that we see for White patients. Do adopt the good habits to exercise and dietary changes, but don’t get too worried about it.’ ”
The study report raises “caution” about using the Framingham, MESA, and other algorithms for evaluating CHD risk. Dr. Pamir explained what that means. “We might be underestimating risk, because what our study showed was that, when we looked at clinically high HDL cholesterol, about 60 mg/dL, it has no benefit for White and Black patients.”
She added, “So that pat on the back we get for patients that have high HDL-C levels? Maybe that pat on the back shouldn’t be there.”
In an invited commentary, Keith C. Ferdinand, MD, of Tulane University in New Orleans, wrote that using HDL cholesterol in risk calculations could inaccurately assess atherosclerotic cardiovascular risk in Black adults “and become a barrier to optimal care.”
In an interview, he said the REGARDS findings call for consideration of other biomarkers for evaluating CHD risk and point to the importance of socioeconomic factors in health outcomes.
“Physicians and other clinicians need to recognize the powerful impact of the social determinants of health and to also recognize the limits of HDL itself as either protective if it’s high or a definitive predictor of risk if it’s low, and focus on some more modern approaches, including coronary artery calcium scoring.”
He also said risk evaluation should include lipoprotein(a), which, he noted in the editorial, the European Atherosclerosis Society recommends measuring. “One of the reasons it’s underutilized is that we really don’t have a specific treatment for it,” he said of Lp(a) in the United States.
In his editorial comment, Dr. Ferdinand called for future research aimed at eliminating health disparities. “Regardless of the development of better tools for the assessment of risk, newer drugs to treat CVD, the use of coronary artery calcium, if we don’t apply evidence-based medicine equally across the population based on race, ethnicity, sex, gender, socioeconomic status, or geography, then the disparities are going to persist,” he said.
The National Institute of Neurological Disorders and Stroke and the National Institute on Aging provided funding for the study. Dr. Pamir has no relevant relationships to disclose. Dr. Ferdinand disclosed relationships with Boehringer Ingelheim, Novartis, Janssen, and Lilly.
High-density lipoprotein cholesterol may not be as effective a biomarker of cardiovascular disease risk as once thought, particularly in Black adults, according to results from a large biracial cohort study that also raised questions about the validity of high HDL cholesterol as a potentially protective factor in White and Black adults alike.
“I think this opens the door to suggest that every biomarker we use might have a race-specific association with disease outcome,” Nathalie Pamir, PhD, an associate professor at Oregon Health & Science University in Portland, said in an interview. “So, something as basic as HDL cholesterol – we’ve known about it since 1970 – has a race signature.”
Dr. Pamir and colleagues reported their findings from the REGARDS (Reasons for Geographic and Racial Differences in Stroke) cohort study that recruited 30,239 Black and White individuals aged 45 years and older from the contiguous United States from 2003 to 2007.
The study found that LDL cholesterol “modestly” predicted coronary heart disease (CHD) risk in Black and White adults. However, low HDL cholesterol, while associated with an increased risk in White patients (hazard ratio, 1.22; 95% confidence interval, 1.05-1.43), did not have a similar association in Blacks (HR, 0.94; 95% CI: 0.78-1.14). And high HDL cholesterol wasn’t found to be predictive in either group (HR, 0.96; 95% CI, 0.79-1.16 for White participants: HR, 0.91; 95% CI, 0.74-1.12 for Black participants).
Among 23,901 study participants who were CHD-risk free over a 10-year follow-up, 664 and 951 CHD events occurred in Black and White participants, respectively. The study cohort was 57.8% White and 58.4% women, with a mean age of 65 years.
The study noted that LDL cholesterol and triglycerides conferred similar risks for CHD in both White and Black participants.
Acknowledging that this study focused on Blacks, Dr. Pamir added that “we need to know about Asian Americans; we need to know about Hispanic Americans.”
Change of approach to lipid management called for
Dr. Pamir noted that the current understanding about HDL cholesterol and CHD risk comes from the Framingham heart study in the 1970s, whose population was 100% White.
Care algorithms derived from the Framingham study as well as the Multi-Ethnic Study of Atherosclerosis incorporate that association between HDL cholesterol and CHD risk, she noted, but these findings from REGARDS should change how cardiologists approach lipid management in Black and White patients.
“The conversation would go something like: High HDL cholesterol levels put you in a higher risk [bracket] but HDL cholesterol levels are not something we treat; we have no drugs for that,” Dr. Pamir said.
“The conversation would continue along the lines that: ‘You need to do more exercise, you need to change your diet, incorporate healthy fats, walnuts, and omega 3s.’
“But what might the conversation be for Black patients? ‘We don’t see the association that we see for White patients. Do adopt the good habits to exercise and dietary changes, but don’t get too worried about it.’ ”
The study report raises “caution” about using the Framingham, MESA, and other algorithms for evaluating CHD risk. Dr. Pamir explained what that means. “We might be underestimating risk, because what our study showed was that, when we looked at clinically high HDL cholesterol, about 60 mg/dL, it has no benefit for White and Black patients.”
She added, “So that pat on the back we get for patients that have high HDL-C levels? Maybe that pat on the back shouldn’t be there.”
In an invited commentary, Keith C. Ferdinand, MD, of Tulane University in New Orleans, wrote that using HDL cholesterol in risk calculations could inaccurately assess atherosclerotic cardiovascular risk in Black adults “and become a barrier to optimal care.”
In an interview, he said the REGARDS findings call for consideration of other biomarkers for evaluating CHD risk and point to the importance of socioeconomic factors in health outcomes.
“Physicians and other clinicians need to recognize the powerful impact of the social determinants of health and to also recognize the limits of HDL itself as either protective if it’s high or a definitive predictor of risk if it’s low, and focus on some more modern approaches, including coronary artery calcium scoring.”
He also said risk evaluation should include lipoprotein(a), which, he noted in the editorial, the European Atherosclerosis Society recommends measuring. “One of the reasons it’s underutilized is that we really don’t have a specific treatment for it,” he said of Lp(a) in the United States.
In his editorial comment, Dr. Ferdinand called for future research aimed at eliminating health disparities. “Regardless of the development of better tools for the assessment of risk, newer drugs to treat CVD, the use of coronary artery calcium, if we don’t apply evidence-based medicine equally across the population based on race, ethnicity, sex, gender, socioeconomic status, or geography, then the disparities are going to persist,” he said.
The National Institute of Neurological Disorders and Stroke and the National Institute on Aging provided funding for the study. Dr. Pamir has no relevant relationships to disclose. Dr. Ferdinand disclosed relationships with Boehringer Ingelheim, Novartis, Janssen, and Lilly.
High-density lipoprotein cholesterol may not be as effective a biomarker of cardiovascular disease risk as once thought, particularly in Black adults, according to results from a large biracial cohort study that also raised questions about the validity of high HDL cholesterol as a potentially protective factor in White and Black adults alike.
“I think this opens the door to suggest that every biomarker we use might have a race-specific association with disease outcome,” Nathalie Pamir, PhD, an associate professor at Oregon Health & Science University in Portland, said in an interview. “So, something as basic as HDL cholesterol – we’ve known about it since 1970 – has a race signature.”
Dr. Pamir and colleagues reported their findings from the REGARDS (Reasons for Geographic and Racial Differences in Stroke) cohort study that recruited 30,239 Black and White individuals aged 45 years and older from the contiguous United States from 2003 to 2007.
The study found that LDL cholesterol “modestly” predicted coronary heart disease (CHD) risk in Black and White adults. However, low HDL cholesterol, while associated with an increased risk in White patients (hazard ratio, 1.22; 95% confidence interval, 1.05-1.43), did not have a similar association in Blacks (HR, 0.94; 95% CI: 0.78-1.14). And high HDL cholesterol wasn’t found to be predictive in either group (HR, 0.96; 95% CI, 0.79-1.16 for White participants: HR, 0.91; 95% CI, 0.74-1.12 for Black participants).
Among 23,901 study participants who were CHD-risk free over a 10-year follow-up, 664 and 951 CHD events occurred in Black and White participants, respectively. The study cohort was 57.8% White and 58.4% women, with a mean age of 65 years.
The study noted that LDL cholesterol and triglycerides conferred similar risks for CHD in both White and Black participants.
Acknowledging that this study focused on Blacks, Dr. Pamir added that “we need to know about Asian Americans; we need to know about Hispanic Americans.”
Change of approach to lipid management called for
Dr. Pamir noted that the current understanding about HDL cholesterol and CHD risk comes from the Framingham heart study in the 1970s, whose population was 100% White.
Care algorithms derived from the Framingham study as well as the Multi-Ethnic Study of Atherosclerosis incorporate that association between HDL cholesterol and CHD risk, she noted, but these findings from REGARDS should change how cardiologists approach lipid management in Black and White patients.
“The conversation would go something like: High HDL cholesterol levels put you in a higher risk [bracket] but HDL cholesterol levels are not something we treat; we have no drugs for that,” Dr. Pamir said.
“The conversation would continue along the lines that: ‘You need to do more exercise, you need to change your diet, incorporate healthy fats, walnuts, and omega 3s.’
“But what might the conversation be for Black patients? ‘We don’t see the association that we see for White patients. Do adopt the good habits to exercise and dietary changes, but don’t get too worried about it.’ ”
The study report raises “caution” about using the Framingham, MESA, and other algorithms for evaluating CHD risk. Dr. Pamir explained what that means. “We might be underestimating risk, because what our study showed was that, when we looked at clinically high HDL cholesterol, about 60 mg/dL, it has no benefit for White and Black patients.”
She added, “So that pat on the back we get for patients that have high HDL-C levels? Maybe that pat on the back shouldn’t be there.”
In an invited commentary, Keith C. Ferdinand, MD, of Tulane University in New Orleans, wrote that using HDL cholesterol in risk calculations could inaccurately assess atherosclerotic cardiovascular risk in Black adults “and become a barrier to optimal care.”
In an interview, he said the REGARDS findings call for consideration of other biomarkers for evaluating CHD risk and point to the importance of socioeconomic factors in health outcomes.
“Physicians and other clinicians need to recognize the powerful impact of the social determinants of health and to also recognize the limits of HDL itself as either protective if it’s high or a definitive predictor of risk if it’s low, and focus on some more modern approaches, including coronary artery calcium scoring.”
He also said risk evaluation should include lipoprotein(a), which, he noted in the editorial, the European Atherosclerosis Society recommends measuring. “One of the reasons it’s underutilized is that we really don’t have a specific treatment for it,” he said of Lp(a) in the United States.
In his editorial comment, Dr. Ferdinand called for future research aimed at eliminating health disparities. “Regardless of the development of better tools for the assessment of risk, newer drugs to treat CVD, the use of coronary artery calcium, if we don’t apply evidence-based medicine equally across the population based on race, ethnicity, sex, gender, socioeconomic status, or geography, then the disparities are going to persist,” he said.
The National Institute of Neurological Disorders and Stroke and the National Institute on Aging provided funding for the study. Dr. Pamir has no relevant relationships to disclose. Dr. Ferdinand disclosed relationships with Boehringer Ingelheim, Novartis, Janssen, and Lilly.
FROM JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Mortality after acute stroke worsened by accompanying acute AFib
The study covered in this summary was published on ResearchSquare.com as a preprint and has not yet been peer-reviewed.
Key takeaway
Why this matters
- A comprehensive understanding of the relationship between acute AF and risk for acute ischemic stroke and prognosis will help improve management and treatment of patients with acute ischemic stroke.
Study design
- The retrospective study included patients with acute ischemic stroke within the prior 24 hours; 12-lead electrocardiogram in the emergency department; and hospitalization and treatment at the hospital stroke center.
- The cohort of 706 patients admitted to a single center in Shanghai, China, from December 2019 to December 2021, included 142 with episodes of acute AF and 564 without such episodes.
- Patients with acute ischemic stroke and acute AF – including AF of new onset, paroxysmal, persistent, or permanent with symptoms such as palpitations or dizziness attributed to rapid ventricular rates – were identified.
- Neurological deficits were assessed using the 7-day National Institutes of Health Stroke Scale/Score (NIHSS). Patients with a 7-day NIHSS score of at least 16 were considered to have moderate to severe stroke.
- Associations between acute AF onset and the severity of early neurological deficits were assessed and related to all-cause mortality within 30 days of the stroke.
Key results
- Patients with acute AF were older than those without acute AF (80.3 years vs. 71.0 years; P < .001).
- Baseline NIHSS scores averaged 16.09 for the stroke patients with acute AF and 8.65 for those without acute AF (P < .001).
- Significantly more patients with acute AF than without acute AF had a 7-day NIHSS score of at least 16 (45.1% vs. 14.4%; P < .001).
- More patients with than without acute AF underwent transcatheter thrombectomy (44.4% vs. 24.5%; P < .001) or received thrombolytic therapy (31.6% vs. 19.7%; P = .005).
- Patients aged 73 years or older showed baseline NIHSS score and acute AF as independent risk factors for early neurological deficits in stroke patients admitted to the emergency department.
- Mortality at 30 days was significantly higher in patients with acute AF than in those without acute AF (30.3% vs. 10.1%; P < .001).
- Baseline NIHSS had an adjusted odds ratio for 30-day mortality of 1.18 (95% confidence interval, 1.15-1.22; P < .001).
- Other independent predictors included acute AF (1.87 [95% CI, 1.09-3.19; P = .022]) and age 73 or older (2.00 [95% CI, 1.18-3.37; P = .01]).
Limitations
- The study was retrospective and didn’t have access to some potentially relevant data, such as duration of AF.
- The single-center study with limited generalizability does not necessarily represent the broad population of stroke patients in China or elsewhere.
Disclosures
- This study was supported by the Cardiovascular Multidisciplinary Integrated Research Fund and Construction of Shanghai Municipal Health Commission.
- The authors report no relevant financial relationships.
This is a summary of a preprint research study, “Acute Atrial Fibrillation During Onset of Stroke Indicates Higher Probability of Post-Stroke Death Outcomes,” written by Yongxia Li, from the Shanghai Sixth People’s Hospital, and colleagues, on ResearchSquare.com. This study has not yet been peer reviewed. The full text of the study can be found on ResearchSquare.com.A version of this article first appeared on Medscape.com.
The study covered in this summary was published on ResearchSquare.com as a preprint and has not yet been peer-reviewed.
Key takeaway
Why this matters
- A comprehensive understanding of the relationship between acute AF and risk for acute ischemic stroke and prognosis will help improve management and treatment of patients with acute ischemic stroke.
Study design
- The retrospective study included patients with acute ischemic stroke within the prior 24 hours; 12-lead electrocardiogram in the emergency department; and hospitalization and treatment at the hospital stroke center.
- The cohort of 706 patients admitted to a single center in Shanghai, China, from December 2019 to December 2021, included 142 with episodes of acute AF and 564 without such episodes.
- Patients with acute ischemic stroke and acute AF – including AF of new onset, paroxysmal, persistent, or permanent with symptoms such as palpitations or dizziness attributed to rapid ventricular rates – were identified.
- Neurological deficits were assessed using the 7-day National Institutes of Health Stroke Scale/Score (NIHSS). Patients with a 7-day NIHSS score of at least 16 were considered to have moderate to severe stroke.
- Associations between acute AF onset and the severity of early neurological deficits were assessed and related to all-cause mortality within 30 days of the stroke.
Key results
- Patients with acute AF were older than those without acute AF (80.3 years vs. 71.0 years; P < .001).
- Baseline NIHSS scores averaged 16.09 for the stroke patients with acute AF and 8.65 for those without acute AF (P < .001).
- Significantly more patients with acute AF than without acute AF had a 7-day NIHSS score of at least 16 (45.1% vs. 14.4%; P < .001).
- More patients with than without acute AF underwent transcatheter thrombectomy (44.4% vs. 24.5%; P < .001) or received thrombolytic therapy (31.6% vs. 19.7%; P = .005).
- Patients aged 73 years or older showed baseline NIHSS score and acute AF as independent risk factors for early neurological deficits in stroke patients admitted to the emergency department.
- Mortality at 30 days was significantly higher in patients with acute AF than in those without acute AF (30.3% vs. 10.1%; P < .001).
- Baseline NIHSS had an adjusted odds ratio for 30-day mortality of 1.18 (95% confidence interval, 1.15-1.22; P < .001).
- Other independent predictors included acute AF (1.87 [95% CI, 1.09-3.19; P = .022]) and age 73 or older (2.00 [95% CI, 1.18-3.37; P = .01]).
Limitations
- The study was retrospective and didn’t have access to some potentially relevant data, such as duration of AF.
- The single-center study with limited generalizability does not necessarily represent the broad population of stroke patients in China or elsewhere.
Disclosures
- This study was supported by the Cardiovascular Multidisciplinary Integrated Research Fund and Construction of Shanghai Municipal Health Commission.
- The authors report no relevant financial relationships.
This is a summary of a preprint research study, “Acute Atrial Fibrillation During Onset of Stroke Indicates Higher Probability of Post-Stroke Death Outcomes,” written by Yongxia Li, from the Shanghai Sixth People’s Hospital, and colleagues, on ResearchSquare.com. This study has not yet been peer reviewed. The full text of the study can be found on ResearchSquare.com.A version of this article first appeared on Medscape.com.
The study covered in this summary was published on ResearchSquare.com as a preprint and has not yet been peer-reviewed.
Key takeaway
Why this matters
- A comprehensive understanding of the relationship between acute AF and risk for acute ischemic stroke and prognosis will help improve management and treatment of patients with acute ischemic stroke.
Study design
- The retrospective study included patients with acute ischemic stroke within the prior 24 hours; 12-lead electrocardiogram in the emergency department; and hospitalization and treatment at the hospital stroke center.
- The cohort of 706 patients admitted to a single center in Shanghai, China, from December 2019 to December 2021, included 142 with episodes of acute AF and 564 without such episodes.
- Patients with acute ischemic stroke and acute AF – including AF of new onset, paroxysmal, persistent, or permanent with symptoms such as palpitations or dizziness attributed to rapid ventricular rates – were identified.
- Neurological deficits were assessed using the 7-day National Institutes of Health Stroke Scale/Score (NIHSS). Patients with a 7-day NIHSS score of at least 16 were considered to have moderate to severe stroke.
- Associations between acute AF onset and the severity of early neurological deficits were assessed and related to all-cause mortality within 30 days of the stroke.
Key results
- Patients with acute AF were older than those without acute AF (80.3 years vs. 71.0 years; P < .001).
- Baseline NIHSS scores averaged 16.09 for the stroke patients with acute AF and 8.65 for those without acute AF (P < .001).
- Significantly more patients with acute AF than without acute AF had a 7-day NIHSS score of at least 16 (45.1% vs. 14.4%; P < .001).
- More patients with than without acute AF underwent transcatheter thrombectomy (44.4% vs. 24.5%; P < .001) or received thrombolytic therapy (31.6% vs. 19.7%; P = .005).
- Patients aged 73 years or older showed baseline NIHSS score and acute AF as independent risk factors for early neurological deficits in stroke patients admitted to the emergency department.
- Mortality at 30 days was significantly higher in patients with acute AF than in those without acute AF (30.3% vs. 10.1%; P < .001).
- Baseline NIHSS had an adjusted odds ratio for 30-day mortality of 1.18 (95% confidence interval, 1.15-1.22; P < .001).
- Other independent predictors included acute AF (1.87 [95% CI, 1.09-3.19; P = .022]) and age 73 or older (2.00 [95% CI, 1.18-3.37; P = .01]).
Limitations
- The study was retrospective and didn’t have access to some potentially relevant data, such as duration of AF.
- The single-center study with limited generalizability does not necessarily represent the broad population of stroke patients in China or elsewhere.
Disclosures
- This study was supported by the Cardiovascular Multidisciplinary Integrated Research Fund and Construction of Shanghai Municipal Health Commission.
- The authors report no relevant financial relationships.
This is a summary of a preprint research study, “Acute Atrial Fibrillation During Onset of Stroke Indicates Higher Probability of Post-Stroke Death Outcomes,” written by Yongxia Li, from the Shanghai Sixth People’s Hospital, and colleagues, on ResearchSquare.com. This study has not yet been peer reviewed. The full text of the study can be found on ResearchSquare.com.A version of this article first appeared on Medscape.com.
IRONMAN galvanizes case for IV iron repletion in heart failure
CHICAGO – Another major study appears to back the use of intravenous iron repletion in patients with heart failure (HF) and iron deficiency, strengthening largely consistent evidence, researchers say, that the treatment may improve symptoms and prevent some HF-related hospital admissions.
To be sure, the IRONMAN trial, which compared intravenous iron versus usual care in such patients – most with reduced ejection fraction and not hospitalized – failed to show a benefit for its primary endpoint. The 18% reduction in risk for HF hospitalization or cardiovascular (CV) death seen in the trial, however encouraging, can only be called a trend (P = .07).
But the intervention showed signs of benefit for some secondary endpoints, including quality of life scores, and hinted at such an effect on HF hospitalization. Risk for the latter endpoint dropped 20% (P = .085) over a median follow-up of 2.7 years.
The findings “build upon the other data we have that correcting iron deficiency can help improve well-being, and particularly reduce the risk of hospitalization, in a broad range of [HF] patients,” said Paul Kalra, MD, of the University of Glasgow and Portsmouth (England) Hospitals University NHS Trust.
The tested regimen “was well tolerated with no safety concerns” and offers “reassurance about the long-term safety” of the intravenous iron it used, ferric derisomaltose (MonoFerric), in patients with HF, Dr. Kalra said at a media briefing on the trial.
The remarks preceded his formal presentation of IRONMAN at the American Heart Association scientific sessions. Dr. Kalra is also lead author on the trial’s publication in The Lancet.
IRONMAN strengthens the base of evidence supporting intravenous iron in HF with iron deficiency, especially chronic HF in outpatients, Dr. Kalra and others said. It also supports efficacy for a form of intravenous iron not previously tested in a major HF trial.
Still, “the totality of data are now supporting intravenous iron per se,” regardless of the iron agent used, said Dr. Kalra. But ferric derisomaltose may have dosing advantages, he observed, “and we’ve now got these long-term safety data.”
The strongest prior support for intravenous iron in HF came from hospitalized patients who received it as ferric carboxymaltose (Ferinject) and were followed only 12 months. That was in the AFFIRM-AHF trial, published 2 years ago, which also missed its primary endpoint – the same one used in IRONMAN. Some outcomes in the two trials were similar.
The risk for HF hospitalization or CV death for intravenous iron therapy, compared with usual care, in AFFIRM-AHF fell 21% (P = .059), missing significance but apparently driven by a 26% drop in risk for HF readmissions (P = .013). But neither that trial nor IRONMAN suggested a benefit for CV mortality on its own.
The COVID effect
In IRONMAN, Dr. Kalra said, usual care could include oral iron supplementation, which 17% of patients in the control group received. That could potentially have kept the intravenous iron group from making a better showing for the primary endpoint, he proposed.
And some iron doses and other treatments were missed by a substantial number of patients in both groups who entered the trial after the United Kingdom’s national lockdown in response to the COVID-19 pandemic, he observed. “Patients were not able to come into hospitals for research visits, or in fact when they were able, may not have wanted to.”
So, the group conducted a “prespecified” sensitivity analysis that excluded the 9% of patients enrolled by the end of March 2020, about the time of the first lockdown, and followed the remainder for another 6 months.
In that analysis, risk for HF hospitalization or CV death declined 24% in the intravenous iron group, a marginal but significant result (P = .047) that was dominated by an improvement in HF hospitalizations.
Effects on guidelines
The intravenous iron recommendations in the European HF guidelines refer only to ferric carboxymaltose without mentioning other forms, such as ferric derisomaltose, “but this is now a class effect given the similarities between AFFIRM-AHF and IRONMAN,” said Gregory D. Lewis, MD, Mass General Brigham, Boston, invited discussant for Dr. Kalra’s presentation at the AHA session.
“In the United States, we relegate IV iron to improvement in functional capacity as a comorbidity of heart failure. Perhaps this role will expand,” added Dr. Lewis, who is medical director of his center’s heart transplant program.
He also wondered aloud whether the purported clinical benefits of intravenous iron in HF patients with iron deficiency, not as yet supported by a significant primary-endpoint showing in one of the major trials, currently justify expansion of its use in practice.
“With the benefits of IV iron on exercise capacity and quality of life, and the safety of administering high doses of IV iron,” potentially reducing HF polypharmacy, he noted, “should we be considering IV iron more commonly for utilization in our patients even if we find that heart failure hospitalizations and mortality are only modestly improved?”
IRONMAN “asked whether there’s benefit to IV iron in the longer term,” Kiran Musunuru, MD, PhD, MPH, University of Pennsylvania,Philadelphia, observed at the media briefing. As the trial was reported, “that does in fact, seem to be the case,” said Dr. Musunuru, who was not involved in IRONMAN.
Therefore, he said, “this study reinforces the message that we should be routinely monitoring our heart failure patients for iron deficiency and supplementing them as needed.”
A commentary linked to the IRONMAN publication agreed. The trial “increases the evidence base for the treatment of iron deficiency with intravenous iron supplementation,” wrote the editorialists, led by Theresa A. McDonagh, MD, King’s College Hospital and School of Cardiovascular Sciences, London.
Patients with acute or chronic HF, iron deficiency, and reduced or mildly reduced ejection fractions “should be offered treatment with intravenous iron to reduce their risk of hospital admission for heart failure,” they concluded.
Mostly reduced-EF outpatients
The open-label, blinded-endpoint IRONMAN trial, conducted at 70 centers in the United Kingdom, entered adults with HF, ejection fractions 45% or lower within the previous 2 years, and iron deficiency defined as transferrin saturation less than 20% or serum ferritin levels below 100 mcg/L, the report states. They were either hospitalized for HF, had such a hospitalization within the past 6 months, or were outpatients with elevated natriuretic peptide levels; the third category accounted for two thirds of the trial population.
Of the 1,137 randomized patients, 569 were assigned to receive intravenous ferric derisomaltose at weight- and hemoglobin-adjusted dosages; 568 went to the usual-care group.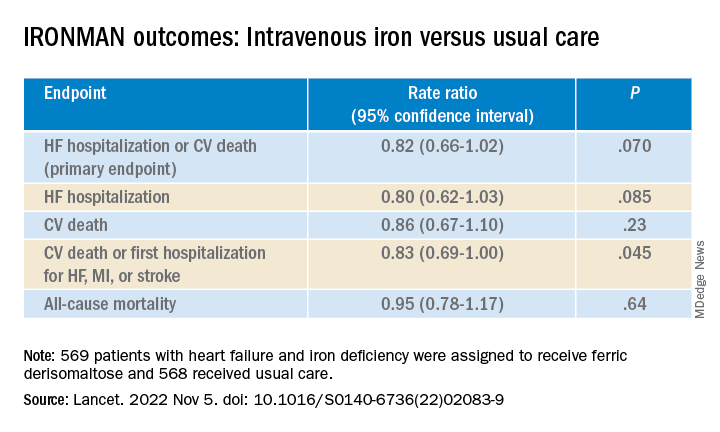
Those receiving intravenous iron visited the trial clinic 4 weeks later and then every 4 months. At those visits, they received a round of ferric derisomaltose if their ferritin levels were below 100 mcg/L, or 400 mcg/L or lower if transferrin saturation was below 25%, the published report states.
Mean scores on the Minnesota Living with Heart Failure Questionnaire improved by a marginally significant 3.33 points (P = .050) at 4 months in the intravenous iron group. The gain receded to a nonsignificant 2.57 points by 20 months (P = .23).
In COVID-related sensitivity analysis, the intravenous iron group showed a significant benefit for the primary endpoint and a trend for improved HF hospitalizations.
- HF hospitalization or CV death: RR, 0.76 (95% confidence interval, 0.58-1.00; P = .047)
- HF hospitalization: RR 0.76 (95% CI, 0.56-1.03; P = .077)
Fewer patients in the intravenous iron group experienced serious cardiac adverse events, 36% compared with 43% in for those on usual care, P = .016.
The recently updated European Society of Cardiology guidelines for HF made it a class 1 recommendation to assess iron status in every patient, Kalra observed. “It doesn›t specify how frequently, but I think we should be thinking about every 4-6 months.”
Dr. Kalra disclosed receiving research grants from Pharmacosmos; and consulting or lecturing for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, Pfizer, Pharmacosmos, Servier, and Vifor Pharma. Dr. Musunuru disclosed significant ownership interest in Verve Therapeutics and Variant Bio. Dr. Lewis disclosed relationships with NXT, American Regent, and RIVUS; and receiving research grants from Cytokinetics and Amgen.
A version of this article first appeared on Medscape.com.
CHICAGO – Another major study appears to back the use of intravenous iron repletion in patients with heart failure (HF) and iron deficiency, strengthening largely consistent evidence, researchers say, that the treatment may improve symptoms and prevent some HF-related hospital admissions.
To be sure, the IRONMAN trial, which compared intravenous iron versus usual care in such patients – most with reduced ejection fraction and not hospitalized – failed to show a benefit for its primary endpoint. The 18% reduction in risk for HF hospitalization or cardiovascular (CV) death seen in the trial, however encouraging, can only be called a trend (P = .07).
But the intervention showed signs of benefit for some secondary endpoints, including quality of life scores, and hinted at such an effect on HF hospitalization. Risk for the latter endpoint dropped 20% (P = .085) over a median follow-up of 2.7 years.
The findings “build upon the other data we have that correcting iron deficiency can help improve well-being, and particularly reduce the risk of hospitalization, in a broad range of [HF] patients,” said Paul Kalra, MD, of the University of Glasgow and Portsmouth (England) Hospitals University NHS Trust.
The tested regimen “was well tolerated with no safety concerns” and offers “reassurance about the long-term safety” of the intravenous iron it used, ferric derisomaltose (MonoFerric), in patients with HF, Dr. Kalra said at a media briefing on the trial.
The remarks preceded his formal presentation of IRONMAN at the American Heart Association scientific sessions. Dr. Kalra is also lead author on the trial’s publication in The Lancet.
IRONMAN strengthens the base of evidence supporting intravenous iron in HF with iron deficiency, especially chronic HF in outpatients, Dr. Kalra and others said. It also supports efficacy for a form of intravenous iron not previously tested in a major HF trial.
Still, “the totality of data are now supporting intravenous iron per se,” regardless of the iron agent used, said Dr. Kalra. But ferric derisomaltose may have dosing advantages, he observed, “and we’ve now got these long-term safety data.”
The strongest prior support for intravenous iron in HF came from hospitalized patients who received it as ferric carboxymaltose (Ferinject) and were followed only 12 months. That was in the AFFIRM-AHF trial, published 2 years ago, which also missed its primary endpoint – the same one used in IRONMAN. Some outcomes in the two trials were similar.
The risk for HF hospitalization or CV death for intravenous iron therapy, compared with usual care, in AFFIRM-AHF fell 21% (P = .059), missing significance but apparently driven by a 26% drop in risk for HF readmissions (P = .013). But neither that trial nor IRONMAN suggested a benefit for CV mortality on its own.
The COVID effect
In IRONMAN, Dr. Kalra said, usual care could include oral iron supplementation, which 17% of patients in the control group received. That could potentially have kept the intravenous iron group from making a better showing for the primary endpoint, he proposed.
And some iron doses and other treatments were missed by a substantial number of patients in both groups who entered the trial after the United Kingdom’s national lockdown in response to the COVID-19 pandemic, he observed. “Patients were not able to come into hospitals for research visits, or in fact when they were able, may not have wanted to.”
So, the group conducted a “prespecified” sensitivity analysis that excluded the 9% of patients enrolled by the end of March 2020, about the time of the first lockdown, and followed the remainder for another 6 months.
In that analysis, risk for HF hospitalization or CV death declined 24% in the intravenous iron group, a marginal but significant result (P = .047) that was dominated by an improvement in HF hospitalizations.
Effects on guidelines
The intravenous iron recommendations in the European HF guidelines refer only to ferric carboxymaltose without mentioning other forms, such as ferric derisomaltose, “but this is now a class effect given the similarities between AFFIRM-AHF and IRONMAN,” said Gregory D. Lewis, MD, Mass General Brigham, Boston, invited discussant for Dr. Kalra’s presentation at the AHA session.
“In the United States, we relegate IV iron to improvement in functional capacity as a comorbidity of heart failure. Perhaps this role will expand,” added Dr. Lewis, who is medical director of his center’s heart transplant program.
He also wondered aloud whether the purported clinical benefits of intravenous iron in HF patients with iron deficiency, not as yet supported by a significant primary-endpoint showing in one of the major trials, currently justify expansion of its use in practice.
“With the benefits of IV iron on exercise capacity and quality of life, and the safety of administering high doses of IV iron,” potentially reducing HF polypharmacy, he noted, “should we be considering IV iron more commonly for utilization in our patients even if we find that heart failure hospitalizations and mortality are only modestly improved?”
IRONMAN “asked whether there’s benefit to IV iron in the longer term,” Kiran Musunuru, MD, PhD, MPH, University of Pennsylvania,Philadelphia, observed at the media briefing. As the trial was reported, “that does in fact, seem to be the case,” said Dr. Musunuru, who was not involved in IRONMAN.
Therefore, he said, “this study reinforces the message that we should be routinely monitoring our heart failure patients for iron deficiency and supplementing them as needed.”
A commentary linked to the IRONMAN publication agreed. The trial “increases the evidence base for the treatment of iron deficiency with intravenous iron supplementation,” wrote the editorialists, led by Theresa A. McDonagh, MD, King’s College Hospital and School of Cardiovascular Sciences, London.
Patients with acute or chronic HF, iron deficiency, and reduced or mildly reduced ejection fractions “should be offered treatment with intravenous iron to reduce their risk of hospital admission for heart failure,” they concluded.
Mostly reduced-EF outpatients
The open-label, blinded-endpoint IRONMAN trial, conducted at 70 centers in the United Kingdom, entered adults with HF, ejection fractions 45% or lower within the previous 2 years, and iron deficiency defined as transferrin saturation less than 20% or serum ferritin levels below 100 mcg/L, the report states. They were either hospitalized for HF, had such a hospitalization within the past 6 months, or were outpatients with elevated natriuretic peptide levels; the third category accounted for two thirds of the trial population.
Of the 1,137 randomized patients, 569 were assigned to receive intravenous ferric derisomaltose at weight- and hemoglobin-adjusted dosages; 568 went to the usual-care group.
Those receiving intravenous iron visited the trial clinic 4 weeks later and then every 4 months. At those visits, they received a round of ferric derisomaltose if their ferritin levels were below 100 mcg/L, or 400 mcg/L or lower if transferrin saturation was below 25%, the published report states.
Mean scores on the Minnesota Living with Heart Failure Questionnaire improved by a marginally significant 3.33 points (P = .050) at 4 months in the intravenous iron group. The gain receded to a nonsignificant 2.57 points by 20 months (P = .23).
In COVID-related sensitivity analysis, the intravenous iron group showed a significant benefit for the primary endpoint and a trend for improved HF hospitalizations.
- HF hospitalization or CV death: RR, 0.76 (95% confidence interval, 0.58-1.00; P = .047)
- HF hospitalization: RR 0.76 (95% CI, 0.56-1.03; P = .077)
Fewer patients in the intravenous iron group experienced serious cardiac adverse events, 36% compared with 43% in for those on usual care, P = .016.
The recently updated European Society of Cardiology guidelines for HF made it a class 1 recommendation to assess iron status in every patient, Kalra observed. “It doesn›t specify how frequently, but I think we should be thinking about every 4-6 months.”
Dr. Kalra disclosed receiving research grants from Pharmacosmos; and consulting or lecturing for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, Pfizer, Pharmacosmos, Servier, and Vifor Pharma. Dr. Musunuru disclosed significant ownership interest in Verve Therapeutics and Variant Bio. Dr. Lewis disclosed relationships with NXT, American Regent, and RIVUS; and receiving research grants from Cytokinetics and Amgen.
A version of this article first appeared on Medscape.com.
CHICAGO – Another major study appears to back the use of intravenous iron repletion in patients with heart failure (HF) and iron deficiency, strengthening largely consistent evidence, researchers say, that the treatment may improve symptoms and prevent some HF-related hospital admissions.
To be sure, the IRONMAN trial, which compared intravenous iron versus usual care in such patients – most with reduced ejection fraction and not hospitalized – failed to show a benefit for its primary endpoint. The 18% reduction in risk for HF hospitalization or cardiovascular (CV) death seen in the trial, however encouraging, can only be called a trend (P = .07).
But the intervention showed signs of benefit for some secondary endpoints, including quality of life scores, and hinted at such an effect on HF hospitalization. Risk for the latter endpoint dropped 20% (P = .085) over a median follow-up of 2.7 years.
The findings “build upon the other data we have that correcting iron deficiency can help improve well-being, and particularly reduce the risk of hospitalization, in a broad range of [HF] patients,” said Paul Kalra, MD, of the University of Glasgow and Portsmouth (England) Hospitals University NHS Trust.
The tested regimen “was well tolerated with no safety concerns” and offers “reassurance about the long-term safety” of the intravenous iron it used, ferric derisomaltose (MonoFerric), in patients with HF, Dr. Kalra said at a media briefing on the trial.
The remarks preceded his formal presentation of IRONMAN at the American Heart Association scientific sessions. Dr. Kalra is also lead author on the trial’s publication in The Lancet.
IRONMAN strengthens the base of evidence supporting intravenous iron in HF with iron deficiency, especially chronic HF in outpatients, Dr. Kalra and others said. It also supports efficacy for a form of intravenous iron not previously tested in a major HF trial.
Still, “the totality of data are now supporting intravenous iron per se,” regardless of the iron agent used, said Dr. Kalra. But ferric derisomaltose may have dosing advantages, he observed, “and we’ve now got these long-term safety data.”
The strongest prior support for intravenous iron in HF came from hospitalized patients who received it as ferric carboxymaltose (Ferinject) and were followed only 12 months. That was in the AFFIRM-AHF trial, published 2 years ago, which also missed its primary endpoint – the same one used in IRONMAN. Some outcomes in the two trials were similar.
The risk for HF hospitalization or CV death for intravenous iron therapy, compared with usual care, in AFFIRM-AHF fell 21% (P = .059), missing significance but apparently driven by a 26% drop in risk for HF readmissions (P = .013). But neither that trial nor IRONMAN suggested a benefit for CV mortality on its own.
The COVID effect
In IRONMAN, Dr. Kalra said, usual care could include oral iron supplementation, which 17% of patients in the control group received. That could potentially have kept the intravenous iron group from making a better showing for the primary endpoint, he proposed.
And some iron doses and other treatments were missed by a substantial number of patients in both groups who entered the trial after the United Kingdom’s national lockdown in response to the COVID-19 pandemic, he observed. “Patients were not able to come into hospitals for research visits, or in fact when they were able, may not have wanted to.”
So, the group conducted a “prespecified” sensitivity analysis that excluded the 9% of patients enrolled by the end of March 2020, about the time of the first lockdown, and followed the remainder for another 6 months.
In that analysis, risk for HF hospitalization or CV death declined 24% in the intravenous iron group, a marginal but significant result (P = .047) that was dominated by an improvement in HF hospitalizations.
Effects on guidelines
The intravenous iron recommendations in the European HF guidelines refer only to ferric carboxymaltose without mentioning other forms, such as ferric derisomaltose, “but this is now a class effect given the similarities between AFFIRM-AHF and IRONMAN,” said Gregory D. Lewis, MD, Mass General Brigham, Boston, invited discussant for Dr. Kalra’s presentation at the AHA session.
“In the United States, we relegate IV iron to improvement in functional capacity as a comorbidity of heart failure. Perhaps this role will expand,” added Dr. Lewis, who is medical director of his center’s heart transplant program.
He also wondered aloud whether the purported clinical benefits of intravenous iron in HF patients with iron deficiency, not as yet supported by a significant primary-endpoint showing in one of the major trials, currently justify expansion of its use in practice.
“With the benefits of IV iron on exercise capacity and quality of life, and the safety of administering high doses of IV iron,” potentially reducing HF polypharmacy, he noted, “should we be considering IV iron more commonly for utilization in our patients even if we find that heart failure hospitalizations and mortality are only modestly improved?”
IRONMAN “asked whether there’s benefit to IV iron in the longer term,” Kiran Musunuru, MD, PhD, MPH, University of Pennsylvania,Philadelphia, observed at the media briefing. As the trial was reported, “that does in fact, seem to be the case,” said Dr. Musunuru, who was not involved in IRONMAN.
Therefore, he said, “this study reinforces the message that we should be routinely monitoring our heart failure patients for iron deficiency and supplementing them as needed.”
A commentary linked to the IRONMAN publication agreed. The trial “increases the evidence base for the treatment of iron deficiency with intravenous iron supplementation,” wrote the editorialists, led by Theresa A. McDonagh, MD, King’s College Hospital and School of Cardiovascular Sciences, London.
Patients with acute or chronic HF, iron deficiency, and reduced or mildly reduced ejection fractions “should be offered treatment with intravenous iron to reduce their risk of hospital admission for heart failure,” they concluded.
Mostly reduced-EF outpatients
The open-label, blinded-endpoint IRONMAN trial, conducted at 70 centers in the United Kingdom, entered adults with HF, ejection fractions 45% or lower within the previous 2 years, and iron deficiency defined as transferrin saturation less than 20% or serum ferritin levels below 100 mcg/L, the report states. They were either hospitalized for HF, had such a hospitalization within the past 6 months, or were outpatients with elevated natriuretic peptide levels; the third category accounted for two thirds of the trial population.
Of the 1,137 randomized patients, 569 were assigned to receive intravenous ferric derisomaltose at weight- and hemoglobin-adjusted dosages; 568 went to the usual-care group.
Those receiving intravenous iron visited the trial clinic 4 weeks later and then every 4 months. At those visits, they received a round of ferric derisomaltose if their ferritin levels were below 100 mcg/L, or 400 mcg/L or lower if transferrin saturation was below 25%, the published report states.
Mean scores on the Minnesota Living with Heart Failure Questionnaire improved by a marginally significant 3.33 points (P = .050) at 4 months in the intravenous iron group. The gain receded to a nonsignificant 2.57 points by 20 months (P = .23).
In COVID-related sensitivity analysis, the intravenous iron group showed a significant benefit for the primary endpoint and a trend for improved HF hospitalizations.
- HF hospitalization or CV death: RR, 0.76 (95% confidence interval, 0.58-1.00; P = .047)
- HF hospitalization: RR 0.76 (95% CI, 0.56-1.03; P = .077)
Fewer patients in the intravenous iron group experienced serious cardiac adverse events, 36% compared with 43% in for those on usual care, P = .016.
The recently updated European Society of Cardiology guidelines for HF made it a class 1 recommendation to assess iron status in every patient, Kalra observed. “It doesn›t specify how frequently, but I think we should be thinking about every 4-6 months.”
Dr. Kalra disclosed receiving research grants from Pharmacosmos; and consulting or lecturing for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, Pfizer, Pharmacosmos, Servier, and Vifor Pharma. Dr. Musunuru disclosed significant ownership interest in Verve Therapeutics and Variant Bio. Dr. Lewis disclosed relationships with NXT, American Regent, and RIVUS; and receiving research grants from Cytokinetics and Amgen.
A version of this article first appeared on Medscape.com.
AT AHA 2022
Be aware, mindfulness training can lower systolic BP: MB-BP
CHICAGO – It’s been said that one can observe a lot just by watching. Turning such observation inward, new evidence suggests, might lead to blood pressure (BP) reductions that approach what’s possible from an antihypertensive agent.
Systolic BP fell over 6 months by almost 6 mm Hg, on average, in people with elevated BP who participated in an 8-week mindful awareness program as part of a randomized trial that included a usual-care control group.
The program taught established mindfulness-training techniques aimed at modifying behaviors regarding diet, exercise, and other controllable influences on the success of antihypertensive therapy.
Participants in the program, called Mindfulness-Based Blood Pressure Reduction (MB-BP), also the name of the single-center study, “showed potentially clinically relevant reductions in systolic blood pressure,” said principal investigator Eric B. Loucks, PhD, Brown University, Providence, R.I.
The phase 2 trial has some limitations, he observed, including on generalizability. For example, it entered about 200 mostly White, college-educated adults from one metropolitan area.
But if these findings are replicated in further studies, “preferably by other research groups, in a larger and broader population, and with longer follow-up,” Dr. Loucks said, the MB-BP intervention could become “an appealing approach to help control blood pressure.”
Dr. Loucks made the comments at a press conference prior to his formal presentation of MB-BP Nov. 6 at American Heart Association (AHA) Scientific Sessions 2022, held in Chicago and virtually.
Mindfulness-based interventions for elevated BP have not been widely studied, “so this is exactly what we need: a well-done trial with a control group to show that it actually works,” Amit Khera, MD, not connected with MB-BP, told this news organization.
The trial is “really important for proof of concept, but it had only 200 people. You need a larger one, and you need longer-term data,” agreed Dr. Khera, who directs the preventive cardiology program at the University of Texas Southwestern Medical Center, in Dallas. “Six months is good, but we want to see if it’s durable.”
Rhian M. Touyz, MBBCh, also not part of MB-BP, agreed that the nearly 6 mm Hg mean systolic BP reduction among program participants is clinically relevant. “I think in the context of global risk and reduction of target organ damage and cardiovascular events, it is significant in terms of events at a population level,” Dr. Touyz, McGill University Health Centre, Montreal, told this news organization.
Many patients on antihypertensive therapy that’s falling short resist the addition of another such agent, she observed, and instead might show further BP reduction from mindfulness training. The intervention probably also “would benefit health in general.” Mindfulness-based approaches could therefore be useful additions to treatment protocols for elevated BP, Dr. Touyz said.
How the training works
The MB-BP program used validated mindfulness-based stress-management techniques, adapted to address elevated BP, that included “personalized feedback and education about hypertension risk factors, mindful awareness training of participants’ relationships with hypertension risk factors, and support for behavior change,” Dr. Loucks and colleagues reported.
Participants were trained in mindfulness skills that included “self-awareness and emotion regulation,” Dr. Loucks said, which they then could apply to their “relationships with the things that we know influence blood pressure, like physical activity, diet, antihypertensive medication adherence, or alcohol consumption.”
One goal is to promote greater “attention control,” he said, “so that there’s some self-awareness that arises in terms of how we feel the next day, after a lot of alcohol consumption, for example, or lack of physical activity.” The process can provide insights that inspire patients to modify behaviors and risk factors that elevate BP, Dr. Loucks explained.
Effects on medication use
Systolic BP responses led some program participants to be managed on fewer or reduced dosages of antihypertensive meds, he told this news organization. Physicians seen outside of the trial could adjust their prescriptions, intensifying or pulling back on meds depending on their assessments of the patient. Any prescription changes would be documented by the researchers at the patient’s next class or trial-clinic visit.
The group that did the training, Dr. Loucks said, was 33% less likely to increase and 30% more likely to decrease their use of BP-lowering medications compared with the control group.
Elevated BP is so common and undertreated that “there is a need for every possible level of intervention, starting from the population level to the individual and everything else in between,” nephrologist Janani Rangaswami, MD, George Washington University, Washington, said at the press conference.
Therefore, “this mindfulness-based approach, in addition to standard of care with pharmacotherapy, is a really welcome addition to the hypertension literature,” said Dr. Rangaswami, who directs her center’s cardiorenal program. The systolic BP reduction seen in the intervention group, she agreed, was “clinically important and meaningful.”
Blinded assessments
The trial entered 201 patients with systolic and diastolic BP greater than 120 mm Hg and 80 mm Hg, respectively; 58.7% were women, 81% were White, and 73% were college-educated, Dr. Loucks reported.
The 100 assigned to the “enhanced usual care” control group received educational materials on controlling high BP. They and the 101 who followed the mindfulness-based program were given and trained on a home BP-monitoring device. They were then followed for the primary endpoint of change in systolic BP at 6 months.
Data management and outcomes assessments were conducted by trialists not involved in the training intervention who were blinded to randomization assignment.
In a prespecified unadjusted analysis by intention-to-treat, systolic BP in the intervention group dropped by a mean of 5.9 mm Hg (P < .001) compared with baseline and 4.5 mm Hg (P = .045), compared with the control group.
A post hoc analysis adjusted for sex and baseline BP showed an average 4.3 mm Hg reduction (P = .056) in those following the MB-BP program, compared with controls.
There were no observed significant effects on diastolic BP.
The study offered clues to how engagement in the MB-BP program might promote reductions in systolic BP, Dr. Loucks observed. For example, it may have led to increased activity levels, reduced sodium intake, and other dietary improvements.
Indeed, program participants averaged about 351 minutes less sedentary time (P = .02) and showed a 0.32-point improvement in Dietary Approaches to Stop Hypertension scores (P = .08), compared with the control group, Dr. Loucks reported. Other modifiable risk factors for elevated BP that could have responded to the mindfulness-based training, he proposed, include obesity, alcohol intake, and reaction to stress.
Dr. Loucks reports that he developed the MB-BP training and was a program instructor but did not receive related financial compensation; he had no other disclosures. Dr. Khera, Dr. Touyz, and Dr. Rangaswami had no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO – It’s been said that one can observe a lot just by watching. Turning such observation inward, new evidence suggests, might lead to blood pressure (BP) reductions that approach what’s possible from an antihypertensive agent.
Systolic BP fell over 6 months by almost 6 mm Hg, on average, in people with elevated BP who participated in an 8-week mindful awareness program as part of a randomized trial that included a usual-care control group.
The program taught established mindfulness-training techniques aimed at modifying behaviors regarding diet, exercise, and other controllable influences on the success of antihypertensive therapy.
Participants in the program, called Mindfulness-Based Blood Pressure Reduction (MB-BP), also the name of the single-center study, “showed potentially clinically relevant reductions in systolic blood pressure,” said principal investigator Eric B. Loucks, PhD, Brown University, Providence, R.I.
The phase 2 trial has some limitations, he observed, including on generalizability. For example, it entered about 200 mostly White, college-educated adults from one metropolitan area.
But if these findings are replicated in further studies, “preferably by other research groups, in a larger and broader population, and with longer follow-up,” Dr. Loucks said, the MB-BP intervention could become “an appealing approach to help control blood pressure.”
Dr. Loucks made the comments at a press conference prior to his formal presentation of MB-BP Nov. 6 at American Heart Association (AHA) Scientific Sessions 2022, held in Chicago and virtually.
Mindfulness-based interventions for elevated BP have not been widely studied, “so this is exactly what we need: a well-done trial with a control group to show that it actually works,” Amit Khera, MD, not connected with MB-BP, told this news organization.
The trial is “really important for proof of concept, but it had only 200 people. You need a larger one, and you need longer-term data,” agreed Dr. Khera, who directs the preventive cardiology program at the University of Texas Southwestern Medical Center, in Dallas. “Six months is good, but we want to see if it’s durable.”
Rhian M. Touyz, MBBCh, also not part of MB-BP, agreed that the nearly 6 mm Hg mean systolic BP reduction among program participants is clinically relevant. “I think in the context of global risk and reduction of target organ damage and cardiovascular events, it is significant in terms of events at a population level,” Dr. Touyz, McGill University Health Centre, Montreal, told this news organization.
Many patients on antihypertensive therapy that’s falling short resist the addition of another such agent, she observed, and instead might show further BP reduction from mindfulness training. The intervention probably also “would benefit health in general.” Mindfulness-based approaches could therefore be useful additions to treatment protocols for elevated BP, Dr. Touyz said.
How the training works
The MB-BP program used validated mindfulness-based stress-management techniques, adapted to address elevated BP, that included “personalized feedback and education about hypertension risk factors, mindful awareness training of participants’ relationships with hypertension risk factors, and support for behavior change,” Dr. Loucks and colleagues reported.
Participants were trained in mindfulness skills that included “self-awareness and emotion regulation,” Dr. Loucks said, which they then could apply to their “relationships with the things that we know influence blood pressure, like physical activity, diet, antihypertensive medication adherence, or alcohol consumption.”
One goal is to promote greater “attention control,” he said, “so that there’s some self-awareness that arises in terms of how we feel the next day, after a lot of alcohol consumption, for example, or lack of physical activity.” The process can provide insights that inspire patients to modify behaviors and risk factors that elevate BP, Dr. Loucks explained.
Effects on medication use
Systolic BP responses led some program participants to be managed on fewer or reduced dosages of antihypertensive meds, he told this news organization. Physicians seen outside of the trial could adjust their prescriptions, intensifying or pulling back on meds depending on their assessments of the patient. Any prescription changes would be documented by the researchers at the patient’s next class or trial-clinic visit.
The group that did the training, Dr. Loucks said, was 33% less likely to increase and 30% more likely to decrease their use of BP-lowering medications compared with the control group.
Elevated BP is so common and undertreated that “there is a need for every possible level of intervention, starting from the population level to the individual and everything else in between,” nephrologist Janani Rangaswami, MD, George Washington University, Washington, said at the press conference.
Therefore, “this mindfulness-based approach, in addition to standard of care with pharmacotherapy, is a really welcome addition to the hypertension literature,” said Dr. Rangaswami, who directs her center’s cardiorenal program. The systolic BP reduction seen in the intervention group, she agreed, was “clinically important and meaningful.”
Blinded assessments
The trial entered 201 patients with systolic and diastolic BP greater than 120 mm Hg and 80 mm Hg, respectively; 58.7% were women, 81% were White, and 73% were college-educated, Dr. Loucks reported.
The 100 assigned to the “enhanced usual care” control group received educational materials on controlling high BP. They and the 101 who followed the mindfulness-based program were given and trained on a home BP-monitoring device. They were then followed for the primary endpoint of change in systolic BP at 6 months.
Data management and outcomes assessments were conducted by trialists not involved in the training intervention who were blinded to randomization assignment.
In a prespecified unadjusted analysis by intention-to-treat, systolic BP in the intervention group dropped by a mean of 5.9 mm Hg (P < .001) compared with baseline and 4.5 mm Hg (P = .045), compared with the control group.
A post hoc analysis adjusted for sex and baseline BP showed an average 4.3 mm Hg reduction (P = .056) in those following the MB-BP program, compared with controls.
There were no observed significant effects on diastolic BP.
The study offered clues to how engagement in the MB-BP program might promote reductions in systolic BP, Dr. Loucks observed. For example, it may have led to increased activity levels, reduced sodium intake, and other dietary improvements.
Indeed, program participants averaged about 351 minutes less sedentary time (P = .02) and showed a 0.32-point improvement in Dietary Approaches to Stop Hypertension scores (P = .08), compared with the control group, Dr. Loucks reported. Other modifiable risk factors for elevated BP that could have responded to the mindfulness-based training, he proposed, include obesity, alcohol intake, and reaction to stress.
Dr. Loucks reports that he developed the MB-BP training and was a program instructor but did not receive related financial compensation; he had no other disclosures. Dr. Khera, Dr. Touyz, and Dr. Rangaswami had no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO – It’s been said that one can observe a lot just by watching. Turning such observation inward, new evidence suggests, might lead to blood pressure (BP) reductions that approach what’s possible from an antihypertensive agent.
Systolic BP fell over 6 months by almost 6 mm Hg, on average, in people with elevated BP who participated in an 8-week mindful awareness program as part of a randomized trial that included a usual-care control group.
The program taught established mindfulness-training techniques aimed at modifying behaviors regarding diet, exercise, and other controllable influences on the success of antihypertensive therapy.
Participants in the program, called Mindfulness-Based Blood Pressure Reduction (MB-BP), also the name of the single-center study, “showed potentially clinically relevant reductions in systolic blood pressure,” said principal investigator Eric B. Loucks, PhD, Brown University, Providence, R.I.
The phase 2 trial has some limitations, he observed, including on generalizability. For example, it entered about 200 mostly White, college-educated adults from one metropolitan area.
But if these findings are replicated in further studies, “preferably by other research groups, in a larger and broader population, and with longer follow-up,” Dr. Loucks said, the MB-BP intervention could become “an appealing approach to help control blood pressure.”
Dr. Loucks made the comments at a press conference prior to his formal presentation of MB-BP Nov. 6 at American Heart Association (AHA) Scientific Sessions 2022, held in Chicago and virtually.
Mindfulness-based interventions for elevated BP have not been widely studied, “so this is exactly what we need: a well-done trial with a control group to show that it actually works,” Amit Khera, MD, not connected with MB-BP, told this news organization.
The trial is “really important for proof of concept, but it had only 200 people. You need a larger one, and you need longer-term data,” agreed Dr. Khera, who directs the preventive cardiology program at the University of Texas Southwestern Medical Center, in Dallas. “Six months is good, but we want to see if it’s durable.”
Rhian M. Touyz, MBBCh, also not part of MB-BP, agreed that the nearly 6 mm Hg mean systolic BP reduction among program participants is clinically relevant. “I think in the context of global risk and reduction of target organ damage and cardiovascular events, it is significant in terms of events at a population level,” Dr. Touyz, McGill University Health Centre, Montreal, told this news organization.
Many patients on antihypertensive therapy that’s falling short resist the addition of another such agent, she observed, and instead might show further BP reduction from mindfulness training. The intervention probably also “would benefit health in general.” Mindfulness-based approaches could therefore be useful additions to treatment protocols for elevated BP, Dr. Touyz said.
How the training works
The MB-BP program used validated mindfulness-based stress-management techniques, adapted to address elevated BP, that included “personalized feedback and education about hypertension risk factors, mindful awareness training of participants’ relationships with hypertension risk factors, and support for behavior change,” Dr. Loucks and colleagues reported.
Participants were trained in mindfulness skills that included “self-awareness and emotion regulation,” Dr. Loucks said, which they then could apply to their “relationships with the things that we know influence blood pressure, like physical activity, diet, antihypertensive medication adherence, or alcohol consumption.”
One goal is to promote greater “attention control,” he said, “so that there’s some self-awareness that arises in terms of how we feel the next day, after a lot of alcohol consumption, for example, or lack of physical activity.” The process can provide insights that inspire patients to modify behaviors and risk factors that elevate BP, Dr. Loucks explained.
Effects on medication use
Systolic BP responses led some program participants to be managed on fewer or reduced dosages of antihypertensive meds, he told this news organization. Physicians seen outside of the trial could adjust their prescriptions, intensifying or pulling back on meds depending on their assessments of the patient. Any prescription changes would be documented by the researchers at the patient’s next class or trial-clinic visit.
The group that did the training, Dr. Loucks said, was 33% less likely to increase and 30% more likely to decrease their use of BP-lowering medications compared with the control group.
Elevated BP is so common and undertreated that “there is a need for every possible level of intervention, starting from the population level to the individual and everything else in between,” nephrologist Janani Rangaswami, MD, George Washington University, Washington, said at the press conference.
Therefore, “this mindfulness-based approach, in addition to standard of care with pharmacotherapy, is a really welcome addition to the hypertension literature,” said Dr. Rangaswami, who directs her center’s cardiorenal program. The systolic BP reduction seen in the intervention group, she agreed, was “clinically important and meaningful.”
Blinded assessments
The trial entered 201 patients with systolic and diastolic BP greater than 120 mm Hg and 80 mm Hg, respectively; 58.7% were women, 81% were White, and 73% were college-educated, Dr. Loucks reported.
The 100 assigned to the “enhanced usual care” control group received educational materials on controlling high BP. They and the 101 who followed the mindfulness-based program were given and trained on a home BP-monitoring device. They were then followed for the primary endpoint of change in systolic BP at 6 months.
Data management and outcomes assessments were conducted by trialists not involved in the training intervention who were blinded to randomization assignment.
In a prespecified unadjusted analysis by intention-to-treat, systolic BP in the intervention group dropped by a mean of 5.9 mm Hg (P < .001) compared with baseline and 4.5 mm Hg (P = .045), compared with the control group.
A post hoc analysis adjusted for sex and baseline BP showed an average 4.3 mm Hg reduction (P = .056) in those following the MB-BP program, compared with controls.
There were no observed significant effects on diastolic BP.
The study offered clues to how engagement in the MB-BP program might promote reductions in systolic BP, Dr. Loucks observed. For example, it may have led to increased activity levels, reduced sodium intake, and other dietary improvements.
Indeed, program participants averaged about 351 minutes less sedentary time (P = .02) and showed a 0.32-point improvement in Dietary Approaches to Stop Hypertension scores (P = .08), compared with the control group, Dr. Loucks reported. Other modifiable risk factors for elevated BP that could have responded to the mindfulness-based training, he proposed, include obesity, alcohol intake, and reaction to stress.
Dr. Loucks reports that he developed the MB-BP training and was a program instructor but did not receive related financial compensation; he had no other disclosures. Dr. Khera, Dr. Touyz, and Dr. Rangaswami had no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AHA 2022
Hypertension linked to risk of severe COVID
U.K. researchers have established that hypertension is associated with a 22% greater risk of severe COVID-19, with the odds of severe COVID-19 unaffected by medication type.
Hypertension “appears to be one of the commonest comorbidities in COVID-19 patients”, explained the authors of a new study, published in PLOS ONE. The authors highlighted that previous research had shown that hypertension was more prevalent in severe and fatal cases compared with all cases of COVID-19.
They pointed out, however, that whether hypertensive individuals have a higher risk of severe COVID-19, compared with nonhypertensives, and whether the absolute level of systolic blood pressure or the type of antihypertensive medication is related to this risk, remained “unclear.”
To try to answer these questions, the research team, led by University of Cambridge researchers, analyzed data from 16,134 individuals who tested positive for COVID-19 (mean age 65.3 years, 47% male, 90% white), 40% were diagnosed with essential hypertension at the analysis baseline – 22% of whom had developed severe COVID-19.
Systolic blood pressure (SBP) was categorized by 10–mm Hg ranges, starting from < 120 mm Hg up to 180+ mm Hg, with the reference category defined as 120-129 mm Hg, based on data from the SPRINT study, which demonstrated that intensive SBP lowering to below 120 mm Hg, as compared with the traditional threshold of 140 mm Hg, was beneficial. Diastolic blood pressure was categorized by 10–mm Hg ranges, starting from < 60 mm Hg up to 100+ mm Hg with 80-90 mm Hg being the reference category.
In their analyses the researchers adjusted for age, sex, body mass index, ethnicity, smoking status, diabetes status, socioeconomic status, and inflammation (C-reactive protein [CRP]), as these were proposed as potential confounders. To assess the direct effect of hypertension on COVID-19, they also adjusted for intermediate variables, including cardiovascular comorbidities and stroke, on the causal pathway between hypertension and severe COVID-19.
Majority of effect of hypertension on severe COVID-19 was direct
The unadjusted odds ratio of the association between hypertension and severe COVID-19 was 2.33 (95% confidence interval, 2.16-2.51), the authors emphasized. They found that, after adjusting for all confounding variables, hypertension was associated with 22% higher odds of severe COVID-19 (OR, 1.22; 95% CI, 1.12-1.33), compared with normotension.
Individuals with severe COVID-19 were marginally older, more likely to be male, and more deprived, the authors said. “They were also more likely to be hypertensive, compared with individuals without severe COVID-19, and a greater proportion of individuals with severe COVID-19 had cardiovascular comorbidities.”
The majority of the effect of hypertension on development of severe COVID-19 was “direct,” they said. However, a modest proportion of the effect was mediated via cardiovascular comorbidities such as peripheral vascular disease, MI, coronary heart disease, arrhythmias, and stroke. Of note, those with a history of stroke had a 47% higher risk of severe COVID-19 and those with a history of other cardiovascular comorbidities had a 30% higher risk of severe COVID-19, the authors commented.
J-shaped relationship
Of the total of 6,517 (40%) individuals who had a diagnosis of essential hypertension at baseline, 67% were treated (41% with monotherapy, 59% with combination therapy), and 33% were untreated.
There were similar numbers of severe COVID-19 in each medication group: ACE inhibitors, 34%; angiotensin receptor blockers (ARBs), 36%; and “other” medications 34%.
In hypertensive individuals receiving antihypertensive medications, there was a “J-shaped relationship” between the level of blood pressure and risk of severe COVID-19 when using a systolic blood pressure level of 120-129 mm Hg as a reference – 150-159 mm Hg versus 120-129 mm Hg (OR 1.91; 95% CI, 1.44-2.53), > 180+ mm Hg versus 120-129 mm Hg (OR 1.93; 95% CI, 1.06-3.51).
The authors commented that there was no evidence of a higher risk of severe COVID-19 until systolic blood pressure “exceeded 150 mm Hg.”
They said it was an interesting finding that “very well-controlled” systolic blood pressure < 120 mm Hg was associated with a 40% (OR, 1.40; 95% CI, 1.11-1.78) greater odds of severe COVID-19. “This may be due to reverse causality, where low systolic blood pressure levels may indicate poorer health, such that the occurrence of severe COVID-19 may be related to underlying disease rather than the level of SBP per se,” they suggested.
The J-shaped association observed remained after multiple adjustments, including presence of known cardiovascular comorbidities, which suggested a possible “real effect” of low SBP on severe COVID-19, “at least in treated hypertensive individuals.”
Their analyses also identified that, compared with a “normal” diastolic blood pressure (80-90 mm Hg), having a diastolic blood pressure higher than 90 mm Hg was associated with higher odds of severe COVID-19.
The association between hypertension and COVID-19 was “amplified” if the individuals were treated and their BP remained uncontrolled, the authors pointed out.
There did not appear to be any difference in the risk of severe COVID-19 between individuals taking ACE inhibitors and those taking ARBs or other antihypertensive medications, the authors said.
Better understanding of underlying mechanisms needed
Individuals with hypertension who tested positive for COVID-19 had “over twice” the risk of developing severe COVID-19, compared with nonhypertensive individuals, the authors said.
They highlighted that their findings also suggest that there are “further effects” influencing the severity of COVID-19 beyond a “dichotomous” diagnosis of hypertension.
“Individuals with a higher-than-target systolic blood pressure may be less healthy, less active, suffering more severe hypertension, or have developed drug-resistant hypertension, all suggesting that the effects of hypertension have already had detrimental physiological effects on the cardiovascular system, which in turn may offer some explanation for the higher risk of severe COVID-19 with uncontrolled SBP,” they explained.
“Hypertension is an important risk factor for COVID-19,” reiterated the authors, who emphasized that a better understanding of the underlying mechanisms driving this increased risk is warranted in case of “more severe strains or other viruses” in the future.
The authors have declared no competing interests.
A version of this article first appeared on Medscape UK.
U.K. researchers have established that hypertension is associated with a 22% greater risk of severe COVID-19, with the odds of severe COVID-19 unaffected by medication type.
Hypertension “appears to be one of the commonest comorbidities in COVID-19 patients”, explained the authors of a new study, published in PLOS ONE. The authors highlighted that previous research had shown that hypertension was more prevalent in severe and fatal cases compared with all cases of COVID-19.
They pointed out, however, that whether hypertensive individuals have a higher risk of severe COVID-19, compared with nonhypertensives, and whether the absolute level of systolic blood pressure or the type of antihypertensive medication is related to this risk, remained “unclear.”
To try to answer these questions, the research team, led by University of Cambridge researchers, analyzed data from 16,134 individuals who tested positive for COVID-19 (mean age 65.3 years, 47% male, 90% white), 40% were diagnosed with essential hypertension at the analysis baseline – 22% of whom had developed severe COVID-19.
Systolic blood pressure (SBP) was categorized by 10–mm Hg ranges, starting from < 120 mm Hg up to 180+ mm Hg, with the reference category defined as 120-129 mm Hg, based on data from the SPRINT study, which demonstrated that intensive SBP lowering to below 120 mm Hg, as compared with the traditional threshold of 140 mm Hg, was beneficial. Diastolic blood pressure was categorized by 10–mm Hg ranges, starting from < 60 mm Hg up to 100+ mm Hg with 80-90 mm Hg being the reference category.
In their analyses the researchers adjusted for age, sex, body mass index, ethnicity, smoking status, diabetes status, socioeconomic status, and inflammation (C-reactive protein [CRP]), as these were proposed as potential confounders. To assess the direct effect of hypertension on COVID-19, they also adjusted for intermediate variables, including cardiovascular comorbidities and stroke, on the causal pathway between hypertension and severe COVID-19.
Majority of effect of hypertension on severe COVID-19 was direct
The unadjusted odds ratio of the association between hypertension and severe COVID-19 was 2.33 (95% confidence interval, 2.16-2.51), the authors emphasized. They found that, after adjusting for all confounding variables, hypertension was associated with 22% higher odds of severe COVID-19 (OR, 1.22; 95% CI, 1.12-1.33), compared with normotension.
Individuals with severe COVID-19 were marginally older, more likely to be male, and more deprived, the authors said. “They were also more likely to be hypertensive, compared with individuals without severe COVID-19, and a greater proportion of individuals with severe COVID-19 had cardiovascular comorbidities.”
The majority of the effect of hypertension on development of severe COVID-19 was “direct,” they said. However, a modest proportion of the effect was mediated via cardiovascular comorbidities such as peripheral vascular disease, MI, coronary heart disease, arrhythmias, and stroke. Of note, those with a history of stroke had a 47% higher risk of severe COVID-19 and those with a history of other cardiovascular comorbidities had a 30% higher risk of severe COVID-19, the authors commented.
J-shaped relationship
Of the total of 6,517 (40%) individuals who had a diagnosis of essential hypertension at baseline, 67% were treated (41% with monotherapy, 59% with combination therapy), and 33% were untreated.
There were similar numbers of severe COVID-19 in each medication group: ACE inhibitors, 34%; angiotensin receptor blockers (ARBs), 36%; and “other” medications 34%.
In hypertensive individuals receiving antihypertensive medications, there was a “J-shaped relationship” between the level of blood pressure and risk of severe COVID-19 when using a systolic blood pressure level of 120-129 mm Hg as a reference – 150-159 mm Hg versus 120-129 mm Hg (OR 1.91; 95% CI, 1.44-2.53), > 180+ mm Hg versus 120-129 mm Hg (OR 1.93; 95% CI, 1.06-3.51).
The authors commented that there was no evidence of a higher risk of severe COVID-19 until systolic blood pressure “exceeded 150 mm Hg.”
They said it was an interesting finding that “very well-controlled” systolic blood pressure < 120 mm Hg was associated with a 40% (OR, 1.40; 95% CI, 1.11-1.78) greater odds of severe COVID-19. “This may be due to reverse causality, where low systolic blood pressure levels may indicate poorer health, such that the occurrence of severe COVID-19 may be related to underlying disease rather than the level of SBP per se,” they suggested.
The J-shaped association observed remained after multiple adjustments, including presence of known cardiovascular comorbidities, which suggested a possible “real effect” of low SBP on severe COVID-19, “at least in treated hypertensive individuals.”
Their analyses also identified that, compared with a “normal” diastolic blood pressure (80-90 mm Hg), having a diastolic blood pressure higher than 90 mm Hg was associated with higher odds of severe COVID-19.
The association between hypertension and COVID-19 was “amplified” if the individuals were treated and their BP remained uncontrolled, the authors pointed out.
There did not appear to be any difference in the risk of severe COVID-19 between individuals taking ACE inhibitors and those taking ARBs or other antihypertensive medications, the authors said.
Better understanding of underlying mechanisms needed
Individuals with hypertension who tested positive for COVID-19 had “over twice” the risk of developing severe COVID-19, compared with nonhypertensive individuals, the authors said.
They highlighted that their findings also suggest that there are “further effects” influencing the severity of COVID-19 beyond a “dichotomous” diagnosis of hypertension.
“Individuals with a higher-than-target systolic blood pressure may be less healthy, less active, suffering more severe hypertension, or have developed drug-resistant hypertension, all suggesting that the effects of hypertension have already had detrimental physiological effects on the cardiovascular system, which in turn may offer some explanation for the higher risk of severe COVID-19 with uncontrolled SBP,” they explained.
“Hypertension is an important risk factor for COVID-19,” reiterated the authors, who emphasized that a better understanding of the underlying mechanisms driving this increased risk is warranted in case of “more severe strains or other viruses” in the future.
The authors have declared no competing interests.
A version of this article first appeared on Medscape UK.
U.K. researchers have established that hypertension is associated with a 22% greater risk of severe COVID-19, with the odds of severe COVID-19 unaffected by medication type.
Hypertension “appears to be one of the commonest comorbidities in COVID-19 patients”, explained the authors of a new study, published in PLOS ONE. The authors highlighted that previous research had shown that hypertension was more prevalent in severe and fatal cases compared with all cases of COVID-19.
They pointed out, however, that whether hypertensive individuals have a higher risk of severe COVID-19, compared with nonhypertensives, and whether the absolute level of systolic blood pressure or the type of antihypertensive medication is related to this risk, remained “unclear.”
To try to answer these questions, the research team, led by University of Cambridge researchers, analyzed data from 16,134 individuals who tested positive for COVID-19 (mean age 65.3 years, 47% male, 90% white), 40% were diagnosed with essential hypertension at the analysis baseline – 22% of whom had developed severe COVID-19.
Systolic blood pressure (SBP) was categorized by 10–mm Hg ranges, starting from < 120 mm Hg up to 180+ mm Hg, with the reference category defined as 120-129 mm Hg, based on data from the SPRINT study, which demonstrated that intensive SBP lowering to below 120 mm Hg, as compared with the traditional threshold of 140 mm Hg, was beneficial. Diastolic blood pressure was categorized by 10–mm Hg ranges, starting from < 60 mm Hg up to 100+ mm Hg with 80-90 mm Hg being the reference category.
In their analyses the researchers adjusted for age, sex, body mass index, ethnicity, smoking status, diabetes status, socioeconomic status, and inflammation (C-reactive protein [CRP]), as these were proposed as potential confounders. To assess the direct effect of hypertension on COVID-19, they also adjusted for intermediate variables, including cardiovascular comorbidities and stroke, on the causal pathway between hypertension and severe COVID-19.
Majority of effect of hypertension on severe COVID-19 was direct
The unadjusted odds ratio of the association between hypertension and severe COVID-19 was 2.33 (95% confidence interval, 2.16-2.51), the authors emphasized. They found that, after adjusting for all confounding variables, hypertension was associated with 22% higher odds of severe COVID-19 (OR, 1.22; 95% CI, 1.12-1.33), compared with normotension.
Individuals with severe COVID-19 were marginally older, more likely to be male, and more deprived, the authors said. “They were also more likely to be hypertensive, compared with individuals without severe COVID-19, and a greater proportion of individuals with severe COVID-19 had cardiovascular comorbidities.”
The majority of the effect of hypertension on development of severe COVID-19 was “direct,” they said. However, a modest proportion of the effect was mediated via cardiovascular comorbidities such as peripheral vascular disease, MI, coronary heart disease, arrhythmias, and stroke. Of note, those with a history of stroke had a 47% higher risk of severe COVID-19 and those with a history of other cardiovascular comorbidities had a 30% higher risk of severe COVID-19, the authors commented.
J-shaped relationship
Of the total of 6,517 (40%) individuals who had a diagnosis of essential hypertension at baseline, 67% were treated (41% with monotherapy, 59% with combination therapy), and 33% were untreated.
There were similar numbers of severe COVID-19 in each medication group: ACE inhibitors, 34%; angiotensin receptor blockers (ARBs), 36%; and “other” medications 34%.
In hypertensive individuals receiving antihypertensive medications, there was a “J-shaped relationship” between the level of blood pressure and risk of severe COVID-19 when using a systolic blood pressure level of 120-129 mm Hg as a reference – 150-159 mm Hg versus 120-129 mm Hg (OR 1.91; 95% CI, 1.44-2.53), > 180+ mm Hg versus 120-129 mm Hg (OR 1.93; 95% CI, 1.06-3.51).
The authors commented that there was no evidence of a higher risk of severe COVID-19 until systolic blood pressure “exceeded 150 mm Hg.”
They said it was an interesting finding that “very well-controlled” systolic blood pressure < 120 mm Hg was associated with a 40% (OR, 1.40; 95% CI, 1.11-1.78) greater odds of severe COVID-19. “This may be due to reverse causality, where low systolic blood pressure levels may indicate poorer health, such that the occurrence of severe COVID-19 may be related to underlying disease rather than the level of SBP per se,” they suggested.
The J-shaped association observed remained after multiple adjustments, including presence of known cardiovascular comorbidities, which suggested a possible “real effect” of low SBP on severe COVID-19, “at least in treated hypertensive individuals.”
Their analyses also identified that, compared with a “normal” diastolic blood pressure (80-90 mm Hg), having a diastolic blood pressure higher than 90 mm Hg was associated with higher odds of severe COVID-19.
The association between hypertension and COVID-19 was “amplified” if the individuals were treated and their BP remained uncontrolled, the authors pointed out.
There did not appear to be any difference in the risk of severe COVID-19 between individuals taking ACE inhibitors and those taking ARBs or other antihypertensive medications, the authors said.
Better understanding of underlying mechanisms needed
Individuals with hypertension who tested positive for COVID-19 had “over twice” the risk of developing severe COVID-19, compared with nonhypertensive individuals, the authors said.
They highlighted that their findings also suggest that there are “further effects” influencing the severity of COVID-19 beyond a “dichotomous” diagnosis of hypertension.
“Individuals with a higher-than-target systolic blood pressure may be less healthy, less active, suffering more severe hypertension, or have developed drug-resistant hypertension, all suggesting that the effects of hypertension have already had detrimental physiological effects on the cardiovascular system, which in turn may offer some explanation for the higher risk of severe COVID-19 with uncontrolled SBP,” they explained.
“Hypertension is an important risk factor for COVID-19,” reiterated the authors, who emphasized that a better understanding of the underlying mechanisms driving this increased risk is warranted in case of “more severe strains or other viruses” in the future.
The authors have declared no competing interests.
A version of this article first appeared on Medscape UK.
FROM PLOS ONE
Medical school culinary medicine programs grow despite limited funding
The way he sees it, the stakes couldn’t be higher. He believes doctors need to see food as medicine to be able to stem the tide of chronic disease.
About 6 in 10 adults in the United States live with chronic diseases, according to the Centers for Disease Control and Prevention, costing $4.1 trillion in annual health care costs. Adult obesity rates are rising, as are obesity-related conditions such as heart disease, stroke, type 2 diabetes, and certain types of cancer.
To turn the tide, Dr. Marvasti created a culinary medicine program in 2020 in collaboration with the University of Arizona Cooperative Extension and local chefs.
Dr. Marvasti, who is board certified in family medicine, graduated from the University of Arizona, Phoenix, where he serves as the director of the medical school’s Culinary Medicine Program.
The program offers an elective course for third- and fourth-year medical students, which introduces the evidence-based field of culinary medicine. Dr Marvasti’s goal is for the course to teach students how to use this science and the joy of cooking to improve long-term health outcomes for their patients.
As part of Dr. Marvasti’s program, students learn cooking fundamentals through chef demonstrations and hands-on practice – to teach students how food can be used to prevent and treat many chronic diseases.
One of the dishes students learn to make includes a quinoa salad made with cucumber, onion, bell peppers, corn, cherry tomatoes, beans, garlic, olive oil, and lemon juice. Another recipe includes a healthier take on dessert: Dark chocolate mousse made with three large, ripe avocados, dark chocolate powder, three tablespoons of agave or maple, coconut cream, nondairy milk, salt, and vanilla. Dr. Marvasti and his team are set to build out the existing program to develop additional resources for medically underserved and rural communities in Arizona, according to a statement from the university. These plans will be funded by a $750,000 grant from Novo Nordisk.
“We’re going to develop an open education curriculum to share, so it’s open access to everyone,” said Dr. Marvasti, who is also director of Public Health, Prevention and Health Promotion and an associate professor at the university. “It can be adaptable at the undergraduate, graduate, and postgraduate level.”
Dr. Marvasti and his colleagues at the University of Arizona aren’t alone. In fact, culinary medicine programs are sprouting some serious legs.
Culinary medicine programs catch on
Jaclyn Albin, MD, CCMS, an associate professor in the departments of internal medicine and pediatrics at UT Southwestern Medical Center, Dallas, conducted a scoping review of the literature on culinary medicine programs for medical students.* Her purpose was to learn how the programs were structured and how they assessed student knowledge and attitudes regarding nutrition counseling for patients.
Dr. Albin and her colleagues performed an initial literature search between June 1 and Aug. 1, 2020, of papers published between Jan. 1, 2012, and Aug. 1, 2020 – excluding some newer programs such as the one at the University of Arizona. The results of their research were published in Academic Medicine.
Ultimately, the authors identified and examined 34 programs offering medical student–focused culinary medicine courses.
Program instructors typically included a team of physicians, dietitians, chefs, and other professionals, the study found.
Most program participants exclusively taught medical students, though the training years of participants varied among programs, and they included first-, second-, third-, and fourth-year students. Some programs allowed students from outside their respective medical school to participate in the trainings.
As for the formats of the program, most included cohorts of 10-20 students attending multiple 2- to 3-hour sessions over the course of several months. The University of Alabama at Birmingham offers one of the longest courses, which spans 4-5 months, according to the paper. In contrast, the University of Rochester (N.Y.) program offers only a 1-day lab divided into four sessions, with each session lasting about 2 hours.
The culinary medicine programs’ course sessions tended to include a 10- to 30-minute didactic session involving videos, research articles, culinary theories, and other lectures, a 60- to 90-minute hands-on cooking session, and a 30-minute discussion around nutrition, culture, and patient care.
Most programs used pre- and post-program surveys to evaluate outcomes, though results varied between programs, according to the study. While each program evaluation had different metrics, the surveys generally revealed students felt more confident discussing dietary interventions with patients and in their own cooking skills following completion.
Course correction
Most of those programs are unfunded or minimally funded, Dr. Albin said.
Her own program, which is immensely popular with medical students, is one she teaches on a volunteer basis.
“I do this for free, in the evenings, because I believe in it,” she said.
Medical school education real estate is limited, so convincing medical schools to add something to the curriculum is difficult, Dr. Albin noted.
But it’s worth it, she said, because nutrition is the underpinning of so many diseases.
“Food is the top risk factor for early death in the U.S.,” Dr. Albin said. “I like to say that five times in a row. People have not digested it.”
During her culinary medicine courses, she also asks her medical students: “Who is comfortable in the kitchen?” Some sheepishly raise their hands, she said. Some don’t. Many don’t know anything about cooking.
Then she teaches students about healthy food and how to make it. As part of her program, medical students are given a pantry starter kit with olive oil and a variety of spices to take home and use.
Some recipes Dr. Albin teaches includes mango chili shrimp salad with lime vinaigrette, eggplant sliders, yellow vegetable curry, and strawberry banana chia pudding.
“If you figure out how to do it for your own busy, everyday life, you are now empowered to tell someone else about it,” she said.
A dietitian’s involvement
Milette Siler, RD, LD, CCMS, works with Dr. Albin to educate medical students and patients about food as medicine. A significant chunk of her job involves teaching future doctors what dietitians do.
When the class starts, many students don’t know two of the five basic things dietitians do, Ms. Siler said. By the end of the class, all students know what a dietitian does.
That’s important as students go on to become doctors.
“For us to remove barriers to care, we have to acknowledge most patients’ entry into health care is their physician,” she said. “The dietitian is often a referral. Doctors need to know enough to do no harm.”
Clinicians are often siloed, she said, and the key to better serving patients is partnership, transparency, and relationships. “I think everybody is at a point where everyone is saying what we’re doing isn’t working,” she said. “The American public deserves better, physicians deserve better, and clinicians deserve better.”
Popular with students
While the old guard has been slow to embrace the shift, her students have helped drive the growth of the culinary medicine field, Dr. Albin said.
“They are not settling for the inadequacy that somehow the rest of us did,” she continued. “I’m so hopeful for the future of the health system. We have a generation of people who will not stand for neglecting the most vital elements.”

Lyndon Bui, a second-year medical student at the University of Arizona, Phoenix, is an example of one of these people.
As a member of a culinary medicine interest group on campus, he said, he has learned a lot about the importance of diet for long-term health. This has given him confidence to talk about food and nutrition.
His group does cooking demos at the Phoenix Farmers Market using food from various local vendors. They usually make a salad from local greens and cook seasonal veggies in a stir fry, he said.
They’ve previously made salad with microgreens – young seedlings of edible vegetables and herbs – and pomegranate seeds with a honey mustard vinaigrette, eggplant or cucumber, and hummus on pita bread, as well as almond butter and honey sandwiches, according to the university.
The group also talks with people in the community, answers questions, and learns about community needs.
Mr. Bui’s participation in this group has helped him cultivate a passion for community outreach that he wants to incorporate into his career.
“I feel like I have the knowledge to provide better advice to patients,” he said. “Knowing all these things about food, I feel more comfortable talking about it and more inclined to refer to a dietitian when maybe I wouldn’t have before.”
Family physician applauds culinary medicine programs
When Angie Neison, MD, CCMS, went to medical school, she was surprised there wasn’t more education on nutrition.
In fact, on average, physicians receive less than 20 hours of nutrition education, according to the University of Arizona.
Now 15 years into her career as a family physician, Dr. Neison says nutrition is a huge part of her practice. She spends time working to bust myths about nutrition for her patients – including that healthy food is boring and bland, that making it is time consuming, and that healthy food is expensive. She also spends time teaching aspects of culinary medicine to her colleagues – many of whom are well into their careers – so they can better serve their patients.
It’s worth it to spend time learning about nutrition, she said, whether that’s as a medical student in a culinary medicine program or a practicing physician taking additional courses.
Nutrition education in medical school hasn’t been a priority, she said, maybe because there is so much to learn, or maybe because there is no money to be made in prevention.
“If doctors learn it, they are able to better guide patients,” she said.
Correction, 11/29/22: An earlier version of this article misstated Dr. Albin's institution.
The way he sees it, the stakes couldn’t be higher. He believes doctors need to see food as medicine to be able to stem the tide of chronic disease.
About 6 in 10 adults in the United States live with chronic diseases, according to the Centers for Disease Control and Prevention, costing $4.1 trillion in annual health care costs. Adult obesity rates are rising, as are obesity-related conditions such as heart disease, stroke, type 2 diabetes, and certain types of cancer.
To turn the tide, Dr. Marvasti created a culinary medicine program in 2020 in collaboration with the University of Arizona Cooperative Extension and local chefs.
Dr. Marvasti, who is board certified in family medicine, graduated from the University of Arizona, Phoenix, where he serves as the director of the medical school’s Culinary Medicine Program.
The program offers an elective course for third- and fourth-year medical students, which introduces the evidence-based field of culinary medicine. Dr Marvasti’s goal is for the course to teach students how to use this science and the joy of cooking to improve long-term health outcomes for their patients.
As part of Dr. Marvasti’s program, students learn cooking fundamentals through chef demonstrations and hands-on practice – to teach students how food can be used to prevent and treat many chronic diseases.
One of the dishes students learn to make includes a quinoa salad made with cucumber, onion, bell peppers, corn, cherry tomatoes, beans, garlic, olive oil, and lemon juice. Another recipe includes a healthier take on dessert: Dark chocolate mousse made with three large, ripe avocados, dark chocolate powder, three tablespoons of agave or maple, coconut cream, nondairy milk, salt, and vanilla. Dr. Marvasti and his team are set to build out the existing program to develop additional resources for medically underserved and rural communities in Arizona, according to a statement from the university. These plans will be funded by a $750,000 grant from Novo Nordisk.
“We’re going to develop an open education curriculum to share, so it’s open access to everyone,” said Dr. Marvasti, who is also director of Public Health, Prevention and Health Promotion and an associate professor at the university. “It can be adaptable at the undergraduate, graduate, and postgraduate level.”
Dr. Marvasti and his colleagues at the University of Arizona aren’t alone. In fact, culinary medicine programs are sprouting some serious legs.
Culinary medicine programs catch on
Jaclyn Albin, MD, CCMS, an associate professor in the departments of internal medicine and pediatrics at UT Southwestern Medical Center, Dallas, conducted a scoping review of the literature on culinary medicine programs for medical students.* Her purpose was to learn how the programs were structured and how they assessed student knowledge and attitudes regarding nutrition counseling for patients.
Dr. Albin and her colleagues performed an initial literature search between June 1 and Aug. 1, 2020, of papers published between Jan. 1, 2012, and Aug. 1, 2020 – excluding some newer programs such as the one at the University of Arizona. The results of their research were published in Academic Medicine.
Ultimately, the authors identified and examined 34 programs offering medical student–focused culinary medicine courses.
Program instructors typically included a team of physicians, dietitians, chefs, and other professionals, the study found.
Most program participants exclusively taught medical students, though the training years of participants varied among programs, and they included first-, second-, third-, and fourth-year students. Some programs allowed students from outside their respective medical school to participate in the trainings.
As for the formats of the program, most included cohorts of 10-20 students attending multiple 2- to 3-hour sessions over the course of several months. The University of Alabama at Birmingham offers one of the longest courses, which spans 4-5 months, according to the paper. In contrast, the University of Rochester (N.Y.) program offers only a 1-day lab divided into four sessions, with each session lasting about 2 hours.
The culinary medicine programs’ course sessions tended to include a 10- to 30-minute didactic session involving videos, research articles, culinary theories, and other lectures, a 60- to 90-minute hands-on cooking session, and a 30-minute discussion around nutrition, culture, and patient care.
Most programs used pre- and post-program surveys to evaluate outcomes, though results varied between programs, according to the study. While each program evaluation had different metrics, the surveys generally revealed students felt more confident discussing dietary interventions with patients and in their own cooking skills following completion.
Course correction
Most of those programs are unfunded or minimally funded, Dr. Albin said.
Her own program, which is immensely popular with medical students, is one she teaches on a volunteer basis.
“I do this for free, in the evenings, because I believe in it,” she said.
Medical school education real estate is limited, so convincing medical schools to add something to the curriculum is difficult, Dr. Albin noted.
But it’s worth it, she said, because nutrition is the underpinning of so many diseases.
“Food is the top risk factor for early death in the U.S.,” Dr. Albin said. “I like to say that five times in a row. People have not digested it.”
During her culinary medicine courses, she also asks her medical students: “Who is comfortable in the kitchen?” Some sheepishly raise their hands, she said. Some don’t. Many don’t know anything about cooking.
Then she teaches students about healthy food and how to make it. As part of her program, medical students are given a pantry starter kit with olive oil and a variety of spices to take home and use.
Some recipes Dr. Albin teaches includes mango chili shrimp salad with lime vinaigrette, eggplant sliders, yellow vegetable curry, and strawberry banana chia pudding.
“If you figure out how to do it for your own busy, everyday life, you are now empowered to tell someone else about it,” she said.
A dietitian’s involvement
Milette Siler, RD, LD, CCMS, works with Dr. Albin to educate medical students and patients about food as medicine. A significant chunk of her job involves teaching future doctors what dietitians do.
When the class starts, many students don’t know two of the five basic things dietitians do, Ms. Siler said. By the end of the class, all students know what a dietitian does.
That’s important as students go on to become doctors.
“For us to remove barriers to care, we have to acknowledge most patients’ entry into health care is their physician,” she said. “The dietitian is often a referral. Doctors need to know enough to do no harm.”
Clinicians are often siloed, she said, and the key to better serving patients is partnership, transparency, and relationships. “I think everybody is at a point where everyone is saying what we’re doing isn’t working,” she said. “The American public deserves better, physicians deserve better, and clinicians deserve better.”
Popular with students
While the old guard has been slow to embrace the shift, her students have helped drive the growth of the culinary medicine field, Dr. Albin said.
“They are not settling for the inadequacy that somehow the rest of us did,” she continued. “I’m so hopeful for the future of the health system. We have a generation of people who will not stand for neglecting the most vital elements.”

Lyndon Bui, a second-year medical student at the University of Arizona, Phoenix, is an example of one of these people.
As a member of a culinary medicine interest group on campus, he said, he has learned a lot about the importance of diet for long-term health. This has given him confidence to talk about food and nutrition.
His group does cooking demos at the Phoenix Farmers Market using food from various local vendors. They usually make a salad from local greens and cook seasonal veggies in a stir fry, he said.
They’ve previously made salad with microgreens – young seedlings of edible vegetables and herbs – and pomegranate seeds with a honey mustard vinaigrette, eggplant or cucumber, and hummus on pita bread, as well as almond butter and honey sandwiches, according to the university.
The group also talks with people in the community, answers questions, and learns about community needs.
Mr. Bui’s participation in this group has helped him cultivate a passion for community outreach that he wants to incorporate into his career.
“I feel like I have the knowledge to provide better advice to patients,” he said. “Knowing all these things about food, I feel more comfortable talking about it and more inclined to refer to a dietitian when maybe I wouldn’t have before.”
Family physician applauds culinary medicine programs
When Angie Neison, MD, CCMS, went to medical school, she was surprised there wasn’t more education on nutrition.
In fact, on average, physicians receive less than 20 hours of nutrition education, according to the University of Arizona.
Now 15 years into her career as a family physician, Dr. Neison says nutrition is a huge part of her practice. She spends time working to bust myths about nutrition for her patients – including that healthy food is boring and bland, that making it is time consuming, and that healthy food is expensive. She also spends time teaching aspects of culinary medicine to her colleagues – many of whom are well into their careers – so they can better serve their patients.
It’s worth it to spend time learning about nutrition, she said, whether that’s as a medical student in a culinary medicine program or a practicing physician taking additional courses.
Nutrition education in medical school hasn’t been a priority, she said, maybe because there is so much to learn, or maybe because there is no money to be made in prevention.
“If doctors learn it, they are able to better guide patients,” she said.
Correction, 11/29/22: An earlier version of this article misstated Dr. Albin's institution.
The way he sees it, the stakes couldn’t be higher. He believes doctors need to see food as medicine to be able to stem the tide of chronic disease.
About 6 in 10 adults in the United States live with chronic diseases, according to the Centers for Disease Control and Prevention, costing $4.1 trillion in annual health care costs. Adult obesity rates are rising, as are obesity-related conditions such as heart disease, stroke, type 2 diabetes, and certain types of cancer.
To turn the tide, Dr. Marvasti created a culinary medicine program in 2020 in collaboration with the University of Arizona Cooperative Extension and local chefs.
Dr. Marvasti, who is board certified in family medicine, graduated from the University of Arizona, Phoenix, where he serves as the director of the medical school’s Culinary Medicine Program.
The program offers an elective course for third- and fourth-year medical students, which introduces the evidence-based field of culinary medicine. Dr Marvasti’s goal is for the course to teach students how to use this science and the joy of cooking to improve long-term health outcomes for their patients.
As part of Dr. Marvasti’s program, students learn cooking fundamentals through chef demonstrations and hands-on practice – to teach students how food can be used to prevent and treat many chronic diseases.
One of the dishes students learn to make includes a quinoa salad made with cucumber, onion, bell peppers, corn, cherry tomatoes, beans, garlic, olive oil, and lemon juice. Another recipe includes a healthier take on dessert: Dark chocolate mousse made with three large, ripe avocados, dark chocolate powder, three tablespoons of agave or maple, coconut cream, nondairy milk, salt, and vanilla. Dr. Marvasti and his team are set to build out the existing program to develop additional resources for medically underserved and rural communities in Arizona, according to a statement from the university. These plans will be funded by a $750,000 grant from Novo Nordisk.
“We’re going to develop an open education curriculum to share, so it’s open access to everyone,” said Dr. Marvasti, who is also director of Public Health, Prevention and Health Promotion and an associate professor at the university. “It can be adaptable at the undergraduate, graduate, and postgraduate level.”
Dr. Marvasti and his colleagues at the University of Arizona aren’t alone. In fact, culinary medicine programs are sprouting some serious legs.
Culinary medicine programs catch on
Jaclyn Albin, MD, CCMS, an associate professor in the departments of internal medicine and pediatrics at UT Southwestern Medical Center, Dallas, conducted a scoping review of the literature on culinary medicine programs for medical students.* Her purpose was to learn how the programs were structured and how they assessed student knowledge and attitudes regarding nutrition counseling for patients.
Dr. Albin and her colleagues performed an initial literature search between June 1 and Aug. 1, 2020, of papers published between Jan. 1, 2012, and Aug. 1, 2020 – excluding some newer programs such as the one at the University of Arizona. The results of their research were published in Academic Medicine.
Ultimately, the authors identified and examined 34 programs offering medical student–focused culinary medicine courses.
Program instructors typically included a team of physicians, dietitians, chefs, and other professionals, the study found.
Most program participants exclusively taught medical students, though the training years of participants varied among programs, and they included first-, second-, third-, and fourth-year students. Some programs allowed students from outside their respective medical school to participate in the trainings.
As for the formats of the program, most included cohorts of 10-20 students attending multiple 2- to 3-hour sessions over the course of several months. The University of Alabama at Birmingham offers one of the longest courses, which spans 4-5 months, according to the paper. In contrast, the University of Rochester (N.Y.) program offers only a 1-day lab divided into four sessions, with each session lasting about 2 hours.
The culinary medicine programs’ course sessions tended to include a 10- to 30-minute didactic session involving videos, research articles, culinary theories, and other lectures, a 60- to 90-minute hands-on cooking session, and a 30-minute discussion around nutrition, culture, and patient care.
Most programs used pre- and post-program surveys to evaluate outcomes, though results varied between programs, according to the study. While each program evaluation had different metrics, the surveys generally revealed students felt more confident discussing dietary interventions with patients and in their own cooking skills following completion.
Course correction
Most of those programs are unfunded or minimally funded, Dr. Albin said.
Her own program, which is immensely popular with medical students, is one she teaches on a volunteer basis.
“I do this for free, in the evenings, because I believe in it,” she said.
Medical school education real estate is limited, so convincing medical schools to add something to the curriculum is difficult, Dr. Albin noted.
But it’s worth it, she said, because nutrition is the underpinning of so many diseases.
“Food is the top risk factor for early death in the U.S.,” Dr. Albin said. “I like to say that five times in a row. People have not digested it.”
During her culinary medicine courses, she also asks her medical students: “Who is comfortable in the kitchen?” Some sheepishly raise their hands, she said. Some don’t. Many don’t know anything about cooking.
Then she teaches students about healthy food and how to make it. As part of her program, medical students are given a pantry starter kit with olive oil and a variety of spices to take home and use.
Some recipes Dr. Albin teaches includes mango chili shrimp salad with lime vinaigrette, eggplant sliders, yellow vegetable curry, and strawberry banana chia pudding.
“If you figure out how to do it for your own busy, everyday life, you are now empowered to tell someone else about it,” she said.
A dietitian’s involvement
Milette Siler, RD, LD, CCMS, works with Dr. Albin to educate medical students and patients about food as medicine. A significant chunk of her job involves teaching future doctors what dietitians do.
When the class starts, many students don’t know two of the five basic things dietitians do, Ms. Siler said. By the end of the class, all students know what a dietitian does.
That’s important as students go on to become doctors.
“For us to remove barriers to care, we have to acknowledge most patients’ entry into health care is their physician,” she said. “The dietitian is often a referral. Doctors need to know enough to do no harm.”
Clinicians are often siloed, she said, and the key to better serving patients is partnership, transparency, and relationships. “I think everybody is at a point where everyone is saying what we’re doing isn’t working,” she said. “The American public deserves better, physicians deserve better, and clinicians deserve better.”
Popular with students
While the old guard has been slow to embrace the shift, her students have helped drive the growth of the culinary medicine field, Dr. Albin said.
“They are not settling for the inadequacy that somehow the rest of us did,” she continued. “I’m so hopeful for the future of the health system. We have a generation of people who will not stand for neglecting the most vital elements.”

Lyndon Bui, a second-year medical student at the University of Arizona, Phoenix, is an example of one of these people.
As a member of a culinary medicine interest group on campus, he said, he has learned a lot about the importance of diet for long-term health. This has given him confidence to talk about food and nutrition.
His group does cooking demos at the Phoenix Farmers Market using food from various local vendors. They usually make a salad from local greens and cook seasonal veggies in a stir fry, he said.
They’ve previously made salad with microgreens – young seedlings of edible vegetables and herbs – and pomegranate seeds with a honey mustard vinaigrette, eggplant or cucumber, and hummus on pita bread, as well as almond butter and honey sandwiches, according to the university.
The group also talks with people in the community, answers questions, and learns about community needs.
Mr. Bui’s participation in this group has helped him cultivate a passion for community outreach that he wants to incorporate into his career.
“I feel like I have the knowledge to provide better advice to patients,” he said. “Knowing all these things about food, I feel more comfortable talking about it and more inclined to refer to a dietitian when maybe I wouldn’t have before.”
Family physician applauds culinary medicine programs
When Angie Neison, MD, CCMS, went to medical school, she was surprised there wasn’t more education on nutrition.
In fact, on average, physicians receive less than 20 hours of nutrition education, according to the University of Arizona.
Now 15 years into her career as a family physician, Dr. Neison says nutrition is a huge part of her practice. She spends time working to bust myths about nutrition for her patients – including that healthy food is boring and bland, that making it is time consuming, and that healthy food is expensive. She also spends time teaching aspects of culinary medicine to her colleagues – many of whom are well into their careers – so they can better serve their patients.
It’s worth it to spend time learning about nutrition, she said, whether that’s as a medical student in a culinary medicine program or a practicing physician taking additional courses.
Nutrition education in medical school hasn’t been a priority, she said, maybe because there is so much to learn, or maybe because there is no money to be made in prevention.
“If doctors learn it, they are able to better guide patients,” she said.
Correction, 11/29/22: An earlier version of this article misstated Dr. Albin's institution.
FROM ACADEMIC MEDICINE
Baxdrostat slashes BP in resistant hypertension: BrigHTN
CHICAGO – An investigational aldosterone synthase inhibitor could be an effective new treatment to reduce blood pressure in patients with treatment-resistant hypertension, reslts of a phase 2 study suggest.
The BrigHTN trial showed systolic blood pressure fell by an average of 20.3 mm Hg, 17.5 mm Hg, and 12.1 mm Hg with baxdrostat 2 mg, 1 mg, and 0.5 mg after 12 weeks follow-up in 248 patients unable to achieve target blood pressure on stable doses of at least three antihypertensive agents, including a diuretic.
After adjustment for the –9.4 mm Hg change observed in the placebo group, there was a statistically significant difference of 11.0 mm Hg in the 2-mg baxdrostat group (P = .0001) and of 8.1 mm Hg in the 1-mg baxdrostat group (P = .003).
The adjusted change in diastolic blood pressure was significant only for the 2-mg dose (–5.2 mm Hg; P = .004).
Once-daily oral baxdrostat had an acceptable side-effect profile and no patients died.
The study, which was stopped early after meeting criteria for overwhelming efficacy, was presented in the final late-breaking science session at the American Heart Association scientific sessions and published simultaneously in the New England Journal of Medicine.
Threading the needle
For at least 20 years, researchers have tried to create a drug that would lower aldosterone levels directly by inhibiting hormone synthesis rather than blocking the mineralocorticoid receptor.
What’s made this extraordinarily difficult is that the enzyme that makes aldosterone synthase and the enzyme required for cortisol synthase, 11-beta-hydroxylase, are 93% sequence similar. Baxdrostat, however, is able to selectively block aldosterone synthase, and thus the production of aldosterone, without also blocking the production of cortisol, explained Mason W. Freeman, MD, lead author of the study and executive vice president of clinical development at CinCor Pharma, which is developing the agent.
“We have beautiful biomarker evidence of not only blood pressure lowering but the mechanism by which that blood pressure reduction is occurring,” he said.
Over 12 weeks of follow-up in the new study, the use of baxdrostat led to decreases in serum aldosterone levels ranging from 3.0 ng/dL with the 0.5-mg dose to 4.9 ng/dL with the 2-mg dose. The 24-hour urinary aldosterone levels decreased with all three doses tested.
Baxdrostat increased plasma renin activity by 3.6, 5.0, and 13.8 mg/mL per hr with the 0.5, 1.0, and 2.0 mg doses, respectively, an indicator of its effect on lowering salt and fluid retention, Dr. Freeman said. Serum cortisol levels were not reduced in any of the baxdrostat groups throughout the study.
‘A bright future’
“It seems to have a bright future in the area of resistant hypertension, particularly in patients who are producing too much aldosterone,” said Suzanne Oparil, MD, invited discussant for the study and director of the Vascular Biology and Hypertension program at the University of Alabama at Birmingham.
She noted that aldosterone is a major contributor to the pathogenesis of resistant hypertension, which afflicts about 20% of the hypertensive population. Aldosterone antagonists are considered by many to be the best add-on treatment for resistant hypertension and do lower blood pressure.
“But they have major problems,” Dr. Oparil added. “Spironolactone, for example, causes hyperkalemia in many patients and adverse effects such as gynecomastia, erectile dysfunction, and feminization.”
Baxdrostat was well tolerated with no serious adverse events deemed related to treatment, Dr. Freeman reported. A total of 18 serious adverse events occurred in 10 patients, 6 of which were in a patient with urosepsis.
Ten adverse events of special interest occurred in eight patients, including one case of hypotension, three cases of hyponatremia, and six cases of hyperkalemia.
Potassium levels ranged from 6.0 to 6.3 mmol/L (6.0-6.3 mEq/L) in three patients and between 5.5 and 5.9 mmol/L (5.5-5.9 mEq/L) on at least two consecutive occasions in three others. Four of the patients were able to resume baxdrostat and complete the trial, whereas two patients discontinued treatment, one of whom was the patient with urosepsis.
Dr. Freeman pointed out that the study population was relatively diverse, with 33%-48% of participants of Hispanic or Latinx ethnicity and 23%-32% being Black.
At baseline, all patients had a seated blood pressure of at least 130/80 mm Hg (average 147.8/87.9 mm Hg) on a background therapy that included a diuretic in 100%, an agent targeting the renin-angiotensin-aldosterone system in 91%-96%, a beta-blocker in 52%-68%, and a calcium channel blocker in 64%-70%.
The study was not designed to test the benefits and risks of aldosterone synthase inhibition beyond 12 weeks and baxdrostat was not compared to alternative antihypertensives, he said. Additional limitations are that medication adherence was based on pill counts rather than drug analysis and enrolling only patients with an estimated glomerular filtration rate over 45 mL/min per 1.73m2 reduced the likelihood of hyperkalemia and other adverse events.
Nevertheless, “we think that these data suggest that baxdrostat has the potential to treat disorders associated with aldosterone excess, including hypertension and primary hyperaldosteronism,” Dr. Freeman said.
The intention is to carry the drug forward into additional phase 2 studies in chronic kidney disease and to begin a phase 3 study in hypertension in 2023, he noted.
The study was funded by CinCor Pharma. Dr. Freeman and three coauthors are employees of CinCor and receive stock-based compensation. The remaining authors have a financial relationship with CinRx Pharma, which has an equity stake in CinCor. Dr. Oparil reports grant/research support from Bayer, Higi, and Novartis; and serving on the scientific advisory board/expert committee for CinCor Pharma and Preventric Diagnostics.
A version of this article first appeared on Medscape.com.
CHICAGO – An investigational aldosterone synthase inhibitor could be an effective new treatment to reduce blood pressure in patients with treatment-resistant hypertension, reslts of a phase 2 study suggest.
The BrigHTN trial showed systolic blood pressure fell by an average of 20.3 mm Hg, 17.5 mm Hg, and 12.1 mm Hg with baxdrostat 2 mg, 1 mg, and 0.5 mg after 12 weeks follow-up in 248 patients unable to achieve target blood pressure on stable doses of at least three antihypertensive agents, including a diuretic.
After adjustment for the –9.4 mm Hg change observed in the placebo group, there was a statistically significant difference of 11.0 mm Hg in the 2-mg baxdrostat group (P = .0001) and of 8.1 mm Hg in the 1-mg baxdrostat group (P = .003).
The adjusted change in diastolic blood pressure was significant only for the 2-mg dose (–5.2 mm Hg; P = .004).
Once-daily oral baxdrostat had an acceptable side-effect profile and no patients died.
The study, which was stopped early after meeting criteria for overwhelming efficacy, was presented in the final late-breaking science session at the American Heart Association scientific sessions and published simultaneously in the New England Journal of Medicine.
Threading the needle
For at least 20 years, researchers have tried to create a drug that would lower aldosterone levels directly by inhibiting hormone synthesis rather than blocking the mineralocorticoid receptor.
What’s made this extraordinarily difficult is that the enzyme that makes aldosterone synthase and the enzyme required for cortisol synthase, 11-beta-hydroxylase, are 93% sequence similar. Baxdrostat, however, is able to selectively block aldosterone synthase, and thus the production of aldosterone, without also blocking the production of cortisol, explained Mason W. Freeman, MD, lead author of the study and executive vice president of clinical development at CinCor Pharma, which is developing the agent.
“We have beautiful biomarker evidence of not only blood pressure lowering but the mechanism by which that blood pressure reduction is occurring,” he said.
Over 12 weeks of follow-up in the new study, the use of baxdrostat led to decreases in serum aldosterone levels ranging from 3.0 ng/dL with the 0.5-mg dose to 4.9 ng/dL with the 2-mg dose. The 24-hour urinary aldosterone levels decreased with all three doses tested.
Baxdrostat increased plasma renin activity by 3.6, 5.0, and 13.8 mg/mL per hr with the 0.5, 1.0, and 2.0 mg doses, respectively, an indicator of its effect on lowering salt and fluid retention, Dr. Freeman said. Serum cortisol levels were not reduced in any of the baxdrostat groups throughout the study.
‘A bright future’
“It seems to have a bright future in the area of resistant hypertension, particularly in patients who are producing too much aldosterone,” said Suzanne Oparil, MD, invited discussant for the study and director of the Vascular Biology and Hypertension program at the University of Alabama at Birmingham.
She noted that aldosterone is a major contributor to the pathogenesis of resistant hypertension, which afflicts about 20% of the hypertensive population. Aldosterone antagonists are considered by many to be the best add-on treatment for resistant hypertension and do lower blood pressure.
“But they have major problems,” Dr. Oparil added. “Spironolactone, for example, causes hyperkalemia in many patients and adverse effects such as gynecomastia, erectile dysfunction, and feminization.”
Baxdrostat was well tolerated with no serious adverse events deemed related to treatment, Dr. Freeman reported. A total of 18 serious adverse events occurred in 10 patients, 6 of which were in a patient with urosepsis.
Ten adverse events of special interest occurred in eight patients, including one case of hypotension, three cases of hyponatremia, and six cases of hyperkalemia.
Potassium levels ranged from 6.0 to 6.3 mmol/L (6.0-6.3 mEq/L) in three patients and between 5.5 and 5.9 mmol/L (5.5-5.9 mEq/L) on at least two consecutive occasions in three others. Four of the patients were able to resume baxdrostat and complete the trial, whereas two patients discontinued treatment, one of whom was the patient with urosepsis.
Dr. Freeman pointed out that the study population was relatively diverse, with 33%-48% of participants of Hispanic or Latinx ethnicity and 23%-32% being Black.
At baseline, all patients had a seated blood pressure of at least 130/80 mm Hg (average 147.8/87.9 mm Hg) on a background therapy that included a diuretic in 100%, an agent targeting the renin-angiotensin-aldosterone system in 91%-96%, a beta-blocker in 52%-68%, and a calcium channel blocker in 64%-70%.
The study was not designed to test the benefits and risks of aldosterone synthase inhibition beyond 12 weeks and baxdrostat was not compared to alternative antihypertensives, he said. Additional limitations are that medication adherence was based on pill counts rather than drug analysis and enrolling only patients with an estimated glomerular filtration rate over 45 mL/min per 1.73m2 reduced the likelihood of hyperkalemia and other adverse events.
Nevertheless, “we think that these data suggest that baxdrostat has the potential to treat disorders associated with aldosterone excess, including hypertension and primary hyperaldosteronism,” Dr. Freeman said.
The intention is to carry the drug forward into additional phase 2 studies in chronic kidney disease and to begin a phase 3 study in hypertension in 2023, he noted.
The study was funded by CinCor Pharma. Dr. Freeman and three coauthors are employees of CinCor and receive stock-based compensation. The remaining authors have a financial relationship with CinRx Pharma, which has an equity stake in CinCor. Dr. Oparil reports grant/research support from Bayer, Higi, and Novartis; and serving on the scientific advisory board/expert committee for CinCor Pharma and Preventric Diagnostics.
A version of this article first appeared on Medscape.com.
CHICAGO – An investigational aldosterone synthase inhibitor could be an effective new treatment to reduce blood pressure in patients with treatment-resistant hypertension, reslts of a phase 2 study suggest.
The BrigHTN trial showed systolic blood pressure fell by an average of 20.3 mm Hg, 17.5 mm Hg, and 12.1 mm Hg with baxdrostat 2 mg, 1 mg, and 0.5 mg after 12 weeks follow-up in 248 patients unable to achieve target blood pressure on stable doses of at least three antihypertensive agents, including a diuretic.
After adjustment for the –9.4 mm Hg change observed in the placebo group, there was a statistically significant difference of 11.0 mm Hg in the 2-mg baxdrostat group (P = .0001) and of 8.1 mm Hg in the 1-mg baxdrostat group (P = .003).
The adjusted change in diastolic blood pressure was significant only for the 2-mg dose (–5.2 mm Hg; P = .004).
Once-daily oral baxdrostat had an acceptable side-effect profile and no patients died.
The study, which was stopped early after meeting criteria for overwhelming efficacy, was presented in the final late-breaking science session at the American Heart Association scientific sessions and published simultaneously in the New England Journal of Medicine.
Threading the needle
For at least 20 years, researchers have tried to create a drug that would lower aldosterone levels directly by inhibiting hormone synthesis rather than blocking the mineralocorticoid receptor.
What’s made this extraordinarily difficult is that the enzyme that makes aldosterone synthase and the enzyme required for cortisol synthase, 11-beta-hydroxylase, are 93% sequence similar. Baxdrostat, however, is able to selectively block aldosterone synthase, and thus the production of aldosterone, without also blocking the production of cortisol, explained Mason W. Freeman, MD, lead author of the study and executive vice president of clinical development at CinCor Pharma, which is developing the agent.
“We have beautiful biomarker evidence of not only blood pressure lowering but the mechanism by which that blood pressure reduction is occurring,” he said.
Over 12 weeks of follow-up in the new study, the use of baxdrostat led to decreases in serum aldosterone levels ranging from 3.0 ng/dL with the 0.5-mg dose to 4.9 ng/dL with the 2-mg dose. The 24-hour urinary aldosterone levels decreased with all three doses tested.
Baxdrostat increased plasma renin activity by 3.6, 5.0, and 13.8 mg/mL per hr with the 0.5, 1.0, and 2.0 mg doses, respectively, an indicator of its effect on lowering salt and fluid retention, Dr. Freeman said. Serum cortisol levels were not reduced in any of the baxdrostat groups throughout the study.
‘A bright future’
“It seems to have a bright future in the area of resistant hypertension, particularly in patients who are producing too much aldosterone,” said Suzanne Oparil, MD, invited discussant for the study and director of the Vascular Biology and Hypertension program at the University of Alabama at Birmingham.
She noted that aldosterone is a major contributor to the pathogenesis of resistant hypertension, which afflicts about 20% of the hypertensive population. Aldosterone antagonists are considered by many to be the best add-on treatment for resistant hypertension and do lower blood pressure.
“But they have major problems,” Dr. Oparil added. “Spironolactone, for example, causes hyperkalemia in many patients and adverse effects such as gynecomastia, erectile dysfunction, and feminization.”
Baxdrostat was well tolerated with no serious adverse events deemed related to treatment, Dr. Freeman reported. A total of 18 serious adverse events occurred in 10 patients, 6 of which were in a patient with urosepsis.
Ten adverse events of special interest occurred in eight patients, including one case of hypotension, three cases of hyponatremia, and six cases of hyperkalemia.
Potassium levels ranged from 6.0 to 6.3 mmol/L (6.0-6.3 mEq/L) in three patients and between 5.5 and 5.9 mmol/L (5.5-5.9 mEq/L) on at least two consecutive occasions in three others. Four of the patients were able to resume baxdrostat and complete the trial, whereas two patients discontinued treatment, one of whom was the patient with urosepsis.
Dr. Freeman pointed out that the study population was relatively diverse, with 33%-48% of participants of Hispanic or Latinx ethnicity and 23%-32% being Black.
At baseline, all patients had a seated blood pressure of at least 130/80 mm Hg (average 147.8/87.9 mm Hg) on a background therapy that included a diuretic in 100%, an agent targeting the renin-angiotensin-aldosterone system in 91%-96%, a beta-blocker in 52%-68%, and a calcium channel blocker in 64%-70%.
The study was not designed to test the benefits and risks of aldosterone synthase inhibition beyond 12 weeks and baxdrostat was not compared to alternative antihypertensives, he said. Additional limitations are that medication adherence was based on pill counts rather than drug analysis and enrolling only patients with an estimated glomerular filtration rate over 45 mL/min per 1.73m2 reduced the likelihood of hyperkalemia and other adverse events.
Nevertheless, “we think that these data suggest that baxdrostat has the potential to treat disorders associated with aldosterone excess, including hypertension and primary hyperaldosteronism,” Dr. Freeman said.
The intention is to carry the drug forward into additional phase 2 studies in chronic kidney disease and to begin a phase 3 study in hypertension in 2023, he noted.
The study was funded by CinCor Pharma. Dr. Freeman and three coauthors are employees of CinCor and receive stock-based compensation. The remaining authors have a financial relationship with CinRx Pharma, which has an equity stake in CinCor. Dr. Oparil reports grant/research support from Bayer, Higi, and Novartis; and serving on the scientific advisory board/expert committee for CinCor Pharma and Preventric Diagnostics.
A version of this article first appeared on Medscape.com.
AT AHA 2022
The Role of Revascularization and Viability Testing in Patients With Multivessel Coronary Artery Disease and Severely Reduced Ejection Fraction
Study 1 Overview (STICHES Investigators)
Objective: To assess the survival benefit of coronary-artery bypass grafting (CABG) added to guideline-directed medical therapy, compared to optimal medical therapy (OMT) alone, in patients with coronary artery disease, heart failure, and severe left ventricular dysfunction. Design: Multicenter, randomized, prospective study with extended follow-up (median duration of 9.8 years).
Setting and participants: A total of 1212 patients with left ventricular ejection fraction (LVEF) of 35% or less and coronary artery disease were randomized to medical therapy plus CABG or OMT alone at 127 clinical sites in 26 countries.
Main outcome measures: The primary endpoint was death from any cause. Main secondary endpoints were death from cardiovascular causes and a composite outcome of death from any cause or hospitalization for cardiovascular causes.
Main results: There were 359 primary outcome all-cause deaths (58.9%) in the CABG group and 398 (66.1%) in the medical therapy group (hazard ratio [HR], 0.84; 95% CI, 0.73-0.97; P = .02). Death from cardiovascular causes was reported in 247 patients (40.5%) in the CABG group and 297 patients (49.3%) in the medical therapy group (HR, 0.79; 95% CI, 0.66-0.93; P < .01). The composite outcome of death from any cause or hospitalization for cardiovascular causes occurred in 467 patients (76.6%) in the CABG group and 467 patients (87.0%) in the medical therapy group (HR, 0.72; 95% CI, 0.64-0.82; P < .01).
Conclusion: Over a median follow-up of 9.8 years in patients with ischemic cardiomyopathy with severely reduced ejection fraction, the rates of death from any cause, death from cardiovascular causes, and the composite of death from any cause or hospitalization for cardiovascular causes were significantly lower in patients undergoing CABG than in patients receiving medical therapy alone.
Study 2 Overview (REVIVED BCIS Trial Group)
Objective: To assess whether percutaneous coronary intervention (PCI) can improve survival and left ventricular function in patients with severe left ventricular systolic dysfunction as compared to OMT alone.
Design: Multicenter, randomized, prospective study.
Setting and participants: A total of 700 patients with LVEF <35% with severe coronary artery disease amendable to PCI and demonstrable myocardial viability were randomly assigned to either PCI plus optimal medical therapy (PCI group) or OMT alone (OMT group).
Main outcome measures: The primary outcome was death from any cause or hospitalization for heart failure. The main secondary outcomes were LVEF at 6 and 12 months and quality of life (QOL) scores.
Main results: Over a median follow-up of 41 months, the primary outcome was reported in 129 patients (37.2%) in the PCI group and in 134 patients (38.0%) in the OMT group (HR, 0.99; 95% CI, 0.78-1.27; P = .96). The LVEF was similar in the 2 groups at 6 months (mean difference, –1.6 percentage points; 95% CI, –3.7 to 0.5) and at 12 months (mean difference, 0.9 percentage points; 95% CI, –1.7 to 3.4). QOL scores at 6 and 12 months favored the PCI group, but the difference had diminished at 24 months.
Conclusion: In patients with severe ischemic cardiomyopathy, revascularization by PCI in addition to OMT did not result in a lower incidence of death from any cause or hospitalization from heart failure.
Commentary
Coronary artery disease is the most common cause of heart failure with reduced ejection fraction and an important cause of mortality.1 Patients with ischemic cardiomyopathy with reduced ejection fraction are often considered for revascularization in addition to OMT and device therapies. Although there have been multiple retrospective studies and registries suggesting that cardiac outcomes and LVEF improve with revascularization, the number of large-scale prospective studies that assessed this clinical question and randomized patients to revascularization plus OMT compared to OMT alone has been limited.
In the Surgical Treatment for Ischemic Heart Failure (STICH) study,2,3 eligible patients had coronary artery disease amendable to CABG and a LVEF of 35% or less. Patients (N = 1212) were randomly assigned to CABG plus OMT or OMT alone between July 2002 and May 2007. The original study, with a median follow-up of 5 years, did not show survival benefit, but the investigators reported that the primary outcome of death from any cause was significantly lower in the CABG group compared to OMT alone when follow-up of the same study population was extended to 9.8 years (58.9% vs 66.1%, P = .02). The findings from this study led to a class I guideline recommendation of CABG over medical therapy in patients with multivessel disease and low ejection fraction.4
Since the STICH trial was designed, there have been significant improvements in devices and techniques used for PCI, and the procedure is now widely performed in patients with multivessel disease.5 The advantages of PCI over CABG include shorter recovery times and lower risk of immediate complications. In this context, the recently reported Revascularization for Ischemic Ventricular Dysfunction (REVIVED) study assessed clinical outcomes in patients with severe coronary artery disease and reduced ejection fraction by randomizing patients to either PCI with OMT or OMT alone.6 At a median follow-up of 3.5 years, the investigators found no difference in the primary outcome of death from any cause or hospitalization for heart failure (37.2% vs 38.0%; 95% CI, 0.78-1.28; P = .96). Moreover, the degree of LVEF improvement, assessed by follow-up echocardiogram read by the core lab, showed no difference in the degree of LVEF improvement between groups at 6 and 12 months. Finally, although results of the QOL assessment using the Kansas City Cardiomyopathy Questionnaire (KCCQ), a validated, patient-reported, heart-failure-specific QOL scale, favored the PCI group at 6 and 12 months of follow-up, the difference had diminished at 24 months.
The main strength of the REVIVED study was that it targeted a patient population with severe coronary artery disease, including left main disease and severely reduced ejection fraction, that historically have been excluded from large-scale randomized controlled studies evaluating PCI with OMT compared to OMT alone.7 However, there are several points to consider when interpreting the results of this study. First, further details of the PCI procedures are necessary. The REVIVED study recommended revascularization of all territories with viable myocardium; the anatomical revascularization index utilizing the British Cardiovascular Intervention Society (BCIS) Jeopardy Score was 71%. It is important to note that this jeopardy score was operator-reported and the core-lab adjudicated anatomical revascularization rate may be lower. Although viability testing primarily utilizing cardiac magnetic resonance imaging was performed in most patients, correlation between the revascularization territory and the viable segments has yet to be reported. Moreover, procedural details such as use of intravascular ultrasound and physiological testing, known to improve clinical outcome, need to be reported.8,9
Second, there is a high prevalence of ischemic cardiomyopathy, and it is important to note that the patients included in this study were highly selected from daily clinical practice, as evidenced by the prolonged enrollment period (8 years). Individuals were largely stable patients with less complex coronary anatomy as evidenced by the median interval from angiography to randomization of 80 days. Taking into consideration the degree of left ventricular dysfunction for patients included in the trial, only 14% of the patients had left main disease and half of the patients only had 2-vessel disease. The severity of the left main disease also needs to be clarified as it is likely that patients the operator determined to be critical were not enrolled in the study. Furthermore, the standard of care based on the STICH trial is to refer patients with severe multivessel coronary artery disease to CABG, making it more likely that patients with more severe and complex disease were not included in this trial. It is also important to note that this study enrolled patients with stable ischemic heart disease, and the data do not apply to patients presenting with acute coronary syndrome.
Third, although the primary outcome was similar between the groups, the secondary outcome of unplanned revascularization was lower in the PCI group. In addition, the rate of acute myocardial infarction (MI) was similar between the 2 groups, but the rate of spontaneous MI was lower in the PCI group compared to the OMT group (5.2% vs 9.3%) as 40% of MI cases in the PCI group were periprocedural MIs. The correlation between periprocedural MI and long-term outcomes has been modest compared to spontaneous MI. Moreover, with the longer follow-up, the number of spontaneous MI cases is expected to rise while the number of periprocedural MI cases is not. Extending the follow-up period is also important, as the STICH extension trial showed a statistically significant difference at 10-year follow up despite negative results at the time of the original publication.
Fourth, the REVIVED trial randomized a significantly lower number of patients compared to the STICH trial, and the authors reported fewer primary-outcome events than the estimated number needed to achieve the power to assess the primary hypothesis. In addition, significant improvements in medical treatment for heart failure with reduced ejection fraction since the STICH trial make comparison of PCI vs CABG in this patient population unfeasible.
Finally, although severe angina was not an exclusion criterion, two-thirds of the patients enrolled had no angina, and only 2% of the patients had baseline severe angina. This is important to consider when interpreting the results of the patient-reported health status as previous studies have shown that patients with worse angina at baseline derive the largest improvement in their QOL,10,11 and symptom improvement is the main indication for PCI in patients with stable ischemic heart disease.
Applications for Clinical Practice and System Implementation
In patients with severe left ventricular systolic dysfunction and multivessel stable ischemic heart disease who are well compensated and have little or no angina at baseline, OMT alone as an initial strategy may be considered against the addition of PCI after careful risk and benefit discussion. Further details about revascularization and extended follow-up data from the REVIVED trial are necessary.
Practice Points
- Patients with ischemic cardiomyopathy with reduced ejection fraction have been an understudied population in previous studies.
- Further studies are necessary to understand the benefits of revascularization and the role of viability testing in this population.
– Taishi Hirai MD, and Ziad Sayed Ahmad, MD
University of Missouri, Columbia, MO
1. Nowbar AN, Gitto M, Howard JP, et al. Mortality from ischemic heart disease. Circ Cardiovasc Qual Outcomes. 2019;12(6):e005375. doi:10.1161/CIRCOUTCOMES
2. Velazquez EJ, Lee KL, Deja MA, et al; for the STICH Investigators. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364(17):1607-1616. doi:10.1056/NEJMoa1100356
3. Velazquez EJ, Lee KL, Jones RH, et al. Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med. 2016;374(16):1511-1520. doi:10.1056/NEJMoa1602001
4. Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(2):e21-e129. doi:10.1016/j.jacc.2021.09.006
5. Kirtane AJ, Doshi D, Leon MB, et al. Treatment of higher-risk patients with an indication for revascularization: evolution within the field of contemporary percutaneous coronary intervention. Circulation. 2016;134(5):422-431. doi:10.1161/CIRCULATIONAHA
6. Perera D, Clayton T, O’Kane PD, et al. Percutaneous revascularization for ischemic left ventricular dysfunction. N Engl J Med. 2022;387(15):1351-1360. doi:10.1056/NEJMoa2206606
7. Maron DJ, Hochman JS, Reynolds HR, et al. Initial invasive or conservative strategy for stable coronary disease. Circulation. 2020;142(18):1725-1735. doi:10.1161/CIRCULATIONAHA
8. De Bruyne B, Pijls NH, Kalesan B, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367(11):991-1001. doi:10.1056/NEJMoa1205361
9. Zhang J, Gao X, Kan J, et al. Intravascular ultrasound versus angiography-guided drug-eluting stent implantation: The ULTIMATE trial. J Am Coll Cardiol. 2018;72(24):3126-3137. doi:10.1016/j.jacc.2018.09.013
10. Spertus JA, Jones PG, Maron DJ, et al. Health-status outcomes with invasive or conservative care in coronary disease. N Engl J Med. 2020;382(15):1408-1419. doi:10.1056/NEJMoa1916370
11. Hirai T, Grantham JA, Sapontis J, et al. Quality of life changes after chronic total occlusion angioplasty in patients with baseline refractory angina. Circ Cardiovasc Interv. 2019;12:e007558. doi:10.1161/CIRCINTERVENTIONS.118.007558
Study 1 Overview (STICHES Investigators)
Objective: To assess the survival benefit of coronary-artery bypass grafting (CABG) added to guideline-directed medical therapy, compared to optimal medical therapy (OMT) alone, in patients with coronary artery disease, heart failure, and severe left ventricular dysfunction. Design: Multicenter, randomized, prospective study with extended follow-up (median duration of 9.8 years).
Setting and participants: A total of 1212 patients with left ventricular ejection fraction (LVEF) of 35% or less and coronary artery disease were randomized to medical therapy plus CABG or OMT alone at 127 clinical sites in 26 countries.
Main outcome measures: The primary endpoint was death from any cause. Main secondary endpoints were death from cardiovascular causes and a composite outcome of death from any cause or hospitalization for cardiovascular causes.
Main results: There were 359 primary outcome all-cause deaths (58.9%) in the CABG group and 398 (66.1%) in the medical therapy group (hazard ratio [HR], 0.84; 95% CI, 0.73-0.97; P = .02). Death from cardiovascular causes was reported in 247 patients (40.5%) in the CABG group and 297 patients (49.3%) in the medical therapy group (HR, 0.79; 95% CI, 0.66-0.93; P < .01). The composite outcome of death from any cause or hospitalization for cardiovascular causes occurred in 467 patients (76.6%) in the CABG group and 467 patients (87.0%) in the medical therapy group (HR, 0.72; 95% CI, 0.64-0.82; P < .01).
Conclusion: Over a median follow-up of 9.8 years in patients with ischemic cardiomyopathy with severely reduced ejection fraction, the rates of death from any cause, death from cardiovascular causes, and the composite of death from any cause or hospitalization for cardiovascular causes were significantly lower in patients undergoing CABG than in patients receiving medical therapy alone.
Study 2 Overview (REVIVED BCIS Trial Group)
Objective: To assess whether percutaneous coronary intervention (PCI) can improve survival and left ventricular function in patients with severe left ventricular systolic dysfunction as compared to OMT alone.
Design: Multicenter, randomized, prospective study.
Setting and participants: A total of 700 patients with LVEF <35% with severe coronary artery disease amendable to PCI and demonstrable myocardial viability were randomly assigned to either PCI plus optimal medical therapy (PCI group) or OMT alone (OMT group).
Main outcome measures: The primary outcome was death from any cause or hospitalization for heart failure. The main secondary outcomes were LVEF at 6 and 12 months and quality of life (QOL) scores.
Main results: Over a median follow-up of 41 months, the primary outcome was reported in 129 patients (37.2%) in the PCI group and in 134 patients (38.0%) in the OMT group (HR, 0.99; 95% CI, 0.78-1.27; P = .96). The LVEF was similar in the 2 groups at 6 months (mean difference, –1.6 percentage points; 95% CI, –3.7 to 0.5) and at 12 months (mean difference, 0.9 percentage points; 95% CI, –1.7 to 3.4). QOL scores at 6 and 12 months favored the PCI group, but the difference had diminished at 24 months.
Conclusion: In patients with severe ischemic cardiomyopathy, revascularization by PCI in addition to OMT did not result in a lower incidence of death from any cause or hospitalization from heart failure.
Commentary
Coronary artery disease is the most common cause of heart failure with reduced ejection fraction and an important cause of mortality.1 Patients with ischemic cardiomyopathy with reduced ejection fraction are often considered for revascularization in addition to OMT and device therapies. Although there have been multiple retrospective studies and registries suggesting that cardiac outcomes and LVEF improve with revascularization, the number of large-scale prospective studies that assessed this clinical question and randomized patients to revascularization plus OMT compared to OMT alone has been limited.
In the Surgical Treatment for Ischemic Heart Failure (STICH) study,2,3 eligible patients had coronary artery disease amendable to CABG and a LVEF of 35% or less. Patients (N = 1212) were randomly assigned to CABG plus OMT or OMT alone between July 2002 and May 2007. The original study, with a median follow-up of 5 years, did not show survival benefit, but the investigators reported that the primary outcome of death from any cause was significantly lower in the CABG group compared to OMT alone when follow-up of the same study population was extended to 9.8 years (58.9% vs 66.1%, P = .02). The findings from this study led to a class I guideline recommendation of CABG over medical therapy in patients with multivessel disease and low ejection fraction.4
Since the STICH trial was designed, there have been significant improvements in devices and techniques used for PCI, and the procedure is now widely performed in patients with multivessel disease.5 The advantages of PCI over CABG include shorter recovery times and lower risk of immediate complications. In this context, the recently reported Revascularization for Ischemic Ventricular Dysfunction (REVIVED) study assessed clinical outcomes in patients with severe coronary artery disease and reduced ejection fraction by randomizing patients to either PCI with OMT or OMT alone.6 At a median follow-up of 3.5 years, the investigators found no difference in the primary outcome of death from any cause or hospitalization for heart failure (37.2% vs 38.0%; 95% CI, 0.78-1.28; P = .96). Moreover, the degree of LVEF improvement, assessed by follow-up echocardiogram read by the core lab, showed no difference in the degree of LVEF improvement between groups at 6 and 12 months. Finally, although results of the QOL assessment using the Kansas City Cardiomyopathy Questionnaire (KCCQ), a validated, patient-reported, heart-failure-specific QOL scale, favored the PCI group at 6 and 12 months of follow-up, the difference had diminished at 24 months.
The main strength of the REVIVED study was that it targeted a patient population with severe coronary artery disease, including left main disease and severely reduced ejection fraction, that historically have been excluded from large-scale randomized controlled studies evaluating PCI with OMT compared to OMT alone.7 However, there are several points to consider when interpreting the results of this study. First, further details of the PCI procedures are necessary. The REVIVED study recommended revascularization of all territories with viable myocardium; the anatomical revascularization index utilizing the British Cardiovascular Intervention Society (BCIS) Jeopardy Score was 71%. It is important to note that this jeopardy score was operator-reported and the core-lab adjudicated anatomical revascularization rate may be lower. Although viability testing primarily utilizing cardiac magnetic resonance imaging was performed in most patients, correlation between the revascularization territory and the viable segments has yet to be reported. Moreover, procedural details such as use of intravascular ultrasound and physiological testing, known to improve clinical outcome, need to be reported.8,9
Second, there is a high prevalence of ischemic cardiomyopathy, and it is important to note that the patients included in this study were highly selected from daily clinical practice, as evidenced by the prolonged enrollment period (8 years). Individuals were largely stable patients with less complex coronary anatomy as evidenced by the median interval from angiography to randomization of 80 days. Taking into consideration the degree of left ventricular dysfunction for patients included in the trial, only 14% of the patients had left main disease and half of the patients only had 2-vessel disease. The severity of the left main disease also needs to be clarified as it is likely that patients the operator determined to be critical were not enrolled in the study. Furthermore, the standard of care based on the STICH trial is to refer patients with severe multivessel coronary artery disease to CABG, making it more likely that patients with more severe and complex disease were not included in this trial. It is also important to note that this study enrolled patients with stable ischemic heart disease, and the data do not apply to patients presenting with acute coronary syndrome.
Third, although the primary outcome was similar between the groups, the secondary outcome of unplanned revascularization was lower in the PCI group. In addition, the rate of acute myocardial infarction (MI) was similar between the 2 groups, but the rate of spontaneous MI was lower in the PCI group compared to the OMT group (5.2% vs 9.3%) as 40% of MI cases in the PCI group were periprocedural MIs. The correlation between periprocedural MI and long-term outcomes has been modest compared to spontaneous MI. Moreover, with the longer follow-up, the number of spontaneous MI cases is expected to rise while the number of periprocedural MI cases is not. Extending the follow-up period is also important, as the STICH extension trial showed a statistically significant difference at 10-year follow up despite negative results at the time of the original publication.
Fourth, the REVIVED trial randomized a significantly lower number of patients compared to the STICH trial, and the authors reported fewer primary-outcome events than the estimated number needed to achieve the power to assess the primary hypothesis. In addition, significant improvements in medical treatment for heart failure with reduced ejection fraction since the STICH trial make comparison of PCI vs CABG in this patient population unfeasible.
Finally, although severe angina was not an exclusion criterion, two-thirds of the patients enrolled had no angina, and only 2% of the patients had baseline severe angina. This is important to consider when interpreting the results of the patient-reported health status as previous studies have shown that patients with worse angina at baseline derive the largest improvement in their QOL,10,11 and symptom improvement is the main indication for PCI in patients with stable ischemic heart disease.
Applications for Clinical Practice and System Implementation
In patients with severe left ventricular systolic dysfunction and multivessel stable ischemic heart disease who are well compensated and have little or no angina at baseline, OMT alone as an initial strategy may be considered against the addition of PCI after careful risk and benefit discussion. Further details about revascularization and extended follow-up data from the REVIVED trial are necessary.
Practice Points
- Patients with ischemic cardiomyopathy with reduced ejection fraction have been an understudied population in previous studies.
- Further studies are necessary to understand the benefits of revascularization and the role of viability testing in this population.
– Taishi Hirai MD, and Ziad Sayed Ahmad, MD
University of Missouri, Columbia, MO
Study 1 Overview (STICHES Investigators)
Objective: To assess the survival benefit of coronary-artery bypass grafting (CABG) added to guideline-directed medical therapy, compared to optimal medical therapy (OMT) alone, in patients with coronary artery disease, heart failure, and severe left ventricular dysfunction. Design: Multicenter, randomized, prospective study with extended follow-up (median duration of 9.8 years).
Setting and participants: A total of 1212 patients with left ventricular ejection fraction (LVEF) of 35% or less and coronary artery disease were randomized to medical therapy plus CABG or OMT alone at 127 clinical sites in 26 countries.
Main outcome measures: The primary endpoint was death from any cause. Main secondary endpoints were death from cardiovascular causes and a composite outcome of death from any cause or hospitalization for cardiovascular causes.
Main results: There were 359 primary outcome all-cause deaths (58.9%) in the CABG group and 398 (66.1%) in the medical therapy group (hazard ratio [HR], 0.84; 95% CI, 0.73-0.97; P = .02). Death from cardiovascular causes was reported in 247 patients (40.5%) in the CABG group and 297 patients (49.3%) in the medical therapy group (HR, 0.79; 95% CI, 0.66-0.93; P < .01). The composite outcome of death from any cause or hospitalization for cardiovascular causes occurred in 467 patients (76.6%) in the CABG group and 467 patients (87.0%) in the medical therapy group (HR, 0.72; 95% CI, 0.64-0.82; P < .01).
Conclusion: Over a median follow-up of 9.8 years in patients with ischemic cardiomyopathy with severely reduced ejection fraction, the rates of death from any cause, death from cardiovascular causes, and the composite of death from any cause or hospitalization for cardiovascular causes were significantly lower in patients undergoing CABG than in patients receiving medical therapy alone.
Study 2 Overview (REVIVED BCIS Trial Group)
Objective: To assess whether percutaneous coronary intervention (PCI) can improve survival and left ventricular function in patients with severe left ventricular systolic dysfunction as compared to OMT alone.
Design: Multicenter, randomized, prospective study.
Setting and participants: A total of 700 patients with LVEF <35% with severe coronary artery disease amendable to PCI and demonstrable myocardial viability were randomly assigned to either PCI plus optimal medical therapy (PCI group) or OMT alone (OMT group).
Main outcome measures: The primary outcome was death from any cause or hospitalization for heart failure. The main secondary outcomes were LVEF at 6 and 12 months and quality of life (QOL) scores.
Main results: Over a median follow-up of 41 months, the primary outcome was reported in 129 patients (37.2%) in the PCI group and in 134 patients (38.0%) in the OMT group (HR, 0.99; 95% CI, 0.78-1.27; P = .96). The LVEF was similar in the 2 groups at 6 months (mean difference, –1.6 percentage points; 95% CI, –3.7 to 0.5) and at 12 months (mean difference, 0.9 percentage points; 95% CI, –1.7 to 3.4). QOL scores at 6 and 12 months favored the PCI group, but the difference had diminished at 24 months.
Conclusion: In patients with severe ischemic cardiomyopathy, revascularization by PCI in addition to OMT did not result in a lower incidence of death from any cause or hospitalization from heart failure.
Commentary
Coronary artery disease is the most common cause of heart failure with reduced ejection fraction and an important cause of mortality.1 Patients with ischemic cardiomyopathy with reduced ejection fraction are often considered for revascularization in addition to OMT and device therapies. Although there have been multiple retrospective studies and registries suggesting that cardiac outcomes and LVEF improve with revascularization, the number of large-scale prospective studies that assessed this clinical question and randomized patients to revascularization plus OMT compared to OMT alone has been limited.
In the Surgical Treatment for Ischemic Heart Failure (STICH) study,2,3 eligible patients had coronary artery disease amendable to CABG and a LVEF of 35% or less. Patients (N = 1212) were randomly assigned to CABG plus OMT or OMT alone between July 2002 and May 2007. The original study, with a median follow-up of 5 years, did not show survival benefit, but the investigators reported that the primary outcome of death from any cause was significantly lower in the CABG group compared to OMT alone when follow-up of the same study population was extended to 9.8 years (58.9% vs 66.1%, P = .02). The findings from this study led to a class I guideline recommendation of CABG over medical therapy in patients with multivessel disease and low ejection fraction.4
Since the STICH trial was designed, there have been significant improvements in devices and techniques used for PCI, and the procedure is now widely performed in patients with multivessel disease.5 The advantages of PCI over CABG include shorter recovery times and lower risk of immediate complications. In this context, the recently reported Revascularization for Ischemic Ventricular Dysfunction (REVIVED) study assessed clinical outcomes in patients with severe coronary artery disease and reduced ejection fraction by randomizing patients to either PCI with OMT or OMT alone.6 At a median follow-up of 3.5 years, the investigators found no difference in the primary outcome of death from any cause or hospitalization for heart failure (37.2% vs 38.0%; 95% CI, 0.78-1.28; P = .96). Moreover, the degree of LVEF improvement, assessed by follow-up echocardiogram read by the core lab, showed no difference in the degree of LVEF improvement between groups at 6 and 12 months. Finally, although results of the QOL assessment using the Kansas City Cardiomyopathy Questionnaire (KCCQ), a validated, patient-reported, heart-failure-specific QOL scale, favored the PCI group at 6 and 12 months of follow-up, the difference had diminished at 24 months.
The main strength of the REVIVED study was that it targeted a patient population with severe coronary artery disease, including left main disease and severely reduced ejection fraction, that historically have been excluded from large-scale randomized controlled studies evaluating PCI with OMT compared to OMT alone.7 However, there are several points to consider when interpreting the results of this study. First, further details of the PCI procedures are necessary. The REVIVED study recommended revascularization of all territories with viable myocardium; the anatomical revascularization index utilizing the British Cardiovascular Intervention Society (BCIS) Jeopardy Score was 71%. It is important to note that this jeopardy score was operator-reported and the core-lab adjudicated anatomical revascularization rate may be lower. Although viability testing primarily utilizing cardiac magnetic resonance imaging was performed in most patients, correlation between the revascularization territory and the viable segments has yet to be reported. Moreover, procedural details such as use of intravascular ultrasound and physiological testing, known to improve clinical outcome, need to be reported.8,9
Second, there is a high prevalence of ischemic cardiomyopathy, and it is important to note that the patients included in this study were highly selected from daily clinical practice, as evidenced by the prolonged enrollment period (8 years). Individuals were largely stable patients with less complex coronary anatomy as evidenced by the median interval from angiography to randomization of 80 days. Taking into consideration the degree of left ventricular dysfunction for patients included in the trial, only 14% of the patients had left main disease and half of the patients only had 2-vessel disease. The severity of the left main disease also needs to be clarified as it is likely that patients the operator determined to be critical were not enrolled in the study. Furthermore, the standard of care based on the STICH trial is to refer patients with severe multivessel coronary artery disease to CABG, making it more likely that patients with more severe and complex disease were not included in this trial. It is also important to note that this study enrolled patients with stable ischemic heart disease, and the data do not apply to patients presenting with acute coronary syndrome.
Third, although the primary outcome was similar between the groups, the secondary outcome of unplanned revascularization was lower in the PCI group. In addition, the rate of acute myocardial infarction (MI) was similar between the 2 groups, but the rate of spontaneous MI was lower in the PCI group compared to the OMT group (5.2% vs 9.3%) as 40% of MI cases in the PCI group were periprocedural MIs. The correlation between periprocedural MI and long-term outcomes has been modest compared to spontaneous MI. Moreover, with the longer follow-up, the number of spontaneous MI cases is expected to rise while the number of periprocedural MI cases is not. Extending the follow-up period is also important, as the STICH extension trial showed a statistically significant difference at 10-year follow up despite negative results at the time of the original publication.
Fourth, the REVIVED trial randomized a significantly lower number of patients compared to the STICH trial, and the authors reported fewer primary-outcome events than the estimated number needed to achieve the power to assess the primary hypothesis. In addition, significant improvements in medical treatment for heart failure with reduced ejection fraction since the STICH trial make comparison of PCI vs CABG in this patient population unfeasible.
Finally, although severe angina was not an exclusion criterion, two-thirds of the patients enrolled had no angina, and only 2% of the patients had baseline severe angina. This is important to consider when interpreting the results of the patient-reported health status as previous studies have shown that patients with worse angina at baseline derive the largest improvement in their QOL,10,11 and symptom improvement is the main indication for PCI in patients with stable ischemic heart disease.
Applications for Clinical Practice and System Implementation
In patients with severe left ventricular systolic dysfunction and multivessel stable ischemic heart disease who are well compensated and have little or no angina at baseline, OMT alone as an initial strategy may be considered against the addition of PCI after careful risk and benefit discussion. Further details about revascularization and extended follow-up data from the REVIVED trial are necessary.
Practice Points
- Patients with ischemic cardiomyopathy with reduced ejection fraction have been an understudied population in previous studies.
- Further studies are necessary to understand the benefits of revascularization and the role of viability testing in this population.
– Taishi Hirai MD, and Ziad Sayed Ahmad, MD
University of Missouri, Columbia, MO
1. Nowbar AN, Gitto M, Howard JP, et al. Mortality from ischemic heart disease. Circ Cardiovasc Qual Outcomes. 2019;12(6):e005375. doi:10.1161/CIRCOUTCOMES
2. Velazquez EJ, Lee KL, Deja MA, et al; for the STICH Investigators. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364(17):1607-1616. doi:10.1056/NEJMoa1100356
3. Velazquez EJ, Lee KL, Jones RH, et al. Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med. 2016;374(16):1511-1520. doi:10.1056/NEJMoa1602001
4. Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(2):e21-e129. doi:10.1016/j.jacc.2021.09.006
5. Kirtane AJ, Doshi D, Leon MB, et al. Treatment of higher-risk patients with an indication for revascularization: evolution within the field of contemporary percutaneous coronary intervention. Circulation. 2016;134(5):422-431. doi:10.1161/CIRCULATIONAHA
6. Perera D, Clayton T, O’Kane PD, et al. Percutaneous revascularization for ischemic left ventricular dysfunction. N Engl J Med. 2022;387(15):1351-1360. doi:10.1056/NEJMoa2206606
7. Maron DJ, Hochman JS, Reynolds HR, et al. Initial invasive or conservative strategy for stable coronary disease. Circulation. 2020;142(18):1725-1735. doi:10.1161/CIRCULATIONAHA
8. De Bruyne B, Pijls NH, Kalesan B, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367(11):991-1001. doi:10.1056/NEJMoa1205361
9. Zhang J, Gao X, Kan J, et al. Intravascular ultrasound versus angiography-guided drug-eluting stent implantation: The ULTIMATE trial. J Am Coll Cardiol. 2018;72(24):3126-3137. doi:10.1016/j.jacc.2018.09.013
10. Spertus JA, Jones PG, Maron DJ, et al. Health-status outcomes with invasive or conservative care in coronary disease. N Engl J Med. 2020;382(15):1408-1419. doi:10.1056/NEJMoa1916370
11. Hirai T, Grantham JA, Sapontis J, et al. Quality of life changes after chronic total occlusion angioplasty in patients with baseline refractory angina. Circ Cardiovasc Interv. 2019;12:e007558. doi:10.1161/CIRCINTERVENTIONS.118.007558
1. Nowbar AN, Gitto M, Howard JP, et al. Mortality from ischemic heart disease. Circ Cardiovasc Qual Outcomes. 2019;12(6):e005375. doi:10.1161/CIRCOUTCOMES
2. Velazquez EJ, Lee KL, Deja MA, et al; for the STICH Investigators. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364(17):1607-1616. doi:10.1056/NEJMoa1100356
3. Velazquez EJ, Lee KL, Jones RH, et al. Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med. 2016;374(16):1511-1520. doi:10.1056/NEJMoa1602001
4. Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(2):e21-e129. doi:10.1016/j.jacc.2021.09.006
5. Kirtane AJ, Doshi D, Leon MB, et al. Treatment of higher-risk patients with an indication for revascularization: evolution within the field of contemporary percutaneous coronary intervention. Circulation. 2016;134(5):422-431. doi:10.1161/CIRCULATIONAHA
6. Perera D, Clayton T, O’Kane PD, et al. Percutaneous revascularization for ischemic left ventricular dysfunction. N Engl J Med. 2022;387(15):1351-1360. doi:10.1056/NEJMoa2206606
7. Maron DJ, Hochman JS, Reynolds HR, et al. Initial invasive or conservative strategy for stable coronary disease. Circulation. 2020;142(18):1725-1735. doi:10.1161/CIRCULATIONAHA
8. De Bruyne B, Pijls NH, Kalesan B, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367(11):991-1001. doi:10.1056/NEJMoa1205361
9. Zhang J, Gao X, Kan J, et al. Intravascular ultrasound versus angiography-guided drug-eluting stent implantation: The ULTIMATE trial. J Am Coll Cardiol. 2018;72(24):3126-3137. doi:10.1016/j.jacc.2018.09.013
10. Spertus JA, Jones PG, Maron DJ, et al. Health-status outcomes with invasive or conservative care in coronary disease. N Engl J Med. 2020;382(15):1408-1419. doi:10.1056/NEJMoa1916370
11. Hirai T, Grantham JA, Sapontis J, et al. Quality of life changes after chronic total occlusion angioplasty in patients with baseline refractory angina. Circ Cardiovasc Interv. 2019;12:e007558. doi:10.1161/CIRCINTERVENTIONS.118.007558
A plane crash interrupts a doctor’s vacation
Emergencies happen anywhere, anytime – and sometimes physicians find themselves in situations where they are the only ones who can help. “Is There a Doctor in the House?” is a new series telling these stories.
When the plane crashed, I was asleep. I had arrived the evening before with my wife and three sons at a house on Kezar Lake on the Maine–New Hampshire border. I jumped out of bed and ran downstairs. My kids had been watching a float plane circling and gliding along the lake. It had crashed into the water and flipped upside down. My oldest brother-in-law jumped into his ski boat and we sped out to the scene.
All we can see are the plane’s pontoons. The rest is underwater. A woman has already surfaced, screaming. I dive in.
I find the woman’s husband and 3-year-old son struggling to get free from the plane through the smashed windshield. They manage to get to the surface. The pilot is dead, impaled through the chest by the left wing strut.
The big problem: A little girl, whom I would learn later is named Lauren, remained trapped. The water is murky but I can see her, a 5- or 6-year-old girl with this long hair, strapped in upside down and unconscious.
The mom and I dive down over and over, pulling and ripping at the door. We cannot get it open. Finally, I’m able to bend the door open enough where I can reach in, but I can’t undo the seatbelt. In my mind, I’m debating, should I try and go through the front windshield? I’m getting really tired, I can tell there’s fuel in the water, and I don’t want to drown in the plane. So I pop up to the surface and yell, “Does anyone have a knife?”
My brother-in-law shoots back to shore in the boat, screaming, “Get a knife!” My niece gets in the boat with one. I’m standing on the pontoon, and my niece is in the front of the boat calling, “Uncle Todd! Uncle Todd!” and she throws the knife. It goes way over my head. I can’t even jump for it, it’s so high.
I have to get the knife. So, I dive into the water to try and find it. Somehow, the black knife has landed on the white wing, 4 or 5 feet under the water. Pure luck. It could have sunk down a hundred feet into the lake. I grab the knife and hand it to the mom, Beth. She’s able to cut the seatbelt, and we both pull Lauren to the surface.
I lay her out on the pontoon. She has no pulse and her pupils are fixed and dilated. Her mom is yelling, “She’s dead, isn’t she?” I start CPR. My skin and eyes are burning from the airplane fuel in the water. I get her breathing, and her heart comes back very quickly. Lauren starts to vomit and I’m trying to keep her airway clear. She’s breathing spontaneously and she has a pulse, so I decide it’s time to move her to shore.
We pull the boat up to the dock and Lauren’s now having anoxic seizures. Her brain has been without oxygen, and now she’s getting perfused again. We get her to shore and lay her on the lawn. I’m still doing mouth-to-mouth, but she’s seizing like crazy, and I don’t have any way to control that. Beth is crying and wants to hold her daughter gently while I’m working.
Someone had called 911, and finally this dude shows up with an ambulance, and it’s like something out of World War II. All he has is an oxygen tank, but the mask is old and cracked. It’s too big for Lauren, but it sort of fits me, so I’m sucking in oxygen and blowing it into the girl’s mouth. I’m doing whatever I can, but I don’t have an IV to start. I have no fluids. I got nothing.
As it happens, I’d done my emergency medicine training at Maine Medical Center, so I tell someone to call them and get a Life Flight chopper. We have to drive somewhere where the chopper can land, so we take the ambulance to the parking lot of the closest store called the Wicked Good Store. That’s a common thing in Maine. Everything is “wicked good.”
The whole town is there by that point. The chopper arrives. The ambulance doors pop open and a woman says, “Todd?” And I say, “Heather?”
Heather is an emergency flight nurse whom I’d trained with many years ago. There’s immediate trust. She has all the right equipment. We put in breathing tubes and IVs. We stop Lauren from seizing. The kid is soon stable.
There is only one extra seat in the chopper, so I tell Beth to go. They take off.
Suddenly, I begin to doubt my decision. Lauren had been underwater for 15 minutes at minimum. I know how long that is. Did I do the right thing? Did I resuscitate a brain-dead child? I didn’t think about it at the time, but if that patient had come to me in the emergency department, I’m honestly not sure what I would have done.
So, I go home. And I don’t get a call. The FAA and sheriff arrive to take statements from us. I don’t hear from anyone.
The next day I start calling. No one will tell me anything, so I finally get to one of the pediatric ICU attendings who had trained me. He says Lauren literally woke up and said, “I have to go pee.” And that was it. She was 100% normal. I couldn’t believe it.
Here’s a theory: In kids, there’s something called the glottic reflex. I think her glottic reflex went off as soon as she hit the water, which basically closed her airway. So when she passed out, she could never get enough water in her lungs and still had enough air in there to keep her alive. Later, I got a call from her uncle. He could barely get the words out because he was in tears. He said Lauren was doing beautifully.
Three days later, I drove to Lauren’s house with my wife and kids. I had her read to me. I watched her play on the jungle gym for motor function. All sorts of stuff. She was totally normal.
Beth told us that the night before the accident, her mother had given the women in her family what she called a “miracle bracelet,” a bracelet that is supposed to give you one miracle in your life. Beth said she had the bracelet on her wrist the day of the accident, and now it’s gone. “Saving Lauren’s life was my miracle,” she said.
Funny thing: For 20 years, I ran all the EMS, police, fire, ambulance, in Boulder, Colo., where I live. I wrote all the protocols, and I would never advise any of my paramedics to dive into jet fuel to save someone. That was risky. But at the time, it was totally automatic. I think it taught me not to give up in certain situations, because you really don’t know.
Dr. Dorfman is an emergency medicine physician in Boulder, Colo., and medical director at Cedalion Health.
A version of this article first appeared on Medscape.com.
Emergencies happen anywhere, anytime – and sometimes physicians find themselves in situations where they are the only ones who can help. “Is There a Doctor in the House?” is a new series telling these stories.
When the plane crashed, I was asleep. I had arrived the evening before with my wife and three sons at a house on Kezar Lake on the Maine–New Hampshire border. I jumped out of bed and ran downstairs. My kids had been watching a float plane circling and gliding along the lake. It had crashed into the water and flipped upside down. My oldest brother-in-law jumped into his ski boat and we sped out to the scene.
All we can see are the plane’s pontoons. The rest is underwater. A woman has already surfaced, screaming. I dive in.
I find the woman’s husband and 3-year-old son struggling to get free from the plane through the smashed windshield. They manage to get to the surface. The pilot is dead, impaled through the chest by the left wing strut.
The big problem: A little girl, whom I would learn later is named Lauren, remained trapped. The water is murky but I can see her, a 5- or 6-year-old girl with this long hair, strapped in upside down and unconscious.
The mom and I dive down over and over, pulling and ripping at the door. We cannot get it open. Finally, I’m able to bend the door open enough where I can reach in, but I can’t undo the seatbelt. In my mind, I’m debating, should I try and go through the front windshield? I’m getting really tired, I can tell there’s fuel in the water, and I don’t want to drown in the plane. So I pop up to the surface and yell, “Does anyone have a knife?”
My brother-in-law shoots back to shore in the boat, screaming, “Get a knife!” My niece gets in the boat with one. I’m standing on the pontoon, and my niece is in the front of the boat calling, “Uncle Todd! Uncle Todd!” and she throws the knife. It goes way over my head. I can’t even jump for it, it’s so high.
I have to get the knife. So, I dive into the water to try and find it. Somehow, the black knife has landed on the white wing, 4 or 5 feet under the water. Pure luck. It could have sunk down a hundred feet into the lake. I grab the knife and hand it to the mom, Beth. She’s able to cut the seatbelt, and we both pull Lauren to the surface.
I lay her out on the pontoon. She has no pulse and her pupils are fixed and dilated. Her mom is yelling, “She’s dead, isn’t she?” I start CPR. My skin and eyes are burning from the airplane fuel in the water. I get her breathing, and her heart comes back very quickly. Lauren starts to vomit and I’m trying to keep her airway clear. She’s breathing spontaneously and she has a pulse, so I decide it’s time to move her to shore.
We pull the boat up to the dock and Lauren’s now having anoxic seizures. Her brain has been without oxygen, and now she’s getting perfused again. We get her to shore and lay her on the lawn. I’m still doing mouth-to-mouth, but she’s seizing like crazy, and I don’t have any way to control that. Beth is crying and wants to hold her daughter gently while I’m working.
Someone had called 911, and finally this dude shows up with an ambulance, and it’s like something out of World War II. All he has is an oxygen tank, but the mask is old and cracked. It’s too big for Lauren, but it sort of fits me, so I’m sucking in oxygen and blowing it into the girl’s mouth. I’m doing whatever I can, but I don’t have an IV to start. I have no fluids. I got nothing.
As it happens, I’d done my emergency medicine training at Maine Medical Center, so I tell someone to call them and get a Life Flight chopper. We have to drive somewhere where the chopper can land, so we take the ambulance to the parking lot of the closest store called the Wicked Good Store. That’s a common thing in Maine. Everything is “wicked good.”
The whole town is there by that point. The chopper arrives. The ambulance doors pop open and a woman says, “Todd?” And I say, “Heather?”
Heather is an emergency flight nurse whom I’d trained with many years ago. There’s immediate trust. She has all the right equipment. We put in breathing tubes and IVs. We stop Lauren from seizing. The kid is soon stable.
There is only one extra seat in the chopper, so I tell Beth to go. They take off.
Suddenly, I begin to doubt my decision. Lauren had been underwater for 15 minutes at minimum. I know how long that is. Did I do the right thing? Did I resuscitate a brain-dead child? I didn’t think about it at the time, but if that patient had come to me in the emergency department, I’m honestly not sure what I would have done.
So, I go home. And I don’t get a call. The FAA and sheriff arrive to take statements from us. I don’t hear from anyone.
The next day I start calling. No one will tell me anything, so I finally get to one of the pediatric ICU attendings who had trained me. He says Lauren literally woke up and said, “I have to go pee.” And that was it. She was 100% normal. I couldn’t believe it.
Here’s a theory: In kids, there’s something called the glottic reflex. I think her glottic reflex went off as soon as she hit the water, which basically closed her airway. So when she passed out, she could never get enough water in her lungs and still had enough air in there to keep her alive. Later, I got a call from her uncle. He could barely get the words out because he was in tears. He said Lauren was doing beautifully.
Three days later, I drove to Lauren’s house with my wife and kids. I had her read to me. I watched her play on the jungle gym for motor function. All sorts of stuff. She was totally normal.
Beth told us that the night before the accident, her mother had given the women in her family what she called a “miracle bracelet,” a bracelet that is supposed to give you one miracle in your life. Beth said she had the bracelet on her wrist the day of the accident, and now it’s gone. “Saving Lauren’s life was my miracle,” she said.
Funny thing: For 20 years, I ran all the EMS, police, fire, ambulance, in Boulder, Colo., where I live. I wrote all the protocols, and I would never advise any of my paramedics to dive into jet fuel to save someone. That was risky. But at the time, it was totally automatic. I think it taught me not to give up in certain situations, because you really don’t know.
Dr. Dorfman is an emergency medicine physician in Boulder, Colo., and medical director at Cedalion Health.
A version of this article first appeared on Medscape.com.
Emergencies happen anywhere, anytime – and sometimes physicians find themselves in situations where they are the only ones who can help. “Is There a Doctor in the House?” is a new series telling these stories.
When the plane crashed, I was asleep. I had arrived the evening before with my wife and three sons at a house on Kezar Lake on the Maine–New Hampshire border. I jumped out of bed and ran downstairs. My kids had been watching a float plane circling and gliding along the lake. It had crashed into the water and flipped upside down. My oldest brother-in-law jumped into his ski boat and we sped out to the scene.
All we can see are the plane’s pontoons. The rest is underwater. A woman has already surfaced, screaming. I dive in.
I find the woman’s husband and 3-year-old son struggling to get free from the plane through the smashed windshield. They manage to get to the surface. The pilot is dead, impaled through the chest by the left wing strut.
The big problem: A little girl, whom I would learn later is named Lauren, remained trapped. The water is murky but I can see her, a 5- or 6-year-old girl with this long hair, strapped in upside down and unconscious.
The mom and I dive down over and over, pulling and ripping at the door. We cannot get it open. Finally, I’m able to bend the door open enough where I can reach in, but I can’t undo the seatbelt. In my mind, I’m debating, should I try and go through the front windshield? I’m getting really tired, I can tell there’s fuel in the water, and I don’t want to drown in the plane. So I pop up to the surface and yell, “Does anyone have a knife?”
My brother-in-law shoots back to shore in the boat, screaming, “Get a knife!” My niece gets in the boat with one. I’m standing on the pontoon, and my niece is in the front of the boat calling, “Uncle Todd! Uncle Todd!” and she throws the knife. It goes way over my head. I can’t even jump for it, it’s so high.
I have to get the knife. So, I dive into the water to try and find it. Somehow, the black knife has landed on the white wing, 4 or 5 feet under the water. Pure luck. It could have sunk down a hundred feet into the lake. I grab the knife and hand it to the mom, Beth. She’s able to cut the seatbelt, and we both pull Lauren to the surface.
I lay her out on the pontoon. She has no pulse and her pupils are fixed and dilated. Her mom is yelling, “She’s dead, isn’t she?” I start CPR. My skin and eyes are burning from the airplane fuel in the water. I get her breathing, and her heart comes back very quickly. Lauren starts to vomit and I’m trying to keep her airway clear. She’s breathing spontaneously and she has a pulse, so I decide it’s time to move her to shore.
We pull the boat up to the dock and Lauren’s now having anoxic seizures. Her brain has been without oxygen, and now she’s getting perfused again. We get her to shore and lay her on the lawn. I’m still doing mouth-to-mouth, but she’s seizing like crazy, and I don’t have any way to control that. Beth is crying and wants to hold her daughter gently while I’m working.
Someone had called 911, and finally this dude shows up with an ambulance, and it’s like something out of World War II. All he has is an oxygen tank, but the mask is old and cracked. It’s too big for Lauren, but it sort of fits me, so I’m sucking in oxygen and blowing it into the girl’s mouth. I’m doing whatever I can, but I don’t have an IV to start. I have no fluids. I got nothing.
As it happens, I’d done my emergency medicine training at Maine Medical Center, so I tell someone to call them and get a Life Flight chopper. We have to drive somewhere where the chopper can land, so we take the ambulance to the parking lot of the closest store called the Wicked Good Store. That’s a common thing in Maine. Everything is “wicked good.”
The whole town is there by that point. The chopper arrives. The ambulance doors pop open and a woman says, “Todd?” And I say, “Heather?”
Heather is an emergency flight nurse whom I’d trained with many years ago. There’s immediate trust. She has all the right equipment. We put in breathing tubes and IVs. We stop Lauren from seizing. The kid is soon stable.
There is only one extra seat in the chopper, so I tell Beth to go. They take off.
Suddenly, I begin to doubt my decision. Lauren had been underwater for 15 minutes at minimum. I know how long that is. Did I do the right thing? Did I resuscitate a brain-dead child? I didn’t think about it at the time, but if that patient had come to me in the emergency department, I’m honestly not sure what I would have done.
So, I go home. And I don’t get a call. The FAA and sheriff arrive to take statements from us. I don’t hear from anyone.
The next day I start calling. No one will tell me anything, so I finally get to one of the pediatric ICU attendings who had trained me. He says Lauren literally woke up and said, “I have to go pee.” And that was it. She was 100% normal. I couldn’t believe it.
Here’s a theory: In kids, there’s something called the glottic reflex. I think her glottic reflex went off as soon as she hit the water, which basically closed her airway. So when she passed out, she could never get enough water in her lungs and still had enough air in there to keep her alive. Later, I got a call from her uncle. He could barely get the words out because he was in tears. He said Lauren was doing beautifully.
Three days later, I drove to Lauren’s house with my wife and kids. I had her read to me. I watched her play on the jungle gym for motor function. All sorts of stuff. She was totally normal.
Beth told us that the night before the accident, her mother had given the women in her family what she called a “miracle bracelet,” a bracelet that is supposed to give you one miracle in your life. Beth said she had the bracelet on her wrist the day of the accident, and now it’s gone. “Saving Lauren’s life was my miracle,” she said.
Funny thing: For 20 years, I ran all the EMS, police, fire, ambulance, in Boulder, Colo., where I live. I wrote all the protocols, and I would never advise any of my paramedics to dive into jet fuel to save someone. That was risky. But at the time, it was totally automatic. I think it taught me not to give up in certain situations, because you really don’t know.
Dr. Dorfman is an emergency medicine physician in Boulder, Colo., and medical director at Cedalion Health.
A version of this article first appeared on Medscape.com.