User login
Home Treatment of Presumed Melanocytic Nevus With Frankincense
To the Editor:
Melanocytic nevi are ubiquitous, and although they are benign, patients often desire to have them removed. We report a patient who presented to our clinic after attempting home removal of a concerning mole on the back with frankincense, a remedy that she found online.
A 43-year-old woman presented with a worrisome mole on the back. She had no personal history of skin cancer, but her father had a history of melanoma in situ in his 60s. The patient reported that she had the mole for years, but approximately 1 month prior to her visit she noticed that it began to bleed and crust, causing concern for melanoma. She read online that the lesion could be removed with topical application of the essential oil frankincense; she applied it directly to the lesion on the back. Within hours she developed a burn where it was applied with associated blistering.
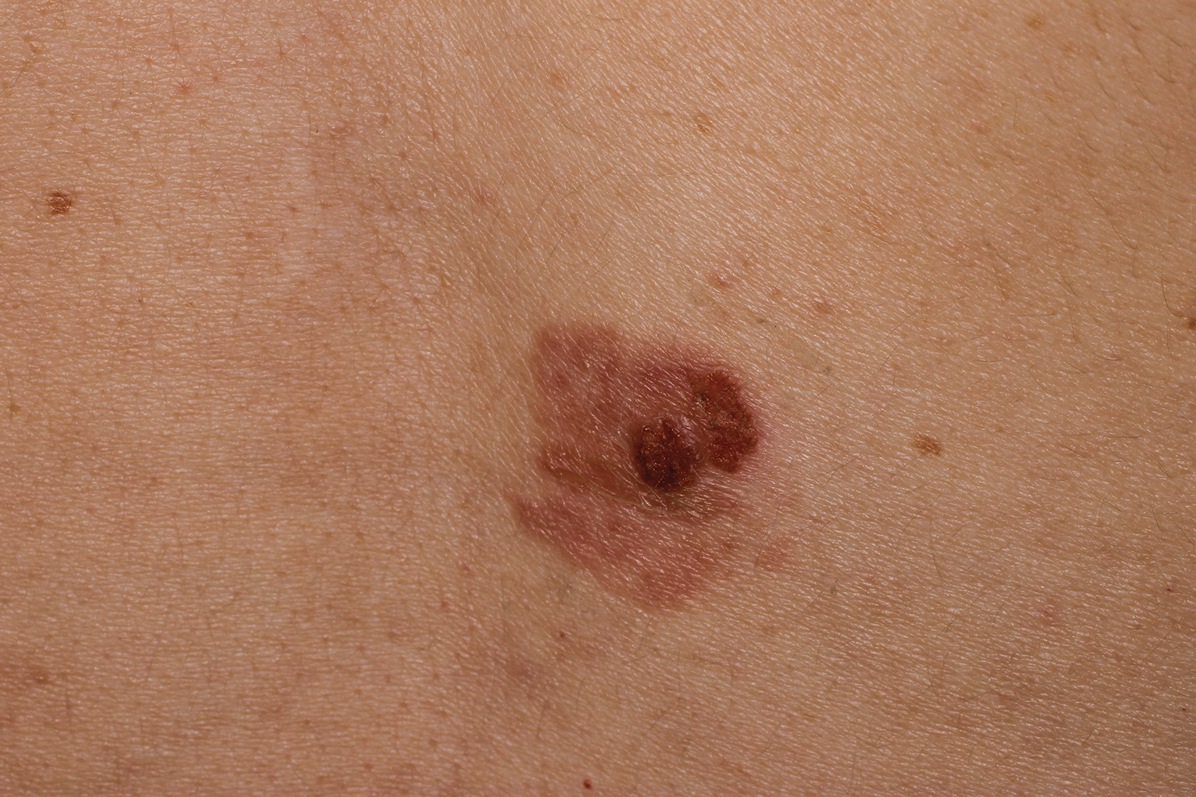
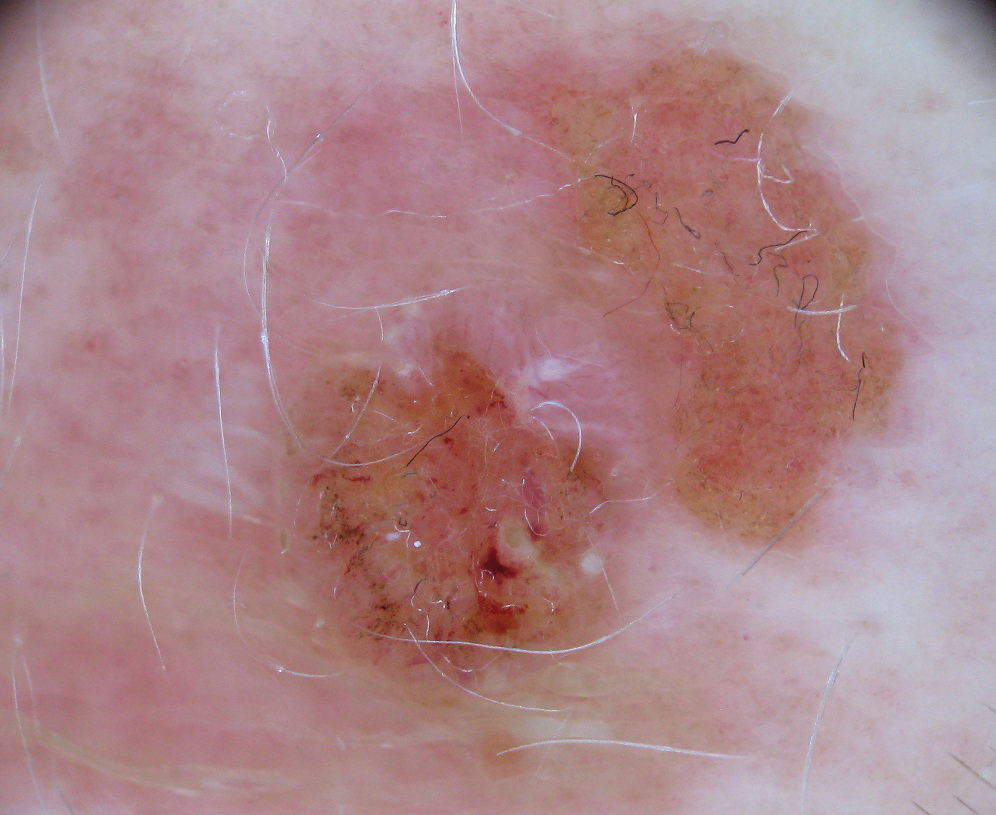
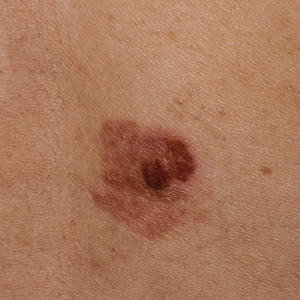
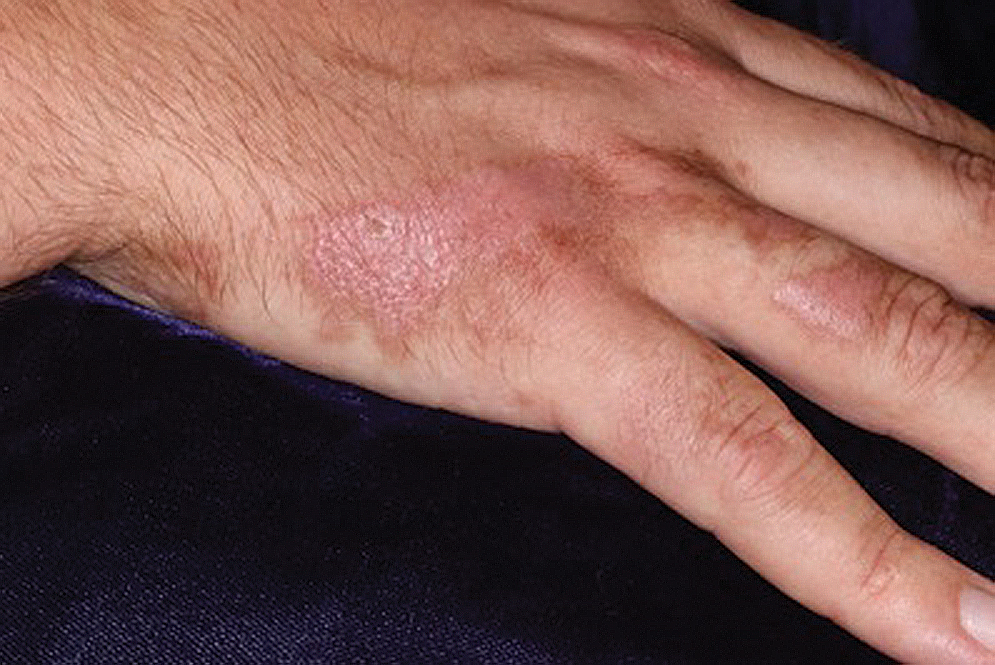
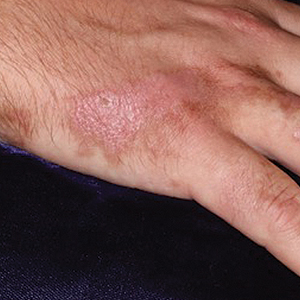
Clinically, the lesion appeared as a darkly pigmented, well-circumscribed papule with hemorrhagic crust overlying a well-demarcated pink plaque (Figure 1). Dermatoscopically, the lesion lacked a pigment network and demonstrated 2 distinct pink papules with peripheral telangiectasia and a pink background with white streaks (Figure 2). A shave biopsy of the lesion demonstrated a nodular basal cell carcinoma extending to the base and margin.
Frankincense is the common name given to oleo-gum-resins of Boswellia species.1 It has been studied extensively for anti-inflammatory and antitumoral properties. It has been demonstrated that high concentrations of its active component, boswellic acid, can have a cytotoxic or cytostatic effect on certain malignant cell lines, such as melanoma, in vitro.2,3 It also has been shown to be antitumoral in mouse models.4 There are limited in vivo studies in the literature assessing the effects of boswellic acid or frankincense on cutaneous melanocytic lesions or other cutaneous malignancies, such as basal cell carcinoma.
A Google search of home remedy mole removal yielded more than 1,000,000 results. At the time of submission, the top 5 results all listed frankincense as a potential treatment along with garlic, iodine, castor oil, onion juice, pineapple juice, banana peels, honey, and aloe vera. None of the results cited evidence for their treatments. Although all recommended dilution of the frankincense prior to application, none warned of potential risks or side effects of its use.
Natural methods of home mole removal have long been sought after. Escharotics are most commonly utilized, including bloodroot (Sanguinaria canadensis), zinc chloride, Chelidonium majus, and Solanum sodomaeum. Many formulations are commercially available online, despite the fact that they can be mutilating and potentially dangerous when used without appropriate supervision.5 This case and an online search demonstrated that these agents are not only potentially harmful home remedies but also are currently falsely advertised as effective therapeutic management for melanocytic nevi.
Approximately 6 million individuals in the United States search the internet for health information daily, and as many as 41% of those do so to learn about alternative medicine.5,6 Although information gleaned from search engines can be useful, it is unregulated and often can be inaccurate. Clinicians generally are unaware of the erroneous material presented online and, therefore, cannot appropriately combat patient misinformation. Our case demonstrates the need to maintain an awareness of common online fallacies to better answer patient questions and guide them to more accurate sources of dermatologic information and appropriate treatment.
- Du Z, Liu Z, Ning Z, et al. Prospects of boswellic acids as potential pharmaceutics. Planta Med. 2015;81:259-271.
- Eichhorn T, Greten HJ, Efferth T. Molecular determinants of the response of tumor cells to boswellic acids. Pharmaceuticals (Basel). 2011;4:1171-1182.
- Zhao W, Entschladen F, Liu H, et al. Boswellic acid acetate induces differentiation and apoptosis in highly metastatic melanoma and fibrosarcoma cell. Cancer Detect Prev. 2003;27:67-75.
- Huang MT, Badmaev V, Ding Y, et al. Anti-tumor and anti-carcinogenic activities of triterpenoid, beta-boswellic acid. Biofactors. 2000;13:225-230.
- Adler BL, Friedman AJ. Safety & efficacy of agents used for home mole removal and skin cancer treatment in the internet age, and analysis of cases. J Drugs Dermatol. 2013;12:1058-1063.
- Kanthawala S, Vermeesch A, Given B, et al. Answers to health questions: internet search results versus online health community responses. J Med Internet Res. 2016;18:E95.
To the Editor:
Melanocytic nevi are ubiquitous, and although they are benign, patients often desire to have them removed. We report a patient who presented to our clinic after attempting home removal of a concerning mole on the back with frankincense, a remedy that she found online.
A 43-year-old woman presented with a worrisome mole on the back. She had no personal history of skin cancer, but her father had a history of melanoma in situ in his 60s. The patient reported that she had the mole for years, but approximately 1 month prior to her visit she noticed that it began to bleed and crust, causing concern for melanoma. She read online that the lesion could be removed with topical application of the essential oil frankincense; she applied it directly to the lesion on the back. Within hours she developed a burn where it was applied with associated blistering.
Clinically, the lesion appeared as a darkly pigmented, well-circumscribed papule with hemorrhagic crust overlying a well-demarcated pink plaque (Figure 1). Dermatoscopically, the lesion lacked a pigment network and demonstrated 2 distinct pink papules with peripheral telangiectasia and a pink background with white streaks (Figure 2). A shave biopsy of the lesion demonstrated a nodular basal cell carcinoma extending to the base and margin.
Frankincense is the common name given to oleo-gum-resins of Boswellia species.1 It has been studied extensively for anti-inflammatory and antitumoral properties. It has been demonstrated that high concentrations of its active component, boswellic acid, can have a cytotoxic or cytostatic effect on certain malignant cell lines, such as melanoma, in vitro.2,3 It also has been shown to be antitumoral in mouse models.4 There are limited in vivo studies in the literature assessing the effects of boswellic acid or frankincense on cutaneous melanocytic lesions or other cutaneous malignancies, such as basal cell carcinoma.
A Google search of home remedy mole removal yielded more than 1,000,000 results. At the time of submission, the top 5 results all listed frankincense as a potential treatment along with garlic, iodine, castor oil, onion juice, pineapple juice, banana peels, honey, and aloe vera. None of the results cited evidence for their treatments. Although all recommended dilution of the frankincense prior to application, none warned of potential risks or side effects of its use.
Natural methods of home mole removal have long been sought after. Escharotics are most commonly utilized, including bloodroot (Sanguinaria canadensis), zinc chloride, Chelidonium majus, and Solanum sodomaeum. Many formulations are commercially available online, despite the fact that they can be mutilating and potentially dangerous when used without appropriate supervision.5 This case and an online search demonstrated that these agents are not only potentially harmful home remedies but also are currently falsely advertised as effective therapeutic management for melanocytic nevi.
Approximately 6 million individuals in the United States search the internet for health information daily, and as many as 41% of those do so to learn about alternative medicine.5,6 Although information gleaned from search engines can be useful, it is unregulated and often can be inaccurate. Clinicians generally are unaware of the erroneous material presented online and, therefore, cannot appropriately combat patient misinformation. Our case demonstrates the need to maintain an awareness of common online fallacies to better answer patient questions and guide them to more accurate sources of dermatologic information and appropriate treatment.
To the Editor:
Melanocytic nevi are ubiquitous, and although they are benign, patients often desire to have them removed. We report a patient who presented to our clinic after attempting home removal of a concerning mole on the back with frankincense, a remedy that she found online.
A 43-year-old woman presented with a worrisome mole on the back. She had no personal history of skin cancer, but her father had a history of melanoma in situ in his 60s. The patient reported that she had the mole for years, but approximately 1 month prior to her visit she noticed that it began to bleed and crust, causing concern for melanoma. She read online that the lesion could be removed with topical application of the essential oil frankincense; she applied it directly to the lesion on the back. Within hours she developed a burn where it was applied with associated blistering.
Clinically, the lesion appeared as a darkly pigmented, well-circumscribed papule with hemorrhagic crust overlying a well-demarcated pink plaque (Figure 1). Dermatoscopically, the lesion lacked a pigment network and demonstrated 2 distinct pink papules with peripheral telangiectasia and a pink background with white streaks (Figure 2). A shave biopsy of the lesion demonstrated a nodular basal cell carcinoma extending to the base and margin.
Frankincense is the common name given to oleo-gum-resins of Boswellia species.1 It has been studied extensively for anti-inflammatory and antitumoral properties. It has been demonstrated that high concentrations of its active component, boswellic acid, can have a cytotoxic or cytostatic effect on certain malignant cell lines, such as melanoma, in vitro.2,3 It also has been shown to be antitumoral in mouse models.4 There are limited in vivo studies in the literature assessing the effects of boswellic acid or frankincense on cutaneous melanocytic lesions or other cutaneous malignancies, such as basal cell carcinoma.
A Google search of home remedy mole removal yielded more than 1,000,000 results. At the time of submission, the top 5 results all listed frankincense as a potential treatment along with garlic, iodine, castor oil, onion juice, pineapple juice, banana peels, honey, and aloe vera. None of the results cited evidence for their treatments. Although all recommended dilution of the frankincense prior to application, none warned of potential risks or side effects of its use.
Natural methods of home mole removal have long been sought after. Escharotics are most commonly utilized, including bloodroot (Sanguinaria canadensis), zinc chloride, Chelidonium majus, and Solanum sodomaeum. Many formulations are commercially available online, despite the fact that they can be mutilating and potentially dangerous when used without appropriate supervision.5 This case and an online search demonstrated that these agents are not only potentially harmful home remedies but also are currently falsely advertised as effective therapeutic management for melanocytic nevi.
Approximately 6 million individuals in the United States search the internet for health information daily, and as many as 41% of those do so to learn about alternative medicine.5,6 Although information gleaned from search engines can be useful, it is unregulated and often can be inaccurate. Clinicians generally are unaware of the erroneous material presented online and, therefore, cannot appropriately combat patient misinformation. Our case demonstrates the need to maintain an awareness of common online fallacies to better answer patient questions and guide them to more accurate sources of dermatologic information and appropriate treatment.
- Du Z, Liu Z, Ning Z, et al. Prospects of boswellic acids as potential pharmaceutics. Planta Med. 2015;81:259-271.
- Eichhorn T, Greten HJ, Efferth T. Molecular determinants of the response of tumor cells to boswellic acids. Pharmaceuticals (Basel). 2011;4:1171-1182.
- Zhao W, Entschladen F, Liu H, et al. Boswellic acid acetate induces differentiation and apoptosis in highly metastatic melanoma and fibrosarcoma cell. Cancer Detect Prev. 2003;27:67-75.
- Huang MT, Badmaev V, Ding Y, et al. Anti-tumor and anti-carcinogenic activities of triterpenoid, beta-boswellic acid. Biofactors. 2000;13:225-230.
- Adler BL, Friedman AJ. Safety & efficacy of agents used for home mole removal and skin cancer treatment in the internet age, and analysis of cases. J Drugs Dermatol. 2013;12:1058-1063.
- Kanthawala S, Vermeesch A, Given B, et al. Answers to health questions: internet search results versus online health community responses. J Med Internet Res. 2016;18:E95.
- Du Z, Liu Z, Ning Z, et al. Prospects of boswellic acids as potential pharmaceutics. Planta Med. 2015;81:259-271.
- Eichhorn T, Greten HJ, Efferth T. Molecular determinants of the response of tumor cells to boswellic acids. Pharmaceuticals (Basel). 2011;4:1171-1182.
- Zhao W, Entschladen F, Liu H, et al. Boswellic acid acetate induces differentiation and apoptosis in highly metastatic melanoma and fibrosarcoma cell. Cancer Detect Prev. 2003;27:67-75.
- Huang MT, Badmaev V, Ding Y, et al. Anti-tumor and anti-carcinogenic activities of triterpenoid, beta-boswellic acid. Biofactors. 2000;13:225-230.
- Adler BL, Friedman AJ. Safety & efficacy of agents used for home mole removal and skin cancer treatment in the internet age, and analysis of cases. J Drugs Dermatol. 2013;12:1058-1063.
- Kanthawala S, Vermeesch A, Given B, et al. Answers to health questions: internet search results versus online health community responses. J Med Internet Res. 2016;18:E95.
Practice Points
- Many patients seek natural methods of home mole removal online, including topical application of essential oils such as frankincense.
- These agents often are unregulated and can be potentially harmful when used without appropriate supervision.
- Dermatologists should be aware of common online fallacies to better answer patient questions and guide them to more accurate sources of dermatologic information and appropriate treatment.
Erythema Multiforme–like Dermatitis Due to Isoniazid Hypersensitivity in a Patient With Psoriasis
To the Editor:
Psoriasis vulgaris is a chronic autoimmune inflammatory disease and biologic agents, such as anti–tumor necrosis factor α (TNF-α), are alternative drugs in case of resistance or adverse events to conventional ones.1 The limitation of these agents is immunosuppression that may cause infections such as tuberculosis (TB). Prophylaxis is indicated to latent TB diseases if the purified protein derivative (tuberculin) skin test is higher than 5 mm before starting these treatments. The challenge in TB treatment is adverse drug reactions (ADRs) that are reported in 4% to 6% of cases.2,3
Erythema multiforme–like dermatitis is a rare skin rash that develops due to isoniazid (INH). The clinical presentation includes erythematoedematous lesions in an acral distribution with no mucosal involvement and systemic exposure to INH. Skin biopsy and patch tests are the supportive diagnostic methods. Isoniazid-associated skin rashes rarely are reported and generally are not severe enough to terminate the drug. We present a patient with psoriasis who received TB prophylaxis before anti–TNF-α use. He presented with erythema multiforme–like dermatitis due to INH. Withdrawal of the drug and treatment of the lesions were the first steps of intolerance, followed by a patch test with the culprit drug after recovery. We discuss the diagnostic drug allergy evaluation and treatment approach.
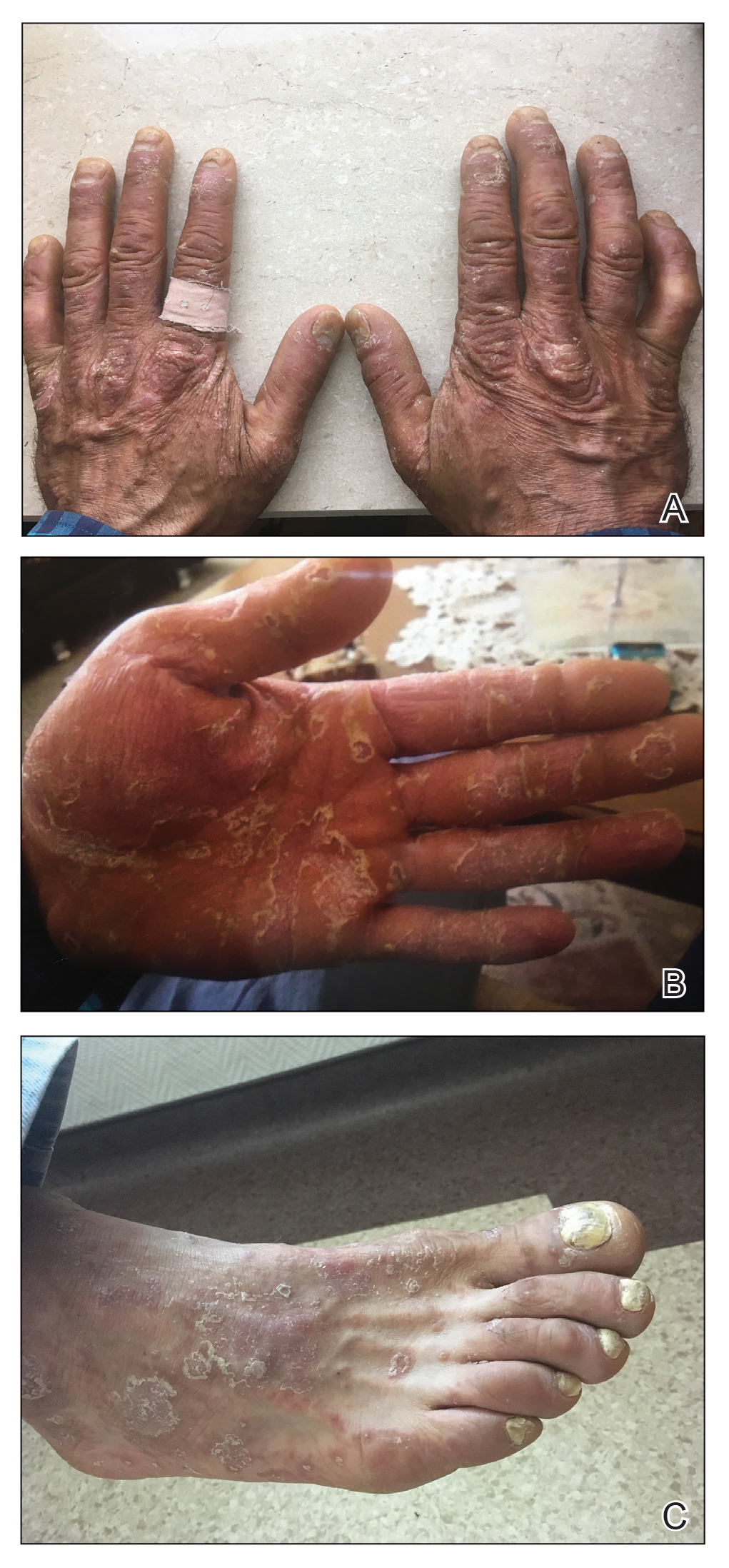
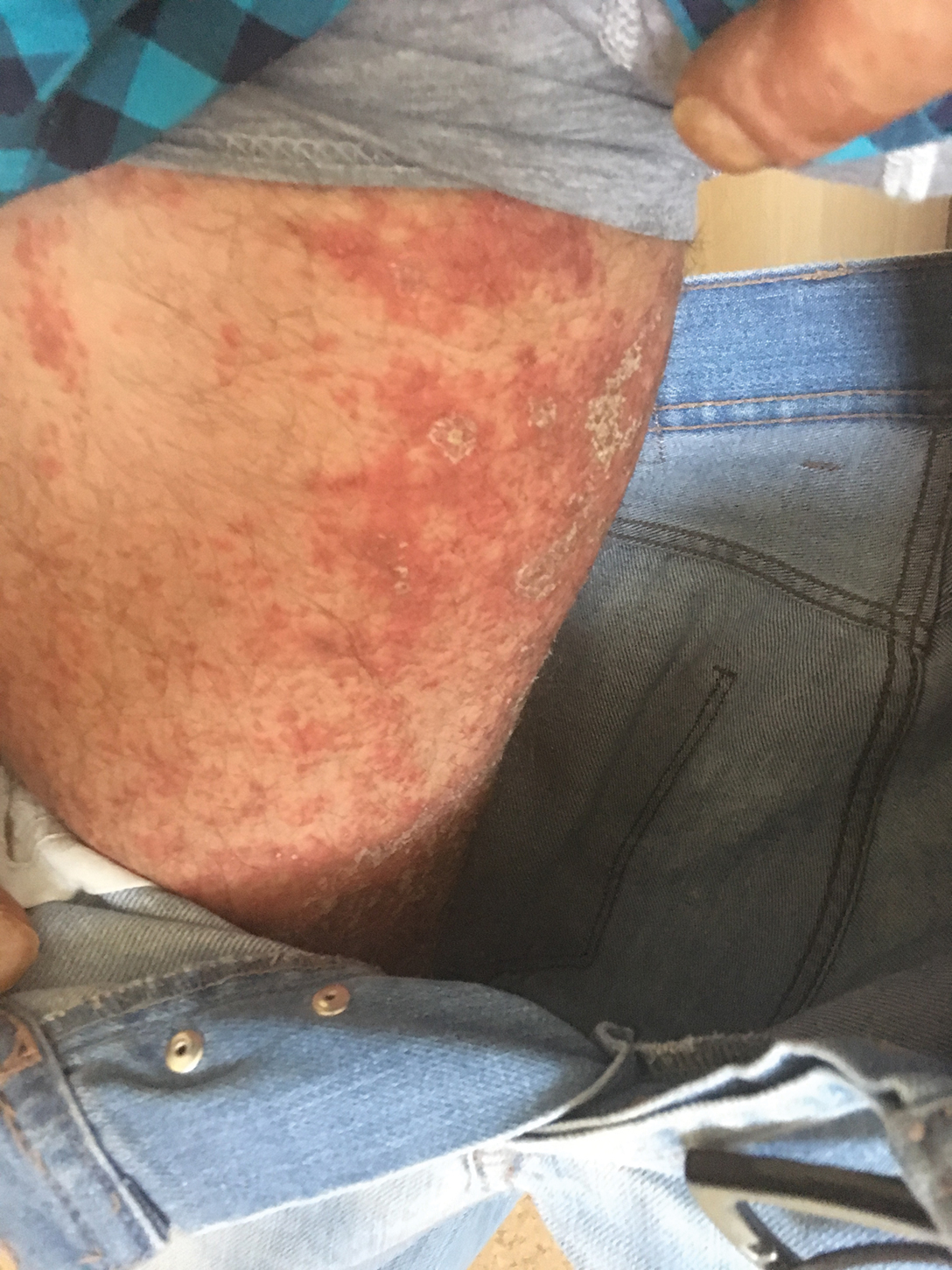
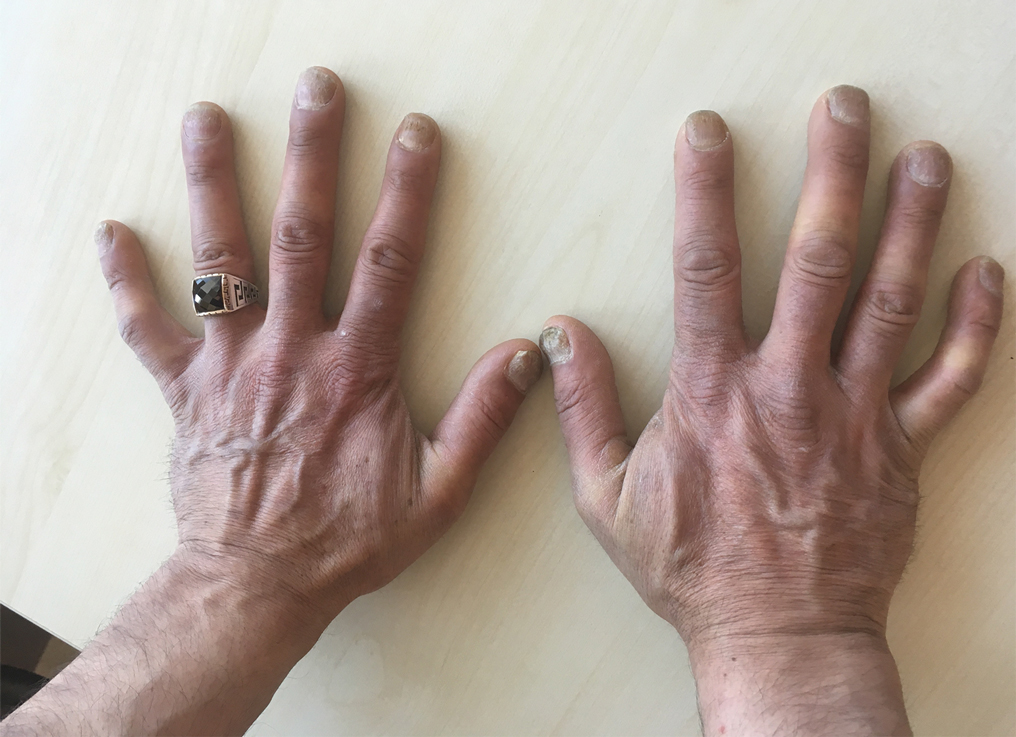
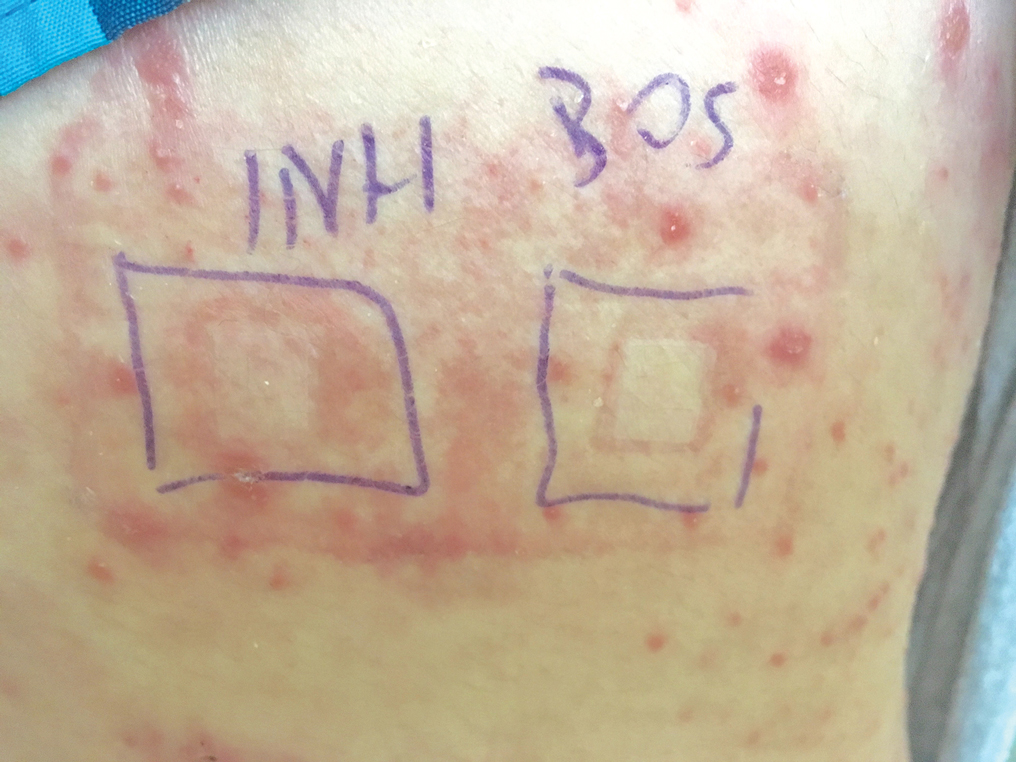
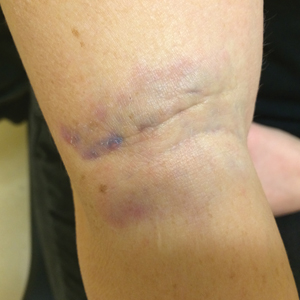
A 37-year-old man presented with a 15-year history of severe psoriasis with frequent flares. He was treated with various topical and systemic agents including acitretin and methotrexate at 4-year intervals. Despite the addition of phototherapy, he underwent a new treatment with anti–TNF-α, as the disease control with other treatments was insufficient. Before starting anti–TNF-α, preventive treatment against TB with INH (300 mg/d) was indicated with 20 mm of purified protein derivative. On approximately the 20th day of treatment, he developed pruritic erythema with desquamation and exfoliation localized to the hands and feet (Figure 1). Isoniazid was discontinued and a topical steroid was initiated. After 3 weeks, the skin lesions were completely improved and INH was reinitiated at the same dose with antihistamine prophylaxis (oral levocetirizine 5 mg/d). Seven days later, similar skin lesions presented that were more extensive on the arms and legs (Figure 2). Complete blood cell counts, renal and hepatic function tests, and hepatitis markers were within reference range in consultation with the allergy division. To distinguish the lesions from a psoriasis attack, a punch biopsy of the eruptive dermatitis showed erythema multiforme–like dermatitis including dermal edema and perivascular lymphocytic infiltration with no relation to psoriasis but consistent with a drug eruption. Isoniazid was discontinued, and the skin lesions resolved after 4 weeks of topical steroid and oral antihistamine use (Figure 3). There was no other drug use except INH, and a skin patch test with INH was positive at 72 hours (Figure 4). Skin tests with INH were done to 5 healthy lesions that were negative. Finally, TB prophylaxis was performed with rifampicin (10 mg/kg/d [600 mg/d]) for 4 months with no ADRs. The patient’s psoriasis lesions improved with anti–TNF-α that was initiated 1 month after starting TB prevention with rifampicin.
This case of erythema multiforme–like dermatitis was diagnosed with acral involvement, a positive patch test to INH, and lymphocytic inflammation in a skin biopsy. It was a drug-induced reaction, as skin lesions developed during INH intake and improved after drug withdrawal.
Isoniazid, also known as isonicotinylhydrazide, is an oral antibiotic used for the treatment of TB and other mycobacteria. Protective treatment against latent TB primarily is done with daily INH for 6 or 9 months; alternatively, INH may be taken weekly with rifapentine for 3 months or daily with rifampicin for 4 months. Daily rifampicin alone for 4 months also is an option. In general, these regimens have similar efficacy; however, in terms of safety, the rifampicin and rifapentine combination regimens have fewer hepatotoxicity events compared to the INH alone regimen, but there are more cutaneous and flulike reactions and gastrointestinal intolerance.4 Cutaneous ADRs to TB treatment such as mild itchiness and cutaneous eruptions usually are observed within 2 months of drug initiation. Pyrazinamide was reported as the most common drug associated with cutaneous ADRs, and INH was the rarest offending drug.5
The frequency of ADRs to INH is approximately 5.4%, and the most prevalent ADRs include asymptomatic elevation of serum liver enzyme concentrations, peripheral neuropathy, and hepatotoxicity, and skin lesions are less common.2 Our patient’s laboratory test results excluded vitamin B deficiency, hepatic and renal dysfunction, and neuropathy.
Previously reported skin reactions related to INH were late-type reactions such as maculopapular rash, dermatitis, erythema multiforme, drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, Stevens-Johnson syndrome, and toxic epidermal necrolysis.5,6 The concerning prediagnosis of psoriatic exacerbation in our patient was ruled out by the absence of typical skin lesions such as well-defined, erythematous plaques and pustules and atypical localization such as the dorsal hands and feet rather than the knees, elbows, lumbosacral region, scalp, and abdomen, which is typical of psoriasis. DRESS syndrome was unlikely with the absence of fever, lymphadenopathy, hypereosinophilia, leukocytosis, and renal and hepatic dysfunction.7 There were no widespread blisters, epidermal detachment, or mucosal involvement on the trunk or face typically associated with Stevens-Johnson syndrome and toxic epidermal necrolysis.7,8 A possible diagnosis of contact dermatitis was suspected with likely skin lesions as exfoliation and chapping, typical localization on the hands and feet, and positive patch test that supported sensitization to the drug. However, the patient’s skin lesions were not eczematous (characterized by erythema, vesiculation, exudation, or bullous edema in the acute phase), and were not localized to areas of irritant exposure.3 In our patient, erythematoedematous lesions in an acral distribution with no mucosal involvement and systemic exposure to INH was compatible with erythema multiforme, whereas the absence of target appearance, positive patch test, and late appearance were incompatible with erythema multiforme.8
Because the clinical picture did not fit contact dermatitis or erythema multiforme, a diagnosis of erythema multiforme–like noneczematous dermatitis was suggested. Noneczematous dermatitis has subtypes that include purpuric, lichenoid, pustular, lymphomatoid, dyshidrosiform, and pigmented, as well as erythema multiforme–like contact eruptions.9 These clinical entities are not associated with contact exposure, but are related to systemic exposure, as seen in our patient.10 The patch test positivity and skin biopsy report also supported the diagnosis of erythema multiforme–like dermatitis. Erythema multiforme–like dermatitis is thought to be caused by medications or infections inducing immunocomplexes and lymphocytic infiltration in the dermis and subepidermis. Nevertheless, the prognosis was self-limiting in both.8 The clinical polymorphism caused by INH in this patient was suggested to be related with individual susceptibility, variability of contact-activating modalities, and the targeted cutaneous structures. Furthermore, among the risk factors for cutaneous ADRs—HIV, polypharmacy, older age, and preexisting renal and liver impairment—the only notable factor in this patient was psoriasis as an autoimmune disorder.
Patients with skin diseases such as psoriasis should be followed up by closer monitoring during INH use. Withdrawal of the drug and symptomatic treatment of the lesions with corticosteroid and antihistamine are the first steps of drug intolerance. After complete recovery and termination of antiallergic drugs, diagnostic tests are recommended if the drug reaction was not life-threatening. Skin prick and intradermal tests are useful in early-type drug reactions, whereas patch testing and late evaluation of an intradermal test may be helpful in the diagnosis of delayed-type reactions. The full dose of INH is avoided in an intradermal test against irritation. A patch test with INH was performed by diluting a 100-mg tablet with 1 mL of distilled water, and used as 1/100, 1/10, and 1/1 dilutions.8 Patch testing with INH also was done in 5 healthy control patients to exclude the irritation effect in this case. The rechallenge of INH was done in a controlled manner in our patient to rule out psoriasis activation since it was a localized skin reaction with no serious ADR. An oral provocation test with the culprit drug is the gold standard of drug allergy diagnosis that should be done in a tertiary hospital with an intensive care unit.
This case of erythema multiforme–like dermatitis due to INH is interesting due to systemic intake of INH, which resulted in dermatitis with localized involvement similar to erythema multiforme but with no immunologic processes or prior sensitization. With the increasing use of anti–TNF-α treatment, INH use will be more prevalent than in the past for the treatment of latent TB. Even though the skin-restricted ADRs of INH are rare and minor, particular attention should be paid to patients with dermatologic diseases. In our case, diagnostic drug allergy evaluation was performed to optimize the second-line treatment of TB infection, in addition to early withdrawal of the culprit drug.
- Vide J, Magina S. Moderate to severe psoriasis treatment challenges through the era of biological drugs.An Bras Dermatol. 2017;92:668-674.
- Gülbay BE, Gürkan OU, Yildiz OA, et al. Side effects due to primary antituberculosis drugs during the initial phase of therapy in 1149 hospitalized patients for tuberculosis. Respir Med. 2006;100:1834-1842.
- Holdiness MR. Contact dermatitis to antituberculosis drugs. Contact Dermatitis. 1986;15:282-288.
- Getahun H, Matteelli A, Abubakar I, et al. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J. 2015;46:1563-1576.
- Tan WC, Ong CK, Kang SC, et al. Two years review of cutaneous adverse drug reaction from first line anti-tuberculous drugs. Med J Malaysia. 2007;62:143-146.
- Özkaya E.Eczematous-type multiple drug allergy from isoniazid and ethambutol with positive patch test results. Cutis. 2013;92:121-124.
- Fernando SL. Drug-reaction eosinophilia and systemic symptoms and drug-induced hypersensitivity syndrome. Australas J Dermatol. 2014;55:15-23.
- Rebollo S, Sanchez P, Vega JM, et al. Hypersensitivity syndrome from isoniazid with positive patch test. Contact Dermatitis. 2001;45:306.
- Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902.
- Bonamonte D, Foti C, Vestita M, et al. Nummular eczema and contact allergy: a retrospective study. Dermatitis. 2012;23:153-157.
To the Editor:
Psoriasis vulgaris is a chronic autoimmune inflammatory disease and biologic agents, such as anti–tumor necrosis factor α (TNF-α), are alternative drugs in case of resistance or adverse events to conventional ones.1 The limitation of these agents is immunosuppression that may cause infections such as tuberculosis (TB). Prophylaxis is indicated to latent TB diseases if the purified protein derivative (tuberculin) skin test is higher than 5 mm before starting these treatments. The challenge in TB treatment is adverse drug reactions (ADRs) that are reported in 4% to 6% of cases.2,3
Erythema multiforme–like dermatitis is a rare skin rash that develops due to isoniazid (INH). The clinical presentation includes erythematoedematous lesions in an acral distribution with no mucosal involvement and systemic exposure to INH. Skin biopsy and patch tests are the supportive diagnostic methods. Isoniazid-associated skin rashes rarely are reported and generally are not severe enough to terminate the drug. We present a patient with psoriasis who received TB prophylaxis before anti–TNF-α use. He presented with erythema multiforme–like dermatitis due to INH. Withdrawal of the drug and treatment of the lesions were the first steps of intolerance, followed by a patch test with the culprit drug after recovery. We discuss the diagnostic drug allergy evaluation and treatment approach.
A 37-year-old man presented with a 15-year history of severe psoriasis with frequent flares. He was treated with various topical and systemic agents including acitretin and methotrexate at 4-year intervals. Despite the addition of phototherapy, he underwent a new treatment with anti–TNF-α, as the disease control with other treatments was insufficient. Before starting anti–TNF-α, preventive treatment against TB with INH (300 mg/d) was indicated with 20 mm of purified protein derivative. On approximately the 20th day of treatment, he developed pruritic erythema with desquamation and exfoliation localized to the hands and feet (Figure 1). Isoniazid was discontinued and a topical steroid was initiated. After 3 weeks, the skin lesions were completely improved and INH was reinitiated at the same dose with antihistamine prophylaxis (oral levocetirizine 5 mg/d). Seven days later, similar skin lesions presented that were more extensive on the arms and legs (Figure 2). Complete blood cell counts, renal and hepatic function tests, and hepatitis markers were within reference range in consultation with the allergy division. To distinguish the lesions from a psoriasis attack, a punch biopsy of the eruptive dermatitis showed erythema multiforme–like dermatitis including dermal edema and perivascular lymphocytic infiltration with no relation to psoriasis but consistent with a drug eruption. Isoniazid was discontinued, and the skin lesions resolved after 4 weeks of topical steroid and oral antihistamine use (Figure 3). There was no other drug use except INH, and a skin patch test with INH was positive at 72 hours (Figure 4). Skin tests with INH were done to 5 healthy lesions that were negative. Finally, TB prophylaxis was performed with rifampicin (10 mg/kg/d [600 mg/d]) for 4 months with no ADRs. The patient’s psoriasis lesions improved with anti–TNF-α that was initiated 1 month after starting TB prevention with rifampicin.
This case of erythema multiforme–like dermatitis was diagnosed with acral involvement, a positive patch test to INH, and lymphocytic inflammation in a skin biopsy. It was a drug-induced reaction, as skin lesions developed during INH intake and improved after drug withdrawal.
Isoniazid, also known as isonicotinylhydrazide, is an oral antibiotic used for the treatment of TB and other mycobacteria. Protective treatment against latent TB primarily is done with daily INH for 6 or 9 months; alternatively, INH may be taken weekly with rifapentine for 3 months or daily with rifampicin for 4 months. Daily rifampicin alone for 4 months also is an option. In general, these regimens have similar efficacy; however, in terms of safety, the rifampicin and rifapentine combination regimens have fewer hepatotoxicity events compared to the INH alone regimen, but there are more cutaneous and flulike reactions and gastrointestinal intolerance.4 Cutaneous ADRs to TB treatment such as mild itchiness and cutaneous eruptions usually are observed within 2 months of drug initiation. Pyrazinamide was reported as the most common drug associated with cutaneous ADRs, and INH was the rarest offending drug.5
The frequency of ADRs to INH is approximately 5.4%, and the most prevalent ADRs include asymptomatic elevation of serum liver enzyme concentrations, peripheral neuropathy, and hepatotoxicity, and skin lesions are less common.2 Our patient’s laboratory test results excluded vitamin B deficiency, hepatic and renal dysfunction, and neuropathy.
Previously reported skin reactions related to INH were late-type reactions such as maculopapular rash, dermatitis, erythema multiforme, drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, Stevens-Johnson syndrome, and toxic epidermal necrolysis.5,6 The concerning prediagnosis of psoriatic exacerbation in our patient was ruled out by the absence of typical skin lesions such as well-defined, erythematous plaques and pustules and atypical localization such as the dorsal hands and feet rather than the knees, elbows, lumbosacral region, scalp, and abdomen, which is typical of psoriasis. DRESS syndrome was unlikely with the absence of fever, lymphadenopathy, hypereosinophilia, leukocytosis, and renal and hepatic dysfunction.7 There were no widespread blisters, epidermal detachment, or mucosal involvement on the trunk or face typically associated with Stevens-Johnson syndrome and toxic epidermal necrolysis.7,8 A possible diagnosis of contact dermatitis was suspected with likely skin lesions as exfoliation and chapping, typical localization on the hands and feet, and positive patch test that supported sensitization to the drug. However, the patient’s skin lesions were not eczematous (characterized by erythema, vesiculation, exudation, or bullous edema in the acute phase), and were not localized to areas of irritant exposure.3 In our patient, erythematoedematous lesions in an acral distribution with no mucosal involvement and systemic exposure to INH was compatible with erythema multiforme, whereas the absence of target appearance, positive patch test, and late appearance were incompatible with erythema multiforme.8
Because the clinical picture did not fit contact dermatitis or erythema multiforme, a diagnosis of erythema multiforme–like noneczematous dermatitis was suggested. Noneczematous dermatitis has subtypes that include purpuric, lichenoid, pustular, lymphomatoid, dyshidrosiform, and pigmented, as well as erythema multiforme–like contact eruptions.9 These clinical entities are not associated with contact exposure, but are related to systemic exposure, as seen in our patient.10 The patch test positivity and skin biopsy report also supported the diagnosis of erythema multiforme–like dermatitis. Erythema multiforme–like dermatitis is thought to be caused by medications or infections inducing immunocomplexes and lymphocytic infiltration in the dermis and subepidermis. Nevertheless, the prognosis was self-limiting in both.8 The clinical polymorphism caused by INH in this patient was suggested to be related with individual susceptibility, variability of contact-activating modalities, and the targeted cutaneous structures. Furthermore, among the risk factors for cutaneous ADRs—HIV, polypharmacy, older age, and preexisting renal and liver impairment—the only notable factor in this patient was psoriasis as an autoimmune disorder.
Patients with skin diseases such as psoriasis should be followed up by closer monitoring during INH use. Withdrawal of the drug and symptomatic treatment of the lesions with corticosteroid and antihistamine are the first steps of drug intolerance. After complete recovery and termination of antiallergic drugs, diagnostic tests are recommended if the drug reaction was not life-threatening. Skin prick and intradermal tests are useful in early-type drug reactions, whereas patch testing and late evaluation of an intradermal test may be helpful in the diagnosis of delayed-type reactions. The full dose of INH is avoided in an intradermal test against irritation. A patch test with INH was performed by diluting a 100-mg tablet with 1 mL of distilled water, and used as 1/100, 1/10, and 1/1 dilutions.8 Patch testing with INH also was done in 5 healthy control patients to exclude the irritation effect in this case. The rechallenge of INH was done in a controlled manner in our patient to rule out psoriasis activation since it was a localized skin reaction with no serious ADR. An oral provocation test with the culprit drug is the gold standard of drug allergy diagnosis that should be done in a tertiary hospital with an intensive care unit.
This case of erythema multiforme–like dermatitis due to INH is interesting due to systemic intake of INH, which resulted in dermatitis with localized involvement similar to erythema multiforme but with no immunologic processes or prior sensitization. With the increasing use of anti–TNF-α treatment, INH use will be more prevalent than in the past for the treatment of latent TB. Even though the skin-restricted ADRs of INH are rare and minor, particular attention should be paid to patients with dermatologic diseases. In our case, diagnostic drug allergy evaluation was performed to optimize the second-line treatment of TB infection, in addition to early withdrawal of the culprit drug.
To the Editor:
Psoriasis vulgaris is a chronic autoimmune inflammatory disease and biologic agents, such as anti–tumor necrosis factor α (TNF-α), are alternative drugs in case of resistance or adverse events to conventional ones.1 The limitation of these agents is immunosuppression that may cause infections such as tuberculosis (TB). Prophylaxis is indicated to latent TB diseases if the purified protein derivative (tuberculin) skin test is higher than 5 mm before starting these treatments. The challenge in TB treatment is adverse drug reactions (ADRs) that are reported in 4% to 6% of cases.2,3
Erythema multiforme–like dermatitis is a rare skin rash that develops due to isoniazid (INH). The clinical presentation includes erythematoedematous lesions in an acral distribution with no mucosal involvement and systemic exposure to INH. Skin biopsy and patch tests are the supportive diagnostic methods. Isoniazid-associated skin rashes rarely are reported and generally are not severe enough to terminate the drug. We present a patient with psoriasis who received TB prophylaxis before anti–TNF-α use. He presented with erythema multiforme–like dermatitis due to INH. Withdrawal of the drug and treatment of the lesions were the first steps of intolerance, followed by a patch test with the culprit drug after recovery. We discuss the diagnostic drug allergy evaluation and treatment approach.
A 37-year-old man presented with a 15-year history of severe psoriasis with frequent flares. He was treated with various topical and systemic agents including acitretin and methotrexate at 4-year intervals. Despite the addition of phototherapy, he underwent a new treatment with anti–TNF-α, as the disease control with other treatments was insufficient. Before starting anti–TNF-α, preventive treatment against TB with INH (300 mg/d) was indicated with 20 mm of purified protein derivative. On approximately the 20th day of treatment, he developed pruritic erythema with desquamation and exfoliation localized to the hands and feet (Figure 1). Isoniazid was discontinued and a topical steroid was initiated. After 3 weeks, the skin lesions were completely improved and INH was reinitiated at the same dose with antihistamine prophylaxis (oral levocetirizine 5 mg/d). Seven days later, similar skin lesions presented that were more extensive on the arms and legs (Figure 2). Complete blood cell counts, renal and hepatic function tests, and hepatitis markers were within reference range in consultation with the allergy division. To distinguish the lesions from a psoriasis attack, a punch biopsy of the eruptive dermatitis showed erythema multiforme–like dermatitis including dermal edema and perivascular lymphocytic infiltration with no relation to psoriasis but consistent with a drug eruption. Isoniazid was discontinued, and the skin lesions resolved after 4 weeks of topical steroid and oral antihistamine use (Figure 3). There was no other drug use except INH, and a skin patch test with INH was positive at 72 hours (Figure 4). Skin tests with INH were done to 5 healthy lesions that were negative. Finally, TB prophylaxis was performed with rifampicin (10 mg/kg/d [600 mg/d]) for 4 months with no ADRs. The patient’s psoriasis lesions improved with anti–TNF-α that was initiated 1 month after starting TB prevention with rifampicin.
This case of erythema multiforme–like dermatitis was diagnosed with acral involvement, a positive patch test to INH, and lymphocytic inflammation in a skin biopsy. It was a drug-induced reaction, as skin lesions developed during INH intake and improved after drug withdrawal.
Isoniazid, also known as isonicotinylhydrazide, is an oral antibiotic used for the treatment of TB and other mycobacteria. Protective treatment against latent TB primarily is done with daily INH for 6 or 9 months; alternatively, INH may be taken weekly with rifapentine for 3 months or daily with rifampicin for 4 months. Daily rifampicin alone for 4 months also is an option. In general, these regimens have similar efficacy; however, in terms of safety, the rifampicin and rifapentine combination regimens have fewer hepatotoxicity events compared to the INH alone regimen, but there are more cutaneous and flulike reactions and gastrointestinal intolerance.4 Cutaneous ADRs to TB treatment such as mild itchiness and cutaneous eruptions usually are observed within 2 months of drug initiation. Pyrazinamide was reported as the most common drug associated with cutaneous ADRs, and INH was the rarest offending drug.5
The frequency of ADRs to INH is approximately 5.4%, and the most prevalent ADRs include asymptomatic elevation of serum liver enzyme concentrations, peripheral neuropathy, and hepatotoxicity, and skin lesions are less common.2 Our patient’s laboratory test results excluded vitamin B deficiency, hepatic and renal dysfunction, and neuropathy.
Previously reported skin reactions related to INH were late-type reactions such as maculopapular rash, dermatitis, erythema multiforme, drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, Stevens-Johnson syndrome, and toxic epidermal necrolysis.5,6 The concerning prediagnosis of psoriatic exacerbation in our patient was ruled out by the absence of typical skin lesions such as well-defined, erythematous plaques and pustules and atypical localization such as the dorsal hands and feet rather than the knees, elbows, lumbosacral region, scalp, and abdomen, which is typical of psoriasis. DRESS syndrome was unlikely with the absence of fever, lymphadenopathy, hypereosinophilia, leukocytosis, and renal and hepatic dysfunction.7 There were no widespread blisters, epidermal detachment, or mucosal involvement on the trunk or face typically associated with Stevens-Johnson syndrome and toxic epidermal necrolysis.7,8 A possible diagnosis of contact dermatitis was suspected with likely skin lesions as exfoliation and chapping, typical localization on the hands and feet, and positive patch test that supported sensitization to the drug. However, the patient’s skin lesions were not eczematous (characterized by erythema, vesiculation, exudation, or bullous edema in the acute phase), and were not localized to areas of irritant exposure.3 In our patient, erythematoedematous lesions in an acral distribution with no mucosal involvement and systemic exposure to INH was compatible with erythema multiforme, whereas the absence of target appearance, positive patch test, and late appearance were incompatible with erythema multiforme.8
Because the clinical picture did not fit contact dermatitis or erythema multiforme, a diagnosis of erythema multiforme–like noneczematous dermatitis was suggested. Noneczematous dermatitis has subtypes that include purpuric, lichenoid, pustular, lymphomatoid, dyshidrosiform, and pigmented, as well as erythema multiforme–like contact eruptions.9 These clinical entities are not associated with contact exposure, but are related to systemic exposure, as seen in our patient.10 The patch test positivity and skin biopsy report also supported the diagnosis of erythema multiforme–like dermatitis. Erythema multiforme–like dermatitis is thought to be caused by medications or infections inducing immunocomplexes and lymphocytic infiltration in the dermis and subepidermis. Nevertheless, the prognosis was self-limiting in both.8 The clinical polymorphism caused by INH in this patient was suggested to be related with individual susceptibility, variability of contact-activating modalities, and the targeted cutaneous structures. Furthermore, among the risk factors for cutaneous ADRs—HIV, polypharmacy, older age, and preexisting renal and liver impairment—the only notable factor in this patient was psoriasis as an autoimmune disorder.
Patients with skin diseases such as psoriasis should be followed up by closer monitoring during INH use. Withdrawal of the drug and symptomatic treatment of the lesions with corticosteroid and antihistamine are the first steps of drug intolerance. After complete recovery and termination of antiallergic drugs, diagnostic tests are recommended if the drug reaction was not life-threatening. Skin prick and intradermal tests are useful in early-type drug reactions, whereas patch testing and late evaluation of an intradermal test may be helpful in the diagnosis of delayed-type reactions. The full dose of INH is avoided in an intradermal test against irritation. A patch test with INH was performed by diluting a 100-mg tablet with 1 mL of distilled water, and used as 1/100, 1/10, and 1/1 dilutions.8 Patch testing with INH also was done in 5 healthy control patients to exclude the irritation effect in this case. The rechallenge of INH was done in a controlled manner in our patient to rule out psoriasis activation since it was a localized skin reaction with no serious ADR. An oral provocation test with the culprit drug is the gold standard of drug allergy diagnosis that should be done in a tertiary hospital with an intensive care unit.
This case of erythema multiforme–like dermatitis due to INH is interesting due to systemic intake of INH, which resulted in dermatitis with localized involvement similar to erythema multiforme but with no immunologic processes or prior sensitization. With the increasing use of anti–TNF-α treatment, INH use will be more prevalent than in the past for the treatment of latent TB. Even though the skin-restricted ADRs of INH are rare and minor, particular attention should be paid to patients with dermatologic diseases. In our case, diagnostic drug allergy evaluation was performed to optimize the second-line treatment of TB infection, in addition to early withdrawal of the culprit drug.
- Vide J, Magina S. Moderate to severe psoriasis treatment challenges through the era of biological drugs.An Bras Dermatol. 2017;92:668-674.
- Gülbay BE, Gürkan OU, Yildiz OA, et al. Side effects due to primary antituberculosis drugs during the initial phase of therapy in 1149 hospitalized patients for tuberculosis. Respir Med. 2006;100:1834-1842.
- Holdiness MR. Contact dermatitis to antituberculosis drugs. Contact Dermatitis. 1986;15:282-288.
- Getahun H, Matteelli A, Abubakar I, et al. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J. 2015;46:1563-1576.
- Tan WC, Ong CK, Kang SC, et al. Two years review of cutaneous adverse drug reaction from first line anti-tuberculous drugs. Med J Malaysia. 2007;62:143-146.
- Özkaya E.Eczematous-type multiple drug allergy from isoniazid and ethambutol with positive patch test results. Cutis. 2013;92:121-124.
- Fernando SL. Drug-reaction eosinophilia and systemic symptoms and drug-induced hypersensitivity syndrome. Australas J Dermatol. 2014;55:15-23.
- Rebollo S, Sanchez P, Vega JM, et al. Hypersensitivity syndrome from isoniazid with positive patch test. Contact Dermatitis. 2001;45:306.
- Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902.
- Bonamonte D, Foti C, Vestita M, et al. Nummular eczema and contact allergy: a retrospective study. Dermatitis. 2012;23:153-157.
- Vide J, Magina S. Moderate to severe psoriasis treatment challenges through the era of biological drugs.An Bras Dermatol. 2017;92:668-674.
- Gülbay BE, Gürkan OU, Yildiz OA, et al. Side effects due to primary antituberculosis drugs during the initial phase of therapy in 1149 hospitalized patients for tuberculosis. Respir Med. 2006;100:1834-1842.
- Holdiness MR. Contact dermatitis to antituberculosis drugs. Contact Dermatitis. 1986;15:282-288.
- Getahun H, Matteelli A, Abubakar I, et al. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J. 2015;46:1563-1576.
- Tan WC, Ong CK, Kang SC, et al. Two years review of cutaneous adverse drug reaction from first line anti-tuberculous drugs. Med J Malaysia. 2007;62:143-146.
- Özkaya E.Eczematous-type multiple drug allergy from isoniazid and ethambutol with positive patch test results. Cutis. 2013;92:121-124.
- Fernando SL. Drug-reaction eosinophilia and systemic symptoms and drug-induced hypersensitivity syndrome. Australas J Dermatol. 2014;55:15-23.
- Rebollo S, Sanchez P, Vega JM, et al. Hypersensitivity syndrome from isoniazid with positive patch test. Contact Dermatitis. 2001;45:306.
- Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902.
- Bonamonte D, Foti C, Vestita M, et al. Nummular eczema and contact allergy: a retrospective study. Dermatitis. 2012;23:153-157.
Practice Points
- Hypersensitivity skin reactions to antituberculosis (TB) drugs are on the rise due to the increasing use of anti–tumor necrosis factor α. Isoniazid (INH) use will be more prevalent than in the past for the treatment of latent TB.
- Even though the skin-restricted adverse events to INH are rare and minor, particular attention should be paid to patients with dermatologic diseases such as psoriasis.
Botanical Briefs: Phytophotodermatitis Is an Occupational and Recreational Dermatosis in the Limelight
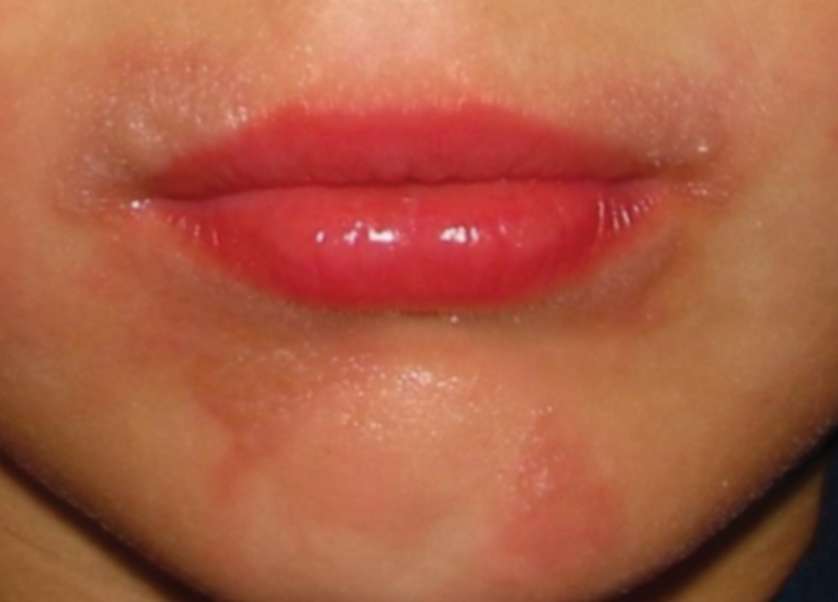
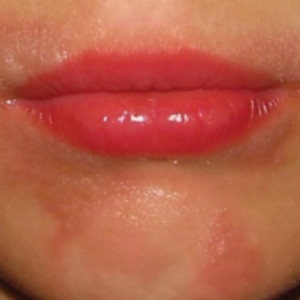
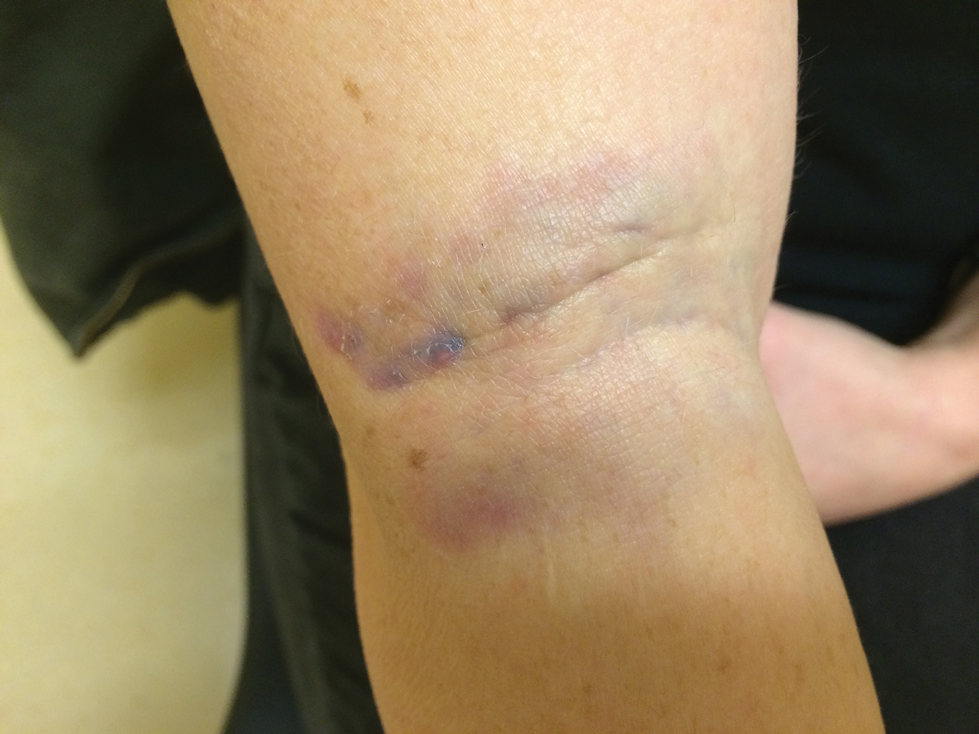
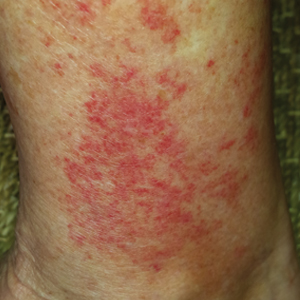
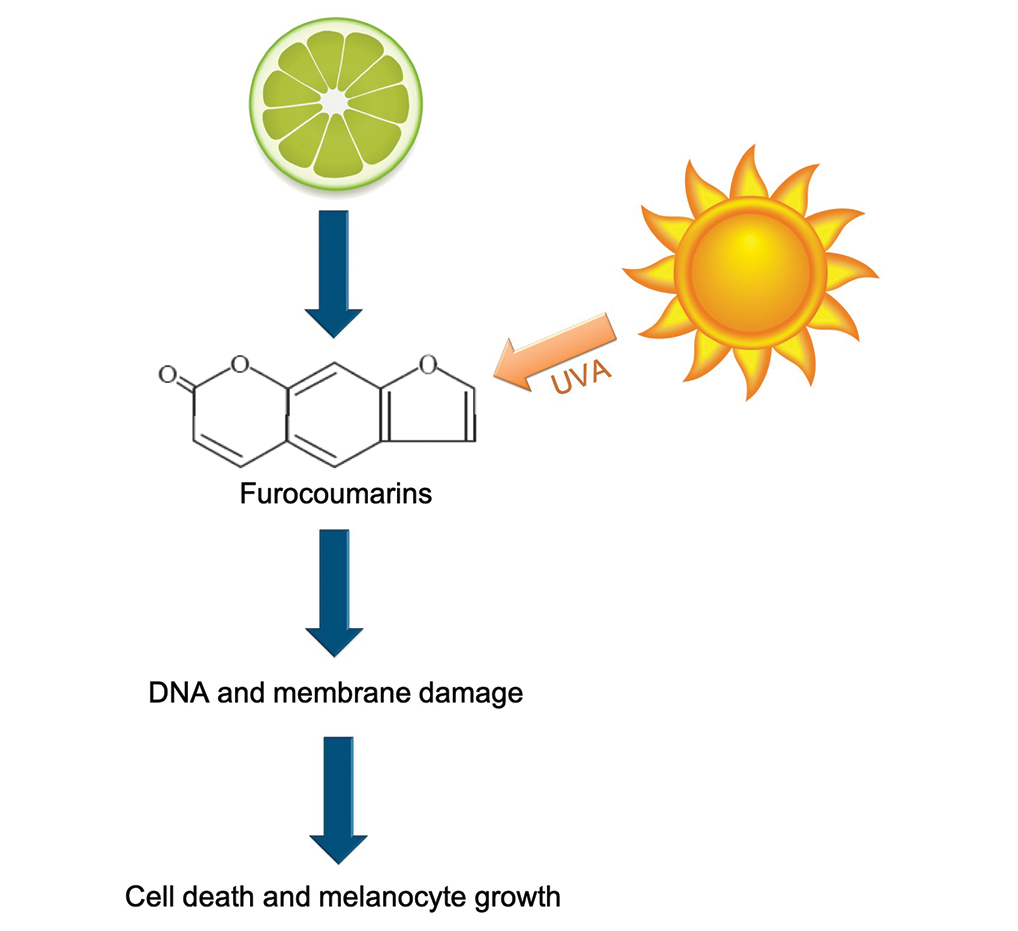
Phytophotodermatitis (PPD) is a nonallergic contact dermatitis and thus is independent of the immune system, so prior sensitization is not required.1-3 It sometimes is known by colorful names such as margarita photodermatitis, in which a slice of lime in a refreshing summer drink may be etiologic,4,5 or berloque dermatitis, caused by exposure to perfumes containing bergapten (5-methoxypsoralen).6,7 Phytophotodermatitis may develop when phototoxic agents such as furocoumarins, which protect plants from fungal pathogens, and psoralens are applied to the skin followed by exposure to UV light, more specifically in the UVA range of 320 to 400 nm. Thus, these chemicals produce a phototoxic rather than photoallergic reaction, leading to cellular damage. Furocoumarins and psoralens often are found in plants such as celery and figs as well as in citrus fruits such as limes, lemons, and grapefruits. Exposure may be cryptic, as the patient may not consider or mention the eruption as possibly caused by activities such as soaking one’s feet in a folk remedy containing fig leaves.7,8 Once these phototoxic agents come in contact with the skin, the symptoms of PPD may arise within 24 hours of exposure, beginning as an acute dermatitis with erythema, edema, vesicles, or bullae accompanied by pain and itching.
Etiology
Phytophotodermatitis is caused by exposure to several different types of plants, including Ficus carica (common fig), the genus Citrus (eg, lime, lemon), or Pastina sativa (wild parsnip). Each of these contain furocoumarins and psoralens—phototoxic agents that cause cellular damage with epidermal necrosis and resultant pain when the skin is exposed to UVA light.1-4 There are 2 types of photochemical reactions in PPD: type I reactions occur in the absence of oxygen, whereas oxygen is present in type II reactions. Both damage cell membranes and DNA, which then results in DNA interstrand cross-linking between the psoralen furan ring and the thymine or cytosine of DNA, activating arachidonic acid metabolic pathways to produce cell death.1
Epidemiology
The incidence of PPD is unknown due to the high variability of reactions in individuals spanning from children to the elderly. It can be caused by many different wild and domestic plants in many areas of the world and can affect any individual regardless of age, race, gender, or ethnicity. Some individuals may be affected by hyperpigmentation without prominent inflammation.8 Diagnosis of PPD can be challenging, and an occupation and recreational history of exposure or recent travel with possible contact with plants may be required.
Occupational Dermatitis
Recreational Dermatitis
Phytophotodermatitis may be caused by exposure to phototoxic agents during leisure activities. Recreational exposure can occur almost anywhere, including in the kitchen, backyard, park, or woods, as well as at the beach. One notable culprit in recreational PPD is cooking with limes, parsley, or parsnips—plants that often are employed as garnishes in dishes, allowing early exposure of juices on the hands. Individuals who garden recreationally should be aware of ornamental plants such as hogweed and figs, which are notorious for causing PPD.13 Children’s camp counselors should have knowledge of PPD, as children have considerable curiosity and may touch or play with attractive plants such as hogweed. Children enjoying sports in parks can accidentally fall onto or be exposed to wild parsnip or hogweed growing nearby and wake up the next day with erythema and burning.14 Photoprotection is important, but sunscreens containing carrot extract can produce PPD.15 Widespread PPD over 80% of the body surface area due to sunbathing after applying fig leaf tea as a tanning agent has been described.16 Eating figs does not cause photosensitization unless the juice is smeared onto the skin. Margarita dermatitis and “Mexican beer dermatitis” can occur due to limes and other citrus fruits being used as ingredients in summer drinks.5 Similarly, preparing sangria may produce PPD from lime and lemon juices.17 In one report, hiking in Corsica resulted in PPD following incidental contact with the endemic plant Peucedanum paniculatum.18
Perfume (Berloque) Dermatitis
Perfume dermatitis, or berloque dermatitis, is a type of PPD for which the name is derived from the German word berlock or the French word berloque meaning trinket or charm; it was first described in 1925 by Rosenthal7 with regard to pendantlike streaks of pigmentation on the neck, face, arms, or trunk. The dermatitis develops due to bergapten, a component of bergamot oil, which is derived from the rind of Citrus bergamia. Many perfumes contain bergamot oil, but the incidence of this condition has been diminished due to use of artificial bergamot oil.6
Clinical Manifestation
Phytophotodermatitis is first evident as erythematous patches that appear within 24 hours of initial exposure to a phototoxic agent and UVA light, sometimes with a burning sensation. Solar exposure within 48 hours of sufficient plant exposure is required. Perfuse sweating may enhance the reaction.19 Rarely, it first may be seen with the sudden appearance of
Differential Diagnosis
Phytophotodermatitis may resemble other types of dermatitis, particularly other forms of contact dermatitis such poison ivy, and occasionally other environmental simulants such as jellyfish stings.1-6,20,21 Photosensitizing disorders including porphyria cutanea tarda, pseudoporphyria, and lupus erythematosus must be distinguished from PPD.22-24 Photosensitizing medications such tetracyclines, thiazide diuretics, sulfonamides, griseofulvin, and sulfonylureas should be considered. Airborne contact dermatitis may resemble PPD, as when poison ivy is burned and is exposed to the skin in sites of airborne contact.20 Excessive solar exposure is popular, particularly among adolescents, so sunburn and sunburnlike reactions can be noteworthy.25,26
Treatment
Phytophotodermatitis can be treated with topical steroids, sometimes adding an oral antihistamine, and occasionally oral steroids.2-4 Localized pain or a burning sensation should respond to therapy. Alternatively, a cold compress applied to the skin can relieve the pain and pruritus, and the burn can be debrided and dressed daily with silver sulfadiazine plus an oral nonsteroidal anti-inflammatory drug. This eruption should be self-limited as long as it is recognized early and the cause avoided. Management of acute exposure includes prompt application of soap and water and avoidance of UV light exposure for 48 to 72 hours to prevent psoralen photoactivation.
Because PPD is essentially a chemical burn, a burn protocol and possible referral to a burn center may be needed, whether the reaction is acute or widespread.11,12,14,27,28 Surgical debridement and skin grafting rarely may be mandated.14 Postinflammatory hyperpigmentation may ensue as the dermatitis resolves but is not common.
The best approach for PPD is prevention (Figure 2). Individuals who are at risk should be aware of their surroundings and potential plants of concern and employ personal protective equipment to shield the skin from plant sap, which should be promptly removed if it comes in contact with the skin.
- Zhang R, Zhu W. Phytophotodermatitis due to Chinese herbal medicine decoction. Indian J Dermatol. 2011;56:329-331.
- Harshman J, Quan Y, Hsiang D. Phytophotodermatitis: rash with many faces. Can Fam Physician. 2017;63:938-940.
- Imen MS, Ahmadabadi A, Tavousi SH, et al. The curious cases of burn by fig tree leaves. Indian J Dermatol. 2019;64:71-73.
- Hankinson A, Lloyd B, Alweis R. Lime-induced phytophotodermatitis [published online September 29, 2014]. J Community Hosp Intern Med Perspect. doi:10.3402/jchimp.v4.25090
- Abramowitz AI, Resnik KS, Cohen KR. Margarita photodermatitis. N Engl J Med. 2013;328:891.
- Quaak MS, Martens H, Hassing RJ, et al. The sunny side of lime. J Travel Med. 2012;19:327-328.
- Rosenthal O. Berloque dermatitis: Berliner Dermatologische Gesellschaft. Dermatol Zeitschrift. 1925;42:295.
- Choi JY, Hwang S, Lee SH, et al. Asymptomatic hyperpigmentation without preceding inflammation as a clinical feature of citrus fruits–induced phytophotodermatitis. Ann Dermatol. 2018;30:75-78.
- Wynn P, Bell S. Phytophotodermatitis in grounds operatives. Occup Med (Lond). 2005;55:393-395.
- Klimaszyk P, Klimaszyk D, Piotrowiak M, et al. Unusual complications after occupational exposure to giant hogweed (Heracleum mantegazzianum): a case report. Int J Occup Med Environ Health. 2014;27:141-144.
- Downs JW, Cumpston KL, Feldman MJ. Giant hogweed phytophotodermatitis. Clin Toxicol (Phila). 2019;57:822-823.
- Maso MJ, Ruszkowski AM, Bauerle J, et al. Celery phytophotodermatitis in a chef. Arch Dermatol. 1991;127:912-913.
- Derraik JG, Rademaker M. Phytophotodermatitis caused by contact with a fig tree (Ficus carica). New Zealand Med J. 2007;120:U2720.
- Chan JC, Sullivan PJ, O’Sullivan MJ, et al. Full thickness burn caused by exposure to giant hogweed: delayed presentation, histological features and surgical management. J Plast Reconstr Aesthet Surg. 2011;64:128-130.
- Bosanac SS, Clark AK, Sivamani RK. Phytophotodermatitis related to carrot extract–containing sunscreen. Dermatol Online J. 2018;24:1-3.
- Sforza M, Andjelkov K, Zaccheddu R. Severe burn on 81% of body surface after sun tanning. Ulus Travma Acil Cerrahi Derg. 2013;19:383-384.
- Mioduszewski M, Beecker J. Phytophotodermatitis from making sangria: a phototoxic reaction to lime and lemon juice. CMAJ. 2015;187:756.
- Torrents R, Schmitt C, Domangé B, et al. Phytophotodermatitis with Peucedanum paniculatum: an endemic species to Corsica. Clin Toxicol (Phila). 2019;57:68-69.
- Sarhane KA, Ibrahim A, Fagan SP, et al. Phytophotodermatitis. Eplasty. 2013;13:ic57.
- DeLeo VA, Suarez SM, Maso MJ. Photoallergic contact dermatitis. results of photopatch testing in New York, 1985 to 1990. Arch Dermatol. 1992;128:1513-1518.
- Kimyon RS, Warshaw EM. Airborne allergic contact dermatitis: management and responsible allergens on the American Contact Dermatitis Society Core Series. Dermatitis. 2019;30:106-115.
- Miteva L, Broshtilova V, Schwartz RA. Unusual clinical manifestations of chronic discoid lupus erythematosus. Serbian J Dermatol Venereol. 2014;6:69-72.
- Handler NS, Handler MZ, Stephany MP, et al. Porphyria cutanea tarda: an intriguing genetic disease and marker. Int J Dermatol. 2017;56:E106-E117.
- Papadopoulos AJ, Schwartz RA, Fekete Z, et al. Pseudoporphyria: an atypical variant resembling toxic epidermal necrolysis. J Cutan Med Surg. 2001;5:479-485.
- Jasterzbski TJ, Janniger EJ, Schwartz RA. Adolescent tanning practices: understanding the popularity of excessive ultraviolet light exposure. In: Or
anje A, Al-Mutairi N, Shwayder T, eds. Practical Pediatric Dermatology. Controversies in Diagnosis and Treatment. Springer Verlag; 2016:177-185. - Lai YC, Janniger EJ, Schwartz RA. Solar protection policy in school children: proposals for progress. In: Oranje A, Al-Mutairi N, Shwayder T, eds. Practical Pediatric Dermatology. Controversies in Diagnosis and Treatment. Springer Verlag; 2016:165-176.
- Lagey K, Duinslaeger L, Vanderkelen A. Burns induced by plants. Burns. 1995;21:542-543.
- Redgrave N, Solomon J. Severe phytophotodermatitis from fig sap: a little known phenomenon. BMJ Case Rep. 2021;14:e238745.
Phytophotodermatitis (PPD) is a nonallergic contact dermatitis and thus is independent of the immune system, so prior sensitization is not required.1-3 It sometimes is known by colorful names such as margarita photodermatitis, in which a slice of lime in a refreshing summer drink may be etiologic,4,5 or berloque dermatitis, caused by exposure to perfumes containing bergapten (5-methoxypsoralen).6,7 Phytophotodermatitis may develop when phototoxic agents such as furocoumarins, which protect plants from fungal pathogens, and psoralens are applied to the skin followed by exposure to UV light, more specifically in the UVA range of 320 to 400 nm. Thus, these chemicals produce a phototoxic rather than photoallergic reaction, leading to cellular damage. Furocoumarins and psoralens often are found in plants such as celery and figs as well as in citrus fruits such as limes, lemons, and grapefruits. Exposure may be cryptic, as the patient may not consider or mention the eruption as possibly caused by activities such as soaking one’s feet in a folk remedy containing fig leaves.7,8 Once these phototoxic agents come in contact with the skin, the symptoms of PPD may arise within 24 hours of exposure, beginning as an acute dermatitis with erythema, edema, vesicles, or bullae accompanied by pain and itching.
Etiology
Phytophotodermatitis is caused by exposure to several different types of plants, including Ficus carica (common fig), the genus Citrus (eg, lime, lemon), or Pastina sativa (wild parsnip). Each of these contain furocoumarins and psoralens—phototoxic agents that cause cellular damage with epidermal necrosis and resultant pain when the skin is exposed to UVA light.1-4 There are 2 types of photochemical reactions in PPD: type I reactions occur in the absence of oxygen, whereas oxygen is present in type II reactions. Both damage cell membranes and DNA, which then results in DNA interstrand cross-linking between the psoralen furan ring and the thymine or cytosine of DNA, activating arachidonic acid metabolic pathways to produce cell death.1
Epidemiology
The incidence of PPD is unknown due to the high variability of reactions in individuals spanning from children to the elderly. It can be caused by many different wild and domestic plants in many areas of the world and can affect any individual regardless of age, race, gender, or ethnicity. Some individuals may be affected by hyperpigmentation without prominent inflammation.8 Diagnosis of PPD can be challenging, and an occupation and recreational history of exposure or recent travel with possible contact with plants may be required.
Occupational Dermatitis
Recreational Dermatitis
Phytophotodermatitis may be caused by exposure to phototoxic agents during leisure activities. Recreational exposure can occur almost anywhere, including in the kitchen, backyard, park, or woods, as well as at the beach. One notable culprit in recreational PPD is cooking with limes, parsley, or parsnips—plants that often are employed as garnishes in dishes, allowing early exposure of juices on the hands. Individuals who garden recreationally should be aware of ornamental plants such as hogweed and figs, which are notorious for causing PPD.13 Children’s camp counselors should have knowledge of PPD, as children have considerable curiosity and may touch or play with attractive plants such as hogweed. Children enjoying sports in parks can accidentally fall onto or be exposed to wild parsnip or hogweed growing nearby and wake up the next day with erythema and burning.14 Photoprotection is important, but sunscreens containing carrot extract can produce PPD.15 Widespread PPD over 80% of the body surface area due to sunbathing after applying fig leaf tea as a tanning agent has been described.16 Eating figs does not cause photosensitization unless the juice is smeared onto the skin. Margarita dermatitis and “Mexican beer dermatitis” can occur due to limes and other citrus fruits being used as ingredients in summer drinks.5 Similarly, preparing sangria may produce PPD from lime and lemon juices.17 In one report, hiking in Corsica resulted in PPD following incidental contact with the endemic plant Peucedanum paniculatum.18
Perfume (Berloque) Dermatitis
Perfume dermatitis, or berloque dermatitis, is a type of PPD for which the name is derived from the German word berlock or the French word berloque meaning trinket or charm; it was first described in 1925 by Rosenthal7 with regard to pendantlike streaks of pigmentation on the neck, face, arms, or trunk. The dermatitis develops due to bergapten, a component of bergamot oil, which is derived from the rind of Citrus bergamia. Many perfumes contain bergamot oil, but the incidence of this condition has been diminished due to use of artificial bergamot oil.6
Clinical Manifestation
Phytophotodermatitis is first evident as erythematous patches that appear within 24 hours of initial exposure to a phototoxic agent and UVA light, sometimes with a burning sensation. Solar exposure within 48 hours of sufficient plant exposure is required. Perfuse sweating may enhance the reaction.19 Rarely, it first may be seen with the sudden appearance of
Differential Diagnosis
Phytophotodermatitis may resemble other types of dermatitis, particularly other forms of contact dermatitis such poison ivy, and occasionally other environmental simulants such as jellyfish stings.1-6,20,21 Photosensitizing disorders including porphyria cutanea tarda, pseudoporphyria, and lupus erythematosus must be distinguished from PPD.22-24 Photosensitizing medications such tetracyclines, thiazide diuretics, sulfonamides, griseofulvin, and sulfonylureas should be considered. Airborne contact dermatitis may resemble PPD, as when poison ivy is burned and is exposed to the skin in sites of airborne contact.20 Excessive solar exposure is popular, particularly among adolescents, so sunburn and sunburnlike reactions can be noteworthy.25,26
Treatment
Phytophotodermatitis can be treated with topical steroids, sometimes adding an oral antihistamine, and occasionally oral steroids.2-4 Localized pain or a burning sensation should respond to therapy. Alternatively, a cold compress applied to the skin can relieve the pain and pruritus, and the burn can be debrided and dressed daily with silver sulfadiazine plus an oral nonsteroidal anti-inflammatory drug. This eruption should be self-limited as long as it is recognized early and the cause avoided. Management of acute exposure includes prompt application of soap and water and avoidance of UV light exposure for 48 to 72 hours to prevent psoralen photoactivation.
Because PPD is essentially a chemical burn, a burn protocol and possible referral to a burn center may be needed, whether the reaction is acute or widespread.11,12,14,27,28 Surgical debridement and skin grafting rarely may be mandated.14 Postinflammatory hyperpigmentation may ensue as the dermatitis resolves but is not common.
The best approach for PPD is prevention (Figure 2). Individuals who are at risk should be aware of their surroundings and potential plants of concern and employ personal protective equipment to shield the skin from plant sap, which should be promptly removed if it comes in contact with the skin.
Phytophotodermatitis (PPD) is a nonallergic contact dermatitis and thus is independent of the immune system, so prior sensitization is not required.1-3 It sometimes is known by colorful names such as margarita photodermatitis, in which a slice of lime in a refreshing summer drink may be etiologic,4,5 or berloque dermatitis, caused by exposure to perfumes containing bergapten (5-methoxypsoralen).6,7 Phytophotodermatitis may develop when phototoxic agents such as furocoumarins, which protect plants from fungal pathogens, and psoralens are applied to the skin followed by exposure to UV light, more specifically in the UVA range of 320 to 400 nm. Thus, these chemicals produce a phototoxic rather than photoallergic reaction, leading to cellular damage. Furocoumarins and psoralens often are found in plants such as celery and figs as well as in citrus fruits such as limes, lemons, and grapefruits. Exposure may be cryptic, as the patient may not consider or mention the eruption as possibly caused by activities such as soaking one’s feet in a folk remedy containing fig leaves.7,8 Once these phototoxic agents come in contact with the skin, the symptoms of PPD may arise within 24 hours of exposure, beginning as an acute dermatitis with erythema, edema, vesicles, or bullae accompanied by pain and itching.
Etiology
Phytophotodermatitis is caused by exposure to several different types of plants, including Ficus carica (common fig), the genus Citrus (eg, lime, lemon), or Pastina sativa (wild parsnip). Each of these contain furocoumarins and psoralens—phototoxic agents that cause cellular damage with epidermal necrosis and resultant pain when the skin is exposed to UVA light.1-4 There are 2 types of photochemical reactions in PPD: type I reactions occur in the absence of oxygen, whereas oxygen is present in type II reactions. Both damage cell membranes and DNA, which then results in DNA interstrand cross-linking between the psoralen furan ring and the thymine or cytosine of DNA, activating arachidonic acid metabolic pathways to produce cell death.1
Epidemiology
The incidence of PPD is unknown due to the high variability of reactions in individuals spanning from children to the elderly. It can be caused by many different wild and domestic plants in many areas of the world and can affect any individual regardless of age, race, gender, or ethnicity. Some individuals may be affected by hyperpigmentation without prominent inflammation.8 Diagnosis of PPD can be challenging, and an occupation and recreational history of exposure or recent travel with possible contact with plants may be required.
Occupational Dermatitis
Recreational Dermatitis
Phytophotodermatitis may be caused by exposure to phototoxic agents during leisure activities. Recreational exposure can occur almost anywhere, including in the kitchen, backyard, park, or woods, as well as at the beach. One notable culprit in recreational PPD is cooking with limes, parsley, or parsnips—plants that often are employed as garnishes in dishes, allowing early exposure of juices on the hands. Individuals who garden recreationally should be aware of ornamental plants such as hogweed and figs, which are notorious for causing PPD.13 Children’s camp counselors should have knowledge of PPD, as children have considerable curiosity and may touch or play with attractive plants such as hogweed. Children enjoying sports in parks can accidentally fall onto or be exposed to wild parsnip or hogweed growing nearby and wake up the next day with erythema and burning.14 Photoprotection is important, but sunscreens containing carrot extract can produce PPD.15 Widespread PPD over 80% of the body surface area due to sunbathing after applying fig leaf tea as a tanning agent has been described.16 Eating figs does not cause photosensitization unless the juice is smeared onto the skin. Margarita dermatitis and “Mexican beer dermatitis” can occur due to limes and other citrus fruits being used as ingredients in summer drinks.5 Similarly, preparing sangria may produce PPD from lime and lemon juices.17 In one report, hiking in Corsica resulted in PPD following incidental contact with the endemic plant Peucedanum paniculatum.18
Perfume (Berloque) Dermatitis
Perfume dermatitis, or berloque dermatitis, is a type of PPD for which the name is derived from the German word berlock or the French word berloque meaning trinket or charm; it was first described in 1925 by Rosenthal7 with regard to pendantlike streaks of pigmentation on the neck, face, arms, or trunk. The dermatitis develops due to bergapten, a component of bergamot oil, which is derived from the rind of Citrus bergamia. Many perfumes contain bergamot oil, but the incidence of this condition has been diminished due to use of artificial bergamot oil.6
Clinical Manifestation
Phytophotodermatitis is first evident as erythematous patches that appear within 24 hours of initial exposure to a phototoxic agent and UVA light, sometimes with a burning sensation. Solar exposure within 48 hours of sufficient plant exposure is required. Perfuse sweating may enhance the reaction.19 Rarely, it first may be seen with the sudden appearance of
Differential Diagnosis
Phytophotodermatitis may resemble other types of dermatitis, particularly other forms of contact dermatitis such poison ivy, and occasionally other environmental simulants such as jellyfish stings.1-6,20,21 Photosensitizing disorders including porphyria cutanea tarda, pseudoporphyria, and lupus erythematosus must be distinguished from PPD.22-24 Photosensitizing medications such tetracyclines, thiazide diuretics, sulfonamides, griseofulvin, and sulfonylureas should be considered. Airborne contact dermatitis may resemble PPD, as when poison ivy is burned and is exposed to the skin in sites of airborne contact.20 Excessive solar exposure is popular, particularly among adolescents, so sunburn and sunburnlike reactions can be noteworthy.25,26
Treatment
Phytophotodermatitis can be treated with topical steroids, sometimes adding an oral antihistamine, and occasionally oral steroids.2-4 Localized pain or a burning sensation should respond to therapy. Alternatively, a cold compress applied to the skin can relieve the pain and pruritus, and the burn can be debrided and dressed daily with silver sulfadiazine plus an oral nonsteroidal anti-inflammatory drug. This eruption should be self-limited as long as it is recognized early and the cause avoided. Management of acute exposure includes prompt application of soap and water and avoidance of UV light exposure for 48 to 72 hours to prevent psoralen photoactivation.
Because PPD is essentially a chemical burn, a burn protocol and possible referral to a burn center may be needed, whether the reaction is acute or widespread.11,12,14,27,28 Surgical debridement and skin grafting rarely may be mandated.14 Postinflammatory hyperpigmentation may ensue as the dermatitis resolves but is not common.
The best approach for PPD is prevention (Figure 2). Individuals who are at risk should be aware of their surroundings and potential plants of concern and employ personal protective equipment to shield the skin from plant sap, which should be promptly removed if it comes in contact with the skin.
- Zhang R, Zhu W. Phytophotodermatitis due to Chinese herbal medicine decoction. Indian J Dermatol. 2011;56:329-331.
- Harshman J, Quan Y, Hsiang D. Phytophotodermatitis: rash with many faces. Can Fam Physician. 2017;63:938-940.
- Imen MS, Ahmadabadi A, Tavousi SH, et al. The curious cases of burn by fig tree leaves. Indian J Dermatol. 2019;64:71-73.
- Hankinson A, Lloyd B, Alweis R. Lime-induced phytophotodermatitis [published online September 29, 2014]. J Community Hosp Intern Med Perspect. doi:10.3402/jchimp.v4.25090
- Abramowitz AI, Resnik KS, Cohen KR. Margarita photodermatitis. N Engl J Med. 2013;328:891.
- Quaak MS, Martens H, Hassing RJ, et al. The sunny side of lime. J Travel Med. 2012;19:327-328.
- Rosenthal O. Berloque dermatitis: Berliner Dermatologische Gesellschaft. Dermatol Zeitschrift. 1925;42:295.
- Choi JY, Hwang S, Lee SH, et al. Asymptomatic hyperpigmentation without preceding inflammation as a clinical feature of citrus fruits–induced phytophotodermatitis. Ann Dermatol. 2018;30:75-78.
- Wynn P, Bell S. Phytophotodermatitis in grounds operatives. Occup Med (Lond). 2005;55:393-395.
- Klimaszyk P, Klimaszyk D, Piotrowiak M, et al. Unusual complications after occupational exposure to giant hogweed (Heracleum mantegazzianum): a case report. Int J Occup Med Environ Health. 2014;27:141-144.
- Downs JW, Cumpston KL, Feldman MJ. Giant hogweed phytophotodermatitis. Clin Toxicol (Phila). 2019;57:822-823.
- Maso MJ, Ruszkowski AM, Bauerle J, et al. Celery phytophotodermatitis in a chef. Arch Dermatol. 1991;127:912-913.
- Derraik JG, Rademaker M. Phytophotodermatitis caused by contact with a fig tree (Ficus carica). New Zealand Med J. 2007;120:U2720.
- Chan JC, Sullivan PJ, O’Sullivan MJ, et al. Full thickness burn caused by exposure to giant hogweed: delayed presentation, histological features and surgical management. J Plast Reconstr Aesthet Surg. 2011;64:128-130.
- Bosanac SS, Clark AK, Sivamani RK. Phytophotodermatitis related to carrot extract–containing sunscreen. Dermatol Online J. 2018;24:1-3.
- Sforza M, Andjelkov K, Zaccheddu R. Severe burn on 81% of body surface after sun tanning. Ulus Travma Acil Cerrahi Derg. 2013;19:383-384.
- Mioduszewski M, Beecker J. Phytophotodermatitis from making sangria: a phototoxic reaction to lime and lemon juice. CMAJ. 2015;187:756.
- Torrents R, Schmitt C, Domangé B, et al. Phytophotodermatitis with Peucedanum paniculatum: an endemic species to Corsica. Clin Toxicol (Phila). 2019;57:68-69.
- Sarhane KA, Ibrahim A, Fagan SP, et al. Phytophotodermatitis. Eplasty. 2013;13:ic57.
- DeLeo VA, Suarez SM, Maso MJ. Photoallergic contact dermatitis. results of photopatch testing in New York, 1985 to 1990. Arch Dermatol. 1992;128:1513-1518.
- Kimyon RS, Warshaw EM. Airborne allergic contact dermatitis: management and responsible allergens on the American Contact Dermatitis Society Core Series. Dermatitis. 2019;30:106-115.
- Miteva L, Broshtilova V, Schwartz RA. Unusual clinical manifestations of chronic discoid lupus erythematosus. Serbian J Dermatol Venereol. 2014;6:69-72.
- Handler NS, Handler MZ, Stephany MP, et al. Porphyria cutanea tarda: an intriguing genetic disease and marker. Int J Dermatol. 2017;56:E106-E117.
- Papadopoulos AJ, Schwartz RA, Fekete Z, et al. Pseudoporphyria: an atypical variant resembling toxic epidermal necrolysis. J Cutan Med Surg. 2001;5:479-485.
- Jasterzbski TJ, Janniger EJ, Schwartz RA. Adolescent tanning practices: understanding the popularity of excessive ultraviolet light exposure. In: Or
anje A, Al-Mutairi N, Shwayder T, eds. Practical Pediatric Dermatology. Controversies in Diagnosis and Treatment. Springer Verlag; 2016:177-185. - Lai YC, Janniger EJ, Schwartz RA. Solar protection policy in school children: proposals for progress. In: Oranje A, Al-Mutairi N, Shwayder T, eds. Practical Pediatric Dermatology. Controversies in Diagnosis and Treatment. Springer Verlag; 2016:165-176.
- Lagey K, Duinslaeger L, Vanderkelen A. Burns induced by plants. Burns. 1995;21:542-543.
- Redgrave N, Solomon J. Severe phytophotodermatitis from fig sap: a little known phenomenon. BMJ Case Rep. 2021;14:e238745.
- Zhang R, Zhu W. Phytophotodermatitis due to Chinese herbal medicine decoction. Indian J Dermatol. 2011;56:329-331.
- Harshman J, Quan Y, Hsiang D. Phytophotodermatitis: rash with many faces. Can Fam Physician. 2017;63:938-940.
- Imen MS, Ahmadabadi A, Tavousi SH, et al. The curious cases of burn by fig tree leaves. Indian J Dermatol. 2019;64:71-73.
- Hankinson A, Lloyd B, Alweis R. Lime-induced phytophotodermatitis [published online September 29, 2014]. J Community Hosp Intern Med Perspect. doi:10.3402/jchimp.v4.25090
- Abramowitz AI, Resnik KS, Cohen KR. Margarita photodermatitis. N Engl J Med. 2013;328:891.
- Quaak MS, Martens H, Hassing RJ, et al. The sunny side of lime. J Travel Med. 2012;19:327-328.
- Rosenthal O. Berloque dermatitis: Berliner Dermatologische Gesellschaft. Dermatol Zeitschrift. 1925;42:295.
- Choi JY, Hwang S, Lee SH, et al. Asymptomatic hyperpigmentation without preceding inflammation as a clinical feature of citrus fruits–induced phytophotodermatitis. Ann Dermatol. 2018;30:75-78.
- Wynn P, Bell S. Phytophotodermatitis in grounds operatives. Occup Med (Lond). 2005;55:393-395.
- Klimaszyk P, Klimaszyk D, Piotrowiak M, et al. Unusual complications after occupational exposure to giant hogweed (Heracleum mantegazzianum): a case report. Int J Occup Med Environ Health. 2014;27:141-144.
- Downs JW, Cumpston KL, Feldman MJ. Giant hogweed phytophotodermatitis. Clin Toxicol (Phila). 2019;57:822-823.
- Maso MJ, Ruszkowski AM, Bauerle J, et al. Celery phytophotodermatitis in a chef. Arch Dermatol. 1991;127:912-913.
- Derraik JG, Rademaker M. Phytophotodermatitis caused by contact with a fig tree (Ficus carica). New Zealand Med J. 2007;120:U2720.
- Chan JC, Sullivan PJ, O’Sullivan MJ, et al. Full thickness burn caused by exposure to giant hogweed: delayed presentation, histological features and surgical management. J Plast Reconstr Aesthet Surg. 2011;64:128-130.
- Bosanac SS, Clark AK, Sivamani RK. Phytophotodermatitis related to carrot extract–containing sunscreen. Dermatol Online J. 2018;24:1-3.
- Sforza M, Andjelkov K, Zaccheddu R. Severe burn on 81% of body surface after sun tanning. Ulus Travma Acil Cerrahi Derg. 2013;19:383-384.
- Mioduszewski M, Beecker J. Phytophotodermatitis from making sangria: a phototoxic reaction to lime and lemon juice. CMAJ. 2015;187:756.
- Torrents R, Schmitt C, Domangé B, et al. Phytophotodermatitis with Peucedanum paniculatum: an endemic species to Corsica. Clin Toxicol (Phila). 2019;57:68-69.
- Sarhane KA, Ibrahim A, Fagan SP, et al. Phytophotodermatitis. Eplasty. 2013;13:ic57.
- DeLeo VA, Suarez SM, Maso MJ. Photoallergic contact dermatitis. results of photopatch testing in New York, 1985 to 1990. Arch Dermatol. 1992;128:1513-1518.
- Kimyon RS, Warshaw EM. Airborne allergic contact dermatitis: management and responsible allergens on the American Contact Dermatitis Society Core Series. Dermatitis. 2019;30:106-115.
- Miteva L, Broshtilova V, Schwartz RA. Unusual clinical manifestations of chronic discoid lupus erythematosus. Serbian J Dermatol Venereol. 2014;6:69-72.
- Handler NS, Handler MZ, Stephany MP, et al. Porphyria cutanea tarda: an intriguing genetic disease and marker. Int J Dermatol. 2017;56:E106-E117.
- Papadopoulos AJ, Schwartz RA, Fekete Z, et al. Pseudoporphyria: an atypical variant resembling toxic epidermal necrolysis. J Cutan Med Surg. 2001;5:479-485.
- Jasterzbski TJ, Janniger EJ, Schwartz RA. Adolescent tanning practices: understanding the popularity of excessive ultraviolet light exposure. In: Or
anje A, Al-Mutairi N, Shwayder T, eds. Practical Pediatric Dermatology. Controversies in Diagnosis and Treatment. Springer Verlag; 2016:177-185. - Lai YC, Janniger EJ, Schwartz RA. Solar protection policy in school children: proposals for progress. In: Oranje A, Al-Mutairi N, Shwayder T, eds. Practical Pediatric Dermatology. Controversies in Diagnosis and Treatment. Springer Verlag; 2016:165-176.
- Lagey K, Duinslaeger L, Vanderkelen A. Burns induced by plants. Burns. 1995;21:542-543.
- Redgrave N, Solomon J. Severe phytophotodermatitis from fig sap: a little known phenomenon. BMJ Case Rep. 2021;14:e238745.
Practice Points
- Phytophotodermatitis (PPD) can be both an occupational and recreational dermatosis.
- Phytophotodermatitis is a nonallergic contact dermatitis and thus is independent of the immune system, so prior sensitization is not required.
- Individuals who work with plants should be aware of PPD and methods of prevention.
- Phytophotodermatitis may be evident only as asymptomatic hyperpigmentation.
Erethism Mercurialis and Reactions to Elemental Mercury
Evidence of human exposure to mercury dates as far back as the Egyptians in 1500
Mercury release in the environment primarily is a function of human activity, including coal-fired power plants, residential heating, and mining.9,10 Mercury from these sources is commonly found in the sediment of lakes and bays, where it is enzymatically converted to methylmercury by aquatic microorganisms; subsequent food chain biomagnification results in elevated mercury levels in apex predators. Substantial release of mercury into the environment also can be attributed to health care facilities from their use of thermometers containing 0.5 to 3 g of elemental mercury,11 blood pressure monitors, and medical waste incinerators.5
Mercury has been reported as the second most common cause of heavy metal poisoning after lead.12 Standards from the US Food and Drug Administration dictate that methylmercury levels in fish and wheat products must not exceed 1 ppm.13 Most plant and animal food sources contain methylmercury at levels between 0.0001 and 0.01 ppm; mercury concentrations are especially high in tuna, averaging 0.4 ppm, while larger predatory fish contain levels in excess of 1 ppm.14 The use of mercury-containing cosmetic products also presents a substantial exposure risk to consumers.5,10 In one study, 3.3% of skin-lightening creams and soaps purchased within the United States contained concentrations of mercury exceeding 1000 ppm.15
We describe a case of mercury toxicity resulting from intentional injection of liquid mercury into the right antecubital fossa in a suicide attempt.
Case Report
A 31-year-old woman presented to the family practice center for evaluation of a firm stained area on the skin of the right arm. She reported increasing anxiety, depression, tremors, irritability, and difficulty concentrating over the last 6 months. She denied headache and joint or muscle pain. Four years earlier, she had broken apart a thermometer and injected approximately 0.7 mL of its contents into the right arm in a suicide attempt. She intended to inject the thermometer’s contents directly into a vein, but the material instead entered the surrounding tissue. She denied notable pain or itching overlying the injection site. Her medications included aripiprazole and buspirone. She noted that she smoked half a pack of cigarettes per day and had a history of methamphetamine abuse. She was homeless and unemployed. Physical examination revealed an anxious tremulous woman with an erythematous to bluish gray, firm plaque on the right antecubital fossa (Figure 1). There were no notable tremors and no gait disturbance.
Her blood mercury level was greater than 100 µg/L and urine mercury was 477 µg/g (reference ranges, 1–8 μg/L and 4–5 μg/L, respectively). A radiograph of the right elbow area revealed scattered punctate foci of increased density within or overlying the anterolateral elbow soft tissues. She was diagnosed with mercury granuloma causing chronic mercury elevation. She underwent excision of the granuloma (Figure 2) with endovascular surgery via an elliptical incision. The patient was subsequently lost to follow-up.
Comment
Elemental mercury is a silver liquid at room temperature that spontaneously evaporates to form mercury vapor, an invisible, odorless, toxic gas. Accidental cutaneous exposure typically is safely managed by washing exposed skin with soap and water,16 though there is a potential risk for systemic absorption, especially when the skin is inflamed. When metallic mercury is subcutaneously injected, it is advised to promptly excise all subcutaneous areas containing mercury, regardless of any symptoms of systemic toxicity. Patients should subsequently be monitored for signs of both central nervous system (CNS) and renal deficits, undergo chelation therapy when systemic effects are apparent, and finally receive psychiatric consultation and treatment when necessary.17
Inorganic mercury compounds are formed when elemental mercury combines with sulfur or oxygen and often take the form of mercury salts, which appear as white crystals.16 These salts occur naturally in the environment and are used in pesticides, antiseptics, and skin-lightening creams and soaps.18
Methylmercury is a highly toxic, organic compound that is capable of crossing the placental and blood-brain barriers. It is the most common organic mercury compound found in the environment.16 Most humans have trace amounts of methylmercury in their bodies, typically as a result of consuming seafood.5
Exposure to mercury most commonly occurs through chronic consumption of methylmercury in seafood or acute inhalation of elemental mercury vapors.9 Iatrogenic cases of mercury exposure via injection also have been reported in the literature, including a case resulting in acute poisoning due to peritoneal lavage with mercury bichloride.19 Acute mercury-induced pulmonary damage typically resolves completely. However, there have been reported cases of exposure progressing to interstitial emphysema, pneumatocele, pneumothorax, pneumomediastinum, interstitial fibrosis, and chronic respiratory insufficiency, with examples of fatal acute respiratory distress syndrome being reported.5,16,20 Although individuals who inhale mercury vapors initially may be unaware of exposure due to little upper airway irritation, symptoms following an initial acute exposure may include ptyalism, a metallic taste, dysphagia, enteritis, diarrhea, nausea, renal damage, and CNS effects.16 Additionally, exposure may lead to confusion with signs and symptoms of metal fume fever, including shortness of breath, pleuritic chest pain, stomatitis, lethargy, and vomiting.20
Chronic exposure to mercury vapor can result in accumulation of mercury in the body, leading to neuropsychiatric, dermatologic, oropharyngeal, and renal manifestations. Sore throat, fever, headache, fatigue, dyspnea, chest pain, and pneumonitis are common.16 Typically, low-level exposure to elemental mercury does not lead to long-lasting health effects. However, individuals exposed to high-level elemental mercury vapors may require hospitalization. Treatment of acute mercury poisoning consists of removing the source of exposure, followed by cardiopulmonary support.16
Specific assays for mercury levels in blood and urine are useful to assess the level of exposure and risk to the patient. Blood mercury concentrations of 20 µg/L or below are considered within reference range; however, once blood and urine concentrations of mercury exceed 100 µg/L, clinical signs of acute mercury poisoning typically manifest.21 Chest radiographs can reveal pulmonary damage, while complete blood cell count, metabolic panel, and urinalysis can assess damage to other organs. Neuropsychiatric testing and nerve conduction studies may provide objective evidence of CNS toxicity. Assays for N-acetyl-β-D-glucosaminidase can provide an indication of early renal tubular dysfunction.16
Elemental mercury is not absorbed from the gastrointestinal tract, posing minimal risk for acute toxicity from ingestion. Generally, less than 10% of ingested inorganic mercury is absorbed from the gut, while elemental mercury is nonabsorbable.10 If an individual ingests a large amount of mercury, it may persist in the gastrointestinal tract for an extended period. Mercury is radiopaque, and abdominal radiographs should be obtained in all cases of ingestion.16
Mercury is toxic to the CNS and peripheral nervous system, resulting in erethism mercurialis, a constellation of neuropsychologic signs and symptoms including restlessness, irritability, insomnia, emotional lability, difficulty concentrating, and impaired memory. In severe cases, delirium and psychosis may develop. Other CNS effects include tremors, paresthesia, dysarthria, neuromuscular changes, headaches, polyneuropathy, and cerebellar ataxia, as well as ophthalmologic and audiologic impairment.5,16
Upon inhalation exposure, patients with respiratory concerns should be given oxygen. Bronchospasms are treated with bronchodilators; however, if multiple chemical exposures are suspected, bronchial-sensitizing agents may pose additional risks. Corticosteroids and antibiotics have been recommended for treatment of chemical pneumonitis, but their efficacy has not been substantiated.16
Skin reactions associated with skin contact to elemental mercury are rare. However, hives and dermatitis have been observed following accidental contact with inorganic mercury compounds.5 Manifestation in children chronically exposed to mercury includes a nonallergic hypersensitivity (acrodynia),5,17 which is characterized by pain and dusky pink discoloration in the hands and feet, most often seen in children chronically exposed to mercury absorbed from vapor inhalation or cutaneous exposure.16
Renal conditions associated with acute inhalation of elemental mercury vapor include proteinuria, nephrotic syndrome, temporary tubular dysfunction, acute tubular necrosis, and oliguric renal failure.16 Chronic exposure to inorganic mercury compounds also has been reported to cause renal damage.5 Chelation therapy should be performed for any symptomatic patient with a clear history of acute elemental mercury exposure.16 The most frequently used chelation agent in cases of acute inorganic mercury exposures is dimercaprol. In rare cases of mercury intoxication, hemodialysis is required in the treatment of renal failure and to expedite removal of dimercaprol-mercury complexes.16
Cardiovascular symptoms associated with acute inhalation of high levels of elemental mercury include tachycardia and hypertension.16 Increases in blood pressure, palpitations, and heart rate also have been observed in instances of acute elemental mercury exposure. Studies show that exposure to mercury increases both the risk for acute myocardial infarction as well as death from coronary heart and cardiovascular diseases.5
Conclusion
Mercury poisoning presents with varied neuropsychologic signs and symptoms. Our case provides insight into a unique route of exposure for mercury toxicity. In addition to the unusual presentation of a mercury granuloma, our case illustrates how surgical techniques can aid in removal of cutaneous reservoirs in the setting of percutaneous exposure.
- History of mercury. Government of Canada website. Modified April 26, 2010. Accessed March 11, 2021. https://www.canada.ca/en/environment-climate-change/services/pollutants/mercury-environment/about/history.html
- Dartmouth Toxic Metals Superfund Research Program website. Accessed March 11, 2021. https://sites.dartmouth.edu/toxmetal/
- Norn S, Permin H, Kruse E, et al. Mercury—a major agent in the history of medicine and alchemy [in Danish]. Dan Medicinhist Arbog. 2008;36:21-40.
- Waldron HA. Did the Mad Hatter have mercury poisoning? Br Med J (Clin Res Ed). 1983;287:1961.
- Poulin J, Gibb H. Mercury: assessing the environmental burden of disease at national and local levels. WHO Environmental Burden of Disease Series No. 16. World Health Organization; 2008.
- Charcot JM. Clinical lectures of the diseases of the nervous system. In: Kinnier Wilson SA. The Landmark Library of Neurology and Neurosurgery. Gryphon Editions; 1994:186.
- Kinnier Wilson SA. Neurology. In: Kinnier Wilson SA. The Landmark Library of Neurology and Neurosurgery. Gryphon Editions; 1994:739-740.
- Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25:1-24.
- Mercury and health. World Health Organization website. Updated March 31, 2017. Accessed March 12, 2021. http://www.whoint/mediacentre/factsheets/fs361/en/
- Olson DA. Mercury toxicity. Updated November 5, 2018. Accessed March 12, 2021.http://emedicine.medscape.com/article/1175560-overview
- Mercury thermometers. Environmental Protection Agency website. Updated June 26, 2018. https://www.epa.gov/mercury/mercury-thermometers
- Jao-Tan C, Pope E. Cutaneous poisoning syndromes in children: a review. Curr Opin Pediatr. 2006;18:410-416.
- US Department of Health and Human Services: Public Health Service Agency for Toxic Substances and Disease Registry. Toxicological profile for mercury: regulations and advisories. Published March 1999. Accessed March 23, 2021. https://www.atsdr.cdc.gov/toxprofiles/tp46.pdf
- US Food and Drug Administration. Mercury levels in commercial fish and shellfish (1990-2012). Updated October 25, 2017. Accessed March 16, 2021. https://www.fda.gov/food/metals-and-your-food/mercury-levels-commercial-fish-and-shellfish-1990-2012
- Hamann CR, Boonchai W, Wen L, et al. Spectrometric analysis of mercury content in 549 skin-lightening products: is mercury toxicity a hidden global health hazard? J Am Acad Dermatol. 2014;70:281-287.e3.
- Mercury. Managing Hazardous Materials Incidents. Agency for Toxic Substances and Disease Registry website. Accessed March 16, 2021. https://www.atsdr.cdc.gov/MHMI/mmg46.pdf
- Krohn IT, Solof A, Mobini J, et al. Subcutaneous injection of metallic mercury. JAMA. 1980;243:548-549.
- Lai O, Parsi KK, Wu D, et al. Mercury toxicity presenting acrodynia and a papulovesicular eruption in a 5-year-old girl. Dermatol Online J. 2016;16;22:13030/qt6444r7nc.
- Dolianiti M, Tasiopoulou K, Kalostou A, et al. Mercury bichloride iatrogenic poisoning: a case report. J Clin Toxicol. 2016;6:2. doi:10.4172/2161-0495.1000290
- Broussard LA, Hammett-Stabler CA, Winecker RE, et al. The toxicology of mercury. Lab Med. 2002;33:614-625. doi:10.1309/5HY1-V3NE-2LFL-P9MT
- Byeong-Jin Y, Byoung-Gwon K, Man-Joong J, et al. Evaluation of mercury exposure levels, clinical diagnosis and treatment for mercury intoxication. Ann Occup Environ Med. 2016;28:5.
Evidence of human exposure to mercury dates as far back as the Egyptians in 1500
Mercury release in the environment primarily is a function of human activity, including coal-fired power plants, residential heating, and mining.9,10 Mercury from these sources is commonly found in the sediment of lakes and bays, where it is enzymatically converted to methylmercury by aquatic microorganisms; subsequent food chain biomagnification results in elevated mercury levels in apex predators. Substantial release of mercury into the environment also can be attributed to health care facilities from their use of thermometers containing 0.5 to 3 g of elemental mercury,11 blood pressure monitors, and medical waste incinerators.5
Mercury has been reported as the second most common cause of heavy metal poisoning after lead.12 Standards from the US Food and Drug Administration dictate that methylmercury levels in fish and wheat products must not exceed 1 ppm.13 Most plant and animal food sources contain methylmercury at levels between 0.0001 and 0.01 ppm; mercury concentrations are especially high in tuna, averaging 0.4 ppm, while larger predatory fish contain levels in excess of 1 ppm.14 The use of mercury-containing cosmetic products also presents a substantial exposure risk to consumers.5,10 In one study, 3.3% of skin-lightening creams and soaps purchased within the United States contained concentrations of mercury exceeding 1000 ppm.15
We describe a case of mercury toxicity resulting from intentional injection of liquid mercury into the right antecubital fossa in a suicide attempt.
Case Report
A 31-year-old woman presented to the family practice center for evaluation of a firm stained area on the skin of the right arm. She reported increasing anxiety, depression, tremors, irritability, and difficulty concentrating over the last 6 months. She denied headache and joint or muscle pain. Four years earlier, she had broken apart a thermometer and injected approximately 0.7 mL of its contents into the right arm in a suicide attempt. She intended to inject the thermometer’s contents directly into a vein, but the material instead entered the surrounding tissue. She denied notable pain or itching overlying the injection site. Her medications included aripiprazole and buspirone. She noted that she smoked half a pack of cigarettes per day and had a history of methamphetamine abuse. She was homeless and unemployed. Physical examination revealed an anxious tremulous woman with an erythematous to bluish gray, firm plaque on the right antecubital fossa (Figure 1). There were no notable tremors and no gait disturbance.
Her blood mercury level was greater than 100 µg/L and urine mercury was 477 µg/g (reference ranges, 1–8 μg/L and 4–5 μg/L, respectively). A radiograph of the right elbow area revealed scattered punctate foci of increased density within or overlying the anterolateral elbow soft tissues. She was diagnosed with mercury granuloma causing chronic mercury elevation. She underwent excision of the granuloma (Figure 2) with endovascular surgery via an elliptical incision. The patient was subsequently lost to follow-up.
Comment
Elemental mercury is a silver liquid at room temperature that spontaneously evaporates to form mercury vapor, an invisible, odorless, toxic gas. Accidental cutaneous exposure typically is safely managed by washing exposed skin with soap and water,16 though there is a potential risk for systemic absorption, especially when the skin is inflamed. When metallic mercury is subcutaneously injected, it is advised to promptly excise all subcutaneous areas containing mercury, regardless of any symptoms of systemic toxicity. Patients should subsequently be monitored for signs of both central nervous system (CNS) and renal deficits, undergo chelation therapy when systemic effects are apparent, and finally receive psychiatric consultation and treatment when necessary.17
Inorganic mercury compounds are formed when elemental mercury combines with sulfur or oxygen and often take the form of mercury salts, which appear as white crystals.16 These salts occur naturally in the environment and are used in pesticides, antiseptics, and skin-lightening creams and soaps.18
Methylmercury is a highly toxic, organic compound that is capable of crossing the placental and blood-brain barriers. It is the most common organic mercury compound found in the environment.16 Most humans have trace amounts of methylmercury in their bodies, typically as a result of consuming seafood.5
Exposure to mercury most commonly occurs through chronic consumption of methylmercury in seafood or acute inhalation of elemental mercury vapors.9 Iatrogenic cases of mercury exposure via injection also have been reported in the literature, including a case resulting in acute poisoning due to peritoneal lavage with mercury bichloride.19 Acute mercury-induced pulmonary damage typically resolves completely. However, there have been reported cases of exposure progressing to interstitial emphysema, pneumatocele, pneumothorax, pneumomediastinum, interstitial fibrosis, and chronic respiratory insufficiency, with examples of fatal acute respiratory distress syndrome being reported.5,16,20 Although individuals who inhale mercury vapors initially may be unaware of exposure due to little upper airway irritation, symptoms following an initial acute exposure may include ptyalism, a metallic taste, dysphagia, enteritis, diarrhea, nausea, renal damage, and CNS effects.16 Additionally, exposure may lead to confusion with signs and symptoms of metal fume fever, including shortness of breath, pleuritic chest pain, stomatitis, lethargy, and vomiting.20
Chronic exposure to mercury vapor can result in accumulation of mercury in the body, leading to neuropsychiatric, dermatologic, oropharyngeal, and renal manifestations. Sore throat, fever, headache, fatigue, dyspnea, chest pain, and pneumonitis are common.16 Typically, low-level exposure to elemental mercury does not lead to long-lasting health effects. However, individuals exposed to high-level elemental mercury vapors may require hospitalization. Treatment of acute mercury poisoning consists of removing the source of exposure, followed by cardiopulmonary support.16
Specific assays for mercury levels in blood and urine are useful to assess the level of exposure and risk to the patient. Blood mercury concentrations of 20 µg/L or below are considered within reference range; however, once blood and urine concentrations of mercury exceed 100 µg/L, clinical signs of acute mercury poisoning typically manifest.21 Chest radiographs can reveal pulmonary damage, while complete blood cell count, metabolic panel, and urinalysis can assess damage to other organs. Neuropsychiatric testing and nerve conduction studies may provide objective evidence of CNS toxicity. Assays for N-acetyl-β-D-glucosaminidase can provide an indication of early renal tubular dysfunction.16
Elemental mercury is not absorbed from the gastrointestinal tract, posing minimal risk for acute toxicity from ingestion. Generally, less than 10% of ingested inorganic mercury is absorbed from the gut, while elemental mercury is nonabsorbable.10 If an individual ingests a large amount of mercury, it may persist in the gastrointestinal tract for an extended period. Mercury is radiopaque, and abdominal radiographs should be obtained in all cases of ingestion.16
Mercury is toxic to the CNS and peripheral nervous system, resulting in erethism mercurialis, a constellation of neuropsychologic signs and symptoms including restlessness, irritability, insomnia, emotional lability, difficulty concentrating, and impaired memory. In severe cases, delirium and psychosis may develop. Other CNS effects include tremors, paresthesia, dysarthria, neuromuscular changes, headaches, polyneuropathy, and cerebellar ataxia, as well as ophthalmologic and audiologic impairment.5,16
Upon inhalation exposure, patients with respiratory concerns should be given oxygen. Bronchospasms are treated with bronchodilators; however, if multiple chemical exposures are suspected, bronchial-sensitizing agents may pose additional risks. Corticosteroids and antibiotics have been recommended for treatment of chemical pneumonitis, but their efficacy has not been substantiated.16
Skin reactions associated with skin contact to elemental mercury are rare. However, hives and dermatitis have been observed following accidental contact with inorganic mercury compounds.5 Manifestation in children chronically exposed to mercury includes a nonallergic hypersensitivity (acrodynia),5,17 which is characterized by pain and dusky pink discoloration in the hands and feet, most often seen in children chronically exposed to mercury absorbed from vapor inhalation or cutaneous exposure.16
Renal conditions associated with acute inhalation of elemental mercury vapor include proteinuria, nephrotic syndrome, temporary tubular dysfunction, acute tubular necrosis, and oliguric renal failure.16 Chronic exposure to inorganic mercury compounds also has been reported to cause renal damage.5 Chelation therapy should be performed for any symptomatic patient with a clear history of acute elemental mercury exposure.16 The most frequently used chelation agent in cases of acute inorganic mercury exposures is dimercaprol. In rare cases of mercury intoxication, hemodialysis is required in the treatment of renal failure and to expedite removal of dimercaprol-mercury complexes.16
Cardiovascular symptoms associated with acute inhalation of high levels of elemental mercury include tachycardia and hypertension.16 Increases in blood pressure, palpitations, and heart rate also have been observed in instances of acute elemental mercury exposure. Studies show that exposure to mercury increases both the risk for acute myocardial infarction as well as death from coronary heart and cardiovascular diseases.5
Conclusion
Mercury poisoning presents with varied neuropsychologic signs and symptoms. Our case provides insight into a unique route of exposure for mercury toxicity. In addition to the unusual presentation of a mercury granuloma, our case illustrates how surgical techniques can aid in removal of cutaneous reservoirs in the setting of percutaneous exposure.
Evidence of human exposure to mercury dates as far back as the Egyptians in 1500
Mercury release in the environment primarily is a function of human activity, including coal-fired power plants, residential heating, and mining.9,10 Mercury from these sources is commonly found in the sediment of lakes and bays, where it is enzymatically converted to methylmercury by aquatic microorganisms; subsequent food chain biomagnification results in elevated mercury levels in apex predators. Substantial release of mercury into the environment also can be attributed to health care facilities from their use of thermometers containing 0.5 to 3 g of elemental mercury,11 blood pressure monitors, and medical waste incinerators.5
Mercury has been reported as the second most common cause of heavy metal poisoning after lead.12 Standards from the US Food and Drug Administration dictate that methylmercury levels in fish and wheat products must not exceed 1 ppm.13 Most plant and animal food sources contain methylmercury at levels between 0.0001 and 0.01 ppm; mercury concentrations are especially high in tuna, averaging 0.4 ppm, while larger predatory fish contain levels in excess of 1 ppm.14 The use of mercury-containing cosmetic products also presents a substantial exposure risk to consumers.5,10 In one study, 3.3% of skin-lightening creams and soaps purchased within the United States contained concentrations of mercury exceeding 1000 ppm.15
We describe a case of mercury toxicity resulting from intentional injection of liquid mercury into the right antecubital fossa in a suicide attempt.
Case Report
A 31-year-old woman presented to the family practice center for evaluation of a firm stained area on the skin of the right arm. She reported increasing anxiety, depression, tremors, irritability, and difficulty concentrating over the last 6 months. She denied headache and joint or muscle pain. Four years earlier, she had broken apart a thermometer and injected approximately 0.7 mL of its contents into the right arm in a suicide attempt. She intended to inject the thermometer’s contents directly into a vein, but the material instead entered the surrounding tissue. She denied notable pain or itching overlying the injection site. Her medications included aripiprazole and buspirone. She noted that she smoked half a pack of cigarettes per day and had a history of methamphetamine abuse. She was homeless and unemployed. Physical examination revealed an anxious tremulous woman with an erythematous to bluish gray, firm plaque on the right antecubital fossa (Figure 1). There were no notable tremors and no gait disturbance.
Her blood mercury level was greater than 100 µg/L and urine mercury was 477 µg/g (reference ranges, 1–8 μg/L and 4–5 μg/L, respectively). A radiograph of the right elbow area revealed scattered punctate foci of increased density within or overlying the anterolateral elbow soft tissues. She was diagnosed with mercury granuloma causing chronic mercury elevation. She underwent excision of the granuloma (Figure 2) with endovascular surgery via an elliptical incision. The patient was subsequently lost to follow-up.
Comment
Elemental mercury is a silver liquid at room temperature that spontaneously evaporates to form mercury vapor, an invisible, odorless, toxic gas. Accidental cutaneous exposure typically is safely managed by washing exposed skin with soap and water,16 though there is a potential risk for systemic absorption, especially when the skin is inflamed. When metallic mercury is subcutaneously injected, it is advised to promptly excise all subcutaneous areas containing mercury, regardless of any symptoms of systemic toxicity. Patients should subsequently be monitored for signs of both central nervous system (CNS) and renal deficits, undergo chelation therapy when systemic effects are apparent, and finally receive psychiatric consultation and treatment when necessary.17
Inorganic mercury compounds are formed when elemental mercury combines with sulfur or oxygen and often take the form of mercury salts, which appear as white crystals.16 These salts occur naturally in the environment and are used in pesticides, antiseptics, and skin-lightening creams and soaps.18
Methylmercury is a highly toxic, organic compound that is capable of crossing the placental and blood-brain barriers. It is the most common organic mercury compound found in the environment.16 Most humans have trace amounts of methylmercury in their bodies, typically as a result of consuming seafood.5
Exposure to mercury most commonly occurs through chronic consumption of methylmercury in seafood or acute inhalation of elemental mercury vapors.9 Iatrogenic cases of mercury exposure via injection also have been reported in the literature, including a case resulting in acute poisoning due to peritoneal lavage with mercury bichloride.19 Acute mercury-induced pulmonary damage typically resolves completely. However, there have been reported cases of exposure progressing to interstitial emphysema, pneumatocele, pneumothorax, pneumomediastinum, interstitial fibrosis, and chronic respiratory insufficiency, with examples of fatal acute respiratory distress syndrome being reported.5,16,20 Although individuals who inhale mercury vapors initially may be unaware of exposure due to little upper airway irritation, symptoms following an initial acute exposure may include ptyalism, a metallic taste, dysphagia, enteritis, diarrhea, nausea, renal damage, and CNS effects.16 Additionally, exposure may lead to confusion with signs and symptoms of metal fume fever, including shortness of breath, pleuritic chest pain, stomatitis, lethargy, and vomiting.20
Chronic exposure to mercury vapor can result in accumulation of mercury in the body, leading to neuropsychiatric, dermatologic, oropharyngeal, and renal manifestations. Sore throat, fever, headache, fatigue, dyspnea, chest pain, and pneumonitis are common.16 Typically, low-level exposure to elemental mercury does not lead to long-lasting health effects. However, individuals exposed to high-level elemental mercury vapors may require hospitalization. Treatment of acute mercury poisoning consists of removing the source of exposure, followed by cardiopulmonary support.16
Specific assays for mercury levels in blood and urine are useful to assess the level of exposure and risk to the patient. Blood mercury concentrations of 20 µg/L or below are considered within reference range; however, once blood and urine concentrations of mercury exceed 100 µg/L, clinical signs of acute mercury poisoning typically manifest.21 Chest radiographs can reveal pulmonary damage, while complete blood cell count, metabolic panel, and urinalysis can assess damage to other organs. Neuropsychiatric testing and nerve conduction studies may provide objective evidence of CNS toxicity. Assays for N-acetyl-β-D-glucosaminidase can provide an indication of early renal tubular dysfunction.16
Elemental mercury is not absorbed from the gastrointestinal tract, posing minimal risk for acute toxicity from ingestion. Generally, less than 10% of ingested inorganic mercury is absorbed from the gut, while elemental mercury is nonabsorbable.10 If an individual ingests a large amount of mercury, it may persist in the gastrointestinal tract for an extended period. Mercury is radiopaque, and abdominal radiographs should be obtained in all cases of ingestion.16
Mercury is toxic to the CNS and peripheral nervous system, resulting in erethism mercurialis, a constellation of neuropsychologic signs and symptoms including restlessness, irritability, insomnia, emotional lability, difficulty concentrating, and impaired memory. In severe cases, delirium and psychosis may develop. Other CNS effects include tremors, paresthesia, dysarthria, neuromuscular changes, headaches, polyneuropathy, and cerebellar ataxia, as well as ophthalmologic and audiologic impairment.5,16
Upon inhalation exposure, patients with respiratory concerns should be given oxygen. Bronchospasms are treated with bronchodilators; however, if multiple chemical exposures are suspected, bronchial-sensitizing agents may pose additional risks. Corticosteroids and antibiotics have been recommended for treatment of chemical pneumonitis, but their efficacy has not been substantiated.16
Skin reactions associated with skin contact to elemental mercury are rare. However, hives and dermatitis have been observed following accidental contact with inorganic mercury compounds.5 Manifestation in children chronically exposed to mercury includes a nonallergic hypersensitivity (acrodynia),5,17 which is characterized by pain and dusky pink discoloration in the hands and feet, most often seen in children chronically exposed to mercury absorbed from vapor inhalation or cutaneous exposure.16
Renal conditions associated with acute inhalation of elemental mercury vapor include proteinuria, nephrotic syndrome, temporary tubular dysfunction, acute tubular necrosis, and oliguric renal failure.16 Chronic exposure to inorganic mercury compounds also has been reported to cause renal damage.5 Chelation therapy should be performed for any symptomatic patient with a clear history of acute elemental mercury exposure.16 The most frequently used chelation agent in cases of acute inorganic mercury exposures is dimercaprol. In rare cases of mercury intoxication, hemodialysis is required in the treatment of renal failure and to expedite removal of dimercaprol-mercury complexes.16
Cardiovascular symptoms associated with acute inhalation of high levels of elemental mercury include tachycardia and hypertension.16 Increases in blood pressure, palpitations, and heart rate also have been observed in instances of acute elemental mercury exposure. Studies show that exposure to mercury increases both the risk for acute myocardial infarction as well as death from coronary heart and cardiovascular diseases.5
Conclusion
Mercury poisoning presents with varied neuropsychologic signs and symptoms. Our case provides insight into a unique route of exposure for mercury toxicity. In addition to the unusual presentation of a mercury granuloma, our case illustrates how surgical techniques can aid in removal of cutaneous reservoirs in the setting of percutaneous exposure.
- History of mercury. Government of Canada website. Modified April 26, 2010. Accessed March 11, 2021. https://www.canada.ca/en/environment-climate-change/services/pollutants/mercury-environment/about/history.html
- Dartmouth Toxic Metals Superfund Research Program website. Accessed March 11, 2021. https://sites.dartmouth.edu/toxmetal/
- Norn S, Permin H, Kruse E, et al. Mercury—a major agent in the history of medicine and alchemy [in Danish]. Dan Medicinhist Arbog. 2008;36:21-40.
- Waldron HA. Did the Mad Hatter have mercury poisoning? Br Med J (Clin Res Ed). 1983;287:1961.
- Poulin J, Gibb H. Mercury: assessing the environmental burden of disease at national and local levels. WHO Environmental Burden of Disease Series No. 16. World Health Organization; 2008.
- Charcot JM. Clinical lectures of the diseases of the nervous system. In: Kinnier Wilson SA. The Landmark Library of Neurology and Neurosurgery. Gryphon Editions; 1994:186.
- Kinnier Wilson SA. Neurology. In: Kinnier Wilson SA. The Landmark Library of Neurology and Neurosurgery. Gryphon Editions; 1994:739-740.
- Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25:1-24.
- Mercury and health. World Health Organization website. Updated March 31, 2017. Accessed March 12, 2021. http://www.whoint/mediacentre/factsheets/fs361/en/
- Olson DA. Mercury toxicity. Updated November 5, 2018. Accessed March 12, 2021.http://emedicine.medscape.com/article/1175560-overview
- Mercury thermometers. Environmental Protection Agency website. Updated June 26, 2018. https://www.epa.gov/mercury/mercury-thermometers
- Jao-Tan C, Pope E. Cutaneous poisoning syndromes in children: a review. Curr Opin Pediatr. 2006;18:410-416.
- US Department of Health and Human Services: Public Health Service Agency for Toxic Substances and Disease Registry. Toxicological profile for mercury: regulations and advisories. Published March 1999. Accessed March 23, 2021. https://www.atsdr.cdc.gov/toxprofiles/tp46.pdf
- US Food and Drug Administration. Mercury levels in commercial fish and shellfish (1990-2012). Updated October 25, 2017. Accessed March 16, 2021. https://www.fda.gov/food/metals-and-your-food/mercury-levels-commercial-fish-and-shellfish-1990-2012
- Hamann CR, Boonchai W, Wen L, et al. Spectrometric analysis of mercury content in 549 skin-lightening products: is mercury toxicity a hidden global health hazard? J Am Acad Dermatol. 2014;70:281-287.e3.
- Mercury. Managing Hazardous Materials Incidents. Agency for Toxic Substances and Disease Registry website. Accessed March 16, 2021. https://www.atsdr.cdc.gov/MHMI/mmg46.pdf
- Krohn IT, Solof A, Mobini J, et al. Subcutaneous injection of metallic mercury. JAMA. 1980;243:548-549.
- Lai O, Parsi KK, Wu D, et al. Mercury toxicity presenting acrodynia and a papulovesicular eruption in a 5-year-old girl. Dermatol Online J. 2016;16;22:13030/qt6444r7nc.
- Dolianiti M, Tasiopoulou K, Kalostou A, et al. Mercury bichloride iatrogenic poisoning: a case report. J Clin Toxicol. 2016;6:2. doi:10.4172/2161-0495.1000290
- Broussard LA, Hammett-Stabler CA, Winecker RE, et al. The toxicology of mercury. Lab Med. 2002;33:614-625. doi:10.1309/5HY1-V3NE-2LFL-P9MT
- Byeong-Jin Y, Byoung-Gwon K, Man-Joong J, et al. Evaluation of mercury exposure levels, clinical diagnosis and treatment for mercury intoxication. Ann Occup Environ Med. 2016;28:5.
- History of mercury. Government of Canada website. Modified April 26, 2010. Accessed March 11, 2021. https://www.canada.ca/en/environment-climate-change/services/pollutants/mercury-environment/about/history.html
- Dartmouth Toxic Metals Superfund Research Program website. Accessed March 11, 2021. https://sites.dartmouth.edu/toxmetal/
- Norn S, Permin H, Kruse E, et al. Mercury—a major agent in the history of medicine and alchemy [in Danish]. Dan Medicinhist Arbog. 2008;36:21-40.
- Waldron HA. Did the Mad Hatter have mercury poisoning? Br Med J (Clin Res Ed). 1983;287:1961.
- Poulin J, Gibb H. Mercury: assessing the environmental burden of disease at national and local levels. WHO Environmental Burden of Disease Series No. 16. World Health Organization; 2008.
- Charcot JM. Clinical lectures of the diseases of the nervous system. In: Kinnier Wilson SA. The Landmark Library of Neurology and Neurosurgery. Gryphon Editions; 1994:186.
- Kinnier Wilson SA. Neurology. In: Kinnier Wilson SA. The Landmark Library of Neurology and Neurosurgery. Gryphon Editions; 1994:739-740.
- Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25:1-24.
- Mercury and health. World Health Organization website. Updated March 31, 2017. Accessed March 12, 2021. http://www.whoint/mediacentre/factsheets/fs361/en/
- Olson DA. Mercury toxicity. Updated November 5, 2018. Accessed March 12, 2021.http://emedicine.medscape.com/article/1175560-overview
- Mercury thermometers. Environmental Protection Agency website. Updated June 26, 2018. https://www.epa.gov/mercury/mercury-thermometers
- Jao-Tan C, Pope E. Cutaneous poisoning syndromes in children: a review. Curr Opin Pediatr. 2006;18:410-416.
- US Department of Health and Human Services: Public Health Service Agency for Toxic Substances and Disease Registry. Toxicological profile for mercury: regulations and advisories. Published March 1999. Accessed March 23, 2021. https://www.atsdr.cdc.gov/toxprofiles/tp46.pdf
- US Food and Drug Administration. Mercury levels in commercial fish and shellfish (1990-2012). Updated October 25, 2017. Accessed March 16, 2021. https://www.fda.gov/food/metals-and-your-food/mercury-levels-commercial-fish-and-shellfish-1990-2012
- Hamann CR, Boonchai W, Wen L, et al. Spectrometric analysis of mercury content in 549 skin-lightening products: is mercury toxicity a hidden global health hazard? J Am Acad Dermatol. 2014;70:281-287.e3.
- Mercury. Managing Hazardous Materials Incidents. Agency for Toxic Substances and Disease Registry website. Accessed March 16, 2021. https://www.atsdr.cdc.gov/MHMI/mmg46.pdf
- Krohn IT, Solof A, Mobini J, et al. Subcutaneous injection of metallic mercury. JAMA. 1980;243:548-549.
- Lai O, Parsi KK, Wu D, et al. Mercury toxicity presenting acrodynia and a papulovesicular eruption in a 5-year-old girl. Dermatol Online J. 2016;16;22:13030/qt6444r7nc.
- Dolianiti M, Tasiopoulou K, Kalostou A, et al. Mercury bichloride iatrogenic poisoning: a case report. J Clin Toxicol. 2016;6:2. doi:10.4172/2161-0495.1000290
- Broussard LA, Hammett-Stabler CA, Winecker RE, et al. The toxicology of mercury. Lab Med. 2002;33:614-625. doi:10.1309/5HY1-V3NE-2LFL-P9MT
- Byeong-Jin Y, Byoung-Gwon K, Man-Joong J, et al. Evaluation of mercury exposure levels, clinical diagnosis and treatment for mercury intoxication. Ann Occup Environ Med. 2016;28:5.
Practice Points
- Chronic mercury granulomas can present as firm, erythematous to bluish gray plaques.
- Accidental skin contact to elemental mercury may cause urticaria and dermatitis.
- Blood mercury concentrations below 20 11µg/L are considered within reference range; once blood and urine concentrations exceed 100 11µg/L, clinical signs of acute mercury poisoning typically manifest.
- Mercury is toxic to the central and peripheral nervous systems, resulting in erethism mercurialis, a constellation of neuropsychologic signs and symptoms including restlessness, irritability, insomnia, emotional lability, difficulty concentrating, and impaired memory.
Contact dermatitis content varies among social media sites
on YouTube, Facebook, Instagram, Google, Twitter, and Reddit.
Data on social media use suggest that approximately 65% of U.S. adults regularly use social media, and 40% of individuals use it in making medical decisions, Morgan Nguyen, a medical student at Northwestern University, Chicago, said at the annual meeting of the American Contact Dermatitis Society, held virtually this year.
“Dermatologists’ awareness of social media discussions can further their understanding of where patients go for information and what they might encounter,” she said. In particular, “contact dermatitis practitioners can tailor their counseling by knowing what their patients are seeing online.”
To characterize the social media landscape for content related to allergic contact dermatitis (ACD), Ms. Nguyen and colleagues assessed metrics on content and authorship on six different platforms.
For YouTube, the authors reviewed 15 videos related to ACD with views ranging from 24,262 to 232,300. Of these videos, two were produced as medical education, four were produced by patients, and nine were produced by physicians. The content of many videos was poor quality, with an average QUEST score of 7.4/28 overall and 8.7 for physician videos. Video quality was not associated with increased views. Video titles included “What to do if you have a rash on your face,” and “Contact dermatitis on lips!”
Overall, Instagram was more popular than Twitter, particularly among patients. The investigators searched using the hashtags #ContactDermatitis, #AllergicContactDermatitis, and #ContactDerm and reviewed the 100 most recent posts for authorship. The most recent 100 posts occurred over 16 days; physicians, patients, and companies each contributed approximately one-third of the content, but patient content was more focused on symptoms, treatment progress, and advice.
For Instagram, the hashtag search phrase made a notable difference in authorship, Ms. Nguyen said. Physicians were disproportionately more likely to use #AllergicContactDermatitis (43%) compared with patients (22%).
On Twitter, the most recent 100 posts were spread over 152 days, and professional organizations and companies generated approximately two-thirds of the posts. The #ContactDermatitis hashtag was the most common, and accounted for 94% of tweets.
Although patient support groups specific to ACD exist on Facebook, the researchers found none on Reddit. These two venues are designed for creating online communities, rather than simply providing information, and the researchers searched for support groups related to contact dermatitis. One of the main differences between the two is that Facebook allows for the creation of private groups, while Reddit is an open forum.
The largest contact dermatitis Facebook group, the “Eczema, Contact Dermatitis and Patch Testing Alliance,” had 4,665 members at the time of the study, and most groups were private. Although no support groups existed on Reddit, titles of Reddit forums discussing ACD included allergies, askdoctors, fragrance, haircarescience, legaladvice, skincareaddicts, beauty, dermatologyquestions, medical_advice, skincare_addiction, tretinoin, and vulvodynia.
For Google, the researchers used terms similar to “contact dermatitis” as generated by the Google Keyword Planner tool, and used Google Adwords data to estimate monthly searches. The top estimated term was “contact dermatitis,” with 8,322 searches, followed by “contact dermatitis pictures,” with 1,666 searches, and “contact dermatitis treatment” with 595 searches. By contrast, “allergic dermatitis” had an estimated 346 monthly searches, and “allergic contact dermatitis” had 194.
Overall, approximately 9,000 searches each month involve “contact dermatitis,” “allergic contact dermatitis,” or “allergic dermatitis,” said Ms. Nguyen. However, these estimated searches seemed comparatively low, given the high burden of ACD, she said. Although ACD ranks eighth among skin diseases based on health care costs, psoriasis (fourteenth based on health care costs) shows an estimated monthly Google search volume of 600,462, she pointed out.
The study findings were limited by several factors including the potential impact of the COVID-19 pandemic on social media use, and by the lack of specificity associated with the search term “contact dermatitis,” which is not unique to ACD, Ms. Nguyen said.
Although more research on quality assessment is needed, the results suggest that social media is a popular venue for ACD patients to seek and share information, Ms. Nguyen emphasized. There is an opportunity for patch testing physicians to create and disperse educational content for patients using these sites, she concluded.
Study highlights education opportunities
“Due to the pandemic, patients have been increasingly interacting with online resources in lieu of coming to a physician’s office,” corresponding author Walter J. Liszewski, MD, of the department of dermatology, Northwestern University, Chicago, said in an interview. “As social media is increasingly used by patients and physicians, it is important to get a sense of its footprint,” he said.
He and Ms. Nguyen were surprised by several of their findings: First, searches for ACD on Google were not particularly common given its relatively high prevalence and economic cost to society. In addition, they found that physicians often used different language than that of patients to describe ACD on Twitter and Instagram. They were also surprised at how often ACD appeared in Reddit posts, which they noted highlights that ACD impacts multiple sections of society.
The greatest challenge in studying social media and medicine is the quality of material available, Dr. Liszewski and Ms. Nguyen observed, emphasizing that while there are numerous videos on ACD on YouTube, the quality is highly variable, and there is a need for more patient-centered, educational materials. However, the results of their study highlight the opportunity for physicians and industry to create medically-accurate educational materials, they added.
Ms. Nguyen and Dr. Liszewski had no financial conflicts to disclose.
on YouTube, Facebook, Instagram, Google, Twitter, and Reddit.
Data on social media use suggest that approximately 65% of U.S. adults regularly use social media, and 40% of individuals use it in making medical decisions, Morgan Nguyen, a medical student at Northwestern University, Chicago, said at the annual meeting of the American Contact Dermatitis Society, held virtually this year.
“Dermatologists’ awareness of social media discussions can further their understanding of where patients go for information and what they might encounter,” she said. In particular, “contact dermatitis practitioners can tailor their counseling by knowing what their patients are seeing online.”
To characterize the social media landscape for content related to allergic contact dermatitis (ACD), Ms. Nguyen and colleagues assessed metrics on content and authorship on six different platforms.
For YouTube, the authors reviewed 15 videos related to ACD with views ranging from 24,262 to 232,300. Of these videos, two were produced as medical education, four were produced by patients, and nine were produced by physicians. The content of many videos was poor quality, with an average QUEST score of 7.4/28 overall and 8.7 for physician videos. Video quality was not associated with increased views. Video titles included “What to do if you have a rash on your face,” and “Contact dermatitis on lips!”
Overall, Instagram was more popular than Twitter, particularly among patients. The investigators searched using the hashtags #ContactDermatitis, #AllergicContactDermatitis, and #ContactDerm and reviewed the 100 most recent posts for authorship. The most recent 100 posts occurred over 16 days; physicians, patients, and companies each contributed approximately one-third of the content, but patient content was more focused on symptoms, treatment progress, and advice.
For Instagram, the hashtag search phrase made a notable difference in authorship, Ms. Nguyen said. Physicians were disproportionately more likely to use #AllergicContactDermatitis (43%) compared with patients (22%).
On Twitter, the most recent 100 posts were spread over 152 days, and professional organizations and companies generated approximately two-thirds of the posts. The #ContactDermatitis hashtag was the most common, and accounted for 94% of tweets.
Although patient support groups specific to ACD exist on Facebook, the researchers found none on Reddit. These two venues are designed for creating online communities, rather than simply providing information, and the researchers searched for support groups related to contact dermatitis. One of the main differences between the two is that Facebook allows for the creation of private groups, while Reddit is an open forum.
The largest contact dermatitis Facebook group, the “Eczema, Contact Dermatitis and Patch Testing Alliance,” had 4,665 members at the time of the study, and most groups were private. Although no support groups existed on Reddit, titles of Reddit forums discussing ACD included allergies, askdoctors, fragrance, haircarescience, legaladvice, skincareaddicts, beauty, dermatologyquestions, medical_advice, skincare_addiction, tretinoin, and vulvodynia.
For Google, the researchers used terms similar to “contact dermatitis” as generated by the Google Keyword Planner tool, and used Google Adwords data to estimate monthly searches. The top estimated term was “contact dermatitis,” with 8,322 searches, followed by “contact dermatitis pictures,” with 1,666 searches, and “contact dermatitis treatment” with 595 searches. By contrast, “allergic dermatitis” had an estimated 346 monthly searches, and “allergic contact dermatitis” had 194.
Overall, approximately 9,000 searches each month involve “contact dermatitis,” “allergic contact dermatitis,” or “allergic dermatitis,” said Ms. Nguyen. However, these estimated searches seemed comparatively low, given the high burden of ACD, she said. Although ACD ranks eighth among skin diseases based on health care costs, psoriasis (fourteenth based on health care costs) shows an estimated monthly Google search volume of 600,462, she pointed out.
The study findings were limited by several factors including the potential impact of the COVID-19 pandemic on social media use, and by the lack of specificity associated with the search term “contact dermatitis,” which is not unique to ACD, Ms. Nguyen said.
Although more research on quality assessment is needed, the results suggest that social media is a popular venue for ACD patients to seek and share information, Ms. Nguyen emphasized. There is an opportunity for patch testing physicians to create and disperse educational content for patients using these sites, she concluded.
Study highlights education opportunities
“Due to the pandemic, patients have been increasingly interacting with online resources in lieu of coming to a physician’s office,” corresponding author Walter J. Liszewski, MD, of the department of dermatology, Northwestern University, Chicago, said in an interview. “As social media is increasingly used by patients and physicians, it is important to get a sense of its footprint,” he said.
He and Ms. Nguyen were surprised by several of their findings: First, searches for ACD on Google were not particularly common given its relatively high prevalence and economic cost to society. In addition, they found that physicians often used different language than that of patients to describe ACD on Twitter and Instagram. They were also surprised at how often ACD appeared in Reddit posts, which they noted highlights that ACD impacts multiple sections of society.
The greatest challenge in studying social media and medicine is the quality of material available, Dr. Liszewski and Ms. Nguyen observed, emphasizing that while there are numerous videos on ACD on YouTube, the quality is highly variable, and there is a need for more patient-centered, educational materials. However, the results of their study highlight the opportunity for physicians and industry to create medically-accurate educational materials, they added.
Ms. Nguyen and Dr. Liszewski had no financial conflicts to disclose.
on YouTube, Facebook, Instagram, Google, Twitter, and Reddit.
Data on social media use suggest that approximately 65% of U.S. adults regularly use social media, and 40% of individuals use it in making medical decisions, Morgan Nguyen, a medical student at Northwestern University, Chicago, said at the annual meeting of the American Contact Dermatitis Society, held virtually this year.
“Dermatologists’ awareness of social media discussions can further their understanding of where patients go for information and what they might encounter,” she said. In particular, “contact dermatitis practitioners can tailor their counseling by knowing what their patients are seeing online.”
To characterize the social media landscape for content related to allergic contact dermatitis (ACD), Ms. Nguyen and colleagues assessed metrics on content and authorship on six different platforms.
For YouTube, the authors reviewed 15 videos related to ACD with views ranging from 24,262 to 232,300. Of these videos, two were produced as medical education, four were produced by patients, and nine were produced by physicians. The content of many videos was poor quality, with an average QUEST score of 7.4/28 overall and 8.7 for physician videos. Video quality was not associated with increased views. Video titles included “What to do if you have a rash on your face,” and “Contact dermatitis on lips!”
Overall, Instagram was more popular than Twitter, particularly among patients. The investigators searched using the hashtags #ContactDermatitis, #AllergicContactDermatitis, and #ContactDerm and reviewed the 100 most recent posts for authorship. The most recent 100 posts occurred over 16 days; physicians, patients, and companies each contributed approximately one-third of the content, but patient content was more focused on symptoms, treatment progress, and advice.
For Instagram, the hashtag search phrase made a notable difference in authorship, Ms. Nguyen said. Physicians were disproportionately more likely to use #AllergicContactDermatitis (43%) compared with patients (22%).
On Twitter, the most recent 100 posts were spread over 152 days, and professional organizations and companies generated approximately two-thirds of the posts. The #ContactDermatitis hashtag was the most common, and accounted for 94% of tweets.
Although patient support groups specific to ACD exist on Facebook, the researchers found none on Reddit. These two venues are designed for creating online communities, rather than simply providing information, and the researchers searched for support groups related to contact dermatitis. One of the main differences between the two is that Facebook allows for the creation of private groups, while Reddit is an open forum.
The largest contact dermatitis Facebook group, the “Eczema, Contact Dermatitis and Patch Testing Alliance,” had 4,665 members at the time of the study, and most groups were private. Although no support groups existed on Reddit, titles of Reddit forums discussing ACD included allergies, askdoctors, fragrance, haircarescience, legaladvice, skincareaddicts, beauty, dermatologyquestions, medical_advice, skincare_addiction, tretinoin, and vulvodynia.
For Google, the researchers used terms similar to “contact dermatitis” as generated by the Google Keyword Planner tool, and used Google Adwords data to estimate monthly searches. The top estimated term was “contact dermatitis,” with 8,322 searches, followed by “contact dermatitis pictures,” with 1,666 searches, and “contact dermatitis treatment” with 595 searches. By contrast, “allergic dermatitis” had an estimated 346 monthly searches, and “allergic contact dermatitis” had 194.
Overall, approximately 9,000 searches each month involve “contact dermatitis,” “allergic contact dermatitis,” or “allergic dermatitis,” said Ms. Nguyen. However, these estimated searches seemed comparatively low, given the high burden of ACD, she said. Although ACD ranks eighth among skin diseases based on health care costs, psoriasis (fourteenth based on health care costs) shows an estimated monthly Google search volume of 600,462, she pointed out.
The study findings were limited by several factors including the potential impact of the COVID-19 pandemic on social media use, and by the lack of specificity associated with the search term “contact dermatitis,” which is not unique to ACD, Ms. Nguyen said.
Although more research on quality assessment is needed, the results suggest that social media is a popular venue for ACD patients to seek and share information, Ms. Nguyen emphasized. There is an opportunity for patch testing physicians to create and disperse educational content for patients using these sites, she concluded.
Study highlights education opportunities
“Due to the pandemic, patients have been increasingly interacting with online resources in lieu of coming to a physician’s office,” corresponding author Walter J. Liszewski, MD, of the department of dermatology, Northwestern University, Chicago, said in an interview. “As social media is increasingly used by patients and physicians, it is important to get a sense of its footprint,” he said.
He and Ms. Nguyen were surprised by several of their findings: First, searches for ACD on Google were not particularly common given its relatively high prevalence and economic cost to society. In addition, they found that physicians often used different language than that of patients to describe ACD on Twitter and Instagram. They were also surprised at how often ACD appeared in Reddit posts, which they noted highlights that ACD impacts multiple sections of society.
The greatest challenge in studying social media and medicine is the quality of material available, Dr. Liszewski and Ms. Nguyen observed, emphasizing that while there are numerous videos on ACD on YouTube, the quality is highly variable, and there is a need for more patient-centered, educational materials. However, the results of their study highlight the opportunity for physicians and industry to create medically-accurate educational materials, they added.
Ms. Nguyen and Dr. Liszewski had no financial conflicts to disclose.
FROM ACDS 2021
Check all components in cases of suspected shoe allergy
according to a retrospective study of more than 30,000 patients.
Contact allergy to shoes remains a common but difficult problem for many reasons, including the limited information from shoe manufacturers, differences in shoe manufacturing processes, and changes in shoe trends, said Raina Bembry, MD, a dermatitis research fellow at Duke University, Durham, N.C., in a presentation at the annual meeting of the American Contact Dermatitis Society.
The North American Contact Dermatitis Group (NACDG) published data on shoe allergens from 2001-2004 in a 2007 review. To update this information to reflect changes in shoe manufacturing and trends, she and her coinvestigators characterized demographics, clinical characteristics, patch test results, and occupational data for NACDG patients with shoe contact allergy. They identified 33,661 patients who were patch tested with the standard series with or without a supplemental allergen between 2005 and 2018; over half were over aged 40.
The primary focus was individuals with a confirmed shoe (defined as shoes, boots, sandals, or slippers) as the source of a screening allergen or supplemental allergen, a positive patch test, and the foot as one of three sites of involvement. A total of 352 individuals met these criteria and had a confirmed final diagnosis of allergic contact dermatitis, Dr. Bembry said. Compared with individuals who had positive patch tests without a confirmed diagnosis, those with confirmed allergic dermatitis were significantly more likely to be male (odds ratio, 3.36) and less likely to be over aged 40 years (OR, 0.49).
The most common NACDG screening allergen, potassium dichromate, was found in 29.8% of the study population, followed by p-tert-butylphenol formaldehyde resin in 20.1%, thiuram mix (13.3%), mixed dialkyl thioureas (12.6%) and carba mix (12%).
Notably, 29.8% of the patients showed positive patch test reactions to supplemental allergens, and 12.2% only reacted to supplemental allergens, Dr. Bembry said.
The results were limited by several factors, including referral selection bias, reliance on clinical judgment for patch test interpretations, and lack of data on the specifics of the supplemental allergens other than the source code, she said. In addition, the study does not identify nonshoe sources of foot contact allergy, and six screening allergens were not testing during this study period.
Overall, the findings were similar to those from previous studies in that patients affected with contact dermatitis from shoe allergens tended to be younger and male, with no occupational relevance to the reaction, said Dr. Bembry.
The finding that almost 20% of allergens were not found with the screening series emphasizes the value of testing not only relevant supplemental allergens, but also patient products and shoe components, she concluded.
Dr. Bembry had no financial conflicts to disclose. Coauthor Amber Atwater, MD, the immediate past president of the ACDS, and associate professor of dermatology at Duke University, disclosed receiving the Pfizer Independent Grant for Learning & Change and consulting for Henkel.
according to a retrospective study of more than 30,000 patients.
Contact allergy to shoes remains a common but difficult problem for many reasons, including the limited information from shoe manufacturers, differences in shoe manufacturing processes, and changes in shoe trends, said Raina Bembry, MD, a dermatitis research fellow at Duke University, Durham, N.C., in a presentation at the annual meeting of the American Contact Dermatitis Society.
The North American Contact Dermatitis Group (NACDG) published data on shoe allergens from 2001-2004 in a 2007 review. To update this information to reflect changes in shoe manufacturing and trends, she and her coinvestigators characterized demographics, clinical characteristics, patch test results, and occupational data for NACDG patients with shoe contact allergy. They identified 33,661 patients who were patch tested with the standard series with or without a supplemental allergen between 2005 and 2018; over half were over aged 40.
The primary focus was individuals with a confirmed shoe (defined as shoes, boots, sandals, or slippers) as the source of a screening allergen or supplemental allergen, a positive patch test, and the foot as one of three sites of involvement. A total of 352 individuals met these criteria and had a confirmed final diagnosis of allergic contact dermatitis, Dr. Bembry said. Compared with individuals who had positive patch tests without a confirmed diagnosis, those with confirmed allergic dermatitis were significantly more likely to be male (odds ratio, 3.36) and less likely to be over aged 40 years (OR, 0.49).
The most common NACDG screening allergen, potassium dichromate, was found in 29.8% of the study population, followed by p-tert-butylphenol formaldehyde resin in 20.1%, thiuram mix (13.3%), mixed dialkyl thioureas (12.6%) and carba mix (12%).
Notably, 29.8% of the patients showed positive patch test reactions to supplemental allergens, and 12.2% only reacted to supplemental allergens, Dr. Bembry said.
The results were limited by several factors, including referral selection bias, reliance on clinical judgment for patch test interpretations, and lack of data on the specifics of the supplemental allergens other than the source code, she said. In addition, the study does not identify nonshoe sources of foot contact allergy, and six screening allergens were not testing during this study period.
Overall, the findings were similar to those from previous studies in that patients affected with contact dermatitis from shoe allergens tended to be younger and male, with no occupational relevance to the reaction, said Dr. Bembry.
The finding that almost 20% of allergens were not found with the screening series emphasizes the value of testing not only relevant supplemental allergens, but also patient products and shoe components, she concluded.
Dr. Bembry had no financial conflicts to disclose. Coauthor Amber Atwater, MD, the immediate past president of the ACDS, and associate professor of dermatology at Duke University, disclosed receiving the Pfizer Independent Grant for Learning & Change and consulting for Henkel.
according to a retrospective study of more than 30,000 patients.
Contact allergy to shoes remains a common but difficult problem for many reasons, including the limited information from shoe manufacturers, differences in shoe manufacturing processes, and changes in shoe trends, said Raina Bembry, MD, a dermatitis research fellow at Duke University, Durham, N.C., in a presentation at the annual meeting of the American Contact Dermatitis Society.
The North American Contact Dermatitis Group (NACDG) published data on shoe allergens from 2001-2004 in a 2007 review. To update this information to reflect changes in shoe manufacturing and trends, she and her coinvestigators characterized demographics, clinical characteristics, patch test results, and occupational data for NACDG patients with shoe contact allergy. They identified 33,661 patients who were patch tested with the standard series with or without a supplemental allergen between 2005 and 2018; over half were over aged 40.
The primary focus was individuals with a confirmed shoe (defined as shoes, boots, sandals, or slippers) as the source of a screening allergen or supplemental allergen, a positive patch test, and the foot as one of three sites of involvement. A total of 352 individuals met these criteria and had a confirmed final diagnosis of allergic contact dermatitis, Dr. Bembry said. Compared with individuals who had positive patch tests without a confirmed diagnosis, those with confirmed allergic dermatitis were significantly more likely to be male (odds ratio, 3.36) and less likely to be over aged 40 years (OR, 0.49).
The most common NACDG screening allergen, potassium dichromate, was found in 29.8% of the study population, followed by p-tert-butylphenol formaldehyde resin in 20.1%, thiuram mix (13.3%), mixed dialkyl thioureas (12.6%) and carba mix (12%).
Notably, 29.8% of the patients showed positive patch test reactions to supplemental allergens, and 12.2% only reacted to supplemental allergens, Dr. Bembry said.
The results were limited by several factors, including referral selection bias, reliance on clinical judgment for patch test interpretations, and lack of data on the specifics of the supplemental allergens other than the source code, she said. In addition, the study does not identify nonshoe sources of foot contact allergy, and six screening allergens were not testing during this study period.
Overall, the findings were similar to those from previous studies in that patients affected with contact dermatitis from shoe allergens tended to be younger and male, with no occupational relevance to the reaction, said Dr. Bembry.
The finding that almost 20% of allergens were not found with the screening series emphasizes the value of testing not only relevant supplemental allergens, but also patient products and shoe components, she concluded.
Dr. Bembry had no financial conflicts to disclose. Coauthor Amber Atwater, MD, the immediate past president of the ACDS, and associate professor of dermatology at Duke University, disclosed receiving the Pfizer Independent Grant for Learning & Change and consulting for Henkel.
FROM ACDS 2021
Contact allergen of the year found in foam in shin guards, footwear
.
The announcement was made by Donald V. Belsito, MD, professor of dermatology, Columbia University, New York, during a presentation at the annual meeting of the American Contact Dermatitis Society, held virtually this year. In his opinion, he said, the most exciting selections occur when international cooperation results in the identification of a new allergen that could become problematic, and acetophenone azine falls into this category.
The chemical formula of acetophenone azine is C16H16N2.
Acetophenone azine was highlighted as a contact allergen in a recent report in Dermatitis. The authors, Nadia Raison-Peyron, MD, from the department of dermatology at the University of Montpelier (France), and Denis Sasseville, MD, from the division of dermatology at McGill University Health Center, Quebec, described publications and reports of about 12 cases of severe allergic contact dermatitis secondary to shin pads or footwear, mainly in children and teens in Europe (one case was in Canada).
A common feature of these cases was the presence of a foam used for cushioning, made of ethyl vinyl acetate (EVA) used in the relevant products.
In one case, a 13-year-old boy who wore shin pads for soccer developed contact dermatitis on both shins that spread, and was described as severe. Patch testing revealed the EVA foam in the shin pads as the only positive reaction. Similar cases have been reported after exposure to EVA-containing products, including shin pads, sneakers, flip-flops, ski boots, insoles, swimming goggles, and bicycle seats, according to the authors.
In some reports, cases related to footwear presented as dyshidrosiform, vesiculobullous eczema, with or without palmar lesions, or presented as plantar hyperkeratotic dermatitis, they wrote. In other cases, patients experienced scarring and postinflammatory hypopigmentation.
The compound is likely not added to EVA intentionally, they added, but instead is thought to result from reactions between additives during the manufacturing process. The presence of acetophenone azine is not well explained, but the current theory is that it results from a combination of “the degradation of the initiator dicumylperoxide and hydrazine from the foaming agent azodicarbonamide,” the authors said.
In the paper, Dr. Raison-Peyron and Dr. Sasseville recommended a patch testing concentration of 0.1% in acetone or petrolatum, as acetophenone azine is not currently available from path test suppliers, although it can be obtained from chemical product distributors.
“Given the recent discovery of this allergen, it is presumed that cases of allergic contact dermatitis would have been missed and labeled irritant contact dermatitis or dyshidrosis,” they noted. To avoid missing more cases, acetophenone azine should be added to the patch testing shoe series, as well as plastics and glues series, they emphasized.
Although no cases of allergic reactions to acetophenone azine have been reported in the United States to date, it is an emerging allergen that should be on the radar for U.S. dermatologists, Amber Atwater, MD, outgoing ACDS president, said in an interview. The lack of reported cases may be in part attributed to the fact that acetophenone azine is not yet available to purchase for testing in the United States, and the allergen could be present in shin guards and other products identified in reported cases, added Dr. Atwater, associate professor of dermatology, Duke University, Durham, N.C.
.
The announcement was made by Donald V. Belsito, MD, professor of dermatology, Columbia University, New York, during a presentation at the annual meeting of the American Contact Dermatitis Society, held virtually this year. In his opinion, he said, the most exciting selections occur when international cooperation results in the identification of a new allergen that could become problematic, and acetophenone azine falls into this category.
The chemical formula of acetophenone azine is C16H16N2.
Acetophenone azine was highlighted as a contact allergen in a recent report in Dermatitis. The authors, Nadia Raison-Peyron, MD, from the department of dermatology at the University of Montpelier (France), and Denis Sasseville, MD, from the division of dermatology at McGill University Health Center, Quebec, described publications and reports of about 12 cases of severe allergic contact dermatitis secondary to shin pads or footwear, mainly in children and teens in Europe (one case was in Canada).
A common feature of these cases was the presence of a foam used for cushioning, made of ethyl vinyl acetate (EVA) used in the relevant products.
In one case, a 13-year-old boy who wore shin pads for soccer developed contact dermatitis on both shins that spread, and was described as severe. Patch testing revealed the EVA foam in the shin pads as the only positive reaction. Similar cases have been reported after exposure to EVA-containing products, including shin pads, sneakers, flip-flops, ski boots, insoles, swimming goggles, and bicycle seats, according to the authors.
In some reports, cases related to footwear presented as dyshidrosiform, vesiculobullous eczema, with or without palmar lesions, or presented as plantar hyperkeratotic dermatitis, they wrote. In other cases, patients experienced scarring and postinflammatory hypopigmentation.
The compound is likely not added to EVA intentionally, they added, but instead is thought to result from reactions between additives during the manufacturing process. The presence of acetophenone azine is not well explained, but the current theory is that it results from a combination of “the degradation of the initiator dicumylperoxide and hydrazine from the foaming agent azodicarbonamide,” the authors said.
In the paper, Dr. Raison-Peyron and Dr. Sasseville recommended a patch testing concentration of 0.1% in acetone or petrolatum, as acetophenone azine is not currently available from path test suppliers, although it can be obtained from chemical product distributors.
“Given the recent discovery of this allergen, it is presumed that cases of allergic contact dermatitis would have been missed and labeled irritant contact dermatitis or dyshidrosis,” they noted. To avoid missing more cases, acetophenone azine should be added to the patch testing shoe series, as well as plastics and glues series, they emphasized.
Although no cases of allergic reactions to acetophenone azine have been reported in the United States to date, it is an emerging allergen that should be on the radar for U.S. dermatologists, Amber Atwater, MD, outgoing ACDS president, said in an interview. The lack of reported cases may be in part attributed to the fact that acetophenone azine is not yet available to purchase for testing in the United States, and the allergen could be present in shin guards and other products identified in reported cases, added Dr. Atwater, associate professor of dermatology, Duke University, Durham, N.C.
.
The announcement was made by Donald V. Belsito, MD, professor of dermatology, Columbia University, New York, during a presentation at the annual meeting of the American Contact Dermatitis Society, held virtually this year. In his opinion, he said, the most exciting selections occur when international cooperation results in the identification of a new allergen that could become problematic, and acetophenone azine falls into this category.
The chemical formula of acetophenone azine is C16H16N2.
Acetophenone azine was highlighted as a contact allergen in a recent report in Dermatitis. The authors, Nadia Raison-Peyron, MD, from the department of dermatology at the University of Montpelier (France), and Denis Sasseville, MD, from the division of dermatology at McGill University Health Center, Quebec, described publications and reports of about 12 cases of severe allergic contact dermatitis secondary to shin pads or footwear, mainly in children and teens in Europe (one case was in Canada).
A common feature of these cases was the presence of a foam used for cushioning, made of ethyl vinyl acetate (EVA) used in the relevant products.
In one case, a 13-year-old boy who wore shin pads for soccer developed contact dermatitis on both shins that spread, and was described as severe. Patch testing revealed the EVA foam in the shin pads as the only positive reaction. Similar cases have been reported after exposure to EVA-containing products, including shin pads, sneakers, flip-flops, ski boots, insoles, swimming goggles, and bicycle seats, according to the authors.
In some reports, cases related to footwear presented as dyshidrosiform, vesiculobullous eczema, with or without palmar lesions, or presented as plantar hyperkeratotic dermatitis, they wrote. In other cases, patients experienced scarring and postinflammatory hypopigmentation.
The compound is likely not added to EVA intentionally, they added, but instead is thought to result from reactions between additives during the manufacturing process. The presence of acetophenone azine is not well explained, but the current theory is that it results from a combination of “the degradation of the initiator dicumylperoxide and hydrazine from the foaming agent azodicarbonamide,” the authors said.
In the paper, Dr. Raison-Peyron and Dr. Sasseville recommended a patch testing concentration of 0.1% in acetone or petrolatum, as acetophenone azine is not currently available from path test suppliers, although it can be obtained from chemical product distributors.
“Given the recent discovery of this allergen, it is presumed that cases of allergic contact dermatitis would have been missed and labeled irritant contact dermatitis or dyshidrosis,” they noted. To avoid missing more cases, acetophenone azine should be added to the patch testing shoe series, as well as plastics and glues series, they emphasized.
Although no cases of allergic reactions to acetophenone azine have been reported in the United States to date, it is an emerging allergen that should be on the radar for U.S. dermatologists, Amber Atwater, MD, outgoing ACDS president, said in an interview. The lack of reported cases may be in part attributed to the fact that acetophenone azine is not yet available to purchase for testing in the United States, and the allergen could be present in shin guards and other products identified in reported cases, added Dr. Atwater, associate professor of dermatology, Duke University, Durham, N.C.
FROM ACDS 2021
Contact Dermatitis of the Hands: Is It Irritant or Allergic?
Hand dermatitis, also known as hand eczema, is common and affects a considerable number of individuals across all ages. The impact of hand dermatitis can be profound, as it can impair one’s ability to perform tasks at home and at work. As a result of the coronavirus disease 2019 (COVID-19) pandemic, there has been an increased focus on hand hygiene and subsequently hand dermatitis. There are many contributors to the severity of hand dermatitis, including genetic factors, immune reactions, and skin barrier disruption. In this column, we will explore irritant contact dermatitis (ICD) and allergic contact dermatitis (ACD) of the hands, including epidemiology, potential causes, clinical characteristics, diagnosis, and management.
Epidemiology
The prevalence of hand dermatitis in the general population is 3% to 4%, with a 1‐year prevalence of 10% and a lifetime prevalence of 15%.1 In a Swedish study of patients self-reporting hand eczema, contact dermatitis comprised 57% of the total cases (N=1385); ICD accounted for 35% of cases followed by ACD in 22%.2 A recent study on hand dermatitis in North American specialty patch test clinics documented that the hands were the primary site of involvement in 24.2% of patients undergoing patch testing (N=37,113).3
The hands are particularly at risk for occupation-related contact dermatitis and are the primary site of involvement in 80% of cases, followed by the wrists and forearms.4 Occupations at greatest risk include cleaning, construction, metalworking, hairdressing, health care, housework, and mechanics.5 Even prior to the COVID-19 pandemic, occupational hand dermatitis was common; in a survey of inpatient nurses, the prevalence was 55% (N=167).6 More recently, a study from China demonstrated a 74.5% prevalence of hand dermatitis in frontline health care workers involved in COVID-19 patient care.7
Etiology of Hand ICD
The pathogenesis of ICD is multifactorial; although traditionally thought to be nonimmunologic, evidence has shown that it involves skin barrier disruption, infiltration by immunocompetent cells, and induction of inflammatory signal molecules. The degree of irritancy is related to the concentration, contact duration, and properties of the irritant. Irritant reactions can be acute, such as those following a single chemical exposure that results in a localized dermatitis, or chronic, such as after repetitive cumulative exposure to mild irritants such as soaps.
Hand hygiene products (eg, soaps, hand sanitizers) can be irritants and have recently gained notoriety given their increased use to prevent COVID-19 transmission.8,9 Specific irritants include iodophors, antimicrobial soaps (chlorhexidine gluconate, chloroxylenol, triclosan), surfactants, and detergents. Wolfe et al10 showed that detergent-based hand cleansing products had the highest association with ICD, which was thought to be due to their propensity to remove protective lipids and reduce moisture content in the stratum corneum. Although hand sanitizers are better tolerated than detergents, they can still contribute to ICD by stripping precious lipids and disrupting the skin barrier.11 Compared to ethanol, isopropanol and N-propanol cause more disruption of the stratum corneum.12 In addition, N-propanol has the same irritant potential as the detergent sodium lauryl sulfate.13 Thus, ethanol-based sanitizers may be better tolerated. Disinfectant surface wipes may include the irritant N-alkyl dimethyl benzyl ammonium chloride. Conversely, hand and baby wipes are formulated specifically for the skin and may be less irritating.11
Occupational contributors to hand ICD include chemical exposures and frequent handwashing. Wet work, mechanical trauma, warm dry air, and prolonged use of occlusive gloves also are well-known irritants.4 Fine or coarse particles encountered in some occupations or hobbies (eg, sand, sawdust, metal filings, plastic) can cause mechanical irritation. Exposure to physical friction from repeated handling of metal components, paper, cardboard, fabric, or steering wheels also has been implicated in hand ICD. Other common categories of occupational irritants include hydrocarbons, such as oils and petroleum.5,14
In addition to environmental factors, atopic dermatitis is an important endogenous factor that increases the risk of ICD due to underlying deficiencies within the main lipid15 and structural16 barrier components. These deficiencies ultimately lead to a lower threshold for the activation of inflammation via water loss and a weakened barrier. Studies have demonstrated that atopic dermatitis increases the risk for developing hand ICD 2- to 4-fold.17
Etiology of Hand ACD
Allergic contact dermatitis is an immune-mediated type IV delayed hypersensitivity reaction. The North American Contact Dermatitis Group reported that the top 5 clinically relevant hand allergens were methylisothiazolinone (MI), nickel, formaldehyde, quaternium-15, and fragrance mix I.3 Similarly, the European Surveillance System on Contact Allergies demonstrated that the most common hand allergens were nickel, preservatives (quaternium-15 and formaldehyde), fragrances, and cobalt.18 In health care workers, rubber accelerators often are relevant in patients with hand ACD.5,19 Hand hygiene products are known to contain potential allergens; a recent study demonstrated that the top 5 allergens in common hand sanitizers were tocopherol, fragrance, propylene glycol, benzoates, and cetylstearyl alcohol,20 whereas the most common allergens in hand cleansers were fragrance, tocopherol, sodium benzoate, chloroxylenol, propylene glycol, and chlorhexidine gluconate.21
Preservatives
Preservatives can contribute to hand ACD. Methylisothiazolinone was the most commonly relevant allergen in a recent North American study of hand contact allergy,3 and a study of North American products confirmed its presence in dishwashing products (64%), shampoos (53%), household cleaners (47%), laundry softeners/additives (30%), soaps and cleansers (29%), and surface disinfectants (27%).22 In addition, in a study of 139 patients with refractory MI contact allergy, the hands were the most common site (69%) and had the highest rate of relapse.23 Because of the common presence of this preservative in liquid-based personal care products, patients with MI hand contact allergy need to be vigilant.
The same North American study highlighted formaldehyde and the formaldehyde releaser quaternium-15 as commonly relevant hand contact allergens.3 Formaldehyde is not commonly found in personal care products, but formaldehyde-releasing preservatives frequently are found in cosmetic products, topical medicaments, detergents, soaps, and metal working fluids. Another study noted that the most relevant contact allergen in health care workers was quaternium-15, possibly due to increased hand hygiene and exposure to medical products used for patient care.24,25
Metals
Nickel is used in metal objects and is found in many workplaces in the form of machines, office supplies, tools, electronics, uniforms, and jewelry. Occupationally related nickel ACD of the hands is most common in hairdressers/barbers/cosmetologists,26 which is not surprising, as hairdressing tools such as scissors and hair clips can release nickel.27,28
Although nickel contact allergy is more common than cobalt, these metals frequently co-react, with up to 25% of nickel-sensitive patients also having positive patch test reactions to cobalt.29 Because cobalt is contained in alloys, the occupations most at risk pertain to hard metal manufacturing. Furthermore, cobalt is used in dentistry for dental tools, fillings, crowns, bridges, and dentures.30 Cobalt also has been identified in leather, and leather gloves have been implicated in hand ACD.31
Fragrances
Fragrances can be added to products to infuse pleasing aromas or mask unpleasant chemical odors. In the North American study of hand ACD, fragrance mix I and balsam of Peru were the sixth and seventh most clinically relevant allergens, respectively.3 In another study, fragrances were found in 50% of waterless cleansers and 95% of rinse-off soaps and were the second most common allergens found in skin disinfectants.21 Fragrance is ubiquitous in personal care and cleansing products, which can make avoidance difficult.
Rubber Accelerators
Contact allergy to rubber additives in medical gloves is the most common cause of occupational hand ACD in health care workers.5,19 Importantly, it usually is rubber accelerators that act as allergens in hand ACD and not natural rubber latex. Rubber accelerators known to cause ACD include thiurams, carbamates, 1,3-diphenylguanidine (DPG), mixed dialkyl thioureas, and benzothiazoles.32 In the setting of hand ACD in North America, reactions to thiuram mix and carba mix were the most common.3 Notably, DPG is a component of carba mix and can be present in rubber gloves. It has been shown that 40.3% of DPG reactions are missed by testing with carba mix alone; therefore, DPG must be patch tested separately.33
Clinical Examination
It can be challenging to differentiate between hand ICD and ACD based on clinical appearance alone, and patch testing often is necessary for diagnosis. In the acute phase, both ICD and ACD can present as erythema, papules, vesicles, bullae, and/or crusting. In the chronic phase, scaling, lichenification, and/or fissures tend to prevail. Both acute and chronic ICD and ACD can be associated with pruritus and pain; however, ICD may be more likely associated with a burning or painful sensation, whereas ACD may be more associated with pruritus.
Other dermatoses may present as hand eruptions and should be kept in the differential diagnosis. Atopic dermatitis, psoriasis, dyshidrotic eczema, hyperkeratotic hand dermatitis, keratolysis exfoliativa, and palmoplantar pustulosis are other common causes of hand eruptions.5,34
Patch Testing for Hand ACD
Consider patch testing for hand dermatitis that is refractory to conservative treatment. Patients with new-onset hand dermatitis without history of atopy and patients with a new worsening of chronic hand dermatitis also may need patch testing.
In addition to a medically appropriate screening series, patients with hand dermatitis often need supplemental patch testing. In a series of 37,113 patients with hand ACD, just over 20% of patients had positive patch test reactions to at least 1 supplemental allergen not on the screening series.3 Supplemental series should be selected based on the patient’s history and exposures; for example, nail salon technicians may need supplemental testing with the nail acrylate series, and massage therapists may need additional testing with the fragrance or essential oil series. Some of the most common supplemental series used for evaluation of hand dermatitis are the rubber, cosmetic, textile and dyes, plant, fragrance, essential oil, oil and coolants, nail or printing acrylates, and hairdressing series. If there is a high suspicion of occupational contact with allergens, obtaining material safety data sheets from the patient’s employer can be helpful to identify relevant allergens for testing.5 The thin-layer rapid use epicutaneous (T.R.U.E.) test may miss several common and relevant hand allergens, including benzalkonium chloride, lanolin, and iodopropynyl butylcarbamate.3
Management
Management of hand ICD requires avoidance of irritants and proper hand hygiene practices.10,34 The hands should be washed using lukewarm water and mild fragrance-free soaps or cleansers,35 keeping in mind that hand sanitizers may be better tolerated due to their lower lipid-stripping effects. The moisturizers with the best efficacy are combinations of humectants (topical urea, glycerin) and occlusive emollients (dimethicone, petrolatum).11 When wet work is necessary, gloves should be worn; however, sweat and humidity from glove use can worsen ICD, and gloves should be changed regularly and applied only when hands are dry. Cotton gloves also can be worn underneath rubber gloves to prevent maceration from sweat.9
The mainstay of hand ACD management is allergen avoidance. The American Contact Dermatitis Society maintains the Contact Allergen Management Program (CAMP), a database that identifies products that do not contain patient allergens. The importance of reading ingredient labels of products should always be emphasized. For patients with rubber accelerator allergies, vinyl or accelerator-free gloves may be used. If the allergen is occupational, communication with the patient’s employer is necessary.5
When hand contact dermatitis does not improve with avoidance of irritants and allergens as well as gentle skin care, topical therapy, phototherapy, and in some cases systemic therapy may be required. High-potency topical corticosteroids or short courses of prednisone may be needed for quick relief. Topical calcineurin inhibitors (tacrolimus and pimecrolimus) and the phosphodiesterase 4 inhibitor crisaborole have shown some efficacy for hand dermatitis and can be used as steroid-sparing agents.36,37 Narrowband UVB and UVA have been used with moderate efficacy to treat resistant hand dermatitis.34,38 Oral immunosuppressant medications such as methotrexate, mycophenolate mofetil, azathioprine, and cyclosporine can be used for more severe cases.34,39,40 Furthermore, oral retinoids have been used for chronic severe hand dermatitis with notable efficacy.41
Our Final Interpretation
The 2 major types of hand contact dermatitis are ICD and ACD. Hand ICD is more common than ACD in both occupational and nonoccupational settings. The hands are the most common sites in the setting of occupational dermatitis; in North American patch test populations, the hands were the primary site of involvement in just under 25% of patients.3 Many hand hygiene products contain irritants and allergens. The lipid-stripping effects of soaps, detergents, and hand sanitizers in conjunction with increased frequency of handwashing can trigger ICD. The most common allergens implicated in hand ACD include MI, nickel, formaldehyde, quaternium-15, and fragrances. Patch testing is important for diagnosis, and supplemental series should be considered. Management includes avoidance of irritants and allergens; liberal use of moisturizers and barrier creams; and prescription topical therapy, phototherapy, or systemic therapy when indicated.
- Thyssen JP, Johansen JD, Linneberg A, et al. The epidemiology of hand eczema in the general population—prevalence and main findings. Contact Dermatitis. 2010;62:75-87.
- Meding B, Swanbeck G. Epidemiology of different types of hand eczema in an industrial city. Acta Derm Venereol. 1989;69:227-233.
- Silverberg JI, Warshaw EM, Atwater AR, et al. Hand dermatitis in adults referred for patch testing: analysis of North American Contact Dermatitis Group data, 2000–2016 [published online November 28, 2020]. J Am Acad Dermatol. https://doi.org/10.1016/j.jaad.2020.11.054
- Sasseville D. Occupational contact dermatitis. Allergy Asthma Clin Immunol. 2008;4:59.
- Lampel HP, Powell HB. Occupational and hand dermatitis: a practical approach. Clin Rev Allergy Immunol. 2019;56:60-71.
- Lampel HP, Patel N, Boyse K, et al. Prevalence of hand dermatitis in inpatient nurses at a United States hospital. Dermatitis. 2007;18:140-142.
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease 2019. J Am Acad Dermatol. 2020;82:1215-1216.
- Wei Tan S, Chiat Oh C. Contact dermatitis from hand hygiene practices in the COVID-19 pandemic. 2020;49:674-676.
- Beiu C, Mihai M, Popa L, et al. Frequent hand washing for COVID-19 prevention can cause hand dermatitis: management tips. Cureus. 2020;12:E7506.
- Wolfe MK, Wells E, Mitro B, et al. Seeking clearer recommendations for hand hygiene in communities facing ebola: a randomized trial investigating the impact of six handwashing methods on skin irritation and dermatitis. PLoS One. 2016;11:e0167378.
- Rundle CW, Presley CL, Militello M, et al. Hand hygiene during COVID-19: recommendations from the American Contact Dermatitis Society. J Am Acad Dermatol. 2020;83:1730-1737.
- Cartner T, Brand N, Tian K, et al. Effect of different alcohols on stratum corneum kallikrein 5 and phospholipase A(2) together with epidermal keratinocytes and skin irritation. Int J Cosmet Sci. 2017;39:188-196.
- Clemmensen A, Andersen F, Petersen TK, et al. The irritant potential of n-propanol (nonanoic acid vehicle) in cumulative skin irritation: a validation study of two different human in vivo test models. Ski Res Technol. 2008;14:277-286.
- McMullen E, Gawkrodger DJ. Physical friction is under-recognized as an irritant that can cause or contribute to contact dermatitis. Br J Dermatol. 2006;154:154-156.
- Macheleidt O, Kaiser HW, Sandhoff K. Deficiency of epidermal protein-bound omega-hydroxyceramides in atopic dermatitis. J Invest Dermatol. 2002;119:166-173.
- Visser MJ, Landeck L, Campbell LE, et al. Impact of atopic dermatitis and loss-of-function mutations in the filaggrin gene on the development of occupational irritant contact dermatitis. Br J Dermatol. 2013;168:326-332.
- Coenraads PJ, Diepgen TL. Risk for hand eczema in employees with past or present atopic dermatitis. Int Arch Occup Environ Health. 1998;71:7-13.
- Oosterhaven JAF, Uter W, Aberer W, et al. European Surveillance System on Contact Allergies (ESSCA): contact allergies in relation to body sites in patients with allergic contact dermatitis. Contact Dermatitis. 2019;80:263-272.
- Goodier MC, Ronkainen SD, Hylwa SA. Rubber accelerators in medical examination and surgical gloves. Dermatitis. 2018;29:66-76.
- Voller LM, Schlarbaum JP, Hylwa SA. Allergenic ingredients in health care hand sanitizers in the United States [published online February 21, 2020]. Dermatitis. doi:10.1097/der.0000000000000567
- Rodriguez-Homs LG, Atwater AR. Allergens in medical hand skin cleansers. Dermatitis. 2019;30:336-341.
- Scheman A, Severson D. American Contact Dermatitis Society Contact Allergy Management Program: an epidemiologic tool to quantify ingredient usage. Dermatitis. 2016;27:11-13.
- Bouschon P, Waton J, Pereira B, et al. Methylisothiazolinone allergic contact dermatitis: assessment of relapses in 139 patients after avoidance advice. Contact Dermatitis. 2019;80:304-310.
- Kadivar S, Belsito DV. Occupational dermatitis in health care workers evaluated for suspected allergic contact dermatitis. Dermatitis. 2015;26:177-183.
- Prodi A, Rui F, Fortina AB, et al. Healthcare workers and skin sensitization: north-eastern Italian database. Occup Med (Chic Ill). 2016;66:72-74.
- Warshaw EM, Schlarbaum JP, Dekoven JG, et al. Occupationally related nickel reactions: a retrospective analysis of the North American Contact Dermatitis Group data 1998-2016. Dermatitis. 2019;30:306-313.
- Thyssen JP, Milting K, Bregnhøj A, et al. Nickel allergy in patch-tested female hairdressers and assessment of nickel release from hairdressers’ scissors and crochet hooks. Contact Dermatitis. 2009;61:281-286.
- Symanzik C, John SM, Strunk M. Nickel release from metal tools in the German hairdressing trade—a current analysis. 2019;80:382-385.
- Rystedt I, Fischer T. Relationship between nickel and cobalt sensitization in hard metal workers. Contact Dermatitis. 1983;9:195-200.
- Kettelarij JAB, Lidén C, Axén E, et al. Cobalt, nickel and chromium release from dental tools and alloys. Contact Dermatitis. 2014;70:3-10.
- Thyssen JP, Johansen JD, Jellesen MS, et al. Consumer leather exposure: an unrecognized cause of cobalt sensitization. 2013;69:276-279.
- Hamnerius N, Svedman C, Bergendorff O, et al. Hand eczema and occupational contact allergies in healthcare workers with a focus on rubber additives. Contact Dermatitis. 2018;79:149-156.
- Warshaw EM, Gupta R, Dekoven JG, et al. Patch testing to diphenylguanidine by the North American Contact Dermatitis Group (2013-2016). Dermatitis. 2020;31:350-358.
- Perry AD, Trafeli JP. Hand dermatitis: review of etiology, diagnosis, and treatment. J Am Board Fam Med. 2009;22:325-330.
- Abtahi-Naeini B. Frequent handwashing amidst the COVID-19 outbreak: prevention of hand irritant contact dermatitis and other considerations. Health Sci Rep. 2020;3:E163.
- Schliemann S, Kelterer D, Bauer A, et al. Tacrolimus ointment in the treatment of occupationally induced chronic hand dermatitis. Contact Dermatitis. 2008;58:299-306. doi:10.1111/j.1600-0536.2007.01314.x
- Lynde CW, Bergman J, Fiorillo L, et al. Use of topical crisaborole for treating dermatitis in a variety of dermatology settings. Skin Therapy Lett. Published June 1, 2020. Accessed February 10, 2021. https://www.skintherapyletter.com/dermatology/topical-crisaborole-dermatitis-treatment/
- Rosén K, Mobacken H, Swanbeck G. Chronic eczematous dermatitis of the hands: a comparison of PUVA and UVB treatment. Acta Derm Venereol. 1987;67:48-54.
- Kwon GP, Tan CZ, Chen JK. Hand dermatitis: utilizing subtype classification to direct intervention. Curr Treat Options Allergy. 2016;3:322-332.
- Warshaw E, Lee G, Storrs FJ. Hand dermatitis: a review of clinical features, therapeutic options, and long-term outcomes. Am J Contact Dermat. 2003;14:119-137.
- Song M, Lee H-J, Lee W-K, et al. Acitretin as a therapeutic option for chronic hand eczema. Ann Dermatol. 2017;29:385-387.
Hand dermatitis, also known as hand eczema, is common and affects a considerable number of individuals across all ages. The impact of hand dermatitis can be profound, as it can impair one’s ability to perform tasks at home and at work. As a result of the coronavirus disease 2019 (COVID-19) pandemic, there has been an increased focus on hand hygiene and subsequently hand dermatitis. There are many contributors to the severity of hand dermatitis, including genetic factors, immune reactions, and skin barrier disruption. In this column, we will explore irritant contact dermatitis (ICD) and allergic contact dermatitis (ACD) of the hands, including epidemiology, potential causes, clinical characteristics, diagnosis, and management.
Epidemiology
The prevalence of hand dermatitis in the general population is 3% to 4%, with a 1‐year prevalence of 10% and a lifetime prevalence of 15%.1 In a Swedish study of patients self-reporting hand eczema, contact dermatitis comprised 57% of the total cases (N=1385); ICD accounted for 35% of cases followed by ACD in 22%.2 A recent study on hand dermatitis in North American specialty patch test clinics documented that the hands were the primary site of involvement in 24.2% of patients undergoing patch testing (N=37,113).3
The hands are particularly at risk for occupation-related contact dermatitis and are the primary site of involvement in 80% of cases, followed by the wrists and forearms.4 Occupations at greatest risk include cleaning, construction, metalworking, hairdressing, health care, housework, and mechanics.5 Even prior to the COVID-19 pandemic, occupational hand dermatitis was common; in a survey of inpatient nurses, the prevalence was 55% (N=167).6 More recently, a study from China demonstrated a 74.5% prevalence of hand dermatitis in frontline health care workers involved in COVID-19 patient care.7
Etiology of Hand ICD
The pathogenesis of ICD is multifactorial; although traditionally thought to be nonimmunologic, evidence has shown that it involves skin barrier disruption, infiltration by immunocompetent cells, and induction of inflammatory signal molecules. The degree of irritancy is related to the concentration, contact duration, and properties of the irritant. Irritant reactions can be acute, such as those following a single chemical exposure that results in a localized dermatitis, or chronic, such as after repetitive cumulative exposure to mild irritants such as soaps.
Hand hygiene products (eg, soaps, hand sanitizers) can be irritants and have recently gained notoriety given their increased use to prevent COVID-19 transmission.8,9 Specific irritants include iodophors, antimicrobial soaps (chlorhexidine gluconate, chloroxylenol, triclosan), surfactants, and detergents. Wolfe et al10 showed that detergent-based hand cleansing products had the highest association with ICD, which was thought to be due to their propensity to remove protective lipids and reduce moisture content in the stratum corneum. Although hand sanitizers are better tolerated than detergents, they can still contribute to ICD by stripping precious lipids and disrupting the skin barrier.11 Compared to ethanol, isopropanol and N-propanol cause more disruption of the stratum corneum.12 In addition, N-propanol has the same irritant potential as the detergent sodium lauryl sulfate.13 Thus, ethanol-based sanitizers may be better tolerated. Disinfectant surface wipes may include the irritant N-alkyl dimethyl benzyl ammonium chloride. Conversely, hand and baby wipes are formulated specifically for the skin and may be less irritating.11
Occupational contributors to hand ICD include chemical exposures and frequent handwashing. Wet work, mechanical trauma, warm dry air, and prolonged use of occlusive gloves also are well-known irritants.4 Fine or coarse particles encountered in some occupations or hobbies (eg, sand, sawdust, metal filings, plastic) can cause mechanical irritation. Exposure to physical friction from repeated handling of metal components, paper, cardboard, fabric, or steering wheels also has been implicated in hand ICD. Other common categories of occupational irritants include hydrocarbons, such as oils and petroleum.5,14
In addition to environmental factors, atopic dermatitis is an important endogenous factor that increases the risk of ICD due to underlying deficiencies within the main lipid15 and structural16 barrier components. These deficiencies ultimately lead to a lower threshold for the activation of inflammation via water loss and a weakened barrier. Studies have demonstrated that atopic dermatitis increases the risk for developing hand ICD 2- to 4-fold.17
Etiology of Hand ACD
Allergic contact dermatitis is an immune-mediated type IV delayed hypersensitivity reaction. The North American Contact Dermatitis Group reported that the top 5 clinically relevant hand allergens were methylisothiazolinone (MI), nickel, formaldehyde, quaternium-15, and fragrance mix I.3 Similarly, the European Surveillance System on Contact Allergies demonstrated that the most common hand allergens were nickel, preservatives (quaternium-15 and formaldehyde), fragrances, and cobalt.18 In health care workers, rubber accelerators often are relevant in patients with hand ACD.5,19 Hand hygiene products are known to contain potential allergens; a recent study demonstrated that the top 5 allergens in common hand sanitizers were tocopherol, fragrance, propylene glycol, benzoates, and cetylstearyl alcohol,20 whereas the most common allergens in hand cleansers were fragrance, tocopherol, sodium benzoate, chloroxylenol, propylene glycol, and chlorhexidine gluconate.21
Preservatives
Preservatives can contribute to hand ACD. Methylisothiazolinone was the most commonly relevant allergen in a recent North American study of hand contact allergy,3 and a study of North American products confirmed its presence in dishwashing products (64%), shampoos (53%), household cleaners (47%), laundry softeners/additives (30%), soaps and cleansers (29%), and surface disinfectants (27%).22 In addition, in a study of 139 patients with refractory MI contact allergy, the hands were the most common site (69%) and had the highest rate of relapse.23 Because of the common presence of this preservative in liquid-based personal care products, patients with MI hand contact allergy need to be vigilant.
The same North American study highlighted formaldehyde and the formaldehyde releaser quaternium-15 as commonly relevant hand contact allergens.3 Formaldehyde is not commonly found in personal care products, but formaldehyde-releasing preservatives frequently are found in cosmetic products, topical medicaments, detergents, soaps, and metal working fluids. Another study noted that the most relevant contact allergen in health care workers was quaternium-15, possibly due to increased hand hygiene and exposure to medical products used for patient care.24,25
Metals
Nickel is used in metal objects and is found in many workplaces in the form of machines, office supplies, tools, electronics, uniforms, and jewelry. Occupationally related nickel ACD of the hands is most common in hairdressers/barbers/cosmetologists,26 which is not surprising, as hairdressing tools such as scissors and hair clips can release nickel.27,28
Although nickel contact allergy is more common than cobalt, these metals frequently co-react, with up to 25% of nickel-sensitive patients also having positive patch test reactions to cobalt.29 Because cobalt is contained in alloys, the occupations most at risk pertain to hard metal manufacturing. Furthermore, cobalt is used in dentistry for dental tools, fillings, crowns, bridges, and dentures.30 Cobalt also has been identified in leather, and leather gloves have been implicated in hand ACD.31
Fragrances
Fragrances can be added to products to infuse pleasing aromas or mask unpleasant chemical odors. In the North American study of hand ACD, fragrance mix I and balsam of Peru were the sixth and seventh most clinically relevant allergens, respectively.3 In another study, fragrances were found in 50% of waterless cleansers and 95% of rinse-off soaps and were the second most common allergens found in skin disinfectants.21 Fragrance is ubiquitous in personal care and cleansing products, which can make avoidance difficult.
Rubber Accelerators
Contact allergy to rubber additives in medical gloves is the most common cause of occupational hand ACD in health care workers.5,19 Importantly, it usually is rubber accelerators that act as allergens in hand ACD and not natural rubber latex. Rubber accelerators known to cause ACD include thiurams, carbamates, 1,3-diphenylguanidine (DPG), mixed dialkyl thioureas, and benzothiazoles.32 In the setting of hand ACD in North America, reactions to thiuram mix and carba mix were the most common.3 Notably, DPG is a component of carba mix and can be present in rubber gloves. It has been shown that 40.3% of DPG reactions are missed by testing with carba mix alone; therefore, DPG must be patch tested separately.33
Clinical Examination
It can be challenging to differentiate between hand ICD and ACD based on clinical appearance alone, and patch testing often is necessary for diagnosis. In the acute phase, both ICD and ACD can present as erythema, papules, vesicles, bullae, and/or crusting. In the chronic phase, scaling, lichenification, and/or fissures tend to prevail. Both acute and chronic ICD and ACD can be associated with pruritus and pain; however, ICD may be more likely associated with a burning or painful sensation, whereas ACD may be more associated with pruritus.
Other dermatoses may present as hand eruptions and should be kept in the differential diagnosis. Atopic dermatitis, psoriasis, dyshidrotic eczema, hyperkeratotic hand dermatitis, keratolysis exfoliativa, and palmoplantar pustulosis are other common causes of hand eruptions.5,34
Patch Testing for Hand ACD
Consider patch testing for hand dermatitis that is refractory to conservative treatment. Patients with new-onset hand dermatitis without history of atopy and patients with a new worsening of chronic hand dermatitis also may need patch testing.
In addition to a medically appropriate screening series, patients with hand dermatitis often need supplemental patch testing. In a series of 37,113 patients with hand ACD, just over 20% of patients had positive patch test reactions to at least 1 supplemental allergen not on the screening series.3 Supplemental series should be selected based on the patient’s history and exposures; for example, nail salon technicians may need supplemental testing with the nail acrylate series, and massage therapists may need additional testing with the fragrance or essential oil series. Some of the most common supplemental series used for evaluation of hand dermatitis are the rubber, cosmetic, textile and dyes, plant, fragrance, essential oil, oil and coolants, nail or printing acrylates, and hairdressing series. If there is a high suspicion of occupational contact with allergens, obtaining material safety data sheets from the patient’s employer can be helpful to identify relevant allergens for testing.5 The thin-layer rapid use epicutaneous (T.R.U.E.) test may miss several common and relevant hand allergens, including benzalkonium chloride, lanolin, and iodopropynyl butylcarbamate.3
Management
Management of hand ICD requires avoidance of irritants and proper hand hygiene practices.10,34 The hands should be washed using lukewarm water and mild fragrance-free soaps or cleansers,35 keeping in mind that hand sanitizers may be better tolerated due to their lower lipid-stripping effects. The moisturizers with the best efficacy are combinations of humectants (topical urea, glycerin) and occlusive emollients (dimethicone, petrolatum).11 When wet work is necessary, gloves should be worn; however, sweat and humidity from glove use can worsen ICD, and gloves should be changed regularly and applied only when hands are dry. Cotton gloves also can be worn underneath rubber gloves to prevent maceration from sweat.9
The mainstay of hand ACD management is allergen avoidance. The American Contact Dermatitis Society maintains the Contact Allergen Management Program (CAMP), a database that identifies products that do not contain patient allergens. The importance of reading ingredient labels of products should always be emphasized. For patients with rubber accelerator allergies, vinyl or accelerator-free gloves may be used. If the allergen is occupational, communication with the patient’s employer is necessary.5
When hand contact dermatitis does not improve with avoidance of irritants and allergens as well as gentle skin care, topical therapy, phototherapy, and in some cases systemic therapy may be required. High-potency topical corticosteroids or short courses of prednisone may be needed for quick relief. Topical calcineurin inhibitors (tacrolimus and pimecrolimus) and the phosphodiesterase 4 inhibitor crisaborole have shown some efficacy for hand dermatitis and can be used as steroid-sparing agents.36,37 Narrowband UVB and UVA have been used with moderate efficacy to treat resistant hand dermatitis.34,38 Oral immunosuppressant medications such as methotrexate, mycophenolate mofetil, azathioprine, and cyclosporine can be used for more severe cases.34,39,40 Furthermore, oral retinoids have been used for chronic severe hand dermatitis with notable efficacy.41
Our Final Interpretation
The 2 major types of hand contact dermatitis are ICD and ACD. Hand ICD is more common than ACD in both occupational and nonoccupational settings. The hands are the most common sites in the setting of occupational dermatitis; in North American patch test populations, the hands were the primary site of involvement in just under 25% of patients.3 Many hand hygiene products contain irritants and allergens. The lipid-stripping effects of soaps, detergents, and hand sanitizers in conjunction with increased frequency of handwashing can trigger ICD. The most common allergens implicated in hand ACD include MI, nickel, formaldehyde, quaternium-15, and fragrances. Patch testing is important for diagnosis, and supplemental series should be considered. Management includes avoidance of irritants and allergens; liberal use of moisturizers and barrier creams; and prescription topical therapy, phototherapy, or systemic therapy when indicated.
Hand dermatitis, also known as hand eczema, is common and affects a considerable number of individuals across all ages. The impact of hand dermatitis can be profound, as it can impair one’s ability to perform tasks at home and at work. As a result of the coronavirus disease 2019 (COVID-19) pandemic, there has been an increased focus on hand hygiene and subsequently hand dermatitis. There are many contributors to the severity of hand dermatitis, including genetic factors, immune reactions, and skin barrier disruption. In this column, we will explore irritant contact dermatitis (ICD) and allergic contact dermatitis (ACD) of the hands, including epidemiology, potential causes, clinical characteristics, diagnosis, and management.
Epidemiology
The prevalence of hand dermatitis in the general population is 3% to 4%, with a 1‐year prevalence of 10% and a lifetime prevalence of 15%.1 In a Swedish study of patients self-reporting hand eczema, contact dermatitis comprised 57% of the total cases (N=1385); ICD accounted for 35% of cases followed by ACD in 22%.2 A recent study on hand dermatitis in North American specialty patch test clinics documented that the hands were the primary site of involvement in 24.2% of patients undergoing patch testing (N=37,113).3
The hands are particularly at risk for occupation-related contact dermatitis and are the primary site of involvement in 80% of cases, followed by the wrists and forearms.4 Occupations at greatest risk include cleaning, construction, metalworking, hairdressing, health care, housework, and mechanics.5 Even prior to the COVID-19 pandemic, occupational hand dermatitis was common; in a survey of inpatient nurses, the prevalence was 55% (N=167).6 More recently, a study from China demonstrated a 74.5% prevalence of hand dermatitis in frontline health care workers involved in COVID-19 patient care.7
Etiology of Hand ICD
The pathogenesis of ICD is multifactorial; although traditionally thought to be nonimmunologic, evidence has shown that it involves skin barrier disruption, infiltration by immunocompetent cells, and induction of inflammatory signal molecules. The degree of irritancy is related to the concentration, contact duration, and properties of the irritant. Irritant reactions can be acute, such as those following a single chemical exposure that results in a localized dermatitis, or chronic, such as after repetitive cumulative exposure to mild irritants such as soaps.
Hand hygiene products (eg, soaps, hand sanitizers) can be irritants and have recently gained notoriety given their increased use to prevent COVID-19 transmission.8,9 Specific irritants include iodophors, antimicrobial soaps (chlorhexidine gluconate, chloroxylenol, triclosan), surfactants, and detergents. Wolfe et al10 showed that detergent-based hand cleansing products had the highest association with ICD, which was thought to be due to their propensity to remove protective lipids and reduce moisture content in the stratum corneum. Although hand sanitizers are better tolerated than detergents, they can still contribute to ICD by stripping precious lipids and disrupting the skin barrier.11 Compared to ethanol, isopropanol and N-propanol cause more disruption of the stratum corneum.12 In addition, N-propanol has the same irritant potential as the detergent sodium lauryl sulfate.13 Thus, ethanol-based sanitizers may be better tolerated. Disinfectant surface wipes may include the irritant N-alkyl dimethyl benzyl ammonium chloride. Conversely, hand and baby wipes are formulated specifically for the skin and may be less irritating.11
Occupational contributors to hand ICD include chemical exposures and frequent handwashing. Wet work, mechanical trauma, warm dry air, and prolonged use of occlusive gloves also are well-known irritants.4 Fine or coarse particles encountered in some occupations or hobbies (eg, sand, sawdust, metal filings, plastic) can cause mechanical irritation. Exposure to physical friction from repeated handling of metal components, paper, cardboard, fabric, or steering wheels also has been implicated in hand ICD. Other common categories of occupational irritants include hydrocarbons, such as oils and petroleum.5,14
In addition to environmental factors, atopic dermatitis is an important endogenous factor that increases the risk of ICD due to underlying deficiencies within the main lipid15 and structural16 barrier components. These deficiencies ultimately lead to a lower threshold for the activation of inflammation via water loss and a weakened barrier. Studies have demonstrated that atopic dermatitis increases the risk for developing hand ICD 2- to 4-fold.17
Etiology of Hand ACD
Allergic contact dermatitis is an immune-mediated type IV delayed hypersensitivity reaction. The North American Contact Dermatitis Group reported that the top 5 clinically relevant hand allergens were methylisothiazolinone (MI), nickel, formaldehyde, quaternium-15, and fragrance mix I.3 Similarly, the European Surveillance System on Contact Allergies demonstrated that the most common hand allergens were nickel, preservatives (quaternium-15 and formaldehyde), fragrances, and cobalt.18 In health care workers, rubber accelerators often are relevant in patients with hand ACD.5,19 Hand hygiene products are known to contain potential allergens; a recent study demonstrated that the top 5 allergens in common hand sanitizers were tocopherol, fragrance, propylene glycol, benzoates, and cetylstearyl alcohol,20 whereas the most common allergens in hand cleansers were fragrance, tocopherol, sodium benzoate, chloroxylenol, propylene glycol, and chlorhexidine gluconate.21
Preservatives
Preservatives can contribute to hand ACD. Methylisothiazolinone was the most commonly relevant allergen in a recent North American study of hand contact allergy,3 and a study of North American products confirmed its presence in dishwashing products (64%), shampoos (53%), household cleaners (47%), laundry softeners/additives (30%), soaps and cleansers (29%), and surface disinfectants (27%).22 In addition, in a study of 139 patients with refractory MI contact allergy, the hands were the most common site (69%) and had the highest rate of relapse.23 Because of the common presence of this preservative in liquid-based personal care products, patients with MI hand contact allergy need to be vigilant.
The same North American study highlighted formaldehyde and the formaldehyde releaser quaternium-15 as commonly relevant hand contact allergens.3 Formaldehyde is not commonly found in personal care products, but formaldehyde-releasing preservatives frequently are found in cosmetic products, topical medicaments, detergents, soaps, and metal working fluids. Another study noted that the most relevant contact allergen in health care workers was quaternium-15, possibly due to increased hand hygiene and exposure to medical products used for patient care.24,25
Metals
Nickel is used in metal objects and is found in many workplaces in the form of machines, office supplies, tools, electronics, uniforms, and jewelry. Occupationally related nickel ACD of the hands is most common in hairdressers/barbers/cosmetologists,26 which is not surprising, as hairdressing tools such as scissors and hair clips can release nickel.27,28
Although nickel contact allergy is more common than cobalt, these metals frequently co-react, with up to 25% of nickel-sensitive patients also having positive patch test reactions to cobalt.29 Because cobalt is contained in alloys, the occupations most at risk pertain to hard metal manufacturing. Furthermore, cobalt is used in dentistry for dental tools, fillings, crowns, bridges, and dentures.30 Cobalt also has been identified in leather, and leather gloves have been implicated in hand ACD.31
Fragrances
Fragrances can be added to products to infuse pleasing aromas or mask unpleasant chemical odors. In the North American study of hand ACD, fragrance mix I and balsam of Peru were the sixth and seventh most clinically relevant allergens, respectively.3 In another study, fragrances were found in 50% of waterless cleansers and 95% of rinse-off soaps and were the second most common allergens found in skin disinfectants.21 Fragrance is ubiquitous in personal care and cleansing products, which can make avoidance difficult.
Rubber Accelerators
Contact allergy to rubber additives in medical gloves is the most common cause of occupational hand ACD in health care workers.5,19 Importantly, it usually is rubber accelerators that act as allergens in hand ACD and not natural rubber latex. Rubber accelerators known to cause ACD include thiurams, carbamates, 1,3-diphenylguanidine (DPG), mixed dialkyl thioureas, and benzothiazoles.32 In the setting of hand ACD in North America, reactions to thiuram mix and carba mix were the most common.3 Notably, DPG is a component of carba mix and can be present in rubber gloves. It has been shown that 40.3% of DPG reactions are missed by testing with carba mix alone; therefore, DPG must be patch tested separately.33
Clinical Examination
It can be challenging to differentiate between hand ICD and ACD based on clinical appearance alone, and patch testing often is necessary for diagnosis. In the acute phase, both ICD and ACD can present as erythema, papules, vesicles, bullae, and/or crusting. In the chronic phase, scaling, lichenification, and/or fissures tend to prevail. Both acute and chronic ICD and ACD can be associated with pruritus and pain; however, ICD may be more likely associated with a burning or painful sensation, whereas ACD may be more associated with pruritus.
Other dermatoses may present as hand eruptions and should be kept in the differential diagnosis. Atopic dermatitis, psoriasis, dyshidrotic eczema, hyperkeratotic hand dermatitis, keratolysis exfoliativa, and palmoplantar pustulosis are other common causes of hand eruptions.5,34
Patch Testing for Hand ACD
Consider patch testing for hand dermatitis that is refractory to conservative treatment. Patients with new-onset hand dermatitis without history of atopy and patients with a new worsening of chronic hand dermatitis also may need patch testing.
In addition to a medically appropriate screening series, patients with hand dermatitis often need supplemental patch testing. In a series of 37,113 patients with hand ACD, just over 20% of patients had positive patch test reactions to at least 1 supplemental allergen not on the screening series.3 Supplemental series should be selected based on the patient’s history and exposures; for example, nail salon technicians may need supplemental testing with the nail acrylate series, and massage therapists may need additional testing with the fragrance or essential oil series. Some of the most common supplemental series used for evaluation of hand dermatitis are the rubber, cosmetic, textile and dyes, plant, fragrance, essential oil, oil and coolants, nail or printing acrylates, and hairdressing series. If there is a high suspicion of occupational contact with allergens, obtaining material safety data sheets from the patient’s employer can be helpful to identify relevant allergens for testing.5 The thin-layer rapid use epicutaneous (T.R.U.E.) test may miss several common and relevant hand allergens, including benzalkonium chloride, lanolin, and iodopropynyl butylcarbamate.3
Management
Management of hand ICD requires avoidance of irritants and proper hand hygiene practices.10,34 The hands should be washed using lukewarm water and mild fragrance-free soaps or cleansers,35 keeping in mind that hand sanitizers may be better tolerated due to their lower lipid-stripping effects. The moisturizers with the best efficacy are combinations of humectants (topical urea, glycerin) and occlusive emollients (dimethicone, petrolatum).11 When wet work is necessary, gloves should be worn; however, sweat and humidity from glove use can worsen ICD, and gloves should be changed regularly and applied only when hands are dry. Cotton gloves also can be worn underneath rubber gloves to prevent maceration from sweat.9
The mainstay of hand ACD management is allergen avoidance. The American Contact Dermatitis Society maintains the Contact Allergen Management Program (CAMP), a database that identifies products that do not contain patient allergens. The importance of reading ingredient labels of products should always be emphasized. For patients with rubber accelerator allergies, vinyl or accelerator-free gloves may be used. If the allergen is occupational, communication with the patient’s employer is necessary.5
When hand contact dermatitis does not improve with avoidance of irritants and allergens as well as gentle skin care, topical therapy, phototherapy, and in some cases systemic therapy may be required. High-potency topical corticosteroids or short courses of prednisone may be needed for quick relief. Topical calcineurin inhibitors (tacrolimus and pimecrolimus) and the phosphodiesterase 4 inhibitor crisaborole have shown some efficacy for hand dermatitis and can be used as steroid-sparing agents.36,37 Narrowband UVB and UVA have been used with moderate efficacy to treat resistant hand dermatitis.34,38 Oral immunosuppressant medications such as methotrexate, mycophenolate mofetil, azathioprine, and cyclosporine can be used for more severe cases.34,39,40 Furthermore, oral retinoids have been used for chronic severe hand dermatitis with notable efficacy.41
Our Final Interpretation
The 2 major types of hand contact dermatitis are ICD and ACD. Hand ICD is more common than ACD in both occupational and nonoccupational settings. The hands are the most common sites in the setting of occupational dermatitis; in North American patch test populations, the hands were the primary site of involvement in just under 25% of patients.3 Many hand hygiene products contain irritants and allergens. The lipid-stripping effects of soaps, detergents, and hand sanitizers in conjunction with increased frequency of handwashing can trigger ICD. The most common allergens implicated in hand ACD include MI, nickel, formaldehyde, quaternium-15, and fragrances. Patch testing is important for diagnosis, and supplemental series should be considered. Management includes avoidance of irritants and allergens; liberal use of moisturizers and barrier creams; and prescription topical therapy, phototherapy, or systemic therapy when indicated.
- Thyssen JP, Johansen JD, Linneberg A, et al. The epidemiology of hand eczema in the general population—prevalence and main findings. Contact Dermatitis. 2010;62:75-87.
- Meding B, Swanbeck G. Epidemiology of different types of hand eczema in an industrial city. Acta Derm Venereol. 1989;69:227-233.
- Silverberg JI, Warshaw EM, Atwater AR, et al. Hand dermatitis in adults referred for patch testing: analysis of North American Contact Dermatitis Group data, 2000–2016 [published online November 28, 2020]. J Am Acad Dermatol. https://doi.org/10.1016/j.jaad.2020.11.054
- Sasseville D. Occupational contact dermatitis. Allergy Asthma Clin Immunol. 2008;4:59.
- Lampel HP, Powell HB. Occupational and hand dermatitis: a practical approach. Clin Rev Allergy Immunol. 2019;56:60-71.
- Lampel HP, Patel N, Boyse K, et al. Prevalence of hand dermatitis in inpatient nurses at a United States hospital. Dermatitis. 2007;18:140-142.
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease 2019. J Am Acad Dermatol. 2020;82:1215-1216.
- Wei Tan S, Chiat Oh C. Contact dermatitis from hand hygiene practices in the COVID-19 pandemic. 2020;49:674-676.
- Beiu C, Mihai M, Popa L, et al. Frequent hand washing for COVID-19 prevention can cause hand dermatitis: management tips. Cureus. 2020;12:E7506.
- Wolfe MK, Wells E, Mitro B, et al. Seeking clearer recommendations for hand hygiene in communities facing ebola: a randomized trial investigating the impact of six handwashing methods on skin irritation and dermatitis. PLoS One. 2016;11:e0167378.
- Rundle CW, Presley CL, Militello M, et al. Hand hygiene during COVID-19: recommendations from the American Contact Dermatitis Society. J Am Acad Dermatol. 2020;83:1730-1737.
- Cartner T, Brand N, Tian K, et al. Effect of different alcohols on stratum corneum kallikrein 5 and phospholipase A(2) together with epidermal keratinocytes and skin irritation. Int J Cosmet Sci. 2017;39:188-196.
- Clemmensen A, Andersen F, Petersen TK, et al. The irritant potential of n-propanol (nonanoic acid vehicle) in cumulative skin irritation: a validation study of two different human in vivo test models. Ski Res Technol. 2008;14:277-286.
- McMullen E, Gawkrodger DJ. Physical friction is under-recognized as an irritant that can cause or contribute to contact dermatitis. Br J Dermatol. 2006;154:154-156.
- Macheleidt O, Kaiser HW, Sandhoff K. Deficiency of epidermal protein-bound omega-hydroxyceramides in atopic dermatitis. J Invest Dermatol. 2002;119:166-173.
- Visser MJ, Landeck L, Campbell LE, et al. Impact of atopic dermatitis and loss-of-function mutations in the filaggrin gene on the development of occupational irritant contact dermatitis. Br J Dermatol. 2013;168:326-332.
- Coenraads PJ, Diepgen TL. Risk for hand eczema in employees with past or present atopic dermatitis. Int Arch Occup Environ Health. 1998;71:7-13.
- Oosterhaven JAF, Uter W, Aberer W, et al. European Surveillance System on Contact Allergies (ESSCA): contact allergies in relation to body sites in patients with allergic contact dermatitis. Contact Dermatitis. 2019;80:263-272.
- Goodier MC, Ronkainen SD, Hylwa SA. Rubber accelerators in medical examination and surgical gloves. Dermatitis. 2018;29:66-76.
- Voller LM, Schlarbaum JP, Hylwa SA. Allergenic ingredients in health care hand sanitizers in the United States [published online February 21, 2020]. Dermatitis. doi:10.1097/der.0000000000000567
- Rodriguez-Homs LG, Atwater AR. Allergens in medical hand skin cleansers. Dermatitis. 2019;30:336-341.
- Scheman A, Severson D. American Contact Dermatitis Society Contact Allergy Management Program: an epidemiologic tool to quantify ingredient usage. Dermatitis. 2016;27:11-13.
- Bouschon P, Waton J, Pereira B, et al. Methylisothiazolinone allergic contact dermatitis: assessment of relapses in 139 patients after avoidance advice. Contact Dermatitis. 2019;80:304-310.
- Kadivar S, Belsito DV. Occupational dermatitis in health care workers evaluated for suspected allergic contact dermatitis. Dermatitis. 2015;26:177-183.
- Prodi A, Rui F, Fortina AB, et al. Healthcare workers and skin sensitization: north-eastern Italian database. Occup Med (Chic Ill). 2016;66:72-74.
- Warshaw EM, Schlarbaum JP, Dekoven JG, et al. Occupationally related nickel reactions: a retrospective analysis of the North American Contact Dermatitis Group data 1998-2016. Dermatitis. 2019;30:306-313.
- Thyssen JP, Milting K, Bregnhøj A, et al. Nickel allergy in patch-tested female hairdressers and assessment of nickel release from hairdressers’ scissors and crochet hooks. Contact Dermatitis. 2009;61:281-286.
- Symanzik C, John SM, Strunk M. Nickel release from metal tools in the German hairdressing trade—a current analysis. 2019;80:382-385.
- Rystedt I, Fischer T. Relationship between nickel and cobalt sensitization in hard metal workers. Contact Dermatitis. 1983;9:195-200.
- Kettelarij JAB, Lidén C, Axén E, et al. Cobalt, nickel and chromium release from dental tools and alloys. Contact Dermatitis. 2014;70:3-10.
- Thyssen JP, Johansen JD, Jellesen MS, et al. Consumer leather exposure: an unrecognized cause of cobalt sensitization. 2013;69:276-279.
- Hamnerius N, Svedman C, Bergendorff O, et al. Hand eczema and occupational contact allergies in healthcare workers with a focus on rubber additives. Contact Dermatitis. 2018;79:149-156.
- Warshaw EM, Gupta R, Dekoven JG, et al. Patch testing to diphenylguanidine by the North American Contact Dermatitis Group (2013-2016). Dermatitis. 2020;31:350-358.
- Perry AD, Trafeli JP. Hand dermatitis: review of etiology, diagnosis, and treatment. J Am Board Fam Med. 2009;22:325-330.
- Abtahi-Naeini B. Frequent handwashing amidst the COVID-19 outbreak: prevention of hand irritant contact dermatitis and other considerations. Health Sci Rep. 2020;3:E163.
- Schliemann S, Kelterer D, Bauer A, et al. Tacrolimus ointment in the treatment of occupationally induced chronic hand dermatitis. Contact Dermatitis. 2008;58:299-306. doi:10.1111/j.1600-0536.2007.01314.x
- Lynde CW, Bergman J, Fiorillo L, et al. Use of topical crisaborole for treating dermatitis in a variety of dermatology settings. Skin Therapy Lett. Published June 1, 2020. Accessed February 10, 2021. https://www.skintherapyletter.com/dermatology/topical-crisaborole-dermatitis-treatment/
- Rosén K, Mobacken H, Swanbeck G. Chronic eczematous dermatitis of the hands: a comparison of PUVA and UVB treatment. Acta Derm Venereol. 1987;67:48-54.
- Kwon GP, Tan CZ, Chen JK. Hand dermatitis: utilizing subtype classification to direct intervention. Curr Treat Options Allergy. 2016;3:322-332.
- Warshaw E, Lee G, Storrs FJ. Hand dermatitis: a review of clinical features, therapeutic options, and long-term outcomes. Am J Contact Dermat. 2003;14:119-137.
- Song M, Lee H-J, Lee W-K, et al. Acitretin as a therapeutic option for chronic hand eczema. Ann Dermatol. 2017;29:385-387.
- Thyssen JP, Johansen JD, Linneberg A, et al. The epidemiology of hand eczema in the general population—prevalence and main findings. Contact Dermatitis. 2010;62:75-87.
- Meding B, Swanbeck G. Epidemiology of different types of hand eczema in an industrial city. Acta Derm Venereol. 1989;69:227-233.
- Silverberg JI, Warshaw EM, Atwater AR, et al. Hand dermatitis in adults referred for patch testing: analysis of North American Contact Dermatitis Group data, 2000–2016 [published online November 28, 2020]. J Am Acad Dermatol. https://doi.org/10.1016/j.jaad.2020.11.054
- Sasseville D. Occupational contact dermatitis. Allergy Asthma Clin Immunol. 2008;4:59.
- Lampel HP, Powell HB. Occupational and hand dermatitis: a practical approach. Clin Rev Allergy Immunol. 2019;56:60-71.
- Lampel HP, Patel N, Boyse K, et al. Prevalence of hand dermatitis in inpatient nurses at a United States hospital. Dermatitis. 2007;18:140-142.
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease 2019. J Am Acad Dermatol. 2020;82:1215-1216.
- Wei Tan S, Chiat Oh C. Contact dermatitis from hand hygiene practices in the COVID-19 pandemic. 2020;49:674-676.
- Beiu C, Mihai M, Popa L, et al. Frequent hand washing for COVID-19 prevention can cause hand dermatitis: management tips. Cureus. 2020;12:E7506.
- Wolfe MK, Wells E, Mitro B, et al. Seeking clearer recommendations for hand hygiene in communities facing ebola: a randomized trial investigating the impact of six handwashing methods on skin irritation and dermatitis. PLoS One. 2016;11:e0167378.
- Rundle CW, Presley CL, Militello M, et al. Hand hygiene during COVID-19: recommendations from the American Contact Dermatitis Society. J Am Acad Dermatol. 2020;83:1730-1737.
- Cartner T, Brand N, Tian K, et al. Effect of different alcohols on stratum corneum kallikrein 5 and phospholipase A(2) together with epidermal keratinocytes and skin irritation. Int J Cosmet Sci. 2017;39:188-196.
- Clemmensen A, Andersen F, Petersen TK, et al. The irritant potential of n-propanol (nonanoic acid vehicle) in cumulative skin irritation: a validation study of two different human in vivo test models. Ski Res Technol. 2008;14:277-286.
- McMullen E, Gawkrodger DJ. Physical friction is under-recognized as an irritant that can cause or contribute to contact dermatitis. Br J Dermatol. 2006;154:154-156.
- Macheleidt O, Kaiser HW, Sandhoff K. Deficiency of epidermal protein-bound omega-hydroxyceramides in atopic dermatitis. J Invest Dermatol. 2002;119:166-173.
- Visser MJ, Landeck L, Campbell LE, et al. Impact of atopic dermatitis and loss-of-function mutations in the filaggrin gene on the development of occupational irritant contact dermatitis. Br J Dermatol. 2013;168:326-332.
- Coenraads PJ, Diepgen TL. Risk for hand eczema in employees with past or present atopic dermatitis. Int Arch Occup Environ Health. 1998;71:7-13.
- Oosterhaven JAF, Uter W, Aberer W, et al. European Surveillance System on Contact Allergies (ESSCA): contact allergies in relation to body sites in patients with allergic contact dermatitis. Contact Dermatitis. 2019;80:263-272.
- Goodier MC, Ronkainen SD, Hylwa SA. Rubber accelerators in medical examination and surgical gloves. Dermatitis. 2018;29:66-76.
- Voller LM, Schlarbaum JP, Hylwa SA. Allergenic ingredients in health care hand sanitizers in the United States [published online February 21, 2020]. Dermatitis. doi:10.1097/der.0000000000000567
- Rodriguez-Homs LG, Atwater AR. Allergens in medical hand skin cleansers. Dermatitis. 2019;30:336-341.
- Scheman A, Severson D. American Contact Dermatitis Society Contact Allergy Management Program: an epidemiologic tool to quantify ingredient usage. Dermatitis. 2016;27:11-13.
- Bouschon P, Waton J, Pereira B, et al. Methylisothiazolinone allergic contact dermatitis: assessment of relapses in 139 patients after avoidance advice. Contact Dermatitis. 2019;80:304-310.
- Kadivar S, Belsito DV. Occupational dermatitis in health care workers evaluated for suspected allergic contact dermatitis. Dermatitis. 2015;26:177-183.
- Prodi A, Rui F, Fortina AB, et al. Healthcare workers and skin sensitization: north-eastern Italian database. Occup Med (Chic Ill). 2016;66:72-74.
- Warshaw EM, Schlarbaum JP, Dekoven JG, et al. Occupationally related nickel reactions: a retrospective analysis of the North American Contact Dermatitis Group data 1998-2016. Dermatitis. 2019;30:306-313.
- Thyssen JP, Milting K, Bregnhøj A, et al. Nickel allergy in patch-tested female hairdressers and assessment of nickel release from hairdressers’ scissors and crochet hooks. Contact Dermatitis. 2009;61:281-286.
- Symanzik C, John SM, Strunk M. Nickel release from metal tools in the German hairdressing trade—a current analysis. 2019;80:382-385.
- Rystedt I, Fischer T. Relationship between nickel and cobalt sensitization in hard metal workers. Contact Dermatitis. 1983;9:195-200.
- Kettelarij JAB, Lidén C, Axén E, et al. Cobalt, nickel and chromium release from dental tools and alloys. Contact Dermatitis. 2014;70:3-10.
- Thyssen JP, Johansen JD, Jellesen MS, et al. Consumer leather exposure: an unrecognized cause of cobalt sensitization. 2013;69:276-279.
- Hamnerius N, Svedman C, Bergendorff O, et al. Hand eczema and occupational contact allergies in healthcare workers with a focus on rubber additives. Contact Dermatitis. 2018;79:149-156.
- Warshaw EM, Gupta R, Dekoven JG, et al. Patch testing to diphenylguanidine by the North American Contact Dermatitis Group (2013-2016). Dermatitis. 2020;31:350-358.
- Perry AD, Trafeli JP. Hand dermatitis: review of etiology, diagnosis, and treatment. J Am Board Fam Med. 2009;22:325-330.
- Abtahi-Naeini B. Frequent handwashing amidst the COVID-19 outbreak: prevention of hand irritant contact dermatitis and other considerations. Health Sci Rep. 2020;3:E163.
- Schliemann S, Kelterer D, Bauer A, et al. Tacrolimus ointment in the treatment of occupationally induced chronic hand dermatitis. Contact Dermatitis. 2008;58:299-306. doi:10.1111/j.1600-0536.2007.01314.x
- Lynde CW, Bergman J, Fiorillo L, et al. Use of topical crisaborole for treating dermatitis in a variety of dermatology settings. Skin Therapy Lett. Published June 1, 2020. Accessed February 10, 2021. https://www.skintherapyletter.com/dermatology/topical-crisaborole-dermatitis-treatment/
- Rosén K, Mobacken H, Swanbeck G. Chronic eczematous dermatitis of the hands: a comparison of PUVA and UVB treatment. Acta Derm Venereol. 1987;67:48-54.
- Kwon GP, Tan CZ, Chen JK. Hand dermatitis: utilizing subtype classification to direct intervention. Curr Treat Options Allergy. 2016;3:322-332.
- Warshaw E, Lee G, Storrs FJ. Hand dermatitis: a review of clinical features, therapeutic options, and long-term outcomes. Am J Contact Dermat. 2003;14:119-137.
- Song M, Lee H-J, Lee W-K, et al. Acitretin as a therapeutic option for chronic hand eczema. Ann Dermatol. 2017;29:385-387.
Practice Points
- For the hands, irritant contact dermatitis (ICD) is more common than allergic contact dermatitis in both occupational and nonoccupational settings. Because of overlapping clinical features, it can be difficult to differentiate between these conditions.
- Use of hand hygiene products, frequent handwashing, wet work, mechanical trauma, and occlusion can contribute to ICD of the hands.
- Common hand contact allergens include preservatives, metals, fragrances, and rubber accelerators.
- Patch testing often is necessary for diagnosis of hand dermatitis, and both screening and supplemental allergen series may be required.
Exercise-Induced Vasculitis in a Patient With Negative Ultrasound Venous Reflux Study: A Mimic of Stasis Dermatitis
To the Editor:
The transient and generic appearance of exercise-induced vasculitis (EIV) makes it a commonly misdiagnosed condition. The lesion often is only encountered through photographs brought by the patient or by taking a thorough history. The lack of findings on clinical inspection and the generic appearance of EIV may lead to misdiagnosis as stasis dermatitis due to its presentation as erythematous lesions on the medial lower legs.
A 68-year-old woman with no notable medical history was referred to our clinic for suspected stasis dermatitis. At presentation, no lesions were identified on the legs, but she brought photographs of an erythematous urticarial eruption on the medial lower legs, extending from just above the sock line to the mid-calves (Figure). The eruptions had occurred over the last 16 years, typically presenting suddenly after playing tennis or an extended period of walking and spontaneously resolving in 4 days. The lesions were painless, restricted to the calves, and were not pruritic, though the initial presentation 16 years prior included pruritic pigmented patches on the anterior thighs. Because the condition spontaneously improved within days, no treatment was attempted. An ultrasound venous reflux study ruled out venous reflux and stasis dermatitis.
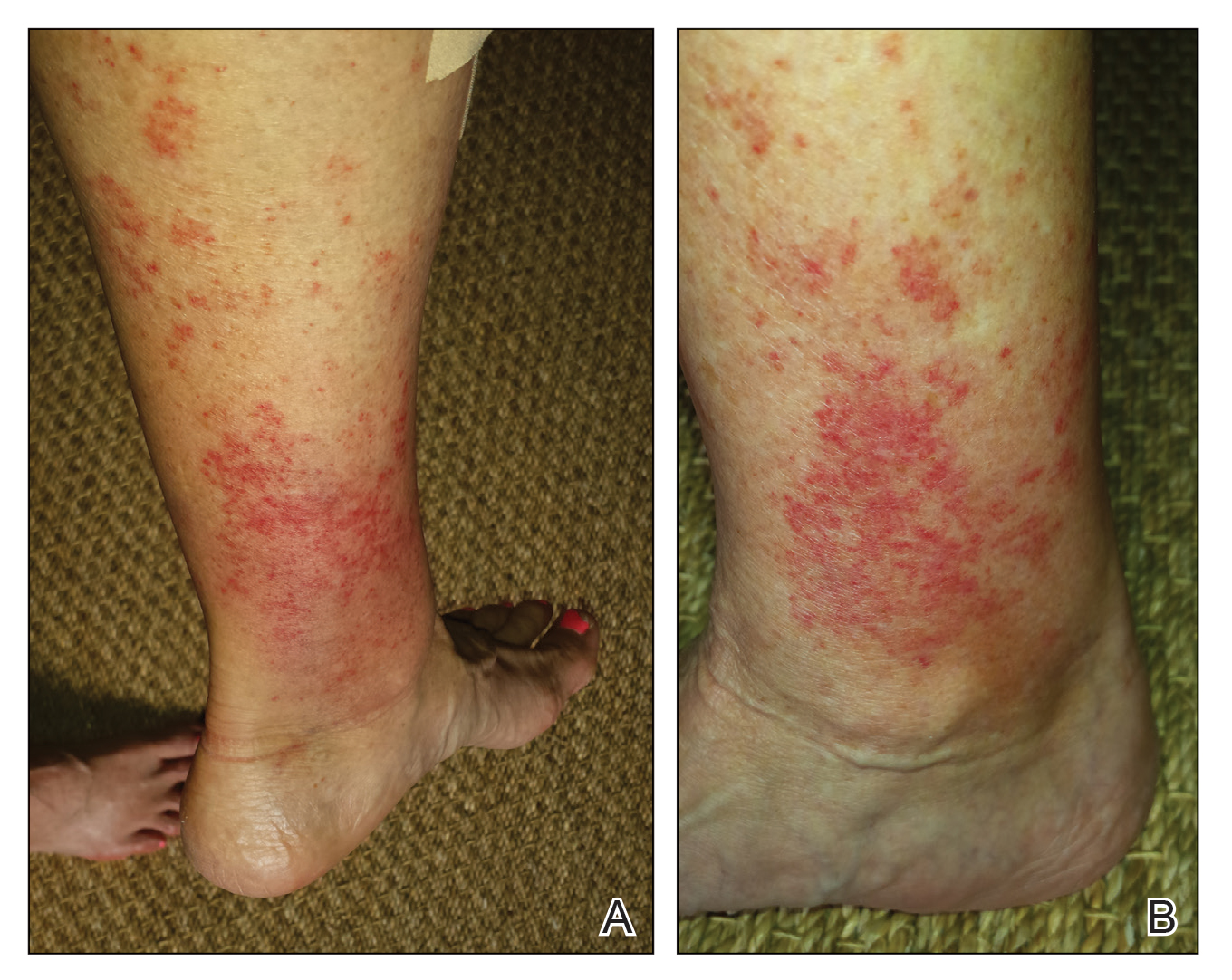
Our patient stated that her 64-year-old sister had reported the same presentation over the last 8 years. Her physical activity was limited strictly to walking, and the lesions occurred after walking for many hours during the day in the heat, involving the medial aspects of the lower legs extending from the ankles to the full length of the calves. Her eruption was warm but was not painful or pruritic. It resolved spontaneously after 5 days with no therapy.
Our patient was advised to wear compression stockings as a preventative measure, but she did not adhere to these recommendations, stating it was impractical to wear compression garments while playing tennis.
Exercise-induced vasculitis most commonly is seen in the medial aspects of the lower extremities as an erythematous urticarial eruption or pigmented purpuric plaque rapidly occurring after a period of exercise.1,2 Lesions often are symmetric and can be pruritic and painful with a lack of systemic symptoms.3 These generic clinical manifestations may lead to a misdiagnosis of stasis dermatitis. One case report included initial treatment of presumptive cellulitis.4 Important clinical findings include a sparing of skin compressed by tight clothing such as socks, a lack of systemic symptoms, rapid appearance after exercise, and spontaneous resolution within a few days. No correlation with chronic venous disease has been demonstrated, as EIV can occur in patients with or without chronic venous insufficiency.5 Duplex ultrasound evaluation showed no venous reflux in our patient.
The pathophysiology of EIV remains unknown, but the concept of exercise-altered microcirculation has been proposed. Heat generated from exercise is normally dissipated by thermoregulatory mechanisms such as cutaneous vasodilation and sweat.1,6 When exercise is extended, done concomitantly in the heat, or performed in legs with preexisting edema or substantial adipose tissue that limit heat attenuation, the thermoregulatory capacity is overloaded and heat-induced muscle fiber breakdown occurs.1,7 Atrophy impairs the skeletal muscle’s ability to pump the increased venous return demanded by exercise to the heart, leading to backflow of venous return and eventual venous stasis.1 Reduction of venous return together with cutaneous vasodilation is thought to induce erythrocyte extravasation.
Histologic examination demonstrates features of leukocytoclastic vasculitis with perivascular lymphocytic and neutrophilic infiltrates.2 Erythrocyte extravasation, IgM deposits, and identification of C3 also have been reported.8,9 The spontaneous resolution of EIV has led to treatment efforts being focused on preventative measures. Several cases have reported some degree of success in preventing EIV with compression therapy, venoactive drugs, systemic steroids, and application of topical steroids before exercise.3
The clinical morphology and lower leg location of EIV leads to a common misdiagnosis of stasis dermatitis. Clinical history of a transient nature is the mainstay in the diagnosis of EIV, and ultrasound venous reflux study may be required in some cases. Preventative measures are superior to treatment and mainly include compression therapy.
- Ramelet AA. Exercise-induced vasculitis. J Eur Acad Dermatol Venereol. 2006;20:423-427.
- Kelly RI, Opie J, Nixon R. Golfer’s vasculitis. Australas J Dermatol. 2005;46:11-14.
- Ramelet AA. Exercise-induced purpura. Dermatology. 2004;208:293-296.
- Cushman D, Rydberg L. A general rehabilitation inpatient with exercise-induced vasculitis. PM R. 2013;5:900-902.
- Veraart JC, Prins M, Hulsmans RF, et al. Influence of endurance exercise on the venous refilling time of the leg. Phlebology. 1994;23:120-123.
- Noakes T. Fluid replacement during marathon running. Clin J Sport Med. 2003;13:309-318.
- Armstrong RB. Muscle damage and endurance events. Sports Med. 1986;3:370-381.
- Prins M, Veraart JC, Vermeulen AH, et al. Leucocytoclastic vasculitis induced by prolonged exercise. Br J Dermatol. 1996;134:915-918.
- Sagdeo A, Gormley RH, Wanat KA, et al. Purpuric eruption on the feet of a healthy young woman. “flip-flop vasculitis” (exercise-induced vasculitis). JAMA Dermatol. 2013;149:751-756.
To the Editor:
The transient and generic appearance of exercise-induced vasculitis (EIV) makes it a commonly misdiagnosed condition. The lesion often is only encountered through photographs brought by the patient or by taking a thorough history. The lack of findings on clinical inspection and the generic appearance of EIV may lead to misdiagnosis as stasis dermatitis due to its presentation as erythematous lesions on the medial lower legs.
A 68-year-old woman with no notable medical history was referred to our clinic for suspected stasis dermatitis. At presentation, no lesions were identified on the legs, but she brought photographs of an erythematous urticarial eruption on the medial lower legs, extending from just above the sock line to the mid-calves (Figure). The eruptions had occurred over the last 16 years, typically presenting suddenly after playing tennis or an extended period of walking and spontaneously resolving in 4 days. The lesions were painless, restricted to the calves, and were not pruritic, though the initial presentation 16 years prior included pruritic pigmented patches on the anterior thighs. Because the condition spontaneously improved within days, no treatment was attempted. An ultrasound venous reflux study ruled out venous reflux and stasis dermatitis.

Our patient stated that her 64-year-old sister had reported the same presentation over the last 8 years. Her physical activity was limited strictly to walking, and the lesions occurred after walking for many hours during the day in the heat, involving the medial aspects of the lower legs extending from the ankles to the full length of the calves. Her eruption was warm but was not painful or pruritic. It resolved spontaneously after 5 days with no therapy.
Our patient was advised to wear compression stockings as a preventative measure, but she did not adhere to these recommendations, stating it was impractical to wear compression garments while playing tennis.
Exercise-induced vasculitis most commonly is seen in the medial aspects of the lower extremities as an erythematous urticarial eruption or pigmented purpuric plaque rapidly occurring after a period of exercise.1,2 Lesions often are symmetric and can be pruritic and painful with a lack of systemic symptoms.3 These generic clinical manifestations may lead to a misdiagnosis of stasis dermatitis. One case report included initial treatment of presumptive cellulitis.4 Important clinical findings include a sparing of skin compressed by tight clothing such as socks, a lack of systemic symptoms, rapid appearance after exercise, and spontaneous resolution within a few days. No correlation with chronic venous disease has been demonstrated, as EIV can occur in patients with or without chronic venous insufficiency.5 Duplex ultrasound evaluation showed no venous reflux in our patient.
The pathophysiology of EIV remains unknown, but the concept of exercise-altered microcirculation has been proposed. Heat generated from exercise is normally dissipated by thermoregulatory mechanisms such as cutaneous vasodilation and sweat.1,6 When exercise is extended, done concomitantly in the heat, or performed in legs with preexisting edema or substantial adipose tissue that limit heat attenuation, the thermoregulatory capacity is overloaded and heat-induced muscle fiber breakdown occurs.1,7 Atrophy impairs the skeletal muscle’s ability to pump the increased venous return demanded by exercise to the heart, leading to backflow of venous return and eventual venous stasis.1 Reduction of venous return together with cutaneous vasodilation is thought to induce erythrocyte extravasation.
Histologic examination demonstrates features of leukocytoclastic vasculitis with perivascular lymphocytic and neutrophilic infiltrates.2 Erythrocyte extravasation, IgM deposits, and identification of C3 also have been reported.8,9 The spontaneous resolution of EIV has led to treatment efforts being focused on preventative measures. Several cases have reported some degree of success in preventing EIV with compression therapy, venoactive drugs, systemic steroids, and application of topical steroids before exercise.3
The clinical morphology and lower leg location of EIV leads to a common misdiagnosis of stasis dermatitis. Clinical history of a transient nature is the mainstay in the diagnosis of EIV, and ultrasound venous reflux study may be required in some cases. Preventative measures are superior to treatment and mainly include compression therapy.
To the Editor:
The transient and generic appearance of exercise-induced vasculitis (EIV) makes it a commonly misdiagnosed condition. The lesion often is only encountered through photographs brought by the patient or by taking a thorough history. The lack of findings on clinical inspection and the generic appearance of EIV may lead to misdiagnosis as stasis dermatitis due to its presentation as erythematous lesions on the medial lower legs.
A 68-year-old woman with no notable medical history was referred to our clinic for suspected stasis dermatitis. At presentation, no lesions were identified on the legs, but she brought photographs of an erythematous urticarial eruption on the medial lower legs, extending from just above the sock line to the mid-calves (Figure). The eruptions had occurred over the last 16 years, typically presenting suddenly after playing tennis or an extended period of walking and spontaneously resolving in 4 days. The lesions were painless, restricted to the calves, and were not pruritic, though the initial presentation 16 years prior included pruritic pigmented patches on the anterior thighs. Because the condition spontaneously improved within days, no treatment was attempted. An ultrasound venous reflux study ruled out venous reflux and stasis dermatitis.

Our patient stated that her 64-year-old sister had reported the same presentation over the last 8 years. Her physical activity was limited strictly to walking, and the lesions occurred after walking for many hours during the day in the heat, involving the medial aspects of the lower legs extending from the ankles to the full length of the calves. Her eruption was warm but was not painful or pruritic. It resolved spontaneously after 5 days with no therapy.
Our patient was advised to wear compression stockings as a preventative measure, but she did not adhere to these recommendations, stating it was impractical to wear compression garments while playing tennis.
Exercise-induced vasculitis most commonly is seen in the medial aspects of the lower extremities as an erythematous urticarial eruption or pigmented purpuric plaque rapidly occurring after a period of exercise.1,2 Lesions often are symmetric and can be pruritic and painful with a lack of systemic symptoms.3 These generic clinical manifestations may lead to a misdiagnosis of stasis dermatitis. One case report included initial treatment of presumptive cellulitis.4 Important clinical findings include a sparing of skin compressed by tight clothing such as socks, a lack of systemic symptoms, rapid appearance after exercise, and spontaneous resolution within a few days. No correlation with chronic venous disease has been demonstrated, as EIV can occur in patients with or without chronic venous insufficiency.5 Duplex ultrasound evaluation showed no venous reflux in our patient.
The pathophysiology of EIV remains unknown, but the concept of exercise-altered microcirculation has been proposed. Heat generated from exercise is normally dissipated by thermoregulatory mechanisms such as cutaneous vasodilation and sweat.1,6 When exercise is extended, done concomitantly in the heat, or performed in legs with preexisting edema or substantial adipose tissue that limit heat attenuation, the thermoregulatory capacity is overloaded and heat-induced muscle fiber breakdown occurs.1,7 Atrophy impairs the skeletal muscle’s ability to pump the increased venous return demanded by exercise to the heart, leading to backflow of venous return and eventual venous stasis.1 Reduction of venous return together with cutaneous vasodilation is thought to induce erythrocyte extravasation.
Histologic examination demonstrates features of leukocytoclastic vasculitis with perivascular lymphocytic and neutrophilic infiltrates.2 Erythrocyte extravasation, IgM deposits, and identification of C3 also have been reported.8,9 The spontaneous resolution of EIV has led to treatment efforts being focused on preventative measures. Several cases have reported some degree of success in preventing EIV with compression therapy, venoactive drugs, systemic steroids, and application of topical steroids before exercise.3
The clinical morphology and lower leg location of EIV leads to a common misdiagnosis of stasis dermatitis. Clinical history of a transient nature is the mainstay in the diagnosis of EIV, and ultrasound venous reflux study may be required in some cases. Preventative measures are superior to treatment and mainly include compression therapy.
- Ramelet AA. Exercise-induced vasculitis. J Eur Acad Dermatol Venereol. 2006;20:423-427.
- Kelly RI, Opie J, Nixon R. Golfer’s vasculitis. Australas J Dermatol. 2005;46:11-14.
- Ramelet AA. Exercise-induced purpura. Dermatology. 2004;208:293-296.
- Cushman D, Rydberg L. A general rehabilitation inpatient with exercise-induced vasculitis. PM R. 2013;5:900-902.
- Veraart JC, Prins M, Hulsmans RF, et al. Influence of endurance exercise on the venous refilling time of the leg. Phlebology. 1994;23:120-123.
- Noakes T. Fluid replacement during marathon running. Clin J Sport Med. 2003;13:309-318.
- Armstrong RB. Muscle damage and endurance events. Sports Med. 1986;3:370-381.
- Prins M, Veraart JC, Vermeulen AH, et al. Leucocytoclastic vasculitis induced by prolonged exercise. Br J Dermatol. 1996;134:915-918.
- Sagdeo A, Gormley RH, Wanat KA, et al. Purpuric eruption on the feet of a healthy young woman. “flip-flop vasculitis” (exercise-induced vasculitis). JAMA Dermatol. 2013;149:751-756.
- Ramelet AA. Exercise-induced vasculitis. J Eur Acad Dermatol Venereol. 2006;20:423-427.
- Kelly RI, Opie J, Nixon R. Golfer’s vasculitis. Australas J Dermatol. 2005;46:11-14.
- Ramelet AA. Exercise-induced purpura. Dermatology. 2004;208:293-296.
- Cushman D, Rydberg L. A general rehabilitation inpatient with exercise-induced vasculitis. PM R. 2013;5:900-902.
- Veraart JC, Prins M, Hulsmans RF, et al. Influence of endurance exercise on the venous refilling time of the leg. Phlebology. 1994;23:120-123.
- Noakes T. Fluid replacement during marathon running. Clin J Sport Med. 2003;13:309-318.
- Armstrong RB. Muscle damage and endurance events. Sports Med. 1986;3:370-381.
- Prins M, Veraart JC, Vermeulen AH, et al. Leucocytoclastic vasculitis induced by prolonged exercise. Br J Dermatol. 1996;134:915-918.
- Sagdeo A, Gormley RH, Wanat KA, et al. Purpuric eruption on the feet of a healthy young woman. “flip-flop vasculitis” (exercise-induced vasculitis). JAMA Dermatol. 2013;149:751-756.
Practice Points
- Clinical history of a transient nature is the mainstay in the diagnosis of exercise-induced vasculitis.
- Exercise-induced vasculitis largely is documented in photographs or by history and may be misdiagnosed as stasis dermatitis due to its clinical morphology and lower leg location.
- Dermatologists should be aware of this disorder and consider performing further workup to rule out stasis dermatitis and diagnose this mimic.
- Preventative measures are superior to treatment and mainly include compression therapy.
Pink Patches With a Hyperpigmented Rim
The Diagnosis: Phytophotodermatitis
A more detailed patient history revealed that there was beer with limes on the boat, but the partygoers neglected to bring a knife. The patient volunteered to tear the limes apart with his bare hands. Because he was clad only in swim trunks, lime juice splattered over various regions of his body.
Phytophotodermatitis is a phototoxic blistering rash that follows topical exposure to plant-derived furocoumarins and sunlight. (Figure) Furocoumarins are photosensitizing substances produced by certain plants, possibly as a defense mechanism against predators.1 They cause a nonimmunologic phototoxic reaction when deposited on the skin and exposed to UVA radiation. Exposure to limes is the most common precipitant of phytophotodermatitis, but other potential culprits include lemons, grapefruit, figs, carrots, parsnips, celery, and dill.2
Lesions associated with phytophotodermatitis classically present as painful erythematous patches and bullae in regions of furocoumarin exposure. Affected areas are well demarcated and irregularly shaped and heal with a characteristic hyperpigmented rim. They often have a downward streak pattern from the dripping juice.3 If the furocoumarins are transferred by touch, lesions can appear in the shape of handprints, which may raise alarms for physical abuse in children.4
Photochemical reactions caused by activated furocoumarins cross-link nuclear DNA and damage cell membranes. These changes lead to cellular death resulting in edema and destruction of the epidermis. Other effects include an increase in keratin and thickening of the stratum corneum. The hyperpigmentation is a result of increased concentration of melanosomes and stimulation of melanocytes by activated furocoumarins.5
Management of phytophotodermatitis depends on the severity of skin injury. Mild cases may not require any treatment, whereas the most severe ones require admission to a burn unit for wound care. Anti-inflammatory medications are the mainstay of therapy. Our patient was prescribed desonide cream 0.05% for application to the affected areas. Sunscreen should be applied to prevent worsening of hyperpigmentation, which may take months to years to fade naturally. If hyperpigmentation is cosmetically troubling to the patient, bleaching agents such as hydroquinone and retinoids or Nd:YAG laser can be used to accelerate the resolution of pigment.5
Phototoxicity differs from less common photoallergic reactions caused by preformed antibodies or a delayed cell-mediated response to a trigger. The classic presentation of photoallergy is apruritic, inflammatory, bullous eruption in a sensitized individual.6 Allergic contact dermatitis more commonly is associated with pruritus than pain, and it presents as a papulovesicular eruption that evolves into lichenified plaques.7 Porphyria cutanea tarda would likely be accompanied by other cutaneous features such as hypertrichosis and sclerodermoid plaques with dystrophic calcification, in addition to wine-colored urine-containing porphyrins.8 Bullous fixed drug eruptions develop within 48 hours of exposure to a causative agent. The patient typically would experience pruritus and burning at the site of clearly demarcated erythematous lesions that healed with hyperpigmentation.9 Lesions of bullous lupus erythematosus may appear in areas without sun exposure, and they would be more likely to leave behind hypopigmentation rather than hyperpigmentation.10
- Pathak MA. Phytophotodermatitis. Clin Dermatol. 1986;4:102-121.
- Egan CL, Sterling G. Phytophotodermatitis: a visit to Margaritaville. Cutis. 1993;51:41-42.
- Hankinson A, Lloyd B, Alweis R. Lime-induced phytophotodermatitis [published online ahead of print September 29, 2014]. J Community Hosp Intern Med Perspect. doi:10.3402/jchimp.v4.25090
- Fitzpatrick JK, Kohlwes J. Lime-induced phytophotodermatitis. J Gen Intern Med. 2018;33:975.
- Weber IC, Davis CP, Greeson DM. Phytophotodermatitis: the other "lime" disease. J Emerg Med. 1999;17:235-237.
- Monteiro AF, Rato M, Martins C. Drug-induced photosensitivity: photoallergic and phototoxic reactions. Clin Dermatol. 2016;34:571-581.
- Tan CH, Rasool S, Johnston GA. Contact dermatitis: allergic and irritant. Clin Dermatol. 2014;32:116-124.
- Dawe R. An overview of the cutaneous porphyrias. F1000Res. 2017;6:1906.
- Bandino JP, Wohltmann WE, Bray DW, et al. Naproxen-induced generalized bullous fixed drug eruption. Dermatol Online J. 2009;15:4.
- Contestable JJ, Edhegard KD, Meyerle JH. Bullous systemic lupus erythematosus: a review and update to diagnosis and treatment. Am J Clin Dermatol. 2014;15:517-524.
The Diagnosis: Phytophotodermatitis
A more detailed patient history revealed that there was beer with limes on the boat, but the partygoers neglected to bring a knife. The patient volunteered to tear the limes apart with his bare hands. Because he was clad only in swim trunks, lime juice splattered over various regions of his body.
Phytophotodermatitis is a phototoxic blistering rash that follows topical exposure to plant-derived furocoumarins and sunlight. (Figure) Furocoumarins are photosensitizing substances produced by certain plants, possibly as a defense mechanism against predators.1 They cause a nonimmunologic phototoxic reaction when deposited on the skin and exposed to UVA radiation. Exposure to limes is the most common precipitant of phytophotodermatitis, but other potential culprits include lemons, grapefruit, figs, carrots, parsnips, celery, and dill.2
Lesions associated with phytophotodermatitis classically present as painful erythematous patches and bullae in regions of furocoumarin exposure. Affected areas are well demarcated and irregularly shaped and heal with a characteristic hyperpigmented rim. They often have a downward streak pattern from the dripping juice.3 If the furocoumarins are transferred by touch, lesions can appear in the shape of handprints, which may raise alarms for physical abuse in children.4
Photochemical reactions caused by activated furocoumarins cross-link nuclear DNA and damage cell membranes. These changes lead to cellular death resulting in edema and destruction of the epidermis. Other effects include an increase in keratin and thickening of the stratum corneum. The hyperpigmentation is a result of increased concentration of melanosomes and stimulation of melanocytes by activated furocoumarins.5
Management of phytophotodermatitis depends on the severity of skin injury. Mild cases may not require any treatment, whereas the most severe ones require admission to a burn unit for wound care. Anti-inflammatory medications are the mainstay of therapy. Our patient was prescribed desonide cream 0.05% for application to the affected areas. Sunscreen should be applied to prevent worsening of hyperpigmentation, which may take months to years to fade naturally. If hyperpigmentation is cosmetically troubling to the patient, bleaching agents such as hydroquinone and retinoids or Nd:YAG laser can be used to accelerate the resolution of pigment.5
Phototoxicity differs from less common photoallergic reactions caused by preformed antibodies or a delayed cell-mediated response to a trigger. The classic presentation of photoallergy is apruritic, inflammatory, bullous eruption in a sensitized individual.6 Allergic contact dermatitis more commonly is associated with pruritus than pain, and it presents as a papulovesicular eruption that evolves into lichenified plaques.7 Porphyria cutanea tarda would likely be accompanied by other cutaneous features such as hypertrichosis and sclerodermoid plaques with dystrophic calcification, in addition to wine-colored urine-containing porphyrins.8 Bullous fixed drug eruptions develop within 48 hours of exposure to a causative agent. The patient typically would experience pruritus and burning at the site of clearly demarcated erythematous lesions that healed with hyperpigmentation.9 Lesions of bullous lupus erythematosus may appear in areas without sun exposure, and they would be more likely to leave behind hypopigmentation rather than hyperpigmentation.10
The Diagnosis: Phytophotodermatitis
A more detailed patient history revealed that there was beer with limes on the boat, but the partygoers neglected to bring a knife. The patient volunteered to tear the limes apart with his bare hands. Because he was clad only in swim trunks, lime juice splattered over various regions of his body.
Phytophotodermatitis is a phototoxic blistering rash that follows topical exposure to plant-derived furocoumarins and sunlight. (Figure) Furocoumarins are photosensitizing substances produced by certain plants, possibly as a defense mechanism against predators.1 They cause a nonimmunologic phototoxic reaction when deposited on the skin and exposed to UVA radiation. Exposure to limes is the most common precipitant of phytophotodermatitis, but other potential culprits include lemons, grapefruit, figs, carrots, parsnips, celery, and dill.2
Lesions associated with phytophotodermatitis classically present as painful erythematous patches and bullae in regions of furocoumarin exposure. Affected areas are well demarcated and irregularly shaped and heal with a characteristic hyperpigmented rim. They often have a downward streak pattern from the dripping juice.3 If the furocoumarins are transferred by touch, lesions can appear in the shape of handprints, which may raise alarms for physical abuse in children.4
Photochemical reactions caused by activated furocoumarins cross-link nuclear DNA and damage cell membranes. These changes lead to cellular death resulting in edema and destruction of the epidermis. Other effects include an increase in keratin and thickening of the stratum corneum. The hyperpigmentation is a result of increased concentration of melanosomes and stimulation of melanocytes by activated furocoumarins.5
Management of phytophotodermatitis depends on the severity of skin injury. Mild cases may not require any treatment, whereas the most severe ones require admission to a burn unit for wound care. Anti-inflammatory medications are the mainstay of therapy. Our patient was prescribed desonide cream 0.05% for application to the affected areas. Sunscreen should be applied to prevent worsening of hyperpigmentation, which may take months to years to fade naturally. If hyperpigmentation is cosmetically troubling to the patient, bleaching agents such as hydroquinone and retinoids or Nd:YAG laser can be used to accelerate the resolution of pigment.5
Phototoxicity differs from less common photoallergic reactions caused by preformed antibodies or a delayed cell-mediated response to a trigger. The classic presentation of photoallergy is apruritic, inflammatory, bullous eruption in a sensitized individual.6 Allergic contact dermatitis more commonly is associated with pruritus than pain, and it presents as a papulovesicular eruption that evolves into lichenified plaques.7 Porphyria cutanea tarda would likely be accompanied by other cutaneous features such as hypertrichosis and sclerodermoid plaques with dystrophic calcification, in addition to wine-colored urine-containing porphyrins.8 Bullous fixed drug eruptions develop within 48 hours of exposure to a causative agent. The patient typically would experience pruritus and burning at the site of clearly demarcated erythematous lesions that healed with hyperpigmentation.9 Lesions of bullous lupus erythematosus may appear in areas without sun exposure, and they would be more likely to leave behind hypopigmentation rather than hyperpigmentation.10
- Pathak MA. Phytophotodermatitis. Clin Dermatol. 1986;4:102-121.
- Egan CL, Sterling G. Phytophotodermatitis: a visit to Margaritaville. Cutis. 1993;51:41-42.
- Hankinson A, Lloyd B, Alweis R. Lime-induced phytophotodermatitis [published online ahead of print September 29, 2014]. J Community Hosp Intern Med Perspect. doi:10.3402/jchimp.v4.25090
- Fitzpatrick JK, Kohlwes J. Lime-induced phytophotodermatitis. J Gen Intern Med. 2018;33:975.
- Weber IC, Davis CP, Greeson DM. Phytophotodermatitis: the other "lime" disease. J Emerg Med. 1999;17:235-237.
- Monteiro AF, Rato M, Martins C. Drug-induced photosensitivity: photoallergic and phototoxic reactions. Clin Dermatol. 2016;34:571-581.
- Tan CH, Rasool S, Johnston GA. Contact dermatitis: allergic and irritant. Clin Dermatol. 2014;32:116-124.
- Dawe R. An overview of the cutaneous porphyrias. F1000Res. 2017;6:1906.
- Bandino JP, Wohltmann WE, Bray DW, et al. Naproxen-induced generalized bullous fixed drug eruption. Dermatol Online J. 2009;15:4.
- Contestable JJ, Edhegard KD, Meyerle JH. Bullous systemic lupus erythematosus: a review and update to diagnosis and treatment. Am J Clin Dermatol. 2014;15:517-524.
- Pathak MA. Phytophotodermatitis. Clin Dermatol. 1986;4:102-121.
- Egan CL, Sterling G. Phytophotodermatitis: a visit to Margaritaville. Cutis. 1993;51:41-42.
- Hankinson A, Lloyd B, Alweis R. Lime-induced phytophotodermatitis [published online ahead of print September 29, 2014]. J Community Hosp Intern Med Perspect. doi:10.3402/jchimp.v4.25090
- Fitzpatrick JK, Kohlwes J. Lime-induced phytophotodermatitis. J Gen Intern Med. 2018;33:975.
- Weber IC, Davis CP, Greeson DM. Phytophotodermatitis: the other "lime" disease. J Emerg Med. 1999;17:235-237.
- Monteiro AF, Rato M, Martins C. Drug-induced photosensitivity: photoallergic and phototoxic reactions. Clin Dermatol. 2016;34:571-581.
- Tan CH, Rasool S, Johnston GA. Contact dermatitis: allergic and irritant. Clin Dermatol. 2014;32:116-124.
- Dawe R. An overview of the cutaneous porphyrias. F1000Res. 2017;6:1906.
- Bandino JP, Wohltmann WE, Bray DW, et al. Naproxen-induced generalized bullous fixed drug eruption. Dermatol Online J. 2009;15:4.
- Contestable JJ, Edhegard KD, Meyerle JH. Bullous systemic lupus erythematosus: a review and update to diagnosis and treatment. Am J Clin Dermatol. 2014;15:517-524.
A 25-year-old man presented with a rash on the right hand, chest, abdomen, right thigh, and ankles of 2 weeks’ duration. He reported that the eruption began with bullous lesions following a boat trip. The bullae ruptured over the next several days, and the lesions evolved to the current appearance. Although the patient had experienced pain at the site of active blisters, he denied any current pain, itching, or bleeding from the lesions. No other medical comorbidities were present.