User login
The Role of Inpatient Dermatology Consultations
Dermatology is an often-underutilized resource in the hospital setting. As the health care landscape has evolved, so has the role of the inpatient dermatologist.1-3 Structural changes in the health system and advances in therapies have shifted dermatology from an admitting service to an almost exclusively outpatient practice. Improved treatment modalities led to decreases in the number of patients requiring admission for chronic dermatoses, and outpatient clinics began offering therapies once limited to hospitals.1,4 Inpatient dermatology consultations emerged and continue to have profound effects on hospitalized patients regardless of their reason for admission.1-11
Inpatient dermatologists supply knowledge in areas primary medical teams lack, and there is evidence that dermatology consultations improve the quality of care while decreasing cost.2,5-7 Establishing correct diagnoses, preventing exposure to unnecessary medications, and reducing hospitalization duration and readmission rates are a few ways dermatology consultations positively impact hospitalized patients.2,5-7,9,10 This study highlights the role of the dermatologist in the care of hospitalized patients at a large academic medical center in an urban setting and reveals how consultation supports the efficiency and efficacy of other services.
Materials and Methods
Study Design—This single-institution, cross-sectional retrospective study included all hospitalized patients at the Thomas Jefferson University Hospital (Philadelphia, Pennsylvania), who received an inpatient dermatology consultation completed by physicians of Jefferson Dermatology Associates between January 1, 2019, and December 31, 2019. The institutional review board at Thomas Jefferson University approved this study.
Data Collection—A list of all inpatient dermatology consultations in 2019 was provided by Jefferson Dermatology Associates. Through a retrospective chart review, data regarding the consultations were collected from the electronic medical record (Epic Systems) and recorded into the Research Electronic Data Capture system. Data on patient demographics, the primary medical team, the dermatology evaluation, and the hospital course of the patient were collected.
Results
Patient Characteristics—Dermatology received 253 inpatient consultation requests during this time period; 53% of patients were female and 47% were male, with a mean age of 55 years. Most patients were White (57%), while 34% were Black. Five percent and 4% of patients were Asian and Hispanic or Latino, respectively (Table 1). The mean duration of hospitalization for all patients was 15 days, and the average number of days to discharge following the first encounter with dermatology was 10 days.
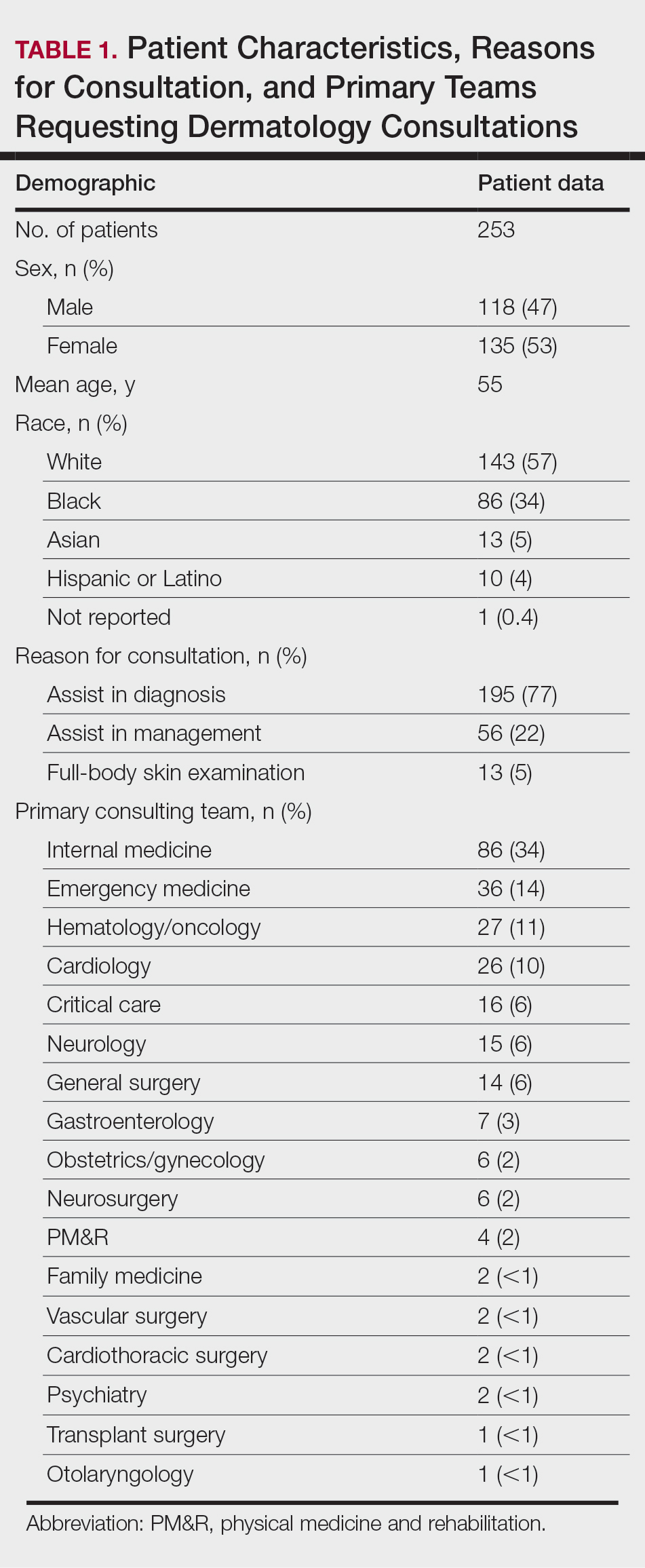
Requesting Team and Reason for Consultation—Internal medicine consulted dermatology most frequently (34% of all consultations), followed by emergency medicine (14%) and a variety of other services (Table 1). Most dermatology consultations were placed to assist in achieving a diagnosis of a cutaneous condition (77%), while a minority were to assist in the management of a previously diagnosed disease (22%). A small fraction of consultations (5%) were to complete full-body skin examinations (FBSEs) to rule out infection or malignancy in candidates for organ transplantation, left ventricular assist devices, or certain chemotherapies. One FBSE was conducted to search for a primary tumor in a patient diagnosed with metastatic melanoma.
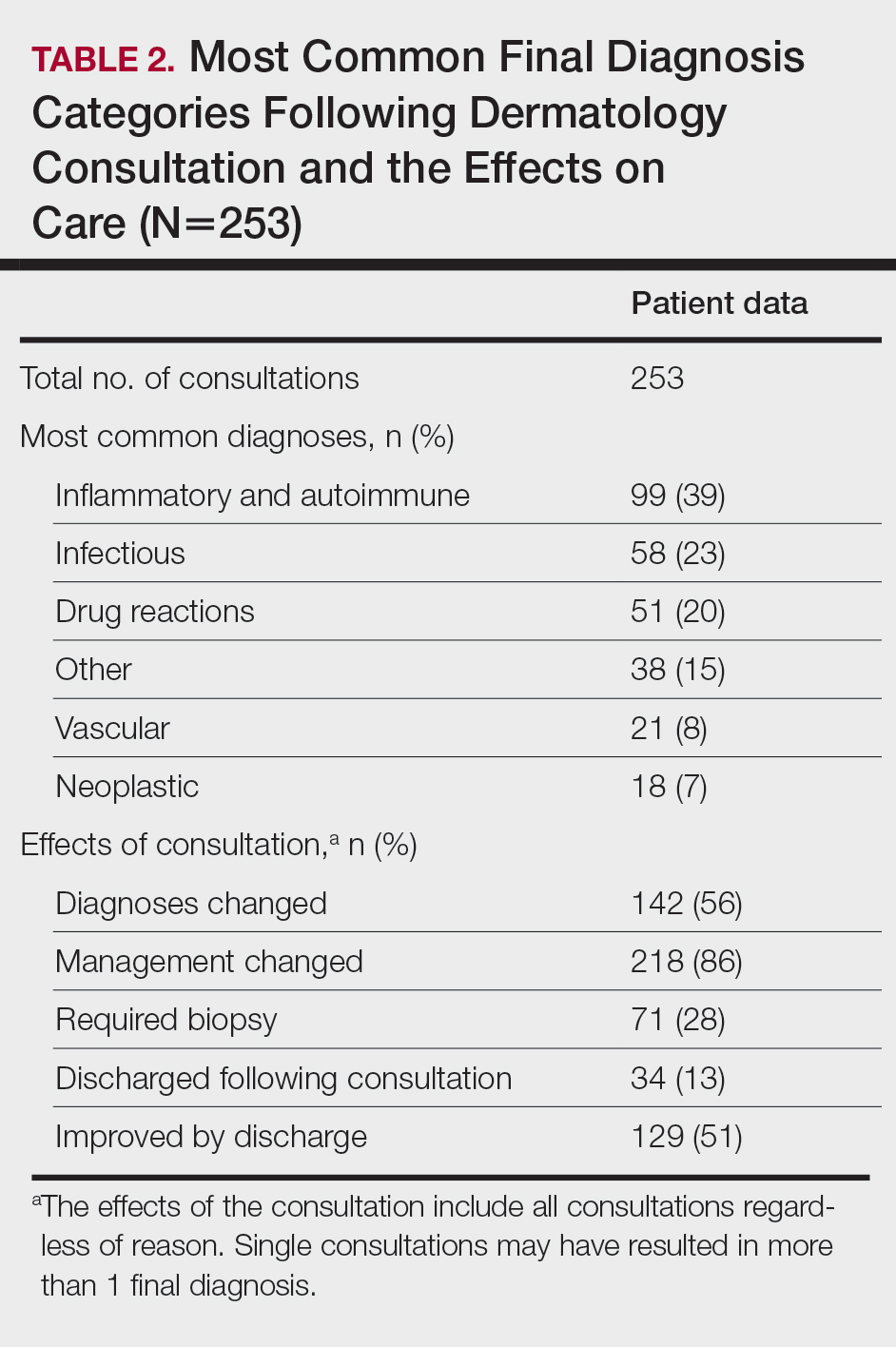
Most Common Final Diagnoses and Consultation Impact—Table 2 lists the most common final diagnosis categories, as well as the effects of the consultation on diagnosis, management, biopsies, hospitalization, and clinical improvement as documented by the primary medical provider. The most common final diagnoses were inflammatory and autoimmune (39%), such as contact dermatitis and seborrheic dermatitis; infectious (23%), such as varicella (primary or zoster) and bacterial furunculosis; drug reactions (20%), such as morbilliform drug eruptions; vascular (8%), such as vasculitis and calciphylaxis; neoplastic (7%), such as keratinocyte carcinomas and leukemia cutis; and other (15%), such as xerosis, keratosis pilaris, and miliaria rubra.
Impact on Diagnosis—Fifty-six percent of all consultations resulted in a change in diagnosis. When dermatology was consulted specifically to assist in the diagnosis of a patient (195 consultations), the working diagnosis of the primary team was changed 69% of the time. Thirty-five of these consultation requests had no preliminary diagnosis, and the primary team listed the working diagnosis as either rash or a morphologic description of the lesion(s). Sixty-three percent of suspected drug eruptions ended with a diagnosis of a form of drug eruption, while 20% of consultations for suspected cellulitis or bacterial infections were confirmed to be cellulitis or soft tissue infections.
Impact on Management—Regardless of the reason for the consultation, most consultations (86%) resulted in a change in management. The remaining 14% consisted of FBSEs with benign findings; cases of cutaneous metastases and leukemia cutis managed by oncology; as well as select cases of purpura fulminans, postfebrile desquamation, and postinflammatory hyperpigmentation.
Changes in management included alterations in medications, requests for additional laboratory work or imaging, additional consultation requests, biopsies, or specific wound care instructions. Seventy-five percent of all consultations were given specific medication recommendations by dermatology. Most (61%) were recommended to be given a topical steroid, antibiotic, or both. However, 45% of all consultations were recommended to initiate a systemic medication, most commonly antihistamines, antibiotics, steroids, antivirals, or immunomodulators. Dermatology recommended discontinuing specific medications in 16% of all consultations, with antibiotics being the most frequent culprit (17 antibiotics discontinued), owing to drug eruptions or misdiagnosed infections. Vancomycin, piperacillin-tazobactam, and trimethoprim-sulfamethoxazole were the most frequently discontinued antibiotics.
Dermatology was consulted for assistance in management of previously diagnosed cutaneous conditions 56 times (22% of all consultations), often regarding complicated cases of hidradenitis suppurativa (9 cases), pyoderma gangrenosum (5 cases), bullous pemphigoid (4 cases), or erythroderma (4 cases). Most of these cases required a single dermatology encounter to provide recommendations (71%), and 21% required 1 additional follow-up. Sixty-three percent of patients consulted for management assistance were noted to have improvement in their cutaneous condition by time of discharge, as documented by the primary provider in the medical record.
Twenty-eight percent of all consultations required at least 1 biopsy. Seventy-two percent of all biopsies were consistent with the dermatologist’s working diagnosis or highest-ranked differential diagnosis, and 16% of biopsy results were consistent with the second- or third-ranked diagnosis. The primary teams requested a biopsy 38 times to assist in diagnosis, as documented in the progress note or consultation request. Only 21 of these consultations (55% of requests) received at least 1 biopsy, as the remaining consultations did not require a biopsy to establish a diagnosis. The most common final diagnoses of consultations receiving biopsies included drug eruptions (5), leukemia cutis (4), vasculopathies (4), vasculitis (4), and calciphylaxis (3).
Impact on Hospitalization and Efficacy—Dermatology performed 217 consultations regarding patients already admitted to the hospital, and 92% remained hospitalized either due to comorbidities or complicated cutaneous conditions following the consultation. The remaining 8% were cleared for discharge. Dermatology received 36 consultation requests from emergency medicine physicians. Fifty-three percent of these patients were admitted, while the remaining 47% were discharged from the emergency department or its observation unit following evaluation.
Fifty-one percent of all consultations were noted to have improvement in their cutaneous condition by the time of discharge, as noted in the physical examination, progress note, or discharge summary of the primary team. Thirty percent of cases remained stable, where improvement was not noted in in the medical record. Most of these cases involved keratinocyte carcinomas scheduled for outpatient excision, benign melanocytic nevi found on FBSE, and benign etiologies that led to immediate discharge following consultation. Three percent of all consultations were noted to have worsened following consultation, including cases of calciphylaxis, vasculopathies, and purpura fulminans, as well as patients who elected for palliative care and hospice. The cutaneous condition by the time of discharge could not be determined from the medical record in 16% of all consultations.
Eighty-five percent of all consultations required a single encounter with dermatology. An additional 10% required a single follow-up with dermatology, while only 5% of patients required 3 or more encounters. Notably, these cases included patients with 1 or more severe cutaneous diseases, such as Sweet syndrome, calciphylaxis, Stevens-Johnson syndrome/toxic epidermal necrolysis, and hidradenitis suppurativa.
Comment
Although dermatology often is viewed as an outpatient specialty, this study provides a glimpse into the ways inpatient dermatology consultations optimize the care of hospitalized patients. Most consultations involved assistance in diagnosing an unknown condition, but several regarded pre-existing skin disorders requiring management aid. As a variety of medical specialties requested consultations, dermatology was able to provide care to a diverse group of patients with conditions varying in complexity and severity. Several specialties benefited from niche dermatologic expertise: hematology and oncology frequently requested dermatology to assist in diagnosis and management of the toxic effects of chemotherapy, cutaneous metastasis, or suspected cutaneous infections in immunocompromised patients. Cardiology patients were frequently evaluated for potential malignancy or infection prior to heart transplantation and initiation of antirejection immunosuppressants. Dermatology was consulted to differentiate cutaneous manifestations of critical illness from underlying systemic disease in the intensive care unit, and patients presenting to the emergency department often were examined to determine if hospital admission was necessary, with 47% of these consultations resulting in a discharge following evaluation by a dermatologist.
Our results were consistent with prior studies1,5,6 that have reported frequent changes in final diagnosis following dermatology consultation, with 69% of working diagnoses changed in this study when consultation was requested for diagnostic assistance. When dermatology was consulted for diagnostic assistance, several of these cases lacked a preliminary differential diagnosis. Although the absence of a documented differential diagnosis may not necessarily reflect a lack of suspicion for a particular etiology, 86% of all consultations included a ranked differential or working diagnosis either in the consultation request or progress note prior to consultation. The final diagnoses of consultations without a preliminary diagnosis varied from the mild and localized to systemic and severe, further suggesting these cases reflected knowledge gaps of the primary medical team.
Integration of dermatology into the care of hospitalized patients could provide an opportunity for education of primary medical teams. With frequent consultation, primary medical teams may become more comfortable diagnosing and managing common cutaneous conditions specific to their specialty or extended hospitalizations.
Several consultations were requested to aid in management of cases of hidradenitis suppurativa, pyoderma gangrenosum, or bullous pemphigoid that either failed outpatient therapy or were complicated by superinfections. Despite the ranges in complexity, the majority of all consultations required a single encounter and led to improvement by the time of discharge, demonstrating the efficacy and efficiency of inpatient dermatologists.
Dermatology consultations often led to changes in management involving medications and additional workup. Changes in management also extended to specific wound care instructions provided by dermatology, as expected for cases of Stevens-Johnson syndrome/toxic epidermal necrolysis, Sweet syndrome, hidradenitis suppurativa, and pyoderma gangrenosum. However, patients with the sequelae of extended hospitalizations, such as chronic wounds, pressure ulcers, and edema bullae, also benefited from this expertise.
When patients required a biopsy, the final diagnoses were consistent with the dermatologist’s number one differential diagnosis or top 3 differential diagnoses 72% and 88% of the time, respectively. Only 55% of cases where the primary team requested a biopsy ultimately required a biopsy, as many involved clinical diagnoses such as urticaria. Not only was dermatology accurate in their preliminary diagnoses, but they decreased cost and morbidity by avoiding unnecessary procedures.
This study provided additional evidence to support the integration of dermatology into the hospital setting for the benefit of patients, primary medical teams, and hospital systems. Dermatology offers high-value care through the efficient diagnosis and management of hospitalized patients, which contributes to decreased cost and improved outcomes.2,5-7,9,10 This study highlighted lesser-known areas of impact, such as the various specialty-specific services dermatology provides as well as the high rates of reported improvement following consultation. Future studies should continue to explore the field’s unique impact on hospitalized medicine as well as other avenues of care delivery, such as telemedicine, that may encourage dermatologists to participate in consultations and increase the volume of patients who may benefit from their care.
- Madigan LM, Fox LP. Where are we now with inpatient consultative dermatology?: assessing the value and evolution of this subspecialty over the past decade. J Am Acad Dermatol. 2019;80:1804-1808. doi:10.1016/j.jaad.2019.01.031
- Noe MH, Rosenbach M. Inpatient dermatologists—crucial for the management of skin diseases in hospitalized patients [editorial]. JAMA Dermatol. 2018;154:524-525. doi:10.1001/jamadermatol.2017.6195
- Strowd LC. Inpatient dermatology: a paradigm shift in the management of skin disease in the hospital. Br J Dermatol. 2019;180:966-967. doi:10.1111/bjd.17778
- Kirsner RS, Yang DG, Kerdel FA. The changing status of inpatient dermatology at American academic dermatology programs. J Am Acad Dermatol. 1999;40:755-757. doi:10.1016/s0190-9622(99)70158-1
- Kroshinsky D, Cotliar J, Hughey LC, et al. Association of dermatology consultation with accuracy of cutaneous disorder diagnoses in hospitalized patients: a multicenter analysis. JAMA Dermatol. 2016;152:477-480. doi:10.1001/jamadermatol.2015.5098
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis. JAMA Dermatol. 2018;154:529-533. doi:10.1001/jamadermatol.2017.6196
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543. doi:10.1001/jamadermatol.2017.6197
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528. doi:10.1001/jamadermatol.2016.6130
- Imadojemu S, Rosenbach M. Dermatologists must take an active role in the diagnosis of cellulitis. JAMA Dermatol. 2017;153:134-135. doi:10.1001/jamadermatol.2016.4230
- Hughey LC. The impact dermatologists can have on misdiagnosis of cellulitis and overuse of antibiotics: closing the gap. JAMA Dermatol. 2014;150:1061-1062. doi:10.1001/jamadermatol.2014.1164
- Ko LN, Kroshinsky D. Dermatology hospitalists: a multicenter survey study characterizing the infrastructure of consultative dermatology in select American hospitals. Int J Dermatol. 2018;57:553-558. doi:10.1111/ijd.13939
Dermatology is an often-underutilized resource in the hospital setting. As the health care landscape has evolved, so has the role of the inpatient dermatologist.1-3 Structural changes in the health system and advances in therapies have shifted dermatology from an admitting service to an almost exclusively outpatient practice. Improved treatment modalities led to decreases in the number of patients requiring admission for chronic dermatoses, and outpatient clinics began offering therapies once limited to hospitals.1,4 Inpatient dermatology consultations emerged and continue to have profound effects on hospitalized patients regardless of their reason for admission.1-11
Inpatient dermatologists supply knowledge in areas primary medical teams lack, and there is evidence that dermatology consultations improve the quality of care while decreasing cost.2,5-7 Establishing correct diagnoses, preventing exposure to unnecessary medications, and reducing hospitalization duration and readmission rates are a few ways dermatology consultations positively impact hospitalized patients.2,5-7,9,10 This study highlights the role of the dermatologist in the care of hospitalized patients at a large academic medical center in an urban setting and reveals how consultation supports the efficiency and efficacy of other services.
Materials and Methods
Study Design—This single-institution, cross-sectional retrospective study included all hospitalized patients at the Thomas Jefferson University Hospital (Philadelphia, Pennsylvania), who received an inpatient dermatology consultation completed by physicians of Jefferson Dermatology Associates between January 1, 2019, and December 31, 2019. The institutional review board at Thomas Jefferson University approved this study.
Data Collection—A list of all inpatient dermatology consultations in 2019 was provided by Jefferson Dermatology Associates. Through a retrospective chart review, data regarding the consultations were collected from the electronic medical record (Epic Systems) and recorded into the Research Electronic Data Capture system. Data on patient demographics, the primary medical team, the dermatology evaluation, and the hospital course of the patient were collected.
Results
Patient Characteristics—Dermatology received 253 inpatient consultation requests during this time period; 53% of patients were female and 47% were male, with a mean age of 55 years. Most patients were White (57%), while 34% were Black. Five percent and 4% of patients were Asian and Hispanic or Latino, respectively (Table 1). The mean duration of hospitalization for all patients was 15 days, and the average number of days to discharge following the first encounter with dermatology was 10 days.

Requesting Team and Reason for Consultation—Internal medicine consulted dermatology most frequently (34% of all consultations), followed by emergency medicine (14%) and a variety of other services (Table 1). Most dermatology consultations were placed to assist in achieving a diagnosis of a cutaneous condition (77%), while a minority were to assist in the management of a previously diagnosed disease (22%). A small fraction of consultations (5%) were to complete full-body skin examinations (FBSEs) to rule out infection or malignancy in candidates for organ transplantation, left ventricular assist devices, or certain chemotherapies. One FBSE was conducted to search for a primary tumor in a patient diagnosed with metastatic melanoma.
Most Common Final Diagnoses and Consultation Impact—Table 2 lists the most common final diagnosis categories, as well as the effects of the consultation on diagnosis, management, biopsies, hospitalization, and clinical improvement as documented by the primary medical provider. The most common final diagnoses were inflammatory and autoimmune (39%), such as contact dermatitis and seborrheic dermatitis; infectious (23%), such as varicella (primary or zoster) and bacterial furunculosis; drug reactions (20%), such as morbilliform drug eruptions; vascular (8%), such as vasculitis and calciphylaxis; neoplastic (7%), such as keratinocyte carcinomas and leukemia cutis; and other (15%), such as xerosis, keratosis pilaris, and miliaria rubra.

Impact on Diagnosis—Fifty-six percent of all consultations resulted in a change in diagnosis. When dermatology was consulted specifically to assist in the diagnosis of a patient (195 consultations), the working diagnosis of the primary team was changed 69% of the time. Thirty-five of these consultation requests had no preliminary diagnosis, and the primary team listed the working diagnosis as either rash or a morphologic description of the lesion(s). Sixty-three percent of suspected drug eruptions ended with a diagnosis of a form of drug eruption, while 20% of consultations for suspected cellulitis or bacterial infections were confirmed to be cellulitis or soft tissue infections.
Impact on Management—Regardless of the reason for the consultation, most consultations (86%) resulted in a change in management. The remaining 14% consisted of FBSEs with benign findings; cases of cutaneous metastases and leukemia cutis managed by oncology; as well as select cases of purpura fulminans, postfebrile desquamation, and postinflammatory hyperpigmentation.
Changes in management included alterations in medications, requests for additional laboratory work or imaging, additional consultation requests, biopsies, or specific wound care instructions. Seventy-five percent of all consultations were given specific medication recommendations by dermatology. Most (61%) were recommended to be given a topical steroid, antibiotic, or both. However, 45% of all consultations were recommended to initiate a systemic medication, most commonly antihistamines, antibiotics, steroids, antivirals, or immunomodulators. Dermatology recommended discontinuing specific medications in 16% of all consultations, with antibiotics being the most frequent culprit (17 antibiotics discontinued), owing to drug eruptions or misdiagnosed infections. Vancomycin, piperacillin-tazobactam, and trimethoprim-sulfamethoxazole were the most frequently discontinued antibiotics.
Dermatology was consulted for assistance in management of previously diagnosed cutaneous conditions 56 times (22% of all consultations), often regarding complicated cases of hidradenitis suppurativa (9 cases), pyoderma gangrenosum (5 cases), bullous pemphigoid (4 cases), or erythroderma (4 cases). Most of these cases required a single dermatology encounter to provide recommendations (71%), and 21% required 1 additional follow-up. Sixty-three percent of patients consulted for management assistance were noted to have improvement in their cutaneous condition by time of discharge, as documented by the primary provider in the medical record.
Twenty-eight percent of all consultations required at least 1 biopsy. Seventy-two percent of all biopsies were consistent with the dermatologist’s working diagnosis or highest-ranked differential diagnosis, and 16% of biopsy results were consistent with the second- or third-ranked diagnosis. The primary teams requested a biopsy 38 times to assist in diagnosis, as documented in the progress note or consultation request. Only 21 of these consultations (55% of requests) received at least 1 biopsy, as the remaining consultations did not require a biopsy to establish a diagnosis. The most common final diagnoses of consultations receiving biopsies included drug eruptions (5), leukemia cutis (4), vasculopathies (4), vasculitis (4), and calciphylaxis (3).
Impact on Hospitalization and Efficacy—Dermatology performed 217 consultations regarding patients already admitted to the hospital, and 92% remained hospitalized either due to comorbidities or complicated cutaneous conditions following the consultation. The remaining 8% were cleared for discharge. Dermatology received 36 consultation requests from emergency medicine physicians. Fifty-three percent of these patients were admitted, while the remaining 47% were discharged from the emergency department or its observation unit following evaluation.
Fifty-one percent of all consultations were noted to have improvement in their cutaneous condition by the time of discharge, as noted in the physical examination, progress note, or discharge summary of the primary team. Thirty percent of cases remained stable, where improvement was not noted in in the medical record. Most of these cases involved keratinocyte carcinomas scheduled for outpatient excision, benign melanocytic nevi found on FBSE, and benign etiologies that led to immediate discharge following consultation. Three percent of all consultations were noted to have worsened following consultation, including cases of calciphylaxis, vasculopathies, and purpura fulminans, as well as patients who elected for palliative care and hospice. The cutaneous condition by the time of discharge could not be determined from the medical record in 16% of all consultations.
Eighty-five percent of all consultations required a single encounter with dermatology. An additional 10% required a single follow-up with dermatology, while only 5% of patients required 3 or more encounters. Notably, these cases included patients with 1 or more severe cutaneous diseases, such as Sweet syndrome, calciphylaxis, Stevens-Johnson syndrome/toxic epidermal necrolysis, and hidradenitis suppurativa.
Comment
Although dermatology often is viewed as an outpatient specialty, this study provides a glimpse into the ways inpatient dermatology consultations optimize the care of hospitalized patients. Most consultations involved assistance in diagnosing an unknown condition, but several regarded pre-existing skin disorders requiring management aid. As a variety of medical specialties requested consultations, dermatology was able to provide care to a diverse group of patients with conditions varying in complexity and severity. Several specialties benefited from niche dermatologic expertise: hematology and oncology frequently requested dermatology to assist in diagnosis and management of the toxic effects of chemotherapy, cutaneous metastasis, or suspected cutaneous infections in immunocompromised patients. Cardiology patients were frequently evaluated for potential malignancy or infection prior to heart transplantation and initiation of antirejection immunosuppressants. Dermatology was consulted to differentiate cutaneous manifestations of critical illness from underlying systemic disease in the intensive care unit, and patients presenting to the emergency department often were examined to determine if hospital admission was necessary, with 47% of these consultations resulting in a discharge following evaluation by a dermatologist.
Our results were consistent with prior studies1,5,6 that have reported frequent changes in final diagnosis following dermatology consultation, with 69% of working diagnoses changed in this study when consultation was requested for diagnostic assistance. When dermatology was consulted for diagnostic assistance, several of these cases lacked a preliminary differential diagnosis. Although the absence of a documented differential diagnosis may not necessarily reflect a lack of suspicion for a particular etiology, 86% of all consultations included a ranked differential or working diagnosis either in the consultation request or progress note prior to consultation. The final diagnoses of consultations without a preliminary diagnosis varied from the mild and localized to systemic and severe, further suggesting these cases reflected knowledge gaps of the primary medical team.
Integration of dermatology into the care of hospitalized patients could provide an opportunity for education of primary medical teams. With frequent consultation, primary medical teams may become more comfortable diagnosing and managing common cutaneous conditions specific to their specialty or extended hospitalizations.
Several consultations were requested to aid in management of cases of hidradenitis suppurativa, pyoderma gangrenosum, or bullous pemphigoid that either failed outpatient therapy or were complicated by superinfections. Despite the ranges in complexity, the majority of all consultations required a single encounter and led to improvement by the time of discharge, demonstrating the efficacy and efficiency of inpatient dermatologists.
Dermatology consultations often led to changes in management involving medications and additional workup. Changes in management also extended to specific wound care instructions provided by dermatology, as expected for cases of Stevens-Johnson syndrome/toxic epidermal necrolysis, Sweet syndrome, hidradenitis suppurativa, and pyoderma gangrenosum. However, patients with the sequelae of extended hospitalizations, such as chronic wounds, pressure ulcers, and edema bullae, also benefited from this expertise.
When patients required a biopsy, the final diagnoses were consistent with the dermatologist’s number one differential diagnosis or top 3 differential diagnoses 72% and 88% of the time, respectively. Only 55% of cases where the primary team requested a biopsy ultimately required a biopsy, as many involved clinical diagnoses such as urticaria. Not only was dermatology accurate in their preliminary diagnoses, but they decreased cost and morbidity by avoiding unnecessary procedures.
This study provided additional evidence to support the integration of dermatology into the hospital setting for the benefit of patients, primary medical teams, and hospital systems. Dermatology offers high-value care through the efficient diagnosis and management of hospitalized patients, which contributes to decreased cost and improved outcomes.2,5-7,9,10 This study highlighted lesser-known areas of impact, such as the various specialty-specific services dermatology provides as well as the high rates of reported improvement following consultation. Future studies should continue to explore the field’s unique impact on hospitalized medicine as well as other avenues of care delivery, such as telemedicine, that may encourage dermatologists to participate in consultations and increase the volume of patients who may benefit from their care.
Dermatology is an often-underutilized resource in the hospital setting. As the health care landscape has evolved, so has the role of the inpatient dermatologist.1-3 Structural changes in the health system and advances in therapies have shifted dermatology from an admitting service to an almost exclusively outpatient practice. Improved treatment modalities led to decreases in the number of patients requiring admission for chronic dermatoses, and outpatient clinics began offering therapies once limited to hospitals.1,4 Inpatient dermatology consultations emerged and continue to have profound effects on hospitalized patients regardless of their reason for admission.1-11
Inpatient dermatologists supply knowledge in areas primary medical teams lack, and there is evidence that dermatology consultations improve the quality of care while decreasing cost.2,5-7 Establishing correct diagnoses, preventing exposure to unnecessary medications, and reducing hospitalization duration and readmission rates are a few ways dermatology consultations positively impact hospitalized patients.2,5-7,9,10 This study highlights the role of the dermatologist in the care of hospitalized patients at a large academic medical center in an urban setting and reveals how consultation supports the efficiency and efficacy of other services.
Materials and Methods
Study Design—This single-institution, cross-sectional retrospective study included all hospitalized patients at the Thomas Jefferson University Hospital (Philadelphia, Pennsylvania), who received an inpatient dermatology consultation completed by physicians of Jefferson Dermatology Associates between January 1, 2019, and December 31, 2019. The institutional review board at Thomas Jefferson University approved this study.
Data Collection—A list of all inpatient dermatology consultations in 2019 was provided by Jefferson Dermatology Associates. Through a retrospective chart review, data regarding the consultations were collected from the electronic medical record (Epic Systems) and recorded into the Research Electronic Data Capture system. Data on patient demographics, the primary medical team, the dermatology evaluation, and the hospital course of the patient were collected.
Results
Patient Characteristics—Dermatology received 253 inpatient consultation requests during this time period; 53% of patients were female and 47% were male, with a mean age of 55 years. Most patients were White (57%), while 34% were Black. Five percent and 4% of patients were Asian and Hispanic or Latino, respectively (Table 1). The mean duration of hospitalization for all patients was 15 days, and the average number of days to discharge following the first encounter with dermatology was 10 days.

Requesting Team and Reason for Consultation—Internal medicine consulted dermatology most frequently (34% of all consultations), followed by emergency medicine (14%) and a variety of other services (Table 1). Most dermatology consultations were placed to assist in achieving a diagnosis of a cutaneous condition (77%), while a minority were to assist in the management of a previously diagnosed disease (22%). A small fraction of consultations (5%) were to complete full-body skin examinations (FBSEs) to rule out infection or malignancy in candidates for organ transplantation, left ventricular assist devices, or certain chemotherapies. One FBSE was conducted to search for a primary tumor in a patient diagnosed with metastatic melanoma.
Most Common Final Diagnoses and Consultation Impact—Table 2 lists the most common final diagnosis categories, as well as the effects of the consultation on diagnosis, management, biopsies, hospitalization, and clinical improvement as documented by the primary medical provider. The most common final diagnoses were inflammatory and autoimmune (39%), such as contact dermatitis and seborrheic dermatitis; infectious (23%), such as varicella (primary or zoster) and bacterial furunculosis; drug reactions (20%), such as morbilliform drug eruptions; vascular (8%), such as vasculitis and calciphylaxis; neoplastic (7%), such as keratinocyte carcinomas and leukemia cutis; and other (15%), such as xerosis, keratosis pilaris, and miliaria rubra.

Impact on Diagnosis—Fifty-six percent of all consultations resulted in a change in diagnosis. When dermatology was consulted specifically to assist in the diagnosis of a patient (195 consultations), the working diagnosis of the primary team was changed 69% of the time. Thirty-five of these consultation requests had no preliminary diagnosis, and the primary team listed the working diagnosis as either rash or a morphologic description of the lesion(s). Sixty-three percent of suspected drug eruptions ended with a diagnosis of a form of drug eruption, while 20% of consultations for suspected cellulitis or bacterial infections were confirmed to be cellulitis or soft tissue infections.
Impact on Management—Regardless of the reason for the consultation, most consultations (86%) resulted in a change in management. The remaining 14% consisted of FBSEs with benign findings; cases of cutaneous metastases and leukemia cutis managed by oncology; as well as select cases of purpura fulminans, postfebrile desquamation, and postinflammatory hyperpigmentation.
Changes in management included alterations in medications, requests for additional laboratory work or imaging, additional consultation requests, biopsies, or specific wound care instructions. Seventy-five percent of all consultations were given specific medication recommendations by dermatology. Most (61%) were recommended to be given a topical steroid, antibiotic, or both. However, 45% of all consultations were recommended to initiate a systemic medication, most commonly antihistamines, antibiotics, steroids, antivirals, or immunomodulators. Dermatology recommended discontinuing specific medications in 16% of all consultations, with antibiotics being the most frequent culprit (17 antibiotics discontinued), owing to drug eruptions or misdiagnosed infections. Vancomycin, piperacillin-tazobactam, and trimethoprim-sulfamethoxazole were the most frequently discontinued antibiotics.
Dermatology was consulted for assistance in management of previously diagnosed cutaneous conditions 56 times (22% of all consultations), often regarding complicated cases of hidradenitis suppurativa (9 cases), pyoderma gangrenosum (5 cases), bullous pemphigoid (4 cases), or erythroderma (4 cases). Most of these cases required a single dermatology encounter to provide recommendations (71%), and 21% required 1 additional follow-up. Sixty-three percent of patients consulted for management assistance were noted to have improvement in their cutaneous condition by time of discharge, as documented by the primary provider in the medical record.
Twenty-eight percent of all consultations required at least 1 biopsy. Seventy-two percent of all biopsies were consistent with the dermatologist’s working diagnosis or highest-ranked differential diagnosis, and 16% of biopsy results were consistent with the second- or third-ranked diagnosis. The primary teams requested a biopsy 38 times to assist in diagnosis, as documented in the progress note or consultation request. Only 21 of these consultations (55% of requests) received at least 1 biopsy, as the remaining consultations did not require a biopsy to establish a diagnosis. The most common final diagnoses of consultations receiving biopsies included drug eruptions (5), leukemia cutis (4), vasculopathies (4), vasculitis (4), and calciphylaxis (3).
Impact on Hospitalization and Efficacy—Dermatology performed 217 consultations regarding patients already admitted to the hospital, and 92% remained hospitalized either due to comorbidities or complicated cutaneous conditions following the consultation. The remaining 8% were cleared for discharge. Dermatology received 36 consultation requests from emergency medicine physicians. Fifty-three percent of these patients were admitted, while the remaining 47% were discharged from the emergency department or its observation unit following evaluation.
Fifty-one percent of all consultations were noted to have improvement in their cutaneous condition by the time of discharge, as noted in the physical examination, progress note, or discharge summary of the primary team. Thirty percent of cases remained stable, where improvement was not noted in in the medical record. Most of these cases involved keratinocyte carcinomas scheduled for outpatient excision, benign melanocytic nevi found on FBSE, and benign etiologies that led to immediate discharge following consultation. Three percent of all consultations were noted to have worsened following consultation, including cases of calciphylaxis, vasculopathies, and purpura fulminans, as well as patients who elected for palliative care and hospice. The cutaneous condition by the time of discharge could not be determined from the medical record in 16% of all consultations.
Eighty-five percent of all consultations required a single encounter with dermatology. An additional 10% required a single follow-up with dermatology, while only 5% of patients required 3 or more encounters. Notably, these cases included patients with 1 or more severe cutaneous diseases, such as Sweet syndrome, calciphylaxis, Stevens-Johnson syndrome/toxic epidermal necrolysis, and hidradenitis suppurativa.
Comment
Although dermatology often is viewed as an outpatient specialty, this study provides a glimpse into the ways inpatient dermatology consultations optimize the care of hospitalized patients. Most consultations involved assistance in diagnosing an unknown condition, but several regarded pre-existing skin disorders requiring management aid. As a variety of medical specialties requested consultations, dermatology was able to provide care to a diverse group of patients with conditions varying in complexity and severity. Several specialties benefited from niche dermatologic expertise: hematology and oncology frequently requested dermatology to assist in diagnosis and management of the toxic effects of chemotherapy, cutaneous metastasis, or suspected cutaneous infections in immunocompromised patients. Cardiology patients were frequently evaluated for potential malignancy or infection prior to heart transplantation and initiation of antirejection immunosuppressants. Dermatology was consulted to differentiate cutaneous manifestations of critical illness from underlying systemic disease in the intensive care unit, and patients presenting to the emergency department often were examined to determine if hospital admission was necessary, with 47% of these consultations resulting in a discharge following evaluation by a dermatologist.
Our results were consistent with prior studies1,5,6 that have reported frequent changes in final diagnosis following dermatology consultation, with 69% of working diagnoses changed in this study when consultation was requested for diagnostic assistance. When dermatology was consulted for diagnostic assistance, several of these cases lacked a preliminary differential diagnosis. Although the absence of a documented differential diagnosis may not necessarily reflect a lack of suspicion for a particular etiology, 86% of all consultations included a ranked differential or working diagnosis either in the consultation request or progress note prior to consultation. The final diagnoses of consultations without a preliminary diagnosis varied from the mild and localized to systemic and severe, further suggesting these cases reflected knowledge gaps of the primary medical team.
Integration of dermatology into the care of hospitalized patients could provide an opportunity for education of primary medical teams. With frequent consultation, primary medical teams may become more comfortable diagnosing and managing common cutaneous conditions specific to their specialty or extended hospitalizations.
Several consultations were requested to aid in management of cases of hidradenitis suppurativa, pyoderma gangrenosum, or bullous pemphigoid that either failed outpatient therapy or were complicated by superinfections. Despite the ranges in complexity, the majority of all consultations required a single encounter and led to improvement by the time of discharge, demonstrating the efficacy and efficiency of inpatient dermatologists.
Dermatology consultations often led to changes in management involving medications and additional workup. Changes in management also extended to specific wound care instructions provided by dermatology, as expected for cases of Stevens-Johnson syndrome/toxic epidermal necrolysis, Sweet syndrome, hidradenitis suppurativa, and pyoderma gangrenosum. However, patients with the sequelae of extended hospitalizations, such as chronic wounds, pressure ulcers, and edema bullae, also benefited from this expertise.
When patients required a biopsy, the final diagnoses were consistent with the dermatologist’s number one differential diagnosis or top 3 differential diagnoses 72% and 88% of the time, respectively. Only 55% of cases where the primary team requested a biopsy ultimately required a biopsy, as many involved clinical diagnoses such as urticaria. Not only was dermatology accurate in their preliminary diagnoses, but they decreased cost and morbidity by avoiding unnecessary procedures.
This study provided additional evidence to support the integration of dermatology into the hospital setting for the benefit of patients, primary medical teams, and hospital systems. Dermatology offers high-value care through the efficient diagnosis and management of hospitalized patients, which contributes to decreased cost and improved outcomes.2,5-7,9,10 This study highlighted lesser-known areas of impact, such as the various specialty-specific services dermatology provides as well as the high rates of reported improvement following consultation. Future studies should continue to explore the field’s unique impact on hospitalized medicine as well as other avenues of care delivery, such as telemedicine, that may encourage dermatologists to participate in consultations and increase the volume of patients who may benefit from their care.
- Madigan LM, Fox LP. Where are we now with inpatient consultative dermatology?: assessing the value and evolution of this subspecialty over the past decade. J Am Acad Dermatol. 2019;80:1804-1808. doi:10.1016/j.jaad.2019.01.031
- Noe MH, Rosenbach M. Inpatient dermatologists—crucial for the management of skin diseases in hospitalized patients [editorial]. JAMA Dermatol. 2018;154:524-525. doi:10.1001/jamadermatol.2017.6195
- Strowd LC. Inpatient dermatology: a paradigm shift in the management of skin disease in the hospital. Br J Dermatol. 2019;180:966-967. doi:10.1111/bjd.17778
- Kirsner RS, Yang DG, Kerdel FA. The changing status of inpatient dermatology at American academic dermatology programs. J Am Acad Dermatol. 1999;40:755-757. doi:10.1016/s0190-9622(99)70158-1
- Kroshinsky D, Cotliar J, Hughey LC, et al. Association of dermatology consultation with accuracy of cutaneous disorder diagnoses in hospitalized patients: a multicenter analysis. JAMA Dermatol. 2016;152:477-480. doi:10.1001/jamadermatol.2015.5098
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis. JAMA Dermatol. 2018;154:529-533. doi:10.1001/jamadermatol.2017.6196
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543. doi:10.1001/jamadermatol.2017.6197
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528. doi:10.1001/jamadermatol.2016.6130
- Imadojemu S, Rosenbach M. Dermatologists must take an active role in the diagnosis of cellulitis. JAMA Dermatol. 2017;153:134-135. doi:10.1001/jamadermatol.2016.4230
- Hughey LC. The impact dermatologists can have on misdiagnosis of cellulitis and overuse of antibiotics: closing the gap. JAMA Dermatol. 2014;150:1061-1062. doi:10.1001/jamadermatol.2014.1164
- Ko LN, Kroshinsky D. Dermatology hospitalists: a multicenter survey study characterizing the infrastructure of consultative dermatology in select American hospitals. Int J Dermatol. 2018;57:553-558. doi:10.1111/ijd.13939
- Madigan LM, Fox LP. Where are we now with inpatient consultative dermatology?: assessing the value and evolution of this subspecialty over the past decade. J Am Acad Dermatol. 2019;80:1804-1808. doi:10.1016/j.jaad.2019.01.031
- Noe MH, Rosenbach M. Inpatient dermatologists—crucial for the management of skin diseases in hospitalized patients [editorial]. JAMA Dermatol. 2018;154:524-525. doi:10.1001/jamadermatol.2017.6195
- Strowd LC. Inpatient dermatology: a paradigm shift in the management of skin disease in the hospital. Br J Dermatol. 2019;180:966-967. doi:10.1111/bjd.17778
- Kirsner RS, Yang DG, Kerdel FA. The changing status of inpatient dermatology at American academic dermatology programs. J Am Acad Dermatol. 1999;40:755-757. doi:10.1016/s0190-9622(99)70158-1
- Kroshinsky D, Cotliar J, Hughey LC, et al. Association of dermatology consultation with accuracy of cutaneous disorder diagnoses in hospitalized patients: a multicenter analysis. JAMA Dermatol. 2016;152:477-480. doi:10.1001/jamadermatol.2015.5098
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis. JAMA Dermatol. 2018;154:529-533. doi:10.1001/jamadermatol.2017.6196
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543. doi:10.1001/jamadermatol.2017.6197
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528. doi:10.1001/jamadermatol.2016.6130
- Imadojemu S, Rosenbach M. Dermatologists must take an active role in the diagnosis of cellulitis. JAMA Dermatol. 2017;153:134-135. doi:10.1001/jamadermatol.2016.4230
- Hughey LC. The impact dermatologists can have on misdiagnosis of cellulitis and overuse of antibiotics: closing the gap. JAMA Dermatol. 2014;150:1061-1062. doi:10.1001/jamadermatol.2014.1164
- Ko LN, Kroshinsky D. Dermatology hospitalists: a multicenter survey study characterizing the infrastructure of consultative dermatology in select American hospitals. Int J Dermatol. 2018;57:553-558. doi:10.1111/ijd.13939
Practice Points
- Inpatient dermatologists fill knowledge gaps that often alter the diagnosis, management, and hospital course of hospitalized patients.
- Several medical specialties benefit from niche expertise of inpatient dermatologists specific to their patient population.
- Integration of inpatient dermatology consultations can prevent unnecessary hospital admissions and medication administration.
Flagellate Shiitake Mushroom Reaction With Histologic Features of Acute Generalized Exanthematous Pustulosis
To the Editor:
A 59-year-old man presented with a severely pruritic rash on the legs, arms, abdomen, groin, and buttocks of 3 days’ duration. He reported subjective fever and chills. Prior to the appearance of the rash, the patient and his family had eaten shiitake mushrooms daily for 3 days. He denied any new medications in the last several months or any recent upper respiratory or gastrointestinal tract illnesses. His medical history included type 2 diabetes mellitus and diabetes-induced end-stage renal disease requiring home peritoneal dialysis. His long-term medications for diabetes mellitus, hypertension, benign prostatic hyperplasia, hyperlipidemia, and insomnia included amlodipine, atorvastatin, finasteride, gabapentin, insulin glargine, linagliptin, metoprolol, and mirtazapine.
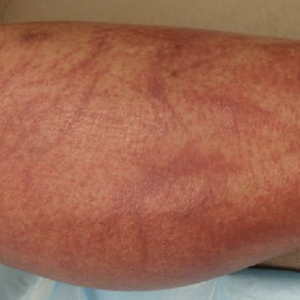
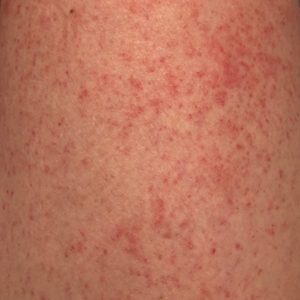
Physical examination revealed an afebrile man with medium brown skin tone and diffuse, bright red, erythematous patches on the lower legs, axillae, medial forearms, lateral trunk, lower abdomen, and groin. There were distinct flagellate, linear, red patches on the lower legs (Figure 1). In addition, small clusters of 1- to 2-mm superficial pustules were present on the right upper medial thigh and left forearm with micropapules grouped in the skin folds.

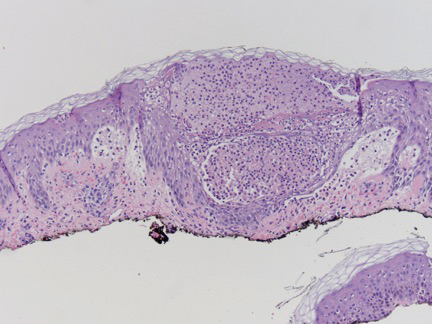
A shave biopsy specimen from a pustule on the right upper medial thigh revealed spongiotic dermatitis with neutrophilic subcorneal pustule formation and frequent eosinophils (Figure 2). The dermis contained scattered mixed inflammatory cells including neutrophils, eosinophils, lymphocytes, and histiocytes (Figure 3). These histologic findings were consistent with acute generalized exanthematous pustulosis (AGEP). No biopsy was performed on the flagellate patches due to its clinically distinct presentation and well-established association with shiitake mushroom ingestion.

The patient was treated with triamcinolone ointment and systemic corticosteroids to reduce pruritus and quickly clear the lesions due to his comorbidities. He recovered completely within 1 week and had no evidence of postinflammatory hyperpigmentation from the flagellate dermatitis.
Flagellate dermatitis is an intensely pruritic dermatitis characterized by 1-mm, disseminated, erythematous papules in a linear grouped arrangement secondary to koebnerization due to the patient scratching. It was first described in 1977 by Nakamura.1 Although it rarely is seen outside of China and Japan, there are well-established associations of flagellate dermatitis with bleomycin and shiitake mushroom (Lentinula edodes) ingestion. One key clinical difference between the two causes is that postinflammatory hyperpigmentation changes usually are seen with bleomycin-induced flagellate dermatitis and typically are not present with shiitake mushroom–induced flagellate dermatitis.2 Following ingestion of shiitake mushrooms, the median time of onset of presentation typically is 24 hours but ranges from 12 hours to 5 days. Most patients completely recover by 3 weeks, with or without treatment.3 Although the pathogenesis of shiitake mushroom–induced flagellate dermatitis is not clear, the most common theory is a toxic reaction to lentinan, a polysaccharide isolated from shiitake mushrooms. However, type I and IV allergic hypersensitivities also have been supported by the time of onset, clearance, severe pruritus, benefit from steroids and antihistamines, and lack of grouped outbreaks in people exposed to shared meals containing shiitake mushrooms.3,4 Furthermore, there is a case of patch test–confirmed allergic contact dermatitis to shiitake mushrooms, demonstrating a 1+ reaction at 96 hours to the cap of a shiitake mushroom but a negative pin-prick test at 20 minutes, suggesting type IV hypersensitivity.5 An additional case revealed a positive skin-prick test with formation of a 4-mm wheal and subsequent pruritic papules and vesicles appearing 48 to 72 hours later at the prick site.6 Subsequent cases have been reported in association with consumption of raw shiitake mushrooms, but cases have been reported after consumption of fully cooked mushrooms, which does not support a toxin-mediated theory, as cooking the mushroom before consumption likely would denature or change the structure of the suspected toxin.2
Acute generalized exanthematous pustulosis is a rare eruption that occurs due to ingestion of a causative agent, usually an antibiotic, and is characterized by the presence of fever and disseminated, erythematous, pinpoint, sterile pustules on the skin and mucous membranes. It affects 1 to 5 persons per million per year, with more than 90% of cases attributed to drug ingestion.7 Spontaneous resolution can be expected within 15 days of its onset; however, there is a mortality rate of up to 5% that occurs most often in those with severe comorbidities or in older patients, for whom systemic corticosteroid therapy may be justified.7,8 A multinational case-control study conducted to evaluate the risk of AGEP associated with certain drugs revealed macrolides (namely pristinamycin); β-lactam antibiotics including penicillin, aminopenicillin, and cephalosporin; quinolones; hydroxychloroquine; anti-infective sulfonamides; terbinafine; and diltiazem as the most strongly associated culprits.9 Our patient’s flagellate dermatitis was unique in that it also showed histologic features of AGEP. The pathogenesis of drug-induced AGEP has been partially elucidated and involves activation of drug-specific CD4+ and CD8+ T cells that migrate to the skin and participate in apoptotic signaling of keratinocytes and recruitment of neutrophils and eosinophils, which form subcorneal sterile pustules.7 In a study of severe cutaneous adverse drug reactions, 50% (7/14) of patients with AGEP had positive patch tests to the causative agent.10 This T cell–dependent response explains why the condition responds to systemic corticosteroids. Additionally, our case report of shiitake mushroom–induced flagellate dermatitis with histologic features of AGEP suggests that the pathogenesis of flagellate dermatitis may be a T cell–mediated type IV hypersensitivity reaction. The time of onset, lack of grouped outbreaks in those sharing shiitake mushroom–containing meals, severe pruritus, lack of cases demonstrating an anaphylactic or wheal and flare response, benefit of steroids, and a case with histologic features of AGEP all lend support to this theory.
We report a case of shiitake mushroom–induced flagellate dermatitis with histologic features of AGEP. The time course, histologic features of AGEP, absence of new medications, and resolution with discontinuation of shiitake mushrooms lends support of the hypothesis that the pathogenesis of shiitake mushroom–induced flagellate dermatitis is similar to AGEP’s type IV hypersensitivity reaction. To further elucidate its pathogenesis, skin prick testing and patch testing with shiitake mushrooms in patients exhibiting shiitake mushroom–induced flagellate dermatitis may prove to be beneficial.
- Nakamura T. Toxicoderma caused by shiitake (Lentinus edodes)[in Japanese]. Jpn J Clin Dermatol. 1977;31:65-68.
- Chu EY, Anand D, Dawn A, et al. Shiitake dermatitis: a report of 3 cases and review of the literature. Cutis. 2013;91:287-290.
- Boels D, Landreau A, Bruneau C, et al. Shiitake dermatitis recorded by French Poison Control Centers—new case series with clinical observations. Clin Toxicol (Phila). 2014;52:625-628.
- Nakamura T. Shiitake (Lentinus edodes) dermatitis. Contact Dermatitis. 1992;27:65-70.
- Curnow P, Tam M. Contact dermatitis to shiitake mushroom. Australas J Dermatol. 2003;44:155-157.
- Lippert U, Martin V, Schwertfeger C, et al. Shiitake dermatitis. Br J Dermatol. 2003;148:178-179.
- Fernando SL. Acute generalised exanthematous pustulosis. Australas J Dermatol. 2012;53:87-92.
- Sidoroff A, Halevy S, Bavinck JN, et al. Acute generalized exanthematous pustulosis (AGEP)—a clinical reaction pattern. J Cutan Pathol. 2001;28:113-119.
- Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)—results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007;157:989-996.
- Wolkenstein P, Chosidow O, Flechet ML, et al. Patch testing in severe cutaneous adverse drug reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Contact Dermatitis. 1996;35:234-236.
To the Editor:
A 59-year-old man presented with a severely pruritic rash on the legs, arms, abdomen, groin, and buttocks of 3 days’ duration. He reported subjective fever and chills. Prior to the appearance of the rash, the patient and his family had eaten shiitake mushrooms daily for 3 days. He denied any new medications in the last several months or any recent upper respiratory or gastrointestinal tract illnesses. His medical history included type 2 diabetes mellitus and diabetes-induced end-stage renal disease requiring home peritoneal dialysis. His long-term medications for diabetes mellitus, hypertension, benign prostatic hyperplasia, hyperlipidemia, and insomnia included amlodipine, atorvastatin, finasteride, gabapentin, insulin glargine, linagliptin, metoprolol, and mirtazapine.
Physical examination revealed an afebrile man with medium brown skin tone and diffuse, bright red, erythematous patches on the lower legs, axillae, medial forearms, lateral trunk, lower abdomen, and groin. There were distinct flagellate, linear, red patches on the lower legs (Figure 1). In addition, small clusters of 1- to 2-mm superficial pustules were present on the right upper medial thigh and left forearm with micropapules grouped in the skin folds.

A shave biopsy specimen from a pustule on the right upper medial thigh revealed spongiotic dermatitis with neutrophilic subcorneal pustule formation and frequent eosinophils (Figure 2). The dermis contained scattered mixed inflammatory cells including neutrophils, eosinophils, lymphocytes, and histiocytes (Figure 3). These histologic findings were consistent with acute generalized exanthematous pustulosis (AGEP). No biopsy was performed on the flagellate patches due to its clinically distinct presentation and well-established association with shiitake mushroom ingestion.

The patient was treated with triamcinolone ointment and systemic corticosteroids to reduce pruritus and quickly clear the lesions due to his comorbidities. He recovered completely within 1 week and had no evidence of postinflammatory hyperpigmentation from the flagellate dermatitis.

Flagellate dermatitis is an intensely pruritic dermatitis characterized by 1-mm, disseminated, erythematous papules in a linear grouped arrangement secondary to koebnerization due to the patient scratching. It was first described in 1977 by Nakamura.1 Although it rarely is seen outside of China and Japan, there are well-established associations of flagellate dermatitis with bleomycin and shiitake mushroom (Lentinula edodes) ingestion. One key clinical difference between the two causes is that postinflammatory hyperpigmentation changes usually are seen with bleomycin-induced flagellate dermatitis and typically are not present with shiitake mushroom–induced flagellate dermatitis.2 Following ingestion of shiitake mushrooms, the median time of onset of presentation typically is 24 hours but ranges from 12 hours to 5 days. Most patients completely recover by 3 weeks, with or without treatment.3 Although the pathogenesis of shiitake mushroom–induced flagellate dermatitis is not clear, the most common theory is a toxic reaction to lentinan, a polysaccharide isolated from shiitake mushrooms. However, type I and IV allergic hypersensitivities also have been supported by the time of onset, clearance, severe pruritus, benefit from steroids and antihistamines, and lack of grouped outbreaks in people exposed to shared meals containing shiitake mushrooms.3,4 Furthermore, there is a case of patch test–confirmed allergic contact dermatitis to shiitake mushrooms, demonstrating a 1+ reaction at 96 hours to the cap of a shiitake mushroom but a negative pin-prick test at 20 minutes, suggesting type IV hypersensitivity.5 An additional case revealed a positive skin-prick test with formation of a 4-mm wheal and subsequent pruritic papules and vesicles appearing 48 to 72 hours later at the prick site.6 Subsequent cases have been reported in association with consumption of raw shiitake mushrooms, but cases have been reported after consumption of fully cooked mushrooms, which does not support a toxin-mediated theory, as cooking the mushroom before consumption likely would denature or change the structure of the suspected toxin.2
Acute generalized exanthematous pustulosis is a rare eruption that occurs due to ingestion of a causative agent, usually an antibiotic, and is characterized by the presence of fever and disseminated, erythematous, pinpoint, sterile pustules on the skin and mucous membranes. It affects 1 to 5 persons per million per year, with more than 90% of cases attributed to drug ingestion.7 Spontaneous resolution can be expected within 15 days of its onset; however, there is a mortality rate of up to 5% that occurs most often in those with severe comorbidities or in older patients, for whom systemic corticosteroid therapy may be justified.7,8 A multinational case-control study conducted to evaluate the risk of AGEP associated with certain drugs revealed macrolides (namely pristinamycin); β-lactam antibiotics including penicillin, aminopenicillin, and cephalosporin; quinolones; hydroxychloroquine; anti-infective sulfonamides; terbinafine; and diltiazem as the most strongly associated culprits.9 Our patient’s flagellate dermatitis was unique in that it also showed histologic features of AGEP. The pathogenesis of drug-induced AGEP has been partially elucidated and involves activation of drug-specific CD4+ and CD8+ T cells that migrate to the skin and participate in apoptotic signaling of keratinocytes and recruitment of neutrophils and eosinophils, which form subcorneal sterile pustules.7 In a study of severe cutaneous adverse drug reactions, 50% (7/14) of patients with AGEP had positive patch tests to the causative agent.10 This T cell–dependent response explains why the condition responds to systemic corticosteroids. Additionally, our case report of shiitake mushroom–induced flagellate dermatitis with histologic features of AGEP suggests that the pathogenesis of flagellate dermatitis may be a T cell–mediated type IV hypersensitivity reaction. The time of onset, lack of grouped outbreaks in those sharing shiitake mushroom–containing meals, severe pruritus, lack of cases demonstrating an anaphylactic or wheal and flare response, benefit of steroids, and a case with histologic features of AGEP all lend support to this theory.
We report a case of shiitake mushroom–induced flagellate dermatitis with histologic features of AGEP. The time course, histologic features of AGEP, absence of new medications, and resolution with discontinuation of shiitake mushrooms lends support of the hypothesis that the pathogenesis of shiitake mushroom–induced flagellate dermatitis is similar to AGEP’s type IV hypersensitivity reaction. To further elucidate its pathogenesis, skin prick testing and patch testing with shiitake mushrooms in patients exhibiting shiitake mushroom–induced flagellate dermatitis may prove to be beneficial.
To the Editor:
A 59-year-old man presented with a severely pruritic rash on the legs, arms, abdomen, groin, and buttocks of 3 days’ duration. He reported subjective fever and chills. Prior to the appearance of the rash, the patient and his family had eaten shiitake mushrooms daily for 3 days. He denied any new medications in the last several months or any recent upper respiratory or gastrointestinal tract illnesses. His medical history included type 2 diabetes mellitus and diabetes-induced end-stage renal disease requiring home peritoneal dialysis. His long-term medications for diabetes mellitus, hypertension, benign prostatic hyperplasia, hyperlipidemia, and insomnia included amlodipine, atorvastatin, finasteride, gabapentin, insulin glargine, linagliptin, metoprolol, and mirtazapine.
Physical examination revealed an afebrile man with medium brown skin tone and diffuse, bright red, erythematous patches on the lower legs, axillae, medial forearms, lateral trunk, lower abdomen, and groin. There were distinct flagellate, linear, red patches on the lower legs (Figure 1). In addition, small clusters of 1- to 2-mm superficial pustules were present on the right upper medial thigh and left forearm with micropapules grouped in the skin folds.

A shave biopsy specimen from a pustule on the right upper medial thigh revealed spongiotic dermatitis with neutrophilic subcorneal pustule formation and frequent eosinophils (Figure 2). The dermis contained scattered mixed inflammatory cells including neutrophils, eosinophils, lymphocytes, and histiocytes (Figure 3). These histologic findings were consistent with acute generalized exanthematous pustulosis (AGEP). No biopsy was performed on the flagellate patches due to its clinically distinct presentation and well-established association with shiitake mushroom ingestion.

The patient was treated with triamcinolone ointment and systemic corticosteroids to reduce pruritus and quickly clear the lesions due to his comorbidities. He recovered completely within 1 week and had no evidence of postinflammatory hyperpigmentation from the flagellate dermatitis.

Flagellate dermatitis is an intensely pruritic dermatitis characterized by 1-mm, disseminated, erythematous papules in a linear grouped arrangement secondary to koebnerization due to the patient scratching. It was first described in 1977 by Nakamura.1 Although it rarely is seen outside of China and Japan, there are well-established associations of flagellate dermatitis with bleomycin and shiitake mushroom (Lentinula edodes) ingestion. One key clinical difference between the two causes is that postinflammatory hyperpigmentation changes usually are seen with bleomycin-induced flagellate dermatitis and typically are not present with shiitake mushroom–induced flagellate dermatitis.2 Following ingestion of shiitake mushrooms, the median time of onset of presentation typically is 24 hours but ranges from 12 hours to 5 days. Most patients completely recover by 3 weeks, with or without treatment.3 Although the pathogenesis of shiitake mushroom–induced flagellate dermatitis is not clear, the most common theory is a toxic reaction to lentinan, a polysaccharide isolated from shiitake mushrooms. However, type I and IV allergic hypersensitivities also have been supported by the time of onset, clearance, severe pruritus, benefit from steroids and antihistamines, and lack of grouped outbreaks in people exposed to shared meals containing shiitake mushrooms.3,4 Furthermore, there is a case of patch test–confirmed allergic contact dermatitis to shiitake mushrooms, demonstrating a 1+ reaction at 96 hours to the cap of a shiitake mushroom but a negative pin-prick test at 20 minutes, suggesting type IV hypersensitivity.5 An additional case revealed a positive skin-prick test with formation of a 4-mm wheal and subsequent pruritic papules and vesicles appearing 48 to 72 hours later at the prick site.6 Subsequent cases have been reported in association with consumption of raw shiitake mushrooms, but cases have been reported after consumption of fully cooked mushrooms, which does not support a toxin-mediated theory, as cooking the mushroom before consumption likely would denature or change the structure of the suspected toxin.2
Acute generalized exanthematous pustulosis is a rare eruption that occurs due to ingestion of a causative agent, usually an antibiotic, and is characterized by the presence of fever and disseminated, erythematous, pinpoint, sterile pustules on the skin and mucous membranes. It affects 1 to 5 persons per million per year, with more than 90% of cases attributed to drug ingestion.7 Spontaneous resolution can be expected within 15 days of its onset; however, there is a mortality rate of up to 5% that occurs most often in those with severe comorbidities or in older patients, for whom systemic corticosteroid therapy may be justified.7,8 A multinational case-control study conducted to evaluate the risk of AGEP associated with certain drugs revealed macrolides (namely pristinamycin); β-lactam antibiotics including penicillin, aminopenicillin, and cephalosporin; quinolones; hydroxychloroquine; anti-infective sulfonamides; terbinafine; and diltiazem as the most strongly associated culprits.9 Our patient’s flagellate dermatitis was unique in that it also showed histologic features of AGEP. The pathogenesis of drug-induced AGEP has been partially elucidated and involves activation of drug-specific CD4+ and CD8+ T cells that migrate to the skin and participate in apoptotic signaling of keratinocytes and recruitment of neutrophils and eosinophils, which form subcorneal sterile pustules.7 In a study of severe cutaneous adverse drug reactions, 50% (7/14) of patients with AGEP had positive patch tests to the causative agent.10 This T cell–dependent response explains why the condition responds to systemic corticosteroids. Additionally, our case report of shiitake mushroom–induced flagellate dermatitis with histologic features of AGEP suggests that the pathogenesis of flagellate dermatitis may be a T cell–mediated type IV hypersensitivity reaction. The time of onset, lack of grouped outbreaks in those sharing shiitake mushroom–containing meals, severe pruritus, lack of cases demonstrating an anaphylactic or wheal and flare response, benefit of steroids, and a case with histologic features of AGEP all lend support to this theory.
We report a case of shiitake mushroom–induced flagellate dermatitis with histologic features of AGEP. The time course, histologic features of AGEP, absence of new medications, and resolution with discontinuation of shiitake mushrooms lends support of the hypothesis that the pathogenesis of shiitake mushroom–induced flagellate dermatitis is similar to AGEP’s type IV hypersensitivity reaction. To further elucidate its pathogenesis, skin prick testing and patch testing with shiitake mushrooms in patients exhibiting shiitake mushroom–induced flagellate dermatitis may prove to be beneficial.
- Nakamura T. Toxicoderma caused by shiitake (Lentinus edodes)[in Japanese]. Jpn J Clin Dermatol. 1977;31:65-68.
- Chu EY, Anand D, Dawn A, et al. Shiitake dermatitis: a report of 3 cases and review of the literature. Cutis. 2013;91:287-290.
- Boels D, Landreau A, Bruneau C, et al. Shiitake dermatitis recorded by French Poison Control Centers—new case series with clinical observations. Clin Toxicol (Phila). 2014;52:625-628.
- Nakamura T. Shiitake (Lentinus edodes) dermatitis. Contact Dermatitis. 1992;27:65-70.
- Curnow P, Tam M. Contact dermatitis to shiitake mushroom. Australas J Dermatol. 2003;44:155-157.
- Lippert U, Martin V, Schwertfeger C, et al. Shiitake dermatitis. Br J Dermatol. 2003;148:178-179.
- Fernando SL. Acute generalised exanthematous pustulosis. Australas J Dermatol. 2012;53:87-92.
- Sidoroff A, Halevy S, Bavinck JN, et al. Acute generalized exanthematous pustulosis (AGEP)—a clinical reaction pattern. J Cutan Pathol. 2001;28:113-119.
- Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)—results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007;157:989-996.
- Wolkenstein P, Chosidow O, Flechet ML, et al. Patch testing in severe cutaneous adverse drug reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Contact Dermatitis. 1996;35:234-236.
- Nakamura T. Toxicoderma caused by shiitake (Lentinus edodes)[in Japanese]. Jpn J Clin Dermatol. 1977;31:65-68.
- Chu EY, Anand D, Dawn A, et al. Shiitake dermatitis: a report of 3 cases and review of the literature. Cutis. 2013;91:287-290.
- Boels D, Landreau A, Bruneau C, et al. Shiitake dermatitis recorded by French Poison Control Centers—new case series with clinical observations. Clin Toxicol (Phila). 2014;52:625-628.
- Nakamura T. Shiitake (Lentinus edodes) dermatitis. Contact Dermatitis. 1992;27:65-70.
- Curnow P, Tam M. Contact dermatitis to shiitake mushroom. Australas J Dermatol. 2003;44:155-157.
- Lippert U, Martin V, Schwertfeger C, et al. Shiitake dermatitis. Br J Dermatol. 2003;148:178-179.
- Fernando SL. Acute generalised exanthematous pustulosis. Australas J Dermatol. 2012;53:87-92.
- Sidoroff A, Halevy S, Bavinck JN, et al. Acute generalized exanthematous pustulosis (AGEP)—a clinical reaction pattern. J Cutan Pathol. 2001;28:113-119.
- Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)—results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007;157:989-996.
- Wolkenstein P, Chosidow O, Flechet ML, et al. Patch testing in severe cutaneous adverse drug reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Contact Dermatitis. 1996;35:234-236.
Practice Points
- Ingestion of shiitake mushrooms and bleomycin is associated with flagellate dermatitis.
- Acute generalized exanthematous pustulosis (AGEP) is a rare condition associated with certain drug ingestion.
Velvety Plaques on the Abdomen and Extremities
The Diagnosis: Dermatitis Neglecta
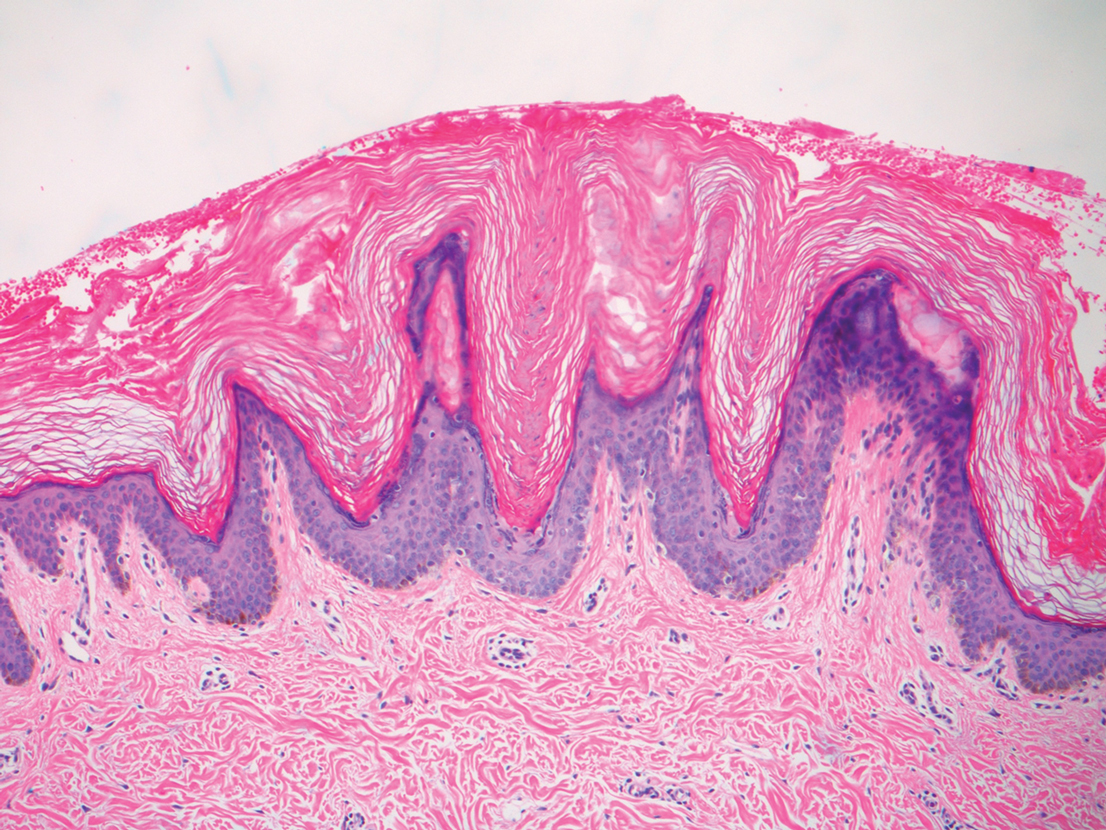
A punch biopsy of the abdomen revealed hyperkeratosis and mild papillomatosis (Figure), which can be seen in dermatitis neglecta (DN) and acanthosis nigricans (AN) as well as confluent and reticulated papillomatosis (CARP). Due to the patient’s history of mood and psychotic disorders, collateral information was obtained from the patient’s family, who reported that the patient had a depressed mood in the last few months and was not showering or caring for herself during this period. There was no additional personal or family history of skin disease. Clinical and histopathologic findings led to a diagnosis of DN. Following recommendations for daily cleansing with soap and water along with topical ammonium lactate, near-complete resolution of the rash was achieved in 3 weeks.
Dermatitis neglecta, or unwashed dermatosis, is a skin condition that occurs secondary to poor hygiene, which was first reported in 1995 by Poskitt et al.1 Avoidance of washing in affected areas can be due to physical disability, pain after injury, neurological deficit, or psychologically induced fear or neglect. Sebum, sweat, corneocytes, and bacteria combine into compact adherent crusts of dirt, which appear as hyperkeratotic plaques with cornflakelike scale.2,3 Despite its innate simplicity, DN is a diagnostic challenge, as it clinically and histologically mimics other dermatoses including AN, terra firmaforme dermatosis, and CARP.2,4 Ultimately, the diagnosis of DN can be made when a history of poor hygiene is probable or elicited, and lesions can be removed with soap and water. Treatment of DN includes daily cleansing with soap and water; however, resistant lesions or extensive disease may require keratolytic agents, as in our patient.2-4 In contrast, terra firma-forme dermatosis, which may look similar, is not due to poor hygiene, and the lesions typically are resistant to soap and water, classically requiring isopropyl alcohol for removal. Overall, maintained awareness of DN is imperative, as early diagnosis can avoid unnecessary biopsies and more complex treatment measures as well as facilitate coordination of care when additional medical or psychiatric concerns are present.
Although the diagnoses of DN and terra firma-forme dermatosis can be distinguished based on the patient’s clinical history and response to simple cleansing measures alone, the alternate diagnoses can be excluded based on different clinical distributions and response to other treatment modalities but sometimes may require clinicopathologic correlation for definitive diagnosis. Our patient had a biopsy diagnosis of psoriasiform dermatitis from an outside provider, but neither her clinical disease nor repeated histopathologic findings supported a diagnosis of psoriasis or other classic psoriasiform dermatoses such as contact dermatitis, dermatophyte/ candidal infection, seborrheic dermatitis, pityriasis rubra pilaris, pityriasis rosea, scabies, or syphilis.
It is imperative to exclude alternative diagnoses because they can have systemic implications and can misguide treatment, as was done initially with our patient. Psoriasis vulgaris in its classic form is a chronic inflammatory skin disease that manifests as sharply demarcated, erythematous plaques with overlying thick silvery scale; it has the additional histologic findings of neutrophilic spongiform pustules in the epidermis, tortuous blood vessels in the papillary dermis, and neutrophils and parakeratosis in the stratum corneum. In its benign form, AN is associated with endocrinopathies, most commonly obesity and insulin-resistant diabetes mellitus, and presents as hyperkeratotic, velvety, hyperpigmented plaques typically limited to the neck and axillae. Malignant AN spontaneously arises in association with systemic malignancy and can be extensive and generalized.5 Treatment of AN primarily focuses on resolution of the underlying systemic disease; however, cosmetic treatment with topical or oral retinoids may hasten resolution of cutaneous disease.6 Confluent and reticulated papillomatosis is characterized by reticulated hyperkeratotic plaques with a common distribution over the central and upper trunk. Unlike DN and AN, which may occur at any age, CARP typically is seen in adolescents and young adults.7 There is no evidence-based gold standard for the management of CARP; however, the successful use of various antibiotics, antifungals, and retinoids—alone or in combination—has been reported.8 Overall, compared to the other entities in the differential diagnosis, DN easily can be prevented with consistent use of soap and water and may be underreported given the asymptomatic nature of the disease and the typical patient population.
- Poskitt L, Wayte J, Wojnarowska F, et al. ‘Dermatitis neglecta’: unwashed dermatosis. Br J Dermatol. 1995;132:827-829.
- Perez-Rodriguez IM, Munoz-Garza FZ, Ocampo-Candiani J. An unusually severe case of dermatosis neglecta: a diagnostic challenge. Case Rep Dermatol. 2014;6:194-199.
- Park JM, Roh MR, Kwon JE, et al. A case of generalized dermatitis neglecta mimicking psoriasis vulgaris. Arch Dermatol. 2010;146:1050-1051.
- Lopes S, Vide J, Antunes I, et al. Dermatitis neglecta: a challenging diagnosis in psychodermatology. Acta Dermatovenerol Alp Pannonica Adriat. 2018;27:109-110.
- Shah KR, Boland CR, Patel M, et al. Cutaneous manifestations of gastrointestinal disease: part I. J Am Acad Dermatol. 2013;68:189. e1-21; quiz 210.
- Patel NU, Roach C, Alinia H, et al. Current treatment options for acanthosis nigricans. Clin Cosmet Investig Dermatol. 2018; 11:407-413.
- Kurtyka DJ, Burke KT, DeKlotz CMC. Use of topical sirolimus (rapamycin) for treating confluent and reticulated papillomatosis. JAMA Dermatol. 2021;157:121-123.
- Mufti A, Sachdeva M, Maliyar K, et al. Treatment outcomes in confluent and reticulated papillomatosis: a systematic review. J Am Acad Dermatol. 2021;84:825-829.
The Diagnosis: Dermatitis Neglecta
A punch biopsy of the abdomen revealed hyperkeratosis and mild papillomatosis (Figure), which can be seen in dermatitis neglecta (DN) and acanthosis nigricans (AN) as well as confluent and reticulated papillomatosis (CARP). Due to the patient’s history of mood and psychotic disorders, collateral information was obtained from the patient’s family, who reported that the patient had a depressed mood in the last few months and was not showering or caring for herself during this period. There was no additional personal or family history of skin disease. Clinical and histopathologic findings led to a diagnosis of DN. Following recommendations for daily cleansing with soap and water along with topical ammonium lactate, near-complete resolution of the rash was achieved in 3 weeks.
Dermatitis neglecta, or unwashed dermatosis, is a skin condition that occurs secondary to poor hygiene, which was first reported in 1995 by Poskitt et al.1 Avoidance of washing in affected areas can be due to physical disability, pain after injury, neurological deficit, or psychologically induced fear or neglect. Sebum, sweat, corneocytes, and bacteria combine into compact adherent crusts of dirt, which appear as hyperkeratotic plaques with cornflakelike scale.2,3 Despite its innate simplicity, DN is a diagnostic challenge, as it clinically and histologically mimics other dermatoses including AN, terra firmaforme dermatosis, and CARP.2,4 Ultimately, the diagnosis of DN can be made when a history of poor hygiene is probable or elicited, and lesions can be removed with soap and water. Treatment of DN includes daily cleansing with soap and water; however, resistant lesions or extensive disease may require keratolytic agents, as in our patient.2-4 In contrast, terra firma-forme dermatosis, which may look similar, is not due to poor hygiene, and the lesions typically are resistant to soap and water, classically requiring isopropyl alcohol for removal. Overall, maintained awareness of DN is imperative, as early diagnosis can avoid unnecessary biopsies and more complex treatment measures as well as facilitate coordination of care when additional medical or psychiatric concerns are present.

Although the diagnoses of DN and terra firma-forme dermatosis can be distinguished based on the patient’s clinical history and response to simple cleansing measures alone, the alternate diagnoses can be excluded based on different clinical distributions and response to other treatment modalities but sometimes may require clinicopathologic correlation for definitive diagnosis. Our patient had a biopsy diagnosis of psoriasiform dermatitis from an outside provider, but neither her clinical disease nor repeated histopathologic findings supported a diagnosis of psoriasis or other classic psoriasiform dermatoses such as contact dermatitis, dermatophyte/ candidal infection, seborrheic dermatitis, pityriasis rubra pilaris, pityriasis rosea, scabies, or syphilis.
It is imperative to exclude alternative diagnoses because they can have systemic implications and can misguide treatment, as was done initially with our patient. Psoriasis vulgaris in its classic form is a chronic inflammatory skin disease that manifests as sharply demarcated, erythematous plaques with overlying thick silvery scale; it has the additional histologic findings of neutrophilic spongiform pustules in the epidermis, tortuous blood vessels in the papillary dermis, and neutrophils and parakeratosis in the stratum corneum. In its benign form, AN is associated with endocrinopathies, most commonly obesity and insulin-resistant diabetes mellitus, and presents as hyperkeratotic, velvety, hyperpigmented plaques typically limited to the neck and axillae. Malignant AN spontaneously arises in association with systemic malignancy and can be extensive and generalized.5 Treatment of AN primarily focuses on resolution of the underlying systemic disease; however, cosmetic treatment with topical or oral retinoids may hasten resolution of cutaneous disease.6 Confluent and reticulated papillomatosis is characterized by reticulated hyperkeratotic plaques with a common distribution over the central and upper trunk. Unlike DN and AN, which may occur at any age, CARP typically is seen in adolescents and young adults.7 There is no evidence-based gold standard for the management of CARP; however, the successful use of various antibiotics, antifungals, and retinoids—alone or in combination—has been reported.8 Overall, compared to the other entities in the differential diagnosis, DN easily can be prevented with consistent use of soap and water and may be underreported given the asymptomatic nature of the disease and the typical patient population.
The Diagnosis: Dermatitis Neglecta
A punch biopsy of the abdomen revealed hyperkeratosis and mild papillomatosis (Figure), which can be seen in dermatitis neglecta (DN) and acanthosis nigricans (AN) as well as confluent and reticulated papillomatosis (CARP). Due to the patient’s history of mood and psychotic disorders, collateral information was obtained from the patient’s family, who reported that the patient had a depressed mood in the last few months and was not showering or caring for herself during this period. There was no additional personal or family history of skin disease. Clinical and histopathologic findings led to a diagnosis of DN. Following recommendations for daily cleansing with soap and water along with topical ammonium lactate, near-complete resolution of the rash was achieved in 3 weeks.
Dermatitis neglecta, or unwashed dermatosis, is a skin condition that occurs secondary to poor hygiene, which was first reported in 1995 by Poskitt et al.1 Avoidance of washing in affected areas can be due to physical disability, pain after injury, neurological deficit, or psychologically induced fear or neglect. Sebum, sweat, corneocytes, and bacteria combine into compact adherent crusts of dirt, which appear as hyperkeratotic plaques with cornflakelike scale.2,3 Despite its innate simplicity, DN is a diagnostic challenge, as it clinically and histologically mimics other dermatoses including AN, terra firmaforme dermatosis, and CARP.2,4 Ultimately, the diagnosis of DN can be made when a history of poor hygiene is probable or elicited, and lesions can be removed with soap and water. Treatment of DN includes daily cleansing with soap and water; however, resistant lesions or extensive disease may require keratolytic agents, as in our patient.2-4 In contrast, terra firma-forme dermatosis, which may look similar, is not due to poor hygiene, and the lesions typically are resistant to soap and water, classically requiring isopropyl alcohol for removal. Overall, maintained awareness of DN is imperative, as early diagnosis can avoid unnecessary biopsies and more complex treatment measures as well as facilitate coordination of care when additional medical or psychiatric concerns are present.

Although the diagnoses of DN and terra firma-forme dermatosis can be distinguished based on the patient’s clinical history and response to simple cleansing measures alone, the alternate diagnoses can be excluded based on different clinical distributions and response to other treatment modalities but sometimes may require clinicopathologic correlation for definitive diagnosis. Our patient had a biopsy diagnosis of psoriasiform dermatitis from an outside provider, but neither her clinical disease nor repeated histopathologic findings supported a diagnosis of psoriasis or other classic psoriasiform dermatoses such as contact dermatitis, dermatophyte/ candidal infection, seborrheic dermatitis, pityriasis rubra pilaris, pityriasis rosea, scabies, or syphilis.
It is imperative to exclude alternative diagnoses because they can have systemic implications and can misguide treatment, as was done initially with our patient. Psoriasis vulgaris in its classic form is a chronic inflammatory skin disease that manifests as sharply demarcated, erythematous plaques with overlying thick silvery scale; it has the additional histologic findings of neutrophilic spongiform pustules in the epidermis, tortuous blood vessels in the papillary dermis, and neutrophils and parakeratosis in the stratum corneum. In its benign form, AN is associated with endocrinopathies, most commonly obesity and insulin-resistant diabetes mellitus, and presents as hyperkeratotic, velvety, hyperpigmented plaques typically limited to the neck and axillae. Malignant AN spontaneously arises in association with systemic malignancy and can be extensive and generalized.5 Treatment of AN primarily focuses on resolution of the underlying systemic disease; however, cosmetic treatment with topical or oral retinoids may hasten resolution of cutaneous disease.6 Confluent and reticulated papillomatosis is characterized by reticulated hyperkeratotic plaques with a common distribution over the central and upper trunk. Unlike DN and AN, which may occur at any age, CARP typically is seen in adolescents and young adults.7 There is no evidence-based gold standard for the management of CARP; however, the successful use of various antibiotics, antifungals, and retinoids—alone or in combination—has been reported.8 Overall, compared to the other entities in the differential diagnosis, DN easily can be prevented with consistent use of soap and water and may be underreported given the asymptomatic nature of the disease and the typical patient population.
- Poskitt L, Wayte J, Wojnarowska F, et al. ‘Dermatitis neglecta’: unwashed dermatosis. Br J Dermatol. 1995;132:827-829.
- Perez-Rodriguez IM, Munoz-Garza FZ, Ocampo-Candiani J. An unusually severe case of dermatosis neglecta: a diagnostic challenge. Case Rep Dermatol. 2014;6:194-199.
- Park JM, Roh MR, Kwon JE, et al. A case of generalized dermatitis neglecta mimicking psoriasis vulgaris. Arch Dermatol. 2010;146:1050-1051.
- Lopes S, Vide J, Antunes I, et al. Dermatitis neglecta: a challenging diagnosis in psychodermatology. Acta Dermatovenerol Alp Pannonica Adriat. 2018;27:109-110.
- Shah KR, Boland CR, Patel M, et al. Cutaneous manifestations of gastrointestinal disease: part I. J Am Acad Dermatol. 2013;68:189. e1-21; quiz 210.
- Patel NU, Roach C, Alinia H, et al. Current treatment options for acanthosis nigricans. Clin Cosmet Investig Dermatol. 2018; 11:407-413.
- Kurtyka DJ, Burke KT, DeKlotz CMC. Use of topical sirolimus (rapamycin) for treating confluent and reticulated papillomatosis. JAMA Dermatol. 2021;157:121-123.
- Mufti A, Sachdeva M, Maliyar K, et al. Treatment outcomes in confluent and reticulated papillomatosis: a systematic review. J Am Acad Dermatol. 2021;84:825-829.
- Poskitt L, Wayte J, Wojnarowska F, et al. ‘Dermatitis neglecta’: unwashed dermatosis. Br J Dermatol. 1995;132:827-829.
- Perez-Rodriguez IM, Munoz-Garza FZ, Ocampo-Candiani J. An unusually severe case of dermatosis neglecta: a diagnostic challenge. Case Rep Dermatol. 2014;6:194-199.
- Park JM, Roh MR, Kwon JE, et al. A case of generalized dermatitis neglecta mimicking psoriasis vulgaris. Arch Dermatol. 2010;146:1050-1051.
- Lopes S, Vide J, Antunes I, et al. Dermatitis neglecta: a challenging diagnosis in psychodermatology. Acta Dermatovenerol Alp Pannonica Adriat. 2018;27:109-110.
- Shah KR, Boland CR, Patel M, et al. Cutaneous manifestations of gastrointestinal disease: part I. J Am Acad Dermatol. 2013;68:189. e1-21; quiz 210.
- Patel NU, Roach C, Alinia H, et al. Current treatment options for acanthosis nigricans. Clin Cosmet Investig Dermatol. 2018; 11:407-413.
- Kurtyka DJ, Burke KT, DeKlotz CMC. Use of topical sirolimus (rapamycin) for treating confluent and reticulated papillomatosis. JAMA Dermatol. 2021;157:121-123.
- Mufti A, Sachdeva M, Maliyar K, et al. Treatment outcomes in confluent and reticulated papillomatosis: a systematic review. J Am Acad Dermatol. 2021;84:825-829.
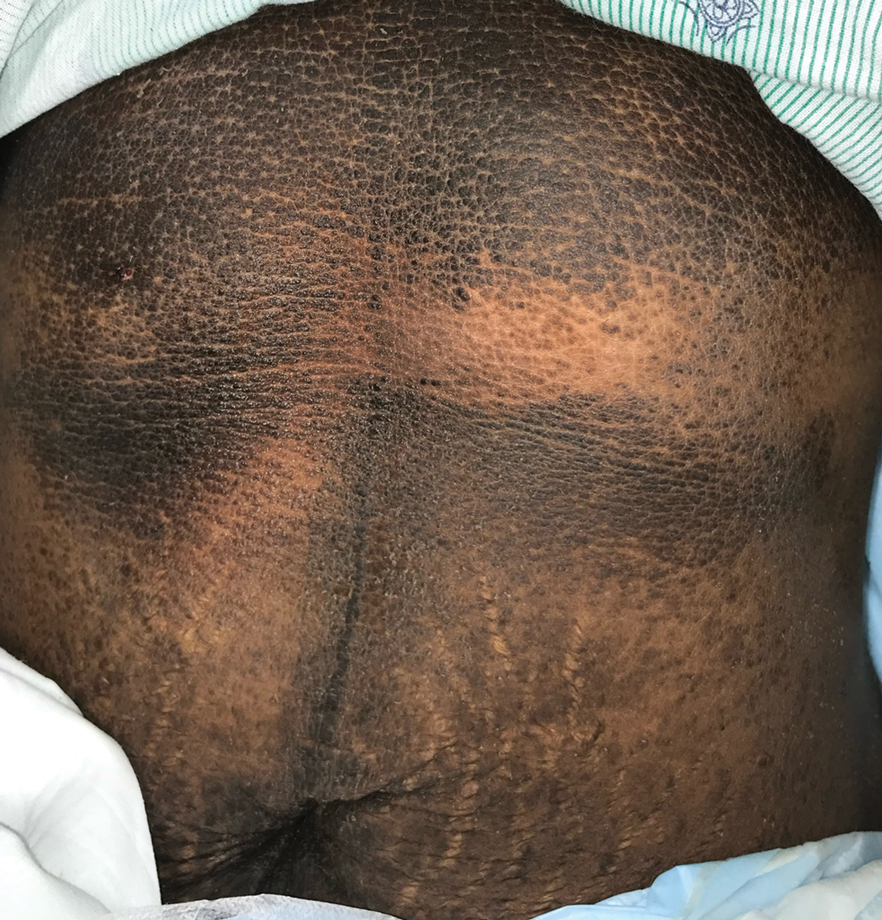
A 28-year-old woman was admitted to the medicine service with bilateral pedal numbness and ataxia, as well as an asymptomatic rash on the neck, chest, abdomen, and extremities of a few months’ duration. The patient was seen by an outside dermatologist for the same rash 1 month prior, at which time a punch biopsy of the right forearm was suggestive of psoriasiform dermatitis; however, the rash failed to improve with topical ammonium lactate and corticosteroids. During the current admission, the patient was found to have low methylmalonic acid and vitamin B1 levels; however, vitamin B12, thyroid studies, rapid plasma reagin test, and inflammatory markers, as well as central and peripheral imaging and nerve conduction studies were normal.
Dermatology was consulted. Physical examination revealed retention hyperkeratosis on the neck that was wipeable with 70% isopropyl alcohol, as well as nonwipeable, thin, reticulated plaques on the mid chest and thick velvety plaques on the abdomen and bilateral extremities. There was notable sparing of areas with natural occlusion such as the back and body folds. A punch biopsy of the abdomen was performed.
Autoeczematization: A Strange Id Reaction of the Skin
Autoeczematization (AE), or id reaction, is a disseminated eczematous reaction that occurs days or weeks after exposure to a primary stimulus, resulting from a release of antigen(s). Whitfield1 first described AE in 1921, when he postulated that the id reaction was due to sensitization of the skin after a primary stimulus. He called it “a form of auto-intoxication derived from changes in the patient’s own tissues.”1 The exact prevalence of id reactions is unknown; one study showed that 17% of patients with dermatophyte infections developed an id reaction, typically tinea pedis linked with vesicles on the palms.2 Tinea capitis is one of the most common causes of AE in children, which is frequently misdiagnosed as a drug reaction. Approximately 37% of patients diagnosed with stasis dermatitis develop an id reaction (Figure 1). A history of contact dermatitis is common in patients presenting with AE.2-6

Pathophysiology of Id Reactions
An abnormal immune response against autologous skin antigens may be responsible for the development of AE. Shelley5 postulated that hair follicles play an important role in id reactions, as Sharquie et al6 recently emphasized for many skin disorders. The pathogenesis of AE is uncertain, but circulating T lymphocytes play a role in this reaction. Normally, T cells are activated by a release of antigens after a primary exposure to a stimulus. However, overactivation of these T cells induces autoimmune reactions such as AE.7 Activated T lymphocytes express HLA-DR and IL-2 receptor, markers elevated in the peripheral blood of patients undergoing id reactions. After treatment, the levels of activated T lymphocytes decline. An increase in the number of CD25+ T cells and a decrease in the number of suppressor T cells in the blood may occur during an id reaction.7-9 Keratinocytes produce proinflammatory cytokines, such as thymic stromal erythropoietin, IL-25, and IL-33, that activate T cells.10-12 Therefore, the most likely pathogenesis of an id reaction is that T lymphocytes are activated at the primary reaction site due to proinflammatory cytokines released by keratinocytes. These activated T cells then travel systemically via hematogenous dissemination. The spread of activated T lymphocytes produces an eczematous reaction at secondary locations distant to the primary site.9
Clinical and Histopathological Features of Id Reactions
Clinically, AE is first evident as a vesicular dissemination that groups to form papules or nummular patches and usually is present on the legs, feet, arms, and/or trunk (Figure 2). The primary dermatitis is localized to the area that was the site of contact to the offending stimuli. This localized eczematous eruption begins with an acute or subacute onset. It has the appearance of small crusted vesicles with erythema (Figure 1). The first sign of AE is vesicles presenting near the primary site on flexural surfaces or on the hands and feet. A classic example is tinea pedis linked with vesicles on the palms and sides of the fingers, resembling dyshidrotic eczema. Sites of prior cutaneous trauma, such as dermatoses, scars, and burns, are common locations for early AE. In later stages, vesicles disseminate to the legs, arms, and trunk, where they group to form papules and nummular patches in a symmetrical pattern.5,13-15 These lesions may be extremely pruritic. The pruritus may be so intense that it interrupts daily activities and disrupts the ability to fall or stay asleep.16
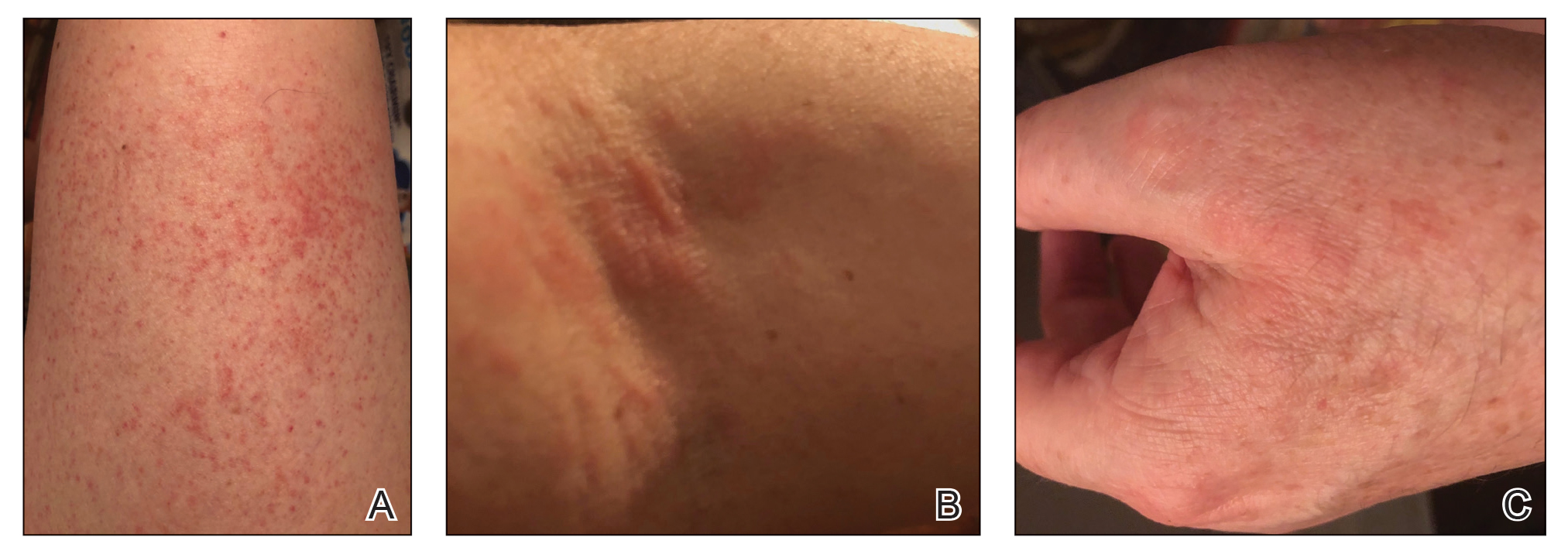
Histologically, biopsy specimens show psoriasiform spongiotic dermatitis with mononuclear cells contained in the vesicles. Interstitial edema and perivascular lymphohistiocytic infiltrates are evident. Eosinophils also may be present. This pattern is not unique toid reactions.17-19 Although AE is a reaction pattern that may be due to a fungal or bacterial infection, the etiologic agent is not evident microscopically within the eczema itself.
Etiology of Id Reactions
Id reactions most commonly occur from either stasis dermatitis or tinea pedis, although a wide variety of other causes should be considered. Evaluation of the primary site rather than the id reaction may identify an infectious or parasitic agent. Sometimes the AE reaction is specifically named: dermatophytid with dermatophytosis, bacterid with a bacterial infectious process, and tuberculid with tuberculosis. Similarly, there may be reactions to underlying candidiasis, sporotrichosis, histoplasmosis, and other fungal infections that can cause a cutaneous id reaction.18,20-22Mycobacterium species, Pseudomonas, Staphylococcus, and Streptococcus are bacterial causes of AE.15,23-26 Viral infections that can cause an id reaction are herpes simplex virus and molluscum contagiosum.27-29 Scabies, leishmaniasis, and pediculosis capitis are parasitic infections that may be etiologic.14,30,31 In addition, noninfectious stimuli besides stasis dermatitis that can produce id reactions include medications, topical creams, tattoo ink, sutures, radiotherapy, and dyshidrotic eczema. The primary reaction to these agents is a localized dermatitis followed by the immunological response that induces a secondary reaction distant from the primary site.17,18,32-38
Differential Diagnoses
Differential diagnoses include other types of eczema and some vesicular eruptions. Irritant contact dermatitis is another dermatosis that presents as a widespread vesicular eruption due to repetitive exposure to toxic irritants. The rash is erythematous with pustules, blisters, and crusts. It is only found in areas directly exposed to irritants, as opposed to AE, which spreads to areas distant to the primary reaction site. Irritant contact dermatitis presents with more of a burning sensation, whereas AE is more pruritic.39,40 Allergic contact dermatitis presents with erythematous vesicles and papules and sometimes with bullae. There is edema and crust formation, which often can spread past the point of contact in later stages. Similar to AE, there is intense pruritus. However, allergic contact dermatitis most commonly is caused by exposure to metals, cosmetics, and fragrances, whereas infectious agents and stasis dermatitis are the most common causes of AE.40,41 It may be challenging to distinguish AE from other causes of widespread eczematous dissemination. Vesicular eruptions sometimes require distinction from AE, including herpetic infections, insect bite reactions, and drug eruptions.18,42
Treatment
The underlying condition should be treated to mitigate the inflammatory response causing the id reaction. If not skillfully orchestrated, the id reaction can reoccur. For infectious causes of AE, an antifungal, antibacterial, antiviral, or antiparasitic should be given. If stasis dermatitis is responsible for the id reaction, compression stockings and leg elevation are indicated. The id reaction itself is treated with systemic or topical corticosteroids and wet compresses if acute. The goal of these treatments is to reduce patient discomfort caused by the inflammation and pruritus.18,43
Conclusion
Id reactions are an unusual phenomenon that commonly occurs after fungal skin infections and stasis dermatitis. T lymphocytes and keratinocytes may play a key role in this reaction, with newer research further delineating the process and possibly providing enhanced treatment options. Therapy focuses on treating the underlying condition, supplemented with corticosteroids for the autoeczema.
- Whitfield A. Lumleian Lectures on Some Points in the Aetiology of Skin Diseases. Delivered before the Royal College of Physicians of London on March 10th, 15th, and 17th, 1921. Lecture II. Lancet. 1921;2:122-127.
- Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128:E453-E457.
- Schrom KP, Kobs A, Nedorost S. Clinical psoriasiform dermatitis following dupilumab use for autoeczematization secondary to chronic stasis dermatitis. Cureus. 2020;12:e7831. doi:10.7759/cureus.7831
- Templeton HJ, Lunsford CJ, Allington HV. Autosensitization dermatitis; report of five cases and protocol of an experiment. Arch Derm Syphilol. 1949;59:68-77.
- Shelley WB. Id reaction. In: Consultations in Dermatology. Saunders; 1972:262-267.
- Sharquie KE, Noaimi AA, Flayih RA. Clinical and histopathological findings in patients with follicular dermatoses: all skin diseases starts in the hair follicles as new hypothesis. Am J Clin Res Rev. 2020;4:17.
- Kasteler JS, Petersen MJ, Vance JE, et al. Circulating activated T lymphocytes in autoeczematization. Arch Dermatol. 1992;128:795-798.
- González-Amaro R, Baranda L, Abud-Mendoza C, et al. Autoeczematization is associated with abnormal immune recognition of autologous skin antigens. J Am Acad Dermatol. 1993;28:56-60.
- Cunningham MJ, Zone JJ, Petersen MJ, et al. Circulating activated (DR-positive) T lymphocytes in a patient with autoeczematization. J Am Acad Dermatol. 1986;14:1039-1041.
- Furue M, Ulzii D, Vu YH, et al. Pathogenesis of atopic dermatitis: current paradigm. Iran J Immunol. 2019;16:97-107.
- Uchi H, Terao H, Koga T, et al. Cytokines and chemokines in the epidermis. J Dermatol Sci. 2000;24(suppl 1):S29-S38.
- Bos JD, Kapsenberg ML. The skin immune system: progress in cutaneous biology. Immunol Today. 1993;14:75-78.
- Young AW Jr. Dynamics of autosensitization dermatitis; a clinical and microscopic concept of autoeczematization. AMA Arch Derm. 1958;77:495-502.
- Brenner S, Wolf R, Landau M. Scabid: an unusual id reaction to scabies. Int J Dermatol. 1993;32:128-129.
- Yamany T, Schwartz RA. Infectious eczematoid dermatitis: a comprehensive review. J Eur Acad Dermatol Venereol. 2015;29:203-208.
- Wang X, Li L, Shi X, et al. Itching and its related factors in subtypes of eczema: a cross-sectional multicenter study in tertiary hospitals of China. Sci Rep. 2018;8:10754.
- Price A, Tavazoie M, Meehan SA, et al. Id reaction associated with red tattoo ink. Cutis. 2018;102:E32-E34.
- Ilkit M, Durdu M, Karaks¸ M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202.
- Kaner SR. Dermatitis venenata of the feet with a generalized “id” reaction. J Am Podiatry Assoc. 1970;60:199-204.
- Jordan L, Jackson NA, Carter-Snell B, et al. Pustular tinea id reaction. Cutis. 2019;103:E3-E4.
- Crum N, Hardaway C, Graham B. Development of an idlike reaction during treatment for acute pulmonary histoplasmosis: a new cutaneous manifestation in histoplasmosis. J Am Acad Dermatol. 2003;48(2 suppl):S5-S6.
- Chirac A, Brzezinski P, Chiriac AE, et al. Autosensitisation (autoeczematisation) reactions in a case of diaper dermatitis candidiasis. Niger Med J. 2014;55:274-275.
- Singh PY, Sinha P, Baveja S, et al. Immune-mediated tuberculous uveitis—a rare association with papulonecrotic tuberculid. Indian J Ophthalmol. 2019;67:1207-1209.
- Urso B, Georgesen C, Harp J. Papulonecrotic tuberculid secondary to Mycobacterium avium complex. Cutis. 2019;104:E11-E13.
- Choudhri SH, Magro CM, Crowson AN, et al. An id reaction to Mycobacterium leprae: first documented case. Cutis. 1994;54:282-286.
- Park JW, Jeong GJ, Seo SJ, et al. Pseudomonas toe web infection and autosensitisation dermatitis: diagnostic and therapeutic challenge. Int Wound J. 2020;17:1543-1544. doi:10.1111/iwj.13386
- Netchiporouk E, Cohen BA. Recognizing and managing eczematous id reactions to molluscum contagiosum virus in children. Pediatrics. 2012;129:E1072-E1075.
- Aurelian L, Ono F, Burnett J. Herpes simplex virus (HSV)-associated erythema multiforme (HAEM): a viral disease with an autoimmune component. Dermatol Online J. 2003;9:1.
- Rocamora V, Romaní J, Puig L, et al. Id reaction to molluscum contagiosum. Pediatr Dermatol. 1996;13:349-350.
- Yes¸ilova Y, Özbilgin A, Turan E, et al. Clinical exacerbation developing during treatment of cutaneous leishmaniasis: an id reaction? Turkiye Parazitol Derg. 2014;38:281-282.
- Connor CJ, Selby JC, Wanat KA. Severe pediculosis capitus: a case of “crusted lice” with autoeczematization. Dermatol Online J. 2016;22:13030/qt7c91z913.
- Shelley WB. The autoimmune mechanism in clinical dermatology. Arch Dermatol. 1962;86:27-34.
- Bosworth A, Hull PR. Disseminated eczema following radiotherapy: a case report. J Cutan Med Surg. 2018;22:353-355.
- Lowther C, Miedler JD, Cockerell CJ. Id-like reaction to BCG therapy for bladder cancer. Cutis. 2013;91:145-151.
- Huerth KA, Glick PL, Glick ZR. Cutaneous id reaction after using cyanoacrylate for wound closure. Cutis. 2020;105:E11-E13.
- Amini S, Burdick AE, Janniger CK. Dyshidrotic eczema (pompholyx). Updated April 22, 2020. Accessed August 23, 2021. https://emedicine.medscape.com/article/1122527-overview
- Sundaresan S, Migden MR, Silapunt S. Stasis dermatitis: pathophysiology, evaluation, and management. Am J Clin Dermatol. 2017;18:383-390.
- Hughes JDM, Pratt MD. Allergic contact dermatitis and autoeczematization to proctosedyl® cream and proctomyxin® cream. Case Rep Dermatol. 2018;10:238-246.
- Bains SN, Nash P, Fonacier L. Irritant contact dermatitis. Clin Rev Allergy Immunol. 2019;56:99-109.
- Novak-Bilic´ G, Vucˇic´ M, Japundžic´ I, et al. Irritant and allergic contact dermatitis—skin lesion characteristics. Acta Clin Croat. 2018;57:713-720.
- Nassau S, Fonacier L. Allergic contact dermatitis. Med Clin North Am. 2020;104:61-76.
- Lewis DJ, Schlichte MJ, Dao H Jr. Atypical disseminated herpes zoster: management guidelines in immunocompromised patients. Cutis. 2017;100:321-330.
- Nedorost S, White S, Rowland DY, et al. Development and implementation of an order set to improve value of care for patients with severe stasis dermatitis. J Am Acad Dermatol. 2019;80:815-817.
Autoeczematization (AE), or id reaction, is a disseminated eczematous reaction that occurs days or weeks after exposure to a primary stimulus, resulting from a release of antigen(s). Whitfield1 first described AE in 1921, when he postulated that the id reaction was due to sensitization of the skin after a primary stimulus. He called it “a form of auto-intoxication derived from changes in the patient’s own tissues.”1 The exact prevalence of id reactions is unknown; one study showed that 17% of patients with dermatophyte infections developed an id reaction, typically tinea pedis linked with vesicles on the palms.2 Tinea capitis is one of the most common causes of AE in children, which is frequently misdiagnosed as a drug reaction. Approximately 37% of patients diagnosed with stasis dermatitis develop an id reaction (Figure 1). A history of contact dermatitis is common in patients presenting with AE.2-6

Pathophysiology of Id Reactions
An abnormal immune response against autologous skin antigens may be responsible for the development of AE. Shelley5 postulated that hair follicles play an important role in id reactions, as Sharquie et al6 recently emphasized for many skin disorders. The pathogenesis of AE is uncertain, but circulating T lymphocytes play a role in this reaction. Normally, T cells are activated by a release of antigens after a primary exposure to a stimulus. However, overactivation of these T cells induces autoimmune reactions such as AE.7 Activated T lymphocytes express HLA-DR and IL-2 receptor, markers elevated in the peripheral blood of patients undergoing id reactions. After treatment, the levels of activated T lymphocytes decline. An increase in the number of CD25+ T cells and a decrease in the number of suppressor T cells in the blood may occur during an id reaction.7-9 Keratinocytes produce proinflammatory cytokines, such as thymic stromal erythropoietin, IL-25, and IL-33, that activate T cells.10-12 Therefore, the most likely pathogenesis of an id reaction is that T lymphocytes are activated at the primary reaction site due to proinflammatory cytokines released by keratinocytes. These activated T cells then travel systemically via hematogenous dissemination. The spread of activated T lymphocytes produces an eczematous reaction at secondary locations distant to the primary site.9
Clinical and Histopathological Features of Id Reactions
Clinically, AE is first evident as a vesicular dissemination that groups to form papules or nummular patches and usually is present on the legs, feet, arms, and/or trunk (Figure 2). The primary dermatitis is localized to the area that was the site of contact to the offending stimuli. This localized eczematous eruption begins with an acute or subacute onset. It has the appearance of small crusted vesicles with erythema (Figure 1). The first sign of AE is vesicles presenting near the primary site on flexural surfaces or on the hands and feet. A classic example is tinea pedis linked with vesicles on the palms and sides of the fingers, resembling dyshidrotic eczema. Sites of prior cutaneous trauma, such as dermatoses, scars, and burns, are common locations for early AE. In later stages, vesicles disseminate to the legs, arms, and trunk, where they group to form papules and nummular patches in a symmetrical pattern.5,13-15 These lesions may be extremely pruritic. The pruritus may be so intense that it interrupts daily activities and disrupts the ability to fall or stay asleep.16

Histologically, biopsy specimens show psoriasiform spongiotic dermatitis with mononuclear cells contained in the vesicles. Interstitial edema and perivascular lymphohistiocytic infiltrates are evident. Eosinophils also may be present. This pattern is not unique toid reactions.17-19 Although AE is a reaction pattern that may be due to a fungal or bacterial infection, the etiologic agent is not evident microscopically within the eczema itself.
Etiology of Id Reactions
Id reactions most commonly occur from either stasis dermatitis or tinea pedis, although a wide variety of other causes should be considered. Evaluation of the primary site rather than the id reaction may identify an infectious or parasitic agent. Sometimes the AE reaction is specifically named: dermatophytid with dermatophytosis, bacterid with a bacterial infectious process, and tuberculid with tuberculosis. Similarly, there may be reactions to underlying candidiasis, sporotrichosis, histoplasmosis, and other fungal infections that can cause a cutaneous id reaction.18,20-22Mycobacterium species, Pseudomonas, Staphylococcus, and Streptococcus are bacterial causes of AE.15,23-26 Viral infections that can cause an id reaction are herpes simplex virus and molluscum contagiosum.27-29 Scabies, leishmaniasis, and pediculosis capitis are parasitic infections that may be etiologic.14,30,31 In addition, noninfectious stimuli besides stasis dermatitis that can produce id reactions include medications, topical creams, tattoo ink, sutures, radiotherapy, and dyshidrotic eczema. The primary reaction to these agents is a localized dermatitis followed by the immunological response that induces a secondary reaction distant from the primary site.17,18,32-38
Differential Diagnoses
Differential diagnoses include other types of eczema and some vesicular eruptions. Irritant contact dermatitis is another dermatosis that presents as a widespread vesicular eruption due to repetitive exposure to toxic irritants. The rash is erythematous with pustules, blisters, and crusts. It is only found in areas directly exposed to irritants, as opposed to AE, which spreads to areas distant to the primary reaction site. Irritant contact dermatitis presents with more of a burning sensation, whereas AE is more pruritic.39,40 Allergic contact dermatitis presents with erythematous vesicles and papules and sometimes with bullae. There is edema and crust formation, which often can spread past the point of contact in later stages. Similar to AE, there is intense pruritus. However, allergic contact dermatitis most commonly is caused by exposure to metals, cosmetics, and fragrances, whereas infectious agents and stasis dermatitis are the most common causes of AE.40,41 It may be challenging to distinguish AE from other causes of widespread eczematous dissemination. Vesicular eruptions sometimes require distinction from AE, including herpetic infections, insect bite reactions, and drug eruptions.18,42
Treatment
The underlying condition should be treated to mitigate the inflammatory response causing the id reaction. If not skillfully orchestrated, the id reaction can reoccur. For infectious causes of AE, an antifungal, antibacterial, antiviral, or antiparasitic should be given. If stasis dermatitis is responsible for the id reaction, compression stockings and leg elevation are indicated. The id reaction itself is treated with systemic or topical corticosteroids and wet compresses if acute. The goal of these treatments is to reduce patient discomfort caused by the inflammation and pruritus.18,43
Conclusion
Id reactions are an unusual phenomenon that commonly occurs after fungal skin infections and stasis dermatitis. T lymphocytes and keratinocytes may play a key role in this reaction, with newer research further delineating the process and possibly providing enhanced treatment options. Therapy focuses on treating the underlying condition, supplemented with corticosteroids for the autoeczema.
Autoeczematization (AE), or id reaction, is a disseminated eczematous reaction that occurs days or weeks after exposure to a primary stimulus, resulting from a release of antigen(s). Whitfield1 first described AE in 1921, when he postulated that the id reaction was due to sensitization of the skin after a primary stimulus. He called it “a form of auto-intoxication derived from changes in the patient’s own tissues.”1 The exact prevalence of id reactions is unknown; one study showed that 17% of patients with dermatophyte infections developed an id reaction, typically tinea pedis linked with vesicles on the palms.2 Tinea capitis is one of the most common causes of AE in children, which is frequently misdiagnosed as a drug reaction. Approximately 37% of patients diagnosed with stasis dermatitis develop an id reaction (Figure 1). A history of contact dermatitis is common in patients presenting with AE.2-6

Pathophysiology of Id Reactions
An abnormal immune response against autologous skin antigens may be responsible for the development of AE. Shelley5 postulated that hair follicles play an important role in id reactions, as Sharquie et al6 recently emphasized for many skin disorders. The pathogenesis of AE is uncertain, but circulating T lymphocytes play a role in this reaction. Normally, T cells are activated by a release of antigens after a primary exposure to a stimulus. However, overactivation of these T cells induces autoimmune reactions such as AE.7 Activated T lymphocytes express HLA-DR and IL-2 receptor, markers elevated in the peripheral blood of patients undergoing id reactions. After treatment, the levels of activated T lymphocytes decline. An increase in the number of CD25+ T cells and a decrease in the number of suppressor T cells in the blood may occur during an id reaction.7-9 Keratinocytes produce proinflammatory cytokines, such as thymic stromal erythropoietin, IL-25, and IL-33, that activate T cells.10-12 Therefore, the most likely pathogenesis of an id reaction is that T lymphocytes are activated at the primary reaction site due to proinflammatory cytokines released by keratinocytes. These activated T cells then travel systemically via hematogenous dissemination. The spread of activated T lymphocytes produces an eczematous reaction at secondary locations distant to the primary site.9
Clinical and Histopathological Features of Id Reactions
Clinically, AE is first evident as a vesicular dissemination that groups to form papules or nummular patches and usually is present on the legs, feet, arms, and/or trunk (Figure 2). The primary dermatitis is localized to the area that was the site of contact to the offending stimuli. This localized eczematous eruption begins with an acute or subacute onset. It has the appearance of small crusted vesicles with erythema (Figure 1). The first sign of AE is vesicles presenting near the primary site on flexural surfaces or on the hands and feet. A classic example is tinea pedis linked with vesicles on the palms and sides of the fingers, resembling dyshidrotic eczema. Sites of prior cutaneous trauma, such as dermatoses, scars, and burns, are common locations for early AE. In later stages, vesicles disseminate to the legs, arms, and trunk, where they group to form papules and nummular patches in a symmetrical pattern.5,13-15 These lesions may be extremely pruritic. The pruritus may be so intense that it interrupts daily activities and disrupts the ability to fall or stay asleep.16

Histologically, biopsy specimens show psoriasiform spongiotic dermatitis with mononuclear cells contained in the vesicles. Interstitial edema and perivascular lymphohistiocytic infiltrates are evident. Eosinophils also may be present. This pattern is not unique toid reactions.17-19 Although AE is a reaction pattern that may be due to a fungal or bacterial infection, the etiologic agent is not evident microscopically within the eczema itself.
Etiology of Id Reactions
Id reactions most commonly occur from either stasis dermatitis or tinea pedis, although a wide variety of other causes should be considered. Evaluation of the primary site rather than the id reaction may identify an infectious or parasitic agent. Sometimes the AE reaction is specifically named: dermatophytid with dermatophytosis, bacterid with a bacterial infectious process, and tuberculid with tuberculosis. Similarly, there may be reactions to underlying candidiasis, sporotrichosis, histoplasmosis, and other fungal infections that can cause a cutaneous id reaction.18,20-22Mycobacterium species, Pseudomonas, Staphylococcus, and Streptococcus are bacterial causes of AE.15,23-26 Viral infections that can cause an id reaction are herpes simplex virus and molluscum contagiosum.27-29 Scabies, leishmaniasis, and pediculosis capitis are parasitic infections that may be etiologic.14,30,31 In addition, noninfectious stimuli besides stasis dermatitis that can produce id reactions include medications, topical creams, tattoo ink, sutures, radiotherapy, and dyshidrotic eczema. The primary reaction to these agents is a localized dermatitis followed by the immunological response that induces a secondary reaction distant from the primary site.17,18,32-38
Differential Diagnoses
Differential diagnoses include other types of eczema and some vesicular eruptions. Irritant contact dermatitis is another dermatosis that presents as a widespread vesicular eruption due to repetitive exposure to toxic irritants. The rash is erythematous with pustules, blisters, and crusts. It is only found in areas directly exposed to irritants, as opposed to AE, which spreads to areas distant to the primary reaction site. Irritant contact dermatitis presents with more of a burning sensation, whereas AE is more pruritic.39,40 Allergic contact dermatitis presents with erythematous vesicles and papules and sometimes with bullae. There is edema and crust formation, which often can spread past the point of contact in later stages. Similar to AE, there is intense pruritus. However, allergic contact dermatitis most commonly is caused by exposure to metals, cosmetics, and fragrances, whereas infectious agents and stasis dermatitis are the most common causes of AE.40,41 It may be challenging to distinguish AE from other causes of widespread eczematous dissemination. Vesicular eruptions sometimes require distinction from AE, including herpetic infections, insect bite reactions, and drug eruptions.18,42
Treatment
The underlying condition should be treated to mitigate the inflammatory response causing the id reaction. If not skillfully orchestrated, the id reaction can reoccur. For infectious causes of AE, an antifungal, antibacterial, antiviral, or antiparasitic should be given. If stasis dermatitis is responsible for the id reaction, compression stockings and leg elevation are indicated. The id reaction itself is treated with systemic or topical corticosteroids and wet compresses if acute. The goal of these treatments is to reduce patient discomfort caused by the inflammation and pruritus.18,43
Conclusion
Id reactions are an unusual phenomenon that commonly occurs after fungal skin infections and stasis dermatitis. T lymphocytes and keratinocytes may play a key role in this reaction, with newer research further delineating the process and possibly providing enhanced treatment options. Therapy focuses on treating the underlying condition, supplemented with corticosteroids for the autoeczema.
- Whitfield A. Lumleian Lectures on Some Points in the Aetiology of Skin Diseases. Delivered before the Royal College of Physicians of London on March 10th, 15th, and 17th, 1921. Lecture II. Lancet. 1921;2:122-127.
- Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128:E453-E457.
- Schrom KP, Kobs A, Nedorost S. Clinical psoriasiform dermatitis following dupilumab use for autoeczematization secondary to chronic stasis dermatitis. Cureus. 2020;12:e7831. doi:10.7759/cureus.7831
- Templeton HJ, Lunsford CJ, Allington HV. Autosensitization dermatitis; report of five cases and protocol of an experiment. Arch Derm Syphilol. 1949;59:68-77.
- Shelley WB. Id reaction. In: Consultations in Dermatology. Saunders; 1972:262-267.
- Sharquie KE, Noaimi AA, Flayih RA. Clinical and histopathological findings in patients with follicular dermatoses: all skin diseases starts in the hair follicles as new hypothesis. Am J Clin Res Rev. 2020;4:17.
- Kasteler JS, Petersen MJ, Vance JE, et al. Circulating activated T lymphocytes in autoeczematization. Arch Dermatol. 1992;128:795-798.
- González-Amaro R, Baranda L, Abud-Mendoza C, et al. Autoeczematization is associated with abnormal immune recognition of autologous skin antigens. J Am Acad Dermatol. 1993;28:56-60.
- Cunningham MJ, Zone JJ, Petersen MJ, et al. Circulating activated (DR-positive) T lymphocytes in a patient with autoeczematization. J Am Acad Dermatol. 1986;14:1039-1041.
- Furue M, Ulzii D, Vu YH, et al. Pathogenesis of atopic dermatitis: current paradigm. Iran J Immunol. 2019;16:97-107.
- Uchi H, Terao H, Koga T, et al. Cytokines and chemokines in the epidermis. J Dermatol Sci. 2000;24(suppl 1):S29-S38.
- Bos JD, Kapsenberg ML. The skin immune system: progress in cutaneous biology. Immunol Today. 1993;14:75-78.
- Young AW Jr. Dynamics of autosensitization dermatitis; a clinical and microscopic concept of autoeczematization. AMA Arch Derm. 1958;77:495-502.
- Brenner S, Wolf R, Landau M. Scabid: an unusual id reaction to scabies. Int J Dermatol. 1993;32:128-129.
- Yamany T, Schwartz RA. Infectious eczematoid dermatitis: a comprehensive review. J Eur Acad Dermatol Venereol. 2015;29:203-208.
- Wang X, Li L, Shi X, et al. Itching and its related factors in subtypes of eczema: a cross-sectional multicenter study in tertiary hospitals of China. Sci Rep. 2018;8:10754.
- Price A, Tavazoie M, Meehan SA, et al. Id reaction associated with red tattoo ink. Cutis. 2018;102:E32-E34.
- Ilkit M, Durdu M, Karaks¸ M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202.
- Kaner SR. Dermatitis venenata of the feet with a generalized “id” reaction. J Am Podiatry Assoc. 1970;60:199-204.
- Jordan L, Jackson NA, Carter-Snell B, et al. Pustular tinea id reaction. Cutis. 2019;103:E3-E4.
- Crum N, Hardaway C, Graham B. Development of an idlike reaction during treatment for acute pulmonary histoplasmosis: a new cutaneous manifestation in histoplasmosis. J Am Acad Dermatol. 2003;48(2 suppl):S5-S6.
- Chirac A, Brzezinski P, Chiriac AE, et al. Autosensitisation (autoeczematisation) reactions in a case of diaper dermatitis candidiasis. Niger Med J. 2014;55:274-275.
- Singh PY, Sinha P, Baveja S, et al. Immune-mediated tuberculous uveitis—a rare association with papulonecrotic tuberculid. Indian J Ophthalmol. 2019;67:1207-1209.
- Urso B, Georgesen C, Harp J. Papulonecrotic tuberculid secondary to Mycobacterium avium complex. Cutis. 2019;104:E11-E13.
- Choudhri SH, Magro CM, Crowson AN, et al. An id reaction to Mycobacterium leprae: first documented case. Cutis. 1994;54:282-286.
- Park JW, Jeong GJ, Seo SJ, et al. Pseudomonas toe web infection and autosensitisation dermatitis: diagnostic and therapeutic challenge. Int Wound J. 2020;17:1543-1544. doi:10.1111/iwj.13386
- Netchiporouk E, Cohen BA. Recognizing and managing eczematous id reactions to molluscum contagiosum virus in children. Pediatrics. 2012;129:E1072-E1075.
- Aurelian L, Ono F, Burnett J. Herpes simplex virus (HSV)-associated erythema multiforme (HAEM): a viral disease with an autoimmune component. Dermatol Online J. 2003;9:1.
- Rocamora V, Romaní J, Puig L, et al. Id reaction to molluscum contagiosum. Pediatr Dermatol. 1996;13:349-350.
- Yes¸ilova Y, Özbilgin A, Turan E, et al. Clinical exacerbation developing during treatment of cutaneous leishmaniasis: an id reaction? Turkiye Parazitol Derg. 2014;38:281-282.
- Connor CJ, Selby JC, Wanat KA. Severe pediculosis capitus: a case of “crusted lice” with autoeczematization. Dermatol Online J. 2016;22:13030/qt7c91z913.
- Shelley WB. The autoimmune mechanism in clinical dermatology. Arch Dermatol. 1962;86:27-34.
- Bosworth A, Hull PR. Disseminated eczema following radiotherapy: a case report. J Cutan Med Surg. 2018;22:353-355.
- Lowther C, Miedler JD, Cockerell CJ. Id-like reaction to BCG therapy for bladder cancer. Cutis. 2013;91:145-151.
- Huerth KA, Glick PL, Glick ZR. Cutaneous id reaction after using cyanoacrylate for wound closure. Cutis. 2020;105:E11-E13.
- Amini S, Burdick AE, Janniger CK. Dyshidrotic eczema (pompholyx). Updated April 22, 2020. Accessed August 23, 2021. https://emedicine.medscape.com/article/1122527-overview
- Sundaresan S, Migden MR, Silapunt S. Stasis dermatitis: pathophysiology, evaluation, and management. Am J Clin Dermatol. 2017;18:383-390.
- Hughes JDM, Pratt MD. Allergic contact dermatitis and autoeczematization to proctosedyl® cream and proctomyxin® cream. Case Rep Dermatol. 2018;10:238-246.
- Bains SN, Nash P, Fonacier L. Irritant contact dermatitis. Clin Rev Allergy Immunol. 2019;56:99-109.
- Novak-Bilic´ G, Vucˇic´ M, Japundžic´ I, et al. Irritant and allergic contact dermatitis—skin lesion characteristics. Acta Clin Croat. 2018;57:713-720.
- Nassau S, Fonacier L. Allergic contact dermatitis. Med Clin North Am. 2020;104:61-76.
- Lewis DJ, Schlichte MJ, Dao H Jr. Atypical disseminated herpes zoster: management guidelines in immunocompromised patients. Cutis. 2017;100:321-330.
- Nedorost S, White S, Rowland DY, et al. Development and implementation of an order set to improve value of care for patients with severe stasis dermatitis. J Am Acad Dermatol. 2019;80:815-817.
- Whitfield A. Lumleian Lectures on Some Points in the Aetiology of Skin Diseases. Delivered before the Royal College of Physicians of London on March 10th, 15th, and 17th, 1921. Lecture II. Lancet. 1921;2:122-127.
- Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128:E453-E457.
- Schrom KP, Kobs A, Nedorost S. Clinical psoriasiform dermatitis following dupilumab use for autoeczematization secondary to chronic stasis dermatitis. Cureus. 2020;12:e7831. doi:10.7759/cureus.7831
- Templeton HJ, Lunsford CJ, Allington HV. Autosensitization dermatitis; report of five cases and protocol of an experiment. Arch Derm Syphilol. 1949;59:68-77.
- Shelley WB. Id reaction. In: Consultations in Dermatology. Saunders; 1972:262-267.
- Sharquie KE, Noaimi AA, Flayih RA. Clinical and histopathological findings in patients with follicular dermatoses: all skin diseases starts in the hair follicles as new hypothesis. Am J Clin Res Rev. 2020;4:17.
- Kasteler JS, Petersen MJ, Vance JE, et al. Circulating activated T lymphocytes in autoeczematization. Arch Dermatol. 1992;128:795-798.
- González-Amaro R, Baranda L, Abud-Mendoza C, et al. Autoeczematization is associated with abnormal immune recognition of autologous skin antigens. J Am Acad Dermatol. 1993;28:56-60.
- Cunningham MJ, Zone JJ, Petersen MJ, et al. Circulating activated (DR-positive) T lymphocytes in a patient with autoeczematization. J Am Acad Dermatol. 1986;14:1039-1041.
- Furue M, Ulzii D, Vu YH, et al. Pathogenesis of atopic dermatitis: current paradigm. Iran J Immunol. 2019;16:97-107.
- Uchi H, Terao H, Koga T, et al. Cytokines and chemokines in the epidermis. J Dermatol Sci. 2000;24(suppl 1):S29-S38.
- Bos JD, Kapsenberg ML. The skin immune system: progress in cutaneous biology. Immunol Today. 1993;14:75-78.
- Young AW Jr. Dynamics of autosensitization dermatitis; a clinical and microscopic concept of autoeczematization. AMA Arch Derm. 1958;77:495-502.
- Brenner S, Wolf R, Landau M. Scabid: an unusual id reaction to scabies. Int J Dermatol. 1993;32:128-129.
- Yamany T, Schwartz RA. Infectious eczematoid dermatitis: a comprehensive review. J Eur Acad Dermatol Venereol. 2015;29:203-208.
- Wang X, Li L, Shi X, et al. Itching and its related factors in subtypes of eczema: a cross-sectional multicenter study in tertiary hospitals of China. Sci Rep. 2018;8:10754.
- Price A, Tavazoie M, Meehan SA, et al. Id reaction associated with red tattoo ink. Cutis. 2018;102:E32-E34.
- Ilkit M, Durdu M, Karaks¸ M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202.
- Kaner SR. Dermatitis venenata of the feet with a generalized “id” reaction. J Am Podiatry Assoc. 1970;60:199-204.
- Jordan L, Jackson NA, Carter-Snell B, et al. Pustular tinea id reaction. Cutis. 2019;103:E3-E4.
- Crum N, Hardaway C, Graham B. Development of an idlike reaction during treatment for acute pulmonary histoplasmosis: a new cutaneous manifestation in histoplasmosis. J Am Acad Dermatol. 2003;48(2 suppl):S5-S6.
- Chirac A, Brzezinski P, Chiriac AE, et al. Autosensitisation (autoeczematisation) reactions in a case of diaper dermatitis candidiasis. Niger Med J. 2014;55:274-275.
- Singh PY, Sinha P, Baveja S, et al. Immune-mediated tuberculous uveitis—a rare association with papulonecrotic tuberculid. Indian J Ophthalmol. 2019;67:1207-1209.
- Urso B, Georgesen C, Harp J. Papulonecrotic tuberculid secondary to Mycobacterium avium complex. Cutis. 2019;104:E11-E13.
- Choudhri SH, Magro CM, Crowson AN, et al. An id reaction to Mycobacterium leprae: first documented case. Cutis. 1994;54:282-286.
- Park JW, Jeong GJ, Seo SJ, et al. Pseudomonas toe web infection and autosensitisation dermatitis: diagnostic and therapeutic challenge. Int Wound J. 2020;17:1543-1544. doi:10.1111/iwj.13386
- Netchiporouk E, Cohen BA. Recognizing and managing eczematous id reactions to molluscum contagiosum virus in children. Pediatrics. 2012;129:E1072-E1075.
- Aurelian L, Ono F, Burnett J. Herpes simplex virus (HSV)-associated erythema multiforme (HAEM): a viral disease with an autoimmune component. Dermatol Online J. 2003;9:1.
- Rocamora V, Romaní J, Puig L, et al. Id reaction to molluscum contagiosum. Pediatr Dermatol. 1996;13:349-350.
- Yes¸ilova Y, Özbilgin A, Turan E, et al. Clinical exacerbation developing during treatment of cutaneous leishmaniasis: an id reaction? Turkiye Parazitol Derg. 2014;38:281-282.
- Connor CJ, Selby JC, Wanat KA. Severe pediculosis capitus: a case of “crusted lice” with autoeczematization. Dermatol Online J. 2016;22:13030/qt7c91z913.
- Shelley WB. The autoimmune mechanism in clinical dermatology. Arch Dermatol. 1962;86:27-34.
- Bosworth A, Hull PR. Disseminated eczema following radiotherapy: a case report. J Cutan Med Surg. 2018;22:353-355.
- Lowther C, Miedler JD, Cockerell CJ. Id-like reaction to BCG therapy for bladder cancer. Cutis. 2013;91:145-151.
- Huerth KA, Glick PL, Glick ZR. Cutaneous id reaction after using cyanoacrylate for wound closure. Cutis. 2020;105:E11-E13.
- Amini S, Burdick AE, Janniger CK. Dyshidrotic eczema (pompholyx). Updated April 22, 2020. Accessed August 23, 2021. https://emedicine.medscape.com/article/1122527-overview
- Sundaresan S, Migden MR, Silapunt S. Stasis dermatitis: pathophysiology, evaluation, and management. Am J Clin Dermatol. 2017;18:383-390.
- Hughes JDM, Pratt MD. Allergic contact dermatitis and autoeczematization to proctosedyl® cream and proctomyxin® cream. Case Rep Dermatol. 2018;10:238-246.
- Bains SN, Nash P, Fonacier L. Irritant contact dermatitis. Clin Rev Allergy Immunol. 2019;56:99-109.
- Novak-Bilic´ G, Vucˇic´ M, Japundžic´ I, et al. Irritant and allergic contact dermatitis—skin lesion characteristics. Acta Clin Croat. 2018;57:713-720.
- Nassau S, Fonacier L. Allergic contact dermatitis. Med Clin North Am. 2020;104:61-76.
- Lewis DJ, Schlichte MJ, Dao H Jr. Atypical disseminated herpes zoster: management guidelines in immunocompromised patients. Cutis. 2017;100:321-330.
- Nedorost S, White S, Rowland DY, et al. Development and implementation of an order set to improve value of care for patients with severe stasis dermatitis. J Am Acad Dermatol. 2019;80:815-817.
Practice Points
- Autoeczematization, or id reaction, is a disseminated reaction of the skin occurring at a site distant to a primary cutaneous infection or stimulus.
- T lymphocytes and keratinocytes are postulated to be involved in the pathogenesis of id reactions.
- Therapy includes treating the underlying pathology while providing topical corticosteroids for the autoeczematous lesions.
Plant Dermatitis: More Than Just Poison Ivy
Plants can contribute to a variety of dermatoses. The Toxicodendron genus, which includes poison ivy, poison oak, and poison sumac, is a well-known and common cause of allergic contact dermatitis (ACD), but many other plants can cause direct or airborne contact dermatitis, especially in gardeners, florists, and farmers. This article provides an overview of different plant-related dermatoses and culprit plants as well as how these dermatoses should be diagnosed and treated.
Epidemiology
Plant dermatoses affect more than 50 million individuals each year.1,2 In the United States, the Toxicodendron genus causes ACD in more than 70% of exposed individuals, leading to medical visits.3 An urgent care visit for a plant-related dermatitis is estimated to cost $168, while an emergency department visit can cost 3 times as much.4 Although less common, Compositae plants are another important culprit of plant dermatitis, particularly in gardeners, florists, and farmers. Data from the 2017-2018 North American Contact Dermatitis Group screening series (N=4947) showed sesquiterpene lactones and Compositae to be positive in 0.5% of patch-tested patients.5
Plant Dermatitis Classifications
Plant dermatitis can be classified into 5 main categories: ACD, mechanical irritant contact dermatitis, chemical irritant contact dermatitis, light-mediated dermatitis, and pseudophytodermatitis.6
Allergic contact dermatitis is an immune-mediated type IV delayed hypersensitivity reaction. The common molecular allergens in plants include phenols, α-methylene-γ-butyrolactones, quinones, terpenes, disulfides, isothiocyanates, and polyacetylenic derivatives.6
Plant contact dermatitis due to mechanical and chemical irritants is precipitated by multiple mechanisms, including disruption of the epidermal barrier and subsequent cytokine release from keratinocytes.7 Nonimmunologic contact urticaria from plants is thought to be a type of irritant reaction precipitated by mechanical or chemical trauma.8
Light-mediated dermatitis includes phytophotodermatitis and photoallergic contact dermatitis. Phytophotodermatitis is a phototoxic reaction triggered by exposure to both plant-derived furanocoumarin and UVA light.9 By contrast, photoallergic contact dermatitis is a delayed hypersensitivity reaction from prior sensitization to a light-activated antigen.10
Pseudophytodermatitis, as its name implies, is not truly mediated by an allergen or irritant intrinsic to the plant but rather by dyes, waxes, insecticides, or arthropods that inhabit the plant or are secondarily applied.6
Common Plant Allergens
Anacardiaceae Family
Most of the allergenic plants within the Anacardiaceae family belong to the Toxicodendron genus, which encompasses poison ivy (Toxicodendron radicans), poison oak (Toxicodendron pubescens,Toxicodendron quercifolium, Toxicodendron diversiloum), and poison sumac (Toxicodendron vernix). Poison ivy is the celebrity of the Anacardiaceae family and contributes to most cases of plant-related ACD. It is found in every state in the continental United States. Poison oak is another common culprit found in the western and southeastern United States.11 Plants within the Anacardiaceae family contain an oleoresin called urushiol, which is the primary sensitizing substance. Although poison ivy and poison oak grow well in full sun to partial shade, poison sumac typically is found in damp swampy areas east of the Rocky Mountains. Most cases of ACD related to Anacardiaceae species are due to direct contact with urushiol from a Toxicodendron plant, but burning of brush containing Toxicodendron can cause airborne exposure when urushiol oil is carried by smoke particles.12 Sensitization to Toxicodendron can cause ACD to other Anacardiaceae species such as the Japanese lacquer tree (Toxicodendron vernicifluum), mango tree (Mangifera indica), cashew tree (Anacardium occidentale), and Indian marking nut tree (Semecarpus anacardium).6 Cross-reactions to components of the ginkgo tree (Ginkgo biloba) also are possible.
Toxicodendron plants can be more easily identified and avoided with knowledge of their characteristic leaf patterns. The most dependable way to identify poison ivy and poison oak species is to look for plants with 3 leaves, giving rise to the common saying, “Leaves of three, leave them be.” Poison sumac plants have groups of 7 to 13 leaves arranged as pairs along a central rib. Another helpful finding is a black deposit that Toxicodendron species leave behind following trauma to the leaves. Urushiol oxidizes when exposed to air and turns into a black deposit that can be seen on damaged leaves themselves or can be demonstrated in a black spot test to verify if a plant is a Toxicodendron species. The test is performed by gathering (carefully, without direct contact) a few leaves in a paper towel and crushing them to release sap. Within minutes, the sap will turn black if the plant is indeed a Toxicodendron species.13Pruritic, edematous, erythematous papules, plaques, and eventual vesicles in a linear distribution are suspicious for Toxicodendron exposure. Although your pet will not develop Toxicodendron ACD, oleoresin-contaminated pets can transfer the oils to their owners after coming into contact with these plants. Toxicodendron dermatitis also can be acquired from oleoresin-contaminated fomites such as clothing and shoes worn in the garden or when hiking. Toxicodendron dermatitis can appear at different sites on the body at different times depending on the amount of oleoresin exposure as well as epidermal thickness. For example, the oleoresin can be transferred from the hands to body areas with a thinner stratum corneum (eg, genitalia) and cause subsequent dermatitis.1
Compositae Family
The Compositae family (also known as Asteraceae) is a large plant family with more than 20,000 species, including numerous weeds, wildflowers, and vegetables. The flowers, leaves, stems, and pollens of the Compositae family are coated by cyclic esters called sesquiterpene lactones. Mitchell and Dupuis14 showed that sesquiterpene lactones are the allergens responsible for ACD to various Compositae plants, including ragweed (Ambrosia), sneezeweed (Helenium), and chrysanthemums (Chrysanthemum). Common Compositae vegetables such as lettuce (Lactuca sativa) have been reported to cause ACD in chefs, grocery store produce handlers, gardeners, and even owners of lettuce-eating pet guinea pigs and turtles.15 Similarly, artichokes (Cynara scolymus) can cause ACD in gardeners.16 Exposure to Compositae species also has been implicated in photoallergic reactions, and studies have demonstrated that some patients with chronic actinic dermatitis also have positive patch test reactions to Compositae species and/or sesquiterpene lactones.17,18
In addition to direct contact with Compositae plants, airborne exposure to sesquiterpene lactones can cause ACD.14 The pattern of airborne contact dermatitis typically involves exposed areas such as the eyelids, central face, and/or neck. The beak sign also can be a clue to airborne contact dermatitis, which involves dermatitis of the face that spares the nasal tip and/or nasal ridge. It is thought that the beak sign may result from increased sebaceous gland concentration on the nose, which prevents penetration of allergens and irritants.19 Unlike photoallergic contact dermatitis, which also can involve the face, airborne ACD frequently involves photoprotected areas such as the submandibular chin and the upper lip. Davies and Kersey20 reported the case of a groundsman who was cutting grass with dandelions (Taraxacum officinale) and was found to have associated airborne ACD of the face, neck, and forearms due to Compositae allergy. In a different setting, the aromas of chamomile (Matricaria chamomilla) have been reported to cause airborne ACD in a tea drinker.21 Paulsen22 found that ingestion of chamomile tea can induce systemic ACD in sensitized individuals.
Alstroemeriaceae, Liliaceae, and Primulaceae
Florists are exposed to many plant species and have a high prevalence of ACD. Thiboutot et al23 found that 15 of 57 (26%) floral workers experienced hand dermatitis that cleared with time away from work. The Peruvian lily (Alstroemeria, Alstroemeriaceae family), which contains tuliposide A, was found to be the leading cause of sensitization.23 Tulips (Tulipa, Liliaceae family), as the flower name suggests, also contain tuliposide A, which along with mechanical irritation from the course tecta fibers on the bulbs lead to a dermatitis known as tulip fingers.24,25 Poison primrose (Primula obconica, Primulaceae family), cultivated for its highly colorful flowers, contains the contact allergen primin.6 A common clinical presentation of ACD for any of these culprit flowers is localized dermatitis of the thumb and index finger in a florist or gardener.
Plants That Cause Irritant Reactions
Cactuses
Although the long spines of the Cactaceae family of cactuses is a warning for passersby, it is the small and nearly invisible barbed hairs (glochids) that inflict a more dramatic cutaneous reaction. The prickly pear cactus (Opuntia species) is a good example of such a plant, as its glochids cause mechanical irritation but also can become embedded in the skin and result in subcutaneous granulomas known as sabra dermatitis.26
Stinging Nettle
The dermatologic term urticaria owes its namesake to the stinging nettle plant, which comes from the family Urticaceae. The stinging nettle has small hairs on its leaves, referred to as stinging trichomes, which have needlelike tips that pierce the skin and inject a mix of histamine, formic acid, and acetylcholine, causing a pruritic dermatitis that may last up to 12 hours.27 The plant is found worldwide and is a common weed in North America.
Phytophotodermatitis
Lemons and limes (Rutaceae family) are common culprits of phytophotodermatitis, often causing what is known as a margarita burn after outdoor consumption or preparation of this tasty citrus beverage.28 An accidental spray of lime juice on the skin while adding it to a beer, guacamole, salsa, or any other food or beverage also can cause phytophotodermatitis.29-31 Although the juice of lemons and limes contains psoralens, the rind can contain a 6- to 186-fold increased concentration.32 Psoralen is the photoactive agent in Rutaceae plants that intercalates in double-stranded DNA and promotes intrastrand cross-links when exposed to UVA light, which ultimately leads to dermatitis.9 Phytophotodermatitis commonly causes erythema, edema, and painful bullae on sun-exposed areas and classically heals with hyperpigmentation.
Pseudophytodermatitis can occur in grain farmers and harvesters who handle wheat and/or barley and incidentally come in contact with insects and chemicals on the plant material. Pseudophytodermatitis from mites in the wheat and/or barley plant can occur at harvest time when contact with the plant material is high. Insects such as the North American itch mite (Pediculoides ventricosus) can cause petechiae, wheals, and pustules. In addition, insecticides such as malathion and arsenical sprays that are applied to plant leaves can cause pseudophytodermatitis, which may be initially diagnosed as dermatitis to the plant itself.6
Patch Testing to Plants
When a patient presents with recurrent or persistent dermatitis and a plant contact allergen is suspected, patch testing is indicated. Most comprehensive patch test series contain various plant allergens, such as sesquiterpene lactones, Compositae mix, and limonene hydroperoxides, and patch testing to a specialized plant series may be necessary. Poison ivy/oak/sumac allergens typically are not included in patch test series because of the high prevalence of allergic reactions to these chemicals and the likelihood of sensitization when patch testing with urushiol. Compositae contact sensitization can be difficult to diagnose because neither sesquiterpene lactone mix 0.1% nor parthenolide 0.1% are sensitive enough to pick up all Compositae allergies.33,34 Paulsen and Andersen34 proposed that if Compositae sensitization is suspected, testing should include sesquiterpene lactone, parthenolide, and Compositae mix II 2.5%, as well as other potential Compositae allergens based on the patient’s history.34
Because plants can have geographic variability and contain potentially unknown allergens,35 testing to plant components may increase the diagnostic yield of patch testing. Dividing the plant into component parts (ie, stem, bulb, leaf, flower) is helpful, as different components have different allergen concentrations. It is important to consult expert resources before proceeding with plant component patch testing because irritant reactions are frequent and may confound the testing.36
Prevention and Treatment
For all plant dermatoses, the mainstay of prevention is to avoid contact with the offending plant material. Gloves can be an important protective tool for plant dermatitis prevention; the correct material depends on the plant species being handled. Rubber gloves should not be worn to protect against Toxicodendron plants since the catechols in urushiol are soluble in rubber; vinyl gloves should be worn instead.6 Marks37 found that tuliposide A, the allergen in the Peruvian lily (Alstroemeria), penetrates both vinyl and latex gloves; it does not penetrate nitrile gloves. If exposed, the risk of dermatitis can be decreased if the allergen is washed away with soap and water as soon as possible. Some allergens such as Toxicodendron are absorbed quickly and need to be washed off within 10 minutes of exposure.6 Importantly, exposed gardening gloves may continue to perpetuate ACD if the allergen is not also washed off the gloves themselves.
For light-mediated dermatoses, sun avoidance or use of an effective sunscreen can reduce symptoms in an individual who has already been exposed.10 UVA light activates psoralen-mediated dermatitis but not until 30 to 120 minutes after absorption into the skin.38
Barrier creams are thought to be protective against plant ACD through a variety of mechanisms. The cream itself is meant to reduce skin contact to an allergen or irritant. Additionally, barrier creams contain active ingredients such as silicone, hydrocarbons, and aluminum chlorohydrate, which are thought to trap or transform offending agents before contacting the skin. When contact with a Toxicodendron species is anticipated, Marks et al39 found that dermatitis was absent or significantly reduced when 144 patients were pretreated with quaternium-18 bentonite lotion 5% (P<.0001).
Although allergen avoidance and use of gloves and barrier creams are the mainstays of preventing plant dermatoses, treatment often is required to control postexposure symptoms. For all plant dermatoses, topical corticosteroids can be used to reduce inflammation and pruritus. In some cases, systemic steroids may be necessary. To prevent rebound of dermatitis, patients often require a 3-week or longer course of oral steroids to quell the reaction, particularly if the dermatitis is vigorous or an id reaction is present.40 Antihistamines and cold compresses also can provide symptomatic relief.
Final Interpretation
Plants can cause a variety of dermatoses. Although Toxicodendron plants are the most frequent cause of ACD, it is important to keep in mind that florists, gardeners, and farmers are exposed to a large variety of allergens, irritants, and phototoxic agents that cause dermatoses as well. Confirmation of plant-induced ACD involves patch testing against suspected species. Prevention involves use of appropriate barriers and avoidance of implicated plants. Treatment includes topical steroids, antihistamines, and prednisone.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128.
- Pariser D, Ceilley R, Lefkovits A, et al. Poison ivy, oak and sumac. Derm Insights. 2003;4:26-28.
- Wolff K, Johnson R. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 6th ed. McGraw Hill Education; 2009.
- Zomorodi N, Butt M, Maczuga S, et al. Cost and diagnostic characteristics of Toxicodendron dermatitis in the USA: a retrospective cross-sectional analysis. Br J Dermatol. 2020;183:772-773.
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123.
- Fowler JF, Zirwas MJ. Fisher’s Contact Dermatitis. 7th ed. Contact Dermatitis Institute; 2019.
- Smith HR, Basketter DA, McFadden JP. Irritant dermatitis, irritancy and its role in allergic contact dermatitis. Clin Exp Dermatol. 2002;27:138-146.
- Wakelin SH. Contact urticaria. Clin Exp Dermatol. 2001;26:132-136.
- Ellis CR, Elston DM. Psoralen-induced phytophotodermatitis. Dermatitis. 2021;32:140-143.
- Deleo VA. Photocontact dermatitis. Dermatol Ther. 2004;17:279-288.
- National Institute for Occupational Safety and Health. Poisonous plants. Centers for Disease Control and Prevention website. Updated June 1, 2018. Accessed August 10, 2021. https://www.cdc.gov/niosh/topics/plants/geographic.html
- Schloemer JA, Zirwas MJ, Burkhart CG. Airborne contact dermatitis: common causes in the USA. Int J Dermatol. 2015;54:271-274.
- Guin JD. The black spot test for recognizing poison ivy and related species. J Am Acad Dermatol. 1980;2:332-333.
- Mitchell J, Dupuis G. Allergic contact dermatitis from sesquiterpenoids of the Compositae family of plants. Br J Dermatol. 1971;84:139-150.
- Paulsen E, Andersen KE. Lettuce contact allergy. Contact Dermatitis. 2016;74:67-75.
- Samaran Q, Clark E, Dereure O, et al. Airborne allergic contact dermatitis caused by artichoke. Contact Dermatitis. 2020;82:395-397.
- Du H, Ross JS, Norris PG, et al. Contact and photocontact sensitization in chronic actinic dermatitis: sesquiterpene lactone mix is an important allergen. Br J Dermatol. 1995;132:543-547.
- Wrangsjo K, Marie Ros A, Walhberg JE. Contact allergy to Compositae plants in patients with summer-exacerbated dermatitis. Contact Dermatitis. 1990;22:148-154.
- Staser K, Ezra N, Sheehan MP, et al. The beak sign: a clinical clue to airborne contact dermatitis. Dermatitis. 2014;25:97-98.
- Davies M, Kersey J. Contact allergy to yarrow and dandelion. Contact Dermatitis. 1986;14:256-257.
- Anzai A, Vázquez Herrera NE, Tosti A. Airborne allergic contact dermatitis caused by chamomile tea. Contact Dermatitis. 2015;72:254-255.
- Paulsen E. Systemic allergic dermatitis caused by sesquiterpene lactones. Contact Dermatitis. 2017;76:1-10.
- Thiboutot DM, Hamory BH, Marks JG. Dermatoses among floral shop workers. J Am Acad Dermatol. 1990;22:54-58.
- Hjorth N, Wilkinson DS. Contact dermatitis IV. tulip fingers, hyacinth itch and lily rash. Br J Dermatol. 1968;80:696-698.
- Guin JD, Franks H. Fingertip dermatitis in a retail florist. Cutis. 2001;67:328-330.
- Magro C, Lipner S. Sabra dermatitis: combined features of delayed hypersensitivity and foreign body reaction to implanted glochidia. Dermatol Online J. 2020;26:13030/qt2157f9g0.
- Cummings AJ, Olsen M. Mechanism of action of stinging nettles. Wilderness Environ Med. 2011;22:136-139.
- Maniam G, Light KML, Wilson J. Margarita burn: recognition and treatment of phytophotodermatitis. J Am Board Fam Med. 2021;34:398-401.
- Flugman SL. Mexican beer dermatitis: a unique variant of lime phytophotodermatitis attributable to contemporary beer-drinking practices. Arch Dermatol. 2010;146:1194-1195.
- Kung AC, Stephens MB, Darling T. Phytophotodermatitis: bulla formation and hyperpigmentation during spring break. Mil Med. 2009;174:657-661.
- Smith LG. Phytophotodermatitis. Images Emerg Med. 2017;1:146-147.
- Wagner AM, Wu JJ, Hansen RC, et al. Bullous phytophotodermatitis associated with high natural concentrations of furanocoumarins in limes. Am J Contact Dermat. 2002;13:10-14.
- Green C, Ferguson J. Sesquiterpene lactone mix is not an adequate screen for Compositae allergy. Contact Dermatitis. 1994;31:151-153.
- Paulsen E, Andersen KE. Screening for Compositae contact sensitization with sesquiterpene lactones and Compositae mix 2.5% pet. Contact Dermatitis. 2019;81:368-373.
- Paulsen E, Andersen KE. Patch testing with constituents of Compositae mixes. Contact Dermatitis. 2012;66:241-246.
- Frosch PJ, Geier J, Uter W, et al. Patch testing with the patients’ own products. Contact Dermatitis. 2011:929-941.
- Marks JG. Allergic contact dermatitis to Alstroemeria. Arch Dermatol. 1988;124:914-916.
- Moreau JF, English JC, Gehris RP. Phytophotodermatitis. J Pediatr Adolesc Gynecol. 2014;27:93-94.
- Marks JG, Fowler JF, Sherertz EF, et al. Prevention of poison ivy and poison oak allergic contact dermatitis by quaternium-18 bentonite. J Am Acad Dermatol. 1995;33:212-216.
- Craig K, Meadows SE. What is the best duration of steroid therapy for contact dermatitis (rhus)? J Fam Pract. 2006;55:166-167.
Plants can contribute to a variety of dermatoses. The Toxicodendron genus, which includes poison ivy, poison oak, and poison sumac, is a well-known and common cause of allergic contact dermatitis (ACD), but many other plants can cause direct or airborne contact dermatitis, especially in gardeners, florists, and farmers. This article provides an overview of different plant-related dermatoses and culprit plants as well as how these dermatoses should be diagnosed and treated.
Epidemiology
Plant dermatoses affect more than 50 million individuals each year.1,2 In the United States, the Toxicodendron genus causes ACD in more than 70% of exposed individuals, leading to medical visits.3 An urgent care visit for a plant-related dermatitis is estimated to cost $168, while an emergency department visit can cost 3 times as much.4 Although less common, Compositae plants are another important culprit of plant dermatitis, particularly in gardeners, florists, and farmers. Data from the 2017-2018 North American Contact Dermatitis Group screening series (N=4947) showed sesquiterpene lactones and Compositae to be positive in 0.5% of patch-tested patients.5
Plant Dermatitis Classifications
Plant dermatitis can be classified into 5 main categories: ACD, mechanical irritant contact dermatitis, chemical irritant contact dermatitis, light-mediated dermatitis, and pseudophytodermatitis.6
Allergic contact dermatitis is an immune-mediated type IV delayed hypersensitivity reaction. The common molecular allergens in plants include phenols, α-methylene-γ-butyrolactones, quinones, terpenes, disulfides, isothiocyanates, and polyacetylenic derivatives.6
Plant contact dermatitis due to mechanical and chemical irritants is precipitated by multiple mechanisms, including disruption of the epidermal barrier and subsequent cytokine release from keratinocytes.7 Nonimmunologic contact urticaria from plants is thought to be a type of irritant reaction precipitated by mechanical or chemical trauma.8
Light-mediated dermatitis includes phytophotodermatitis and photoallergic contact dermatitis. Phytophotodermatitis is a phototoxic reaction triggered by exposure to both plant-derived furanocoumarin and UVA light.9 By contrast, photoallergic contact dermatitis is a delayed hypersensitivity reaction from prior sensitization to a light-activated antigen.10
Pseudophytodermatitis, as its name implies, is not truly mediated by an allergen or irritant intrinsic to the plant but rather by dyes, waxes, insecticides, or arthropods that inhabit the plant or are secondarily applied.6
Common Plant Allergens
Anacardiaceae Family
Most of the allergenic plants within the Anacardiaceae family belong to the Toxicodendron genus, which encompasses poison ivy (Toxicodendron radicans), poison oak (Toxicodendron pubescens,Toxicodendron quercifolium, Toxicodendron diversiloum), and poison sumac (Toxicodendron vernix). Poison ivy is the celebrity of the Anacardiaceae family and contributes to most cases of plant-related ACD. It is found in every state in the continental United States. Poison oak is another common culprit found in the western and southeastern United States.11 Plants within the Anacardiaceae family contain an oleoresin called urushiol, which is the primary sensitizing substance. Although poison ivy and poison oak grow well in full sun to partial shade, poison sumac typically is found in damp swampy areas east of the Rocky Mountains. Most cases of ACD related to Anacardiaceae species are due to direct contact with urushiol from a Toxicodendron plant, but burning of brush containing Toxicodendron can cause airborne exposure when urushiol oil is carried by smoke particles.12 Sensitization to Toxicodendron can cause ACD to other Anacardiaceae species such as the Japanese lacquer tree (Toxicodendron vernicifluum), mango tree (Mangifera indica), cashew tree (Anacardium occidentale), and Indian marking nut tree (Semecarpus anacardium).6 Cross-reactions to components of the ginkgo tree (Ginkgo biloba) also are possible.
Toxicodendron plants can be more easily identified and avoided with knowledge of their characteristic leaf patterns. The most dependable way to identify poison ivy and poison oak species is to look for plants with 3 leaves, giving rise to the common saying, “Leaves of three, leave them be.” Poison sumac plants have groups of 7 to 13 leaves arranged as pairs along a central rib. Another helpful finding is a black deposit that Toxicodendron species leave behind following trauma to the leaves. Urushiol oxidizes when exposed to air and turns into a black deposit that can be seen on damaged leaves themselves or can be demonstrated in a black spot test to verify if a plant is a Toxicodendron species. The test is performed by gathering (carefully, without direct contact) a few leaves in a paper towel and crushing them to release sap. Within minutes, the sap will turn black if the plant is indeed a Toxicodendron species.13Pruritic, edematous, erythematous papules, plaques, and eventual vesicles in a linear distribution are suspicious for Toxicodendron exposure. Although your pet will not develop Toxicodendron ACD, oleoresin-contaminated pets can transfer the oils to their owners after coming into contact with these plants. Toxicodendron dermatitis also can be acquired from oleoresin-contaminated fomites such as clothing and shoes worn in the garden or when hiking. Toxicodendron dermatitis can appear at different sites on the body at different times depending on the amount of oleoresin exposure as well as epidermal thickness. For example, the oleoresin can be transferred from the hands to body areas with a thinner stratum corneum (eg, genitalia) and cause subsequent dermatitis.1
Compositae Family
The Compositae family (also known as Asteraceae) is a large plant family with more than 20,000 species, including numerous weeds, wildflowers, and vegetables. The flowers, leaves, stems, and pollens of the Compositae family are coated by cyclic esters called sesquiterpene lactones. Mitchell and Dupuis14 showed that sesquiterpene lactones are the allergens responsible for ACD to various Compositae plants, including ragweed (Ambrosia), sneezeweed (Helenium), and chrysanthemums (Chrysanthemum). Common Compositae vegetables such as lettuce (Lactuca sativa) have been reported to cause ACD in chefs, grocery store produce handlers, gardeners, and even owners of lettuce-eating pet guinea pigs and turtles.15 Similarly, artichokes (Cynara scolymus) can cause ACD in gardeners.16 Exposure to Compositae species also has been implicated in photoallergic reactions, and studies have demonstrated that some patients with chronic actinic dermatitis also have positive patch test reactions to Compositae species and/or sesquiterpene lactones.17,18
In addition to direct contact with Compositae plants, airborne exposure to sesquiterpene lactones can cause ACD.14 The pattern of airborne contact dermatitis typically involves exposed areas such as the eyelids, central face, and/or neck. The beak sign also can be a clue to airborne contact dermatitis, which involves dermatitis of the face that spares the nasal tip and/or nasal ridge. It is thought that the beak sign may result from increased sebaceous gland concentration on the nose, which prevents penetration of allergens and irritants.19 Unlike photoallergic contact dermatitis, which also can involve the face, airborne ACD frequently involves photoprotected areas such as the submandibular chin and the upper lip. Davies and Kersey20 reported the case of a groundsman who was cutting grass with dandelions (Taraxacum officinale) and was found to have associated airborne ACD of the face, neck, and forearms due to Compositae allergy. In a different setting, the aromas of chamomile (Matricaria chamomilla) have been reported to cause airborne ACD in a tea drinker.21 Paulsen22 found that ingestion of chamomile tea can induce systemic ACD in sensitized individuals.
Alstroemeriaceae, Liliaceae, and Primulaceae
Florists are exposed to many plant species and have a high prevalence of ACD. Thiboutot et al23 found that 15 of 57 (26%) floral workers experienced hand dermatitis that cleared with time away from work. The Peruvian lily (Alstroemeria, Alstroemeriaceae family), which contains tuliposide A, was found to be the leading cause of sensitization.23 Tulips (Tulipa, Liliaceae family), as the flower name suggests, also contain tuliposide A, which along with mechanical irritation from the course tecta fibers on the bulbs lead to a dermatitis known as tulip fingers.24,25 Poison primrose (Primula obconica, Primulaceae family), cultivated for its highly colorful flowers, contains the contact allergen primin.6 A common clinical presentation of ACD for any of these culprit flowers is localized dermatitis of the thumb and index finger in a florist or gardener.
Plants That Cause Irritant Reactions
Cactuses
Although the long spines of the Cactaceae family of cactuses is a warning for passersby, it is the small and nearly invisible barbed hairs (glochids) that inflict a more dramatic cutaneous reaction. The prickly pear cactus (Opuntia species) is a good example of such a plant, as its glochids cause mechanical irritation but also can become embedded in the skin and result in subcutaneous granulomas known as sabra dermatitis.26
Stinging Nettle
The dermatologic term urticaria owes its namesake to the stinging nettle plant, which comes from the family Urticaceae. The stinging nettle has small hairs on its leaves, referred to as stinging trichomes, which have needlelike tips that pierce the skin and inject a mix of histamine, formic acid, and acetylcholine, causing a pruritic dermatitis that may last up to 12 hours.27 The plant is found worldwide and is a common weed in North America.
Phytophotodermatitis
Lemons and limes (Rutaceae family) are common culprits of phytophotodermatitis, often causing what is known as a margarita burn after outdoor consumption or preparation of this tasty citrus beverage.28 An accidental spray of lime juice on the skin while adding it to a beer, guacamole, salsa, or any other food or beverage also can cause phytophotodermatitis.29-31 Although the juice of lemons and limes contains psoralens, the rind can contain a 6- to 186-fold increased concentration.32 Psoralen is the photoactive agent in Rutaceae plants that intercalates in double-stranded DNA and promotes intrastrand cross-links when exposed to UVA light, which ultimately leads to dermatitis.9 Phytophotodermatitis commonly causes erythema, edema, and painful bullae on sun-exposed areas and classically heals with hyperpigmentation.
Pseudophytodermatitis can occur in grain farmers and harvesters who handle wheat and/or barley and incidentally come in contact with insects and chemicals on the plant material. Pseudophytodermatitis from mites in the wheat and/or barley plant can occur at harvest time when contact with the plant material is high. Insects such as the North American itch mite (Pediculoides ventricosus) can cause petechiae, wheals, and pustules. In addition, insecticides such as malathion and arsenical sprays that are applied to plant leaves can cause pseudophytodermatitis, which may be initially diagnosed as dermatitis to the plant itself.6
Patch Testing to Plants
When a patient presents with recurrent or persistent dermatitis and a plant contact allergen is suspected, patch testing is indicated. Most comprehensive patch test series contain various plant allergens, such as sesquiterpene lactones, Compositae mix, and limonene hydroperoxides, and patch testing to a specialized plant series may be necessary. Poison ivy/oak/sumac allergens typically are not included in patch test series because of the high prevalence of allergic reactions to these chemicals and the likelihood of sensitization when patch testing with urushiol. Compositae contact sensitization can be difficult to diagnose because neither sesquiterpene lactone mix 0.1% nor parthenolide 0.1% are sensitive enough to pick up all Compositae allergies.33,34 Paulsen and Andersen34 proposed that if Compositae sensitization is suspected, testing should include sesquiterpene lactone, parthenolide, and Compositae mix II 2.5%, as well as other potential Compositae allergens based on the patient’s history.34
Because plants can have geographic variability and contain potentially unknown allergens,35 testing to plant components may increase the diagnostic yield of patch testing. Dividing the plant into component parts (ie, stem, bulb, leaf, flower) is helpful, as different components have different allergen concentrations. It is important to consult expert resources before proceeding with plant component patch testing because irritant reactions are frequent and may confound the testing.36
Prevention and Treatment
For all plant dermatoses, the mainstay of prevention is to avoid contact with the offending plant material. Gloves can be an important protective tool for plant dermatitis prevention; the correct material depends on the plant species being handled. Rubber gloves should not be worn to protect against Toxicodendron plants since the catechols in urushiol are soluble in rubber; vinyl gloves should be worn instead.6 Marks37 found that tuliposide A, the allergen in the Peruvian lily (Alstroemeria), penetrates both vinyl and latex gloves; it does not penetrate nitrile gloves. If exposed, the risk of dermatitis can be decreased if the allergen is washed away with soap and water as soon as possible. Some allergens such as Toxicodendron are absorbed quickly and need to be washed off within 10 minutes of exposure.6 Importantly, exposed gardening gloves may continue to perpetuate ACD if the allergen is not also washed off the gloves themselves.
For light-mediated dermatoses, sun avoidance or use of an effective sunscreen can reduce symptoms in an individual who has already been exposed.10 UVA light activates psoralen-mediated dermatitis but not until 30 to 120 minutes after absorption into the skin.38
Barrier creams are thought to be protective against plant ACD through a variety of mechanisms. The cream itself is meant to reduce skin contact to an allergen or irritant. Additionally, barrier creams contain active ingredients such as silicone, hydrocarbons, and aluminum chlorohydrate, which are thought to trap or transform offending agents before contacting the skin. When contact with a Toxicodendron species is anticipated, Marks et al39 found that dermatitis was absent or significantly reduced when 144 patients were pretreated with quaternium-18 bentonite lotion 5% (P<.0001).
Although allergen avoidance and use of gloves and barrier creams are the mainstays of preventing plant dermatoses, treatment often is required to control postexposure symptoms. For all plant dermatoses, topical corticosteroids can be used to reduce inflammation and pruritus. In some cases, systemic steroids may be necessary. To prevent rebound of dermatitis, patients often require a 3-week or longer course of oral steroids to quell the reaction, particularly if the dermatitis is vigorous or an id reaction is present.40 Antihistamines and cold compresses also can provide symptomatic relief.
Final Interpretation
Plants can cause a variety of dermatoses. Although Toxicodendron plants are the most frequent cause of ACD, it is important to keep in mind that florists, gardeners, and farmers are exposed to a large variety of allergens, irritants, and phototoxic agents that cause dermatoses as well. Confirmation of plant-induced ACD involves patch testing against suspected species. Prevention involves use of appropriate barriers and avoidance of implicated plants. Treatment includes topical steroids, antihistamines, and prednisone.
Plants can contribute to a variety of dermatoses. The Toxicodendron genus, which includes poison ivy, poison oak, and poison sumac, is a well-known and common cause of allergic contact dermatitis (ACD), but many other plants can cause direct or airborne contact dermatitis, especially in gardeners, florists, and farmers. This article provides an overview of different plant-related dermatoses and culprit plants as well as how these dermatoses should be diagnosed and treated.
Epidemiology
Plant dermatoses affect more than 50 million individuals each year.1,2 In the United States, the Toxicodendron genus causes ACD in more than 70% of exposed individuals, leading to medical visits.3 An urgent care visit for a plant-related dermatitis is estimated to cost $168, while an emergency department visit can cost 3 times as much.4 Although less common, Compositae plants are another important culprit of plant dermatitis, particularly in gardeners, florists, and farmers. Data from the 2017-2018 North American Contact Dermatitis Group screening series (N=4947) showed sesquiterpene lactones and Compositae to be positive in 0.5% of patch-tested patients.5
Plant Dermatitis Classifications
Plant dermatitis can be classified into 5 main categories: ACD, mechanical irritant contact dermatitis, chemical irritant contact dermatitis, light-mediated dermatitis, and pseudophytodermatitis.6
Allergic contact dermatitis is an immune-mediated type IV delayed hypersensitivity reaction. The common molecular allergens in plants include phenols, α-methylene-γ-butyrolactones, quinones, terpenes, disulfides, isothiocyanates, and polyacetylenic derivatives.6
Plant contact dermatitis due to mechanical and chemical irritants is precipitated by multiple mechanisms, including disruption of the epidermal barrier and subsequent cytokine release from keratinocytes.7 Nonimmunologic contact urticaria from plants is thought to be a type of irritant reaction precipitated by mechanical or chemical trauma.8
Light-mediated dermatitis includes phytophotodermatitis and photoallergic contact dermatitis. Phytophotodermatitis is a phototoxic reaction triggered by exposure to both plant-derived furanocoumarin and UVA light.9 By contrast, photoallergic contact dermatitis is a delayed hypersensitivity reaction from prior sensitization to a light-activated antigen.10
Pseudophytodermatitis, as its name implies, is not truly mediated by an allergen or irritant intrinsic to the plant but rather by dyes, waxes, insecticides, or arthropods that inhabit the plant or are secondarily applied.6
Common Plant Allergens
Anacardiaceae Family
Most of the allergenic plants within the Anacardiaceae family belong to the Toxicodendron genus, which encompasses poison ivy (Toxicodendron radicans), poison oak (Toxicodendron pubescens,Toxicodendron quercifolium, Toxicodendron diversiloum), and poison sumac (Toxicodendron vernix). Poison ivy is the celebrity of the Anacardiaceae family and contributes to most cases of plant-related ACD. It is found in every state in the continental United States. Poison oak is another common culprit found in the western and southeastern United States.11 Plants within the Anacardiaceae family contain an oleoresin called urushiol, which is the primary sensitizing substance. Although poison ivy and poison oak grow well in full sun to partial shade, poison sumac typically is found in damp swampy areas east of the Rocky Mountains. Most cases of ACD related to Anacardiaceae species are due to direct contact with urushiol from a Toxicodendron plant, but burning of brush containing Toxicodendron can cause airborne exposure when urushiol oil is carried by smoke particles.12 Sensitization to Toxicodendron can cause ACD to other Anacardiaceae species such as the Japanese lacquer tree (Toxicodendron vernicifluum), mango tree (Mangifera indica), cashew tree (Anacardium occidentale), and Indian marking nut tree (Semecarpus anacardium).6 Cross-reactions to components of the ginkgo tree (Ginkgo biloba) also are possible.
Toxicodendron plants can be more easily identified and avoided with knowledge of their characteristic leaf patterns. The most dependable way to identify poison ivy and poison oak species is to look for plants with 3 leaves, giving rise to the common saying, “Leaves of three, leave them be.” Poison sumac plants have groups of 7 to 13 leaves arranged as pairs along a central rib. Another helpful finding is a black deposit that Toxicodendron species leave behind following trauma to the leaves. Urushiol oxidizes when exposed to air and turns into a black deposit that can be seen on damaged leaves themselves or can be demonstrated in a black spot test to verify if a plant is a Toxicodendron species. The test is performed by gathering (carefully, without direct contact) a few leaves in a paper towel and crushing them to release sap. Within minutes, the sap will turn black if the plant is indeed a Toxicodendron species.13Pruritic, edematous, erythematous papules, plaques, and eventual vesicles in a linear distribution are suspicious for Toxicodendron exposure. Although your pet will not develop Toxicodendron ACD, oleoresin-contaminated pets can transfer the oils to their owners after coming into contact with these plants. Toxicodendron dermatitis also can be acquired from oleoresin-contaminated fomites such as clothing and shoes worn in the garden or when hiking. Toxicodendron dermatitis can appear at different sites on the body at different times depending on the amount of oleoresin exposure as well as epidermal thickness. For example, the oleoresin can be transferred from the hands to body areas with a thinner stratum corneum (eg, genitalia) and cause subsequent dermatitis.1
Compositae Family
The Compositae family (also known as Asteraceae) is a large plant family with more than 20,000 species, including numerous weeds, wildflowers, and vegetables. The flowers, leaves, stems, and pollens of the Compositae family are coated by cyclic esters called sesquiterpene lactones. Mitchell and Dupuis14 showed that sesquiterpene lactones are the allergens responsible for ACD to various Compositae plants, including ragweed (Ambrosia), sneezeweed (Helenium), and chrysanthemums (Chrysanthemum). Common Compositae vegetables such as lettuce (Lactuca sativa) have been reported to cause ACD in chefs, grocery store produce handlers, gardeners, and even owners of lettuce-eating pet guinea pigs and turtles.15 Similarly, artichokes (Cynara scolymus) can cause ACD in gardeners.16 Exposure to Compositae species also has been implicated in photoallergic reactions, and studies have demonstrated that some patients with chronic actinic dermatitis also have positive patch test reactions to Compositae species and/or sesquiterpene lactones.17,18
In addition to direct contact with Compositae plants, airborne exposure to sesquiterpene lactones can cause ACD.14 The pattern of airborne contact dermatitis typically involves exposed areas such as the eyelids, central face, and/or neck. The beak sign also can be a clue to airborne contact dermatitis, which involves dermatitis of the face that spares the nasal tip and/or nasal ridge. It is thought that the beak sign may result from increased sebaceous gland concentration on the nose, which prevents penetration of allergens and irritants.19 Unlike photoallergic contact dermatitis, which also can involve the face, airborne ACD frequently involves photoprotected areas such as the submandibular chin and the upper lip. Davies and Kersey20 reported the case of a groundsman who was cutting grass with dandelions (Taraxacum officinale) and was found to have associated airborne ACD of the face, neck, and forearms due to Compositae allergy. In a different setting, the aromas of chamomile (Matricaria chamomilla) have been reported to cause airborne ACD in a tea drinker.21 Paulsen22 found that ingestion of chamomile tea can induce systemic ACD in sensitized individuals.
Alstroemeriaceae, Liliaceae, and Primulaceae
Florists are exposed to many plant species and have a high prevalence of ACD. Thiboutot et al23 found that 15 of 57 (26%) floral workers experienced hand dermatitis that cleared with time away from work. The Peruvian lily (Alstroemeria, Alstroemeriaceae family), which contains tuliposide A, was found to be the leading cause of sensitization.23 Tulips (Tulipa, Liliaceae family), as the flower name suggests, also contain tuliposide A, which along with mechanical irritation from the course tecta fibers on the bulbs lead to a dermatitis known as tulip fingers.24,25 Poison primrose (Primula obconica, Primulaceae family), cultivated for its highly colorful flowers, contains the contact allergen primin.6 A common clinical presentation of ACD for any of these culprit flowers is localized dermatitis of the thumb and index finger in a florist or gardener.
Plants That Cause Irritant Reactions
Cactuses
Although the long spines of the Cactaceae family of cactuses is a warning for passersby, it is the small and nearly invisible barbed hairs (glochids) that inflict a more dramatic cutaneous reaction. The prickly pear cactus (Opuntia species) is a good example of such a plant, as its glochids cause mechanical irritation but also can become embedded in the skin and result in subcutaneous granulomas known as sabra dermatitis.26
Stinging Nettle
The dermatologic term urticaria owes its namesake to the stinging nettle plant, which comes from the family Urticaceae. The stinging nettle has small hairs on its leaves, referred to as stinging trichomes, which have needlelike tips that pierce the skin and inject a mix of histamine, formic acid, and acetylcholine, causing a pruritic dermatitis that may last up to 12 hours.27 The plant is found worldwide and is a common weed in North America.
Phytophotodermatitis
Lemons and limes (Rutaceae family) are common culprits of phytophotodermatitis, often causing what is known as a margarita burn after outdoor consumption or preparation of this tasty citrus beverage.28 An accidental spray of lime juice on the skin while adding it to a beer, guacamole, salsa, or any other food or beverage also can cause phytophotodermatitis.29-31 Although the juice of lemons and limes contains psoralens, the rind can contain a 6- to 186-fold increased concentration.32 Psoralen is the photoactive agent in Rutaceae plants that intercalates in double-stranded DNA and promotes intrastrand cross-links when exposed to UVA light, which ultimately leads to dermatitis.9 Phytophotodermatitis commonly causes erythema, edema, and painful bullae on sun-exposed areas and classically heals with hyperpigmentation.
Pseudophytodermatitis can occur in grain farmers and harvesters who handle wheat and/or barley and incidentally come in contact with insects and chemicals on the plant material. Pseudophytodermatitis from mites in the wheat and/or barley plant can occur at harvest time when contact with the plant material is high. Insects such as the North American itch mite (Pediculoides ventricosus) can cause petechiae, wheals, and pustules. In addition, insecticides such as malathion and arsenical sprays that are applied to plant leaves can cause pseudophytodermatitis, which may be initially diagnosed as dermatitis to the plant itself.6
Patch Testing to Plants
When a patient presents with recurrent or persistent dermatitis and a plant contact allergen is suspected, patch testing is indicated. Most comprehensive patch test series contain various plant allergens, such as sesquiterpene lactones, Compositae mix, and limonene hydroperoxides, and patch testing to a specialized plant series may be necessary. Poison ivy/oak/sumac allergens typically are not included in patch test series because of the high prevalence of allergic reactions to these chemicals and the likelihood of sensitization when patch testing with urushiol. Compositae contact sensitization can be difficult to diagnose because neither sesquiterpene lactone mix 0.1% nor parthenolide 0.1% are sensitive enough to pick up all Compositae allergies.33,34 Paulsen and Andersen34 proposed that if Compositae sensitization is suspected, testing should include sesquiterpene lactone, parthenolide, and Compositae mix II 2.5%, as well as other potential Compositae allergens based on the patient’s history.34
Because plants can have geographic variability and contain potentially unknown allergens,35 testing to plant components may increase the diagnostic yield of patch testing. Dividing the plant into component parts (ie, stem, bulb, leaf, flower) is helpful, as different components have different allergen concentrations. It is important to consult expert resources before proceeding with plant component patch testing because irritant reactions are frequent and may confound the testing.36
Prevention and Treatment
For all plant dermatoses, the mainstay of prevention is to avoid contact with the offending plant material. Gloves can be an important protective tool for plant dermatitis prevention; the correct material depends on the plant species being handled. Rubber gloves should not be worn to protect against Toxicodendron plants since the catechols in urushiol are soluble in rubber; vinyl gloves should be worn instead.6 Marks37 found that tuliposide A, the allergen in the Peruvian lily (Alstroemeria), penetrates both vinyl and latex gloves; it does not penetrate nitrile gloves. If exposed, the risk of dermatitis can be decreased if the allergen is washed away with soap and water as soon as possible. Some allergens such as Toxicodendron are absorbed quickly and need to be washed off within 10 minutes of exposure.6 Importantly, exposed gardening gloves may continue to perpetuate ACD if the allergen is not also washed off the gloves themselves.
For light-mediated dermatoses, sun avoidance or use of an effective sunscreen can reduce symptoms in an individual who has already been exposed.10 UVA light activates psoralen-mediated dermatitis but not until 30 to 120 minutes after absorption into the skin.38
Barrier creams are thought to be protective against plant ACD through a variety of mechanisms. The cream itself is meant to reduce skin contact to an allergen or irritant. Additionally, barrier creams contain active ingredients such as silicone, hydrocarbons, and aluminum chlorohydrate, which are thought to trap or transform offending agents before contacting the skin. When contact with a Toxicodendron species is anticipated, Marks et al39 found that dermatitis was absent or significantly reduced when 144 patients were pretreated with quaternium-18 bentonite lotion 5% (P<.0001).
Although allergen avoidance and use of gloves and barrier creams are the mainstays of preventing plant dermatoses, treatment often is required to control postexposure symptoms. For all plant dermatoses, topical corticosteroids can be used to reduce inflammation and pruritus. In some cases, systemic steroids may be necessary. To prevent rebound of dermatitis, patients often require a 3-week or longer course of oral steroids to quell the reaction, particularly if the dermatitis is vigorous or an id reaction is present.40 Antihistamines and cold compresses also can provide symptomatic relief.
Final Interpretation
Plants can cause a variety of dermatoses. Although Toxicodendron plants are the most frequent cause of ACD, it is important to keep in mind that florists, gardeners, and farmers are exposed to a large variety of allergens, irritants, and phototoxic agents that cause dermatoses as well. Confirmation of plant-induced ACD involves patch testing against suspected species. Prevention involves use of appropriate barriers and avoidance of implicated plants. Treatment includes topical steroids, antihistamines, and prednisone.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128.
- Pariser D, Ceilley R, Lefkovits A, et al. Poison ivy, oak and sumac. Derm Insights. 2003;4:26-28.
- Wolff K, Johnson R. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 6th ed. McGraw Hill Education; 2009.
- Zomorodi N, Butt M, Maczuga S, et al. Cost and diagnostic characteristics of Toxicodendron dermatitis in the USA: a retrospective cross-sectional analysis. Br J Dermatol. 2020;183:772-773.
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123.
- Fowler JF, Zirwas MJ. Fisher’s Contact Dermatitis. 7th ed. Contact Dermatitis Institute; 2019.
- Smith HR, Basketter DA, McFadden JP. Irritant dermatitis, irritancy and its role in allergic contact dermatitis. Clin Exp Dermatol. 2002;27:138-146.
- Wakelin SH. Contact urticaria. Clin Exp Dermatol. 2001;26:132-136.
- Ellis CR, Elston DM. Psoralen-induced phytophotodermatitis. Dermatitis. 2021;32:140-143.
- Deleo VA. Photocontact dermatitis. Dermatol Ther. 2004;17:279-288.
- National Institute for Occupational Safety and Health. Poisonous plants. Centers for Disease Control and Prevention website. Updated June 1, 2018. Accessed August 10, 2021. https://www.cdc.gov/niosh/topics/plants/geographic.html
- Schloemer JA, Zirwas MJ, Burkhart CG. Airborne contact dermatitis: common causes in the USA. Int J Dermatol. 2015;54:271-274.
- Guin JD. The black spot test for recognizing poison ivy and related species. J Am Acad Dermatol. 1980;2:332-333.
- Mitchell J, Dupuis G. Allergic contact dermatitis from sesquiterpenoids of the Compositae family of plants. Br J Dermatol. 1971;84:139-150.
- Paulsen E, Andersen KE. Lettuce contact allergy. Contact Dermatitis. 2016;74:67-75.
- Samaran Q, Clark E, Dereure O, et al. Airborne allergic contact dermatitis caused by artichoke. Contact Dermatitis. 2020;82:395-397.
- Du H, Ross JS, Norris PG, et al. Contact and photocontact sensitization in chronic actinic dermatitis: sesquiterpene lactone mix is an important allergen. Br J Dermatol. 1995;132:543-547.
- Wrangsjo K, Marie Ros A, Walhberg JE. Contact allergy to Compositae plants in patients with summer-exacerbated dermatitis. Contact Dermatitis. 1990;22:148-154.
- Staser K, Ezra N, Sheehan MP, et al. The beak sign: a clinical clue to airborne contact dermatitis. Dermatitis. 2014;25:97-98.
- Davies M, Kersey J. Contact allergy to yarrow and dandelion. Contact Dermatitis. 1986;14:256-257.
- Anzai A, Vázquez Herrera NE, Tosti A. Airborne allergic contact dermatitis caused by chamomile tea. Contact Dermatitis. 2015;72:254-255.
- Paulsen E. Systemic allergic dermatitis caused by sesquiterpene lactones. Contact Dermatitis. 2017;76:1-10.
- Thiboutot DM, Hamory BH, Marks JG. Dermatoses among floral shop workers. J Am Acad Dermatol. 1990;22:54-58.
- Hjorth N, Wilkinson DS. Contact dermatitis IV. tulip fingers, hyacinth itch and lily rash. Br J Dermatol. 1968;80:696-698.
- Guin JD, Franks H. Fingertip dermatitis in a retail florist. Cutis. 2001;67:328-330.
- Magro C, Lipner S. Sabra dermatitis: combined features of delayed hypersensitivity and foreign body reaction to implanted glochidia. Dermatol Online J. 2020;26:13030/qt2157f9g0.
- Cummings AJ, Olsen M. Mechanism of action of stinging nettles. Wilderness Environ Med. 2011;22:136-139.
- Maniam G, Light KML, Wilson J. Margarita burn: recognition and treatment of phytophotodermatitis. J Am Board Fam Med. 2021;34:398-401.
- Flugman SL. Mexican beer dermatitis: a unique variant of lime phytophotodermatitis attributable to contemporary beer-drinking practices. Arch Dermatol. 2010;146:1194-1195.
- Kung AC, Stephens MB, Darling T. Phytophotodermatitis: bulla formation and hyperpigmentation during spring break. Mil Med. 2009;174:657-661.
- Smith LG. Phytophotodermatitis. Images Emerg Med. 2017;1:146-147.
- Wagner AM, Wu JJ, Hansen RC, et al. Bullous phytophotodermatitis associated with high natural concentrations of furanocoumarins in limes. Am J Contact Dermat. 2002;13:10-14.
- Green C, Ferguson J. Sesquiterpene lactone mix is not an adequate screen for Compositae allergy. Contact Dermatitis. 1994;31:151-153.
- Paulsen E, Andersen KE. Screening for Compositae contact sensitization with sesquiterpene lactones and Compositae mix 2.5% pet. Contact Dermatitis. 2019;81:368-373.
- Paulsen E, Andersen KE. Patch testing with constituents of Compositae mixes. Contact Dermatitis. 2012;66:241-246.
- Frosch PJ, Geier J, Uter W, et al. Patch testing with the patients’ own products. Contact Dermatitis. 2011:929-941.
- Marks JG. Allergic contact dermatitis to Alstroemeria. Arch Dermatol. 1988;124:914-916.
- Moreau JF, English JC, Gehris RP. Phytophotodermatitis. J Pediatr Adolesc Gynecol. 2014;27:93-94.
- Marks JG, Fowler JF, Sherertz EF, et al. Prevention of poison ivy and poison oak allergic contact dermatitis by quaternium-18 bentonite. J Am Acad Dermatol. 1995;33:212-216.
- Craig K, Meadows SE. What is the best duration of steroid therapy for contact dermatitis (rhus)? J Fam Pract. 2006;55:166-167.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128.
- Pariser D, Ceilley R, Lefkovits A, et al. Poison ivy, oak and sumac. Derm Insights. 2003;4:26-28.
- Wolff K, Johnson R. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 6th ed. McGraw Hill Education; 2009.
- Zomorodi N, Butt M, Maczuga S, et al. Cost and diagnostic characteristics of Toxicodendron dermatitis in the USA: a retrospective cross-sectional analysis. Br J Dermatol. 2020;183:772-773.
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123.
- Fowler JF, Zirwas MJ. Fisher’s Contact Dermatitis. 7th ed. Contact Dermatitis Institute; 2019.
- Smith HR, Basketter DA, McFadden JP. Irritant dermatitis, irritancy and its role in allergic contact dermatitis. Clin Exp Dermatol. 2002;27:138-146.
- Wakelin SH. Contact urticaria. Clin Exp Dermatol. 2001;26:132-136.
- Ellis CR, Elston DM. Psoralen-induced phytophotodermatitis. Dermatitis. 2021;32:140-143.
- Deleo VA. Photocontact dermatitis. Dermatol Ther. 2004;17:279-288.
- National Institute for Occupational Safety and Health. Poisonous plants. Centers for Disease Control and Prevention website. Updated June 1, 2018. Accessed August 10, 2021. https://www.cdc.gov/niosh/topics/plants/geographic.html
- Schloemer JA, Zirwas MJ, Burkhart CG. Airborne contact dermatitis: common causes in the USA. Int J Dermatol. 2015;54:271-274.
- Guin JD. The black spot test for recognizing poison ivy and related species. J Am Acad Dermatol. 1980;2:332-333.
- Mitchell J, Dupuis G. Allergic contact dermatitis from sesquiterpenoids of the Compositae family of plants. Br J Dermatol. 1971;84:139-150.
- Paulsen E, Andersen KE. Lettuce contact allergy. Contact Dermatitis. 2016;74:67-75.
- Samaran Q, Clark E, Dereure O, et al. Airborne allergic contact dermatitis caused by artichoke. Contact Dermatitis. 2020;82:395-397.
- Du H, Ross JS, Norris PG, et al. Contact and photocontact sensitization in chronic actinic dermatitis: sesquiterpene lactone mix is an important allergen. Br J Dermatol. 1995;132:543-547.
- Wrangsjo K, Marie Ros A, Walhberg JE. Contact allergy to Compositae plants in patients with summer-exacerbated dermatitis. Contact Dermatitis. 1990;22:148-154.
- Staser K, Ezra N, Sheehan MP, et al. The beak sign: a clinical clue to airborne contact dermatitis. Dermatitis. 2014;25:97-98.
- Davies M, Kersey J. Contact allergy to yarrow and dandelion. Contact Dermatitis. 1986;14:256-257.
- Anzai A, Vázquez Herrera NE, Tosti A. Airborne allergic contact dermatitis caused by chamomile tea. Contact Dermatitis. 2015;72:254-255.
- Paulsen E. Systemic allergic dermatitis caused by sesquiterpene lactones. Contact Dermatitis. 2017;76:1-10.
- Thiboutot DM, Hamory BH, Marks JG. Dermatoses among floral shop workers. J Am Acad Dermatol. 1990;22:54-58.
- Hjorth N, Wilkinson DS. Contact dermatitis IV. tulip fingers, hyacinth itch and lily rash. Br J Dermatol. 1968;80:696-698.
- Guin JD, Franks H. Fingertip dermatitis in a retail florist. Cutis. 2001;67:328-330.
- Magro C, Lipner S. Sabra dermatitis: combined features of delayed hypersensitivity and foreign body reaction to implanted glochidia. Dermatol Online J. 2020;26:13030/qt2157f9g0.
- Cummings AJ, Olsen M. Mechanism of action of stinging nettles. Wilderness Environ Med. 2011;22:136-139.
- Maniam G, Light KML, Wilson J. Margarita burn: recognition and treatment of phytophotodermatitis. J Am Board Fam Med. 2021;34:398-401.
- Flugman SL. Mexican beer dermatitis: a unique variant of lime phytophotodermatitis attributable to contemporary beer-drinking practices. Arch Dermatol. 2010;146:1194-1195.
- Kung AC, Stephens MB, Darling T. Phytophotodermatitis: bulla formation and hyperpigmentation during spring break. Mil Med. 2009;174:657-661.
- Smith LG. Phytophotodermatitis. Images Emerg Med. 2017;1:146-147.
- Wagner AM, Wu JJ, Hansen RC, et al. Bullous phytophotodermatitis associated with high natural concentrations of furanocoumarins in limes. Am J Contact Dermat. 2002;13:10-14.
- Green C, Ferguson J. Sesquiterpene lactone mix is not an adequate screen for Compositae allergy. Contact Dermatitis. 1994;31:151-153.
- Paulsen E, Andersen KE. Screening for Compositae contact sensitization with sesquiterpene lactones and Compositae mix 2.5% pet. Contact Dermatitis. 2019;81:368-373.
- Paulsen E, Andersen KE. Patch testing with constituents of Compositae mixes. Contact Dermatitis. 2012;66:241-246.
- Frosch PJ, Geier J, Uter W, et al. Patch testing with the patients’ own products. Contact Dermatitis. 2011:929-941.
- Marks JG. Allergic contact dermatitis to Alstroemeria. Arch Dermatol. 1988;124:914-916.
- Moreau JF, English JC, Gehris RP. Phytophotodermatitis. J Pediatr Adolesc Gynecol. 2014;27:93-94.
- Marks JG, Fowler JF, Sherertz EF, et al. Prevention of poison ivy and poison oak allergic contact dermatitis by quaternium-18 bentonite. J Am Acad Dermatol. 1995;33:212-216.
- Craig K, Meadows SE. What is the best duration of steroid therapy for contact dermatitis (rhus)? J Fam Pract. 2006;55:166-167.
Practice Points
- Gardeners, florists, farmers, and outdoor enthusiasts are at risk for various plant dermatoses, which can be classified into 5 main categories: allergic contact dermatitis (ACD), mechanical irritant contact dermatitis, chemical irritant contact dermatitis, light-mediated dermatitis, and pseudophytodermatitis.
- Poison ivy, from the Toxicodendron genus, is the leading cause of plant ACD; however, a myriad of other plants also can cause dermatoses.
- Patch testing can be used to identify the source of immune-mediated type IV delayed hypersensitivity reactions to various plant species in individuals with recurrent or persistent dermatitis.
- Treatment options for all plant dermatoses can include topical steroids, antihistamines, and oral prednisone. Prevention involves avoidance or use of an effective barrier.
Aquatic Antagonists: Sea Cucumbers (Holothuroidea)
Sea cucumbers—commonly known as trepang in Indonesia, namako in Japan, and hai shen in China, where they are treasured as a food delicacy—are sea creatures belonging to the phylum Echinodermata, class Holothuridea, and family Cucumariidae . 1,2 They are an integral part of a variety of marine habitats, serving as cleaners as they filter through sediment for nutrients. They can be found on the ocean floor under hundreds of feet of water or in shallow sandy waters along the coast, but they most commonly are found living among coral reefs. Sea cucumbers look just as they sound—shaped like cucumbers or sausages, ranging from under 1 inch to upwards of 6 feet in length depending on the specific species (Figure 1). They have a group of tentacles around the mouth used for filtering sediment, and they move about the ocean floor on tubular feet protruding through the body wall, similar to a sea star.

Beneficial Properties and Cultural Relevance
Although more than 1200 species of sea cucumbers have been identified thus far, only about 20 of these are edible.2 The most common of the edible species is Stichopus japonicus, which can be found off the coasts of Korea, China, Japan, and Russia. This particular species most commonly is used in traditional dishes and is divided into 3 groups based on the color: red, green, or black. The price and taste of sea cucumbers varies based on the color, with red being the most expensive.2 The body wall of the sea cucumber is cleaned, repeatedly boiled, and dried until edible. It is considered a delicacy, not only in food but also in pharmaceutical forms, as it is comprised of a variety of vitamins, minerals, and other nutrients that are thought to provide anticancer, anticoagulant, antioxidant, antifungal, and anti-inflammatory properties. Components of the body wall include collagen, mucopolysaccharides, peptides, gelatin, glycosaminoglycans, glycosides (including various holotoxins), hydroxylates, saponins, and fatty acids.2 The regenerative properties of the sea cucumber also are important in future biomedical developments.
Toxic Properties
Although sea cucumbers have proven to have many beneficial properties, at least 30 species also produce potent toxins that pose a danger to both humans and other wildlife.3 The toxins are collectively referred to as holothurin; however, specific species actually produce a variety of holothurin toxins with unique chemical structures. Each toxin is a variation of a specific triterpene glycoside called saponins, which are common glycosides in the plant world. Holothurin was the first saponin to be found in animals. The only animals known to contain holothurin are the echinoderms, including sea cucumbers and sea stars.1 Holothurins A and B are the 2 groups of holothurin toxins produced specifically by sea cucumbers. The toxins are composed of roughly 60% glycosides and pigment; 30% free amino acids (alanine, arginine, cysteine, glycine, glutamic acid, histidine, serine, and valine); 5% to 10% insoluble proteins; and 1% cholesterol, salts, and polypeptides.3
Holothurins are concentrated in granules within specialized structures of the sea cucumber called Cuvierian tubules, which freely float in the posterior coelomic cavity of the sea cucumber and are attached at the base of the respiratory tree. It is with these tubules that sea cucumbers utilize a unique defensive mechanism. Upon disturbance, the sea cucumber will turn its posterior end to the threat and squeeze its body in a series of violent contractions, inducing a tear in the cloacal wall.4 The tubules pass through this tear, are autotomized from the attachment point at the respiratory tree, and are finally expelled through the anus onto the predator and into the surrounding waters. The tubules are both sticky on contact and poisonous due to the holothurin, allowing the sea cucumber to crawl away from the threat unscathed. Over time, the tubules will regenerate, allowing the sea cucumber to protect itself again in the face of future danger.
Aside from direct disturbance by a threat, sea cucumbers also are known to undergo evisceration due to high temperatures and oxygen deficiency.3 Species that lack Cuvierian tubules can still produce holothurin toxins, though the toxins are secreted onto the outer surface of the body wall and mainly pose a risk with direct contact undiluted by seawater.5 The toxin induces a neural blockade in other sea creatures through its interaction with ion channels. On Asian islands, sea cucumbers have been exploited for this ability and commonly are thrown into tidal pools by fishermen to paralyze fish for easier capture.1
Effects on Human Skin
In humans, the holothurin toxins of sea cucumbers cause an acute irritant dermatitis upon contact with the skin.6 Fishermen or divers handling sea cucumbers without gloves may present with an irritant contact dermatitis characterized by marked erythema and swelling (Figure 2).6-8 Additionally, holothurin toxins can cause irritation of the mucous membranes of the eyes and mouth. Contact with the mucous membranes of the eyes can induce a painful conjunctivitis that may result in blindness.6,8 Ingestion of large quantities of sea cucumber can produce an anticoagulant effect, and toxins in some species act similar to cardiac glycosides.3,9
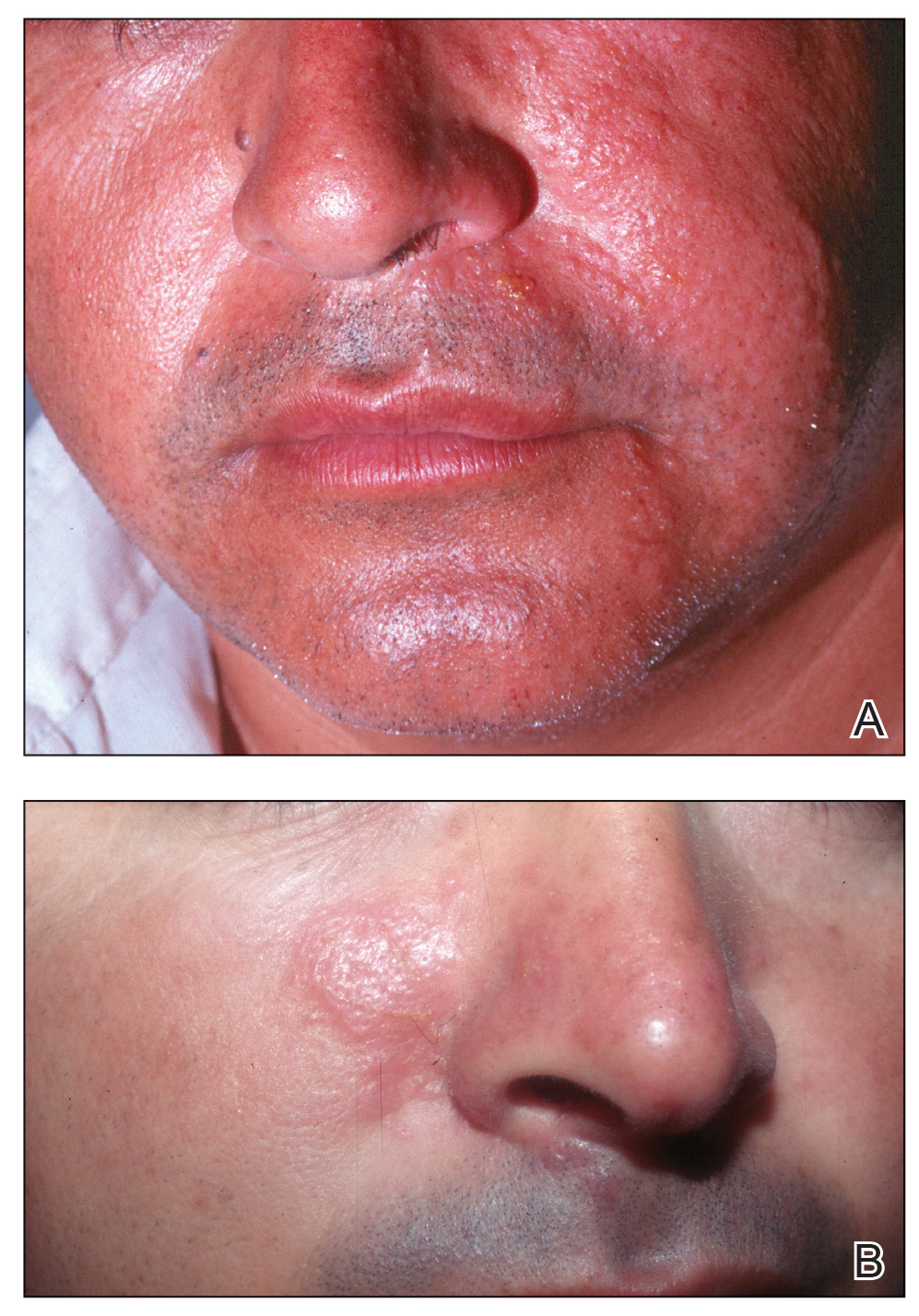
In addition to their own toxins, sea cucumbers also can secrete undigested nematocysts of previously consumed cnidarians through the integument.7,10 In this case, the result of direct contact with the body wall is similar to a jellyfish sting in addition to the irritant contact dermatitis caused by the holothurin toxin.
Treatment and Prevention
Irritant dermatitis resulting from contact with a holothurin toxin is first treated with cleansing of the affected area at the time of exposure with generous amounts of seawater or preferably hot seawater and soap. Most marine toxins are inactivated by heat, but holothurin is partially heat stable. Vinegar or isopropyl alcohol also have been used.9 The result is removal of the slime containing the holothurin toxin rather than deactivation of the toxin. Although this alone may relieve symptoms, dermatitis also may be addressed with topical anesthetics, corticosteroids, or, if a severe reaction has occurred, systemic steroids.9
Conjunctivitis should be addressed with copious irrigation with tap water and topical anesthesia. Following proper irrigation, providers may choose to follow up with fluorescein staining to rule out corneal injury.10
The dermatologic effects of holothurin toxins can be prevented with the use of gloves and diving masks or goggles. Proper protective wear should be utilized not only when directly handling sea cucumbers but also when swimming in water where sea cucumbers may be present. Systemic toxicity can be prevented by proper cooking, as holothurin toxins are only partially heat resistant and also are hydrolyzed into nontoxic products by gastric acid. Additionally, the species of the sea cucumber should be confirmed prior to consumption, as edible species are known to contain less toxin.1
Conclusion
Although sea cucumbers have ecologic, culinary, and pharmaceutical value, they also can pose a threat to both humans and wildlife. The holothurin toxins produced by sea cucumbers cause a painful contact dermatitis and can lead to conjunctivitis and even blindness following eye exposure. Although the toxin is broken down into nontoxic metabolites by gastric acid, large amounts of potent variants can induce systemic effects. Individuals who come in contact with sea cucumbers, such as fishermen and divers, should utilize proper protection including gloves and protective eyewear.
- Burnett K, Fenner P, Williamson J. Venomous and Poisonous Marine Animals: A Medical and Biological Handbook. University of New South Wales Press; 1996.
- Oh GW, Ko SC, Lee DH, et al. Biological activities and biomedical potential of sea cucumber (Stichopus japonicus): a review. Fisheries Aquatic Sci. 2017;20:28.
- Nigrelli RF, Jakowska S. Effects of holothurian, a steroid saponin from the Bahamian sea cucumber (Actinopyga agassizi), on various biological systems. Ann NY Acad Sci. 1960;90:884-892.
- Demeuldre M, Hennebert E, Bonneel M, et al. Mechanical adaptability of sea cucumber Cuvierian tubules involves a mutable collagenous tissue. J Exp Biol. 2017;220:2108-2119.
- Matranga V, ed. Echinodermata: Progress in Molecular and Subcellular Biology. Springer; 2005.
- Tlougan, BE, Podjasek, JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Bonamonte D, Verni P, Filoni A, et al. Dermatitis caused by echinoderms. In: Bonamonte D, Angelini G, eds. Springer; 2016:59-72.
- Haddad V Jr. Medical Emergencies Caused by Aquatic Animals: A Zoological and Clinical Guide. Springer International Publishing; 2016.
- French LK, Horowitz BZ. Marine vertebrates, cnidarians, and mollusks. In: Brent J, Burkhart K, Dargan P, et al, eds. Critical Care Toxicology. Springer; 2017:1-30.
- Smith ML. Skin problems from marine echinoderms. Dermatol Ther. 2002;15:30-33.
Sea cucumbers—commonly known as trepang in Indonesia, namako in Japan, and hai shen in China, where they are treasured as a food delicacy—are sea creatures belonging to the phylum Echinodermata, class Holothuridea, and family Cucumariidae . 1,2 They are an integral part of a variety of marine habitats, serving as cleaners as they filter through sediment for nutrients. They can be found on the ocean floor under hundreds of feet of water or in shallow sandy waters along the coast, but they most commonly are found living among coral reefs. Sea cucumbers look just as they sound—shaped like cucumbers or sausages, ranging from under 1 inch to upwards of 6 feet in length depending on the specific species (Figure 1). They have a group of tentacles around the mouth used for filtering sediment, and they move about the ocean floor on tubular feet protruding through the body wall, similar to a sea star.

Beneficial Properties and Cultural Relevance
Although more than 1200 species of sea cucumbers have been identified thus far, only about 20 of these are edible.2 The most common of the edible species is Stichopus japonicus, which can be found off the coasts of Korea, China, Japan, and Russia. This particular species most commonly is used in traditional dishes and is divided into 3 groups based on the color: red, green, or black. The price and taste of sea cucumbers varies based on the color, with red being the most expensive.2 The body wall of the sea cucumber is cleaned, repeatedly boiled, and dried until edible. It is considered a delicacy, not only in food but also in pharmaceutical forms, as it is comprised of a variety of vitamins, minerals, and other nutrients that are thought to provide anticancer, anticoagulant, antioxidant, antifungal, and anti-inflammatory properties. Components of the body wall include collagen, mucopolysaccharides, peptides, gelatin, glycosaminoglycans, glycosides (including various holotoxins), hydroxylates, saponins, and fatty acids.2 The regenerative properties of the sea cucumber also are important in future biomedical developments.
Toxic Properties
Although sea cucumbers have proven to have many beneficial properties, at least 30 species also produce potent toxins that pose a danger to both humans and other wildlife.3 The toxins are collectively referred to as holothurin; however, specific species actually produce a variety of holothurin toxins with unique chemical structures. Each toxin is a variation of a specific triterpene glycoside called saponins, which are common glycosides in the plant world. Holothurin was the first saponin to be found in animals. The only animals known to contain holothurin are the echinoderms, including sea cucumbers and sea stars.1 Holothurins A and B are the 2 groups of holothurin toxins produced specifically by sea cucumbers. The toxins are composed of roughly 60% glycosides and pigment; 30% free amino acids (alanine, arginine, cysteine, glycine, glutamic acid, histidine, serine, and valine); 5% to 10% insoluble proteins; and 1% cholesterol, salts, and polypeptides.3
Holothurins are concentrated in granules within specialized structures of the sea cucumber called Cuvierian tubules, which freely float in the posterior coelomic cavity of the sea cucumber and are attached at the base of the respiratory tree. It is with these tubules that sea cucumbers utilize a unique defensive mechanism. Upon disturbance, the sea cucumber will turn its posterior end to the threat and squeeze its body in a series of violent contractions, inducing a tear in the cloacal wall.4 The tubules pass through this tear, are autotomized from the attachment point at the respiratory tree, and are finally expelled through the anus onto the predator and into the surrounding waters. The tubules are both sticky on contact and poisonous due to the holothurin, allowing the sea cucumber to crawl away from the threat unscathed. Over time, the tubules will regenerate, allowing the sea cucumber to protect itself again in the face of future danger.
Aside from direct disturbance by a threat, sea cucumbers also are known to undergo evisceration due to high temperatures and oxygen deficiency.3 Species that lack Cuvierian tubules can still produce holothurin toxins, though the toxins are secreted onto the outer surface of the body wall and mainly pose a risk with direct contact undiluted by seawater.5 The toxin induces a neural blockade in other sea creatures through its interaction with ion channels. On Asian islands, sea cucumbers have been exploited for this ability and commonly are thrown into tidal pools by fishermen to paralyze fish for easier capture.1
Effects on Human Skin
In humans, the holothurin toxins of sea cucumbers cause an acute irritant dermatitis upon contact with the skin.6 Fishermen or divers handling sea cucumbers without gloves may present with an irritant contact dermatitis characterized by marked erythema and swelling (Figure 2).6-8 Additionally, holothurin toxins can cause irritation of the mucous membranes of the eyes and mouth. Contact with the mucous membranes of the eyes can induce a painful conjunctivitis that may result in blindness.6,8 Ingestion of large quantities of sea cucumber can produce an anticoagulant effect, and toxins in some species act similar to cardiac glycosides.3,9

In addition to their own toxins, sea cucumbers also can secrete undigested nematocysts of previously consumed cnidarians through the integument.7,10 In this case, the result of direct contact with the body wall is similar to a jellyfish sting in addition to the irritant contact dermatitis caused by the holothurin toxin.
Treatment and Prevention
Irritant dermatitis resulting from contact with a holothurin toxin is first treated with cleansing of the affected area at the time of exposure with generous amounts of seawater or preferably hot seawater and soap. Most marine toxins are inactivated by heat, but holothurin is partially heat stable. Vinegar or isopropyl alcohol also have been used.9 The result is removal of the slime containing the holothurin toxin rather than deactivation of the toxin. Although this alone may relieve symptoms, dermatitis also may be addressed with topical anesthetics, corticosteroids, or, if a severe reaction has occurred, systemic steroids.9
Conjunctivitis should be addressed with copious irrigation with tap water and topical anesthesia. Following proper irrigation, providers may choose to follow up with fluorescein staining to rule out corneal injury.10
The dermatologic effects of holothurin toxins can be prevented with the use of gloves and diving masks or goggles. Proper protective wear should be utilized not only when directly handling sea cucumbers but also when swimming in water where sea cucumbers may be present. Systemic toxicity can be prevented by proper cooking, as holothurin toxins are only partially heat resistant and also are hydrolyzed into nontoxic products by gastric acid. Additionally, the species of the sea cucumber should be confirmed prior to consumption, as edible species are known to contain less toxin.1
Conclusion
Although sea cucumbers have ecologic, culinary, and pharmaceutical value, they also can pose a threat to both humans and wildlife. The holothurin toxins produced by sea cucumbers cause a painful contact dermatitis and can lead to conjunctivitis and even blindness following eye exposure. Although the toxin is broken down into nontoxic metabolites by gastric acid, large amounts of potent variants can induce systemic effects. Individuals who come in contact with sea cucumbers, such as fishermen and divers, should utilize proper protection including gloves and protective eyewear.
Sea cucumbers—commonly known as trepang in Indonesia, namako in Japan, and hai shen in China, where they are treasured as a food delicacy—are sea creatures belonging to the phylum Echinodermata, class Holothuridea, and family Cucumariidae . 1,2 They are an integral part of a variety of marine habitats, serving as cleaners as they filter through sediment for nutrients. They can be found on the ocean floor under hundreds of feet of water or in shallow sandy waters along the coast, but they most commonly are found living among coral reefs. Sea cucumbers look just as they sound—shaped like cucumbers or sausages, ranging from under 1 inch to upwards of 6 feet in length depending on the specific species (Figure 1). They have a group of tentacles around the mouth used for filtering sediment, and they move about the ocean floor on tubular feet protruding through the body wall, similar to a sea star.

Beneficial Properties and Cultural Relevance
Although more than 1200 species of sea cucumbers have been identified thus far, only about 20 of these are edible.2 The most common of the edible species is Stichopus japonicus, which can be found off the coasts of Korea, China, Japan, and Russia. This particular species most commonly is used in traditional dishes and is divided into 3 groups based on the color: red, green, or black. The price and taste of sea cucumbers varies based on the color, with red being the most expensive.2 The body wall of the sea cucumber is cleaned, repeatedly boiled, and dried until edible. It is considered a delicacy, not only in food but also in pharmaceutical forms, as it is comprised of a variety of vitamins, minerals, and other nutrients that are thought to provide anticancer, anticoagulant, antioxidant, antifungal, and anti-inflammatory properties. Components of the body wall include collagen, mucopolysaccharides, peptides, gelatin, glycosaminoglycans, glycosides (including various holotoxins), hydroxylates, saponins, and fatty acids.2 The regenerative properties of the sea cucumber also are important in future biomedical developments.
Toxic Properties
Although sea cucumbers have proven to have many beneficial properties, at least 30 species also produce potent toxins that pose a danger to both humans and other wildlife.3 The toxins are collectively referred to as holothurin; however, specific species actually produce a variety of holothurin toxins with unique chemical structures. Each toxin is a variation of a specific triterpene glycoside called saponins, which are common glycosides in the plant world. Holothurin was the first saponin to be found in animals. The only animals known to contain holothurin are the echinoderms, including sea cucumbers and sea stars.1 Holothurins A and B are the 2 groups of holothurin toxins produced specifically by sea cucumbers. The toxins are composed of roughly 60% glycosides and pigment; 30% free amino acids (alanine, arginine, cysteine, glycine, glutamic acid, histidine, serine, and valine); 5% to 10% insoluble proteins; and 1% cholesterol, salts, and polypeptides.3
Holothurins are concentrated in granules within specialized structures of the sea cucumber called Cuvierian tubules, which freely float in the posterior coelomic cavity of the sea cucumber and are attached at the base of the respiratory tree. It is with these tubules that sea cucumbers utilize a unique defensive mechanism. Upon disturbance, the sea cucumber will turn its posterior end to the threat and squeeze its body in a series of violent contractions, inducing a tear in the cloacal wall.4 The tubules pass through this tear, are autotomized from the attachment point at the respiratory tree, and are finally expelled through the anus onto the predator and into the surrounding waters. The tubules are both sticky on contact and poisonous due to the holothurin, allowing the sea cucumber to crawl away from the threat unscathed. Over time, the tubules will regenerate, allowing the sea cucumber to protect itself again in the face of future danger.
Aside from direct disturbance by a threat, sea cucumbers also are known to undergo evisceration due to high temperatures and oxygen deficiency.3 Species that lack Cuvierian tubules can still produce holothurin toxins, though the toxins are secreted onto the outer surface of the body wall and mainly pose a risk with direct contact undiluted by seawater.5 The toxin induces a neural blockade in other sea creatures through its interaction with ion channels. On Asian islands, sea cucumbers have been exploited for this ability and commonly are thrown into tidal pools by fishermen to paralyze fish for easier capture.1
Effects on Human Skin
In humans, the holothurin toxins of sea cucumbers cause an acute irritant dermatitis upon contact with the skin.6 Fishermen or divers handling sea cucumbers without gloves may present with an irritant contact dermatitis characterized by marked erythema and swelling (Figure 2).6-8 Additionally, holothurin toxins can cause irritation of the mucous membranes of the eyes and mouth. Contact with the mucous membranes of the eyes can induce a painful conjunctivitis that may result in blindness.6,8 Ingestion of large quantities of sea cucumber can produce an anticoagulant effect, and toxins in some species act similar to cardiac glycosides.3,9

In addition to their own toxins, sea cucumbers also can secrete undigested nematocysts of previously consumed cnidarians through the integument.7,10 In this case, the result of direct contact with the body wall is similar to a jellyfish sting in addition to the irritant contact dermatitis caused by the holothurin toxin.
Treatment and Prevention
Irritant dermatitis resulting from contact with a holothurin toxin is first treated with cleansing of the affected area at the time of exposure with generous amounts of seawater or preferably hot seawater and soap. Most marine toxins are inactivated by heat, but holothurin is partially heat stable. Vinegar or isopropyl alcohol also have been used.9 The result is removal of the slime containing the holothurin toxin rather than deactivation of the toxin. Although this alone may relieve symptoms, dermatitis also may be addressed with topical anesthetics, corticosteroids, or, if a severe reaction has occurred, systemic steroids.9
Conjunctivitis should be addressed with copious irrigation with tap water and topical anesthesia. Following proper irrigation, providers may choose to follow up with fluorescein staining to rule out corneal injury.10
The dermatologic effects of holothurin toxins can be prevented with the use of gloves and diving masks or goggles. Proper protective wear should be utilized not only when directly handling sea cucumbers but also when swimming in water where sea cucumbers may be present. Systemic toxicity can be prevented by proper cooking, as holothurin toxins are only partially heat resistant and also are hydrolyzed into nontoxic products by gastric acid. Additionally, the species of the sea cucumber should be confirmed prior to consumption, as edible species are known to contain less toxin.1
Conclusion
Although sea cucumbers have ecologic, culinary, and pharmaceutical value, they also can pose a threat to both humans and wildlife. The holothurin toxins produced by sea cucumbers cause a painful contact dermatitis and can lead to conjunctivitis and even blindness following eye exposure. Although the toxin is broken down into nontoxic metabolites by gastric acid, large amounts of potent variants can induce systemic effects. Individuals who come in contact with sea cucumbers, such as fishermen and divers, should utilize proper protection including gloves and protective eyewear.
- Burnett K, Fenner P, Williamson J. Venomous and Poisonous Marine Animals: A Medical and Biological Handbook. University of New South Wales Press; 1996.
- Oh GW, Ko SC, Lee DH, et al. Biological activities and biomedical potential of sea cucumber (Stichopus japonicus): a review. Fisheries Aquatic Sci. 2017;20:28.
- Nigrelli RF, Jakowska S. Effects of holothurian, a steroid saponin from the Bahamian sea cucumber (Actinopyga agassizi), on various biological systems. Ann NY Acad Sci. 1960;90:884-892.
- Demeuldre M, Hennebert E, Bonneel M, et al. Mechanical adaptability of sea cucumber Cuvierian tubules involves a mutable collagenous tissue. J Exp Biol. 2017;220:2108-2119.
- Matranga V, ed. Echinodermata: Progress in Molecular and Subcellular Biology. Springer; 2005.
- Tlougan, BE, Podjasek, JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Bonamonte D, Verni P, Filoni A, et al. Dermatitis caused by echinoderms. In: Bonamonte D, Angelini G, eds. Springer; 2016:59-72.
- Haddad V Jr. Medical Emergencies Caused by Aquatic Animals: A Zoological and Clinical Guide. Springer International Publishing; 2016.
- French LK, Horowitz BZ. Marine vertebrates, cnidarians, and mollusks. In: Brent J, Burkhart K, Dargan P, et al, eds. Critical Care Toxicology. Springer; 2017:1-30.
- Smith ML. Skin problems from marine echinoderms. Dermatol Ther. 2002;15:30-33.
- Burnett K, Fenner P, Williamson J. Venomous and Poisonous Marine Animals: A Medical and Biological Handbook. University of New South Wales Press; 1996.
- Oh GW, Ko SC, Lee DH, et al. Biological activities and biomedical potential of sea cucumber (Stichopus japonicus): a review. Fisheries Aquatic Sci. 2017;20:28.
- Nigrelli RF, Jakowska S. Effects of holothurian, a steroid saponin from the Bahamian sea cucumber (Actinopyga agassizi), on various biological systems. Ann NY Acad Sci. 1960;90:884-892.
- Demeuldre M, Hennebert E, Bonneel M, et al. Mechanical adaptability of sea cucumber Cuvierian tubules involves a mutable collagenous tissue. J Exp Biol. 2017;220:2108-2119.
- Matranga V, ed. Echinodermata: Progress in Molecular and Subcellular Biology. Springer; 2005.
- Tlougan, BE, Podjasek, JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Bonamonte D, Verni P, Filoni A, et al. Dermatitis caused by echinoderms. In: Bonamonte D, Angelini G, eds. Springer; 2016:59-72.
- Haddad V Jr. Medical Emergencies Caused by Aquatic Animals: A Zoological and Clinical Guide. Springer International Publishing; 2016.
- French LK, Horowitz BZ. Marine vertebrates, cnidarians, and mollusks. In: Brent J, Burkhart K, Dargan P, et al, eds. Critical Care Toxicology. Springer; 2017:1-30.
- Smith ML. Skin problems from marine echinoderms. Dermatol Ther. 2002;15:30-33.
Practice Points
- Sea cucumbers produce a toxin known as holothurin, which is contained in specialized structures called Cuvierian tubules and secreted onto the outer surface of the body wall. Some species also eject portions of their toxic inner organs through the anus as a defensive mechanism.
- In humans, the holothurin toxins cause an acute irritant dermatitis upon contact with the skin and a painful chemical conjunctivitis upon contact with the eyes.
- In addition to their own toxin, sea cucumbers also can secrete undigested nematocysts of previously consumed cnidarians through their integument, causing additional effects on human skin.
- The dermatologic effects of sea cucumbers can be prevented with the use of gloves and swim masks or goggles.
Patch Test–Directed Dietary Avoidance in the Management of Irritable Bowel Syndrome
Irritable bowel syndrome (IBS) is one of the most common disorders managed by primary care physicians and gastroenterologists.1 Characterized by abdominal pain coinciding with altered stool form and/or frequency as defined by the Rome IV diagnostic criteria,2 symptoms range from mild to debilitating and may remarkably impair quality of life and work productivity.1
The cause of IBS is poorly understood. Proposed pathophysiologic factors include impaired mucosal function, microbial imbalance, visceral hypersensitivity, psychologic dysfunction, genetic factors, neurotransmitter imbalance, postinfectious gastroenteritis, inflammation, and food intolerance, any or all of which may lead to the development and maintenance of IBS symptoms.3 More recent observations of inflammation in the intestinal lining4,5 and proinflammatory peripherally circulating cytokines6 challenge its traditional classification as a functional disorder.
The cause of this inflammation is of intense interest, with speculation that the bacterial microbiota, bile acids, association with postinfectious gastroenteritis and inflammatory bowel disease cases, and/or foods may contribute. Although approximately 50% of individuals with IBS report that foods aggravate their symptoms,7 studies investigating type I antibody–mediated immediate hypersensitivity have largely failed to demonstrate a substantial link, prompting many authorities to regard these associations as food “intolerances” rather than true allergies. Based on this body of literature, a large 2010 consensus report on all aspects of food allergies advises against food allergy testing for IBS.8
In contrast, by utilizing type IV food allergen skin patch testing, 2 proof-of-concept studies9,10 investigated a different allergic mechanism in IBS, namely cell-mediated delayed-type hypersensitivity. Because many foods and food additives are known to cause allergic contact dermatitis,11 it was hypothesized that these foods may elicit a similar delayed-type hypersensitivity response in the intestinal lining in previously sensitized individuals. By following a patch test–guided food avoidance diet, a large subpopulation of patients with IBS experienced partial or complete IBS symptom relief.9,10 Our study further investigates a role for food-related delayed-type hypersensitivities in the pathogenesis of IBS.
Methods
Patient Selection
This study was conducted in a secondary care community-based setting. All patients were self-referred over an 18-month period ending in October 2019, had physician-diagnosed IBS, and/or met the Rome IV criteria for IBS and presented expressly for the food patch testing on a fee-for-service basis. Subtype of IBS was determined on presentation by the self-reported historically predominant symptom. Duration of IBS symptoms was self-reported and was rounded to the nearest year for purposes of data collection.
Exclusion criteria included pregnancy, known allergy to adhesive tape or any of the food allergens used in the study, severe skin rash, symptoms that had a known cause other than IBS, or active treatment with systemic immunosuppressive medications.
Patch Testing
Skin patch testing was initiated using an extensive panel of 117 type IV food allergens (eTable)11 identified in the literature,12 most of which utilized standard compounded formulations13 or were available from reputable patch test manufacturers (Brial Allergen GmbH; Chemotechnique Diagnostics). This panel was not approved by the US Food and Drug Administration. The freeze-dried vegetable formulations were taken from the 2018 report.9 Standard skin patch test procedure protocols12 were used, affixing the patches to the upper aspect of the back.
Following patch test application on day 1, two follow-up visits occurred on day 3 and either day 4 or day 5. On day 3, patches were removed, and the initial results were read by a board-certified dermatologist according to a standard grading system.14 Interpretation of patch tests included no reaction, questionable reaction consisting of macular erythema, weak reaction consisting of erythema and slight edema, or strong reaction consisting of erythema and marked edema. On day 4 or day 5, the final patch test reading was performed, and patients were informed of their results. Patients were advised to avoid ingestion of all foods that elicited a questionable or positive patch test response for at least 3 months, and information about the foods and their avoidance also was distributed and reviewed.
Food Avoidance Questionnaire
Patients with questionable or positive patch tests at 72 or 96 hours were advised of their eligibility to participate in an institutional review board–approved food avoidance questionnaire study investigating the utility of patch test–guided food avoidance on IBS symptoms. The questionnaire assessed the following: (1) baseline average abdominal pain prior to patch test–guided avoidance diet (0=no symptoms; 10=very severe); (2) average abdominal pain since initiation of patch test–guided avoidance diet (0=no symptoms; 10=very severe); (3) degree of improvement in overall IBS symptoms by the end of the food avoidance period (0=no improvement; 10=great improvement); (4) compliance with the avoidance diet for the duration of the avoidance period (completely, partially, not at all, or not sure).
Questionnaires and informed consent were mailed to patients via the US Postal Service 3 months after completing the patch testing. The questionnaire and consent were to be completed and returned after dietary avoidance of the identified allergens for at least 3 months. Patients were not compensated for participation in the study.
Statistical Analysis
Statistical analysis of data collected from study questionnaires was performed with Microsoft Excel. Mean abdominal pain and mean global improvement scores were reported along with 1 SD of the mean. For comparison of mean abdominal pain and improvement in global IBS symptoms from baseline to after 3 months of identified allergen avoidance, a Mann-Whitney U test was performed, with P<.05 being considered statistically significant.
Results
Thirty-seven consecutive patients underwent the testing and were eligible for the study. Nineteen patients were included in the study by virtue of completing and returning their posttest food avoidance questionnaire and informed consent. Eighteen patients were White and 1 was Asian. Subcategories of IBS were diarrhea predominant (9 [47.4%]), constipation predominant (3 [15.8%]), mixed type (5 [26.3%]), and undetermined type (2 [10.5%]). Questionnaire answers were reported after a mean (SD) duration of patch test–directed food avoidance of 4.5 (3.0) months (Table 1).
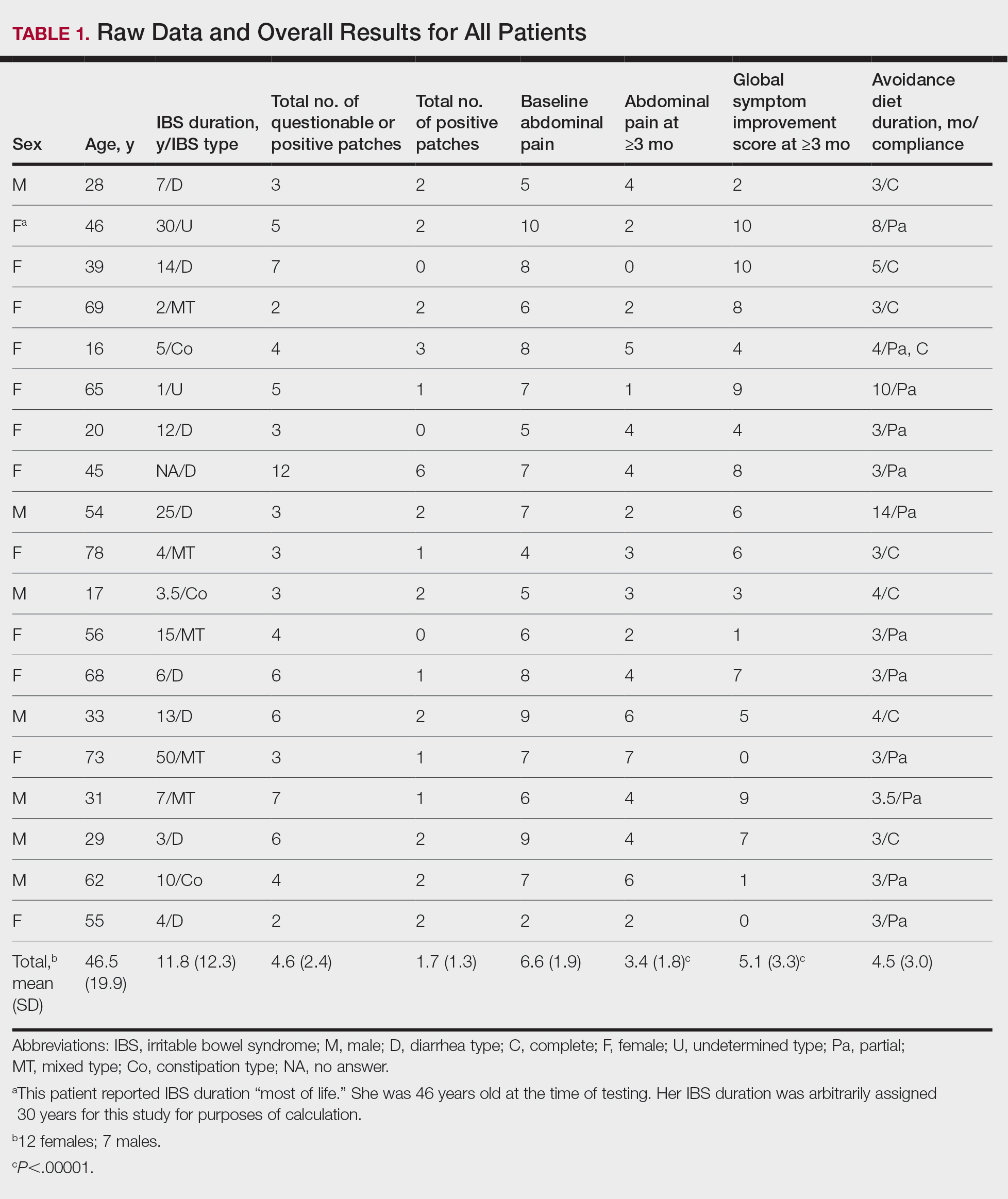
Overall Improvement
Fifteen (78.9%) patients reported at least slight to great improvement in their global IBS symptoms, and 4 (21.1%) reported no improvement (Table 2), with a mean (SD) improvement score of 5.1 (3.3)(P<.00001).
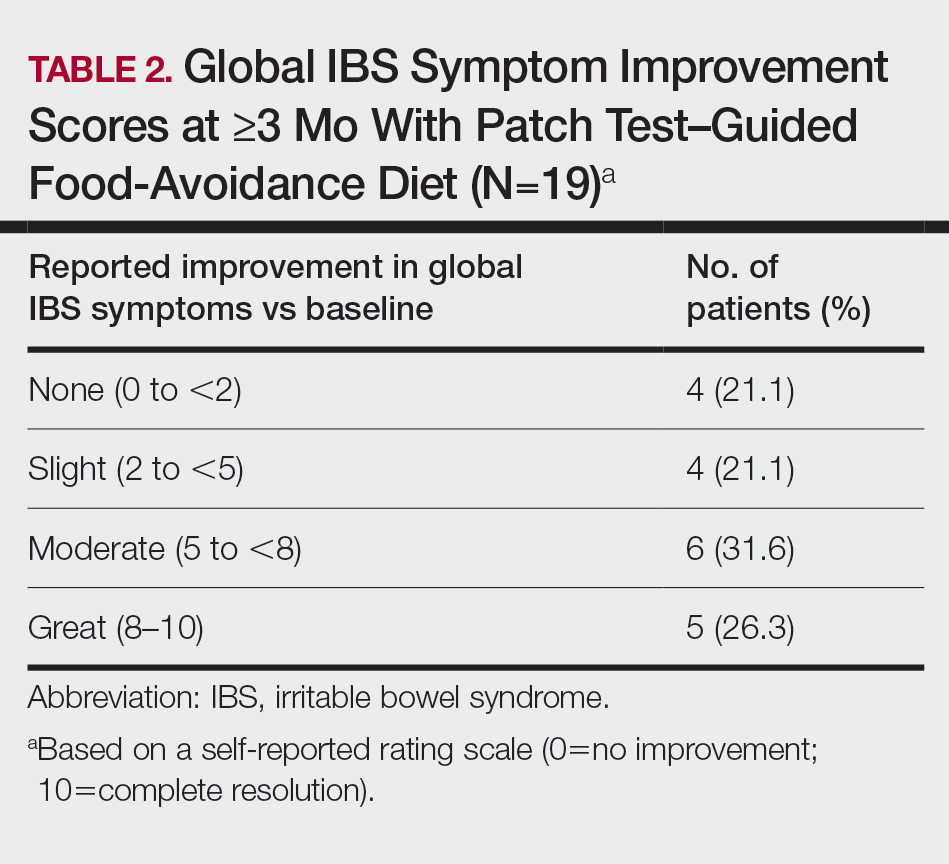
Abdominal Pain
All 19 patients reported mild to marked abdominal pain at baseline. The mean (SD) baseline pain score was 6.6 (1.9). The mean (SD) pain score was 3.4 (1.8)(P<.00001) after an average patch test–guided dietary avoidance of 4.5 (3.0) months (Table 3).
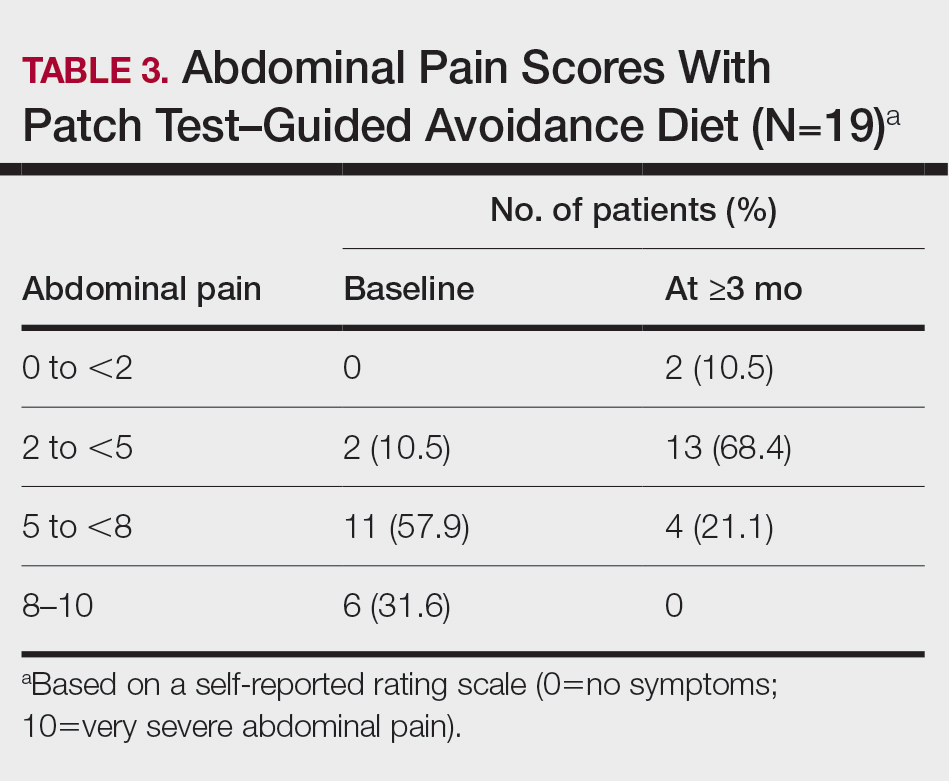
Comment
Despite intense research interest and a growing number of new medications for IBS approved by the US Food and Drug Administration, there remains a large void in the search for cost-effective and efficacious approaches for IBS evaluation and treatment. In addition to major disturbances in quality of life,14,15 the cost to society in direct medical expenses and indirect costs associated with loss of productivity and work absenteeism is considerable; estimates range from $21 billion or more annually.16
Food Hypersensitivities Triggering IBS
This study further evaluated a role for skin patch testing to identify delayed-type (type IV) food hypersensitivities that trigger IBS symptoms and differed from the prior investigations9,10 in that the symptoms used to define IBS were updated from the Rome III17 to the newer Rome IV2 criteria. The data presented here show moderate to great improvement in global IBS symptoms in 58% (11/19) of patients, which is in line with a 2018 report of 40 study participants for whom follow-up at 3 or more months was available,9 providing additional support for a role for type IV food allergies in causing the same gastrointestinal tract symptoms that define IBS. The distinction between food-related studies, including this one, that implicate food allergies9,10 and prior studies that did not support a role for food allergies in IBS pathogenesis8 can be accounted for by the type of allergy investigated. Conclusions that IBS flares after food ingestion were attributable to intolerance rather than true allergy were based on results investigating only the humoral arm and failed to consider the cell-mediated arm of the immune system. As such, foods that appear to trigger IBS symptoms on an allergic basis in our study are recognized in the literature12 as type IV allergens that elicit cell-mediated immunologic responses rather than more widely recognized type I allergens, such as peanuts and shellfish, that elicit immediate-type hypersensitivity responses. Although any type IV food allergen(s) could be responsible, a pattern emerged in this study and the study published in 2018.9 Namely, some foods stood out as more frequently inducing patch test reactions, with the 3 most common being carmine, cinnamon bark oil, and sodium bisulfite (eTable). The sample size is relatively small, but the results raise the question of whether these foods are the most likely to trigger IBS symptoms in the general population. If so, is it the result of a higher innate sensitizing potential and/or a higher frequency of exposure in commonly eaten foods? Larger randomized clinical trials are needed.
Immune Response and IBS
There is mounting evidence that the immune system may play a role in the pathophysiology of IBS.18 Both lymphocyte infiltration of the myenteric plexus and an increase in intestinal mucosal T lymphocytes have been observed, and it is generally accepted that the mucosal immune system seems to be activated, at least in a subset of patients with IBS.19 Irritable bowel syndrome associations with quiescent inflammatory bowel disease or postinfectious gastroenteritis provide 2 potential causes for the inflammation, but most IBS patients have had neither.20 The mucosal lining of the intestine and immune system have vast exposure to intraluminal allergens in transit, and it is hypothesized that the same delayed-type hypersensitivity response elicited in the skin by patch testing is elicited in the intestine, resulting in the inflammation that triggers IBS symptoms.10 The results here add to the growing body of evidence that ingestion of type IV food allergens by previously sensitized individuals could, in fact, be the primary source of the inflammation observed in a large subpopulation of individuals who carry a diagnosis of IBS.
Food Allergens in Patch Testing
Many of the food allergens used in this study are commonly found in various nonfood products that may contact the skin. For example, many flavorings are used as fragrances, and many preservatives, binders, thickeners, emulsifiers, and stabilizers serve the same role in moisturizers, cosmetics, and topical medications. Likewise, nickel sulfate hexahydrate, ubiquitous in foods that arise from the earth, often is found in metal in jewelry, clothing components, and cell phones. All are potential sensitizers. Thus, the question may arise whether the causal relationship between the food allergens identified by patch testing and IBS symptoms might be more of a systemic effect akin to systemic contact dermatitis as sometimes follows ingestion of an allergen to which an individual has been topically sensitized, rather than the proposed localized immunologic response in the intestinal lining. We were unaware of patient history of allergic contact dermatitis to any of the patch test allergens in this study, but the dermatologist author here (M.S.) has unpublished experience with 2 other patients with IBS who have benefited from low-nickel diets after having had positive patch tests to nickel sulfate hexahydrate and who, in retrospect, did report a history of earring dermatitis. Future investigations using pre– and post–food challenge histologic assessments of the intestinal mucosa in patients who benefit from patch test–guided food avoidance diets should help to better define the mechanism.
Because IBS has not been traditionally associated with structural or biochemical abnormalities detectable with current routine diagnostic tools, it has long been viewed as a functional disorder. The findings published more recently,9,10 in addition to this study’s results, would negate this functional classification in the subset of patients with IBS symptoms who experience sustained relief of their symptoms by patch test–directed food avoidance. The underlying delayed-type hypersensitivity pathogenesis of the IBS-like symptoms in these individuals would mandate an organic classification, aptly named allergic contact enteritis.10
Follow-up Data
The mean (SD) follow-up duration for this study and the 2018 report9 was 4.5 (3.0) months and 7.6 (3.9) months, respectively. The placebo effect is a concern for disorders such as IBS in which primarily subjective outcome measures are available,21 and in a retrospective analysis of 25 randomized, placebo-controlled IBS clinical trials, Spiller22 concluded the optimum length of such trials to be more than 3 months, which these studies exceed. Although not blinded or placebo controlled, the length of follow-up in the 2018 report9 and here enhances the validity of the results.
Limitation
The retrospective manner in which the self-assessments were reported in this study introduces the potential for recall bias, a variable that could affect results. The presence and direction of bias by any given individual cannot be known, making it difficult to determine any effect it may have had. Further investigation should include daily assessments and refine the primary study end points to include both abdominal pain and the defecation considerations that define IBS.
Conclusion
Food patch testing has the potential to offer a safe, cost-effective approach to the evaluation and management of IBS symptoms. Randomized clinical trials are needed to further investigate the validity of the proof-of-concept results to date. For patients who benefit from a patch test–guided avoidance diet, invasive and costly endoscopic, radiologic, and laboratory testing and pharmacologic management could be averted. Symptomatic relief could be attained simply by avoiding the implicated foods, essentially doing more by doing less.
- Enck P, Aziz Q, Barbara G, et al. Irritable bowel syndrome. Nat Rev Dis Primers. 2016;2:1-24.
- Lacy BE, Patel NK. Rome criteria and a diagnostic approach to irritable bowel syndrome. J Clin Med. 2017;6:99.
- Barbara G, De Giorgio R, Stanghellini V, et al. New pathophysiological mechanisms in irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20(suppl 2):1-9
- Chadwick VS, Chen W, Shu D, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology 2002;122:1778-1783.
- Tornblom H, Lindberg G, Nyberg B, et al. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology. 2002;123:1972-1979.
- O’Mahony L, McCarthy J, Kelly
P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541-551. - Ragnarsson G, Bodemar G. Pain is temporally related to eating but not to defecation in the irritable bowel syndrome (IBS): patients’ description of diarrhea, constipation and symptom variation during a prospective 6-week study. Eur J Gastroenterol Hepatol. 1998;10:415-421.
- Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 suppl):S1-S58.
- Shin GH, Smith MS, Toro B, et al. Utility of food patch testing in the evaluation and management of irritable bowel syndrome. Skin. 2018;2:1-15.
- Stierstorfer MB, Sha CT. Food patch testing for irritable bowel syndrome. J Am Acad Dermatol. 2013;68:377-384.
- Marks JG, Belsito DV, DeLeo MD, et al. North American Contact Dermatitis Group patch test results for the detection of delayed-type hypersensitivity to topical allergens. J Am Acad Dermatol. 1998;38:911-918.
- Rietschel RL, Fowler JF Jr. Fisher’s Contact Dermatitis. BC Decker; 2008.
- DeGroot AC. Patch Testing. acdegroot Publishing; 2008.
- Gralnek IM, Hays RD, Kilbourne A, et al. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology. 2000;119:654-660.
- Halder SL, Lock GR, Talley NJ, et al. Impact of functional gastrointestinal disorders on health-related quality of life: a population-based case–control study. Aliment Pharmacol Ther. 2004;19:233-242.
- International Foundation for Gastrointestinal Disorders. About IBS. statistics. Accessed July 20, 2021. https://www.aboutibs.org/facts-about-ibs/statistics.html
- Rome Foundation. Guidelines—Rome III diagnostic criteria for functional gastrointestinal disorders. J Gastrointestin Liver Dis. 2006;15:307-312.
- Collins SM. Is the irritable gut an inflamed gut? Scand J Gastroenterol. 1992;192(suppl):102-105.
- Park MI, Camilleri M. Is there a role of food allergy in irritable bowel syndrome and functional dyspepsia? a systemic review. Neurogastroenterol Motil. 2006;18:595-607.
- Grover M, Herfarth H, Drossman DA. The functional-organic dichotomy: postinfectious irritable bowel syndrome and inflammatory bowel disease–irritable bowel syndrome. Clin Gastroenterol Hepatol. 2009;7:48-53.
- Hrobiartsson A, Gotzsche PC. Is the placebo powerless? an analysis of clinical trials comparing placebo with no treatment. N Engl J Med. 2001;344:1594-1602.
- Spiller RC. Problems and challenges in the design of irritable bowel syndrome clinical trials: experience from published trials. Am J Med. 1999;107:91S-97S.
Irritable bowel syndrome (IBS) is one of the most common disorders managed by primary care physicians and gastroenterologists.1 Characterized by abdominal pain coinciding with altered stool form and/or frequency as defined by the Rome IV diagnostic criteria,2 symptoms range from mild to debilitating and may remarkably impair quality of life and work productivity.1
The cause of IBS is poorly understood. Proposed pathophysiologic factors include impaired mucosal function, microbial imbalance, visceral hypersensitivity, psychologic dysfunction, genetic factors, neurotransmitter imbalance, postinfectious gastroenteritis, inflammation, and food intolerance, any or all of which may lead to the development and maintenance of IBS symptoms.3 More recent observations of inflammation in the intestinal lining4,5 and proinflammatory peripherally circulating cytokines6 challenge its traditional classification as a functional disorder.
The cause of this inflammation is of intense interest, with speculation that the bacterial microbiota, bile acids, association with postinfectious gastroenteritis and inflammatory bowel disease cases, and/or foods may contribute. Although approximately 50% of individuals with IBS report that foods aggravate their symptoms,7 studies investigating type I antibody–mediated immediate hypersensitivity have largely failed to demonstrate a substantial link, prompting many authorities to regard these associations as food “intolerances” rather than true allergies. Based on this body of literature, a large 2010 consensus report on all aspects of food allergies advises against food allergy testing for IBS.8
In contrast, by utilizing type IV food allergen skin patch testing, 2 proof-of-concept studies9,10 investigated a different allergic mechanism in IBS, namely cell-mediated delayed-type hypersensitivity. Because many foods and food additives are known to cause allergic contact dermatitis,11 it was hypothesized that these foods may elicit a similar delayed-type hypersensitivity response in the intestinal lining in previously sensitized individuals. By following a patch test–guided food avoidance diet, a large subpopulation of patients with IBS experienced partial or complete IBS symptom relief.9,10 Our study further investigates a role for food-related delayed-type hypersensitivities in the pathogenesis of IBS.
Methods
Patient Selection
This study was conducted in a secondary care community-based setting. All patients were self-referred over an 18-month period ending in October 2019, had physician-diagnosed IBS, and/or met the Rome IV criteria for IBS and presented expressly for the food patch testing on a fee-for-service basis. Subtype of IBS was determined on presentation by the self-reported historically predominant symptom. Duration of IBS symptoms was self-reported and was rounded to the nearest year for purposes of data collection.
Exclusion criteria included pregnancy, known allergy to adhesive tape or any of the food allergens used in the study, severe skin rash, symptoms that had a known cause other than IBS, or active treatment with systemic immunosuppressive medications.
Patch Testing
Skin patch testing was initiated using an extensive panel of 117 type IV food allergens (eTable)11 identified in the literature,12 most of which utilized standard compounded formulations13 or were available from reputable patch test manufacturers (Brial Allergen GmbH; Chemotechnique Diagnostics). This panel was not approved by the US Food and Drug Administration. The freeze-dried vegetable formulations were taken from the 2018 report.9 Standard skin patch test procedure protocols12 were used, affixing the patches to the upper aspect of the back.
Following patch test application on day 1, two follow-up visits occurred on day 3 and either day 4 or day 5. On day 3, patches were removed, and the initial results were read by a board-certified dermatologist according to a standard grading system.14 Interpretation of patch tests included no reaction, questionable reaction consisting of macular erythema, weak reaction consisting of erythema and slight edema, or strong reaction consisting of erythema and marked edema. On day 4 or day 5, the final patch test reading was performed, and patients were informed of their results. Patients were advised to avoid ingestion of all foods that elicited a questionable or positive patch test response for at least 3 months, and information about the foods and their avoidance also was distributed and reviewed.
Food Avoidance Questionnaire
Patients with questionable or positive patch tests at 72 or 96 hours were advised of their eligibility to participate in an institutional review board–approved food avoidance questionnaire study investigating the utility of patch test–guided food avoidance on IBS symptoms. The questionnaire assessed the following: (1) baseline average abdominal pain prior to patch test–guided avoidance diet (0=no symptoms; 10=very severe); (2) average abdominal pain since initiation of patch test–guided avoidance diet (0=no symptoms; 10=very severe); (3) degree of improvement in overall IBS symptoms by the end of the food avoidance period (0=no improvement; 10=great improvement); (4) compliance with the avoidance diet for the duration of the avoidance period (completely, partially, not at all, or not sure).
Questionnaires and informed consent were mailed to patients via the US Postal Service 3 months after completing the patch testing. The questionnaire and consent were to be completed and returned after dietary avoidance of the identified allergens for at least 3 months. Patients were not compensated for participation in the study.
Statistical Analysis
Statistical analysis of data collected from study questionnaires was performed with Microsoft Excel. Mean abdominal pain and mean global improvement scores were reported along with 1 SD of the mean. For comparison of mean abdominal pain and improvement in global IBS symptoms from baseline to after 3 months of identified allergen avoidance, a Mann-Whitney U test was performed, with P<.05 being considered statistically significant.
Results
Thirty-seven consecutive patients underwent the testing and were eligible for the study. Nineteen patients were included in the study by virtue of completing and returning their posttest food avoidance questionnaire and informed consent. Eighteen patients were White and 1 was Asian. Subcategories of IBS were diarrhea predominant (9 [47.4%]), constipation predominant (3 [15.8%]), mixed type (5 [26.3%]), and undetermined type (2 [10.5%]). Questionnaire answers were reported after a mean (SD) duration of patch test–directed food avoidance of 4.5 (3.0) months (Table 1).

Overall Improvement
Fifteen (78.9%) patients reported at least slight to great improvement in their global IBS symptoms, and 4 (21.1%) reported no improvement (Table 2), with a mean (SD) improvement score of 5.1 (3.3)(P<.00001).

Abdominal Pain
All 19 patients reported mild to marked abdominal pain at baseline. The mean (SD) baseline pain score was 6.6 (1.9). The mean (SD) pain score was 3.4 (1.8)(P<.00001) after an average patch test–guided dietary avoidance of 4.5 (3.0) months (Table 3).

Comment
Despite intense research interest and a growing number of new medications for IBS approved by the US Food and Drug Administration, there remains a large void in the search for cost-effective and efficacious approaches for IBS evaluation and treatment. In addition to major disturbances in quality of life,14,15 the cost to society in direct medical expenses and indirect costs associated with loss of productivity and work absenteeism is considerable; estimates range from $21 billion or more annually.16
Food Hypersensitivities Triggering IBS
This study further evaluated a role for skin patch testing to identify delayed-type (type IV) food hypersensitivities that trigger IBS symptoms and differed from the prior investigations9,10 in that the symptoms used to define IBS were updated from the Rome III17 to the newer Rome IV2 criteria. The data presented here show moderate to great improvement in global IBS symptoms in 58% (11/19) of patients, which is in line with a 2018 report of 40 study participants for whom follow-up at 3 or more months was available,9 providing additional support for a role for type IV food allergies in causing the same gastrointestinal tract symptoms that define IBS. The distinction between food-related studies, including this one, that implicate food allergies9,10 and prior studies that did not support a role for food allergies in IBS pathogenesis8 can be accounted for by the type of allergy investigated. Conclusions that IBS flares after food ingestion were attributable to intolerance rather than true allergy were based on results investigating only the humoral arm and failed to consider the cell-mediated arm of the immune system. As such, foods that appear to trigger IBS symptoms on an allergic basis in our study are recognized in the literature12 as type IV allergens that elicit cell-mediated immunologic responses rather than more widely recognized type I allergens, such as peanuts and shellfish, that elicit immediate-type hypersensitivity responses. Although any type IV food allergen(s) could be responsible, a pattern emerged in this study and the study published in 2018.9 Namely, some foods stood out as more frequently inducing patch test reactions, with the 3 most common being carmine, cinnamon bark oil, and sodium bisulfite (eTable). The sample size is relatively small, but the results raise the question of whether these foods are the most likely to trigger IBS symptoms in the general population. If so, is it the result of a higher innate sensitizing potential and/or a higher frequency of exposure in commonly eaten foods? Larger randomized clinical trials are needed.
Immune Response and IBS
There is mounting evidence that the immune system may play a role in the pathophysiology of IBS.18 Both lymphocyte infiltration of the myenteric plexus and an increase in intestinal mucosal T lymphocytes have been observed, and it is generally accepted that the mucosal immune system seems to be activated, at least in a subset of patients with IBS.19 Irritable bowel syndrome associations with quiescent inflammatory bowel disease or postinfectious gastroenteritis provide 2 potential causes for the inflammation, but most IBS patients have had neither.20 The mucosal lining of the intestine and immune system have vast exposure to intraluminal allergens in transit, and it is hypothesized that the same delayed-type hypersensitivity response elicited in the skin by patch testing is elicited in the intestine, resulting in the inflammation that triggers IBS symptoms.10 The results here add to the growing body of evidence that ingestion of type IV food allergens by previously sensitized individuals could, in fact, be the primary source of the inflammation observed in a large subpopulation of individuals who carry a diagnosis of IBS.
Food Allergens in Patch Testing
Many of the food allergens used in this study are commonly found in various nonfood products that may contact the skin. For example, many flavorings are used as fragrances, and many preservatives, binders, thickeners, emulsifiers, and stabilizers serve the same role in moisturizers, cosmetics, and topical medications. Likewise, nickel sulfate hexahydrate, ubiquitous in foods that arise from the earth, often is found in metal in jewelry, clothing components, and cell phones. All are potential sensitizers. Thus, the question may arise whether the causal relationship between the food allergens identified by patch testing and IBS symptoms might be more of a systemic effect akin to systemic contact dermatitis as sometimes follows ingestion of an allergen to which an individual has been topically sensitized, rather than the proposed localized immunologic response in the intestinal lining. We were unaware of patient history of allergic contact dermatitis to any of the patch test allergens in this study, but the dermatologist author here (M.S.) has unpublished experience with 2 other patients with IBS who have benefited from low-nickel diets after having had positive patch tests to nickel sulfate hexahydrate and who, in retrospect, did report a history of earring dermatitis. Future investigations using pre– and post–food challenge histologic assessments of the intestinal mucosa in patients who benefit from patch test–guided food avoidance diets should help to better define the mechanism.
Because IBS has not been traditionally associated with structural or biochemical abnormalities detectable with current routine diagnostic tools, it has long been viewed as a functional disorder. The findings published more recently,9,10 in addition to this study’s results, would negate this functional classification in the subset of patients with IBS symptoms who experience sustained relief of their symptoms by patch test–directed food avoidance. The underlying delayed-type hypersensitivity pathogenesis of the IBS-like symptoms in these individuals would mandate an organic classification, aptly named allergic contact enteritis.10
Follow-up Data
The mean (SD) follow-up duration for this study and the 2018 report9 was 4.5 (3.0) months and 7.6 (3.9) months, respectively. The placebo effect is a concern for disorders such as IBS in which primarily subjective outcome measures are available,21 and in a retrospective analysis of 25 randomized, placebo-controlled IBS clinical trials, Spiller22 concluded the optimum length of such trials to be more than 3 months, which these studies exceed. Although not blinded or placebo controlled, the length of follow-up in the 2018 report9 and here enhances the validity of the results.
Limitation
The retrospective manner in which the self-assessments were reported in this study introduces the potential for recall bias, a variable that could affect results. The presence and direction of bias by any given individual cannot be known, making it difficult to determine any effect it may have had. Further investigation should include daily assessments and refine the primary study end points to include both abdominal pain and the defecation considerations that define IBS.
Conclusion
Food patch testing has the potential to offer a safe, cost-effective approach to the evaluation and management of IBS symptoms. Randomized clinical trials are needed to further investigate the validity of the proof-of-concept results to date. For patients who benefit from a patch test–guided avoidance diet, invasive and costly endoscopic, radiologic, and laboratory testing and pharmacologic management could be averted. Symptomatic relief could be attained simply by avoiding the implicated foods, essentially doing more by doing less.
Irritable bowel syndrome (IBS) is one of the most common disorders managed by primary care physicians and gastroenterologists.1 Characterized by abdominal pain coinciding with altered stool form and/or frequency as defined by the Rome IV diagnostic criteria,2 symptoms range from mild to debilitating and may remarkably impair quality of life and work productivity.1
The cause of IBS is poorly understood. Proposed pathophysiologic factors include impaired mucosal function, microbial imbalance, visceral hypersensitivity, psychologic dysfunction, genetic factors, neurotransmitter imbalance, postinfectious gastroenteritis, inflammation, and food intolerance, any or all of which may lead to the development and maintenance of IBS symptoms.3 More recent observations of inflammation in the intestinal lining4,5 and proinflammatory peripherally circulating cytokines6 challenge its traditional classification as a functional disorder.
The cause of this inflammation is of intense interest, with speculation that the bacterial microbiota, bile acids, association with postinfectious gastroenteritis and inflammatory bowel disease cases, and/or foods may contribute. Although approximately 50% of individuals with IBS report that foods aggravate their symptoms,7 studies investigating type I antibody–mediated immediate hypersensitivity have largely failed to demonstrate a substantial link, prompting many authorities to regard these associations as food “intolerances” rather than true allergies. Based on this body of literature, a large 2010 consensus report on all aspects of food allergies advises against food allergy testing for IBS.8
In contrast, by utilizing type IV food allergen skin patch testing, 2 proof-of-concept studies9,10 investigated a different allergic mechanism in IBS, namely cell-mediated delayed-type hypersensitivity. Because many foods and food additives are known to cause allergic contact dermatitis,11 it was hypothesized that these foods may elicit a similar delayed-type hypersensitivity response in the intestinal lining in previously sensitized individuals. By following a patch test–guided food avoidance diet, a large subpopulation of patients with IBS experienced partial or complete IBS symptom relief.9,10 Our study further investigates a role for food-related delayed-type hypersensitivities in the pathogenesis of IBS.
Methods
Patient Selection
This study was conducted in a secondary care community-based setting. All patients were self-referred over an 18-month period ending in October 2019, had physician-diagnosed IBS, and/or met the Rome IV criteria for IBS and presented expressly for the food patch testing on a fee-for-service basis. Subtype of IBS was determined on presentation by the self-reported historically predominant symptom. Duration of IBS symptoms was self-reported and was rounded to the nearest year for purposes of data collection.
Exclusion criteria included pregnancy, known allergy to adhesive tape or any of the food allergens used in the study, severe skin rash, symptoms that had a known cause other than IBS, or active treatment with systemic immunosuppressive medications.
Patch Testing
Skin patch testing was initiated using an extensive panel of 117 type IV food allergens (eTable)11 identified in the literature,12 most of which utilized standard compounded formulations13 or were available from reputable patch test manufacturers (Brial Allergen GmbH; Chemotechnique Diagnostics). This panel was not approved by the US Food and Drug Administration. The freeze-dried vegetable formulations were taken from the 2018 report.9 Standard skin patch test procedure protocols12 were used, affixing the patches to the upper aspect of the back.
Following patch test application on day 1, two follow-up visits occurred on day 3 and either day 4 or day 5. On day 3, patches were removed, and the initial results were read by a board-certified dermatologist according to a standard grading system.14 Interpretation of patch tests included no reaction, questionable reaction consisting of macular erythema, weak reaction consisting of erythema and slight edema, or strong reaction consisting of erythema and marked edema. On day 4 or day 5, the final patch test reading was performed, and patients were informed of their results. Patients were advised to avoid ingestion of all foods that elicited a questionable or positive patch test response for at least 3 months, and information about the foods and their avoidance also was distributed and reviewed.
Food Avoidance Questionnaire
Patients with questionable or positive patch tests at 72 or 96 hours were advised of their eligibility to participate in an institutional review board–approved food avoidance questionnaire study investigating the utility of patch test–guided food avoidance on IBS symptoms. The questionnaire assessed the following: (1) baseline average abdominal pain prior to patch test–guided avoidance diet (0=no symptoms; 10=very severe); (2) average abdominal pain since initiation of patch test–guided avoidance diet (0=no symptoms; 10=very severe); (3) degree of improvement in overall IBS symptoms by the end of the food avoidance period (0=no improvement; 10=great improvement); (4) compliance with the avoidance diet for the duration of the avoidance period (completely, partially, not at all, or not sure).
Questionnaires and informed consent were mailed to patients via the US Postal Service 3 months after completing the patch testing. The questionnaire and consent were to be completed and returned after dietary avoidance of the identified allergens for at least 3 months. Patients were not compensated for participation in the study.
Statistical Analysis
Statistical analysis of data collected from study questionnaires was performed with Microsoft Excel. Mean abdominal pain and mean global improvement scores were reported along with 1 SD of the mean. For comparison of mean abdominal pain and improvement in global IBS symptoms from baseline to after 3 months of identified allergen avoidance, a Mann-Whitney U test was performed, with P<.05 being considered statistically significant.
Results
Thirty-seven consecutive patients underwent the testing and were eligible for the study. Nineteen patients were included in the study by virtue of completing and returning their posttest food avoidance questionnaire and informed consent. Eighteen patients were White and 1 was Asian. Subcategories of IBS were diarrhea predominant (9 [47.4%]), constipation predominant (3 [15.8%]), mixed type (5 [26.3%]), and undetermined type (2 [10.5%]). Questionnaire answers were reported after a mean (SD) duration of patch test–directed food avoidance of 4.5 (3.0) months (Table 1).

Overall Improvement
Fifteen (78.9%) patients reported at least slight to great improvement in their global IBS symptoms, and 4 (21.1%) reported no improvement (Table 2), with a mean (SD) improvement score of 5.1 (3.3)(P<.00001).

Abdominal Pain
All 19 patients reported mild to marked abdominal pain at baseline. The mean (SD) baseline pain score was 6.6 (1.9). The mean (SD) pain score was 3.4 (1.8)(P<.00001) after an average patch test–guided dietary avoidance of 4.5 (3.0) months (Table 3).

Comment
Despite intense research interest and a growing number of new medications for IBS approved by the US Food and Drug Administration, there remains a large void in the search for cost-effective and efficacious approaches for IBS evaluation and treatment. In addition to major disturbances in quality of life,14,15 the cost to society in direct medical expenses and indirect costs associated with loss of productivity and work absenteeism is considerable; estimates range from $21 billion or more annually.16
Food Hypersensitivities Triggering IBS
This study further evaluated a role for skin patch testing to identify delayed-type (type IV) food hypersensitivities that trigger IBS symptoms and differed from the prior investigations9,10 in that the symptoms used to define IBS were updated from the Rome III17 to the newer Rome IV2 criteria. The data presented here show moderate to great improvement in global IBS symptoms in 58% (11/19) of patients, which is in line with a 2018 report of 40 study participants for whom follow-up at 3 or more months was available,9 providing additional support for a role for type IV food allergies in causing the same gastrointestinal tract symptoms that define IBS. The distinction between food-related studies, including this one, that implicate food allergies9,10 and prior studies that did not support a role for food allergies in IBS pathogenesis8 can be accounted for by the type of allergy investigated. Conclusions that IBS flares after food ingestion were attributable to intolerance rather than true allergy were based on results investigating only the humoral arm and failed to consider the cell-mediated arm of the immune system. As such, foods that appear to trigger IBS symptoms on an allergic basis in our study are recognized in the literature12 as type IV allergens that elicit cell-mediated immunologic responses rather than more widely recognized type I allergens, such as peanuts and shellfish, that elicit immediate-type hypersensitivity responses. Although any type IV food allergen(s) could be responsible, a pattern emerged in this study and the study published in 2018.9 Namely, some foods stood out as more frequently inducing patch test reactions, with the 3 most common being carmine, cinnamon bark oil, and sodium bisulfite (eTable). The sample size is relatively small, but the results raise the question of whether these foods are the most likely to trigger IBS symptoms in the general population. If so, is it the result of a higher innate sensitizing potential and/or a higher frequency of exposure in commonly eaten foods? Larger randomized clinical trials are needed.
Immune Response and IBS
There is mounting evidence that the immune system may play a role in the pathophysiology of IBS.18 Both lymphocyte infiltration of the myenteric plexus and an increase in intestinal mucosal T lymphocytes have been observed, and it is generally accepted that the mucosal immune system seems to be activated, at least in a subset of patients with IBS.19 Irritable bowel syndrome associations with quiescent inflammatory bowel disease or postinfectious gastroenteritis provide 2 potential causes for the inflammation, but most IBS patients have had neither.20 The mucosal lining of the intestine and immune system have vast exposure to intraluminal allergens in transit, and it is hypothesized that the same delayed-type hypersensitivity response elicited in the skin by patch testing is elicited in the intestine, resulting in the inflammation that triggers IBS symptoms.10 The results here add to the growing body of evidence that ingestion of type IV food allergens by previously sensitized individuals could, in fact, be the primary source of the inflammation observed in a large subpopulation of individuals who carry a diagnosis of IBS.
Food Allergens in Patch Testing
Many of the food allergens used in this study are commonly found in various nonfood products that may contact the skin. For example, many flavorings are used as fragrances, and many preservatives, binders, thickeners, emulsifiers, and stabilizers serve the same role in moisturizers, cosmetics, and topical medications. Likewise, nickel sulfate hexahydrate, ubiquitous in foods that arise from the earth, often is found in metal in jewelry, clothing components, and cell phones. All are potential sensitizers. Thus, the question may arise whether the causal relationship between the food allergens identified by patch testing and IBS symptoms might be more of a systemic effect akin to systemic contact dermatitis as sometimes follows ingestion of an allergen to which an individual has been topically sensitized, rather than the proposed localized immunologic response in the intestinal lining. We were unaware of patient history of allergic contact dermatitis to any of the patch test allergens in this study, but the dermatologist author here (M.S.) has unpublished experience with 2 other patients with IBS who have benefited from low-nickel diets after having had positive patch tests to nickel sulfate hexahydrate and who, in retrospect, did report a history of earring dermatitis. Future investigations using pre– and post–food challenge histologic assessments of the intestinal mucosa in patients who benefit from patch test–guided food avoidance diets should help to better define the mechanism.
Because IBS has not been traditionally associated with structural or biochemical abnormalities detectable with current routine diagnostic tools, it has long been viewed as a functional disorder. The findings published more recently,9,10 in addition to this study’s results, would negate this functional classification in the subset of patients with IBS symptoms who experience sustained relief of their symptoms by patch test–directed food avoidance. The underlying delayed-type hypersensitivity pathogenesis of the IBS-like symptoms in these individuals would mandate an organic classification, aptly named allergic contact enteritis.10
Follow-up Data
The mean (SD) follow-up duration for this study and the 2018 report9 was 4.5 (3.0) months and 7.6 (3.9) months, respectively. The placebo effect is a concern for disorders such as IBS in which primarily subjective outcome measures are available,21 and in a retrospective analysis of 25 randomized, placebo-controlled IBS clinical trials, Spiller22 concluded the optimum length of such trials to be more than 3 months, which these studies exceed. Although not blinded or placebo controlled, the length of follow-up in the 2018 report9 and here enhances the validity of the results.
Limitation
The retrospective manner in which the self-assessments were reported in this study introduces the potential for recall bias, a variable that could affect results. The presence and direction of bias by any given individual cannot be known, making it difficult to determine any effect it may have had. Further investigation should include daily assessments and refine the primary study end points to include both abdominal pain and the defecation considerations that define IBS.
Conclusion
Food patch testing has the potential to offer a safe, cost-effective approach to the evaluation and management of IBS symptoms. Randomized clinical trials are needed to further investigate the validity of the proof-of-concept results to date. For patients who benefit from a patch test–guided avoidance diet, invasive and costly endoscopic, radiologic, and laboratory testing and pharmacologic management could be averted. Symptomatic relief could be attained simply by avoiding the implicated foods, essentially doing more by doing less.
- Enck P, Aziz Q, Barbara G, et al. Irritable bowel syndrome. Nat Rev Dis Primers. 2016;2:1-24.
- Lacy BE, Patel NK. Rome criteria and a diagnostic approach to irritable bowel syndrome. J Clin Med. 2017;6:99.
- Barbara G, De Giorgio R, Stanghellini V, et al. New pathophysiological mechanisms in irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20(suppl 2):1-9
- Chadwick VS, Chen W, Shu D, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology 2002;122:1778-1783.
- Tornblom H, Lindberg G, Nyberg B, et al. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology. 2002;123:1972-1979.
- O’Mahony L, McCarthy J, Kelly
P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541-551. - Ragnarsson G, Bodemar G. Pain is temporally related to eating but not to defecation in the irritable bowel syndrome (IBS): patients’ description of diarrhea, constipation and symptom variation during a prospective 6-week study. Eur J Gastroenterol Hepatol. 1998;10:415-421.
- Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 suppl):S1-S58.
- Shin GH, Smith MS, Toro B, et al. Utility of food patch testing in the evaluation and management of irritable bowel syndrome. Skin. 2018;2:1-15.
- Stierstorfer MB, Sha CT. Food patch testing for irritable bowel syndrome. J Am Acad Dermatol. 2013;68:377-384.
- Marks JG, Belsito DV, DeLeo MD, et al. North American Contact Dermatitis Group patch test results for the detection of delayed-type hypersensitivity to topical allergens. J Am Acad Dermatol. 1998;38:911-918.
- Rietschel RL, Fowler JF Jr. Fisher’s Contact Dermatitis. BC Decker; 2008.
- DeGroot AC. Patch Testing. acdegroot Publishing; 2008.
- Gralnek IM, Hays RD, Kilbourne A, et al. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology. 2000;119:654-660.
- Halder SL, Lock GR, Talley NJ, et al. Impact of functional gastrointestinal disorders on health-related quality of life: a population-based case–control study. Aliment Pharmacol Ther. 2004;19:233-242.
- International Foundation for Gastrointestinal Disorders. About IBS. statistics. Accessed July 20, 2021. https://www.aboutibs.org/facts-about-ibs/statistics.html
- Rome Foundation. Guidelines—Rome III diagnostic criteria for functional gastrointestinal disorders. J Gastrointestin Liver Dis. 2006;15:307-312.
- Collins SM. Is the irritable gut an inflamed gut? Scand J Gastroenterol. 1992;192(suppl):102-105.
- Park MI, Camilleri M. Is there a role of food allergy in irritable bowel syndrome and functional dyspepsia? a systemic review. Neurogastroenterol Motil. 2006;18:595-607.
- Grover M, Herfarth H, Drossman DA. The functional-organic dichotomy: postinfectious irritable bowel syndrome and inflammatory bowel disease–irritable bowel syndrome. Clin Gastroenterol Hepatol. 2009;7:48-53.
- Hrobiartsson A, Gotzsche PC. Is the placebo powerless? an analysis of clinical trials comparing placebo with no treatment. N Engl J Med. 2001;344:1594-1602.
- Spiller RC. Problems and challenges in the design of irritable bowel syndrome clinical trials: experience from published trials. Am J Med. 1999;107:91S-97S.
- Enck P, Aziz Q, Barbara G, et al. Irritable bowel syndrome. Nat Rev Dis Primers. 2016;2:1-24.
- Lacy BE, Patel NK. Rome criteria and a diagnostic approach to irritable bowel syndrome. J Clin Med. 2017;6:99.
- Barbara G, De Giorgio R, Stanghellini V, et al. New pathophysiological mechanisms in irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20(suppl 2):1-9
- Chadwick VS, Chen W, Shu D, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology 2002;122:1778-1783.
- Tornblom H, Lindberg G, Nyberg B, et al. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology. 2002;123:1972-1979.
- O’Mahony L, McCarthy J, Kelly
P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541-551. - Ragnarsson G, Bodemar G. Pain is temporally related to eating but not to defecation in the irritable bowel syndrome (IBS): patients’ description of diarrhea, constipation and symptom variation during a prospective 6-week study. Eur J Gastroenterol Hepatol. 1998;10:415-421.
- Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 suppl):S1-S58.
- Shin GH, Smith MS, Toro B, et al. Utility of food patch testing in the evaluation and management of irritable bowel syndrome. Skin. 2018;2:1-15.
- Stierstorfer MB, Sha CT. Food patch testing for irritable bowel syndrome. J Am Acad Dermatol. 2013;68:377-384.
- Marks JG, Belsito DV, DeLeo MD, et al. North American Contact Dermatitis Group patch test results for the detection of delayed-type hypersensitivity to topical allergens. J Am Acad Dermatol. 1998;38:911-918.
- Rietschel RL, Fowler JF Jr. Fisher’s Contact Dermatitis. BC Decker; 2008.
- DeGroot AC. Patch Testing. acdegroot Publishing; 2008.
- Gralnek IM, Hays RD, Kilbourne A, et al. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology. 2000;119:654-660.
- Halder SL, Lock GR, Talley NJ, et al. Impact of functional gastrointestinal disorders on health-related quality of life: a population-based case–control study. Aliment Pharmacol Ther. 2004;19:233-242.
- International Foundation for Gastrointestinal Disorders. About IBS. statistics. Accessed July 20, 2021. https://www.aboutibs.org/facts-about-ibs/statistics.html
- Rome Foundation. Guidelines—Rome III diagnostic criteria for functional gastrointestinal disorders. J Gastrointestin Liver Dis. 2006;15:307-312.
- Collins SM. Is the irritable gut an inflamed gut? Scand J Gastroenterol. 1992;192(suppl):102-105.
- Park MI, Camilleri M. Is there a role of food allergy in irritable bowel syndrome and functional dyspepsia? a systemic review. Neurogastroenterol Motil. 2006;18:595-607.
- Grover M, Herfarth H, Drossman DA. The functional-organic dichotomy: postinfectious irritable bowel syndrome and inflammatory bowel disease–irritable bowel syndrome. Clin Gastroenterol Hepatol. 2009;7:48-53.
- Hrobiartsson A, Gotzsche PC. Is the placebo powerless? an analysis of clinical trials comparing placebo with no treatment. N Engl J Med. 2001;344:1594-1602.
- Spiller RC. Problems and challenges in the design of irritable bowel syndrome clinical trials: experience from published trials. Am J Med. 1999;107:91S-97S.
Practice Points
- Recent observations of inflammation in irritable bowel syndrome (IBS) challenge its traditional classification as a functional disorder.
- Delayed-type food hypersensitivities, as detectable by skin patch testing, to type IV food allergens are one plausible cause for intestinal inflammation.
- Patch test–directed food avoidance improves IBS symptoms in some patients and offers a new approach to the evaluation and management of this condition.
- Dermatologists and other health care practitioners with expertise in patch testing are uniquely positioned to utilize these skills to help patients with IBS.
Formaldehyde-Induced Contact Dermatitis From an N95 Respirator Mask
The COVID-19 pandemic has overwhelmed health care facilities and health care providers (HCPs) due to the limited resources available to treat a rapidly expanding patient population. Health care providers have been required to work long hours and put themselves at increased risk of infection by coming into frequent contact with infected patients. In addition to the risk of becoming infected with severe acute respiratory syndrome coronavirus 2, HCPs might be required to wear personal protective equipment (PPE) for the entirety of the workday, which can cause a variety of adverse effects.
During the COVID-19 pandemic, there has been an increase in reported cases of facial acne, pressure injury, urticaria, allergic contact dermatitis (ACD), irritant contact dermatitis (ICD), and exacerbation of underlying cutaneous conditions among health care workers.1-4 This increase in dermatologic disorders among HCPs has been associated with the increased utilization of and duration of exposure to PPE—particularly N95 respirator masks and surgical masks.5-7 Most studies of these reactions have attributed them to local pressure, friction, hyperhydration, elevated pH, and occlusion caused by prolonged wearing of the masks, resulting ultimately in acne and other rashes8-10; however, a few studies have suggested that formaldehyde is a potential culprit underlying the increase in skin reactions to face masks.11-14
Formaldehyde is a known skin irritant and has been found to cause ACD and ICD from exposure to textiles and cosmetics treated with this chemical.15-18 Both N95 and surgical masks previously have been found to contain sufficient levels of formaldehyde or formaldehyde-releasing resins (FRRs) to induce ACD or ICD in susceptible people.12-14 In this article, we focus on the role of formaldehyde in N95 masks as a potential cause of ACD and ICD in HCPs who have been wearing PPE during the COVID-19 pandemic.
Formaldehyde: Benefits With Significant Problems
Formaldehyde is nearly ubiquitous in the textile industry because it confers advantageous properties, including resistance to flames, water, and wrinkling.15 Despite these advantages, it has long been established that consumers can become sensitized to formaldehyde and FRRs in textiles after chronic exposure.15-18
A study of Australian HCPs found that 5.2% of those tested had ACD in response to formaldehyde, which was attributed to their PPE.11 In a case report of ACD caused by FRRs, Donovan and Skotnicki-Grant12 suggested that individuals who are sensitive to formaldehyde are vulnerable to reactions that are exacerbated by friction, warmth, moisture, and tight-fitting materials—all of which can occur when wearing an N95 mask. In that report, a formaldehyde-sensitive patient had a strong positive reaction on patch testing to melamine formaldehyde and to a piece of her N95 mask while taking prednisone 8 mg/d, suggesting that some sensitized patients have a strong reaction to their mask even when they are immunosuppressed.12
This finding, along with the known formaldehyde content of some N95 masks, suggests that these masks might be a cause of contact dermatitis in some HCPs. Somewhat complicating the situation is that false-negative patch testing can occur in and might contribute to the underdiagnosis of formaldehyde-induced N95 mask facial dermatitis.12,13 Some HCPs have reported mild respiratory symptoms and eye irritation associated with the use of an N95 mask—symptoms that are consistent with formaldehyde exposure. In some cases, those symptoms have caused discomfort sufficient to prompt HCPs to take leave from work.13,14
Development of contact dermatitis in response to an N95 mask is not novel; this problem also was observed during the severe acute respiratory syndrome pandemic of the early 2000s.9,17 Some HCPs noticed onset of skin reactions after they were required to wear an N95 mask in the workplace, which some studies attributed to material in the mask increasing the likelihood of developing an adverse reaction.2,6,8 The components of N95 masks and the materials from which they are manufactured are listed in the Table.19

Other studies have shown that formaldehyde-sensitive individuals had positive patch test reactions to the fabric of N95 and surgical masks, which was found to contain free formaldehyde or FRRs.12-14 However, there are limited reports in the literature confirming the presence of formaldehyde in N95 masks, suggesting the need for (1) more patch testing of N95 mask fabric and (2) correlative high-performance liquid chromatography analysis of the masks to confirm that formaldehyde-sensitive individuals are at risk of formaldehyde-related dermatosis in response to an N95 mask. The absence of any regulatory requirements to list the chemical components of N95 masks makes it impossible for mask users to avoid exposure to potential irritants or carcinogens.
Face Masks, Adverse Reactions, and Formaldehyde
Allergic contact dermatitis and ICD typically are rare responses to wearing facial masks, but the recent COVID-19 pandemic has forced HCPs to wear masks for longer than 6 hours at a time and to reuse a single mask, which has been shown to increase the likelihood of adverse reactions.1,4,6 Additionally, humid environments, tight-fitting materials, and skin abrasions—all of which can be induced by wearing an N95 mask—have been found to increase the likelihood of formaldehyde-related contact dermatitis by increasing the release of free formaldehyde or by enhancing its penetration into the skin.6,20,21
Formaldehyde is an ubiquitous chemical agent that is part of indoor and outdoor working and residential environments. Health care professionals have many opportunities to be exposed to formaldehyde, which is a well-known mucous membrane irritant and a primary skin-sensitizing agent associated with both contact dermatitis (type IV hypersensitivity reaction), and an immediate anaphylactic reaction (type I hypersensitivity reaction).22-25 Exposure to formaldehyde by inhalation has been identified as a potential cause of asthma.26,27 More studies on the prevalence of formaldehyde-induced hypersensitivity reactions would be beneficial to HCPs for early diagnosis of hypersensitivity, adequate prophylaxis, and occupational risk assessment.
N95 mask dermatitis also heightens the potential for breaches of PPE protocols. The discomfort that HCPs experience in response to adverse skin reactions to masks can cause an increased rate of inappropriate mask-wearing, face-touching during mask adjustment, and removal of the mask in the health care setting.28 These acts of face-touching and PPE adjustment have been shown to increase microbial transmission and to reduce the efficacy of PPE in blocking pathogens.29,30
Considering the mounting evidence that widespread use of masks effectively prevents viral transmission, it is crucial that all HCPs wear appropriate PPE when treating patients during the COVID-19 pandemic.31,32 The recent surge in ACD and ICD among HCPs in response to wearing N95 masks creates a need to determine the underlying cause of these dermatoses and find methods of mitigating sensitization of HCPs to the offending agents. The current epidemiology of COVID-19 in the United States suggests that PPE will be necessary for much longer than originally anticipated and will continue to be worn for long hours by HCPs.
Formaldehyde-Free Alternatives?
Some researchers have proposed that using materials that are free of allergens like formaldehyde might be a long-term solution to the development of contact dermatitis.15,33 Formaldehyde is used in the finishing process of N95 masks for wrinkle and crease resistance and to prevent mildew. It is possible that formaldehyde could be completely removed from the manufacturing process, although no studies on the effects of such alternatives on mask efficacy have been performed.
Formaldehyde-free alternatives that would confer similar properties on textiles have been explored; the most promising alternative to formaldehyde in cross-linking cellulose fibers is polycarboxylic acid in combination with sodium hypophosphite, which can help avoid the adverse health outcomes and environmental impact of formaldehyde.34-36 Studies of such alternatives in the manufacturing of N95 masks would be needed to establish the efficacy and durability of formaldehyde-free PPE.
Final Thoughts
Additional studies are needed to confirm the presence of formaldehyde in N95 masks and to confirm that the mask material yields a positive patch test in sensitized individuals. The paucity of available studies that quantify formaldehyde or FRR content of N95 and surgical masks makes it difficult to establish an association between the chemical content of masks and the prevalence of mask dermatitis among HCPs; however, available reports of skin reactions, including contact dermatitis, from PPE suggest that formaldehyde sensitivity might be at least part of the problem. As such, we propose that manufacturers of N95 and surgical masks be required to reveal the chemical components of their products so that consumers can make educated purchasing decisions.
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease-2019. letter. J Am Acad Dermatol. 2020;82:1215-1216. doi:10.1016/j.jaad.2020.03.014
- Yan Y, Chen H, Chen L, et al. Consensus of Chinese experts on protection of skin and mucous membrane barrier for health-care workers fighting against coronavirus disease 2019. Dermatol Ther. 2020;33:e13310. doi:10.1111/dth.13310
- Elston DM. Occupational skin disease among health care workers during the coronavirus (COVID-19) epidemic. J Am Acad Dermatol. 2020;82:1085-1086. doi:10.1016/j.jaad.2020.03.012
- Balato A, Ayala F, Bruze M, et al. European Task Force on Contact Dermatitis statement on coronavirus disease-19 (COVID-19) outbreak and the risk of adverse cutaneous reactions. J Eur Acad Dermatol Venereol. 2020;34:E353-E354. doi:10.1111/jdv.16557
- Hu K, Fan J, Li X, et al. The adverse skin reactions of health care workers using personal protective equipment for COVID-19. Medicine (Baltimore). 2020;99:e20603. doi:10.1097/MD.0000000000020603
- Singh M, Pawar M, Bothra A, et al. Personal protective equipment induced facial dermatoses in healthcare workers managing coronavirus disease 2019. J Eur Acad Dermatol Venereol. 2020;34:E378-E380. doi:10.1111/jdv.16628
- Zhou P, Huang Z, Xiao Y, et al. Protecting Chinese healthcare workers while combating the 2019 novel coronavirus. Infect Control Hosp Epidemiol. 2020;41:745-746. doi:10.1017/ice.2020.60
- Hua W, Zuo Y, Wan R, et al. Short-term skin reactions following use of N95 respirators and medical masks. Contact Dermatitis. 2020;83:115-121. doi:10.1111/cod.13601
- Foo CCI, Goon ATJ, Leow Y-H, et al. Adverse skin reactions to personal protective equipment against severe acute respiratory syndrome—a descriptive study in Singapore. Contact Dermatitis. 2006;55:291-294. doi:10.1111/j.1600-0536.2006.00953.x
- Zuo Y, Hua W, Luo Y, et al. Skin reactions of N95 masks and medial masks among health-care personnel: a self‐report questionnaire survey in China. Contact Dermatitis. 2020;83:145-147. doi:10.1111/cod.13555
- Higgins CL, Palmer AM, Cahill JL, et al. Occupational skin disease among Australian healthcare workers: a retrospective analysis from an occupational dermatology clinic, 1993-2014. Contact Dermatitis. 2016;75:213-222. doi:10.1111/cod.12616
- Donovan J, Skotnicki-Grant S. Allergic contact dermatitis from formaldehyde textile resins in surgical uniforms and nonwoven textile masks. Dermatitis. 2007;18:40-44. doi:10.2310/6620.2007.05003
- Donovan J, Kudla I, Holness LD, et al. Skin reactions following use of N95 facial masks. meeting abstract. Dermatitis. 2007;18:104.
- Aerts O, Dendooven E, Foubert K, et al. Surgical mask dermatitis caused by formaldehyde (releasers) during the COVID-19 pandemic. Contact Dermatitis. 2020;83:172-1173. doi:10.1111/cod.13626
- Fowler JF. Formaldehyde as a textile allergen. Curr Probl Dermatol. 2003;31:156-165. doi:10.1159/000072245
- Schorr WF, Keran E, Plotka E. Formaldehyde allergy: the quantitative analysis of American clothing for free formaldehyde and its relevance in clinical practice. Arch Dermatol. 1974;110:73-76.
- Slodownik D, Williams J, Tate B, et al. Textile allergy—the Melbourne experience. Contact Dermatitis. 2011;65:38-42. doi:10.1111/j.1600-0536.2010.01861.x
- O’Quinn SE, Kennedy CB. Contact dermatitis due to formaldehyde in clothing textiles. JAMA. 1965;194:593-596.
- Technical specification sheet—3M™ Particulate Respirator 8210, N95. Published 2018. 3M website. Accessed July 12, 2021. https://multimedia.3m.com/mws/media/1425070O/3m-particulate-respirator-8210-n95-technical-specifications.pdf
- Bhoyrul B, Lecamwasam K, Wilkinson M, et al. A review of non‐glove personal protective equipment‐related occupational dermatoses reported to EPIDERM between 1993 and 2013. Contact Dermatitis. 2019;80:217-221. doi: 10.1111/cod.13177
- Lyapina M, Kissselova-Yaneva A, Krasteva A, et al. Allergic contact dermatitis from formaldehyde exposure. Journal of IMAB - Annual Proceeding (Scientific Papers). 2012;18:255-262. doi:10.5272/jimab.2012184.255
- Foussereau J, Cavelier C, Selig D. Occupational eczema from para-tertiary-butylphenol formaldehyde resins: a review of the sensitizing resins. Contact Dermatitis. 1976;2:254-258. doi:10.1111/j.1600-0536.1976.tb03043.x
- Frølich KW, Andersen LM, Knutsen A, et al. Phenoxyethanol as a nontoxic substitute for formaldehyde in long-term preservation of human anatomical specimens for dissection and demonstration purposes. Anat Rec. 1984;208:271-278. doi:10.1002/ar.1092080214
- Bolt HM. Experimental toxicology of formaldehyde. J Cancer Res Clin Oncol. 1987;113:305-309. doi:10.1007/BF00397713
- Arts JHE, Rennen MAJ, de Heer C. Inhaled formaldehyde: evaluation of sensory irritation in relation to carcinogenicity. Regul Toxicol Pharmacol. 2006;44:144-160. doi:10.1016/j.yrtph.2005.11.006
- Kim CW, Song JS, Ahn YS, et al. Occupational asthma due to formaldehyde. Yonsei Med J. 2001;42:440-445. doi:10.3349/ymj.2001.42.4.440
- Nordman H, Keskinen H, Tuppurainen M. Formaldehyde asthma—rare or overlooked? J Allergy Clin Immunol. 1985;75(1 pt 1):91-99. doi:10.1016/0091-6749(85)90018-1
- Kantor J. Behavioral considerations and impact on personal protective equipment use: early lessons from the coronavirus (COVID-19) pandemic. J Am Acad Dermatol. 2020;82:1087-1088. doi:10.1016/j.jaad.2020.03.013
- Kwok YLA, Gralton J, McLaws M-L. Face touching: a frequent habit that has implications for hand hygiene. Am J Infect Control. 2015;43:112-114. doi:10.1016/j.ajic.2014.10.015
- Nicas M, Best D. A study quantifying the hand-to-face contact rate and its potential application to predicting respiratory tract infection. J Occup Environ Hyg. 2008;5:347-352. doi:10.1080/15459620802003896
- MacIntyre CR, Chughtai AA. A rapid systematic review of the efficacy of face masks and respirators against coronaviruses and other respiratory transmissible viruses for the community, healthcare workers and sick patients. Int J Nurs Stud. 2020;108:103629. doi:10.1016/j.ijnurstu.2020.103629
- Garcia Godoy LR, Jones AE, Anderson TN, et al. Facial protection for healthcare workers during pandemics: a scoping review. BMJ Glob Health. 2020;5:e002553. doi:10.1136/bmjgh-2020-002553
- Svedman C, Engfeldt M, Malinauskiene L. Textile contact dermatitis: how fabrics can induce ermatitis. Curr Treat Options Allergy. 2019;6:103-111. doi:10.1007/s40521-019-0197-5
- Yang CQ, Wang X, Kang I-S. Ester crosslinking of cotton fabric by polymeric carboxylic acids and citric acid. Textile Res J. 1997;67:334-342. https://doi.org/10.1177/004051759706700505
- Welch CM. Formaldehyde-free durable-press finishes. Rev Prog Coloration Related Top. 1992;22:32-41. https://doi.org/10.1111/j.1478-4408.1992.tb00087.x
- Peng H, Yang CQ, Wang S. Nonformaldehyde durable press finishing of cotton fabrics using the combination of maleic acid and sodium hypophosphite. Carbohydrate Polymers. 2012;87:491-499. doi:10.1016/j.carbpol.2011.08.013
The COVID-19 pandemic has overwhelmed health care facilities and health care providers (HCPs) due to the limited resources available to treat a rapidly expanding patient population. Health care providers have been required to work long hours and put themselves at increased risk of infection by coming into frequent contact with infected patients. In addition to the risk of becoming infected with severe acute respiratory syndrome coronavirus 2, HCPs might be required to wear personal protective equipment (PPE) for the entirety of the workday, which can cause a variety of adverse effects.
During the COVID-19 pandemic, there has been an increase in reported cases of facial acne, pressure injury, urticaria, allergic contact dermatitis (ACD), irritant contact dermatitis (ICD), and exacerbation of underlying cutaneous conditions among health care workers.1-4 This increase in dermatologic disorders among HCPs has been associated with the increased utilization of and duration of exposure to PPE—particularly N95 respirator masks and surgical masks.5-7 Most studies of these reactions have attributed them to local pressure, friction, hyperhydration, elevated pH, and occlusion caused by prolonged wearing of the masks, resulting ultimately in acne and other rashes8-10; however, a few studies have suggested that formaldehyde is a potential culprit underlying the increase in skin reactions to face masks.11-14
Formaldehyde is a known skin irritant and has been found to cause ACD and ICD from exposure to textiles and cosmetics treated with this chemical.15-18 Both N95 and surgical masks previously have been found to contain sufficient levels of formaldehyde or formaldehyde-releasing resins (FRRs) to induce ACD or ICD in susceptible people.12-14 In this article, we focus on the role of formaldehyde in N95 masks as a potential cause of ACD and ICD in HCPs who have been wearing PPE during the COVID-19 pandemic.
Formaldehyde: Benefits With Significant Problems
Formaldehyde is nearly ubiquitous in the textile industry because it confers advantageous properties, including resistance to flames, water, and wrinkling.15 Despite these advantages, it has long been established that consumers can become sensitized to formaldehyde and FRRs in textiles after chronic exposure.15-18
A study of Australian HCPs found that 5.2% of those tested had ACD in response to formaldehyde, which was attributed to their PPE.11 In a case report of ACD caused by FRRs, Donovan and Skotnicki-Grant12 suggested that individuals who are sensitive to formaldehyde are vulnerable to reactions that are exacerbated by friction, warmth, moisture, and tight-fitting materials—all of which can occur when wearing an N95 mask. In that report, a formaldehyde-sensitive patient had a strong positive reaction on patch testing to melamine formaldehyde and to a piece of her N95 mask while taking prednisone 8 mg/d, suggesting that some sensitized patients have a strong reaction to their mask even when they are immunosuppressed.12
This finding, along with the known formaldehyde content of some N95 masks, suggests that these masks might be a cause of contact dermatitis in some HCPs. Somewhat complicating the situation is that false-negative patch testing can occur in and might contribute to the underdiagnosis of formaldehyde-induced N95 mask facial dermatitis.12,13 Some HCPs have reported mild respiratory symptoms and eye irritation associated with the use of an N95 mask—symptoms that are consistent with formaldehyde exposure. In some cases, those symptoms have caused discomfort sufficient to prompt HCPs to take leave from work.13,14
Development of contact dermatitis in response to an N95 mask is not novel; this problem also was observed during the severe acute respiratory syndrome pandemic of the early 2000s.9,17 Some HCPs noticed onset of skin reactions after they were required to wear an N95 mask in the workplace, which some studies attributed to material in the mask increasing the likelihood of developing an adverse reaction.2,6,8 The components of N95 masks and the materials from which they are manufactured are listed in the Table.19

Other studies have shown that formaldehyde-sensitive individuals had positive patch test reactions to the fabric of N95 and surgical masks, which was found to contain free formaldehyde or FRRs.12-14 However, there are limited reports in the literature confirming the presence of formaldehyde in N95 masks, suggesting the need for (1) more patch testing of N95 mask fabric and (2) correlative high-performance liquid chromatography analysis of the masks to confirm that formaldehyde-sensitive individuals are at risk of formaldehyde-related dermatosis in response to an N95 mask. The absence of any regulatory requirements to list the chemical components of N95 masks makes it impossible for mask users to avoid exposure to potential irritants or carcinogens.
Face Masks, Adverse Reactions, and Formaldehyde
Allergic contact dermatitis and ICD typically are rare responses to wearing facial masks, but the recent COVID-19 pandemic has forced HCPs to wear masks for longer than 6 hours at a time and to reuse a single mask, which has been shown to increase the likelihood of adverse reactions.1,4,6 Additionally, humid environments, tight-fitting materials, and skin abrasions—all of which can be induced by wearing an N95 mask—have been found to increase the likelihood of formaldehyde-related contact dermatitis by increasing the release of free formaldehyde or by enhancing its penetration into the skin.6,20,21
Formaldehyde is an ubiquitous chemical agent that is part of indoor and outdoor working and residential environments. Health care professionals have many opportunities to be exposed to formaldehyde, which is a well-known mucous membrane irritant and a primary skin-sensitizing agent associated with both contact dermatitis (type IV hypersensitivity reaction), and an immediate anaphylactic reaction (type I hypersensitivity reaction).22-25 Exposure to formaldehyde by inhalation has been identified as a potential cause of asthma.26,27 More studies on the prevalence of formaldehyde-induced hypersensitivity reactions would be beneficial to HCPs for early diagnosis of hypersensitivity, adequate prophylaxis, and occupational risk assessment.
N95 mask dermatitis also heightens the potential for breaches of PPE protocols. The discomfort that HCPs experience in response to adverse skin reactions to masks can cause an increased rate of inappropriate mask-wearing, face-touching during mask adjustment, and removal of the mask in the health care setting.28 These acts of face-touching and PPE adjustment have been shown to increase microbial transmission and to reduce the efficacy of PPE in blocking pathogens.29,30
Considering the mounting evidence that widespread use of masks effectively prevents viral transmission, it is crucial that all HCPs wear appropriate PPE when treating patients during the COVID-19 pandemic.31,32 The recent surge in ACD and ICD among HCPs in response to wearing N95 masks creates a need to determine the underlying cause of these dermatoses and find methods of mitigating sensitization of HCPs to the offending agents. The current epidemiology of COVID-19 in the United States suggests that PPE will be necessary for much longer than originally anticipated and will continue to be worn for long hours by HCPs.
Formaldehyde-Free Alternatives?
Some researchers have proposed that using materials that are free of allergens like formaldehyde might be a long-term solution to the development of contact dermatitis.15,33 Formaldehyde is used in the finishing process of N95 masks for wrinkle and crease resistance and to prevent mildew. It is possible that formaldehyde could be completely removed from the manufacturing process, although no studies on the effects of such alternatives on mask efficacy have been performed.
Formaldehyde-free alternatives that would confer similar properties on textiles have been explored; the most promising alternative to formaldehyde in cross-linking cellulose fibers is polycarboxylic acid in combination with sodium hypophosphite, which can help avoid the adverse health outcomes and environmental impact of formaldehyde.34-36 Studies of such alternatives in the manufacturing of N95 masks would be needed to establish the efficacy and durability of formaldehyde-free PPE.
Final Thoughts
Additional studies are needed to confirm the presence of formaldehyde in N95 masks and to confirm that the mask material yields a positive patch test in sensitized individuals. The paucity of available studies that quantify formaldehyde or FRR content of N95 and surgical masks makes it difficult to establish an association between the chemical content of masks and the prevalence of mask dermatitis among HCPs; however, available reports of skin reactions, including contact dermatitis, from PPE suggest that formaldehyde sensitivity might be at least part of the problem. As such, we propose that manufacturers of N95 and surgical masks be required to reveal the chemical components of their products so that consumers can make educated purchasing decisions.
The COVID-19 pandemic has overwhelmed health care facilities and health care providers (HCPs) due to the limited resources available to treat a rapidly expanding patient population. Health care providers have been required to work long hours and put themselves at increased risk of infection by coming into frequent contact with infected patients. In addition to the risk of becoming infected with severe acute respiratory syndrome coronavirus 2, HCPs might be required to wear personal protective equipment (PPE) for the entirety of the workday, which can cause a variety of adverse effects.
During the COVID-19 pandemic, there has been an increase in reported cases of facial acne, pressure injury, urticaria, allergic contact dermatitis (ACD), irritant contact dermatitis (ICD), and exacerbation of underlying cutaneous conditions among health care workers.1-4 This increase in dermatologic disorders among HCPs has been associated with the increased utilization of and duration of exposure to PPE—particularly N95 respirator masks and surgical masks.5-7 Most studies of these reactions have attributed them to local pressure, friction, hyperhydration, elevated pH, and occlusion caused by prolonged wearing of the masks, resulting ultimately in acne and other rashes8-10; however, a few studies have suggested that formaldehyde is a potential culprit underlying the increase in skin reactions to face masks.11-14
Formaldehyde is a known skin irritant and has been found to cause ACD and ICD from exposure to textiles and cosmetics treated with this chemical.15-18 Both N95 and surgical masks previously have been found to contain sufficient levels of formaldehyde or formaldehyde-releasing resins (FRRs) to induce ACD or ICD in susceptible people.12-14 In this article, we focus on the role of formaldehyde in N95 masks as a potential cause of ACD and ICD in HCPs who have been wearing PPE during the COVID-19 pandemic.
Formaldehyde: Benefits With Significant Problems
Formaldehyde is nearly ubiquitous in the textile industry because it confers advantageous properties, including resistance to flames, water, and wrinkling.15 Despite these advantages, it has long been established that consumers can become sensitized to formaldehyde and FRRs in textiles after chronic exposure.15-18
A study of Australian HCPs found that 5.2% of those tested had ACD in response to formaldehyde, which was attributed to their PPE.11 In a case report of ACD caused by FRRs, Donovan and Skotnicki-Grant12 suggested that individuals who are sensitive to formaldehyde are vulnerable to reactions that are exacerbated by friction, warmth, moisture, and tight-fitting materials—all of which can occur when wearing an N95 mask. In that report, a formaldehyde-sensitive patient had a strong positive reaction on patch testing to melamine formaldehyde and to a piece of her N95 mask while taking prednisone 8 mg/d, suggesting that some sensitized patients have a strong reaction to their mask even when they are immunosuppressed.12
This finding, along with the known formaldehyde content of some N95 masks, suggests that these masks might be a cause of contact dermatitis in some HCPs. Somewhat complicating the situation is that false-negative patch testing can occur in and might contribute to the underdiagnosis of formaldehyde-induced N95 mask facial dermatitis.12,13 Some HCPs have reported mild respiratory symptoms and eye irritation associated with the use of an N95 mask—symptoms that are consistent with formaldehyde exposure. In some cases, those symptoms have caused discomfort sufficient to prompt HCPs to take leave from work.13,14
Development of contact dermatitis in response to an N95 mask is not novel; this problem also was observed during the severe acute respiratory syndrome pandemic of the early 2000s.9,17 Some HCPs noticed onset of skin reactions after they were required to wear an N95 mask in the workplace, which some studies attributed to material in the mask increasing the likelihood of developing an adverse reaction.2,6,8 The components of N95 masks and the materials from which they are manufactured are listed in the Table.19

Other studies have shown that formaldehyde-sensitive individuals had positive patch test reactions to the fabric of N95 and surgical masks, which was found to contain free formaldehyde or FRRs.12-14 However, there are limited reports in the literature confirming the presence of formaldehyde in N95 masks, suggesting the need for (1) more patch testing of N95 mask fabric and (2) correlative high-performance liquid chromatography analysis of the masks to confirm that formaldehyde-sensitive individuals are at risk of formaldehyde-related dermatosis in response to an N95 mask. The absence of any regulatory requirements to list the chemical components of N95 masks makes it impossible for mask users to avoid exposure to potential irritants or carcinogens.
Face Masks, Adverse Reactions, and Formaldehyde
Allergic contact dermatitis and ICD typically are rare responses to wearing facial masks, but the recent COVID-19 pandemic has forced HCPs to wear masks for longer than 6 hours at a time and to reuse a single mask, which has been shown to increase the likelihood of adverse reactions.1,4,6 Additionally, humid environments, tight-fitting materials, and skin abrasions—all of which can be induced by wearing an N95 mask—have been found to increase the likelihood of formaldehyde-related contact dermatitis by increasing the release of free formaldehyde or by enhancing its penetration into the skin.6,20,21
Formaldehyde is an ubiquitous chemical agent that is part of indoor and outdoor working and residential environments. Health care professionals have many opportunities to be exposed to formaldehyde, which is a well-known mucous membrane irritant and a primary skin-sensitizing agent associated with both contact dermatitis (type IV hypersensitivity reaction), and an immediate anaphylactic reaction (type I hypersensitivity reaction).22-25 Exposure to formaldehyde by inhalation has been identified as a potential cause of asthma.26,27 More studies on the prevalence of formaldehyde-induced hypersensitivity reactions would be beneficial to HCPs for early diagnosis of hypersensitivity, adequate prophylaxis, and occupational risk assessment.
N95 mask dermatitis also heightens the potential for breaches of PPE protocols. The discomfort that HCPs experience in response to adverse skin reactions to masks can cause an increased rate of inappropriate mask-wearing, face-touching during mask adjustment, and removal of the mask in the health care setting.28 These acts of face-touching and PPE adjustment have been shown to increase microbial transmission and to reduce the efficacy of PPE in blocking pathogens.29,30
Considering the mounting evidence that widespread use of masks effectively prevents viral transmission, it is crucial that all HCPs wear appropriate PPE when treating patients during the COVID-19 pandemic.31,32 The recent surge in ACD and ICD among HCPs in response to wearing N95 masks creates a need to determine the underlying cause of these dermatoses and find methods of mitigating sensitization of HCPs to the offending agents. The current epidemiology of COVID-19 in the United States suggests that PPE will be necessary for much longer than originally anticipated and will continue to be worn for long hours by HCPs.
Formaldehyde-Free Alternatives?
Some researchers have proposed that using materials that are free of allergens like formaldehyde might be a long-term solution to the development of contact dermatitis.15,33 Formaldehyde is used in the finishing process of N95 masks for wrinkle and crease resistance and to prevent mildew. It is possible that formaldehyde could be completely removed from the manufacturing process, although no studies on the effects of such alternatives on mask efficacy have been performed.
Formaldehyde-free alternatives that would confer similar properties on textiles have been explored; the most promising alternative to formaldehyde in cross-linking cellulose fibers is polycarboxylic acid in combination with sodium hypophosphite, which can help avoid the adverse health outcomes and environmental impact of formaldehyde.34-36 Studies of such alternatives in the manufacturing of N95 masks would be needed to establish the efficacy and durability of formaldehyde-free PPE.
Final Thoughts
Additional studies are needed to confirm the presence of formaldehyde in N95 masks and to confirm that the mask material yields a positive patch test in sensitized individuals. The paucity of available studies that quantify formaldehyde or FRR content of N95 and surgical masks makes it difficult to establish an association between the chemical content of masks and the prevalence of mask dermatitis among HCPs; however, available reports of skin reactions, including contact dermatitis, from PPE suggest that formaldehyde sensitivity might be at least part of the problem. As such, we propose that manufacturers of N95 and surgical masks be required to reveal the chemical components of their products so that consumers can make educated purchasing decisions.
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease-2019. letter. J Am Acad Dermatol. 2020;82:1215-1216. doi:10.1016/j.jaad.2020.03.014
- Yan Y, Chen H, Chen L, et al. Consensus of Chinese experts on protection of skin and mucous membrane barrier for health-care workers fighting against coronavirus disease 2019. Dermatol Ther. 2020;33:e13310. doi:10.1111/dth.13310
- Elston DM. Occupational skin disease among health care workers during the coronavirus (COVID-19) epidemic. J Am Acad Dermatol. 2020;82:1085-1086. doi:10.1016/j.jaad.2020.03.012
- Balato A, Ayala F, Bruze M, et al. European Task Force on Contact Dermatitis statement on coronavirus disease-19 (COVID-19) outbreak and the risk of adverse cutaneous reactions. J Eur Acad Dermatol Venereol. 2020;34:E353-E354. doi:10.1111/jdv.16557
- Hu K, Fan J, Li X, et al. The adverse skin reactions of health care workers using personal protective equipment for COVID-19. Medicine (Baltimore). 2020;99:e20603. doi:10.1097/MD.0000000000020603
- Singh M, Pawar M, Bothra A, et al. Personal protective equipment induced facial dermatoses in healthcare workers managing coronavirus disease 2019. J Eur Acad Dermatol Venereol. 2020;34:E378-E380. doi:10.1111/jdv.16628
- Zhou P, Huang Z, Xiao Y, et al. Protecting Chinese healthcare workers while combating the 2019 novel coronavirus. Infect Control Hosp Epidemiol. 2020;41:745-746. doi:10.1017/ice.2020.60
- Hua W, Zuo Y, Wan R, et al. Short-term skin reactions following use of N95 respirators and medical masks. Contact Dermatitis. 2020;83:115-121. doi:10.1111/cod.13601
- Foo CCI, Goon ATJ, Leow Y-H, et al. Adverse skin reactions to personal protective equipment against severe acute respiratory syndrome—a descriptive study in Singapore. Contact Dermatitis. 2006;55:291-294. doi:10.1111/j.1600-0536.2006.00953.x
- Zuo Y, Hua W, Luo Y, et al. Skin reactions of N95 masks and medial masks among health-care personnel: a self‐report questionnaire survey in China. Contact Dermatitis. 2020;83:145-147. doi:10.1111/cod.13555
- Higgins CL, Palmer AM, Cahill JL, et al. Occupational skin disease among Australian healthcare workers: a retrospective analysis from an occupational dermatology clinic, 1993-2014. Contact Dermatitis. 2016;75:213-222. doi:10.1111/cod.12616
- Donovan J, Skotnicki-Grant S. Allergic contact dermatitis from formaldehyde textile resins in surgical uniforms and nonwoven textile masks. Dermatitis. 2007;18:40-44. doi:10.2310/6620.2007.05003
- Donovan J, Kudla I, Holness LD, et al. Skin reactions following use of N95 facial masks. meeting abstract. Dermatitis. 2007;18:104.
- Aerts O, Dendooven E, Foubert K, et al. Surgical mask dermatitis caused by formaldehyde (releasers) during the COVID-19 pandemic. Contact Dermatitis. 2020;83:172-1173. doi:10.1111/cod.13626
- Fowler JF. Formaldehyde as a textile allergen. Curr Probl Dermatol. 2003;31:156-165. doi:10.1159/000072245
- Schorr WF, Keran E, Plotka E. Formaldehyde allergy: the quantitative analysis of American clothing for free formaldehyde and its relevance in clinical practice. Arch Dermatol. 1974;110:73-76.
- Slodownik D, Williams J, Tate B, et al. Textile allergy—the Melbourne experience. Contact Dermatitis. 2011;65:38-42. doi:10.1111/j.1600-0536.2010.01861.x
- O’Quinn SE, Kennedy CB. Contact dermatitis due to formaldehyde in clothing textiles. JAMA. 1965;194:593-596.
- Technical specification sheet—3M™ Particulate Respirator 8210, N95. Published 2018. 3M website. Accessed July 12, 2021. https://multimedia.3m.com/mws/media/1425070O/3m-particulate-respirator-8210-n95-technical-specifications.pdf
- Bhoyrul B, Lecamwasam K, Wilkinson M, et al. A review of non‐glove personal protective equipment‐related occupational dermatoses reported to EPIDERM between 1993 and 2013. Contact Dermatitis. 2019;80:217-221. doi: 10.1111/cod.13177
- Lyapina M, Kissselova-Yaneva A, Krasteva A, et al. Allergic contact dermatitis from formaldehyde exposure. Journal of IMAB - Annual Proceeding (Scientific Papers). 2012;18:255-262. doi:10.5272/jimab.2012184.255
- Foussereau J, Cavelier C, Selig D. Occupational eczema from para-tertiary-butylphenol formaldehyde resins: a review of the sensitizing resins. Contact Dermatitis. 1976;2:254-258. doi:10.1111/j.1600-0536.1976.tb03043.x
- Frølich KW, Andersen LM, Knutsen A, et al. Phenoxyethanol as a nontoxic substitute for formaldehyde in long-term preservation of human anatomical specimens for dissection and demonstration purposes. Anat Rec. 1984;208:271-278. doi:10.1002/ar.1092080214
- Bolt HM. Experimental toxicology of formaldehyde. J Cancer Res Clin Oncol. 1987;113:305-309. doi:10.1007/BF00397713
- Arts JHE, Rennen MAJ, de Heer C. Inhaled formaldehyde: evaluation of sensory irritation in relation to carcinogenicity. Regul Toxicol Pharmacol. 2006;44:144-160. doi:10.1016/j.yrtph.2005.11.006
- Kim CW, Song JS, Ahn YS, et al. Occupational asthma due to formaldehyde. Yonsei Med J. 2001;42:440-445. doi:10.3349/ymj.2001.42.4.440
- Nordman H, Keskinen H, Tuppurainen M. Formaldehyde asthma—rare or overlooked? J Allergy Clin Immunol. 1985;75(1 pt 1):91-99. doi:10.1016/0091-6749(85)90018-1
- Kantor J. Behavioral considerations and impact on personal protective equipment use: early lessons from the coronavirus (COVID-19) pandemic. J Am Acad Dermatol. 2020;82:1087-1088. doi:10.1016/j.jaad.2020.03.013
- Kwok YLA, Gralton J, McLaws M-L. Face touching: a frequent habit that has implications for hand hygiene. Am J Infect Control. 2015;43:112-114. doi:10.1016/j.ajic.2014.10.015
- Nicas M, Best D. A study quantifying the hand-to-face contact rate and its potential application to predicting respiratory tract infection. J Occup Environ Hyg. 2008;5:347-352. doi:10.1080/15459620802003896
- MacIntyre CR, Chughtai AA. A rapid systematic review of the efficacy of face masks and respirators against coronaviruses and other respiratory transmissible viruses for the community, healthcare workers and sick patients. Int J Nurs Stud. 2020;108:103629. doi:10.1016/j.ijnurstu.2020.103629
- Garcia Godoy LR, Jones AE, Anderson TN, et al. Facial protection for healthcare workers during pandemics: a scoping review. BMJ Glob Health. 2020;5:e002553. doi:10.1136/bmjgh-2020-002553
- Svedman C, Engfeldt M, Malinauskiene L. Textile contact dermatitis: how fabrics can induce ermatitis. Curr Treat Options Allergy. 2019;6:103-111. doi:10.1007/s40521-019-0197-5
- Yang CQ, Wang X, Kang I-S. Ester crosslinking of cotton fabric by polymeric carboxylic acids and citric acid. Textile Res J. 1997;67:334-342. https://doi.org/10.1177/004051759706700505
- Welch CM. Formaldehyde-free durable-press finishes. Rev Prog Coloration Related Top. 1992;22:32-41. https://doi.org/10.1111/j.1478-4408.1992.tb00087.x
- Peng H, Yang CQ, Wang S. Nonformaldehyde durable press finishing of cotton fabrics using the combination of maleic acid and sodium hypophosphite. Carbohydrate Polymers. 2012;87:491-499. doi:10.1016/j.carbpol.2011.08.013
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease-2019. letter. J Am Acad Dermatol. 2020;82:1215-1216. doi:10.1016/j.jaad.2020.03.014
- Yan Y, Chen H, Chen L, et al. Consensus of Chinese experts on protection of skin and mucous membrane barrier for health-care workers fighting against coronavirus disease 2019. Dermatol Ther. 2020;33:e13310. doi:10.1111/dth.13310
- Elston DM. Occupational skin disease among health care workers during the coronavirus (COVID-19) epidemic. J Am Acad Dermatol. 2020;82:1085-1086. doi:10.1016/j.jaad.2020.03.012
- Balato A, Ayala F, Bruze M, et al. European Task Force on Contact Dermatitis statement on coronavirus disease-19 (COVID-19) outbreak and the risk of adverse cutaneous reactions. J Eur Acad Dermatol Venereol. 2020;34:E353-E354. doi:10.1111/jdv.16557
- Hu K, Fan J, Li X, et al. The adverse skin reactions of health care workers using personal protective equipment for COVID-19. Medicine (Baltimore). 2020;99:e20603. doi:10.1097/MD.0000000000020603
- Singh M, Pawar M, Bothra A, et al. Personal protective equipment induced facial dermatoses in healthcare workers managing coronavirus disease 2019. J Eur Acad Dermatol Venereol. 2020;34:E378-E380. doi:10.1111/jdv.16628
- Zhou P, Huang Z, Xiao Y, et al. Protecting Chinese healthcare workers while combating the 2019 novel coronavirus. Infect Control Hosp Epidemiol. 2020;41:745-746. doi:10.1017/ice.2020.60
- Hua W, Zuo Y, Wan R, et al. Short-term skin reactions following use of N95 respirators and medical masks. Contact Dermatitis. 2020;83:115-121. doi:10.1111/cod.13601
- Foo CCI, Goon ATJ, Leow Y-H, et al. Adverse skin reactions to personal protective equipment against severe acute respiratory syndrome—a descriptive study in Singapore. Contact Dermatitis. 2006;55:291-294. doi:10.1111/j.1600-0536.2006.00953.x
- Zuo Y, Hua W, Luo Y, et al. Skin reactions of N95 masks and medial masks among health-care personnel: a self‐report questionnaire survey in China. Contact Dermatitis. 2020;83:145-147. doi:10.1111/cod.13555
- Higgins CL, Palmer AM, Cahill JL, et al. Occupational skin disease among Australian healthcare workers: a retrospective analysis from an occupational dermatology clinic, 1993-2014. Contact Dermatitis. 2016;75:213-222. doi:10.1111/cod.12616
- Donovan J, Skotnicki-Grant S. Allergic contact dermatitis from formaldehyde textile resins in surgical uniforms and nonwoven textile masks. Dermatitis. 2007;18:40-44. doi:10.2310/6620.2007.05003
- Donovan J, Kudla I, Holness LD, et al. Skin reactions following use of N95 facial masks. meeting abstract. Dermatitis. 2007;18:104.
- Aerts O, Dendooven E, Foubert K, et al. Surgical mask dermatitis caused by formaldehyde (releasers) during the COVID-19 pandemic. Contact Dermatitis. 2020;83:172-1173. doi:10.1111/cod.13626
- Fowler JF. Formaldehyde as a textile allergen. Curr Probl Dermatol. 2003;31:156-165. doi:10.1159/000072245
- Schorr WF, Keran E, Plotka E. Formaldehyde allergy: the quantitative analysis of American clothing for free formaldehyde and its relevance in clinical practice. Arch Dermatol. 1974;110:73-76.
- Slodownik D, Williams J, Tate B, et al. Textile allergy—the Melbourne experience. Contact Dermatitis. 2011;65:38-42. doi:10.1111/j.1600-0536.2010.01861.x
- O’Quinn SE, Kennedy CB. Contact dermatitis due to formaldehyde in clothing textiles. JAMA. 1965;194:593-596.
- Technical specification sheet—3M™ Particulate Respirator 8210, N95. Published 2018. 3M website. Accessed July 12, 2021. https://multimedia.3m.com/mws/media/1425070O/3m-particulate-respirator-8210-n95-technical-specifications.pdf
- Bhoyrul B, Lecamwasam K, Wilkinson M, et al. A review of non‐glove personal protective equipment‐related occupational dermatoses reported to EPIDERM between 1993 and 2013. Contact Dermatitis. 2019;80:217-221. doi: 10.1111/cod.13177
- Lyapina M, Kissselova-Yaneva A, Krasteva A, et al. Allergic contact dermatitis from formaldehyde exposure. Journal of IMAB - Annual Proceeding (Scientific Papers). 2012;18:255-262. doi:10.5272/jimab.2012184.255
- Foussereau J, Cavelier C, Selig D. Occupational eczema from para-tertiary-butylphenol formaldehyde resins: a review of the sensitizing resins. Contact Dermatitis. 1976;2:254-258. doi:10.1111/j.1600-0536.1976.tb03043.x
- Frølich KW, Andersen LM, Knutsen A, et al. Phenoxyethanol as a nontoxic substitute for formaldehyde in long-term preservation of human anatomical specimens for dissection and demonstration purposes. Anat Rec. 1984;208:271-278. doi:10.1002/ar.1092080214
- Bolt HM. Experimental toxicology of formaldehyde. J Cancer Res Clin Oncol. 1987;113:305-309. doi:10.1007/BF00397713
- Arts JHE, Rennen MAJ, de Heer C. Inhaled formaldehyde: evaluation of sensory irritation in relation to carcinogenicity. Regul Toxicol Pharmacol. 2006;44:144-160. doi:10.1016/j.yrtph.2005.11.006
- Kim CW, Song JS, Ahn YS, et al. Occupational asthma due to formaldehyde. Yonsei Med J. 2001;42:440-445. doi:10.3349/ymj.2001.42.4.440
- Nordman H, Keskinen H, Tuppurainen M. Formaldehyde asthma—rare or overlooked? J Allergy Clin Immunol. 1985;75(1 pt 1):91-99. doi:10.1016/0091-6749(85)90018-1
- Kantor J. Behavioral considerations and impact on personal protective equipment use: early lessons from the coronavirus (COVID-19) pandemic. J Am Acad Dermatol. 2020;82:1087-1088. doi:10.1016/j.jaad.2020.03.013
- Kwok YLA, Gralton J, McLaws M-L. Face touching: a frequent habit that has implications for hand hygiene. Am J Infect Control. 2015;43:112-114. doi:10.1016/j.ajic.2014.10.015
- Nicas M, Best D. A study quantifying the hand-to-face contact rate and its potential application to predicting respiratory tract infection. J Occup Environ Hyg. 2008;5:347-352. doi:10.1080/15459620802003896
- MacIntyre CR, Chughtai AA. A rapid systematic review of the efficacy of face masks and respirators against coronaviruses and other respiratory transmissible viruses for the community, healthcare workers and sick patients. Int J Nurs Stud. 2020;108:103629. doi:10.1016/j.ijnurstu.2020.103629
- Garcia Godoy LR, Jones AE, Anderson TN, et al. Facial protection for healthcare workers during pandemics: a scoping review. BMJ Glob Health. 2020;5:e002553. doi:10.1136/bmjgh-2020-002553
- Svedman C, Engfeldt M, Malinauskiene L. Textile contact dermatitis: how fabrics can induce ermatitis. Curr Treat Options Allergy. 2019;6:103-111. doi:10.1007/s40521-019-0197-5
- Yang CQ, Wang X, Kang I-S. Ester crosslinking of cotton fabric by polymeric carboxylic acids and citric acid. Textile Res J. 1997;67:334-342. https://doi.org/10.1177/004051759706700505
- Welch CM. Formaldehyde-free durable-press finishes. Rev Prog Coloration Related Top. 1992;22:32-41. https://doi.org/10.1111/j.1478-4408.1992.tb00087.x
- Peng H, Yang CQ, Wang S. Nonformaldehyde durable press finishing of cotton fabrics using the combination of maleic acid and sodium hypophosphite. Carbohydrate Polymers. 2012;87:491-499. doi:10.1016/j.carbpol.2011.08.013
Practice Points
- Prolonged wearing of N95 respirator masks has been associated with causing or complicating a number of facial inflammatory dermatoses.
- Consider the possibility of contact dermatitis secondary to formaldehyde exposure in individuals wearing N95 masks for prolonged periods.
- Information on the chemical components of N95 masks would be useful for clinicians tasked with evaluating patients with facial inflammatory dermatoses.
Phototherapy: Safe and Effective for Challenging Skin Conditions in Older Adults
Identifying safe, effective, and affordable evidence-based dermatologic treatments for older adults can be challenging because of age-related changes in the skin, comorbidities, polypharmacy, mobility issues, and cognitive changes. Phototherapy has been shown to be an effective nonpharmacologic treatment option for multiple challenging dermatologic conditions1-8; however, few studies have specifically examined its effectiveness in older adults. The challenge for older patients with psoriasis and dermatitis is that the conditions can be difficult to control and often require multiple treatment modalities.9,10 Patients with psoriasis also have a higher risk for diabetes, dyslipidemia, and cardiovascular disease compared to other older patients,11,12 which poses treatment challenges and makes nonpharmacologic treatments even more appealing.
Recent studies show that phototherapy can help decrease the use of dermatologic medications. Foerster and colleagues2 found that adults with psoriasis who were treated with phototherapy significantly decreased their use of topical steroids (24.5% fewer patients required steroid creams and 31.1% fewer patients required psoriasis-specific topicals)(P<.01) while their use of non–psoriasis-specific medications did not change. Click and colleagues13 identified a decrease in medication costs, health care utilization, and risk for immunosuppression in patients treated with phototherapy when compared to those treated with biologics and apremilast. Methotrexate is a common dermatologic medication that is highly associated with increased risks in elderly patients because of impaired immune system function and the presence of comorbidities (eg, kidney disease, obesity, diabetes, fatty liver),14 which increase in prevalence with age. Combining phototherapy with methotrexate can substantially decrease the amount of methotrexate needed to achieve disease control,15 thereby decreasing the methotrexate-associated risks. Findings from these studies suggest that a safe, effective, cost-effective, and well-tolerated nonpharmacologic alternative, such as phototherapy, is highly desirable and should be optimized. Unfortunately, most studies that report the effectiveness of phototherapy are in younger populations.
This retrospective study aimed to (1) identify the most common dermatologic conditions treated with phototherapy in older adults, (2) examine the effectiveness and safety of phototherapy in older adults
Methods
Design, Setting, Sample, and Statistical Analysis
The institutional review boards of Kaiser Permanente Washington Health Research Institute, Seattle, and the University of Washington, Seattle, approved this study. It was conducted in a large US multispecialty health care system (Group Health, Seattle, Washington [now Kaiser Permanente Washington]) serving approximately 600,000 patients, using billing records to identify all patients treated with phototherapy between January 1, 2015, and December 31, 2015, all who received narrowband UVB (NB-UVB) phototherapy. All adults 65 years and older who received phototherapy treatment during the 12-month study period were included. Patients were included regardless of comorbidities and other dermatologic treatments to maintain as much uniformity as possible between the present study and 2 prior studies examining phototherapy in older adult populations in the United Kingdom16 and Turkey.17 Demographic and clinical factors were presented using frequencies (percentages) or means and medians as appropriate. Comparisons of dermatologic conditions and clearance levels used a Fisher exact test. The number of phototherapy treatments to clearance and total number of treatments were compared between groups of patients using independent sample t tests.
Phototherapy Protocol
All patients received treatments administered by specially trained phototherapy nurses using a Daavlin UV Series (The Daavlin Company) or an Ultralite unit (Ultralite Enterprises, Inc), both with 48 lamps. All phototherapy nurses had been previously trained to provide treatments based on standardized protocols (Table 1) and to determine the patient’s level of disease clearance using a high to low clearance scale (Table 2). Daavlin’s treatment protocols were built into the software that accompanied the units and were developed based on the American Academy of Dermatology guidelines. The starting dose for an individual patient was determined based on the estimated
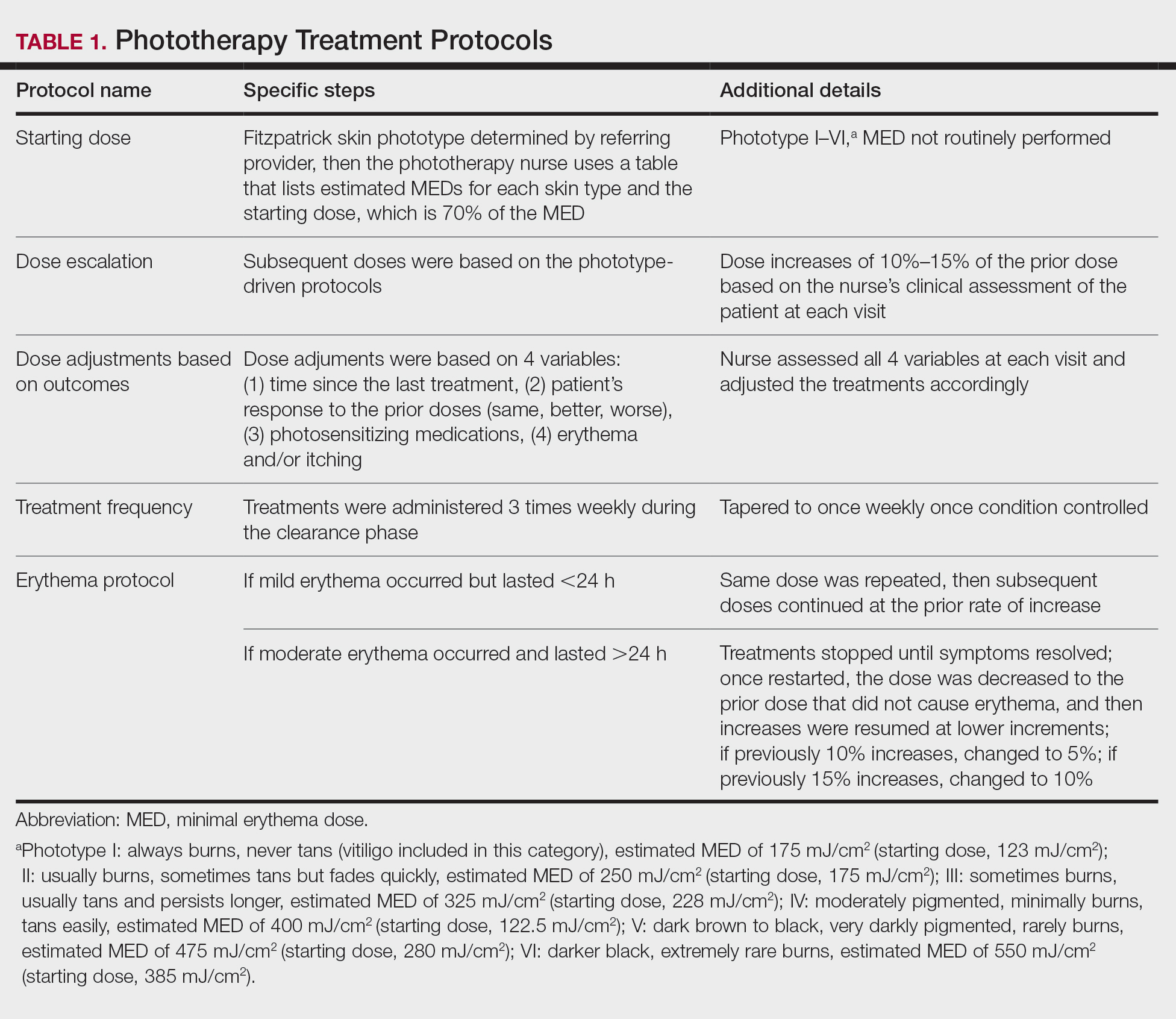
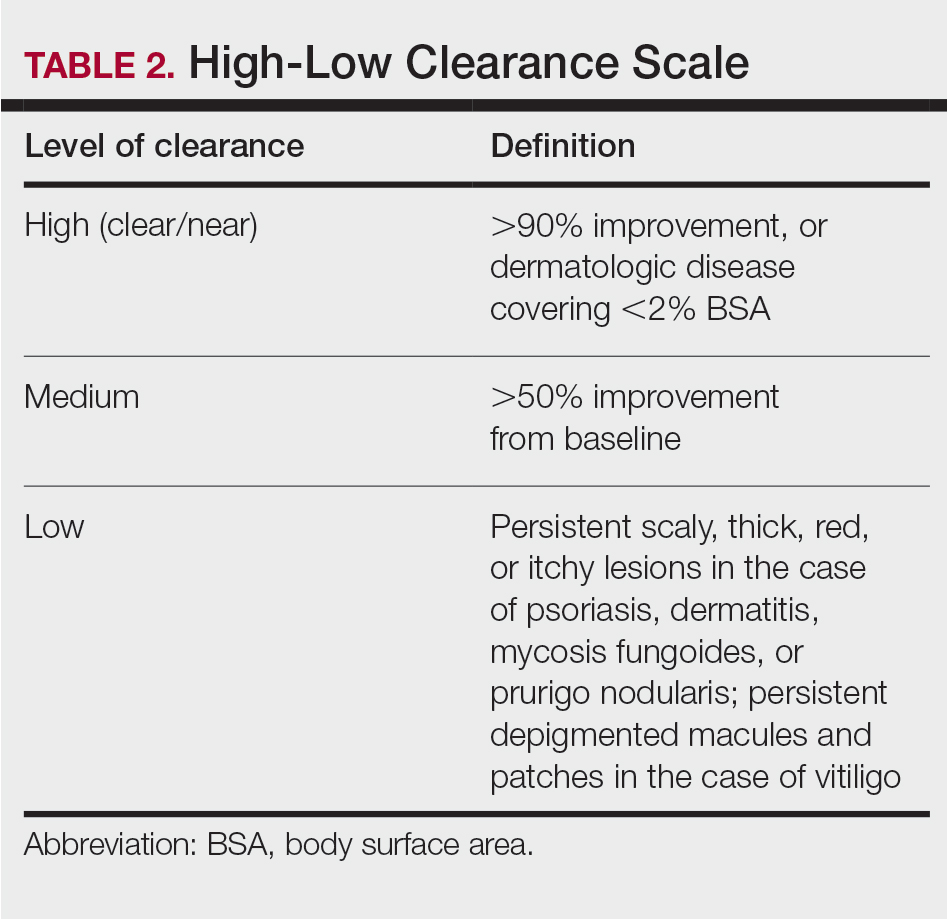
Results
Patients
Billing records identified 229 total patients who received phototherapy in 2015, of whom 52 (22.7%) were at least 65 years old. The median age was 70 years (range, 65–91 years). Twenty-nine (56%) were men and 35 (67%) had previously received phototherapy treatments.
Dermatologic Conditions Treated With Phototherapy
Our primary aim was to identify the most common dermatologic conditions treated with phototherapy in older adults. Psoriasis and dermatitis were the most common conditions treated in the sample (50% [26/52] and 21% [11/52], respectively), with mycosis fungoides being the third most common (10% [5/52]) and vitiligo tied with prurigo nodularis as fourth most common (6% [3/52])(Figure 1).

Effectiveness and Safety of Phototherapy
Our secondary aim was to examine the effectiveness and safety of phototherapy in older adults. Phototherapy was effective in this population, with 50 of 52 patients (96%) achieving a high or medium level of clearance. The degree of clearance for each of the dermatologic conditions is shown in Figure 2. Psoriasis and dermatitis achieved high clearance rates in 81% (21/26) and 82% (9/11) of patients, respectively. Overall, conditions did not have significant differences in clearances rates (Fisher exact test, P=.10). On average, it took patients 33 treatments to achieve medium or high rates of clearance. Psoriasis cleared more quickly, with an average of 30.4 treatments vs 36.1 treatments for other conditions, but the difference was not significant (t test, P=.26). Patients received an average of 98 total phototherapy treatments; the median number of treatments was 81 due to many being on maintenance therapy over several months. There was no relationship between a history of treatment with phototherapy and the total number of treatments needed to achieve clearance (t test, P=.40), but interestingly, those who had a history of phototherapy took approximately 5 more treatments to achieve clearance. The present study found that a slightly larger number of men were being treated for psoriasis (15 men vs 11 women), but there was no significant difference in response rate based on gender.
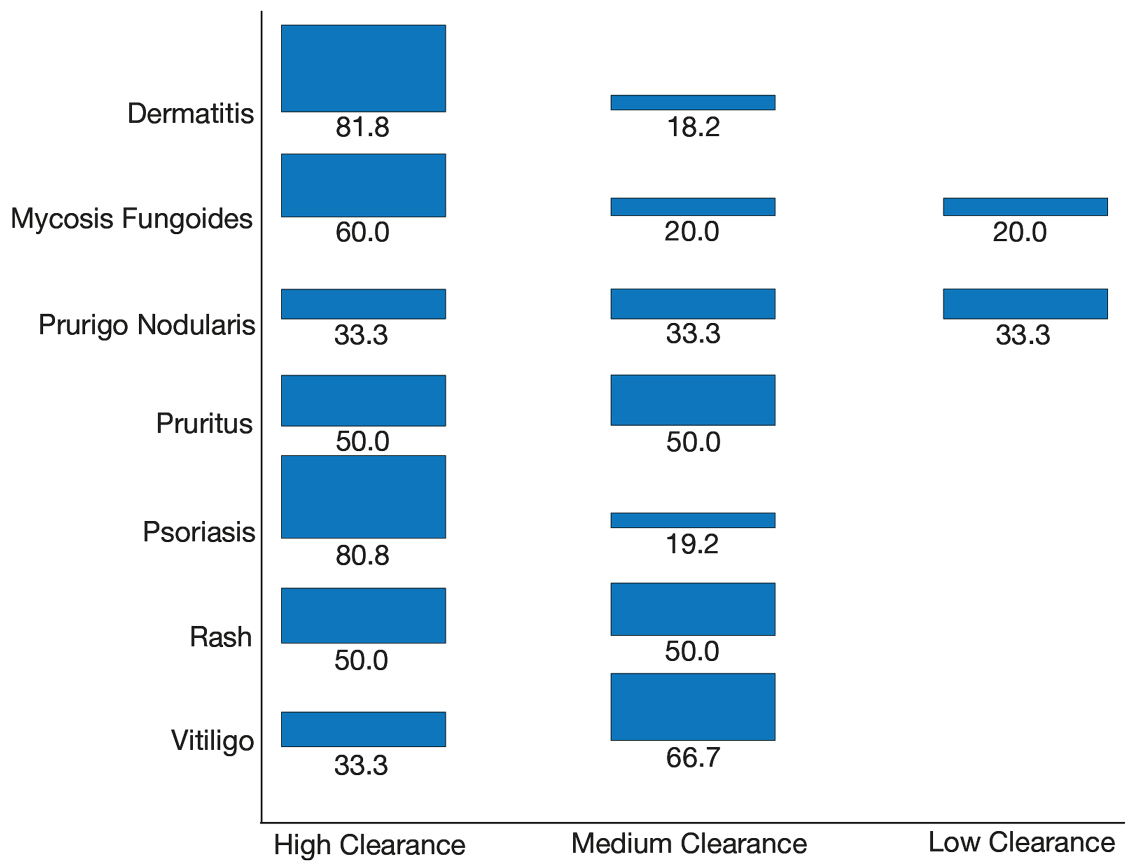
Side effects from phototherapy were minimal; 24 patients (46%) experienced grade 1 (mild) erythema at some point during their treatment course. Thirteen (25%) patients experienced grade 2 erythema, but this was a rare event for most patients. Only 1 (2%) patient experienced grade 3 erythema 1 time. Three patients experienced increased itching (6%). Thirteen (25%) patients had no side effects. None developed severe erythema or blisters, and none discontinued phototherapy because of side effects. Over the course of the study year, we found a high degree of acceptance of phototherapy treatments by older patients: 22 (42%) completed therapy after achieving clearance, 10 (19%) were continuing ongoing treatments (maintenance), and 15 (29%) stopped because of life circumstances (eg, other health issues, moving out of the area). Only 4 (8%) stopped because of a lack of effectiveness, and 1 (2%) patient because the treatments were burdensome.
Comparison of Outcomes
Our third aim was to compare the outcomes with similar studies in the United Kingdom16 and Turkey.17 This study confirmed that phototherapy is being used in older adults (22.7% of this study’s total patients) and is an effective treatment for older patients experiencing a range of challenging inflammatory and proliferative skin diseases similar to studies in the general population. Prior phototherapy studies in elderly patients also found psoriasis to be the most common skin condition treated, with 1 study finding that 51% (19/37) of older phototherapy patients had psoriasis,16 while another reported 58% (37/95) of older phototherapy patients had psoriasis.17 These numbers are similar to those in our study, which showed 50% (26/52) of elderly phototherapy patients had psoriasis. Psoriasis is the main indication for treatment with NB-UVB phototherapy in the general population,19 and because the risk for psoriasis increases with age,20 it is not surprising that all 3 studies found psoriasis to be the most common indication in elderly phototherapy patients. Table 3 provides further details on conditions treated in all 3 studies.
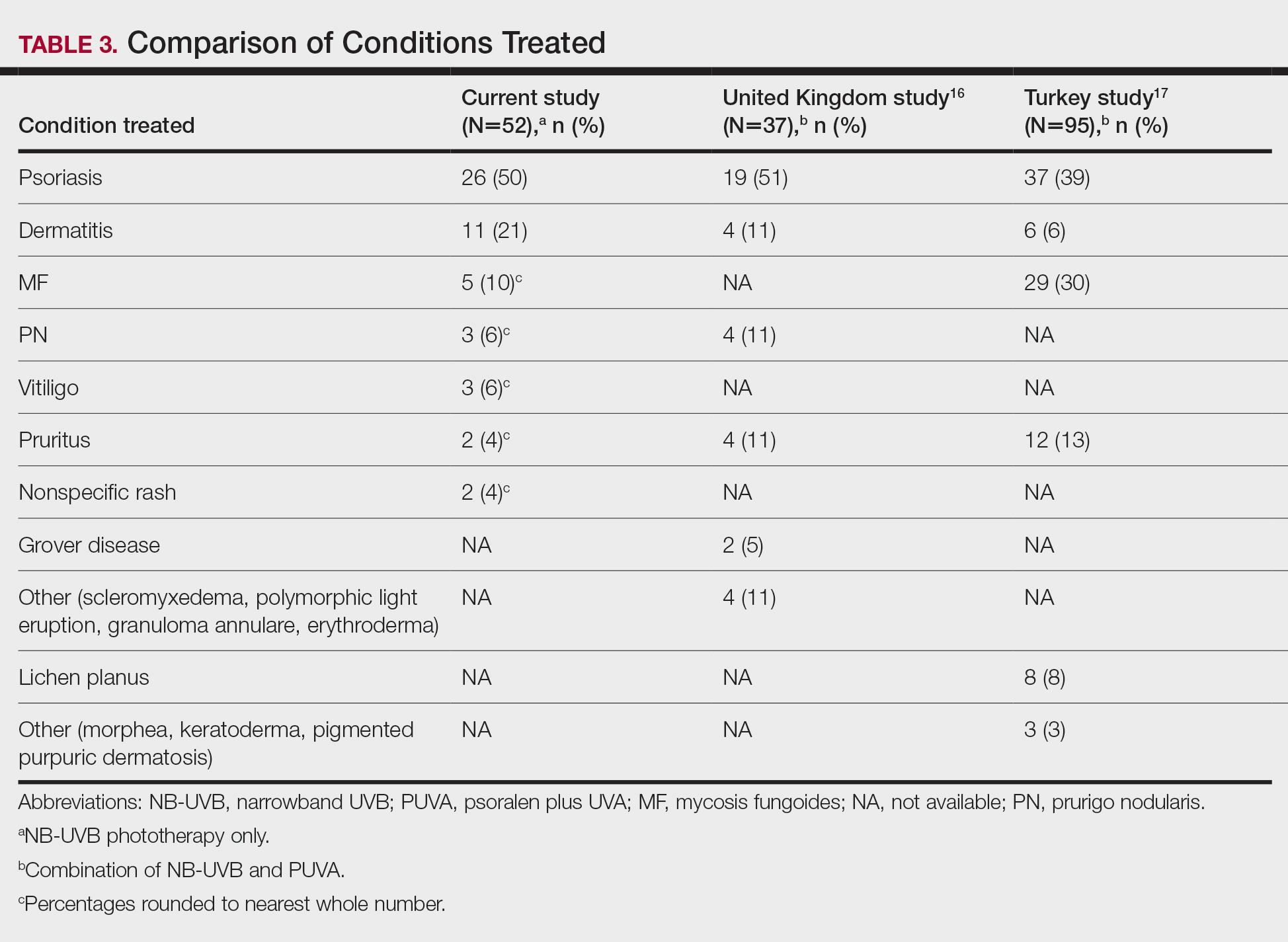
Comment
Our study found that 94% of patients with psoriasis achieved clearance with an average of 30.4 treatments, which is comparable to the reported 91% response rate with an average of 30 treatments in the United Kingdom.16 The other similar study in Turkey17 reported 73.7% of psoriasis patients achieved a 75% or more improvement from baseline with an average of 42 treatments, which may reflect underlying differences in regional skin type. Of note, the scatter chart (Figure 3) shows that several patients in the present study’s analysis are listed as not clear, but many of those patients had low treatment numbers below the mean time to clearance. Thus, the present study’s response rate may have been underestimated.
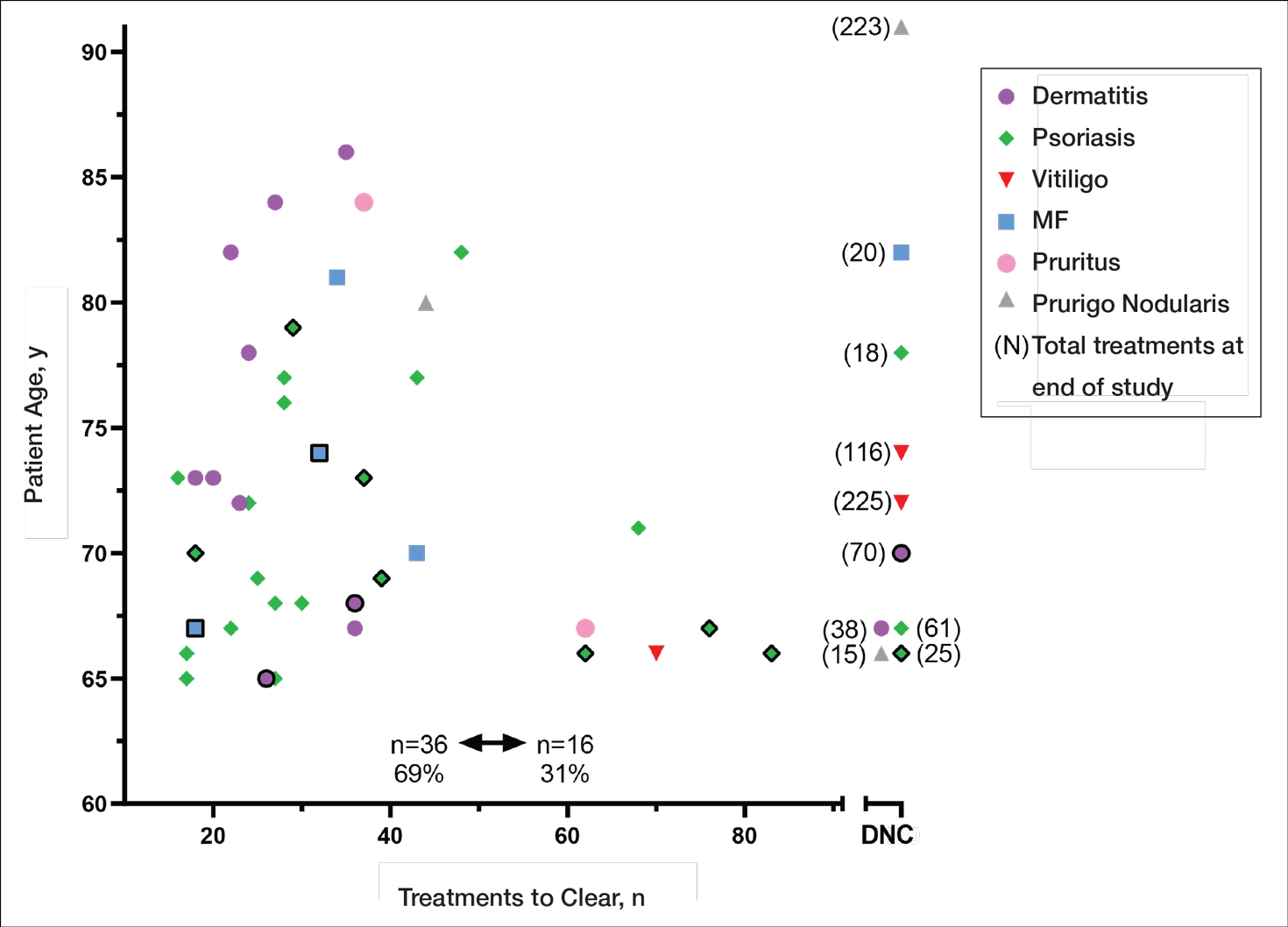
In the general population, studies show that psoriasis treated with standardized phototherapy protocols typically clears with an average of 20.6 treatments.21 The levels of clearance were similar in our study’s older population, but more treatments were required to achieve those results, with an average of 10 more treatments needed (an additional 3.3 weeks). Similar results were found in this sample for dermatitis and mycosis fungoides, indicating comparable clearance rates and levels but a need for more treatments to achieve similar results compared to the general population.
Additionally, in the current study more patients experienced grade 1 (mild) erythema (46%) and grade 2 erythema (25%) at some point in their treatment compared with the United Kingdom16 (1.89%) and Turkey17 (35%) studies, though these side effects did not impact the clearance rate. Interestingly, the current study’s scatter chart (Figure 3) illustrates that this side effect did not seem to increase with aging in this population. If anything, the erythema response was more prevalent in the median or younger patients in the sample. Erythema may have been due to the frequent use of photosensitizing medications in older adults in the United States, some of which typically get discontinued in patients 75 years and older (eg, statins). Other potential causes might include the use of phototype vs minimal erythema dose–driven protocols, the standard utilization of protocols originally designed for psoriasis vs other condition-specific protocols, missed treatments leading to increased sensitivity, or possibly shielding mishaps (eg, not wearing a prescribed face shield). Given the number of potential causes and the possibility of overlapping factors, careful analysis is important. With NB-UVB phototherapy, near-erythemogenic doses are optimal to achieve effective treatments, but this delicate balance may be more problematic for older adults. Future studies are needed to fully determine the factors at play for this population. In the interim, it is important for phototherapy-trained nurses to consider this risk carefully in the older population. They must follow the prescribed protocols that guide them to query patients about their responses to the prior treatment (eg, erythema, tenderness, itching), photosensitizing medications, missed treatments, and placement of shielding, and then adjust the treatment dosing accordingly.
Limitations
This study had several limitations. Although clinical outcomes were recorded prospectively, the analysis was retrospective, unblinded, and not placebo controlled. It was conducted in a single organization (Group Health [now Kaiser Permanente Washington]) but did analyze data from 4 medical centers in different cities with diverse demographics and a variety of nursing staff providing the treatments. Although the vitiligo treatment protocol likely slowed the response rate for those patients with vitiligo, the numbers were small (ie, only 3 of 52 patients), so the researchers chose to include them in the current study. The sample population was relatively small, but when these data are evaluated alongside the studies in the United Kingdom16 and Turkey,17 they show a consistent picture illustrating the effectiveness and safety of phototherapy in the older population. Further epidemiologic studies could be helpful to further describe the usefulness of this modality compared with other treatments for a variety of dermatoses in this age group. Supplementary analysis specifically examining the relationship between the number and type of photosensitizing medications, frequency of erythema, and time to clearance also could be useful.
Conclusion
Older adults with a variety of dermatoses respond well to phototherapy and should have the opportunity to use it, particularly considering the potential for increased complications and costs from other treatment modalities, such as commonly used immunosuppressive pharmaceuticals. However, the current study and the comparison studies indicate that it is important to carefully consider the slower clearance rates and the potential risk for increased erythema in this population and adjust patient education and treatment dosing accordingly.
Unfortunately, many dermatology centers do not offer phototherapy because of infrastructure limitations such as space and specially trained nursing staff. Increasing accessibility of phototherapy for older adults through home treatments may be an alternative, given its effectiveness in the general population.22,23 In addition, home phototherapy may be worth pursuing for the older population considering the challenges they may face with transportation to the clinic setting and their increased risk for serious illness if exposed to infections such as COVID-19. The COVID-19 pandemic has brought to light the need for reliable, safe, and effective treatments that can be utilized in the safety of patients’ homes and should therefore be considered as an option for older adults. Issues such as mobility and cognitive decline could pose some complicating factors, but with the help of a well-trained family member or caregiver, home phototherapy could be a viable option that improves accessibility for older patients. Future research opportunities include further examination of the slower but ultimately equivalent response to phototherapy in the older population, the influence of photosensitizing medications on phototherapy effects, and the impact of phototherapy on utilization of immunosuppressive pharmaceuticals in older adults.
- British Photodermatology Group. An appraisal of narrowband (TL-01) UVB phototherapy. British Photodermatology Group Workshop Report (April 1996). Br J Dermatol. 1997;137:327-330.
Foerster J, Boswell K, West J, et al. Narrowband UVB treatment is highly effective and causes a strong reduction in the use of steroid and other creams in psoriasis patients in clinical practice. PLoS ONE. 2017;12:e0181813. doi:10.1371/journal.pone.0181813 - Fernández-Guarino M, Aboin-Gonzalez S, Barchino L, et al. Treatment of moderate and severe adult chronic atopic dermatitis with narrow-band UVB and the combination of narrow-band UVB/UVA phototherapy. Dermatol Ther. 2015;29:19-23.
- Ryu HH, Choe YS, Jo S, et al. Remission period in psoriasis after multiple cycles of narrowband ultraviolet B phototherapy. J Dermatol. 2014;41:622-627.
Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128:583-593. - Gambichler T, Breuckmann F, Boms S, et al. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
- Martin JA, Laube S, Edwards C, et al. Rate of acute adverse events for narrow-band UVB and psoralen-UVA phototherapy. Photodermatol Photoimmunol Photomed. 2007;23:68-72.
- Mokos ZB, Jovic A, Ceovic R, et al. Therapeutic challenges in the mature patient. Clin Dermatol. 2018;36:128-139.
- Di Lernia V, Goldust M. An overview of the efficacy and safety of systemic treatments for psoriasis in the elderly. Exp Opin Biol Ther. 2018;18:897-903.
- Napolitano M, Balato N, Ayala F, et al. Psoriasis in elderly and non-elderly population: clinical and molecular features. G Ital Dermatol Venereol. 2016;151:587-595.
- Grozdev IS, Van Voorhees AS, Gottlieb AB, et al. Psoriasis in the elderly: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2011;65:537-545.
- Click J, Alabaster A, Postlethwaite D, et al. Effect of availability of at-home phototherapy on the use of systemic medications for psoriasis.
Photodermatol Photoimmunol Photomed. 2017;33:345-346. - Piaserico S, Conti A, Lo Console F, et al.
Efficacy and safety of systemic treatments for psoriasis in elderly. Acta Derm Venereol. 2014;94:293-297. - Soliman A, Nofal E, Nofal A, et al. Combination therapy of methotrexate plus NB-UVB phototherapy is more effective than methotrexate monotherapy in the treatment of chronic plaque psoriasis. J Dermatol Treat. 2015;26:528-534.
- Powell JB, Gach JE. Phototherapy in the elderly. Clin Exp Dermatol. 2015;40:605-610.
- Bulur I, Erdogan HK, Aksu AE, et al. The efficacy and safety of phototherapy in geriatric patients: a retrospective study. An Bras Dermatol. 2018;93:33-38.
- Madigan LM, Al-Jamal M, Hamzavi I. Exploring the gaps in the evidence-based application of narrowband UVB for the treatment of vitiligo. Photodermatol Photoimmunol Photomed. 2016;32:66-80.
- Ibbotson SH. A perspective on the use of NB-UVB phototherapy vs. PUVA photochemotherapy. Front Med (Lausanne). 2018;5:184.
- Bell LM, Sedlack R, Beard CM, et al. Incidence of psoriasis in Rochester, Minn, 1980-1983. Arch Dermatol. 1991;127:1184-1187.
- Totonchy MB, Chiu MW. UV-based therapy. Dermatol Clin. 2014;32:399-413.
- Cameron H, Yule S, Dawe RS, et al. Review of an established UK home phototherapy service 1998-2011: improving access to a cost-effective treatment for chronic skin disease. Public Health. 2014;128:317-324.
- Matthews SW, Simmer M, Williams L, et al. Transition of patients with psoriasis from office-based phototherapy to nurse-supported home phototherapy: a pilot study. JDNA. 2018;10:29-41.
Identifying safe, effective, and affordable evidence-based dermatologic treatments for older adults can be challenging because of age-related changes in the skin, comorbidities, polypharmacy, mobility issues, and cognitive changes. Phototherapy has been shown to be an effective nonpharmacologic treatment option for multiple challenging dermatologic conditions1-8; however, few studies have specifically examined its effectiveness in older adults. The challenge for older patients with psoriasis and dermatitis is that the conditions can be difficult to control and often require multiple treatment modalities.9,10 Patients with psoriasis also have a higher risk for diabetes, dyslipidemia, and cardiovascular disease compared to other older patients,11,12 which poses treatment challenges and makes nonpharmacologic treatments even more appealing.
Recent studies show that phototherapy can help decrease the use of dermatologic medications. Foerster and colleagues2 found that adults with psoriasis who were treated with phototherapy significantly decreased their use of topical steroids (24.5% fewer patients required steroid creams and 31.1% fewer patients required psoriasis-specific topicals)(P<.01) while their use of non–psoriasis-specific medications did not change. Click and colleagues13 identified a decrease in medication costs, health care utilization, and risk for immunosuppression in patients treated with phototherapy when compared to those treated with biologics and apremilast. Methotrexate is a common dermatologic medication that is highly associated with increased risks in elderly patients because of impaired immune system function and the presence of comorbidities (eg, kidney disease, obesity, diabetes, fatty liver),14 which increase in prevalence with age. Combining phototherapy with methotrexate can substantially decrease the amount of methotrexate needed to achieve disease control,15 thereby decreasing the methotrexate-associated risks. Findings from these studies suggest that a safe, effective, cost-effective, and well-tolerated nonpharmacologic alternative, such as phototherapy, is highly desirable and should be optimized. Unfortunately, most studies that report the effectiveness of phototherapy are in younger populations.
This retrospective study aimed to (1) identify the most common dermatologic conditions treated with phototherapy in older adults, (2) examine the effectiveness and safety of phototherapy in older adults
Methods
Design, Setting, Sample, and Statistical Analysis
The institutional review boards of Kaiser Permanente Washington Health Research Institute, Seattle, and the University of Washington, Seattle, approved this study. It was conducted in a large US multispecialty health care system (Group Health, Seattle, Washington [now Kaiser Permanente Washington]) serving approximately 600,000 patients, using billing records to identify all patients treated with phototherapy between January 1, 2015, and December 31, 2015, all who received narrowband UVB (NB-UVB) phototherapy. All adults 65 years and older who received phototherapy treatment during the 12-month study period were included. Patients were included regardless of comorbidities and other dermatologic treatments to maintain as much uniformity as possible between the present study and 2 prior studies examining phototherapy in older adult populations in the United Kingdom16 and Turkey.17 Demographic and clinical factors were presented using frequencies (percentages) or means and medians as appropriate. Comparisons of dermatologic conditions and clearance levels used a Fisher exact test. The number of phototherapy treatments to clearance and total number of treatments were compared between groups of patients using independent sample t tests.
Phototherapy Protocol
All patients received treatments administered by specially trained phototherapy nurses using a Daavlin UV Series (The Daavlin Company) or an Ultralite unit (Ultralite Enterprises, Inc), both with 48 lamps. All phototherapy nurses had been previously trained to provide treatments based on standardized protocols (Table 1) and to determine the patient’s level of disease clearance using a high to low clearance scale (Table 2). Daavlin’s treatment protocols were built into the software that accompanied the units and were developed based on the American Academy of Dermatology guidelines. The starting dose for an individual patient was determined based on the estimated


Results
Patients
Billing records identified 229 total patients who received phototherapy in 2015, of whom 52 (22.7%) were at least 65 years old. The median age was 70 years (range, 65–91 years). Twenty-nine (56%) were men and 35 (67%) had previously received phototherapy treatments.
Dermatologic Conditions Treated With Phototherapy
Our primary aim was to identify the most common dermatologic conditions treated with phototherapy in older adults. Psoriasis and dermatitis were the most common conditions treated in the sample (50% [26/52] and 21% [11/52], respectively), with mycosis fungoides being the third most common (10% [5/52]) and vitiligo tied with prurigo nodularis as fourth most common (6% [3/52])(Figure 1).

Effectiveness and Safety of Phototherapy
Our secondary aim was to examine the effectiveness and safety of phototherapy in older adults. Phototherapy was effective in this population, with 50 of 52 patients (96%) achieving a high or medium level of clearance. The degree of clearance for each of the dermatologic conditions is shown in Figure 2. Psoriasis and dermatitis achieved high clearance rates in 81% (21/26) and 82% (9/11) of patients, respectively. Overall, conditions did not have significant differences in clearances rates (Fisher exact test, P=.10). On average, it took patients 33 treatments to achieve medium or high rates of clearance. Psoriasis cleared more quickly, with an average of 30.4 treatments vs 36.1 treatments for other conditions, but the difference was not significant (t test, P=.26). Patients received an average of 98 total phototherapy treatments; the median number of treatments was 81 due to many being on maintenance therapy over several months. There was no relationship between a history of treatment with phototherapy and the total number of treatments needed to achieve clearance (t test, P=.40), but interestingly, those who had a history of phototherapy took approximately 5 more treatments to achieve clearance. The present study found that a slightly larger number of men were being treated for psoriasis (15 men vs 11 women), but there was no significant difference in response rate based on gender.

Side effects from phototherapy were minimal; 24 patients (46%) experienced grade 1 (mild) erythema at some point during their treatment course. Thirteen (25%) patients experienced grade 2 erythema, but this was a rare event for most patients. Only 1 (2%) patient experienced grade 3 erythema 1 time. Three patients experienced increased itching (6%). Thirteen (25%) patients had no side effects. None developed severe erythema or blisters, and none discontinued phototherapy because of side effects. Over the course of the study year, we found a high degree of acceptance of phototherapy treatments by older patients: 22 (42%) completed therapy after achieving clearance, 10 (19%) were continuing ongoing treatments (maintenance), and 15 (29%) stopped because of life circumstances (eg, other health issues, moving out of the area). Only 4 (8%) stopped because of a lack of effectiveness, and 1 (2%) patient because the treatments were burdensome.
Comparison of Outcomes
Our third aim was to compare the outcomes with similar studies in the United Kingdom16 and Turkey.17 This study confirmed that phototherapy is being used in older adults (22.7% of this study’s total patients) and is an effective treatment for older patients experiencing a range of challenging inflammatory and proliferative skin diseases similar to studies in the general population. Prior phototherapy studies in elderly patients also found psoriasis to be the most common skin condition treated, with 1 study finding that 51% (19/37) of older phototherapy patients had psoriasis,16 while another reported 58% (37/95) of older phototherapy patients had psoriasis.17 These numbers are similar to those in our study, which showed 50% (26/52) of elderly phototherapy patients had psoriasis. Psoriasis is the main indication for treatment with NB-UVB phototherapy in the general population,19 and because the risk for psoriasis increases with age,20 it is not surprising that all 3 studies found psoriasis to be the most common indication in elderly phototherapy patients. Table 3 provides further details on conditions treated in all 3 studies.

Comment
Our study found that 94% of patients with psoriasis achieved clearance with an average of 30.4 treatments, which is comparable to the reported 91% response rate with an average of 30 treatments in the United Kingdom.16 The other similar study in Turkey17 reported 73.7% of psoriasis patients achieved a 75% or more improvement from baseline with an average of 42 treatments, which may reflect underlying differences in regional skin type. Of note, the scatter chart (Figure 3) shows that several patients in the present study’s analysis are listed as not clear, but many of those patients had low treatment numbers below the mean time to clearance. Thus, the present study’s response rate may have been underestimated.

In the general population, studies show that psoriasis treated with standardized phototherapy protocols typically clears with an average of 20.6 treatments.21 The levels of clearance were similar in our study’s older population, but more treatments were required to achieve those results, with an average of 10 more treatments needed (an additional 3.3 weeks). Similar results were found in this sample for dermatitis and mycosis fungoides, indicating comparable clearance rates and levels but a need for more treatments to achieve similar results compared to the general population.
Additionally, in the current study more patients experienced grade 1 (mild) erythema (46%) and grade 2 erythema (25%) at some point in their treatment compared with the United Kingdom16 (1.89%) and Turkey17 (35%) studies, though these side effects did not impact the clearance rate. Interestingly, the current study’s scatter chart (Figure 3) illustrates that this side effect did not seem to increase with aging in this population. If anything, the erythema response was more prevalent in the median or younger patients in the sample. Erythema may have been due to the frequent use of photosensitizing medications in older adults in the United States, some of which typically get discontinued in patients 75 years and older (eg, statins). Other potential causes might include the use of phototype vs minimal erythema dose–driven protocols, the standard utilization of protocols originally designed for psoriasis vs other condition-specific protocols, missed treatments leading to increased sensitivity, or possibly shielding mishaps (eg, not wearing a prescribed face shield). Given the number of potential causes and the possibility of overlapping factors, careful analysis is important. With NB-UVB phototherapy, near-erythemogenic doses are optimal to achieve effective treatments, but this delicate balance may be more problematic for older adults. Future studies are needed to fully determine the factors at play for this population. In the interim, it is important for phototherapy-trained nurses to consider this risk carefully in the older population. They must follow the prescribed protocols that guide them to query patients about their responses to the prior treatment (eg, erythema, tenderness, itching), photosensitizing medications, missed treatments, and placement of shielding, and then adjust the treatment dosing accordingly.
Limitations
This study had several limitations. Although clinical outcomes were recorded prospectively, the analysis was retrospective, unblinded, and not placebo controlled. It was conducted in a single organization (Group Health [now Kaiser Permanente Washington]) but did analyze data from 4 medical centers in different cities with diverse demographics and a variety of nursing staff providing the treatments. Although the vitiligo treatment protocol likely slowed the response rate for those patients with vitiligo, the numbers were small (ie, only 3 of 52 patients), so the researchers chose to include them in the current study. The sample population was relatively small, but when these data are evaluated alongside the studies in the United Kingdom16 and Turkey,17 they show a consistent picture illustrating the effectiveness and safety of phototherapy in the older population. Further epidemiologic studies could be helpful to further describe the usefulness of this modality compared with other treatments for a variety of dermatoses in this age group. Supplementary analysis specifically examining the relationship between the number and type of photosensitizing medications, frequency of erythema, and time to clearance also could be useful.
Conclusion
Older adults with a variety of dermatoses respond well to phototherapy and should have the opportunity to use it, particularly considering the potential for increased complications and costs from other treatment modalities, such as commonly used immunosuppressive pharmaceuticals. However, the current study and the comparison studies indicate that it is important to carefully consider the slower clearance rates and the potential risk for increased erythema in this population and adjust patient education and treatment dosing accordingly.
Unfortunately, many dermatology centers do not offer phototherapy because of infrastructure limitations such as space and specially trained nursing staff. Increasing accessibility of phototherapy for older adults through home treatments may be an alternative, given its effectiveness in the general population.22,23 In addition, home phototherapy may be worth pursuing for the older population considering the challenges they may face with transportation to the clinic setting and their increased risk for serious illness if exposed to infections such as COVID-19. The COVID-19 pandemic has brought to light the need for reliable, safe, and effective treatments that can be utilized in the safety of patients’ homes and should therefore be considered as an option for older adults. Issues such as mobility and cognitive decline could pose some complicating factors, but with the help of a well-trained family member or caregiver, home phototherapy could be a viable option that improves accessibility for older patients. Future research opportunities include further examination of the slower but ultimately equivalent response to phototherapy in the older population, the influence of photosensitizing medications on phototherapy effects, and the impact of phototherapy on utilization of immunosuppressive pharmaceuticals in older adults.
Identifying safe, effective, and affordable evidence-based dermatologic treatments for older adults can be challenging because of age-related changes in the skin, comorbidities, polypharmacy, mobility issues, and cognitive changes. Phototherapy has been shown to be an effective nonpharmacologic treatment option for multiple challenging dermatologic conditions1-8; however, few studies have specifically examined its effectiveness in older adults. The challenge for older patients with psoriasis and dermatitis is that the conditions can be difficult to control and often require multiple treatment modalities.9,10 Patients with psoriasis also have a higher risk for diabetes, dyslipidemia, and cardiovascular disease compared to other older patients,11,12 which poses treatment challenges and makes nonpharmacologic treatments even more appealing.
Recent studies show that phototherapy can help decrease the use of dermatologic medications. Foerster and colleagues2 found that adults with psoriasis who were treated with phototherapy significantly decreased their use of topical steroids (24.5% fewer patients required steroid creams and 31.1% fewer patients required psoriasis-specific topicals)(P<.01) while their use of non–psoriasis-specific medications did not change. Click and colleagues13 identified a decrease in medication costs, health care utilization, and risk for immunosuppression in patients treated with phototherapy when compared to those treated with biologics and apremilast. Methotrexate is a common dermatologic medication that is highly associated with increased risks in elderly patients because of impaired immune system function and the presence of comorbidities (eg, kidney disease, obesity, diabetes, fatty liver),14 which increase in prevalence with age. Combining phototherapy with methotrexate can substantially decrease the amount of methotrexate needed to achieve disease control,15 thereby decreasing the methotrexate-associated risks. Findings from these studies suggest that a safe, effective, cost-effective, and well-tolerated nonpharmacologic alternative, such as phototherapy, is highly desirable and should be optimized. Unfortunately, most studies that report the effectiveness of phototherapy are in younger populations.
This retrospective study aimed to (1) identify the most common dermatologic conditions treated with phototherapy in older adults, (2) examine the effectiveness and safety of phototherapy in older adults
Methods
Design, Setting, Sample, and Statistical Analysis
The institutional review boards of Kaiser Permanente Washington Health Research Institute, Seattle, and the University of Washington, Seattle, approved this study. It was conducted in a large US multispecialty health care system (Group Health, Seattle, Washington [now Kaiser Permanente Washington]) serving approximately 600,000 patients, using billing records to identify all patients treated with phototherapy between January 1, 2015, and December 31, 2015, all who received narrowband UVB (NB-UVB) phototherapy. All adults 65 years and older who received phototherapy treatment during the 12-month study period were included. Patients were included regardless of comorbidities and other dermatologic treatments to maintain as much uniformity as possible between the present study and 2 prior studies examining phototherapy in older adult populations in the United Kingdom16 and Turkey.17 Demographic and clinical factors were presented using frequencies (percentages) or means and medians as appropriate. Comparisons of dermatologic conditions and clearance levels used a Fisher exact test. The number of phototherapy treatments to clearance and total number of treatments were compared between groups of patients using independent sample t tests.
Phototherapy Protocol
All patients received treatments administered by specially trained phototherapy nurses using a Daavlin UV Series (The Daavlin Company) or an Ultralite unit (Ultralite Enterprises, Inc), both with 48 lamps. All phototherapy nurses had been previously trained to provide treatments based on standardized protocols (Table 1) and to determine the patient’s level of disease clearance using a high to low clearance scale (Table 2). Daavlin’s treatment protocols were built into the software that accompanied the units and were developed based on the American Academy of Dermatology guidelines. The starting dose for an individual patient was determined based on the estimated


Results
Patients
Billing records identified 229 total patients who received phototherapy in 2015, of whom 52 (22.7%) were at least 65 years old. The median age was 70 years (range, 65–91 years). Twenty-nine (56%) were men and 35 (67%) had previously received phototherapy treatments.
Dermatologic Conditions Treated With Phototherapy
Our primary aim was to identify the most common dermatologic conditions treated with phototherapy in older adults. Psoriasis and dermatitis were the most common conditions treated in the sample (50% [26/52] and 21% [11/52], respectively), with mycosis fungoides being the third most common (10% [5/52]) and vitiligo tied with prurigo nodularis as fourth most common (6% [3/52])(Figure 1).

Effectiveness and Safety of Phototherapy
Our secondary aim was to examine the effectiveness and safety of phototherapy in older adults. Phototherapy was effective in this population, with 50 of 52 patients (96%) achieving a high or medium level of clearance. The degree of clearance for each of the dermatologic conditions is shown in Figure 2. Psoriasis and dermatitis achieved high clearance rates in 81% (21/26) and 82% (9/11) of patients, respectively. Overall, conditions did not have significant differences in clearances rates (Fisher exact test, P=.10). On average, it took patients 33 treatments to achieve medium or high rates of clearance. Psoriasis cleared more quickly, with an average of 30.4 treatments vs 36.1 treatments for other conditions, but the difference was not significant (t test, P=.26). Patients received an average of 98 total phototherapy treatments; the median number of treatments was 81 due to many being on maintenance therapy over several months. There was no relationship between a history of treatment with phototherapy and the total number of treatments needed to achieve clearance (t test, P=.40), but interestingly, those who had a history of phototherapy took approximately 5 more treatments to achieve clearance. The present study found that a slightly larger number of men were being treated for psoriasis (15 men vs 11 women), but there was no significant difference in response rate based on gender.

Side effects from phototherapy were minimal; 24 patients (46%) experienced grade 1 (mild) erythema at some point during their treatment course. Thirteen (25%) patients experienced grade 2 erythema, but this was a rare event for most patients. Only 1 (2%) patient experienced grade 3 erythema 1 time. Three patients experienced increased itching (6%). Thirteen (25%) patients had no side effects. None developed severe erythema or blisters, and none discontinued phototherapy because of side effects. Over the course of the study year, we found a high degree of acceptance of phototherapy treatments by older patients: 22 (42%) completed therapy after achieving clearance, 10 (19%) were continuing ongoing treatments (maintenance), and 15 (29%) stopped because of life circumstances (eg, other health issues, moving out of the area). Only 4 (8%) stopped because of a lack of effectiveness, and 1 (2%) patient because the treatments were burdensome.
Comparison of Outcomes
Our third aim was to compare the outcomes with similar studies in the United Kingdom16 and Turkey.17 This study confirmed that phototherapy is being used in older adults (22.7% of this study’s total patients) and is an effective treatment for older patients experiencing a range of challenging inflammatory and proliferative skin diseases similar to studies in the general population. Prior phototherapy studies in elderly patients also found psoriasis to be the most common skin condition treated, with 1 study finding that 51% (19/37) of older phototherapy patients had psoriasis,16 while another reported 58% (37/95) of older phototherapy patients had psoriasis.17 These numbers are similar to those in our study, which showed 50% (26/52) of elderly phototherapy patients had psoriasis. Psoriasis is the main indication for treatment with NB-UVB phototherapy in the general population,19 and because the risk for psoriasis increases with age,20 it is not surprising that all 3 studies found psoriasis to be the most common indication in elderly phototherapy patients. Table 3 provides further details on conditions treated in all 3 studies.

Comment
Our study found that 94% of patients with psoriasis achieved clearance with an average of 30.4 treatments, which is comparable to the reported 91% response rate with an average of 30 treatments in the United Kingdom.16 The other similar study in Turkey17 reported 73.7% of psoriasis patients achieved a 75% or more improvement from baseline with an average of 42 treatments, which may reflect underlying differences in regional skin type. Of note, the scatter chart (Figure 3) shows that several patients in the present study’s analysis are listed as not clear, but many of those patients had low treatment numbers below the mean time to clearance. Thus, the present study’s response rate may have been underestimated.

In the general population, studies show that psoriasis treated with standardized phototherapy protocols typically clears with an average of 20.6 treatments.21 The levels of clearance were similar in our study’s older population, but more treatments were required to achieve those results, with an average of 10 more treatments needed (an additional 3.3 weeks). Similar results were found in this sample for dermatitis and mycosis fungoides, indicating comparable clearance rates and levels but a need for more treatments to achieve similar results compared to the general population.
Additionally, in the current study more patients experienced grade 1 (mild) erythema (46%) and grade 2 erythema (25%) at some point in their treatment compared with the United Kingdom16 (1.89%) and Turkey17 (35%) studies, though these side effects did not impact the clearance rate. Interestingly, the current study’s scatter chart (Figure 3) illustrates that this side effect did not seem to increase with aging in this population. If anything, the erythema response was more prevalent in the median or younger patients in the sample. Erythema may have been due to the frequent use of photosensitizing medications in older adults in the United States, some of which typically get discontinued in patients 75 years and older (eg, statins). Other potential causes might include the use of phototype vs minimal erythema dose–driven protocols, the standard utilization of protocols originally designed for psoriasis vs other condition-specific protocols, missed treatments leading to increased sensitivity, or possibly shielding mishaps (eg, not wearing a prescribed face shield). Given the number of potential causes and the possibility of overlapping factors, careful analysis is important. With NB-UVB phototherapy, near-erythemogenic doses are optimal to achieve effective treatments, but this delicate balance may be more problematic for older adults. Future studies are needed to fully determine the factors at play for this population. In the interim, it is important for phototherapy-trained nurses to consider this risk carefully in the older population. They must follow the prescribed protocols that guide them to query patients about their responses to the prior treatment (eg, erythema, tenderness, itching), photosensitizing medications, missed treatments, and placement of shielding, and then adjust the treatment dosing accordingly.
Limitations
This study had several limitations. Although clinical outcomes were recorded prospectively, the analysis was retrospective, unblinded, and not placebo controlled. It was conducted in a single organization (Group Health [now Kaiser Permanente Washington]) but did analyze data from 4 medical centers in different cities with diverse demographics and a variety of nursing staff providing the treatments. Although the vitiligo treatment protocol likely slowed the response rate for those patients with vitiligo, the numbers were small (ie, only 3 of 52 patients), so the researchers chose to include them in the current study. The sample population was relatively small, but when these data are evaluated alongside the studies in the United Kingdom16 and Turkey,17 they show a consistent picture illustrating the effectiveness and safety of phototherapy in the older population. Further epidemiologic studies could be helpful to further describe the usefulness of this modality compared with other treatments for a variety of dermatoses in this age group. Supplementary analysis specifically examining the relationship between the number and type of photosensitizing medications, frequency of erythema, and time to clearance also could be useful.
Conclusion
Older adults with a variety of dermatoses respond well to phototherapy and should have the opportunity to use it, particularly considering the potential for increased complications and costs from other treatment modalities, such as commonly used immunosuppressive pharmaceuticals. However, the current study and the comparison studies indicate that it is important to carefully consider the slower clearance rates and the potential risk for increased erythema in this population and adjust patient education and treatment dosing accordingly.
Unfortunately, many dermatology centers do not offer phototherapy because of infrastructure limitations such as space and specially trained nursing staff. Increasing accessibility of phototherapy for older adults through home treatments may be an alternative, given its effectiveness in the general population.22,23 In addition, home phototherapy may be worth pursuing for the older population considering the challenges they may face with transportation to the clinic setting and their increased risk for serious illness if exposed to infections such as COVID-19. The COVID-19 pandemic has brought to light the need for reliable, safe, and effective treatments that can be utilized in the safety of patients’ homes and should therefore be considered as an option for older adults. Issues such as mobility and cognitive decline could pose some complicating factors, but with the help of a well-trained family member or caregiver, home phototherapy could be a viable option that improves accessibility for older patients. Future research opportunities include further examination of the slower but ultimately equivalent response to phototherapy in the older population, the influence of photosensitizing medications on phototherapy effects, and the impact of phototherapy on utilization of immunosuppressive pharmaceuticals in older adults.
- British Photodermatology Group. An appraisal of narrowband (TL-01) UVB phototherapy. British Photodermatology Group Workshop Report (April 1996). Br J Dermatol. 1997;137:327-330.
Foerster J, Boswell K, West J, et al. Narrowband UVB treatment is highly effective and causes a strong reduction in the use of steroid and other creams in psoriasis patients in clinical practice. PLoS ONE. 2017;12:e0181813. doi:10.1371/journal.pone.0181813 - Fernández-Guarino M, Aboin-Gonzalez S, Barchino L, et al. Treatment of moderate and severe adult chronic atopic dermatitis with narrow-band UVB and the combination of narrow-band UVB/UVA phototherapy. Dermatol Ther. 2015;29:19-23.
- Ryu HH, Choe YS, Jo S, et al. Remission period in psoriasis after multiple cycles of narrowband ultraviolet B phototherapy. J Dermatol. 2014;41:622-627.
Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128:583-593. - Gambichler T, Breuckmann F, Boms S, et al. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
- Martin JA, Laube S, Edwards C, et al. Rate of acute adverse events for narrow-band UVB and psoralen-UVA phototherapy. Photodermatol Photoimmunol Photomed. 2007;23:68-72.
- Mokos ZB, Jovic A, Ceovic R, et al. Therapeutic challenges in the mature patient. Clin Dermatol. 2018;36:128-139.
- Di Lernia V, Goldust M. An overview of the efficacy and safety of systemic treatments for psoriasis in the elderly. Exp Opin Biol Ther. 2018;18:897-903.
- Napolitano M, Balato N, Ayala F, et al. Psoriasis in elderly and non-elderly population: clinical and molecular features. G Ital Dermatol Venereol. 2016;151:587-595.
- Grozdev IS, Van Voorhees AS, Gottlieb AB, et al. Psoriasis in the elderly: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2011;65:537-545.
- Click J, Alabaster A, Postlethwaite D, et al. Effect of availability of at-home phototherapy on the use of systemic medications for psoriasis.
Photodermatol Photoimmunol Photomed. 2017;33:345-346. - Piaserico S, Conti A, Lo Console F, et al.
Efficacy and safety of systemic treatments for psoriasis in elderly. Acta Derm Venereol. 2014;94:293-297. - Soliman A, Nofal E, Nofal A, et al. Combination therapy of methotrexate plus NB-UVB phototherapy is more effective than methotrexate monotherapy in the treatment of chronic plaque psoriasis. J Dermatol Treat. 2015;26:528-534.
- Powell JB, Gach JE. Phototherapy in the elderly. Clin Exp Dermatol. 2015;40:605-610.
- Bulur I, Erdogan HK, Aksu AE, et al. The efficacy and safety of phototherapy in geriatric patients: a retrospective study. An Bras Dermatol. 2018;93:33-38.
- Madigan LM, Al-Jamal M, Hamzavi I. Exploring the gaps in the evidence-based application of narrowband UVB for the treatment of vitiligo. Photodermatol Photoimmunol Photomed. 2016;32:66-80.
- Ibbotson SH. A perspective on the use of NB-UVB phototherapy vs. PUVA photochemotherapy. Front Med (Lausanne). 2018;5:184.
- Bell LM, Sedlack R, Beard CM, et al. Incidence of psoriasis in Rochester, Minn, 1980-1983. Arch Dermatol. 1991;127:1184-1187.
- Totonchy MB, Chiu MW. UV-based therapy. Dermatol Clin. 2014;32:399-413.
- Cameron H, Yule S, Dawe RS, et al. Review of an established UK home phototherapy service 1998-2011: improving access to a cost-effective treatment for chronic skin disease. Public Health. 2014;128:317-324.
- Matthews SW, Simmer M, Williams L, et al. Transition of patients with psoriasis from office-based phototherapy to nurse-supported home phototherapy: a pilot study. JDNA. 2018;10:29-41.
- British Photodermatology Group. An appraisal of narrowband (TL-01) UVB phototherapy. British Photodermatology Group Workshop Report (April 1996). Br J Dermatol. 1997;137:327-330.
Foerster J, Boswell K, West J, et al. Narrowband UVB treatment is highly effective and causes a strong reduction in the use of steroid and other creams in psoriasis patients in clinical practice. PLoS ONE. 2017;12:e0181813. doi:10.1371/journal.pone.0181813 - Fernández-Guarino M, Aboin-Gonzalez S, Barchino L, et al. Treatment of moderate and severe adult chronic atopic dermatitis with narrow-band UVB and the combination of narrow-band UVB/UVA phototherapy. Dermatol Ther. 2015;29:19-23.
- Ryu HH, Choe YS, Jo S, et al. Remission period in psoriasis after multiple cycles of narrowband ultraviolet B phototherapy. J Dermatol. 2014;41:622-627.
Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128:583-593. - Gambichler T, Breuckmann F, Boms S, et al. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
- Martin JA, Laube S, Edwards C, et al. Rate of acute adverse events for narrow-band UVB and psoralen-UVA phototherapy. Photodermatol Photoimmunol Photomed. 2007;23:68-72.
- Mokos ZB, Jovic A, Ceovic R, et al. Therapeutic challenges in the mature patient. Clin Dermatol. 2018;36:128-139.
- Di Lernia V, Goldust M. An overview of the efficacy and safety of systemic treatments for psoriasis in the elderly. Exp Opin Biol Ther. 2018;18:897-903.
- Napolitano M, Balato N, Ayala F, et al. Psoriasis in elderly and non-elderly population: clinical and molecular features. G Ital Dermatol Venereol. 2016;151:587-595.
- Grozdev IS, Van Voorhees AS, Gottlieb AB, et al. Psoriasis in the elderly: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2011;65:537-545.
- Click J, Alabaster A, Postlethwaite D, et al. Effect of availability of at-home phototherapy on the use of systemic medications for psoriasis.
Photodermatol Photoimmunol Photomed. 2017;33:345-346. - Piaserico S, Conti A, Lo Console F, et al.
Efficacy and safety of systemic treatments for psoriasis in elderly. Acta Derm Venereol. 2014;94:293-297. - Soliman A, Nofal E, Nofal A, et al. Combination therapy of methotrexate plus NB-UVB phototherapy is more effective than methotrexate monotherapy in the treatment of chronic plaque psoriasis. J Dermatol Treat. 2015;26:528-534.
- Powell JB, Gach JE. Phototherapy in the elderly. Clin Exp Dermatol. 2015;40:605-610.
- Bulur I, Erdogan HK, Aksu AE, et al. The efficacy and safety of phototherapy in geriatric patients: a retrospective study. An Bras Dermatol. 2018;93:33-38.
- Madigan LM, Al-Jamal M, Hamzavi I. Exploring the gaps in the evidence-based application of narrowband UVB for the treatment of vitiligo. Photodermatol Photoimmunol Photomed. 2016;32:66-80.
- Ibbotson SH. A perspective on the use of NB-UVB phototherapy vs. PUVA photochemotherapy. Front Med (Lausanne). 2018;5:184.
- Bell LM, Sedlack R, Beard CM, et al. Incidence of psoriasis in Rochester, Minn, 1980-1983. Arch Dermatol. 1991;127:1184-1187.
- Totonchy MB, Chiu MW. UV-based therapy. Dermatol Clin. 2014;32:399-413.
- Cameron H, Yule S, Dawe RS, et al. Review of an established UK home phototherapy service 1998-2011: improving access to a cost-effective treatment for chronic skin disease. Public Health. 2014;128:317-324.
- Matthews SW, Simmer M, Williams L, et al. Transition of patients with psoriasis from office-based phototherapy to nurse-supported home phototherapy: a pilot study. JDNA. 2018;10:29-41.
Practice Points
- With appropriate nursing care, phototherapy can be safe and effective for a variety of conditions in elderly patients.
- Compared to younger patients, elderly patients may need more sessions to achieve comparable clearance rates.
- The increased prevalence of photosensitizing medications in the elderly population will require careful adjustments in dosing.
Face mask–related injuries rose dramatically in 2020
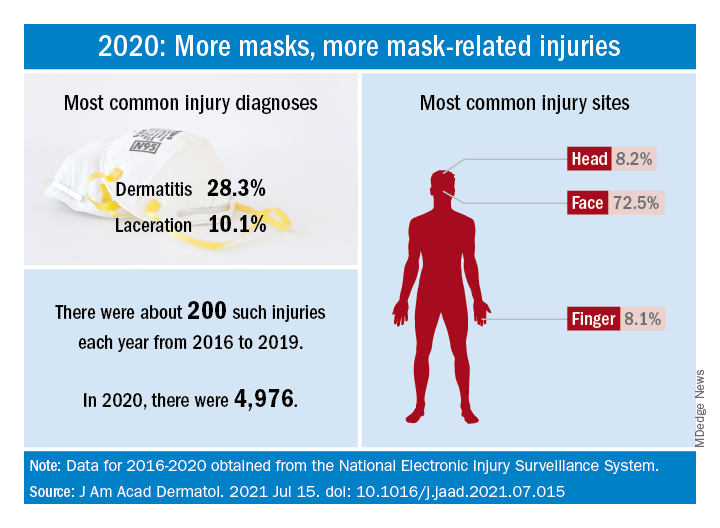
How dramatic? The number of mask-related injuries treated in U.S. emergency departments averaged about 200 per year from 2016 to 2019. In 2020, that figure soared to 4,976 – an increase of almost 2,400%, Gerald McGwin Jr., PhD, and associates said in a research letter published in the Journal to the American Academy of Dermatology.
“Prior to the COVID-19 pandemic the use of respiratory protection equipment was largely limited to healthcare and industrial settings. As [face mask] use by the general population increased, so too have reports of dermatologic reactions,” said Dr. McGwin and associates of the department of epidemiology at the University of Alabama at Birmingham.
Dermatitis was the most common mask-related injury treated last year, affecting 28.3% of those presenting to EDs, followed by lacerations at 10.1%. Injuries were more common in women than men, but while and black patients “were equally represented,” they noted, based on data from the National Electronic Injury Surveillance System, which includes about 100 hospitals and EDs.
Most injuries were caused by rashes/allergic reactions (38%) from prolonged use, poorly fitting masks (19%), and obscured vision (14%). “There was a small (5%) but meaningful number of injuries, all among children, attributable to consuming pieces of a mask or inserting dismantled pieces of a mask into body orifices,” the investigators said.
Guidance from the Centers for Disease Control and Prevention is available “to aid in the choice and proper fit of face masks,” they wrote, and “increased awareness of these resources [could] minimize the future occurrence of mask-related injuries.”
There was no funding source for the study, and the investigators did not declare any conflicts of interest.

How dramatic? The number of mask-related injuries treated in U.S. emergency departments averaged about 200 per year from 2016 to 2019. In 2020, that figure soared to 4,976 – an increase of almost 2,400%, Gerald McGwin Jr., PhD, and associates said in a research letter published in the Journal to the American Academy of Dermatology.
“Prior to the COVID-19 pandemic the use of respiratory protection equipment was largely limited to healthcare and industrial settings. As [face mask] use by the general population increased, so too have reports of dermatologic reactions,” said Dr. McGwin and associates of the department of epidemiology at the University of Alabama at Birmingham.
Dermatitis was the most common mask-related injury treated last year, affecting 28.3% of those presenting to EDs, followed by lacerations at 10.1%. Injuries were more common in women than men, but while and black patients “were equally represented,” they noted, based on data from the National Electronic Injury Surveillance System, which includes about 100 hospitals and EDs.
Most injuries were caused by rashes/allergic reactions (38%) from prolonged use, poorly fitting masks (19%), and obscured vision (14%). “There was a small (5%) but meaningful number of injuries, all among children, attributable to consuming pieces of a mask or inserting dismantled pieces of a mask into body orifices,” the investigators said.
Guidance from the Centers for Disease Control and Prevention is available “to aid in the choice and proper fit of face masks,” they wrote, and “increased awareness of these resources [could] minimize the future occurrence of mask-related injuries.”
There was no funding source for the study, and the investigators did not declare any conflicts of interest.

How dramatic? The number of mask-related injuries treated in U.S. emergency departments averaged about 200 per year from 2016 to 2019. In 2020, that figure soared to 4,976 – an increase of almost 2,400%, Gerald McGwin Jr., PhD, and associates said in a research letter published in the Journal to the American Academy of Dermatology.
“Prior to the COVID-19 pandemic the use of respiratory protection equipment was largely limited to healthcare and industrial settings. As [face mask] use by the general population increased, so too have reports of dermatologic reactions,” said Dr. McGwin and associates of the department of epidemiology at the University of Alabama at Birmingham.
Dermatitis was the most common mask-related injury treated last year, affecting 28.3% of those presenting to EDs, followed by lacerations at 10.1%. Injuries were more common in women than men, but while and black patients “were equally represented,” they noted, based on data from the National Electronic Injury Surveillance System, which includes about 100 hospitals and EDs.
Most injuries were caused by rashes/allergic reactions (38%) from prolonged use, poorly fitting masks (19%), and obscured vision (14%). “There was a small (5%) but meaningful number of injuries, all among children, attributable to consuming pieces of a mask or inserting dismantled pieces of a mask into body orifices,” the investigators said.
Guidance from the Centers for Disease Control and Prevention is available “to aid in the choice and proper fit of face masks,” they wrote, and “increased awareness of these resources [could] minimize the future occurrence of mask-related injuries.”
There was no funding source for the study, and the investigators did not declare any conflicts of interest.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY