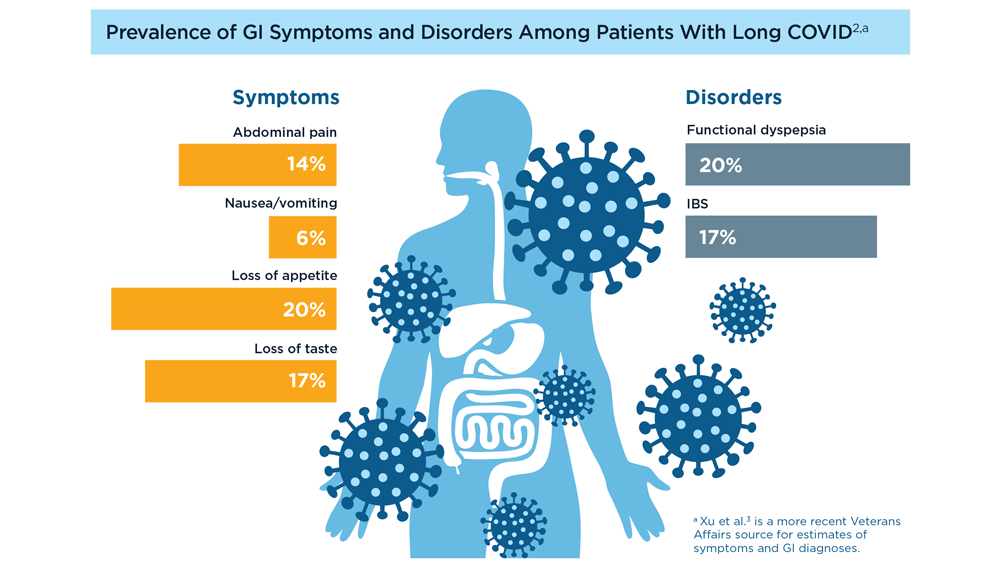
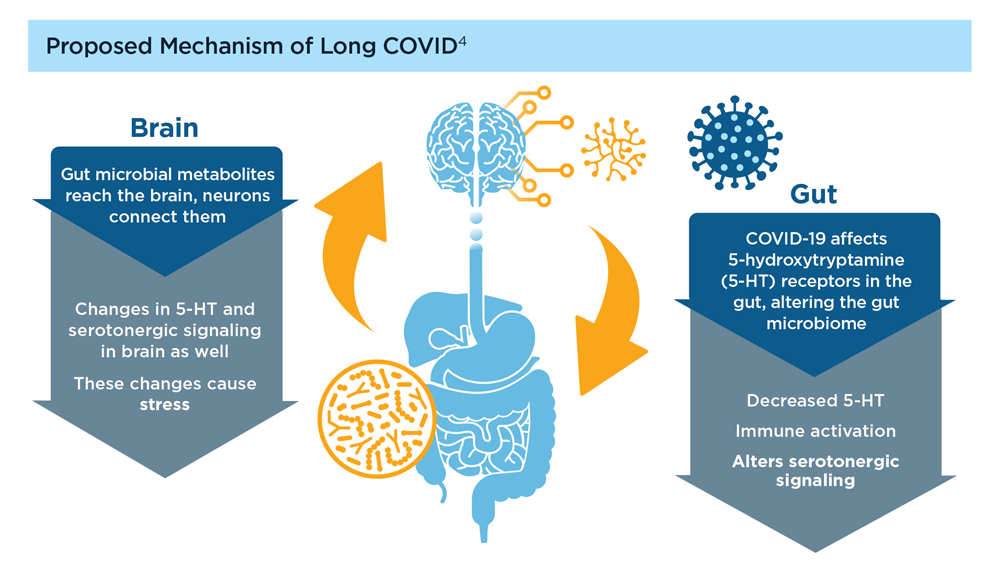
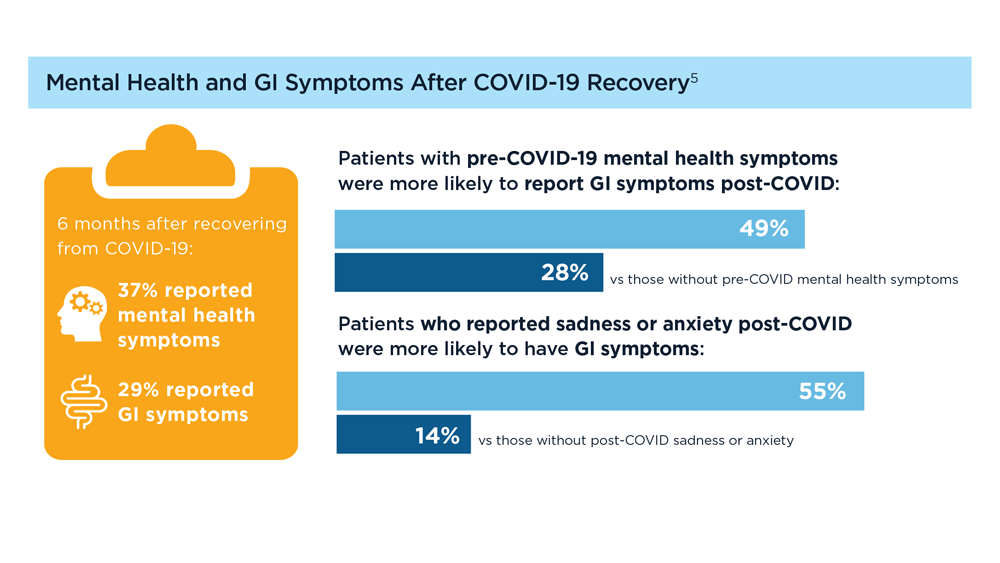
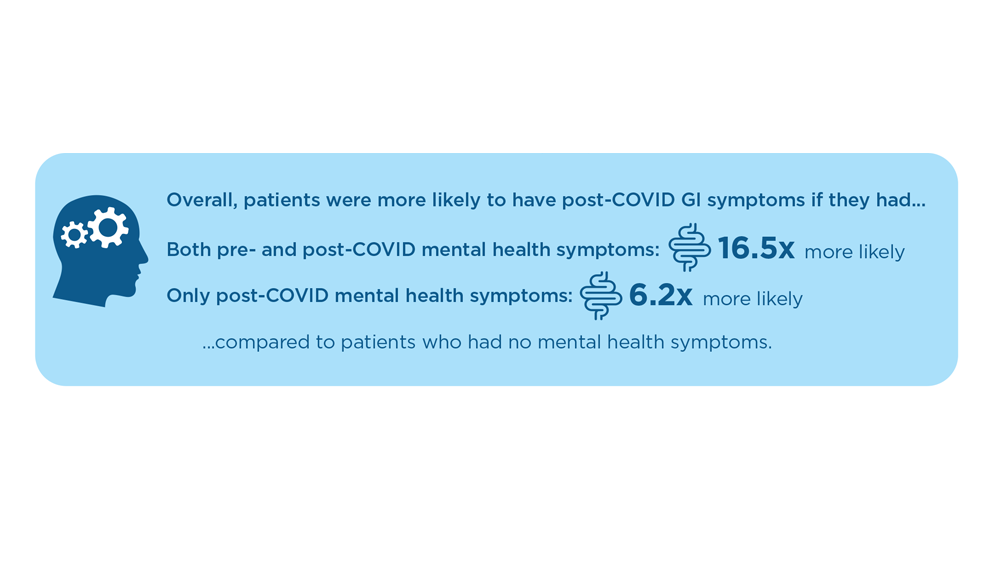
User login
COVID, no matter the severity, linked with urologic effects in men
SARS-CoV-2 infection is linked in men with increased incidence of urinary retention, urinary tract infection (UTI), and blood in the urine, a new study finds.
Authors of the study, led by Alex Qinyang Liu, of S.H. Ho Urology Centre, at The Chinese University of Hong Kong, highlighted the clinical implications.
“Clinicians should be aware of the significantly higher incidence of LUTS [lower urinary tract symptoms] complications with COVID-19 in this patient group and understand that these urological manifestations can occur regardless of COVID-19 severity,” the authors wrote.
Findings were published online in the Journal of Internal Medicine.
They explained that current literature has included only small case series and observational studies assessing the connection between COVID-19 and male LUTS.
Nearly 18,000 patients in study
Included in this study were all male patients who used the public health care system in Hong Kong who received alpha-blocker monotherapy for LUTS from 2021 to 2022. After propensity score matching, 17,986 patients were included. Half had polymerase chain reaction–confirmed SARS-CoV-2 infection (n = 8,993).
The retrospective study compared urologic outcomes, including male benign prostatic hyperplasia (BPH) complications, and changes in medical treatment in the two groups. They compared male patients with SARS-CoV-2 infection who were taking baseline alpha blocker monotherapy for LUTS with a control group who had no SARS-CoV-2 infection.
They found that, compared with controls, the SARS-CoV-2–infected group had significantly higher incidence of retention of urine (4.55% vs. 0.86%, P < .001), hematuria (1.36% vs. 0.41%, P < .001), clinical UTI (4.31% vs. 1.49%, P < .001), culture-proven bacteriuria (9.02% vs. 1.97%, P < .001), and addition of 5-alpha reductase inhibitors (0.50% vs. 0.02%, P < .001).
Similar side effects even with asymptomatic infection
The researchers pointed out that similar incidence of retention of urine, hematuria, and addition of medication were seen even when patients had asymptomatic infection.
They added that their findings have biological plausibility because the coexpression of the proteins ACE2 and TMPRSS2 in the prostate makes it a target for SARS-CoV-2, which leads to inflammation and may help explain the primary outcomes.
“Given the high infectivity and unprecedented scale of the COVID-19 pandemic, these urological symptoms and complications represent a significant clinical burden that clinicians and urologists should be aware of,” the authors wrote.
The authors noted that the prevalence of BPH and LUTS rises with age and are among the most common urologic conditions affecting older men. “Incidentally, male patients of advanced age are also more significantly affected by COVID-19.”
The authors declare no relevant financial relationships.
SARS-CoV-2 infection is linked in men with increased incidence of urinary retention, urinary tract infection (UTI), and blood in the urine, a new study finds.
Authors of the study, led by Alex Qinyang Liu, of S.H. Ho Urology Centre, at The Chinese University of Hong Kong, highlighted the clinical implications.
“Clinicians should be aware of the significantly higher incidence of LUTS [lower urinary tract symptoms] complications with COVID-19 in this patient group and understand that these urological manifestations can occur regardless of COVID-19 severity,” the authors wrote.
Findings were published online in the Journal of Internal Medicine.
They explained that current literature has included only small case series and observational studies assessing the connection between COVID-19 and male LUTS.
Nearly 18,000 patients in study
Included in this study were all male patients who used the public health care system in Hong Kong who received alpha-blocker monotherapy for LUTS from 2021 to 2022. After propensity score matching, 17,986 patients were included. Half had polymerase chain reaction–confirmed SARS-CoV-2 infection (n = 8,993).
The retrospective study compared urologic outcomes, including male benign prostatic hyperplasia (BPH) complications, and changes in medical treatment in the two groups. They compared male patients with SARS-CoV-2 infection who were taking baseline alpha blocker monotherapy for LUTS with a control group who had no SARS-CoV-2 infection.
They found that, compared with controls, the SARS-CoV-2–infected group had significantly higher incidence of retention of urine (4.55% vs. 0.86%, P < .001), hematuria (1.36% vs. 0.41%, P < .001), clinical UTI (4.31% vs. 1.49%, P < .001), culture-proven bacteriuria (9.02% vs. 1.97%, P < .001), and addition of 5-alpha reductase inhibitors (0.50% vs. 0.02%, P < .001).
Similar side effects even with asymptomatic infection
The researchers pointed out that similar incidence of retention of urine, hematuria, and addition of medication were seen even when patients had asymptomatic infection.
They added that their findings have biological plausibility because the coexpression of the proteins ACE2 and TMPRSS2 in the prostate makes it a target for SARS-CoV-2, which leads to inflammation and may help explain the primary outcomes.
“Given the high infectivity and unprecedented scale of the COVID-19 pandemic, these urological symptoms and complications represent a significant clinical burden that clinicians and urologists should be aware of,” the authors wrote.
The authors noted that the prevalence of BPH and LUTS rises with age and are among the most common urologic conditions affecting older men. “Incidentally, male patients of advanced age are also more significantly affected by COVID-19.”
The authors declare no relevant financial relationships.
SARS-CoV-2 infection is linked in men with increased incidence of urinary retention, urinary tract infection (UTI), and blood in the urine, a new study finds.
Authors of the study, led by Alex Qinyang Liu, of S.H. Ho Urology Centre, at The Chinese University of Hong Kong, highlighted the clinical implications.
“Clinicians should be aware of the significantly higher incidence of LUTS [lower urinary tract symptoms] complications with COVID-19 in this patient group and understand that these urological manifestations can occur regardless of COVID-19 severity,” the authors wrote.
Findings were published online in the Journal of Internal Medicine.
They explained that current literature has included only small case series and observational studies assessing the connection between COVID-19 and male LUTS.
Nearly 18,000 patients in study
Included in this study were all male patients who used the public health care system in Hong Kong who received alpha-blocker monotherapy for LUTS from 2021 to 2022. After propensity score matching, 17,986 patients were included. Half had polymerase chain reaction–confirmed SARS-CoV-2 infection (n = 8,993).
The retrospective study compared urologic outcomes, including male benign prostatic hyperplasia (BPH) complications, and changes in medical treatment in the two groups. They compared male patients with SARS-CoV-2 infection who were taking baseline alpha blocker monotherapy for LUTS with a control group who had no SARS-CoV-2 infection.
They found that, compared with controls, the SARS-CoV-2–infected group had significantly higher incidence of retention of urine (4.55% vs. 0.86%, P < .001), hematuria (1.36% vs. 0.41%, P < .001), clinical UTI (4.31% vs. 1.49%, P < .001), culture-proven bacteriuria (9.02% vs. 1.97%, P < .001), and addition of 5-alpha reductase inhibitors (0.50% vs. 0.02%, P < .001).
Similar side effects even with asymptomatic infection
The researchers pointed out that similar incidence of retention of urine, hematuria, and addition of medication were seen even when patients had asymptomatic infection.
They added that their findings have biological plausibility because the coexpression of the proteins ACE2 and TMPRSS2 in the prostate makes it a target for SARS-CoV-2, which leads to inflammation and may help explain the primary outcomes.
“Given the high infectivity and unprecedented scale of the COVID-19 pandemic, these urological symptoms and complications represent a significant clinical burden that clinicians and urologists should be aware of,” the authors wrote.
The authors noted that the prevalence of BPH and LUTS rises with age and are among the most common urologic conditions affecting older men. “Incidentally, male patients of advanced age are also more significantly affected by COVID-19.”
The authors declare no relevant financial relationships.
FROM THE JOURNAL OF INTERNAL MEDICINE
Paxlovid tied to benefits in high-risk patients with COVID
In a cohort study from British Columbia that included nearly 7,000 patients with COVID-19, nirmatrelvir-ritonavir was associated with a 2.5% reduction in risk for death or emergency hospitalization in clinically extremely vulnerable (CEV) patients who were severely immunocompromised. No significant benefit was observed in patients who were not immunocompromised.
“This finding could help substantially limit unnecessary use of nirmatrelvir and ritonavir in older, otherwise healthy individuals,” lead author Colin R. Dormuth, ScD, associate professor of anesthesiology, pharmacology, and therapeutics at the University of British Columbia, Vancouver, told this news organization. “Another finding that was surprising and might help place the role of nirmatrelvir and ritonavir in context is that even in severely immunocompromised individuals who did not take [the drug], the risk of death or hospitalization with COVID-19 was less than 4% in our study population.”
The study was published online in JAMA Network Open.
Who benefits?
The investigators analyzed medical records for 6,866 patients in British Columbia (median age, 70 years; 57% women) who presented between Feb. 1, 2022, and Feb. 3, 2023. Eligible patients belonged to one of four higher-risk groups who received priority for COVID-19 vaccination.
Two groups included CEV patients who were severely (CEV1) or moderately (CEV2) immunocompromised. The CEV3 group was not immunocompromised but had medical conditions associated with a high risk for complications from COVID-19. A fourth expanded eligibility (EXEL) group included higher-risk patients who were not in one of the other groups, such as unvaccinated patients older than age 70 years.
The investigators matched treated patients to untreated patients in the same vulnerability group according to age, sex, and month of infection. The primary outcome was death from any cause or emergency hospitalization with COVID-19 within 28 days.
Treatment with nirmatrelvir-ritonavir was associated with statistically significant relative reductions in the primary outcome, compared with no treatment, for patients in the CEV1 (risk difference, −2.5%) and CEV2 (RD, −1.7%) groups. In the CEV3 group, the RD of −1.3% was not statistically significant. In the EXEL group, treatment was associated with a higher risk for the primary outcome (RD, 1.0%), but the result was not statistically significant.
The results were “robust across sex and older vs. younger age,” the authors note. “No reduction in the primary outcome was observed in lower-risk individuals, including those aged 70 years or older without serious comorbidities.”
The combination of nirmatrelvir-ritonavir was approved for use in Canada based on interim efficacy and safety data from the Evaluation of Inhibition for COVID-19 in High-Risk Patients (EPIC-HR) trial, said Dr. Dormuth.
British Columbia’s eligibility criteria for nirmatrelvir-ritonavir coverage differ substantially from the criteria for participants in the EPIC-HR trial, he noted. Those patients were unvaccinated, had no natural immunity from a previous COVID-19 infection, and were infected with COVID-19 variants that were different from those now circulating. The current study was prompted by the need to look at a broader population of individuals in British Columbia with varying risks of complications from COVID-19 infection.
Before the study, a common view was that patients aged 70 and older would benefit from the drug, said Dr. Dormuth. “Our study, which accounted for medical conditions related to an individual’s vulnerability to complications, showed that older age on its own was not a reason to use nirmatrelvir and ritonavir once relevant medical conditions were taken into consideration.”
The researchers are working on a study to identify with greater specificity which comorbid conditions are most associated with nirmatrelvir-ritonavir effectiveness, he added. “It could be that a relatively small number of conditions can be used to identify most individuals who would benefit from the drug.”
‘Signal toward benefit’
Commenting on the findings for this news organization, Abhijit Duggal, MD, vice chair of critical care at the Cleveland Clinic, who was not involved in this study, said, “I’m always very wary when we look at observational data and we start saying the effectiveness is not really as high as was seen in other studies. We are seeing an effect with all these studies that seems to be in the right direction.
“Having said that,” he added, “is the effect going to be potentially more in patients at higher risk? Absolutely. I think these postmarket studies are really showing that after vaccination, if someone does get infected, this is a secondary option available to us that can prevent progression of the disease, which would likely be more severe in immunocompromised patients.”
Dr. Duggal was a coinvestigator on a recent study of more than 68,000 patients that showed that nirmatrelvir-ritonavir or molnupiravir was associated with reductions in mortality and hospitalization in nonhospitalized patients infected with the Omicron variant, regardless of age, race and ethnicity, virus strain, vaccination status, previous infection status, or coexisting conditions.
“In all groups, there was a signal toward benefit,” said Dr. Duggal. “These studies tell us that these drugs do remain valid options. But their use needs to be discussed on a case-by-case basis with patients we feel are deteriorating or at a higher risk because of underlying disease processes.”
The study was supported by funding from the British Columbia Ministry of Health. Dr. Dormuth and Dr. Duggal report no relevant financial relationships.
A version of this article appeared on Medscape.com.
In a cohort study from British Columbia that included nearly 7,000 patients with COVID-19, nirmatrelvir-ritonavir was associated with a 2.5% reduction in risk for death or emergency hospitalization in clinically extremely vulnerable (CEV) patients who were severely immunocompromised. No significant benefit was observed in patients who were not immunocompromised.
“This finding could help substantially limit unnecessary use of nirmatrelvir and ritonavir in older, otherwise healthy individuals,” lead author Colin R. Dormuth, ScD, associate professor of anesthesiology, pharmacology, and therapeutics at the University of British Columbia, Vancouver, told this news organization. “Another finding that was surprising and might help place the role of nirmatrelvir and ritonavir in context is that even in severely immunocompromised individuals who did not take [the drug], the risk of death or hospitalization with COVID-19 was less than 4% in our study population.”
The study was published online in JAMA Network Open.
Who benefits?
The investigators analyzed medical records for 6,866 patients in British Columbia (median age, 70 years; 57% women) who presented between Feb. 1, 2022, and Feb. 3, 2023. Eligible patients belonged to one of four higher-risk groups who received priority for COVID-19 vaccination.
Two groups included CEV patients who were severely (CEV1) or moderately (CEV2) immunocompromised. The CEV3 group was not immunocompromised but had medical conditions associated with a high risk for complications from COVID-19. A fourth expanded eligibility (EXEL) group included higher-risk patients who were not in one of the other groups, such as unvaccinated patients older than age 70 years.
The investigators matched treated patients to untreated patients in the same vulnerability group according to age, sex, and month of infection. The primary outcome was death from any cause or emergency hospitalization with COVID-19 within 28 days.
Treatment with nirmatrelvir-ritonavir was associated with statistically significant relative reductions in the primary outcome, compared with no treatment, for patients in the CEV1 (risk difference, −2.5%) and CEV2 (RD, −1.7%) groups. In the CEV3 group, the RD of −1.3% was not statistically significant. In the EXEL group, treatment was associated with a higher risk for the primary outcome (RD, 1.0%), but the result was not statistically significant.
The results were “robust across sex and older vs. younger age,” the authors note. “No reduction in the primary outcome was observed in lower-risk individuals, including those aged 70 years or older without serious comorbidities.”
The combination of nirmatrelvir-ritonavir was approved for use in Canada based on interim efficacy and safety data from the Evaluation of Inhibition for COVID-19 in High-Risk Patients (EPIC-HR) trial, said Dr. Dormuth.
British Columbia’s eligibility criteria for nirmatrelvir-ritonavir coverage differ substantially from the criteria for participants in the EPIC-HR trial, he noted. Those patients were unvaccinated, had no natural immunity from a previous COVID-19 infection, and were infected with COVID-19 variants that were different from those now circulating. The current study was prompted by the need to look at a broader population of individuals in British Columbia with varying risks of complications from COVID-19 infection.
Before the study, a common view was that patients aged 70 and older would benefit from the drug, said Dr. Dormuth. “Our study, which accounted for medical conditions related to an individual’s vulnerability to complications, showed that older age on its own was not a reason to use nirmatrelvir and ritonavir once relevant medical conditions were taken into consideration.”
The researchers are working on a study to identify with greater specificity which comorbid conditions are most associated with nirmatrelvir-ritonavir effectiveness, he added. “It could be that a relatively small number of conditions can be used to identify most individuals who would benefit from the drug.”
‘Signal toward benefit’
Commenting on the findings for this news organization, Abhijit Duggal, MD, vice chair of critical care at the Cleveland Clinic, who was not involved in this study, said, “I’m always very wary when we look at observational data and we start saying the effectiveness is not really as high as was seen in other studies. We are seeing an effect with all these studies that seems to be in the right direction.
“Having said that,” he added, “is the effect going to be potentially more in patients at higher risk? Absolutely. I think these postmarket studies are really showing that after vaccination, if someone does get infected, this is a secondary option available to us that can prevent progression of the disease, which would likely be more severe in immunocompromised patients.”
Dr. Duggal was a coinvestigator on a recent study of more than 68,000 patients that showed that nirmatrelvir-ritonavir or molnupiravir was associated with reductions in mortality and hospitalization in nonhospitalized patients infected with the Omicron variant, regardless of age, race and ethnicity, virus strain, vaccination status, previous infection status, or coexisting conditions.
“In all groups, there was a signal toward benefit,” said Dr. Duggal. “These studies tell us that these drugs do remain valid options. But their use needs to be discussed on a case-by-case basis with patients we feel are deteriorating or at a higher risk because of underlying disease processes.”
The study was supported by funding from the British Columbia Ministry of Health. Dr. Dormuth and Dr. Duggal report no relevant financial relationships.
A version of this article appeared on Medscape.com.
In a cohort study from British Columbia that included nearly 7,000 patients with COVID-19, nirmatrelvir-ritonavir was associated with a 2.5% reduction in risk for death or emergency hospitalization in clinically extremely vulnerable (CEV) patients who were severely immunocompromised. No significant benefit was observed in patients who were not immunocompromised.
“This finding could help substantially limit unnecessary use of nirmatrelvir and ritonavir in older, otherwise healthy individuals,” lead author Colin R. Dormuth, ScD, associate professor of anesthesiology, pharmacology, and therapeutics at the University of British Columbia, Vancouver, told this news organization. “Another finding that was surprising and might help place the role of nirmatrelvir and ritonavir in context is that even in severely immunocompromised individuals who did not take [the drug], the risk of death or hospitalization with COVID-19 was less than 4% in our study population.”
The study was published online in JAMA Network Open.
Who benefits?
The investigators analyzed medical records for 6,866 patients in British Columbia (median age, 70 years; 57% women) who presented between Feb. 1, 2022, and Feb. 3, 2023. Eligible patients belonged to one of four higher-risk groups who received priority for COVID-19 vaccination.
Two groups included CEV patients who were severely (CEV1) or moderately (CEV2) immunocompromised. The CEV3 group was not immunocompromised but had medical conditions associated with a high risk for complications from COVID-19. A fourth expanded eligibility (EXEL) group included higher-risk patients who were not in one of the other groups, such as unvaccinated patients older than age 70 years.
The investigators matched treated patients to untreated patients in the same vulnerability group according to age, sex, and month of infection. The primary outcome was death from any cause or emergency hospitalization with COVID-19 within 28 days.
Treatment with nirmatrelvir-ritonavir was associated with statistically significant relative reductions in the primary outcome, compared with no treatment, for patients in the CEV1 (risk difference, −2.5%) and CEV2 (RD, −1.7%) groups. In the CEV3 group, the RD of −1.3% was not statistically significant. In the EXEL group, treatment was associated with a higher risk for the primary outcome (RD, 1.0%), but the result was not statistically significant.
The results were “robust across sex and older vs. younger age,” the authors note. “No reduction in the primary outcome was observed in lower-risk individuals, including those aged 70 years or older without serious comorbidities.”
The combination of nirmatrelvir-ritonavir was approved for use in Canada based on interim efficacy and safety data from the Evaluation of Inhibition for COVID-19 in High-Risk Patients (EPIC-HR) trial, said Dr. Dormuth.
British Columbia’s eligibility criteria for nirmatrelvir-ritonavir coverage differ substantially from the criteria for participants in the EPIC-HR trial, he noted. Those patients were unvaccinated, had no natural immunity from a previous COVID-19 infection, and were infected with COVID-19 variants that were different from those now circulating. The current study was prompted by the need to look at a broader population of individuals in British Columbia with varying risks of complications from COVID-19 infection.
Before the study, a common view was that patients aged 70 and older would benefit from the drug, said Dr. Dormuth. “Our study, which accounted for medical conditions related to an individual’s vulnerability to complications, showed that older age on its own was not a reason to use nirmatrelvir and ritonavir once relevant medical conditions were taken into consideration.”
The researchers are working on a study to identify with greater specificity which comorbid conditions are most associated with nirmatrelvir-ritonavir effectiveness, he added. “It could be that a relatively small number of conditions can be used to identify most individuals who would benefit from the drug.”
‘Signal toward benefit’
Commenting on the findings for this news organization, Abhijit Duggal, MD, vice chair of critical care at the Cleveland Clinic, who was not involved in this study, said, “I’m always very wary when we look at observational data and we start saying the effectiveness is not really as high as was seen in other studies. We are seeing an effect with all these studies that seems to be in the right direction.
“Having said that,” he added, “is the effect going to be potentially more in patients at higher risk? Absolutely. I think these postmarket studies are really showing that after vaccination, if someone does get infected, this is a secondary option available to us that can prevent progression of the disease, which would likely be more severe in immunocompromised patients.”
Dr. Duggal was a coinvestigator on a recent study of more than 68,000 patients that showed that nirmatrelvir-ritonavir or molnupiravir was associated with reductions in mortality and hospitalization in nonhospitalized patients infected with the Omicron variant, regardless of age, race and ethnicity, virus strain, vaccination status, previous infection status, or coexisting conditions.
“In all groups, there was a signal toward benefit,” said Dr. Duggal. “These studies tell us that these drugs do remain valid options. But their use needs to be discussed on a case-by-case basis with patients we feel are deteriorating or at a higher risk because of underlying disease processes.”
The study was supported by funding from the British Columbia Ministry of Health. Dr. Dormuth and Dr. Duggal report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Don’t fear POTS: Tips for diagnosis and treatment
This transcript has been edited for clarity.
Michelle L. O’Donoghue, MD, MPH: I’m here in Amsterdam at the European Society of Cardiology (ESC) Congress 2023. Joining me for a great discussion is my friend Dr. Pam Taub, who is a cardiologist and a professor of medicine at UC San Diego. She has a particular interest in postural orthostatic tachycardia syndrome (POTS), so that’s what we’ll be talking about today.
Thanks for joining me, Pam. When we think about POTS, for those who are not familiar with the term, what does it actually mean and how do you diagnose it?
No tilt table required
Pam R. Taub, MD: That’s typically associated with symptoms such as lightheadedness, dizziness, and cognitive difficulties such as brain fog. The diagnosis can be made by tilt-table testing, but it can also be made in the office with simple orthostats.
In my clinic, I have people lie down for 3-5 minutes. At the end of that period, you get a heart rate and blood pressure. Then you have them stand up for 3-5 minutes and then get heart rate and blood pressure, and you look at the differences. If the heart rate goes up by 30 points – so maybe they’re 80 beats/min when they’re lying down and when they stand up, it goes to 110 beats/min – that’s POTS, so very objective criteria. Typically, these people don’t have what we call orthostatic hypotension, where there is a significant decrease in the blood pressure. It’s more a heart rate issue.
Dr. O’Donoghue: How symptomatically do they usually present?
Dr. Taub: It’s a spectrum. Some people have mild symptoms. After they’re in the upright position for maybe 10 minutes, they get symptoms. There are some people who, when they go from a lying to standing position, they’re extremely symptomatic and can’t really do any activities. There are some people that are even wheelchair-bound because the symptoms are so debilitating. There’s a wide spectrum.
Dr. O’Donoghue: There has been more discussion, I feel like, about the rising prevalence of POTS as a diagnosis, and in particular since the COVID pandemic. What’s our understanding of the relationship between COVID and POTS and what the mechanism might be?
Dr. Taub: We’ve known that POTS can be triggered by a viral infection. Before COVID, we knew that in certain individuals that we think have an underlying genetic predisposition, usually some autoimmune substrate, when they get certain types of infections, whether it’s influenza or mononucleosis, they get POTS.
Typically, when they get an infection, they start getting deconditioned. They don’t feel well, so they’re on bed rest. When they get long periods of bed rest, when they start to become active, they start to have overactivation of their sympathetic nervous system, and they have a large amount of cardiovascular deconditioning. It’s a cycle that is often triggered after an infection.
A huge increase of POTS has been seen after COVID-19 because we had so many people exposed to this virus. With COVID-19, there is a period where people don’t feel great and they are getting bed rest, so they’re getting deconditioned. We’ve seen so many patients referred for post-COVID POTS and also long COVID or the post-acute sequelae of COVID-19, where POTS is a part of that presentation.
Female sex and autoimmune conditions
Dr. O’Donoghue: We know that POTS seems to disproportionately affect women. Is that understood? Is it thought that that’s related to the perhaps the autoimmune component of that illness?
Dr. Taub: Yes. The theory is because women tend to have more autoimmune conditions, that’s why they’re more predisposed. There’s a large amount of genetic susceptibility. For instance, we know that there’s an association between POTS and conditions like Ehlers-Danlos syndrome and between POTS and mast cell activation. Some of those conditions are more prevalent in women as well.
Dr. O’Donoghue: I feel like many physicians don’t know how to manage POTS, and they’re actually a little fearful perhaps to take it on. Fortunately, there have been a growing number of POTS clinics with specialists that focus on that area. For the average practitioner who maybe can’t refer to a POTS clinic, how should they approach that?
Dr. Taub: The first thing is its diagnosis. When someone tells you that they have symptoms of orthostatic intolerance – so, activities that involve standing – you need to first have that on your differential diagnosis. You can make the diagnosis in the office with orthostats. You don’t need a tilt table. It’s sometimes helpful if you’re unsure about the diagnosis, but you can make the diagnosis.
Many times, you’re finding people that have very mild symptoms. You can treat that with some good lifestyle recommendations, such as increased hydration, increasing salt in their diet, and compression. And the exercise component is really important.
Many people with POTS are told to go exercise, go for a run, or go for a walk. That’s incorrect, because these people have symptoms when they’re in the upright position. The type of exercise they need to do initially is exercise in the lying or seated position – so exercises like rowing or a seated bike, and strength training. As they start to feel better, then they can do upright exercise.
You should never tell a person that has POTS to just initially start with upright exercise, because they’re going to feel so much worse and then they’re never going to want to exercise. It’s really important to give them the right exercise recommendations. I find that for many of these mild cases, if they do the right exercise and engage in the right lifestyle strategies, they get better.
Compression wear and drug therapy
Dr. O’Donoghue: When it comes to compression stockings, do you usually start with a particular length?
Dr. Taub: It’s interesting. There are many different compression stockings, medical grade. Through patients with POTS, I’ve gotten feedback on certain types of athletic wear that have built-in compression, and that’s a little bit easier for people to wear every day because they can do their errands and it doesn’t look like they’re wearing medical-grade compression stockings.
Basically, I’ve collected all the different recommendations that patients say help, and I give them a list. The medical-grade compression stockings sometimes are very challenging to put on, and sometimes people just need light compression or even just socks. Any kind of compression is going to help.
Dr. O’Donoghue: That’s a great tip, because I know there are many patients who refuse to wear the compression stockings. If there’s a fashionable alternative, that’s always good to reach for.
Dr. Taub: Another thing that patients have told me is that abdominal compression is also very helpful. There are many commercially available abdominal compression options, like shapewear. Many patients with POTS use that and that helps, too.
Dr. O’Donoghue: Good. For those patients with POTS that is refractory to the measures you’ve already discussed, what are the next steps after that?
Dr. Taub: Pharmacotherapy is very synergistic with lifestyle, and there are many different pharmacotherapy options. One of the first things that you want to think about is lowering that heart rate. The reason people feel horrible is because their heart rate is usually very high when they’re upright. If they’re upright for long periods of time and they’re having very high heart rates, they’re going to get really tired because it’s like they’re exercising for hours when they’re upright.
Heart rate lowering is the cornerstone of therapy. Traditionally, we’ve used beta-blockers for heart rate lowering. The problem is they also lower blood pressure. They can also cause fatigue, so not the ideal agent for patients with POTS.
One of the clinical trials that I led was with a drug called ivabradine, which selectively works on the SA node and decreases heart rate without affecting blood pressure. What’s really elegant about ivabradine is it has a more potent effect when the heart rate is higher. When the patient is standing, it’s going to have a more potent effect on heart rate lowering. It’s really well tolerated in patients with POTS. In our study, we showed an improvement in quality of life metrics. That’s one of the first-line drugs that I use for patients with POTS.
The other thing is some of them will also have a concomitant lowering of blood pressure. You can think about medications that increase blood pressure, like midodrine, fludrocortisone, and droxidopa. Sometimes that combination of a heart rate-lowering medication and a medication that increases blood pressure really works well.
Dr. O’Donoghue: That’s very helpful. I think that those kinds of practical tips are the ones that practitioners really want to reach for, because they need to have that algorithm in their mind to take on this condition. Thanks again for walking us through that.
I think it’s a very interesting space, and there’s more that we’re going to be learning over the next few years as we further flesh out these post-COVID cases and what we learn from that as well.
Dr. Taub: There are many clinical trials now starting in POTS, so it’s exciting.
Dr. O’Donoghue: Absolutely. Thank you again for joining me today. Signing off, this is Dr Michelle O’Donoghue.
Dr. O’Donoghue is a cardiologist at Brigham and Women’s Hospital and senior investigator with the TIMI Study Group. A strong believer in evidence-based medicine, she relishes discussions about the published literature. A native Canadian, Dr. O’Donoghue loves spending time outdoors with her family but admits with shame that she’s never strapped on hockey skates. She disclosed ties with Amgen, AstraZeneca Pharmaceuticals LP, CVS Minute Clinic, Eisai, GlaxoSmithKline, Janssen Pharmaceuticals, Merck, Novartis, and The Medicines Company. Dr. Taub is professor of Medicine, University of California San Diego Health, La Jolla. She disclosed ties with Amgen, Bayer, Boehringer Ingelheim, Medtronic, Merck, Novartis, Novo Nordisk, and Sanofi.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Michelle L. O’Donoghue, MD, MPH: I’m here in Amsterdam at the European Society of Cardiology (ESC) Congress 2023. Joining me for a great discussion is my friend Dr. Pam Taub, who is a cardiologist and a professor of medicine at UC San Diego. She has a particular interest in postural orthostatic tachycardia syndrome (POTS), so that’s what we’ll be talking about today.
Thanks for joining me, Pam. When we think about POTS, for those who are not familiar with the term, what does it actually mean and how do you diagnose it?
No tilt table required
Pam R. Taub, MD: That’s typically associated with symptoms such as lightheadedness, dizziness, and cognitive difficulties such as brain fog. The diagnosis can be made by tilt-table testing, but it can also be made in the office with simple orthostats.
In my clinic, I have people lie down for 3-5 minutes. At the end of that period, you get a heart rate and blood pressure. Then you have them stand up for 3-5 minutes and then get heart rate and blood pressure, and you look at the differences. If the heart rate goes up by 30 points – so maybe they’re 80 beats/min when they’re lying down and when they stand up, it goes to 110 beats/min – that’s POTS, so very objective criteria. Typically, these people don’t have what we call orthostatic hypotension, where there is a significant decrease in the blood pressure. It’s more a heart rate issue.
Dr. O’Donoghue: How symptomatically do they usually present?
Dr. Taub: It’s a spectrum. Some people have mild symptoms. After they’re in the upright position for maybe 10 minutes, they get symptoms. There are some people who, when they go from a lying to standing position, they’re extremely symptomatic and can’t really do any activities. There are some people that are even wheelchair-bound because the symptoms are so debilitating. There’s a wide spectrum.
Dr. O’Donoghue: There has been more discussion, I feel like, about the rising prevalence of POTS as a diagnosis, and in particular since the COVID pandemic. What’s our understanding of the relationship between COVID and POTS and what the mechanism might be?
Dr. Taub: We’ve known that POTS can be triggered by a viral infection. Before COVID, we knew that in certain individuals that we think have an underlying genetic predisposition, usually some autoimmune substrate, when they get certain types of infections, whether it’s influenza or mononucleosis, they get POTS.
Typically, when they get an infection, they start getting deconditioned. They don’t feel well, so they’re on bed rest. When they get long periods of bed rest, when they start to become active, they start to have overactivation of their sympathetic nervous system, and they have a large amount of cardiovascular deconditioning. It’s a cycle that is often triggered after an infection.
A huge increase of POTS has been seen after COVID-19 because we had so many people exposed to this virus. With COVID-19, there is a period where people don’t feel great and they are getting bed rest, so they’re getting deconditioned. We’ve seen so many patients referred for post-COVID POTS and also long COVID or the post-acute sequelae of COVID-19, where POTS is a part of that presentation.
Female sex and autoimmune conditions
Dr. O’Donoghue: We know that POTS seems to disproportionately affect women. Is that understood? Is it thought that that’s related to the perhaps the autoimmune component of that illness?
Dr. Taub: Yes. The theory is because women tend to have more autoimmune conditions, that’s why they’re more predisposed. There’s a large amount of genetic susceptibility. For instance, we know that there’s an association between POTS and conditions like Ehlers-Danlos syndrome and between POTS and mast cell activation. Some of those conditions are more prevalent in women as well.
Dr. O’Donoghue: I feel like many physicians don’t know how to manage POTS, and they’re actually a little fearful perhaps to take it on. Fortunately, there have been a growing number of POTS clinics with specialists that focus on that area. For the average practitioner who maybe can’t refer to a POTS clinic, how should they approach that?
Dr. Taub: The first thing is its diagnosis. When someone tells you that they have symptoms of orthostatic intolerance – so, activities that involve standing – you need to first have that on your differential diagnosis. You can make the diagnosis in the office with orthostats. You don’t need a tilt table. It’s sometimes helpful if you’re unsure about the diagnosis, but you can make the diagnosis.
Many times, you’re finding people that have very mild symptoms. You can treat that with some good lifestyle recommendations, such as increased hydration, increasing salt in their diet, and compression. And the exercise component is really important.
Many people with POTS are told to go exercise, go for a run, or go for a walk. That’s incorrect, because these people have symptoms when they’re in the upright position. The type of exercise they need to do initially is exercise in the lying or seated position – so exercises like rowing or a seated bike, and strength training. As they start to feel better, then they can do upright exercise.
You should never tell a person that has POTS to just initially start with upright exercise, because they’re going to feel so much worse and then they’re never going to want to exercise. It’s really important to give them the right exercise recommendations. I find that for many of these mild cases, if they do the right exercise and engage in the right lifestyle strategies, they get better.
Compression wear and drug therapy
Dr. O’Donoghue: When it comes to compression stockings, do you usually start with a particular length?
Dr. Taub: It’s interesting. There are many different compression stockings, medical grade. Through patients with POTS, I’ve gotten feedback on certain types of athletic wear that have built-in compression, and that’s a little bit easier for people to wear every day because they can do their errands and it doesn’t look like they’re wearing medical-grade compression stockings.
Basically, I’ve collected all the different recommendations that patients say help, and I give them a list. The medical-grade compression stockings sometimes are very challenging to put on, and sometimes people just need light compression or even just socks. Any kind of compression is going to help.
Dr. O’Donoghue: That’s a great tip, because I know there are many patients who refuse to wear the compression stockings. If there’s a fashionable alternative, that’s always good to reach for.
Dr. Taub: Another thing that patients have told me is that abdominal compression is also very helpful. There are many commercially available abdominal compression options, like shapewear. Many patients with POTS use that and that helps, too.
Dr. O’Donoghue: Good. For those patients with POTS that is refractory to the measures you’ve already discussed, what are the next steps after that?
Dr. Taub: Pharmacotherapy is very synergistic with lifestyle, and there are many different pharmacotherapy options. One of the first things that you want to think about is lowering that heart rate. The reason people feel horrible is because their heart rate is usually very high when they’re upright. If they’re upright for long periods of time and they’re having very high heart rates, they’re going to get really tired because it’s like they’re exercising for hours when they’re upright.
Heart rate lowering is the cornerstone of therapy. Traditionally, we’ve used beta-blockers for heart rate lowering. The problem is they also lower blood pressure. They can also cause fatigue, so not the ideal agent for patients with POTS.
One of the clinical trials that I led was with a drug called ivabradine, which selectively works on the SA node and decreases heart rate without affecting blood pressure. What’s really elegant about ivabradine is it has a more potent effect when the heart rate is higher. When the patient is standing, it’s going to have a more potent effect on heart rate lowering. It’s really well tolerated in patients with POTS. In our study, we showed an improvement in quality of life metrics. That’s one of the first-line drugs that I use for patients with POTS.
The other thing is some of them will also have a concomitant lowering of blood pressure. You can think about medications that increase blood pressure, like midodrine, fludrocortisone, and droxidopa. Sometimes that combination of a heart rate-lowering medication and a medication that increases blood pressure really works well.
Dr. O’Donoghue: That’s very helpful. I think that those kinds of practical tips are the ones that practitioners really want to reach for, because they need to have that algorithm in their mind to take on this condition. Thanks again for walking us through that.
I think it’s a very interesting space, and there’s more that we’re going to be learning over the next few years as we further flesh out these post-COVID cases and what we learn from that as well.
Dr. Taub: There are many clinical trials now starting in POTS, so it’s exciting.
Dr. O’Donoghue: Absolutely. Thank you again for joining me today. Signing off, this is Dr Michelle O’Donoghue.
Dr. O’Donoghue is a cardiologist at Brigham and Women’s Hospital and senior investigator with the TIMI Study Group. A strong believer in evidence-based medicine, she relishes discussions about the published literature. A native Canadian, Dr. O’Donoghue loves spending time outdoors with her family but admits with shame that she’s never strapped on hockey skates. She disclosed ties with Amgen, AstraZeneca Pharmaceuticals LP, CVS Minute Clinic, Eisai, GlaxoSmithKline, Janssen Pharmaceuticals, Merck, Novartis, and The Medicines Company. Dr. Taub is professor of Medicine, University of California San Diego Health, La Jolla. She disclosed ties with Amgen, Bayer, Boehringer Ingelheim, Medtronic, Merck, Novartis, Novo Nordisk, and Sanofi.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Michelle L. O’Donoghue, MD, MPH: I’m here in Amsterdam at the European Society of Cardiology (ESC) Congress 2023. Joining me for a great discussion is my friend Dr. Pam Taub, who is a cardiologist and a professor of medicine at UC San Diego. She has a particular interest in postural orthostatic tachycardia syndrome (POTS), so that’s what we’ll be talking about today.
Thanks for joining me, Pam. When we think about POTS, for those who are not familiar with the term, what does it actually mean and how do you diagnose it?
No tilt table required
Pam R. Taub, MD: That’s typically associated with symptoms such as lightheadedness, dizziness, and cognitive difficulties such as brain fog. The diagnosis can be made by tilt-table testing, but it can also be made in the office with simple orthostats.
In my clinic, I have people lie down for 3-5 minutes. At the end of that period, you get a heart rate and blood pressure. Then you have them stand up for 3-5 minutes and then get heart rate and blood pressure, and you look at the differences. If the heart rate goes up by 30 points – so maybe they’re 80 beats/min when they’re lying down and when they stand up, it goes to 110 beats/min – that’s POTS, so very objective criteria. Typically, these people don’t have what we call orthostatic hypotension, where there is a significant decrease in the blood pressure. It’s more a heart rate issue.
Dr. O’Donoghue: How symptomatically do they usually present?
Dr. Taub: It’s a spectrum. Some people have mild symptoms. After they’re in the upright position for maybe 10 minutes, they get symptoms. There are some people who, when they go from a lying to standing position, they’re extremely symptomatic and can’t really do any activities. There are some people that are even wheelchair-bound because the symptoms are so debilitating. There’s a wide spectrum.
Dr. O’Donoghue: There has been more discussion, I feel like, about the rising prevalence of POTS as a diagnosis, and in particular since the COVID pandemic. What’s our understanding of the relationship between COVID and POTS and what the mechanism might be?
Dr. Taub: We’ve known that POTS can be triggered by a viral infection. Before COVID, we knew that in certain individuals that we think have an underlying genetic predisposition, usually some autoimmune substrate, when they get certain types of infections, whether it’s influenza or mononucleosis, they get POTS.
Typically, when they get an infection, they start getting deconditioned. They don’t feel well, so they’re on bed rest. When they get long periods of bed rest, when they start to become active, they start to have overactivation of their sympathetic nervous system, and they have a large amount of cardiovascular deconditioning. It’s a cycle that is often triggered after an infection.
A huge increase of POTS has been seen after COVID-19 because we had so many people exposed to this virus. With COVID-19, there is a period where people don’t feel great and they are getting bed rest, so they’re getting deconditioned. We’ve seen so many patients referred for post-COVID POTS and also long COVID or the post-acute sequelae of COVID-19, where POTS is a part of that presentation.
Female sex and autoimmune conditions
Dr. O’Donoghue: We know that POTS seems to disproportionately affect women. Is that understood? Is it thought that that’s related to the perhaps the autoimmune component of that illness?
Dr. Taub: Yes. The theory is because women tend to have more autoimmune conditions, that’s why they’re more predisposed. There’s a large amount of genetic susceptibility. For instance, we know that there’s an association between POTS and conditions like Ehlers-Danlos syndrome and between POTS and mast cell activation. Some of those conditions are more prevalent in women as well.
Dr. O’Donoghue: I feel like many physicians don’t know how to manage POTS, and they’re actually a little fearful perhaps to take it on. Fortunately, there have been a growing number of POTS clinics with specialists that focus on that area. For the average practitioner who maybe can’t refer to a POTS clinic, how should they approach that?
Dr. Taub: The first thing is its diagnosis. When someone tells you that they have symptoms of orthostatic intolerance – so, activities that involve standing – you need to first have that on your differential diagnosis. You can make the diagnosis in the office with orthostats. You don’t need a tilt table. It’s sometimes helpful if you’re unsure about the diagnosis, but you can make the diagnosis.
Many times, you’re finding people that have very mild symptoms. You can treat that with some good lifestyle recommendations, such as increased hydration, increasing salt in their diet, and compression. And the exercise component is really important.
Many people with POTS are told to go exercise, go for a run, or go for a walk. That’s incorrect, because these people have symptoms when they’re in the upright position. The type of exercise they need to do initially is exercise in the lying or seated position – so exercises like rowing or a seated bike, and strength training. As they start to feel better, then they can do upright exercise.
You should never tell a person that has POTS to just initially start with upright exercise, because they’re going to feel so much worse and then they’re never going to want to exercise. It’s really important to give them the right exercise recommendations. I find that for many of these mild cases, if they do the right exercise and engage in the right lifestyle strategies, they get better.
Compression wear and drug therapy
Dr. O’Donoghue: When it comes to compression stockings, do you usually start with a particular length?
Dr. Taub: It’s interesting. There are many different compression stockings, medical grade. Through patients with POTS, I’ve gotten feedback on certain types of athletic wear that have built-in compression, and that’s a little bit easier for people to wear every day because they can do their errands and it doesn’t look like they’re wearing medical-grade compression stockings.
Basically, I’ve collected all the different recommendations that patients say help, and I give them a list. The medical-grade compression stockings sometimes are very challenging to put on, and sometimes people just need light compression or even just socks. Any kind of compression is going to help.
Dr. O’Donoghue: That’s a great tip, because I know there are many patients who refuse to wear the compression stockings. If there’s a fashionable alternative, that’s always good to reach for.
Dr. Taub: Another thing that patients have told me is that abdominal compression is also very helpful. There are many commercially available abdominal compression options, like shapewear. Many patients with POTS use that and that helps, too.
Dr. O’Donoghue: Good. For those patients with POTS that is refractory to the measures you’ve already discussed, what are the next steps after that?
Dr. Taub: Pharmacotherapy is very synergistic with lifestyle, and there are many different pharmacotherapy options. One of the first things that you want to think about is lowering that heart rate. The reason people feel horrible is because their heart rate is usually very high when they’re upright. If they’re upright for long periods of time and they’re having very high heart rates, they’re going to get really tired because it’s like they’re exercising for hours when they’re upright.
Heart rate lowering is the cornerstone of therapy. Traditionally, we’ve used beta-blockers for heart rate lowering. The problem is they also lower blood pressure. They can also cause fatigue, so not the ideal agent for patients with POTS.
One of the clinical trials that I led was with a drug called ivabradine, which selectively works on the SA node and decreases heart rate without affecting blood pressure. What’s really elegant about ivabradine is it has a more potent effect when the heart rate is higher. When the patient is standing, it’s going to have a more potent effect on heart rate lowering. It’s really well tolerated in patients with POTS. In our study, we showed an improvement in quality of life metrics. That’s one of the first-line drugs that I use for patients with POTS.
The other thing is some of them will also have a concomitant lowering of blood pressure. You can think about medications that increase blood pressure, like midodrine, fludrocortisone, and droxidopa. Sometimes that combination of a heart rate-lowering medication and a medication that increases blood pressure really works well.
Dr. O’Donoghue: That’s very helpful. I think that those kinds of practical tips are the ones that practitioners really want to reach for, because they need to have that algorithm in their mind to take on this condition. Thanks again for walking us through that.
I think it’s a very interesting space, and there’s more that we’re going to be learning over the next few years as we further flesh out these post-COVID cases and what we learn from that as well.
Dr. Taub: There are many clinical trials now starting in POTS, so it’s exciting.
Dr. O’Donoghue: Absolutely. Thank you again for joining me today. Signing off, this is Dr Michelle O’Donoghue.
Dr. O’Donoghue is a cardiologist at Brigham and Women’s Hospital and senior investigator with the TIMI Study Group. A strong believer in evidence-based medicine, she relishes discussions about the published literature. A native Canadian, Dr. O’Donoghue loves spending time outdoors with her family but admits with shame that she’s never strapped on hockey skates. She disclosed ties with Amgen, AstraZeneca Pharmaceuticals LP, CVS Minute Clinic, Eisai, GlaxoSmithKline, Janssen Pharmaceuticals, Merck, Novartis, and The Medicines Company. Dr. Taub is professor of Medicine, University of California San Diego Health, La Jolla. She disclosed ties with Amgen, Bayer, Boehringer Ingelheim, Medtronic, Merck, Novartis, Novo Nordisk, and Sanofi.
A version of this article appeared on Medscape.com.
More evidence shows COVID-19’s link to risk for autoimmune disease
TOPLINE:
Research from South Korea provides additional evidence for the connection between COVID-19 and an increased risk for autoimmune conditions post infection.
METHODOLOGY:
- In this retrospective study, researchers identified 354,527 individuals diagnosed with COVID-19 via polymerase chain reaction (PCR) testing from Oct. 8, 2020, to Dec. 31, 2021.
- Researchers compared the COVID-19 group with 6,134,940 healthy individuals who had no evidence of COVID-19 to quantify the risk for autoimmune and autoinflammatory connective tissue disorders.
- Patients were followed until diagnosis, death, or end of study period (Dec. 31, 2021).
TAKEAWAY:
- Risks for alopecia areata, alopecia totalis, antineutrophil cytoplasmic antibody–associated vasculitis, Crohn’s disease, and sarcoidosis were higher in the COVID-19 group.
- Patients with more severe COVID-19 (admitted to the ICU) were at greater risk for many autoimmune conditions, including alopecia totalis, psoriasis, vitiligo, and vasculitis.
IN PRACTICE:
“Our results emphasize the need to focus on managing not only the acute stages of COVID-19 itself but also autoimmune diseases as complications of COVID-19,” the authors wrote.
SOURCE:
Sung Ha Lim, MD, of Yonsei University, Wonju, South Korea, was the first author of the study, published in JAMA Network Open.
LIMITATIONS:
The study was retrospective and was composed almost exclusively of individuals from a single ethnicity. The study could have included individuals with COVID-19 in the control group who did not undergo PCR testing. The analysis did not include detailed information on each patient, including genetic information, that could have contributed to autoimmune disease risk.
DISCLOSURES:
The study was supported by a fund from the research program of the Korea Medical Institute and by grants from the Korea Health Industry Development Institute, the Korean Ministry of Health & Welfare, and the National Research Foundation of Korea. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Research from South Korea provides additional evidence for the connection between COVID-19 and an increased risk for autoimmune conditions post infection.
METHODOLOGY:
- In this retrospective study, researchers identified 354,527 individuals diagnosed with COVID-19 via polymerase chain reaction (PCR) testing from Oct. 8, 2020, to Dec. 31, 2021.
- Researchers compared the COVID-19 group with 6,134,940 healthy individuals who had no evidence of COVID-19 to quantify the risk for autoimmune and autoinflammatory connective tissue disorders.
- Patients were followed until diagnosis, death, or end of study period (Dec. 31, 2021).
TAKEAWAY:
- Risks for alopecia areata, alopecia totalis, antineutrophil cytoplasmic antibody–associated vasculitis, Crohn’s disease, and sarcoidosis were higher in the COVID-19 group.
- Patients with more severe COVID-19 (admitted to the ICU) were at greater risk for many autoimmune conditions, including alopecia totalis, psoriasis, vitiligo, and vasculitis.
IN PRACTICE:
“Our results emphasize the need to focus on managing not only the acute stages of COVID-19 itself but also autoimmune diseases as complications of COVID-19,” the authors wrote.
SOURCE:
Sung Ha Lim, MD, of Yonsei University, Wonju, South Korea, was the first author of the study, published in JAMA Network Open.
LIMITATIONS:
The study was retrospective and was composed almost exclusively of individuals from a single ethnicity. The study could have included individuals with COVID-19 in the control group who did not undergo PCR testing. The analysis did not include detailed information on each patient, including genetic information, that could have contributed to autoimmune disease risk.
DISCLOSURES:
The study was supported by a fund from the research program of the Korea Medical Institute and by grants from the Korea Health Industry Development Institute, the Korean Ministry of Health & Welfare, and the National Research Foundation of Korea. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Research from South Korea provides additional evidence for the connection between COVID-19 and an increased risk for autoimmune conditions post infection.
METHODOLOGY:
- In this retrospective study, researchers identified 354,527 individuals diagnosed with COVID-19 via polymerase chain reaction (PCR) testing from Oct. 8, 2020, to Dec. 31, 2021.
- Researchers compared the COVID-19 group with 6,134,940 healthy individuals who had no evidence of COVID-19 to quantify the risk for autoimmune and autoinflammatory connective tissue disorders.
- Patients were followed until diagnosis, death, or end of study period (Dec. 31, 2021).
TAKEAWAY:
- Risks for alopecia areata, alopecia totalis, antineutrophil cytoplasmic antibody–associated vasculitis, Crohn’s disease, and sarcoidosis were higher in the COVID-19 group.
- Patients with more severe COVID-19 (admitted to the ICU) were at greater risk for many autoimmune conditions, including alopecia totalis, psoriasis, vitiligo, and vasculitis.
IN PRACTICE:
“Our results emphasize the need to focus on managing not only the acute stages of COVID-19 itself but also autoimmune diseases as complications of COVID-19,” the authors wrote.
SOURCE:
Sung Ha Lim, MD, of Yonsei University, Wonju, South Korea, was the first author of the study, published in JAMA Network Open.
LIMITATIONS:
The study was retrospective and was composed almost exclusively of individuals from a single ethnicity. The study could have included individuals with COVID-19 in the control group who did not undergo PCR testing. The analysis did not include detailed information on each patient, including genetic information, that could have contributed to autoimmune disease risk.
DISCLOSURES:
The study was supported by a fund from the research program of the Korea Medical Institute and by grants from the Korea Health Industry Development Institute, the Korean Ministry of Health & Welfare, and the National Research Foundation of Korea. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Preparing for the viral trifecta: RSV, influenza, and COVID-19
New armamentaria available to fight an old disease.
In July 2023, nirsevimab (Beyfortus), a monoclonal antibody, was approved by the Food and Drug Administration for the prevention of respiratory syncytial virus (RSV) disease in infants and children younger than 2 years of age. On Aug. 3, 2023, the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention recommended routine use of it for all infants younger than 8 months of age born during or entering their first RSV season. Its use is also recommended for certain children 8-19 months of age who are at increased risk for severe RSV disease at the start of their second RSV season. Hearing the approval, I immediately had a flashback to residency, recalling the multiple infants admitted each fall and winter exhibiting classic symptoms including cough, rhinorrhea, nasal flaring, retractions, and wheezing with many having oxygen requirements and others needing intubation. Only supportive care was available.
RSV is the leading cause of infant hospitalizations. Annually, the CDC estimates there are 50,000-80,000 RSV hospitalizations and 100-300 RSV-related deaths in the United States in persons younger than 5 years of age. While premature infants have the highest rates of hospitalization (three times a term infant) about 79% of hospitalized children younger than 2 years have no underlying medical risks.1 The majority of children will experience RSV as an upper respiratory infection within the first 2 years of life. However, severe disease requiring hospitalization is more likely to occur in premature infants and children younger than 6 months; children younger than 2 with congenital heart disease and/or chronic lung disease; children with severe cystic fibrosis; as well as the immunocompromised child and individuals with neuromuscular disorders that preclude clearing mucous secretions or have difficulty swallowing.
Palivizumab (Synagis), the first monoclonal antibody to prevent RSV in infants was licensed in 1998. Its use was limited to infants meeting specific criteria developed by the American Academy of Pediatrics. Only 5% of infants had access to it. It was a short-acting agent requiring monthly injections, which were very costly ($1,661-$2,584 per dose). Eligible infants could receive up to five injections per season. Several studies proved its use was not cost beneficial.
What are the advantages of nirsevimab? It’s a long-acting monoclonal antibody. Only one dose is required per season. Costs will significantly diminish. It is recommended for all infants younger than 8 months of age born during RSV season. Those children 8-19 months at risk for severe RSV disease can receive it prior to the start of their second RSV season. During RSV season (October 1 to March 31), the initial dose should be administered to newborns just prior to hospital discharge. Older infants and newborns who did not receive it prior to hospital discharge can receive it at their medical home. Newborns should receive it within the first week of life. It is covered by the Vaccine for Children Program. Simultaneous administration with routine childhood immunizations is recommended. Finally, RSV season may vary in tropical areas (Southern Florida, Puerto Rico. etc.) and Alaska. The timing of nirsevimab administration should be based on local RSV activity provided by state and local authorities.
In addition, the FDA approved an RSV vaccine (Abrysvo) for use in adults at least 60 years of age and in pregnant women at 32-36 weeks’ gestation. The latter is administered to prevent lower respiratory tract infection in infants from birth to 6 months. Recommendations have been published for administration in nonpregnant adults. Specific information is forthcoming in terms timing of administration of nirsevimab in infants whose mothers receive Abrysvo.
RSV season is quickly approaching. Detailed recommendations for administration and FAQ questions related to nirsevimab and palivizumab can be found at https://www.aap.org or https://www.cdc.gov/vaccines/hcp/acip-recs/index.html.
Influenza
So, what about influenza? Vaccine composition has been tweaked to match the circulating viruses but the recommended age for annual routine administration remains unchanged. All persons at least 6 months of age should be vaccinated. Children between 6 months and 8 years need two doses at least 4 weeks apart when receiving vaccine for the first time. Immunizing everyone in the household is encouraged especially if there are household contacts at risk for developing severe disease, including infants too young to be vaccinated. Keep in mind children may be coinfected with multiple viruses. Adams and colleagues reviewed the prevalence of coinfection of influenza and Sars-CoV-2 in persons younger than 18 years reported to three CDC surveillance platforms during the 2021-2022 season.2 Thirty-two of 575 hospitalized (6%) coinfections were analyzed and 7 of 44 (16%) deaths. Compared with patients without coinfections, the coinfected patients were more likely to require mechanical ventilation (13% vs. 4%) or CPAP (16% vs. 6%). Only 4 of 23 who were influenza vaccine eligible were vaccinated. Of seven coinfected children who died, none had received influenza vaccine and only one received an antiviral. Only 5 of 31 (16%) infected only with influenza were vaccinated.3
Influenza activity was lower than usual during the 2021-2022 season. However, this report revealed underuse of both influenza vaccine and antiviral therapy, both of which are routinely recommended.
COVID-19
What’s new with COVID-19? On Sept. 12, 2023, ACIP recommended that everyone at least 6 months of age receive the 2023-2024 (monovalent, XBB containing) COVID-19 vaccines. Children at least 5 years of age need one dose and those younger need one or two doses depending on the number of doses previously received. Why the change? Circulating variants continue to change. There is a current uptick in cases including hospitalizations (7.7%) and deaths (4.5%) and it’s just the beginning of the season.4 Symptoms, risk groups and complications have not changed. The primary goal is to prevent infection, hospitalization, long term complications, and death.
We are now armed with the most up-to-date interventions to help prevent the acquisition of these three viruses. Our next step is recommending and delivering them to our patients.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She reported no relevant financial disclosures.
References
1.Suh M et al. J Infect Dis. 2022;226(Suppl 2):S154-36. doi: 10.1093/infdis/jiac120.
2. Adams K et al. MMWR Morb Mortal Wkly Rep. 2022;71:1589-96. doi: http://dx.doi.org/10.15585/mmwr.mm7150a4.
3. Pingali C et al. MMWR Morb Mortal Wkly Rep. 2023 Aug 25;72:912-9. doi: http://dx.doi.org/10.15585/mmwr.mm7234a3.
4. CDC Covid Data Tracker.
New armamentaria available to fight an old disease.
New armamentaria available to fight an old disease.
In July 2023, nirsevimab (Beyfortus), a monoclonal antibody, was approved by the Food and Drug Administration for the prevention of respiratory syncytial virus (RSV) disease in infants and children younger than 2 years of age. On Aug. 3, 2023, the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention recommended routine use of it for all infants younger than 8 months of age born during or entering their first RSV season. Its use is also recommended for certain children 8-19 months of age who are at increased risk for severe RSV disease at the start of their second RSV season. Hearing the approval, I immediately had a flashback to residency, recalling the multiple infants admitted each fall and winter exhibiting classic symptoms including cough, rhinorrhea, nasal flaring, retractions, and wheezing with many having oxygen requirements and others needing intubation. Only supportive care was available.
RSV is the leading cause of infant hospitalizations. Annually, the CDC estimates there are 50,000-80,000 RSV hospitalizations and 100-300 RSV-related deaths in the United States in persons younger than 5 years of age. While premature infants have the highest rates of hospitalization (three times a term infant) about 79% of hospitalized children younger than 2 years have no underlying medical risks.1 The majority of children will experience RSV as an upper respiratory infection within the first 2 years of life. However, severe disease requiring hospitalization is more likely to occur in premature infants and children younger than 6 months; children younger than 2 with congenital heart disease and/or chronic lung disease; children with severe cystic fibrosis; as well as the immunocompromised child and individuals with neuromuscular disorders that preclude clearing mucous secretions or have difficulty swallowing.
Palivizumab (Synagis), the first monoclonal antibody to prevent RSV in infants was licensed in 1998. Its use was limited to infants meeting specific criteria developed by the American Academy of Pediatrics. Only 5% of infants had access to it. It was a short-acting agent requiring monthly injections, which were very costly ($1,661-$2,584 per dose). Eligible infants could receive up to five injections per season. Several studies proved its use was not cost beneficial.
What are the advantages of nirsevimab? It’s a long-acting monoclonal antibody. Only one dose is required per season. Costs will significantly diminish. It is recommended for all infants younger than 8 months of age born during RSV season. Those children 8-19 months at risk for severe RSV disease can receive it prior to the start of their second RSV season. During RSV season (October 1 to March 31), the initial dose should be administered to newborns just prior to hospital discharge. Older infants and newborns who did not receive it prior to hospital discharge can receive it at their medical home. Newborns should receive it within the first week of life. It is covered by the Vaccine for Children Program. Simultaneous administration with routine childhood immunizations is recommended. Finally, RSV season may vary in tropical areas (Southern Florida, Puerto Rico. etc.) and Alaska. The timing of nirsevimab administration should be based on local RSV activity provided by state and local authorities.
In addition, the FDA approved an RSV vaccine (Abrysvo) for use in adults at least 60 years of age and in pregnant women at 32-36 weeks’ gestation. The latter is administered to prevent lower respiratory tract infection in infants from birth to 6 months. Recommendations have been published for administration in nonpregnant adults. Specific information is forthcoming in terms timing of administration of nirsevimab in infants whose mothers receive Abrysvo.
RSV season is quickly approaching. Detailed recommendations for administration and FAQ questions related to nirsevimab and palivizumab can be found at https://www.aap.org or https://www.cdc.gov/vaccines/hcp/acip-recs/index.html.
Influenza
So, what about influenza? Vaccine composition has been tweaked to match the circulating viruses but the recommended age for annual routine administration remains unchanged. All persons at least 6 months of age should be vaccinated. Children between 6 months and 8 years need two doses at least 4 weeks apart when receiving vaccine for the first time. Immunizing everyone in the household is encouraged especially if there are household contacts at risk for developing severe disease, including infants too young to be vaccinated. Keep in mind children may be coinfected with multiple viruses. Adams and colleagues reviewed the prevalence of coinfection of influenza and Sars-CoV-2 in persons younger than 18 years reported to three CDC surveillance platforms during the 2021-2022 season.2 Thirty-two of 575 hospitalized (6%) coinfections were analyzed and 7 of 44 (16%) deaths. Compared with patients without coinfections, the coinfected patients were more likely to require mechanical ventilation (13% vs. 4%) or CPAP (16% vs. 6%). Only 4 of 23 who were influenza vaccine eligible were vaccinated. Of seven coinfected children who died, none had received influenza vaccine and only one received an antiviral. Only 5 of 31 (16%) infected only with influenza were vaccinated.3
Influenza activity was lower than usual during the 2021-2022 season. However, this report revealed underuse of both influenza vaccine and antiviral therapy, both of which are routinely recommended.
COVID-19
What’s new with COVID-19? On Sept. 12, 2023, ACIP recommended that everyone at least 6 months of age receive the 2023-2024 (monovalent, XBB containing) COVID-19 vaccines. Children at least 5 years of age need one dose and those younger need one or two doses depending on the number of doses previously received. Why the change? Circulating variants continue to change. There is a current uptick in cases including hospitalizations (7.7%) and deaths (4.5%) and it’s just the beginning of the season.4 Symptoms, risk groups and complications have not changed. The primary goal is to prevent infection, hospitalization, long term complications, and death.
We are now armed with the most up-to-date interventions to help prevent the acquisition of these three viruses. Our next step is recommending and delivering them to our patients.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She reported no relevant financial disclosures.
References
1.Suh M et al. J Infect Dis. 2022;226(Suppl 2):S154-36. doi: 10.1093/infdis/jiac120.
2. Adams K et al. MMWR Morb Mortal Wkly Rep. 2022;71:1589-96. doi: http://dx.doi.org/10.15585/mmwr.mm7150a4.
3. Pingali C et al. MMWR Morb Mortal Wkly Rep. 2023 Aug 25;72:912-9. doi: http://dx.doi.org/10.15585/mmwr.mm7234a3.
4. CDC Covid Data Tracker.
In July 2023, nirsevimab (Beyfortus), a monoclonal antibody, was approved by the Food and Drug Administration for the prevention of respiratory syncytial virus (RSV) disease in infants and children younger than 2 years of age. On Aug. 3, 2023, the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention recommended routine use of it for all infants younger than 8 months of age born during or entering their first RSV season. Its use is also recommended for certain children 8-19 months of age who are at increased risk for severe RSV disease at the start of their second RSV season. Hearing the approval, I immediately had a flashback to residency, recalling the multiple infants admitted each fall and winter exhibiting classic symptoms including cough, rhinorrhea, nasal flaring, retractions, and wheezing with many having oxygen requirements and others needing intubation. Only supportive care was available.
RSV is the leading cause of infant hospitalizations. Annually, the CDC estimates there are 50,000-80,000 RSV hospitalizations and 100-300 RSV-related deaths in the United States in persons younger than 5 years of age. While premature infants have the highest rates of hospitalization (three times a term infant) about 79% of hospitalized children younger than 2 years have no underlying medical risks.1 The majority of children will experience RSV as an upper respiratory infection within the first 2 years of life. However, severe disease requiring hospitalization is more likely to occur in premature infants and children younger than 6 months; children younger than 2 with congenital heart disease and/or chronic lung disease; children with severe cystic fibrosis; as well as the immunocompromised child and individuals with neuromuscular disorders that preclude clearing mucous secretions or have difficulty swallowing.
Palivizumab (Synagis), the first monoclonal antibody to prevent RSV in infants was licensed in 1998. Its use was limited to infants meeting specific criteria developed by the American Academy of Pediatrics. Only 5% of infants had access to it. It was a short-acting agent requiring monthly injections, which were very costly ($1,661-$2,584 per dose). Eligible infants could receive up to five injections per season. Several studies proved its use was not cost beneficial.
What are the advantages of nirsevimab? It’s a long-acting monoclonal antibody. Only one dose is required per season. Costs will significantly diminish. It is recommended for all infants younger than 8 months of age born during RSV season. Those children 8-19 months at risk for severe RSV disease can receive it prior to the start of their second RSV season. During RSV season (October 1 to March 31), the initial dose should be administered to newborns just prior to hospital discharge. Older infants and newborns who did not receive it prior to hospital discharge can receive it at their medical home. Newborns should receive it within the first week of life. It is covered by the Vaccine for Children Program. Simultaneous administration with routine childhood immunizations is recommended. Finally, RSV season may vary in tropical areas (Southern Florida, Puerto Rico. etc.) and Alaska. The timing of nirsevimab administration should be based on local RSV activity provided by state and local authorities.
In addition, the FDA approved an RSV vaccine (Abrysvo) for use in adults at least 60 years of age and in pregnant women at 32-36 weeks’ gestation. The latter is administered to prevent lower respiratory tract infection in infants from birth to 6 months. Recommendations have been published for administration in nonpregnant adults. Specific information is forthcoming in terms timing of administration of nirsevimab in infants whose mothers receive Abrysvo.
RSV season is quickly approaching. Detailed recommendations for administration and FAQ questions related to nirsevimab and palivizumab can be found at https://www.aap.org or https://www.cdc.gov/vaccines/hcp/acip-recs/index.html.
Influenza
So, what about influenza? Vaccine composition has been tweaked to match the circulating viruses but the recommended age for annual routine administration remains unchanged. All persons at least 6 months of age should be vaccinated. Children between 6 months and 8 years need two doses at least 4 weeks apart when receiving vaccine for the first time. Immunizing everyone in the household is encouraged especially if there are household contacts at risk for developing severe disease, including infants too young to be vaccinated. Keep in mind children may be coinfected with multiple viruses. Adams and colleagues reviewed the prevalence of coinfection of influenza and Sars-CoV-2 in persons younger than 18 years reported to three CDC surveillance platforms during the 2021-2022 season.2 Thirty-two of 575 hospitalized (6%) coinfections were analyzed and 7 of 44 (16%) deaths. Compared with patients without coinfections, the coinfected patients were more likely to require mechanical ventilation (13% vs. 4%) or CPAP (16% vs. 6%). Only 4 of 23 who were influenza vaccine eligible were vaccinated. Of seven coinfected children who died, none had received influenza vaccine and only one received an antiviral. Only 5 of 31 (16%) infected only with influenza were vaccinated.3
Influenza activity was lower than usual during the 2021-2022 season. However, this report revealed underuse of both influenza vaccine and antiviral therapy, both of which are routinely recommended.
COVID-19
What’s new with COVID-19? On Sept. 12, 2023, ACIP recommended that everyone at least 6 months of age receive the 2023-2024 (monovalent, XBB containing) COVID-19 vaccines. Children at least 5 years of age need one dose and those younger need one or two doses depending on the number of doses previously received. Why the change? Circulating variants continue to change. There is a current uptick in cases including hospitalizations (7.7%) and deaths (4.5%) and it’s just the beginning of the season.4 Symptoms, risk groups and complications have not changed. The primary goal is to prevent infection, hospitalization, long term complications, and death.
We are now armed with the most up-to-date interventions to help prevent the acquisition of these three viruses. Our next step is recommending and delivering them to our patients.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She reported no relevant financial disclosures.
References
1.Suh M et al. J Infect Dis. 2022;226(Suppl 2):S154-36. doi: 10.1093/infdis/jiac120.
2. Adams K et al. MMWR Morb Mortal Wkly Rep. 2022;71:1589-96. doi: http://dx.doi.org/10.15585/mmwr.mm7150a4.
3. Pingali C et al. MMWR Morb Mortal Wkly Rep. 2023 Aug 25;72:912-9. doi: http://dx.doi.org/10.15585/mmwr.mm7234a3.
4. CDC Covid Data Tracker.
COVID-19 virus infects coronary vasculature
TOPLINE:
, which could help explain why people with COVID-19 have an increased risk for ischemic cardiovascular complications up to 1 year after infection.
METHODOLOGY:
- Researchers obtained 27 coronary autopsy specimens from eight patients who died from COVID-19, mean age 70 years and 75% male. All had coronary artery disease and most had cardiovascular risk factors such as hypertension, were overweight or obese, and had hyperlipidemia and type 2 diabetes.
- All but one patient, who was pronounced dead before hospital admission, were hospitalized for an average of 17.6 days.
- To identify SARS-CoV-2 viral RNA (vRNA) in the autoptic coronary vasculature, researchers performed RNA fluorescence in situ hybridization (RNA-FISH) analysis for the vRNA encoding the spike (S) protein; they also probed the antisense strand of the S gene (S antisense), which is only produced during viral replication.
TAKEAWAY:
- The study found evidence of SARS-CoV-2 replication in all analyzed human autopsy coronaries regardless of their pathological classification, although viral replication was highest in early-stage lesions that progress to more advanced atherosclerotic plaques.
- Findings indicated that more than 79% of macrophages (white blood cells that help remove lipids) and more than 90% of foam cells (lipid-laden macrophages that are a hallmark of atherosclerosis at all stages of the disease) are S+, and more than 40% of both cell types are S antisense+, indicating SARS-CoV-2 can infect macrophages at a high rate.
- SARS-CoV-2 induced a strong inflammatory response as evidenced by release of cytokines (including interleukin-1 beta and interluekin-6 that are linked to myocardial infarction) in both macrophages and foam cells, which may contribute to the ischemic cardiovascular complications in patients with COVID-19.
IN PRACTICE:
“Our data conclusively demonstrate that SARS-CoV-2 is capable of infecting and replicating in macrophages within the coronary vasculature of patients with COVID-19,” write the authors, adding that SARS-CoV-2 preferentially replicates in foam cells, compared with other macrophages, suggesting these cells “might act as a reservoir of SARS-CoV-2 viral debris in the atherosclerotic plaque.”
SOURCE:
The study was led by Natalia Eberhardt, PhD, postdoctoral fellow, department of medicine, division of cardiology, New York University, and colleagues. It was published online in Nature Cardiovascular Research.
LIMITATIONS:
Findings are relevant only to the original strains of SARS-CoV-2 that circulated in New York between May 2020 and May 2021, and are not generalizable to patients younger and healthier than those from whom samples were obtained for the study.
DISCLOSURES:
The study received support from the National Institutes of Health. The authors report no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
, which could help explain why people with COVID-19 have an increased risk for ischemic cardiovascular complications up to 1 year after infection.
METHODOLOGY:
- Researchers obtained 27 coronary autopsy specimens from eight patients who died from COVID-19, mean age 70 years and 75% male. All had coronary artery disease and most had cardiovascular risk factors such as hypertension, were overweight or obese, and had hyperlipidemia and type 2 diabetes.
- All but one patient, who was pronounced dead before hospital admission, were hospitalized for an average of 17.6 days.
- To identify SARS-CoV-2 viral RNA (vRNA) in the autoptic coronary vasculature, researchers performed RNA fluorescence in situ hybridization (RNA-FISH) analysis for the vRNA encoding the spike (S) protein; they also probed the antisense strand of the S gene (S antisense), which is only produced during viral replication.
TAKEAWAY:
- The study found evidence of SARS-CoV-2 replication in all analyzed human autopsy coronaries regardless of their pathological classification, although viral replication was highest in early-stage lesions that progress to more advanced atherosclerotic plaques.
- Findings indicated that more than 79% of macrophages (white blood cells that help remove lipids) and more than 90% of foam cells (lipid-laden macrophages that are a hallmark of atherosclerosis at all stages of the disease) are S+, and more than 40% of both cell types are S antisense+, indicating SARS-CoV-2 can infect macrophages at a high rate.
- SARS-CoV-2 induced a strong inflammatory response as evidenced by release of cytokines (including interleukin-1 beta and interluekin-6 that are linked to myocardial infarction) in both macrophages and foam cells, which may contribute to the ischemic cardiovascular complications in patients with COVID-19.
IN PRACTICE:
“Our data conclusively demonstrate that SARS-CoV-2 is capable of infecting and replicating in macrophages within the coronary vasculature of patients with COVID-19,” write the authors, adding that SARS-CoV-2 preferentially replicates in foam cells, compared with other macrophages, suggesting these cells “might act as a reservoir of SARS-CoV-2 viral debris in the atherosclerotic plaque.”
SOURCE:
The study was led by Natalia Eberhardt, PhD, postdoctoral fellow, department of medicine, division of cardiology, New York University, and colleagues. It was published online in Nature Cardiovascular Research.
LIMITATIONS:
Findings are relevant only to the original strains of SARS-CoV-2 that circulated in New York between May 2020 and May 2021, and are not generalizable to patients younger and healthier than those from whom samples were obtained for the study.
DISCLOSURES:
The study received support from the National Institutes of Health. The authors report no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
, which could help explain why people with COVID-19 have an increased risk for ischemic cardiovascular complications up to 1 year after infection.
METHODOLOGY:
- Researchers obtained 27 coronary autopsy specimens from eight patients who died from COVID-19, mean age 70 years and 75% male. All had coronary artery disease and most had cardiovascular risk factors such as hypertension, were overweight or obese, and had hyperlipidemia and type 2 diabetes.
- All but one patient, who was pronounced dead before hospital admission, were hospitalized for an average of 17.6 days.
- To identify SARS-CoV-2 viral RNA (vRNA) in the autoptic coronary vasculature, researchers performed RNA fluorescence in situ hybridization (RNA-FISH) analysis for the vRNA encoding the spike (S) protein; they also probed the antisense strand of the S gene (S antisense), which is only produced during viral replication.
TAKEAWAY:
- The study found evidence of SARS-CoV-2 replication in all analyzed human autopsy coronaries regardless of their pathological classification, although viral replication was highest in early-stage lesions that progress to more advanced atherosclerotic plaques.
- Findings indicated that more than 79% of macrophages (white blood cells that help remove lipids) and more than 90% of foam cells (lipid-laden macrophages that are a hallmark of atherosclerosis at all stages of the disease) are S+, and more than 40% of both cell types are S antisense+, indicating SARS-CoV-2 can infect macrophages at a high rate.
- SARS-CoV-2 induced a strong inflammatory response as evidenced by release of cytokines (including interleukin-1 beta and interluekin-6 that are linked to myocardial infarction) in both macrophages and foam cells, which may contribute to the ischemic cardiovascular complications in patients with COVID-19.
IN PRACTICE:
“Our data conclusively demonstrate that SARS-CoV-2 is capable of infecting and replicating in macrophages within the coronary vasculature of patients with COVID-19,” write the authors, adding that SARS-CoV-2 preferentially replicates in foam cells, compared with other macrophages, suggesting these cells “might act as a reservoir of SARS-CoV-2 viral debris in the atherosclerotic plaque.”
SOURCE:
The study was led by Natalia Eberhardt, PhD, postdoctoral fellow, department of medicine, division of cardiology, New York University, and colleagues. It was published online in Nature Cardiovascular Research.
LIMITATIONS:
Findings are relevant only to the original strains of SARS-CoV-2 that circulated in New York between May 2020 and May 2021, and are not generalizable to patients younger and healthier than those from whom samples were obtained for the study.
DISCLOSURES:
The study received support from the National Institutes of Health. The authors report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Study: Unexpected vaginal bleeding rises after COVID vaccination
The researchers suggested it could have been connected to the SARS-CoV-2 spike protein in the vaccines. The study was published in Science Advances.
After vaccinations became widely available, many women reported heavier menstrual bleeding than normal. Researchers at the Norwegian Institute of Public Health in Oslo examined the data, particularly among women who do not have periods, such as those who have been through menopause or are taking contraceptives.
The researchers used an ongoing population health survey called the Norwegian Mother, Father, and Child Cohort Study, Nature reported. They examined more than 21,000 responses from postmenopausal, perimenopausal, and nonmenstruating premenopausal women. Some were on long-term hormonal contraceptives.
They learned that 252 postmenopausal women, 1,008 perimenopausal women, and 924 premenopausal women reported having unexpected vaginal bleeding.
About half said the bleeding occurred within 4 weeks of the first or second shot or both. The risk of bleeding was up three to five times for premenopausal and perimenopausal women, and two to three times for postmenopausal women, the researchers found.
Postmenopausal bleeding is usually serious and can be a sign of cancer. “Knowing a patient’s vaccination status could put their bleeding incidence into context,” said Kate Clancy, a biological anthropologist at the University of Illinois at Urbana-Champaign.
The study received funding through the Norwegian Institute of Public Health and Research Council of Norway. The researchers reported no conflicts of interest.
A version of this article first appeared on WebMD.com.
The researchers suggested it could have been connected to the SARS-CoV-2 spike protein in the vaccines. The study was published in Science Advances.
After vaccinations became widely available, many women reported heavier menstrual bleeding than normal. Researchers at the Norwegian Institute of Public Health in Oslo examined the data, particularly among women who do not have periods, such as those who have been through menopause or are taking contraceptives.
The researchers used an ongoing population health survey called the Norwegian Mother, Father, and Child Cohort Study, Nature reported. They examined more than 21,000 responses from postmenopausal, perimenopausal, and nonmenstruating premenopausal women. Some were on long-term hormonal contraceptives.
They learned that 252 postmenopausal women, 1,008 perimenopausal women, and 924 premenopausal women reported having unexpected vaginal bleeding.
About half said the bleeding occurred within 4 weeks of the first or second shot or both. The risk of bleeding was up three to five times for premenopausal and perimenopausal women, and two to three times for postmenopausal women, the researchers found.
Postmenopausal bleeding is usually serious and can be a sign of cancer. “Knowing a patient’s vaccination status could put their bleeding incidence into context,” said Kate Clancy, a biological anthropologist at the University of Illinois at Urbana-Champaign.
The study received funding through the Norwegian Institute of Public Health and Research Council of Norway. The researchers reported no conflicts of interest.
A version of this article first appeared on WebMD.com.
The researchers suggested it could have been connected to the SARS-CoV-2 spike protein in the vaccines. The study was published in Science Advances.
After vaccinations became widely available, many women reported heavier menstrual bleeding than normal. Researchers at the Norwegian Institute of Public Health in Oslo examined the data, particularly among women who do not have periods, such as those who have been through menopause or are taking contraceptives.
The researchers used an ongoing population health survey called the Norwegian Mother, Father, and Child Cohort Study, Nature reported. They examined more than 21,000 responses from postmenopausal, perimenopausal, and nonmenstruating premenopausal women. Some were on long-term hormonal contraceptives.
They learned that 252 postmenopausal women, 1,008 perimenopausal women, and 924 premenopausal women reported having unexpected vaginal bleeding.
About half said the bleeding occurred within 4 weeks of the first or second shot or both. The risk of bleeding was up three to five times for premenopausal and perimenopausal women, and two to three times for postmenopausal women, the researchers found.
Postmenopausal bleeding is usually serious and can be a sign of cancer. “Knowing a patient’s vaccination status could put their bleeding incidence into context,” said Kate Clancy, a biological anthropologist at the University of Illinois at Urbana-Champaign.
The study received funding through the Norwegian Institute of Public Health and Research Council of Norway. The researchers reported no conflicts of interest.
A version of this article first appeared on WebMD.com.
FROM SCIENCE ADVANCES
People with long COVID have specific blood biomarkers, study says
The findings may be a step toward creating blood tests to positively identify people with long COVID so specialized treatments can be employed, researchers said.
“This is a decisive step forward in the development of valid and reliable blood testing protocols for long COVID,” said David Putrino, PhD., lead author and professor of rehabilitation and human performance and director of the Abilities Research Center at Icahn Mount Sinai Health System, New York.
Researchers from the Icahn School of Medicine at Mount Sinai and Yale School of Medicine looked at blood samples from about 270 people between January 2021 and June 2022. The people had never been infected with COVID, had fully recovered from an infection, or still showed symptoms at least four months after infection.
Using machine learning, the research teams were able to differentiate between people with and without long COVID with 96% accuracy based on distinctive features in the blood samples, according to a news release from Mount Sinai.
People with long COVID had abnormal T-cell activity and low levels of the hormone cortisol. Cortisol helps people feel alert and awake, which would explain why people with long COVID often report fatigue, NBC News said in a report on the study.
“It was one of the findings that most definitively separated the folks with long Covid from the people without long Covid,” Dr. Putrino told NBC News.
The study also found that long COVID appears to reactivate latent viruses including Epstein-Barr and mononucleosis, the study said.
The blood tests could allow doctors to come up with specialized treatments in people who report a wide variety of long COVID symptoms, Dr. Putrino said.
“There is no ‘silver bullet’ for treating long COVID, because it is an illness that infiltrates complex systems such as the immune and hormonal regulation,” he said.
The Centers for Disease Control and Prevention says about one in five Americans who had COVID still have long COVID. Symptoms include fatigue, brain fog, dizziness, digestive problems, and loss of smell and taste.
A version of this article appeared on WebMD.com.
The findings may be a step toward creating blood tests to positively identify people with long COVID so specialized treatments can be employed, researchers said.
“This is a decisive step forward in the development of valid and reliable blood testing protocols for long COVID,” said David Putrino, PhD., lead author and professor of rehabilitation and human performance and director of the Abilities Research Center at Icahn Mount Sinai Health System, New York.
Researchers from the Icahn School of Medicine at Mount Sinai and Yale School of Medicine looked at blood samples from about 270 people between January 2021 and June 2022. The people had never been infected with COVID, had fully recovered from an infection, or still showed symptoms at least four months after infection.
Using machine learning, the research teams were able to differentiate between people with and without long COVID with 96% accuracy based on distinctive features in the blood samples, according to a news release from Mount Sinai.
People with long COVID had abnormal T-cell activity and low levels of the hormone cortisol. Cortisol helps people feel alert and awake, which would explain why people with long COVID often report fatigue, NBC News said in a report on the study.
“It was one of the findings that most definitively separated the folks with long Covid from the people without long Covid,” Dr. Putrino told NBC News.
The study also found that long COVID appears to reactivate latent viruses including Epstein-Barr and mononucleosis, the study said.
The blood tests could allow doctors to come up with specialized treatments in people who report a wide variety of long COVID symptoms, Dr. Putrino said.
“There is no ‘silver bullet’ for treating long COVID, because it is an illness that infiltrates complex systems such as the immune and hormonal regulation,” he said.
The Centers for Disease Control and Prevention says about one in five Americans who had COVID still have long COVID. Symptoms include fatigue, brain fog, dizziness, digestive problems, and loss of smell and taste.
A version of this article appeared on WebMD.com.
The findings may be a step toward creating blood tests to positively identify people with long COVID so specialized treatments can be employed, researchers said.
“This is a decisive step forward in the development of valid and reliable blood testing protocols for long COVID,” said David Putrino, PhD., lead author and professor of rehabilitation and human performance and director of the Abilities Research Center at Icahn Mount Sinai Health System, New York.
Researchers from the Icahn School of Medicine at Mount Sinai and Yale School of Medicine looked at blood samples from about 270 people between January 2021 and June 2022. The people had never been infected with COVID, had fully recovered from an infection, or still showed symptoms at least four months after infection.
Using machine learning, the research teams were able to differentiate between people with and without long COVID with 96% accuracy based on distinctive features in the blood samples, according to a news release from Mount Sinai.
People with long COVID had abnormal T-cell activity and low levels of the hormone cortisol. Cortisol helps people feel alert and awake, which would explain why people with long COVID often report fatigue, NBC News said in a report on the study.
“It was one of the findings that most definitively separated the folks with long Covid from the people without long Covid,” Dr. Putrino told NBC News.
The study also found that long COVID appears to reactivate latent viruses including Epstein-Barr and mononucleosis, the study said.
The blood tests could allow doctors to come up with specialized treatments in people who report a wide variety of long COVID symptoms, Dr. Putrino said.
“There is no ‘silver bullet’ for treating long COVID, because it is an illness that infiltrates complex systems such as the immune and hormonal regulation,” he said.
The Centers for Disease Control and Prevention says about one in five Americans who had COVID still have long COVID. Symptoms include fatigue, brain fog, dizziness, digestive problems, and loss of smell and taste.
A version of this article appeared on WebMD.com.
Study: Antiviral med linked to COVID mutations that can spread
published in the online journal Nature.
There’s no evidence that molnupiravir, sold under the brand name Lagevrio, has caused the creation of more transmissible or severe variants of COVID, the study says, but researchers called for more scrutiny of the drug.
Researchers looked at 15 million COVID genomes and discovered that hallmark mutations linked to molnupiravir increased in 2022, especially in places where the drug was widely used, such as the United States and the United Kingdom. Levels of the mutations were also found in populations where the drug was heavily prescribed, such as seniors.
Molnupiravir is an antiviral given to people after they show signs of having COVID-19. It interferes with the COVID-19 virus’s ability to make copies of itself, thus stopping the spread of the virus throughout the body and keeping the virus level low.
The study found the virus can sometimes survive molnupiravir, resulting in mutations that have spread to other people.
Theo Sanderson, PhD, the lead author on the study and a postdoctoral researcher at the Francis Crick Institute in London, told The Guardian that the implications of the mutations were unclear.
“The signature is very clear, but there aren’t any widely circulating variants that have the signature. At the moment there’s nothing that’s transmitted very widely that’s due to molnupiravir,” he said.
The study doesn’t say people should not use molnupiravir but calls for public health officials to scrutinize it.
“The observation that molnupiravir treatment has left a visible trace in global sequencing databases, including onwards transmission of molnupiravir-derived sequences, will be an important consideration for assessing the effects and evolutionary safety of this drug,” the researchers concluded.
When reached for comment, Merck questioned the evidence.
“The authors assume these mutations were associated with viral spread from molnupiravir-treated patients without documented evidence of that transmission. Instead, the authors rely on circumstantial associations between the region from which the sequence was identified and time frame of sequence collection in countries where molnupiravir is available to draw their conclusions,” the company said.
The Food and Drug Administration authorized the use of molnupiravir for the treatment of mild to moderate COVID-19 in adults in December 2021. The FDA has also authorized the use of nirmatrelvir/ritonavir (Paxlovid), an antiviral made by Pfizer.
A version of this article appeared on WebMD.com.
published in the online journal Nature.
There’s no evidence that molnupiravir, sold under the brand name Lagevrio, has caused the creation of more transmissible or severe variants of COVID, the study says, but researchers called for more scrutiny of the drug.
Researchers looked at 15 million COVID genomes and discovered that hallmark mutations linked to molnupiravir increased in 2022, especially in places where the drug was widely used, such as the United States and the United Kingdom. Levels of the mutations were also found in populations where the drug was heavily prescribed, such as seniors.
Molnupiravir is an antiviral given to people after they show signs of having COVID-19. It interferes with the COVID-19 virus’s ability to make copies of itself, thus stopping the spread of the virus throughout the body and keeping the virus level low.
The study found the virus can sometimes survive molnupiravir, resulting in mutations that have spread to other people.
Theo Sanderson, PhD, the lead author on the study and a postdoctoral researcher at the Francis Crick Institute in London, told The Guardian that the implications of the mutations were unclear.
“The signature is very clear, but there aren’t any widely circulating variants that have the signature. At the moment there’s nothing that’s transmitted very widely that’s due to molnupiravir,” he said.
The study doesn’t say people should not use molnupiravir but calls for public health officials to scrutinize it.
“The observation that molnupiravir treatment has left a visible trace in global sequencing databases, including onwards transmission of molnupiravir-derived sequences, will be an important consideration for assessing the effects and evolutionary safety of this drug,” the researchers concluded.
When reached for comment, Merck questioned the evidence.
“The authors assume these mutations were associated with viral spread from molnupiravir-treated patients without documented evidence of that transmission. Instead, the authors rely on circumstantial associations between the region from which the sequence was identified and time frame of sequence collection in countries where molnupiravir is available to draw their conclusions,” the company said.
The Food and Drug Administration authorized the use of molnupiravir for the treatment of mild to moderate COVID-19 in adults in December 2021. The FDA has also authorized the use of nirmatrelvir/ritonavir (Paxlovid), an antiviral made by Pfizer.
A version of this article appeared on WebMD.com.
published in the online journal Nature.
There’s no evidence that molnupiravir, sold under the brand name Lagevrio, has caused the creation of more transmissible or severe variants of COVID, the study says, but researchers called for more scrutiny of the drug.
Researchers looked at 15 million COVID genomes and discovered that hallmark mutations linked to molnupiravir increased in 2022, especially in places where the drug was widely used, such as the United States and the United Kingdom. Levels of the mutations were also found in populations where the drug was heavily prescribed, such as seniors.
Molnupiravir is an antiviral given to people after they show signs of having COVID-19. It interferes with the COVID-19 virus’s ability to make copies of itself, thus stopping the spread of the virus throughout the body and keeping the virus level low.
The study found the virus can sometimes survive molnupiravir, resulting in mutations that have spread to other people.
Theo Sanderson, PhD, the lead author on the study and a postdoctoral researcher at the Francis Crick Institute in London, told The Guardian that the implications of the mutations were unclear.
“The signature is very clear, but there aren’t any widely circulating variants that have the signature. At the moment there’s nothing that’s transmitted very widely that’s due to molnupiravir,” he said.
The study doesn’t say people should not use molnupiravir but calls for public health officials to scrutinize it.
“The observation that molnupiravir treatment has left a visible trace in global sequencing databases, including onwards transmission of molnupiravir-derived sequences, will be an important consideration for assessing the effects and evolutionary safety of this drug,” the researchers concluded.
When reached for comment, Merck questioned the evidence.
“The authors assume these mutations were associated with viral spread from molnupiravir-treated patients without documented evidence of that transmission. Instead, the authors rely on circumstantial associations between the region from which the sequence was identified and time frame of sequence collection in countries where molnupiravir is available to draw their conclusions,” the company said.
The Food and Drug Administration authorized the use of molnupiravir for the treatment of mild to moderate COVID-19 in adults in December 2021. The FDA has also authorized the use of nirmatrelvir/ritonavir (Paxlovid), an antiviral made by Pfizer.
A version of this article appeared on WebMD.com.
FROM NATURE
Long COVID and the Gastrointestinal System: Emerging Evidence
- Lutchmansingh DD et al. Semin Respir Crit Care Med. 2023;44(1):130-142. doi:10.1055/s-0042-1759568
- Choudhury A et al. Therap Adv Gastroenterol. 2022;15:17562848221118403. doi:10.1177/17562848221118403
- Xu E et al. Nat Commun. 2023;14(1):983. doi:10.1038/s41467-023-36223-7
- Freedberg DE, Chang L. Curr Opin Gastroenterol. 2022;38(6):555-561. doi:10.1097/MOG.0000000000000876
- Blackett JW et al. Gastroenterology. 2022;162(2):648-650.e2. doi:10.1053/j.gastro.2021.10.040
- Chey WD et al. Gastroenterology. 2021;160(1):47-62. doi:10.1053/j.gastro.2020.06.099
- Líška D et al. Front Public Health. 2022;10:975992. doi:10.3389/fpubh.2022.975992
- Moens M et al. Front Public Health. 2022;10:991572. doi:10.3389/fpubh.2022.991572
- Cutler DM. The economic cost of long COVID: an update. Scholars at Harvard. Published July 2022. Accessed July 20, 2023. https://scholar.harvard.edu/sites/scholar.harvard.edu/files/cutler/files/long_covid_update_7-22.pdf
- National Center for Education Statistics (2023). Public School Expenditures. Condition of Education. US Department of Education, Institute of Education Sciences. Accessed August 4, 2023. https://nces.ed.gov/programs/coe/indicator/cmb
- Lutchmansingh DD et al. Semin Respir Crit Care Med. 2023;44(1):130-142. doi:10.1055/s-0042-1759568
- Choudhury A et al. Therap Adv Gastroenterol. 2022;15:17562848221118403. doi:10.1177/17562848221118403
- Xu E et al. Nat Commun. 2023;14(1):983. doi:10.1038/s41467-023-36223-7
- Freedberg DE, Chang L. Curr Opin Gastroenterol. 2022;38(6):555-561. doi:10.1097/MOG.0000000000000876
- Blackett JW et al. Gastroenterology. 2022;162(2):648-650.e2. doi:10.1053/j.gastro.2021.10.040
- Chey WD et al. Gastroenterology. 2021;160(1):47-62. doi:10.1053/j.gastro.2020.06.099
- Líška D et al. Front Public Health. 2022;10:975992. doi:10.3389/fpubh.2022.975992
- Moens M et al. Front Public Health. 2022;10:991572. doi:10.3389/fpubh.2022.991572
- Cutler DM. The economic cost of long COVID: an update. Scholars at Harvard. Published July 2022. Accessed July 20, 2023. https://scholar.harvard.edu/sites/scholar.harvard.edu/files/cutler/files/long_covid_update_7-22.pdf
- National Center for Education Statistics (2023). Public School Expenditures. Condition of Education. US Department of Education, Institute of Education Sciences. Accessed August 4, 2023. https://nces.ed.gov/programs/coe/indicator/cmb
- Lutchmansingh DD et al. Semin Respir Crit Care Med. 2023;44(1):130-142. doi:10.1055/s-0042-1759568
- Choudhury A et al. Therap Adv Gastroenterol. 2022;15:17562848221118403. doi:10.1177/17562848221118403
- Xu E et al. Nat Commun. 2023;14(1):983. doi:10.1038/s41467-023-36223-7
- Freedberg DE, Chang L. Curr Opin Gastroenterol. 2022;38(6):555-561. doi:10.1097/MOG.0000000000000876
- Blackett JW et al. Gastroenterology. 2022;162(2):648-650.e2. doi:10.1053/j.gastro.2021.10.040
- Chey WD et al. Gastroenterology. 2021;160(1):47-62. doi:10.1053/j.gastro.2020.06.099
- Líška D et al. Front Public Health. 2022;10:975992. doi:10.3389/fpubh.2022.975992
- Moens M et al. Front Public Health. 2022;10:991572. doi:10.3389/fpubh.2022.991572
- Cutler DM. The economic cost of long COVID: an update. Scholars at Harvard. Published July 2022. Accessed July 20, 2023. https://scholar.harvard.edu/sites/scholar.harvard.edu/files/cutler/files/long_covid_update_7-22.pdf
- National Center for Education Statistics (2023). Public School Expenditures. Condition of Education. US Department of Education, Institute of Education Sciences. Accessed August 4, 2023. https://nces.ed.gov/programs/coe/indicator/cmb