User login
Testes may ‘serve as viral sanctuary’ for SARS-CoV-2, small study shows
, raising questions about potential consequences for reproductive health among those infected.
The study, published online Feb. 8 on the preprint server MedRxiv, found that “patients who become critically ill exhibit severe damages and may harbor the active virus in testes,” which can “serve as a viral sanctuary.”
Guilherme M.J. Costa, PhD, a professor at Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, led the study, which has not yet been peer-reviewed.
“A critical point of this article is that the virus was active in the patient’s testis after a long period of infection, indicating that the testis is able to maintain the viable virus for extended periods. It happens for many kinds of viruses in this genital organ,” Dr. Costa said in an interview.
Brian Keith McNeil, MD, vice-chair, department of urology at SUNY Downstate Health Sciences University in New York, told this news organization that the topic of COVID-19 and fertility has been discussed but data are sparse on the subject.
“The question this raises is whether or not COVID can live in the testes, and based on this it seems it can,” he said, adding that it also raises the question of whether COVID-19 could be transmitted through semen. “It leads one to wonder whether this could have a long-term impact on fertility in men and women.”
The authors wrote that deep testicular evaluation of patients who have been infected with COVID-19 is critical because the testes have one of the highest expressions of angiotensin converting enzyme 2 (ACE2) receptors, which play a large role in entrance of the virus into cells.
“A direct influence of SARS-CoV-2 in testicular cells might deregulate ACE2, elevating the levels of angiotensin II, a potent pro-inflammatory and angiogenic peptide,” the authors wrote.
Sperm-producing cells infected
In 2021, the researchers enrolled 11 male patients deceased from COVID-19 complications; none had received a vaccine. Infection was confirmed by SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR) performed during their hospital stay. All 11 patients were admitted to the intensive care unit with severe pulmonary symptoms.
All but one of the patients had children and none had scrotal symptoms or complaints during their time in the hospital. Their clinical histories revealed no testicular disorders.
Dr. Costa said they found that detecting SARS-CoV-2 mRNA in testes is difficult in a conventional RT-PCR test.
Therefore, “We modified the protocol of the RT-PCR and used nanosensors. We observed that SARS-CoV-2 has a huge tropism for the testes in this context,” he said.
He said the team performed stainings and “discovered that macrophages and germ cells are highly infected.”
That’s important, he said, because an immune cell, which is supposed to fight the virus, is infected in the tissue. Also, the germ cell, responsible for sperm production, is infected.
“This reopens the worries about the presence of SARS-CoV-2 in semen, as other authors mentioned,” he said.
New findings
The team also found that the testes are a good place for viral replication.
The authors say they are the first to show:
- The longer the severe condition, the lower the number of surviving germ cells.
- There was fluctuation in several essential testicular genes.
- The intratesticular testosterone levels are 30 times reduced in the testes of COVID-19 patients.
The control group was composed of six patients who had undergone testicle removal after prostate cancer was suspected. Collection of both testicles from the test group was performed within 3 hours of death after a family member signed an informed consent document.
Recent research on semen demonstrates that patients who recovered from COVID-19 reestablish their sperm quality after 3 months of infection.
That study, in Fertility and Sterility, found that sperm quality was initially reduced for months in some men after recovery from COVID-19.
The team studied semen samples from 120 Belgian men (mean age, 35 years) at an average 52 days after their last COVID-19 symptoms. The semen was not found to be infectious.
But among 35 men who provided samples within a month after infection, reductions in sperm motility were evident in 60% and sperm counts were reduced in 37%, according to the report.
Testicular damage
The results [of the Costa et al. paper] emphasize the importance of testicular damage in severe COVID-19,” Rafael Kroon Campos, PhD, a postdoctoral fellow in the department of microbiology & immunology at the University of Texas Medical Branch at Galveston, said in an interview.
He noted that other viruses have also been shown to infect or otherwise cause testicular damage or orchitis, such as Zika, Ebola, and the closely related SARS-CoV-1. Sexual transmission has been documented for Zika and Ebola viruses.
Dr. Campos said with SARS-CoV-2, it is unclear whether sexual transmission plays a role.
“Some reports found evidence of viral RNA in semen, but these were rare occurrences. The study by Costa and colleagues used a combination of sensitive techniques and they were able to detect a small amount of viral RNA and viral protein in the testicular tissue of the deceased patients, as well as show viral factories indicating replication of the virus by electron microscopy,” he said.
Dr. Campos said the findings are particularly important and concerning because of the large number of severe cases of COVID-19.
“It is critical to continue to investigate the impact of the disease in testes, including the impact of different variants of concern on testicular damage,” he said.
Dr. McNeil and Dr. Campos have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, raising questions about potential consequences for reproductive health among those infected.
The study, published online Feb. 8 on the preprint server MedRxiv, found that “patients who become critically ill exhibit severe damages and may harbor the active virus in testes,” which can “serve as a viral sanctuary.”
Guilherme M.J. Costa, PhD, a professor at Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, led the study, which has not yet been peer-reviewed.
“A critical point of this article is that the virus was active in the patient’s testis after a long period of infection, indicating that the testis is able to maintain the viable virus for extended periods. It happens for many kinds of viruses in this genital organ,” Dr. Costa said in an interview.
Brian Keith McNeil, MD, vice-chair, department of urology at SUNY Downstate Health Sciences University in New York, told this news organization that the topic of COVID-19 and fertility has been discussed but data are sparse on the subject.
“The question this raises is whether or not COVID can live in the testes, and based on this it seems it can,” he said, adding that it also raises the question of whether COVID-19 could be transmitted through semen. “It leads one to wonder whether this could have a long-term impact on fertility in men and women.”
The authors wrote that deep testicular evaluation of patients who have been infected with COVID-19 is critical because the testes have one of the highest expressions of angiotensin converting enzyme 2 (ACE2) receptors, which play a large role in entrance of the virus into cells.
“A direct influence of SARS-CoV-2 in testicular cells might deregulate ACE2, elevating the levels of angiotensin II, a potent pro-inflammatory and angiogenic peptide,” the authors wrote.
Sperm-producing cells infected
In 2021, the researchers enrolled 11 male patients deceased from COVID-19 complications; none had received a vaccine. Infection was confirmed by SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR) performed during their hospital stay. All 11 patients were admitted to the intensive care unit with severe pulmonary symptoms.
All but one of the patients had children and none had scrotal symptoms or complaints during their time in the hospital. Their clinical histories revealed no testicular disorders.
Dr. Costa said they found that detecting SARS-CoV-2 mRNA in testes is difficult in a conventional RT-PCR test.
Therefore, “We modified the protocol of the RT-PCR and used nanosensors. We observed that SARS-CoV-2 has a huge tropism for the testes in this context,” he said.
He said the team performed stainings and “discovered that macrophages and germ cells are highly infected.”
That’s important, he said, because an immune cell, which is supposed to fight the virus, is infected in the tissue. Also, the germ cell, responsible for sperm production, is infected.
“This reopens the worries about the presence of SARS-CoV-2 in semen, as other authors mentioned,” he said.
New findings
The team also found that the testes are a good place for viral replication.
The authors say they are the first to show:
- The longer the severe condition, the lower the number of surviving germ cells.
- There was fluctuation in several essential testicular genes.
- The intratesticular testosterone levels are 30 times reduced in the testes of COVID-19 patients.
The control group was composed of six patients who had undergone testicle removal after prostate cancer was suspected. Collection of both testicles from the test group was performed within 3 hours of death after a family member signed an informed consent document.
Recent research on semen demonstrates that patients who recovered from COVID-19 reestablish their sperm quality after 3 months of infection.
That study, in Fertility and Sterility, found that sperm quality was initially reduced for months in some men after recovery from COVID-19.
The team studied semen samples from 120 Belgian men (mean age, 35 years) at an average 52 days after their last COVID-19 symptoms. The semen was not found to be infectious.
But among 35 men who provided samples within a month after infection, reductions in sperm motility were evident in 60% and sperm counts were reduced in 37%, according to the report.
Testicular damage
The results [of the Costa et al. paper] emphasize the importance of testicular damage in severe COVID-19,” Rafael Kroon Campos, PhD, a postdoctoral fellow in the department of microbiology & immunology at the University of Texas Medical Branch at Galveston, said in an interview.
He noted that other viruses have also been shown to infect or otherwise cause testicular damage or orchitis, such as Zika, Ebola, and the closely related SARS-CoV-1. Sexual transmission has been documented for Zika and Ebola viruses.
Dr. Campos said with SARS-CoV-2, it is unclear whether sexual transmission plays a role.
“Some reports found evidence of viral RNA in semen, but these were rare occurrences. The study by Costa and colleagues used a combination of sensitive techniques and they were able to detect a small amount of viral RNA and viral protein in the testicular tissue of the deceased patients, as well as show viral factories indicating replication of the virus by electron microscopy,” he said.
Dr. Campos said the findings are particularly important and concerning because of the large number of severe cases of COVID-19.
“It is critical to continue to investigate the impact of the disease in testes, including the impact of different variants of concern on testicular damage,” he said.
Dr. McNeil and Dr. Campos have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, raising questions about potential consequences for reproductive health among those infected.
The study, published online Feb. 8 on the preprint server MedRxiv, found that “patients who become critically ill exhibit severe damages and may harbor the active virus in testes,” which can “serve as a viral sanctuary.”
Guilherme M.J. Costa, PhD, a professor at Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, led the study, which has not yet been peer-reviewed.
“A critical point of this article is that the virus was active in the patient’s testis after a long period of infection, indicating that the testis is able to maintain the viable virus for extended periods. It happens for many kinds of viruses in this genital organ,” Dr. Costa said in an interview.
Brian Keith McNeil, MD, vice-chair, department of urology at SUNY Downstate Health Sciences University in New York, told this news organization that the topic of COVID-19 and fertility has been discussed but data are sparse on the subject.
“The question this raises is whether or not COVID can live in the testes, and based on this it seems it can,” he said, adding that it also raises the question of whether COVID-19 could be transmitted through semen. “It leads one to wonder whether this could have a long-term impact on fertility in men and women.”
The authors wrote that deep testicular evaluation of patients who have been infected with COVID-19 is critical because the testes have one of the highest expressions of angiotensin converting enzyme 2 (ACE2) receptors, which play a large role in entrance of the virus into cells.
“A direct influence of SARS-CoV-2 in testicular cells might deregulate ACE2, elevating the levels of angiotensin II, a potent pro-inflammatory and angiogenic peptide,” the authors wrote.
Sperm-producing cells infected
In 2021, the researchers enrolled 11 male patients deceased from COVID-19 complications; none had received a vaccine. Infection was confirmed by SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR) performed during their hospital stay. All 11 patients were admitted to the intensive care unit with severe pulmonary symptoms.
All but one of the patients had children and none had scrotal symptoms or complaints during their time in the hospital. Their clinical histories revealed no testicular disorders.
Dr. Costa said they found that detecting SARS-CoV-2 mRNA in testes is difficult in a conventional RT-PCR test.
Therefore, “We modified the protocol of the RT-PCR and used nanosensors. We observed that SARS-CoV-2 has a huge tropism for the testes in this context,” he said.
He said the team performed stainings and “discovered that macrophages and germ cells are highly infected.”
That’s important, he said, because an immune cell, which is supposed to fight the virus, is infected in the tissue. Also, the germ cell, responsible for sperm production, is infected.
“This reopens the worries about the presence of SARS-CoV-2 in semen, as other authors mentioned,” he said.
New findings
The team also found that the testes are a good place for viral replication.
The authors say they are the first to show:
- The longer the severe condition, the lower the number of surviving germ cells.
- There was fluctuation in several essential testicular genes.
- The intratesticular testosterone levels are 30 times reduced in the testes of COVID-19 patients.
The control group was composed of six patients who had undergone testicle removal after prostate cancer was suspected. Collection of both testicles from the test group was performed within 3 hours of death after a family member signed an informed consent document.
Recent research on semen demonstrates that patients who recovered from COVID-19 reestablish their sperm quality after 3 months of infection.
That study, in Fertility and Sterility, found that sperm quality was initially reduced for months in some men after recovery from COVID-19.
The team studied semen samples from 120 Belgian men (mean age, 35 years) at an average 52 days after their last COVID-19 symptoms. The semen was not found to be infectious.
But among 35 men who provided samples within a month after infection, reductions in sperm motility were evident in 60% and sperm counts were reduced in 37%, according to the report.
Testicular damage
The results [of the Costa et al. paper] emphasize the importance of testicular damage in severe COVID-19,” Rafael Kroon Campos, PhD, a postdoctoral fellow in the department of microbiology & immunology at the University of Texas Medical Branch at Galveston, said in an interview.
He noted that other viruses have also been shown to infect or otherwise cause testicular damage or orchitis, such as Zika, Ebola, and the closely related SARS-CoV-1. Sexual transmission has been documented for Zika and Ebola viruses.
Dr. Campos said with SARS-CoV-2, it is unclear whether sexual transmission plays a role.
“Some reports found evidence of viral RNA in semen, but these were rare occurrences. The study by Costa and colleagues used a combination of sensitive techniques and they were able to detect a small amount of viral RNA and viral protein in the testicular tissue of the deceased patients, as well as show viral factories indicating replication of the virus by electron microscopy,” he said.
Dr. Campos said the findings are particularly important and concerning because of the large number of severe cases of COVID-19.
“It is critical to continue to investigate the impact of the disease in testes, including the impact of different variants of concern on testicular damage,” he said.
Dr. McNeil and Dr. Campos have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MEDRXIV
Perinatal deaths from COVID show ‘extensive’ placental damage
Recent evidence has shown that women who contract COVID-19 during pregnancy are at increased risk for pregnancy loss and neonatal death. Now, an analysis of pathology data from dozens of perinatal deaths shows how.
Unlike numerous pathogens that kill the fetus by infecting it directly, SARS-CoV-2 causes “widespread and severe” destruction of the placenta that deprives the fetus of oxygen, a team of 44 researchers in 12 countries concluded after examining 64 stillbirths and four neonatal deaths in which the placentas were infected with the virus. They noted that such damage occurs in a small percentage of pregnant women with COVID and that all the women in the study had not been vaccinated against the disease.
The findings were published online Feb. 10 in the Archives of Pathology & Laboratory Medicine.
Nearly all placentas had each of three features that pathologists have dubbed SARS-CoV-2 placentitis: large deposits of fibrin, a clotting protein that obstructs the flow of blood, death of cells in the trophoblast, and an unusual form of inflammation called chronic histiocytic intervillositis. Some had other abnormalities that could have exacerbated the condition.
The researchers called the extent of damage “striking,” affecting 77.7% of the placenta on average. The virus did not appear to harm fetal tissue, but placental damage “was extensive and highly destructive,” they write. Notably, none of the women in the analysis were known to have severe COVID.
Virus seen ‘chewing up the placenta’
David Schwartz, MD, a pathologist in Atlanta, and the lead author of the study, said COVID appears to be unique in destroying the placenta.
“I don’t know of any infection that does that to this degree or with this uniformity,” Dr. Schwartz told this news organization. “The simple message is that this infection is chewing up the placenta and destroying its capability to oxygenate the fetus.”
In November, the Centers for Disease Control and Prevention reported that maternal COVID increases the risk of losing a pregnancy. From March 2020 to September 2021, 8,154 stillbirths were reported, affecting 0.65% of births by women without COVID and 1.26% of births by women with COVID, for a relative risk of 1.90 (95% confidence interval, 1.69-2.15).
Delta, the variant that dominated in mid-2021, appears to have been particularly harmful. The CDC reported that the relative risk for stillbirth for mothers with COVID-19 during that period increased to 4.04 (95% CI, 3.28-4.97). Many cases in the new analysis coincided with Delta.
Dr. Schwartz and his colleagues said immunization, along with antiviral therapy, might reduce the chance of SARS-CoV-2 infecting the placenta. None of the mothers in the analysis was vaccinated, and Dr. Schwartz said he is not aware of a single case in a vaccinated woman.
The analysis comes on the heels of a study from the National Institutes of Health linking severe to moderate COVID infection to greater risk of other pregnancy complications: cesarean and preterm delivery, death during childbirth, postpartum hemorrhaging, and non-COVID infections.
Diana Bianchi, MD, director of NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development, said those findings underscore the need for pregnant women to be vaccinated. (The shots have been shown to be safe for pregnant women.)
Denise Jamieson, MD, MPH, chair of the department of gynecology and obstetrics at Emory University, Atlanta, who was not involved in the new analysis, said the findings may have important clinical implications. In addition to ensuring that pregnant patients are fully vaccinated, she said “there may be opportunities to more closely monitor the placenta during pregnancy using imaging modalities such as ultrasound.”
Even in the presence of severe abnormalities, a fetus that has reached a viable gestational age could potentially be delivered prior to stillbirth, Dr. Jamieson said. The 64 stillbirths in the analysis ranged from 15 to 39.2 weeks of gestation, with an average of 30 weeks. Eight were delivered at full term.
However, additional studies are needed to support monitoring of placental changes, she said: “It is not ready for prime time now.”
Christopher Zahn, MD, vice president of practice activities the American College of Obstetricians and Gynecologists, cautioned that data on COVID and pregnancy complications remain limited.
The findings in this analysis “do not prove the association between COVID-19 infection and neonatal outcomes,” Dr. Zahn said. “While stillbirth could potentially be another adverse outcome for pregnant people who contract COVID-19, currently we don’t have enough data to confirm that a COVID-19 infection at any point in pregnancy indicates increased risk of stillbirth.”
He added that ACOG continues to strongly recommend vaccination against COVID for women who are pregnant, recently pregnant, or planning to be pregnant.
Dr. Schwartz and Dr. Jamieson have disclosed no relevant financial relationships. One author reported receiving financial support from the Slovak Research and Development Agency. Another reported funding from the Belgian Fund for Scientific Research and the Fetus for Life charity.
A version of this article first appeared on Medscape.com.
Recent evidence has shown that women who contract COVID-19 during pregnancy are at increased risk for pregnancy loss and neonatal death. Now, an analysis of pathology data from dozens of perinatal deaths shows how.
Unlike numerous pathogens that kill the fetus by infecting it directly, SARS-CoV-2 causes “widespread and severe” destruction of the placenta that deprives the fetus of oxygen, a team of 44 researchers in 12 countries concluded after examining 64 stillbirths and four neonatal deaths in which the placentas were infected with the virus. They noted that such damage occurs in a small percentage of pregnant women with COVID and that all the women in the study had not been vaccinated against the disease.
The findings were published online Feb. 10 in the Archives of Pathology & Laboratory Medicine.
Nearly all placentas had each of three features that pathologists have dubbed SARS-CoV-2 placentitis: large deposits of fibrin, a clotting protein that obstructs the flow of blood, death of cells in the trophoblast, and an unusual form of inflammation called chronic histiocytic intervillositis. Some had other abnormalities that could have exacerbated the condition.
The researchers called the extent of damage “striking,” affecting 77.7% of the placenta on average. The virus did not appear to harm fetal tissue, but placental damage “was extensive and highly destructive,” they write. Notably, none of the women in the analysis were known to have severe COVID.
Virus seen ‘chewing up the placenta’
David Schwartz, MD, a pathologist in Atlanta, and the lead author of the study, said COVID appears to be unique in destroying the placenta.
“I don’t know of any infection that does that to this degree or with this uniformity,” Dr. Schwartz told this news organization. “The simple message is that this infection is chewing up the placenta and destroying its capability to oxygenate the fetus.”
In November, the Centers for Disease Control and Prevention reported that maternal COVID increases the risk of losing a pregnancy. From March 2020 to September 2021, 8,154 stillbirths were reported, affecting 0.65% of births by women without COVID and 1.26% of births by women with COVID, for a relative risk of 1.90 (95% confidence interval, 1.69-2.15).
Delta, the variant that dominated in mid-2021, appears to have been particularly harmful. The CDC reported that the relative risk for stillbirth for mothers with COVID-19 during that period increased to 4.04 (95% CI, 3.28-4.97). Many cases in the new analysis coincided with Delta.
Dr. Schwartz and his colleagues said immunization, along with antiviral therapy, might reduce the chance of SARS-CoV-2 infecting the placenta. None of the mothers in the analysis was vaccinated, and Dr. Schwartz said he is not aware of a single case in a vaccinated woman.
The analysis comes on the heels of a study from the National Institutes of Health linking severe to moderate COVID infection to greater risk of other pregnancy complications: cesarean and preterm delivery, death during childbirth, postpartum hemorrhaging, and non-COVID infections.
Diana Bianchi, MD, director of NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development, said those findings underscore the need for pregnant women to be vaccinated. (The shots have been shown to be safe for pregnant women.)
Denise Jamieson, MD, MPH, chair of the department of gynecology and obstetrics at Emory University, Atlanta, who was not involved in the new analysis, said the findings may have important clinical implications. In addition to ensuring that pregnant patients are fully vaccinated, she said “there may be opportunities to more closely monitor the placenta during pregnancy using imaging modalities such as ultrasound.”
Even in the presence of severe abnormalities, a fetus that has reached a viable gestational age could potentially be delivered prior to stillbirth, Dr. Jamieson said. The 64 stillbirths in the analysis ranged from 15 to 39.2 weeks of gestation, with an average of 30 weeks. Eight were delivered at full term.
However, additional studies are needed to support monitoring of placental changes, she said: “It is not ready for prime time now.”
Christopher Zahn, MD, vice president of practice activities the American College of Obstetricians and Gynecologists, cautioned that data on COVID and pregnancy complications remain limited.
The findings in this analysis “do not prove the association between COVID-19 infection and neonatal outcomes,” Dr. Zahn said. “While stillbirth could potentially be another adverse outcome for pregnant people who contract COVID-19, currently we don’t have enough data to confirm that a COVID-19 infection at any point in pregnancy indicates increased risk of stillbirth.”
He added that ACOG continues to strongly recommend vaccination against COVID for women who are pregnant, recently pregnant, or planning to be pregnant.
Dr. Schwartz and Dr. Jamieson have disclosed no relevant financial relationships. One author reported receiving financial support from the Slovak Research and Development Agency. Another reported funding from the Belgian Fund for Scientific Research and the Fetus for Life charity.
A version of this article first appeared on Medscape.com.
Recent evidence has shown that women who contract COVID-19 during pregnancy are at increased risk for pregnancy loss and neonatal death. Now, an analysis of pathology data from dozens of perinatal deaths shows how.
Unlike numerous pathogens that kill the fetus by infecting it directly, SARS-CoV-2 causes “widespread and severe” destruction of the placenta that deprives the fetus of oxygen, a team of 44 researchers in 12 countries concluded after examining 64 stillbirths and four neonatal deaths in which the placentas were infected with the virus. They noted that such damage occurs in a small percentage of pregnant women with COVID and that all the women in the study had not been vaccinated against the disease.
The findings were published online Feb. 10 in the Archives of Pathology & Laboratory Medicine.
Nearly all placentas had each of three features that pathologists have dubbed SARS-CoV-2 placentitis: large deposits of fibrin, a clotting protein that obstructs the flow of blood, death of cells in the trophoblast, and an unusual form of inflammation called chronic histiocytic intervillositis. Some had other abnormalities that could have exacerbated the condition.
The researchers called the extent of damage “striking,” affecting 77.7% of the placenta on average. The virus did not appear to harm fetal tissue, but placental damage “was extensive and highly destructive,” they write. Notably, none of the women in the analysis were known to have severe COVID.
Virus seen ‘chewing up the placenta’
David Schwartz, MD, a pathologist in Atlanta, and the lead author of the study, said COVID appears to be unique in destroying the placenta.
“I don’t know of any infection that does that to this degree or with this uniformity,” Dr. Schwartz told this news organization. “The simple message is that this infection is chewing up the placenta and destroying its capability to oxygenate the fetus.”
In November, the Centers for Disease Control and Prevention reported that maternal COVID increases the risk of losing a pregnancy. From March 2020 to September 2021, 8,154 stillbirths were reported, affecting 0.65% of births by women without COVID and 1.26% of births by women with COVID, for a relative risk of 1.90 (95% confidence interval, 1.69-2.15).
Delta, the variant that dominated in mid-2021, appears to have been particularly harmful. The CDC reported that the relative risk for stillbirth for mothers with COVID-19 during that period increased to 4.04 (95% CI, 3.28-4.97). Many cases in the new analysis coincided with Delta.
Dr. Schwartz and his colleagues said immunization, along with antiviral therapy, might reduce the chance of SARS-CoV-2 infecting the placenta. None of the mothers in the analysis was vaccinated, and Dr. Schwartz said he is not aware of a single case in a vaccinated woman.
The analysis comes on the heels of a study from the National Institutes of Health linking severe to moderate COVID infection to greater risk of other pregnancy complications: cesarean and preterm delivery, death during childbirth, postpartum hemorrhaging, and non-COVID infections.
Diana Bianchi, MD, director of NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development, said those findings underscore the need for pregnant women to be vaccinated. (The shots have been shown to be safe for pregnant women.)
Denise Jamieson, MD, MPH, chair of the department of gynecology and obstetrics at Emory University, Atlanta, who was not involved in the new analysis, said the findings may have important clinical implications. In addition to ensuring that pregnant patients are fully vaccinated, she said “there may be opportunities to more closely monitor the placenta during pregnancy using imaging modalities such as ultrasound.”
Even in the presence of severe abnormalities, a fetus that has reached a viable gestational age could potentially be delivered prior to stillbirth, Dr. Jamieson said. The 64 stillbirths in the analysis ranged from 15 to 39.2 weeks of gestation, with an average of 30 weeks. Eight were delivered at full term.
However, additional studies are needed to support monitoring of placental changes, she said: “It is not ready for prime time now.”
Christopher Zahn, MD, vice president of practice activities the American College of Obstetricians and Gynecologists, cautioned that data on COVID and pregnancy complications remain limited.
The findings in this analysis “do not prove the association between COVID-19 infection and neonatal outcomes,” Dr. Zahn said. “While stillbirth could potentially be another adverse outcome for pregnant people who contract COVID-19, currently we don’t have enough data to confirm that a COVID-19 infection at any point in pregnancy indicates increased risk of stillbirth.”
He added that ACOG continues to strongly recommend vaccination against COVID for women who are pregnant, recently pregnant, or planning to be pregnant.
Dr. Schwartz and Dr. Jamieson have disclosed no relevant financial relationships. One author reported receiving financial support from the Slovak Research and Development Agency. Another reported funding from the Belgian Fund for Scientific Research and the Fetus for Life charity.
A version of this article first appeared on Medscape.com.
‘Substantial’ CVD risks, burden up to a year after COVID-19
People who have had COVID-19 have an increased risk for, and 12-month burden of, cardiovascular disease (CVD) that is substantial and spans an array of cardiovascular disorders, a deep dive into federal data suggests.
“I went into this thinking that this is most likely happening in people to start with who have a higher risk of cardiovascular disorders, smokers, people with high BMI, diabetes, but what we found is something different,” Ziyad Al-Aly, MD, said in an interview. “It’s evident in people at high risk, but it was also as clear as the sun even in people who have no cardiovascular risk whatsoever.”
Rates were increased in younger adults, never smokers, White and Black people, and males and females, he said. “So the risk confirmed by the SARS-CoV-2 virus seems to spare almost no one.”
Although cardiovascular outcomes increased with the severity of the acute infection, the excess risks and burdens were also evident in those who never required hospitalization, a group that represents the majority of people with COVID-19, observed Dr. Al-Aly, who directs the Clinical Epidemiology Center at the Veterans Affairs St. Louis Health Care System.
“This study is very important because it underscores not just the acute cardiovascular risk associated with COVID but the increased risk of chronic cardiovascular outcomes as well,” cardiologist C. Michael Gibson, MD, professor of medicine, Harvard Medical School, Boston, said in an interview. “Given the number of patients in the U.S. who have been infected with COVID, this could represent a significant chronic burden on the health care system, particularly as health care professionals leave the profession.”
For the study, the investigators used national VA databases to build a cohort of 153,760 veterans who were alive 30 days after testing positive for COVID-19 between March 1, 2020, and January 2021. They were compared with a contemporary cohort of 5.6 million veterans with no evidence of SARS-CoV-2 infection and a historical cohort of 5.8 million veterans using the system in 2017 prior to the pandemic. Median follow-up was 347, 348, and 347 days, respectively.
As reported in Nature Medicine, the risk for a major adverse cardiovascular event, a composite of myocardial infarction, stroke, and all-cause mortality, was 4% higher in people who had been infected with COVID-19 than in those who had not.
“People say 4% is small, but actually it’s really, really big if you think about it in the context of the huge number of people who have had COVID-19 in the United States, and also globally,” Dr. Al-Aly said.
Compared with the contemporary control group, people who had COVID-19 had an increased risk (hazard ratio [HR]) and burden per 1,000 people at 1 year for the following cardiovascular outcomes:
- Stroke: HR, 1.52; burden, 4.03
- Transient ischemic attack: HR, 1.49; burden, 1.84
- Dysrhythmias: HR, 1.69; burden, 19.86
- Ischemic heart disease: HR, 1.66; burden, 7.28
- Heart failure: HR, 1.72; burden, 11.61
- Nonischemic cardiomyopathy: HR, 1.62; burden 3.56
- Pulmonary embolism: HR, 2.93; burden, 5.47
- Deep vein thrombosis: HR, 2.09; burden, 4.18
- Pericarditis: HR, 1.85, burden, 0.98
- Myocarditis: HR, 5.38; burden, 0.31
Recent reports have raised concerns about an association between COVID-19 vaccines and myocarditis and pericarditis, particularly in young males. Although very few of the participants were vaccinated prior to becoming infected, as vaccines were not yet widely available, the researchers performed two analyses censoring participants at the time of the first dose of any COVID-19 vaccine and adjusting for vaccination as a time-varying covariate.
The absolute numbers of myocarditis and pericarditis were still higher than the contemporary and historical cohorts. These numbers are much larger than those reported for myocarditis after vaccines, which are generally around 40 cases per 1 million people, observed Dr. Al-Aly.
The overall results were also consistent when compared with the historical control subjects.
“What we’re seeing in our report and others is that SARS-CoV-2 can leave a sort of scar or imprint on people, and some of these conditions are likely chronic conditions,” Dr. Al-Aly said. “So you’re going to have a generation of people who will bear the scar of COVID for their lifetime and I think that requires recognition and attention, so we’re aware of the magnitude of the problem and prepared to deal with it.”
With more than 76 million COVID-19 cases in the United States, that effort will likely have to be at the federal level, similar to President Joe Biden’s recent relaunch of the “Cancer Moonshot,” he added. “We need a greater and broader recognition at the federal level to try and recognize that when you have an earthquake, you don’t just deal with the earthquake when the earth is shaking, but you also need to deal with the aftermath.”
Dr. Gibson pointed out that this was a study of predominantly males and, thus, it’s unclear if the results can be extended to females. Nevertheless, he added, “long COVID may include outcomes beyond the central nervous system and we should educate patients about the risk of late cardiovascular outcomes.”
The authors noted the largely White, male cohort may limit generalizability of the findings. Other limitations include the possibility that some people may have had COVID-19 but were not tested, the datasets lacked information on cause of death, and possible residual confounding not accounted for in the adjusted analyses.
The research was funded by the U.S. Department of Veterans Affairs and two American Society of Nephrology and Kidney Cure fellowship awards. The authors declared no competing interests. Dr. Gibson reports having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
People who have had COVID-19 have an increased risk for, and 12-month burden of, cardiovascular disease (CVD) that is substantial and spans an array of cardiovascular disorders, a deep dive into federal data suggests.
“I went into this thinking that this is most likely happening in people to start with who have a higher risk of cardiovascular disorders, smokers, people with high BMI, diabetes, but what we found is something different,” Ziyad Al-Aly, MD, said in an interview. “It’s evident in people at high risk, but it was also as clear as the sun even in people who have no cardiovascular risk whatsoever.”
Rates were increased in younger adults, never smokers, White and Black people, and males and females, he said. “So the risk confirmed by the SARS-CoV-2 virus seems to spare almost no one.”
Although cardiovascular outcomes increased with the severity of the acute infection, the excess risks and burdens were also evident in those who never required hospitalization, a group that represents the majority of people with COVID-19, observed Dr. Al-Aly, who directs the Clinical Epidemiology Center at the Veterans Affairs St. Louis Health Care System.
“This study is very important because it underscores not just the acute cardiovascular risk associated with COVID but the increased risk of chronic cardiovascular outcomes as well,” cardiologist C. Michael Gibson, MD, professor of medicine, Harvard Medical School, Boston, said in an interview. “Given the number of patients in the U.S. who have been infected with COVID, this could represent a significant chronic burden on the health care system, particularly as health care professionals leave the profession.”
For the study, the investigators used national VA databases to build a cohort of 153,760 veterans who were alive 30 days after testing positive for COVID-19 between March 1, 2020, and January 2021. They were compared with a contemporary cohort of 5.6 million veterans with no evidence of SARS-CoV-2 infection and a historical cohort of 5.8 million veterans using the system in 2017 prior to the pandemic. Median follow-up was 347, 348, and 347 days, respectively.
As reported in Nature Medicine, the risk for a major adverse cardiovascular event, a composite of myocardial infarction, stroke, and all-cause mortality, was 4% higher in people who had been infected with COVID-19 than in those who had not.
“People say 4% is small, but actually it’s really, really big if you think about it in the context of the huge number of people who have had COVID-19 in the United States, and also globally,” Dr. Al-Aly said.
Compared with the contemporary control group, people who had COVID-19 had an increased risk (hazard ratio [HR]) and burden per 1,000 people at 1 year for the following cardiovascular outcomes:
- Stroke: HR, 1.52; burden, 4.03
- Transient ischemic attack: HR, 1.49; burden, 1.84
- Dysrhythmias: HR, 1.69; burden, 19.86
- Ischemic heart disease: HR, 1.66; burden, 7.28
- Heart failure: HR, 1.72; burden, 11.61
- Nonischemic cardiomyopathy: HR, 1.62; burden 3.56
- Pulmonary embolism: HR, 2.93; burden, 5.47
- Deep vein thrombosis: HR, 2.09; burden, 4.18
- Pericarditis: HR, 1.85, burden, 0.98
- Myocarditis: HR, 5.38; burden, 0.31
Recent reports have raised concerns about an association between COVID-19 vaccines and myocarditis and pericarditis, particularly in young males. Although very few of the participants were vaccinated prior to becoming infected, as vaccines were not yet widely available, the researchers performed two analyses censoring participants at the time of the first dose of any COVID-19 vaccine and adjusting for vaccination as a time-varying covariate.
The absolute numbers of myocarditis and pericarditis were still higher than the contemporary and historical cohorts. These numbers are much larger than those reported for myocarditis after vaccines, which are generally around 40 cases per 1 million people, observed Dr. Al-Aly.
The overall results were also consistent when compared with the historical control subjects.
“What we’re seeing in our report and others is that SARS-CoV-2 can leave a sort of scar or imprint on people, and some of these conditions are likely chronic conditions,” Dr. Al-Aly said. “So you’re going to have a generation of people who will bear the scar of COVID for their lifetime and I think that requires recognition and attention, so we’re aware of the magnitude of the problem and prepared to deal with it.”
With more than 76 million COVID-19 cases in the United States, that effort will likely have to be at the federal level, similar to President Joe Biden’s recent relaunch of the “Cancer Moonshot,” he added. “We need a greater and broader recognition at the federal level to try and recognize that when you have an earthquake, you don’t just deal with the earthquake when the earth is shaking, but you also need to deal with the aftermath.”
Dr. Gibson pointed out that this was a study of predominantly males and, thus, it’s unclear if the results can be extended to females. Nevertheless, he added, “long COVID may include outcomes beyond the central nervous system and we should educate patients about the risk of late cardiovascular outcomes.”
The authors noted the largely White, male cohort may limit generalizability of the findings. Other limitations include the possibility that some people may have had COVID-19 but were not tested, the datasets lacked information on cause of death, and possible residual confounding not accounted for in the adjusted analyses.
The research was funded by the U.S. Department of Veterans Affairs and two American Society of Nephrology and Kidney Cure fellowship awards. The authors declared no competing interests. Dr. Gibson reports having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
People who have had COVID-19 have an increased risk for, and 12-month burden of, cardiovascular disease (CVD) that is substantial and spans an array of cardiovascular disorders, a deep dive into federal data suggests.
“I went into this thinking that this is most likely happening in people to start with who have a higher risk of cardiovascular disorders, smokers, people with high BMI, diabetes, but what we found is something different,” Ziyad Al-Aly, MD, said in an interview. “It’s evident in people at high risk, but it was also as clear as the sun even in people who have no cardiovascular risk whatsoever.”
Rates were increased in younger adults, never smokers, White and Black people, and males and females, he said. “So the risk confirmed by the SARS-CoV-2 virus seems to spare almost no one.”
Although cardiovascular outcomes increased with the severity of the acute infection, the excess risks and burdens were also evident in those who never required hospitalization, a group that represents the majority of people with COVID-19, observed Dr. Al-Aly, who directs the Clinical Epidemiology Center at the Veterans Affairs St. Louis Health Care System.
“This study is very important because it underscores not just the acute cardiovascular risk associated with COVID but the increased risk of chronic cardiovascular outcomes as well,” cardiologist C. Michael Gibson, MD, professor of medicine, Harvard Medical School, Boston, said in an interview. “Given the number of patients in the U.S. who have been infected with COVID, this could represent a significant chronic burden on the health care system, particularly as health care professionals leave the profession.”
For the study, the investigators used national VA databases to build a cohort of 153,760 veterans who were alive 30 days after testing positive for COVID-19 between March 1, 2020, and January 2021. They were compared with a contemporary cohort of 5.6 million veterans with no evidence of SARS-CoV-2 infection and a historical cohort of 5.8 million veterans using the system in 2017 prior to the pandemic. Median follow-up was 347, 348, and 347 days, respectively.
As reported in Nature Medicine, the risk for a major adverse cardiovascular event, a composite of myocardial infarction, stroke, and all-cause mortality, was 4% higher in people who had been infected with COVID-19 than in those who had not.
“People say 4% is small, but actually it’s really, really big if you think about it in the context of the huge number of people who have had COVID-19 in the United States, and also globally,” Dr. Al-Aly said.
Compared with the contemporary control group, people who had COVID-19 had an increased risk (hazard ratio [HR]) and burden per 1,000 people at 1 year for the following cardiovascular outcomes:
- Stroke: HR, 1.52; burden, 4.03
- Transient ischemic attack: HR, 1.49; burden, 1.84
- Dysrhythmias: HR, 1.69; burden, 19.86
- Ischemic heart disease: HR, 1.66; burden, 7.28
- Heart failure: HR, 1.72; burden, 11.61
- Nonischemic cardiomyopathy: HR, 1.62; burden 3.56
- Pulmonary embolism: HR, 2.93; burden, 5.47
- Deep vein thrombosis: HR, 2.09; burden, 4.18
- Pericarditis: HR, 1.85, burden, 0.98
- Myocarditis: HR, 5.38; burden, 0.31
Recent reports have raised concerns about an association between COVID-19 vaccines and myocarditis and pericarditis, particularly in young males. Although very few of the participants were vaccinated prior to becoming infected, as vaccines were not yet widely available, the researchers performed two analyses censoring participants at the time of the first dose of any COVID-19 vaccine and adjusting for vaccination as a time-varying covariate.
The absolute numbers of myocarditis and pericarditis were still higher than the contemporary and historical cohorts. These numbers are much larger than those reported for myocarditis after vaccines, which are generally around 40 cases per 1 million people, observed Dr. Al-Aly.
The overall results were also consistent when compared with the historical control subjects.
“What we’re seeing in our report and others is that SARS-CoV-2 can leave a sort of scar or imprint on people, and some of these conditions are likely chronic conditions,” Dr. Al-Aly said. “So you’re going to have a generation of people who will bear the scar of COVID for their lifetime and I think that requires recognition and attention, so we’re aware of the magnitude of the problem and prepared to deal with it.”
With more than 76 million COVID-19 cases in the United States, that effort will likely have to be at the federal level, similar to President Joe Biden’s recent relaunch of the “Cancer Moonshot,” he added. “We need a greater and broader recognition at the federal level to try and recognize that when you have an earthquake, you don’t just deal with the earthquake when the earth is shaking, but you also need to deal with the aftermath.”
Dr. Gibson pointed out that this was a study of predominantly males and, thus, it’s unclear if the results can be extended to females. Nevertheless, he added, “long COVID may include outcomes beyond the central nervous system and we should educate patients about the risk of late cardiovascular outcomes.”
The authors noted the largely White, male cohort may limit generalizability of the findings. Other limitations include the possibility that some people may have had COVID-19 but were not tested, the datasets lacked information on cause of death, and possible residual confounding not accounted for in the adjusted analyses.
The research was funded by the U.S. Department of Veterans Affairs and two American Society of Nephrology and Kidney Cure fellowship awards. The authors declared no competing interests. Dr. Gibson reports having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Scientists see hope in new therapy for COVID-19 brain fog patients
People with long-COVID “brain fog” may be able to recover mental abilities that were dulled or stolen from them by the virus through an approach that has improved the effects of stroke, traumatic brain injury, and other post-viral disorders, doctors and scientists say.
For a lucky portion of the population, COVID-19 lasts a handful of days with minor symptoms. But for an estimated 37% who contract the virus, symptoms can linger for weeks, months, or even years. One of the most common symptoms of long COVID is brain fog: a life-altering condition characterized by slow thinking, confusion, difficulty remembering things, and poor concentration.
The approaches are based on the concept of neuroplasticity: The ability of neural networks in the brain to change, adapt, and strengthen, much like a muscle in the body that has been trained and exercised.
“The brain’s ability to bounce back from injury is what neuroplasticity is, and I’ve worked with people in our rehab clinic who have had brain tumors or suffer the effects of surgery or radiation on the brain, and people who have had West Nile virus, HIV, and meningitis,” said Tom Bergquist, PhD, clinical neuropsychologist at Mayo Clinic in Rochester, Minn. “There’s not a week that goes by that I don’t see someone recovering from COVID-19.”
One of the approaches used in the clinic is errorless learning, or having a patient with memory problems repeat information a certain number of times without error. The repetition helps rebuild those memory skills that were weakened during infection, Dr. Bergquist says.
People who have experienced brain fog after other viral infections have seen improvements with these approaches. Ben Ahrens, co-founder and CEO of re-origin – a company that offers neuroplasticity therapy – says he had long-term cognitive issues after a Lyme disease infection. Posttreatment Lyme disease syndrome, or chronic Lyme disease, occurs in about 1 in 10 people who are infected.
Mr. Ahrens says he was struck with Lyme 10 years ago and had brain fog, joint pain, and brain lesions detectable on scans for several years after infection.
According to Mr. Ahrens, neuroplasticity-based therapies help combat what researchers have found may be a lingering memory of past infections that lead to a heightened immune response, causing lingering symptoms.
“Essentially, what we believe is happening here, is the brain has learned that these symptoms are life-threatening – because, in fact, they can be,” Mr. Ahrens said. “The brain’s one job is to protect the body, and once it’s learned to associate these symptoms with that potentially very dangerous pathogen, even after it’s gone, things like a normal headache can trigger an immune cascade.”
Studies are underway at the University of Alabama at Birmingham to examine whether constraint-induced therapy – an approach rooted in neuroplasticity and historically used for loss of limb and speech function – is also effective for cognitive impairments like brain fog.
One technique they use is called shaping, which requires a person to repeatedly carry out their personal best function of impaired use – for example, remembering household tasks they have previously forgotten. That is done multiple times over several weeks in the clinic, and patients are given ways to transfer those skills to real-life use.
So far, the results are promising, said Edward Taub, PhD, researcher and professor of psychology at the University of Alabama at Birmingham.
When used in the past for physical impairments, researchers have noted not just clinical improvements, but structural changes. It led to an increase in the brain’s gray matter – which allows individuals to control movement, memory, and emotions – and improved white matter, which helps communication between gray matter areas.
Though results of the cognitive studies have not been published, Dr. Taub said patients with brain fog have shown improvement after just 35 hours of therapy and are nearly 100% improved after 6 months.
“The idea behind this is that the brain is responsive to use,” Dr. Taub said. “The amount of brain territory that’s dedicated to supporting or mediating a given behavioral function depends on the demands placed on the brain.”
A version of this article first appeared on WebMD.com.
People with long-COVID “brain fog” may be able to recover mental abilities that were dulled or stolen from them by the virus through an approach that has improved the effects of stroke, traumatic brain injury, and other post-viral disorders, doctors and scientists say.
For a lucky portion of the population, COVID-19 lasts a handful of days with minor symptoms. But for an estimated 37% who contract the virus, symptoms can linger for weeks, months, or even years. One of the most common symptoms of long COVID is brain fog: a life-altering condition characterized by slow thinking, confusion, difficulty remembering things, and poor concentration.
The approaches are based on the concept of neuroplasticity: The ability of neural networks in the brain to change, adapt, and strengthen, much like a muscle in the body that has been trained and exercised.
“The brain’s ability to bounce back from injury is what neuroplasticity is, and I’ve worked with people in our rehab clinic who have had brain tumors or suffer the effects of surgery or radiation on the brain, and people who have had West Nile virus, HIV, and meningitis,” said Tom Bergquist, PhD, clinical neuropsychologist at Mayo Clinic in Rochester, Minn. “There’s not a week that goes by that I don’t see someone recovering from COVID-19.”
One of the approaches used in the clinic is errorless learning, or having a patient with memory problems repeat information a certain number of times without error. The repetition helps rebuild those memory skills that were weakened during infection, Dr. Bergquist says.
People who have experienced brain fog after other viral infections have seen improvements with these approaches. Ben Ahrens, co-founder and CEO of re-origin – a company that offers neuroplasticity therapy – says he had long-term cognitive issues after a Lyme disease infection. Posttreatment Lyme disease syndrome, or chronic Lyme disease, occurs in about 1 in 10 people who are infected.
Mr. Ahrens says he was struck with Lyme 10 years ago and had brain fog, joint pain, and brain lesions detectable on scans for several years after infection.
According to Mr. Ahrens, neuroplasticity-based therapies help combat what researchers have found may be a lingering memory of past infections that lead to a heightened immune response, causing lingering symptoms.
“Essentially, what we believe is happening here, is the brain has learned that these symptoms are life-threatening – because, in fact, they can be,” Mr. Ahrens said. “The brain’s one job is to protect the body, and once it’s learned to associate these symptoms with that potentially very dangerous pathogen, even after it’s gone, things like a normal headache can trigger an immune cascade.”
Studies are underway at the University of Alabama at Birmingham to examine whether constraint-induced therapy – an approach rooted in neuroplasticity and historically used for loss of limb and speech function – is also effective for cognitive impairments like brain fog.
One technique they use is called shaping, which requires a person to repeatedly carry out their personal best function of impaired use – for example, remembering household tasks they have previously forgotten. That is done multiple times over several weeks in the clinic, and patients are given ways to transfer those skills to real-life use.
So far, the results are promising, said Edward Taub, PhD, researcher and professor of psychology at the University of Alabama at Birmingham.
When used in the past for physical impairments, researchers have noted not just clinical improvements, but structural changes. It led to an increase in the brain’s gray matter – which allows individuals to control movement, memory, and emotions – and improved white matter, which helps communication between gray matter areas.
Though results of the cognitive studies have not been published, Dr. Taub said patients with brain fog have shown improvement after just 35 hours of therapy and are nearly 100% improved after 6 months.
“The idea behind this is that the brain is responsive to use,” Dr. Taub said. “The amount of brain territory that’s dedicated to supporting or mediating a given behavioral function depends on the demands placed on the brain.”
A version of this article first appeared on WebMD.com.
People with long-COVID “brain fog” may be able to recover mental abilities that were dulled or stolen from them by the virus through an approach that has improved the effects of stroke, traumatic brain injury, and other post-viral disorders, doctors and scientists say.
For a lucky portion of the population, COVID-19 lasts a handful of days with minor symptoms. But for an estimated 37% who contract the virus, symptoms can linger for weeks, months, or even years. One of the most common symptoms of long COVID is brain fog: a life-altering condition characterized by slow thinking, confusion, difficulty remembering things, and poor concentration.
The approaches are based on the concept of neuroplasticity: The ability of neural networks in the brain to change, adapt, and strengthen, much like a muscle in the body that has been trained and exercised.
“The brain’s ability to bounce back from injury is what neuroplasticity is, and I’ve worked with people in our rehab clinic who have had brain tumors or suffer the effects of surgery or radiation on the brain, and people who have had West Nile virus, HIV, and meningitis,” said Tom Bergquist, PhD, clinical neuropsychologist at Mayo Clinic in Rochester, Minn. “There’s not a week that goes by that I don’t see someone recovering from COVID-19.”
One of the approaches used in the clinic is errorless learning, or having a patient with memory problems repeat information a certain number of times without error. The repetition helps rebuild those memory skills that were weakened during infection, Dr. Bergquist says.
People who have experienced brain fog after other viral infections have seen improvements with these approaches. Ben Ahrens, co-founder and CEO of re-origin – a company that offers neuroplasticity therapy – says he had long-term cognitive issues after a Lyme disease infection. Posttreatment Lyme disease syndrome, or chronic Lyme disease, occurs in about 1 in 10 people who are infected.
Mr. Ahrens says he was struck with Lyme 10 years ago and had brain fog, joint pain, and brain lesions detectable on scans for several years after infection.
According to Mr. Ahrens, neuroplasticity-based therapies help combat what researchers have found may be a lingering memory of past infections that lead to a heightened immune response, causing lingering symptoms.
“Essentially, what we believe is happening here, is the brain has learned that these symptoms are life-threatening – because, in fact, they can be,” Mr. Ahrens said. “The brain’s one job is to protect the body, and once it’s learned to associate these symptoms with that potentially very dangerous pathogen, even after it’s gone, things like a normal headache can trigger an immune cascade.”
Studies are underway at the University of Alabama at Birmingham to examine whether constraint-induced therapy – an approach rooted in neuroplasticity and historically used for loss of limb and speech function – is also effective for cognitive impairments like brain fog.
One technique they use is called shaping, which requires a person to repeatedly carry out their personal best function of impaired use – for example, remembering household tasks they have previously forgotten. That is done multiple times over several weeks in the clinic, and patients are given ways to transfer those skills to real-life use.
So far, the results are promising, said Edward Taub, PhD, researcher and professor of psychology at the University of Alabama at Birmingham.
When used in the past for physical impairments, researchers have noted not just clinical improvements, but structural changes. It led to an increase in the brain’s gray matter – which allows individuals to control movement, memory, and emotions – and improved white matter, which helps communication between gray matter areas.
Though results of the cognitive studies have not been published, Dr. Taub said patients with brain fog have shown improvement after just 35 hours of therapy and are nearly 100% improved after 6 months.
“The idea behind this is that the brain is responsive to use,” Dr. Taub said. “The amount of brain territory that’s dedicated to supporting or mediating a given behavioral function depends on the demands placed on the brain.”
A version of this article first appeared on WebMD.com.
Seniors face higher risk of other medical conditions after COVID-19
The findings of the observational study, which were published in the BMJ, show the risk of a new condition being triggered by COVID is more than twice as high in seniors, compared with younger patients. Plus, the researchers observed an even higher risk among those who were hospitalized, with nearly half (46%) of patients having developed new conditions after the acute COVID-19 infection period.
Respiratory failure with shortness of breath was the most common postacute sequela, but a wide range of heart, kidney, lung, liver, cognitive, mental health, and other conditions were diagnosed at least 3 weeks after initial infection and persisted beyond 30 days.
This is one of the first studies to specifically describe the incidence and severity of new conditions triggered by COVID-19 infection in a general sample of older adults, said study author Ken Cohen MD, FACP, executive director of translational research at Optum Labs and national senior medical director at Optum Care.
“Much of what has been published on the postacute sequelae of COVID-19 has been predominantly from a younger population, and many of the patients had been hospitalized,” Dr. Cohen noted. “This was the first study to focus on a large population of seniors, most of whom did not require hospitalization.”
Dr. Cohen and colleagues reviewed the health insurance records of more than 133,000 Medicare beneficiaries aged 65 or older who were diagnosed with COVID-19 before April 2020. They also matched individuals by age, race, sex, hospitalization status, and other factors to comparison groups without COVID-19 (one from 2020 and one from 2019), and to a group diagnosed with other lower respiratory tract viral infections before the pandemic.
Risk of developing new conditions was higher in hospitalized
After acute COVID-19 infection, 32% of seniors sought medical care for at least one new medical condition in 2020, compared with 21% of uninfected people in the same year.
The most commonly observed conditions included:
- Respiratory failure (7.55% higher risk).
- Fatigue (5.66% higher risk).
- High blood pressure (4.43% higher risk).
- Memory problems (2.63% higher risk).
- Kidney injury (2.59% higher risk).
- Mental health diagnoses (2.5% higher risk).
- Blood-clotting disorders (1.47 % higher risk).
- Heart rhythm disorders (2.9% higher risk).
The risk of developing new conditions was even higher among those 23,486 who were hospitalized in 2020. Those individuals showed a 23.6% higher risk for developing at least one new condition, compared with uninfected seniors in the same year. Also, patients older than 75 had a higher risk for neurological disorders, including dementia, encephalopathy, and memory problems. The researchers also found that respiratory failure and kidney injury were significantly more likely to affect men and Black patients.
When those who had COVID were compared with the group with other lower respiratory viral infections before the pandemic, only the risks of respiratory failure (2.39% higher), dementia (0.71% higher), and fatigue (0.18% higher) were higher.
Primary care providers can learn from these data to better evaluate and manage their geriatric patients with COVID-19 infection, said Amit Shah, MD, a geriatrician with the Mayo Clinic in Phoenix, in an interview.
“We must assess older patients who have had COVID-19 for more than just improvement from the respiratory symptoms of COVID-19 in post-COVID follow-up visits,” he said. “Older individuals with frailty have vulnerability to subsequent complications from severe illnesses and it is common to see post-illness diagnoses, such as new diagnosis of delirium; dementia; or renal, respiratory, or cardiac issues that is precipitated by the original illness. This study confirms that this is likely the case with COVID-19 as well.
“Primary care physicians should be vigilant for these complications, including attention to the rehabilitation needs of older patients with longer-term postviral fatigue from COVID-19,” Dr. Shah added.
Data predates ‘Omicron wave’
It remains uncertain whether sequelae will differ with the Omicron variant, but the findings remain applicable, Dr. Cohen said.
“We know that illness from the Omicron variant is on average less severe in those that have been vaccinated. However, throughout the Omicron wave, individuals who have not been vaccinated continue to have significant rates of serious illness and hospitalization,” he said.
“Our findings showed that serious illness with hospitalization was associated with a higher rate of sequelae. It can therefore be inferred that the rates of sequelae seen in our study would continue to occur in unvaccinated individuals who contract Omicron, but might occur less frequently in vaccinated individuals who contract Omicron and have less severe illness.”
Dr. Cohen serves as a consultant for Pfizer. Dr. Shah has disclosed no relevant financial relationships.
The findings of the observational study, which were published in the BMJ, show the risk of a new condition being triggered by COVID is more than twice as high in seniors, compared with younger patients. Plus, the researchers observed an even higher risk among those who were hospitalized, with nearly half (46%) of patients having developed new conditions after the acute COVID-19 infection period.
Respiratory failure with shortness of breath was the most common postacute sequela, but a wide range of heart, kidney, lung, liver, cognitive, mental health, and other conditions were diagnosed at least 3 weeks after initial infection and persisted beyond 30 days.
This is one of the first studies to specifically describe the incidence and severity of new conditions triggered by COVID-19 infection in a general sample of older adults, said study author Ken Cohen MD, FACP, executive director of translational research at Optum Labs and national senior medical director at Optum Care.
“Much of what has been published on the postacute sequelae of COVID-19 has been predominantly from a younger population, and many of the patients had been hospitalized,” Dr. Cohen noted. “This was the first study to focus on a large population of seniors, most of whom did not require hospitalization.”
Dr. Cohen and colleagues reviewed the health insurance records of more than 133,000 Medicare beneficiaries aged 65 or older who were diagnosed with COVID-19 before April 2020. They also matched individuals by age, race, sex, hospitalization status, and other factors to comparison groups without COVID-19 (one from 2020 and one from 2019), and to a group diagnosed with other lower respiratory tract viral infections before the pandemic.
Risk of developing new conditions was higher in hospitalized
After acute COVID-19 infection, 32% of seniors sought medical care for at least one new medical condition in 2020, compared with 21% of uninfected people in the same year.
The most commonly observed conditions included:
- Respiratory failure (7.55% higher risk).
- Fatigue (5.66% higher risk).
- High blood pressure (4.43% higher risk).
- Memory problems (2.63% higher risk).
- Kidney injury (2.59% higher risk).
- Mental health diagnoses (2.5% higher risk).
- Blood-clotting disorders (1.47 % higher risk).
- Heart rhythm disorders (2.9% higher risk).
The risk of developing new conditions was even higher among those 23,486 who were hospitalized in 2020. Those individuals showed a 23.6% higher risk for developing at least one new condition, compared with uninfected seniors in the same year. Also, patients older than 75 had a higher risk for neurological disorders, including dementia, encephalopathy, and memory problems. The researchers also found that respiratory failure and kidney injury were significantly more likely to affect men and Black patients.
When those who had COVID were compared with the group with other lower respiratory viral infections before the pandemic, only the risks of respiratory failure (2.39% higher), dementia (0.71% higher), and fatigue (0.18% higher) were higher.
Primary care providers can learn from these data to better evaluate and manage their geriatric patients with COVID-19 infection, said Amit Shah, MD, a geriatrician with the Mayo Clinic in Phoenix, in an interview.
“We must assess older patients who have had COVID-19 for more than just improvement from the respiratory symptoms of COVID-19 in post-COVID follow-up visits,” he said. “Older individuals with frailty have vulnerability to subsequent complications from severe illnesses and it is common to see post-illness diagnoses, such as new diagnosis of delirium; dementia; or renal, respiratory, or cardiac issues that is precipitated by the original illness. This study confirms that this is likely the case with COVID-19 as well.
“Primary care physicians should be vigilant for these complications, including attention to the rehabilitation needs of older patients with longer-term postviral fatigue from COVID-19,” Dr. Shah added.
Data predates ‘Omicron wave’
It remains uncertain whether sequelae will differ with the Omicron variant, but the findings remain applicable, Dr. Cohen said.
“We know that illness from the Omicron variant is on average less severe in those that have been vaccinated. However, throughout the Omicron wave, individuals who have not been vaccinated continue to have significant rates of serious illness and hospitalization,” he said.
“Our findings showed that serious illness with hospitalization was associated with a higher rate of sequelae. It can therefore be inferred that the rates of sequelae seen in our study would continue to occur in unvaccinated individuals who contract Omicron, but might occur less frequently in vaccinated individuals who contract Omicron and have less severe illness.”
Dr. Cohen serves as a consultant for Pfizer. Dr. Shah has disclosed no relevant financial relationships.
The findings of the observational study, which were published in the BMJ, show the risk of a new condition being triggered by COVID is more than twice as high in seniors, compared with younger patients. Plus, the researchers observed an even higher risk among those who were hospitalized, with nearly half (46%) of patients having developed new conditions after the acute COVID-19 infection period.
Respiratory failure with shortness of breath was the most common postacute sequela, but a wide range of heart, kidney, lung, liver, cognitive, mental health, and other conditions were diagnosed at least 3 weeks after initial infection and persisted beyond 30 days.
This is one of the first studies to specifically describe the incidence and severity of new conditions triggered by COVID-19 infection in a general sample of older adults, said study author Ken Cohen MD, FACP, executive director of translational research at Optum Labs and national senior medical director at Optum Care.
“Much of what has been published on the postacute sequelae of COVID-19 has been predominantly from a younger population, and many of the patients had been hospitalized,” Dr. Cohen noted. “This was the first study to focus on a large population of seniors, most of whom did not require hospitalization.”
Dr. Cohen and colleagues reviewed the health insurance records of more than 133,000 Medicare beneficiaries aged 65 or older who were diagnosed with COVID-19 before April 2020. They also matched individuals by age, race, sex, hospitalization status, and other factors to comparison groups without COVID-19 (one from 2020 and one from 2019), and to a group diagnosed with other lower respiratory tract viral infections before the pandemic.
Risk of developing new conditions was higher in hospitalized
After acute COVID-19 infection, 32% of seniors sought medical care for at least one new medical condition in 2020, compared with 21% of uninfected people in the same year.
The most commonly observed conditions included:
- Respiratory failure (7.55% higher risk).
- Fatigue (5.66% higher risk).
- High blood pressure (4.43% higher risk).
- Memory problems (2.63% higher risk).
- Kidney injury (2.59% higher risk).
- Mental health diagnoses (2.5% higher risk).
- Blood-clotting disorders (1.47 % higher risk).
- Heart rhythm disorders (2.9% higher risk).
The risk of developing new conditions was even higher among those 23,486 who were hospitalized in 2020. Those individuals showed a 23.6% higher risk for developing at least one new condition, compared with uninfected seniors in the same year. Also, patients older than 75 had a higher risk for neurological disorders, including dementia, encephalopathy, and memory problems. The researchers also found that respiratory failure and kidney injury were significantly more likely to affect men and Black patients.
When those who had COVID were compared with the group with other lower respiratory viral infections before the pandemic, only the risks of respiratory failure (2.39% higher), dementia (0.71% higher), and fatigue (0.18% higher) were higher.
Primary care providers can learn from these data to better evaluate and manage their geriatric patients with COVID-19 infection, said Amit Shah, MD, a geriatrician with the Mayo Clinic in Phoenix, in an interview.
“We must assess older patients who have had COVID-19 for more than just improvement from the respiratory symptoms of COVID-19 in post-COVID follow-up visits,” he said. “Older individuals with frailty have vulnerability to subsequent complications from severe illnesses and it is common to see post-illness diagnoses, such as new diagnosis of delirium; dementia; or renal, respiratory, or cardiac issues that is precipitated by the original illness. This study confirms that this is likely the case with COVID-19 as well.
“Primary care physicians should be vigilant for these complications, including attention to the rehabilitation needs of older patients with longer-term postviral fatigue from COVID-19,” Dr. Shah added.
Data predates ‘Omicron wave’
It remains uncertain whether sequelae will differ with the Omicron variant, but the findings remain applicable, Dr. Cohen said.
“We know that illness from the Omicron variant is on average less severe in those that have been vaccinated. However, throughout the Omicron wave, individuals who have not been vaccinated continue to have significant rates of serious illness and hospitalization,” he said.
“Our findings showed that serious illness with hospitalization was associated with a higher rate of sequelae. It can therefore be inferred that the rates of sequelae seen in our study would continue to occur in unvaccinated individuals who contract Omicron, but might occur less frequently in vaccinated individuals who contract Omicron and have less severe illness.”
Dr. Cohen serves as a consultant for Pfizer. Dr. Shah has disclosed no relevant financial relationships.
FROM BMJ
J&J pauses production of COVID vaccine
Johnson & Johnson stopped making its COVID-19 vaccine at a key facility in the Netherlands.
The Johnson & Johnson shot is seen as a critical vaccine for poorer countries. , people familiar with the decision told The New York Times.
The plant, located in Leiden, has been making an experimental but potentially more profitable vaccine instead. The experimental vaccine is for an unrelated virus -- respiratory syncytial virus, or RSV -- that will be used for a clinical trial.
The pause is said to be temporary. The Leiden plant is expected to restart production of the COVID-19 vaccine next month. The company has said that it has millions of COVID-19 doses in inventory, though it’s unclear whether the pause has affected vaccine supplies.
The interruption could reduce the supply of Johnson & Johnson’s COVID-19 vaccine by a few hundred million doses, one of the sources told the newspaper, since the doses made from renewed production won’t likely ship until May or June. Other facilities have been hired to produce the vaccine but aren’t running yet or haven’t received regulatory approval to ship doses for packaging.
Jake Sargent, a spokesman for Johnson & Johnson, told the Times that the company is “focused on ensuring our vaccine is available where people are in need” and that its global production network “is working day and night.” He said that the company has millions of doses in inventory and is continuing to deliver vaccine batches to facilities that package doses.
The pause has surprised officials at two main recipients of the Johnson & Johnson shots -- the African Union and Covax, the organization that coordinates COVID-19 vaccines for poorer countries. Leaders of the two organizations learned about the halt in production from reporters at the Times.
“This is not the time to be switching production lines of anything, when the lives of people across the developing world hang in the balance,” Ayoade Alakija, coleader of the African Union’s vaccine delivery program, told the newspaper.
Poorer countries rely on Johnson & Johnson’s vaccine because it doesn’t require ultracold refrigeration. The vaccine is also less expensive than others and easy to provide to hard-to-reach populations.
“In many low- and middle-income countries, our vaccine is the most important and sometimes only option,” Penny Heaton, MD, a Johnson & Johnson executive, said in December during a meeting with the CDC’s vaccine advisory committee.
“We have a global vaccine, and the world is depending on us,” she said.
A version of this article first appeared on WebMD.com.
Johnson & Johnson stopped making its COVID-19 vaccine at a key facility in the Netherlands.
The Johnson & Johnson shot is seen as a critical vaccine for poorer countries. , people familiar with the decision told The New York Times.
The plant, located in Leiden, has been making an experimental but potentially more profitable vaccine instead. The experimental vaccine is for an unrelated virus -- respiratory syncytial virus, or RSV -- that will be used for a clinical trial.
The pause is said to be temporary. The Leiden plant is expected to restart production of the COVID-19 vaccine next month. The company has said that it has millions of COVID-19 doses in inventory, though it’s unclear whether the pause has affected vaccine supplies.
The interruption could reduce the supply of Johnson & Johnson’s COVID-19 vaccine by a few hundred million doses, one of the sources told the newspaper, since the doses made from renewed production won’t likely ship until May or June. Other facilities have been hired to produce the vaccine but aren’t running yet or haven’t received regulatory approval to ship doses for packaging.
Jake Sargent, a spokesman for Johnson & Johnson, told the Times that the company is “focused on ensuring our vaccine is available where people are in need” and that its global production network “is working day and night.” He said that the company has millions of doses in inventory and is continuing to deliver vaccine batches to facilities that package doses.
The pause has surprised officials at two main recipients of the Johnson & Johnson shots -- the African Union and Covax, the organization that coordinates COVID-19 vaccines for poorer countries. Leaders of the two organizations learned about the halt in production from reporters at the Times.
“This is not the time to be switching production lines of anything, when the lives of people across the developing world hang in the balance,” Ayoade Alakija, coleader of the African Union’s vaccine delivery program, told the newspaper.
Poorer countries rely on Johnson & Johnson’s vaccine because it doesn’t require ultracold refrigeration. The vaccine is also less expensive than others and easy to provide to hard-to-reach populations.
“In many low- and middle-income countries, our vaccine is the most important and sometimes only option,” Penny Heaton, MD, a Johnson & Johnson executive, said in December during a meeting with the CDC’s vaccine advisory committee.
“We have a global vaccine, and the world is depending on us,” she said.
A version of this article first appeared on WebMD.com.
Johnson & Johnson stopped making its COVID-19 vaccine at a key facility in the Netherlands.
The Johnson & Johnson shot is seen as a critical vaccine for poorer countries. , people familiar with the decision told The New York Times.
The plant, located in Leiden, has been making an experimental but potentially more profitable vaccine instead. The experimental vaccine is for an unrelated virus -- respiratory syncytial virus, or RSV -- that will be used for a clinical trial.
The pause is said to be temporary. The Leiden plant is expected to restart production of the COVID-19 vaccine next month. The company has said that it has millions of COVID-19 doses in inventory, though it’s unclear whether the pause has affected vaccine supplies.
The interruption could reduce the supply of Johnson & Johnson’s COVID-19 vaccine by a few hundred million doses, one of the sources told the newspaper, since the doses made from renewed production won’t likely ship until May or June. Other facilities have been hired to produce the vaccine but aren’t running yet or haven’t received regulatory approval to ship doses for packaging.
Jake Sargent, a spokesman for Johnson & Johnson, told the Times that the company is “focused on ensuring our vaccine is available where people are in need” and that its global production network “is working day and night.” He said that the company has millions of doses in inventory and is continuing to deliver vaccine batches to facilities that package doses.
The pause has surprised officials at two main recipients of the Johnson & Johnson shots -- the African Union and Covax, the organization that coordinates COVID-19 vaccines for poorer countries. Leaders of the two organizations learned about the halt in production from reporters at the Times.
“This is not the time to be switching production lines of anything, when the lives of people across the developing world hang in the balance,” Ayoade Alakija, coleader of the African Union’s vaccine delivery program, told the newspaper.
Poorer countries rely on Johnson & Johnson’s vaccine because it doesn’t require ultracold refrigeration. The vaccine is also less expensive than others and easy to provide to hard-to-reach populations.
“In many low- and middle-income countries, our vaccine is the most important and sometimes only option,” Penny Heaton, MD, a Johnson & Johnson executive, said in December during a meeting with the CDC’s vaccine advisory committee.
“We have a global vaccine, and the world is depending on us,” she said.
A version of this article first appeared on WebMD.com.
Agreement reached for research definition of ‘long COVID’ in children and young people
Long COVID can affect adults, young people, and children, and now for the first time, in a landmark study accepted for publication in the Archives of Disease in Childhood, formal agreement has been made on a research definition for post–acute COVID-19, or “long COVID” as it is commonly known, in children and young people.
The researchers charged themselves with a single objective – to derive a research definition for long COVID (post–acute COVID-19) in children and young people to allow comparisons between research studies. Specifically, so studies on prevalence, course, and outcome of long COVID in this age group can be reliably compared, because to date there has been no consensus. In fact, the authors pointed out how the “slew of definitions” currently used all differ in number, type, and duration of symptoms, which hampers research efforts. In addition, the lack of definition consensus has contributed to very wide reported variations in the estimated prevalence of long COVID in children of 1%-51%, with the authors saying that a “consistently applied definition of long COVID will help reduce the variability of prevalence estimates.”
Statements sequentially whittled down
“Using robust consensus methodology,” the authors said, “we derived a research definition for long COVID in children and young people.”
To achieve the definition consensus, a three-phase online Delphi process was used, followed by a virtual consensus meeting. The 123 participants registered to take part in the study included 23 people (19%) in a lived experience panel, 50 (42%) in the researcher or researcher/service delivery combined panel and 47 (39%) in the service delivery panel. Of 120 registered participants, 105 (88%) completed phase 1, 86 eligible participants (82% of those completing phase 1) completed phase 2 and 77 eligible participants (90% of those completing phase 2) completed phase 3. Seventeen participants attended and voted at the consensus meeting – 4 (23%) from the service delivery panel, 11 (65%) from the researcher panel, and 2 (12%) from the lived experience panel.
Presented with 49 statements in each phase, participants scored these from 1-9 based on how important they were perceived to be with regards inclusion in the research definition of long COVID in children and young people. Having been sequentially whittled down in three phases, 10 statements were discussed at the consensus meeting, and a panel of eight 11- to 17-year-olds affected by long COVID also reviewed the statements to reach a final agreement.
Five of the statements were agreed to be included in the definition, which stated that long COVID in children and young people is a condition in which a child or young person has symptoms (at least one of which is a physical symptom) that have continued or developed after a diagnosis of COVID-19 (confirmed with one or more positive COVID tests); impact their physical, mental, or social well-being; are interfering with some aspect of daily living (for example, school, work, home, or relationships); and persist for a minimum duration of 12 weeks after initial testing for COVID-19 (even if symptoms have waxed and waned over that period).
David Strain, MBChB, MD, chair of the BMA board of science and clinical senior lecturer and honorary consultant, University of Exeter (England), told the Science Media Centre: “A Delphi study builds a consensus from the world’s experts by presenting a series of statements and continuing to refine them until there is agreement as to what the definition of pediatric long COVID should be.” He added: “This is vitally important in order to align the global research effort into long COVID.”
Reassuringly similar
From the agreed five statements, a further research definition was proposed to align with the World Health Organization definition for adults: “Post–COVID-19 condition occurs in young people with a history of confirmed SARS CoV-2 infection, with at least one persisting physical symptom for a minimum duration of 12 weeks after initial testing that cannot be explained by an alternative diagnosis. The symptoms have an impact on everyday functioning, may continue or develop after COVID-19 infection, and may fluctuate or relapse over time.”
The authors concluded: “This is the first research definition of long COVID (post–COVID-19 condition) in children and young people and complements the clinical case definition in adults proposed by WHO,” adding that the two definitions are “reassuringly similar.”
They reiterated how widespread adoption of this definition would allow comparisons between studies such that a core outcome set can be developed and the prevalence, course and outcome of long COVID in children and young people can be reliably evaluated, which “will substantially help strengthen the evidence base on this debilitating condition.”
In addition, the authors said that a consistently applied definition of long COVID will help to provide a “more accurate picture on the true impact of the condition.”
The researchers emphasized the need to differentiate between a clinical case definition and a research definition of long COVID and explained: “It is understandable that the patient groups representing people with long COVID are concerned about a definition that could restrict access to services that are needed.”
They went on to say that in their view the decision whether a child or young person can see a health care professional, access any support needed, or be referred, investigated, or treated for long COVID should be a “shared decision involving the young person, their carers, and clinicians.”
Dr. Strain reinforced that it was important that the definition was a research one and not a clinical one, pointing out that the 12-week period in the research definition “does not necessarily mean that a child or young person should need to wait 3 months before being offered help or assistance from their health care team, indeed a 3-month delay in offering support to a child or young person, at this vitally important period of their educational development, could have lasting long-term impacts.”
A version of this article first appeared on Medscape.co.uk.
Long COVID can affect adults, young people, and children, and now for the first time, in a landmark study accepted for publication in the Archives of Disease in Childhood, formal agreement has been made on a research definition for post–acute COVID-19, or “long COVID” as it is commonly known, in children and young people.
The researchers charged themselves with a single objective – to derive a research definition for long COVID (post–acute COVID-19) in children and young people to allow comparisons between research studies. Specifically, so studies on prevalence, course, and outcome of long COVID in this age group can be reliably compared, because to date there has been no consensus. In fact, the authors pointed out how the “slew of definitions” currently used all differ in number, type, and duration of symptoms, which hampers research efforts. In addition, the lack of definition consensus has contributed to very wide reported variations in the estimated prevalence of long COVID in children of 1%-51%, with the authors saying that a “consistently applied definition of long COVID will help reduce the variability of prevalence estimates.”
Statements sequentially whittled down
“Using robust consensus methodology,” the authors said, “we derived a research definition for long COVID in children and young people.”
To achieve the definition consensus, a three-phase online Delphi process was used, followed by a virtual consensus meeting. The 123 participants registered to take part in the study included 23 people (19%) in a lived experience panel, 50 (42%) in the researcher or researcher/service delivery combined panel and 47 (39%) in the service delivery panel. Of 120 registered participants, 105 (88%) completed phase 1, 86 eligible participants (82% of those completing phase 1) completed phase 2 and 77 eligible participants (90% of those completing phase 2) completed phase 3. Seventeen participants attended and voted at the consensus meeting – 4 (23%) from the service delivery panel, 11 (65%) from the researcher panel, and 2 (12%) from the lived experience panel.
Presented with 49 statements in each phase, participants scored these from 1-9 based on how important they were perceived to be with regards inclusion in the research definition of long COVID in children and young people. Having been sequentially whittled down in three phases, 10 statements were discussed at the consensus meeting, and a panel of eight 11- to 17-year-olds affected by long COVID also reviewed the statements to reach a final agreement.
Five of the statements were agreed to be included in the definition, which stated that long COVID in children and young people is a condition in which a child or young person has symptoms (at least one of which is a physical symptom) that have continued or developed after a diagnosis of COVID-19 (confirmed with one or more positive COVID tests); impact their physical, mental, or social well-being; are interfering with some aspect of daily living (for example, school, work, home, or relationships); and persist for a minimum duration of 12 weeks after initial testing for COVID-19 (even if symptoms have waxed and waned over that period).
David Strain, MBChB, MD, chair of the BMA board of science and clinical senior lecturer and honorary consultant, University of Exeter (England), told the Science Media Centre: “A Delphi study builds a consensus from the world’s experts by presenting a series of statements and continuing to refine them until there is agreement as to what the definition of pediatric long COVID should be.” He added: “This is vitally important in order to align the global research effort into long COVID.”
Reassuringly similar
From the agreed five statements, a further research definition was proposed to align with the World Health Organization definition for adults: “Post–COVID-19 condition occurs in young people with a history of confirmed SARS CoV-2 infection, with at least one persisting physical symptom for a minimum duration of 12 weeks after initial testing that cannot be explained by an alternative diagnosis. The symptoms have an impact on everyday functioning, may continue or develop after COVID-19 infection, and may fluctuate or relapse over time.”
The authors concluded: “This is the first research definition of long COVID (post–COVID-19 condition) in children and young people and complements the clinical case definition in adults proposed by WHO,” adding that the two definitions are “reassuringly similar.”
They reiterated how widespread adoption of this definition would allow comparisons between studies such that a core outcome set can be developed and the prevalence, course and outcome of long COVID in children and young people can be reliably evaluated, which “will substantially help strengthen the evidence base on this debilitating condition.”
In addition, the authors said that a consistently applied definition of long COVID will help to provide a “more accurate picture on the true impact of the condition.”
The researchers emphasized the need to differentiate between a clinical case definition and a research definition of long COVID and explained: “It is understandable that the patient groups representing people with long COVID are concerned about a definition that could restrict access to services that are needed.”
They went on to say that in their view the decision whether a child or young person can see a health care professional, access any support needed, or be referred, investigated, or treated for long COVID should be a “shared decision involving the young person, their carers, and clinicians.”
Dr. Strain reinforced that it was important that the definition was a research one and not a clinical one, pointing out that the 12-week period in the research definition “does not necessarily mean that a child or young person should need to wait 3 months before being offered help or assistance from their health care team, indeed a 3-month delay in offering support to a child or young person, at this vitally important period of their educational development, could have lasting long-term impacts.”
A version of this article first appeared on Medscape.co.uk.
Long COVID can affect adults, young people, and children, and now for the first time, in a landmark study accepted for publication in the Archives of Disease in Childhood, formal agreement has been made on a research definition for post–acute COVID-19, or “long COVID” as it is commonly known, in children and young people.
The researchers charged themselves with a single objective – to derive a research definition for long COVID (post–acute COVID-19) in children and young people to allow comparisons between research studies. Specifically, so studies on prevalence, course, and outcome of long COVID in this age group can be reliably compared, because to date there has been no consensus. In fact, the authors pointed out how the “slew of definitions” currently used all differ in number, type, and duration of symptoms, which hampers research efforts. In addition, the lack of definition consensus has contributed to very wide reported variations in the estimated prevalence of long COVID in children of 1%-51%, with the authors saying that a “consistently applied definition of long COVID will help reduce the variability of prevalence estimates.”
Statements sequentially whittled down
“Using robust consensus methodology,” the authors said, “we derived a research definition for long COVID in children and young people.”
To achieve the definition consensus, a three-phase online Delphi process was used, followed by a virtual consensus meeting. The 123 participants registered to take part in the study included 23 people (19%) in a lived experience panel, 50 (42%) in the researcher or researcher/service delivery combined panel and 47 (39%) in the service delivery panel. Of 120 registered participants, 105 (88%) completed phase 1, 86 eligible participants (82% of those completing phase 1) completed phase 2 and 77 eligible participants (90% of those completing phase 2) completed phase 3. Seventeen participants attended and voted at the consensus meeting – 4 (23%) from the service delivery panel, 11 (65%) from the researcher panel, and 2 (12%) from the lived experience panel.
Presented with 49 statements in each phase, participants scored these from 1-9 based on how important they were perceived to be with regards inclusion in the research definition of long COVID in children and young people. Having been sequentially whittled down in three phases, 10 statements were discussed at the consensus meeting, and a panel of eight 11- to 17-year-olds affected by long COVID also reviewed the statements to reach a final agreement.
Five of the statements were agreed to be included in the definition, which stated that long COVID in children and young people is a condition in which a child or young person has symptoms (at least one of which is a physical symptom) that have continued or developed after a diagnosis of COVID-19 (confirmed with one or more positive COVID tests); impact their physical, mental, or social well-being; are interfering with some aspect of daily living (for example, school, work, home, or relationships); and persist for a minimum duration of 12 weeks after initial testing for COVID-19 (even if symptoms have waxed and waned over that period).
David Strain, MBChB, MD, chair of the BMA board of science and clinical senior lecturer and honorary consultant, University of Exeter (England), told the Science Media Centre: “A Delphi study builds a consensus from the world’s experts by presenting a series of statements and continuing to refine them until there is agreement as to what the definition of pediatric long COVID should be.” He added: “This is vitally important in order to align the global research effort into long COVID.”
Reassuringly similar
From the agreed five statements, a further research definition was proposed to align with the World Health Organization definition for adults: “Post–COVID-19 condition occurs in young people with a history of confirmed SARS CoV-2 infection, with at least one persisting physical symptom for a minimum duration of 12 weeks after initial testing that cannot be explained by an alternative diagnosis. The symptoms have an impact on everyday functioning, may continue or develop after COVID-19 infection, and may fluctuate or relapse over time.”
The authors concluded: “This is the first research definition of long COVID (post–COVID-19 condition) in children and young people and complements the clinical case definition in adults proposed by WHO,” adding that the two definitions are “reassuringly similar.”
They reiterated how widespread adoption of this definition would allow comparisons between studies such that a core outcome set can be developed and the prevalence, course and outcome of long COVID in children and young people can be reliably evaluated, which “will substantially help strengthen the evidence base on this debilitating condition.”
In addition, the authors said that a consistently applied definition of long COVID will help to provide a “more accurate picture on the true impact of the condition.”
The researchers emphasized the need to differentiate between a clinical case definition and a research definition of long COVID and explained: “It is understandable that the patient groups representing people with long COVID are concerned about a definition that could restrict access to services that are needed.”
They went on to say that in their view the decision whether a child or young person can see a health care professional, access any support needed, or be referred, investigated, or treated for long COVID should be a “shared decision involving the young person, their carers, and clinicians.”
Dr. Strain reinforced that it was important that the definition was a research one and not a clinical one, pointing out that the 12-week period in the research definition “does not necessarily mean that a child or young person should need to wait 3 months before being offered help or assistance from their health care team, indeed a 3-month delay in offering support to a child or young person, at this vitally important period of their educational development, could have lasting long-term impacts.”
A version of this article first appeared on Medscape.co.uk.
FROM THE ARCHIVES OF DISEASE IN CHILDHOOD
Promising leads to crack long COVID discovered
It’s a story of promise at a time of urgent need.
They proposed many theories on what might be driving long COVID. A role for a virus “cryptic reservoir” that could reactivate at any time, “viral remnants” that trigger chronic inflammation, and action by “autoimmune antibodies” that cause ongoing symptoms are possibilities.
In fact, it’s likely that research will show long COVID is a condition with more than one cause, the experts said during a recent webinar.
People might experience post-infection problems, including organ damage that takes time to heal after initial COVID-19 illness. Or they may be living with post-immune factors, including ongoing immune system responses triggered by autoantibodies.
Determining the cause or causes of long COVID is essential for treatment. For example, if one person’s symptoms persist because of an overactive immune system, “we need to provide immunosuppressant therapies,” Akiko Iwasaki, PhD, said. “But we don’t want to give that to someone who has a persistent virus reservoir,” meaning remnants of the virus remain in their bodies.
Interestingly, a study preprint, which has not been peer reviewed, found dogs were accurate more than half the time in sniffing out long COVID, said Dr. Iwasaki, professor of immunobiology and developmental biology at Yale University, New Haven, Conn.
The dogs were tasked with identifying 45 people with long COVID versus 188 people without it. The findings suggest the presence of a unique chemical in the sweat of people with long COVID that could someday lead to a diagnostic test.
Viral persistence possible
If one of the main theories holds, it could be that the coronavirus somehow remains in the body in some form for some people after COVID-19.
Mady Hornig, MD, agreed this is a possibility that needs to be investigated further.
“A weakened immune response to an infection may mean that you have cryptic reservoirs of virus that are continuing to cause symptoms,” she said during the briefing. Dr. Hornig is a doctor-scientist specializing in epidemiology at Columbia University, New York.
“That may explain why some patients with long COVID feel better after vaccination,” because the vaccine creates a strong antibody response to fight COVID-19, Dr. Iwasaki said.
Researchers are unearthing additional potential factors contributing to long COVID.
Viral persistence could also reactivate other dormant viruses in the body, such as Epstein-Barr virus (EBV), said Lawrence Purpura, MD, MPH, an infectious disease specialist at New York Presbyterian/Columbia University. Reactivation of Epstein-Barr is one of four identifying signs of long COVID revealed in a Jan. 25 study published in the journal Cell.
Immune overactivation also possible?
For other people with long COVID, it’s not the virus sticking around but the body’s reaction that’s the issue.
Investigators suggest autoimmunity plays a role, and they point to the presence of autoantibodies, for example.
When these autoantibodies persist, they can cause tissue and organ damage over time.
Other investigators are proposing “immune exhaustion” in long COVID because of similarities to chronic fatigue syndrome, Dr. Hornig said.
“It should be ‘all hands on deck’ for research into long COVID,” she said. “The number of disabled individuals who will likely qualify for a diagnosis of [chronic fatigue syndrome] is growing by the second.”
Forging ahead on future research
It’s clear there is more work to do. There are investigators working on banking tissue samples from people with long COVID to learn more, for example.
Also, finding a biomarker unique to long COVID could vastly improve the precision of diagnosing long COVID, especially if the dog sniffing option does not pan out.
Of the thousands of biomarker possibilities, Dr. Hornig said, “maybe that’s one or two that ultimately make a real impact on patient care. So it’s going to be critical to find those quickly, translate them, and make them available.”
In the meantime, some answers might come from a large study sponsored by the National Institutes of Health. The NIH is funding the “Researching COVID to Enhance Recovery” project using $470 million from the American Rescue Plan. Investigators at NYU Langone Health are leading the effort and plan to share the wealth by funding more than 100 researchers at more than 30 institutions to create a “metacohort” to study long COVID. More information is available at recovercovid.org.
“Fortunately, through the global research effort, we are now really starting to expand our understanding of how long COVID manifests, how common it is, and what the underlying mechanisms may be,” Dr. Purpura said.
A version of this article first appeared on WebMD.com.
It’s a story of promise at a time of urgent need.
They proposed many theories on what might be driving long COVID. A role for a virus “cryptic reservoir” that could reactivate at any time, “viral remnants” that trigger chronic inflammation, and action by “autoimmune antibodies” that cause ongoing symptoms are possibilities.
In fact, it’s likely that research will show long COVID is a condition with more than one cause, the experts said during a recent webinar.
People might experience post-infection problems, including organ damage that takes time to heal after initial COVID-19 illness. Or they may be living with post-immune factors, including ongoing immune system responses triggered by autoantibodies.
Determining the cause or causes of long COVID is essential for treatment. For example, if one person’s symptoms persist because of an overactive immune system, “we need to provide immunosuppressant therapies,” Akiko Iwasaki, PhD, said. “But we don’t want to give that to someone who has a persistent virus reservoir,” meaning remnants of the virus remain in their bodies.
Interestingly, a study preprint, which has not been peer reviewed, found dogs were accurate more than half the time in sniffing out long COVID, said Dr. Iwasaki, professor of immunobiology and developmental biology at Yale University, New Haven, Conn.
The dogs were tasked with identifying 45 people with long COVID versus 188 people without it. The findings suggest the presence of a unique chemical in the sweat of people with long COVID that could someday lead to a diagnostic test.
Viral persistence possible
If one of the main theories holds, it could be that the coronavirus somehow remains in the body in some form for some people after COVID-19.
Mady Hornig, MD, agreed this is a possibility that needs to be investigated further.
“A weakened immune response to an infection may mean that you have cryptic reservoirs of virus that are continuing to cause symptoms,” she said during the briefing. Dr. Hornig is a doctor-scientist specializing in epidemiology at Columbia University, New York.
“That may explain why some patients with long COVID feel better after vaccination,” because the vaccine creates a strong antibody response to fight COVID-19, Dr. Iwasaki said.
Researchers are unearthing additional potential factors contributing to long COVID.
Viral persistence could also reactivate other dormant viruses in the body, such as Epstein-Barr virus (EBV), said Lawrence Purpura, MD, MPH, an infectious disease specialist at New York Presbyterian/Columbia University. Reactivation of Epstein-Barr is one of four identifying signs of long COVID revealed in a Jan. 25 study published in the journal Cell.
Immune overactivation also possible?
For other people with long COVID, it’s not the virus sticking around but the body’s reaction that’s the issue.
Investigators suggest autoimmunity plays a role, and they point to the presence of autoantibodies, for example.
When these autoantibodies persist, they can cause tissue and organ damage over time.
Other investigators are proposing “immune exhaustion” in long COVID because of similarities to chronic fatigue syndrome, Dr. Hornig said.
“It should be ‘all hands on deck’ for research into long COVID,” she said. “The number of disabled individuals who will likely qualify for a diagnosis of [chronic fatigue syndrome] is growing by the second.”
Forging ahead on future research
It’s clear there is more work to do. There are investigators working on banking tissue samples from people with long COVID to learn more, for example.
Also, finding a biomarker unique to long COVID could vastly improve the precision of diagnosing long COVID, especially if the dog sniffing option does not pan out.
Of the thousands of biomarker possibilities, Dr. Hornig said, “maybe that’s one or two that ultimately make a real impact on patient care. So it’s going to be critical to find those quickly, translate them, and make them available.”
In the meantime, some answers might come from a large study sponsored by the National Institutes of Health. The NIH is funding the “Researching COVID to Enhance Recovery” project using $470 million from the American Rescue Plan. Investigators at NYU Langone Health are leading the effort and plan to share the wealth by funding more than 100 researchers at more than 30 institutions to create a “metacohort” to study long COVID. More information is available at recovercovid.org.
“Fortunately, through the global research effort, we are now really starting to expand our understanding of how long COVID manifests, how common it is, and what the underlying mechanisms may be,” Dr. Purpura said.
A version of this article first appeared on WebMD.com.
It’s a story of promise at a time of urgent need.
They proposed many theories on what might be driving long COVID. A role for a virus “cryptic reservoir” that could reactivate at any time, “viral remnants” that trigger chronic inflammation, and action by “autoimmune antibodies” that cause ongoing symptoms are possibilities.
In fact, it’s likely that research will show long COVID is a condition with more than one cause, the experts said during a recent webinar.
People might experience post-infection problems, including organ damage that takes time to heal after initial COVID-19 illness. Or they may be living with post-immune factors, including ongoing immune system responses triggered by autoantibodies.
Determining the cause or causes of long COVID is essential for treatment. For example, if one person’s symptoms persist because of an overactive immune system, “we need to provide immunosuppressant therapies,” Akiko Iwasaki, PhD, said. “But we don’t want to give that to someone who has a persistent virus reservoir,” meaning remnants of the virus remain in their bodies.
Interestingly, a study preprint, which has not been peer reviewed, found dogs were accurate more than half the time in sniffing out long COVID, said Dr. Iwasaki, professor of immunobiology and developmental biology at Yale University, New Haven, Conn.
The dogs were tasked with identifying 45 people with long COVID versus 188 people without it. The findings suggest the presence of a unique chemical in the sweat of people with long COVID that could someday lead to a diagnostic test.
Viral persistence possible
If one of the main theories holds, it could be that the coronavirus somehow remains in the body in some form for some people after COVID-19.
Mady Hornig, MD, agreed this is a possibility that needs to be investigated further.
“A weakened immune response to an infection may mean that you have cryptic reservoirs of virus that are continuing to cause symptoms,” she said during the briefing. Dr. Hornig is a doctor-scientist specializing in epidemiology at Columbia University, New York.
“That may explain why some patients with long COVID feel better after vaccination,” because the vaccine creates a strong antibody response to fight COVID-19, Dr. Iwasaki said.
Researchers are unearthing additional potential factors contributing to long COVID.
Viral persistence could also reactivate other dormant viruses in the body, such as Epstein-Barr virus (EBV), said Lawrence Purpura, MD, MPH, an infectious disease specialist at New York Presbyterian/Columbia University. Reactivation of Epstein-Barr is one of four identifying signs of long COVID revealed in a Jan. 25 study published in the journal Cell.
Immune overactivation also possible?
For other people with long COVID, it’s not the virus sticking around but the body’s reaction that’s the issue.
Investigators suggest autoimmunity plays a role, and they point to the presence of autoantibodies, for example.
When these autoantibodies persist, they can cause tissue and organ damage over time.
Other investigators are proposing “immune exhaustion” in long COVID because of similarities to chronic fatigue syndrome, Dr. Hornig said.
“It should be ‘all hands on deck’ for research into long COVID,” she said. “The number of disabled individuals who will likely qualify for a diagnosis of [chronic fatigue syndrome] is growing by the second.”
Forging ahead on future research
It’s clear there is more work to do. There are investigators working on banking tissue samples from people with long COVID to learn more, for example.
Also, finding a biomarker unique to long COVID could vastly improve the precision of diagnosing long COVID, especially if the dog sniffing option does not pan out.
Of the thousands of biomarker possibilities, Dr. Hornig said, “maybe that’s one or two that ultimately make a real impact on patient care. So it’s going to be critical to find those quickly, translate them, and make them available.”
In the meantime, some answers might come from a large study sponsored by the National Institutes of Health. The NIH is funding the “Researching COVID to Enhance Recovery” project using $470 million from the American Rescue Plan. Investigators at NYU Langone Health are leading the effort and plan to share the wealth by funding more than 100 researchers at more than 30 institutions to create a “metacohort” to study long COVID. More information is available at recovercovid.org.
“Fortunately, through the global research effort, we are now really starting to expand our understanding of how long COVID manifests, how common it is, and what the underlying mechanisms may be,” Dr. Purpura said.
A version of this article first appeared on WebMD.com.
Children and COVID: New cases down again, but still ‘extremely high’
The indication of an Omicron decline has become a trend: New cases of COVID-19 in children were down for a second consecutive week in the United States, according to the American Academy of Pediatrics and the Children’s Hospital Association.
but the nearly 632,000 cases reported were down by 22% from the previous week and by 45% from what appears to be the peak of the Omicron surge during the week of Jan. 14-20, the AAP/CHA data show.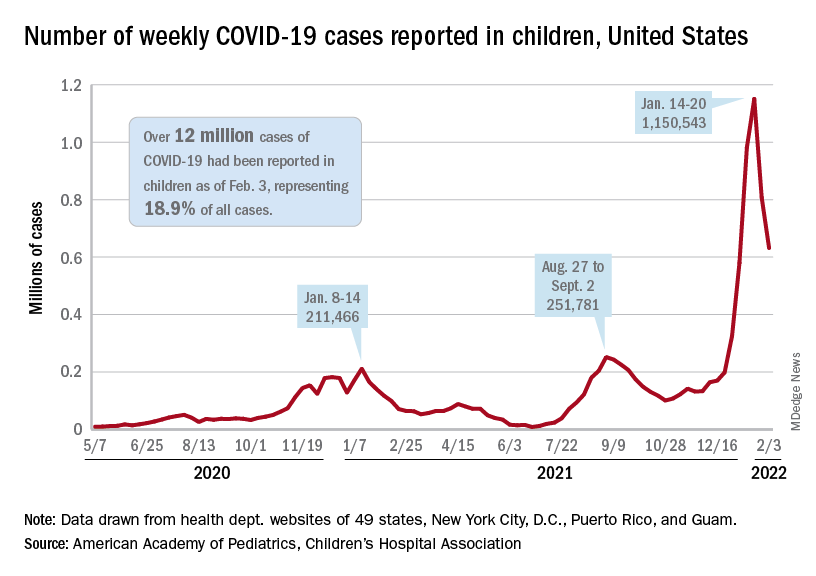
To put the effect of the Delta and Omicron variants into some sort of perspective, the total number of COVID-19 cases among children passed 5 million at the beginning of September 2021, about a year and a half into the pandemic. In the last 5 months, the cumulative count has more than doubled and now stands at 12 million, the AAP and CHA said in their weekly COVID report.
Hospital admissions and emergency department visits followed the same downward trend over the last week. The rate of new hospitalizations fell to 0.81 per 100,000 children aged 0-17 years as of Feb. 2 (down from a peak of 1.25 per 100,000 on Jan. 15), and ED visits with diagnosed COVID-19 dropped to 1.8% (peak was 14.1%), 1.9% (peak was 14.3%), and 3.4% (peak was 14%) of all visits for children aged 16-17, 12-15, and 0-11 years, respectively, the Centers for Disease Control and Prevention reported.
The vaccination response
The surge of infections brought about by the Omicron variant, however, did not translate into increased vaccination, at least for the youngest eligible children. Vaccine initiation rose slightly among children aged 5-11 in early and mid-January but, by early February, new vaccinations had declined to their lowest point since approval in early November of 2021, the AAP said in its weekly COVID vaccination report.
As a result, the 5- to 11-year-olds are well behind the pace set by those aged 12-15 for the first 3 months of their vaccination experience. Through the first 13 weeks after the COVID vaccine was approved for children aged 12-15 in early May, 44.5% had received at least one dose and 32.3% were fully vaccinated. Among children aged 5-11, the corresponding figures through 13 weeks were 31% and 22.5%, according to CDC data.
The vaccination reaction to Omicron was somewhat more robust for children aged 12-17, compared with the younger group, but initiations dropped at the same time that new cases began to decline. In terms of total volume, the response among 12- to 17-year-olds was much smaller than that seen in July and August of 2021 as the Delta surge was hitting the United States, the AAP vaccination report shows.
All those vaccinations add up to this: Over 16.8 million children aged 12-17 and almost 9 million aged 5-11 had received at least one dose of vaccine as of Feb. 7, which works out to 66.6% of the older group and 31.2% of the younger cohort. Almost 14.3 million (56.4%) of those aged 12-17 are fully vaccinated, as are 6.6 million (22.9%) of the 5- to 11-year-olds, the CDC said on its COVID Data Tracker.
The indication of an Omicron decline has become a trend: New cases of COVID-19 in children were down for a second consecutive week in the United States, according to the American Academy of Pediatrics and the Children’s Hospital Association.
but the nearly 632,000 cases reported were down by 22% from the previous week and by 45% from what appears to be the peak of the Omicron surge during the week of Jan. 14-20, the AAP/CHA data show.
To put the effect of the Delta and Omicron variants into some sort of perspective, the total number of COVID-19 cases among children passed 5 million at the beginning of September 2021, about a year and a half into the pandemic. In the last 5 months, the cumulative count has more than doubled and now stands at 12 million, the AAP and CHA said in their weekly COVID report.
Hospital admissions and emergency department visits followed the same downward trend over the last week. The rate of new hospitalizations fell to 0.81 per 100,000 children aged 0-17 years as of Feb. 2 (down from a peak of 1.25 per 100,000 on Jan. 15), and ED visits with diagnosed COVID-19 dropped to 1.8% (peak was 14.1%), 1.9% (peak was 14.3%), and 3.4% (peak was 14%) of all visits for children aged 16-17, 12-15, and 0-11 years, respectively, the Centers for Disease Control and Prevention reported.
The vaccination response
The surge of infections brought about by the Omicron variant, however, did not translate into increased vaccination, at least for the youngest eligible children. Vaccine initiation rose slightly among children aged 5-11 in early and mid-January but, by early February, new vaccinations had declined to their lowest point since approval in early November of 2021, the AAP said in its weekly COVID vaccination report.
As a result, the 5- to 11-year-olds are well behind the pace set by those aged 12-15 for the first 3 months of their vaccination experience. Through the first 13 weeks after the COVID vaccine was approved for children aged 12-15 in early May, 44.5% had received at least one dose and 32.3% were fully vaccinated. Among children aged 5-11, the corresponding figures through 13 weeks were 31% and 22.5%, according to CDC data.
The vaccination reaction to Omicron was somewhat more robust for children aged 12-17, compared with the younger group, but initiations dropped at the same time that new cases began to decline. In terms of total volume, the response among 12- to 17-year-olds was much smaller than that seen in July and August of 2021 as the Delta surge was hitting the United States, the AAP vaccination report shows.
All those vaccinations add up to this: Over 16.8 million children aged 12-17 and almost 9 million aged 5-11 had received at least one dose of vaccine as of Feb. 7, which works out to 66.6% of the older group and 31.2% of the younger cohort. Almost 14.3 million (56.4%) of those aged 12-17 are fully vaccinated, as are 6.6 million (22.9%) of the 5- to 11-year-olds, the CDC said on its COVID Data Tracker.
The indication of an Omicron decline has become a trend: New cases of COVID-19 in children were down for a second consecutive week in the United States, according to the American Academy of Pediatrics and the Children’s Hospital Association.
but the nearly 632,000 cases reported were down by 22% from the previous week and by 45% from what appears to be the peak of the Omicron surge during the week of Jan. 14-20, the AAP/CHA data show.
To put the effect of the Delta and Omicron variants into some sort of perspective, the total number of COVID-19 cases among children passed 5 million at the beginning of September 2021, about a year and a half into the pandemic. In the last 5 months, the cumulative count has more than doubled and now stands at 12 million, the AAP and CHA said in their weekly COVID report.
Hospital admissions and emergency department visits followed the same downward trend over the last week. The rate of new hospitalizations fell to 0.81 per 100,000 children aged 0-17 years as of Feb. 2 (down from a peak of 1.25 per 100,000 on Jan. 15), and ED visits with diagnosed COVID-19 dropped to 1.8% (peak was 14.1%), 1.9% (peak was 14.3%), and 3.4% (peak was 14%) of all visits for children aged 16-17, 12-15, and 0-11 years, respectively, the Centers for Disease Control and Prevention reported.
The vaccination response
The surge of infections brought about by the Omicron variant, however, did not translate into increased vaccination, at least for the youngest eligible children. Vaccine initiation rose slightly among children aged 5-11 in early and mid-January but, by early February, new vaccinations had declined to their lowest point since approval in early November of 2021, the AAP said in its weekly COVID vaccination report.
As a result, the 5- to 11-year-olds are well behind the pace set by those aged 12-15 for the first 3 months of their vaccination experience. Through the first 13 weeks after the COVID vaccine was approved for children aged 12-15 in early May, 44.5% had received at least one dose and 32.3% were fully vaccinated. Among children aged 5-11, the corresponding figures through 13 weeks were 31% and 22.5%, according to CDC data.
The vaccination reaction to Omicron was somewhat more robust for children aged 12-17, compared with the younger group, but initiations dropped at the same time that new cases began to decline. In terms of total volume, the response among 12- to 17-year-olds was much smaller than that seen in July and August of 2021 as the Delta surge was hitting the United States, the AAP vaccination report shows.
All those vaccinations add up to this: Over 16.8 million children aged 12-17 and almost 9 million aged 5-11 had received at least one dose of vaccine as of Feb. 7, which works out to 66.6% of the older group and 31.2% of the younger cohort. Almost 14.3 million (56.4%) of those aged 12-17 are fully vaccinated, as are 6.6 million (22.9%) of the 5- to 11-year-olds, the CDC said on its COVID Data Tracker.
Enough is enough: the pandemic and loss of female oncologists
Imagine this: As a young girl, you decide you want to become a doctor when you grow up. You spend countless hours studying, researching, and volunteering to eventually make it into medical school. Four years later, you graduate top of your class and match into your first-choice residency program. You are so proud of yourself!
During your last year of residency, a pandemic takes the entire world by storm. You persevere through your last 14 months of residency that included additional time in the ICU, not seeing your colleagues, and interviewing for your new job all from your own living room. After all of this, you finally get to start doing what you have been waiting to do for the past decade: train with the brilliant minds in hematology and oncology.
All of a sudden, You start to question: If these incredible women have decided that the sacrifice this career requires is too much, then (1) How will I survive? and (2) Did I make a huge mistake in my career decision? Spoiler alert: This girl is me.
The World Health Organization defines burnout as a “syndrome conceptualized as resulting from chronic workplace stress that has not been successfully managed. It is characterized by energy depletion or exhaustion, increased mental distance from one’s job, and reduced professional efficacy.”
We know that 33% of oncologists are feeling burned out right now, according to the Medscape National Physician Burnout & Suicide Report 2021. Of the 51% of female physicians that are burned out, work-life balance has been identified as the biggest workplace concern to them. Research has shown that hours per week devoted to direct patient care is the dominant predictor of burnout for practicing oncologists. But in academic oncology, that is followed by grant deadlines, manuscript rejections, and the constant reminders that you are a new face in oncology, a specialty that was previously male-dominated.
In less than a year, we have had several key female oncologists leave our cancer center. While some made the decision to retire early, two of them chose to pivot their careers and leave clinical medicine to assist with drug development and clinical trials. Although this is extremely important work for cancer care, I was shocked to hear that these amazing and successful clinicians were choosing to remove all direct patient care from their practice, when for many of them, patient care was what motivated them to pursue medicine in the first place. They were loved by their patients, respected as researchers, and well known as educators within the division.
One shared that she no longer felt like she could be a good mother, wife, or daughter with what was currently being demanded of her to have a successful academic career. In hearing this news, I was saddened to have to say goodbye to a mentor of mine and immediately started second-guessing my career choice. I felt that my goal of having an impactful career and prosperous home life was not only unattainable but potentially unrealistic.
While we know that female physicians already experience a greater degree of burnout, the pandemic has only added fuel to the fire. This is especially true in cancer care. It has been estimated that new cancer diagnosis have decreased by as much as 23% since the beginning of the pandemic. This delay in diagnosis will lead to patients presenting with more advanced disease, busier clinic schedules, and worsened clinical outcomes for years to come. With no end in sight, I worry what this will mean for women currently in oncology, in addition to those in training or deciding if they should pursue this as a career.
Extrapolating evidence from prior epidemics, physicians are at increased risk for burnout due to immediate and long-term effects from this pandemic. We need to act now to not only continue addressing previously existing individual and organizational causes of burnout but also develop strategies to provide support for the COVID-19–specific impacts on oncologists’ well-being. An editorial published by the American Society of Clinical Oncology provides helpful suggestions on how to do this.
A recent cross-sectional survey found that 22% of academic female oncologists were likely or very likely to pursue a career outside of academia in the next 5 years. Losing these women would be detrimental to the field. This would mean a significant number of patients losing their long-term oncologists with whom they have years of care, trainees losing their professional and research mentors to guide and help mold them into successful independent practitioners and researchers, and arguably most important, little girls losing role models to show them that regardless of their gender, they can become an oncologist.Dr. Poterala is a current hematology and oncology fellow at the University of Wisconsin Carbone Cancer Center, Madison. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Imagine this: As a young girl, you decide you want to become a doctor when you grow up. You spend countless hours studying, researching, and volunteering to eventually make it into medical school. Four years later, you graduate top of your class and match into your first-choice residency program. You are so proud of yourself!
During your last year of residency, a pandemic takes the entire world by storm. You persevere through your last 14 months of residency that included additional time in the ICU, not seeing your colleagues, and interviewing for your new job all from your own living room. After all of this, you finally get to start doing what you have been waiting to do for the past decade: train with the brilliant minds in hematology and oncology.
All of a sudden, You start to question: If these incredible women have decided that the sacrifice this career requires is too much, then (1) How will I survive? and (2) Did I make a huge mistake in my career decision? Spoiler alert: This girl is me.
The World Health Organization defines burnout as a “syndrome conceptualized as resulting from chronic workplace stress that has not been successfully managed. It is characterized by energy depletion or exhaustion, increased mental distance from one’s job, and reduced professional efficacy.”
We know that 33% of oncologists are feeling burned out right now, according to the Medscape National Physician Burnout & Suicide Report 2021. Of the 51% of female physicians that are burned out, work-life balance has been identified as the biggest workplace concern to them. Research has shown that hours per week devoted to direct patient care is the dominant predictor of burnout for practicing oncologists. But in academic oncology, that is followed by grant deadlines, manuscript rejections, and the constant reminders that you are a new face in oncology, a specialty that was previously male-dominated.
In less than a year, we have had several key female oncologists leave our cancer center. While some made the decision to retire early, two of them chose to pivot their careers and leave clinical medicine to assist with drug development and clinical trials. Although this is extremely important work for cancer care, I was shocked to hear that these amazing and successful clinicians were choosing to remove all direct patient care from their practice, when for many of them, patient care was what motivated them to pursue medicine in the first place. They were loved by their patients, respected as researchers, and well known as educators within the division.
One shared that she no longer felt like she could be a good mother, wife, or daughter with what was currently being demanded of her to have a successful academic career. In hearing this news, I was saddened to have to say goodbye to a mentor of mine and immediately started second-guessing my career choice. I felt that my goal of having an impactful career and prosperous home life was not only unattainable but potentially unrealistic.
While we know that female physicians already experience a greater degree of burnout, the pandemic has only added fuel to the fire. This is especially true in cancer care. It has been estimated that new cancer diagnosis have decreased by as much as 23% since the beginning of the pandemic. This delay in diagnosis will lead to patients presenting with more advanced disease, busier clinic schedules, and worsened clinical outcomes for years to come. With no end in sight, I worry what this will mean for women currently in oncology, in addition to those in training or deciding if they should pursue this as a career.
Extrapolating evidence from prior epidemics, physicians are at increased risk for burnout due to immediate and long-term effects from this pandemic. We need to act now to not only continue addressing previously existing individual and organizational causes of burnout but also develop strategies to provide support for the COVID-19–specific impacts on oncologists’ well-being. An editorial published by the American Society of Clinical Oncology provides helpful suggestions on how to do this.
A recent cross-sectional survey found that 22% of academic female oncologists were likely or very likely to pursue a career outside of academia in the next 5 years. Losing these women would be detrimental to the field. This would mean a significant number of patients losing their long-term oncologists with whom they have years of care, trainees losing their professional and research mentors to guide and help mold them into successful independent practitioners and researchers, and arguably most important, little girls losing role models to show them that regardless of their gender, they can become an oncologist.Dr. Poterala is a current hematology and oncology fellow at the University of Wisconsin Carbone Cancer Center, Madison. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Imagine this: As a young girl, you decide you want to become a doctor when you grow up. You spend countless hours studying, researching, and volunteering to eventually make it into medical school. Four years later, you graduate top of your class and match into your first-choice residency program. You are so proud of yourself!
During your last year of residency, a pandemic takes the entire world by storm. You persevere through your last 14 months of residency that included additional time in the ICU, not seeing your colleagues, and interviewing for your new job all from your own living room. After all of this, you finally get to start doing what you have been waiting to do for the past decade: train with the brilliant minds in hematology and oncology.
All of a sudden, You start to question: If these incredible women have decided that the sacrifice this career requires is too much, then (1) How will I survive? and (2) Did I make a huge mistake in my career decision? Spoiler alert: This girl is me.
The World Health Organization defines burnout as a “syndrome conceptualized as resulting from chronic workplace stress that has not been successfully managed. It is characterized by energy depletion or exhaustion, increased mental distance from one’s job, and reduced professional efficacy.”
We know that 33% of oncologists are feeling burned out right now, according to the Medscape National Physician Burnout & Suicide Report 2021. Of the 51% of female physicians that are burned out, work-life balance has been identified as the biggest workplace concern to them. Research has shown that hours per week devoted to direct patient care is the dominant predictor of burnout for practicing oncologists. But in academic oncology, that is followed by grant deadlines, manuscript rejections, and the constant reminders that you are a new face in oncology, a specialty that was previously male-dominated.
In less than a year, we have had several key female oncologists leave our cancer center. While some made the decision to retire early, two of them chose to pivot their careers and leave clinical medicine to assist with drug development and clinical trials. Although this is extremely important work for cancer care, I was shocked to hear that these amazing and successful clinicians were choosing to remove all direct patient care from their practice, when for many of them, patient care was what motivated them to pursue medicine in the first place. They were loved by their patients, respected as researchers, and well known as educators within the division.
One shared that she no longer felt like she could be a good mother, wife, or daughter with what was currently being demanded of her to have a successful academic career. In hearing this news, I was saddened to have to say goodbye to a mentor of mine and immediately started second-guessing my career choice. I felt that my goal of having an impactful career and prosperous home life was not only unattainable but potentially unrealistic.
While we know that female physicians already experience a greater degree of burnout, the pandemic has only added fuel to the fire. This is especially true in cancer care. It has been estimated that new cancer diagnosis have decreased by as much as 23% since the beginning of the pandemic. This delay in diagnosis will lead to patients presenting with more advanced disease, busier clinic schedules, and worsened clinical outcomes for years to come. With no end in sight, I worry what this will mean for women currently in oncology, in addition to those in training or deciding if they should pursue this as a career.
Extrapolating evidence from prior epidemics, physicians are at increased risk for burnout due to immediate and long-term effects from this pandemic. We need to act now to not only continue addressing previously existing individual and organizational causes of burnout but also develop strategies to provide support for the COVID-19–specific impacts on oncologists’ well-being. An editorial published by the American Society of Clinical Oncology provides helpful suggestions on how to do this.
A recent cross-sectional survey found that 22% of academic female oncologists were likely or very likely to pursue a career outside of academia in the next 5 years. Losing these women would be detrimental to the field. This would mean a significant number of patients losing their long-term oncologists with whom they have years of care, trainees losing their professional and research mentors to guide and help mold them into successful independent practitioners and researchers, and arguably most important, little girls losing role models to show them that regardless of their gender, they can become an oncologist.Dr. Poterala is a current hematology and oncology fellow at the University of Wisconsin Carbone Cancer Center, Madison. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.