User login
COVID-19 and the psychiatrist/psychoanalyst: My experience
COVID-19 affected all aspects of psychiatric care. As a psychiatrist who is also a psychoanalyst, I faced some unique challenges to caring for my patients during the pandemic. In this article, I describe how COVID-19 impacted my practice, and how I adjusted to ensure that my patients received the best possible care.
The loss of ‘normal’
Our recognition of the loss was not immediate since no one knew what to expect. From March 11, 2020 through the end of the warm weather, when we could be outdoors, personal life was still gratifying. There was even a new spirit of togetherness in my neighborhood, with people seamlessly cooperating by crossing the street to avoid getting too close to one another, practicing proper social distancing in the grocery line, and smiling at everyone.
November 2020 through Spring 2021 was an unprecedented period of no socialization and spending time exclusively with my husband. By the end, I was finally aware of the exhaustion I felt trying to work with patients via phone and video sessions. Beyond that, we were (and still are) conducting administrative meetings and national organization meetings by video.
Spring 2021 until the arrival of cold weather felt more relaxed, as socializing outside again became possible. But from Winter 2021 to now has been a weary repeat of isolation, and a realization that my work life might never go back to “normal.” I would have to make peace with various sorts of losses of gratification in my work.
Life before COVID-19
I am a psychiatrist and psychoanalyst in a group private practice near the University of Cincinnati Medical Center. As a former full-time faculty member there, I maintain some teaching and supervision of residents. I typically see patients from 8:30 AM until 6:30 PM, and for years have had an average of 5 patients in psychoanalysis on the couch for 3 to 4 sessions per week. I see some psychotherapy patients weekly or twice a week and have some hours for new diagnostic evaluations and medication management. In addition, as a faculty member of the Cincinnati Psychoanalytic Institute, I take part in several committees, teach in the psychotherapy program and psychoanalytic training program, and supervise students and candidates. Most weeks, I see between 35 and 40 patients, with 4 to 6 weeks of vacation time per year.
Major changes with the onset of the pandemic
Once the threat from COVID-19 became clear in March 2020, I thought through my options. My office comprises 5 professional offices, a waiting room, and an administrative area. Our administrative assistant and 1 or 2 practitioners were in the office with me most days. We maintained appropriate distance from each another and wore masks in common areas. The practice group was exemplary in immediately setting up safe practices. I learned a few colleagues were seeing patients outside using lawn chairs in the back of our lot where there was some privacy, but many stopped coming to the building altogether.
I felt real sadness having to tell patients I could no longer see them in my office. However, I was relieved to find how quickly many patients made an immediate transition to telephone or video sessions. Since I was alone in my office and not distracted by barking dogs, ringing doorbells, or loud lawnmowers, I continued to come to the office, and never switched to working from home.
Since I was not vis-à-vis with patients on the couch, those sessions shifted to the telephone. I offered psychotherapy patients the option of video sessions via the Health Insurance Portability and Accountability Act–compliant Doximity app (doxy.me) or telephone, and found that approximately 75% preferred video. When I used the telephone, I used a professional-grade headset, which made it less onerous than being tied to a receiver, and I occasionally used the speaker option. I also installed a desk platform that allows me to raise and lower my computer from sitting to standing height.
I worried a great deal about patients I felt would do poorly with video or telephone sessions: older adults who found comfort in human contact that was sometimes curative, less well-integrated individuals who needed real contact in order to feel there was a treatment process, those with serious mental illnesses who needed reassurance at their reality-testing, and new patients who I couldn’t fully assess without in-person meetings.
In the beginning of the pandemic, as we were still learning about the virus, nothing seemed safe. We were washing our hands constantly, afraid to touch doorknobs, mail, or groceries. Thankfully, we learned that COVID-19 transmission occurs primarily through inhalation of droplets and particles containing the virus.1 Masks, good ventilation, and adequate distance from others considerably cut infection rates. By January 2021, the availability of a vaccine made an enormous difference in vulnerability to severe illness.
When I stopped seeing patients in my office, I set up the conference room that had doors on either end so I could sit on one end of a table and have the patient at the other end, keeping about 8 feet between us. I also kept a fan blowing air away from me and parallel to the patient. After each session, I opened both doors to allow for full ventilation of the room. This provided a solution for the patients I knew I needed to meet with in person.
Continue to: Case examples: How it worked...
Case examples: How it worked
The following case examples illustrate how I provided care during this time. To protect patient anonymity, these vignettes are composites.
Psychotherapy patients
Established patients in psychotherapy have seemed to work well with video or telephone sessions. The video option added a new element I never appreciated: seeing patients in their homes or cars allowed me to gain a new set of impressions about them. The use of technology is clearly another element I would not have identified before. Less technically adept older patients are likely to join a video session with only the top of their head visible, or with insufficient lighting. In some cases, I coached patients to rearrange their computer so I could see their faces, but only if it seemed that doing so would not cause them greater distress.
Ms. A, age 74, is a widow who retired from a high-level professional position 5 years ago. She was brought to the hospital due to ongoing anxiety, especially about her health. Ms. A maintained a wide range of relationships with friends, colleagues she mentored, and neighbors who provided a satisfying social network, and she continued to contribute to her field via scholarly writing projects. Before the pandemic, she found occasional sessions helpful in putting her health fears into perspective. When the pandemic led her to isolate at home, Ms. A became anxious and depressed to an unprecedented extent. Video sessions were unsatisfying, and she was terrified of taking tranquilizers or other medications. Once COVID-19 vaccinations became available and both she and I received both doses, we switched to meeting in the conference room every 2 to 3 weeks, with considerably better results.
Mr. B, age 41, is a single male who I diagnosed with schizophrenia at age 19 when he developed paranoid delusions and auditory hallucinations. Mr. B was not interested in taking antipsychotic medications, and his situation did not improve even when he did try taking them. He volunteered at a local emergency department doing odd jobs—moving gurneys, cleaning rooms, hauling boxes of supplies—for many years, and had always been employed in jobs such as grocery stocking or janitorial work that did not involve extensive interactions with people. He repeatedly enrolled in programs that would provide a skill such as phlebotomy or medical billing, only to find that he was never hired for such work. We talked once a month for 30 minutes about his frustrations trying to find women to date and marry, and how he was repeatedly taken advantage of (one “date” from an escort service took him to an ATM and got him to withdraw most of the money in his account).
Coincident with COVID-19, Mr. B’s father died from widespread metastatic cancer. His father had been Mr. B’s guide, friend, payee for Social Security Disability Insurance funds, and source of advice. To provide humane and somewhat effective treatment, I saw Mr. B in the conference room. His capacity to express grief and distress at the loss of his father has been impressive, as has his initiative in finding a grief group to attend, which he has done consistently.
Several patients who had been seeing me for weekly psychotherapy chose not to continue, many without specifically informing me of their decision. I understood the situation was in flux, and it would not be clear to anyone what to expect for the future. To avoid pressuring anyone, I chose not to contact patients to inquire about their plans.
Ms. C, age 50, is a professional with 3 children whose marriage had been highly dissatisfying for years, and she was now ready to investigate it. She was very successful in her career, having taken on a leadership role in her firm and earning a high income, while her husband was erratic, unreliable, and self-absorbed. Though he was well-educated and competent in his field, he could not maintain employment in a corporate environment and worked as a consultant with relatively little success. Along with the hours she spent working, Ms. C took responsibility for the family finances, was the chief wage earner, managed the needs of their children, made sure meals were prepared, and took on many other responsibilities.
Continue to: Case examples: How it worked (cont.)...
We agreed to a weekly session that fit Ms. C’s schedule, and she seemed able to relax and talk about herself. I found Ms. C quite likeable and enjoyed meeting with her, though I worried about whether we would need a greater intensity to get at the reasons such a successful and intelligent woman would fear setting limits with her husband or even considering ending the relationship. The reasons were clear as we put together the story of her early life, but conviction only develops with full emotional awareness (transference provides this in psychoanalysis).
The pandemic started approximately 18 months into our work, and Ms. C disappeared. She called my administrative assistant to cancel further appointments but did not ask to speak with me directly. While I knew this might represent resistance, I also felt unwilling to pressure Ms. C if she chose not to continue. I remain hopeful that I will hear from her once again; if not, I will send a note by mail to say that I enjoyed working with her, am happy to see her again, and hope she found some benefit from our work.
Mr. D contacted me for psychotherapy following the death of his father, who I had seen as a patient many years earlier. I was aware of the likely impact of his father’s outsized personality and emotional dysregulation on Mr. D and agreed to meet with him. He had taken over the family business and had made it an even greater success, but had trouble feeling confident about setting limits with employees who he knew took advantage of his avoidance.
Mr. D and I met weekly for several months and then moved to every other week, a form of resistance I expected as we got closer to his feeling pain. At the same time, I recognize that many patients use this tactic to “dose” themselves with the intensity they can tolerate, and Mr. D was quite observant and able to pick up themes where we’d left off.
When the pandemic shut down office visits, Mr. D immediately agreed to video sessions, which he has continued at roughly the same frequency. While I miss sitting with him, we continue to make progress towards his goal of learning to see himself as able to compete with his father.
Psychoanalysis patients
I found that patients in psychoanalysis had no trouble with the transition to telephone sessions, and the intensity of the work was not diluted. In some ways, audio-only communication is more intimate and might encourage patients to talk about topics they may not have otherwise brought up. I have not seen any evidence of less progress among these patients.
Dr. E, age 45, is a divorced physician who began psychoanalysis 3 times per week on the couch in 2018 for problems with frustration and confusion about his career, his identity as a father, and intense loneliness. He had worked up to 80 hours per week to earn as much money as he could, but also to avoid time at home with his then-wife and young children. The lack of time to recover led him to hate his work, left no time for social connections, and led to binges of heavy drinking. Our work had begun to allow him to develop a narrative about his early life that had never been considered, and to identify patterns of repetition of old defensive strategies that had never served him well.
At the onset of the pandemic, I told Dr. E that we would have to switch to telephone sessions, and he agreed immediately. In fact, he came to prefer telephone work since it spared him the 2 hours per day he had spent coming to my office. While I found it less satisfying than working in person, we have continued the same schedule and with the same intensity and trajectory established before the pandemic.
Continue to: Working with new patients...
Working with new patients
Seeing new patients for diagnostic evaluation is always best done in person, because the information I gain from the patient’s appearance, clothing, demeanor, gait, postures, gesturing, and facial expressions (among other elements) gives me important impressions I miss with video or telephone. In many cases, patients gain a sense of who I am from sitting in my office, and using the conference room eliminates that benefit. I attempted to create a warm environment in the conference room by obtaining lamps that produce warmer indirect light and hanging artwork that reflects my tastes. There are clocks in places that allow me and my patient to keep track of time. In meeting new patients by video, I get some impressions about their surroundings that add to the information I get through our interview. I have done many diagnostic evaluations during the pandemic and gotten treatments (whether medication, psychotherapy, or both) underway without discernible problems in the outcomes. Patients who started with me in person have mostly wanted to continue with in-person meetings, but as many have told me, interspersed video sessions save them travel time.
What about vaccination?
Once COVID-19 vaccinations were widely available, I assumed patients would be as eager to get them as I had been. When I began asking patients about whether they had gotten their vaccines, I was surprised to hear that a few were not going to get vaccinated, clearly based on political views and misinformation about the danger of vaccines. (The topic of political beliefs and their impact on psychological treatment is beyond the scope of this commentary.) I tried to counter obvious misinformation, repeated my recommendation that the patient get vaccinated, and then turned to other topics. I later decided to tell all patients that vaccination was required to enter the office. Only 1 patient who had been coming to the office dropped out, and she eventually returned to meeting by video.
COVID-19’s toll on the therapist
While the first several months of the pandemic were so full of uncertainty about the future, once vaccinations were available, it seemed cause for hope of a return to normalcy. As time went on, however, it became clear that normal was still a long way off. With vaccine refusal and new variants upending my naïve view that we were near the end, I began to feel aware of the impact this had on me, and began to focus on self-care (Box). I had always seen myself as unusually lucky to have a full practice, a supportive partnership with my husband, grown children who didn’t need me to homeschool them, a strong social network of friends who could share the burden and cheer each other up at outdoor gatherings, and a wonderful group of siblings and in-laws (all in different cities) who stayed in touch via video calls and quarantined in advance of getting together in someone’s home.
Box
Self-care has always been a requirement of doing psychotherapeutic work, and I encourage practitioners to be sure they are attending to themselves. We can’t be effective as listeners, empathizers, diagnosticians, and problem-solvers if we ourselves aren’t healthy. We evaluate our patients in terms of mood, outlook, sleep, appetite, energy, motivation, and energy; we also investigate their capacity for relationships that are sustaining. Self-care is the same, taking care of both our physical and relationship beings. Getting enough sleep, exercising daily, cooking healthy meals, and making time to relax are all ways of caring for our physical identities that should have been in place before COVID-19. Making personal time for ourselves in the face of constant demands for time from patients, colleagues, partners, children, parents, siblings, and friends never happens without the resolve to do it. As a psychiatrist who is used to sitting for up to 10 hours per day, I strongly recommend making a daily habit of walking, running, biking, or using an elliptical trainer, treadmill, or stationary bike for 30 minutes or more. Sleep is necessary for adequate concentration and attention to patient after patient. If you have trouble sleeping, talk with your doctor about remedies. If you use a sleep aid, I strongly recommend alternating medications so you don’t develop tolerance to any of them. Plan your food and cooking ahead of time so you aren’t tempted to order out. If you cook simple meals yourself (ideally with your partner helping or in range so you can chat), you will consume fewer calories, less sodium, and more nutrients. Even if you have a spouse and young children at home, work out a plan with your partner that allows each of you time for exercise or to recoup after a long day with patients. Babysitters allow you to take the time to be with each other that is necessary to sustaining a connection. Think about time for sexual intimacy if that has dropped off the calendar. Relationships with others, such as parents, siblings and their families, and friends are invaluable. The time spent with others might seem inconsequential, but is critical to our internal sense of security, even in the face of external disorder.
Staying busy and engaged with my practice, spouse, family, and friends kept sadness away most of the time. But I surprised myself a few months ago when I sat down to reflect and check in with myself. I felt enormous loss, resentment, and exhaustion at the privations of the pandemic: every trip to the grocery story felt dangerous. I hadn’t seen the inside of a concert hall, movie theater, restaurant, or museum in nearly 2 years. Travel for meetings and visits to family and friends and various adventures had been abruptly stopped. I lost both parents (not to COVID-19) during 2020; both were older adults living in senior communities that could not allow visitors. The usual grieving process would include attending services at my synagogue where I could say Kaddish for them, and video services were simply not tolerable.
Most of us have become experts at video meetings and likely have come to despise them. While our Institute has always held classes with some out-of-town students joining by video, with a very sophisticated system that provides excellent sound and visual fidelity, teaching entirely by video is another matter. I now teach students I have never met in person and might not recognize if I passed them in public. The art of creating discussion around a table is much more difficult on a computer screen. The first class I taught to residents during the pandemic was completely disorienting as I faced a wall of black screens with names and silence. Each student had turned off their camera and muted their microphone, so I was lecturing to a computer. That never happened again after I insisted on seeing everyone’s face and hearing their voices.
Thankfully, my usual experience of a long day seeing patients followed by chatting while cooking dinner with my husband and walking the dogs before settling down to read didn’t change. But the pleasure of sitting with patients was replaced by the daily grind of figuring out who will need a video link, who will be on the telephone, and who will come to the office, and it doesn’t feel the same. Again, in the big picture, I realize how fortunate I have been, but it’s been a big change in the world of the psychotherapist.
1. Centers for Disease Control and Prevention. COVID-19 frequently asked questions. Accessed March 8, 2022. https://www.cdc.gov/coronavirus/2019-ncov/faq.html#Spread
COVID-19 affected all aspects of psychiatric care. As a psychiatrist who is also a psychoanalyst, I faced some unique challenges to caring for my patients during the pandemic. In this article, I describe how COVID-19 impacted my practice, and how I adjusted to ensure that my patients received the best possible care.
The loss of ‘normal’
Our recognition of the loss was not immediate since no one knew what to expect. From March 11, 2020 through the end of the warm weather, when we could be outdoors, personal life was still gratifying. There was even a new spirit of togetherness in my neighborhood, with people seamlessly cooperating by crossing the street to avoid getting too close to one another, practicing proper social distancing in the grocery line, and smiling at everyone.
November 2020 through Spring 2021 was an unprecedented period of no socialization and spending time exclusively with my husband. By the end, I was finally aware of the exhaustion I felt trying to work with patients via phone and video sessions. Beyond that, we were (and still are) conducting administrative meetings and national organization meetings by video.
Spring 2021 until the arrival of cold weather felt more relaxed, as socializing outside again became possible. But from Winter 2021 to now has been a weary repeat of isolation, and a realization that my work life might never go back to “normal.” I would have to make peace with various sorts of losses of gratification in my work.
Life before COVID-19
I am a psychiatrist and psychoanalyst in a group private practice near the University of Cincinnati Medical Center. As a former full-time faculty member there, I maintain some teaching and supervision of residents. I typically see patients from 8:30 AM until 6:30 PM, and for years have had an average of 5 patients in psychoanalysis on the couch for 3 to 4 sessions per week. I see some psychotherapy patients weekly or twice a week and have some hours for new diagnostic evaluations and medication management. In addition, as a faculty member of the Cincinnati Psychoanalytic Institute, I take part in several committees, teach in the psychotherapy program and psychoanalytic training program, and supervise students and candidates. Most weeks, I see between 35 and 40 patients, with 4 to 6 weeks of vacation time per year.
Major changes with the onset of the pandemic
Once the threat from COVID-19 became clear in March 2020, I thought through my options. My office comprises 5 professional offices, a waiting room, and an administrative area. Our administrative assistant and 1 or 2 practitioners were in the office with me most days. We maintained appropriate distance from each another and wore masks in common areas. The practice group was exemplary in immediately setting up safe practices. I learned a few colleagues were seeing patients outside using lawn chairs in the back of our lot where there was some privacy, but many stopped coming to the building altogether.
I felt real sadness having to tell patients I could no longer see them in my office. However, I was relieved to find how quickly many patients made an immediate transition to telephone or video sessions. Since I was alone in my office and not distracted by barking dogs, ringing doorbells, or loud lawnmowers, I continued to come to the office, and never switched to working from home.
Since I was not vis-à-vis with patients on the couch, those sessions shifted to the telephone. I offered psychotherapy patients the option of video sessions via the Health Insurance Portability and Accountability Act–compliant Doximity app (doxy.me) or telephone, and found that approximately 75% preferred video. When I used the telephone, I used a professional-grade headset, which made it less onerous than being tied to a receiver, and I occasionally used the speaker option. I also installed a desk platform that allows me to raise and lower my computer from sitting to standing height.
I worried a great deal about patients I felt would do poorly with video or telephone sessions: older adults who found comfort in human contact that was sometimes curative, less well-integrated individuals who needed real contact in order to feel there was a treatment process, those with serious mental illnesses who needed reassurance at their reality-testing, and new patients who I couldn’t fully assess without in-person meetings.
In the beginning of the pandemic, as we were still learning about the virus, nothing seemed safe. We were washing our hands constantly, afraid to touch doorknobs, mail, or groceries. Thankfully, we learned that COVID-19 transmission occurs primarily through inhalation of droplets and particles containing the virus.1 Masks, good ventilation, and adequate distance from others considerably cut infection rates. By January 2021, the availability of a vaccine made an enormous difference in vulnerability to severe illness.
When I stopped seeing patients in my office, I set up the conference room that had doors on either end so I could sit on one end of a table and have the patient at the other end, keeping about 8 feet between us. I also kept a fan blowing air away from me and parallel to the patient. After each session, I opened both doors to allow for full ventilation of the room. This provided a solution for the patients I knew I needed to meet with in person.
Continue to: Case examples: How it worked...
Case examples: How it worked
The following case examples illustrate how I provided care during this time. To protect patient anonymity, these vignettes are composites.
Psychotherapy patients
Established patients in psychotherapy have seemed to work well with video or telephone sessions. The video option added a new element I never appreciated: seeing patients in their homes or cars allowed me to gain a new set of impressions about them. The use of technology is clearly another element I would not have identified before. Less technically adept older patients are likely to join a video session with only the top of their head visible, or with insufficient lighting. In some cases, I coached patients to rearrange their computer so I could see their faces, but only if it seemed that doing so would not cause them greater distress.
Ms. A, age 74, is a widow who retired from a high-level professional position 5 years ago. She was brought to the hospital due to ongoing anxiety, especially about her health. Ms. A maintained a wide range of relationships with friends, colleagues she mentored, and neighbors who provided a satisfying social network, and she continued to contribute to her field via scholarly writing projects. Before the pandemic, she found occasional sessions helpful in putting her health fears into perspective. When the pandemic led her to isolate at home, Ms. A became anxious and depressed to an unprecedented extent. Video sessions were unsatisfying, and she was terrified of taking tranquilizers or other medications. Once COVID-19 vaccinations became available and both she and I received both doses, we switched to meeting in the conference room every 2 to 3 weeks, with considerably better results.
Mr. B, age 41, is a single male who I diagnosed with schizophrenia at age 19 when he developed paranoid delusions and auditory hallucinations. Mr. B was not interested in taking antipsychotic medications, and his situation did not improve even when he did try taking them. He volunteered at a local emergency department doing odd jobs—moving gurneys, cleaning rooms, hauling boxes of supplies—for many years, and had always been employed in jobs such as grocery stocking or janitorial work that did not involve extensive interactions with people. He repeatedly enrolled in programs that would provide a skill such as phlebotomy or medical billing, only to find that he was never hired for such work. We talked once a month for 30 minutes about his frustrations trying to find women to date and marry, and how he was repeatedly taken advantage of (one “date” from an escort service took him to an ATM and got him to withdraw most of the money in his account).
Coincident with COVID-19, Mr. B’s father died from widespread metastatic cancer. His father had been Mr. B’s guide, friend, payee for Social Security Disability Insurance funds, and source of advice. To provide humane and somewhat effective treatment, I saw Mr. B in the conference room. His capacity to express grief and distress at the loss of his father has been impressive, as has his initiative in finding a grief group to attend, which he has done consistently.
Several patients who had been seeing me for weekly psychotherapy chose not to continue, many without specifically informing me of their decision. I understood the situation was in flux, and it would not be clear to anyone what to expect for the future. To avoid pressuring anyone, I chose not to contact patients to inquire about their plans.
Ms. C, age 50, is a professional with 3 children whose marriage had been highly dissatisfying for years, and she was now ready to investigate it. She was very successful in her career, having taken on a leadership role in her firm and earning a high income, while her husband was erratic, unreliable, and self-absorbed. Though he was well-educated and competent in his field, he could not maintain employment in a corporate environment and worked as a consultant with relatively little success. Along with the hours she spent working, Ms. C took responsibility for the family finances, was the chief wage earner, managed the needs of their children, made sure meals were prepared, and took on many other responsibilities.
Continue to: Case examples: How it worked (cont.)...
We agreed to a weekly session that fit Ms. C’s schedule, and she seemed able to relax and talk about herself. I found Ms. C quite likeable and enjoyed meeting with her, though I worried about whether we would need a greater intensity to get at the reasons such a successful and intelligent woman would fear setting limits with her husband or even considering ending the relationship. The reasons were clear as we put together the story of her early life, but conviction only develops with full emotional awareness (transference provides this in psychoanalysis).
The pandemic started approximately 18 months into our work, and Ms. C disappeared. She called my administrative assistant to cancel further appointments but did not ask to speak with me directly. While I knew this might represent resistance, I also felt unwilling to pressure Ms. C if she chose not to continue. I remain hopeful that I will hear from her once again; if not, I will send a note by mail to say that I enjoyed working with her, am happy to see her again, and hope she found some benefit from our work.
Mr. D contacted me for psychotherapy following the death of his father, who I had seen as a patient many years earlier. I was aware of the likely impact of his father’s outsized personality and emotional dysregulation on Mr. D and agreed to meet with him. He had taken over the family business and had made it an even greater success, but had trouble feeling confident about setting limits with employees who he knew took advantage of his avoidance.
Mr. D and I met weekly for several months and then moved to every other week, a form of resistance I expected as we got closer to his feeling pain. At the same time, I recognize that many patients use this tactic to “dose” themselves with the intensity they can tolerate, and Mr. D was quite observant and able to pick up themes where we’d left off.
When the pandemic shut down office visits, Mr. D immediately agreed to video sessions, which he has continued at roughly the same frequency. While I miss sitting with him, we continue to make progress towards his goal of learning to see himself as able to compete with his father.
Psychoanalysis patients
I found that patients in psychoanalysis had no trouble with the transition to telephone sessions, and the intensity of the work was not diluted. In some ways, audio-only communication is more intimate and might encourage patients to talk about topics they may not have otherwise brought up. I have not seen any evidence of less progress among these patients.
Dr. E, age 45, is a divorced physician who began psychoanalysis 3 times per week on the couch in 2018 for problems with frustration and confusion about his career, his identity as a father, and intense loneliness. He had worked up to 80 hours per week to earn as much money as he could, but also to avoid time at home with his then-wife and young children. The lack of time to recover led him to hate his work, left no time for social connections, and led to binges of heavy drinking. Our work had begun to allow him to develop a narrative about his early life that had never been considered, and to identify patterns of repetition of old defensive strategies that had never served him well.
At the onset of the pandemic, I told Dr. E that we would have to switch to telephone sessions, and he agreed immediately. In fact, he came to prefer telephone work since it spared him the 2 hours per day he had spent coming to my office. While I found it less satisfying than working in person, we have continued the same schedule and with the same intensity and trajectory established before the pandemic.
Continue to: Working with new patients...
Working with new patients
Seeing new patients for diagnostic evaluation is always best done in person, because the information I gain from the patient’s appearance, clothing, demeanor, gait, postures, gesturing, and facial expressions (among other elements) gives me important impressions I miss with video or telephone. In many cases, patients gain a sense of who I am from sitting in my office, and using the conference room eliminates that benefit. I attempted to create a warm environment in the conference room by obtaining lamps that produce warmer indirect light and hanging artwork that reflects my tastes. There are clocks in places that allow me and my patient to keep track of time. In meeting new patients by video, I get some impressions about their surroundings that add to the information I get through our interview. I have done many diagnostic evaluations during the pandemic and gotten treatments (whether medication, psychotherapy, or both) underway without discernible problems in the outcomes. Patients who started with me in person have mostly wanted to continue with in-person meetings, but as many have told me, interspersed video sessions save them travel time.
What about vaccination?
Once COVID-19 vaccinations were widely available, I assumed patients would be as eager to get them as I had been. When I began asking patients about whether they had gotten their vaccines, I was surprised to hear that a few were not going to get vaccinated, clearly based on political views and misinformation about the danger of vaccines. (The topic of political beliefs and their impact on psychological treatment is beyond the scope of this commentary.) I tried to counter obvious misinformation, repeated my recommendation that the patient get vaccinated, and then turned to other topics. I later decided to tell all patients that vaccination was required to enter the office. Only 1 patient who had been coming to the office dropped out, and she eventually returned to meeting by video.
COVID-19’s toll on the therapist
While the first several months of the pandemic were so full of uncertainty about the future, once vaccinations were available, it seemed cause for hope of a return to normalcy. As time went on, however, it became clear that normal was still a long way off. With vaccine refusal and new variants upending my naïve view that we were near the end, I began to feel aware of the impact this had on me, and began to focus on self-care (Box). I had always seen myself as unusually lucky to have a full practice, a supportive partnership with my husband, grown children who didn’t need me to homeschool them, a strong social network of friends who could share the burden and cheer each other up at outdoor gatherings, and a wonderful group of siblings and in-laws (all in different cities) who stayed in touch via video calls and quarantined in advance of getting together in someone’s home.
Box
Self-care has always been a requirement of doing psychotherapeutic work, and I encourage practitioners to be sure they are attending to themselves. We can’t be effective as listeners, empathizers, diagnosticians, and problem-solvers if we ourselves aren’t healthy. We evaluate our patients in terms of mood, outlook, sleep, appetite, energy, motivation, and energy; we also investigate their capacity for relationships that are sustaining. Self-care is the same, taking care of both our physical and relationship beings. Getting enough sleep, exercising daily, cooking healthy meals, and making time to relax are all ways of caring for our physical identities that should have been in place before COVID-19. Making personal time for ourselves in the face of constant demands for time from patients, colleagues, partners, children, parents, siblings, and friends never happens without the resolve to do it. As a psychiatrist who is used to sitting for up to 10 hours per day, I strongly recommend making a daily habit of walking, running, biking, or using an elliptical trainer, treadmill, or stationary bike for 30 minutes or more. Sleep is necessary for adequate concentration and attention to patient after patient. If you have trouble sleeping, talk with your doctor about remedies. If you use a sleep aid, I strongly recommend alternating medications so you don’t develop tolerance to any of them. Plan your food and cooking ahead of time so you aren’t tempted to order out. If you cook simple meals yourself (ideally with your partner helping or in range so you can chat), you will consume fewer calories, less sodium, and more nutrients. Even if you have a spouse and young children at home, work out a plan with your partner that allows each of you time for exercise or to recoup after a long day with patients. Babysitters allow you to take the time to be with each other that is necessary to sustaining a connection. Think about time for sexual intimacy if that has dropped off the calendar. Relationships with others, such as parents, siblings and their families, and friends are invaluable. The time spent with others might seem inconsequential, but is critical to our internal sense of security, even in the face of external disorder.
Staying busy and engaged with my practice, spouse, family, and friends kept sadness away most of the time. But I surprised myself a few months ago when I sat down to reflect and check in with myself. I felt enormous loss, resentment, and exhaustion at the privations of the pandemic: every trip to the grocery story felt dangerous. I hadn’t seen the inside of a concert hall, movie theater, restaurant, or museum in nearly 2 years. Travel for meetings and visits to family and friends and various adventures had been abruptly stopped. I lost both parents (not to COVID-19) during 2020; both were older adults living in senior communities that could not allow visitors. The usual grieving process would include attending services at my synagogue where I could say Kaddish for them, and video services were simply not tolerable.
Most of us have become experts at video meetings and likely have come to despise them. While our Institute has always held classes with some out-of-town students joining by video, with a very sophisticated system that provides excellent sound and visual fidelity, teaching entirely by video is another matter. I now teach students I have never met in person and might not recognize if I passed them in public. The art of creating discussion around a table is much more difficult on a computer screen. The first class I taught to residents during the pandemic was completely disorienting as I faced a wall of black screens with names and silence. Each student had turned off their camera and muted their microphone, so I was lecturing to a computer. That never happened again after I insisted on seeing everyone’s face and hearing their voices.
Thankfully, my usual experience of a long day seeing patients followed by chatting while cooking dinner with my husband and walking the dogs before settling down to read didn’t change. But the pleasure of sitting with patients was replaced by the daily grind of figuring out who will need a video link, who will be on the telephone, and who will come to the office, and it doesn’t feel the same. Again, in the big picture, I realize how fortunate I have been, but it’s been a big change in the world of the psychotherapist.
COVID-19 affected all aspects of psychiatric care. As a psychiatrist who is also a psychoanalyst, I faced some unique challenges to caring for my patients during the pandemic. In this article, I describe how COVID-19 impacted my practice, and how I adjusted to ensure that my patients received the best possible care.
The loss of ‘normal’
Our recognition of the loss was not immediate since no one knew what to expect. From March 11, 2020 through the end of the warm weather, when we could be outdoors, personal life was still gratifying. There was even a new spirit of togetherness in my neighborhood, with people seamlessly cooperating by crossing the street to avoid getting too close to one another, practicing proper social distancing in the grocery line, and smiling at everyone.
November 2020 through Spring 2021 was an unprecedented period of no socialization and spending time exclusively with my husband. By the end, I was finally aware of the exhaustion I felt trying to work with patients via phone and video sessions. Beyond that, we were (and still are) conducting administrative meetings and national organization meetings by video.
Spring 2021 until the arrival of cold weather felt more relaxed, as socializing outside again became possible. But from Winter 2021 to now has been a weary repeat of isolation, and a realization that my work life might never go back to “normal.” I would have to make peace with various sorts of losses of gratification in my work.
Life before COVID-19
I am a psychiatrist and psychoanalyst in a group private practice near the University of Cincinnati Medical Center. As a former full-time faculty member there, I maintain some teaching and supervision of residents. I typically see patients from 8:30 AM until 6:30 PM, and for years have had an average of 5 patients in psychoanalysis on the couch for 3 to 4 sessions per week. I see some psychotherapy patients weekly or twice a week and have some hours for new diagnostic evaluations and medication management. In addition, as a faculty member of the Cincinnati Psychoanalytic Institute, I take part in several committees, teach in the psychotherapy program and psychoanalytic training program, and supervise students and candidates. Most weeks, I see between 35 and 40 patients, with 4 to 6 weeks of vacation time per year.
Major changes with the onset of the pandemic
Once the threat from COVID-19 became clear in March 2020, I thought through my options. My office comprises 5 professional offices, a waiting room, and an administrative area. Our administrative assistant and 1 or 2 practitioners were in the office with me most days. We maintained appropriate distance from each another and wore masks in common areas. The practice group was exemplary in immediately setting up safe practices. I learned a few colleagues were seeing patients outside using lawn chairs in the back of our lot where there was some privacy, but many stopped coming to the building altogether.
I felt real sadness having to tell patients I could no longer see them in my office. However, I was relieved to find how quickly many patients made an immediate transition to telephone or video sessions. Since I was alone in my office and not distracted by barking dogs, ringing doorbells, or loud lawnmowers, I continued to come to the office, and never switched to working from home.
Since I was not vis-à-vis with patients on the couch, those sessions shifted to the telephone. I offered psychotherapy patients the option of video sessions via the Health Insurance Portability and Accountability Act–compliant Doximity app (doxy.me) or telephone, and found that approximately 75% preferred video. When I used the telephone, I used a professional-grade headset, which made it less onerous than being tied to a receiver, and I occasionally used the speaker option. I also installed a desk platform that allows me to raise and lower my computer from sitting to standing height.
I worried a great deal about patients I felt would do poorly with video or telephone sessions: older adults who found comfort in human contact that was sometimes curative, less well-integrated individuals who needed real contact in order to feel there was a treatment process, those with serious mental illnesses who needed reassurance at their reality-testing, and new patients who I couldn’t fully assess without in-person meetings.
In the beginning of the pandemic, as we were still learning about the virus, nothing seemed safe. We were washing our hands constantly, afraid to touch doorknobs, mail, or groceries. Thankfully, we learned that COVID-19 transmission occurs primarily through inhalation of droplets and particles containing the virus.1 Masks, good ventilation, and adequate distance from others considerably cut infection rates. By January 2021, the availability of a vaccine made an enormous difference in vulnerability to severe illness.
When I stopped seeing patients in my office, I set up the conference room that had doors on either end so I could sit on one end of a table and have the patient at the other end, keeping about 8 feet between us. I also kept a fan blowing air away from me and parallel to the patient. After each session, I opened both doors to allow for full ventilation of the room. This provided a solution for the patients I knew I needed to meet with in person.
Continue to: Case examples: How it worked...
Case examples: How it worked
The following case examples illustrate how I provided care during this time. To protect patient anonymity, these vignettes are composites.
Psychotherapy patients
Established patients in psychotherapy have seemed to work well with video or telephone sessions. The video option added a new element I never appreciated: seeing patients in their homes or cars allowed me to gain a new set of impressions about them. The use of technology is clearly another element I would not have identified before. Less technically adept older patients are likely to join a video session with only the top of their head visible, or with insufficient lighting. In some cases, I coached patients to rearrange their computer so I could see their faces, but only if it seemed that doing so would not cause them greater distress.
Ms. A, age 74, is a widow who retired from a high-level professional position 5 years ago. She was brought to the hospital due to ongoing anxiety, especially about her health. Ms. A maintained a wide range of relationships with friends, colleagues she mentored, and neighbors who provided a satisfying social network, and she continued to contribute to her field via scholarly writing projects. Before the pandemic, she found occasional sessions helpful in putting her health fears into perspective. When the pandemic led her to isolate at home, Ms. A became anxious and depressed to an unprecedented extent. Video sessions were unsatisfying, and she was terrified of taking tranquilizers or other medications. Once COVID-19 vaccinations became available and both she and I received both doses, we switched to meeting in the conference room every 2 to 3 weeks, with considerably better results.
Mr. B, age 41, is a single male who I diagnosed with schizophrenia at age 19 when he developed paranoid delusions and auditory hallucinations. Mr. B was not interested in taking antipsychotic medications, and his situation did not improve even when he did try taking them. He volunteered at a local emergency department doing odd jobs—moving gurneys, cleaning rooms, hauling boxes of supplies—for many years, and had always been employed in jobs such as grocery stocking or janitorial work that did not involve extensive interactions with people. He repeatedly enrolled in programs that would provide a skill such as phlebotomy or medical billing, only to find that he was never hired for such work. We talked once a month for 30 minutes about his frustrations trying to find women to date and marry, and how he was repeatedly taken advantage of (one “date” from an escort service took him to an ATM and got him to withdraw most of the money in his account).
Coincident with COVID-19, Mr. B’s father died from widespread metastatic cancer. His father had been Mr. B’s guide, friend, payee for Social Security Disability Insurance funds, and source of advice. To provide humane and somewhat effective treatment, I saw Mr. B in the conference room. His capacity to express grief and distress at the loss of his father has been impressive, as has his initiative in finding a grief group to attend, which he has done consistently.
Several patients who had been seeing me for weekly psychotherapy chose not to continue, many without specifically informing me of their decision. I understood the situation was in flux, and it would not be clear to anyone what to expect for the future. To avoid pressuring anyone, I chose not to contact patients to inquire about their plans.
Ms. C, age 50, is a professional with 3 children whose marriage had been highly dissatisfying for years, and she was now ready to investigate it. She was very successful in her career, having taken on a leadership role in her firm and earning a high income, while her husband was erratic, unreliable, and self-absorbed. Though he was well-educated and competent in his field, he could not maintain employment in a corporate environment and worked as a consultant with relatively little success. Along with the hours she spent working, Ms. C took responsibility for the family finances, was the chief wage earner, managed the needs of their children, made sure meals were prepared, and took on many other responsibilities.
Continue to: Case examples: How it worked (cont.)...
We agreed to a weekly session that fit Ms. C’s schedule, and she seemed able to relax and talk about herself. I found Ms. C quite likeable and enjoyed meeting with her, though I worried about whether we would need a greater intensity to get at the reasons such a successful and intelligent woman would fear setting limits with her husband or even considering ending the relationship. The reasons were clear as we put together the story of her early life, but conviction only develops with full emotional awareness (transference provides this in psychoanalysis).
The pandemic started approximately 18 months into our work, and Ms. C disappeared. She called my administrative assistant to cancel further appointments but did not ask to speak with me directly. While I knew this might represent resistance, I also felt unwilling to pressure Ms. C if she chose not to continue. I remain hopeful that I will hear from her once again; if not, I will send a note by mail to say that I enjoyed working with her, am happy to see her again, and hope she found some benefit from our work.
Mr. D contacted me for psychotherapy following the death of his father, who I had seen as a patient many years earlier. I was aware of the likely impact of his father’s outsized personality and emotional dysregulation on Mr. D and agreed to meet with him. He had taken over the family business and had made it an even greater success, but had trouble feeling confident about setting limits with employees who he knew took advantage of his avoidance.
Mr. D and I met weekly for several months and then moved to every other week, a form of resistance I expected as we got closer to his feeling pain. At the same time, I recognize that many patients use this tactic to “dose” themselves with the intensity they can tolerate, and Mr. D was quite observant and able to pick up themes where we’d left off.
When the pandemic shut down office visits, Mr. D immediately agreed to video sessions, which he has continued at roughly the same frequency. While I miss sitting with him, we continue to make progress towards his goal of learning to see himself as able to compete with his father.
Psychoanalysis patients
I found that patients in psychoanalysis had no trouble with the transition to telephone sessions, and the intensity of the work was not diluted. In some ways, audio-only communication is more intimate and might encourage patients to talk about topics they may not have otherwise brought up. I have not seen any evidence of less progress among these patients.
Dr. E, age 45, is a divorced physician who began psychoanalysis 3 times per week on the couch in 2018 for problems with frustration and confusion about his career, his identity as a father, and intense loneliness. He had worked up to 80 hours per week to earn as much money as he could, but also to avoid time at home with his then-wife and young children. The lack of time to recover led him to hate his work, left no time for social connections, and led to binges of heavy drinking. Our work had begun to allow him to develop a narrative about his early life that had never been considered, and to identify patterns of repetition of old defensive strategies that had never served him well.
At the onset of the pandemic, I told Dr. E that we would have to switch to telephone sessions, and he agreed immediately. In fact, he came to prefer telephone work since it spared him the 2 hours per day he had spent coming to my office. While I found it less satisfying than working in person, we have continued the same schedule and with the same intensity and trajectory established before the pandemic.
Continue to: Working with new patients...
Working with new patients
Seeing new patients for diagnostic evaluation is always best done in person, because the information I gain from the patient’s appearance, clothing, demeanor, gait, postures, gesturing, and facial expressions (among other elements) gives me important impressions I miss with video or telephone. In many cases, patients gain a sense of who I am from sitting in my office, and using the conference room eliminates that benefit. I attempted to create a warm environment in the conference room by obtaining lamps that produce warmer indirect light and hanging artwork that reflects my tastes. There are clocks in places that allow me and my patient to keep track of time. In meeting new patients by video, I get some impressions about their surroundings that add to the information I get through our interview. I have done many diagnostic evaluations during the pandemic and gotten treatments (whether medication, psychotherapy, or both) underway without discernible problems in the outcomes. Patients who started with me in person have mostly wanted to continue with in-person meetings, but as many have told me, interspersed video sessions save them travel time.
What about vaccination?
Once COVID-19 vaccinations were widely available, I assumed patients would be as eager to get them as I had been. When I began asking patients about whether they had gotten their vaccines, I was surprised to hear that a few were not going to get vaccinated, clearly based on political views and misinformation about the danger of vaccines. (The topic of political beliefs and their impact on psychological treatment is beyond the scope of this commentary.) I tried to counter obvious misinformation, repeated my recommendation that the patient get vaccinated, and then turned to other topics. I later decided to tell all patients that vaccination was required to enter the office. Only 1 patient who had been coming to the office dropped out, and she eventually returned to meeting by video.
COVID-19’s toll on the therapist
While the first several months of the pandemic were so full of uncertainty about the future, once vaccinations were available, it seemed cause for hope of a return to normalcy. As time went on, however, it became clear that normal was still a long way off. With vaccine refusal and new variants upending my naïve view that we were near the end, I began to feel aware of the impact this had on me, and began to focus on self-care (Box). I had always seen myself as unusually lucky to have a full practice, a supportive partnership with my husband, grown children who didn’t need me to homeschool them, a strong social network of friends who could share the burden and cheer each other up at outdoor gatherings, and a wonderful group of siblings and in-laws (all in different cities) who stayed in touch via video calls and quarantined in advance of getting together in someone’s home.
Box
Self-care has always been a requirement of doing psychotherapeutic work, and I encourage practitioners to be sure they are attending to themselves. We can’t be effective as listeners, empathizers, diagnosticians, and problem-solvers if we ourselves aren’t healthy. We evaluate our patients in terms of mood, outlook, sleep, appetite, energy, motivation, and energy; we also investigate their capacity for relationships that are sustaining. Self-care is the same, taking care of both our physical and relationship beings. Getting enough sleep, exercising daily, cooking healthy meals, and making time to relax are all ways of caring for our physical identities that should have been in place before COVID-19. Making personal time for ourselves in the face of constant demands for time from patients, colleagues, partners, children, parents, siblings, and friends never happens without the resolve to do it. As a psychiatrist who is used to sitting for up to 10 hours per day, I strongly recommend making a daily habit of walking, running, biking, or using an elliptical trainer, treadmill, or stationary bike for 30 minutes or more. Sleep is necessary for adequate concentration and attention to patient after patient. If you have trouble sleeping, talk with your doctor about remedies. If you use a sleep aid, I strongly recommend alternating medications so you don’t develop tolerance to any of them. Plan your food and cooking ahead of time so you aren’t tempted to order out. If you cook simple meals yourself (ideally with your partner helping or in range so you can chat), you will consume fewer calories, less sodium, and more nutrients. Even if you have a spouse and young children at home, work out a plan with your partner that allows each of you time for exercise or to recoup after a long day with patients. Babysitters allow you to take the time to be with each other that is necessary to sustaining a connection. Think about time for sexual intimacy if that has dropped off the calendar. Relationships with others, such as parents, siblings and their families, and friends are invaluable. The time spent with others might seem inconsequential, but is critical to our internal sense of security, even in the face of external disorder.
Staying busy and engaged with my practice, spouse, family, and friends kept sadness away most of the time. But I surprised myself a few months ago when I sat down to reflect and check in with myself. I felt enormous loss, resentment, and exhaustion at the privations of the pandemic: every trip to the grocery story felt dangerous. I hadn’t seen the inside of a concert hall, movie theater, restaurant, or museum in nearly 2 years. Travel for meetings and visits to family and friends and various adventures had been abruptly stopped. I lost both parents (not to COVID-19) during 2020; both were older adults living in senior communities that could not allow visitors. The usual grieving process would include attending services at my synagogue where I could say Kaddish for them, and video services were simply not tolerable.
Most of us have become experts at video meetings and likely have come to despise them. While our Institute has always held classes with some out-of-town students joining by video, with a very sophisticated system that provides excellent sound and visual fidelity, teaching entirely by video is another matter. I now teach students I have never met in person and might not recognize if I passed them in public. The art of creating discussion around a table is much more difficult on a computer screen. The first class I taught to residents during the pandemic was completely disorienting as I faced a wall of black screens with names and silence. Each student had turned off their camera and muted their microphone, so I was lecturing to a computer. That never happened again after I insisted on seeing everyone’s face and hearing their voices.
Thankfully, my usual experience of a long day seeing patients followed by chatting while cooking dinner with my husband and walking the dogs before settling down to read didn’t change. But the pleasure of sitting with patients was replaced by the daily grind of figuring out who will need a video link, who will be on the telephone, and who will come to the office, and it doesn’t feel the same. Again, in the big picture, I realize how fortunate I have been, but it’s been a big change in the world of the psychotherapist.
1. Centers for Disease Control and Prevention. COVID-19 frequently asked questions. Accessed March 8, 2022. https://www.cdc.gov/coronavirus/2019-ncov/faq.html#Spread
1. Centers for Disease Control and Prevention. COVID-19 frequently asked questions. Accessed March 8, 2022. https://www.cdc.gov/coronavirus/2019-ncov/faq.html#Spread
Managing a COVID-19–positive psychiatric patient on a medical unit
With the COVID-19 pandemic turning the world on its head, we have seen more first-episode psychotic breaks and quick deterioration in previously stable patients. Early in the pandemic, care was particularly complicated for psychiatric patients who had been infected with the virus. Many of these patients required immediate psychiatric hospitalization. At that time, many community hospital psychiatric inpatient units did not have the capacity, staffing, or infrastructure to safely admit such patients, so they needed to be managed on a medical unit. Here, I discuss the case of a COVID-19–positive woman with psychiatric illness who we managed while she was in quarantine on a medical unit.
Case report
Early in the COVID-19 pandemic, Ms. B, a 35-year-old teacher with a history of depression, was evaluated in the emergency department for bizarre behavior and paranoid delusions regarding her family. Initial laboratory and imaging testing was negative for any potential medical causes of her psychiatric symptoms. Psychiatric hospitalization was recommended, but before Ms. B could be transferred to the psychiatric unit, she tested positive for COVID-19. At that time, our community hospital did not have a designated wing on our psychiatric unit for patients infected with COVID-19. Thus, Ms. B was admitted to the medical floor, where she was quarantined in her room. She would need to remain asymptomatic and test negative for COVID-19 before she could be transferred to the psychiatric unit.
Upon arriving at the medical unit, Ms. B was hostile and uncooperative. She frequently attempted to leave her room and required restraints throughout the day. Our consultation-liaison (CL) team was consulted to assist in managing her. During the initial interview, we noticed that she had covered all 4 walls of her room with papers filled with handwritten notes. Ms. B had cut her gown to expose her stomach and legs. She had pressured speech, tangential thinking, and was religiously preoccupied. She denied any visual and auditory hallucinations, but her persecutory delusions involving her family persisted. We believed that her signs and symptoms were consistent with a manic episode from underlying, and likely undiagnosed, bipolar I disorder that was precipitated by her COVID-19 infection.
We first addressed Ms. B’s and the staff’s safety by transferring her to a larger room with a vestibule at the end of the hallway so she had more room to walk and minimal exposure to the stimuli of the medical unit. We initiated one-on-one observation to redirect her and prevent elopement. We incentivized her cooperation with staff by providing her with paper, pencils, reading material, and phone privileges. We started oral risperidone 2 mg twice daily and lorazepam 2 mg 3 times daily for short-term behavioral control and acute treatment of her symptoms, with the goal of deferring additional treatment decisions to the inpatient psychiatry team after she was transferred to the psychiatric unit. Ms. B’s agitation and impulsivity improved. She began participating with the medical team and was eventually transferred out of our medical unit to a psychiatric unit at a different facility.
COVID-19 and psychiatric illness: Clinical concerns
While infection from COVID-19 and widespread social distancing of the general population have been linked to depression and anxiety, manic and psychotic symptoms secondary to the COVID-19 pandemic have not been well described. The association between influenza infection and psychosis has been reported since the Spanish Flu pandemic,1 but there is limited data on the association between COVID-19 and psychosis. A review of 14 studies found that 0.9% to 4% of people exposed to a virus during an epidemic or pandemic develop psychosis or psychotic symptoms.1 Psychosis was associated with viral exposure, treatments used to manage the infection (steroid therapy), and psychosocial stress. This study also found that treatment with low doses of antipsychotic medication—notably aripiprazole—seemed to have been effective.1
Nonetheless, it is important to keep in mind a thorough differential diagnosis and rule out any potential organic etiologies in a COVID-19–positive patient who presents with psychiatric symptoms.2 For Ms. B, we began by ruling out drug-induced psychosis and electrolyte imbalance, and obtained brain imaging to rule out malignancy. We considered an interictal behavior syndrome of temporal lobe epilepsy, a neuropsychiatric disorder characterized by alterations in sexual behavior, religiosity, and extensive and compulsive writing and drawing.3 Neurology was consulted to evaluate the patient and possibly use EEG to detect interictal spikes, a tall task given the patient’s restlessness and paranoia. Ultimately, we determined the patient was most likely exhibiting symptoms of previously undetected bipolar disorder.
Managing patients with psychiatric illness on a medical floor during a pandemic such as COVID-19 requires the psychiatrist to truly serve as a consultant and liaison between the patient and the treatment team.4 Clinical management should address both infection control and psychiatric symptoms.5 We visited with Ms. B frequently, provided psychoeducation, engaged her in treatment, and updated her on the treatment plan.
As the medical world continues to adjust to treating patients during the pandemic, CL psychiatrists may be tasked with managing patients with acute psychiatric illness on the medical unit while they await transfer to a psychiatric unit. A creative, multifaceted, and team-based approach is key to ensure effective care and safety for all involved.
1. Brown E, Gray R, Lo Monaco S, et al. The potential impact of COVID-19 on psychosis: a rapid review of contemporary epidemic and pandemic research. Schizophr Res. 2020;222:79-87. doi:10.1016/j.schres.2020.05.005
2. Byrne P. Managing the acute psychotic episode. BMJ. 2007;334(7595):686-692. doi:10.1136/bmj.39148.668160.80
3. Waxman SG, Geschwind N. The interictal behavior syndrome of temporal lobe epilepsy. Arch Gen Psychiatry. 1975;32(12):1580-1586. doi:10.1001/archpsyc.1975.01760300118011
4. Stern TA, Freudenreich O, Smith FA, et al. Psychotic patients. In: Massachusetts General Hospital: Handbook of General Hospital Psychiatry. Mosby; 1997:109-121.
5. Deshpande S, Livingstone A. First-onset psychosis in older adults: social isolation influence during COVID pandemic—a UK case series. Progress in Neurology and Psychiatry. 2021;25(1):14-18. doi:10.1002/pnp.692
With the COVID-19 pandemic turning the world on its head, we have seen more first-episode psychotic breaks and quick deterioration in previously stable patients. Early in the pandemic, care was particularly complicated for psychiatric patients who had been infected with the virus. Many of these patients required immediate psychiatric hospitalization. At that time, many community hospital psychiatric inpatient units did not have the capacity, staffing, or infrastructure to safely admit such patients, so they needed to be managed on a medical unit. Here, I discuss the case of a COVID-19–positive woman with psychiatric illness who we managed while she was in quarantine on a medical unit.
Case report
Early in the COVID-19 pandemic, Ms. B, a 35-year-old teacher with a history of depression, was evaluated in the emergency department for bizarre behavior and paranoid delusions regarding her family. Initial laboratory and imaging testing was negative for any potential medical causes of her psychiatric symptoms. Psychiatric hospitalization was recommended, but before Ms. B could be transferred to the psychiatric unit, she tested positive for COVID-19. At that time, our community hospital did not have a designated wing on our psychiatric unit for patients infected with COVID-19. Thus, Ms. B was admitted to the medical floor, where she was quarantined in her room. She would need to remain asymptomatic and test negative for COVID-19 before she could be transferred to the psychiatric unit.
Upon arriving at the medical unit, Ms. B was hostile and uncooperative. She frequently attempted to leave her room and required restraints throughout the day. Our consultation-liaison (CL) team was consulted to assist in managing her. During the initial interview, we noticed that she had covered all 4 walls of her room with papers filled with handwritten notes. Ms. B had cut her gown to expose her stomach and legs. She had pressured speech, tangential thinking, and was religiously preoccupied. She denied any visual and auditory hallucinations, but her persecutory delusions involving her family persisted. We believed that her signs and symptoms were consistent with a manic episode from underlying, and likely undiagnosed, bipolar I disorder that was precipitated by her COVID-19 infection.
We first addressed Ms. B’s and the staff’s safety by transferring her to a larger room with a vestibule at the end of the hallway so she had more room to walk and minimal exposure to the stimuli of the medical unit. We initiated one-on-one observation to redirect her and prevent elopement. We incentivized her cooperation with staff by providing her with paper, pencils, reading material, and phone privileges. We started oral risperidone 2 mg twice daily and lorazepam 2 mg 3 times daily for short-term behavioral control and acute treatment of her symptoms, with the goal of deferring additional treatment decisions to the inpatient psychiatry team after she was transferred to the psychiatric unit. Ms. B’s agitation and impulsivity improved. She began participating with the medical team and was eventually transferred out of our medical unit to a psychiatric unit at a different facility.
COVID-19 and psychiatric illness: Clinical concerns
While infection from COVID-19 and widespread social distancing of the general population have been linked to depression and anxiety, manic and psychotic symptoms secondary to the COVID-19 pandemic have not been well described. The association between influenza infection and psychosis has been reported since the Spanish Flu pandemic,1 but there is limited data on the association between COVID-19 and psychosis. A review of 14 studies found that 0.9% to 4% of people exposed to a virus during an epidemic or pandemic develop psychosis or psychotic symptoms.1 Psychosis was associated with viral exposure, treatments used to manage the infection (steroid therapy), and psychosocial stress. This study also found that treatment with low doses of antipsychotic medication—notably aripiprazole—seemed to have been effective.1
Nonetheless, it is important to keep in mind a thorough differential diagnosis and rule out any potential organic etiologies in a COVID-19–positive patient who presents with psychiatric symptoms.2 For Ms. B, we began by ruling out drug-induced psychosis and electrolyte imbalance, and obtained brain imaging to rule out malignancy. We considered an interictal behavior syndrome of temporal lobe epilepsy, a neuropsychiatric disorder characterized by alterations in sexual behavior, religiosity, and extensive and compulsive writing and drawing.3 Neurology was consulted to evaluate the patient and possibly use EEG to detect interictal spikes, a tall task given the patient’s restlessness and paranoia. Ultimately, we determined the patient was most likely exhibiting symptoms of previously undetected bipolar disorder.
Managing patients with psychiatric illness on a medical floor during a pandemic such as COVID-19 requires the psychiatrist to truly serve as a consultant and liaison between the patient and the treatment team.4 Clinical management should address both infection control and psychiatric symptoms.5 We visited with Ms. B frequently, provided psychoeducation, engaged her in treatment, and updated her on the treatment plan.
As the medical world continues to adjust to treating patients during the pandemic, CL psychiatrists may be tasked with managing patients with acute psychiatric illness on the medical unit while they await transfer to a psychiatric unit. A creative, multifaceted, and team-based approach is key to ensure effective care and safety for all involved.
With the COVID-19 pandemic turning the world on its head, we have seen more first-episode psychotic breaks and quick deterioration in previously stable patients. Early in the pandemic, care was particularly complicated for psychiatric patients who had been infected with the virus. Many of these patients required immediate psychiatric hospitalization. At that time, many community hospital psychiatric inpatient units did not have the capacity, staffing, or infrastructure to safely admit such patients, so they needed to be managed on a medical unit. Here, I discuss the case of a COVID-19–positive woman with psychiatric illness who we managed while she was in quarantine on a medical unit.
Case report
Early in the COVID-19 pandemic, Ms. B, a 35-year-old teacher with a history of depression, was evaluated in the emergency department for bizarre behavior and paranoid delusions regarding her family. Initial laboratory and imaging testing was negative for any potential medical causes of her psychiatric symptoms. Psychiatric hospitalization was recommended, but before Ms. B could be transferred to the psychiatric unit, she tested positive for COVID-19. At that time, our community hospital did not have a designated wing on our psychiatric unit for patients infected with COVID-19. Thus, Ms. B was admitted to the medical floor, where she was quarantined in her room. She would need to remain asymptomatic and test negative for COVID-19 before she could be transferred to the psychiatric unit.
Upon arriving at the medical unit, Ms. B was hostile and uncooperative. She frequently attempted to leave her room and required restraints throughout the day. Our consultation-liaison (CL) team was consulted to assist in managing her. During the initial interview, we noticed that she had covered all 4 walls of her room with papers filled with handwritten notes. Ms. B had cut her gown to expose her stomach and legs. She had pressured speech, tangential thinking, and was religiously preoccupied. She denied any visual and auditory hallucinations, but her persecutory delusions involving her family persisted. We believed that her signs and symptoms were consistent with a manic episode from underlying, and likely undiagnosed, bipolar I disorder that was precipitated by her COVID-19 infection.
We first addressed Ms. B’s and the staff’s safety by transferring her to a larger room with a vestibule at the end of the hallway so she had more room to walk and minimal exposure to the stimuli of the medical unit. We initiated one-on-one observation to redirect her and prevent elopement. We incentivized her cooperation with staff by providing her with paper, pencils, reading material, and phone privileges. We started oral risperidone 2 mg twice daily and lorazepam 2 mg 3 times daily for short-term behavioral control and acute treatment of her symptoms, with the goal of deferring additional treatment decisions to the inpatient psychiatry team after she was transferred to the psychiatric unit. Ms. B’s agitation and impulsivity improved. She began participating with the medical team and was eventually transferred out of our medical unit to a psychiatric unit at a different facility.
COVID-19 and psychiatric illness: Clinical concerns
While infection from COVID-19 and widespread social distancing of the general population have been linked to depression and anxiety, manic and psychotic symptoms secondary to the COVID-19 pandemic have not been well described. The association between influenza infection and psychosis has been reported since the Spanish Flu pandemic,1 but there is limited data on the association between COVID-19 and psychosis. A review of 14 studies found that 0.9% to 4% of people exposed to a virus during an epidemic or pandemic develop psychosis or psychotic symptoms.1 Psychosis was associated with viral exposure, treatments used to manage the infection (steroid therapy), and psychosocial stress. This study also found that treatment with low doses of antipsychotic medication—notably aripiprazole—seemed to have been effective.1
Nonetheless, it is important to keep in mind a thorough differential diagnosis and rule out any potential organic etiologies in a COVID-19–positive patient who presents with psychiatric symptoms.2 For Ms. B, we began by ruling out drug-induced psychosis and electrolyte imbalance, and obtained brain imaging to rule out malignancy. We considered an interictal behavior syndrome of temporal lobe epilepsy, a neuropsychiatric disorder characterized by alterations in sexual behavior, religiosity, and extensive and compulsive writing and drawing.3 Neurology was consulted to evaluate the patient and possibly use EEG to detect interictal spikes, a tall task given the patient’s restlessness and paranoia. Ultimately, we determined the patient was most likely exhibiting symptoms of previously undetected bipolar disorder.
Managing patients with psychiatric illness on a medical floor during a pandemic such as COVID-19 requires the psychiatrist to truly serve as a consultant and liaison between the patient and the treatment team.4 Clinical management should address both infection control and psychiatric symptoms.5 We visited with Ms. B frequently, provided psychoeducation, engaged her in treatment, and updated her on the treatment plan.
As the medical world continues to adjust to treating patients during the pandemic, CL psychiatrists may be tasked with managing patients with acute psychiatric illness on the medical unit while they await transfer to a psychiatric unit. A creative, multifaceted, and team-based approach is key to ensure effective care and safety for all involved.
1. Brown E, Gray R, Lo Monaco S, et al. The potential impact of COVID-19 on psychosis: a rapid review of contemporary epidemic and pandemic research. Schizophr Res. 2020;222:79-87. doi:10.1016/j.schres.2020.05.005
2. Byrne P. Managing the acute psychotic episode. BMJ. 2007;334(7595):686-692. doi:10.1136/bmj.39148.668160.80
3. Waxman SG, Geschwind N. The interictal behavior syndrome of temporal lobe epilepsy. Arch Gen Psychiatry. 1975;32(12):1580-1586. doi:10.1001/archpsyc.1975.01760300118011
4. Stern TA, Freudenreich O, Smith FA, et al. Psychotic patients. In: Massachusetts General Hospital: Handbook of General Hospital Psychiatry. Mosby; 1997:109-121.
5. Deshpande S, Livingstone A. First-onset psychosis in older adults: social isolation influence during COVID pandemic—a UK case series. Progress in Neurology and Psychiatry. 2021;25(1):14-18. doi:10.1002/pnp.692
1. Brown E, Gray R, Lo Monaco S, et al. The potential impact of COVID-19 on psychosis: a rapid review of contemporary epidemic and pandemic research. Schizophr Res. 2020;222:79-87. doi:10.1016/j.schres.2020.05.005
2. Byrne P. Managing the acute psychotic episode. BMJ. 2007;334(7595):686-692. doi:10.1136/bmj.39148.668160.80
3. Waxman SG, Geschwind N. The interictal behavior syndrome of temporal lobe epilepsy. Arch Gen Psychiatry. 1975;32(12):1580-1586. doi:10.1001/archpsyc.1975.01760300118011
4. Stern TA, Freudenreich O, Smith FA, et al. Psychotic patients. In: Massachusetts General Hospital: Handbook of General Hospital Psychiatry. Mosby; 1997:109-121.
5. Deshpande S, Livingstone A. First-onset psychosis in older adults: social isolation influence during COVID pandemic—a UK case series. Progress in Neurology and Psychiatry. 2021;25(1):14-18. doi:10.1002/pnp.692
Skin reactions to first COVID-19 vaccine don’t justify forgoing second dose
BOSTON – Requests for a according to an analysis of several large sets of data presented at the annual meeting of the American Academy of Dermatology.
According to the data, “there are no serious adverse consequences from these cutaneous reactions,” said Esther Freeman, MD, PhD, director of Global Health Dermatology, Massachusetts General Hospital, Boston.
This is important because the risk of vaccine hesitancy goes up dramatically in patients who experience reactions to the first vaccine dose, according to follow-up of more than 50,000 employees vaccinated in the Mass General Brigham Healthcare System (MGBHS). According to Dr. Freeman, there was almost a fourfold increase in the rate of second-dose refusals for those with cutaneous reactions and a more than fourfold increase in those who developed angioedema.
Before the data were available, skin reactions were a source of concern among dermatologists and others involved in monitoring vaccine-related adverse events. Injection site reactions (ISRs) are associated with essentially every injectable vaccine, so these were expected, but a small proportion of patients developed large red plaques in the injection arm 7-8 days after the inoculation.
“These delayed reactions caused a lot of initial panic,” said Dr. Freeman, who counted herself among those alarmed about what the reactions might signify. “Was this cellulitis? Would the next dose cause anaphylaxis? We were concerned.”
This concern dissipated with the availability of more data. In a global registry that has so far captured more than 1,000 cutaneous reactions from 52 participating countries, it appears that about 2% of patients have a cutaneous reaction other than an ISR after the first dose. All resolve with minimal skin care or no treatment.
After the second dose, the proportion is lower. If there is a reaction, it typically occurs earlier and resolves more quickly.
“What we have learned is that fewer than half of patients who had a reaction to the first dose have a reaction to the second, and those who did have a reaction had a milder course,” said Dr. Freeman.
These data are “incredibly reassuring” on many levels, she explained. In addition, it allows clinicians to confidently explain to patients that there are no serious sequelae from the rashes, whether immediate or delayed, from the available COVID-19 vaccines.
“Every skin reaction I have seen is something we can treat through,” she added, noting that most reactions resolve with little or no supportive care. Following skin reactions, particularly the delayed lesions, it is not uncommon for patients to refuse a second shot. Some request a medical waiver to avoid further vaccine exposure. According to Dr. Freeman, this is unwarranted.
“I have granted exactly zero waivers,” she said. She explains to patients that these reactions have not been predictive of serious events, such as anaphylaxis. Although the trigger of the hypersensitivity reaction remains unknown, there is no evidence of serious consequences.
Delayed skin reactions are more commonly associated with the Moderna than the Pfizer vaccine. One notable difference between these vaccines is the greater content of mRNA in the Moderna formulation, but Freeman said that this is only one potential hypothesis for higher frequency of reactions to this version of the vaccine.
Patients with a history of allergic disease are more likely to develop a reaction but not significantly more likely to have a reaction that is more difficult to manage, according to Kimberly G. Blumenthal, MD, quality and safety officer for allergy, and codirector of the clinical epidemiology program in the division of rheumatology, allergy, and immunology at Mass General.
Anaphylaxis has been associated with COVD-19 vaccines just as it has with essentially every injectable vaccine, Dr. Blumenthal said during the same session. But the risk is very low, and it stays low even among those with a history of severe hypersensitivity reactions in the past.
Among the data collected from more than 52,000 vaccinated MGBHS employees, 0.9% had a history of severe allergic reaction to a prior vaccine. Of these, 11.6% had an allergic reaction to the COVID-19 vaccine. This was more than twice the 4.6% rate of allergic reactions among employees without a history of allergic reactions, but serious consequences were rare in both groups.
Of those with a reaction to the first dose, all but 2.4% took a subsequent dose. Again, serious reactions were exceedingly rare. These serious reactions did include anaphylaxis and hospitalization in 3% of patients, but there were no fatalities and all resolved.
The absence of serious sequelae from a reaction to a COVID-19 vaccine must be considered within the context of the benefit, which includes protection from death and hospitalization from the virus, according to Dr. Blumenthal. Citing the evidence that first-shot reactions are a source of vaccine hesitancy, she agreed that it is important to educate patients about relative risks.
“Even in our own cohort of MGBHS employees, we have people, including those who had been provaccine in the past, become hesitant,” commented Dr. Blumenthal, who said there are data from the Kaiser Permanente System showing similar vaccine reluctance following a first-shot reaction.
After more than 500 million doses of the Moderna and Pfizer vaccines had been administered worldwide, there was not a single reported death from anaphylaxis. Although Dr. Blumenthal said that an unconfirmed death of this type had been recently reported, she emphasized that this single death, if valid, is dwarfed by the lives saved with vaccination.
Asked about her strategy for counseling patients with vaccine hesitancy, Dr. Freeman said the body of safety data is large and compelling. There is overwhelming evidence of a favorable benefit-to-risk ratio overall and among those with a first-shot reaction.
“I can reassure them on the basis of the data,” Dr. Freeman said in an interview. “Less than half will have a reaction to the second shot and even if they do have a reaction, it is likely to be less severe.”
Although the main message is that vaccination is potentially lifesaving and far outweighs any risks, Freeman specifically gives this message to those hesitant to take a second shot after a first-shot reaction: “I can get you through it.”
Dr. Freeman encouraged health care professionals to report cases of COVID-19 vaccine–related dermatologic side effects to the American Academy of Dermatology / International League of Dermatologic Societies COVID-19 dermatology registry. Dermatologic manifestations of COVID-19 can also be reported to the registry.
Dr. Freeman disclosed receiving grants/research funding from the International League of Dermatologic Societies and from the National Institutes of Health. Dr. Blumenthal disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BOSTON – Requests for a according to an analysis of several large sets of data presented at the annual meeting of the American Academy of Dermatology.
According to the data, “there are no serious adverse consequences from these cutaneous reactions,” said Esther Freeman, MD, PhD, director of Global Health Dermatology, Massachusetts General Hospital, Boston.
This is important because the risk of vaccine hesitancy goes up dramatically in patients who experience reactions to the first vaccine dose, according to follow-up of more than 50,000 employees vaccinated in the Mass General Brigham Healthcare System (MGBHS). According to Dr. Freeman, there was almost a fourfold increase in the rate of second-dose refusals for those with cutaneous reactions and a more than fourfold increase in those who developed angioedema.
Before the data were available, skin reactions were a source of concern among dermatologists and others involved in monitoring vaccine-related adverse events. Injection site reactions (ISRs) are associated with essentially every injectable vaccine, so these were expected, but a small proportion of patients developed large red plaques in the injection arm 7-8 days after the inoculation.
“These delayed reactions caused a lot of initial panic,” said Dr. Freeman, who counted herself among those alarmed about what the reactions might signify. “Was this cellulitis? Would the next dose cause anaphylaxis? We were concerned.”
This concern dissipated with the availability of more data. In a global registry that has so far captured more than 1,000 cutaneous reactions from 52 participating countries, it appears that about 2% of patients have a cutaneous reaction other than an ISR after the first dose. All resolve with minimal skin care or no treatment.
After the second dose, the proportion is lower. If there is a reaction, it typically occurs earlier and resolves more quickly.
“What we have learned is that fewer than half of patients who had a reaction to the first dose have a reaction to the second, and those who did have a reaction had a milder course,” said Dr. Freeman.
These data are “incredibly reassuring” on many levels, she explained. In addition, it allows clinicians to confidently explain to patients that there are no serious sequelae from the rashes, whether immediate or delayed, from the available COVID-19 vaccines.
“Every skin reaction I have seen is something we can treat through,” she added, noting that most reactions resolve with little or no supportive care. Following skin reactions, particularly the delayed lesions, it is not uncommon for patients to refuse a second shot. Some request a medical waiver to avoid further vaccine exposure. According to Dr. Freeman, this is unwarranted.
“I have granted exactly zero waivers,” she said. She explains to patients that these reactions have not been predictive of serious events, such as anaphylaxis. Although the trigger of the hypersensitivity reaction remains unknown, there is no evidence of serious consequences.
Delayed skin reactions are more commonly associated with the Moderna than the Pfizer vaccine. One notable difference between these vaccines is the greater content of mRNA in the Moderna formulation, but Freeman said that this is only one potential hypothesis for higher frequency of reactions to this version of the vaccine.
Patients with a history of allergic disease are more likely to develop a reaction but not significantly more likely to have a reaction that is more difficult to manage, according to Kimberly G. Blumenthal, MD, quality and safety officer for allergy, and codirector of the clinical epidemiology program in the division of rheumatology, allergy, and immunology at Mass General.
Anaphylaxis has been associated with COVD-19 vaccines just as it has with essentially every injectable vaccine, Dr. Blumenthal said during the same session. But the risk is very low, and it stays low even among those with a history of severe hypersensitivity reactions in the past.
Among the data collected from more than 52,000 vaccinated MGBHS employees, 0.9% had a history of severe allergic reaction to a prior vaccine. Of these, 11.6% had an allergic reaction to the COVID-19 vaccine. This was more than twice the 4.6% rate of allergic reactions among employees without a history of allergic reactions, but serious consequences were rare in both groups.
Of those with a reaction to the first dose, all but 2.4% took a subsequent dose. Again, serious reactions were exceedingly rare. These serious reactions did include anaphylaxis and hospitalization in 3% of patients, but there were no fatalities and all resolved.
The absence of serious sequelae from a reaction to a COVID-19 vaccine must be considered within the context of the benefit, which includes protection from death and hospitalization from the virus, according to Dr. Blumenthal. Citing the evidence that first-shot reactions are a source of vaccine hesitancy, she agreed that it is important to educate patients about relative risks.
“Even in our own cohort of MGBHS employees, we have people, including those who had been provaccine in the past, become hesitant,” commented Dr. Blumenthal, who said there are data from the Kaiser Permanente System showing similar vaccine reluctance following a first-shot reaction.
After more than 500 million doses of the Moderna and Pfizer vaccines had been administered worldwide, there was not a single reported death from anaphylaxis. Although Dr. Blumenthal said that an unconfirmed death of this type had been recently reported, she emphasized that this single death, if valid, is dwarfed by the lives saved with vaccination.
Asked about her strategy for counseling patients with vaccine hesitancy, Dr. Freeman said the body of safety data is large and compelling. There is overwhelming evidence of a favorable benefit-to-risk ratio overall and among those with a first-shot reaction.
“I can reassure them on the basis of the data,” Dr. Freeman said in an interview. “Less than half will have a reaction to the second shot and even if they do have a reaction, it is likely to be less severe.”
Although the main message is that vaccination is potentially lifesaving and far outweighs any risks, Freeman specifically gives this message to those hesitant to take a second shot after a first-shot reaction: “I can get you through it.”
Dr. Freeman encouraged health care professionals to report cases of COVID-19 vaccine–related dermatologic side effects to the American Academy of Dermatology / International League of Dermatologic Societies COVID-19 dermatology registry. Dermatologic manifestations of COVID-19 can also be reported to the registry.
Dr. Freeman disclosed receiving grants/research funding from the International League of Dermatologic Societies and from the National Institutes of Health. Dr. Blumenthal disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BOSTON – Requests for a according to an analysis of several large sets of data presented at the annual meeting of the American Academy of Dermatology.
According to the data, “there are no serious adverse consequences from these cutaneous reactions,” said Esther Freeman, MD, PhD, director of Global Health Dermatology, Massachusetts General Hospital, Boston.
This is important because the risk of vaccine hesitancy goes up dramatically in patients who experience reactions to the first vaccine dose, according to follow-up of more than 50,000 employees vaccinated in the Mass General Brigham Healthcare System (MGBHS). According to Dr. Freeman, there was almost a fourfold increase in the rate of second-dose refusals for those with cutaneous reactions and a more than fourfold increase in those who developed angioedema.
Before the data were available, skin reactions were a source of concern among dermatologists and others involved in monitoring vaccine-related adverse events. Injection site reactions (ISRs) are associated with essentially every injectable vaccine, so these were expected, but a small proportion of patients developed large red plaques in the injection arm 7-8 days after the inoculation.
“These delayed reactions caused a lot of initial panic,” said Dr. Freeman, who counted herself among those alarmed about what the reactions might signify. “Was this cellulitis? Would the next dose cause anaphylaxis? We were concerned.”
This concern dissipated with the availability of more data. In a global registry that has so far captured more than 1,000 cutaneous reactions from 52 participating countries, it appears that about 2% of patients have a cutaneous reaction other than an ISR after the first dose. All resolve with minimal skin care or no treatment.
After the second dose, the proportion is lower. If there is a reaction, it typically occurs earlier and resolves more quickly.
“What we have learned is that fewer than half of patients who had a reaction to the first dose have a reaction to the second, and those who did have a reaction had a milder course,” said Dr. Freeman.
These data are “incredibly reassuring” on many levels, she explained. In addition, it allows clinicians to confidently explain to patients that there are no serious sequelae from the rashes, whether immediate or delayed, from the available COVID-19 vaccines.
“Every skin reaction I have seen is something we can treat through,” she added, noting that most reactions resolve with little or no supportive care. Following skin reactions, particularly the delayed lesions, it is not uncommon for patients to refuse a second shot. Some request a medical waiver to avoid further vaccine exposure. According to Dr. Freeman, this is unwarranted.
“I have granted exactly zero waivers,” she said. She explains to patients that these reactions have not been predictive of serious events, such as anaphylaxis. Although the trigger of the hypersensitivity reaction remains unknown, there is no evidence of serious consequences.
Delayed skin reactions are more commonly associated with the Moderna than the Pfizer vaccine. One notable difference between these vaccines is the greater content of mRNA in the Moderna formulation, but Freeman said that this is only one potential hypothesis for higher frequency of reactions to this version of the vaccine.
Patients with a history of allergic disease are more likely to develop a reaction but not significantly more likely to have a reaction that is more difficult to manage, according to Kimberly G. Blumenthal, MD, quality and safety officer for allergy, and codirector of the clinical epidemiology program in the division of rheumatology, allergy, and immunology at Mass General.
Anaphylaxis has been associated with COVD-19 vaccines just as it has with essentially every injectable vaccine, Dr. Blumenthal said during the same session. But the risk is very low, and it stays low even among those with a history of severe hypersensitivity reactions in the past.
Among the data collected from more than 52,000 vaccinated MGBHS employees, 0.9% had a history of severe allergic reaction to a prior vaccine. Of these, 11.6% had an allergic reaction to the COVID-19 vaccine. This was more than twice the 4.6% rate of allergic reactions among employees without a history of allergic reactions, but serious consequences were rare in both groups.
Of those with a reaction to the first dose, all but 2.4% took a subsequent dose. Again, serious reactions were exceedingly rare. These serious reactions did include anaphylaxis and hospitalization in 3% of patients, but there were no fatalities and all resolved.
The absence of serious sequelae from a reaction to a COVID-19 vaccine must be considered within the context of the benefit, which includes protection from death and hospitalization from the virus, according to Dr. Blumenthal. Citing the evidence that first-shot reactions are a source of vaccine hesitancy, she agreed that it is important to educate patients about relative risks.
“Even in our own cohort of MGBHS employees, we have people, including those who had been provaccine in the past, become hesitant,” commented Dr. Blumenthal, who said there are data from the Kaiser Permanente System showing similar vaccine reluctance following a first-shot reaction.
After more than 500 million doses of the Moderna and Pfizer vaccines had been administered worldwide, there was not a single reported death from anaphylaxis. Although Dr. Blumenthal said that an unconfirmed death of this type had been recently reported, she emphasized that this single death, if valid, is dwarfed by the lives saved with vaccination.
Asked about her strategy for counseling patients with vaccine hesitancy, Dr. Freeman said the body of safety data is large and compelling. There is overwhelming evidence of a favorable benefit-to-risk ratio overall and among those with a first-shot reaction.
“I can reassure them on the basis of the data,” Dr. Freeman said in an interview. “Less than half will have a reaction to the second shot and even if they do have a reaction, it is likely to be less severe.”
Although the main message is that vaccination is potentially lifesaving and far outweighs any risks, Freeman specifically gives this message to those hesitant to take a second shot after a first-shot reaction: “I can get you through it.”
Dr. Freeman encouraged health care professionals to report cases of COVID-19 vaccine–related dermatologic side effects to the American Academy of Dermatology / International League of Dermatologic Societies COVID-19 dermatology registry. Dermatologic manifestations of COVID-19 can also be reported to the registry.
Dr. Freeman disclosed receiving grants/research funding from the International League of Dermatologic Societies and from the National Institutes of Health. Dr. Blumenthal disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AAD 2022
Fingers take the fight to COVID-19
Pointing a finger at COVID-19
The battle against COVID-19 is seemingly never ending. It’s been 2 years and still we struggle against the virus. But now, a new hero rises against the eternal menace, a powerful weapon against this scourge of humanity. And that weapon? Finger length.
Before you break out the sad trombone, hear us out. One of the big questions around COVID-19 is the role testosterone plays in its severity: Does low testosterone increase or decrease the odds of contracting severe COVID-19? To help answer that question, English researchers have published a study analyzing finger length ratios in both COVID-19 patients and a healthy control group. That seems random, but high testosterone in the womb leads to longer ring fingers in adulthood, while high estrogen leads to longer index fingers.
According to the researchers, those who had significant left hand–right hand differences in the ratio between the second and fourth digits, as well as the third and fifth digits, were significantly more likely to have severe COVID-19 compared with those with more even ratios. Those with “feminized” short little fingers were also at risk. Those large ratio differences indicate low testosterone and high estrogen, which may explain why elderly men are at such high risk for severe COVID-19. Testosterone naturally falls off as men get older.
The results add credence to clinical trials looking to use testosterone-boosting drugs against COVID-19, the researchers said. It also gives credence to LOTME’s brand-new 12-step finger strength fitness routine and our branded finger weights. Now just $19.95! It’s the bargain of the century! Boost your testosterone naturally and protect yourself from COVID-19! We promise it’s not a scam.
Some emergencies need a superhero
Last week, we learned about the most boring person in the world. This week just happens to be opposite week, so we’re looking at a candidate for the most interesting person. Someone who can swoop down from the sky to save the injured and helpless. Someone who can go where helicopters fear to tread. Someone with jet engines for arms. Superhero-type stuff.
The Great North Air Ambulance Service (GNAAS), a charitable organization located in the United Kingdom, recently announced that one of its members has completed training on the Gravity Industries Jet Suit. The suit “has two engines on each arm and a larger engine on the back [that] provide up to 317 pounds of thrust,” Interesting Engineering explained.
GNAAS is putting the suit into operation in England’s Lake District National Park, which includes mountainous terrain that is not very hospitable to helicopter landings. A paramedic using the suit can reach hikers stranded on mountainsides much faster than rescuers who have to run or hike from the nearest helicopter landing site.
“Everyone looks at the wow factor and the fact we are the world’s first jet suit paramedics, but for us, it’s about delivering patient care,” GNAAS’ Andy Mawson told Interesting Engineering. Sounds like superhero-speak to us.
So if you’re in the Lake District and have taken a bit of a tumble, you can call a superhero on your cell phone or you can use this to summon one.
Why we’re rejecting food as medicine
Humans have been using food to treat ailments much longer than we’ve had the advances of modern medicine. So why have we rejected its worth in our treatment processes? And what can be done to change that? The Center for Food as Medicine and the Hunter College NYC Food Policy Center just released a 335-page report that answers those questions.
First, the why: Meals in health care settings are not medically designed to help with the specific needs of the patient. Produce-prescription and nutrition-incentive programs don’t have the government funds to fully support them. And a lot of medical schools don’t even require students to take a basic nutrition course. So there’s a lack of knowledge and a disconnect between health care providers and food as a resource.
Then there’s a lack of trust in the food industry and their validity. Social media uses food as a means of promoting “pseudoscientific alternative medicine” or spreading false info, pushing away legitimate providers. The food industry has had its fingers in food science studies and an almost mafia-esque chokehold on American dietary guidelines. No wonder food for medicine is getting the boot!
To change the situation, the report offers 10 key recommendations on how to advance the idea of incorporating food into medicine for treatment and prevention. They include boosting the funding for research, making hospitals more food-as-medicine focused, expanding federal programs, and improving public awareness on the role nutrition can play in medical treatment or prevention.
So maybe instead of rejecting food outright, we should be looking a little deeper at how we can use it to our advantage. Just a thought: Ice cream as an antidepressant.
Being rude is a good thing, apparently
If you’ve ever been called argumentative, stubborn, or unpleasant, then this LOTME is for you. Researchers at the University of Geneva have found that people who are more stubborn and hate to conform have brains that are more protected against Alzheimer’s disease. That type of personality seems to preserve the part of the brain that usually deteriorates as we grow older.
The original hypothesis that personality may have a protective effect against brain degeneration led the investigators to conduct cognitive and personality assessments of 65 elderly participants over a 5-year period. Researchers have been attempting to create vaccines to protect against Alzheimer’s disease, but these new findings offer a nonbiological way to help.
“For a long time, the brain is able to compensate by activating alternative networks; when the first clinical signs appear, however, it is unfortunately often too late. The identification of early biomarkers is therefore essential for … effective disease management,” lead author Panteleimon Giannakopoulos, MD, said in a Study Finds report.
You may be wondering how people with more agreeable and less confrontational personalities can seek help. Well, researchers are working on that, too. It’s a complex situation, but as always, we’re rooting for you, science!
At least now you can take solace in the fact that your elderly next-door neighbor who yells at you for stepping on his lawn is probably more protected against Alzheimer’s disease.
Pointing a finger at COVID-19
The battle against COVID-19 is seemingly never ending. It’s been 2 years and still we struggle against the virus. But now, a new hero rises against the eternal menace, a powerful weapon against this scourge of humanity. And that weapon? Finger length.
Before you break out the sad trombone, hear us out. One of the big questions around COVID-19 is the role testosterone plays in its severity: Does low testosterone increase or decrease the odds of contracting severe COVID-19? To help answer that question, English researchers have published a study analyzing finger length ratios in both COVID-19 patients and a healthy control group. That seems random, but high testosterone in the womb leads to longer ring fingers in adulthood, while high estrogen leads to longer index fingers.
According to the researchers, those who had significant left hand–right hand differences in the ratio between the second and fourth digits, as well as the third and fifth digits, were significantly more likely to have severe COVID-19 compared with those with more even ratios. Those with “feminized” short little fingers were also at risk. Those large ratio differences indicate low testosterone and high estrogen, which may explain why elderly men are at such high risk for severe COVID-19. Testosterone naturally falls off as men get older.
The results add credence to clinical trials looking to use testosterone-boosting drugs against COVID-19, the researchers said. It also gives credence to LOTME’s brand-new 12-step finger strength fitness routine and our branded finger weights. Now just $19.95! It’s the bargain of the century! Boost your testosterone naturally and protect yourself from COVID-19! We promise it’s not a scam.
Some emergencies need a superhero
Last week, we learned about the most boring person in the world. This week just happens to be opposite week, so we’re looking at a candidate for the most interesting person. Someone who can swoop down from the sky to save the injured and helpless. Someone who can go where helicopters fear to tread. Someone with jet engines for arms. Superhero-type stuff.
The Great North Air Ambulance Service (GNAAS), a charitable organization located in the United Kingdom, recently announced that one of its members has completed training on the Gravity Industries Jet Suit. The suit “has two engines on each arm and a larger engine on the back [that] provide up to 317 pounds of thrust,” Interesting Engineering explained.
GNAAS is putting the suit into operation in England’s Lake District National Park, which includes mountainous terrain that is not very hospitable to helicopter landings. A paramedic using the suit can reach hikers stranded on mountainsides much faster than rescuers who have to run or hike from the nearest helicopter landing site.
“Everyone looks at the wow factor and the fact we are the world’s first jet suit paramedics, but for us, it’s about delivering patient care,” GNAAS’ Andy Mawson told Interesting Engineering. Sounds like superhero-speak to us.
So if you’re in the Lake District and have taken a bit of a tumble, you can call a superhero on your cell phone or you can use this to summon one.
Why we’re rejecting food as medicine
Humans have been using food to treat ailments much longer than we’ve had the advances of modern medicine. So why have we rejected its worth in our treatment processes? And what can be done to change that? The Center for Food as Medicine and the Hunter College NYC Food Policy Center just released a 335-page report that answers those questions.
First, the why: Meals in health care settings are not medically designed to help with the specific needs of the patient. Produce-prescription and nutrition-incentive programs don’t have the government funds to fully support them. And a lot of medical schools don’t even require students to take a basic nutrition course. So there’s a lack of knowledge and a disconnect between health care providers and food as a resource.
Then there’s a lack of trust in the food industry and their validity. Social media uses food as a means of promoting “pseudoscientific alternative medicine” or spreading false info, pushing away legitimate providers. The food industry has had its fingers in food science studies and an almost mafia-esque chokehold on American dietary guidelines. No wonder food for medicine is getting the boot!
To change the situation, the report offers 10 key recommendations on how to advance the idea of incorporating food into medicine for treatment and prevention. They include boosting the funding for research, making hospitals more food-as-medicine focused, expanding federal programs, and improving public awareness on the role nutrition can play in medical treatment or prevention.
So maybe instead of rejecting food outright, we should be looking a little deeper at how we can use it to our advantage. Just a thought: Ice cream as an antidepressant.
Being rude is a good thing, apparently
If you’ve ever been called argumentative, stubborn, or unpleasant, then this LOTME is for you. Researchers at the University of Geneva have found that people who are more stubborn and hate to conform have brains that are more protected against Alzheimer’s disease. That type of personality seems to preserve the part of the brain that usually deteriorates as we grow older.
The original hypothesis that personality may have a protective effect against brain degeneration led the investigators to conduct cognitive and personality assessments of 65 elderly participants over a 5-year period. Researchers have been attempting to create vaccines to protect against Alzheimer’s disease, but these new findings offer a nonbiological way to help.
“For a long time, the brain is able to compensate by activating alternative networks; when the first clinical signs appear, however, it is unfortunately often too late. The identification of early biomarkers is therefore essential for … effective disease management,” lead author Panteleimon Giannakopoulos, MD, said in a Study Finds report.
You may be wondering how people with more agreeable and less confrontational personalities can seek help. Well, researchers are working on that, too. It’s a complex situation, but as always, we’re rooting for you, science!
At least now you can take solace in the fact that your elderly next-door neighbor who yells at you for stepping on his lawn is probably more protected against Alzheimer’s disease.
Pointing a finger at COVID-19
The battle against COVID-19 is seemingly never ending. It’s been 2 years and still we struggle against the virus. But now, a new hero rises against the eternal menace, a powerful weapon against this scourge of humanity. And that weapon? Finger length.
Before you break out the sad trombone, hear us out. One of the big questions around COVID-19 is the role testosterone plays in its severity: Does low testosterone increase or decrease the odds of contracting severe COVID-19? To help answer that question, English researchers have published a study analyzing finger length ratios in both COVID-19 patients and a healthy control group. That seems random, but high testosterone in the womb leads to longer ring fingers in adulthood, while high estrogen leads to longer index fingers.
According to the researchers, those who had significant left hand–right hand differences in the ratio between the second and fourth digits, as well as the third and fifth digits, were significantly more likely to have severe COVID-19 compared with those with more even ratios. Those with “feminized” short little fingers were also at risk. Those large ratio differences indicate low testosterone and high estrogen, which may explain why elderly men are at such high risk for severe COVID-19. Testosterone naturally falls off as men get older.
The results add credence to clinical trials looking to use testosterone-boosting drugs against COVID-19, the researchers said. It also gives credence to LOTME’s brand-new 12-step finger strength fitness routine and our branded finger weights. Now just $19.95! It’s the bargain of the century! Boost your testosterone naturally and protect yourself from COVID-19! We promise it’s not a scam.
Some emergencies need a superhero
Last week, we learned about the most boring person in the world. This week just happens to be opposite week, so we’re looking at a candidate for the most interesting person. Someone who can swoop down from the sky to save the injured and helpless. Someone who can go where helicopters fear to tread. Someone with jet engines for arms. Superhero-type stuff.
The Great North Air Ambulance Service (GNAAS), a charitable organization located in the United Kingdom, recently announced that one of its members has completed training on the Gravity Industries Jet Suit. The suit “has two engines on each arm and a larger engine on the back [that] provide up to 317 pounds of thrust,” Interesting Engineering explained.
GNAAS is putting the suit into operation in England’s Lake District National Park, which includes mountainous terrain that is not very hospitable to helicopter landings. A paramedic using the suit can reach hikers stranded on mountainsides much faster than rescuers who have to run or hike from the nearest helicopter landing site.
“Everyone looks at the wow factor and the fact we are the world’s first jet suit paramedics, but for us, it’s about delivering patient care,” GNAAS’ Andy Mawson told Interesting Engineering. Sounds like superhero-speak to us.
So if you’re in the Lake District and have taken a bit of a tumble, you can call a superhero on your cell phone or you can use this to summon one.
Why we’re rejecting food as medicine
Humans have been using food to treat ailments much longer than we’ve had the advances of modern medicine. So why have we rejected its worth in our treatment processes? And what can be done to change that? The Center for Food as Medicine and the Hunter College NYC Food Policy Center just released a 335-page report that answers those questions.
First, the why: Meals in health care settings are not medically designed to help with the specific needs of the patient. Produce-prescription and nutrition-incentive programs don’t have the government funds to fully support them. And a lot of medical schools don’t even require students to take a basic nutrition course. So there’s a lack of knowledge and a disconnect between health care providers and food as a resource.
Then there’s a lack of trust in the food industry and their validity. Social media uses food as a means of promoting “pseudoscientific alternative medicine” or spreading false info, pushing away legitimate providers. The food industry has had its fingers in food science studies and an almost mafia-esque chokehold on American dietary guidelines. No wonder food for medicine is getting the boot!
To change the situation, the report offers 10 key recommendations on how to advance the idea of incorporating food into medicine for treatment and prevention. They include boosting the funding for research, making hospitals more food-as-medicine focused, expanding federal programs, and improving public awareness on the role nutrition can play in medical treatment or prevention.
So maybe instead of rejecting food outright, we should be looking a little deeper at how we can use it to our advantage. Just a thought: Ice cream as an antidepressant.
Being rude is a good thing, apparently
If you’ve ever been called argumentative, stubborn, or unpleasant, then this LOTME is for you. Researchers at the University of Geneva have found that people who are more stubborn and hate to conform have brains that are more protected against Alzheimer’s disease. That type of personality seems to preserve the part of the brain that usually deteriorates as we grow older.
The original hypothesis that personality may have a protective effect against brain degeneration led the investigators to conduct cognitive and personality assessments of 65 elderly participants over a 5-year period. Researchers have been attempting to create vaccines to protect against Alzheimer’s disease, but these new findings offer a nonbiological way to help.
“For a long time, the brain is able to compensate by activating alternative networks; when the first clinical signs appear, however, it is unfortunately often too late. The identification of early biomarkers is therefore essential for … effective disease management,” lead author Panteleimon Giannakopoulos, MD, said in a Study Finds report.
You may be wondering how people with more agreeable and less confrontational personalities can seek help. Well, researchers are working on that, too. It’s a complex situation, but as always, we’re rooting for you, science!
At least now you can take solace in the fact that your elderly next-door neighbor who yells at you for stepping on his lawn is probably more protected against Alzheimer’s disease.
Psychotropic med use tied to ‘striking’ post-COVID dementia risk
, new research suggests.
Results from a large study of more than 1,700 patients who had been hospitalized with COVID showed a greater than twofold increased risk for post-COVID dementia in those taking antipsychotics and mood stabilizers/anticonvulsants – medications often used to treat schizophrenia, psychosis, bipolar disorder, and seizures.
“We know that pre-existing psychiatric illness is associated with poor COVID-19 outcomes, but our study is the first to show an association with certain psychiatric medications and dementia,” co-investigator Liron Sinvani, MD, the Feinstein Institutes for Medical Research, Manhasset, New York, said in an interview.
“Our study highlights the potential interaction between baseline neuropsychiatric disease, psychotropic medications, COVID-19, and dementia,” Dr. Sinvani added.
The findings were published online March 18 in Frontiers in Medicine.
‘Striking’ dementia rate
Using electronic health records, the researchers evaluated pre-COVID psychotropic medication use and post-COVID dementia onset in 1,755 adults aged 65 and older. All were hospitalized with COVID-19 at Northwell Health between March 1 and April 20, 2020.
A “striking” 13% of the participants (n = 223) developed dementia within 1-year of follow-up, the investigators report.
Among the 438 patients (25%) exposed to at least one psychotropic medication before COVID-19, 105 (24%) developed dementia in the year following COVID versus 118 of 1,317 (9%) patients with no pre-COVID exposure to psychotropic medication (odds ratio, 3.2; 95% confidence interval, 2.37-4.32).
Both pre-COVID psychotropic medication use (OR, 2.7; 95% CI, 1.8-4.0, P < .001) and delirium (OR, 3.0; 95% CI, 1.9-4.6, P < .001) were significantly associated with post-COVID dementia at 1 year.
In a sensitivity analysis in the subset of 423 patients with at least one documented neurologic or psychiatric diagnosis at the time of COVID admission, and after adjusting for confounding factors, pre-COVID psychotropic medication use remained significantly linked to post-COVID dementia onset (OR, 3.09; 95% CI, 1.5-6.6, P = .002).
Drug classes most strongly associated with 1-year post-COVID dementia onset were antipsychotics (OR, 2.8, 95% CI, 1.7-4.4, P < .001) and mood stabilizers/anticonvulsants (OR, 2.4, 95% CI, 1.39-4.02, P = .001).
In a further exploratory analysis, the psychotropics valproic acid (multiple brands) and haloperidol (Haldol) had the largest association with post-COVID dementia.
Antidepressants as a class were not associated with post-COVID dementia, but the potential effects of two commonly prescribed antidepressants in older adults, mirtazapine (Remeron) and escitalopram (Lexapro), “warrant further investigation,” the researchers note.
Predictive risk marker?
“This research shows that psychotropic medications can be considered a predictive risk marker for post-COVID dementia. In patients taking psychotropic medications, COVID-19 could have accelerated progression of dementia after hospitalization,” lead author Yun Freudenberg-Hua, MD, the Feinstein Institutes, said in a news release.
It is unclear why psychotropic medications may raise the risk for dementia onset after COVID, the investigators note.
“It is intuitive that psychotropic medications indicate pre-existing neuropsychiatric conditions in which COVID-19 occurs. It is possible that psychotropic medications may potentiate the neurostructural changes that have been found in the brain of those who have recovered from COVID-19,” they write.
The sensitivity analysis in patients with documented neurologic and psychiatric diagnoses supports this interpretation.
COVID-19 may also accelerate the underlying brain disorders for which psychotropic medications were prescribed, leading to the greater incidence of post-COVID dementia, the researchers write.
“It is important to note that this study is in no way recommending people should stop taking antipsychotics but simply that clinicians need to factor in a patient’s medication history while considering post-COVID aftereffects,” Dr. Freudenberg-Hua said.
“Given that the number of patients with dementia is projected to triple in the next 30 years, these findings have significant public health implications,” Dr. Sinvani added.
She noted that “care partners and health care professionals” should look for early signs of dementia, such as forgetfulness and depressive symptoms, in their patients.
“Future studies must continue to evaluate these associations, which are key for potential future interventions to prevent dementia,” Dr. Sinvani said.
The study was funded by the National Institutes of Health. Dr. Freudenberg-Hua co-owns stock and stock options from Regeneron Pharmaceuticals. Dr. Sinvani has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a large study of more than 1,700 patients who had been hospitalized with COVID showed a greater than twofold increased risk for post-COVID dementia in those taking antipsychotics and mood stabilizers/anticonvulsants – medications often used to treat schizophrenia, psychosis, bipolar disorder, and seizures.
“We know that pre-existing psychiatric illness is associated with poor COVID-19 outcomes, but our study is the first to show an association with certain psychiatric medications and dementia,” co-investigator Liron Sinvani, MD, the Feinstein Institutes for Medical Research, Manhasset, New York, said in an interview.
“Our study highlights the potential interaction between baseline neuropsychiatric disease, psychotropic medications, COVID-19, and dementia,” Dr. Sinvani added.
The findings were published online March 18 in Frontiers in Medicine.
‘Striking’ dementia rate
Using electronic health records, the researchers evaluated pre-COVID psychotropic medication use and post-COVID dementia onset in 1,755 adults aged 65 and older. All were hospitalized with COVID-19 at Northwell Health between March 1 and April 20, 2020.
A “striking” 13% of the participants (n = 223) developed dementia within 1-year of follow-up, the investigators report.
Among the 438 patients (25%) exposed to at least one psychotropic medication before COVID-19, 105 (24%) developed dementia in the year following COVID versus 118 of 1,317 (9%) patients with no pre-COVID exposure to psychotropic medication (odds ratio, 3.2; 95% confidence interval, 2.37-4.32).
Both pre-COVID psychotropic medication use (OR, 2.7; 95% CI, 1.8-4.0, P < .001) and delirium (OR, 3.0; 95% CI, 1.9-4.6, P < .001) were significantly associated with post-COVID dementia at 1 year.
In a sensitivity analysis in the subset of 423 patients with at least one documented neurologic or psychiatric diagnosis at the time of COVID admission, and after adjusting for confounding factors, pre-COVID psychotropic medication use remained significantly linked to post-COVID dementia onset (OR, 3.09; 95% CI, 1.5-6.6, P = .002).
Drug classes most strongly associated with 1-year post-COVID dementia onset were antipsychotics (OR, 2.8, 95% CI, 1.7-4.4, P < .001) and mood stabilizers/anticonvulsants (OR, 2.4, 95% CI, 1.39-4.02, P = .001).
In a further exploratory analysis, the psychotropics valproic acid (multiple brands) and haloperidol (Haldol) had the largest association with post-COVID dementia.
Antidepressants as a class were not associated with post-COVID dementia, but the potential effects of two commonly prescribed antidepressants in older adults, mirtazapine (Remeron) and escitalopram (Lexapro), “warrant further investigation,” the researchers note.
Predictive risk marker?
“This research shows that psychotropic medications can be considered a predictive risk marker for post-COVID dementia. In patients taking psychotropic medications, COVID-19 could have accelerated progression of dementia after hospitalization,” lead author Yun Freudenberg-Hua, MD, the Feinstein Institutes, said in a news release.
It is unclear why psychotropic medications may raise the risk for dementia onset after COVID, the investigators note.
“It is intuitive that psychotropic medications indicate pre-existing neuropsychiatric conditions in which COVID-19 occurs. It is possible that psychotropic medications may potentiate the neurostructural changes that have been found in the brain of those who have recovered from COVID-19,” they write.
The sensitivity analysis in patients with documented neurologic and psychiatric diagnoses supports this interpretation.
COVID-19 may also accelerate the underlying brain disorders for which psychotropic medications were prescribed, leading to the greater incidence of post-COVID dementia, the researchers write.
“It is important to note that this study is in no way recommending people should stop taking antipsychotics but simply that clinicians need to factor in a patient’s medication history while considering post-COVID aftereffects,” Dr. Freudenberg-Hua said.
“Given that the number of patients with dementia is projected to triple in the next 30 years, these findings have significant public health implications,” Dr. Sinvani added.
She noted that “care partners and health care professionals” should look for early signs of dementia, such as forgetfulness and depressive symptoms, in their patients.
“Future studies must continue to evaluate these associations, which are key for potential future interventions to prevent dementia,” Dr. Sinvani said.
The study was funded by the National Institutes of Health. Dr. Freudenberg-Hua co-owns stock and stock options from Regeneron Pharmaceuticals. Dr. Sinvani has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a large study of more than 1,700 patients who had been hospitalized with COVID showed a greater than twofold increased risk for post-COVID dementia in those taking antipsychotics and mood stabilizers/anticonvulsants – medications often used to treat schizophrenia, psychosis, bipolar disorder, and seizures.
“We know that pre-existing psychiatric illness is associated with poor COVID-19 outcomes, but our study is the first to show an association with certain psychiatric medications and dementia,” co-investigator Liron Sinvani, MD, the Feinstein Institutes for Medical Research, Manhasset, New York, said in an interview.
“Our study highlights the potential interaction between baseline neuropsychiatric disease, psychotropic medications, COVID-19, and dementia,” Dr. Sinvani added.
The findings were published online March 18 in Frontiers in Medicine.
‘Striking’ dementia rate
Using electronic health records, the researchers evaluated pre-COVID psychotropic medication use and post-COVID dementia onset in 1,755 adults aged 65 and older. All were hospitalized with COVID-19 at Northwell Health between March 1 and April 20, 2020.
A “striking” 13% of the participants (n = 223) developed dementia within 1-year of follow-up, the investigators report.
Among the 438 patients (25%) exposed to at least one psychotropic medication before COVID-19, 105 (24%) developed dementia in the year following COVID versus 118 of 1,317 (9%) patients with no pre-COVID exposure to psychotropic medication (odds ratio, 3.2; 95% confidence interval, 2.37-4.32).
Both pre-COVID psychotropic medication use (OR, 2.7; 95% CI, 1.8-4.0, P < .001) and delirium (OR, 3.0; 95% CI, 1.9-4.6, P < .001) were significantly associated with post-COVID dementia at 1 year.
In a sensitivity analysis in the subset of 423 patients with at least one documented neurologic or psychiatric diagnosis at the time of COVID admission, and after adjusting for confounding factors, pre-COVID psychotropic medication use remained significantly linked to post-COVID dementia onset (OR, 3.09; 95% CI, 1.5-6.6, P = .002).
Drug classes most strongly associated with 1-year post-COVID dementia onset were antipsychotics (OR, 2.8, 95% CI, 1.7-4.4, P < .001) and mood stabilizers/anticonvulsants (OR, 2.4, 95% CI, 1.39-4.02, P = .001).
In a further exploratory analysis, the psychotropics valproic acid (multiple brands) and haloperidol (Haldol) had the largest association with post-COVID dementia.
Antidepressants as a class were not associated with post-COVID dementia, but the potential effects of two commonly prescribed antidepressants in older adults, mirtazapine (Remeron) and escitalopram (Lexapro), “warrant further investigation,” the researchers note.
Predictive risk marker?
“This research shows that psychotropic medications can be considered a predictive risk marker for post-COVID dementia. In patients taking psychotropic medications, COVID-19 could have accelerated progression of dementia after hospitalization,” lead author Yun Freudenberg-Hua, MD, the Feinstein Institutes, said in a news release.
It is unclear why psychotropic medications may raise the risk for dementia onset after COVID, the investigators note.
“It is intuitive that psychotropic medications indicate pre-existing neuropsychiatric conditions in which COVID-19 occurs. It is possible that psychotropic medications may potentiate the neurostructural changes that have been found in the brain of those who have recovered from COVID-19,” they write.
The sensitivity analysis in patients with documented neurologic and psychiatric diagnoses supports this interpretation.
COVID-19 may also accelerate the underlying brain disorders for which psychotropic medications were prescribed, leading to the greater incidence of post-COVID dementia, the researchers write.
“It is important to note that this study is in no way recommending people should stop taking antipsychotics but simply that clinicians need to factor in a patient’s medication history while considering post-COVID aftereffects,” Dr. Freudenberg-Hua said.
“Given that the number of patients with dementia is projected to triple in the next 30 years, these findings have significant public health implications,” Dr. Sinvani added.
She noted that “care partners and health care professionals” should look for early signs of dementia, such as forgetfulness and depressive symptoms, in their patients.
“Future studies must continue to evaluate these associations, which are key for potential future interventions to prevent dementia,” Dr. Sinvani said.
The study was funded by the National Institutes of Health. Dr. Freudenberg-Hua co-owns stock and stock options from Regeneron Pharmaceuticals. Dr. Sinvani has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM FRONTIERS IN MEDICINE
Global registry tracks COVID-19 outcomes in atopic dermatitis patients
BOSTON – , results from a global registry demonstrated.
Moreover, combination systemic treatment, especially those that included systemic corticosteroids, was associated with the highest risk of COVID-19–related hospitalization.
“Patients with inflammatory skin diseases such as AD may be at higher risk of COVID-19,” Annelie H. Musters, MD, said during a late-breaking abstract session at the annual meeting of the American Academy of Dermatology. “Another factor to consider is that AD patients are often treated with systemic immunomodulatory therapy, including systemic corticosteroids and nonsteroidal immunosuppressants such as methotrexate, cyclosporin, biologics, and Janus kinase inhibitors. Different mechanisms of action and levels of immunosuppression may impart variable risks of serious infections.”
On the other hand, some degree of immunomodulation may have beneficial effects on the course of COVID-19 in AD patients, said Dr. Musters, of the department of dermatology at Academic Medical Center, University of Amsterdam. Targeting of specific immune pathways could reduce the development of a hyperinflammatory state in severe COVID-19. Dual blockade of interleukin (IL)-4 and IL-13 with dupilumab may have a protective effect in the context of COVID-19 infection, because expression of Th2 cytokines, including IL-4 and IL-13, may be increased during COVID-19.
“At the start of the pandemic, many of us were faced with important questions, like do systemic immunomodulatory treatments influence outcomes of COVID-19 in patients with AD?” she said. “Do patients on dupilumab or other novel systemics fare better than those on conventional systemic treatment?”
To answer these questions, she and her colleagues launched a web-based registry in April 2020 to investigate COVID-19 outcomes in patients with AD treated with or without systemic immunomodulatory treatments. For the registry, known as Surveillance Epidemiology of Coronavirus Under Research Exclusion for Atopic Dermatitis (SECURE-AD), clinicians in 27 countries used a web-based form to enter anonymized data after patients had fully recovered from COVID-19. Eligibility criteria included having proven or highly suspected COVID-19, and there were no restrictions on age nor the type of AD treatment they were receiving.
Dr. Musters reported results from 442 patients who were recruited between April 2, 2020, and Oct. 31, 2021. Their mean age was 35.6 years, their median body mass index was 23.7 kg/m2, and there was an even sex distribution. Most patients were White and were recruited from Italy. Of the 442 patients, 216 (48.8%) received dupilumab monotherapy, 131 (29.6%) received topical treatments, and 14 (3.16%) received combination systemic treatments, including systemic corticosteroids. About 12% presented to the emergency department and 6% were hospitalized. Of those hospitalized, 2% required intensive care and/or ventilation, and no deaths have occurred in the registry to date.
By treatment group, hospitalization rates were highest among those on combination treatments (35.7%), followed by systemic corticosteroids (14.3%), topical treatments only (9.9%), other conventional systemics (3.6%), methotrexate (3.3%), and dupilumab (2.3%).
To further explore the differences between hospitalization rates in treatment groups, the researchers performed a multivariable logistic regression analysis, adjusted for age, sex, ethnicity, and comorbidity score. Compared with those who received dupilumab, the adjusted odds ratios (ORs) for hospitalization were highest among those who received topical treatments (OR, 4.95), followed by those who received systemic corticosteroids (OR, 2.81), and those who received other conventional systemic treatments (OR, 2.36).
Dr. Musters and colleagues also found that compared with patients on nonsteroidal immunosuppressive therapy, patients on combination systemic therapy had a significantly higher odds of hospitalization, specifically an OR of 45.75 for those on combination treatment including corticosteroids, an OR of 37.57 for those on combination treatment not including steroids, and an OR of 1.87 for those on systemic corticosteroids as monotherapy.
“Overall, the risk of COVID-19 complications appears to be low in patients with AD, even when treated with systemic immunomodulatory agents,” Dr. Musters concluded. “Dupilumab monotherapy was associated with lower odds of hospitalizations compared with other therapies. Moreover, combination systemic treatment, especially combinations including systemic corticosteroids, was associated with the highest risk of severe COVID-19.”
She added that other population-based study designs are more suitable to answer other important questions, such as whether the overall risk of COVID-19 in patients with AD is higher or lower compared to healthy controls.
Amy S. Paller, MD, professor and chair of the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study, characterized the results as reassuring. In this patient population, “we expected that dupilumab would not cause any problems,” she said. “We wouldn’t necessarily expect it to [confer] a benefit, but I think it’s because the patients who need a systemic medication are going on something that’s very targeted (dupilumab) rather than something that has a broader immunosuppressing function. It was interesting but not surprising that those on systemic steroids had more of a problem. Get them on something that’s very targeted if you can and don’t suppress the immune systems that might be handling COVID-19.”
Dr. Musters reported having no disclosures. Dr. Paller disclosed that she is consultant to and/or an investigator for many pharmaceutical companies.
BOSTON – , results from a global registry demonstrated.
Moreover, combination systemic treatment, especially those that included systemic corticosteroids, was associated with the highest risk of COVID-19–related hospitalization.
“Patients with inflammatory skin diseases such as AD may be at higher risk of COVID-19,” Annelie H. Musters, MD, said during a late-breaking abstract session at the annual meeting of the American Academy of Dermatology. “Another factor to consider is that AD patients are often treated with systemic immunomodulatory therapy, including systemic corticosteroids and nonsteroidal immunosuppressants such as methotrexate, cyclosporin, biologics, and Janus kinase inhibitors. Different mechanisms of action and levels of immunosuppression may impart variable risks of serious infections.”
On the other hand, some degree of immunomodulation may have beneficial effects on the course of COVID-19 in AD patients, said Dr. Musters, of the department of dermatology at Academic Medical Center, University of Amsterdam. Targeting of specific immune pathways could reduce the development of a hyperinflammatory state in severe COVID-19. Dual blockade of interleukin (IL)-4 and IL-13 with dupilumab may have a protective effect in the context of COVID-19 infection, because expression of Th2 cytokines, including IL-4 and IL-13, may be increased during COVID-19.
“At the start of the pandemic, many of us were faced with important questions, like do systemic immunomodulatory treatments influence outcomes of COVID-19 in patients with AD?” she said. “Do patients on dupilumab or other novel systemics fare better than those on conventional systemic treatment?”
To answer these questions, she and her colleagues launched a web-based registry in April 2020 to investigate COVID-19 outcomes in patients with AD treated with or without systemic immunomodulatory treatments. For the registry, known as Surveillance Epidemiology of Coronavirus Under Research Exclusion for Atopic Dermatitis (SECURE-AD), clinicians in 27 countries used a web-based form to enter anonymized data after patients had fully recovered from COVID-19. Eligibility criteria included having proven or highly suspected COVID-19, and there were no restrictions on age nor the type of AD treatment they were receiving.
Dr. Musters reported results from 442 patients who were recruited between April 2, 2020, and Oct. 31, 2021. Their mean age was 35.6 years, their median body mass index was 23.7 kg/m2, and there was an even sex distribution. Most patients were White and were recruited from Italy. Of the 442 patients, 216 (48.8%) received dupilumab monotherapy, 131 (29.6%) received topical treatments, and 14 (3.16%) received combination systemic treatments, including systemic corticosteroids. About 12% presented to the emergency department and 6% were hospitalized. Of those hospitalized, 2% required intensive care and/or ventilation, and no deaths have occurred in the registry to date.
By treatment group, hospitalization rates were highest among those on combination treatments (35.7%), followed by systemic corticosteroids (14.3%), topical treatments only (9.9%), other conventional systemics (3.6%), methotrexate (3.3%), and dupilumab (2.3%).
To further explore the differences between hospitalization rates in treatment groups, the researchers performed a multivariable logistic regression analysis, adjusted for age, sex, ethnicity, and comorbidity score. Compared with those who received dupilumab, the adjusted odds ratios (ORs) for hospitalization were highest among those who received topical treatments (OR, 4.95), followed by those who received systemic corticosteroids (OR, 2.81), and those who received other conventional systemic treatments (OR, 2.36).
Dr. Musters and colleagues also found that compared with patients on nonsteroidal immunosuppressive therapy, patients on combination systemic therapy had a significantly higher odds of hospitalization, specifically an OR of 45.75 for those on combination treatment including corticosteroids, an OR of 37.57 for those on combination treatment not including steroids, and an OR of 1.87 for those on systemic corticosteroids as monotherapy.
“Overall, the risk of COVID-19 complications appears to be low in patients with AD, even when treated with systemic immunomodulatory agents,” Dr. Musters concluded. “Dupilumab monotherapy was associated with lower odds of hospitalizations compared with other therapies. Moreover, combination systemic treatment, especially combinations including systemic corticosteroids, was associated with the highest risk of severe COVID-19.”
She added that other population-based study designs are more suitable to answer other important questions, such as whether the overall risk of COVID-19 in patients with AD is higher or lower compared to healthy controls.
Amy S. Paller, MD, professor and chair of the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study, characterized the results as reassuring. In this patient population, “we expected that dupilumab would not cause any problems,” she said. “We wouldn’t necessarily expect it to [confer] a benefit, but I think it’s because the patients who need a systemic medication are going on something that’s very targeted (dupilumab) rather than something that has a broader immunosuppressing function. It was interesting but not surprising that those on systemic steroids had more of a problem. Get them on something that’s very targeted if you can and don’t suppress the immune systems that might be handling COVID-19.”
Dr. Musters reported having no disclosures. Dr. Paller disclosed that she is consultant to and/or an investigator for many pharmaceutical companies.
BOSTON – , results from a global registry demonstrated.
Moreover, combination systemic treatment, especially those that included systemic corticosteroids, was associated with the highest risk of COVID-19–related hospitalization.
“Patients with inflammatory skin diseases such as AD may be at higher risk of COVID-19,” Annelie H. Musters, MD, said during a late-breaking abstract session at the annual meeting of the American Academy of Dermatology. “Another factor to consider is that AD patients are often treated with systemic immunomodulatory therapy, including systemic corticosteroids and nonsteroidal immunosuppressants such as methotrexate, cyclosporin, biologics, and Janus kinase inhibitors. Different mechanisms of action and levels of immunosuppression may impart variable risks of serious infections.”
On the other hand, some degree of immunomodulation may have beneficial effects on the course of COVID-19 in AD patients, said Dr. Musters, of the department of dermatology at Academic Medical Center, University of Amsterdam. Targeting of specific immune pathways could reduce the development of a hyperinflammatory state in severe COVID-19. Dual blockade of interleukin (IL)-4 and IL-13 with dupilumab may have a protective effect in the context of COVID-19 infection, because expression of Th2 cytokines, including IL-4 and IL-13, may be increased during COVID-19.
“At the start of the pandemic, many of us were faced with important questions, like do systemic immunomodulatory treatments influence outcomes of COVID-19 in patients with AD?” she said. “Do patients on dupilumab or other novel systemics fare better than those on conventional systemic treatment?”
To answer these questions, she and her colleagues launched a web-based registry in April 2020 to investigate COVID-19 outcomes in patients with AD treated with or without systemic immunomodulatory treatments. For the registry, known as Surveillance Epidemiology of Coronavirus Under Research Exclusion for Atopic Dermatitis (SECURE-AD), clinicians in 27 countries used a web-based form to enter anonymized data after patients had fully recovered from COVID-19. Eligibility criteria included having proven or highly suspected COVID-19, and there were no restrictions on age nor the type of AD treatment they were receiving.
Dr. Musters reported results from 442 patients who were recruited between April 2, 2020, and Oct. 31, 2021. Their mean age was 35.6 years, their median body mass index was 23.7 kg/m2, and there was an even sex distribution. Most patients were White and were recruited from Italy. Of the 442 patients, 216 (48.8%) received dupilumab monotherapy, 131 (29.6%) received topical treatments, and 14 (3.16%) received combination systemic treatments, including systemic corticosteroids. About 12% presented to the emergency department and 6% were hospitalized. Of those hospitalized, 2% required intensive care and/or ventilation, and no deaths have occurred in the registry to date.
By treatment group, hospitalization rates were highest among those on combination treatments (35.7%), followed by systemic corticosteroids (14.3%), topical treatments only (9.9%), other conventional systemics (3.6%), methotrexate (3.3%), and dupilumab (2.3%).
To further explore the differences between hospitalization rates in treatment groups, the researchers performed a multivariable logistic regression analysis, adjusted for age, sex, ethnicity, and comorbidity score. Compared with those who received dupilumab, the adjusted odds ratios (ORs) for hospitalization were highest among those who received topical treatments (OR, 4.95), followed by those who received systemic corticosteroids (OR, 2.81), and those who received other conventional systemic treatments (OR, 2.36).
Dr. Musters and colleagues also found that compared with patients on nonsteroidal immunosuppressive therapy, patients on combination systemic therapy had a significantly higher odds of hospitalization, specifically an OR of 45.75 for those on combination treatment including corticosteroids, an OR of 37.57 for those on combination treatment not including steroids, and an OR of 1.87 for those on systemic corticosteroids as monotherapy.
“Overall, the risk of COVID-19 complications appears to be low in patients with AD, even when treated with systemic immunomodulatory agents,” Dr. Musters concluded. “Dupilumab monotherapy was associated with lower odds of hospitalizations compared with other therapies. Moreover, combination systemic treatment, especially combinations including systemic corticosteroids, was associated with the highest risk of severe COVID-19.”
She added that other population-based study designs are more suitable to answer other important questions, such as whether the overall risk of COVID-19 in patients with AD is higher or lower compared to healthy controls.
Amy S. Paller, MD, professor and chair of the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study, characterized the results as reassuring. In this patient population, “we expected that dupilumab would not cause any problems,” she said. “We wouldn’t necessarily expect it to [confer] a benefit, but I think it’s because the patients who need a systemic medication are going on something that’s very targeted (dupilumab) rather than something that has a broader immunosuppressing function. It was interesting but not surprising that those on systemic steroids had more of a problem. Get them on something that’s very targeted if you can and don’t suppress the immune systems that might be handling COVID-19.”
Dr. Musters reported having no disclosures. Dr. Paller disclosed that she is consultant to and/or an investigator for many pharmaceutical companies.
AT AAD 22
Children and COVID: The long goodbye continues
COVID-19 continues to be a diminishing issue for U.S. children, as the number of new cases declined for the ninth consecutive week, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
the AAP and CHA said in their weekly COVID report. The most recently infected children brought the total number of COVID-19 cases to just over 12.8 million since the pandemic began.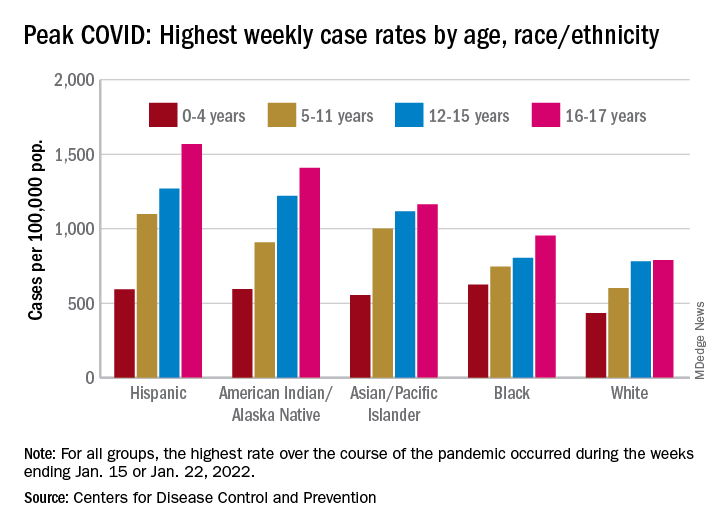
Other measures of COVID occurrence in children, such as hospital admissions and emergency department visits, also followed recent downward trends, although the sizes of the declines are beginning to decrease. Admissions dropped by 13.3% during the week ending March 26, but that followed declines of 25%, 20%, 26.5% and 24.4% for the 4 previous weeks, data from the Centers for Disease Control and Prevention show.
The slowdown in ED visits started a couple of weeks earlier, but the decline is still ongoing. As of March 25, ED visits with a confirmed COVID diagnosis represented just 0.4% of all visits for children aged 0-11 years, down from 1.1% on Feb. 25 and a peak of 14.3% on Jan. 15. For children aged 12-15, the latest figure is just 0.2%, compared with 0.5% on Feb. 25 and a peak of 14.3% on Jan. 9, the CDC reported on its COVID Data Tracker.
Although he was speaking of the nation as a whole and not specifically of children, Anthony Fauci, MD, the director of the National Institute of Allergy and Infectious Diseases, recently told the Washington Post that, “unless something changes dramatically,” another major surge isn’t on the horizon.
That sentiment, however, was not entirely shared by Moderna’s chief medical officer, Paul Burton, MD, PhD. In an interview with WebMD, he said that another COVID wave is inevitable and that it’s too soon to dismantle the vaccine infrastructure: “We’ve come so far. We’ve put so much into this to now take our foot off the gas. I think it would be a mistake for public health worldwide.”
Disparities during the Omicron surge
As the country puts Omicron in its rear view mirror, a quick look back at the CDC data shows some differences in how children were affected. At the surge’s peak in early to mid-January, Hispanic children were the most likely to get COVID-19, with incidence highest in the older groups. (See graph.)
At their peak week of Jan. 2-8, Hispanic children aged 16-17 years had a COVID rate of 1,568 cases per 100,000 population, versus 790 per 100,000 for White children, whose peak occurred a week later, from Jan. 9 to 15. Hispanic children aged 5-11 (1,098 per 100,000) and 12-15 (1,269 per 100,000) also had the highest recorded rates of the largest racial/ethnic groups, while Black children had the highest one-week rate, 625 per 100,000, among the 0- to 4-year-olds, according to the CDC.
COVID-19 continues to be a diminishing issue for U.S. children, as the number of new cases declined for the ninth consecutive week, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
the AAP and CHA said in their weekly COVID report. The most recently infected children brought the total number of COVID-19 cases to just over 12.8 million since the pandemic began.
Other measures of COVID occurrence in children, such as hospital admissions and emergency department visits, also followed recent downward trends, although the sizes of the declines are beginning to decrease. Admissions dropped by 13.3% during the week ending March 26, but that followed declines of 25%, 20%, 26.5% and 24.4% for the 4 previous weeks, data from the Centers for Disease Control and Prevention show.
The slowdown in ED visits started a couple of weeks earlier, but the decline is still ongoing. As of March 25, ED visits with a confirmed COVID diagnosis represented just 0.4% of all visits for children aged 0-11 years, down from 1.1% on Feb. 25 and a peak of 14.3% on Jan. 15. For children aged 12-15, the latest figure is just 0.2%, compared with 0.5% on Feb. 25 and a peak of 14.3% on Jan. 9, the CDC reported on its COVID Data Tracker.
Although he was speaking of the nation as a whole and not specifically of children, Anthony Fauci, MD, the director of the National Institute of Allergy and Infectious Diseases, recently told the Washington Post that, “unless something changes dramatically,” another major surge isn’t on the horizon.
That sentiment, however, was not entirely shared by Moderna’s chief medical officer, Paul Burton, MD, PhD. In an interview with WebMD, he said that another COVID wave is inevitable and that it’s too soon to dismantle the vaccine infrastructure: “We’ve come so far. We’ve put so much into this to now take our foot off the gas. I think it would be a mistake for public health worldwide.”
Disparities during the Omicron surge
As the country puts Omicron in its rear view mirror, a quick look back at the CDC data shows some differences in how children were affected. At the surge’s peak in early to mid-January, Hispanic children were the most likely to get COVID-19, with incidence highest in the older groups. (See graph.)
At their peak week of Jan. 2-8, Hispanic children aged 16-17 years had a COVID rate of 1,568 cases per 100,000 population, versus 790 per 100,000 for White children, whose peak occurred a week later, from Jan. 9 to 15. Hispanic children aged 5-11 (1,098 per 100,000) and 12-15 (1,269 per 100,000) also had the highest recorded rates of the largest racial/ethnic groups, while Black children had the highest one-week rate, 625 per 100,000, among the 0- to 4-year-olds, according to the CDC.
COVID-19 continues to be a diminishing issue for U.S. children, as the number of new cases declined for the ninth consecutive week, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
the AAP and CHA said in their weekly COVID report. The most recently infected children brought the total number of COVID-19 cases to just over 12.8 million since the pandemic began.
Other measures of COVID occurrence in children, such as hospital admissions and emergency department visits, also followed recent downward trends, although the sizes of the declines are beginning to decrease. Admissions dropped by 13.3% during the week ending March 26, but that followed declines of 25%, 20%, 26.5% and 24.4% for the 4 previous weeks, data from the Centers for Disease Control and Prevention show.
The slowdown in ED visits started a couple of weeks earlier, but the decline is still ongoing. As of March 25, ED visits with a confirmed COVID diagnosis represented just 0.4% of all visits for children aged 0-11 years, down from 1.1% on Feb. 25 and a peak of 14.3% on Jan. 15. For children aged 12-15, the latest figure is just 0.2%, compared with 0.5% on Feb. 25 and a peak of 14.3% on Jan. 9, the CDC reported on its COVID Data Tracker.
Although he was speaking of the nation as a whole and not specifically of children, Anthony Fauci, MD, the director of the National Institute of Allergy and Infectious Diseases, recently told the Washington Post that, “unless something changes dramatically,” another major surge isn’t on the horizon.
That sentiment, however, was not entirely shared by Moderna’s chief medical officer, Paul Burton, MD, PhD. In an interview with WebMD, he said that another COVID wave is inevitable and that it’s too soon to dismantle the vaccine infrastructure: “We’ve come so far. We’ve put so much into this to now take our foot off the gas. I think it would be a mistake for public health worldwide.”
Disparities during the Omicron surge
As the country puts Omicron in its rear view mirror, a quick look back at the CDC data shows some differences in how children were affected. At the surge’s peak in early to mid-January, Hispanic children were the most likely to get COVID-19, with incidence highest in the older groups. (See graph.)
At their peak week of Jan. 2-8, Hispanic children aged 16-17 years had a COVID rate of 1,568 cases per 100,000 population, versus 790 per 100,000 for White children, whose peak occurred a week later, from Jan. 9 to 15. Hispanic children aged 5-11 (1,098 per 100,000) and 12-15 (1,269 per 100,000) also had the highest recorded rates of the largest racial/ethnic groups, while Black children had the highest one-week rate, 625 per 100,000, among the 0- to 4-year-olds, according to the CDC.
As FDA OKs another COVID booster, some experts question need
, even though many top infectious disease experts questioned the need before the agency’s decision.
The FDA granted emergency use authorization for both Pfizer and Moderna to offer the second booster – and fourth shot overall – for adults over 50 as well as those over 18 with compromised immune systems.
The Centers for Control and Prevention must still sign off before those doses start reaching American arms. That approval could come at any time.
“The general consensus, certainly the CDC’s consensus, is that the current vaccines are still really quite effective against Omicron and this new BA.2 variant in keeping people out of the hospital, and preventing the development of severe disease,” William Schaffner, MD, an infectious disease specialist at Vanderbilt University in Nashville said prior to the FDA’s announcement March 29.
Of the 217.4 million Americans who are “fully vaccinated,” i.e., received two doses of either Pfizer or Moderna’s vaccines or one dose of the Johnson & Johnson vaccine, only 45% have also received a booster shot, according to the CDC.
“Given that, there’s no need at the moment for the general population to get a fourth inoculation,” Dr. Schaffner says. “Our current focus ought to be on making sure that as many people as possible get that [first] booster who are eligible.”
Monica Gandhi, MD, an infectious disease specialist at the University of California, San Francisco, agreed that another booster for everyone was unnecessary. The only people who would need a fourth shot (or third, if they had the Johnson & Johnson vaccine initially) are those over age 65 or 70 years, Dr. Gandhi says.
“Older people need those antibodies up high because they’re more susceptible to severe breakthroughs,” she said, also before the latest development.
To boost or not to boost
Daniel Kuritzkes, MD, chief of infectious diseases at Brigham & Women’s Hospital in Boston, said the timing of a booster and who should be eligible depends on what the nation is trying to achieve with its vaccination strategy.
“Is the goal to prevent any symptomatic infection with COVID-19, is the goal to prevent the spread of COVID-19, or is the goal to prevent severe disease that requires hospitalization?” asked Dr. Kuritzkes.
The current vaccine — with a booster — has prevented severe disease, he said.
An Israeli study showed, for instance, that a third Pfizer dose was 93% effective against hospitalization, 92% effective against severe illness, and 81% effective against death.
A just-published study in the New England Journal of Medicine found that a booster of the Pfizer vaccine was 95% effective against COVID-19 infection and that it did not raise any new safety issues.
A small Israeli study, also published in NEJM, of a fourth Pfizer dose given to health care workers found that it prevented symptomatic infection and illness, but that it was much less effective than previous doses — maybe 65% effective against symptomatic illness, the authors write.
Giving Americans another booster now — which has been shown to lose some effectiveness after about 4 months — means it might not offer protection this fall and winter, when there could be a seasonal surge of the virus, Dr. Kuritzkes says.
And, even if people receive boosters every few months, they are still likely to get a mild respiratory virus infection, he said.
“I’m pretty convinced that we cannot boost ourselves out of this pandemic,” said Dr. Kuritzkes. “We need to first of all ensure there’s global immunization so that all the people who have not been vaccinated at all get vaccinated. That’s far more important than boosting people a fourth time.”
Booster confusion
The April 6 FDA meeting of the agency’s Vaccines and Related Biological Products Advisory Committee comes as the two major COVID vaccine makers — Pfizer and Moderna — have applied for emergency use authorization for an additional booster.
Pfizer had asked for authorization for a fourth shot in patients over age 65 years, while Moderna wanted a booster to be available to all Americans over 18. The FDA instead granted authorization to both companies for those over 50 and anyone 18 or older who is immunocompromised.
What this means for the committee’s April 6 meeting is not clear. The original agenda says the committee will consider the evidence on safety and effectiveness of the additional vaccine doses and discuss how to set up a process — similar to that used for the influenza vaccine — to be able to determine the makeup of COVID vaccines as new variants emerge. That could lay the groundwork for an annual COVID shot, if needed.
The FDA advisers will not make recommendations nor vote on whether — and which — Americans should get a COVID booster. That is the job of the CDC’s Advisory Committee on Immunization Practices (ACIP).
The last time a booster was considered, CDC Director Rochelle Walensky, MD, overrode the committee and recommended that all Americans — not just older individuals — get an additional COVID shot, which became the first booster.
That past action worries Dr. Gandhi, who calls it confusing, and says it may have contributed to the fact that less than half of Americans have since chosen to get a booster.
Dr. Schaffner says he expects the FDA to authorize emergency use for fourth doses of the Pfizer and Moderna vaccines, but he doesn’t think the CDC committee will recommend routine use. As was seen before, however, the CDC director does not have to follow the committee’s advice.
The members of ACIP “might be more conservative or narrower in scope in terms of recommending who needs to be boosted and when boosting is appropriate,” Dr. Kuritzkes says.
Dr. Gandhi says she’s concerned the FDA’s deliberations could be swayed by Moderna and Pfizer’s influence and that “pharmaceutical companies are going to have more of a say than they should in the scientific process.”
There are similar worries for Dr. Schaffner. He says he’s “a bit grumpy” that the vaccine makers have been using press releases to argue for boosters.
“Press releases are no way to make vaccine recommendations,” Dr. Schaffner said, adding that he “would advise [vaccine makers] to sit down and be quiet and let the FDA and CDC advisory committee do their thing.”
Moderna Chief Medical Officer Paul Burton, MD, however, told WebMD last week that the signs point to why a fourth shot may be needed.
“We see waning of effectiveness, antibody levels come down, and certainly effectiveness against Omicron comes down in 3 to 6 months,” Burton said. “The natural history, from what we’re seeing around the world, is that BA.2 is definitely here, it’s highly transmissible, and I think we are going to get an additional wave of BA.2 here in the United States.”
Another wave is coming, he said, and “I think there will be waning of effectiveness. We need to be prepared for that, so that’s why we need the fourth dose.”
Supply issues?
Meanwhile, the United Kingdom has begun offering boosters to anyone over 75, and Sweden’s health authority has recommended a fourth shot to people over age 80.
That puts pressure on the United States — at least on its politicians and policymakers — to, in a sense, keep up, said the infectious disease specialists.
Indeed, the White House has been keeping fourth shots in the news, warning that it is running out of money to ensure that all Americans would have access to one, if recommended.
On March 23, outgoing White House COVID-19 Response Coordinator Jeff Zients said the federal government had enough vaccine for the immunocompromised to get a fourth dose “and, if authorized in the coming weeks, enough supply for fourth doses for our most vulnerable, including seniors.”
But he warned that without congressional approval of a COVID-19 funding package, “We can’t procure the necessary vaccine supply to support fourth shots for all Americans.”
Mr. Zients also noted that other countries, including Japan, Vietnam, and the Philippines had already secured future booster doses and added, “We should be securing additional supply right now.”
Dr. Schaffner says that while it would be nice to “have a booster on the shelf,” the United States needs to put more effort into creating a globally-coordinated process for ensuring that vaccines match circulating strains and that they are manufactured on a timely basis.
He says he and others “have been reminding the public that the COVID pandemic may indeed be diminishing and moving into the endemic, but that doesn’t mean COVID is over or finished or disappeared.”
Dr. Schaffner says that it may be that “perhaps we’d need a periodic reminder to our immune system to remain protected. In other words, we might have to get boosted perhaps annually like we do with influenza.”
A version of this article first appeared on WebMD.com.
, even though many top infectious disease experts questioned the need before the agency’s decision.
The FDA granted emergency use authorization for both Pfizer and Moderna to offer the second booster – and fourth shot overall – for adults over 50 as well as those over 18 with compromised immune systems.
The Centers for Control and Prevention must still sign off before those doses start reaching American arms. That approval could come at any time.
“The general consensus, certainly the CDC’s consensus, is that the current vaccines are still really quite effective against Omicron and this new BA.2 variant in keeping people out of the hospital, and preventing the development of severe disease,” William Schaffner, MD, an infectious disease specialist at Vanderbilt University in Nashville said prior to the FDA’s announcement March 29.
Of the 217.4 million Americans who are “fully vaccinated,” i.e., received two doses of either Pfizer or Moderna’s vaccines or one dose of the Johnson & Johnson vaccine, only 45% have also received a booster shot, according to the CDC.
“Given that, there’s no need at the moment for the general population to get a fourth inoculation,” Dr. Schaffner says. “Our current focus ought to be on making sure that as many people as possible get that [first] booster who are eligible.”
Monica Gandhi, MD, an infectious disease specialist at the University of California, San Francisco, agreed that another booster for everyone was unnecessary. The only people who would need a fourth shot (or third, if they had the Johnson & Johnson vaccine initially) are those over age 65 or 70 years, Dr. Gandhi says.
“Older people need those antibodies up high because they’re more susceptible to severe breakthroughs,” she said, also before the latest development.
To boost or not to boost
Daniel Kuritzkes, MD, chief of infectious diseases at Brigham & Women’s Hospital in Boston, said the timing of a booster and who should be eligible depends on what the nation is trying to achieve with its vaccination strategy.
“Is the goal to prevent any symptomatic infection with COVID-19, is the goal to prevent the spread of COVID-19, or is the goal to prevent severe disease that requires hospitalization?” asked Dr. Kuritzkes.
The current vaccine — with a booster — has prevented severe disease, he said.
An Israeli study showed, for instance, that a third Pfizer dose was 93% effective against hospitalization, 92% effective against severe illness, and 81% effective against death.
A just-published study in the New England Journal of Medicine found that a booster of the Pfizer vaccine was 95% effective against COVID-19 infection and that it did not raise any new safety issues.
A small Israeli study, also published in NEJM, of a fourth Pfizer dose given to health care workers found that it prevented symptomatic infection and illness, but that it was much less effective than previous doses — maybe 65% effective against symptomatic illness, the authors write.
Giving Americans another booster now — which has been shown to lose some effectiveness after about 4 months — means it might not offer protection this fall and winter, when there could be a seasonal surge of the virus, Dr. Kuritzkes says.
And, even if people receive boosters every few months, they are still likely to get a mild respiratory virus infection, he said.
“I’m pretty convinced that we cannot boost ourselves out of this pandemic,” said Dr. Kuritzkes. “We need to first of all ensure there’s global immunization so that all the people who have not been vaccinated at all get vaccinated. That’s far more important than boosting people a fourth time.”
Booster confusion
The April 6 FDA meeting of the agency’s Vaccines and Related Biological Products Advisory Committee comes as the two major COVID vaccine makers — Pfizer and Moderna — have applied for emergency use authorization for an additional booster.
Pfizer had asked for authorization for a fourth shot in patients over age 65 years, while Moderna wanted a booster to be available to all Americans over 18. The FDA instead granted authorization to both companies for those over 50 and anyone 18 or older who is immunocompromised.
What this means for the committee’s April 6 meeting is not clear. The original agenda says the committee will consider the evidence on safety and effectiveness of the additional vaccine doses and discuss how to set up a process — similar to that used for the influenza vaccine — to be able to determine the makeup of COVID vaccines as new variants emerge. That could lay the groundwork for an annual COVID shot, if needed.
The FDA advisers will not make recommendations nor vote on whether — and which — Americans should get a COVID booster. That is the job of the CDC’s Advisory Committee on Immunization Practices (ACIP).
The last time a booster was considered, CDC Director Rochelle Walensky, MD, overrode the committee and recommended that all Americans — not just older individuals — get an additional COVID shot, which became the first booster.
That past action worries Dr. Gandhi, who calls it confusing, and says it may have contributed to the fact that less than half of Americans have since chosen to get a booster.
Dr. Schaffner says he expects the FDA to authorize emergency use for fourth doses of the Pfizer and Moderna vaccines, but he doesn’t think the CDC committee will recommend routine use. As was seen before, however, the CDC director does not have to follow the committee’s advice.
The members of ACIP “might be more conservative or narrower in scope in terms of recommending who needs to be boosted and when boosting is appropriate,” Dr. Kuritzkes says.
Dr. Gandhi says she’s concerned the FDA’s deliberations could be swayed by Moderna and Pfizer’s influence and that “pharmaceutical companies are going to have more of a say than they should in the scientific process.”
There are similar worries for Dr. Schaffner. He says he’s “a bit grumpy” that the vaccine makers have been using press releases to argue for boosters.
“Press releases are no way to make vaccine recommendations,” Dr. Schaffner said, adding that he “would advise [vaccine makers] to sit down and be quiet and let the FDA and CDC advisory committee do their thing.”
Moderna Chief Medical Officer Paul Burton, MD, however, told WebMD last week that the signs point to why a fourth shot may be needed.
“We see waning of effectiveness, antibody levels come down, and certainly effectiveness against Omicron comes down in 3 to 6 months,” Burton said. “The natural history, from what we’re seeing around the world, is that BA.2 is definitely here, it’s highly transmissible, and I think we are going to get an additional wave of BA.2 here in the United States.”
Another wave is coming, he said, and “I think there will be waning of effectiveness. We need to be prepared for that, so that’s why we need the fourth dose.”
Supply issues?
Meanwhile, the United Kingdom has begun offering boosters to anyone over 75, and Sweden’s health authority has recommended a fourth shot to people over age 80.
That puts pressure on the United States — at least on its politicians and policymakers — to, in a sense, keep up, said the infectious disease specialists.
Indeed, the White House has been keeping fourth shots in the news, warning that it is running out of money to ensure that all Americans would have access to one, if recommended.
On March 23, outgoing White House COVID-19 Response Coordinator Jeff Zients said the federal government had enough vaccine for the immunocompromised to get a fourth dose “and, if authorized in the coming weeks, enough supply for fourth doses for our most vulnerable, including seniors.”
But he warned that without congressional approval of a COVID-19 funding package, “We can’t procure the necessary vaccine supply to support fourth shots for all Americans.”
Mr. Zients also noted that other countries, including Japan, Vietnam, and the Philippines had already secured future booster doses and added, “We should be securing additional supply right now.”
Dr. Schaffner says that while it would be nice to “have a booster on the shelf,” the United States needs to put more effort into creating a globally-coordinated process for ensuring that vaccines match circulating strains and that they are manufactured on a timely basis.
He says he and others “have been reminding the public that the COVID pandemic may indeed be diminishing and moving into the endemic, but that doesn’t mean COVID is over or finished or disappeared.”
Dr. Schaffner says that it may be that “perhaps we’d need a periodic reminder to our immune system to remain protected. In other words, we might have to get boosted perhaps annually like we do with influenza.”
A version of this article first appeared on WebMD.com.
, even though many top infectious disease experts questioned the need before the agency’s decision.
The FDA granted emergency use authorization for both Pfizer and Moderna to offer the second booster – and fourth shot overall – for adults over 50 as well as those over 18 with compromised immune systems.
The Centers for Control and Prevention must still sign off before those doses start reaching American arms. That approval could come at any time.
“The general consensus, certainly the CDC’s consensus, is that the current vaccines are still really quite effective against Omicron and this new BA.2 variant in keeping people out of the hospital, and preventing the development of severe disease,” William Schaffner, MD, an infectious disease specialist at Vanderbilt University in Nashville said prior to the FDA’s announcement March 29.
Of the 217.4 million Americans who are “fully vaccinated,” i.e., received two doses of either Pfizer or Moderna’s vaccines or one dose of the Johnson & Johnson vaccine, only 45% have also received a booster shot, according to the CDC.
“Given that, there’s no need at the moment for the general population to get a fourth inoculation,” Dr. Schaffner says. “Our current focus ought to be on making sure that as many people as possible get that [first] booster who are eligible.”
Monica Gandhi, MD, an infectious disease specialist at the University of California, San Francisco, agreed that another booster for everyone was unnecessary. The only people who would need a fourth shot (or third, if they had the Johnson & Johnson vaccine initially) are those over age 65 or 70 years, Dr. Gandhi says.
“Older people need those antibodies up high because they’re more susceptible to severe breakthroughs,” she said, also before the latest development.
To boost or not to boost
Daniel Kuritzkes, MD, chief of infectious diseases at Brigham & Women’s Hospital in Boston, said the timing of a booster and who should be eligible depends on what the nation is trying to achieve with its vaccination strategy.
“Is the goal to prevent any symptomatic infection with COVID-19, is the goal to prevent the spread of COVID-19, or is the goal to prevent severe disease that requires hospitalization?” asked Dr. Kuritzkes.
The current vaccine — with a booster — has prevented severe disease, he said.
An Israeli study showed, for instance, that a third Pfizer dose was 93% effective against hospitalization, 92% effective against severe illness, and 81% effective against death.
A just-published study in the New England Journal of Medicine found that a booster of the Pfizer vaccine was 95% effective against COVID-19 infection and that it did not raise any new safety issues.
A small Israeli study, also published in NEJM, of a fourth Pfizer dose given to health care workers found that it prevented symptomatic infection and illness, but that it was much less effective than previous doses — maybe 65% effective against symptomatic illness, the authors write.
Giving Americans another booster now — which has been shown to lose some effectiveness after about 4 months — means it might not offer protection this fall and winter, when there could be a seasonal surge of the virus, Dr. Kuritzkes says.
And, even if people receive boosters every few months, they are still likely to get a mild respiratory virus infection, he said.
“I’m pretty convinced that we cannot boost ourselves out of this pandemic,” said Dr. Kuritzkes. “We need to first of all ensure there’s global immunization so that all the people who have not been vaccinated at all get vaccinated. That’s far more important than boosting people a fourth time.”
Booster confusion
The April 6 FDA meeting of the agency’s Vaccines and Related Biological Products Advisory Committee comes as the two major COVID vaccine makers — Pfizer and Moderna — have applied for emergency use authorization for an additional booster.
Pfizer had asked for authorization for a fourth shot in patients over age 65 years, while Moderna wanted a booster to be available to all Americans over 18. The FDA instead granted authorization to both companies for those over 50 and anyone 18 or older who is immunocompromised.
What this means for the committee’s April 6 meeting is not clear. The original agenda says the committee will consider the evidence on safety and effectiveness of the additional vaccine doses and discuss how to set up a process — similar to that used for the influenza vaccine — to be able to determine the makeup of COVID vaccines as new variants emerge. That could lay the groundwork for an annual COVID shot, if needed.
The FDA advisers will not make recommendations nor vote on whether — and which — Americans should get a COVID booster. That is the job of the CDC’s Advisory Committee on Immunization Practices (ACIP).
The last time a booster was considered, CDC Director Rochelle Walensky, MD, overrode the committee and recommended that all Americans — not just older individuals — get an additional COVID shot, which became the first booster.
That past action worries Dr. Gandhi, who calls it confusing, and says it may have contributed to the fact that less than half of Americans have since chosen to get a booster.
Dr. Schaffner says he expects the FDA to authorize emergency use for fourth doses of the Pfizer and Moderna vaccines, but he doesn’t think the CDC committee will recommend routine use. As was seen before, however, the CDC director does not have to follow the committee’s advice.
The members of ACIP “might be more conservative or narrower in scope in terms of recommending who needs to be boosted and when boosting is appropriate,” Dr. Kuritzkes says.
Dr. Gandhi says she’s concerned the FDA’s deliberations could be swayed by Moderna and Pfizer’s influence and that “pharmaceutical companies are going to have more of a say than they should in the scientific process.”
There are similar worries for Dr. Schaffner. He says he’s “a bit grumpy” that the vaccine makers have been using press releases to argue for boosters.
“Press releases are no way to make vaccine recommendations,” Dr. Schaffner said, adding that he “would advise [vaccine makers] to sit down and be quiet and let the FDA and CDC advisory committee do their thing.”
Moderna Chief Medical Officer Paul Burton, MD, however, told WebMD last week that the signs point to why a fourth shot may be needed.
“We see waning of effectiveness, antibody levels come down, and certainly effectiveness against Omicron comes down in 3 to 6 months,” Burton said. “The natural history, from what we’re seeing around the world, is that BA.2 is definitely here, it’s highly transmissible, and I think we are going to get an additional wave of BA.2 here in the United States.”
Another wave is coming, he said, and “I think there will be waning of effectiveness. We need to be prepared for that, so that’s why we need the fourth dose.”
Supply issues?
Meanwhile, the United Kingdom has begun offering boosters to anyone over 75, and Sweden’s health authority has recommended a fourth shot to people over age 80.
That puts pressure on the United States — at least on its politicians and policymakers — to, in a sense, keep up, said the infectious disease specialists.
Indeed, the White House has been keeping fourth shots in the news, warning that it is running out of money to ensure that all Americans would have access to one, if recommended.
On March 23, outgoing White House COVID-19 Response Coordinator Jeff Zients said the federal government had enough vaccine for the immunocompromised to get a fourth dose “and, if authorized in the coming weeks, enough supply for fourth doses for our most vulnerable, including seniors.”
But he warned that without congressional approval of a COVID-19 funding package, “We can’t procure the necessary vaccine supply to support fourth shots for all Americans.”
Mr. Zients also noted that other countries, including Japan, Vietnam, and the Philippines had already secured future booster doses and added, “We should be securing additional supply right now.”
Dr. Schaffner says that while it would be nice to “have a booster on the shelf,” the United States needs to put more effort into creating a globally-coordinated process for ensuring that vaccines match circulating strains and that they are manufactured on a timely basis.
He says he and others “have been reminding the public that the COVID pandemic may indeed be diminishing and moving into the endemic, but that doesn’t mean COVID is over or finished or disappeared.”
Dr. Schaffner says that it may be that “perhaps we’d need a periodic reminder to our immune system to remain protected. In other words, we might have to get boosted perhaps annually like we do with influenza.”
A version of this article first appeared on WebMD.com.
‘Staggeringly high’ rates of psychiatric symptoms after COVID-19
DENVER – Neurocognitive and psychiatric symptoms of mental illness, including posttraumatic stress disorder, are alarmingly high among patients who have previously had COVID-19 – even among those who were not hospitalized with the virus, new research shows.
The findings are from an online survey of more than 800 respondents.
“Regardless of how long ago they had been infected with COVID-19, all respondents had persistent symptoms,” co-investigator Beth Patterson, MSc, adjunct clinical professor at McMaster University, MacAnxiety Research Centre, Hamilton, Ont., told this news organization.
“The take-home message for clinicians is to be aware that if you have patients who had COVID-19, it’s quite likely that they may also experience a psychiatric issue and that they may have reduced resilience and lower quality-of-life [issues],” Ms. Patterson said.
The survey results were presented here at the Anxiety and Depression Association of America (ADAA) Conference 2022.
100% report symptoms
The study included 827 respondents (81% women) to an online survey who had contracted COVID.
Using validated symptom severity scores, respondents were assessed for mental health and neurocognitive issues, as well as some physical and quality-of-life factors.
Remarkably, all participants (100%) reported having current, persistent symptoms of COVID. In addition, 88% (n = 729) reported persistent neurocognitive symptoms, even though only 15.5% reported they had been hospitalized for COVID.
Of those hospitalized, 28.9% were treated in the intensive care unit; 42.2% stayed in hospital for less than 1 week; and 13.3% remained hospitalized for at least 3 weeks.
Data were not available on how long it had been since the patients were diagnosed or hospitalized, but most participants (68%) said they had not returned to normal functioning since contracting COVID.
The most common persistent symptoms were fatigue (75.9%), brain fog (67.9%), difficulty concentrating (61%), and weakness (51.2%).
More than half of respondents reported neurocognitive symptoms, including poor memory (57.4%) and word-finding problems in processing information (46.9%). Only 11% reported no persistent neurocognitive symptoms.
A total of 41.7% of respondents reported anxiety using the Generalized Anxiety Disorder-7 (GAD-7) scale, and rates of depression were 61.4% as assessed with the Patient Health Questionnaire (PHQ-9).
Rates of probable posttraumatic stress disorder were 40.5% as assessed via the PTSD checklist (PCL-5).
Although it wasn’t possible to use diagnostic screens, the assessment scores suggest strikingly high rates of mental health disorders among the respondents, Ms. Patterson said.
“When we look at the mean scores on the validated scales, we see percentages of probable diagnoses that are staggeringly higher than you would find in the population,” she added.
Of note, about 44% of respondents reported having had mental health treatment in the past, and 33.7% were receiving current mental health treatment.
Although the study had no control group, the findings are consistent with larger studies that have had comparator groups, including research recently published in the BMJ.
Poor understanding of COVID’s fallout
In an editorial accompanying the BMJ study, Scott Weich, MD, Mental Health Research Unit, School of Health and Related Research, University of Sheffield, United Kingdom, emphasized the need to better understand the lingering mental health aspects of COVID-19 infection.
“Our attachment to syndromal phenotypes means that we have learned remarkably little about the causes of mental ill health – in this case psychopathology associated with a viral pandemic,” Dr. Weich writes.
Dr. Weich called for improved efforts to understanding long COVID, as well as the establishment of more effective responses to the mental health fallout from the pandemic.
Commenting on the current study, Dr. Weich elaborated on the challenges in disentangling the causes of mental health effects in illness.
“In terms of other viruses, etc., there is a long history of debate and pitched battles between those that attribute mental health effects to predominantly biological processes, [involving] immunological and other responses, and those who understand these responses are mediated by psychological and social processes,” he noted.
“The story of myalgic encephalomyelitis/chronic fatigue syndrome speaks volumes about these different positions, and how difficult it can be to find a middle ground,” he said.
“This has been going on for centuries and may never be fully resolved, at least until we have clearer and more definitive evidence of pathophysiology, though this seems incredibly elusive,” Dr. Weich said.
The authors and Dr. Weich have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
DENVER – Neurocognitive and psychiatric symptoms of mental illness, including posttraumatic stress disorder, are alarmingly high among patients who have previously had COVID-19 – even among those who were not hospitalized with the virus, new research shows.
The findings are from an online survey of more than 800 respondents.
“Regardless of how long ago they had been infected with COVID-19, all respondents had persistent symptoms,” co-investigator Beth Patterson, MSc, adjunct clinical professor at McMaster University, MacAnxiety Research Centre, Hamilton, Ont., told this news organization.
“The take-home message for clinicians is to be aware that if you have patients who had COVID-19, it’s quite likely that they may also experience a psychiatric issue and that they may have reduced resilience and lower quality-of-life [issues],” Ms. Patterson said.
The survey results were presented here at the Anxiety and Depression Association of America (ADAA) Conference 2022.
100% report symptoms
The study included 827 respondents (81% women) to an online survey who had contracted COVID.
Using validated symptom severity scores, respondents were assessed for mental health and neurocognitive issues, as well as some physical and quality-of-life factors.
Remarkably, all participants (100%) reported having current, persistent symptoms of COVID. In addition, 88% (n = 729) reported persistent neurocognitive symptoms, even though only 15.5% reported they had been hospitalized for COVID.
Of those hospitalized, 28.9% were treated in the intensive care unit; 42.2% stayed in hospital for less than 1 week; and 13.3% remained hospitalized for at least 3 weeks.
Data were not available on how long it had been since the patients were diagnosed or hospitalized, but most participants (68%) said they had not returned to normal functioning since contracting COVID.
The most common persistent symptoms were fatigue (75.9%), brain fog (67.9%), difficulty concentrating (61%), and weakness (51.2%).
More than half of respondents reported neurocognitive symptoms, including poor memory (57.4%) and word-finding problems in processing information (46.9%). Only 11% reported no persistent neurocognitive symptoms.
A total of 41.7% of respondents reported anxiety using the Generalized Anxiety Disorder-7 (GAD-7) scale, and rates of depression were 61.4% as assessed with the Patient Health Questionnaire (PHQ-9).
Rates of probable posttraumatic stress disorder were 40.5% as assessed via the PTSD checklist (PCL-5).
Although it wasn’t possible to use diagnostic screens, the assessment scores suggest strikingly high rates of mental health disorders among the respondents, Ms. Patterson said.
“When we look at the mean scores on the validated scales, we see percentages of probable diagnoses that are staggeringly higher than you would find in the population,” she added.
Of note, about 44% of respondents reported having had mental health treatment in the past, and 33.7% were receiving current mental health treatment.
Although the study had no control group, the findings are consistent with larger studies that have had comparator groups, including research recently published in the BMJ.
Poor understanding of COVID’s fallout
In an editorial accompanying the BMJ study, Scott Weich, MD, Mental Health Research Unit, School of Health and Related Research, University of Sheffield, United Kingdom, emphasized the need to better understand the lingering mental health aspects of COVID-19 infection.
“Our attachment to syndromal phenotypes means that we have learned remarkably little about the causes of mental ill health – in this case psychopathology associated with a viral pandemic,” Dr. Weich writes.
Dr. Weich called for improved efforts to understanding long COVID, as well as the establishment of more effective responses to the mental health fallout from the pandemic.
Commenting on the current study, Dr. Weich elaborated on the challenges in disentangling the causes of mental health effects in illness.
“In terms of other viruses, etc., there is a long history of debate and pitched battles between those that attribute mental health effects to predominantly biological processes, [involving] immunological and other responses, and those who understand these responses are mediated by psychological and social processes,” he noted.
“The story of myalgic encephalomyelitis/chronic fatigue syndrome speaks volumes about these different positions, and how difficult it can be to find a middle ground,” he said.
“This has been going on for centuries and may never be fully resolved, at least until we have clearer and more definitive evidence of pathophysiology, though this seems incredibly elusive,” Dr. Weich said.
The authors and Dr. Weich have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
DENVER – Neurocognitive and psychiatric symptoms of mental illness, including posttraumatic stress disorder, are alarmingly high among patients who have previously had COVID-19 – even among those who were not hospitalized with the virus, new research shows.
The findings are from an online survey of more than 800 respondents.
“Regardless of how long ago they had been infected with COVID-19, all respondents had persistent symptoms,” co-investigator Beth Patterson, MSc, adjunct clinical professor at McMaster University, MacAnxiety Research Centre, Hamilton, Ont., told this news organization.
“The take-home message for clinicians is to be aware that if you have patients who had COVID-19, it’s quite likely that they may also experience a psychiatric issue and that they may have reduced resilience and lower quality-of-life [issues],” Ms. Patterson said.
The survey results were presented here at the Anxiety and Depression Association of America (ADAA) Conference 2022.
100% report symptoms
The study included 827 respondents (81% women) to an online survey who had contracted COVID.
Using validated symptom severity scores, respondents were assessed for mental health and neurocognitive issues, as well as some physical and quality-of-life factors.
Remarkably, all participants (100%) reported having current, persistent symptoms of COVID. In addition, 88% (n = 729) reported persistent neurocognitive symptoms, even though only 15.5% reported they had been hospitalized for COVID.
Of those hospitalized, 28.9% were treated in the intensive care unit; 42.2% stayed in hospital for less than 1 week; and 13.3% remained hospitalized for at least 3 weeks.
Data were not available on how long it had been since the patients were diagnosed or hospitalized, but most participants (68%) said they had not returned to normal functioning since contracting COVID.
The most common persistent symptoms were fatigue (75.9%), brain fog (67.9%), difficulty concentrating (61%), and weakness (51.2%).
More than half of respondents reported neurocognitive symptoms, including poor memory (57.4%) and word-finding problems in processing information (46.9%). Only 11% reported no persistent neurocognitive symptoms.
A total of 41.7% of respondents reported anxiety using the Generalized Anxiety Disorder-7 (GAD-7) scale, and rates of depression were 61.4% as assessed with the Patient Health Questionnaire (PHQ-9).
Rates of probable posttraumatic stress disorder were 40.5% as assessed via the PTSD checklist (PCL-5).
Although it wasn’t possible to use diagnostic screens, the assessment scores suggest strikingly high rates of mental health disorders among the respondents, Ms. Patterson said.
“When we look at the mean scores on the validated scales, we see percentages of probable diagnoses that are staggeringly higher than you would find in the population,” she added.
Of note, about 44% of respondents reported having had mental health treatment in the past, and 33.7% were receiving current mental health treatment.
Although the study had no control group, the findings are consistent with larger studies that have had comparator groups, including research recently published in the BMJ.
Poor understanding of COVID’s fallout
In an editorial accompanying the BMJ study, Scott Weich, MD, Mental Health Research Unit, School of Health and Related Research, University of Sheffield, United Kingdom, emphasized the need to better understand the lingering mental health aspects of COVID-19 infection.
“Our attachment to syndromal phenotypes means that we have learned remarkably little about the causes of mental ill health – in this case psychopathology associated with a viral pandemic,” Dr. Weich writes.
Dr. Weich called for improved efforts to understanding long COVID, as well as the establishment of more effective responses to the mental health fallout from the pandemic.
Commenting on the current study, Dr. Weich elaborated on the challenges in disentangling the causes of mental health effects in illness.
“In terms of other viruses, etc., there is a long history of debate and pitched battles between those that attribute mental health effects to predominantly biological processes, [involving] immunological and other responses, and those who understand these responses are mediated by psychological and social processes,” he noted.
“The story of myalgic encephalomyelitis/chronic fatigue syndrome speaks volumes about these different positions, and how difficult it can be to find a middle ground,” he said.
“This has been going on for centuries and may never be fully resolved, at least until we have clearer and more definitive evidence of pathophysiology, though this seems incredibly elusive,” Dr. Weich said.
The authors and Dr. Weich have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ADAA 2022
Different variants may cause different long COVID symptoms: Study
Long COVID symptoms may differ depending on which SARS-CoV-2 variant is behind a person’s infection, a new study shows.
The data from Italy compared long COVID symptoms reported by patients infected with SARS-CoV-2 from March to December 2020 (when the original, or “Wuhan,” variant was dominant) with those reported by patients infected from January to April 2021 (B.1.1.7-, or Alpha variant-dominant). It showed a substantial change in the pattern of neurological and cognitive/emotional problems – the latter mostly seen with the Alpha variant.
Infectious disease specialist Michele Spinicci, MD, from the University of Florence and Careggi University Hospital, Italy, led the work. “Many of the symptoms reported in this study have been measured [before], but this is the first time they have been linked to different COVID-19 variants,” he told this news organization. “Findings in patients with long COVID were focused on neurological and psychological difficulties.”
However, he pointed out that much remains to be understood about long COVID in terms of symptoms, diagnosis, and treatment.
“Long COVID is a huge area that involves many different fields of medicine, so there is not one single piece of advice to give on management. There’s lots to consider when evaluating a long COVID patient,” he said.
Results showed that when the Alpha variant was the dominant variant, the prevalence of myalgia (10%), dyspnea (42%), brain fog/mental confusion (17%), and anxiety/depression (13%) significantly increased relative to the wild-type (original, Wuhan) variant, while anosmia (2%), dysgeusia (4%), and impaired hearing (1%) were less common.
When the wild-type (original, Wuhan) variant was dominant, fatigue (37%), insomnia (16%), dysgeusia (11%), and impaired hearing (5%) were all more common than with the Alpha variant. Dyspnea (33%), brain fog (10%), myalgia (4%), and anxiety/depression (6%) were less common.
Overall, 76% of the patients in the trial reported at least one persistent symptom, while the most common reported symptoms were dyspnea (37%) and chronic fatigue (36%), followed by insomnia (16%), visual disorders (13%), and brain fog (13%).
The findings come from an early-release abstract that will be presented at the European Congress of Clinical Microbiology & Infectious Diseases (ECCMID) 2022, in Lisbon, Portugal, in a few weeks’ time.
‘The take-home point’
Michael A. Horberg, MD, associate medical director, Kaiser Permanente – Mid-Atlantic Permanente Medical Group, Rockville, Maryland, has recently presented data on symptoms seen with long COVID in over 28,000 people, as reported by this news organization, at the Conference on Retroviruses and Opportunistic Infections 2022. These people were infected with the wild-type virus.
Commenting on the study by Dr. Spinicci, he said: “The issue is that as we go along the COVID lifespan from acute to long COVID, what prompts patients to seek medical attention may change. If symptoms are not severe or were not well publicized previously, patients may not see the need to seek care or evaluation. As such, it doesn’t surprise me to find these changes over time, independent of any potential biological activity of the virus or its consequences.”
Dr. Horberg noted that their own study results are consistent with those of Dr. Spinicci et al. from March to December 2020 (original, Wuhan variant). “To me, the take-home point is long COVID is real, and physicians need to be on the lookout for it. However, not all symptoms are due to long COVID, and we need to keep the time course of symptoms during evaluation of such patients.”
Also providing comment on the findings was Debby Bogaert, MD, chair of Pediatric Medicine, University of Edinburgh. Reflecting on whether the symptoms were due to long COVID or another underlying disease, she said: “The number of patients with ongoing symptoms is very high, therefore [it is] unlikely that all of this is re-emergence of underlying or previous health problems. The type of symptoms reported are also as reported by other cohorts, so not unexpected. And irrespective of the root cause, they require care.”
Dr. Bogaert also noted that the data reiterate that COVID-19 is a new disease, and that “new variants might show shifting clinical pictures, not only regarding severity and symptoms of acute disease, but possibly also regarding sequela,” and that this, “underlines the importance of ongoing surveillance of variants, and ongoing evaluation of the acute and long-term clinical picture accompanying these, to ensure we adapt our public health approaches, clinical treatment plans, and long-term follow-up when and where needed.”
Dr. Bogaert stressed that only by keeping track of the changes in symptoms both acute and long-term – by patients and doctors – would the best patient care be provided.
“Patients need to know so they can report these back to their doctors, and doctors need to know over time that the picture of sequela might shift, so sequela are recognized early, and these patients receive the appropriate follow-up treatment,” she said. These shifting patterns might also apply to community patients as well as those hospitalized with COVID-19.
Study details
The retrospective, observational study included 428 patients, 59% men, with a mean age of 64 years, who had been treated at the Careggi University Hospital’s post-COVID outpatient service between June 2020 and June 2021, when the original form of SARS-CoV-2, and later the Alpha variant, were circulating, with some overlap.
All patients had been hospitalized with COVID-19 and discharged 4-12 weeks prior to attending the outpatient post-COVID service. They were asked to complete a questionnaire on persistent symptoms at the median of 53 days after being discharged from the hospital. In addition, data on medical history, microbiological and clinical COVID-19 course, self-reported symptoms (at the point of the follow-up visit), and patient demographics were obtained from electronic medical records.
Newer variants being studied
Upon analysis of long COVID symptoms according to treatment given during the acute phase using multivariate analysis, increasing oxygen support (odds ratio, 1.4; 95% confidence interval, 1.1-1.8), use of immunosuppressant drugs (OR, 6.4; 95% CI, 1.5-28), and female sex (OR, 1.8; 95% CI, 1.1-2.9) were associated with a higher risk for long COVID symptoms, while patients with type 2 diabetes (OR, 0.4; 95% CI, 0.2-0.7) had a lower risk of developing long COVID symptoms.
When asked whether the increased anxiety and depression seen with the Alpha variant might be also linked to the fact that people are living through hard times, with lockdowns, economic difficulties, possible illness, and even fatalities among family and friends due to COVID, Dr. Spinicci pointed out that “it’s a preliminary study, and there are lots of factors that we didn’t explore. It’s difficult to arrive at definite conclusions about long COVID because so much remains unknown. There are lots of external and environmental factors in the general population that might contribute to these findings.”
Dr. Spinicci has continued to enroll patients from later periods of the pandemic, including patients who were infected with the Delta and Omicron variants of SARS-CoV-2.
“We’re interested in finding out if these other variants are also associated with different phenotypes of long COVID. This study is part of our follow-up program here in the hospital where lots of different specialties are following patients for 20 months,” he said.
Dr. Horberg noted that one criticism of this study is that it was unclear whether the researchers accounted for pre-existing conditions. “They note the co-morbidities in the table 1, but don’t say how they accounted for that in their analyses. We found a lot of what patients were calling ‘long COVID’ were exacerbations of co-morbidities but not a new condition.”
Dr. Spinicci and his coauthors acknowledged that the study was observational. And, as such, it does not prove cause and effect, and they could not confirm which variant of the virus caused the infection in different patients, which may limit the conclusions that can be drawn.
“Future research should focus on the potential impacts of variants of concern and vaccination status on ongoing symptoms,” Spinicci said.
Early release of an abstract will be presented at the European Congress of Clinical Microbiology & Infectious Diseases (ECCMID) 2022, in Lisbon, Portugal, April 23-26, 2022. Abstract 02768.
Dr. Spinicci and Dr. Horberg have disclosed no relevant financial relationships. Dr. Bogaert declared that she is on the program committee of ECCMID; she has been a member of SIGN/NICE COVID-19 rapid guideline: managing the long-term effects of COVID-19; and she is involved in multiple ongoing COVID-related studies, both acute and long-term sequela (funding MRC, CSO, ZonMw).
A version of this article first appeared on Medscape.com.
Long COVID symptoms may differ depending on which SARS-CoV-2 variant is behind a person’s infection, a new study shows.
The data from Italy compared long COVID symptoms reported by patients infected with SARS-CoV-2 from March to December 2020 (when the original, or “Wuhan,” variant was dominant) with those reported by patients infected from January to April 2021 (B.1.1.7-, or Alpha variant-dominant). It showed a substantial change in the pattern of neurological and cognitive/emotional problems – the latter mostly seen with the Alpha variant.
Infectious disease specialist Michele Spinicci, MD, from the University of Florence and Careggi University Hospital, Italy, led the work. “Many of the symptoms reported in this study have been measured [before], but this is the first time they have been linked to different COVID-19 variants,” he told this news organization. “Findings in patients with long COVID were focused on neurological and psychological difficulties.”
However, he pointed out that much remains to be understood about long COVID in terms of symptoms, diagnosis, and treatment.
“Long COVID is a huge area that involves many different fields of medicine, so there is not one single piece of advice to give on management. There’s lots to consider when evaluating a long COVID patient,” he said.
Results showed that when the Alpha variant was the dominant variant, the prevalence of myalgia (10%), dyspnea (42%), brain fog/mental confusion (17%), and anxiety/depression (13%) significantly increased relative to the wild-type (original, Wuhan) variant, while anosmia (2%), dysgeusia (4%), and impaired hearing (1%) were less common.
When the wild-type (original, Wuhan) variant was dominant, fatigue (37%), insomnia (16%), dysgeusia (11%), and impaired hearing (5%) were all more common than with the Alpha variant. Dyspnea (33%), brain fog (10%), myalgia (4%), and anxiety/depression (6%) were less common.
Overall, 76% of the patients in the trial reported at least one persistent symptom, while the most common reported symptoms were dyspnea (37%) and chronic fatigue (36%), followed by insomnia (16%), visual disorders (13%), and brain fog (13%).
The findings come from an early-release abstract that will be presented at the European Congress of Clinical Microbiology & Infectious Diseases (ECCMID) 2022, in Lisbon, Portugal, in a few weeks’ time.
‘The take-home point’
Michael A. Horberg, MD, associate medical director, Kaiser Permanente – Mid-Atlantic Permanente Medical Group, Rockville, Maryland, has recently presented data on symptoms seen with long COVID in over 28,000 people, as reported by this news organization, at the Conference on Retroviruses and Opportunistic Infections 2022. These people were infected with the wild-type virus.
Commenting on the study by Dr. Spinicci, he said: “The issue is that as we go along the COVID lifespan from acute to long COVID, what prompts patients to seek medical attention may change. If symptoms are not severe or were not well publicized previously, patients may not see the need to seek care or evaluation. As such, it doesn’t surprise me to find these changes over time, independent of any potential biological activity of the virus or its consequences.”
Dr. Horberg noted that their own study results are consistent with those of Dr. Spinicci et al. from March to December 2020 (original, Wuhan variant). “To me, the take-home point is long COVID is real, and physicians need to be on the lookout for it. However, not all symptoms are due to long COVID, and we need to keep the time course of symptoms during evaluation of such patients.”
Also providing comment on the findings was Debby Bogaert, MD, chair of Pediatric Medicine, University of Edinburgh. Reflecting on whether the symptoms were due to long COVID or another underlying disease, she said: “The number of patients with ongoing symptoms is very high, therefore [it is] unlikely that all of this is re-emergence of underlying or previous health problems. The type of symptoms reported are also as reported by other cohorts, so not unexpected. And irrespective of the root cause, they require care.”
Dr. Bogaert also noted that the data reiterate that COVID-19 is a new disease, and that “new variants might show shifting clinical pictures, not only regarding severity and symptoms of acute disease, but possibly also regarding sequela,” and that this, “underlines the importance of ongoing surveillance of variants, and ongoing evaluation of the acute and long-term clinical picture accompanying these, to ensure we adapt our public health approaches, clinical treatment plans, and long-term follow-up when and where needed.”
Dr. Bogaert stressed that only by keeping track of the changes in symptoms both acute and long-term – by patients and doctors – would the best patient care be provided.
“Patients need to know so they can report these back to their doctors, and doctors need to know over time that the picture of sequela might shift, so sequela are recognized early, and these patients receive the appropriate follow-up treatment,” she said. These shifting patterns might also apply to community patients as well as those hospitalized with COVID-19.
Study details
The retrospective, observational study included 428 patients, 59% men, with a mean age of 64 years, who had been treated at the Careggi University Hospital’s post-COVID outpatient service between June 2020 and June 2021, when the original form of SARS-CoV-2, and later the Alpha variant, were circulating, with some overlap.
All patients had been hospitalized with COVID-19 and discharged 4-12 weeks prior to attending the outpatient post-COVID service. They were asked to complete a questionnaire on persistent symptoms at the median of 53 days after being discharged from the hospital. In addition, data on medical history, microbiological and clinical COVID-19 course, self-reported symptoms (at the point of the follow-up visit), and patient demographics were obtained from electronic medical records.
Newer variants being studied
Upon analysis of long COVID symptoms according to treatment given during the acute phase using multivariate analysis, increasing oxygen support (odds ratio, 1.4; 95% confidence interval, 1.1-1.8), use of immunosuppressant drugs (OR, 6.4; 95% CI, 1.5-28), and female sex (OR, 1.8; 95% CI, 1.1-2.9) were associated with a higher risk for long COVID symptoms, while patients with type 2 diabetes (OR, 0.4; 95% CI, 0.2-0.7) had a lower risk of developing long COVID symptoms.
When asked whether the increased anxiety and depression seen with the Alpha variant might be also linked to the fact that people are living through hard times, with lockdowns, economic difficulties, possible illness, and even fatalities among family and friends due to COVID, Dr. Spinicci pointed out that “it’s a preliminary study, and there are lots of factors that we didn’t explore. It’s difficult to arrive at definite conclusions about long COVID because so much remains unknown. There are lots of external and environmental factors in the general population that might contribute to these findings.”
Dr. Spinicci has continued to enroll patients from later periods of the pandemic, including patients who were infected with the Delta and Omicron variants of SARS-CoV-2.
“We’re interested in finding out if these other variants are also associated with different phenotypes of long COVID. This study is part of our follow-up program here in the hospital where lots of different specialties are following patients for 20 months,” he said.
Dr. Horberg noted that one criticism of this study is that it was unclear whether the researchers accounted for pre-existing conditions. “They note the co-morbidities in the table 1, but don’t say how they accounted for that in their analyses. We found a lot of what patients were calling ‘long COVID’ were exacerbations of co-morbidities but not a new condition.”
Dr. Spinicci and his coauthors acknowledged that the study was observational. And, as such, it does not prove cause and effect, and they could not confirm which variant of the virus caused the infection in different patients, which may limit the conclusions that can be drawn.
“Future research should focus on the potential impacts of variants of concern and vaccination status on ongoing symptoms,” Spinicci said.
Early release of an abstract will be presented at the European Congress of Clinical Microbiology & Infectious Diseases (ECCMID) 2022, in Lisbon, Portugal, April 23-26, 2022. Abstract 02768.
Dr. Spinicci and Dr. Horberg have disclosed no relevant financial relationships. Dr. Bogaert declared that she is on the program committee of ECCMID; she has been a member of SIGN/NICE COVID-19 rapid guideline: managing the long-term effects of COVID-19; and she is involved in multiple ongoing COVID-related studies, both acute and long-term sequela (funding MRC, CSO, ZonMw).
A version of this article first appeared on Medscape.com.
Long COVID symptoms may differ depending on which SARS-CoV-2 variant is behind a person’s infection, a new study shows.
The data from Italy compared long COVID symptoms reported by patients infected with SARS-CoV-2 from March to December 2020 (when the original, or “Wuhan,” variant was dominant) with those reported by patients infected from January to April 2021 (B.1.1.7-, or Alpha variant-dominant). It showed a substantial change in the pattern of neurological and cognitive/emotional problems – the latter mostly seen with the Alpha variant.
Infectious disease specialist Michele Spinicci, MD, from the University of Florence and Careggi University Hospital, Italy, led the work. “Many of the symptoms reported in this study have been measured [before], but this is the first time they have been linked to different COVID-19 variants,” he told this news organization. “Findings in patients with long COVID were focused on neurological and psychological difficulties.”
However, he pointed out that much remains to be understood about long COVID in terms of symptoms, diagnosis, and treatment.
“Long COVID is a huge area that involves many different fields of medicine, so there is not one single piece of advice to give on management. There’s lots to consider when evaluating a long COVID patient,” he said.
Results showed that when the Alpha variant was the dominant variant, the prevalence of myalgia (10%), dyspnea (42%), brain fog/mental confusion (17%), and anxiety/depression (13%) significantly increased relative to the wild-type (original, Wuhan) variant, while anosmia (2%), dysgeusia (4%), and impaired hearing (1%) were less common.
When the wild-type (original, Wuhan) variant was dominant, fatigue (37%), insomnia (16%), dysgeusia (11%), and impaired hearing (5%) were all more common than with the Alpha variant. Dyspnea (33%), brain fog (10%), myalgia (4%), and anxiety/depression (6%) were less common.
Overall, 76% of the patients in the trial reported at least one persistent symptom, while the most common reported symptoms were dyspnea (37%) and chronic fatigue (36%), followed by insomnia (16%), visual disorders (13%), and brain fog (13%).
The findings come from an early-release abstract that will be presented at the European Congress of Clinical Microbiology & Infectious Diseases (ECCMID) 2022, in Lisbon, Portugal, in a few weeks’ time.
‘The take-home point’
Michael A. Horberg, MD, associate medical director, Kaiser Permanente – Mid-Atlantic Permanente Medical Group, Rockville, Maryland, has recently presented data on symptoms seen with long COVID in over 28,000 people, as reported by this news organization, at the Conference on Retroviruses and Opportunistic Infections 2022. These people were infected with the wild-type virus.
Commenting on the study by Dr. Spinicci, he said: “The issue is that as we go along the COVID lifespan from acute to long COVID, what prompts patients to seek medical attention may change. If symptoms are not severe or were not well publicized previously, patients may not see the need to seek care or evaluation. As such, it doesn’t surprise me to find these changes over time, independent of any potential biological activity of the virus or its consequences.”
Dr. Horberg noted that their own study results are consistent with those of Dr. Spinicci et al. from March to December 2020 (original, Wuhan variant). “To me, the take-home point is long COVID is real, and physicians need to be on the lookout for it. However, not all symptoms are due to long COVID, and we need to keep the time course of symptoms during evaluation of such patients.”
Also providing comment on the findings was Debby Bogaert, MD, chair of Pediatric Medicine, University of Edinburgh. Reflecting on whether the symptoms were due to long COVID or another underlying disease, she said: “The number of patients with ongoing symptoms is very high, therefore [it is] unlikely that all of this is re-emergence of underlying or previous health problems. The type of symptoms reported are also as reported by other cohorts, so not unexpected. And irrespective of the root cause, they require care.”
Dr. Bogaert also noted that the data reiterate that COVID-19 is a new disease, and that “new variants might show shifting clinical pictures, not only regarding severity and symptoms of acute disease, but possibly also regarding sequela,” and that this, “underlines the importance of ongoing surveillance of variants, and ongoing evaluation of the acute and long-term clinical picture accompanying these, to ensure we adapt our public health approaches, clinical treatment plans, and long-term follow-up when and where needed.”
Dr. Bogaert stressed that only by keeping track of the changes in symptoms both acute and long-term – by patients and doctors – would the best patient care be provided.
“Patients need to know so they can report these back to their doctors, and doctors need to know over time that the picture of sequela might shift, so sequela are recognized early, and these patients receive the appropriate follow-up treatment,” she said. These shifting patterns might also apply to community patients as well as those hospitalized with COVID-19.
Study details
The retrospective, observational study included 428 patients, 59% men, with a mean age of 64 years, who had been treated at the Careggi University Hospital’s post-COVID outpatient service between June 2020 and June 2021, when the original form of SARS-CoV-2, and later the Alpha variant, were circulating, with some overlap.
All patients had been hospitalized with COVID-19 and discharged 4-12 weeks prior to attending the outpatient post-COVID service. They were asked to complete a questionnaire on persistent symptoms at the median of 53 days after being discharged from the hospital. In addition, data on medical history, microbiological and clinical COVID-19 course, self-reported symptoms (at the point of the follow-up visit), and patient demographics were obtained from electronic medical records.
Newer variants being studied
Upon analysis of long COVID symptoms according to treatment given during the acute phase using multivariate analysis, increasing oxygen support (odds ratio, 1.4; 95% confidence interval, 1.1-1.8), use of immunosuppressant drugs (OR, 6.4; 95% CI, 1.5-28), and female sex (OR, 1.8; 95% CI, 1.1-2.9) were associated with a higher risk for long COVID symptoms, while patients with type 2 diabetes (OR, 0.4; 95% CI, 0.2-0.7) had a lower risk of developing long COVID symptoms.
When asked whether the increased anxiety and depression seen with the Alpha variant might be also linked to the fact that people are living through hard times, with lockdowns, economic difficulties, possible illness, and even fatalities among family and friends due to COVID, Dr. Spinicci pointed out that “it’s a preliminary study, and there are lots of factors that we didn’t explore. It’s difficult to arrive at definite conclusions about long COVID because so much remains unknown. There are lots of external and environmental factors in the general population that might contribute to these findings.”
Dr. Spinicci has continued to enroll patients from later periods of the pandemic, including patients who were infected with the Delta and Omicron variants of SARS-CoV-2.
“We’re interested in finding out if these other variants are also associated with different phenotypes of long COVID. This study is part of our follow-up program here in the hospital where lots of different specialties are following patients for 20 months,” he said.
Dr. Horberg noted that one criticism of this study is that it was unclear whether the researchers accounted for pre-existing conditions. “They note the co-morbidities in the table 1, but don’t say how they accounted for that in their analyses. We found a lot of what patients were calling ‘long COVID’ were exacerbations of co-morbidities but not a new condition.”
Dr. Spinicci and his coauthors acknowledged that the study was observational. And, as such, it does not prove cause and effect, and they could not confirm which variant of the virus caused the infection in different patients, which may limit the conclusions that can be drawn.
“Future research should focus on the potential impacts of variants of concern and vaccination status on ongoing symptoms,” Spinicci said.
Early release of an abstract will be presented at the European Congress of Clinical Microbiology & Infectious Diseases (ECCMID) 2022, in Lisbon, Portugal, April 23-26, 2022. Abstract 02768.
Dr. Spinicci and Dr. Horberg have disclosed no relevant financial relationships. Dr. Bogaert declared that she is on the program committee of ECCMID; she has been a member of SIGN/NICE COVID-19 rapid guideline: managing the long-term effects of COVID-19; and she is involved in multiple ongoing COVID-related studies, both acute and long-term sequela (funding MRC, CSO, ZonMw).
A version of this article first appeared on Medscape.com.
CROI 2022