User login
Disaster Preparedness in Dermatology Residency Programs
In an age of changing climate and emerging global pandemics, the ability of residency programs to prepare for and adapt to potential disasters may be paramount in preserving the training of physicians. The current literature regarding residency program disaster preparedness, which focuses predominantly on hurricanes and COVID-19,1-8 is lacking in recommendations specific to dermatology residency programs. Likewise, the Accreditation Council for Graduate Medical Education (ACGME) guidelines9 do not address dermatology-specific concerns in disaster preparedness or response. Herein, we propose recommendations to mitigate the impact of various types of disasters on dermatology residency programs and their trainees with regard to resident safety and wellness, resident education, and patient care (Table).
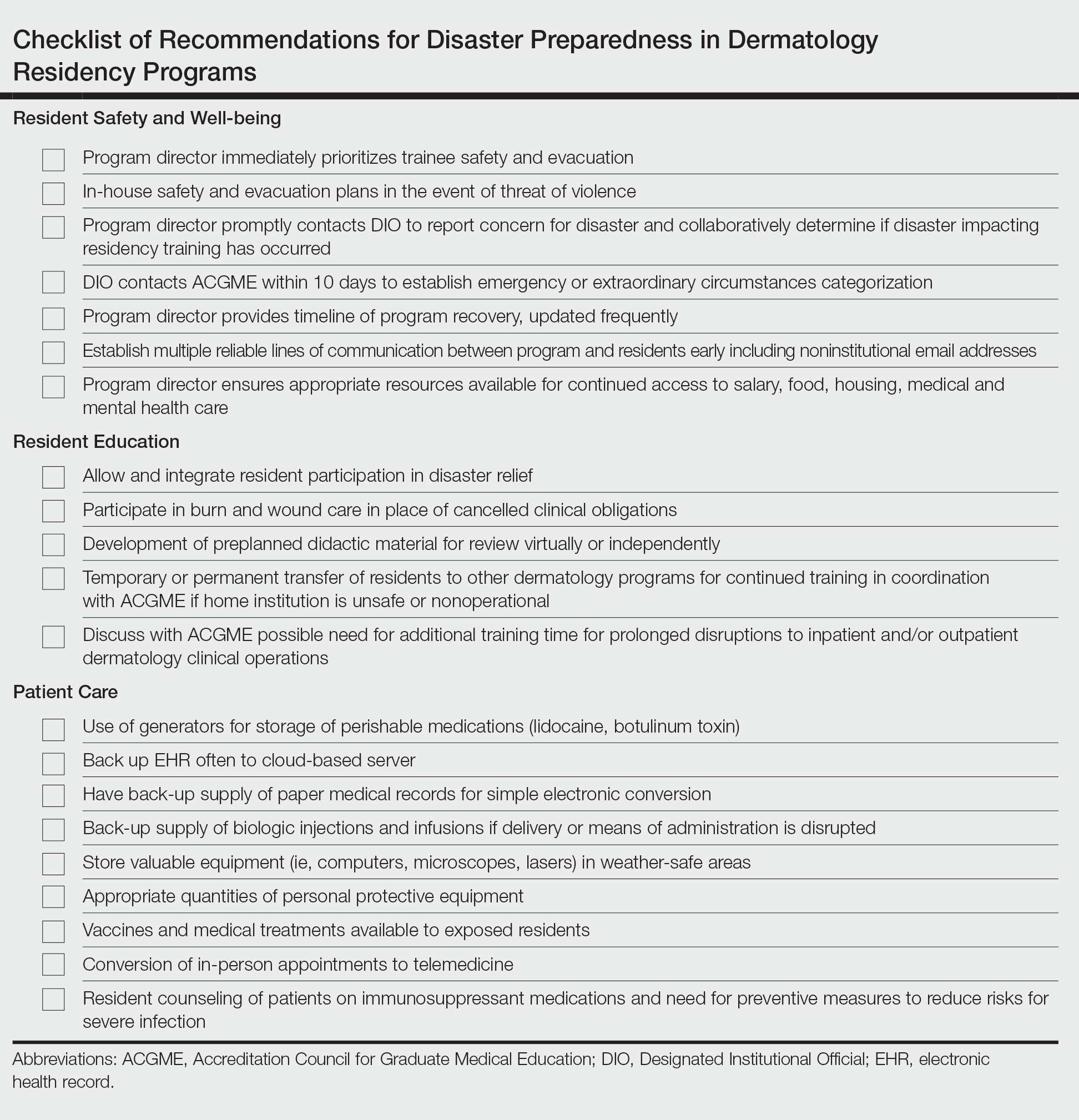
Resident Safety and Wellness
Role of the Program Director—The role of the program director is critical, serving as a figure of structure and reassurance.4,7,10 Once concern of disaster arises, the program director should contact the Designated Institutional Official (DIO) to express concerns about possible disruptions to resident training. The DIO should then contact the ACGME within 10 days to report the disaster and submit a request for emergency (eg, pandemic) or extraordinary circumstances (eg, natural disaster) categorization.4,9 Program directors should promptly prepare plans for program reconfiguration and resident transfers in alignment with ACGME requirements to maintain evaluation and completion of core competencies of training during disasters.9 Program directors should prioritize the safety of trainees during the immediate threat with clear guidelines on sheltering, evacuations, or quarantines; a timeline of program recovery based on communication with residents, faculty, and administration should then be established.10,11
Communication—Establishing a strong line of communication between program directors and residents is paramount. Collection of emergency noninstitutional contact information, establishment of a centralized website for information dissemination, use of noninstitutional email and proxy servers outside of the location of impact, social media updates, on-site use of 2-way radios, and program-wide conference calls when possible should be strongly considered as part of the disaster response.2-4,12,13
Resident Accommodations and Mental Health—If training is disrupted, residents should be reassured of continued access to salary, housing, food, or other resources as necessary.3,4,11 There should be clear contingency plans if residents need to leave the program for extended periods of time due to injury, illness, or personal circumstances. Although relevant in all types of disasters, resident mental health and response to trauma also must be addressed. Access to counseling, morale-building opportunities (eg, resident social events), and screening for depression or posttraumatic stress disorder may help promote well-being among residents following traumatic events.14
Resident Education
Participation in Disaster Relief—Residents may seek to aid in the disaster response, which may prove challenging in the setting of programs with high patient volume.4 In coordination with the ACGME and graduate medical education governing bodies, program directors should consider how residents may fulfill dermatology training requirements in conjunction with disaster relief efforts, such as working in an inpatient setting or providing wound care.10
Continued Didactic Education—The use of online learning and conference calls for continuing the dermatology curriculum is an efficient means to maintaining resident education when meeting in person poses risks to residents.15 Projections of microscopy images, clinical photographs, or other instructional materials allow for continued instruction on resident examination, histopathology, and diagnostic skills.
Continued Clinical Training—If the home institution cannot support the operation of dermatology clinics, residents should be guaranteed continued training at other institutions. Agreements with other dermatology programs, community hospitals, or private dermatology practices should be established in advance, with consideration given to the number of residents a program can support, funding transfers, and credentialing requirements.2,4,5
Prolonged Disruptions—Nonessential departments of medical institutions may cease to function during war or mass casualty disasters, and it may be unsafe to send dermatology residents to other institutions or clinical areas. If the threat is prolonged, programs may need to consider allowing current residents a longer duration of training despite potential overlap with incoming dermatology residents.7
Patient Care
Disruptions to Clinic Operations—Regarding threats of violence, dangerous exposures, or natural disasters, there should be clear guidelines on sheltering in the clinical setting or stabilizing patients during a procedure.11 Equipment used by residents such as laptops, microscopes, and treatment devices (eg, lasers) should be stored in weather-safe locations that would not be notably impacted by moisture or structural damage to the clinic building. If electricity or internet access are compromised, paper medical records should be available to residents to continue clinical operations. Electronic health records used by residents should regularly be backed up on remote servers or cloud storage to allow continued access to patient health information if on-site servers are not functional.12 If disruptions are prolonged, residency program administration should coordinate with the institution to ensure there is adequate supply and storage of medications (eg, lidocaine, botulinum toxin) as well as a continued means of delivering biologic medications to patients and an ability to obtain laboratory or dermatopathology services.
In-Person Appointments vs Telemedicine—There are benefits to both residency training and patient care when physicians are able to perform in-person examinations, biopsies, and in-office treatments.16 Programs should ensure an adequate supply of personal protective equipment to continue in-office appointments, vaccinations, and medical care if a resident or other members of the team are exposed to an infectious disease.7 If in-person appointments are limited or impossible, telemedicine capabilities may still allow residents to meet program requirements.7,10,15 However, reduced patient volume due to decreased elective visits or procedures may complicate the fulfillment of clinical requirements, which may need to be adjusted in the wake of a disaster.7
Use of Immunosuppressive Therapies—Residency programs should address the risks of prescribing immunosuppressive therapies (eg, biologics) during an infectious threat with their residents and encourage trainees to counsel patients on the importance of preventative measures to reduce risks for severe infection.17
Final Thoughts
- Davis W. Hurricane Katrina: the challenge to graduate medical education. Ochsner J. 2006;6:39.
- Cefalu CA, Schwartz RS. Salvaging a geriatric medicine academic program in disaster mode—the LSU training program post-Katrina.J Natl Med Assoc. 2007;99:590-596.
- Ayyala R. Lessons from Katrina: a program director’s perspective. Ophthalmology. 2007;114:1425-1426.
- Wiese JG. Leadership in graduate medical education: eleven steps instrumental in recovering residency programs after a disaster. Am J Med Sci. 2008;336:168-173.
- Griffies WS. Post-Katrina stabilization of the LSU/Ochsner Psychiatry Residency Program: caveats for disaster preparedness. Acad Psychiatry. 2009;33:418-422.
- Kearns DG, Chat VS, Uppal S, et al. Applying to dermatology residency during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:1214-1215.
- Matthews JB, Blair PG, Ellison EC, et al. Checklist framework for surgical education disaster plans. J Am Coll Surg. 2021;233:557-563.
- Litchman GH, Marson JW, Rigel DS. The continuing impact of COVID-19 on dermatology practice: office workflow, economics, and future implications. J Am Acad Dermatol. 2021;84:576-579.
- Accreditation Council for Graduate Medical Education. Sponsoring institution emergency categorization. Accessed October 20, 2022. https://www.acgme.org/covid-19/sponsoring-institution-emergency-categorization/
- Li YM, Galimberti F, Abrouk M, et al. US dermatology resident responses about the COVID-19 pandemic: results from a nationwide survey. South Med J. 2020;113:462-465.
- Newman B, Gallion C. Hurricane Harvey: firsthand perspectives for disaster preparedness in graduate medical education. Acad Med. 2019;94:1267-1269.
- Pero CD, Pou AM, Arriaga MA, et al. Post-Katrina: study in crisis-related program adaptability. Otolaryngol Head Neck Surg. 2008;138:394-397.
- Hattaway R, Singh N, Rais-Bahrami S, et al. Adaptations of dermatology residency programs to changes in medical education amid the COVID-19 pandemic: virtual opportunities and social media. SKIN. 2021;5:94-100.
- Hillier K, Paskaradevan J, Wilkes JK, et al. Disaster plans: resident involvement and well-being during Hurricane Harvey. J Grad Med Educ. 2019;11:129-131.
- Samimi S, Choi J, Rosman IS, et al. Impact of COVID-19 on dermatology residency. Dermatol Clin. 2021;39:609-618.
- Bastola M, Locatis C, Fontelo P. Diagnostic reliability of in-person versus remote dermatology: a meta-analysis. Telemed J E Health. 2021;27:247-250.
- Bashyam AM, Feldman SR. Should patients stop their biologic treatment during the COVID-19 pandemic? J Dermatolog Treat. 2020;31:317-318.
In an age of changing climate and emerging global pandemics, the ability of residency programs to prepare for and adapt to potential disasters may be paramount in preserving the training of physicians. The current literature regarding residency program disaster preparedness, which focuses predominantly on hurricanes and COVID-19,1-8 is lacking in recommendations specific to dermatology residency programs. Likewise, the Accreditation Council for Graduate Medical Education (ACGME) guidelines9 do not address dermatology-specific concerns in disaster preparedness or response. Herein, we propose recommendations to mitigate the impact of various types of disasters on dermatology residency programs and their trainees with regard to resident safety and wellness, resident education, and patient care (Table).

Resident Safety and Wellness
Role of the Program Director—The role of the program director is critical, serving as a figure of structure and reassurance.4,7,10 Once concern of disaster arises, the program director should contact the Designated Institutional Official (DIO) to express concerns about possible disruptions to resident training. The DIO should then contact the ACGME within 10 days to report the disaster and submit a request for emergency (eg, pandemic) or extraordinary circumstances (eg, natural disaster) categorization.4,9 Program directors should promptly prepare plans for program reconfiguration and resident transfers in alignment with ACGME requirements to maintain evaluation and completion of core competencies of training during disasters.9 Program directors should prioritize the safety of trainees during the immediate threat with clear guidelines on sheltering, evacuations, or quarantines; a timeline of program recovery based on communication with residents, faculty, and administration should then be established.10,11
Communication—Establishing a strong line of communication between program directors and residents is paramount. Collection of emergency noninstitutional contact information, establishment of a centralized website for information dissemination, use of noninstitutional email and proxy servers outside of the location of impact, social media updates, on-site use of 2-way radios, and program-wide conference calls when possible should be strongly considered as part of the disaster response.2-4,12,13
Resident Accommodations and Mental Health—If training is disrupted, residents should be reassured of continued access to salary, housing, food, or other resources as necessary.3,4,11 There should be clear contingency plans if residents need to leave the program for extended periods of time due to injury, illness, or personal circumstances. Although relevant in all types of disasters, resident mental health and response to trauma also must be addressed. Access to counseling, morale-building opportunities (eg, resident social events), and screening for depression or posttraumatic stress disorder may help promote well-being among residents following traumatic events.14
Resident Education
Participation in Disaster Relief—Residents may seek to aid in the disaster response, which may prove challenging in the setting of programs with high patient volume.4 In coordination with the ACGME and graduate medical education governing bodies, program directors should consider how residents may fulfill dermatology training requirements in conjunction with disaster relief efforts, such as working in an inpatient setting or providing wound care.10
Continued Didactic Education—The use of online learning and conference calls for continuing the dermatology curriculum is an efficient means to maintaining resident education when meeting in person poses risks to residents.15 Projections of microscopy images, clinical photographs, or other instructional materials allow for continued instruction on resident examination, histopathology, and diagnostic skills.
Continued Clinical Training—If the home institution cannot support the operation of dermatology clinics, residents should be guaranteed continued training at other institutions. Agreements with other dermatology programs, community hospitals, or private dermatology practices should be established in advance, with consideration given to the number of residents a program can support, funding transfers, and credentialing requirements.2,4,5
Prolonged Disruptions—Nonessential departments of medical institutions may cease to function during war or mass casualty disasters, and it may be unsafe to send dermatology residents to other institutions or clinical areas. If the threat is prolonged, programs may need to consider allowing current residents a longer duration of training despite potential overlap with incoming dermatology residents.7
Patient Care
Disruptions to Clinic Operations—Regarding threats of violence, dangerous exposures, or natural disasters, there should be clear guidelines on sheltering in the clinical setting or stabilizing patients during a procedure.11 Equipment used by residents such as laptops, microscopes, and treatment devices (eg, lasers) should be stored in weather-safe locations that would not be notably impacted by moisture or structural damage to the clinic building. If electricity or internet access are compromised, paper medical records should be available to residents to continue clinical operations. Electronic health records used by residents should regularly be backed up on remote servers or cloud storage to allow continued access to patient health information if on-site servers are not functional.12 If disruptions are prolonged, residency program administration should coordinate with the institution to ensure there is adequate supply and storage of medications (eg, lidocaine, botulinum toxin) as well as a continued means of delivering biologic medications to patients and an ability to obtain laboratory or dermatopathology services.
In-Person Appointments vs Telemedicine—There are benefits to both residency training and patient care when physicians are able to perform in-person examinations, biopsies, and in-office treatments.16 Programs should ensure an adequate supply of personal protective equipment to continue in-office appointments, vaccinations, and medical care if a resident or other members of the team are exposed to an infectious disease.7 If in-person appointments are limited or impossible, telemedicine capabilities may still allow residents to meet program requirements.7,10,15 However, reduced patient volume due to decreased elective visits or procedures may complicate the fulfillment of clinical requirements, which may need to be adjusted in the wake of a disaster.7
Use of Immunosuppressive Therapies—Residency programs should address the risks of prescribing immunosuppressive therapies (eg, biologics) during an infectious threat with their residents and encourage trainees to counsel patients on the importance of preventative measures to reduce risks for severe infection.17
Final Thoughts
In an age of changing climate and emerging global pandemics, the ability of residency programs to prepare for and adapt to potential disasters may be paramount in preserving the training of physicians. The current literature regarding residency program disaster preparedness, which focuses predominantly on hurricanes and COVID-19,1-8 is lacking in recommendations specific to dermatology residency programs. Likewise, the Accreditation Council for Graduate Medical Education (ACGME) guidelines9 do not address dermatology-specific concerns in disaster preparedness or response. Herein, we propose recommendations to mitigate the impact of various types of disasters on dermatology residency programs and their trainees with regard to resident safety and wellness, resident education, and patient care (Table).

Resident Safety and Wellness
Role of the Program Director—The role of the program director is critical, serving as a figure of structure and reassurance.4,7,10 Once concern of disaster arises, the program director should contact the Designated Institutional Official (DIO) to express concerns about possible disruptions to resident training. The DIO should then contact the ACGME within 10 days to report the disaster and submit a request for emergency (eg, pandemic) or extraordinary circumstances (eg, natural disaster) categorization.4,9 Program directors should promptly prepare plans for program reconfiguration and resident transfers in alignment with ACGME requirements to maintain evaluation and completion of core competencies of training during disasters.9 Program directors should prioritize the safety of trainees during the immediate threat with clear guidelines on sheltering, evacuations, or quarantines; a timeline of program recovery based on communication with residents, faculty, and administration should then be established.10,11
Communication—Establishing a strong line of communication between program directors and residents is paramount. Collection of emergency noninstitutional contact information, establishment of a centralized website for information dissemination, use of noninstitutional email and proxy servers outside of the location of impact, social media updates, on-site use of 2-way radios, and program-wide conference calls when possible should be strongly considered as part of the disaster response.2-4,12,13
Resident Accommodations and Mental Health—If training is disrupted, residents should be reassured of continued access to salary, housing, food, or other resources as necessary.3,4,11 There should be clear contingency plans if residents need to leave the program for extended periods of time due to injury, illness, or personal circumstances. Although relevant in all types of disasters, resident mental health and response to trauma also must be addressed. Access to counseling, morale-building opportunities (eg, resident social events), and screening for depression or posttraumatic stress disorder may help promote well-being among residents following traumatic events.14
Resident Education
Participation in Disaster Relief—Residents may seek to aid in the disaster response, which may prove challenging in the setting of programs with high patient volume.4 In coordination with the ACGME and graduate medical education governing bodies, program directors should consider how residents may fulfill dermatology training requirements in conjunction with disaster relief efforts, such as working in an inpatient setting or providing wound care.10
Continued Didactic Education—The use of online learning and conference calls for continuing the dermatology curriculum is an efficient means to maintaining resident education when meeting in person poses risks to residents.15 Projections of microscopy images, clinical photographs, or other instructional materials allow for continued instruction on resident examination, histopathology, and diagnostic skills.
Continued Clinical Training—If the home institution cannot support the operation of dermatology clinics, residents should be guaranteed continued training at other institutions. Agreements with other dermatology programs, community hospitals, or private dermatology practices should be established in advance, with consideration given to the number of residents a program can support, funding transfers, and credentialing requirements.2,4,5
Prolonged Disruptions—Nonessential departments of medical institutions may cease to function during war or mass casualty disasters, and it may be unsafe to send dermatology residents to other institutions or clinical areas. If the threat is prolonged, programs may need to consider allowing current residents a longer duration of training despite potential overlap with incoming dermatology residents.7
Patient Care
Disruptions to Clinic Operations—Regarding threats of violence, dangerous exposures, or natural disasters, there should be clear guidelines on sheltering in the clinical setting or stabilizing patients during a procedure.11 Equipment used by residents such as laptops, microscopes, and treatment devices (eg, lasers) should be stored in weather-safe locations that would not be notably impacted by moisture or structural damage to the clinic building. If electricity or internet access are compromised, paper medical records should be available to residents to continue clinical operations. Electronic health records used by residents should regularly be backed up on remote servers or cloud storage to allow continued access to patient health information if on-site servers are not functional.12 If disruptions are prolonged, residency program administration should coordinate with the institution to ensure there is adequate supply and storage of medications (eg, lidocaine, botulinum toxin) as well as a continued means of delivering biologic medications to patients and an ability to obtain laboratory or dermatopathology services.
In-Person Appointments vs Telemedicine—There are benefits to both residency training and patient care when physicians are able to perform in-person examinations, biopsies, and in-office treatments.16 Programs should ensure an adequate supply of personal protective equipment to continue in-office appointments, vaccinations, and medical care if a resident or other members of the team are exposed to an infectious disease.7 If in-person appointments are limited or impossible, telemedicine capabilities may still allow residents to meet program requirements.7,10,15 However, reduced patient volume due to decreased elective visits or procedures may complicate the fulfillment of clinical requirements, which may need to be adjusted in the wake of a disaster.7
Use of Immunosuppressive Therapies—Residency programs should address the risks of prescribing immunosuppressive therapies (eg, biologics) during an infectious threat with their residents and encourage trainees to counsel patients on the importance of preventative measures to reduce risks for severe infection.17
Final Thoughts
- Davis W. Hurricane Katrina: the challenge to graduate medical education. Ochsner J. 2006;6:39.
- Cefalu CA, Schwartz RS. Salvaging a geriatric medicine academic program in disaster mode—the LSU training program post-Katrina.J Natl Med Assoc. 2007;99:590-596.
- Ayyala R. Lessons from Katrina: a program director’s perspective. Ophthalmology. 2007;114:1425-1426.
- Wiese JG. Leadership in graduate medical education: eleven steps instrumental in recovering residency programs after a disaster. Am J Med Sci. 2008;336:168-173.
- Griffies WS. Post-Katrina stabilization of the LSU/Ochsner Psychiatry Residency Program: caveats for disaster preparedness. Acad Psychiatry. 2009;33:418-422.
- Kearns DG, Chat VS, Uppal S, et al. Applying to dermatology residency during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:1214-1215.
- Matthews JB, Blair PG, Ellison EC, et al. Checklist framework for surgical education disaster plans. J Am Coll Surg. 2021;233:557-563.
- Litchman GH, Marson JW, Rigel DS. The continuing impact of COVID-19 on dermatology practice: office workflow, economics, and future implications. J Am Acad Dermatol. 2021;84:576-579.
- Accreditation Council for Graduate Medical Education. Sponsoring institution emergency categorization. Accessed October 20, 2022. https://www.acgme.org/covid-19/sponsoring-institution-emergency-categorization/
- Li YM, Galimberti F, Abrouk M, et al. US dermatology resident responses about the COVID-19 pandemic: results from a nationwide survey. South Med J. 2020;113:462-465.
- Newman B, Gallion C. Hurricane Harvey: firsthand perspectives for disaster preparedness in graduate medical education. Acad Med. 2019;94:1267-1269.
- Pero CD, Pou AM, Arriaga MA, et al. Post-Katrina: study in crisis-related program adaptability. Otolaryngol Head Neck Surg. 2008;138:394-397.
- Hattaway R, Singh N, Rais-Bahrami S, et al. Adaptations of dermatology residency programs to changes in medical education amid the COVID-19 pandemic: virtual opportunities and social media. SKIN. 2021;5:94-100.
- Hillier K, Paskaradevan J, Wilkes JK, et al. Disaster plans: resident involvement and well-being during Hurricane Harvey. J Grad Med Educ. 2019;11:129-131.
- Samimi S, Choi J, Rosman IS, et al. Impact of COVID-19 on dermatology residency. Dermatol Clin. 2021;39:609-618.
- Bastola M, Locatis C, Fontelo P. Diagnostic reliability of in-person versus remote dermatology: a meta-analysis. Telemed J E Health. 2021;27:247-250.
- Bashyam AM, Feldman SR. Should patients stop their biologic treatment during the COVID-19 pandemic? J Dermatolog Treat. 2020;31:317-318.
- Davis W. Hurricane Katrina: the challenge to graduate medical education. Ochsner J. 2006;6:39.
- Cefalu CA, Schwartz RS. Salvaging a geriatric medicine academic program in disaster mode—the LSU training program post-Katrina.J Natl Med Assoc. 2007;99:590-596.
- Ayyala R. Lessons from Katrina: a program director’s perspective. Ophthalmology. 2007;114:1425-1426.
- Wiese JG. Leadership in graduate medical education: eleven steps instrumental in recovering residency programs after a disaster. Am J Med Sci. 2008;336:168-173.
- Griffies WS. Post-Katrina stabilization of the LSU/Ochsner Psychiatry Residency Program: caveats for disaster preparedness. Acad Psychiatry. 2009;33:418-422.
- Kearns DG, Chat VS, Uppal S, et al. Applying to dermatology residency during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:1214-1215.
- Matthews JB, Blair PG, Ellison EC, et al. Checklist framework for surgical education disaster plans. J Am Coll Surg. 2021;233:557-563.
- Litchman GH, Marson JW, Rigel DS. The continuing impact of COVID-19 on dermatology practice: office workflow, economics, and future implications. J Am Acad Dermatol. 2021;84:576-579.
- Accreditation Council for Graduate Medical Education. Sponsoring institution emergency categorization. Accessed October 20, 2022. https://www.acgme.org/covid-19/sponsoring-institution-emergency-categorization/
- Li YM, Galimberti F, Abrouk M, et al. US dermatology resident responses about the COVID-19 pandemic: results from a nationwide survey. South Med J. 2020;113:462-465.
- Newman B, Gallion C. Hurricane Harvey: firsthand perspectives for disaster preparedness in graduate medical education. Acad Med. 2019;94:1267-1269.
- Pero CD, Pou AM, Arriaga MA, et al. Post-Katrina: study in crisis-related program adaptability. Otolaryngol Head Neck Surg. 2008;138:394-397.
- Hattaway R, Singh N, Rais-Bahrami S, et al. Adaptations of dermatology residency programs to changes in medical education amid the COVID-19 pandemic: virtual opportunities and social media. SKIN. 2021;5:94-100.
- Hillier K, Paskaradevan J, Wilkes JK, et al. Disaster plans: resident involvement and well-being during Hurricane Harvey. J Grad Med Educ. 2019;11:129-131.
- Samimi S, Choi J, Rosman IS, et al. Impact of COVID-19 on dermatology residency. Dermatol Clin. 2021;39:609-618.
- Bastola M, Locatis C, Fontelo P. Diagnostic reliability of in-person versus remote dermatology: a meta-analysis. Telemed J E Health. 2021;27:247-250.
- Bashyam AM, Feldman SR. Should patients stop their biologic treatment during the COVID-19 pandemic? J Dermatolog Treat. 2020;31:317-318.
Practice Points
- Dermatology residency programs should prioritize the development of disaster preparedness plans prior to the onset of disasters.
- Comprehensive disaster preparedness addresses many possible disruptions to dermatology resident training and clinic operations, including natural and manmade disasters and threats of widespread infectious disease.
- Safety being paramount, dermatology residency programs may be tasked with maintaining resident wellness, continuing resident education—potentially in unconventional ways—and adapting clinical operations to continue patient care.
Man with COVID finally tests negative after 411 days
according to experts in the United Kingdom.
The man was treated with a mixture of neutralizing monoclonal antibodies, King’s College London said in a news release.
The man, 59, tested positive in December 2020 and tested negative in January 2022. He had a weakened immune system because of a previous kidney transplant. He received three doses of vaccine and his symptoms lessened, but he kept testing positive for COVID.
To find out if the man had a persistent infection or had been infected several times, doctors did a genetic analysis of the virus.
“This revealed that the patient’s infection was a persistent infection with an early COVID variant – a variation of the original Wuhan variant that was dominant in the United Kingdom in the later months of 2020. Analysis found the patient’s virus had multiple mutations since he was first infected,” King’s College said.
The doctors treated him with a Regeneron treatment that is no longer widely used because it’s not effective against newer COVID variants.
“Some new variants of the virus are resistant to all the antibody treatments available in the United Kingdom and Europe. Some people with weakened immune systems are still at risk of severe illness and becoming persistently infected. We are still working to understand the best way to protect and treat them,” Luke Snell, MD, from the King’s College School of Immunology & Microbial Sciences, said in the news release.
This is one of the longest known cases of COVID infection. Another man in England was infected with COVID for 505 days before his death, which King’s College said was the longest known COVID infection.
A version of this article first appeared on WebMD.com.
according to experts in the United Kingdom.
The man was treated with a mixture of neutralizing monoclonal antibodies, King’s College London said in a news release.
The man, 59, tested positive in December 2020 and tested negative in January 2022. He had a weakened immune system because of a previous kidney transplant. He received three doses of vaccine and his symptoms lessened, but he kept testing positive for COVID.
To find out if the man had a persistent infection or had been infected several times, doctors did a genetic analysis of the virus.
“This revealed that the patient’s infection was a persistent infection with an early COVID variant – a variation of the original Wuhan variant that was dominant in the United Kingdom in the later months of 2020. Analysis found the patient’s virus had multiple mutations since he was first infected,” King’s College said.
The doctors treated him with a Regeneron treatment that is no longer widely used because it’s not effective against newer COVID variants.
“Some new variants of the virus are resistant to all the antibody treatments available in the United Kingdom and Europe. Some people with weakened immune systems are still at risk of severe illness and becoming persistently infected. We are still working to understand the best way to protect and treat them,” Luke Snell, MD, from the King’s College School of Immunology & Microbial Sciences, said in the news release.
This is one of the longest known cases of COVID infection. Another man in England was infected with COVID for 505 days before his death, which King’s College said was the longest known COVID infection.
A version of this article first appeared on WebMD.com.
according to experts in the United Kingdom.
The man was treated with a mixture of neutralizing monoclonal antibodies, King’s College London said in a news release.
The man, 59, tested positive in December 2020 and tested negative in January 2022. He had a weakened immune system because of a previous kidney transplant. He received three doses of vaccine and his symptoms lessened, but he kept testing positive for COVID.
To find out if the man had a persistent infection or had been infected several times, doctors did a genetic analysis of the virus.
“This revealed that the patient’s infection was a persistent infection with an early COVID variant – a variation of the original Wuhan variant that was dominant in the United Kingdom in the later months of 2020. Analysis found the patient’s virus had multiple mutations since he was first infected,” King’s College said.
The doctors treated him with a Regeneron treatment that is no longer widely used because it’s not effective against newer COVID variants.
“Some new variants of the virus are resistant to all the antibody treatments available in the United Kingdom and Europe. Some people with weakened immune systems are still at risk of severe illness and becoming persistently infected. We are still working to understand the best way to protect and treat them,” Luke Snell, MD, from the King’s College School of Immunology & Microbial Sciences, said in the news release.
This is one of the longest known cases of COVID infection. Another man in England was infected with COVID for 505 days before his death, which King’s College said was the longest known COVID infection.
A version of this article first appeared on WebMD.com.
COVID bivalent booster better vs. recent Omicron subvariants: Pfizer
the company reported on Nov. 4, supporting calls by public health officials for eligible people to get this booster before a potential COVID-19 surge this winter.
The company’s ongoing phase 2/3 study of their Omicron BA.4 and BA.5 bivalent – which targets both the virus’ original strain and the two subvariants – shows that the vaccine offered the strongest protection in people older than 55 years.
One month after receiving a 30-mcg booster with the bivalent vaccine, those older than 55 had four times more neutralizing antibodies against these Omicron subvariants, compared with people who received the original monovalent vaccine as a booster in the study.
Researchers compared the geometric mean titer (GMT) levels of these antibodies in three groups before and 1 month after boosting. The 36 people older than 55 years in the released study findings had an GMT level of 896 with the bivalent booster, a level 13 times higher than before this immunization.
For the 38 adults ages 18-55 in the study, the GMT level increased to 606 at 1 month after the bivalent booster, an increase of almost 10-fold, compared with baseline. In a comparator group of 40 people receiving the original vaccine as a fourth dose, the GMT level was 236, or threefold higher than before their booster shot.
The newly released data is “very encouraging and consistent now with three studies all showing a substantial 3-4 fold increased level of neutralizing antibodies versus BA.5 as compared with the original booster,” said Eric Topol, MD, director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape Medical News.
Pfizer and BioNTech announced the updated findings in a Nov. 4 press release.
A booster dose of the BA.4/BA.5-adapted bivalent vaccine is authorized for emergency use by the Food and Drug Administration for ages 5 years and older. The safety and tolerability profile of the Pfizer/BioNTech bivalent booster remains favorable and similar to the original COVID-19 vaccine, the company reported.
Until recently, the BA.5 Omicron variant was the dominant strain in the United States, but is now getting elbowed out by the subvariants BQ.1.1, BQ.1, and BA.4.6, which together make up almost 45% of the circulating virus.
Some skepticism
“It is important to note that these data are press-release level, which does not allow a view of the data totality,” Hana El Sahly, MD, professor of molecular virology and microbiology, Baylor College of Medicine, Houston, said in an interview.
“For example, there may be significant differences between the groups, and the release mentions at least one difference that is of importance: the interval since the last vaccination which often affects the response to subsequent boosting,” she said.
Dr. El Sahly added that the findings are not surprising. “In the short term, a variant-specific vaccine produces a higher level of antibody against the variant in the vaccine than the vaccines based on the ancestral strains.”
More researcher results are warranted. “These data do not indicate that these differences between the two vaccines translate into a meaningful clinical benefit at a population level,” Dr. El Sahly said.
An uncertain winter ahead
“As we head into the holiday season, we hope these updated data will encourage people to seek out a COVID-19 bivalent booster as soon as they are eligible in order to maintain high levels of protection against the widely circulating Omicron BA.4 and BA.5 sublineages,” Albert Bourla, Pfizer chairman and CEO, stated in the release.
The updated data from the Pfizer/BioNTech study are “all the more reason to get a booster, with added protection also versus BQ.1.1, which will soon become dominant in the U.S.,” Dr. Topol predicted.
It is unclear when the next surge will happen, as COVID-19 does not always follow a seasonal pattern, at least not yet, Dr. El Sahly said. “Regardless, it is reasonable to recommend additional vaccine doses to immunocompromised and frail or older persons. More importantly, influenza vaccination and being up to date on pneumococcal vaccines are highly recommended as soon as feasible, given the early and intense flu season.”
A version of this article first appeared on Medscape.com.
the company reported on Nov. 4, supporting calls by public health officials for eligible people to get this booster before a potential COVID-19 surge this winter.
The company’s ongoing phase 2/3 study of their Omicron BA.4 and BA.5 bivalent – which targets both the virus’ original strain and the two subvariants – shows that the vaccine offered the strongest protection in people older than 55 years.
One month after receiving a 30-mcg booster with the bivalent vaccine, those older than 55 had four times more neutralizing antibodies against these Omicron subvariants, compared with people who received the original monovalent vaccine as a booster in the study.
Researchers compared the geometric mean titer (GMT) levels of these antibodies in three groups before and 1 month after boosting. The 36 people older than 55 years in the released study findings had an GMT level of 896 with the bivalent booster, a level 13 times higher than before this immunization.
For the 38 adults ages 18-55 in the study, the GMT level increased to 606 at 1 month after the bivalent booster, an increase of almost 10-fold, compared with baseline. In a comparator group of 40 people receiving the original vaccine as a fourth dose, the GMT level was 236, or threefold higher than before their booster shot.
The newly released data is “very encouraging and consistent now with three studies all showing a substantial 3-4 fold increased level of neutralizing antibodies versus BA.5 as compared with the original booster,” said Eric Topol, MD, director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape Medical News.
Pfizer and BioNTech announced the updated findings in a Nov. 4 press release.
A booster dose of the BA.4/BA.5-adapted bivalent vaccine is authorized for emergency use by the Food and Drug Administration for ages 5 years and older. The safety and tolerability profile of the Pfizer/BioNTech bivalent booster remains favorable and similar to the original COVID-19 vaccine, the company reported.
Until recently, the BA.5 Omicron variant was the dominant strain in the United States, but is now getting elbowed out by the subvariants BQ.1.1, BQ.1, and BA.4.6, which together make up almost 45% of the circulating virus.
Some skepticism
“It is important to note that these data are press-release level, which does not allow a view of the data totality,” Hana El Sahly, MD, professor of molecular virology and microbiology, Baylor College of Medicine, Houston, said in an interview.
“For example, there may be significant differences between the groups, and the release mentions at least one difference that is of importance: the interval since the last vaccination which often affects the response to subsequent boosting,” she said.
Dr. El Sahly added that the findings are not surprising. “In the short term, a variant-specific vaccine produces a higher level of antibody against the variant in the vaccine than the vaccines based on the ancestral strains.”
More researcher results are warranted. “These data do not indicate that these differences between the two vaccines translate into a meaningful clinical benefit at a population level,” Dr. El Sahly said.
An uncertain winter ahead
“As we head into the holiday season, we hope these updated data will encourage people to seek out a COVID-19 bivalent booster as soon as they are eligible in order to maintain high levels of protection against the widely circulating Omicron BA.4 and BA.5 sublineages,” Albert Bourla, Pfizer chairman and CEO, stated in the release.
The updated data from the Pfizer/BioNTech study are “all the more reason to get a booster, with added protection also versus BQ.1.1, which will soon become dominant in the U.S.,” Dr. Topol predicted.
It is unclear when the next surge will happen, as COVID-19 does not always follow a seasonal pattern, at least not yet, Dr. El Sahly said. “Regardless, it is reasonable to recommend additional vaccine doses to immunocompromised and frail or older persons. More importantly, influenza vaccination and being up to date on pneumococcal vaccines are highly recommended as soon as feasible, given the early and intense flu season.”
A version of this article first appeared on Medscape.com.
the company reported on Nov. 4, supporting calls by public health officials for eligible people to get this booster before a potential COVID-19 surge this winter.
The company’s ongoing phase 2/3 study of their Omicron BA.4 and BA.5 bivalent – which targets both the virus’ original strain and the two subvariants – shows that the vaccine offered the strongest protection in people older than 55 years.
One month after receiving a 30-mcg booster with the bivalent vaccine, those older than 55 had four times more neutralizing antibodies against these Omicron subvariants, compared with people who received the original monovalent vaccine as a booster in the study.
Researchers compared the geometric mean titer (GMT) levels of these antibodies in three groups before and 1 month after boosting. The 36 people older than 55 years in the released study findings had an GMT level of 896 with the bivalent booster, a level 13 times higher than before this immunization.
For the 38 adults ages 18-55 in the study, the GMT level increased to 606 at 1 month after the bivalent booster, an increase of almost 10-fold, compared with baseline. In a comparator group of 40 people receiving the original vaccine as a fourth dose, the GMT level was 236, or threefold higher than before their booster shot.
The newly released data is “very encouraging and consistent now with three studies all showing a substantial 3-4 fold increased level of neutralizing antibodies versus BA.5 as compared with the original booster,” said Eric Topol, MD, director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape Medical News.
Pfizer and BioNTech announced the updated findings in a Nov. 4 press release.
A booster dose of the BA.4/BA.5-adapted bivalent vaccine is authorized for emergency use by the Food and Drug Administration for ages 5 years and older. The safety and tolerability profile of the Pfizer/BioNTech bivalent booster remains favorable and similar to the original COVID-19 vaccine, the company reported.
Until recently, the BA.5 Omicron variant was the dominant strain in the United States, but is now getting elbowed out by the subvariants BQ.1.1, BQ.1, and BA.4.6, which together make up almost 45% of the circulating virus.
Some skepticism
“It is important to note that these data are press-release level, which does not allow a view of the data totality,” Hana El Sahly, MD, professor of molecular virology and microbiology, Baylor College of Medicine, Houston, said in an interview.
“For example, there may be significant differences between the groups, and the release mentions at least one difference that is of importance: the interval since the last vaccination which often affects the response to subsequent boosting,” she said.
Dr. El Sahly added that the findings are not surprising. “In the short term, a variant-specific vaccine produces a higher level of antibody against the variant in the vaccine than the vaccines based on the ancestral strains.”
More researcher results are warranted. “These data do not indicate that these differences between the two vaccines translate into a meaningful clinical benefit at a population level,” Dr. El Sahly said.
An uncertain winter ahead
“As we head into the holiday season, we hope these updated data will encourage people to seek out a COVID-19 bivalent booster as soon as they are eligible in order to maintain high levels of protection against the widely circulating Omicron BA.4 and BA.5 sublineages,” Albert Bourla, Pfizer chairman and CEO, stated in the release.
The updated data from the Pfizer/BioNTech study are “all the more reason to get a booster, with added protection also versus BQ.1.1, which will soon become dominant in the U.S.,” Dr. Topol predicted.
It is unclear when the next surge will happen, as COVID-19 does not always follow a seasonal pattern, at least not yet, Dr. El Sahly said. “Regardless, it is reasonable to recommend additional vaccine doses to immunocompromised and frail or older persons. More importantly, influenza vaccination and being up to date on pneumococcal vaccines are highly recommended as soon as feasible, given the early and intense flu season.”
A version of this article first appeared on Medscape.com.
Working while sick: Why doctors don’t stay home when ill
The reasons are likely as varied as, “you weren’t feeling bad enough to miss work,” “you couldn’t afford to miss pay,” “you had too many patients to see,” or “too much work to do.”
In Medscape’s Employed Physicians Report: Loving the Focus, Hating the Bureaucracy, 61% of physicians reported that they sometimes or often come to work sick. Only 2% of respondents said they never come to work unwell.
Medscape wanted to know more about how often you call in sick, how often you come to work feeling unwell, what symptoms you have, and the dogma of your workplace culture regarding sick days. Not to mention the brutal ethos that starts in medical school, in which calling in sick shows weakness or is unacceptable.
So, we polled 2,347 physicians in the United States and abroad and asked them about their sniffling, sneezing, cold, flu, and fever symptoms, and, of course, COVID. Results were split about 50-50 among male and female physicians. The poll ran from Sept. 28 through Oct. 11.
Coming to work sick
It’s no surprise that the majority of physicians who were polled (85%) have come to work sick in 2022. In the last prepandemic year (2019), about 70% came to work feeling sick one to five times, and 13% worked while sick six to ten times.
When asked about the symptoms that they’ve previously come to work with, 48% of U.S. physicians said multiple symptoms. They gave high marks for runny nose, cough, congestion, and sore throat. Only 27% have worked with a fever, 22% have worked with other symptoms, and 7% have worked with both strep throat and COVID.
“My workplace, especially in the COVID years, accommodates persons who honestly do not feel well enough to report. Sooner or later, everyone covers for someone else who has to be out,” says Kenneth Abbott, MD, an oncologist in Maryland.
The culture of working while sick
Why doctors come to work when they’re sick is complicated. The overwhelming majority of U.S. respondents cited professional obligations; 73% noted that they feel a professional obligation to their patients, and 72% feel a professional obligation to their co-workers. Half of the polled U.S. physicians said they didn’t feel bad enough to stay home, while 48% said they had too much work to do to stay home.
Some 45% said the expectation at their workplace is to come to work unless seriously ill; 43% had too many patients to see; and 18% didn’t think they were contagious when they headed to work sick. Unfortunately, 15% chose to work while sick because otherwise they would lose pay.
In light of these responses, it’s not surprising that 93% reported they’d seen other medical professionals working when sick.
“My schedule is almost always booked weeks in advance. If someone misses or has to cancel their appointment, they typically have 2-4 weeks to wait to get back in. If I was sick and a full day of patients (or God forbid more than a day) had to be canceled because I called in, it’s so much more work when I return,” says Caitlin Briggs, MD, a psychiatrist in Lexington, Ky.
Doctors’ workplace sick day policy
Most employees’ benefits allow at least a few sick days, but doctors who treat society’s ill patients don’t seem to stay home from work when they’re suffering. So, we asked physicians, official policy aside, whether they thought going to work sick was expected in their workplace. The majority (76%) said yes, while 24% said no.
“Unless I’m dying or extremely contagious, I usually work. At least now, I have the telehealth option. Not saying any of this is right, but it’s the reality we deal with and the choice we must make,” says Dr. Briggs.
Additionally, 58% of polled physicians said their workplace did not have a clearly defined policy against coming to work sick, while 20% said theirs did, and 22% weren’t sure.
“The first thing I heard on the subject as a medical student was that sick people come to the hospital, so if you’re sick, then you come to the hospital too ... to work. If you can’t work, then you will be admitted. Another aphorism was from Churchill, that ‘most of the world’s work is done by people who don’t feel very well,’ ” says Paul Andreason, MD, a psychiatrist in Bethesda, Md.
Working in the time of COVID
Working while ill during ordinary times is one thing, but what about working in the time of COVID? Has the pandemic changed the culture of coming to work sick because medical facilities, such as doctor’s offices and hospitals, don’t want their staff coming in when they have COVID?
Surprisingly, when we asked physicians whether the pandemic has made it more or less acceptable to come to work sick, only 61% thought COVID has made it less acceptable to work while sick, while 16% thought it made it more acceptable, and 23% said there’s no change.
“I draw the line at fevers/chills, feeling like you’ve just been run over, or significant enteritis,” says Dr. Abbott. “Also, if I have to take palliative meds that interfere with alertness, I’m not doing my patients any favors.”
While a minority of physicians may call in sick, most still suffer through their sneezing, coughing, chills, and fever while seeing patients as usual.
A version of this article first appeared on Medscape.com.
The reasons are likely as varied as, “you weren’t feeling bad enough to miss work,” “you couldn’t afford to miss pay,” “you had too many patients to see,” or “too much work to do.”
In Medscape’s Employed Physicians Report: Loving the Focus, Hating the Bureaucracy, 61% of physicians reported that they sometimes or often come to work sick. Only 2% of respondents said they never come to work unwell.
Medscape wanted to know more about how often you call in sick, how often you come to work feeling unwell, what symptoms you have, and the dogma of your workplace culture regarding sick days. Not to mention the brutal ethos that starts in medical school, in which calling in sick shows weakness or is unacceptable.
So, we polled 2,347 physicians in the United States and abroad and asked them about their sniffling, sneezing, cold, flu, and fever symptoms, and, of course, COVID. Results were split about 50-50 among male and female physicians. The poll ran from Sept. 28 through Oct. 11.
Coming to work sick
It’s no surprise that the majority of physicians who were polled (85%) have come to work sick in 2022. In the last prepandemic year (2019), about 70% came to work feeling sick one to five times, and 13% worked while sick six to ten times.
When asked about the symptoms that they’ve previously come to work with, 48% of U.S. physicians said multiple symptoms. They gave high marks for runny nose, cough, congestion, and sore throat. Only 27% have worked with a fever, 22% have worked with other symptoms, and 7% have worked with both strep throat and COVID.
“My workplace, especially in the COVID years, accommodates persons who honestly do not feel well enough to report. Sooner or later, everyone covers for someone else who has to be out,” says Kenneth Abbott, MD, an oncologist in Maryland.
The culture of working while sick
Why doctors come to work when they’re sick is complicated. The overwhelming majority of U.S. respondents cited professional obligations; 73% noted that they feel a professional obligation to their patients, and 72% feel a professional obligation to their co-workers. Half of the polled U.S. physicians said they didn’t feel bad enough to stay home, while 48% said they had too much work to do to stay home.
Some 45% said the expectation at their workplace is to come to work unless seriously ill; 43% had too many patients to see; and 18% didn’t think they were contagious when they headed to work sick. Unfortunately, 15% chose to work while sick because otherwise they would lose pay.
In light of these responses, it’s not surprising that 93% reported they’d seen other medical professionals working when sick.
“My schedule is almost always booked weeks in advance. If someone misses or has to cancel their appointment, they typically have 2-4 weeks to wait to get back in. If I was sick and a full day of patients (or God forbid more than a day) had to be canceled because I called in, it’s so much more work when I return,” says Caitlin Briggs, MD, a psychiatrist in Lexington, Ky.
Doctors’ workplace sick day policy
Most employees’ benefits allow at least a few sick days, but doctors who treat society’s ill patients don’t seem to stay home from work when they’re suffering. So, we asked physicians, official policy aside, whether they thought going to work sick was expected in their workplace. The majority (76%) said yes, while 24% said no.
“Unless I’m dying or extremely contagious, I usually work. At least now, I have the telehealth option. Not saying any of this is right, but it’s the reality we deal with and the choice we must make,” says Dr. Briggs.
Additionally, 58% of polled physicians said their workplace did not have a clearly defined policy against coming to work sick, while 20% said theirs did, and 22% weren’t sure.
“The first thing I heard on the subject as a medical student was that sick people come to the hospital, so if you’re sick, then you come to the hospital too ... to work. If you can’t work, then you will be admitted. Another aphorism was from Churchill, that ‘most of the world’s work is done by people who don’t feel very well,’ ” says Paul Andreason, MD, a psychiatrist in Bethesda, Md.
Working in the time of COVID
Working while ill during ordinary times is one thing, but what about working in the time of COVID? Has the pandemic changed the culture of coming to work sick because medical facilities, such as doctor’s offices and hospitals, don’t want their staff coming in when they have COVID?
Surprisingly, when we asked physicians whether the pandemic has made it more or less acceptable to come to work sick, only 61% thought COVID has made it less acceptable to work while sick, while 16% thought it made it more acceptable, and 23% said there’s no change.
“I draw the line at fevers/chills, feeling like you’ve just been run over, or significant enteritis,” says Dr. Abbott. “Also, if I have to take palliative meds that interfere with alertness, I’m not doing my patients any favors.”
While a minority of physicians may call in sick, most still suffer through their sneezing, coughing, chills, and fever while seeing patients as usual.
A version of this article first appeared on Medscape.com.
The reasons are likely as varied as, “you weren’t feeling bad enough to miss work,” “you couldn’t afford to miss pay,” “you had too many patients to see,” or “too much work to do.”
In Medscape’s Employed Physicians Report: Loving the Focus, Hating the Bureaucracy, 61% of physicians reported that they sometimes or often come to work sick. Only 2% of respondents said they never come to work unwell.
Medscape wanted to know more about how often you call in sick, how often you come to work feeling unwell, what symptoms you have, and the dogma of your workplace culture regarding sick days. Not to mention the brutal ethos that starts in medical school, in which calling in sick shows weakness or is unacceptable.
So, we polled 2,347 physicians in the United States and abroad and asked them about their sniffling, sneezing, cold, flu, and fever symptoms, and, of course, COVID. Results were split about 50-50 among male and female physicians. The poll ran from Sept. 28 through Oct. 11.
Coming to work sick
It’s no surprise that the majority of physicians who were polled (85%) have come to work sick in 2022. In the last prepandemic year (2019), about 70% came to work feeling sick one to five times, and 13% worked while sick six to ten times.
When asked about the symptoms that they’ve previously come to work with, 48% of U.S. physicians said multiple symptoms. They gave high marks for runny nose, cough, congestion, and sore throat. Only 27% have worked with a fever, 22% have worked with other symptoms, and 7% have worked with both strep throat and COVID.
“My workplace, especially in the COVID years, accommodates persons who honestly do not feel well enough to report. Sooner or later, everyone covers for someone else who has to be out,” says Kenneth Abbott, MD, an oncologist in Maryland.
The culture of working while sick
Why doctors come to work when they’re sick is complicated. The overwhelming majority of U.S. respondents cited professional obligations; 73% noted that they feel a professional obligation to their patients, and 72% feel a professional obligation to their co-workers. Half of the polled U.S. physicians said they didn’t feel bad enough to stay home, while 48% said they had too much work to do to stay home.
Some 45% said the expectation at their workplace is to come to work unless seriously ill; 43% had too many patients to see; and 18% didn’t think they were contagious when they headed to work sick. Unfortunately, 15% chose to work while sick because otherwise they would lose pay.
In light of these responses, it’s not surprising that 93% reported they’d seen other medical professionals working when sick.
“My schedule is almost always booked weeks in advance. If someone misses or has to cancel their appointment, they typically have 2-4 weeks to wait to get back in. If I was sick and a full day of patients (or God forbid more than a day) had to be canceled because I called in, it’s so much more work when I return,” says Caitlin Briggs, MD, a psychiatrist in Lexington, Ky.
Doctors’ workplace sick day policy
Most employees’ benefits allow at least a few sick days, but doctors who treat society’s ill patients don’t seem to stay home from work when they’re suffering. So, we asked physicians, official policy aside, whether they thought going to work sick was expected in their workplace. The majority (76%) said yes, while 24% said no.
“Unless I’m dying or extremely contagious, I usually work. At least now, I have the telehealth option. Not saying any of this is right, but it’s the reality we deal with and the choice we must make,” says Dr. Briggs.
Additionally, 58% of polled physicians said their workplace did not have a clearly defined policy against coming to work sick, while 20% said theirs did, and 22% weren’t sure.
“The first thing I heard on the subject as a medical student was that sick people come to the hospital, so if you’re sick, then you come to the hospital too ... to work. If you can’t work, then you will be admitted. Another aphorism was from Churchill, that ‘most of the world’s work is done by people who don’t feel very well,’ ” says Paul Andreason, MD, a psychiatrist in Bethesda, Md.
Working in the time of COVID
Working while ill during ordinary times is one thing, but what about working in the time of COVID? Has the pandemic changed the culture of coming to work sick because medical facilities, such as doctor’s offices and hospitals, don’t want their staff coming in when they have COVID?
Surprisingly, when we asked physicians whether the pandemic has made it more or less acceptable to come to work sick, only 61% thought COVID has made it less acceptable to work while sick, while 16% thought it made it more acceptable, and 23% said there’s no change.
“I draw the line at fevers/chills, feeling like you’ve just been run over, or significant enteritis,” says Dr. Abbott. “Also, if I have to take palliative meds that interfere with alertness, I’m not doing my patients any favors.”
While a minority of physicians may call in sick, most still suffer through their sneezing, coughing, chills, and fever while seeing patients as usual.
A version of this article first appeared on Medscape.com.
Mid-October flulike illness cases higher than past 5 years
Outpatient visits for influenzalike illness (ILI), which includes influenza, SARS-CoV-2, and RSV, were higher after 3 weeks than for any of the previous five flu seasons: 3.3% of visits reported through the CDC’s Outpatient Influenza-like Illness Surveillance Network involved ILI as of Oct. 22. The highest comparable rate in the previous 5 years was the 1.9% recorded in late October of 2021, shortly after the definition of ILI was changed to also include illnesses other than influenza.
This season’s higher flu activity is in contrast to the previous two, which were unusually mild. The change, however, is not unexpected, as William Schaffner, MD, an infectious disease expert and professor of preventive medicine at Vanderbilt University, recently told CNN.
“Here we are in the middle of October – not the middle of November – we’re already seeing scattered influenza cases, even hospitalized influenza cases, around the country,” he said. “So we know that this virus is now spreading out in the community already. It’s gathering speed already. It looks to me to be about a month early.”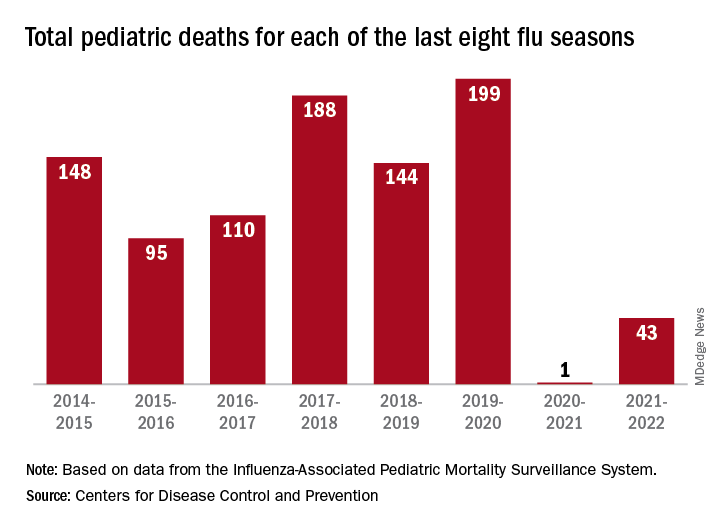
One indication of the mildness of the previous two flu seasons was the number of deaths, both pediatric and overall. Influenza-associated pediatric deaths had averaged about 110 per season over the previous eight seasons, compared with just 1 for 2020-2021 and 43 in 2021-2022. Overall flu deaths never reached 1% of all weekly deaths for either season, well below baseline levels for the flu, which range from 5.5% to 6.8%, CDC data show.
Other indicators of early severity
This season’s early rise in viral activity also can be seen in hospitalizations. The cumulative rate of flu-related admissions was 1.5 per 100,000 population as of Oct. 22, higher than the rate observed in the comparable week of previous seasons going back to 2010-2011, according to the CDC’s Influenza Hospitalization Surveillance Network.
A look at state reports of ILI outpatient visit rates shows that the District of Columbia and South Carolina are already in the very high range of the CDC’s severity scale, while 11 states are in the high range. Again going back to 2010-2011, no jurisdiction has ever been in the very high range this early in the season, based on data from the Outpatient Influenza-like Illness Surveillance Network.
Outpatient visits for influenzalike illness (ILI), which includes influenza, SARS-CoV-2, and RSV, were higher after 3 weeks than for any of the previous five flu seasons: 3.3% of visits reported through the CDC’s Outpatient Influenza-like Illness Surveillance Network involved ILI as of Oct. 22. The highest comparable rate in the previous 5 years was the 1.9% recorded in late October of 2021, shortly after the definition of ILI was changed to also include illnesses other than influenza.
This season’s higher flu activity is in contrast to the previous two, which were unusually mild. The change, however, is not unexpected, as William Schaffner, MD, an infectious disease expert and professor of preventive medicine at Vanderbilt University, recently told CNN.
“Here we are in the middle of October – not the middle of November – we’re already seeing scattered influenza cases, even hospitalized influenza cases, around the country,” he said. “So we know that this virus is now spreading out in the community already. It’s gathering speed already. It looks to me to be about a month early.”
One indication of the mildness of the previous two flu seasons was the number of deaths, both pediatric and overall. Influenza-associated pediatric deaths had averaged about 110 per season over the previous eight seasons, compared with just 1 for 2020-2021 and 43 in 2021-2022. Overall flu deaths never reached 1% of all weekly deaths for either season, well below baseline levels for the flu, which range from 5.5% to 6.8%, CDC data show.
Other indicators of early severity
This season’s early rise in viral activity also can be seen in hospitalizations. The cumulative rate of flu-related admissions was 1.5 per 100,000 population as of Oct. 22, higher than the rate observed in the comparable week of previous seasons going back to 2010-2011, according to the CDC’s Influenza Hospitalization Surveillance Network.
A look at state reports of ILI outpatient visit rates shows that the District of Columbia and South Carolina are already in the very high range of the CDC’s severity scale, while 11 states are in the high range. Again going back to 2010-2011, no jurisdiction has ever been in the very high range this early in the season, based on data from the Outpatient Influenza-like Illness Surveillance Network.
Outpatient visits for influenzalike illness (ILI), which includes influenza, SARS-CoV-2, and RSV, were higher after 3 weeks than for any of the previous five flu seasons: 3.3% of visits reported through the CDC’s Outpatient Influenza-like Illness Surveillance Network involved ILI as of Oct. 22. The highest comparable rate in the previous 5 years was the 1.9% recorded in late October of 2021, shortly after the definition of ILI was changed to also include illnesses other than influenza.
This season’s higher flu activity is in contrast to the previous two, which were unusually mild. The change, however, is not unexpected, as William Schaffner, MD, an infectious disease expert and professor of preventive medicine at Vanderbilt University, recently told CNN.
“Here we are in the middle of October – not the middle of November – we’re already seeing scattered influenza cases, even hospitalized influenza cases, around the country,” he said. “So we know that this virus is now spreading out in the community already. It’s gathering speed already. It looks to me to be about a month early.”
One indication of the mildness of the previous two flu seasons was the number of deaths, both pediatric and overall. Influenza-associated pediatric deaths had averaged about 110 per season over the previous eight seasons, compared with just 1 for 2020-2021 and 43 in 2021-2022. Overall flu deaths never reached 1% of all weekly deaths for either season, well below baseline levels for the flu, which range from 5.5% to 6.8%, CDC data show.
Other indicators of early severity
This season’s early rise in viral activity also can be seen in hospitalizations. The cumulative rate of flu-related admissions was 1.5 per 100,000 population as of Oct. 22, higher than the rate observed in the comparable week of previous seasons going back to 2010-2011, according to the CDC’s Influenza Hospitalization Surveillance Network.
A look at state reports of ILI outpatient visit rates shows that the District of Columbia and South Carolina are already in the very high range of the CDC’s severity scale, while 11 states are in the high range. Again going back to 2010-2011, no jurisdiction has ever been in the very high range this early in the season, based on data from the Outpatient Influenza-like Illness Surveillance Network.
You and the skeptical patient: Who’s the doctor here?
“I spoke to him on many occasions about the dangers of COVID, but he just didn’t believe me,” said Dr. Hood, an internist in Lexington, Ky. “He just didn’t give me enough time to help him. He waited to let me know he was ill with COVID and took days to pick up the medicine. Unfortunately, he then passed away.”
The rise of the skeptical patient
It can be extremely frustrating for doctors when patients question or disbelieve their physician’s medical advice and explanations. And many physicians resent the amount of time they spend trying to explain or make their case, especially during a busy day. But patients’ skepticism about the validity of some treatments seems to be increasing.
“Patients are now more likely to have their own medical explanation for their complaint than they used to, and that can be bad for their health,” Dr. Hood said.
Dr. Hood sees medical cynicism as part of Americans’ growing distrust of experts, leveraged by easy access to the internet. “When people Google, they tend to look for support of their opinions, rather than arrive at a fully educated decision.”
Only about half of patients believe their physicians “provide fair and accurate treatment information all or most of the time,” according to a 2019 survey by the Pew Research Center.
Patients’ distrust has become more obvious during the COVID-19 pandemic, said John Schumann, MD, an internist with Oak Street Health, a practice with more than 500 physicians and other providers in 20 states, treating almost exclusively Medicare patients.
“The skeptics became more entrenched during the pandemic,” said Dr. Schumann, who is based in Tulsa, Okla. “They may think the COVID vaccines were approved too quickly, or believe the pandemic itself is a hoax.”
“There’s a lot of antiscience rhetoric now,” Dr. Schumann added. “I’d say about half of my patients are comfortable with science-based decisions and the other half are not.”
What are patients mistrustful about?
Patients’ suspicions of certain therapies began long before the pandemic. In dermatology, for example, some patients refuse to take topical steroids, said Steven R. Feldman, MD, a dermatologist in Winston-Salem, N.C.
“Their distrust is usually based on anecdotal stories they read about,” he noted. “Patients in other specialties are dead set against vaccinations.”
In addition to refusing treatments and inoculations, some patients ask for questionable regimens mentioned in the news. “Some patients have demanded hydroxychloroquine or Noromectin, drugs that are unproven in the treatment of COVID,” Dr. Schumann said. “We refuse to prescribe them.”
Dr. Hood said patients’ reluctance to follow medical advice can often be based on cost. “I have a patient who was more willing to save $20 than to save his life. But when the progression of his test results fit my predictions, he became more willing to take treatments. I had to wait for the opportune moment to convince him.”
Many naysayer patients keep their views to themselves, and physicians may be unaware that the patients are stonewalling. A 2006 study estimated that about 10%-16% of primary care patients actively resist medical authority.
Dr. Schumann cited patients who don’t want to hear an upsetting diagnosis. “Some patients might refuse to take a biopsy to see if they have cancer because they don’t want to know,” he said. “In many cases, they simply won’t get the biopsy and won’t tell the doctor that they didn’t.”
Sometimes skeptics’ arguments have merit
Some patients’ concerns can be valid, such as when they refuse to go on statins, said Zain Hakeem, DO, a physician in Austin, Tex.
“In some cases, I feel that statins are not necessary,” he said. “The science on statins for primary prevention is not strong, although they should be used for exceedingly high-risk patients.”
Certain patients, especially those with chronic conditions, do a great deal of research, using legitimate sources on the Web, and their research is well supported.
However, these patients can be overconfident in their conclusions. Several studies have shown that with just a little experience, people can replace beginners’ caution with a false sense of competence.
For example, “Patients may not weigh the risks correctly,” Dr. Hakeem said. “They can be more concerned about the risk of having their colon perforated during a colonoscopy, while the risk of cancer if they don’t have a colonoscopy is much higher.”
Some highly successful people may be more likely to trust their own medical instincts. When Steve Jobs, the founder of Apple, was diagnosed with pancreatic cancer in 2003, he put off surgery for 9 months while he tried to cure his disease with a vegan diet, acupuncture, herbs, bowel cleansings, and other remedies he read about. He died in 2011. Some experts believe that delay hastened his death.
Of course, not all physicians’ diagnoses or treatments are correct. One study indicated doctors’ diagnostic error rate could be as high as 15%. And just as patients can be overconfident in their conclusions, so can doctors. Another study found that physicians’ stated confidence in their diagnosis was only slightly affected by the inaccuracy of that diagnosis or the difficulty of the case.
Best ways to deal with cynical patients
Patients’ skepticism can frustrate doctors, reduce the efficiency of care delivery, and interfere with recovery. What can doctors do to deal with these problems?
1. Build the patient’s trust in you. “Getting patients to adhere to your advice involves making sure they feel they have a caring doctor whom they trust,” Dr. Feldman said.
“I want to show patients that I am entirely focused on them,” he added. “For example, I may rush to the door of the exam room from my last appointment, but I open the door very slowly and deliberately, because I want the patient to see that I won’t hurry with them.”
2. Spend time with the patient. Familiarity builds trust. Dr. Schumann said doctors at Oak Street Health see their patients an average of six to eight times a year, an unusually high number. “The more patients see their physicians, the more likely they are to trust them.”
3. Keep up to date. “I make sure I’m up to date with the literature, and I try to present a truthful message,” Dr. Hood said. “For instance, my research showed that inflammation played a strong role in developing complications from COVID, so I wrote a detailed treatment protocol aimed at the inflammation and the immune response, which has been very effective.”
4. Confront patients tactfully. Patients who do research on the Web don’t want to be scolded, Dr. Feldman said. In fact, he praises them, even if he doesn’t agree with their findings. “I might say: ‘What a relief to finally find patients who’ve taken the time to educate themselves before coming here.’ ”
Dr. Feldman is careful not to dispute patients’ conclusions. “Debating the issues is not an effective approach to get patients to trust you. The last thing you want to tell a patient is: ‘Listen to me! I’m an expert.’ People just dig in.”
However, it does help to give patients feedback. “I’m a big fan of patients arguing with me,” Dr. Hakeem said. “It means you can straighten out misunderstandings and improve decision-making.”
5. Explain your reasoning. “You need to communicate clearly and show them your thinking,” Dr. Hood said. “For instance, I’ll explain why a patient has a strong risk for heart attack.”
6. Acknowledge uncertainties. “The doctor may present the science as far more certain than it is,” Dr. Hakeem said. “If you don’t acknowledge the uncertainties, you could break the patient’s trust in you.”
7. Don’t use a lot of numbers. “Data is not a good tool to convince patients,” Dr. Feldman said. “The human brain isn’t designed to work that way.”
If you want to use numbers to show clinical risk, Dr. Hakeem advisd using natural frequencies, such as 10 out of 10,000, which is less confusing to the patient than the equivalent percentage of 0.1%.
It can be helpful to refer to familiar concepts. One way to understand a risk is to compare it with risks in daily life, such as the dangers of driving or falling in the shower, Dr. Hakeem added.
Dr. Feldman often refers to another person’s experience when presenting his medical advice. “I might say to the patient: ‘You remind me of another patient I had. They were sitting in the same chair you’re sitting in. They did really well on this drug, and I think it’s probably the best choice for you, too.’ ”
8. Adopt shared decision-making. This approach involves empowering the patient to become an equal partner in medical decisions. The patient is given information through portals and is encouraged to do research. Critics, however, say that most patients don’t want this degree of empowerment and would rather depend on the doctor’s advice.
Conclusion
It’s often impossible to get through to a skeptical patient, which can be disheartening for doctors. “Physicians want to do what is best for the patient, so when the patient doesn’t listen, they may take it personally,” Dr. Hood said. “But you always have to remember, the patient is the one with disease, and it’s up to the patient to open the door.”
Still, some skeptical patients ultimately change their minds. Dr. Schumann said patients who initially declined the COVID vaccine eventually decided to get it. “It often took them more than a year. but it’s never too late.”
A version of this article first appeared on Medscape.com.
“I spoke to him on many occasions about the dangers of COVID, but he just didn’t believe me,” said Dr. Hood, an internist in Lexington, Ky. “He just didn’t give me enough time to help him. He waited to let me know he was ill with COVID and took days to pick up the medicine. Unfortunately, he then passed away.”
The rise of the skeptical patient
It can be extremely frustrating for doctors when patients question or disbelieve their physician’s medical advice and explanations. And many physicians resent the amount of time they spend trying to explain or make their case, especially during a busy day. But patients’ skepticism about the validity of some treatments seems to be increasing.
“Patients are now more likely to have their own medical explanation for their complaint than they used to, and that can be bad for their health,” Dr. Hood said.
Dr. Hood sees medical cynicism as part of Americans’ growing distrust of experts, leveraged by easy access to the internet. “When people Google, they tend to look for support of their opinions, rather than arrive at a fully educated decision.”
Only about half of patients believe their physicians “provide fair and accurate treatment information all or most of the time,” according to a 2019 survey by the Pew Research Center.
Patients’ distrust has become more obvious during the COVID-19 pandemic, said John Schumann, MD, an internist with Oak Street Health, a practice with more than 500 physicians and other providers in 20 states, treating almost exclusively Medicare patients.
“The skeptics became more entrenched during the pandemic,” said Dr. Schumann, who is based in Tulsa, Okla. “They may think the COVID vaccines were approved too quickly, or believe the pandemic itself is a hoax.”
“There’s a lot of antiscience rhetoric now,” Dr. Schumann added. “I’d say about half of my patients are comfortable with science-based decisions and the other half are not.”
What are patients mistrustful about?
Patients’ suspicions of certain therapies began long before the pandemic. In dermatology, for example, some patients refuse to take topical steroids, said Steven R. Feldman, MD, a dermatologist in Winston-Salem, N.C.
“Their distrust is usually based on anecdotal stories they read about,” he noted. “Patients in other specialties are dead set against vaccinations.”
In addition to refusing treatments and inoculations, some patients ask for questionable regimens mentioned in the news. “Some patients have demanded hydroxychloroquine or Noromectin, drugs that are unproven in the treatment of COVID,” Dr. Schumann said. “We refuse to prescribe them.”
Dr. Hood said patients’ reluctance to follow medical advice can often be based on cost. “I have a patient who was more willing to save $20 than to save his life. But when the progression of his test results fit my predictions, he became more willing to take treatments. I had to wait for the opportune moment to convince him.”
Many naysayer patients keep their views to themselves, and physicians may be unaware that the patients are stonewalling. A 2006 study estimated that about 10%-16% of primary care patients actively resist medical authority.
Dr. Schumann cited patients who don’t want to hear an upsetting diagnosis. “Some patients might refuse to take a biopsy to see if they have cancer because they don’t want to know,” he said. “In many cases, they simply won’t get the biopsy and won’t tell the doctor that they didn’t.”
Sometimes skeptics’ arguments have merit
Some patients’ concerns can be valid, such as when they refuse to go on statins, said Zain Hakeem, DO, a physician in Austin, Tex.
“In some cases, I feel that statins are not necessary,” he said. “The science on statins for primary prevention is not strong, although they should be used for exceedingly high-risk patients.”
Certain patients, especially those with chronic conditions, do a great deal of research, using legitimate sources on the Web, and their research is well supported.
However, these patients can be overconfident in their conclusions. Several studies have shown that with just a little experience, people can replace beginners’ caution with a false sense of competence.
For example, “Patients may not weigh the risks correctly,” Dr. Hakeem said. “They can be more concerned about the risk of having their colon perforated during a colonoscopy, while the risk of cancer if they don’t have a colonoscopy is much higher.”
Some highly successful people may be more likely to trust their own medical instincts. When Steve Jobs, the founder of Apple, was diagnosed with pancreatic cancer in 2003, he put off surgery for 9 months while he tried to cure his disease with a vegan diet, acupuncture, herbs, bowel cleansings, and other remedies he read about. He died in 2011. Some experts believe that delay hastened his death.
Of course, not all physicians’ diagnoses or treatments are correct. One study indicated doctors’ diagnostic error rate could be as high as 15%. And just as patients can be overconfident in their conclusions, so can doctors. Another study found that physicians’ stated confidence in their diagnosis was only slightly affected by the inaccuracy of that diagnosis or the difficulty of the case.
Best ways to deal with cynical patients
Patients’ skepticism can frustrate doctors, reduce the efficiency of care delivery, and interfere with recovery. What can doctors do to deal with these problems?
1. Build the patient’s trust in you. “Getting patients to adhere to your advice involves making sure they feel they have a caring doctor whom they trust,” Dr. Feldman said.
“I want to show patients that I am entirely focused on them,” he added. “For example, I may rush to the door of the exam room from my last appointment, but I open the door very slowly and deliberately, because I want the patient to see that I won’t hurry with them.”
2. Spend time with the patient. Familiarity builds trust. Dr. Schumann said doctors at Oak Street Health see their patients an average of six to eight times a year, an unusually high number. “The more patients see their physicians, the more likely they are to trust them.”
3. Keep up to date. “I make sure I’m up to date with the literature, and I try to present a truthful message,” Dr. Hood said. “For instance, my research showed that inflammation played a strong role in developing complications from COVID, so I wrote a detailed treatment protocol aimed at the inflammation and the immune response, which has been very effective.”
4. Confront patients tactfully. Patients who do research on the Web don’t want to be scolded, Dr. Feldman said. In fact, he praises them, even if he doesn’t agree with their findings. “I might say: ‘What a relief to finally find patients who’ve taken the time to educate themselves before coming here.’ ”
Dr. Feldman is careful not to dispute patients’ conclusions. “Debating the issues is not an effective approach to get patients to trust you. The last thing you want to tell a patient is: ‘Listen to me! I’m an expert.’ People just dig in.”
However, it does help to give patients feedback. “I’m a big fan of patients arguing with me,” Dr. Hakeem said. “It means you can straighten out misunderstandings and improve decision-making.”
5. Explain your reasoning. “You need to communicate clearly and show them your thinking,” Dr. Hood said. “For instance, I’ll explain why a patient has a strong risk for heart attack.”
6. Acknowledge uncertainties. “The doctor may present the science as far more certain than it is,” Dr. Hakeem said. “If you don’t acknowledge the uncertainties, you could break the patient’s trust in you.”
7. Don’t use a lot of numbers. “Data is not a good tool to convince patients,” Dr. Feldman said. “The human brain isn’t designed to work that way.”
If you want to use numbers to show clinical risk, Dr. Hakeem advisd using natural frequencies, such as 10 out of 10,000, which is less confusing to the patient than the equivalent percentage of 0.1%.
It can be helpful to refer to familiar concepts. One way to understand a risk is to compare it with risks in daily life, such as the dangers of driving or falling in the shower, Dr. Hakeem added.
Dr. Feldman often refers to another person’s experience when presenting his medical advice. “I might say to the patient: ‘You remind me of another patient I had. They were sitting in the same chair you’re sitting in. They did really well on this drug, and I think it’s probably the best choice for you, too.’ ”
8. Adopt shared decision-making. This approach involves empowering the patient to become an equal partner in medical decisions. The patient is given information through portals and is encouraged to do research. Critics, however, say that most patients don’t want this degree of empowerment and would rather depend on the doctor’s advice.
Conclusion
It’s often impossible to get through to a skeptical patient, which can be disheartening for doctors. “Physicians want to do what is best for the patient, so when the patient doesn’t listen, they may take it personally,” Dr. Hood said. “But you always have to remember, the patient is the one with disease, and it’s up to the patient to open the door.”
Still, some skeptical patients ultimately change their minds. Dr. Schumann said patients who initially declined the COVID vaccine eventually decided to get it. “It often took them more than a year. but it’s never too late.”
A version of this article first appeared on Medscape.com.
“I spoke to him on many occasions about the dangers of COVID, but he just didn’t believe me,” said Dr. Hood, an internist in Lexington, Ky. “He just didn’t give me enough time to help him. He waited to let me know he was ill with COVID and took days to pick up the medicine. Unfortunately, he then passed away.”
The rise of the skeptical patient
It can be extremely frustrating for doctors when patients question or disbelieve their physician’s medical advice and explanations. And many physicians resent the amount of time they spend trying to explain or make their case, especially during a busy day. But patients’ skepticism about the validity of some treatments seems to be increasing.
“Patients are now more likely to have their own medical explanation for their complaint than they used to, and that can be bad for their health,” Dr. Hood said.
Dr. Hood sees medical cynicism as part of Americans’ growing distrust of experts, leveraged by easy access to the internet. “When people Google, they tend to look for support of their opinions, rather than arrive at a fully educated decision.”
Only about half of patients believe their physicians “provide fair and accurate treatment information all or most of the time,” according to a 2019 survey by the Pew Research Center.
Patients’ distrust has become more obvious during the COVID-19 pandemic, said John Schumann, MD, an internist with Oak Street Health, a practice with more than 500 physicians and other providers in 20 states, treating almost exclusively Medicare patients.
“The skeptics became more entrenched during the pandemic,” said Dr. Schumann, who is based in Tulsa, Okla. “They may think the COVID vaccines were approved too quickly, or believe the pandemic itself is a hoax.”
“There’s a lot of antiscience rhetoric now,” Dr. Schumann added. “I’d say about half of my patients are comfortable with science-based decisions and the other half are not.”
What are patients mistrustful about?
Patients’ suspicions of certain therapies began long before the pandemic. In dermatology, for example, some patients refuse to take topical steroids, said Steven R. Feldman, MD, a dermatologist in Winston-Salem, N.C.
“Their distrust is usually based on anecdotal stories they read about,” he noted. “Patients in other specialties are dead set against vaccinations.”
In addition to refusing treatments and inoculations, some patients ask for questionable regimens mentioned in the news. “Some patients have demanded hydroxychloroquine or Noromectin, drugs that are unproven in the treatment of COVID,” Dr. Schumann said. “We refuse to prescribe them.”
Dr. Hood said patients’ reluctance to follow medical advice can often be based on cost. “I have a patient who was more willing to save $20 than to save his life. But when the progression of his test results fit my predictions, he became more willing to take treatments. I had to wait for the opportune moment to convince him.”
Many naysayer patients keep their views to themselves, and physicians may be unaware that the patients are stonewalling. A 2006 study estimated that about 10%-16% of primary care patients actively resist medical authority.
Dr. Schumann cited patients who don’t want to hear an upsetting diagnosis. “Some patients might refuse to take a biopsy to see if they have cancer because they don’t want to know,” he said. “In many cases, they simply won’t get the biopsy and won’t tell the doctor that they didn’t.”
Sometimes skeptics’ arguments have merit
Some patients’ concerns can be valid, such as when they refuse to go on statins, said Zain Hakeem, DO, a physician in Austin, Tex.
“In some cases, I feel that statins are not necessary,” he said. “The science on statins for primary prevention is not strong, although they should be used for exceedingly high-risk patients.”
Certain patients, especially those with chronic conditions, do a great deal of research, using legitimate sources on the Web, and their research is well supported.
However, these patients can be overconfident in their conclusions. Several studies have shown that with just a little experience, people can replace beginners’ caution with a false sense of competence.
For example, “Patients may not weigh the risks correctly,” Dr. Hakeem said. “They can be more concerned about the risk of having their colon perforated during a colonoscopy, while the risk of cancer if they don’t have a colonoscopy is much higher.”
Some highly successful people may be more likely to trust their own medical instincts. When Steve Jobs, the founder of Apple, was diagnosed with pancreatic cancer in 2003, he put off surgery for 9 months while he tried to cure his disease with a vegan diet, acupuncture, herbs, bowel cleansings, and other remedies he read about. He died in 2011. Some experts believe that delay hastened his death.
Of course, not all physicians’ diagnoses or treatments are correct. One study indicated doctors’ diagnostic error rate could be as high as 15%. And just as patients can be overconfident in their conclusions, so can doctors. Another study found that physicians’ stated confidence in their diagnosis was only slightly affected by the inaccuracy of that diagnosis or the difficulty of the case.
Best ways to deal with cynical patients
Patients’ skepticism can frustrate doctors, reduce the efficiency of care delivery, and interfere with recovery. What can doctors do to deal with these problems?
1. Build the patient’s trust in you. “Getting patients to adhere to your advice involves making sure they feel they have a caring doctor whom they trust,” Dr. Feldman said.
“I want to show patients that I am entirely focused on them,” he added. “For example, I may rush to the door of the exam room from my last appointment, but I open the door very slowly and deliberately, because I want the patient to see that I won’t hurry with them.”
2. Spend time with the patient. Familiarity builds trust. Dr. Schumann said doctors at Oak Street Health see their patients an average of six to eight times a year, an unusually high number. “The more patients see their physicians, the more likely they are to trust them.”
3. Keep up to date. “I make sure I’m up to date with the literature, and I try to present a truthful message,” Dr. Hood said. “For instance, my research showed that inflammation played a strong role in developing complications from COVID, so I wrote a detailed treatment protocol aimed at the inflammation and the immune response, which has been very effective.”
4. Confront patients tactfully. Patients who do research on the Web don’t want to be scolded, Dr. Feldman said. In fact, he praises them, even if he doesn’t agree with their findings. “I might say: ‘What a relief to finally find patients who’ve taken the time to educate themselves before coming here.’ ”
Dr. Feldman is careful not to dispute patients’ conclusions. “Debating the issues is not an effective approach to get patients to trust you. The last thing you want to tell a patient is: ‘Listen to me! I’m an expert.’ People just dig in.”
However, it does help to give patients feedback. “I’m a big fan of patients arguing with me,” Dr. Hakeem said. “It means you can straighten out misunderstandings and improve decision-making.”
5. Explain your reasoning. “You need to communicate clearly and show them your thinking,” Dr. Hood said. “For instance, I’ll explain why a patient has a strong risk for heart attack.”
6. Acknowledge uncertainties. “The doctor may present the science as far more certain than it is,” Dr. Hakeem said. “If you don’t acknowledge the uncertainties, you could break the patient’s trust in you.”
7. Don’t use a lot of numbers. “Data is not a good tool to convince patients,” Dr. Feldman said. “The human brain isn’t designed to work that way.”
If you want to use numbers to show clinical risk, Dr. Hakeem advisd using natural frequencies, such as 10 out of 10,000, which is less confusing to the patient than the equivalent percentage of 0.1%.
It can be helpful to refer to familiar concepts. One way to understand a risk is to compare it with risks in daily life, such as the dangers of driving or falling in the shower, Dr. Hakeem added.
Dr. Feldman often refers to another person’s experience when presenting his medical advice. “I might say to the patient: ‘You remind me of another patient I had. They were sitting in the same chair you’re sitting in. They did really well on this drug, and I think it’s probably the best choice for you, too.’ ”
8. Adopt shared decision-making. This approach involves empowering the patient to become an equal partner in medical decisions. The patient is given information through portals and is encouraged to do research. Critics, however, say that most patients don’t want this degree of empowerment and would rather depend on the doctor’s advice.
Conclusion
It’s often impossible to get through to a skeptical patient, which can be disheartening for doctors. “Physicians want to do what is best for the patient, so when the patient doesn’t listen, they may take it personally,” Dr. Hood said. “But you always have to remember, the patient is the one with disease, and it’s up to the patient to open the door.”
Still, some skeptical patients ultimately change their minds. Dr. Schumann said patients who initially declined the COVID vaccine eventually decided to get it. “It often took them more than a year. but it’s never too late.”
A version of this article first appeared on Medscape.com.
The marked contrast in pandemic outcomes between Japan and the United States
This article was originally published Oct. 8 on Medscape Editor-In-Chief Eric Topol’s “Ground Truths” column on Substack.
Over time it has the least cumulative deaths per capita of any major country in the world. That’s without a zero-Covid policy or any national lockdowns, which is why I have not included China as a comparator.
Before we get into that data, let’s take a look at the age pyramids for Japan and the United States. The No. 1 risk factor for death from COVID-19 is advanced age, and you can see that in Japan about 25% of the population is age 65 and older, whereas in the United States that proportion is substantially reduced at 15%. Sure there are differences in comorbidities such as obesity and diabetes, but there is also the trade-off of a much higher population density in Japan.
Besides masks, which were distributed early on by the government to the population in Japan, there was the “Avoid the 3Cs” cluster-busting strategy, widely disseminated in the spring of 2020, leveraging Pareto’s 80-20 principle, long before there were any vaccines available. For a good portion of the pandemic, the Ministry of Foreign Affairs of Japan maintained a strict policy for border control, which while hard to quantify, may certainly have contributed to its success.
Besides these factors, once vaccines became available, Japan got the population with the primary series to 83% rapidly, even after getting a late start by many months compared with the United States, which has peaked at 68%. That’s a big gap.
But that gap got much worse when it came to boosters. Ninety-five percent of Japanese eligible compared with 40.8% of Americans have had a booster shot. Of note, that 95% in Japan pertains to the whole population. In the United States the percentage of people age 65 and older who have had two boosters is currently only 42%. I’ve previously reviewed the important lifesaving impact of two boosters among people age 65 and older from five independent studies during Omicron waves throughout the world.
Now let’s turn to cumulative fatalities in the two countries. There’s a huge, nearly ninefold difference, per capita. Using today’s Covid-19 Dashboard, there are cumulatively 45,533 deaths in Japan and 1,062,560 American deaths. That translates to 1 in 2,758 people in Japan compared with 1 in 315 Americans dying of COVID.
And if we look at excess mortality instead of confirmed COVID deaths, that enormous gap doesn’t change.
Obviously it would be good to have data for other COVID outcomes, such as hospitalizations, ICUs, and Long COVID, but they are not accessible.
Comparing Japan, the country that has fared the best, with the United States, one of the worst pandemic outcome results, leaves us with a sense that Prof Ian MacKay’s “Swiss cheese model” is the best explanation. It’s not just one thing. Masks, consistent evidence-based communication (3Cs) with attention to ventilation and air quality, and the outstanding uptake of vaccines and boosters all contributed to Japan’s success.
There is another factor to add to that model – Paxlovid. Its benefit of reducing hospitalizations and deaths for people over age 65 is unquestionable.
That’s why I had previously modified the Swiss cheese model to add Paxlovid.
But in the United States, where 15% of the population is 65 and older, they account for over 75% of the daily death toll, still in the range of 400 per day. Here, with a very high proportion of people age 65 and older left vulnerable without boosters, or primary vaccines, Paxlovid is only being given to less than 25% of the eligible (age 50+), and less people age 80 and older are getting Paxlovid than those age 45. The reasons that doctors are not prescribing it – worried about interactions for a 5-day course and rebound – are not substantiated.
Bottom line: In the United States we are not protecting our population anywhere near as well as Japan, as grossly evident by the fatalities among people at the highest risk. There needs to be far better uptake of boosters and use of Paxlovid in the age 65+ group, but the need for amped up protection is not at all restricted to this age subgroup. Across all age groups age 18 and over there is an 81% reduction of hospitalizations with two boosters with the most updated CDC data available, through the Omicron BA.5 wave.
No less the previous data through May 2022 showing protection from death across all ages with two boosters
And please don’t forget that around the world, over 20 million lives were saved, just in 2021, the first year of vaccines.
We can learn so much from a model country like Japan. Yes, we need nasal and variant-proof vaccines to effectively deal with the new variants that are already getting legs in places like XBB in Singapore and ones not on the radar yet. But right now we’ve got to do far better for people getting boosters and, when a person age 65 or older gets COVID, Paxlovid. Take a look at the Chris Hayes video segment when he pleaded for Americans to get a booster shot. Every day that vaccine waning of the U.S. population exceeds the small percentage of people who get a booster, our vulnerability increases. If we don’t get that on track, it’s likely going to be a rough winter ahead.
Dr. Topol is director of the Scripps Translational Science Institute in La Jolla, Calif. He has received research grants from the National Institutes of Health and reported conflicts of interest involving Dexcom, Illumina, Molecular Stethoscope, Quest Diagnostics, and Blue Cross Blue Shield Association. A version of this article appeared on Medscape.com.
This article was originally published Oct. 8 on Medscape Editor-In-Chief Eric Topol’s “Ground Truths” column on Substack.
Over time it has the least cumulative deaths per capita of any major country in the world. That’s without a zero-Covid policy or any national lockdowns, which is why I have not included China as a comparator.
Before we get into that data, let’s take a look at the age pyramids for Japan and the United States. The No. 1 risk factor for death from COVID-19 is advanced age, and you can see that in Japan about 25% of the population is age 65 and older, whereas in the United States that proportion is substantially reduced at 15%. Sure there are differences in comorbidities such as obesity and diabetes, but there is also the trade-off of a much higher population density in Japan.
Besides masks, which were distributed early on by the government to the population in Japan, there was the “Avoid the 3Cs” cluster-busting strategy, widely disseminated in the spring of 2020, leveraging Pareto’s 80-20 principle, long before there were any vaccines available. For a good portion of the pandemic, the Ministry of Foreign Affairs of Japan maintained a strict policy for border control, which while hard to quantify, may certainly have contributed to its success.
Besides these factors, once vaccines became available, Japan got the population with the primary series to 83% rapidly, even after getting a late start by many months compared with the United States, which has peaked at 68%. That’s a big gap.
But that gap got much worse when it came to boosters. Ninety-five percent of Japanese eligible compared with 40.8% of Americans have had a booster shot. Of note, that 95% in Japan pertains to the whole population. In the United States the percentage of people age 65 and older who have had two boosters is currently only 42%. I’ve previously reviewed the important lifesaving impact of two boosters among people age 65 and older from five independent studies during Omicron waves throughout the world.
Now let’s turn to cumulative fatalities in the two countries. There’s a huge, nearly ninefold difference, per capita. Using today’s Covid-19 Dashboard, there are cumulatively 45,533 deaths in Japan and 1,062,560 American deaths. That translates to 1 in 2,758 people in Japan compared with 1 in 315 Americans dying of COVID.
And if we look at excess mortality instead of confirmed COVID deaths, that enormous gap doesn’t change.
Obviously it would be good to have data for other COVID outcomes, such as hospitalizations, ICUs, and Long COVID, but they are not accessible.
Comparing Japan, the country that has fared the best, with the United States, one of the worst pandemic outcome results, leaves us with a sense that Prof Ian MacKay’s “Swiss cheese model” is the best explanation. It’s not just one thing. Masks, consistent evidence-based communication (3Cs) with attention to ventilation and air quality, and the outstanding uptake of vaccines and boosters all contributed to Japan’s success.
There is another factor to add to that model – Paxlovid. Its benefit of reducing hospitalizations and deaths for people over age 65 is unquestionable.
That’s why I had previously modified the Swiss cheese model to add Paxlovid.
But in the United States, where 15% of the population is 65 and older, they account for over 75% of the daily death toll, still in the range of 400 per day. Here, with a very high proportion of people age 65 and older left vulnerable without boosters, or primary vaccines, Paxlovid is only being given to less than 25% of the eligible (age 50+), and less people age 80 and older are getting Paxlovid than those age 45. The reasons that doctors are not prescribing it – worried about interactions for a 5-day course and rebound – are not substantiated.
Bottom line: In the United States we are not protecting our population anywhere near as well as Japan, as grossly evident by the fatalities among people at the highest risk. There needs to be far better uptake of boosters and use of Paxlovid in the age 65+ group, but the need for amped up protection is not at all restricted to this age subgroup. Across all age groups age 18 and over there is an 81% reduction of hospitalizations with two boosters with the most updated CDC data available, through the Omicron BA.5 wave.
No less the previous data through May 2022 showing protection from death across all ages with two boosters
And please don’t forget that around the world, over 20 million lives were saved, just in 2021, the first year of vaccines.
We can learn so much from a model country like Japan. Yes, we need nasal and variant-proof vaccines to effectively deal with the new variants that are already getting legs in places like XBB in Singapore and ones not on the radar yet. But right now we’ve got to do far better for people getting boosters and, when a person age 65 or older gets COVID, Paxlovid. Take a look at the Chris Hayes video segment when he pleaded for Americans to get a booster shot. Every day that vaccine waning of the U.S. population exceeds the small percentage of people who get a booster, our vulnerability increases. If we don’t get that on track, it’s likely going to be a rough winter ahead.
Dr. Topol is director of the Scripps Translational Science Institute in La Jolla, Calif. He has received research grants from the National Institutes of Health and reported conflicts of interest involving Dexcom, Illumina, Molecular Stethoscope, Quest Diagnostics, and Blue Cross Blue Shield Association. A version of this article appeared on Medscape.com.
This article was originally published Oct. 8 on Medscape Editor-In-Chief Eric Topol’s “Ground Truths” column on Substack.
Over time it has the least cumulative deaths per capita of any major country in the world. That’s without a zero-Covid policy or any national lockdowns, which is why I have not included China as a comparator.
Before we get into that data, let’s take a look at the age pyramids for Japan and the United States. The No. 1 risk factor for death from COVID-19 is advanced age, and you can see that in Japan about 25% of the population is age 65 and older, whereas in the United States that proportion is substantially reduced at 15%. Sure there are differences in comorbidities such as obesity and diabetes, but there is also the trade-off of a much higher population density in Japan.
Besides masks, which were distributed early on by the government to the population in Japan, there was the “Avoid the 3Cs” cluster-busting strategy, widely disseminated in the spring of 2020, leveraging Pareto’s 80-20 principle, long before there were any vaccines available. For a good portion of the pandemic, the Ministry of Foreign Affairs of Japan maintained a strict policy for border control, which while hard to quantify, may certainly have contributed to its success.
Besides these factors, once vaccines became available, Japan got the population with the primary series to 83% rapidly, even after getting a late start by many months compared with the United States, which has peaked at 68%. That’s a big gap.
But that gap got much worse when it came to boosters. Ninety-five percent of Japanese eligible compared with 40.8% of Americans have had a booster shot. Of note, that 95% in Japan pertains to the whole population. In the United States the percentage of people age 65 and older who have had two boosters is currently only 42%. I’ve previously reviewed the important lifesaving impact of two boosters among people age 65 and older from five independent studies during Omicron waves throughout the world.
Now let’s turn to cumulative fatalities in the two countries. There’s a huge, nearly ninefold difference, per capita. Using today’s Covid-19 Dashboard, there are cumulatively 45,533 deaths in Japan and 1,062,560 American deaths. That translates to 1 in 2,758 people in Japan compared with 1 in 315 Americans dying of COVID.
And if we look at excess mortality instead of confirmed COVID deaths, that enormous gap doesn’t change.
Obviously it would be good to have data for other COVID outcomes, such as hospitalizations, ICUs, and Long COVID, but they are not accessible.
Comparing Japan, the country that has fared the best, with the United States, one of the worst pandemic outcome results, leaves us with a sense that Prof Ian MacKay’s “Swiss cheese model” is the best explanation. It’s not just one thing. Masks, consistent evidence-based communication (3Cs) with attention to ventilation and air quality, and the outstanding uptake of vaccines and boosters all contributed to Japan’s success.
There is another factor to add to that model – Paxlovid. Its benefit of reducing hospitalizations and deaths for people over age 65 is unquestionable.
That’s why I had previously modified the Swiss cheese model to add Paxlovid.
But in the United States, where 15% of the population is 65 and older, they account for over 75% of the daily death toll, still in the range of 400 per day. Here, with a very high proportion of people age 65 and older left vulnerable without boosters, or primary vaccines, Paxlovid is only being given to less than 25% of the eligible (age 50+), and less people age 80 and older are getting Paxlovid than those age 45. The reasons that doctors are not prescribing it – worried about interactions for a 5-day course and rebound – are not substantiated.
Bottom line: In the United States we are not protecting our population anywhere near as well as Japan, as grossly evident by the fatalities among people at the highest risk. There needs to be far better uptake of boosters and use of Paxlovid in the age 65+ group, but the need for amped up protection is not at all restricted to this age subgroup. Across all age groups age 18 and over there is an 81% reduction of hospitalizations with two boosters with the most updated CDC data available, through the Omicron BA.5 wave.
No less the previous data through May 2022 showing protection from death across all ages with two boosters
And please don’t forget that around the world, over 20 million lives were saved, just in 2021, the first year of vaccines.
We can learn so much from a model country like Japan. Yes, we need nasal and variant-proof vaccines to effectively deal with the new variants that are already getting legs in places like XBB in Singapore and ones not on the radar yet. But right now we’ve got to do far better for people getting boosters and, when a person age 65 or older gets COVID, Paxlovid. Take a look at the Chris Hayes video segment when he pleaded for Americans to get a booster shot. Every day that vaccine waning of the U.S. population exceeds the small percentage of people who get a booster, our vulnerability increases. If we don’t get that on track, it’s likely going to be a rough winter ahead.
Dr. Topol is director of the Scripps Translational Science Institute in La Jolla, Calif. He has received research grants from the National Institutes of Health and reported conflicts of interest involving Dexcom, Illumina, Molecular Stethoscope, Quest Diagnostics, and Blue Cross Blue Shield Association. A version of this article appeared on Medscape.com.
For many, long COVID’s impacts go on and on, major study says
in the same time frame, a large study out of Scotland found.
Multiple studies are evaluating people with long COVID in the hopes of figuring out why some people experience debilitating symptoms long after their primary infection ends and others either do not or recover more quickly.
This current study is notable for its large size – 96,238 people. Researchers checked in with participants at 6, 12, and 18 months, and included a group of people never infected with the coronavirus to help investigators make a stronger case.
“A lot of the symptoms of long COVID are nonspecific and therefore can occur in people never infected,” says senior study author Jill P. Pell, MD, head of the School of Health and Wellbeing at the University of Glasgow in Scotland.
Ruling out coincidence
This study shows that people experienced a wide range of symptoms after becoming infected with COVID-19 at a significantly higher rate than those who were never infected, “thereby confirming that they were genuinely associated with COVID and not merely a coincidence,” she said.
Among 21,525 people who had COVID-19 and had symptoms, tiredness, headache and muscle aches or muscle weakness were the most common ongoing symptoms.
Loss of smell was almost nine times more likely in this group compared to the never-infected group in one analysis where researchers controlled for other possible factors. The risk for loss of taste was almost six times greater, followed by risk of breathlessness at three times higher.
Long COVID risk was highest after a severe original infection and among older people, women, Black, and South Asian populations, people with socioeconomic disadvantages, and those with more than one underlying health condition.
Adding up the 6% with no recovery after 18 months and 42% with partial recovery means that between 6 and 18 months following symptomatic coronavirus infection, almost half of those infected still experience persistent symptoms.
Vaccination validated
On the plus side, people vaccinated against COVID-19 before getting infected had a lower risk for some persistent symptoms. In addition, Dr. Pell and colleagues found no evidence that people who experienced asymptomatic infection were likely to experience long COVID symptoms or challenges with activities of daily living.
The findings of the Long-COVID in Scotland Study (Long-CISS) were published in the journal Nature Communications.
‘More long COVID than ever before’
“Unfortunately, these long COVID symptoms are not getting better as the cases of COVID get milder,” said Thomas Gut, DO, medical director for the post-COVID recovery program at Staten Island (N.Y.) University Hospital. “Quite the opposite – this infection has become so common in a community because it’s so mild and spreading so rapidly that we’re seeing more long COVID symptoms than ever before.”
Although most patients he sees with long COVID resolve their symptoms within 3-6 months, “We do see some patients who require short-term disability because their symptoms continue past 6 months and out to 2 years,” said Dr. Gut, a hospitalist at Staten Island University Hospital, a member hospital of Northwell Health.
Patients with fatigue and neurocognitive symptoms “have a very tough time going back to work. Short-term disability gives them the time and finances to pursue specialty care with cardiology, pulmonary, and neurocognitive testing,” he said.
Support the whole person
The burden of living with long COVID goes beyond the persistent symptoms. “Long COVID can have wide-ranging impacts – not only on health but also quality of life and activities of daily living [including] work, mobility, self-care and more,” Dr. Pell said. “So, people with long COVID need support relevant to their individual needs and this may extend beyond the health care sector, for example including social services, school or workplace.”
Still, Lisa Penziner, RN, founder of the COVID Long Haulers Support Group in Westchester and Long Island, N.Y., said while people with the most severe cases of COVID-19 tended to have the worst long COVID symptoms, they’re not the only ones.
“We saw many post-COVID members who had mild cases and their long-haul symptoms were worse weeks later than the virus itself,” said Md. Penziner.
She estimates that 80%-90% of her support group members recover within 6 months. “However, there are others who were experiencing symptoms for much longer.”
Respiratory treatment, physical therapy, and other follow-up doctor visits are common after 6 months, for example.
“Additionally, there is a mental health component to recovery as well, meaning that the patient must learn to live while experiencing lingering, long-haul COVID symptoms in work and daily life,” said Ms. Penziner, director of special projects at North Westchester Restorative Therapy & Nursing.
In addition to ongoing medical care, people with long COVID need understanding, she said.
“While long-haul symptoms do not happen to everyone, it is proven that many do experience long-haul symptoms, and the support of the community in understanding is important.”
Limitations of the study
Dr. Pell and colleagues noted some strengths and weaknesses to their study. For example, “as a general population study, our findings provide a better indication of the overall risk and burden of long COVID than hospitalized cohorts,” they noted.
Also, the Scottish population is 96% White, so other long COVID studies with more diverse participants are warranted.
Another potential weakness is the response rate of 16% among those invited to participate in the study, which Dr. Pell and colleagues addressed: “Our cohort included a large sample (33,281) of people previously infected and the response rate of 16% overall and 20% among people who had symptomatic infection was consistent with previous studies that have used SMS text invitations as the sole method of recruitment.”
“We tell patients this should last 3-6 months, but some patients have longer recovery periods,” Dr. Gut said. “We’re here for them. We have a lot of services available to help get them through the recovery process, and we have a lot of options to help support them.”
“What we found most helpful is when there is peer-to-peer support, reaffirming to the member that they are not alone in the long-haul battle, which has been a major benefit of the support group,” Ms. Penziner said.
A version of this article first appeared on WebMD.com.
in the same time frame, a large study out of Scotland found.
Multiple studies are evaluating people with long COVID in the hopes of figuring out why some people experience debilitating symptoms long after their primary infection ends and others either do not or recover more quickly.
This current study is notable for its large size – 96,238 people. Researchers checked in with participants at 6, 12, and 18 months, and included a group of people never infected with the coronavirus to help investigators make a stronger case.
“A lot of the symptoms of long COVID are nonspecific and therefore can occur in people never infected,” says senior study author Jill P. Pell, MD, head of the School of Health and Wellbeing at the University of Glasgow in Scotland.
Ruling out coincidence
This study shows that people experienced a wide range of symptoms after becoming infected with COVID-19 at a significantly higher rate than those who were never infected, “thereby confirming that they were genuinely associated with COVID and not merely a coincidence,” she said.
Among 21,525 people who had COVID-19 and had symptoms, tiredness, headache and muscle aches or muscle weakness were the most common ongoing symptoms.
Loss of smell was almost nine times more likely in this group compared to the never-infected group in one analysis where researchers controlled for other possible factors. The risk for loss of taste was almost six times greater, followed by risk of breathlessness at three times higher.
Long COVID risk was highest after a severe original infection and among older people, women, Black, and South Asian populations, people with socioeconomic disadvantages, and those with more than one underlying health condition.
Adding up the 6% with no recovery after 18 months and 42% with partial recovery means that between 6 and 18 months following symptomatic coronavirus infection, almost half of those infected still experience persistent symptoms.
Vaccination validated
On the plus side, people vaccinated against COVID-19 before getting infected had a lower risk for some persistent symptoms. In addition, Dr. Pell and colleagues found no evidence that people who experienced asymptomatic infection were likely to experience long COVID symptoms or challenges with activities of daily living.
The findings of the Long-COVID in Scotland Study (Long-CISS) were published in the journal Nature Communications.
‘More long COVID than ever before’
“Unfortunately, these long COVID symptoms are not getting better as the cases of COVID get milder,” said Thomas Gut, DO, medical director for the post-COVID recovery program at Staten Island (N.Y.) University Hospital. “Quite the opposite – this infection has become so common in a community because it’s so mild and spreading so rapidly that we’re seeing more long COVID symptoms than ever before.”
Although most patients he sees with long COVID resolve their symptoms within 3-6 months, “We do see some patients who require short-term disability because their symptoms continue past 6 months and out to 2 years,” said Dr. Gut, a hospitalist at Staten Island University Hospital, a member hospital of Northwell Health.
Patients with fatigue and neurocognitive symptoms “have a very tough time going back to work. Short-term disability gives them the time and finances to pursue specialty care with cardiology, pulmonary, and neurocognitive testing,” he said.
Support the whole person
The burden of living with long COVID goes beyond the persistent symptoms. “Long COVID can have wide-ranging impacts – not only on health but also quality of life and activities of daily living [including] work, mobility, self-care and more,” Dr. Pell said. “So, people with long COVID need support relevant to their individual needs and this may extend beyond the health care sector, for example including social services, school or workplace.”
Still, Lisa Penziner, RN, founder of the COVID Long Haulers Support Group in Westchester and Long Island, N.Y., said while people with the most severe cases of COVID-19 tended to have the worst long COVID symptoms, they’re not the only ones.
“We saw many post-COVID members who had mild cases and their long-haul symptoms were worse weeks later than the virus itself,” said Md. Penziner.
She estimates that 80%-90% of her support group members recover within 6 months. “However, there are others who were experiencing symptoms for much longer.”
Respiratory treatment, physical therapy, and other follow-up doctor visits are common after 6 months, for example.
“Additionally, there is a mental health component to recovery as well, meaning that the patient must learn to live while experiencing lingering, long-haul COVID symptoms in work and daily life,” said Ms. Penziner, director of special projects at North Westchester Restorative Therapy & Nursing.
In addition to ongoing medical care, people with long COVID need understanding, she said.
“While long-haul symptoms do not happen to everyone, it is proven that many do experience long-haul symptoms, and the support of the community in understanding is important.”
Limitations of the study
Dr. Pell and colleagues noted some strengths and weaknesses to their study. For example, “as a general population study, our findings provide a better indication of the overall risk and burden of long COVID than hospitalized cohorts,” they noted.
Also, the Scottish population is 96% White, so other long COVID studies with more diverse participants are warranted.
Another potential weakness is the response rate of 16% among those invited to participate in the study, which Dr. Pell and colleagues addressed: “Our cohort included a large sample (33,281) of people previously infected and the response rate of 16% overall and 20% among people who had symptomatic infection was consistent with previous studies that have used SMS text invitations as the sole method of recruitment.”
“We tell patients this should last 3-6 months, but some patients have longer recovery periods,” Dr. Gut said. “We’re here for them. We have a lot of services available to help get them through the recovery process, and we have a lot of options to help support them.”
“What we found most helpful is when there is peer-to-peer support, reaffirming to the member that they are not alone in the long-haul battle, which has been a major benefit of the support group,” Ms. Penziner said.
A version of this article first appeared on WebMD.com.
in the same time frame, a large study out of Scotland found.
Multiple studies are evaluating people with long COVID in the hopes of figuring out why some people experience debilitating symptoms long after their primary infection ends and others either do not or recover more quickly.
This current study is notable for its large size – 96,238 people. Researchers checked in with participants at 6, 12, and 18 months, and included a group of people never infected with the coronavirus to help investigators make a stronger case.
“A lot of the symptoms of long COVID are nonspecific and therefore can occur in people never infected,” says senior study author Jill P. Pell, MD, head of the School of Health and Wellbeing at the University of Glasgow in Scotland.
Ruling out coincidence
This study shows that people experienced a wide range of symptoms after becoming infected with COVID-19 at a significantly higher rate than those who were never infected, “thereby confirming that they were genuinely associated with COVID and not merely a coincidence,” she said.
Among 21,525 people who had COVID-19 and had symptoms, tiredness, headache and muscle aches or muscle weakness were the most common ongoing symptoms.
Loss of smell was almost nine times more likely in this group compared to the never-infected group in one analysis where researchers controlled for other possible factors. The risk for loss of taste was almost six times greater, followed by risk of breathlessness at three times higher.
Long COVID risk was highest after a severe original infection and among older people, women, Black, and South Asian populations, people with socioeconomic disadvantages, and those with more than one underlying health condition.
Adding up the 6% with no recovery after 18 months and 42% with partial recovery means that between 6 and 18 months following symptomatic coronavirus infection, almost half of those infected still experience persistent symptoms.
Vaccination validated
On the plus side, people vaccinated against COVID-19 before getting infected had a lower risk for some persistent symptoms. In addition, Dr. Pell and colleagues found no evidence that people who experienced asymptomatic infection were likely to experience long COVID symptoms or challenges with activities of daily living.
The findings of the Long-COVID in Scotland Study (Long-CISS) were published in the journal Nature Communications.
‘More long COVID than ever before’
“Unfortunately, these long COVID symptoms are not getting better as the cases of COVID get milder,” said Thomas Gut, DO, medical director for the post-COVID recovery program at Staten Island (N.Y.) University Hospital. “Quite the opposite – this infection has become so common in a community because it’s so mild and spreading so rapidly that we’re seeing more long COVID symptoms than ever before.”
Although most patients he sees with long COVID resolve their symptoms within 3-6 months, “We do see some patients who require short-term disability because their symptoms continue past 6 months and out to 2 years,” said Dr. Gut, a hospitalist at Staten Island University Hospital, a member hospital of Northwell Health.
Patients with fatigue and neurocognitive symptoms “have a very tough time going back to work. Short-term disability gives them the time and finances to pursue specialty care with cardiology, pulmonary, and neurocognitive testing,” he said.
Support the whole person
The burden of living with long COVID goes beyond the persistent symptoms. “Long COVID can have wide-ranging impacts – not only on health but also quality of life and activities of daily living [including] work, mobility, self-care and more,” Dr. Pell said. “So, people with long COVID need support relevant to their individual needs and this may extend beyond the health care sector, for example including social services, school or workplace.”
Still, Lisa Penziner, RN, founder of the COVID Long Haulers Support Group in Westchester and Long Island, N.Y., said while people with the most severe cases of COVID-19 tended to have the worst long COVID symptoms, they’re not the only ones.
“We saw many post-COVID members who had mild cases and their long-haul symptoms were worse weeks later than the virus itself,” said Md. Penziner.
She estimates that 80%-90% of her support group members recover within 6 months. “However, there are others who were experiencing symptoms for much longer.”
Respiratory treatment, physical therapy, and other follow-up doctor visits are common after 6 months, for example.
“Additionally, there is a mental health component to recovery as well, meaning that the patient must learn to live while experiencing lingering, long-haul COVID symptoms in work and daily life,” said Ms. Penziner, director of special projects at North Westchester Restorative Therapy & Nursing.
In addition to ongoing medical care, people with long COVID need understanding, she said.
“While long-haul symptoms do not happen to everyone, it is proven that many do experience long-haul symptoms, and the support of the community in understanding is important.”
Limitations of the study
Dr. Pell and colleagues noted some strengths and weaknesses to their study. For example, “as a general population study, our findings provide a better indication of the overall risk and burden of long COVID than hospitalized cohorts,” they noted.
Also, the Scottish population is 96% White, so other long COVID studies with more diverse participants are warranted.
Another potential weakness is the response rate of 16% among those invited to participate in the study, which Dr. Pell and colleagues addressed: “Our cohort included a large sample (33,281) of people previously infected and the response rate of 16% overall and 20% among people who had symptomatic infection was consistent with previous studies that have used SMS text invitations as the sole method of recruitment.”
“We tell patients this should last 3-6 months, but some patients have longer recovery periods,” Dr. Gut said. “We’re here for them. We have a lot of services available to help get them through the recovery process, and we have a lot of options to help support them.”
“What we found most helpful is when there is peer-to-peer support, reaffirming to the member that they are not alone in the long-haul battle, which has been a major benefit of the support group,” Ms. Penziner said.
A version of this article first appeared on WebMD.com.
FROM NATURE COMMUNICATIONS
Mother-to-child transmission of SARS-CoV-2 may be underestimated
ANAHEIM, CALIF. – The rate of mother-to-child transmission of SARS-CoV-2 infection is likely higher than the current estimate of 2%-8%, suggests a recent study using cord blood serology to determine incidence. The study was presented at the American Academy of Pediatrics National Conference.
“Cord blood screening is a potential tool to identify SARS-CoV-2 infected and/or exposed neonates who should then be followed for long-term consequences of mother-to-child transmission,” Amy Yeh, MD, an assistant professor of clinical pediatrics at the University of Southern California, Los Angeles, told attendees at the meeting.
Dr. Yeh and her colleagues collected cord blood from more than 500 mothers at LAC+USC Medical Center from October 2021 to April 2022 and tested them for IgG antibodies against three SARS-CoV-2 antigens: nucleoprotein (N), receptor-binding domain (RBD), and spike protein (S1). Results with an IgG mean fluorescence intensity (MFI) above 700 were considered positive for IgG antibodies. A positive result for N as well as RBD or S1 indicated a natural infection while a positive result for only RBD or S1 indicated a vaccine response or past infection.
The researchers also tested a subset of the IgG positive samples for IgM and IgA antibodies against N, S1, and RBD, with an IgM MFI greater than 24 and an IgA MFI greater than 102 used as the thresholds for positive results.
Among 384 cord blood samples analyzed, 85.4% were positive for IgG against RBD, indicating that the mother had SARS-CoV-2 immunity from either a past infection or vaccination. Of these anti-RBD positive samples, 60.7% were anti-N IgG negative, suggesting that N had waned since vaccination or the past infection.
Since the other 39.3% that were anti-N IgG positive suggest a past maternal infection, the researchers assessed these 129 samples for IgM and IgA antibodies against RBD. They found that 16 of them had high levels of anti-RBD IgA and/or IgM antibodies, pointing to a rate of mother-to-child-transmission of up to 12.4%.
Sallie Permar, MD, PhD, a professor and the chair of pediatrics at Weill Cornell Medicine in New York, who was not involved in the research, said most studies of placental transmission have focused on virologic testing, such as PCR. “Serologic tests for congenital infections are inherently challenged by the transfer of maternal IgG across the placenta and therefore must rely on non-IgG isotype response detection, which have inherently been more susceptible to false-positive results than IgG-based tests,” Dr. Permar said.
Also, “it is unclear if virologic testing was performed in the infants, which, if positive in the same infants for which cord blood IgM/IgA responses were identified, could further validate positive serologic findings,” added Dr. Permar, who is also pediatrician-in-chief at New York-Presbyterian Komansky Children’s Hospital.
Given these limitations, Dr. Permar reiterated that diagnostics for congenital SARS-CoV-2 continue to evolve, even if congenital SARS-CoV-2 infection currently appears rare. Dr. Permar said she agreed with Dr. Yeh that following those who do develop this infection is important.
“There have been initial reports of neurodevelopmental and other outcomes from long-term follow-up cohorts of infants exposed to SARS-CoV-2 infection in utero with variable results and it should continue to be pursued using cohorts both enrolled early in the pandemic and those enrolled more recently after population-level immunity to SARS-CoV-2 was achieved,” said Dr. Permar.
Dr. Permar serves as a consultant to Moderna, Pfizer, Merck, Dynavax, and Hoopika on their CMV vaccine programs and has led sponsored research programs with Moderna and Merck. Information on study funding and on disclosures for Dr. Yeh was unavailable.
ANAHEIM, CALIF. – The rate of mother-to-child transmission of SARS-CoV-2 infection is likely higher than the current estimate of 2%-8%, suggests a recent study using cord blood serology to determine incidence. The study was presented at the American Academy of Pediatrics National Conference.
“Cord blood screening is a potential tool to identify SARS-CoV-2 infected and/or exposed neonates who should then be followed for long-term consequences of mother-to-child transmission,” Amy Yeh, MD, an assistant professor of clinical pediatrics at the University of Southern California, Los Angeles, told attendees at the meeting.
Dr. Yeh and her colleagues collected cord blood from more than 500 mothers at LAC+USC Medical Center from October 2021 to April 2022 and tested them for IgG antibodies against three SARS-CoV-2 antigens: nucleoprotein (N), receptor-binding domain (RBD), and spike protein (S1). Results with an IgG mean fluorescence intensity (MFI) above 700 were considered positive for IgG antibodies. A positive result for N as well as RBD or S1 indicated a natural infection while a positive result for only RBD or S1 indicated a vaccine response or past infection.
The researchers also tested a subset of the IgG positive samples for IgM and IgA antibodies against N, S1, and RBD, with an IgM MFI greater than 24 and an IgA MFI greater than 102 used as the thresholds for positive results.
Among 384 cord blood samples analyzed, 85.4% were positive for IgG against RBD, indicating that the mother had SARS-CoV-2 immunity from either a past infection or vaccination. Of these anti-RBD positive samples, 60.7% were anti-N IgG negative, suggesting that N had waned since vaccination or the past infection.
Since the other 39.3% that were anti-N IgG positive suggest a past maternal infection, the researchers assessed these 129 samples for IgM and IgA antibodies against RBD. They found that 16 of them had high levels of anti-RBD IgA and/or IgM antibodies, pointing to a rate of mother-to-child-transmission of up to 12.4%.
Sallie Permar, MD, PhD, a professor and the chair of pediatrics at Weill Cornell Medicine in New York, who was not involved in the research, said most studies of placental transmission have focused on virologic testing, such as PCR. “Serologic tests for congenital infections are inherently challenged by the transfer of maternal IgG across the placenta and therefore must rely on non-IgG isotype response detection, which have inherently been more susceptible to false-positive results than IgG-based tests,” Dr. Permar said.
Also, “it is unclear if virologic testing was performed in the infants, which, if positive in the same infants for which cord blood IgM/IgA responses were identified, could further validate positive serologic findings,” added Dr. Permar, who is also pediatrician-in-chief at New York-Presbyterian Komansky Children’s Hospital.
Given these limitations, Dr. Permar reiterated that diagnostics for congenital SARS-CoV-2 continue to evolve, even if congenital SARS-CoV-2 infection currently appears rare. Dr. Permar said she agreed with Dr. Yeh that following those who do develop this infection is important.
“There have been initial reports of neurodevelopmental and other outcomes from long-term follow-up cohorts of infants exposed to SARS-CoV-2 infection in utero with variable results and it should continue to be pursued using cohorts both enrolled early in the pandemic and those enrolled more recently after population-level immunity to SARS-CoV-2 was achieved,” said Dr. Permar.
Dr. Permar serves as a consultant to Moderna, Pfizer, Merck, Dynavax, and Hoopika on their CMV vaccine programs and has led sponsored research programs with Moderna and Merck. Information on study funding and on disclosures for Dr. Yeh was unavailable.
ANAHEIM, CALIF. – The rate of mother-to-child transmission of SARS-CoV-2 infection is likely higher than the current estimate of 2%-8%, suggests a recent study using cord blood serology to determine incidence. The study was presented at the American Academy of Pediatrics National Conference.
“Cord blood screening is a potential tool to identify SARS-CoV-2 infected and/or exposed neonates who should then be followed for long-term consequences of mother-to-child transmission,” Amy Yeh, MD, an assistant professor of clinical pediatrics at the University of Southern California, Los Angeles, told attendees at the meeting.
Dr. Yeh and her colleagues collected cord blood from more than 500 mothers at LAC+USC Medical Center from October 2021 to April 2022 and tested them for IgG antibodies against three SARS-CoV-2 antigens: nucleoprotein (N), receptor-binding domain (RBD), and spike protein (S1). Results with an IgG mean fluorescence intensity (MFI) above 700 were considered positive for IgG antibodies. A positive result for N as well as RBD or S1 indicated a natural infection while a positive result for only RBD or S1 indicated a vaccine response or past infection.
The researchers also tested a subset of the IgG positive samples for IgM and IgA antibodies against N, S1, and RBD, with an IgM MFI greater than 24 and an IgA MFI greater than 102 used as the thresholds for positive results.
Among 384 cord blood samples analyzed, 85.4% were positive for IgG against RBD, indicating that the mother had SARS-CoV-2 immunity from either a past infection or vaccination. Of these anti-RBD positive samples, 60.7% were anti-N IgG negative, suggesting that N had waned since vaccination or the past infection.
Since the other 39.3% that were anti-N IgG positive suggest a past maternal infection, the researchers assessed these 129 samples for IgM and IgA antibodies against RBD. They found that 16 of them had high levels of anti-RBD IgA and/or IgM antibodies, pointing to a rate of mother-to-child-transmission of up to 12.4%.
Sallie Permar, MD, PhD, a professor and the chair of pediatrics at Weill Cornell Medicine in New York, who was not involved in the research, said most studies of placental transmission have focused on virologic testing, such as PCR. “Serologic tests for congenital infections are inherently challenged by the transfer of maternal IgG across the placenta and therefore must rely on non-IgG isotype response detection, which have inherently been more susceptible to false-positive results than IgG-based tests,” Dr. Permar said.
Also, “it is unclear if virologic testing was performed in the infants, which, if positive in the same infants for which cord blood IgM/IgA responses were identified, could further validate positive serologic findings,” added Dr. Permar, who is also pediatrician-in-chief at New York-Presbyterian Komansky Children’s Hospital.
Given these limitations, Dr. Permar reiterated that diagnostics for congenital SARS-CoV-2 continue to evolve, even if congenital SARS-CoV-2 infection currently appears rare. Dr. Permar said she agreed with Dr. Yeh that following those who do develop this infection is important.
“There have been initial reports of neurodevelopmental and other outcomes from long-term follow-up cohorts of infants exposed to SARS-CoV-2 infection in utero with variable results and it should continue to be pursued using cohorts both enrolled early in the pandemic and those enrolled more recently after population-level immunity to SARS-CoV-2 was achieved,” said Dr. Permar.
Dr. Permar serves as a consultant to Moderna, Pfizer, Merck, Dynavax, and Hoopika on their CMV vaccine programs and has led sponsored research programs with Moderna and Merck. Information on study funding and on disclosures for Dr. Yeh was unavailable.
AT AAP 2022
COVID-19 linked to increased Alzheimer’s risk
The study of more than 6 million people aged 65 years or older found a 50%-80% increased risk for AD in the year after COVID-19; the risk was especially high for women older than 85 years.
However, the investigators were quick to point out that the observational retrospective study offers no evidence that COVID-19 causes AD. There could be a viral etiology at play, or the connection could be related to inflammation in neural tissue from the SARS-CoV-2 infection. Or it could simply be that exposure to the health care system for COVID-19 increased the odds of detection of existing undiagnosed AD cases.
Whatever the case, these findings point to a potential spike in AD cases, which is a cause for concern, study investigator Pamela Davis, MD, PhD, a professor in the Center for Community Health Integration at Case Western Reserve University, Cleveland, said in an interview.
“COVID may be giving us a legacy of ongoing medical difficulties,” Dr. Davis said. “We were already concerned about having a very large care burden and cost burden from Alzheimer’s disease. If this is another burden that’s increased by COVID, this is something we’re really going to have to prepare for.”
The findings were published online in Journal of Alzheimer’s Disease.
Increased risk
Earlier research points to a potential link between COVID-19 and increased risk for AD and Parkinson’s disease.
For the current study, researchers analyzed anonymous electronic health records of 6.2 million adults aged 65 years or older who received medical treatment between February 2020 and May 2021 and had no prior diagnosis of AD. The database includes information on almost 30% of the entire U.S. population.
Overall, there were 410,748 cases of COVID-19 during the study period.
The overall risk for new diagnosis of AD in the COVID-19 cohort was close to double that of those who did not have COVID-19 (0.68% vs. 0.35%, respectively).
After propensity-score matching, those who have had COVID-19 had a significantly higher risk for an AD diagnosis compared with those who were not infected (hazard ratio [HR], 1.69; 95% confidence interval [CI],1.53-1.72).
Risk for AD was elevated in all age groups, regardless of gender or ethnicity. Researchers did not collect data on COVID-19 severity, and the medical codes for long COVID were not published until after the study had ended.
Those with the highest risk were individuals older than 85 years (HR, 1.89; 95% CI, 1.73-2.07) and women (HR, 1.82; 95% CI, 1.69-1.97).
“We expected to see some impact, but I was surprised that it was as potent as it was,” Dr. Davis said.
Association, not causation
Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, who commented on the findings for this article, called the study interesting but emphasized caution in interpreting the results.
“Because this study only showed an association through medical records, we cannot know what the underlying mechanisms driving this association are without more research,” Dr. Snyder said. “If you have had COVID-19, it doesn’t mean you’re going to get dementia. But if you have had COVID-19 and are experiencing long-term symptoms including cognitive difficulties, talk to your doctor.”
Dr. Davis agreed, noting that this type of study offers information on association, but not causation. “I do think that this makes it imperative that we continue to follow the population for what’s going on in various neurodegenerative diseases,” Dr. Davis said.
The study was funded by the National Institute of Aging, National Institute on Alcohol Abuse and Alcoholism, the Clinical and Translational Science Collaborative of Cleveland, and the National Cancer Institute. Dr. Synder reports no relevant financial conflicts.
A version of this article first appeared on Medscape.com.
The study of more than 6 million people aged 65 years or older found a 50%-80% increased risk for AD in the year after COVID-19; the risk was especially high for women older than 85 years.
However, the investigators were quick to point out that the observational retrospective study offers no evidence that COVID-19 causes AD. There could be a viral etiology at play, or the connection could be related to inflammation in neural tissue from the SARS-CoV-2 infection. Or it could simply be that exposure to the health care system for COVID-19 increased the odds of detection of existing undiagnosed AD cases.
Whatever the case, these findings point to a potential spike in AD cases, which is a cause for concern, study investigator Pamela Davis, MD, PhD, a professor in the Center for Community Health Integration at Case Western Reserve University, Cleveland, said in an interview.
“COVID may be giving us a legacy of ongoing medical difficulties,” Dr. Davis said. “We were already concerned about having a very large care burden and cost burden from Alzheimer’s disease. If this is another burden that’s increased by COVID, this is something we’re really going to have to prepare for.”
The findings were published online in Journal of Alzheimer’s Disease.
Increased risk
Earlier research points to a potential link between COVID-19 and increased risk for AD and Parkinson’s disease.
For the current study, researchers analyzed anonymous electronic health records of 6.2 million adults aged 65 years or older who received medical treatment between February 2020 and May 2021 and had no prior diagnosis of AD. The database includes information on almost 30% of the entire U.S. population.
Overall, there were 410,748 cases of COVID-19 during the study period.
The overall risk for new diagnosis of AD in the COVID-19 cohort was close to double that of those who did not have COVID-19 (0.68% vs. 0.35%, respectively).
After propensity-score matching, those who have had COVID-19 had a significantly higher risk for an AD diagnosis compared with those who were not infected (hazard ratio [HR], 1.69; 95% confidence interval [CI],1.53-1.72).
Risk for AD was elevated in all age groups, regardless of gender or ethnicity. Researchers did not collect data on COVID-19 severity, and the medical codes for long COVID were not published until after the study had ended.
Those with the highest risk were individuals older than 85 years (HR, 1.89; 95% CI, 1.73-2.07) and women (HR, 1.82; 95% CI, 1.69-1.97).
“We expected to see some impact, but I was surprised that it was as potent as it was,” Dr. Davis said.
Association, not causation
Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, who commented on the findings for this article, called the study interesting but emphasized caution in interpreting the results.
“Because this study only showed an association through medical records, we cannot know what the underlying mechanisms driving this association are without more research,” Dr. Snyder said. “If you have had COVID-19, it doesn’t mean you’re going to get dementia. But if you have had COVID-19 and are experiencing long-term symptoms including cognitive difficulties, talk to your doctor.”
Dr. Davis agreed, noting that this type of study offers information on association, but not causation. “I do think that this makes it imperative that we continue to follow the population for what’s going on in various neurodegenerative diseases,” Dr. Davis said.
The study was funded by the National Institute of Aging, National Institute on Alcohol Abuse and Alcoholism, the Clinical and Translational Science Collaborative of Cleveland, and the National Cancer Institute. Dr. Synder reports no relevant financial conflicts.
A version of this article first appeared on Medscape.com.
The study of more than 6 million people aged 65 years or older found a 50%-80% increased risk for AD in the year after COVID-19; the risk was especially high for women older than 85 years.
However, the investigators were quick to point out that the observational retrospective study offers no evidence that COVID-19 causes AD. There could be a viral etiology at play, or the connection could be related to inflammation in neural tissue from the SARS-CoV-2 infection. Or it could simply be that exposure to the health care system for COVID-19 increased the odds of detection of existing undiagnosed AD cases.
Whatever the case, these findings point to a potential spike in AD cases, which is a cause for concern, study investigator Pamela Davis, MD, PhD, a professor in the Center for Community Health Integration at Case Western Reserve University, Cleveland, said in an interview.
“COVID may be giving us a legacy of ongoing medical difficulties,” Dr. Davis said. “We were already concerned about having a very large care burden and cost burden from Alzheimer’s disease. If this is another burden that’s increased by COVID, this is something we’re really going to have to prepare for.”
The findings were published online in Journal of Alzheimer’s Disease.
Increased risk
Earlier research points to a potential link between COVID-19 and increased risk for AD and Parkinson’s disease.
For the current study, researchers analyzed anonymous electronic health records of 6.2 million adults aged 65 years or older who received medical treatment between February 2020 and May 2021 and had no prior diagnosis of AD. The database includes information on almost 30% of the entire U.S. population.
Overall, there were 410,748 cases of COVID-19 during the study period.
The overall risk for new diagnosis of AD in the COVID-19 cohort was close to double that of those who did not have COVID-19 (0.68% vs. 0.35%, respectively).
After propensity-score matching, those who have had COVID-19 had a significantly higher risk for an AD diagnosis compared with those who were not infected (hazard ratio [HR], 1.69; 95% confidence interval [CI],1.53-1.72).
Risk for AD was elevated in all age groups, regardless of gender or ethnicity. Researchers did not collect data on COVID-19 severity, and the medical codes for long COVID were not published until after the study had ended.
Those with the highest risk were individuals older than 85 years (HR, 1.89; 95% CI, 1.73-2.07) and women (HR, 1.82; 95% CI, 1.69-1.97).
“We expected to see some impact, but I was surprised that it was as potent as it was,” Dr. Davis said.
Association, not causation
Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, who commented on the findings for this article, called the study interesting but emphasized caution in interpreting the results.
“Because this study only showed an association through medical records, we cannot know what the underlying mechanisms driving this association are without more research,” Dr. Snyder said. “If you have had COVID-19, it doesn’t mean you’re going to get dementia. But if you have had COVID-19 and are experiencing long-term symptoms including cognitive difficulties, talk to your doctor.”
Dr. Davis agreed, noting that this type of study offers information on association, but not causation. “I do think that this makes it imperative that we continue to follow the population for what’s going on in various neurodegenerative diseases,” Dr. Davis said.
The study was funded by the National Institute of Aging, National Institute on Alcohol Abuse and Alcoholism, the Clinical and Translational Science Collaborative of Cleveland, and the National Cancer Institute. Dr. Synder reports no relevant financial conflicts.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ALZHEIMER’S DISEASE

