User login
Direct-to-Consumer Teledermatology Growth: A Review and Outlook for the Future
In recent years, direct-to-consumer (DTC) teledermatology platforms have gained popularity as telehealth business models, allowing patients to directly initiate visits with physicians and purchase medications from single platforms. A shortage of dermatologists, improved technology, drug patent expirations, and rising health care costs accelerated the growth of DTC dermatology.1 During the COVID-19 pandemic, teledermatology adoption surged due to the need to provide care while social distancing and minimizing viral exposure. These needs prompted additional federal funding and loosened regulatory provisions.2 As the userbase of these companies has grown, so have their valuations.3 Although the DTC model has attracted the attention of patients and investors, its rise provokes many questions about patients acting as consumers in health care. Indeed, DTC telemedicine offers greater autonomy and convenience for patients, but it may impact the quality of care and the nature of physician-patient relationships, perhaps making them more transactional.
Evolution of DTC in Health Care
The DTC model emphasizes individual choice and accessible health care. Although the definition has evolved, the core idea is not new.4 Over decades, pharmaceutical companies have spent billions of dollars on DTC advertising, circumventing physicians by directly reaching patients with campaigns on prescription drugs and laboratory tests and shaping public definitions of diseases.5
The DTC model of care is fundamentally different from traditional care models in that it changes the roles of the patient and physician. Whereas early telehealth models required a health care provider to initiate teleconsultations with specialists, DTC telemedicine bypasses this step (eg, the patient can consult a dermatologist without needing a primary care provider’s input first). This care can then be provided by dermatologists with whom patients may or may not have pre-established relationships.4,6
Dermatology was an early adopter of DTC telemedicine. The shortage of dermatologists in the United States created demand for increasing accessibility to dermatologic care. Additionally, the visual nature of diagnosing dermatologic disease was ideal for platforms supporting image sharing.7 Early DTC providers were primarily individual companies offering teledermatology. However, many dermatologists can now offer DTC capabilities via companies such as Amwell and Teladoc Health.8
Over the last 2 decades, start-ups such as Warby Parker (eyeglasses) and Casper (mattresses) defined the DTC industry using borrowed supply chains, cohesive branding, heavy social media marketing, and web-only retail. Scalability, lack of competition, and abundant venture capital created competition across numerous markets.9 Health care capitalized on this DTC model, creating a $700 billion market for products ranging from hearing aids to over-the-counter medications.10
Borrowing from this DTC playbook, platforms were created to offer delivery of generic prescription drugs to patients’ doorsteps. However, unlike with other products bought online, a consumer cannot simply add prescription drugs to their shopping cart and check out. In all models of American medical practice, physicians still serve as gatekeepers, providing a safeguard for patients to ensure appropriate prescription and avoid negative consequences of unnecessary drug use. This new model effectively streamlines diagnosis, prescription, and drug delivery without the patient ever having to leave home. Combining the prescribing and selling of medications (2 tasks that traditionally have been separated) potentially creates financial conflicts of interest (COIs). Additionally, high utilization of health care, including more prescriptions and visits, does not necessarily equal high quality of care. The companies stand to benefit from extra care regardless of need, and thus these models must be scrutinized for any incentives driving unnecessary care and prescriptions.
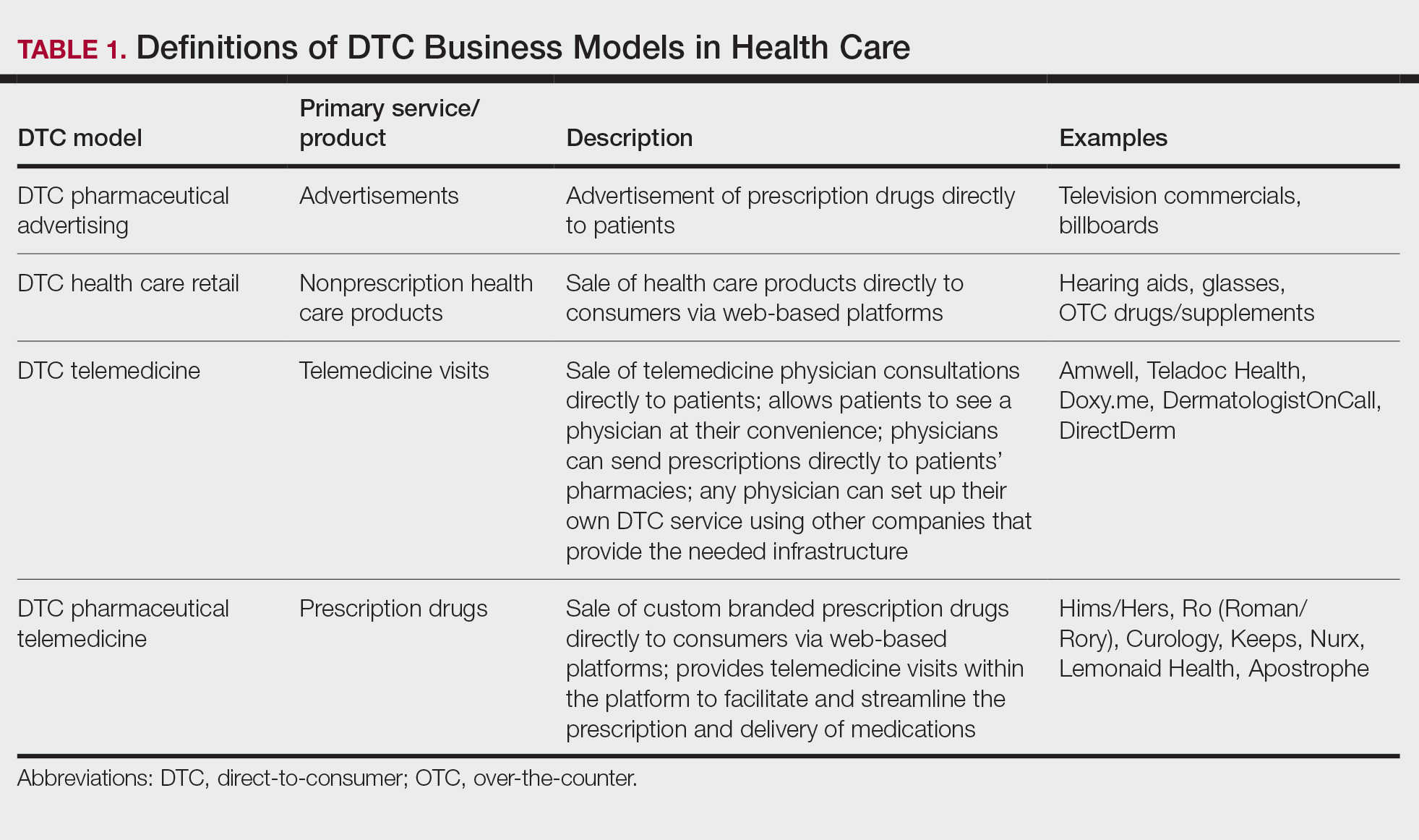
Ultimately, DTC has evolved to encompass multiple definitions in health care (Table 1). Although all models provide health care, each offers a different modality of delivery. The primary service may be the sale of prescription drugs or simply telemedicine visits. This review primarily discusses DTC pharmaceutical telemedicine platforms that sell private-label drugs and also offer telemedicine services to streamline care. However, the history, risks, and benefits discussed may apply to all models.
The DTC Landscape
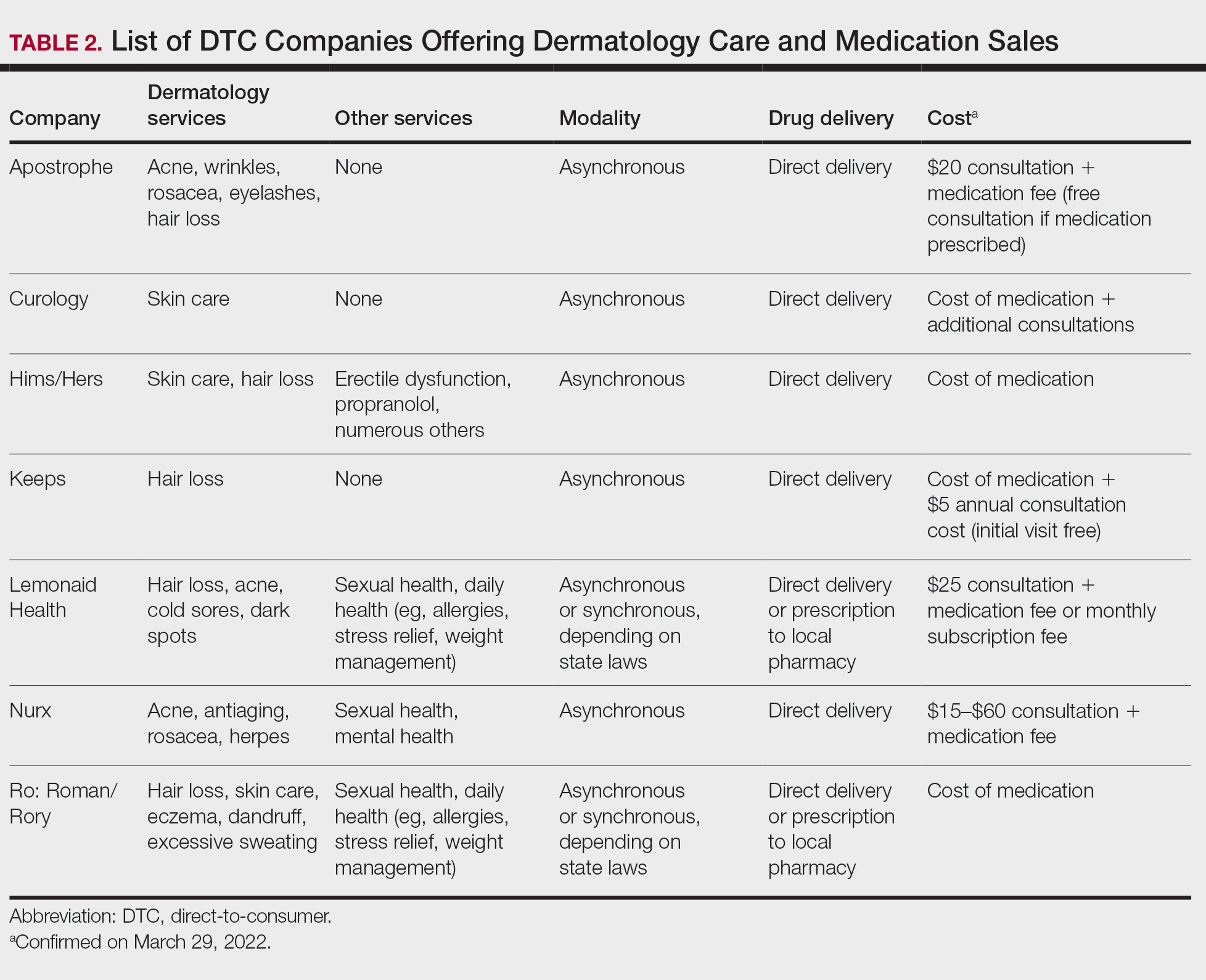
Most DTC companies employ variations on a model with the same 3 main components: a triage questionnaire, telehealth services, and prescription/drug delivery (Figure). The triage questionnaire elicits a history of the patient’s presentation and medical history. Some companies may use artificial intelligence (AI) algorithms to tailor questions to patient needs. There are 2 modalities for patient-provider communication: synchronous and asynchronous. Synchronous communication entails real-time patient-physician conversations via audio only or video call. Asynchronous (or store-and-forward) communication refers to consultations provided via messaging or text-based modality, where a provider may respond to a patient within 24 hours.6 Direct-to-consumer platforms primarily use asynchronous visits (Table 2). However, some also use synchronous modalities if the provider deems it necessary or if state laws require it.
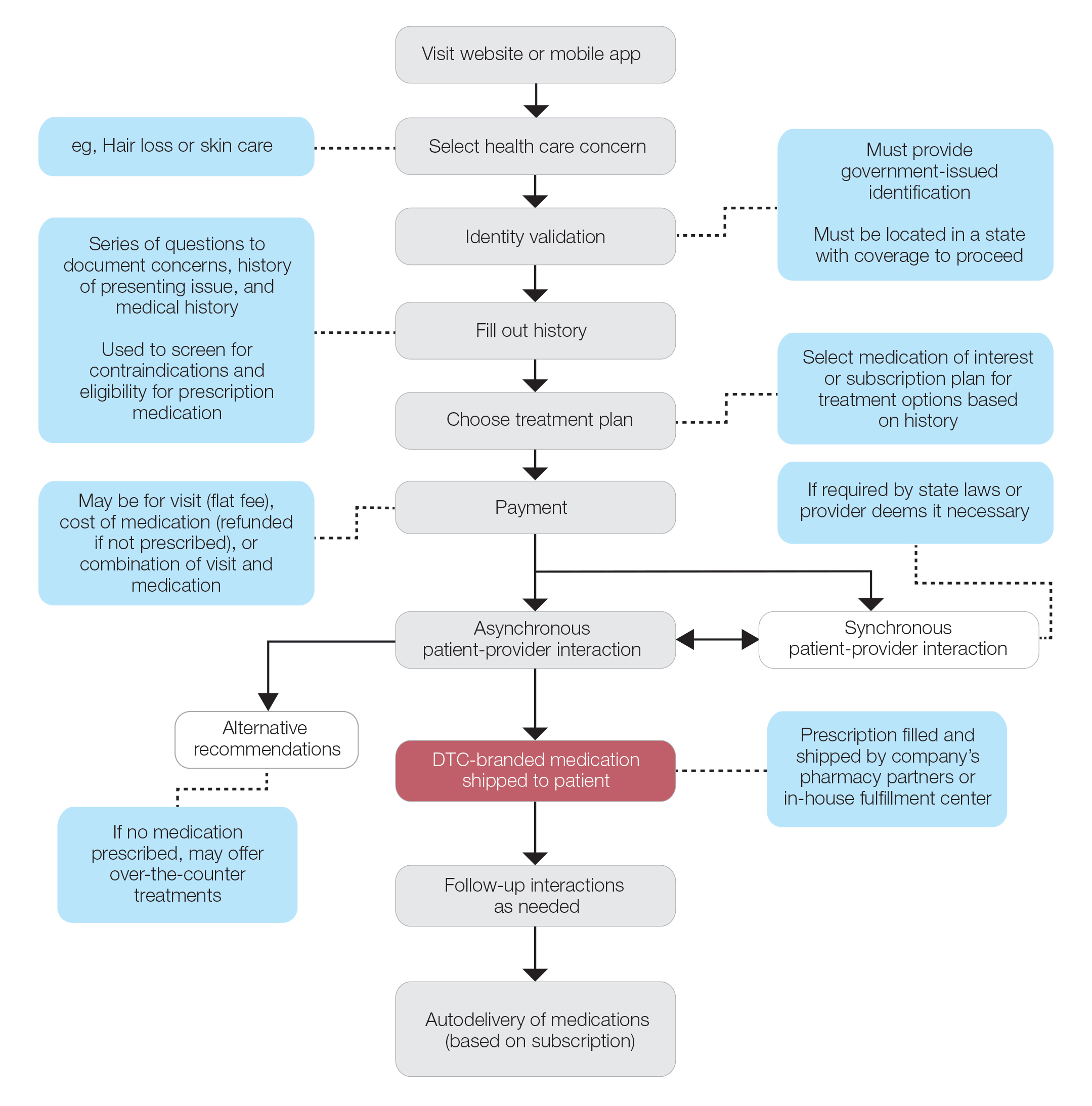
Once a provider has consulted with the patient, they can prescribe medication as needed. In certain cases, with adequate history, a prescription may be issued without a full physician visit. Furthermore, DTC companies require purchase of their custom-branded generic drugs. Prescriptions are fulfilled by the company’s pharmacy network and directly shipped to patients; few will allow patients to transfer a prescription to a pharmacy of their choice. Some platforms also sell supplements and over-the-counter medications.
Payment models vary among these companies, and most do not accept insurance (Table 2). Select models may provide free consultations and only require payment for pharmaceuticals. Others charge for consultations but reallocate payment to the cost of medication if prescribed. Another model involves flat rates for consultations and additional charges for drugs but unlimited messaging with providers for the duration of the prescription. Moreover, patients can subscribe to monthly deliveries of their medications.
Foundation of DTC
Technological advances have enabled patients to receive remote treatment from a single platform offering video calls, AI, electronic medical record interoperability, and integration of drug supply chains. Even in its simplest form, AI is increasingly used, as it allows for programs and chatbots to screen and triage patients.11 Technology also has improved at targeted mass marketing through social media platforms and search engines (eg, companies can use age, interests, location, and other parameters to target individuals likely needing acne treatment).
Drug patent expirations are a key catalyst for the rise of DTC companies, creating an attractive business model with generic drugs as the core product. Since 2008, patents for medications treating chronic conditions, such as erectile dysfunction, have expired. These patent expirations are responsible for $198 billion in projected prescription sales between 2019 and 2024.1 Thus, it follows that DTC companies have seized this opportunity to act as middlemen, taking advantage of these generic medications’ lower costs to create platforms focused on personalization and accessibility.
Rising deductibles have led patients to consider cheaper out-of-pocket alternatives that are not covered by insurance.1 For example, insurers typically do not cover finasteride treatment for conditions deemed cosmetic, such as androgenetic alopecia.12 The low cost of generic drugs creates an attractive business model for patients and investors. According to GoodRx, the average retail price for a 30-day supply of brand-name finasteride (Propecia [Merck]) is $135.92, whereas generic finasteride is $75.24.13 Direct-to-consumer pharmaceutical companies offer a 30-day supply of generic finasteride ranging from $8.33 to $30.14 The average wholesale cost for retailers is an estimated $2.31 for 30 days.15 Although profit margins on generic medications may be lower, more affordable drugs increase the size of the total market. These prescriptions are available as subscription plans, resulting in recurring revenue.
Lax US pharmaceutical marketing regulations allow direct advertising to the general public.16 In 1997, the US Food and Drug Administration allowed DTC advertisements to replace summaries of serious and common adverse effects with short statements covering important risks or referrals to other sources for complete information. In 2015, the US Food and Drug Administration guidelines preventing encouragement of self-diagnosis and self-treatment were withdrawn.5 These changes enable DTC companies to launch large advertising campaigns and to accelerate customer acquisition, as the industry often describes it, with ease.
Rapid Growth and Implications
Increasing generic drug availability and improving telemedicine capabilities have the potential to reduce costs and barriers but also have the potential for financial gain. Venture capital funds have recognized this opportunity, reflected by millions of dollars of investments, and accelerated the growth of DTC health care start-ups. For example, Ro has raised $376 million from venture capital, valuing the company at $1.5 billion.3
Direct-to-consumer companies require a heavy focus on marketing campaigns for customer acquisition. Their aesthetically pleasing websites and aggressive campaigns target specific audiences based on demographics, digital use habits, and purchasing behavior.4 Some campaigns celebrate the ease of obtaining prescriptions.17 Companies have been effective in recruiting so-called millennial and Generation Z patients, known to search the internet for remedies prior to seeking physician consultations.18 Recognizing these needs, some platforms offer guides on diseases they treat, creating effective customer-acquisition funnels. Recruitment of these technology-friendly patients has proven effective, especially given the largely positive media coverage of DTC platforms––potentially serving as a surrogate for medical credibility for patients.18
Some DTC companies also market physically; skin care ads may be strategically placed in social media feeds, or even found near mirrors in public bathrooms.19 Marketing campaigns also involve disease awareness; such efforts serve to increase diagnoses and prescribed treatments while destigmatizing diseases. Although DTC companies argue this strategy empowers patients, these marketing habits have the potential to take advantage of uninformed patients. Campaigns could potentially medicalize normal experiences and expand disease definitions resulting in overdiagnosis, overtreatment, and wasted resources.5 For example, off-label propranolol use has been advertised to attract patients who might have “nerves that come creeping before an important presentation.”17 Disease awareness campaigns also may lead people to falsely believe unproven drug benefits.5 According to studies, DTC pharmaceutical advertisements are low in informational quality and result in increased patient visits and prescriptions despite cost-effective alternatives.5,20-22
Fragmentation of the health care system is another possible complication of DTC teledermatology. These companies operate as for-profit organizations separated from the rest of the health care system, raising concerns about care coordination.8 Vital health data may not be conveyed as patients move among different providers and pharmacies. One study found DTC teledermatology rarely offered to provide medical records or facilitate a referral to a local physician.23 Such a lack of communication is concerning, as medication errors are the leading cause of avoidable harm in health care.24
Direct-to-consumer care models also seemingly redefine the physician-patient relationship by turning patients into consumers. Patient interactions may seem transactional and streamlined toward sales. For these platforms, a visit often is set up as an evaluation of a patient’s suitability for a prescription, not necessarily for the best treatment modality for the problem. These companies primarily make money through the sale of prescription drugs, creating a potential COI that may undermine the patient-physician relationship. Although some companies have made it clear that medical care and pharmaceutical sales are provided by legally separate business entities and that they do not pay physicians on commission, a conflict may still exist given the financial importance of physicians prescribing medication to the success of the business.16
Even as DTC models advertise upon expanded access and choice, the companies largely prohibit patients from choosing their own pharmacy. Instead, they encourage patients to fill prescriptions with the company’s pharmacy network by claiming lower costs compared with competitors. One DTC company, Hims, is launching a prescription-fulfillment center to further consolidate their business.17,19,25 The inherent COI of issuing and fulfilling prescriptions raises concerns of patient harm.26 For example, when Dermatology.com launched as a DTC prescription skin medication shop backed by Bausch Health Companies Inc, its model included telemedicine consultation. Although consultations were provided by RxDefine, a third party, only Dermatology.com drugs were prescribed. Given the poor quality of care and obvious financial COI, an uproar in the dermatology community and advocacy by the American Academy of Dermatology led to the shutdown of Dermatology.com’s online prescription services.26
The quality of care among DTC telemedicine platforms has been equivocal. Some studies have reported equivalent care in person and online, while others have reported poor adherence to guidelines, overuse of antibiotics, and misdiagnosis.8,23 A vital portion of the DTC experience is the history questionnaire, which is geared to diagnosis and risk assessment.25 Resneck et al23 found diagnostic quality to be adequate for simple dermatologic clinical scenarios but poor for scenarios requiring more than basic histories. Although Ro has reported leveraging data from millions of interactions to ask the right questions and streamline visits, it is still unclear whether history questionnaires are adequate.17,27 Additionally, consultations may lack sufficient counseling on adverse effects, risks, or pregnancy warnings, as well as discussions on alternative treatments and preventative care.17,23 Finally, patients often are limited in their choice of dermatologist; the lack of a fully developed relationship increases concerns of follow-up and monitoring practices. Although some DTC platforms offer unlimited interactions with physicians for the duration of a prescription, it is unknown how often these services are utilized or how adequate the quality of these interactions is. This potential for lax follow-up is especially concerning for prescriptions that autorenew on a monthly basis and could result in unnecessary overtreatment.
Postpandemic and Future Outlook
The COVID-19 pandemic dramatically impacted the use of telemedicine. To minimize COVID-19 transmission, the Centers for Medicare & Medicaid Services and private payers expanded telehealth coverage and eliminated reimbursement and licensing barriers.28 A decade’s worth of regulatory changes and consumer adoption was accelerated to weeks, resulting in telemedicine companies reaching record-high visit numbers.29 McKinsey & Company estimated that telehealth visit numbers surged 50- to 175-fold compared with pre–COVID-19 numbers. Additionally, 76% of patients were interested in future telehealth use, and 64% of providers were more comfortable using telehealth than before the pandemic.30 For their part, US dermatologists reported an increase in telemedicine use from 14.1% to 96.9% since COVID-19.31
Exactly how much DTC pharmaceutical telemedicine companies are growing is unclear, but private investments may be an indication. A record $14.7 billion was invested in the digital health sector in the first half of 2021; the majority went to telehealth companies.30 Ro, which reported $230 million in revenue in 2020 and has served 6 million visits, raised $200 milllion in July 2020 and $500 million in March 2021.32 Although post–COVID-19 health care will certainly involve increased telemedicine, the extent remains unclear, as telehealth vendors saw decreased usage upon reopening of state economies. Ultimately, the postpandemic regulatory landscape is hard to predict.30
Although COVID-19 appears to have caused rapid growth for DTC platforms, it also may have spurred competition. Telemedicine providers have given independent dermatologists and health care systems the infrastructure to implement custom DTC services.33 Although systems do not directly sell prescription drugs, the target market is essentially the same: patients looking for instant virtual dermatologic care. Therefore, sustained telemedicine services offered by traditional practices and systems may prove detrimental to DTC companies. However, unlike most telemedicine services, DTC models are less affected by certain changes in regulation since they do not rely on insurance. If regulations are tightened and reimbursements for telehealth are not attractive for dermatologists, teledermatology services may see an overall decrease. If so, patients who appreciate teledermatology may shift to using DTC platforms, even if their insurance does not cover them. Still, a nationwide survey found 56% of respondents felt an established relationship with a physician prior to a telemedicine visit is important, which may create a barrier for DTC adoption.34
Conclusion
Direct-to-consumer teledermatology represents a growing for-profit model of health care that provides patients with seemingly affordable and convenient care. However, there is potential for overtreatment, misdiagnosis, and fragmentation of health care. It will be important to monitor and evaluate the quality of care that DTC teledermatology offers and advocate for appropriate regulations and oversight. Eventually, more patients will have medications prescribed and dermatologic care administered through DTC companies. Dermatologists will benefit from this knowledge of DTC models to properly counsel patients on the risks and benefits of their use.
- Vennare J. The DTC healthcare report. Fitt Insider. September 15, 2019. Accessed February 23, 2022. https://insider.fitt.co/direct-to-consumer-healthcare-startups/
- Kannampallil T, Ma J. Digital translucence: adapting telemedicine delivery post-COVID-19. Telemed J E Health. 2020;26:1120-1122.
- Farr C. Ro, a 3-year-old online health provider, just raised a new round that values it at $1.5 billion. CNBC. July 27, 2020. Accessed February 23, 2022. https://www.cnbc.com/2020/07/27/ro-raises-200-million-at-1point5-billion-valuation-250-million-sales.html
- Elliott T, Shih J. Direct to consumer telemedicine. Curr Allergy Asthma Rep. 2019;19:1.
- Schwartz LM, Woloshin S. Medical marketing in the United States, 1997-2016. JAMA. 2019;321:80-96.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Coates SJ, Kvedar J, Granstein RD. Teledermatology: from historical perspective to emerging techniques of the modern era. J Am Acad Dermatol. 2015;72:563-574.
- Rheuban KS, Krupinski EA, eds. Understanding Telehealth. McGraw-Hill Education; 2017.
- Schlesinger LA, Higgins M, Roseman S. Reinventing the direct-to-consumer business model. Harvard Business Review. March 31, 2020. Accessed February 23, 2022. https://hbr.org/2020/03/reinventing-the-direct-to-consumer-business-model
- Cohen AB, Mathews SC, Dorsey ER, et al. Direct-to-consumer digital health. Lancet Digit Health. 2020;2:E163-E165.
- 6 telehealth trends for 2020. Wolters Kluwer. Published January 27, 2021. Accessed February 23, 2022. https://www.wolterskluwer.com/en/expert-insights/6-telehealth-trends-for-2020
- Jadoo SA, Lipoff JB. Prescribing to save patients money: ethical considerations. J Am Acad Dermatol. 2018;78:826-828.
- Propecia. GoodRx. Accessed February 23, 2022. https://www.goodrx.com/propecia
- Lauer A. The truth about online hair-loss treatments like Roman and Hims, according to a dermatologist. InsideHook. January 13, 2020. Accessed February 23, 2022. https://www.insidehook.com/article/grooming/men-hair-loss-treatments-dermatologist-review
- Friedman Y. Drug price trends for NDC 16729-0089. DrugPatentWatch. Accessed February 23, 2022. https://www.drugpatentwatch.com/p/drug-price/ndc/index.php?query=16729-0089
- Curtis H, Milner J. Ethical concerns with online direct-to-consumer pharmaceutical companies. J Med Ethics. 2020;46:168-171.
- Jain T, Lu RJ, Mehrotra A. Prescriptions on demand: the growth of direct-to-consumer telemedicine companies. JAMA. 2019;322:925-926.
- Shahinyan RH, Amighi A, Carey AN, et al. Direct-to-consumer internet prescription platforms overlook crucial pathology found during traditional office evaluation of young men with erectile dysfunction. Urology. 2020;143:165-172.
- Ali M. Andrew Dudum—bold strategies that propelled Hims & Hers into unicorn status. Exit Strategy with Moiz Ali. Published April 2020. Accessed February 23, 2022. https://open.spotify.com/episode/6DtaJxwZDjvZSJI88DTf24?si=b3FHQiUIQY62YjfRHmnJBQ
- Klara K, Kim J, Ross JS. Direct-to-consumer broadcast advertisements for pharmaceuticals: off-label promotion and adherence to FDA guidelines. J Gen Intern Med. 2018;33:651-658.
- Sullivan HW, Aikin KJ, Poehlman J. Communicating risk information in direct-to-consumer prescription drug television ads: a content analysis. Health Commun. 2019;34:212-219.
- Applequist J, Ball JG. An updated analysis of direct-to-consumer television advertisements for prescription drugs. Ann Fam Med. 2018;16:211-216.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Patient safety. World Health Organization. Published September 13, 2019. Accessed February 1, 2022. https://www.who.int/news-room/fact-sheets/detail/patient-safety
- Bollmeier SG, Stevenson E, Finnegan P, et al. Direct to consumer telemedicine: is healthcare from home best? Mo Med. 2020;117:303-309.
26. Court E. Bausch yanked online prescribing after dermatologist backlash. Bloomberg.com. Published March 11, 2020. Accessed September 25, 2020. https://www.bloomberg.com/news/articles/2020-03-11/bausch-yanked-online-prescribing-after-dermatologist-backlash
27. Reitano Z. The future of healthcare: how Ro helps providers treat patients 2 minutes, 2 days, 2 weeks, and 2 years at a time. Medium. Published March 4, 2019. Accessed February 1, 2022. https://medium.com/ro-co/the-future-of-healthcare-how-ro-helps-providers-treat-patients-2-mins-2-days-2-weeks-and-2-10efc0679d7
28. Lee I, Kovarik C, Tejasvi T, et al. Telehealth: helping your patients and practice survive and thrive during the COVID-19 crisis with rapid quality implementation. J Am Acad Dermatol. 2020;82:1213-1214.
29. Pifer R. “Weeks where decades happen”: telehealth 6 months into COVID-19. Healthcare Dive. Published July 27, 2020. Accessed February 23, 2022. https://www.healthcaredive.com/news/telehealth-6-months-coronavirus/581447/
30. Bestsennyy O, Gilbert G, Harris A, et al. Telehealth: a quarter-trillion-dollar post-COVID-19 reality? McKinsey & Company. Updated July 9, 2021. Accessed February 23, 2022. https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality
31. Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
32. Jennings K. Digital health startup Ro raised $500 million at $5 billion valuation. Forbes. March 22, 2021. Accessed March 29, 2022. https://www.forbes.com/sites/katiejennings/2021/03/22/digital-health-startup-ro-raised-500-million-at-5-billion-valuation/?sh=695be0e462f5
33. Hollander JE, Carr BG. Virtually perfect? telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
34. Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
In recent years, direct-to-consumer (DTC) teledermatology platforms have gained popularity as telehealth business models, allowing patients to directly initiate visits with physicians and purchase medications from single platforms. A shortage of dermatologists, improved technology, drug patent expirations, and rising health care costs accelerated the growth of DTC dermatology.1 During the COVID-19 pandemic, teledermatology adoption surged due to the need to provide care while social distancing and minimizing viral exposure. These needs prompted additional federal funding and loosened regulatory provisions.2 As the userbase of these companies has grown, so have their valuations.3 Although the DTC model has attracted the attention of patients and investors, its rise provokes many questions about patients acting as consumers in health care. Indeed, DTC telemedicine offers greater autonomy and convenience for patients, but it may impact the quality of care and the nature of physician-patient relationships, perhaps making them more transactional.
Evolution of DTC in Health Care
The DTC model emphasizes individual choice and accessible health care. Although the definition has evolved, the core idea is not new.4 Over decades, pharmaceutical companies have spent billions of dollars on DTC advertising, circumventing physicians by directly reaching patients with campaigns on prescription drugs and laboratory tests and shaping public definitions of diseases.5
The DTC model of care is fundamentally different from traditional care models in that it changes the roles of the patient and physician. Whereas early telehealth models required a health care provider to initiate teleconsultations with specialists, DTC telemedicine bypasses this step (eg, the patient can consult a dermatologist without needing a primary care provider’s input first). This care can then be provided by dermatologists with whom patients may or may not have pre-established relationships.4,6
Dermatology was an early adopter of DTC telemedicine. The shortage of dermatologists in the United States created demand for increasing accessibility to dermatologic care. Additionally, the visual nature of diagnosing dermatologic disease was ideal for platforms supporting image sharing.7 Early DTC providers were primarily individual companies offering teledermatology. However, many dermatologists can now offer DTC capabilities via companies such as Amwell and Teladoc Health.8
Over the last 2 decades, start-ups such as Warby Parker (eyeglasses) and Casper (mattresses) defined the DTC industry using borrowed supply chains, cohesive branding, heavy social media marketing, and web-only retail. Scalability, lack of competition, and abundant venture capital created competition across numerous markets.9 Health care capitalized on this DTC model, creating a $700 billion market for products ranging from hearing aids to over-the-counter medications.10
Borrowing from this DTC playbook, platforms were created to offer delivery of generic prescription drugs to patients’ doorsteps. However, unlike with other products bought online, a consumer cannot simply add prescription drugs to their shopping cart and check out. In all models of American medical practice, physicians still serve as gatekeepers, providing a safeguard for patients to ensure appropriate prescription and avoid negative consequences of unnecessary drug use. This new model effectively streamlines diagnosis, prescription, and drug delivery without the patient ever having to leave home. Combining the prescribing and selling of medications (2 tasks that traditionally have been separated) potentially creates financial conflicts of interest (COIs). Additionally, high utilization of health care, including more prescriptions and visits, does not necessarily equal high quality of care. The companies stand to benefit from extra care regardless of need, and thus these models must be scrutinized for any incentives driving unnecessary care and prescriptions.
Ultimately, DTC has evolved to encompass multiple definitions in health care (Table 1). Although all models provide health care, each offers a different modality of delivery. The primary service may be the sale of prescription drugs or simply telemedicine visits. This review primarily discusses DTC pharmaceutical telemedicine platforms that sell private-label drugs and also offer telemedicine services to streamline care. However, the history, risks, and benefits discussed may apply to all models.

The DTC Landscape
Most DTC companies employ variations on a model with the same 3 main components: a triage questionnaire, telehealth services, and prescription/drug delivery (Figure). The triage questionnaire elicits a history of the patient’s presentation and medical history. Some companies may use artificial intelligence (AI) algorithms to tailor questions to patient needs. There are 2 modalities for patient-provider communication: synchronous and asynchronous. Synchronous communication entails real-time patient-physician conversations via audio only or video call. Asynchronous (or store-and-forward) communication refers to consultations provided via messaging or text-based modality, where a provider may respond to a patient within 24 hours.6 Direct-to-consumer platforms primarily use asynchronous visits (Table 2). However, some also use synchronous modalities if the provider deems it necessary or if state laws require it.

Once a provider has consulted with the patient, they can prescribe medication as needed. In certain cases, with adequate history, a prescription may be issued without a full physician visit. Furthermore, DTC companies require purchase of their custom-branded generic drugs. Prescriptions are fulfilled by the company’s pharmacy network and directly shipped to patients; few will allow patients to transfer a prescription to a pharmacy of their choice. Some platforms also sell supplements and over-the-counter medications.

Payment models vary among these companies, and most do not accept insurance (Table 2). Select models may provide free consultations and only require payment for pharmaceuticals. Others charge for consultations but reallocate payment to the cost of medication if prescribed. Another model involves flat rates for consultations and additional charges for drugs but unlimited messaging with providers for the duration of the prescription. Moreover, patients can subscribe to monthly deliveries of their medications.
Foundation of DTC
Technological advances have enabled patients to receive remote treatment from a single platform offering video calls, AI, electronic medical record interoperability, and integration of drug supply chains. Even in its simplest form, AI is increasingly used, as it allows for programs and chatbots to screen and triage patients.11 Technology also has improved at targeted mass marketing through social media platforms and search engines (eg, companies can use age, interests, location, and other parameters to target individuals likely needing acne treatment).
Drug patent expirations are a key catalyst for the rise of DTC companies, creating an attractive business model with generic drugs as the core product. Since 2008, patents for medications treating chronic conditions, such as erectile dysfunction, have expired. These patent expirations are responsible for $198 billion in projected prescription sales between 2019 and 2024.1 Thus, it follows that DTC companies have seized this opportunity to act as middlemen, taking advantage of these generic medications’ lower costs to create platforms focused on personalization and accessibility.
Rising deductibles have led patients to consider cheaper out-of-pocket alternatives that are not covered by insurance.1 For example, insurers typically do not cover finasteride treatment for conditions deemed cosmetic, such as androgenetic alopecia.12 The low cost of generic drugs creates an attractive business model for patients and investors. According to GoodRx, the average retail price for a 30-day supply of brand-name finasteride (Propecia [Merck]) is $135.92, whereas generic finasteride is $75.24.13 Direct-to-consumer pharmaceutical companies offer a 30-day supply of generic finasteride ranging from $8.33 to $30.14 The average wholesale cost for retailers is an estimated $2.31 for 30 days.15 Although profit margins on generic medications may be lower, more affordable drugs increase the size of the total market. These prescriptions are available as subscription plans, resulting in recurring revenue.
Lax US pharmaceutical marketing regulations allow direct advertising to the general public.16 In 1997, the US Food and Drug Administration allowed DTC advertisements to replace summaries of serious and common adverse effects with short statements covering important risks or referrals to other sources for complete information. In 2015, the US Food and Drug Administration guidelines preventing encouragement of self-diagnosis and self-treatment were withdrawn.5 These changes enable DTC companies to launch large advertising campaigns and to accelerate customer acquisition, as the industry often describes it, with ease.
Rapid Growth and Implications
Increasing generic drug availability and improving telemedicine capabilities have the potential to reduce costs and barriers but also have the potential for financial gain. Venture capital funds have recognized this opportunity, reflected by millions of dollars of investments, and accelerated the growth of DTC health care start-ups. For example, Ro has raised $376 million from venture capital, valuing the company at $1.5 billion.3
Direct-to-consumer companies require a heavy focus on marketing campaigns for customer acquisition. Their aesthetically pleasing websites and aggressive campaigns target specific audiences based on demographics, digital use habits, and purchasing behavior.4 Some campaigns celebrate the ease of obtaining prescriptions.17 Companies have been effective in recruiting so-called millennial and Generation Z patients, known to search the internet for remedies prior to seeking physician consultations.18 Recognizing these needs, some platforms offer guides on diseases they treat, creating effective customer-acquisition funnels. Recruitment of these technology-friendly patients has proven effective, especially given the largely positive media coverage of DTC platforms––potentially serving as a surrogate for medical credibility for patients.18
Some DTC companies also market physically; skin care ads may be strategically placed in social media feeds, or even found near mirrors in public bathrooms.19 Marketing campaigns also involve disease awareness; such efforts serve to increase diagnoses and prescribed treatments while destigmatizing diseases. Although DTC companies argue this strategy empowers patients, these marketing habits have the potential to take advantage of uninformed patients. Campaigns could potentially medicalize normal experiences and expand disease definitions resulting in overdiagnosis, overtreatment, and wasted resources.5 For example, off-label propranolol use has been advertised to attract patients who might have “nerves that come creeping before an important presentation.”17 Disease awareness campaigns also may lead people to falsely believe unproven drug benefits.5 According to studies, DTC pharmaceutical advertisements are low in informational quality and result in increased patient visits and prescriptions despite cost-effective alternatives.5,20-22
Fragmentation of the health care system is another possible complication of DTC teledermatology. These companies operate as for-profit organizations separated from the rest of the health care system, raising concerns about care coordination.8 Vital health data may not be conveyed as patients move among different providers and pharmacies. One study found DTC teledermatology rarely offered to provide medical records or facilitate a referral to a local physician.23 Such a lack of communication is concerning, as medication errors are the leading cause of avoidable harm in health care.24
Direct-to-consumer care models also seemingly redefine the physician-patient relationship by turning patients into consumers. Patient interactions may seem transactional and streamlined toward sales. For these platforms, a visit often is set up as an evaluation of a patient’s suitability for a prescription, not necessarily for the best treatment modality for the problem. These companies primarily make money through the sale of prescription drugs, creating a potential COI that may undermine the patient-physician relationship. Although some companies have made it clear that medical care and pharmaceutical sales are provided by legally separate business entities and that they do not pay physicians on commission, a conflict may still exist given the financial importance of physicians prescribing medication to the success of the business.16
Even as DTC models advertise upon expanded access and choice, the companies largely prohibit patients from choosing their own pharmacy. Instead, they encourage patients to fill prescriptions with the company’s pharmacy network by claiming lower costs compared with competitors. One DTC company, Hims, is launching a prescription-fulfillment center to further consolidate their business.17,19,25 The inherent COI of issuing and fulfilling prescriptions raises concerns of patient harm.26 For example, when Dermatology.com launched as a DTC prescription skin medication shop backed by Bausch Health Companies Inc, its model included telemedicine consultation. Although consultations were provided by RxDefine, a third party, only Dermatology.com drugs were prescribed. Given the poor quality of care and obvious financial COI, an uproar in the dermatology community and advocacy by the American Academy of Dermatology led to the shutdown of Dermatology.com’s online prescription services.26
The quality of care among DTC telemedicine platforms has been equivocal. Some studies have reported equivalent care in person and online, while others have reported poor adherence to guidelines, overuse of antibiotics, and misdiagnosis.8,23 A vital portion of the DTC experience is the history questionnaire, which is geared to diagnosis and risk assessment.25 Resneck et al23 found diagnostic quality to be adequate for simple dermatologic clinical scenarios but poor for scenarios requiring more than basic histories. Although Ro has reported leveraging data from millions of interactions to ask the right questions and streamline visits, it is still unclear whether history questionnaires are adequate.17,27 Additionally, consultations may lack sufficient counseling on adverse effects, risks, or pregnancy warnings, as well as discussions on alternative treatments and preventative care.17,23 Finally, patients often are limited in their choice of dermatologist; the lack of a fully developed relationship increases concerns of follow-up and monitoring practices. Although some DTC platforms offer unlimited interactions with physicians for the duration of a prescription, it is unknown how often these services are utilized or how adequate the quality of these interactions is. This potential for lax follow-up is especially concerning for prescriptions that autorenew on a monthly basis and could result in unnecessary overtreatment.
Postpandemic and Future Outlook
The COVID-19 pandemic dramatically impacted the use of telemedicine. To minimize COVID-19 transmission, the Centers for Medicare & Medicaid Services and private payers expanded telehealth coverage and eliminated reimbursement and licensing barriers.28 A decade’s worth of regulatory changes and consumer adoption was accelerated to weeks, resulting in telemedicine companies reaching record-high visit numbers.29 McKinsey & Company estimated that telehealth visit numbers surged 50- to 175-fold compared with pre–COVID-19 numbers. Additionally, 76% of patients were interested in future telehealth use, and 64% of providers were more comfortable using telehealth than before the pandemic.30 For their part, US dermatologists reported an increase in telemedicine use from 14.1% to 96.9% since COVID-19.31
Exactly how much DTC pharmaceutical telemedicine companies are growing is unclear, but private investments may be an indication. A record $14.7 billion was invested in the digital health sector in the first half of 2021; the majority went to telehealth companies.30 Ro, which reported $230 million in revenue in 2020 and has served 6 million visits, raised $200 milllion in July 2020 and $500 million in March 2021.32 Although post–COVID-19 health care will certainly involve increased telemedicine, the extent remains unclear, as telehealth vendors saw decreased usage upon reopening of state economies. Ultimately, the postpandemic regulatory landscape is hard to predict.30
Although COVID-19 appears to have caused rapid growth for DTC platforms, it also may have spurred competition. Telemedicine providers have given independent dermatologists and health care systems the infrastructure to implement custom DTC services.33 Although systems do not directly sell prescription drugs, the target market is essentially the same: patients looking for instant virtual dermatologic care. Therefore, sustained telemedicine services offered by traditional practices and systems may prove detrimental to DTC companies. However, unlike most telemedicine services, DTC models are less affected by certain changes in regulation since they do not rely on insurance. If regulations are tightened and reimbursements for telehealth are not attractive for dermatologists, teledermatology services may see an overall decrease. If so, patients who appreciate teledermatology may shift to using DTC platforms, even if their insurance does not cover them. Still, a nationwide survey found 56% of respondents felt an established relationship with a physician prior to a telemedicine visit is important, which may create a barrier for DTC adoption.34
Conclusion
Direct-to-consumer teledermatology represents a growing for-profit model of health care that provides patients with seemingly affordable and convenient care. However, there is potential for overtreatment, misdiagnosis, and fragmentation of health care. It will be important to monitor and evaluate the quality of care that DTC teledermatology offers and advocate for appropriate regulations and oversight. Eventually, more patients will have medications prescribed and dermatologic care administered through DTC companies. Dermatologists will benefit from this knowledge of DTC models to properly counsel patients on the risks and benefits of their use.
In recent years, direct-to-consumer (DTC) teledermatology platforms have gained popularity as telehealth business models, allowing patients to directly initiate visits with physicians and purchase medications from single platforms. A shortage of dermatologists, improved technology, drug patent expirations, and rising health care costs accelerated the growth of DTC dermatology.1 During the COVID-19 pandemic, teledermatology adoption surged due to the need to provide care while social distancing and minimizing viral exposure. These needs prompted additional federal funding and loosened regulatory provisions.2 As the userbase of these companies has grown, so have their valuations.3 Although the DTC model has attracted the attention of patients and investors, its rise provokes many questions about patients acting as consumers in health care. Indeed, DTC telemedicine offers greater autonomy and convenience for patients, but it may impact the quality of care and the nature of physician-patient relationships, perhaps making them more transactional.
Evolution of DTC in Health Care
The DTC model emphasizes individual choice and accessible health care. Although the definition has evolved, the core idea is not new.4 Over decades, pharmaceutical companies have spent billions of dollars on DTC advertising, circumventing physicians by directly reaching patients with campaigns on prescription drugs and laboratory tests and shaping public definitions of diseases.5
The DTC model of care is fundamentally different from traditional care models in that it changes the roles of the patient and physician. Whereas early telehealth models required a health care provider to initiate teleconsultations with specialists, DTC telemedicine bypasses this step (eg, the patient can consult a dermatologist without needing a primary care provider’s input first). This care can then be provided by dermatologists with whom patients may or may not have pre-established relationships.4,6
Dermatology was an early adopter of DTC telemedicine. The shortage of dermatologists in the United States created demand for increasing accessibility to dermatologic care. Additionally, the visual nature of diagnosing dermatologic disease was ideal for platforms supporting image sharing.7 Early DTC providers were primarily individual companies offering teledermatology. However, many dermatologists can now offer DTC capabilities via companies such as Amwell and Teladoc Health.8
Over the last 2 decades, start-ups such as Warby Parker (eyeglasses) and Casper (mattresses) defined the DTC industry using borrowed supply chains, cohesive branding, heavy social media marketing, and web-only retail. Scalability, lack of competition, and abundant venture capital created competition across numerous markets.9 Health care capitalized on this DTC model, creating a $700 billion market for products ranging from hearing aids to over-the-counter medications.10
Borrowing from this DTC playbook, platforms were created to offer delivery of generic prescription drugs to patients’ doorsteps. However, unlike with other products bought online, a consumer cannot simply add prescription drugs to their shopping cart and check out. In all models of American medical practice, physicians still serve as gatekeepers, providing a safeguard for patients to ensure appropriate prescription and avoid negative consequences of unnecessary drug use. This new model effectively streamlines diagnosis, prescription, and drug delivery without the patient ever having to leave home. Combining the prescribing and selling of medications (2 tasks that traditionally have been separated) potentially creates financial conflicts of interest (COIs). Additionally, high utilization of health care, including more prescriptions and visits, does not necessarily equal high quality of care. The companies stand to benefit from extra care regardless of need, and thus these models must be scrutinized for any incentives driving unnecessary care and prescriptions.
Ultimately, DTC has evolved to encompass multiple definitions in health care (Table 1). Although all models provide health care, each offers a different modality of delivery. The primary service may be the sale of prescription drugs or simply telemedicine visits. This review primarily discusses DTC pharmaceutical telemedicine platforms that sell private-label drugs and also offer telemedicine services to streamline care. However, the history, risks, and benefits discussed may apply to all models.

The DTC Landscape
Most DTC companies employ variations on a model with the same 3 main components: a triage questionnaire, telehealth services, and prescription/drug delivery (Figure). The triage questionnaire elicits a history of the patient’s presentation and medical history. Some companies may use artificial intelligence (AI) algorithms to tailor questions to patient needs. There are 2 modalities for patient-provider communication: synchronous and asynchronous. Synchronous communication entails real-time patient-physician conversations via audio only or video call. Asynchronous (or store-and-forward) communication refers to consultations provided via messaging or text-based modality, where a provider may respond to a patient within 24 hours.6 Direct-to-consumer platforms primarily use asynchronous visits (Table 2). However, some also use synchronous modalities if the provider deems it necessary or if state laws require it.

Once a provider has consulted with the patient, they can prescribe medication as needed. In certain cases, with adequate history, a prescription may be issued without a full physician visit. Furthermore, DTC companies require purchase of their custom-branded generic drugs. Prescriptions are fulfilled by the company’s pharmacy network and directly shipped to patients; few will allow patients to transfer a prescription to a pharmacy of their choice. Some platforms also sell supplements and over-the-counter medications.

Payment models vary among these companies, and most do not accept insurance (Table 2). Select models may provide free consultations and only require payment for pharmaceuticals. Others charge for consultations but reallocate payment to the cost of medication if prescribed. Another model involves flat rates for consultations and additional charges for drugs but unlimited messaging with providers for the duration of the prescription. Moreover, patients can subscribe to monthly deliveries of their medications.
Foundation of DTC
Technological advances have enabled patients to receive remote treatment from a single platform offering video calls, AI, electronic medical record interoperability, and integration of drug supply chains. Even in its simplest form, AI is increasingly used, as it allows for programs and chatbots to screen and triage patients.11 Technology also has improved at targeted mass marketing through social media platforms and search engines (eg, companies can use age, interests, location, and other parameters to target individuals likely needing acne treatment).
Drug patent expirations are a key catalyst for the rise of DTC companies, creating an attractive business model with generic drugs as the core product. Since 2008, patents for medications treating chronic conditions, such as erectile dysfunction, have expired. These patent expirations are responsible for $198 billion in projected prescription sales between 2019 and 2024.1 Thus, it follows that DTC companies have seized this opportunity to act as middlemen, taking advantage of these generic medications’ lower costs to create platforms focused on personalization and accessibility.
Rising deductibles have led patients to consider cheaper out-of-pocket alternatives that are not covered by insurance.1 For example, insurers typically do not cover finasteride treatment for conditions deemed cosmetic, such as androgenetic alopecia.12 The low cost of generic drugs creates an attractive business model for patients and investors. According to GoodRx, the average retail price for a 30-day supply of brand-name finasteride (Propecia [Merck]) is $135.92, whereas generic finasteride is $75.24.13 Direct-to-consumer pharmaceutical companies offer a 30-day supply of generic finasteride ranging from $8.33 to $30.14 The average wholesale cost for retailers is an estimated $2.31 for 30 days.15 Although profit margins on generic medications may be lower, more affordable drugs increase the size of the total market. These prescriptions are available as subscription plans, resulting in recurring revenue.
Lax US pharmaceutical marketing regulations allow direct advertising to the general public.16 In 1997, the US Food and Drug Administration allowed DTC advertisements to replace summaries of serious and common adverse effects with short statements covering important risks or referrals to other sources for complete information. In 2015, the US Food and Drug Administration guidelines preventing encouragement of self-diagnosis and self-treatment were withdrawn.5 These changes enable DTC companies to launch large advertising campaigns and to accelerate customer acquisition, as the industry often describes it, with ease.
Rapid Growth and Implications
Increasing generic drug availability and improving telemedicine capabilities have the potential to reduce costs and barriers but also have the potential for financial gain. Venture capital funds have recognized this opportunity, reflected by millions of dollars of investments, and accelerated the growth of DTC health care start-ups. For example, Ro has raised $376 million from venture capital, valuing the company at $1.5 billion.3
Direct-to-consumer companies require a heavy focus on marketing campaigns for customer acquisition. Their aesthetically pleasing websites and aggressive campaigns target specific audiences based on demographics, digital use habits, and purchasing behavior.4 Some campaigns celebrate the ease of obtaining prescriptions.17 Companies have been effective in recruiting so-called millennial and Generation Z patients, known to search the internet for remedies prior to seeking physician consultations.18 Recognizing these needs, some platforms offer guides on diseases they treat, creating effective customer-acquisition funnels. Recruitment of these technology-friendly patients has proven effective, especially given the largely positive media coverage of DTC platforms––potentially serving as a surrogate for medical credibility for patients.18
Some DTC companies also market physically; skin care ads may be strategically placed in social media feeds, or even found near mirrors in public bathrooms.19 Marketing campaigns also involve disease awareness; such efforts serve to increase diagnoses and prescribed treatments while destigmatizing diseases. Although DTC companies argue this strategy empowers patients, these marketing habits have the potential to take advantage of uninformed patients. Campaigns could potentially medicalize normal experiences and expand disease definitions resulting in overdiagnosis, overtreatment, and wasted resources.5 For example, off-label propranolol use has been advertised to attract patients who might have “nerves that come creeping before an important presentation.”17 Disease awareness campaigns also may lead people to falsely believe unproven drug benefits.5 According to studies, DTC pharmaceutical advertisements are low in informational quality and result in increased patient visits and prescriptions despite cost-effective alternatives.5,20-22
Fragmentation of the health care system is another possible complication of DTC teledermatology. These companies operate as for-profit organizations separated from the rest of the health care system, raising concerns about care coordination.8 Vital health data may not be conveyed as patients move among different providers and pharmacies. One study found DTC teledermatology rarely offered to provide medical records or facilitate a referral to a local physician.23 Such a lack of communication is concerning, as medication errors are the leading cause of avoidable harm in health care.24
Direct-to-consumer care models also seemingly redefine the physician-patient relationship by turning patients into consumers. Patient interactions may seem transactional and streamlined toward sales. For these platforms, a visit often is set up as an evaluation of a patient’s suitability for a prescription, not necessarily for the best treatment modality for the problem. These companies primarily make money through the sale of prescription drugs, creating a potential COI that may undermine the patient-physician relationship. Although some companies have made it clear that medical care and pharmaceutical sales are provided by legally separate business entities and that they do not pay physicians on commission, a conflict may still exist given the financial importance of physicians prescribing medication to the success of the business.16
Even as DTC models advertise upon expanded access and choice, the companies largely prohibit patients from choosing their own pharmacy. Instead, they encourage patients to fill prescriptions with the company’s pharmacy network by claiming lower costs compared with competitors. One DTC company, Hims, is launching a prescription-fulfillment center to further consolidate their business.17,19,25 The inherent COI of issuing and fulfilling prescriptions raises concerns of patient harm.26 For example, when Dermatology.com launched as a DTC prescription skin medication shop backed by Bausch Health Companies Inc, its model included telemedicine consultation. Although consultations were provided by RxDefine, a third party, only Dermatology.com drugs were prescribed. Given the poor quality of care and obvious financial COI, an uproar in the dermatology community and advocacy by the American Academy of Dermatology led to the shutdown of Dermatology.com’s online prescription services.26
The quality of care among DTC telemedicine platforms has been equivocal. Some studies have reported equivalent care in person and online, while others have reported poor adherence to guidelines, overuse of antibiotics, and misdiagnosis.8,23 A vital portion of the DTC experience is the history questionnaire, which is geared to diagnosis and risk assessment.25 Resneck et al23 found diagnostic quality to be adequate for simple dermatologic clinical scenarios but poor for scenarios requiring more than basic histories. Although Ro has reported leveraging data from millions of interactions to ask the right questions and streamline visits, it is still unclear whether history questionnaires are adequate.17,27 Additionally, consultations may lack sufficient counseling on adverse effects, risks, or pregnancy warnings, as well as discussions on alternative treatments and preventative care.17,23 Finally, patients often are limited in their choice of dermatologist; the lack of a fully developed relationship increases concerns of follow-up and monitoring practices. Although some DTC platforms offer unlimited interactions with physicians for the duration of a prescription, it is unknown how often these services are utilized or how adequate the quality of these interactions is. This potential for lax follow-up is especially concerning for prescriptions that autorenew on a monthly basis and could result in unnecessary overtreatment.
Postpandemic and Future Outlook
The COVID-19 pandemic dramatically impacted the use of telemedicine. To minimize COVID-19 transmission, the Centers for Medicare & Medicaid Services and private payers expanded telehealth coverage and eliminated reimbursement and licensing barriers.28 A decade’s worth of regulatory changes and consumer adoption was accelerated to weeks, resulting in telemedicine companies reaching record-high visit numbers.29 McKinsey & Company estimated that telehealth visit numbers surged 50- to 175-fold compared with pre–COVID-19 numbers. Additionally, 76% of patients were interested in future telehealth use, and 64% of providers were more comfortable using telehealth than before the pandemic.30 For their part, US dermatologists reported an increase in telemedicine use from 14.1% to 96.9% since COVID-19.31
Exactly how much DTC pharmaceutical telemedicine companies are growing is unclear, but private investments may be an indication. A record $14.7 billion was invested in the digital health sector in the first half of 2021; the majority went to telehealth companies.30 Ro, which reported $230 million in revenue in 2020 and has served 6 million visits, raised $200 milllion in July 2020 and $500 million in March 2021.32 Although post–COVID-19 health care will certainly involve increased telemedicine, the extent remains unclear, as telehealth vendors saw decreased usage upon reopening of state economies. Ultimately, the postpandemic regulatory landscape is hard to predict.30
Although COVID-19 appears to have caused rapid growth for DTC platforms, it also may have spurred competition. Telemedicine providers have given independent dermatologists and health care systems the infrastructure to implement custom DTC services.33 Although systems do not directly sell prescription drugs, the target market is essentially the same: patients looking for instant virtual dermatologic care. Therefore, sustained telemedicine services offered by traditional practices and systems may prove detrimental to DTC companies. However, unlike most telemedicine services, DTC models are less affected by certain changes in regulation since they do not rely on insurance. If regulations are tightened and reimbursements for telehealth are not attractive for dermatologists, teledermatology services may see an overall decrease. If so, patients who appreciate teledermatology may shift to using DTC platforms, even if their insurance does not cover them. Still, a nationwide survey found 56% of respondents felt an established relationship with a physician prior to a telemedicine visit is important, which may create a barrier for DTC adoption.34
Conclusion
Direct-to-consumer teledermatology represents a growing for-profit model of health care that provides patients with seemingly affordable and convenient care. However, there is potential for overtreatment, misdiagnosis, and fragmentation of health care. It will be important to monitor and evaluate the quality of care that DTC teledermatology offers and advocate for appropriate regulations and oversight. Eventually, more patients will have medications prescribed and dermatologic care administered through DTC companies. Dermatologists will benefit from this knowledge of DTC models to properly counsel patients on the risks and benefits of their use.
- Vennare J. The DTC healthcare report. Fitt Insider. September 15, 2019. Accessed February 23, 2022. https://insider.fitt.co/direct-to-consumer-healthcare-startups/
- Kannampallil T, Ma J. Digital translucence: adapting telemedicine delivery post-COVID-19. Telemed J E Health. 2020;26:1120-1122.
- Farr C. Ro, a 3-year-old online health provider, just raised a new round that values it at $1.5 billion. CNBC. July 27, 2020. Accessed February 23, 2022. https://www.cnbc.com/2020/07/27/ro-raises-200-million-at-1point5-billion-valuation-250-million-sales.html
- Elliott T, Shih J. Direct to consumer telemedicine. Curr Allergy Asthma Rep. 2019;19:1.
- Schwartz LM, Woloshin S. Medical marketing in the United States, 1997-2016. JAMA. 2019;321:80-96.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Coates SJ, Kvedar J, Granstein RD. Teledermatology: from historical perspective to emerging techniques of the modern era. J Am Acad Dermatol. 2015;72:563-574.
- Rheuban KS, Krupinski EA, eds. Understanding Telehealth. McGraw-Hill Education; 2017.
- Schlesinger LA, Higgins M, Roseman S. Reinventing the direct-to-consumer business model. Harvard Business Review. March 31, 2020. Accessed February 23, 2022. https://hbr.org/2020/03/reinventing-the-direct-to-consumer-business-model
- Cohen AB, Mathews SC, Dorsey ER, et al. Direct-to-consumer digital health. Lancet Digit Health. 2020;2:E163-E165.
- 6 telehealth trends for 2020. Wolters Kluwer. Published January 27, 2021. Accessed February 23, 2022. https://www.wolterskluwer.com/en/expert-insights/6-telehealth-trends-for-2020
- Jadoo SA, Lipoff JB. Prescribing to save patients money: ethical considerations. J Am Acad Dermatol. 2018;78:826-828.
- Propecia. GoodRx. Accessed February 23, 2022. https://www.goodrx.com/propecia
- Lauer A. The truth about online hair-loss treatments like Roman and Hims, according to a dermatologist. InsideHook. January 13, 2020. Accessed February 23, 2022. https://www.insidehook.com/article/grooming/men-hair-loss-treatments-dermatologist-review
- Friedman Y. Drug price trends for NDC 16729-0089. DrugPatentWatch. Accessed February 23, 2022. https://www.drugpatentwatch.com/p/drug-price/ndc/index.php?query=16729-0089
- Curtis H, Milner J. Ethical concerns with online direct-to-consumer pharmaceutical companies. J Med Ethics. 2020;46:168-171.
- Jain T, Lu RJ, Mehrotra A. Prescriptions on demand: the growth of direct-to-consumer telemedicine companies. JAMA. 2019;322:925-926.
- Shahinyan RH, Amighi A, Carey AN, et al. Direct-to-consumer internet prescription platforms overlook crucial pathology found during traditional office evaluation of young men with erectile dysfunction. Urology. 2020;143:165-172.
- Ali M. Andrew Dudum—bold strategies that propelled Hims & Hers into unicorn status. Exit Strategy with Moiz Ali. Published April 2020. Accessed February 23, 2022. https://open.spotify.com/episode/6DtaJxwZDjvZSJI88DTf24?si=b3FHQiUIQY62YjfRHmnJBQ
- Klara K, Kim J, Ross JS. Direct-to-consumer broadcast advertisements for pharmaceuticals: off-label promotion and adherence to FDA guidelines. J Gen Intern Med. 2018;33:651-658.
- Sullivan HW, Aikin KJ, Poehlman J. Communicating risk information in direct-to-consumer prescription drug television ads: a content analysis. Health Commun. 2019;34:212-219.
- Applequist J, Ball JG. An updated analysis of direct-to-consumer television advertisements for prescription drugs. Ann Fam Med. 2018;16:211-216.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Patient safety. World Health Organization. Published September 13, 2019. Accessed February 1, 2022. https://www.who.int/news-room/fact-sheets/detail/patient-safety
- Bollmeier SG, Stevenson E, Finnegan P, et al. Direct to consumer telemedicine: is healthcare from home best? Mo Med. 2020;117:303-309.
26. Court E. Bausch yanked online prescribing after dermatologist backlash. Bloomberg.com. Published March 11, 2020. Accessed September 25, 2020. https://www.bloomberg.com/news/articles/2020-03-11/bausch-yanked-online-prescribing-after-dermatologist-backlash
27. Reitano Z. The future of healthcare: how Ro helps providers treat patients 2 minutes, 2 days, 2 weeks, and 2 years at a time. Medium. Published March 4, 2019. Accessed February 1, 2022. https://medium.com/ro-co/the-future-of-healthcare-how-ro-helps-providers-treat-patients-2-mins-2-days-2-weeks-and-2-10efc0679d7
28. Lee I, Kovarik C, Tejasvi T, et al. Telehealth: helping your patients and practice survive and thrive during the COVID-19 crisis with rapid quality implementation. J Am Acad Dermatol. 2020;82:1213-1214.
29. Pifer R. “Weeks where decades happen”: telehealth 6 months into COVID-19. Healthcare Dive. Published July 27, 2020. Accessed February 23, 2022. https://www.healthcaredive.com/news/telehealth-6-months-coronavirus/581447/
30. Bestsennyy O, Gilbert G, Harris A, et al. Telehealth: a quarter-trillion-dollar post-COVID-19 reality? McKinsey & Company. Updated July 9, 2021. Accessed February 23, 2022. https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality
31. Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
32. Jennings K. Digital health startup Ro raised $500 million at $5 billion valuation. Forbes. March 22, 2021. Accessed March 29, 2022. https://www.forbes.com/sites/katiejennings/2021/03/22/digital-health-startup-ro-raised-500-million-at-5-billion-valuation/?sh=695be0e462f5
33. Hollander JE, Carr BG. Virtually perfect? telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
34. Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
- Vennare J. The DTC healthcare report. Fitt Insider. September 15, 2019. Accessed February 23, 2022. https://insider.fitt.co/direct-to-consumer-healthcare-startups/
- Kannampallil T, Ma J. Digital translucence: adapting telemedicine delivery post-COVID-19. Telemed J E Health. 2020;26:1120-1122.
- Farr C. Ro, a 3-year-old online health provider, just raised a new round that values it at $1.5 billion. CNBC. July 27, 2020. Accessed February 23, 2022. https://www.cnbc.com/2020/07/27/ro-raises-200-million-at-1point5-billion-valuation-250-million-sales.html
- Elliott T, Shih J. Direct to consumer telemedicine. Curr Allergy Asthma Rep. 2019;19:1.
- Schwartz LM, Woloshin S. Medical marketing in the United States, 1997-2016. JAMA. 2019;321:80-96.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Coates SJ, Kvedar J, Granstein RD. Teledermatology: from historical perspective to emerging techniques of the modern era. J Am Acad Dermatol. 2015;72:563-574.
- Rheuban KS, Krupinski EA, eds. Understanding Telehealth. McGraw-Hill Education; 2017.
- Schlesinger LA, Higgins M, Roseman S. Reinventing the direct-to-consumer business model. Harvard Business Review. March 31, 2020. Accessed February 23, 2022. https://hbr.org/2020/03/reinventing-the-direct-to-consumer-business-model
- Cohen AB, Mathews SC, Dorsey ER, et al. Direct-to-consumer digital health. Lancet Digit Health. 2020;2:E163-E165.
- 6 telehealth trends for 2020. Wolters Kluwer. Published January 27, 2021. Accessed February 23, 2022. https://www.wolterskluwer.com/en/expert-insights/6-telehealth-trends-for-2020
- Jadoo SA, Lipoff JB. Prescribing to save patients money: ethical considerations. J Am Acad Dermatol. 2018;78:826-828.
- Propecia. GoodRx. Accessed February 23, 2022. https://www.goodrx.com/propecia
- Lauer A. The truth about online hair-loss treatments like Roman and Hims, according to a dermatologist. InsideHook. January 13, 2020. Accessed February 23, 2022. https://www.insidehook.com/article/grooming/men-hair-loss-treatments-dermatologist-review
- Friedman Y. Drug price trends for NDC 16729-0089. DrugPatentWatch. Accessed February 23, 2022. https://www.drugpatentwatch.com/p/drug-price/ndc/index.php?query=16729-0089
- Curtis H, Milner J. Ethical concerns with online direct-to-consumer pharmaceutical companies. J Med Ethics. 2020;46:168-171.
- Jain T, Lu RJ, Mehrotra A. Prescriptions on demand: the growth of direct-to-consumer telemedicine companies. JAMA. 2019;322:925-926.
- Shahinyan RH, Amighi A, Carey AN, et al. Direct-to-consumer internet prescription platforms overlook crucial pathology found during traditional office evaluation of young men with erectile dysfunction. Urology. 2020;143:165-172.
- Ali M. Andrew Dudum—bold strategies that propelled Hims & Hers into unicorn status. Exit Strategy with Moiz Ali. Published April 2020. Accessed February 23, 2022. https://open.spotify.com/episode/6DtaJxwZDjvZSJI88DTf24?si=b3FHQiUIQY62YjfRHmnJBQ
- Klara K, Kim J, Ross JS. Direct-to-consumer broadcast advertisements for pharmaceuticals: off-label promotion and adherence to FDA guidelines. J Gen Intern Med. 2018;33:651-658.
- Sullivan HW, Aikin KJ, Poehlman J. Communicating risk information in direct-to-consumer prescription drug television ads: a content analysis. Health Commun. 2019;34:212-219.
- Applequist J, Ball JG. An updated analysis of direct-to-consumer television advertisements for prescription drugs. Ann Fam Med. 2018;16:211-216.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Patient safety. World Health Organization. Published September 13, 2019. Accessed February 1, 2022. https://www.who.int/news-room/fact-sheets/detail/patient-safety
- Bollmeier SG, Stevenson E, Finnegan P, et al. Direct to consumer telemedicine: is healthcare from home best? Mo Med. 2020;117:303-309.
26. Court E. Bausch yanked online prescribing after dermatologist backlash. Bloomberg.com. Published March 11, 2020. Accessed September 25, 2020. https://www.bloomberg.com/news/articles/2020-03-11/bausch-yanked-online-prescribing-after-dermatologist-backlash
27. Reitano Z. The future of healthcare: how Ro helps providers treat patients 2 minutes, 2 days, 2 weeks, and 2 years at a time. Medium. Published March 4, 2019. Accessed February 1, 2022. https://medium.com/ro-co/the-future-of-healthcare-how-ro-helps-providers-treat-patients-2-mins-2-days-2-weeks-and-2-10efc0679d7
28. Lee I, Kovarik C, Tejasvi T, et al. Telehealth: helping your patients and practice survive and thrive during the COVID-19 crisis with rapid quality implementation. J Am Acad Dermatol. 2020;82:1213-1214.
29. Pifer R. “Weeks where decades happen”: telehealth 6 months into COVID-19. Healthcare Dive. Published July 27, 2020. Accessed February 23, 2022. https://www.healthcaredive.com/news/telehealth-6-months-coronavirus/581447/
30. Bestsennyy O, Gilbert G, Harris A, et al. Telehealth: a quarter-trillion-dollar post-COVID-19 reality? McKinsey & Company. Updated July 9, 2021. Accessed February 23, 2022. https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality
31. Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
32. Jennings K. Digital health startup Ro raised $500 million at $5 billion valuation. Forbes. March 22, 2021. Accessed March 29, 2022. https://www.forbes.com/sites/katiejennings/2021/03/22/digital-health-startup-ro-raised-500-million-at-5-billion-valuation/?sh=695be0e462f5
33. Hollander JE, Carr BG. Virtually perfect? telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
34. Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
Practice Points
- Direct-to-consumer (DTC) teledermatology platforms are for-profit companies that provide telemedicine visits and sell prescription drugs directly to patients.
- Although they are growing in popularity, DTC teledermatology platforms may lead to overdiagnosis, overtreatment, and fragmentation of health care. Knowledge of teledermatology will be vital to counsel patients on the risks and benefits of these platforms.
FDA to decide by June on future of COVID vaccines
April 6.
But members of the panel also acknowledged that it will be an uphill battle to reach that goal, especially given how quickly the virus continues to change.
The members of the Vaccines and Related Biological Products Advisory Committee said they want to find the balance that makes sure Americans are protected against severe illness and death but doesn’t wear them out with constant recommendations for boosters.
“We don’t feel comfortable with multiple boosters every 8 weeks,” said committee chairman Arnold Monto, MD, professor emeritus of public health at the University of Michigan, Ann Arbor. “We’d love to see an annual vaccination similar to influenza but realize that the evolution of the virus will dictate how we respond in terms of additional vaccine doses.”
The virus itself will dictate vaccination plans, he said.
The government must also keep its focus on convincing Americans who haven’t been vaccinated to join the club, said committee member Henry H. Bernstein, DO, given that “it seems quite obvious that those who are vaccinated do better than those who aren’t vaccinated.”
The government should clearly communicate to the public the goals of vaccination, he said.
“I would suggest that our overall aim is to prevent severe disease, hospitalization, and death more than just infection prevention,” said Dr. Bernstein, professor of pediatrics at Hofstra University, Hempstead, N.Y.
The FDA called the meeting of its advisers to discuss overall booster and vaccine strategy, even though it already authorized a fourth dose of the Pfizer and Moderna vaccines for certain immune compromised adults and for everyone over age 50.
Early in the all-day meeting, temporary committee member James Hildreth, MD, the president of Meharry Medical College, Nashville, Tenn., asked why that authorization was given without the panel’s input. Peter Marks, MD, the director of FDA’s Center for Biologics Evaluation and Research, said the decision was based on data from the United Kingdom and Israel that suggested immunity from a third shot was already waning.
Dr. Marks later said the fourth dose was “authorized as a stopgap measure until we could get something else in place,” because the aim was to protect older Americans who had died at a higher rate than younger individuals.
“I think we’re very much on board that we simply can’t be boosting people as frequently as we are,” said Dr. Marks.
Not enough information to make broader plan
The meeting was meant to be a larger conversation about how to keep pace with the evolving virus and to set up a vaccine selection and development process to better and more quickly respond to changes, such as new variants.
But committee members said they felt stymied by a lack of information. They wanted more data from vaccine manufacturers’ clinical trials. And they noted that so far, there’s no objective, reliable lab-based measurement of COVID-19 vaccine effectiveness – known as a correlate of immunity. Instead, public health officials have looked at rates of hospitalizations and deaths to measure whether the vaccine is still offering protection.
“The question is, what is insufficient protection?” asked H. Cody Meissner, MD, director of pediatric infectious disease at Tufts Medical Center in Boston. “At what point will we say the vaccine isn’t working well enough?”
Centers for Disease Control and Prevention officials presented data showing that a third shot has been more effective than a two-shot regimen in preventing serious disease and death, and that the three shots were significantly more protective than being unvaccinated.
In February, as the Omicron variant continued to rage, unvaccinated Americans aged 5 years and older had an almost three times higher risk of testing positive, and nine times higher risk of dying, compared with those who were considered fully vaccinated, said Heather Scobie, PhD, MPH, a member of the CDC’s COVID-19 Emergency Response team.
But only 98 million Americans – about half of those aged 12 years or older – have received a third dose, Dr. Scobie said.
It’s also still not clear how much more protection a fourth shot adds, or how long it will last. The committee heard data on a just-published study of a fourth dose of the Pfizer vaccine given to some 600,000 Israelis during the Omicron wave from January to March. The rate of severe COVID-19 was 3.5 times lower in the group that received a fourth dose, compared with those who had gotten only three shots, and protection lasted for at least 12 weeks.
Still, study authors said, any protection against infection itself was “short lived.”
More like flu vaccine?
The advisers discussed the possibility of making COVID-19 vaccine development similar to the process for the flu vaccine but acknowledged many difficulties.
The flu predictably hits during the winter in each hemisphere and a global surveillance network helps the World Health Organization decide on the vaccine strains each year. Then each nation’s regulatory and public health officials choose the strains for their shot and vaccine makers begin what is typically a 6-month-long manufacturing process.
COVID outbreaks have happened during all seasons and new variants haven’t always hit every country in a similar fashion. The COVID virus has mutated at five times the speed of the flu virus – producing a new dominant strain in a year, compared with the 3-5 years it takes for the flu virus to do so, said Trevor Bedford, PhD, a professor in the vaccine and infectious disease division at the Fred Hutchinson Cancer Research Center in Seattle.
Global COVID surveillance is patchy and the WHO has not yet created a program to help select strains for a COVID-19 vaccine but is working on a process. Currently, vaccine makers seem to be driving vaccine strain selection, said panelist Paul Offit, MD, professor of paediatrics at Children’s Hospital of Philadelphia. “I feel like to some extent the companies dictate the conversation. It shouldn’t come from them. It should come from us.”
“The important thing is that the public understands how complex this is,” said temporary committee member Oveta A. Fuller, PhD, associate professor of microbiology and immunology at the University of Michigan. “We didn’t get to understand influenza in 2 years. It’s taken years to get an imperfect but useful process to deal with flu.”
A version of this article first appeared on WebMD.com.
April 6.
But members of the panel also acknowledged that it will be an uphill battle to reach that goal, especially given how quickly the virus continues to change.
The members of the Vaccines and Related Biological Products Advisory Committee said they want to find the balance that makes sure Americans are protected against severe illness and death but doesn’t wear them out with constant recommendations for boosters.
“We don’t feel comfortable with multiple boosters every 8 weeks,” said committee chairman Arnold Monto, MD, professor emeritus of public health at the University of Michigan, Ann Arbor. “We’d love to see an annual vaccination similar to influenza but realize that the evolution of the virus will dictate how we respond in terms of additional vaccine doses.”
The virus itself will dictate vaccination plans, he said.
The government must also keep its focus on convincing Americans who haven’t been vaccinated to join the club, said committee member Henry H. Bernstein, DO, given that “it seems quite obvious that those who are vaccinated do better than those who aren’t vaccinated.”
The government should clearly communicate to the public the goals of vaccination, he said.
“I would suggest that our overall aim is to prevent severe disease, hospitalization, and death more than just infection prevention,” said Dr. Bernstein, professor of pediatrics at Hofstra University, Hempstead, N.Y.
The FDA called the meeting of its advisers to discuss overall booster and vaccine strategy, even though it already authorized a fourth dose of the Pfizer and Moderna vaccines for certain immune compromised adults and for everyone over age 50.
Early in the all-day meeting, temporary committee member James Hildreth, MD, the president of Meharry Medical College, Nashville, Tenn., asked why that authorization was given without the panel’s input. Peter Marks, MD, the director of FDA’s Center for Biologics Evaluation and Research, said the decision was based on data from the United Kingdom and Israel that suggested immunity from a third shot was already waning.
Dr. Marks later said the fourth dose was “authorized as a stopgap measure until we could get something else in place,” because the aim was to protect older Americans who had died at a higher rate than younger individuals.
“I think we’re very much on board that we simply can’t be boosting people as frequently as we are,” said Dr. Marks.
Not enough information to make broader plan
The meeting was meant to be a larger conversation about how to keep pace with the evolving virus and to set up a vaccine selection and development process to better and more quickly respond to changes, such as new variants.
But committee members said they felt stymied by a lack of information. They wanted more data from vaccine manufacturers’ clinical trials. And they noted that so far, there’s no objective, reliable lab-based measurement of COVID-19 vaccine effectiveness – known as a correlate of immunity. Instead, public health officials have looked at rates of hospitalizations and deaths to measure whether the vaccine is still offering protection.
“The question is, what is insufficient protection?” asked H. Cody Meissner, MD, director of pediatric infectious disease at Tufts Medical Center in Boston. “At what point will we say the vaccine isn’t working well enough?”
Centers for Disease Control and Prevention officials presented data showing that a third shot has been more effective than a two-shot regimen in preventing serious disease and death, and that the three shots were significantly more protective than being unvaccinated.
In February, as the Omicron variant continued to rage, unvaccinated Americans aged 5 years and older had an almost three times higher risk of testing positive, and nine times higher risk of dying, compared with those who were considered fully vaccinated, said Heather Scobie, PhD, MPH, a member of the CDC’s COVID-19 Emergency Response team.
But only 98 million Americans – about half of those aged 12 years or older – have received a third dose, Dr. Scobie said.
It’s also still not clear how much more protection a fourth shot adds, or how long it will last. The committee heard data on a just-published study of a fourth dose of the Pfizer vaccine given to some 600,000 Israelis during the Omicron wave from January to March. The rate of severe COVID-19 was 3.5 times lower in the group that received a fourth dose, compared with those who had gotten only three shots, and protection lasted for at least 12 weeks.
Still, study authors said, any protection against infection itself was “short lived.”
More like flu vaccine?
The advisers discussed the possibility of making COVID-19 vaccine development similar to the process for the flu vaccine but acknowledged many difficulties.
The flu predictably hits during the winter in each hemisphere and a global surveillance network helps the World Health Organization decide on the vaccine strains each year. Then each nation’s regulatory and public health officials choose the strains for their shot and vaccine makers begin what is typically a 6-month-long manufacturing process.
COVID outbreaks have happened during all seasons and new variants haven’t always hit every country in a similar fashion. The COVID virus has mutated at five times the speed of the flu virus – producing a new dominant strain in a year, compared with the 3-5 years it takes for the flu virus to do so, said Trevor Bedford, PhD, a professor in the vaccine and infectious disease division at the Fred Hutchinson Cancer Research Center in Seattle.
Global COVID surveillance is patchy and the WHO has not yet created a program to help select strains for a COVID-19 vaccine but is working on a process. Currently, vaccine makers seem to be driving vaccine strain selection, said panelist Paul Offit, MD, professor of paediatrics at Children’s Hospital of Philadelphia. “I feel like to some extent the companies dictate the conversation. It shouldn’t come from them. It should come from us.”
“The important thing is that the public understands how complex this is,” said temporary committee member Oveta A. Fuller, PhD, associate professor of microbiology and immunology at the University of Michigan. “We didn’t get to understand influenza in 2 years. It’s taken years to get an imperfect but useful process to deal with flu.”
A version of this article first appeared on WebMD.com.
April 6.
But members of the panel also acknowledged that it will be an uphill battle to reach that goal, especially given how quickly the virus continues to change.
The members of the Vaccines and Related Biological Products Advisory Committee said they want to find the balance that makes sure Americans are protected against severe illness and death but doesn’t wear them out with constant recommendations for boosters.
“We don’t feel comfortable with multiple boosters every 8 weeks,” said committee chairman Arnold Monto, MD, professor emeritus of public health at the University of Michigan, Ann Arbor. “We’d love to see an annual vaccination similar to influenza but realize that the evolution of the virus will dictate how we respond in terms of additional vaccine doses.”
The virus itself will dictate vaccination plans, he said.
The government must also keep its focus on convincing Americans who haven’t been vaccinated to join the club, said committee member Henry H. Bernstein, DO, given that “it seems quite obvious that those who are vaccinated do better than those who aren’t vaccinated.”
The government should clearly communicate to the public the goals of vaccination, he said.
“I would suggest that our overall aim is to prevent severe disease, hospitalization, and death more than just infection prevention,” said Dr. Bernstein, professor of pediatrics at Hofstra University, Hempstead, N.Y.
The FDA called the meeting of its advisers to discuss overall booster and vaccine strategy, even though it already authorized a fourth dose of the Pfizer and Moderna vaccines for certain immune compromised adults and for everyone over age 50.
Early in the all-day meeting, temporary committee member James Hildreth, MD, the president of Meharry Medical College, Nashville, Tenn., asked why that authorization was given without the panel’s input. Peter Marks, MD, the director of FDA’s Center for Biologics Evaluation and Research, said the decision was based on data from the United Kingdom and Israel that suggested immunity from a third shot was already waning.
Dr. Marks later said the fourth dose was “authorized as a stopgap measure until we could get something else in place,” because the aim was to protect older Americans who had died at a higher rate than younger individuals.
“I think we’re very much on board that we simply can’t be boosting people as frequently as we are,” said Dr. Marks.
Not enough information to make broader plan
The meeting was meant to be a larger conversation about how to keep pace with the evolving virus and to set up a vaccine selection and development process to better and more quickly respond to changes, such as new variants.
But committee members said they felt stymied by a lack of information. They wanted more data from vaccine manufacturers’ clinical trials. And they noted that so far, there’s no objective, reliable lab-based measurement of COVID-19 vaccine effectiveness – known as a correlate of immunity. Instead, public health officials have looked at rates of hospitalizations and deaths to measure whether the vaccine is still offering protection.
“The question is, what is insufficient protection?” asked H. Cody Meissner, MD, director of pediatric infectious disease at Tufts Medical Center in Boston. “At what point will we say the vaccine isn’t working well enough?”
Centers for Disease Control and Prevention officials presented data showing that a third shot has been more effective than a two-shot regimen in preventing serious disease and death, and that the three shots were significantly more protective than being unvaccinated.
In February, as the Omicron variant continued to rage, unvaccinated Americans aged 5 years and older had an almost three times higher risk of testing positive, and nine times higher risk of dying, compared with those who were considered fully vaccinated, said Heather Scobie, PhD, MPH, a member of the CDC’s COVID-19 Emergency Response team.
But only 98 million Americans – about half of those aged 12 years or older – have received a third dose, Dr. Scobie said.
It’s also still not clear how much more protection a fourth shot adds, or how long it will last. The committee heard data on a just-published study of a fourth dose of the Pfizer vaccine given to some 600,000 Israelis during the Omicron wave from January to March. The rate of severe COVID-19 was 3.5 times lower in the group that received a fourth dose, compared with those who had gotten only three shots, and protection lasted for at least 12 weeks.
Still, study authors said, any protection against infection itself was “short lived.”
More like flu vaccine?
The advisers discussed the possibility of making COVID-19 vaccine development similar to the process for the flu vaccine but acknowledged many difficulties.
The flu predictably hits during the winter in each hemisphere and a global surveillance network helps the World Health Organization decide on the vaccine strains each year. Then each nation’s regulatory and public health officials choose the strains for their shot and vaccine makers begin what is typically a 6-month-long manufacturing process.
COVID outbreaks have happened during all seasons and new variants haven’t always hit every country in a similar fashion. The COVID virus has mutated at five times the speed of the flu virus – producing a new dominant strain in a year, compared with the 3-5 years it takes for the flu virus to do so, said Trevor Bedford, PhD, a professor in the vaccine and infectious disease division at the Fred Hutchinson Cancer Research Center in Seattle.
Global COVID surveillance is patchy and the WHO has not yet created a program to help select strains for a COVID-19 vaccine but is working on a process. Currently, vaccine makers seem to be driving vaccine strain selection, said panelist Paul Offit, MD, professor of paediatrics at Children’s Hospital of Philadelphia. “I feel like to some extent the companies dictate the conversation. It shouldn’t come from them. It should come from us.”
“The important thing is that the public understands how complex this is,” said temporary committee member Oveta A. Fuller, PhD, associate professor of microbiology and immunology at the University of Michigan. “We didn’t get to understand influenza in 2 years. It’s taken years to get an imperfect but useful process to deal with flu.”
A version of this article first appeared on WebMD.com.
U.S. pulls COVID drug as Omicron subvariant spreads
F, the Omicron subvariant that now accounts for most new cases in the United States, The Associated Press reports.
The Food and Drug Administration announced that the antibody drug sotrovimab is no longer authorized to treat patients in U.S. states or territories. The decision was expected, as the FDA restricted the drug’s use across the country throughout March as BA.2 became dominant in certain regions, the AP reported.
The BA.2 subvariant now accounts for 72% of new COVID-19 cases sequenced by health authorities, according to the latest CDC data updated April 5. The FDA cited the CDC data in its reason for pulling back on the authorization of the drug.
The GlaxoSmithKline drug is the latest antibody medication to be pulled due to coronavirus mutations. In January, the FDA halted the use of antibody drugs from Regeneron and Eli Lilly because they didn’t work against the Omicron variant.
The FDA’s decision means that one antibody drug is still authorized for use against routine COVID-19 cases, the AP reported. A different Eli Lilly drug – bebtelovimab – still appears to work against BA.2.
Doctors can also prescribe antiviral pills, which typically affect the coronavirus spike protein and aren’t affected by mutations, to treat mild to moderate COVID-19, the AP reported. The authorized pills from Pfizer and Merck – Paxlovid and Lagevrio – have been shipped to pharmacy chains and medical clinics in hopes of getting them to patients early enough to work.
The federal government purchased nearly $2 billion worth of the GlaxoSmithKline drug and shipped more than 900,000 doses to states last fall, the AP reported. In March, the company announced that it was studying a higher dose that could be effective against BA.2, which would require FDA approval before resuming use in the United States.
The antibody drugs mimic the virus-blocking proteins found in the human body, the AP reported. They’re designed to attack a specific virus and need to be updated as the coronavirus mutates.
A version of this article first appeared on WebMD.com.
F, the Omicron subvariant that now accounts for most new cases in the United States, The Associated Press reports.
The Food and Drug Administration announced that the antibody drug sotrovimab is no longer authorized to treat patients in U.S. states or territories. The decision was expected, as the FDA restricted the drug’s use across the country throughout March as BA.2 became dominant in certain regions, the AP reported.
The BA.2 subvariant now accounts for 72% of new COVID-19 cases sequenced by health authorities, according to the latest CDC data updated April 5. The FDA cited the CDC data in its reason for pulling back on the authorization of the drug.
The GlaxoSmithKline drug is the latest antibody medication to be pulled due to coronavirus mutations. In January, the FDA halted the use of antibody drugs from Regeneron and Eli Lilly because they didn’t work against the Omicron variant.
The FDA’s decision means that one antibody drug is still authorized for use against routine COVID-19 cases, the AP reported. A different Eli Lilly drug – bebtelovimab – still appears to work against BA.2.
Doctors can also prescribe antiviral pills, which typically affect the coronavirus spike protein and aren’t affected by mutations, to treat mild to moderate COVID-19, the AP reported. The authorized pills from Pfizer and Merck – Paxlovid and Lagevrio – have been shipped to pharmacy chains and medical clinics in hopes of getting them to patients early enough to work.
The federal government purchased nearly $2 billion worth of the GlaxoSmithKline drug and shipped more than 900,000 doses to states last fall, the AP reported. In March, the company announced that it was studying a higher dose that could be effective against BA.2, which would require FDA approval before resuming use in the United States.
The antibody drugs mimic the virus-blocking proteins found in the human body, the AP reported. They’re designed to attack a specific virus and need to be updated as the coronavirus mutates.
A version of this article first appeared on WebMD.com.
F, the Omicron subvariant that now accounts for most new cases in the United States, The Associated Press reports.
The Food and Drug Administration announced that the antibody drug sotrovimab is no longer authorized to treat patients in U.S. states or territories. The decision was expected, as the FDA restricted the drug’s use across the country throughout March as BA.2 became dominant in certain regions, the AP reported.
The BA.2 subvariant now accounts for 72% of new COVID-19 cases sequenced by health authorities, according to the latest CDC data updated April 5. The FDA cited the CDC data in its reason for pulling back on the authorization of the drug.
The GlaxoSmithKline drug is the latest antibody medication to be pulled due to coronavirus mutations. In January, the FDA halted the use of antibody drugs from Regeneron and Eli Lilly because they didn’t work against the Omicron variant.
The FDA’s decision means that one antibody drug is still authorized for use against routine COVID-19 cases, the AP reported. A different Eli Lilly drug – bebtelovimab – still appears to work against BA.2.
Doctors can also prescribe antiviral pills, which typically affect the coronavirus spike protein and aren’t affected by mutations, to treat mild to moderate COVID-19, the AP reported. The authorized pills from Pfizer and Merck – Paxlovid and Lagevrio – have been shipped to pharmacy chains and medical clinics in hopes of getting them to patients early enough to work.
The federal government purchased nearly $2 billion worth of the GlaxoSmithKline drug and shipped more than 900,000 doses to states last fall, the AP reported. In March, the company announced that it was studying a higher dose that could be effective against BA.2, which would require FDA approval before resuming use in the United States.
The antibody drugs mimic the virus-blocking proteins found in the human body, the AP reported. They’re designed to attack a specific virus and need to be updated as the coronavirus mutates.
A version of this article first appeared on WebMD.com.
Preterm C-sections, induced deliveries dropped during COVID-19 pandemic
Premature births from cesarean (C-section) and induced deliveries dropped abruptly by 6.5% from the projected number in the first month of the COVID-19 pandemic and stayed at the lower rate consistently throughout the year, researchers have found.
Results of the study, led by Daniel Dench, PhD, assistant professor at the Georgia Institute of Technology School of Economics in Atlanta, were published online in Pediatrics.
The authors say their findings help answer the question of whether numbers of preterm (less than 37 weeks gestation) C-sections and induced deliveries would change if women didn’t see their physicians during pregnancy as often, especially in person, and raise the question of whether some birth interventions by physicians may not be necessary. The pandemic gave researchers a natural, ethical way to study the question.
The researchers found that in March 2020 – the start of business closures and stay-at-home orders around the country – preterm births from C-sections or induced deliveries immediately fell from the forecast number for the month by 0.4 percentage points. For the rest of 2020, the number remained on average 0.35 percentage points below the numbers predicted.
That means 350 fewer preterm C-sections and induced deliveries per 100,000 live births, or 10,000 fewer overall, the authors said.
Dr. Dench told this publication the numbers for those births had been steady from January 2010 to February 2020, but the pattern “diverges from this trend very clearly beginning exactly in March 2020 and does not return to trend by December 2020.”
Meanwhile, during the study period, the number of full-term cesarean and induced deliveries stayed steady and started to increase slightly in 2020. Researchers also adjusted for seasonality as, for example, preterm births are higher on average in February than in March.
So far, Dr. Dench said in a press release, it’s not clear whether the lower numbers mean physicians didn’t deliver babies that ended up surviving in the womb anyway or if they missed some that would die in the womb without intervention.
To better understand those implications, Dr. Dench says he is turning to fetal death records for March-December 2020 and he said he expects to have those results analyzed by the end of the year.
If there was no change in fetal deaths at the same time as the drop in preterm births, Dr. Dench said, that could point to physician interventions that may not have been necessary.
Mya R. Zapata, MD, an obstetrician-gynecologist with UCLA Health, who was not involved with the study, told this publication that checking the fetal deaths is a good start and an objective outcome in answering the question, but she points out there are other outcomes that will take a deeper analysis, such as whether there are differences later in developmental outcomes after fewer physician visits.
“It’s always a good question for health care,” she said, “are we doing more than we need to?”
Dr. Zapata is the obstetrics service chief for UCLA’s labor and delivery unit and was an integral part of decision-making as to what services were essential and for which patients. She said the fewer visits and fewer ultrasounds the researchers describe fit with what ob.gyns. at UCLA experienced as the pandemic hit.
“We really tried to hone in on people who were at highest risk for an adverse outcome,” she said. “I still have the question of whether there were things we missed in low-risk people. It will take time to get the entire answer. But it does make us reflect that perhaps less intervention could be better for patients and easier. It’s our job in medicine to keep asking the question of what is essential and safe and not just continue with current practice because that’s what we’ve always done.”
The amount of data gave the researchers an unusual view. They studied 38,891,271 singleton births in the United States from 2010 to 2020 with data from the National Center for Health Statistics.
“If you look at 1,000 births in a single hospital, or even at 30,000 births across a hospital system, you wouldn’t be able to see the drop as clearly,” Dr. Dench said. “The drop we detected is a huge change, but you might miss it in a small sample.”
The researchers acknowledge a limitation of the study is that half of all preterm C-sections and induced deliveries happen because of a ruptured membrane, a spontaneous cause. Those instances can’t be distinguished from the ones caused by doctors’ interventions in this study.
“Still, these findings are significant because the causes for preterm births are not always known,” the authors wrote in the press release.
The study authors and Dr. Zapata reported no relevant financial relationships.
Premature births from cesarean (C-section) and induced deliveries dropped abruptly by 6.5% from the projected number in the first month of the COVID-19 pandemic and stayed at the lower rate consistently throughout the year, researchers have found.
Results of the study, led by Daniel Dench, PhD, assistant professor at the Georgia Institute of Technology School of Economics in Atlanta, were published online in Pediatrics.
The authors say their findings help answer the question of whether numbers of preterm (less than 37 weeks gestation) C-sections and induced deliveries would change if women didn’t see their physicians during pregnancy as often, especially in person, and raise the question of whether some birth interventions by physicians may not be necessary. The pandemic gave researchers a natural, ethical way to study the question.
The researchers found that in March 2020 – the start of business closures and stay-at-home orders around the country – preterm births from C-sections or induced deliveries immediately fell from the forecast number for the month by 0.4 percentage points. For the rest of 2020, the number remained on average 0.35 percentage points below the numbers predicted.
That means 350 fewer preterm C-sections and induced deliveries per 100,000 live births, or 10,000 fewer overall, the authors said.
Dr. Dench told this publication the numbers for those births had been steady from January 2010 to February 2020, but the pattern “diverges from this trend very clearly beginning exactly in March 2020 and does not return to trend by December 2020.”
Meanwhile, during the study period, the number of full-term cesarean and induced deliveries stayed steady and started to increase slightly in 2020. Researchers also adjusted for seasonality as, for example, preterm births are higher on average in February than in March.
So far, Dr. Dench said in a press release, it’s not clear whether the lower numbers mean physicians didn’t deliver babies that ended up surviving in the womb anyway or if they missed some that would die in the womb without intervention.
To better understand those implications, Dr. Dench says he is turning to fetal death records for March-December 2020 and he said he expects to have those results analyzed by the end of the year.
If there was no change in fetal deaths at the same time as the drop in preterm births, Dr. Dench said, that could point to physician interventions that may not have been necessary.
Mya R. Zapata, MD, an obstetrician-gynecologist with UCLA Health, who was not involved with the study, told this publication that checking the fetal deaths is a good start and an objective outcome in answering the question, but she points out there are other outcomes that will take a deeper analysis, such as whether there are differences later in developmental outcomes after fewer physician visits.
“It’s always a good question for health care,” she said, “are we doing more than we need to?”
Dr. Zapata is the obstetrics service chief for UCLA’s labor and delivery unit and was an integral part of decision-making as to what services were essential and for which patients. She said the fewer visits and fewer ultrasounds the researchers describe fit with what ob.gyns. at UCLA experienced as the pandemic hit.
“We really tried to hone in on people who were at highest risk for an adverse outcome,” she said. “I still have the question of whether there were things we missed in low-risk people. It will take time to get the entire answer. But it does make us reflect that perhaps less intervention could be better for patients and easier. It’s our job in medicine to keep asking the question of what is essential and safe and not just continue with current practice because that’s what we’ve always done.”
The amount of data gave the researchers an unusual view. They studied 38,891,271 singleton births in the United States from 2010 to 2020 with data from the National Center for Health Statistics.
“If you look at 1,000 births in a single hospital, or even at 30,000 births across a hospital system, you wouldn’t be able to see the drop as clearly,” Dr. Dench said. “The drop we detected is a huge change, but you might miss it in a small sample.”
The researchers acknowledge a limitation of the study is that half of all preterm C-sections and induced deliveries happen because of a ruptured membrane, a spontaneous cause. Those instances can’t be distinguished from the ones caused by doctors’ interventions in this study.
“Still, these findings are significant because the causes for preterm births are not always known,” the authors wrote in the press release.
The study authors and Dr. Zapata reported no relevant financial relationships.
Premature births from cesarean (C-section) and induced deliveries dropped abruptly by 6.5% from the projected number in the first month of the COVID-19 pandemic and stayed at the lower rate consistently throughout the year, researchers have found.
Results of the study, led by Daniel Dench, PhD, assistant professor at the Georgia Institute of Technology School of Economics in Atlanta, were published online in Pediatrics.
The authors say their findings help answer the question of whether numbers of preterm (less than 37 weeks gestation) C-sections and induced deliveries would change if women didn’t see their physicians during pregnancy as often, especially in person, and raise the question of whether some birth interventions by physicians may not be necessary. The pandemic gave researchers a natural, ethical way to study the question.
The researchers found that in March 2020 – the start of business closures and stay-at-home orders around the country – preterm births from C-sections or induced deliveries immediately fell from the forecast number for the month by 0.4 percentage points. For the rest of 2020, the number remained on average 0.35 percentage points below the numbers predicted.
That means 350 fewer preterm C-sections and induced deliveries per 100,000 live births, or 10,000 fewer overall, the authors said.
Dr. Dench told this publication the numbers for those births had been steady from January 2010 to February 2020, but the pattern “diverges from this trend very clearly beginning exactly in March 2020 and does not return to trend by December 2020.”
Meanwhile, during the study period, the number of full-term cesarean and induced deliveries stayed steady and started to increase slightly in 2020. Researchers also adjusted for seasonality as, for example, preterm births are higher on average in February than in March.
So far, Dr. Dench said in a press release, it’s not clear whether the lower numbers mean physicians didn’t deliver babies that ended up surviving in the womb anyway or if they missed some that would die in the womb without intervention.
To better understand those implications, Dr. Dench says he is turning to fetal death records for March-December 2020 and he said he expects to have those results analyzed by the end of the year.
If there was no change in fetal deaths at the same time as the drop in preterm births, Dr. Dench said, that could point to physician interventions that may not have been necessary.
Mya R. Zapata, MD, an obstetrician-gynecologist with UCLA Health, who was not involved with the study, told this publication that checking the fetal deaths is a good start and an objective outcome in answering the question, but she points out there are other outcomes that will take a deeper analysis, such as whether there are differences later in developmental outcomes after fewer physician visits.
“It’s always a good question for health care,” she said, “are we doing more than we need to?”
Dr. Zapata is the obstetrics service chief for UCLA’s labor and delivery unit and was an integral part of decision-making as to what services were essential and for which patients. She said the fewer visits and fewer ultrasounds the researchers describe fit with what ob.gyns. at UCLA experienced as the pandemic hit.
“We really tried to hone in on people who were at highest risk for an adverse outcome,” she said. “I still have the question of whether there were things we missed in low-risk people. It will take time to get the entire answer. But it does make us reflect that perhaps less intervention could be better for patients and easier. It’s our job in medicine to keep asking the question of what is essential and safe and not just continue with current practice because that’s what we’ve always done.”
The amount of data gave the researchers an unusual view. They studied 38,891,271 singleton births in the United States from 2010 to 2020 with data from the National Center for Health Statistics.
“If you look at 1,000 births in a single hospital, or even at 30,000 births across a hospital system, you wouldn’t be able to see the drop as clearly,” Dr. Dench said. “The drop we detected is a huge change, but you might miss it in a small sample.”
The researchers acknowledge a limitation of the study is that half of all preterm C-sections and induced deliveries happen because of a ruptured membrane, a spontaneous cause. Those instances can’t be distinguished from the ones caused by doctors’ interventions in this study.
“Still, these findings are significant because the causes for preterm births are not always known,” the authors wrote in the press release.
The study authors and Dr. Zapata reported no relevant financial relationships.
FROM PEDIATRICS
White House announces long-COVID action plan
The National Research Action Plan on Long COVID will gather experts from various agencies, including the Department of Defense and the Department of Veterans Affairs, to expand existing long-COVID clinics and broaden research on symptoms of the virus that persist long after infection.
“We’ll collaborate with academic, industry, state and local partners to better understand long COVID,” Health and Human Services Secretary Xavier Becerra said at a White House briefing April 5. “We need to work as aggressively as we can to make sure no American is left behind.”
The plan will build on the RECOVER Initiative, a $1.15 billion effort announced last year that will study long COVID.
The COVID-19 Response Team also announced that the United States will donate tens of millions of pediatric coronavirus vaccines to other countries. More than 20 countries have asked for the donations, the team said.
The United States has delivered more than 500 million vaccine doses to 114 countries.
Meanwhile, national COVID-19 numbers continue to fall. CDC Director Rochelle Walensky, MD, reported that average daily cases are down 4% this week to 25,000; hospitalizations have dropped 17% to 1,400 per day; and daily deaths are down to 570 a day, which is a decrease of about 17%.
New national estimates show that Omicron’s subvariant BA.2 now accounts for 72% of circulating variants nationally, she said.
Top infectious disease expert Anthony Fauci, MD, reported that recent data supports the need for a second booster among certain people 50 and older – a move authorized by the Food and Drug Administration and Centers for Disease Control and Prevention last week.
“The effectiveness of the first booster dose we know wanes over time, and growing evidence shows a second dose can restore vaccine effectiveness for certain populations,” he said.
Dr. Fauci reported findings from an Israeli study of more than 1 million people 60 and older, which showed that an additional booster dose after 4 months lowered the rate of infection by two times and lowered the rate of severe infection by more than four times.
Another study from Israeli scientists showed that out of half a million people 60 and older, a second booster after 4 months brought a 78% reduction in death, compared to those who received only the first boost.
A version of this article first appeared on WebMD.com.
The National Research Action Plan on Long COVID will gather experts from various agencies, including the Department of Defense and the Department of Veterans Affairs, to expand existing long-COVID clinics and broaden research on symptoms of the virus that persist long after infection.
“We’ll collaborate with academic, industry, state and local partners to better understand long COVID,” Health and Human Services Secretary Xavier Becerra said at a White House briefing April 5. “We need to work as aggressively as we can to make sure no American is left behind.”
The plan will build on the RECOVER Initiative, a $1.15 billion effort announced last year that will study long COVID.
The COVID-19 Response Team also announced that the United States will donate tens of millions of pediatric coronavirus vaccines to other countries. More than 20 countries have asked for the donations, the team said.
The United States has delivered more than 500 million vaccine doses to 114 countries.
Meanwhile, national COVID-19 numbers continue to fall. CDC Director Rochelle Walensky, MD, reported that average daily cases are down 4% this week to 25,000; hospitalizations have dropped 17% to 1,400 per day; and daily deaths are down to 570 a day, which is a decrease of about 17%.
New national estimates show that Omicron’s subvariant BA.2 now accounts for 72% of circulating variants nationally, she said.
Top infectious disease expert Anthony Fauci, MD, reported that recent data supports the need for a second booster among certain people 50 and older – a move authorized by the Food and Drug Administration and Centers for Disease Control and Prevention last week.
“The effectiveness of the first booster dose we know wanes over time, and growing evidence shows a second dose can restore vaccine effectiveness for certain populations,” he said.
Dr. Fauci reported findings from an Israeli study of more than 1 million people 60 and older, which showed that an additional booster dose after 4 months lowered the rate of infection by two times and lowered the rate of severe infection by more than four times.
Another study from Israeli scientists showed that out of half a million people 60 and older, a second booster after 4 months brought a 78% reduction in death, compared to those who received only the first boost.
A version of this article first appeared on WebMD.com.
The National Research Action Plan on Long COVID will gather experts from various agencies, including the Department of Defense and the Department of Veterans Affairs, to expand existing long-COVID clinics and broaden research on symptoms of the virus that persist long after infection.
“We’ll collaborate with academic, industry, state and local partners to better understand long COVID,” Health and Human Services Secretary Xavier Becerra said at a White House briefing April 5. “We need to work as aggressively as we can to make sure no American is left behind.”
The plan will build on the RECOVER Initiative, a $1.15 billion effort announced last year that will study long COVID.
The COVID-19 Response Team also announced that the United States will donate tens of millions of pediatric coronavirus vaccines to other countries. More than 20 countries have asked for the donations, the team said.
The United States has delivered more than 500 million vaccine doses to 114 countries.
Meanwhile, national COVID-19 numbers continue to fall. CDC Director Rochelle Walensky, MD, reported that average daily cases are down 4% this week to 25,000; hospitalizations have dropped 17% to 1,400 per day; and daily deaths are down to 570 a day, which is a decrease of about 17%.
New national estimates show that Omicron’s subvariant BA.2 now accounts for 72% of circulating variants nationally, she said.
Top infectious disease expert Anthony Fauci, MD, reported that recent data supports the need for a second booster among certain people 50 and older – a move authorized by the Food and Drug Administration and Centers for Disease Control and Prevention last week.
“The effectiveness of the first booster dose we know wanes over time, and growing evidence shows a second dose can restore vaccine effectiveness for certain populations,” he said.
Dr. Fauci reported findings from an Israeli study of more than 1 million people 60 and older, which showed that an additional booster dose after 4 months lowered the rate of infection by two times and lowered the rate of severe infection by more than four times.
Another study from Israeli scientists showed that out of half a million people 60 and older, a second booster after 4 months brought a 78% reduction in death, compared to those who received only the first boost.
A version of this article first appeared on WebMD.com.
New COVID combo-variant XE found in U.K.
As of last week, the U.K. Health Security Agency had found 637 cases of the variant, known as XE. The earliest case was found Jan. 19.
The new strain is known as a recombinant, which means it is a combination of two variants or viruses.
XE makes up less than 1% of sequenced cases in the United Kingdom so far, and there is no evidence yet that the strain leads to more severe disease or less vaccine protection.
“Right now, there’s really no public health concern,” John Brownstein, PhD, an epidemiologist and chief innovation officer at Boston Children’s Hospital, told ABC. “Recombinant variants happen over and over. In fact, the reason that this is the XE variant recombinant is that we’ve had XA, XB, XC, XD already, and none of those have turned out to be any real concern.”
A World Health Organization update published March 29 notes XE’s high transmissibility and says it may have a growth advantage of 10% over the BA.2 subvariant that now makes up more than 70% of cases in the United States.
A version of this article first appeared on WebMD.com.
As of last week, the U.K. Health Security Agency had found 637 cases of the variant, known as XE. The earliest case was found Jan. 19.
The new strain is known as a recombinant, which means it is a combination of two variants or viruses.
XE makes up less than 1% of sequenced cases in the United Kingdom so far, and there is no evidence yet that the strain leads to more severe disease or less vaccine protection.
“Right now, there’s really no public health concern,” John Brownstein, PhD, an epidemiologist and chief innovation officer at Boston Children’s Hospital, told ABC. “Recombinant variants happen over and over. In fact, the reason that this is the XE variant recombinant is that we’ve had XA, XB, XC, XD already, and none of those have turned out to be any real concern.”
A World Health Organization update published March 29 notes XE’s high transmissibility and says it may have a growth advantage of 10% over the BA.2 subvariant that now makes up more than 70% of cases in the United States.
A version of this article first appeared on WebMD.com.
As of last week, the U.K. Health Security Agency had found 637 cases of the variant, known as XE. The earliest case was found Jan. 19.
The new strain is known as a recombinant, which means it is a combination of two variants or viruses.
XE makes up less than 1% of sequenced cases in the United Kingdom so far, and there is no evidence yet that the strain leads to more severe disease or less vaccine protection.
“Right now, there’s really no public health concern,” John Brownstein, PhD, an epidemiologist and chief innovation officer at Boston Children’s Hospital, told ABC. “Recombinant variants happen over and over. In fact, the reason that this is the XE variant recombinant is that we’ve had XA, XB, XC, XD already, and none of those have turned out to be any real concern.”
A World Health Organization update published March 29 notes XE’s high transmissibility and says it may have a growth advantage of 10% over the BA.2 subvariant that now makes up more than 70% of cases in the United States.
A version of this article first appeared on WebMD.com.
Children and COVID-19: Decline in new cases may be leveling off
Even as a number of states see increases in new COVID-19 cases among all ages, the trend remains downward for children, albeit at a slower pace than in recent weeks, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
New pediatric cases in the United States totaled 27,521 for the most recent week, March 25-31, down by 5.2% from the previous week. Earlier weekly declines, going backward through March and into late February, were 9.3%, 23%, 39.5%, and 46%, according to data collected by the AAP and CHA from state and territorial health agencies. The lowest weekly total recorded since the initial wave in 2020 was just under 8,500 during the week of June 18-24, 2021.
Reported COVID-19 cases in children now total over 12.8 million since the beginning of the pandemic in March 2020, and those infections represent 19.0% of all cases. That share of new cases has not increased in the last 7 weeks, the AAP and CHA noted in their weekly COVID report, suggesting that children have not been bearing a disproportionate share of the declining Omicron burden.
As for Omicron, the BA.2 subvariant now makes up about 55% of COVID-19 infections, the Centers for Disease Control and Prevention said in its COVID Data Tracker Weekly Review, and New York, Massachusetts, and New Jersey are among the states reporting BA.2-driven increases in new cases of as much as 30%, the New York Times said.
Rates of new cases for the latest week available (March 27 to April 2) and at their Omicron peaks in January were 11.3 per 100,000 and 1,011 per 100,000 (ages 0-4 years), 12.5 and 1,505 per 100,000 (5-11 years), 12.7 and 1,779 per 100,000 (12-15 years), and 13.1 and 1,982 per 100,000 (16-17 years), the CDC said on its COVID Data Tracker.
Hospitalization rates, however, were a bit of a mixed bag. The last 2 weeks (March 13-19 and March 20-26) of data available from the CDC’s COVID-NET show that hospitalizations were up slightly in children aged 0-4 years (1.3 per 100,000 to 1.4 per 100,000), down for 5- to 11-year-olds (0.6 to 0.2), and steady for those aged 12-17 (0.4 to 0.4). COVID-NET collects data from nearly 100 counties in 10 states and from a separate four-state network.
Vaccinations got a small boost in the last week, the first one since early February. Initial doses and completions climbed slightly in the 12- to 17-year-olds, while just first doses were up a bit among the 5- to 11-year-olds during the week of March 24-30, compared with the previous week, although both groups are still well below the highest counts recorded so far in 2022, which are, in turn, far short of 2021’s peaks, according to CDC data analyzed by the AAP.
Even as a number of states see increases in new COVID-19 cases among all ages, the trend remains downward for children, albeit at a slower pace than in recent weeks, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
New pediatric cases in the United States totaled 27,521 for the most recent week, March 25-31, down by 5.2% from the previous week. Earlier weekly declines, going backward through March and into late February, were 9.3%, 23%, 39.5%, and 46%, according to data collected by the AAP and CHA from state and territorial health agencies. The lowest weekly total recorded since the initial wave in 2020 was just under 8,500 during the week of June 18-24, 2021.
Reported COVID-19 cases in children now total over 12.8 million since the beginning of the pandemic in March 2020, and those infections represent 19.0% of all cases. That share of new cases has not increased in the last 7 weeks, the AAP and CHA noted in their weekly COVID report, suggesting that children have not been bearing a disproportionate share of the declining Omicron burden.
As for Omicron, the BA.2 subvariant now makes up about 55% of COVID-19 infections, the Centers for Disease Control and Prevention said in its COVID Data Tracker Weekly Review, and New York, Massachusetts, and New Jersey are among the states reporting BA.2-driven increases in new cases of as much as 30%, the New York Times said.
Rates of new cases for the latest week available (March 27 to April 2) and at their Omicron peaks in January were 11.3 per 100,000 and 1,011 per 100,000 (ages 0-4 years), 12.5 and 1,505 per 100,000 (5-11 years), 12.7 and 1,779 per 100,000 (12-15 years), and 13.1 and 1,982 per 100,000 (16-17 years), the CDC said on its COVID Data Tracker.
Hospitalization rates, however, were a bit of a mixed bag. The last 2 weeks (March 13-19 and March 20-26) of data available from the CDC’s COVID-NET show that hospitalizations were up slightly in children aged 0-4 years (1.3 per 100,000 to 1.4 per 100,000), down for 5- to 11-year-olds (0.6 to 0.2), and steady for those aged 12-17 (0.4 to 0.4). COVID-NET collects data from nearly 100 counties in 10 states and from a separate four-state network.
Vaccinations got a small boost in the last week, the first one since early February. Initial doses and completions climbed slightly in the 12- to 17-year-olds, while just first doses were up a bit among the 5- to 11-year-olds during the week of March 24-30, compared with the previous week, although both groups are still well below the highest counts recorded so far in 2022, which are, in turn, far short of 2021’s peaks, according to CDC data analyzed by the AAP.
Even as a number of states see increases in new COVID-19 cases among all ages, the trend remains downward for children, albeit at a slower pace than in recent weeks, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
New pediatric cases in the United States totaled 27,521 for the most recent week, March 25-31, down by 5.2% from the previous week. Earlier weekly declines, going backward through March and into late February, were 9.3%, 23%, 39.5%, and 46%, according to data collected by the AAP and CHA from state and territorial health agencies. The lowest weekly total recorded since the initial wave in 2020 was just under 8,500 during the week of June 18-24, 2021.
Reported COVID-19 cases in children now total over 12.8 million since the beginning of the pandemic in March 2020, and those infections represent 19.0% of all cases. That share of new cases has not increased in the last 7 weeks, the AAP and CHA noted in their weekly COVID report, suggesting that children have not been bearing a disproportionate share of the declining Omicron burden.
As for Omicron, the BA.2 subvariant now makes up about 55% of COVID-19 infections, the Centers for Disease Control and Prevention said in its COVID Data Tracker Weekly Review, and New York, Massachusetts, and New Jersey are among the states reporting BA.2-driven increases in new cases of as much as 30%, the New York Times said.
Rates of new cases for the latest week available (March 27 to April 2) and at their Omicron peaks in January were 11.3 per 100,000 and 1,011 per 100,000 (ages 0-4 years), 12.5 and 1,505 per 100,000 (5-11 years), 12.7 and 1,779 per 100,000 (12-15 years), and 13.1 and 1,982 per 100,000 (16-17 years), the CDC said on its COVID Data Tracker.
Hospitalization rates, however, were a bit of a mixed bag. The last 2 weeks (March 13-19 and March 20-26) of data available from the CDC’s COVID-NET show that hospitalizations were up slightly in children aged 0-4 years (1.3 per 100,000 to 1.4 per 100,000), down for 5- to 11-year-olds (0.6 to 0.2), and steady for those aged 12-17 (0.4 to 0.4). COVID-NET collects data from nearly 100 counties in 10 states and from a separate four-state network.
Vaccinations got a small boost in the last week, the first one since early February. Initial doses and completions climbed slightly in the 12- to 17-year-olds, while just first doses were up a bit among the 5- to 11-year-olds during the week of March 24-30, compared with the previous week, although both groups are still well below the highest counts recorded so far in 2022, which are, in turn, far short of 2021’s peaks, according to CDC data analyzed by the AAP.
More evidence that COVID ‘brain fog’ is biologically based
Researchers found elevated levels of CSF immune activation and immunovascular markers in individuals with cognitive postacute sequelae of SARS-CoV-2 infection (PASC). Patients whose cognitive symptoms developed during the acute phase of COVID-19 had the highest levels of brain inflammation.
The findings add to a growing body of evidence that suggests the condition often referred to as “brain fog” has a neurologic basis, said lead author Joanna Hellmuth, MD, MHS, assistant professor of neurology at the University of California, San Francisco Weill Institute of Neurosciences and the UCSF Memory and Aging Center.
The findings will be presented at the 2022 annual meeting of the American Academy of Neurology.
Inflammatory response
There are no effective diagnostic tests or treatments for cognitive PASC, which prompted the investigators to study inflammation in patients with the condition. Initial findings were reported earlier in 2022, which showed abnormalities in the CSF in 77% of patients with cognitive impairment. Patients without cognitive impairments had normal CSF.
Extending that work in this new study, researchers studied patients from the Long-term Impact of Infection With Novel Coronavirus (LIINC) study with confirmed SARS-CoV-2 infection who were not hospitalized. They conducted 2-hour neurocognitive interviews and identified 23 people with new, persistent cognitive symptoms (cognitive PASC) and 10 with no cognitive symptoms who served as controls.
All participants underwent additional neurologic examination and neuropsychological testing, and half agreed to a lumbar puncture to allow researchers to collect CSF samples. The CSF was collected a median of 10.2 months after initial COVID symptoms began.
Participants with cognitive PASC had higher median levels of CSF acute phase reactants C-reactive protein (0.007 mg/L vs. 0.000 mg/L; P =.004) and serum amyloid A (0.001 mg/L vs. 0.000 mg/L; P = .001), compared with COVID controls.
The PASC group also had elevated levels of CSF immune activation markers interferon gamma–inducible protein (IP-10), interleukin-8, and immunovascular markers vascular endothelial growth factor-C and VEGFR-1, although the differences with the control group were not statistically significant.
The timing of the onset of cognitive problems was also associated with higher levels of immune activation and immunovascular markers. Patients with brain fog that developed during the acute phase of COVID-19 had higher levels of CSF VEGF-C, compared with patients whose cognitive symptoms developed more than a month after initial COVID symptoms (173 pg/mL vs. 99 pg/mL; P = .048) and COVID controls (79 pg/mL; P = .048).
Acute onset cognitive PASC participants had higher CSF levels of IP-10 (P = .030), IL-8 (P = .048), placental growth factor (P = .030) and intercellular adhesion molecule-1 (P = .045), compared with COVID controls.
Researchers believe these new findings could mean that intrathecal immune activation and endothelial activation/dysfunction may contribute to cognitive PASC and that the mechanisms involved may be different in patients with acute cognitive PASC versus those with delayed onset.
“Our data suggests that perhaps in these people with more acute cognitive changes they don’t have the return to homeostasis,” Dr. Hellmuth said, while patients with delayed onset cognitive PASC had levels more in line with COVID patients who had no cognitive issues.
Moving the needle forward
Commenting on the findings, William Schaffner, MD, professor of infectious diseases, Vanderbilt University Medical Center, Nashville, Tenn., said that, while the study doesn’t rule out a possible psychological basis for cognitive PASC, it adds more weight to the biological argument.
“When you have nonspecific symptoms for which specific tests are unavailable,” Dr. Schaffner explained, “there is a natural question that always comes up: Is this principally a biologically induced phenomenon or psychological? This moves the needle substantially in the direction of a biological phenomenon.”
Another important element to the study, Dr. Schaffner said, is that the patients involved had mild COVID.
“Not every patient with long COVID symptoms had been hospitalized with severe disease,” he said. “There are inflammatory phenomenon in various organ systems such that even if the inflammatory response in the lung was not severe enough to get you into the hospital, there were inflammatory responses in other organ systems that could persist once the acute infection resolved.”
Although the small size of the study is a limitation, Dr. Schaffner said that shouldn’t minimize the importance of these findings.
“That it’s small doesn’t diminish its value,” he said. “The next step forward might be to try to associate the markers more specifically with COVID. The more precise we can be, the more convincing the story will become.”
The study was funded by the National Institutes of Health. Dr. Hellmuth received grant support from the National Institutes of Health/National Institute of Mental Health supporting this work and personal fees for medical-legal consultation outside of the submitted work. Dr. Schaffner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers found elevated levels of CSF immune activation and immunovascular markers in individuals with cognitive postacute sequelae of SARS-CoV-2 infection (PASC). Patients whose cognitive symptoms developed during the acute phase of COVID-19 had the highest levels of brain inflammation.
The findings add to a growing body of evidence that suggests the condition often referred to as “brain fog” has a neurologic basis, said lead author Joanna Hellmuth, MD, MHS, assistant professor of neurology at the University of California, San Francisco Weill Institute of Neurosciences and the UCSF Memory and Aging Center.
The findings will be presented at the 2022 annual meeting of the American Academy of Neurology.
Inflammatory response
There are no effective diagnostic tests or treatments for cognitive PASC, which prompted the investigators to study inflammation in patients with the condition. Initial findings were reported earlier in 2022, which showed abnormalities in the CSF in 77% of patients with cognitive impairment. Patients without cognitive impairments had normal CSF.
Extending that work in this new study, researchers studied patients from the Long-term Impact of Infection With Novel Coronavirus (LIINC) study with confirmed SARS-CoV-2 infection who were not hospitalized. They conducted 2-hour neurocognitive interviews and identified 23 people with new, persistent cognitive symptoms (cognitive PASC) and 10 with no cognitive symptoms who served as controls.
All participants underwent additional neurologic examination and neuropsychological testing, and half agreed to a lumbar puncture to allow researchers to collect CSF samples. The CSF was collected a median of 10.2 months after initial COVID symptoms began.
Participants with cognitive PASC had higher median levels of CSF acute phase reactants C-reactive protein (0.007 mg/L vs. 0.000 mg/L; P =.004) and serum amyloid A (0.001 mg/L vs. 0.000 mg/L; P = .001), compared with COVID controls.
The PASC group also had elevated levels of CSF immune activation markers interferon gamma–inducible protein (IP-10), interleukin-8, and immunovascular markers vascular endothelial growth factor-C and VEGFR-1, although the differences with the control group were not statistically significant.
The timing of the onset of cognitive problems was also associated with higher levels of immune activation and immunovascular markers. Patients with brain fog that developed during the acute phase of COVID-19 had higher levels of CSF VEGF-C, compared with patients whose cognitive symptoms developed more than a month after initial COVID symptoms (173 pg/mL vs. 99 pg/mL; P = .048) and COVID controls (79 pg/mL; P = .048).
Acute onset cognitive PASC participants had higher CSF levels of IP-10 (P = .030), IL-8 (P = .048), placental growth factor (P = .030) and intercellular adhesion molecule-1 (P = .045), compared with COVID controls.
Researchers believe these new findings could mean that intrathecal immune activation and endothelial activation/dysfunction may contribute to cognitive PASC and that the mechanisms involved may be different in patients with acute cognitive PASC versus those with delayed onset.
“Our data suggests that perhaps in these people with more acute cognitive changes they don’t have the return to homeostasis,” Dr. Hellmuth said, while patients with delayed onset cognitive PASC had levels more in line with COVID patients who had no cognitive issues.
Moving the needle forward
Commenting on the findings, William Schaffner, MD, professor of infectious diseases, Vanderbilt University Medical Center, Nashville, Tenn., said that, while the study doesn’t rule out a possible psychological basis for cognitive PASC, it adds more weight to the biological argument.
“When you have nonspecific symptoms for which specific tests are unavailable,” Dr. Schaffner explained, “there is a natural question that always comes up: Is this principally a biologically induced phenomenon or psychological? This moves the needle substantially in the direction of a biological phenomenon.”
Another important element to the study, Dr. Schaffner said, is that the patients involved had mild COVID.
“Not every patient with long COVID symptoms had been hospitalized with severe disease,” he said. “There are inflammatory phenomenon in various organ systems such that even if the inflammatory response in the lung was not severe enough to get you into the hospital, there were inflammatory responses in other organ systems that could persist once the acute infection resolved.”
Although the small size of the study is a limitation, Dr. Schaffner said that shouldn’t minimize the importance of these findings.
“That it’s small doesn’t diminish its value,” he said. “The next step forward might be to try to associate the markers more specifically with COVID. The more precise we can be, the more convincing the story will become.”
The study was funded by the National Institutes of Health. Dr. Hellmuth received grant support from the National Institutes of Health/National Institute of Mental Health supporting this work and personal fees for medical-legal consultation outside of the submitted work. Dr. Schaffner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers found elevated levels of CSF immune activation and immunovascular markers in individuals with cognitive postacute sequelae of SARS-CoV-2 infection (PASC). Patients whose cognitive symptoms developed during the acute phase of COVID-19 had the highest levels of brain inflammation.
The findings add to a growing body of evidence that suggests the condition often referred to as “brain fog” has a neurologic basis, said lead author Joanna Hellmuth, MD, MHS, assistant professor of neurology at the University of California, San Francisco Weill Institute of Neurosciences and the UCSF Memory and Aging Center.
The findings will be presented at the 2022 annual meeting of the American Academy of Neurology.
Inflammatory response
There are no effective diagnostic tests or treatments for cognitive PASC, which prompted the investigators to study inflammation in patients with the condition. Initial findings were reported earlier in 2022, which showed abnormalities in the CSF in 77% of patients with cognitive impairment. Patients without cognitive impairments had normal CSF.
Extending that work in this new study, researchers studied patients from the Long-term Impact of Infection With Novel Coronavirus (LIINC) study with confirmed SARS-CoV-2 infection who were not hospitalized. They conducted 2-hour neurocognitive interviews and identified 23 people with new, persistent cognitive symptoms (cognitive PASC) and 10 with no cognitive symptoms who served as controls.
All participants underwent additional neurologic examination and neuropsychological testing, and half agreed to a lumbar puncture to allow researchers to collect CSF samples. The CSF was collected a median of 10.2 months after initial COVID symptoms began.
Participants with cognitive PASC had higher median levels of CSF acute phase reactants C-reactive protein (0.007 mg/L vs. 0.000 mg/L; P =.004) and serum amyloid A (0.001 mg/L vs. 0.000 mg/L; P = .001), compared with COVID controls.
The PASC group also had elevated levels of CSF immune activation markers interferon gamma–inducible protein (IP-10), interleukin-8, and immunovascular markers vascular endothelial growth factor-C and VEGFR-1, although the differences with the control group were not statistically significant.
The timing of the onset of cognitive problems was also associated with higher levels of immune activation and immunovascular markers. Patients with brain fog that developed during the acute phase of COVID-19 had higher levels of CSF VEGF-C, compared with patients whose cognitive symptoms developed more than a month after initial COVID symptoms (173 pg/mL vs. 99 pg/mL; P = .048) and COVID controls (79 pg/mL; P = .048).
Acute onset cognitive PASC participants had higher CSF levels of IP-10 (P = .030), IL-8 (P = .048), placental growth factor (P = .030) and intercellular adhesion molecule-1 (P = .045), compared with COVID controls.
Researchers believe these new findings could mean that intrathecal immune activation and endothelial activation/dysfunction may contribute to cognitive PASC and that the mechanisms involved may be different in patients with acute cognitive PASC versus those with delayed onset.
“Our data suggests that perhaps in these people with more acute cognitive changes they don’t have the return to homeostasis,” Dr. Hellmuth said, while patients with delayed onset cognitive PASC had levels more in line with COVID patients who had no cognitive issues.
Moving the needle forward
Commenting on the findings, William Schaffner, MD, professor of infectious diseases, Vanderbilt University Medical Center, Nashville, Tenn., said that, while the study doesn’t rule out a possible psychological basis for cognitive PASC, it adds more weight to the biological argument.
“When you have nonspecific symptoms for which specific tests are unavailable,” Dr. Schaffner explained, “there is a natural question that always comes up: Is this principally a biologically induced phenomenon or psychological? This moves the needle substantially in the direction of a biological phenomenon.”
Another important element to the study, Dr. Schaffner said, is that the patients involved had mild COVID.
“Not every patient with long COVID symptoms had been hospitalized with severe disease,” he said. “There are inflammatory phenomenon in various organ systems such that even if the inflammatory response in the lung was not severe enough to get you into the hospital, there were inflammatory responses in other organ systems that could persist once the acute infection resolved.”
Although the small size of the study is a limitation, Dr. Schaffner said that shouldn’t minimize the importance of these findings.
“That it’s small doesn’t diminish its value,” he said. “The next step forward might be to try to associate the markers more specifically with COVID. The more precise we can be, the more convincing the story will become.”
The study was funded by the National Institutes of Health. Dr. Hellmuth received grant support from the National Institutes of Health/National Institute of Mental Health supporting this work and personal fees for medical-legal consultation outside of the submitted work. Dr. Schaffner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAN 2022
First COVID-19 human challenge study provides insights
A small droplet that contains the coronavirus can infect someone with COVID-19, according to recent results from the first COVID-19 human challenge study, which were published in Nature Medicine.
Human challenge trials deliberately infect healthy volunteers to understand how an infection occurs and develops. In the first human challenge study for COVID-19, people were infected with the SARS-CoV-2 virus to better understand what has happened during the pandemic.
“Really, there’s no other type of study where you can do that, because normally, patients only come to your attention if they have developed symptoms, and so you miss all of those preceding days when the infection is brewing,” Christopher Chiu, MD, PhD, the lead study author and an infectious disease doctor and immunologist at Imperial College London, told CNN.
Starting in March 2021, Dr. Chiu and colleagues carefully selected 36 volunteers aged 18-30 years who didn’t have any risk factors for severe COVID-19, such as being overweight or having kidney, liver, heart, lung or blood problems. Participants also signed an extensive informed consent form, CNN reported.
The researchers conducted the trial in phases for safety. The first 10 participants who were infected received remdesivir, the antiviral drug, to reduce their chances of progressing to severe COVID-19. The research team also had monoclonal antibodies on hand in case any volunteers developed more severe symptoms. Ultimately, the researchers said, remdesivir was unnecessary, and they didn’t need to use the antibodies.
As part of the study, the participants had a small droplet of fluid that contained the original coronavirus strain inserted into their nose through a long tube. They stayed at London’s Royal Free Hospital for 2 weeks and were monitored by doctors 24 hours a day in rooms that had special air flow to keep the virus from spreading.
Of the 36 participants, 18 became infected, including two who never developed symptoms. The others had mild cases with symptoms such as congestion, sneezing, stuffy nose, and sore throat. Some also had headaches, muscle and joint pain, fatigue, and fever.
About 83% of participants who contracted COVID-19 lost their sense of smell to some degree, and nine people couldn’t smell at all. The symptom improved for most participants within 90 days, though one person still hadn’t fully regained their sense of smell about six months after the study ended.
The research team reported several other findings:
- Small amounts of the virus can make someone sick. About 10 mcm, or the amount in a single droplet that someone sneezes or coughs, can lead to infection.
- About 40 hours after the virus was inserted into a participant’s nose, the virus could be detected in the back of the throat.
- It took about 58 hours for the virus to appear on swabs from the nose, where the viral load eventually increased even more.
- COVID-19 has a short incubation period. It takes about 2 days after infection for someone to begin shedding the virus to others.
- People become contagious and shed high amounts of the virus before they show symptoms.
- In addition, infected people can shed high levels of the virus even if they don’t develop any symptoms.
- The study volunteers shed the virus for about 6 days on average, though some shed the virus for up to 12 days, even if they didn’t have symptoms.
- Lateral flow tests, which are used for rapid at-home tests, work well when an infected person is contagious. These tests could diagnose infection before 70%-80% of the viable virus had been generated.
The findings emphasized the importance of contagious people covering their mouth and nose when sick to protect others, Dr. Chiu told CNN.
None of the study volunteers developed lung issues as part of their infection, CNN reported. Dr. Chiu said that’s likely because they were young, healthy and received tiny amounts of the virus. All of the participants will be followed for a year to monitor for potential long-term effects.
Throughout the study, the research team also conducted cognitive tests to check the participants’ short-term memory and reaction time. The researchers are still analyzing the data, but the results “will really be informative,” Dr. Chiu told CNN.
Now the research team will conduct another human challenge trial, which will include vaccinated people who will be infected with the Delta variant. The researchers intend to study participants’ immune responses, which could provide valuable insights about new variants and vaccines.
“While there are differences in transmissibility due to the emergence of variants, such as Delta and Omicron, fundamentally, this is the same disease and the same factors will be responsible for protecting it,” Dr. Chiu said in a statement.
The research team will also study the 18 participants who didn’t get sick in the first human challenge trial. They didn’t develop antibodies, Dr. Chiu told CNN, despite receiving the same dose of the virus as those who got sick.
Before the study, all of the participants were screened for antibodies to other viruses, such as the original SARS virus. That means the volunteers weren’t cross-protected, and other factors may play into why some people don’t contract COVID-19. Future studies could help researchers provide better advice about protection if new variants emerge or a future pandemic occurs.
“There are lots of other things that help protect us,” Dr. Chiu said. “There are barriers in the nose. There are different kinds of proteins and things which are very ancient, primordial, protective systems ... and we’re really interested in trying to understand what those are.”
A version of this article first appeared on WebMD.com.
A small droplet that contains the coronavirus can infect someone with COVID-19, according to recent results from the first COVID-19 human challenge study, which were published in Nature Medicine.
Human challenge trials deliberately infect healthy volunteers to understand how an infection occurs and develops. In the first human challenge study for COVID-19, people were infected with the SARS-CoV-2 virus to better understand what has happened during the pandemic.
“Really, there’s no other type of study where you can do that, because normally, patients only come to your attention if they have developed symptoms, and so you miss all of those preceding days when the infection is brewing,” Christopher Chiu, MD, PhD, the lead study author and an infectious disease doctor and immunologist at Imperial College London, told CNN.
Starting in March 2021, Dr. Chiu and colleagues carefully selected 36 volunteers aged 18-30 years who didn’t have any risk factors for severe COVID-19, such as being overweight or having kidney, liver, heart, lung or blood problems. Participants also signed an extensive informed consent form, CNN reported.
The researchers conducted the trial in phases for safety. The first 10 participants who were infected received remdesivir, the antiviral drug, to reduce their chances of progressing to severe COVID-19. The research team also had monoclonal antibodies on hand in case any volunteers developed more severe symptoms. Ultimately, the researchers said, remdesivir was unnecessary, and they didn’t need to use the antibodies.
As part of the study, the participants had a small droplet of fluid that contained the original coronavirus strain inserted into their nose through a long tube. They stayed at London’s Royal Free Hospital for 2 weeks and were monitored by doctors 24 hours a day in rooms that had special air flow to keep the virus from spreading.
Of the 36 participants, 18 became infected, including two who never developed symptoms. The others had mild cases with symptoms such as congestion, sneezing, stuffy nose, and sore throat. Some also had headaches, muscle and joint pain, fatigue, and fever.
About 83% of participants who contracted COVID-19 lost their sense of smell to some degree, and nine people couldn’t smell at all. The symptom improved for most participants within 90 days, though one person still hadn’t fully regained their sense of smell about six months after the study ended.
The research team reported several other findings:
- Small amounts of the virus can make someone sick. About 10 mcm, or the amount in a single droplet that someone sneezes or coughs, can lead to infection.
- About 40 hours after the virus was inserted into a participant’s nose, the virus could be detected in the back of the throat.
- It took about 58 hours for the virus to appear on swabs from the nose, where the viral load eventually increased even more.
- COVID-19 has a short incubation period. It takes about 2 days after infection for someone to begin shedding the virus to others.
- People become contagious and shed high amounts of the virus before they show symptoms.
- In addition, infected people can shed high levels of the virus even if they don’t develop any symptoms.
- The study volunteers shed the virus for about 6 days on average, though some shed the virus for up to 12 days, even if they didn’t have symptoms.
- Lateral flow tests, which are used for rapid at-home tests, work well when an infected person is contagious. These tests could diagnose infection before 70%-80% of the viable virus had been generated.
The findings emphasized the importance of contagious people covering their mouth and nose when sick to protect others, Dr. Chiu told CNN.
None of the study volunteers developed lung issues as part of their infection, CNN reported. Dr. Chiu said that’s likely because they were young, healthy and received tiny amounts of the virus. All of the participants will be followed for a year to monitor for potential long-term effects.
Throughout the study, the research team also conducted cognitive tests to check the participants’ short-term memory and reaction time. The researchers are still analyzing the data, but the results “will really be informative,” Dr. Chiu told CNN.
Now the research team will conduct another human challenge trial, which will include vaccinated people who will be infected with the Delta variant. The researchers intend to study participants’ immune responses, which could provide valuable insights about new variants and vaccines.
“While there are differences in transmissibility due to the emergence of variants, such as Delta and Omicron, fundamentally, this is the same disease and the same factors will be responsible for protecting it,” Dr. Chiu said in a statement.
The research team will also study the 18 participants who didn’t get sick in the first human challenge trial. They didn’t develop antibodies, Dr. Chiu told CNN, despite receiving the same dose of the virus as those who got sick.
Before the study, all of the participants were screened for antibodies to other viruses, such as the original SARS virus. That means the volunteers weren’t cross-protected, and other factors may play into why some people don’t contract COVID-19. Future studies could help researchers provide better advice about protection if new variants emerge or a future pandemic occurs.
“There are lots of other things that help protect us,” Dr. Chiu said. “There are barriers in the nose. There are different kinds of proteins and things which are very ancient, primordial, protective systems ... and we’re really interested in trying to understand what those are.”
A version of this article first appeared on WebMD.com.
A small droplet that contains the coronavirus can infect someone with COVID-19, according to recent results from the first COVID-19 human challenge study, which were published in Nature Medicine.
Human challenge trials deliberately infect healthy volunteers to understand how an infection occurs and develops. In the first human challenge study for COVID-19, people were infected with the SARS-CoV-2 virus to better understand what has happened during the pandemic.
“Really, there’s no other type of study where you can do that, because normally, patients only come to your attention if they have developed symptoms, and so you miss all of those preceding days when the infection is brewing,” Christopher Chiu, MD, PhD, the lead study author and an infectious disease doctor and immunologist at Imperial College London, told CNN.
Starting in March 2021, Dr. Chiu and colleagues carefully selected 36 volunteers aged 18-30 years who didn’t have any risk factors for severe COVID-19, such as being overweight or having kidney, liver, heart, lung or blood problems. Participants also signed an extensive informed consent form, CNN reported.
The researchers conducted the trial in phases for safety. The first 10 participants who were infected received remdesivir, the antiviral drug, to reduce their chances of progressing to severe COVID-19. The research team also had monoclonal antibodies on hand in case any volunteers developed more severe symptoms. Ultimately, the researchers said, remdesivir was unnecessary, and they didn’t need to use the antibodies.
As part of the study, the participants had a small droplet of fluid that contained the original coronavirus strain inserted into their nose through a long tube. They stayed at London’s Royal Free Hospital for 2 weeks and were monitored by doctors 24 hours a day in rooms that had special air flow to keep the virus from spreading.
Of the 36 participants, 18 became infected, including two who never developed symptoms. The others had mild cases with symptoms such as congestion, sneezing, stuffy nose, and sore throat. Some also had headaches, muscle and joint pain, fatigue, and fever.
About 83% of participants who contracted COVID-19 lost their sense of smell to some degree, and nine people couldn’t smell at all. The symptom improved for most participants within 90 days, though one person still hadn’t fully regained their sense of smell about six months after the study ended.
The research team reported several other findings:
- Small amounts of the virus can make someone sick. About 10 mcm, or the amount in a single droplet that someone sneezes or coughs, can lead to infection.
- About 40 hours after the virus was inserted into a participant’s nose, the virus could be detected in the back of the throat.
- It took about 58 hours for the virus to appear on swabs from the nose, where the viral load eventually increased even more.
- COVID-19 has a short incubation period. It takes about 2 days after infection for someone to begin shedding the virus to others.
- People become contagious and shed high amounts of the virus before they show symptoms.
- In addition, infected people can shed high levels of the virus even if they don’t develop any symptoms.
- The study volunteers shed the virus for about 6 days on average, though some shed the virus for up to 12 days, even if they didn’t have symptoms.
- Lateral flow tests, which are used for rapid at-home tests, work well when an infected person is contagious. These tests could diagnose infection before 70%-80% of the viable virus had been generated.
The findings emphasized the importance of contagious people covering their mouth and nose when sick to protect others, Dr. Chiu told CNN.
None of the study volunteers developed lung issues as part of their infection, CNN reported. Dr. Chiu said that’s likely because they were young, healthy and received tiny amounts of the virus. All of the participants will be followed for a year to monitor for potential long-term effects.
Throughout the study, the research team also conducted cognitive tests to check the participants’ short-term memory and reaction time. The researchers are still analyzing the data, but the results “will really be informative,” Dr. Chiu told CNN.
Now the research team will conduct another human challenge trial, which will include vaccinated people who will be infected with the Delta variant. The researchers intend to study participants’ immune responses, which could provide valuable insights about new variants and vaccines.
“While there are differences in transmissibility due to the emergence of variants, such as Delta and Omicron, fundamentally, this is the same disease and the same factors will be responsible for protecting it,” Dr. Chiu said in a statement.
The research team will also study the 18 participants who didn’t get sick in the first human challenge trial. They didn’t develop antibodies, Dr. Chiu told CNN, despite receiving the same dose of the virus as those who got sick.
Before the study, all of the participants were screened for antibodies to other viruses, such as the original SARS virus. That means the volunteers weren’t cross-protected, and other factors may play into why some people don’t contract COVID-19. Future studies could help researchers provide better advice about protection if new variants emerge or a future pandemic occurs.
“There are lots of other things that help protect us,” Dr. Chiu said. “There are barriers in the nose. There are different kinds of proteins and things which are very ancient, primordial, protective systems ... and we’re really interested in trying to understand what those are.”
A version of this article first appeared on WebMD.com.
FROM NATURE MEDICINE
Ivermectin doesn’t help treat COVID-19, large study finds
according to results from a large clinical trial published in the New England Journal of Medicine.
The findings pretty much rule out the drug as a treatment for COVID-19, the study authors wrote.
“There’s really no sign of any benefit,” David Boulware, MD, one of the coauthors and an infectious disease specialist at the University of Minnesota, Minneapolis, told the New York Times.
The researchers shared a summary of the results in August 2021 during an online presentation hosted by the National Institutes of Health. The full data hadn’t been published until now.
“Now that people can dive into the details and the data, hopefully that will steer the majority of doctors away from ivermectin toward other therapies,” Dr. Boulware said.
In the trial, the research team compared more than 1,350 people infected with the coronavirus in Brazil who received either ivermectin or a placebo as treatment.
Between March and August 2021, 679 patients received a daily dose of ivermectin over the course of 3 days. The researchers found that ivermectin didn’t reduce the risk that people with COVID-19 would be hospitalized or go to an ED within 28 days after treatment.
In addition, the researchers looked at particular groups to understand if some patients benefited for some reason, such as taking ivermectin sooner after testing positive for COVID-19. But those who took the drug during the first 3 days after a positive coronavirus test ended up doing worse than those in the placebo group. The drug also didn’t help patients recover sooner.
The researchers found “no important effects” of treatment with ivermectin on the number of days people spent in the hospital, the number of days hospitalized people needed mechanical ventilation, or the risk of death.
Ivermectin has become a controversial focal point during the pandemic.
For decades, the drug has been widely used to treat parasitic infections. At the beginning of the pandemic, researchers checked thousands of existing drugs against the coronavirus to determine if a potential treatment already existed. Laboratory experiments on cells suggested that ivermectin might work, the New York Times reported.
But some researchers noted that the experiments worked because a high concentration of ivermectin was used, a much higher dose than would be safe for people. Despite the concerns, some doctors began prescribing ivermectin to patients. After receiving reports of people who needed medical attention, particularly after using formulations intended for livestock, the Food and Drug Administration issued a warning that the drug wasn’t approved to be used for COVID-19.
Researchers around the world have done small clinical trials to understand whether ivermectin treats COVID-19, the newspaper reported. At the end of 2020, Andrew Hill, MD, a virologist at the University of Liverpool in England, reviewed the results from 23 trials and concluded that the drug could lower the risk of death from COVID-19. He published the results in July 2021, but later reports found that many of the studies were flawed, and at least one was fraudulent.
Dr. Hill retracted his original study and began another analysis, which was published in January 2022. In this review, he and his colleagues focused on studies that were least likely to be biased. They found that ivermectin was not helpful.
Recently, Dr. Hill and associates ran another analysis using the new data from the Brazil trial, and once again they saw no benefit.
Several clinical trials are still testing ivermectin as a treatment, the New York Times reported, with results expected in upcoming months. After reviewing the data from the Brazil trial, which tested ivermectin and a variety of other drugs against COVID-19, some infectious disease experts say they’ll likely see more of the same – that ivermectin doesn’t help people with COVID-19.
“I welcome the results of the other clinical trials and will view them with an open mind,” Paul Sax, MD, an infectious disease expert at Brigham and Women’s Hospital, Boston, who has been watching the data on the drug throughout the pandemic, told the New York Times.
“But at some point, it will become a waste of resources to continue studying an unpromising approach,” he said.
A version of this article first appeared on WebMD.com.
according to results from a large clinical trial published in the New England Journal of Medicine.
The findings pretty much rule out the drug as a treatment for COVID-19, the study authors wrote.
“There’s really no sign of any benefit,” David Boulware, MD, one of the coauthors and an infectious disease specialist at the University of Minnesota, Minneapolis, told the New York Times.
The researchers shared a summary of the results in August 2021 during an online presentation hosted by the National Institutes of Health. The full data hadn’t been published until now.
“Now that people can dive into the details and the data, hopefully that will steer the majority of doctors away from ivermectin toward other therapies,” Dr. Boulware said.
In the trial, the research team compared more than 1,350 people infected with the coronavirus in Brazil who received either ivermectin or a placebo as treatment.
Between March and August 2021, 679 patients received a daily dose of ivermectin over the course of 3 days. The researchers found that ivermectin didn’t reduce the risk that people with COVID-19 would be hospitalized or go to an ED within 28 days after treatment.
In addition, the researchers looked at particular groups to understand if some patients benefited for some reason, such as taking ivermectin sooner after testing positive for COVID-19. But those who took the drug during the first 3 days after a positive coronavirus test ended up doing worse than those in the placebo group. The drug also didn’t help patients recover sooner.
The researchers found “no important effects” of treatment with ivermectin on the number of days people spent in the hospital, the number of days hospitalized people needed mechanical ventilation, or the risk of death.
Ivermectin has become a controversial focal point during the pandemic.
For decades, the drug has been widely used to treat parasitic infections. At the beginning of the pandemic, researchers checked thousands of existing drugs against the coronavirus to determine if a potential treatment already existed. Laboratory experiments on cells suggested that ivermectin might work, the New York Times reported.
But some researchers noted that the experiments worked because a high concentration of ivermectin was used, a much higher dose than would be safe for people. Despite the concerns, some doctors began prescribing ivermectin to patients. After receiving reports of people who needed medical attention, particularly after using formulations intended for livestock, the Food and Drug Administration issued a warning that the drug wasn’t approved to be used for COVID-19.
Researchers around the world have done small clinical trials to understand whether ivermectin treats COVID-19, the newspaper reported. At the end of 2020, Andrew Hill, MD, a virologist at the University of Liverpool in England, reviewed the results from 23 trials and concluded that the drug could lower the risk of death from COVID-19. He published the results in July 2021, but later reports found that many of the studies were flawed, and at least one was fraudulent.
Dr. Hill retracted his original study and began another analysis, which was published in January 2022. In this review, he and his colleagues focused on studies that were least likely to be biased. They found that ivermectin was not helpful.
Recently, Dr. Hill and associates ran another analysis using the new data from the Brazil trial, and once again they saw no benefit.
Several clinical trials are still testing ivermectin as a treatment, the New York Times reported, with results expected in upcoming months. After reviewing the data from the Brazil trial, which tested ivermectin and a variety of other drugs against COVID-19, some infectious disease experts say they’ll likely see more of the same – that ivermectin doesn’t help people with COVID-19.
“I welcome the results of the other clinical trials and will view them with an open mind,” Paul Sax, MD, an infectious disease expert at Brigham and Women’s Hospital, Boston, who has been watching the data on the drug throughout the pandemic, told the New York Times.
“But at some point, it will become a waste of resources to continue studying an unpromising approach,” he said.
A version of this article first appeared on WebMD.com.
according to results from a large clinical trial published in the New England Journal of Medicine.
The findings pretty much rule out the drug as a treatment for COVID-19, the study authors wrote.
“There’s really no sign of any benefit,” David Boulware, MD, one of the coauthors and an infectious disease specialist at the University of Minnesota, Minneapolis, told the New York Times.
The researchers shared a summary of the results in August 2021 during an online presentation hosted by the National Institutes of Health. The full data hadn’t been published until now.
“Now that people can dive into the details and the data, hopefully that will steer the majority of doctors away from ivermectin toward other therapies,” Dr. Boulware said.
In the trial, the research team compared more than 1,350 people infected with the coronavirus in Brazil who received either ivermectin or a placebo as treatment.
Between March and August 2021, 679 patients received a daily dose of ivermectin over the course of 3 days. The researchers found that ivermectin didn’t reduce the risk that people with COVID-19 would be hospitalized or go to an ED within 28 days after treatment.
In addition, the researchers looked at particular groups to understand if some patients benefited for some reason, such as taking ivermectin sooner after testing positive for COVID-19. But those who took the drug during the first 3 days after a positive coronavirus test ended up doing worse than those in the placebo group. The drug also didn’t help patients recover sooner.
The researchers found “no important effects” of treatment with ivermectin on the number of days people spent in the hospital, the number of days hospitalized people needed mechanical ventilation, or the risk of death.
Ivermectin has become a controversial focal point during the pandemic.
For decades, the drug has been widely used to treat parasitic infections. At the beginning of the pandemic, researchers checked thousands of existing drugs against the coronavirus to determine if a potential treatment already existed. Laboratory experiments on cells suggested that ivermectin might work, the New York Times reported.
But some researchers noted that the experiments worked because a high concentration of ivermectin was used, a much higher dose than would be safe for people. Despite the concerns, some doctors began prescribing ivermectin to patients. After receiving reports of people who needed medical attention, particularly after using formulations intended for livestock, the Food and Drug Administration issued a warning that the drug wasn’t approved to be used for COVID-19.
Researchers around the world have done small clinical trials to understand whether ivermectin treats COVID-19, the newspaper reported. At the end of 2020, Andrew Hill, MD, a virologist at the University of Liverpool in England, reviewed the results from 23 trials and concluded that the drug could lower the risk of death from COVID-19. He published the results in July 2021, but later reports found that many of the studies were flawed, and at least one was fraudulent.
Dr. Hill retracted his original study and began another analysis, which was published in January 2022. In this review, he and his colleagues focused on studies that were least likely to be biased. They found that ivermectin was not helpful.
Recently, Dr. Hill and associates ran another analysis using the new data from the Brazil trial, and once again they saw no benefit.
Several clinical trials are still testing ivermectin as a treatment, the New York Times reported, with results expected in upcoming months. After reviewing the data from the Brazil trial, which tested ivermectin and a variety of other drugs against COVID-19, some infectious disease experts say they’ll likely see more of the same – that ivermectin doesn’t help people with COVID-19.
“I welcome the results of the other clinical trials and will view them with an open mind,” Paul Sax, MD, an infectious disease expert at Brigham and Women’s Hospital, Boston, who has been watching the data on the drug throughout the pandemic, told the New York Times.
“But at some point, it will become a waste of resources to continue studying an unpromising approach,” he said.
A version of this article first appeared on WebMD.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE

