User login
Repetitive TMS effective for comorbid depression, substance use
In a retrospective observational study, participants receiving 20-30 rTMS sessions delivered over a course of 4-6 weeks showed significant reductions in both craving and depression symptom scores.
In addition, the researchers found that the number of rTMS sessions significantly predicted the number of days of drug abstinence, even after controlling for confounders.
“For each additional TMS session, there was an additional 10 days of abstinence in the community,” principal investigator Wael Foad, MD, medical director, Erada Center for Treatment and Rehabilitation, Dubai, United Arab Emirates, told this news organization.
However, Dr. Foad noted that he would need to construct a randomized controlled trial to further explore that “interesting” finding.
The results were published in the Annals of Clinical Psychiatry.
Inpatient program
The researchers retrospectively analyzed medical records of men admitted to the inpatient unit at the Erada Center between June 2019 and September 2020. The vast majority were native to the UAE.
The inpatient program focuses on treating patients with SUDs and is the only dedicated addiction rehabilitation service in Dubai, the investigators noted.
They analyzed outcomes for 55 men with mild to moderate MDD who received rTMS as standard treatment.
Participants were excluded from the data analysis if they had another comorbid diagnosis from the DSM-5 other than SUD or MDD. They were also excluded if they used an illicit substance 2 weeks before the study or used certain medications, including antipsychotics, benzodiazepines, or mood stabilizers.
When patients first arrived on the unit, they were detoxed for a period of time before they began receiving rTMS sessions.
The 55 men received 20-30 high-frequency rTMS sessions over the course of 4-6 weeks in the area of the dorsolateral prefrontal cortex. Each session consisted of 3,000 pulses delivered over a period of 37.5 minutes. Severity of depression was measured with the Clinical Global Impression–Severity Scale (CGI-S), which uses a 7-point Likert scale.
In addition, participants’ scores were tracked on the Brief Substance Craving Scale (BSCS), a self-report scale that measures craving for primary and secondary substances of abuse over a 24-hr period.
Of all participants, 47% said opiates and 35% said methamphetamine were their primary substances of abuse.
Significant improvement
Results showed a statistically significant improvement (P < .05) between baseline and post-rTMS treatment scores in severity of depression and drug craving, as measured by the BSCS and the CGI-S.
The researchers noted that eight participants dropped out of the study after their first rTMS session for various reasons.
Dr. Foad explained that investigators contracted with study participants to receive 20 rTMS sessions; if the sessions were not fully completed during the inpatient stay, the rTMS sessions were continued on an outpatient basis. A study clinician closely monitored patients until they finished their sessions.
For each additional rTMS session the patients completed beyond 20 sessions, there was an associated excess of 10 more days of abstinence from the primary drug in the community.
The investigators speculated that rTMS may reduce drug craving by increasing dopaminergic binding in the striatum, or by releasing dopamine in the caudate nucleus.
Study limitations cited include the lack of a control group and the fact that the study sample was limited to male inpatients, which limits generalizability of the findings to other populations.
Promising intervention
Commenting on the study, Colleen Ann Hanlon, PhD, noted that, from years of work using TMS for depression, “we know that more sessions of TMS during the acute treatment phase tends to lead to stronger and possibly more durable results long-term.”
Dr. Hanlon, who was not involved with the current research, formerly headed a clinical neuromodulation lab at Wake Forest University, Winston-Salem, N.C. She is now vice president of medical affairs at BrainsWay, an international health technology company specializing in Deep TMS.
She noted that Deep TMS was approved by the Food and Drug Administration for smoking cessation in 2020, “which was a tremendous win for our field at large, and requires only 15 acute sessions followed by 3 weekly sessions” of deep TMS.
“I suspect this is just the beginning of a new era in neuromodulation-based therapeutics for people struggling with drug and alcohol use disorders,” Dr. Hanlon said.
The study behind the FDA approval for smoking approval was a large double-blind, sham-controlled multisite clinical trial where investigators used an H4 coil – a TMS coil that modulates multiple brain areas involved in addictive behaviors simultaneously.
Results from that study showed that 15 sessions of deep TMS significantly improved smoking cessation rates relative to sham (10 Hz, 120% motor threshold, H4 coil, 1,800 pulses/session).
“The difference in cigarette consumption and craving was significant as early as 2 weeks after treatment initiation,” said Dr. Hanlon. “I am looking forward to the future of this field for all people suffering from drug and alcohol use disorders.”
The study and services provided through the Erada Center were funded by the government of Dubai. The investigators reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a retrospective observational study, participants receiving 20-30 rTMS sessions delivered over a course of 4-6 weeks showed significant reductions in both craving and depression symptom scores.
In addition, the researchers found that the number of rTMS sessions significantly predicted the number of days of drug abstinence, even after controlling for confounders.
“For each additional TMS session, there was an additional 10 days of abstinence in the community,” principal investigator Wael Foad, MD, medical director, Erada Center for Treatment and Rehabilitation, Dubai, United Arab Emirates, told this news organization.
However, Dr. Foad noted that he would need to construct a randomized controlled trial to further explore that “interesting” finding.
The results were published in the Annals of Clinical Psychiatry.
Inpatient program
The researchers retrospectively analyzed medical records of men admitted to the inpatient unit at the Erada Center between June 2019 and September 2020. The vast majority were native to the UAE.
The inpatient program focuses on treating patients with SUDs and is the only dedicated addiction rehabilitation service in Dubai, the investigators noted.
They analyzed outcomes for 55 men with mild to moderate MDD who received rTMS as standard treatment.
Participants were excluded from the data analysis if they had another comorbid diagnosis from the DSM-5 other than SUD or MDD. They were also excluded if they used an illicit substance 2 weeks before the study or used certain medications, including antipsychotics, benzodiazepines, or mood stabilizers.
When patients first arrived on the unit, they were detoxed for a period of time before they began receiving rTMS sessions.
The 55 men received 20-30 high-frequency rTMS sessions over the course of 4-6 weeks in the area of the dorsolateral prefrontal cortex. Each session consisted of 3,000 pulses delivered over a period of 37.5 minutes. Severity of depression was measured with the Clinical Global Impression–Severity Scale (CGI-S), which uses a 7-point Likert scale.
In addition, participants’ scores were tracked on the Brief Substance Craving Scale (BSCS), a self-report scale that measures craving for primary and secondary substances of abuse over a 24-hr period.
Of all participants, 47% said opiates and 35% said methamphetamine were their primary substances of abuse.
Significant improvement
Results showed a statistically significant improvement (P < .05) between baseline and post-rTMS treatment scores in severity of depression and drug craving, as measured by the BSCS and the CGI-S.
The researchers noted that eight participants dropped out of the study after their first rTMS session for various reasons.
Dr. Foad explained that investigators contracted with study participants to receive 20 rTMS sessions; if the sessions were not fully completed during the inpatient stay, the rTMS sessions were continued on an outpatient basis. A study clinician closely monitored patients until they finished their sessions.
For each additional rTMS session the patients completed beyond 20 sessions, there was an associated excess of 10 more days of abstinence from the primary drug in the community.
The investigators speculated that rTMS may reduce drug craving by increasing dopaminergic binding in the striatum, or by releasing dopamine in the caudate nucleus.
Study limitations cited include the lack of a control group and the fact that the study sample was limited to male inpatients, which limits generalizability of the findings to other populations.
Promising intervention
Commenting on the study, Colleen Ann Hanlon, PhD, noted that, from years of work using TMS for depression, “we know that more sessions of TMS during the acute treatment phase tends to lead to stronger and possibly more durable results long-term.”
Dr. Hanlon, who was not involved with the current research, formerly headed a clinical neuromodulation lab at Wake Forest University, Winston-Salem, N.C. She is now vice president of medical affairs at BrainsWay, an international health technology company specializing in Deep TMS.
She noted that Deep TMS was approved by the Food and Drug Administration for smoking cessation in 2020, “which was a tremendous win for our field at large, and requires only 15 acute sessions followed by 3 weekly sessions” of deep TMS.
“I suspect this is just the beginning of a new era in neuromodulation-based therapeutics for people struggling with drug and alcohol use disorders,” Dr. Hanlon said.
The study behind the FDA approval for smoking approval was a large double-blind, sham-controlled multisite clinical trial where investigators used an H4 coil – a TMS coil that modulates multiple brain areas involved in addictive behaviors simultaneously.
Results from that study showed that 15 sessions of deep TMS significantly improved smoking cessation rates relative to sham (10 Hz, 120% motor threshold, H4 coil, 1,800 pulses/session).
“The difference in cigarette consumption and craving was significant as early as 2 weeks after treatment initiation,” said Dr. Hanlon. “I am looking forward to the future of this field for all people suffering from drug and alcohol use disorders.”
The study and services provided through the Erada Center were funded by the government of Dubai. The investigators reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a retrospective observational study, participants receiving 20-30 rTMS sessions delivered over a course of 4-6 weeks showed significant reductions in both craving and depression symptom scores.
In addition, the researchers found that the number of rTMS sessions significantly predicted the number of days of drug abstinence, even after controlling for confounders.
“For each additional TMS session, there was an additional 10 days of abstinence in the community,” principal investigator Wael Foad, MD, medical director, Erada Center for Treatment and Rehabilitation, Dubai, United Arab Emirates, told this news organization.
However, Dr. Foad noted that he would need to construct a randomized controlled trial to further explore that “interesting” finding.
The results were published in the Annals of Clinical Psychiatry.
Inpatient program
The researchers retrospectively analyzed medical records of men admitted to the inpatient unit at the Erada Center between June 2019 and September 2020. The vast majority were native to the UAE.
The inpatient program focuses on treating patients with SUDs and is the only dedicated addiction rehabilitation service in Dubai, the investigators noted.
They analyzed outcomes for 55 men with mild to moderate MDD who received rTMS as standard treatment.
Participants were excluded from the data analysis if they had another comorbid diagnosis from the DSM-5 other than SUD or MDD. They were also excluded if they used an illicit substance 2 weeks before the study or used certain medications, including antipsychotics, benzodiazepines, or mood stabilizers.
When patients first arrived on the unit, they were detoxed for a period of time before they began receiving rTMS sessions.
The 55 men received 20-30 high-frequency rTMS sessions over the course of 4-6 weeks in the area of the dorsolateral prefrontal cortex. Each session consisted of 3,000 pulses delivered over a period of 37.5 minutes. Severity of depression was measured with the Clinical Global Impression–Severity Scale (CGI-S), which uses a 7-point Likert scale.
In addition, participants’ scores were tracked on the Brief Substance Craving Scale (BSCS), a self-report scale that measures craving for primary and secondary substances of abuse over a 24-hr period.
Of all participants, 47% said opiates and 35% said methamphetamine were their primary substances of abuse.
Significant improvement
Results showed a statistically significant improvement (P < .05) between baseline and post-rTMS treatment scores in severity of depression and drug craving, as measured by the BSCS and the CGI-S.
The researchers noted that eight participants dropped out of the study after their first rTMS session for various reasons.
Dr. Foad explained that investigators contracted with study participants to receive 20 rTMS sessions; if the sessions were not fully completed during the inpatient stay, the rTMS sessions were continued on an outpatient basis. A study clinician closely monitored patients until they finished their sessions.
For each additional rTMS session the patients completed beyond 20 sessions, there was an associated excess of 10 more days of abstinence from the primary drug in the community.
The investigators speculated that rTMS may reduce drug craving by increasing dopaminergic binding in the striatum, or by releasing dopamine in the caudate nucleus.
Study limitations cited include the lack of a control group and the fact that the study sample was limited to male inpatients, which limits generalizability of the findings to other populations.
Promising intervention
Commenting on the study, Colleen Ann Hanlon, PhD, noted that, from years of work using TMS for depression, “we know that more sessions of TMS during the acute treatment phase tends to lead to stronger and possibly more durable results long-term.”
Dr. Hanlon, who was not involved with the current research, formerly headed a clinical neuromodulation lab at Wake Forest University, Winston-Salem, N.C. She is now vice president of medical affairs at BrainsWay, an international health technology company specializing in Deep TMS.
She noted that Deep TMS was approved by the Food and Drug Administration for smoking cessation in 2020, “which was a tremendous win for our field at large, and requires only 15 acute sessions followed by 3 weekly sessions” of deep TMS.
“I suspect this is just the beginning of a new era in neuromodulation-based therapeutics for people struggling with drug and alcohol use disorders,” Dr. Hanlon said.
The study behind the FDA approval for smoking approval was a large double-blind, sham-controlled multisite clinical trial where investigators used an H4 coil – a TMS coil that modulates multiple brain areas involved in addictive behaviors simultaneously.
Results from that study showed that 15 sessions of deep TMS significantly improved smoking cessation rates relative to sham (10 Hz, 120% motor threshold, H4 coil, 1,800 pulses/session).
“The difference in cigarette consumption and craving was significant as early as 2 weeks after treatment initiation,” said Dr. Hanlon. “I am looking forward to the future of this field for all people suffering from drug and alcohol use disorders.”
The study and services provided through the Erada Center were funded by the government of Dubai. The investigators reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE ANNALS OF CLINICAL PSYCHIATRY
Bright light therapy boosts therapeutic response
Both depression and bipolar disorder are leading causes of disability worldwide, and data show that only 50%-60% of these patients respond to first-line antidepressants, wrote Alessandro Cuomo, MD, of the University of Siena Medical Center, Italy, and colleagues.
Bright light therapy (BLT) was originally introduced as a treatment for seasonal affective disorder, but its use has been expanded to treat nonseasonal depression and bipolar disorder, they said. However, the impact of BLT on depressive symptoms in bipolar depression in particular has not been examined, they noted.
In a study published in the Journal of Affective Disorders, the researchers identified 18 men and 23 women aged 18 years and older with bipolar depression based on DSM-5 criteria who had already been treated with antidepressants. The participants were randomized to antidepressants combined with BLT or antidepressants combined with red light exposure (controls). The participants were positioned at 30-80 cm from the 10,000-lux light source for 30 minutes daily. The mean age of the participants was 49.1 years.
The primary outcome was scores on the Montgomery-Åsberg Depression Scale (MADRS), Hamilton Depression Rating Scale (HAMD-17), and CGI-Severity of illness (CGI-S), Fatigue Severity Scale (FSS), and Quality of Life Scale (QOLS) after the 8 weeks of treatment.
After 4 weeks, MADRS scores and HAMD-17 scores were significantly lower in the treatment group, compared with the controls (20 and 18 vs. 27.5 and 24.9, respectively; P < .001). Quality of life scores increased in the treatment group, compared with controls, with median scores of 39 vs. 29.50, respectively.
After 8 weeks, the treatment group continued to show significant improvement, compared with the control group, with scores on the MADRS, HAMD-17, CGI-S, and QOLS of 14.0, 9.0, 1.0, and 62.0 vs. 16.0, 15.5, 2.0, and 40.0, respectively. No side effects were reported.
“From our findings, BLT [proved] particularly effective in bipolar patients without triggering any manic switch, as evidenced instead in some similar studies,” the researchers wrote in their discussion.
Although the mechanism of action for BLT remains unclear, the current study findings confirm the existing knowledge of BLT, they noted. The positive effect of BLT on quality of life “might be attributable to the ability of BLT to reduce the latency times of antidepressants and increase the production of serotonin and melatonin,” as shown in previous work, they said.
The study findings were limited by several factors including the small sample size, which prevents definitive conclusions about the effectiveness of BLT in combination with different antidepressants, and the heterogeneity of the antidepressant treatments, the researchers noted. Larger, prospective studies and randomized, controlled trials are needed, as are studies of special populations such as older adults or those with degenerative diseases, they said.
However, the results suggest BLT has value as a safe and effective treatment and a way to boost therapeutic response and reduce the impact of long-lasting therapies, they concluded.
The study received no outside funding. Dr. Cuomo disclosed serving as a consultant and/or a speaker for Angelini, Glaxo Smith Kline, Lundbeck, Janssen, Otsuka, Pfizer, and Recordati.
Both depression and bipolar disorder are leading causes of disability worldwide, and data show that only 50%-60% of these patients respond to first-line antidepressants, wrote Alessandro Cuomo, MD, of the University of Siena Medical Center, Italy, and colleagues.
Bright light therapy (BLT) was originally introduced as a treatment for seasonal affective disorder, but its use has been expanded to treat nonseasonal depression and bipolar disorder, they said. However, the impact of BLT on depressive symptoms in bipolar depression in particular has not been examined, they noted.
In a study published in the Journal of Affective Disorders, the researchers identified 18 men and 23 women aged 18 years and older with bipolar depression based on DSM-5 criteria who had already been treated with antidepressants. The participants were randomized to antidepressants combined with BLT or antidepressants combined with red light exposure (controls). The participants were positioned at 30-80 cm from the 10,000-lux light source for 30 minutes daily. The mean age of the participants was 49.1 years.
The primary outcome was scores on the Montgomery-Åsberg Depression Scale (MADRS), Hamilton Depression Rating Scale (HAMD-17), and CGI-Severity of illness (CGI-S), Fatigue Severity Scale (FSS), and Quality of Life Scale (QOLS) after the 8 weeks of treatment.
After 4 weeks, MADRS scores and HAMD-17 scores were significantly lower in the treatment group, compared with the controls (20 and 18 vs. 27.5 and 24.9, respectively; P < .001). Quality of life scores increased in the treatment group, compared with controls, with median scores of 39 vs. 29.50, respectively.
After 8 weeks, the treatment group continued to show significant improvement, compared with the control group, with scores on the MADRS, HAMD-17, CGI-S, and QOLS of 14.0, 9.0, 1.0, and 62.0 vs. 16.0, 15.5, 2.0, and 40.0, respectively. No side effects were reported.
“From our findings, BLT [proved] particularly effective in bipolar patients without triggering any manic switch, as evidenced instead in some similar studies,” the researchers wrote in their discussion.
Although the mechanism of action for BLT remains unclear, the current study findings confirm the existing knowledge of BLT, they noted. The positive effect of BLT on quality of life “might be attributable to the ability of BLT to reduce the latency times of antidepressants and increase the production of serotonin and melatonin,” as shown in previous work, they said.
The study findings were limited by several factors including the small sample size, which prevents definitive conclusions about the effectiveness of BLT in combination with different antidepressants, and the heterogeneity of the antidepressant treatments, the researchers noted. Larger, prospective studies and randomized, controlled trials are needed, as are studies of special populations such as older adults or those with degenerative diseases, they said.
However, the results suggest BLT has value as a safe and effective treatment and a way to boost therapeutic response and reduce the impact of long-lasting therapies, they concluded.
The study received no outside funding. Dr. Cuomo disclosed serving as a consultant and/or a speaker for Angelini, Glaxo Smith Kline, Lundbeck, Janssen, Otsuka, Pfizer, and Recordati.
Both depression and bipolar disorder are leading causes of disability worldwide, and data show that only 50%-60% of these patients respond to first-line antidepressants, wrote Alessandro Cuomo, MD, of the University of Siena Medical Center, Italy, and colleagues.
Bright light therapy (BLT) was originally introduced as a treatment for seasonal affective disorder, but its use has been expanded to treat nonseasonal depression and bipolar disorder, they said. However, the impact of BLT on depressive symptoms in bipolar depression in particular has not been examined, they noted.
In a study published in the Journal of Affective Disorders, the researchers identified 18 men and 23 women aged 18 years and older with bipolar depression based on DSM-5 criteria who had already been treated with antidepressants. The participants were randomized to antidepressants combined with BLT or antidepressants combined with red light exposure (controls). The participants were positioned at 30-80 cm from the 10,000-lux light source for 30 minutes daily. The mean age of the participants was 49.1 years.
The primary outcome was scores on the Montgomery-Åsberg Depression Scale (MADRS), Hamilton Depression Rating Scale (HAMD-17), and CGI-Severity of illness (CGI-S), Fatigue Severity Scale (FSS), and Quality of Life Scale (QOLS) after the 8 weeks of treatment.
After 4 weeks, MADRS scores and HAMD-17 scores were significantly lower in the treatment group, compared with the controls (20 and 18 vs. 27.5 and 24.9, respectively; P < .001). Quality of life scores increased in the treatment group, compared with controls, with median scores of 39 vs. 29.50, respectively.
After 8 weeks, the treatment group continued to show significant improvement, compared with the control group, with scores on the MADRS, HAMD-17, CGI-S, and QOLS of 14.0, 9.0, 1.0, and 62.0 vs. 16.0, 15.5, 2.0, and 40.0, respectively. No side effects were reported.
“From our findings, BLT [proved] particularly effective in bipolar patients without triggering any manic switch, as evidenced instead in some similar studies,” the researchers wrote in their discussion.
Although the mechanism of action for BLT remains unclear, the current study findings confirm the existing knowledge of BLT, they noted. The positive effect of BLT on quality of life “might be attributable to the ability of BLT to reduce the latency times of antidepressants and increase the production of serotonin and melatonin,” as shown in previous work, they said.
The study findings were limited by several factors including the small sample size, which prevents definitive conclusions about the effectiveness of BLT in combination with different antidepressants, and the heterogeneity of the antidepressant treatments, the researchers noted. Larger, prospective studies and randomized, controlled trials are needed, as are studies of special populations such as older adults or those with degenerative diseases, they said.
However, the results suggest BLT has value as a safe and effective treatment and a way to boost therapeutic response and reduce the impact of long-lasting therapies, they concluded.
The study received no outside funding. Dr. Cuomo disclosed serving as a consultant and/or a speaker for Angelini, Glaxo Smith Kline, Lundbeck, Janssen, Otsuka, Pfizer, and Recordati.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Mental health system failing kids leaving ED
Only 56% of children enrolled in Medicaid received any outpatient follow-up within 30 days after a mental health emergency department discharge, according to results of a large study released in Pediatrics.
Fewer than one-third (31.2%) had an outpatient visit within a week after a mental health ED discharge.
Researchers conducted a retrospective study of 28,551 children ages 6-17 years old who had mental health discharges from EDs from January 2018 to June 2019.
The researchers, led by Jennifer A. Hoffmann, MD, MS, with the division of emergency medicine, Ann & Robert H. Lurie Children’s Hospital of Chicago and Northwestern University, Chicago, also analyzed the effect that having a timely follow-up had on whether the child was likely to return to the ED.
Follow-up within 30 days cuts risk of quick return to ED
They found that follow-up within 30 days was linked with a 26% decreased risk of return within 5 days of the initial ED discharge (hazard ratio, 0.74; 95% confidence interval, 0.63-0.91).
The researchers also found racial disparities in the data. The odds for getting follow-up outpatient care were lower for non-Hispanic Black children, for children with fee-for-service insurance, and for children with no previous mental health outpatient visits.
The numbers were particularly striking for Black children, who were 10% less likely to get outpatient follow-up than their White counterparts.
In addition, 27% of all children in this sample returned to the ED for mental health-related symptoms within 6 months, 20% spent more than 48 hours in the ED for their initial mental health visit, and children with 14 or more mental health outpatient visits had five times higher adjusted odds of follow-up within 7 days and 9.5 times higher adjusted odds of follow-up within 30 days, compared with children with no outpatient mental health visits in the previous year.
A ‘mental health system of care in crisis’
In an accompanying editorial, Hannah E. Karpman, MSW, PhD, with the department of pediatrics, University of Massachusetts, Worcester, and colleagues said those statistics help expose other signs of “a pediatric mental health system of care in crisis.”
If one in five children are spending more than 2 days in the ED for their initial mental health visit, they wrote, that signals the follow-up care they need is not readily available.
The 27% returning to the ED shows that, even if the children are getting outpatient services, that environment is failing them, they noted.
Additionally, 28% of children presented with more than four mental health diagnoses, “suggesting poor diagnostic specificity or perhaps inadequate diagnostic categories to characterize their needs.”
The authors called for interventions that link patients to outpatient care within 5 days of a mental health ED discharge.
The editorialists wrote: “We believe it is time for a “child mental health moonshot,” and call on the field and its funders to come together to launch the next wave of bold mental health research for the benefit of these children and their families who so desperately need our support.”
Things may even be worse in light of COVID
David Rettew, MD, a child and adolescent psychiatrist with Lane County Behavioral Health in Eugene, Ore., and Oregon Health & Science University, Portland, said in an interview the numbers won’t surprise clinicians who support these children or the patients’ families.
He added that he wouldn’t be surprised if things are even worse now after this study’s data collection, “as COVID and other factors have driven more mental health professionals away from many of the people who need them the most.”
The study does present new evidence that quick access to care is particularly tough for young people who aren’t already established in care, he noted.
“As wait lists grow at outpatient clinics, we are seeing ever stronger need for centers willing and able to provide actual mental health assessment and treatment for people right ‘off the street,’” he said.
Dr. Rettew emphasized that, because mental health conditions rarely improve quickly, having a timely follow-up appointment is important, but won’t likely bring quick improvement.
He agreed with the editorialists’ argument and emphasized, “not only do we need to focus on more rapid care, but also more comprehensive and effective care.
“For an adolescent in crisis, achieving stability often involves more than a medication tweak and a supportive conversation,” Dr. Rettew said. “Rather, it can require an intensive multimodal approach that addresses things like family financial stressors, parental mental health and substance use concerns, school supports, and health promotion or lifestyle changes. What we desperately need are more teams that can quickly intervene on all these levels.”
Addressing problems before crisis is essential
Ideally, teams would address these issues before a crisis. That helps support the “moonshot” charge the editorialists suggest, which “would significantly disrupt the current way we value different components of our health care system,” Dr. Rettew said.
He highlighted a statistic that may get lost in the data: Nearly 40% of youth in enough danger to need an ED visit had no more than one health-related appointment of any kind in the previous year.
“To me, this speaks volumes about the need for earlier involvement before things escalate to the level of an emergency,” Dr. Rettew said.
The authors and editorialists declared no relevant financial relationships. Dr. Rettew is author of the book, “Parenting Made Complicated: What Science Really Knows about the Greatest Debates of Early Childhood.”
Only 56% of children enrolled in Medicaid received any outpatient follow-up within 30 days after a mental health emergency department discharge, according to results of a large study released in Pediatrics.
Fewer than one-third (31.2%) had an outpatient visit within a week after a mental health ED discharge.
Researchers conducted a retrospective study of 28,551 children ages 6-17 years old who had mental health discharges from EDs from January 2018 to June 2019.
The researchers, led by Jennifer A. Hoffmann, MD, MS, with the division of emergency medicine, Ann & Robert H. Lurie Children’s Hospital of Chicago and Northwestern University, Chicago, also analyzed the effect that having a timely follow-up had on whether the child was likely to return to the ED.
Follow-up within 30 days cuts risk of quick return to ED
They found that follow-up within 30 days was linked with a 26% decreased risk of return within 5 days of the initial ED discharge (hazard ratio, 0.74; 95% confidence interval, 0.63-0.91).
The researchers also found racial disparities in the data. The odds for getting follow-up outpatient care were lower for non-Hispanic Black children, for children with fee-for-service insurance, and for children with no previous mental health outpatient visits.
The numbers were particularly striking for Black children, who were 10% less likely to get outpatient follow-up than their White counterparts.
In addition, 27% of all children in this sample returned to the ED for mental health-related symptoms within 6 months, 20% spent more than 48 hours in the ED for their initial mental health visit, and children with 14 or more mental health outpatient visits had five times higher adjusted odds of follow-up within 7 days and 9.5 times higher adjusted odds of follow-up within 30 days, compared with children with no outpatient mental health visits in the previous year.
A ‘mental health system of care in crisis’
In an accompanying editorial, Hannah E. Karpman, MSW, PhD, with the department of pediatrics, University of Massachusetts, Worcester, and colleagues said those statistics help expose other signs of “a pediatric mental health system of care in crisis.”
If one in five children are spending more than 2 days in the ED for their initial mental health visit, they wrote, that signals the follow-up care they need is not readily available.
The 27% returning to the ED shows that, even if the children are getting outpatient services, that environment is failing them, they noted.
Additionally, 28% of children presented with more than four mental health diagnoses, “suggesting poor diagnostic specificity or perhaps inadequate diagnostic categories to characterize their needs.”
The authors called for interventions that link patients to outpatient care within 5 days of a mental health ED discharge.
The editorialists wrote: “We believe it is time for a “child mental health moonshot,” and call on the field and its funders to come together to launch the next wave of bold mental health research for the benefit of these children and their families who so desperately need our support.”
Things may even be worse in light of COVID
David Rettew, MD, a child and adolescent psychiatrist with Lane County Behavioral Health in Eugene, Ore., and Oregon Health & Science University, Portland, said in an interview the numbers won’t surprise clinicians who support these children or the patients’ families.
He added that he wouldn’t be surprised if things are even worse now after this study’s data collection, “as COVID and other factors have driven more mental health professionals away from many of the people who need them the most.”
The study does present new evidence that quick access to care is particularly tough for young people who aren’t already established in care, he noted.
“As wait lists grow at outpatient clinics, we are seeing ever stronger need for centers willing and able to provide actual mental health assessment and treatment for people right ‘off the street,’” he said.
Dr. Rettew emphasized that, because mental health conditions rarely improve quickly, having a timely follow-up appointment is important, but won’t likely bring quick improvement.
He agreed with the editorialists’ argument and emphasized, “not only do we need to focus on more rapid care, but also more comprehensive and effective care.
“For an adolescent in crisis, achieving stability often involves more than a medication tweak and a supportive conversation,” Dr. Rettew said. “Rather, it can require an intensive multimodal approach that addresses things like family financial stressors, parental mental health and substance use concerns, school supports, and health promotion or lifestyle changes. What we desperately need are more teams that can quickly intervene on all these levels.”
Addressing problems before crisis is essential
Ideally, teams would address these issues before a crisis. That helps support the “moonshot” charge the editorialists suggest, which “would significantly disrupt the current way we value different components of our health care system,” Dr. Rettew said.
He highlighted a statistic that may get lost in the data: Nearly 40% of youth in enough danger to need an ED visit had no more than one health-related appointment of any kind in the previous year.
“To me, this speaks volumes about the need for earlier involvement before things escalate to the level of an emergency,” Dr. Rettew said.
The authors and editorialists declared no relevant financial relationships. Dr. Rettew is author of the book, “Parenting Made Complicated: What Science Really Knows about the Greatest Debates of Early Childhood.”
Only 56% of children enrolled in Medicaid received any outpatient follow-up within 30 days after a mental health emergency department discharge, according to results of a large study released in Pediatrics.
Fewer than one-third (31.2%) had an outpatient visit within a week after a mental health ED discharge.
Researchers conducted a retrospective study of 28,551 children ages 6-17 years old who had mental health discharges from EDs from January 2018 to June 2019.
The researchers, led by Jennifer A. Hoffmann, MD, MS, with the division of emergency medicine, Ann & Robert H. Lurie Children’s Hospital of Chicago and Northwestern University, Chicago, also analyzed the effect that having a timely follow-up had on whether the child was likely to return to the ED.
Follow-up within 30 days cuts risk of quick return to ED
They found that follow-up within 30 days was linked with a 26% decreased risk of return within 5 days of the initial ED discharge (hazard ratio, 0.74; 95% confidence interval, 0.63-0.91).
The researchers also found racial disparities in the data. The odds for getting follow-up outpatient care were lower for non-Hispanic Black children, for children with fee-for-service insurance, and for children with no previous mental health outpatient visits.
The numbers were particularly striking for Black children, who were 10% less likely to get outpatient follow-up than their White counterparts.
In addition, 27% of all children in this sample returned to the ED for mental health-related symptoms within 6 months, 20% spent more than 48 hours in the ED for their initial mental health visit, and children with 14 or more mental health outpatient visits had five times higher adjusted odds of follow-up within 7 days and 9.5 times higher adjusted odds of follow-up within 30 days, compared with children with no outpatient mental health visits in the previous year.
A ‘mental health system of care in crisis’
In an accompanying editorial, Hannah E. Karpman, MSW, PhD, with the department of pediatrics, University of Massachusetts, Worcester, and colleagues said those statistics help expose other signs of “a pediatric mental health system of care in crisis.”
If one in five children are spending more than 2 days in the ED for their initial mental health visit, they wrote, that signals the follow-up care they need is not readily available.
The 27% returning to the ED shows that, even if the children are getting outpatient services, that environment is failing them, they noted.
Additionally, 28% of children presented with more than four mental health diagnoses, “suggesting poor diagnostic specificity or perhaps inadequate diagnostic categories to characterize their needs.”
The authors called for interventions that link patients to outpatient care within 5 days of a mental health ED discharge.
The editorialists wrote: “We believe it is time for a “child mental health moonshot,” and call on the field and its funders to come together to launch the next wave of bold mental health research for the benefit of these children and their families who so desperately need our support.”
Things may even be worse in light of COVID
David Rettew, MD, a child and adolescent psychiatrist with Lane County Behavioral Health in Eugene, Ore., and Oregon Health & Science University, Portland, said in an interview the numbers won’t surprise clinicians who support these children or the patients’ families.
He added that he wouldn’t be surprised if things are even worse now after this study’s data collection, “as COVID and other factors have driven more mental health professionals away from many of the people who need them the most.”
The study does present new evidence that quick access to care is particularly tough for young people who aren’t already established in care, he noted.
“As wait lists grow at outpatient clinics, we are seeing ever stronger need for centers willing and able to provide actual mental health assessment and treatment for people right ‘off the street,’” he said.
Dr. Rettew emphasized that, because mental health conditions rarely improve quickly, having a timely follow-up appointment is important, but won’t likely bring quick improvement.
He agreed with the editorialists’ argument and emphasized, “not only do we need to focus on more rapid care, but also more comprehensive and effective care.
“For an adolescent in crisis, achieving stability often involves more than a medication tweak and a supportive conversation,” Dr. Rettew said. “Rather, it can require an intensive multimodal approach that addresses things like family financial stressors, parental mental health and substance use concerns, school supports, and health promotion or lifestyle changes. What we desperately need are more teams that can quickly intervene on all these levels.”
Addressing problems before crisis is essential
Ideally, teams would address these issues before a crisis. That helps support the “moonshot” charge the editorialists suggest, which “would significantly disrupt the current way we value different components of our health care system,” Dr. Rettew said.
He highlighted a statistic that may get lost in the data: Nearly 40% of youth in enough danger to need an ED visit had no more than one health-related appointment of any kind in the previous year.
“To me, this speaks volumes about the need for earlier involvement before things escalate to the level of an emergency,” Dr. Rettew said.
The authors and editorialists declared no relevant financial relationships. Dr. Rettew is author of the book, “Parenting Made Complicated: What Science Really Knows about the Greatest Debates of Early Childhood.”
FROM PEDIATRICS
Finding catatonia requires knowing what to look for
Catatonia is a psychomotor syndrome identified by its clinical phenotype. Unlike common psychiatric syndromes such as major depression that are characterized by self-report of symptoms, catatonia is identified chiefly by empirically evaluated signs on clinical evaluation. Its signs are recognized through observation, physical examination, or elicitation by clinical maneuvers or the presentation of stimuli. However, catatonia is often overlooked even though its clinical signs are often visibly apparent, including to the casual observer.
Why is catatonia underdiagnosed? A key modifiable factor appears to be a prevalent misunderstanding over what catatonia looks like.1 We have sought to address this in a few ways.
First identified was the need for comprehensive educational resources on how to assess for and recognize catatonia. Using the Bush-Francis Catatonia Rating Scale – the most widely used scale for catatonia in both research and clinical settings and the most cited publication in the catatonia literature – our team developed the BFCRS Training Manual and Coding Guide.2,3 This manual expands on the definitions of each BFCRS item based on how it was originally operationalized by the scale’s authors. Subsequently, we created a comprehensive set of educational resources including videos illustrating how to assess for catatonia, a video for each of the 23 items on the BFCRS, and self-assessment tools. All resources are freely available online at https://bfcrs.urmc.edu.4
Through this project it became apparent that there are many discrepancies across the field regarding the phenotype of catatonia. Specifically, a recent review inspired by this project set about to characterize the scope of distinctions across diagnostic systems and rating scales.5 For instance, each diagnostic system and rating scale includes a unique set of signs, approaches diagnostic thresholds differently, and often operationalizes clinical features in ways that lead either to criterion overlap (for example, combativeness would be scored both as combativeness and agitation on ICD-11) or contradictions with other systems or scales (for example, varied definitions of waxy flexibility). In the face of so many inconsistencies, what is a clinician to do? What follows is a discussion of how to apply the insights from this recent review in clinical and research settings.
Starting with DSM-5-TR and ICD-11 – the current editions of the two leading diagnostic systems – one might ask: How do they compare?6,7 Overall, these two systems are broadly aligned in terms of the catatonic syndrome. Both systems identify individual clinical signs (as opposed to symptom complexes). Both require three features as a diagnostic threshold. Most of the same clinical signs are included in both systems, and the definitions of individual items are largely equivalent. Additionally, both systems allow for diagnosis of catatonia in association with psychiatric and medical conditions and include a category for unspecified catatonia.
Despite these core agreements, though, there are several important distinctions. First, whereas all 12 signs included in DSM-5-TR count toward an ICD-11 catatonia diagnosis, the opposite cannot be said. ICD-11 includes several features that are not in DSM-5-TR: rigidity, verbigeration, withdrawal, staring, ambitendency, impulsivity, and combativeness. Next, autonomic abnormality, which signifies the most severe type of catatonia called malignant catatonia, is included as a potential comorbidity in ICD-11 but not mentioned in DSM-5-TR. Third, ICD-11 includes a separate diagnosis for substance-induced catatonia, whereas this condition would be diagnosed as unspecified catatonia in DSM-5-TR.
There are also elements missing from both systems. The most notable of these is that neither system specifies the period over which findings must be present for diagnosis. By clinical convention, the practical definition of 24 hours is appropriate in most instances. The clinical features identified during direct evaluation are usually sufficient for diagnosis, but additional signs observed or documented over the prior 24 hours should be incorporated as part of the clinical evaluation. Another distinction is how to handle clinical features before and after lorazepam challenge. As noted in the BFCRS Training Manual, it would be appropriate to compare “state assessments” (that is, restricted to features identified only during direct, in-person assessment) from before and after lorazepam administration to document improvement.4
Whereas DSM-5-TR and ICD-11 are broadly in agreement, comparing these systems with catatonia rating scales reveals many sources of potential confusion, but also concrete guidance on operationalizing individual items.5 How exactly should each of catatonia’s clinical signs be defined? Descriptions differ, and thresholds of duration and frequency vary considerably across scales. As a result, clinicians who use different scales and then convert these results to diagnostic criteria are liable to come to different clinical conclusions. For instance, both echophenomena and negativism must be elicited more than five times to be scored per Northoff,8 but even a single convincing instance of either would be scored on the BFCRS as “occasional.”2
Such discrepancies are important because, whereas the psychometric properties of several catatonia scales have been documented, there are no analogous studies on the DSM-5-TR and ICD-11 criteria. Therefore, it is essential for clinicians and researchers to document how diagnostic criteria have been operationalized. The most practical and evidence-based way to do this is to use a clinically validated scale and convert these to diagnostic criteria, yet in doing so a few modifications will be necessary.
Of the available clinical scales, the BFCRS is best positioned for clinical use. The BFCRS has been validated clinically and has good reliability, detailed item definitions and audiovisual examples available. In addition, it is the only scale with a published semistructured evaluation (see initial paper and Training Manual), which takes about 5 minutes.2,4 In terms of utility, all 12 signs included by DSM-5-TR are among the first 14 items on the BFCRS, which constitutes a standalone tool known as the Bush-Francis Catatonia Screening Instrument (BFCSI, see Table).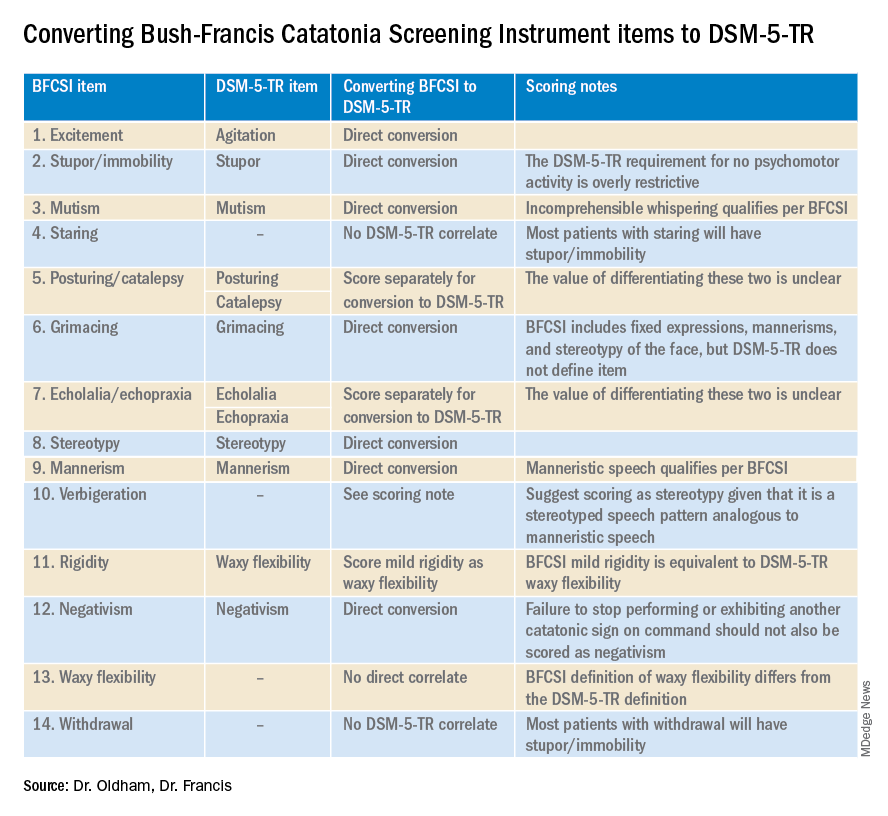
Many fundamental questions remain about catatonia,but the importance of a shared understanding of its clinical features is clear.9 Catatonia should be on the differential whenever a patient exhibits a markedly altered level of activity or grossly abnormal behavior, especially when inappropriate to context. We encourage readers to familiarize themselves with the phenotype of catatonia through online educational resources4 because the optimal care of patients with catatonia requires – at a minimum – that we know what we’re looking for.
Dr. Oldham is assistant professor of psychiatry at the University of Rochester (N.Y.) Medical Center. Dr. Francis is professor of psychiatry at Penn State University, Hershey. The authors declare no relevant conflicts of interest. Funding for the educational project hosted at https://bfcrs.urmc.edu was provided by the department of psychiatry at the University of Rochester Medical Center. Dr. Oldham is currently supported by a K23 career development award from the National Institute on Aging (AG072383). The educational resources referenced in this piece could not have been created were it not for the intellectual and thespian collaboration of Joshua R. Wortzel, MD, who is currently a fellow in child and adolescent psychiatry at Brown University, Providence, R.I. The authors are also indebted to Hochang B. Lee, MD, for his gracious support of this project.
References
1. Wortzel JR et al. J Clin Psychiatry. 2021 Aug 17;82(5):21m14025. doi: 10.4088/JCP.21m14025.
2. Bush G et al. Acta Psychiatr Scand. 1996 Feb;93(2):129-36. doi: 10.1111/j.1600-0447.1996.tb09814.x.
3. Weleff J et al. J Acad Consult Liaison Psychiatry. 2023 Jan-Feb;64(1):13-27. doi:10.1016/j.jaclp.2022.07.002.
4. Oldham MA et al. Bush-Francis Catatonia Rating Scale Assessment Resources. University of Rochester Medical Center, Department of Psychiatry. https://bfcrs.urmc.edu.
5. Oldham MA. Schizophr Res. 2022 Aug 19;S0920-9964(22)00294-8. doi: 10.1016/j.schres.2022.08.002.
6. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5-TR. Washington, D.C.: American Psychiatric Association Publishing, 2022.
7. World Health Organization. ICD-11 for Mortality and Morbidity Stastistics. 2022. https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/486722075.
8. Northoff G et al. Mov Disord. May 1999;14(3):404-16. doi: 10.1002/1531-8257(199905)14:3<404::AID-MDS1004>3.0.CO;2-5.
9. Walther S et al. The Lancet Psychiatry. 2019 Jul;6(7):610-9. doi: 10.1016/S2215-0366(18)30474-7.
Catatonia is a psychomotor syndrome identified by its clinical phenotype. Unlike common psychiatric syndromes such as major depression that are characterized by self-report of symptoms, catatonia is identified chiefly by empirically evaluated signs on clinical evaluation. Its signs are recognized through observation, physical examination, or elicitation by clinical maneuvers or the presentation of stimuli. However, catatonia is often overlooked even though its clinical signs are often visibly apparent, including to the casual observer.
Why is catatonia underdiagnosed? A key modifiable factor appears to be a prevalent misunderstanding over what catatonia looks like.1 We have sought to address this in a few ways.
First identified was the need for comprehensive educational resources on how to assess for and recognize catatonia. Using the Bush-Francis Catatonia Rating Scale – the most widely used scale for catatonia in both research and clinical settings and the most cited publication in the catatonia literature – our team developed the BFCRS Training Manual and Coding Guide.2,3 This manual expands on the definitions of each BFCRS item based on how it was originally operationalized by the scale’s authors. Subsequently, we created a comprehensive set of educational resources including videos illustrating how to assess for catatonia, a video for each of the 23 items on the BFCRS, and self-assessment tools. All resources are freely available online at https://bfcrs.urmc.edu.4
Through this project it became apparent that there are many discrepancies across the field regarding the phenotype of catatonia. Specifically, a recent review inspired by this project set about to characterize the scope of distinctions across diagnostic systems and rating scales.5 For instance, each diagnostic system and rating scale includes a unique set of signs, approaches diagnostic thresholds differently, and often operationalizes clinical features in ways that lead either to criterion overlap (for example, combativeness would be scored both as combativeness and agitation on ICD-11) or contradictions with other systems or scales (for example, varied definitions of waxy flexibility). In the face of so many inconsistencies, what is a clinician to do? What follows is a discussion of how to apply the insights from this recent review in clinical and research settings.
Starting with DSM-5-TR and ICD-11 – the current editions of the two leading diagnostic systems – one might ask: How do they compare?6,7 Overall, these two systems are broadly aligned in terms of the catatonic syndrome. Both systems identify individual clinical signs (as opposed to symptom complexes). Both require three features as a diagnostic threshold. Most of the same clinical signs are included in both systems, and the definitions of individual items are largely equivalent. Additionally, both systems allow for diagnosis of catatonia in association with psychiatric and medical conditions and include a category for unspecified catatonia.
Despite these core agreements, though, there are several important distinctions. First, whereas all 12 signs included in DSM-5-TR count toward an ICD-11 catatonia diagnosis, the opposite cannot be said. ICD-11 includes several features that are not in DSM-5-TR: rigidity, verbigeration, withdrawal, staring, ambitendency, impulsivity, and combativeness. Next, autonomic abnormality, which signifies the most severe type of catatonia called malignant catatonia, is included as a potential comorbidity in ICD-11 but not mentioned in DSM-5-TR. Third, ICD-11 includes a separate diagnosis for substance-induced catatonia, whereas this condition would be diagnosed as unspecified catatonia in DSM-5-TR.
There are also elements missing from both systems. The most notable of these is that neither system specifies the period over which findings must be present for diagnosis. By clinical convention, the practical definition of 24 hours is appropriate in most instances. The clinical features identified during direct evaluation are usually sufficient for diagnosis, but additional signs observed or documented over the prior 24 hours should be incorporated as part of the clinical evaluation. Another distinction is how to handle clinical features before and after lorazepam challenge. As noted in the BFCRS Training Manual, it would be appropriate to compare “state assessments” (that is, restricted to features identified only during direct, in-person assessment) from before and after lorazepam administration to document improvement.4
Whereas DSM-5-TR and ICD-11 are broadly in agreement, comparing these systems with catatonia rating scales reveals many sources of potential confusion, but also concrete guidance on operationalizing individual items.5 How exactly should each of catatonia’s clinical signs be defined? Descriptions differ, and thresholds of duration and frequency vary considerably across scales. As a result, clinicians who use different scales and then convert these results to diagnostic criteria are liable to come to different clinical conclusions. For instance, both echophenomena and negativism must be elicited more than five times to be scored per Northoff,8 but even a single convincing instance of either would be scored on the BFCRS as “occasional.”2
Such discrepancies are important because, whereas the psychometric properties of several catatonia scales have been documented, there are no analogous studies on the DSM-5-TR and ICD-11 criteria. Therefore, it is essential for clinicians and researchers to document how diagnostic criteria have been operationalized. The most practical and evidence-based way to do this is to use a clinically validated scale and convert these to diagnostic criteria, yet in doing so a few modifications will be necessary.
Of the available clinical scales, the BFCRS is best positioned for clinical use. The BFCRS has been validated clinically and has good reliability, detailed item definitions and audiovisual examples available. In addition, it is the only scale with a published semistructured evaluation (see initial paper and Training Manual), which takes about 5 minutes.2,4 In terms of utility, all 12 signs included by DSM-5-TR are among the first 14 items on the BFCRS, which constitutes a standalone tool known as the Bush-Francis Catatonia Screening Instrument (BFCSI, see Table).
Many fundamental questions remain about catatonia,but the importance of a shared understanding of its clinical features is clear.9 Catatonia should be on the differential whenever a patient exhibits a markedly altered level of activity or grossly abnormal behavior, especially when inappropriate to context. We encourage readers to familiarize themselves with the phenotype of catatonia through online educational resources4 because the optimal care of patients with catatonia requires – at a minimum – that we know what we’re looking for.
Dr. Oldham is assistant professor of psychiatry at the University of Rochester (N.Y.) Medical Center. Dr. Francis is professor of psychiatry at Penn State University, Hershey. The authors declare no relevant conflicts of interest. Funding for the educational project hosted at https://bfcrs.urmc.edu was provided by the department of psychiatry at the University of Rochester Medical Center. Dr. Oldham is currently supported by a K23 career development award from the National Institute on Aging (AG072383). The educational resources referenced in this piece could not have been created were it not for the intellectual and thespian collaboration of Joshua R. Wortzel, MD, who is currently a fellow in child and adolescent psychiatry at Brown University, Providence, R.I. The authors are also indebted to Hochang B. Lee, MD, for his gracious support of this project.
References
1. Wortzel JR et al. J Clin Psychiatry. 2021 Aug 17;82(5):21m14025. doi: 10.4088/JCP.21m14025.
2. Bush G et al. Acta Psychiatr Scand. 1996 Feb;93(2):129-36. doi: 10.1111/j.1600-0447.1996.tb09814.x.
3. Weleff J et al. J Acad Consult Liaison Psychiatry. 2023 Jan-Feb;64(1):13-27. doi:10.1016/j.jaclp.2022.07.002.
4. Oldham MA et al. Bush-Francis Catatonia Rating Scale Assessment Resources. University of Rochester Medical Center, Department of Psychiatry. https://bfcrs.urmc.edu.
5. Oldham MA. Schizophr Res. 2022 Aug 19;S0920-9964(22)00294-8. doi: 10.1016/j.schres.2022.08.002.
6. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5-TR. Washington, D.C.: American Psychiatric Association Publishing, 2022.
7. World Health Organization. ICD-11 for Mortality and Morbidity Stastistics. 2022. https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/486722075.
8. Northoff G et al. Mov Disord. May 1999;14(3):404-16. doi: 10.1002/1531-8257(199905)14:3<404::AID-MDS1004>3.0.CO;2-5.
9. Walther S et al. The Lancet Psychiatry. 2019 Jul;6(7):610-9. doi: 10.1016/S2215-0366(18)30474-7.
Catatonia is a psychomotor syndrome identified by its clinical phenotype. Unlike common psychiatric syndromes such as major depression that are characterized by self-report of symptoms, catatonia is identified chiefly by empirically evaluated signs on clinical evaluation. Its signs are recognized through observation, physical examination, or elicitation by clinical maneuvers or the presentation of stimuli. However, catatonia is often overlooked even though its clinical signs are often visibly apparent, including to the casual observer.
Why is catatonia underdiagnosed? A key modifiable factor appears to be a prevalent misunderstanding over what catatonia looks like.1 We have sought to address this in a few ways.
First identified was the need for comprehensive educational resources on how to assess for and recognize catatonia. Using the Bush-Francis Catatonia Rating Scale – the most widely used scale for catatonia in both research and clinical settings and the most cited publication in the catatonia literature – our team developed the BFCRS Training Manual and Coding Guide.2,3 This manual expands on the definitions of each BFCRS item based on how it was originally operationalized by the scale’s authors. Subsequently, we created a comprehensive set of educational resources including videos illustrating how to assess for catatonia, a video for each of the 23 items on the BFCRS, and self-assessment tools. All resources are freely available online at https://bfcrs.urmc.edu.4
Through this project it became apparent that there are many discrepancies across the field regarding the phenotype of catatonia. Specifically, a recent review inspired by this project set about to characterize the scope of distinctions across diagnostic systems and rating scales.5 For instance, each diagnostic system and rating scale includes a unique set of signs, approaches diagnostic thresholds differently, and often operationalizes clinical features in ways that lead either to criterion overlap (for example, combativeness would be scored both as combativeness and agitation on ICD-11) or contradictions with other systems or scales (for example, varied definitions of waxy flexibility). In the face of so many inconsistencies, what is a clinician to do? What follows is a discussion of how to apply the insights from this recent review in clinical and research settings.
Starting with DSM-5-TR and ICD-11 – the current editions of the two leading diagnostic systems – one might ask: How do they compare?6,7 Overall, these two systems are broadly aligned in terms of the catatonic syndrome. Both systems identify individual clinical signs (as opposed to symptom complexes). Both require three features as a diagnostic threshold. Most of the same clinical signs are included in both systems, and the definitions of individual items are largely equivalent. Additionally, both systems allow for diagnosis of catatonia in association with psychiatric and medical conditions and include a category for unspecified catatonia.
Despite these core agreements, though, there are several important distinctions. First, whereas all 12 signs included in DSM-5-TR count toward an ICD-11 catatonia diagnosis, the opposite cannot be said. ICD-11 includes several features that are not in DSM-5-TR: rigidity, verbigeration, withdrawal, staring, ambitendency, impulsivity, and combativeness. Next, autonomic abnormality, which signifies the most severe type of catatonia called malignant catatonia, is included as a potential comorbidity in ICD-11 but not mentioned in DSM-5-TR. Third, ICD-11 includes a separate diagnosis for substance-induced catatonia, whereas this condition would be diagnosed as unspecified catatonia in DSM-5-TR.
There are also elements missing from both systems. The most notable of these is that neither system specifies the period over which findings must be present for diagnosis. By clinical convention, the practical definition of 24 hours is appropriate in most instances. The clinical features identified during direct evaluation are usually sufficient for diagnosis, but additional signs observed or documented over the prior 24 hours should be incorporated as part of the clinical evaluation. Another distinction is how to handle clinical features before and after lorazepam challenge. As noted in the BFCRS Training Manual, it would be appropriate to compare “state assessments” (that is, restricted to features identified only during direct, in-person assessment) from before and after lorazepam administration to document improvement.4
Whereas DSM-5-TR and ICD-11 are broadly in agreement, comparing these systems with catatonia rating scales reveals many sources of potential confusion, but also concrete guidance on operationalizing individual items.5 How exactly should each of catatonia’s clinical signs be defined? Descriptions differ, and thresholds of duration and frequency vary considerably across scales. As a result, clinicians who use different scales and then convert these results to diagnostic criteria are liable to come to different clinical conclusions. For instance, both echophenomena and negativism must be elicited more than five times to be scored per Northoff,8 but even a single convincing instance of either would be scored on the BFCRS as “occasional.”2
Such discrepancies are important because, whereas the psychometric properties of several catatonia scales have been documented, there are no analogous studies on the DSM-5-TR and ICD-11 criteria. Therefore, it is essential for clinicians and researchers to document how diagnostic criteria have been operationalized. The most practical and evidence-based way to do this is to use a clinically validated scale and convert these to diagnostic criteria, yet in doing so a few modifications will be necessary.
Of the available clinical scales, the BFCRS is best positioned for clinical use. The BFCRS has been validated clinically and has good reliability, detailed item definitions and audiovisual examples available. In addition, it is the only scale with a published semistructured evaluation (see initial paper and Training Manual), which takes about 5 minutes.2,4 In terms of utility, all 12 signs included by DSM-5-TR are among the first 14 items on the BFCRS, which constitutes a standalone tool known as the Bush-Francis Catatonia Screening Instrument (BFCSI, see Table).
Many fundamental questions remain about catatonia,but the importance of a shared understanding of its clinical features is clear.9 Catatonia should be on the differential whenever a patient exhibits a markedly altered level of activity or grossly abnormal behavior, especially when inappropriate to context. We encourage readers to familiarize themselves with the phenotype of catatonia through online educational resources4 because the optimal care of patients with catatonia requires – at a minimum – that we know what we’re looking for.
Dr. Oldham is assistant professor of psychiatry at the University of Rochester (N.Y.) Medical Center. Dr. Francis is professor of psychiatry at Penn State University, Hershey. The authors declare no relevant conflicts of interest. Funding for the educational project hosted at https://bfcrs.urmc.edu was provided by the department of psychiatry at the University of Rochester Medical Center. Dr. Oldham is currently supported by a K23 career development award from the National Institute on Aging (AG072383). The educational resources referenced in this piece could not have been created were it not for the intellectual and thespian collaboration of Joshua R. Wortzel, MD, who is currently a fellow in child and adolescent psychiatry at Brown University, Providence, R.I. The authors are also indebted to Hochang B. Lee, MD, for his gracious support of this project.
References
1. Wortzel JR et al. J Clin Psychiatry. 2021 Aug 17;82(5):21m14025. doi: 10.4088/JCP.21m14025.
2. Bush G et al. Acta Psychiatr Scand. 1996 Feb;93(2):129-36. doi: 10.1111/j.1600-0447.1996.tb09814.x.
3. Weleff J et al. J Acad Consult Liaison Psychiatry. 2023 Jan-Feb;64(1):13-27. doi:10.1016/j.jaclp.2022.07.002.
4. Oldham MA et al. Bush-Francis Catatonia Rating Scale Assessment Resources. University of Rochester Medical Center, Department of Psychiatry. https://bfcrs.urmc.edu.
5. Oldham MA. Schizophr Res. 2022 Aug 19;S0920-9964(22)00294-8. doi: 10.1016/j.schres.2022.08.002.
6. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5-TR. Washington, D.C.: American Psychiatric Association Publishing, 2022.
7. World Health Organization. ICD-11 for Mortality and Morbidity Stastistics. 2022. https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/486722075.
8. Northoff G et al. Mov Disord. May 1999;14(3):404-16. doi: 10.1002/1531-8257(199905)14:3<404::AID-MDS1004>3.0.CO;2-5.
9. Walther S et al. The Lancet Psychiatry. 2019 Jul;6(7):610-9. doi: 10.1016/S2215-0366(18)30474-7.
TMS tied to ‘marked’ antidepressant, anxiolytic effects in anxious depression
In an analysis of data from more than 1,800 patients with a diagnosis of major depressive disorder (MDD), more than 75% also had anxiety. Following TMS, those with anxious depression showed reductions from baseline of at least 50% on anxiety and depression scores.
In addition, the anxious and nonanxious groups had equivalent absolute improvement in scores measuring depression.
“The ultimate message is that TMS is quite effective in the more difficult-to-treat and more disabled group of anxious depressives,” coinvestigator Scott Aaronson, MD, chief science officer, Institute for Advanced Diagnostics and Therapeutics, and director of the Psychedelic Center of Excellence, Sheppard Pratt, Towson, Md., told this news organization.
The findings were published online in the Journal of Clinical Psychiatry.
Large cohort
Dr. Aaronson noted that between 50% and 75% of patients with depression also have significant anxiety symptoms.
“The presence of significant anxiety in a depressed person significantly increases depression symptom severity, functional impairment, chronicity, and suicidality,” he said.
In general, “when patients with anxious depression are identified in a treatment study, they are less likely to respond to the index treatment and are frequently excluded from some treatment trials,” he added.
Dr. Aaronson noted that previously reported outcomes from TMS for anxious depression have been “suggestive of efficacy but have not been well studied within a large cohort.”
To investigate these issues, the current investigators turned to the NeuroStar Advanced Therapy System Clinical Outcomes Registry. It is the largest database of patients with difficult-to-treat depression, all of whom had undergone TMS.
This “extraordinary” database was able to provide previous insight into how often TMS works, whether some of the treatment parameters can be altered while still preserving efficacy, and whether bilateral TMS works better than unilateral TMS in patients with MDD, Dr. Aaronson said.
In the current study, researchers retrospectively analyzed data on 1,820 patients with MDD. All had completed the Patient Health Questinonaire–9 (PHQ-9) and the Generalized Anxiety Disorder–7 (GAD-7) at baseline and following at least one TMS intervention.
Most patients (n = 1,514) had anxious depression, defined as a baseline GAD-7 score of 10 or higher, and 306 had nonanxious depression, defined as a GAD-7 score below that threshold.
The investigators assessed the total sample of these patients who had been treated with any TMS protocol, as well as a subsample of patients (n = 625) who had been treated only with high-frequency left dorsolateral prefrontal cortex (HF-LUL) stimulation.
Patients were also subdivided into intent-to-treat and Completer samples (n = 1,820 and 1,429, respectively).
Consistent effects
There was no difference in gender distribution between the anxious and nonanxious group.
However, the anxious group was significantly younger (by about 5 years), compared with the nonanxious group. They also reported higher severity of depressive symptoms at baseline, with PHQ-9 scores approximately 2.5 points higher.
This was a “notable finding, since the PHQ-9 does not contain items directly assessing anxiety,” the researchers wrote.
There were also differences between the groups in the type of TMS protocol they received, with exclusive HF-LUL more common in the nonanxious depression group compared with other types of TMS protocols or unclassified protocols in the anxious depression group.
“Anxiolytic and antidepressant effects were consistent across the [intent-to-treat] and completed samples and patients who received any TMS protocol or only HF-LUL TMS,” the investigators reported.
GAD-7 scores “decreased markedly” in the anxious depression group. GAD-7 response rates ranged from 47.8% to 60.6% and GAD-7 remission rates ranged from 26.4% to 38.0% (P < .0001 for both).
There were no between-group differences in PHQ-9 scores in the magnitude of change pre- to post treatment. The anxious group scored about 2.5 points higher both pre- and post treatment, compared with the anxious group – with an effect size for change ranging from 1.46 to 1.74 in the anxious group and from 1.66 to 1.95 in the nonanxious group.
Response, remission rates
Notably, the anxious and nonanxious groups both showed “marked antidepressant effects,” with response and remission rates in the anxious group ranging from 55.2% to 66.8% and from 24.0% to 33.2%, respectively.
However, response and remission rates were significantly higher in the nonanxious versus the anxious group.
“Thus, despite manifesting the same degree of change in the PHQ-9 scores, the higher baseline and post-TMS scores in the anxious group resulted in significantly lower response and remission rates,” the investigators wrote.
They noted that the difference in post-TMS adjusted means was “small” and the groups also “did not differ in the absolute extent of symptoms improvement after multivariate adjustment.”
The relationship changes in the GAD-7 and the PHQ-9 scores “covaried” for the total IT sample (r1818 = 0.69, P < .001), although the relation was more “robust” in the anxious depression group versus the nonanxious depression group (r1512 = .75 vs. r304 = 0.50; P < .001 for both).
“The anxious depressed folks were sicker and had higher scores on scales capturing the severity of their illness,” Dr. Aaronson said. However, their “outcomes were similar, taking into account the higher baseline scores which had the effect of lowering the percent of anxious participants who met response and remission criteria.”
He reported that the average decline in depression rating scale scores was not significantly different between the groups, and the decline in depression scores tracked similarly to the decline in anxiety scores, “meaning they strongly covaried.”
The authors noted that a limitation was that, although the data was prospectively gathered, the analyses were retrospective.
Settles the debate?
Commenting on the study, Shan Siddiqi, MD, assistant professor of psychiatry at Harvard Medical School, Boston, said clinicians know that patients with comorbid anxiety are less likely to be referred for TMS, “probably because of the longstanding perception that TMS doesn’t work as well for them.”
This perception “has persisted, despite several small studies to the contrary, perhaps because we know that these patients are less responsive to other treatments,” said Dr. Siddiqi, who is also director of psychiatric neuromodulation research at Brigham and Women’s Center for Brain Circuit Therapeutics in Boston. He was not involved with the current research.
“This new study will hopefully settle that debate and let us move on to a new question: How do we optimize the treatment for this important patient population that has largely been excluded from many of our prior studies?”
The NeuroStar Advanced Therapy System Clinical Outcomes Registry, analysis of the registry data, and the drafting of this manuscript were supported by Neuronetics Inc. Dr. Aaronson serves as a scientific adviser to Genomind, LivaNova, Neuronetics, Janssen Pharmaceuticals, and Sage Therapeutics; and has received research support from Compass Pathways and Neuronetics. Dr. Siddiqi is a scientific consultant for Magnus Medical; a clinical consultant for Acacia Mental Health, Kaizen Brain Center, and Boston Precision Neurotherapeutics; and has received investigator-initiated research funding from Neuronetics and BrainsWay. He has also served as a speaker for BrainsWay and PsychU.org, owns stock in BrainsWay and Magnus Medical, and owns intellectual property involving the use of functional connectivity to target TMS.
A version of this article first appeared on Medscape.com.
In an analysis of data from more than 1,800 patients with a diagnosis of major depressive disorder (MDD), more than 75% also had anxiety. Following TMS, those with anxious depression showed reductions from baseline of at least 50% on anxiety and depression scores.
In addition, the anxious and nonanxious groups had equivalent absolute improvement in scores measuring depression.
“The ultimate message is that TMS is quite effective in the more difficult-to-treat and more disabled group of anxious depressives,” coinvestigator Scott Aaronson, MD, chief science officer, Institute for Advanced Diagnostics and Therapeutics, and director of the Psychedelic Center of Excellence, Sheppard Pratt, Towson, Md., told this news organization.
The findings were published online in the Journal of Clinical Psychiatry.
Large cohort
Dr. Aaronson noted that between 50% and 75% of patients with depression also have significant anxiety symptoms.
“The presence of significant anxiety in a depressed person significantly increases depression symptom severity, functional impairment, chronicity, and suicidality,” he said.
In general, “when patients with anxious depression are identified in a treatment study, they are less likely to respond to the index treatment and are frequently excluded from some treatment trials,” he added.
Dr. Aaronson noted that previously reported outcomes from TMS for anxious depression have been “suggestive of efficacy but have not been well studied within a large cohort.”
To investigate these issues, the current investigators turned to the NeuroStar Advanced Therapy System Clinical Outcomes Registry. It is the largest database of patients with difficult-to-treat depression, all of whom had undergone TMS.
This “extraordinary” database was able to provide previous insight into how often TMS works, whether some of the treatment parameters can be altered while still preserving efficacy, and whether bilateral TMS works better than unilateral TMS in patients with MDD, Dr. Aaronson said.
In the current study, researchers retrospectively analyzed data on 1,820 patients with MDD. All had completed the Patient Health Questinonaire–9 (PHQ-9) and the Generalized Anxiety Disorder–7 (GAD-7) at baseline and following at least one TMS intervention.
Most patients (n = 1,514) had anxious depression, defined as a baseline GAD-7 score of 10 or higher, and 306 had nonanxious depression, defined as a GAD-7 score below that threshold.
The investigators assessed the total sample of these patients who had been treated with any TMS protocol, as well as a subsample of patients (n = 625) who had been treated only with high-frequency left dorsolateral prefrontal cortex (HF-LUL) stimulation.
Patients were also subdivided into intent-to-treat and Completer samples (n = 1,820 and 1,429, respectively).
Consistent effects
There was no difference in gender distribution between the anxious and nonanxious group.
However, the anxious group was significantly younger (by about 5 years), compared with the nonanxious group. They also reported higher severity of depressive symptoms at baseline, with PHQ-9 scores approximately 2.5 points higher.
This was a “notable finding, since the PHQ-9 does not contain items directly assessing anxiety,” the researchers wrote.
There were also differences between the groups in the type of TMS protocol they received, with exclusive HF-LUL more common in the nonanxious depression group compared with other types of TMS protocols or unclassified protocols in the anxious depression group.
“Anxiolytic and antidepressant effects were consistent across the [intent-to-treat] and completed samples and patients who received any TMS protocol or only HF-LUL TMS,” the investigators reported.
GAD-7 scores “decreased markedly” in the anxious depression group. GAD-7 response rates ranged from 47.8% to 60.6% and GAD-7 remission rates ranged from 26.4% to 38.0% (P < .0001 for both).
There were no between-group differences in PHQ-9 scores in the magnitude of change pre- to post treatment. The anxious group scored about 2.5 points higher both pre- and post treatment, compared with the anxious group – with an effect size for change ranging from 1.46 to 1.74 in the anxious group and from 1.66 to 1.95 in the nonanxious group.
Response, remission rates
Notably, the anxious and nonanxious groups both showed “marked antidepressant effects,” with response and remission rates in the anxious group ranging from 55.2% to 66.8% and from 24.0% to 33.2%, respectively.
However, response and remission rates were significantly higher in the nonanxious versus the anxious group.
“Thus, despite manifesting the same degree of change in the PHQ-9 scores, the higher baseline and post-TMS scores in the anxious group resulted in significantly lower response and remission rates,” the investigators wrote.
They noted that the difference in post-TMS adjusted means was “small” and the groups also “did not differ in the absolute extent of symptoms improvement after multivariate adjustment.”
The relationship changes in the GAD-7 and the PHQ-9 scores “covaried” for the total IT sample (r1818 = 0.69, P < .001), although the relation was more “robust” in the anxious depression group versus the nonanxious depression group (r1512 = .75 vs. r304 = 0.50; P < .001 for both).
“The anxious depressed folks were sicker and had higher scores on scales capturing the severity of their illness,” Dr. Aaronson said. However, their “outcomes were similar, taking into account the higher baseline scores which had the effect of lowering the percent of anxious participants who met response and remission criteria.”
He reported that the average decline in depression rating scale scores was not significantly different between the groups, and the decline in depression scores tracked similarly to the decline in anxiety scores, “meaning they strongly covaried.”
The authors noted that a limitation was that, although the data was prospectively gathered, the analyses were retrospective.
Settles the debate?
Commenting on the study, Shan Siddiqi, MD, assistant professor of psychiatry at Harvard Medical School, Boston, said clinicians know that patients with comorbid anxiety are less likely to be referred for TMS, “probably because of the longstanding perception that TMS doesn’t work as well for them.”
This perception “has persisted, despite several small studies to the contrary, perhaps because we know that these patients are less responsive to other treatments,” said Dr. Siddiqi, who is also director of psychiatric neuromodulation research at Brigham and Women’s Center for Brain Circuit Therapeutics in Boston. He was not involved with the current research.
“This new study will hopefully settle that debate and let us move on to a new question: How do we optimize the treatment for this important patient population that has largely been excluded from many of our prior studies?”
The NeuroStar Advanced Therapy System Clinical Outcomes Registry, analysis of the registry data, and the drafting of this manuscript were supported by Neuronetics Inc. Dr. Aaronson serves as a scientific adviser to Genomind, LivaNova, Neuronetics, Janssen Pharmaceuticals, and Sage Therapeutics; and has received research support from Compass Pathways and Neuronetics. Dr. Siddiqi is a scientific consultant for Magnus Medical; a clinical consultant for Acacia Mental Health, Kaizen Brain Center, and Boston Precision Neurotherapeutics; and has received investigator-initiated research funding from Neuronetics and BrainsWay. He has also served as a speaker for BrainsWay and PsychU.org, owns stock in BrainsWay and Magnus Medical, and owns intellectual property involving the use of functional connectivity to target TMS.
A version of this article first appeared on Medscape.com.
In an analysis of data from more than 1,800 patients with a diagnosis of major depressive disorder (MDD), more than 75% also had anxiety. Following TMS, those with anxious depression showed reductions from baseline of at least 50% on anxiety and depression scores.
In addition, the anxious and nonanxious groups had equivalent absolute improvement in scores measuring depression.
“The ultimate message is that TMS is quite effective in the more difficult-to-treat and more disabled group of anxious depressives,” coinvestigator Scott Aaronson, MD, chief science officer, Institute for Advanced Diagnostics and Therapeutics, and director of the Psychedelic Center of Excellence, Sheppard Pratt, Towson, Md., told this news organization.
The findings were published online in the Journal of Clinical Psychiatry.
Large cohort
Dr. Aaronson noted that between 50% and 75% of patients with depression also have significant anxiety symptoms.
“The presence of significant anxiety in a depressed person significantly increases depression symptom severity, functional impairment, chronicity, and suicidality,” he said.
In general, “when patients with anxious depression are identified in a treatment study, they are less likely to respond to the index treatment and are frequently excluded from some treatment trials,” he added.
Dr. Aaronson noted that previously reported outcomes from TMS for anxious depression have been “suggestive of efficacy but have not been well studied within a large cohort.”
To investigate these issues, the current investigators turned to the NeuroStar Advanced Therapy System Clinical Outcomes Registry. It is the largest database of patients with difficult-to-treat depression, all of whom had undergone TMS.
This “extraordinary” database was able to provide previous insight into how often TMS works, whether some of the treatment parameters can be altered while still preserving efficacy, and whether bilateral TMS works better than unilateral TMS in patients with MDD, Dr. Aaronson said.
In the current study, researchers retrospectively analyzed data on 1,820 patients with MDD. All had completed the Patient Health Questinonaire–9 (PHQ-9) and the Generalized Anxiety Disorder–7 (GAD-7) at baseline and following at least one TMS intervention.
Most patients (n = 1,514) had anxious depression, defined as a baseline GAD-7 score of 10 or higher, and 306 had nonanxious depression, defined as a GAD-7 score below that threshold.
The investigators assessed the total sample of these patients who had been treated with any TMS protocol, as well as a subsample of patients (n = 625) who had been treated only with high-frequency left dorsolateral prefrontal cortex (HF-LUL) stimulation.
Patients were also subdivided into intent-to-treat and Completer samples (n = 1,820 and 1,429, respectively).
Consistent effects
There was no difference in gender distribution between the anxious and nonanxious group.
However, the anxious group was significantly younger (by about 5 years), compared with the nonanxious group. They also reported higher severity of depressive symptoms at baseline, with PHQ-9 scores approximately 2.5 points higher.
This was a “notable finding, since the PHQ-9 does not contain items directly assessing anxiety,” the researchers wrote.
There were also differences between the groups in the type of TMS protocol they received, with exclusive HF-LUL more common in the nonanxious depression group compared with other types of TMS protocols or unclassified protocols in the anxious depression group.
“Anxiolytic and antidepressant effects were consistent across the [intent-to-treat] and completed samples and patients who received any TMS protocol or only HF-LUL TMS,” the investigators reported.
GAD-7 scores “decreased markedly” in the anxious depression group. GAD-7 response rates ranged from 47.8% to 60.6% and GAD-7 remission rates ranged from 26.4% to 38.0% (P < .0001 for both).
There were no between-group differences in PHQ-9 scores in the magnitude of change pre- to post treatment. The anxious group scored about 2.5 points higher both pre- and post treatment, compared with the anxious group – with an effect size for change ranging from 1.46 to 1.74 in the anxious group and from 1.66 to 1.95 in the nonanxious group.
Response, remission rates
Notably, the anxious and nonanxious groups both showed “marked antidepressant effects,” with response and remission rates in the anxious group ranging from 55.2% to 66.8% and from 24.0% to 33.2%, respectively.
However, response and remission rates were significantly higher in the nonanxious versus the anxious group.
“Thus, despite manifesting the same degree of change in the PHQ-9 scores, the higher baseline and post-TMS scores in the anxious group resulted in significantly lower response and remission rates,” the investigators wrote.
They noted that the difference in post-TMS adjusted means was “small” and the groups also “did not differ in the absolute extent of symptoms improvement after multivariate adjustment.”
The relationship changes in the GAD-7 and the PHQ-9 scores “covaried” for the total IT sample (r1818 = 0.69, P < .001), although the relation was more “robust” in the anxious depression group versus the nonanxious depression group (r1512 = .75 vs. r304 = 0.50; P < .001 for both).
“The anxious depressed folks were sicker and had higher scores on scales capturing the severity of their illness,” Dr. Aaronson said. However, their “outcomes were similar, taking into account the higher baseline scores which had the effect of lowering the percent of anxious participants who met response and remission criteria.”
He reported that the average decline in depression rating scale scores was not significantly different between the groups, and the decline in depression scores tracked similarly to the decline in anxiety scores, “meaning they strongly covaried.”
The authors noted that a limitation was that, although the data was prospectively gathered, the analyses were retrospective.
Settles the debate?
Commenting on the study, Shan Siddiqi, MD, assistant professor of psychiatry at Harvard Medical School, Boston, said clinicians know that patients with comorbid anxiety are less likely to be referred for TMS, “probably because of the longstanding perception that TMS doesn’t work as well for them.”
This perception “has persisted, despite several small studies to the contrary, perhaps because we know that these patients are less responsive to other treatments,” said Dr. Siddiqi, who is also director of psychiatric neuromodulation research at Brigham and Women’s Center for Brain Circuit Therapeutics in Boston. He was not involved with the current research.
“This new study will hopefully settle that debate and let us move on to a new question: How do we optimize the treatment for this important patient population that has largely been excluded from many of our prior studies?”
The NeuroStar Advanced Therapy System Clinical Outcomes Registry, analysis of the registry data, and the drafting of this manuscript were supported by Neuronetics Inc. Dr. Aaronson serves as a scientific adviser to Genomind, LivaNova, Neuronetics, Janssen Pharmaceuticals, and Sage Therapeutics; and has received research support from Compass Pathways and Neuronetics. Dr. Siddiqi is a scientific consultant for Magnus Medical; a clinical consultant for Acacia Mental Health, Kaizen Brain Center, and Boston Precision Neurotherapeutics; and has received investigator-initiated research funding from Neuronetics and BrainsWay. He has also served as a speaker for BrainsWay and PsychU.org, owns stock in BrainsWay and Magnus Medical, and owns intellectual property involving the use of functional connectivity to target TMS.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
Primary care providers are increasingly addressing mental health concerns
particularly anxiety and stress-related diagnoses, based on a recent study.
These findings point to a sizable gap in psychiatric care that has likely been exacerbated by the pandemic, reported lead author Lisa S. Rotenstein, MD, MBA, assistant professor of medicine at Harvard Medical School and Medical Director of Population Health at Brigham and Women’s Hospital, both in Boston, and colleagues.
To ensure that PCPs can effectively manage this burden, innovative approaches are needed, such as value-based care models, billing codes for integrated behavioral health, and e-consultations with psychiatric colleagues, they added.
“Previous studies demonstrated that the rate of adult mental health outpatient visits increased between 1995 and 2010,” Dr. Rotenstein and colleagues wrote in Health Affairs. “However, more than a decade later, the extent to which the rate of primary care visits addressing mental health concerns has changed is unclear, with multiple health care delivery trends potentially influencing a further increase in prevalence.”
To address this knowledge gap, the investigators turned to the 2006-2018 National Ambulatory Medical Care Surveys, a nationally representative, serial, cross-sectional dataset. The present analysis included 109,898 visits representing 3,891,233,060 weighted visits.
Over the study period, the proportion of PCP visits that addressed mental health concerns rose from 10.7% to 15.9%.
This latter figure has probably increased since the onset of the pandemic, the investigators wrote, while availability of psychiatric care hasn’t kept pace, meaning PCPs are increasingly on the hook for managing mental illness.
“Even before the pandemic, one in five Americans lived with a mental health condition,” Dr. Rotenstein said in a written comment. “The COVID pandemic has only accelerated demand for mental health treatment. ... We know that there aren’t enough psychiatrists to meet this demand.”
Over the course of the study period, the rate of depression and affective disorders diagnoses slowed while anxiety and stress-related disorders were increasingly diagnosed.
“Particularly given the common co-occurrence of anxiety and depression, the trends we identified may represent physicians’ greater comfort over time with accurately diagnosing anxiety in the primary care setting, potentially for diagnoses that previously would have been classified as depression,” the investigators wrote, noting these findings align with a 2014 study by Olfson and colleagues.
Multiple factors associated with primary care mental health visits
Several variables were associated with significantly greater likelihood that a mental health concern would be addressed at a given visit, including female sex, younger age, payment via Medicare or Medicaid, and the physician being the patient’s regular physician.
“Our study demonstrated that mental health concerns were significantly more likely to be addressed in a visit with one’s usual primary care physician,” Dr. Rotenstein said. “This finding emphasizes the value of the longitudinal, supportive relationship developed in primary care for raising and addressing the full continuum of a patient’s needs, including mental health concerns.”
The investigators also observed significant associations between race/ethnicity and likelihood of addressing a mental health concern.
Compared with White patients, Black patients were 40% less likely to have a primary care visit with a mental health concern (odds ratio, 0.6; P less than .001). Similarly, Hispanic patients were 40% less likely than non-Hispanic patients to have a visit with a mental health concern (OR, 0.6; P less than .001).
“Unfortunately, our data don’t give us insight into why Black and Hispanic patients were less likely to have a mental health concern addressed in the context of a primary care visit,” Dr. Rotenstein said. “However, the data do suggest an urgent need to better understand and subsequently address the underlying causes of these disparities.”
She suggested several possible explanations, including differences in rates of screening, issues with access to care, insurance coverage disparities, and communication or cultural barriers.
Stuck in the reimbursement trap
Michael Klinkman, MD , professor of family medicine and learning health sciences at the University of Michigan Medical School, Ann Arbor, said the data align with his own clinical experience.
“The proportion of visits where depression was addresed went down, but the baseline is going up, so I don’t think we’re dealing with any less depression,” Dr. Klinkman said in an interview. “It’s just that there’s a lot more anxiety and stress that we’re finding and dealing with in primary care.”
While most family doctors are comfortable with best practices in managing these conditions, they may feel increasingly overburdened by the sheer number of patients with mental illness under their care alone, according to Dr. Klinkman.
“Primary care docs are increasingly feeling like they’re on their own in dealing with mental health problems,” he said.
While he agreed in theory with the interventions proposed by Dr. Rotenstein and colleagues, some solutions, like billing code changes, may ultimately worsen the burden on primary care providers.
“My fear in all of this, frankly, is that we’re going to create a better sense of the need for primary care practice in general to address mental health and social care issues, and we’re just going to create a lot more work and more widget-counting around doing that,” said Dr. Klinkman.
Value-based care appears to be a better solution, he said, since “we’re trying to take care of a human being, not the 1,050 pieces of that human being’s care that we’re trying to bundle up with different codes.”
A flat-fee, per-patient model, however, is unlikely to gain traction in the United States.
Dr. Klinkman has been involved in health care system reform up to the federal level, where he has encountered politicians who understood the issues but were incapable of helping because of partisan gridlock, he said. “It’s just politically near impossible to make changes in this basic health care business model.”
Policymakers advised Dr. Klinkman and his colleagues to strive for incremental changes, leaving them to grapple with increasingly complex reimbursement rules.
“We’re kind of stuck in this trap of trying to create new codes for services that we think ought to be better reimbursed,” Dr. Klinkman said. “We’re missing the person in all of this – the human being we’re trying to serve.”
The investigators, Dr. Cain, and Dr. Klinkman disclosed no conflicts of interest.
*This article was updated on 2/27/2023.
particularly anxiety and stress-related diagnoses, based on a recent study.
These findings point to a sizable gap in psychiatric care that has likely been exacerbated by the pandemic, reported lead author Lisa S. Rotenstein, MD, MBA, assistant professor of medicine at Harvard Medical School and Medical Director of Population Health at Brigham and Women’s Hospital, both in Boston, and colleagues.
To ensure that PCPs can effectively manage this burden, innovative approaches are needed, such as value-based care models, billing codes for integrated behavioral health, and e-consultations with psychiatric colleagues, they added.
“Previous studies demonstrated that the rate of adult mental health outpatient visits increased between 1995 and 2010,” Dr. Rotenstein and colleagues wrote in Health Affairs. “However, more than a decade later, the extent to which the rate of primary care visits addressing mental health concerns has changed is unclear, with multiple health care delivery trends potentially influencing a further increase in prevalence.”
To address this knowledge gap, the investigators turned to the 2006-2018 National Ambulatory Medical Care Surveys, a nationally representative, serial, cross-sectional dataset. The present analysis included 109,898 visits representing 3,891,233,060 weighted visits.
Over the study period, the proportion of PCP visits that addressed mental health concerns rose from 10.7% to 15.9%.
This latter figure has probably increased since the onset of the pandemic, the investigators wrote, while availability of psychiatric care hasn’t kept pace, meaning PCPs are increasingly on the hook for managing mental illness.
“Even before the pandemic, one in five Americans lived with a mental health condition,” Dr. Rotenstein said in a written comment. “The COVID pandemic has only accelerated demand for mental health treatment. ... We know that there aren’t enough psychiatrists to meet this demand.”
Over the course of the study period, the rate of depression and affective disorders diagnoses slowed while anxiety and stress-related disorders were increasingly diagnosed.
“Particularly given the common co-occurrence of anxiety and depression, the trends we identified may represent physicians’ greater comfort over time with accurately diagnosing anxiety in the primary care setting, potentially for diagnoses that previously would have been classified as depression,” the investigators wrote, noting these findings align with a 2014 study by Olfson and colleagues.
Multiple factors associated with primary care mental health visits
Several variables were associated with significantly greater likelihood that a mental health concern would be addressed at a given visit, including female sex, younger age, payment via Medicare or Medicaid, and the physician being the patient’s regular physician.
“Our study demonstrated that mental health concerns were significantly more likely to be addressed in a visit with one’s usual primary care physician,” Dr. Rotenstein said. “This finding emphasizes the value of the longitudinal, supportive relationship developed in primary care for raising and addressing the full continuum of a patient’s needs, including mental health concerns.”
The investigators also observed significant associations between race/ethnicity and likelihood of addressing a mental health concern.
Compared with White patients, Black patients were 40% less likely to have a primary care visit with a mental health concern (odds ratio, 0.6; P less than .001). Similarly, Hispanic patients were 40% less likely than non-Hispanic patients to have a visit with a mental health concern (OR, 0.6; P less than .001).
“Unfortunately, our data don’t give us insight into why Black and Hispanic patients were less likely to have a mental health concern addressed in the context of a primary care visit,” Dr. Rotenstein said. “However, the data do suggest an urgent need to better understand and subsequently address the underlying causes of these disparities.”
She suggested several possible explanations, including differences in rates of screening, issues with access to care, insurance coverage disparities, and communication or cultural barriers.
Stuck in the reimbursement trap
Michael Klinkman, MD , professor of family medicine and learning health sciences at the University of Michigan Medical School, Ann Arbor, said the data align with his own clinical experience.
“The proportion of visits where depression was addresed went down, but the baseline is going up, so I don’t think we’re dealing with any less depression,” Dr. Klinkman said in an interview. “It’s just that there’s a lot more anxiety and stress that we’re finding and dealing with in primary care.”
While most family doctors are comfortable with best practices in managing these conditions, they may feel increasingly overburdened by the sheer number of patients with mental illness under their care alone, according to Dr. Klinkman.
“Primary care docs are increasingly feeling like they’re on their own in dealing with mental health problems,” he said.
While he agreed in theory with the interventions proposed by Dr. Rotenstein and colleagues, some solutions, like billing code changes, may ultimately worsen the burden on primary care providers.
“My fear in all of this, frankly, is that we’re going to create a better sense of the need for primary care practice in general to address mental health and social care issues, and we’re just going to create a lot more work and more widget-counting around doing that,” said Dr. Klinkman.
Value-based care appears to be a better solution, he said, since “we’re trying to take care of a human being, not the 1,050 pieces of that human being’s care that we’re trying to bundle up with different codes.”
A flat-fee, per-patient model, however, is unlikely to gain traction in the United States.
Dr. Klinkman has been involved in health care system reform up to the federal level, where he has encountered politicians who understood the issues but were incapable of helping because of partisan gridlock, he said. “It’s just politically near impossible to make changes in this basic health care business model.”
Policymakers advised Dr. Klinkman and his colleagues to strive for incremental changes, leaving them to grapple with increasingly complex reimbursement rules.
“We’re kind of stuck in this trap of trying to create new codes for services that we think ought to be better reimbursed,” Dr. Klinkman said. “We’re missing the person in all of this – the human being we’re trying to serve.”
The investigators, Dr. Cain, and Dr. Klinkman disclosed no conflicts of interest.
*This article was updated on 2/27/2023.
particularly anxiety and stress-related diagnoses, based on a recent study.
These findings point to a sizable gap in psychiatric care that has likely been exacerbated by the pandemic, reported lead author Lisa S. Rotenstein, MD, MBA, assistant professor of medicine at Harvard Medical School and Medical Director of Population Health at Brigham and Women’s Hospital, both in Boston, and colleagues.
To ensure that PCPs can effectively manage this burden, innovative approaches are needed, such as value-based care models, billing codes for integrated behavioral health, and e-consultations with psychiatric colleagues, they added.
“Previous studies demonstrated that the rate of adult mental health outpatient visits increased between 1995 and 2010,” Dr. Rotenstein and colleagues wrote in Health Affairs. “However, more than a decade later, the extent to which the rate of primary care visits addressing mental health concerns has changed is unclear, with multiple health care delivery trends potentially influencing a further increase in prevalence.”
To address this knowledge gap, the investigators turned to the 2006-2018 National Ambulatory Medical Care Surveys, a nationally representative, serial, cross-sectional dataset. The present analysis included 109,898 visits representing 3,891,233,060 weighted visits.
Over the study period, the proportion of PCP visits that addressed mental health concerns rose from 10.7% to 15.9%.
This latter figure has probably increased since the onset of the pandemic, the investigators wrote, while availability of psychiatric care hasn’t kept pace, meaning PCPs are increasingly on the hook for managing mental illness.
“Even before the pandemic, one in five Americans lived with a mental health condition,” Dr. Rotenstein said in a written comment. “The COVID pandemic has only accelerated demand for mental health treatment. ... We know that there aren’t enough psychiatrists to meet this demand.”
Over the course of the study period, the rate of depression and affective disorders diagnoses slowed while anxiety and stress-related disorders were increasingly diagnosed.
“Particularly given the common co-occurrence of anxiety and depression, the trends we identified may represent physicians’ greater comfort over time with accurately diagnosing anxiety in the primary care setting, potentially for diagnoses that previously would have been classified as depression,” the investigators wrote, noting these findings align with a 2014 study by Olfson and colleagues.
Multiple factors associated with primary care mental health visits
Several variables were associated with significantly greater likelihood that a mental health concern would be addressed at a given visit, including female sex, younger age, payment via Medicare or Medicaid, and the physician being the patient’s regular physician.
“Our study demonstrated that mental health concerns were significantly more likely to be addressed in a visit with one’s usual primary care physician,” Dr. Rotenstein said. “This finding emphasizes the value of the longitudinal, supportive relationship developed in primary care for raising and addressing the full continuum of a patient’s needs, including mental health concerns.”
The investigators also observed significant associations between race/ethnicity and likelihood of addressing a mental health concern.
Compared with White patients, Black patients were 40% less likely to have a primary care visit with a mental health concern (odds ratio, 0.6; P less than .001). Similarly, Hispanic patients were 40% less likely than non-Hispanic patients to have a visit with a mental health concern (OR, 0.6; P less than .001).
“Unfortunately, our data don’t give us insight into why Black and Hispanic patients were less likely to have a mental health concern addressed in the context of a primary care visit,” Dr. Rotenstein said. “However, the data do suggest an urgent need to better understand and subsequently address the underlying causes of these disparities.”
She suggested several possible explanations, including differences in rates of screening, issues with access to care, insurance coverage disparities, and communication or cultural barriers.
Stuck in the reimbursement trap
Michael Klinkman, MD , professor of family medicine and learning health sciences at the University of Michigan Medical School, Ann Arbor, said the data align with his own clinical experience.
“The proportion of visits where depression was addresed went down, but the baseline is going up, so I don’t think we’re dealing with any less depression,” Dr. Klinkman said in an interview. “It’s just that there’s a lot more anxiety and stress that we’re finding and dealing with in primary care.”
While most family doctors are comfortable with best practices in managing these conditions, they may feel increasingly overburdened by the sheer number of patients with mental illness under their care alone, according to Dr. Klinkman.
“Primary care docs are increasingly feeling like they’re on their own in dealing with mental health problems,” he said.
While he agreed in theory with the interventions proposed by Dr. Rotenstein and colleagues, some solutions, like billing code changes, may ultimately worsen the burden on primary care providers.
“My fear in all of this, frankly, is that we’re going to create a better sense of the need for primary care practice in general to address mental health and social care issues, and we’re just going to create a lot more work and more widget-counting around doing that,” said Dr. Klinkman.
Value-based care appears to be a better solution, he said, since “we’re trying to take care of a human being, not the 1,050 pieces of that human being’s care that we’re trying to bundle up with different codes.”
A flat-fee, per-patient model, however, is unlikely to gain traction in the United States.
Dr. Klinkman has been involved in health care system reform up to the federal level, where he has encountered politicians who understood the issues but were incapable of helping because of partisan gridlock, he said. “It’s just politically near impossible to make changes in this basic health care business model.”
Policymakers advised Dr. Klinkman and his colleagues to strive for incremental changes, leaving them to grapple with increasingly complex reimbursement rules.
“We’re kind of stuck in this trap of trying to create new codes for services that we think ought to be better reimbursed,” Dr. Klinkman said. “We’re missing the person in all of this – the human being we’re trying to serve.”
The investigators, Dr. Cain, and Dr. Klinkman disclosed no conflicts of interest.
*This article was updated on 2/27/2023.
FROM HEALTH AFFAIRS
Loneliness risk elevated among young cancer survivors
findings from a large retrospective study suggest.
Young cancer survivors were more than twice as likely to report loneliness at study baseline and follow-up. Loneliness at these times was associated with an almost 10-fold increased risk for anxiety and a nearly 18-fold increased risk for depression.
“We observed an elevated prevalence of loneliness in survivors, compared to sibling controls, and found that loneliness was associated with emotional, behavioral, and physical health morbidities,” lead study author Chiara Papini, PhD, of St. Jude Children’s Research Hospital, Memphis, and her colleagues write. “Our results highlight the importance of identifying and screening young adult survivors of childhood cancer for loneliness and the need for targeted interventions to reduce loneliness.”
The article was published online in the journal Cancer.
Most young cancer survivors in the United States reach adulthood and need to play catch-up: make up for missed school and work, become reacquainted with old friends, and develop new friendships, social networks, and intimate relationships. Meeting these needs may be hindered by adverse physical and psychosocial problems that linger or develop after treatment, which may leave cancer survivors feeling isolated.
“Young adult survivors of childhood cancer are navigating a developmental period marked by increased social expectations, during which loneliness may have significant impact on physical and mental health,” Dr. Papini and colleagues say.
To better understand the risks for loneliness among young cancer survivors, Dr. Papini and her colleagues analyzed data from the retrospective Childhood Cancer Survivor Study, which followed young survivors who had been diagnosed with a range of cancers before age 21 years. Study participants had been treated at one of 31 study sites in North America and had survived 5 years or longer after diagnosis.
The 9,664 survivors and 2,221 randomly sampled siblings ranged in age from 19 to 39 years at the time they completed a survey that assessed emotional distress at baseline and at follow‐up a median of 6.6 years. At baseline, the median age of the survivors was 27 years, and a median of 17.5 years had passed from the time of their diagnosis.
The most common diagnoses were leukemia (35%), Hodgkin lymphoma (15%), central nervous system (CNS) tumors (14%), and bone tumors (10%). More than half (56%) had received radiation therapy.
Using multivariable models, the researchers found that survivors were more likely than siblings to report moderate to extreme loneliness at either baseline or follow‐up (prevalence ratio, 1.04) and were more than two times more likely to report loneliness at both baseline and follow‐up (PR, 2.21).
Loneliness at baseline and follow‐up was associated with a much greater risk for anxiety (relative risk, 9.75) and depression (RR, 17.86). Loneliness at follow‐up was linked with increased risks for suicidal ideation (RR, 1.52), heavy or risky alcohol consumption (RR, 1.27), and any grade 2-4 new‐onset chronic health condition (RR, 1.29), especially those that were neurologic (RR, 4.37).
Survivors of CNS tumors (odds ratio, 2.59) and leukemia (OR, 2.52) were most likely to report loneliness at both baseline and follow‐up, though survivors of four other cancer types also faced an elevated risk for loneliness: neuroblastoma (OR, 2.32), bone tumor (OR, 2.12), soft tissue sarcoma (OR, 1.78), and Hodgkin lymphoma (OR, 1.69).
Treatment type appeared to matter as well. Survivors who underwent amputation (OR, 1.82) or were treated with cranial radiation greater than or equal to 20 Gy (OR, 1.56) or corticosteroids (OR, 1.31) were more likely to report loneliness at baseline and follow‐up, compared with those who reported no loneliness at both time points.
The authors acknowledge limitations to the study, including the fact that roughly 90% of survivors and siblings were White, which limits the applicability of their results to diverse groups. In addition, the responses were self-reported without external validation.
Overall, though, the findings provide a framework for clinicians to understand and identify loneliness among young cancer survivors and help them cope with their emotions.
“The Childhood Cancer Survivor Study provides the largest and the most comprehensive dataset on childhood cancer survivors and healthy-sibling comparisons, giving us powerful data on survivorship, late effects, and psychosocial and health outcomes,” Rachel M. Moore, PhD, child psychologist at Children’s Mercy Kansas City, Mo., said in an interview.
Asking a simple question – “Are you feeling lonely?” – can identify at-risk survivors and enable health care teams to provide timely interventions that address young patients’ physical and psychological needs, said Dr. Moore, who was not involved in the study.
Dr. Moore noted that within her clinical practice, “adolescent and young adult survivors frequently discuss loneliness in their daily lives. They feel different from their peers and misunderstood. Having a conversation early in survivorship care about the experience of loneliness as a product of cancer treatment can open the door to regular screening and destigmatizing mental health services.”
Supporting young people throughout their survivorship journey is important, said Rusha Bhandari, MD, medical director of the Childhood, Adolescent, and Young Adult Cancer Survivorship Program at City of Hope, Duarte, Calif. This study can help ensure that clinicians “provide comprehensive care, including psychosocial screening and support, to meet the unique needs of our young adult survivors,” said Dr. Bhandari, who also was not involved in the research.
The National Cancer Institute and the American Lebanese Syrian Associated Charities supported the study. One co-author reported receiving corporate consulting fees. Dr. Papini, the remaining co-authors, Dr. Moore, and Dr. Bhandari report no relevant financial involvements.
A version of this article first appeared on Medscape.com.
findings from a large retrospective study suggest.
Young cancer survivors were more than twice as likely to report loneliness at study baseline and follow-up. Loneliness at these times was associated with an almost 10-fold increased risk for anxiety and a nearly 18-fold increased risk for depression.
“We observed an elevated prevalence of loneliness in survivors, compared to sibling controls, and found that loneliness was associated with emotional, behavioral, and physical health morbidities,” lead study author Chiara Papini, PhD, of St. Jude Children’s Research Hospital, Memphis, and her colleagues write. “Our results highlight the importance of identifying and screening young adult survivors of childhood cancer for loneliness and the need for targeted interventions to reduce loneliness.”
The article was published online in the journal Cancer.
Most young cancer survivors in the United States reach adulthood and need to play catch-up: make up for missed school and work, become reacquainted with old friends, and develop new friendships, social networks, and intimate relationships. Meeting these needs may be hindered by adverse physical and psychosocial problems that linger or develop after treatment, which may leave cancer survivors feeling isolated.
“Young adult survivors of childhood cancer are navigating a developmental period marked by increased social expectations, during which loneliness may have significant impact on physical and mental health,” Dr. Papini and colleagues say.
To better understand the risks for loneliness among young cancer survivors, Dr. Papini and her colleagues analyzed data from the retrospective Childhood Cancer Survivor Study, which followed young survivors who had been diagnosed with a range of cancers before age 21 years. Study participants had been treated at one of 31 study sites in North America and had survived 5 years or longer after diagnosis.
The 9,664 survivors and 2,221 randomly sampled siblings ranged in age from 19 to 39 years at the time they completed a survey that assessed emotional distress at baseline and at follow‐up a median of 6.6 years. At baseline, the median age of the survivors was 27 years, and a median of 17.5 years had passed from the time of their diagnosis.
The most common diagnoses were leukemia (35%), Hodgkin lymphoma (15%), central nervous system (CNS) tumors (14%), and bone tumors (10%). More than half (56%) had received radiation therapy.
Using multivariable models, the researchers found that survivors were more likely than siblings to report moderate to extreme loneliness at either baseline or follow‐up (prevalence ratio, 1.04) and were more than two times more likely to report loneliness at both baseline and follow‐up (PR, 2.21).
Loneliness at baseline and follow‐up was associated with a much greater risk for anxiety (relative risk, 9.75) and depression (RR, 17.86). Loneliness at follow‐up was linked with increased risks for suicidal ideation (RR, 1.52), heavy or risky alcohol consumption (RR, 1.27), and any grade 2-4 new‐onset chronic health condition (RR, 1.29), especially those that were neurologic (RR, 4.37).
Survivors of CNS tumors (odds ratio, 2.59) and leukemia (OR, 2.52) were most likely to report loneliness at both baseline and follow‐up, though survivors of four other cancer types also faced an elevated risk for loneliness: neuroblastoma (OR, 2.32), bone tumor (OR, 2.12), soft tissue sarcoma (OR, 1.78), and Hodgkin lymphoma (OR, 1.69).
Treatment type appeared to matter as well. Survivors who underwent amputation (OR, 1.82) or were treated with cranial radiation greater than or equal to 20 Gy (OR, 1.56) or corticosteroids (OR, 1.31) were more likely to report loneliness at baseline and follow‐up, compared with those who reported no loneliness at both time points.
The authors acknowledge limitations to the study, including the fact that roughly 90% of survivors and siblings were White, which limits the applicability of their results to diverse groups. In addition, the responses were self-reported without external validation.
Overall, though, the findings provide a framework for clinicians to understand and identify loneliness among young cancer survivors and help them cope with their emotions.
“The Childhood Cancer Survivor Study provides the largest and the most comprehensive dataset on childhood cancer survivors and healthy-sibling comparisons, giving us powerful data on survivorship, late effects, and psychosocial and health outcomes,” Rachel M. Moore, PhD, child psychologist at Children’s Mercy Kansas City, Mo., said in an interview.
Asking a simple question – “Are you feeling lonely?” – can identify at-risk survivors and enable health care teams to provide timely interventions that address young patients’ physical and psychological needs, said Dr. Moore, who was not involved in the study.
Dr. Moore noted that within her clinical practice, “adolescent and young adult survivors frequently discuss loneliness in their daily lives. They feel different from their peers and misunderstood. Having a conversation early in survivorship care about the experience of loneliness as a product of cancer treatment can open the door to regular screening and destigmatizing mental health services.”
Supporting young people throughout their survivorship journey is important, said Rusha Bhandari, MD, medical director of the Childhood, Adolescent, and Young Adult Cancer Survivorship Program at City of Hope, Duarte, Calif. This study can help ensure that clinicians “provide comprehensive care, including psychosocial screening and support, to meet the unique needs of our young adult survivors,” said Dr. Bhandari, who also was not involved in the research.
The National Cancer Institute and the American Lebanese Syrian Associated Charities supported the study. One co-author reported receiving corporate consulting fees. Dr. Papini, the remaining co-authors, Dr. Moore, and Dr. Bhandari report no relevant financial involvements.
A version of this article first appeared on Medscape.com.
findings from a large retrospective study suggest.
Young cancer survivors were more than twice as likely to report loneliness at study baseline and follow-up. Loneliness at these times was associated with an almost 10-fold increased risk for anxiety and a nearly 18-fold increased risk for depression.
“We observed an elevated prevalence of loneliness in survivors, compared to sibling controls, and found that loneliness was associated with emotional, behavioral, and physical health morbidities,” lead study author Chiara Papini, PhD, of St. Jude Children’s Research Hospital, Memphis, and her colleagues write. “Our results highlight the importance of identifying and screening young adult survivors of childhood cancer for loneliness and the need for targeted interventions to reduce loneliness.”
The article was published online in the journal Cancer.
Most young cancer survivors in the United States reach adulthood and need to play catch-up: make up for missed school and work, become reacquainted with old friends, and develop new friendships, social networks, and intimate relationships. Meeting these needs may be hindered by adverse physical and psychosocial problems that linger or develop after treatment, which may leave cancer survivors feeling isolated.
“Young adult survivors of childhood cancer are navigating a developmental period marked by increased social expectations, during which loneliness may have significant impact on physical and mental health,” Dr. Papini and colleagues say.
To better understand the risks for loneliness among young cancer survivors, Dr. Papini and her colleagues analyzed data from the retrospective Childhood Cancer Survivor Study, which followed young survivors who had been diagnosed with a range of cancers before age 21 years. Study participants had been treated at one of 31 study sites in North America and had survived 5 years or longer after diagnosis.
The 9,664 survivors and 2,221 randomly sampled siblings ranged in age from 19 to 39 years at the time they completed a survey that assessed emotional distress at baseline and at follow‐up a median of 6.6 years. At baseline, the median age of the survivors was 27 years, and a median of 17.5 years had passed from the time of their diagnosis.
The most common diagnoses were leukemia (35%), Hodgkin lymphoma (15%), central nervous system (CNS) tumors (14%), and bone tumors (10%). More than half (56%) had received radiation therapy.
Using multivariable models, the researchers found that survivors were more likely than siblings to report moderate to extreme loneliness at either baseline or follow‐up (prevalence ratio, 1.04) and were more than two times more likely to report loneliness at both baseline and follow‐up (PR, 2.21).
Loneliness at baseline and follow‐up was associated with a much greater risk for anxiety (relative risk, 9.75) and depression (RR, 17.86). Loneliness at follow‐up was linked with increased risks for suicidal ideation (RR, 1.52), heavy or risky alcohol consumption (RR, 1.27), and any grade 2-4 new‐onset chronic health condition (RR, 1.29), especially those that were neurologic (RR, 4.37).
Survivors of CNS tumors (odds ratio, 2.59) and leukemia (OR, 2.52) were most likely to report loneliness at both baseline and follow‐up, though survivors of four other cancer types also faced an elevated risk for loneliness: neuroblastoma (OR, 2.32), bone tumor (OR, 2.12), soft tissue sarcoma (OR, 1.78), and Hodgkin lymphoma (OR, 1.69).
Treatment type appeared to matter as well. Survivors who underwent amputation (OR, 1.82) or were treated with cranial radiation greater than or equal to 20 Gy (OR, 1.56) or corticosteroids (OR, 1.31) were more likely to report loneliness at baseline and follow‐up, compared with those who reported no loneliness at both time points.
The authors acknowledge limitations to the study, including the fact that roughly 90% of survivors and siblings were White, which limits the applicability of their results to diverse groups. In addition, the responses were self-reported without external validation.
Overall, though, the findings provide a framework for clinicians to understand and identify loneliness among young cancer survivors and help them cope with their emotions.
“The Childhood Cancer Survivor Study provides the largest and the most comprehensive dataset on childhood cancer survivors and healthy-sibling comparisons, giving us powerful data on survivorship, late effects, and psychosocial and health outcomes,” Rachel M. Moore, PhD, child psychologist at Children’s Mercy Kansas City, Mo., said in an interview.
Asking a simple question – “Are you feeling lonely?” – can identify at-risk survivors and enable health care teams to provide timely interventions that address young patients’ physical and psychological needs, said Dr. Moore, who was not involved in the study.
Dr. Moore noted that within her clinical practice, “adolescent and young adult survivors frequently discuss loneliness in their daily lives. They feel different from their peers and misunderstood. Having a conversation early in survivorship care about the experience of loneliness as a product of cancer treatment can open the door to regular screening and destigmatizing mental health services.”
Supporting young people throughout their survivorship journey is important, said Rusha Bhandari, MD, medical director of the Childhood, Adolescent, and Young Adult Cancer Survivorship Program at City of Hope, Duarte, Calif. This study can help ensure that clinicians “provide comprehensive care, including psychosocial screening and support, to meet the unique needs of our young adult survivors,” said Dr. Bhandari, who also was not involved in the research.
The National Cancer Institute and the American Lebanese Syrian Associated Charities supported the study. One co-author reported receiving corporate consulting fees. Dr. Papini, the remaining co-authors, Dr. Moore, and Dr. Bhandari report no relevant financial involvements.
A version of this article first appeared on Medscape.com.
FROM CANCER
Perceived barriers to accessing psychiatric electroceutical interventions for depression
Psychiatric electroceutical interventions (PEIs) – including Food and Drug Administration–approved therapies like electroconvulsive therapy (ECT) and repetitive transcranial magnetic stimulation (rTMS), as well as experimental interventions such as deep brain stimulation (DBS) and adaptive brain implants (ABI) – offer therapeutic promise for patients suffering with major depressive disorder (MDD). Yet there remain many open questions regarding their use, even in cases where their safety and effectiveness is well established.
Our research aims to better understand how different stakeholder groups view these interventions. We conducted a series of interviews with psychiatrists, patients with MDD, and members of the public to more fully comprehend these groups’ perceptions of barriers to using these therapies.1 They raised concerns about limitations to access posed by the limited geographic availability of these treatments, their cost, and lack of insurance coverage. In addition, each stakeholder group cited lack of knowledge about PEIs as a perceived barrier to their wider implementation in depression care.
Our participants recognized there are significant geographic limitations to accessing PEIs, as many of these treatments are available only in large, well-resourced cities. This is especially true for DBS and ABIs as they remain investigational, require neurosurgery, and currently are offered only during clinical research trials. However, even for established therapies like ECT and rTMS, access often remains limited to larger treatment centers. Further, training on the proper implementation and use of these modalities is limited in the United States. Current requirements from the Accreditation Council for Graduate Medical Education state only that psychiatry residents demonstrate knowledge of these therapies and their indications, falling short of requiring first-hand experience in referring or administering them.2
Our participants also perceived the cost of these therapies as a significant barrier affecting a large proportion of patients who could potentially benefit from them. Another frequently mentioned barrier is the lack of insurance coverage for existing PEIs, particularly rTMS. Even when insurance covers treatment with an approved PEI (for example, ECT, rTMS), there may be a requirement to have tried and failed multiple antidepressant medications first. These insurance requirements may contribute to a lack of general clarity about when these treatments should be used. The psychiatrists we interviewed, for example, were almost evenly split between believing that ECT and/or rTMS should be offered earlier in the course of therapy and believing that they should be reserved only for patients with treatment-resistant depression.
Further, some psychiatrists we interviewed stated that they wanted more information about the appropriate use of these treatments. This is unsurprising, as the available guidelines for the approved electroceutical treatments are outdated. Although the American Psychiatric Association Task Force is due to publish updated guidelines for ECT, it has been more than 20 years since the current guidelines were published.3 More recent guidelines, such as those issued in 2016 by the Canadian Network for Mood and Anxiety Treatments cite studies that were even then several years old.4 For rTMS, newer guidelines are available, but they have not yet been revised to include recent developments such as the SAINT protocol.5,6
While useful, clinical guidelines do not provide all of the information psychiatrists require for clinical decision-making. They are only as good as the evidence available and to the extent that they include all of the considerations important to psychiatrists and the specific patients they are treating.7,8 We asked the psychiatrists in our interviews what practical information they would like to see included in treatment guidelines. They offered a range of suggestions: better guidance about which patients would be most likely to benefit, when to offer the treatments, and how to combine these therapies with other interventions.
For the experimental PEIs (DBS and ABIs), similar questions and concerns arise. In the current research context, psychiatrists may not be aware of which patients are good candidates for referral to clinical trials. If these therapies are approved, similar questions about patient selection and place in treatment (for example, first line, second line, etc.) remain.9
Finally, each of our participant groups believed that patients and the public lack adequate knowledge about electroceutical interventions, and they emphasized the importance of giving potential patients sufficient information to enable them to provide valid informed consent. This is important in the case of the approved electroceutical therapies (ECT and rTMS), in part because of the potential for decision-making to be influenced unduly by misinformation and controversy – especially given that the media’s depiction of these interventions might influence patients’ willingness to receive helpful therapies such as ECT.10
Our interviews were used to inform the development of a national survey of these four stakeholder groups, which will provide further information about perceived barriers to accessing PEIs.
Dr. Bluhm is associate professor of philosophy at Michigan State University, East Lansing. Dr. Achtyes is director of the division of psychiatry and behavioral medicine at Michigan State University, Grand Rapids. Dr. McCright is chair of the department of sociology at Michigan State University. Dr. Cabrera is Dorothy Foehr Huck and J. Lloyd Huck Chair in Neuroethics at the Huck Institutes of the Life Sciences, Penn State University, University Park.
References
1. Cabrera LY et al. Psychiatry Res. 2022 Jul;313:114612. doi: 10.1016/j.psychres.2022.114612.
2. Accreditation Council for Graduate Medical Education. Psychiatry – Program Requirements and FAQs. https://www.acgme.org/specialties/psychiatry/program-requirements-and-faqs-and-applications/
3. American Psychiatric Association. The Practice of Electroconvulsive Therapy, Second Edition: Recommendations for Treatment, Training, and Privileging. 2001.
4. Miley RV et al. Can J Psychiatry. 2016 Sep;61(9):561-75. doi: 10.1177/0706743716660033.
5. Perera T et al. Brain Stimul. 2016 May-Jun;9(3):336-46. doi: 10.1016/j.brs.2016.03.010.
6. Cole EJ et al. Am J Psychiatry. 2020 Aug 1;177(8):716-26. doi: 10.1176/appi.ajp.2019.19070720.
7. Gabriel FC et al. PLoS One. 2020 Apr 21;15(4):e0231700. doi: 10.1371/journal.pone.0231700.
8. Woolf SH et al. BMJ. 1999 Feb 20;318(7182):527-30. doi: 10.1136/bmj.318.7182.527.
9. Widge AS et al. Biol Psychiatry. 2016 Feb 15;79(4):e9-10. doi: 10.1016/j.biopsych.2015.06.005.
10. Sienaert P. Brain Stimul. 2016 Nov-Dec;9(6):882-91. doi: 10.1016/j.brs.2016.07.005.
Psychiatric electroceutical interventions (PEIs) – including Food and Drug Administration–approved therapies like electroconvulsive therapy (ECT) and repetitive transcranial magnetic stimulation (rTMS), as well as experimental interventions such as deep brain stimulation (DBS) and adaptive brain implants (ABI) – offer therapeutic promise for patients suffering with major depressive disorder (MDD). Yet there remain many open questions regarding their use, even in cases where their safety and effectiveness is well established.
Our research aims to better understand how different stakeholder groups view these interventions. We conducted a series of interviews with psychiatrists, patients with MDD, and members of the public to more fully comprehend these groups’ perceptions of barriers to using these therapies.1 They raised concerns about limitations to access posed by the limited geographic availability of these treatments, their cost, and lack of insurance coverage. In addition, each stakeholder group cited lack of knowledge about PEIs as a perceived barrier to their wider implementation in depression care.
Our participants recognized there are significant geographic limitations to accessing PEIs, as many of these treatments are available only in large, well-resourced cities. This is especially true for DBS and ABIs as they remain investigational, require neurosurgery, and currently are offered only during clinical research trials. However, even for established therapies like ECT and rTMS, access often remains limited to larger treatment centers. Further, training on the proper implementation and use of these modalities is limited in the United States. Current requirements from the Accreditation Council for Graduate Medical Education state only that psychiatry residents demonstrate knowledge of these therapies and their indications, falling short of requiring first-hand experience in referring or administering them.2
Our participants also perceived the cost of these therapies as a significant barrier affecting a large proportion of patients who could potentially benefit from them. Another frequently mentioned barrier is the lack of insurance coverage for existing PEIs, particularly rTMS. Even when insurance covers treatment with an approved PEI (for example, ECT, rTMS), there may be a requirement to have tried and failed multiple antidepressant medications first. These insurance requirements may contribute to a lack of general clarity about when these treatments should be used. The psychiatrists we interviewed, for example, were almost evenly split between believing that ECT and/or rTMS should be offered earlier in the course of therapy and believing that they should be reserved only for patients with treatment-resistant depression.
Further, some psychiatrists we interviewed stated that they wanted more information about the appropriate use of these treatments. This is unsurprising, as the available guidelines for the approved electroceutical treatments are outdated. Although the American Psychiatric Association Task Force is due to publish updated guidelines for ECT, it has been more than 20 years since the current guidelines were published.3 More recent guidelines, such as those issued in 2016 by the Canadian Network for Mood and Anxiety Treatments cite studies that were even then several years old.4 For rTMS, newer guidelines are available, but they have not yet been revised to include recent developments such as the SAINT protocol.5,6
While useful, clinical guidelines do not provide all of the information psychiatrists require for clinical decision-making. They are only as good as the evidence available and to the extent that they include all of the considerations important to psychiatrists and the specific patients they are treating.7,8 We asked the psychiatrists in our interviews what practical information they would like to see included in treatment guidelines. They offered a range of suggestions: better guidance about which patients would be most likely to benefit, when to offer the treatments, and how to combine these therapies with other interventions.
For the experimental PEIs (DBS and ABIs), similar questions and concerns arise. In the current research context, psychiatrists may not be aware of which patients are good candidates for referral to clinical trials. If these therapies are approved, similar questions about patient selection and place in treatment (for example, first line, second line, etc.) remain.9
Finally, each of our participant groups believed that patients and the public lack adequate knowledge about electroceutical interventions, and they emphasized the importance of giving potential patients sufficient information to enable them to provide valid informed consent. This is important in the case of the approved electroceutical therapies (ECT and rTMS), in part because of the potential for decision-making to be influenced unduly by misinformation and controversy – especially given that the media’s depiction of these interventions might influence patients’ willingness to receive helpful therapies such as ECT.10
Our interviews were used to inform the development of a national survey of these four stakeholder groups, which will provide further information about perceived barriers to accessing PEIs.
Dr. Bluhm is associate professor of philosophy at Michigan State University, East Lansing. Dr. Achtyes is director of the division of psychiatry and behavioral medicine at Michigan State University, Grand Rapids. Dr. McCright is chair of the department of sociology at Michigan State University. Dr. Cabrera is Dorothy Foehr Huck and J. Lloyd Huck Chair in Neuroethics at the Huck Institutes of the Life Sciences, Penn State University, University Park.
References
1. Cabrera LY et al. Psychiatry Res. 2022 Jul;313:114612. doi: 10.1016/j.psychres.2022.114612.
2. Accreditation Council for Graduate Medical Education. Psychiatry – Program Requirements and FAQs. https://www.acgme.org/specialties/psychiatry/program-requirements-and-faqs-and-applications/
3. American Psychiatric Association. The Practice of Electroconvulsive Therapy, Second Edition: Recommendations for Treatment, Training, and Privileging. 2001.
4. Miley RV et al. Can J Psychiatry. 2016 Sep;61(9):561-75. doi: 10.1177/0706743716660033.
5. Perera T et al. Brain Stimul. 2016 May-Jun;9(3):336-46. doi: 10.1016/j.brs.2016.03.010.
6. Cole EJ et al. Am J Psychiatry. 2020 Aug 1;177(8):716-26. doi: 10.1176/appi.ajp.2019.19070720.
7. Gabriel FC et al. PLoS One. 2020 Apr 21;15(4):e0231700. doi: 10.1371/journal.pone.0231700.
8. Woolf SH et al. BMJ. 1999 Feb 20;318(7182):527-30. doi: 10.1136/bmj.318.7182.527.
9. Widge AS et al. Biol Psychiatry. 2016 Feb 15;79(4):e9-10. doi: 10.1016/j.biopsych.2015.06.005.
10. Sienaert P. Brain Stimul. 2016 Nov-Dec;9(6):882-91. doi: 10.1016/j.brs.2016.07.005.
Psychiatric electroceutical interventions (PEIs) – including Food and Drug Administration–approved therapies like electroconvulsive therapy (ECT) and repetitive transcranial magnetic stimulation (rTMS), as well as experimental interventions such as deep brain stimulation (DBS) and adaptive brain implants (ABI) – offer therapeutic promise for patients suffering with major depressive disorder (MDD). Yet there remain many open questions regarding their use, even in cases where their safety and effectiveness is well established.
Our research aims to better understand how different stakeholder groups view these interventions. We conducted a series of interviews with psychiatrists, patients with MDD, and members of the public to more fully comprehend these groups’ perceptions of barriers to using these therapies.1 They raised concerns about limitations to access posed by the limited geographic availability of these treatments, their cost, and lack of insurance coverage. In addition, each stakeholder group cited lack of knowledge about PEIs as a perceived barrier to their wider implementation in depression care.
Our participants recognized there are significant geographic limitations to accessing PEIs, as many of these treatments are available only in large, well-resourced cities. This is especially true for DBS and ABIs as they remain investigational, require neurosurgery, and currently are offered only during clinical research trials. However, even for established therapies like ECT and rTMS, access often remains limited to larger treatment centers. Further, training on the proper implementation and use of these modalities is limited in the United States. Current requirements from the Accreditation Council for Graduate Medical Education state only that psychiatry residents demonstrate knowledge of these therapies and their indications, falling short of requiring first-hand experience in referring or administering them.2
Our participants also perceived the cost of these therapies as a significant barrier affecting a large proportion of patients who could potentially benefit from them. Another frequently mentioned barrier is the lack of insurance coverage for existing PEIs, particularly rTMS. Even when insurance covers treatment with an approved PEI (for example, ECT, rTMS), there may be a requirement to have tried and failed multiple antidepressant medications first. These insurance requirements may contribute to a lack of general clarity about when these treatments should be used. The psychiatrists we interviewed, for example, were almost evenly split between believing that ECT and/or rTMS should be offered earlier in the course of therapy and believing that they should be reserved only for patients with treatment-resistant depression.
Further, some psychiatrists we interviewed stated that they wanted more information about the appropriate use of these treatments. This is unsurprising, as the available guidelines for the approved electroceutical treatments are outdated. Although the American Psychiatric Association Task Force is due to publish updated guidelines for ECT, it has been more than 20 years since the current guidelines were published.3 More recent guidelines, such as those issued in 2016 by the Canadian Network for Mood and Anxiety Treatments cite studies that were even then several years old.4 For rTMS, newer guidelines are available, but they have not yet been revised to include recent developments such as the SAINT protocol.5,6
While useful, clinical guidelines do not provide all of the information psychiatrists require for clinical decision-making. They are only as good as the evidence available and to the extent that they include all of the considerations important to psychiatrists and the specific patients they are treating.7,8 We asked the psychiatrists in our interviews what practical information they would like to see included in treatment guidelines. They offered a range of suggestions: better guidance about which patients would be most likely to benefit, when to offer the treatments, and how to combine these therapies with other interventions.
For the experimental PEIs (DBS and ABIs), similar questions and concerns arise. In the current research context, psychiatrists may not be aware of which patients are good candidates for referral to clinical trials. If these therapies are approved, similar questions about patient selection and place in treatment (for example, first line, second line, etc.) remain.9
Finally, each of our participant groups believed that patients and the public lack adequate knowledge about electroceutical interventions, and they emphasized the importance of giving potential patients sufficient information to enable them to provide valid informed consent. This is important in the case of the approved electroceutical therapies (ECT and rTMS), in part because of the potential for decision-making to be influenced unduly by misinformation and controversy – especially given that the media’s depiction of these interventions might influence patients’ willingness to receive helpful therapies such as ECT.10
Our interviews were used to inform the development of a national survey of these four stakeholder groups, which will provide further information about perceived barriers to accessing PEIs.
Dr. Bluhm is associate professor of philosophy at Michigan State University, East Lansing. Dr. Achtyes is director of the division of psychiatry and behavioral medicine at Michigan State University, Grand Rapids. Dr. McCright is chair of the department of sociology at Michigan State University. Dr. Cabrera is Dorothy Foehr Huck and J. Lloyd Huck Chair in Neuroethics at the Huck Institutes of the Life Sciences, Penn State University, University Park.
References
1. Cabrera LY et al. Psychiatry Res. 2022 Jul;313:114612. doi: 10.1016/j.psychres.2022.114612.
2. Accreditation Council for Graduate Medical Education. Psychiatry – Program Requirements and FAQs. https://www.acgme.org/specialties/psychiatry/program-requirements-and-faqs-and-applications/
3. American Psychiatric Association. The Practice of Electroconvulsive Therapy, Second Edition: Recommendations for Treatment, Training, and Privileging. 2001.
4. Miley RV et al. Can J Psychiatry. 2016 Sep;61(9):561-75. doi: 10.1177/0706743716660033.
5. Perera T et al. Brain Stimul. 2016 May-Jun;9(3):336-46. doi: 10.1016/j.brs.2016.03.010.
6. Cole EJ et al. Am J Psychiatry. 2020 Aug 1;177(8):716-26. doi: 10.1176/appi.ajp.2019.19070720.
7. Gabriel FC et al. PLoS One. 2020 Apr 21;15(4):e0231700. doi: 10.1371/journal.pone.0231700.
8. Woolf SH et al. BMJ. 1999 Feb 20;318(7182):527-30. doi: 10.1136/bmj.318.7182.527.
9. Widge AS et al. Biol Psychiatry. 2016 Feb 15;79(4):e9-10. doi: 10.1016/j.biopsych.2015.06.005.
10. Sienaert P. Brain Stimul. 2016 Nov-Dec;9(6):882-91. doi: 10.1016/j.brs.2016.07.005.
Long-term depression may hasten brain aging in midlife
Previous research suggests a possible link between depression and increased risk of dementia in older adults, but the association between depression and brain health in early adulthood and midlife has not been well studied, wrote Christina S. Dintica, PhD, of the University of California, San Francisco, and colleagues.
In a study published in the Journal of Affective Disorders, the researchers identified 649 individuals aged 23-36 at baseline who were part of the Coronary Artery Risk Development in Young Adults (CARDIA) study. All participants underwent brain MRI and cognitive testing. Depressive symptoms were assessed six times over a 25-year period using the Center for Epidemiological Studies Depression scale (CES–D), and the scores were analyzed as time-weighted averages (TWA). Elevated depressive symptoms were defined as CES-D scores of 16 or higher. Brain age was assessed via high-dimensional neuroimaging. Approximately half of the participants were female, and half were Black.
Overall, each 5-point increment in TWA depression symptoms over 25 years was associated with a 1-year increase in brain age, and individuals with elevated TWA depression averaged a 3-year increase in brain age compared with those with lower levels of depression after controlling for factors including chronological age, sex, education, race, MRI scanning site, and intracranial volume, they said. The association was attenuated in a model controlling for antidepressant use, and further attenuated after adjusting for smoking, alcohol consumption, income, body mass index, diabetes, and physical exercise.
The researchers also investigated the impact of the age period of elevated depressive symptoms on brain age. Compared with low depressive symptoms, elevated TWA CES-D at ages 30-39 years, 40-49 years, and 50-59 years was associated with increased brain ages of 2.43, 3.19, and 1.82.
In addition, elevated depressive symptoms were associated with a threefold increase in the odds of poor cognitive function at midlife (odds ratio, 3.30), although these odds were reduced after adjusting for use of antidepressants (OR, 1.47).
The mechanisms of action for the link between depression and accelerated brain aging remains uncertain, the researchers wrote in their discussion. “Studies over the last 20 years have demonstrated that increased inflammation and hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis are two of the most consistent biological findings in major depression, which have been linked to premature aging,” they noted. “Alternative explanations for the link between depression and adverse brain health could be underlying factors that explain both outcomes rather independently, such as low socioeconomic status, childhood maltreatment, or shared genetic effects,” they added.
Adjustment for antidepressant use had little effect overall on the association between depressive symptom severity and brain age, they said.
The current study findings were limited by the single assessment of brain age, which prevented evaluation of the temporality of the association between brain aging and depression, the researchers noted.
However, the results were strengthened by the large and diverse cohort, long-term follow-up, and use of high-dimensional neuroimaging, they said. Longitudinal studies are needed to explore mechanisms of action and the potential benefits of antidepressants, they added.
In the meantime, monitoring and treating depressive symptoms in young adults may help promote brain health in midlife and older age, they concluded.
The CARDIA study was supported by the National Heart, Lung, and Blood Institute, the National Institute on Aging, and the Alzheimer’s Association. The researchers had no financial conflicts to disclose.
Previous research suggests a possible link between depression and increased risk of dementia in older adults, but the association between depression and brain health in early adulthood and midlife has not been well studied, wrote Christina S. Dintica, PhD, of the University of California, San Francisco, and colleagues.
In a study published in the Journal of Affective Disorders, the researchers identified 649 individuals aged 23-36 at baseline who were part of the Coronary Artery Risk Development in Young Adults (CARDIA) study. All participants underwent brain MRI and cognitive testing. Depressive symptoms were assessed six times over a 25-year period using the Center for Epidemiological Studies Depression scale (CES–D), and the scores were analyzed as time-weighted averages (TWA). Elevated depressive symptoms were defined as CES-D scores of 16 or higher. Brain age was assessed via high-dimensional neuroimaging. Approximately half of the participants were female, and half were Black.
Overall, each 5-point increment in TWA depression symptoms over 25 years was associated with a 1-year increase in brain age, and individuals with elevated TWA depression averaged a 3-year increase in brain age compared with those with lower levels of depression after controlling for factors including chronological age, sex, education, race, MRI scanning site, and intracranial volume, they said. The association was attenuated in a model controlling for antidepressant use, and further attenuated after adjusting for smoking, alcohol consumption, income, body mass index, diabetes, and physical exercise.
The researchers also investigated the impact of the age period of elevated depressive symptoms on brain age. Compared with low depressive symptoms, elevated TWA CES-D at ages 30-39 years, 40-49 years, and 50-59 years was associated with increased brain ages of 2.43, 3.19, and 1.82.
In addition, elevated depressive symptoms were associated with a threefold increase in the odds of poor cognitive function at midlife (odds ratio, 3.30), although these odds were reduced after adjusting for use of antidepressants (OR, 1.47).
The mechanisms of action for the link between depression and accelerated brain aging remains uncertain, the researchers wrote in their discussion. “Studies over the last 20 years have demonstrated that increased inflammation and hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis are two of the most consistent biological findings in major depression, which have been linked to premature aging,” they noted. “Alternative explanations for the link between depression and adverse brain health could be underlying factors that explain both outcomes rather independently, such as low socioeconomic status, childhood maltreatment, or shared genetic effects,” they added.
Adjustment for antidepressant use had little effect overall on the association between depressive symptom severity and brain age, they said.
The current study findings were limited by the single assessment of brain age, which prevented evaluation of the temporality of the association between brain aging and depression, the researchers noted.
However, the results were strengthened by the large and diverse cohort, long-term follow-up, and use of high-dimensional neuroimaging, they said. Longitudinal studies are needed to explore mechanisms of action and the potential benefits of antidepressants, they added.
In the meantime, monitoring and treating depressive symptoms in young adults may help promote brain health in midlife and older age, they concluded.
The CARDIA study was supported by the National Heart, Lung, and Blood Institute, the National Institute on Aging, and the Alzheimer’s Association. The researchers had no financial conflicts to disclose.
Previous research suggests a possible link between depression and increased risk of dementia in older adults, but the association between depression and brain health in early adulthood and midlife has not been well studied, wrote Christina S. Dintica, PhD, of the University of California, San Francisco, and colleagues.
In a study published in the Journal of Affective Disorders, the researchers identified 649 individuals aged 23-36 at baseline who were part of the Coronary Artery Risk Development in Young Adults (CARDIA) study. All participants underwent brain MRI and cognitive testing. Depressive symptoms were assessed six times over a 25-year period using the Center for Epidemiological Studies Depression scale (CES–D), and the scores were analyzed as time-weighted averages (TWA). Elevated depressive symptoms were defined as CES-D scores of 16 or higher. Brain age was assessed via high-dimensional neuroimaging. Approximately half of the participants were female, and half were Black.
Overall, each 5-point increment in TWA depression symptoms over 25 years was associated with a 1-year increase in brain age, and individuals with elevated TWA depression averaged a 3-year increase in brain age compared with those with lower levels of depression after controlling for factors including chronological age, sex, education, race, MRI scanning site, and intracranial volume, they said. The association was attenuated in a model controlling for antidepressant use, and further attenuated after adjusting for smoking, alcohol consumption, income, body mass index, diabetes, and physical exercise.
The researchers also investigated the impact of the age period of elevated depressive symptoms on brain age. Compared with low depressive symptoms, elevated TWA CES-D at ages 30-39 years, 40-49 years, and 50-59 years was associated with increased brain ages of 2.43, 3.19, and 1.82.
In addition, elevated depressive symptoms were associated with a threefold increase in the odds of poor cognitive function at midlife (odds ratio, 3.30), although these odds were reduced after adjusting for use of antidepressants (OR, 1.47).
The mechanisms of action for the link between depression and accelerated brain aging remains uncertain, the researchers wrote in their discussion. “Studies over the last 20 years have demonstrated that increased inflammation and hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis are two of the most consistent biological findings in major depression, which have been linked to premature aging,” they noted. “Alternative explanations for the link between depression and adverse brain health could be underlying factors that explain both outcomes rather independently, such as low socioeconomic status, childhood maltreatment, or shared genetic effects,” they added.
Adjustment for antidepressant use had little effect overall on the association between depressive symptom severity and brain age, they said.
The current study findings were limited by the single assessment of brain age, which prevented evaluation of the temporality of the association between brain aging and depression, the researchers noted.
However, the results were strengthened by the large and diverse cohort, long-term follow-up, and use of high-dimensional neuroimaging, they said. Longitudinal studies are needed to explore mechanisms of action and the potential benefits of antidepressants, they added.
In the meantime, monitoring and treating depressive symptoms in young adults may help promote brain health in midlife and older age, they concluded.
The CARDIA study was supported by the National Heart, Lung, and Blood Institute, the National Institute on Aging, and the Alzheimer’s Association. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Positive top-line results for novel psychedelic in major depression
Top-line results from a phase 2a study of SPL026 (intravenous N,N-Dimethyltryptamine [DMT]) showed a 57% remission rate 3 months after participants received a single dose of the drug, the developer reports.
Small Pharma noted in a press release that this is the first placebo-controlled efficacy trial of a short-duration psychedelic for depression completed to date.
Investigators reported significant improvement in depression symptoms 2 weeks after dosing, which was the primary endpoint, and the improvement persisted at week 12.
“We now have the first evidence that SPL026 DMT, combined with supportive therapy, may be effective for people suffering from MDD,” chief investigator David Erritzoe, MD, PhD, clinical psychiatrist at Imperial College London, said in a statement.
“For patients who are unfortunate to experience little benefit from existing antidepressants, the potential for rapid and durable relief from a single treatment, as shown in this trial, is very promising,” Dr. Erritzoe added.
Randomized trial results
The blinded, randomized, placebo-controlled, two-staged phase 2a study included 34 patients with moderate to severe MDD. Those who were taking pharmacological antidepressant medication at baseline stopped taking the medication prior to dosing with SPL026.
Patients received a placebo (n = 17) or active treatment (n = 17). The latter consisted of a short IV infusion of 21.5 mg of SPL026, resulting in a 20- to 30-minute psychedelic experience, and supportive therapy.
The dose was selected based on data analysis from the company’s phase 1 study in healthy volunteers.
Efficacy was assessed using the Montgomery-Asberg Depression Rating Scale (MADRS) to measure changes in MDD symptoms.
Two weeks after dosing, those receiving the novel therapy showed a significant reduction in depressive symptoms, demonstrating a –7.4-point difference versus the placebo group in MADRS score (P = .02).
Analysis of key secondary endpoints showed a rapid onset of antidepressant effect 1 week post-dose, with a statistically significant difference in MADRS score between the active and placebo groups of –10.8 points (P = .002).
Next steps?
All participants were subsequently enrolled into an open-label phase of the trial where they received a single dose of SPL026 with supportive therapy. They were then followed for a further 12 weeks.
In the open-label phase, patients who received at least one active dose of SPL026 with supportive therapy reported a durable improvement in depression symptoms.
No apparent difference in antidepressant effect was observed between a one- or two-dose regimen of SPL026.
“SPL026 with supportive therapy was shown to have a significant antidepressant effect that was rapid and durable,” Carol Routledge, PhD, chief medical and scientific officer at Small Pharma, said in the statement.
“The results are clinically meaningful and enable us to progress into an international multisite phase 2b study where we seek to further explore the efficacy and safety profile of SPL026 in a larger MDD patient population,” Dr. Routledge added.
A version of this article first appeared on Medscape.com.
Top-line results from a phase 2a study of SPL026 (intravenous N,N-Dimethyltryptamine [DMT]) showed a 57% remission rate 3 months after participants received a single dose of the drug, the developer reports.
Small Pharma noted in a press release that this is the first placebo-controlled efficacy trial of a short-duration psychedelic for depression completed to date.
Investigators reported significant improvement in depression symptoms 2 weeks after dosing, which was the primary endpoint, and the improvement persisted at week 12.
“We now have the first evidence that SPL026 DMT, combined with supportive therapy, may be effective for people suffering from MDD,” chief investigator David Erritzoe, MD, PhD, clinical psychiatrist at Imperial College London, said in a statement.
“For patients who are unfortunate to experience little benefit from existing antidepressants, the potential for rapid and durable relief from a single treatment, as shown in this trial, is very promising,” Dr. Erritzoe added.
Randomized trial results
The blinded, randomized, placebo-controlled, two-staged phase 2a study included 34 patients with moderate to severe MDD. Those who were taking pharmacological antidepressant medication at baseline stopped taking the medication prior to dosing with SPL026.
Patients received a placebo (n = 17) or active treatment (n = 17). The latter consisted of a short IV infusion of 21.5 mg of SPL026, resulting in a 20- to 30-minute psychedelic experience, and supportive therapy.
The dose was selected based on data analysis from the company’s phase 1 study in healthy volunteers.
Efficacy was assessed using the Montgomery-Asberg Depression Rating Scale (MADRS) to measure changes in MDD symptoms.
Two weeks after dosing, those receiving the novel therapy showed a significant reduction in depressive symptoms, demonstrating a –7.4-point difference versus the placebo group in MADRS score (P = .02).
Analysis of key secondary endpoints showed a rapid onset of antidepressant effect 1 week post-dose, with a statistically significant difference in MADRS score between the active and placebo groups of –10.8 points (P = .002).
Next steps?
All participants were subsequently enrolled into an open-label phase of the trial where they received a single dose of SPL026 with supportive therapy. They were then followed for a further 12 weeks.
In the open-label phase, patients who received at least one active dose of SPL026 with supportive therapy reported a durable improvement in depression symptoms.
No apparent difference in antidepressant effect was observed between a one- or two-dose regimen of SPL026.
“SPL026 with supportive therapy was shown to have a significant antidepressant effect that was rapid and durable,” Carol Routledge, PhD, chief medical and scientific officer at Small Pharma, said in the statement.
“The results are clinically meaningful and enable us to progress into an international multisite phase 2b study where we seek to further explore the efficacy and safety profile of SPL026 in a larger MDD patient population,” Dr. Routledge added.
A version of this article first appeared on Medscape.com.
Top-line results from a phase 2a study of SPL026 (intravenous N,N-Dimethyltryptamine [DMT]) showed a 57% remission rate 3 months after participants received a single dose of the drug, the developer reports.
Small Pharma noted in a press release that this is the first placebo-controlled efficacy trial of a short-duration psychedelic for depression completed to date.
Investigators reported significant improvement in depression symptoms 2 weeks after dosing, which was the primary endpoint, and the improvement persisted at week 12.
“We now have the first evidence that SPL026 DMT, combined with supportive therapy, may be effective for people suffering from MDD,” chief investigator David Erritzoe, MD, PhD, clinical psychiatrist at Imperial College London, said in a statement.
“For patients who are unfortunate to experience little benefit from existing antidepressants, the potential for rapid and durable relief from a single treatment, as shown in this trial, is very promising,” Dr. Erritzoe added.
Randomized trial results
The blinded, randomized, placebo-controlled, two-staged phase 2a study included 34 patients with moderate to severe MDD. Those who were taking pharmacological antidepressant medication at baseline stopped taking the medication prior to dosing with SPL026.
Patients received a placebo (n = 17) or active treatment (n = 17). The latter consisted of a short IV infusion of 21.5 mg of SPL026, resulting in a 20- to 30-minute psychedelic experience, and supportive therapy.
The dose was selected based on data analysis from the company’s phase 1 study in healthy volunteers.
Efficacy was assessed using the Montgomery-Asberg Depression Rating Scale (MADRS) to measure changes in MDD symptoms.
Two weeks after dosing, those receiving the novel therapy showed a significant reduction in depressive symptoms, demonstrating a –7.4-point difference versus the placebo group in MADRS score (P = .02).
Analysis of key secondary endpoints showed a rapid onset of antidepressant effect 1 week post-dose, with a statistically significant difference in MADRS score between the active and placebo groups of –10.8 points (P = .002).
Next steps?
All participants were subsequently enrolled into an open-label phase of the trial where they received a single dose of SPL026 with supportive therapy. They were then followed for a further 12 weeks.
In the open-label phase, patients who received at least one active dose of SPL026 with supportive therapy reported a durable improvement in depression symptoms.
No apparent difference in antidepressant effect was observed between a one- or two-dose regimen of SPL026.
“SPL026 with supportive therapy was shown to have a significant antidepressant effect that was rapid and durable,” Carol Routledge, PhD, chief medical and scientific officer at Small Pharma, said in the statement.
“The results are clinically meaningful and enable us to progress into an international multisite phase 2b study where we seek to further explore the efficacy and safety profile of SPL026 in a larger MDD patient population,” Dr. Routledge added.
A version of this article first appeared on Medscape.com.




















