User login
How heat kills: Deadly weather ‘cooking’ people from within
Millions of Americans have been languishing for weeks in the oppressive heat and humidity of a merciless summer. Deadly heat has already taken the lives of hundreds in the Pacific Northwest alone, with numbers likely to grow as the full impact of heat-related deaths eventually comes to light.
In the final week of July, the National Weather Service issued excessive heat warnings for 17 states, stretching from the West Coast, across the Midwest, down south into Louisiana and Georgia. Temperatures 10° to 15° F above average threaten the lives and livelihoods of people all across the country.
After a scorching heat wave in late June, residents of the Pacific Northwest are once again likely to see triple-digit temperatures in the coming days. With the heat, hospitals may face another surge of people with heat-related illnesses.
Erika Moseson, MD, a lung and intensive care specialist, witnessed firsthand the life-threatening impacts of soaring temperatures. She happened to be running her 10-bed intensive care unit in a suburban hospital in Gresham, Ore., about 15 miles east of Portland, the weekend of June 26. Within 12 hours, almost half her ICU beds were filled with people found unconscious on the street, in the bushes, or in their own beds, all because their body’s defenses had become overwhelmed by heat.
“It was unidentified person after unidentified person, coming in, same story, temperatures through the roof, comatose,” Dr. Moseson recalled. Young people in their 20s with muscle breakdown markers through the roof, a sign of rhabdomyolysis; people with no other medical problems that would have put them in a high-risk category.
As a lifelong Oregonian, she’d never seen anything like this before. “We’re all trained for it. I know what happens to you if you have heatstroke, I know how to treat it,” she trailed off, still finding it hard to believe. Still reeling from the number of cases in just a few hours. Still shocked that this happened on what’s supposed to be the cooler, rainforest side of Oregon.
Among those she treated and resuscitated, the memory of a patient that she lost continues to gnaw at her.
“I’ve gone back to it day after day since it happened,” she reflected.
Adults, in their 50s, living at home with their children. Just 1 hour prior, they’d all said goodnight. Then 1 hour later, when a child came to check in, both parents were unconscious.
Dr. Moseson shared how her team tried everything in their power for 18 hours to save the parent that was brought to her ICU. But like hundreds of others who went through the heat wave that weekend, her patient didn’t survive.
It was too late. From Dr. Moseson’s experience, it’s what happens “if you’re cooking a human.”
How heat kills
Regardless of where we live on the planet, humans maintain a consistent internal temperature around 98° F for our systems to function properly.
Our bodies have an entire temperature-regulating system to balance heat gain with heat loss so we don’t stray too far from our ideal range. The hypothalamus functions as the thermostat, communicating with heat sensors in our skin, muscles, and spinal cord. Based on signals about our core body temperature, our nervous system makes many decisions for us – opening up blood vessels in the peripheral parts of our body, pushing more blood toward the skin, and activating sweat glands to produce more sweat.
Sweat is one of the most powerful tools we have to maintain a safe internal temperature. Of course, there are some things under our control, such as removing clothing, drinking more water, and finding shade (or preferably air conditioning). But beyond that, it’s our ability to sweat that keeps us cool. When sweat evaporates into the air, heat from our skin goes with it, cooling us off.
Over time, our sweat response can work better as we get used to warmer environments, a process that’s known as acclimatization. Over the period of a few days to weeks, the sweat glands of acclimated people can start making sweat at lower temperatures, produce more sweat, and absorb more salt back into our system, all to make us more efficient “sweaters.”
While someone who’s not used to the heat may only produce 1 liter of sweat per hour, people who have become acclimated can produce 2-3 liters every hour, allowing evaporation to eliminate more than two times the amount of heat.
Because the process of acclimatization can take some time, typically it’s the first throes of summer, or heat waves in places where people don’t typically see high temperatures, that are the most deadly. And of course, the right infrastructure, like access to air conditioning, also plays a large role in limiting heat-related death and hospitalization.
A 2019 study showed that heat-related hospitalizations peak at different temperatures in different places. For example, hospitalizations typically peak in Texas when the temperature hits 105° F. But they might be highest in the Pacific Northwest at just 81° F.
Even with acclimatization, there are limits to how much our bodies can adapt to heat. When the humidity goes up past 75%, there’s already so much moisture in the air that heat loss through evaporation no longer occurs.
It’s this connection between heat and humidity that can be deadly. This is why the heat index (a measure that takes into account temperature and relative humidity) and wet bulb globe temperature (a measure commonly used by the military and competitive athletes that takes into account temperature, humidity, wind speed, sun angle, and cloud cover) are both better at showing how dangerous the heat may be for our health, compared to temperature alone.
Kristie L. Ebi, PhD, a professor in the Center for Health and the Global Environment at the University of Washington, Seattle, has been studying the effects of heat and other climate-sensitive conditions on health for over 20 years. She stresses that it’s not just the recorded temperatures, but the prolonged exposure that kills.
If you never get a chance to bring down that core body temperature, if your internal temperatures stay above the range where your cells and your organs can work well for a long time, that’s when you can have the most dangerous effects of heat.
“It depends then on your age, your fitness, your individual physiology, underlying medical conditions, to how quickly that could affect the functioning of those organs. There’s lots of variability in there,” Dr. Ebi said.
Our hearts take on the brunt of the early response, working harder to pump blood toward the skin. Water and salt loss through our skin can start to cause electrolyte changes that can cause heat cramps and heat exhaustion. We feel tired, nauseated, dizzy. With enough water loss, we may become dehydrated, limiting the blood flow to our brains, causing us to pass out.
These early signs are like a car’s check engine light – systems are already being damaged, but resting, refueling, and, most importantly, turning off the heat are critical steps to prevent fatal injury.
If hazardous heat exposure continues and our internal temperatures continue to rise, nerves stop talking to each other, the proteins in our body unfold and lose their shape, and the cells of our organs disintegrate. This in turn sets off a fire alarm in our blood vessels, where a variety of chemical messengers, including “heat-shock proteins,” are released. The release of these inflammatory proteins, coupled with the loss of blood flow, eventually leads to the death of cells throughout the body, from the brain, to the heart, the muscles, and the kidneys.
This process is referred to as heatstroke. In essence, we melt from the inside.
At a certain point, this cascade can’t be reversed. Just like when you cool a melting block of ice, the parts that have melted will not go back to their original shape. It’s a similar process in our bodies, so delays in cooling and treatment can lead to death rates as high as 80%.
On the outside, we see people who look confused and disoriented, with hot skin and rapid breathing, and they may eventually become unconscious. Core body temperatures over 105° F clinch the diagnosis, but at the first sign of feeling unwell, cooling should be started.
There is no fancier or more effective treatment than that: Cool right away. In emergency rooms in Washington State, doctors used body bags filled with ice and water to cool victims of the heat wave in late June.
“It was all from heat ... that’s the thing, you feel so idiotic ... you’re like, ‘I’ve given you ice’ ... you bring their temperature down. But it’s already set off this cascade that you can’t stop,” Dr. Moseson said.
By the time Dr. Moseson’s patient made it to her, cooling with ice was just the beginning of the attempts to resuscitate and revive. The patient was already showing evidence of a process causing widespread bleeding and clotting, known as disseminated intravascular coagulation, along with damage to the heart and failing kidneys. Over 18 hours, her team cooled the patient, flooded the blood vessels with fluids and blood products, attempted to start dialysis, and inserted a breathing tube – all of the technology that is used to save people from serious cardiovascular collapse from other conditions. But nothing could reverse the melting that had already occurred.
Deaths from heat are 100% preventable. Until they’re not.
No respite
As Dr. Ebi says, the key to preventing heat-related death is to cool down enough to stabilize our internal cells and proteins before the irreversible cascade begins.
But for close to 80% of Americans who live in urban areas, temperatures can be even higher and more intolerable compared to surrounding areas because of the way we’ve designed our cities. In effect, we have unintentionally created hot zones called “urban heat islands.”
Jeremy Hoffman, PhD, chief scientist for the Science Museum of Virginia, explains that things like bricks, asphalt, and parking lots absorb more of the sun’s energy throughout the day and then emit that back into the air as heat throughout the afternoon and into the evening. This raises the air and surface temperatures in cities, relative to rural areas. When temperatures don’t cool enough at night, there’s no way to recover from the day’s heat. You start the next day still depleted, with less reserve to face the heat of a new day.
When you dig even deeper, it turns out that even within the same city, there are huge “thermal inequities,” as Dr. Hoffman calls them. In a 2019 study, he found that wealthier parts of cities had more natural spaces such as parks and tree-lined streets, compared to areas that had been intentionally “redlined,” or systematically deprived of investment. This pattern repeats itself in over 100 urban areas across the country and translates to huge temperature differences on the order of 10-20 degrees Fahrenheit within the same city, at the exact same time during a heat wave.
“In some ways, the way that we’ve decided to plan and build our cities physically turns up the thermostat by several tens of degrees during heat waves in particular neighborhoods,” Dr. Hoffman said.
Dr. Hoffman’s work showed that the city of Portland (where the death toll from the heat wave in late June was the highest) had some of the most intense differences between formerly redlined vs. tree-lined areas out of the more than 100 cities that he studied.
“Watching it play out, I was really concerned, not only as a climate scientist, but as a human. Understanding the urban heat island effect and the extreme nature of the inequity in our cities, thermally and otherwise, once you start to really recognize it, you can’t forget it.”
The most vulnerable
When it comes to identifying and protecting the people most vulnerable to heat stress and heat-related death, there is an ever-growing list of those most at risk. Unfortunately, very few recognize when they themselves are at risk, often until it’s too late.
According to Linda McCauley, PhD, dean of the Emory University School of Nursing in Atlanta, “the scope of who is vulnerable is quickly increasing.”
For example, we’re used to recognizing that pregnant women and young children are at risk. Public health campaigns have long advised us not to leave young children and pets in hot cars. We know that adolescents who play sports during hot summer months are at high risk for heat-related events and even death.
In Georgia, a 15-year-old boy collapsed and died after his first day back at football practice when the heat index was 105° F on July 26, even as it appears that all protocols for heat safety were being followed.
We recognize that outdoor workers face devastating consequences from prolonged exertion in the heat and must have safer working conditions.
The elderly and those with long-term medical and mental health conditions are also more vulnerable to heat. The elderly may not have the same warning signs and may not recognize that they are dehydrated until it is too late. In addition, their sweating mechanism weakens, and they may be taking medicines that interfere with their ability to regulate their temperature.
Poverty and inadequate housing are risk factors, especially for those in urban heat islands. For many people, their housing does not have enough cooling to protect them, and they can’t safely get themselves to cooling shelters.
These patterns for the most vulnerable fit for the majority of deaths in Oregon during the late June heat wave. Most victims were older, lived alone, and didn’t have air conditioning. But with climate change, the predictions are that temperatures will go higher and heat waves will last longer.
“There’s probably very few people today that are ‘immune’ to the effects of heat-related stress with climate change. All of us can be put in situations where we are susceptible,” Dr. McCauley said.
Dr. Moseson agreed. Many of her patients fit none of these risk categories – she treated people with no health problems in their 20s in her ICU, and the patient she lost would not traditionally have been thought of as high risk. That 50-something patient had no long-standing medical problems, and lived with family in a newly renovated suburban home that had air conditioning. The only problem was that the air conditioner had broken and there had been no rush to fix it based on past experience with Oregon summers.
Preventing heat deaths
Protecting ourselves and our families means monitoring the “simple things.” The first three rules are to make sure we’re drinking plenty of water – this means drinking whether we feel thirsty or not. If we’re not in an air-conditioned place, we’ve got to look for shade. And we need to take regular rest breaks.
Inside a home without air conditioning, placing ice in front of a fan to cool the air can work, but realistically, if you are in a place without air conditioning and the temperatures are approaching 90° F, it’s safest to find another place to stay, if possible.
For those playing sports, there are usually 1-week to 2-week protocols that allow for acclimatization when the season begins – this means starting slowly, without gear, and ramping up activity. Still, parents and coaches should watch advanced weather reports to make sure it’s safe to practice outside.
How we dress can also help us, so light clothing is key. And if we’re able to schedule activities for times when it is cooler, that can also protect us from overheating.
If anyone shows early signs of heat stress, removing clothing, cooling their bodies with cold water, and getting them out of the heat is critical. Any evidence of heatstroke is an emergency, and 911 should be called without delay. The faster the core temperature can be dropped, the better the chances for recovery.
On the level of communities, access to natural air conditioning in the form of healthy tree canopies, and trees at bus stops to provide shade can help a lot. According to Dr. Hoffman, these investments help almost right away. Reimagining our cities to remove the “hot zones” that we have created is another key to protecting ourselves as our climate changes.
Reaching our limits in a changing climate
Already, we are seeing more intense, more frequent, and longer-lasting heat waves throughout the country and across the globe.
Dr. Ebi, a coauthor of a recently released scientific analysis that found that the late June Pacific Northwest heat wave would have been virtually impossible without climate change, herself lived through the scorching temperatures in Seattle. Her work shows that the changing climate is killing us right now.
We are approaching a time where extreme temperatures and humidity will make it almost impossible for people to be outside in many parts of the world. Researchers have found that periods of extreme humid heat have more than doubled since 1979, and some places have already had wet-bulb temperatures at the limits of what scientists think humans can tolerate under ideal conditions, meaning for people in perfect health, completely unclothed, in gale-force winds, performing no activity. Obviously that’s less than ideal for most of us and helps explain why thousands of people die at temperatures much lower than our upper limit.
Dr. Ebi pointed out that the good news is that many local communities with a long history of managing high temperatures have a lot of knowledge to share with regions that are newly dealing with these conditions. This includes how local areas develop early warning and response systems with specific action plans.
But, she cautions, it’s going to take a lot of coordination and a lot of behavior change to stabilize the earth’s climate, understand our weak points, and protect our health.
For Dr. Moseson, this reality has hit home.
“I already spent the year being terrified that I as an ICU doctor was going to be the one who gave my mom COVID. Finally I’m vaccinated, she’s vaccinated. Now I’ve watched someone die because they don’t have AC. And my parents, they’re old-school Oregonians, they don’t have AC.”
A version of this article originally appeared on WebMD.com.
Millions of Americans have been languishing for weeks in the oppressive heat and humidity of a merciless summer. Deadly heat has already taken the lives of hundreds in the Pacific Northwest alone, with numbers likely to grow as the full impact of heat-related deaths eventually comes to light.
In the final week of July, the National Weather Service issued excessive heat warnings for 17 states, stretching from the West Coast, across the Midwest, down south into Louisiana and Georgia. Temperatures 10° to 15° F above average threaten the lives and livelihoods of people all across the country.
After a scorching heat wave in late June, residents of the Pacific Northwest are once again likely to see triple-digit temperatures in the coming days. With the heat, hospitals may face another surge of people with heat-related illnesses.
Erika Moseson, MD, a lung and intensive care specialist, witnessed firsthand the life-threatening impacts of soaring temperatures. She happened to be running her 10-bed intensive care unit in a suburban hospital in Gresham, Ore., about 15 miles east of Portland, the weekend of June 26. Within 12 hours, almost half her ICU beds were filled with people found unconscious on the street, in the bushes, or in their own beds, all because their body’s defenses had become overwhelmed by heat.
“It was unidentified person after unidentified person, coming in, same story, temperatures through the roof, comatose,” Dr. Moseson recalled. Young people in their 20s with muscle breakdown markers through the roof, a sign of rhabdomyolysis; people with no other medical problems that would have put them in a high-risk category.
As a lifelong Oregonian, she’d never seen anything like this before. “We’re all trained for it. I know what happens to you if you have heatstroke, I know how to treat it,” she trailed off, still finding it hard to believe. Still reeling from the number of cases in just a few hours. Still shocked that this happened on what’s supposed to be the cooler, rainforest side of Oregon.
Among those she treated and resuscitated, the memory of a patient that she lost continues to gnaw at her.
“I’ve gone back to it day after day since it happened,” she reflected.
Adults, in their 50s, living at home with their children. Just 1 hour prior, they’d all said goodnight. Then 1 hour later, when a child came to check in, both parents were unconscious.
Dr. Moseson shared how her team tried everything in their power for 18 hours to save the parent that was brought to her ICU. But like hundreds of others who went through the heat wave that weekend, her patient didn’t survive.
It was too late. From Dr. Moseson’s experience, it’s what happens “if you’re cooking a human.”
How heat kills
Regardless of where we live on the planet, humans maintain a consistent internal temperature around 98° F for our systems to function properly.
Our bodies have an entire temperature-regulating system to balance heat gain with heat loss so we don’t stray too far from our ideal range. The hypothalamus functions as the thermostat, communicating with heat sensors in our skin, muscles, and spinal cord. Based on signals about our core body temperature, our nervous system makes many decisions for us – opening up blood vessels in the peripheral parts of our body, pushing more blood toward the skin, and activating sweat glands to produce more sweat.
Sweat is one of the most powerful tools we have to maintain a safe internal temperature. Of course, there are some things under our control, such as removing clothing, drinking more water, and finding shade (or preferably air conditioning). But beyond that, it’s our ability to sweat that keeps us cool. When sweat evaporates into the air, heat from our skin goes with it, cooling us off.
Over time, our sweat response can work better as we get used to warmer environments, a process that’s known as acclimatization. Over the period of a few days to weeks, the sweat glands of acclimated people can start making sweat at lower temperatures, produce more sweat, and absorb more salt back into our system, all to make us more efficient “sweaters.”
While someone who’s not used to the heat may only produce 1 liter of sweat per hour, people who have become acclimated can produce 2-3 liters every hour, allowing evaporation to eliminate more than two times the amount of heat.
Because the process of acclimatization can take some time, typically it’s the first throes of summer, or heat waves in places where people don’t typically see high temperatures, that are the most deadly. And of course, the right infrastructure, like access to air conditioning, also plays a large role in limiting heat-related death and hospitalization.
A 2019 study showed that heat-related hospitalizations peak at different temperatures in different places. For example, hospitalizations typically peak in Texas when the temperature hits 105° F. But they might be highest in the Pacific Northwest at just 81° F.
Even with acclimatization, there are limits to how much our bodies can adapt to heat. When the humidity goes up past 75%, there’s already so much moisture in the air that heat loss through evaporation no longer occurs.
It’s this connection between heat and humidity that can be deadly. This is why the heat index (a measure that takes into account temperature and relative humidity) and wet bulb globe temperature (a measure commonly used by the military and competitive athletes that takes into account temperature, humidity, wind speed, sun angle, and cloud cover) are both better at showing how dangerous the heat may be for our health, compared to temperature alone.
Kristie L. Ebi, PhD, a professor in the Center for Health and the Global Environment at the University of Washington, Seattle, has been studying the effects of heat and other climate-sensitive conditions on health for over 20 years. She stresses that it’s not just the recorded temperatures, but the prolonged exposure that kills.
If you never get a chance to bring down that core body temperature, if your internal temperatures stay above the range where your cells and your organs can work well for a long time, that’s when you can have the most dangerous effects of heat.
“It depends then on your age, your fitness, your individual physiology, underlying medical conditions, to how quickly that could affect the functioning of those organs. There’s lots of variability in there,” Dr. Ebi said.
Our hearts take on the brunt of the early response, working harder to pump blood toward the skin. Water and salt loss through our skin can start to cause electrolyte changes that can cause heat cramps and heat exhaustion. We feel tired, nauseated, dizzy. With enough water loss, we may become dehydrated, limiting the blood flow to our brains, causing us to pass out.
These early signs are like a car’s check engine light – systems are already being damaged, but resting, refueling, and, most importantly, turning off the heat are critical steps to prevent fatal injury.
If hazardous heat exposure continues and our internal temperatures continue to rise, nerves stop talking to each other, the proteins in our body unfold and lose their shape, and the cells of our organs disintegrate. This in turn sets off a fire alarm in our blood vessels, where a variety of chemical messengers, including “heat-shock proteins,” are released. The release of these inflammatory proteins, coupled with the loss of blood flow, eventually leads to the death of cells throughout the body, from the brain, to the heart, the muscles, and the kidneys.
This process is referred to as heatstroke. In essence, we melt from the inside.
At a certain point, this cascade can’t be reversed. Just like when you cool a melting block of ice, the parts that have melted will not go back to their original shape. It’s a similar process in our bodies, so delays in cooling and treatment can lead to death rates as high as 80%.
On the outside, we see people who look confused and disoriented, with hot skin and rapid breathing, and they may eventually become unconscious. Core body temperatures over 105° F clinch the diagnosis, but at the first sign of feeling unwell, cooling should be started.
There is no fancier or more effective treatment than that: Cool right away. In emergency rooms in Washington State, doctors used body bags filled with ice and water to cool victims of the heat wave in late June.
“It was all from heat ... that’s the thing, you feel so idiotic ... you’re like, ‘I’ve given you ice’ ... you bring their temperature down. But it’s already set off this cascade that you can’t stop,” Dr. Moseson said.
By the time Dr. Moseson’s patient made it to her, cooling with ice was just the beginning of the attempts to resuscitate and revive. The patient was already showing evidence of a process causing widespread bleeding and clotting, known as disseminated intravascular coagulation, along with damage to the heart and failing kidneys. Over 18 hours, her team cooled the patient, flooded the blood vessels with fluids and blood products, attempted to start dialysis, and inserted a breathing tube – all of the technology that is used to save people from serious cardiovascular collapse from other conditions. But nothing could reverse the melting that had already occurred.
Deaths from heat are 100% preventable. Until they’re not.
No respite
As Dr. Ebi says, the key to preventing heat-related death is to cool down enough to stabilize our internal cells and proteins before the irreversible cascade begins.
But for close to 80% of Americans who live in urban areas, temperatures can be even higher and more intolerable compared to surrounding areas because of the way we’ve designed our cities. In effect, we have unintentionally created hot zones called “urban heat islands.”
Jeremy Hoffman, PhD, chief scientist for the Science Museum of Virginia, explains that things like bricks, asphalt, and parking lots absorb more of the sun’s energy throughout the day and then emit that back into the air as heat throughout the afternoon and into the evening. This raises the air and surface temperatures in cities, relative to rural areas. When temperatures don’t cool enough at night, there’s no way to recover from the day’s heat. You start the next day still depleted, with less reserve to face the heat of a new day.
When you dig even deeper, it turns out that even within the same city, there are huge “thermal inequities,” as Dr. Hoffman calls them. In a 2019 study, he found that wealthier parts of cities had more natural spaces such as parks and tree-lined streets, compared to areas that had been intentionally “redlined,” or systematically deprived of investment. This pattern repeats itself in over 100 urban areas across the country and translates to huge temperature differences on the order of 10-20 degrees Fahrenheit within the same city, at the exact same time during a heat wave.
“In some ways, the way that we’ve decided to plan and build our cities physically turns up the thermostat by several tens of degrees during heat waves in particular neighborhoods,” Dr. Hoffman said.
Dr. Hoffman’s work showed that the city of Portland (where the death toll from the heat wave in late June was the highest) had some of the most intense differences between formerly redlined vs. tree-lined areas out of the more than 100 cities that he studied.
“Watching it play out, I was really concerned, not only as a climate scientist, but as a human. Understanding the urban heat island effect and the extreme nature of the inequity in our cities, thermally and otherwise, once you start to really recognize it, you can’t forget it.”
The most vulnerable
When it comes to identifying and protecting the people most vulnerable to heat stress and heat-related death, there is an ever-growing list of those most at risk. Unfortunately, very few recognize when they themselves are at risk, often until it’s too late.
According to Linda McCauley, PhD, dean of the Emory University School of Nursing in Atlanta, “the scope of who is vulnerable is quickly increasing.”
For example, we’re used to recognizing that pregnant women and young children are at risk. Public health campaigns have long advised us not to leave young children and pets in hot cars. We know that adolescents who play sports during hot summer months are at high risk for heat-related events and even death.
In Georgia, a 15-year-old boy collapsed and died after his first day back at football practice when the heat index was 105° F on July 26, even as it appears that all protocols for heat safety were being followed.
We recognize that outdoor workers face devastating consequences from prolonged exertion in the heat and must have safer working conditions.
The elderly and those with long-term medical and mental health conditions are also more vulnerable to heat. The elderly may not have the same warning signs and may not recognize that they are dehydrated until it is too late. In addition, their sweating mechanism weakens, and they may be taking medicines that interfere with their ability to regulate their temperature.
Poverty and inadequate housing are risk factors, especially for those in urban heat islands. For many people, their housing does not have enough cooling to protect them, and they can’t safely get themselves to cooling shelters.
These patterns for the most vulnerable fit for the majority of deaths in Oregon during the late June heat wave. Most victims were older, lived alone, and didn’t have air conditioning. But with climate change, the predictions are that temperatures will go higher and heat waves will last longer.
“There’s probably very few people today that are ‘immune’ to the effects of heat-related stress with climate change. All of us can be put in situations where we are susceptible,” Dr. McCauley said.
Dr. Moseson agreed. Many of her patients fit none of these risk categories – she treated people with no health problems in their 20s in her ICU, and the patient she lost would not traditionally have been thought of as high risk. That 50-something patient had no long-standing medical problems, and lived with family in a newly renovated suburban home that had air conditioning. The only problem was that the air conditioner had broken and there had been no rush to fix it based on past experience with Oregon summers.
Preventing heat deaths
Protecting ourselves and our families means monitoring the “simple things.” The first three rules are to make sure we’re drinking plenty of water – this means drinking whether we feel thirsty or not. If we’re not in an air-conditioned place, we’ve got to look for shade. And we need to take regular rest breaks.
Inside a home without air conditioning, placing ice in front of a fan to cool the air can work, but realistically, if you are in a place without air conditioning and the temperatures are approaching 90° F, it’s safest to find another place to stay, if possible.
For those playing sports, there are usually 1-week to 2-week protocols that allow for acclimatization when the season begins – this means starting slowly, without gear, and ramping up activity. Still, parents and coaches should watch advanced weather reports to make sure it’s safe to practice outside.
How we dress can also help us, so light clothing is key. And if we’re able to schedule activities for times when it is cooler, that can also protect us from overheating.
If anyone shows early signs of heat stress, removing clothing, cooling their bodies with cold water, and getting them out of the heat is critical. Any evidence of heatstroke is an emergency, and 911 should be called without delay. The faster the core temperature can be dropped, the better the chances for recovery.
On the level of communities, access to natural air conditioning in the form of healthy tree canopies, and trees at bus stops to provide shade can help a lot. According to Dr. Hoffman, these investments help almost right away. Reimagining our cities to remove the “hot zones” that we have created is another key to protecting ourselves as our climate changes.
Reaching our limits in a changing climate
Already, we are seeing more intense, more frequent, and longer-lasting heat waves throughout the country and across the globe.
Dr. Ebi, a coauthor of a recently released scientific analysis that found that the late June Pacific Northwest heat wave would have been virtually impossible without climate change, herself lived through the scorching temperatures in Seattle. Her work shows that the changing climate is killing us right now.
We are approaching a time where extreme temperatures and humidity will make it almost impossible for people to be outside in many parts of the world. Researchers have found that periods of extreme humid heat have more than doubled since 1979, and some places have already had wet-bulb temperatures at the limits of what scientists think humans can tolerate under ideal conditions, meaning for people in perfect health, completely unclothed, in gale-force winds, performing no activity. Obviously that’s less than ideal for most of us and helps explain why thousands of people die at temperatures much lower than our upper limit.
Dr. Ebi pointed out that the good news is that many local communities with a long history of managing high temperatures have a lot of knowledge to share with regions that are newly dealing with these conditions. This includes how local areas develop early warning and response systems with specific action plans.
But, she cautions, it’s going to take a lot of coordination and a lot of behavior change to stabilize the earth’s climate, understand our weak points, and protect our health.
For Dr. Moseson, this reality has hit home.
“I already spent the year being terrified that I as an ICU doctor was going to be the one who gave my mom COVID. Finally I’m vaccinated, she’s vaccinated. Now I’ve watched someone die because they don’t have AC. And my parents, they’re old-school Oregonians, they don’t have AC.”
A version of this article originally appeared on WebMD.com.
Millions of Americans have been languishing for weeks in the oppressive heat and humidity of a merciless summer. Deadly heat has already taken the lives of hundreds in the Pacific Northwest alone, with numbers likely to grow as the full impact of heat-related deaths eventually comes to light.
In the final week of July, the National Weather Service issued excessive heat warnings for 17 states, stretching from the West Coast, across the Midwest, down south into Louisiana and Georgia. Temperatures 10° to 15° F above average threaten the lives and livelihoods of people all across the country.
After a scorching heat wave in late June, residents of the Pacific Northwest are once again likely to see triple-digit temperatures in the coming days. With the heat, hospitals may face another surge of people with heat-related illnesses.
Erika Moseson, MD, a lung and intensive care specialist, witnessed firsthand the life-threatening impacts of soaring temperatures. She happened to be running her 10-bed intensive care unit in a suburban hospital in Gresham, Ore., about 15 miles east of Portland, the weekend of June 26. Within 12 hours, almost half her ICU beds were filled with people found unconscious on the street, in the bushes, or in their own beds, all because their body’s defenses had become overwhelmed by heat.
“It was unidentified person after unidentified person, coming in, same story, temperatures through the roof, comatose,” Dr. Moseson recalled. Young people in their 20s with muscle breakdown markers through the roof, a sign of rhabdomyolysis; people with no other medical problems that would have put them in a high-risk category.
As a lifelong Oregonian, she’d never seen anything like this before. “We’re all trained for it. I know what happens to you if you have heatstroke, I know how to treat it,” she trailed off, still finding it hard to believe. Still reeling from the number of cases in just a few hours. Still shocked that this happened on what’s supposed to be the cooler, rainforest side of Oregon.
Among those she treated and resuscitated, the memory of a patient that she lost continues to gnaw at her.
“I’ve gone back to it day after day since it happened,” she reflected.
Adults, in their 50s, living at home with their children. Just 1 hour prior, they’d all said goodnight. Then 1 hour later, when a child came to check in, both parents were unconscious.
Dr. Moseson shared how her team tried everything in their power for 18 hours to save the parent that was brought to her ICU. But like hundreds of others who went through the heat wave that weekend, her patient didn’t survive.
It was too late. From Dr. Moseson’s experience, it’s what happens “if you’re cooking a human.”
How heat kills
Regardless of where we live on the planet, humans maintain a consistent internal temperature around 98° F for our systems to function properly.
Our bodies have an entire temperature-regulating system to balance heat gain with heat loss so we don’t stray too far from our ideal range. The hypothalamus functions as the thermostat, communicating with heat sensors in our skin, muscles, and spinal cord. Based on signals about our core body temperature, our nervous system makes many decisions for us – opening up blood vessels in the peripheral parts of our body, pushing more blood toward the skin, and activating sweat glands to produce more sweat.
Sweat is one of the most powerful tools we have to maintain a safe internal temperature. Of course, there are some things under our control, such as removing clothing, drinking more water, and finding shade (or preferably air conditioning). But beyond that, it’s our ability to sweat that keeps us cool. When sweat evaporates into the air, heat from our skin goes with it, cooling us off.
Over time, our sweat response can work better as we get used to warmer environments, a process that’s known as acclimatization. Over the period of a few days to weeks, the sweat glands of acclimated people can start making sweat at lower temperatures, produce more sweat, and absorb more salt back into our system, all to make us more efficient “sweaters.”
While someone who’s not used to the heat may only produce 1 liter of sweat per hour, people who have become acclimated can produce 2-3 liters every hour, allowing evaporation to eliminate more than two times the amount of heat.
Because the process of acclimatization can take some time, typically it’s the first throes of summer, or heat waves in places where people don’t typically see high temperatures, that are the most deadly. And of course, the right infrastructure, like access to air conditioning, also plays a large role in limiting heat-related death and hospitalization.
A 2019 study showed that heat-related hospitalizations peak at different temperatures in different places. For example, hospitalizations typically peak in Texas when the temperature hits 105° F. But they might be highest in the Pacific Northwest at just 81° F.
Even with acclimatization, there are limits to how much our bodies can adapt to heat. When the humidity goes up past 75%, there’s already so much moisture in the air that heat loss through evaporation no longer occurs.
It’s this connection between heat and humidity that can be deadly. This is why the heat index (a measure that takes into account temperature and relative humidity) and wet bulb globe temperature (a measure commonly used by the military and competitive athletes that takes into account temperature, humidity, wind speed, sun angle, and cloud cover) are both better at showing how dangerous the heat may be for our health, compared to temperature alone.
Kristie L. Ebi, PhD, a professor in the Center for Health and the Global Environment at the University of Washington, Seattle, has been studying the effects of heat and other climate-sensitive conditions on health for over 20 years. She stresses that it’s not just the recorded temperatures, but the prolonged exposure that kills.
If you never get a chance to bring down that core body temperature, if your internal temperatures stay above the range where your cells and your organs can work well for a long time, that’s when you can have the most dangerous effects of heat.
“It depends then on your age, your fitness, your individual physiology, underlying medical conditions, to how quickly that could affect the functioning of those organs. There’s lots of variability in there,” Dr. Ebi said.
Our hearts take on the brunt of the early response, working harder to pump blood toward the skin. Water and salt loss through our skin can start to cause electrolyte changes that can cause heat cramps and heat exhaustion. We feel tired, nauseated, dizzy. With enough water loss, we may become dehydrated, limiting the blood flow to our brains, causing us to pass out.
These early signs are like a car’s check engine light – systems are already being damaged, but resting, refueling, and, most importantly, turning off the heat are critical steps to prevent fatal injury.
If hazardous heat exposure continues and our internal temperatures continue to rise, nerves stop talking to each other, the proteins in our body unfold and lose their shape, and the cells of our organs disintegrate. This in turn sets off a fire alarm in our blood vessels, where a variety of chemical messengers, including “heat-shock proteins,” are released. The release of these inflammatory proteins, coupled with the loss of blood flow, eventually leads to the death of cells throughout the body, from the brain, to the heart, the muscles, and the kidneys.
This process is referred to as heatstroke. In essence, we melt from the inside.
At a certain point, this cascade can’t be reversed. Just like when you cool a melting block of ice, the parts that have melted will not go back to their original shape. It’s a similar process in our bodies, so delays in cooling and treatment can lead to death rates as high as 80%.
On the outside, we see people who look confused and disoriented, with hot skin and rapid breathing, and they may eventually become unconscious. Core body temperatures over 105° F clinch the diagnosis, but at the first sign of feeling unwell, cooling should be started.
There is no fancier or more effective treatment than that: Cool right away. In emergency rooms in Washington State, doctors used body bags filled with ice and water to cool victims of the heat wave in late June.
“It was all from heat ... that’s the thing, you feel so idiotic ... you’re like, ‘I’ve given you ice’ ... you bring their temperature down. But it’s already set off this cascade that you can’t stop,” Dr. Moseson said.
By the time Dr. Moseson’s patient made it to her, cooling with ice was just the beginning of the attempts to resuscitate and revive. The patient was already showing evidence of a process causing widespread bleeding and clotting, known as disseminated intravascular coagulation, along with damage to the heart and failing kidneys. Over 18 hours, her team cooled the patient, flooded the blood vessels with fluids and blood products, attempted to start dialysis, and inserted a breathing tube – all of the technology that is used to save people from serious cardiovascular collapse from other conditions. But nothing could reverse the melting that had already occurred.
Deaths from heat are 100% preventable. Until they’re not.
No respite
As Dr. Ebi says, the key to preventing heat-related death is to cool down enough to stabilize our internal cells and proteins before the irreversible cascade begins.
But for close to 80% of Americans who live in urban areas, temperatures can be even higher and more intolerable compared to surrounding areas because of the way we’ve designed our cities. In effect, we have unintentionally created hot zones called “urban heat islands.”
Jeremy Hoffman, PhD, chief scientist for the Science Museum of Virginia, explains that things like bricks, asphalt, and parking lots absorb more of the sun’s energy throughout the day and then emit that back into the air as heat throughout the afternoon and into the evening. This raises the air and surface temperatures in cities, relative to rural areas. When temperatures don’t cool enough at night, there’s no way to recover from the day’s heat. You start the next day still depleted, with less reserve to face the heat of a new day.
When you dig even deeper, it turns out that even within the same city, there are huge “thermal inequities,” as Dr. Hoffman calls them. In a 2019 study, he found that wealthier parts of cities had more natural spaces such as parks and tree-lined streets, compared to areas that had been intentionally “redlined,” or systematically deprived of investment. This pattern repeats itself in over 100 urban areas across the country and translates to huge temperature differences on the order of 10-20 degrees Fahrenheit within the same city, at the exact same time during a heat wave.
“In some ways, the way that we’ve decided to plan and build our cities physically turns up the thermostat by several tens of degrees during heat waves in particular neighborhoods,” Dr. Hoffman said.
Dr. Hoffman’s work showed that the city of Portland (where the death toll from the heat wave in late June was the highest) had some of the most intense differences between formerly redlined vs. tree-lined areas out of the more than 100 cities that he studied.
“Watching it play out, I was really concerned, not only as a climate scientist, but as a human. Understanding the urban heat island effect and the extreme nature of the inequity in our cities, thermally and otherwise, once you start to really recognize it, you can’t forget it.”
The most vulnerable
When it comes to identifying and protecting the people most vulnerable to heat stress and heat-related death, there is an ever-growing list of those most at risk. Unfortunately, very few recognize when they themselves are at risk, often until it’s too late.
According to Linda McCauley, PhD, dean of the Emory University School of Nursing in Atlanta, “the scope of who is vulnerable is quickly increasing.”
For example, we’re used to recognizing that pregnant women and young children are at risk. Public health campaigns have long advised us not to leave young children and pets in hot cars. We know that adolescents who play sports during hot summer months are at high risk for heat-related events and even death.
In Georgia, a 15-year-old boy collapsed and died after his first day back at football practice when the heat index was 105° F on July 26, even as it appears that all protocols for heat safety were being followed.
We recognize that outdoor workers face devastating consequences from prolonged exertion in the heat and must have safer working conditions.
The elderly and those with long-term medical and mental health conditions are also more vulnerable to heat. The elderly may not have the same warning signs and may not recognize that they are dehydrated until it is too late. In addition, their sweating mechanism weakens, and they may be taking medicines that interfere with their ability to regulate their temperature.
Poverty and inadequate housing are risk factors, especially for those in urban heat islands. For many people, their housing does not have enough cooling to protect them, and they can’t safely get themselves to cooling shelters.
These patterns for the most vulnerable fit for the majority of deaths in Oregon during the late June heat wave. Most victims were older, lived alone, and didn’t have air conditioning. But with climate change, the predictions are that temperatures will go higher and heat waves will last longer.
“There’s probably very few people today that are ‘immune’ to the effects of heat-related stress with climate change. All of us can be put in situations where we are susceptible,” Dr. McCauley said.
Dr. Moseson agreed. Many of her patients fit none of these risk categories – she treated people with no health problems in their 20s in her ICU, and the patient she lost would not traditionally have been thought of as high risk. That 50-something patient had no long-standing medical problems, and lived with family in a newly renovated suburban home that had air conditioning. The only problem was that the air conditioner had broken and there had been no rush to fix it based on past experience with Oregon summers.
Preventing heat deaths
Protecting ourselves and our families means monitoring the “simple things.” The first three rules are to make sure we’re drinking plenty of water – this means drinking whether we feel thirsty or not. If we’re not in an air-conditioned place, we’ve got to look for shade. And we need to take regular rest breaks.
Inside a home without air conditioning, placing ice in front of a fan to cool the air can work, but realistically, if you are in a place without air conditioning and the temperatures are approaching 90° F, it’s safest to find another place to stay, if possible.
For those playing sports, there are usually 1-week to 2-week protocols that allow for acclimatization when the season begins – this means starting slowly, without gear, and ramping up activity. Still, parents and coaches should watch advanced weather reports to make sure it’s safe to practice outside.
How we dress can also help us, so light clothing is key. And if we’re able to schedule activities for times when it is cooler, that can also protect us from overheating.
If anyone shows early signs of heat stress, removing clothing, cooling their bodies with cold water, and getting them out of the heat is critical. Any evidence of heatstroke is an emergency, and 911 should be called without delay. The faster the core temperature can be dropped, the better the chances for recovery.
On the level of communities, access to natural air conditioning in the form of healthy tree canopies, and trees at bus stops to provide shade can help a lot. According to Dr. Hoffman, these investments help almost right away. Reimagining our cities to remove the “hot zones” that we have created is another key to protecting ourselves as our climate changes.
Reaching our limits in a changing climate
Already, we are seeing more intense, more frequent, and longer-lasting heat waves throughout the country and across the globe.
Dr. Ebi, a coauthor of a recently released scientific analysis that found that the late June Pacific Northwest heat wave would have been virtually impossible without climate change, herself lived through the scorching temperatures in Seattle. Her work shows that the changing climate is killing us right now.
We are approaching a time where extreme temperatures and humidity will make it almost impossible for people to be outside in many parts of the world. Researchers have found that periods of extreme humid heat have more than doubled since 1979, and some places have already had wet-bulb temperatures at the limits of what scientists think humans can tolerate under ideal conditions, meaning for people in perfect health, completely unclothed, in gale-force winds, performing no activity. Obviously that’s less than ideal for most of us and helps explain why thousands of people die at temperatures much lower than our upper limit.
Dr. Ebi pointed out that the good news is that many local communities with a long history of managing high temperatures have a lot of knowledge to share with regions that are newly dealing with these conditions. This includes how local areas develop early warning and response systems with specific action plans.
But, she cautions, it’s going to take a lot of coordination and a lot of behavior change to stabilize the earth’s climate, understand our weak points, and protect our health.
For Dr. Moseson, this reality has hit home.
“I already spent the year being terrified that I as an ICU doctor was going to be the one who gave my mom COVID. Finally I’m vaccinated, she’s vaccinated. Now I’ve watched someone die because they don’t have AC. And my parents, they’re old-school Oregonians, they don’t have AC.”
A version of this article originally appeared on WebMD.com.
Traumatic Fractures Should Trigger Osteoporosis Assessment in Postmenopausal Women
Study Overview
Objective. To compare the risk of subsequent fractures after an initial traumatic or nontraumatic fracture in postmenopausal women.
Design. A prospective observational study utilizing data from the Women’s Health Initiative (WHI) Study, WHI Clinical Trials (WHI-CT), and WHI Bone Density Substudy to evaluate rates at which patients who suffered a traumatic fracture vs nontraumatic fracture develop a subsequent fracture.
Setting and participants. The WHI study, implemented at 40 United States clinical sites, enrolled 161 808 postmenopausal women aged 50 to 79 years at baseline between 1993 and 1998. The study cohort consisted of 75 335 patients who had self-reported fractures from September 1994 to December 1998 that were confirmed by the WHI Bone Density Substudy and WHI-CT. Of these participants, 253 (0.3%) were excluded because of a lack of follow-up information regarding incident fractures, and 8208 (10.9%) were excluded due to incomplete information on covariates, thus resulting in an analytic sample of 66 874 (88.8%) participants. Prospective fracture ascertainment with participants was conducted at least annually and the mechanism of fracture was assessed to differentiate traumatic vs nontraumatic incident fractures. Traumatic fractures were defined as fractures caused by motor vehicle collisions, falls from a height, falls downstairs, or sports injury. Nontraumatic fractures were defined as fractures caused by a trip and fall.
Main outcome measures. The primary outcome was an incident fracture at an anatomically distinct body part. Fractures were classified as upper extremity (carpal, elbow, lower or upper end of humerus, shaft of humerus, upper radius/ulna, or radius/ulna), lower extremity (ankle, hip, patella, pelvis, shaft of femur, tibia/fibula, or tibial plateau), or spine (lumbar and/or thoracic spine). Self-reported fractures were verified via medical chart review by WHI study physicians; hip fractures were confirmed by review of written reports of radiographic studies; and nonhip fractures were confirmed by review of radiography reports or clinical documentations.
Main results. In total, 66 874 women in the study (mean [SD] age) 63.1 (7.0) years without clinical fracture and 65.3 (7.2) years with clinical fracture at baseline were followed for 8.1 (1.6) years. Of these participants, 7142 (10.7%) experienced incident fracture during the study follow-up period (13.9 per 1000 person-years), and 721 (10.1%) of whom had a subsequent fracture. The adjusted hazard ratio (aHR) of subsequent fracture after an initial fracture was 1.49 (95% CI, 1.38-1.61, P < .001). Covariates adjusted were age, race, ethnicity, body mass index, treated diabetes, frequency of falls in the previous year, and physical function and activity. In women with initial traumatic fracture, the association between initial and subsequent fracture was increased (aHR, 1.25; 95% CI, 1.06-1.48, P = .01). Among women with initial nontraumatic fracture, the association between initial and subsequent fracture was also increased (aHR, 1.52; 95% CI, 1.37-1.68, P < .001). The confidence intervals for the 2 preceding associations for traumatic and nontraumatic initial fracture strata were overlapping.
Conclusion. Fractures, regardless of mechanism of injury, are similarly associated with an increased risk of subsequent fractures in postmenopausal women aged 50 years and older. Findings from this study provide evidence to support reevaluation of current clinical guidelines to include traumatic fracture as a trigger for osteoporosis screening.
Commentary
Osteoporosis is one of the most common age-associated disease that affects 1 in 4 women and 1 in 20 men over the age of 65.1 It increases the risk of fracture, and its clinical sequelae include reduced mobility, health decline, and increased all-cause mortality. The high prevalence of osteoporosis poses a clinical challenge as the global population continues to age. Pharmacological treatments such as bisphosphonates are highly effective in preventing or slowing bone mineral density (BMD) loss and reducing risk of fragility fractures (eg, nontraumatic fractures of the vertebra, hip, and femur) and are commonly used to mitigate adverse effects of degenerative bone changes secondary to osteoporosis.1
The high prevalence of osteoporosis and effectiveness of bisphosphonates raises the question of how to optimally identify adults at risk for osteoporosis so that pharmacologic therapy can be promptly initiated to prevent disease progression. Multiple osteoporosis screening guidelines, including those from the United States Preventive Services Task Force (USPSTF), American Association of Family Physicians, and National Osteoporosis Foundation, are widely used in the clinical setting to address this important clinical question. In general, the prevailing wisdom is to screen osteoporosis in postmenopausal women over the age of 65, women under the age of 65 who have a significant 10-year fracture risk, or women over the age of 50 who have experienced a fragility fracture.1 In the study reported by Crandall et al, it was shown that the risks of having subsequent fractures were similar after an initial traumatic or nontraumatic (fragility) fracture in postmenopausal women aged 50 years and older.2 This finding brings into question whether traumatic fractures should be viewed any differently than nontraumatic fractures in women over the age of 50 in light of evaluation for osteoporosis. Furthermore, these results suggest that most fractures in postmenopausal women may indicate decreased bone integrity, thus adding to the rationale that osteoporosis screening needs to be considered and expanded to include postmenopausal women under the age of 65 who endured a traumatic fracture.
Per current guidelines, a woman under the age of 65 is recommended for osteoporosis screening only if she has an increased 10-year fracture risk compared to women aged 65 years and older. This risk is calculated based on the World Health Organization fracture-risk algorithm (WHO FRAX) tool which uses multiple factors such as age, weight, and history of fragility fractures to predict whether an individual is at risk of developing a fracture in the next 10 years. The WHO FRAX tool does not include traumatic fractures in its risk calculation and current clinical guidelines do not account for traumatic fractures as a red flag to initiate osteoporosis screening. Therefore, postmenopausal women under the age of 65 are less likely to be screened for osteoporosis when they experience a traumatic fracture compared to a fragility fracture, despite being at a demonstrably higher risk for subsequent fracture. As an unintended consequence, this may lead to the under diagnosis of osteoporosis in postmenopausal women under the age of 65. Thus, Crandall et al conclude that a fracture due to any cause warrants follow up evaluation for osteoporosis including BMD testing in women older than 50 years of age.
Older men constitute another population who are commonly under screened for osteoporosis. The current USPSTF guidelines indicate that there is an insufficient body of evidence to screen men for osteoporosis given its lower prevalence.1 However, it is important to note that men have significantly increased mortality after a hip fracture, are less likely to be on pharmacological treatment for osteoporosis, and are under diagnosed for osteoporosis.3 Consistent with findings from the current study, Leslie et al showed that high-trauma and low-trauma fractures have similarly elevated subsequent fracture risk in both men and women over the age of 40 in a Canadian study.4 Moreover, in the same study, BMD was decreased in both men and women who suffered a fracture regardless of the injury mechanism. This finding further underscores a need to consider traumatic fractures as a risk factor for osteoporosis. Taken together, given that men are under screened and treated for osteoporosis but have increased mortality post-fracture, considerations to initiate osteoporosis evaluation should be similarly given to men who endured a traumatic fracture.
The study conducted by Crandall et al has several strengths. It is noteworthy for the large size of the WHI cohort with participants from across the United States which enables the capture of a wider range of age groups as women under the age of 65 are not common participants of osteoporosis studies. Additionally, data ascertainment and outcome adjudication utilizing medical records and physician review assure data quality. A limitation of the study is that the study cohort consists exclusively of women and therefore the findings are not generalizable to men. However, findings from this study echo those from other studies that investigate the relationship between fracture mechanisms and subsequent fracture risk in men and women.3,4 Collectively, these comparable findings highlight the need for additional research to validate traumatic fracture as a risk factor for osteoporosis and to incorporate it into clinical guidelines for osteoporosis screening.
Applications for Clinical Practice
The findings from the current study indicate that traumatic and fragility fractures may be more alike than previously recognized in regards to bone health and subsequent fracture prevention in postmenopausal women. If validated, these results may lead to changes in clinical practice whereby all fractures in postmenopausal women could trigger osteoporosis screening, assessment, and treatment if indicated for the secondary prevention of fractures.
1. US Preventive Services Task Force, Curry SJ, Krist Ah, et al. Screening for Osteoporosis to Prevent Fractures: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319(24):2521–2531. doi:10.1001/jama.2018.7498
2. Crandall CJ, Larson JC, LaCroix AZ, et al. Risk of Subsequent Fractures in Postmenopausal Women After Nontraumatic vs Traumatic Fractures. JAMA Intern Med. Published online June 7, 2021. doi:10.1001/jamainternmed.2021.2617
3. Mackey DC, Lui L, Cawthon PM, et al. High-Trauma Fractures and Low Bone Mineral Density in Older Women and Men. JAMA. 2007;298(20):2381–2388. doi:10.1001/jama.298.20.2381
4. Leslie WD, Schousboe JT, Morin SN, et al. Fracture risk following high-trauma versus low-trauma fracture: a registry-based cohort study. Osteoporos Int. 2020;31(6):1059–1067. doi:10.1007/s00198-019-05274-2
Study Overview
Objective. To compare the risk of subsequent fractures after an initial traumatic or nontraumatic fracture in postmenopausal women.
Design. A prospective observational study utilizing data from the Women’s Health Initiative (WHI) Study, WHI Clinical Trials (WHI-CT), and WHI Bone Density Substudy to evaluate rates at which patients who suffered a traumatic fracture vs nontraumatic fracture develop a subsequent fracture.
Setting and participants. The WHI study, implemented at 40 United States clinical sites, enrolled 161 808 postmenopausal women aged 50 to 79 years at baseline between 1993 and 1998. The study cohort consisted of 75 335 patients who had self-reported fractures from September 1994 to December 1998 that were confirmed by the WHI Bone Density Substudy and WHI-CT. Of these participants, 253 (0.3%) were excluded because of a lack of follow-up information regarding incident fractures, and 8208 (10.9%) were excluded due to incomplete information on covariates, thus resulting in an analytic sample of 66 874 (88.8%) participants. Prospective fracture ascertainment with participants was conducted at least annually and the mechanism of fracture was assessed to differentiate traumatic vs nontraumatic incident fractures. Traumatic fractures were defined as fractures caused by motor vehicle collisions, falls from a height, falls downstairs, or sports injury. Nontraumatic fractures were defined as fractures caused by a trip and fall.
Main outcome measures. The primary outcome was an incident fracture at an anatomically distinct body part. Fractures were classified as upper extremity (carpal, elbow, lower or upper end of humerus, shaft of humerus, upper radius/ulna, or radius/ulna), lower extremity (ankle, hip, patella, pelvis, shaft of femur, tibia/fibula, or tibial plateau), or spine (lumbar and/or thoracic spine). Self-reported fractures were verified via medical chart review by WHI study physicians; hip fractures were confirmed by review of written reports of radiographic studies; and nonhip fractures were confirmed by review of radiography reports or clinical documentations.
Main results. In total, 66 874 women in the study (mean [SD] age) 63.1 (7.0) years without clinical fracture and 65.3 (7.2) years with clinical fracture at baseline were followed for 8.1 (1.6) years. Of these participants, 7142 (10.7%) experienced incident fracture during the study follow-up period (13.9 per 1000 person-years), and 721 (10.1%) of whom had a subsequent fracture. The adjusted hazard ratio (aHR) of subsequent fracture after an initial fracture was 1.49 (95% CI, 1.38-1.61, P < .001). Covariates adjusted were age, race, ethnicity, body mass index, treated diabetes, frequency of falls in the previous year, and physical function and activity. In women with initial traumatic fracture, the association between initial and subsequent fracture was increased (aHR, 1.25; 95% CI, 1.06-1.48, P = .01). Among women with initial nontraumatic fracture, the association between initial and subsequent fracture was also increased (aHR, 1.52; 95% CI, 1.37-1.68, P < .001). The confidence intervals for the 2 preceding associations for traumatic and nontraumatic initial fracture strata were overlapping.
Conclusion. Fractures, regardless of mechanism of injury, are similarly associated with an increased risk of subsequent fractures in postmenopausal women aged 50 years and older. Findings from this study provide evidence to support reevaluation of current clinical guidelines to include traumatic fracture as a trigger for osteoporosis screening.
Commentary
Osteoporosis is one of the most common age-associated disease that affects 1 in 4 women and 1 in 20 men over the age of 65.1 It increases the risk of fracture, and its clinical sequelae include reduced mobility, health decline, and increased all-cause mortality. The high prevalence of osteoporosis poses a clinical challenge as the global population continues to age. Pharmacological treatments such as bisphosphonates are highly effective in preventing or slowing bone mineral density (BMD) loss and reducing risk of fragility fractures (eg, nontraumatic fractures of the vertebra, hip, and femur) and are commonly used to mitigate adverse effects of degenerative bone changes secondary to osteoporosis.1
The high prevalence of osteoporosis and effectiveness of bisphosphonates raises the question of how to optimally identify adults at risk for osteoporosis so that pharmacologic therapy can be promptly initiated to prevent disease progression. Multiple osteoporosis screening guidelines, including those from the United States Preventive Services Task Force (USPSTF), American Association of Family Physicians, and National Osteoporosis Foundation, are widely used in the clinical setting to address this important clinical question. In general, the prevailing wisdom is to screen osteoporosis in postmenopausal women over the age of 65, women under the age of 65 who have a significant 10-year fracture risk, or women over the age of 50 who have experienced a fragility fracture.1 In the study reported by Crandall et al, it was shown that the risks of having subsequent fractures were similar after an initial traumatic or nontraumatic (fragility) fracture in postmenopausal women aged 50 years and older.2 This finding brings into question whether traumatic fractures should be viewed any differently than nontraumatic fractures in women over the age of 50 in light of evaluation for osteoporosis. Furthermore, these results suggest that most fractures in postmenopausal women may indicate decreased bone integrity, thus adding to the rationale that osteoporosis screening needs to be considered and expanded to include postmenopausal women under the age of 65 who endured a traumatic fracture.
Per current guidelines, a woman under the age of 65 is recommended for osteoporosis screening only if she has an increased 10-year fracture risk compared to women aged 65 years and older. This risk is calculated based on the World Health Organization fracture-risk algorithm (WHO FRAX) tool which uses multiple factors such as age, weight, and history of fragility fractures to predict whether an individual is at risk of developing a fracture in the next 10 years. The WHO FRAX tool does not include traumatic fractures in its risk calculation and current clinical guidelines do not account for traumatic fractures as a red flag to initiate osteoporosis screening. Therefore, postmenopausal women under the age of 65 are less likely to be screened for osteoporosis when they experience a traumatic fracture compared to a fragility fracture, despite being at a demonstrably higher risk for subsequent fracture. As an unintended consequence, this may lead to the under diagnosis of osteoporosis in postmenopausal women under the age of 65. Thus, Crandall et al conclude that a fracture due to any cause warrants follow up evaluation for osteoporosis including BMD testing in women older than 50 years of age.
Older men constitute another population who are commonly under screened for osteoporosis. The current USPSTF guidelines indicate that there is an insufficient body of evidence to screen men for osteoporosis given its lower prevalence.1 However, it is important to note that men have significantly increased mortality after a hip fracture, are less likely to be on pharmacological treatment for osteoporosis, and are under diagnosed for osteoporosis.3 Consistent with findings from the current study, Leslie et al showed that high-trauma and low-trauma fractures have similarly elevated subsequent fracture risk in both men and women over the age of 40 in a Canadian study.4 Moreover, in the same study, BMD was decreased in both men and women who suffered a fracture regardless of the injury mechanism. This finding further underscores a need to consider traumatic fractures as a risk factor for osteoporosis. Taken together, given that men are under screened and treated for osteoporosis but have increased mortality post-fracture, considerations to initiate osteoporosis evaluation should be similarly given to men who endured a traumatic fracture.
The study conducted by Crandall et al has several strengths. It is noteworthy for the large size of the WHI cohort with participants from across the United States which enables the capture of a wider range of age groups as women under the age of 65 are not common participants of osteoporosis studies. Additionally, data ascertainment and outcome adjudication utilizing medical records and physician review assure data quality. A limitation of the study is that the study cohort consists exclusively of women and therefore the findings are not generalizable to men. However, findings from this study echo those from other studies that investigate the relationship between fracture mechanisms and subsequent fracture risk in men and women.3,4 Collectively, these comparable findings highlight the need for additional research to validate traumatic fracture as a risk factor for osteoporosis and to incorporate it into clinical guidelines for osteoporosis screening.
Applications for Clinical Practice
The findings from the current study indicate that traumatic and fragility fractures may be more alike than previously recognized in regards to bone health and subsequent fracture prevention in postmenopausal women. If validated, these results may lead to changes in clinical practice whereby all fractures in postmenopausal women could trigger osteoporosis screening, assessment, and treatment if indicated for the secondary prevention of fractures.
Study Overview
Objective. To compare the risk of subsequent fractures after an initial traumatic or nontraumatic fracture in postmenopausal women.
Design. A prospective observational study utilizing data from the Women’s Health Initiative (WHI) Study, WHI Clinical Trials (WHI-CT), and WHI Bone Density Substudy to evaluate rates at which patients who suffered a traumatic fracture vs nontraumatic fracture develop a subsequent fracture.
Setting and participants. The WHI study, implemented at 40 United States clinical sites, enrolled 161 808 postmenopausal women aged 50 to 79 years at baseline between 1993 and 1998. The study cohort consisted of 75 335 patients who had self-reported fractures from September 1994 to December 1998 that were confirmed by the WHI Bone Density Substudy and WHI-CT. Of these participants, 253 (0.3%) were excluded because of a lack of follow-up information regarding incident fractures, and 8208 (10.9%) were excluded due to incomplete information on covariates, thus resulting in an analytic sample of 66 874 (88.8%) participants. Prospective fracture ascertainment with participants was conducted at least annually and the mechanism of fracture was assessed to differentiate traumatic vs nontraumatic incident fractures. Traumatic fractures were defined as fractures caused by motor vehicle collisions, falls from a height, falls downstairs, or sports injury. Nontraumatic fractures were defined as fractures caused by a trip and fall.
Main outcome measures. The primary outcome was an incident fracture at an anatomically distinct body part. Fractures were classified as upper extremity (carpal, elbow, lower or upper end of humerus, shaft of humerus, upper radius/ulna, or radius/ulna), lower extremity (ankle, hip, patella, pelvis, shaft of femur, tibia/fibula, or tibial plateau), or spine (lumbar and/or thoracic spine). Self-reported fractures were verified via medical chart review by WHI study physicians; hip fractures were confirmed by review of written reports of radiographic studies; and nonhip fractures were confirmed by review of radiography reports or clinical documentations.
Main results. In total, 66 874 women in the study (mean [SD] age) 63.1 (7.0) years without clinical fracture and 65.3 (7.2) years with clinical fracture at baseline were followed for 8.1 (1.6) years. Of these participants, 7142 (10.7%) experienced incident fracture during the study follow-up period (13.9 per 1000 person-years), and 721 (10.1%) of whom had a subsequent fracture. The adjusted hazard ratio (aHR) of subsequent fracture after an initial fracture was 1.49 (95% CI, 1.38-1.61, P < .001). Covariates adjusted were age, race, ethnicity, body mass index, treated diabetes, frequency of falls in the previous year, and physical function and activity. In women with initial traumatic fracture, the association between initial and subsequent fracture was increased (aHR, 1.25; 95% CI, 1.06-1.48, P = .01). Among women with initial nontraumatic fracture, the association between initial and subsequent fracture was also increased (aHR, 1.52; 95% CI, 1.37-1.68, P < .001). The confidence intervals for the 2 preceding associations for traumatic and nontraumatic initial fracture strata were overlapping.
Conclusion. Fractures, regardless of mechanism of injury, are similarly associated with an increased risk of subsequent fractures in postmenopausal women aged 50 years and older. Findings from this study provide evidence to support reevaluation of current clinical guidelines to include traumatic fracture as a trigger for osteoporosis screening.
Commentary
Osteoporosis is one of the most common age-associated disease that affects 1 in 4 women and 1 in 20 men over the age of 65.1 It increases the risk of fracture, and its clinical sequelae include reduced mobility, health decline, and increased all-cause mortality. The high prevalence of osteoporosis poses a clinical challenge as the global population continues to age. Pharmacological treatments such as bisphosphonates are highly effective in preventing or slowing bone mineral density (BMD) loss and reducing risk of fragility fractures (eg, nontraumatic fractures of the vertebra, hip, and femur) and are commonly used to mitigate adverse effects of degenerative bone changes secondary to osteoporosis.1
The high prevalence of osteoporosis and effectiveness of bisphosphonates raises the question of how to optimally identify adults at risk for osteoporosis so that pharmacologic therapy can be promptly initiated to prevent disease progression. Multiple osteoporosis screening guidelines, including those from the United States Preventive Services Task Force (USPSTF), American Association of Family Physicians, and National Osteoporosis Foundation, are widely used in the clinical setting to address this important clinical question. In general, the prevailing wisdom is to screen osteoporosis in postmenopausal women over the age of 65, women under the age of 65 who have a significant 10-year fracture risk, or women over the age of 50 who have experienced a fragility fracture.1 In the study reported by Crandall et al, it was shown that the risks of having subsequent fractures were similar after an initial traumatic or nontraumatic (fragility) fracture in postmenopausal women aged 50 years and older.2 This finding brings into question whether traumatic fractures should be viewed any differently than nontraumatic fractures in women over the age of 50 in light of evaluation for osteoporosis. Furthermore, these results suggest that most fractures in postmenopausal women may indicate decreased bone integrity, thus adding to the rationale that osteoporosis screening needs to be considered and expanded to include postmenopausal women under the age of 65 who endured a traumatic fracture.
Per current guidelines, a woman under the age of 65 is recommended for osteoporosis screening only if she has an increased 10-year fracture risk compared to women aged 65 years and older. This risk is calculated based on the World Health Organization fracture-risk algorithm (WHO FRAX) tool which uses multiple factors such as age, weight, and history of fragility fractures to predict whether an individual is at risk of developing a fracture in the next 10 years. The WHO FRAX tool does not include traumatic fractures in its risk calculation and current clinical guidelines do not account for traumatic fractures as a red flag to initiate osteoporosis screening. Therefore, postmenopausal women under the age of 65 are less likely to be screened for osteoporosis when they experience a traumatic fracture compared to a fragility fracture, despite being at a demonstrably higher risk for subsequent fracture. As an unintended consequence, this may lead to the under diagnosis of osteoporosis in postmenopausal women under the age of 65. Thus, Crandall et al conclude that a fracture due to any cause warrants follow up evaluation for osteoporosis including BMD testing in women older than 50 years of age.
Older men constitute another population who are commonly under screened for osteoporosis. The current USPSTF guidelines indicate that there is an insufficient body of evidence to screen men for osteoporosis given its lower prevalence.1 However, it is important to note that men have significantly increased mortality after a hip fracture, are less likely to be on pharmacological treatment for osteoporosis, and are under diagnosed for osteoporosis.3 Consistent with findings from the current study, Leslie et al showed that high-trauma and low-trauma fractures have similarly elevated subsequent fracture risk in both men and women over the age of 40 in a Canadian study.4 Moreover, in the same study, BMD was decreased in both men and women who suffered a fracture regardless of the injury mechanism. This finding further underscores a need to consider traumatic fractures as a risk factor for osteoporosis. Taken together, given that men are under screened and treated for osteoporosis but have increased mortality post-fracture, considerations to initiate osteoporosis evaluation should be similarly given to men who endured a traumatic fracture.
The study conducted by Crandall et al has several strengths. It is noteworthy for the large size of the WHI cohort with participants from across the United States which enables the capture of a wider range of age groups as women under the age of 65 are not common participants of osteoporosis studies. Additionally, data ascertainment and outcome adjudication utilizing medical records and physician review assure data quality. A limitation of the study is that the study cohort consists exclusively of women and therefore the findings are not generalizable to men. However, findings from this study echo those from other studies that investigate the relationship between fracture mechanisms and subsequent fracture risk in men and women.3,4 Collectively, these comparable findings highlight the need for additional research to validate traumatic fracture as a risk factor for osteoporosis and to incorporate it into clinical guidelines for osteoporosis screening.
Applications for Clinical Practice
The findings from the current study indicate that traumatic and fragility fractures may be more alike than previously recognized in regards to bone health and subsequent fracture prevention in postmenopausal women. If validated, these results may lead to changes in clinical practice whereby all fractures in postmenopausal women could trigger osteoporosis screening, assessment, and treatment if indicated for the secondary prevention of fractures.
1. US Preventive Services Task Force, Curry SJ, Krist Ah, et al. Screening for Osteoporosis to Prevent Fractures: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319(24):2521–2531. doi:10.1001/jama.2018.7498
2. Crandall CJ, Larson JC, LaCroix AZ, et al. Risk of Subsequent Fractures in Postmenopausal Women After Nontraumatic vs Traumatic Fractures. JAMA Intern Med. Published online June 7, 2021. doi:10.1001/jamainternmed.2021.2617
3. Mackey DC, Lui L, Cawthon PM, et al. High-Trauma Fractures and Low Bone Mineral Density in Older Women and Men. JAMA. 2007;298(20):2381–2388. doi:10.1001/jama.298.20.2381
4. Leslie WD, Schousboe JT, Morin SN, et al. Fracture risk following high-trauma versus low-trauma fracture: a registry-based cohort study. Osteoporos Int. 2020;31(6):1059–1067. doi:10.1007/s00198-019-05274-2
1. US Preventive Services Task Force, Curry SJ, Krist Ah, et al. Screening for Osteoporosis to Prevent Fractures: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319(24):2521–2531. doi:10.1001/jama.2018.7498
2. Crandall CJ, Larson JC, LaCroix AZ, et al. Risk of Subsequent Fractures in Postmenopausal Women After Nontraumatic vs Traumatic Fractures. JAMA Intern Med. Published online June 7, 2021. doi:10.1001/jamainternmed.2021.2617
3. Mackey DC, Lui L, Cawthon PM, et al. High-Trauma Fractures and Low Bone Mineral Density in Older Women and Men. JAMA. 2007;298(20):2381–2388. doi:10.1001/jama.298.20.2381
4. Leslie WD, Schousboe JT, Morin SN, et al. Fracture risk following high-trauma versus low-trauma fracture: a registry-based cohort study. Osteoporos Int. 2020;31(6):1059–1067. doi:10.1007/s00198-019-05274-2
I Never Wanted To Be a Hero
I have been in the business of medicine for more than 15 years and I will never forget the initial surge of the COVID-19 pandemic in Massachusetts.
As a hospitalist, I admitted patients infected with COVID-19, followed them on the floor, and, since I had some experience working in an intensive care unit (ICU), was assigned to cover a “COVID ICU.” This wing of the hospital used to be a fancy orthopedic floor that our institution was lucky enough to have. So began the most life-changing experience in my career as a physician.
In this role, we witness death more than any of us would care to discuss. It comes with the territory, and we never expected this to change once COVID hit. However, so many patients succumbed to this disease, especially during the first surge, which made it difficult to handle emotionally. Patients that fell ill initially stayed isolated at home, optimistic they would turn the corner only to enter the hospital a week later after their conditioned worsened. After requiring a couple of liters of supplemental oxygen in the emergency room, they eventually ended up on a high flow nasal cannula in just a matter of hours.
Patients slowly got sicker and felt more helpless as the days passed, leading us to prescribe drugs that eventually proved to have no benefit. We checked countless inflammatory markers, most of which we were not even sure what to do with. Many times, we hosted a family meeting via FaceTime, holding a patient’s hand in one hand and an iPad in the other to discuss goals of care. Too often, a dark cloud hung over these discussions, a realization that there was not much else we could do.
I have always felt that helping someone have a decent and peaceful death is important, especially when the prognosis is grim, and that patient is suffering. But the sheer number of times this happened during the initial surge of the pandemic was difficult to handle. It felt like I had more of those discussions in 3 months than I did during my entire career as a hospitalist.
We helped plenty of people get better, with some heading home in a week. They thanked us, painted rocks and the sidewalks in front of the hospital displaying messages of gratitude, and sent lunches. Others, though, left the hospital 2 months later with a tube in their stomach so they could receive some form of nutrition and another in their neck to help them breathe.
These struggles were by no means special to me; other hospitalists around the world faced similar situations at one point or another during the pandemic. Working overtime, coming home late, exhausted, undressing in the garage, trying to be there for my 3 kids who were full of energy after a whole day of Zoom and doing the usual kid stuff. My house used to have strict rules about screen time. No more.
The summer months provided a bit of a COVID break, with only 1 or 2 infected patients entering my care. We went to outdoor restaurants and tried to get our lives back to “normal.” As the weather turned cold, however, things went south again. This time no more hydroxychloroquine, a drug used to fight malaria but also treat other autoimmune diseases, as it was proven eventually over many studies that it is not helpful and was potentially harmful. We instead shifted our focus to remdesivir—an antiviral drug that displayed some benefits—tocilizumab, and dexamethasone, anti-inflammatory drugs with the latter providing some positive outcomes on mortality.
Patient survival rates improved slightly, likely due to a combination of factors. We were more experienced at fighting the disease, which led to things in the hospital not being as chaotic and more time available to spend with the patients. Personal protective equipment (PPE) and tests were more readily available, and the population getting hit by the disease changed slightly with fewer elderly people from nursing homes falling ill because of social distancing, other safety measures, or having already fought the disease. Our attention turned instead to more young people that had returned to work and their social lives.
The arrival of the vaccines brought considerable relief. I remember a few decades ago debating and sometimes fighting with friends and family over who was better: Iron Man or Spider-Man. Now I found myself having the same conversation about the Pfizer and Moderna COVID vaccines.
Summer 2021 holds significantly more promise. Most of the adult population is getting vaccinated, and I am very hopeful that we are approaching the end of this nightmare. In June, our office received word that we could remove our masks if we were fully vaccinated. It felt weird, but represented another sign that things are improving. I took my kids to the mall and removed my mask. It felt odd considering how that little blue thing became part of me during the pandemic. It also felt strange to not prescribe a single dose of remdesivir for an entire month.
It feels good—and normal—to care for the patients that we neglected for a year. It has been a needed boost to see patients return to their health care providers for their colonoscopy screenings, mammograms, and managing chronic problems like coronary artery disease, congestive heart failure, or receiving chemotherapy.
I learned plenty from this pandemic and hope I am not alone. I learned to be humble. We started with a drug that was harmful, moved on to a drug that is probably neutral and eventually were able to come up with a drug that seems to decrease mortality at least in some COVID patients. I learned it is fine to try new therapies based on the best data in the hope they result in positive clinical outcomes. However, it is critical that we all keep an eye on the rapidly evolving literature and adjust our behavior accordingly.
I also learned, or relearned, that if people are desperate enough, they will drink bleach to see if it works. Others are convinced that the purpose of vaccination is to inject a microchip allowing ourselves to be tracked by some higher power. I learned that we must take the first step to prepare for the next pandemic by having a decent reserve of PPE.
It is clear synthetic messenger RNA (mRNA) technology is here to stay, and I believe it has a huge potential to change many areas of medicine. mRNA vaccines proved to be much faster to develop and probably much easier to change as the pathogen, in this case coronavirus, changes.
The technology could be used against a variety of infectious diseases to make vaccines against malaria, tuberculosis, HIV, or hepatitis. It can also be very useful for faster vaccine development needed in future possible pandemics such as influenza, Ebola, or severe acute respiratory syndrome. It may also be used for cancer treatment.
As John P. Cooke, MD, PhD, the medical director for the Center of RNA Therapeutics Program at the Houston Methodist Research Institute, said, “Most vaccines today are still viral vaccines – they are inactivated virus, so it’s potentially infectious and you have to have virus on hand. With mRNA, you’re just writing code which is going to tell the cell to make a viral protein – one part of a viral protein to stimulate an immune response. And, here’s the wonderful thing, you don’t even need the virus in hand, just its DNA code.”1
Corresponding author: Dragos Vesbianu, MD, Attending Hospitalist, Newton-Wellesley Hospital, 2014 Washington St, Newton, MA 02462; [email protected].
Financial dislosures: None.
1. Houston Methodist. Messenger RNA – the Therapy of the Future. Newswise. November 16, 2020. Accessed June 25, 2021. https://www.newswise.com/coronavirus/messenger-rna-the-therapy-of-the-future/
I have been in the business of medicine for more than 15 years and I will never forget the initial surge of the COVID-19 pandemic in Massachusetts.
As a hospitalist, I admitted patients infected with COVID-19, followed them on the floor, and, since I had some experience working in an intensive care unit (ICU), was assigned to cover a “COVID ICU.” This wing of the hospital used to be a fancy orthopedic floor that our institution was lucky enough to have. So began the most life-changing experience in my career as a physician.
In this role, we witness death more than any of us would care to discuss. It comes with the territory, and we never expected this to change once COVID hit. However, so many patients succumbed to this disease, especially during the first surge, which made it difficult to handle emotionally. Patients that fell ill initially stayed isolated at home, optimistic they would turn the corner only to enter the hospital a week later after their conditioned worsened. After requiring a couple of liters of supplemental oxygen in the emergency room, they eventually ended up on a high flow nasal cannula in just a matter of hours.
Patients slowly got sicker and felt more helpless as the days passed, leading us to prescribe drugs that eventually proved to have no benefit. We checked countless inflammatory markers, most of which we were not even sure what to do with. Many times, we hosted a family meeting via FaceTime, holding a patient’s hand in one hand and an iPad in the other to discuss goals of care. Too often, a dark cloud hung over these discussions, a realization that there was not much else we could do.
I have always felt that helping someone have a decent and peaceful death is important, especially when the prognosis is grim, and that patient is suffering. But the sheer number of times this happened during the initial surge of the pandemic was difficult to handle. It felt like I had more of those discussions in 3 months than I did during my entire career as a hospitalist.
We helped plenty of people get better, with some heading home in a week. They thanked us, painted rocks and the sidewalks in front of the hospital displaying messages of gratitude, and sent lunches. Others, though, left the hospital 2 months later with a tube in their stomach so they could receive some form of nutrition and another in their neck to help them breathe.
These struggles were by no means special to me; other hospitalists around the world faced similar situations at one point or another during the pandemic. Working overtime, coming home late, exhausted, undressing in the garage, trying to be there for my 3 kids who were full of energy after a whole day of Zoom and doing the usual kid stuff. My house used to have strict rules about screen time. No more.
The summer months provided a bit of a COVID break, with only 1 or 2 infected patients entering my care. We went to outdoor restaurants and tried to get our lives back to “normal.” As the weather turned cold, however, things went south again. This time no more hydroxychloroquine, a drug used to fight malaria but also treat other autoimmune diseases, as it was proven eventually over many studies that it is not helpful and was potentially harmful. We instead shifted our focus to remdesivir—an antiviral drug that displayed some benefits—tocilizumab, and dexamethasone, anti-inflammatory drugs with the latter providing some positive outcomes on mortality.
Patient survival rates improved slightly, likely due to a combination of factors. We were more experienced at fighting the disease, which led to things in the hospital not being as chaotic and more time available to spend with the patients. Personal protective equipment (PPE) and tests were more readily available, and the population getting hit by the disease changed slightly with fewer elderly people from nursing homes falling ill because of social distancing, other safety measures, or having already fought the disease. Our attention turned instead to more young people that had returned to work and their social lives.
The arrival of the vaccines brought considerable relief. I remember a few decades ago debating and sometimes fighting with friends and family over who was better: Iron Man or Spider-Man. Now I found myself having the same conversation about the Pfizer and Moderna COVID vaccines.
Summer 2021 holds significantly more promise. Most of the adult population is getting vaccinated, and I am very hopeful that we are approaching the end of this nightmare. In June, our office received word that we could remove our masks if we were fully vaccinated. It felt weird, but represented another sign that things are improving. I took my kids to the mall and removed my mask. It felt odd considering how that little blue thing became part of me during the pandemic. It also felt strange to not prescribe a single dose of remdesivir for an entire month.
It feels good—and normal—to care for the patients that we neglected for a year. It has been a needed boost to see patients return to their health care providers for their colonoscopy screenings, mammograms, and managing chronic problems like coronary artery disease, congestive heart failure, or receiving chemotherapy.
I learned plenty from this pandemic and hope I am not alone. I learned to be humble. We started with a drug that was harmful, moved on to a drug that is probably neutral and eventually were able to come up with a drug that seems to decrease mortality at least in some COVID patients. I learned it is fine to try new therapies based on the best data in the hope they result in positive clinical outcomes. However, it is critical that we all keep an eye on the rapidly evolving literature and adjust our behavior accordingly.
I also learned, or relearned, that if people are desperate enough, they will drink bleach to see if it works. Others are convinced that the purpose of vaccination is to inject a microchip allowing ourselves to be tracked by some higher power. I learned that we must take the first step to prepare for the next pandemic by having a decent reserve of PPE.
It is clear synthetic messenger RNA (mRNA) technology is here to stay, and I believe it has a huge potential to change many areas of medicine. mRNA vaccines proved to be much faster to develop and probably much easier to change as the pathogen, in this case coronavirus, changes.
The technology could be used against a variety of infectious diseases to make vaccines against malaria, tuberculosis, HIV, or hepatitis. It can also be very useful for faster vaccine development needed in future possible pandemics such as influenza, Ebola, or severe acute respiratory syndrome. It may also be used for cancer treatment.
As John P. Cooke, MD, PhD, the medical director for the Center of RNA Therapeutics Program at the Houston Methodist Research Institute, said, “Most vaccines today are still viral vaccines – they are inactivated virus, so it’s potentially infectious and you have to have virus on hand. With mRNA, you’re just writing code which is going to tell the cell to make a viral protein – one part of a viral protein to stimulate an immune response. And, here’s the wonderful thing, you don’t even need the virus in hand, just its DNA code.”1
Corresponding author: Dragos Vesbianu, MD, Attending Hospitalist, Newton-Wellesley Hospital, 2014 Washington St, Newton, MA 02462; [email protected].
Financial dislosures: None.
I have been in the business of medicine for more than 15 years and I will never forget the initial surge of the COVID-19 pandemic in Massachusetts.
As a hospitalist, I admitted patients infected with COVID-19, followed them on the floor, and, since I had some experience working in an intensive care unit (ICU), was assigned to cover a “COVID ICU.” This wing of the hospital used to be a fancy orthopedic floor that our institution was lucky enough to have. So began the most life-changing experience in my career as a physician.
In this role, we witness death more than any of us would care to discuss. It comes with the territory, and we never expected this to change once COVID hit. However, so many patients succumbed to this disease, especially during the first surge, which made it difficult to handle emotionally. Patients that fell ill initially stayed isolated at home, optimistic they would turn the corner only to enter the hospital a week later after their conditioned worsened. After requiring a couple of liters of supplemental oxygen in the emergency room, they eventually ended up on a high flow nasal cannula in just a matter of hours.
Patients slowly got sicker and felt more helpless as the days passed, leading us to prescribe drugs that eventually proved to have no benefit. We checked countless inflammatory markers, most of which we were not even sure what to do with. Many times, we hosted a family meeting via FaceTime, holding a patient’s hand in one hand and an iPad in the other to discuss goals of care. Too often, a dark cloud hung over these discussions, a realization that there was not much else we could do.
I have always felt that helping someone have a decent and peaceful death is important, especially when the prognosis is grim, and that patient is suffering. But the sheer number of times this happened during the initial surge of the pandemic was difficult to handle. It felt like I had more of those discussions in 3 months than I did during my entire career as a hospitalist.
We helped plenty of people get better, with some heading home in a week. They thanked us, painted rocks and the sidewalks in front of the hospital displaying messages of gratitude, and sent lunches. Others, though, left the hospital 2 months later with a tube in their stomach so they could receive some form of nutrition and another in their neck to help them breathe.
These struggles were by no means special to me; other hospitalists around the world faced similar situations at one point or another during the pandemic. Working overtime, coming home late, exhausted, undressing in the garage, trying to be there for my 3 kids who were full of energy after a whole day of Zoom and doing the usual kid stuff. My house used to have strict rules about screen time. No more.
The summer months provided a bit of a COVID break, with only 1 or 2 infected patients entering my care. We went to outdoor restaurants and tried to get our lives back to “normal.” As the weather turned cold, however, things went south again. This time no more hydroxychloroquine, a drug used to fight malaria but also treat other autoimmune diseases, as it was proven eventually over many studies that it is not helpful and was potentially harmful. We instead shifted our focus to remdesivir—an antiviral drug that displayed some benefits—tocilizumab, and dexamethasone, anti-inflammatory drugs with the latter providing some positive outcomes on mortality.
Patient survival rates improved slightly, likely due to a combination of factors. We were more experienced at fighting the disease, which led to things in the hospital not being as chaotic and more time available to spend with the patients. Personal protective equipment (PPE) and tests were more readily available, and the population getting hit by the disease changed slightly with fewer elderly people from nursing homes falling ill because of social distancing, other safety measures, or having already fought the disease. Our attention turned instead to more young people that had returned to work and their social lives.
The arrival of the vaccines brought considerable relief. I remember a few decades ago debating and sometimes fighting with friends and family over who was better: Iron Man or Spider-Man. Now I found myself having the same conversation about the Pfizer and Moderna COVID vaccines.
Summer 2021 holds significantly more promise. Most of the adult population is getting vaccinated, and I am very hopeful that we are approaching the end of this nightmare. In June, our office received word that we could remove our masks if we were fully vaccinated. It felt weird, but represented another sign that things are improving. I took my kids to the mall and removed my mask. It felt odd considering how that little blue thing became part of me during the pandemic. It also felt strange to not prescribe a single dose of remdesivir for an entire month.
It feels good—and normal—to care for the patients that we neglected for a year. It has been a needed boost to see patients return to their health care providers for their colonoscopy screenings, mammograms, and managing chronic problems like coronary artery disease, congestive heart failure, or receiving chemotherapy.
I learned plenty from this pandemic and hope I am not alone. I learned to be humble. We started with a drug that was harmful, moved on to a drug that is probably neutral and eventually were able to come up with a drug that seems to decrease mortality at least in some COVID patients. I learned it is fine to try new therapies based on the best data in the hope they result in positive clinical outcomes. However, it is critical that we all keep an eye on the rapidly evolving literature and adjust our behavior accordingly.
I also learned, or relearned, that if people are desperate enough, they will drink bleach to see if it works. Others are convinced that the purpose of vaccination is to inject a microchip allowing ourselves to be tracked by some higher power. I learned that we must take the first step to prepare for the next pandemic by having a decent reserve of PPE.
It is clear synthetic messenger RNA (mRNA) technology is here to stay, and I believe it has a huge potential to change many areas of medicine. mRNA vaccines proved to be much faster to develop and probably much easier to change as the pathogen, in this case coronavirus, changes.
The technology could be used against a variety of infectious diseases to make vaccines against malaria, tuberculosis, HIV, or hepatitis. It can also be very useful for faster vaccine development needed in future possible pandemics such as influenza, Ebola, or severe acute respiratory syndrome. It may also be used for cancer treatment.
As John P. Cooke, MD, PhD, the medical director for the Center of RNA Therapeutics Program at the Houston Methodist Research Institute, said, “Most vaccines today are still viral vaccines – they are inactivated virus, so it’s potentially infectious and you have to have virus on hand. With mRNA, you’re just writing code which is going to tell the cell to make a viral protein – one part of a viral protein to stimulate an immune response. And, here’s the wonderful thing, you don’t even need the virus in hand, just its DNA code.”1
Corresponding author: Dragos Vesbianu, MD, Attending Hospitalist, Newton-Wellesley Hospital, 2014 Washington St, Newton, MA 02462; [email protected].
Financial dislosures: None.
1. Houston Methodist. Messenger RNA – the Therapy of the Future. Newswise. November 16, 2020. Accessed June 25, 2021. https://www.newswise.com/coronavirus/messenger-rna-the-therapy-of-the-future/
1. Houston Methodist. Messenger RNA – the Therapy of the Future. Newswise. November 16, 2020. Accessed June 25, 2021. https://www.newswise.com/coronavirus/messenger-rna-the-therapy-of-the-future/
Cost Comparison of 2 Video Laryngoscopes in a Large Academic Center
From the Department of Anesthesiology, Thomas Jefferson University and Hospitals, Sidney Kimmel Medical College, Philadelphia, PA, and Sidney Kimmel Medical College at Thomas Jefferson University, Philadelphia, PA.
Objective: Retrospective study examining hospital cost information of patients requiring endotracheal intubation with video laryngoscopy. Provide a practical cost assessment on use of the McGRATH and GlideScope video laryngoscopes (VLs).
Methods: This study examined 52 hospital locations within a single, large university hospital, with most of those locations being hospital operating rooms. A total of 34 600 endotracheal intubations performed over 24 months, of which 11 345 were video laryngoscopies. Electronic medical records containing demographic data and information related to endotracheal intubation procedures, with monthly breakdowns between GlideScope and McGRATH intubations, were reviewed. Cost information calculated for equipment, blades, batteries, repairs, and subsequent analysis performed to determine cost differences between those 2 instruments during the COVID-19 period.
Results: A total of 5501 video laryngoscopy procedures were performed using the McGRATH VL and 5305 were performed using the GlideScope VL. Costs over 24 months were $181 093 lower (55.5%) for McGRATH compared to GlideScope. The mean (SD) monthly costs for GlideScope blades were $3837 ($1050) and $3236 ($538) for years 1 and 2, respectively, vs $1652 ($663) and $2933 ($585) for McGRATH blades (P < .001). Most total cost differences were attributed to equipment and blade purchases, which were $202 595 (65.0%) higher for GlideScope. During the COVID-19 period, the use of the McGRATH increased to 61% of all video laryngoscopy cases, compared to 37% for GlideScope (P < .001). Blade cost difference for the COVID-19 period was $128 higher for the McGRATH even though 293 more intubations were performed with that device.
Conclusions: Use of the McGRATH resulted in a cost savings of 55% compared to the GlideScope, and its use was highest during the COVID-19 period, which may be explained by its more portable and practical features.
Keywords: video laryngoscope; McGRATH; GlideScope; endotracheal intubation; hospital costs; COVID-19.
Hospitals have come to rely on video laryngoscopes (VLs) for tracheal intubation as necessary tools for better visualization of airways. Modern video laryngoscopy developed in the 2000s1 as a progression from direct laryngoscopy, which began in 1852 when Horace Green used a bent tongue spatula and sunlight to examine a child.2 VLs have seen many improvements and adaptations of their own, resulting in many different styles and types circulating around hospitals. The GlideScope (Verathon Inc, Bothell, WA) and the McGRATH (Medtronic, Minneapolis, MN) are examples of such instruments, which are now widely used in the US and are the 2 VLs of choice at our institution.
A few studies have compared VLs to direct laryngoscopes. In their systematic review, Lewis et al have shown the numerous benefits of using a VL over a direct laryngoscope. Some general conclusions were that the use of video laryngoscopy reduced the number of failed intubations, decreased laryngeal trauma, and provided improved visualizations.3 Other studies have compared the different types of VLs, including the McGRATH and the GlideScope, examining factors such as intubation time and display quality of the image. Two studies found that medical students were equally successful at using both the McGRATH and the GlideScope,4,5 while another study found that care providers using the GlideScope had quicker intubation times.6 Lastly, Savoldelli et al concluded that more providers preferred the McGRATH, which provided better laryngeal views,7 while their subsequent study showed more favorable learning curves of the Airtraq compared to the McGRATH and other VLs.8
Although there have been no reported differences in safety and effectiveness of the McGRATH and GlideScope devices, cost data on the use of these 2 popular laryngoscopes are lacking. Such information is important considering the increasing costs of medical technologies and the significant financial losses experienced by health care systems due to the COVID-19 crisis. The purpose of this retrospective cohort study was to compare the cost efficiency of the McGRATH MAC and GlideScope Core VLs at a large academic center.
Methods
This retrospective study was performed under exemption from the Thomas Jefferson University Institutional Review Board. The primary data sources consisted of hospital electronic patient records (EPIC) and cost information from the device manufacturers and hospital staff. The electronic patient data were provided by the EPIC Enterprise Analytics Business Intelligence group at Thomas Jefferson University Hospital (Center City Campus, Philadelphia, PA), while device costs were obtained from Verathon, Medtronic, and departmental staff responsible for purchasing equipment. Monthly data were obtained over a 24-month period (June 2018 through May 2020) when the McGRATH VL was placed into use in the department of anesthesiology. The 2 types of VLs were made available for use in a total of 52 locations, with the majority being hospital operating rooms.
The following variables were recorded: number of endotracheal intubations performed each month with breakdown between video laryngoscopy and flexible bronchoscopy airways, frequency of use for each type of laryngoscope, blades used, and equipment costs for use of each laryngoscope. Hospital cost estimates for both the McGRATH and GlideScope laryngoscopes included batteries, handles, blades, and the devices themselves. Cost data were also collected on frequency of device failure, maintenance, and replacement of parts and lost equipment.
Analysis
De-identified electronic medical records consisted of nominal and quantitative variables, with demographic data and information related to the endotracheal intubation procedure. All data were in chronological order and sorted by date after which coding was applied, to identify device type and allocate pertinent cost information. Descriptive statistics were reported as mean (SD) and sum for costs; frequency tables were generated for intubation procedures according to device type and time periods. Data were analyzed using the χ2 test, the student t test, and the Wilcoxon Mann-Whitney U test, with a P value set at .05 for statistical significance. SPSS version 26 and GraphPad Prism version 6 were used for all statistical analyses.
Results
A total of 34 600 endotracheal intubations were performed over the 24-month study period, and 11 345 (32.8%) were video laryngoscopy procedures. Out of all video laryngoscopy procedures, 5501 (48.5%) were performed using the McGRATH VL and 5305 (46.8%) were conducted using the GlideScope VL. The difference of 539 (4.8%) cases accounts for flexible bronchoscopy procedures and endotracheal intubations using other video laryngoscopy equipment. The mean (SD) monthly number of video laryngoscopy procedures for the 24 months was 221 (54) and 229 (89) for the GlideScope and McGRATH devices, respectively. Monthly endotracheal intubation distributions over 24 months trended upward for the McGRATH VL and downward for the GlideScope, but there was no statistically significant (P = .71) difference in overall use between the 2 instruments (Figure 1).
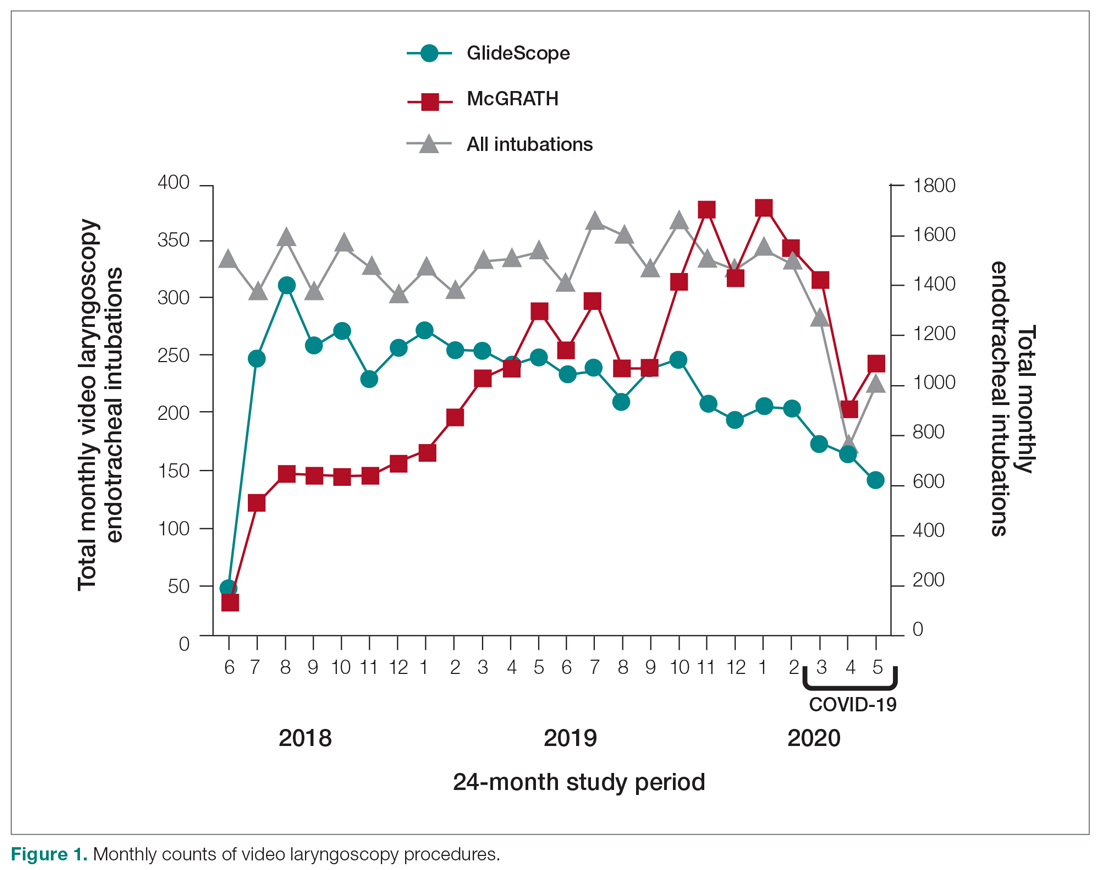
To examine the observed usage trends between the 2 VL during the first and last 12 months, a univariate ANOVA was conducted with the 2 time periods entered as predictors in the model. Video laryngoscopy intubations were performed (P = .001) more frequently with the GlideScope during the first 12 months; however, use of the McGRATH VL increased (P < .001) during the following 12 months compared to GlideScope. The GlideScope accounted for 54% of all VL intubations during the first 12 months, with the McGRATH accounting for 58% of all video laryngoscopy procedures for months 12 to 24. Additionally, the increase in video laryngoscopy procedures with the McGRATH during the last 3 months of the study period was despite an overall reduction in surgical volume due to the COVID-19 crisis, defined for this study as March 1, 2020, to May 31, 2020 (Figure 1). There was a statistically significant (P < .001) difference in the case distribution between use of the McGRATH and GlideScope VL for that period. The anesthesia personnel’s use of the McGRATH VL increased to 61% of all video laryngoscopy cases, compared to 37% for the GlideScope (Figure 2).
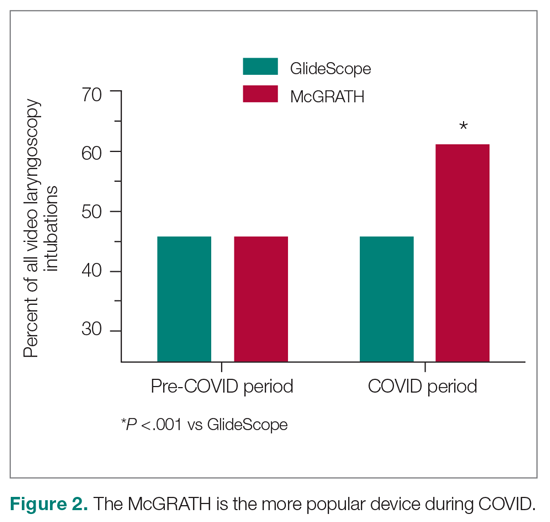
The total costs calculated for equipment, blades, and repairs are presented in Table 1 and yearly total costs are shown in Figure 3. Overall costs were $181 093 lower (55.5%) for the McGRATH VL compared to the GlideScope over the 24-month period. The mean (SD) monthly costs for GlideScope VL blades were $3837 ($1050) and $3236 ($538) for years 1 and 2, respectively, vs $1652 ($663) and $2933 ($585) for the McGRATH VL blades. Most of the total cost differences were attributed to equipment and blade purchases, which were $202 595 (65.0%) higher for the GlideScope compared to the McGRATH VL. The monthly blade costs alone were higher (P < .001) for the GlideScope over the 2-year period; however, the McGRATH VL required use of disposable stylets at a cost of $10 177 for all endotracheal intubations, compared to $700 for the GlideScope device.
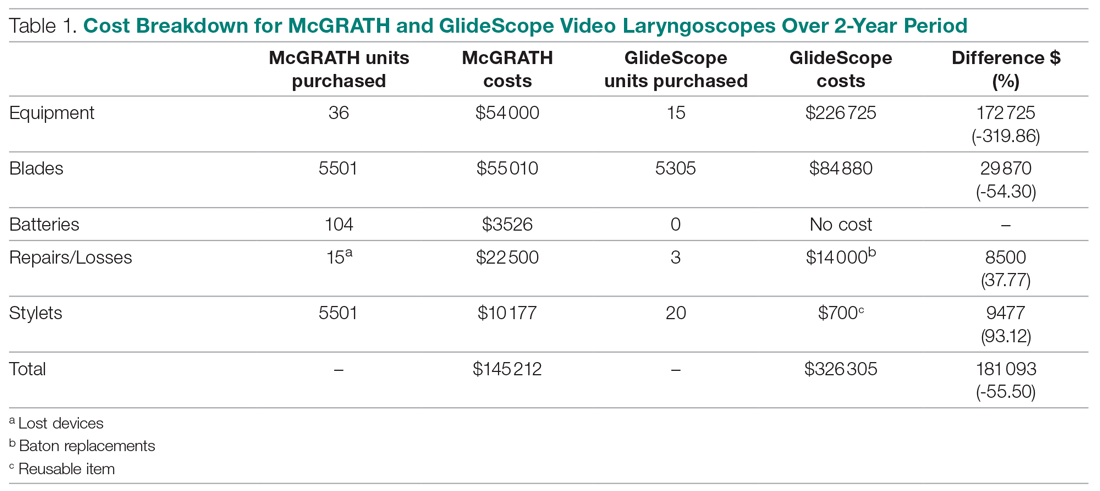
An analysis was performed to determine whether costs differed between those 2 instruments during the COVID-19 period. There was a statistically significant (P < .001) difference in the case distribution between use of the McGRATH and GlideScope VLs during that period. The calculated blade cost difference for the COVID period was $128 higher for the McGRATH even though 293 more intubations were performed with that device (Table 2).
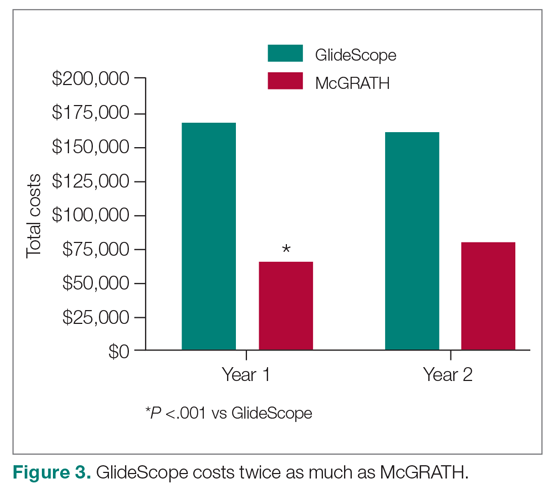
Discussion
We attempted to provide useful cost estimates by presenting pricing data reflecting the approximate cost that most large institutional anesthesia practices would incur for using those 2 specific devices and related peripherals. The main findings of our analysis showed that use of the McGRATH MAC VL resulted in a 55% cost savings compared to the GlideScope, with a similar number of cases performed with each device over the 24-month study period. We believe this represents a substantial savings to the department and institution, which has prompted internal review on the use of video laryngoscopy equipment. None of the McGRATH units failed; however, the GlideScope required 3 baton replacements.
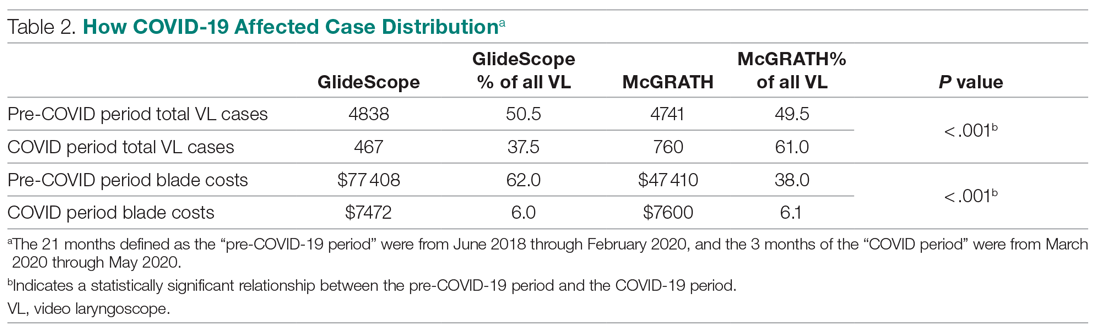
Of note, use of the McGRATH MAC increased during the COVID-19 period, which may be explained by the fact that the operators found it to be a more portable device. Several physicians in the department commented that its smaller size made the McGRATH MAC more practical during the time when a plexiglass box was being used around the patient’s head to shield the intubator from aerosolized viral particles.
Although this study demonstrated the cost-saving value of the McGRATH over the GlideScope, a suggested next step would be to examine resource utilization related to video laryngoscopy use. The more dynamic tracking of the use of these devices should facilitate the assessment of existing related resources and decision making, to optimize the benefits of this initiative. We would anticipate reduced use of anesthesia personnel, such as technicians to assist with the management of this device which could be significant. As new respiratory viruses are appearing each year, video laryngoscopy will continue to gain increasing use in operating rooms and acute care locations. The adding of protective barriers between patients and providers calls for use of the most practical and effective VL devices, to protect personnel who are at high risk of contamination from airway secretions and aerosolized particles.9,10
The COVID-19 pandemic has demonstrated the value of anesthesiology in regards to analyzing and finding solutions to effectively manage infected patients or those suspected of infection in the perioperative environment. Inexpensive products are often avoided because cheaper devices are associated with being of lower quality. However, the association with cost and quality—and the assumption that a higher price is positively correlated with higher quality—is overall inconsistent in the medical literature.11 A more effective or higher quality treatment does not necessarily cost more and may actually end up costing less,12 as was the case in this study. We have been able to directly cut departmental expenses by using a more efficient and cost-effective device for intubations, without compromising safety and efficacy. Future studies should determine whether this significant reduction in costs from video laryngoscopy intubations with the McGRATH VL will be sustained across anesthesiology departments in the Jefferson Health Enterprise Hospitals, or other health systems, as well as its impact on workflow and personnel resources.
This analysis was restricted to one of the campuses of the Jefferson Health Enterprise. However, this is the largest anesthesia practice, encompassing several locations, which should reflect the general practice patterns across other anesthesiology departments in this large institution. The costs for the devices and peripherals may vary across anesthesia practices depending on volume and contracts negotiated with the suppliers. It was not possible to estimate this variability, which could change the total costs by a few percentage points. We recognize that there may be other costs associated with securing the McGRATH VL to prevent loss from theft or misplacement, which were not included in the study. Lastly, the inability to obtain randomized samples for the 2 groups treated with each device opens up the possibility of selection bias. There were, however, multiple intubators who were free to select 1 of the devices for endotracheal intubation, which may have reduced the effect of selection bias.
Conclusion
This study demonstrated that over a 24-month period use of the McGRATH MAC VL resulted in a cost reduction of around 55% compared to using the GlideScope for endotracheal intubation procedures performed at a major academic center. Over the first 3 months of the COVID-19 crisis, which our study included, use of the McGRATH VL increased while GlideScope use decreased. This was most likely related to the portability and smaller size of the McGRATH, which better facilitated intubations of COVID-19 patients.
Acknowledgements: The authors thank Craig Smith, Senior Anesthesia Technician, for his assistance with the cost information and excellent record-keeping related to the use of video laryngoscopes.
Corresponding author: Marc C. Torjman, PhD, Professor, Department of Anesthesiology, Sidney Kimmel Medical College at Thomas Jefferson University, 111 South 11th St, Suite G-8290, Philadelphia, PA 19107; [email protected].
Financial disclosures: Dr. Thaler has served as a consultant for Medtronic since September 2020. He has participated in 2 webinars on the routine use of video laryngoscopy.
Funding: This study was supported by the Department of Anesthesiology at Thomas Jefferson University.
1. Channa AB. Video laryngoscopes. Saudi J Anaesth. 2011;5(4):357-359.
2. Pieters BM, Eindhoven GB, Acott C, Van Zundert AAJ. Pioneers of laryngoscopy: indirect, direct and video laryngoscopy. Anaesth Intensive Care. 2015;43(suppl):4-11.
3. Lewis SR, Butler AR, Parker J, et al. Videolaryngoscopy versus direct laryngoscopy for adult patients requiring tracheal intubation. Cochrane Database Syst Rev. 2016;11(11):CD011136.
4. Kim W, Choi HJ, Lim T, Kang BS. Can the new McGrath laryngoscope rival the GlideScope Ranger portable video laryngoscope? A randomized manikin study. Am J Emerg Med. 2014;32(10):1225-1229.
5. Kim W, Choi HJ, Lim T, et al. Is McGrath MAC better than Glidescope Ranger for novice providers in the simulated difficult airway? A randomized manikin study. Resuscitation. 2014;85(suppl 1):S32.
6. Jeon WJ, Kim KH, Yeom JH, et al. A comparison of the Glidescope to the McGrath videolaryngoscope in patients. Korean J Anesthesiol. 2011;61(1):19-23.
7. Savoldelli GL, Schiffer E, Abegg C, et al. Comparison of the Glidescope, the McGrath, the Airtraq and the Macintosh laryngoscopes in simulated difficult airways. Anaesthesia. 2008;63(12):1358-1364.
8. Savoldelli GL, Schiffer E, Abegg C, et al. Learning curves of the Glidescope, the McGrath and the Airtraq laryngoscopes: a manikin study. Eur J Anaesthesiol. 2009;26(7):554-558.
9. Schumacher J, Arlidge J, Dudley D, et al. The impact of respiratory protective equipment on difficult airway management: a randomised, crossover, simulation study. Anaesthesia. 2020;75(10):1301-1306.
10. De Jong A, Pardo E, Rolle A, et al. Airway management for COVID-19: a move towards universal videolaryngoscope? Lancet Respir Med. 2020;8(6):555.
11. Hussey PS, Wertheimer S, Mehrotra A. The association between health care quality and cost: a systematic review. Ann Intern Med. 2013;158(1):27-34.
12. Mitton C, Dionne F, Peacock S, Sheps S. Quality and cost in healthcare: a relationship worth examining. Appl Health Econ Health Policy. 2006;5(4):201-208.
From the Department of Anesthesiology, Thomas Jefferson University and Hospitals, Sidney Kimmel Medical College, Philadelphia, PA, and Sidney Kimmel Medical College at Thomas Jefferson University, Philadelphia, PA.
Objective: Retrospective study examining hospital cost information of patients requiring endotracheal intubation with video laryngoscopy. Provide a practical cost assessment on use of the McGRATH and GlideScope video laryngoscopes (VLs).
Methods: This study examined 52 hospital locations within a single, large university hospital, with most of those locations being hospital operating rooms. A total of 34 600 endotracheal intubations performed over 24 months, of which 11 345 were video laryngoscopies. Electronic medical records containing demographic data and information related to endotracheal intubation procedures, with monthly breakdowns between GlideScope and McGRATH intubations, were reviewed. Cost information calculated for equipment, blades, batteries, repairs, and subsequent analysis performed to determine cost differences between those 2 instruments during the COVID-19 period.
Results: A total of 5501 video laryngoscopy procedures were performed using the McGRATH VL and 5305 were performed using the GlideScope VL. Costs over 24 months were $181 093 lower (55.5%) for McGRATH compared to GlideScope. The mean (SD) monthly costs for GlideScope blades were $3837 ($1050) and $3236 ($538) for years 1 and 2, respectively, vs $1652 ($663) and $2933 ($585) for McGRATH blades (P < .001). Most total cost differences were attributed to equipment and blade purchases, which were $202 595 (65.0%) higher for GlideScope. During the COVID-19 period, the use of the McGRATH increased to 61% of all video laryngoscopy cases, compared to 37% for GlideScope (P < .001). Blade cost difference for the COVID-19 period was $128 higher for the McGRATH even though 293 more intubations were performed with that device.
Conclusions: Use of the McGRATH resulted in a cost savings of 55% compared to the GlideScope, and its use was highest during the COVID-19 period, which may be explained by its more portable and practical features.
Keywords: video laryngoscope; McGRATH; GlideScope; endotracheal intubation; hospital costs; COVID-19.
Hospitals have come to rely on video laryngoscopes (VLs) for tracheal intubation as necessary tools for better visualization of airways. Modern video laryngoscopy developed in the 2000s1 as a progression from direct laryngoscopy, which began in 1852 when Horace Green used a bent tongue spatula and sunlight to examine a child.2 VLs have seen many improvements and adaptations of their own, resulting in many different styles and types circulating around hospitals. The GlideScope (Verathon Inc, Bothell, WA) and the McGRATH (Medtronic, Minneapolis, MN) are examples of such instruments, which are now widely used in the US and are the 2 VLs of choice at our institution.
A few studies have compared VLs to direct laryngoscopes. In their systematic review, Lewis et al have shown the numerous benefits of using a VL over a direct laryngoscope. Some general conclusions were that the use of video laryngoscopy reduced the number of failed intubations, decreased laryngeal trauma, and provided improved visualizations.3 Other studies have compared the different types of VLs, including the McGRATH and the GlideScope, examining factors such as intubation time and display quality of the image. Two studies found that medical students were equally successful at using both the McGRATH and the GlideScope,4,5 while another study found that care providers using the GlideScope had quicker intubation times.6 Lastly, Savoldelli et al concluded that more providers preferred the McGRATH, which provided better laryngeal views,7 while their subsequent study showed more favorable learning curves of the Airtraq compared to the McGRATH and other VLs.8
Although there have been no reported differences in safety and effectiveness of the McGRATH and GlideScope devices, cost data on the use of these 2 popular laryngoscopes are lacking. Such information is important considering the increasing costs of medical technologies and the significant financial losses experienced by health care systems due to the COVID-19 crisis. The purpose of this retrospective cohort study was to compare the cost efficiency of the McGRATH MAC and GlideScope Core VLs at a large academic center.
Methods
This retrospective study was performed under exemption from the Thomas Jefferson University Institutional Review Board. The primary data sources consisted of hospital electronic patient records (EPIC) and cost information from the device manufacturers and hospital staff. The electronic patient data were provided by the EPIC Enterprise Analytics Business Intelligence group at Thomas Jefferson University Hospital (Center City Campus, Philadelphia, PA), while device costs were obtained from Verathon, Medtronic, and departmental staff responsible for purchasing equipment. Monthly data were obtained over a 24-month period (June 2018 through May 2020) when the McGRATH VL was placed into use in the department of anesthesiology. The 2 types of VLs were made available for use in a total of 52 locations, with the majority being hospital operating rooms.
The following variables were recorded: number of endotracheal intubations performed each month with breakdown between video laryngoscopy and flexible bronchoscopy airways, frequency of use for each type of laryngoscope, blades used, and equipment costs for use of each laryngoscope. Hospital cost estimates for both the McGRATH and GlideScope laryngoscopes included batteries, handles, blades, and the devices themselves. Cost data were also collected on frequency of device failure, maintenance, and replacement of parts and lost equipment.
Analysis
De-identified electronic medical records consisted of nominal and quantitative variables, with demographic data and information related to the endotracheal intubation procedure. All data were in chronological order and sorted by date after which coding was applied, to identify device type and allocate pertinent cost information. Descriptive statistics were reported as mean (SD) and sum for costs; frequency tables were generated for intubation procedures according to device type and time periods. Data were analyzed using the χ2 test, the student t test, and the Wilcoxon Mann-Whitney U test, with a P value set at .05 for statistical significance. SPSS version 26 and GraphPad Prism version 6 were used for all statistical analyses.
Results
A total of 34 600 endotracheal intubations were performed over the 24-month study period, and 11 345 (32.8%) were video laryngoscopy procedures. Out of all video laryngoscopy procedures, 5501 (48.5%) were performed using the McGRATH VL and 5305 (46.8%) were conducted using the GlideScope VL. The difference of 539 (4.8%) cases accounts for flexible bronchoscopy procedures and endotracheal intubations using other video laryngoscopy equipment. The mean (SD) monthly number of video laryngoscopy procedures for the 24 months was 221 (54) and 229 (89) for the GlideScope and McGRATH devices, respectively. Monthly endotracheal intubation distributions over 24 months trended upward for the McGRATH VL and downward for the GlideScope, but there was no statistically significant (P = .71) difference in overall use between the 2 instruments (Figure 1).

To examine the observed usage trends between the 2 VL during the first and last 12 months, a univariate ANOVA was conducted with the 2 time periods entered as predictors in the model. Video laryngoscopy intubations were performed (P = .001) more frequently with the GlideScope during the first 12 months; however, use of the McGRATH VL increased (P < .001) during the following 12 months compared to GlideScope. The GlideScope accounted for 54% of all VL intubations during the first 12 months, with the McGRATH accounting for 58% of all video laryngoscopy procedures for months 12 to 24. Additionally, the increase in video laryngoscopy procedures with the McGRATH during the last 3 months of the study period was despite an overall reduction in surgical volume due to the COVID-19 crisis, defined for this study as March 1, 2020, to May 31, 2020 (Figure 1). There was a statistically significant (P < .001) difference in the case distribution between use of the McGRATH and GlideScope VL for that period. The anesthesia personnel’s use of the McGRATH VL increased to 61% of all video laryngoscopy cases, compared to 37% for the GlideScope (Figure 2).

The total costs calculated for equipment, blades, and repairs are presented in Table 1 and yearly total costs are shown in Figure 3. Overall costs were $181 093 lower (55.5%) for the McGRATH VL compared to the GlideScope over the 24-month period. The mean (SD) monthly costs for GlideScope VL blades were $3837 ($1050) and $3236 ($538) for years 1 and 2, respectively, vs $1652 ($663) and $2933 ($585) for the McGRATH VL blades. Most of the total cost differences were attributed to equipment and blade purchases, which were $202 595 (65.0%) higher for the GlideScope compared to the McGRATH VL. The monthly blade costs alone were higher (P < .001) for the GlideScope over the 2-year period; however, the McGRATH VL required use of disposable stylets at a cost of $10 177 for all endotracheal intubations, compared to $700 for the GlideScope device.

An analysis was performed to determine whether costs differed between those 2 instruments during the COVID-19 period. There was a statistically significant (P < .001) difference in the case distribution between use of the McGRATH and GlideScope VLs during that period. The calculated blade cost difference for the COVID period was $128 higher for the McGRATH even though 293 more intubations were performed with that device (Table 2).

Discussion
We attempted to provide useful cost estimates by presenting pricing data reflecting the approximate cost that most large institutional anesthesia practices would incur for using those 2 specific devices and related peripherals. The main findings of our analysis showed that use of the McGRATH MAC VL resulted in a 55% cost savings compared to the GlideScope, with a similar number of cases performed with each device over the 24-month study period. We believe this represents a substantial savings to the department and institution, which has prompted internal review on the use of video laryngoscopy equipment. None of the McGRATH units failed; however, the GlideScope required 3 baton replacements.

Of note, use of the McGRATH MAC increased during the COVID-19 period, which may be explained by the fact that the operators found it to be a more portable device. Several physicians in the department commented that its smaller size made the McGRATH MAC more practical during the time when a plexiglass box was being used around the patient’s head to shield the intubator from aerosolized viral particles.
Although this study demonstrated the cost-saving value of the McGRATH over the GlideScope, a suggested next step would be to examine resource utilization related to video laryngoscopy use. The more dynamic tracking of the use of these devices should facilitate the assessment of existing related resources and decision making, to optimize the benefits of this initiative. We would anticipate reduced use of anesthesia personnel, such as technicians to assist with the management of this device which could be significant. As new respiratory viruses are appearing each year, video laryngoscopy will continue to gain increasing use in operating rooms and acute care locations. The adding of protective barriers between patients and providers calls for use of the most practical and effective VL devices, to protect personnel who are at high risk of contamination from airway secretions and aerosolized particles.9,10
The COVID-19 pandemic has demonstrated the value of anesthesiology in regards to analyzing and finding solutions to effectively manage infected patients or those suspected of infection in the perioperative environment. Inexpensive products are often avoided because cheaper devices are associated with being of lower quality. However, the association with cost and quality—and the assumption that a higher price is positively correlated with higher quality—is overall inconsistent in the medical literature.11 A more effective or higher quality treatment does not necessarily cost more and may actually end up costing less,12 as was the case in this study. We have been able to directly cut departmental expenses by using a more efficient and cost-effective device for intubations, without compromising safety and efficacy. Future studies should determine whether this significant reduction in costs from video laryngoscopy intubations with the McGRATH VL will be sustained across anesthesiology departments in the Jefferson Health Enterprise Hospitals, or other health systems, as well as its impact on workflow and personnel resources.
This analysis was restricted to one of the campuses of the Jefferson Health Enterprise. However, this is the largest anesthesia practice, encompassing several locations, which should reflect the general practice patterns across other anesthesiology departments in this large institution. The costs for the devices and peripherals may vary across anesthesia practices depending on volume and contracts negotiated with the suppliers. It was not possible to estimate this variability, which could change the total costs by a few percentage points. We recognize that there may be other costs associated with securing the McGRATH VL to prevent loss from theft or misplacement, which were not included in the study. Lastly, the inability to obtain randomized samples for the 2 groups treated with each device opens up the possibility of selection bias. There were, however, multiple intubators who were free to select 1 of the devices for endotracheal intubation, which may have reduced the effect of selection bias.
Conclusion
This study demonstrated that over a 24-month period use of the McGRATH MAC VL resulted in a cost reduction of around 55% compared to using the GlideScope for endotracheal intubation procedures performed at a major academic center. Over the first 3 months of the COVID-19 crisis, which our study included, use of the McGRATH VL increased while GlideScope use decreased. This was most likely related to the portability and smaller size of the McGRATH, which better facilitated intubations of COVID-19 patients.
Acknowledgements: The authors thank Craig Smith, Senior Anesthesia Technician, for his assistance with the cost information and excellent record-keeping related to the use of video laryngoscopes.
Corresponding author: Marc C. Torjman, PhD, Professor, Department of Anesthesiology, Sidney Kimmel Medical College at Thomas Jefferson University, 111 South 11th St, Suite G-8290, Philadelphia, PA 19107; [email protected].
Financial disclosures: Dr. Thaler has served as a consultant for Medtronic since September 2020. He has participated in 2 webinars on the routine use of video laryngoscopy.
Funding: This study was supported by the Department of Anesthesiology at Thomas Jefferson University.
From the Department of Anesthesiology, Thomas Jefferson University and Hospitals, Sidney Kimmel Medical College, Philadelphia, PA, and Sidney Kimmel Medical College at Thomas Jefferson University, Philadelphia, PA.
Objective: Retrospective study examining hospital cost information of patients requiring endotracheal intubation with video laryngoscopy. Provide a practical cost assessment on use of the McGRATH and GlideScope video laryngoscopes (VLs).
Methods: This study examined 52 hospital locations within a single, large university hospital, with most of those locations being hospital operating rooms. A total of 34 600 endotracheal intubations performed over 24 months, of which 11 345 were video laryngoscopies. Electronic medical records containing demographic data and information related to endotracheal intubation procedures, with monthly breakdowns between GlideScope and McGRATH intubations, were reviewed. Cost information calculated for equipment, blades, batteries, repairs, and subsequent analysis performed to determine cost differences between those 2 instruments during the COVID-19 period.
Results: A total of 5501 video laryngoscopy procedures were performed using the McGRATH VL and 5305 were performed using the GlideScope VL. Costs over 24 months were $181 093 lower (55.5%) for McGRATH compared to GlideScope. The mean (SD) monthly costs for GlideScope blades were $3837 ($1050) and $3236 ($538) for years 1 and 2, respectively, vs $1652 ($663) and $2933 ($585) for McGRATH blades (P < .001). Most total cost differences were attributed to equipment and blade purchases, which were $202 595 (65.0%) higher for GlideScope. During the COVID-19 period, the use of the McGRATH increased to 61% of all video laryngoscopy cases, compared to 37% for GlideScope (P < .001). Blade cost difference for the COVID-19 period was $128 higher for the McGRATH even though 293 more intubations were performed with that device.
Conclusions: Use of the McGRATH resulted in a cost savings of 55% compared to the GlideScope, and its use was highest during the COVID-19 period, which may be explained by its more portable and practical features.
Keywords: video laryngoscope; McGRATH; GlideScope; endotracheal intubation; hospital costs; COVID-19.
Hospitals have come to rely on video laryngoscopes (VLs) for tracheal intubation as necessary tools for better visualization of airways. Modern video laryngoscopy developed in the 2000s1 as a progression from direct laryngoscopy, which began in 1852 when Horace Green used a bent tongue spatula and sunlight to examine a child.2 VLs have seen many improvements and adaptations of their own, resulting in many different styles and types circulating around hospitals. The GlideScope (Verathon Inc, Bothell, WA) and the McGRATH (Medtronic, Minneapolis, MN) are examples of such instruments, which are now widely used in the US and are the 2 VLs of choice at our institution.
A few studies have compared VLs to direct laryngoscopes. In their systematic review, Lewis et al have shown the numerous benefits of using a VL over a direct laryngoscope. Some general conclusions were that the use of video laryngoscopy reduced the number of failed intubations, decreased laryngeal trauma, and provided improved visualizations.3 Other studies have compared the different types of VLs, including the McGRATH and the GlideScope, examining factors such as intubation time and display quality of the image. Two studies found that medical students were equally successful at using both the McGRATH and the GlideScope,4,5 while another study found that care providers using the GlideScope had quicker intubation times.6 Lastly, Savoldelli et al concluded that more providers preferred the McGRATH, which provided better laryngeal views,7 while their subsequent study showed more favorable learning curves of the Airtraq compared to the McGRATH and other VLs.8
Although there have been no reported differences in safety and effectiveness of the McGRATH and GlideScope devices, cost data on the use of these 2 popular laryngoscopes are lacking. Such information is important considering the increasing costs of medical technologies and the significant financial losses experienced by health care systems due to the COVID-19 crisis. The purpose of this retrospective cohort study was to compare the cost efficiency of the McGRATH MAC and GlideScope Core VLs at a large academic center.
Methods
This retrospective study was performed under exemption from the Thomas Jefferson University Institutional Review Board. The primary data sources consisted of hospital electronic patient records (EPIC) and cost information from the device manufacturers and hospital staff. The electronic patient data were provided by the EPIC Enterprise Analytics Business Intelligence group at Thomas Jefferson University Hospital (Center City Campus, Philadelphia, PA), while device costs were obtained from Verathon, Medtronic, and departmental staff responsible for purchasing equipment. Monthly data were obtained over a 24-month period (June 2018 through May 2020) when the McGRATH VL was placed into use in the department of anesthesiology. The 2 types of VLs were made available for use in a total of 52 locations, with the majority being hospital operating rooms.
The following variables were recorded: number of endotracheal intubations performed each month with breakdown between video laryngoscopy and flexible bronchoscopy airways, frequency of use for each type of laryngoscope, blades used, and equipment costs for use of each laryngoscope. Hospital cost estimates for both the McGRATH and GlideScope laryngoscopes included batteries, handles, blades, and the devices themselves. Cost data were also collected on frequency of device failure, maintenance, and replacement of parts and lost equipment.
Analysis
De-identified electronic medical records consisted of nominal and quantitative variables, with demographic data and information related to the endotracheal intubation procedure. All data were in chronological order and sorted by date after which coding was applied, to identify device type and allocate pertinent cost information. Descriptive statistics were reported as mean (SD) and sum for costs; frequency tables were generated for intubation procedures according to device type and time periods. Data were analyzed using the χ2 test, the student t test, and the Wilcoxon Mann-Whitney U test, with a P value set at .05 for statistical significance. SPSS version 26 and GraphPad Prism version 6 were used for all statistical analyses.
Results
A total of 34 600 endotracheal intubations were performed over the 24-month study period, and 11 345 (32.8%) were video laryngoscopy procedures. Out of all video laryngoscopy procedures, 5501 (48.5%) were performed using the McGRATH VL and 5305 (46.8%) were conducted using the GlideScope VL. The difference of 539 (4.8%) cases accounts for flexible bronchoscopy procedures and endotracheal intubations using other video laryngoscopy equipment. The mean (SD) monthly number of video laryngoscopy procedures for the 24 months was 221 (54) and 229 (89) for the GlideScope and McGRATH devices, respectively. Monthly endotracheal intubation distributions over 24 months trended upward for the McGRATH VL and downward for the GlideScope, but there was no statistically significant (P = .71) difference in overall use between the 2 instruments (Figure 1).

To examine the observed usage trends between the 2 VL during the first and last 12 months, a univariate ANOVA was conducted with the 2 time periods entered as predictors in the model. Video laryngoscopy intubations were performed (P = .001) more frequently with the GlideScope during the first 12 months; however, use of the McGRATH VL increased (P < .001) during the following 12 months compared to GlideScope. The GlideScope accounted for 54% of all VL intubations during the first 12 months, with the McGRATH accounting for 58% of all video laryngoscopy procedures for months 12 to 24. Additionally, the increase in video laryngoscopy procedures with the McGRATH during the last 3 months of the study period was despite an overall reduction in surgical volume due to the COVID-19 crisis, defined for this study as March 1, 2020, to May 31, 2020 (Figure 1). There was a statistically significant (P < .001) difference in the case distribution between use of the McGRATH and GlideScope VL for that period. The anesthesia personnel’s use of the McGRATH VL increased to 61% of all video laryngoscopy cases, compared to 37% for the GlideScope (Figure 2).

The total costs calculated for equipment, blades, and repairs are presented in Table 1 and yearly total costs are shown in Figure 3. Overall costs were $181 093 lower (55.5%) for the McGRATH VL compared to the GlideScope over the 24-month period. The mean (SD) monthly costs for GlideScope VL blades were $3837 ($1050) and $3236 ($538) for years 1 and 2, respectively, vs $1652 ($663) and $2933 ($585) for the McGRATH VL blades. Most of the total cost differences were attributed to equipment and blade purchases, which were $202 595 (65.0%) higher for the GlideScope compared to the McGRATH VL. The monthly blade costs alone were higher (P < .001) for the GlideScope over the 2-year period; however, the McGRATH VL required use of disposable stylets at a cost of $10 177 for all endotracheal intubations, compared to $700 for the GlideScope device.

An analysis was performed to determine whether costs differed between those 2 instruments during the COVID-19 period. There was a statistically significant (P < .001) difference in the case distribution between use of the McGRATH and GlideScope VLs during that period. The calculated blade cost difference for the COVID period was $128 higher for the McGRATH even though 293 more intubations were performed with that device (Table 2).

Discussion
We attempted to provide useful cost estimates by presenting pricing data reflecting the approximate cost that most large institutional anesthesia practices would incur for using those 2 specific devices and related peripherals. The main findings of our analysis showed that use of the McGRATH MAC VL resulted in a 55% cost savings compared to the GlideScope, with a similar number of cases performed with each device over the 24-month study period. We believe this represents a substantial savings to the department and institution, which has prompted internal review on the use of video laryngoscopy equipment. None of the McGRATH units failed; however, the GlideScope required 3 baton replacements.

Of note, use of the McGRATH MAC increased during the COVID-19 period, which may be explained by the fact that the operators found it to be a more portable device. Several physicians in the department commented that its smaller size made the McGRATH MAC more practical during the time when a plexiglass box was being used around the patient’s head to shield the intubator from aerosolized viral particles.
Although this study demonstrated the cost-saving value of the McGRATH over the GlideScope, a suggested next step would be to examine resource utilization related to video laryngoscopy use. The more dynamic tracking of the use of these devices should facilitate the assessment of existing related resources and decision making, to optimize the benefits of this initiative. We would anticipate reduced use of anesthesia personnel, such as technicians to assist with the management of this device which could be significant. As new respiratory viruses are appearing each year, video laryngoscopy will continue to gain increasing use in operating rooms and acute care locations. The adding of protective barriers between patients and providers calls for use of the most practical and effective VL devices, to protect personnel who are at high risk of contamination from airway secretions and aerosolized particles.9,10
The COVID-19 pandemic has demonstrated the value of anesthesiology in regards to analyzing and finding solutions to effectively manage infected patients or those suspected of infection in the perioperative environment. Inexpensive products are often avoided because cheaper devices are associated with being of lower quality. However, the association with cost and quality—and the assumption that a higher price is positively correlated with higher quality—is overall inconsistent in the medical literature.11 A more effective or higher quality treatment does not necessarily cost more and may actually end up costing less,12 as was the case in this study. We have been able to directly cut departmental expenses by using a more efficient and cost-effective device for intubations, without compromising safety and efficacy. Future studies should determine whether this significant reduction in costs from video laryngoscopy intubations with the McGRATH VL will be sustained across anesthesiology departments in the Jefferson Health Enterprise Hospitals, or other health systems, as well as its impact on workflow and personnel resources.
This analysis was restricted to one of the campuses of the Jefferson Health Enterprise. However, this is the largest anesthesia practice, encompassing several locations, which should reflect the general practice patterns across other anesthesiology departments in this large institution. The costs for the devices and peripherals may vary across anesthesia practices depending on volume and contracts negotiated with the suppliers. It was not possible to estimate this variability, which could change the total costs by a few percentage points. We recognize that there may be other costs associated with securing the McGRATH VL to prevent loss from theft or misplacement, which were not included in the study. Lastly, the inability to obtain randomized samples for the 2 groups treated with each device opens up the possibility of selection bias. There were, however, multiple intubators who were free to select 1 of the devices for endotracheal intubation, which may have reduced the effect of selection bias.
Conclusion
This study demonstrated that over a 24-month period use of the McGRATH MAC VL resulted in a cost reduction of around 55% compared to using the GlideScope for endotracheal intubation procedures performed at a major academic center. Over the first 3 months of the COVID-19 crisis, which our study included, use of the McGRATH VL increased while GlideScope use decreased. This was most likely related to the portability and smaller size of the McGRATH, which better facilitated intubations of COVID-19 patients.
Acknowledgements: The authors thank Craig Smith, Senior Anesthesia Technician, for his assistance with the cost information and excellent record-keeping related to the use of video laryngoscopes.
Corresponding author: Marc C. Torjman, PhD, Professor, Department of Anesthesiology, Sidney Kimmel Medical College at Thomas Jefferson University, 111 South 11th St, Suite G-8290, Philadelphia, PA 19107; [email protected].
Financial disclosures: Dr. Thaler has served as a consultant for Medtronic since September 2020. He has participated in 2 webinars on the routine use of video laryngoscopy.
Funding: This study was supported by the Department of Anesthesiology at Thomas Jefferson University.
1. Channa AB. Video laryngoscopes. Saudi J Anaesth. 2011;5(4):357-359.
2. Pieters BM, Eindhoven GB, Acott C, Van Zundert AAJ. Pioneers of laryngoscopy: indirect, direct and video laryngoscopy. Anaesth Intensive Care. 2015;43(suppl):4-11.
3. Lewis SR, Butler AR, Parker J, et al. Videolaryngoscopy versus direct laryngoscopy for adult patients requiring tracheal intubation. Cochrane Database Syst Rev. 2016;11(11):CD011136.
4. Kim W, Choi HJ, Lim T, Kang BS. Can the new McGrath laryngoscope rival the GlideScope Ranger portable video laryngoscope? A randomized manikin study. Am J Emerg Med. 2014;32(10):1225-1229.
5. Kim W, Choi HJ, Lim T, et al. Is McGrath MAC better than Glidescope Ranger for novice providers in the simulated difficult airway? A randomized manikin study. Resuscitation. 2014;85(suppl 1):S32.
6. Jeon WJ, Kim KH, Yeom JH, et al. A comparison of the Glidescope to the McGrath videolaryngoscope in patients. Korean J Anesthesiol. 2011;61(1):19-23.
7. Savoldelli GL, Schiffer E, Abegg C, et al. Comparison of the Glidescope, the McGrath, the Airtraq and the Macintosh laryngoscopes in simulated difficult airways. Anaesthesia. 2008;63(12):1358-1364.
8. Savoldelli GL, Schiffer E, Abegg C, et al. Learning curves of the Glidescope, the McGrath and the Airtraq laryngoscopes: a manikin study. Eur J Anaesthesiol. 2009;26(7):554-558.
9. Schumacher J, Arlidge J, Dudley D, et al. The impact of respiratory protective equipment on difficult airway management: a randomised, crossover, simulation study. Anaesthesia. 2020;75(10):1301-1306.
10. De Jong A, Pardo E, Rolle A, et al. Airway management for COVID-19: a move towards universal videolaryngoscope? Lancet Respir Med. 2020;8(6):555.
11. Hussey PS, Wertheimer S, Mehrotra A. The association between health care quality and cost: a systematic review. Ann Intern Med. 2013;158(1):27-34.
12. Mitton C, Dionne F, Peacock S, Sheps S. Quality and cost in healthcare: a relationship worth examining. Appl Health Econ Health Policy. 2006;5(4):201-208.
1. Channa AB. Video laryngoscopes. Saudi J Anaesth. 2011;5(4):357-359.
2. Pieters BM, Eindhoven GB, Acott C, Van Zundert AAJ. Pioneers of laryngoscopy: indirect, direct and video laryngoscopy. Anaesth Intensive Care. 2015;43(suppl):4-11.
3. Lewis SR, Butler AR, Parker J, et al. Videolaryngoscopy versus direct laryngoscopy for adult patients requiring tracheal intubation. Cochrane Database Syst Rev. 2016;11(11):CD011136.
4. Kim W, Choi HJ, Lim T, Kang BS. Can the new McGrath laryngoscope rival the GlideScope Ranger portable video laryngoscope? A randomized manikin study. Am J Emerg Med. 2014;32(10):1225-1229.
5. Kim W, Choi HJ, Lim T, et al. Is McGrath MAC better than Glidescope Ranger for novice providers in the simulated difficult airway? A randomized manikin study. Resuscitation. 2014;85(suppl 1):S32.
6. Jeon WJ, Kim KH, Yeom JH, et al. A comparison of the Glidescope to the McGrath videolaryngoscope in patients. Korean J Anesthesiol. 2011;61(1):19-23.
7. Savoldelli GL, Schiffer E, Abegg C, et al. Comparison of the Glidescope, the McGrath, the Airtraq and the Macintosh laryngoscopes in simulated difficult airways. Anaesthesia. 2008;63(12):1358-1364.
8. Savoldelli GL, Schiffer E, Abegg C, et al. Learning curves of the Glidescope, the McGrath and the Airtraq laryngoscopes: a manikin study. Eur J Anaesthesiol. 2009;26(7):554-558.
9. Schumacher J, Arlidge J, Dudley D, et al. The impact of respiratory protective equipment on difficult airway management: a randomised, crossover, simulation study. Anaesthesia. 2020;75(10):1301-1306.
10. De Jong A, Pardo E, Rolle A, et al. Airway management for COVID-19: a move towards universal videolaryngoscope? Lancet Respir Med. 2020;8(6):555.
11. Hussey PS, Wertheimer S, Mehrotra A. The association between health care quality and cost: a systematic review. Ann Intern Med. 2013;158(1):27-34.
12. Mitton C, Dionne F, Peacock S, Sheps S. Quality and cost in healthcare: a relationship worth examining. Appl Health Econ Health Policy. 2006;5(4):201-208.
Practical Application of Self-Determination Theory to Achieve a Reduction in Postoperative Hypothermia Rate: A Quality Improvement Project
From Children’s Health System of Texas, Division of Pediatric Anesthesiology, Dallas, TX (Drs. Sakhai, Bocanegra, Chandran, Kimatian, and Kiss), UT Southwestern Medical Center, Department of Anesthesiology and Pain Management, Dallas, TX (Drs. Bocanegra, Chandran, Kimatian, and Kiss), and UT Southwestern Medical Center, Department of Population and Data Sciences, Dallas, TX (Dr. Reisch).
Objective: Policy-driven changes in medical practice have long been the norm. Seldom are changes in clinical practice sought to be brought about by a person’s tendency toward growth or self‐actualization. Many hospitals have instituted hypothermia bundles to help reduce the incidence of unanticipated postoperative hypothermia. Although successful in the short-term, sustained changes are difficult to maintain. We implemented a quality-improvement project focused on addressing the affective components of self-determination theory (SDT) to create sustainable behavioral change while satisfying providers’ basic psychological needs for autonomy, competence, and relatedness.
Methods: A total of 3 Plan-Do-Study-Act (PDSA) cycles were enacted over the span of 14 months at a major tertiary care pediatric hospital to recruit and motivate anesthesia providers and perioperative team members to reduce the percentage of hypothermic postsurgical patients by 50%. As an optional initial incentive for participation, anesthesiologists would qualify for American Board of Anesthesiology Maintenance of Certification in Anesthesiology (MOCA) Part 4 Quality Improvement credits for monitoring their own temperature data and participating in project-related meetings. Providers were given autonomy to develop a personal plan for achieving the desired goals.
Results: The median rate of hypothermia was reduced from 6.9% to 1.6% in July 2019 and was reduced again in July 2020 to 1.3%, an 81% reduction overall. A low hypothermia rate was successfully maintained for at least 21 subsequent months after participants received their MOCA credits in July 2019.
Conclusions: Using an approach that focused on the elements of competency, autonomy, and relatedness central to the principles of SDT, we observed the development of a new culture of vigilance for prevention of hypothermia that successfully endured beyond the project end date.
Keywords: postoperative hypothermia; self-determination theory; motivation; quality improvement.
Perioperative hypothermia, generally accepted as a core temperature less than 36 °C in clinical practice, is a common complication in the pediatric surgical population and is associated with poor postoperative outcomes.1 Hypothermic patients may develop respiratory depression, hypoglycemia, and metabolic acidosis that may lead to decreased oxygen delivery and end organ tissue hypoxia.2-4 Other potential detrimental effects of failing to maintain normal body temperature are impaired clotting factor enzyme function and platelet dysfunction, increasing the risk for postoperative bleeding.5,6 In addition, there are financial implications when hypothermic patients require care and resources postoperatively because of delayed emergence or shivering.7
The American Society of Anesthesiologists recommends intraoperative temperature monitoring for procedures when clinically significant changes in body temperature are anticipated.8 Maintenance of normothermia in the pediatric population is especially challenging owing to a larger skin-surface area compared with body mass ratio and less subcutaneous fat content than in adults. Preventing postoperative hypothermia starts preoperatively with parental education and can be as simple as covering the child with a blanket and setting the preoperative room to an acceptably warm temperature.9,10 Intraoperatively, maintaining operating room (OR) temperatures at or above 21.1 °C and using active warming devices and radiant warmers when appropriate are important techniques to preserve the child’s body temperature.11,12
Despite the knowledge of these risks and vigilant avoidance of hypothermia, unplanned perioperative hypothermia can occur in up to 70% of surgical patients.1 Beyond the clinical benefits, as health care marches toward a value-based payment methodology, quality indicators such as avoiding hypothermia may be linked directly to payment.
Self-determination theory (SDT) was first developed in 1980 by Deci and Ryan.13 The central premise of the theory states that people develop their full potential if circumstances allow them to satisfy their basic psychological needs: autonomy, competence, and relatedness. Under these conditions, people’s natural inclination toward growth can be realized, and they are more likely to internalize external goals. Under an extrinsic reward system, motivation can waver, as people may perceive rewards as controlling.
Many institutions have implemented hypothermia bundles to help decrease the rate of hypothermic patients, but while initially successful, the effectiveness of these interventions tends to fade over time as participants settle into old, comfortable routines.14 With SDT in mind, we designed our quality-improvement (QI) project with interventions to allow clinicians autonomy without instituting rigid guidelines or punitive actions. We aimed to directly address the affective components central to motivation and engagement so that we could bring about long-term meaningful changes in our practice.
Methods
Setting
The hypothermia QI intervention was instituted at a major tertiary care children’s hospital that performs more than 40 000 pediatric general anesthetics annually. Our division of pediatric anesthesiology consists of 66 fellowship-trained pediatric anesthesiologists, 15 or more rotating trainees per month, 13 anesthesiology assistants, 15 anesthesia technicians, and more than 50 perioperative nurses.
The most frequent pediatric surgeries include, but are not limited to, general surgery, otolaryngology, urology, gastroenterology, plastic surgery, neurosurgery, and dentistry. The surgeries are conducted in the hospital’s main operative floor, which consists of 15 ORs and 2 gastroenterology procedure rooms. Although the implementation of the QI project included several operating sites, we focused on collecting temperature data from surgical patients at our main campus recovery unit. We obtained the patients’ initial temperatures upon arrival to the recovery unit from a retrospective electronic health record review of all patients who underwent anesthesia from January 2016 through April 2021.
Postoperative hypothermia was identified as an area of potential improvement after several patients were reported to be hypothermic upon arrival to the recovery unit in the later part of 2018. Further review revealed significant heterogeneity of practices and lack of standardization of patient-warming methods. By comparing the temperatures pre- and postintervention, we could measure the effectiveness of the QI initiative. Prior to the start of our project, the hypothermia rate in our patient population was not actively tracked, and the effectiveness of our variable practice was not measured.
The cutoff for hypothermia for our QI project was defined as body temperature below 36 °C, since this value has been previously used in the literature and is commonly accepted in anesthesia practice as the delineation for hypothermia in patients undergoing general anesthesia.1
Interventions
This QI project was designed and modeled after the Institute for Healthcare Improvement Model for Improvement.15 Three cycles of Plan-Do-Study-Act (PDSA) were developed and instituted over a 14-month period until December 2019 (Table 1).
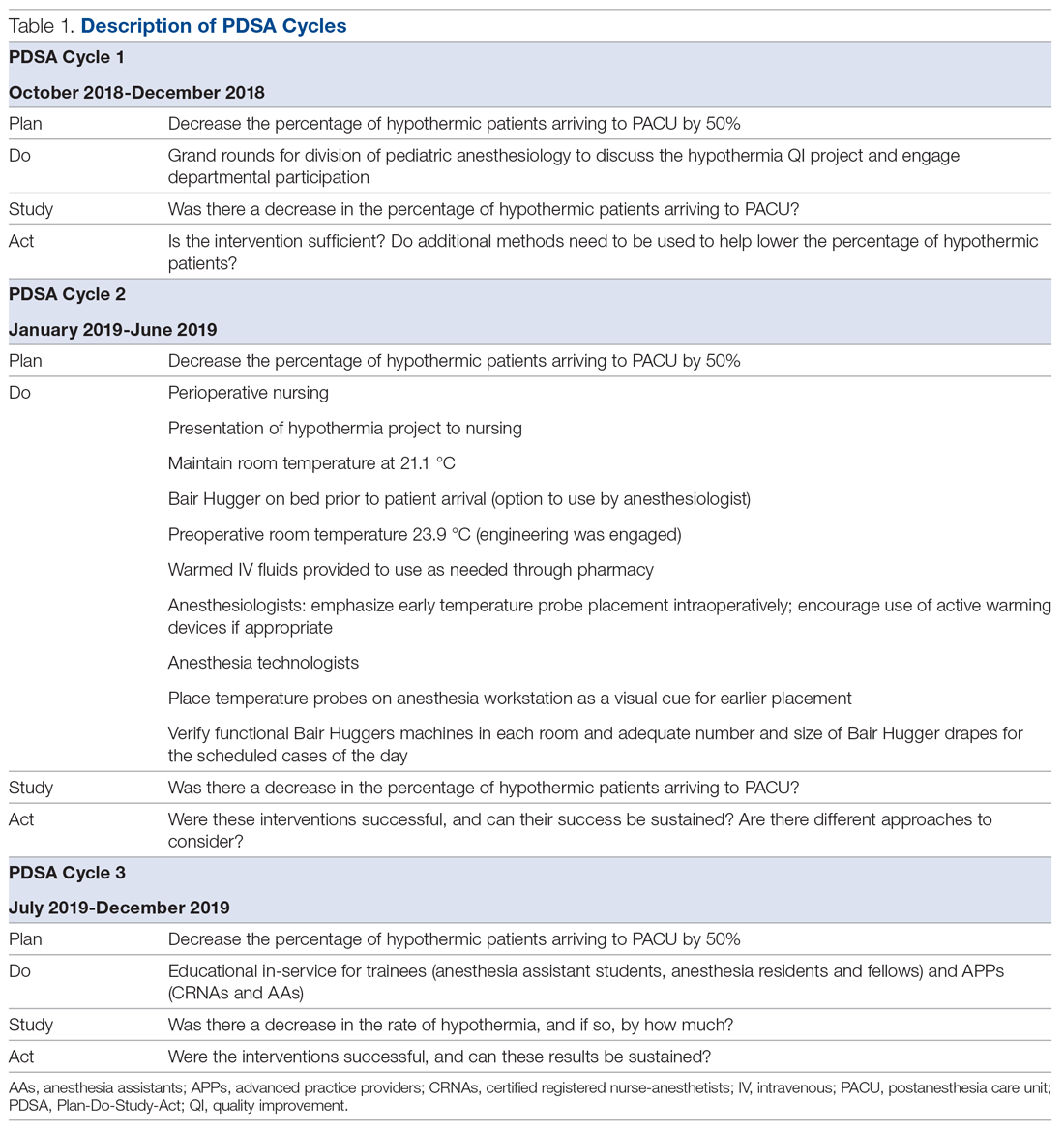
A retrospective review was conducted to determine the percentage of surgical patients arriving to our recovery units with an initial temperature reading of less than 36 °C. A project key driver diagram and smart aim were created and approved by the hospital’s continuing medical education (CME) committee for credit via the American Board of Medical Specialties (ABMS) Multi-Specialty Portfolio Program, Maintenance of Certification in Anesthesiology (MOCA) Part 4.
The first PDSA cycle involved introducing the QI project and sharing the aims of the project at a department grand rounds in the latter part of October 2018. Enrollment to participate in the project was open to all anesthesiologists in the division, and participants could earn up to 20 hours of MOCA Part 4 credits. A spreadsheet was developed and maintained to track each anesthesiologist’s monthly percentage of hypothermic patients. The de-identified patient data were shared with the division via monthly emails. In addition, individual providers with a hypothermic patient in the recovery room received a notification email.
The anesthesiologists participated in the QI project by reviewing their personal percentage of hypothermic patients on an ongoing basis to earn the credit. There was no explicit requirement to decrease their own rate of patients with body temperature less than 36 °C or expectation to achieve a predetermined goal, so the participants could not “fail.”
Because of the large interest in this project, a hypothermia committee was formed that consisted of 36 anesthesiologists. This group reviewed the data and exchanged ideas for improvement in November 2018 as part of the first PDSA cycle. The committee met monthly and was responsible for actively engaging other members of the department and perioperative staff to help in this multidisciplinary effort of combating hypothermia in our surgical pediatric population.
PDSA cycle 2 involved several major initiatives, including direct incorporation of the rest of the perioperative team. The perioperative nursing team was educated on the risks of hypothermia and engaged to take an active role by maintaining the operating suite temperature at 21.1 °C and turning on the Bair Hugger (3M) blanket to 43 °C on the OR bed prior to patient arrival to the OR. Additionally, anesthesia technicians (ATs) were tasked with ensuring an adequate supply of Bair Hugger drapes for all cases of the day. The facility’s engineering team was engaged to move the preoperative room temperature controls away from families (who frequently made the rooms cold) and instead set it at a consistent temperature of 23.9 °C. ATs were also asked to place axillary and nasal temperature probes on the anesthesia workstations as a visual reminder to facilitate temperature monitoring closer to the start of anesthesia (instead of the anesthesia provider having to remember to retrieve a temperature probe out of a drawer and place it on the patient). Furthermore, anesthesiologists were instructed via the aforementioned monthly emails and at monthly department meetings to place the temperature probes as early as possible in order to recognize and respond to intraoperative hypothermia in a timelier manner. Finally, supply chain leaders were informed of our expected increase in the use of the blankets and probes and proportionally increased ordering of these supplies to make sure availability would not present an obstacle.
In PDSA cycle 3, trainees (anesthesia assistant students, anesthesia residents and fellows) and advanced practice providers (APPs) (certified registered nurse-anesthetists [CRNAs] and certified anesthesia assistants [C-AAs]) were informed of the QI project. This initiative was guided toward improving vigilance for hypothermia in the rest of the anesthesia team members. The trainees and APPs usually set up the anesthesia area prior to patient arrival, so their recruitment in support of this effort would ensure appropriate OR temperature, active warming device deployment, and the availability and early placement of the correct temperature probe for the case. To facilitate personal accountability, the trainees and APPs were also emailed their own patients’ rate of hypothermia.
Along the course of the project, quarterly committee meetings and departmental monthly meetings served as venues to express concerns and look for areas of improvement, such as specific patterns or trends leading to hypothermic patients. One specific example was the identification of the gastrointestinal endoscopic patients having a rate of hypothermia that was 2% higher than average. Directed education on the importance of Bair Hugger blankets and using warm intravenous fluids worked well to decrease the rate of hypothermia in these patients. This collection of data was shared at regular intervals during monthly department meetings as well and more frequently using departmental emails. The hospital’s secure intranet SharePoint (Microsoft) site was used to share the data among providers.
Study of the interventions and measures
To study the effectiveness and impact of the project to motivate our anesthesiologists and other team members, we compared the first temperatures obtained in the recovery unit prior to the start of the intervention with those collected after the start of the QI project in November 2018. Because of the variability of temperature monitoring intraoperatively (nasal, axillary, rectal), we decided to use the temperature obtained by the nurse in the recovery room upon the patient’s arrival. Over the years analyzed, the nurse’s technique of measuring the temperature remained consistent. All patient temperature measurements were performed using the TAT-5000 (Exergen Corporation). This temporal artery thermometer has been previously shown to correlate well with bladder temperatures (70% of measurements differ by no more than 0.5 °C, as reported by Langham et al16).
Admittedly, we could not measure the degree of motivation or internalization of the project goals by our cohort, but we could measure the reduction in the rate of hypothermia and subjectively gauge engagement in the project by the various groups of participants and the sustainability of the results. In addition, all participating anesthesiologists received MOCA Part 4 credits in July 2019. We continued our data collection until April 2021 to determine if our project had brought about sustainable changes in practice that would continue past the initial motivator of obtaining CME credit.
Analysis
Data analysis was performed using Excel (Microsoft) and SAS, version 9.4 (SAS Institute).
The median of the monthly percentage of patients with a temperature of less than 36.0 °C was also determined for the preintervention time frame. This served as our baseline hypothermia rate, and we aimed to lower it by 50%. Run charts, a well-described methodology to gauge the effectiveness of the QI project, were constructed with the collected data.17
We performed additional analysis to adjust for different time periods throughout the year. The time period between January 2016 and October 2018 was considered preintervention. We considered November 2018 the start of our intervention, or more specifically, the start of our PDSA cycles. October 2018 was analyzed as part of the preintervention data. To account for seasonal temperature variations, the statistical analysis focused on the comparisons of the same calendar quarters for before and after starting intervention using Wilcoxon Mann-Whitney U tests. To reach an overall conclusion, the probabilities for the 4 quarters were combined for each criterion separately utilizing the Fisher χ2 combined probability method.
The hypothermia QI project was reviewed by the institutional review board and determined to be exempt.
Results
The temperatures of 40 875 patients were available for analysis for the preintervention period between January 2016 and October 2018. The median percentage of patients with temperatures less than 36.0 °C was 6.9% (interquartile range [IQR], 5.8%-8.4%). The highest percentage was in February 2016 (9.9%), and the lowest was in March 2018 (3.4%). Following the start of the first PDSA cycle, the next 6 consecutive rates of hypothermia were below the median preintervention value, and a new median for these percentages was calculated at 3.4% (IQR, 2.6%-4.3%). In July 2019, the proportion of hypothermic patients decreased once more for 6 consecutive months, yielding a new median of 1.6% (IQR, 1.2%-1.8%) and again in July 2020, to yield a median of 1.3% (IQR, 1.2%-1.5%) (Figure). In all, 33 799 patients were analyzed after the start of the project from November 2018 to the end of the data collection period through April 2021.
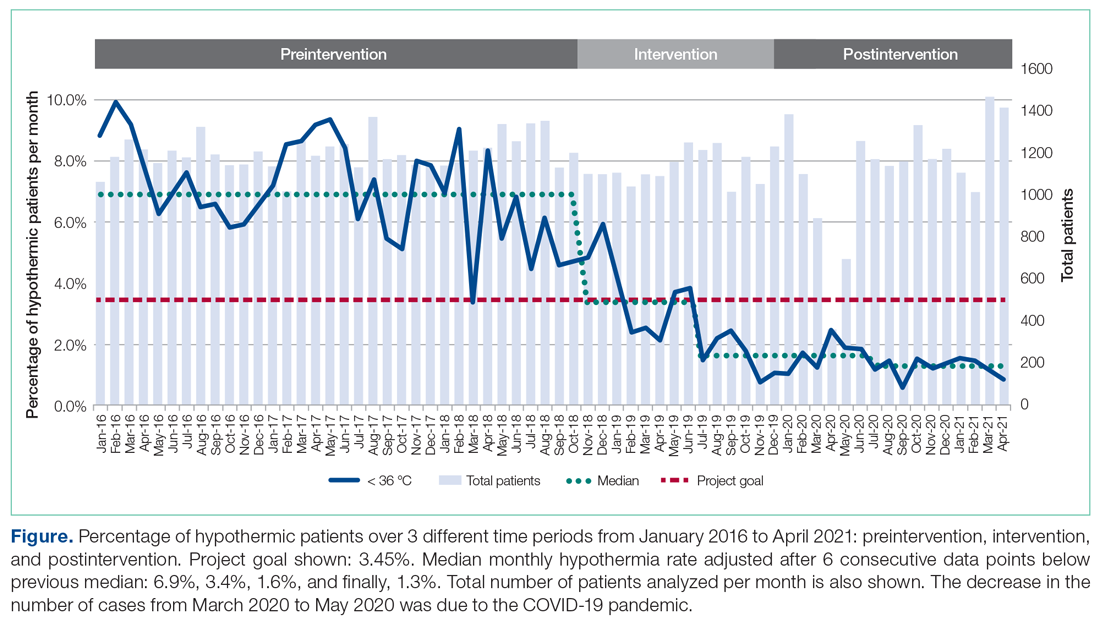
The preintervention monthly rates of hypothermia were compared, quarter to quarter, with those starting in November 2018 using the Wilcoxon Mann-Whitney U test. The decrease in proportion of hypothermic patients after the start of the intervention was statistically significant (P < .001). In addition, the percentage of patients with temperatures greater than 38 °C was not significantly different between the pre- and postintervention time periods (P < .25) (Table 2). The decrease in the number of patients available for analysis from March 2020 to May 2020 was due to the COVID-19 pandemic.
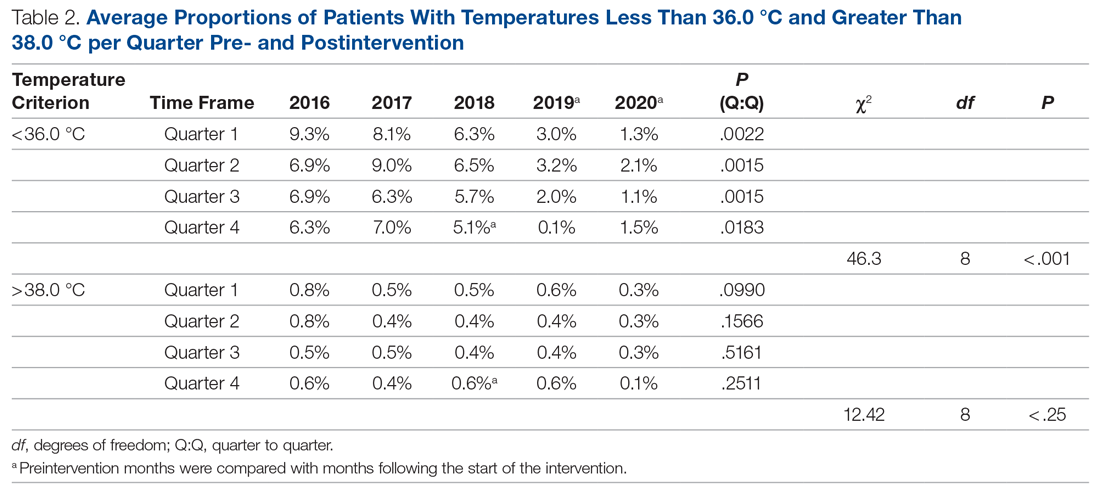
Subjectively, we did not experience any notable resistance to our efforts, and the experience was largely positive for everyone involved. Clinicians identified as having high monthly rates of hypothermia (5% or higher) corrected their numbers the following month after being notified via email or in person.
Discussion
To achieve changes in practice, the health care industry has relied on instituting guidelines, regulations, and policies, often with punitive consequences. We call into question this long-standing framework and propose a novel approach to help evolve the field of QI. Studies in human psychology have long demonstrated the demotivation power of a reward system and the negative response to attempts by authority to use incentives to control or coerce. In our QI project, we instituted 3 PDSA cycles and applied elements from SDT to motivate people’s behaviors. We demonstrate how a new culture focused on maintaining intraoperative normothermia was developed and brought about a measurable and significant decrease in the rate of hypothermia. The relevance of SDT, a widely accepted unifying theory that bridges and links social and personality psychology, should not be understated in health care. Authorities wishing to have long-standing influence should consider a person’s right to make their own decisions and, if possible, a unique way of doing things.
Positively reinforcing behavior has been shown to have a paradoxical effect by dampening an individual’s intrinsic motivation or desire to perform certain tasks.18 Deadlines, surveillance, and authoritative commands are also deterrents.19,20 We focused on providing the tools and information to the clinicians and relied on their innate need for autonomy, growth, and self-actualization to bring about change in clinical practice.21 Group meetings served as a construct for exchanging ideas and to encourage participation, but without the implementation of rigid guidelines or policies. Intraoperative active warming devices and temperature probes were made available, but their use was not mandated. The use of these devices was intentionally not audited to avoid any overbearing control. Providers were, however, given monthly temperature data to help individually assess the effectiveness of their interventions. We did not impose any negative or punitive actions for those clinicians who had high rates of hypothermic patients, and we did not reward those who had low rates of hypothermia. We wanted the participants to feel that the inner self was the source of their behavior, and this was in parallel with their own interests and values. If providers could feel their need for competency could be realized, we hoped they would continue to adhere to the measures we provided to maintain a low rate of hypothermia.
The effectiveness of our efforts was demonstrated by a decrease in the prevalence of postoperative hypothermia in our surgical patients. The initial decrease of the median rate of hypothermia from 6.9% to 3.4% occurred shortly into the start of the first PDSA cycle. The second PDSA cycle started in January 2019 with a multimodal approach and included almost all parties involved in the perioperative care of our surgical patients. Not only was this intervention responsible for a continued downward trend in the percentage of hypothermic patients, but it set the stage for the third and final PDSA cycle, which started in July 2019. The architecture was in place to integrate trainees and APPs to reinforce our initiative. Subsequently, the new median percentage of hypothermic patients was further decreased to an all-time low of 1.6% per month, satisfying and surpassing the goal of the QI project of decreasing the rate of hypothermia by only 50%. Our organization thereafter maintained a monthly hypothermia rate below 2%, except for April 2020, when it reached 2.5%. Our lowest median percentage was obtained after July 2020, reaching 1.3%.
To account for seasonal variations in temperatures and types of surgeries performed, we compared the percentage of hypothermic patients before and after the start of intervention, quarter by quarter. The decrease in the proportion of hypothermic patients after the start of intervention was statistically significant (P < .001). In addition, the data failed to prove any statistical difference for temperatures above 38 °C between the 2 periods, indicating that our interventions did not result in significant overwarming of patients. The clinical implications of decreasing the percentage of hypothermic patients from 6.9% to 1.3% is likely clinically important when considering the large number of patients who undergo surgery at large tertiary care pediatric centers. Even if simple interventions reduce hypothermia in only a handful of patients, routine applications of simple measures to keep patients normothermic is likely best clinical practice.
Anesthesiologists who participated in the hypothermia QI project by tracking the incidence of hypothermia in their patients were able to collect MOCA Part 4 credits in July 2019. There was no requirement for the individual anesthesiologist to reduce the rate of hypothermia or apply any of the encouraged strategies to obtain credit. As previously stated, there were also no rewards for obtaining low hypothermia rates for the providers. The temperature data continued to be collected through April 2021, 21 months after the credits were distributed, to demonstrate a continued, meaningful change, at least in the short-term. While the MOCA Part 4 credits likely served as an initial motivating factor to encourage participation in the QI project, they certainly were not responsible for the sustained low hypothermia rate after July 2019. We showed that the low rate of hypothermia was successfully maintained, indicating that the change in providers’ behavior was independent of the external motivator of obtaining the credit hours. Mere participation in the project by reviewing one’s temperature data was all that was required to obtain the credit. The Organismic Integration Theory, a mini-theory within SDT, best explains this phenomenon by describing any motivated behavior on a continuum ranging from controlled to autonomous.22 Do people perform the task resentfully, on their own volition because they believe it is the correct action, or somewhere in between? We explain the sustained low rates of hypothermia after the MOCA credits were distributed due to a shift to the autonomous end of the continuum with the clinician’s active willingness to meet the challenges and apply intrinsically motivated behaviors to lower the rate of hypothermia. The internalization of external motivators is difficult to prove, but the evidence supports that the methods we used to motivate individuals were effective and have resulted in a significant downward trend in our hypothermia rate.
There are several limitations to our QI project. The first involves the measuring of postoperative temperature in the recovery units. The temperatures were obtained using the same medical-grade infrared thermometer for all the patients, but other variables, such as timing and techniques, were not standardized. Secondly, overall surgical outcomes related to hypothermia were not tracked because we were unable to control for other confounding variables in our large cohort of patients, so we cannot say if the drop in the hypothermia rate had a clinically significant outcome. Thirdly, we propose that SDT offers a compellingly fitting explanation of the psychology of motivation in our efforts, but it may be possible that other theories may offer equally fitting explanations. The ability to measure the degree of motivation is lacking, and we did not explicitly ask participants what their specific source of motivation was. Aside from SDT, the reduction in hypothermia rate could also be attributed to the ease and availability of warming equipment that was made in each OR. This QI project was successfully applied to only 1 institution, so its ability to be widely applicable remains uncertain. In addition, data collection continued during the COVID-19 pandemic when case volumes decreased. However, by June 2020, the number of surgical cases at our institution had largely returned to prepandemic levels. Additional data collection beyond April 2021 would be helpful to determine if the reduction in hypothermia rates is truly sustained.
Conclusion
Overall, the importance of maintaining perioperative normothermia was well disseminated and agreed upon by all departments involved. Despite the limitations of the project, there was a significant reduction in rates of hypothermia, and sustainability of outcomes was consistently demonstrated in the poststudy period.
Using 3 cycles of the PDSA method, we successfully decreased the median rate of postoperative hypothermia in our pediatric surgical population from a preintervention value of 6.9% to 1.3%—a reduction of more than 81.2%. We provided motivation for members of our anesthesiology staff to participate by offering MOCA 2.0 Part 4 credits, but the lower rate of hypothermic patients was maintained for 15 months after the credits were distributed. Over the course of the project, there was a shift in culture, and extra vigilance was given to temperature monitoring and assessment. We attribute this sustained cultural change to the deliberate incorporation of the principles of competency, autonomy, and relatedness central to SDT to the structure of the interventions, avoiding rigid guidelines and pathways in favor of affective engagement to establish intrinsic motivation.
Acknowledgements: The authors thank Joan Reisch, PhD, for her assistance with the statistical analysis.
Corresponding author: Edgar Erold Kiss, MD, 1935 Medical District Dr, Dallas, TX 75235; [email protected].
Financial disclosures: None.
1. Leslie K, Sessler DI. Perioperative hypothermia in the high-risk surgical patient. Best Pract Res Clin Anaesthesiol. 2003;17(4):485-498.
2. Sessler DI. Forced-air warming in infants and children. Paediatr Anaesth. 2013;23(6):467-468.
3. Wetzel RC. Evaluation of children. In: Longnecker DE, Tinker JH, Morgan Jr GE, eds. Principles and Practice of Anesthesiology. 2nd ed. Mosby Publishers; 1999:445-447.
4. Witt L, Dennhardt N, Eich C, et al. Prevention of intraoperative hypothermia in neonates and infants: results of a prospective multicenter observational study with a new forced-air warming system with increased warm air flow. Paediatr Anaesth. 2013;23(6):469-474.
5. Blum R, Cote C. Pediatric equipment. In: Blum R, Cote C, eds. A Practice of Anaesthesia for Infants and Children. Saunders Elsevier; 2009:1099-1101.
6. Doufas AG. Consequences of inadvertent perioperative hypothermia. Best Pract Res Clin Anaesthesiol. 2003;17(4):535-549.
7. Mahoney CB, Odom J. Maintaining intraoperative normothermia: a meta-analysis of outcomes with costs. AANA J. 1999;67(2):155-163.
8. American Society of Anesthesiologists Committee on Standards and Practice Parameters. Standards for Basic Anesthetic Monitoring. Approved by the ASA House of Delegates October 21, 1986; last amended October 20, 2010; last affirmed October 28, 2015.
9. Horn E-P, Bein B, Böhm R, et al. The effect of short time periods of pre-operative warming in the prevention of peri-operative hypothermia. Anaesthesia. 2012;67(6):612-617.
10. Andrzejowski J, Hoyle J, Eapen G, Turnbull D. Effect of prewarming on post-induction core temperature and the incidence of inadvertent perioperative hypothermia in patients undergoing general anaesthesia. Br J Anaesth. 2008;101(5):627-631.
11. Sessler DI. Complications and treatment of mild hypothermia. Anesthesiology. 2001;95(2):531-543.
12. Bräuer A, English MJM, Steinmetz N, et al. Efficacy of forced-air warming systems with full body blankets. Can J Anaesth. 2007;54(1):34-41.
13. Deci EL, Ryan RM. The “what” and “why” of goal pursuits: human needs and the self‐determination of behavior. Psychol Inquiry. 2000;11(4):227-268.
14. Al-Shamari M, Puttha R, Yuen S, et al. G9 Can introduction of a hypothermia bundle reduce hypothermia in the newborns? Arch Dis Childhood. 2019;104(suppl 2):A4.1-A4.
15. Institute for Healthcare Improvement. How to improve. Accessed May 12, 2021. http://www.ihi.org/resources/Pages/HowtoImprove/default.aspx
16. Langham GE, Meheshwari A, You J, et al. Noninvasive temperature monitoring in postanesthesia care units. Anesthesiology. 2009;111(1):90-96.
17. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning from variation in healthcare processes. BMJ Qual Saf. 2011;20(1):46-51.
18. Deci EL. Effects of externally mediated rewards on intrinsic motivation. J Pers Soc Psychol. 1971;18(1):105-115.
19. Deci EL, Koestner R, Ryan RM. A meta-analytic review of experiments examining the effects of extrinsic rewards on intrinsic motivation. Psychol Bull. 1999;125(6):627-668.
20. Deci EL, Koestner R, Ryan RM. The undermining effect is a reality after all—extrinsic rewards, task interest, and self-determination: Reply to Eisenberger, Pierce, and Cameron (1999) and Lepper, Henderlong, and Gingras (1999). Psychol Bull. 1999;125(6):692-700.
21. Maslow A. The Farther Reaches of Human Nature. Viking Press; 1971.
22. Sheldon KM, Prentice M. Self-determination theory as a foundation for personality researchers. J Pers. 2019;87(1):5-14.
From Children’s Health System of Texas, Division of Pediatric Anesthesiology, Dallas, TX (Drs. Sakhai, Bocanegra, Chandran, Kimatian, and Kiss), UT Southwestern Medical Center, Department of Anesthesiology and Pain Management, Dallas, TX (Drs. Bocanegra, Chandran, Kimatian, and Kiss), and UT Southwestern Medical Center, Department of Population and Data Sciences, Dallas, TX (Dr. Reisch).
Objective: Policy-driven changes in medical practice have long been the norm. Seldom are changes in clinical practice sought to be brought about by a person’s tendency toward growth or self‐actualization. Many hospitals have instituted hypothermia bundles to help reduce the incidence of unanticipated postoperative hypothermia. Although successful in the short-term, sustained changes are difficult to maintain. We implemented a quality-improvement project focused on addressing the affective components of self-determination theory (SDT) to create sustainable behavioral change while satisfying providers’ basic psychological needs for autonomy, competence, and relatedness.
Methods: A total of 3 Plan-Do-Study-Act (PDSA) cycles were enacted over the span of 14 months at a major tertiary care pediatric hospital to recruit and motivate anesthesia providers and perioperative team members to reduce the percentage of hypothermic postsurgical patients by 50%. As an optional initial incentive for participation, anesthesiologists would qualify for American Board of Anesthesiology Maintenance of Certification in Anesthesiology (MOCA) Part 4 Quality Improvement credits for monitoring their own temperature data and participating in project-related meetings. Providers were given autonomy to develop a personal plan for achieving the desired goals.
Results: The median rate of hypothermia was reduced from 6.9% to 1.6% in July 2019 and was reduced again in July 2020 to 1.3%, an 81% reduction overall. A low hypothermia rate was successfully maintained for at least 21 subsequent months after participants received their MOCA credits in July 2019.
Conclusions: Using an approach that focused on the elements of competency, autonomy, and relatedness central to the principles of SDT, we observed the development of a new culture of vigilance for prevention of hypothermia that successfully endured beyond the project end date.
Keywords: postoperative hypothermia; self-determination theory; motivation; quality improvement.
Perioperative hypothermia, generally accepted as a core temperature less than 36 °C in clinical practice, is a common complication in the pediatric surgical population and is associated with poor postoperative outcomes.1 Hypothermic patients may develop respiratory depression, hypoglycemia, and metabolic acidosis that may lead to decreased oxygen delivery and end organ tissue hypoxia.2-4 Other potential detrimental effects of failing to maintain normal body temperature are impaired clotting factor enzyme function and platelet dysfunction, increasing the risk for postoperative bleeding.5,6 In addition, there are financial implications when hypothermic patients require care and resources postoperatively because of delayed emergence or shivering.7
The American Society of Anesthesiologists recommends intraoperative temperature monitoring for procedures when clinically significant changes in body temperature are anticipated.8 Maintenance of normothermia in the pediatric population is especially challenging owing to a larger skin-surface area compared with body mass ratio and less subcutaneous fat content than in adults. Preventing postoperative hypothermia starts preoperatively with parental education and can be as simple as covering the child with a blanket and setting the preoperative room to an acceptably warm temperature.9,10 Intraoperatively, maintaining operating room (OR) temperatures at or above 21.1 °C and using active warming devices and radiant warmers when appropriate are important techniques to preserve the child’s body temperature.11,12
Despite the knowledge of these risks and vigilant avoidance of hypothermia, unplanned perioperative hypothermia can occur in up to 70% of surgical patients.1 Beyond the clinical benefits, as health care marches toward a value-based payment methodology, quality indicators such as avoiding hypothermia may be linked directly to payment.
Self-determination theory (SDT) was first developed in 1980 by Deci and Ryan.13 The central premise of the theory states that people develop their full potential if circumstances allow them to satisfy their basic psychological needs: autonomy, competence, and relatedness. Under these conditions, people’s natural inclination toward growth can be realized, and they are more likely to internalize external goals. Under an extrinsic reward system, motivation can waver, as people may perceive rewards as controlling.
Many institutions have implemented hypothermia bundles to help decrease the rate of hypothermic patients, but while initially successful, the effectiveness of these interventions tends to fade over time as participants settle into old, comfortable routines.14 With SDT in mind, we designed our quality-improvement (QI) project with interventions to allow clinicians autonomy without instituting rigid guidelines or punitive actions. We aimed to directly address the affective components central to motivation and engagement so that we could bring about long-term meaningful changes in our practice.
Methods
Setting
The hypothermia QI intervention was instituted at a major tertiary care children’s hospital that performs more than 40 000 pediatric general anesthetics annually. Our division of pediatric anesthesiology consists of 66 fellowship-trained pediatric anesthesiologists, 15 or more rotating trainees per month, 13 anesthesiology assistants, 15 anesthesia technicians, and more than 50 perioperative nurses.
The most frequent pediatric surgeries include, but are not limited to, general surgery, otolaryngology, urology, gastroenterology, plastic surgery, neurosurgery, and dentistry. The surgeries are conducted in the hospital’s main operative floor, which consists of 15 ORs and 2 gastroenterology procedure rooms. Although the implementation of the QI project included several operating sites, we focused on collecting temperature data from surgical patients at our main campus recovery unit. We obtained the patients’ initial temperatures upon arrival to the recovery unit from a retrospective electronic health record review of all patients who underwent anesthesia from January 2016 through April 2021.
Postoperative hypothermia was identified as an area of potential improvement after several patients were reported to be hypothermic upon arrival to the recovery unit in the later part of 2018. Further review revealed significant heterogeneity of practices and lack of standardization of patient-warming methods. By comparing the temperatures pre- and postintervention, we could measure the effectiveness of the QI initiative. Prior to the start of our project, the hypothermia rate in our patient population was not actively tracked, and the effectiveness of our variable practice was not measured.
The cutoff for hypothermia for our QI project was defined as body temperature below 36 °C, since this value has been previously used in the literature and is commonly accepted in anesthesia practice as the delineation for hypothermia in patients undergoing general anesthesia.1
Interventions
This QI project was designed and modeled after the Institute for Healthcare Improvement Model for Improvement.15 Three cycles of Plan-Do-Study-Act (PDSA) were developed and instituted over a 14-month period until December 2019 (Table 1).

A retrospective review was conducted to determine the percentage of surgical patients arriving to our recovery units with an initial temperature reading of less than 36 °C. A project key driver diagram and smart aim were created and approved by the hospital’s continuing medical education (CME) committee for credit via the American Board of Medical Specialties (ABMS) Multi-Specialty Portfolio Program, Maintenance of Certification in Anesthesiology (MOCA) Part 4.
The first PDSA cycle involved introducing the QI project and sharing the aims of the project at a department grand rounds in the latter part of October 2018. Enrollment to participate in the project was open to all anesthesiologists in the division, and participants could earn up to 20 hours of MOCA Part 4 credits. A spreadsheet was developed and maintained to track each anesthesiologist’s monthly percentage of hypothermic patients. The de-identified patient data were shared with the division via monthly emails. In addition, individual providers with a hypothermic patient in the recovery room received a notification email.
The anesthesiologists participated in the QI project by reviewing their personal percentage of hypothermic patients on an ongoing basis to earn the credit. There was no explicit requirement to decrease their own rate of patients with body temperature less than 36 °C or expectation to achieve a predetermined goal, so the participants could not “fail.”
Because of the large interest in this project, a hypothermia committee was formed that consisted of 36 anesthesiologists. This group reviewed the data and exchanged ideas for improvement in November 2018 as part of the first PDSA cycle. The committee met monthly and was responsible for actively engaging other members of the department and perioperative staff to help in this multidisciplinary effort of combating hypothermia in our surgical pediatric population.
PDSA cycle 2 involved several major initiatives, including direct incorporation of the rest of the perioperative team. The perioperative nursing team was educated on the risks of hypothermia and engaged to take an active role by maintaining the operating suite temperature at 21.1 °C and turning on the Bair Hugger (3M) blanket to 43 °C on the OR bed prior to patient arrival to the OR. Additionally, anesthesia technicians (ATs) were tasked with ensuring an adequate supply of Bair Hugger drapes for all cases of the day. The facility’s engineering team was engaged to move the preoperative room temperature controls away from families (who frequently made the rooms cold) and instead set it at a consistent temperature of 23.9 °C. ATs were also asked to place axillary and nasal temperature probes on the anesthesia workstations as a visual reminder to facilitate temperature monitoring closer to the start of anesthesia (instead of the anesthesia provider having to remember to retrieve a temperature probe out of a drawer and place it on the patient). Furthermore, anesthesiologists were instructed via the aforementioned monthly emails and at monthly department meetings to place the temperature probes as early as possible in order to recognize and respond to intraoperative hypothermia in a timelier manner. Finally, supply chain leaders were informed of our expected increase in the use of the blankets and probes and proportionally increased ordering of these supplies to make sure availability would not present an obstacle.
In PDSA cycle 3, trainees (anesthesia assistant students, anesthesia residents and fellows) and advanced practice providers (APPs) (certified registered nurse-anesthetists [CRNAs] and certified anesthesia assistants [C-AAs]) were informed of the QI project. This initiative was guided toward improving vigilance for hypothermia in the rest of the anesthesia team members. The trainees and APPs usually set up the anesthesia area prior to patient arrival, so their recruitment in support of this effort would ensure appropriate OR temperature, active warming device deployment, and the availability and early placement of the correct temperature probe for the case. To facilitate personal accountability, the trainees and APPs were also emailed their own patients’ rate of hypothermia.
Along the course of the project, quarterly committee meetings and departmental monthly meetings served as venues to express concerns and look for areas of improvement, such as specific patterns or trends leading to hypothermic patients. One specific example was the identification of the gastrointestinal endoscopic patients having a rate of hypothermia that was 2% higher than average. Directed education on the importance of Bair Hugger blankets and using warm intravenous fluids worked well to decrease the rate of hypothermia in these patients. This collection of data was shared at regular intervals during monthly department meetings as well and more frequently using departmental emails. The hospital’s secure intranet SharePoint (Microsoft) site was used to share the data among providers.
Study of the interventions and measures
To study the effectiveness and impact of the project to motivate our anesthesiologists and other team members, we compared the first temperatures obtained in the recovery unit prior to the start of the intervention with those collected after the start of the QI project in November 2018. Because of the variability of temperature monitoring intraoperatively (nasal, axillary, rectal), we decided to use the temperature obtained by the nurse in the recovery room upon the patient’s arrival. Over the years analyzed, the nurse’s technique of measuring the temperature remained consistent. All patient temperature measurements were performed using the TAT-5000 (Exergen Corporation). This temporal artery thermometer has been previously shown to correlate well with bladder temperatures (70% of measurements differ by no more than 0.5 °C, as reported by Langham et al16).
Admittedly, we could not measure the degree of motivation or internalization of the project goals by our cohort, but we could measure the reduction in the rate of hypothermia and subjectively gauge engagement in the project by the various groups of participants and the sustainability of the results. In addition, all participating anesthesiologists received MOCA Part 4 credits in July 2019. We continued our data collection until April 2021 to determine if our project had brought about sustainable changes in practice that would continue past the initial motivator of obtaining CME credit.
Analysis
Data analysis was performed using Excel (Microsoft) and SAS, version 9.4 (SAS Institute).
The median of the monthly percentage of patients with a temperature of less than 36.0 °C was also determined for the preintervention time frame. This served as our baseline hypothermia rate, and we aimed to lower it by 50%. Run charts, a well-described methodology to gauge the effectiveness of the QI project, were constructed with the collected data.17
We performed additional analysis to adjust for different time periods throughout the year. The time period between January 2016 and October 2018 was considered preintervention. We considered November 2018 the start of our intervention, or more specifically, the start of our PDSA cycles. October 2018 was analyzed as part of the preintervention data. To account for seasonal temperature variations, the statistical analysis focused on the comparisons of the same calendar quarters for before and after starting intervention using Wilcoxon Mann-Whitney U tests. To reach an overall conclusion, the probabilities for the 4 quarters were combined for each criterion separately utilizing the Fisher χ2 combined probability method.
The hypothermia QI project was reviewed by the institutional review board and determined to be exempt.
Results
The temperatures of 40 875 patients were available for analysis for the preintervention period between January 2016 and October 2018. The median percentage of patients with temperatures less than 36.0 °C was 6.9% (interquartile range [IQR], 5.8%-8.4%). The highest percentage was in February 2016 (9.9%), and the lowest was in March 2018 (3.4%). Following the start of the first PDSA cycle, the next 6 consecutive rates of hypothermia were below the median preintervention value, and a new median for these percentages was calculated at 3.4% (IQR, 2.6%-4.3%). In July 2019, the proportion of hypothermic patients decreased once more for 6 consecutive months, yielding a new median of 1.6% (IQR, 1.2%-1.8%) and again in July 2020, to yield a median of 1.3% (IQR, 1.2%-1.5%) (Figure). In all, 33 799 patients were analyzed after the start of the project from November 2018 to the end of the data collection period through April 2021.

The preintervention monthly rates of hypothermia were compared, quarter to quarter, with those starting in November 2018 using the Wilcoxon Mann-Whitney U test. The decrease in proportion of hypothermic patients after the start of the intervention was statistically significant (P < .001). In addition, the percentage of patients with temperatures greater than 38 °C was not significantly different between the pre- and postintervention time periods (P < .25) (Table 2). The decrease in the number of patients available for analysis from March 2020 to May 2020 was due to the COVID-19 pandemic.

Subjectively, we did not experience any notable resistance to our efforts, and the experience was largely positive for everyone involved. Clinicians identified as having high monthly rates of hypothermia (5% or higher) corrected their numbers the following month after being notified via email or in person.
Discussion
To achieve changes in practice, the health care industry has relied on instituting guidelines, regulations, and policies, often with punitive consequences. We call into question this long-standing framework and propose a novel approach to help evolve the field of QI. Studies in human psychology have long demonstrated the demotivation power of a reward system and the negative response to attempts by authority to use incentives to control or coerce. In our QI project, we instituted 3 PDSA cycles and applied elements from SDT to motivate people’s behaviors. We demonstrate how a new culture focused on maintaining intraoperative normothermia was developed and brought about a measurable and significant decrease in the rate of hypothermia. The relevance of SDT, a widely accepted unifying theory that bridges and links social and personality psychology, should not be understated in health care. Authorities wishing to have long-standing influence should consider a person’s right to make their own decisions and, if possible, a unique way of doing things.
Positively reinforcing behavior has been shown to have a paradoxical effect by dampening an individual’s intrinsic motivation or desire to perform certain tasks.18 Deadlines, surveillance, and authoritative commands are also deterrents.19,20 We focused on providing the tools and information to the clinicians and relied on their innate need for autonomy, growth, and self-actualization to bring about change in clinical practice.21 Group meetings served as a construct for exchanging ideas and to encourage participation, but without the implementation of rigid guidelines or policies. Intraoperative active warming devices and temperature probes were made available, but their use was not mandated. The use of these devices was intentionally not audited to avoid any overbearing control. Providers were, however, given monthly temperature data to help individually assess the effectiveness of their interventions. We did not impose any negative or punitive actions for those clinicians who had high rates of hypothermic patients, and we did not reward those who had low rates of hypothermia. We wanted the participants to feel that the inner self was the source of their behavior, and this was in parallel with their own interests and values. If providers could feel their need for competency could be realized, we hoped they would continue to adhere to the measures we provided to maintain a low rate of hypothermia.
The effectiveness of our efforts was demonstrated by a decrease in the prevalence of postoperative hypothermia in our surgical patients. The initial decrease of the median rate of hypothermia from 6.9% to 3.4% occurred shortly into the start of the first PDSA cycle. The second PDSA cycle started in January 2019 with a multimodal approach and included almost all parties involved in the perioperative care of our surgical patients. Not only was this intervention responsible for a continued downward trend in the percentage of hypothermic patients, but it set the stage for the third and final PDSA cycle, which started in July 2019. The architecture was in place to integrate trainees and APPs to reinforce our initiative. Subsequently, the new median percentage of hypothermic patients was further decreased to an all-time low of 1.6% per month, satisfying and surpassing the goal of the QI project of decreasing the rate of hypothermia by only 50%. Our organization thereafter maintained a monthly hypothermia rate below 2%, except for April 2020, when it reached 2.5%. Our lowest median percentage was obtained after July 2020, reaching 1.3%.
To account for seasonal variations in temperatures and types of surgeries performed, we compared the percentage of hypothermic patients before and after the start of intervention, quarter by quarter. The decrease in the proportion of hypothermic patients after the start of intervention was statistically significant (P < .001). In addition, the data failed to prove any statistical difference for temperatures above 38 °C between the 2 periods, indicating that our interventions did not result in significant overwarming of patients. The clinical implications of decreasing the percentage of hypothermic patients from 6.9% to 1.3% is likely clinically important when considering the large number of patients who undergo surgery at large tertiary care pediatric centers. Even if simple interventions reduce hypothermia in only a handful of patients, routine applications of simple measures to keep patients normothermic is likely best clinical practice.
Anesthesiologists who participated in the hypothermia QI project by tracking the incidence of hypothermia in their patients were able to collect MOCA Part 4 credits in July 2019. There was no requirement for the individual anesthesiologist to reduce the rate of hypothermia or apply any of the encouraged strategies to obtain credit. As previously stated, there were also no rewards for obtaining low hypothermia rates for the providers. The temperature data continued to be collected through April 2021, 21 months after the credits were distributed, to demonstrate a continued, meaningful change, at least in the short-term. While the MOCA Part 4 credits likely served as an initial motivating factor to encourage participation in the QI project, they certainly were not responsible for the sustained low hypothermia rate after July 2019. We showed that the low rate of hypothermia was successfully maintained, indicating that the change in providers’ behavior was independent of the external motivator of obtaining the credit hours. Mere participation in the project by reviewing one’s temperature data was all that was required to obtain the credit. The Organismic Integration Theory, a mini-theory within SDT, best explains this phenomenon by describing any motivated behavior on a continuum ranging from controlled to autonomous.22 Do people perform the task resentfully, on their own volition because they believe it is the correct action, or somewhere in between? We explain the sustained low rates of hypothermia after the MOCA credits were distributed due to a shift to the autonomous end of the continuum with the clinician’s active willingness to meet the challenges and apply intrinsically motivated behaviors to lower the rate of hypothermia. The internalization of external motivators is difficult to prove, but the evidence supports that the methods we used to motivate individuals were effective and have resulted in a significant downward trend in our hypothermia rate.
There are several limitations to our QI project. The first involves the measuring of postoperative temperature in the recovery units. The temperatures were obtained using the same medical-grade infrared thermometer for all the patients, but other variables, such as timing and techniques, were not standardized. Secondly, overall surgical outcomes related to hypothermia were not tracked because we were unable to control for other confounding variables in our large cohort of patients, so we cannot say if the drop in the hypothermia rate had a clinically significant outcome. Thirdly, we propose that SDT offers a compellingly fitting explanation of the psychology of motivation in our efforts, but it may be possible that other theories may offer equally fitting explanations. The ability to measure the degree of motivation is lacking, and we did not explicitly ask participants what their specific source of motivation was. Aside from SDT, the reduction in hypothermia rate could also be attributed to the ease and availability of warming equipment that was made in each OR. This QI project was successfully applied to only 1 institution, so its ability to be widely applicable remains uncertain. In addition, data collection continued during the COVID-19 pandemic when case volumes decreased. However, by June 2020, the number of surgical cases at our institution had largely returned to prepandemic levels. Additional data collection beyond April 2021 would be helpful to determine if the reduction in hypothermia rates is truly sustained.
Conclusion
Overall, the importance of maintaining perioperative normothermia was well disseminated and agreed upon by all departments involved. Despite the limitations of the project, there was a significant reduction in rates of hypothermia, and sustainability of outcomes was consistently demonstrated in the poststudy period.
Using 3 cycles of the PDSA method, we successfully decreased the median rate of postoperative hypothermia in our pediatric surgical population from a preintervention value of 6.9% to 1.3%—a reduction of more than 81.2%. We provided motivation for members of our anesthesiology staff to participate by offering MOCA 2.0 Part 4 credits, but the lower rate of hypothermic patients was maintained for 15 months after the credits were distributed. Over the course of the project, there was a shift in culture, and extra vigilance was given to temperature monitoring and assessment. We attribute this sustained cultural change to the deliberate incorporation of the principles of competency, autonomy, and relatedness central to SDT to the structure of the interventions, avoiding rigid guidelines and pathways in favor of affective engagement to establish intrinsic motivation.
Acknowledgements: The authors thank Joan Reisch, PhD, for her assistance with the statistical analysis.
Corresponding author: Edgar Erold Kiss, MD, 1935 Medical District Dr, Dallas, TX 75235; [email protected].
Financial disclosures: None.
From Children’s Health System of Texas, Division of Pediatric Anesthesiology, Dallas, TX (Drs. Sakhai, Bocanegra, Chandran, Kimatian, and Kiss), UT Southwestern Medical Center, Department of Anesthesiology and Pain Management, Dallas, TX (Drs. Bocanegra, Chandran, Kimatian, and Kiss), and UT Southwestern Medical Center, Department of Population and Data Sciences, Dallas, TX (Dr. Reisch).
Objective: Policy-driven changes in medical practice have long been the norm. Seldom are changes in clinical practice sought to be brought about by a person’s tendency toward growth or self‐actualization. Many hospitals have instituted hypothermia bundles to help reduce the incidence of unanticipated postoperative hypothermia. Although successful in the short-term, sustained changes are difficult to maintain. We implemented a quality-improvement project focused on addressing the affective components of self-determination theory (SDT) to create sustainable behavioral change while satisfying providers’ basic psychological needs for autonomy, competence, and relatedness.
Methods: A total of 3 Plan-Do-Study-Act (PDSA) cycles were enacted over the span of 14 months at a major tertiary care pediatric hospital to recruit and motivate anesthesia providers and perioperative team members to reduce the percentage of hypothermic postsurgical patients by 50%. As an optional initial incentive for participation, anesthesiologists would qualify for American Board of Anesthesiology Maintenance of Certification in Anesthesiology (MOCA) Part 4 Quality Improvement credits for monitoring their own temperature data and participating in project-related meetings. Providers were given autonomy to develop a personal plan for achieving the desired goals.
Results: The median rate of hypothermia was reduced from 6.9% to 1.6% in July 2019 and was reduced again in July 2020 to 1.3%, an 81% reduction overall. A low hypothermia rate was successfully maintained for at least 21 subsequent months after participants received their MOCA credits in July 2019.
Conclusions: Using an approach that focused on the elements of competency, autonomy, and relatedness central to the principles of SDT, we observed the development of a new culture of vigilance for prevention of hypothermia that successfully endured beyond the project end date.
Keywords: postoperative hypothermia; self-determination theory; motivation; quality improvement.
Perioperative hypothermia, generally accepted as a core temperature less than 36 °C in clinical practice, is a common complication in the pediatric surgical population and is associated with poor postoperative outcomes.1 Hypothermic patients may develop respiratory depression, hypoglycemia, and metabolic acidosis that may lead to decreased oxygen delivery and end organ tissue hypoxia.2-4 Other potential detrimental effects of failing to maintain normal body temperature are impaired clotting factor enzyme function and platelet dysfunction, increasing the risk for postoperative bleeding.5,6 In addition, there are financial implications when hypothermic patients require care and resources postoperatively because of delayed emergence or shivering.7
The American Society of Anesthesiologists recommends intraoperative temperature monitoring for procedures when clinically significant changes in body temperature are anticipated.8 Maintenance of normothermia in the pediatric population is especially challenging owing to a larger skin-surface area compared with body mass ratio and less subcutaneous fat content than in adults. Preventing postoperative hypothermia starts preoperatively with parental education and can be as simple as covering the child with a blanket and setting the preoperative room to an acceptably warm temperature.9,10 Intraoperatively, maintaining operating room (OR) temperatures at or above 21.1 °C and using active warming devices and radiant warmers when appropriate are important techniques to preserve the child’s body temperature.11,12
Despite the knowledge of these risks and vigilant avoidance of hypothermia, unplanned perioperative hypothermia can occur in up to 70% of surgical patients.1 Beyond the clinical benefits, as health care marches toward a value-based payment methodology, quality indicators such as avoiding hypothermia may be linked directly to payment.
Self-determination theory (SDT) was first developed in 1980 by Deci and Ryan.13 The central premise of the theory states that people develop their full potential if circumstances allow them to satisfy their basic psychological needs: autonomy, competence, and relatedness. Under these conditions, people’s natural inclination toward growth can be realized, and they are more likely to internalize external goals. Under an extrinsic reward system, motivation can waver, as people may perceive rewards as controlling.
Many institutions have implemented hypothermia bundles to help decrease the rate of hypothermic patients, but while initially successful, the effectiveness of these interventions tends to fade over time as participants settle into old, comfortable routines.14 With SDT in mind, we designed our quality-improvement (QI) project with interventions to allow clinicians autonomy without instituting rigid guidelines or punitive actions. We aimed to directly address the affective components central to motivation and engagement so that we could bring about long-term meaningful changes in our practice.
Methods
Setting
The hypothermia QI intervention was instituted at a major tertiary care children’s hospital that performs more than 40 000 pediatric general anesthetics annually. Our division of pediatric anesthesiology consists of 66 fellowship-trained pediatric anesthesiologists, 15 or more rotating trainees per month, 13 anesthesiology assistants, 15 anesthesia technicians, and more than 50 perioperative nurses.
The most frequent pediatric surgeries include, but are not limited to, general surgery, otolaryngology, urology, gastroenterology, plastic surgery, neurosurgery, and dentistry. The surgeries are conducted in the hospital’s main operative floor, which consists of 15 ORs and 2 gastroenterology procedure rooms. Although the implementation of the QI project included several operating sites, we focused on collecting temperature data from surgical patients at our main campus recovery unit. We obtained the patients’ initial temperatures upon arrival to the recovery unit from a retrospective electronic health record review of all patients who underwent anesthesia from January 2016 through April 2021.
Postoperative hypothermia was identified as an area of potential improvement after several patients were reported to be hypothermic upon arrival to the recovery unit in the later part of 2018. Further review revealed significant heterogeneity of practices and lack of standardization of patient-warming methods. By comparing the temperatures pre- and postintervention, we could measure the effectiveness of the QI initiative. Prior to the start of our project, the hypothermia rate in our patient population was not actively tracked, and the effectiveness of our variable practice was not measured.
The cutoff for hypothermia for our QI project was defined as body temperature below 36 °C, since this value has been previously used in the literature and is commonly accepted in anesthesia practice as the delineation for hypothermia in patients undergoing general anesthesia.1
Interventions
This QI project was designed and modeled after the Institute for Healthcare Improvement Model for Improvement.15 Three cycles of Plan-Do-Study-Act (PDSA) were developed and instituted over a 14-month period until December 2019 (Table 1).

A retrospective review was conducted to determine the percentage of surgical patients arriving to our recovery units with an initial temperature reading of less than 36 °C. A project key driver diagram and smart aim were created and approved by the hospital’s continuing medical education (CME) committee for credit via the American Board of Medical Specialties (ABMS) Multi-Specialty Portfolio Program, Maintenance of Certification in Anesthesiology (MOCA) Part 4.
The first PDSA cycle involved introducing the QI project and sharing the aims of the project at a department grand rounds in the latter part of October 2018. Enrollment to participate in the project was open to all anesthesiologists in the division, and participants could earn up to 20 hours of MOCA Part 4 credits. A spreadsheet was developed and maintained to track each anesthesiologist’s monthly percentage of hypothermic patients. The de-identified patient data were shared with the division via monthly emails. In addition, individual providers with a hypothermic patient in the recovery room received a notification email.
The anesthesiologists participated in the QI project by reviewing their personal percentage of hypothermic patients on an ongoing basis to earn the credit. There was no explicit requirement to decrease their own rate of patients with body temperature less than 36 °C or expectation to achieve a predetermined goal, so the participants could not “fail.”
Because of the large interest in this project, a hypothermia committee was formed that consisted of 36 anesthesiologists. This group reviewed the data and exchanged ideas for improvement in November 2018 as part of the first PDSA cycle. The committee met monthly and was responsible for actively engaging other members of the department and perioperative staff to help in this multidisciplinary effort of combating hypothermia in our surgical pediatric population.
PDSA cycle 2 involved several major initiatives, including direct incorporation of the rest of the perioperative team. The perioperative nursing team was educated on the risks of hypothermia and engaged to take an active role by maintaining the operating suite temperature at 21.1 °C and turning on the Bair Hugger (3M) blanket to 43 °C on the OR bed prior to patient arrival to the OR. Additionally, anesthesia technicians (ATs) were tasked with ensuring an adequate supply of Bair Hugger drapes for all cases of the day. The facility’s engineering team was engaged to move the preoperative room temperature controls away from families (who frequently made the rooms cold) and instead set it at a consistent temperature of 23.9 °C. ATs were also asked to place axillary and nasal temperature probes on the anesthesia workstations as a visual reminder to facilitate temperature monitoring closer to the start of anesthesia (instead of the anesthesia provider having to remember to retrieve a temperature probe out of a drawer and place it on the patient). Furthermore, anesthesiologists were instructed via the aforementioned monthly emails and at monthly department meetings to place the temperature probes as early as possible in order to recognize and respond to intraoperative hypothermia in a timelier manner. Finally, supply chain leaders were informed of our expected increase in the use of the blankets and probes and proportionally increased ordering of these supplies to make sure availability would not present an obstacle.
In PDSA cycle 3, trainees (anesthesia assistant students, anesthesia residents and fellows) and advanced practice providers (APPs) (certified registered nurse-anesthetists [CRNAs] and certified anesthesia assistants [C-AAs]) were informed of the QI project. This initiative was guided toward improving vigilance for hypothermia in the rest of the anesthesia team members. The trainees and APPs usually set up the anesthesia area prior to patient arrival, so their recruitment in support of this effort would ensure appropriate OR temperature, active warming device deployment, and the availability and early placement of the correct temperature probe for the case. To facilitate personal accountability, the trainees and APPs were also emailed their own patients’ rate of hypothermia.
Along the course of the project, quarterly committee meetings and departmental monthly meetings served as venues to express concerns and look for areas of improvement, such as specific patterns or trends leading to hypothermic patients. One specific example was the identification of the gastrointestinal endoscopic patients having a rate of hypothermia that was 2% higher than average. Directed education on the importance of Bair Hugger blankets and using warm intravenous fluids worked well to decrease the rate of hypothermia in these patients. This collection of data was shared at regular intervals during monthly department meetings as well and more frequently using departmental emails. The hospital’s secure intranet SharePoint (Microsoft) site was used to share the data among providers.
Study of the interventions and measures
To study the effectiveness and impact of the project to motivate our anesthesiologists and other team members, we compared the first temperatures obtained in the recovery unit prior to the start of the intervention with those collected after the start of the QI project in November 2018. Because of the variability of temperature monitoring intraoperatively (nasal, axillary, rectal), we decided to use the temperature obtained by the nurse in the recovery room upon the patient’s arrival. Over the years analyzed, the nurse’s technique of measuring the temperature remained consistent. All patient temperature measurements were performed using the TAT-5000 (Exergen Corporation). This temporal artery thermometer has been previously shown to correlate well with bladder temperatures (70% of measurements differ by no more than 0.5 °C, as reported by Langham et al16).
Admittedly, we could not measure the degree of motivation or internalization of the project goals by our cohort, but we could measure the reduction in the rate of hypothermia and subjectively gauge engagement in the project by the various groups of participants and the sustainability of the results. In addition, all participating anesthesiologists received MOCA Part 4 credits in July 2019. We continued our data collection until April 2021 to determine if our project had brought about sustainable changes in practice that would continue past the initial motivator of obtaining CME credit.
Analysis
Data analysis was performed using Excel (Microsoft) and SAS, version 9.4 (SAS Institute).
The median of the monthly percentage of patients with a temperature of less than 36.0 °C was also determined for the preintervention time frame. This served as our baseline hypothermia rate, and we aimed to lower it by 50%. Run charts, a well-described methodology to gauge the effectiveness of the QI project, were constructed with the collected data.17
We performed additional analysis to adjust for different time periods throughout the year. The time period between January 2016 and October 2018 was considered preintervention. We considered November 2018 the start of our intervention, or more specifically, the start of our PDSA cycles. October 2018 was analyzed as part of the preintervention data. To account for seasonal temperature variations, the statistical analysis focused on the comparisons of the same calendar quarters for before and after starting intervention using Wilcoxon Mann-Whitney U tests. To reach an overall conclusion, the probabilities for the 4 quarters were combined for each criterion separately utilizing the Fisher χ2 combined probability method.
The hypothermia QI project was reviewed by the institutional review board and determined to be exempt.
Results
The temperatures of 40 875 patients were available for analysis for the preintervention period between January 2016 and October 2018. The median percentage of patients with temperatures less than 36.0 °C was 6.9% (interquartile range [IQR], 5.8%-8.4%). The highest percentage was in February 2016 (9.9%), and the lowest was in March 2018 (3.4%). Following the start of the first PDSA cycle, the next 6 consecutive rates of hypothermia were below the median preintervention value, and a new median for these percentages was calculated at 3.4% (IQR, 2.6%-4.3%). In July 2019, the proportion of hypothermic patients decreased once more for 6 consecutive months, yielding a new median of 1.6% (IQR, 1.2%-1.8%) and again in July 2020, to yield a median of 1.3% (IQR, 1.2%-1.5%) (Figure). In all, 33 799 patients were analyzed after the start of the project from November 2018 to the end of the data collection period through April 2021.

The preintervention monthly rates of hypothermia were compared, quarter to quarter, with those starting in November 2018 using the Wilcoxon Mann-Whitney U test. The decrease in proportion of hypothermic patients after the start of the intervention was statistically significant (P < .001). In addition, the percentage of patients with temperatures greater than 38 °C was not significantly different between the pre- and postintervention time periods (P < .25) (Table 2). The decrease in the number of patients available for analysis from March 2020 to May 2020 was due to the COVID-19 pandemic.

Subjectively, we did not experience any notable resistance to our efforts, and the experience was largely positive for everyone involved. Clinicians identified as having high monthly rates of hypothermia (5% or higher) corrected their numbers the following month after being notified via email or in person.
Discussion
To achieve changes in practice, the health care industry has relied on instituting guidelines, regulations, and policies, often with punitive consequences. We call into question this long-standing framework and propose a novel approach to help evolve the field of QI. Studies in human psychology have long demonstrated the demotivation power of a reward system and the negative response to attempts by authority to use incentives to control or coerce. In our QI project, we instituted 3 PDSA cycles and applied elements from SDT to motivate people’s behaviors. We demonstrate how a new culture focused on maintaining intraoperative normothermia was developed and brought about a measurable and significant decrease in the rate of hypothermia. The relevance of SDT, a widely accepted unifying theory that bridges and links social and personality psychology, should not be understated in health care. Authorities wishing to have long-standing influence should consider a person’s right to make their own decisions and, if possible, a unique way of doing things.
Positively reinforcing behavior has been shown to have a paradoxical effect by dampening an individual’s intrinsic motivation or desire to perform certain tasks.18 Deadlines, surveillance, and authoritative commands are also deterrents.19,20 We focused on providing the tools and information to the clinicians and relied on their innate need for autonomy, growth, and self-actualization to bring about change in clinical practice.21 Group meetings served as a construct for exchanging ideas and to encourage participation, but without the implementation of rigid guidelines or policies. Intraoperative active warming devices and temperature probes were made available, but their use was not mandated. The use of these devices was intentionally not audited to avoid any overbearing control. Providers were, however, given monthly temperature data to help individually assess the effectiveness of their interventions. We did not impose any negative or punitive actions for those clinicians who had high rates of hypothermic patients, and we did not reward those who had low rates of hypothermia. We wanted the participants to feel that the inner self was the source of their behavior, and this was in parallel with their own interests and values. If providers could feel their need for competency could be realized, we hoped they would continue to adhere to the measures we provided to maintain a low rate of hypothermia.
The effectiveness of our efforts was demonstrated by a decrease in the prevalence of postoperative hypothermia in our surgical patients. The initial decrease of the median rate of hypothermia from 6.9% to 3.4% occurred shortly into the start of the first PDSA cycle. The second PDSA cycle started in January 2019 with a multimodal approach and included almost all parties involved in the perioperative care of our surgical patients. Not only was this intervention responsible for a continued downward trend in the percentage of hypothermic patients, but it set the stage for the third and final PDSA cycle, which started in July 2019. The architecture was in place to integrate trainees and APPs to reinforce our initiative. Subsequently, the new median percentage of hypothermic patients was further decreased to an all-time low of 1.6% per month, satisfying and surpassing the goal of the QI project of decreasing the rate of hypothermia by only 50%. Our organization thereafter maintained a monthly hypothermia rate below 2%, except for April 2020, when it reached 2.5%. Our lowest median percentage was obtained after July 2020, reaching 1.3%.
To account for seasonal variations in temperatures and types of surgeries performed, we compared the percentage of hypothermic patients before and after the start of intervention, quarter by quarter. The decrease in the proportion of hypothermic patients after the start of intervention was statistically significant (P < .001). In addition, the data failed to prove any statistical difference for temperatures above 38 °C between the 2 periods, indicating that our interventions did not result in significant overwarming of patients. The clinical implications of decreasing the percentage of hypothermic patients from 6.9% to 1.3% is likely clinically important when considering the large number of patients who undergo surgery at large tertiary care pediatric centers. Even if simple interventions reduce hypothermia in only a handful of patients, routine applications of simple measures to keep patients normothermic is likely best clinical practice.
Anesthesiologists who participated in the hypothermia QI project by tracking the incidence of hypothermia in their patients were able to collect MOCA Part 4 credits in July 2019. There was no requirement for the individual anesthesiologist to reduce the rate of hypothermia or apply any of the encouraged strategies to obtain credit. As previously stated, there were also no rewards for obtaining low hypothermia rates for the providers. The temperature data continued to be collected through April 2021, 21 months after the credits were distributed, to demonstrate a continued, meaningful change, at least in the short-term. While the MOCA Part 4 credits likely served as an initial motivating factor to encourage participation in the QI project, they certainly were not responsible for the sustained low hypothermia rate after July 2019. We showed that the low rate of hypothermia was successfully maintained, indicating that the change in providers’ behavior was independent of the external motivator of obtaining the credit hours. Mere participation in the project by reviewing one’s temperature data was all that was required to obtain the credit. The Organismic Integration Theory, a mini-theory within SDT, best explains this phenomenon by describing any motivated behavior on a continuum ranging from controlled to autonomous.22 Do people perform the task resentfully, on their own volition because they believe it is the correct action, or somewhere in between? We explain the sustained low rates of hypothermia after the MOCA credits were distributed due to a shift to the autonomous end of the continuum with the clinician’s active willingness to meet the challenges and apply intrinsically motivated behaviors to lower the rate of hypothermia. The internalization of external motivators is difficult to prove, but the evidence supports that the methods we used to motivate individuals were effective and have resulted in a significant downward trend in our hypothermia rate.
There are several limitations to our QI project. The first involves the measuring of postoperative temperature in the recovery units. The temperatures were obtained using the same medical-grade infrared thermometer for all the patients, but other variables, such as timing and techniques, were not standardized. Secondly, overall surgical outcomes related to hypothermia were not tracked because we were unable to control for other confounding variables in our large cohort of patients, so we cannot say if the drop in the hypothermia rate had a clinically significant outcome. Thirdly, we propose that SDT offers a compellingly fitting explanation of the psychology of motivation in our efforts, but it may be possible that other theories may offer equally fitting explanations. The ability to measure the degree of motivation is lacking, and we did not explicitly ask participants what their specific source of motivation was. Aside from SDT, the reduction in hypothermia rate could also be attributed to the ease and availability of warming equipment that was made in each OR. This QI project was successfully applied to only 1 institution, so its ability to be widely applicable remains uncertain. In addition, data collection continued during the COVID-19 pandemic when case volumes decreased. However, by June 2020, the number of surgical cases at our institution had largely returned to prepandemic levels. Additional data collection beyond April 2021 would be helpful to determine if the reduction in hypothermia rates is truly sustained.
Conclusion
Overall, the importance of maintaining perioperative normothermia was well disseminated and agreed upon by all departments involved. Despite the limitations of the project, there was a significant reduction in rates of hypothermia, and sustainability of outcomes was consistently demonstrated in the poststudy period.
Using 3 cycles of the PDSA method, we successfully decreased the median rate of postoperative hypothermia in our pediatric surgical population from a preintervention value of 6.9% to 1.3%—a reduction of more than 81.2%. We provided motivation for members of our anesthesiology staff to participate by offering MOCA 2.0 Part 4 credits, but the lower rate of hypothermic patients was maintained for 15 months after the credits were distributed. Over the course of the project, there was a shift in culture, and extra vigilance was given to temperature monitoring and assessment. We attribute this sustained cultural change to the deliberate incorporation of the principles of competency, autonomy, and relatedness central to SDT to the structure of the interventions, avoiding rigid guidelines and pathways in favor of affective engagement to establish intrinsic motivation.
Acknowledgements: The authors thank Joan Reisch, PhD, for her assistance with the statistical analysis.
Corresponding author: Edgar Erold Kiss, MD, 1935 Medical District Dr, Dallas, TX 75235; [email protected].
Financial disclosures: None.
1. Leslie K, Sessler DI. Perioperative hypothermia in the high-risk surgical patient. Best Pract Res Clin Anaesthesiol. 2003;17(4):485-498.
2. Sessler DI. Forced-air warming in infants and children. Paediatr Anaesth. 2013;23(6):467-468.
3. Wetzel RC. Evaluation of children. In: Longnecker DE, Tinker JH, Morgan Jr GE, eds. Principles and Practice of Anesthesiology. 2nd ed. Mosby Publishers; 1999:445-447.
4. Witt L, Dennhardt N, Eich C, et al. Prevention of intraoperative hypothermia in neonates and infants: results of a prospective multicenter observational study with a new forced-air warming system with increased warm air flow. Paediatr Anaesth. 2013;23(6):469-474.
5. Blum R, Cote C. Pediatric equipment. In: Blum R, Cote C, eds. A Practice of Anaesthesia for Infants and Children. Saunders Elsevier; 2009:1099-1101.
6. Doufas AG. Consequences of inadvertent perioperative hypothermia. Best Pract Res Clin Anaesthesiol. 2003;17(4):535-549.
7. Mahoney CB, Odom J. Maintaining intraoperative normothermia: a meta-analysis of outcomes with costs. AANA J. 1999;67(2):155-163.
8. American Society of Anesthesiologists Committee on Standards and Practice Parameters. Standards for Basic Anesthetic Monitoring. Approved by the ASA House of Delegates October 21, 1986; last amended October 20, 2010; last affirmed October 28, 2015.
9. Horn E-P, Bein B, Böhm R, et al. The effect of short time periods of pre-operative warming in the prevention of peri-operative hypothermia. Anaesthesia. 2012;67(6):612-617.
10. Andrzejowski J, Hoyle J, Eapen G, Turnbull D. Effect of prewarming on post-induction core temperature and the incidence of inadvertent perioperative hypothermia in patients undergoing general anaesthesia. Br J Anaesth. 2008;101(5):627-631.
11. Sessler DI. Complications and treatment of mild hypothermia. Anesthesiology. 2001;95(2):531-543.
12. Bräuer A, English MJM, Steinmetz N, et al. Efficacy of forced-air warming systems with full body blankets. Can J Anaesth. 2007;54(1):34-41.
13. Deci EL, Ryan RM. The “what” and “why” of goal pursuits: human needs and the self‐determination of behavior. Psychol Inquiry. 2000;11(4):227-268.
14. Al-Shamari M, Puttha R, Yuen S, et al. G9 Can introduction of a hypothermia bundle reduce hypothermia in the newborns? Arch Dis Childhood. 2019;104(suppl 2):A4.1-A4.
15. Institute for Healthcare Improvement. How to improve. Accessed May 12, 2021. http://www.ihi.org/resources/Pages/HowtoImprove/default.aspx
16. Langham GE, Meheshwari A, You J, et al. Noninvasive temperature monitoring in postanesthesia care units. Anesthesiology. 2009;111(1):90-96.
17. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning from variation in healthcare processes. BMJ Qual Saf. 2011;20(1):46-51.
18. Deci EL. Effects of externally mediated rewards on intrinsic motivation. J Pers Soc Psychol. 1971;18(1):105-115.
19. Deci EL, Koestner R, Ryan RM. A meta-analytic review of experiments examining the effects of extrinsic rewards on intrinsic motivation. Psychol Bull. 1999;125(6):627-668.
20. Deci EL, Koestner R, Ryan RM. The undermining effect is a reality after all—extrinsic rewards, task interest, and self-determination: Reply to Eisenberger, Pierce, and Cameron (1999) and Lepper, Henderlong, and Gingras (1999). Psychol Bull. 1999;125(6):692-700.
21. Maslow A. The Farther Reaches of Human Nature. Viking Press; 1971.
22. Sheldon KM, Prentice M. Self-determination theory as a foundation for personality researchers. J Pers. 2019;87(1):5-14.
1. Leslie K, Sessler DI. Perioperative hypothermia in the high-risk surgical patient. Best Pract Res Clin Anaesthesiol. 2003;17(4):485-498.
2. Sessler DI. Forced-air warming in infants and children. Paediatr Anaesth. 2013;23(6):467-468.
3. Wetzel RC. Evaluation of children. In: Longnecker DE, Tinker JH, Morgan Jr GE, eds. Principles and Practice of Anesthesiology. 2nd ed. Mosby Publishers; 1999:445-447.
4. Witt L, Dennhardt N, Eich C, et al. Prevention of intraoperative hypothermia in neonates and infants: results of a prospective multicenter observational study with a new forced-air warming system with increased warm air flow. Paediatr Anaesth. 2013;23(6):469-474.
5. Blum R, Cote C. Pediatric equipment. In: Blum R, Cote C, eds. A Practice of Anaesthesia for Infants and Children. Saunders Elsevier; 2009:1099-1101.
6. Doufas AG. Consequences of inadvertent perioperative hypothermia. Best Pract Res Clin Anaesthesiol. 2003;17(4):535-549.
7. Mahoney CB, Odom J. Maintaining intraoperative normothermia: a meta-analysis of outcomes with costs. AANA J. 1999;67(2):155-163.
8. American Society of Anesthesiologists Committee on Standards and Practice Parameters. Standards for Basic Anesthetic Monitoring. Approved by the ASA House of Delegates October 21, 1986; last amended October 20, 2010; last affirmed October 28, 2015.
9. Horn E-P, Bein B, Böhm R, et al. The effect of short time periods of pre-operative warming in the prevention of peri-operative hypothermia. Anaesthesia. 2012;67(6):612-617.
10. Andrzejowski J, Hoyle J, Eapen G, Turnbull D. Effect of prewarming on post-induction core temperature and the incidence of inadvertent perioperative hypothermia in patients undergoing general anaesthesia. Br J Anaesth. 2008;101(5):627-631.
11. Sessler DI. Complications and treatment of mild hypothermia. Anesthesiology. 2001;95(2):531-543.
12. Bräuer A, English MJM, Steinmetz N, et al. Efficacy of forced-air warming systems with full body blankets. Can J Anaesth. 2007;54(1):34-41.
13. Deci EL, Ryan RM. The “what” and “why” of goal pursuits: human needs and the self‐determination of behavior. Psychol Inquiry. 2000;11(4):227-268.
14. Al-Shamari M, Puttha R, Yuen S, et al. G9 Can introduction of a hypothermia bundle reduce hypothermia in the newborns? Arch Dis Childhood. 2019;104(suppl 2):A4.1-A4.
15. Institute for Healthcare Improvement. How to improve. Accessed May 12, 2021. http://www.ihi.org/resources/Pages/HowtoImprove/default.aspx
16. Langham GE, Meheshwari A, You J, et al. Noninvasive temperature monitoring in postanesthesia care units. Anesthesiology. 2009;111(1):90-96.
17. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning from variation in healthcare processes. BMJ Qual Saf. 2011;20(1):46-51.
18. Deci EL. Effects of externally mediated rewards on intrinsic motivation. J Pers Soc Psychol. 1971;18(1):105-115.
19. Deci EL, Koestner R, Ryan RM. A meta-analytic review of experiments examining the effects of extrinsic rewards on intrinsic motivation. Psychol Bull. 1999;125(6):627-668.
20. Deci EL, Koestner R, Ryan RM. The undermining effect is a reality after all—extrinsic rewards, task interest, and self-determination: Reply to Eisenberger, Pierce, and Cameron (1999) and Lepper, Henderlong, and Gingras (1999). Psychol Bull. 1999;125(6):692-700.
21. Maslow A. The Farther Reaches of Human Nature. Viking Press; 1971.
22. Sheldon KM, Prentice M. Self-determination theory as a foundation for personality researchers. J Pers. 2019;87(1):5-14.
Long COVID seen in patients with severe and mild disease
Findings from the cohort, composed of 113 COVID-19 survivors who developed ARDS after admission to a single center before to April 16, 2020, were presented online at the 31st European Congress of Clinical Microbiology & Infectious Diseases by Judit Aranda, MD, from Complex Hospitalari Moisés Broggi in Barcelona.
Median age of the participants was 64 years, and 70% were male. At least one persistent symptom was experienced during follow-up by 81% of the cohort, with 45% reporting shortness of breath, 50% reporting muscle pain, 43% reporting memory impairment, and 46% reporting physical weakness of at least 5 on a 10-point scale.
Of the 104 participants who completed a 6-minute walk test, 30% had a decrease in oxygen saturation level of at least 4%, and 5% had an initial or final level below 88%. Of the 46 participants who underwent a pulmonary function test, 15% had a forced expiratory volume in 1 second below 70%.
And of the 49% of participants with pathologic findings on chest x-ray, most were bilateral interstitial infiltrates (88%).
In addition, more than 90% of participants developed depression, anxiety, or PTSD, Dr. Aranda reported.
Not the whole picture
This study shows that sicker people – “those in intensive care units with acute respiratory distress syndrome” – are “more likely to be struggling with more severe symptoms,” said Christopher Terndrup, MD, from the division of general internal medicine and geriatrics at Oregon Health & Science University, Portland.
But a Swiss study, also presented at the meeting, “shows how even mild COVID cases can lead to debilitating symptoms,” Dr. Terndrup said in an interview.
The investigation of long-term COVID symptoms in outpatients was presented online by Florian Desgranges, MD, from Lausanne (Switzerland) University Hospital. He and his colleagues found that more than half of those with a mild to moderate disease had persistent symptoms at least 3 months after diagnosis.
The prevalence of long COVID has varied in previous research, from 15% in a study of health care workers, to 46% in a study of patients with mild COVID, 52% in a study of young COVID outpatients, and 76% in a study of patients hospitalized with COVID.
Dr. Desgranges and colleagues evaluated patients seen in an ED or outpatient clinic from February to April 2020.
The 418 patients with a confirmed COVID-19 diagnosis were compared with a control group of 89 patients who presented to the same centers during the same time frame with similar symptoms – cough, shortness of breath, or fever – but had a negative SARS-CoV-2 test.
The number of patients with comorbidities was similar in the COVID and control groups (34% vs. 36%), as was median age (41 vs. 36 years) and the prevalence of women (62% vs 64%), but the proportion of health care workers was lower in the COVID group (64% vs 82%; P =.006).
Symptoms that persisted for at least 3 months were more common in the COVID than in the control group (53% vs. 37%). And patients in the COVID group reported more symptoms than those in the control group after adjustment for age, gender, smoking status, comorbidities, and timing of the survey phone call.
Levels of sleeping problems and headache were similar in the two groups.
“We have to remember that with COVID-19 came the psychosocial changes of the pandemic situation” Dr. Desgranges said.
This study suggests that some long-COVID symptoms – such as the fatigue, headache, and sleep disorders reported in the control group – could be related to the pandemic itself, which has caused psychosocial distress, Dr. Terndrup said.
Another study that looked at outpatients “has some fantastic long-term follow-up data, and shows that many patients are still engaging in rehabilitation programs nearly a year after their diagnosis,” he explained.
The COVID HOME study
That prospective longitudinal COVID HOME study, which assessed long-term symptoms in people who were never hospitalized for COVID, was presented online by Adriana Tami, MD, PhD, from the University Medical Center Groningen (the Netherlands).
The researchers visited the homes of patients to collect data, blood samples, and perform polymerase chain reaction (PCR) testing 1, 2, and 3 weeks after a diagnosis of COVID-19. If their PCR test was still positive, testing continued until week 6 or a negative test. In addition, participants completed questionnaires at week 2 and at months 3, 6 and 12 to assess fatigue, quality of life, and symptoms of depression and anxiety.
Three-month follow-up data were available for 134 of the 276 people initially enrolled in the study. Questionnaires were completed by 85 participants at 3 months, 62 participants at 6 months, and 10 participants at 12 months.
At least 40% of participants reported long-lasting symptoms at some point during follow-up, and at least 30% said they didn’t feel fully recovered at 12 months. The most common symptom was persistent fatigue, reported at 3, 6, and 12 months by at least 44% of participants. Other common symptoms – reported by at least 20% of respondents at 3, 6, and 12 months – were headache, mental or neurologic symptoms, and sleep disorders, shortness of breath, lack of smell or taste, and severe fatigue.
“We have a high proportion of nonhospitalized individuals who suffer from long COVID after more than 12 months,” Dr. Tami concluded, adding that the study is ongoing. “We have other variables that we want to look at, including duration viral shedding and serological results and variants.”
“These cohort studies are very helpful, but they can lead to inaccurate conclusions,” Dr. Terndrup cautioned.
They only provide pieces of the big picture, but they “do add to a growing body of knowledge about a significant portion of COVID patients still struggling with symptoms long after their initial infection. The symptoms can be quite variable but are dominated by both physical and mental fatigue, and tend to be worse in patients who were sicker at initial infection,” he said in an interview.
As a whole, these studies reinforce the need for treatment programs to help patients who suffer from long COVID, he added, but “I advise caution to folks suffering out there who seek ‘miracle cures’; across the world, we are collaborating to find solutions that are safe and effective.”
We are in desperate need of an equity lens in these studies.
“There is still a great deal to learn about long COVID,” said Dr. Terndrup. Data on underrepresented populations – such as Black, Indigenous, and people of color – are lacking from these and others studies, he explained. “We are in desperate need of an equity lens in these studies,” particularly in the United States, where there are “significant disparities” in the treatment of different populations.
However, “I do hope that this work can lead to a better understanding of how other viral infections can cause long-lasting symptoms,” said Dr. Terndrup.
“We have long proposed that after acute presentation, some microbes can cause chronic symptoms, like fatigue and widespread pain. Perhaps we can learn how to better care for these patients after learning from COVID’s significant impact on our societies across the globe.”
Dr. Aranda and Dr. Desgranges have disclosed no relevant financial relationships or study funding. The study by Dr. Tami’s team was funded by the University Medical Center Groningen Organization for Health Research and Development, and Connecting European Cohorts to Increase Common and Effective Response to SARS-CoV-2 Pandemic. Dr. Terndrup disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Findings from the cohort, composed of 113 COVID-19 survivors who developed ARDS after admission to a single center before to April 16, 2020, were presented online at the 31st European Congress of Clinical Microbiology & Infectious Diseases by Judit Aranda, MD, from Complex Hospitalari Moisés Broggi in Barcelona.
Median age of the participants was 64 years, and 70% were male. At least one persistent symptom was experienced during follow-up by 81% of the cohort, with 45% reporting shortness of breath, 50% reporting muscle pain, 43% reporting memory impairment, and 46% reporting physical weakness of at least 5 on a 10-point scale.
Of the 104 participants who completed a 6-minute walk test, 30% had a decrease in oxygen saturation level of at least 4%, and 5% had an initial or final level below 88%. Of the 46 participants who underwent a pulmonary function test, 15% had a forced expiratory volume in 1 second below 70%.
And of the 49% of participants with pathologic findings on chest x-ray, most were bilateral interstitial infiltrates (88%).
In addition, more than 90% of participants developed depression, anxiety, or PTSD, Dr. Aranda reported.
Not the whole picture
This study shows that sicker people – “those in intensive care units with acute respiratory distress syndrome” – are “more likely to be struggling with more severe symptoms,” said Christopher Terndrup, MD, from the division of general internal medicine and geriatrics at Oregon Health & Science University, Portland.
But a Swiss study, also presented at the meeting, “shows how even mild COVID cases can lead to debilitating symptoms,” Dr. Terndrup said in an interview.
The investigation of long-term COVID symptoms in outpatients was presented online by Florian Desgranges, MD, from Lausanne (Switzerland) University Hospital. He and his colleagues found that more than half of those with a mild to moderate disease had persistent symptoms at least 3 months after diagnosis.
The prevalence of long COVID has varied in previous research, from 15% in a study of health care workers, to 46% in a study of patients with mild COVID, 52% in a study of young COVID outpatients, and 76% in a study of patients hospitalized with COVID.
Dr. Desgranges and colleagues evaluated patients seen in an ED or outpatient clinic from February to April 2020.
The 418 patients with a confirmed COVID-19 diagnosis were compared with a control group of 89 patients who presented to the same centers during the same time frame with similar symptoms – cough, shortness of breath, or fever – but had a negative SARS-CoV-2 test.
The number of patients with comorbidities was similar in the COVID and control groups (34% vs. 36%), as was median age (41 vs. 36 years) and the prevalence of women (62% vs 64%), but the proportion of health care workers was lower in the COVID group (64% vs 82%; P =.006).
Symptoms that persisted for at least 3 months were more common in the COVID than in the control group (53% vs. 37%). And patients in the COVID group reported more symptoms than those in the control group after adjustment for age, gender, smoking status, comorbidities, and timing of the survey phone call.
Levels of sleeping problems and headache were similar in the two groups.
“We have to remember that with COVID-19 came the psychosocial changes of the pandemic situation” Dr. Desgranges said.
This study suggests that some long-COVID symptoms – such as the fatigue, headache, and sleep disorders reported in the control group – could be related to the pandemic itself, which has caused psychosocial distress, Dr. Terndrup said.
Another study that looked at outpatients “has some fantastic long-term follow-up data, and shows that many patients are still engaging in rehabilitation programs nearly a year after their diagnosis,” he explained.
The COVID HOME study
That prospective longitudinal COVID HOME study, which assessed long-term symptoms in people who were never hospitalized for COVID, was presented online by Adriana Tami, MD, PhD, from the University Medical Center Groningen (the Netherlands).
The researchers visited the homes of patients to collect data, blood samples, and perform polymerase chain reaction (PCR) testing 1, 2, and 3 weeks after a diagnosis of COVID-19. If their PCR test was still positive, testing continued until week 6 or a negative test. In addition, participants completed questionnaires at week 2 and at months 3, 6 and 12 to assess fatigue, quality of life, and symptoms of depression and anxiety.
Three-month follow-up data were available for 134 of the 276 people initially enrolled in the study. Questionnaires were completed by 85 participants at 3 months, 62 participants at 6 months, and 10 participants at 12 months.
At least 40% of participants reported long-lasting symptoms at some point during follow-up, and at least 30% said they didn’t feel fully recovered at 12 months. The most common symptom was persistent fatigue, reported at 3, 6, and 12 months by at least 44% of participants. Other common symptoms – reported by at least 20% of respondents at 3, 6, and 12 months – were headache, mental or neurologic symptoms, and sleep disorders, shortness of breath, lack of smell or taste, and severe fatigue.
“We have a high proportion of nonhospitalized individuals who suffer from long COVID after more than 12 months,” Dr. Tami concluded, adding that the study is ongoing. “We have other variables that we want to look at, including duration viral shedding and serological results and variants.”
“These cohort studies are very helpful, but they can lead to inaccurate conclusions,” Dr. Terndrup cautioned.
They only provide pieces of the big picture, but they “do add to a growing body of knowledge about a significant portion of COVID patients still struggling with symptoms long after their initial infection. The symptoms can be quite variable but are dominated by both physical and mental fatigue, and tend to be worse in patients who were sicker at initial infection,” he said in an interview.
As a whole, these studies reinforce the need for treatment programs to help patients who suffer from long COVID, he added, but “I advise caution to folks suffering out there who seek ‘miracle cures’; across the world, we are collaborating to find solutions that are safe and effective.”
We are in desperate need of an equity lens in these studies.
“There is still a great deal to learn about long COVID,” said Dr. Terndrup. Data on underrepresented populations – such as Black, Indigenous, and people of color – are lacking from these and others studies, he explained. “We are in desperate need of an equity lens in these studies,” particularly in the United States, where there are “significant disparities” in the treatment of different populations.
However, “I do hope that this work can lead to a better understanding of how other viral infections can cause long-lasting symptoms,” said Dr. Terndrup.
“We have long proposed that after acute presentation, some microbes can cause chronic symptoms, like fatigue and widespread pain. Perhaps we can learn how to better care for these patients after learning from COVID’s significant impact on our societies across the globe.”
Dr. Aranda and Dr. Desgranges have disclosed no relevant financial relationships or study funding. The study by Dr. Tami’s team was funded by the University Medical Center Groningen Organization for Health Research and Development, and Connecting European Cohorts to Increase Common and Effective Response to SARS-CoV-2 Pandemic. Dr. Terndrup disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Findings from the cohort, composed of 113 COVID-19 survivors who developed ARDS after admission to a single center before to April 16, 2020, were presented online at the 31st European Congress of Clinical Microbiology & Infectious Diseases by Judit Aranda, MD, from Complex Hospitalari Moisés Broggi in Barcelona.
Median age of the participants was 64 years, and 70% were male. At least one persistent symptom was experienced during follow-up by 81% of the cohort, with 45% reporting shortness of breath, 50% reporting muscle pain, 43% reporting memory impairment, and 46% reporting physical weakness of at least 5 on a 10-point scale.
Of the 104 participants who completed a 6-minute walk test, 30% had a decrease in oxygen saturation level of at least 4%, and 5% had an initial or final level below 88%. Of the 46 participants who underwent a pulmonary function test, 15% had a forced expiratory volume in 1 second below 70%.
And of the 49% of participants with pathologic findings on chest x-ray, most were bilateral interstitial infiltrates (88%).
In addition, more than 90% of participants developed depression, anxiety, or PTSD, Dr. Aranda reported.
Not the whole picture
This study shows that sicker people – “those in intensive care units with acute respiratory distress syndrome” – are “more likely to be struggling with more severe symptoms,” said Christopher Terndrup, MD, from the division of general internal medicine and geriatrics at Oregon Health & Science University, Portland.
But a Swiss study, also presented at the meeting, “shows how even mild COVID cases can lead to debilitating symptoms,” Dr. Terndrup said in an interview.
The investigation of long-term COVID symptoms in outpatients was presented online by Florian Desgranges, MD, from Lausanne (Switzerland) University Hospital. He and his colleagues found that more than half of those with a mild to moderate disease had persistent symptoms at least 3 months after diagnosis.
The prevalence of long COVID has varied in previous research, from 15% in a study of health care workers, to 46% in a study of patients with mild COVID, 52% in a study of young COVID outpatients, and 76% in a study of patients hospitalized with COVID.
Dr. Desgranges and colleagues evaluated patients seen in an ED or outpatient clinic from February to April 2020.
The 418 patients with a confirmed COVID-19 diagnosis were compared with a control group of 89 patients who presented to the same centers during the same time frame with similar symptoms – cough, shortness of breath, or fever – but had a negative SARS-CoV-2 test.
The number of patients with comorbidities was similar in the COVID and control groups (34% vs. 36%), as was median age (41 vs. 36 years) and the prevalence of women (62% vs 64%), but the proportion of health care workers was lower in the COVID group (64% vs 82%; P =.006).
Symptoms that persisted for at least 3 months were more common in the COVID than in the control group (53% vs. 37%). And patients in the COVID group reported more symptoms than those in the control group after adjustment for age, gender, smoking status, comorbidities, and timing of the survey phone call.
Levels of sleeping problems and headache were similar in the two groups.
“We have to remember that with COVID-19 came the psychosocial changes of the pandemic situation” Dr. Desgranges said.
This study suggests that some long-COVID symptoms – such as the fatigue, headache, and sleep disorders reported in the control group – could be related to the pandemic itself, which has caused psychosocial distress, Dr. Terndrup said.
Another study that looked at outpatients “has some fantastic long-term follow-up data, and shows that many patients are still engaging in rehabilitation programs nearly a year after their diagnosis,” he explained.
The COVID HOME study
That prospective longitudinal COVID HOME study, which assessed long-term symptoms in people who were never hospitalized for COVID, was presented online by Adriana Tami, MD, PhD, from the University Medical Center Groningen (the Netherlands).
The researchers visited the homes of patients to collect data, blood samples, and perform polymerase chain reaction (PCR) testing 1, 2, and 3 weeks after a diagnosis of COVID-19. If their PCR test was still positive, testing continued until week 6 or a negative test. In addition, participants completed questionnaires at week 2 and at months 3, 6 and 12 to assess fatigue, quality of life, and symptoms of depression and anxiety.
Three-month follow-up data were available for 134 of the 276 people initially enrolled in the study. Questionnaires were completed by 85 participants at 3 months, 62 participants at 6 months, and 10 participants at 12 months.
At least 40% of participants reported long-lasting symptoms at some point during follow-up, and at least 30% said they didn’t feel fully recovered at 12 months. The most common symptom was persistent fatigue, reported at 3, 6, and 12 months by at least 44% of participants. Other common symptoms – reported by at least 20% of respondents at 3, 6, and 12 months – were headache, mental or neurologic symptoms, and sleep disorders, shortness of breath, lack of smell or taste, and severe fatigue.
“We have a high proportion of nonhospitalized individuals who suffer from long COVID after more than 12 months,” Dr. Tami concluded, adding that the study is ongoing. “We have other variables that we want to look at, including duration viral shedding and serological results and variants.”
“These cohort studies are very helpful, but they can lead to inaccurate conclusions,” Dr. Terndrup cautioned.
They only provide pieces of the big picture, but they “do add to a growing body of knowledge about a significant portion of COVID patients still struggling with symptoms long after their initial infection. The symptoms can be quite variable but are dominated by both physical and mental fatigue, and tend to be worse in patients who were sicker at initial infection,” he said in an interview.
As a whole, these studies reinforce the need for treatment programs to help patients who suffer from long COVID, he added, but “I advise caution to folks suffering out there who seek ‘miracle cures’; across the world, we are collaborating to find solutions that are safe and effective.”
We are in desperate need of an equity lens in these studies.
“There is still a great deal to learn about long COVID,” said Dr. Terndrup. Data on underrepresented populations – such as Black, Indigenous, and people of color – are lacking from these and others studies, he explained. “We are in desperate need of an equity lens in these studies,” particularly in the United States, where there are “significant disparities” in the treatment of different populations.
However, “I do hope that this work can lead to a better understanding of how other viral infections can cause long-lasting symptoms,” said Dr. Terndrup.
“We have long proposed that after acute presentation, some microbes can cause chronic symptoms, like fatigue and widespread pain. Perhaps we can learn how to better care for these patients after learning from COVID’s significant impact on our societies across the globe.”
Dr. Aranda and Dr. Desgranges have disclosed no relevant financial relationships or study funding. The study by Dr. Tami’s team was funded by the University Medical Center Groningen Organization for Health Research and Development, and Connecting European Cohorts to Increase Common and Effective Response to SARS-CoV-2 Pandemic. Dr. Terndrup disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EUROPEAN CONGRESS OF CLINICAL MICROBIOLOGY & INFECTIOUS DISEASES
Early high-dose vitamin D3 did not reduce mortality in critically ill, vitamin D–deficient patients
Background: Critically ill patients are often vitamin D deficient, but no large randomized trials have investigated whether early vitamin D supplementation can affect clinical outcomes.
Study design: Multicenter, randomized, double-blind, placebo-controlled phase 3 trial.Setting: 44 U.S. hospitals, during April 2017–July 2018.
Synopsis: The study enrolled 1,078 patients with 25-hydroxyvitamin D levels < 20 ng/mL who were critically ill (defined as patients being admitted to the ICU with one or more risk factor for lung injury or death). Participants were randomized to early administration of a single dose of 540,000 IUs of enteral vitamin D3 or placebo. The authors did not identify a statistically significant difference in the 90-day all-cause mortality between the two groups. Additionally, there were no significant differences in length of stay, ventilator-free days or serious adverse outcomes between the two groups.
Bottom line: Early administration of high-dose enteral vitamin D3 did not decrease 90-day all-cause mortality in critically ill, vitamin D–deficient patients.
Citation: Ginde A et al. Early high-dose vitamin D3 for critically ill, vitamin D–deficient patients. N Engl J Med. 2019 Dec 26; 281:2529-40.
Dr. Persaud is a hospitalist, Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, both in Boston.
Background: Critically ill patients are often vitamin D deficient, but no large randomized trials have investigated whether early vitamin D supplementation can affect clinical outcomes.
Study design: Multicenter, randomized, double-blind, placebo-controlled phase 3 trial.Setting: 44 U.S. hospitals, during April 2017–July 2018.
Synopsis: The study enrolled 1,078 patients with 25-hydroxyvitamin D levels < 20 ng/mL who were critically ill (defined as patients being admitted to the ICU with one or more risk factor for lung injury or death). Participants were randomized to early administration of a single dose of 540,000 IUs of enteral vitamin D3 or placebo. The authors did not identify a statistically significant difference in the 90-day all-cause mortality between the two groups. Additionally, there were no significant differences in length of stay, ventilator-free days or serious adverse outcomes between the two groups.
Bottom line: Early administration of high-dose enteral vitamin D3 did not decrease 90-day all-cause mortality in critically ill, vitamin D–deficient patients.
Citation: Ginde A et al. Early high-dose vitamin D3 for critically ill, vitamin D–deficient patients. N Engl J Med. 2019 Dec 26; 281:2529-40.
Dr. Persaud is a hospitalist, Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, both in Boston.
Background: Critically ill patients are often vitamin D deficient, but no large randomized trials have investigated whether early vitamin D supplementation can affect clinical outcomes.
Study design: Multicenter, randomized, double-blind, placebo-controlled phase 3 trial.Setting: 44 U.S. hospitals, during April 2017–July 2018.
Synopsis: The study enrolled 1,078 patients with 25-hydroxyvitamin D levels < 20 ng/mL who were critically ill (defined as patients being admitted to the ICU with one or more risk factor for lung injury or death). Participants were randomized to early administration of a single dose of 540,000 IUs of enteral vitamin D3 or placebo. The authors did not identify a statistically significant difference in the 90-day all-cause mortality between the two groups. Additionally, there were no significant differences in length of stay, ventilator-free days or serious adverse outcomes between the two groups.
Bottom line: Early administration of high-dose enteral vitamin D3 did not decrease 90-day all-cause mortality in critically ill, vitamin D–deficient patients.
Citation: Ginde A et al. Early high-dose vitamin D3 for critically ill, vitamin D–deficient patients. N Engl J Med. 2019 Dec 26; 281:2529-40.
Dr. Persaud is a hospitalist, Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, both in Boston.
Revised dispatch system boosts bystander CPR in those with limited English
The improved Los Angeles medical dispatch system prompted more callers with limited English proficiency to initiate telecommunicator-assisted cardiopulmonary resuscitation (T-CPR), compared with the previous system, a new study shows.
The Los Angeles Tiered Dispatch System (LA-TDS), adopted in late 2014, used simplified questions aimed at identifying cardiac arrest, compared with the city’s earlier Medical Priority Dispatch System (MPDS).
The result was substantially decreased call processing times, decreased “undertriage” of out-of-hospital cardiac arrest (OHCA), and improved overall T-CPR rates (Resuscitation. 2020 Oct;155:74-81).
But now, a secondary analysis of the data shows there was a much higher jump in T-CPR rates among a small subset of callers with limited English proficiency, compared with those proficient in English (JAMA Network Open. 2021;4[6]:e216827).
“This was an unanticipated, significant, and disproportionate change, but fortunately a very good change,” lead author Stephen Sanko, MD, said in an interview.
While the T-CPR rate among English-proficient callers increased from 55% with the MPDS to 67% with the LA-TDS (odds ratio, 1.66; P = .007), it rose from 28% to 69% (OR, 5.66; P = .003) among callers with limited English proficiency. In the adjusted analysis, the new LA-TDS was associated with a 69% higher prevalence of T-CPR among English-proficient callers, compared with a 350% greater prevalence among callers with limited English proficiency.
“The emergency communication process between a caller and 911 telecommunicator is more complex than we thought, and likely constitutes a unique subsubspecialty that interacts with fields as diverse as medicine, health equity, linguistics, sociology, consumer behavior and others,” said Dr. Sanko, who is from the division of emergency medical services at the University of Southern California in Los Angeles.
“Yet in spite of this complexity, we’re starting to be able to reproducibly classify elements of the emergency conversation that we believe are tied to outcomes we all care about. ... Modulators of health disparities are present as early as the dispatch conversation, and, importantly, they can be intervened upon to promote improved outcomes,” he continued.
The retrospective cohort study was a predefined secondary analysis of a previously published study comparing telecommunicator management of out-of-hospital cardiac arrest over 3 months with the MPDS versus 3 months with the LA-TDS. The primary outcome was the number of patients who received telecommunicator-assisted chest compressions from callers with limited English proficiency.
Of the 597 emergency calls that met the inclusion criteria, 289 (48%) were in the MPDS cohort and 308 (52%) were in the LA-TDS cohort. In the MPDS cohort, 263 callers had English proficiency and 26 had limited proficiency; in the latter cohort, those figures were 273 and 35, respectively.
There were no significant differences between cohorts in the use of real-time translation services, which were employed 27%-31% of the time.
The reason for the overall T-CPR improvement is likely that the LA-TDS was tailored to the community needs, said Dr. Sanko. “Most people, including doctors, think of 911 dispatch as something simple and straightforward, like ordering a pizza or calling a ride share. [But] LA-TDS is a ‘home grown’ dispatch system whose structure, questions, and emergency instructions were all developed by EMS medical directors and telecommunicators with extensive experience in our community.”
That being said, the researchers acknowledge that the reason behind the bigger T-CPR boost in LEP callers remains unclear. Although the link between language and system was statistically significant, they noted “it was not an a priori hypothesis and appeared to be largely attributable to the low T-CPR rates for callers with limited English proficiency using MPDS.” Additionally, such callers were “remarkably under-represented” in the sample, “which included approximately 600 calls over two quarters in a large city,” said Dr Sanko.
“We hypothesize that a more direct structure, earlier commitment to treating patients with abnormal life status indicators as being suspected cardiac arrest cases, and earlier reassurance may have improved caller confidence that telecommunicators knew what they were doing. This in turn may have translated into an increased likelihood of bystander caller willingness to perform immediate life-saving maneuvers.”
Despite a number of limitations, “the study is important and highlights instructive topics for discussion that suggest potential next-step opportunities,” noted Richard Chocron, MD, PhD, Miranda Lewis, MD, and Thomas Rea, MD, MPH, in an invited commentary that accompanied the publication. Dr. Chocron is from the Paris University, Paris Research Cardiovascular Center, INSERM; Dr. Lewis is from the Georges Pompidou European Hospital in Paris; and Dr. Rea is from the Division of Emergency Medical Services, Public Health–Seattle & King County. Both Dr. Lewis and Dr. Rea are also at the University of Washington, Seattle.
“Sanko et al. found that approximately 10% of all emergency calls were classified as limited English proficiency calls in a community in which 19% of the population was considered to have limited English proficiency,” they added. “This finding suggests the possibility that populations with limited English proficiency are less likely to activate 911 for incidence of cardiac arrest. If true, this finding would compound the health disparity observed among those with limited English proficiency. This topic is important in that it transcends the role of EMS personnel and engages a broad spectrum of societal stakeholders. We must listen, learn, and ultimately deliver public safety resources to groups who have not been well served by conventional approaches.”
None of the authors or editorialists reported any conflicts of interest.
The improved Los Angeles medical dispatch system prompted more callers with limited English proficiency to initiate telecommunicator-assisted cardiopulmonary resuscitation (T-CPR), compared with the previous system, a new study shows.
The Los Angeles Tiered Dispatch System (LA-TDS), adopted in late 2014, used simplified questions aimed at identifying cardiac arrest, compared with the city’s earlier Medical Priority Dispatch System (MPDS).
The result was substantially decreased call processing times, decreased “undertriage” of out-of-hospital cardiac arrest (OHCA), and improved overall T-CPR rates (Resuscitation. 2020 Oct;155:74-81).
But now, a secondary analysis of the data shows there was a much higher jump in T-CPR rates among a small subset of callers with limited English proficiency, compared with those proficient in English (JAMA Network Open. 2021;4[6]:e216827).
“This was an unanticipated, significant, and disproportionate change, but fortunately a very good change,” lead author Stephen Sanko, MD, said in an interview.
While the T-CPR rate among English-proficient callers increased from 55% with the MPDS to 67% with the LA-TDS (odds ratio, 1.66; P = .007), it rose from 28% to 69% (OR, 5.66; P = .003) among callers with limited English proficiency. In the adjusted analysis, the new LA-TDS was associated with a 69% higher prevalence of T-CPR among English-proficient callers, compared with a 350% greater prevalence among callers with limited English proficiency.
“The emergency communication process between a caller and 911 telecommunicator is more complex than we thought, and likely constitutes a unique subsubspecialty that interacts with fields as diverse as medicine, health equity, linguistics, sociology, consumer behavior and others,” said Dr. Sanko, who is from the division of emergency medical services at the University of Southern California in Los Angeles.
“Yet in spite of this complexity, we’re starting to be able to reproducibly classify elements of the emergency conversation that we believe are tied to outcomes we all care about. ... Modulators of health disparities are present as early as the dispatch conversation, and, importantly, they can be intervened upon to promote improved outcomes,” he continued.
The retrospective cohort study was a predefined secondary analysis of a previously published study comparing telecommunicator management of out-of-hospital cardiac arrest over 3 months with the MPDS versus 3 months with the LA-TDS. The primary outcome was the number of patients who received telecommunicator-assisted chest compressions from callers with limited English proficiency.
Of the 597 emergency calls that met the inclusion criteria, 289 (48%) were in the MPDS cohort and 308 (52%) were in the LA-TDS cohort. In the MPDS cohort, 263 callers had English proficiency and 26 had limited proficiency; in the latter cohort, those figures were 273 and 35, respectively.
There were no significant differences between cohorts in the use of real-time translation services, which were employed 27%-31% of the time.
The reason for the overall T-CPR improvement is likely that the LA-TDS was tailored to the community needs, said Dr. Sanko. “Most people, including doctors, think of 911 dispatch as something simple and straightforward, like ordering a pizza or calling a ride share. [But] LA-TDS is a ‘home grown’ dispatch system whose structure, questions, and emergency instructions were all developed by EMS medical directors and telecommunicators with extensive experience in our community.”
That being said, the researchers acknowledge that the reason behind the bigger T-CPR boost in LEP callers remains unclear. Although the link between language and system was statistically significant, they noted “it was not an a priori hypothesis and appeared to be largely attributable to the low T-CPR rates for callers with limited English proficiency using MPDS.” Additionally, such callers were “remarkably under-represented” in the sample, “which included approximately 600 calls over two quarters in a large city,” said Dr Sanko.
“We hypothesize that a more direct structure, earlier commitment to treating patients with abnormal life status indicators as being suspected cardiac arrest cases, and earlier reassurance may have improved caller confidence that telecommunicators knew what they were doing. This in turn may have translated into an increased likelihood of bystander caller willingness to perform immediate life-saving maneuvers.”
Despite a number of limitations, “the study is important and highlights instructive topics for discussion that suggest potential next-step opportunities,” noted Richard Chocron, MD, PhD, Miranda Lewis, MD, and Thomas Rea, MD, MPH, in an invited commentary that accompanied the publication. Dr. Chocron is from the Paris University, Paris Research Cardiovascular Center, INSERM; Dr. Lewis is from the Georges Pompidou European Hospital in Paris; and Dr. Rea is from the Division of Emergency Medical Services, Public Health–Seattle & King County. Both Dr. Lewis and Dr. Rea are also at the University of Washington, Seattle.
“Sanko et al. found that approximately 10% of all emergency calls were classified as limited English proficiency calls in a community in which 19% of the population was considered to have limited English proficiency,” they added. “This finding suggests the possibility that populations with limited English proficiency are less likely to activate 911 for incidence of cardiac arrest. If true, this finding would compound the health disparity observed among those with limited English proficiency. This topic is important in that it transcends the role of EMS personnel and engages a broad spectrum of societal stakeholders. We must listen, learn, and ultimately deliver public safety resources to groups who have not been well served by conventional approaches.”
None of the authors or editorialists reported any conflicts of interest.
The improved Los Angeles medical dispatch system prompted more callers with limited English proficiency to initiate telecommunicator-assisted cardiopulmonary resuscitation (T-CPR), compared with the previous system, a new study shows.
The Los Angeles Tiered Dispatch System (LA-TDS), adopted in late 2014, used simplified questions aimed at identifying cardiac arrest, compared with the city’s earlier Medical Priority Dispatch System (MPDS).
The result was substantially decreased call processing times, decreased “undertriage” of out-of-hospital cardiac arrest (OHCA), and improved overall T-CPR rates (Resuscitation. 2020 Oct;155:74-81).
But now, a secondary analysis of the data shows there was a much higher jump in T-CPR rates among a small subset of callers with limited English proficiency, compared with those proficient in English (JAMA Network Open. 2021;4[6]:e216827).
“This was an unanticipated, significant, and disproportionate change, but fortunately a very good change,” lead author Stephen Sanko, MD, said in an interview.
While the T-CPR rate among English-proficient callers increased from 55% with the MPDS to 67% with the LA-TDS (odds ratio, 1.66; P = .007), it rose from 28% to 69% (OR, 5.66; P = .003) among callers with limited English proficiency. In the adjusted analysis, the new LA-TDS was associated with a 69% higher prevalence of T-CPR among English-proficient callers, compared with a 350% greater prevalence among callers with limited English proficiency.
“The emergency communication process between a caller and 911 telecommunicator is more complex than we thought, and likely constitutes a unique subsubspecialty that interacts with fields as diverse as medicine, health equity, linguistics, sociology, consumer behavior and others,” said Dr. Sanko, who is from the division of emergency medical services at the University of Southern California in Los Angeles.
“Yet in spite of this complexity, we’re starting to be able to reproducibly classify elements of the emergency conversation that we believe are tied to outcomes we all care about. ... Modulators of health disparities are present as early as the dispatch conversation, and, importantly, they can be intervened upon to promote improved outcomes,” he continued.
The retrospective cohort study was a predefined secondary analysis of a previously published study comparing telecommunicator management of out-of-hospital cardiac arrest over 3 months with the MPDS versus 3 months with the LA-TDS. The primary outcome was the number of patients who received telecommunicator-assisted chest compressions from callers with limited English proficiency.
Of the 597 emergency calls that met the inclusion criteria, 289 (48%) were in the MPDS cohort and 308 (52%) were in the LA-TDS cohort. In the MPDS cohort, 263 callers had English proficiency and 26 had limited proficiency; in the latter cohort, those figures were 273 and 35, respectively.
There were no significant differences between cohorts in the use of real-time translation services, which were employed 27%-31% of the time.
The reason for the overall T-CPR improvement is likely that the LA-TDS was tailored to the community needs, said Dr. Sanko. “Most people, including doctors, think of 911 dispatch as something simple and straightforward, like ordering a pizza or calling a ride share. [But] LA-TDS is a ‘home grown’ dispatch system whose structure, questions, and emergency instructions were all developed by EMS medical directors and telecommunicators with extensive experience in our community.”
That being said, the researchers acknowledge that the reason behind the bigger T-CPR boost in LEP callers remains unclear. Although the link between language and system was statistically significant, they noted “it was not an a priori hypothesis and appeared to be largely attributable to the low T-CPR rates for callers with limited English proficiency using MPDS.” Additionally, such callers were “remarkably under-represented” in the sample, “which included approximately 600 calls over two quarters in a large city,” said Dr Sanko.
“We hypothesize that a more direct structure, earlier commitment to treating patients with abnormal life status indicators as being suspected cardiac arrest cases, and earlier reassurance may have improved caller confidence that telecommunicators knew what they were doing. This in turn may have translated into an increased likelihood of bystander caller willingness to perform immediate life-saving maneuvers.”
Despite a number of limitations, “the study is important and highlights instructive topics for discussion that suggest potential next-step opportunities,” noted Richard Chocron, MD, PhD, Miranda Lewis, MD, and Thomas Rea, MD, MPH, in an invited commentary that accompanied the publication. Dr. Chocron is from the Paris University, Paris Research Cardiovascular Center, INSERM; Dr. Lewis is from the Georges Pompidou European Hospital in Paris; and Dr. Rea is from the Division of Emergency Medical Services, Public Health–Seattle & King County. Both Dr. Lewis and Dr. Rea are also at the University of Washington, Seattle.
“Sanko et al. found that approximately 10% of all emergency calls were classified as limited English proficiency calls in a community in which 19% of the population was considered to have limited English proficiency,” they added. “This finding suggests the possibility that populations with limited English proficiency are less likely to activate 911 for incidence of cardiac arrest. If true, this finding would compound the health disparity observed among those with limited English proficiency. This topic is important in that it transcends the role of EMS personnel and engages a broad spectrum of societal stakeholders. We must listen, learn, and ultimately deliver public safety resources to groups who have not been well served by conventional approaches.”
None of the authors or editorialists reported any conflicts of interest.
FROM JAMA NETWORK OPEN
In-hospital resuscitation: Focus on effective chest pumps, prompt shocks
The keys to effective resuscitation in the hospital setting include effective compression and early defibrillation, according to Jessica Nave Allen, MD, FHM, a hospitalist with Emory University Hospital in Atlanta. She spoke about best practices in resuscitation medicine recently at SHM Converge, the annual conference of the Society of Hospital Medicine.
“We know CPR [cardiopulmonary resuscitation] and shocking are the two biggest determinants of outcomes, so really strive to make those chest compressions really high quality,” said Dr. Allen. She urged hospitalists to consider mechanical piston compressions and even “reverse CPR” when appropriate.
Dr. Allen offered several other tips about effective in-hospital resuscitation.
Don’t overcrowd the hospital room
There shouldn’t be more than eight people inside the room during a code, she said. If you’re the code leader, “make sure that somebody has already started high-quality chest compressions. You want to make sure that somebody is already on the airway. It’s usually two people, one person to actually hold the mask down to make sure there’s a good seal, and the other person to deliver the breaths.”
Two to three people should be assigned to chest compressions, Dr. Allen said, “and you need one or two nurses for medication delivery and grabbing things from the runners. And then you need to have a recorder and the code leader. Everyone else who’s not in one of those formalized roles needs to be outside the room. That includes the pharmacist, who usually stands at the door if you don’t have a code pharmacist at your institution.”
A helpful mnemonic for the resuscitation process is I(CA)RAMBO, which was developed at Tufts Medical Center and published in 2020, she said. The mnemonic stands for the following:
- I: Identify yourself as code leader.
- CA: Compression, Airway.
- R: Roles (assign roles in the resuscitation).
- A: Access (intravenous access is preferred to intraosseous, per the American Heart Association’s , unless intravenous access is unavailable, Dr. Allen noted).
- M: Monitor (make sure pads are placed correctly; turn the defibrillator on).
- B: Backboard.
- O: Oxygen.
Focus on high-quality chest compressions
The number of chest compressions must be 100-120 per minute, Dr. Allen said. You can time them to the beat of a song, such as “Stayin’ Alive,” or with a metronome, she said, “but whatever it is, you need to stay in that window.”
The correct compression depth is 2-2.4 inches. “That’s very difficult to do during the middle of a code, which is why it’s important to allow full recoil,” she said. “This doesn’t mean taking your hands off of the chest: You should actually never take your hands off of the chest. But you should allow the chest wall to return to its normal state. Also, make sure you aren’t off the chest for more for 10 seconds whenever you’re doing a rhythm check.”
Audiovisual feedback devices can provide insight into the quality of chest compressions. For example, some defibrillators are equipped with sensors that urge users to push harder and faster when appropriate. “Studies have shown that the quality of chest compressions goes up when you use these devices,” she said.
Don’t be afraid of mechanical chest compression
Although early research raised questions about the quality of resuscitation outcomes when mechanical piston chest compression devices are used, a 2015 systematic review and meta-analysis found that “man was equal to machine,” Dr. Allen said. “The bottom line is that these devices may be a reasonable alternative to conventional CPR in specific settings.”
American Heart Association guidelines state that mechanical compressions may be appropriate in certain specific situations “where the delivery of high-quality manual compressions may be challenging or dangerous for the provider.”
According to Dr. Allen, “there are times when it’s useful,” such as for a patient with COVID-19, in the cath lab, or in a medical helicopter.
Move quickly to defibrillation
“Most of us know that you want to shock as early as possible in shockable rhythms,” Dr. Allen said. Support, she said, comes from a 2008 study that linked delayed defibrillation to lower survival rates. “We want to shock as soon as possible, because your chances of surviving go down for every minute you wait.”
Take special care for patients with confirmed or suspected COVID-19
“Not surprisingly, the goals here are to minimize exposure to staff,” Dr. Allen said.
Put on personal protective equipment before entering the room even if care is delayed, she advised, and reduce the number of staff members in the room below the typical maximum of eight. “In COVID, it should be a maximum of six, and some institutions have even gotten it down to four where the code leaders are outside the room with an iPad.”
Use mechanical compression devices, she advised, and place patients on ventilators as soon as possible. She added: “Use a HEPA [high-efficiency particulate air] filter for all your airway modalities.”
CPR may be challenging in some cases, such as when a large, intubated patient is prone and cannot be quickly or safely flipped over. In those cases, consider posterior chest compressions, also known as reverse CPR, at vertebral positions T7-T10. “We have done reverse CPR on several COVID patients throughout the Emory system,” she said.
Debrief right after codes
“You really want to debrief with the code team,” Dr. Allen said. “If you don’t already have a policy in place at your institution, you should help come up with one where you sit down with the team and talk about what could you have done better as a group. It’s not a time to place blame. It’s a time to learn.”
Dr. Allen has disclosed no relevant financial relationships.
This article was updated 7/26/21.
A version of this article first appeared on Medscape.com.
The keys to effective resuscitation in the hospital setting include effective compression and early defibrillation, according to Jessica Nave Allen, MD, FHM, a hospitalist with Emory University Hospital in Atlanta. She spoke about best practices in resuscitation medicine recently at SHM Converge, the annual conference of the Society of Hospital Medicine.
“We know CPR [cardiopulmonary resuscitation] and shocking are the two biggest determinants of outcomes, so really strive to make those chest compressions really high quality,” said Dr. Allen. She urged hospitalists to consider mechanical piston compressions and even “reverse CPR” when appropriate.
Dr. Allen offered several other tips about effective in-hospital resuscitation.
Don’t overcrowd the hospital room
There shouldn’t be more than eight people inside the room during a code, she said. If you’re the code leader, “make sure that somebody has already started high-quality chest compressions. You want to make sure that somebody is already on the airway. It’s usually two people, one person to actually hold the mask down to make sure there’s a good seal, and the other person to deliver the breaths.”
Two to three people should be assigned to chest compressions, Dr. Allen said, “and you need one or two nurses for medication delivery and grabbing things from the runners. And then you need to have a recorder and the code leader. Everyone else who’s not in one of those formalized roles needs to be outside the room. That includes the pharmacist, who usually stands at the door if you don’t have a code pharmacist at your institution.”
A helpful mnemonic for the resuscitation process is I(CA)RAMBO, which was developed at Tufts Medical Center and published in 2020, she said. The mnemonic stands for the following:
- I: Identify yourself as code leader.
- CA: Compression, Airway.
- R: Roles (assign roles in the resuscitation).
- A: Access (intravenous access is preferred to intraosseous, per the American Heart Association’s , unless intravenous access is unavailable, Dr. Allen noted).
- M: Monitor (make sure pads are placed correctly; turn the defibrillator on).
- B: Backboard.
- O: Oxygen.
Focus on high-quality chest compressions
The number of chest compressions must be 100-120 per minute, Dr. Allen said. You can time them to the beat of a song, such as “Stayin’ Alive,” or with a metronome, she said, “but whatever it is, you need to stay in that window.”
The correct compression depth is 2-2.4 inches. “That’s very difficult to do during the middle of a code, which is why it’s important to allow full recoil,” she said. “This doesn’t mean taking your hands off of the chest: You should actually never take your hands off of the chest. But you should allow the chest wall to return to its normal state. Also, make sure you aren’t off the chest for more for 10 seconds whenever you’re doing a rhythm check.”
Audiovisual feedback devices can provide insight into the quality of chest compressions. For example, some defibrillators are equipped with sensors that urge users to push harder and faster when appropriate. “Studies have shown that the quality of chest compressions goes up when you use these devices,” she said.
Don’t be afraid of mechanical chest compression
Although early research raised questions about the quality of resuscitation outcomes when mechanical piston chest compression devices are used, a 2015 systematic review and meta-analysis found that “man was equal to machine,” Dr. Allen said. “The bottom line is that these devices may be a reasonable alternative to conventional CPR in specific settings.”
American Heart Association guidelines state that mechanical compressions may be appropriate in certain specific situations “where the delivery of high-quality manual compressions may be challenging or dangerous for the provider.”
According to Dr. Allen, “there are times when it’s useful,” such as for a patient with COVID-19, in the cath lab, or in a medical helicopter.
Move quickly to defibrillation
“Most of us know that you want to shock as early as possible in shockable rhythms,” Dr. Allen said. Support, she said, comes from a 2008 study that linked delayed defibrillation to lower survival rates. “We want to shock as soon as possible, because your chances of surviving go down for every minute you wait.”
Take special care for patients with confirmed or suspected COVID-19
“Not surprisingly, the goals here are to minimize exposure to staff,” Dr. Allen said.
Put on personal protective equipment before entering the room even if care is delayed, she advised, and reduce the number of staff members in the room below the typical maximum of eight. “In COVID, it should be a maximum of six, and some institutions have even gotten it down to four where the code leaders are outside the room with an iPad.”
Use mechanical compression devices, she advised, and place patients on ventilators as soon as possible. She added: “Use a HEPA [high-efficiency particulate air] filter for all your airway modalities.”
CPR may be challenging in some cases, such as when a large, intubated patient is prone and cannot be quickly or safely flipped over. In those cases, consider posterior chest compressions, also known as reverse CPR, at vertebral positions T7-T10. “We have done reverse CPR on several COVID patients throughout the Emory system,” she said.
Debrief right after codes
“You really want to debrief with the code team,” Dr. Allen said. “If you don’t already have a policy in place at your institution, you should help come up with one where you sit down with the team and talk about what could you have done better as a group. It’s not a time to place blame. It’s a time to learn.”
Dr. Allen has disclosed no relevant financial relationships.
This article was updated 7/26/21.
A version of this article first appeared on Medscape.com.
The keys to effective resuscitation in the hospital setting include effective compression and early defibrillation, according to Jessica Nave Allen, MD, FHM, a hospitalist with Emory University Hospital in Atlanta. She spoke about best practices in resuscitation medicine recently at SHM Converge, the annual conference of the Society of Hospital Medicine.
“We know CPR [cardiopulmonary resuscitation] and shocking are the two biggest determinants of outcomes, so really strive to make those chest compressions really high quality,” said Dr. Allen. She urged hospitalists to consider mechanical piston compressions and even “reverse CPR” when appropriate.
Dr. Allen offered several other tips about effective in-hospital resuscitation.
Don’t overcrowd the hospital room
There shouldn’t be more than eight people inside the room during a code, she said. If you’re the code leader, “make sure that somebody has already started high-quality chest compressions. You want to make sure that somebody is already on the airway. It’s usually two people, one person to actually hold the mask down to make sure there’s a good seal, and the other person to deliver the breaths.”
Two to three people should be assigned to chest compressions, Dr. Allen said, “and you need one or two nurses for medication delivery and grabbing things from the runners. And then you need to have a recorder and the code leader. Everyone else who’s not in one of those formalized roles needs to be outside the room. That includes the pharmacist, who usually stands at the door if you don’t have a code pharmacist at your institution.”
A helpful mnemonic for the resuscitation process is I(CA)RAMBO, which was developed at Tufts Medical Center and published in 2020, she said. The mnemonic stands for the following:
- I: Identify yourself as code leader.
- CA: Compression, Airway.
- R: Roles (assign roles in the resuscitation).
- A: Access (intravenous access is preferred to intraosseous, per the American Heart Association’s , unless intravenous access is unavailable, Dr. Allen noted).
- M: Monitor (make sure pads are placed correctly; turn the defibrillator on).
- B: Backboard.
- O: Oxygen.
Focus on high-quality chest compressions
The number of chest compressions must be 100-120 per minute, Dr. Allen said. You can time them to the beat of a song, such as “Stayin’ Alive,” or with a metronome, she said, “but whatever it is, you need to stay in that window.”
The correct compression depth is 2-2.4 inches. “That’s very difficult to do during the middle of a code, which is why it’s important to allow full recoil,” she said. “This doesn’t mean taking your hands off of the chest: You should actually never take your hands off of the chest. But you should allow the chest wall to return to its normal state. Also, make sure you aren’t off the chest for more for 10 seconds whenever you’re doing a rhythm check.”
Audiovisual feedback devices can provide insight into the quality of chest compressions. For example, some defibrillators are equipped with sensors that urge users to push harder and faster when appropriate. “Studies have shown that the quality of chest compressions goes up when you use these devices,” she said.
Don’t be afraid of mechanical chest compression
Although early research raised questions about the quality of resuscitation outcomes when mechanical piston chest compression devices are used, a 2015 systematic review and meta-analysis found that “man was equal to machine,” Dr. Allen said. “The bottom line is that these devices may be a reasonable alternative to conventional CPR in specific settings.”
American Heart Association guidelines state that mechanical compressions may be appropriate in certain specific situations “where the delivery of high-quality manual compressions may be challenging or dangerous for the provider.”
According to Dr. Allen, “there are times when it’s useful,” such as for a patient with COVID-19, in the cath lab, or in a medical helicopter.
Move quickly to defibrillation
“Most of us know that you want to shock as early as possible in shockable rhythms,” Dr. Allen said. Support, she said, comes from a 2008 study that linked delayed defibrillation to lower survival rates. “We want to shock as soon as possible, because your chances of surviving go down for every minute you wait.”
Take special care for patients with confirmed or suspected COVID-19
“Not surprisingly, the goals here are to minimize exposure to staff,” Dr. Allen said.
Put on personal protective equipment before entering the room even if care is delayed, she advised, and reduce the number of staff members in the room below the typical maximum of eight. “In COVID, it should be a maximum of six, and some institutions have even gotten it down to four where the code leaders are outside the room with an iPad.”
Use mechanical compression devices, she advised, and place patients on ventilators as soon as possible. She added: “Use a HEPA [high-efficiency particulate air] filter for all your airway modalities.”
CPR may be challenging in some cases, such as when a large, intubated patient is prone and cannot be quickly or safely flipped over. In those cases, consider posterior chest compressions, also known as reverse CPR, at vertebral positions T7-T10. “We have done reverse CPR on several COVID patients throughout the Emory system,” she said.
Debrief right after codes
“You really want to debrief with the code team,” Dr. Allen said. “If you don’t already have a policy in place at your institution, you should help come up with one where you sit down with the team and talk about what could you have done better as a group. It’s not a time to place blame. It’s a time to learn.”
Dr. Allen has disclosed no relevant financial relationships.
This article was updated 7/26/21.
A version of this article first appeared on Medscape.com.
FROM SHM CONVERGE 2021
Avoiding excess oxygen in mechanically ventilated patients ‘seems sensible’
The respiratory therapists at Mount Sinai Beth Israel, New York, know when Lina Miyakawa, MD, starts a week in the ICU, because she turns down the fraction of inspired oxygen (FiO2) levels if patients tolerate it.
“Hyperoxia in mechanical ventilation is a topic that’s near and dear to my heart,” Dr. Miyakawa, a pulmonary and critical care medicine specialist at Mount Sinai Beth Israel, said during SHM Converge, the annual conference of the Society of Hospital Medicine. “You can always find ‘wean down FiO2’ in my consult notes.”
While it is believed that humans have built up evolutionary defenses against hypoxia but not against hyperoxia, medical literature on the topic of hyperoxia with supplemental oxygen is fairly young. “In medical school we were taught to give oxygen for anybody with chest pain and concern about acute coronary syndrome,” she said. “This was until recent data suggested harm from liberal oxygen use.”
In a single-center trial of 434 critical care patients with an ICU length of stay of 72 hours or longer, Italian researchers examined the effects of a conservative protocol for oxygen therapy versus conventional therapy on ICU mortality (JAMA. 2016;316[15]:1583-9). The trial was stopped because the patients who were assigned to receive conservative therapy had a significantly lower mortality than the ones who received usual care (P = .01). “The study was not perfect, and the premature stoppage likely exaggerated the effect size,” said Dr. Miyakawa, who was not affiliated with the trial. “However, subsequent retrospective studies continue to support a benefit with conservative oxygen use, especially in different groups of patients. One of note is hyperoxia following cardiac arrest. There’s something called a two-hit model that speaks to worsening ischemia with reperfusion injury after the initial hypoxic event from the cardiac arrest itself” (See Intensive Care Med. 2015;41:534-6).
In a multicenter cohort study that drew from the Project IMPACT critical care database of ICUs at 120 U.S. hospitals between 2001 and 2005, researchers led by J. Hope Kilgannon, MD, tested the hypothesis that post-resuscitation hyperoxia is associated with increased in-hospital mortality (JAMA. 2010;303[21]:2165-71). The study population consisted of 6,326 patients who were divided into three groups: the hypoxic group (a PaO2 of less than 60 mm Hg); the normoxic group (a PaO2 of 60-299 mm Hg), and the hyperoxic group (a PaO2 of over 300 mm Hg). The mortality for the hyperoxic group was 63%, the hypoxic group at 57%, and the normoxic group at 45%.
More recently, the ICU-ROX Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group evaluated conservative versus liberal approaches in providing oxygen to 965 patients who were mechanically ventilated between 2015 and 2018 at 21 ICUs (N Eng J Med. 2020;382:989-98). Of the 965 patients, 484 were randomly assigned to the conservative oxygen group (defined as an SpO2 of 97% or lower) and 481 were assigned to the usual oxygen group (defined as having no specific measures limiting FiO2 or the SpO2). The primary outcome was the number of ventilator-free days from randomization until day 28, while the secondary outcome was mortality at 180 days. The researchers also performed a subgroup analysis of patients at risk for hypoxic-ischemic encephalopathy.
No significant differences were observed in the number of ventilator days between the two group (a median of 21 days in the conservative oxygen group versus 22 days in the usual oxygen group, respectively; P = .80) nor in mortality at 180 days (35.7% vs. 34.5%). However, in the subgroup analysis, patients with hypoxic-ischemic encephalopathy were noted to have more ventilator-free days (21 vs. 0 days), improved 180-day mortality (43% vs. 59%), and less functional impairment (55% vs. 68%) in the conservative-oxygen group.
“The results of this study suggest that conservative oxygen therapy has no additional advantage over standard oxygen therapy, but there may be benefits in those vulnerable to hyperoxia, which warrants further investigation,” Dr. Miyakawa said. “There are a few points to note on this topic. First, many of the previous studies had more liberal oxygen strategies than the ones used in this study, which could be the reason why we are seeing these results. In addition, O2 titration relies on imperfect approximations. PaO2 cannot be measured continuously; we really depend on the SpO2 on a minute-by-minute basis. Critically ill patients can also undergo episodes of hypoperfusion and shock state minute-by-minute. That’s when they’re at risk for hypoxemia. This would not be captured continuously with just O2 saturations.”
Dr. Miyakawa also highlighted the Liberal Oxygenation versus Conservative Oxygenation in Acute Respiratory Distress Syndrome trial (LOCO2) a prospective, multicenter, randomized, open-label trial involving patients with ARDS. It was carried out at 13 ICUs in France between June 2016 and September 2018 in an effort determine whether conservative oxygenation would reduce mortality at 28 days compared with the usual liberal-oxygen strategy (N Eng J Med. 2020;382:999-1008). The researchers detected a signal of increased mortality in the conservative oxygen group (34% vs. 27%), which led to a premature stoppage of the trial. “I’d like to postulate that the higher incidence of proning in the liberal oxygenation group compared to the conservative oxygen group (51% to 34%) may be the reason for the difference in mortality,” said Dr. Miyakawa, who was not affiliated with LOCO2. “This is supported from the 2013 PROSEVA Study Group, which reported that prone positioning in ARDS significantly decreases 28- and 90-day mortality” (see N Engl J Med. 2013; 368:2159-68).
She said that future trials on this topic “will have to address how a particular [oxygenation] target is both set and achieved in each group of patients, particularly those with specific organ injuries. In the meantime, in my opinion, avoiding excess oxygen seems sensible.”
Dr. Miyakawa reported having no financial disclosures.
The respiratory therapists at Mount Sinai Beth Israel, New York, know when Lina Miyakawa, MD, starts a week in the ICU, because she turns down the fraction of inspired oxygen (FiO2) levels if patients tolerate it.
“Hyperoxia in mechanical ventilation is a topic that’s near and dear to my heart,” Dr. Miyakawa, a pulmonary and critical care medicine specialist at Mount Sinai Beth Israel, said during SHM Converge, the annual conference of the Society of Hospital Medicine. “You can always find ‘wean down FiO2’ in my consult notes.”
While it is believed that humans have built up evolutionary defenses against hypoxia but not against hyperoxia, medical literature on the topic of hyperoxia with supplemental oxygen is fairly young. “In medical school we were taught to give oxygen for anybody with chest pain and concern about acute coronary syndrome,” she said. “This was until recent data suggested harm from liberal oxygen use.”
In a single-center trial of 434 critical care patients with an ICU length of stay of 72 hours or longer, Italian researchers examined the effects of a conservative protocol for oxygen therapy versus conventional therapy on ICU mortality (JAMA. 2016;316[15]:1583-9). The trial was stopped because the patients who were assigned to receive conservative therapy had a significantly lower mortality than the ones who received usual care (P = .01). “The study was not perfect, and the premature stoppage likely exaggerated the effect size,” said Dr. Miyakawa, who was not affiliated with the trial. “However, subsequent retrospective studies continue to support a benefit with conservative oxygen use, especially in different groups of patients. One of note is hyperoxia following cardiac arrest. There’s something called a two-hit model that speaks to worsening ischemia with reperfusion injury after the initial hypoxic event from the cardiac arrest itself” (See Intensive Care Med. 2015;41:534-6).
In a multicenter cohort study that drew from the Project IMPACT critical care database of ICUs at 120 U.S. hospitals between 2001 and 2005, researchers led by J. Hope Kilgannon, MD, tested the hypothesis that post-resuscitation hyperoxia is associated with increased in-hospital mortality (JAMA. 2010;303[21]:2165-71). The study population consisted of 6,326 patients who were divided into three groups: the hypoxic group (a PaO2 of less than 60 mm Hg); the normoxic group (a PaO2 of 60-299 mm Hg), and the hyperoxic group (a PaO2 of over 300 mm Hg). The mortality for the hyperoxic group was 63%, the hypoxic group at 57%, and the normoxic group at 45%.
More recently, the ICU-ROX Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group evaluated conservative versus liberal approaches in providing oxygen to 965 patients who were mechanically ventilated between 2015 and 2018 at 21 ICUs (N Eng J Med. 2020;382:989-98). Of the 965 patients, 484 were randomly assigned to the conservative oxygen group (defined as an SpO2 of 97% or lower) and 481 were assigned to the usual oxygen group (defined as having no specific measures limiting FiO2 or the SpO2). The primary outcome was the number of ventilator-free days from randomization until day 28, while the secondary outcome was mortality at 180 days. The researchers also performed a subgroup analysis of patients at risk for hypoxic-ischemic encephalopathy.
No significant differences were observed in the number of ventilator days between the two group (a median of 21 days in the conservative oxygen group versus 22 days in the usual oxygen group, respectively; P = .80) nor in mortality at 180 days (35.7% vs. 34.5%). However, in the subgroup analysis, patients with hypoxic-ischemic encephalopathy were noted to have more ventilator-free days (21 vs. 0 days), improved 180-day mortality (43% vs. 59%), and less functional impairment (55% vs. 68%) in the conservative-oxygen group.
“The results of this study suggest that conservative oxygen therapy has no additional advantage over standard oxygen therapy, but there may be benefits in those vulnerable to hyperoxia, which warrants further investigation,” Dr. Miyakawa said. “There are a few points to note on this topic. First, many of the previous studies had more liberal oxygen strategies than the ones used in this study, which could be the reason why we are seeing these results. In addition, O2 titration relies on imperfect approximations. PaO2 cannot be measured continuously; we really depend on the SpO2 on a minute-by-minute basis. Critically ill patients can also undergo episodes of hypoperfusion and shock state minute-by-minute. That’s when they’re at risk for hypoxemia. This would not be captured continuously with just O2 saturations.”
Dr. Miyakawa also highlighted the Liberal Oxygenation versus Conservative Oxygenation in Acute Respiratory Distress Syndrome trial (LOCO2) a prospective, multicenter, randomized, open-label trial involving patients with ARDS. It was carried out at 13 ICUs in France between June 2016 and September 2018 in an effort determine whether conservative oxygenation would reduce mortality at 28 days compared with the usual liberal-oxygen strategy (N Eng J Med. 2020;382:999-1008). The researchers detected a signal of increased mortality in the conservative oxygen group (34% vs. 27%), which led to a premature stoppage of the trial. “I’d like to postulate that the higher incidence of proning in the liberal oxygenation group compared to the conservative oxygen group (51% to 34%) may be the reason for the difference in mortality,” said Dr. Miyakawa, who was not affiliated with LOCO2. “This is supported from the 2013 PROSEVA Study Group, which reported that prone positioning in ARDS significantly decreases 28- and 90-day mortality” (see N Engl J Med. 2013; 368:2159-68).
She said that future trials on this topic “will have to address how a particular [oxygenation] target is both set and achieved in each group of patients, particularly those with specific organ injuries. In the meantime, in my opinion, avoiding excess oxygen seems sensible.”
Dr. Miyakawa reported having no financial disclosures.
The respiratory therapists at Mount Sinai Beth Israel, New York, know when Lina Miyakawa, MD, starts a week in the ICU, because she turns down the fraction of inspired oxygen (FiO2) levels if patients tolerate it.
“Hyperoxia in mechanical ventilation is a topic that’s near and dear to my heart,” Dr. Miyakawa, a pulmonary and critical care medicine specialist at Mount Sinai Beth Israel, said during SHM Converge, the annual conference of the Society of Hospital Medicine. “You can always find ‘wean down FiO2’ in my consult notes.”
While it is believed that humans have built up evolutionary defenses against hypoxia but not against hyperoxia, medical literature on the topic of hyperoxia with supplemental oxygen is fairly young. “In medical school we were taught to give oxygen for anybody with chest pain and concern about acute coronary syndrome,” she said. “This was until recent data suggested harm from liberal oxygen use.”
In a single-center trial of 434 critical care patients with an ICU length of stay of 72 hours or longer, Italian researchers examined the effects of a conservative protocol for oxygen therapy versus conventional therapy on ICU mortality (JAMA. 2016;316[15]:1583-9). The trial was stopped because the patients who were assigned to receive conservative therapy had a significantly lower mortality than the ones who received usual care (P = .01). “The study was not perfect, and the premature stoppage likely exaggerated the effect size,” said Dr. Miyakawa, who was not affiliated with the trial. “However, subsequent retrospective studies continue to support a benefit with conservative oxygen use, especially in different groups of patients. One of note is hyperoxia following cardiac arrest. There’s something called a two-hit model that speaks to worsening ischemia with reperfusion injury after the initial hypoxic event from the cardiac arrest itself” (See Intensive Care Med. 2015;41:534-6).
In a multicenter cohort study that drew from the Project IMPACT critical care database of ICUs at 120 U.S. hospitals between 2001 and 2005, researchers led by J. Hope Kilgannon, MD, tested the hypothesis that post-resuscitation hyperoxia is associated with increased in-hospital mortality (JAMA. 2010;303[21]:2165-71). The study population consisted of 6,326 patients who were divided into three groups: the hypoxic group (a PaO2 of less than 60 mm Hg); the normoxic group (a PaO2 of 60-299 mm Hg), and the hyperoxic group (a PaO2 of over 300 mm Hg). The mortality for the hyperoxic group was 63%, the hypoxic group at 57%, and the normoxic group at 45%.
More recently, the ICU-ROX Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group evaluated conservative versus liberal approaches in providing oxygen to 965 patients who were mechanically ventilated between 2015 and 2018 at 21 ICUs (N Eng J Med. 2020;382:989-98). Of the 965 patients, 484 were randomly assigned to the conservative oxygen group (defined as an SpO2 of 97% or lower) and 481 were assigned to the usual oxygen group (defined as having no specific measures limiting FiO2 or the SpO2). The primary outcome was the number of ventilator-free days from randomization until day 28, while the secondary outcome was mortality at 180 days. The researchers also performed a subgroup analysis of patients at risk for hypoxic-ischemic encephalopathy.
No significant differences were observed in the number of ventilator days between the two group (a median of 21 days in the conservative oxygen group versus 22 days in the usual oxygen group, respectively; P = .80) nor in mortality at 180 days (35.7% vs. 34.5%). However, in the subgroup analysis, patients with hypoxic-ischemic encephalopathy were noted to have more ventilator-free days (21 vs. 0 days), improved 180-day mortality (43% vs. 59%), and less functional impairment (55% vs. 68%) in the conservative-oxygen group.
“The results of this study suggest that conservative oxygen therapy has no additional advantage over standard oxygen therapy, but there may be benefits in those vulnerable to hyperoxia, which warrants further investigation,” Dr. Miyakawa said. “There are a few points to note on this topic. First, many of the previous studies had more liberal oxygen strategies than the ones used in this study, which could be the reason why we are seeing these results. In addition, O2 titration relies on imperfect approximations. PaO2 cannot be measured continuously; we really depend on the SpO2 on a minute-by-minute basis. Critically ill patients can also undergo episodes of hypoperfusion and shock state minute-by-minute. That’s when they’re at risk for hypoxemia. This would not be captured continuously with just O2 saturations.”
Dr. Miyakawa also highlighted the Liberal Oxygenation versus Conservative Oxygenation in Acute Respiratory Distress Syndrome trial (LOCO2) a prospective, multicenter, randomized, open-label trial involving patients with ARDS. It was carried out at 13 ICUs in France between June 2016 and September 2018 in an effort determine whether conservative oxygenation would reduce mortality at 28 days compared with the usual liberal-oxygen strategy (N Eng J Med. 2020;382:999-1008). The researchers detected a signal of increased mortality in the conservative oxygen group (34% vs. 27%), which led to a premature stoppage of the trial. “I’d like to postulate that the higher incidence of proning in the liberal oxygenation group compared to the conservative oxygen group (51% to 34%) may be the reason for the difference in mortality,” said Dr. Miyakawa, who was not affiliated with LOCO2. “This is supported from the 2013 PROSEVA Study Group, which reported that prone positioning in ARDS significantly decreases 28- and 90-day mortality” (see N Engl J Med. 2013; 368:2159-68).
She said that future trials on this topic “will have to address how a particular [oxygenation] target is both set and achieved in each group of patients, particularly those with specific organ injuries. In the meantime, in my opinion, avoiding excess oxygen seems sensible.”
Dr. Miyakawa reported having no financial disclosures.
FROM SHM CONVERGE 2021






