User login
Contact Dermatitis of the Hands: Is It Irritant or Allergic?
Hand dermatitis, also known as hand eczema, is common and affects a considerable number of individuals across all ages. The impact of hand dermatitis can be profound, as it can impair one’s ability to perform tasks at home and at work. As a result of the coronavirus disease 2019 (COVID-19) pandemic, there has been an increased focus on hand hygiene and subsequently hand dermatitis. There are many contributors to the severity of hand dermatitis, including genetic factors, immune reactions, and skin barrier disruption. In this column, we will explore irritant contact dermatitis (ICD) and allergic contact dermatitis (ACD) of the hands, including epidemiology, potential causes, clinical characteristics, diagnosis, and management.
Epidemiology
The prevalence of hand dermatitis in the general population is 3% to 4%, with a 1‐year prevalence of 10% and a lifetime prevalence of 15%.1 In a Swedish study of patients self-reporting hand eczema, contact dermatitis comprised 57% of the total cases (N=1385); ICD accounted for 35% of cases followed by ACD in 22%.2 A recent study on hand dermatitis in North American specialty patch test clinics documented that the hands were the primary site of involvement in 24.2% of patients undergoing patch testing (N=37,113).3
The hands are particularly at risk for occupation-related contact dermatitis and are the primary site of involvement in 80% of cases, followed by the wrists and forearms.4 Occupations at greatest risk include cleaning, construction, metalworking, hairdressing, health care, housework, and mechanics.5 Even prior to the COVID-19 pandemic, occupational hand dermatitis was common; in a survey of inpatient nurses, the prevalence was 55% (N=167).6 More recently, a study from China demonstrated a 74.5% prevalence of hand dermatitis in frontline health care workers involved in COVID-19 patient care.7
Etiology of Hand ICD
The pathogenesis of ICD is multifactorial; although traditionally thought to be nonimmunologic, evidence has shown that it involves skin barrier disruption, infiltration by immunocompetent cells, and induction of inflammatory signal molecules. The degree of irritancy is related to the concentration, contact duration, and properties of the irritant. Irritant reactions can be acute, such as those following a single chemical exposure that results in a localized dermatitis, or chronic, such as after repetitive cumulative exposure to mild irritants such as soaps.
Hand hygiene products (eg, soaps, hand sanitizers) can be irritants and have recently gained notoriety given their increased use to prevent COVID-19 transmission.8,9 Specific irritants include iodophors, antimicrobial soaps (chlorhexidine gluconate, chloroxylenol, triclosan), surfactants, and detergents. Wolfe et al10 showed that detergent-based hand cleansing products had the highest association with ICD, which was thought to be due to their propensity to remove protective lipids and reduce moisture content in the stratum corneum. Although hand sanitizers are better tolerated than detergents, they can still contribute to ICD by stripping precious lipids and disrupting the skin barrier.11 Compared to ethanol, isopropanol and N-propanol cause more disruption of the stratum corneum.12 In addition, N-propanol has the same irritant potential as the detergent sodium lauryl sulfate.13 Thus, ethanol-based sanitizers may be better tolerated. Disinfectant surface wipes may include the irritant N-alkyl dimethyl benzyl ammonium chloride. Conversely, hand and baby wipes are formulated specifically for the skin and may be less irritating.11
Occupational contributors to hand ICD include chemical exposures and frequent handwashing. Wet work, mechanical trauma, warm dry air, and prolonged use of occlusive gloves also are well-known irritants.4 Fine or coarse particles encountered in some occupations or hobbies (eg, sand, sawdust, metal filings, plastic) can cause mechanical irritation. Exposure to physical friction from repeated handling of metal components, paper, cardboard, fabric, or steering wheels also has been implicated in hand ICD. Other common categories of occupational irritants include hydrocarbons, such as oils and petroleum.5,14
In addition to environmental factors, atopic dermatitis is an important endogenous factor that increases the risk of ICD due to underlying deficiencies within the main lipid15 and structural16 barrier components. These deficiencies ultimately lead to a lower threshold for the activation of inflammation via water loss and a weakened barrier. Studies have demonstrated that atopic dermatitis increases the risk for developing hand ICD 2- to 4-fold.17
Etiology of Hand ACD
Allergic contact dermatitis is an immune-mediated type IV delayed hypersensitivity reaction. The North American Contact Dermatitis Group reported that the top 5 clinically relevant hand allergens were methylisothiazolinone (MI), nickel, formaldehyde, quaternium-15, and fragrance mix I.3 Similarly, the European Surveillance System on Contact Allergies demonstrated that the most common hand allergens were nickel, preservatives (quaternium-15 and formaldehyde), fragrances, and cobalt.18 In health care workers, rubber accelerators often are relevant in patients with hand ACD.5,19 Hand hygiene products are known to contain potential allergens; a recent study demonstrated that the top 5 allergens in common hand sanitizers were tocopherol, fragrance, propylene glycol, benzoates, and cetylstearyl alcohol,20 whereas the most common allergens in hand cleansers were fragrance, tocopherol, sodium benzoate, chloroxylenol, propylene glycol, and chlorhexidine gluconate.21
Preservatives
Preservatives can contribute to hand ACD. Methylisothiazolinone was the most commonly relevant allergen in a recent North American study of hand contact allergy,3 and a study of North American products confirmed its presence in dishwashing products (64%), shampoos (53%), household cleaners (47%), laundry softeners/additives (30%), soaps and cleansers (29%), and surface disinfectants (27%).22 In addition, in a study of 139 patients with refractory MI contact allergy, the hands were the most common site (69%) and had the highest rate of relapse.23 Because of the common presence of this preservative in liquid-based personal care products, patients with MI hand contact allergy need to be vigilant.
The same North American study highlighted formaldehyde and the formaldehyde releaser quaternium-15 as commonly relevant hand contact allergens.3 Formaldehyde is not commonly found in personal care products, but formaldehyde-releasing preservatives frequently are found in cosmetic products, topical medicaments, detergents, soaps, and metal working fluids. Another study noted that the most relevant contact allergen in health care workers was quaternium-15, possibly due to increased hand hygiene and exposure to medical products used for patient care.24,25
Metals
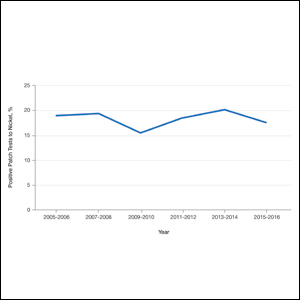
Nickel is used in metal objects and is found in many workplaces in the form of machines, office supplies, tools, electronics, uniforms, and jewelry. Occupationally related nickel ACD of the hands is most common in hairdressers/barbers/cosmetologists,26 which is not surprising, as hairdressing tools such as scissors and hair clips can release nickel.27,28
Although nickel contact allergy is more common than cobalt, these metals frequently co-react, with up to 25% of nickel-sensitive patients also having positive patch test reactions to cobalt.29 Because cobalt is contained in alloys, the occupations most at risk pertain to hard metal manufacturing. Furthermore, cobalt is used in dentistry for dental tools, fillings, crowns, bridges, and dentures.30 Cobalt also has been identified in leather, and leather gloves have been implicated in hand ACD.31
Fragrances
Fragrances can be added to products to infuse pleasing aromas or mask unpleasant chemical odors. In the North American study of hand ACD, fragrance mix I and balsam of Peru were the sixth and seventh most clinically relevant allergens, respectively.3 In another study, fragrances were found in 50% of waterless cleansers and 95% of rinse-off soaps and were the second most common allergens found in skin disinfectants.21 Fragrance is ubiquitous in personal care and cleansing products, which can make avoidance difficult.
Rubber Accelerators
Contact allergy to rubber additives in medical gloves is the most common cause of occupational hand ACD in health care workers.5,19 Importantly, it usually is rubber accelerators that act as allergens in hand ACD and not natural rubber latex. Rubber accelerators known to cause ACD include thiurams, carbamates, 1,3-diphenylguanidine (DPG), mixed dialkyl thioureas, and benzothiazoles.32 In the setting of hand ACD in North America, reactions to thiuram mix and carba mix were the most common.3 Notably, DPG is a component of carba mix and can be present in rubber gloves. It has been shown that 40.3% of DPG reactions are missed by testing with carba mix alone; therefore, DPG must be patch tested separately.33
Clinical Examination
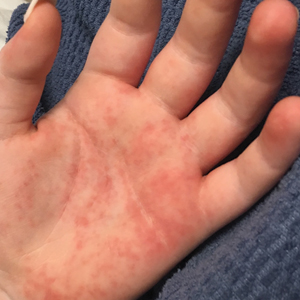
It can be challenging to differentiate between hand ICD and ACD based on clinical appearance alone, and patch testing often is necessary for diagnosis. In the acute phase, both ICD and ACD can present as erythema, papules, vesicles, bullae, and/or crusting. In the chronic phase, scaling, lichenification, and/or fissures tend to prevail. Both acute and chronic ICD and ACD can be associated with pruritus and pain; however, ICD may be more likely associated with a burning or painful sensation, whereas ACD may be more associated with pruritus.
Other dermatoses may present as hand eruptions and should be kept in the differential diagnosis. Atopic dermatitis, psoriasis, dyshidrotic eczema, hyperkeratotic hand dermatitis, keratolysis exfoliativa, and palmoplantar pustulosis are other common causes of hand eruptions.5,34
Patch Testing for Hand ACD
Consider patch testing for hand dermatitis that is refractory to conservative treatment. Patients with new-onset hand dermatitis without history of atopy and patients with a new worsening of chronic hand dermatitis also may need patch testing.
In addition to a medically appropriate screening series, patients with hand dermatitis often need supplemental patch testing. In a series of 37,113 patients with hand ACD, just over 20% of patients had positive patch test reactions to at least 1 supplemental allergen not on the screening series.3 Supplemental series should be selected based on the patient’s history and exposures; for example, nail salon technicians may need supplemental testing with the nail acrylate series, and massage therapists may need additional testing with the fragrance or essential oil series. Some of the most common supplemental series used for evaluation of hand dermatitis are the rubber, cosmetic, textile and dyes, plant, fragrance, essential oil, oil and coolants, nail or printing acrylates, and hairdressing series. If there is a high suspicion of occupational contact with allergens, obtaining material safety data sheets from the patient’s employer can be helpful to identify relevant allergens for testing.5 The thin-layer rapid use epicutaneous (T.R.U.E.) test may miss several common and relevant hand allergens, including benzalkonium chloride, lanolin, and iodopropynyl butylcarbamate.3
Management
Management of hand ICD requires avoidance of irritants and proper hand hygiene practices.10,34 The hands should be washed using lukewarm water and mild fragrance-free soaps or cleansers,35 keeping in mind that hand sanitizers may be better tolerated due to their lower lipid-stripping effects. The moisturizers with the best efficacy are combinations of humectants (topical urea, glycerin) and occlusive emollients (dimethicone, petrolatum).11 When wet work is necessary, gloves should be worn; however, sweat and humidity from glove use can worsen ICD, and gloves should be changed regularly and applied only when hands are dry. Cotton gloves also can be worn underneath rubber gloves to prevent maceration from sweat.9
The mainstay of hand ACD management is allergen avoidance. The American Contact Dermatitis Society maintains the Contact Allergen Management Program (CAMP), a database that identifies products that do not contain patient allergens. The importance of reading ingredient labels of products should always be emphasized. For patients with rubber accelerator allergies, vinyl or accelerator-free gloves may be used. If the allergen is occupational, communication with the patient’s employer is necessary.5
When hand contact dermatitis does not improve with avoidance of irritants and allergens as well as gentle skin care, topical therapy, phototherapy, and in some cases systemic therapy may be required. High-potency topical corticosteroids or short courses of prednisone may be needed for quick relief. Topical calcineurin inhibitors (tacrolimus and pimecrolimus) and the phosphodiesterase 4 inhibitor crisaborole have shown some efficacy for hand dermatitis and can be used as steroid-sparing agents.36,37 Narrowband UVB and UVA have been used with moderate efficacy to treat resistant hand dermatitis.34,38 Oral immunosuppressant medications such as methotrexate, mycophenolate mofetil, azathioprine, and cyclosporine can be used for more severe cases.34,39,40 Furthermore, oral retinoids have been used for chronic severe hand dermatitis with notable efficacy.41
Our Final Interpretation
The 2 major types of hand contact dermatitis are ICD and ACD. Hand ICD is more common than ACD in both occupational and nonoccupational settings. The hands are the most common sites in the setting of occupational dermatitis; in North American patch test populations, the hands were the primary site of involvement in just under 25% of patients.3 Many hand hygiene products contain irritants and allergens. The lipid-stripping effects of soaps, detergents, and hand sanitizers in conjunction with increased frequency of handwashing can trigger ICD. The most common allergens implicated in hand ACD include MI, nickel, formaldehyde, quaternium-15, and fragrances. Patch testing is important for diagnosis, and supplemental series should be considered. Management includes avoidance of irritants and allergens; liberal use of moisturizers and barrier creams; and prescription topical therapy, phototherapy, or systemic therapy when indicated.
- Thyssen JP, Johansen JD, Linneberg A, et al. The epidemiology of hand eczema in the general population—prevalence and main findings. Contact Dermatitis. 2010;62:75-87.
- Meding B, Swanbeck G. Epidemiology of different types of hand eczema in an industrial city. Acta Derm Venereol. 1989;69:227-233.
- Silverberg JI, Warshaw EM, Atwater AR, et al. Hand dermatitis in adults referred for patch testing: analysis of North American Contact Dermatitis Group data, 2000–2016 [published online November 28, 2020]. J Am Acad Dermatol. https://doi.org/10.1016/j.jaad.2020.11.054
- Sasseville D. Occupational contact dermatitis. Allergy Asthma Clin Immunol. 2008;4:59.
- Lampel HP, Powell HB. Occupational and hand dermatitis: a practical approach. Clin Rev Allergy Immunol. 2019;56:60-71.
- Lampel HP, Patel N, Boyse K, et al. Prevalence of hand dermatitis in inpatient nurses at a United States hospital. Dermatitis. 2007;18:140-142.
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease 2019. J Am Acad Dermatol. 2020;82:1215-1216.
- Wei Tan S, Chiat Oh C. Contact dermatitis from hand hygiene practices in the COVID-19 pandemic. 2020;49:674-676.
- Beiu C, Mihai M, Popa L, et al. Frequent hand washing for COVID-19 prevention can cause hand dermatitis: management tips. Cureus. 2020;12:E7506.
- Wolfe MK, Wells E, Mitro B, et al. Seeking clearer recommendations for hand hygiene in communities facing ebola: a randomized trial investigating the impact of six handwashing methods on skin irritation and dermatitis. PLoS One. 2016;11:e0167378.
- Rundle CW, Presley CL, Militello M, et al. Hand hygiene during COVID-19: recommendations from the American Contact Dermatitis Society. J Am Acad Dermatol. 2020;83:1730-1737.
- Cartner T, Brand N, Tian K, et al. Effect of different alcohols on stratum corneum kallikrein 5 and phospholipase A(2) together with epidermal keratinocytes and skin irritation. Int J Cosmet Sci. 2017;39:188-196.
- Clemmensen A, Andersen F, Petersen TK, et al. The irritant potential of n-propanol (nonanoic acid vehicle) in cumulative skin irritation: a validation study of two different human in vivo test models. Ski Res Technol. 2008;14:277-286.
- McMullen E, Gawkrodger DJ. Physical friction is under-recognized as an irritant that can cause or contribute to contact dermatitis. Br J Dermatol. 2006;154:154-156.
- Macheleidt O, Kaiser HW, Sandhoff K. Deficiency of epidermal protein-bound omega-hydroxyceramides in atopic dermatitis. J Invest Dermatol. 2002;119:166-173.
- Visser MJ, Landeck L, Campbell LE, et al. Impact of atopic dermatitis and loss-of-function mutations in the filaggrin gene on the development of occupational irritant contact dermatitis. Br J Dermatol. 2013;168:326-332.
- Coenraads PJ, Diepgen TL. Risk for hand eczema in employees with past or present atopic dermatitis. Int Arch Occup Environ Health. 1998;71:7-13.
- Oosterhaven JAF, Uter W, Aberer W, et al. European Surveillance System on Contact Allergies (ESSCA): contact allergies in relation to body sites in patients with allergic contact dermatitis. Contact Dermatitis. 2019;80:263-272.
- Goodier MC, Ronkainen SD, Hylwa SA. Rubber accelerators in medical examination and surgical gloves. Dermatitis. 2018;29:66-76.
- Voller LM, Schlarbaum JP, Hylwa SA. Allergenic ingredients in health care hand sanitizers in the United States [published online February 21, 2020]. Dermatitis. doi:10.1097/der.0000000000000567
- Rodriguez-Homs LG, Atwater AR. Allergens in medical hand skin cleansers. Dermatitis. 2019;30:336-341.
- Scheman A, Severson D. American Contact Dermatitis Society Contact Allergy Management Program: an epidemiologic tool to quantify ingredient usage. Dermatitis. 2016;27:11-13.
- Bouschon P, Waton J, Pereira B, et al. Methylisothiazolinone allergic contact dermatitis: assessment of relapses in 139 patients after avoidance advice. Contact Dermatitis. 2019;80:304-310.
- Kadivar S, Belsito DV. Occupational dermatitis in health care workers evaluated for suspected allergic contact dermatitis. Dermatitis. 2015;26:177-183.
- Prodi A, Rui F, Fortina AB, et al. Healthcare workers and skin sensitization: north-eastern Italian database. Occup Med (Chic Ill). 2016;66:72-74.
- Warshaw EM, Schlarbaum JP, Dekoven JG, et al. Occupationally related nickel reactions: a retrospective analysis of the North American Contact Dermatitis Group data 1998-2016. Dermatitis. 2019;30:306-313.
- Thyssen JP, Milting K, Bregnhøj A, et al. Nickel allergy in patch-tested female hairdressers and assessment of nickel release from hairdressers’ scissors and crochet hooks. Contact Dermatitis. 2009;61:281-286.
- Symanzik C, John SM, Strunk M. Nickel release from metal tools in the German hairdressing trade—a current analysis. 2019;80:382-385.
- Rystedt I, Fischer T. Relationship between nickel and cobalt sensitization in hard metal workers. Contact Dermatitis. 1983;9:195-200.
- Kettelarij JAB, Lidén C, Axén E, et al. Cobalt, nickel and chromium release from dental tools and alloys. Contact Dermatitis. 2014;70:3-10.
- Thyssen JP, Johansen JD, Jellesen MS, et al. Consumer leather exposure: an unrecognized cause of cobalt sensitization. 2013;69:276-279.
- Hamnerius N, Svedman C, Bergendorff O, et al. Hand eczema and occupational contact allergies in healthcare workers with a focus on rubber additives. Contact Dermatitis. 2018;79:149-156.
- Warshaw EM, Gupta R, Dekoven JG, et al. Patch testing to diphenylguanidine by the North American Contact Dermatitis Group (2013-2016). Dermatitis. 2020;31:350-358.
- Perry AD, Trafeli JP. Hand dermatitis: review of etiology, diagnosis, and treatment. J Am Board Fam Med. 2009;22:325-330.
- Abtahi-Naeini B. Frequent handwashing amidst the COVID-19 outbreak: prevention of hand irritant contact dermatitis and other considerations. Health Sci Rep. 2020;3:E163.
- Schliemann S, Kelterer D, Bauer A, et al. Tacrolimus ointment in the treatment of occupationally induced chronic hand dermatitis. Contact Dermatitis. 2008;58:299-306. doi:10.1111/j.1600-0536.2007.01314.x
- Lynde CW, Bergman J, Fiorillo L, et al. Use of topical crisaborole for treating dermatitis in a variety of dermatology settings. Skin Therapy Lett. Published June 1, 2020. Accessed February 10, 2021. https://www.skintherapyletter.com/dermatology/topical-crisaborole-dermatitis-treatment/
- Rosén K, Mobacken H, Swanbeck G. Chronic eczematous dermatitis of the hands: a comparison of PUVA and UVB treatment. Acta Derm Venereol. 1987;67:48-54.
- Kwon GP, Tan CZ, Chen JK. Hand dermatitis: utilizing subtype classification to direct intervention. Curr Treat Options Allergy. 2016;3:322-332.
- Warshaw E, Lee G, Storrs FJ. Hand dermatitis: a review of clinical features, therapeutic options, and long-term outcomes. Am J Contact Dermat. 2003;14:119-137.
- Song M, Lee H-J, Lee W-K, et al. Acitretin as a therapeutic option for chronic hand eczema. Ann Dermatol. 2017;29:385-387.
Hand dermatitis, also known as hand eczema, is common and affects a considerable number of individuals across all ages. The impact of hand dermatitis can be profound, as it can impair one’s ability to perform tasks at home and at work. As a result of the coronavirus disease 2019 (COVID-19) pandemic, there has been an increased focus on hand hygiene and subsequently hand dermatitis. There are many contributors to the severity of hand dermatitis, including genetic factors, immune reactions, and skin barrier disruption. In this column, we will explore irritant contact dermatitis (ICD) and allergic contact dermatitis (ACD) of the hands, including epidemiology, potential causes, clinical characteristics, diagnosis, and management.
Epidemiology
The prevalence of hand dermatitis in the general population is 3% to 4%, with a 1‐year prevalence of 10% and a lifetime prevalence of 15%.1 In a Swedish study of patients self-reporting hand eczema, contact dermatitis comprised 57% of the total cases (N=1385); ICD accounted for 35% of cases followed by ACD in 22%.2 A recent study on hand dermatitis in North American specialty patch test clinics documented that the hands were the primary site of involvement in 24.2% of patients undergoing patch testing (N=37,113).3
The hands are particularly at risk for occupation-related contact dermatitis and are the primary site of involvement in 80% of cases, followed by the wrists and forearms.4 Occupations at greatest risk include cleaning, construction, metalworking, hairdressing, health care, housework, and mechanics.5 Even prior to the COVID-19 pandemic, occupational hand dermatitis was common; in a survey of inpatient nurses, the prevalence was 55% (N=167).6 More recently, a study from China demonstrated a 74.5% prevalence of hand dermatitis in frontline health care workers involved in COVID-19 patient care.7
Etiology of Hand ICD
The pathogenesis of ICD is multifactorial; although traditionally thought to be nonimmunologic, evidence has shown that it involves skin barrier disruption, infiltration by immunocompetent cells, and induction of inflammatory signal molecules. The degree of irritancy is related to the concentration, contact duration, and properties of the irritant. Irritant reactions can be acute, such as those following a single chemical exposure that results in a localized dermatitis, or chronic, such as after repetitive cumulative exposure to mild irritants such as soaps.
Hand hygiene products (eg, soaps, hand sanitizers) can be irritants and have recently gained notoriety given their increased use to prevent COVID-19 transmission.8,9 Specific irritants include iodophors, antimicrobial soaps (chlorhexidine gluconate, chloroxylenol, triclosan), surfactants, and detergents. Wolfe et al10 showed that detergent-based hand cleansing products had the highest association with ICD, which was thought to be due to their propensity to remove protective lipids and reduce moisture content in the stratum corneum. Although hand sanitizers are better tolerated than detergents, they can still contribute to ICD by stripping precious lipids and disrupting the skin barrier.11 Compared to ethanol, isopropanol and N-propanol cause more disruption of the stratum corneum.12 In addition, N-propanol has the same irritant potential as the detergent sodium lauryl sulfate.13 Thus, ethanol-based sanitizers may be better tolerated. Disinfectant surface wipes may include the irritant N-alkyl dimethyl benzyl ammonium chloride. Conversely, hand and baby wipes are formulated specifically for the skin and may be less irritating.11
Occupational contributors to hand ICD include chemical exposures and frequent handwashing. Wet work, mechanical trauma, warm dry air, and prolonged use of occlusive gloves also are well-known irritants.4 Fine or coarse particles encountered in some occupations or hobbies (eg, sand, sawdust, metal filings, plastic) can cause mechanical irritation. Exposure to physical friction from repeated handling of metal components, paper, cardboard, fabric, or steering wheels also has been implicated in hand ICD. Other common categories of occupational irritants include hydrocarbons, such as oils and petroleum.5,14
In addition to environmental factors, atopic dermatitis is an important endogenous factor that increases the risk of ICD due to underlying deficiencies within the main lipid15 and structural16 barrier components. These deficiencies ultimately lead to a lower threshold for the activation of inflammation via water loss and a weakened barrier. Studies have demonstrated that atopic dermatitis increases the risk for developing hand ICD 2- to 4-fold.17
Etiology of Hand ACD
Allergic contact dermatitis is an immune-mediated type IV delayed hypersensitivity reaction. The North American Contact Dermatitis Group reported that the top 5 clinically relevant hand allergens were methylisothiazolinone (MI), nickel, formaldehyde, quaternium-15, and fragrance mix I.3 Similarly, the European Surveillance System on Contact Allergies demonstrated that the most common hand allergens were nickel, preservatives (quaternium-15 and formaldehyde), fragrances, and cobalt.18 In health care workers, rubber accelerators often are relevant in patients with hand ACD.5,19 Hand hygiene products are known to contain potential allergens; a recent study demonstrated that the top 5 allergens in common hand sanitizers were tocopherol, fragrance, propylene glycol, benzoates, and cetylstearyl alcohol,20 whereas the most common allergens in hand cleansers were fragrance, tocopherol, sodium benzoate, chloroxylenol, propylene glycol, and chlorhexidine gluconate.21
Preservatives
Preservatives can contribute to hand ACD. Methylisothiazolinone was the most commonly relevant allergen in a recent North American study of hand contact allergy,3 and a study of North American products confirmed its presence in dishwashing products (64%), shampoos (53%), household cleaners (47%), laundry softeners/additives (30%), soaps and cleansers (29%), and surface disinfectants (27%).22 In addition, in a study of 139 patients with refractory MI contact allergy, the hands were the most common site (69%) and had the highest rate of relapse.23 Because of the common presence of this preservative in liquid-based personal care products, patients with MI hand contact allergy need to be vigilant.
The same North American study highlighted formaldehyde and the formaldehyde releaser quaternium-15 as commonly relevant hand contact allergens.3 Formaldehyde is not commonly found in personal care products, but formaldehyde-releasing preservatives frequently are found in cosmetic products, topical medicaments, detergents, soaps, and metal working fluids. Another study noted that the most relevant contact allergen in health care workers was quaternium-15, possibly due to increased hand hygiene and exposure to medical products used for patient care.24,25
Metals
Nickel is used in metal objects and is found in many workplaces in the form of machines, office supplies, tools, electronics, uniforms, and jewelry. Occupationally related nickel ACD of the hands is most common in hairdressers/barbers/cosmetologists,26 which is not surprising, as hairdressing tools such as scissors and hair clips can release nickel.27,28
Although nickel contact allergy is more common than cobalt, these metals frequently co-react, with up to 25% of nickel-sensitive patients also having positive patch test reactions to cobalt.29 Because cobalt is contained in alloys, the occupations most at risk pertain to hard metal manufacturing. Furthermore, cobalt is used in dentistry for dental tools, fillings, crowns, bridges, and dentures.30 Cobalt also has been identified in leather, and leather gloves have been implicated in hand ACD.31
Fragrances
Fragrances can be added to products to infuse pleasing aromas or mask unpleasant chemical odors. In the North American study of hand ACD, fragrance mix I and balsam of Peru were the sixth and seventh most clinically relevant allergens, respectively.3 In another study, fragrances were found in 50% of waterless cleansers and 95% of rinse-off soaps and were the second most common allergens found in skin disinfectants.21 Fragrance is ubiquitous in personal care and cleansing products, which can make avoidance difficult.
Rubber Accelerators
Contact allergy to rubber additives in medical gloves is the most common cause of occupational hand ACD in health care workers.5,19 Importantly, it usually is rubber accelerators that act as allergens in hand ACD and not natural rubber latex. Rubber accelerators known to cause ACD include thiurams, carbamates, 1,3-diphenylguanidine (DPG), mixed dialkyl thioureas, and benzothiazoles.32 In the setting of hand ACD in North America, reactions to thiuram mix and carba mix were the most common.3 Notably, DPG is a component of carba mix and can be present in rubber gloves. It has been shown that 40.3% of DPG reactions are missed by testing with carba mix alone; therefore, DPG must be patch tested separately.33
Clinical Examination
It can be challenging to differentiate between hand ICD and ACD based on clinical appearance alone, and patch testing often is necessary for diagnosis. In the acute phase, both ICD and ACD can present as erythema, papules, vesicles, bullae, and/or crusting. In the chronic phase, scaling, lichenification, and/or fissures tend to prevail. Both acute and chronic ICD and ACD can be associated with pruritus and pain; however, ICD may be more likely associated with a burning or painful sensation, whereas ACD may be more associated with pruritus.
Other dermatoses may present as hand eruptions and should be kept in the differential diagnosis. Atopic dermatitis, psoriasis, dyshidrotic eczema, hyperkeratotic hand dermatitis, keratolysis exfoliativa, and palmoplantar pustulosis are other common causes of hand eruptions.5,34
Patch Testing for Hand ACD
Consider patch testing for hand dermatitis that is refractory to conservative treatment. Patients with new-onset hand dermatitis without history of atopy and patients with a new worsening of chronic hand dermatitis also may need patch testing.
In addition to a medically appropriate screening series, patients with hand dermatitis often need supplemental patch testing. In a series of 37,113 patients with hand ACD, just over 20% of patients had positive patch test reactions to at least 1 supplemental allergen not on the screening series.3 Supplemental series should be selected based on the patient’s history and exposures; for example, nail salon technicians may need supplemental testing with the nail acrylate series, and massage therapists may need additional testing with the fragrance or essential oil series. Some of the most common supplemental series used for evaluation of hand dermatitis are the rubber, cosmetic, textile and dyes, plant, fragrance, essential oil, oil and coolants, nail or printing acrylates, and hairdressing series. If there is a high suspicion of occupational contact with allergens, obtaining material safety data sheets from the patient’s employer can be helpful to identify relevant allergens for testing.5 The thin-layer rapid use epicutaneous (T.R.U.E.) test may miss several common and relevant hand allergens, including benzalkonium chloride, lanolin, and iodopropynyl butylcarbamate.3
Management
Management of hand ICD requires avoidance of irritants and proper hand hygiene practices.10,34 The hands should be washed using lukewarm water and mild fragrance-free soaps or cleansers,35 keeping in mind that hand sanitizers may be better tolerated due to their lower lipid-stripping effects. The moisturizers with the best efficacy are combinations of humectants (topical urea, glycerin) and occlusive emollients (dimethicone, petrolatum).11 When wet work is necessary, gloves should be worn; however, sweat and humidity from glove use can worsen ICD, and gloves should be changed regularly and applied only when hands are dry. Cotton gloves also can be worn underneath rubber gloves to prevent maceration from sweat.9
The mainstay of hand ACD management is allergen avoidance. The American Contact Dermatitis Society maintains the Contact Allergen Management Program (CAMP), a database that identifies products that do not contain patient allergens. The importance of reading ingredient labels of products should always be emphasized. For patients with rubber accelerator allergies, vinyl or accelerator-free gloves may be used. If the allergen is occupational, communication with the patient’s employer is necessary.5
When hand contact dermatitis does not improve with avoidance of irritants and allergens as well as gentle skin care, topical therapy, phototherapy, and in some cases systemic therapy may be required. High-potency topical corticosteroids or short courses of prednisone may be needed for quick relief. Topical calcineurin inhibitors (tacrolimus and pimecrolimus) and the phosphodiesterase 4 inhibitor crisaborole have shown some efficacy for hand dermatitis and can be used as steroid-sparing agents.36,37 Narrowband UVB and UVA have been used with moderate efficacy to treat resistant hand dermatitis.34,38 Oral immunosuppressant medications such as methotrexate, mycophenolate mofetil, azathioprine, and cyclosporine can be used for more severe cases.34,39,40 Furthermore, oral retinoids have been used for chronic severe hand dermatitis with notable efficacy.41
Our Final Interpretation
The 2 major types of hand contact dermatitis are ICD and ACD. Hand ICD is more common than ACD in both occupational and nonoccupational settings. The hands are the most common sites in the setting of occupational dermatitis; in North American patch test populations, the hands were the primary site of involvement in just under 25% of patients.3 Many hand hygiene products contain irritants and allergens. The lipid-stripping effects of soaps, detergents, and hand sanitizers in conjunction with increased frequency of handwashing can trigger ICD. The most common allergens implicated in hand ACD include MI, nickel, formaldehyde, quaternium-15, and fragrances. Patch testing is important for diagnosis, and supplemental series should be considered. Management includes avoidance of irritants and allergens; liberal use of moisturizers and barrier creams; and prescription topical therapy, phototherapy, or systemic therapy when indicated.
Hand dermatitis, also known as hand eczema, is common and affects a considerable number of individuals across all ages. The impact of hand dermatitis can be profound, as it can impair one’s ability to perform tasks at home and at work. As a result of the coronavirus disease 2019 (COVID-19) pandemic, there has been an increased focus on hand hygiene and subsequently hand dermatitis. There are many contributors to the severity of hand dermatitis, including genetic factors, immune reactions, and skin barrier disruption. In this column, we will explore irritant contact dermatitis (ICD) and allergic contact dermatitis (ACD) of the hands, including epidemiology, potential causes, clinical characteristics, diagnosis, and management.
Epidemiology
The prevalence of hand dermatitis in the general population is 3% to 4%, with a 1‐year prevalence of 10% and a lifetime prevalence of 15%.1 In a Swedish study of patients self-reporting hand eczema, contact dermatitis comprised 57% of the total cases (N=1385); ICD accounted for 35% of cases followed by ACD in 22%.2 A recent study on hand dermatitis in North American specialty patch test clinics documented that the hands were the primary site of involvement in 24.2% of patients undergoing patch testing (N=37,113).3
The hands are particularly at risk for occupation-related contact dermatitis and are the primary site of involvement in 80% of cases, followed by the wrists and forearms.4 Occupations at greatest risk include cleaning, construction, metalworking, hairdressing, health care, housework, and mechanics.5 Even prior to the COVID-19 pandemic, occupational hand dermatitis was common; in a survey of inpatient nurses, the prevalence was 55% (N=167).6 More recently, a study from China demonstrated a 74.5% prevalence of hand dermatitis in frontline health care workers involved in COVID-19 patient care.7
Etiology of Hand ICD
The pathogenesis of ICD is multifactorial; although traditionally thought to be nonimmunologic, evidence has shown that it involves skin barrier disruption, infiltration by immunocompetent cells, and induction of inflammatory signal molecules. The degree of irritancy is related to the concentration, contact duration, and properties of the irritant. Irritant reactions can be acute, such as those following a single chemical exposure that results in a localized dermatitis, or chronic, such as after repetitive cumulative exposure to mild irritants such as soaps.
Hand hygiene products (eg, soaps, hand sanitizers) can be irritants and have recently gained notoriety given their increased use to prevent COVID-19 transmission.8,9 Specific irritants include iodophors, antimicrobial soaps (chlorhexidine gluconate, chloroxylenol, triclosan), surfactants, and detergents. Wolfe et al10 showed that detergent-based hand cleansing products had the highest association with ICD, which was thought to be due to their propensity to remove protective lipids and reduce moisture content in the stratum corneum. Although hand sanitizers are better tolerated than detergents, they can still contribute to ICD by stripping precious lipids and disrupting the skin barrier.11 Compared to ethanol, isopropanol and N-propanol cause more disruption of the stratum corneum.12 In addition, N-propanol has the same irritant potential as the detergent sodium lauryl sulfate.13 Thus, ethanol-based sanitizers may be better tolerated. Disinfectant surface wipes may include the irritant N-alkyl dimethyl benzyl ammonium chloride. Conversely, hand and baby wipes are formulated specifically for the skin and may be less irritating.11
Occupational contributors to hand ICD include chemical exposures and frequent handwashing. Wet work, mechanical trauma, warm dry air, and prolonged use of occlusive gloves also are well-known irritants.4 Fine or coarse particles encountered in some occupations or hobbies (eg, sand, sawdust, metal filings, plastic) can cause mechanical irritation. Exposure to physical friction from repeated handling of metal components, paper, cardboard, fabric, or steering wheels also has been implicated in hand ICD. Other common categories of occupational irritants include hydrocarbons, such as oils and petroleum.5,14
In addition to environmental factors, atopic dermatitis is an important endogenous factor that increases the risk of ICD due to underlying deficiencies within the main lipid15 and structural16 barrier components. These deficiencies ultimately lead to a lower threshold for the activation of inflammation via water loss and a weakened barrier. Studies have demonstrated that atopic dermatitis increases the risk for developing hand ICD 2- to 4-fold.17
Etiology of Hand ACD
Allergic contact dermatitis is an immune-mediated type IV delayed hypersensitivity reaction. The North American Contact Dermatitis Group reported that the top 5 clinically relevant hand allergens were methylisothiazolinone (MI), nickel, formaldehyde, quaternium-15, and fragrance mix I.3 Similarly, the European Surveillance System on Contact Allergies demonstrated that the most common hand allergens were nickel, preservatives (quaternium-15 and formaldehyde), fragrances, and cobalt.18 In health care workers, rubber accelerators often are relevant in patients with hand ACD.5,19 Hand hygiene products are known to contain potential allergens; a recent study demonstrated that the top 5 allergens in common hand sanitizers were tocopherol, fragrance, propylene glycol, benzoates, and cetylstearyl alcohol,20 whereas the most common allergens in hand cleansers were fragrance, tocopherol, sodium benzoate, chloroxylenol, propylene glycol, and chlorhexidine gluconate.21
Preservatives
Preservatives can contribute to hand ACD. Methylisothiazolinone was the most commonly relevant allergen in a recent North American study of hand contact allergy,3 and a study of North American products confirmed its presence in dishwashing products (64%), shampoos (53%), household cleaners (47%), laundry softeners/additives (30%), soaps and cleansers (29%), and surface disinfectants (27%).22 In addition, in a study of 139 patients with refractory MI contact allergy, the hands were the most common site (69%) and had the highest rate of relapse.23 Because of the common presence of this preservative in liquid-based personal care products, patients with MI hand contact allergy need to be vigilant.
The same North American study highlighted formaldehyde and the formaldehyde releaser quaternium-15 as commonly relevant hand contact allergens.3 Formaldehyde is not commonly found in personal care products, but formaldehyde-releasing preservatives frequently are found in cosmetic products, topical medicaments, detergents, soaps, and metal working fluids. Another study noted that the most relevant contact allergen in health care workers was quaternium-15, possibly due to increased hand hygiene and exposure to medical products used for patient care.24,25
Metals
Nickel is used in metal objects and is found in many workplaces in the form of machines, office supplies, tools, electronics, uniforms, and jewelry. Occupationally related nickel ACD of the hands is most common in hairdressers/barbers/cosmetologists,26 which is not surprising, as hairdressing tools such as scissors and hair clips can release nickel.27,28
Although nickel contact allergy is more common than cobalt, these metals frequently co-react, with up to 25% of nickel-sensitive patients also having positive patch test reactions to cobalt.29 Because cobalt is contained in alloys, the occupations most at risk pertain to hard metal manufacturing. Furthermore, cobalt is used in dentistry for dental tools, fillings, crowns, bridges, and dentures.30 Cobalt also has been identified in leather, and leather gloves have been implicated in hand ACD.31
Fragrances
Fragrances can be added to products to infuse pleasing aromas or mask unpleasant chemical odors. In the North American study of hand ACD, fragrance mix I and balsam of Peru were the sixth and seventh most clinically relevant allergens, respectively.3 In another study, fragrances were found in 50% of waterless cleansers and 95% of rinse-off soaps and were the second most common allergens found in skin disinfectants.21 Fragrance is ubiquitous in personal care and cleansing products, which can make avoidance difficult.
Rubber Accelerators
Contact allergy to rubber additives in medical gloves is the most common cause of occupational hand ACD in health care workers.5,19 Importantly, it usually is rubber accelerators that act as allergens in hand ACD and not natural rubber latex. Rubber accelerators known to cause ACD include thiurams, carbamates, 1,3-diphenylguanidine (DPG), mixed dialkyl thioureas, and benzothiazoles.32 In the setting of hand ACD in North America, reactions to thiuram mix and carba mix were the most common.3 Notably, DPG is a component of carba mix and can be present in rubber gloves. It has been shown that 40.3% of DPG reactions are missed by testing with carba mix alone; therefore, DPG must be patch tested separately.33
Clinical Examination
It can be challenging to differentiate between hand ICD and ACD based on clinical appearance alone, and patch testing often is necessary for diagnosis. In the acute phase, both ICD and ACD can present as erythema, papules, vesicles, bullae, and/or crusting. In the chronic phase, scaling, lichenification, and/or fissures tend to prevail. Both acute and chronic ICD and ACD can be associated with pruritus and pain; however, ICD may be more likely associated with a burning or painful sensation, whereas ACD may be more associated with pruritus.
Other dermatoses may present as hand eruptions and should be kept in the differential diagnosis. Atopic dermatitis, psoriasis, dyshidrotic eczema, hyperkeratotic hand dermatitis, keratolysis exfoliativa, and palmoplantar pustulosis are other common causes of hand eruptions.5,34
Patch Testing for Hand ACD
Consider patch testing for hand dermatitis that is refractory to conservative treatment. Patients with new-onset hand dermatitis without history of atopy and patients with a new worsening of chronic hand dermatitis also may need patch testing.
In addition to a medically appropriate screening series, patients with hand dermatitis often need supplemental patch testing. In a series of 37,113 patients with hand ACD, just over 20% of patients had positive patch test reactions to at least 1 supplemental allergen not on the screening series.3 Supplemental series should be selected based on the patient’s history and exposures; for example, nail salon technicians may need supplemental testing with the nail acrylate series, and massage therapists may need additional testing with the fragrance or essential oil series. Some of the most common supplemental series used for evaluation of hand dermatitis are the rubber, cosmetic, textile and dyes, plant, fragrance, essential oil, oil and coolants, nail or printing acrylates, and hairdressing series. If there is a high suspicion of occupational contact with allergens, obtaining material safety data sheets from the patient’s employer can be helpful to identify relevant allergens for testing.5 The thin-layer rapid use epicutaneous (T.R.U.E.) test may miss several common and relevant hand allergens, including benzalkonium chloride, lanolin, and iodopropynyl butylcarbamate.3
Management
Management of hand ICD requires avoidance of irritants and proper hand hygiene practices.10,34 The hands should be washed using lukewarm water and mild fragrance-free soaps or cleansers,35 keeping in mind that hand sanitizers may be better tolerated due to their lower lipid-stripping effects. The moisturizers with the best efficacy are combinations of humectants (topical urea, glycerin) and occlusive emollients (dimethicone, petrolatum).11 When wet work is necessary, gloves should be worn; however, sweat and humidity from glove use can worsen ICD, and gloves should be changed regularly and applied only when hands are dry. Cotton gloves also can be worn underneath rubber gloves to prevent maceration from sweat.9
The mainstay of hand ACD management is allergen avoidance. The American Contact Dermatitis Society maintains the Contact Allergen Management Program (CAMP), a database that identifies products that do not contain patient allergens. The importance of reading ingredient labels of products should always be emphasized. For patients with rubber accelerator allergies, vinyl or accelerator-free gloves may be used. If the allergen is occupational, communication with the patient’s employer is necessary.5
When hand contact dermatitis does not improve with avoidance of irritants and allergens as well as gentle skin care, topical therapy, phototherapy, and in some cases systemic therapy may be required. High-potency topical corticosteroids or short courses of prednisone may be needed for quick relief. Topical calcineurin inhibitors (tacrolimus and pimecrolimus) and the phosphodiesterase 4 inhibitor crisaborole have shown some efficacy for hand dermatitis and can be used as steroid-sparing agents.36,37 Narrowband UVB and UVA have been used with moderate efficacy to treat resistant hand dermatitis.34,38 Oral immunosuppressant medications such as methotrexate, mycophenolate mofetil, azathioprine, and cyclosporine can be used for more severe cases.34,39,40 Furthermore, oral retinoids have been used for chronic severe hand dermatitis with notable efficacy.41
Our Final Interpretation
The 2 major types of hand contact dermatitis are ICD and ACD. Hand ICD is more common than ACD in both occupational and nonoccupational settings. The hands are the most common sites in the setting of occupational dermatitis; in North American patch test populations, the hands were the primary site of involvement in just under 25% of patients.3 Many hand hygiene products contain irritants and allergens. The lipid-stripping effects of soaps, detergents, and hand sanitizers in conjunction with increased frequency of handwashing can trigger ICD. The most common allergens implicated in hand ACD include MI, nickel, formaldehyde, quaternium-15, and fragrances. Patch testing is important for diagnosis, and supplemental series should be considered. Management includes avoidance of irritants and allergens; liberal use of moisturizers and barrier creams; and prescription topical therapy, phototherapy, or systemic therapy when indicated.
- Thyssen JP, Johansen JD, Linneberg A, et al. The epidemiology of hand eczema in the general population—prevalence and main findings. Contact Dermatitis. 2010;62:75-87.
- Meding B, Swanbeck G. Epidemiology of different types of hand eczema in an industrial city. Acta Derm Venereol. 1989;69:227-233.
- Silverberg JI, Warshaw EM, Atwater AR, et al. Hand dermatitis in adults referred for patch testing: analysis of North American Contact Dermatitis Group data, 2000–2016 [published online November 28, 2020]. J Am Acad Dermatol. https://doi.org/10.1016/j.jaad.2020.11.054
- Sasseville D. Occupational contact dermatitis. Allergy Asthma Clin Immunol. 2008;4:59.
- Lampel HP, Powell HB. Occupational and hand dermatitis: a practical approach. Clin Rev Allergy Immunol. 2019;56:60-71.
- Lampel HP, Patel N, Boyse K, et al. Prevalence of hand dermatitis in inpatient nurses at a United States hospital. Dermatitis. 2007;18:140-142.
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease 2019. J Am Acad Dermatol. 2020;82:1215-1216.
- Wei Tan S, Chiat Oh C. Contact dermatitis from hand hygiene practices in the COVID-19 pandemic. 2020;49:674-676.
- Beiu C, Mihai M, Popa L, et al. Frequent hand washing for COVID-19 prevention can cause hand dermatitis: management tips. Cureus. 2020;12:E7506.
- Wolfe MK, Wells E, Mitro B, et al. Seeking clearer recommendations for hand hygiene in communities facing ebola: a randomized trial investigating the impact of six handwashing methods on skin irritation and dermatitis. PLoS One. 2016;11:e0167378.
- Rundle CW, Presley CL, Militello M, et al. Hand hygiene during COVID-19: recommendations from the American Contact Dermatitis Society. J Am Acad Dermatol. 2020;83:1730-1737.
- Cartner T, Brand N, Tian K, et al. Effect of different alcohols on stratum corneum kallikrein 5 and phospholipase A(2) together with epidermal keratinocytes and skin irritation. Int J Cosmet Sci. 2017;39:188-196.
- Clemmensen A, Andersen F, Petersen TK, et al. The irritant potential of n-propanol (nonanoic acid vehicle) in cumulative skin irritation: a validation study of two different human in vivo test models. Ski Res Technol. 2008;14:277-286.
- McMullen E, Gawkrodger DJ. Physical friction is under-recognized as an irritant that can cause or contribute to contact dermatitis. Br J Dermatol. 2006;154:154-156.
- Macheleidt O, Kaiser HW, Sandhoff K. Deficiency of epidermal protein-bound omega-hydroxyceramides in atopic dermatitis. J Invest Dermatol. 2002;119:166-173.
- Visser MJ, Landeck L, Campbell LE, et al. Impact of atopic dermatitis and loss-of-function mutations in the filaggrin gene on the development of occupational irritant contact dermatitis. Br J Dermatol. 2013;168:326-332.
- Coenraads PJ, Diepgen TL. Risk for hand eczema in employees with past or present atopic dermatitis. Int Arch Occup Environ Health. 1998;71:7-13.
- Oosterhaven JAF, Uter W, Aberer W, et al. European Surveillance System on Contact Allergies (ESSCA): contact allergies in relation to body sites in patients with allergic contact dermatitis. Contact Dermatitis. 2019;80:263-272.
- Goodier MC, Ronkainen SD, Hylwa SA. Rubber accelerators in medical examination and surgical gloves. Dermatitis. 2018;29:66-76.
- Voller LM, Schlarbaum JP, Hylwa SA. Allergenic ingredients in health care hand sanitizers in the United States [published online February 21, 2020]. Dermatitis. doi:10.1097/der.0000000000000567
- Rodriguez-Homs LG, Atwater AR. Allergens in medical hand skin cleansers. Dermatitis. 2019;30:336-341.
- Scheman A, Severson D. American Contact Dermatitis Society Contact Allergy Management Program: an epidemiologic tool to quantify ingredient usage. Dermatitis. 2016;27:11-13.
- Bouschon P, Waton J, Pereira B, et al. Methylisothiazolinone allergic contact dermatitis: assessment of relapses in 139 patients after avoidance advice. Contact Dermatitis. 2019;80:304-310.
- Kadivar S, Belsito DV. Occupational dermatitis in health care workers evaluated for suspected allergic contact dermatitis. Dermatitis. 2015;26:177-183.
- Prodi A, Rui F, Fortina AB, et al. Healthcare workers and skin sensitization: north-eastern Italian database. Occup Med (Chic Ill). 2016;66:72-74.
- Warshaw EM, Schlarbaum JP, Dekoven JG, et al. Occupationally related nickel reactions: a retrospective analysis of the North American Contact Dermatitis Group data 1998-2016. Dermatitis. 2019;30:306-313.
- Thyssen JP, Milting K, Bregnhøj A, et al. Nickel allergy in patch-tested female hairdressers and assessment of nickel release from hairdressers’ scissors and crochet hooks. Contact Dermatitis. 2009;61:281-286.
- Symanzik C, John SM, Strunk M. Nickel release from metal tools in the German hairdressing trade—a current analysis. 2019;80:382-385.
- Rystedt I, Fischer T. Relationship between nickel and cobalt sensitization in hard metal workers. Contact Dermatitis. 1983;9:195-200.
- Kettelarij JAB, Lidén C, Axén E, et al. Cobalt, nickel and chromium release from dental tools and alloys. Contact Dermatitis. 2014;70:3-10.
- Thyssen JP, Johansen JD, Jellesen MS, et al. Consumer leather exposure: an unrecognized cause of cobalt sensitization. 2013;69:276-279.
- Hamnerius N, Svedman C, Bergendorff O, et al. Hand eczema and occupational contact allergies in healthcare workers with a focus on rubber additives. Contact Dermatitis. 2018;79:149-156.
- Warshaw EM, Gupta R, Dekoven JG, et al. Patch testing to diphenylguanidine by the North American Contact Dermatitis Group (2013-2016). Dermatitis. 2020;31:350-358.
- Perry AD, Trafeli JP. Hand dermatitis: review of etiology, diagnosis, and treatment. J Am Board Fam Med. 2009;22:325-330.
- Abtahi-Naeini B. Frequent handwashing amidst the COVID-19 outbreak: prevention of hand irritant contact dermatitis and other considerations. Health Sci Rep. 2020;3:E163.
- Schliemann S, Kelterer D, Bauer A, et al. Tacrolimus ointment in the treatment of occupationally induced chronic hand dermatitis. Contact Dermatitis. 2008;58:299-306. doi:10.1111/j.1600-0536.2007.01314.x
- Lynde CW, Bergman J, Fiorillo L, et al. Use of topical crisaborole for treating dermatitis in a variety of dermatology settings. Skin Therapy Lett. Published June 1, 2020. Accessed February 10, 2021. https://www.skintherapyletter.com/dermatology/topical-crisaborole-dermatitis-treatment/
- Rosén K, Mobacken H, Swanbeck G. Chronic eczematous dermatitis of the hands: a comparison of PUVA and UVB treatment. Acta Derm Venereol. 1987;67:48-54.
- Kwon GP, Tan CZ, Chen JK. Hand dermatitis: utilizing subtype classification to direct intervention. Curr Treat Options Allergy. 2016;3:322-332.
- Warshaw E, Lee G, Storrs FJ. Hand dermatitis: a review of clinical features, therapeutic options, and long-term outcomes. Am J Contact Dermat. 2003;14:119-137.
- Song M, Lee H-J, Lee W-K, et al. Acitretin as a therapeutic option for chronic hand eczema. Ann Dermatol. 2017;29:385-387.
- Thyssen JP, Johansen JD, Linneberg A, et al. The epidemiology of hand eczema in the general population—prevalence and main findings. Contact Dermatitis. 2010;62:75-87.
- Meding B, Swanbeck G. Epidemiology of different types of hand eczema in an industrial city. Acta Derm Venereol. 1989;69:227-233.
- Silverberg JI, Warshaw EM, Atwater AR, et al. Hand dermatitis in adults referred for patch testing: analysis of North American Contact Dermatitis Group data, 2000–2016 [published online November 28, 2020]. J Am Acad Dermatol. https://doi.org/10.1016/j.jaad.2020.11.054
- Sasseville D. Occupational contact dermatitis. Allergy Asthma Clin Immunol. 2008;4:59.
- Lampel HP, Powell HB. Occupational and hand dermatitis: a practical approach. Clin Rev Allergy Immunol. 2019;56:60-71.
- Lampel HP, Patel N, Boyse K, et al. Prevalence of hand dermatitis in inpatient nurses at a United States hospital. Dermatitis. 2007;18:140-142.
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease 2019. J Am Acad Dermatol. 2020;82:1215-1216.
- Wei Tan S, Chiat Oh C. Contact dermatitis from hand hygiene practices in the COVID-19 pandemic. 2020;49:674-676.
- Beiu C, Mihai M, Popa L, et al. Frequent hand washing for COVID-19 prevention can cause hand dermatitis: management tips. Cureus. 2020;12:E7506.
- Wolfe MK, Wells E, Mitro B, et al. Seeking clearer recommendations for hand hygiene in communities facing ebola: a randomized trial investigating the impact of six handwashing methods on skin irritation and dermatitis. PLoS One. 2016;11:e0167378.
- Rundle CW, Presley CL, Militello M, et al. Hand hygiene during COVID-19: recommendations from the American Contact Dermatitis Society. J Am Acad Dermatol. 2020;83:1730-1737.
- Cartner T, Brand N, Tian K, et al. Effect of different alcohols on stratum corneum kallikrein 5 and phospholipase A(2) together with epidermal keratinocytes and skin irritation. Int J Cosmet Sci. 2017;39:188-196.
- Clemmensen A, Andersen F, Petersen TK, et al. The irritant potential of n-propanol (nonanoic acid vehicle) in cumulative skin irritation: a validation study of two different human in vivo test models. Ski Res Technol. 2008;14:277-286.
- McMullen E, Gawkrodger DJ. Physical friction is under-recognized as an irritant that can cause or contribute to contact dermatitis. Br J Dermatol. 2006;154:154-156.
- Macheleidt O, Kaiser HW, Sandhoff K. Deficiency of epidermal protein-bound omega-hydroxyceramides in atopic dermatitis. J Invest Dermatol. 2002;119:166-173.
- Visser MJ, Landeck L, Campbell LE, et al. Impact of atopic dermatitis and loss-of-function mutations in the filaggrin gene on the development of occupational irritant contact dermatitis. Br J Dermatol. 2013;168:326-332.
- Coenraads PJ, Diepgen TL. Risk for hand eczema in employees with past or present atopic dermatitis. Int Arch Occup Environ Health. 1998;71:7-13.
- Oosterhaven JAF, Uter W, Aberer W, et al. European Surveillance System on Contact Allergies (ESSCA): contact allergies in relation to body sites in patients with allergic contact dermatitis. Contact Dermatitis. 2019;80:263-272.
- Goodier MC, Ronkainen SD, Hylwa SA. Rubber accelerators in medical examination and surgical gloves. Dermatitis. 2018;29:66-76.
- Voller LM, Schlarbaum JP, Hylwa SA. Allergenic ingredients in health care hand sanitizers in the United States [published online February 21, 2020]. Dermatitis. doi:10.1097/der.0000000000000567
- Rodriguez-Homs LG, Atwater AR. Allergens in medical hand skin cleansers. Dermatitis. 2019;30:336-341.
- Scheman A, Severson D. American Contact Dermatitis Society Contact Allergy Management Program: an epidemiologic tool to quantify ingredient usage. Dermatitis. 2016;27:11-13.
- Bouschon P, Waton J, Pereira B, et al. Methylisothiazolinone allergic contact dermatitis: assessment of relapses in 139 patients after avoidance advice. Contact Dermatitis. 2019;80:304-310.
- Kadivar S, Belsito DV. Occupational dermatitis in health care workers evaluated for suspected allergic contact dermatitis. Dermatitis. 2015;26:177-183.
- Prodi A, Rui F, Fortina AB, et al. Healthcare workers and skin sensitization: north-eastern Italian database. Occup Med (Chic Ill). 2016;66:72-74.
- Warshaw EM, Schlarbaum JP, Dekoven JG, et al. Occupationally related nickel reactions: a retrospective analysis of the North American Contact Dermatitis Group data 1998-2016. Dermatitis. 2019;30:306-313.
- Thyssen JP, Milting K, Bregnhøj A, et al. Nickel allergy in patch-tested female hairdressers and assessment of nickel release from hairdressers’ scissors and crochet hooks. Contact Dermatitis. 2009;61:281-286.
- Symanzik C, John SM, Strunk M. Nickel release from metal tools in the German hairdressing trade—a current analysis. 2019;80:382-385.
- Rystedt I, Fischer T. Relationship between nickel and cobalt sensitization in hard metal workers. Contact Dermatitis. 1983;9:195-200.
- Kettelarij JAB, Lidén C, Axén E, et al. Cobalt, nickel and chromium release from dental tools and alloys. Contact Dermatitis. 2014;70:3-10.
- Thyssen JP, Johansen JD, Jellesen MS, et al. Consumer leather exposure: an unrecognized cause of cobalt sensitization. 2013;69:276-279.
- Hamnerius N, Svedman C, Bergendorff O, et al. Hand eczema and occupational contact allergies in healthcare workers with a focus on rubber additives. Contact Dermatitis. 2018;79:149-156.
- Warshaw EM, Gupta R, Dekoven JG, et al. Patch testing to diphenylguanidine by the North American Contact Dermatitis Group (2013-2016). Dermatitis. 2020;31:350-358.
- Perry AD, Trafeli JP. Hand dermatitis: review of etiology, diagnosis, and treatment. J Am Board Fam Med. 2009;22:325-330.
- Abtahi-Naeini B. Frequent handwashing amidst the COVID-19 outbreak: prevention of hand irritant contact dermatitis and other considerations. Health Sci Rep. 2020;3:E163.
- Schliemann S, Kelterer D, Bauer A, et al. Tacrolimus ointment in the treatment of occupationally induced chronic hand dermatitis. Contact Dermatitis. 2008;58:299-306. doi:10.1111/j.1600-0536.2007.01314.x
- Lynde CW, Bergman J, Fiorillo L, et al. Use of topical crisaborole for treating dermatitis in a variety of dermatology settings. Skin Therapy Lett. Published June 1, 2020. Accessed February 10, 2021. https://www.skintherapyletter.com/dermatology/topical-crisaborole-dermatitis-treatment/
- Rosén K, Mobacken H, Swanbeck G. Chronic eczematous dermatitis of the hands: a comparison of PUVA and UVB treatment. Acta Derm Venereol. 1987;67:48-54.
- Kwon GP, Tan CZ, Chen JK. Hand dermatitis: utilizing subtype classification to direct intervention. Curr Treat Options Allergy. 2016;3:322-332.
- Warshaw E, Lee G, Storrs FJ. Hand dermatitis: a review of clinical features, therapeutic options, and long-term outcomes. Am J Contact Dermat. 2003;14:119-137.
- Song M, Lee H-J, Lee W-K, et al. Acitretin as a therapeutic option for chronic hand eczema. Ann Dermatol. 2017;29:385-387.
Practice Points
- For the hands, irritant contact dermatitis (ICD) is more common than allergic contact dermatitis in both occupational and nonoccupational settings. Because of overlapping clinical features, it can be difficult to differentiate between these conditions.
- Use of hand hygiene products, frequent handwashing, wet work, mechanical trauma, and occlusion can contribute to ICD of the hands.
- Common hand contact allergens include preservatives, metals, fragrances, and rubber accelerators.
- Patch testing often is necessary for diagnosis of hand dermatitis, and both screening and supplemental allergen series may be required.
Exercise-Induced Vasculitis in a Patient With Negative Ultrasound Venous Reflux Study: A Mimic of Stasis Dermatitis
To the Editor:
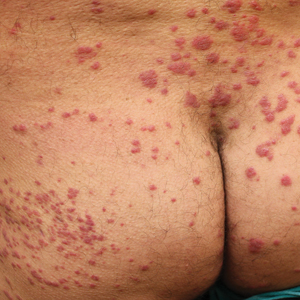
The transient and generic appearance of exercise-induced vasculitis (EIV) makes it a commonly misdiagnosed condition. The lesion often is only encountered through photographs brought by the patient or by taking a thorough history. The lack of findings on clinical inspection and the generic appearance of EIV may lead to misdiagnosis as stasis dermatitis due to its presentation as erythematous lesions on the medial lower legs.
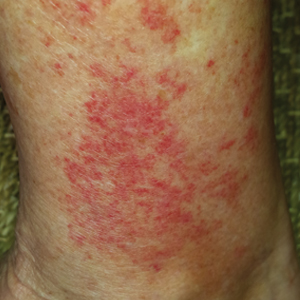
A 68-year-old woman with no notable medical history was referred to our clinic for suspected stasis dermatitis. At presentation, no lesions were identified on the legs, but she brought photographs of an erythematous urticarial eruption on the medial lower legs, extending from just above the sock line to the mid-calves (Figure). The eruptions had occurred over the last 16 years, typically presenting suddenly after playing tennis or an extended period of walking and spontaneously resolving in 4 days. The lesions were painless, restricted to the calves, and were not pruritic, though the initial presentation 16 years prior included pruritic pigmented patches on the anterior thighs. Because the condition spontaneously improved within days, no treatment was attempted. An ultrasound venous reflux study ruled out venous reflux and stasis dermatitis.
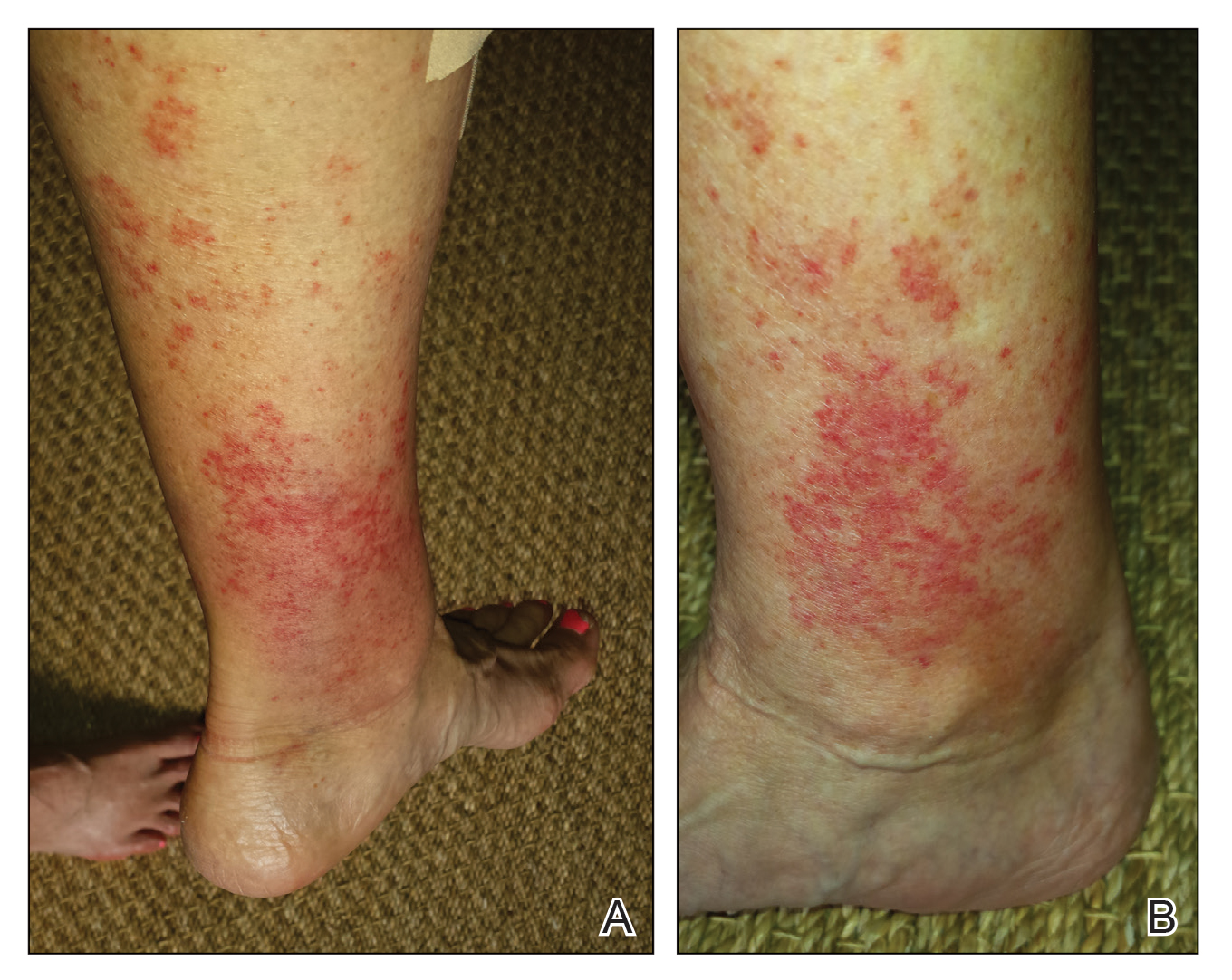
Our patient stated that her 64-year-old sister had reported the same presentation over the last 8 years. Her physical activity was limited strictly to walking, and the lesions occurred after walking for many hours during the day in the heat, involving the medial aspects of the lower legs extending from the ankles to the full length of the calves. Her eruption was warm but was not painful or pruritic. It resolved spontaneously after 5 days with no therapy.
Our patient was advised to wear compression stockings as a preventative measure, but she did not adhere to these recommendations, stating it was impractical to wear compression garments while playing tennis.
Exercise-induced vasculitis most commonly is seen in the medial aspects of the lower extremities as an erythematous urticarial eruption or pigmented purpuric plaque rapidly occurring after a period of exercise.1,2 Lesions often are symmetric and can be pruritic and painful with a lack of systemic symptoms.3 These generic clinical manifestations may lead to a misdiagnosis of stasis dermatitis. One case report included initial treatment of presumptive cellulitis.4 Important clinical findings include a sparing of skin compressed by tight clothing such as socks, a lack of systemic symptoms, rapid appearance after exercise, and spontaneous resolution within a few days. No correlation with chronic venous disease has been demonstrated, as EIV can occur in patients with or without chronic venous insufficiency.5 Duplex ultrasound evaluation showed no venous reflux in our patient.
The pathophysiology of EIV remains unknown, but the concept of exercise-altered microcirculation has been proposed. Heat generated from exercise is normally dissipated by thermoregulatory mechanisms such as cutaneous vasodilation and sweat.1,6 When exercise is extended, done concomitantly in the heat, or performed in legs with preexisting edema or substantial adipose tissue that limit heat attenuation, the thermoregulatory capacity is overloaded and heat-induced muscle fiber breakdown occurs.1,7 Atrophy impairs the skeletal muscle’s ability to pump the increased venous return demanded by exercise to the heart, leading to backflow of venous return and eventual venous stasis.1 Reduction of venous return together with cutaneous vasodilation is thought to induce erythrocyte extravasation.
Histologic examination demonstrates features of leukocytoclastic vasculitis with perivascular lymphocytic and neutrophilic infiltrates.2 Erythrocyte extravasation, IgM deposits, and identification of C3 also have been reported.8,9 The spontaneous resolution of EIV has led to treatment efforts being focused on preventative measures. Several cases have reported some degree of success in preventing EIV with compression therapy, venoactive drugs, systemic steroids, and application of topical steroids before exercise.3
The clinical morphology and lower leg location of EIV leads to a common misdiagnosis of stasis dermatitis. Clinical history of a transient nature is the mainstay in the diagnosis of EIV, and ultrasound venous reflux study may be required in some cases. Preventative measures are superior to treatment and mainly include compression therapy.
- Ramelet AA. Exercise-induced vasculitis. J Eur Acad Dermatol Venereol. 2006;20:423-427.
- Kelly RI, Opie J, Nixon R. Golfer’s vasculitis. Australas J Dermatol. 2005;46:11-14.
- Ramelet AA. Exercise-induced purpura. Dermatology. 2004;208:293-296.
- Cushman D, Rydberg L. A general rehabilitation inpatient with exercise-induced vasculitis. PM R. 2013;5:900-902.
- Veraart JC, Prins M, Hulsmans RF, et al. Influence of endurance exercise on the venous refilling time of the leg. Phlebology. 1994;23:120-123.
- Noakes T. Fluid replacement during marathon running. Clin J Sport Med. 2003;13:309-318.
- Armstrong RB. Muscle damage and endurance events. Sports Med. 1986;3:370-381.
- Prins M, Veraart JC, Vermeulen AH, et al. Leucocytoclastic vasculitis induced by prolonged exercise. Br J Dermatol. 1996;134:915-918.
- Sagdeo A, Gormley RH, Wanat KA, et al. Purpuric eruption on the feet of a healthy young woman. “flip-flop vasculitis” (exercise-induced vasculitis). JAMA Dermatol. 2013;149:751-756.
To the Editor:
The transient and generic appearance of exercise-induced vasculitis (EIV) makes it a commonly misdiagnosed condition. The lesion often is only encountered through photographs brought by the patient or by taking a thorough history. The lack of findings on clinical inspection and the generic appearance of EIV may lead to misdiagnosis as stasis dermatitis due to its presentation as erythematous lesions on the medial lower legs.
A 68-year-old woman with no notable medical history was referred to our clinic for suspected stasis dermatitis. At presentation, no lesions were identified on the legs, but she brought photographs of an erythematous urticarial eruption on the medial lower legs, extending from just above the sock line to the mid-calves (Figure). The eruptions had occurred over the last 16 years, typically presenting suddenly after playing tennis or an extended period of walking and spontaneously resolving in 4 days. The lesions were painless, restricted to the calves, and were not pruritic, though the initial presentation 16 years prior included pruritic pigmented patches on the anterior thighs. Because the condition spontaneously improved within days, no treatment was attempted. An ultrasound venous reflux study ruled out venous reflux and stasis dermatitis.

Our patient stated that her 64-year-old sister had reported the same presentation over the last 8 years. Her physical activity was limited strictly to walking, and the lesions occurred after walking for many hours during the day in the heat, involving the medial aspects of the lower legs extending from the ankles to the full length of the calves. Her eruption was warm but was not painful or pruritic. It resolved spontaneously after 5 days with no therapy.
Our patient was advised to wear compression stockings as a preventative measure, but she did not adhere to these recommendations, stating it was impractical to wear compression garments while playing tennis.
Exercise-induced vasculitis most commonly is seen in the medial aspects of the lower extremities as an erythematous urticarial eruption or pigmented purpuric plaque rapidly occurring after a period of exercise.1,2 Lesions often are symmetric and can be pruritic and painful with a lack of systemic symptoms.3 These generic clinical manifestations may lead to a misdiagnosis of stasis dermatitis. One case report included initial treatment of presumptive cellulitis.4 Important clinical findings include a sparing of skin compressed by tight clothing such as socks, a lack of systemic symptoms, rapid appearance after exercise, and spontaneous resolution within a few days. No correlation with chronic venous disease has been demonstrated, as EIV can occur in patients with or without chronic venous insufficiency.5 Duplex ultrasound evaluation showed no venous reflux in our patient.
The pathophysiology of EIV remains unknown, but the concept of exercise-altered microcirculation has been proposed. Heat generated from exercise is normally dissipated by thermoregulatory mechanisms such as cutaneous vasodilation and sweat.1,6 When exercise is extended, done concomitantly in the heat, or performed in legs with preexisting edema or substantial adipose tissue that limit heat attenuation, the thermoregulatory capacity is overloaded and heat-induced muscle fiber breakdown occurs.1,7 Atrophy impairs the skeletal muscle’s ability to pump the increased venous return demanded by exercise to the heart, leading to backflow of venous return and eventual venous stasis.1 Reduction of venous return together with cutaneous vasodilation is thought to induce erythrocyte extravasation.
Histologic examination demonstrates features of leukocytoclastic vasculitis with perivascular lymphocytic and neutrophilic infiltrates.2 Erythrocyte extravasation, IgM deposits, and identification of C3 also have been reported.8,9 The spontaneous resolution of EIV has led to treatment efforts being focused on preventative measures. Several cases have reported some degree of success in preventing EIV with compression therapy, venoactive drugs, systemic steroids, and application of topical steroids before exercise.3
The clinical morphology and lower leg location of EIV leads to a common misdiagnosis of stasis dermatitis. Clinical history of a transient nature is the mainstay in the diagnosis of EIV, and ultrasound venous reflux study may be required in some cases. Preventative measures are superior to treatment and mainly include compression therapy.
To the Editor:
The transient and generic appearance of exercise-induced vasculitis (EIV) makes it a commonly misdiagnosed condition. The lesion often is only encountered through photographs brought by the patient or by taking a thorough history. The lack of findings on clinical inspection and the generic appearance of EIV may lead to misdiagnosis as stasis dermatitis due to its presentation as erythematous lesions on the medial lower legs.
A 68-year-old woman with no notable medical history was referred to our clinic for suspected stasis dermatitis. At presentation, no lesions were identified on the legs, but she brought photographs of an erythematous urticarial eruption on the medial lower legs, extending from just above the sock line to the mid-calves (Figure). The eruptions had occurred over the last 16 years, typically presenting suddenly after playing tennis or an extended period of walking and spontaneously resolving in 4 days. The lesions were painless, restricted to the calves, and were not pruritic, though the initial presentation 16 years prior included pruritic pigmented patches on the anterior thighs. Because the condition spontaneously improved within days, no treatment was attempted. An ultrasound venous reflux study ruled out venous reflux and stasis dermatitis.

Our patient stated that her 64-year-old sister had reported the same presentation over the last 8 years. Her physical activity was limited strictly to walking, and the lesions occurred after walking for many hours during the day in the heat, involving the medial aspects of the lower legs extending from the ankles to the full length of the calves. Her eruption was warm but was not painful or pruritic. It resolved spontaneously after 5 days with no therapy.
Our patient was advised to wear compression stockings as a preventative measure, but she did not adhere to these recommendations, stating it was impractical to wear compression garments while playing tennis.
Exercise-induced vasculitis most commonly is seen in the medial aspects of the lower extremities as an erythematous urticarial eruption or pigmented purpuric plaque rapidly occurring after a period of exercise.1,2 Lesions often are symmetric and can be pruritic and painful with a lack of systemic symptoms.3 These generic clinical manifestations may lead to a misdiagnosis of stasis dermatitis. One case report included initial treatment of presumptive cellulitis.4 Important clinical findings include a sparing of skin compressed by tight clothing such as socks, a lack of systemic symptoms, rapid appearance after exercise, and spontaneous resolution within a few days. No correlation with chronic venous disease has been demonstrated, as EIV can occur in patients with or without chronic venous insufficiency.5 Duplex ultrasound evaluation showed no venous reflux in our patient.
The pathophysiology of EIV remains unknown, but the concept of exercise-altered microcirculation has been proposed. Heat generated from exercise is normally dissipated by thermoregulatory mechanisms such as cutaneous vasodilation and sweat.1,6 When exercise is extended, done concomitantly in the heat, or performed in legs with preexisting edema or substantial adipose tissue that limit heat attenuation, the thermoregulatory capacity is overloaded and heat-induced muscle fiber breakdown occurs.1,7 Atrophy impairs the skeletal muscle’s ability to pump the increased venous return demanded by exercise to the heart, leading to backflow of venous return and eventual venous stasis.1 Reduction of venous return together with cutaneous vasodilation is thought to induce erythrocyte extravasation.
Histologic examination demonstrates features of leukocytoclastic vasculitis with perivascular lymphocytic and neutrophilic infiltrates.2 Erythrocyte extravasation, IgM deposits, and identification of C3 also have been reported.8,9 The spontaneous resolution of EIV has led to treatment efforts being focused on preventative measures. Several cases have reported some degree of success in preventing EIV with compression therapy, venoactive drugs, systemic steroids, and application of topical steroids before exercise.3
The clinical morphology and lower leg location of EIV leads to a common misdiagnosis of stasis dermatitis. Clinical history of a transient nature is the mainstay in the diagnosis of EIV, and ultrasound venous reflux study may be required in some cases. Preventative measures are superior to treatment and mainly include compression therapy.
- Ramelet AA. Exercise-induced vasculitis. J Eur Acad Dermatol Venereol. 2006;20:423-427.
- Kelly RI, Opie J, Nixon R. Golfer’s vasculitis. Australas J Dermatol. 2005;46:11-14.
- Ramelet AA. Exercise-induced purpura. Dermatology. 2004;208:293-296.
- Cushman D, Rydberg L. A general rehabilitation inpatient with exercise-induced vasculitis. PM R. 2013;5:900-902.
- Veraart JC, Prins M, Hulsmans RF, et al. Influence of endurance exercise on the venous refilling time of the leg. Phlebology. 1994;23:120-123.
- Noakes T. Fluid replacement during marathon running. Clin J Sport Med. 2003;13:309-318.
- Armstrong RB. Muscle damage and endurance events. Sports Med. 1986;3:370-381.
- Prins M, Veraart JC, Vermeulen AH, et al. Leucocytoclastic vasculitis induced by prolonged exercise. Br J Dermatol. 1996;134:915-918.
- Sagdeo A, Gormley RH, Wanat KA, et al. Purpuric eruption on the feet of a healthy young woman. “flip-flop vasculitis” (exercise-induced vasculitis). JAMA Dermatol. 2013;149:751-756.
- Ramelet AA. Exercise-induced vasculitis. J Eur Acad Dermatol Venereol. 2006;20:423-427.
- Kelly RI, Opie J, Nixon R. Golfer’s vasculitis. Australas J Dermatol. 2005;46:11-14.
- Ramelet AA. Exercise-induced purpura. Dermatology. 2004;208:293-296.
- Cushman D, Rydberg L. A general rehabilitation inpatient with exercise-induced vasculitis. PM R. 2013;5:900-902.
- Veraart JC, Prins M, Hulsmans RF, et al. Influence of endurance exercise on the venous refilling time of the leg. Phlebology. 1994;23:120-123.
- Noakes T. Fluid replacement during marathon running. Clin J Sport Med. 2003;13:309-318.
- Armstrong RB. Muscle damage and endurance events. Sports Med. 1986;3:370-381.
- Prins M, Veraart JC, Vermeulen AH, et al. Leucocytoclastic vasculitis induced by prolonged exercise. Br J Dermatol. 1996;134:915-918.
- Sagdeo A, Gormley RH, Wanat KA, et al. Purpuric eruption on the feet of a healthy young woman. “flip-flop vasculitis” (exercise-induced vasculitis). JAMA Dermatol. 2013;149:751-756.
Practice Points
- Clinical history of a transient nature is the mainstay in the diagnosis of exercise-induced vasculitis.
- Exercise-induced vasculitis largely is documented in photographs or by history and may be misdiagnosed as stasis dermatitis due to its clinical morphology and lower leg location.
- Dermatologists should be aware of this disorder and consider performing further workup to rule out stasis dermatitis and diagnose this mimic.
- Preventative measures are superior to treatment and mainly include compression therapy.
Pink Patches With a Hyperpigmented Rim
The Diagnosis: Phytophotodermatitis
A more detailed patient history revealed that there was beer with limes on the boat, but the partygoers neglected to bring a knife. The patient volunteered to tear the limes apart with his bare hands. Because he was clad only in swim trunks, lime juice splattered over various regions of his body.
Phytophotodermatitis is a phototoxic blistering rash that follows topical exposure to plant-derived furocoumarins and sunlight. (Figure) Furocoumarins are photosensitizing substances produced by certain plants, possibly as a defense mechanism against predators.1 They cause a nonimmunologic phototoxic reaction when deposited on the skin and exposed to UVA radiation. Exposure to limes is the most common precipitant of phytophotodermatitis, but other potential culprits include lemons, grapefruit, figs, carrots, parsnips, celery, and dill.2
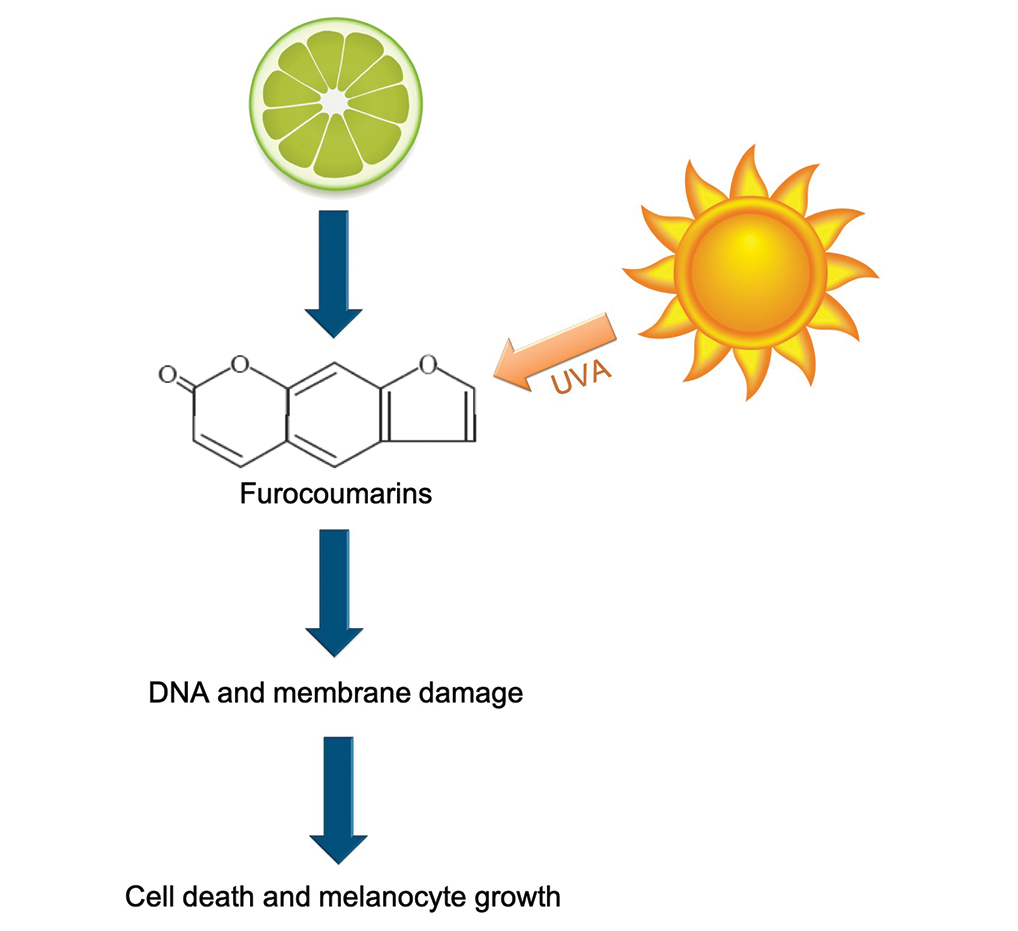
Lesions associated with phytophotodermatitis classically present as painful erythematous patches and bullae in regions of furocoumarin exposure. Affected areas are well demarcated and irregularly shaped and heal with a characteristic hyperpigmented rim. They often have a downward streak pattern from the dripping juice.3 If the furocoumarins are transferred by touch, lesions can appear in the shape of handprints, which may raise alarms for physical abuse in children.4
Photochemical reactions caused by activated furocoumarins cross-link nuclear DNA and damage cell membranes. These changes lead to cellular death resulting in edema and destruction of the epidermis. Other effects include an increase in keratin and thickening of the stratum corneum. The hyperpigmentation is a result of increased concentration of melanosomes and stimulation of melanocytes by activated furocoumarins.5
Management of phytophotodermatitis depends on the severity of skin injury. Mild cases may not require any treatment, whereas the most severe ones require admission to a burn unit for wound care. Anti-inflammatory medications are the mainstay of therapy. Our patient was prescribed desonide cream 0.05% for application to the affected areas. Sunscreen should be applied to prevent worsening of hyperpigmentation, which may take months to years to fade naturally. If hyperpigmentation is cosmetically troubling to the patient, bleaching agents such as hydroquinone and retinoids or Nd:YAG laser can be used to accelerate the resolution of pigment.5
Phototoxicity differs from less common photoallergic reactions caused by preformed antibodies or a delayed cell-mediated response to a trigger. The classic presentation of photoallergy is apruritic, inflammatory, bullous eruption in a sensitized individual.6 Allergic contact dermatitis more commonly is associated with pruritus than pain, and it presents as a papulovesicular eruption that evolves into lichenified plaques.7 Porphyria cutanea tarda would likely be accompanied by other cutaneous features such as hypertrichosis and sclerodermoid plaques with dystrophic calcification, in addition to wine-colored urine-containing porphyrins.8 Bullous fixed drug eruptions develop within 48 hours of exposure to a causative agent. The patient typically would experience pruritus and burning at the site of clearly demarcated erythematous lesions that healed with hyperpigmentation.9 Lesions of bullous lupus erythematosus may appear in areas without sun exposure, and they would be more likely to leave behind hypopigmentation rather than hyperpigmentation.10
- Pathak MA. Phytophotodermatitis. Clin Dermatol. 1986;4:102-121.
- Egan CL, Sterling G. Phytophotodermatitis: a visit to Margaritaville. Cutis. 1993;51:41-42.
- Hankinson A, Lloyd B, Alweis R. Lime-induced phytophotodermatitis [published online ahead of print September 29, 2014]. J Community Hosp Intern Med Perspect. doi:10.3402/jchimp.v4.25090
- Fitzpatrick JK, Kohlwes J. Lime-induced phytophotodermatitis. J Gen Intern Med. 2018;33:975.
- Weber IC, Davis CP, Greeson DM. Phytophotodermatitis: the other "lime" disease. J Emerg Med. 1999;17:235-237.
- Monteiro AF, Rato M, Martins C. Drug-induced photosensitivity: photoallergic and phototoxic reactions. Clin Dermatol. 2016;34:571-581.
- Tan CH, Rasool S, Johnston GA. Contact dermatitis: allergic and irritant. Clin Dermatol. 2014;32:116-124.
- Dawe R. An overview of the cutaneous porphyrias. F1000Res. 2017;6:1906.
- Bandino JP, Wohltmann WE, Bray DW, et al. Naproxen-induced generalized bullous fixed drug eruption. Dermatol Online J. 2009;15:4.
- Contestable JJ, Edhegard KD, Meyerle JH. Bullous systemic lupus erythematosus: a review and update to diagnosis and treatment. Am J Clin Dermatol. 2014;15:517-524.
The Diagnosis: Phytophotodermatitis
A more detailed patient history revealed that there was beer with limes on the boat, but the partygoers neglected to bring a knife. The patient volunteered to tear the limes apart with his bare hands. Because he was clad only in swim trunks, lime juice splattered over various regions of his body.
Phytophotodermatitis is a phototoxic blistering rash that follows topical exposure to plant-derived furocoumarins and sunlight. (Figure) Furocoumarins are photosensitizing substances produced by certain plants, possibly as a defense mechanism against predators.1 They cause a nonimmunologic phototoxic reaction when deposited on the skin and exposed to UVA radiation. Exposure to limes is the most common precipitant of phytophotodermatitis, but other potential culprits include lemons, grapefruit, figs, carrots, parsnips, celery, and dill.2

Lesions associated with phytophotodermatitis classically present as painful erythematous patches and bullae in regions of furocoumarin exposure. Affected areas are well demarcated and irregularly shaped and heal with a characteristic hyperpigmented rim. They often have a downward streak pattern from the dripping juice.3 If the furocoumarins are transferred by touch, lesions can appear in the shape of handprints, which may raise alarms for physical abuse in children.4
Photochemical reactions caused by activated furocoumarins cross-link nuclear DNA and damage cell membranes. These changes lead to cellular death resulting in edema and destruction of the epidermis. Other effects include an increase in keratin and thickening of the stratum corneum. The hyperpigmentation is a result of increased concentration of melanosomes and stimulation of melanocytes by activated furocoumarins.5
Management of phytophotodermatitis depends on the severity of skin injury. Mild cases may not require any treatment, whereas the most severe ones require admission to a burn unit for wound care. Anti-inflammatory medications are the mainstay of therapy. Our patient was prescribed desonide cream 0.05% for application to the affected areas. Sunscreen should be applied to prevent worsening of hyperpigmentation, which may take months to years to fade naturally. If hyperpigmentation is cosmetically troubling to the patient, bleaching agents such as hydroquinone and retinoids or Nd:YAG laser can be used to accelerate the resolution of pigment.5
Phototoxicity differs from less common photoallergic reactions caused by preformed antibodies or a delayed cell-mediated response to a trigger. The classic presentation of photoallergy is apruritic, inflammatory, bullous eruption in a sensitized individual.6 Allergic contact dermatitis more commonly is associated with pruritus than pain, and it presents as a papulovesicular eruption that evolves into lichenified plaques.7 Porphyria cutanea tarda would likely be accompanied by other cutaneous features such as hypertrichosis and sclerodermoid plaques with dystrophic calcification, in addition to wine-colored urine-containing porphyrins.8 Bullous fixed drug eruptions develop within 48 hours of exposure to a causative agent. The patient typically would experience pruritus and burning at the site of clearly demarcated erythematous lesions that healed with hyperpigmentation.9 Lesions of bullous lupus erythematosus may appear in areas without sun exposure, and they would be more likely to leave behind hypopigmentation rather than hyperpigmentation.10
The Diagnosis: Phytophotodermatitis
A more detailed patient history revealed that there was beer with limes on the boat, but the partygoers neglected to bring a knife. The patient volunteered to tear the limes apart with his bare hands. Because he was clad only in swim trunks, lime juice splattered over various regions of his body.
Phytophotodermatitis is a phototoxic blistering rash that follows topical exposure to plant-derived furocoumarins and sunlight. (Figure) Furocoumarins are photosensitizing substances produced by certain plants, possibly as a defense mechanism against predators.1 They cause a nonimmunologic phototoxic reaction when deposited on the skin and exposed to UVA radiation. Exposure to limes is the most common precipitant of phytophotodermatitis, but other potential culprits include lemons, grapefruit, figs, carrots, parsnips, celery, and dill.2

Lesions associated with phytophotodermatitis classically present as painful erythematous patches and bullae in regions of furocoumarin exposure. Affected areas are well demarcated and irregularly shaped and heal with a characteristic hyperpigmented rim. They often have a downward streak pattern from the dripping juice.3 If the furocoumarins are transferred by touch, lesions can appear in the shape of handprints, which may raise alarms for physical abuse in children.4
Photochemical reactions caused by activated furocoumarins cross-link nuclear DNA and damage cell membranes. These changes lead to cellular death resulting in edema and destruction of the epidermis. Other effects include an increase in keratin and thickening of the stratum corneum. The hyperpigmentation is a result of increased concentration of melanosomes and stimulation of melanocytes by activated furocoumarins.5
Management of phytophotodermatitis depends on the severity of skin injury. Mild cases may not require any treatment, whereas the most severe ones require admission to a burn unit for wound care. Anti-inflammatory medications are the mainstay of therapy. Our patient was prescribed desonide cream 0.05% for application to the affected areas. Sunscreen should be applied to prevent worsening of hyperpigmentation, which may take months to years to fade naturally. If hyperpigmentation is cosmetically troubling to the patient, bleaching agents such as hydroquinone and retinoids or Nd:YAG laser can be used to accelerate the resolution of pigment.5
Phototoxicity differs from less common photoallergic reactions caused by preformed antibodies or a delayed cell-mediated response to a trigger. The classic presentation of photoallergy is apruritic, inflammatory, bullous eruption in a sensitized individual.6 Allergic contact dermatitis more commonly is associated with pruritus than pain, and it presents as a papulovesicular eruption that evolves into lichenified plaques.7 Porphyria cutanea tarda would likely be accompanied by other cutaneous features such as hypertrichosis and sclerodermoid plaques with dystrophic calcification, in addition to wine-colored urine-containing porphyrins.8 Bullous fixed drug eruptions develop within 48 hours of exposure to a causative agent. The patient typically would experience pruritus and burning at the site of clearly demarcated erythematous lesions that healed with hyperpigmentation.9 Lesions of bullous lupus erythematosus may appear in areas without sun exposure, and they would be more likely to leave behind hypopigmentation rather than hyperpigmentation.10
- Pathak MA. Phytophotodermatitis. Clin Dermatol. 1986;4:102-121.
- Egan CL, Sterling G. Phytophotodermatitis: a visit to Margaritaville. Cutis. 1993;51:41-42.
- Hankinson A, Lloyd B, Alweis R. Lime-induced phytophotodermatitis [published online ahead of print September 29, 2014]. J Community Hosp Intern Med Perspect. doi:10.3402/jchimp.v4.25090
- Fitzpatrick JK, Kohlwes J. Lime-induced phytophotodermatitis. J Gen Intern Med. 2018;33:975.
- Weber IC, Davis CP, Greeson DM. Phytophotodermatitis: the other "lime" disease. J Emerg Med. 1999;17:235-237.
- Monteiro AF, Rato M, Martins C. Drug-induced photosensitivity: photoallergic and phototoxic reactions. Clin Dermatol. 2016;34:571-581.
- Tan CH, Rasool S, Johnston GA. Contact dermatitis: allergic and irritant. Clin Dermatol. 2014;32:116-124.
- Dawe R. An overview of the cutaneous porphyrias. F1000Res. 2017;6:1906.
- Bandino JP, Wohltmann WE, Bray DW, et al. Naproxen-induced generalized bullous fixed drug eruption. Dermatol Online J. 2009;15:4.
- Contestable JJ, Edhegard KD, Meyerle JH. Bullous systemic lupus erythematosus: a review and update to diagnosis and treatment. Am J Clin Dermatol. 2014;15:517-524.
- Pathak MA. Phytophotodermatitis. Clin Dermatol. 1986;4:102-121.
- Egan CL, Sterling G. Phytophotodermatitis: a visit to Margaritaville. Cutis. 1993;51:41-42.
- Hankinson A, Lloyd B, Alweis R. Lime-induced phytophotodermatitis [published online ahead of print September 29, 2014]. J Community Hosp Intern Med Perspect. doi:10.3402/jchimp.v4.25090
- Fitzpatrick JK, Kohlwes J. Lime-induced phytophotodermatitis. J Gen Intern Med. 2018;33:975.
- Weber IC, Davis CP, Greeson DM. Phytophotodermatitis: the other "lime" disease. J Emerg Med. 1999;17:235-237.
- Monteiro AF, Rato M, Martins C. Drug-induced photosensitivity: photoallergic and phototoxic reactions. Clin Dermatol. 2016;34:571-581.
- Tan CH, Rasool S, Johnston GA. Contact dermatitis: allergic and irritant. Clin Dermatol. 2014;32:116-124.
- Dawe R. An overview of the cutaneous porphyrias. F1000Res. 2017;6:1906.
- Bandino JP, Wohltmann WE, Bray DW, et al. Naproxen-induced generalized bullous fixed drug eruption. Dermatol Online J. 2009;15:4.
- Contestable JJ, Edhegard KD, Meyerle JH. Bullous systemic lupus erythematosus: a review and update to diagnosis and treatment. Am J Clin Dermatol. 2014;15:517-524.
A 25-year-old man presented with a rash on the right hand, chest, abdomen, right thigh, and ankles of 2 weeks’ duration. He reported that the eruption began with bullous lesions following a boat trip. The bullae ruptured over the next several days, and the lesions evolved to the current appearance. Although the patient had experienced pain at the site of active blisters, he denied any current pain, itching, or bleeding from the lesions. No other medical comorbidities were present.
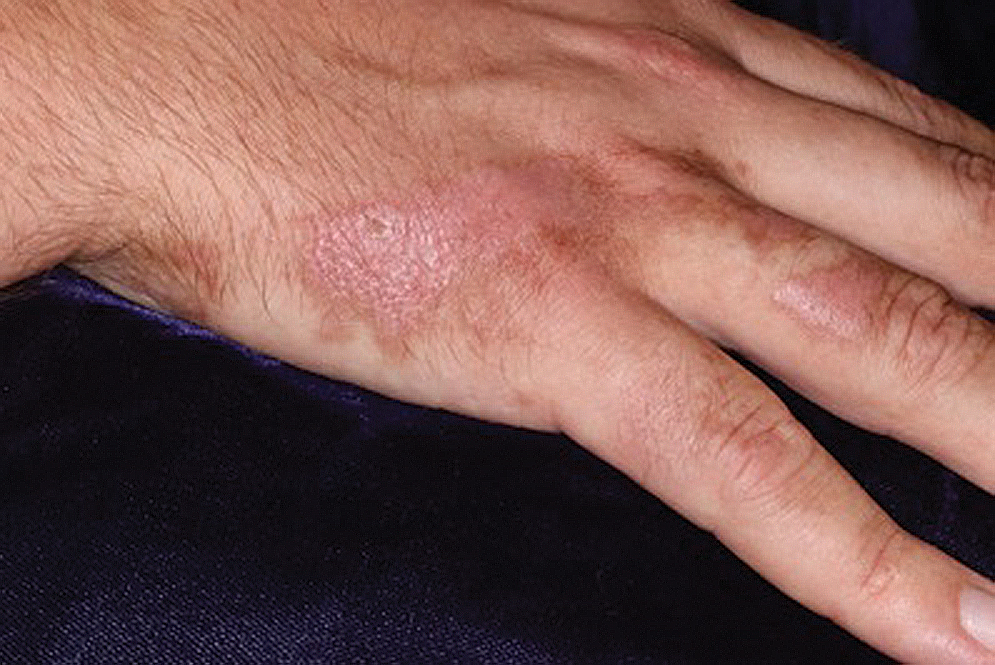
What’s Eating You? Black Butterfly (Hylesia nigricans)
The order Lepidoptera (phylum Arthropoda, class Hexapoda) is comprised of moths and butterflies.1 Lepidopterism refers to a range of adverse medical conditions resulting from contact with these insects, typically during the caterpillar (larval) stage. It involves multiple pathologic mechanisms, including direct toxicity of venom and mechanical irritant effects.2 Erucism has been defined as any reaction caused by contact with caterpillars or any reaction limited to the skin caused by contact with caterpillars, butterflies, or moths. Lepidopterism can mean any reaction to caterpillars or moths, referring only to reactions from contact with scales or hairs from adult moths or butterflies, or referring only to cases with systemic signs and symptoms (eg, rhinoconjunctival or asthmatic symptoms, angioedema and anaphylaxis, hemorrhagic diathesis) with or without cutaneous findings, resulting from contact with any lepidopteran source.1 Strictly speaking, erucism should refer to any reaction from caterpillars and lepidopterism to reactions from moths or butterflies. Because reactions to both larval and adult lepidoptera can cause a variety of either cutaneous and/or systemic symptoms, classifying reactions into erucism or lepidopterism is only of academic interest.1
We report a documented case of lepidopterism in a patient with acute cutaneous lesions following exposure to an adult-stage black butterfly (Hylesia nigricans).
Case Report
A 21-year-old oil well worker presented with pruritic skin lesions on the right arm and flank of 3 hours’ duration. He reported that a black butterfly had perched on his arm while he was working and left a considerable number of small yellowish hairs on the skin, after which an intense pruritus and skin lesions began to develop. He denied other associated symptoms. Physical examination revealed numerous 1- to 2-mm, nonconfluent, erythematous and edematous papules on the right forearm, arm (Figure 1A), and flank. Some abrasions of the skin due to scratching and crusting were noted (Figure 1B). A skin biopsy from the right arm showed a superficial perivascular dermatitis with a mixed infiltrate of polymorphonuclear predominance with eosinophils (Figure 2A). Importantly, a structure resembling an urticating spicule was identified in the stratum corneum (Figure 2B); spicules are located on the abdomen of arthropods and are associated with an inflammatory response in human skin.
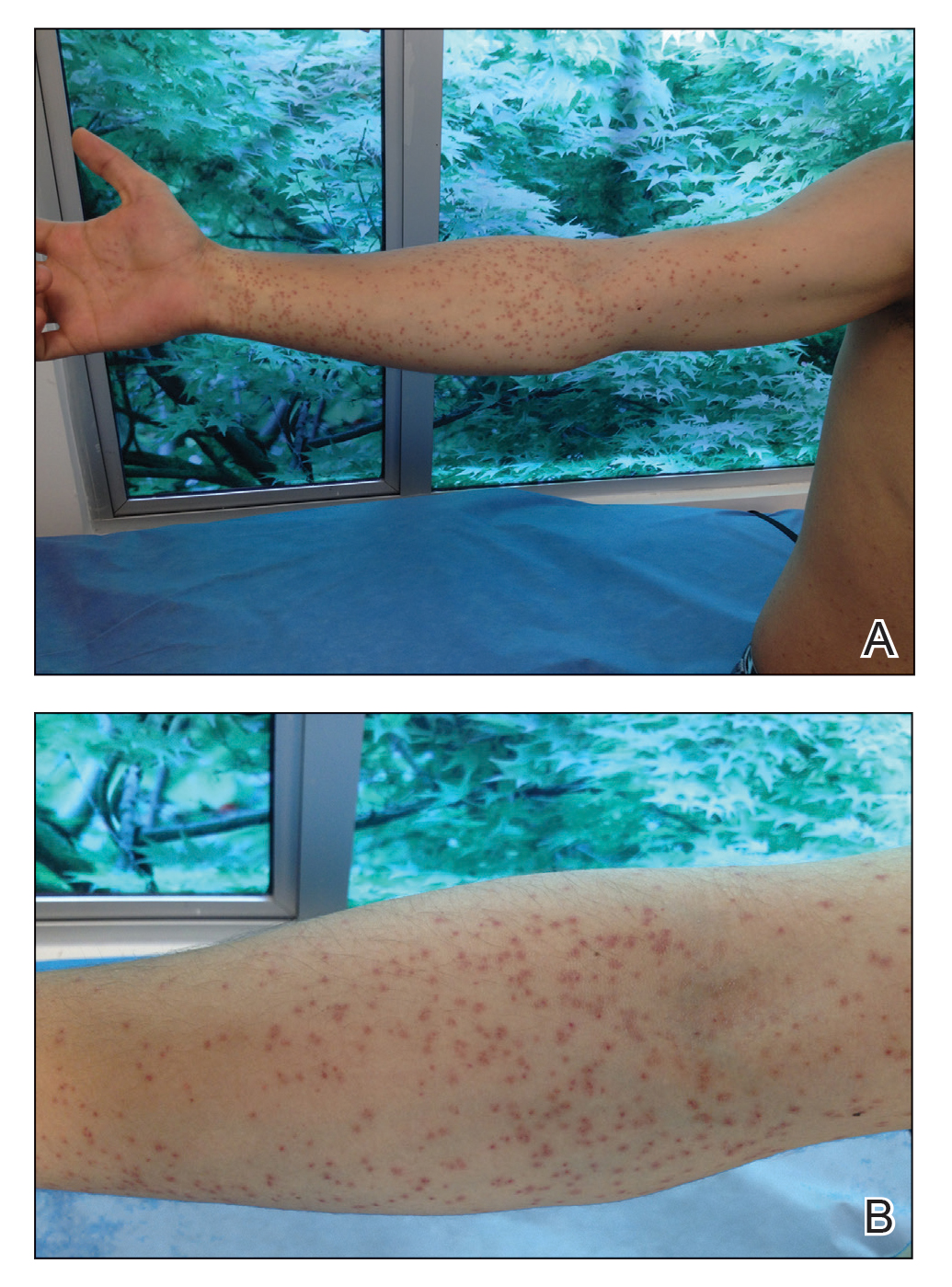
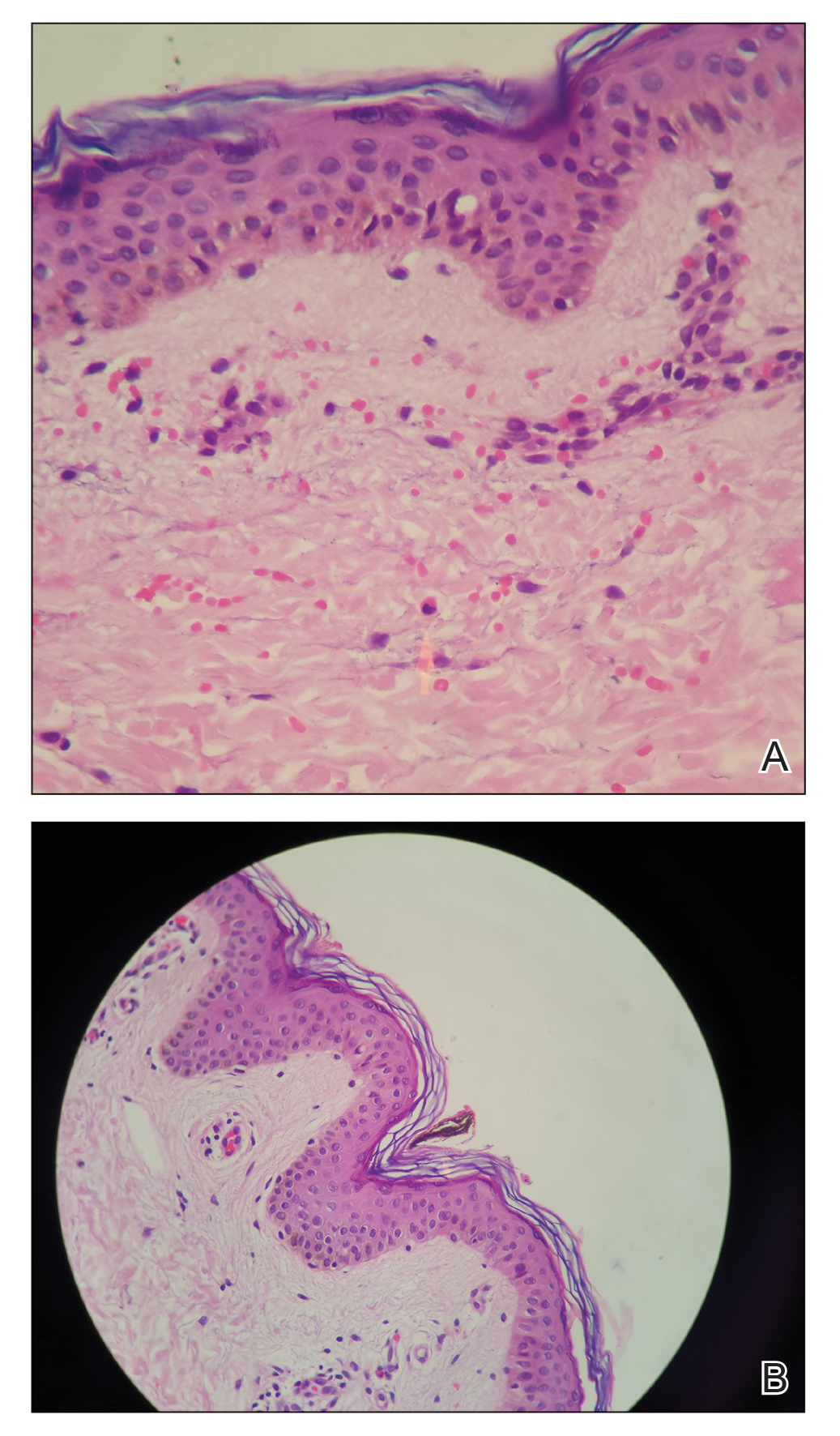
Based on the patient’s history of butterfly exposure, clinical presentation of the lesions, and histopathologic findings demonstrating the presence of the spicules, the diagnosis of lepidopterism was confirmed. The patient was treated with oral antihistamines and topical steroids for 1 week with complete resolution of the lesions.
Comment
Epidemiology of Envenomation
Although many tropical insects carry infectious diseases, cutaneous injury can occur by other mechanisms, such as dermatitis caused by contact with the skin (erucism or lepidopterism). Caterpillar envenomation is common, but this phenomenon rarely has been studied due to few reported cases, which hinders a complete understanding of the problem.3
The order Lepidoptera comprises 2 suborders: Rhopalocera, with adult specimens that fly during the daytime (butterflies), and Heterocera, which are largely nocturnal (moths). The stages of development include egg, larva (caterpillar), pupa (chrysalis), and adult (imago), constituting a holometabolic life cycle.4 Adult butterflies and moths represent the reproductive stage of lepidoptera.
The pathology of lepidopterism is attributed to contact with fluids such as hemolymph and secretions from the spicules, with histamine being identified as the main causative component.3 During the reproductive stage, female insects approach light sources and release clouds of bristles from their abdomens that can penetrate human skin and cause an irritating dermatitis.5 Lepidopterism can occur following contact with bristles from insects of the Hylesia genus (Saturniidae family), such as in our patient.3,6 Epidemic outbreaks have been reported in several countries, mainly Argentina, Brazil, and Venezuela.5 The patient was located in Colombia, a country without any reported cases of lepidopterism from the black butterfly (H nigricans). Cutaneous reactions to lepidoptera insects come in many forms, most commonly presenting as a mild stinging reaction with a papular eruption, pruritic urticarial papules and plaques, or scaly erythematous papules and plaques in exposed areas.7
Histopathologic Findings
The histology of lepidoptera exposure is nonspecific, typically demonstrating epidermal edema, superficial perivascular lymphocytic infiltrate, and eosinophils. Epidermal necrosis and vasculitis rarely are seen. Embedded spines from Hylesia insects have been described.7 The histopathologic examination generally reveals a foreign body reaction in addition to granulomas.3
Therapy
The use of oral antihistamines is indicated for the control of pruritus, and topical treatment with cold compresses, baths, and corticosteroid creams is recommended.3,8,9
Conclusion
We report the case of a patient with lepidopterism, a rare entity confirmed histologically with documentation of a spicule in the stratum corneum in the patient’s biopsy. Changes due to urbanization and industrialization have a closer relationship with various animal species that are pathogenic to humans; therefore, we encourage dermatologists to be aware of these diseases.
- Hossler EW. Caterpillars and moths: part I. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:666.
- Redd JT, Voorhees RE, Török TJ. Outbreak of lepidopterism at a Boy Scout camp. J Am Acad Dermatol. 2007;56:952-955.
- Haddad V Jr, Cardoso JL, Lupi O, et al. Tropical dermatology: venomous arthropods and human skin: part I. Insecta. J Am Acad Dermatol. 2012;67:331.
- Cardoso AEC, Haddad V Jr. Accidents caused by lepidopterans (moth larvae and adult): study on the epidemiological, clinical and therapeutic aspects. An Bras Dermatol. 2005;80:571-578.
- Salomón AD, Simón D, Rimoldi JC, et al. Lepidopterism due to the butterfly Hylesia nigricans. preventive research-intervention in Buenos Aires. Medicina (B Aires). 2005;65:241-246.
- Moreira SC, Lima JC, Silva L, et al. Description of an outbreak of lepidopterism (dermatitis associated with contact with moths) among sailors in Salvador, State of Bahia. Rev Soc Bras Med Trop. 2007;40:591-593.
- Hossler EW. Caterpillars and moths: part II. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:666.
- Maier H, Spiegel W, Kinaciyan T, et al. The oak processionary caterpillar as the cause of an epidemic airborne disease: survey and analysis. Br J Dermatol. 2003;149:990-997.
- Herrera-Chaumont C, Sojo-Milano M, Pérez-Ybarra LM. Knowledge and practices on lepidopterism by Hylesia metabus (Cramer, 1775)(Lepidoptera: Saturniidae) in Yaguaraparo parish, Sucre state, northeastern Venezuela. Revista Biomédica. 2016;27:11-23.
The order Lepidoptera (phylum Arthropoda, class Hexapoda) is comprised of moths and butterflies.1 Lepidopterism refers to a range of adverse medical conditions resulting from contact with these insects, typically during the caterpillar (larval) stage. It involves multiple pathologic mechanisms, including direct toxicity of venom and mechanical irritant effects.2 Erucism has been defined as any reaction caused by contact with caterpillars or any reaction limited to the skin caused by contact with caterpillars, butterflies, or moths. Lepidopterism can mean any reaction to caterpillars or moths, referring only to reactions from contact with scales or hairs from adult moths or butterflies, or referring only to cases with systemic signs and symptoms (eg, rhinoconjunctival or asthmatic symptoms, angioedema and anaphylaxis, hemorrhagic diathesis) with or without cutaneous findings, resulting from contact with any lepidopteran source.1 Strictly speaking, erucism should refer to any reaction from caterpillars and lepidopterism to reactions from moths or butterflies. Because reactions to both larval and adult lepidoptera can cause a variety of either cutaneous and/or systemic symptoms, classifying reactions into erucism or lepidopterism is only of academic interest.1
We report a documented case of lepidopterism in a patient with acute cutaneous lesions following exposure to an adult-stage black butterfly (Hylesia nigricans).
Case Report
A 21-year-old oil well worker presented with pruritic skin lesions on the right arm and flank of 3 hours’ duration. He reported that a black butterfly had perched on his arm while he was working and left a considerable number of small yellowish hairs on the skin, after which an intense pruritus and skin lesions began to develop. He denied other associated symptoms. Physical examination revealed numerous 1- to 2-mm, nonconfluent, erythematous and edematous papules on the right forearm, arm (Figure 1A), and flank. Some abrasions of the skin due to scratching and crusting were noted (Figure 1B). A skin biopsy from the right arm showed a superficial perivascular dermatitis with a mixed infiltrate of polymorphonuclear predominance with eosinophils (Figure 2A). Importantly, a structure resembling an urticating spicule was identified in the stratum corneum (Figure 2B); spicules are located on the abdomen of arthropods and are associated with an inflammatory response in human skin.


Based on the patient’s history of butterfly exposure, clinical presentation of the lesions, and histopathologic findings demonstrating the presence of the spicules, the diagnosis of lepidopterism was confirmed. The patient was treated with oral antihistamines and topical steroids for 1 week with complete resolution of the lesions.
Comment
Epidemiology of Envenomation
Although many tropical insects carry infectious diseases, cutaneous injury can occur by other mechanisms, such as dermatitis caused by contact with the skin (erucism or lepidopterism). Caterpillar envenomation is common, but this phenomenon rarely has been studied due to few reported cases, which hinders a complete understanding of the problem.3
The order Lepidoptera comprises 2 suborders: Rhopalocera, with adult specimens that fly during the daytime (butterflies), and Heterocera, which are largely nocturnal (moths). The stages of development include egg, larva (caterpillar), pupa (chrysalis), and adult (imago), constituting a holometabolic life cycle.4 Adult butterflies and moths represent the reproductive stage of lepidoptera.
The pathology of lepidopterism is attributed to contact with fluids such as hemolymph and secretions from the spicules, with histamine being identified as the main causative component.3 During the reproductive stage, female insects approach light sources and release clouds of bristles from their abdomens that can penetrate human skin and cause an irritating dermatitis.5 Lepidopterism can occur following contact with bristles from insects of the Hylesia genus (Saturniidae family), such as in our patient.3,6 Epidemic outbreaks have been reported in several countries, mainly Argentina, Brazil, and Venezuela.5 The patient was located in Colombia, a country without any reported cases of lepidopterism from the black butterfly (H nigricans). Cutaneous reactions to lepidoptera insects come in many forms, most commonly presenting as a mild stinging reaction with a papular eruption, pruritic urticarial papules and plaques, or scaly erythematous papules and plaques in exposed areas.7
Histopathologic Findings
The histology of lepidoptera exposure is nonspecific, typically demonstrating epidermal edema, superficial perivascular lymphocytic infiltrate, and eosinophils. Epidermal necrosis and vasculitis rarely are seen. Embedded spines from Hylesia insects have been described.7 The histopathologic examination generally reveals a foreign body reaction in addition to granulomas.3
Therapy
The use of oral antihistamines is indicated for the control of pruritus, and topical treatment with cold compresses, baths, and corticosteroid creams is recommended.3,8,9
Conclusion
We report the case of a patient with lepidopterism, a rare entity confirmed histologically with documentation of a spicule in the stratum corneum in the patient’s biopsy. Changes due to urbanization and industrialization have a closer relationship with various animal species that are pathogenic to humans; therefore, we encourage dermatologists to be aware of these diseases.
The order Lepidoptera (phylum Arthropoda, class Hexapoda) is comprised of moths and butterflies.1 Lepidopterism refers to a range of adverse medical conditions resulting from contact with these insects, typically during the caterpillar (larval) stage. It involves multiple pathologic mechanisms, including direct toxicity of venom and mechanical irritant effects.2 Erucism has been defined as any reaction caused by contact with caterpillars or any reaction limited to the skin caused by contact with caterpillars, butterflies, or moths. Lepidopterism can mean any reaction to caterpillars or moths, referring only to reactions from contact with scales or hairs from adult moths or butterflies, or referring only to cases with systemic signs and symptoms (eg, rhinoconjunctival or asthmatic symptoms, angioedema and anaphylaxis, hemorrhagic diathesis) with or without cutaneous findings, resulting from contact with any lepidopteran source.1 Strictly speaking, erucism should refer to any reaction from caterpillars and lepidopterism to reactions from moths or butterflies. Because reactions to both larval and adult lepidoptera can cause a variety of either cutaneous and/or systemic symptoms, classifying reactions into erucism or lepidopterism is only of academic interest.1
We report a documented case of lepidopterism in a patient with acute cutaneous lesions following exposure to an adult-stage black butterfly (Hylesia nigricans).
Case Report
A 21-year-old oil well worker presented with pruritic skin lesions on the right arm and flank of 3 hours’ duration. He reported that a black butterfly had perched on his arm while he was working and left a considerable number of small yellowish hairs on the skin, after which an intense pruritus and skin lesions began to develop. He denied other associated symptoms. Physical examination revealed numerous 1- to 2-mm, nonconfluent, erythematous and edematous papules on the right forearm, arm (Figure 1A), and flank. Some abrasions of the skin due to scratching and crusting were noted (Figure 1B). A skin biopsy from the right arm showed a superficial perivascular dermatitis with a mixed infiltrate of polymorphonuclear predominance with eosinophils (Figure 2A). Importantly, a structure resembling an urticating spicule was identified in the stratum corneum (Figure 2B); spicules are located on the abdomen of arthropods and are associated with an inflammatory response in human skin.


Based on the patient’s history of butterfly exposure, clinical presentation of the lesions, and histopathologic findings demonstrating the presence of the spicules, the diagnosis of lepidopterism was confirmed. The patient was treated with oral antihistamines and topical steroids for 1 week with complete resolution of the lesions.
Comment
Epidemiology of Envenomation
Although many tropical insects carry infectious diseases, cutaneous injury can occur by other mechanisms, such as dermatitis caused by contact with the skin (erucism or lepidopterism). Caterpillar envenomation is common, but this phenomenon rarely has been studied due to few reported cases, which hinders a complete understanding of the problem.3
The order Lepidoptera comprises 2 suborders: Rhopalocera, with adult specimens that fly during the daytime (butterflies), and Heterocera, which are largely nocturnal (moths). The stages of development include egg, larva (caterpillar), pupa (chrysalis), and adult (imago), constituting a holometabolic life cycle.4 Adult butterflies and moths represent the reproductive stage of lepidoptera.
The pathology of lepidopterism is attributed to contact with fluids such as hemolymph and secretions from the spicules, with histamine being identified as the main causative component.3 During the reproductive stage, female insects approach light sources and release clouds of bristles from their abdomens that can penetrate human skin and cause an irritating dermatitis.5 Lepidopterism can occur following contact with bristles from insects of the Hylesia genus (Saturniidae family), such as in our patient.3,6 Epidemic outbreaks have been reported in several countries, mainly Argentina, Brazil, and Venezuela.5 The patient was located in Colombia, a country without any reported cases of lepidopterism from the black butterfly (H nigricans). Cutaneous reactions to lepidoptera insects come in many forms, most commonly presenting as a mild stinging reaction with a papular eruption, pruritic urticarial papules and plaques, or scaly erythematous papules and plaques in exposed areas.7
Histopathologic Findings
The histology of lepidoptera exposure is nonspecific, typically demonstrating epidermal edema, superficial perivascular lymphocytic infiltrate, and eosinophils. Epidermal necrosis and vasculitis rarely are seen. Embedded spines from Hylesia insects have been described.7 The histopathologic examination generally reveals a foreign body reaction in addition to granulomas.3
Therapy
The use of oral antihistamines is indicated for the control of pruritus, and topical treatment with cold compresses, baths, and corticosteroid creams is recommended.3,8,9
Conclusion
We report the case of a patient with lepidopterism, a rare entity confirmed histologically with documentation of a spicule in the stratum corneum in the patient’s biopsy. Changes due to urbanization and industrialization have a closer relationship with various animal species that are pathogenic to humans; therefore, we encourage dermatologists to be aware of these diseases.
- Hossler EW. Caterpillars and moths: part I. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:666.
- Redd JT, Voorhees RE, Török TJ. Outbreak of lepidopterism at a Boy Scout camp. J Am Acad Dermatol. 2007;56:952-955.
- Haddad V Jr, Cardoso JL, Lupi O, et al. Tropical dermatology: venomous arthropods and human skin: part I. Insecta. J Am Acad Dermatol. 2012;67:331.
- Cardoso AEC, Haddad V Jr. Accidents caused by lepidopterans (moth larvae and adult): study on the epidemiological, clinical and therapeutic aspects. An Bras Dermatol. 2005;80:571-578.
- Salomón AD, Simón D, Rimoldi JC, et al. Lepidopterism due to the butterfly Hylesia nigricans. preventive research-intervention in Buenos Aires. Medicina (B Aires). 2005;65:241-246.
- Moreira SC, Lima JC, Silva L, et al. Description of an outbreak of lepidopterism (dermatitis associated with contact with moths) among sailors in Salvador, State of Bahia. Rev Soc Bras Med Trop. 2007;40:591-593.
- Hossler EW. Caterpillars and moths: part II. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:666.
- Maier H, Spiegel W, Kinaciyan T, et al. The oak processionary caterpillar as the cause of an epidemic airborne disease: survey and analysis. Br J Dermatol. 2003;149:990-997.
- Herrera-Chaumont C, Sojo-Milano M, Pérez-Ybarra LM. Knowledge and practices on lepidopterism by Hylesia metabus (Cramer, 1775)(Lepidoptera: Saturniidae) in Yaguaraparo parish, Sucre state, northeastern Venezuela. Revista Biomédica. 2016;27:11-23.
- Hossler EW. Caterpillars and moths: part I. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:666.
- Redd JT, Voorhees RE, Török TJ. Outbreak of lepidopterism at a Boy Scout camp. J Am Acad Dermatol. 2007;56:952-955.
- Haddad V Jr, Cardoso JL, Lupi O, et al. Tropical dermatology: venomous arthropods and human skin: part I. Insecta. J Am Acad Dermatol. 2012;67:331.
- Cardoso AEC, Haddad V Jr. Accidents caused by lepidopterans (moth larvae and adult): study on the epidemiological, clinical and therapeutic aspects. An Bras Dermatol. 2005;80:571-578.
- Salomón AD, Simón D, Rimoldi JC, et al. Lepidopterism due to the butterfly Hylesia nigricans. preventive research-intervention in Buenos Aires. Medicina (B Aires). 2005;65:241-246.
- Moreira SC, Lima JC, Silva L, et al. Description of an outbreak of lepidopterism (dermatitis associated with contact with moths) among sailors in Salvador, State of Bahia. Rev Soc Bras Med Trop. 2007;40:591-593.
- Hossler EW. Caterpillars and moths: part II. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:666.
- Maier H, Spiegel W, Kinaciyan T, et al. The oak processionary caterpillar as the cause of an epidemic airborne disease: survey and analysis. Br J Dermatol. 2003;149:990-997.
- Herrera-Chaumont C, Sojo-Milano M, Pérez-Ybarra LM. Knowledge and practices on lepidopterism by Hylesia metabus (Cramer, 1775)(Lepidoptera: Saturniidae) in Yaguaraparo parish, Sucre state, northeastern Venezuela. Revista Biomédica. 2016;27:11-23.
Practice Points
- When contact with caterpillars, butterflies, or moths occurs, patients should be advised not to rub or scratch the area or attempt to remove or crush the insect with a bare hand, as this may further spread irritating setae or spines.
- Careful removal of the larva with forceps or a similar instrument, combined with tape stripping of the area and immediate washing with soap and water, can be effective in minimizing exposure.
- Contaminated clothing should be removed and laundered thoroughly.
Testosterone Pellet–Induced Generalized Drug Eruption
To the Editor:
Testosterone-replacement therapy (TRT) is indicated for hypogonadism. The benefits of TRT are well documented, with multiple options available for delivery. Testosterone pellet implantation (TPI) is an effective treatment option for hypogonadism with minimal adverse reactions. Availability of TRT is increasing, as facilities are offering off-label applications. Although TPI generally is well tolerated, cutaneous reactions have been documented. We present a patient with drug-induced dermatitis following TPI.
A 51-year-old man with hypogonadism presented with an extremely pruritic rash that began on the left buttock 3 days after receiving his fourth TPI. The patient had received subcutaneous insertions of 8 testosterone pellets (75 mg per pellet every 6 months) to the left buttock. He denied any history of a similar rash. His medical history was remarkable for hyperlipidemia, which was controlled with niacin and omega-3 fatty acids (fish oil). Other medications included glucosamine. Before presenting to our clinic, he was given a 40-mg intramuscular injection of triamcinolone acetonide and trimethoprim-sulfamethoxazole twice daily for 7 days, a methylprednisolone dose pack, and triamcinolone ointment 0.1% twice daily by his primary care physician, all without improvement of the rash.
Physical examination revealed multiple well-circumscribed, coalescing clusters of darkly erythematous papules and dermal plaques of varying size on the buttocks with extension to the lower back, abdomen, and thighs (Figure 1). The differential diagnosis included lichenoid eruption, pseudolymphoma, sarcoidosis, and granuloma annulare.
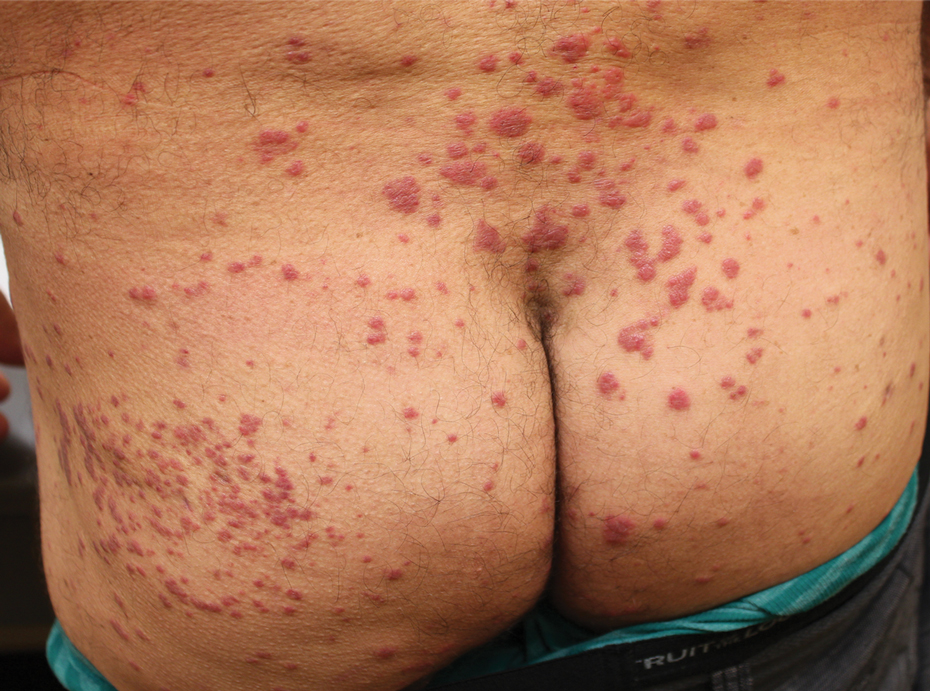
Histologic examination of a punch biopsy revealed an epidermis with a normal stratum corneum and subtle cell-poor vacuolar interface dermatitis with rare necrotic keratinocytes. There was a mild perivascular lymphocytic infiltrate with slight edema within the dermis without notable eosinophils or findings indicative of a vasculitic process (Figure 2).
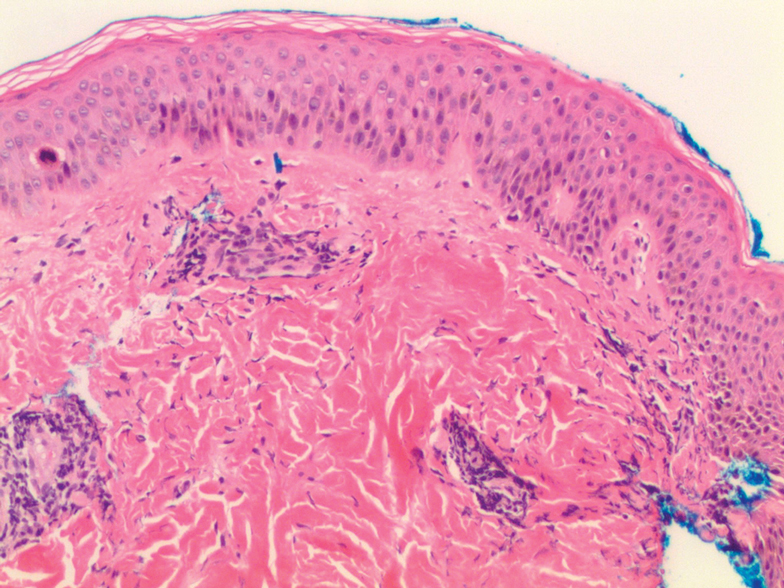
Oral prednisone 60 mg daily and betamethasone ointment 0.05% applied twice daily were started, with notable improvement of the rash in 1 week (Figure 3). Given the temporal relationship of the TPI, histologic findings suggestive of drug eruption, and resolution of symptoms shortly after treatment, a diagnosis of testosterone pellet–induced generalized dermatitis was established.
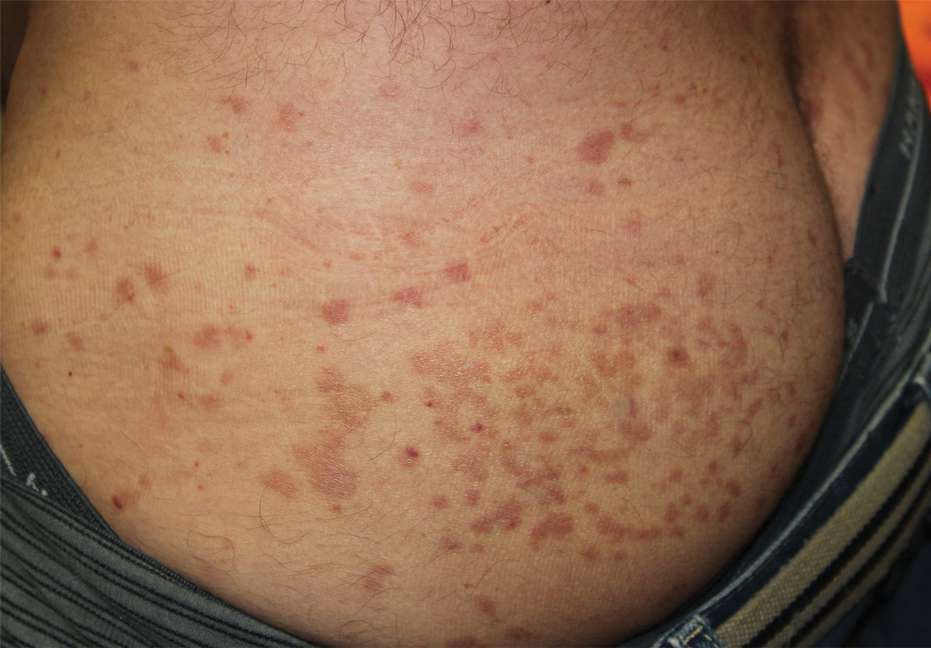
Testosterone-replacement therapy is the principal treatment of male pathologic hypoandrogenism, but off-label prescription frequently occurs for age-related hypogonadism and hypoactive sexual desire disorder.1 Testosterone-replacement therapy also can enhance sexual desire and function and improve mood in premenopausal and postmenopausal women with testosterone deficiency.2 Delivery options include topicals, intramuscular injections, oral formulations, transdermal patches and gels, and subcutaneous placement of testosterone pellets (TPI).Cutaneous reactions to TPI are rare. Hirsutism, male-pattern hair loss, and acne are possible cutaneous adverse reactions.3 In addition, a localized erythematous pruritic eruption at the implantation site and an immunologic foreign-body reaction to testosterone pellets have been reported.4
In one case report, a man developed recurrent ill-defined, erythematous, scaly plaques and patches over the buttocks and thighs, consistent with testosterone-induced eczematous dermatitis, subsequent to his second TPI. The patient presented with the eruption within 4 weeks after the most recent implantation, similar to our case, but differed temporally in initial presentation, presenting after the second implantation.5 Our case differed in morphologic presentation (dermal plaques as opposed to eczematous change) and refractoriness to triamcinolone injection.
Testosterone-replacement therapy is becoming more widely available. Lack of regulation of proper marketing by such facilities as medical spas that offer TPI for off-label applications has led to a rampant increase in TRT prescribing, possibly foreshadowing an increase in adverse cutaneous reactions to TRT.6
Our case of histologically consistent testosterone pellet–induced dermatitis highlights a rare cutaneous adverse reaction that can occur subsequent to TPI and illustrates the efficacy of high-dose oral steroids as a treatment option. With increased use of TRT, physicians should be cognizant of the potential adverse cutaneous effects related to this treatment and counsel patients appropriately prior to initiating treatment.
Acknowledgment
We thank the patient for granting permission to publish this case.
- Clayton AH, Kingsberg SA, Goldstein I. Evaluation and management of hypoactive sexual desire disorder. Sex Med. 2018;6:59-74.
- Glaser R, Dimitrakakis C. Testosterone therapy in women: myths and misconceptions. Maturitas. 2013;74:230-234.
- Testopel (testosterone pellet) [package insert]. Endo Pharmaceuticals, Inc; 2016. Accessed December 16, 2020. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=a1741a0b-3d4c-42dc-880d-a06e96cce9ef&type=display
- Cavender RK, Fairall M. Subcutaneous testosterone pellet implant (Testopel) therapy for men with testosterone deficiency syndrome: a single-site retrospective safety analysis. J Sex Med. 2009;6:3177-3192.
- Heldt Manica LA, Cohen PR. Testosterone pellet associated dermatitis: report and review of Testopel-related cutaneous adverse effects. Cureus. 2017;9:e1560.
- Mintzes B. The marketing of testosterone treatments for age-related low testosterone or ‘Low T’. Curr Opin Endocrinol Diabetes Obes. 2018;25:224-230.
To the Editor:
Testosterone-replacement therapy (TRT) is indicated for hypogonadism. The benefits of TRT are well documented, with multiple options available for delivery. Testosterone pellet implantation (TPI) is an effective treatment option for hypogonadism with minimal adverse reactions. Availability of TRT is increasing, as facilities are offering off-label applications. Although TPI generally is well tolerated, cutaneous reactions have been documented. We present a patient with drug-induced dermatitis following TPI.
A 51-year-old man with hypogonadism presented with an extremely pruritic rash that began on the left buttock 3 days after receiving his fourth TPI. The patient had received subcutaneous insertions of 8 testosterone pellets (75 mg per pellet every 6 months) to the left buttock. He denied any history of a similar rash. His medical history was remarkable for hyperlipidemia, which was controlled with niacin and omega-3 fatty acids (fish oil). Other medications included glucosamine. Before presenting to our clinic, he was given a 40-mg intramuscular injection of triamcinolone acetonide and trimethoprim-sulfamethoxazole twice daily for 7 days, a methylprednisolone dose pack, and triamcinolone ointment 0.1% twice daily by his primary care physician, all without improvement of the rash.
Physical examination revealed multiple well-circumscribed, coalescing clusters of darkly erythematous papules and dermal plaques of varying size on the buttocks with extension to the lower back, abdomen, and thighs (Figure 1). The differential diagnosis included lichenoid eruption, pseudolymphoma, sarcoidosis, and granuloma annulare.

Histologic examination of a punch biopsy revealed an epidermis with a normal stratum corneum and subtle cell-poor vacuolar interface dermatitis with rare necrotic keratinocytes. There was a mild perivascular lymphocytic infiltrate with slight edema within the dermis without notable eosinophils or findings indicative of a vasculitic process (Figure 2).

Oral prednisone 60 mg daily and betamethasone ointment 0.05% applied twice daily were started, with notable improvement of the rash in 1 week (Figure 3). Given the temporal relationship of the TPI, histologic findings suggestive of drug eruption, and resolution of symptoms shortly after treatment, a diagnosis of testosterone pellet–induced generalized dermatitis was established.

Testosterone-replacement therapy is the principal treatment of male pathologic hypoandrogenism, but off-label prescription frequently occurs for age-related hypogonadism and hypoactive sexual desire disorder.1 Testosterone-replacement therapy also can enhance sexual desire and function and improve mood in premenopausal and postmenopausal women with testosterone deficiency.2 Delivery options include topicals, intramuscular injections, oral formulations, transdermal patches and gels, and subcutaneous placement of testosterone pellets (TPI).Cutaneous reactions to TPI are rare. Hirsutism, male-pattern hair loss, and acne are possible cutaneous adverse reactions.3 In addition, a localized erythematous pruritic eruption at the implantation site and an immunologic foreign-body reaction to testosterone pellets have been reported.4
In one case report, a man developed recurrent ill-defined, erythematous, scaly plaques and patches over the buttocks and thighs, consistent with testosterone-induced eczematous dermatitis, subsequent to his second TPI. The patient presented with the eruption within 4 weeks after the most recent implantation, similar to our case, but differed temporally in initial presentation, presenting after the second implantation.5 Our case differed in morphologic presentation (dermal plaques as opposed to eczematous change) and refractoriness to triamcinolone injection.
Testosterone-replacement therapy is becoming more widely available. Lack of regulation of proper marketing by such facilities as medical spas that offer TPI for off-label applications has led to a rampant increase in TRT prescribing, possibly foreshadowing an increase in adverse cutaneous reactions to TRT.6
Our case of histologically consistent testosterone pellet–induced dermatitis highlights a rare cutaneous adverse reaction that can occur subsequent to TPI and illustrates the efficacy of high-dose oral steroids as a treatment option. With increased use of TRT, physicians should be cognizant of the potential adverse cutaneous effects related to this treatment and counsel patients appropriately prior to initiating treatment.
Acknowledgment
We thank the patient for granting permission to publish this case.
To the Editor:
Testosterone-replacement therapy (TRT) is indicated for hypogonadism. The benefits of TRT are well documented, with multiple options available for delivery. Testosterone pellet implantation (TPI) is an effective treatment option for hypogonadism with minimal adverse reactions. Availability of TRT is increasing, as facilities are offering off-label applications. Although TPI generally is well tolerated, cutaneous reactions have been documented. We present a patient with drug-induced dermatitis following TPI.
A 51-year-old man with hypogonadism presented with an extremely pruritic rash that began on the left buttock 3 days after receiving his fourth TPI. The patient had received subcutaneous insertions of 8 testosterone pellets (75 mg per pellet every 6 months) to the left buttock. He denied any history of a similar rash. His medical history was remarkable for hyperlipidemia, which was controlled with niacin and omega-3 fatty acids (fish oil). Other medications included glucosamine. Before presenting to our clinic, he was given a 40-mg intramuscular injection of triamcinolone acetonide and trimethoprim-sulfamethoxazole twice daily for 7 days, a methylprednisolone dose pack, and triamcinolone ointment 0.1% twice daily by his primary care physician, all without improvement of the rash.
Physical examination revealed multiple well-circumscribed, coalescing clusters of darkly erythematous papules and dermal plaques of varying size on the buttocks with extension to the lower back, abdomen, and thighs (Figure 1). The differential diagnosis included lichenoid eruption, pseudolymphoma, sarcoidosis, and granuloma annulare.

Histologic examination of a punch biopsy revealed an epidermis with a normal stratum corneum and subtle cell-poor vacuolar interface dermatitis with rare necrotic keratinocytes. There was a mild perivascular lymphocytic infiltrate with slight edema within the dermis without notable eosinophils or findings indicative of a vasculitic process (Figure 2).

Oral prednisone 60 mg daily and betamethasone ointment 0.05% applied twice daily were started, with notable improvement of the rash in 1 week (Figure 3). Given the temporal relationship of the TPI, histologic findings suggestive of drug eruption, and resolution of symptoms shortly after treatment, a diagnosis of testosterone pellet–induced generalized dermatitis was established.

Testosterone-replacement therapy is the principal treatment of male pathologic hypoandrogenism, but off-label prescription frequently occurs for age-related hypogonadism and hypoactive sexual desire disorder.1 Testosterone-replacement therapy also can enhance sexual desire and function and improve mood in premenopausal and postmenopausal women with testosterone deficiency.2 Delivery options include topicals, intramuscular injections, oral formulations, transdermal patches and gels, and subcutaneous placement of testosterone pellets (TPI).Cutaneous reactions to TPI are rare. Hirsutism, male-pattern hair loss, and acne are possible cutaneous adverse reactions.3 In addition, a localized erythematous pruritic eruption at the implantation site and an immunologic foreign-body reaction to testosterone pellets have been reported.4
In one case report, a man developed recurrent ill-defined, erythematous, scaly plaques and patches over the buttocks and thighs, consistent with testosterone-induced eczematous dermatitis, subsequent to his second TPI. The patient presented with the eruption within 4 weeks after the most recent implantation, similar to our case, but differed temporally in initial presentation, presenting after the second implantation.5 Our case differed in morphologic presentation (dermal plaques as opposed to eczematous change) and refractoriness to triamcinolone injection.
Testosterone-replacement therapy is becoming more widely available. Lack of regulation of proper marketing by such facilities as medical spas that offer TPI for off-label applications has led to a rampant increase in TRT prescribing, possibly foreshadowing an increase in adverse cutaneous reactions to TRT.6
Our case of histologically consistent testosterone pellet–induced dermatitis highlights a rare cutaneous adverse reaction that can occur subsequent to TPI and illustrates the efficacy of high-dose oral steroids as a treatment option. With increased use of TRT, physicians should be cognizant of the potential adverse cutaneous effects related to this treatment and counsel patients appropriately prior to initiating treatment.
Acknowledgment
We thank the patient for granting permission to publish this case.
- Clayton AH, Kingsberg SA, Goldstein I. Evaluation and management of hypoactive sexual desire disorder. Sex Med. 2018;6:59-74.
- Glaser R, Dimitrakakis C. Testosterone therapy in women: myths and misconceptions. Maturitas. 2013;74:230-234.
- Testopel (testosterone pellet) [package insert]. Endo Pharmaceuticals, Inc; 2016. Accessed December 16, 2020. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=a1741a0b-3d4c-42dc-880d-a06e96cce9ef&type=display
- Cavender RK, Fairall M. Subcutaneous testosterone pellet implant (Testopel) therapy for men with testosterone deficiency syndrome: a single-site retrospective safety analysis. J Sex Med. 2009;6:3177-3192.
- Heldt Manica LA, Cohen PR. Testosterone pellet associated dermatitis: report and review of Testopel-related cutaneous adverse effects. Cureus. 2017;9:e1560.
- Mintzes B. The marketing of testosterone treatments for age-related low testosterone or ‘Low T’. Curr Opin Endocrinol Diabetes Obes. 2018;25:224-230.
- Clayton AH, Kingsberg SA, Goldstein I. Evaluation and management of hypoactive sexual desire disorder. Sex Med. 2018;6:59-74.
- Glaser R, Dimitrakakis C. Testosterone therapy in women: myths and misconceptions. Maturitas. 2013;74:230-234.
- Testopel (testosterone pellet) [package insert]. Endo Pharmaceuticals, Inc; 2016. Accessed December 16, 2020. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=a1741a0b-3d4c-42dc-880d-a06e96cce9ef&type=display
- Cavender RK, Fairall M. Subcutaneous testosterone pellet implant (Testopel) therapy for men with testosterone deficiency syndrome: a single-site retrospective safety analysis. J Sex Med. 2009;6:3177-3192.
- Heldt Manica LA, Cohen PR. Testosterone pellet associated dermatitis: report and review of Testopel-related cutaneous adverse effects. Cureus. 2017;9:e1560.
- Mintzes B. The marketing of testosterone treatments for age-related low testosterone or ‘Low T’. Curr Opin Endocrinol Diabetes Obes. 2018;25:224-230.
Practice Points
- Dermatologists should be aware that testosterone pellet implantation can cause dermatitis overlying the implantation site, which can generalize and differ in morphologic presentation.
- For patients presenting with a suspected case of testosterone pellet–induced dermatitis, a high-dose oral corticosteroid can be deployed as an effective therapy.
Contact Allergy to Nickel: Still #1 After All These Years
Nickel is unrivaled as the most common cause of contact allergy worldwide.1 Nickel is commonly used as a hardening agent in metal products, and complete avoidance is challenging due to numerous potential exposures (eg, direct contact, airborne, dietary, medical implantation). Allergic contact dermatitis to nickel (Ni-ACD) can lead to decreased quality of life, inability to work, and considerable health care expenses.1 Here, we review the epidemiology of nickel allergy, regulation of nickel in the United States and Europe, common clinical presentations, and pearls on avoidance.
Epidemiology
Nickel continues to be the most common cause of contact allergy worldwide. Data from the 2015-2016 North American Contact Dermatitis Group patch test cycle (N=5597) showed nickel sulfate to be positive in 17.5% of patients patch tested to nickel.2 The prevalence of nickel allergy has been relatively stable in North America since 2005 (Figure 1). Although Ni-ACD historically was identified as an occupational disease of the hands in male nickel platers, the epidemiology of nickel allergy has shifted.1 Today, most cases are nonoccupational and affect women more often than men,3 in part due to improved industrial hygiene, pervasive incorporation of nickel in consumer items, and differences in cultural practices such as piercings.1,3 Piercings in particular have been implicated as important sources of nickel exposure, as this practice disrupts normal skin barrier function and is a potentially sensitizing event. Multiple studies including a large-scale epidemiologic analysis from 2017 have found piercings to be associated with an increased frequency of Ni-ACD (24.4% with piercing vs 9.6% without piercing). Interestingly, the degree of nickel sensitivity also was found to increase with the number of piercings (14.3% with 1 piercing vs 34.0% with ≥5 piercings).4

Regulation
Nickel content has been regulated in parts of the European Union (EU) since the 1990s, but regulation in the United States is lacking. In an attempt to reduce the prevalence of nickel allergy, the EU limits the level of nickel release from consumer items intended to be in direct and prolonged contact with the skin. These limits were first introduced in Denmark in 1990, followed closely by the EU Nickel Directive in 1994, which has resulted in consistent patterns of decreasing prevalence of Ni-ACD in multiple European countries.5 Notably, a Danish study comparing the prevalence of sensitization between girls with ears pierced before vs after implementation of nickel regulation found a decrease in prevalence from 17.1% to 3.9%.6 Additionally, this initiative has greatly reduced the economic burden of nickel dermatitis. It is estimated that Denmark alone has saved US $2 billion over a 20-year period in both direct and indirect health care costs.7
However, a policy is only effective if it is enforced, and it has been reported in the EU that 8% to 32% of tested jewelry exceeds the limit placed on nickel release, with imported jewelry being especially problematic.5 Also of interest, the 1 and 2 euro coins are known to release more nickel than pure nickel itself, releasing 240 to 320 times more than is allowed under the EU Nickel Directive (Figure 2).8 Although coins are not explicitly mentioned as items having prolonged contact with the skin, they can and do exacerbate allergic contact dermatitis of the hands, especially in occupational groups such as cashiers.9 Unsurprisingly, during the discussions to determine the composition of coins prior to the mass adoption of the euro in the EU in 2002, dermatologists and nickel industry experts remained divided in their recommendations.10 However, the EU regulation is considered a public health success overall, and the trends of Ni-ACD and economic burden are opposite of the United States, where legislation has yet to be adopted.

Patch Testing to Nickel
In North America, the 2 available patch test systems are the chamber method and the Thin-layer Rapid Use Epicutaneous (T.R.U.E.) test (SmartPractice). In the T.R.U.E. test, nickel sulfate is used to formulate the patch at 200 µg/cm2 using hydroxypropyl cellulose as the gel vehicle. In the chamber method, nickel sulfate is used on either an aluminum or plastic chamber, most commonly at concentrations of 2.5% or 5% in petrolatum. Nickel sulfate 2.5% is most frequently used in US-based patch test clinics. A 2018 study (N=205) comparing the sensitivities of the 2.5% and 5% concentrations of nickel found 5% to be more sensitive; 31% of the cohort tested positive at 5% but only 20% at 2.5%, suggesting the 5% formulation is superior at detecting nickel allergy.11
Similar to other metals, nickel may react later than other allergens. A 2019 analysis of the prevalence of new patch test reactions on day 7 showed that 17% of 607 patients were negative on day 3 but were positive on day 7, further emphasizing the importance of a properly timed delayed reading.12
Clinical Presentation
Localized
The classic presentation of Ni-ACD is a scaly erythematous dermatitis in a typical distribution (eg, earlobes [earrings], wrists [watch], periumbilical [belt]). These scenarios usually can be diagnosed by the astute clinician without patch testing; however, the source of exposure may be less obvious if the nickel-releasing item has intermittent contact with the skin (eg, coins in the pocket, furniture hardware, personal grooming devices).13 Other reported exposures include facial dermatitis from mobile phones, dermatitis of the ulnar hands from laptop use, and hand dermatitis from gaming controllers,14-16 perhaps another reason for some to unplug.
Systemic
Sensitized individuals also may present with systemic contact dermatitis after airborne, oral, mucosal, or intravenous exposure. Presentations vary but have been reported to manifest as flare-up reactions in previously affected areas, pompholyx, diffuse dermatitis, flexural dermatitis, and baboon syndrome.17 Although it is unknown if airborne exposure alone is sufficient for sensitization, cases have been reported in occupational settings.18 One report described a man presenting with widespread dermatitis involving the extremities, chest, and genital area after his first day working at an electroplating plant.19
Systemic contact dermatitis from foods high in nickel (eg, chocolate, sunflower seeds, whole-grain flour, dried beans) and occasionally nonfood items (eg, coins) also has occurred. The so-called Easter egg hunt dermatitis has been described in children with Ni-ACD after candy ingestion.20 Another case described an 8-year-old girl and budding illusionist with severe diffuse dermatitis; a thorough history revealed the dermatitis began after she ingested a coin while performing a magic trick.21
Cases of nickel systemic contact dermatitis have been reported following medical device implantation, including reactions to cardiac devices, orthopedic implants, neurosurgery materials, and others.22 In addition, both intraoral and extraoral manifestations following application of orthodontic materials and dental implants have been reported.23,24 Although nickel-containing medical devices generally are well tolerated even in nickel-sensitive individuals, the development of systemic Ni-ACD has at times required device or hardware removal.22,23
After the Patch Test: Avoidance of Nickel
Counseling patients on nickel avoidance is critical to clinical improvement. Common nickel-containing items include jewelry, metal on clothing (eg, zippers, clasps, grommets), belt buckles, watches, glasses, furniture, coins, and keys. Numerous personal care products may also contain nickel, including nail clippers, eyelash curlers, tweezers, mascara tubes, and razors.25,26 Patients should be made aware that nickel-free alternatives are available for the majority of these products. Internet-based tips such as painting nail polish on products or iron-on patches tend to be of limited use in our experience. Patients may consider purchasing a nickel spot test to detect nickel in their environment; the dimethylglyoxime nickel spot test is inexpensive, rapid, and easy-to-use. To use the test, a small amount of the chemical is rubbed on the metal item using a cotton swab; a pink color indicates nickel release. Patients can be reassured that dimethylglyoxime does not harm jewelry.
Some general advice for patients regarding jewelry, the most common source of nickel exposure, is to only wear jewelry that is made from metals such as surgical-grade stainless steel, pure sterling silver, or platinum. If yellow gold is the preferred metal, it is prudent to be aware that lower karat items could potentially contain nickel. White gold should be avoided, as it often contains nickel to contribute to its color. Finally, gold-plated jewelry should be avoided, as the plating can wear off and expose a possibly nickel-containing base.
A low-nickel diet may be of benefit in select patients. A meta-analysis assessing systemic contact dermatitis from nickel ingestion found that 1% of nickel-sensitive individuals may be expected to react to nickel found in a normal diet.27 However, as with any diet, adherence can be difficult. Thankfully, Mislankar and Zirwas28 have developed a simple point-based system to help increase compliance. Additionally, a free mobile application is now available; Nickel Navigator can be used to track daily nickel intake and may be especially convenient for our more tech-savvy patients. In conjunction with a low-nickel diet, some authors also recommend eating meals high in vitamin C or supplementation with vitamin C, as co-ingestion has been shown to reduce nickel absorption.29
Final Interpretation
Nickel allergy remains common, found in up to 17.5% of patch tested patients. Despite regulation in the EU, nickel continues to have high prevalence of positive patch test reactions around the world. Nickel is not only found in jewelry and belt buckles but also in personal care products, electronics, and food. Allergen avoidance is key and requires knowledge of common items containing nickel and a low nickel diet for select patients.
- Ahlström MG, Thyssen JP, Wennervaldt M, et al. Nickel allergy and allergic contact dermatitis: a clinical review of immunology, epidemiology, exposure, and treatment. Contact Dermatitis. 2019;81:227-241.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group Patch Test Results: 2015-2016. Dermatitis. 2018;29:297-309.
- Thyssen JP, Menné T. Metal allergy—a review on exposures, penetration, genetics, prevalence, and clinical implications. Chem Res Toxicol. 2010;23:309-318.
- Warshaw EM, Aschenbeck KA, DeKoven JG, et al. Piercing and metal sensitivity: extended analysis of the North American Contact Dermatitis Group data, 2007-2014. Dermatitis. 2017;28:333-341.
- Ahlström MG, Thyssen JP, Menné T, et al. Prevalence of nickel allergy in Europe following the EU Nickel Directive—a review. Contact Dermatitis. 2017;77:193-200.
- Jensen CS, Lisby S, Baadsgaard O, et al. Decrease in nickel sensitization in a Danish schoolgirl population with ears pierced after implementation of a nickel-exposure regulation. Br J Dermatol. 2002;146:636-642.
- Serup-Hansen N, Gudum A, Sørensen MM. Valuation of Chemical Related Health Impacts. Danish Environmental Protection Agency. Published 2004. Accessed December 14, 2020. https://www2.mst.dk/udgiv/publications/2004/87-7614-295-7/pdf/87-7614-296-5.pdf
- Nestle FO, Speidel H, Speidel MO. Metallurgy: high nickel release from 1- and 2-euro coins. Nature. 2002;419:132.
- Kanerva L, Estlander T, Jolanki R. Bank clerk’s occupational allergic nickel and cobalt contact dermatitis from coins. Contact Dermatitis. 1998;38:217-218.
- Aberer W. Platitudes in allergy—based on the example of the euro. Contact Dermatitis. 2001;45:254-255.
- Goldminz AM, Scheinman PL. Comparison of nickel sulfate 2.5% and nickel sulfate 5% for detecting nickel contact allergy. Dermatitis. 2018;29:321-323.
- van Amerongen CCA, Ofenloch R, Dittmar D, et al. New positive patch test reactions on day 7—the additional value of the day 7 patch test reading. Contact Dermatitis. 2019;81:280-287.
- Silverberg NB, Pelletier JL, Jacob SE, et al; Section of Dermatology, Section on Allergy and Immunology. Nickel allergic contact dermatitis: identification, treatment, and prevention. Pediatrics. 2020;145:E20200628.
- Aquino M, Mucci T, Chong M, et al. Mobile phones: potential sources of nickel and cobalt exposure for metal allergic patients. Pediatr Allergy Immunol Pulmonol. 2013;26:181-186.
- Jensen P, Jellesen MS, Møller P, et al. Nickel allergy and dermatitis following use of a laptop computer. J Am Acad Dermatol. 2012;67:E170-E171.
- Jacob SE. Xbox—a source of nickel exposure in children. Pediatr Dermatol. 2014;31:115-116.
- Menné T, Veien NK. Systemic contact dermatitis. In: Rycroft RJG, Menné T, Frosch PJ, et al, eds. Textbook of Contact Dermatitis. Springer; 2001:355-366.
- Mann E, Ranft U, Eberwein G, et al. Does airborne nickel exposure induce nickel sensitization? Contact Dermatitis. 2010;62:355-362.
- Candura SM, Locatelli C, Butera R, et al. Widespread nickel dermatitis from inhalation. Contact Dermatitis. 2001;45:174-175.
- Jacob SE, Hamann D, Goldenberg A, et al. Easter egg hunt dermatitis: systemic allergic contact dermatitis associated with chocolate ingestion. Pediatr Dermatol. 2015;32:231-233.
- Mahdi G, Israel DM, Hassall E. Nickel dermatitis and associated gastritis after coin ingestion. J Pediatr Gastroenterol Nutr. 1996;23:74-76.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Schultz JC, Connelly E, Glesne L, et al. Cutaneous and oral eruption from oral exposure to nickel in dental braces. Dermatitis. 2004;15:154-157.
- Pigatto PD, Brambilla L, Ferrucci S, et al. Systemic allergic contact dermatitis associated with allergy to intraoral metals. Dermatol Online J. 2014;20:13030/qt74632201.
- Brandrup F. Nickel eyelid dermatitis from an eyelash curler. Contact Dermatitis. 1991;25:77.
- Walsh G, Wilkinson SM. Materials and allergens within spectacle frames: a review. Contact Dermatitis. 2006;55:130-139.
- Bergman D, Goldenberg A, Rundle C, et al. Low nickel diet: a patient-centered review [published May 24, 2016]. J Clin Exp Dermatol Res. doi:10.4172/2155-9554.1000355
- Mislankar M, Zirwas MJ. Low-nickel diet scoring system for systemic nickel allergy. Dermatitis. 2013;24:190-195.
- Zirwas MJ, Molenda MA. Dietary nickel as a cause of systemic contact dermatitis. J Clin Aesthet Dermatol. 2009;2:39-43.
Nickel is unrivaled as the most common cause of contact allergy worldwide.1 Nickel is commonly used as a hardening agent in metal products, and complete avoidance is challenging due to numerous potential exposures (eg, direct contact, airborne, dietary, medical implantation). Allergic contact dermatitis to nickel (Ni-ACD) can lead to decreased quality of life, inability to work, and considerable health care expenses.1 Here, we review the epidemiology of nickel allergy, regulation of nickel in the United States and Europe, common clinical presentations, and pearls on avoidance.
Epidemiology
Nickel continues to be the most common cause of contact allergy worldwide. Data from the 2015-2016 North American Contact Dermatitis Group patch test cycle (N=5597) showed nickel sulfate to be positive in 17.5% of patients patch tested to nickel.2 The prevalence of nickel allergy has been relatively stable in North America since 2005 (Figure 1). Although Ni-ACD historically was identified as an occupational disease of the hands in male nickel platers, the epidemiology of nickel allergy has shifted.1 Today, most cases are nonoccupational and affect women more often than men,3 in part due to improved industrial hygiene, pervasive incorporation of nickel in consumer items, and differences in cultural practices such as piercings.1,3 Piercings in particular have been implicated as important sources of nickel exposure, as this practice disrupts normal skin barrier function and is a potentially sensitizing event. Multiple studies including a large-scale epidemiologic analysis from 2017 have found piercings to be associated with an increased frequency of Ni-ACD (24.4% with piercing vs 9.6% without piercing). Interestingly, the degree of nickel sensitivity also was found to increase with the number of piercings (14.3% with 1 piercing vs 34.0% with ≥5 piercings).4

Regulation
Nickel content has been regulated in parts of the European Union (EU) since the 1990s, but regulation in the United States is lacking. In an attempt to reduce the prevalence of nickel allergy, the EU limits the level of nickel release from consumer items intended to be in direct and prolonged contact with the skin. These limits were first introduced in Denmark in 1990, followed closely by the EU Nickel Directive in 1994, which has resulted in consistent patterns of decreasing prevalence of Ni-ACD in multiple European countries.5 Notably, a Danish study comparing the prevalence of sensitization between girls with ears pierced before vs after implementation of nickel regulation found a decrease in prevalence from 17.1% to 3.9%.6 Additionally, this initiative has greatly reduced the economic burden of nickel dermatitis. It is estimated that Denmark alone has saved US $2 billion over a 20-year period in both direct and indirect health care costs.7
However, a policy is only effective if it is enforced, and it has been reported in the EU that 8% to 32% of tested jewelry exceeds the limit placed on nickel release, with imported jewelry being especially problematic.5 Also of interest, the 1 and 2 euro coins are known to release more nickel than pure nickel itself, releasing 240 to 320 times more than is allowed under the EU Nickel Directive (Figure 2).8 Although coins are not explicitly mentioned as items having prolonged contact with the skin, they can and do exacerbate allergic contact dermatitis of the hands, especially in occupational groups such as cashiers.9 Unsurprisingly, during the discussions to determine the composition of coins prior to the mass adoption of the euro in the EU in 2002, dermatologists and nickel industry experts remained divided in their recommendations.10 However, the EU regulation is considered a public health success overall, and the trends of Ni-ACD and economic burden are opposite of the United States, where legislation has yet to be adopted.

Patch Testing to Nickel
In North America, the 2 available patch test systems are the chamber method and the Thin-layer Rapid Use Epicutaneous (T.R.U.E.) test (SmartPractice). In the T.R.U.E. test, nickel sulfate is used to formulate the patch at 200 µg/cm2 using hydroxypropyl cellulose as the gel vehicle. In the chamber method, nickel sulfate is used on either an aluminum or plastic chamber, most commonly at concentrations of 2.5% or 5% in petrolatum. Nickel sulfate 2.5% is most frequently used in US-based patch test clinics. A 2018 study (N=205) comparing the sensitivities of the 2.5% and 5% concentrations of nickel found 5% to be more sensitive; 31% of the cohort tested positive at 5% but only 20% at 2.5%, suggesting the 5% formulation is superior at detecting nickel allergy.11
Similar to other metals, nickel may react later than other allergens. A 2019 analysis of the prevalence of new patch test reactions on day 7 showed that 17% of 607 patients were negative on day 3 but were positive on day 7, further emphasizing the importance of a properly timed delayed reading.12
Clinical Presentation
Localized
The classic presentation of Ni-ACD is a scaly erythematous dermatitis in a typical distribution (eg, earlobes [earrings], wrists [watch], periumbilical [belt]). These scenarios usually can be diagnosed by the astute clinician without patch testing; however, the source of exposure may be less obvious if the nickel-releasing item has intermittent contact with the skin (eg, coins in the pocket, furniture hardware, personal grooming devices).13 Other reported exposures include facial dermatitis from mobile phones, dermatitis of the ulnar hands from laptop use, and hand dermatitis from gaming controllers,14-16 perhaps another reason for some to unplug.
Systemic
Sensitized individuals also may present with systemic contact dermatitis after airborne, oral, mucosal, or intravenous exposure. Presentations vary but have been reported to manifest as flare-up reactions in previously affected areas, pompholyx, diffuse dermatitis, flexural dermatitis, and baboon syndrome.17 Although it is unknown if airborne exposure alone is sufficient for sensitization, cases have been reported in occupational settings.18 One report described a man presenting with widespread dermatitis involving the extremities, chest, and genital area after his first day working at an electroplating plant.19
Systemic contact dermatitis from foods high in nickel (eg, chocolate, sunflower seeds, whole-grain flour, dried beans) and occasionally nonfood items (eg, coins) also has occurred. The so-called Easter egg hunt dermatitis has been described in children with Ni-ACD after candy ingestion.20 Another case described an 8-year-old girl and budding illusionist with severe diffuse dermatitis; a thorough history revealed the dermatitis began after she ingested a coin while performing a magic trick.21
Cases of nickel systemic contact dermatitis have been reported following medical device implantation, including reactions to cardiac devices, orthopedic implants, neurosurgery materials, and others.22 In addition, both intraoral and extraoral manifestations following application of orthodontic materials and dental implants have been reported.23,24 Although nickel-containing medical devices generally are well tolerated even in nickel-sensitive individuals, the development of systemic Ni-ACD has at times required device or hardware removal.22,23
After the Patch Test: Avoidance of Nickel
Counseling patients on nickel avoidance is critical to clinical improvement. Common nickel-containing items include jewelry, metal on clothing (eg, zippers, clasps, grommets), belt buckles, watches, glasses, furniture, coins, and keys. Numerous personal care products may also contain nickel, including nail clippers, eyelash curlers, tweezers, mascara tubes, and razors.25,26 Patients should be made aware that nickel-free alternatives are available for the majority of these products. Internet-based tips such as painting nail polish on products or iron-on patches tend to be of limited use in our experience. Patients may consider purchasing a nickel spot test to detect nickel in their environment; the dimethylglyoxime nickel spot test is inexpensive, rapid, and easy-to-use. To use the test, a small amount of the chemical is rubbed on the metal item using a cotton swab; a pink color indicates nickel release. Patients can be reassured that dimethylglyoxime does not harm jewelry.
Some general advice for patients regarding jewelry, the most common source of nickel exposure, is to only wear jewelry that is made from metals such as surgical-grade stainless steel, pure sterling silver, or platinum. If yellow gold is the preferred metal, it is prudent to be aware that lower karat items could potentially contain nickel. White gold should be avoided, as it often contains nickel to contribute to its color. Finally, gold-plated jewelry should be avoided, as the plating can wear off and expose a possibly nickel-containing base.
A low-nickel diet may be of benefit in select patients. A meta-analysis assessing systemic contact dermatitis from nickel ingestion found that 1% of nickel-sensitive individuals may be expected to react to nickel found in a normal diet.27 However, as with any diet, adherence can be difficult. Thankfully, Mislankar and Zirwas28 have developed a simple point-based system to help increase compliance. Additionally, a free mobile application is now available; Nickel Navigator can be used to track daily nickel intake and may be especially convenient for our more tech-savvy patients. In conjunction with a low-nickel diet, some authors also recommend eating meals high in vitamin C or supplementation with vitamin C, as co-ingestion has been shown to reduce nickel absorption.29
Final Interpretation
Nickel allergy remains common, found in up to 17.5% of patch tested patients. Despite regulation in the EU, nickel continues to have high prevalence of positive patch test reactions around the world. Nickel is not only found in jewelry and belt buckles but also in personal care products, electronics, and food. Allergen avoidance is key and requires knowledge of common items containing nickel and a low nickel diet for select patients.
Nickel is unrivaled as the most common cause of contact allergy worldwide.1 Nickel is commonly used as a hardening agent in metal products, and complete avoidance is challenging due to numerous potential exposures (eg, direct contact, airborne, dietary, medical implantation). Allergic contact dermatitis to nickel (Ni-ACD) can lead to decreased quality of life, inability to work, and considerable health care expenses.1 Here, we review the epidemiology of nickel allergy, regulation of nickel in the United States and Europe, common clinical presentations, and pearls on avoidance.
Epidemiology
Nickel continues to be the most common cause of contact allergy worldwide. Data from the 2015-2016 North American Contact Dermatitis Group patch test cycle (N=5597) showed nickel sulfate to be positive in 17.5% of patients patch tested to nickel.2 The prevalence of nickel allergy has been relatively stable in North America since 2005 (Figure 1). Although Ni-ACD historically was identified as an occupational disease of the hands in male nickel platers, the epidemiology of nickel allergy has shifted.1 Today, most cases are nonoccupational and affect women more often than men,3 in part due to improved industrial hygiene, pervasive incorporation of nickel in consumer items, and differences in cultural practices such as piercings.1,3 Piercings in particular have been implicated as important sources of nickel exposure, as this practice disrupts normal skin barrier function and is a potentially sensitizing event. Multiple studies including a large-scale epidemiologic analysis from 2017 have found piercings to be associated with an increased frequency of Ni-ACD (24.4% with piercing vs 9.6% without piercing). Interestingly, the degree of nickel sensitivity also was found to increase with the number of piercings (14.3% with 1 piercing vs 34.0% with ≥5 piercings).4

Regulation
Nickel content has been regulated in parts of the European Union (EU) since the 1990s, but regulation in the United States is lacking. In an attempt to reduce the prevalence of nickel allergy, the EU limits the level of nickel release from consumer items intended to be in direct and prolonged contact with the skin. These limits were first introduced in Denmark in 1990, followed closely by the EU Nickel Directive in 1994, which has resulted in consistent patterns of decreasing prevalence of Ni-ACD in multiple European countries.5 Notably, a Danish study comparing the prevalence of sensitization between girls with ears pierced before vs after implementation of nickel regulation found a decrease in prevalence from 17.1% to 3.9%.6 Additionally, this initiative has greatly reduced the economic burden of nickel dermatitis. It is estimated that Denmark alone has saved US $2 billion over a 20-year period in both direct and indirect health care costs.7
However, a policy is only effective if it is enforced, and it has been reported in the EU that 8% to 32% of tested jewelry exceeds the limit placed on nickel release, with imported jewelry being especially problematic.5 Also of interest, the 1 and 2 euro coins are known to release more nickel than pure nickel itself, releasing 240 to 320 times more than is allowed under the EU Nickel Directive (Figure 2).8 Although coins are not explicitly mentioned as items having prolonged contact with the skin, they can and do exacerbate allergic contact dermatitis of the hands, especially in occupational groups such as cashiers.9 Unsurprisingly, during the discussions to determine the composition of coins prior to the mass adoption of the euro in the EU in 2002, dermatologists and nickel industry experts remained divided in their recommendations.10 However, the EU regulation is considered a public health success overall, and the trends of Ni-ACD and economic burden are opposite of the United States, where legislation has yet to be adopted.

Patch Testing to Nickel
In North America, the 2 available patch test systems are the chamber method and the Thin-layer Rapid Use Epicutaneous (T.R.U.E.) test (SmartPractice). In the T.R.U.E. test, nickel sulfate is used to formulate the patch at 200 µg/cm2 using hydroxypropyl cellulose as the gel vehicle. In the chamber method, nickel sulfate is used on either an aluminum or plastic chamber, most commonly at concentrations of 2.5% or 5% in petrolatum. Nickel sulfate 2.5% is most frequently used in US-based patch test clinics. A 2018 study (N=205) comparing the sensitivities of the 2.5% and 5% concentrations of nickel found 5% to be more sensitive; 31% of the cohort tested positive at 5% but only 20% at 2.5%, suggesting the 5% formulation is superior at detecting nickel allergy.11
Similar to other metals, nickel may react later than other allergens. A 2019 analysis of the prevalence of new patch test reactions on day 7 showed that 17% of 607 patients were negative on day 3 but were positive on day 7, further emphasizing the importance of a properly timed delayed reading.12
Clinical Presentation
Localized
The classic presentation of Ni-ACD is a scaly erythematous dermatitis in a typical distribution (eg, earlobes [earrings], wrists [watch], periumbilical [belt]). These scenarios usually can be diagnosed by the astute clinician without patch testing; however, the source of exposure may be less obvious if the nickel-releasing item has intermittent contact with the skin (eg, coins in the pocket, furniture hardware, personal grooming devices).13 Other reported exposures include facial dermatitis from mobile phones, dermatitis of the ulnar hands from laptop use, and hand dermatitis from gaming controllers,14-16 perhaps another reason for some to unplug.
Systemic
Sensitized individuals also may present with systemic contact dermatitis after airborne, oral, mucosal, or intravenous exposure. Presentations vary but have been reported to manifest as flare-up reactions in previously affected areas, pompholyx, diffuse dermatitis, flexural dermatitis, and baboon syndrome.17 Although it is unknown if airborne exposure alone is sufficient for sensitization, cases have been reported in occupational settings.18 One report described a man presenting with widespread dermatitis involving the extremities, chest, and genital area after his first day working at an electroplating plant.19
Systemic contact dermatitis from foods high in nickel (eg, chocolate, sunflower seeds, whole-grain flour, dried beans) and occasionally nonfood items (eg, coins) also has occurred. The so-called Easter egg hunt dermatitis has been described in children with Ni-ACD after candy ingestion.20 Another case described an 8-year-old girl and budding illusionist with severe diffuse dermatitis; a thorough history revealed the dermatitis began after she ingested a coin while performing a magic trick.21
Cases of nickel systemic contact dermatitis have been reported following medical device implantation, including reactions to cardiac devices, orthopedic implants, neurosurgery materials, and others.22 In addition, both intraoral and extraoral manifestations following application of orthodontic materials and dental implants have been reported.23,24 Although nickel-containing medical devices generally are well tolerated even in nickel-sensitive individuals, the development of systemic Ni-ACD has at times required device or hardware removal.22,23
After the Patch Test: Avoidance of Nickel
Counseling patients on nickel avoidance is critical to clinical improvement. Common nickel-containing items include jewelry, metal on clothing (eg, zippers, clasps, grommets), belt buckles, watches, glasses, furniture, coins, and keys. Numerous personal care products may also contain nickel, including nail clippers, eyelash curlers, tweezers, mascara tubes, and razors.25,26 Patients should be made aware that nickel-free alternatives are available for the majority of these products. Internet-based tips such as painting nail polish on products or iron-on patches tend to be of limited use in our experience. Patients may consider purchasing a nickel spot test to detect nickel in their environment; the dimethylglyoxime nickel spot test is inexpensive, rapid, and easy-to-use. To use the test, a small amount of the chemical is rubbed on the metal item using a cotton swab; a pink color indicates nickel release. Patients can be reassured that dimethylglyoxime does not harm jewelry.
Some general advice for patients regarding jewelry, the most common source of nickel exposure, is to only wear jewelry that is made from metals such as surgical-grade stainless steel, pure sterling silver, or platinum. If yellow gold is the preferred metal, it is prudent to be aware that lower karat items could potentially contain nickel. White gold should be avoided, as it often contains nickel to contribute to its color. Finally, gold-plated jewelry should be avoided, as the plating can wear off and expose a possibly nickel-containing base.
A low-nickel diet may be of benefit in select patients. A meta-analysis assessing systemic contact dermatitis from nickel ingestion found that 1% of nickel-sensitive individuals may be expected to react to nickel found in a normal diet.27 However, as with any diet, adherence can be difficult. Thankfully, Mislankar and Zirwas28 have developed a simple point-based system to help increase compliance. Additionally, a free mobile application is now available; Nickel Navigator can be used to track daily nickel intake and may be especially convenient for our more tech-savvy patients. In conjunction with a low-nickel diet, some authors also recommend eating meals high in vitamin C or supplementation with vitamin C, as co-ingestion has been shown to reduce nickel absorption.29
Final Interpretation
Nickel allergy remains common, found in up to 17.5% of patch tested patients. Despite regulation in the EU, nickel continues to have high prevalence of positive patch test reactions around the world. Nickel is not only found in jewelry and belt buckles but also in personal care products, electronics, and food. Allergen avoidance is key and requires knowledge of common items containing nickel and a low nickel diet for select patients.
- Ahlström MG, Thyssen JP, Wennervaldt M, et al. Nickel allergy and allergic contact dermatitis: a clinical review of immunology, epidemiology, exposure, and treatment. Contact Dermatitis. 2019;81:227-241.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group Patch Test Results: 2015-2016. Dermatitis. 2018;29:297-309.
- Thyssen JP, Menné T. Metal allergy—a review on exposures, penetration, genetics, prevalence, and clinical implications. Chem Res Toxicol. 2010;23:309-318.
- Warshaw EM, Aschenbeck KA, DeKoven JG, et al. Piercing and metal sensitivity: extended analysis of the North American Contact Dermatitis Group data, 2007-2014. Dermatitis. 2017;28:333-341.
- Ahlström MG, Thyssen JP, Menné T, et al. Prevalence of nickel allergy in Europe following the EU Nickel Directive—a review. Contact Dermatitis. 2017;77:193-200.
- Jensen CS, Lisby S, Baadsgaard O, et al. Decrease in nickel sensitization in a Danish schoolgirl population with ears pierced after implementation of a nickel-exposure regulation. Br J Dermatol. 2002;146:636-642.
- Serup-Hansen N, Gudum A, Sørensen MM. Valuation of Chemical Related Health Impacts. Danish Environmental Protection Agency. Published 2004. Accessed December 14, 2020. https://www2.mst.dk/udgiv/publications/2004/87-7614-295-7/pdf/87-7614-296-5.pdf
- Nestle FO, Speidel H, Speidel MO. Metallurgy: high nickel release from 1- and 2-euro coins. Nature. 2002;419:132.
- Kanerva L, Estlander T, Jolanki R. Bank clerk’s occupational allergic nickel and cobalt contact dermatitis from coins. Contact Dermatitis. 1998;38:217-218.
- Aberer W. Platitudes in allergy—based on the example of the euro. Contact Dermatitis. 2001;45:254-255.
- Goldminz AM, Scheinman PL. Comparison of nickel sulfate 2.5% and nickel sulfate 5% for detecting nickel contact allergy. Dermatitis. 2018;29:321-323.
- van Amerongen CCA, Ofenloch R, Dittmar D, et al. New positive patch test reactions on day 7—the additional value of the day 7 patch test reading. Contact Dermatitis. 2019;81:280-287.
- Silverberg NB, Pelletier JL, Jacob SE, et al; Section of Dermatology, Section on Allergy and Immunology. Nickel allergic contact dermatitis: identification, treatment, and prevention. Pediatrics. 2020;145:E20200628.
- Aquino M, Mucci T, Chong M, et al. Mobile phones: potential sources of nickel and cobalt exposure for metal allergic patients. Pediatr Allergy Immunol Pulmonol. 2013;26:181-186.
- Jensen P, Jellesen MS, Møller P, et al. Nickel allergy and dermatitis following use of a laptop computer. J Am Acad Dermatol. 2012;67:E170-E171.
- Jacob SE. Xbox—a source of nickel exposure in children. Pediatr Dermatol. 2014;31:115-116.
- Menné T, Veien NK. Systemic contact dermatitis. In: Rycroft RJG, Menné T, Frosch PJ, et al, eds. Textbook of Contact Dermatitis. Springer; 2001:355-366.
- Mann E, Ranft U, Eberwein G, et al. Does airborne nickel exposure induce nickel sensitization? Contact Dermatitis. 2010;62:355-362.
- Candura SM, Locatelli C, Butera R, et al. Widespread nickel dermatitis from inhalation. Contact Dermatitis. 2001;45:174-175.
- Jacob SE, Hamann D, Goldenberg A, et al. Easter egg hunt dermatitis: systemic allergic contact dermatitis associated with chocolate ingestion. Pediatr Dermatol. 2015;32:231-233.
- Mahdi G, Israel DM, Hassall E. Nickel dermatitis and associated gastritis after coin ingestion. J Pediatr Gastroenterol Nutr. 1996;23:74-76.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Schultz JC, Connelly E, Glesne L, et al. Cutaneous and oral eruption from oral exposure to nickel in dental braces. Dermatitis. 2004;15:154-157.
- Pigatto PD, Brambilla L, Ferrucci S, et al. Systemic allergic contact dermatitis associated with allergy to intraoral metals. Dermatol Online J. 2014;20:13030/qt74632201.
- Brandrup F. Nickel eyelid dermatitis from an eyelash curler. Contact Dermatitis. 1991;25:77.
- Walsh G, Wilkinson SM. Materials and allergens within spectacle frames: a review. Contact Dermatitis. 2006;55:130-139.
- Bergman D, Goldenberg A, Rundle C, et al. Low nickel diet: a patient-centered review [published May 24, 2016]. J Clin Exp Dermatol Res. doi:10.4172/2155-9554.1000355
- Mislankar M, Zirwas MJ. Low-nickel diet scoring system for systemic nickel allergy. Dermatitis. 2013;24:190-195.
- Zirwas MJ, Molenda MA. Dietary nickel as a cause of systemic contact dermatitis. J Clin Aesthet Dermatol. 2009;2:39-43.
- Ahlström MG, Thyssen JP, Wennervaldt M, et al. Nickel allergy and allergic contact dermatitis: a clinical review of immunology, epidemiology, exposure, and treatment. Contact Dermatitis. 2019;81:227-241.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group Patch Test Results: 2015-2016. Dermatitis. 2018;29:297-309.
- Thyssen JP, Menné T. Metal allergy—a review on exposures, penetration, genetics, prevalence, and clinical implications. Chem Res Toxicol. 2010;23:309-318.
- Warshaw EM, Aschenbeck KA, DeKoven JG, et al. Piercing and metal sensitivity: extended analysis of the North American Contact Dermatitis Group data, 2007-2014. Dermatitis. 2017;28:333-341.
- Ahlström MG, Thyssen JP, Menné T, et al. Prevalence of nickel allergy in Europe following the EU Nickel Directive—a review. Contact Dermatitis. 2017;77:193-200.
- Jensen CS, Lisby S, Baadsgaard O, et al. Decrease in nickel sensitization in a Danish schoolgirl population with ears pierced after implementation of a nickel-exposure regulation. Br J Dermatol. 2002;146:636-642.
- Serup-Hansen N, Gudum A, Sørensen MM. Valuation of Chemical Related Health Impacts. Danish Environmental Protection Agency. Published 2004. Accessed December 14, 2020. https://www2.mst.dk/udgiv/publications/2004/87-7614-295-7/pdf/87-7614-296-5.pdf
- Nestle FO, Speidel H, Speidel MO. Metallurgy: high nickel release from 1- and 2-euro coins. Nature. 2002;419:132.
- Kanerva L, Estlander T, Jolanki R. Bank clerk’s occupational allergic nickel and cobalt contact dermatitis from coins. Contact Dermatitis. 1998;38:217-218.
- Aberer W. Platitudes in allergy—based on the example of the euro. Contact Dermatitis. 2001;45:254-255.
- Goldminz AM, Scheinman PL. Comparison of nickel sulfate 2.5% and nickel sulfate 5% for detecting nickel contact allergy. Dermatitis. 2018;29:321-323.
- van Amerongen CCA, Ofenloch R, Dittmar D, et al. New positive patch test reactions on day 7—the additional value of the day 7 patch test reading. Contact Dermatitis. 2019;81:280-287.
- Silverberg NB, Pelletier JL, Jacob SE, et al; Section of Dermatology, Section on Allergy and Immunology. Nickel allergic contact dermatitis: identification, treatment, and prevention. Pediatrics. 2020;145:E20200628.
- Aquino M, Mucci T, Chong M, et al. Mobile phones: potential sources of nickel and cobalt exposure for metal allergic patients. Pediatr Allergy Immunol Pulmonol. 2013;26:181-186.
- Jensen P, Jellesen MS, Møller P, et al. Nickel allergy and dermatitis following use of a laptop computer. J Am Acad Dermatol. 2012;67:E170-E171.
- Jacob SE. Xbox—a source of nickel exposure in children. Pediatr Dermatol. 2014;31:115-116.
- Menné T, Veien NK. Systemic contact dermatitis. In: Rycroft RJG, Menné T, Frosch PJ, et al, eds. Textbook of Contact Dermatitis. Springer; 2001:355-366.
- Mann E, Ranft U, Eberwein G, et al. Does airborne nickel exposure induce nickel sensitization? Contact Dermatitis. 2010;62:355-362.
- Candura SM, Locatelli C, Butera R, et al. Widespread nickel dermatitis from inhalation. Contact Dermatitis. 2001;45:174-175.
- Jacob SE, Hamann D, Goldenberg A, et al. Easter egg hunt dermatitis: systemic allergic contact dermatitis associated with chocolate ingestion. Pediatr Dermatol. 2015;32:231-233.
- Mahdi G, Israel DM, Hassall E. Nickel dermatitis and associated gastritis after coin ingestion. J Pediatr Gastroenterol Nutr. 1996;23:74-76.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Schultz JC, Connelly E, Glesne L, et al. Cutaneous and oral eruption from oral exposure to nickel in dental braces. Dermatitis. 2004;15:154-157.
- Pigatto PD, Brambilla L, Ferrucci S, et al. Systemic allergic contact dermatitis associated with allergy to intraoral metals. Dermatol Online J. 2014;20:13030/qt74632201.
- Brandrup F. Nickel eyelid dermatitis from an eyelash curler. Contact Dermatitis. 1991;25:77.
- Walsh G, Wilkinson SM. Materials and allergens within spectacle frames: a review. Contact Dermatitis. 2006;55:130-139.
- Bergman D, Goldenberg A, Rundle C, et al. Low nickel diet: a patient-centered review [published May 24, 2016]. J Clin Exp Dermatol Res. doi:10.4172/2155-9554.1000355
- Mislankar M, Zirwas MJ. Low-nickel diet scoring system for systemic nickel allergy. Dermatitis. 2013;24:190-195.
- Zirwas MJ, Molenda MA. Dietary nickel as a cause of systemic contact dermatitis. J Clin Aesthet Dermatol. 2009;2:39-43.
Practice Points
- Nickel is the most common cause of contact allergy worldwide. It is ubiquitous in our daily environment, making avoidance challenging.
- Nickel allergic contact dermatitis typically presents in a localized distribution but also can present as systemic contact dermatitis.
- Nickel regulation has been adopted in Europe, but similar legislation does not exist in the United States.
Patch Testing 101, Part 2: After the Patch Test
The first part of this 2-part series addressed the basics of patch testing, including patch test systems, allergens, and patch test readings. In the second part of this series, we examine the incredibly important and absolutely vital steps that come after the patch test: determining relevance, patient counseling, and identifying allergen-free products for patient use. Let’s dive in!
Determining Relevance
The purpose of determining relevance is to assess whether the positive patch test explains the patient’s dermatitis. It is important to consider all of the patient’s exposures, including at home, at work, and during recreational activities. Several relevance grading scales exist. The North American Contact Dermatitis Group grades relevance as current, past, or unknown. Current relevance is further divided into definite, probable, and possible.1 Table 1 includes explanations and clinical examples of each relevance type.
True relevance is only known weeks or months after patch testing is complete. If the patient avoids allergens and is subsequently free of dermatitis, the allergens identified through patch testing were relevant. However, if the patient avoids allergens and sees no improvement in dermatitis, the allergens were not relevant. Gipson et al2 analyzed relevance as documented by the physician at final patch test reading vs patient opinion of relevance 30 days to 3 years after the final reading and found that there was variable agreement between the 2 groups; percentage agreement for formaldehyde-releasing preservatives was 88%, neomycin was 78%, nickel was 71%, fragrances was 65%, and gold was 56%. These differences underscore the need for ongoing research on patch test methods, determination of relevance, and standards for patient follow-up.2
Patient Counseling
Patient counseling is one of the most important and complex parts of patch testing. We have consulted with patients who had already completed patch testing with other providers but did not receive comprehensive allergen counseling and therefore did not improve. It is up to you to explain positive allergens to your patients in a way that they understand, can retain long-term, and can use to their advantage to keep their skin free of dermatitis, which is an incredibly difficult feat to accomplish. The resources that we describe next are the very basic requirements for proficient patch testing.
There are several tools that can be utilized to develop patch test counseling skills (Table 2). Membership with the American Contact Dermatitis Society (ACDS) includes opportunities for virtual and in-person (post–coronavirus disease 2019) lectures and conferences, videos, patch test support information, and patient resources. The European Society of Contact Dermatitis is similar, with a focus on European-based patch testers. Both societies are affiliated with academic journals—Dermatitis and Contact Dermatitis, respectively—which are phenomenal educational resources. Dermatitis Academy (https://www.dermatitisacademy.com) and Contact Dermatitis Institute (https://www.contactdermatitisinstitute.com) are websites that are privately designed and managed by US-based patch test experts.

Allergen Information Handouts
Allergen information should be presented in both verbal and written formats as well as in the patient’s preferred language and education level. Patch test counseling is detailed and complex. Patients rarely remember everything that is discussed; written information allows them to review again when necessary. Allergen information sheets typically include the name of the allergen, alternative names, types of products that might contain the allergen, and other pertinent facts. They also can be helpful for the physician who does not patch test full time; in this case, they can be used as a quick reference to guide patient counseling. It is helpful to highlight or underline important points and make notes when relevant. Importantly, reviewing information sheets with the patient allows time for questions.
Allergen information sheets are provided by manufacturers of patch test materials, including SmartPractice (allergEAZE, T.R.U.E. Test) and Chemotechnique (Dormer)(Table 2). The ACDS also provides a selection of allergen information sheets for members to share with their patients. The ACDS allergen handouts are designed for patient use, are vetted by practicing patch test dermatologists, and contain up-to-date information for patients. We recommend that you choose the handout(s) that are most appropriate for your patient; this decision can be made based on patient education or reading level, the region of the world where you are patch testing or where the patient lives, the patient’s primary language, and the specific allergen. Information on rare or new allergens may not be available on every website resource.
Identification of Allergen-Free Products
We ask patients to bring their personal care products to their patch test reading visit, and once positive allergens are known, we search for the presence of that allergen in their products. It is helpful for patients if products that are “safe” and “not safe” are sorted for them. We frequently emphasize that just one exposure to an allergen in a personal care product can be the source of the dermatitis. If a product label does not include ingredients, they often can be identified with a quick web search (use your favorite search engine or see Table 2 for websites); however, caution is advised, as lists found online may not match those found on in-store products.3 Reviewing the patient’s own products in the clinic is preferred over searching for ingredient lists online. If the product’s ingredients cannot be found (eg, ingredients that are found on external packaging), the patient has several choices: do not use, complete repeat open application testing if it is a leave-on product, or check to see if it is on a product database safe list.
We explain to patients that once they have confirmed that they are using only “safe” allergen-free products, it can take up to 6 to 8 weeks for dermatitis to improve, and at that point, the skin may only be about 75% to 80% clear. A clear description of what to expect and when is needed for a strong patient-physician partnership. For example, if the patient expects to be clear in 2 days but is not and stops avoiding their allergens because they think the process has failed, their dermatitis will not improve.
Product Databases
Because allergens sometimes have multiple different chemical names and cross-reactivity is abundant, avoidance of both the allergen and cross-reactors can be daunting for many patients (and dermatologists!). The use of a product database to aid in product selection is an invaluable resource. Product databases help patients avoid not only their allergens but also common cross-reactors by relying on complex cross-reactor programming. The ACDS owns and maintains the Contact Allergy Management Program (CAMP). Another resource is SkinSafe, which is powered by HER Inc and developed with the Mayo Clinic. Both CAMP and SkinSafe have mobile apps and update product lists frequently; they allow for much easier shopping and identification of safe products.
We typically use CAMP for generation of patient safe lists. We enter the patient’s allergens into the database, and a safe list is generated and shared with the patient. Next, we educate the patient on how to use the safe list. It is vital that the concept of exact product matching be explained to patients, as not all products from one brand or type of product is necessarily safe for a given individual. We also share information on how to download the CAMP app onto mobile devices and tablets.
Product safe lists are important resources for patients to be successful in avoiding allergens but are not a substitute for reading labels. Both CAMP and SkinSafe can potentially contain ingredient list errors due to companies frequently changing their product formulations.3 Although safe lists are an important part in selecting safe skin care products, they are not a substitute for label reading.
Counseling Pitfalls and Pearls
Language
Chemotechnique handouts are available in English, Swedish, French, and Spanish, and ACDS handouts are available in English and Spanish. If language interpretation is needed, inform the interpreter before the visit begins that you will be discussing patch test information and products so they can carefully interpret the details of the discussion.
Barriers to Allergen Avoidance
There are several barriers to long-term avoidance of contact allergy. In a European-based study of methylisothiazolinone (MI) contact allergy 2 to 5 years after patch testing, challenges described by patients included label reading, verifying products, difficulty obtaining ingredients of industrial products, the need to have their “safe” products always available for use, remembering allergen name, avoiding workplace allergens, finding acceptable MI-free products, and navigating the cost of MI-free products.4
Patient allergen recall is a well-documented long-term concern. In the previously mentioned European study (N=139), 11% of patients identified remembering the allergen name as a contributor to difficulty with avoidance.4 A Swedish study evaluated patient allergen recall at 1, 5, and 10 years after patch testing was completed; 96% of 252 patients remembered that they had completed patch testing, 79% (111/141) remembered that they had positive results, and only 29% (41/141) correctly recalled their allergens.5 Patients who had completed patch testing 10 years prior were less likely to correctly recall their allergens (P=.0045). Recall also was less likely if there was more than 1 allergen as well as in males.5 Korkmaz and Boyvat6 analyzed outcomes 6 months after patch testing in Turkey and found that 38 of 51 (74.5%) correctly recalled their allergens. Patients with more than 1 positive allergen were less likely to recall their allergens (P=.046), and patients with higher baseline investigator global assessment (P=.036) and dermatology life quality index (P=.041) scores were more likely to recall their allergens.6 A US-based study (N=757) noted that 34.1% of patients correctly recalled all of their allergens.7 Patients were less likely to remember if they had 3 or more positives but were more likely to remember if they were aged 50 to 59 years (compared to other age groups) or female as well as if their occupation was nursing (as compared to other occupations).
Additional barriers include hidden sources of allergens, as has been reported in the cases of undeclared MI8 and formaldehyde9 in personal care products. Although this phenomenon is thought to be the exception and not the rule, possible reasons for the presence of these undeclared allergens include their use as preservatives in raw materials,8,9 or in the case of formaldehyde, theorized release from product packaging or auto-oxidation and degradation of other chemicals present within the product.9
Readers may recall that we mentioned the option of identifying product ingredients with online search engines or databases, but it is not a perfect system. Comstock and Reeder3 reviewed and compared online ingredient lists from Amazon and several product databases to products taken off shelves at Target and Walgreens and found that 27.7% of online ingredient lists did not match the in-store labels.3 These differences likely are due to changes in product formulations, ingredient variability based on production site, outdated product on store shelves, or data entry error and may not be entirely avoidable. Regardless, patch test experts should be aware of this possibility. When in doubt, always check the product’s original packaging.
Finally, the elephant in the room: We challenge you, as dermatologists and patch test enthusiasts, to name all of the formaldehyde releasers or perhaps declare whether linalool and hydroxycitronellol are fragrances, preservatives, or surfactants. How about naming the relationship between cocamidopropyl betaine, amidoamine, and dimethylaminopropylamine? Difficult stuff, right? And we are medical specialists. It is downright impossible for many of our patients to memorize the names of these chemicals, let alone know their cross-reactors or other important chemical relationships. We mention that providing a safe list is part of patient counseling, but we bring up this knowledge gap to illustrate that patch testing without providing resources to select safe care products is almost as bad as not patch testing at all because in many cases patients may be left without the tools they need to be successful. Do not let this be your downfall!
Final Interpretation
The most challenging and nuanced part of patch testing happens after the actual patch test: assessment of relevance, allergen counseling, and identification of appropriate products for patient use. You now have the tools to successfully counsel your patients after patch testing; get to it!
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Gipson KA, Carlson SW, Nedorost ST. Physician-patient agreement in the assessment of allergen relevance. Dermatitis. 2010;21:275-279.
- Comstock JR, Reeder MJ. Accuracy of product ingredient labeling: comparing drugstore products with online databases and online retailers. Dermatitis. 2020;31:106-111.
- Bouschon P, Waton J, Pereira B, et al. Methylisothiazolinone allergic contact dermatitis: assessment of relapses in 139 patients after avoidance advice. Contact Dermatitis. 2019;80:304-310.
- Jamil WN, Erikssohn I, Lindberg M. How well is the outcome of patch testing remembered by the patients? a 10-year follow-up of testing with the Swedish baseline series at the department of dermatology in Örebro, Sweden. Contact Dermatitis. 2012;66:215-220.
- Korkmaz P, Boyvat A. Effect of patch testing on the course of allergic contact dermatitis and prognostic factors that influence outcomes. Dermatitis. 2019;30:135-141.
- Scalf LA, Genebriera J, Davis MD, et al. Patients’ perceptions of the usefulness and outcome of patch testing. J Am Acad Dermatol. 2007;56:928-932.
- Kerre S, Naessens T, Theunis M, et al. Facial dermatitis caused by undeclared methylisothiazolinone in a gel mask: is the preservation of raw materials in cosmetics a cause of concern? Contact Dermatitis. 2018;78:421-424.
- Nikle A, Ericson M, Warshaw E. Formaldehyde release from personal care products: chromotropic acid method analysis. Dermatitis. 2019;30:67-73.
The first part of this 2-part series addressed the basics of patch testing, including patch test systems, allergens, and patch test readings. In the second part of this series, we examine the incredibly important and absolutely vital steps that come after the patch test: determining relevance, patient counseling, and identifying allergen-free products for patient use. Let’s dive in!
Determining Relevance
The purpose of determining relevance is to assess whether the positive patch test explains the patient’s dermatitis. It is important to consider all of the patient’s exposures, including at home, at work, and during recreational activities. Several relevance grading scales exist. The North American Contact Dermatitis Group grades relevance as current, past, or unknown. Current relevance is further divided into definite, probable, and possible.1 Table 1 includes explanations and clinical examples of each relevance type.

True relevance is only known weeks or months after patch testing is complete. If the patient avoids allergens and is subsequently free of dermatitis, the allergens identified through patch testing were relevant. However, if the patient avoids allergens and sees no improvement in dermatitis, the allergens were not relevant. Gipson et al2 analyzed relevance as documented by the physician at final patch test reading vs patient opinion of relevance 30 days to 3 years after the final reading and found that there was variable agreement between the 2 groups; percentage agreement for formaldehyde-releasing preservatives was 88%, neomycin was 78%, nickel was 71%, fragrances was 65%, and gold was 56%. These differences underscore the need for ongoing research on patch test methods, determination of relevance, and standards for patient follow-up.2
Patient Counseling
Patient counseling is one of the most important and complex parts of patch testing. We have consulted with patients who had already completed patch testing with other providers but did not receive comprehensive allergen counseling and therefore did not improve. It is up to you to explain positive allergens to your patients in a way that they understand, can retain long-term, and can use to their advantage to keep their skin free of dermatitis, which is an incredibly difficult feat to accomplish. The resources that we describe next are the very basic requirements for proficient patch testing.
There are several tools that can be utilized to develop patch test counseling skills (Table 2). Membership with the American Contact Dermatitis Society (ACDS) includes opportunities for virtual and in-person (post–coronavirus disease 2019) lectures and conferences, videos, patch test support information, and patient resources. The European Society of Contact Dermatitis is similar, with a focus on European-based patch testers. Both societies are affiliated with academic journals—Dermatitis and Contact Dermatitis, respectively—which are phenomenal educational resources. Dermatitis Academy (https://www.dermatitisacademy.com) and Contact Dermatitis Institute (https://www.contactdermatitisinstitute.com) are websites that are privately designed and managed by US-based patch test experts.

Allergen Information Handouts
Allergen information should be presented in both verbal and written formats as well as in the patient’s preferred language and education level. Patch test counseling is detailed and complex. Patients rarely remember everything that is discussed; written information allows them to review again when necessary. Allergen information sheets typically include the name of the allergen, alternative names, types of products that might contain the allergen, and other pertinent facts. They also can be helpful for the physician who does not patch test full time; in this case, they can be used as a quick reference to guide patient counseling. It is helpful to highlight or underline important points and make notes when relevant. Importantly, reviewing information sheets with the patient allows time for questions.
Allergen information sheets are provided by manufacturers of patch test materials, including SmartPractice (allergEAZE, T.R.U.E. Test) and Chemotechnique (Dormer)(Table 2). The ACDS also provides a selection of allergen information sheets for members to share with their patients. The ACDS allergen handouts are designed for patient use, are vetted by practicing patch test dermatologists, and contain up-to-date information for patients. We recommend that you choose the handout(s) that are most appropriate for your patient; this decision can be made based on patient education or reading level, the region of the world where you are patch testing or where the patient lives, the patient’s primary language, and the specific allergen. Information on rare or new allergens may not be available on every website resource.
Identification of Allergen-Free Products
We ask patients to bring their personal care products to their patch test reading visit, and once positive allergens are known, we search for the presence of that allergen in their products. It is helpful for patients if products that are “safe” and “not safe” are sorted for them. We frequently emphasize that just one exposure to an allergen in a personal care product can be the source of the dermatitis. If a product label does not include ingredients, they often can be identified with a quick web search (use your favorite search engine or see Table 2 for websites); however, caution is advised, as lists found online may not match those found on in-store products.3 Reviewing the patient’s own products in the clinic is preferred over searching for ingredient lists online. If the product’s ingredients cannot be found (eg, ingredients that are found on external packaging), the patient has several choices: do not use, complete repeat open application testing if it is a leave-on product, or check to see if it is on a product database safe list.
We explain to patients that once they have confirmed that they are using only “safe” allergen-free products, it can take up to 6 to 8 weeks for dermatitis to improve, and at that point, the skin may only be about 75% to 80% clear. A clear description of what to expect and when is needed for a strong patient-physician partnership. For example, if the patient expects to be clear in 2 days but is not and stops avoiding their allergens because they think the process has failed, their dermatitis will not improve.
Product Databases
Because allergens sometimes have multiple different chemical names and cross-reactivity is abundant, avoidance of both the allergen and cross-reactors can be daunting for many patients (and dermatologists!). The use of a product database to aid in product selection is an invaluable resource. Product databases help patients avoid not only their allergens but also common cross-reactors by relying on complex cross-reactor programming. The ACDS owns and maintains the Contact Allergy Management Program (CAMP). Another resource is SkinSafe, which is powered by HER Inc and developed with the Mayo Clinic. Both CAMP and SkinSafe have mobile apps and update product lists frequently; they allow for much easier shopping and identification of safe products.
We typically use CAMP for generation of patient safe lists. We enter the patient’s allergens into the database, and a safe list is generated and shared with the patient. Next, we educate the patient on how to use the safe list. It is vital that the concept of exact product matching be explained to patients, as not all products from one brand or type of product is necessarily safe for a given individual. We also share information on how to download the CAMP app onto mobile devices and tablets.
Product safe lists are important resources for patients to be successful in avoiding allergens but are not a substitute for reading labels. Both CAMP and SkinSafe can potentially contain ingredient list errors due to companies frequently changing their product formulations.3 Although safe lists are an important part in selecting safe skin care products, they are not a substitute for label reading.
Counseling Pitfalls and Pearls
Language
Chemotechnique handouts are available in English, Swedish, French, and Spanish, and ACDS handouts are available in English and Spanish. If language interpretation is needed, inform the interpreter before the visit begins that you will be discussing patch test information and products so they can carefully interpret the details of the discussion.
Barriers to Allergen Avoidance
There are several barriers to long-term avoidance of contact allergy. In a European-based study of methylisothiazolinone (MI) contact allergy 2 to 5 years after patch testing, challenges described by patients included label reading, verifying products, difficulty obtaining ingredients of industrial products, the need to have their “safe” products always available for use, remembering allergen name, avoiding workplace allergens, finding acceptable MI-free products, and navigating the cost of MI-free products.4
Patient allergen recall is a well-documented long-term concern. In the previously mentioned European study (N=139), 11% of patients identified remembering the allergen name as a contributor to difficulty with avoidance.4 A Swedish study evaluated patient allergen recall at 1, 5, and 10 years after patch testing was completed; 96% of 252 patients remembered that they had completed patch testing, 79% (111/141) remembered that they had positive results, and only 29% (41/141) correctly recalled their allergens.5 Patients who had completed patch testing 10 years prior were less likely to correctly recall their allergens (P=.0045). Recall also was less likely if there was more than 1 allergen as well as in males.5 Korkmaz and Boyvat6 analyzed outcomes 6 months after patch testing in Turkey and found that 38 of 51 (74.5%) correctly recalled their allergens. Patients with more than 1 positive allergen were less likely to recall their allergens (P=.046), and patients with higher baseline investigator global assessment (P=.036) and dermatology life quality index (P=.041) scores were more likely to recall their allergens.6 A US-based study (N=757) noted that 34.1% of patients correctly recalled all of their allergens.7 Patients were less likely to remember if they had 3 or more positives but were more likely to remember if they were aged 50 to 59 years (compared to other age groups) or female as well as if their occupation was nursing (as compared to other occupations).
Additional barriers include hidden sources of allergens, as has been reported in the cases of undeclared MI8 and formaldehyde9 in personal care products. Although this phenomenon is thought to be the exception and not the rule, possible reasons for the presence of these undeclared allergens include their use as preservatives in raw materials,8,9 or in the case of formaldehyde, theorized release from product packaging or auto-oxidation and degradation of other chemicals present within the product.9
Readers may recall that we mentioned the option of identifying product ingredients with online search engines or databases, but it is not a perfect system. Comstock and Reeder3 reviewed and compared online ingredient lists from Amazon and several product databases to products taken off shelves at Target and Walgreens and found that 27.7% of online ingredient lists did not match the in-store labels.3 These differences likely are due to changes in product formulations, ingredient variability based on production site, outdated product on store shelves, or data entry error and may not be entirely avoidable. Regardless, patch test experts should be aware of this possibility. When in doubt, always check the product’s original packaging.
Finally, the elephant in the room: We challenge you, as dermatologists and patch test enthusiasts, to name all of the formaldehyde releasers or perhaps declare whether linalool and hydroxycitronellol are fragrances, preservatives, or surfactants. How about naming the relationship between cocamidopropyl betaine, amidoamine, and dimethylaminopropylamine? Difficult stuff, right? And we are medical specialists. It is downright impossible for many of our patients to memorize the names of these chemicals, let alone know their cross-reactors or other important chemical relationships. We mention that providing a safe list is part of patient counseling, but we bring up this knowledge gap to illustrate that patch testing without providing resources to select safe care products is almost as bad as not patch testing at all because in many cases patients may be left without the tools they need to be successful. Do not let this be your downfall!
Final Interpretation
The most challenging and nuanced part of patch testing happens after the actual patch test: assessment of relevance, allergen counseling, and identification of appropriate products for patient use. You now have the tools to successfully counsel your patients after patch testing; get to it!
The first part of this 2-part series addressed the basics of patch testing, including patch test systems, allergens, and patch test readings. In the second part of this series, we examine the incredibly important and absolutely vital steps that come after the patch test: determining relevance, patient counseling, and identifying allergen-free products for patient use. Let’s dive in!
Determining Relevance
The purpose of determining relevance is to assess whether the positive patch test explains the patient’s dermatitis. It is important to consider all of the patient’s exposures, including at home, at work, and during recreational activities. Several relevance grading scales exist. The North American Contact Dermatitis Group grades relevance as current, past, or unknown. Current relevance is further divided into definite, probable, and possible.1 Table 1 includes explanations and clinical examples of each relevance type.

True relevance is only known weeks or months after patch testing is complete. If the patient avoids allergens and is subsequently free of dermatitis, the allergens identified through patch testing were relevant. However, if the patient avoids allergens and sees no improvement in dermatitis, the allergens were not relevant. Gipson et al2 analyzed relevance as documented by the physician at final patch test reading vs patient opinion of relevance 30 days to 3 years after the final reading and found that there was variable agreement between the 2 groups; percentage agreement for formaldehyde-releasing preservatives was 88%, neomycin was 78%, nickel was 71%, fragrances was 65%, and gold was 56%. These differences underscore the need for ongoing research on patch test methods, determination of relevance, and standards for patient follow-up.2
Patient Counseling
Patient counseling is one of the most important and complex parts of patch testing. We have consulted with patients who had already completed patch testing with other providers but did not receive comprehensive allergen counseling and therefore did not improve. It is up to you to explain positive allergens to your patients in a way that they understand, can retain long-term, and can use to their advantage to keep their skin free of dermatitis, which is an incredibly difficult feat to accomplish. The resources that we describe next are the very basic requirements for proficient patch testing.
There are several tools that can be utilized to develop patch test counseling skills (Table 2). Membership with the American Contact Dermatitis Society (ACDS) includes opportunities for virtual and in-person (post–coronavirus disease 2019) lectures and conferences, videos, patch test support information, and patient resources. The European Society of Contact Dermatitis is similar, with a focus on European-based patch testers. Both societies are affiliated with academic journals—Dermatitis and Contact Dermatitis, respectively—which are phenomenal educational resources. Dermatitis Academy (https://www.dermatitisacademy.com) and Contact Dermatitis Institute (https://www.contactdermatitisinstitute.com) are websites that are privately designed and managed by US-based patch test experts.

Allergen Information Handouts
Allergen information should be presented in both verbal and written formats as well as in the patient’s preferred language and education level. Patch test counseling is detailed and complex. Patients rarely remember everything that is discussed; written information allows them to review again when necessary. Allergen information sheets typically include the name of the allergen, alternative names, types of products that might contain the allergen, and other pertinent facts. They also can be helpful for the physician who does not patch test full time; in this case, they can be used as a quick reference to guide patient counseling. It is helpful to highlight or underline important points and make notes when relevant. Importantly, reviewing information sheets with the patient allows time for questions.
Allergen information sheets are provided by manufacturers of patch test materials, including SmartPractice (allergEAZE, T.R.U.E. Test) and Chemotechnique (Dormer)(Table 2). The ACDS also provides a selection of allergen information sheets for members to share with their patients. The ACDS allergen handouts are designed for patient use, are vetted by practicing patch test dermatologists, and contain up-to-date information for patients. We recommend that you choose the handout(s) that are most appropriate for your patient; this decision can be made based on patient education or reading level, the region of the world where you are patch testing or where the patient lives, the patient’s primary language, and the specific allergen. Information on rare or new allergens may not be available on every website resource.
Identification of Allergen-Free Products
We ask patients to bring their personal care products to their patch test reading visit, and once positive allergens are known, we search for the presence of that allergen in their products. It is helpful for patients if products that are “safe” and “not safe” are sorted for them. We frequently emphasize that just one exposure to an allergen in a personal care product can be the source of the dermatitis. If a product label does not include ingredients, they often can be identified with a quick web search (use your favorite search engine or see Table 2 for websites); however, caution is advised, as lists found online may not match those found on in-store products.3 Reviewing the patient’s own products in the clinic is preferred over searching for ingredient lists online. If the product’s ingredients cannot be found (eg, ingredients that are found on external packaging), the patient has several choices: do not use, complete repeat open application testing if it is a leave-on product, or check to see if it is on a product database safe list.
We explain to patients that once they have confirmed that they are using only “safe” allergen-free products, it can take up to 6 to 8 weeks for dermatitis to improve, and at that point, the skin may only be about 75% to 80% clear. A clear description of what to expect and when is needed for a strong patient-physician partnership. For example, if the patient expects to be clear in 2 days but is not and stops avoiding their allergens because they think the process has failed, their dermatitis will not improve.
Product Databases
Because allergens sometimes have multiple different chemical names and cross-reactivity is abundant, avoidance of both the allergen and cross-reactors can be daunting for many patients (and dermatologists!). The use of a product database to aid in product selection is an invaluable resource. Product databases help patients avoid not only their allergens but also common cross-reactors by relying on complex cross-reactor programming. The ACDS owns and maintains the Contact Allergy Management Program (CAMP). Another resource is SkinSafe, which is powered by HER Inc and developed with the Mayo Clinic. Both CAMP and SkinSafe have mobile apps and update product lists frequently; they allow for much easier shopping and identification of safe products.
We typically use CAMP for generation of patient safe lists. We enter the patient’s allergens into the database, and a safe list is generated and shared with the patient. Next, we educate the patient on how to use the safe list. It is vital that the concept of exact product matching be explained to patients, as not all products from one brand or type of product is necessarily safe for a given individual. We also share information on how to download the CAMP app onto mobile devices and tablets.
Product safe lists are important resources for patients to be successful in avoiding allergens but are not a substitute for reading labels. Both CAMP and SkinSafe can potentially contain ingredient list errors due to companies frequently changing their product formulations.3 Although safe lists are an important part in selecting safe skin care products, they are not a substitute for label reading.
Counseling Pitfalls and Pearls
Language
Chemotechnique handouts are available in English, Swedish, French, and Spanish, and ACDS handouts are available in English and Spanish. If language interpretation is needed, inform the interpreter before the visit begins that you will be discussing patch test information and products so they can carefully interpret the details of the discussion.
Barriers to Allergen Avoidance
There are several barriers to long-term avoidance of contact allergy. In a European-based study of methylisothiazolinone (MI) contact allergy 2 to 5 years after patch testing, challenges described by patients included label reading, verifying products, difficulty obtaining ingredients of industrial products, the need to have their “safe” products always available for use, remembering allergen name, avoiding workplace allergens, finding acceptable MI-free products, and navigating the cost of MI-free products.4
Patient allergen recall is a well-documented long-term concern. In the previously mentioned European study (N=139), 11% of patients identified remembering the allergen name as a contributor to difficulty with avoidance.4 A Swedish study evaluated patient allergen recall at 1, 5, and 10 years after patch testing was completed; 96% of 252 patients remembered that they had completed patch testing, 79% (111/141) remembered that they had positive results, and only 29% (41/141) correctly recalled their allergens.5 Patients who had completed patch testing 10 years prior were less likely to correctly recall their allergens (P=.0045). Recall also was less likely if there was more than 1 allergen as well as in males.5 Korkmaz and Boyvat6 analyzed outcomes 6 months after patch testing in Turkey and found that 38 of 51 (74.5%) correctly recalled their allergens. Patients with more than 1 positive allergen were less likely to recall their allergens (P=.046), and patients with higher baseline investigator global assessment (P=.036) and dermatology life quality index (P=.041) scores were more likely to recall their allergens.6 A US-based study (N=757) noted that 34.1% of patients correctly recalled all of their allergens.7 Patients were less likely to remember if they had 3 or more positives but were more likely to remember if they were aged 50 to 59 years (compared to other age groups) or female as well as if their occupation was nursing (as compared to other occupations).
Additional barriers include hidden sources of allergens, as has been reported in the cases of undeclared MI8 and formaldehyde9 in personal care products. Although this phenomenon is thought to be the exception and not the rule, possible reasons for the presence of these undeclared allergens include their use as preservatives in raw materials,8,9 or in the case of formaldehyde, theorized release from product packaging or auto-oxidation and degradation of other chemicals present within the product.9
Readers may recall that we mentioned the option of identifying product ingredients with online search engines or databases, but it is not a perfect system. Comstock and Reeder3 reviewed and compared online ingredient lists from Amazon and several product databases to products taken off shelves at Target and Walgreens and found that 27.7% of online ingredient lists did not match the in-store labels.3 These differences likely are due to changes in product formulations, ingredient variability based on production site, outdated product on store shelves, or data entry error and may not be entirely avoidable. Regardless, patch test experts should be aware of this possibility. When in doubt, always check the product’s original packaging.
Finally, the elephant in the room: We challenge you, as dermatologists and patch test enthusiasts, to name all of the formaldehyde releasers or perhaps declare whether linalool and hydroxycitronellol are fragrances, preservatives, or surfactants. How about naming the relationship between cocamidopropyl betaine, amidoamine, and dimethylaminopropylamine? Difficult stuff, right? And we are medical specialists. It is downright impossible for many of our patients to memorize the names of these chemicals, let alone know their cross-reactors or other important chemical relationships. We mention that providing a safe list is part of patient counseling, but we bring up this knowledge gap to illustrate that patch testing without providing resources to select safe care products is almost as bad as not patch testing at all because in many cases patients may be left without the tools they need to be successful. Do not let this be your downfall!
Final Interpretation
The most challenging and nuanced part of patch testing happens after the actual patch test: assessment of relevance, allergen counseling, and identification of appropriate products for patient use. You now have the tools to successfully counsel your patients after patch testing; get to it!
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Gipson KA, Carlson SW, Nedorost ST. Physician-patient agreement in the assessment of allergen relevance. Dermatitis. 2010;21:275-279.
- Comstock JR, Reeder MJ. Accuracy of product ingredient labeling: comparing drugstore products with online databases and online retailers. Dermatitis. 2020;31:106-111.
- Bouschon P, Waton J, Pereira B, et al. Methylisothiazolinone allergic contact dermatitis: assessment of relapses in 139 patients after avoidance advice. Contact Dermatitis. 2019;80:304-310.
- Jamil WN, Erikssohn I, Lindberg M. How well is the outcome of patch testing remembered by the patients? a 10-year follow-up of testing with the Swedish baseline series at the department of dermatology in Örebro, Sweden. Contact Dermatitis. 2012;66:215-220.
- Korkmaz P, Boyvat A. Effect of patch testing on the course of allergic contact dermatitis and prognostic factors that influence outcomes. Dermatitis. 2019;30:135-141.
- Scalf LA, Genebriera J, Davis MD, et al. Patients’ perceptions of the usefulness and outcome of patch testing. J Am Acad Dermatol. 2007;56:928-932.
- Kerre S, Naessens T, Theunis M, et al. Facial dermatitis caused by undeclared methylisothiazolinone in a gel mask: is the preservation of raw materials in cosmetics a cause of concern? Contact Dermatitis. 2018;78:421-424.
- Nikle A, Ericson M, Warshaw E. Formaldehyde release from personal care products: chromotropic acid method analysis. Dermatitis. 2019;30:67-73.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Gipson KA, Carlson SW, Nedorost ST. Physician-patient agreement in the assessment of allergen relevance. Dermatitis. 2010;21:275-279.
- Comstock JR, Reeder MJ. Accuracy of product ingredient labeling: comparing drugstore products with online databases and online retailers. Dermatitis. 2020;31:106-111.
- Bouschon P, Waton J, Pereira B, et al. Methylisothiazolinone allergic contact dermatitis: assessment of relapses in 139 patients after avoidance advice. Contact Dermatitis. 2019;80:304-310.
- Jamil WN, Erikssohn I, Lindberg M. How well is the outcome of patch testing remembered by the patients? a 10-year follow-up of testing with the Swedish baseline series at the department of dermatology in Örebro, Sweden. Contact Dermatitis. 2012;66:215-220.
- Korkmaz P, Boyvat A. Effect of patch testing on the course of allergic contact dermatitis and prognostic factors that influence outcomes. Dermatitis. 2019;30:135-141.
- Scalf LA, Genebriera J, Davis MD, et al. Patients’ perceptions of the usefulness and outcome of patch testing. J Am Acad Dermatol. 2007;56:928-932.
- Kerre S, Naessens T, Theunis M, et al. Facial dermatitis caused by undeclared methylisothiazolinone in a gel mask: is the preservation of raw materials in cosmetics a cause of concern? Contact Dermatitis. 2018;78:421-424.
- Nikle A, Ericson M, Warshaw E. Formaldehyde release from personal care products: chromotropic acid method analysis. Dermatitis. 2019;30:67-73.
Practice Points
- Positive patch test reactions must be interpreted in the context of the patient’s exposures, both current and past.
- Allergen information sheets and product database safe lists are invaluable tools to help patients select safe skin care products.
Foreign-Body Reaction to Orthopedic Hardware a Decade After Implantation
To the Editor:
Cutaneous reactions to implantable devices, such as dental implants, intracoronary stents, prosthetic valves, endovascular prostheses, gynecologic devices, and spinal cord stimulator devices, occur with varying frequency and include infectious, hypersensitivity, allergic, and foreign-body reactions. Manifestations have included contact dermatitis; urticarial, vasculitic, and bullous eruptions; extrusion; and granuloma formation.1,2 Immune complex reactions around implants causing pain, inflammation, and loosening of hardwarealso have been reported.3,4 Most reported cutaneous reactions typically occur within the first weeks or months after implantation; a reaction rarely presents several years after implantation. We report a cutaneous reaction to an orthopedic appliance almost 10 years after implantation.
A 67-year-old man presented with 2 painful nodules on the right clavicle that were present for several months. The patient denied fever, chills, weight loss, enlarged lymph nodes, or night sweats. Approximately 10 years prior to the appearance of the nodules, the patient fractured the right clavicle and underwent placement of a metal plate. His medical history included resection of the right tonsil and soft-palate carcinoma with radical neck dissection and postoperative radiation, which was completed approximately 4 years prior to placement of the metal plate. The patient recently completed 4 to 6 weeks of fluorouracil for shave biopsy–proven actinic keratosis overlying the entire irradiated area.
Physical examination revealed 2 pink friable nodules measuring 1.5 to 2.5 cm in diameter and leaking serous fluid within the irradiated area (Figure 1). The differential diagnosis included pyogenic granuloma, cutaneous recurrent metastasis, and atypical basal cell carcinoma. A skin biopsy specimen showed hemorrhagic ulcerated skin with acute and chronic inflammation and abscess.
The patient presented for excisional biopsy of these areas on the right medial clavicle 1 week later. Physical examination revealed the 2 nodules had decreased in diameter; now, however, the patient had 4 discrete lesions measuring 4 to 7 mm in diameter, which were similar in appearance to the earlier nodules (Figure 2). He reported a low-grade fever, erythema, and increased tenderness of the area.
Underlying loosened orthopedic hardware screws were revealed upon punch biopsies of the involved areas (Figure 3). Wound cultures showed abundant Staphylococcus aureus and moderate group B Streptococcus; cultures for Mycobacterium were negative. The C-reactive protein level was elevated (5.47 mg/dL [reference range, ≤0.7 mg/dL]), and the erythrocyte sedimentation rate was increased (68 mm/h [reference range, 0–15 mm/h]). A complete blood cell count was within reference range, except for a mildly elevated eosinophil count (6.7% [reference range, 0%–5%]). The patient was admitted to the hospital, and antibiotics were started. Two days later, the orthopedic surgery service removed the hardware. At 3-week follow-up, physical examination revealed near closure of the wounds.
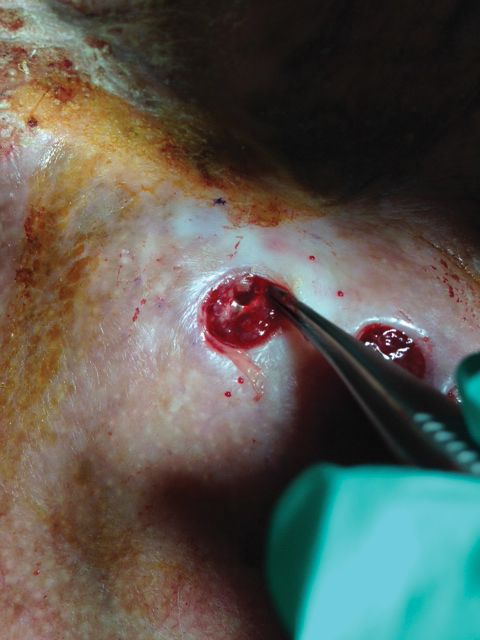
Cutaneous reactions to orthopedic implants include dermatitis, as well as urticarial, vasculitic, and bullous eruptions. Immune complex reactions can develop around implants, causing pain, inflammation, and loosening of hardware.1,3 Most inflammatory reactions take place within several months after implantation.3 Our patient’s reaction to hardware 10 years after implantation highlights the importance of taking a detailedand thorough history that includes queries about distant surgery.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Chaudhry ZA, Najib U, Bajwa ZH, et al. Detailed analysis of allergic cutaneous reactions to spinal cord stimulator devices. J Pain Res. 2013;6:617-623.
- Huber M, Reinisch G, Trettenhahn G, et al. Presence of corrosion products and hypersensitivity-associated reactions in periprosthetic tissue after aseptic loosening of total hip replacements with metal bearing surfaces. Acta Biomater. 2009;5:172-180.
- Poncet-Wallet C, Ormezzano Y, Ernst E, et al. Study of a case of cochlear implant with recurrent cutaneous extrusion. Ann Otolaryngol Chir Cervicofac. 2009;126:264-268.
To the Editor:
Cutaneous reactions to implantable devices, such as dental implants, intracoronary stents, prosthetic valves, endovascular prostheses, gynecologic devices, and spinal cord stimulator devices, occur with varying frequency and include infectious, hypersensitivity, allergic, and foreign-body reactions. Manifestations have included contact dermatitis; urticarial, vasculitic, and bullous eruptions; extrusion; and granuloma formation.1,2 Immune complex reactions around implants causing pain, inflammation, and loosening of hardwarealso have been reported.3,4 Most reported cutaneous reactions typically occur within the first weeks or months after implantation; a reaction rarely presents several years after implantation. We report a cutaneous reaction to an orthopedic appliance almost 10 years after implantation.
A 67-year-old man presented with 2 painful nodules on the right clavicle that were present for several months. The patient denied fever, chills, weight loss, enlarged lymph nodes, or night sweats. Approximately 10 years prior to the appearance of the nodules, the patient fractured the right clavicle and underwent placement of a metal plate. His medical history included resection of the right tonsil and soft-palate carcinoma with radical neck dissection and postoperative radiation, which was completed approximately 4 years prior to placement of the metal plate. The patient recently completed 4 to 6 weeks of fluorouracil for shave biopsy–proven actinic keratosis overlying the entire irradiated area.
Physical examination revealed 2 pink friable nodules measuring 1.5 to 2.5 cm in diameter and leaking serous fluid within the irradiated area (Figure 1). The differential diagnosis included pyogenic granuloma, cutaneous recurrent metastasis, and atypical basal cell carcinoma. A skin biopsy specimen showed hemorrhagic ulcerated skin with acute and chronic inflammation and abscess.

The patient presented for excisional biopsy of these areas on the right medial clavicle 1 week later. Physical examination revealed the 2 nodules had decreased in diameter; now, however, the patient had 4 discrete lesions measuring 4 to 7 mm in diameter, which were similar in appearance to the earlier nodules (Figure 2). He reported a low-grade fever, erythema, and increased tenderness of the area.

Underlying loosened orthopedic hardware screws were revealed upon punch biopsies of the involved areas (Figure 3). Wound cultures showed abundant Staphylococcus aureus and moderate group B Streptococcus; cultures for Mycobacterium were negative. The C-reactive protein level was elevated (5.47 mg/dL [reference range, ≤0.7 mg/dL]), and the erythrocyte sedimentation rate was increased (68 mm/h [reference range, 0–15 mm/h]). A complete blood cell count was within reference range, except for a mildly elevated eosinophil count (6.7% [reference range, 0%–5%]). The patient was admitted to the hospital, and antibiotics were started. Two days later, the orthopedic surgery service removed the hardware. At 3-week follow-up, physical examination revealed near closure of the wounds.

Cutaneous reactions to orthopedic implants include dermatitis, as well as urticarial, vasculitic, and bullous eruptions. Immune complex reactions can develop around implants, causing pain, inflammation, and loosening of hardware.1,3 Most inflammatory reactions take place within several months after implantation.3 Our patient’s reaction to hardware 10 years after implantation highlights the importance of taking a detailedand thorough history that includes queries about distant surgery.
To the Editor:
Cutaneous reactions to implantable devices, such as dental implants, intracoronary stents, prosthetic valves, endovascular prostheses, gynecologic devices, and spinal cord stimulator devices, occur with varying frequency and include infectious, hypersensitivity, allergic, and foreign-body reactions. Manifestations have included contact dermatitis; urticarial, vasculitic, and bullous eruptions; extrusion; and granuloma formation.1,2 Immune complex reactions around implants causing pain, inflammation, and loosening of hardwarealso have been reported.3,4 Most reported cutaneous reactions typically occur within the first weeks or months after implantation; a reaction rarely presents several years after implantation. We report a cutaneous reaction to an orthopedic appliance almost 10 years after implantation.
A 67-year-old man presented with 2 painful nodules on the right clavicle that were present for several months. The patient denied fever, chills, weight loss, enlarged lymph nodes, or night sweats. Approximately 10 years prior to the appearance of the nodules, the patient fractured the right clavicle and underwent placement of a metal plate. His medical history included resection of the right tonsil and soft-palate carcinoma with radical neck dissection and postoperative radiation, which was completed approximately 4 years prior to placement of the metal plate. The patient recently completed 4 to 6 weeks of fluorouracil for shave biopsy–proven actinic keratosis overlying the entire irradiated area.
Physical examination revealed 2 pink friable nodules measuring 1.5 to 2.5 cm in diameter and leaking serous fluid within the irradiated area (Figure 1). The differential diagnosis included pyogenic granuloma, cutaneous recurrent metastasis, and atypical basal cell carcinoma. A skin biopsy specimen showed hemorrhagic ulcerated skin with acute and chronic inflammation and abscess.

The patient presented for excisional biopsy of these areas on the right medial clavicle 1 week later. Physical examination revealed the 2 nodules had decreased in diameter; now, however, the patient had 4 discrete lesions measuring 4 to 7 mm in diameter, which were similar in appearance to the earlier nodules (Figure 2). He reported a low-grade fever, erythema, and increased tenderness of the area.

Underlying loosened orthopedic hardware screws were revealed upon punch biopsies of the involved areas (Figure 3). Wound cultures showed abundant Staphylococcus aureus and moderate group B Streptococcus; cultures for Mycobacterium were negative. The C-reactive protein level was elevated (5.47 mg/dL [reference range, ≤0.7 mg/dL]), and the erythrocyte sedimentation rate was increased (68 mm/h [reference range, 0–15 mm/h]). A complete blood cell count was within reference range, except for a mildly elevated eosinophil count (6.7% [reference range, 0%–5%]). The patient was admitted to the hospital, and antibiotics were started. Two days later, the orthopedic surgery service removed the hardware. At 3-week follow-up, physical examination revealed near closure of the wounds.

Cutaneous reactions to orthopedic implants include dermatitis, as well as urticarial, vasculitic, and bullous eruptions. Immune complex reactions can develop around implants, causing pain, inflammation, and loosening of hardware.1,3 Most inflammatory reactions take place within several months after implantation.3 Our patient’s reaction to hardware 10 years after implantation highlights the importance of taking a detailedand thorough history that includes queries about distant surgery.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Chaudhry ZA, Najib U, Bajwa ZH, et al. Detailed analysis of allergic cutaneous reactions to spinal cord stimulator devices. J Pain Res. 2013;6:617-623.
- Huber M, Reinisch G, Trettenhahn G, et al. Presence of corrosion products and hypersensitivity-associated reactions in periprosthetic tissue after aseptic loosening of total hip replacements with metal bearing surfaces. Acta Biomater. 2009;5:172-180.
- Poncet-Wallet C, Ormezzano Y, Ernst E, et al. Study of a case of cochlear implant with recurrent cutaneous extrusion. Ann Otolaryngol Chir Cervicofac. 2009;126:264-268.
- Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- Chaudhry ZA, Najib U, Bajwa ZH, et al. Detailed analysis of allergic cutaneous reactions to spinal cord stimulator devices. J Pain Res. 2013;6:617-623.
- Huber M, Reinisch G, Trettenhahn G, et al. Presence of corrosion products and hypersensitivity-associated reactions in periprosthetic tissue after aseptic loosening of total hip replacements with metal bearing surfaces. Acta Biomater. 2009;5:172-180.
- Poncet-Wallet C, Ormezzano Y, Ernst E, et al. Study of a case of cochlear implant with recurrent cutaneous extrusion. Ann Otolaryngol Chir Cervicofac. 2009;126:264-268.
Practice Points
- Cutaneous reactions to implantable devices occur with varying frequency and include infectious, hypersensitivity, allergic, and foreign-body reactions.
- Most reactions typically occur within the first weeks or months after implantation; however, a reaction rarely may present several years after implantation.
Umbilicated Neoplasm on the Chest
Dermoscopy showed polylobular, whitish yellow, amorphous structures at the center of the lesion surrounded by a crown of vessels (Figure 1). Histopathology revealed hyperplastic crateriform lesions containing large eosinophilic intracytoplasmic inclusion bodies within keratinocytes (Figure 2). At follow-up 2 weeks after the biopsy, the patient presented with approximately 20 more reddish papules of varying sizes on the abdomen and back that presented as dome-shaped papules and had a typical umbilicated center. The clinical manifestations, dermoscopy, and pathology findings were consistent with molluscum contagiosum (MC).
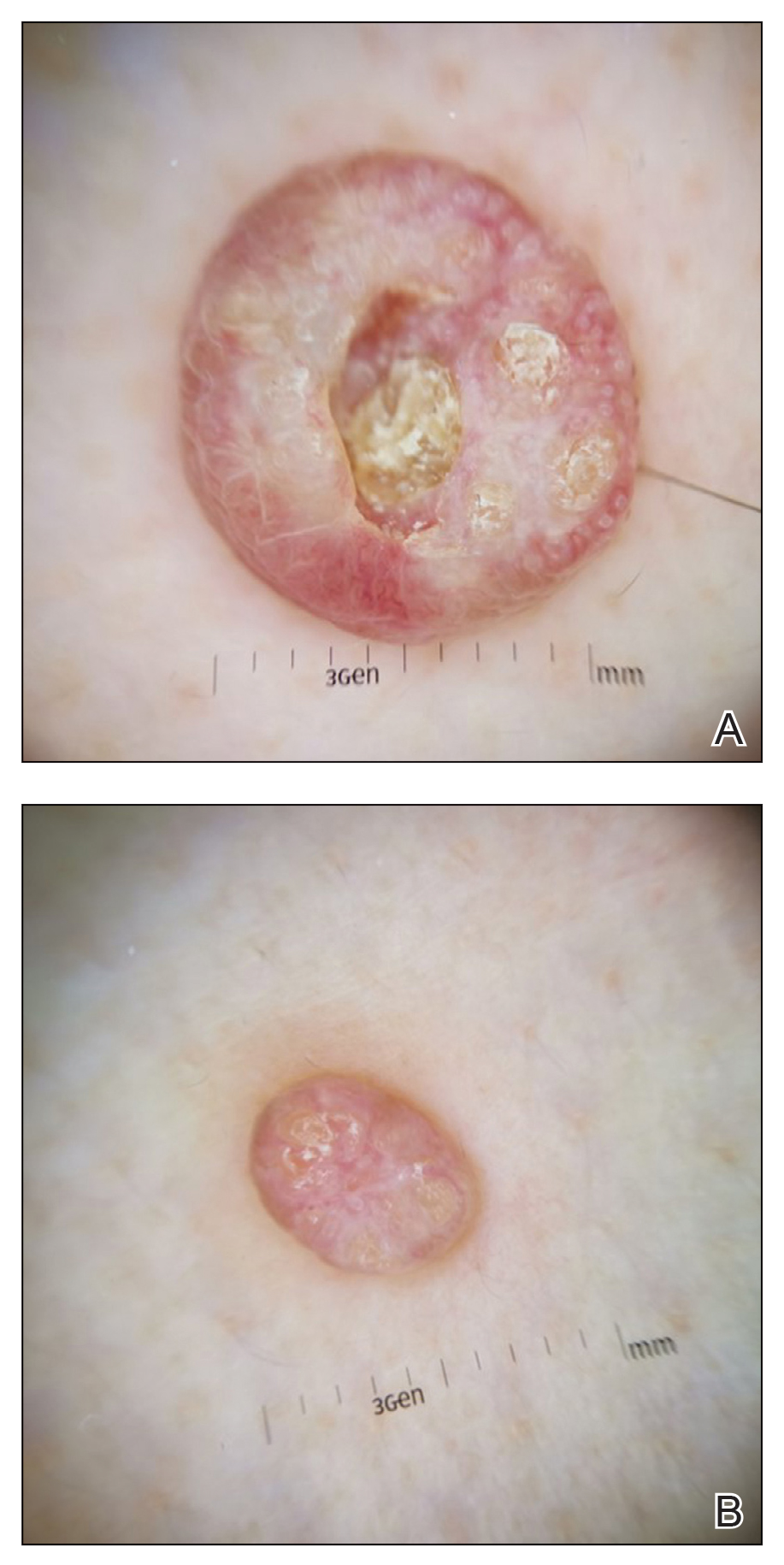
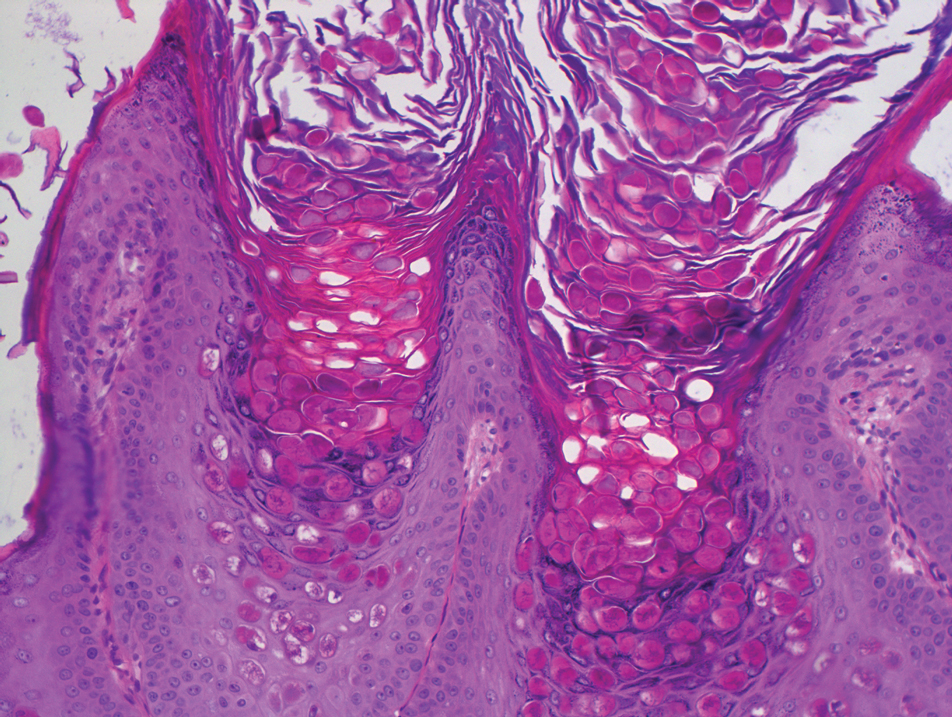
Molluscum contagiosum was first described in 1814. It is a benign cutaneous infectious disease caused by a double-stranded DNA virus of the poxvirus family. Molluscum contagiosum lesions usually manifest clinically as dome-shaped, flesh-colored or translucent, umbilicated papules measuring 1 to 5 mm in diameter that are commonly distributed over the face, trunk, and extremities and usually are self-limiting.1
Giant MC is rare and can be seen either in patients on immunosuppressive therapy or in those with diseases that can cause immunosuppression, such as human immunodeficiency virus, leukemia, atopic dermatitis, Wiskott-Aldrich syndrome, and sarcoidosis. In these instances, MC often is greater than 1 cm in diameter. Atypical variants may have an eczematous presentation or a lesion with secondary abscess formation and also can be spread widely over the body.2 Due to these atypical appearances and large dimensions in immunocompromised patients, other dermatologic diseases should be considered in the differential diagnosis, such as basal cell carcinoma, keratoacanthoma, squamous cell carcinoma, cutaneous horn, cutaneous cryptococcosis, histoplasmosis, and xanthomatosis.3
In our patient, the differential diagnosis included keratoacanthoma, which may present as a solitary, discrete, round to oval, flesh-colored, umbilicated nodule with a central keratin-filled crater and has a rapid clinical evolution, usually regressing within 4 to 6 months.
Squamous cell carcinoma may appear as scaly red patches, open sores, warts, or elevated growths with a central depression and may crust or bleed. Basal cell carcinoma typically may appear as a dome-shaped skin nodule with visible blood vessels or sometimes presents as a red patch similar to eczema. Xanthomatosis often appears as yellow to orange, mostly asymptomatic, supple patches or plaques, usually with sharp and distinctive edges.
Ancillary diagnostic modalities such as dermoscopy may be used to improve diagnostic accuracy. The best known capillaroscopic feature of MC is the peripheral crown of vessels in a radial distribution. A study of 258 MC lesions highlighted that crown and crown plus radial arrangements are the most common vascular structure patterns under dermoscopy. In addition, polylobular amorphous white structures in the center of the lesions tend to be a feature of larger MC papules.4 Histologically, MC shows lobulated crateriform lesions, thickening of the epidermis into the dermis, and the typical appearance of large eosinophilic intracytoplasmic inclusion bodies within keratinocytes.5
There are several treatment options available for MC. Common modalities include liquid nitrogen cryospray, curettage, and electrocauterization. In immunocompromised patients, MC lesions usually are resistant to ordinary therapy. The efficacy of topical agents such as imiquimod, which can induce high levels of IFN-α and other cytokines, has been demonstrated in these patients.6 Cidofovir, a nucleoside analog that has potent antiviral properties, also can be included as a therapeutic option.3 Our patient’s largest MC lesion was treated with surgical excision, the 2 large lesions on the left side of the chest with cryotherapy, and the other small lesions with curettage.
- Hanson D, Diven DG. Molluscum contagiosum. Dermatol Online J. 2003;9:2.
- Singh S, Swain M, Shukla S, et al. An unusual presentation of giant molluscum contagiosum diagnosed on cytology. Diagn Cytopathol. 2018;46:794-796.
- Mansur AT, Goktay F, Gunduz S, et al. Multiple giant molluscum contagiosum in a renal transplant recipient. Transpl Infect Dis. 2004;6:120-123.
- Ku SH, Cho EB, Park EJ, et al. Dermoscopic features of molluscum contagiosum based on white structures and their correlation with histopathological findings. Clin Exp Dermatol. 2015;40:208-210.
- Trčko K, Hošnjak L, Kušar B, et al. Clinical, histopathological, and virological evaluation of 203 patients with a clinical diagnosis of molluscum contagiosum [published online November 12, 2018]. Open Forum Infect Dis. 2018;5.
- Gardner LS, Ormond PJ. Treatment of multiple giant molluscum contagiosum in a renal transplant patient with imiquimod 5% cream. Clin Exp Dermatol. 2010;31:452-453.
Dermoscopy showed polylobular, whitish yellow, amorphous structures at the center of the lesion surrounded by a crown of vessels (Figure 1). Histopathology revealed hyperplastic crateriform lesions containing large eosinophilic intracytoplasmic inclusion bodies within keratinocytes (Figure 2). At follow-up 2 weeks after the biopsy, the patient presented with approximately 20 more reddish papules of varying sizes on the abdomen and back that presented as dome-shaped papules and had a typical umbilicated center. The clinical manifestations, dermoscopy, and pathology findings were consistent with molluscum contagiosum (MC).


Molluscum contagiosum was first described in 1814. It is a benign cutaneous infectious disease caused by a double-stranded DNA virus of the poxvirus family. Molluscum contagiosum lesions usually manifest clinically as dome-shaped, flesh-colored or translucent, umbilicated papules measuring 1 to 5 mm in diameter that are commonly distributed over the face, trunk, and extremities and usually are self-limiting.1
Giant MC is rare and can be seen either in patients on immunosuppressive therapy or in those with diseases that can cause immunosuppression, such as human immunodeficiency virus, leukemia, atopic dermatitis, Wiskott-Aldrich syndrome, and sarcoidosis. In these instances, MC often is greater than 1 cm in diameter. Atypical variants may have an eczematous presentation or a lesion with secondary abscess formation and also can be spread widely over the body.2 Due to these atypical appearances and large dimensions in immunocompromised patients, other dermatologic diseases should be considered in the differential diagnosis, such as basal cell carcinoma, keratoacanthoma, squamous cell carcinoma, cutaneous horn, cutaneous cryptococcosis, histoplasmosis, and xanthomatosis.3
In our patient, the differential diagnosis included keratoacanthoma, which may present as a solitary, discrete, round to oval, flesh-colored, umbilicated nodule with a central keratin-filled crater and has a rapid clinical evolution, usually regressing within 4 to 6 months.
Squamous cell carcinoma may appear as scaly red patches, open sores, warts, or elevated growths with a central depression and may crust or bleed. Basal cell carcinoma typically may appear as a dome-shaped skin nodule with visible blood vessels or sometimes presents as a red patch similar to eczema. Xanthomatosis often appears as yellow to orange, mostly asymptomatic, supple patches or plaques, usually with sharp and distinctive edges.
Ancillary diagnostic modalities such as dermoscopy may be used to improve diagnostic accuracy. The best known capillaroscopic feature of MC is the peripheral crown of vessels in a radial distribution. A study of 258 MC lesions highlighted that crown and crown plus radial arrangements are the most common vascular structure patterns under dermoscopy. In addition, polylobular amorphous white structures in the center of the lesions tend to be a feature of larger MC papules.4 Histologically, MC shows lobulated crateriform lesions, thickening of the epidermis into the dermis, and the typical appearance of large eosinophilic intracytoplasmic inclusion bodies within keratinocytes.5
There are several treatment options available for MC. Common modalities include liquid nitrogen cryospray, curettage, and electrocauterization. In immunocompromised patients, MC lesions usually are resistant to ordinary therapy. The efficacy of topical agents such as imiquimod, which can induce high levels of IFN-α and other cytokines, has been demonstrated in these patients.6 Cidofovir, a nucleoside analog that has potent antiviral properties, also can be included as a therapeutic option.3 Our patient’s largest MC lesion was treated with surgical excision, the 2 large lesions on the left side of the chest with cryotherapy, and the other small lesions with curettage.
Dermoscopy showed polylobular, whitish yellow, amorphous structures at the center of the lesion surrounded by a crown of vessels (Figure 1). Histopathology revealed hyperplastic crateriform lesions containing large eosinophilic intracytoplasmic inclusion bodies within keratinocytes (Figure 2). At follow-up 2 weeks after the biopsy, the patient presented with approximately 20 more reddish papules of varying sizes on the abdomen and back that presented as dome-shaped papules and had a typical umbilicated center. The clinical manifestations, dermoscopy, and pathology findings were consistent with molluscum contagiosum (MC).


Molluscum contagiosum was first described in 1814. It is a benign cutaneous infectious disease caused by a double-stranded DNA virus of the poxvirus family. Molluscum contagiosum lesions usually manifest clinically as dome-shaped, flesh-colored or translucent, umbilicated papules measuring 1 to 5 mm in diameter that are commonly distributed over the face, trunk, and extremities and usually are self-limiting.1
Giant MC is rare and can be seen either in patients on immunosuppressive therapy or in those with diseases that can cause immunosuppression, such as human immunodeficiency virus, leukemia, atopic dermatitis, Wiskott-Aldrich syndrome, and sarcoidosis. In these instances, MC often is greater than 1 cm in diameter. Atypical variants may have an eczematous presentation or a lesion with secondary abscess formation and also can be spread widely over the body.2 Due to these atypical appearances and large dimensions in immunocompromised patients, other dermatologic diseases should be considered in the differential diagnosis, such as basal cell carcinoma, keratoacanthoma, squamous cell carcinoma, cutaneous horn, cutaneous cryptococcosis, histoplasmosis, and xanthomatosis.3
In our patient, the differential diagnosis included keratoacanthoma, which may present as a solitary, discrete, round to oval, flesh-colored, umbilicated nodule with a central keratin-filled crater and has a rapid clinical evolution, usually regressing within 4 to 6 months.
Squamous cell carcinoma may appear as scaly red patches, open sores, warts, or elevated growths with a central depression and may crust or bleed. Basal cell carcinoma typically may appear as a dome-shaped skin nodule with visible blood vessels or sometimes presents as a red patch similar to eczema. Xanthomatosis often appears as yellow to orange, mostly asymptomatic, supple patches or plaques, usually with sharp and distinctive edges.
Ancillary diagnostic modalities such as dermoscopy may be used to improve diagnostic accuracy. The best known capillaroscopic feature of MC is the peripheral crown of vessels in a radial distribution. A study of 258 MC lesions highlighted that crown and crown plus radial arrangements are the most common vascular structure patterns under dermoscopy. In addition, polylobular amorphous white structures in the center of the lesions tend to be a feature of larger MC papules.4 Histologically, MC shows lobulated crateriform lesions, thickening of the epidermis into the dermis, and the typical appearance of large eosinophilic intracytoplasmic inclusion bodies within keratinocytes.5
There are several treatment options available for MC. Common modalities include liquid nitrogen cryospray, curettage, and electrocauterization. In immunocompromised patients, MC lesions usually are resistant to ordinary therapy. The efficacy of topical agents such as imiquimod, which can induce high levels of IFN-α and other cytokines, has been demonstrated in these patients.6 Cidofovir, a nucleoside analog that has potent antiviral properties, also can be included as a therapeutic option.3 Our patient’s largest MC lesion was treated with surgical excision, the 2 large lesions on the left side of the chest with cryotherapy, and the other small lesions with curettage.
- Hanson D, Diven DG. Molluscum contagiosum. Dermatol Online J. 2003;9:2.
- Singh S, Swain M, Shukla S, et al. An unusual presentation of giant molluscum contagiosum diagnosed on cytology. Diagn Cytopathol. 2018;46:794-796.
- Mansur AT, Goktay F, Gunduz S, et al. Multiple giant molluscum contagiosum in a renal transplant recipient. Transpl Infect Dis. 2004;6:120-123.
- Ku SH, Cho EB, Park EJ, et al. Dermoscopic features of molluscum contagiosum based on white structures and their correlation with histopathological findings. Clin Exp Dermatol. 2015;40:208-210.
- Trčko K, Hošnjak L, Kušar B, et al. Clinical, histopathological, and virological evaluation of 203 patients with a clinical diagnosis of molluscum contagiosum [published online November 12, 2018]. Open Forum Infect Dis. 2018;5.
- Gardner LS, Ormond PJ. Treatment of multiple giant molluscum contagiosum in a renal transplant patient with imiquimod 5% cream. Clin Exp Dermatol. 2010;31:452-453.
- Hanson D, Diven DG. Molluscum contagiosum. Dermatol Online J. 2003;9:2.
- Singh S, Swain M, Shukla S, et al. An unusual presentation of giant molluscum contagiosum diagnosed on cytology. Diagn Cytopathol. 2018;46:794-796.
- Mansur AT, Goktay F, Gunduz S, et al. Multiple giant molluscum contagiosum in a renal transplant recipient. Transpl Infect Dis. 2004;6:120-123.
- Ku SH, Cho EB, Park EJ, et al. Dermoscopic features of molluscum contagiosum based on white structures and their correlation with histopathological findings. Clin Exp Dermatol. 2015;40:208-210.
- Trčko K, Hošnjak L, Kušar B, et al. Clinical, histopathological, and virological evaluation of 203 patients with a clinical diagnosis of molluscum contagiosum [published online November 12, 2018]. Open Forum Infect Dis. 2018;5.
- Gardner LS, Ormond PJ. Treatment of multiple giant molluscum contagiosum in a renal transplant patient with imiquimod 5% cream. Clin Exp Dermatol. 2010;31:452-453.
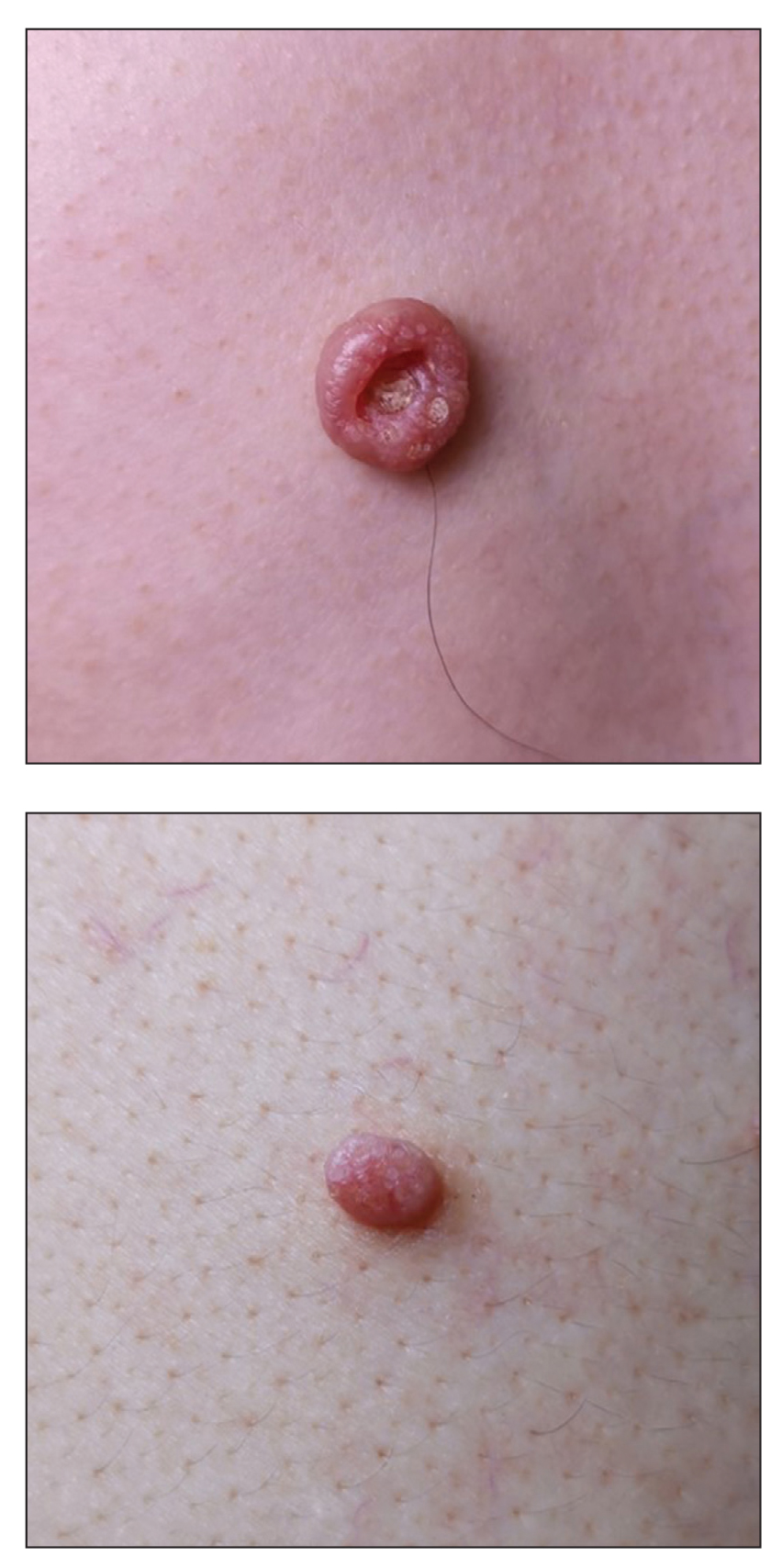
A 49-year-old man presented with a slow-growing mass on the chest of 1 year’s duration. The neoplasm started as a small papule that gradually increased in size. The patient denied pain, itching, bleeding, or discharge. He had a history of end-stage renal disease with a kidney transplant 8 years prior. His medication history included long-term use of oral tacrolimus, mycophenolate mofetil, and prednisone. Physical examination revealed a yellowish red, exogenous, pedunculated neoplasm on the right side of the chest measuring 1 cm in diameter with an umbilicated center and keratotic material (top). There were 2 more yellowish red papules on the left side of the chest measuring 0.5 cm in diameter without an umbilicated center (bottom). Dermoscopy and a biopsy were performed.
Palmoplantar Eruption in a Patient With Mercury Poisoning
Mercury poisoning affects multiple body systems, leading to variable clinical presentations. Mercury intoxication at low levels frequently presents with weakness, fatigue, weight loss, and abdominal pain. At higher levels of mercury intoxication, tremors and neurologic dysfunction are more prevalent.1 Dermatologic manifestations of mercury exposure vary and include pink disease (acrodynia), mercury exanthem, contact dermatitis, and cutaneous granulomas. Untreated mercury poisoning may result in severe complications, including renal tubular necrosis, pneumonitis, persistent neurologic dysfunction, and fatality in some cases.1,2
Pink disease is a rare disease that typically arises in infants and young children from chronic mercury exposure.3 We report a unique presentation of pink disease occurring in an 18-year-old woman following mercury exposure.
Case Report
An 18-year-old woman who was previously healthy presented to the hospital for evaluation of body aches and back pain. She reported a transient rash on the torso 2 weeks prior, but at the current presentation, only the distal upper and lower extremities were involved. A review of systems revealed myalgia, most severe in the lower back; muscle spasms; stiffness in the fingers; abdominal pain; constipation; paresthesia in the hands and feet; hyperhidrosis; and generalized weakness.
Vitals on admission revealed tachycardia (112 beats per minute). Physical examination revealed the patient was pale and fatigued; she appeared to be in pain, with observable facial grimacing and muscle spasms in the legs. She had poorly demarcated pink macules and papules scattered on the left palm (Figure 1), right forearm, right wrist, and dorsal aspects of the feet including the soles. A few pinpoint pustules were present on the left fifth digit.
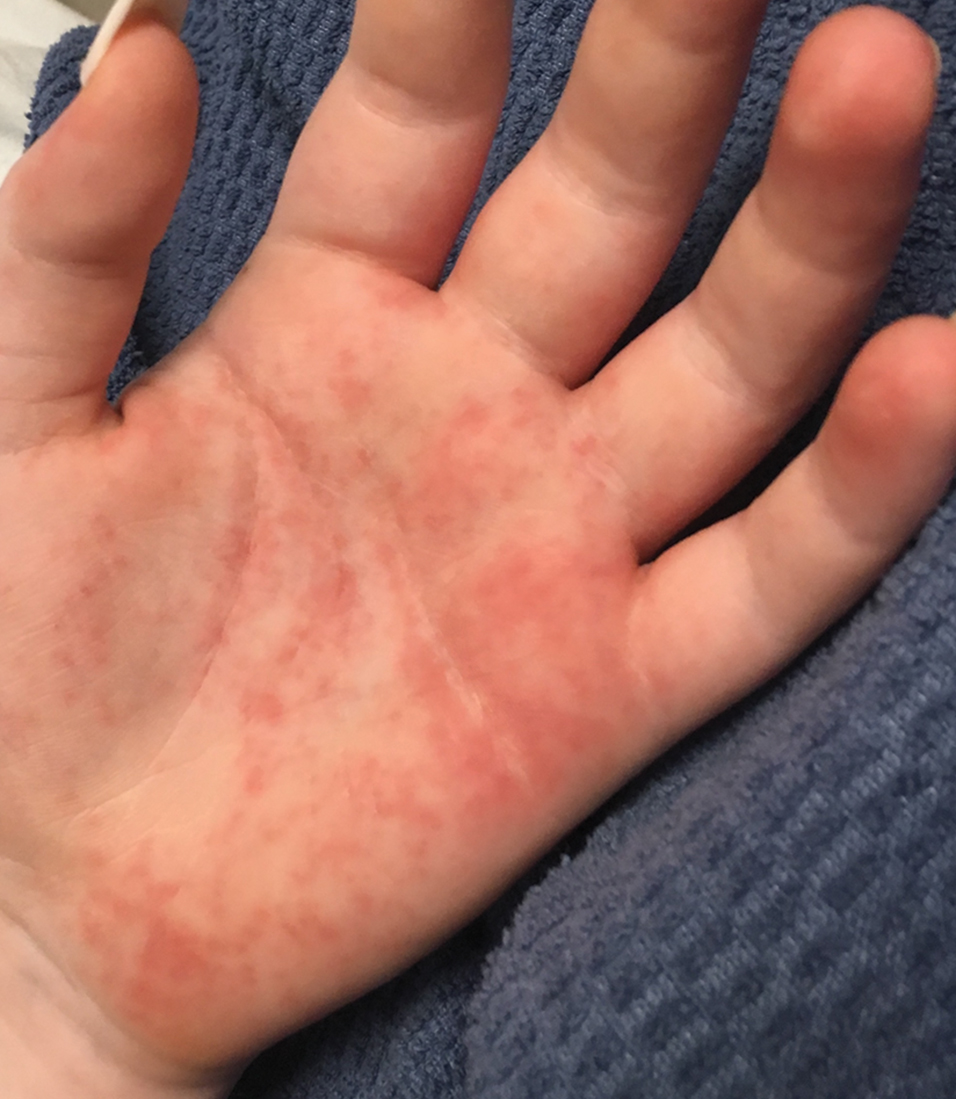
An extensive workup was initiated to rule out infectious, autoimmune, or toxic etiologies. Two 4-mm punch biopsies of the left palm were performed for hematoxylin and eosin staining and tissue culture. Findings on hematoxylin and eosin stain were nonspecific, showing acanthosis, orthokeratosis, and a mild interface and perivascular lymphocytic infiltrate (Figure 2); superficial bacterial colonization was present, but the tissue culture was negative.
Laboratory studies showed mild transaminitis, and stool was positive for Campylobacter antigen. Electromyography showed myokymia (fascicular muscle contractions). A heavy metal serum panel and urine screen were positive for elevated mercury levels, with a serum mercury level of 23 µg/L (reference range, 0.0–14.9 µg/L) and a urine mercury level of 76 µg/L (reference range, 0–19 µg/L).
Upon further questioning, it was discovered that the patient’s brother and neighbor found a glass bottle containing mercury in their house 10 days prior. They played with the mercury beads with their hands, throwing them around the room and spilling them around the house, which led to mercury exposure in multiple individuals, including our patient. Of note, her brother and neighbor also were hospitalized at the same time as our patient with similar symptoms.
A diagnosis of mercury poisoning was made along with a component of postinfectious reactive arthropathy due to Campylobacter. The myokymia and skin eruption were believed to be secondary to mercury poisoning. The patient was started on ciprofloxacin (750 mg twice daily), intravenous immunoglobulin for Campylobacter, a 2-week treatment regimen with the chelating agent succimer (500 mg twice daily) for mercury poisoning, and a 3-day regimen of pulse intravenous steroids (intravenous methylprednisolone 500 mg once daily) to reduce inflammation. Repeat mercury levels showed a downward trend, and the rash improved with time. All family members were advised to undergo testing for mercury exposure.
Comment
Manifestations of Mercury Poisoning
Dermatologic manifestations of mercury exposure are varied. The most common—allergic contact dermatitis—presents after repeat systemic or topical exposure.4 Mercury exanthem is an acute systemic contact dermatitis most commonly triggered by mercury vapor inhalation. It manifests as an erythematous maculopapular eruption predominantly involving the flexural areas and the anterior thighs in a V-shaped distribution.5 Purpura may be seen in severe cases. Cutaneous granulomas after direct injection of mercury also have been reported as well as cutaneous hyperpigmentation after chronic mercury absorption.6
Presentation of Pink Disease
Pink disease occurs in children after chronic mercury exposure. It was a common pediatric disorder in the 19th century due to the presence of mercury in certain anthelmintics and teething powders.7 However, prevalence drastically decreased after the removal of mercury from these products.3 Although pink disease classically was associated with mercury ingestion, cases also occurred secondary to external application of mercury.7 Additionally, in 1988 a case was reported in a 14-month-old girl after inhalation of mercury vapor from a spilled bottle of mercury.3
Pink disease begins with pink discoloration of the fingertips, nose, and toes, and later progresses to involvement of the hands and feet. Erythema, edema, and desquamation of the hands and feet are seen, along with irritability and autonomic dysfunction that manifests as profuse perspiration, tachycardia, and hypertension.3
Diagnosis of Pink Disease
The differential diagnosis of palmoplantar rash is broad and includes rickettsial disease; syphilis; scabies; toxic shock syndrome; infective endocarditis; meningococcal infection; hand-foot-and-mouth disease; dermatophytosis; and palmoplantar keratodermas. The involvement of the hands and feet in our patient, along with hyperhidrosis, tachycardia, and paresthesia, led us to believe that her condition was a variation of pink disease. The patient’s age at presentation (18 years) was unique, as it is atypical for pink disease. Although the polyarthropathy was attributed to Campylobacter, it is important to note that high levels of mercury exposure also have been associated with polyarthritis,8 polyneuropathy,4 and neuromuscular abnormalities on electromyography.4 Therefore, it is possible that the presence of these symptoms in our patient was either secondary to or compounded by mercury exposure.
Mercury Poisoning
Diagnosis of mercury poisoning can be made by assessing blood, urine, hair, or nail concentrations. However, as mercury deposits in multiple organs, individual concentrations do not correlate with total-body mercury levels.1 Currently, no universal diagnostic criteria for mercury toxicity exist, though a provocation test with the chelating agent 2,
Elemental mercury, as found in some thermometers, dental amalgams, and electrical appliances (eg, certain switches, fluorescent light bulbs), can be converted to inorganic mercury in the body.9 Elemental mercury is vaporized at room temperature; the predominant route of exposure is by subsequent inhalation and lung absorbtion.10 Cutaneous absorption of high concentrations of elementary mercury in either liquid or vapor form may occur, though the rate is slow and absorption is poor. In cases of accidental exposure, contaminated clothing should be removed and immediately decontaminated or disposed. Exposed skin should be washed with a mild soap and water and rinsed thoroughly.10
The treatment of inorganic mercury poisoning is accomplished with the chelating agents succimer, dimercaptopropanesulfonate, dimercaprol, or D-penicillamine.1 In symptomatic cases with high clinical suspicion, the first dose of chelation treatment should be initiated early without delay for laboratory confirmation, as treatment efficacy decreases with an increased interim between exposure and onset of chelation.11 Combination chelation therapy also may be used in treatment. Plasma exchange or hemodialysis are treatment options for extreme, life-threatening cases.1
Conclusion
Mercury exposure should be included in the differential diagnosis of patients presenting with a rash on the palms and soles, especially in young patients with systemic symptoms. A high level of suspicion and a thorough history can prevent a delay in treatment and an unnecessarily extensive and expensive workup. An emphasis on early diagnosis and treatment is important for optimal outcomes and can prevent the severe and potentially devastating consequences of mercury toxicity.
- Bernhoft RA. Mercury toxicity and treatment: a review of the literature. J Environ Public Health. 2012;2012:460508.
- Kamensky OL, Horton D, Kingsley DP, et al. A case of accidental mercury intoxication. J Emerg Med. 2019;56:275-278.
- Dinehart SM, Dillard R, Raimer SS, et al. Cutaneous manifestations of acrodynia (pink disease). Arch Dermatol. 1988;124:107-109.
- Malek A, Aouad K, El Khoury R, et al. Chronic mercury intoxication masquerading as systemic disease: a case report and review of the literature. Eur J Case Rep Intern Med. 2017;4:000632.
- Nakayama H, Niki F, Shono M, et al. Mercury exanthem. Contact Dermatitis. 1983;9:411-417.
- Boyd AS, Seger D, Vannucci S, et al. Mercury exposure and cutaneous disease. J Am Acad Dermatol. 2000;43:81-90.
- Warkany J. Acrodynia—postmortem of a disease. Am J Dis Child. 1966;112:147-156.
- Karatas¸ GK, Tosun AK, Karacehennem E, et al. Mercury poisoning: an unusual cause of polyarthritis. Clin Rheumatol. 2002;21:73-75.
- Mercury Factsheet. Centers for Disease Control and Prevention website. https://www.cdc.gov/biomonitoring/Mercury_FactSheet.html. Reviewed April 7, 2017. Accessed October 21, 2020.
- Medical management guidelines for mercury. Agency for Toxic Substances & Disease Registry website. https://www.atsdr.cdc .gov/MMG/MMG.asp?id=106&tid=24. Update October 21, 2014. Accessed September 11, 2020.
- Kosnett MJ. The role of chelation in the treatment of arsenic and mercury poisoning. J Med Toxicol. 2013;9:347-354.
Mercury poisoning affects multiple body systems, leading to variable clinical presentations. Mercury intoxication at low levels frequently presents with weakness, fatigue, weight loss, and abdominal pain. At higher levels of mercury intoxication, tremors and neurologic dysfunction are more prevalent.1 Dermatologic manifestations of mercury exposure vary and include pink disease (acrodynia), mercury exanthem, contact dermatitis, and cutaneous granulomas. Untreated mercury poisoning may result in severe complications, including renal tubular necrosis, pneumonitis, persistent neurologic dysfunction, and fatality in some cases.1,2
Pink disease is a rare disease that typically arises in infants and young children from chronic mercury exposure.3 We report a unique presentation of pink disease occurring in an 18-year-old woman following mercury exposure.
Case Report
An 18-year-old woman who was previously healthy presented to the hospital for evaluation of body aches and back pain. She reported a transient rash on the torso 2 weeks prior, but at the current presentation, only the distal upper and lower extremities were involved. A review of systems revealed myalgia, most severe in the lower back; muscle spasms; stiffness in the fingers; abdominal pain; constipation; paresthesia in the hands and feet; hyperhidrosis; and generalized weakness.
Vitals on admission revealed tachycardia (112 beats per minute). Physical examination revealed the patient was pale and fatigued; she appeared to be in pain, with observable facial grimacing and muscle spasms in the legs. She had poorly demarcated pink macules and papules scattered on the left palm (Figure 1), right forearm, right wrist, and dorsal aspects of the feet including the soles. A few pinpoint pustules were present on the left fifth digit.

An extensive workup was initiated to rule out infectious, autoimmune, or toxic etiologies. Two 4-mm punch biopsies of the left palm were performed for hematoxylin and eosin staining and tissue culture. Findings on hematoxylin and eosin stain were nonspecific, showing acanthosis, orthokeratosis, and a mild interface and perivascular lymphocytic infiltrate (Figure 2); superficial bacterial colonization was present, but the tissue culture was negative.

Laboratory studies showed mild transaminitis, and stool was positive for Campylobacter antigen. Electromyography showed myokymia (fascicular muscle contractions). A heavy metal serum panel and urine screen were positive for elevated mercury levels, with a serum mercury level of 23 µg/L (reference range, 0.0–14.9 µg/L) and a urine mercury level of 76 µg/L (reference range, 0–19 µg/L).
Upon further questioning, it was discovered that the patient’s brother and neighbor found a glass bottle containing mercury in their house 10 days prior. They played with the mercury beads with their hands, throwing them around the room and spilling them around the house, which led to mercury exposure in multiple individuals, including our patient. Of note, her brother and neighbor also were hospitalized at the same time as our patient with similar symptoms.
A diagnosis of mercury poisoning was made along with a component of postinfectious reactive arthropathy due to Campylobacter. The myokymia and skin eruption were believed to be secondary to mercury poisoning. The patient was started on ciprofloxacin (750 mg twice daily), intravenous immunoglobulin for Campylobacter, a 2-week treatment regimen with the chelating agent succimer (500 mg twice daily) for mercury poisoning, and a 3-day regimen of pulse intravenous steroids (intravenous methylprednisolone 500 mg once daily) to reduce inflammation. Repeat mercury levels showed a downward trend, and the rash improved with time. All family members were advised to undergo testing for mercury exposure.
Comment
Manifestations of Mercury Poisoning
Dermatologic manifestations of mercury exposure are varied. The most common—allergic contact dermatitis—presents after repeat systemic or topical exposure.4 Mercury exanthem is an acute systemic contact dermatitis most commonly triggered by mercury vapor inhalation. It manifests as an erythematous maculopapular eruption predominantly involving the flexural areas and the anterior thighs in a V-shaped distribution.5 Purpura may be seen in severe cases. Cutaneous granulomas after direct injection of mercury also have been reported as well as cutaneous hyperpigmentation after chronic mercury absorption.6
Presentation of Pink Disease
Pink disease occurs in children after chronic mercury exposure. It was a common pediatric disorder in the 19th century due to the presence of mercury in certain anthelmintics and teething powders.7 However, prevalence drastically decreased after the removal of mercury from these products.3 Although pink disease classically was associated with mercury ingestion, cases also occurred secondary to external application of mercury.7 Additionally, in 1988 a case was reported in a 14-month-old girl after inhalation of mercury vapor from a spilled bottle of mercury.3
Pink disease begins with pink discoloration of the fingertips, nose, and toes, and later progresses to involvement of the hands and feet. Erythema, edema, and desquamation of the hands and feet are seen, along with irritability and autonomic dysfunction that manifests as profuse perspiration, tachycardia, and hypertension.3
Diagnosis of Pink Disease
The differential diagnosis of palmoplantar rash is broad and includes rickettsial disease; syphilis; scabies; toxic shock syndrome; infective endocarditis; meningococcal infection; hand-foot-and-mouth disease; dermatophytosis; and palmoplantar keratodermas. The involvement of the hands and feet in our patient, along with hyperhidrosis, tachycardia, and paresthesia, led us to believe that her condition was a variation of pink disease. The patient’s age at presentation (18 years) was unique, as it is atypical for pink disease. Although the polyarthropathy was attributed to Campylobacter, it is important to note that high levels of mercury exposure also have been associated with polyarthritis,8 polyneuropathy,4 and neuromuscular abnormalities on electromyography.4 Therefore, it is possible that the presence of these symptoms in our patient was either secondary to or compounded by mercury exposure.
Mercury Poisoning
Diagnosis of mercury poisoning can be made by assessing blood, urine, hair, or nail concentrations. However, as mercury deposits in multiple organs, individual concentrations do not correlate with total-body mercury levels.1 Currently, no universal diagnostic criteria for mercury toxicity exist, though a provocation test with the chelating agent 2,
Elemental mercury, as found in some thermometers, dental amalgams, and electrical appliances (eg, certain switches, fluorescent light bulbs), can be converted to inorganic mercury in the body.9 Elemental mercury is vaporized at room temperature; the predominant route of exposure is by subsequent inhalation and lung absorbtion.10 Cutaneous absorption of high concentrations of elementary mercury in either liquid or vapor form may occur, though the rate is slow and absorption is poor. In cases of accidental exposure, contaminated clothing should be removed and immediately decontaminated or disposed. Exposed skin should be washed with a mild soap and water and rinsed thoroughly.10
The treatment of inorganic mercury poisoning is accomplished with the chelating agents succimer, dimercaptopropanesulfonate, dimercaprol, or D-penicillamine.1 In symptomatic cases with high clinical suspicion, the first dose of chelation treatment should be initiated early without delay for laboratory confirmation, as treatment efficacy decreases with an increased interim between exposure and onset of chelation.11 Combination chelation therapy also may be used in treatment. Plasma exchange or hemodialysis are treatment options for extreme, life-threatening cases.1
Conclusion
Mercury exposure should be included in the differential diagnosis of patients presenting with a rash on the palms and soles, especially in young patients with systemic symptoms. A high level of suspicion and a thorough history can prevent a delay in treatment and an unnecessarily extensive and expensive workup. An emphasis on early diagnosis and treatment is important for optimal outcomes and can prevent the severe and potentially devastating consequences of mercury toxicity.
Mercury poisoning affects multiple body systems, leading to variable clinical presentations. Mercury intoxication at low levels frequently presents with weakness, fatigue, weight loss, and abdominal pain. At higher levels of mercury intoxication, tremors and neurologic dysfunction are more prevalent.1 Dermatologic manifestations of mercury exposure vary and include pink disease (acrodynia), mercury exanthem, contact dermatitis, and cutaneous granulomas. Untreated mercury poisoning may result in severe complications, including renal tubular necrosis, pneumonitis, persistent neurologic dysfunction, and fatality in some cases.1,2
Pink disease is a rare disease that typically arises in infants and young children from chronic mercury exposure.3 We report a unique presentation of pink disease occurring in an 18-year-old woman following mercury exposure.
Case Report
An 18-year-old woman who was previously healthy presented to the hospital for evaluation of body aches and back pain. She reported a transient rash on the torso 2 weeks prior, but at the current presentation, only the distal upper and lower extremities were involved. A review of systems revealed myalgia, most severe in the lower back; muscle spasms; stiffness in the fingers; abdominal pain; constipation; paresthesia in the hands and feet; hyperhidrosis; and generalized weakness.
Vitals on admission revealed tachycardia (112 beats per minute). Physical examination revealed the patient was pale and fatigued; she appeared to be in pain, with observable facial grimacing and muscle spasms in the legs. She had poorly demarcated pink macules and papules scattered on the left palm (Figure 1), right forearm, right wrist, and dorsal aspects of the feet including the soles. A few pinpoint pustules were present on the left fifth digit.

An extensive workup was initiated to rule out infectious, autoimmune, or toxic etiologies. Two 4-mm punch biopsies of the left palm were performed for hematoxylin and eosin staining and tissue culture. Findings on hematoxylin and eosin stain were nonspecific, showing acanthosis, orthokeratosis, and a mild interface and perivascular lymphocytic infiltrate (Figure 2); superficial bacterial colonization was present, but the tissue culture was negative.

Laboratory studies showed mild transaminitis, and stool was positive for Campylobacter antigen. Electromyography showed myokymia (fascicular muscle contractions). A heavy metal serum panel and urine screen were positive for elevated mercury levels, with a serum mercury level of 23 µg/L (reference range, 0.0–14.9 µg/L) and a urine mercury level of 76 µg/L (reference range, 0–19 µg/L).
Upon further questioning, it was discovered that the patient’s brother and neighbor found a glass bottle containing mercury in their house 10 days prior. They played with the mercury beads with their hands, throwing them around the room and spilling them around the house, which led to mercury exposure in multiple individuals, including our patient. Of note, her brother and neighbor also were hospitalized at the same time as our patient with similar symptoms.
A diagnosis of mercury poisoning was made along with a component of postinfectious reactive arthropathy due to Campylobacter. The myokymia and skin eruption were believed to be secondary to mercury poisoning. The patient was started on ciprofloxacin (750 mg twice daily), intravenous immunoglobulin for Campylobacter, a 2-week treatment regimen with the chelating agent succimer (500 mg twice daily) for mercury poisoning, and a 3-day regimen of pulse intravenous steroids (intravenous methylprednisolone 500 mg once daily) to reduce inflammation. Repeat mercury levels showed a downward trend, and the rash improved with time. All family members were advised to undergo testing for mercury exposure.
Comment
Manifestations of Mercury Poisoning
Dermatologic manifestations of mercury exposure are varied. The most common—allergic contact dermatitis—presents after repeat systemic or topical exposure.4 Mercury exanthem is an acute systemic contact dermatitis most commonly triggered by mercury vapor inhalation. It manifests as an erythematous maculopapular eruption predominantly involving the flexural areas and the anterior thighs in a V-shaped distribution.5 Purpura may be seen in severe cases. Cutaneous granulomas after direct injection of mercury also have been reported as well as cutaneous hyperpigmentation after chronic mercury absorption.6
Presentation of Pink Disease
Pink disease occurs in children after chronic mercury exposure. It was a common pediatric disorder in the 19th century due to the presence of mercury in certain anthelmintics and teething powders.7 However, prevalence drastically decreased after the removal of mercury from these products.3 Although pink disease classically was associated with mercury ingestion, cases also occurred secondary to external application of mercury.7 Additionally, in 1988 a case was reported in a 14-month-old girl after inhalation of mercury vapor from a spilled bottle of mercury.3
Pink disease begins with pink discoloration of the fingertips, nose, and toes, and later progresses to involvement of the hands and feet. Erythema, edema, and desquamation of the hands and feet are seen, along with irritability and autonomic dysfunction that manifests as profuse perspiration, tachycardia, and hypertension.3
Diagnosis of Pink Disease
The differential diagnosis of palmoplantar rash is broad and includes rickettsial disease; syphilis; scabies; toxic shock syndrome; infective endocarditis; meningococcal infection; hand-foot-and-mouth disease; dermatophytosis; and palmoplantar keratodermas. The involvement of the hands and feet in our patient, along with hyperhidrosis, tachycardia, and paresthesia, led us to believe that her condition was a variation of pink disease. The patient’s age at presentation (18 years) was unique, as it is atypical for pink disease. Although the polyarthropathy was attributed to Campylobacter, it is important to note that high levels of mercury exposure also have been associated with polyarthritis,8 polyneuropathy,4 and neuromuscular abnormalities on electromyography.4 Therefore, it is possible that the presence of these symptoms in our patient was either secondary to or compounded by mercury exposure.
Mercury Poisoning
Diagnosis of mercury poisoning can be made by assessing blood, urine, hair, or nail concentrations. However, as mercury deposits in multiple organs, individual concentrations do not correlate with total-body mercury levels.1 Currently, no universal diagnostic criteria for mercury toxicity exist, though a provocation test with the chelating agent 2,
Elemental mercury, as found in some thermometers, dental amalgams, and electrical appliances (eg, certain switches, fluorescent light bulbs), can be converted to inorganic mercury in the body.9 Elemental mercury is vaporized at room temperature; the predominant route of exposure is by subsequent inhalation and lung absorbtion.10 Cutaneous absorption of high concentrations of elementary mercury in either liquid or vapor form may occur, though the rate is slow and absorption is poor. In cases of accidental exposure, contaminated clothing should be removed and immediately decontaminated or disposed. Exposed skin should be washed with a mild soap and water and rinsed thoroughly.10
The treatment of inorganic mercury poisoning is accomplished with the chelating agents succimer, dimercaptopropanesulfonate, dimercaprol, or D-penicillamine.1 In symptomatic cases with high clinical suspicion, the first dose of chelation treatment should be initiated early without delay for laboratory confirmation, as treatment efficacy decreases with an increased interim between exposure and onset of chelation.11 Combination chelation therapy also may be used in treatment. Plasma exchange or hemodialysis are treatment options for extreme, life-threatening cases.1
Conclusion
Mercury exposure should be included in the differential diagnosis of patients presenting with a rash on the palms and soles, especially in young patients with systemic symptoms. A high level of suspicion and a thorough history can prevent a delay in treatment and an unnecessarily extensive and expensive workup. An emphasis on early diagnosis and treatment is important for optimal outcomes and can prevent the severe and potentially devastating consequences of mercury toxicity.
- Bernhoft RA. Mercury toxicity and treatment: a review of the literature. J Environ Public Health. 2012;2012:460508.
- Kamensky OL, Horton D, Kingsley DP, et al. A case of accidental mercury intoxication. J Emerg Med. 2019;56:275-278.
- Dinehart SM, Dillard R, Raimer SS, et al. Cutaneous manifestations of acrodynia (pink disease). Arch Dermatol. 1988;124:107-109.
- Malek A, Aouad K, El Khoury R, et al. Chronic mercury intoxication masquerading as systemic disease: a case report and review of the literature. Eur J Case Rep Intern Med. 2017;4:000632.
- Nakayama H, Niki F, Shono M, et al. Mercury exanthem. Contact Dermatitis. 1983;9:411-417.
- Boyd AS, Seger D, Vannucci S, et al. Mercury exposure and cutaneous disease. J Am Acad Dermatol. 2000;43:81-90.
- Warkany J. Acrodynia—postmortem of a disease. Am J Dis Child. 1966;112:147-156.
- Karatas¸ GK, Tosun AK, Karacehennem E, et al. Mercury poisoning: an unusual cause of polyarthritis. Clin Rheumatol. 2002;21:73-75.
- Mercury Factsheet. Centers for Disease Control and Prevention website. https://www.cdc.gov/biomonitoring/Mercury_FactSheet.html. Reviewed April 7, 2017. Accessed October 21, 2020.
- Medical management guidelines for mercury. Agency for Toxic Substances & Disease Registry website. https://www.atsdr.cdc .gov/MMG/MMG.asp?id=106&tid=24. Update October 21, 2014. Accessed September 11, 2020.
- Kosnett MJ. The role of chelation in the treatment of arsenic and mercury poisoning. J Med Toxicol. 2013;9:347-354.
- Bernhoft RA. Mercury toxicity and treatment: a review of the literature. J Environ Public Health. 2012;2012:460508.
- Kamensky OL, Horton D, Kingsley DP, et al. A case of accidental mercury intoxication. J Emerg Med. 2019;56:275-278.
- Dinehart SM, Dillard R, Raimer SS, et al. Cutaneous manifestations of acrodynia (pink disease). Arch Dermatol. 1988;124:107-109.
- Malek A, Aouad K, El Khoury R, et al. Chronic mercury intoxication masquerading as systemic disease: a case report and review of the literature. Eur J Case Rep Intern Med. 2017;4:000632.
- Nakayama H, Niki F, Shono M, et al. Mercury exanthem. Contact Dermatitis. 1983;9:411-417.
- Boyd AS, Seger D, Vannucci S, et al. Mercury exposure and cutaneous disease. J Am Acad Dermatol. 2000;43:81-90.
- Warkany J. Acrodynia—postmortem of a disease. Am J Dis Child. 1966;112:147-156.
- Karatas¸ GK, Tosun AK, Karacehennem E, et al. Mercury poisoning: an unusual cause of polyarthritis. Clin Rheumatol. 2002;21:73-75.
- Mercury Factsheet. Centers for Disease Control and Prevention website. https://www.cdc.gov/biomonitoring/Mercury_FactSheet.html. Reviewed April 7, 2017. Accessed October 21, 2020.
- Medical management guidelines for mercury. Agency for Toxic Substances & Disease Registry website. https://www.atsdr.cdc .gov/MMG/MMG.asp?id=106&tid=24. Update October 21, 2014. Accessed September 11, 2020.
- Kosnett MJ. The role of chelation in the treatment of arsenic and mercury poisoning. J Med Toxicol. 2013;9:347-354.
Practice Points
- The dermatologic and histologic presentation of mercury exposure may be nonspecific, requiring a high degree of clinical suspicion to make a diagnosis.
- Mercury exposure should be included in the differential diagnosis in patients presenting with a rash of the palms and soles, especially in young patients with systemic symptoms.