User login
Plastic IUD placement instruments prevent uterine perforations
Key clinical point: In a biomechanical ex vivo analysis, metal uterine sounds caused uterine perforation, but the manufacturer’s plastic intrauterine device placement rod did not.
Major finding: The lowest mean maximum force generated for IUD placement was 12.3 Newtons with the levonorgestrel intrauterine system placement instrument, followed by 14.1 Newtons with the copper T380A intrauterine device placement instrument 14.1 Newtons; the highest mean maximum force of 17.9 N occurred with the metal sound (P < 0.01).
Study details: The data come from 16 premenopausal women with benign conditions who provided hysterectomy sections at a single center.
Disclosures: The study was funded indirectly through grants to the University of Utah from Bayer, Bioceptive, Sebela, Medicines 360, Merck, and Cooper Surgical. Lead author Dr. Duncan had no financial conflicts to disclose.
Source: Duncan J et al. BMC Womens Health. 2021 Apr 7. doi: 10.1186/s12905-021-01285-6.
Key clinical point: In a biomechanical ex vivo analysis, metal uterine sounds caused uterine perforation, but the manufacturer’s plastic intrauterine device placement rod did not.
Major finding: The lowest mean maximum force generated for IUD placement was 12.3 Newtons with the levonorgestrel intrauterine system placement instrument, followed by 14.1 Newtons with the copper T380A intrauterine device placement instrument 14.1 Newtons; the highest mean maximum force of 17.9 N occurred with the metal sound (P < 0.01).
Study details: The data come from 16 premenopausal women with benign conditions who provided hysterectomy sections at a single center.
Disclosures: The study was funded indirectly through grants to the University of Utah from Bayer, Bioceptive, Sebela, Medicines 360, Merck, and Cooper Surgical. Lead author Dr. Duncan had no financial conflicts to disclose.
Source: Duncan J et al. BMC Womens Health. 2021 Apr 7. doi: 10.1186/s12905-021-01285-6.
Key clinical point: In a biomechanical ex vivo analysis, metal uterine sounds caused uterine perforation, but the manufacturer’s plastic intrauterine device placement rod did not.
Major finding: The lowest mean maximum force generated for IUD placement was 12.3 Newtons with the levonorgestrel intrauterine system placement instrument, followed by 14.1 Newtons with the copper T380A intrauterine device placement instrument 14.1 Newtons; the highest mean maximum force of 17.9 N occurred with the metal sound (P < 0.01).
Study details: The data come from 16 premenopausal women with benign conditions who provided hysterectomy sections at a single center.
Disclosures: The study was funded indirectly through grants to the University of Utah from Bayer, Bioceptive, Sebela, Medicines 360, Merck, and Cooper Surgical. Lead author Dr. Duncan had no financial conflicts to disclose.
Source: Duncan J et al. BMC Womens Health. 2021 Apr 7. doi: 10.1186/s12905-021-01285-6.
Oral contraceptive use shows no impact on later heart failure risk
Key clinical point: Use of oral contraceptives by women of reproductive age was not associated with increased risk of heart failure later in life, but the potential impact of different formulations and dosages deserves further research.
Major finding: Over an average of 12 years’ follow-up, the researchers identified 138 incident cases of heart failure. The incidence of heart failure was not significantly associated with heart failure in multivariate analysis (hazard ratio 0.96); however, any OC use was positively associated with left ventricular end-diastolic mass (P = 0.006) and stroke volume (P = 0.01 and P = 0.005 for left and right ventricles, respectively).
Study details: The data come from a retrospective study of 3,594 women with an average age of 62 years who were enrolled in the Multi‐Ethnic Study of Atherosclerosis and could provide data on oral contraceptive use.
Disclosures: The study was supported by the Guangdong Peak Project, the Science and Technology Planning Project of Guangdong Province, Natural Science Foundation of Guangdong Province, and the National Key Research and Development Program of China. The researchers had no financial conflicts to disclose.
Source: Luo D et al. ESC Heart Fail. 2021 Apr 9. doi: 10.1002/ehf2.13328.
Key clinical point: Use of oral contraceptives by women of reproductive age was not associated with increased risk of heart failure later in life, but the potential impact of different formulations and dosages deserves further research.
Major finding: Over an average of 12 years’ follow-up, the researchers identified 138 incident cases of heart failure. The incidence of heart failure was not significantly associated with heart failure in multivariate analysis (hazard ratio 0.96); however, any OC use was positively associated with left ventricular end-diastolic mass (P = 0.006) and stroke volume (P = 0.01 and P = 0.005 for left and right ventricles, respectively).
Study details: The data come from a retrospective study of 3,594 women with an average age of 62 years who were enrolled in the Multi‐Ethnic Study of Atherosclerosis and could provide data on oral contraceptive use.
Disclosures: The study was supported by the Guangdong Peak Project, the Science and Technology Planning Project of Guangdong Province, Natural Science Foundation of Guangdong Province, and the National Key Research and Development Program of China. The researchers had no financial conflicts to disclose.
Source: Luo D et al. ESC Heart Fail. 2021 Apr 9. doi: 10.1002/ehf2.13328.
Key clinical point: Use of oral contraceptives by women of reproductive age was not associated with increased risk of heart failure later in life, but the potential impact of different formulations and dosages deserves further research.
Major finding: Over an average of 12 years’ follow-up, the researchers identified 138 incident cases of heart failure. The incidence of heart failure was not significantly associated with heart failure in multivariate analysis (hazard ratio 0.96); however, any OC use was positively associated with left ventricular end-diastolic mass (P = 0.006) and stroke volume (P = 0.01 and P = 0.005 for left and right ventricles, respectively).
Study details: The data come from a retrospective study of 3,594 women with an average age of 62 years who were enrolled in the Multi‐Ethnic Study of Atherosclerosis and could provide data on oral contraceptive use.
Disclosures: The study was supported by the Guangdong Peak Project, the Science and Technology Planning Project of Guangdong Province, Natural Science Foundation of Guangdong Province, and the National Key Research and Development Program of China. The researchers had no financial conflicts to disclose.
Source: Luo D et al. ESC Heart Fail. 2021 Apr 9. doi: 10.1002/ehf2.13328.
Oral contraceptive with new estrogen earns approval
The Food and Drug Administration has approved a new estrogen for the first time in more than 50 years.
The novel combined oral contraceptive, marketed as Nextstellis, contains 3 mg drospirenone (DRSP) and 14.2 mg of estetrol (E4) in tablet form. Estetrol is an estrogen that is naturally produced during pregnancy, but will now be produced from a plant source; it has not previously been used in oral contraceptives.
Approval of the unique estetrol/drospirenone combination was based on data from a pair of phase 3 clinical trials including 3,725 women. Overall, Nextstellis was safe and effective while meeting its primary endpoint of pregnancy prevention, according to a company press release. Participants also reported favorable results on secondary endpoints including cycle control, bleeding profile, safety, and tolerability.
Although many women take short-acting contraceptives containing estrogen and progestin, concerns persist about side effects, said Mitchell Creinin, MD, of the University of California, in the press release. In addition to providing effective contraception, the drug showed minimal impact on specific markers of concern, including triglycerides, cholesterol, and glucose, as well as weight and endocrine markers, Dr. Creinin said.
Nextstellis was developed by the Belgian biotech company Mithra Pharmaceuticals, and the drug is licensed for distribution in Australia and the United States by Mayne Pharma, with an expected launch at the end of June 2021.
The Food and Drug Administration has approved a new estrogen for the first time in more than 50 years.
The novel combined oral contraceptive, marketed as Nextstellis, contains 3 mg drospirenone (DRSP) and 14.2 mg of estetrol (E4) in tablet form. Estetrol is an estrogen that is naturally produced during pregnancy, but will now be produced from a plant source; it has not previously been used in oral contraceptives.
Approval of the unique estetrol/drospirenone combination was based on data from a pair of phase 3 clinical trials including 3,725 women. Overall, Nextstellis was safe and effective while meeting its primary endpoint of pregnancy prevention, according to a company press release. Participants also reported favorable results on secondary endpoints including cycle control, bleeding profile, safety, and tolerability.
Although many women take short-acting contraceptives containing estrogen and progestin, concerns persist about side effects, said Mitchell Creinin, MD, of the University of California, in the press release. In addition to providing effective contraception, the drug showed minimal impact on specific markers of concern, including triglycerides, cholesterol, and glucose, as well as weight and endocrine markers, Dr. Creinin said.
Nextstellis was developed by the Belgian biotech company Mithra Pharmaceuticals, and the drug is licensed for distribution in Australia and the United States by Mayne Pharma, with an expected launch at the end of June 2021.
The Food and Drug Administration has approved a new estrogen for the first time in more than 50 years.
The novel combined oral contraceptive, marketed as Nextstellis, contains 3 mg drospirenone (DRSP) and 14.2 mg of estetrol (E4) in tablet form. Estetrol is an estrogen that is naturally produced during pregnancy, but will now be produced from a plant source; it has not previously been used in oral contraceptives.
Approval of the unique estetrol/drospirenone combination was based on data from a pair of phase 3 clinical trials including 3,725 women. Overall, Nextstellis was safe and effective while meeting its primary endpoint of pregnancy prevention, according to a company press release. Participants also reported favorable results on secondary endpoints including cycle control, bleeding profile, safety, and tolerability.
Although many women take short-acting contraceptives containing estrogen and progestin, concerns persist about side effects, said Mitchell Creinin, MD, of the University of California, in the press release. In addition to providing effective contraception, the drug showed minimal impact on specific markers of concern, including triglycerides, cholesterol, and glucose, as well as weight and endocrine markers, Dr. Creinin said.
Nextstellis was developed by the Belgian biotech company Mithra Pharmaceuticals, and the drug is licensed for distribution in Australia and the United States by Mayne Pharma, with an expected launch at the end of June 2021.
Patient-centered contraceptive care for medically complex patients
CASE Patient-centered counseling for contraception
A 19-year-old woman (G0) with moderately well-controlled seizure disorder while taking levetiracetam, who reports migraines, and has a BMI of 32 kg/m2 presents to your office seeking contraception. She is currently sexually active with her second lifetime partner and uses condoms inconsistently. She is otherwise healthy and has no problems to report. Her last menstrual period (LMP) was 1 week ago, and a pregnancy test today is negative. How do you approach counseling for this patient?
The modern contraceptive patient
Our patients are becoming increasingly medically and socially complicated. Meeting the contraceptive needs of patients with multiple comorbidities can be a daunting task. Doing so in a patient-centered way that also recognizes the social contexts and intimacy inherent to contraceptive care can feel overwhelming. However, by employing a systematic approach to each patient, we can provide safe, effective, individualized care to our medically complex patients. Having a few “go-to tools” can streamline the process.
Medically complex patients are often told that they need to avoid pregnancy or optimize their health conditions prior to becoming pregnant, but they may not receive medically-appropriate contraception.1-3 Additionally, obesity rates in women of reproductive age in the United States are increasing, along with related medical complexities.4 Disparities in contraceptive access and use of particular methods exist by socioeconomic status, body mass index (BMI), age, and geography. 5,6 Evidence-based, shared decision making can improve contraceptive satisfaction.7
Clinicians need to stay attuned to all options. Staying current on available contraceptive methods can broaden clinicians’ thinking and allow patients more choices that are compatible with their medical needs. In the last 2 years alone, a 1-year combined estrogen-progestin vaginal ring, a drospirinone-only pill, and a nonhormonal spermicide have become available for prescription.8-10 Both 52 mg levonorgestrel-containing intrauterine devices (IUDs) are now US Food and Drug Administration (FDA)-approved for 6 years, and there is excellent data for off-label use to 7 years.11
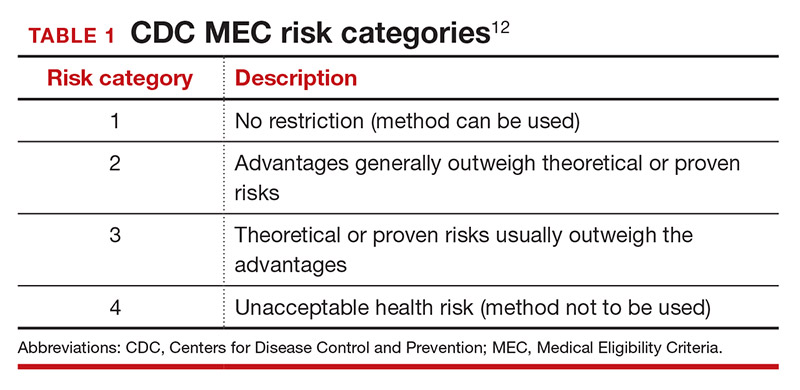
Tools are available for use. To ensure patient safety, we must evaluate the relative risks of each method given their specific medical history. The Centers for Disease Control and Prevention (CDC) Medical Eligibility Criteria (MEC) provides a comprehensive reference for using each contraceptive method category with preexisting medical conditions on a scale from 1 (no restrictions) to 4 (unacceptable health risk) (TABLE 1).12 It is important to remember that pregnancy often poses a larger risk even than category 4 methods. With proper counseling and documentation, a category 3 method may be appropriate in some circumstances. The CDC MEC can serve as an excellent counseling tool and is available as a free smartphone app. The app can be downloaded via https://www.cdc.gov/reproductivehealth/contraception/mmwr/mec/summary.html (TABLE 2).
In a shared decision-making model, we contribute our medical knowledge, and the patient provides expertise on her own values and social context.13 By starting the contraceptive conversation with open-ended questions, we invite the patient to lead the discussion. We partner with them in finding a safe, effective method that is compatible with both the medical history and stated preferences. Bedsider.org has an interactive tool that allows patients to explore different contraceptive methods and compare their various characteristics. While tiered efficacy models may help us to organize our thinking as clinicians, it is important to recognize that patients may consider side effect profiles, nonreliance on clinicians for discontinuation, or other priorities above effectiveness.
Continue to: How to craft your approach...
How to craft your approach
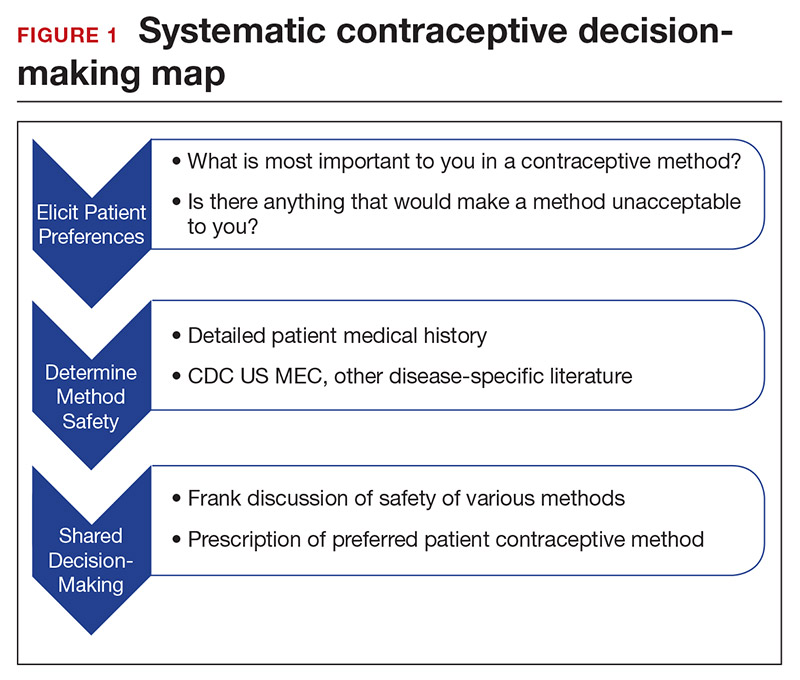
Developing a systematic approach to the medically complex patient seeking contraception can help to change an initially daunting task into a fulfilling experience (FIGURE 1). Begin by eliciting patient priorities. Then frame the discussion around them, rather than around efficacy. Although anecdotal reasoning can initially be frustrating (“My best friend’s IUD was really painful and I don’t want anything like that inside me!”), learning about these experiences prior to counseling can be incredibly informative. Ask detailed questions about medical comorbidities, as these subtleties may change the relative safety of each method. Finally, engage the patient in a frank discussion of the relative merits, safety, and use of all medically appropriate contraceptive methods. The right method is the method that the patient will use.
CASE Continued: Applying our counseling method
Upon open-ended questioning, the patient tells you that she absolutely cannot be on a contraceptive method that will make her gain weight. She has several friends who told her that they gained weight on “the shot” and “the implant.” She wants to avoid these at all costs and thinks she might want to take “the pill.” She also tells you that she is in college and that her daily routine varies significantly between weekdays and weekends. She definitely does not want to get pregnant until she has completed her education, which will be at least 3 years from now.
To best counsel this patient and arrive at the most appropriate contraceptive option for her, clarify her medical history and employ shared decision-making for her chosen method.
Probe her seizure history
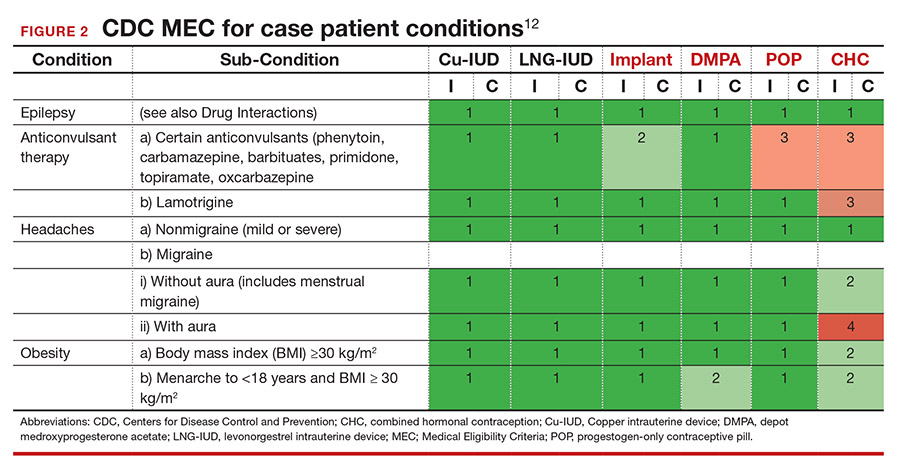
She tells you that she has had seizures since she was a child, and the last one occurred 4 months ago when she ran out of her anticonvulsant medication. Her seizures have never been associated with her menses. This is an important piece of information. The frequency of catamenial seizures can be decreased with use of any method that suppresses ovulation, such as depot-medroxyprogesterone (DMPA) injections, continuous combined hormonal contraceptive (CHC) pills or ring, or the implant. Noncatamenial seizures also can be suppressed by DMPA, which increases the seizure threshold.14 Many anticonvulsants are metabolized through cytochrome P450 in the liver and, therefore, interact with all oral contraceptive formulations. However, levetiracetam is not among them and may be safely taken with progestin-only pills. At this point, all contraceptive methods remain CDC MEC category 1 (FIGURE 2).12
Ask migraine specifics
It is important to clarify whether or not the patient experiences aura with her migraines. She says that she always knows when a migraine is coming on because she sees floaters in her vision for about 30 minutes prior to the onset of excruciating headache. One tool that may aid in the diagnosis of aura is the Visual Aura Rating Scale (VARS).15 The presence of aura renders all CHCs category 4 by the CDC MEC.12 (See FIGURE 2.)
Discuss contraceptive pros and cons
Have a frank discussion about the relative risks and benefits of each method. For instance, although DMPA may improve the patient’s seizures, she has expressed a desire to avoid weight gain, and DMPA is the only method consistently shown in studies to do so.16 Her seizures are not associated with menses, so menstrual suppression is neither beneficial nor deleterious. Although her current medication levetiracetam does not influence the metabolism of contraceptive methods, many anticonvulsants do. Offer anticipatory guidance around seeking gynecologic consultation with any future seizure medication changes.
Allow for shared decision-making on a final choice
The patient indicated that she had been considering “the pill” when she made this appointment, but you have explained that CHCs are contraindicated for her. She is concerned that she will not be able to stick to the strict dosing schedule of a progestin-only pill. Although you inform her that the drospirinone-only pill has a more forgiving window, the patient decides that she wants a “set it and forget it” method and opts for an IUD.
CASE Resolved
Following recommendations from the American College of Obstetricians and Gynecologists (ACOG), you provide for same-day insertion of a 52-mg levonorgestrel IUD.17 You use a paracervical block in addition to ibuprofen for pain control.18 The patient undergoes same-day testing for gonorrhea and chlamydia, and she understands that if a test is found to be positive, she can be treated without removing the IUD. You provide instruction on the importance of dual contraceptive use with barrier methods for the prevention of STIs. The patient is instructed on self-string checks, and she acknowledges that she will call if she has any concerns; no routine follow-up is required. She leaves her visit satisfied with her preferred, safe, effective contraceptive method in situ. ●
- Lauring JR, Lehman EB, Deimling TA, et al. Combined hormonal contraception use in reproductive-age women with contraindications to estrogen use. Am J Obstet Gynecol. 2016;215:330.e1-e7.
- Mendel A, Bernatsky S, Pineau CA, et al. Use of combined hormonal contraceptives among women with systemic lupus erythematosus with and without medical contraindications to oestrogen. Rheumatology (Oxford). 2019;58:1259-1267.
- Judge CP, Zhao X, Sileanu FE, et al. Medical contraindications to estrogen and contraceptive use among women veterans. Am J Obstet Gynecol. 2018;218:234.e1-234.e9.
- Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. 2020;360:1-8.
- Guttmacher Institute. Contraceptive use in the United States. April 2020. . Accessed March 22, 2021.
- Mosher WD, Lantos H, Burke AE. Obesity and contraceptive use among women 20–44 years of age in the United States: results from the 2011–15 National Survey of Family Growth (NSFG). Contraception. 2018:97:392-398.
- Dehlendorf C, Grumbach K, Schmittdiel JA, et al. Shared decision making in contraceptive counseling. Contraception. 2017;95:452-455.
- Annovera [package insert]. Boca Raton, FL: TherapeuticsMD, Inc; 2020.
- Slynd [package insert]. Florham Park, NJ: Exeltis; 2019.
- Phexxi [package insert]. San Diego, CA: Evofem; 2020.
- , et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93:498-506.
- Centers for Disease Control and Prevention. US Medical Eligibility Criteria (US MEC) for Contraceptive Use, 2016. . Accessed March 23, 2021.
- Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44:681-692.
- Dutton C, Foldvary‐Schaefer N. Contraception in women with epilepsy: pharmacokinetic interactions, contraceptive options, and management. Int Rev Neurobiol. 83;2008:113-134.
- Eriksen MK, Thomsen LL, Olesen J. The visual aura rating scale (VARS) for migraine aura diagnosis. Cephalalgia. 2005;25:801-810.
- ME, , , et al. Prospective study of weight change in new adolescent users of DMPA, NET-EN, COCs, nonusers and discontinuers of hormonal contraception. Contraception. 2010;81:30-34.
- Espey E, Hofler L. Long-acting reversible contraception: implants and intrauterine devices. Practice bulletin 186. Obstet Gynecol. 2017;130:e251-269.
- Akers AY, Steinway C, Sonalkar S, et al. Reducing pain during intrauterine device insertion: a randomized controlled trial in adolescents and young women. Obstet Gynecol. 2017;130:795-802.
CASE Patient-centered counseling for contraception
A 19-year-old woman (G0) with moderately well-controlled seizure disorder while taking levetiracetam, who reports migraines, and has a BMI of 32 kg/m2 presents to your office seeking contraception. She is currently sexually active with her second lifetime partner and uses condoms inconsistently. She is otherwise healthy and has no problems to report. Her last menstrual period (LMP) was 1 week ago, and a pregnancy test today is negative. How do you approach counseling for this patient?
The modern contraceptive patient
Our patients are becoming increasingly medically and socially complicated. Meeting the contraceptive needs of patients with multiple comorbidities can be a daunting task. Doing so in a patient-centered way that also recognizes the social contexts and intimacy inherent to contraceptive care can feel overwhelming. However, by employing a systematic approach to each patient, we can provide safe, effective, individualized care to our medically complex patients. Having a few “go-to tools” can streamline the process.
Medically complex patients are often told that they need to avoid pregnancy or optimize their health conditions prior to becoming pregnant, but they may not receive medically-appropriate contraception.1-3 Additionally, obesity rates in women of reproductive age in the United States are increasing, along with related medical complexities.4 Disparities in contraceptive access and use of particular methods exist by socioeconomic status, body mass index (BMI), age, and geography. 5,6 Evidence-based, shared decision making can improve contraceptive satisfaction.7
Clinicians need to stay attuned to all options. Staying current on available contraceptive methods can broaden clinicians’ thinking and allow patients more choices that are compatible with their medical needs. In the last 2 years alone, a 1-year combined estrogen-progestin vaginal ring, a drospirinone-only pill, and a nonhormonal spermicide have become available for prescription.8-10 Both 52 mg levonorgestrel-containing intrauterine devices (IUDs) are now US Food and Drug Administration (FDA)-approved for 6 years, and there is excellent data for off-label use to 7 years.11
Tools are available for use. To ensure patient safety, we must evaluate the relative risks of each method given their specific medical history. The Centers for Disease Control and Prevention (CDC) Medical Eligibility Criteria (MEC) provides a comprehensive reference for using each contraceptive method category with preexisting medical conditions on a scale from 1 (no restrictions) to 4 (unacceptable health risk) (TABLE 1).12 It is important to remember that pregnancy often poses a larger risk even than category 4 methods. With proper counseling and documentation, a category 3 method may be appropriate in some circumstances. The CDC MEC can serve as an excellent counseling tool and is available as a free smartphone app. The app can be downloaded via https://www.cdc.gov/reproductivehealth/contraception/mmwr/mec/summary.html (TABLE 2).


In a shared decision-making model, we contribute our medical knowledge, and the patient provides expertise on her own values and social context.13 By starting the contraceptive conversation with open-ended questions, we invite the patient to lead the discussion. We partner with them in finding a safe, effective method that is compatible with both the medical history and stated preferences. Bedsider.org has an interactive tool that allows patients to explore different contraceptive methods and compare their various characteristics. While tiered efficacy models may help us to organize our thinking as clinicians, it is important to recognize that patients may consider side effect profiles, nonreliance on clinicians for discontinuation, or other priorities above effectiveness.
Continue to: How to craft your approach...
How to craft your approach
Developing a systematic approach to the medically complex patient seeking contraception can help to change an initially daunting task into a fulfilling experience (FIGURE 1). Begin by eliciting patient priorities. Then frame the discussion around them, rather than around efficacy. Although anecdotal reasoning can initially be frustrating (“My best friend’s IUD was really painful and I don’t want anything like that inside me!”), learning about these experiences prior to counseling can be incredibly informative. Ask detailed questions about medical comorbidities, as these subtleties may change the relative safety of each method. Finally, engage the patient in a frank discussion of the relative merits, safety, and use of all medically appropriate contraceptive methods. The right method is the method that the patient will use.

CASE Continued: Applying our counseling method
Upon open-ended questioning, the patient tells you that she absolutely cannot be on a contraceptive method that will make her gain weight. She has several friends who told her that they gained weight on “the shot” and “the implant.” She wants to avoid these at all costs and thinks she might want to take “the pill.” She also tells you that she is in college and that her daily routine varies significantly between weekdays and weekends. She definitely does not want to get pregnant until she has completed her education, which will be at least 3 years from now.
To best counsel this patient and arrive at the most appropriate contraceptive option for her, clarify her medical history and employ shared decision-making for her chosen method.
Probe her seizure history
She tells you that she has had seizures since she was a child, and the last one occurred 4 months ago when she ran out of her anticonvulsant medication. Her seizures have never been associated with her menses. This is an important piece of information. The frequency of catamenial seizures can be decreased with use of any method that suppresses ovulation, such as depot-medroxyprogesterone (DMPA) injections, continuous combined hormonal contraceptive (CHC) pills or ring, or the implant. Noncatamenial seizures also can be suppressed by DMPA, which increases the seizure threshold.14 Many anticonvulsants are metabolized through cytochrome P450 in the liver and, therefore, interact with all oral contraceptive formulations. However, levetiracetam is not among them and may be safely taken with progestin-only pills. At this point, all contraceptive methods remain CDC MEC category 1 (FIGURE 2).12

Ask migraine specifics
It is important to clarify whether or not the patient experiences aura with her migraines. She says that she always knows when a migraine is coming on because she sees floaters in her vision for about 30 minutes prior to the onset of excruciating headache. One tool that may aid in the diagnosis of aura is the Visual Aura Rating Scale (VARS).15 The presence of aura renders all CHCs category 4 by the CDC MEC.12 (See FIGURE 2.)
Discuss contraceptive pros and cons
Have a frank discussion about the relative risks and benefits of each method. For instance, although DMPA may improve the patient’s seizures, she has expressed a desire to avoid weight gain, and DMPA is the only method consistently shown in studies to do so.16 Her seizures are not associated with menses, so menstrual suppression is neither beneficial nor deleterious. Although her current medication levetiracetam does not influence the metabolism of contraceptive methods, many anticonvulsants do. Offer anticipatory guidance around seeking gynecologic consultation with any future seizure medication changes.
Allow for shared decision-making on a final choice
The patient indicated that she had been considering “the pill” when she made this appointment, but you have explained that CHCs are contraindicated for her. She is concerned that she will not be able to stick to the strict dosing schedule of a progestin-only pill. Although you inform her that the drospirinone-only pill has a more forgiving window, the patient decides that she wants a “set it and forget it” method and opts for an IUD.
CASE Resolved
Following recommendations from the American College of Obstetricians and Gynecologists (ACOG), you provide for same-day insertion of a 52-mg levonorgestrel IUD.17 You use a paracervical block in addition to ibuprofen for pain control.18 The patient undergoes same-day testing for gonorrhea and chlamydia, and she understands that if a test is found to be positive, she can be treated without removing the IUD. You provide instruction on the importance of dual contraceptive use with barrier methods for the prevention of STIs. The patient is instructed on self-string checks, and she acknowledges that she will call if she has any concerns; no routine follow-up is required. She leaves her visit satisfied with her preferred, safe, effective contraceptive method in situ. ●
CASE Patient-centered counseling for contraception
A 19-year-old woman (G0) with moderately well-controlled seizure disorder while taking levetiracetam, who reports migraines, and has a BMI of 32 kg/m2 presents to your office seeking contraception. She is currently sexually active with her second lifetime partner and uses condoms inconsistently. She is otherwise healthy and has no problems to report. Her last menstrual period (LMP) was 1 week ago, and a pregnancy test today is negative. How do you approach counseling for this patient?
The modern contraceptive patient
Our patients are becoming increasingly medically and socially complicated. Meeting the contraceptive needs of patients with multiple comorbidities can be a daunting task. Doing so in a patient-centered way that also recognizes the social contexts and intimacy inherent to contraceptive care can feel overwhelming. However, by employing a systematic approach to each patient, we can provide safe, effective, individualized care to our medically complex patients. Having a few “go-to tools” can streamline the process.
Medically complex patients are often told that they need to avoid pregnancy or optimize their health conditions prior to becoming pregnant, but they may not receive medically-appropriate contraception.1-3 Additionally, obesity rates in women of reproductive age in the United States are increasing, along with related medical complexities.4 Disparities in contraceptive access and use of particular methods exist by socioeconomic status, body mass index (BMI), age, and geography. 5,6 Evidence-based, shared decision making can improve contraceptive satisfaction.7
Clinicians need to stay attuned to all options. Staying current on available contraceptive methods can broaden clinicians’ thinking and allow patients more choices that are compatible with their medical needs. In the last 2 years alone, a 1-year combined estrogen-progestin vaginal ring, a drospirinone-only pill, and a nonhormonal spermicide have become available for prescription.8-10 Both 52 mg levonorgestrel-containing intrauterine devices (IUDs) are now US Food and Drug Administration (FDA)-approved for 6 years, and there is excellent data for off-label use to 7 years.11
Tools are available for use. To ensure patient safety, we must evaluate the relative risks of each method given their specific medical history. The Centers for Disease Control and Prevention (CDC) Medical Eligibility Criteria (MEC) provides a comprehensive reference for using each contraceptive method category with preexisting medical conditions on a scale from 1 (no restrictions) to 4 (unacceptable health risk) (TABLE 1).12 It is important to remember that pregnancy often poses a larger risk even than category 4 methods. With proper counseling and documentation, a category 3 method may be appropriate in some circumstances. The CDC MEC can serve as an excellent counseling tool and is available as a free smartphone app. The app can be downloaded via https://www.cdc.gov/reproductivehealth/contraception/mmwr/mec/summary.html (TABLE 2).


In a shared decision-making model, we contribute our medical knowledge, and the patient provides expertise on her own values and social context.13 By starting the contraceptive conversation with open-ended questions, we invite the patient to lead the discussion. We partner with them in finding a safe, effective method that is compatible with both the medical history and stated preferences. Bedsider.org has an interactive tool that allows patients to explore different contraceptive methods and compare their various characteristics. While tiered efficacy models may help us to organize our thinking as clinicians, it is important to recognize that patients may consider side effect profiles, nonreliance on clinicians for discontinuation, or other priorities above effectiveness.
Continue to: How to craft your approach...
How to craft your approach
Developing a systematic approach to the medically complex patient seeking contraception can help to change an initially daunting task into a fulfilling experience (FIGURE 1). Begin by eliciting patient priorities. Then frame the discussion around them, rather than around efficacy. Although anecdotal reasoning can initially be frustrating (“My best friend’s IUD was really painful and I don’t want anything like that inside me!”), learning about these experiences prior to counseling can be incredibly informative. Ask detailed questions about medical comorbidities, as these subtleties may change the relative safety of each method. Finally, engage the patient in a frank discussion of the relative merits, safety, and use of all medically appropriate contraceptive methods. The right method is the method that the patient will use.

CASE Continued: Applying our counseling method
Upon open-ended questioning, the patient tells you that she absolutely cannot be on a contraceptive method that will make her gain weight. She has several friends who told her that they gained weight on “the shot” and “the implant.” She wants to avoid these at all costs and thinks she might want to take “the pill.” She also tells you that she is in college and that her daily routine varies significantly between weekdays and weekends. She definitely does not want to get pregnant until she has completed her education, which will be at least 3 years from now.
To best counsel this patient and arrive at the most appropriate contraceptive option for her, clarify her medical history and employ shared decision-making for her chosen method.
Probe her seizure history
She tells you that she has had seizures since she was a child, and the last one occurred 4 months ago when she ran out of her anticonvulsant medication. Her seizures have never been associated with her menses. This is an important piece of information. The frequency of catamenial seizures can be decreased with use of any method that suppresses ovulation, such as depot-medroxyprogesterone (DMPA) injections, continuous combined hormonal contraceptive (CHC) pills or ring, or the implant. Noncatamenial seizures also can be suppressed by DMPA, which increases the seizure threshold.14 Many anticonvulsants are metabolized through cytochrome P450 in the liver and, therefore, interact with all oral contraceptive formulations. However, levetiracetam is not among them and may be safely taken with progestin-only pills. At this point, all contraceptive methods remain CDC MEC category 1 (FIGURE 2).12

Ask migraine specifics
It is important to clarify whether or not the patient experiences aura with her migraines. She says that she always knows when a migraine is coming on because she sees floaters in her vision for about 30 minutes prior to the onset of excruciating headache. One tool that may aid in the diagnosis of aura is the Visual Aura Rating Scale (VARS).15 The presence of aura renders all CHCs category 4 by the CDC MEC.12 (See FIGURE 2.)
Discuss contraceptive pros and cons
Have a frank discussion about the relative risks and benefits of each method. For instance, although DMPA may improve the patient’s seizures, she has expressed a desire to avoid weight gain, and DMPA is the only method consistently shown in studies to do so.16 Her seizures are not associated with menses, so menstrual suppression is neither beneficial nor deleterious. Although her current medication levetiracetam does not influence the metabolism of contraceptive methods, many anticonvulsants do. Offer anticipatory guidance around seeking gynecologic consultation with any future seizure medication changes.
Allow for shared decision-making on a final choice
The patient indicated that she had been considering “the pill” when she made this appointment, but you have explained that CHCs are contraindicated for her. She is concerned that she will not be able to stick to the strict dosing schedule of a progestin-only pill. Although you inform her that the drospirinone-only pill has a more forgiving window, the patient decides that she wants a “set it and forget it” method and opts for an IUD.
CASE Resolved
Following recommendations from the American College of Obstetricians and Gynecologists (ACOG), you provide for same-day insertion of a 52-mg levonorgestrel IUD.17 You use a paracervical block in addition to ibuprofen for pain control.18 The patient undergoes same-day testing for gonorrhea and chlamydia, and she understands that if a test is found to be positive, she can be treated without removing the IUD. You provide instruction on the importance of dual contraceptive use with barrier methods for the prevention of STIs. The patient is instructed on self-string checks, and she acknowledges that she will call if she has any concerns; no routine follow-up is required. She leaves her visit satisfied with her preferred, safe, effective contraceptive method in situ. ●
- Lauring JR, Lehman EB, Deimling TA, et al. Combined hormonal contraception use in reproductive-age women with contraindications to estrogen use. Am J Obstet Gynecol. 2016;215:330.e1-e7.
- Mendel A, Bernatsky S, Pineau CA, et al. Use of combined hormonal contraceptives among women with systemic lupus erythematosus with and without medical contraindications to oestrogen. Rheumatology (Oxford). 2019;58:1259-1267.
- Judge CP, Zhao X, Sileanu FE, et al. Medical contraindications to estrogen and contraceptive use among women veterans. Am J Obstet Gynecol. 2018;218:234.e1-234.e9.
- Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. 2020;360:1-8.
- Guttmacher Institute. Contraceptive use in the United States. April 2020. . Accessed March 22, 2021.
- Mosher WD, Lantos H, Burke AE. Obesity and contraceptive use among women 20–44 years of age in the United States: results from the 2011–15 National Survey of Family Growth (NSFG). Contraception. 2018:97:392-398.
- Dehlendorf C, Grumbach K, Schmittdiel JA, et al. Shared decision making in contraceptive counseling. Contraception. 2017;95:452-455.
- Annovera [package insert]. Boca Raton, FL: TherapeuticsMD, Inc; 2020.
- Slynd [package insert]. Florham Park, NJ: Exeltis; 2019.
- Phexxi [package insert]. San Diego, CA: Evofem; 2020.
- , et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93:498-506.
- Centers for Disease Control and Prevention. US Medical Eligibility Criteria (US MEC) for Contraceptive Use, 2016. . Accessed March 23, 2021.
- Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44:681-692.
- Dutton C, Foldvary‐Schaefer N. Contraception in women with epilepsy: pharmacokinetic interactions, contraceptive options, and management. Int Rev Neurobiol. 83;2008:113-134.
- Eriksen MK, Thomsen LL, Olesen J. The visual aura rating scale (VARS) for migraine aura diagnosis. Cephalalgia. 2005;25:801-810.
- ME, , , et al. Prospective study of weight change in new adolescent users of DMPA, NET-EN, COCs, nonusers and discontinuers of hormonal contraception. Contraception. 2010;81:30-34.
- Espey E, Hofler L. Long-acting reversible contraception: implants and intrauterine devices. Practice bulletin 186. Obstet Gynecol. 2017;130:e251-269.
- Akers AY, Steinway C, Sonalkar S, et al. Reducing pain during intrauterine device insertion: a randomized controlled trial in adolescents and young women. Obstet Gynecol. 2017;130:795-802.
- Lauring JR, Lehman EB, Deimling TA, et al. Combined hormonal contraception use in reproductive-age women with contraindications to estrogen use. Am J Obstet Gynecol. 2016;215:330.e1-e7.
- Mendel A, Bernatsky S, Pineau CA, et al. Use of combined hormonal contraceptives among women with systemic lupus erythematosus with and without medical contraindications to oestrogen. Rheumatology (Oxford). 2019;58:1259-1267.
- Judge CP, Zhao X, Sileanu FE, et al. Medical contraindications to estrogen and contraceptive use among women veterans. Am J Obstet Gynecol. 2018;218:234.e1-234.e9.
- Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. 2020;360:1-8.
- Guttmacher Institute. Contraceptive use in the United States. April 2020. . Accessed March 22, 2021.
- Mosher WD, Lantos H, Burke AE. Obesity and contraceptive use among women 20–44 years of age in the United States: results from the 2011–15 National Survey of Family Growth (NSFG). Contraception. 2018:97:392-398.
- Dehlendorf C, Grumbach K, Schmittdiel JA, et al. Shared decision making in contraceptive counseling. Contraception. 2017;95:452-455.
- Annovera [package insert]. Boca Raton, FL: TherapeuticsMD, Inc; 2020.
- Slynd [package insert]. Florham Park, NJ: Exeltis; 2019.
- Phexxi [package insert]. San Diego, CA: Evofem; 2020.
- , et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93:498-506.
- Centers for Disease Control and Prevention. US Medical Eligibility Criteria (US MEC) for Contraceptive Use, 2016. . Accessed March 23, 2021.
- Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44:681-692.
- Dutton C, Foldvary‐Schaefer N. Contraception in women with epilepsy: pharmacokinetic interactions, contraceptive options, and management. Int Rev Neurobiol. 83;2008:113-134.
- Eriksen MK, Thomsen LL, Olesen J. The visual aura rating scale (VARS) for migraine aura diagnosis. Cephalalgia. 2005;25:801-810.
- ME, , , et al. Prospective study of weight change in new adolescent users of DMPA, NET-EN, COCs, nonusers and discontinuers of hormonal contraception. Contraception. 2010;81:30-34.
- Espey E, Hofler L. Long-acting reversible contraception: implants and intrauterine devices. Practice bulletin 186. Obstet Gynecol. 2017;130:e251-269.
- Akers AY, Steinway C, Sonalkar S, et al. Reducing pain during intrauterine device insertion: a randomized controlled trial in adolescents and young women. Obstet Gynecol. 2017;130:795-802.
Optimize your treatment of endometriosis by using an FDA-approved hormonal medication
Women with endometriosis often present for medical care for one or more of the following health issues: pelvic pain, infertility, and/or an adnexal cyst (endometrioma). For women with moderate or severe pelvic pain and laparoscopically diagnosed endometriosis, hormone therapy is often necessary to achieve maximal long-term reduction in pain and optimize health. I focus on opportunities to optimize hormonal treatment of endometriosis in this editorial.
When plan A is not working, move expeditiously to plan B
Cyclic or continuous combination estrogen-progestin contraceptives are commonly prescribed to treat pelvic pain caused by endometriosis. Although endometriosis pain may initially improve with estrogen-progestin contraceptives, many women on this medication will eventually report that they have worsening pelvic pain that adversely impacts their daily activities. Surprisingly, clinicians often continue to prescribe estrogen-progestin contraceptives even after the patient reports that the treatment is not effective, and their pain continues to be bothersome.
Patients benefit when they have access to the full range of hormone treatments that have been approved by the FDA for the treatment of moderate to severe pelvic pain caused by endometriosis (TABLE). In the situation where an estrogen-progestin contraceptive is no longer effective at reducing the pelvic pain, I will often offer the patient the option of norethindrone acetate (NEA) or elagolix treatment. My experience is that stopping the estrogen-progestin contraceptive and starting NEA or elagolix will result in a significant decrease in pain symptoms and improvement in the patient’s quality of life.

Other FDA-approved options to treat pelvic pain caused by endometriosis include depot medroxyprogesterone acetate injectable suspension, depot leuprolide acetate, goserelin implant, and danazol. I do not routinely prescribe depot medroxyprogesterone acetate because some patients report new onset or worsening symptoms of depression on the medication. I prescribe depot-leuprolide acetate less often than in the past, because many patients report moderate to severe hypoestrogenic symptoms on this medication. In women taking depot-leuprolide acetate, moderate to severe vasomotor symptoms can be improved by prescribing NEA pills, but the alternative of norethindrone monotherapy is less expensive. I seldom use goserelin or danazol in my practice. The needle required to place the goserelin implant has a diameter of approximately 1.7 mm (16 gauge) or 2.1 mm (14 gauge), for the 3.6 mg and 10 mg doses, respectively. The large diameter of the needle can cause pain and bruising at the implant site. As a comparison, the progestin subdermal implant needle is approximately 2.1 mm in diameter. Danazol is associated with weight gain, and most women prefer to avoid this side effect.
Continue to: Norethindrone acetate...
Norethindrone acetate
NEA 5 mg daily is approved by the FDA to treat endometriosis.1 NEA was approved at a time when large controlled clinical trials were not routinely required for a medicine to be approved. The data to support NEA treatment of pelvic pain caused by endometriosis is based on cohort studies. In a study of 194 women, median age 21 years with moderate to severe pelvic pain and surgically proven endometriosis, the effect of NEA on pelvic pain was explored.2 The initial dose of NEA was 5 mg daily. If the patient did not achieve a reduction in pelvic pain and amenorrhea on the NEA dose of 5 mg daily, the dose was increased by 2.5 mg every 2 weeks, up to a maximum of 15 mg, until amenorrhea and/or a decrease in pelvic pain was achieved. Ninety-five percent of the women in this cohort had previously been treated with an estrogen-progestin contraceptive or a GnRH antagonist and had discontinued those medications because of inadequate control of pelvic pain or because of side effects of the medication.
In this large cohort, 65% of women reported significant improvement in pelvic pain, with a median pain score of 5 before treatment and 0 following NEA treatment. About 55% of the women reported no side effects. The most commonly reported side effects were weight gain (16%; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%). (In this study women could report more than one side effect.)
In another cohort study of 52 women with pelvic pain and surgically confirmed endometriosis, NEA treatment resulted in pain relief in 94% of the women.3 Breakthrough bleeding was a common side effect, reported by 58% of participants. The investigators concluded that NEA treatment was a “cost-effective alternative with relatively mild side effects in the treatment of symptomatic endometriosis.” A conclusion which I endorse.
NEA has been reported to effectively treat ovarian endometriomas and rectovaginal endometriosis.4,5 In a cohort of 18 women who had previously had the surgical resection of an ovarian endometriosis cyst and had postoperative recurrence of pelvic pain and ovarian endometriosis, treatment was initiated with an escalating NEA regimen.4 Treatment was initiated with NEA 5 mg daily, with the dosage increased every 2 weeks by 2.5 mg until amenorrhea was established. Most women achieved amenorrhea with NEA 5 mg daily, and 89% had reduced pelvic pain. The investigators reported complete regression of the endometriosis cyst(s) in 74% of the women. In my experience, NEA does not result in complete regression of endometriosis cysts, but it does cause a reduction in cyst diameter and total volume.
In a retrospective cohort study, 61 women with pelvic pain and rectovaginal endometriosis had 5 years of treatment with NEA 2.5 mg or 5.0 mg daily.5 NEA treatment resulted in a decrease in dysmenorrhea, deep dyspareunia, and dyschezia. The most common side effects attributed to NEA treatment were weight gain (30%), vaginal bleeding (23%), decreased libido (11%), headache (9%), bloating or swelling (8%), depression (7%), and acne (5%). In women who had sequential imaging studies, NEA treatment resulted in a decrease in rectovaginal lesion volume, stable disease volume, or an increase in lesion volume in 56%, 32%, and 12% of the women, respectively. The investigators concluded that for women with rectovaginal endometriosis, NEA treatment is a low-cost option for long-term treatment.
In my practice, I do not prescribe NEA at doses greater than 5 mg daily. There are case reports that NEA at a dose of ≥10 mg daily is associated with the development of a hepatic adenoma,6 elevated liver transaminase concentration,7 and jaundice.8 If NEA 5 mg daily is not effective in controlling pelvic pain caused by endometriosis, I stop the NEA and start a GnRH analogue, most often elagolix.
NEA 5 mg is not FDA approved as a contraceptive. However, norethindrone 0.35 mg daily, also known as the “mini-pill”, is approved as a progestin-only contraceptive.9 NEA is rapidly and completely deacetylated to norethindrone, and the disposition of oral NEA is indistinguishable from that of norethindrone.1 Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg has contraceptive properties if taken daily.
Continue to: Elagolix...
Elagolix
Elagolix is FDA approved for the treatment of pelvic pain caused by endometriosis. I reviewed the key studies resulting in FDA approval in the November 2018 issue of
In the Elaris Endometriosis-I study, 872 women with endometriosis and pelvic pain were randomly assigned to treatment with 1 of 2 doses of elagolix (high-dose [200 mg twice daily] and low-dose [150 mg once daily]) or placebo.11 After 3 months of therapy, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). After 3 months of therapy, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.01 low-dose elagolix vs placebo and P<.001 high-dose elagolix vs placebo).
Hot flashes that were severe enough to be reported as an adverse event by the study participants were reported by 42%, 24%, and 7% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47% and hip femoral neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively.
Another large clinical trial of elagolix for the treatment of pelvic pain caused by endometriosis, Elaris EM-II, involving 817 women, produced results very similar to those reported in Elaris EM-I. The elagolix continuation studies, Elaris EM-III and -IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.12
In my 2018 review,10 I noted that elagolix dose adjustment can be utilized to attempt to achieve maximal pain relief with minimal vasomotor symptoms. Elagolix at 200 mg twice daily produces a mean estradiol concentration of 12 pg/mL, whereas elagolix at 150 mg daily resulted in a mean estradiol concentration of 41 pg/mL.13 The estrogen threshold hypothesis posits that in women with endometriosis a stable estradiol concentration of 20 to 30 pg/mL is often associated with decreased pain and fewer vasomotor events.14 To achieve the target estradiol range of 20 to 30 pg/mL, I often initiate elagolix treatment with 200 mg twice daily. This enables a rapid onset of amenorrhea and a reduction in pelvic pain. Once amenorrhea has been achieved and a decrease in pelvic pain has occurred, I adjust the dose downward to 200 mg twice daily on even calendar days of each month and 200 mg once daily on odd calendar days each month. Some women will have continued pain relief and amenorrhea when the dose is further decreased to 200 mg once daily. If bothersome bleeding recurs and/or pain symptoms increase in severity, the dose can be increased to 200 mg twice daily or an alternating regimen of 200 mg twice daily and 200 mg once daily, every 2 days. An alternative to dose adjustment is to combine elagolix with NEA, which can reduce the severity of hot flashes and reduce bone loss caused by hypoestrogenism.15,16
Health insurers and pharmacy benefits managers may require a prior authorization before approving and dispensing elagolix. The prior authorization process can be burdensome for clinicians, consuming limited healthcare resources, contributing to burnout and frustrating patients.17 Elagolix is less expensive than depot-leuprolide acetate and nafarelin nasal spray and somewhat more expensive than a goserelin implant.18,19
Elagolix is not approved as a contraceptive. In the Elaris EM-I and -II trials women were advised to use 2 forms of contraception, although pregnancies did occur. There were 6 pregnancies among 475 women taking elagolix 150 mg daily and 2 pregnancies among 477 women taking elagolix 200 mg twice daily.20 Women taking elagolix should be advised to use a contraceptive, but not an estrogen-progestin contraceptive.
Continue to: Do not use opioids to treat chronic pelvic pain caused by endometriosis...
Do not use opioids to treat chronic pelvic pain caused by endometriosis
One of the greatest public health tragedies of our era is the opioid misuse epidemic. Hundreds of thousands of deaths have been caused by opioid misuse. The Centers for Disease Control and Prevention reported that for the 12-month period ending in May 2020, there were 81,000 opioid-related deaths, the greatest number ever reported in a 12-month period.21 Many authorities believe that in the United States opioid medications have been over-prescribed, contributing to the opioid misuse epidemic. There is little evidence that chronic pelvic pain is optimally managed by chronic treatment with an opioid.22,23 Prescribing opioids to vulnerable individuals to treat chronic pelvic pain may result in opioid dependency and adversely affect the patient’s health. It is best to pledge not to prescribe an opioid medication for a woman with chronic pelvic pain caused by endometriosis. In situations when pelvic pain is difficult to control with hormonal therapy and nonopioid pain medications, referral to a specialty pain practice may be warranted.
Post–conservative surgery hormone treatment reduces pelvic pain recurrence
In a meta-analysis of 14 studies that reported on endometriosis recurrence rates following conservative surgery, recurrence (defined as recurrent pelvic pain or an imaging study showing recurrent endometriosis) was significantly reduced with the use of hormone treatment compared with expectant management or placebo treatment.24 The postoperative relative risk of endometriosis recurrence was reduced by 83% with progestin treatment, 64% with estrogen-progestin contraceptive treatment, and 38% with GnRH analogue treatment. Overall, the number of patients that needed to be treated to prevent one endometriosis recurrence was 10, assuming a recurrence rate of 25% in the placebo treatment or expectant management groups.
For women with pelvic pain caused by endometriosis who develop a recurrence of pelvic pain while on postoperative hormone treatment, it is important for the prescribing clinician to be flexible and consider changing the hormone regimen. For example, if a postoperative patient is treated with a continuous estrogen-progestin contraceptive and develops recurrent pain, I will stop the contraceptive and initiate treatment with either NEA or elagolix.
Capitalize on opportunities to improve the medical care of women with endometriosis
Early diagnosis of endometriosis can be facilitated by recognizing that the condition is a common cause of moderate to severe dysmenorrhea. In 5 studies involving 1,187 women, the mean length of time from onset of pelvic pain symptoms to diagnosis of endometriosis was 8.6 years.25 If a woman with pelvic pain caused by endometriosis has not had sufficient pain relief with one brand of continuous estrogen-progestin contraceptive, it is best not to prescribe an alternative brand but rather to switch to a progestin-only treatment or a GnRH antagonist. If plan A is not working, move expeditiously to plan B. ●
- Aygestin [package insert]. Barr Laboratories: Pomona, NY; 2007.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108.
- Muneyyirci-Delale O, Karacan M. Effect of norethindrone acetate in the treatment of symptomatic endometriosis. Int J Fertil Womens Med. 1998;43:24-27.
- Muneyyirci-Delale O, Anopa J, Charles C, et al. Medical management of recurrent endometrioma with long-term norethindrone acetate. Int J Women Health. 2012;4:149-154.
- Morotti M, Venturini PL, Biscaldi E, et al. Efficacy and acceptability of long-term norethindrone acetate for the treatment of rectovaginal endometriosis. Eur J Obstet Gynecol Repro Biol. 2017;213:4-10.
- Brady PC, Missmer SA, Laufer MR. Hepatic adenomas in adolescents and young women with endometriosis treated with norethindrone acetate. J Pediatr Adolesc Gynecol. 2017;30:422-424.
- Choudhary NS, Bodh V, Chaudhari S, et al. Norethisterone related drug induced liver injury: a series of 3 cases. J Clin Exp Hepatol. 2017;7:266- 268.
- Perez-Mera RA, Shields CE. Jaundice associated with norethindrone acetate therapy. N Engl J Med. 1962;267:1137-1138.
- Camila [package insert]. Mayne Pharma Inc: Greenville, NC; 2018.
- Barbieri RL. Elagolix: a new treatment for pelvic pain caused by endometriosis. OBG Manag. 2018;30:10,12-14, 20.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Orilissa [package insert]. AbbVie Inc; North Chicago, IL; 2018.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Gallagher JS, Missmer SA, Hornstein MD, et al. Long-term effects of gonadotropin-releasing hormone agonists and add-back in adolescent endometriosis. J Pediatr Adolesc Gynecol. 2018;31:376- 381.
- Miller A, Shor R, Waites T, et al. Prior authorization reform for better patient care. J Am Coll Cardiol. 2018;71:1937-1939.
- Depot-leuprolide acetate. Good Rx website. https://www.goodrx.com/. Accessed January 22, 2021.
- Goserelin. Good Rx website. https://www .goodrx.com/. Accessed January 22, 2021
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID19. https://www.cdc.gov/media/releases/2020 /p1218-overdose-deaths-covid-19.html. Reviewed December 18, 2020. Accessed March 24, 2021.
- Till SR, As-Sanie S. 3 cases of chronic pelvic pain with nonsurgical, nonopioid therapies. OBG Manag. 2018;30:41-48.
- Steele A. Opioid use and depression in chronic pelvic pain. Obstet Gynecol Clin North Am. 2014;41:491-501.
- Zakhari A, Delpero E, McKeown S, et al. Endometriosis recurrence following post-operative hormonal suppression: a systematic review and meta-analysis. Hum Reprod Update. 2021;27:96- 107.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8, 10-11, 16.
Women with endometriosis often present for medical care for one or more of the following health issues: pelvic pain, infertility, and/or an adnexal cyst (endometrioma). For women with moderate or severe pelvic pain and laparoscopically diagnosed endometriosis, hormone therapy is often necessary to achieve maximal long-term reduction in pain and optimize health. I focus on opportunities to optimize hormonal treatment of endometriosis in this editorial.
When plan A is not working, move expeditiously to plan B
Cyclic or continuous combination estrogen-progestin contraceptives are commonly prescribed to treat pelvic pain caused by endometriosis. Although endometriosis pain may initially improve with estrogen-progestin contraceptives, many women on this medication will eventually report that they have worsening pelvic pain that adversely impacts their daily activities. Surprisingly, clinicians often continue to prescribe estrogen-progestin contraceptives even after the patient reports that the treatment is not effective, and their pain continues to be bothersome.
Patients benefit when they have access to the full range of hormone treatments that have been approved by the FDA for the treatment of moderate to severe pelvic pain caused by endometriosis (TABLE). In the situation where an estrogen-progestin contraceptive is no longer effective at reducing the pelvic pain, I will often offer the patient the option of norethindrone acetate (NEA) or elagolix treatment. My experience is that stopping the estrogen-progestin contraceptive and starting NEA or elagolix will result in a significant decrease in pain symptoms and improvement in the patient’s quality of life.

Other FDA-approved options to treat pelvic pain caused by endometriosis include depot medroxyprogesterone acetate injectable suspension, depot leuprolide acetate, goserelin implant, and danazol. I do not routinely prescribe depot medroxyprogesterone acetate because some patients report new onset or worsening symptoms of depression on the medication. I prescribe depot-leuprolide acetate less often than in the past, because many patients report moderate to severe hypoestrogenic symptoms on this medication. In women taking depot-leuprolide acetate, moderate to severe vasomotor symptoms can be improved by prescribing NEA pills, but the alternative of norethindrone monotherapy is less expensive. I seldom use goserelin or danazol in my practice. The needle required to place the goserelin implant has a diameter of approximately 1.7 mm (16 gauge) or 2.1 mm (14 gauge), for the 3.6 mg and 10 mg doses, respectively. The large diameter of the needle can cause pain and bruising at the implant site. As a comparison, the progestin subdermal implant needle is approximately 2.1 mm in diameter. Danazol is associated with weight gain, and most women prefer to avoid this side effect.
Continue to: Norethindrone acetate...
Norethindrone acetate
NEA 5 mg daily is approved by the FDA to treat endometriosis.1 NEA was approved at a time when large controlled clinical trials were not routinely required for a medicine to be approved. The data to support NEA treatment of pelvic pain caused by endometriosis is based on cohort studies. In a study of 194 women, median age 21 years with moderate to severe pelvic pain and surgically proven endometriosis, the effect of NEA on pelvic pain was explored.2 The initial dose of NEA was 5 mg daily. If the patient did not achieve a reduction in pelvic pain and amenorrhea on the NEA dose of 5 mg daily, the dose was increased by 2.5 mg every 2 weeks, up to a maximum of 15 mg, until amenorrhea and/or a decrease in pelvic pain was achieved. Ninety-five percent of the women in this cohort had previously been treated with an estrogen-progestin contraceptive or a GnRH antagonist and had discontinued those medications because of inadequate control of pelvic pain or because of side effects of the medication.
In this large cohort, 65% of women reported significant improvement in pelvic pain, with a median pain score of 5 before treatment and 0 following NEA treatment. About 55% of the women reported no side effects. The most commonly reported side effects were weight gain (16%; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%). (In this study women could report more than one side effect.)
In another cohort study of 52 women with pelvic pain and surgically confirmed endometriosis, NEA treatment resulted in pain relief in 94% of the women.3 Breakthrough bleeding was a common side effect, reported by 58% of participants. The investigators concluded that NEA treatment was a “cost-effective alternative with relatively mild side effects in the treatment of symptomatic endometriosis.” A conclusion which I endorse.
NEA has been reported to effectively treat ovarian endometriomas and rectovaginal endometriosis.4,5 In a cohort of 18 women who had previously had the surgical resection of an ovarian endometriosis cyst and had postoperative recurrence of pelvic pain and ovarian endometriosis, treatment was initiated with an escalating NEA regimen.4 Treatment was initiated with NEA 5 mg daily, with the dosage increased every 2 weeks by 2.5 mg until amenorrhea was established. Most women achieved amenorrhea with NEA 5 mg daily, and 89% had reduced pelvic pain. The investigators reported complete regression of the endometriosis cyst(s) in 74% of the women. In my experience, NEA does not result in complete regression of endometriosis cysts, but it does cause a reduction in cyst diameter and total volume.
In a retrospective cohort study, 61 women with pelvic pain and rectovaginal endometriosis had 5 years of treatment with NEA 2.5 mg or 5.0 mg daily.5 NEA treatment resulted in a decrease in dysmenorrhea, deep dyspareunia, and dyschezia. The most common side effects attributed to NEA treatment were weight gain (30%), vaginal bleeding (23%), decreased libido (11%), headache (9%), bloating or swelling (8%), depression (7%), and acne (5%). In women who had sequential imaging studies, NEA treatment resulted in a decrease in rectovaginal lesion volume, stable disease volume, or an increase in lesion volume in 56%, 32%, and 12% of the women, respectively. The investigators concluded that for women with rectovaginal endometriosis, NEA treatment is a low-cost option for long-term treatment.
In my practice, I do not prescribe NEA at doses greater than 5 mg daily. There are case reports that NEA at a dose of ≥10 mg daily is associated with the development of a hepatic adenoma,6 elevated liver transaminase concentration,7 and jaundice.8 If NEA 5 mg daily is not effective in controlling pelvic pain caused by endometriosis, I stop the NEA and start a GnRH analogue, most often elagolix.
NEA 5 mg is not FDA approved as a contraceptive. However, norethindrone 0.35 mg daily, also known as the “mini-pill”, is approved as a progestin-only contraceptive.9 NEA is rapidly and completely deacetylated to norethindrone, and the disposition of oral NEA is indistinguishable from that of norethindrone.1 Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg has contraceptive properties if taken daily.
Continue to: Elagolix...
Elagolix
Elagolix is FDA approved for the treatment of pelvic pain caused by endometriosis. I reviewed the key studies resulting in FDA approval in the November 2018 issue of
In the Elaris Endometriosis-I study, 872 women with endometriosis and pelvic pain were randomly assigned to treatment with 1 of 2 doses of elagolix (high-dose [200 mg twice daily] and low-dose [150 mg once daily]) or placebo.11 After 3 months of therapy, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). After 3 months of therapy, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.01 low-dose elagolix vs placebo and P<.001 high-dose elagolix vs placebo).
Hot flashes that were severe enough to be reported as an adverse event by the study participants were reported by 42%, 24%, and 7% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47% and hip femoral neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively.
Another large clinical trial of elagolix for the treatment of pelvic pain caused by endometriosis, Elaris EM-II, involving 817 women, produced results very similar to those reported in Elaris EM-I. The elagolix continuation studies, Elaris EM-III and -IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.12
In my 2018 review,10 I noted that elagolix dose adjustment can be utilized to attempt to achieve maximal pain relief with minimal vasomotor symptoms. Elagolix at 200 mg twice daily produces a mean estradiol concentration of 12 pg/mL, whereas elagolix at 150 mg daily resulted in a mean estradiol concentration of 41 pg/mL.13 The estrogen threshold hypothesis posits that in women with endometriosis a stable estradiol concentration of 20 to 30 pg/mL is often associated with decreased pain and fewer vasomotor events.14 To achieve the target estradiol range of 20 to 30 pg/mL, I often initiate elagolix treatment with 200 mg twice daily. This enables a rapid onset of amenorrhea and a reduction in pelvic pain. Once amenorrhea has been achieved and a decrease in pelvic pain has occurred, I adjust the dose downward to 200 mg twice daily on even calendar days of each month and 200 mg once daily on odd calendar days each month. Some women will have continued pain relief and amenorrhea when the dose is further decreased to 200 mg once daily. If bothersome bleeding recurs and/or pain symptoms increase in severity, the dose can be increased to 200 mg twice daily or an alternating regimen of 200 mg twice daily and 200 mg once daily, every 2 days. An alternative to dose adjustment is to combine elagolix with NEA, which can reduce the severity of hot flashes and reduce bone loss caused by hypoestrogenism.15,16
Health insurers and pharmacy benefits managers may require a prior authorization before approving and dispensing elagolix. The prior authorization process can be burdensome for clinicians, consuming limited healthcare resources, contributing to burnout and frustrating patients.17 Elagolix is less expensive than depot-leuprolide acetate and nafarelin nasal spray and somewhat more expensive than a goserelin implant.18,19
Elagolix is not approved as a contraceptive. In the Elaris EM-I and -II trials women were advised to use 2 forms of contraception, although pregnancies did occur. There were 6 pregnancies among 475 women taking elagolix 150 mg daily and 2 pregnancies among 477 women taking elagolix 200 mg twice daily.20 Women taking elagolix should be advised to use a contraceptive, but not an estrogen-progestin contraceptive.
Continue to: Do not use opioids to treat chronic pelvic pain caused by endometriosis...
Do not use opioids to treat chronic pelvic pain caused by endometriosis
One of the greatest public health tragedies of our era is the opioid misuse epidemic. Hundreds of thousands of deaths have been caused by opioid misuse. The Centers for Disease Control and Prevention reported that for the 12-month period ending in May 2020, there were 81,000 opioid-related deaths, the greatest number ever reported in a 12-month period.21 Many authorities believe that in the United States opioid medications have been over-prescribed, contributing to the opioid misuse epidemic. There is little evidence that chronic pelvic pain is optimally managed by chronic treatment with an opioid.22,23 Prescribing opioids to vulnerable individuals to treat chronic pelvic pain may result in opioid dependency and adversely affect the patient’s health. It is best to pledge not to prescribe an opioid medication for a woman with chronic pelvic pain caused by endometriosis. In situations when pelvic pain is difficult to control with hormonal therapy and nonopioid pain medications, referral to a specialty pain practice may be warranted.
Post–conservative surgery hormone treatment reduces pelvic pain recurrence
In a meta-analysis of 14 studies that reported on endometriosis recurrence rates following conservative surgery, recurrence (defined as recurrent pelvic pain or an imaging study showing recurrent endometriosis) was significantly reduced with the use of hormone treatment compared with expectant management or placebo treatment.24 The postoperative relative risk of endometriosis recurrence was reduced by 83% with progestin treatment, 64% with estrogen-progestin contraceptive treatment, and 38% with GnRH analogue treatment. Overall, the number of patients that needed to be treated to prevent one endometriosis recurrence was 10, assuming a recurrence rate of 25% in the placebo treatment or expectant management groups.
For women with pelvic pain caused by endometriosis who develop a recurrence of pelvic pain while on postoperative hormone treatment, it is important for the prescribing clinician to be flexible and consider changing the hormone regimen. For example, if a postoperative patient is treated with a continuous estrogen-progestin contraceptive and develops recurrent pain, I will stop the contraceptive and initiate treatment with either NEA or elagolix.
Capitalize on opportunities to improve the medical care of women with endometriosis
Early diagnosis of endometriosis can be facilitated by recognizing that the condition is a common cause of moderate to severe dysmenorrhea. In 5 studies involving 1,187 women, the mean length of time from onset of pelvic pain symptoms to diagnosis of endometriosis was 8.6 years.25 If a woman with pelvic pain caused by endometriosis has not had sufficient pain relief with one brand of continuous estrogen-progestin contraceptive, it is best not to prescribe an alternative brand but rather to switch to a progestin-only treatment or a GnRH antagonist. If plan A is not working, move expeditiously to plan B. ●
Women with endometriosis often present for medical care for one or more of the following health issues: pelvic pain, infertility, and/or an adnexal cyst (endometrioma). For women with moderate or severe pelvic pain and laparoscopically diagnosed endometriosis, hormone therapy is often necessary to achieve maximal long-term reduction in pain and optimize health. I focus on opportunities to optimize hormonal treatment of endometriosis in this editorial.
When plan A is not working, move expeditiously to plan B
Cyclic or continuous combination estrogen-progestin contraceptives are commonly prescribed to treat pelvic pain caused by endometriosis. Although endometriosis pain may initially improve with estrogen-progestin contraceptives, many women on this medication will eventually report that they have worsening pelvic pain that adversely impacts their daily activities. Surprisingly, clinicians often continue to prescribe estrogen-progestin contraceptives even after the patient reports that the treatment is not effective, and their pain continues to be bothersome.
Patients benefit when they have access to the full range of hormone treatments that have been approved by the FDA for the treatment of moderate to severe pelvic pain caused by endometriosis (TABLE). In the situation where an estrogen-progestin contraceptive is no longer effective at reducing the pelvic pain, I will often offer the patient the option of norethindrone acetate (NEA) or elagolix treatment. My experience is that stopping the estrogen-progestin contraceptive and starting NEA or elagolix will result in a significant decrease in pain symptoms and improvement in the patient’s quality of life.

Other FDA-approved options to treat pelvic pain caused by endometriosis include depot medroxyprogesterone acetate injectable suspension, depot leuprolide acetate, goserelin implant, and danazol. I do not routinely prescribe depot medroxyprogesterone acetate because some patients report new onset or worsening symptoms of depression on the medication. I prescribe depot-leuprolide acetate less often than in the past, because many patients report moderate to severe hypoestrogenic symptoms on this medication. In women taking depot-leuprolide acetate, moderate to severe vasomotor symptoms can be improved by prescribing NEA pills, but the alternative of norethindrone monotherapy is less expensive. I seldom use goserelin or danazol in my practice. The needle required to place the goserelin implant has a diameter of approximately 1.7 mm (16 gauge) or 2.1 mm (14 gauge), for the 3.6 mg and 10 mg doses, respectively. The large diameter of the needle can cause pain and bruising at the implant site. As a comparison, the progestin subdermal implant needle is approximately 2.1 mm in diameter. Danazol is associated with weight gain, and most women prefer to avoid this side effect.
Continue to: Norethindrone acetate...
Norethindrone acetate
NEA 5 mg daily is approved by the FDA to treat endometriosis.1 NEA was approved at a time when large controlled clinical trials were not routinely required for a medicine to be approved. The data to support NEA treatment of pelvic pain caused by endometriosis is based on cohort studies. In a study of 194 women, median age 21 years with moderate to severe pelvic pain and surgically proven endometriosis, the effect of NEA on pelvic pain was explored.2 The initial dose of NEA was 5 mg daily. If the patient did not achieve a reduction in pelvic pain and amenorrhea on the NEA dose of 5 mg daily, the dose was increased by 2.5 mg every 2 weeks, up to a maximum of 15 mg, until amenorrhea and/or a decrease in pelvic pain was achieved. Ninety-five percent of the women in this cohort had previously been treated with an estrogen-progestin contraceptive or a GnRH antagonist and had discontinued those medications because of inadequate control of pelvic pain or because of side effects of the medication.
In this large cohort, 65% of women reported significant improvement in pelvic pain, with a median pain score of 5 before treatment and 0 following NEA treatment. About 55% of the women reported no side effects. The most commonly reported side effects were weight gain (16%; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%). (In this study women could report more than one side effect.)
In another cohort study of 52 women with pelvic pain and surgically confirmed endometriosis, NEA treatment resulted in pain relief in 94% of the women.3 Breakthrough bleeding was a common side effect, reported by 58% of participants. The investigators concluded that NEA treatment was a “cost-effective alternative with relatively mild side effects in the treatment of symptomatic endometriosis.” A conclusion which I endorse.
NEA has been reported to effectively treat ovarian endometriomas and rectovaginal endometriosis.4,5 In a cohort of 18 women who had previously had the surgical resection of an ovarian endometriosis cyst and had postoperative recurrence of pelvic pain and ovarian endometriosis, treatment was initiated with an escalating NEA regimen.4 Treatment was initiated with NEA 5 mg daily, with the dosage increased every 2 weeks by 2.5 mg until amenorrhea was established. Most women achieved amenorrhea with NEA 5 mg daily, and 89% had reduced pelvic pain. The investigators reported complete regression of the endometriosis cyst(s) in 74% of the women. In my experience, NEA does not result in complete regression of endometriosis cysts, but it does cause a reduction in cyst diameter and total volume.
In a retrospective cohort study, 61 women with pelvic pain and rectovaginal endometriosis had 5 years of treatment with NEA 2.5 mg or 5.0 mg daily.5 NEA treatment resulted in a decrease in dysmenorrhea, deep dyspareunia, and dyschezia. The most common side effects attributed to NEA treatment were weight gain (30%), vaginal bleeding (23%), decreased libido (11%), headache (9%), bloating or swelling (8%), depression (7%), and acne (5%). In women who had sequential imaging studies, NEA treatment resulted in a decrease in rectovaginal lesion volume, stable disease volume, or an increase in lesion volume in 56%, 32%, and 12% of the women, respectively. The investigators concluded that for women with rectovaginal endometriosis, NEA treatment is a low-cost option for long-term treatment.
In my practice, I do not prescribe NEA at doses greater than 5 mg daily. There are case reports that NEA at a dose of ≥10 mg daily is associated with the development of a hepatic adenoma,6 elevated liver transaminase concentration,7 and jaundice.8 If NEA 5 mg daily is not effective in controlling pelvic pain caused by endometriosis, I stop the NEA and start a GnRH analogue, most often elagolix.
NEA 5 mg is not FDA approved as a contraceptive. However, norethindrone 0.35 mg daily, also known as the “mini-pill”, is approved as a progestin-only contraceptive.9 NEA is rapidly and completely deacetylated to norethindrone, and the disposition of oral NEA is indistinguishable from that of norethindrone.1 Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg has contraceptive properties if taken daily.
Continue to: Elagolix...
Elagolix
Elagolix is FDA approved for the treatment of pelvic pain caused by endometriosis. I reviewed the key studies resulting in FDA approval in the November 2018 issue of
In the Elaris Endometriosis-I study, 872 women with endometriosis and pelvic pain were randomly assigned to treatment with 1 of 2 doses of elagolix (high-dose [200 mg twice daily] and low-dose [150 mg once daily]) or placebo.11 After 3 months of therapy, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). After 3 months of therapy, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.01 low-dose elagolix vs placebo and P<.001 high-dose elagolix vs placebo).
Hot flashes that were severe enough to be reported as an adverse event by the study participants were reported by 42%, 24%, and 7% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47% and hip femoral neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively.
Another large clinical trial of elagolix for the treatment of pelvic pain caused by endometriosis, Elaris EM-II, involving 817 women, produced results very similar to those reported in Elaris EM-I. The elagolix continuation studies, Elaris EM-III and -IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.12
In my 2018 review,10 I noted that elagolix dose adjustment can be utilized to attempt to achieve maximal pain relief with minimal vasomotor symptoms. Elagolix at 200 mg twice daily produces a mean estradiol concentration of 12 pg/mL, whereas elagolix at 150 mg daily resulted in a mean estradiol concentration of 41 pg/mL.13 The estrogen threshold hypothesis posits that in women with endometriosis a stable estradiol concentration of 20 to 30 pg/mL is often associated with decreased pain and fewer vasomotor events.14 To achieve the target estradiol range of 20 to 30 pg/mL, I often initiate elagolix treatment with 200 mg twice daily. This enables a rapid onset of amenorrhea and a reduction in pelvic pain. Once amenorrhea has been achieved and a decrease in pelvic pain has occurred, I adjust the dose downward to 200 mg twice daily on even calendar days of each month and 200 mg once daily on odd calendar days each month. Some women will have continued pain relief and amenorrhea when the dose is further decreased to 200 mg once daily. If bothersome bleeding recurs and/or pain symptoms increase in severity, the dose can be increased to 200 mg twice daily or an alternating regimen of 200 mg twice daily and 200 mg once daily, every 2 days. An alternative to dose adjustment is to combine elagolix with NEA, which can reduce the severity of hot flashes and reduce bone loss caused by hypoestrogenism.15,16
Health insurers and pharmacy benefits managers may require a prior authorization before approving and dispensing elagolix. The prior authorization process can be burdensome for clinicians, consuming limited healthcare resources, contributing to burnout and frustrating patients.17 Elagolix is less expensive than depot-leuprolide acetate and nafarelin nasal spray and somewhat more expensive than a goserelin implant.18,19
Elagolix is not approved as a contraceptive. In the Elaris EM-I and -II trials women were advised to use 2 forms of contraception, although pregnancies did occur. There were 6 pregnancies among 475 women taking elagolix 150 mg daily and 2 pregnancies among 477 women taking elagolix 200 mg twice daily.20 Women taking elagolix should be advised to use a contraceptive, but not an estrogen-progestin contraceptive.
Continue to: Do not use opioids to treat chronic pelvic pain caused by endometriosis...
Do not use opioids to treat chronic pelvic pain caused by endometriosis
One of the greatest public health tragedies of our era is the opioid misuse epidemic. Hundreds of thousands of deaths have been caused by opioid misuse. The Centers for Disease Control and Prevention reported that for the 12-month period ending in May 2020, there were 81,000 opioid-related deaths, the greatest number ever reported in a 12-month period.21 Many authorities believe that in the United States opioid medications have been over-prescribed, contributing to the opioid misuse epidemic. There is little evidence that chronic pelvic pain is optimally managed by chronic treatment with an opioid.22,23 Prescribing opioids to vulnerable individuals to treat chronic pelvic pain may result in opioid dependency and adversely affect the patient’s health. It is best to pledge not to prescribe an opioid medication for a woman with chronic pelvic pain caused by endometriosis. In situations when pelvic pain is difficult to control with hormonal therapy and nonopioid pain medications, referral to a specialty pain practice may be warranted.
Post–conservative surgery hormone treatment reduces pelvic pain recurrence
In a meta-analysis of 14 studies that reported on endometriosis recurrence rates following conservative surgery, recurrence (defined as recurrent pelvic pain or an imaging study showing recurrent endometriosis) was significantly reduced with the use of hormone treatment compared with expectant management or placebo treatment.24 The postoperative relative risk of endometriosis recurrence was reduced by 83% with progestin treatment, 64% with estrogen-progestin contraceptive treatment, and 38% with GnRH analogue treatment. Overall, the number of patients that needed to be treated to prevent one endometriosis recurrence was 10, assuming a recurrence rate of 25% in the placebo treatment or expectant management groups.
For women with pelvic pain caused by endometriosis who develop a recurrence of pelvic pain while on postoperative hormone treatment, it is important for the prescribing clinician to be flexible and consider changing the hormone regimen. For example, if a postoperative patient is treated with a continuous estrogen-progestin contraceptive and develops recurrent pain, I will stop the contraceptive and initiate treatment with either NEA or elagolix.
Capitalize on opportunities to improve the medical care of women with endometriosis
Early diagnosis of endometriosis can be facilitated by recognizing that the condition is a common cause of moderate to severe dysmenorrhea. In 5 studies involving 1,187 women, the mean length of time from onset of pelvic pain symptoms to diagnosis of endometriosis was 8.6 years.25 If a woman with pelvic pain caused by endometriosis has not had sufficient pain relief with one brand of continuous estrogen-progestin contraceptive, it is best not to prescribe an alternative brand but rather to switch to a progestin-only treatment or a GnRH antagonist. If plan A is not working, move expeditiously to plan B. ●
- Aygestin [package insert]. Barr Laboratories: Pomona, NY; 2007.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108.
- Muneyyirci-Delale O, Karacan M. Effect of norethindrone acetate in the treatment of symptomatic endometriosis. Int J Fertil Womens Med. 1998;43:24-27.
- Muneyyirci-Delale O, Anopa J, Charles C, et al. Medical management of recurrent endometrioma with long-term norethindrone acetate. Int J Women Health. 2012;4:149-154.
- Morotti M, Venturini PL, Biscaldi E, et al. Efficacy and acceptability of long-term norethindrone acetate for the treatment of rectovaginal endometriosis. Eur J Obstet Gynecol Repro Biol. 2017;213:4-10.
- Brady PC, Missmer SA, Laufer MR. Hepatic adenomas in adolescents and young women with endometriosis treated with norethindrone acetate. J Pediatr Adolesc Gynecol. 2017;30:422-424.
- Choudhary NS, Bodh V, Chaudhari S, et al. Norethisterone related drug induced liver injury: a series of 3 cases. J Clin Exp Hepatol. 2017;7:266- 268.
- Perez-Mera RA, Shields CE. Jaundice associated with norethindrone acetate therapy. N Engl J Med. 1962;267:1137-1138.
- Camila [package insert]. Mayne Pharma Inc: Greenville, NC; 2018.
- Barbieri RL. Elagolix: a new treatment for pelvic pain caused by endometriosis. OBG Manag. 2018;30:10,12-14, 20.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Orilissa [package insert]. AbbVie Inc; North Chicago, IL; 2018.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Gallagher JS, Missmer SA, Hornstein MD, et al. Long-term effects of gonadotropin-releasing hormone agonists and add-back in adolescent endometriosis. J Pediatr Adolesc Gynecol. 2018;31:376- 381.
- Miller A, Shor R, Waites T, et al. Prior authorization reform for better patient care. J Am Coll Cardiol. 2018;71:1937-1939.
- Depot-leuprolide acetate. Good Rx website. https://www.goodrx.com/. Accessed January 22, 2021.
- Goserelin. Good Rx website. https://www .goodrx.com/. Accessed January 22, 2021
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID19. https://www.cdc.gov/media/releases/2020 /p1218-overdose-deaths-covid-19.html. Reviewed December 18, 2020. Accessed March 24, 2021.
- Till SR, As-Sanie S. 3 cases of chronic pelvic pain with nonsurgical, nonopioid therapies. OBG Manag. 2018;30:41-48.
- Steele A. Opioid use and depression in chronic pelvic pain. Obstet Gynecol Clin North Am. 2014;41:491-501.
- Zakhari A, Delpero E, McKeown S, et al. Endometriosis recurrence following post-operative hormonal suppression: a systematic review and meta-analysis. Hum Reprod Update. 2021;27:96- 107.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8, 10-11, 16.
- Aygestin [package insert]. Barr Laboratories: Pomona, NY; 2007.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108.
- Muneyyirci-Delale O, Karacan M. Effect of norethindrone acetate in the treatment of symptomatic endometriosis. Int J Fertil Womens Med. 1998;43:24-27.
- Muneyyirci-Delale O, Anopa J, Charles C, et al. Medical management of recurrent endometrioma with long-term norethindrone acetate. Int J Women Health. 2012;4:149-154.
- Morotti M, Venturini PL, Biscaldi E, et al. Efficacy and acceptability of long-term norethindrone acetate for the treatment of rectovaginal endometriosis. Eur J Obstet Gynecol Repro Biol. 2017;213:4-10.
- Brady PC, Missmer SA, Laufer MR. Hepatic adenomas in adolescents and young women with endometriosis treated with norethindrone acetate. J Pediatr Adolesc Gynecol. 2017;30:422-424.
- Choudhary NS, Bodh V, Chaudhari S, et al. Norethisterone related drug induced liver injury: a series of 3 cases. J Clin Exp Hepatol. 2017;7:266- 268.
- Perez-Mera RA, Shields CE. Jaundice associated with norethindrone acetate therapy. N Engl J Med. 1962;267:1137-1138.
- Camila [package insert]. Mayne Pharma Inc: Greenville, NC; 2018.
- Barbieri RL. Elagolix: a new treatment for pelvic pain caused by endometriosis. OBG Manag. 2018;30:10,12-14, 20.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Orilissa [package insert]. AbbVie Inc; North Chicago, IL; 2018.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Gallagher JS, Missmer SA, Hornstein MD, et al. Long-term effects of gonadotropin-releasing hormone agonists and add-back in adolescent endometriosis. J Pediatr Adolesc Gynecol. 2018;31:376- 381.
- Miller A, Shor R, Waites T, et al. Prior authorization reform for better patient care. J Am Coll Cardiol. 2018;71:1937-1939.
- Depot-leuprolide acetate. Good Rx website. https://www.goodrx.com/. Accessed January 22, 2021.
- Goserelin. Good Rx website. https://www .goodrx.com/. Accessed January 22, 2021
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID19. https://www.cdc.gov/media/releases/2020 /p1218-overdose-deaths-covid-19.html. Reviewed December 18, 2020. Accessed March 24, 2021.
- Till SR, As-Sanie S. 3 cases of chronic pelvic pain with nonsurgical, nonopioid therapies. OBG Manag. 2018;30:41-48.
- Steele A. Opioid use and depression in chronic pelvic pain. Obstet Gynecol Clin North Am. 2014;41:491-501.
- Zakhari A, Delpero E, McKeown S, et al. Endometriosis recurrence following post-operative hormonal suppression: a systematic review and meta-analysis. Hum Reprod Update. 2021;27:96- 107.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8, 10-11, 16.
How physicians can provide better care to transgender patients
People who identify as transgender experience many health disparities, in addition to lack of access to quality care. The most commonly cited barrier is the lack of providers who are knowledgeable about transgender health care, according to past surveys.
Even those who do seek care often have unpleasant experiences. A 2015 survey conducted by the National Center for Transgender Equality found that 33% of those who saw a health care provider reported at least one unfavorable experience related to being transgender, such as being verbally harassed or refused treatment because of their gender identity. In fact, 23% of those surveyed say they did not seek health care they needed in the past year because of fear of being mistreated as a transgender person.
This interview has been edited for length and clarity.
Question: Surveys have shown that many people who identify as transgender will seek only transition care, not primary or preventive care. Why is that?
Dr. Brandt: My answer is multifactorial. Transgender patients do seek primary care – just not as readily. There’s a lot of misconceptions about health care needs for the LGBT community in general. For example, lesbian or bisexual women may be not as well informed about the need for Pap smears compared with their heterosexual counterparts. These misconceptions are further exacerbated in the transgender community.
The fact that a lot of patients seek only transition-related care, but not preventive services, such as primary care and gynecologic care, is also related to fears of discrimination and lack of education of providers. These patients are afraid when they walk into an office that they will be misgendered or their physician won’t be familiar with their health care needs.
What can clinics and clinicians do to create a safe and welcoming environment?
Dr. Brandt: It starts with educating office staff about terminology and gender identities.
A key feature of our EHR is the sexual orientation and gender identity platform, which asks questions about a patient’s gender identity, sexual orientation, sex assigned at birth, and organ inventory. These data are then found in the patient information tab and are just as relevant as their insurance status, age, and date of birth.
There are many ways a doctor’s office can signal to patients that they are inclusive. They can hang LGBTQ-friendly flags or symbols or a sign saying, “We have an anti-discrimination policy” in the waiting room. A welcoming environment can also be achieved by revising patient questionnaires or forms so that they aren’t gender-specific or binary.
Given that the patient may have limited contact with a primary care clinician, how do you prioritize what you address during the visit?
Dr. Brandt: Similar to cisgender patients, it depends initially on the age of the patient and the reason for the visit. The priorities of an otherwise healthy transgender patient in their 20s are going to be largely the same as for a cisgender patient of the same age. As patients age in the primary care world, you’re addressing more issues, such as colorectal screening, lipid disorders, and mammograms, and that doesn’t change. For the most part, the problems that you address should be specific for that age group.
It becomes more complicated when you add in factors such as hormone therapy and whether patients have had any type of gender-affirming surgery. Those things can change the usual recommendations for screening or risk assessment. We try to figure out what routine health maintenance and cancer screening a patient needs based on age and risk factors, in addition to hormone status and surgical state.
Do you think that many physicians are educated about the care of underserved populations such as transgender patients?
Dr. Brandt: Yes and no. We are definitely getting better at it. For example, the American College of Obstetricians and Gynecologists published a committee opinion highlighting transgender care. So organizations are starting to prioritize these populations and recognize that they are, in fact, underserved and they have special health care needs.
However, the knowledge gaps are still pretty big. I get calls daily from providers asking questions about how to manage patients on hormones, or how to examine a patient who has undergone a vaginoplasty. I hear a lot of horror stories from transgender patients who had their hormones stopped for absurd and medically misinformed reasons.
But I definitely think it’s getting better and it’s being addressed at all levels – the medical school level, the residency level, and the attending level. It just takes time to inform people and for people to get used to the health care needs of these patients.
What should physicians keep in mind when treating patients who identify as transgender?
Dr. Brandt: First and foremost, understanding the terminology and the difference between gender identity, sex, and sexual orientation. Being familiar with that language and being able to speak that language very comfortably and not being awkward about it is a really important thing for primary care physicians and indeed any physician who treats transgender patients.
Physicians should also be aware that any underserved population has higher rates of mental health issues, such as depression and anxiety. Obviously, that goes along with being underserved and the stigma and the disparities that exist for these patients. Having providers educate themselves about what those disparities are and how they impact a patient’s daily life and health is paramount to knowing how to treat patients.
What are your top health concerns for these patients and how do you address them?
Dr. Brandt: I think mental health and safety is probably the number one for me. About 41% of transgender adults have attempted suicide. That number is roughly 51% in transgender youth. That is an astonishing number. These patients have much higher rates of domestic violence, intimate partner violence, and sexual assault, especially trans women and trans women of color. So understanding those statistics is huge.
Obesity, smoking, and substance abuse are my next three. Again, those are things that should be addressed at any visit, regardless of the gender identity or sexual orientation of the patient, but those rates are particularly high in this population.
Fertility and long-term care for patients should be addressed. Many patients who identify as transgender are told they can’t have a family. As a primary care physician, you may see a patient before they are seen by an ob.gyn. or surgeon. Talking about what a patient’s long-term life goals are with fertility and family planning, and what that looks like for them, is a big thing for me. Other providers may not feel that’s a concern, but I believe it should be discussed before initiation of hormone therapy, which can significantly impact fertility in some patients.
Are there nuances to the physical examination that primary care physicians should be aware of when dealing with transmasculine patients vs. transfeminine patients?
Dr. Brandt: Absolutely. And this interview can’t cover the scope of those nuances. An example that comes to mind is the genital exam. For transgender women who have undergone a vaginoplasty, the pelvic exam can be very affirming. Whereas for transgender men, a gynecologic exam can significantly exacerbate dysphoria and there are ways to conduct the exam to limit this discomfort and avoid creating a traumatic experience for the patient. It’s important to be aware that the genital exam, or any type of genitourinary exam, can be either affirming or not affirming.
Sexually transmitted infections are up in the general population, and the trans population is at even higher risk. What should physicians think about when they assess this risk?
Dr. Brandt: It’s really important for primary care clinicians and for gynecologists to learn to be comfortable talking about sexual practices, because what people do behind closed doors is really a key to how to counsel patients about safe sex.
People are well aware of the need to have safe sex. However, depending on the type of sex that you’re having, what body parts go where, what is truly safe can vary and people may not know, for example, to wear a condom when sex toys are involved or that a transgender male on testosterone can become pregnant during penile-vaginal intercourse. Providers really should be very educated on the array of sexual practices that people have and how to counsel them about those. They should know how to ask patients the gender identity of their sexual partners, the sexual orientation of their partners, and what parts go where during sex.
Providers should also talk to patients about PrEP [pre-exposure prophylaxis], whether they identify as cisgender or transgender. My trans patients tend to be a lot more educated about PrEP than other patients. It’s something that many of the residents, even in a standard gynecologic clinic, for example, don’t talk to cisgender patients about because of the stigma surrounding HIV. Many providers still think that the only people who are at risk for HIV are men who have sex with men. And while those rates are higher in some populations, depending on sexual practices, those aren’t the only patients who qualify for PrEP.
Overall, in order to counsel patients about STIs and safe sexual practices, providers should learn to be comfortable talking about sex.
Do you have any strategies on how to make the appointment more successful in addressing those issues?
Dr. Brandt: Bedside manner is a hard thing to teach, and comfort in talking about sex, gender identity, and sexual orientation can vary – but there are a lot of continuing medical education courses that physicians can utilize through the World Professional Association for Transgender Health.
If providers start to notice an influx of patients who identify as transgender or if they want to start seeing transgender patients, it’s really important for them to have that training before they start interacting with patients. In all of medicine, we sort of learn as we go, but this patient population has been subjected to discrimination, violence, error, and misgendering. They have dealt with providers who didn’t understand their health care needs. While this field is evolving, knowing how to appropriately address a patient (using their correct name, pronouns, etc.) is an absolute must.
That needs to be part of a provider’s routine vernacular and not something that they sort of stumble through. You can scare a patient away as soon as they walk into the office with an uneducated front desk staff and things that are seen in the office. Seeking out those educational tools, being aware of your own deficits as a provider and the educational needs of your office, and addressing those needs is really key.
A version of this article first appeared on Medscape.com.
People who identify as transgender experience many health disparities, in addition to lack of access to quality care. The most commonly cited barrier is the lack of providers who are knowledgeable about transgender health care, according to past surveys.
Even those who do seek care often have unpleasant experiences. A 2015 survey conducted by the National Center for Transgender Equality found that 33% of those who saw a health care provider reported at least one unfavorable experience related to being transgender, such as being verbally harassed or refused treatment because of their gender identity. In fact, 23% of those surveyed say they did not seek health care they needed in the past year because of fear of being mistreated as a transgender person.
This interview has been edited for length and clarity.
Question: Surveys have shown that many people who identify as transgender will seek only transition care, not primary or preventive care. Why is that?
Dr. Brandt: My answer is multifactorial. Transgender patients do seek primary care – just not as readily. There’s a lot of misconceptions about health care needs for the LGBT community in general. For example, lesbian or bisexual women may be not as well informed about the need for Pap smears compared with their heterosexual counterparts. These misconceptions are further exacerbated in the transgender community.
The fact that a lot of patients seek only transition-related care, but not preventive services, such as primary care and gynecologic care, is also related to fears of discrimination and lack of education of providers. These patients are afraid when they walk into an office that they will be misgendered or their physician won’t be familiar with their health care needs.
What can clinics and clinicians do to create a safe and welcoming environment?
Dr. Brandt: It starts with educating office staff about terminology and gender identities.
A key feature of our EHR is the sexual orientation and gender identity platform, which asks questions about a patient’s gender identity, sexual orientation, sex assigned at birth, and organ inventory. These data are then found in the patient information tab and are just as relevant as their insurance status, age, and date of birth.
There are many ways a doctor’s office can signal to patients that they are inclusive. They can hang LGBTQ-friendly flags or symbols or a sign saying, “We have an anti-discrimination policy” in the waiting room. A welcoming environment can also be achieved by revising patient questionnaires or forms so that they aren’t gender-specific or binary.
Given that the patient may have limited contact with a primary care clinician, how do you prioritize what you address during the visit?
Dr. Brandt: Similar to cisgender patients, it depends initially on the age of the patient and the reason for the visit. The priorities of an otherwise healthy transgender patient in their 20s are going to be largely the same as for a cisgender patient of the same age. As patients age in the primary care world, you’re addressing more issues, such as colorectal screening, lipid disorders, and mammograms, and that doesn’t change. For the most part, the problems that you address should be specific for that age group.
It becomes more complicated when you add in factors such as hormone therapy and whether patients have had any type of gender-affirming surgery. Those things can change the usual recommendations for screening or risk assessment. We try to figure out what routine health maintenance and cancer screening a patient needs based on age and risk factors, in addition to hormone status and surgical state.
Do you think that many physicians are educated about the care of underserved populations such as transgender patients?
Dr. Brandt: Yes and no. We are definitely getting better at it. For example, the American College of Obstetricians and Gynecologists published a committee opinion highlighting transgender care. So organizations are starting to prioritize these populations and recognize that they are, in fact, underserved and they have special health care needs.
However, the knowledge gaps are still pretty big. I get calls daily from providers asking questions about how to manage patients on hormones, or how to examine a patient who has undergone a vaginoplasty. I hear a lot of horror stories from transgender patients who had their hormones stopped for absurd and medically misinformed reasons.
But I definitely think it’s getting better and it’s being addressed at all levels – the medical school level, the residency level, and the attending level. It just takes time to inform people and for people to get used to the health care needs of these patients.
What should physicians keep in mind when treating patients who identify as transgender?
Dr. Brandt: First and foremost, understanding the terminology and the difference between gender identity, sex, and sexual orientation. Being familiar with that language and being able to speak that language very comfortably and not being awkward about it is a really important thing for primary care physicians and indeed any physician who treats transgender patients.
Physicians should also be aware that any underserved population has higher rates of mental health issues, such as depression and anxiety. Obviously, that goes along with being underserved and the stigma and the disparities that exist for these patients. Having providers educate themselves about what those disparities are and how they impact a patient’s daily life and health is paramount to knowing how to treat patients.
What are your top health concerns for these patients and how do you address them?
Dr. Brandt: I think mental health and safety is probably the number one for me. About 41% of transgender adults have attempted suicide. That number is roughly 51% in transgender youth. That is an astonishing number. These patients have much higher rates of domestic violence, intimate partner violence, and sexual assault, especially trans women and trans women of color. So understanding those statistics is huge.
Obesity, smoking, and substance abuse are my next three. Again, those are things that should be addressed at any visit, regardless of the gender identity or sexual orientation of the patient, but those rates are particularly high in this population.
Fertility and long-term care for patients should be addressed. Many patients who identify as transgender are told they can’t have a family. As a primary care physician, you may see a patient before they are seen by an ob.gyn. or surgeon. Talking about what a patient’s long-term life goals are with fertility and family planning, and what that looks like for them, is a big thing for me. Other providers may not feel that’s a concern, but I believe it should be discussed before initiation of hormone therapy, which can significantly impact fertility in some patients.
Are there nuances to the physical examination that primary care physicians should be aware of when dealing with transmasculine patients vs. transfeminine patients?
Dr. Brandt: Absolutely. And this interview can’t cover the scope of those nuances. An example that comes to mind is the genital exam. For transgender women who have undergone a vaginoplasty, the pelvic exam can be very affirming. Whereas for transgender men, a gynecologic exam can significantly exacerbate dysphoria and there are ways to conduct the exam to limit this discomfort and avoid creating a traumatic experience for the patient. It’s important to be aware that the genital exam, or any type of genitourinary exam, can be either affirming or not affirming.
Sexually transmitted infections are up in the general population, and the trans population is at even higher risk. What should physicians think about when they assess this risk?
Dr. Brandt: It’s really important for primary care clinicians and for gynecologists to learn to be comfortable talking about sexual practices, because what people do behind closed doors is really a key to how to counsel patients about safe sex.
People are well aware of the need to have safe sex. However, depending on the type of sex that you’re having, what body parts go where, what is truly safe can vary and people may not know, for example, to wear a condom when sex toys are involved or that a transgender male on testosterone can become pregnant during penile-vaginal intercourse. Providers really should be very educated on the array of sexual practices that people have and how to counsel them about those. They should know how to ask patients the gender identity of their sexual partners, the sexual orientation of their partners, and what parts go where during sex.
Providers should also talk to patients about PrEP [pre-exposure prophylaxis], whether they identify as cisgender or transgender. My trans patients tend to be a lot more educated about PrEP than other patients. It’s something that many of the residents, even in a standard gynecologic clinic, for example, don’t talk to cisgender patients about because of the stigma surrounding HIV. Many providers still think that the only people who are at risk for HIV are men who have sex with men. And while those rates are higher in some populations, depending on sexual practices, those aren’t the only patients who qualify for PrEP.
Overall, in order to counsel patients about STIs and safe sexual practices, providers should learn to be comfortable talking about sex.
Do you have any strategies on how to make the appointment more successful in addressing those issues?
Dr. Brandt: Bedside manner is a hard thing to teach, and comfort in talking about sex, gender identity, and sexual orientation can vary – but there are a lot of continuing medical education courses that physicians can utilize through the World Professional Association for Transgender Health.
If providers start to notice an influx of patients who identify as transgender or if they want to start seeing transgender patients, it’s really important for them to have that training before they start interacting with patients. In all of medicine, we sort of learn as we go, but this patient population has been subjected to discrimination, violence, error, and misgendering. They have dealt with providers who didn’t understand their health care needs. While this field is evolving, knowing how to appropriately address a patient (using their correct name, pronouns, etc.) is an absolute must.
That needs to be part of a provider’s routine vernacular and not something that they sort of stumble through. You can scare a patient away as soon as they walk into the office with an uneducated front desk staff and things that are seen in the office. Seeking out those educational tools, being aware of your own deficits as a provider and the educational needs of your office, and addressing those needs is really key.
A version of this article first appeared on Medscape.com.
People who identify as transgender experience many health disparities, in addition to lack of access to quality care. The most commonly cited barrier is the lack of providers who are knowledgeable about transgender health care, according to past surveys.
Even those who do seek care often have unpleasant experiences. A 2015 survey conducted by the National Center for Transgender Equality found that 33% of those who saw a health care provider reported at least one unfavorable experience related to being transgender, such as being verbally harassed or refused treatment because of their gender identity. In fact, 23% of those surveyed say they did not seek health care they needed in the past year because of fear of being mistreated as a transgender person.
This interview has been edited for length and clarity.
Question: Surveys have shown that many people who identify as transgender will seek only transition care, not primary or preventive care. Why is that?
Dr. Brandt: My answer is multifactorial. Transgender patients do seek primary care – just not as readily. There’s a lot of misconceptions about health care needs for the LGBT community in general. For example, lesbian or bisexual women may be not as well informed about the need for Pap smears compared with their heterosexual counterparts. These misconceptions are further exacerbated in the transgender community.
The fact that a lot of patients seek only transition-related care, but not preventive services, such as primary care and gynecologic care, is also related to fears of discrimination and lack of education of providers. These patients are afraid when they walk into an office that they will be misgendered or their physician won’t be familiar with their health care needs.
What can clinics and clinicians do to create a safe and welcoming environment?
Dr. Brandt: It starts with educating office staff about terminology and gender identities.
A key feature of our EHR is the sexual orientation and gender identity platform, which asks questions about a patient’s gender identity, sexual orientation, sex assigned at birth, and organ inventory. These data are then found in the patient information tab and are just as relevant as their insurance status, age, and date of birth.
There are many ways a doctor’s office can signal to patients that they are inclusive. They can hang LGBTQ-friendly flags or symbols or a sign saying, “We have an anti-discrimination policy” in the waiting room. A welcoming environment can also be achieved by revising patient questionnaires or forms so that they aren’t gender-specific or binary.
Given that the patient may have limited contact with a primary care clinician, how do you prioritize what you address during the visit?
Dr. Brandt: Similar to cisgender patients, it depends initially on the age of the patient and the reason for the visit. The priorities of an otherwise healthy transgender patient in their 20s are going to be largely the same as for a cisgender patient of the same age. As patients age in the primary care world, you’re addressing more issues, such as colorectal screening, lipid disorders, and mammograms, and that doesn’t change. For the most part, the problems that you address should be specific for that age group.
It becomes more complicated when you add in factors such as hormone therapy and whether patients have had any type of gender-affirming surgery. Those things can change the usual recommendations for screening or risk assessment. We try to figure out what routine health maintenance and cancer screening a patient needs based on age and risk factors, in addition to hormone status and surgical state.
Do you think that many physicians are educated about the care of underserved populations such as transgender patients?
Dr. Brandt: Yes and no. We are definitely getting better at it. For example, the American College of Obstetricians and Gynecologists published a committee opinion highlighting transgender care. So organizations are starting to prioritize these populations and recognize that they are, in fact, underserved and they have special health care needs.
However, the knowledge gaps are still pretty big. I get calls daily from providers asking questions about how to manage patients on hormones, or how to examine a patient who has undergone a vaginoplasty. I hear a lot of horror stories from transgender patients who had their hormones stopped for absurd and medically misinformed reasons.
But I definitely think it’s getting better and it’s being addressed at all levels – the medical school level, the residency level, and the attending level. It just takes time to inform people and for people to get used to the health care needs of these patients.
What should physicians keep in mind when treating patients who identify as transgender?
Dr. Brandt: First and foremost, understanding the terminology and the difference between gender identity, sex, and sexual orientation. Being familiar with that language and being able to speak that language very comfortably and not being awkward about it is a really important thing for primary care physicians and indeed any physician who treats transgender patients.
Physicians should also be aware that any underserved population has higher rates of mental health issues, such as depression and anxiety. Obviously, that goes along with being underserved and the stigma and the disparities that exist for these patients. Having providers educate themselves about what those disparities are and how they impact a patient’s daily life and health is paramount to knowing how to treat patients.
What are your top health concerns for these patients and how do you address them?
Dr. Brandt: I think mental health and safety is probably the number one for me. About 41% of transgender adults have attempted suicide. That number is roughly 51% in transgender youth. That is an astonishing number. These patients have much higher rates of domestic violence, intimate partner violence, and sexual assault, especially trans women and trans women of color. So understanding those statistics is huge.
Obesity, smoking, and substance abuse are my next three. Again, those are things that should be addressed at any visit, regardless of the gender identity or sexual orientation of the patient, but those rates are particularly high in this population.
Fertility and long-term care for patients should be addressed. Many patients who identify as transgender are told they can’t have a family. As a primary care physician, you may see a patient before they are seen by an ob.gyn. or surgeon. Talking about what a patient’s long-term life goals are with fertility and family planning, and what that looks like for them, is a big thing for me. Other providers may not feel that’s a concern, but I believe it should be discussed before initiation of hormone therapy, which can significantly impact fertility in some patients.
Are there nuances to the physical examination that primary care physicians should be aware of when dealing with transmasculine patients vs. transfeminine patients?
Dr. Brandt: Absolutely. And this interview can’t cover the scope of those nuances. An example that comes to mind is the genital exam. For transgender women who have undergone a vaginoplasty, the pelvic exam can be very affirming. Whereas for transgender men, a gynecologic exam can significantly exacerbate dysphoria and there are ways to conduct the exam to limit this discomfort and avoid creating a traumatic experience for the patient. It’s important to be aware that the genital exam, or any type of genitourinary exam, can be either affirming or not affirming.
Sexually transmitted infections are up in the general population, and the trans population is at even higher risk. What should physicians think about when they assess this risk?
Dr. Brandt: It’s really important for primary care clinicians and for gynecologists to learn to be comfortable talking about sexual practices, because what people do behind closed doors is really a key to how to counsel patients about safe sex.
People are well aware of the need to have safe sex. However, depending on the type of sex that you’re having, what body parts go where, what is truly safe can vary and people may not know, for example, to wear a condom when sex toys are involved or that a transgender male on testosterone can become pregnant during penile-vaginal intercourse. Providers really should be very educated on the array of sexual practices that people have and how to counsel them about those. They should know how to ask patients the gender identity of their sexual partners, the sexual orientation of their partners, and what parts go where during sex.
Providers should also talk to patients about PrEP [pre-exposure prophylaxis], whether they identify as cisgender or transgender. My trans patients tend to be a lot more educated about PrEP than other patients. It’s something that many of the residents, even in a standard gynecologic clinic, for example, don’t talk to cisgender patients about because of the stigma surrounding HIV. Many providers still think that the only people who are at risk for HIV are men who have sex with men. And while those rates are higher in some populations, depending on sexual practices, those aren’t the only patients who qualify for PrEP.
Overall, in order to counsel patients about STIs and safe sexual practices, providers should learn to be comfortable talking about sex.
Do you have any strategies on how to make the appointment more successful in addressing those issues?
Dr. Brandt: Bedside manner is a hard thing to teach, and comfort in talking about sex, gender identity, and sexual orientation can vary – but there are a lot of continuing medical education courses that physicians can utilize through the World Professional Association for Transgender Health.
If providers start to notice an influx of patients who identify as transgender or if they want to start seeing transgender patients, it’s really important for them to have that training before they start interacting with patients. In all of medicine, we sort of learn as we go, but this patient population has been subjected to discrimination, violence, error, and misgendering. They have dealt with providers who didn’t understand their health care needs. While this field is evolving, knowing how to appropriately address a patient (using their correct name, pronouns, etc.) is an absolute must.
That needs to be part of a provider’s routine vernacular and not something that they sort of stumble through. You can scare a patient away as soon as they walk into the office with an uneducated front desk staff and things that are seen in the office. Seeking out those educational tools, being aware of your own deficits as a provider and the educational needs of your office, and addressing those needs is really key.
A version of this article first appeared on Medscape.com.
COVID-19 pandemic hinders access to contraception
The pandemic has affected reproductive health because of barriers to contraception access, potentially increasing unwanted pregnancies, reported Tracy Kuo Lin, PhD, of the University of California, San Francisco, and associates.
During the pandemic, women have experienced an increased desire to avoid pregnancy, and when that desire is coupled with loss of income, accessing contraception becomes more difficult, Dr. Lin and colleagues observed in a cross-sectional survey published in the journal Contraception.
The study aimed to quantify the effect of COVID-19 on women’s economic status and reproductive health decisions related to childbearing and pregnancy. Women aged 18-49 who resided in the U.S. were targeted via Facebook and Instagram advertisements from May 16 to June 16, 2020. In all, 554 racially diverse respondents from 43 states were selected: 41% aged 18-24, 37% aged 25-34, and 23% aged 35-49.
Income losses affect nearly half of respondents
In determining risk of unwanted pregnancy, the researchers considered the influence of COVID-19 on a number of factors related to economic well-being as well as respondents’ sexual activity, intimate partner violence, overall desire for pregnancy, and access to contraception as issues affecting their interest in becoming pregnant and their ability to access medical care and contraception. Overall, 46% experienced a reduction in income, 43% reported no change, and 10% experienced an increase in income.
Difficulty in being able to afford food, transportation, and housing doubled among respondents from 8% to 16% as a result of the pandemic. The study authors cited education, race/ethnicity, federal poverty level, and change in income as predictors of inability to provide for these basic needs.
A total of 83% of respondents reported having sex within the past month; 54% of those had sex with someone they lived with, compared with 29% who had sex with someone they did not live with. The pandemic had no impact on sexual desire for 37% of respondents, compared with 32% who experienced a decrease in desire, and 29% who experienced more desire for sex. The presence of shelter-in-place orders had no effect on frequency of or desire for sex. Among the respondents, 4% noted intimate partner violence, which increased slightly from 3% before the pandemic.
Among respondents using contraception, the study authors noted that 17% reported greater difficulty accessing birth control during the pandemic compared with 4% who felt access had become easier. Of those citing increased difficulties, 9% noted increasing challenges getting to a pharmacy, 4% were less able to afford birth control, 3% said it had become harder to obtain a prescription, and 1% cited difficulties having long-acting reversible contraceptives removed.
Despite the pandemic’s overall impact on quality of life, 41% of respondents reported a stronger desire to become pregnant, compared with 25% who had a reduced desire, and 34% whose interest in pregnancy was unchanged by the pandemic.
More than one-third of respondents (37%) admitted that COVID-19 contributed to their fears of becoming pregnant while 13% indicated their fear of pregnancy stemmed from concerns over being able to afford the cost of having a child. Not surprisingly, the decrease in desire for pregnancy was twice as high in those who reported they were unable to afford food, transportation, and/or housing compared with those who saw no change in their ability to afford basic needs.
“In these uncertain economic times, it is of utmost importance to create policies that will ensure access to and comprehensive coverage of core sexual and reproductive health services,” Dr. Lin and colleagues urged. “By doing so, we safeguard people’s ability to make decisions that support their reproductive health goals.”
Will COVID-19 drive needed practice and policy changes?
“This study highlights the economic and reproductive health toll of COVID-19 and the pressing need for improved contraception access,” Eve Espey, MD, MPH, said in an interview.
“Ob.gyns. and other practitioners can use this information to consider evidence-based practice changes that incorporate telemedicine visits, extended refills on contraceptive methods, and a focus on postpartum and postabortion initiation of contraception,” noted Dr. Espey, of the University of New Mexico, Albuquerque. For women who are experiencing economic hardship, consulting with state-based programs that offer pharmacy access and online access to contraceptives may offer a reasonable alternative, she added.
The study was funded by the University of California, San Francisco’s National Center of Excellence in Women’s Health. Dr. Lin received funding from Lazarex Cancer Foundation. The remaining authors had no conflicts of interest and reported no disclosures.
The pandemic has affected reproductive health because of barriers to contraception access, potentially increasing unwanted pregnancies, reported Tracy Kuo Lin, PhD, of the University of California, San Francisco, and associates.
During the pandemic, women have experienced an increased desire to avoid pregnancy, and when that desire is coupled with loss of income, accessing contraception becomes more difficult, Dr. Lin and colleagues observed in a cross-sectional survey published in the journal Contraception.
The study aimed to quantify the effect of COVID-19 on women’s economic status and reproductive health decisions related to childbearing and pregnancy. Women aged 18-49 who resided in the U.S. were targeted via Facebook and Instagram advertisements from May 16 to June 16, 2020. In all, 554 racially diverse respondents from 43 states were selected: 41% aged 18-24, 37% aged 25-34, and 23% aged 35-49.
Income losses affect nearly half of respondents
In determining risk of unwanted pregnancy, the researchers considered the influence of COVID-19 on a number of factors related to economic well-being as well as respondents’ sexual activity, intimate partner violence, overall desire for pregnancy, and access to contraception as issues affecting their interest in becoming pregnant and their ability to access medical care and contraception. Overall, 46% experienced a reduction in income, 43% reported no change, and 10% experienced an increase in income.
Difficulty in being able to afford food, transportation, and housing doubled among respondents from 8% to 16% as a result of the pandemic. The study authors cited education, race/ethnicity, federal poverty level, and change in income as predictors of inability to provide for these basic needs.
A total of 83% of respondents reported having sex within the past month; 54% of those had sex with someone they lived with, compared with 29% who had sex with someone they did not live with. The pandemic had no impact on sexual desire for 37% of respondents, compared with 32% who experienced a decrease in desire, and 29% who experienced more desire for sex. The presence of shelter-in-place orders had no effect on frequency of or desire for sex. Among the respondents, 4% noted intimate partner violence, which increased slightly from 3% before the pandemic.
Among respondents using contraception, the study authors noted that 17% reported greater difficulty accessing birth control during the pandemic compared with 4% who felt access had become easier. Of those citing increased difficulties, 9% noted increasing challenges getting to a pharmacy, 4% were less able to afford birth control, 3% said it had become harder to obtain a prescription, and 1% cited difficulties having long-acting reversible contraceptives removed.
Despite the pandemic’s overall impact on quality of life, 41% of respondents reported a stronger desire to become pregnant, compared with 25% who had a reduced desire, and 34% whose interest in pregnancy was unchanged by the pandemic.
More than one-third of respondents (37%) admitted that COVID-19 contributed to their fears of becoming pregnant while 13% indicated their fear of pregnancy stemmed from concerns over being able to afford the cost of having a child. Not surprisingly, the decrease in desire for pregnancy was twice as high in those who reported they were unable to afford food, transportation, and/or housing compared with those who saw no change in their ability to afford basic needs.
“In these uncertain economic times, it is of utmost importance to create policies that will ensure access to and comprehensive coverage of core sexual and reproductive health services,” Dr. Lin and colleagues urged. “By doing so, we safeguard people’s ability to make decisions that support their reproductive health goals.”
Will COVID-19 drive needed practice and policy changes?
“This study highlights the economic and reproductive health toll of COVID-19 and the pressing need for improved contraception access,” Eve Espey, MD, MPH, said in an interview.
“Ob.gyns. and other practitioners can use this information to consider evidence-based practice changes that incorporate telemedicine visits, extended refills on contraceptive methods, and a focus on postpartum and postabortion initiation of contraception,” noted Dr. Espey, of the University of New Mexico, Albuquerque. For women who are experiencing economic hardship, consulting with state-based programs that offer pharmacy access and online access to contraceptives may offer a reasonable alternative, she added.
The study was funded by the University of California, San Francisco’s National Center of Excellence in Women’s Health. Dr. Lin received funding from Lazarex Cancer Foundation. The remaining authors had no conflicts of interest and reported no disclosures.
The pandemic has affected reproductive health because of barriers to contraception access, potentially increasing unwanted pregnancies, reported Tracy Kuo Lin, PhD, of the University of California, San Francisco, and associates.
During the pandemic, women have experienced an increased desire to avoid pregnancy, and when that desire is coupled with loss of income, accessing contraception becomes more difficult, Dr. Lin and colleagues observed in a cross-sectional survey published in the journal Contraception.
The study aimed to quantify the effect of COVID-19 on women’s economic status and reproductive health decisions related to childbearing and pregnancy. Women aged 18-49 who resided in the U.S. were targeted via Facebook and Instagram advertisements from May 16 to June 16, 2020. In all, 554 racially diverse respondents from 43 states were selected: 41% aged 18-24, 37% aged 25-34, and 23% aged 35-49.
Income losses affect nearly half of respondents
In determining risk of unwanted pregnancy, the researchers considered the influence of COVID-19 on a number of factors related to economic well-being as well as respondents’ sexual activity, intimate partner violence, overall desire for pregnancy, and access to contraception as issues affecting their interest in becoming pregnant and their ability to access medical care and contraception. Overall, 46% experienced a reduction in income, 43% reported no change, and 10% experienced an increase in income.
Difficulty in being able to afford food, transportation, and housing doubled among respondents from 8% to 16% as a result of the pandemic. The study authors cited education, race/ethnicity, federal poverty level, and change in income as predictors of inability to provide for these basic needs.
A total of 83% of respondents reported having sex within the past month; 54% of those had sex with someone they lived with, compared with 29% who had sex with someone they did not live with. The pandemic had no impact on sexual desire for 37% of respondents, compared with 32% who experienced a decrease in desire, and 29% who experienced more desire for sex. The presence of shelter-in-place orders had no effect on frequency of or desire for sex. Among the respondents, 4% noted intimate partner violence, which increased slightly from 3% before the pandemic.
Among respondents using contraception, the study authors noted that 17% reported greater difficulty accessing birth control during the pandemic compared with 4% who felt access had become easier. Of those citing increased difficulties, 9% noted increasing challenges getting to a pharmacy, 4% were less able to afford birth control, 3% said it had become harder to obtain a prescription, and 1% cited difficulties having long-acting reversible contraceptives removed.
Despite the pandemic’s overall impact on quality of life, 41% of respondents reported a stronger desire to become pregnant, compared with 25% who had a reduced desire, and 34% whose interest in pregnancy was unchanged by the pandemic.
More than one-third of respondents (37%) admitted that COVID-19 contributed to their fears of becoming pregnant while 13% indicated their fear of pregnancy stemmed from concerns over being able to afford the cost of having a child. Not surprisingly, the decrease in desire for pregnancy was twice as high in those who reported they were unable to afford food, transportation, and/or housing compared with those who saw no change in their ability to afford basic needs.
“In these uncertain economic times, it is of utmost importance to create policies that will ensure access to and comprehensive coverage of core sexual and reproductive health services,” Dr. Lin and colleagues urged. “By doing so, we safeguard people’s ability to make decisions that support their reproductive health goals.”
Will COVID-19 drive needed practice and policy changes?
“This study highlights the economic and reproductive health toll of COVID-19 and the pressing need for improved contraception access,” Eve Espey, MD, MPH, said in an interview.
“Ob.gyns. and other practitioners can use this information to consider evidence-based practice changes that incorporate telemedicine visits, extended refills on contraceptive methods, and a focus on postpartum and postabortion initiation of contraception,” noted Dr. Espey, of the University of New Mexico, Albuquerque. For women who are experiencing economic hardship, consulting with state-based programs that offer pharmacy access and online access to contraceptives may offer a reasonable alternative, she added.
The study was funded by the University of California, San Francisco’s National Center of Excellence in Women’s Health. Dr. Lin received funding from Lazarex Cancer Foundation. The remaining authors had no conflicts of interest and reported no disclosures.
FROM CONTRACEPTION
Levonorgestrel IUD effective as emergency contraception
A levonorgestrel 52-mg intrauterine device is noninferior to a copper IUD for emergency contraception, according to randomized trial results published online in the New England Journal of Medicine.
Although the trial didn’t directly compare emergency oral contraception to the hormonal IUD, the authors speculated, on the basis of prior findings, that the levonorgestrel IUD is more effective than oral emergency contraceptive pills. In addition, there is no delay in providing ongoing contraception as there is when ulipristal acetate is used for emergency contraception.
Prior research has found that copper IUDs are a highly effective method of emergency contraception, but studies of the use of other IUDs as emergency contraception have been lacking.
To examine whether the levonorgestrel IUD is noninferior to the copper IUD as emergency contraception after unprotected sexual intercourse during the previous 5 days, David K. Turok, MD, MPH, associate professor of obstetrics and gynecology at the University of Utah Health, Salt Lake City, and colleagues conducted a trial at six Planned Parenthood health centers in Utah.
Researchers enrolled patients between August 2016 and December 2019. Trial sites purchased levonorgestrel 52-mg IUDs (Liletta) and copper T380A IUDs (ParaGard) for the study. The companies that distribute the IUDs were not involved in the trial.
Pregnancy rates were 1 of 317 participants (0.3%) among those who received the levonorgestrel IUD, and 0 of 321 (0%) among those who received the copper IUD. The difference between the two arms was well within the prespecified noninferiority margin of 2.5%.
Adverse event rates were generally similar between the two groups, with 5.2% of participants in the levonorgestrel IUD group seeking medical care in the month after IUD placement, compared with 4.9% in the copper IUD group.
A welcome option
The study “benefits women by allowing us to introduce a new option into the method mix of emergency contraception,” commented Wing Kay Fok, MD, a clinical assistant professor of obstetrics and gynecology at Weill Cornell Medicine, New York.
Specialists in family planning had followed preliminary data from this study and were anticipating the final results. Clinicians who are comfortable placing a copper IUD for emergency contraception are likely to be comfortable placing a levonorgestrel 52-mg IUD, given these data, Dr. Fok said.
“This is definitely – from what we can tell – a more effective method than the pill,” she said.
Gabriela Aguilar, MD, MPH, fellow and clinical instructor in the department of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn., said she is reassured by the data and is prepared to offer the 52-mg levonorgestrel IUD as emergency contraception.
The trial is “an important clinical study that has the ability to significantly change clinical practice,” Dr. Aguilar said. She credited the University of Utah and Planned Parenthood for their roles in it.
“Anytime that there are more options, ideally, that means that access is also increased,” Dr. Aguilar said.
Many patients will still prefer oral emergency contraceptive pills, she said. “But for those who are interested in an IUD ... for the immediate and ongoing birth control after that, now they have the two options instead of just the one IUD option.”
One pregnancy
The trial included women aged 18-35 years who requested emergency contraception after unprotected sexual intercourse within the previous 5 days. Other inclusion criteria were that participants had a desire to initiate use of an IUD; a desire to prevent pregnancy for at least 1 year; a negative result on urine pregnancy testing; a history of regular menstrual cycles; and a known date of the last menstrual period. The investigators did not exclude individuals who had unprotected sexual intercourse more than 5 days before IUD placement.
Participants were unaware of their assigned intervention. The nurse practitioners and certified nurse midwives who performed the IUD insertions were aware of the IUD type.
The primary outcome was pregnancy, as determined by a positive result on urine pregnancy testing 1 month after IUD insertion or by a review of survey and health record data.
One pregnancy “occurred in a participant who reported a single episode of unprotected sexual intercourse 48 hours before IUD placement,” the study authors wrote. “Pregnancy dating by an ultrasound examination at 10 weeks was consistent with conception occurring as a result of an emergency contraception failure. The pregnancy ended in a spontaneous abortion at 10 weeks with the IUD still in place.”
“We hope that providers can begin to deliver this method to everyone who wants and needs it and that people considering both emergency contraception and an ongoing method of birth control know that they now have the option of a hormonal IUD in addition to the nonhormonal, copper IUD,” Dr. Turok said in a news release from Planned Parenthood.
The study used a hormonal IUD manufactured by Liletta; Mirena also manufactures a levonorgestrel 52-mg IUD. The results of the study would apply to Mirena’s product too, according to Planned Parenthood.
“There are various IUDs on the market that are at lower doses, and so those IUDs may not demonstrate similar results,” Dr. Aguilar said.
The research was supported by the National Institutes of Health and the University of Utah. Dr. Turok is the director of surgical services for Planned Parenthood Association of Utah; the trial was conducted at PPAU centers, but Dr. Turok does not work at the sites where the study was conducted. Dr. Turok has consulted for Sebela Pharmaceuticals as the principal investigator for two phase 3 studies that assessed novel IUDs. Dr. Turok and one coauthor received grant support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Fok and Dr. Aguilar disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A levonorgestrel 52-mg intrauterine device is noninferior to a copper IUD for emergency contraception, according to randomized trial results published online in the New England Journal of Medicine.
Although the trial didn’t directly compare emergency oral contraception to the hormonal IUD, the authors speculated, on the basis of prior findings, that the levonorgestrel IUD is more effective than oral emergency contraceptive pills. In addition, there is no delay in providing ongoing contraception as there is when ulipristal acetate is used for emergency contraception.
Prior research has found that copper IUDs are a highly effective method of emergency contraception, but studies of the use of other IUDs as emergency contraception have been lacking.
To examine whether the levonorgestrel IUD is noninferior to the copper IUD as emergency contraception after unprotected sexual intercourse during the previous 5 days, David K. Turok, MD, MPH, associate professor of obstetrics and gynecology at the University of Utah Health, Salt Lake City, and colleagues conducted a trial at six Planned Parenthood health centers in Utah.
Researchers enrolled patients between August 2016 and December 2019. Trial sites purchased levonorgestrel 52-mg IUDs (Liletta) and copper T380A IUDs (ParaGard) for the study. The companies that distribute the IUDs were not involved in the trial.
Pregnancy rates were 1 of 317 participants (0.3%) among those who received the levonorgestrel IUD, and 0 of 321 (0%) among those who received the copper IUD. The difference between the two arms was well within the prespecified noninferiority margin of 2.5%.
Adverse event rates were generally similar between the two groups, with 5.2% of participants in the levonorgestrel IUD group seeking medical care in the month after IUD placement, compared with 4.9% in the copper IUD group.
A welcome option
The study “benefits women by allowing us to introduce a new option into the method mix of emergency contraception,” commented Wing Kay Fok, MD, a clinical assistant professor of obstetrics and gynecology at Weill Cornell Medicine, New York.
Specialists in family planning had followed preliminary data from this study and were anticipating the final results. Clinicians who are comfortable placing a copper IUD for emergency contraception are likely to be comfortable placing a levonorgestrel 52-mg IUD, given these data, Dr. Fok said.
“This is definitely – from what we can tell – a more effective method than the pill,” she said.
Gabriela Aguilar, MD, MPH, fellow and clinical instructor in the department of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn., said she is reassured by the data and is prepared to offer the 52-mg levonorgestrel IUD as emergency contraception.
The trial is “an important clinical study that has the ability to significantly change clinical practice,” Dr. Aguilar said. She credited the University of Utah and Planned Parenthood for their roles in it.
“Anytime that there are more options, ideally, that means that access is also increased,” Dr. Aguilar said.
Many patients will still prefer oral emergency contraceptive pills, she said. “But for those who are interested in an IUD ... for the immediate and ongoing birth control after that, now they have the two options instead of just the one IUD option.”
One pregnancy
The trial included women aged 18-35 years who requested emergency contraception after unprotected sexual intercourse within the previous 5 days. Other inclusion criteria were that participants had a desire to initiate use of an IUD; a desire to prevent pregnancy for at least 1 year; a negative result on urine pregnancy testing; a history of regular menstrual cycles; and a known date of the last menstrual period. The investigators did not exclude individuals who had unprotected sexual intercourse more than 5 days before IUD placement.
Participants were unaware of their assigned intervention. The nurse practitioners and certified nurse midwives who performed the IUD insertions were aware of the IUD type.
The primary outcome was pregnancy, as determined by a positive result on urine pregnancy testing 1 month after IUD insertion or by a review of survey and health record data.
One pregnancy “occurred in a participant who reported a single episode of unprotected sexual intercourse 48 hours before IUD placement,” the study authors wrote. “Pregnancy dating by an ultrasound examination at 10 weeks was consistent with conception occurring as a result of an emergency contraception failure. The pregnancy ended in a spontaneous abortion at 10 weeks with the IUD still in place.”
“We hope that providers can begin to deliver this method to everyone who wants and needs it and that people considering both emergency contraception and an ongoing method of birth control know that they now have the option of a hormonal IUD in addition to the nonhormonal, copper IUD,” Dr. Turok said in a news release from Planned Parenthood.
The study used a hormonal IUD manufactured by Liletta; Mirena also manufactures a levonorgestrel 52-mg IUD. The results of the study would apply to Mirena’s product too, according to Planned Parenthood.
“There are various IUDs on the market that are at lower doses, and so those IUDs may not demonstrate similar results,” Dr. Aguilar said.
The research was supported by the National Institutes of Health and the University of Utah. Dr. Turok is the director of surgical services for Planned Parenthood Association of Utah; the trial was conducted at PPAU centers, but Dr. Turok does not work at the sites where the study was conducted. Dr. Turok has consulted for Sebela Pharmaceuticals as the principal investigator for two phase 3 studies that assessed novel IUDs. Dr. Turok and one coauthor received grant support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Fok and Dr. Aguilar disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A levonorgestrel 52-mg intrauterine device is noninferior to a copper IUD for emergency contraception, according to randomized trial results published online in the New England Journal of Medicine.
Although the trial didn’t directly compare emergency oral contraception to the hormonal IUD, the authors speculated, on the basis of prior findings, that the levonorgestrel IUD is more effective than oral emergency contraceptive pills. In addition, there is no delay in providing ongoing contraception as there is when ulipristal acetate is used for emergency contraception.
Prior research has found that copper IUDs are a highly effective method of emergency contraception, but studies of the use of other IUDs as emergency contraception have been lacking.
To examine whether the levonorgestrel IUD is noninferior to the copper IUD as emergency contraception after unprotected sexual intercourse during the previous 5 days, David K. Turok, MD, MPH, associate professor of obstetrics and gynecology at the University of Utah Health, Salt Lake City, and colleagues conducted a trial at six Planned Parenthood health centers in Utah.
Researchers enrolled patients between August 2016 and December 2019. Trial sites purchased levonorgestrel 52-mg IUDs (Liletta) and copper T380A IUDs (ParaGard) for the study. The companies that distribute the IUDs were not involved in the trial.
Pregnancy rates were 1 of 317 participants (0.3%) among those who received the levonorgestrel IUD, and 0 of 321 (0%) among those who received the copper IUD. The difference between the two arms was well within the prespecified noninferiority margin of 2.5%.
Adverse event rates were generally similar between the two groups, with 5.2% of participants in the levonorgestrel IUD group seeking medical care in the month after IUD placement, compared with 4.9% in the copper IUD group.
A welcome option
The study “benefits women by allowing us to introduce a new option into the method mix of emergency contraception,” commented Wing Kay Fok, MD, a clinical assistant professor of obstetrics and gynecology at Weill Cornell Medicine, New York.
Specialists in family planning had followed preliminary data from this study and were anticipating the final results. Clinicians who are comfortable placing a copper IUD for emergency contraception are likely to be comfortable placing a levonorgestrel 52-mg IUD, given these data, Dr. Fok said.
“This is definitely – from what we can tell – a more effective method than the pill,” she said.
Gabriela Aguilar, MD, MPH, fellow and clinical instructor in the department of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn., said she is reassured by the data and is prepared to offer the 52-mg levonorgestrel IUD as emergency contraception.
The trial is “an important clinical study that has the ability to significantly change clinical practice,” Dr. Aguilar said. She credited the University of Utah and Planned Parenthood for their roles in it.
“Anytime that there are more options, ideally, that means that access is also increased,” Dr. Aguilar said.
Many patients will still prefer oral emergency contraceptive pills, she said. “But for those who are interested in an IUD ... for the immediate and ongoing birth control after that, now they have the two options instead of just the one IUD option.”
One pregnancy
The trial included women aged 18-35 years who requested emergency contraception after unprotected sexual intercourse within the previous 5 days. Other inclusion criteria were that participants had a desire to initiate use of an IUD; a desire to prevent pregnancy for at least 1 year; a negative result on urine pregnancy testing; a history of regular menstrual cycles; and a known date of the last menstrual period. The investigators did not exclude individuals who had unprotected sexual intercourse more than 5 days before IUD placement.
Participants were unaware of their assigned intervention. The nurse practitioners and certified nurse midwives who performed the IUD insertions were aware of the IUD type.
The primary outcome was pregnancy, as determined by a positive result on urine pregnancy testing 1 month after IUD insertion or by a review of survey and health record data.
One pregnancy “occurred in a participant who reported a single episode of unprotected sexual intercourse 48 hours before IUD placement,” the study authors wrote. “Pregnancy dating by an ultrasound examination at 10 weeks was consistent with conception occurring as a result of an emergency contraception failure. The pregnancy ended in a spontaneous abortion at 10 weeks with the IUD still in place.”
“We hope that providers can begin to deliver this method to everyone who wants and needs it and that people considering both emergency contraception and an ongoing method of birth control know that they now have the option of a hormonal IUD in addition to the nonhormonal, copper IUD,” Dr. Turok said in a news release from Planned Parenthood.
The study used a hormonal IUD manufactured by Liletta; Mirena also manufactures a levonorgestrel 52-mg IUD. The results of the study would apply to Mirena’s product too, according to Planned Parenthood.
“There are various IUDs on the market that are at lower doses, and so those IUDs may not demonstrate similar results,” Dr. Aguilar said.
The research was supported by the National Institutes of Health and the University of Utah. Dr. Turok is the director of surgical services for Planned Parenthood Association of Utah; the trial was conducted at PPAU centers, but Dr. Turok does not work at the sites where the study was conducted. Dr. Turok has consulted for Sebela Pharmaceuticals as the principal investigator for two phase 3 studies that assessed novel IUDs. Dr. Turok and one coauthor received grant support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Fok and Dr. Aguilar disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Oral contraceptives may reduce ovarian and endometrial cancer risk 35 years after discontinuation
At the same time, oral contraceptive use is associated with a short-term increased risk of breast cancer after discontinuation, although the lifetime risk of breast cancer is not significantly different, the researchers found.
The absolute risk of breast cancer after discontinuation is “extremely small” and should be a limited factor when deciding whether to start oral contraceptive pills (OCPs), a doctor said.
The study was conducted by Torgny Karlsson, PhD, a researcher in the department of immunology, genetics, and pathology at Uppsala (Sweden) University, and colleagues and published online in Cancer Research.
Reinforcing and extending knowledge
“These findings are generally consistent with what is known, but extend that knowledge, most notably by the longer-term follow-up for the cohort,” commented Nancy L. Keating, MD, MPH, professor of health care policy and medicine at Harvard Medical School and a physician at Brigham and Women’s Hospital, both in Boston. “Other studies have also shown that OCPs lower risk of ovarian and endometrial cancer. This study suggests that this protective benefit extends up to 30-35 years after discontinuing OCPs.”
The results “reinforce the message to patients of the protective effect of OCPs on risk of ovarian and endometrial cancer,” Dr. Keating said. “Women concerned about these cancers can be reassured that this protective effect appears to persist for decades after discontinuing use.”
Prior studies have indicated that oral contraceptives may be associated with an increased risk of breast cancer.
In terms of breast cancer risk, the study “again extends follow-up and shows that risk of breast cancer was higher for current and ever users through age 50,” although the lifetime risk was not elevated, Dr. Keating said.
“The counseling regarding the effect on breast cancer is more complex,” she said. “I tell women about the very small increased risk of breast cancer during and immediately after use. Because cancer is very rare among women at the ages when OCPs are typically prescribed, the absolute risk increase is extremely small. This paper adds reassurance that this small increase in risk does not persist.”
For certain patients, the association may be more relevant.
“For most women, this risk is so small that it should be a limited factor in their decision to start OCPs,” Dr. Keating said. “However, for women with a substantially higher risk of breast cancer, or a family history of breast cancer at a young age, the small increased risk of breast cancer during and immediately after OCP use is more relevant, and counseling should include carefully weighing the benefits and harms of OCPs with other forms of contraception (and no contraception).”
Although the protective effects of oral contraceptives on ovarian and endometrial cancer were well known, the study describes long-term outcomes that can further inform patient counseling, said Samuel S. Badalian, MD, PhD, chief of the department of obstetrics and gynecology at Bassett Medical Center in Cooperstown, N.Y., and clinical professor of obstetrics and gynecology at the State University of New York, Syracuse.
“Women with individual or family risk factors of ovarian or endometrial cancers will need to know about the protective effects of oral contraceptives and long-term benefits related with their use (30-35 years after discontinuation),” Dr. Badalian said. “Women with family history of breast cancer need to know that lifetime risk of breast cancer might not differ between ever and never users, even if there is an increased short-term risk.”
Data from the U.K. Biobank
To examine the time-dependent effects between long-term oral contraceptive use and cancer risk, the researchers examined data from 256,661 women from the U.K. Biobank who were born between 1939 and 1970. The researchers identified cancer diagnoses using information from national registers and self-reported data until March 2019.
Of the women included in the study, 82% had used or still were using oral contraceptives, whereas 18% had never used oral contraceptives. Overall, ever users were younger, more frequently smokers, and had a lower body mass index, compared with never users. Most women started using oral contraceptives between 1969 and 1978. Last use of oral contraceptives occurred on average 10.7 years after starting.
The researchers adjusted for covariates and used logistic regression analyses to measure the cumulative risk of cancer. They used Cox regression analysis to examine instantaneous risk, measured using hazard ratios.
In all, there were 17,739 cases of breast cancer (6.9%), 1,966 cases of ovarian cancer (0.76%), and 2,462 cases of endometrial cancer (0.96%).
Among ever users, the likelihood of ovarian cancer (OR, 0.72) and endometrial cancer (OR, 0.68) was lower, compared with never users. “However, we did not see a significant association between oral contraceptive use and breast cancer” for the study period as a whole, the researchers reported. When the researchers limited follow-up to age 50 years, however, the odds ratio for breast cancer was increased (OR, 1.09).
“Surprisingly, we only found a small increased risk of breast cancer among oral contraceptive users, and the increased risk disappeared within a few years after discontinuation,” Åsa Johansson, PhD, a researcher in the department of immunology, genetics, and pathology at Uppsala University and one of the study authors, said in a news release. “Our results suggest that the lifetime risk of breast cancer might not differ between ever and never users, even if there is an increased short-term risk.”
Oral contraceptives today typically use lower doses of estrogen and other types of progesterone, compared with formulas commonly used when participants in the study started taking them, so the results may not directly apply to patients currently taking oral contraceptives, the researchers noted.
The study was supported by the Swedish Research Council, the Swedish Cancer Society, and the Kjell and Märta Beijers, the Marcus Borgström, the Åke Wiberg, and the A and M Rudbergs foundations. The authors, Dr. Keating, and Dr. Badalian had no conflicts of interest.
At the same time, oral contraceptive use is associated with a short-term increased risk of breast cancer after discontinuation, although the lifetime risk of breast cancer is not significantly different, the researchers found.
The absolute risk of breast cancer after discontinuation is “extremely small” and should be a limited factor when deciding whether to start oral contraceptive pills (OCPs), a doctor said.
The study was conducted by Torgny Karlsson, PhD, a researcher in the department of immunology, genetics, and pathology at Uppsala (Sweden) University, and colleagues and published online in Cancer Research.
Reinforcing and extending knowledge
“These findings are generally consistent with what is known, but extend that knowledge, most notably by the longer-term follow-up for the cohort,” commented Nancy L. Keating, MD, MPH, professor of health care policy and medicine at Harvard Medical School and a physician at Brigham and Women’s Hospital, both in Boston. “Other studies have also shown that OCPs lower risk of ovarian and endometrial cancer. This study suggests that this protective benefit extends up to 30-35 years after discontinuing OCPs.”
The results “reinforce the message to patients of the protective effect of OCPs on risk of ovarian and endometrial cancer,” Dr. Keating said. “Women concerned about these cancers can be reassured that this protective effect appears to persist for decades after discontinuing use.”
Prior studies have indicated that oral contraceptives may be associated with an increased risk of breast cancer.
In terms of breast cancer risk, the study “again extends follow-up and shows that risk of breast cancer was higher for current and ever users through age 50,” although the lifetime risk was not elevated, Dr. Keating said.
“The counseling regarding the effect on breast cancer is more complex,” she said. “I tell women about the very small increased risk of breast cancer during and immediately after use. Because cancer is very rare among women at the ages when OCPs are typically prescribed, the absolute risk increase is extremely small. This paper adds reassurance that this small increase in risk does not persist.”
For certain patients, the association may be more relevant.
“For most women, this risk is so small that it should be a limited factor in their decision to start OCPs,” Dr. Keating said. “However, for women with a substantially higher risk of breast cancer, or a family history of breast cancer at a young age, the small increased risk of breast cancer during and immediately after OCP use is more relevant, and counseling should include carefully weighing the benefits and harms of OCPs with other forms of contraception (and no contraception).”
Although the protective effects of oral contraceptives on ovarian and endometrial cancer were well known, the study describes long-term outcomes that can further inform patient counseling, said Samuel S. Badalian, MD, PhD, chief of the department of obstetrics and gynecology at Bassett Medical Center in Cooperstown, N.Y., and clinical professor of obstetrics and gynecology at the State University of New York, Syracuse.
“Women with individual or family risk factors of ovarian or endometrial cancers will need to know about the protective effects of oral contraceptives and long-term benefits related with their use (30-35 years after discontinuation),” Dr. Badalian said. “Women with family history of breast cancer need to know that lifetime risk of breast cancer might not differ between ever and never users, even if there is an increased short-term risk.”
Data from the U.K. Biobank
To examine the time-dependent effects between long-term oral contraceptive use and cancer risk, the researchers examined data from 256,661 women from the U.K. Biobank who were born between 1939 and 1970. The researchers identified cancer diagnoses using information from national registers and self-reported data until March 2019.
Of the women included in the study, 82% had used or still were using oral contraceptives, whereas 18% had never used oral contraceptives. Overall, ever users were younger, more frequently smokers, and had a lower body mass index, compared with never users. Most women started using oral contraceptives between 1969 and 1978. Last use of oral contraceptives occurred on average 10.7 years after starting.
The researchers adjusted for covariates and used logistic regression analyses to measure the cumulative risk of cancer. They used Cox regression analysis to examine instantaneous risk, measured using hazard ratios.
In all, there were 17,739 cases of breast cancer (6.9%), 1,966 cases of ovarian cancer (0.76%), and 2,462 cases of endometrial cancer (0.96%).
Among ever users, the likelihood of ovarian cancer (OR, 0.72) and endometrial cancer (OR, 0.68) was lower, compared with never users. “However, we did not see a significant association between oral contraceptive use and breast cancer” for the study period as a whole, the researchers reported. When the researchers limited follow-up to age 50 years, however, the odds ratio for breast cancer was increased (OR, 1.09).
“Surprisingly, we only found a small increased risk of breast cancer among oral contraceptive users, and the increased risk disappeared within a few years after discontinuation,” Åsa Johansson, PhD, a researcher in the department of immunology, genetics, and pathology at Uppsala University and one of the study authors, said in a news release. “Our results suggest that the lifetime risk of breast cancer might not differ between ever and never users, even if there is an increased short-term risk.”
Oral contraceptives today typically use lower doses of estrogen and other types of progesterone, compared with formulas commonly used when participants in the study started taking them, so the results may not directly apply to patients currently taking oral contraceptives, the researchers noted.
The study was supported by the Swedish Research Council, the Swedish Cancer Society, and the Kjell and Märta Beijers, the Marcus Borgström, the Åke Wiberg, and the A and M Rudbergs foundations. The authors, Dr. Keating, and Dr. Badalian had no conflicts of interest.
At the same time, oral contraceptive use is associated with a short-term increased risk of breast cancer after discontinuation, although the lifetime risk of breast cancer is not significantly different, the researchers found.
The absolute risk of breast cancer after discontinuation is “extremely small” and should be a limited factor when deciding whether to start oral contraceptive pills (OCPs), a doctor said.
The study was conducted by Torgny Karlsson, PhD, a researcher in the department of immunology, genetics, and pathology at Uppsala (Sweden) University, and colleagues and published online in Cancer Research.
Reinforcing and extending knowledge
“These findings are generally consistent with what is known, but extend that knowledge, most notably by the longer-term follow-up for the cohort,” commented Nancy L. Keating, MD, MPH, professor of health care policy and medicine at Harvard Medical School and a physician at Brigham and Women’s Hospital, both in Boston. “Other studies have also shown that OCPs lower risk of ovarian and endometrial cancer. This study suggests that this protective benefit extends up to 30-35 years after discontinuing OCPs.”
The results “reinforce the message to patients of the protective effect of OCPs on risk of ovarian and endometrial cancer,” Dr. Keating said. “Women concerned about these cancers can be reassured that this protective effect appears to persist for decades after discontinuing use.”
Prior studies have indicated that oral contraceptives may be associated with an increased risk of breast cancer.
In terms of breast cancer risk, the study “again extends follow-up and shows that risk of breast cancer was higher for current and ever users through age 50,” although the lifetime risk was not elevated, Dr. Keating said.
“The counseling regarding the effect on breast cancer is more complex,” she said. “I tell women about the very small increased risk of breast cancer during and immediately after use. Because cancer is very rare among women at the ages when OCPs are typically prescribed, the absolute risk increase is extremely small. This paper adds reassurance that this small increase in risk does not persist.”
For certain patients, the association may be more relevant.
“For most women, this risk is so small that it should be a limited factor in their decision to start OCPs,” Dr. Keating said. “However, for women with a substantially higher risk of breast cancer, or a family history of breast cancer at a young age, the small increased risk of breast cancer during and immediately after OCP use is more relevant, and counseling should include carefully weighing the benefits and harms of OCPs with other forms of contraception (and no contraception).”
Although the protective effects of oral contraceptives on ovarian and endometrial cancer were well known, the study describes long-term outcomes that can further inform patient counseling, said Samuel S. Badalian, MD, PhD, chief of the department of obstetrics and gynecology at Bassett Medical Center in Cooperstown, N.Y., and clinical professor of obstetrics and gynecology at the State University of New York, Syracuse.
“Women with individual or family risk factors of ovarian or endometrial cancers will need to know about the protective effects of oral contraceptives and long-term benefits related with their use (30-35 years after discontinuation),” Dr. Badalian said. “Women with family history of breast cancer need to know that lifetime risk of breast cancer might not differ between ever and never users, even if there is an increased short-term risk.”
Data from the U.K. Biobank
To examine the time-dependent effects between long-term oral contraceptive use and cancer risk, the researchers examined data from 256,661 women from the U.K. Biobank who were born between 1939 and 1970. The researchers identified cancer diagnoses using information from national registers and self-reported data until March 2019.
Of the women included in the study, 82% had used or still were using oral contraceptives, whereas 18% had never used oral contraceptives. Overall, ever users were younger, more frequently smokers, and had a lower body mass index, compared with never users. Most women started using oral contraceptives between 1969 and 1978. Last use of oral contraceptives occurred on average 10.7 years after starting.
The researchers adjusted for covariates and used logistic regression analyses to measure the cumulative risk of cancer. They used Cox regression analysis to examine instantaneous risk, measured using hazard ratios.
In all, there were 17,739 cases of breast cancer (6.9%), 1,966 cases of ovarian cancer (0.76%), and 2,462 cases of endometrial cancer (0.96%).
Among ever users, the likelihood of ovarian cancer (OR, 0.72) and endometrial cancer (OR, 0.68) was lower, compared with never users. “However, we did not see a significant association between oral contraceptive use and breast cancer” for the study period as a whole, the researchers reported. When the researchers limited follow-up to age 50 years, however, the odds ratio for breast cancer was increased (OR, 1.09).
“Surprisingly, we only found a small increased risk of breast cancer among oral contraceptive users, and the increased risk disappeared within a few years after discontinuation,” Åsa Johansson, PhD, a researcher in the department of immunology, genetics, and pathology at Uppsala University and one of the study authors, said in a news release. “Our results suggest that the lifetime risk of breast cancer might not differ between ever and never users, even if there is an increased short-term risk.”
Oral contraceptives today typically use lower doses of estrogen and other types of progesterone, compared with formulas commonly used when participants in the study started taking them, so the results may not directly apply to patients currently taking oral contraceptives, the researchers noted.
The study was supported by the Swedish Research Council, the Swedish Cancer Society, and the Kjell and Märta Beijers, the Marcus Borgström, the Åke Wiberg, and the A and M Rudbergs foundations. The authors, Dr. Keating, and Dr. Badalian had no conflicts of interest.
FROM CANCER RESEARCH
Does last contraceptive method used impact the return of normal fertility?
Yland JJ, Bresnick KA, Hatch EE, et al. Pregravid contraceptive use and fecundability: prospective cohort study. BMJ. 2020;371:m3966.
EXPERT COMMENTARY
Most US women aged 15 to 49 currently use contraception, with long-acting reversible contraception (LARC)—IUDs and the contraceptive implant—increasing in popularity over the last decade.1 Oral contraceptive pills, male condoms, and LARC are the most common reversible methods used.1 While the efficacy and safety of contraception have been established, few studies have examined the effect of recent contraceptive use on fertility.
Fecundability is the probability of pregnancy during a single menstrual cycle for a couple engaging in regular intercourse and not using contraception.2 Small studies have found short-term reductions in fecundability after discontinuing combined oral contraceptives and larger reductions after stopping injectable contraceptives, with no long-term differences among methods.3,4
Data are limited regarding the effects of other forms of contraception on fecundability, particularly LARC methods. A recent study was designed to evaluate the association between the last contraceptive method used and subsequent fecundability.2
Details of the study
Yland and colleagues pooled data from 3 prospective cohort studies of 17,954 women planning pregnancies in Denmark, Canada, and the United States. Participants reported the contraceptive method used most recently before trying to conceive. They completed questionnaires every 2 months for 12 months or until they reported a pregnancy. Women were excluded if they tried to conceive for more than 6 menstrual cycles at study entry.
The authors calculated the fecundability ratio—the average probability of conception per cycle for a specific contraceptive method compared with a reference method—using proportional probability models adjusted for potential confounders. They also calculated pregnancy attempt time using participant-reported menstrual cycle length and date of last menstrual period during follow-up questionnaires.
Continue to: Injectable contraceptives associated with longest delayed fertility return...
Injectable contraceptives associated with longest delayed fertility return
After adjusting for personal factors, medical history, lifestyle characteristics, and indicators of underlying fertility, the authors found that injectable contraceptive use was associated with decreased fecundability compared with barrier method use (fecundability ratio [FR], 0.65; 95% confidence interval [CI], 0.47–0.89). Hormonal IUD use was associated with slight increases in fecundability compared with barrier method use (FR, 1.14; 95% CI, 1.07–1.22) and copper IUD use (FR, 1.18; 95% CI, 1.05–1.33). All other contraceptive methods were not significantly different from barrier methods.
LARC method use was associated with the shortest delay in return of normal fertility (2 cycles), followed by oral and ring contraceptives (3 cycles) and patch (4 cycles). Women using injectable contraceptives experienced the longest delay (5–8 menstrual cycles). Lifetime duration of contraceptive use did not impact fecundability in the North American cohort.
Study strengths and limitations
This large, prospective study contributes useful information about fecundability after stopping contraceptive methods. It confirms earlier studies’ findings that showed decreased fecundability after stopping injectable contraceptives. Study participants’ most recent method used was similar to overall US method distribution.1
Study limitations include online recruitment of self-selecting participants, which introduces selection bias. The study population was overwhelmingly white (92%) and highly educated (70% with college degrees), quite different from the US population. These findings may therefore have limited generalizability. Additionally, injectable contraceptive users had higher body mass index and were more likely to smoke and have diabetes, infertility, or irregular menstrual cycles. IUD users were more likely to be parous and have a history of unplanned pregnancy, indicating possible higher baseline fertility. Even after adjusting, possible unmeasured factors could impact study results. ●
This is the largest study to date to evaluate fecundability after stopping different contraceptive methods among women planning pregnancies. The study confirms previous research that associated injectable contraceptives with delayed return of normal fertility. It provides reassurance for counseling users of IUDs, implants, oral contraception, ring, and patch: those methods were not associated with reduced fecundability compared with barrier methods. The study also suggests long-term contraceptive use does not decrease fecundability.
Women may ask when to stop their contraceptive method to optimally time a pregnancy. In this study, measurements of return to normal fertility were imprecise. Individualized counseling, accounting for personal circumstances, is still best when advising when to stop contraception for couples planning pregnancy.
LISA HOFLER, MD, MPH, MBA, AND LINDSAY DALE, MD
- Daniels K, Abma JC. Current contraceptive status among women aged 15–49: United States, 2017–2019. NCHS Data Brief, no. 388. Hyattsville, MD: National Center for Health Statistics; 2020.
- Yland JJ, Bresnick KA, Hatch EE, et al. Pregravid contraceptive use and fecundability: prospective cohort study. BMJ. 2020;371:m3966.
- Hassan MA, Killick SR. Is previous use of hormonal contraception associated with a detrimental effect on subsequent fecundity? Hum Reprod. 2004;19:344-351.
- Mansour D, Gemzell-Danielsson K, Inki P, et al. Fertility after discontinuation of contraception: a comprehensive review of the literature. Contraception. 2011;84:465-477.
Yland JJ, Bresnick KA, Hatch EE, et al. Pregravid contraceptive use and fecundability: prospective cohort study. BMJ. 2020;371:m3966.
EXPERT COMMENTARY
Most US women aged 15 to 49 currently use contraception, with long-acting reversible contraception (LARC)—IUDs and the contraceptive implant—increasing in popularity over the last decade.1 Oral contraceptive pills, male condoms, and LARC are the most common reversible methods used.1 While the efficacy and safety of contraception have been established, few studies have examined the effect of recent contraceptive use on fertility.
Fecundability is the probability of pregnancy during a single menstrual cycle for a couple engaging in regular intercourse and not using contraception.2 Small studies have found short-term reductions in fecundability after discontinuing combined oral contraceptives and larger reductions after stopping injectable contraceptives, with no long-term differences among methods.3,4
Data are limited regarding the effects of other forms of contraception on fecundability, particularly LARC methods. A recent study was designed to evaluate the association between the last contraceptive method used and subsequent fecundability.2
Details of the study
Yland and colleagues pooled data from 3 prospective cohort studies of 17,954 women planning pregnancies in Denmark, Canada, and the United States. Participants reported the contraceptive method used most recently before trying to conceive. They completed questionnaires every 2 months for 12 months or until they reported a pregnancy. Women were excluded if they tried to conceive for more than 6 menstrual cycles at study entry.
The authors calculated the fecundability ratio—the average probability of conception per cycle for a specific contraceptive method compared with a reference method—using proportional probability models adjusted for potential confounders. They also calculated pregnancy attempt time using participant-reported menstrual cycle length and date of last menstrual period during follow-up questionnaires.
Continue to: Injectable contraceptives associated with longest delayed fertility return...
Injectable contraceptives associated with longest delayed fertility return
After adjusting for personal factors, medical history, lifestyle characteristics, and indicators of underlying fertility, the authors found that injectable contraceptive use was associated with decreased fecundability compared with barrier method use (fecundability ratio [FR], 0.65; 95% confidence interval [CI], 0.47–0.89). Hormonal IUD use was associated with slight increases in fecundability compared with barrier method use (FR, 1.14; 95% CI, 1.07–1.22) and copper IUD use (FR, 1.18; 95% CI, 1.05–1.33). All other contraceptive methods were not significantly different from barrier methods.
LARC method use was associated with the shortest delay in return of normal fertility (2 cycles), followed by oral and ring contraceptives (3 cycles) and patch (4 cycles). Women using injectable contraceptives experienced the longest delay (5–8 menstrual cycles). Lifetime duration of contraceptive use did not impact fecundability in the North American cohort.
Study strengths and limitations
This large, prospective study contributes useful information about fecundability after stopping contraceptive methods. It confirms earlier studies’ findings that showed decreased fecundability after stopping injectable contraceptives. Study participants’ most recent method used was similar to overall US method distribution.1
Study limitations include online recruitment of self-selecting participants, which introduces selection bias. The study population was overwhelmingly white (92%) and highly educated (70% with college degrees), quite different from the US population. These findings may therefore have limited generalizability. Additionally, injectable contraceptive users had higher body mass index and were more likely to smoke and have diabetes, infertility, or irregular menstrual cycles. IUD users were more likely to be parous and have a history of unplanned pregnancy, indicating possible higher baseline fertility. Even after adjusting, possible unmeasured factors could impact study results. ●
This is the largest study to date to evaluate fecundability after stopping different contraceptive methods among women planning pregnancies. The study confirms previous research that associated injectable contraceptives with delayed return of normal fertility. It provides reassurance for counseling users of IUDs, implants, oral contraception, ring, and patch: those methods were not associated with reduced fecundability compared with barrier methods. The study also suggests long-term contraceptive use does not decrease fecundability.
Women may ask when to stop their contraceptive method to optimally time a pregnancy. In this study, measurements of return to normal fertility were imprecise. Individualized counseling, accounting for personal circumstances, is still best when advising when to stop contraception for couples planning pregnancy.
LISA HOFLER, MD, MPH, MBA, AND LINDSAY DALE, MD
Yland JJ, Bresnick KA, Hatch EE, et al. Pregravid contraceptive use and fecundability: prospective cohort study. BMJ. 2020;371:m3966.
EXPERT COMMENTARY
Most US women aged 15 to 49 currently use contraception, with long-acting reversible contraception (LARC)—IUDs and the contraceptive implant—increasing in popularity over the last decade.1 Oral contraceptive pills, male condoms, and LARC are the most common reversible methods used.1 While the efficacy and safety of contraception have been established, few studies have examined the effect of recent contraceptive use on fertility.
Fecundability is the probability of pregnancy during a single menstrual cycle for a couple engaging in regular intercourse and not using contraception.2 Small studies have found short-term reductions in fecundability after discontinuing combined oral contraceptives and larger reductions after stopping injectable contraceptives, with no long-term differences among methods.3,4
Data are limited regarding the effects of other forms of contraception on fecundability, particularly LARC methods. A recent study was designed to evaluate the association between the last contraceptive method used and subsequent fecundability.2
Details of the study
Yland and colleagues pooled data from 3 prospective cohort studies of 17,954 women planning pregnancies in Denmark, Canada, and the United States. Participants reported the contraceptive method used most recently before trying to conceive. They completed questionnaires every 2 months for 12 months or until they reported a pregnancy. Women were excluded if they tried to conceive for more than 6 menstrual cycles at study entry.
The authors calculated the fecundability ratio—the average probability of conception per cycle for a specific contraceptive method compared with a reference method—using proportional probability models adjusted for potential confounders. They also calculated pregnancy attempt time using participant-reported menstrual cycle length and date of last menstrual period during follow-up questionnaires.
Continue to: Injectable contraceptives associated with longest delayed fertility return...
Injectable contraceptives associated with longest delayed fertility return
After adjusting for personal factors, medical history, lifestyle characteristics, and indicators of underlying fertility, the authors found that injectable contraceptive use was associated with decreased fecundability compared with barrier method use (fecundability ratio [FR], 0.65; 95% confidence interval [CI], 0.47–0.89). Hormonal IUD use was associated with slight increases in fecundability compared with barrier method use (FR, 1.14; 95% CI, 1.07–1.22) and copper IUD use (FR, 1.18; 95% CI, 1.05–1.33). All other contraceptive methods were not significantly different from barrier methods.
LARC method use was associated with the shortest delay in return of normal fertility (2 cycles), followed by oral and ring contraceptives (3 cycles) and patch (4 cycles). Women using injectable contraceptives experienced the longest delay (5–8 menstrual cycles). Lifetime duration of contraceptive use did not impact fecundability in the North American cohort.
Study strengths and limitations
This large, prospective study contributes useful information about fecundability after stopping contraceptive methods. It confirms earlier studies’ findings that showed decreased fecundability after stopping injectable contraceptives. Study participants’ most recent method used was similar to overall US method distribution.1
Study limitations include online recruitment of self-selecting participants, which introduces selection bias. The study population was overwhelmingly white (92%) and highly educated (70% with college degrees), quite different from the US population. These findings may therefore have limited generalizability. Additionally, injectable contraceptive users had higher body mass index and were more likely to smoke and have diabetes, infertility, or irregular menstrual cycles. IUD users were more likely to be parous and have a history of unplanned pregnancy, indicating possible higher baseline fertility. Even after adjusting, possible unmeasured factors could impact study results. ●
This is the largest study to date to evaluate fecundability after stopping different contraceptive methods among women planning pregnancies. The study confirms previous research that associated injectable contraceptives with delayed return of normal fertility. It provides reassurance for counseling users of IUDs, implants, oral contraception, ring, and patch: those methods were not associated with reduced fecundability compared with barrier methods. The study also suggests long-term contraceptive use does not decrease fecundability.
Women may ask when to stop their contraceptive method to optimally time a pregnancy. In this study, measurements of return to normal fertility were imprecise. Individualized counseling, accounting for personal circumstances, is still best when advising when to stop contraception for couples planning pregnancy.
LISA HOFLER, MD, MPH, MBA, AND LINDSAY DALE, MD
- Daniels K, Abma JC. Current contraceptive status among women aged 15–49: United States, 2017–2019. NCHS Data Brief, no. 388. Hyattsville, MD: National Center for Health Statistics; 2020.
- Yland JJ, Bresnick KA, Hatch EE, et al. Pregravid contraceptive use and fecundability: prospective cohort study. BMJ. 2020;371:m3966.
- Hassan MA, Killick SR. Is previous use of hormonal contraception associated with a detrimental effect on subsequent fecundity? Hum Reprod. 2004;19:344-351.
- Mansour D, Gemzell-Danielsson K, Inki P, et al. Fertility after discontinuation of contraception: a comprehensive review of the literature. Contraception. 2011;84:465-477.
- Daniels K, Abma JC. Current contraceptive status among women aged 15–49: United States, 2017–2019. NCHS Data Brief, no. 388. Hyattsville, MD: National Center for Health Statistics; 2020.
- Yland JJ, Bresnick KA, Hatch EE, et al. Pregravid contraceptive use and fecundability: prospective cohort study. BMJ. 2020;371:m3966.
- Hassan MA, Killick SR. Is previous use of hormonal contraception associated with a detrimental effect on subsequent fecundity? Hum Reprod. 2004;19:344-351.
- Mansour D, Gemzell-Danielsson K, Inki P, et al. Fertility after discontinuation of contraception: a comprehensive review of the literature. Contraception. 2011;84:465-477.







