User login
Study results support screening rosacea patients for cardiometabolic disease
according to the results of a meta-analysis of more than 50,000 patients.
To date, “mounting comorbidities of rosacea have been identified, suggesting that rosacea is not simply a skin disease but has links to multiple systemic illnesses,” wrote Qi Chen, MD, of Central South University, Changsha, China, and colleagues. The association with rosacea and cardiometabolic disease has been controversial, they added.
In a study published in the Journal of the American Academy of Dermatology, they identified 13 studies including 50,442 rosacea patients and 1,525,864 controls. Approximately 71% of the rosacea patients were women.
Overall, patients with rosacea showed a statistically significant association for hypertension (risk ratio, 1.20; 95% confidence interval, 1.08-1.34; P = .001) and dyslipidemia (RR, 1.32; 95% CI, 1.10-1.58; P = .002). Specifically, rosacea patients averaged higher standard mean differences of systolic and diastolic blood pressure, total cholesterol, HDL cholesterol and LDL cholesterol, and triglycerides, compared with controls.
Rosacea was not significantly associated with an increased risk for ischemic heart disease, stroke, or diabetes, although the rosacea patients showed significantly increased risk of higher fasting blood glucose, compared with controls.
Findings don’t show causality
The study findings were limited by several factors, including the observational nature of some of the studies and the inability to perform subgroup analyses based on subtype and disease severity, the researchers noted. In addition, most of the rosacea patients were outpatients. “Further investigations are warranted to identify the relationship between rosacea and [cardiometabolic disease] in general populations to further validate the significance of our findings.”
However, the results support the value of screening for cardiometabolic disease in rosacea patients to facilitate diagnosis and treatment of disease at an early stage, they concluded.
“Rosacea has been linked statistically to many comorbidities including depression, anxiety, hypertension, and diabetes mellitus,” Julie Harper, MD, of the Dermatology and Skin Care Center of Birmingham (Alabama), said in an interview.
“This study looked more specifically at cardiometabolic disease and found a statistically significant correlation between rosacea and hypertension, higher total cholesterol, higher triglycerides and higher fasting blood glucose,” she said. However, “while there is an association present in this meta-analysis, we cannot assume a cause-and-effect relationship.”
Although the analysis does not prove causality, the key message for clinicians is that cardiometabolic disease is quite common in rosacea patients, and risk factors should be identified and treated early, said Dr. Harper. “Our patients with and without rosacea will benefit from age-appropriate screening, physical examination, and laboratory evaluation with a primary care physician. For rosacea patients in particular, we can advise them that early research suggests that individuals with rosacea might have an increased risk of hypertension and/or high cholesterol and triglycerides. It never hurts to make an appointment with primary care and to be checked.”
“We need more confirmatory studies that minimize the influence of confounding,” Dr. Harper added. Rosacea also has also been linked to obesity, which is another risk factor for cardiometabolic disease.
The study was supported by multiple grants from the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose. Dr. Harper had no relevant financial conflicts to disclose.
SOURCE: Chen Q et al. J Am Acad Dermatol. 2020 Nov;83(5):1331-40.
according to the results of a meta-analysis of more than 50,000 patients.
To date, “mounting comorbidities of rosacea have been identified, suggesting that rosacea is not simply a skin disease but has links to multiple systemic illnesses,” wrote Qi Chen, MD, of Central South University, Changsha, China, and colleagues. The association with rosacea and cardiometabolic disease has been controversial, they added.
In a study published in the Journal of the American Academy of Dermatology, they identified 13 studies including 50,442 rosacea patients and 1,525,864 controls. Approximately 71% of the rosacea patients were women.
Overall, patients with rosacea showed a statistically significant association for hypertension (risk ratio, 1.20; 95% confidence interval, 1.08-1.34; P = .001) and dyslipidemia (RR, 1.32; 95% CI, 1.10-1.58; P = .002). Specifically, rosacea patients averaged higher standard mean differences of systolic and diastolic blood pressure, total cholesterol, HDL cholesterol and LDL cholesterol, and triglycerides, compared with controls.
Rosacea was not significantly associated with an increased risk for ischemic heart disease, stroke, or diabetes, although the rosacea patients showed significantly increased risk of higher fasting blood glucose, compared with controls.
Findings don’t show causality
The study findings were limited by several factors, including the observational nature of some of the studies and the inability to perform subgroup analyses based on subtype and disease severity, the researchers noted. In addition, most of the rosacea patients were outpatients. “Further investigations are warranted to identify the relationship between rosacea and [cardiometabolic disease] in general populations to further validate the significance of our findings.”
However, the results support the value of screening for cardiometabolic disease in rosacea patients to facilitate diagnosis and treatment of disease at an early stage, they concluded.
“Rosacea has been linked statistically to many comorbidities including depression, anxiety, hypertension, and diabetes mellitus,” Julie Harper, MD, of the Dermatology and Skin Care Center of Birmingham (Alabama), said in an interview.
“This study looked more specifically at cardiometabolic disease and found a statistically significant correlation between rosacea and hypertension, higher total cholesterol, higher triglycerides and higher fasting blood glucose,” she said. However, “while there is an association present in this meta-analysis, we cannot assume a cause-and-effect relationship.”
Although the analysis does not prove causality, the key message for clinicians is that cardiometabolic disease is quite common in rosacea patients, and risk factors should be identified and treated early, said Dr. Harper. “Our patients with and without rosacea will benefit from age-appropriate screening, physical examination, and laboratory evaluation with a primary care physician. For rosacea patients in particular, we can advise them that early research suggests that individuals with rosacea might have an increased risk of hypertension and/or high cholesterol and triglycerides. It never hurts to make an appointment with primary care and to be checked.”
“We need more confirmatory studies that minimize the influence of confounding,” Dr. Harper added. Rosacea also has also been linked to obesity, which is another risk factor for cardiometabolic disease.
The study was supported by multiple grants from the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose. Dr. Harper had no relevant financial conflicts to disclose.
SOURCE: Chen Q et al. J Am Acad Dermatol. 2020 Nov;83(5):1331-40.
according to the results of a meta-analysis of more than 50,000 patients.
To date, “mounting comorbidities of rosacea have been identified, suggesting that rosacea is not simply a skin disease but has links to multiple systemic illnesses,” wrote Qi Chen, MD, of Central South University, Changsha, China, and colleagues. The association with rosacea and cardiometabolic disease has been controversial, they added.
In a study published in the Journal of the American Academy of Dermatology, they identified 13 studies including 50,442 rosacea patients and 1,525,864 controls. Approximately 71% of the rosacea patients were women.
Overall, patients with rosacea showed a statistically significant association for hypertension (risk ratio, 1.20; 95% confidence interval, 1.08-1.34; P = .001) and dyslipidemia (RR, 1.32; 95% CI, 1.10-1.58; P = .002). Specifically, rosacea patients averaged higher standard mean differences of systolic and diastolic blood pressure, total cholesterol, HDL cholesterol and LDL cholesterol, and triglycerides, compared with controls.
Rosacea was not significantly associated with an increased risk for ischemic heart disease, stroke, or diabetes, although the rosacea patients showed significantly increased risk of higher fasting blood glucose, compared with controls.
Findings don’t show causality
The study findings were limited by several factors, including the observational nature of some of the studies and the inability to perform subgroup analyses based on subtype and disease severity, the researchers noted. In addition, most of the rosacea patients were outpatients. “Further investigations are warranted to identify the relationship between rosacea and [cardiometabolic disease] in general populations to further validate the significance of our findings.”
However, the results support the value of screening for cardiometabolic disease in rosacea patients to facilitate diagnosis and treatment of disease at an early stage, they concluded.
“Rosacea has been linked statistically to many comorbidities including depression, anxiety, hypertension, and diabetes mellitus,” Julie Harper, MD, of the Dermatology and Skin Care Center of Birmingham (Alabama), said in an interview.
“This study looked more specifically at cardiometabolic disease and found a statistically significant correlation between rosacea and hypertension, higher total cholesterol, higher triglycerides and higher fasting blood glucose,” she said. However, “while there is an association present in this meta-analysis, we cannot assume a cause-and-effect relationship.”
Although the analysis does not prove causality, the key message for clinicians is that cardiometabolic disease is quite common in rosacea patients, and risk factors should be identified and treated early, said Dr. Harper. “Our patients with and without rosacea will benefit from age-appropriate screening, physical examination, and laboratory evaluation with a primary care physician. For rosacea patients in particular, we can advise them that early research suggests that individuals with rosacea might have an increased risk of hypertension and/or high cholesterol and triglycerides. It never hurts to make an appointment with primary care and to be checked.”
“We need more confirmatory studies that minimize the influence of confounding,” Dr. Harper added. Rosacea also has also been linked to obesity, which is another risk factor for cardiometabolic disease.
The study was supported by multiple grants from the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose. Dr. Harper had no relevant financial conflicts to disclose.
SOURCE: Chen Q et al. J Am Acad Dermatol. 2020 Nov;83(5):1331-40.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Moving from subtypes to phenotypes is simplifying management of rosacea
When a new phenotype approach to the diagnosis of rosacea was proposed 2 years ago, this simpler and more accurate method was accompanied by several corollary advantages, including a more rational approach to treatment and better methods of measuring treatment efficacy, according to an expert speaking at the annual Coastal Dermatology Symposium, held virtually.
“By looking at rosacea in a more simple way – but a more accurate way – we are able to track what happens [to key features] over time,” explained Jerry Tan, MD, of the University of Western Ontario, London.
With the previous method of subtyping, many rosacea patients failed to fit neatly into any of the four categories, producing confusion and diverting attention from troublesome symptoms.
“Rosacea patients often present with a range of features that span multiple subtypes or progress between them,” Dr. Tan explained. The risk is not just a delay in diagnosis but a failure to focus on symptoms patients find most bothersome.
The previous diagnostic criteria for rosacea, published in 2002, identified primary and secondary symptoms within its four subtypes. The new diagnostic criteria, endorsed by the National Rosacea Society and published in 2018, rely on phenotypes defined by diagnostic, major, and minor symptoms. Rather than the four previous subtypes, which were erythematotelangiectatic, papulopustular, phymatous, and ocular, the phenotypes facilitate diagnosis in patients with mixed features.
By replacing “the old thought process of subtyping” with a newer focus on phenotypes, the updated criteria were “aimed toward accuracy, simplicity and practicality,” Dr. Tan said.
Moreover, without squeezing patients into subgroups where they do not neatly fit, the new criteria draw attention to the specific symptoms that bring patients to the clinician.
The phenotype approach to treatment strategies was reflected in a systematic review of treatments based on phenotypes that was published in 2019, not long after the new classification system became available. In this review, coauthored by Dr. Tan, the GRADE certainty-of-evidence approach was employed to identify effective therapies, matching specific symptoms with specific therapies such as low-dose isotretinoin for papules or omega-3 fatty acids for dry eyes.
Based on a patient-centric approach that emphasizes control of key symptoms, Dr. Tan also described a method of documenting the severity of major and minor symptoms at each visit. With this method, called a rosacea patient tracker, patients and physicians can determine whether therapies are effective against the signs and symptoms of disease that they find most burdensome, according to Dr. Tan, who was the first author of an article he cited as a reference to this phenotype-based methodology.
Overall, the phenotype approach to rosacea “rationalizes treatment,” he said.
Specifically, the heterogeneity of symptoms in rosacea is mirrored in the heterogeneity of underlying pathophysiology. According to Dr. Tan, the upregulation of cytokines for inflammation, of angiogenic pathways for vascular symptoms, and of matrix metalloproteinases for tissue remodeling are all implicated in rosacea but drive different types of symptoms. While appropriate skin care and efforts to identify and minimize symptom triggers is appropriate for all patients, phenotypes provide a guide to the most appropriate therapies.
He said he hopes that the focus on phenotypes will draw attention to differences in these pathophysiological mechanisms. According to Dr. Tan, evaluating rosacea from the perspective of phenotypes has represented an important paradigm shift that extends beyond diagnosis.
“The move to the phenotype approach is hopefully simpler, more accurate, and more relevant,” Dr. Tan said.
This same approach has been advocated by others, including Esther J. van Zurren, MD, professor of dermatology at Leiden University Medical Centre in the Netherlands, the lead author of the 2018 systematic review article discussed by Dr. Tan. In this review article on the phenotype approach, specific strategies were recommended for specific symptoms on the basis of grading by an international group of experts that included Dr. Tan, a coauthor.
“These strategies should be directed toward achieving improvements in general well-being by targeting those aspects most bothersome to the patient,” the article advises. Like Dr. Tan, she considers this phenotype-based approach to diagnosis and treatment to be a meaningful clinical advance over the guidelines published in 2002.
“Management strategies for people with rosacea should include phenotype-based treatments,” she agreed, adding that specific choices should be made on the basis of these phenotypes “instead of the previous subtype classification.”
The meeting was jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are owned by the same parent company.
When a new phenotype approach to the diagnosis of rosacea was proposed 2 years ago, this simpler and more accurate method was accompanied by several corollary advantages, including a more rational approach to treatment and better methods of measuring treatment efficacy, according to an expert speaking at the annual Coastal Dermatology Symposium, held virtually.
“By looking at rosacea in a more simple way – but a more accurate way – we are able to track what happens [to key features] over time,” explained Jerry Tan, MD, of the University of Western Ontario, London.
With the previous method of subtyping, many rosacea patients failed to fit neatly into any of the four categories, producing confusion and diverting attention from troublesome symptoms.
“Rosacea patients often present with a range of features that span multiple subtypes or progress between them,” Dr. Tan explained. The risk is not just a delay in diagnosis but a failure to focus on symptoms patients find most bothersome.
The previous diagnostic criteria for rosacea, published in 2002, identified primary and secondary symptoms within its four subtypes. The new diagnostic criteria, endorsed by the National Rosacea Society and published in 2018, rely on phenotypes defined by diagnostic, major, and minor symptoms. Rather than the four previous subtypes, which were erythematotelangiectatic, papulopustular, phymatous, and ocular, the phenotypes facilitate diagnosis in patients with mixed features.
By replacing “the old thought process of subtyping” with a newer focus on phenotypes, the updated criteria were “aimed toward accuracy, simplicity and practicality,” Dr. Tan said.
Moreover, without squeezing patients into subgroups where they do not neatly fit, the new criteria draw attention to the specific symptoms that bring patients to the clinician.
The phenotype approach to treatment strategies was reflected in a systematic review of treatments based on phenotypes that was published in 2019, not long after the new classification system became available. In this review, coauthored by Dr. Tan, the GRADE certainty-of-evidence approach was employed to identify effective therapies, matching specific symptoms with specific therapies such as low-dose isotretinoin for papules or omega-3 fatty acids for dry eyes.
Based on a patient-centric approach that emphasizes control of key symptoms, Dr. Tan also described a method of documenting the severity of major and minor symptoms at each visit. With this method, called a rosacea patient tracker, patients and physicians can determine whether therapies are effective against the signs and symptoms of disease that they find most burdensome, according to Dr. Tan, who was the first author of an article he cited as a reference to this phenotype-based methodology.
Overall, the phenotype approach to rosacea “rationalizes treatment,” he said.
Specifically, the heterogeneity of symptoms in rosacea is mirrored in the heterogeneity of underlying pathophysiology. According to Dr. Tan, the upregulation of cytokines for inflammation, of angiogenic pathways for vascular symptoms, and of matrix metalloproteinases for tissue remodeling are all implicated in rosacea but drive different types of symptoms. While appropriate skin care and efforts to identify and minimize symptom triggers is appropriate for all patients, phenotypes provide a guide to the most appropriate therapies.
He said he hopes that the focus on phenotypes will draw attention to differences in these pathophysiological mechanisms. According to Dr. Tan, evaluating rosacea from the perspective of phenotypes has represented an important paradigm shift that extends beyond diagnosis.
“The move to the phenotype approach is hopefully simpler, more accurate, and more relevant,” Dr. Tan said.
This same approach has been advocated by others, including Esther J. van Zurren, MD, professor of dermatology at Leiden University Medical Centre in the Netherlands, the lead author of the 2018 systematic review article discussed by Dr. Tan. In this review article on the phenotype approach, specific strategies were recommended for specific symptoms on the basis of grading by an international group of experts that included Dr. Tan, a coauthor.
“These strategies should be directed toward achieving improvements in general well-being by targeting those aspects most bothersome to the patient,” the article advises. Like Dr. Tan, she considers this phenotype-based approach to diagnosis and treatment to be a meaningful clinical advance over the guidelines published in 2002.
“Management strategies for people with rosacea should include phenotype-based treatments,” she agreed, adding that specific choices should be made on the basis of these phenotypes “instead of the previous subtype classification.”
The meeting was jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are owned by the same parent company.
When a new phenotype approach to the diagnosis of rosacea was proposed 2 years ago, this simpler and more accurate method was accompanied by several corollary advantages, including a more rational approach to treatment and better methods of measuring treatment efficacy, according to an expert speaking at the annual Coastal Dermatology Symposium, held virtually.
“By looking at rosacea in a more simple way – but a more accurate way – we are able to track what happens [to key features] over time,” explained Jerry Tan, MD, of the University of Western Ontario, London.
With the previous method of subtyping, many rosacea patients failed to fit neatly into any of the four categories, producing confusion and diverting attention from troublesome symptoms.
“Rosacea patients often present with a range of features that span multiple subtypes or progress between them,” Dr. Tan explained. The risk is not just a delay in diagnosis but a failure to focus on symptoms patients find most bothersome.
The previous diagnostic criteria for rosacea, published in 2002, identified primary and secondary symptoms within its four subtypes. The new diagnostic criteria, endorsed by the National Rosacea Society and published in 2018, rely on phenotypes defined by diagnostic, major, and minor symptoms. Rather than the four previous subtypes, which were erythematotelangiectatic, papulopustular, phymatous, and ocular, the phenotypes facilitate diagnosis in patients with mixed features.
By replacing “the old thought process of subtyping” with a newer focus on phenotypes, the updated criteria were “aimed toward accuracy, simplicity and practicality,” Dr. Tan said.
Moreover, without squeezing patients into subgroups where they do not neatly fit, the new criteria draw attention to the specific symptoms that bring patients to the clinician.
The phenotype approach to treatment strategies was reflected in a systematic review of treatments based on phenotypes that was published in 2019, not long after the new classification system became available. In this review, coauthored by Dr. Tan, the GRADE certainty-of-evidence approach was employed to identify effective therapies, matching specific symptoms with specific therapies such as low-dose isotretinoin for papules or omega-3 fatty acids for dry eyes.
Based on a patient-centric approach that emphasizes control of key symptoms, Dr. Tan also described a method of documenting the severity of major and minor symptoms at each visit. With this method, called a rosacea patient tracker, patients and physicians can determine whether therapies are effective against the signs and symptoms of disease that they find most burdensome, according to Dr. Tan, who was the first author of an article he cited as a reference to this phenotype-based methodology.
Overall, the phenotype approach to rosacea “rationalizes treatment,” he said.
Specifically, the heterogeneity of symptoms in rosacea is mirrored in the heterogeneity of underlying pathophysiology. According to Dr. Tan, the upregulation of cytokines for inflammation, of angiogenic pathways for vascular symptoms, and of matrix metalloproteinases for tissue remodeling are all implicated in rosacea but drive different types of symptoms. While appropriate skin care and efforts to identify and minimize symptom triggers is appropriate for all patients, phenotypes provide a guide to the most appropriate therapies.
He said he hopes that the focus on phenotypes will draw attention to differences in these pathophysiological mechanisms. According to Dr. Tan, evaluating rosacea from the perspective of phenotypes has represented an important paradigm shift that extends beyond diagnosis.
“The move to the phenotype approach is hopefully simpler, more accurate, and more relevant,” Dr. Tan said.
This same approach has been advocated by others, including Esther J. van Zurren, MD, professor of dermatology at Leiden University Medical Centre in the Netherlands, the lead author of the 2018 systematic review article discussed by Dr. Tan. In this review article on the phenotype approach, specific strategies were recommended for specific symptoms on the basis of grading by an international group of experts that included Dr. Tan, a coauthor.
“These strategies should be directed toward achieving improvements in general well-being by targeting those aspects most bothersome to the patient,” the article advises. Like Dr. Tan, she considers this phenotype-based approach to diagnosis and treatment to be a meaningful clinical advance over the guidelines published in 2002.
“Management strategies for people with rosacea should include phenotype-based treatments,” she agreed, adding that specific choices should be made on the basis of these phenotypes “instead of the previous subtype classification.”
The meeting was jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are owned by the same parent company.
FROM COASTAL DERM
Phase 1 study: Beta-blocker may improve melanoma treatment response
Response rates were high without dose-limiting toxicities in a small phase 1 study that evaluated the addition of propranolol to pembrolizumab in treatment-naive patients with metastatic melanoma.
“To our knowledge, this effort is the,” wrote the two co-first authors, Shipra Gandhi, MD, and Manu Pandey, MBBS, from the Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., and coauthors.
The need for combinations built on anti-PD1 checkpoint inhibitor therapy strategies in metastatic melanoma that safely improve outcomes is underscored by the high (59%) grade 3 or 4 treatment-related adverse event (TRAE) rates when an anti-CTLA4 agent (ipilimumab) was added to an anti-PD-1 agent (nivolumab), they noted. In contrast, a TRAE rate of only 17% has been reported with pembrolizumab monotherapy.
The phase 1b study was stimulated by preclinical, retrospective observations of improved overall survival (OS) in cancer patients treated with beta-blockers. These were preceded by murine melanoma studies showing decreased tumor growth and metastasis with the nonselective beta-blocker propranolol. “Propranolol exerts an antitumor effect,” the authors stated, “by favorably modulating the tumor microenvironment (TME) by decreasing myeloid-derived suppressor cells and increasing CD8+ T-cell and natural killer cells in the TME.” Other research in a melanoma model in chronically-stressed mice has demonstrated synergy between an anti-PD1 antibody and propranolol.
“We know that stress can have a significant negative effect on health, but the extent to which stress may impact the outcome of cancer therapy is not well understood at all,” Dr. Ghandi said in a statement provided by Roswell Park. “We set out to better understand this relationship and to explore its implications for cancer treatment.”
The investigators recruited nine White adults (median age 65 years) with treatment-naive, histologically confirmed unresectable stage III or IV melanoma and Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 to the open-label, single arm, nonrandomized, single-center, dose-finding study. Patients received standard of care intravenous pembrolizumab 200 mg every 3 weeks and, in three groups, propranolol doses of 10 mg, 20 mg, or 30 mg twice a day until 2 years on study or disease progression or the development of dose-limiting toxicities (DLTs). Assessing the safety and efficacy (overall response rate [ORR] within 6 months of starting therapy) of pembrolizumab with the increasing doses of propranolol and selecting the recommended phase 2 dose were the study’s primary objectives.
Objective responses (complete or partial responses) were reported in seven of the nine patients, with partial tumor responses in two patients in the propranolol 10-mg group, two partial responses in the 20-mg group, and three partial responses in the 30-mg group.
While all patients experienced TRAEs, only one was above grade 2. The most commonly reported TRAEs were fatigue, rash and vitiligo, reported in four of the nine patients. Two patients in the 20-mg twice-a-day group discontinued therapy because of TRAEs (hemophagocytic lymphohistiocytosis and labyrinthitis). No DLTs were observed at any of the three dose levels, and no deaths occurred on study treatment.
The authors said that propranolol 30 mg twice a day was chosen as the recommended phase 2 dose, because in combination with pembrolizumab, there were no DLTs, and preliminary antitumor efficacy was observed in all three patients. Also, in all three patients, the investigators observed a trend toward higher CD8+T-cell percentage, higher ratios of CD8+T-cell/ Treg and CD8+T-cell/ polymorphonuclear myeloid-derived suppressor cells. They underscored, however, that the small size and significant heterogeneity in biomarkers made a statistically sound and meaningful interpretation of biomarkers for deciding the phase 2 dose difficult.
“In repurposing propranolol,” Dr. Pandey said in the Roswell statement, “we’ve gained important insights on how to manage stress in people with cancer – who can face dangerously elevated levels of mental and physical stress related to their diagnosis and treatment.”
In an interview, one of the two senior authors, Elizabeth Repasky, PhD, professor of oncology and immunology at Roswell Park, said, “it’s exciting that an extremely inexpensive drug like propranolol that could be used in every country around the world could have an impact on cancer by blocking stress, especially chronic stress.” Her murine research showing that adding propranolol to immunotherapy or radiotherapy or chemotherapy improved tumor growth control provided rationale for the current study.
“The breakthrough in this study is that it reveals the immune system as the best target to look at, and shows that what stress reduction is doing is improving a patient’s immune response to his or her own tumor,” Dr. Repasky said. “The mind/body connection is so important, but we have not had a handle on how to study it,” she added.
Further research funded by Herd of Hope grants at Roswell will look at tumor effects of propranolol and nonpharmacological reducers of chronic stress such as exercise, meditation, yoga, and Tai Chi, with first studies in breast cancer.
The study was funded by Roswell Park, private, and NIH grants. The authors had no disclosures.
SOURCE: Gandhi S et al. Clin Cancer Res. 2020 Oct 30. doi: 10.1158/1078-0432.CCR-20-2381
Response rates were high without dose-limiting toxicities in a small phase 1 study that evaluated the addition of propranolol to pembrolizumab in treatment-naive patients with metastatic melanoma.
“To our knowledge, this effort is the,” wrote the two co-first authors, Shipra Gandhi, MD, and Manu Pandey, MBBS, from the Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., and coauthors.
The need for combinations built on anti-PD1 checkpoint inhibitor therapy strategies in metastatic melanoma that safely improve outcomes is underscored by the high (59%) grade 3 or 4 treatment-related adverse event (TRAE) rates when an anti-CTLA4 agent (ipilimumab) was added to an anti-PD-1 agent (nivolumab), they noted. In contrast, a TRAE rate of only 17% has been reported with pembrolizumab monotherapy.
The phase 1b study was stimulated by preclinical, retrospective observations of improved overall survival (OS) in cancer patients treated with beta-blockers. These were preceded by murine melanoma studies showing decreased tumor growth and metastasis with the nonselective beta-blocker propranolol. “Propranolol exerts an antitumor effect,” the authors stated, “by favorably modulating the tumor microenvironment (TME) by decreasing myeloid-derived suppressor cells and increasing CD8+ T-cell and natural killer cells in the TME.” Other research in a melanoma model in chronically-stressed mice has demonstrated synergy between an anti-PD1 antibody and propranolol.
“We know that stress can have a significant negative effect on health, but the extent to which stress may impact the outcome of cancer therapy is not well understood at all,” Dr. Ghandi said in a statement provided by Roswell Park. “We set out to better understand this relationship and to explore its implications for cancer treatment.”
The investigators recruited nine White adults (median age 65 years) with treatment-naive, histologically confirmed unresectable stage III or IV melanoma and Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 to the open-label, single arm, nonrandomized, single-center, dose-finding study. Patients received standard of care intravenous pembrolizumab 200 mg every 3 weeks and, in three groups, propranolol doses of 10 mg, 20 mg, or 30 mg twice a day until 2 years on study or disease progression or the development of dose-limiting toxicities (DLTs). Assessing the safety and efficacy (overall response rate [ORR] within 6 months of starting therapy) of pembrolizumab with the increasing doses of propranolol and selecting the recommended phase 2 dose were the study’s primary objectives.
Objective responses (complete or partial responses) were reported in seven of the nine patients, with partial tumor responses in two patients in the propranolol 10-mg group, two partial responses in the 20-mg group, and three partial responses in the 30-mg group.
While all patients experienced TRAEs, only one was above grade 2. The most commonly reported TRAEs were fatigue, rash and vitiligo, reported in four of the nine patients. Two patients in the 20-mg twice-a-day group discontinued therapy because of TRAEs (hemophagocytic lymphohistiocytosis and labyrinthitis). No DLTs were observed at any of the three dose levels, and no deaths occurred on study treatment.
The authors said that propranolol 30 mg twice a day was chosen as the recommended phase 2 dose, because in combination with pembrolizumab, there were no DLTs, and preliminary antitumor efficacy was observed in all three patients. Also, in all three patients, the investigators observed a trend toward higher CD8+T-cell percentage, higher ratios of CD8+T-cell/ Treg and CD8+T-cell/ polymorphonuclear myeloid-derived suppressor cells. They underscored, however, that the small size and significant heterogeneity in biomarkers made a statistically sound and meaningful interpretation of biomarkers for deciding the phase 2 dose difficult.
“In repurposing propranolol,” Dr. Pandey said in the Roswell statement, “we’ve gained important insights on how to manage stress in people with cancer – who can face dangerously elevated levels of mental and physical stress related to their diagnosis and treatment.”
In an interview, one of the two senior authors, Elizabeth Repasky, PhD, professor of oncology and immunology at Roswell Park, said, “it’s exciting that an extremely inexpensive drug like propranolol that could be used in every country around the world could have an impact on cancer by blocking stress, especially chronic stress.” Her murine research showing that adding propranolol to immunotherapy or radiotherapy or chemotherapy improved tumor growth control provided rationale for the current study.
“The breakthrough in this study is that it reveals the immune system as the best target to look at, and shows that what stress reduction is doing is improving a patient’s immune response to his or her own tumor,” Dr. Repasky said. “The mind/body connection is so important, but we have not had a handle on how to study it,” she added.
Further research funded by Herd of Hope grants at Roswell will look at tumor effects of propranolol and nonpharmacological reducers of chronic stress such as exercise, meditation, yoga, and Tai Chi, with first studies in breast cancer.
The study was funded by Roswell Park, private, and NIH grants. The authors had no disclosures.
SOURCE: Gandhi S et al. Clin Cancer Res. 2020 Oct 30. doi: 10.1158/1078-0432.CCR-20-2381
Response rates were high without dose-limiting toxicities in a small phase 1 study that evaluated the addition of propranolol to pembrolizumab in treatment-naive patients with metastatic melanoma.
“To our knowledge, this effort is the,” wrote the two co-first authors, Shipra Gandhi, MD, and Manu Pandey, MBBS, from the Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., and coauthors.
The need for combinations built on anti-PD1 checkpoint inhibitor therapy strategies in metastatic melanoma that safely improve outcomes is underscored by the high (59%) grade 3 or 4 treatment-related adverse event (TRAE) rates when an anti-CTLA4 agent (ipilimumab) was added to an anti-PD-1 agent (nivolumab), they noted. In contrast, a TRAE rate of only 17% has been reported with pembrolizumab monotherapy.
The phase 1b study was stimulated by preclinical, retrospective observations of improved overall survival (OS) in cancer patients treated with beta-blockers. These were preceded by murine melanoma studies showing decreased tumor growth and metastasis with the nonselective beta-blocker propranolol. “Propranolol exerts an antitumor effect,” the authors stated, “by favorably modulating the tumor microenvironment (TME) by decreasing myeloid-derived suppressor cells and increasing CD8+ T-cell and natural killer cells in the TME.” Other research in a melanoma model in chronically-stressed mice has demonstrated synergy between an anti-PD1 antibody and propranolol.
“We know that stress can have a significant negative effect on health, but the extent to which stress may impact the outcome of cancer therapy is not well understood at all,” Dr. Ghandi said in a statement provided by Roswell Park. “We set out to better understand this relationship and to explore its implications for cancer treatment.”
The investigators recruited nine White adults (median age 65 years) with treatment-naive, histologically confirmed unresectable stage III or IV melanoma and Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 to the open-label, single arm, nonrandomized, single-center, dose-finding study. Patients received standard of care intravenous pembrolizumab 200 mg every 3 weeks and, in three groups, propranolol doses of 10 mg, 20 mg, or 30 mg twice a day until 2 years on study or disease progression or the development of dose-limiting toxicities (DLTs). Assessing the safety and efficacy (overall response rate [ORR] within 6 months of starting therapy) of pembrolizumab with the increasing doses of propranolol and selecting the recommended phase 2 dose were the study’s primary objectives.
Objective responses (complete or partial responses) were reported in seven of the nine patients, with partial tumor responses in two patients in the propranolol 10-mg group, two partial responses in the 20-mg group, and three partial responses in the 30-mg group.
While all patients experienced TRAEs, only one was above grade 2. The most commonly reported TRAEs were fatigue, rash and vitiligo, reported in four of the nine patients. Two patients in the 20-mg twice-a-day group discontinued therapy because of TRAEs (hemophagocytic lymphohistiocytosis and labyrinthitis). No DLTs were observed at any of the three dose levels, and no deaths occurred on study treatment.
The authors said that propranolol 30 mg twice a day was chosen as the recommended phase 2 dose, because in combination with pembrolizumab, there were no DLTs, and preliminary antitumor efficacy was observed in all three patients. Also, in all three patients, the investigators observed a trend toward higher CD8+T-cell percentage, higher ratios of CD8+T-cell/ Treg and CD8+T-cell/ polymorphonuclear myeloid-derived suppressor cells. They underscored, however, that the small size and significant heterogeneity in biomarkers made a statistically sound and meaningful interpretation of biomarkers for deciding the phase 2 dose difficult.
“In repurposing propranolol,” Dr. Pandey said in the Roswell statement, “we’ve gained important insights on how to manage stress in people with cancer – who can face dangerously elevated levels of mental and physical stress related to their diagnosis and treatment.”
In an interview, one of the two senior authors, Elizabeth Repasky, PhD, professor of oncology and immunology at Roswell Park, said, “it’s exciting that an extremely inexpensive drug like propranolol that could be used in every country around the world could have an impact on cancer by blocking stress, especially chronic stress.” Her murine research showing that adding propranolol to immunotherapy or radiotherapy or chemotherapy improved tumor growth control provided rationale for the current study.
“The breakthrough in this study is that it reveals the immune system as the best target to look at, and shows that what stress reduction is doing is improving a patient’s immune response to his or her own tumor,” Dr. Repasky said. “The mind/body connection is so important, but we have not had a handle on how to study it,” she added.
Further research funded by Herd of Hope grants at Roswell will look at tumor effects of propranolol and nonpharmacological reducers of chronic stress such as exercise, meditation, yoga, and Tai Chi, with first studies in breast cancer.
The study was funded by Roswell Park, private, and NIH grants. The authors had no disclosures.
SOURCE: Gandhi S et al. Clin Cancer Res. 2020 Oct 30. doi: 10.1158/1078-0432.CCR-20-2381
FROM CLINICAL CANCER RESEARCH
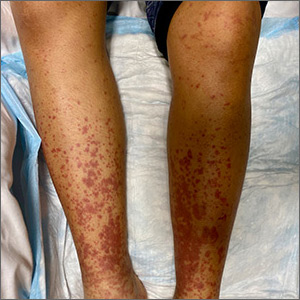
Painful, lower extremity rash
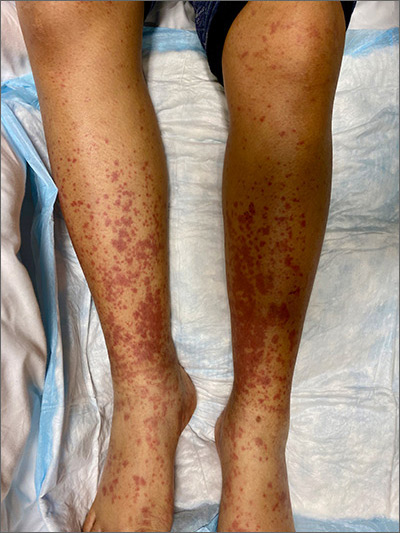
This woman’s palpable purpura with edema in her lower extremities was consistent with cutaneous leukocytoclastic vasculitis (LCV).
LCV is characterized by the circulation of immune complexes that promote activation of complement, leading to endothelial injury and palpable purpura. Pain, arthralgia, cutaneous ulceration, and constitutional symptoms may be observed. About 50% of LCV cases are idiopathic. Identified causes include infection (including syphilis infection), drugs, malignancy, and connective tissue disease.
Systemic involvement must be ruled out in any patient with cutaneous LCV. The work-up is based on the individual patient assessment and may include a complete blood count with differential, complete metabolic panel, inflammatory markers, urinalysis, hepatitis panel, anti-nuclear antibody, rheumatoid factor, anti-neutrophil cytoplasmic antibodies, cryoglobulins, serum protein electrophoresis, and serum complement. A cutaneous punch biopsy for both hematoxylin and eosin (H&E) and direct immunofluorescence (DIF) confirms the diagnosis of LCV.
For uncomplicated LCV cases without systemic involvement, treatment is generally supportive. Any identified underlying cause should be addressed. Analgesics may be considered for pain. Systemic therapy is indicated for patients with cutaneous ulceration, systemic vasculitis, or recurrent cases; this therapy may include colchicine, dapsone, corticosteroids, mycophenolate mofetil, and methotrexate.
In this patient’s case, a punch biopsy of the left lower extremity showed findings consistent with cutaneous LCV. She denied a history of intravenous drug use or initiation of new medications. Labs were notable for an elevated erythrocyte sedimentation rate, c-reactive protein, and elevated creatinine.
Incidentally, she was found to be 32-weeks pregnant, went into pre-term labor while admitted, and delivered her baby without complication.
She had a reactive treponemal antibody, with rapid plasma reagin titer of 1:128, which confirmed a diagnosis of syphilis. She was treated with 1 dose of intra-muscular penicillin G while an inpatient. Her arthralgias improved during her hospitalization without initiation of steroids or other immunomodulatory therapy. Outpatient follow-up was expected to consist of completion of 3 total doses of IM penicillin, as well as renal studies, given her elevated creatinine.
Photo courtesy of Cyrelle R. Fermin, MD, and text courtesy of Cyrelle R. Fermin, MD, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Goeser MR, Laniosz V, Wetter DA. A practical approach to the diagnosis, evaluation, and management of cutaneous small-vessel vasculitis. Am J Clin Dermatol. 2014;15:299-306.

This woman’s palpable purpura with edema in her lower extremities was consistent with cutaneous leukocytoclastic vasculitis (LCV).
LCV is characterized by the circulation of immune complexes that promote activation of complement, leading to endothelial injury and palpable purpura. Pain, arthralgia, cutaneous ulceration, and constitutional symptoms may be observed. About 50% of LCV cases are idiopathic. Identified causes include infection (including syphilis infection), drugs, malignancy, and connective tissue disease.
Systemic involvement must be ruled out in any patient with cutaneous LCV. The work-up is based on the individual patient assessment and may include a complete blood count with differential, complete metabolic panel, inflammatory markers, urinalysis, hepatitis panel, anti-nuclear antibody, rheumatoid factor, anti-neutrophil cytoplasmic antibodies, cryoglobulins, serum protein electrophoresis, and serum complement. A cutaneous punch biopsy for both hematoxylin and eosin (H&E) and direct immunofluorescence (DIF) confirms the diagnosis of LCV.
For uncomplicated LCV cases without systemic involvement, treatment is generally supportive. Any identified underlying cause should be addressed. Analgesics may be considered for pain. Systemic therapy is indicated for patients with cutaneous ulceration, systemic vasculitis, or recurrent cases; this therapy may include colchicine, dapsone, corticosteroids, mycophenolate mofetil, and methotrexate.
In this patient’s case, a punch biopsy of the left lower extremity showed findings consistent with cutaneous LCV. She denied a history of intravenous drug use or initiation of new medications. Labs were notable for an elevated erythrocyte sedimentation rate, c-reactive protein, and elevated creatinine.
Incidentally, she was found to be 32-weeks pregnant, went into pre-term labor while admitted, and delivered her baby without complication.
She had a reactive treponemal antibody, with rapid plasma reagin titer of 1:128, which confirmed a diagnosis of syphilis. She was treated with 1 dose of intra-muscular penicillin G while an inpatient. Her arthralgias improved during her hospitalization without initiation of steroids or other immunomodulatory therapy. Outpatient follow-up was expected to consist of completion of 3 total doses of IM penicillin, as well as renal studies, given her elevated creatinine.
Photo courtesy of Cyrelle R. Fermin, MD, and text courtesy of Cyrelle R. Fermin, MD, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

This woman’s palpable purpura with edema in her lower extremities was consistent with cutaneous leukocytoclastic vasculitis (LCV).
LCV is characterized by the circulation of immune complexes that promote activation of complement, leading to endothelial injury and palpable purpura. Pain, arthralgia, cutaneous ulceration, and constitutional symptoms may be observed. About 50% of LCV cases are idiopathic. Identified causes include infection (including syphilis infection), drugs, malignancy, and connective tissue disease.
Systemic involvement must be ruled out in any patient with cutaneous LCV. The work-up is based on the individual patient assessment and may include a complete blood count with differential, complete metabolic panel, inflammatory markers, urinalysis, hepatitis panel, anti-nuclear antibody, rheumatoid factor, anti-neutrophil cytoplasmic antibodies, cryoglobulins, serum protein electrophoresis, and serum complement. A cutaneous punch biopsy for both hematoxylin and eosin (H&E) and direct immunofluorescence (DIF) confirms the diagnosis of LCV.
For uncomplicated LCV cases without systemic involvement, treatment is generally supportive. Any identified underlying cause should be addressed. Analgesics may be considered for pain. Systemic therapy is indicated for patients with cutaneous ulceration, systemic vasculitis, or recurrent cases; this therapy may include colchicine, dapsone, corticosteroids, mycophenolate mofetil, and methotrexate.
In this patient’s case, a punch biopsy of the left lower extremity showed findings consistent with cutaneous LCV. She denied a history of intravenous drug use or initiation of new medications. Labs were notable for an elevated erythrocyte sedimentation rate, c-reactive protein, and elevated creatinine.
Incidentally, she was found to be 32-weeks pregnant, went into pre-term labor while admitted, and delivered her baby without complication.
She had a reactive treponemal antibody, with rapid plasma reagin titer of 1:128, which confirmed a diagnosis of syphilis. She was treated with 1 dose of intra-muscular penicillin G while an inpatient. Her arthralgias improved during her hospitalization without initiation of steroids or other immunomodulatory therapy. Outpatient follow-up was expected to consist of completion of 3 total doses of IM penicillin, as well as renal studies, given her elevated creatinine.
Photo courtesy of Cyrelle R. Fermin, MD, and text courtesy of Cyrelle R. Fermin, MD, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Goeser MR, Laniosz V, Wetter DA. A practical approach to the diagnosis, evaluation, and management of cutaneous small-vessel vasculitis. Am J Clin Dermatol. 2014;15:299-306.
Goeser MR, Laniosz V, Wetter DA. A practical approach to the diagnosis, evaluation, and management of cutaneous small-vessel vasculitis. Am J Clin Dermatol. 2014;15:299-306.
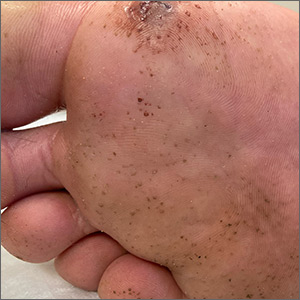
“Polka-dotted” feet
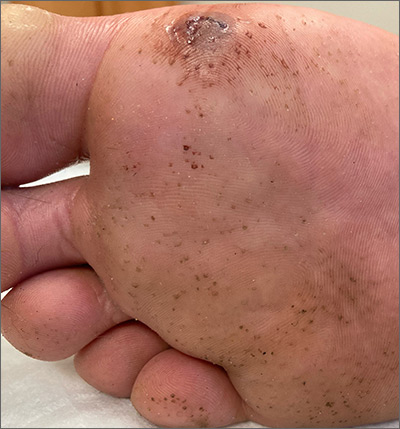
This man has pitted keratolysis (PK), characterized by multiple small pits on the soles of the feet. PK is often associated with hyperhidrosis and significant odor. The lesions usually have a punched-out appearance and are flesh-colored. The dark color of these lesions was due to the patient’s footwear.
PK is caused by bacterial overgrowth in the stratum corneum. Corynebacterium is the most common bacterial culprit, but Kytococcus, Actinomyces, and Dermatophilus have also been implicated. The bacterial infection is thought to be secondary to hyperhidrosis or as a result of hygiene, footwear, or other conditions that retain moisture and promote maceration of the soles of the feet. Therefore, treatment includes a 2-pronged approach: Resolve the bacterial infection and reduce excess moisture. Effective antibacterials include topical clindamycin, erythromycin, fusidic acid, and benzoyl peroxide. Oral antibiotics are not often required.
Hyperhidrosis can be treated with prescription strength 20% aluminum chloride antiperspirant applied to the feet in a tapering schedule, first daily and then 2 or 3 times weekly. Aluminum chloride is frequently not covered by insurance companies, but over-the-counter (OTC) 12% formulations (Certain DRI) usually suffice. Additionally, changing socks and using moisture-wicking shoes or socks are helpful measures to keep feet dry.
One study treated PK with topical erythromycin 3% gel twice daily, without the use of aluminum chloride antiperspirants, and found that the hyperhidrosis greatly improved. The authors theorized that the gram-positive bacterial infection upregulated eccrine sweat glands causing hyperhidrosis as a secondary, rather than the primary, cause of PK.
This patient was prescribed topical erythromycin gel twice daily for the soles of his feet. For his hyperhidrosis, he was advised to purchase OTC aluminum chloride antiperspirants to apply to his feet daily for the first week and to then decrease to 2 or 3 times per week. He was counseled to take an extra pair of socks for changing midway through his workday and to return for reevaluation if his skin did not improve.
Image courtesy of Sarah Friedberg, MD, and text courtesy of Daniel Stulberg, MD, FAAFP, and Sarah Friedberg, MD, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Pranteda G, Carlesimo M, Pranteda G, et al. Pitted keratolysis, erythromycin, and hyperhidrosis. Dermatol Ther. 2014;27:101-104.

This man has pitted keratolysis (PK), characterized by multiple small pits on the soles of the feet. PK is often associated with hyperhidrosis and significant odor. The lesions usually have a punched-out appearance and are flesh-colored. The dark color of these lesions was due to the patient’s footwear.
PK is caused by bacterial overgrowth in the stratum corneum. Corynebacterium is the most common bacterial culprit, but Kytococcus, Actinomyces, and Dermatophilus have also been implicated. The bacterial infection is thought to be secondary to hyperhidrosis or as a result of hygiene, footwear, or other conditions that retain moisture and promote maceration of the soles of the feet. Therefore, treatment includes a 2-pronged approach: Resolve the bacterial infection and reduce excess moisture. Effective antibacterials include topical clindamycin, erythromycin, fusidic acid, and benzoyl peroxide. Oral antibiotics are not often required.
Hyperhidrosis can be treated with prescription strength 20% aluminum chloride antiperspirant applied to the feet in a tapering schedule, first daily and then 2 or 3 times weekly. Aluminum chloride is frequently not covered by insurance companies, but over-the-counter (OTC) 12% formulations (Certain DRI) usually suffice. Additionally, changing socks and using moisture-wicking shoes or socks are helpful measures to keep feet dry.
One study treated PK with topical erythromycin 3% gel twice daily, without the use of aluminum chloride antiperspirants, and found that the hyperhidrosis greatly improved. The authors theorized that the gram-positive bacterial infection upregulated eccrine sweat glands causing hyperhidrosis as a secondary, rather than the primary, cause of PK.
This patient was prescribed topical erythromycin gel twice daily for the soles of his feet. For his hyperhidrosis, he was advised to purchase OTC aluminum chloride antiperspirants to apply to his feet daily for the first week and to then decrease to 2 or 3 times per week. He was counseled to take an extra pair of socks for changing midway through his workday and to return for reevaluation if his skin did not improve.
Image courtesy of Sarah Friedberg, MD, and text courtesy of Daniel Stulberg, MD, FAAFP, and Sarah Friedberg, MD, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

This man has pitted keratolysis (PK), characterized by multiple small pits on the soles of the feet. PK is often associated with hyperhidrosis and significant odor. The lesions usually have a punched-out appearance and are flesh-colored. The dark color of these lesions was due to the patient’s footwear.
PK is caused by bacterial overgrowth in the stratum corneum. Corynebacterium is the most common bacterial culprit, but Kytococcus, Actinomyces, and Dermatophilus have also been implicated. The bacterial infection is thought to be secondary to hyperhidrosis or as a result of hygiene, footwear, or other conditions that retain moisture and promote maceration of the soles of the feet. Therefore, treatment includes a 2-pronged approach: Resolve the bacterial infection and reduce excess moisture. Effective antibacterials include topical clindamycin, erythromycin, fusidic acid, and benzoyl peroxide. Oral antibiotics are not often required.
Hyperhidrosis can be treated with prescription strength 20% aluminum chloride antiperspirant applied to the feet in a tapering schedule, first daily and then 2 or 3 times weekly. Aluminum chloride is frequently not covered by insurance companies, but over-the-counter (OTC) 12% formulations (Certain DRI) usually suffice. Additionally, changing socks and using moisture-wicking shoes or socks are helpful measures to keep feet dry.
One study treated PK with topical erythromycin 3% gel twice daily, without the use of aluminum chloride antiperspirants, and found that the hyperhidrosis greatly improved. The authors theorized that the gram-positive bacterial infection upregulated eccrine sweat glands causing hyperhidrosis as a secondary, rather than the primary, cause of PK.
This patient was prescribed topical erythromycin gel twice daily for the soles of his feet. For his hyperhidrosis, he was advised to purchase OTC aluminum chloride antiperspirants to apply to his feet daily for the first week and to then decrease to 2 or 3 times per week. He was counseled to take an extra pair of socks for changing midway through his workday and to return for reevaluation if his skin did not improve.
Image courtesy of Sarah Friedberg, MD, and text courtesy of Daniel Stulberg, MD, FAAFP, and Sarah Friedberg, MD, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Pranteda G, Carlesimo M, Pranteda G, et al. Pitted keratolysis, erythromycin, and hyperhidrosis. Dermatol Ther. 2014;27:101-104.
Pranteda G, Carlesimo M, Pranteda G, et al. Pitted keratolysis, erythromycin, and hyperhidrosis. Dermatol Ther. 2014;27:101-104.
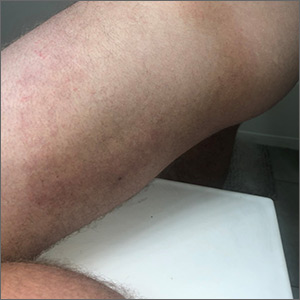
Large circular thigh rash
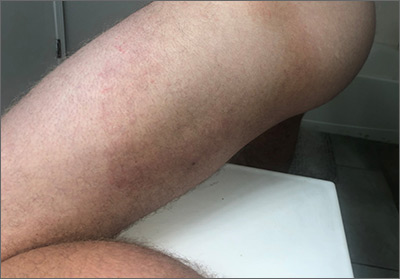
This patient had the deep form of erythema annulare centrifugum (EAC). As the name implies, it manifests as an expanding, red circular pattern that often clears in the middle. There is usually a ring of scale that trails behind the advancing border. However, in the deep form, it may be more subtle than the pronounced scale of the superficial form. Pruritus is a very common symptom associated with this condition.
EAC is a hypersensitivity reaction, which can be in response to several stimuli including underlying malignancy, medications, fungal and dermatophyte infections, inflammatory conditions, and pregnancy. A careful history and physical exam can be helpful in determining if a work-up for malignancy is warranted.
Since many medications including nonsteroidal anti-inflammatory drugs (NSAIDs), antidepressants, and biologicals can cause this condition, a history of which medications were started within the previous several months may be helpful.
When EAC is due to an underlying malignancy, it is called paraneoplastic erythema annulare centrifugum. It can be secondary to solid tumors or lymphoproliferative disorders.
More than 50% percent of the cases are idiopathic, and no underlying condition is identified. The skin findings may last for weeks—and even years.
If an underlying cause is found, treatment is directed at that condition, and the skin findings usually improve with resolution of the instigating condition. If no specific cause is found, the itching can be managed with systemic antihistamines or topical steroids. Some case studies have reported success with the use of systemic antibiotics, including erythromycin. Improvement with antibiotics may be due to treatment of an occult underlying bacterial process or owing to the anti-inflammatory effects of many antibiotics.
Since the patient in this case had onychomycosis of his toenails, and fungal and dermatophyte infections are a common trigger, he was placed on a 12-week course of oral terbinafine 250 mg/d. The plan was to biopsy the rash if it didn’t resolve. At 3 weeks, the rash had resolved, and the patient was asymptomatic.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
McDaniel B, Cook C. Erythema annulare centrifugum. In: Abai B, Abu-Ghosh A, Acharya AB, et al, eds. StatPearls. Treasure Island, FL; 2020. https://www.ncbi.nlm.nih.gov/books/NBK482494/. Accessed December 2, 2020.

This patient had the deep form of erythema annulare centrifugum (EAC). As the name implies, it manifests as an expanding, red circular pattern that often clears in the middle. There is usually a ring of scale that trails behind the advancing border. However, in the deep form, it may be more subtle than the pronounced scale of the superficial form. Pruritus is a very common symptom associated with this condition.
EAC is a hypersensitivity reaction, which can be in response to several stimuli including underlying malignancy, medications, fungal and dermatophyte infections, inflammatory conditions, and pregnancy. A careful history and physical exam can be helpful in determining if a work-up for malignancy is warranted.
Since many medications including nonsteroidal anti-inflammatory drugs (NSAIDs), antidepressants, and biologicals can cause this condition, a history of which medications were started within the previous several months may be helpful.
When EAC is due to an underlying malignancy, it is called paraneoplastic erythema annulare centrifugum. It can be secondary to solid tumors or lymphoproliferative disorders.
More than 50% percent of the cases are idiopathic, and no underlying condition is identified. The skin findings may last for weeks—and even years.
If an underlying cause is found, treatment is directed at that condition, and the skin findings usually improve with resolution of the instigating condition. If no specific cause is found, the itching can be managed with systemic antihistamines or topical steroids. Some case studies have reported success with the use of systemic antibiotics, including erythromycin. Improvement with antibiotics may be due to treatment of an occult underlying bacterial process or owing to the anti-inflammatory effects of many antibiotics.
Since the patient in this case had onychomycosis of his toenails, and fungal and dermatophyte infections are a common trigger, he was placed on a 12-week course of oral terbinafine 250 mg/d. The plan was to biopsy the rash if it didn’t resolve. At 3 weeks, the rash had resolved, and the patient was asymptomatic.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

This patient had the deep form of erythema annulare centrifugum (EAC). As the name implies, it manifests as an expanding, red circular pattern that often clears in the middle. There is usually a ring of scale that trails behind the advancing border. However, in the deep form, it may be more subtle than the pronounced scale of the superficial form. Pruritus is a very common symptom associated with this condition.
EAC is a hypersensitivity reaction, which can be in response to several stimuli including underlying malignancy, medications, fungal and dermatophyte infections, inflammatory conditions, and pregnancy. A careful history and physical exam can be helpful in determining if a work-up for malignancy is warranted.
Since many medications including nonsteroidal anti-inflammatory drugs (NSAIDs), antidepressants, and biologicals can cause this condition, a history of which medications were started within the previous several months may be helpful.
When EAC is due to an underlying malignancy, it is called paraneoplastic erythema annulare centrifugum. It can be secondary to solid tumors or lymphoproliferative disorders.
More than 50% percent of the cases are idiopathic, and no underlying condition is identified. The skin findings may last for weeks—and even years.
If an underlying cause is found, treatment is directed at that condition, and the skin findings usually improve with resolution of the instigating condition. If no specific cause is found, the itching can be managed with systemic antihistamines or topical steroids. Some case studies have reported success with the use of systemic antibiotics, including erythromycin. Improvement with antibiotics may be due to treatment of an occult underlying bacterial process or owing to the anti-inflammatory effects of many antibiotics.
Since the patient in this case had onychomycosis of his toenails, and fungal and dermatophyte infections are a common trigger, he was placed on a 12-week course of oral terbinafine 250 mg/d. The plan was to biopsy the rash if it didn’t resolve. At 3 weeks, the rash had resolved, and the patient was asymptomatic.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
McDaniel B, Cook C. Erythema annulare centrifugum. In: Abai B, Abu-Ghosh A, Acharya AB, et al, eds. StatPearls. Treasure Island, FL; 2020. https://www.ncbi.nlm.nih.gov/books/NBK482494/. Accessed December 2, 2020.
McDaniel B, Cook C. Erythema annulare centrifugum. In: Abai B, Abu-Ghosh A, Acharya AB, et al, eds. StatPearls. Treasure Island, FL; 2020. https://www.ncbi.nlm.nih.gov/books/NBK482494/. Accessed December 2, 2020.
Read This Rorschach Chest
ANSWER
The correct answer is Grover disease (choice “c”).
DISCUSSION
Grover disease—also known as transient acantholytic dermatosis—was first described in 1975 by Ralph Grover, MD, and is now recognized as a relatively common condition, especially in White men older than 40. Its appearance on darker skin is unusual. The morphologic presentation seen in this case is fairly typical, though most patients complain more about itching than this patient did.
The actual cause of Grover disease is unknown. Heat, sweat, and sunlight are suspected triggers. Its appearance can be associated with certain medications, recent treatment with ionizing radiation, or end-stage renal failure.
Biopsy is the key to diagnosis of Grover disease, especially in the context of the clinical presentation. This method also serves to rule out the other items in the differential.
Tinea corporis (choice “a”) was ruled out by the KOH as well as the rash’s appearance. Darier disease (choice “b”)—otherwise known as keratosis follicularis—is an inherited dermatosis that presents with multiple findings on skin, nails, and mucous membranes; however, the papulosquamous rash would be far more coarse than what manifests in Grover disease, and it would have manifested at a much earlier age. While folliculitis (choice “d”) was a possibility in this patient, the biopsy ruled it out.
TREATMENT
Grover disease is notoriously difficult to treat. Although it is described as a “transient” dermatosis, it can last months to years despite all therapeutic efforts.
This patient was treated with a stronger topical steroid (0.05% betamethasone cream) twice a day and minocycline (100 mg/d) for 2 months. These cleared his rash.
ANSWER
The correct answer is Grover disease (choice “c”).
DISCUSSION
Grover disease—also known as transient acantholytic dermatosis—was first described in 1975 by Ralph Grover, MD, and is now recognized as a relatively common condition, especially in White men older than 40. Its appearance on darker skin is unusual. The morphologic presentation seen in this case is fairly typical, though most patients complain more about itching than this patient did.
The actual cause of Grover disease is unknown. Heat, sweat, and sunlight are suspected triggers. Its appearance can be associated with certain medications, recent treatment with ionizing radiation, or end-stage renal failure.
Biopsy is the key to diagnosis of Grover disease, especially in the context of the clinical presentation. This method also serves to rule out the other items in the differential.
Tinea corporis (choice “a”) was ruled out by the KOH as well as the rash’s appearance. Darier disease (choice “b”)—otherwise known as keratosis follicularis—is an inherited dermatosis that presents with multiple findings on skin, nails, and mucous membranes; however, the papulosquamous rash would be far more coarse than what manifests in Grover disease, and it would have manifested at a much earlier age. While folliculitis (choice “d”) was a possibility in this patient, the biopsy ruled it out.
TREATMENT
Grover disease is notoriously difficult to treat. Although it is described as a “transient” dermatosis, it can last months to years despite all therapeutic efforts.
This patient was treated with a stronger topical steroid (0.05% betamethasone cream) twice a day and minocycline (100 mg/d) for 2 months. These cleared his rash.
ANSWER
The correct answer is Grover disease (choice “c”).
DISCUSSION
Grover disease—also known as transient acantholytic dermatosis—was first described in 1975 by Ralph Grover, MD, and is now recognized as a relatively common condition, especially in White men older than 40. Its appearance on darker skin is unusual. The morphologic presentation seen in this case is fairly typical, though most patients complain more about itching than this patient did.
The actual cause of Grover disease is unknown. Heat, sweat, and sunlight are suspected triggers. Its appearance can be associated with certain medications, recent treatment with ionizing radiation, or end-stage renal failure.
Biopsy is the key to diagnosis of Grover disease, especially in the context of the clinical presentation. This method also serves to rule out the other items in the differential.
Tinea corporis (choice “a”) was ruled out by the KOH as well as the rash’s appearance. Darier disease (choice “b”)—otherwise known as keratosis follicularis—is an inherited dermatosis that presents with multiple findings on skin, nails, and mucous membranes; however, the papulosquamous rash would be far more coarse than what manifests in Grover disease, and it would have manifested at a much earlier age. While folliculitis (choice “d”) was a possibility in this patient, the biopsy ruled it out.
TREATMENT
Grover disease is notoriously difficult to treat. Although it is described as a “transient” dermatosis, it can last months to years despite all therapeutic efforts.
This patient was treated with a stronger topical steroid (0.05% betamethasone cream) twice a day and minocycline (100 mg/d) for 2 months. These cleared his rash.
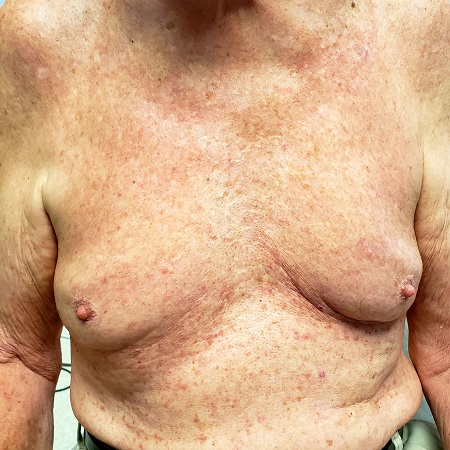
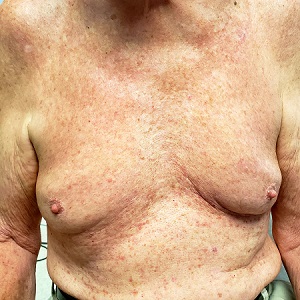
Several months ago, an asymptomatic rash manifested on the 63-year-old man’s chest. His primary care provider diagnosed this as eczema, though the patient has no history of atopic conditions. Initial treatment included 0.025% triamcinolone cream, which was later switched to 0.1%. This improved the appearance but failed to clear the condition, prompting a referral to dermatology.
The patient is in otherwise good health. He has no history of seasonal allergies, asthma, urticaria, or eczema. He takes no medications that could have caused the rash. There is no family history of atopy or any other skin disease.
The florid, papulovesicular rash covers his chest, from the upper sternum to the mid-abdomen. Palpation reveals a rough texture to the condition that is faintly erythematous. The rash also affects small areas of his triceps, but the rest of his skin—including back, legs, and buttocks—are clear. His hair and nails appear normal.
A KOH prep shows fungal elements, but the lab results are negative. Longitudinal shave biopsy shows subcorneal separation along with acantholysis (destruction of connections between keratinocytes).
Acute-on-chronic itch is new frontier in atopic dermatitis
Brian S. Kim, MD, said at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Recent years have brought enormous progress in understanding how chronic itch in patients with atopic dermatitis (AD) is mediated by type 2 cytokines, including interleukin-13, IL-4, and IL-31, as well as by Janus kinase (JAK) signaling. This has led to development of potent therapies targeting these mediators, including dupilumab (Dupixent) and the investigational agents tralokinumab, lebrikizumab, abrocitinib, upadacitinib, baricitinib, and the IL-31 inhibitor nemolizumab.
“This is now one of the most active areas in the field of dermatology,” observed Dr. Kim, a dermatologist and codirector of the Center for the Study of Itch and Sensory Disorders at Washington University in St. Louis.
He has figured prominently in this effort. He and his coinvestigators conducted translational studies in mouse models which unraveled key mechanisms by which the immune system responsible for skin inflammation in AD communicates with the nervous system to trigger the neural sensation of itch. He also led a phase 2 randomized trial in 307 patients with AD, which demonstrated that the investigational JAK1/JAK2 inhibitor ruxolitinib cream markedly improved itch within 36 hours, well before subsequent improvement in skin inflammation – and the topical JAK inhibitor did so with minimal systemic absorption.
Compared with chronic itch, much less research attention has been devoted to the phenomenon of acute itch flares superimposed upon the chronic itch of AD. These acute-on-chronic itch flares are a common feature of the disease. In a soon-to-be-published study of 159 AD patients in the placebo arm of a clinical trial, Dr. Kim and coinvestigators found that 26% exhibited a pattern of acute itch flares during the course of a single month. During the next month, 3.1% of patients under study went from an acute-on-chronic itch pattern in month 1 to a nonflare pattern, 20% went from a nonflare pattern in month 1 to acute itch flares in month 2, and 23% of the overall study population retained their pattern of acute itch flares through both months.
“This does not seem to be just a static phenotype, but rather these patients can evolve over time. And we think that this can be driven by allergen-specific IgE,” according to Dr. Kim.
Indeed, the investigators found that patients with allergen-specific IgE in their serum were roughly twice as likely to exhibit the acute-on-chronic itch flare pattern than those without allergen-specific IgE.
The classical thinking has been that IgE binds to its receptors on mast cells, causing mast cell degranulation and release of histamine and other itch-inducing molecules. Yet antihistamines have proven notoriously ineffective for the treatment of AD.
Circulating basophils capable of working their way into inflamed skin also have IgE receptors. Dr. Kim and colleagues have shown that allergen-specific IgE in mice binds to those receptors, causing the basophils to degenerate, releasing itch-promoting chemicals. They have subsequently carried over this work into the clinical arena.
“We’ve found that patients with atopic dermatitis have significantly higher expression of receptors for IgE in their basophils than in the basophils of healthy controls, indicating perhaps that the basophils in patients with atopic dermatitis are much more prone to stimulation by allergen by way of IgE. This is a new concept that we’re exploring,” Dr. Kim said.
“We haven’t really known before what IgE does in atopic dermatitis, but it turns out that it may actually play a very important role in triggering acute flares of itch,” the dermatologist explained. “What’s been surprising is that the IgE activity is not mediated so much by mast cells, which are tissue-resident; the predominant means appears to be that IgE acts on basophils. That then creates release not of histamine, but of leukotriene C4, which is a very potent pruritogen. This may be responsible for those acute itch flares.”
Asked during an audience Q&A how allergen-specific IgE–mediated basophil activation might be targeted therapeutically in order to prevent acute-on-chronic itch flares in patients with AD, Dr. Kim mentioned two possibilities. One is treatment with potent anti-IgE agents, which to date have not been adequately tested for their antipruritic prowess in AD.
“Also, there’s another molecule that seems to be relatively basophil-selective and -specific that’s just been discovered by my colleague Xinzhong Dong at Johns Hopkins University [in Baltimore] – called MRGPRX2 – that may actually be a potentially viable way to go after basophils, maybe even by depleting them if you had an antibody against that,” Dr. Kim said. He was a coinvestigator in Dr. Dong’s recent study characterizing MRGPRX2, the mast-cell-expressed Mas-related G-protein–coupled receptor activator.
Dr. Kim reported receiving research funding from Cara Therapeutics and LEO Pharma, holding a patent for the use of JAK inhibitors in chronic itch, and serving as a consultant to numerous pharmaceutical companies.
MedscapeLive and this news organization are owned by the same parent company.
Brian S. Kim, MD, said at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Recent years have brought enormous progress in understanding how chronic itch in patients with atopic dermatitis (AD) is mediated by type 2 cytokines, including interleukin-13, IL-4, and IL-31, as well as by Janus kinase (JAK) signaling. This has led to development of potent therapies targeting these mediators, including dupilumab (Dupixent) and the investigational agents tralokinumab, lebrikizumab, abrocitinib, upadacitinib, baricitinib, and the IL-31 inhibitor nemolizumab.
“This is now one of the most active areas in the field of dermatology,” observed Dr. Kim, a dermatologist and codirector of the Center for the Study of Itch and Sensory Disorders at Washington University in St. Louis.
He has figured prominently in this effort. He and his coinvestigators conducted translational studies in mouse models which unraveled key mechanisms by which the immune system responsible for skin inflammation in AD communicates with the nervous system to trigger the neural sensation of itch. He also led a phase 2 randomized trial in 307 patients with AD, which demonstrated that the investigational JAK1/JAK2 inhibitor ruxolitinib cream markedly improved itch within 36 hours, well before subsequent improvement in skin inflammation – and the topical JAK inhibitor did so with minimal systemic absorption.
Compared with chronic itch, much less research attention has been devoted to the phenomenon of acute itch flares superimposed upon the chronic itch of AD. These acute-on-chronic itch flares are a common feature of the disease. In a soon-to-be-published study of 159 AD patients in the placebo arm of a clinical trial, Dr. Kim and coinvestigators found that 26% exhibited a pattern of acute itch flares during the course of a single month. During the next month, 3.1% of patients under study went from an acute-on-chronic itch pattern in month 1 to a nonflare pattern, 20% went from a nonflare pattern in month 1 to acute itch flares in month 2, and 23% of the overall study population retained their pattern of acute itch flares through both months.
“This does not seem to be just a static phenotype, but rather these patients can evolve over time. And we think that this can be driven by allergen-specific IgE,” according to Dr. Kim.
Indeed, the investigators found that patients with allergen-specific IgE in their serum were roughly twice as likely to exhibit the acute-on-chronic itch flare pattern than those without allergen-specific IgE.
The classical thinking has been that IgE binds to its receptors on mast cells, causing mast cell degranulation and release of histamine and other itch-inducing molecules. Yet antihistamines have proven notoriously ineffective for the treatment of AD.
Circulating basophils capable of working their way into inflamed skin also have IgE receptors. Dr. Kim and colleagues have shown that allergen-specific IgE in mice binds to those receptors, causing the basophils to degenerate, releasing itch-promoting chemicals. They have subsequently carried over this work into the clinical arena.
“We’ve found that patients with atopic dermatitis have significantly higher expression of receptors for IgE in their basophils than in the basophils of healthy controls, indicating perhaps that the basophils in patients with atopic dermatitis are much more prone to stimulation by allergen by way of IgE. This is a new concept that we’re exploring,” Dr. Kim said.
“We haven’t really known before what IgE does in atopic dermatitis, but it turns out that it may actually play a very important role in triggering acute flares of itch,” the dermatologist explained. “What’s been surprising is that the IgE activity is not mediated so much by mast cells, which are tissue-resident; the predominant means appears to be that IgE acts on basophils. That then creates release not of histamine, but of leukotriene C4, which is a very potent pruritogen. This may be responsible for those acute itch flares.”
Asked during an audience Q&A how allergen-specific IgE–mediated basophil activation might be targeted therapeutically in order to prevent acute-on-chronic itch flares in patients with AD, Dr. Kim mentioned two possibilities. One is treatment with potent anti-IgE agents, which to date have not been adequately tested for their antipruritic prowess in AD.
“Also, there’s another molecule that seems to be relatively basophil-selective and -specific that’s just been discovered by my colleague Xinzhong Dong at Johns Hopkins University [in Baltimore] – called MRGPRX2 – that may actually be a potentially viable way to go after basophils, maybe even by depleting them if you had an antibody against that,” Dr. Kim said. He was a coinvestigator in Dr. Dong’s recent study characterizing MRGPRX2, the mast-cell-expressed Mas-related G-protein–coupled receptor activator.
Dr. Kim reported receiving research funding from Cara Therapeutics and LEO Pharma, holding a patent for the use of JAK inhibitors in chronic itch, and serving as a consultant to numerous pharmaceutical companies.
MedscapeLive and this news organization are owned by the same parent company.
Brian S. Kim, MD, said at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Recent years have brought enormous progress in understanding how chronic itch in patients with atopic dermatitis (AD) is mediated by type 2 cytokines, including interleukin-13, IL-4, and IL-31, as well as by Janus kinase (JAK) signaling. This has led to development of potent therapies targeting these mediators, including dupilumab (Dupixent) and the investigational agents tralokinumab, lebrikizumab, abrocitinib, upadacitinib, baricitinib, and the IL-31 inhibitor nemolizumab.
“This is now one of the most active areas in the field of dermatology,” observed Dr. Kim, a dermatologist and codirector of the Center for the Study of Itch and Sensory Disorders at Washington University in St. Louis.
He has figured prominently in this effort. He and his coinvestigators conducted translational studies in mouse models which unraveled key mechanisms by which the immune system responsible for skin inflammation in AD communicates with the nervous system to trigger the neural sensation of itch. He also led a phase 2 randomized trial in 307 patients with AD, which demonstrated that the investigational JAK1/JAK2 inhibitor ruxolitinib cream markedly improved itch within 36 hours, well before subsequent improvement in skin inflammation – and the topical JAK inhibitor did so with minimal systemic absorption.
Compared with chronic itch, much less research attention has been devoted to the phenomenon of acute itch flares superimposed upon the chronic itch of AD. These acute-on-chronic itch flares are a common feature of the disease. In a soon-to-be-published study of 159 AD patients in the placebo arm of a clinical trial, Dr. Kim and coinvestigators found that 26% exhibited a pattern of acute itch flares during the course of a single month. During the next month, 3.1% of patients under study went from an acute-on-chronic itch pattern in month 1 to a nonflare pattern, 20% went from a nonflare pattern in month 1 to acute itch flares in month 2, and 23% of the overall study population retained their pattern of acute itch flares through both months.
“This does not seem to be just a static phenotype, but rather these patients can evolve over time. And we think that this can be driven by allergen-specific IgE,” according to Dr. Kim.
Indeed, the investigators found that patients with allergen-specific IgE in their serum were roughly twice as likely to exhibit the acute-on-chronic itch flare pattern than those without allergen-specific IgE.
The classical thinking has been that IgE binds to its receptors on mast cells, causing mast cell degranulation and release of histamine and other itch-inducing molecules. Yet antihistamines have proven notoriously ineffective for the treatment of AD.
Circulating basophils capable of working their way into inflamed skin also have IgE receptors. Dr. Kim and colleagues have shown that allergen-specific IgE in mice binds to those receptors, causing the basophils to degenerate, releasing itch-promoting chemicals. They have subsequently carried over this work into the clinical arena.
“We’ve found that patients with atopic dermatitis have significantly higher expression of receptors for IgE in their basophils than in the basophils of healthy controls, indicating perhaps that the basophils in patients with atopic dermatitis are much more prone to stimulation by allergen by way of IgE. This is a new concept that we’re exploring,” Dr. Kim said.
“We haven’t really known before what IgE does in atopic dermatitis, but it turns out that it may actually play a very important role in triggering acute flares of itch,” the dermatologist explained. “What’s been surprising is that the IgE activity is not mediated so much by mast cells, which are tissue-resident; the predominant means appears to be that IgE acts on basophils. That then creates release not of histamine, but of leukotriene C4, which is a very potent pruritogen. This may be responsible for those acute itch flares.”
Asked during an audience Q&A how allergen-specific IgE–mediated basophil activation might be targeted therapeutically in order to prevent acute-on-chronic itch flares in patients with AD, Dr. Kim mentioned two possibilities. One is treatment with potent anti-IgE agents, which to date have not been adequately tested for their antipruritic prowess in AD.
“Also, there’s another molecule that seems to be relatively basophil-selective and -specific that’s just been discovered by my colleague Xinzhong Dong at Johns Hopkins University [in Baltimore] – called MRGPRX2 – that may actually be a potentially viable way to go after basophils, maybe even by depleting them if you had an antibody against that,” Dr. Kim said. He was a coinvestigator in Dr. Dong’s recent study characterizing MRGPRX2, the mast-cell-expressed Mas-related G-protein–coupled receptor activator.
Dr. Kim reported receiving research funding from Cara Therapeutics and LEO Pharma, holding a patent for the use of JAK inhibitors in chronic itch, and serving as a consultant to numerous pharmaceutical companies.
MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Novel topical acne combo hits marks in phase 3 trials
A novel proprietary James Del Rosso, MD, reported at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Sol-Gel Technologies, the Israeli company developing the fixed-dose cream, called Twyneo, has applied to the Food and Drug Administration for marketing approval.
The product combines two workhorse topical agents for the treatment of acne, which are ordinarily incompatible, since benzoyl peroxide degrades tretinoin and reduces its effectiveness. The company’s silica-based microencapsulation technology overcomes that obstacle, explained Dr. Del Rosso, a dermatologist at JDR Research in Las Vegas.
The two identical phase 3, randomized, double-blind, vehicle-controlled clinical trials included a total of 858 patients ages 9 years and older with moderate to severe acne enrolled at 63 U.S. sites. Participants were randomized 2:1 to once-daily application of Twyneo or its vehicle cream for 12 weeks.
In one trial, the coprimary endpoint of at least a two-grade reduction and clear or almost clear skin at week 12 on a 5-point Investigator Global Assessment (IGA) scale was achieved in 38.5% of patients on Twyneo and 11.5% of controls. In the other trial, the IGA success rates were 25.4% and 14.7%. In both trials, the between-group difference was statistically significant.
The other coprimary endpoints were the absolute change from baseline in inflammatory and noninflammatory lesion counts. Inflammatory lesions were reduced by 21.6% and 16.2% in the active treatment arms of the two trials, compared with 14.8% and 14.1% reductions in the control groups. Noninflammatory lesion counts fell by 29.7% and 24.2% in patients on active treatment, versus 19.8% and 17.4% reductions in controls. The between-group differences were statistically significant.
Skin tolerability of Twyneo was “very good” and similar to vehicle, according to Dr. Del Rosso.
He reported receiving research funding from Sol-Gel, the studies’ sponsor.
MedscapeLive and this news organization are owned by the same parent company.
[email protected]
A novel proprietary James Del Rosso, MD, reported at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Sol-Gel Technologies, the Israeli company developing the fixed-dose cream, called Twyneo, has applied to the Food and Drug Administration for marketing approval.
The product combines two workhorse topical agents for the treatment of acne, which are ordinarily incompatible, since benzoyl peroxide degrades tretinoin and reduces its effectiveness. The company’s silica-based microencapsulation technology overcomes that obstacle, explained Dr. Del Rosso, a dermatologist at JDR Research in Las Vegas.
The two identical phase 3, randomized, double-blind, vehicle-controlled clinical trials included a total of 858 patients ages 9 years and older with moderate to severe acne enrolled at 63 U.S. sites. Participants were randomized 2:1 to once-daily application of Twyneo or its vehicle cream for 12 weeks.
In one trial, the coprimary endpoint of at least a two-grade reduction and clear or almost clear skin at week 12 on a 5-point Investigator Global Assessment (IGA) scale was achieved in 38.5% of patients on Twyneo and 11.5% of controls. In the other trial, the IGA success rates were 25.4% and 14.7%. In both trials, the between-group difference was statistically significant.
The other coprimary endpoints were the absolute change from baseline in inflammatory and noninflammatory lesion counts. Inflammatory lesions were reduced by 21.6% and 16.2% in the active treatment arms of the two trials, compared with 14.8% and 14.1% reductions in the control groups. Noninflammatory lesion counts fell by 29.7% and 24.2% in patients on active treatment, versus 19.8% and 17.4% reductions in controls. The between-group differences were statistically significant.
Skin tolerability of Twyneo was “very good” and similar to vehicle, according to Dr. Del Rosso.
He reported receiving research funding from Sol-Gel, the studies’ sponsor.
MedscapeLive and this news organization are owned by the same parent company.
[email protected]
A novel proprietary James Del Rosso, MD, reported at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Sol-Gel Technologies, the Israeli company developing the fixed-dose cream, called Twyneo, has applied to the Food and Drug Administration for marketing approval.
The product combines two workhorse topical agents for the treatment of acne, which are ordinarily incompatible, since benzoyl peroxide degrades tretinoin and reduces its effectiveness. The company’s silica-based microencapsulation technology overcomes that obstacle, explained Dr. Del Rosso, a dermatologist at JDR Research in Las Vegas.
The two identical phase 3, randomized, double-blind, vehicle-controlled clinical trials included a total of 858 patients ages 9 years and older with moderate to severe acne enrolled at 63 U.S. sites. Participants were randomized 2:1 to once-daily application of Twyneo or its vehicle cream for 12 weeks.
In one trial, the coprimary endpoint of at least a two-grade reduction and clear or almost clear skin at week 12 on a 5-point Investigator Global Assessment (IGA) scale was achieved in 38.5% of patients on Twyneo and 11.5% of controls. In the other trial, the IGA success rates were 25.4% and 14.7%. In both trials, the between-group difference was statistically significant.
The other coprimary endpoints were the absolute change from baseline in inflammatory and noninflammatory lesion counts. Inflammatory lesions were reduced by 21.6% and 16.2% in the active treatment arms of the two trials, compared with 14.8% and 14.1% reductions in the control groups. Noninflammatory lesion counts fell by 29.7% and 24.2% in patients on active treatment, versus 19.8% and 17.4% reductions in controls. The between-group differences were statistically significant.
Skin tolerability of Twyneo was “very good” and similar to vehicle, according to Dr. Del Rosso.
He reported receiving research funding from Sol-Gel, the studies’ sponsor.
MedscapeLive and this news organization are owned by the same parent company.
[email protected]
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Daily sunscreen use will prevent more melanoma deaths than early detection
The dramatic advances in targeted therapies for late-stage melanoma capture the headlines, but a recent Australian study quietly concluded that according to Laura Korb Ferris, MD, PhD, a dermatologist and director of clinical trials in the department of dermatology at the University of Pittsburgh.
“I think it’s really important that we recognize the importance of preventing skin cancer, and not just early detection, not just treatment of late disease,” Dr. Ferris said at a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medical Education.
She highlighted the Australian cost-effectiveness analysis, which used Markov modeling of data from two published population-based, randomized controlled trials carried out in Queensland, Australia.
The cost-effectiveness study compared the estimated long-term impact of three different approaches to control of melanoma: a primary prevention strategy, which basically consisted of promoting daily sunscreen use and other forms of sun protection; early detection through annual whole-body skin examinations by physicians starting at age 50; and no intervention. The analysis provided estimates of the number of cases of melanoma, deaths caused by melanoma, nonmelanoma skin cancers, and quality of life outcomes over the course of 30 years starting in 50-year-old men and women.
Primary prevention through sun protection was the clear winner, as shown by the results:
- A 44% reduction in the incidence of melanoma, compared with early detection via annual physician skin examinations.
- A 39% reduction in projected melanoma deaths compared with early detection, which in turn achieved only a 2% reduction when compared with no intervention.
- 27% fewer keratinocyte cancers excised than with annual skin examinations.
- A 21.7% reduction in societal costs, compared with an early-detection program.
Daily sunscreen use for primary prevention was also associated with a modest 0.1% increase in quality-adjusted life-years. “Prevention is low cost, low risk, and effective,” Dr. Ferris observed.
The investigators noted that, while residents of the Australian state of Queensland are mainly fair-skinned and confront high UV radiation levels throughout the year, somewhat limiting the generalizability of the study findings, the relationships between the costs of interventional strategies and their outcomes should be proportional in other countries.
True enough, but a strategy of annual skin examinations starting at age 50 years as modeled in the Australian study is not the most productive way to conduct a melanoma early-detection program, Dr. Ferris said. She noted that data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program show that the median age at diagnosis of melanoma in the United States is 65 years, while the median age at death caused by the malignancy is 71 years. That information is helpful in formulating strategies to improve early detection through more focused, higher-yield screening.
Case in point: European investigators have estimated that, by screening everyone age 50 years and older, 475 people need to be screened and an average of 19.6 lesions must be biopsied in order to detect one melanoma. But by reserving screening for those age 50 years and up who have any one of three risk factors – a personal history of melanoma, atypical nevi, or at least 40 common nevi – those numbers drop dramatically: 98 people need to be screened and 13.5 lesions biopsied to detect one melanoma. And by further narrowing the screened population to those age 65 years or older with any of the three risk factors, 63 seniors would need to be screened and 9.2 lesions excised to find one melanoma.
Total-body skin examinations are time-consuming for dermatologists. In a recent U.S. study, investigators determined that the additional face-to-face time required per skin cancer detected by doing a total-body skin exam in adults who present to a dermatologist for another reason is 4.5 hours. And that’s just the time involved in detecting any type of skin cancer.
“To get that number for melanoma, multiply by 15 to 20,” Dr. Ferris said.
The investigators also determined that, for each decade of advancing age and increment in lighter skin phototype, the number-needed-to-examine in order to identify one skin cancer of any type decreased.
“By focusing on patients who are older and have fair skin types we can get that time down to about 1 hour,” commented Dr. Ferris, who penned an editorial perspective on the study.
While many dermatologists recommend that people with a high common nevus count undergo frequent screening for melanoma because they are at particularly high risk for invasive disease, a couple of recent studies challenge that notion, she pointed out. One was a retrospective study of 326 consecutive new melanoma patients which found that patients with a higher nevus count had thinner melanomas and a greater likelihood of in situ melanoma. Patients who presented with invasive melanoma had a mean total nevus count of 31.5 lesions, while those with in situ melanoma averaged 57.2 nevi. Each additional nevus was associated with a 4% reduction in the likelihood of invasive melanoma, independent of age and sex.
The other study included 566 newly diagnosed melanoma patients in two U.S. centers. Among the 56% of patients who were younger than 60 years, those who had more than 50 total nevi were 68% less likely to have a thick melanoma in a logistic regression analysis that controlled for demographic factors, as well as anatomic location of the melanoma, histologic subtype, and skin cancer screening frequency. In contrast, younger patients with more than 5 atypical nevi were 2.43-fold more likely to have thicker melanomas than were those with no such lesions. The lesson, according to the investigators, is that total nevus count isn’t a reliable determinant of a patient’s risk status or the need for skin examinations.
Dr. Ferris reported no financial conflicts of interest regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
The dramatic advances in targeted therapies for late-stage melanoma capture the headlines, but a recent Australian study quietly concluded that according to Laura Korb Ferris, MD, PhD, a dermatologist and director of clinical trials in the department of dermatology at the University of Pittsburgh.
“I think it’s really important that we recognize the importance of preventing skin cancer, and not just early detection, not just treatment of late disease,” Dr. Ferris said at a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medical Education.
She highlighted the Australian cost-effectiveness analysis, which used Markov modeling of data from two published population-based, randomized controlled trials carried out in Queensland, Australia.
The cost-effectiveness study compared the estimated long-term impact of three different approaches to control of melanoma: a primary prevention strategy, which basically consisted of promoting daily sunscreen use and other forms of sun protection; early detection through annual whole-body skin examinations by physicians starting at age 50; and no intervention. The analysis provided estimates of the number of cases of melanoma, deaths caused by melanoma, nonmelanoma skin cancers, and quality of life outcomes over the course of 30 years starting in 50-year-old men and women.
Primary prevention through sun protection was the clear winner, as shown by the results:
- A 44% reduction in the incidence of melanoma, compared with early detection via annual physician skin examinations.
- A 39% reduction in projected melanoma deaths compared with early detection, which in turn achieved only a 2% reduction when compared with no intervention.
- 27% fewer keratinocyte cancers excised than with annual skin examinations.
- A 21.7% reduction in societal costs, compared with an early-detection program.
Daily sunscreen use for primary prevention was also associated with a modest 0.1% increase in quality-adjusted life-years. “Prevention is low cost, low risk, and effective,” Dr. Ferris observed.
The investigators noted that, while residents of the Australian state of Queensland are mainly fair-skinned and confront high UV radiation levels throughout the year, somewhat limiting the generalizability of the study findings, the relationships between the costs of interventional strategies and their outcomes should be proportional in other countries.
True enough, but a strategy of annual skin examinations starting at age 50 years as modeled in the Australian study is not the most productive way to conduct a melanoma early-detection program, Dr. Ferris said. She noted that data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program show that the median age at diagnosis of melanoma in the United States is 65 years, while the median age at death caused by the malignancy is 71 years. That information is helpful in formulating strategies to improve early detection through more focused, higher-yield screening.
Case in point: European investigators have estimated that, by screening everyone age 50 years and older, 475 people need to be screened and an average of 19.6 lesions must be biopsied in order to detect one melanoma. But by reserving screening for those age 50 years and up who have any one of three risk factors – a personal history of melanoma, atypical nevi, or at least 40 common nevi – those numbers drop dramatically: 98 people need to be screened and 13.5 lesions biopsied to detect one melanoma. And by further narrowing the screened population to those age 65 years or older with any of the three risk factors, 63 seniors would need to be screened and 9.2 lesions excised to find one melanoma.
Total-body skin examinations are time-consuming for dermatologists. In a recent U.S. study, investigators determined that the additional face-to-face time required per skin cancer detected by doing a total-body skin exam in adults who present to a dermatologist for another reason is 4.5 hours. And that’s just the time involved in detecting any type of skin cancer.
“To get that number for melanoma, multiply by 15 to 20,” Dr. Ferris said.
The investigators also determined that, for each decade of advancing age and increment in lighter skin phototype, the number-needed-to-examine in order to identify one skin cancer of any type decreased.
“By focusing on patients who are older and have fair skin types we can get that time down to about 1 hour,” commented Dr. Ferris, who penned an editorial perspective on the study.
While many dermatologists recommend that people with a high common nevus count undergo frequent screening for melanoma because they are at particularly high risk for invasive disease, a couple of recent studies challenge that notion, she pointed out. One was a retrospective study of 326 consecutive new melanoma patients which found that patients with a higher nevus count had thinner melanomas and a greater likelihood of in situ melanoma. Patients who presented with invasive melanoma had a mean total nevus count of 31.5 lesions, while those with in situ melanoma averaged 57.2 nevi. Each additional nevus was associated with a 4% reduction in the likelihood of invasive melanoma, independent of age and sex.
The other study included 566 newly diagnosed melanoma patients in two U.S. centers. Among the 56% of patients who were younger than 60 years, those who had more than 50 total nevi were 68% less likely to have a thick melanoma in a logistic regression analysis that controlled for demographic factors, as well as anatomic location of the melanoma, histologic subtype, and skin cancer screening frequency. In contrast, younger patients with more than 5 atypical nevi were 2.43-fold more likely to have thicker melanomas than were those with no such lesions. The lesson, according to the investigators, is that total nevus count isn’t a reliable determinant of a patient’s risk status or the need for skin examinations.
Dr. Ferris reported no financial conflicts of interest regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
The dramatic advances in targeted therapies for late-stage melanoma capture the headlines, but a recent Australian study quietly concluded that according to Laura Korb Ferris, MD, PhD, a dermatologist and director of clinical trials in the department of dermatology at the University of Pittsburgh.
“I think it’s really important that we recognize the importance of preventing skin cancer, and not just early detection, not just treatment of late disease,” Dr. Ferris said at a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medical Education.
She highlighted the Australian cost-effectiveness analysis, which used Markov modeling of data from two published population-based, randomized controlled trials carried out in Queensland, Australia.
The cost-effectiveness study compared the estimated long-term impact of three different approaches to control of melanoma: a primary prevention strategy, which basically consisted of promoting daily sunscreen use and other forms of sun protection; early detection through annual whole-body skin examinations by physicians starting at age 50; and no intervention. The analysis provided estimates of the number of cases of melanoma, deaths caused by melanoma, nonmelanoma skin cancers, and quality of life outcomes over the course of 30 years starting in 50-year-old men and women.
Primary prevention through sun protection was the clear winner, as shown by the results:
- A 44% reduction in the incidence of melanoma, compared with early detection via annual physician skin examinations.
- A 39% reduction in projected melanoma deaths compared with early detection, which in turn achieved only a 2% reduction when compared with no intervention.
- 27% fewer keratinocyte cancers excised than with annual skin examinations.
- A 21.7% reduction in societal costs, compared with an early-detection program.
Daily sunscreen use for primary prevention was also associated with a modest 0.1% increase in quality-adjusted life-years. “Prevention is low cost, low risk, and effective,” Dr. Ferris observed.
The investigators noted that, while residents of the Australian state of Queensland are mainly fair-skinned and confront high UV radiation levels throughout the year, somewhat limiting the generalizability of the study findings, the relationships between the costs of interventional strategies and their outcomes should be proportional in other countries.
True enough, but a strategy of annual skin examinations starting at age 50 years as modeled in the Australian study is not the most productive way to conduct a melanoma early-detection program, Dr. Ferris said. She noted that data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program show that the median age at diagnosis of melanoma in the United States is 65 years, while the median age at death caused by the malignancy is 71 years. That information is helpful in formulating strategies to improve early detection through more focused, higher-yield screening.
Case in point: European investigators have estimated that, by screening everyone age 50 years and older, 475 people need to be screened and an average of 19.6 lesions must be biopsied in order to detect one melanoma. But by reserving screening for those age 50 years and up who have any one of three risk factors – a personal history of melanoma, atypical nevi, or at least 40 common nevi – those numbers drop dramatically: 98 people need to be screened and 13.5 lesions biopsied to detect one melanoma. And by further narrowing the screened population to those age 65 years or older with any of the three risk factors, 63 seniors would need to be screened and 9.2 lesions excised to find one melanoma.
Total-body skin examinations are time-consuming for dermatologists. In a recent U.S. study, investigators determined that the additional face-to-face time required per skin cancer detected by doing a total-body skin exam in adults who present to a dermatologist for another reason is 4.5 hours. And that’s just the time involved in detecting any type of skin cancer.
“To get that number for melanoma, multiply by 15 to 20,” Dr. Ferris said.
The investigators also determined that, for each decade of advancing age and increment in lighter skin phototype, the number-needed-to-examine in order to identify one skin cancer of any type decreased.
“By focusing on patients who are older and have fair skin types we can get that time down to about 1 hour,” commented Dr. Ferris, who penned an editorial perspective on the study.
While many dermatologists recommend that people with a high common nevus count undergo frequent screening for melanoma because they are at particularly high risk for invasive disease, a couple of recent studies challenge that notion, she pointed out. One was a retrospective study of 326 consecutive new melanoma patients which found that patients with a higher nevus count had thinner melanomas and a greater likelihood of in situ melanoma. Patients who presented with invasive melanoma had a mean total nevus count of 31.5 lesions, while those with in situ melanoma averaged 57.2 nevi. Each additional nevus was associated with a 4% reduction in the likelihood of invasive melanoma, independent of age and sex.
The other study included 566 newly diagnosed melanoma patients in two U.S. centers. Among the 56% of patients who were younger than 60 years, those who had more than 50 total nevi were 68% less likely to have a thick melanoma in a logistic regression analysis that controlled for demographic factors, as well as anatomic location of the melanoma, histologic subtype, and skin cancer screening frequency. In contrast, younger patients with more than 5 atypical nevi were 2.43-fold more likely to have thicker melanomas than were those with no such lesions. The lesson, according to the investigators, is that total nevus count isn’t a reliable determinant of a patient’s risk status or the need for skin examinations.
Dr. Ferris reported no financial conflicts of interest regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
FROM THE CUTANEOUS MALIGNANCIES FORUM