User login
COVID Levels Decline, but Other Viruses Remain High
COVID-19 may be headed toward a springtime retreat.
The indication comes from declining levels of SARS-CoV-2 being detected in wastewater over the past 3 weeks. Virus levels are already considered “low” throughout western U.S. states. Detections are at medium levels in the Midwest and South, while high levels persist in the Northeast, according to WastewaterSCAN.
But it’s not time to let your guard down because high levels of other viruses that cause stomach and respiratory illnesses continue to circulate widely nationwide. Wastewater data currently shows threats from flu, RSV, norovirus, and rotavirus.
The rate of positive flu tests reported to the CDC had been a downward trend since peaking around a rate of 16% in mid-January, but positive test rates are now climbing again, with the most recent weekly rate back around 15%. So far this flu season, 116 children and an estimated 20,000 adults have died from the flu, according to the CDC’s weekly flu publication, FluView.
RSV wastewater detection remains high, especially in the Midwest and Northeast, WastewaterSCAN data shows. But positive RSV test results reported to the CDC are at the lowest point of the 2023 to 2024 season, with less than 2,000 positive results listed for the week of March 9, down from a peak of more than 14,000 cases around Christmas.
About 12% of norovirus tests reported to the CDC in the last 3 weeks of February were positive, mirroring an upward trend observed during the same time period last year. In 2023, norovirus peaked in the U.S. in March with a positive test rate around 16%, CDC data show.
Last year, COVID also followed a downward springtime trend. Around this time last year, there were about 20,000 weekly hospital admissions due to COVID-19, compared to just over 13,000 in early March this year. All COVID metrics, including the positive test rate, hospitalizations, and ER visits, are currently trending downward, the CDC’s COVID Data Tracker indicates. The positive COVID test rate is 5%, and just 1% of ER visits in the U.S. involve a COVID-19 diagnosis.
“We’re seeing a downward trend, which is fantastic,” Marlene Wolfe, PhD, WastewaterSCAN’s program director, told USA Today. “Hopefully, that pattern continues as we enjoy some warmer weather and longer daylight.”
A version of this article appeared on WebMD.com.
COVID-19 may be headed toward a springtime retreat.
The indication comes from declining levels of SARS-CoV-2 being detected in wastewater over the past 3 weeks. Virus levels are already considered “low” throughout western U.S. states. Detections are at medium levels in the Midwest and South, while high levels persist in the Northeast, according to WastewaterSCAN.
But it’s not time to let your guard down because high levels of other viruses that cause stomach and respiratory illnesses continue to circulate widely nationwide. Wastewater data currently shows threats from flu, RSV, norovirus, and rotavirus.
The rate of positive flu tests reported to the CDC had been a downward trend since peaking around a rate of 16% in mid-January, but positive test rates are now climbing again, with the most recent weekly rate back around 15%. So far this flu season, 116 children and an estimated 20,000 adults have died from the flu, according to the CDC’s weekly flu publication, FluView.
RSV wastewater detection remains high, especially in the Midwest and Northeast, WastewaterSCAN data shows. But positive RSV test results reported to the CDC are at the lowest point of the 2023 to 2024 season, with less than 2,000 positive results listed for the week of March 9, down from a peak of more than 14,000 cases around Christmas.
About 12% of norovirus tests reported to the CDC in the last 3 weeks of February were positive, mirroring an upward trend observed during the same time period last year. In 2023, norovirus peaked in the U.S. in March with a positive test rate around 16%, CDC data show.
Last year, COVID also followed a downward springtime trend. Around this time last year, there were about 20,000 weekly hospital admissions due to COVID-19, compared to just over 13,000 in early March this year. All COVID metrics, including the positive test rate, hospitalizations, and ER visits, are currently trending downward, the CDC’s COVID Data Tracker indicates. The positive COVID test rate is 5%, and just 1% of ER visits in the U.S. involve a COVID-19 diagnosis.
“We’re seeing a downward trend, which is fantastic,” Marlene Wolfe, PhD, WastewaterSCAN’s program director, told USA Today. “Hopefully, that pattern continues as we enjoy some warmer weather and longer daylight.”
A version of this article appeared on WebMD.com.
COVID-19 may be headed toward a springtime retreat.
The indication comes from declining levels of SARS-CoV-2 being detected in wastewater over the past 3 weeks. Virus levels are already considered “low” throughout western U.S. states. Detections are at medium levels in the Midwest and South, while high levels persist in the Northeast, according to WastewaterSCAN.
But it’s not time to let your guard down because high levels of other viruses that cause stomach and respiratory illnesses continue to circulate widely nationwide. Wastewater data currently shows threats from flu, RSV, norovirus, and rotavirus.
The rate of positive flu tests reported to the CDC had been a downward trend since peaking around a rate of 16% in mid-January, but positive test rates are now climbing again, with the most recent weekly rate back around 15%. So far this flu season, 116 children and an estimated 20,000 adults have died from the flu, according to the CDC’s weekly flu publication, FluView.
RSV wastewater detection remains high, especially in the Midwest and Northeast, WastewaterSCAN data shows. But positive RSV test results reported to the CDC are at the lowest point of the 2023 to 2024 season, with less than 2,000 positive results listed for the week of March 9, down from a peak of more than 14,000 cases around Christmas.
About 12% of norovirus tests reported to the CDC in the last 3 weeks of February were positive, mirroring an upward trend observed during the same time period last year. In 2023, norovirus peaked in the U.S. in March with a positive test rate around 16%, CDC data show.
Last year, COVID also followed a downward springtime trend. Around this time last year, there were about 20,000 weekly hospital admissions due to COVID-19, compared to just over 13,000 in early March this year. All COVID metrics, including the positive test rate, hospitalizations, and ER visits, are currently trending downward, the CDC’s COVID Data Tracker indicates. The positive COVID test rate is 5%, and just 1% of ER visits in the U.S. involve a COVID-19 diagnosis.
“We’re seeing a downward trend, which is fantastic,” Marlene Wolfe, PhD, WastewaterSCAN’s program director, told USA Today. “Hopefully, that pattern continues as we enjoy some warmer weather and longer daylight.”
A version of this article appeared on WebMD.com.
Immunomodulators Do Not Affect COVID-19 Vaccine Efficacy
TOPLINE:
The results of a recent study suggest that biologics and small molecule inhibitors (SMIs) do not impair the protective effect of COVID-19 vaccine against hospitalization in patients with psoriasis and hidradenitis suppurativa (HS).
METHODOLOGY:
- It remains unknown whether immunomodulatory therapies impair COVID-19 vaccine efficacy and increase hospitalization rates linked to COVID-19 in patients with inflammatory skin conditions such as psoriasis or HS.
- Researchers conducted a cross-sectional study using data from the Epic Cosmos database from January 2020 to October 2023, identifying 30,845 patients with psoriasis or HS.
- Overall, 22,293 patients with documented completion of their primary COVID-19 vaccine series were included in the analysis.
- Of the vaccinated patients, they compared 7046 patients with psoriasis on SMIs and 2033 with psoriasis or HS on biologics with 13,214 patients who did not receive biologics or SMIs.
- The primary outcome was the COVID-19 hospitalization rate.
- Treatment with biologics did not increase COVID-19-related hospitalization rates in vaccinated patients with psoriasis or HS (hospitalization rate, 6.0% for both those taking and those not taking a biologic; P > .99).
- Similarly, hospitalization rates did not significantly differ between vaccinated patients who received SMIs vs those who did not (7.1% vs 6.0%; P = .0596).
IN PRACTICE:
These findings “encourage dermatologists to continue treating [psoriasis]/HS confidently despite the ongoing COVID-19 pandemic,” the authors concluded.
SOURCE:
The study led by Bella R. Lee from Ohio State University Wexner Medical Center, Columbus, was published online on March 13, 2024, in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Multivariable adjustments could not be performed in this study due to unavailability of individual-level data, and hospital admissions that occurred outside the Epic system were not captured.
DISCLOSURES:
The study did not receive any funding. All authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
The results of a recent study suggest that biologics and small molecule inhibitors (SMIs) do not impair the protective effect of COVID-19 vaccine against hospitalization in patients with psoriasis and hidradenitis suppurativa (HS).
METHODOLOGY:
- It remains unknown whether immunomodulatory therapies impair COVID-19 vaccine efficacy and increase hospitalization rates linked to COVID-19 in patients with inflammatory skin conditions such as psoriasis or HS.
- Researchers conducted a cross-sectional study using data from the Epic Cosmos database from January 2020 to October 2023, identifying 30,845 patients with psoriasis or HS.
- Overall, 22,293 patients with documented completion of their primary COVID-19 vaccine series were included in the analysis.
- Of the vaccinated patients, they compared 7046 patients with psoriasis on SMIs and 2033 with psoriasis or HS on biologics with 13,214 patients who did not receive biologics or SMIs.
- The primary outcome was the COVID-19 hospitalization rate.
- Treatment with biologics did not increase COVID-19-related hospitalization rates in vaccinated patients with psoriasis or HS (hospitalization rate, 6.0% for both those taking and those not taking a biologic; P > .99).
- Similarly, hospitalization rates did not significantly differ between vaccinated patients who received SMIs vs those who did not (7.1% vs 6.0%; P = .0596).
IN PRACTICE:
These findings “encourage dermatologists to continue treating [psoriasis]/HS confidently despite the ongoing COVID-19 pandemic,” the authors concluded.
SOURCE:
The study led by Bella R. Lee from Ohio State University Wexner Medical Center, Columbus, was published online on March 13, 2024, in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Multivariable adjustments could not be performed in this study due to unavailability of individual-level data, and hospital admissions that occurred outside the Epic system were not captured.
DISCLOSURES:
The study did not receive any funding. All authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
The results of a recent study suggest that biologics and small molecule inhibitors (SMIs) do not impair the protective effect of COVID-19 vaccine against hospitalization in patients with psoriasis and hidradenitis suppurativa (HS).
METHODOLOGY:
- It remains unknown whether immunomodulatory therapies impair COVID-19 vaccine efficacy and increase hospitalization rates linked to COVID-19 in patients with inflammatory skin conditions such as psoriasis or HS.
- Researchers conducted a cross-sectional study using data from the Epic Cosmos database from January 2020 to October 2023, identifying 30,845 patients with psoriasis or HS.
- Overall, 22,293 patients with documented completion of their primary COVID-19 vaccine series were included in the analysis.
- Of the vaccinated patients, they compared 7046 patients with psoriasis on SMIs and 2033 with psoriasis or HS on biologics with 13,214 patients who did not receive biologics or SMIs.
- The primary outcome was the COVID-19 hospitalization rate.
- Treatment with biologics did not increase COVID-19-related hospitalization rates in vaccinated patients with psoriasis or HS (hospitalization rate, 6.0% for both those taking and those not taking a biologic; P > .99).
- Similarly, hospitalization rates did not significantly differ between vaccinated patients who received SMIs vs those who did not (7.1% vs 6.0%; P = .0596).
IN PRACTICE:
These findings “encourage dermatologists to continue treating [psoriasis]/HS confidently despite the ongoing COVID-19 pandemic,” the authors concluded.
SOURCE:
The study led by Bella R. Lee from Ohio State University Wexner Medical Center, Columbus, was published online on March 13, 2024, in the Journal of the American Academy of Dermatology.
LIMITATIONS:
Multivariable adjustments could not be performed in this study due to unavailability of individual-level data, and hospital admissions that occurred outside the Epic system were not captured.
DISCLOSURES:
The study did not receive any funding. All authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
Hormones and Viruses Influence Each Other: Consider These Connections in Your Patients
Stefan Bornstein, MD, PhD, professor, made it clear during a press conference at the 67th Congress of the German Society of Endocrinology (DGE) that there is more than one interaction between them. Nowadays, one can almost speak of an “endocrine virology and even of the virome as an additional, hormonally metabolically active gland,” said Dr. Bornstein, who will receive the Berthold Medal from the DGE in 2024.
Many questions remain unanswered: “We need a better understanding of the interaction of hormone systems with infectious agents — from basics to therapeutic applications,” emphasized the director of the Medical Clinic and Polyclinic III and the Center for Internal Medicine at the Carl Gustav Carus University Hospital, Dresden, Germany.
If infectious diseases could trigger diabetes and other metabolic diseases, this means that “through vaccination programs, we may be able to prevent the occurrence of common metabolic diseases such as diabetes,” said Dr. Bornstein. He highlighted that many people who experienced severe COVID-19 during the pandemic, or died from it, exhibited diabetes or a pre-metabolic syndrome.
“SARS-CoV-2 has utilized an endocrine signaling pathway to invade our cells and cause damage in the organ systems and inflammation,” said Dr. Bornstein. Conversely, it is now known that infections with coronaviruses or other infectious agents like influenza can significantly worsen metabolic status, diabetes, and other endocrine diseases.
SARS-CoV-2 Infects the Beta Cells
Data from the COVID-19 pandemic showed that the likelihood of developing type 1 diabetes significantly increases with a SARS-CoV-2 infection. Researchers led by Dr. Bornstein demonstrated in 2021 that SARS-CoV-2 can infect the insulin-producing cells of the organ. They examined pancreatic tissue from 20 patients who died from COVID-19 using immunofluorescence, immunohistochemistry, RNA in situ hybridization, and electron microscopy.
They found viral SARS-CoV-2 infiltration of the beta cells in all patients. In 11 patients with COVID-19, the expression of ACE2, TMPRSS, and other receptors and factors like DPP4, HMBG1, and NRP1 that can facilitate virus entry was examined. They found that even in the absence of manifest newly onset diabetes, necroptotic cell death, immune cell infiltration, and SARS-CoV-2 infection of the pancreas beta cells can contribute to varying degrees of metabolic disturbance in patients with COVID-19.
In a report published in October 2020, Tim Hollstein, MD, from the Institute for Diabetology and Clinical Metabolic Research at UKSH in Kiel, Germany, and colleagues described the case of a 19-year-old man who developed symptoms of insulin-dependent diabetes after a SARS-CoV-2 infection, without the presence of autoantibodies typical for type 1 diabetes.
The man presented to the emergency department with diabetic ketoacidosis, a C-peptide level of 0.62 µg/L, a blood glucose concentration of 30.6 mmol/L (552 mg/dL), and an A1c level of 16.8%. The patient’s history revealed a probable SARS-CoV-2 infection 5-7 weeks before admission, based on a positive antibody test against SARS-CoV-2.
Some Viruses Produce Insulin-Like Proteins
Recent studies have shown that some viruses can produce insulin-like proteins or hormones that interfere with the metabolism of the affected organism, reported Dr. Bornstein. In addition to metabolic regulation, these “viral hormones” also seem to influence cell turnover and cell death.
Dr. Bornstein pointed out that antiviral medications can delay the onset of type 1 diabetes by preserving the function of insulin-producing beta cells. It has also been shown that conventional medications used to treat hormonal disorders can reduce the susceptibility of the organism to infections — such as antidiabetic preparations like DPP-4 inhibitors or metformin.
In a review published in 2023, Nikolaos Perakakis, MD, professor, research group leader at the Paul Langerhans Institute Dresden, Dresden, Germany, Dr. Bornstein, and colleagues discussed scientific evidence for a close mutual dependence between various virus infections and metabolic diseases. They discussed how viruses can lead to the development or progression of metabolic diseases and vice versa and how metabolic diseases can increase the severity of a virus infection.
Viruses Favor Metabolic Diseases...
Viruses can favor metabolic diseases by, for example, influencing the regulation of cell survival and specific signaling pathways relevant for cell death, proliferation, or dedifferentiation in important endocrine and metabolic organs. Viruses are also capable of controlling cellular glucose metabolism by modulating glucose transporters, altering glucose uptake, regulating signaling pathways, and stimulating glycolysis in infected cells.
Due to the destruction of beta cells, enteroviruses, but also the mumps virus, parainfluenza virus, or human herpes virus 6, are associated with the development of diabetes. The timing of infection often precedes or coincides with the peak of development of islet autoantibodies. The fact that only a small proportion of patients actually develop type 1 diabetes suggests that genetic background, and likely the timing of infection, play an important role.
...And Metabolic Diseases Influence the Course of Infection
Infection with hepatitis C virus (HCV), on the other hand, is associated with an increased risk for type 2 diabetes, with the risk being higher for older individuals with a family history of diabetes. The negative effects of HCV on glucose balance are mainly attributed to increased insulin resistance in the liver. HCV reduces hepatic glucose uptake by downregulating the expression of glucose transporters and additionally impairs insulin signal transduction by inhibiting the PI3K/Akt signaling pathway.
People with obesity, diabetes, or insulin resistance show significant changes in the innate and adaptive functions of the immune system. Regarding the innate immune system, impaired chemotaxis and phagocytosis of neutrophils have been observed in patients with type 2 diabetes.
In the case of obesity, the number of natural killer T cells in adipose tissue decreases, whereas B cells accumulate in adipose tissue and secrete more proinflammatory cytokines. Longitudinal multiomics analyses of various biopsies from individuals with insulin resistance showed a delayed immune response to respiratory virus infections compared with individuals with normal insulin sensitivity.
This story was translated from Medscape Germany using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Stefan Bornstein, MD, PhD, professor, made it clear during a press conference at the 67th Congress of the German Society of Endocrinology (DGE) that there is more than one interaction between them. Nowadays, one can almost speak of an “endocrine virology and even of the virome as an additional, hormonally metabolically active gland,” said Dr. Bornstein, who will receive the Berthold Medal from the DGE in 2024.
Many questions remain unanswered: “We need a better understanding of the interaction of hormone systems with infectious agents — from basics to therapeutic applications,” emphasized the director of the Medical Clinic and Polyclinic III and the Center for Internal Medicine at the Carl Gustav Carus University Hospital, Dresden, Germany.
If infectious diseases could trigger diabetes and other metabolic diseases, this means that “through vaccination programs, we may be able to prevent the occurrence of common metabolic diseases such as diabetes,” said Dr. Bornstein. He highlighted that many people who experienced severe COVID-19 during the pandemic, or died from it, exhibited diabetes or a pre-metabolic syndrome.
“SARS-CoV-2 has utilized an endocrine signaling pathway to invade our cells and cause damage in the organ systems and inflammation,” said Dr. Bornstein. Conversely, it is now known that infections with coronaviruses or other infectious agents like influenza can significantly worsen metabolic status, diabetes, and other endocrine diseases.
SARS-CoV-2 Infects the Beta Cells
Data from the COVID-19 pandemic showed that the likelihood of developing type 1 diabetes significantly increases with a SARS-CoV-2 infection. Researchers led by Dr. Bornstein demonstrated in 2021 that SARS-CoV-2 can infect the insulin-producing cells of the organ. They examined pancreatic tissue from 20 patients who died from COVID-19 using immunofluorescence, immunohistochemistry, RNA in situ hybridization, and electron microscopy.
They found viral SARS-CoV-2 infiltration of the beta cells in all patients. In 11 patients with COVID-19, the expression of ACE2, TMPRSS, and other receptors and factors like DPP4, HMBG1, and NRP1 that can facilitate virus entry was examined. They found that even in the absence of manifest newly onset diabetes, necroptotic cell death, immune cell infiltration, and SARS-CoV-2 infection of the pancreas beta cells can contribute to varying degrees of metabolic disturbance in patients with COVID-19.
In a report published in October 2020, Tim Hollstein, MD, from the Institute for Diabetology and Clinical Metabolic Research at UKSH in Kiel, Germany, and colleagues described the case of a 19-year-old man who developed symptoms of insulin-dependent diabetes after a SARS-CoV-2 infection, without the presence of autoantibodies typical for type 1 diabetes.
The man presented to the emergency department with diabetic ketoacidosis, a C-peptide level of 0.62 µg/L, a blood glucose concentration of 30.6 mmol/L (552 mg/dL), and an A1c level of 16.8%. The patient’s history revealed a probable SARS-CoV-2 infection 5-7 weeks before admission, based on a positive antibody test against SARS-CoV-2.
Some Viruses Produce Insulin-Like Proteins
Recent studies have shown that some viruses can produce insulin-like proteins or hormones that interfere with the metabolism of the affected organism, reported Dr. Bornstein. In addition to metabolic regulation, these “viral hormones” also seem to influence cell turnover and cell death.
Dr. Bornstein pointed out that antiviral medications can delay the onset of type 1 diabetes by preserving the function of insulin-producing beta cells. It has also been shown that conventional medications used to treat hormonal disorders can reduce the susceptibility of the organism to infections — such as antidiabetic preparations like DPP-4 inhibitors or metformin.
In a review published in 2023, Nikolaos Perakakis, MD, professor, research group leader at the Paul Langerhans Institute Dresden, Dresden, Germany, Dr. Bornstein, and colleagues discussed scientific evidence for a close mutual dependence between various virus infections and metabolic diseases. They discussed how viruses can lead to the development or progression of metabolic diseases and vice versa and how metabolic diseases can increase the severity of a virus infection.
Viruses Favor Metabolic Diseases...
Viruses can favor metabolic diseases by, for example, influencing the regulation of cell survival and specific signaling pathways relevant for cell death, proliferation, or dedifferentiation in important endocrine and metabolic organs. Viruses are also capable of controlling cellular glucose metabolism by modulating glucose transporters, altering glucose uptake, regulating signaling pathways, and stimulating glycolysis in infected cells.
Due to the destruction of beta cells, enteroviruses, but also the mumps virus, parainfluenza virus, or human herpes virus 6, are associated with the development of diabetes. The timing of infection often precedes or coincides with the peak of development of islet autoantibodies. The fact that only a small proportion of patients actually develop type 1 diabetes suggests that genetic background, and likely the timing of infection, play an important role.
...And Metabolic Diseases Influence the Course of Infection
Infection with hepatitis C virus (HCV), on the other hand, is associated with an increased risk for type 2 diabetes, with the risk being higher for older individuals with a family history of diabetes. The negative effects of HCV on glucose balance are mainly attributed to increased insulin resistance in the liver. HCV reduces hepatic glucose uptake by downregulating the expression of glucose transporters and additionally impairs insulin signal transduction by inhibiting the PI3K/Akt signaling pathway.
People with obesity, diabetes, or insulin resistance show significant changes in the innate and adaptive functions of the immune system. Regarding the innate immune system, impaired chemotaxis and phagocytosis of neutrophils have been observed in patients with type 2 diabetes.
In the case of obesity, the number of natural killer T cells in adipose tissue decreases, whereas B cells accumulate in adipose tissue and secrete more proinflammatory cytokines. Longitudinal multiomics analyses of various biopsies from individuals with insulin resistance showed a delayed immune response to respiratory virus infections compared with individuals with normal insulin sensitivity.
This story was translated from Medscape Germany using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Stefan Bornstein, MD, PhD, professor, made it clear during a press conference at the 67th Congress of the German Society of Endocrinology (DGE) that there is more than one interaction between them. Nowadays, one can almost speak of an “endocrine virology and even of the virome as an additional, hormonally metabolically active gland,” said Dr. Bornstein, who will receive the Berthold Medal from the DGE in 2024.
Many questions remain unanswered: “We need a better understanding of the interaction of hormone systems with infectious agents — from basics to therapeutic applications,” emphasized the director of the Medical Clinic and Polyclinic III and the Center for Internal Medicine at the Carl Gustav Carus University Hospital, Dresden, Germany.
If infectious diseases could trigger diabetes and other metabolic diseases, this means that “through vaccination programs, we may be able to prevent the occurrence of common metabolic diseases such as diabetes,” said Dr. Bornstein. He highlighted that many people who experienced severe COVID-19 during the pandemic, or died from it, exhibited diabetes or a pre-metabolic syndrome.
“SARS-CoV-2 has utilized an endocrine signaling pathway to invade our cells and cause damage in the organ systems and inflammation,” said Dr. Bornstein. Conversely, it is now known that infections with coronaviruses or other infectious agents like influenza can significantly worsen metabolic status, diabetes, and other endocrine diseases.
SARS-CoV-2 Infects the Beta Cells
Data from the COVID-19 pandemic showed that the likelihood of developing type 1 diabetes significantly increases with a SARS-CoV-2 infection. Researchers led by Dr. Bornstein demonstrated in 2021 that SARS-CoV-2 can infect the insulin-producing cells of the organ. They examined pancreatic tissue from 20 patients who died from COVID-19 using immunofluorescence, immunohistochemistry, RNA in situ hybridization, and electron microscopy.
They found viral SARS-CoV-2 infiltration of the beta cells in all patients. In 11 patients with COVID-19, the expression of ACE2, TMPRSS, and other receptors and factors like DPP4, HMBG1, and NRP1 that can facilitate virus entry was examined. They found that even in the absence of manifest newly onset diabetes, necroptotic cell death, immune cell infiltration, and SARS-CoV-2 infection of the pancreas beta cells can contribute to varying degrees of metabolic disturbance in patients with COVID-19.
In a report published in October 2020, Tim Hollstein, MD, from the Institute for Diabetology and Clinical Metabolic Research at UKSH in Kiel, Germany, and colleagues described the case of a 19-year-old man who developed symptoms of insulin-dependent diabetes after a SARS-CoV-2 infection, without the presence of autoantibodies typical for type 1 diabetes.
The man presented to the emergency department with diabetic ketoacidosis, a C-peptide level of 0.62 µg/L, a blood glucose concentration of 30.6 mmol/L (552 mg/dL), and an A1c level of 16.8%. The patient’s history revealed a probable SARS-CoV-2 infection 5-7 weeks before admission, based on a positive antibody test against SARS-CoV-2.
Some Viruses Produce Insulin-Like Proteins
Recent studies have shown that some viruses can produce insulin-like proteins or hormones that interfere with the metabolism of the affected organism, reported Dr. Bornstein. In addition to metabolic regulation, these “viral hormones” also seem to influence cell turnover and cell death.
Dr. Bornstein pointed out that antiviral medications can delay the onset of type 1 diabetes by preserving the function of insulin-producing beta cells. It has also been shown that conventional medications used to treat hormonal disorders can reduce the susceptibility of the organism to infections — such as antidiabetic preparations like DPP-4 inhibitors or metformin.
In a review published in 2023, Nikolaos Perakakis, MD, professor, research group leader at the Paul Langerhans Institute Dresden, Dresden, Germany, Dr. Bornstein, and colleagues discussed scientific evidence for a close mutual dependence between various virus infections and metabolic diseases. They discussed how viruses can lead to the development or progression of metabolic diseases and vice versa and how metabolic diseases can increase the severity of a virus infection.
Viruses Favor Metabolic Diseases...
Viruses can favor metabolic diseases by, for example, influencing the regulation of cell survival and specific signaling pathways relevant for cell death, proliferation, or dedifferentiation in important endocrine and metabolic organs. Viruses are also capable of controlling cellular glucose metabolism by modulating glucose transporters, altering glucose uptake, regulating signaling pathways, and stimulating glycolysis in infected cells.
Due to the destruction of beta cells, enteroviruses, but also the mumps virus, parainfluenza virus, or human herpes virus 6, are associated with the development of diabetes. The timing of infection often precedes or coincides with the peak of development of islet autoantibodies. The fact that only a small proportion of patients actually develop type 1 diabetes suggests that genetic background, and likely the timing of infection, play an important role.
...And Metabolic Diseases Influence the Course of Infection
Infection with hepatitis C virus (HCV), on the other hand, is associated with an increased risk for type 2 diabetes, with the risk being higher for older individuals with a family history of diabetes. The negative effects of HCV on glucose balance are mainly attributed to increased insulin resistance in the liver. HCV reduces hepatic glucose uptake by downregulating the expression of glucose transporters and additionally impairs insulin signal transduction by inhibiting the PI3K/Akt signaling pathway.
People with obesity, diabetes, or insulin resistance show significant changes in the innate and adaptive functions of the immune system. Regarding the innate immune system, impaired chemotaxis and phagocytosis of neutrophils have been observed in patients with type 2 diabetes.
In the case of obesity, the number of natural killer T cells in adipose tissue decreases, whereas B cells accumulate in adipose tissue and secrete more proinflammatory cytokines. Longitudinal multiomics analyses of various biopsies from individuals with insulin resistance showed a delayed immune response to respiratory virus infections compared with individuals with normal insulin sensitivity.
This story was translated from Medscape Germany using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Epilepsy Linked to Higher COVID Hospitalization, Death Rates
, data from two linked studies showed.
Results showed that individuals with epilepsy had a 60% higher risk for hospitalization and a 33% higher risk of dying from COVID-19 than those without the disorder. However, during the pandemic, the number of hospitalizations and ER visits by people with epilepsy dropped by as much as 30%.
“The neurotropic effects of Sars-CoV-2 might explain some of this increased risk for people with epilepsy, or epilepsy might be associated with alterations in the immune system, predisposing to more severe COVID-19,” wrote the investigators, led by Owen Pickrell, MBBChirm, PhD, Swansea University, United Kingdom.
The findings were published online March 5 in Epilepsia.
Skill Shifting
Epilepsy is one of the most common neurological conditions and affects approximately 50 million people worldwide, with significant comorbidity and an increased risk for early death.
During the pandemic, clinicians treating people with epilepsy and other conditions shifted their skills to treat an ever-increasing number of patients with COVID-19, which may have hindered epilepsy-specific services for a time.
To further explore how the COVID-19 pandemic may have affected the health of this patient population, researchers analyzed health records from a large database with information about hospital admissions, primary care visits, COVID-19 vaccination status, and demographics of 90% of Welsh residents.
Those living with epilepsy before or during the study period (March 1, 2020, to June 31, 2021) were identified and compared with controls without epilepsy.
The analysis included approximately 27,280 people with epilepsy and 136,400 matched controls. Among those with epilepsy, there were 158 deaths (0.58%) and 933 hospitalizations (3.4%). In comparison, there were 370 deaths (0.27%) and 1871 hospitalizations (1.4%) in the control group.
Unadjusted analyses showed the risk of dying from COVID-19 for those with epilepsy vs controls was more than twofold higher (hazard ratio [HR], 2.15; 95% CI; 1.78-2.59) and the increase in the risk for hospitalization was similar (HR, 2.15; 95% CI; 1.94-2.37).
After adjusting for 40 comorbidities, including serious mental illness, asthma, and diabetes, those with epilepsy had a 60% increased risk for hospitalization (adjusted HR [aHR], 1.60) and a 33% increased risk for death (aHR, 1.33) than those without epilepsy (all P < .0001).
The findings “may have implications for prioritizing future COVID-19 treatments and vaccinations for people with epilepsy,” the investigators wrote.
Study limitations included the inability to account for the effect of vaccinations or prior infections with SARS-CoV-2. Moreover, the study did not account for geographical or temporal variations in prevalence and COVID-19 variants.
Consultations Canceled
In the related study, researchers analyzed healthcare utilization by people with epilepsy before and after the pandemic using the same database. Results showed hospital admissions, ER visits, and outpatient visits significantly decreased during the pandemic.
In the year before the pandemic, people with epilepsy had double the rate of ER visits (rate ratio [RR], 2.36), hospital admissions (RR, 2.08), and outpatient appointments (RR, 1.92) compared with matched controls.
However, during the pandemic there was a greater reduction in hospital admissions (RR, 0.70; 95% CI, 0.69-0.72) and ER visits (RR, 0.78; 95% CI, 0.77-0.70) in those with epilepsy versus matched controls (RR, 0.82; 95% CI, 0.81-0.83) as well as hospital visits and ER visits (RR, 0.87; 95% CI, 0.86-0.88; all P < .0001). New epilepsy diagnoses also decreased during the pandemic (RR, 0.73; P < .0001)
The redeployment of epileptologists during the pandemic also meant that epilepsy consultations and investigations were canceled, making it harder for people with epilepsy to access specialty care, the researchers noted.
“Our research also showed that there were fewer new diagnoses of epilepsy and fewer contacts with health services by people with epilepsy, during the period we examined,” Huw Strafford, lead data analyst for the studies, said in a release.
Both studies were funded by Health and Care Research Wales. Dr. Pickrell reported receiving speaker fees from UCB Pharma and Angelini Pharma, travel grants from Angelini Pharma, and an unrestricted grant from UCB Pharma.
A version of this article appeared on Medscape.com .
, data from two linked studies showed.
Results showed that individuals with epilepsy had a 60% higher risk for hospitalization and a 33% higher risk of dying from COVID-19 than those without the disorder. However, during the pandemic, the number of hospitalizations and ER visits by people with epilepsy dropped by as much as 30%.
“The neurotropic effects of Sars-CoV-2 might explain some of this increased risk for people with epilepsy, or epilepsy might be associated with alterations in the immune system, predisposing to more severe COVID-19,” wrote the investigators, led by Owen Pickrell, MBBChirm, PhD, Swansea University, United Kingdom.
The findings were published online March 5 in Epilepsia.
Skill Shifting
Epilepsy is one of the most common neurological conditions and affects approximately 50 million people worldwide, with significant comorbidity and an increased risk for early death.
During the pandemic, clinicians treating people with epilepsy and other conditions shifted their skills to treat an ever-increasing number of patients with COVID-19, which may have hindered epilepsy-specific services for a time.
To further explore how the COVID-19 pandemic may have affected the health of this patient population, researchers analyzed health records from a large database with information about hospital admissions, primary care visits, COVID-19 vaccination status, and demographics of 90% of Welsh residents.
Those living with epilepsy before or during the study period (March 1, 2020, to June 31, 2021) were identified and compared with controls without epilepsy.
The analysis included approximately 27,280 people with epilepsy and 136,400 matched controls. Among those with epilepsy, there were 158 deaths (0.58%) and 933 hospitalizations (3.4%). In comparison, there were 370 deaths (0.27%) and 1871 hospitalizations (1.4%) in the control group.
Unadjusted analyses showed the risk of dying from COVID-19 for those with epilepsy vs controls was more than twofold higher (hazard ratio [HR], 2.15; 95% CI; 1.78-2.59) and the increase in the risk for hospitalization was similar (HR, 2.15; 95% CI; 1.94-2.37).
After adjusting for 40 comorbidities, including serious mental illness, asthma, and diabetes, those with epilepsy had a 60% increased risk for hospitalization (adjusted HR [aHR], 1.60) and a 33% increased risk for death (aHR, 1.33) than those without epilepsy (all P < .0001).
The findings “may have implications for prioritizing future COVID-19 treatments and vaccinations for people with epilepsy,” the investigators wrote.
Study limitations included the inability to account for the effect of vaccinations or prior infections with SARS-CoV-2. Moreover, the study did not account for geographical or temporal variations in prevalence and COVID-19 variants.
Consultations Canceled
In the related study, researchers analyzed healthcare utilization by people with epilepsy before and after the pandemic using the same database. Results showed hospital admissions, ER visits, and outpatient visits significantly decreased during the pandemic.
In the year before the pandemic, people with epilepsy had double the rate of ER visits (rate ratio [RR], 2.36), hospital admissions (RR, 2.08), and outpatient appointments (RR, 1.92) compared with matched controls.
However, during the pandemic there was a greater reduction in hospital admissions (RR, 0.70; 95% CI, 0.69-0.72) and ER visits (RR, 0.78; 95% CI, 0.77-0.70) in those with epilepsy versus matched controls (RR, 0.82; 95% CI, 0.81-0.83) as well as hospital visits and ER visits (RR, 0.87; 95% CI, 0.86-0.88; all P < .0001). New epilepsy diagnoses also decreased during the pandemic (RR, 0.73; P < .0001)
The redeployment of epileptologists during the pandemic also meant that epilepsy consultations and investigations were canceled, making it harder for people with epilepsy to access specialty care, the researchers noted.
“Our research also showed that there were fewer new diagnoses of epilepsy and fewer contacts with health services by people with epilepsy, during the period we examined,” Huw Strafford, lead data analyst for the studies, said in a release.
Both studies were funded by Health and Care Research Wales. Dr. Pickrell reported receiving speaker fees from UCB Pharma and Angelini Pharma, travel grants from Angelini Pharma, and an unrestricted grant from UCB Pharma.
A version of this article appeared on Medscape.com .
, data from two linked studies showed.
Results showed that individuals with epilepsy had a 60% higher risk for hospitalization and a 33% higher risk of dying from COVID-19 than those without the disorder. However, during the pandemic, the number of hospitalizations and ER visits by people with epilepsy dropped by as much as 30%.
“The neurotropic effects of Sars-CoV-2 might explain some of this increased risk for people with epilepsy, or epilepsy might be associated with alterations in the immune system, predisposing to more severe COVID-19,” wrote the investigators, led by Owen Pickrell, MBBChirm, PhD, Swansea University, United Kingdom.
The findings were published online March 5 in Epilepsia.
Skill Shifting
Epilepsy is one of the most common neurological conditions and affects approximately 50 million people worldwide, with significant comorbidity and an increased risk for early death.
During the pandemic, clinicians treating people with epilepsy and other conditions shifted their skills to treat an ever-increasing number of patients with COVID-19, which may have hindered epilepsy-specific services for a time.
To further explore how the COVID-19 pandemic may have affected the health of this patient population, researchers analyzed health records from a large database with information about hospital admissions, primary care visits, COVID-19 vaccination status, and demographics of 90% of Welsh residents.
Those living with epilepsy before or during the study period (March 1, 2020, to June 31, 2021) were identified and compared with controls without epilepsy.
The analysis included approximately 27,280 people with epilepsy and 136,400 matched controls. Among those with epilepsy, there were 158 deaths (0.58%) and 933 hospitalizations (3.4%). In comparison, there were 370 deaths (0.27%) and 1871 hospitalizations (1.4%) in the control group.
Unadjusted analyses showed the risk of dying from COVID-19 for those with epilepsy vs controls was more than twofold higher (hazard ratio [HR], 2.15; 95% CI; 1.78-2.59) and the increase in the risk for hospitalization was similar (HR, 2.15; 95% CI; 1.94-2.37).
After adjusting for 40 comorbidities, including serious mental illness, asthma, and diabetes, those with epilepsy had a 60% increased risk for hospitalization (adjusted HR [aHR], 1.60) and a 33% increased risk for death (aHR, 1.33) than those without epilepsy (all P < .0001).
The findings “may have implications for prioritizing future COVID-19 treatments and vaccinations for people with epilepsy,” the investigators wrote.
Study limitations included the inability to account for the effect of vaccinations or prior infections with SARS-CoV-2. Moreover, the study did not account for geographical or temporal variations in prevalence and COVID-19 variants.
Consultations Canceled
In the related study, researchers analyzed healthcare utilization by people with epilepsy before and after the pandemic using the same database. Results showed hospital admissions, ER visits, and outpatient visits significantly decreased during the pandemic.
In the year before the pandemic, people with epilepsy had double the rate of ER visits (rate ratio [RR], 2.36), hospital admissions (RR, 2.08), and outpatient appointments (RR, 1.92) compared with matched controls.
However, during the pandemic there was a greater reduction in hospital admissions (RR, 0.70; 95% CI, 0.69-0.72) and ER visits (RR, 0.78; 95% CI, 0.77-0.70) in those with epilepsy versus matched controls (RR, 0.82; 95% CI, 0.81-0.83) as well as hospital visits and ER visits (RR, 0.87; 95% CI, 0.86-0.88; all P < .0001). New epilepsy diagnoses also decreased during the pandemic (RR, 0.73; P < .0001)
The redeployment of epileptologists during the pandemic also meant that epilepsy consultations and investigations were canceled, making it harder for people with epilepsy to access specialty care, the researchers noted.
“Our research also showed that there were fewer new diagnoses of epilepsy and fewer contacts with health services by people with epilepsy, during the period we examined,” Huw Strafford, lead data analyst for the studies, said in a release.
Both studies were funded by Health and Care Research Wales. Dr. Pickrell reported receiving speaker fees from UCB Pharma and Angelini Pharma, travel grants from Angelini Pharma, and an unrestricted grant from UCB Pharma.
A version of this article appeared on Medscape.com .
FROM EPILEPSIA
Cognitive Deficits After Most Severe COVID Cases Associated With 9-Point IQ Drop
A new study from the United Kingdom provides greater clarity on how SARS-CoV-2 infection can affect cognition and memory, including novel data on how long brain fog may last after the illness resolves and which cognitive functions are most vulnerable.
In a large community sample, researchers found that on average, people who had recovered from COVID-19 showed small cognitive deficits equivalent to a 3-point loss in IQ for up to 1 year or more after recovering from the acute illness compared with peers who never had COVID-19.
However, people who had more severe cases, requiring treatment in a hospital intensive care unit, had cognitive deficits equivalent to a 9-point drop in IQ.
“People with ongoing persistent symptoms, indicative of long COVID, had larger cognitive deficits than people whose symptoms had resolved,” first author Adam Hampshire, PhD, with Imperial College London, London, England, told this news organization.
The largest deficits among cognitive tasks were in memory, reasoning, and executive function, he added.
“That is, people who had had COVID-19 were both slower and less accurate when performing tasks that measure those abilities,” Dr. Hampshire said. “The group with the largest cognitive deficits were patients who had been in intensive care for COVID-19.”
The study was published online in The New England Journal of Medicine.
Lingering Brain Fog
Cognitive symptoms after SARS-CoV-2 infection are well recognized, but whether objectively measurable cognitive deficits exist and how long they persist remains unclear.
To investigate, researchers invited 800,000 adults from the REACT study of SARS-CoV-2 transmission in England to complete an online assessment for cognitive function with eight domains.
Altogether, 141,583 participants started the cognitive battery by completing at least one task, and 112,964 completed all eight tasks.
The researchers estimated global cognitive scores among participants who had been previously infected with SARS-CoV-2 with symptoms that persisted for at least 12 weeks, whether or not resolved, and among uninfected participants.
Compared with uninfected adults, those who had COVID-19 that resolved had a small cognitive deficit, corresponding to a 3-point loss in IQ, the researchers found.
Adults with unresolved persistent COVID-19 symptoms had the equivalent of a 6-point loss in IQ, and those who had been admitted to the intensive care unit had the equivalent of a 9-point loss in IQ, in line with previous findings of cognitive deficits in patients hospitalized in a critical care unit, the researchers report.
Larger cognitive deficits were evident in adults infected early in the pandemic by the original SARS-CoV-2 virus or the B.1.1.7 variant, whereas peers infected later in the pandemic (eg., in the Omicron period), showed smaller cognitive deficits. This finding is in line with other studies suggesting that the association between COVID-19–associated cognitive deficits attenuated as the pandemic progressed, the researchers noted.
They also found that people who had COVID-19 after receiving two or more vaccinations showed better cognitive performance compared with those who had not been vaccinated.
The memory, reasoning, and executive function tasks were among the most sensitive to COVID-19–related cognitive differences and performance on these tasks differed according to illness duration and hospitalization.
Dr. Hampshire said that more research is needed to determine whether the cognitive deficits resolve with time.
“The implications of longer-term persistence of cognitive deficits and their clinical relevance remain unclear and warrant ongoing surveillance,” he said.
Larger Cognitive Deficits Likely?
These results are “a concern and the broader implications require evaluation,” wrote Ziyad Al-Aly, MD, with Washington University School of Medicine in St. Louis, Missouri, and Clifford Rosen, MD, with Tufts University School of Medicine in Boston, Massachusetts, in an accompanying editorial.
In their view, several outstanding questions remain, including what the potential functional implications of a 3-point loss in IQ may be and whether COVID-19–related cognitive deficits predispose to a higher risk for dementia later in life.
“A deeper understanding of the biology of cognitive dysfunction after SARS-CoV-2 infection and how best to prevent and treat it are critical for addressing the needs of affected persons and preserving the cognitive health of populations,” Drs. Al-Aly and Rosen concluded.
Commenting on the study for this news organization, Jacqueline Becker, PhD, clinical neuropsychologist and assistant professor of medicine, Icahn School of Medicine at Mount Sinai, New York City, noted that “one important caveat” is that the study used an online assessment tool for cognitive function and therefore the findings should be taken with “a grain of salt.”
“That said, this is a large sample, and the findings are generally consistent with what we’ve seen in terms of cognitive deficits post-COVID,” Dr. Becker said.
It’s likely that this study “underestimates” the degree of cognitive deficits that would be seen on validated neuropsychological tests, she added.
In a recent study, Dr. Becker and her colleagues investigated rates of cognitive impairment in 740 COVID-19 patients who recovered and were treated in outpatient, emergency department, or inpatient hospital settings.
Using validated neuropsychological measures, they found a relatively high frequency of cognitive impairment several months after patients contracted COVID-19. Impairments in executive functioning, processing speed, category fluency, memory encoding, and recall were predominant among hospitalized patients.
Dr. Becker noted that in her experience, cognition typically will improve in some patients 12-18 months post COVID.
Support for the study was provided by the National Institute for Health and Care Research and UK Research and Innovation and by the Department of Health and Social Care in England and the Huo Family Foundation. Disclosures for authors and editorial writers are available at NEJM.org. Dr. Becker has no relevant disclosures.
A version of this article appeared on Medscape.com.
A new study from the United Kingdom provides greater clarity on how SARS-CoV-2 infection can affect cognition and memory, including novel data on how long brain fog may last after the illness resolves and which cognitive functions are most vulnerable.
In a large community sample, researchers found that on average, people who had recovered from COVID-19 showed small cognitive deficits equivalent to a 3-point loss in IQ for up to 1 year or more after recovering from the acute illness compared with peers who never had COVID-19.
However, people who had more severe cases, requiring treatment in a hospital intensive care unit, had cognitive deficits equivalent to a 9-point drop in IQ.
“People with ongoing persistent symptoms, indicative of long COVID, had larger cognitive deficits than people whose symptoms had resolved,” first author Adam Hampshire, PhD, with Imperial College London, London, England, told this news organization.
The largest deficits among cognitive tasks were in memory, reasoning, and executive function, he added.
“That is, people who had had COVID-19 were both slower and less accurate when performing tasks that measure those abilities,” Dr. Hampshire said. “The group with the largest cognitive deficits were patients who had been in intensive care for COVID-19.”
The study was published online in The New England Journal of Medicine.
Lingering Brain Fog
Cognitive symptoms after SARS-CoV-2 infection are well recognized, but whether objectively measurable cognitive deficits exist and how long they persist remains unclear.
To investigate, researchers invited 800,000 adults from the REACT study of SARS-CoV-2 transmission in England to complete an online assessment for cognitive function with eight domains.
Altogether, 141,583 participants started the cognitive battery by completing at least one task, and 112,964 completed all eight tasks.
The researchers estimated global cognitive scores among participants who had been previously infected with SARS-CoV-2 with symptoms that persisted for at least 12 weeks, whether or not resolved, and among uninfected participants.
Compared with uninfected adults, those who had COVID-19 that resolved had a small cognitive deficit, corresponding to a 3-point loss in IQ, the researchers found.
Adults with unresolved persistent COVID-19 symptoms had the equivalent of a 6-point loss in IQ, and those who had been admitted to the intensive care unit had the equivalent of a 9-point loss in IQ, in line with previous findings of cognitive deficits in patients hospitalized in a critical care unit, the researchers report.
Larger cognitive deficits were evident in adults infected early in the pandemic by the original SARS-CoV-2 virus or the B.1.1.7 variant, whereas peers infected later in the pandemic (eg., in the Omicron period), showed smaller cognitive deficits. This finding is in line with other studies suggesting that the association between COVID-19–associated cognitive deficits attenuated as the pandemic progressed, the researchers noted.
They also found that people who had COVID-19 after receiving two or more vaccinations showed better cognitive performance compared with those who had not been vaccinated.
The memory, reasoning, and executive function tasks were among the most sensitive to COVID-19–related cognitive differences and performance on these tasks differed according to illness duration and hospitalization.
Dr. Hampshire said that more research is needed to determine whether the cognitive deficits resolve with time.
“The implications of longer-term persistence of cognitive deficits and their clinical relevance remain unclear and warrant ongoing surveillance,” he said.
Larger Cognitive Deficits Likely?
These results are “a concern and the broader implications require evaluation,” wrote Ziyad Al-Aly, MD, with Washington University School of Medicine in St. Louis, Missouri, and Clifford Rosen, MD, with Tufts University School of Medicine in Boston, Massachusetts, in an accompanying editorial.
In their view, several outstanding questions remain, including what the potential functional implications of a 3-point loss in IQ may be and whether COVID-19–related cognitive deficits predispose to a higher risk for dementia later in life.
“A deeper understanding of the biology of cognitive dysfunction after SARS-CoV-2 infection and how best to prevent and treat it are critical for addressing the needs of affected persons and preserving the cognitive health of populations,” Drs. Al-Aly and Rosen concluded.
Commenting on the study for this news organization, Jacqueline Becker, PhD, clinical neuropsychologist and assistant professor of medicine, Icahn School of Medicine at Mount Sinai, New York City, noted that “one important caveat” is that the study used an online assessment tool for cognitive function and therefore the findings should be taken with “a grain of salt.”
“That said, this is a large sample, and the findings are generally consistent with what we’ve seen in terms of cognitive deficits post-COVID,” Dr. Becker said.
It’s likely that this study “underestimates” the degree of cognitive deficits that would be seen on validated neuropsychological tests, she added.
In a recent study, Dr. Becker and her colleagues investigated rates of cognitive impairment in 740 COVID-19 patients who recovered and were treated in outpatient, emergency department, or inpatient hospital settings.
Using validated neuropsychological measures, they found a relatively high frequency of cognitive impairment several months after patients contracted COVID-19. Impairments in executive functioning, processing speed, category fluency, memory encoding, and recall were predominant among hospitalized patients.
Dr. Becker noted that in her experience, cognition typically will improve in some patients 12-18 months post COVID.
Support for the study was provided by the National Institute for Health and Care Research and UK Research and Innovation and by the Department of Health and Social Care in England and the Huo Family Foundation. Disclosures for authors and editorial writers are available at NEJM.org. Dr. Becker has no relevant disclosures.
A version of this article appeared on Medscape.com.
A new study from the United Kingdom provides greater clarity on how SARS-CoV-2 infection can affect cognition and memory, including novel data on how long brain fog may last after the illness resolves and which cognitive functions are most vulnerable.
In a large community sample, researchers found that on average, people who had recovered from COVID-19 showed small cognitive deficits equivalent to a 3-point loss in IQ for up to 1 year or more after recovering from the acute illness compared with peers who never had COVID-19.
However, people who had more severe cases, requiring treatment in a hospital intensive care unit, had cognitive deficits equivalent to a 9-point drop in IQ.
“People with ongoing persistent symptoms, indicative of long COVID, had larger cognitive deficits than people whose symptoms had resolved,” first author Adam Hampshire, PhD, with Imperial College London, London, England, told this news organization.
The largest deficits among cognitive tasks were in memory, reasoning, and executive function, he added.
“That is, people who had had COVID-19 were both slower and less accurate when performing tasks that measure those abilities,” Dr. Hampshire said. “The group with the largest cognitive deficits were patients who had been in intensive care for COVID-19.”
The study was published online in The New England Journal of Medicine.
Lingering Brain Fog
Cognitive symptoms after SARS-CoV-2 infection are well recognized, but whether objectively measurable cognitive deficits exist and how long they persist remains unclear.
To investigate, researchers invited 800,000 adults from the REACT study of SARS-CoV-2 transmission in England to complete an online assessment for cognitive function with eight domains.
Altogether, 141,583 participants started the cognitive battery by completing at least one task, and 112,964 completed all eight tasks.
The researchers estimated global cognitive scores among participants who had been previously infected with SARS-CoV-2 with symptoms that persisted for at least 12 weeks, whether or not resolved, and among uninfected participants.
Compared with uninfected adults, those who had COVID-19 that resolved had a small cognitive deficit, corresponding to a 3-point loss in IQ, the researchers found.
Adults with unresolved persistent COVID-19 symptoms had the equivalent of a 6-point loss in IQ, and those who had been admitted to the intensive care unit had the equivalent of a 9-point loss in IQ, in line with previous findings of cognitive deficits in patients hospitalized in a critical care unit, the researchers report.
Larger cognitive deficits were evident in adults infected early in the pandemic by the original SARS-CoV-2 virus or the B.1.1.7 variant, whereas peers infected later in the pandemic (eg., in the Omicron period), showed smaller cognitive deficits. This finding is in line with other studies suggesting that the association between COVID-19–associated cognitive deficits attenuated as the pandemic progressed, the researchers noted.
They also found that people who had COVID-19 after receiving two or more vaccinations showed better cognitive performance compared with those who had not been vaccinated.
The memory, reasoning, and executive function tasks were among the most sensitive to COVID-19–related cognitive differences and performance on these tasks differed according to illness duration and hospitalization.
Dr. Hampshire said that more research is needed to determine whether the cognitive deficits resolve with time.
“The implications of longer-term persistence of cognitive deficits and their clinical relevance remain unclear and warrant ongoing surveillance,” he said.
Larger Cognitive Deficits Likely?
These results are “a concern and the broader implications require evaluation,” wrote Ziyad Al-Aly, MD, with Washington University School of Medicine in St. Louis, Missouri, and Clifford Rosen, MD, with Tufts University School of Medicine in Boston, Massachusetts, in an accompanying editorial.
In their view, several outstanding questions remain, including what the potential functional implications of a 3-point loss in IQ may be and whether COVID-19–related cognitive deficits predispose to a higher risk for dementia later in life.
“A deeper understanding of the biology of cognitive dysfunction after SARS-CoV-2 infection and how best to prevent and treat it are critical for addressing the needs of affected persons and preserving the cognitive health of populations,” Drs. Al-Aly and Rosen concluded.
Commenting on the study for this news organization, Jacqueline Becker, PhD, clinical neuropsychologist and assistant professor of medicine, Icahn School of Medicine at Mount Sinai, New York City, noted that “one important caveat” is that the study used an online assessment tool for cognitive function and therefore the findings should be taken with “a grain of salt.”
“That said, this is a large sample, and the findings are generally consistent with what we’ve seen in terms of cognitive deficits post-COVID,” Dr. Becker said.
It’s likely that this study “underestimates” the degree of cognitive deficits that would be seen on validated neuropsychological tests, she added.
In a recent study, Dr. Becker and her colleagues investigated rates of cognitive impairment in 740 COVID-19 patients who recovered and were treated in outpatient, emergency department, or inpatient hospital settings.
Using validated neuropsychological measures, they found a relatively high frequency of cognitive impairment several months after patients contracted COVID-19. Impairments in executive functioning, processing speed, category fluency, memory encoding, and recall were predominant among hospitalized patients.
Dr. Becker noted that in her experience, cognition typically will improve in some patients 12-18 months post COVID.
Support for the study was provided by the National Institute for Health and Care Research and UK Research and Innovation and by the Department of Health and Social Care in England and the Huo Family Foundation. Disclosures for authors and editorial writers are available at NEJM.org. Dr. Becker has no relevant disclosures.
A version of this article appeared on Medscape.com.
COVID Virus Can Remain in the Body Over a Year
Scientists at the University of California, San Francisco, have discovered that remnants of the COVID-19 virus can linger in blood and tissue for more than a year after a person is first infected.
In their research on long COVID, the scientists found COVID antigens in the blood for up to 14 months after infection, and in tissue samples for more than 2 years after infection.
“These two studies provide some of the strongest evidence so far that COVID antigens can persist in some people, even though we think they have normal immune responses,” Michael Peluso, MD, an infectious disease researcher in the UCSF School of Medicine, who led both studies, said in a statement.
Scientists don’t know what causes long COVID, in which symptoms of the illness persist months or years after recovery. The most common symptoms are extreme fatigue, shortness of breath, loss of smell, and muscle aches.
The UCSF research team examined blood samples from 171 infected people and found the COVID “spike” protein was still present up to 14 months after infection in some people. The antigens were found more often in people who were hospitalized with COVID or who reported being very sick but were not hospitalized.
Researchers next looked at the UCSF Long COVID Tissue Bank, which contains samples donated by patients with and without long COVID.
They found portions of viral RNA in the tissue up to 2 years after people were infected, though there was no evidence of reinfection. Those viral fragments were found in connective tissue where immune cells are, suggesting that the fragments caused the immune system to attack, according to the researchers.
The UCSF team is running clinical trials to find out if monoclonal antibodies or antiviral drugs can remove the virus.
The findings were presented in Denver this week at the Conference on Retroviruses and Opportunistic Infections.
A version of this article appeared on WebMD.com.
Scientists at the University of California, San Francisco, have discovered that remnants of the COVID-19 virus can linger in blood and tissue for more than a year after a person is first infected.
In their research on long COVID, the scientists found COVID antigens in the blood for up to 14 months after infection, and in tissue samples for more than 2 years after infection.
“These two studies provide some of the strongest evidence so far that COVID antigens can persist in some people, even though we think they have normal immune responses,” Michael Peluso, MD, an infectious disease researcher in the UCSF School of Medicine, who led both studies, said in a statement.
Scientists don’t know what causes long COVID, in which symptoms of the illness persist months or years after recovery. The most common symptoms are extreme fatigue, shortness of breath, loss of smell, and muscle aches.
The UCSF research team examined blood samples from 171 infected people and found the COVID “spike” protein was still present up to 14 months after infection in some people. The antigens were found more often in people who were hospitalized with COVID or who reported being very sick but were not hospitalized.
Researchers next looked at the UCSF Long COVID Tissue Bank, which contains samples donated by patients with and without long COVID.
They found portions of viral RNA in the tissue up to 2 years after people were infected, though there was no evidence of reinfection. Those viral fragments were found in connective tissue where immune cells are, suggesting that the fragments caused the immune system to attack, according to the researchers.
The UCSF team is running clinical trials to find out if monoclonal antibodies or antiviral drugs can remove the virus.
The findings were presented in Denver this week at the Conference on Retroviruses and Opportunistic Infections.
A version of this article appeared on WebMD.com.
Scientists at the University of California, San Francisco, have discovered that remnants of the COVID-19 virus can linger in blood and tissue for more than a year after a person is first infected.
In their research on long COVID, the scientists found COVID antigens in the blood for up to 14 months after infection, and in tissue samples for more than 2 years after infection.
“These two studies provide some of the strongest evidence so far that COVID antigens can persist in some people, even though we think they have normal immune responses,” Michael Peluso, MD, an infectious disease researcher in the UCSF School of Medicine, who led both studies, said in a statement.
Scientists don’t know what causes long COVID, in which symptoms of the illness persist months or years after recovery. The most common symptoms are extreme fatigue, shortness of breath, loss of smell, and muscle aches.
The UCSF research team examined blood samples from 171 infected people and found the COVID “spike” protein was still present up to 14 months after infection in some people. The antigens were found more often in people who were hospitalized with COVID or who reported being very sick but were not hospitalized.
Researchers next looked at the UCSF Long COVID Tissue Bank, which contains samples donated by patients with and without long COVID.
They found portions of viral RNA in the tissue up to 2 years after people were infected, though there was no evidence of reinfection. Those viral fragments were found in connective tissue where immune cells are, suggesting that the fragments caused the immune system to attack, according to the researchers.
The UCSF team is running clinical trials to find out if monoclonal antibodies or antiviral drugs can remove the virus.
The findings were presented in Denver this week at the Conference on Retroviruses and Opportunistic Infections.
A version of this article appeared on WebMD.com.
New Data on Mild COVID’s Risk for Neurologic, Psychiatric Disorders
While severe COVID-19 is associated with a significantly higher risk for psychiatric and neurologic disorders a year after infection, mild does not carry the same risk, a new study shows.
However, less severe COVID-19 was not linked to a higher incidence of psychiatric diagnoses and was associated with only a slightly higher risk for neurologic disorders.
The new research challenges previous findings of long-term risk for psychiatric and neurologic disorders associated with SARS-CoV-2 in patients who had not been hospitalized for the condition.
“Our study does not support previous findings of substantial post-acute neurologic and psychiatric morbidities among the general population of SARS-CoV-2-infected individuals but does corroborate an elevated risk among the most severe cases with COVID-19,” the authors wrote.
The study was published online on February 21 in Neurology.
‘Alarming’ Findings
Previous studies have reported nervous system symptoms in patients who have experienced COVID-19, which may persist for several weeks or months after the acute phase, even in milder cases.
But these findings haven’t been consistent across all studies, and few studies have addressed the potential effect of different viral variants and vaccination status on post-acute psychiatric and neurologic morbidities.
“Our study was partly motivated by our strong research interest in the associations between infectious disease and later chronic disease and partly by international studies, such as those conducted in the US Veterans Health databases, that have suggested substantial risks of psychiatric and neurological conditions associated with infection,” senior author Anders Hviid, MSc, DrMedSci, head of the department and professor of pharmacoepidemiology, Statens Serum Institut, Copenhagen, Denmark, told this news organization.
Investigators drew on data from the Danish National Patient Registry to compare the risk for neurologic and psychiatric disorders during the 12 months after acute COVID-19 infection to risk among people who never tested positive.
They examined data on all recorded hospital contacts between January 2005 and January 2023 for a discharge diagnosis of at least one of 11 psychiatric illnesses or at least one of 30 neurologic disorders.
The researchers compared the incidence of each disorder within 1-12 months after infection with those of COVID-naive individuals and stratified analyses according to time since infection, vaccination status, variant period, age, sex, and infection severity.
The final study cohort included 1.8 million individuals who tested positive during the study period and 1.5 who didn’t. Three quarters of those who tested positive were infected primarily with the Omicron variant.
Hospitalized vs Nonhospitalized
Overall, individuals who tested positive had a 24% lower risk for psychiatric disorders during the post-acute period (incident rate ratio [IRR], 0.76; 95% CI, 0.74-0.78) compared with the control group, but a 5% higher risk for any neurologic disorder (IRR, 1.05; 95% CI, 1.04-1.07).
Age, sex, and variant had less influence on risk than infection severity, where the differences between hospitalized and nonhospitalized patients were significant.
Compared with COVID-negative individuals, the risk for any psychiatric disorder was double for hospitalized patients (IRR, 2.05; 95% CI, 1.78-2.37) but was 25% lower among nonhospitalized patients (IRR, 0.75; 95% CI, 0.73-0.77).
For neurologic disorders, the IRR for hospitalized patients was 2.44 (95% CI, 2.29-2.60) compared with COVID-negative individuals vs an IRR of only 1.02 (95% CI, 1.01-1.04) among nonhospitalized patients.
“In a general population, there was little support for clinically relevant post-acute risk increases of psychiatric and neurologic disorders associated with SARS-CoV-2 infection without hospitalization. This was particularly true for vaccinated individuals and for the more recent variants,” the authors wrote, adding that the only exception was for change in sense and smell.
‘Flaws’ in Previous Studies?
The findings in hospitalized patients were in line with previous findings, but those in nonhospitalized patients stand out, they added.
Previous studies were done predominantly in older males with comorbidities and those who were more socioeconomically disadvantaged, which could lead to a bias, Dr. Hviid said.
Those other studies “had a number of fundamental flaws that we do not believe our study has,” Dr. Hviid said. “Our study was conducted in the general population, with free and universal testing and healthcare.”
Researchers stress that sequelae after infection are predominantly associated with severe illness.
“Today, a healthy vaccinated adult having an asymptomatic or mild bout of COVID-19 with the current variants shouldn’t fear developing serious psychiatric or neurologic disorders in the months or years after infection.”
One limitation is that only hospital contacts were included, omitting possible diagnoses given outside hospital settings.
‘Extreme Caution’ Required
The link between COVID-19 and brain health is “complex,” and the new findings should be viewed cautiously, said Maxime Taquet, MRCPsych, PhD, National Institute for Health and Care Research clinical lecturer and specialty registrar in Psychiatry, Oxford Health NHS Foundation Trust, England, who commented on the findings.
Previous research by Dr. Taquet, who was not involved in the current study, found an increased risk for neurologic and psychiatric diagnoses during the first 6 months after COVID-19 diagnosis.
The current study “contributes to better understanding this link by providing data from another country with a different organization of healthcare provision than the US, where most of the existing data come from,” Dr. Taquet said.
However, “some observations — for example, that COVID-19 is associated with a 50% reduction in the risk of autism, a condition present from very early in life — call for extreme caution in the interpretation of the findings, as they suggest that residual bias has not been accounted for,” Dr. Taquet continued.
Authors of an accompanying editorial, Eric Chow, MD, MS, MPH, of the Division of Allergy and Infectious Diseases, University of Washington, School of Public Health, and Anita Chopra, MD, of the post-COVID Clinic, University of Washington, Seattle, called the study a “critical contribution to the published literature.”
The association of neurologic and psychiatric diagnoses with severe disease “is a reminder of the importance of risk reduction by combining vaccinations with improved indoor ventilation and masking,” they concluded.
The study was supported by a grant from the Independent Research Fund Denmark. Dr. Hviid and coauthors, Dr. Chopra, and Dr. Taquet reported no relevant financial relationships. Dr. Chow received a travel award from the Infectious Diseases Society of America to attend ID Week 2022.
A version of this article appeared on Medscape.com.
While severe COVID-19 is associated with a significantly higher risk for psychiatric and neurologic disorders a year after infection, mild does not carry the same risk, a new study shows.
However, less severe COVID-19 was not linked to a higher incidence of psychiatric diagnoses and was associated with only a slightly higher risk for neurologic disorders.
The new research challenges previous findings of long-term risk for psychiatric and neurologic disorders associated with SARS-CoV-2 in patients who had not been hospitalized for the condition.
“Our study does not support previous findings of substantial post-acute neurologic and psychiatric morbidities among the general population of SARS-CoV-2-infected individuals but does corroborate an elevated risk among the most severe cases with COVID-19,” the authors wrote.
The study was published online on February 21 in Neurology.
‘Alarming’ Findings
Previous studies have reported nervous system symptoms in patients who have experienced COVID-19, which may persist for several weeks or months after the acute phase, even in milder cases.
But these findings haven’t been consistent across all studies, and few studies have addressed the potential effect of different viral variants and vaccination status on post-acute psychiatric and neurologic morbidities.
“Our study was partly motivated by our strong research interest in the associations between infectious disease and later chronic disease and partly by international studies, such as those conducted in the US Veterans Health databases, that have suggested substantial risks of psychiatric and neurological conditions associated with infection,” senior author Anders Hviid, MSc, DrMedSci, head of the department and professor of pharmacoepidemiology, Statens Serum Institut, Copenhagen, Denmark, told this news organization.
Investigators drew on data from the Danish National Patient Registry to compare the risk for neurologic and psychiatric disorders during the 12 months after acute COVID-19 infection to risk among people who never tested positive.
They examined data on all recorded hospital contacts between January 2005 and January 2023 for a discharge diagnosis of at least one of 11 psychiatric illnesses or at least one of 30 neurologic disorders.
The researchers compared the incidence of each disorder within 1-12 months after infection with those of COVID-naive individuals and stratified analyses according to time since infection, vaccination status, variant period, age, sex, and infection severity.
The final study cohort included 1.8 million individuals who tested positive during the study period and 1.5 who didn’t. Three quarters of those who tested positive were infected primarily with the Omicron variant.
Hospitalized vs Nonhospitalized
Overall, individuals who tested positive had a 24% lower risk for psychiatric disorders during the post-acute period (incident rate ratio [IRR], 0.76; 95% CI, 0.74-0.78) compared with the control group, but a 5% higher risk for any neurologic disorder (IRR, 1.05; 95% CI, 1.04-1.07).
Age, sex, and variant had less influence on risk than infection severity, where the differences between hospitalized and nonhospitalized patients were significant.
Compared with COVID-negative individuals, the risk for any psychiatric disorder was double for hospitalized patients (IRR, 2.05; 95% CI, 1.78-2.37) but was 25% lower among nonhospitalized patients (IRR, 0.75; 95% CI, 0.73-0.77).
For neurologic disorders, the IRR for hospitalized patients was 2.44 (95% CI, 2.29-2.60) compared with COVID-negative individuals vs an IRR of only 1.02 (95% CI, 1.01-1.04) among nonhospitalized patients.
“In a general population, there was little support for clinically relevant post-acute risk increases of psychiatric and neurologic disorders associated with SARS-CoV-2 infection without hospitalization. This was particularly true for vaccinated individuals and for the more recent variants,” the authors wrote, adding that the only exception was for change in sense and smell.
‘Flaws’ in Previous Studies?
The findings in hospitalized patients were in line with previous findings, but those in nonhospitalized patients stand out, they added.
Previous studies were done predominantly in older males with comorbidities and those who were more socioeconomically disadvantaged, which could lead to a bias, Dr. Hviid said.
Those other studies “had a number of fundamental flaws that we do not believe our study has,” Dr. Hviid said. “Our study was conducted in the general population, with free and universal testing and healthcare.”
Researchers stress that sequelae after infection are predominantly associated with severe illness.
“Today, a healthy vaccinated adult having an asymptomatic or mild bout of COVID-19 with the current variants shouldn’t fear developing serious psychiatric or neurologic disorders in the months or years after infection.”
One limitation is that only hospital contacts were included, omitting possible diagnoses given outside hospital settings.
‘Extreme Caution’ Required
The link between COVID-19 and brain health is “complex,” and the new findings should be viewed cautiously, said Maxime Taquet, MRCPsych, PhD, National Institute for Health and Care Research clinical lecturer and specialty registrar in Psychiatry, Oxford Health NHS Foundation Trust, England, who commented on the findings.
Previous research by Dr. Taquet, who was not involved in the current study, found an increased risk for neurologic and psychiatric diagnoses during the first 6 months after COVID-19 diagnosis.
The current study “contributes to better understanding this link by providing data from another country with a different organization of healthcare provision than the US, where most of the existing data come from,” Dr. Taquet said.
However, “some observations — for example, that COVID-19 is associated with a 50% reduction in the risk of autism, a condition present from very early in life — call for extreme caution in the interpretation of the findings, as they suggest that residual bias has not been accounted for,” Dr. Taquet continued.
Authors of an accompanying editorial, Eric Chow, MD, MS, MPH, of the Division of Allergy and Infectious Diseases, University of Washington, School of Public Health, and Anita Chopra, MD, of the post-COVID Clinic, University of Washington, Seattle, called the study a “critical contribution to the published literature.”
The association of neurologic and psychiatric diagnoses with severe disease “is a reminder of the importance of risk reduction by combining vaccinations with improved indoor ventilation and masking,” they concluded.
The study was supported by a grant from the Independent Research Fund Denmark. Dr. Hviid and coauthors, Dr. Chopra, and Dr. Taquet reported no relevant financial relationships. Dr. Chow received a travel award from the Infectious Diseases Society of America to attend ID Week 2022.
A version of this article appeared on Medscape.com.
While severe COVID-19 is associated with a significantly higher risk for psychiatric and neurologic disorders a year after infection, mild does not carry the same risk, a new study shows.
However, less severe COVID-19 was not linked to a higher incidence of psychiatric diagnoses and was associated with only a slightly higher risk for neurologic disorders.
The new research challenges previous findings of long-term risk for psychiatric and neurologic disorders associated with SARS-CoV-2 in patients who had not been hospitalized for the condition.
“Our study does not support previous findings of substantial post-acute neurologic and psychiatric morbidities among the general population of SARS-CoV-2-infected individuals but does corroborate an elevated risk among the most severe cases with COVID-19,” the authors wrote.
The study was published online on February 21 in Neurology.
‘Alarming’ Findings
Previous studies have reported nervous system symptoms in patients who have experienced COVID-19, which may persist for several weeks or months after the acute phase, even in milder cases.
But these findings haven’t been consistent across all studies, and few studies have addressed the potential effect of different viral variants and vaccination status on post-acute psychiatric and neurologic morbidities.
“Our study was partly motivated by our strong research interest in the associations between infectious disease and later chronic disease and partly by international studies, such as those conducted in the US Veterans Health databases, that have suggested substantial risks of psychiatric and neurological conditions associated with infection,” senior author Anders Hviid, MSc, DrMedSci, head of the department and professor of pharmacoepidemiology, Statens Serum Institut, Copenhagen, Denmark, told this news organization.
Investigators drew on data from the Danish National Patient Registry to compare the risk for neurologic and psychiatric disorders during the 12 months after acute COVID-19 infection to risk among people who never tested positive.
They examined data on all recorded hospital contacts between January 2005 and January 2023 for a discharge diagnosis of at least one of 11 psychiatric illnesses or at least one of 30 neurologic disorders.
The researchers compared the incidence of each disorder within 1-12 months after infection with those of COVID-naive individuals and stratified analyses according to time since infection, vaccination status, variant period, age, sex, and infection severity.
The final study cohort included 1.8 million individuals who tested positive during the study period and 1.5 who didn’t. Three quarters of those who tested positive were infected primarily with the Omicron variant.
Hospitalized vs Nonhospitalized
Overall, individuals who tested positive had a 24% lower risk for psychiatric disorders during the post-acute period (incident rate ratio [IRR], 0.76; 95% CI, 0.74-0.78) compared with the control group, but a 5% higher risk for any neurologic disorder (IRR, 1.05; 95% CI, 1.04-1.07).
Age, sex, and variant had less influence on risk than infection severity, where the differences between hospitalized and nonhospitalized patients were significant.
Compared with COVID-negative individuals, the risk for any psychiatric disorder was double for hospitalized patients (IRR, 2.05; 95% CI, 1.78-2.37) but was 25% lower among nonhospitalized patients (IRR, 0.75; 95% CI, 0.73-0.77).
For neurologic disorders, the IRR for hospitalized patients was 2.44 (95% CI, 2.29-2.60) compared with COVID-negative individuals vs an IRR of only 1.02 (95% CI, 1.01-1.04) among nonhospitalized patients.
“In a general population, there was little support for clinically relevant post-acute risk increases of psychiatric and neurologic disorders associated with SARS-CoV-2 infection without hospitalization. This was particularly true for vaccinated individuals and for the more recent variants,” the authors wrote, adding that the only exception was for change in sense and smell.
‘Flaws’ in Previous Studies?
The findings in hospitalized patients were in line with previous findings, but those in nonhospitalized patients stand out, they added.
Previous studies were done predominantly in older males with comorbidities and those who were more socioeconomically disadvantaged, which could lead to a bias, Dr. Hviid said.
Those other studies “had a number of fundamental flaws that we do not believe our study has,” Dr. Hviid said. “Our study was conducted in the general population, with free and universal testing and healthcare.”
Researchers stress that sequelae after infection are predominantly associated with severe illness.
“Today, a healthy vaccinated adult having an asymptomatic or mild bout of COVID-19 with the current variants shouldn’t fear developing serious psychiatric or neurologic disorders in the months or years after infection.”
One limitation is that only hospital contacts were included, omitting possible diagnoses given outside hospital settings.
‘Extreme Caution’ Required
The link between COVID-19 and brain health is “complex,” and the new findings should be viewed cautiously, said Maxime Taquet, MRCPsych, PhD, National Institute for Health and Care Research clinical lecturer and specialty registrar in Psychiatry, Oxford Health NHS Foundation Trust, England, who commented on the findings.
Previous research by Dr. Taquet, who was not involved in the current study, found an increased risk for neurologic and psychiatric diagnoses during the first 6 months after COVID-19 diagnosis.
The current study “contributes to better understanding this link by providing data from another country with a different organization of healthcare provision than the US, where most of the existing data come from,” Dr. Taquet said.
However, “some observations — for example, that COVID-19 is associated with a 50% reduction in the risk of autism, a condition present from very early in life — call for extreme caution in the interpretation of the findings, as they suggest that residual bias has not been accounted for,” Dr. Taquet continued.
Authors of an accompanying editorial, Eric Chow, MD, MS, MPH, of the Division of Allergy and Infectious Diseases, University of Washington, School of Public Health, and Anita Chopra, MD, of the post-COVID Clinic, University of Washington, Seattle, called the study a “critical contribution to the published literature.”
The association of neurologic and psychiatric diagnoses with severe disease “is a reminder of the importance of risk reduction by combining vaccinations with improved indoor ventilation and masking,” they concluded.
The study was supported by a grant from the Independent Research Fund Denmark. Dr. Hviid and coauthors, Dr. Chopra, and Dr. Taquet reported no relevant financial relationships. Dr. Chow received a travel award from the Infectious Diseases Society of America to attend ID Week 2022.
A version of this article appeared on Medscape.com.
COVID-19 Is a Very Weird Virus
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
In the early days of the pandemic, before we really understood what COVID was, two specialties in the hospital had a foreboding sense that something was very strange about this virus. The first was the pulmonologists, who noticed the striking levels of hypoxemia — low oxygen in the blood — and the rapidity with which patients who had previously been stable would crash in the intensive care unit.
The second, and I mark myself among this group, were the nephrologists. The dialysis machines stopped working right. I remember rounding on patients in the hospital who were on dialysis for kidney failure in the setting of severe COVID infection and seeing clots forming on the dialysis filters. Some patients could barely get in a full treatment because the filters would clog so quickly.
We knew it was worse than flu because of the mortality rates, but these oddities made us realize that it was different too — not just a particularly nasty respiratory virus but one that had effects on the body that we hadn’t really seen before.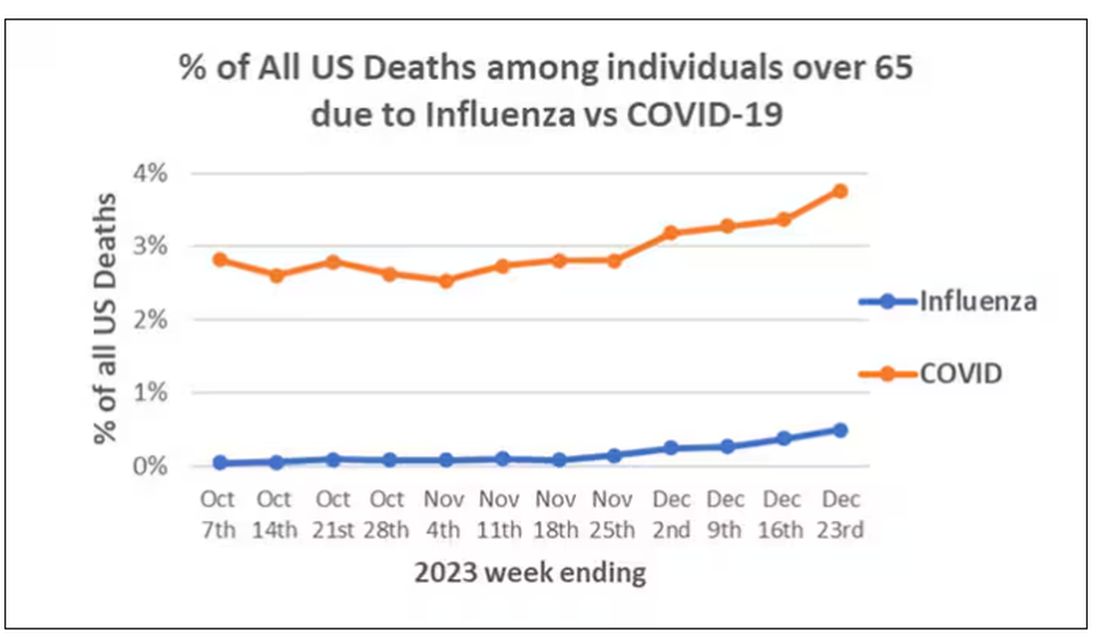
That’s why I’ve always been interested in studies that compare what happens to patients after COVID infection vs what happens to patients after other respiratory infections. This week, we’ll look at an intriguing study that suggests that COVID may lead to autoimmune diseases like rheumatoid arthritis, lupus, and vasculitis.
The study appears in the Annals of Internal Medicine and is made possible by the universal electronic health record systems of South Korea and Japan, who collaborated to create a truly staggering cohort of more than 20 million individuals living in those countries from 2020 to 2021.
The exposure of interest? COVID infection, experienced by just under 5% of that cohort over the study period. (Remember, there was a time when COVID infections were relatively controlled, particularly in some countries.)
The researchers wanted to compare the risk for autoimmune disease among COVID-infected individuals against two control groups. The first control group was the general population. This is interesting but a difficult analysis, because people who become infected with COVID might be very different from the general population. The second control group was people infected with influenza. I like this a lot better; the risk factors for COVID and influenza are quite similar, and the fact that this group was diagnosed with flu means at least that they are getting medical care and are sort of “in the system,” so to speak.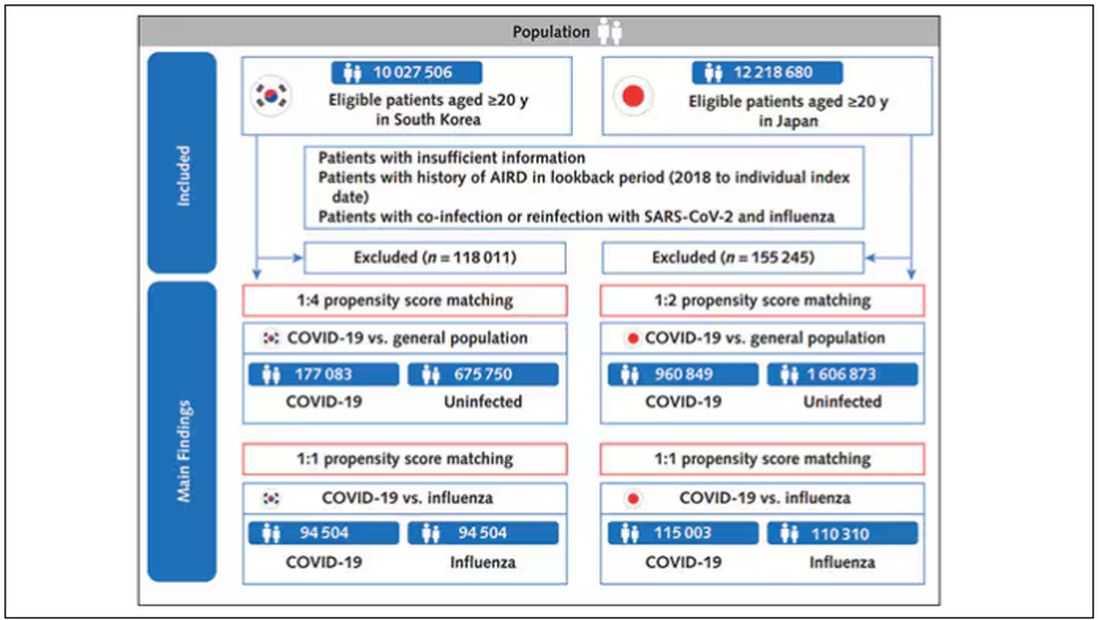
But it’s not enough to simply identify these folks and see who ends up with more autoimmune disease. The authors used propensity score matching to pair individuals infected with COVID with individuals from the control groups who were very similar to them. I’ve talked about this strategy before, but the basic idea is that you build a model predicting the likelihood of infection with COVID, based on a slew of factors — and the slew these authors used is pretty big, as shown below — and then stick people with similar risk for COVID together, with one member of the pair having had COVID and the other having eluded it (at least for the study period).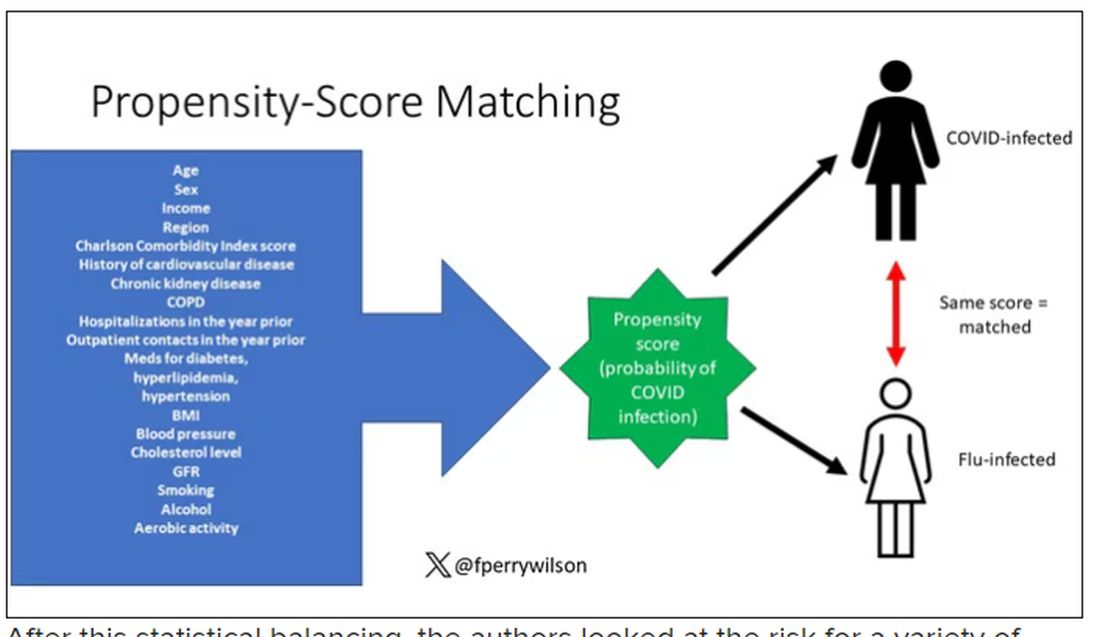
After this statistical balancing, the authors looked at the risk for a variety of autoimmune diseases.
Compared with those infected with flu, those infected with COVID were more likely to be diagnosed with any autoimmune condition, connective tissue disease, and, in Japan at least, inflammatory arthritis.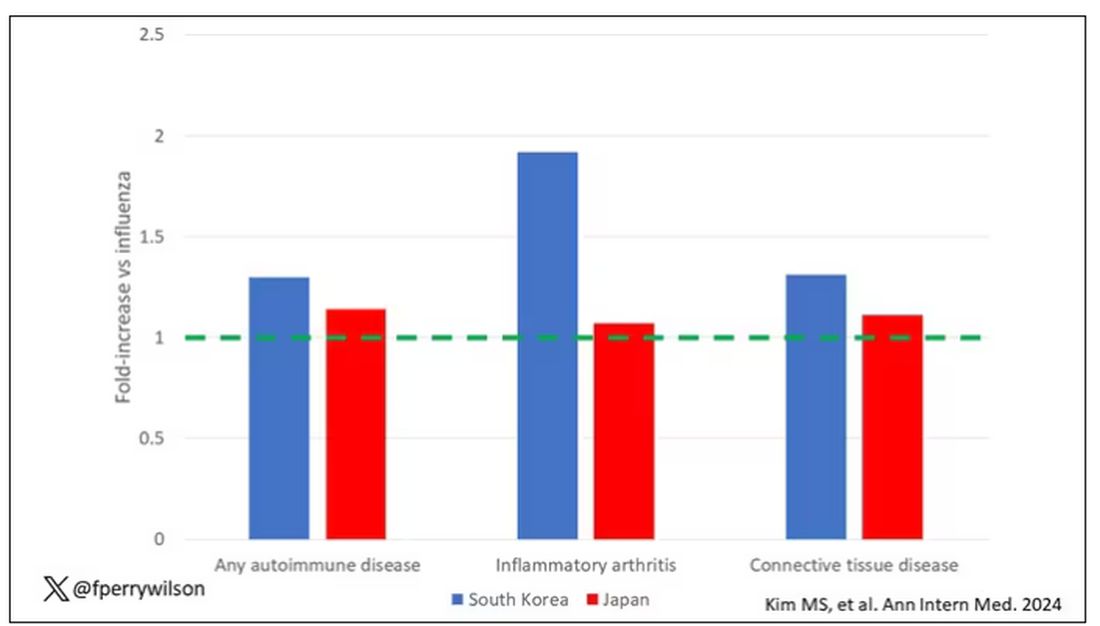
The authors acknowledge that being diagnosed with a disease might not be the same as actually having the disease, so in another analysis they looked only at people who received treatment for the autoimmune conditions, and the signals were even stronger in that group.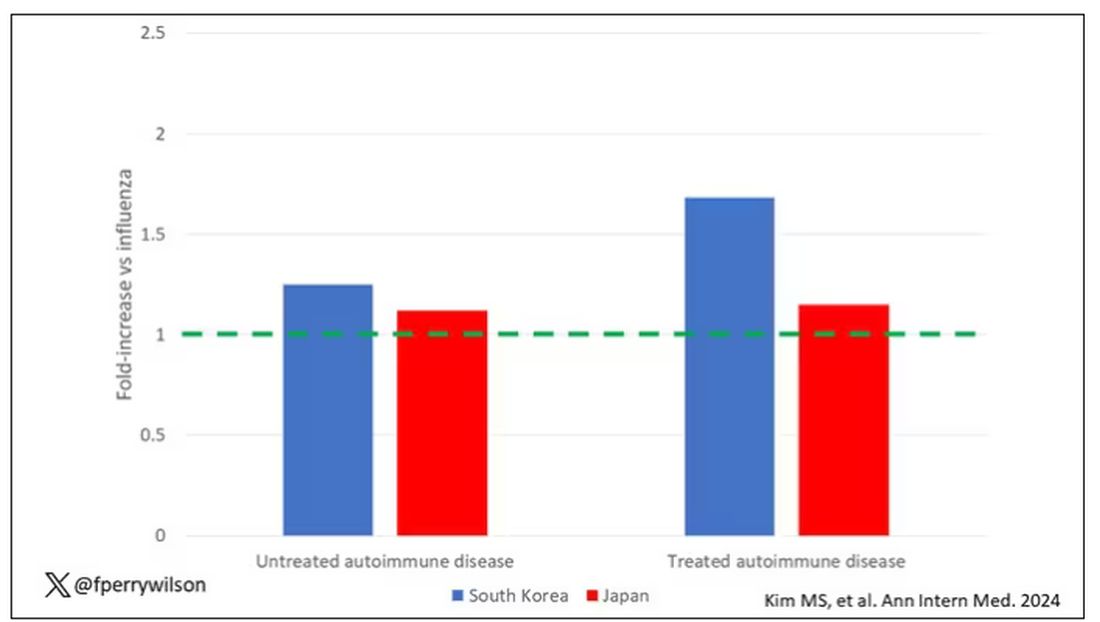
This risk seemed to be highest in the 6 months following the COVID infection, which makes sense biologically if we think that the infection is somehow screwing up the immune system.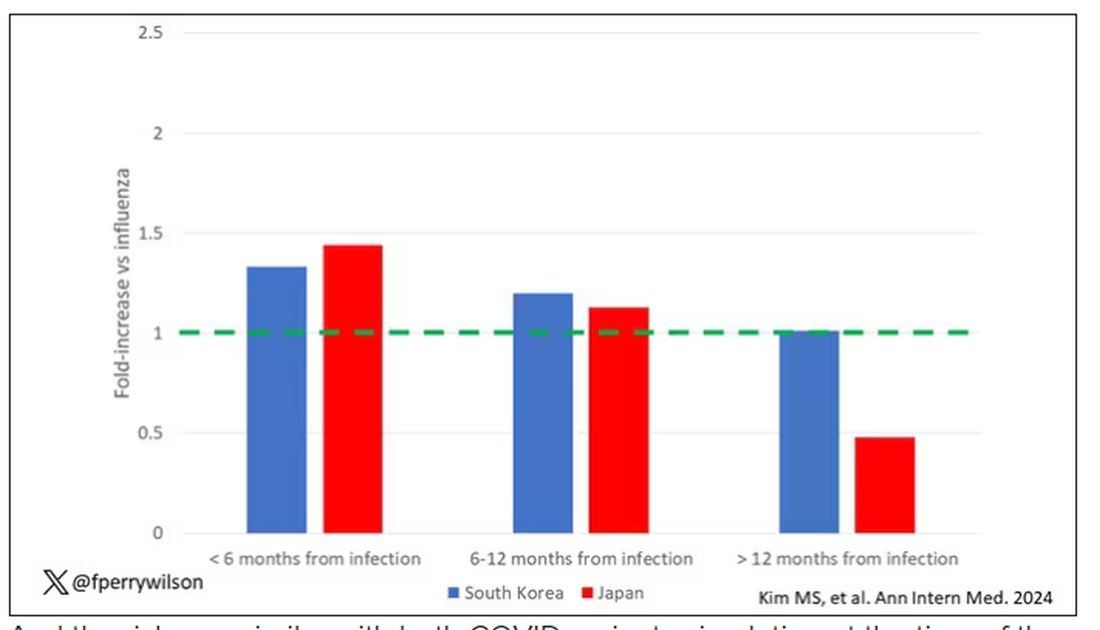
And the risk was similar with both COVID variants circulating at the time of the study.
The only factor that reduced the risk? You guessed it: vaccination. This is a particularly interesting finding because the exposure cohort was defined by having been infected with COVID. Therefore, the mechanism of protection is not prevention of infection; it’s something else. Perhaps vaccination helps to get the immune system in a state to respond to COVID infection more… appropriately?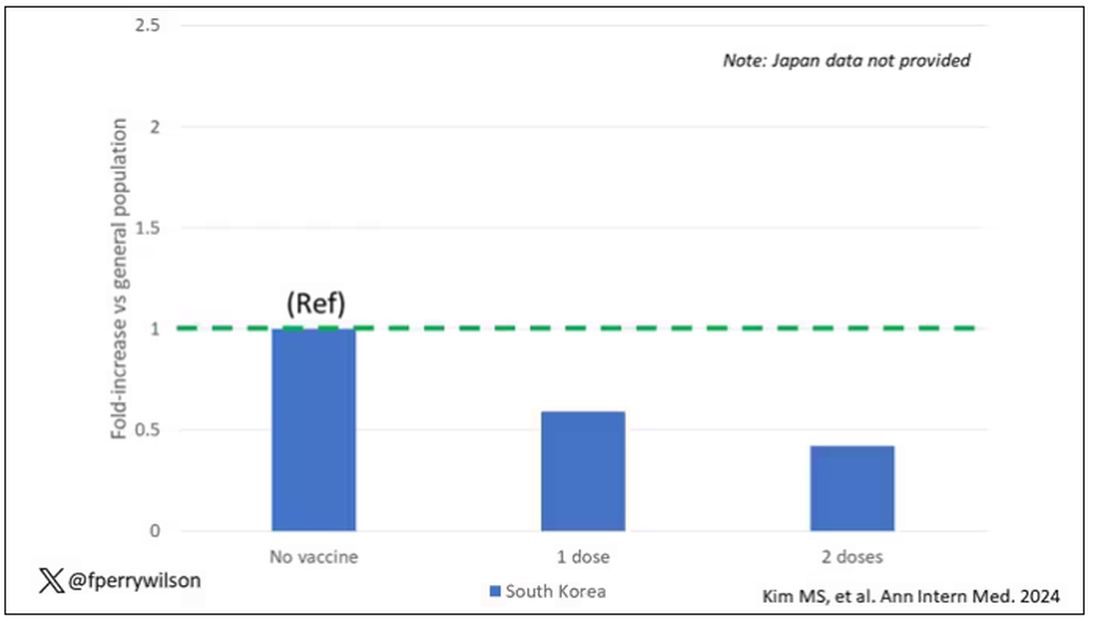
Yes, this study is observational. We can’t draw causal conclusions here. But it does reinforce my long-held belief that COVID is a weird virus, one with effects that are different from the respiratory viruses we are used to. I can’t say for certain whether COVID causes immune system dysfunction that puts someone at risk for autoimmunity — not from this study. But I can say it wouldn’t surprise me.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
In the early days of the pandemic, before we really understood what COVID was, two specialties in the hospital had a foreboding sense that something was very strange about this virus. The first was the pulmonologists, who noticed the striking levels of hypoxemia — low oxygen in the blood — and the rapidity with which patients who had previously been stable would crash in the intensive care unit.
The second, and I mark myself among this group, were the nephrologists. The dialysis machines stopped working right. I remember rounding on patients in the hospital who were on dialysis for kidney failure in the setting of severe COVID infection and seeing clots forming on the dialysis filters. Some patients could barely get in a full treatment because the filters would clog so quickly.
We knew it was worse than flu because of the mortality rates, but these oddities made us realize that it was different too — not just a particularly nasty respiratory virus but one that had effects on the body that we hadn’t really seen before.
That’s why I’ve always been interested in studies that compare what happens to patients after COVID infection vs what happens to patients after other respiratory infections. This week, we’ll look at an intriguing study that suggests that COVID may lead to autoimmune diseases like rheumatoid arthritis, lupus, and vasculitis.
The study appears in the Annals of Internal Medicine and is made possible by the universal electronic health record systems of South Korea and Japan, who collaborated to create a truly staggering cohort of more than 20 million individuals living in those countries from 2020 to 2021.
The exposure of interest? COVID infection, experienced by just under 5% of that cohort over the study period. (Remember, there was a time when COVID infections were relatively controlled, particularly in some countries.)
The researchers wanted to compare the risk for autoimmune disease among COVID-infected individuals against two control groups. The first control group was the general population. This is interesting but a difficult analysis, because people who become infected with COVID might be very different from the general population. The second control group was people infected with influenza. I like this a lot better; the risk factors for COVID and influenza are quite similar, and the fact that this group was diagnosed with flu means at least that they are getting medical care and are sort of “in the system,” so to speak.
But it’s not enough to simply identify these folks and see who ends up with more autoimmune disease. The authors used propensity score matching to pair individuals infected with COVID with individuals from the control groups who were very similar to them. I’ve talked about this strategy before, but the basic idea is that you build a model predicting the likelihood of infection with COVID, based on a slew of factors — and the slew these authors used is pretty big, as shown below — and then stick people with similar risk for COVID together, with one member of the pair having had COVID and the other having eluded it (at least for the study period).
After this statistical balancing, the authors looked at the risk for a variety of autoimmune diseases.
Compared with those infected with flu, those infected with COVID were more likely to be diagnosed with any autoimmune condition, connective tissue disease, and, in Japan at least, inflammatory arthritis.
The authors acknowledge that being diagnosed with a disease might not be the same as actually having the disease, so in another analysis they looked only at people who received treatment for the autoimmune conditions, and the signals were even stronger in that group.
This risk seemed to be highest in the 6 months following the COVID infection, which makes sense biologically if we think that the infection is somehow screwing up the immune system.
And the risk was similar with both COVID variants circulating at the time of the study.
The only factor that reduced the risk? You guessed it: vaccination. This is a particularly interesting finding because the exposure cohort was defined by having been infected with COVID. Therefore, the mechanism of protection is not prevention of infection; it’s something else. Perhaps vaccination helps to get the immune system in a state to respond to COVID infection more… appropriately?
Yes, this study is observational. We can’t draw causal conclusions here. But it does reinforce my long-held belief that COVID is a weird virus, one with effects that are different from the respiratory viruses we are used to. I can’t say for certain whether COVID causes immune system dysfunction that puts someone at risk for autoimmunity — not from this study. But I can say it wouldn’t surprise me.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
In the early days of the pandemic, before we really understood what COVID was, two specialties in the hospital had a foreboding sense that something was very strange about this virus. The first was the pulmonologists, who noticed the striking levels of hypoxemia — low oxygen in the blood — and the rapidity with which patients who had previously been stable would crash in the intensive care unit.
The second, and I mark myself among this group, were the nephrologists. The dialysis machines stopped working right. I remember rounding on patients in the hospital who were on dialysis for kidney failure in the setting of severe COVID infection and seeing clots forming on the dialysis filters. Some patients could barely get in a full treatment because the filters would clog so quickly.
We knew it was worse than flu because of the mortality rates, but these oddities made us realize that it was different too — not just a particularly nasty respiratory virus but one that had effects on the body that we hadn’t really seen before.
That’s why I’ve always been interested in studies that compare what happens to patients after COVID infection vs what happens to patients after other respiratory infections. This week, we’ll look at an intriguing study that suggests that COVID may lead to autoimmune diseases like rheumatoid arthritis, lupus, and vasculitis.
The study appears in the Annals of Internal Medicine and is made possible by the universal electronic health record systems of South Korea and Japan, who collaborated to create a truly staggering cohort of more than 20 million individuals living in those countries from 2020 to 2021.
The exposure of interest? COVID infection, experienced by just under 5% of that cohort over the study period. (Remember, there was a time when COVID infections were relatively controlled, particularly in some countries.)
The researchers wanted to compare the risk for autoimmune disease among COVID-infected individuals against two control groups. The first control group was the general population. This is interesting but a difficult analysis, because people who become infected with COVID might be very different from the general population. The second control group was people infected with influenza. I like this a lot better; the risk factors for COVID and influenza are quite similar, and the fact that this group was diagnosed with flu means at least that they are getting medical care and are sort of “in the system,” so to speak.
But it’s not enough to simply identify these folks and see who ends up with more autoimmune disease. The authors used propensity score matching to pair individuals infected with COVID with individuals from the control groups who were very similar to them. I’ve talked about this strategy before, but the basic idea is that you build a model predicting the likelihood of infection with COVID, based on a slew of factors — and the slew these authors used is pretty big, as shown below — and then stick people with similar risk for COVID together, with one member of the pair having had COVID and the other having eluded it (at least for the study period).
After this statistical balancing, the authors looked at the risk for a variety of autoimmune diseases.
Compared with those infected with flu, those infected with COVID were more likely to be diagnosed with any autoimmune condition, connective tissue disease, and, in Japan at least, inflammatory arthritis.
The authors acknowledge that being diagnosed with a disease might not be the same as actually having the disease, so in another analysis they looked only at people who received treatment for the autoimmune conditions, and the signals were even stronger in that group.
This risk seemed to be highest in the 6 months following the COVID infection, which makes sense biologically if we think that the infection is somehow screwing up the immune system.
And the risk was similar with both COVID variants circulating at the time of the study.
The only factor that reduced the risk? You guessed it: vaccination. This is a particularly interesting finding because the exposure cohort was defined by having been infected with COVID. Therefore, the mechanism of protection is not prevention of infection; it’s something else. Perhaps vaccination helps to get the immune system in a state to respond to COVID infection more… appropriately?
Yes, this study is observational. We can’t draw causal conclusions here. But it does reinforce my long-held belief that COVID is a weird virus, one with effects that are different from the respiratory viruses we are used to. I can’t say for certain whether COVID causes immune system dysfunction that puts someone at risk for autoimmunity — not from this study. But I can say it wouldn’t surprise me.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Increased Risk of New Rheumatic Disease Follows COVID-19 Infection
The risk of developing a new autoimmune inflammatory rheumatic disease (AIRD) is greater following a COVID-19 infection than after an influenza infection or in the general population, according to a study published March 5 in Annals of Internal Medicine. More severe COVID-19 infections were linked to a greater risk of incident rheumatic disease, but vaccination appeared protective against development of a new AIRD.
“Importantly, this study shows the value of vaccination to prevent severe disease and these types of sequelae,” Anne Davidson, MBBS, a professor in the Institute of Molecular Medicine at The Feinstein Institutes for Medical Research in Manhasset, New York, who was not involved in the study, said in an interview.
Previous research had already identified the likelihood of an association between SARS-CoV-2 infection and subsequent development of a new AIRD. This new study, however, includes much larger cohorts from two different countries and relies on more robust methodology than previous studies, experts said.
“Unique steps were taken by the study authors to make sure that what they were looking at in terms of signal was most likely true,” Alfred Kim, MD, PhD, assistant professor of medicine in rheumatology at Washington University in St. Louis, who was not involved in the study, said in an interview. Dr. Davidson agreed, noting that these authors “were a bit more rigorous with ascertainment of the autoimmune diagnosis, using two codes and also checking that appropriate medications were administered.”
More Robust and Rigorous Research
Past cohort studies finding an increased risk of rheumatic disease after COVID-19 “based their findings solely on comparisons between infected and uninfected groups, which could be influenced by ascertainment bias due to disparities in care, differences in health-seeking tendencies, and inherent risks among the groups,” Min Seo Kim, MD, of the Broad Institute of MIT and Harvard, Cambridge, Massachusetts, and his colleagues reported. Their study, however, required at least two claims with codes for rheumatic disease and compared patients with COVID-19 to those with flu “to adjust for the potentially heightened detection of AIRD in SARS-CoV-2–infected persons owing to their interactions with the health care system.”
Dr. Alfred Kim said the fact that they used at least two claims codes “gives a little more credence that the patients were actually experiencing some sort of autoimmune inflammatory condition as opposed to a very transient issue post COVID that just went away on its own.”
He acknowledged that the previous research was reasonably strong, “especially in light of the fact that there has been so much work done on a molecular level demonstrating that COVID-19 is associated with a substantial increase in autoantibodies in a significant proportion of patients, so this always opened up the possibility that this could associate with some sort of autoimmune disease downstream.”
While the study is well done with a large population, “it still has limitations that might overestimate the effect,” Kevin W. Byram, MD, associate professor of medicine in rheumatology and immunology at Vanderbilt University Medical Center in Nashville, Tennessee, who was not involved in the study, said in an interview. “We certainly have seen individual cases of new rheumatic disease where COVID-19 infection is likely the trigger,” but the phenomenon is not new, he added.
“Many autoimmune diseases are spurred by a loss of tolerance that might be induced by a pathogen of some sort,” Dr. Byram said. “The study is right to point out different forms of bias that might be at play. One in particular that is important to consider in a study like this is the lack of case-level adjudication regarding the diagnosis of rheumatic disease” since the study relied on available ICD-10 codes and medication prescriptions.
The researchers used national claims data to compare risk of incident AIRD in 10,027,506 South Korean and 12,218,680 Japanese adults, aged 20 and older, at 1 month, 6 months, and 12 months after COVID-19 infection, influenza infection, or a matched index date for uninfected control participants. Only patients with at least two claims for AIRD were considered to have a new diagnosis.
Patients who had COVID-19 between January 2020 and December 2021, confirmed by PCR or antigen testing, were matched 1:1 with patients who had test-confirmed influenza during that time and 1:4 with uninfected control participants, whose index date was set to the infection date of their matched COVID-19 patient.
The propensity score matching was based on age, sex, household income, urban versus rural residence, and various clinical characteristics and history: body mass index; blood pressure; fasting blood glucose; glomerular filtration rate; smoking status; alcohol consumption; weekly aerobic physical activity; comorbidity index; hospitalizations and outpatient visits in the previous year; past use of diabetes, hyperlipidemia, or hypertension medication; and history of cardiovascular disease, chronic kidney disease, chronic obstructive pulmonary disease, or respiratory infectious disease.
Patients with a history of AIRD or with coinfection or reinfection of COVID-19 and influenza were excluded, as were patients diagnosed with rheumatic disease within a month of COVID-19 infection.
Risk Varied With Disease Severity and Vaccination Status
Among the Korean patients, 3.9% had a COVID-19 infection and 0.98% had an influenza infection. After matching, the comparison populations included 94,504 patients with COVID-19 versus 94,504 patients with flu, and 177,083 patients with COVID-19 versus 675,750 uninfected controls.
The risk of developing an AIRD at least 1 month after infection in South Korean patients with COVID-19 was 25% higher than in uninfected control participants (adjusted hazard ratio [aHR], 1.25; 95% CI, 1.18–1.31; P < .05) and 30% higher than in influenza patients (aHR, 1.3; 95% CI, 1.02–1.59; P < .05). Specifically, risk in South Korean patients with COVID-19 was significantly increased for connective tissue disease and both treated and untreated AIRD but not for inflammatory arthritis.
Among the Japanese patients, 8.2% had COVID-19 and 0.99% had flu, resulting in matched populations of 115,003 with COVID-19 versus 110,310 with flu, and 960,849 with COVID-19 versus 1,606,873 uninfected patients. The effect size was larger in Japanese patients, with a 79% increased risk for AIRD in patients with COVID-19, compared with the general population (aHR, 1.79; 95% CI, 1.77–1.82; P < .05) and a 14% increased risk, compared with patients with influenza infection (aHR, 1.14; 95% CI, 1.10–1.17; P < .05). In Japanese patients, risk was increased across all four categories, including a doubled risk for inflammatory arthritis (aHR, 2.02; 95% CI, 1.96–2.07; P < .05), compared with the general population.
The researchers had data only from the South Korean cohort to calculate risk based on vaccination status, SARS-CoV-2 variant (wild type versus Delta), and COVID-19 severity. Researchers determined a COVID-19 infection to be moderate-to-severe based on billing codes for ICU admission or requiring oxygen therapy, extracorporeal membrane oxygenation, renal replacement, or CPR.
Infection with both the original strain and the Delta variant were linked to similar increased risks for AIRD, but moderate to severe COVID-19 infections had greater risk of subsequent AIRD (aHR, 1.42; P < .05) than mild infections (aHR, 1.22; P < .05). Vaccination was linked to a lower risk of AIRD within the COVID-19 patient population: One dose was linked to a 41% reduced risk (HR, 0.59; P < .05) and two doses were linked to a 58% reduced risk (HR, 0.42; P < .05), regardless of the vaccine type, compared with unvaccinated patients with COVID-19. The apparent protective effect of vaccination was true only for patients with mild COVID-19, not those with moderate to severe infection.
“One has to wonder whether or not these people were at much higher risk of developing autoimmune disease that just got exposed because they got COVID, so that a fraction of these would have gotten an autoimmune disease downstream,” Dr. Alfred Kim said. Regardless, one clinical implication of the findings is the reduced risk in vaccinated patients, regardless of the vaccine type, given the fact that “mRNA vaccination in particular has not been associated with any autoantibody development,” he said.
Though the correlations in the study cannot translate to causation, several mechanisms might be at play in a viral infection contributing to autoimmune risk, Dr. Davidson said. Given that viral nucleic acids also recognize self-nucleic acids, “a large load of viral nucleic acid may break tolerance,” or “viral proteins could also mimic self-proteins,” she said. “In addition, tolerance may be broken by a highly inflammatory environment associated with the release of cytokines and other inflammatory mediators.”
The association between new-onset autoimmune disease and severe COVID-19 infection suggests multiple mechanisms may be involved in excess immune stimulation, Dr. Davidson said. But she added that it’s unclear how these findings, involving the original strain and Delta variant of SARS-CoV-2, might relate to currently circulating variants.
The research was funded by the National Research Foundation of Korea, the Korea Health Industry Development Institute, and the Ministry of Food and Drug Safety of the Republic of Korea. The authors reported no relevant financial relationships with industry. Dr. Alfred Kim has sponsored research agreements with AstraZeneca, Bristol-Myers Squibb, and Novartis; receives royalties from a patent with Kypha Inc.; and has done consulting or speaking for Amgen, ANI Pharmaceuticals, Aurinia Pharmaceuticals, Exagen Diagnostics, GlaxoSmithKline, Kypha, Miltenyi Biotech, Pfizer, Rheumatology & Arthritis Learning Network, Synthekine, Techtonic Therapeutics, and UpToDate. Dr. Byram reported consulting for TenSixteen Bio. Dr. Davidson had no disclosures.
The risk of developing a new autoimmune inflammatory rheumatic disease (AIRD) is greater following a COVID-19 infection than after an influenza infection or in the general population, according to a study published March 5 in Annals of Internal Medicine. More severe COVID-19 infections were linked to a greater risk of incident rheumatic disease, but vaccination appeared protective against development of a new AIRD.
“Importantly, this study shows the value of vaccination to prevent severe disease and these types of sequelae,” Anne Davidson, MBBS, a professor in the Institute of Molecular Medicine at The Feinstein Institutes for Medical Research in Manhasset, New York, who was not involved in the study, said in an interview.
Previous research had already identified the likelihood of an association between SARS-CoV-2 infection and subsequent development of a new AIRD. This new study, however, includes much larger cohorts from two different countries and relies on more robust methodology than previous studies, experts said.
“Unique steps were taken by the study authors to make sure that what they were looking at in terms of signal was most likely true,” Alfred Kim, MD, PhD, assistant professor of medicine in rheumatology at Washington University in St. Louis, who was not involved in the study, said in an interview. Dr. Davidson agreed, noting that these authors “were a bit more rigorous with ascertainment of the autoimmune diagnosis, using two codes and also checking that appropriate medications were administered.”
More Robust and Rigorous Research
Past cohort studies finding an increased risk of rheumatic disease after COVID-19 “based their findings solely on comparisons between infected and uninfected groups, which could be influenced by ascertainment bias due to disparities in care, differences in health-seeking tendencies, and inherent risks among the groups,” Min Seo Kim, MD, of the Broad Institute of MIT and Harvard, Cambridge, Massachusetts, and his colleagues reported. Their study, however, required at least two claims with codes for rheumatic disease and compared patients with COVID-19 to those with flu “to adjust for the potentially heightened detection of AIRD in SARS-CoV-2–infected persons owing to their interactions with the health care system.”
Dr. Alfred Kim said the fact that they used at least two claims codes “gives a little more credence that the patients were actually experiencing some sort of autoimmune inflammatory condition as opposed to a very transient issue post COVID that just went away on its own.”
He acknowledged that the previous research was reasonably strong, “especially in light of the fact that there has been so much work done on a molecular level demonstrating that COVID-19 is associated with a substantial increase in autoantibodies in a significant proportion of patients, so this always opened up the possibility that this could associate with some sort of autoimmune disease downstream.”
While the study is well done with a large population, “it still has limitations that might overestimate the effect,” Kevin W. Byram, MD, associate professor of medicine in rheumatology and immunology at Vanderbilt University Medical Center in Nashville, Tennessee, who was not involved in the study, said in an interview. “We certainly have seen individual cases of new rheumatic disease where COVID-19 infection is likely the trigger,” but the phenomenon is not new, he added.
“Many autoimmune diseases are spurred by a loss of tolerance that might be induced by a pathogen of some sort,” Dr. Byram said. “The study is right to point out different forms of bias that might be at play. One in particular that is important to consider in a study like this is the lack of case-level adjudication regarding the diagnosis of rheumatic disease” since the study relied on available ICD-10 codes and medication prescriptions.
The researchers used national claims data to compare risk of incident AIRD in 10,027,506 South Korean and 12,218,680 Japanese adults, aged 20 and older, at 1 month, 6 months, and 12 months after COVID-19 infection, influenza infection, or a matched index date for uninfected control participants. Only patients with at least two claims for AIRD were considered to have a new diagnosis.
Patients who had COVID-19 between January 2020 and December 2021, confirmed by PCR or antigen testing, were matched 1:1 with patients who had test-confirmed influenza during that time and 1:4 with uninfected control participants, whose index date was set to the infection date of their matched COVID-19 patient.
The propensity score matching was based on age, sex, household income, urban versus rural residence, and various clinical characteristics and history: body mass index; blood pressure; fasting blood glucose; glomerular filtration rate; smoking status; alcohol consumption; weekly aerobic physical activity; comorbidity index; hospitalizations and outpatient visits in the previous year; past use of diabetes, hyperlipidemia, or hypertension medication; and history of cardiovascular disease, chronic kidney disease, chronic obstructive pulmonary disease, or respiratory infectious disease.
Patients with a history of AIRD or with coinfection or reinfection of COVID-19 and influenza were excluded, as were patients diagnosed with rheumatic disease within a month of COVID-19 infection.
Risk Varied With Disease Severity and Vaccination Status
Among the Korean patients, 3.9% had a COVID-19 infection and 0.98% had an influenza infection. After matching, the comparison populations included 94,504 patients with COVID-19 versus 94,504 patients with flu, and 177,083 patients with COVID-19 versus 675,750 uninfected controls.
The risk of developing an AIRD at least 1 month after infection in South Korean patients with COVID-19 was 25% higher than in uninfected control participants (adjusted hazard ratio [aHR], 1.25; 95% CI, 1.18–1.31; P < .05) and 30% higher than in influenza patients (aHR, 1.3; 95% CI, 1.02–1.59; P < .05). Specifically, risk in South Korean patients with COVID-19 was significantly increased for connective tissue disease and both treated and untreated AIRD but not for inflammatory arthritis.
Among the Japanese patients, 8.2% had COVID-19 and 0.99% had flu, resulting in matched populations of 115,003 with COVID-19 versus 110,310 with flu, and 960,849 with COVID-19 versus 1,606,873 uninfected patients. The effect size was larger in Japanese patients, with a 79% increased risk for AIRD in patients with COVID-19, compared with the general population (aHR, 1.79; 95% CI, 1.77–1.82; P < .05) and a 14% increased risk, compared with patients with influenza infection (aHR, 1.14; 95% CI, 1.10–1.17; P < .05). In Japanese patients, risk was increased across all four categories, including a doubled risk for inflammatory arthritis (aHR, 2.02; 95% CI, 1.96–2.07; P < .05), compared with the general population.
The researchers had data only from the South Korean cohort to calculate risk based on vaccination status, SARS-CoV-2 variant (wild type versus Delta), and COVID-19 severity. Researchers determined a COVID-19 infection to be moderate-to-severe based on billing codes for ICU admission or requiring oxygen therapy, extracorporeal membrane oxygenation, renal replacement, or CPR.
Infection with both the original strain and the Delta variant were linked to similar increased risks for AIRD, but moderate to severe COVID-19 infections had greater risk of subsequent AIRD (aHR, 1.42; P < .05) than mild infections (aHR, 1.22; P < .05). Vaccination was linked to a lower risk of AIRD within the COVID-19 patient population: One dose was linked to a 41% reduced risk (HR, 0.59; P < .05) and two doses were linked to a 58% reduced risk (HR, 0.42; P < .05), regardless of the vaccine type, compared with unvaccinated patients with COVID-19. The apparent protective effect of vaccination was true only for patients with mild COVID-19, not those with moderate to severe infection.
“One has to wonder whether or not these people were at much higher risk of developing autoimmune disease that just got exposed because they got COVID, so that a fraction of these would have gotten an autoimmune disease downstream,” Dr. Alfred Kim said. Regardless, one clinical implication of the findings is the reduced risk in vaccinated patients, regardless of the vaccine type, given the fact that “mRNA vaccination in particular has not been associated with any autoantibody development,” he said.
Though the correlations in the study cannot translate to causation, several mechanisms might be at play in a viral infection contributing to autoimmune risk, Dr. Davidson said. Given that viral nucleic acids also recognize self-nucleic acids, “a large load of viral nucleic acid may break tolerance,” or “viral proteins could also mimic self-proteins,” she said. “In addition, tolerance may be broken by a highly inflammatory environment associated with the release of cytokines and other inflammatory mediators.”
The association between new-onset autoimmune disease and severe COVID-19 infection suggests multiple mechanisms may be involved in excess immune stimulation, Dr. Davidson said. But she added that it’s unclear how these findings, involving the original strain and Delta variant of SARS-CoV-2, might relate to currently circulating variants.
The research was funded by the National Research Foundation of Korea, the Korea Health Industry Development Institute, and the Ministry of Food and Drug Safety of the Republic of Korea. The authors reported no relevant financial relationships with industry. Dr. Alfred Kim has sponsored research agreements with AstraZeneca, Bristol-Myers Squibb, and Novartis; receives royalties from a patent with Kypha Inc.; and has done consulting or speaking for Amgen, ANI Pharmaceuticals, Aurinia Pharmaceuticals, Exagen Diagnostics, GlaxoSmithKline, Kypha, Miltenyi Biotech, Pfizer, Rheumatology & Arthritis Learning Network, Synthekine, Techtonic Therapeutics, and UpToDate. Dr. Byram reported consulting for TenSixteen Bio. Dr. Davidson had no disclosures.
The risk of developing a new autoimmune inflammatory rheumatic disease (AIRD) is greater following a COVID-19 infection than after an influenza infection or in the general population, according to a study published March 5 in Annals of Internal Medicine. More severe COVID-19 infections were linked to a greater risk of incident rheumatic disease, but vaccination appeared protective against development of a new AIRD.
“Importantly, this study shows the value of vaccination to prevent severe disease and these types of sequelae,” Anne Davidson, MBBS, a professor in the Institute of Molecular Medicine at The Feinstein Institutes for Medical Research in Manhasset, New York, who was not involved in the study, said in an interview.
Previous research had already identified the likelihood of an association between SARS-CoV-2 infection and subsequent development of a new AIRD. This new study, however, includes much larger cohorts from two different countries and relies on more robust methodology than previous studies, experts said.
“Unique steps were taken by the study authors to make sure that what they were looking at in terms of signal was most likely true,” Alfred Kim, MD, PhD, assistant professor of medicine in rheumatology at Washington University in St. Louis, who was not involved in the study, said in an interview. Dr. Davidson agreed, noting that these authors “were a bit more rigorous with ascertainment of the autoimmune diagnosis, using two codes and also checking that appropriate medications were administered.”
More Robust and Rigorous Research
Past cohort studies finding an increased risk of rheumatic disease after COVID-19 “based their findings solely on comparisons between infected and uninfected groups, which could be influenced by ascertainment bias due to disparities in care, differences in health-seeking tendencies, and inherent risks among the groups,” Min Seo Kim, MD, of the Broad Institute of MIT and Harvard, Cambridge, Massachusetts, and his colleagues reported. Their study, however, required at least two claims with codes for rheumatic disease and compared patients with COVID-19 to those with flu “to adjust for the potentially heightened detection of AIRD in SARS-CoV-2–infected persons owing to their interactions with the health care system.”
Dr. Alfred Kim said the fact that they used at least two claims codes “gives a little more credence that the patients were actually experiencing some sort of autoimmune inflammatory condition as opposed to a very transient issue post COVID that just went away on its own.”
He acknowledged that the previous research was reasonably strong, “especially in light of the fact that there has been so much work done on a molecular level demonstrating that COVID-19 is associated with a substantial increase in autoantibodies in a significant proportion of patients, so this always opened up the possibility that this could associate with some sort of autoimmune disease downstream.”
While the study is well done with a large population, “it still has limitations that might overestimate the effect,” Kevin W. Byram, MD, associate professor of medicine in rheumatology and immunology at Vanderbilt University Medical Center in Nashville, Tennessee, who was not involved in the study, said in an interview. “We certainly have seen individual cases of new rheumatic disease where COVID-19 infection is likely the trigger,” but the phenomenon is not new, he added.
“Many autoimmune diseases are spurred by a loss of tolerance that might be induced by a pathogen of some sort,” Dr. Byram said. “The study is right to point out different forms of bias that might be at play. One in particular that is important to consider in a study like this is the lack of case-level adjudication regarding the diagnosis of rheumatic disease” since the study relied on available ICD-10 codes and medication prescriptions.
The researchers used national claims data to compare risk of incident AIRD in 10,027,506 South Korean and 12,218,680 Japanese adults, aged 20 and older, at 1 month, 6 months, and 12 months after COVID-19 infection, influenza infection, or a matched index date for uninfected control participants. Only patients with at least two claims for AIRD were considered to have a new diagnosis.
Patients who had COVID-19 between January 2020 and December 2021, confirmed by PCR or antigen testing, were matched 1:1 with patients who had test-confirmed influenza during that time and 1:4 with uninfected control participants, whose index date was set to the infection date of their matched COVID-19 patient.
The propensity score matching was based on age, sex, household income, urban versus rural residence, and various clinical characteristics and history: body mass index; blood pressure; fasting blood glucose; glomerular filtration rate; smoking status; alcohol consumption; weekly aerobic physical activity; comorbidity index; hospitalizations and outpatient visits in the previous year; past use of diabetes, hyperlipidemia, or hypertension medication; and history of cardiovascular disease, chronic kidney disease, chronic obstructive pulmonary disease, or respiratory infectious disease.
Patients with a history of AIRD or with coinfection or reinfection of COVID-19 and influenza were excluded, as were patients diagnosed with rheumatic disease within a month of COVID-19 infection.
Risk Varied With Disease Severity and Vaccination Status
Among the Korean patients, 3.9% had a COVID-19 infection and 0.98% had an influenza infection. After matching, the comparison populations included 94,504 patients with COVID-19 versus 94,504 patients with flu, and 177,083 patients with COVID-19 versus 675,750 uninfected controls.
The risk of developing an AIRD at least 1 month after infection in South Korean patients with COVID-19 was 25% higher than in uninfected control participants (adjusted hazard ratio [aHR], 1.25; 95% CI, 1.18–1.31; P < .05) and 30% higher than in influenza patients (aHR, 1.3; 95% CI, 1.02–1.59; P < .05). Specifically, risk in South Korean patients with COVID-19 was significantly increased for connective tissue disease and both treated and untreated AIRD but not for inflammatory arthritis.
Among the Japanese patients, 8.2% had COVID-19 and 0.99% had flu, resulting in matched populations of 115,003 with COVID-19 versus 110,310 with flu, and 960,849 with COVID-19 versus 1,606,873 uninfected patients. The effect size was larger in Japanese patients, with a 79% increased risk for AIRD in patients with COVID-19, compared with the general population (aHR, 1.79; 95% CI, 1.77–1.82; P < .05) and a 14% increased risk, compared with patients with influenza infection (aHR, 1.14; 95% CI, 1.10–1.17; P < .05). In Japanese patients, risk was increased across all four categories, including a doubled risk for inflammatory arthritis (aHR, 2.02; 95% CI, 1.96–2.07; P < .05), compared with the general population.
The researchers had data only from the South Korean cohort to calculate risk based on vaccination status, SARS-CoV-2 variant (wild type versus Delta), and COVID-19 severity. Researchers determined a COVID-19 infection to be moderate-to-severe based on billing codes for ICU admission or requiring oxygen therapy, extracorporeal membrane oxygenation, renal replacement, or CPR.
Infection with both the original strain and the Delta variant were linked to similar increased risks for AIRD, but moderate to severe COVID-19 infections had greater risk of subsequent AIRD (aHR, 1.42; P < .05) than mild infections (aHR, 1.22; P < .05). Vaccination was linked to a lower risk of AIRD within the COVID-19 patient population: One dose was linked to a 41% reduced risk (HR, 0.59; P < .05) and two doses were linked to a 58% reduced risk (HR, 0.42; P < .05), regardless of the vaccine type, compared with unvaccinated patients with COVID-19. The apparent protective effect of vaccination was true only for patients with mild COVID-19, not those with moderate to severe infection.
“One has to wonder whether or not these people were at much higher risk of developing autoimmune disease that just got exposed because they got COVID, so that a fraction of these would have gotten an autoimmune disease downstream,” Dr. Alfred Kim said. Regardless, one clinical implication of the findings is the reduced risk in vaccinated patients, regardless of the vaccine type, given the fact that “mRNA vaccination in particular has not been associated with any autoantibody development,” he said.
Though the correlations in the study cannot translate to causation, several mechanisms might be at play in a viral infection contributing to autoimmune risk, Dr. Davidson said. Given that viral nucleic acids also recognize self-nucleic acids, “a large load of viral nucleic acid may break tolerance,” or “viral proteins could also mimic self-proteins,” she said. “In addition, tolerance may be broken by a highly inflammatory environment associated with the release of cytokines and other inflammatory mediators.”
The association between new-onset autoimmune disease and severe COVID-19 infection suggests multiple mechanisms may be involved in excess immune stimulation, Dr. Davidson said. But she added that it’s unclear how these findings, involving the original strain and Delta variant of SARS-CoV-2, might relate to currently circulating variants.
The research was funded by the National Research Foundation of Korea, the Korea Health Industry Development Institute, and the Ministry of Food and Drug Safety of the Republic of Korea. The authors reported no relevant financial relationships with industry. Dr. Alfred Kim has sponsored research agreements with AstraZeneca, Bristol-Myers Squibb, and Novartis; receives royalties from a patent with Kypha Inc.; and has done consulting or speaking for Amgen, ANI Pharmaceuticals, Aurinia Pharmaceuticals, Exagen Diagnostics, GlaxoSmithKline, Kypha, Miltenyi Biotech, Pfizer, Rheumatology & Arthritis Learning Network, Synthekine, Techtonic Therapeutics, and UpToDate. Dr. Byram reported consulting for TenSixteen Bio. Dr. Davidson had no disclosures.
FROM ANNALS OF INTERNAL MEDICINE
Cognitive Deficits After Most Severe COVID Cases Associated With 9-Point IQ Drop
and which cognitive functions are most vulnerable.
In a large community sample, researchers found that on average, people who had recovered from COVID-19 showed small cognitive deficits equivalent to a 3-point loss in IQ for up to 1 year or more after recovering from the acute illness compared with peers who never had COVID-19.
However, people who had more severe cases, requiring treatment in a hospital intensive care unit, had cognitive deficits equivalent to a 9-point drop in IQ.
“People with ongoing persistent symptoms, indicative of long COVID, had larger cognitive deficits than people whose symptoms had resolved,” first author Adam Hampshire, PhD, with Imperial College London, told this news organization.
The largest deficits among cognitive tasks were in memory, reasoning, and executive function, he added.
“That is, people who had had COVID-19 were both slower and less accurate when performing tasks that measure those abilities,” Dr. Hampshire said. “The group with the largest cognitive deficits were patients who had been in intensive care for COVID-19.”
The study was published online in The New England Journal of Medicine.
Lingering Brain Fog
Cognitive symptoms after SARS-CoV-2 infection are well recognized, but whether objectively measurable cognitive deficits exist and how long they persist remains unclear.
To investigate, researchers invited 800,000 adults from the REACT study of SARS-CoV-2 transmission in England to complete an online assessment for cognitive function with eight domains.
Altogether, 141,583 participants started the cognitive battery by completing at least one task, and 112,964 completed all eight tasks.
The researchers estimated global cognitive scores among participants who had been previously infected with SARS-CoV-2 with symptoms that persisted for at least 12 weeks, whether or not resolved, and among uninfected participants.
Compared with uninfected adults, those who had COVID-19 that resolved had a small cognitive deficit, corresponding to a 3-point loss in IQ, the researchers found.
Adults with unresolved persistent COVID-19 symptoms had the equivalent of a 6-point loss in IQ, and those who had been admitted to the intensive care unit had the equivalent of a 9-point loss in IQ, in line with previous findings of cognitive deficits in patients hospitalized in a critical care unit, the researchers report.
Larger cognitive deficits were evident in adults infected early in the pandemic by the original SARS-CoV-2 virus or the B.1.1.7 variant, whereas peers infected later in the pandemic (eg, in the Omicron period), showed smaller cognitive deficits. This finding is in line with other studies suggesting that the association between COVID-19–associated cognitive deficits attenuated as the pandemic progressed, the researchers noted.
They also found that people who had COVID-19 after receiving two or more vaccinations showed better cognitive performance compared with those who had not been vaccinated.
The memory, reasoning, and executive function tasks were among the most sensitive to COVID-19–related cognitive differences and performance on these tasks differed according to illness duration and hospitalization.
Dr. Hampshire said that more research is needed to determine whether the cognitive deficits resolve with time.
“The implications of longer-term persistence of cognitive deficits and their clinical relevance remain unclear and warrant ongoing surveillance,” he said.
Larger Cognitive Deficits Likely?
These results are “a concern and the broader implications require evaluation,” wrote Ziyad Al-Aly, MD, with Washington University School of Medicine in St. Louis, and Clifford Rosen, MD, with Tufts University School of Medicine in Boston, in an accompanying editorial.
In their view, several outstanding questions remain, including what the potential functional implications of a 3-point loss in IQ may be and whether COVID-19–related cognitive deficits predispose to a higher risk for dementia later in life.
“A deeper understanding of the biology of cognitive dysfunction after SARS-CoV-2 infection and how best to prevent and treat it are critical for addressing the needs of affected persons and preserving the cognitive health of populations,” Drs. Al-Aly and Rosen concluded.
Commenting on the study for this news organization, Jacqueline Becker, PhD, clinical neuropsychologist and assistant professor of medicine, Icahn School of Medicine at Mount Sinai, New York City, noted that “one important caveat” is that the study used an online assessment tool for cognitive function and therefore the findings should be taken with “a grain of salt.”
“That said, this is a large sample, and the findings are generally consistent with what we’ve seen in terms of cognitive deficits post-COVID,” Dr. Becker said.
It’s likely that this study “underestimates” the degree of cognitive deficits that would be seen on validated neuropsychological tests, she added.
In a recent study, Dr. Becker and her colleagues investigated rates of cognitive impairment in 740 COVID-19 patients who recovered and were treated in outpatient, emergency department, or inpatient hospital settings.
Using validated neuropsychological measures, they found a relatively high frequency of cognitive impairment several months after patients contracted COVID-19. Impairments in executive functioning, processing speed, category fluency, memory encoding, and recall were predominant among hospitalized patients.
Dr. Becker noted that in her experience, cognition typically will improve in some patients 12-18 months post-COVID.
Support for the study was provided by the National Institute for Health and Care Research and UK Research and Innovation and by the Department of Health and Social Care in England and the Huo Family Foundation. Disclosures for authors and editorial writers are available at NEJM.org. Dr. Becker has no relevant disclosures.
A version of this article appeared on Medscape.com.
and which cognitive functions are most vulnerable.
In a large community sample, researchers found that on average, people who had recovered from COVID-19 showed small cognitive deficits equivalent to a 3-point loss in IQ for up to 1 year or more after recovering from the acute illness compared with peers who never had COVID-19.
However, people who had more severe cases, requiring treatment in a hospital intensive care unit, had cognitive deficits equivalent to a 9-point drop in IQ.
“People with ongoing persistent symptoms, indicative of long COVID, had larger cognitive deficits than people whose symptoms had resolved,” first author Adam Hampshire, PhD, with Imperial College London, told this news organization.
The largest deficits among cognitive tasks were in memory, reasoning, and executive function, he added.
“That is, people who had had COVID-19 were both slower and less accurate when performing tasks that measure those abilities,” Dr. Hampshire said. “The group with the largest cognitive deficits were patients who had been in intensive care for COVID-19.”
The study was published online in The New England Journal of Medicine.
Lingering Brain Fog
Cognitive symptoms after SARS-CoV-2 infection are well recognized, but whether objectively measurable cognitive deficits exist and how long they persist remains unclear.
To investigate, researchers invited 800,000 adults from the REACT study of SARS-CoV-2 transmission in England to complete an online assessment for cognitive function with eight domains.
Altogether, 141,583 participants started the cognitive battery by completing at least one task, and 112,964 completed all eight tasks.
The researchers estimated global cognitive scores among participants who had been previously infected with SARS-CoV-2 with symptoms that persisted for at least 12 weeks, whether or not resolved, and among uninfected participants.
Compared with uninfected adults, those who had COVID-19 that resolved had a small cognitive deficit, corresponding to a 3-point loss in IQ, the researchers found.
Adults with unresolved persistent COVID-19 symptoms had the equivalent of a 6-point loss in IQ, and those who had been admitted to the intensive care unit had the equivalent of a 9-point loss in IQ, in line with previous findings of cognitive deficits in patients hospitalized in a critical care unit, the researchers report.
Larger cognitive deficits were evident in adults infected early in the pandemic by the original SARS-CoV-2 virus or the B.1.1.7 variant, whereas peers infected later in the pandemic (eg, in the Omicron period), showed smaller cognitive deficits. This finding is in line with other studies suggesting that the association between COVID-19–associated cognitive deficits attenuated as the pandemic progressed, the researchers noted.
They also found that people who had COVID-19 after receiving two or more vaccinations showed better cognitive performance compared with those who had not been vaccinated.
The memory, reasoning, and executive function tasks were among the most sensitive to COVID-19–related cognitive differences and performance on these tasks differed according to illness duration and hospitalization.
Dr. Hampshire said that more research is needed to determine whether the cognitive deficits resolve with time.
“The implications of longer-term persistence of cognitive deficits and their clinical relevance remain unclear and warrant ongoing surveillance,” he said.
Larger Cognitive Deficits Likely?
These results are “a concern and the broader implications require evaluation,” wrote Ziyad Al-Aly, MD, with Washington University School of Medicine in St. Louis, and Clifford Rosen, MD, with Tufts University School of Medicine in Boston, in an accompanying editorial.
In their view, several outstanding questions remain, including what the potential functional implications of a 3-point loss in IQ may be and whether COVID-19–related cognitive deficits predispose to a higher risk for dementia later in life.
“A deeper understanding of the biology of cognitive dysfunction after SARS-CoV-2 infection and how best to prevent and treat it are critical for addressing the needs of affected persons and preserving the cognitive health of populations,” Drs. Al-Aly and Rosen concluded.
Commenting on the study for this news organization, Jacqueline Becker, PhD, clinical neuropsychologist and assistant professor of medicine, Icahn School of Medicine at Mount Sinai, New York City, noted that “one important caveat” is that the study used an online assessment tool for cognitive function and therefore the findings should be taken with “a grain of salt.”
“That said, this is a large sample, and the findings are generally consistent with what we’ve seen in terms of cognitive deficits post-COVID,” Dr. Becker said.
It’s likely that this study “underestimates” the degree of cognitive deficits that would be seen on validated neuropsychological tests, she added.
In a recent study, Dr. Becker and her colleagues investigated rates of cognitive impairment in 740 COVID-19 patients who recovered and were treated in outpatient, emergency department, or inpatient hospital settings.
Using validated neuropsychological measures, they found a relatively high frequency of cognitive impairment several months after patients contracted COVID-19. Impairments in executive functioning, processing speed, category fluency, memory encoding, and recall were predominant among hospitalized patients.
Dr. Becker noted that in her experience, cognition typically will improve in some patients 12-18 months post-COVID.
Support for the study was provided by the National Institute for Health and Care Research and UK Research and Innovation and by the Department of Health and Social Care in England and the Huo Family Foundation. Disclosures for authors and editorial writers are available at NEJM.org. Dr. Becker has no relevant disclosures.
A version of this article appeared on Medscape.com.
and which cognitive functions are most vulnerable.
In a large community sample, researchers found that on average, people who had recovered from COVID-19 showed small cognitive deficits equivalent to a 3-point loss in IQ for up to 1 year or more after recovering from the acute illness compared with peers who never had COVID-19.
However, people who had more severe cases, requiring treatment in a hospital intensive care unit, had cognitive deficits equivalent to a 9-point drop in IQ.
“People with ongoing persistent symptoms, indicative of long COVID, had larger cognitive deficits than people whose symptoms had resolved,” first author Adam Hampshire, PhD, with Imperial College London, told this news organization.
The largest deficits among cognitive tasks were in memory, reasoning, and executive function, he added.
“That is, people who had had COVID-19 were both slower and less accurate when performing tasks that measure those abilities,” Dr. Hampshire said. “The group with the largest cognitive deficits were patients who had been in intensive care for COVID-19.”
The study was published online in The New England Journal of Medicine.
Lingering Brain Fog
Cognitive symptoms after SARS-CoV-2 infection are well recognized, but whether objectively measurable cognitive deficits exist and how long they persist remains unclear.
To investigate, researchers invited 800,000 adults from the REACT study of SARS-CoV-2 transmission in England to complete an online assessment for cognitive function with eight domains.
Altogether, 141,583 participants started the cognitive battery by completing at least one task, and 112,964 completed all eight tasks.
The researchers estimated global cognitive scores among participants who had been previously infected with SARS-CoV-2 with symptoms that persisted for at least 12 weeks, whether or not resolved, and among uninfected participants.
Compared with uninfected adults, those who had COVID-19 that resolved had a small cognitive deficit, corresponding to a 3-point loss in IQ, the researchers found.
Adults with unresolved persistent COVID-19 symptoms had the equivalent of a 6-point loss in IQ, and those who had been admitted to the intensive care unit had the equivalent of a 9-point loss in IQ, in line with previous findings of cognitive deficits in patients hospitalized in a critical care unit, the researchers report.
Larger cognitive deficits were evident in adults infected early in the pandemic by the original SARS-CoV-2 virus or the B.1.1.7 variant, whereas peers infected later in the pandemic (eg, in the Omicron period), showed smaller cognitive deficits. This finding is in line with other studies suggesting that the association between COVID-19–associated cognitive deficits attenuated as the pandemic progressed, the researchers noted.
They also found that people who had COVID-19 after receiving two or more vaccinations showed better cognitive performance compared with those who had not been vaccinated.
The memory, reasoning, and executive function tasks were among the most sensitive to COVID-19–related cognitive differences and performance on these tasks differed according to illness duration and hospitalization.
Dr. Hampshire said that more research is needed to determine whether the cognitive deficits resolve with time.
“The implications of longer-term persistence of cognitive deficits and their clinical relevance remain unclear and warrant ongoing surveillance,” he said.
Larger Cognitive Deficits Likely?
These results are “a concern and the broader implications require evaluation,” wrote Ziyad Al-Aly, MD, with Washington University School of Medicine in St. Louis, and Clifford Rosen, MD, with Tufts University School of Medicine in Boston, in an accompanying editorial.
In their view, several outstanding questions remain, including what the potential functional implications of a 3-point loss in IQ may be and whether COVID-19–related cognitive deficits predispose to a higher risk for dementia later in life.
“A deeper understanding of the biology of cognitive dysfunction after SARS-CoV-2 infection and how best to prevent and treat it are critical for addressing the needs of affected persons and preserving the cognitive health of populations,” Drs. Al-Aly and Rosen concluded.
Commenting on the study for this news organization, Jacqueline Becker, PhD, clinical neuropsychologist and assistant professor of medicine, Icahn School of Medicine at Mount Sinai, New York City, noted that “one important caveat” is that the study used an online assessment tool for cognitive function and therefore the findings should be taken with “a grain of salt.”
“That said, this is a large sample, and the findings are generally consistent with what we’ve seen in terms of cognitive deficits post-COVID,” Dr. Becker said.
It’s likely that this study “underestimates” the degree of cognitive deficits that would be seen on validated neuropsychological tests, she added.
In a recent study, Dr. Becker and her colleagues investigated rates of cognitive impairment in 740 COVID-19 patients who recovered and were treated in outpatient, emergency department, or inpatient hospital settings.
Using validated neuropsychological measures, they found a relatively high frequency of cognitive impairment several months after patients contracted COVID-19. Impairments in executive functioning, processing speed, category fluency, memory encoding, and recall were predominant among hospitalized patients.
Dr. Becker noted that in her experience, cognition typically will improve in some patients 12-18 months post-COVID.
Support for the study was provided by the National Institute for Health and Care Research and UK Research and Innovation and by the Department of Health and Social Care in England and the Huo Family Foundation. Disclosures for authors and editorial writers are available at NEJM.org. Dr. Becker has no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE



